User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Keep menstrual cramps away the dietary prevention way
Foods for thought: Menstrual cramp prevention
For those who menstruate, it’s typical for that time of the month to bring cravings for things that may give a serotonin boost that eases the rise in stress hormones. Chocolate and other foods high in sugar fall into that category, but they could actually be adding to the problem.
About 90% of adolescent girls have menstrual pain, and it’s the leading cause of school absences for the demographic. Muscle relaxers and PMS pills are usually the recommended solution to alleviating menstrual cramps, but what if the patient doesn’t want to take any medicine?
Serah Sannoh of Rutgers University wanted to find another way to relieve her menstrual pains. The literature review she presented at the annual meeting of the North American Menopause Society found multiple studies that examined dietary patterns that resulted in menstrual pain.
In Ms. Sannoh’s analysis, she looked at how certain foods have an effect on cramps. Do they contribute to the pain or reduce it? Diets high in processed foods, oils, sugars, salt, and omega-6 fatty acids promote inflammation in the muscles around the uterus. Thus, cramps.
The answer, sometimes, is not to add a medicine but to change our daily practices, she suggested. Foods high in omega-3 fatty acids helped reduce pain, and those who practiced a vegan diet had the lowest muscle inflammation rates. So more salmon and fewer Swedish Fish.
Stage 1 of the robot apocalypse is already upon us
The mere mention of a robot apocalypse is enough to conjure images of terrifying robot soldiers with Austrian accents harvesting and killing humanity while the survivors live blissfully in a simulation and do low-gravity kung fu with high-profile Hollywood actors. They’ll even take over the navy.
Reality is often less exciting than the movies, but rest assured, the robots will not be denied their dominion of Earth. Our future robot overlords are simply taking a more subtle, less dramatic route toward their ultimate subjugation of mankind: They’re making us all sad and burned out.
The research pulls from work conducted in multiple countries to paint a picture of a humanity filled with anxiety about jobs as robotic automation grows more common. In India, a survey of automobile manufacturing works showed that working alongside industrial robots was linked with greater reports of burnout and workplace incivility. In Singapore, a group of college students randomly assigned to read one of three articles – one about the use of robots in business, a generic article about robots, or an article unrelated to robots – were then surveyed about their job security concerns. Three guesses as to which group was most worried.
In addition, the researchers analyzed 185 U.S. metropolitan areas for robot prevalence alongside use of job-recruiting websites and found that the more robots a city used, the more common job searches were. Unemployment rates weren’t affected, suggesting people had job insecurity because of robots. Sure, there could be other, nonrobotic reasons for this, but that’s no fun. We’re here because we fear our future android rulers.
It’s not all doom and gloom, fortunately. In an online experiment, the study authors found that self-affirmation exercises, such as writing down characteristics or values important to us, can overcome the existential fears and lessen concern about robots in the workplace. One of the authors noted that, while some fear is justified, “media reports on new technologies like robots and algorithms tend to be apocalyptic in nature, so people may develop an irrational fear about them.”
Oops. Our bad.
Apocalypse, stage 2: Leaping oral superorganisms
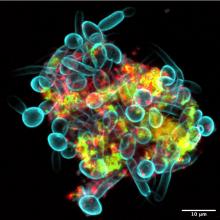
The terms of our secret agreement with the shadowy-but-powerful dental-industrial complex stipulate that LOTME can only cover tooth-related news once a year. This is that once a year.
Since we’ve already dealt with a robot apocalypse, how about a sci-fi horror story? A story with a “cross-kingdom partnership” in which assemblages of bacteria and fungi perform feats greater than either could achieve on its own. A story in which new microscopy technologies allow “scientists to visualize the behavior of living microbes in real time,” according to a statement from the University of Pennsylvania, Philadelphia.
While looking at saliva samples from toddlers with severe tooth decay, lead author Zhi Ren and associates “noticed the bacteria and fungi forming these assemblages and developing motions we never thought they would possess: a ‘walking-like’ and ‘leaping-like’ mobility. … It’s almost like a new organism – a superorganism – with new functions,” said senior author Hyun Koo, DDS, PhD, of Penn Dental Medicine.
Did he say “mobility”? He did, didn’t he?
To study these alleged superorganisms, they set up a laboratory system “using the bacteria, fungi, and a tooth-like material, all incubated in human saliva,” the university explained.
“Incubated in human saliva.” There’s a phrase you don’t see every day.
It only took a few hours for the investigators to observe the bacterial/fungal assemblages making leaps of more than 100 microns across the tooth-like material. “That is more than 200 times their own body length,” Dr. Ren said, “making them even better than most vertebrates, relative to body size. For example, tree frogs and grasshoppers can leap forward about 50 times and 20 times their own body length, respectively.”
So, will it be the robots or the evil superorganisms? Let us give you a word of advice: Always bet on bacteria.
Foods for thought: Menstrual cramp prevention
For those who menstruate, it’s typical for that time of the month to bring cravings for things that may give a serotonin boost that eases the rise in stress hormones. Chocolate and other foods high in sugar fall into that category, but they could actually be adding to the problem.
About 90% of adolescent girls have menstrual pain, and it’s the leading cause of school absences for the demographic. Muscle relaxers and PMS pills are usually the recommended solution to alleviating menstrual cramps, but what if the patient doesn’t want to take any medicine?
Serah Sannoh of Rutgers University wanted to find another way to relieve her menstrual pains. The literature review she presented at the annual meeting of the North American Menopause Society found multiple studies that examined dietary patterns that resulted in menstrual pain.
In Ms. Sannoh’s analysis, she looked at how certain foods have an effect on cramps. Do they contribute to the pain or reduce it? Diets high in processed foods, oils, sugars, salt, and omega-6 fatty acids promote inflammation in the muscles around the uterus. Thus, cramps.
The answer, sometimes, is not to add a medicine but to change our daily practices, she suggested. Foods high in omega-3 fatty acids helped reduce pain, and those who practiced a vegan diet had the lowest muscle inflammation rates. So more salmon and fewer Swedish Fish.
Stage 1 of the robot apocalypse is already upon us
The mere mention of a robot apocalypse is enough to conjure images of terrifying robot soldiers with Austrian accents harvesting and killing humanity while the survivors live blissfully in a simulation and do low-gravity kung fu with high-profile Hollywood actors. They’ll even take over the navy.
Reality is often less exciting than the movies, but rest assured, the robots will not be denied their dominion of Earth. Our future robot overlords are simply taking a more subtle, less dramatic route toward their ultimate subjugation of mankind: They’re making us all sad and burned out.
The research pulls from work conducted in multiple countries to paint a picture of a humanity filled with anxiety about jobs as robotic automation grows more common. In India, a survey of automobile manufacturing works showed that working alongside industrial robots was linked with greater reports of burnout and workplace incivility. In Singapore, a group of college students randomly assigned to read one of three articles – one about the use of robots in business, a generic article about robots, or an article unrelated to robots – were then surveyed about their job security concerns. Three guesses as to which group was most worried.
In addition, the researchers analyzed 185 U.S. metropolitan areas for robot prevalence alongside use of job-recruiting websites and found that the more robots a city used, the more common job searches were. Unemployment rates weren’t affected, suggesting people had job insecurity because of robots. Sure, there could be other, nonrobotic reasons for this, but that’s no fun. We’re here because we fear our future android rulers.
It’s not all doom and gloom, fortunately. In an online experiment, the study authors found that self-affirmation exercises, such as writing down characteristics or values important to us, can overcome the existential fears and lessen concern about robots in the workplace. One of the authors noted that, while some fear is justified, “media reports on new technologies like robots and algorithms tend to be apocalyptic in nature, so people may develop an irrational fear about them.”
Oops. Our bad.
Apocalypse, stage 2: Leaping oral superorganisms
The terms of our secret agreement with the shadowy-but-powerful dental-industrial complex stipulate that LOTME can only cover tooth-related news once a year. This is that once a year.
Since we’ve already dealt with a robot apocalypse, how about a sci-fi horror story? A story with a “cross-kingdom partnership” in which assemblages of bacteria and fungi perform feats greater than either could achieve on its own. A story in which new microscopy technologies allow “scientists to visualize the behavior of living microbes in real time,” according to a statement from the University of Pennsylvania, Philadelphia.
While looking at saliva samples from toddlers with severe tooth decay, lead author Zhi Ren and associates “noticed the bacteria and fungi forming these assemblages and developing motions we never thought they would possess: a ‘walking-like’ and ‘leaping-like’ mobility. … It’s almost like a new organism – a superorganism – with new functions,” said senior author Hyun Koo, DDS, PhD, of Penn Dental Medicine.
Did he say “mobility”? He did, didn’t he?
To study these alleged superorganisms, they set up a laboratory system “using the bacteria, fungi, and a tooth-like material, all incubated in human saliva,” the university explained.
“Incubated in human saliva.” There’s a phrase you don’t see every day.
It only took a few hours for the investigators to observe the bacterial/fungal assemblages making leaps of more than 100 microns across the tooth-like material. “That is more than 200 times their own body length,” Dr. Ren said, “making them even better than most vertebrates, relative to body size. For example, tree frogs and grasshoppers can leap forward about 50 times and 20 times their own body length, respectively.”
So, will it be the robots or the evil superorganisms? Let us give you a word of advice: Always bet on bacteria.
Foods for thought: Menstrual cramp prevention
For those who menstruate, it’s typical for that time of the month to bring cravings for things that may give a serotonin boost that eases the rise in stress hormones. Chocolate and other foods high in sugar fall into that category, but they could actually be adding to the problem.
About 90% of adolescent girls have menstrual pain, and it’s the leading cause of school absences for the demographic. Muscle relaxers and PMS pills are usually the recommended solution to alleviating menstrual cramps, but what if the patient doesn’t want to take any medicine?
Serah Sannoh of Rutgers University wanted to find another way to relieve her menstrual pains. The literature review she presented at the annual meeting of the North American Menopause Society found multiple studies that examined dietary patterns that resulted in menstrual pain.
In Ms. Sannoh’s analysis, she looked at how certain foods have an effect on cramps. Do they contribute to the pain or reduce it? Diets high in processed foods, oils, sugars, salt, and omega-6 fatty acids promote inflammation in the muscles around the uterus. Thus, cramps.
The answer, sometimes, is not to add a medicine but to change our daily practices, she suggested. Foods high in omega-3 fatty acids helped reduce pain, and those who practiced a vegan diet had the lowest muscle inflammation rates. So more salmon and fewer Swedish Fish.
Stage 1 of the robot apocalypse is already upon us
The mere mention of a robot apocalypse is enough to conjure images of terrifying robot soldiers with Austrian accents harvesting and killing humanity while the survivors live blissfully in a simulation and do low-gravity kung fu with high-profile Hollywood actors. They’ll even take over the navy.
Reality is often less exciting than the movies, but rest assured, the robots will not be denied their dominion of Earth. Our future robot overlords are simply taking a more subtle, less dramatic route toward their ultimate subjugation of mankind: They’re making us all sad and burned out.
The research pulls from work conducted in multiple countries to paint a picture of a humanity filled with anxiety about jobs as robotic automation grows more common. In India, a survey of automobile manufacturing works showed that working alongside industrial robots was linked with greater reports of burnout and workplace incivility. In Singapore, a group of college students randomly assigned to read one of three articles – one about the use of robots in business, a generic article about robots, or an article unrelated to robots – were then surveyed about their job security concerns. Three guesses as to which group was most worried.
In addition, the researchers analyzed 185 U.S. metropolitan areas for robot prevalence alongside use of job-recruiting websites and found that the more robots a city used, the more common job searches were. Unemployment rates weren’t affected, suggesting people had job insecurity because of robots. Sure, there could be other, nonrobotic reasons for this, but that’s no fun. We’re here because we fear our future android rulers.
It’s not all doom and gloom, fortunately. In an online experiment, the study authors found that self-affirmation exercises, such as writing down characteristics or values important to us, can overcome the existential fears and lessen concern about robots in the workplace. One of the authors noted that, while some fear is justified, “media reports on new technologies like robots and algorithms tend to be apocalyptic in nature, so people may develop an irrational fear about them.”
Oops. Our bad.
Apocalypse, stage 2: Leaping oral superorganisms
The terms of our secret agreement with the shadowy-but-powerful dental-industrial complex stipulate that LOTME can only cover tooth-related news once a year. This is that once a year.
Since we’ve already dealt with a robot apocalypse, how about a sci-fi horror story? A story with a “cross-kingdom partnership” in which assemblages of bacteria and fungi perform feats greater than either could achieve on its own. A story in which new microscopy technologies allow “scientists to visualize the behavior of living microbes in real time,” according to a statement from the University of Pennsylvania, Philadelphia.
While looking at saliva samples from toddlers with severe tooth decay, lead author Zhi Ren and associates “noticed the bacteria and fungi forming these assemblages and developing motions we never thought they would possess: a ‘walking-like’ and ‘leaping-like’ mobility. … It’s almost like a new organism – a superorganism – with new functions,” said senior author Hyun Koo, DDS, PhD, of Penn Dental Medicine.
Did he say “mobility”? He did, didn’t he?
To study these alleged superorganisms, they set up a laboratory system “using the bacteria, fungi, and a tooth-like material, all incubated in human saliva,” the university explained.
“Incubated in human saliva.” There’s a phrase you don’t see every day.
It only took a few hours for the investigators to observe the bacterial/fungal assemblages making leaps of more than 100 microns across the tooth-like material. “That is more than 200 times their own body length,” Dr. Ren said, “making them even better than most vertebrates, relative to body size. For example, tree frogs and grasshoppers can leap forward about 50 times and 20 times their own body length, respectively.”
So, will it be the robots or the evil superorganisms? Let us give you a word of advice: Always bet on bacteria.
Check biases when caring for children with obesity
Counting calories should not be the focus of weight-loss strategies for children with obesity, according to an expert who said pediatricians need to change the way they discuss weight with their patients.
During a plenary session of the American Academy of Pediatrics National Conference, Joseph A. Skelton, MD, professor of pediatrics at Wake Forest University School of Medicine, Winston-Salem, N.C., said pediatricians should recognize the behavioral, physical, environmental, and genetic factors that contribute to obesity. For instance, food deserts are on the rise, and they undermine the ability of parents to feed their children healthy meals. In addition, more children are less physically active.
“Obesity is a lot more complex than calories in, calories out,” Dr. Skelton said. “We choose to treat issues of obesity as personal responsibility – ‘you did this to yourself’ – but when you look at how we move around and live our lives, our food systems, our policies, the social and environmental changes have caused shifts in our behavior.”
According to Dr. Skelton, bias against children with obesity can harm their self-image and weaken their motivations for losing weight. In addition, doctors may change how they deliver care on the basis of stereotypes regarding obese children. These stereotypes are often reinforced in media portrayals, Dr. Skelton said.
“When children or when adults who have excess weight or obesity are portrayed, they are portrayed typically in a negative fashion,” Dr. Skelton said. “There’s increasing evidence that weight bias and weight discrimination are increasing the morbidity we see in patients who develop obesity.”
For many children with obesity, visits to the pediatrician often center on weight, regardless of the reason for the appointment. Weight stigma and bias on the part of health care providers can increase stress, as well as adverse health outcomes in children, according to a 2019 study (Curr Opin Endocrinol Diabetes Obes. 2019 Feb 1. doi: 10.1097/MED.0000000000000453). Dr. Skelton recommended that pediatricians listen to their patients’ concerns and make a personalized care plan.
Dr. Skelton said doctors can pull from projects such as Health at Every Size, which offers templates for personalized health plans for children with obesity. It has a heavy focus on a weight-neutral approach to pediatric health.
“There are various ways to manage weight in a healthy and safe way,” Dr. Skelton said.
Evidence-based methods of treating obesity include focusing on health and healthy behaviors rather than weight and using the body mass index as a screening tool for further conversations about overall health, rather than as an indicator of health based on weight.
Dr. Skelton also encouraged pediatricians to be on the alert for indicators of disordered eating, which can include dieting, teasing, or talking excessively about weight at home and can involve reading misinformation about dieting online.
“Your job is to educate people on the dangers of following unscientific information online,” Dr. Skelton said. “We can address issues of weight health in a way that is patient centered and is very safe, without unintended consequences.” Brooke Sweeney, MD, professor of internal medicine and pediatrics at University of Missouri–Kansas City, said problems with weight bias in society and in clinical practice can lead to false assumptions about people who have obesity.
“It’s normal to gain adipose, or fat tissue, at different times in life, during puberty or pregnancy, and some people normally gain more weight than others,” Dr. Sweeney said.
The body will try to maintain a weight set point. That set point is influenced by many factors, such as genetics, environment, and lifestyle.
“When you lose weight, your body tries to get you back to the set point, decreasing energy expenditure and increasing hunger and reward pathways,” she said. “We have gained so much knowledge through research to better understand the pathophysiology of obesity, and we are making good progress on improving advanced treatments for increased weight in children.”
Dr. Skelton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Counting calories should not be the focus of weight-loss strategies for children with obesity, according to an expert who said pediatricians need to change the way they discuss weight with their patients.
During a plenary session of the American Academy of Pediatrics National Conference, Joseph A. Skelton, MD, professor of pediatrics at Wake Forest University School of Medicine, Winston-Salem, N.C., said pediatricians should recognize the behavioral, physical, environmental, and genetic factors that contribute to obesity. For instance, food deserts are on the rise, and they undermine the ability of parents to feed their children healthy meals. In addition, more children are less physically active.
“Obesity is a lot more complex than calories in, calories out,” Dr. Skelton said. “We choose to treat issues of obesity as personal responsibility – ‘you did this to yourself’ – but when you look at how we move around and live our lives, our food systems, our policies, the social and environmental changes have caused shifts in our behavior.”
According to Dr. Skelton, bias against children with obesity can harm their self-image and weaken their motivations for losing weight. In addition, doctors may change how they deliver care on the basis of stereotypes regarding obese children. These stereotypes are often reinforced in media portrayals, Dr. Skelton said.
“When children or when adults who have excess weight or obesity are portrayed, they are portrayed typically in a negative fashion,” Dr. Skelton said. “There’s increasing evidence that weight bias and weight discrimination are increasing the morbidity we see in patients who develop obesity.”
For many children with obesity, visits to the pediatrician often center on weight, regardless of the reason for the appointment. Weight stigma and bias on the part of health care providers can increase stress, as well as adverse health outcomes in children, according to a 2019 study (Curr Opin Endocrinol Diabetes Obes. 2019 Feb 1. doi: 10.1097/MED.0000000000000453). Dr. Skelton recommended that pediatricians listen to their patients’ concerns and make a personalized care plan.
Dr. Skelton said doctors can pull from projects such as Health at Every Size, which offers templates for personalized health plans for children with obesity. It has a heavy focus on a weight-neutral approach to pediatric health.
“There are various ways to manage weight in a healthy and safe way,” Dr. Skelton said.
Evidence-based methods of treating obesity include focusing on health and healthy behaviors rather than weight and using the body mass index as a screening tool for further conversations about overall health, rather than as an indicator of health based on weight.
Dr. Skelton also encouraged pediatricians to be on the alert for indicators of disordered eating, which can include dieting, teasing, or talking excessively about weight at home and can involve reading misinformation about dieting online.
“Your job is to educate people on the dangers of following unscientific information online,” Dr. Skelton said. “We can address issues of weight health in a way that is patient centered and is very safe, without unintended consequences.” Brooke Sweeney, MD, professor of internal medicine and pediatrics at University of Missouri–Kansas City, said problems with weight bias in society and in clinical practice can lead to false assumptions about people who have obesity.
“It’s normal to gain adipose, or fat tissue, at different times in life, during puberty or pregnancy, and some people normally gain more weight than others,” Dr. Sweeney said.
The body will try to maintain a weight set point. That set point is influenced by many factors, such as genetics, environment, and lifestyle.
“When you lose weight, your body tries to get you back to the set point, decreasing energy expenditure and increasing hunger and reward pathways,” she said. “We have gained so much knowledge through research to better understand the pathophysiology of obesity, and we are making good progress on improving advanced treatments for increased weight in children.”
Dr. Skelton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Counting calories should not be the focus of weight-loss strategies for children with obesity, according to an expert who said pediatricians need to change the way they discuss weight with their patients.
During a plenary session of the American Academy of Pediatrics National Conference, Joseph A. Skelton, MD, professor of pediatrics at Wake Forest University School of Medicine, Winston-Salem, N.C., said pediatricians should recognize the behavioral, physical, environmental, and genetic factors that contribute to obesity. For instance, food deserts are on the rise, and they undermine the ability of parents to feed their children healthy meals. In addition, more children are less physically active.
“Obesity is a lot more complex than calories in, calories out,” Dr. Skelton said. “We choose to treat issues of obesity as personal responsibility – ‘you did this to yourself’ – but when you look at how we move around and live our lives, our food systems, our policies, the social and environmental changes have caused shifts in our behavior.”
According to Dr. Skelton, bias against children with obesity can harm their self-image and weaken their motivations for losing weight. In addition, doctors may change how they deliver care on the basis of stereotypes regarding obese children. These stereotypes are often reinforced in media portrayals, Dr. Skelton said.
“When children or when adults who have excess weight or obesity are portrayed, they are portrayed typically in a negative fashion,” Dr. Skelton said. “There’s increasing evidence that weight bias and weight discrimination are increasing the morbidity we see in patients who develop obesity.”
For many children with obesity, visits to the pediatrician often center on weight, regardless of the reason for the appointment. Weight stigma and bias on the part of health care providers can increase stress, as well as adverse health outcomes in children, according to a 2019 study (Curr Opin Endocrinol Diabetes Obes. 2019 Feb 1. doi: 10.1097/MED.0000000000000453). Dr. Skelton recommended that pediatricians listen to their patients’ concerns and make a personalized care plan.
Dr. Skelton said doctors can pull from projects such as Health at Every Size, which offers templates for personalized health plans for children with obesity. It has a heavy focus on a weight-neutral approach to pediatric health.
“There are various ways to manage weight in a healthy and safe way,” Dr. Skelton said.
Evidence-based methods of treating obesity include focusing on health and healthy behaviors rather than weight and using the body mass index as a screening tool for further conversations about overall health, rather than as an indicator of health based on weight.
Dr. Skelton also encouraged pediatricians to be on the alert for indicators of disordered eating, which can include dieting, teasing, or talking excessively about weight at home and can involve reading misinformation about dieting online.
“Your job is to educate people on the dangers of following unscientific information online,” Dr. Skelton said. “We can address issues of weight health in a way that is patient centered and is very safe, without unintended consequences.” Brooke Sweeney, MD, professor of internal medicine and pediatrics at University of Missouri–Kansas City, said problems with weight bias in society and in clinical practice can lead to false assumptions about people who have obesity.
“It’s normal to gain adipose, or fat tissue, at different times in life, during puberty or pregnancy, and some people normally gain more weight than others,” Dr. Sweeney said.
The body will try to maintain a weight set point. That set point is influenced by many factors, such as genetics, environment, and lifestyle.
“When you lose weight, your body tries to get you back to the set point, decreasing energy expenditure and increasing hunger and reward pathways,” she said. “We have gained so much knowledge through research to better understand the pathophysiology of obesity, and we are making good progress on improving advanced treatments for increased weight in children.”
Dr. Skelton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAP 2022
With sleuth work, pediatricians can identify genetic disorders
Jennifer Kalish, MD, PhD, fields as many as 10 inquiries a month from pediatricians who spot an unusual feature during a clinical exam, and wonder if they should refer the family to a geneticist.
“There are hundreds of rare disorders, and for a pediatrician, they can be hard to recognize,” Dr. Kalish said. “That’s why we’re here as geneticists – to partner so that we can help.”
Pediatricians play a key role in spotting signs of rare genetic diseases, but may need guidance for recognizing the more subtle presentations of a disorder, according to Dr. Kalish, a geneticist and director of the Beckwith-Wiedemann Syndrome Clinic at Children’s Hospital of Philadelphia, who spoke at the American Academy of Pediatrics National Conference.
Spectrums of disease
Pediatricians may struggle with deciding whether to make a referral, in part because genetic syndromes “do not always look like the textbook,” she said.
With many conditions, “we’re starting to understand that there’s really a spectrum of how affected versus less affected one can be,” by genetic and epigenetic changes, which have led to recognition that many cases are more subtle and harder to diagnose, she said.
Beckwith-Wiedemann syndrome is a prime example. The overgrowth disorder affects an estimated 1 in 10,340 infants, and is associated with a heightened risk of Wilms tumors, a form of kidney cancer, and hepatoblastomas. Children diagnosed with these conditions typically undergo frequent screenings to detect tumors to jumpstart treatment.
Some researchers believe Beckwith-Wiedemann syndrome is underdiagnosed because it can present in many different ways because of variations in the distributions of affected cells in the body, known as mosaicism.
To address the complexity, Dr. Kalish guided development of a scoring system for determining whether molecular testing is warranted. Primary features such as an enlarged tongue and lateralized overgrowth carry more points, whereas suggestive features like ear creases or large birth weight carry fewer points.
Diagnostic advances have occurred for other syndromes, as well. For example, researchers have created a scoring system for Russell-Silver syndrome, a less common disorder characterized by slow growth before and after birth, in which mosaicism is also present.
Early diagnosis and intervention of Russell-Silver syndrome can ensure that patients grow to their maximum potential and address problems such as feeding issues.
Spotting a “compilation of features”
Although tools are available, Dr. Kalish said pediatricians don’t need to make a diagnosis, and instead can refer patients to a geneticist after recognizing clinical features that hint at a genetic etiology.
For pediatricians, the process of deciding whether to refer a patient to a geneticist may entail ruling out nongenetic causes, considering patient and family history, and ultimately deciding whether there is a “compilation of features” that falls outside the norm, she said. Unfortunately, she added, there’s “not a simple list I could just hand out saying, ‘If you see these things, call me.’ ”
Dr. Kalish said pediatricians should be aware that two children with similar features can have different syndromes. She presented case studies of two infants, who both had enlarged tongues and older mothers.
One child had hallmarks that pointed to Beckwith-Wiedemann syndrome: conception with in vitro fertilization, length in the 98th percentile, a long umbilical cord, nevus simplex birthmarks, and labial and leg asymmetry.
The other baby had features aligned with Down syndrome: a heart murmur, upward slanting eyes, and a single crease on the palm.
In some cases, isolated features such as the shape, slant, or spacing of eyes, or the presence of creases on the ears, may simply be familial or inherited traits, Dr. Kalish said.
She noted that “there’s been a lot of work in genetics in the past few years to show what syndromes look like” in diverse populations. The American Journal of Medical Genetics Part A has published a series of reports on the topic.
Dr. Kalish reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Jennifer Kalish, MD, PhD, fields as many as 10 inquiries a month from pediatricians who spot an unusual feature during a clinical exam, and wonder if they should refer the family to a geneticist.
“There are hundreds of rare disorders, and for a pediatrician, they can be hard to recognize,” Dr. Kalish said. “That’s why we’re here as geneticists – to partner so that we can help.”
Pediatricians play a key role in spotting signs of rare genetic diseases, but may need guidance for recognizing the more subtle presentations of a disorder, according to Dr. Kalish, a geneticist and director of the Beckwith-Wiedemann Syndrome Clinic at Children’s Hospital of Philadelphia, who spoke at the American Academy of Pediatrics National Conference.
Spectrums of disease
Pediatricians may struggle with deciding whether to make a referral, in part because genetic syndromes “do not always look like the textbook,” she said.
With many conditions, “we’re starting to understand that there’s really a spectrum of how affected versus less affected one can be,” by genetic and epigenetic changes, which have led to recognition that many cases are more subtle and harder to diagnose, she said.
Beckwith-Wiedemann syndrome is a prime example. The overgrowth disorder affects an estimated 1 in 10,340 infants, and is associated with a heightened risk of Wilms tumors, a form of kidney cancer, and hepatoblastomas. Children diagnosed with these conditions typically undergo frequent screenings to detect tumors to jumpstart treatment.
Some researchers believe Beckwith-Wiedemann syndrome is underdiagnosed because it can present in many different ways because of variations in the distributions of affected cells in the body, known as mosaicism.
To address the complexity, Dr. Kalish guided development of a scoring system for determining whether molecular testing is warranted. Primary features such as an enlarged tongue and lateralized overgrowth carry more points, whereas suggestive features like ear creases or large birth weight carry fewer points.
Diagnostic advances have occurred for other syndromes, as well. For example, researchers have created a scoring system for Russell-Silver syndrome, a less common disorder characterized by slow growth before and after birth, in which mosaicism is also present.
Early diagnosis and intervention of Russell-Silver syndrome can ensure that patients grow to their maximum potential and address problems such as feeding issues.
Spotting a “compilation of features”
Although tools are available, Dr. Kalish said pediatricians don’t need to make a diagnosis, and instead can refer patients to a geneticist after recognizing clinical features that hint at a genetic etiology.
For pediatricians, the process of deciding whether to refer a patient to a geneticist may entail ruling out nongenetic causes, considering patient and family history, and ultimately deciding whether there is a “compilation of features” that falls outside the norm, she said. Unfortunately, she added, there’s “not a simple list I could just hand out saying, ‘If you see these things, call me.’ ”
Dr. Kalish said pediatricians should be aware that two children with similar features can have different syndromes. She presented case studies of two infants, who both had enlarged tongues and older mothers.
One child had hallmarks that pointed to Beckwith-Wiedemann syndrome: conception with in vitro fertilization, length in the 98th percentile, a long umbilical cord, nevus simplex birthmarks, and labial and leg asymmetry.
The other baby had features aligned with Down syndrome: a heart murmur, upward slanting eyes, and a single crease on the palm.
In some cases, isolated features such as the shape, slant, or spacing of eyes, or the presence of creases on the ears, may simply be familial or inherited traits, Dr. Kalish said.
She noted that “there’s been a lot of work in genetics in the past few years to show what syndromes look like” in diverse populations. The American Journal of Medical Genetics Part A has published a series of reports on the topic.
Dr. Kalish reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Jennifer Kalish, MD, PhD, fields as many as 10 inquiries a month from pediatricians who spot an unusual feature during a clinical exam, and wonder if they should refer the family to a geneticist.
“There are hundreds of rare disorders, and for a pediatrician, they can be hard to recognize,” Dr. Kalish said. “That’s why we’re here as geneticists – to partner so that we can help.”
Pediatricians play a key role in spotting signs of rare genetic diseases, but may need guidance for recognizing the more subtle presentations of a disorder, according to Dr. Kalish, a geneticist and director of the Beckwith-Wiedemann Syndrome Clinic at Children’s Hospital of Philadelphia, who spoke at the American Academy of Pediatrics National Conference.
Spectrums of disease
Pediatricians may struggle with deciding whether to make a referral, in part because genetic syndromes “do not always look like the textbook,” she said.
With many conditions, “we’re starting to understand that there’s really a spectrum of how affected versus less affected one can be,” by genetic and epigenetic changes, which have led to recognition that many cases are more subtle and harder to diagnose, she said.
Beckwith-Wiedemann syndrome is a prime example. The overgrowth disorder affects an estimated 1 in 10,340 infants, and is associated with a heightened risk of Wilms tumors, a form of kidney cancer, and hepatoblastomas. Children diagnosed with these conditions typically undergo frequent screenings to detect tumors to jumpstart treatment.
Some researchers believe Beckwith-Wiedemann syndrome is underdiagnosed because it can present in many different ways because of variations in the distributions of affected cells in the body, known as mosaicism.
To address the complexity, Dr. Kalish guided development of a scoring system for determining whether molecular testing is warranted. Primary features such as an enlarged tongue and lateralized overgrowth carry more points, whereas suggestive features like ear creases or large birth weight carry fewer points.
Diagnostic advances have occurred for other syndromes, as well. For example, researchers have created a scoring system for Russell-Silver syndrome, a less common disorder characterized by slow growth before and after birth, in which mosaicism is also present.
Early diagnosis and intervention of Russell-Silver syndrome can ensure that patients grow to their maximum potential and address problems such as feeding issues.
Spotting a “compilation of features”
Although tools are available, Dr. Kalish said pediatricians don’t need to make a diagnosis, and instead can refer patients to a geneticist after recognizing clinical features that hint at a genetic etiology.
For pediatricians, the process of deciding whether to refer a patient to a geneticist may entail ruling out nongenetic causes, considering patient and family history, and ultimately deciding whether there is a “compilation of features” that falls outside the norm, she said. Unfortunately, she added, there’s “not a simple list I could just hand out saying, ‘If you see these things, call me.’ ”
Dr. Kalish said pediatricians should be aware that two children with similar features can have different syndromes. She presented case studies of two infants, who both had enlarged tongues and older mothers.
One child had hallmarks that pointed to Beckwith-Wiedemann syndrome: conception with in vitro fertilization, length in the 98th percentile, a long umbilical cord, nevus simplex birthmarks, and labial and leg asymmetry.
The other baby had features aligned with Down syndrome: a heart murmur, upward slanting eyes, and a single crease on the palm.
In some cases, isolated features such as the shape, slant, or spacing of eyes, or the presence of creases on the ears, may simply be familial or inherited traits, Dr. Kalish said.
She noted that “there’s been a lot of work in genetics in the past few years to show what syndromes look like” in diverse populations. The American Journal of Medical Genetics Part A has published a series of reports on the topic.
Dr. Kalish reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAP 2022
63% of long COVID patients are women, study says
according to a new study published in JAMA.
The global study also found that about 6% of people with symptomatic infections had long COVID in 2020 and 2021. The risk for long COVID seemed to be greater among those who needed hospitalization, especially those who needed intensive care.
“Quantifying the number of individuals with long COVID may help policy makers ensure adequate access to services to guide people toward recovery, return to the workplace or school, and restore their mental health and social life,” the researchers wrote.
The study team, which included dozens of researchers across nearly every continent, analyzed data from 54 studies and two databases for more than 1 million patients in 22 countries who had symptomatic COVID infections in 2020 and 2021. They looked at three long COVID symptom types: persistent fatigue with bodily pain or mood swings, ongoing respiratory problems, and cognitive issues. The study included people aged 4-66.
Overall, 6.2% of people reported one of the long COVID symptom types, including 3.7% with ongoing respiratory problems, 3.2% with persistent fatigue and bodily pain or mood swings, and 2.2% with cognitive problems. Among those with long COVID, 38% of people reported more than one symptom cluster.
At 3 months after infection, long COVID symptoms were nearly twice as common in women who were at least 20 years old at 10.6%, compared with men who were at least 20 years old at 5.4%.
Children and teens appeared to have lower risks of long COVID. About 2.8% of patients under age 20 with symptomatic infection developed long-term issues.
The estimated average duration of long COVID symptoms was 9 months among hospitalized patients and 4 months among those who weren’t hospitalized. About 15% of people with long COVID symptoms 3 months after the initial infection continued to have symptoms at 12 months.
The study was largely based on detailed data from ongoing COVID-19 studies in the United States, Austria, the Faroe Islands, Germany, Iran, Italy, the Netherlands, Russia, Sweden, and Switzerland, according to UPI. It was supplemented by published data and research conducted as part of the Global Burden of Diseases, Injuries and Risk Factors Study. The dozens of researchers are referred to as “Global Burden of Disease Long COVID Collaborators.”
The study had limitations, the researchers said, including the assumption that long COVID follows a similar course in all countries. Additional studies may show how long COVID symptoms and severity may vary in different countries and continents.
Ultimately, ongoing studies of large numbers of people with long COVID could help scientists and public health officials understand risk factors and ways to treat the debilitating condition, the study authors wrote, noting that “postinfection fatigue syndrome” has been reported before, namely during the 1918 flu pandemic, after the SARS outbreak in 2003, and after the Ebola epidemic in West Africa in 2014.
“Similar symptoms have been reported after other viral infections, including the Epstein-Barr virus, mononucleosis, and dengue, as well as after nonviral infections such as Q fever, Lyme disease and giardiasis,” they wrote.
Several study investigators reported receiving grants and personal fees from a variety of sources.
A version of this article first appeared on Medscape.com.
according to a new study published in JAMA.
The global study also found that about 6% of people with symptomatic infections had long COVID in 2020 and 2021. The risk for long COVID seemed to be greater among those who needed hospitalization, especially those who needed intensive care.
“Quantifying the number of individuals with long COVID may help policy makers ensure adequate access to services to guide people toward recovery, return to the workplace or school, and restore their mental health and social life,” the researchers wrote.
The study team, which included dozens of researchers across nearly every continent, analyzed data from 54 studies and two databases for more than 1 million patients in 22 countries who had symptomatic COVID infections in 2020 and 2021. They looked at three long COVID symptom types: persistent fatigue with bodily pain or mood swings, ongoing respiratory problems, and cognitive issues. The study included people aged 4-66.
Overall, 6.2% of people reported one of the long COVID symptom types, including 3.7% with ongoing respiratory problems, 3.2% with persistent fatigue and bodily pain or mood swings, and 2.2% with cognitive problems. Among those with long COVID, 38% of people reported more than one symptom cluster.
At 3 months after infection, long COVID symptoms were nearly twice as common in women who were at least 20 years old at 10.6%, compared with men who were at least 20 years old at 5.4%.
Children and teens appeared to have lower risks of long COVID. About 2.8% of patients under age 20 with symptomatic infection developed long-term issues.
The estimated average duration of long COVID symptoms was 9 months among hospitalized patients and 4 months among those who weren’t hospitalized. About 15% of people with long COVID symptoms 3 months after the initial infection continued to have symptoms at 12 months.
The study was largely based on detailed data from ongoing COVID-19 studies in the United States, Austria, the Faroe Islands, Germany, Iran, Italy, the Netherlands, Russia, Sweden, and Switzerland, according to UPI. It was supplemented by published data and research conducted as part of the Global Burden of Diseases, Injuries and Risk Factors Study. The dozens of researchers are referred to as “Global Burden of Disease Long COVID Collaborators.”
The study had limitations, the researchers said, including the assumption that long COVID follows a similar course in all countries. Additional studies may show how long COVID symptoms and severity may vary in different countries and continents.
Ultimately, ongoing studies of large numbers of people with long COVID could help scientists and public health officials understand risk factors and ways to treat the debilitating condition, the study authors wrote, noting that “postinfection fatigue syndrome” has been reported before, namely during the 1918 flu pandemic, after the SARS outbreak in 2003, and after the Ebola epidemic in West Africa in 2014.
“Similar symptoms have been reported after other viral infections, including the Epstein-Barr virus, mononucleosis, and dengue, as well as after nonviral infections such as Q fever, Lyme disease and giardiasis,” they wrote.
Several study investigators reported receiving grants and personal fees from a variety of sources.
A version of this article first appeared on Medscape.com.
according to a new study published in JAMA.
The global study also found that about 6% of people with symptomatic infections had long COVID in 2020 and 2021. The risk for long COVID seemed to be greater among those who needed hospitalization, especially those who needed intensive care.
“Quantifying the number of individuals with long COVID may help policy makers ensure adequate access to services to guide people toward recovery, return to the workplace or school, and restore their mental health and social life,” the researchers wrote.
The study team, which included dozens of researchers across nearly every continent, analyzed data from 54 studies and two databases for more than 1 million patients in 22 countries who had symptomatic COVID infections in 2020 and 2021. They looked at three long COVID symptom types: persistent fatigue with bodily pain or mood swings, ongoing respiratory problems, and cognitive issues. The study included people aged 4-66.
Overall, 6.2% of people reported one of the long COVID symptom types, including 3.7% with ongoing respiratory problems, 3.2% with persistent fatigue and bodily pain or mood swings, and 2.2% with cognitive problems. Among those with long COVID, 38% of people reported more than one symptom cluster.
At 3 months after infection, long COVID symptoms were nearly twice as common in women who were at least 20 years old at 10.6%, compared with men who were at least 20 years old at 5.4%.
Children and teens appeared to have lower risks of long COVID. About 2.8% of patients under age 20 with symptomatic infection developed long-term issues.
The estimated average duration of long COVID symptoms was 9 months among hospitalized patients and 4 months among those who weren’t hospitalized. About 15% of people with long COVID symptoms 3 months after the initial infection continued to have symptoms at 12 months.
The study was largely based on detailed data from ongoing COVID-19 studies in the United States, Austria, the Faroe Islands, Germany, Iran, Italy, the Netherlands, Russia, Sweden, and Switzerland, according to UPI. It was supplemented by published data and research conducted as part of the Global Burden of Diseases, Injuries and Risk Factors Study. The dozens of researchers are referred to as “Global Burden of Disease Long COVID Collaborators.”
The study had limitations, the researchers said, including the assumption that long COVID follows a similar course in all countries. Additional studies may show how long COVID symptoms and severity may vary in different countries and continents.
Ultimately, ongoing studies of large numbers of people with long COVID could help scientists and public health officials understand risk factors and ways to treat the debilitating condition, the study authors wrote, noting that “postinfection fatigue syndrome” has been reported before, namely during the 1918 flu pandemic, after the SARS outbreak in 2003, and after the Ebola epidemic in West Africa in 2014.
“Similar symptoms have been reported after other viral infections, including the Epstein-Barr virus, mononucleosis, and dengue, as well as after nonviral infections such as Q fever, Lyme disease and giardiasis,” they wrote.
Several study investigators reported receiving grants and personal fees from a variety of sources.
A version of this article first appeared on Medscape.com.
FROM JAMA
Older diabetes drugs linked to dementia risk -- one lower, one higher
a new observational study in patients with type 2 diabetes suggests.
The data, obtained from nationwide electronic medical records from the Department of Veterans Affairs, yielded a 22% lower risk of dementia with TZD monotherapy and a 12% elevated risk with sulfonylurea monotherapy, compared with metformin monotherapy. The apparent protective effects of TZDs were greater among individuals with overweight or obesity.
“Our findings provide additional information to aid clinicians’ selection of [glucose-lowering medications] for patients with mild or moderate type 2 diabetes and [who] are at high risk of dementia,” Xin Tang and colleagues wrote in their article, published online in BMJ Open Diabetes Research & Care.
The results “add substantially to the literature concerning the effects of [glucose-lowering medications] on dementia where previous findings have been inconsistent. Studies with a follow-up time of less than 3 years have mainly reported null associations, while studies with longer a follow-up time typically yielded protective findings. With a mean follow-up time of 6.8 years, we had a sufficient duration to detect treatment differences,” the investigators wrote.
“Supplementing [a] sulfonylurea with either metformin or [a] TZD may partially offset its prodementia effects. These findings may help inform medication selection for elderly patients with T2D at high risk of dementia,” they added.
Randomized trials needed to determine cause and effect
Ivan Koychev, PhD, a senior clinical researcher in the department of psychiatry at the University of Oxford (England), told the UK Science Media Centre: “This is a large, well-conducted real-world data study that highlights the importance of checking whether already prescribed medications may be useful for preventing dementia.”
The findings regarding TZDs, also known as glitazones, are in line with existing literature suggesting dementia protection with other drugs prescribed for type 2 diabetes that weren’t examined in the current study, such as newer agents like glucagonlike peptide–1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. Koychev said.
“The main limitations of this study is that following the initial 2-year period the authors were interested in, the participants may have been prescribed one of the other type 2 diabetes drugs [GLP-1 agonists or SGLT2 inhibitors] that have been found to reduce dementia risk, thus potentially making the direct glitazone [TZD] effect more difficult to discern,” Dr. Koychev noted.
And, he pointed out that the study design limits attribution of causality. “It is also important to note that people with type 2 diabetes do run a higher risk of both dementia and cognitive deficits and that these medications are only prescribed in these patients, so all this data is from this patient group rather than the general population.”
James Connell, PhD, head of translational science at Alzheimer’s Research UK, agreed. “While this observational study found that those with type 2 diabetes taking thiazolidinedione had a lower dementia risk than those on the most common medication for type 2 diabetes, it only shows an association between taking the drug and dementia risk and not a causal relationship.
“Double-blind and placebo-controlled clinical trials are needed to see whether the drug [TDZ] could help lower dementia risk in people with and without diabetes. Anyone with any questions about what treatments they are receiving should speak to their doctor,” he told the UK Science Media Centre.
Opposite effects of sulfonylureas, TZDs versus metformin
The study authors analyzed 559,106 VA patients with type 2 diabetes who initiated glucose-lowering medication during 2001-2017 and took it for at least a year. They were aged 60 years or older and did not have dementia at baseline. Most were White (76.8%) and male (96.9%), two-thirds (63.1%) had obesity, and mean hemoglobin A1c was 6.8%.
Overall, 31,125 developed all-cause dementia. The incidence rate was 8.2 cases per 1,000 person-years, ranging from 6.2 cases per 1,000 person-years among those taking metformin monotherapy to 13.4 cases per 1,000 person-years in those taking both sulfonylurea and a TZD.
Compared with metformin monotherapy, the hazard ratio for all-cause dementia for sulfonylurea monotherapy was a significant 1.12. The increased risk was also seen for vascular dementia, with an HR of 1.14.
In contrast, TZD monotherapy was associated with a significantly lower risk for all-cause dementia (HR, 0.78), as well as for Alzheimer’s disease (HR, 0.89) and vascular dementia (HR, 0.43), compared with metformin monotherapy.
The combination of metformin and TZD also lowered the risk of all-cause dementia, while regimens including sulfonylureas raised the risks for all-cause and vascular dementia.
Most of the results didn’t change significantly when the drug exposure window was extended to 2 years.
Effects more pronounced in those with obesity
The protective 1-year effects of TZD monotherapy and of metformin plus TZD, compared with metformin alone, were more significant among participants aged 75 or younger and with a body mass index above 25 kg/m2, compared with those who were older than 75 years and with normal BMIs, respectively.
On the other hand, the greater risk for dementia incurred with sulfonylureas was further increased among those with higher BMI.
This research was partially funded by grants from the National Human Genome Research Institute, the National Science Foundation, the National Institute of Diabetes and Digestive and Kidney Disease, and the National Heart, Lung, and Blood Institute. Dr. Koychev is chief investigator for a trial, sponsored by Oxford University and funded by Novo Nordisk, testing whether the GLP-1 agonist semaglutide reduces the risk for dementia in aging adults.
A version of this article first appeared on Medscape.com.
a new observational study in patients with type 2 diabetes suggests.
The data, obtained from nationwide electronic medical records from the Department of Veterans Affairs, yielded a 22% lower risk of dementia with TZD monotherapy and a 12% elevated risk with sulfonylurea monotherapy, compared with metformin monotherapy. The apparent protective effects of TZDs were greater among individuals with overweight or obesity.
“Our findings provide additional information to aid clinicians’ selection of [glucose-lowering medications] for patients with mild or moderate type 2 diabetes and [who] are at high risk of dementia,” Xin Tang and colleagues wrote in their article, published online in BMJ Open Diabetes Research & Care.
The results “add substantially to the literature concerning the effects of [glucose-lowering medications] on dementia where previous findings have been inconsistent. Studies with a follow-up time of less than 3 years have mainly reported null associations, while studies with longer a follow-up time typically yielded protective findings. With a mean follow-up time of 6.8 years, we had a sufficient duration to detect treatment differences,” the investigators wrote.
“Supplementing [a] sulfonylurea with either metformin or [a] TZD may partially offset its prodementia effects. These findings may help inform medication selection for elderly patients with T2D at high risk of dementia,” they added.
Randomized trials needed to determine cause and effect
Ivan Koychev, PhD, a senior clinical researcher in the department of psychiatry at the University of Oxford (England), told the UK Science Media Centre: “This is a large, well-conducted real-world data study that highlights the importance of checking whether already prescribed medications may be useful for preventing dementia.”
The findings regarding TZDs, also known as glitazones, are in line with existing literature suggesting dementia protection with other drugs prescribed for type 2 diabetes that weren’t examined in the current study, such as newer agents like glucagonlike peptide–1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. Koychev said.
“The main limitations of this study is that following the initial 2-year period the authors were interested in, the participants may have been prescribed one of the other type 2 diabetes drugs [GLP-1 agonists or SGLT2 inhibitors] that have been found to reduce dementia risk, thus potentially making the direct glitazone [TZD] effect more difficult to discern,” Dr. Koychev noted.
And, he pointed out that the study design limits attribution of causality. “It is also important to note that people with type 2 diabetes do run a higher risk of both dementia and cognitive deficits and that these medications are only prescribed in these patients, so all this data is from this patient group rather than the general population.”
James Connell, PhD, head of translational science at Alzheimer’s Research UK, agreed. “While this observational study found that those with type 2 diabetes taking thiazolidinedione had a lower dementia risk than those on the most common medication for type 2 diabetes, it only shows an association between taking the drug and dementia risk and not a causal relationship.
“Double-blind and placebo-controlled clinical trials are needed to see whether the drug [TDZ] could help lower dementia risk in people with and without diabetes. Anyone with any questions about what treatments they are receiving should speak to their doctor,” he told the UK Science Media Centre.
Opposite effects of sulfonylureas, TZDs versus metformin
The study authors analyzed 559,106 VA patients with type 2 diabetes who initiated glucose-lowering medication during 2001-2017 and took it for at least a year. They were aged 60 years or older and did not have dementia at baseline. Most were White (76.8%) and male (96.9%), two-thirds (63.1%) had obesity, and mean hemoglobin A1c was 6.8%.
Overall, 31,125 developed all-cause dementia. The incidence rate was 8.2 cases per 1,000 person-years, ranging from 6.2 cases per 1,000 person-years among those taking metformin monotherapy to 13.4 cases per 1,000 person-years in those taking both sulfonylurea and a TZD.
Compared with metformin monotherapy, the hazard ratio for all-cause dementia for sulfonylurea monotherapy was a significant 1.12. The increased risk was also seen for vascular dementia, with an HR of 1.14.
In contrast, TZD monotherapy was associated with a significantly lower risk for all-cause dementia (HR, 0.78), as well as for Alzheimer’s disease (HR, 0.89) and vascular dementia (HR, 0.43), compared with metformin monotherapy.
The combination of metformin and TZD also lowered the risk of all-cause dementia, while regimens including sulfonylureas raised the risks for all-cause and vascular dementia.
Most of the results didn’t change significantly when the drug exposure window was extended to 2 years.
Effects more pronounced in those with obesity
The protective 1-year effects of TZD monotherapy and of metformin plus TZD, compared with metformin alone, were more significant among participants aged 75 or younger and with a body mass index above 25 kg/m2, compared with those who were older than 75 years and with normal BMIs, respectively.
On the other hand, the greater risk for dementia incurred with sulfonylureas was further increased among those with higher BMI.
This research was partially funded by grants from the National Human Genome Research Institute, the National Science Foundation, the National Institute of Diabetes and Digestive and Kidney Disease, and the National Heart, Lung, and Blood Institute. Dr. Koychev is chief investigator for a trial, sponsored by Oxford University and funded by Novo Nordisk, testing whether the GLP-1 agonist semaglutide reduces the risk for dementia in aging adults.
A version of this article first appeared on Medscape.com.
a new observational study in patients with type 2 diabetes suggests.
The data, obtained from nationwide electronic medical records from the Department of Veterans Affairs, yielded a 22% lower risk of dementia with TZD monotherapy and a 12% elevated risk with sulfonylurea monotherapy, compared with metformin monotherapy. The apparent protective effects of TZDs were greater among individuals with overweight or obesity.
“Our findings provide additional information to aid clinicians’ selection of [glucose-lowering medications] for patients with mild or moderate type 2 diabetes and [who] are at high risk of dementia,” Xin Tang and colleagues wrote in their article, published online in BMJ Open Diabetes Research & Care.
The results “add substantially to the literature concerning the effects of [glucose-lowering medications] on dementia where previous findings have been inconsistent. Studies with a follow-up time of less than 3 years have mainly reported null associations, while studies with longer a follow-up time typically yielded protective findings. With a mean follow-up time of 6.8 years, we had a sufficient duration to detect treatment differences,” the investigators wrote.
“Supplementing [a] sulfonylurea with either metformin or [a] TZD may partially offset its prodementia effects. These findings may help inform medication selection for elderly patients with T2D at high risk of dementia,” they added.
Randomized trials needed to determine cause and effect
Ivan Koychev, PhD, a senior clinical researcher in the department of psychiatry at the University of Oxford (England), told the UK Science Media Centre: “This is a large, well-conducted real-world data study that highlights the importance of checking whether already prescribed medications may be useful for preventing dementia.”
The findings regarding TZDs, also known as glitazones, are in line with existing literature suggesting dementia protection with other drugs prescribed for type 2 diabetes that weren’t examined in the current study, such as newer agents like glucagonlike peptide–1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. Koychev said.
“The main limitations of this study is that following the initial 2-year period the authors were interested in, the participants may have been prescribed one of the other type 2 diabetes drugs [GLP-1 agonists or SGLT2 inhibitors] that have been found to reduce dementia risk, thus potentially making the direct glitazone [TZD] effect more difficult to discern,” Dr. Koychev noted.
And, he pointed out that the study design limits attribution of causality. “It is also important to note that people with type 2 diabetes do run a higher risk of both dementia and cognitive deficits and that these medications are only prescribed in these patients, so all this data is from this patient group rather than the general population.”
James Connell, PhD, head of translational science at Alzheimer’s Research UK, agreed. “While this observational study found that those with type 2 diabetes taking thiazolidinedione had a lower dementia risk than those on the most common medication for type 2 diabetes, it only shows an association between taking the drug and dementia risk and not a causal relationship.
“Double-blind and placebo-controlled clinical trials are needed to see whether the drug [TDZ] could help lower dementia risk in people with and without diabetes. Anyone with any questions about what treatments they are receiving should speak to their doctor,” he told the UK Science Media Centre.
Opposite effects of sulfonylureas, TZDs versus metformin
The study authors analyzed 559,106 VA patients with type 2 diabetes who initiated glucose-lowering medication during 2001-2017 and took it for at least a year. They were aged 60 years or older and did not have dementia at baseline. Most were White (76.8%) and male (96.9%), two-thirds (63.1%) had obesity, and mean hemoglobin A1c was 6.8%.
Overall, 31,125 developed all-cause dementia. The incidence rate was 8.2 cases per 1,000 person-years, ranging from 6.2 cases per 1,000 person-years among those taking metformin monotherapy to 13.4 cases per 1,000 person-years in those taking both sulfonylurea and a TZD.
Compared with metformin monotherapy, the hazard ratio for all-cause dementia for sulfonylurea monotherapy was a significant 1.12. The increased risk was also seen for vascular dementia, with an HR of 1.14.
In contrast, TZD monotherapy was associated with a significantly lower risk for all-cause dementia (HR, 0.78), as well as for Alzheimer’s disease (HR, 0.89) and vascular dementia (HR, 0.43), compared with metformin monotherapy.
The combination of metformin and TZD also lowered the risk of all-cause dementia, while regimens including sulfonylureas raised the risks for all-cause and vascular dementia.
Most of the results didn’t change significantly when the drug exposure window was extended to 2 years.
Effects more pronounced in those with obesity
The protective 1-year effects of TZD monotherapy and of metformin plus TZD, compared with metformin alone, were more significant among participants aged 75 or younger and with a body mass index above 25 kg/m2, compared with those who were older than 75 years and with normal BMIs, respectively.
On the other hand, the greater risk for dementia incurred with sulfonylureas was further increased among those with higher BMI.
This research was partially funded by grants from the National Human Genome Research Institute, the National Science Foundation, the National Institute of Diabetes and Digestive and Kidney Disease, and the National Heart, Lung, and Blood Institute. Dr. Koychev is chief investigator for a trial, sponsored by Oxford University and funded by Novo Nordisk, testing whether the GLP-1 agonist semaglutide reduces the risk for dementia in aging adults.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN DIABETES RESEARCH & CARE
New advice on artificial pancreas insulin delivery systems
A new consensus statement summarizes the benefits, limitations, and challenges of using automated insulin delivery (AID) systems and provides recommendations for use by people with diabetes.
“Automated insulin delivery systems” is becoming the standard terminology – including by the U.S. Food and Drug Administration – to refer to systems that integrate data from a continuous glucose monitoring (CGM) system via a control algorithm into an insulin pump in order to automate subcutaneous insulin delivery. “Hybrid AID” or “hybrid closed-loop” refers to the current status of these systems, which still require some degree of user input to control glucose levels.
The term “artificial pancreas” was used interchangeably with AID in the past, but it doesn’t take into account exocrine pancreatic function. The term “bionic pancreas” refers to a specific system in development that would ultimately include glucagon along with insulin.
The new consensus report, titled “Automated insulin delivery: Benefits, challenges, and recommendations,” was published online in Diabetes Care and Diabetologia.
The document is geared toward not only diabetologists and other specialists, but also diabetes nurses and specialist dietitians. Colleagues working at regulatory agencies, health care organizations, and related media might also benefit from reading it.
It is endorsed by two professional societies – the European Association for the Study of Diabetes and the American Diabetes Association – and contrasts with other statements about AID systems that are sponsored by their manufacturers, noted document co-author Mark Evans, PhD, professor of diabetic medicine, University of Cambridge, England, in a statement.
“Many clinically relevant aspects, including safety, are addressed in this report. The aim ... is to encourage ongoing improvement of this technology, its safe and effective use, and its accessibility to all who can benefit from it,” Dr. Evans said.
Lead author Jennifer Sherr, MD, PhD, pediatric endocrinology, Yale University, New Haven, Conn., commented that the report “addresses the clinical usage of AID systems from a practical point of view rather than as ... a meta-analysis or a review of all relevant clinical studies. ... As such, the benefits and limitations of systems are discussed while also considering safety, regulatory pathways, and access to this technology.”
AID systems do not mean diabetes is “cured”
Separate recommendations provided at the end of the document are aimed at specific stakeholders, including health care providers, patients and their caregivers, manufacturers, regulatory agencies, and the research community.
The authors make clear in the introduction that, while representing “a significant movement toward optimizing glucose management for individuals with diabetes,” the use of AID systems doesn’t mean that diabetes is “cured.” Rather, “expectations need to be set adequately so that individuals with diabetes and providers understand what such systems can and cannot do.”
In particular, current commercially available AID systems require user input for mealtime insulin dosing and sometimes for correction doses of high blood glucose levels, although the systems at least partially automate that.
“When integrated into care, AID systems hold promise to relieve some of the daily burdens of diabetes care,” the authors write.
The statement also details problems that may arise with the physical devices, including skin irritation from adhesives, occlusion of insulin infusion sets, early CGM sensor failure, and inadequate dosing algorithms.
“Individuals with diabetes who are considering this type of advanced diabetes therapy should not only have appropriate technical understanding of the system but also be able to revert to standard diabetes treatment (that is, nonautomated subcutaneous insulin delivery by pump or injections) in case the AID system fails. They should be able to independently troubleshoot and have access to their health care provider if needed.”
To monitor the impact of the technology, the authors emphasize the importance of the time-in-range metric derived from CGM, with the goal of achieving 70% or greater time in target blood glucose range.
Separate sections of the document address the benefits and limitations of AID systems, education and expectations for both patients and providers, and patient and provider perspectives, including how to handle urgent questions.
Other sections cover special populations such as pregnant women and people with type 2 diabetes, considerations for patient selection for current AID systems, safety, improving access to the technology, liability, and do-it-yourself systems.
Recommendations for health care professionals
A table near the end of the document provides specific recommendations for health care professionals, including the following:
- Be knowledgeable about AID systems and nuances of different systems, including their distinguishing features as well as strengths and weaknesses.
- Inform patients with diabetes about AID systems, including review of currently available systems, and create realistic expectations for device use.
- Involve patients with diabetes in shared decision-making when considering use of AID systems.
- Share information with patients with diabetes, as well as their peers, about general standards set by national and international guidelines on AID systems.
- Provide an on-call number or method by which a person with diabetes can always access support from a health care provider at the practice, including weekends and nights.
- Implement, potentially, protocols on times when AID systems should not be used.
- Use an individual’s health data to improve quality of care and health outcomes.
Most members of the ADA/EASD Diabetes Technology Working Group work with industry, but industry had no input on the project. Dr. Sherr has reported conducting clinical trials for Eli Lilly, Insulet, and Medtronic, and has received in-kind support for research studies from Dexcom and Medtronic. She has also reported consulting for Eli Lilly, Lexicon, Medtronic, and Sanofi, and being an advisory board member for Bigfoot Biomedical, Cecelia Health, Eli Lilly, Insulet, T1D Fund, and Vertex Pharmaceuticals. Dr. Evans has reported conducting clinical trials or research collaborations for, serving on advisory boards for, or receiving speakers fees or travel support from Medtronic, Roche, Abbott Diabetes Care, Dexcom, Novo Nordisk, Eli Lilly, Sanofi, Zucara Therapeutics, Pila Pharma, and AstraZeneca. The University of Cambridge has received salary support for Dr. Evans from the National Health Service.
A version of this article first appeared on Medscape.com.
A new consensus statement summarizes the benefits, limitations, and challenges of using automated insulin delivery (AID) systems and provides recommendations for use by people with diabetes.
“Automated insulin delivery systems” is becoming the standard terminology – including by the U.S. Food and Drug Administration – to refer to systems that integrate data from a continuous glucose monitoring (CGM) system via a control algorithm into an insulin pump in order to automate subcutaneous insulin delivery. “Hybrid AID” or “hybrid closed-loop” refers to the current status of these systems, which still require some degree of user input to control glucose levels.
The term “artificial pancreas” was used interchangeably with AID in the past, but it doesn’t take into account exocrine pancreatic function. The term “bionic pancreas” refers to a specific system in development that would ultimately include glucagon along with insulin.
The new consensus report, titled “Automated insulin delivery: Benefits, challenges, and recommendations,” was published online in Diabetes Care and Diabetologia.
The document is geared toward not only diabetologists and other specialists, but also diabetes nurses and specialist dietitians. Colleagues working at regulatory agencies, health care organizations, and related media might also benefit from reading it.
It is endorsed by two professional societies – the European Association for the Study of Diabetes and the American Diabetes Association – and contrasts with other statements about AID systems that are sponsored by their manufacturers, noted document co-author Mark Evans, PhD, professor of diabetic medicine, University of Cambridge, England, in a statement.
“Many clinically relevant aspects, including safety, are addressed in this report. The aim ... is to encourage ongoing improvement of this technology, its safe and effective use, and its accessibility to all who can benefit from it,” Dr. Evans said.
Lead author Jennifer Sherr, MD, PhD, pediatric endocrinology, Yale University, New Haven, Conn., commented that the report “addresses the clinical usage of AID systems from a practical point of view rather than as ... a meta-analysis or a review of all relevant clinical studies. ... As such, the benefits and limitations of systems are discussed while also considering safety, regulatory pathways, and access to this technology.”
AID systems do not mean diabetes is “cured”
Separate recommendations provided at the end of the document are aimed at specific stakeholders, including health care providers, patients and their caregivers, manufacturers, regulatory agencies, and the research community.
The authors make clear in the introduction that, while representing “a significant movement toward optimizing glucose management for individuals with diabetes,” the use of AID systems doesn’t mean that diabetes is “cured.” Rather, “expectations need to be set adequately so that individuals with diabetes and providers understand what such systems can and cannot do.”
In particular, current commercially available AID systems require user input for mealtime insulin dosing and sometimes for correction doses of high blood glucose levels, although the systems at least partially automate that.
“When integrated into care, AID systems hold promise to relieve some of the daily burdens of diabetes care,” the authors write.
The statement also details problems that may arise with the physical devices, including skin irritation from adhesives, occlusion of insulin infusion sets, early CGM sensor failure, and inadequate dosing algorithms.
“Individuals with diabetes who are considering this type of advanced diabetes therapy should not only have appropriate technical understanding of the system but also be able to revert to standard diabetes treatment (that is, nonautomated subcutaneous insulin delivery by pump or injections) in case the AID system fails. They should be able to independently troubleshoot and have access to their health care provider if needed.”
To monitor the impact of the technology, the authors emphasize the importance of the time-in-range metric derived from CGM, with the goal of achieving 70% or greater time in target blood glucose range.
Separate sections of the document address the benefits and limitations of AID systems, education and expectations for both patients and providers, and patient and provider perspectives, including how to handle urgent questions.
Other sections cover special populations such as pregnant women and people with type 2 diabetes, considerations for patient selection for current AID systems, safety, improving access to the technology, liability, and do-it-yourself systems.
Recommendations for health care professionals
A table near the end of the document provides specific recommendations for health care professionals, including the following:
- Be knowledgeable about AID systems and nuances of different systems, including their distinguishing features as well as strengths and weaknesses.
- Inform patients with diabetes about AID systems, including review of currently available systems, and create realistic expectations for device use.
- Involve patients with diabetes in shared decision-making when considering use of AID systems.
- Share information with patients with diabetes, as well as their peers, about general standards set by national and international guidelines on AID systems.
- Provide an on-call number or method by which a person with diabetes can always access support from a health care provider at the practice, including weekends and nights.
- Implement, potentially, protocols on times when AID systems should not be used.
- Use an individual’s health data to improve quality of care and health outcomes.
Most members of the ADA/EASD Diabetes Technology Working Group work with industry, but industry had no input on the project. Dr. Sherr has reported conducting clinical trials for Eli Lilly, Insulet, and Medtronic, and has received in-kind support for research studies from Dexcom and Medtronic. She has also reported consulting for Eli Lilly, Lexicon, Medtronic, and Sanofi, and being an advisory board member for Bigfoot Biomedical, Cecelia Health, Eli Lilly, Insulet, T1D Fund, and Vertex Pharmaceuticals. Dr. Evans has reported conducting clinical trials or research collaborations for, serving on advisory boards for, or receiving speakers fees or travel support from Medtronic, Roche, Abbott Diabetes Care, Dexcom, Novo Nordisk, Eli Lilly, Sanofi, Zucara Therapeutics, Pila Pharma, and AstraZeneca. The University of Cambridge has received salary support for Dr. Evans from the National Health Service.
A version of this article first appeared on Medscape.com.
A new consensus statement summarizes the benefits, limitations, and challenges of using automated insulin delivery (AID) systems and provides recommendations for use by people with diabetes.
“Automated insulin delivery systems” is becoming the standard terminology – including by the U.S. Food and Drug Administration – to refer to systems that integrate data from a continuous glucose monitoring (CGM) system via a control algorithm into an insulin pump in order to automate subcutaneous insulin delivery. “Hybrid AID” or “hybrid closed-loop” refers to the current status of these systems, which still require some degree of user input to control glucose levels.
The term “artificial pancreas” was used interchangeably with AID in the past, but it doesn’t take into account exocrine pancreatic function. The term “bionic pancreas” refers to a specific system in development that would ultimately include glucagon along with insulin.
The new consensus report, titled “Automated insulin delivery: Benefits, challenges, and recommendations,” was published online in Diabetes Care and Diabetologia.
The document is geared toward not only diabetologists and other specialists, but also diabetes nurses and specialist dietitians. Colleagues working at regulatory agencies, health care organizations, and related media might also benefit from reading it.
It is endorsed by two professional societies – the European Association for the Study of Diabetes and the American Diabetes Association – and contrasts with other statements about AID systems that are sponsored by their manufacturers, noted document co-author Mark Evans, PhD, professor of diabetic medicine, University of Cambridge, England, in a statement.
“Many clinically relevant aspects, including safety, are addressed in this report. The aim ... is to encourage ongoing improvement of this technology, its safe and effective use, and its accessibility to all who can benefit from it,” Dr. Evans said.
Lead author Jennifer Sherr, MD, PhD, pediatric endocrinology, Yale University, New Haven, Conn., commented that the report “addresses the clinical usage of AID systems from a practical point of view rather than as ... a meta-analysis or a review of all relevant clinical studies. ... As such, the benefits and limitations of systems are discussed while also considering safety, regulatory pathways, and access to this technology.”
AID systems do not mean diabetes is “cured”
Separate recommendations provided at the end of the document are aimed at specific stakeholders, including health care providers, patients and their caregivers, manufacturers, regulatory agencies, and the research community.
The authors make clear in the introduction that, while representing “a significant movement toward optimizing glucose management for individuals with diabetes,” the use of AID systems doesn’t mean that diabetes is “cured.” Rather, “expectations need to be set adequately so that individuals with diabetes and providers understand what such systems can and cannot do.”
In particular, current commercially available AID systems require user input for mealtime insulin dosing and sometimes for correction doses of high blood glucose levels, although the systems at least partially automate that.
“When integrated into care, AID systems hold promise to relieve some of the daily burdens of diabetes care,” the authors write.
The statement also details problems that may arise with the physical devices, including skin irritation from adhesives, occlusion of insulin infusion sets, early CGM sensor failure, and inadequate dosing algorithms.
“Individuals with diabetes who are considering this type of advanced diabetes therapy should not only have appropriate technical understanding of the system but also be able to revert to standard diabetes treatment (that is, nonautomated subcutaneous insulin delivery by pump or injections) in case the AID system fails. They should be able to independently troubleshoot and have access to their health care provider if needed.”
To monitor the impact of the technology, the authors emphasize the importance of the time-in-range metric derived from CGM, with the goal of achieving 70% or greater time in target blood glucose range.
Separate sections of the document address the benefits and limitations of AID systems, education and expectations for both patients and providers, and patient and provider perspectives, including how to handle urgent questions.
Other sections cover special populations such as pregnant women and people with type 2 diabetes, considerations for patient selection for current AID systems, safety, improving access to the technology, liability, and do-it-yourself systems.
Recommendations for health care professionals
A table near the end of the document provides specific recommendations for health care professionals, including the following:
- Be knowledgeable about AID systems and nuances of different systems, including their distinguishing features as well as strengths and weaknesses.
- Inform patients with diabetes about AID systems, including review of currently available systems, and create realistic expectations for device use.
- Involve patients with diabetes in shared decision-making when considering use of AID systems.
- Share information with patients with diabetes, as well as their peers, about general standards set by national and international guidelines on AID systems.
- Provide an on-call number or method by which a person with diabetes can always access support from a health care provider at the practice, including weekends and nights.
- Implement, potentially, protocols on times when AID systems should not be used.
- Use an individual’s health data to improve quality of care and health outcomes.
Most members of the ADA/EASD Diabetes Technology Working Group work with industry, but industry had no input on the project. Dr. Sherr has reported conducting clinical trials for Eli Lilly, Insulet, and Medtronic, and has received in-kind support for research studies from Dexcom and Medtronic. She has also reported consulting for Eli Lilly, Lexicon, Medtronic, and Sanofi, and being an advisory board member for Bigfoot Biomedical, Cecelia Health, Eli Lilly, Insulet, T1D Fund, and Vertex Pharmaceuticals. Dr. Evans has reported conducting clinical trials or research collaborations for, serving on advisory boards for, or receiving speakers fees or travel support from Medtronic, Roche, Abbott Diabetes Care, Dexcom, Novo Nordisk, Eli Lilly, Sanofi, Zucara Therapeutics, Pila Pharma, and AstraZeneca. The University of Cambridge has received salary support for Dr. Evans from the National Health Service.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE AND DIABETOLOGIA
The truth about the ‘happy hormone’: Why we shouldn’t mess with dopamine
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Like texting and driving: The human cost of AI
A recent medical meeting I attended included multiple sessions on the use of artificial intelligence (AI), a mere preview, I suspect, of what is to come for both patients and physicians.
I vow not to be a contrarian, but I have concerns. If we’d known how cell phones would permeate nearly every waking moment of our lives, would we have built in more protections from the onset?
Although anyone can see the enormous potential of AI in medicine, harnessing the wonders of it without guarding against the dangers could be paramount to texting and driving.
A palpable disruption in the common work-a-day human interaction is a given. CEOs who mind the bottom line will seek every opportunity to cut personnel whenever machine learning can deliver. As our dependence on algorithms increases, our need to understand electrocardiogram interpretation and echocardiographic calculations will wane. Subtle case information will go undetected. Nuanced subconscious alerts regarding the patient condition will go unnoticed.
These realities are never reflected in the pronouncements of companies who promote and develop AI.
The 2-minute echo
In September 2020, Carolyn Lam, MBBS, PhD, and James Hare, MBA, founders of the AI tech company US2.AI, told Healthcare Transformers that AI advances in echocardiology will turn “a manual process of 30 minutes, 250 clicks, with up to 21% variability among fully trained sonographers analyzing the same exam, into an AI-automated process taking 2 minutes, 1 click, with 0% variability.”
Let’s contrast this 2-minute human-machine interaction with the standard 20- to 30-minute human-to-human echocardiography procedure.
Take Mrs. Smith, for instance. She is referred for echocardiography for shortness of breath. She’s shown to a room and instructed to lie down on a table, where she undergoes a brief AI-directed acquisition of images and then a cheery dismissal from the imaging lab. Medical corporate chief financial officers will salivate at the efficiency, the decrease in cost for personnel, and the sharp increase in put-through for the echo lab schedule.
But what if Mrs. Smith gets a standard 30-minute sonographer-directed exam and the astute echocardiographer notes a left ventricular ejection fraction of 38%. A conversation with the patient reveals that she lost her son a few weeks ago. Upon completion of the study, the patient stands up and then adds, “I hope I can sleep in my bed tonight.” Thinking there may be more to the patient’s insomnia than grief-driven anxiety, the sonographer asks her to explain. “I had to sleep in a chair last night because I couldn’t breathe,” Mrs. Smith replies.
The sonographer reasons correctly that Mrs. Smith is likely a few weeks past an acute coronary syndrome for which she didn’t seek attention and is now in heart failure. The consulting cardiologist is alerted. Mrs. Smith is worked into the office schedule a week earlier than planned, and a costly in-patient stay for acute heart failure or worse is avoided.
Here’s a true-life example (some details have been changed to protect the patient’s identity): Mr. Rodriquez was referred for echocardiography because of dizziness. The sonographer notes significant mitral regurgitation and a decline in left ventricular ejection fraction from moderately impaired to severely reduced. When the sonographer inquires about a fresh bruise over Mr. Rodriguez’s left eye, he replies that he “must have fallen, but can’t remember.” The sonographer also notes runs of nonsustained ventricular tachycardia on the echo telemetry, and after a phone call from the echo lab to the ordering physician, Mr. Rodriquez is admitted. Instead of chancing a sudden death at home while awaiting follow-up, he undergoes catheterization and gets an implantable cardioverter defibrillator.
These scenarios illustrate that a 2-minute visit for AI-directed acquisition of echocardiogram images will never garner the protections of a conversation with a human. Any attempts at downplaying the importance of these human interactions are misguided.
Sometimes we embrace the latest advances in medicine while failing to tend to the most rudimentary necessities of data analysis and reporting. Catherine M. Otto, MD, director of the heart valve clinic and a professor of cardiology at the University of Washington Medical Center, Seattle, is a fan of the basics.
At the recent annual congress of the European Society of Cardiology, she commented on the AI-ENHANCED trial, which used an AI decision support algorithm to identify patients with moderate to severe aortic stenosis, which is associated with poor survival if left untreated. She correctly highlighted that while we are discussing the merits of AI-driven assessment of aortic stenosis, we are doing so in an era when many echo interpreters exclude critical information. The vital findings of aortic valve area, Vmax, and ejection fraction are often nowhere to be seen on reports. We should attend to our basic flaws in interpretation and reporting before we shift our focus to AI.
Flawed algorithms
Incorrect AI algorithms that are broadly adopted could negatively affect the health of millions.
Perhaps the most unsettling claim is made by causaLens: “Causal AI is the only technology that can reason and make choices like humans do,” the website states. A tantalizing tag line that is categorically untrue.
Our mysterious and complex neurophysiological function of reasoning still eludes understanding, but one thing is certain: medical reasoning originates with listening, seeing, and touching.
As AI infiltrates mainstream medicine, opportunities for hearing, observing, and palpating will be greatly reduced.
Folkert Asselbergs from University Medical Center Utrecht, the Netherlands, who has cautioned against overhyping AI, was the discussant for an ESC study on the use of causal AI to improve cardiovascular risk estimation.
He flashed a slide of a 2019 Science article on racial bias in an algorithm that U.S. health care systems use. Remedying that bias “would increase the percentage of Black people receiving additional help from 17.7% to 46.5%,” according to the authors.
Successful integration of AI-driven technology will come only if we build human interaction into every patient encounter.
I hope I don’t live to see the rise of the physician cyborg.
Artificial intelligence could be the greatest boon since the invention of the stethoscope, but it will be our downfall if we stop administering a healthy dose of humanity to every patient encounter.
Melissa Walton-Shirley, MD, is a clinical cardiologist in Nashville, Tenn., who has retired from full-time invasive cardiology. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A recent medical meeting I attended included multiple sessions on the use of artificial intelligence (AI), a mere preview, I suspect, of what is to come for both patients and physicians.
I vow not to be a contrarian, but I have concerns. If we’d known how cell phones would permeate nearly every waking moment of our lives, would we have built in more protections from the onset?
Although anyone can see the enormous potential of AI in medicine, harnessing the wonders of it without guarding against the dangers could be paramount to texting and driving.
A palpable disruption in the common work-a-day human interaction is a given. CEOs who mind the bottom line will seek every opportunity to cut personnel whenever machine learning can deliver. As our dependence on algorithms increases, our need to understand electrocardiogram interpretation and echocardiographic calculations will wane. Subtle case information will go undetected. Nuanced subconscious alerts regarding the patient condition will go unnoticed.
These realities are never reflected in the pronouncements of companies who promote and develop AI.
The 2-minute echo
In September 2020, Carolyn Lam, MBBS, PhD, and James Hare, MBA, founders of the AI tech company US2.AI, told Healthcare Transformers that AI advances in echocardiology will turn “a manual process of 30 minutes, 250 clicks, with up to 21% variability among fully trained sonographers analyzing the same exam, into an AI-automated process taking 2 minutes, 1 click, with 0% variability.”
Let’s contrast this 2-minute human-machine interaction with the standard 20- to 30-minute human-to-human echocardiography procedure.
Take Mrs. Smith, for instance. She is referred for echocardiography for shortness of breath. She’s shown to a room and instructed to lie down on a table, where she undergoes a brief AI-directed acquisition of images and then a cheery dismissal from the imaging lab. Medical corporate chief financial officers will salivate at the efficiency, the decrease in cost for personnel, and the sharp increase in put-through for the echo lab schedule.
But what if Mrs. Smith gets a standard 30-minute sonographer-directed exam and the astute echocardiographer notes a left ventricular ejection fraction of 38%. A conversation with the patient reveals that she lost her son a few weeks ago. Upon completion of the study, the patient stands up and then adds, “I hope I can sleep in my bed tonight.” Thinking there may be more to the patient’s insomnia than grief-driven anxiety, the sonographer asks her to explain. “I had to sleep in a chair last night because I couldn’t breathe,” Mrs. Smith replies.
The sonographer reasons correctly that Mrs. Smith is likely a few weeks past an acute coronary syndrome for which she didn’t seek attention and is now in heart failure. The consulting cardiologist is alerted. Mrs. Smith is worked into the office schedule a week earlier than planned, and a costly in-patient stay for acute heart failure or worse is avoided.
Here’s a true-life example (some details have been changed to protect the patient’s identity): Mr. Rodriquez was referred for echocardiography because of dizziness. The sonographer notes significant mitral regurgitation and a decline in left ventricular ejection fraction from moderately impaired to severely reduced. When the sonographer inquires about a fresh bruise over Mr. Rodriguez’s left eye, he replies that he “must have fallen, but can’t remember.” The sonographer also notes runs of nonsustained ventricular tachycardia on the echo telemetry, and after a phone call from the echo lab to the ordering physician, Mr. Rodriquez is admitted. Instead of chancing a sudden death at home while awaiting follow-up, he undergoes catheterization and gets an implantable cardioverter defibrillator.
These scenarios illustrate that a 2-minute visit for AI-directed acquisition of echocardiogram images will never garner the protections of a conversation with a human. Any attempts at downplaying the importance of these human interactions are misguided.
Sometimes we embrace the latest advances in medicine while failing to tend to the most rudimentary necessities of data analysis and reporting. Catherine M. Otto, MD, director of the heart valve clinic and a professor of cardiology at the University of Washington Medical Center, Seattle, is a fan of the basics.
At the recent annual congress of the European Society of Cardiology, she commented on the AI-ENHANCED trial, which used an AI decision support algorithm to identify patients with moderate to severe aortic stenosis, which is associated with poor survival if left untreated. She correctly highlighted that while we are discussing the merits of AI-driven assessment of aortic stenosis, we are doing so in an era when many echo interpreters exclude critical information. The vital findings of aortic valve area, Vmax, and ejection fraction are often nowhere to be seen on reports. We should attend to our basic flaws in interpretation and reporting before we shift our focus to AI.
Flawed algorithms
Incorrect AI algorithms that are broadly adopted could negatively affect the health of millions.
Perhaps the most unsettling claim is made by causaLens: “Causal AI is the only technology that can reason and make choices like humans do,” the website states. A tantalizing tag line that is categorically untrue.
Our mysterious and complex neurophysiological function of reasoning still eludes understanding, but one thing is certain: medical reasoning originates with listening, seeing, and touching.
As AI infiltrates mainstream medicine, opportunities for hearing, observing, and palpating will be greatly reduced.
Folkert Asselbergs from University Medical Center Utrecht, the Netherlands, who has cautioned against overhyping AI, was the discussant for an ESC study on the use of causal AI to improve cardiovascular risk estimation.
He flashed a slide of a 2019 Science article on racial bias in an algorithm that U.S. health care systems use. Remedying that bias “would increase the percentage of Black people receiving additional help from 17.7% to 46.5%,” according to the authors.
Successful integration of AI-driven technology will come only if we build human interaction into every patient encounter.
I hope I don’t live to see the rise of the physician cyborg.
Artificial intelligence could be the greatest boon since the invention of the stethoscope, but it will be our downfall if we stop administering a healthy dose of humanity to every patient encounter.
Melissa Walton-Shirley, MD, is a clinical cardiologist in Nashville, Tenn., who has retired from full-time invasive cardiology. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A recent medical meeting I attended included multiple sessions on the use of artificial intelligence (AI), a mere preview, I suspect, of what is to come for both patients and physicians.
I vow not to be a contrarian, but I have concerns. If we’d known how cell phones would permeate nearly every waking moment of our lives, would we have built in more protections from the onset?
Although anyone can see the enormous potential of AI in medicine, harnessing the wonders of it without guarding against the dangers could be paramount to texting and driving.
A palpable disruption in the common work-a-day human interaction is a given. CEOs who mind the bottom line will seek every opportunity to cut personnel whenever machine learning can deliver. As our dependence on algorithms increases, our need to understand electrocardiogram interpretation and echocardiographic calculations will wane. Subtle case information will go undetected. Nuanced subconscious alerts regarding the patient condition will go unnoticed.
These realities are never reflected in the pronouncements of companies who promote and develop AI.
The 2-minute echo
In September 2020, Carolyn Lam, MBBS, PhD, and James Hare, MBA, founders of the AI tech company US2.AI, told Healthcare Transformers that AI advances in echocardiology will turn “a manual process of 30 minutes, 250 clicks, with up to 21% variability among fully trained sonographers analyzing the same exam, into an AI-automated process taking 2 minutes, 1 click, with 0% variability.”
Let’s contrast this 2-minute human-machine interaction with the standard 20- to 30-minute human-to-human echocardiography procedure.
Take Mrs. Smith, for instance. She is referred for echocardiography for shortness of breath. She’s shown to a room and instructed to lie down on a table, where she undergoes a brief AI-directed acquisition of images and then a cheery dismissal from the imaging lab. Medical corporate chief financial officers will salivate at the efficiency, the decrease in cost for personnel, and the sharp increase in put-through for the echo lab schedule.
But what if Mrs. Smith gets a standard 30-minute sonographer-directed exam and the astute echocardiographer notes a left ventricular ejection fraction of 38%. A conversation with the patient reveals that she lost her son a few weeks ago. Upon completion of the study, the patient stands up and then adds, “I hope I can sleep in my bed tonight.” Thinking there may be more to the patient’s insomnia than grief-driven anxiety, the sonographer asks her to explain. “I had to sleep in a chair last night because I couldn’t breathe,” Mrs. Smith replies.
The sonographer reasons correctly that Mrs. Smith is likely a few weeks past an acute coronary syndrome for which she didn’t seek attention and is now in heart failure. The consulting cardiologist is alerted. Mrs. Smith is worked into the office schedule a week earlier than planned, and a costly in-patient stay for acute heart failure or worse is avoided.
Here’s a true-life example (some details have been changed to protect the patient’s identity): Mr. Rodriquez was referred for echocardiography because of dizziness. The sonographer notes significant mitral regurgitation and a decline in left ventricular ejection fraction from moderately impaired to severely reduced. When the sonographer inquires about a fresh bruise over Mr. Rodriguez’s left eye, he replies that he “must have fallen, but can’t remember.” The sonographer also notes runs of nonsustained ventricular tachycardia on the echo telemetry, and after a phone call from the echo lab to the ordering physician, Mr. Rodriquez is admitted. Instead of chancing a sudden death at home while awaiting follow-up, he undergoes catheterization and gets an implantable cardioverter defibrillator.
These scenarios illustrate that a 2-minute visit for AI-directed acquisition of echocardiogram images will never garner the protections of a conversation with a human. Any attempts at downplaying the importance of these human interactions are misguided.
Sometimes we embrace the latest advances in medicine while failing to tend to the most rudimentary necessities of data analysis and reporting. Catherine M. Otto, MD, director of the heart valve clinic and a professor of cardiology at the University of Washington Medical Center, Seattle, is a fan of the basics.
At the recent annual congress of the European Society of Cardiology, she commented on the AI-ENHANCED trial, which used an AI decision support algorithm to identify patients with moderate to severe aortic stenosis, which is associated with poor survival if left untreated. She correctly highlighted that while we are discussing the merits of AI-driven assessment of aortic stenosis, we are doing so in an era when many echo interpreters exclude critical information. The vital findings of aortic valve area, Vmax, and ejection fraction are often nowhere to be seen on reports. We should attend to our basic flaws in interpretation and reporting before we shift our focus to AI.
Flawed algorithms
Incorrect AI algorithms that are broadly adopted could negatively affect the health of millions.
Perhaps the most unsettling claim is made by causaLens: “Causal AI is the only technology that can reason and make choices like humans do,” the website states. A tantalizing tag line that is categorically untrue.
Our mysterious and complex neurophysiological function of reasoning still eludes understanding, but one thing is certain: medical reasoning originates with listening, seeing, and touching.
As AI infiltrates mainstream medicine, opportunities for hearing, observing, and palpating will be greatly reduced.
Folkert Asselbergs from University Medical Center Utrecht, the Netherlands, who has cautioned against overhyping AI, was the discussant for an ESC study on the use of causal AI to improve cardiovascular risk estimation.
He flashed a slide of a 2019 Science article on racial bias in an algorithm that U.S. health care systems use. Remedying that bias “would increase the percentage of Black people receiving additional help from 17.7% to 46.5%,” according to the authors.
Successful integration of AI-driven technology will come only if we build human interaction into every patient encounter.
I hope I don’t live to see the rise of the physician cyborg.
Artificial intelligence could be the greatest boon since the invention of the stethoscope, but it will be our downfall if we stop administering a healthy dose of humanity to every patient encounter.
Melissa Walton-Shirley, MD, is a clinical cardiologist in Nashville, Tenn., who has retired from full-time invasive cardiology. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Dapagliflozin DELIVERs regardless of systolic pressure in HFpEF
Whatever the mechanism of benefit from dapagliflozin (Farxiga) in patients with heart failure (HF) – and potentially also other sodium-glucose cotransporter 2 (SGLT2) inhibitors – its blood pressure lowering effects aren’t likely to contribute much.
Indeed, at least in patients with HF and non-reduced ejection fractions, dapagliflozin has only a modest BP-lowering effect and cuts cardiovascular (CV) risk regardless of baseline pressure or change in systolic BP, suggests a secondary analysis from the large placebo-controlled DELIVER trial.
Systolic BP fell over 1 month by just under 2 mmHg, on average, in trial patients with either mildly reduced or preserved ejection fraction (HFmrEF or HFpEF, respectively) assigned to take dapagliflozin versus placebo.
The effect was achieved without increasing the risk for adverse events from dapagliflozin, even among patients with the lowest baseline systolic pressures. Adverse outcomes overall, however, were more common at the lowest systolic BP level than at higher pressures, researchers reported.
They say the findings should help alleviate long-standing concerns that initiating SGLT2 inhibitors, with their recognized diuretic effects, might present a hazard in patients with HF and low systolic BP.
“It is a consistent theme in heart failure trials that the blood pressure–lowering effect of SGLT2 inhibitors is more modest than it is in non–heart-failure populations,” Senthil Selvaraj, MD, Duke University, Durham, N.C., told this news organization.
Changes to antihypertensive drug therapy throughout the trial, which presumably enhanced BP responses and “might occur more frequently in the placebo group,” Dr. Selvaraj said, “might explain why the blood pressure effect is a little bit more modest in this population.”
Dr. Selvaraj presented the analysis at the Annual Scientific Meeting of the Heart Failure Society of America, held in National Harbor, Md., and is lead author on its same-day publication in JACC: Heart Failure.
The findings “reinforce the clinical benefits of SGLT2 inhibitors in patients with heart failure across the full spectrum of ejection fractions and large range of systolic blood pressures,” said Gregg C. Fonarow, MD, University of California, Los Angeles Medical Center, who was not part of the DELIVER analysis.
The study’s greater adjusted risks for CV and all-cause mortality risks at the lowest baseline systolic pressures “parallels a series of observational analyses from registries, including OPTIMIZE-HF,” Dr. Fonarow observed.
In those prior studies of patients with established HFpEF, “systolic BP less than 120 mmHg or even 130 mmHg was associated with worse outcomes than those with higher systolic BP.”
The current findings, therefore, “highlight how optimal blood pressure targets in patients with established heart failure have not been well established,” Dr. Fonarow said.
The analysis included all 6,263 participants in DELIVER, outpatients or patients hospitalized for worsening HF who were in NYHA class 2-4 with a left ventricular ejection fraction (LVEF) greater than 40%. They averaged 72 in age, and 44% were women. Their mean baseline systolic BP was 128 mmHg.
After 1 month, mean systolic BP had fallen by 1.8 mmHg (P < .001) in patients who had been randomly assigned to dapagliflozin versus placebo. The effect was consistent (interaction P = .16) across all systolic BP categories (less than 120 mmHg, 120-129 mmHg, 130-139 mmHg, and 140 mmHg or higher).
The effect was similarly independent of estimated glomerular filtration rate (eGFR) and LVEF (interaction P = .30 and P = .33, respectively), Dr. Selvaraj reported.
In an analysis adjusted for both baseline and 1-month change in systolic BP, the effect of dapagliflozin on the primary endpoint was “minimally attenuated,” compared with the primary analysis, he said. That suggests the clinical benefits “did not significantly relate to the blood pressure–lowering effect” of the SGLT2 inhibitor.
In that analysis, the hazard ratio for CV death or worsening HF for dapagliflozin versus placebo was 0.85 (95% confidence interval, 0.75-0.96; P = .010). The HR had been 0.82 (95% CI, 0.73-0.92; P < .001) overall in the DELIVER primary analysis.
The current study doesn’t shed further light on the main SGLT2 inhibitor mechanism of clinical benefit in nondiabetics with HF, which remains a mystery.
“There is a diuretic effect, but it’s not incredibly robust,” Dr. Selvaraj observed. It may contribute to the drugs’ benefits, “but it’s definitely more than that – a lot more than that.”
DELIVER was funded by AstraZeneca. Dr. Selvaraj reported no relevant conflicts. Disclosures for the other authors are in the report. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Whatever the mechanism of benefit from dapagliflozin (Farxiga) in patients with heart failure (HF) – and potentially also other sodium-glucose cotransporter 2 (SGLT2) inhibitors – its blood pressure lowering effects aren’t likely to contribute much.
Indeed, at least in patients with HF and non-reduced ejection fractions, dapagliflozin has only a modest BP-lowering effect and cuts cardiovascular (CV) risk regardless of baseline pressure or change in systolic BP, suggests a secondary analysis from the large placebo-controlled DELIVER trial.
Systolic BP fell over 1 month by just under 2 mmHg, on average, in trial patients with either mildly reduced or preserved ejection fraction (HFmrEF or HFpEF, respectively) assigned to take dapagliflozin versus placebo.
The effect was achieved without increasing the risk for adverse events from dapagliflozin, even among patients with the lowest baseline systolic pressures. Adverse outcomes overall, however, were more common at the lowest systolic BP level than at higher pressures, researchers reported.
They say the findings should help alleviate long-standing concerns that initiating SGLT2 inhibitors, with their recognized diuretic effects, might present a hazard in patients with HF and low systolic BP.
“It is a consistent theme in heart failure trials that the blood pressure–lowering effect of SGLT2 inhibitors is more modest than it is in non–heart-failure populations,” Senthil Selvaraj, MD, Duke University, Durham, N.C., told this news organization.
Changes to antihypertensive drug therapy throughout the trial, which presumably enhanced BP responses and “might occur more frequently in the placebo group,” Dr. Selvaraj said, “might explain why the blood pressure effect is a little bit more modest in this population.”
Dr. Selvaraj presented the analysis at the Annual Scientific Meeting of the Heart Failure Society of America, held in National Harbor, Md., and is lead author on its same-day publication in JACC: Heart Failure.
The findings “reinforce the clinical benefits of SGLT2 inhibitors in patients with heart failure across the full spectrum of ejection fractions and large range of systolic blood pressures,” said Gregg C. Fonarow, MD, University of California, Los Angeles Medical Center, who was not part of the DELIVER analysis.
The study’s greater adjusted risks for CV and all-cause mortality risks at the lowest baseline systolic pressures “parallels a series of observational analyses from registries, including OPTIMIZE-HF,” Dr. Fonarow observed.
In those prior studies of patients with established HFpEF, “systolic BP less than 120 mmHg or even 130 mmHg was associated with worse outcomes than those with higher systolic BP.”
The current findings, therefore, “highlight how optimal blood pressure targets in patients with established heart failure have not been well established,” Dr. Fonarow said.
The analysis included all 6,263 participants in DELIVER, outpatients or patients hospitalized for worsening HF who were in NYHA class 2-4 with a left ventricular ejection fraction (LVEF) greater than 40%. They averaged 72 in age, and 44% were women. Their mean baseline systolic BP was 128 mmHg.
After 1 month, mean systolic BP had fallen by 1.8 mmHg (P < .001) in patients who had been randomly assigned to dapagliflozin versus placebo. The effect was consistent (interaction P = .16) across all systolic BP categories (less than 120 mmHg, 120-129 mmHg, 130-139 mmHg, and 140 mmHg or higher).
The effect was similarly independent of estimated glomerular filtration rate (eGFR) and LVEF (interaction P = .30 and P = .33, respectively), Dr. Selvaraj reported.
In an analysis adjusted for both baseline and 1-month change in systolic BP, the effect of dapagliflozin on the primary endpoint was “minimally attenuated,” compared with the primary analysis, he said. That suggests the clinical benefits “did not significantly relate to the blood pressure–lowering effect” of the SGLT2 inhibitor.
In that analysis, the hazard ratio for CV death or worsening HF for dapagliflozin versus placebo was 0.85 (95% confidence interval, 0.75-0.96; P = .010). The HR had been 0.82 (95% CI, 0.73-0.92; P < .001) overall in the DELIVER primary analysis.
The current study doesn’t shed further light on the main SGLT2 inhibitor mechanism of clinical benefit in nondiabetics with HF, which remains a mystery.
“There is a diuretic effect, but it’s not incredibly robust,” Dr. Selvaraj observed. It may contribute to the drugs’ benefits, “but it’s definitely more than that – a lot more than that.”
DELIVER was funded by AstraZeneca. Dr. Selvaraj reported no relevant conflicts. Disclosures for the other authors are in the report. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Whatever the mechanism of benefit from dapagliflozin (Farxiga) in patients with heart failure (HF) – and potentially also other sodium-glucose cotransporter 2 (SGLT2) inhibitors – its blood pressure lowering effects aren’t likely to contribute much.
Indeed, at least in patients with HF and non-reduced ejection fractions, dapagliflozin has only a modest BP-lowering effect and cuts cardiovascular (CV) risk regardless of baseline pressure or change in systolic BP, suggests a secondary analysis from the large placebo-controlled DELIVER trial.
Systolic BP fell over 1 month by just under 2 mmHg, on average, in trial patients with either mildly reduced or preserved ejection fraction (HFmrEF or HFpEF, respectively) assigned to take dapagliflozin versus placebo.
The effect was achieved without increasing the risk for adverse events from dapagliflozin, even among patients with the lowest baseline systolic pressures. Adverse outcomes overall, however, were more common at the lowest systolic BP level than at higher pressures, researchers reported.
They say the findings should help alleviate long-standing concerns that initiating SGLT2 inhibitors, with their recognized diuretic effects, might present a hazard in patients with HF and low systolic BP.
“It is a consistent theme in heart failure trials that the blood pressure–lowering effect of SGLT2 inhibitors is more modest than it is in non–heart-failure populations,” Senthil Selvaraj, MD, Duke University, Durham, N.C., told this news organization.
Changes to antihypertensive drug therapy throughout the trial, which presumably enhanced BP responses and “might occur more frequently in the placebo group,” Dr. Selvaraj said, “might explain why the blood pressure effect is a little bit more modest in this population.”
Dr. Selvaraj presented the analysis at the Annual Scientific Meeting of the Heart Failure Society of America, held in National Harbor, Md., and is lead author on its same-day publication in JACC: Heart Failure.
The findings “reinforce the clinical benefits of SGLT2 inhibitors in patients with heart failure across the full spectrum of ejection fractions and large range of systolic blood pressures,” said Gregg C. Fonarow, MD, University of California, Los Angeles Medical Center, who was not part of the DELIVER analysis.
The study’s greater adjusted risks for CV and all-cause mortality risks at the lowest baseline systolic pressures “parallels a series of observational analyses from registries, including OPTIMIZE-HF,” Dr. Fonarow observed.
In those prior studies of patients with established HFpEF, “systolic BP less than 120 mmHg or even 130 mmHg was associated with worse outcomes than those with higher systolic BP.”
The current findings, therefore, “highlight how optimal blood pressure targets in patients with established heart failure have not been well established,” Dr. Fonarow said.
The analysis included all 6,263 participants in DELIVER, outpatients or patients hospitalized for worsening HF who were in NYHA class 2-4 with a left ventricular ejection fraction (LVEF) greater than 40%. They averaged 72 in age, and 44% were women. Their mean baseline systolic BP was 128 mmHg.
After 1 month, mean systolic BP had fallen by 1.8 mmHg (P < .001) in patients who had been randomly assigned to dapagliflozin versus placebo. The effect was consistent (interaction P = .16) across all systolic BP categories (less than 120 mmHg, 120-129 mmHg, 130-139 mmHg, and 140 mmHg or higher).
The effect was similarly independent of estimated glomerular filtration rate (eGFR) and LVEF (interaction P = .30 and P = .33, respectively), Dr. Selvaraj reported.
In an analysis adjusted for both baseline and 1-month change in systolic BP, the effect of dapagliflozin on the primary endpoint was “minimally attenuated,” compared with the primary analysis, he said. That suggests the clinical benefits “did not significantly relate to the blood pressure–lowering effect” of the SGLT2 inhibitor.
In that analysis, the hazard ratio for CV death or worsening HF for dapagliflozin versus placebo was 0.85 (95% confidence interval, 0.75-0.96; P = .010). The HR had been 0.82 (95% CI, 0.73-0.92; P < .001) overall in the DELIVER primary analysis.
The current study doesn’t shed further light on the main SGLT2 inhibitor mechanism of clinical benefit in nondiabetics with HF, which remains a mystery.
“There is a diuretic effect, but it’s not incredibly robust,” Dr. Selvaraj observed. It may contribute to the drugs’ benefits, “but it’s definitely more than that – a lot more than that.”
DELIVER was funded by AstraZeneca. Dr. Selvaraj reported no relevant conflicts. Disclosures for the other authors are in the report. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Bariatric surgery prompts visceral fat reduction, cardiac changes
Weight loss after bariatric surgery was linked with visceral fat reduction as well as reduced blood pressure, fasting glucose, and left ventricular remodeling, based an imaging study in 213 patients.
“We found that ventricular function measured by strain imaging improved in both the left and right sides of the heart, but function measured in the traditional method using endocardial motion [in other words, ejection fraction] actually worsened,” senior investigator Barry A. Borlaug, MD, said in an interview.
Although previous studies have shown positive effects of weight loss on the heart after bariatric surgery, most have been short term and have not specifically examined the effects of visceral fat reduction, wrote the investigators.
“We are in the middle of an increasing epidemic of obesity worldwide, but particularly in the United States, where it is currently projected that one in two adults will be obese by 2030,” added Dr. Borlaug of Mayo Clinic, Rochester, Minn. “Heart failure with preserved ejection fraction (HFpEF) is growing in tandem, and numerous recent studies have shown that obesity is one of the strongest risk factors for developing HFpEF, and that the severity of HFpEF is intimately linked to excess body fat. This suggests that therapies to reduce body fat could improve the cardiac abnormalities that cause HFpEF, which was our focus in this study,” he explained.
In the study, published in the Journal of the American College of Cardiology, the researchers reviewed echocardiography data from 213 obese patients before and more than 180 days after bariatric surgery. They also measured abdominal visceral adipose tissue (VAT) of 52 patients via computed tomography. The average age of the patients was 54 years, the average body mass index was 45 kg/m2, and 67% were women. Comorbidities included hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.
The primary outcome was changes in cardiac structure and function.
After a median follow-up of 5.3 years, patients overall averaged a 23% reduction in body weight and a 22% reduction in BMI. In the 52 patients with abdominal scans, the VAT area decreased by 30% overall. Changes in left ventricular mass were significantly correlated to changes in the VAT.
Epicardial adipose thickness decreased by 14% overall. Left and right ventricular longitudinal strains improved at follow-up, but left atrial strain deteriorated, the researchers noted.
Although the mechanism of action remains unclear, the results suggest that left ventricular remodeling was associated with visceral adiposity rather than subcutaneous fat, the researchers wrote.
They also found that right ventricular strain was negatively correlated with VAT, but not with body weight or BMI.
“These findings suggest that weight loss, particularly reduction in visceral adiposity, benefits [right ventricular] structure and function in a manner akin to that observed in the [left ventricle],” the researchers noted.
Some surprises and limitations
Dr. Borlaug said he found some, but not all, of the results surprising. “Earlier studies had shown evidence for benefit from weight loss on cardiac structure and function, but had been limited by smaller sample sizes, shorter durations of evaluation, and variable methods used,” he said in an interview.
The findings that strain imaging showed both left and right ventricular function improved while EF declined “shows some of the problems with using EF, as it is affected by chamber size and geometry. We have previously shown that patients with HFpEF display an increase in fat around the heart, and this affects cardiac function and interaction between the left and right sides of the heart, so we expected to see that this fat depot would be reduced, and this was indeed the case,” Dr. Borlaug added.
In the current study, “visceral fat was most strongly tied to the heart remodeling in obesity, and changes in visceral fat were most strongly tied to improvements in cardiac structure following weight loss,” Dr. Borlaug told this news organization. “This further supports this concept that excess visceral fat plays a key role in HFpEF, especially in the abdomen and around the heart,” he said.
However, “The biggest surprise was the discordant effects in the left atrium,” Dr. Borlaug said. “Left atrial remodeling and dysfunction play a crucial role in HFpEF as well, and we expected that this would improve following weight loss, but in fact we observed that left atrial function deteriorated, and other indicators of atrial myopathy worsened, including higher estimates of left atrial pressures and increased prevalence of atrial fibrillation,” he said.
This difference emphasizes that weight loss may not address all abnormalities that lead to HFpEF, although a key limitation of the current study was the lack of a control group of patients with the same degree of obesity and no weight-loss intervention, and the deterioration in left atrial function might have been even greater in the absence of weight loss, Dr. Borlaug added.
Larger numbers support effects
Previous research shows that structural heart changes associated with obesity can be reversed through weight loss, but the current study fills a gap by providing long-term data in a larger sample than previously studied, wrote Paul Heidenreich, MD, of Stanford (Calif.) University in an accompanying editorial).
“There has been uncertainty regarding the prolonged effect of weight loss on cardiac function; this study was larger than many prior studies and provided a longer follow-up,” Dr. Heidenreich said in an interview.
“One unusual finding was that, while weight loss led to left ventricle reverse remodeling (reduction in wall thickness), the same effect was not seen for the left atrium; the left atrial size continued to increase,” he said. “I would have expected the left atrial changes to mirror the changes in the left ventricle,” he noted.
The findings support the greater cardiac risk of visceral vs. subcutaneous adipose tissue, and although body mass index will retain prognostic value, measures of central obesity are more likely predictors of cardiac structural changes and events and should be reported in clinical studies, Dr. Heidenreich wrote.
However, “We need a better understanding of the factors that influence left atrial remodeling and reverse remodeling,” Dr. Heidenreich told this news organization. “While left ventricular compliance and pressure play a role, there are other factors that need to be elucidated,” he said.
Studies in progress may inform practice
The current data call for further study to test novel treatments to facilitate weight loss in patients with HFpEF and those at risk for HFpEF, and some of these studies with medicines are underway, Dr. Borlaug said in the interview.
“Until such studies are completed, we will not truly understand the effects of weight loss on the heart, but the present data certainly provide strong support that patients who have obesity and HFpEF or are at risk for HFpEF should try to lose weight through lifestyle interventions,” he said.
Whether the cardiac changes seen in the current study would be different with nonsurgical weight loss remains a key question because many obese patients are reluctant to undergo bariatric surgery, Dr. Borlaug said. “We cannot assess whether the effects would differ with nonsurgical weight loss, and this requires further study,” he added.
As for additional research, “Randomized, controlled trials of weight-loss interventions, with appropriate controls and comprehensive assessments of cardiac structure, function, and hemodynamics will be most informative,” said Dr. Borlaug. “Larger trials powered to evaluate cardiovascular outcomes such as heart failure hospitalization or cardiovascular death also are critically important to better understand the role of weight loss to treat and prevent HFpEF, the ultimate form of obesity-related heart disease,” he emphasized.
The study was supported in part by grants to lead author Dr. Hidemi Sorimachi of the Mayo Clinic from the Uehara Memorial Foundation, Japan, and to corresponding author Dr. Borlaug from the National Institutes of Health. Dr. Borlaug also disclosed previous grants from National Institutes of Health/National Heart, Lung, and Blood Institute, AstraZeneca, Corvia, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, and Tenax Therapeutics; and consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. Dr. Heidenreich had no financial disclosures.
Weight loss after bariatric surgery was linked with visceral fat reduction as well as reduced blood pressure, fasting glucose, and left ventricular remodeling, based an imaging study in 213 patients.
“We found that ventricular function measured by strain imaging improved in both the left and right sides of the heart, but function measured in the traditional method using endocardial motion [in other words, ejection fraction] actually worsened,” senior investigator Barry A. Borlaug, MD, said in an interview.
Although previous studies have shown positive effects of weight loss on the heart after bariatric surgery, most have been short term and have not specifically examined the effects of visceral fat reduction, wrote the investigators.
“We are in the middle of an increasing epidemic of obesity worldwide, but particularly in the United States, where it is currently projected that one in two adults will be obese by 2030,” added Dr. Borlaug of Mayo Clinic, Rochester, Minn. “Heart failure with preserved ejection fraction (HFpEF) is growing in tandem, and numerous recent studies have shown that obesity is one of the strongest risk factors for developing HFpEF, and that the severity of HFpEF is intimately linked to excess body fat. This suggests that therapies to reduce body fat could improve the cardiac abnormalities that cause HFpEF, which was our focus in this study,” he explained.
In the study, published in the Journal of the American College of Cardiology, the researchers reviewed echocardiography data from 213 obese patients before and more than 180 days after bariatric surgery. They also measured abdominal visceral adipose tissue (VAT) of 52 patients via computed tomography. The average age of the patients was 54 years, the average body mass index was 45 kg/m2, and 67% were women. Comorbidities included hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.
The primary outcome was changes in cardiac structure and function.
After a median follow-up of 5.3 years, patients overall averaged a 23% reduction in body weight and a 22% reduction in BMI. In the 52 patients with abdominal scans, the VAT area decreased by 30% overall. Changes in left ventricular mass were significantly correlated to changes in the VAT.
Epicardial adipose thickness decreased by 14% overall. Left and right ventricular longitudinal strains improved at follow-up, but left atrial strain deteriorated, the researchers noted.
Although the mechanism of action remains unclear, the results suggest that left ventricular remodeling was associated with visceral adiposity rather than subcutaneous fat, the researchers wrote.
They also found that right ventricular strain was negatively correlated with VAT, but not with body weight or BMI.
“These findings suggest that weight loss, particularly reduction in visceral adiposity, benefits [right ventricular] structure and function in a manner akin to that observed in the [left ventricle],” the researchers noted.
Some surprises and limitations
Dr. Borlaug said he found some, but not all, of the results surprising. “Earlier studies had shown evidence for benefit from weight loss on cardiac structure and function, but had been limited by smaller sample sizes, shorter durations of evaluation, and variable methods used,” he said in an interview.
The findings that strain imaging showed both left and right ventricular function improved while EF declined “shows some of the problems with using EF, as it is affected by chamber size and geometry. We have previously shown that patients with HFpEF display an increase in fat around the heart, and this affects cardiac function and interaction between the left and right sides of the heart, so we expected to see that this fat depot would be reduced, and this was indeed the case,” Dr. Borlaug added.
In the current study, “visceral fat was most strongly tied to the heart remodeling in obesity, and changes in visceral fat were most strongly tied to improvements in cardiac structure following weight loss,” Dr. Borlaug told this news organization. “This further supports this concept that excess visceral fat plays a key role in HFpEF, especially in the abdomen and around the heart,” he said.
However, “The biggest surprise was the discordant effects in the left atrium,” Dr. Borlaug said. “Left atrial remodeling and dysfunction play a crucial role in HFpEF as well, and we expected that this would improve following weight loss, but in fact we observed that left atrial function deteriorated, and other indicators of atrial myopathy worsened, including higher estimates of left atrial pressures and increased prevalence of atrial fibrillation,” he said.
This difference emphasizes that weight loss may not address all abnormalities that lead to HFpEF, although a key limitation of the current study was the lack of a control group of patients with the same degree of obesity and no weight-loss intervention, and the deterioration in left atrial function might have been even greater in the absence of weight loss, Dr. Borlaug added.
Larger numbers support effects
Previous research shows that structural heart changes associated with obesity can be reversed through weight loss, but the current study fills a gap by providing long-term data in a larger sample than previously studied, wrote Paul Heidenreich, MD, of Stanford (Calif.) University in an accompanying editorial).
“There has been uncertainty regarding the prolonged effect of weight loss on cardiac function; this study was larger than many prior studies and provided a longer follow-up,” Dr. Heidenreich said in an interview.
“One unusual finding was that, while weight loss led to left ventricle reverse remodeling (reduction in wall thickness), the same effect was not seen for the left atrium; the left atrial size continued to increase,” he said. “I would have expected the left atrial changes to mirror the changes in the left ventricle,” he noted.
The findings support the greater cardiac risk of visceral vs. subcutaneous adipose tissue, and although body mass index will retain prognostic value, measures of central obesity are more likely predictors of cardiac structural changes and events and should be reported in clinical studies, Dr. Heidenreich wrote.
However, “We need a better understanding of the factors that influence left atrial remodeling and reverse remodeling,” Dr. Heidenreich told this news organization. “While left ventricular compliance and pressure play a role, there are other factors that need to be elucidated,” he said.
Studies in progress may inform practice
The current data call for further study to test novel treatments to facilitate weight loss in patients with HFpEF and those at risk for HFpEF, and some of these studies with medicines are underway, Dr. Borlaug said in the interview.
“Until such studies are completed, we will not truly understand the effects of weight loss on the heart, but the present data certainly provide strong support that patients who have obesity and HFpEF or are at risk for HFpEF should try to lose weight through lifestyle interventions,” he said.
Whether the cardiac changes seen in the current study would be different with nonsurgical weight loss remains a key question because many obese patients are reluctant to undergo bariatric surgery, Dr. Borlaug said. “We cannot assess whether the effects would differ with nonsurgical weight loss, and this requires further study,” he added.
As for additional research, “Randomized, controlled trials of weight-loss interventions, with appropriate controls and comprehensive assessments of cardiac structure, function, and hemodynamics will be most informative,” said Dr. Borlaug. “Larger trials powered to evaluate cardiovascular outcomes such as heart failure hospitalization or cardiovascular death also are critically important to better understand the role of weight loss to treat and prevent HFpEF, the ultimate form of obesity-related heart disease,” he emphasized.
The study was supported in part by grants to lead author Dr. Hidemi Sorimachi of the Mayo Clinic from the Uehara Memorial Foundation, Japan, and to corresponding author Dr. Borlaug from the National Institutes of Health. Dr. Borlaug also disclosed previous grants from National Institutes of Health/National Heart, Lung, and Blood Institute, AstraZeneca, Corvia, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, and Tenax Therapeutics; and consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. Dr. Heidenreich had no financial disclosures.
Weight loss after bariatric surgery was linked with visceral fat reduction as well as reduced blood pressure, fasting glucose, and left ventricular remodeling, based an imaging study in 213 patients.
“We found that ventricular function measured by strain imaging improved in both the left and right sides of the heart, but function measured in the traditional method using endocardial motion [in other words, ejection fraction] actually worsened,” senior investigator Barry A. Borlaug, MD, said in an interview.
Although previous studies have shown positive effects of weight loss on the heart after bariatric surgery, most have been short term and have not specifically examined the effects of visceral fat reduction, wrote the investigators.
“We are in the middle of an increasing epidemic of obesity worldwide, but particularly in the United States, where it is currently projected that one in two adults will be obese by 2030,” added Dr. Borlaug of Mayo Clinic, Rochester, Minn. “Heart failure with preserved ejection fraction (HFpEF) is growing in tandem, and numerous recent studies have shown that obesity is one of the strongest risk factors for developing HFpEF, and that the severity of HFpEF is intimately linked to excess body fat. This suggests that therapies to reduce body fat could improve the cardiac abnormalities that cause HFpEF, which was our focus in this study,” he explained.
In the study, published in the Journal of the American College of Cardiology, the researchers reviewed echocardiography data from 213 obese patients before and more than 180 days after bariatric surgery. They also measured abdominal visceral adipose tissue (VAT) of 52 patients via computed tomography. The average age of the patients was 54 years, the average body mass index was 45 kg/m2, and 67% were women. Comorbidities included hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.
The primary outcome was changes in cardiac structure and function.
After a median follow-up of 5.3 years, patients overall averaged a 23% reduction in body weight and a 22% reduction in BMI. In the 52 patients with abdominal scans, the VAT area decreased by 30% overall. Changes in left ventricular mass were significantly correlated to changes in the VAT.
Epicardial adipose thickness decreased by 14% overall. Left and right ventricular longitudinal strains improved at follow-up, but left atrial strain deteriorated, the researchers noted.
Although the mechanism of action remains unclear, the results suggest that left ventricular remodeling was associated with visceral adiposity rather than subcutaneous fat, the researchers wrote.
They also found that right ventricular strain was negatively correlated with VAT, but not with body weight or BMI.
“These findings suggest that weight loss, particularly reduction in visceral adiposity, benefits [right ventricular] structure and function in a manner akin to that observed in the [left ventricle],” the researchers noted.
Some surprises and limitations
Dr. Borlaug said he found some, but not all, of the results surprising. “Earlier studies had shown evidence for benefit from weight loss on cardiac structure and function, but had been limited by smaller sample sizes, shorter durations of evaluation, and variable methods used,” he said in an interview.
The findings that strain imaging showed both left and right ventricular function improved while EF declined “shows some of the problems with using EF, as it is affected by chamber size and geometry. We have previously shown that patients with HFpEF display an increase in fat around the heart, and this affects cardiac function and interaction between the left and right sides of the heart, so we expected to see that this fat depot would be reduced, and this was indeed the case,” Dr. Borlaug added.
In the current study, “visceral fat was most strongly tied to the heart remodeling in obesity, and changes in visceral fat were most strongly tied to improvements in cardiac structure following weight loss,” Dr. Borlaug told this news organization. “This further supports this concept that excess visceral fat plays a key role in HFpEF, especially in the abdomen and around the heart,” he said.
However, “The biggest surprise was the discordant effects in the left atrium,” Dr. Borlaug said. “Left atrial remodeling and dysfunction play a crucial role in HFpEF as well, and we expected that this would improve following weight loss, but in fact we observed that left atrial function deteriorated, and other indicators of atrial myopathy worsened, including higher estimates of left atrial pressures and increased prevalence of atrial fibrillation,” he said.
This difference emphasizes that weight loss may not address all abnormalities that lead to HFpEF, although a key limitation of the current study was the lack of a control group of patients with the same degree of obesity and no weight-loss intervention, and the deterioration in left atrial function might have been even greater in the absence of weight loss, Dr. Borlaug added.
Larger numbers support effects
Previous research shows that structural heart changes associated with obesity can be reversed through weight loss, but the current study fills a gap by providing long-term data in a larger sample than previously studied, wrote Paul Heidenreich, MD, of Stanford (Calif.) University in an accompanying editorial).
“There has been uncertainty regarding the prolonged effect of weight loss on cardiac function; this study was larger than many prior studies and provided a longer follow-up,” Dr. Heidenreich said in an interview.
“One unusual finding was that, while weight loss led to left ventricle reverse remodeling (reduction in wall thickness), the same effect was not seen for the left atrium; the left atrial size continued to increase,” he said. “I would have expected the left atrial changes to mirror the changes in the left ventricle,” he noted.
The findings support the greater cardiac risk of visceral vs. subcutaneous adipose tissue, and although body mass index will retain prognostic value, measures of central obesity are more likely predictors of cardiac structural changes and events and should be reported in clinical studies, Dr. Heidenreich wrote.
However, “We need a better understanding of the factors that influence left atrial remodeling and reverse remodeling,” Dr. Heidenreich told this news organization. “While left ventricular compliance and pressure play a role, there are other factors that need to be elucidated,” he said.
Studies in progress may inform practice
The current data call for further study to test novel treatments to facilitate weight loss in patients with HFpEF and those at risk for HFpEF, and some of these studies with medicines are underway, Dr. Borlaug said in the interview.
“Until such studies are completed, we will not truly understand the effects of weight loss on the heart, but the present data certainly provide strong support that patients who have obesity and HFpEF or are at risk for HFpEF should try to lose weight through lifestyle interventions,” he said.
Whether the cardiac changes seen in the current study would be different with nonsurgical weight loss remains a key question because many obese patients are reluctant to undergo bariatric surgery, Dr. Borlaug said. “We cannot assess whether the effects would differ with nonsurgical weight loss, and this requires further study,” he added.
As for additional research, “Randomized, controlled trials of weight-loss interventions, with appropriate controls and comprehensive assessments of cardiac structure, function, and hemodynamics will be most informative,” said Dr. Borlaug. “Larger trials powered to evaluate cardiovascular outcomes such as heart failure hospitalization or cardiovascular death also are critically important to better understand the role of weight loss to treat and prevent HFpEF, the ultimate form of obesity-related heart disease,” he emphasized.
The study was supported in part by grants to lead author Dr. Hidemi Sorimachi of the Mayo Clinic from the Uehara Memorial Foundation, Japan, and to corresponding author Dr. Borlaug from the National Institutes of Health. Dr. Borlaug also disclosed previous grants from National Institutes of Health/National Heart, Lung, and Blood Institute, AstraZeneca, Corvia, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, and Tenax Therapeutics; and consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. Dr. Heidenreich had no financial disclosures.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY