User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Legacy of neutral renal denervation trial recast by long-term outcomes: SYMPLICITY HTN-3
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.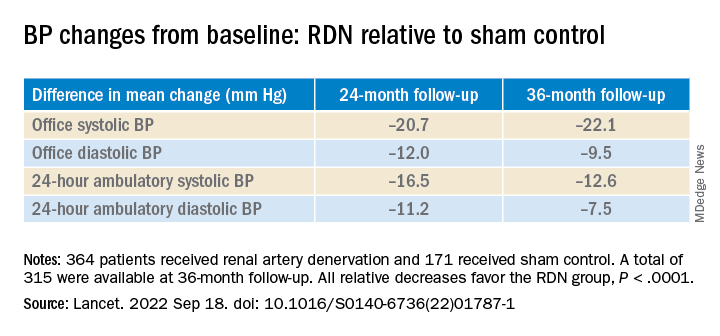
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
AT TCT 2022
Docs gain new flexibility treating osteoporosis from steroids
Doctors caring for patients taking steroids now have broader flexibility for which drugs to use to prevent osteoporosis associated with the medications.
The American College of Rheumatology (ACR) has released an updated guideline that advises treatment providers on when and how long to prescribe therapies that prevent or treat glucocorticoid-induced osteoporosis (GIOP). Since the ACR last updated the guideline in 2017, the Food and Drug Administration has approved new treatments for osteoporosis, which are now included in the recommendations.
The new guideline also advises physicians that they may need to transition patients to a second treatment after concluding a first course – so-called sequential therapy – to better protect them against bone loss and fracture. It also offers detailed instructions for which drugs to use, when, and how long these medications should be administered for patients taking glucocorticoids over a long period of time.
The guideline’s inclusion of sequential therapy is significant and will be helpful to practicing clinicians, according to S.B. Tanner IV, MD, director of the Osteoporosis Clinic at Vanderbilt Health, Nashville, Tenn.
“For the first time, the ACR has offered guidance for starting and stopping treatments,” Dr. Tanner said. “This guideline supports awareness that osteoporosis is lifelong – something that will consistently need monitoring.”
An estimated 2.5 million Americans use glucocorticoids, according to a 2013 study in Arthritis Care & Research. Meanwhile, a 2019 study of residents in Denmark found 3% of people in the country were prescribed glucocorticoids annually. That study estimated 54% of glucocorticoid users were female and found the percentage of people taking glucocorticoids increased with age.
Glucocorticoids are used to treat a variety of inflammatory conditions, from multiple sclerosis to lupus, and often are prescribed to transplant patients to prevent their immune systems from rejecting new organs. When taken over time these medications can cause osteoporosis, which in turn raises the risk of fracture.
More than 10% of patients who receive long-term glucocorticoid treatment are diagnosed with clinical fractures. In addition, even low-dose glucocorticoid therapy is associated with a bone loss rate of 10% per year for a patient.
Osteoporosis prevention
After stopping some prevention therapies for GIOP, a high risk of bone loss or fracture still persists, according to Linda A. Russell, MD, director of the Osteoporosis and Metabolic Bone Health Center for the Hospital for Special Surgery, New York, and co-principal investigator of the new guideline.
“We wanted to be sure the need for sequential treatment is adequately communicated, including to patients who might not know they need to start a second medication,” Dr. Russell said.
Physicians and patients must be aware that when completing a course of one GIOP treatment, another drug for the condition should be started, as specified in the guideline.
“Early intervention can prevent glucocorticoid-induced fractures that can lead to substantial morbidity and increased mortality,” said Mary Beth Humphrey, MD, PhD, interim vice president for research at the University of Oklahoma Health Sciences Center in Oklahoma City and co-principal investigator of the ACR guideline.
Janet Rubin, MD, vice chair for research in the Department of medicine at the University of North Carolina at Chapel Hill, said she is hopeful the guideline will change practice.”The risk of bone loss, fractures, and osteoporosis due to glucocorticoids has been known since the beginning of time, but the guideline reinforces the risk and treatment strategies for rheumatologists,” she said. “Such recommendations are known to influence doctor prescribing habits.”
Anyone can fracture
While age and other risk factors, including menopause, increase the risk of developing GIOP, bone loss can occur rapidly for a patient of any age.
Even a glucocorticoid dose as low as 2.5 mg will increase the risk of vertebral fractures, with some occurring as soon as 3 months after treatment starts, Dr. Humphrey said. For patients taking up to 7.5 mg daily, the risk of vertebral fracture doubles. Doses greater than 10 mg daily for more than 3 months raise the likelihood of a vertebral fracture by a factor of 14, and result in a 300% increase in the likelihood of hip fractures, according to Dr. Humphrey.
“When on steroids, even patients with high bone density scores can fracture,” Dr. Tanner said. “The 2017 guideline was almost too elaborate in its effort to calculate risk. The updated guideline acknowledges moderate risk and suggests that this is a group of patients who need treatment.”
Rank ordering adds flexibility
The updated ACR guideline no longer ranks medications based on patient fracture data, side effects, cost care, and whether the drug is provided through injection, pill, or IV.
All of the preventive treatments the panel recommends reduce the risk of steroid-induced bone loss, Dr. Humphrey said.
“We thought the 2017 guideline was too restrictive,” Dr. Russell said. “We’re giving physicians and patients more leeway to choose a medication based on their preferences.”
Patient preference of delivery mechanism – such as a desire for pills only – can now be weighed more heavily into drug treatment decisions.
“In the exam room, there are three dynamics going on: What the patient wants, what the doctor knows is most effective, and what the insurer will pay,” Dr. Tanner said. “Doing away with rank ordering opens up the conversation beyond cost to consider all those factors.”
The guideline team conducted a systematic literature review for clinical questions on nonpharmacologic and pharmacologic treatment addressed in the 2017 guideline, and for questions on new pharmacologic treatments, discontinuation of medications, and sequential and combination therapy. The voting panel consisted of two patient representatives and 13 experts representing adult and pediatric rheumatology and endocrinology, nephrology, and gastroenterology.
A full manuscript has been submitted for publication in Arthritis & Rheumatology and Arthritis Care and Research for peer review, and is expected to publish in early 2023.
Dr. Humphrey and Dr. Russell, the co-principal investigators for the guideline, and Dr. Rubin have disclosed no relevant financial relationships. Dr. Tanner reported a current research grant funded by Amgen through the University of Alabama at Birmingham and being a paid course instructor for the International Society for Clinical Densitometry bone density course, Osteoporosis Essentials.
A version of this article first appeared on Medscape.com.
Doctors caring for patients taking steroids now have broader flexibility for which drugs to use to prevent osteoporosis associated with the medications.
The American College of Rheumatology (ACR) has released an updated guideline that advises treatment providers on when and how long to prescribe therapies that prevent or treat glucocorticoid-induced osteoporosis (GIOP). Since the ACR last updated the guideline in 2017, the Food and Drug Administration has approved new treatments for osteoporosis, which are now included in the recommendations.
The new guideline also advises physicians that they may need to transition patients to a second treatment after concluding a first course – so-called sequential therapy – to better protect them against bone loss and fracture. It also offers detailed instructions for which drugs to use, when, and how long these medications should be administered for patients taking glucocorticoids over a long period of time.
The guideline’s inclusion of sequential therapy is significant and will be helpful to practicing clinicians, according to S.B. Tanner IV, MD, director of the Osteoporosis Clinic at Vanderbilt Health, Nashville, Tenn.
“For the first time, the ACR has offered guidance for starting and stopping treatments,” Dr. Tanner said. “This guideline supports awareness that osteoporosis is lifelong – something that will consistently need monitoring.”
An estimated 2.5 million Americans use glucocorticoids, according to a 2013 study in Arthritis Care & Research. Meanwhile, a 2019 study of residents in Denmark found 3% of people in the country were prescribed glucocorticoids annually. That study estimated 54% of glucocorticoid users were female and found the percentage of people taking glucocorticoids increased with age.
Glucocorticoids are used to treat a variety of inflammatory conditions, from multiple sclerosis to lupus, and often are prescribed to transplant patients to prevent their immune systems from rejecting new organs. When taken over time these medications can cause osteoporosis, which in turn raises the risk of fracture.
More than 10% of patients who receive long-term glucocorticoid treatment are diagnosed with clinical fractures. In addition, even low-dose glucocorticoid therapy is associated with a bone loss rate of 10% per year for a patient.
Osteoporosis prevention
After stopping some prevention therapies for GIOP, a high risk of bone loss or fracture still persists, according to Linda A. Russell, MD, director of the Osteoporosis and Metabolic Bone Health Center for the Hospital for Special Surgery, New York, and co-principal investigator of the new guideline.
“We wanted to be sure the need for sequential treatment is adequately communicated, including to patients who might not know they need to start a second medication,” Dr. Russell said.
Physicians and patients must be aware that when completing a course of one GIOP treatment, another drug for the condition should be started, as specified in the guideline.
“Early intervention can prevent glucocorticoid-induced fractures that can lead to substantial morbidity and increased mortality,” said Mary Beth Humphrey, MD, PhD, interim vice president for research at the University of Oklahoma Health Sciences Center in Oklahoma City and co-principal investigator of the ACR guideline.
Janet Rubin, MD, vice chair for research in the Department of medicine at the University of North Carolina at Chapel Hill, said she is hopeful the guideline will change practice.”The risk of bone loss, fractures, and osteoporosis due to glucocorticoids has been known since the beginning of time, but the guideline reinforces the risk and treatment strategies for rheumatologists,” she said. “Such recommendations are known to influence doctor prescribing habits.”
Anyone can fracture
While age and other risk factors, including menopause, increase the risk of developing GIOP, bone loss can occur rapidly for a patient of any age.
Even a glucocorticoid dose as low as 2.5 mg will increase the risk of vertebral fractures, with some occurring as soon as 3 months after treatment starts, Dr. Humphrey said. For patients taking up to 7.5 mg daily, the risk of vertebral fracture doubles. Doses greater than 10 mg daily for more than 3 months raise the likelihood of a vertebral fracture by a factor of 14, and result in a 300% increase in the likelihood of hip fractures, according to Dr. Humphrey.
“When on steroids, even patients with high bone density scores can fracture,” Dr. Tanner said. “The 2017 guideline was almost too elaborate in its effort to calculate risk. The updated guideline acknowledges moderate risk and suggests that this is a group of patients who need treatment.”
Rank ordering adds flexibility
The updated ACR guideline no longer ranks medications based on patient fracture data, side effects, cost care, and whether the drug is provided through injection, pill, or IV.
All of the preventive treatments the panel recommends reduce the risk of steroid-induced bone loss, Dr. Humphrey said.
“We thought the 2017 guideline was too restrictive,” Dr. Russell said. “We’re giving physicians and patients more leeway to choose a medication based on their preferences.”
Patient preference of delivery mechanism – such as a desire for pills only – can now be weighed more heavily into drug treatment decisions.
“In the exam room, there are three dynamics going on: What the patient wants, what the doctor knows is most effective, and what the insurer will pay,” Dr. Tanner said. “Doing away with rank ordering opens up the conversation beyond cost to consider all those factors.”
The guideline team conducted a systematic literature review for clinical questions on nonpharmacologic and pharmacologic treatment addressed in the 2017 guideline, and for questions on new pharmacologic treatments, discontinuation of medications, and sequential and combination therapy. The voting panel consisted of two patient representatives and 13 experts representing adult and pediatric rheumatology and endocrinology, nephrology, and gastroenterology.
A full manuscript has been submitted for publication in Arthritis & Rheumatology and Arthritis Care and Research for peer review, and is expected to publish in early 2023.
Dr. Humphrey and Dr. Russell, the co-principal investigators for the guideline, and Dr. Rubin have disclosed no relevant financial relationships. Dr. Tanner reported a current research grant funded by Amgen through the University of Alabama at Birmingham and being a paid course instructor for the International Society for Clinical Densitometry bone density course, Osteoporosis Essentials.
A version of this article first appeared on Medscape.com.
Doctors caring for patients taking steroids now have broader flexibility for which drugs to use to prevent osteoporosis associated with the medications.
The American College of Rheumatology (ACR) has released an updated guideline that advises treatment providers on when and how long to prescribe therapies that prevent or treat glucocorticoid-induced osteoporosis (GIOP). Since the ACR last updated the guideline in 2017, the Food and Drug Administration has approved new treatments for osteoporosis, which are now included in the recommendations.
The new guideline also advises physicians that they may need to transition patients to a second treatment after concluding a first course – so-called sequential therapy – to better protect them against bone loss and fracture. It also offers detailed instructions for which drugs to use, when, and how long these medications should be administered for patients taking glucocorticoids over a long period of time.
The guideline’s inclusion of sequential therapy is significant and will be helpful to practicing clinicians, according to S.B. Tanner IV, MD, director of the Osteoporosis Clinic at Vanderbilt Health, Nashville, Tenn.
“For the first time, the ACR has offered guidance for starting and stopping treatments,” Dr. Tanner said. “This guideline supports awareness that osteoporosis is lifelong – something that will consistently need monitoring.”
An estimated 2.5 million Americans use glucocorticoids, according to a 2013 study in Arthritis Care & Research. Meanwhile, a 2019 study of residents in Denmark found 3% of people in the country were prescribed glucocorticoids annually. That study estimated 54% of glucocorticoid users were female and found the percentage of people taking glucocorticoids increased with age.
Glucocorticoids are used to treat a variety of inflammatory conditions, from multiple sclerosis to lupus, and often are prescribed to transplant patients to prevent their immune systems from rejecting new organs. When taken over time these medications can cause osteoporosis, which in turn raises the risk of fracture.
More than 10% of patients who receive long-term glucocorticoid treatment are diagnosed with clinical fractures. In addition, even low-dose glucocorticoid therapy is associated with a bone loss rate of 10% per year for a patient.
Osteoporosis prevention
After stopping some prevention therapies for GIOP, a high risk of bone loss or fracture still persists, according to Linda A. Russell, MD, director of the Osteoporosis and Metabolic Bone Health Center for the Hospital for Special Surgery, New York, and co-principal investigator of the new guideline.
“We wanted to be sure the need for sequential treatment is adequately communicated, including to patients who might not know they need to start a second medication,” Dr. Russell said.
Physicians and patients must be aware that when completing a course of one GIOP treatment, another drug for the condition should be started, as specified in the guideline.
“Early intervention can prevent glucocorticoid-induced fractures that can lead to substantial morbidity and increased mortality,” said Mary Beth Humphrey, MD, PhD, interim vice president for research at the University of Oklahoma Health Sciences Center in Oklahoma City and co-principal investigator of the ACR guideline.
Janet Rubin, MD, vice chair for research in the Department of medicine at the University of North Carolina at Chapel Hill, said she is hopeful the guideline will change practice.”The risk of bone loss, fractures, and osteoporosis due to glucocorticoids has been known since the beginning of time, but the guideline reinforces the risk and treatment strategies for rheumatologists,” she said. “Such recommendations are known to influence doctor prescribing habits.”
Anyone can fracture
While age and other risk factors, including menopause, increase the risk of developing GIOP, bone loss can occur rapidly for a patient of any age.
Even a glucocorticoid dose as low as 2.5 mg will increase the risk of vertebral fractures, with some occurring as soon as 3 months after treatment starts, Dr. Humphrey said. For patients taking up to 7.5 mg daily, the risk of vertebral fracture doubles. Doses greater than 10 mg daily for more than 3 months raise the likelihood of a vertebral fracture by a factor of 14, and result in a 300% increase in the likelihood of hip fractures, according to Dr. Humphrey.
“When on steroids, even patients with high bone density scores can fracture,” Dr. Tanner said. “The 2017 guideline was almost too elaborate in its effort to calculate risk. The updated guideline acknowledges moderate risk and suggests that this is a group of patients who need treatment.”
Rank ordering adds flexibility
The updated ACR guideline no longer ranks medications based on patient fracture data, side effects, cost care, and whether the drug is provided through injection, pill, or IV.
All of the preventive treatments the panel recommends reduce the risk of steroid-induced bone loss, Dr. Humphrey said.
“We thought the 2017 guideline was too restrictive,” Dr. Russell said. “We’re giving physicians and patients more leeway to choose a medication based on their preferences.”
Patient preference of delivery mechanism – such as a desire for pills only – can now be weighed more heavily into drug treatment decisions.
“In the exam room, there are three dynamics going on: What the patient wants, what the doctor knows is most effective, and what the insurer will pay,” Dr. Tanner said. “Doing away with rank ordering opens up the conversation beyond cost to consider all those factors.”
The guideline team conducted a systematic literature review for clinical questions on nonpharmacologic and pharmacologic treatment addressed in the 2017 guideline, and for questions on new pharmacologic treatments, discontinuation of medications, and sequential and combination therapy. The voting panel consisted of two patient representatives and 13 experts representing adult and pediatric rheumatology and endocrinology, nephrology, and gastroenterology.
A full manuscript has been submitted for publication in Arthritis & Rheumatology and Arthritis Care and Research for peer review, and is expected to publish in early 2023.
Dr. Humphrey and Dr. Russell, the co-principal investigators for the guideline, and Dr. Rubin have disclosed no relevant financial relationships. Dr. Tanner reported a current research grant funded by Amgen through the University of Alabama at Birmingham and being a paid course instructor for the International Society for Clinical Densitometry bone density course, Osteoporosis Essentials.
A version of this article first appeared on Medscape.com.
Unsure on the best T2D drug choice? Let patients decide
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
AT EASD 2022
Early age at hysterectomy ups type 2 diabetes risk
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
FROM EASD 2022
Limiting antibiotic overprescription in pandemics: New guidelines
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
FROM INFECTION CONTROL & HOSPITAL EPIDEMIOLOGY
Type 1 diabetes complication risk rises with A1c, duration
Long-term A1c from the time of type 1 diabetes diagnosis strongly predicts the development of severe retinopathy and nephropathy, new data suggest.
“[Weighted] HbA1c followed from diagnosis is a very strong biomarker for pan-retinal laser-treated diabetic retinopathy (PDR) and nephropathy, [and] the prevalence of both is still increasing 32 years after diagnosis,” say Hans J. Arnqvist, MD, and colleagues in their study published online Sept. 12 in Diabetes Care.
The results are from a 32-year follow-up of 447 patients from time of diagnosis of type 1 diabetes at age 0-34 in the Vascular Diabetic Complications in Southeast Sweden study.
“To avoid PDR and macroalbuminuria in patients with type 1 diabetes, A1c less than 7.0% (53 mmol/mol) and as normal as possible should be recommended when achievable without severe hypoglycemia and with good quality of life,” stress Dr. Arnqvist, department of endocrinology, Linköping University (Sweden), and coauthors.
At the time of the 20- to 24-year VISS follow-up, severe eye complications, defined as PDR, or nephropathy, defined as macroalbuminuria, were not present in participants with a long-term weighted mean A1c less than 7.6% (60 mmol/mol), they write.
Is explanation an increase in glycemic burden with diabetes duration?
By years 32-36, the prevalence of PDR had risen from 14% to 27%, and macroalbuminuria from 4% to 8%, with prevalence strongly correlated with A1c levels. At the same time, the threshold for the appearance of those severe complications dropped, with the lowest A1c values for appearance of PDR decreasing from 7.6% to 7.3% and for macroalbuminuria from 8.4% to 8.1%.
“A possible explanation for the lowered threshold for development of severe microangiopathy is the increase in ‘glycemic burden’ with diabetes duration,” the authors speculate.
In all A1c categories above 6.7% (> 50 mmol/mol), the cumulative proportion with PDR and/or macroproteinuria continued to increase up to at least 32 years of diabetes duration.
At the highest A1c quintile, greater than 9.5% (> 80mmol/mol), 75% had developed PDR and 44.2% had macroalbuminuria.
These findings align with guidelines from both the International Society for Pediatric and Adolescent Diabetes, which recommend A1c less than 7% (53 mmol/mol) as a treatment goal, and the UK National Institute for Health and Care Excellence, which advises a target A1c of 6.5% (48 mmol/mol) or lower in children and adults with type 1 diabetes.
The American Diabetes Association recommends individualized A1c targets ranging from 6.5% to 8.0%.
The study was supported by Barndiabetesfonden (Swedish Children’s Diabetes Foundation) and Region Ostergotlands Stiftelsefonder. The authors reported no further disclosures.
A version of this article first appeared on Medscape.com.
Long-term A1c from the time of type 1 diabetes diagnosis strongly predicts the development of severe retinopathy and nephropathy, new data suggest.
“[Weighted] HbA1c followed from diagnosis is a very strong biomarker for pan-retinal laser-treated diabetic retinopathy (PDR) and nephropathy, [and] the prevalence of both is still increasing 32 years after diagnosis,” say Hans J. Arnqvist, MD, and colleagues in their study published online Sept. 12 in Diabetes Care.
The results are from a 32-year follow-up of 447 patients from time of diagnosis of type 1 diabetes at age 0-34 in the Vascular Diabetic Complications in Southeast Sweden study.
“To avoid PDR and macroalbuminuria in patients with type 1 diabetes, A1c less than 7.0% (53 mmol/mol) and as normal as possible should be recommended when achievable without severe hypoglycemia and with good quality of life,” stress Dr. Arnqvist, department of endocrinology, Linköping University (Sweden), and coauthors.
At the time of the 20- to 24-year VISS follow-up, severe eye complications, defined as PDR, or nephropathy, defined as macroalbuminuria, were not present in participants with a long-term weighted mean A1c less than 7.6% (60 mmol/mol), they write.
Is explanation an increase in glycemic burden with diabetes duration?
By years 32-36, the prevalence of PDR had risen from 14% to 27%, and macroalbuminuria from 4% to 8%, with prevalence strongly correlated with A1c levels. At the same time, the threshold for the appearance of those severe complications dropped, with the lowest A1c values for appearance of PDR decreasing from 7.6% to 7.3% and for macroalbuminuria from 8.4% to 8.1%.
“A possible explanation for the lowered threshold for development of severe microangiopathy is the increase in ‘glycemic burden’ with diabetes duration,” the authors speculate.
In all A1c categories above 6.7% (> 50 mmol/mol), the cumulative proportion with PDR and/or macroproteinuria continued to increase up to at least 32 years of diabetes duration.
At the highest A1c quintile, greater than 9.5% (> 80mmol/mol), 75% had developed PDR and 44.2% had macroalbuminuria.
These findings align with guidelines from both the International Society for Pediatric and Adolescent Diabetes, which recommend A1c less than 7% (53 mmol/mol) as a treatment goal, and the UK National Institute for Health and Care Excellence, which advises a target A1c of 6.5% (48 mmol/mol) or lower in children and adults with type 1 diabetes.
The American Diabetes Association recommends individualized A1c targets ranging from 6.5% to 8.0%.
The study was supported by Barndiabetesfonden (Swedish Children’s Diabetes Foundation) and Region Ostergotlands Stiftelsefonder. The authors reported no further disclosures.
A version of this article first appeared on Medscape.com.
Long-term A1c from the time of type 1 diabetes diagnosis strongly predicts the development of severe retinopathy and nephropathy, new data suggest.
“[Weighted] HbA1c followed from diagnosis is a very strong biomarker for pan-retinal laser-treated diabetic retinopathy (PDR) and nephropathy, [and] the prevalence of both is still increasing 32 years after diagnosis,” say Hans J. Arnqvist, MD, and colleagues in their study published online Sept. 12 in Diabetes Care.
The results are from a 32-year follow-up of 447 patients from time of diagnosis of type 1 diabetes at age 0-34 in the Vascular Diabetic Complications in Southeast Sweden study.
“To avoid PDR and macroalbuminuria in patients with type 1 diabetes, A1c less than 7.0% (53 mmol/mol) and as normal as possible should be recommended when achievable without severe hypoglycemia and with good quality of life,” stress Dr. Arnqvist, department of endocrinology, Linköping University (Sweden), and coauthors.
At the time of the 20- to 24-year VISS follow-up, severe eye complications, defined as PDR, or nephropathy, defined as macroalbuminuria, were not present in participants with a long-term weighted mean A1c less than 7.6% (60 mmol/mol), they write.
Is explanation an increase in glycemic burden with diabetes duration?
By years 32-36, the prevalence of PDR had risen from 14% to 27%, and macroalbuminuria from 4% to 8%, with prevalence strongly correlated with A1c levels. At the same time, the threshold for the appearance of those severe complications dropped, with the lowest A1c values for appearance of PDR decreasing from 7.6% to 7.3% and for macroalbuminuria from 8.4% to 8.1%.
“A possible explanation for the lowered threshold for development of severe microangiopathy is the increase in ‘glycemic burden’ with diabetes duration,” the authors speculate.
In all A1c categories above 6.7% (> 50 mmol/mol), the cumulative proportion with PDR and/or macroproteinuria continued to increase up to at least 32 years of diabetes duration.
At the highest A1c quintile, greater than 9.5% (> 80mmol/mol), 75% had developed PDR and 44.2% had macroalbuminuria.
These findings align with guidelines from both the International Society for Pediatric and Adolescent Diabetes, which recommend A1c less than 7% (53 mmol/mol) as a treatment goal, and the UK National Institute for Health and Care Excellence, which advises a target A1c of 6.5% (48 mmol/mol) or lower in children and adults with type 1 diabetes.
The American Diabetes Association recommends individualized A1c targets ranging from 6.5% to 8.0%.
The study was supported by Barndiabetesfonden (Swedish Children’s Diabetes Foundation) and Region Ostergotlands Stiftelsefonder. The authors reported no further disclosures.
A version of this article first appeared on Medscape.com.
Could cold exposure, especially shivering, combat type 2 diabetes?
STOCKHOLM – Shivering upon repeated short exposures to cold improves glucose tolerance, decreases fasting blood glucose and lipid levels, and markedly reduces blood pressure, show new study results in adults with obesity and overweight.
Presenting the preliminary findings at the annual meeting of the European Association for the Study of Diabetes, Adam Sellers, a PhD student from Maastricht (the Netherlands) University, said: “The results are highly promising and may eventually suggest an alternative treatment or preventative measure for type 2 diabetes.”
Dr. Sellers found that 10 daily 1-hour sessions of shivering at 10° C led to 85% of participants showing a drop in fasting glucose, and a 32% drop in lipid levels, as well as a blood pressure drop of around 8% overall.
Although cold exposure is known to increase brown fat, Dr. Sellers doesn’t believe this explains his findings. “This research, in addition to two other prior studies, suggest that shivering and skeletal muscle may play a more important role than brown fat,” he said.
“Muscle can contract mechanically – [the concept of the] shivers – thereby generating heat, and there is considerably more muscle than brown fat in a human, so shivering can burn more calories and produce more heat,” he explained.
He added that, in the future, “in a similar way to saunas and steam rooms, there might be cold rooms where people go and sit in the cold room and shiver, or possibly patients attend hospital and shivering is induced.”
Audience member Anna Krook, PhD, professor of integrative physiology at the Karolinska Institute, Stockholm, commented on the work, saying the results are “potent” and demonstrate the metabolic effect of shivering. “One thing that struck me was, given the time the subject had to spend – 1 hour shivering over 10 days, I wonder if 1 hour of exercise would show similarly potent effects, and perhaps for those people who cannot perform exercise for whatever reason this might be a good alternative.”
She pointed out that, in terms of translation into practice, it “really depends on how tolerable this is. It also shows how important our muscle is in regulating metabolism. The study showed that you had to be shivering, and it wasn’t just enough to be cold, which has implications for the role of brown fat, especially when we consider the small amount of brown fat we have compared to muscle, which can be half of body weight.”
And Denis P. Blondin, PhD, said: “The reality is that we know it can be difficult and even painful for individuals with obesity to perform exercise, and therefore, cold exposure offers a passive way of improving our metabolic profile and cardiovascular health.”
“Some will argue that it is unrealistic to propose cold exposure as a therapy, but people overlook the fact that cold exposure [mostly through cold-water immersion] has increased in popularity over the past 5 years and has also been a cultural staple for many Nordic countries, albeit often performed with heat exposure as well [see the use of saunas and cold-water swimming in Finland and other Nordic countries],” added Dr. Blondin, of the faculty of medicine and health sciences, University of Sherbrooke (Que.)
“While it can certainly be uncomfortable at first (like starting an exercise program), we adapt very quickly,” he added.
1 hour in a cold-water suit to induce shivering
In the current study, Dr. Sellers exposed adults (aged 40-75 years; 11 men and 4 postmenopausal women) with overweight/obesity (body mass index, 27-35 kg/m2) to 10 consecutive cold exposures of at least 1 hour of shivering per cold exposure.
“The shivering in this new research was more intense [than in prior studies] and was induced with a different cold exposure method – a 10° C water-perfused suit [compared with a prior study of 14-15° C, 6 hours/day]. This facilitated a shorter cold exposure duration, deemed feasible for the participants,” explained Dr. Sellers.
“At baseline, participants had glucose and A1c levels at the upper end of the normal criteria [5.5 mmol/l and 5.4%, respectively],” he said, referring to measurements that were suggestive of possible progression to type 2 diabetes.
He explained how the cold exposure was applied. “We induced the cold with a water-perfused suit worn by the participant, through which water flows at 10° C, and this cools the participant. Eventually, the participant starts to shiver, and does so for at least 1 hour every morning for 10 days.”
Participants’ shivering-induced heat production was measured via surface electromyography and visual observation to confirm the presence of shivering. Both before and after the 10-day course of shivering, physiological measurements were taken in the morning while participants were at rest in an overnight fasted state, and under thermoneutral conditions. Blood pressure and fasting blood glucose were measured.
A 2-hour oral glucose tolerance test (OGTT) was conducted twice for each participant: on the morning before the 10-day course of shivering and again on the morning after the final 10th day of shivering.
The primary endpoint was change from before to after the 10-day shivering intervention, as represented by the total area under the curve of glucose levels over time during the OGTT.
“This provides a measure of the glucose concentrations in the blood before and after the 10 shivering sessions over the 10 days.”
Fasting glucose and blood lipids fall, glucose tolerance improves
After 10 shivering sessions, mean fasting plasma glucose decreased significantly in 13 out of the 15 participants, compared with before the first session (from 5.84 mmol/L to 5.67 mmol/L; P = .013).
Glucose tolerance during the OGTT improved by 6% (P = .041). “We can see that this was not due to a change in their insulin concentrations in the blood,” remarked Dr. Sellers, referring to the finding that plasma insulin concentrations at baseline and during the OGTT did not change.
Fasting plasma triglyceride and free-fatty acid concentrations also decreased significantly by 32% (P = .001) and 11% (P = .036), respectively.
“This is important because free-fatty acids are involved in the role of insulin resistance,” said Dr. Sellers. “In addition, the large reduction in serum triglycerides could have implications for atherosclerosis, which may also be beneficial.”
Dr. Sellers also found that systolic blood pressure decreased by 10 mm Hg or 7.4% (P < .001), while diastolic blood pressure decreased by 7 mm Hg or 8.1% (P < .001) on average. This lowering was seen in all participants.
“Again, quite strikingly, all participants showed” a reduction in blood pressure, said Dr. Sellers, which he noted relates to a decrease in resting heart rate (P = .062).
Brown fat or skeletal muscle contraction?
Dr. Sellers pointed out that, despite nonshivering thermogenesis being involved in mild cold acclimation, the data so far suggest that some level of mild muscle activity or shivering appears crucial in provoking the beneficial metabolic effects of cold acclimation.
“Brown fat is a metabolic heating system inside our bodies, burning calories”, explained Dr. Sellers. “This generates heat and prevents calories from being deposited as normal white fat. Brown fat is activated during cold and when we eat, but its activity is less in older adults and in individuals with obesity and diabetes.”
“Going forward, we might investigate the effects of shorter duration – so more intense shivering – to try and elucidate more precisely the optimum duration and intensity of shivering needed,” said Dr. Sellers.
“Our findings are promising and may have important health implications. In future studies, we plan to assess the effect of shivering in adults with type 2 diabetes,” he concluded.
Dr. Seller and Dr. Krook have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Shivering upon repeated short exposures to cold improves glucose tolerance, decreases fasting blood glucose and lipid levels, and markedly reduces blood pressure, show new study results in adults with obesity and overweight.
Presenting the preliminary findings at the annual meeting of the European Association for the Study of Diabetes, Adam Sellers, a PhD student from Maastricht (the Netherlands) University, said: “The results are highly promising and may eventually suggest an alternative treatment or preventative measure for type 2 diabetes.”
Dr. Sellers found that 10 daily 1-hour sessions of shivering at 10° C led to 85% of participants showing a drop in fasting glucose, and a 32% drop in lipid levels, as well as a blood pressure drop of around 8% overall.
Although cold exposure is known to increase brown fat, Dr. Sellers doesn’t believe this explains his findings. “This research, in addition to two other prior studies, suggest that shivering and skeletal muscle may play a more important role than brown fat,” he said.
“Muscle can contract mechanically – [the concept of the] shivers – thereby generating heat, and there is considerably more muscle than brown fat in a human, so shivering can burn more calories and produce more heat,” he explained.
He added that, in the future, “in a similar way to saunas and steam rooms, there might be cold rooms where people go and sit in the cold room and shiver, or possibly patients attend hospital and shivering is induced.”
Audience member Anna Krook, PhD, professor of integrative physiology at the Karolinska Institute, Stockholm, commented on the work, saying the results are “potent” and demonstrate the metabolic effect of shivering. “One thing that struck me was, given the time the subject had to spend – 1 hour shivering over 10 days, I wonder if 1 hour of exercise would show similarly potent effects, and perhaps for those people who cannot perform exercise for whatever reason this might be a good alternative.”
She pointed out that, in terms of translation into practice, it “really depends on how tolerable this is. It also shows how important our muscle is in regulating metabolism. The study showed that you had to be shivering, and it wasn’t just enough to be cold, which has implications for the role of brown fat, especially when we consider the small amount of brown fat we have compared to muscle, which can be half of body weight.”
And Denis P. Blondin, PhD, said: “The reality is that we know it can be difficult and even painful for individuals with obesity to perform exercise, and therefore, cold exposure offers a passive way of improving our metabolic profile and cardiovascular health.”
“Some will argue that it is unrealistic to propose cold exposure as a therapy, but people overlook the fact that cold exposure [mostly through cold-water immersion] has increased in popularity over the past 5 years and has also been a cultural staple for many Nordic countries, albeit often performed with heat exposure as well [see the use of saunas and cold-water swimming in Finland and other Nordic countries],” added Dr. Blondin, of the faculty of medicine and health sciences, University of Sherbrooke (Que.)
“While it can certainly be uncomfortable at first (like starting an exercise program), we adapt very quickly,” he added.
1 hour in a cold-water suit to induce shivering
In the current study, Dr. Sellers exposed adults (aged 40-75 years; 11 men and 4 postmenopausal women) with overweight/obesity (body mass index, 27-35 kg/m2) to 10 consecutive cold exposures of at least 1 hour of shivering per cold exposure.
“The shivering in this new research was more intense [than in prior studies] and was induced with a different cold exposure method – a 10° C water-perfused suit [compared with a prior study of 14-15° C, 6 hours/day]. This facilitated a shorter cold exposure duration, deemed feasible for the participants,” explained Dr. Sellers.
“At baseline, participants had glucose and A1c levels at the upper end of the normal criteria [5.5 mmol/l and 5.4%, respectively],” he said, referring to measurements that were suggestive of possible progression to type 2 diabetes.
He explained how the cold exposure was applied. “We induced the cold with a water-perfused suit worn by the participant, through which water flows at 10° C, and this cools the participant. Eventually, the participant starts to shiver, and does so for at least 1 hour every morning for 10 days.”
Participants’ shivering-induced heat production was measured via surface electromyography and visual observation to confirm the presence of shivering. Both before and after the 10-day course of shivering, physiological measurements were taken in the morning while participants were at rest in an overnight fasted state, and under thermoneutral conditions. Blood pressure and fasting blood glucose were measured.
A 2-hour oral glucose tolerance test (OGTT) was conducted twice for each participant: on the morning before the 10-day course of shivering and again on the morning after the final 10th day of shivering.
The primary endpoint was change from before to after the 10-day shivering intervention, as represented by the total area under the curve of glucose levels over time during the OGTT.
“This provides a measure of the glucose concentrations in the blood before and after the 10 shivering sessions over the 10 days.”
Fasting glucose and blood lipids fall, glucose tolerance improves
After 10 shivering sessions, mean fasting plasma glucose decreased significantly in 13 out of the 15 participants, compared with before the first session (from 5.84 mmol/L to 5.67 mmol/L; P = .013).
Glucose tolerance during the OGTT improved by 6% (P = .041). “We can see that this was not due to a change in their insulin concentrations in the blood,” remarked Dr. Sellers, referring to the finding that plasma insulin concentrations at baseline and during the OGTT did not change.
Fasting plasma triglyceride and free-fatty acid concentrations also decreased significantly by 32% (P = .001) and 11% (P = .036), respectively.
“This is important because free-fatty acids are involved in the role of insulin resistance,” said Dr. Sellers. “In addition, the large reduction in serum triglycerides could have implications for atherosclerosis, which may also be beneficial.”
Dr. Sellers also found that systolic blood pressure decreased by 10 mm Hg or 7.4% (P < .001), while diastolic blood pressure decreased by 7 mm Hg or 8.1% (P < .001) on average. This lowering was seen in all participants.
“Again, quite strikingly, all participants showed” a reduction in blood pressure, said Dr. Sellers, which he noted relates to a decrease in resting heart rate (P = .062).
Brown fat or skeletal muscle contraction?
Dr. Sellers pointed out that, despite nonshivering thermogenesis being involved in mild cold acclimation, the data so far suggest that some level of mild muscle activity or shivering appears crucial in provoking the beneficial metabolic effects of cold acclimation.
“Brown fat is a metabolic heating system inside our bodies, burning calories”, explained Dr. Sellers. “This generates heat and prevents calories from being deposited as normal white fat. Brown fat is activated during cold and when we eat, but its activity is less in older adults and in individuals with obesity and diabetes.”
“Going forward, we might investigate the effects of shorter duration – so more intense shivering – to try and elucidate more precisely the optimum duration and intensity of shivering needed,” said Dr. Sellers.
“Our findings are promising and may have important health implications. In future studies, we plan to assess the effect of shivering in adults with type 2 diabetes,” he concluded.
Dr. Seller and Dr. Krook have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Shivering upon repeated short exposures to cold improves glucose tolerance, decreases fasting blood glucose and lipid levels, and markedly reduces blood pressure, show new study results in adults with obesity and overweight.
Presenting the preliminary findings at the annual meeting of the European Association for the Study of Diabetes, Adam Sellers, a PhD student from Maastricht (the Netherlands) University, said: “The results are highly promising and may eventually suggest an alternative treatment or preventative measure for type 2 diabetes.”
Dr. Sellers found that 10 daily 1-hour sessions of shivering at 10° C led to 85% of participants showing a drop in fasting glucose, and a 32% drop in lipid levels, as well as a blood pressure drop of around 8% overall.
Although cold exposure is known to increase brown fat, Dr. Sellers doesn’t believe this explains his findings. “This research, in addition to two other prior studies, suggest that shivering and skeletal muscle may play a more important role than brown fat,” he said.
“Muscle can contract mechanically – [the concept of the] shivers – thereby generating heat, and there is considerably more muscle than brown fat in a human, so shivering can burn more calories and produce more heat,” he explained.
He added that, in the future, “in a similar way to saunas and steam rooms, there might be cold rooms where people go and sit in the cold room and shiver, or possibly patients attend hospital and shivering is induced.”
Audience member Anna Krook, PhD, professor of integrative physiology at the Karolinska Institute, Stockholm, commented on the work, saying the results are “potent” and demonstrate the metabolic effect of shivering. “One thing that struck me was, given the time the subject had to spend – 1 hour shivering over 10 days, I wonder if 1 hour of exercise would show similarly potent effects, and perhaps for those people who cannot perform exercise for whatever reason this might be a good alternative.”
She pointed out that, in terms of translation into practice, it “really depends on how tolerable this is. It also shows how important our muscle is in regulating metabolism. The study showed that you had to be shivering, and it wasn’t just enough to be cold, which has implications for the role of brown fat, especially when we consider the small amount of brown fat we have compared to muscle, which can be half of body weight.”
And Denis P. Blondin, PhD, said: “The reality is that we know it can be difficult and even painful for individuals with obesity to perform exercise, and therefore, cold exposure offers a passive way of improving our metabolic profile and cardiovascular health.”
“Some will argue that it is unrealistic to propose cold exposure as a therapy, but people overlook the fact that cold exposure [mostly through cold-water immersion] has increased in popularity over the past 5 years and has also been a cultural staple for many Nordic countries, albeit often performed with heat exposure as well [see the use of saunas and cold-water swimming in Finland and other Nordic countries],” added Dr. Blondin, of the faculty of medicine and health sciences, University of Sherbrooke (Que.)
“While it can certainly be uncomfortable at first (like starting an exercise program), we adapt very quickly,” he added.
1 hour in a cold-water suit to induce shivering
In the current study, Dr. Sellers exposed adults (aged 40-75 years; 11 men and 4 postmenopausal women) with overweight/obesity (body mass index, 27-35 kg/m2) to 10 consecutive cold exposures of at least 1 hour of shivering per cold exposure.
“The shivering in this new research was more intense [than in prior studies] and was induced with a different cold exposure method – a 10° C water-perfused suit [compared with a prior study of 14-15° C, 6 hours/day]. This facilitated a shorter cold exposure duration, deemed feasible for the participants,” explained Dr. Sellers.
“At baseline, participants had glucose and A1c levels at the upper end of the normal criteria [5.5 mmol/l and 5.4%, respectively],” he said, referring to measurements that were suggestive of possible progression to type 2 diabetes.
He explained how the cold exposure was applied. “We induced the cold with a water-perfused suit worn by the participant, through which water flows at 10° C, and this cools the participant. Eventually, the participant starts to shiver, and does so for at least 1 hour every morning for 10 days.”
Participants’ shivering-induced heat production was measured via surface electromyography and visual observation to confirm the presence of shivering. Both before and after the 10-day course of shivering, physiological measurements were taken in the morning while participants were at rest in an overnight fasted state, and under thermoneutral conditions. Blood pressure and fasting blood glucose were measured.
A 2-hour oral glucose tolerance test (OGTT) was conducted twice for each participant: on the morning before the 10-day course of shivering and again on the morning after the final 10th day of shivering.
The primary endpoint was change from before to after the 10-day shivering intervention, as represented by the total area under the curve of glucose levels over time during the OGTT.
“This provides a measure of the glucose concentrations in the blood before and after the 10 shivering sessions over the 10 days.”
Fasting glucose and blood lipids fall, glucose tolerance improves
After 10 shivering sessions, mean fasting plasma glucose decreased significantly in 13 out of the 15 participants, compared with before the first session (from 5.84 mmol/L to 5.67 mmol/L; P = .013).
Glucose tolerance during the OGTT improved by 6% (P = .041). “We can see that this was not due to a change in their insulin concentrations in the blood,” remarked Dr. Sellers, referring to the finding that plasma insulin concentrations at baseline and during the OGTT did not change.
Fasting plasma triglyceride and free-fatty acid concentrations also decreased significantly by 32% (P = .001) and 11% (P = .036), respectively.
“This is important because free-fatty acids are involved in the role of insulin resistance,” said Dr. Sellers. “In addition, the large reduction in serum triglycerides could have implications for atherosclerosis, which may also be beneficial.”
Dr. Sellers also found that systolic blood pressure decreased by 10 mm Hg or 7.4% (P < .001), while diastolic blood pressure decreased by 7 mm Hg or 8.1% (P < .001) on average. This lowering was seen in all participants.
“Again, quite strikingly, all participants showed” a reduction in blood pressure, said Dr. Sellers, which he noted relates to a decrease in resting heart rate (P = .062).
Brown fat or skeletal muscle contraction?
Dr. Sellers pointed out that, despite nonshivering thermogenesis being involved in mild cold acclimation, the data so far suggest that some level of mild muscle activity or shivering appears crucial in provoking the beneficial metabolic effects of cold acclimation.
“Brown fat is a metabolic heating system inside our bodies, burning calories”, explained Dr. Sellers. “This generates heat and prevents calories from being deposited as normal white fat. Brown fat is activated during cold and when we eat, but its activity is less in older adults and in individuals with obesity and diabetes.”
“Going forward, we might investigate the effects of shorter duration – so more intense shivering – to try and elucidate more precisely the optimum duration and intensity of shivering needed,” said Dr. Sellers.
“Our findings are promising and may have important health implications. In future studies, we plan to assess the effect of shivering in adults with type 2 diabetes,” he concluded.
Dr. Seller and Dr. Krook have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Mothers’ diabetes linked to ADHD in their children
Children born to women who develop diabetes either before or during their pregnancy could be at risk for developing attention-deficit/hyperactivity disorder, data from a large multinational cohort study appear to show.
Considering more than 4.5 million mother-child pairs, it was found that children whose mothers had diabetes around the time of their pregnancy were 16% more likely to have ADHD diagnosed than were those whose mothers did not.
An increased risk was seen regardless of the type of diabetes, and regardless of whether or not the diabetes was present before or appeared during the pregnancy.
“We found a small increased risk of ADHD in children born to mothers with diabetes, including pregestational diabetes and gestational diabetes,” Carolyn Cesta, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Cesta, a postdoctoral researcher in the Centre for Pharmacoepidemiology at the Karolinska Institutet in Stockholm noted that the effect sizes seen were lower than had been reported previously.
“This may be because we adjusted for a large number of covariates, including maternal ADHD and psychiatric disorders,” Dr. Cesta said.
ADHD and diabetes
“Previous studies have reported an increase in the risk of ADHD in children born to mothers with diabetes,” explained Dr. Cesta.
However, “these studies have been limited by the use of self-reported data, small sample sizes, lack of adjustment for important confounders, and they’re often limited to [White] populations,” she added. “There’s a lot of heterogeneity between these studies,” she said.
To try to iron out the differences seen in the prior studies, Dr. Cesta and associates looked at data from several databases based in Hong Kong (Clinical Data Analysis and Reporting System), four Nordic countries (Population Health Registers for Finland, Iceland, Norway, and Sweden), and Taiwan (National Health Insurance Database).
To create the matched mother-child pairs, the databases were searched to find women who had children born between 2001 and 2018, and who had follow-up data available up to 2020 on not only their diabetes status and child’s ADHD status, but also other parameters, such as other maternal diagnoses, maternal medications, and a host of sociodemographic factors.
More than 24 potentially confounding or covariates were considered in the analysis, which used Cox proportional hazard regression modeling and propensity score analysis to calculate hazard ratios with 95% confidence intervals.
“We looked at whether [mothers] had a diagnosis of ADHD themselves, or other psychiatric disorders, because there is high heritability for these disorders,” Dr. Cesta said, indicating that all bases had endeavored to be covered.
Main findings
Results showed some differences in the prevalence of diabetes and ADHD between the three cohorts used in the analysis. The prevalence of any maternal diabetes ranged from 8.8% in the Hong Kong cohort to 3.3% in the Taiwan cohort, with a prevalence of 6.8% for the Nordic cohort.
Rates of pregestational diabetes were lowest in the Taiwan and Hong Kong cohorts, at 0.2% and 0.5%, respectively, and 2.2% in the Nordic cohort. Gestational diabetes rates were a respective 3.1%, 7.8%, and 4.6%.
The highest rate of ADHD in children was seen in the Taiwan cohort, at 9.6%, followed by 4.2% for the Hong Kong cohort, and 2.6% for the Nordic cohort.
The hazard ratio for having childhood ADHD was 1.16 when comparing any maternal diabetes to no maternal diabetes, 1.40 comparing mothers with and without pregestational diabetes, and a respective 1.36 and 1.37 comparing those with and without type 1 diabetes, and those with and without type 2 diabetes.
The HR for childhood ADHD comparing mothers with and without gestational diabetes was 1.13.
“Within the analysis for gestational diabetes, we had enough numbers to look at siblings that are discordant for maternal gestational diabetes,” Dr. Cesta said. Essentially “we’re comparing two siblings from the same mother, one that was exposed to gestational diabetes, one that wasn’t,” she explained.
Interestingly there was no association between ADHD and maternal gestational diabetes in the sibling analysis (HR, 1.0).
“When it comes to gestational diabetes, the evidence from our sibling analysis indicate that the association may actually be confounded by shared genetics and environmental factors,” said Dr. Cesta.
“So, future studies should explore the role of specific genetic factors in glycemic control during pregnancy and the relationship between maternal diabetes and ADHD.”
Answering long-standing questions
These data will help a lot in answering questions that clinicians have been asking themselves a long time, commented Jardena Puder, MD, who chaired the session.
“It still remains a bit puzzling that genetic and environmental factors could be responsible, if you see the same effect in type 1 [diabetes], and in type 2 [diabetes], and gestational diabetes,” said Dr. Puder, who is an endocrinologist and diabetologist at the woman-mother-child department at the Vaud University Hospital Center, Lausanne, Switzerland.
Type 1 and type 2 are “very distinct” in terms of the genetic and environmental factors involved, “so, the fact that you see [the effect] in both remains a bit puzzling,” said Dr. Puder.
“I wish we had the numbers to be able to do the sibling analysis for type 1 and type 2, just to see if we could tease anything out,” said Dr. Cesta.
“I do think this is part of the bigger question of what the relationship is between, like, metabolic disorders and psychiatric disorders, because even outside of pregnancy, we see that there’s often a comorbidity with them. So, it’s a good point.”
The next step is to look at the role of treatment and what effects glycemic control might have on the small, but still apparent, association between maternal diabetes and ADHD.
The study had multiple funders including the Hong Kong Research Grant Council, NordForsk, the Research Council of Norway, the Norwegian ADHD Research Network, the Hong Kong Innovation and Technology Commission, and European Horizon 2020.
Dr. Cesta had no conflicts of interest to disclose. Dr. Puder chaired the session in which the findings were presented and made no specific disclosures.
Children born to women who develop diabetes either before or during their pregnancy could be at risk for developing attention-deficit/hyperactivity disorder, data from a large multinational cohort study appear to show.
Considering more than 4.5 million mother-child pairs, it was found that children whose mothers had diabetes around the time of their pregnancy were 16% more likely to have ADHD diagnosed than were those whose mothers did not.
An increased risk was seen regardless of the type of diabetes, and regardless of whether or not the diabetes was present before or appeared during the pregnancy.
“We found a small increased risk of ADHD in children born to mothers with diabetes, including pregestational diabetes and gestational diabetes,” Carolyn Cesta, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Cesta, a postdoctoral researcher in the Centre for Pharmacoepidemiology at the Karolinska Institutet in Stockholm noted that the effect sizes seen were lower than had been reported previously.
“This may be because we adjusted for a large number of covariates, including maternal ADHD and psychiatric disorders,” Dr. Cesta said.
ADHD and diabetes
“Previous studies have reported an increase in the risk of ADHD in children born to mothers with diabetes,” explained Dr. Cesta.
However, “these studies have been limited by the use of self-reported data, small sample sizes, lack of adjustment for important confounders, and they’re often limited to [White] populations,” she added. “There’s a lot of heterogeneity between these studies,” she said.
To try to iron out the differences seen in the prior studies, Dr. Cesta and associates looked at data from several databases based in Hong Kong (Clinical Data Analysis and Reporting System), four Nordic countries (Population Health Registers for Finland, Iceland, Norway, and Sweden), and Taiwan (National Health Insurance Database).
To create the matched mother-child pairs, the databases were searched to find women who had children born between 2001 and 2018, and who had follow-up data available up to 2020 on not only their diabetes status and child’s ADHD status, but also other parameters, such as other maternal diagnoses, maternal medications, and a host of sociodemographic factors.
More than 24 potentially confounding or covariates were considered in the analysis, which used Cox proportional hazard regression modeling and propensity score analysis to calculate hazard ratios with 95% confidence intervals.
“We looked at whether [mothers] had a diagnosis of ADHD themselves, or other psychiatric disorders, because there is high heritability for these disorders,” Dr. Cesta said, indicating that all bases had endeavored to be covered.
Main findings
Results showed some differences in the prevalence of diabetes and ADHD between the three cohorts used in the analysis. The prevalence of any maternal diabetes ranged from 8.8% in the Hong Kong cohort to 3.3% in the Taiwan cohort, with a prevalence of 6.8% for the Nordic cohort.
Rates of pregestational diabetes were lowest in the Taiwan and Hong Kong cohorts, at 0.2% and 0.5%, respectively, and 2.2% in the Nordic cohort. Gestational diabetes rates were a respective 3.1%, 7.8%, and 4.6%.
The highest rate of ADHD in children was seen in the Taiwan cohort, at 9.6%, followed by 4.2% for the Hong Kong cohort, and 2.6% for the Nordic cohort.
The hazard ratio for having childhood ADHD was 1.16 when comparing any maternal diabetes to no maternal diabetes, 1.40 comparing mothers with and without pregestational diabetes, and a respective 1.36 and 1.37 comparing those with and without type 1 diabetes, and those with and without type 2 diabetes.
The HR for childhood ADHD comparing mothers with and without gestational diabetes was 1.13.
“Within the analysis for gestational diabetes, we had enough numbers to look at siblings that are discordant for maternal gestational diabetes,” Dr. Cesta said. Essentially “we’re comparing two siblings from the same mother, one that was exposed to gestational diabetes, one that wasn’t,” she explained.
Interestingly there was no association between ADHD and maternal gestational diabetes in the sibling analysis (HR, 1.0).
“When it comes to gestational diabetes, the evidence from our sibling analysis indicate that the association may actually be confounded by shared genetics and environmental factors,” said Dr. Cesta.
“So, future studies should explore the role of specific genetic factors in glycemic control during pregnancy and the relationship between maternal diabetes and ADHD.”
Answering long-standing questions
These data will help a lot in answering questions that clinicians have been asking themselves a long time, commented Jardena Puder, MD, who chaired the session.
“It still remains a bit puzzling that genetic and environmental factors could be responsible, if you see the same effect in type 1 [diabetes], and in type 2 [diabetes], and gestational diabetes,” said Dr. Puder, who is an endocrinologist and diabetologist at the woman-mother-child department at the Vaud University Hospital Center, Lausanne, Switzerland.
Type 1 and type 2 are “very distinct” in terms of the genetic and environmental factors involved, “so, the fact that you see [the effect] in both remains a bit puzzling,” said Dr. Puder.
“I wish we had the numbers to be able to do the sibling analysis for type 1 and type 2, just to see if we could tease anything out,” said Dr. Cesta.
“I do think this is part of the bigger question of what the relationship is between, like, metabolic disorders and psychiatric disorders, because even outside of pregnancy, we see that there’s often a comorbidity with them. So, it’s a good point.”
The next step is to look at the role of treatment and what effects glycemic control might have on the small, but still apparent, association between maternal diabetes and ADHD.
The study had multiple funders including the Hong Kong Research Grant Council, NordForsk, the Research Council of Norway, the Norwegian ADHD Research Network, the Hong Kong Innovation and Technology Commission, and European Horizon 2020.
Dr. Cesta had no conflicts of interest to disclose. Dr. Puder chaired the session in which the findings were presented and made no specific disclosures.
Children born to women who develop diabetes either before or during their pregnancy could be at risk for developing attention-deficit/hyperactivity disorder, data from a large multinational cohort study appear to show.
Considering more than 4.5 million mother-child pairs, it was found that children whose mothers had diabetes around the time of their pregnancy were 16% more likely to have ADHD diagnosed than were those whose mothers did not.
An increased risk was seen regardless of the type of diabetes, and regardless of whether or not the diabetes was present before or appeared during the pregnancy.
“We found a small increased risk of ADHD in children born to mothers with diabetes, including pregestational diabetes and gestational diabetes,” Carolyn Cesta, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Cesta, a postdoctoral researcher in the Centre for Pharmacoepidemiology at the Karolinska Institutet in Stockholm noted that the effect sizes seen were lower than had been reported previously.
“This may be because we adjusted for a large number of covariates, including maternal ADHD and psychiatric disorders,” Dr. Cesta said.
ADHD and diabetes
“Previous studies have reported an increase in the risk of ADHD in children born to mothers with diabetes,” explained Dr. Cesta.
However, “these studies have been limited by the use of self-reported data, small sample sizes, lack of adjustment for important confounders, and they’re often limited to [White] populations,” she added. “There’s a lot of heterogeneity between these studies,” she said.
To try to iron out the differences seen in the prior studies, Dr. Cesta and associates looked at data from several databases based in Hong Kong (Clinical Data Analysis and Reporting System), four Nordic countries (Population Health Registers for Finland, Iceland, Norway, and Sweden), and Taiwan (National Health Insurance Database).
To create the matched mother-child pairs, the databases were searched to find women who had children born between 2001 and 2018, and who had follow-up data available up to 2020 on not only their diabetes status and child’s ADHD status, but also other parameters, such as other maternal diagnoses, maternal medications, and a host of sociodemographic factors.
More than 24 potentially confounding or covariates were considered in the analysis, which used Cox proportional hazard regression modeling and propensity score analysis to calculate hazard ratios with 95% confidence intervals.
“We looked at whether [mothers] had a diagnosis of ADHD themselves, or other psychiatric disorders, because there is high heritability for these disorders,” Dr. Cesta said, indicating that all bases had endeavored to be covered.
Main findings
Results showed some differences in the prevalence of diabetes and ADHD between the three cohorts used in the analysis. The prevalence of any maternal diabetes ranged from 8.8% in the Hong Kong cohort to 3.3% in the Taiwan cohort, with a prevalence of 6.8% for the Nordic cohort.
Rates of pregestational diabetes were lowest in the Taiwan and Hong Kong cohorts, at 0.2% and 0.5%, respectively, and 2.2% in the Nordic cohort. Gestational diabetes rates were a respective 3.1%, 7.8%, and 4.6%.
The highest rate of ADHD in children was seen in the Taiwan cohort, at 9.6%, followed by 4.2% for the Hong Kong cohort, and 2.6% for the Nordic cohort.
The hazard ratio for having childhood ADHD was 1.16 when comparing any maternal diabetes to no maternal diabetes, 1.40 comparing mothers with and without pregestational diabetes, and a respective 1.36 and 1.37 comparing those with and without type 1 diabetes, and those with and without type 2 diabetes.
The HR for childhood ADHD comparing mothers with and without gestational diabetes was 1.13.
“Within the analysis for gestational diabetes, we had enough numbers to look at siblings that are discordant for maternal gestational diabetes,” Dr. Cesta said. Essentially “we’re comparing two siblings from the same mother, one that was exposed to gestational diabetes, one that wasn’t,” she explained.
Interestingly there was no association between ADHD and maternal gestational diabetes in the sibling analysis (HR, 1.0).
“When it comes to gestational diabetes, the evidence from our sibling analysis indicate that the association may actually be confounded by shared genetics and environmental factors,” said Dr. Cesta.
“So, future studies should explore the role of specific genetic factors in glycemic control during pregnancy and the relationship between maternal diabetes and ADHD.”
Answering long-standing questions
These data will help a lot in answering questions that clinicians have been asking themselves a long time, commented Jardena Puder, MD, who chaired the session.
“It still remains a bit puzzling that genetic and environmental factors could be responsible, if you see the same effect in type 1 [diabetes], and in type 2 [diabetes], and gestational diabetes,” said Dr. Puder, who is an endocrinologist and diabetologist at the woman-mother-child department at the Vaud University Hospital Center, Lausanne, Switzerland.
Type 1 and type 2 are “very distinct” in terms of the genetic and environmental factors involved, “so, the fact that you see [the effect] in both remains a bit puzzling,” said Dr. Puder.
“I wish we had the numbers to be able to do the sibling analysis for type 1 and type 2, just to see if we could tease anything out,” said Dr. Cesta.
“I do think this is part of the bigger question of what the relationship is between, like, metabolic disorders and psychiatric disorders, because even outside of pregnancy, we see that there’s often a comorbidity with them. So, it’s a good point.”
The next step is to look at the role of treatment and what effects glycemic control might have on the small, but still apparent, association between maternal diabetes and ADHD.
The study had multiple funders including the Hong Kong Research Grant Council, NordForsk, the Research Council of Norway, the Norwegian ADHD Research Network, the Hong Kong Innovation and Technology Commission, and European Horizon 2020.
Dr. Cesta had no conflicts of interest to disclose. Dr. Puder chaired the session in which the findings were presented and made no specific disclosures.
FROM EASD 2022
Triple threat: Novel agent shows potent T2D weight loss in phase 1
STOCKHOLM – First came the GLP-1 receptor agonists as treatments for patients with type 2 diabetes, then came tirzepatide (Mounjaro) which added a second incretin agonism for the receptor to the glucose-dependent insulinotropic polypeptide (GIP). Now coming onto the clinical scene is a molecule with triple agonism to the GLP-1 receptor, the GIP receptor, and to the glucagon receptor.
That molecule, LY3437943, showed reasonable safety and tolerability and an apparent incremental uptick in weight loss compared with the approved incretin-based agents for people with type 2 diabetes in a 12-week, dose-ranging study involving a 52 patients with type 2 diabetes who received the new agent.
The 12 people who uptitrated for a total of 12 weeks and reached the highest tested dose of LY3437943, 12 mg, injected once weekly during the final 4 weeks, showed an average weight loss of 8.65 kg, while the 11 patients who maxed out at a weekly dose of 6 mg of LY3437943 had an average 12-week weight loss of 7.52 kg, Zvonko Milicevic, MD, reported at the annual meeting of the European Association for the Study of Diabetes.
Fifteen more participants received placebo and five received a comparator GLP-1 receptor agonist. All 72 patients in the study were also already on treatment with metformin when they entered, and they were maintained on metformin throughout the study period.
The new agent showed “greater weight loss efficacy than currently approved medications,” said Dr. Milicevic, a staff researcher who works in Vienna for Eli Lilly, the company developing LY3437943.
‘Really impressive’ weight loss
Martin Haluzik, MD, who chaired the session where Dr. Milicevic spoke, agreed. “The data, especially for weight reduction, were really impressive,” Dr. Haluzik said in an interview. “It looks stronger than the best we have at the moment,” the dual incretin agonist tirzepatide, he added.
Cross-study comparisons are very unreliable, but to put the weight loss seen with LY3437943 in perspective, the 12-week weight reduction that occurred with the highest dose of tirzepatide tested (15 mg/weekly) in the pivotal SURPASS-2 trial with 1,879 randomized patients with type 2 diabetes was an average of roughly 5 kg, while the comparator of 1 mg weekly of semaglutide (Ozempic) tested in the same study produced an average weight loss of about 4 kg.
Other notable efficacy results for LY3437943 after 12 weeks on treatment included an average reduction in hemoglobin A1c from baseline of 1.90%, achieved in the group that received 6 mg weekly as their maximum dose for 8 weeks after a 4-week run-in at a lower dose; a reduction in systolic blood pressure of 7.99 mm Hg on the 6-mg maximum weekly dose and of 12.06 mm Hg on the maximum 12-mg weekly dose; and “robust” reductions in lipids including cuts from baseline of about 40% for both triglycerides and very-LDL cholesterol, Dr. Milicevic reported.
Adverse effects resemble approved incretin-based agents
The study, which ran at four U.S. sites, had a primary objective of safety assessment, and Dr. Milicevic said the results showed acceptable safety and tolerability consistent with the glucagon-like peptide-1 (GLP-1) receptor agonists and tirzepatide. Like those agents, LY3437943 caused primarily mild gastrointestinal adverse effects such as nausea and diarrhea. Of the 52 patients in the study who received the triple agonist, 4 discontinued treatment because of a treatment-emergent adverse effect, including 1 patient in the subgroup who received the maximum dose.
The only concerning adverse effect noted by Dr. Haluzik was the average increase in heart rate from baseline of 10.26 beats/min in the subgroup that received the maximum dose, roughly twice the increase seen with tirzepatide and semaglutide in SURPASS-2. The average heart rate increase was about half that, 5.30 beats/min compared with baseline, in the subgroup that received a maximum weekly dose of 6 mg.
Overall, the results showed “no major adverse effects that might hamper use,” said Dr. Haluzik, an endocrinologist and professor at Charles University in Prague.
Two phase 2 studies of LY3437943 are underway and are scheduled to finish before the end of 2022. They include a study of about 300 people with type 2 diabetes that’s running at 43 U.S. sites, and a second study of about 500 people with overweight or obesity running at 28 U.S. sites.
The study was sponsored by Eli Lilly, the company developing LY3437943. Dr. Milicevic is an employee of and stockholder of Eli Lilly. Dr. Haluzik has been an adviser to, consultant to, and received honoraria and research support from Eli Lilly. He has had similar relationships with Amgen, AstraZeneca, Boehringer Ingelheim, BristolMyersSquibb, Janssen, Johnson & Johnson, Mundipharma, Novo Nordisk, and Sanofi.
STOCKHOLM – First came the GLP-1 receptor agonists as treatments for patients with type 2 diabetes, then came tirzepatide (Mounjaro) which added a second incretin agonism for the receptor to the glucose-dependent insulinotropic polypeptide (GIP). Now coming onto the clinical scene is a molecule with triple agonism to the GLP-1 receptor, the GIP receptor, and to the glucagon receptor.
That molecule, LY3437943, showed reasonable safety and tolerability and an apparent incremental uptick in weight loss compared with the approved incretin-based agents for people with type 2 diabetes in a 12-week, dose-ranging study involving a 52 patients with type 2 diabetes who received the new agent.
The 12 people who uptitrated for a total of 12 weeks and reached the highest tested dose of LY3437943, 12 mg, injected once weekly during the final 4 weeks, showed an average weight loss of 8.65 kg, while the 11 patients who maxed out at a weekly dose of 6 mg of LY3437943 had an average 12-week weight loss of 7.52 kg, Zvonko Milicevic, MD, reported at the annual meeting of the European Association for the Study of Diabetes.
Fifteen more participants received placebo and five received a comparator GLP-1 receptor agonist. All 72 patients in the study were also already on treatment with metformin when they entered, and they were maintained on metformin throughout the study period.
The new agent showed “greater weight loss efficacy than currently approved medications,” said Dr. Milicevic, a staff researcher who works in Vienna for Eli Lilly, the company developing LY3437943.
‘Really impressive’ weight loss
Martin Haluzik, MD, who chaired the session where Dr. Milicevic spoke, agreed. “The data, especially for weight reduction, were really impressive,” Dr. Haluzik said in an interview. “It looks stronger than the best we have at the moment,” the dual incretin agonist tirzepatide, he added.
Cross-study comparisons are very unreliable, but to put the weight loss seen with LY3437943 in perspective, the 12-week weight reduction that occurred with the highest dose of tirzepatide tested (15 mg/weekly) in the pivotal SURPASS-2 trial with 1,879 randomized patients with type 2 diabetes was an average of roughly 5 kg, while the comparator of 1 mg weekly of semaglutide (Ozempic) tested in the same study produced an average weight loss of about 4 kg.
Other notable efficacy results for LY3437943 after 12 weeks on treatment included an average reduction in hemoglobin A1c from baseline of 1.90%, achieved in the group that received 6 mg weekly as their maximum dose for 8 weeks after a 4-week run-in at a lower dose; a reduction in systolic blood pressure of 7.99 mm Hg on the 6-mg maximum weekly dose and of 12.06 mm Hg on the maximum 12-mg weekly dose; and “robust” reductions in lipids including cuts from baseline of about 40% for both triglycerides and very-LDL cholesterol, Dr. Milicevic reported.
Adverse effects resemble approved incretin-based agents
The study, which ran at four U.S. sites, had a primary objective of safety assessment, and Dr. Milicevic said the results showed acceptable safety and tolerability consistent with the glucagon-like peptide-1 (GLP-1) receptor agonists and tirzepatide. Like those agents, LY3437943 caused primarily mild gastrointestinal adverse effects such as nausea and diarrhea. Of the 52 patients in the study who received the triple agonist, 4 discontinued treatment because of a treatment-emergent adverse effect, including 1 patient in the subgroup who received the maximum dose.
The only concerning adverse effect noted by Dr. Haluzik was the average increase in heart rate from baseline of 10.26 beats/min in the subgroup that received the maximum dose, roughly twice the increase seen with tirzepatide and semaglutide in SURPASS-2. The average heart rate increase was about half that, 5.30 beats/min compared with baseline, in the subgroup that received a maximum weekly dose of 6 mg.
Overall, the results showed “no major adverse effects that might hamper use,” said Dr. Haluzik, an endocrinologist and professor at Charles University in Prague.
Two phase 2 studies of LY3437943 are underway and are scheduled to finish before the end of 2022. They include a study of about 300 people with type 2 diabetes that’s running at 43 U.S. sites, and a second study of about 500 people with overweight or obesity running at 28 U.S. sites.
The study was sponsored by Eli Lilly, the company developing LY3437943. Dr. Milicevic is an employee of and stockholder of Eli Lilly. Dr. Haluzik has been an adviser to, consultant to, and received honoraria and research support from Eli Lilly. He has had similar relationships with Amgen, AstraZeneca, Boehringer Ingelheim, BristolMyersSquibb, Janssen, Johnson & Johnson, Mundipharma, Novo Nordisk, and Sanofi.
STOCKHOLM – First came the GLP-1 receptor agonists as treatments for patients with type 2 diabetes, then came tirzepatide (Mounjaro) which added a second incretin agonism for the receptor to the glucose-dependent insulinotropic polypeptide (GIP). Now coming onto the clinical scene is a molecule with triple agonism to the GLP-1 receptor, the GIP receptor, and to the glucagon receptor.
That molecule, LY3437943, showed reasonable safety and tolerability and an apparent incremental uptick in weight loss compared with the approved incretin-based agents for people with type 2 diabetes in a 12-week, dose-ranging study involving a 52 patients with type 2 diabetes who received the new agent.
The 12 people who uptitrated for a total of 12 weeks and reached the highest tested dose of LY3437943, 12 mg, injected once weekly during the final 4 weeks, showed an average weight loss of 8.65 kg, while the 11 patients who maxed out at a weekly dose of 6 mg of LY3437943 had an average 12-week weight loss of 7.52 kg, Zvonko Milicevic, MD, reported at the annual meeting of the European Association for the Study of Diabetes.
Fifteen more participants received placebo and five received a comparator GLP-1 receptor agonist. All 72 patients in the study were also already on treatment with metformin when they entered, and they were maintained on metformin throughout the study period.
The new agent showed “greater weight loss efficacy than currently approved medications,” said Dr. Milicevic, a staff researcher who works in Vienna for Eli Lilly, the company developing LY3437943.
‘Really impressive’ weight loss
Martin Haluzik, MD, who chaired the session where Dr. Milicevic spoke, agreed. “The data, especially for weight reduction, were really impressive,” Dr. Haluzik said in an interview. “It looks stronger than the best we have at the moment,” the dual incretin agonist tirzepatide, he added.
Cross-study comparisons are very unreliable, but to put the weight loss seen with LY3437943 in perspective, the 12-week weight reduction that occurred with the highest dose of tirzepatide tested (15 mg/weekly) in the pivotal SURPASS-2 trial with 1,879 randomized patients with type 2 diabetes was an average of roughly 5 kg, while the comparator of 1 mg weekly of semaglutide (Ozempic) tested in the same study produced an average weight loss of about 4 kg.
Other notable efficacy results for LY3437943 after 12 weeks on treatment included an average reduction in hemoglobin A1c from baseline of 1.90%, achieved in the group that received 6 mg weekly as their maximum dose for 8 weeks after a 4-week run-in at a lower dose; a reduction in systolic blood pressure of 7.99 mm Hg on the 6-mg maximum weekly dose and of 12.06 mm Hg on the maximum 12-mg weekly dose; and “robust” reductions in lipids including cuts from baseline of about 40% for both triglycerides and very-LDL cholesterol, Dr. Milicevic reported.
Adverse effects resemble approved incretin-based agents
The study, which ran at four U.S. sites, had a primary objective of safety assessment, and Dr. Milicevic said the results showed acceptable safety and tolerability consistent with the glucagon-like peptide-1 (GLP-1) receptor agonists and tirzepatide. Like those agents, LY3437943 caused primarily mild gastrointestinal adverse effects such as nausea and diarrhea. Of the 52 patients in the study who received the triple agonist, 4 discontinued treatment because of a treatment-emergent adverse effect, including 1 patient in the subgroup who received the maximum dose.
The only concerning adverse effect noted by Dr. Haluzik was the average increase in heart rate from baseline of 10.26 beats/min in the subgroup that received the maximum dose, roughly twice the increase seen with tirzepatide and semaglutide in SURPASS-2. The average heart rate increase was about half that, 5.30 beats/min compared with baseline, in the subgroup that received a maximum weekly dose of 6 mg.
Overall, the results showed “no major adverse effects that might hamper use,” said Dr. Haluzik, an endocrinologist and professor at Charles University in Prague.
Two phase 2 studies of LY3437943 are underway and are scheduled to finish before the end of 2022. They include a study of about 300 people with type 2 diabetes that’s running at 43 U.S. sites, and a second study of about 500 people with overweight or obesity running at 28 U.S. sites.
The study was sponsored by Eli Lilly, the company developing LY3437943. Dr. Milicevic is an employee of and stockholder of Eli Lilly. Dr. Haluzik has been an adviser to, consultant to, and received honoraria and research support from Eli Lilly. He has had similar relationships with Amgen, AstraZeneca, Boehringer Ingelheim, BristolMyersSquibb, Janssen, Johnson & Johnson, Mundipharma, Novo Nordisk, and Sanofi.
AT EASD 2022
Early bird gets the worm, night owl gets the diabetes
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.