User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Does vitamin D benefit only those who are deficient?
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
U.S. obesity rates soar in early adulthood
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.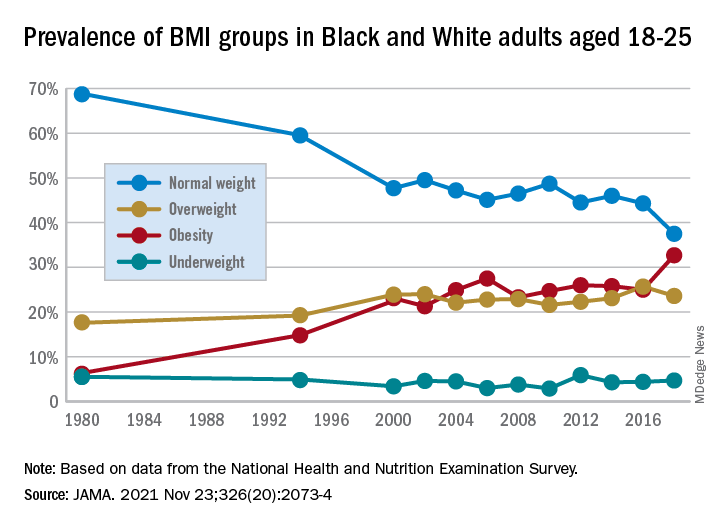
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fueling an ‘already raging fire’: Fifth COVID surge approaches
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
Swell in off-label antipsychotic prescribing ‘not harmless’
A growing trend of off-label, low-dose antipsychotic prescribing to treat disorders such as anxiety and insomnia has been tied to an increased risk of cardiometabolic death, new research shows.
Investigators studied data from large Swedish registries on over 420,000 individuals without previous psychotic, bipolar, or cardiometabolic disorders and found that off-label treatment with olanzapine or quetiapine for 6 to 12 months – even at a low dose – was associated with an almost twofold higher risk of cardiometabolic mortality, compared to no treatment. The risk remained elevated after 12 months, but the finding was not deemed significant.
“Clinicians should be made aware that low-dose treatment with these drugs is probably not a harmless choice for insomnia and anxiety, and while they have the benefit of not being addictive and [are] seemingly effective, they might come at a cost of shortening patients’ life span,” study investigator Jonas Berge, MD, PhD, associate professor and resident psychiatrist, Lund University, Sweden, said in an interview.
“Clinicians should take this information into account when prescribing the drugs and also monitor the patients with regular physical examinations and blood tests in the same way as when treating patients with psychosis with higher doses of these drugs,” he said.
The study was published online Nov. 9 in the Journal of Psychiatric Research.
A growing trend
Use of low-dose antipsychotics to treat a variety of psychiatric and behavioral disturbances, including anxiety, insomnia, and agitation, has “surged in popularity,” the authors wrote.
Quetiapine and olanzapine “rank as two of the most frequently prescribed second-generation antipsychotics and, next to clozapine, are considered to exhort the highest risk for cardiometabolic sequelae, including components of metabolic syndrome,” they added.
Previous research examining the association between second-generation antipsychotics and placebo has either not focused on cardiometabolic-specific causes or has examined only cohorts with severe mental illness, so those findings “do not necessarily generalize to others treated off-label,” they noted.
“The motivation for the study came from my work as a psychiatrist, in which I’ve noticed that the off-label use of these medications [olanzapine and quetiapine] for anxiety and insomnia seems highly prevalent, and that many patients seem to gain a lot of weight, despite low doses,” Dr. Berge said.
There is “evidence to suggest that clinicians may underappreciate cardiometabolic risks owing to antipsychotic treatment, as routine screening is often incomplete or inconsistent,” the authors noted.
“To do a risk-benefit analysis of these drugs in low doses, the risks involved – as well as the effects, of course – need to be studied,” Dr. Berge stated.
To investigate the question, the researchers turned to three large cross-linked Swedish registers: the National Patient Register, containing demographic and medical data; the Prescribed Drug Register; and the Cause of Death Register.
They identified all individuals aged 18 years and older with at least one psychiatric visit (inpatient or outpatient) between July 1, 2006, and Dec. 31, 2016, to see how many were prescribed low-dose olanzapine or quetiapine (defined as ≤ 5 mg/day of olanzapine or olanzapine equivalent [OE]), which was used as a proxy marker for off-label treatment, since this dose is considered subtherapeutic for severe mental illness.
They calculated two time-dependent variables – cumulative dose and past annual average dose – and then used those to compute three different exposure valuables: those treated with low-dose OE; cumulative exposure (i.e., period treated with an average 5 mg/day); and a continuous variable “corresponding to each year exposed OE 5 mg/day.”
The primary outcome was set as mortality from cardiometabolic-related disorders, while secondary outcomes were disease-specific and all-cause mortality.
‘Weak’ association
The final cohort consisted of 428,525 individuals (mean [SD] age, 36.8 [15.4] years, 52.7% female) at baseline, with observation taking place over a mean of 4.8 years [range, 1 day to 10.5 years]) or a total of over 2 million (2,062,241) person-years.
Of the cohort, 4.3% (n = 18,317) had at least two prescriptions for either olanzapine or quetiapine (although subsequently, 86.5% were censored for exceeding the average OE dose of 5 mg/day).
By the end of the study, 3.1% of the cohort had died during the observation time, and of these, 69.5% were from disease-specific causes, while close to one-fifth (19.5%) were from cardiometabolic-specific causes.
On the whole, treatment status (i.e., treated vs. untreated) was not significantly associated with cardiometabolic mortality (adjusted hazard ratio [HR], .86 [95% confidence interval, 0.64-1.15]; P = .307).
Compared to no treatment, treatment with olanzapine or quetiapine for less than 6 months was significantly associated with a reduced risk of cardiovascular mortality (adjusted HR, .56 [.37 – .87]; P = .010). On the other hand, treatment for 6-12 months was significantly associated with an almost twofold increased risk (adjusted HR, 1.89 [1.22-2.92]; P = .004). The increased risk continued beyond 12 months, although the difference no longer remained significant.
“In the subgroup analysis consisting of individuals who had ever been treated with olanzapine/quetiapine, starting at the date of their first prescription, the hazard for cardiometabolic mortality increased significantly by 45% (6%-99%; P = .019) for every year exposed to an average 5 mg/day,” the authors reported.
The authors concluded that the association between low-dose olanzapine/quetiapine treatment and cardiometabolic mortality was present, but “weak.”
The hazard for disease-specific mortality also significantly increased with each year exposed to an average of 5 mg/day of OE (HR, 1.24 [1.03-1.50]; P = .026).
Treatment status similarly was associated with all-cause mortality (HR, 1.16 [1.03-1.30]; P = .012), although the increased hazard for all-cause mortality with each year of exposure was not considered significant.
“The findings of this study are consistent with the hypothesis that continuous low-dose treatment with these drugs is associated with increased cardiometabolic mortality, but ,” Dr. Berge said.
Seek alternatives
Commenting on the study for this news organization, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, called it a “timely paper” and “an important concept [because] low-doses of these antipsychotics are frequently prescribed across America and there has been less data on the safety [of these antipsychotics at lower doses].”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, said that this “important report reminds us that there are metabolic safety concerns, even at low doses, where these medications are often used off label.”
He advised clinicians to “seek alternatives, and alternatives that are on-label, for conditions like anxiety and sleep disturbances.”
This work was supported by the South Region Board ALF, Sweden. Dr. Berge and coauthors have disclosed no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; and speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and AbbVie. Dr. McIntyre is CEO of AltMed.
A version of this article first appeared on Medscape.com.
A growing trend of off-label, low-dose antipsychotic prescribing to treat disorders such as anxiety and insomnia has been tied to an increased risk of cardiometabolic death, new research shows.
Investigators studied data from large Swedish registries on over 420,000 individuals without previous psychotic, bipolar, or cardiometabolic disorders and found that off-label treatment with olanzapine or quetiapine for 6 to 12 months – even at a low dose – was associated with an almost twofold higher risk of cardiometabolic mortality, compared to no treatment. The risk remained elevated after 12 months, but the finding was not deemed significant.
“Clinicians should be made aware that low-dose treatment with these drugs is probably not a harmless choice for insomnia and anxiety, and while they have the benefit of not being addictive and [are] seemingly effective, they might come at a cost of shortening patients’ life span,” study investigator Jonas Berge, MD, PhD, associate professor and resident psychiatrist, Lund University, Sweden, said in an interview.
“Clinicians should take this information into account when prescribing the drugs and also monitor the patients with regular physical examinations and blood tests in the same way as when treating patients with psychosis with higher doses of these drugs,” he said.
The study was published online Nov. 9 in the Journal of Psychiatric Research.
A growing trend
Use of low-dose antipsychotics to treat a variety of psychiatric and behavioral disturbances, including anxiety, insomnia, and agitation, has “surged in popularity,” the authors wrote.
Quetiapine and olanzapine “rank as two of the most frequently prescribed second-generation antipsychotics and, next to clozapine, are considered to exhort the highest risk for cardiometabolic sequelae, including components of metabolic syndrome,” they added.
Previous research examining the association between second-generation antipsychotics and placebo has either not focused on cardiometabolic-specific causes or has examined only cohorts with severe mental illness, so those findings “do not necessarily generalize to others treated off-label,” they noted.
“The motivation for the study came from my work as a psychiatrist, in which I’ve noticed that the off-label use of these medications [olanzapine and quetiapine] for anxiety and insomnia seems highly prevalent, and that many patients seem to gain a lot of weight, despite low doses,” Dr. Berge said.
There is “evidence to suggest that clinicians may underappreciate cardiometabolic risks owing to antipsychotic treatment, as routine screening is often incomplete or inconsistent,” the authors noted.
“To do a risk-benefit analysis of these drugs in low doses, the risks involved – as well as the effects, of course – need to be studied,” Dr. Berge stated.
To investigate the question, the researchers turned to three large cross-linked Swedish registers: the National Patient Register, containing demographic and medical data; the Prescribed Drug Register; and the Cause of Death Register.
They identified all individuals aged 18 years and older with at least one psychiatric visit (inpatient or outpatient) between July 1, 2006, and Dec. 31, 2016, to see how many were prescribed low-dose olanzapine or quetiapine (defined as ≤ 5 mg/day of olanzapine or olanzapine equivalent [OE]), which was used as a proxy marker for off-label treatment, since this dose is considered subtherapeutic for severe mental illness.
They calculated two time-dependent variables – cumulative dose and past annual average dose – and then used those to compute three different exposure valuables: those treated with low-dose OE; cumulative exposure (i.e., period treated with an average 5 mg/day); and a continuous variable “corresponding to each year exposed OE 5 mg/day.”
The primary outcome was set as mortality from cardiometabolic-related disorders, while secondary outcomes were disease-specific and all-cause mortality.
‘Weak’ association
The final cohort consisted of 428,525 individuals (mean [SD] age, 36.8 [15.4] years, 52.7% female) at baseline, with observation taking place over a mean of 4.8 years [range, 1 day to 10.5 years]) or a total of over 2 million (2,062,241) person-years.
Of the cohort, 4.3% (n = 18,317) had at least two prescriptions for either olanzapine or quetiapine (although subsequently, 86.5% were censored for exceeding the average OE dose of 5 mg/day).
By the end of the study, 3.1% of the cohort had died during the observation time, and of these, 69.5% were from disease-specific causes, while close to one-fifth (19.5%) were from cardiometabolic-specific causes.
On the whole, treatment status (i.e., treated vs. untreated) was not significantly associated with cardiometabolic mortality (adjusted hazard ratio [HR], .86 [95% confidence interval, 0.64-1.15]; P = .307).
Compared to no treatment, treatment with olanzapine or quetiapine for less than 6 months was significantly associated with a reduced risk of cardiovascular mortality (adjusted HR, .56 [.37 – .87]; P = .010). On the other hand, treatment for 6-12 months was significantly associated with an almost twofold increased risk (adjusted HR, 1.89 [1.22-2.92]; P = .004). The increased risk continued beyond 12 months, although the difference no longer remained significant.
“In the subgroup analysis consisting of individuals who had ever been treated with olanzapine/quetiapine, starting at the date of their first prescription, the hazard for cardiometabolic mortality increased significantly by 45% (6%-99%; P = .019) for every year exposed to an average 5 mg/day,” the authors reported.
The authors concluded that the association between low-dose olanzapine/quetiapine treatment and cardiometabolic mortality was present, but “weak.”
The hazard for disease-specific mortality also significantly increased with each year exposed to an average of 5 mg/day of OE (HR, 1.24 [1.03-1.50]; P = .026).
Treatment status similarly was associated with all-cause mortality (HR, 1.16 [1.03-1.30]; P = .012), although the increased hazard for all-cause mortality with each year of exposure was not considered significant.
“The findings of this study are consistent with the hypothesis that continuous low-dose treatment with these drugs is associated with increased cardiometabolic mortality, but ,” Dr. Berge said.
Seek alternatives
Commenting on the study for this news organization, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, called it a “timely paper” and “an important concept [because] low-doses of these antipsychotics are frequently prescribed across America and there has been less data on the safety [of these antipsychotics at lower doses].”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, said that this “important report reminds us that there are metabolic safety concerns, even at low doses, where these medications are often used off label.”
He advised clinicians to “seek alternatives, and alternatives that are on-label, for conditions like anxiety and sleep disturbances.”
This work was supported by the South Region Board ALF, Sweden. Dr. Berge and coauthors have disclosed no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; and speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and AbbVie. Dr. McIntyre is CEO of AltMed.
A version of this article first appeared on Medscape.com.
A growing trend of off-label, low-dose antipsychotic prescribing to treat disorders such as anxiety and insomnia has been tied to an increased risk of cardiometabolic death, new research shows.
Investigators studied data from large Swedish registries on over 420,000 individuals without previous psychotic, bipolar, or cardiometabolic disorders and found that off-label treatment with olanzapine or quetiapine for 6 to 12 months – even at a low dose – was associated with an almost twofold higher risk of cardiometabolic mortality, compared to no treatment. The risk remained elevated after 12 months, but the finding was not deemed significant.
“Clinicians should be made aware that low-dose treatment with these drugs is probably not a harmless choice for insomnia and anxiety, and while they have the benefit of not being addictive and [are] seemingly effective, they might come at a cost of shortening patients’ life span,” study investigator Jonas Berge, MD, PhD, associate professor and resident psychiatrist, Lund University, Sweden, said in an interview.
“Clinicians should take this information into account when prescribing the drugs and also monitor the patients with regular physical examinations and blood tests in the same way as when treating patients with psychosis with higher doses of these drugs,” he said.
The study was published online Nov. 9 in the Journal of Psychiatric Research.
A growing trend
Use of low-dose antipsychotics to treat a variety of psychiatric and behavioral disturbances, including anxiety, insomnia, and agitation, has “surged in popularity,” the authors wrote.
Quetiapine and olanzapine “rank as two of the most frequently prescribed second-generation antipsychotics and, next to clozapine, are considered to exhort the highest risk for cardiometabolic sequelae, including components of metabolic syndrome,” they added.
Previous research examining the association between second-generation antipsychotics and placebo has either not focused on cardiometabolic-specific causes or has examined only cohorts with severe mental illness, so those findings “do not necessarily generalize to others treated off-label,” they noted.
“The motivation for the study came from my work as a psychiatrist, in which I’ve noticed that the off-label use of these medications [olanzapine and quetiapine] for anxiety and insomnia seems highly prevalent, and that many patients seem to gain a lot of weight, despite low doses,” Dr. Berge said.
There is “evidence to suggest that clinicians may underappreciate cardiometabolic risks owing to antipsychotic treatment, as routine screening is often incomplete or inconsistent,” the authors noted.
“To do a risk-benefit analysis of these drugs in low doses, the risks involved – as well as the effects, of course – need to be studied,” Dr. Berge stated.
To investigate the question, the researchers turned to three large cross-linked Swedish registers: the National Patient Register, containing demographic and medical data; the Prescribed Drug Register; and the Cause of Death Register.
They identified all individuals aged 18 years and older with at least one psychiatric visit (inpatient or outpatient) between July 1, 2006, and Dec. 31, 2016, to see how many were prescribed low-dose olanzapine or quetiapine (defined as ≤ 5 mg/day of olanzapine or olanzapine equivalent [OE]), which was used as a proxy marker for off-label treatment, since this dose is considered subtherapeutic for severe mental illness.
They calculated two time-dependent variables – cumulative dose and past annual average dose – and then used those to compute three different exposure valuables: those treated with low-dose OE; cumulative exposure (i.e., period treated with an average 5 mg/day); and a continuous variable “corresponding to each year exposed OE 5 mg/day.”
The primary outcome was set as mortality from cardiometabolic-related disorders, while secondary outcomes were disease-specific and all-cause mortality.
‘Weak’ association
The final cohort consisted of 428,525 individuals (mean [SD] age, 36.8 [15.4] years, 52.7% female) at baseline, with observation taking place over a mean of 4.8 years [range, 1 day to 10.5 years]) or a total of over 2 million (2,062,241) person-years.
Of the cohort, 4.3% (n = 18,317) had at least two prescriptions for either olanzapine or quetiapine (although subsequently, 86.5% were censored for exceeding the average OE dose of 5 mg/day).
By the end of the study, 3.1% of the cohort had died during the observation time, and of these, 69.5% were from disease-specific causes, while close to one-fifth (19.5%) were from cardiometabolic-specific causes.
On the whole, treatment status (i.e., treated vs. untreated) was not significantly associated with cardiometabolic mortality (adjusted hazard ratio [HR], .86 [95% confidence interval, 0.64-1.15]; P = .307).
Compared to no treatment, treatment with olanzapine or quetiapine for less than 6 months was significantly associated with a reduced risk of cardiovascular mortality (adjusted HR, .56 [.37 – .87]; P = .010). On the other hand, treatment for 6-12 months was significantly associated with an almost twofold increased risk (adjusted HR, 1.89 [1.22-2.92]; P = .004). The increased risk continued beyond 12 months, although the difference no longer remained significant.
“In the subgroup analysis consisting of individuals who had ever been treated with olanzapine/quetiapine, starting at the date of their first prescription, the hazard for cardiometabolic mortality increased significantly by 45% (6%-99%; P = .019) for every year exposed to an average 5 mg/day,” the authors reported.
The authors concluded that the association between low-dose olanzapine/quetiapine treatment and cardiometabolic mortality was present, but “weak.”
The hazard for disease-specific mortality also significantly increased with each year exposed to an average of 5 mg/day of OE (HR, 1.24 [1.03-1.50]; P = .026).
Treatment status similarly was associated with all-cause mortality (HR, 1.16 [1.03-1.30]; P = .012), although the increased hazard for all-cause mortality with each year of exposure was not considered significant.
“The findings of this study are consistent with the hypothesis that continuous low-dose treatment with these drugs is associated with increased cardiometabolic mortality, but ,” Dr. Berge said.
Seek alternatives
Commenting on the study for this news organization, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, called it a “timely paper” and “an important concept [because] low-doses of these antipsychotics are frequently prescribed across America and there has been less data on the safety [of these antipsychotics at lower doses].”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, said that this “important report reminds us that there are metabolic safety concerns, even at low doses, where these medications are often used off label.”
He advised clinicians to “seek alternatives, and alternatives that are on-label, for conditions like anxiety and sleep disturbances.”
This work was supported by the South Region Board ALF, Sweden. Dr. Berge and coauthors have disclosed no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; and speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and AbbVie. Dr. McIntyre is CEO of AltMed.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Firefighters’ blood pressure surges when they are called to action
In response to a 911 alert or page, firefighters’ systolic and diastolic blood pressure surges and their heart rate accelerates, with a similar response whether the call is for a fire or medical emergency, a small study suggests.
On average, the 41 firefighters monitored in the study, who were middle-aged and overweight, had a 9% increase in systolic blood pressure when called to a fire, a 9% increase in diastolic blood pressure when called to a medical emergency, and a 16% increase in heart rate for both types of calls.
Senior study author Deborah Feairheller, PhD, presented these results at the virtual American Heart Association scientific sessions.
Firefighters have a higher prevalence of cardiovascular disease (CVD) than that of the general population, explained Dr. Feairheller, director of the Hypertension and Endothelial Function with Aerobic and Resistance Training (HEART) Lab and clinical associate professor of kinesiology at the University of New Hampshire, Durham.
More than 50% of firefighter deaths in the line of duty are from CVD, she noted. Moreover, almost 75% of firefighters have hypertension and fewer than 25% have it under control.
The study findings show that all emergency and first responders “should know what their typical blood pressure level is and be aware of how it fluctuates,” Dr. Feairheller said in a press release from the AHA. “Most important, if they have high blood pressure, they should make sure it is well controlled,” she said.
“I really hope that fire departments everywhere see these data, rise to the occasion, and advocate for BP awareness in their crews,” Dr. Feairheller, a volunteer firefighter, said in an interview.
“I do think this has value to any occupation that wears a pager,” she added. “Clinicians, physicians, other emergency responders, all of those occupations are stressful and could place people at risk if they have undiagnosed or uncontrolled hypertension.”
Invited to comment, Comilla Sasson, MD, PhD, an emergency department physician who was not involved with this research, said in an interview that she saw parallels between stress experienced by firefighters and, for example, emergency department physicians.
The transient increases in BP, both systolic and diastolic, along with the heart rate are likely due to the body’s natural fight or flight response to an emergency call, including increases in epinephrine and cortisol, said Dr. Sasson, vice president of science and innovation for emergency cardiovascular care at the American Heart Association.
“The thing that is most interesting to me,” said Dr. Sasson, who can be subject to a series of high-stress situations on a shift, such as multiple trauma victims, a stroke victim, or a person in cardiac arrest, is “what is the cumulative impact of this over time?”
She said she wonders if “having to be ‘ready to go’ at any time, along with disruptions in sleep/wake schedules, and poorer eating and working-out habits when you are on shift, has long-term sequelae on the body.”
Stress-related surges in blood pressure “could be a reason for worse health outcomes in this group,” Dr. Sasson said, adding that this needs to be investigated further.
Firefighters with high normal BP, high BMI
Dr. Feairheller and colleagues recruited 41 volunteer and employee firefighters from suburban Philadelphia and Dover, N.H.
On average, the 37 men and 4 women had a mean age of 41 years, had been working as firefighters for 16.9 years, and had a mean body mass index of 30.3 kg/m2.
They wore ambulatory blood pressure monitors during an on-call work shift for at least 12 consecutive hours.
In addition to the automatic readings, the participants were instructed to prompt the machine to take a reading whenever a pager or emergency call sounded or when they felt they were entering a stressful situation.
Over the 12-hour shift, on average, participants had a blood pressure of 131/79.3 mm Hg and a heart rate of 75.7 bpm.
When they were alerted go to a fire, their blood pressure surged by 19.2/10.5 mm Hg, and their heart rate rose to 85.5 bpm.
Similarly, when they were alerted to go to a medical emergency, their blood pressure jumped up by 18.7/16.5 mm Hg and their heart rate climbed to 90.5 bpm.
The surges in blood pressure and heart rate were similar when participants were riding in the fire truck to a call or when the call turned out to be a false alarm.
What can be done?
“If we can increase awareness and identify specific risk factors in firefighters,” Dr. Feairheller said, this could “save a life of someone who spends their day saving lives and property.”
To start, “regular, in-station or home BP monitoring should be encouraged,” she said. “Firefighters should start to track their BP levels in the morning, at night, at work. Being a volunteer firefighter myself, I know the stress and anxiety and sadness and heavy work that comes with the job,” she said. “I want to be able to do what I can to help make the crews healthier.”
Dr. Sasson suggested that ways to increase awareness and improve the health of firefighters might include “counseling, appropriate breaks, possibly food service/delivery to provide better nutritional options, built-in time for exercise (gym or cardio equipment on site), and discussions about how stress can impact the body over time.”
It is important to advocate for better mental health care, because people may have PTSD, depression, substance abuse, or other mental health conditions brought on by their stressful jobs, she said.
“Also, it would be interesting to know what is the current state of health monitoring (both physical, mental, and emotional) that occurs for firefighters,” she said.
The American Heart Association funded the study. The authors and Dr. Sasson report no disclosures.
A version of this article first appeared on Medscape.com.
In response to a 911 alert or page, firefighters’ systolic and diastolic blood pressure surges and their heart rate accelerates, with a similar response whether the call is for a fire or medical emergency, a small study suggests.
On average, the 41 firefighters monitored in the study, who were middle-aged and overweight, had a 9% increase in systolic blood pressure when called to a fire, a 9% increase in diastolic blood pressure when called to a medical emergency, and a 16% increase in heart rate for both types of calls.
Senior study author Deborah Feairheller, PhD, presented these results at the virtual American Heart Association scientific sessions.
Firefighters have a higher prevalence of cardiovascular disease (CVD) than that of the general population, explained Dr. Feairheller, director of the Hypertension and Endothelial Function with Aerobic and Resistance Training (HEART) Lab and clinical associate professor of kinesiology at the University of New Hampshire, Durham.
More than 50% of firefighter deaths in the line of duty are from CVD, she noted. Moreover, almost 75% of firefighters have hypertension and fewer than 25% have it under control.
The study findings show that all emergency and first responders “should know what their typical blood pressure level is and be aware of how it fluctuates,” Dr. Feairheller said in a press release from the AHA. “Most important, if they have high blood pressure, they should make sure it is well controlled,” she said.
“I really hope that fire departments everywhere see these data, rise to the occasion, and advocate for BP awareness in their crews,” Dr. Feairheller, a volunteer firefighter, said in an interview.
“I do think this has value to any occupation that wears a pager,” she added. “Clinicians, physicians, other emergency responders, all of those occupations are stressful and could place people at risk if they have undiagnosed or uncontrolled hypertension.”
Invited to comment, Comilla Sasson, MD, PhD, an emergency department physician who was not involved with this research, said in an interview that she saw parallels between stress experienced by firefighters and, for example, emergency department physicians.
The transient increases in BP, both systolic and diastolic, along with the heart rate are likely due to the body’s natural fight or flight response to an emergency call, including increases in epinephrine and cortisol, said Dr. Sasson, vice president of science and innovation for emergency cardiovascular care at the American Heart Association.
“The thing that is most interesting to me,” said Dr. Sasson, who can be subject to a series of high-stress situations on a shift, such as multiple trauma victims, a stroke victim, or a person in cardiac arrest, is “what is the cumulative impact of this over time?”
She said she wonders if “having to be ‘ready to go’ at any time, along with disruptions in sleep/wake schedules, and poorer eating and working-out habits when you are on shift, has long-term sequelae on the body.”
Stress-related surges in blood pressure “could be a reason for worse health outcomes in this group,” Dr. Sasson said, adding that this needs to be investigated further.
Firefighters with high normal BP, high BMI
Dr. Feairheller and colleagues recruited 41 volunteer and employee firefighters from suburban Philadelphia and Dover, N.H.
On average, the 37 men and 4 women had a mean age of 41 years, had been working as firefighters for 16.9 years, and had a mean body mass index of 30.3 kg/m2.
They wore ambulatory blood pressure monitors during an on-call work shift for at least 12 consecutive hours.
In addition to the automatic readings, the participants were instructed to prompt the machine to take a reading whenever a pager or emergency call sounded or when they felt they were entering a stressful situation.
Over the 12-hour shift, on average, participants had a blood pressure of 131/79.3 mm Hg and a heart rate of 75.7 bpm.
When they were alerted go to a fire, their blood pressure surged by 19.2/10.5 mm Hg, and their heart rate rose to 85.5 bpm.
Similarly, when they were alerted to go to a medical emergency, their blood pressure jumped up by 18.7/16.5 mm Hg and their heart rate climbed to 90.5 bpm.
The surges in blood pressure and heart rate were similar when participants were riding in the fire truck to a call or when the call turned out to be a false alarm.
What can be done?
“If we can increase awareness and identify specific risk factors in firefighters,” Dr. Feairheller said, this could “save a life of someone who spends their day saving lives and property.”
To start, “regular, in-station or home BP monitoring should be encouraged,” she said. “Firefighters should start to track their BP levels in the morning, at night, at work. Being a volunteer firefighter myself, I know the stress and anxiety and sadness and heavy work that comes with the job,” she said. “I want to be able to do what I can to help make the crews healthier.”
Dr. Sasson suggested that ways to increase awareness and improve the health of firefighters might include “counseling, appropriate breaks, possibly food service/delivery to provide better nutritional options, built-in time for exercise (gym or cardio equipment on site), and discussions about how stress can impact the body over time.”
It is important to advocate for better mental health care, because people may have PTSD, depression, substance abuse, or other mental health conditions brought on by their stressful jobs, she said.
“Also, it would be interesting to know what is the current state of health monitoring (both physical, mental, and emotional) that occurs for firefighters,” she said.
The American Heart Association funded the study. The authors and Dr. Sasson report no disclosures.
A version of this article first appeared on Medscape.com.
In response to a 911 alert or page, firefighters’ systolic and diastolic blood pressure surges and their heart rate accelerates, with a similar response whether the call is for a fire or medical emergency, a small study suggests.
On average, the 41 firefighters monitored in the study, who were middle-aged and overweight, had a 9% increase in systolic blood pressure when called to a fire, a 9% increase in diastolic blood pressure when called to a medical emergency, and a 16% increase in heart rate for both types of calls.
Senior study author Deborah Feairheller, PhD, presented these results at the virtual American Heart Association scientific sessions.
Firefighters have a higher prevalence of cardiovascular disease (CVD) than that of the general population, explained Dr. Feairheller, director of the Hypertension and Endothelial Function with Aerobic and Resistance Training (HEART) Lab and clinical associate professor of kinesiology at the University of New Hampshire, Durham.
More than 50% of firefighter deaths in the line of duty are from CVD, she noted. Moreover, almost 75% of firefighters have hypertension and fewer than 25% have it under control.
The study findings show that all emergency and first responders “should know what their typical blood pressure level is and be aware of how it fluctuates,” Dr. Feairheller said in a press release from the AHA. “Most important, if they have high blood pressure, they should make sure it is well controlled,” she said.
“I really hope that fire departments everywhere see these data, rise to the occasion, and advocate for BP awareness in their crews,” Dr. Feairheller, a volunteer firefighter, said in an interview.
“I do think this has value to any occupation that wears a pager,” she added. “Clinicians, physicians, other emergency responders, all of those occupations are stressful and could place people at risk if they have undiagnosed or uncontrolled hypertension.”
Invited to comment, Comilla Sasson, MD, PhD, an emergency department physician who was not involved with this research, said in an interview that she saw parallels between stress experienced by firefighters and, for example, emergency department physicians.
The transient increases in BP, both systolic and diastolic, along with the heart rate are likely due to the body’s natural fight or flight response to an emergency call, including increases in epinephrine and cortisol, said Dr. Sasson, vice president of science and innovation for emergency cardiovascular care at the American Heart Association.
“The thing that is most interesting to me,” said Dr. Sasson, who can be subject to a series of high-stress situations on a shift, such as multiple trauma victims, a stroke victim, or a person in cardiac arrest, is “what is the cumulative impact of this over time?”
She said she wonders if “having to be ‘ready to go’ at any time, along with disruptions in sleep/wake schedules, and poorer eating and working-out habits when you are on shift, has long-term sequelae on the body.”
Stress-related surges in blood pressure “could be a reason for worse health outcomes in this group,” Dr. Sasson said, adding that this needs to be investigated further.
Firefighters with high normal BP, high BMI
Dr. Feairheller and colleagues recruited 41 volunteer and employee firefighters from suburban Philadelphia and Dover, N.H.
On average, the 37 men and 4 women had a mean age of 41 years, had been working as firefighters for 16.9 years, and had a mean body mass index of 30.3 kg/m2.
They wore ambulatory blood pressure monitors during an on-call work shift for at least 12 consecutive hours.
In addition to the automatic readings, the participants were instructed to prompt the machine to take a reading whenever a pager or emergency call sounded or when they felt they were entering a stressful situation.
Over the 12-hour shift, on average, participants had a blood pressure of 131/79.3 mm Hg and a heart rate of 75.7 bpm.
When they were alerted go to a fire, their blood pressure surged by 19.2/10.5 mm Hg, and their heart rate rose to 85.5 bpm.
Similarly, when they were alerted to go to a medical emergency, their blood pressure jumped up by 18.7/16.5 mm Hg and their heart rate climbed to 90.5 bpm.
The surges in blood pressure and heart rate were similar when participants were riding in the fire truck to a call or when the call turned out to be a false alarm.
What can be done?
“If we can increase awareness and identify specific risk factors in firefighters,” Dr. Feairheller said, this could “save a life of someone who spends their day saving lives and property.”
To start, “regular, in-station or home BP monitoring should be encouraged,” she said. “Firefighters should start to track their BP levels in the morning, at night, at work. Being a volunteer firefighter myself, I know the stress and anxiety and sadness and heavy work that comes with the job,” she said. “I want to be able to do what I can to help make the crews healthier.”
Dr. Sasson suggested that ways to increase awareness and improve the health of firefighters might include “counseling, appropriate breaks, possibly food service/delivery to provide better nutritional options, built-in time for exercise (gym or cardio equipment on site), and discussions about how stress can impact the body over time.”
It is important to advocate for better mental health care, because people may have PTSD, depression, substance abuse, or other mental health conditions brought on by their stressful jobs, she said.
“Also, it would be interesting to know what is the current state of health monitoring (both physical, mental, and emotional) that occurs for firefighters,” she said.
The American Heart Association funded the study. The authors and Dr. Sasson report no disclosures.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Type 2 diabetes remission can happen naturally in 1 in 20
Roughly 5% of adults with type 2 diabetes achieve remission of their disease, often unbeknownst to the patient and without aggressive weight-loss interventions, according to a new analysis of data from more than 160,000 people in a national diabetes registry in Scotland.
“One of our key new findings is that a reasonably large proportion of people [with type 2 diabetes] were able to achieve remission in routine care, without undergoing bariatric surgery and prior to the introduction of very-low-calorie interventions in routine care,” said Mireille Captieux, MBChB, lead author of the report, in an interview.
The findings “support previous reports that weight loss is associated with type 2 diabetes remission,” said Dr. Captieux, a diabetes researcher at the University of Edinburgh (Scotland).
In her analysis, two of the strongest correlates of remission related to weight loss.
First, a history of bariatric surgery, which included a scant 488 people (0.3% of the study cohort), was associated with a 13-fold increase in the rate of remission, compared with those who did not undergo bariatric surgery. Second, weight loss of 15 kg (33 lb) or more at the time of remission detection in 2019, in comparison with their weight at initial diabetes diagnosis, was linked with a greater than fourfold increase in the rate of remission, compared with those who did not have this amount of weight loss.
But “even losing a small amount of weight increased the chances of remission,” highlights Dr. Captieux. “This finding offers a counterbalance to the pessimistic assumption that almost all people find it very difficult to lose weight.”
Hopeful message, but which people achieve diabetes remission?
“What’s encouraging here is that you have people who probably did not do anything radical, and yet they went into remission. The next step is to find out who these people are and what they did to go into remission,” commented Julia Lawton, PhD, a professor of health and social science at the University of Edinburgh whose research focuses on how patients with diabetes manage their disorder.
“If we can understand who the patients are who can achieve remission without taking extreme measures, it could help people in the health professions get beyond their presumptions about who is, or is not, a good candidate for achieving diabetes remission,” said Dr. Lawton, who was not involved with the study.
The message from this study is “very hopeful,” Dr. Lawton said in an interview. “How can we make this opportunity [for diabetes remission] available to more people? What can we learn from these patients that we could then apply to other patients?”
Dr. Captieux agrees. Given her findings, an important next step is to find out more about the population in remission to better understand “their perspectives on the challenges and benefits of supporting weight loss.
“Obesity is a complex issue, and therefore weight loss interventions that target individual actions and behaviors are much more likely to be effective if they are accompanied by multiple interventions at different levels,” Dr. Captieux said.
In addition, “more evidence is needed to assess the sustainability of diabetes remission and the effect of different durations of remission for a clinically relevant definition.”
Duration, definition of diabetes remission
Dr. Captieux noted that the new international consensus definition of type 2 diabetes remission – which specifies a minimum 3-month duration of glycemic control to qualify as remission – means that people with diabetes “may frequently oscillate” between remission and active disease.
This makes it important to better define the effect of duration of diabetes remission regarding various diabetes complications.
Another issue raised by the new findings is the importance of distinguishing people who lose weight because of a healthier diet and increased activity from those who lose weight because of chronic illness or frailty that’s followed by long-term adverse outcomes.
If these two populations are not distinguished in an observational cohort study – such as the one run by Dr. Captieux and her associates – then the people with chronic illness might appear to have worse outcomes following diabetes remission.
Dr. Captieux and her coauthors used data collected in the Scottish Care Information–Diabetes registry, which includes almost all people diagnosed with diabetes in Scotland. They focused on people with diabetes who had first been diagnosed with diabetes during 2004-2018, who were at least 30 years old at the time of their initial diagnosis, and who had received care in the national health system during 2019.
This yielded a study cohort of 162,316 people, of whom 7,710 (4.8%) were identified by the researchers as being in remission in 2019.
Patients in remission were defined as those whose hemoglobin A1c level was less than 6.5% at their index reading in 2019 and whose A1c level could be documented as being lower than 6.5% for at least 1 year prior to the 2019 measurement.
In a primary logistic regression analysis, the authors identified five variables that were significantly linked with remission: age of at least 65 years (the association was even stronger for age older than 75 years), a lower A1c level at the time of initial diabetes diagnosis, weight loss, prior bariatric surgery, and no prior treatment with a glucose-lowering therapy.
The strongest association was with having had no prior treatment with a glucose-lowering therapy in 2019. People who met this criterion were nearly 15 times more likely to be in remission in 2019, compared with those who had received at least one of these agents.
The study received no commercial funding. Dr. Captieux and Dr. Lawton have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Roughly 5% of adults with type 2 diabetes achieve remission of their disease, often unbeknownst to the patient and without aggressive weight-loss interventions, according to a new analysis of data from more than 160,000 people in a national diabetes registry in Scotland.
“One of our key new findings is that a reasonably large proportion of people [with type 2 diabetes] were able to achieve remission in routine care, without undergoing bariatric surgery and prior to the introduction of very-low-calorie interventions in routine care,” said Mireille Captieux, MBChB, lead author of the report, in an interview.
The findings “support previous reports that weight loss is associated with type 2 diabetes remission,” said Dr. Captieux, a diabetes researcher at the University of Edinburgh (Scotland).
In her analysis, two of the strongest correlates of remission related to weight loss.
First, a history of bariatric surgery, which included a scant 488 people (0.3% of the study cohort), was associated with a 13-fold increase in the rate of remission, compared with those who did not undergo bariatric surgery. Second, weight loss of 15 kg (33 lb) or more at the time of remission detection in 2019, in comparison with their weight at initial diabetes diagnosis, was linked with a greater than fourfold increase in the rate of remission, compared with those who did not have this amount of weight loss.
But “even losing a small amount of weight increased the chances of remission,” highlights Dr. Captieux. “This finding offers a counterbalance to the pessimistic assumption that almost all people find it very difficult to lose weight.”
Hopeful message, but which people achieve diabetes remission?
“What’s encouraging here is that you have people who probably did not do anything radical, and yet they went into remission. The next step is to find out who these people are and what they did to go into remission,” commented Julia Lawton, PhD, a professor of health and social science at the University of Edinburgh whose research focuses on how patients with diabetes manage their disorder.
“If we can understand who the patients are who can achieve remission without taking extreme measures, it could help people in the health professions get beyond their presumptions about who is, or is not, a good candidate for achieving diabetes remission,” said Dr. Lawton, who was not involved with the study.
The message from this study is “very hopeful,” Dr. Lawton said in an interview. “How can we make this opportunity [for diabetes remission] available to more people? What can we learn from these patients that we could then apply to other patients?”
Dr. Captieux agrees. Given her findings, an important next step is to find out more about the population in remission to better understand “their perspectives on the challenges and benefits of supporting weight loss.
“Obesity is a complex issue, and therefore weight loss interventions that target individual actions and behaviors are much more likely to be effective if they are accompanied by multiple interventions at different levels,” Dr. Captieux said.
In addition, “more evidence is needed to assess the sustainability of diabetes remission and the effect of different durations of remission for a clinically relevant definition.”
Duration, definition of diabetes remission
Dr. Captieux noted that the new international consensus definition of type 2 diabetes remission – which specifies a minimum 3-month duration of glycemic control to qualify as remission – means that people with diabetes “may frequently oscillate” between remission and active disease.
This makes it important to better define the effect of duration of diabetes remission regarding various diabetes complications.
Another issue raised by the new findings is the importance of distinguishing people who lose weight because of a healthier diet and increased activity from those who lose weight because of chronic illness or frailty that’s followed by long-term adverse outcomes.
If these two populations are not distinguished in an observational cohort study – such as the one run by Dr. Captieux and her associates – then the people with chronic illness might appear to have worse outcomes following diabetes remission.
Dr. Captieux and her coauthors used data collected in the Scottish Care Information–Diabetes registry, which includes almost all people diagnosed with diabetes in Scotland. They focused on people with diabetes who had first been diagnosed with diabetes during 2004-2018, who were at least 30 years old at the time of their initial diagnosis, and who had received care in the national health system during 2019.
This yielded a study cohort of 162,316 people, of whom 7,710 (4.8%) were identified by the researchers as being in remission in 2019.
Patients in remission were defined as those whose hemoglobin A1c level was less than 6.5% at their index reading in 2019 and whose A1c level could be documented as being lower than 6.5% for at least 1 year prior to the 2019 measurement.
In a primary logistic regression analysis, the authors identified five variables that were significantly linked with remission: age of at least 65 years (the association was even stronger for age older than 75 years), a lower A1c level at the time of initial diabetes diagnosis, weight loss, prior bariatric surgery, and no prior treatment with a glucose-lowering therapy.
The strongest association was with having had no prior treatment with a glucose-lowering therapy in 2019. People who met this criterion were nearly 15 times more likely to be in remission in 2019, compared with those who had received at least one of these agents.
The study received no commercial funding. Dr. Captieux and Dr. Lawton have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Roughly 5% of adults with type 2 diabetes achieve remission of their disease, often unbeknownst to the patient and without aggressive weight-loss interventions, according to a new analysis of data from more than 160,000 people in a national diabetes registry in Scotland.
“One of our key new findings is that a reasonably large proportion of people [with type 2 diabetes] were able to achieve remission in routine care, without undergoing bariatric surgery and prior to the introduction of very-low-calorie interventions in routine care,” said Mireille Captieux, MBChB, lead author of the report, in an interview.
The findings “support previous reports that weight loss is associated with type 2 diabetes remission,” said Dr. Captieux, a diabetes researcher at the University of Edinburgh (Scotland).
In her analysis, two of the strongest correlates of remission related to weight loss.
First, a history of bariatric surgery, which included a scant 488 people (0.3% of the study cohort), was associated with a 13-fold increase in the rate of remission, compared with those who did not undergo bariatric surgery. Second, weight loss of 15 kg (33 lb) or more at the time of remission detection in 2019, in comparison with their weight at initial diabetes diagnosis, was linked with a greater than fourfold increase in the rate of remission, compared with those who did not have this amount of weight loss.
But “even losing a small amount of weight increased the chances of remission,” highlights Dr. Captieux. “This finding offers a counterbalance to the pessimistic assumption that almost all people find it very difficult to lose weight.”
Hopeful message, but which people achieve diabetes remission?
“What’s encouraging here is that you have people who probably did not do anything radical, and yet they went into remission. The next step is to find out who these people are and what they did to go into remission,” commented Julia Lawton, PhD, a professor of health and social science at the University of Edinburgh whose research focuses on how patients with diabetes manage their disorder.
“If we can understand who the patients are who can achieve remission without taking extreme measures, it could help people in the health professions get beyond their presumptions about who is, or is not, a good candidate for achieving diabetes remission,” said Dr. Lawton, who was not involved with the study.
The message from this study is “very hopeful,” Dr. Lawton said in an interview. “How can we make this opportunity [for diabetes remission] available to more people? What can we learn from these patients that we could then apply to other patients?”
Dr. Captieux agrees. Given her findings, an important next step is to find out more about the population in remission to better understand “their perspectives on the challenges and benefits of supporting weight loss.
“Obesity is a complex issue, and therefore weight loss interventions that target individual actions and behaviors are much more likely to be effective if they are accompanied by multiple interventions at different levels,” Dr. Captieux said.
In addition, “more evidence is needed to assess the sustainability of diabetes remission and the effect of different durations of remission for a clinically relevant definition.”
Duration, definition of diabetes remission
Dr. Captieux noted that the new international consensus definition of type 2 diabetes remission – which specifies a minimum 3-month duration of glycemic control to qualify as remission – means that people with diabetes “may frequently oscillate” between remission and active disease.
This makes it important to better define the effect of duration of diabetes remission regarding various diabetes complications.
Another issue raised by the new findings is the importance of distinguishing people who lose weight because of a healthier diet and increased activity from those who lose weight because of chronic illness or frailty that’s followed by long-term adverse outcomes.
If these two populations are not distinguished in an observational cohort study – such as the one run by Dr. Captieux and her associates – then the people with chronic illness might appear to have worse outcomes following diabetes remission.
Dr. Captieux and her coauthors used data collected in the Scottish Care Information–Diabetes registry, which includes almost all people diagnosed with diabetes in Scotland. They focused on people with diabetes who had first been diagnosed with diabetes during 2004-2018, who were at least 30 years old at the time of their initial diagnosis, and who had received care in the national health system during 2019.
This yielded a study cohort of 162,316 people, of whom 7,710 (4.8%) were identified by the researchers as being in remission in 2019.
Patients in remission were defined as those whose hemoglobin A1c level was less than 6.5% at their index reading in 2019 and whose A1c level could be documented as being lower than 6.5% for at least 1 year prior to the 2019 measurement.
In a primary logistic regression analysis, the authors identified five variables that were significantly linked with remission: age of at least 65 years (the association was even stronger for age older than 75 years), a lower A1c level at the time of initial diabetes diagnosis, weight loss, prior bariatric surgery, and no prior treatment with a glucose-lowering therapy.
The strongest association was with having had no prior treatment with a glucose-lowering therapy in 2019. People who met this criterion were nearly 15 times more likely to be in remission in 2019, compared with those who had received at least one of these agents.
The study received no commercial funding. Dr. Captieux and Dr. Lawton have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID surge in Europe: A preview of what’s ahead for the U.S.?
Health experts are warning the United States could be headed for another COVID-19 surge just as we enter the holiday season, following a massive new wave of infections in Europe – a troubling pattern seen throughout the pandemic.
Eighteen months into the global health crisis that has killed 5.1 million people worldwide including more than 767,000 Americans, Europe has become the epicenter of the global health crisis once again.
And some infectious disease specialists say the United States may be next.
“It’s déjà vu, yet again,” says Eric Topol, M.D., founder and director of the Scripps Research Translational Institute. In a new analysis published in The Guardian, the professor of molecular medicine argues that it’s “wishful thinking” for U.S. authorities to believe the nation is “immune” to what’s happening in Europe.
Dr. Topol is also editor-in-chief of Medscape, MDedge’s sister site for medical professionals.
Three times over the past 18 months coronavirus surges in the United States followed similar spikes in Europe, where COVID-19 deaths grew by 10% this month.
Dr. Topol argues another wave may be in store for the states, as European countries implement new lockdowns. COVID-19 spikes are hitting some regions of the continent hard, including areas with high vaccination rates and strict control measures.
Eastern Europe and Russia, where vaccination rates are low, have experienced the worst of it. But even western countries, such as Germany, Austria and the United Kingdom, are reporting some of the highest daily infection figures in the world today.
Countries are responding in increasingly drastic ways.
In Russia, President Vladimir Putin ordered tens of thousands of workers to stay home earlier this month.
In the Dutch city of Utrecht, traditional Christmas celebrations have been canceled as the country is headed for a partial lockdown.
Austria announced a 20-day lockdown beginning Nov. 22 and on Nov. 19 leaders there announced that all 9 million residents will be required to be vaccinated by February. Leaders there are telling unvaccinated individuals to stay at home and out of restaurants, cafes, and other shops in hard-hit regions of the country.
And in Germany, where daily new-infection rates now stand at 50,000, officials have introduced stricter mask mandates and made proof of vaccination or past infection mandatory for entry to many venues. Berlin is also eyeing proposals to shut down the city’s traditional Christmas markets while authorities in Cologne have already called off holiday celebrations, after the ceremonial head of festivities tested positive for COVID-19. Bavaria canceled its popular Christmas markets and will order lockdowns in particularly vulnerable districts, while unvaccinated people will face serious restrictions on where they can go.
Former FDA Commissioner Scott Gottlieb, MD, says what’s happening across the European continent is troubling.
But he also believes it’s possible the United States may be better prepared to head off a similar surge this time around, with increased testing, vaccination and new therapies such as monoclonal antibodies, and antiviral therapeutics.
“Germany’s challenges are [a] caution to [the] world, the COVID pandemic isn’t over globally, won’t be for long time,” he says. “But [the] U.S. is further along than many other countries, in part because we already suffered more spread, in part because we’re making progress on vaccines, therapeutics, testing.”
Other experts agree the United States may not be as vulnerable to another wave of COVID-19 in coming weeks but have stopped short of suggesting we’re out of the woods.
“I don’t think that what we’re seeing in Europe necessarily means that we’re in for a huge surge of serious illness and death the way that we saw last year here in the states,” says David Dowdy, MD, PhD, an associate professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health and a general internist with Baltimore Medical Services.
“But I think anyone who says that they can predict the course of the pandemic for the next few months or few years has been proven wrong in the past and will probably be proven wrong in the future,” Dr. Dowdy says. “None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness.”
Looking back, and forward
What’s happening in Europe today mirrors past COVID-19 spikes that presaged big upticks in cases, hospitalizations, and deaths in the United States.
When the pandemic first hit Europe in March 2020, then-President Donald Trump downplayed the threat of the virus despite the warnings of his own advisors and independent public health experts who said COVID-19 could have dire impacts without an aggressive federal action plan.
By late spring the United States had become the epicenter of the pandemic, when case totals eclipsed those of other countries and New York City became a hot zone, according to data compiled by the Johns Hopkins Coronavirus Resource Center. Over the summer, spread of the disease slowed in New York, after tough control measures were instituted, but steadily increased in other states.
Then, later in the year, the Alpha variant of the virus took hold in the United Kingdom and the United States was again unprepared. By winter, the number of cases accelerated in every state in a major second surge that kept millions of Americans from traveling and gathering for the winter holidays.
With the rollout of COVID vaccines last December, cases in the United States – and in many parts of the world – began to fall. Some experts even suggested we’d turned a corner on the pandemic.
But then, last spring and summer, the Delta variant popped up in India and spread to the United Kingdom in a third major wave of COVID. Once again, the United States was unprepared, with 4 in 10 Americans refusing the vaccine and even some vaccinated individuals succumbing to breakthrough Delta infections.
The resulting Delta surge swept the country, preventing many businesses and schools from fully reopening and stressing hospitals in some areas of the country – particularly southern states – with new influxes of COVID-19 patients.
Now, Europe is facing another rise in COVID, with about 350 cases per 100,000 people and many countries hitting new record highs.
What’s driving the European resurgence?
So, what’s behind the new COVID-19 wave in Europe and what might it mean for the United States?
Shaun Truelove, PhD, an infectious disease epidemiologist and faculty member of the Johns Hopkins School of Public Health, says experts are examining several likely factors:
Waning immunity from the vaccines. Data from Johns Hopkins shows infections rising in nations with lower vaccination rates.
The impact of the Delta variant, which is three times more transmissible than the original virus and can even sicken some vaccinated individuals.
The spread of COVID-19 among teens and children; the easing of precautions (such as masking and social distancing); differences in the types of vaccines used in European nations and the United States.
“These are all possibilities,” says Dr. Truelove. “There are so many factors and so it’s difficult to pinpoint exactly what’s driving it and what effect each of those things might be having.”
As a result, it’s difficult to predict and prepare for what might lie ahead for the United States, he says.
“There’s a ton of uncertainty and we’re trying to understand what’s going to happen here over the next 6 months,” he says.
Even so, Dr. Truelove adds that what’s happening overseas might not be “super predictive” of a new wave of COVID in the United States.
For one thing, he says, the Pfizer and Moderna vaccines, the two mRNA vaccines used predominantly in the United States, are far more effective – 94-95% – than the Oxford/AstraZeneca COVID shot (63%) widely administered across Europe.
Secondly, European countries have imposed much stronger and stricter control measures throughout the pandemic than the United States. That might actually be driving the new surges because fewer unvaccinated people have been exposed to the virus, which means they have lower “natural immunity” from prior COVID infection.
Dr. Truelove explains: “Stronger and stricter control measures … have the consequence of leaving a lot more susceptible individuals in the population, [because] the stronger the controls, the fewer people get infected. And so, you have more individuals remaining in the population who are more susceptible and at risk of getting infected in the future.”
By contrast, he notes, a “large chunk” of the United States has not put strict lockdowns in place.
“So, what we’ve seen over the past couple months with the Delta wave is that in a lot of those states with lower vaccination coverage and lower controls this virus has really burned through a lot of the susceptible population. As a result, we’re seeing the curves coming down and what really looks like a lot of the built-up immunity in these states, especially southern states.”
But whether these differences will be enough for the United States to dodge another COVID-19 bullet this winter is uncertain.
“I don’t want to say that the [Europe] surge is NOT a predictor of what might come in the U.S., because I think that it very well could be,” Dr. Truelove says. “And so, people need to be aware of that, and be cautious and be sure get their vaccines and everything else.
“But I’m hopeful that because of some of the differences that maybe we’ll have a little bit of a different situation.”
The takeaway: How best to prepare?
Dr. Dowdy agrees that Europe’s current troubles might not necessarily mean a major new winter surge in the United States.
But he also points out that cases are beginning to head up again in New England, the Midwest, and other regions of the country that are just experiencing the first chill of winter.
“After reaching a low point about 3 weeks ago, cases due to COVID-19 have started to rise again in the United States,” he says. “Cases were falling consistently until mid-October, but over the last 3 weeks, cases have started to rise again in most states.
“Cases in Eastern and Central Europe have more than doubled during that time, meaning that the possibility of a winter surge here is very real.”
Even so, Dr. Dowdy believes the rising rates of vaccination could limit the number of Americans who will be hospitalized with severe disease or die this winter.
Still, he warns against being too optimistic, as Americans travel and get together for the winter holidays.
None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness, Dr. Dowdy says.”
The upshot?
“People need to realize that it’s not quite over,” Dr. Truelove says. “We still have a substantial amount of infection in our country. We’re still above 200 cases per million [and] 500,000 incident cases per week or so. That’s a lot of death and a lot of hospitalizations. So, we still have to be concerned and do our best to reduce transmission … by wearing masks, getting vaccinated, getting a booster shot, and getting your children vaccinated.”
Johns Hopkins social and behavioral scientist Rupali Limaye, PhD, MPH, adds that while COVID vaccines have been a “game changer” in the pandemic, more than a third of Americans have yet to receive one.
“That’s really what we need to be messaging around -- that people can still get COVID, there can still be breakthrough infections,” says Dr. Limaye, a health communications scholar. “But the great news is if you have been vaccinated, you are very much less likely, I think it’s 12 times, to be hospitalized or have severe COVID compared to those that are un-vaccinated.”
Dr. Topol agrees, adding: “Now is the time for the U.S. to heed the European signal for the first time, to pull out all the stops. Promote primary vaccination and boosters like there’s no tomorrow. Aggressively counter the pervasive misinformation and disinformation. Accelerate and expand the vaccine mandates ...
“Instead of succumbing to yet another major rise in cases and their sequelae, this is a chance for America to finally rise to the occasion, showing an ability to lead and execute.”
A version of this article first appeared on WebMD.com.
Health experts are warning the United States could be headed for another COVID-19 surge just as we enter the holiday season, following a massive new wave of infections in Europe – a troubling pattern seen throughout the pandemic.
Eighteen months into the global health crisis that has killed 5.1 million people worldwide including more than 767,000 Americans, Europe has become the epicenter of the global health crisis once again.
And some infectious disease specialists say the United States may be next.
“It’s déjà vu, yet again,” says Eric Topol, M.D., founder and director of the Scripps Research Translational Institute. In a new analysis published in The Guardian, the professor of molecular medicine argues that it’s “wishful thinking” for U.S. authorities to believe the nation is “immune” to what’s happening in Europe.
Dr. Topol is also editor-in-chief of Medscape, MDedge’s sister site for medical professionals.
Three times over the past 18 months coronavirus surges in the United States followed similar spikes in Europe, where COVID-19 deaths grew by 10% this month.
Dr. Topol argues another wave may be in store for the states, as European countries implement new lockdowns. COVID-19 spikes are hitting some regions of the continent hard, including areas with high vaccination rates and strict control measures.
Eastern Europe and Russia, where vaccination rates are low, have experienced the worst of it. But even western countries, such as Germany, Austria and the United Kingdom, are reporting some of the highest daily infection figures in the world today.
Countries are responding in increasingly drastic ways.
In Russia, President Vladimir Putin ordered tens of thousands of workers to stay home earlier this month.
In the Dutch city of Utrecht, traditional Christmas celebrations have been canceled as the country is headed for a partial lockdown.
Austria announced a 20-day lockdown beginning Nov. 22 and on Nov. 19 leaders there announced that all 9 million residents will be required to be vaccinated by February. Leaders there are telling unvaccinated individuals to stay at home and out of restaurants, cafes, and other shops in hard-hit regions of the country.
And in Germany, where daily new-infection rates now stand at 50,000, officials have introduced stricter mask mandates and made proof of vaccination or past infection mandatory for entry to many venues. Berlin is also eyeing proposals to shut down the city’s traditional Christmas markets while authorities in Cologne have already called off holiday celebrations, after the ceremonial head of festivities tested positive for COVID-19. Bavaria canceled its popular Christmas markets and will order lockdowns in particularly vulnerable districts, while unvaccinated people will face serious restrictions on where they can go.
Former FDA Commissioner Scott Gottlieb, MD, says what’s happening across the European continent is troubling.
But he also believes it’s possible the United States may be better prepared to head off a similar surge this time around, with increased testing, vaccination and new therapies such as monoclonal antibodies, and antiviral therapeutics.
“Germany’s challenges are [a] caution to [the] world, the COVID pandemic isn’t over globally, won’t be for long time,” he says. “But [the] U.S. is further along than many other countries, in part because we already suffered more spread, in part because we’re making progress on vaccines, therapeutics, testing.”
Other experts agree the United States may not be as vulnerable to another wave of COVID-19 in coming weeks but have stopped short of suggesting we’re out of the woods.
“I don’t think that what we’re seeing in Europe necessarily means that we’re in for a huge surge of serious illness and death the way that we saw last year here in the states,” says David Dowdy, MD, PhD, an associate professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health and a general internist with Baltimore Medical Services.
“But I think anyone who says that they can predict the course of the pandemic for the next few months or few years has been proven wrong in the past and will probably be proven wrong in the future,” Dr. Dowdy says. “None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness.”
Looking back, and forward
What’s happening in Europe today mirrors past COVID-19 spikes that presaged big upticks in cases, hospitalizations, and deaths in the United States.
When the pandemic first hit Europe in March 2020, then-President Donald Trump downplayed the threat of the virus despite the warnings of his own advisors and independent public health experts who said COVID-19 could have dire impacts without an aggressive federal action plan.
By late spring the United States had become the epicenter of the pandemic, when case totals eclipsed those of other countries and New York City became a hot zone, according to data compiled by the Johns Hopkins Coronavirus Resource Center. Over the summer, spread of the disease slowed in New York, after tough control measures were instituted, but steadily increased in other states.
Then, later in the year, the Alpha variant of the virus took hold in the United Kingdom and the United States was again unprepared. By winter, the number of cases accelerated in every state in a major second surge that kept millions of Americans from traveling and gathering for the winter holidays.
With the rollout of COVID vaccines last December, cases in the United States – and in many parts of the world – began to fall. Some experts even suggested we’d turned a corner on the pandemic.
But then, last spring and summer, the Delta variant popped up in India and spread to the United Kingdom in a third major wave of COVID. Once again, the United States was unprepared, with 4 in 10 Americans refusing the vaccine and even some vaccinated individuals succumbing to breakthrough Delta infections.
The resulting Delta surge swept the country, preventing many businesses and schools from fully reopening and stressing hospitals in some areas of the country – particularly southern states – with new influxes of COVID-19 patients.
Now, Europe is facing another rise in COVID, with about 350 cases per 100,000 people and many countries hitting new record highs.
What’s driving the European resurgence?
So, what’s behind the new COVID-19 wave in Europe and what might it mean for the United States?
Shaun Truelove, PhD, an infectious disease epidemiologist and faculty member of the Johns Hopkins School of Public Health, says experts are examining several likely factors:
Waning immunity from the vaccines. Data from Johns Hopkins shows infections rising in nations with lower vaccination rates.
The impact of the Delta variant, which is three times more transmissible than the original virus and can even sicken some vaccinated individuals.
The spread of COVID-19 among teens and children; the easing of precautions (such as masking and social distancing); differences in the types of vaccines used in European nations and the United States.
“These are all possibilities,” says Dr. Truelove. “There are so many factors and so it’s difficult to pinpoint exactly what’s driving it and what effect each of those things might be having.”
As a result, it’s difficult to predict and prepare for what might lie ahead for the United States, he says.
“There’s a ton of uncertainty and we’re trying to understand what’s going to happen here over the next 6 months,” he says.
Even so, Dr. Truelove adds that what’s happening overseas might not be “super predictive” of a new wave of COVID in the United States.
For one thing, he says, the Pfizer and Moderna vaccines, the two mRNA vaccines used predominantly in the United States, are far more effective – 94-95% – than the Oxford/AstraZeneca COVID shot (63%) widely administered across Europe.
Secondly, European countries have imposed much stronger and stricter control measures throughout the pandemic than the United States. That might actually be driving the new surges because fewer unvaccinated people have been exposed to the virus, which means they have lower “natural immunity” from prior COVID infection.
Dr. Truelove explains: “Stronger and stricter control measures … have the consequence of leaving a lot more susceptible individuals in the population, [because] the stronger the controls, the fewer people get infected. And so, you have more individuals remaining in the population who are more susceptible and at risk of getting infected in the future.”
By contrast, he notes, a “large chunk” of the United States has not put strict lockdowns in place.
“So, what we’ve seen over the past couple months with the Delta wave is that in a lot of those states with lower vaccination coverage and lower controls this virus has really burned through a lot of the susceptible population. As a result, we’re seeing the curves coming down and what really looks like a lot of the built-up immunity in these states, especially southern states.”
But whether these differences will be enough for the United States to dodge another COVID-19 bullet this winter is uncertain.
“I don’t want to say that the [Europe] surge is NOT a predictor of what might come in the U.S., because I think that it very well could be,” Dr. Truelove says. “And so, people need to be aware of that, and be cautious and be sure get their vaccines and everything else.
“But I’m hopeful that because of some of the differences that maybe we’ll have a little bit of a different situation.”
The takeaway: How best to prepare?
Dr. Dowdy agrees that Europe’s current troubles might not necessarily mean a major new winter surge in the United States.
But he also points out that cases are beginning to head up again in New England, the Midwest, and other regions of the country that are just experiencing the first chill of winter.
“After reaching a low point about 3 weeks ago, cases due to COVID-19 have started to rise again in the United States,” he says. “Cases were falling consistently until mid-October, but over the last 3 weeks, cases have started to rise again in most states.
“Cases in Eastern and Central Europe have more than doubled during that time, meaning that the possibility of a winter surge here is very real.”
Even so, Dr. Dowdy believes the rising rates of vaccination could limit the number of Americans who will be hospitalized with severe disease or die this winter.
Still, he warns against being too optimistic, as Americans travel and get together for the winter holidays.
None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness, Dr. Dowdy says.”
The upshot?
“People need to realize that it’s not quite over,” Dr. Truelove says. “We still have a substantial amount of infection in our country. We’re still above 200 cases per million [and] 500,000 incident cases per week or so. That’s a lot of death and a lot of hospitalizations. So, we still have to be concerned and do our best to reduce transmission … by wearing masks, getting vaccinated, getting a booster shot, and getting your children vaccinated.”
Johns Hopkins social and behavioral scientist Rupali Limaye, PhD, MPH, adds that while COVID vaccines have been a “game changer” in the pandemic, more than a third of Americans have yet to receive one.
“That’s really what we need to be messaging around -- that people can still get COVID, there can still be breakthrough infections,” says Dr. Limaye, a health communications scholar. “But the great news is if you have been vaccinated, you are very much less likely, I think it’s 12 times, to be hospitalized or have severe COVID compared to those that are un-vaccinated.”
Dr. Topol agrees, adding: “Now is the time for the U.S. to heed the European signal for the first time, to pull out all the stops. Promote primary vaccination and boosters like there’s no tomorrow. Aggressively counter the pervasive misinformation and disinformation. Accelerate and expand the vaccine mandates ...
“Instead of succumbing to yet another major rise in cases and their sequelae, this is a chance for America to finally rise to the occasion, showing an ability to lead and execute.”
A version of this article first appeared on WebMD.com.
Health experts are warning the United States could be headed for another COVID-19 surge just as we enter the holiday season, following a massive new wave of infections in Europe – a troubling pattern seen throughout the pandemic.
Eighteen months into the global health crisis that has killed 5.1 million people worldwide including more than 767,000 Americans, Europe has become the epicenter of the global health crisis once again.
And some infectious disease specialists say the United States may be next.
“It’s déjà vu, yet again,” says Eric Topol, M.D., founder and director of the Scripps Research Translational Institute. In a new analysis published in The Guardian, the professor of molecular medicine argues that it’s “wishful thinking” for U.S. authorities to believe the nation is “immune” to what’s happening in Europe.
Dr. Topol is also editor-in-chief of Medscape, MDedge’s sister site for medical professionals.
Three times over the past 18 months coronavirus surges in the United States followed similar spikes in Europe, where COVID-19 deaths grew by 10% this month.
Dr. Topol argues another wave may be in store for the states, as European countries implement new lockdowns. COVID-19 spikes are hitting some regions of the continent hard, including areas with high vaccination rates and strict control measures.
Eastern Europe and Russia, where vaccination rates are low, have experienced the worst of it. But even western countries, such as Germany, Austria and the United Kingdom, are reporting some of the highest daily infection figures in the world today.
Countries are responding in increasingly drastic ways.
In Russia, President Vladimir Putin ordered tens of thousands of workers to stay home earlier this month.
In the Dutch city of Utrecht, traditional Christmas celebrations have been canceled as the country is headed for a partial lockdown.
Austria announced a 20-day lockdown beginning Nov. 22 and on Nov. 19 leaders there announced that all 9 million residents will be required to be vaccinated by February. Leaders there are telling unvaccinated individuals to stay at home and out of restaurants, cafes, and other shops in hard-hit regions of the country.
And in Germany, where daily new-infection rates now stand at 50,000, officials have introduced stricter mask mandates and made proof of vaccination or past infection mandatory for entry to many venues. Berlin is also eyeing proposals to shut down the city’s traditional Christmas markets while authorities in Cologne have already called off holiday celebrations, after the ceremonial head of festivities tested positive for COVID-19. Bavaria canceled its popular Christmas markets and will order lockdowns in particularly vulnerable districts, while unvaccinated people will face serious restrictions on where they can go.
Former FDA Commissioner Scott Gottlieb, MD, says what’s happening across the European continent is troubling.
But he also believes it’s possible the United States may be better prepared to head off a similar surge this time around, with increased testing, vaccination and new therapies such as monoclonal antibodies, and antiviral therapeutics.
“Germany’s challenges are [a] caution to [the] world, the COVID pandemic isn’t over globally, won’t be for long time,” he says. “But [the] U.S. is further along than many other countries, in part because we already suffered more spread, in part because we’re making progress on vaccines, therapeutics, testing.”
Other experts agree the United States may not be as vulnerable to another wave of COVID-19 in coming weeks but have stopped short of suggesting we’re out of the woods.
“I don’t think that what we’re seeing in Europe necessarily means that we’re in for a huge surge of serious illness and death the way that we saw last year here in the states,” says David Dowdy, MD, PhD, an associate professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health and a general internist with Baltimore Medical Services.
“But I think anyone who says that they can predict the course of the pandemic for the next few months or few years has been proven wrong in the past and will probably be proven wrong in the future,” Dr. Dowdy says. “None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness.”
Looking back, and forward
What’s happening in Europe today mirrors past COVID-19 spikes that presaged big upticks in cases, hospitalizations, and deaths in the United States.
When the pandemic first hit Europe in March 2020, then-President Donald Trump downplayed the threat of the virus despite the warnings of his own advisors and independent public health experts who said COVID-19 could have dire impacts without an aggressive federal action plan.
By late spring the United States had become the epicenter of the pandemic, when case totals eclipsed those of other countries and New York City became a hot zone, according to data compiled by the Johns Hopkins Coronavirus Resource Center. Over the summer, spread of the disease slowed in New York, after tough control measures were instituted, but steadily increased in other states.
Then, later in the year, the Alpha variant of the virus took hold in the United Kingdom and the United States was again unprepared. By winter, the number of cases accelerated in every state in a major second surge that kept millions of Americans from traveling and gathering for the winter holidays.
With the rollout of COVID vaccines last December, cases in the United States – and in many parts of the world – began to fall. Some experts even suggested we’d turned a corner on the pandemic.
But then, last spring and summer, the Delta variant popped up in India and spread to the United Kingdom in a third major wave of COVID. Once again, the United States was unprepared, with 4 in 10 Americans refusing the vaccine and even some vaccinated individuals succumbing to breakthrough Delta infections.
The resulting Delta surge swept the country, preventing many businesses and schools from fully reopening and stressing hospitals in some areas of the country – particularly southern states – with new influxes of COVID-19 patients.
Now, Europe is facing another rise in COVID, with about 350 cases per 100,000 people and many countries hitting new record highs.
What’s driving the European resurgence?
So, what’s behind the new COVID-19 wave in Europe and what might it mean for the United States?
Shaun Truelove, PhD, an infectious disease epidemiologist and faculty member of the Johns Hopkins School of Public Health, says experts are examining several likely factors:
Waning immunity from the vaccines. Data from Johns Hopkins shows infections rising in nations with lower vaccination rates.
The impact of the Delta variant, which is three times more transmissible than the original virus and can even sicken some vaccinated individuals.
The spread of COVID-19 among teens and children; the easing of precautions (such as masking and social distancing); differences in the types of vaccines used in European nations and the United States.
“These are all possibilities,” says Dr. Truelove. “There are so many factors and so it’s difficult to pinpoint exactly what’s driving it and what effect each of those things might be having.”
As a result, it’s difficult to predict and prepare for what might lie ahead for the United States, he says.
“There’s a ton of uncertainty and we’re trying to understand what’s going to happen here over the next 6 months,” he says.
Even so, Dr. Truelove adds that what’s happening overseas might not be “super predictive” of a new wave of COVID in the United States.
For one thing, he says, the Pfizer and Moderna vaccines, the two mRNA vaccines used predominantly in the United States, are far more effective – 94-95% – than the Oxford/AstraZeneca COVID shot (63%) widely administered across Europe.
Secondly, European countries have imposed much stronger and stricter control measures throughout the pandemic than the United States. That might actually be driving the new surges because fewer unvaccinated people have been exposed to the virus, which means they have lower “natural immunity” from prior COVID infection.
Dr. Truelove explains: “Stronger and stricter control measures … have the consequence of leaving a lot more susceptible individuals in the population, [because] the stronger the controls, the fewer people get infected. And so, you have more individuals remaining in the population who are more susceptible and at risk of getting infected in the future.”
By contrast, he notes, a “large chunk” of the United States has not put strict lockdowns in place.
“So, what we’ve seen over the past couple months with the Delta wave is that in a lot of those states with lower vaccination coverage and lower controls this virus has really burned through a lot of the susceptible population. As a result, we’re seeing the curves coming down and what really looks like a lot of the built-up immunity in these states, especially southern states.”
But whether these differences will be enough for the United States to dodge another COVID-19 bullet this winter is uncertain.
“I don’t want to say that the [Europe] surge is NOT a predictor of what might come in the U.S., because I think that it very well could be,” Dr. Truelove says. “And so, people need to be aware of that, and be cautious and be sure get their vaccines and everything else.
“But I’m hopeful that because of some of the differences that maybe we’ll have a little bit of a different situation.”
The takeaway: How best to prepare?
Dr. Dowdy agrees that Europe’s current troubles might not necessarily mean a major new winter surge in the United States.
But he also points out that cases are beginning to head up again in New England, the Midwest, and other regions of the country that are just experiencing the first chill of winter.
“After reaching a low point about 3 weeks ago, cases due to COVID-19 have started to rise again in the United States,” he says. “Cases were falling consistently until mid-October, but over the last 3 weeks, cases have started to rise again in most states.
“Cases in Eastern and Central Europe have more than doubled during that time, meaning that the possibility of a winter surge here is very real.”
Even so, Dr. Dowdy believes the rising rates of vaccination could limit the number of Americans who will be hospitalized with severe disease or die this winter.
Still, he warns against being too optimistic, as Americans travel and get together for the winter holidays.
None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness, Dr. Dowdy says.”
The upshot?
“People need to realize that it’s not quite over,” Dr. Truelove says. “We still have a substantial amount of infection in our country. We’re still above 200 cases per million [and] 500,000 incident cases per week or so. That’s a lot of death and a lot of hospitalizations. So, we still have to be concerned and do our best to reduce transmission … by wearing masks, getting vaccinated, getting a booster shot, and getting your children vaccinated.”
Johns Hopkins social and behavioral scientist Rupali Limaye, PhD, MPH, adds that while COVID vaccines have been a “game changer” in the pandemic, more than a third of Americans have yet to receive one.
“That’s really what we need to be messaging around -- that people can still get COVID, there can still be breakthrough infections,” says Dr. Limaye, a health communications scholar. “But the great news is if you have been vaccinated, you are very much less likely, I think it’s 12 times, to be hospitalized or have severe COVID compared to those that are un-vaccinated.”
Dr. Topol agrees, adding: “Now is the time for the U.S. to heed the European signal for the first time, to pull out all the stops. Promote primary vaccination and boosters like there’s no tomorrow. Aggressively counter the pervasive misinformation and disinformation. Accelerate and expand the vaccine mandates ...
“Instead of succumbing to yet another major rise in cases and their sequelae, this is a chance for America to finally rise to the occasion, showing an ability to lead and execute.”
A version of this article first appeared on WebMD.com.
In pill or food form, healthy fatty acids reduce liver fat
For patients with nonalcoholic fatty liver disease (NAFLD) who supplement their diets with polyunsaturated fatty acids (PUFA), liver and metabolic parameters improve, results of a systematic review and meta-analysis suggest.
Data from randomized clinical trials show that, for participants with NAFLD who used PUFA supplements with or without additional dietary interventions, hepatic steatosis and lobular inflammation decreased, and in one study, fibrosis decreased. There were also improvements in liver enzyme levels, said Saleh Alqahtani, MBChB, associate professor of medicine at Johns Hopkins University, Baltimore, during a presentation at the annual meeting of the American Association for the Study of Liver Diseases.
“Since there’s no effective medical therapy for NAFLD, weight loss through lifestyle modifications becomes the most important focused intervention for patients with NAFLD,” he said. “However, the majority of patients fail to achieve or to maintain weight loss for long-term therapy. Therefore, dietary intervention or supplementation might help reduce the prevalence of NAFLD and decrease the progression of nonalcoholic steatohepatitis [NASH] and liver cirrhosis.
“More clinical trials are warranted to determine the long-term efficacy of the Mediterranean diet and polyunsaturated fatty acid supplementation among adult patients with NAFLD,” he added.
RCTs and case-control studies
It’s well documented that consumption of PUFAs, found in fatty fish and in canola, grapeseed, corn, and soybean oils, as well as monounsaturated fatty acids, found in olive oil and peanut oil, can contribute to improvement of NALFD, Dr. Alqahtani said.
In contrast, foods high in saturated fatty acids, such as butter, as well as trans fats and cholesterol can contribute to NAFLD progression, he said.
In their studies of intrahepatic triglyceride content, Dr. Alqahtani and colleagues found that fatty acids in the liver come from three major sources: dietary fatty acids, which account for about 15% of liver fat, tissue lipolysis, and de novo hepatic lipogenesis.
Previous systematic reviews and meta-analyses of the relationship between diet and NAFLD have focused on marine-based (n-3) PUFAs, but “the data regarding the evidence of unsaturated fatty acids through supplements or monounsaturated fatty acids through dietary supplementation are lacking,” he said.
To summarize the effects of dietary or supplemental fatty acids on liver and metabolic parameters in adults with NAFLD, Dr. Alqahtani and colleagues conducted a systematic review and meta-analysis, concentrating on studies that included specifics about interventions and outcomes.
They identified a total of 18 randomized controlled trials and 4 case-control studies that met their criteria. The studies were published from 2008 to 2020.
Regarding the effects of interventions on the components of NASH, they found that, in 1 or more of 12 randomized trials of PUFA supplementation with or without dietary interventions, there were associations with decreased hepatic steatosis, lobular inflammation, and fibrosis and declines in ALT and AST levels.
In three trials of dietary-only interventions, there were decreases in hepatic steatosis and ALT and/or AST levels. In two studies of the effects of healthy cooking oils only, hepatic steatosis decreased, but there was no effect on ALT or AST levels.
All three interventions were associated with improvements in fasting glucose levels and insulin metabolism, as well as decreases in total cholesterol, triglycerides, and LDL cholesterol and increases in HDL cholesterol.
Better understanding of dietary composition
“We’ve known for a while that dietary composition may impact NAFLD and NASH,” said Manal F. Abdelmalek, MD, professor of medicine at Duke University, Durham, N.C., who commented on the study.
“What [Dr. Alqahtani and colleagues] have shown is that supplementation with healthy fatty acids improves fatty liver. This really does extend our knowledge of what we understand about dietary composition, particularly the recommendations that support higher fish consumption and a Mediterranean-style diet,” she said.
“It’s not just about the fat but the type of fat that’s consumed, and drilling down to the particulars of dietary composition beyond calories alone,” she added.
No source of funding for the study has been disclosed. Dr. Alqahtani and Dr. Abdelmalek have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with nonalcoholic fatty liver disease (NAFLD) who supplement their diets with polyunsaturated fatty acids (PUFA), liver and metabolic parameters improve, results of a systematic review and meta-analysis suggest.
Data from randomized clinical trials show that, for participants with NAFLD who used PUFA supplements with or without additional dietary interventions, hepatic steatosis and lobular inflammation decreased, and in one study, fibrosis decreased. There were also improvements in liver enzyme levels, said Saleh Alqahtani, MBChB, associate professor of medicine at Johns Hopkins University, Baltimore, during a presentation at the annual meeting of the American Association for the Study of Liver Diseases.
“Since there’s no effective medical therapy for NAFLD, weight loss through lifestyle modifications becomes the most important focused intervention for patients with NAFLD,” he said. “However, the majority of patients fail to achieve or to maintain weight loss for long-term therapy. Therefore, dietary intervention or supplementation might help reduce the prevalence of NAFLD and decrease the progression of nonalcoholic steatohepatitis [NASH] and liver cirrhosis.
“More clinical trials are warranted to determine the long-term efficacy of the Mediterranean diet and polyunsaturated fatty acid supplementation among adult patients with NAFLD,” he added.
RCTs and case-control studies
It’s well documented that consumption of PUFAs, found in fatty fish and in canola, grapeseed, corn, and soybean oils, as well as monounsaturated fatty acids, found in olive oil and peanut oil, can contribute to improvement of NALFD, Dr. Alqahtani said.
In contrast, foods high in saturated fatty acids, such as butter, as well as trans fats and cholesterol can contribute to NAFLD progression, he said.
In their studies of intrahepatic triglyceride content, Dr. Alqahtani and colleagues found that fatty acids in the liver come from three major sources: dietary fatty acids, which account for about 15% of liver fat, tissue lipolysis, and de novo hepatic lipogenesis.
Previous systematic reviews and meta-analyses of the relationship between diet and NAFLD have focused on marine-based (n-3) PUFAs, but “the data regarding the evidence of unsaturated fatty acids through supplements or monounsaturated fatty acids through dietary supplementation are lacking,” he said.
To summarize the effects of dietary or supplemental fatty acids on liver and metabolic parameters in adults with NAFLD, Dr. Alqahtani and colleagues conducted a systematic review and meta-analysis, concentrating on studies that included specifics about interventions and outcomes.
They identified a total of 18 randomized controlled trials and 4 case-control studies that met their criteria. The studies were published from 2008 to 2020.
Regarding the effects of interventions on the components of NASH, they found that, in 1 or more of 12 randomized trials of PUFA supplementation with or without dietary interventions, there were associations with decreased hepatic steatosis, lobular inflammation, and fibrosis and declines in ALT and AST levels.
In three trials of dietary-only interventions, there were decreases in hepatic steatosis and ALT and/or AST levels. In two studies of the effects of healthy cooking oils only, hepatic steatosis decreased, but there was no effect on ALT or AST levels.
All three interventions were associated with improvements in fasting glucose levels and insulin metabolism, as well as decreases in total cholesterol, triglycerides, and LDL cholesterol and increases in HDL cholesterol.
Better understanding of dietary composition
“We’ve known for a while that dietary composition may impact NAFLD and NASH,” said Manal F. Abdelmalek, MD, professor of medicine at Duke University, Durham, N.C., who commented on the study.
“What [Dr. Alqahtani and colleagues] have shown is that supplementation with healthy fatty acids improves fatty liver. This really does extend our knowledge of what we understand about dietary composition, particularly the recommendations that support higher fish consumption and a Mediterranean-style diet,” she said.
“It’s not just about the fat but the type of fat that’s consumed, and drilling down to the particulars of dietary composition beyond calories alone,” she added.
No source of funding for the study has been disclosed. Dr. Alqahtani and Dr. Abdelmalek have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with nonalcoholic fatty liver disease (NAFLD) who supplement their diets with polyunsaturated fatty acids (PUFA), liver and metabolic parameters improve, results of a systematic review and meta-analysis suggest.
Data from randomized clinical trials show that, for participants with NAFLD who used PUFA supplements with or without additional dietary interventions, hepatic steatosis and lobular inflammation decreased, and in one study, fibrosis decreased. There were also improvements in liver enzyme levels, said Saleh Alqahtani, MBChB, associate professor of medicine at Johns Hopkins University, Baltimore, during a presentation at the annual meeting of the American Association for the Study of Liver Diseases.
“Since there’s no effective medical therapy for NAFLD, weight loss through lifestyle modifications becomes the most important focused intervention for patients with NAFLD,” he said. “However, the majority of patients fail to achieve or to maintain weight loss for long-term therapy. Therefore, dietary intervention or supplementation might help reduce the prevalence of NAFLD and decrease the progression of nonalcoholic steatohepatitis [NASH] and liver cirrhosis.
“More clinical trials are warranted to determine the long-term efficacy of the Mediterranean diet and polyunsaturated fatty acid supplementation among adult patients with NAFLD,” he added.
RCTs and case-control studies
It’s well documented that consumption of PUFAs, found in fatty fish and in canola, grapeseed, corn, and soybean oils, as well as monounsaturated fatty acids, found in olive oil and peanut oil, can contribute to improvement of NALFD, Dr. Alqahtani said.
In contrast, foods high in saturated fatty acids, such as butter, as well as trans fats and cholesterol can contribute to NAFLD progression, he said.
In their studies of intrahepatic triglyceride content, Dr. Alqahtani and colleagues found that fatty acids in the liver come from three major sources: dietary fatty acids, which account for about 15% of liver fat, tissue lipolysis, and de novo hepatic lipogenesis.
Previous systematic reviews and meta-analyses of the relationship between diet and NAFLD have focused on marine-based (n-3) PUFAs, but “the data regarding the evidence of unsaturated fatty acids through supplements or monounsaturated fatty acids through dietary supplementation are lacking,” he said.
To summarize the effects of dietary or supplemental fatty acids on liver and metabolic parameters in adults with NAFLD, Dr. Alqahtani and colleagues conducted a systematic review and meta-analysis, concentrating on studies that included specifics about interventions and outcomes.
They identified a total of 18 randomized controlled trials and 4 case-control studies that met their criteria. The studies were published from 2008 to 2020.
Regarding the effects of interventions on the components of NASH, they found that, in 1 or more of 12 randomized trials of PUFA supplementation with or without dietary interventions, there were associations with decreased hepatic steatosis, lobular inflammation, and fibrosis and declines in ALT and AST levels.
In three trials of dietary-only interventions, there were decreases in hepatic steatosis and ALT and/or AST levels. In two studies of the effects of healthy cooking oils only, hepatic steatosis decreased, but there was no effect on ALT or AST levels.
All three interventions were associated with improvements in fasting glucose levels and insulin metabolism, as well as decreases in total cholesterol, triglycerides, and LDL cholesterol and increases in HDL cholesterol.
Better understanding of dietary composition
“We’ve known for a while that dietary composition may impact NAFLD and NASH,” said Manal F. Abdelmalek, MD, professor of medicine at Duke University, Durham, N.C., who commented on the study.
“What [Dr. Alqahtani and colleagues] have shown is that supplementation with healthy fatty acids improves fatty liver. This really does extend our knowledge of what we understand about dietary composition, particularly the recommendations that support higher fish consumption and a Mediterranean-style diet,” she said.
“It’s not just about the fat but the type of fat that’s consumed, and drilling down to the particulars of dietary composition beyond calories alone,” she added.
No source of funding for the study has been disclosed. Dr. Alqahtani and Dr. Abdelmalek have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LIVER MEETING 2021
Patient whips out smartphone and starts recording: Trouble ahead?
Joe Lindsey, a 48-year old Colorado-based journalist, has dealt with complex hearing loss for about 15 years. which has led to countless doctor’s visits, treatments, and even surgery in hopes of finding improvement. As time went on and Mr. Lindsey’s hearing deteriorated, he began recording his appointments in order to retain important information.
Mr. Lindsey had positive intentions, but not every patient does.
With smartphones everywhere, recording medical appointments can be fraught with downsides too. While there are clear-cut reasons for recording doctor visits, patients’ goals and how they carry out the taping are key. Audio only? Or also video? With the physician’s knowledge and permission, or without?
These are the legal and ethical weeds doctors find themselves in today, so it’s important to understand all sides of the issue.
The medical world is divided on its sentiments about patients recording their visits. The American Medical Association, in fact, failed to make progress on a recent policy (resolution 007) proposal to encourage that any “audio or video recording made during a medical encounter should require both physician and patient notification and consent.” Rather than voting on the resolution, the AMA house of delegates tabled it and chose to gather more information on the issue.
In most cases, patients are recording their visits in good faith, says Jeffrey Segal, MD, JD, the CEO and founder of Medical Justice, a risk mitigation and reputation management firm for healthcare clinicians. “When it comes to ‘Team, let’s record this,’ I’m a fan,” he says. “The most common reason patients record visits is that there’s a lot of information transferred from the doctor to the patient, and there’s just not enough time to absorb it all.”
While the option is there for patients to take notes, in the give-and-take nature of conversation, this can get difficult. “If they record the visit, they can then digest it all down the road,” says Dr. Segal. “A compliant patient is one who understands what’s expected. That’s the charitable explanation for recording, and I support it.”
It’s that question of good intent, however, that concerns some physicians in today’s highly litigious society. “The worry is that there’s a small subset of patients with an ulterior motive,” says Dr. Segal.
“Some patients do record in case of an event down the road,” he adds. “They want the recording to potentially talk to a lawyer, or to file a board complaint.”
Laws in the United States surrounding recordings are confusing, with variations from state to state. Currently, 39 U.S. states allow for one-party consent — meaning a patient can record a visit without consenting with the physician.
Monica Verduzco-Gutierrez, MD, professor and chair of rehabilitation medicine at University of Texas Health, San Antonio, resides in Texas, which is one of the 39 one-consent states. “Physicians must be aware of this fact and consider how it might be used against them,” she says. “A good practice is to set expectations with the patient from the start. Also, know your hospital’s policy — some may have boundaries surrounding recordings.”
The first step is to know what type of state you practice in. Regardless of whether you are in a one- or two-party consent state — but especially a one-party state — it’s a smart move to add a sign at your office saying that you support the recording of visits, provided the patient is open and transparent about it. “Let the patient know that if they plan to record, they should ask your permission,” says Dr. Segal. “Let them know it’s not appropriate if they haven’t received your permission.”
There are, of course, the occasional horror stories involving surreptitious recordings. “I remember a case where a patient left a phone actively recording in his bag of clothing, which went into the OR with him,” he says. “The background conversation was not flattering to the patient, who happened to be an employee of the hospital. When he came to and listened to the recording, he sued, winning his case.”
The age of video and telehealth
What about the rare situation when a patient pulls out a phone and begins to videotape a conversation? It can be a big slippery slope. “Patients can abuse a video recording with editing, and the recording becomes one-dimensional, which is unfair to the physician,” adds Dr. Segal.
Patients sometimes have other motives as well. “I’m aware of occasions where a doctor/patient visit got heated and the patient took out the phone to video record, sharing it to social media,” says Dr. Segal. “Once someone uses a phone to take video, just stop the conversation. Tell the patient, ‘We’re having a disagreement,’ and that it’s time to put an end to it.”
He adds that from the physician side, a video can be a protagonist in a conversation. “Frankly, a camera on your face changes the nature of things,” Dr. Segal says. “It’s much easier to have the phone sitting in a corner, quietly recording.”
Other scenarios might involve a patient’s family member accompanying the patient and bringing out their phone to record. “Doctors should consider how this might be used against them — it can blow up,” says Dr. Verduzco-Gutierrez. “Draw boundaries on this behavior, using your hospital’s policy if it has one.”
In today’s pandemic landscape, this is particularly important, she adds. “There’s generally more mistrust in the medical system right now,” says Dr. Verduzco-Gutierrez. “People are getting misinformation from sources that aren’t credible, and then want to record their visits because they aren’t receiving the treatment they want, for instance.”
COVID has also added the tricky element of telehealth, which has exploded since 2020. “You don’t know what a patient is doing on the other side of the screen,” Dr. Verduzco-Gutierrez explains. “Face-to-face, you might see them with their phones out, but anything goes with telehealth. You have to be open and communicative with your patients about your policies from the start to avoid any negative connotations.”
How taping can help patients
Mr. Lindsey, the Colorado journalist, is far from alone in his desire to use visit recordings in order to retain valuable information — and with good reason. According to the Dartmouth Institute for Health Policy and Clinical Practice’s Open Recordings Project, at least 1 in 10 patients records their doctor’s visits.
“I realized I was missing things and in a medical setting, that matters,” Mr. Lindsey says. “Last year, once COVID hit and we all began wearing masks, I lost my ability to read lips, one of my coping mechanisms. It became even more important that I had a backup recording to ensure I understood everything.”
Even if a patient doesn’t have hearing loss like Mr. Lindsey, having an audio record of a visit can be useful. According to a 2018 study on patient recall of key information 1 week out from their visits, 49% of decisions and recommendations were recalled accurately without prompting; 36% recalled with a prompt; and 15% recalled erroneously or not at all.
This squares with the personal experiences of Dr. Verduzco-Gutierrez. “I even see this with my mom, who doesn’t remember many details of her doctor’s visits when I ask her,” she says. “This can definitely impact treatment.”
For better or worse
Dr. Verduzco-Gutierrez says that often it comes down to how a patient learns best. “I teach my residents to keep this in mind and to ask the patient in advance what works best for them,” she says. “If a patient is a visual learner, they might want to take notes or have access to the appointment notes after the visit. If they will learn and retain the information best with an audio recording, then offer that option.”
Mr. Lindsey makes it a habit to inform his physicians that he will be making an audio recording of his visits. “I always let them know that I’m recording for accuracy and not to catch them in some sort of falsehood,” he says. “I can get the doctor’s notes, but those are often short and to the point; I can get more information by going back over the recording.”
To date, Mr. Lindsey hasn’t experienced any pushback from his physicians. “No one has balked at the idea or acted surprised that I want to do it,” he explains. “I think most doctors appreciate that we have a tool we can make use of for better care.”
In past coverage of the topic, some healthcare providers weighed in with support for recordings, usually citing personal reasons. “I am so very grateful for the physicians that allowed me to record the medical appointments that I attended with my parents,” said one. “As their adult daughter, I was painfully aware that my parents struggled to process and understand all of the new information coming their way.”
Another expressed support as well, stating that as a patient, he prefers recordings to notes, because the latter “bears little resemblance to the content of the meeting and discussion with the physician. If the patient straightforwardly asks for permission to record, then why not honor the good intent expressed thereby?”
More often than not, patients have good intentions when they decide to hit the record button in a medical visit. A little preparation goes a long way, however, says Dr. Segal: “Assume you’re being recorded, and act accordingly.”
A version of this article first appeared on Medscape.com.
Joe Lindsey, a 48-year old Colorado-based journalist, has dealt with complex hearing loss for about 15 years. which has led to countless doctor’s visits, treatments, and even surgery in hopes of finding improvement. As time went on and Mr. Lindsey’s hearing deteriorated, he began recording his appointments in order to retain important information.
Mr. Lindsey had positive intentions, but not every patient does.
With smartphones everywhere, recording medical appointments can be fraught with downsides too. While there are clear-cut reasons for recording doctor visits, patients’ goals and how they carry out the taping are key. Audio only? Or also video? With the physician’s knowledge and permission, or without?
These are the legal and ethical weeds doctors find themselves in today, so it’s important to understand all sides of the issue.
The medical world is divided on its sentiments about patients recording their visits. The American Medical Association, in fact, failed to make progress on a recent policy (resolution 007) proposal to encourage that any “audio or video recording made during a medical encounter should require both physician and patient notification and consent.” Rather than voting on the resolution, the AMA house of delegates tabled it and chose to gather more information on the issue.
In most cases, patients are recording their visits in good faith, says Jeffrey Segal, MD, JD, the CEO and founder of Medical Justice, a risk mitigation and reputation management firm for healthcare clinicians. “When it comes to ‘Team, let’s record this,’ I’m a fan,” he says. “The most common reason patients record visits is that there’s a lot of information transferred from the doctor to the patient, and there’s just not enough time to absorb it all.”
While the option is there for patients to take notes, in the give-and-take nature of conversation, this can get difficult. “If they record the visit, they can then digest it all down the road,” says Dr. Segal. “A compliant patient is one who understands what’s expected. That’s the charitable explanation for recording, and I support it.”
It’s that question of good intent, however, that concerns some physicians in today’s highly litigious society. “The worry is that there’s a small subset of patients with an ulterior motive,” says Dr. Segal.
“Some patients do record in case of an event down the road,” he adds. “They want the recording to potentially talk to a lawyer, or to file a board complaint.”
Laws in the United States surrounding recordings are confusing, with variations from state to state. Currently, 39 U.S. states allow for one-party consent — meaning a patient can record a visit without consenting with the physician.
Monica Verduzco-Gutierrez, MD, professor and chair of rehabilitation medicine at University of Texas Health, San Antonio, resides in Texas, which is one of the 39 one-consent states. “Physicians must be aware of this fact and consider how it might be used against them,” she says. “A good practice is to set expectations with the patient from the start. Also, know your hospital’s policy — some may have boundaries surrounding recordings.”
The first step is to know what type of state you practice in. Regardless of whether you are in a one- or two-party consent state — but especially a one-party state — it’s a smart move to add a sign at your office saying that you support the recording of visits, provided the patient is open and transparent about it. “Let the patient know that if they plan to record, they should ask your permission,” says Dr. Segal. “Let them know it’s not appropriate if they haven’t received your permission.”
There are, of course, the occasional horror stories involving surreptitious recordings. “I remember a case where a patient left a phone actively recording in his bag of clothing, which went into the OR with him,” he says. “The background conversation was not flattering to the patient, who happened to be an employee of the hospital. When he came to and listened to the recording, he sued, winning his case.”
The age of video and telehealth
What about the rare situation when a patient pulls out a phone and begins to videotape a conversation? It can be a big slippery slope. “Patients can abuse a video recording with editing, and the recording becomes one-dimensional, which is unfair to the physician,” adds Dr. Segal.
Patients sometimes have other motives as well. “I’m aware of occasions where a doctor/patient visit got heated and the patient took out the phone to video record, sharing it to social media,” says Dr. Segal. “Once someone uses a phone to take video, just stop the conversation. Tell the patient, ‘We’re having a disagreement,’ and that it’s time to put an end to it.”
He adds that from the physician side, a video can be a protagonist in a conversation. “Frankly, a camera on your face changes the nature of things,” Dr. Segal says. “It’s much easier to have the phone sitting in a corner, quietly recording.”
Other scenarios might involve a patient’s family member accompanying the patient and bringing out their phone to record. “Doctors should consider how this might be used against them — it can blow up,” says Dr. Verduzco-Gutierrez. “Draw boundaries on this behavior, using your hospital’s policy if it has one.”
In today’s pandemic landscape, this is particularly important, she adds. “There’s generally more mistrust in the medical system right now,” says Dr. Verduzco-Gutierrez. “People are getting misinformation from sources that aren’t credible, and then want to record their visits because they aren’t receiving the treatment they want, for instance.”
COVID has also added the tricky element of telehealth, which has exploded since 2020. “You don’t know what a patient is doing on the other side of the screen,” Dr. Verduzco-Gutierrez explains. “Face-to-face, you might see them with their phones out, but anything goes with telehealth. You have to be open and communicative with your patients about your policies from the start to avoid any negative connotations.”
How taping can help patients
Mr. Lindsey, the Colorado journalist, is far from alone in his desire to use visit recordings in order to retain valuable information — and with good reason. According to the Dartmouth Institute for Health Policy and Clinical Practice’s Open Recordings Project, at least 1 in 10 patients records their doctor’s visits.
“I realized I was missing things and in a medical setting, that matters,” Mr. Lindsey says. “Last year, once COVID hit and we all began wearing masks, I lost my ability to read lips, one of my coping mechanisms. It became even more important that I had a backup recording to ensure I understood everything.”
Even if a patient doesn’t have hearing loss like Mr. Lindsey, having an audio record of a visit can be useful. According to a 2018 study on patient recall of key information 1 week out from their visits, 49% of decisions and recommendations were recalled accurately without prompting; 36% recalled with a prompt; and 15% recalled erroneously or not at all.
This squares with the personal experiences of Dr. Verduzco-Gutierrez. “I even see this with my mom, who doesn’t remember many details of her doctor’s visits when I ask her,” she says. “This can definitely impact treatment.”
For better or worse
Dr. Verduzco-Gutierrez says that often it comes down to how a patient learns best. “I teach my residents to keep this in mind and to ask the patient in advance what works best for them,” she says. “If a patient is a visual learner, they might want to take notes or have access to the appointment notes after the visit. If they will learn and retain the information best with an audio recording, then offer that option.”
Mr. Lindsey makes it a habit to inform his physicians that he will be making an audio recording of his visits. “I always let them know that I’m recording for accuracy and not to catch them in some sort of falsehood,” he says. “I can get the doctor’s notes, but those are often short and to the point; I can get more information by going back over the recording.”
To date, Mr. Lindsey hasn’t experienced any pushback from his physicians. “No one has balked at the idea or acted surprised that I want to do it,” he explains. “I think most doctors appreciate that we have a tool we can make use of for better care.”
In past coverage of the topic, some healthcare providers weighed in with support for recordings, usually citing personal reasons. “I am so very grateful for the physicians that allowed me to record the medical appointments that I attended with my parents,” said one. “As their adult daughter, I was painfully aware that my parents struggled to process and understand all of the new information coming their way.”
Another expressed support as well, stating that as a patient, he prefers recordings to notes, because the latter “bears little resemblance to the content of the meeting and discussion with the physician. If the patient straightforwardly asks for permission to record, then why not honor the good intent expressed thereby?”
More often than not, patients have good intentions when they decide to hit the record button in a medical visit. A little preparation goes a long way, however, says Dr. Segal: “Assume you’re being recorded, and act accordingly.”
A version of this article first appeared on Medscape.com.
Joe Lindsey, a 48-year old Colorado-based journalist, has dealt with complex hearing loss for about 15 years. which has led to countless doctor’s visits, treatments, and even surgery in hopes of finding improvement. As time went on and Mr. Lindsey’s hearing deteriorated, he began recording his appointments in order to retain important information.
Mr. Lindsey had positive intentions, but not every patient does.
With smartphones everywhere, recording medical appointments can be fraught with downsides too. While there are clear-cut reasons for recording doctor visits, patients’ goals and how they carry out the taping are key. Audio only? Or also video? With the physician’s knowledge and permission, or without?
These are the legal and ethical weeds doctors find themselves in today, so it’s important to understand all sides of the issue.
The medical world is divided on its sentiments about patients recording their visits. The American Medical Association, in fact, failed to make progress on a recent policy (resolution 007) proposal to encourage that any “audio or video recording made during a medical encounter should require both physician and patient notification and consent.” Rather than voting on the resolution, the AMA house of delegates tabled it and chose to gather more information on the issue.
In most cases, patients are recording their visits in good faith, says Jeffrey Segal, MD, JD, the CEO and founder of Medical Justice, a risk mitigation and reputation management firm for healthcare clinicians. “When it comes to ‘Team, let’s record this,’ I’m a fan,” he says. “The most common reason patients record visits is that there’s a lot of information transferred from the doctor to the patient, and there’s just not enough time to absorb it all.”
While the option is there for patients to take notes, in the give-and-take nature of conversation, this can get difficult. “If they record the visit, they can then digest it all down the road,” says Dr. Segal. “A compliant patient is one who understands what’s expected. That’s the charitable explanation for recording, and I support it.”
It’s that question of good intent, however, that concerns some physicians in today’s highly litigious society. “The worry is that there’s a small subset of patients with an ulterior motive,” says Dr. Segal.
“Some patients do record in case of an event down the road,” he adds. “They want the recording to potentially talk to a lawyer, or to file a board complaint.”
Laws in the United States surrounding recordings are confusing, with variations from state to state. Currently, 39 U.S. states allow for one-party consent — meaning a patient can record a visit without consenting with the physician.
Monica Verduzco-Gutierrez, MD, professor and chair of rehabilitation medicine at University of Texas Health, San Antonio, resides in Texas, which is one of the 39 one-consent states. “Physicians must be aware of this fact and consider how it might be used against them,” she says. “A good practice is to set expectations with the patient from the start. Also, know your hospital’s policy — some may have boundaries surrounding recordings.”
The first step is to know what type of state you practice in. Regardless of whether you are in a one- or two-party consent state — but especially a one-party state — it’s a smart move to add a sign at your office saying that you support the recording of visits, provided the patient is open and transparent about it. “Let the patient know that if they plan to record, they should ask your permission,” says Dr. Segal. “Let them know it’s not appropriate if they haven’t received your permission.”
There are, of course, the occasional horror stories involving surreptitious recordings. “I remember a case where a patient left a phone actively recording in his bag of clothing, which went into the OR with him,” he says. “The background conversation was not flattering to the patient, who happened to be an employee of the hospital. When he came to and listened to the recording, he sued, winning his case.”
The age of video and telehealth
What about the rare situation when a patient pulls out a phone and begins to videotape a conversation? It can be a big slippery slope. “Patients can abuse a video recording with editing, and the recording becomes one-dimensional, which is unfair to the physician,” adds Dr. Segal.
Patients sometimes have other motives as well. “I’m aware of occasions where a doctor/patient visit got heated and the patient took out the phone to video record, sharing it to social media,” says Dr. Segal. “Once someone uses a phone to take video, just stop the conversation. Tell the patient, ‘We’re having a disagreement,’ and that it’s time to put an end to it.”
He adds that from the physician side, a video can be a protagonist in a conversation. “Frankly, a camera on your face changes the nature of things,” Dr. Segal says. “It’s much easier to have the phone sitting in a corner, quietly recording.”
Other scenarios might involve a patient’s family member accompanying the patient and bringing out their phone to record. “Doctors should consider how this might be used against them — it can blow up,” says Dr. Verduzco-Gutierrez. “Draw boundaries on this behavior, using your hospital’s policy if it has one.”
In today’s pandemic landscape, this is particularly important, she adds. “There’s generally more mistrust in the medical system right now,” says Dr. Verduzco-Gutierrez. “People are getting misinformation from sources that aren’t credible, and then want to record their visits because they aren’t receiving the treatment they want, for instance.”
COVID has also added the tricky element of telehealth, which has exploded since 2020. “You don’t know what a patient is doing on the other side of the screen,” Dr. Verduzco-Gutierrez explains. “Face-to-face, you might see them with their phones out, but anything goes with telehealth. You have to be open and communicative with your patients about your policies from the start to avoid any negative connotations.”
How taping can help patients
Mr. Lindsey, the Colorado journalist, is far from alone in his desire to use visit recordings in order to retain valuable information — and with good reason. According to the Dartmouth Institute for Health Policy and Clinical Practice’s Open Recordings Project, at least 1 in 10 patients records their doctor’s visits.
“I realized I was missing things and in a medical setting, that matters,” Mr. Lindsey says. “Last year, once COVID hit and we all began wearing masks, I lost my ability to read lips, one of my coping mechanisms. It became even more important that I had a backup recording to ensure I understood everything.”
Even if a patient doesn’t have hearing loss like Mr. Lindsey, having an audio record of a visit can be useful. According to a 2018 study on patient recall of key information 1 week out from their visits, 49% of decisions and recommendations were recalled accurately without prompting; 36% recalled with a prompt; and 15% recalled erroneously or not at all.
This squares with the personal experiences of Dr. Verduzco-Gutierrez. “I even see this with my mom, who doesn’t remember many details of her doctor’s visits when I ask her,” she says. “This can definitely impact treatment.”
For better or worse
Dr. Verduzco-Gutierrez says that often it comes down to how a patient learns best. “I teach my residents to keep this in mind and to ask the patient in advance what works best for them,” she says. “If a patient is a visual learner, they might want to take notes or have access to the appointment notes after the visit. If they will learn and retain the information best with an audio recording, then offer that option.”
Mr. Lindsey makes it a habit to inform his physicians that he will be making an audio recording of his visits. “I always let them know that I’m recording for accuracy and not to catch them in some sort of falsehood,” he says. “I can get the doctor’s notes, but those are often short and to the point; I can get more information by going back over the recording.”
To date, Mr. Lindsey hasn’t experienced any pushback from his physicians. “No one has balked at the idea or acted surprised that I want to do it,” he explains. “I think most doctors appreciate that we have a tool we can make use of for better care.”
In past coverage of the topic, some healthcare providers weighed in with support for recordings, usually citing personal reasons. “I am so very grateful for the physicians that allowed me to record the medical appointments that I attended with my parents,” said one. “As their adult daughter, I was painfully aware that my parents struggled to process and understand all of the new information coming their way.”
Another expressed support as well, stating that as a patient, he prefers recordings to notes, because the latter “bears little resemblance to the content of the meeting and discussion with the physician. If the patient straightforwardly asks for permission to record, then why not honor the good intent expressed thereby?”
More often than not, patients have good intentions when they decide to hit the record button in a medical visit. A little preparation goes a long way, however, says Dr. Segal: “Assume you’re being recorded, and act accordingly.”
A version of this article first appeared on Medscape.com.
CDC: All adults should be eligible for Pfizer, Moderna boosters
on its vaccine recommendations.
The Advisory Committee on Immunization Practices, or ACIP, recommended that all adults be eligible for a third dose of a Pfizer or Moderna mRNA vaccine, at least 6 months after their second dose.
They also strengthened a recommendation that everyone over the age of 50 should get a third dose, whether or not they have an underlying health condition that may increase their risk from a COVID-19 infection.
The committee voted 11 to 0 in favor of both policies.
CDC Director Rochelle Walensky, MD, must now sign off on both policies, which she is expected to do.
More than 70 million adults are now eligible for booster shots in the United States, but only about 31 million people have received one. About half of those who have been boosted are over the age of 65.
In a recent survey, the Kaiser Family Foundation found that about 4 in 10 younger adults said they were unsure if they qualified for a booster.
Under the current policy, boosters are recommended for everyone age 65 and older. But people who are younger than age 65 are eligible for boosters if they have an underlying health condition or live or work in a high-risk situation—something individuals have to determine on their own. Experts said that shading of the policy had created confusion that was holding people back.
Nirav Shah, MD, JD, president of the Association of State and Territorial Health Officials, noted that public health officials have been swamped with calls from people who are trying to figure out if they are eligible to get a booster dose.
He said that in a call the evening of Nov. 18 with state health departments, “There was not a single state that voiced opposition to this move,” he told the ACIP.
Dr. Shah said that the current guidelines were well intentioned, but “in pursuit of precision, they create confusion.”
“Our concern is that eligible individuals are not receiving boosters right now as a result of this confusion,” he said.
The committee based its decision on the results of a new study of boosters in Pfizer vaccine recipients, as well as reassuring safety information that’s being collected through the CDC and FDA’s monitoring systems.
Pfizer presented the early results from a study of 10,000 people who had all received two doses of its vaccine. Half of the study participants received a third shot, or booster. The other half got a placebo.
The study is ongoing, but so far, six of the people in the booster group have gotten a COVID-19 infection with symptoms compared to 123 people who got COVID-19 in the placebo group, making boosters 95% effective at keeping people from getting sick. Most people in the study had gotten their original doses about 10 months earlier. They’ve been followed for about 10 weeks since their booster. Importantly, there were no study participants hospitalized for COVID-19 infections in either the placebo or booster group, indicating that the first two doses were still very effective at preventing severe outcomes from infection.
The majority of side effects after a third Pfizer dose were mild and temporary. Side effects like sore arms, swelling, fever, headache, and fatigue were more common in the booster group — affecting about 1 in 4 people who got a third shot. Vaccination side effects were less common after boosters than have been seen after the second dose of the vaccine.
Some cases of myocarditis and pericarditis have been reported after people received vaccine boosters, but the risk for this heart inflammation appears to be extremely low, about two cases for every million doses given. There were 54 cases of myocarditis reported so far to the Vaccine Adverse Event Reporting System, or VAERS. So far, only 12 have met the case definition and are considered related to vaccination. Most of the reported cases are still being studied.
on its vaccine recommendations.
The Advisory Committee on Immunization Practices, or ACIP, recommended that all adults be eligible for a third dose of a Pfizer or Moderna mRNA vaccine, at least 6 months after their second dose.
They also strengthened a recommendation that everyone over the age of 50 should get a third dose, whether or not they have an underlying health condition that may increase their risk from a COVID-19 infection.
The committee voted 11 to 0 in favor of both policies.
CDC Director Rochelle Walensky, MD, must now sign off on both policies, which she is expected to do.
More than 70 million adults are now eligible for booster shots in the United States, but only about 31 million people have received one. About half of those who have been boosted are over the age of 65.
In a recent survey, the Kaiser Family Foundation found that about 4 in 10 younger adults said they were unsure if they qualified for a booster.
Under the current policy, boosters are recommended for everyone age 65 and older. But people who are younger than age 65 are eligible for boosters if they have an underlying health condition or live or work in a high-risk situation—something individuals have to determine on their own. Experts said that shading of the policy had created confusion that was holding people back.
Nirav Shah, MD, JD, president of the Association of State and Territorial Health Officials, noted that public health officials have been swamped with calls from people who are trying to figure out if they are eligible to get a booster dose.
He said that in a call the evening of Nov. 18 with state health departments, “There was not a single state that voiced opposition to this move,” he told the ACIP.
Dr. Shah said that the current guidelines were well intentioned, but “in pursuit of precision, they create confusion.”
“Our concern is that eligible individuals are not receiving boosters right now as a result of this confusion,” he said.
The committee based its decision on the results of a new study of boosters in Pfizer vaccine recipients, as well as reassuring safety information that’s being collected through the CDC and FDA’s monitoring systems.
Pfizer presented the early results from a study of 10,000 people who had all received two doses of its vaccine. Half of the study participants received a third shot, or booster. The other half got a placebo.
The study is ongoing, but so far, six of the people in the booster group have gotten a COVID-19 infection with symptoms compared to 123 people who got COVID-19 in the placebo group, making boosters 95% effective at keeping people from getting sick. Most people in the study had gotten their original doses about 10 months earlier. They’ve been followed for about 10 weeks since their booster. Importantly, there were no study participants hospitalized for COVID-19 infections in either the placebo or booster group, indicating that the first two doses were still very effective at preventing severe outcomes from infection.
The majority of side effects after a third Pfizer dose were mild and temporary. Side effects like sore arms, swelling, fever, headache, and fatigue were more common in the booster group — affecting about 1 in 4 people who got a third shot. Vaccination side effects were less common after boosters than have been seen after the second dose of the vaccine.
Some cases of myocarditis and pericarditis have been reported after people received vaccine boosters, but the risk for this heart inflammation appears to be extremely low, about two cases for every million doses given. There were 54 cases of myocarditis reported so far to the Vaccine Adverse Event Reporting System, or VAERS. So far, only 12 have met the case definition and are considered related to vaccination. Most of the reported cases are still being studied.
on its vaccine recommendations.
The Advisory Committee on Immunization Practices, or ACIP, recommended that all adults be eligible for a third dose of a Pfizer or Moderna mRNA vaccine, at least 6 months after their second dose.
They also strengthened a recommendation that everyone over the age of 50 should get a third dose, whether or not they have an underlying health condition that may increase their risk from a COVID-19 infection.
The committee voted 11 to 0 in favor of both policies.
CDC Director Rochelle Walensky, MD, must now sign off on both policies, which she is expected to do.
More than 70 million adults are now eligible for booster shots in the United States, but only about 31 million people have received one. About half of those who have been boosted are over the age of 65.
In a recent survey, the Kaiser Family Foundation found that about 4 in 10 younger adults said they were unsure if they qualified for a booster.
Under the current policy, boosters are recommended for everyone age 65 and older. But people who are younger than age 65 are eligible for boosters if they have an underlying health condition or live or work in a high-risk situation—something individuals have to determine on their own. Experts said that shading of the policy had created confusion that was holding people back.
Nirav Shah, MD, JD, president of the Association of State and Territorial Health Officials, noted that public health officials have been swamped with calls from people who are trying to figure out if they are eligible to get a booster dose.
He said that in a call the evening of Nov. 18 with state health departments, “There was not a single state that voiced opposition to this move,” he told the ACIP.
Dr. Shah said that the current guidelines were well intentioned, but “in pursuit of precision, they create confusion.”
“Our concern is that eligible individuals are not receiving boosters right now as a result of this confusion,” he said.
The committee based its decision on the results of a new study of boosters in Pfizer vaccine recipients, as well as reassuring safety information that’s being collected through the CDC and FDA’s monitoring systems.
Pfizer presented the early results from a study of 10,000 people who had all received two doses of its vaccine. Half of the study participants received a third shot, or booster. The other half got a placebo.
The study is ongoing, but so far, six of the people in the booster group have gotten a COVID-19 infection with symptoms compared to 123 people who got COVID-19 in the placebo group, making boosters 95% effective at keeping people from getting sick. Most people in the study had gotten their original doses about 10 months earlier. They’ve been followed for about 10 weeks since their booster. Importantly, there were no study participants hospitalized for COVID-19 infections in either the placebo or booster group, indicating that the first two doses were still very effective at preventing severe outcomes from infection.
The majority of side effects after a third Pfizer dose were mild and temporary. Side effects like sore arms, swelling, fever, headache, and fatigue were more common in the booster group — affecting about 1 in 4 people who got a third shot. Vaccination side effects were less common after boosters than have been seen after the second dose of the vaccine.
Some cases of myocarditis and pericarditis have been reported after people received vaccine boosters, but the risk for this heart inflammation appears to be extremely low, about two cases for every million doses given. There were 54 cases of myocarditis reported so far to the Vaccine Adverse Event Reporting System, or VAERS. So far, only 12 have met the case definition and are considered related to vaccination. Most of the reported cases are still being studied.



