User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Aspirin primary prevention benefit in those with raised Lp(a)?
Aspirin may be of specific benefit for the primary prevention of cardiovascular disease in individuals with raised Lp(a) levels, a new study has suggested.
The study analyzed data from the ASPREE (ASPirin in Reducing Events in the Elderly) trial, which randomized 19,000 individuals aged 70 years or older without a history of cardiovascular disease to aspirin (100 mg/day) or placebo. While the main results, reported previously, showed no net benefit of aspirin in the overall population, the current analysis suggests there may be a benefit in individuals with raised Lp(a) levels.
The current analysis was published online in the Journal of the American College of Cardiology.
“Our study provides evidence that aspirin may specifically benefit older individuals with genotypes for elevated plasma Lp(a) in the setting of high-risk primary prevention of cardiovascular events and that overall benefit may outweigh harm related to major bleeding,” the authors, led by Paul Lacaze, PhD, Monash University, Melbourne, conclude.
They also point out that similar observations have been previously seen in another large aspirin primary prevention study conducted in younger women, the Women’s Health Study, and the current analysis provides validation of those findings.
“Our results provide new evidence to support the potential use of aspirin to target individuals with elevated Lp(a) for the primary prevention of cardiovascular events,” the researchers say.
They acknowledge that these results would be strengthened by the use of directly measured plasma Lp(a) levels, in addition to Lp(a) genotypes.
But they add: “Nonetheless, given the lack of any currently approved therapies for targeting elevated Lp(a), our findings may have widespread clinical implications, adding evidence to the rationale that aspirin may be a viable option for reducing Lp(a)-mediated cardiovascular risk.”
Dr. Lacaze and colleagues explain that elevated plasma Lp(a) levels confer up to fourfold increased risk of cardiovascular disease, with around 20%-30% of the general population affected. Despite the high burden and prevalence of elevated plasma Lp(a), there are currently no approved pharmacologic therapies targeting this lipoprotein. Although promising candidates are in development for the secondary prevention of Lp(a)-mediated cardiovascular disease, it will be many years before these candidates are assessed for primary prevention.
For the current study, researchers analyzed data from 12,815 ASPREE participants who had undergone genotyping and compared outcomes with aspirin versus placebo in those with and without genotypes associated with elevated Lp(a) levels.
Results showed that individuals with elevated Lp(a)-associated genotypes, defined in two different ways, showed a reduction in ischemic events with aspirin versus placebo, and this benefit was not outweighed by an increased bleeding risk.
Specifically, in the placebo group, individuals who carried the rs3798220-C allele, which is known to be associated with raised Lp(a) levels, making up 3.2% of the genotyped population in the study, had an almost twofold increased risk of major adverse cardiovascular events than those not carrying this genotype. However, the risk was attenuated in the aspirin group, with carriers of the rs3798220-C allele actually having a lower rate of cardiovascular events than noncarriers.
In addition, rs3798220-C carrier status was not significantly associated with increased risk of clinically significant bleeding events in the aspirin group.
Similar results were seen with the second way of identifying patients with a high risk of elevated Lp(a) levels using a 43-variant genetic risk score (LPA-GRS).
In the whole study population, aspirin reduced major adverse cardiovascular events by 1.7 events per 1,000 person-years and increased clinically significant bleeding events by 1.7 events per 1,000 person-years, suggesting parity between overall benefit versus harm.
However, in the rs3798220-C subgroup, aspirin reduced major adverse cardiovascular events by 11.4 events per 1,000 person-years (a more than sixfold higher magnitude of cardiovascular disease risk reduction than in the overall cohort), with a bleeding risk of 3.3 events per 1,000 person-years, the researchers report.
“Hence in rs3798220-C carriers, aspirin appeared to have a net benefit of 8.1 events per 1,000 person-years,” they state.
In the highest LPA-GRS quintile, aspirin reduced major adverse cardiovascular events by 3.3 events per 1,000 person-years (approximately twofold higher magnitude of risk reduction, compared with the overall cohort), with an increase in bleeding risk of 1.6 events per 1,000 person-years (almost identical bleeding risk to the overall cohort). This shifted the benefit versus harm balance in the highest LPA-GRS quintile to a net benefit of 1.7 events per 1,000 person-years.
Similar findings in the Women’s Health Study
Dr. Lacaze and colleagues point out that similar results have also been seen in another large aspirin primary prevention study – the Women’s Health Study (WHS).
The WHS compared aspirin 100 mg every other day with placebo in initially healthy younger women. Previously reported results showed that women carrying the rs3798220-C variant, associated with highly elevated Lp(a) levels, had a twofold higher risk of cardiovascular events than noncarrier women in the placebo group, but this risk was reduced in the aspirin group. And there was no increased risk of bleeding in women with elevated Lp(a).
“These results, in the absence of any other randomized controlled trial evidence or approved therapy for treating Lp(a)-associated risk, have been used by some physicians as justification for prescribing aspirin in patients with elevated Lp(a),” Dr. Lacaze and colleagues note.
“In the present study of the ASPREE trial population, our results were consistent with the WHS analysis, despite randomizing older individuals (both men and women),” they add.
They say this validation of the WHS result provides evidence that a very high-risk subgroup of individuals with highly elevated Lp(a) – those carrying the rs3798220-C allele – may benefit from low-dose aspirin for the primary prevention of cardiovascular events. Further, the benefits in this subgroup specifically may outweigh any bleeding risk.
But they point out that rs3798220-C carriers comprise only a small portion of all individuals with elevated Lp(a) in the general population, while the polygenic LPA-GRS explains about 60% of the variation in directly measured plasma Lp(a) levels and has the potential advantage of being able to identify a larger group of individuals at increased risk.
The researchers note, however, that it is not clear to what extent the LPA-GRS results add further evidence to suggest that individuals with elevated Lp(a), beyond rs3798220-C carriers, may be more likely to benefit from aspirin.
“If the benefit of aspirin extends beyond very high-risk rs3798220-C carriers alone, to the broader 20%-30% of individuals with elevated Lp(a), the potential utility of aspirin for the primary prevention of cardiovascular events would increase substantially,” they say.
‘Very high clinical relevance’
In an accompanying editorial, Ana Devesa, MD, Borja Ibanez, MD, PhD, and Valentin Fuster, MD, PhD, The National Center for Cardiovascular Research, Madrid, say that: “[Dr.] Lacaze et al. are to be congratulated for a study of very high clinical relevance that represents a first indication for primary prevention for patients at high cardiovascular risk.”
They explain that the pathogenic mechanism of Lp(a) is believed to be a combination of prothrombotic and proatherogenic effects, and the current findings support the hypothesis that the prothrombotic mechanism of Lp(a) is mediated by platelet aggregation.
This would explain the occurrence of thrombotic events in the presence of atherosclerosis in that elevated Lp(a) levels may induce platelet adhesion and aggregation to the activated atherosclerotic plaque, thus enhancing the atherothrombotic process. Moreover, activated platelets release several mediators that result in cell adhesion and attraction of chemokines and proinflammatory cytokines, driving an inflammatory response and mediating atherosclerosis progression, they add.
The editorialists highlight the limitations of the study already acknowledged by the authors: The analysis used genotypes rather than elevated Lp(a) levels and included only those of European ancestry, meaning the results are difficult to extrapolate to other populations.
“The next steps in clinical practice should be defined, and there are still questions to be answered,” they conclude. “Will every patient benefit from antithrombotic therapies? Should all patients who have elevated Lp(a) levels be treated with aspirin?”
The ASPREE Biobank is supported by grants from the Commonwealth Scientific and Industrial Research Organisation, Monash University, Menzies Research Institute, Australian National University, University of Melbourne, National Institutes of Health, National Health and Medical Research Council of Australia, and the Victorian Cancer Agency. Dr. Lacaze is supported by a National Heart Foundation Future Leader Fellowship.
A version of this article first appeared on Medscape.com.
Aspirin may be of specific benefit for the primary prevention of cardiovascular disease in individuals with raised Lp(a) levels, a new study has suggested.
The study analyzed data from the ASPREE (ASPirin in Reducing Events in the Elderly) trial, which randomized 19,000 individuals aged 70 years or older without a history of cardiovascular disease to aspirin (100 mg/day) or placebo. While the main results, reported previously, showed no net benefit of aspirin in the overall population, the current analysis suggests there may be a benefit in individuals with raised Lp(a) levels.
The current analysis was published online in the Journal of the American College of Cardiology.
“Our study provides evidence that aspirin may specifically benefit older individuals with genotypes for elevated plasma Lp(a) in the setting of high-risk primary prevention of cardiovascular events and that overall benefit may outweigh harm related to major bleeding,” the authors, led by Paul Lacaze, PhD, Monash University, Melbourne, conclude.
They also point out that similar observations have been previously seen in another large aspirin primary prevention study conducted in younger women, the Women’s Health Study, and the current analysis provides validation of those findings.
“Our results provide new evidence to support the potential use of aspirin to target individuals with elevated Lp(a) for the primary prevention of cardiovascular events,” the researchers say.
They acknowledge that these results would be strengthened by the use of directly measured plasma Lp(a) levels, in addition to Lp(a) genotypes.
But they add: “Nonetheless, given the lack of any currently approved therapies for targeting elevated Lp(a), our findings may have widespread clinical implications, adding evidence to the rationale that aspirin may be a viable option for reducing Lp(a)-mediated cardiovascular risk.”
Dr. Lacaze and colleagues explain that elevated plasma Lp(a) levels confer up to fourfold increased risk of cardiovascular disease, with around 20%-30% of the general population affected. Despite the high burden and prevalence of elevated plasma Lp(a), there are currently no approved pharmacologic therapies targeting this lipoprotein. Although promising candidates are in development for the secondary prevention of Lp(a)-mediated cardiovascular disease, it will be many years before these candidates are assessed for primary prevention.
For the current study, researchers analyzed data from 12,815 ASPREE participants who had undergone genotyping and compared outcomes with aspirin versus placebo in those with and without genotypes associated with elevated Lp(a) levels.
Results showed that individuals with elevated Lp(a)-associated genotypes, defined in two different ways, showed a reduction in ischemic events with aspirin versus placebo, and this benefit was not outweighed by an increased bleeding risk.
Specifically, in the placebo group, individuals who carried the rs3798220-C allele, which is known to be associated with raised Lp(a) levels, making up 3.2% of the genotyped population in the study, had an almost twofold increased risk of major adverse cardiovascular events than those not carrying this genotype. However, the risk was attenuated in the aspirin group, with carriers of the rs3798220-C allele actually having a lower rate of cardiovascular events than noncarriers.
In addition, rs3798220-C carrier status was not significantly associated with increased risk of clinically significant bleeding events in the aspirin group.
Similar results were seen with the second way of identifying patients with a high risk of elevated Lp(a) levels using a 43-variant genetic risk score (LPA-GRS).
In the whole study population, aspirin reduced major adverse cardiovascular events by 1.7 events per 1,000 person-years and increased clinically significant bleeding events by 1.7 events per 1,000 person-years, suggesting parity between overall benefit versus harm.
However, in the rs3798220-C subgroup, aspirin reduced major adverse cardiovascular events by 11.4 events per 1,000 person-years (a more than sixfold higher magnitude of cardiovascular disease risk reduction than in the overall cohort), with a bleeding risk of 3.3 events per 1,000 person-years, the researchers report.
“Hence in rs3798220-C carriers, aspirin appeared to have a net benefit of 8.1 events per 1,000 person-years,” they state.
In the highest LPA-GRS quintile, aspirin reduced major adverse cardiovascular events by 3.3 events per 1,000 person-years (approximately twofold higher magnitude of risk reduction, compared with the overall cohort), with an increase in bleeding risk of 1.6 events per 1,000 person-years (almost identical bleeding risk to the overall cohort). This shifted the benefit versus harm balance in the highest LPA-GRS quintile to a net benefit of 1.7 events per 1,000 person-years.
Similar findings in the Women’s Health Study
Dr. Lacaze and colleagues point out that similar results have also been seen in another large aspirin primary prevention study – the Women’s Health Study (WHS).
The WHS compared aspirin 100 mg every other day with placebo in initially healthy younger women. Previously reported results showed that women carrying the rs3798220-C variant, associated with highly elevated Lp(a) levels, had a twofold higher risk of cardiovascular events than noncarrier women in the placebo group, but this risk was reduced in the aspirin group. And there was no increased risk of bleeding in women with elevated Lp(a).
“These results, in the absence of any other randomized controlled trial evidence or approved therapy for treating Lp(a)-associated risk, have been used by some physicians as justification for prescribing aspirin in patients with elevated Lp(a),” Dr. Lacaze and colleagues note.
“In the present study of the ASPREE trial population, our results were consistent with the WHS analysis, despite randomizing older individuals (both men and women),” they add.
They say this validation of the WHS result provides evidence that a very high-risk subgroup of individuals with highly elevated Lp(a) – those carrying the rs3798220-C allele – may benefit from low-dose aspirin for the primary prevention of cardiovascular events. Further, the benefits in this subgroup specifically may outweigh any bleeding risk.
But they point out that rs3798220-C carriers comprise only a small portion of all individuals with elevated Lp(a) in the general population, while the polygenic LPA-GRS explains about 60% of the variation in directly measured plasma Lp(a) levels and has the potential advantage of being able to identify a larger group of individuals at increased risk.
The researchers note, however, that it is not clear to what extent the LPA-GRS results add further evidence to suggest that individuals with elevated Lp(a), beyond rs3798220-C carriers, may be more likely to benefit from aspirin.
“If the benefit of aspirin extends beyond very high-risk rs3798220-C carriers alone, to the broader 20%-30% of individuals with elevated Lp(a), the potential utility of aspirin for the primary prevention of cardiovascular events would increase substantially,” they say.
‘Very high clinical relevance’
In an accompanying editorial, Ana Devesa, MD, Borja Ibanez, MD, PhD, and Valentin Fuster, MD, PhD, The National Center for Cardiovascular Research, Madrid, say that: “[Dr.] Lacaze et al. are to be congratulated for a study of very high clinical relevance that represents a first indication for primary prevention for patients at high cardiovascular risk.”
They explain that the pathogenic mechanism of Lp(a) is believed to be a combination of prothrombotic and proatherogenic effects, and the current findings support the hypothesis that the prothrombotic mechanism of Lp(a) is mediated by platelet aggregation.
This would explain the occurrence of thrombotic events in the presence of atherosclerosis in that elevated Lp(a) levels may induce platelet adhesion and aggregation to the activated atherosclerotic plaque, thus enhancing the atherothrombotic process. Moreover, activated platelets release several mediators that result in cell adhesion and attraction of chemokines and proinflammatory cytokines, driving an inflammatory response and mediating atherosclerosis progression, they add.
The editorialists highlight the limitations of the study already acknowledged by the authors: The analysis used genotypes rather than elevated Lp(a) levels and included only those of European ancestry, meaning the results are difficult to extrapolate to other populations.
“The next steps in clinical practice should be defined, and there are still questions to be answered,” they conclude. “Will every patient benefit from antithrombotic therapies? Should all patients who have elevated Lp(a) levels be treated with aspirin?”
The ASPREE Biobank is supported by grants from the Commonwealth Scientific and Industrial Research Organisation, Monash University, Menzies Research Institute, Australian National University, University of Melbourne, National Institutes of Health, National Health and Medical Research Council of Australia, and the Victorian Cancer Agency. Dr. Lacaze is supported by a National Heart Foundation Future Leader Fellowship.
A version of this article first appeared on Medscape.com.
Aspirin may be of specific benefit for the primary prevention of cardiovascular disease in individuals with raised Lp(a) levels, a new study has suggested.
The study analyzed data from the ASPREE (ASPirin in Reducing Events in the Elderly) trial, which randomized 19,000 individuals aged 70 years or older without a history of cardiovascular disease to aspirin (100 mg/day) or placebo. While the main results, reported previously, showed no net benefit of aspirin in the overall population, the current analysis suggests there may be a benefit in individuals with raised Lp(a) levels.
The current analysis was published online in the Journal of the American College of Cardiology.
“Our study provides evidence that aspirin may specifically benefit older individuals with genotypes for elevated plasma Lp(a) in the setting of high-risk primary prevention of cardiovascular events and that overall benefit may outweigh harm related to major bleeding,” the authors, led by Paul Lacaze, PhD, Monash University, Melbourne, conclude.
They also point out that similar observations have been previously seen in another large aspirin primary prevention study conducted in younger women, the Women’s Health Study, and the current analysis provides validation of those findings.
“Our results provide new evidence to support the potential use of aspirin to target individuals with elevated Lp(a) for the primary prevention of cardiovascular events,” the researchers say.
They acknowledge that these results would be strengthened by the use of directly measured plasma Lp(a) levels, in addition to Lp(a) genotypes.
But they add: “Nonetheless, given the lack of any currently approved therapies for targeting elevated Lp(a), our findings may have widespread clinical implications, adding evidence to the rationale that aspirin may be a viable option for reducing Lp(a)-mediated cardiovascular risk.”
Dr. Lacaze and colleagues explain that elevated plasma Lp(a) levels confer up to fourfold increased risk of cardiovascular disease, with around 20%-30% of the general population affected. Despite the high burden and prevalence of elevated plasma Lp(a), there are currently no approved pharmacologic therapies targeting this lipoprotein. Although promising candidates are in development for the secondary prevention of Lp(a)-mediated cardiovascular disease, it will be many years before these candidates are assessed for primary prevention.
For the current study, researchers analyzed data from 12,815 ASPREE participants who had undergone genotyping and compared outcomes with aspirin versus placebo in those with and without genotypes associated with elevated Lp(a) levels.
Results showed that individuals with elevated Lp(a)-associated genotypes, defined in two different ways, showed a reduction in ischemic events with aspirin versus placebo, and this benefit was not outweighed by an increased bleeding risk.
Specifically, in the placebo group, individuals who carried the rs3798220-C allele, which is known to be associated with raised Lp(a) levels, making up 3.2% of the genotyped population in the study, had an almost twofold increased risk of major adverse cardiovascular events than those not carrying this genotype. However, the risk was attenuated in the aspirin group, with carriers of the rs3798220-C allele actually having a lower rate of cardiovascular events than noncarriers.
In addition, rs3798220-C carrier status was not significantly associated with increased risk of clinically significant bleeding events in the aspirin group.
Similar results were seen with the second way of identifying patients with a high risk of elevated Lp(a) levels using a 43-variant genetic risk score (LPA-GRS).
In the whole study population, aspirin reduced major adverse cardiovascular events by 1.7 events per 1,000 person-years and increased clinically significant bleeding events by 1.7 events per 1,000 person-years, suggesting parity between overall benefit versus harm.
However, in the rs3798220-C subgroup, aspirin reduced major adverse cardiovascular events by 11.4 events per 1,000 person-years (a more than sixfold higher magnitude of cardiovascular disease risk reduction than in the overall cohort), with a bleeding risk of 3.3 events per 1,000 person-years, the researchers report.
“Hence in rs3798220-C carriers, aspirin appeared to have a net benefit of 8.1 events per 1,000 person-years,” they state.
In the highest LPA-GRS quintile, aspirin reduced major adverse cardiovascular events by 3.3 events per 1,000 person-years (approximately twofold higher magnitude of risk reduction, compared with the overall cohort), with an increase in bleeding risk of 1.6 events per 1,000 person-years (almost identical bleeding risk to the overall cohort). This shifted the benefit versus harm balance in the highest LPA-GRS quintile to a net benefit of 1.7 events per 1,000 person-years.
Similar findings in the Women’s Health Study
Dr. Lacaze and colleagues point out that similar results have also been seen in another large aspirin primary prevention study – the Women’s Health Study (WHS).
The WHS compared aspirin 100 mg every other day with placebo in initially healthy younger women. Previously reported results showed that women carrying the rs3798220-C variant, associated with highly elevated Lp(a) levels, had a twofold higher risk of cardiovascular events than noncarrier women in the placebo group, but this risk was reduced in the aspirin group. And there was no increased risk of bleeding in women with elevated Lp(a).
“These results, in the absence of any other randomized controlled trial evidence or approved therapy for treating Lp(a)-associated risk, have been used by some physicians as justification for prescribing aspirin in patients with elevated Lp(a),” Dr. Lacaze and colleagues note.
“In the present study of the ASPREE trial population, our results were consistent with the WHS analysis, despite randomizing older individuals (both men and women),” they add.
They say this validation of the WHS result provides evidence that a very high-risk subgroup of individuals with highly elevated Lp(a) – those carrying the rs3798220-C allele – may benefit from low-dose aspirin for the primary prevention of cardiovascular events. Further, the benefits in this subgroup specifically may outweigh any bleeding risk.
But they point out that rs3798220-C carriers comprise only a small portion of all individuals with elevated Lp(a) in the general population, while the polygenic LPA-GRS explains about 60% of the variation in directly measured plasma Lp(a) levels and has the potential advantage of being able to identify a larger group of individuals at increased risk.
The researchers note, however, that it is not clear to what extent the LPA-GRS results add further evidence to suggest that individuals with elevated Lp(a), beyond rs3798220-C carriers, may be more likely to benefit from aspirin.
“If the benefit of aspirin extends beyond very high-risk rs3798220-C carriers alone, to the broader 20%-30% of individuals with elevated Lp(a), the potential utility of aspirin for the primary prevention of cardiovascular events would increase substantially,” they say.
‘Very high clinical relevance’
In an accompanying editorial, Ana Devesa, MD, Borja Ibanez, MD, PhD, and Valentin Fuster, MD, PhD, The National Center for Cardiovascular Research, Madrid, say that: “[Dr.] Lacaze et al. are to be congratulated for a study of very high clinical relevance that represents a first indication for primary prevention for patients at high cardiovascular risk.”
They explain that the pathogenic mechanism of Lp(a) is believed to be a combination of prothrombotic and proatherogenic effects, and the current findings support the hypothesis that the prothrombotic mechanism of Lp(a) is mediated by platelet aggregation.
This would explain the occurrence of thrombotic events in the presence of atherosclerosis in that elevated Lp(a) levels may induce platelet adhesion and aggregation to the activated atherosclerotic plaque, thus enhancing the atherothrombotic process. Moreover, activated platelets release several mediators that result in cell adhesion and attraction of chemokines and proinflammatory cytokines, driving an inflammatory response and mediating atherosclerosis progression, they add.
The editorialists highlight the limitations of the study already acknowledged by the authors: The analysis used genotypes rather than elevated Lp(a) levels and included only those of European ancestry, meaning the results are difficult to extrapolate to other populations.
“The next steps in clinical practice should be defined, and there are still questions to be answered,” they conclude. “Will every patient benefit from antithrombotic therapies? Should all patients who have elevated Lp(a) levels be treated with aspirin?”
The ASPREE Biobank is supported by grants from the Commonwealth Scientific and Industrial Research Organisation, Monash University, Menzies Research Institute, Australian National University, University of Melbourne, National Institutes of Health, National Health and Medical Research Council of Australia, and the Victorian Cancer Agency. Dr. Lacaze is supported by a National Heart Foundation Future Leader Fellowship.
A version of this article first appeared on Medscape.com.
Severe COVID-19–related outcomes found worse in men with RA
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
FROM THE LANCET SUMMIT ON SEX AND GENDER IN RHEUMATOLOGY
COVID pandemic associated with anorexia in Canadian youth
, data suggest.
Preliminary results of the Canadian Paediatric Surveillance Program (CPSP) indicate that the pandemic has been a precipitating factor in the development of anorexia nervosa in almost half of children and adolescents studied. The pandemic also has precipitated hospitalizations for anorexia in more than one-third of cases.
“Data globally, and certainly our data here in Canada, have shown a real increase in health care utilization with the onset of the COVID-19 pandemic,” study author Debra Katzman, MD, professor of pediatrics at the Hospital for Sick Children in Toronto and the University of Toronto, said in an interview. “And when I talk about health care utilization, I’m talking about hospitalizations for eating disorders.”
The data were included in the 2021 results of the CPSP.
Focus on appearance
CPSP is a collaboration between the Public Health Agency of Canada and the Canadian Pediatric Society that consists of a network of 2,800 pediatricians and pediatric subspecialists across Canada. The latest results include surveillance studies on 14 diseases and conditions, with data collected during various periods.
From April 2020 to May 2021, researchers identified 1,800 COVID-19 cases in children and collected detailed information on 1,456 of them, including 405 cases hospitalized with pediatric inflammatory multisystem syndrome (PIMS). The median age of hospitalized cases was 3.2 years for SARS-CoV-2 infection and 5.4 years for PIMS.
Dr. Katzman and colleagues observed 118 first-time hospitalizations for anorexia nervosa between Sept. 1 and Dec. 31, 2021. More than 90% of reported cases were female, with 66% of verified cases in teens aged 14-17 years and the remainder in adolescents aged 11-13 years.
In 49% of cases, the reporting physician identified the COVID-19 pandemic as a precipitating factor in the development of anorexia nervosa. In 37% of cases, the reporting physician identified the pandemic as having precipitated the anorexia-related hospitalization.
Last year, a cross-sectional analysis of children in Canada reported that monthly hospitalizations for anorexia nervosa increased from 7.5 to 20 from March through November 2020. The monthly rate in the CPSP study was closer to 30 for first-time hospitalizations.
Dr. Katzman said that the findings about anorexia nervosa didn’t surprise her. “There was so much disruption and [so many] restrictions to young peoples’ daily routines – closures of schools and recreational activities – they lost regular connection with their peers, and they lost extracurricular and social activities,” she said. “That led to heightened anxiety and depression and really a lack of control.”
Adolescents and teens were also spending more time on social media than they were before the pandemic, she noted. “They were looking at themselves all the time, so they were getting preoccupied with their body image. There was a heightened focus on appearance, and I think that things like public-health mitigation strategies – things like hand washing, social distancing, mask wearing – may have impacted the psychological well-being of young people.”
The closure of outpatient facilities, long waiting lists to get into facilities that were opened, and “coronaphobia” about going to physicians’ offices and emergency departments compounded the problem, Dr. Katzman added.
The long-term effects of COVID and eating disorders in children are unknown, Dr. Katzman said. “This is sort of a wake-up call for the health care system that during times of stress or pandemics or crises, these kinds of things can happen, and we need to be prepared to provide the resources for vulnerable populations moving forward,” she said.
Heightened anxiety
Commenting on the data, Margaret Thew, APNP, director of the eating disorders program at Children’s Wisconsin in Milwaukee, said that isolation due to school closures and negative social media messages created the “perfect storm” for eating disorders in adolescents and teenagers because of higher rates of anxiety and depression. Ms. Thew was not involved in the research.
The storm is not over yet, she said. “What everyone needs to keep in mind is that we still have this very heightened state of anxiety and depression ... for adolescents, teenagers, and preteens alike,” Ms. Thew said in an interview, “and we know that many of them are not coping with their anxiety very well.”
In her experience, since the start of the pandemic, the average age of pediatric patients with eating disorders declined from 16 to 15 years, and the youngest age declined from 12 to 11 years.
Overall, the CPSP results show that children are affected by mental health issues at an earlier age than before the pandemic, said Ms. Thew. “Years ago, we wouldn’t have thought that an 8-year-old needed to be screened for some of these risk factors, but now we’re definitely getting more younger children who are struggling, and I think it’s taking too long for them to get the care they need because it’s being overlooked,” she said.
The report was funded by the Public Health Agency of Canada, Health Canada, Alberta Children’s Hospital Research Institute, Bethanys Hope Foundation, CHEO Research Institute, and Children’s Hospital Research Institute of Manitoba. Dr. Katzman and Ms. Thew have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, data suggest.
Preliminary results of the Canadian Paediatric Surveillance Program (CPSP) indicate that the pandemic has been a precipitating factor in the development of anorexia nervosa in almost half of children and adolescents studied. The pandemic also has precipitated hospitalizations for anorexia in more than one-third of cases.
“Data globally, and certainly our data here in Canada, have shown a real increase in health care utilization with the onset of the COVID-19 pandemic,” study author Debra Katzman, MD, professor of pediatrics at the Hospital for Sick Children in Toronto and the University of Toronto, said in an interview. “And when I talk about health care utilization, I’m talking about hospitalizations for eating disorders.”
The data were included in the 2021 results of the CPSP.
Focus on appearance
CPSP is a collaboration between the Public Health Agency of Canada and the Canadian Pediatric Society that consists of a network of 2,800 pediatricians and pediatric subspecialists across Canada. The latest results include surveillance studies on 14 diseases and conditions, with data collected during various periods.
From April 2020 to May 2021, researchers identified 1,800 COVID-19 cases in children and collected detailed information on 1,456 of them, including 405 cases hospitalized with pediatric inflammatory multisystem syndrome (PIMS). The median age of hospitalized cases was 3.2 years for SARS-CoV-2 infection and 5.4 years for PIMS.
Dr. Katzman and colleagues observed 118 first-time hospitalizations for anorexia nervosa between Sept. 1 and Dec. 31, 2021. More than 90% of reported cases were female, with 66% of verified cases in teens aged 14-17 years and the remainder in adolescents aged 11-13 years.
In 49% of cases, the reporting physician identified the COVID-19 pandemic as a precipitating factor in the development of anorexia nervosa. In 37% of cases, the reporting physician identified the pandemic as having precipitated the anorexia-related hospitalization.
Last year, a cross-sectional analysis of children in Canada reported that monthly hospitalizations for anorexia nervosa increased from 7.5 to 20 from March through November 2020. The monthly rate in the CPSP study was closer to 30 for first-time hospitalizations.
Dr. Katzman said that the findings about anorexia nervosa didn’t surprise her. “There was so much disruption and [so many] restrictions to young peoples’ daily routines – closures of schools and recreational activities – they lost regular connection with their peers, and they lost extracurricular and social activities,” she said. “That led to heightened anxiety and depression and really a lack of control.”
Adolescents and teens were also spending more time on social media than they were before the pandemic, she noted. “They were looking at themselves all the time, so they were getting preoccupied with their body image. There was a heightened focus on appearance, and I think that things like public-health mitigation strategies – things like hand washing, social distancing, mask wearing – may have impacted the psychological well-being of young people.”
The closure of outpatient facilities, long waiting lists to get into facilities that were opened, and “coronaphobia” about going to physicians’ offices and emergency departments compounded the problem, Dr. Katzman added.
The long-term effects of COVID and eating disorders in children are unknown, Dr. Katzman said. “This is sort of a wake-up call for the health care system that during times of stress or pandemics or crises, these kinds of things can happen, and we need to be prepared to provide the resources for vulnerable populations moving forward,” she said.
Heightened anxiety
Commenting on the data, Margaret Thew, APNP, director of the eating disorders program at Children’s Wisconsin in Milwaukee, said that isolation due to school closures and negative social media messages created the “perfect storm” for eating disorders in adolescents and teenagers because of higher rates of anxiety and depression. Ms. Thew was not involved in the research.
The storm is not over yet, she said. “What everyone needs to keep in mind is that we still have this very heightened state of anxiety and depression ... for adolescents, teenagers, and preteens alike,” Ms. Thew said in an interview, “and we know that many of them are not coping with their anxiety very well.”
In her experience, since the start of the pandemic, the average age of pediatric patients with eating disorders declined from 16 to 15 years, and the youngest age declined from 12 to 11 years.
Overall, the CPSP results show that children are affected by mental health issues at an earlier age than before the pandemic, said Ms. Thew. “Years ago, we wouldn’t have thought that an 8-year-old needed to be screened for some of these risk factors, but now we’re definitely getting more younger children who are struggling, and I think it’s taking too long for them to get the care they need because it’s being overlooked,” she said.
The report was funded by the Public Health Agency of Canada, Health Canada, Alberta Children’s Hospital Research Institute, Bethanys Hope Foundation, CHEO Research Institute, and Children’s Hospital Research Institute of Manitoba. Dr. Katzman and Ms. Thew have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, data suggest.
Preliminary results of the Canadian Paediatric Surveillance Program (CPSP) indicate that the pandemic has been a precipitating factor in the development of anorexia nervosa in almost half of children and adolescents studied. The pandemic also has precipitated hospitalizations for anorexia in more than one-third of cases.
“Data globally, and certainly our data here in Canada, have shown a real increase in health care utilization with the onset of the COVID-19 pandemic,” study author Debra Katzman, MD, professor of pediatrics at the Hospital for Sick Children in Toronto and the University of Toronto, said in an interview. “And when I talk about health care utilization, I’m talking about hospitalizations for eating disorders.”
The data were included in the 2021 results of the CPSP.
Focus on appearance
CPSP is a collaboration between the Public Health Agency of Canada and the Canadian Pediatric Society that consists of a network of 2,800 pediatricians and pediatric subspecialists across Canada. The latest results include surveillance studies on 14 diseases and conditions, with data collected during various periods.
From April 2020 to May 2021, researchers identified 1,800 COVID-19 cases in children and collected detailed information on 1,456 of them, including 405 cases hospitalized with pediatric inflammatory multisystem syndrome (PIMS). The median age of hospitalized cases was 3.2 years for SARS-CoV-2 infection and 5.4 years for PIMS.
Dr. Katzman and colleagues observed 118 first-time hospitalizations for anorexia nervosa between Sept. 1 and Dec. 31, 2021. More than 90% of reported cases were female, with 66% of verified cases in teens aged 14-17 years and the remainder in adolescents aged 11-13 years.
In 49% of cases, the reporting physician identified the COVID-19 pandemic as a precipitating factor in the development of anorexia nervosa. In 37% of cases, the reporting physician identified the pandemic as having precipitated the anorexia-related hospitalization.
Last year, a cross-sectional analysis of children in Canada reported that monthly hospitalizations for anorexia nervosa increased from 7.5 to 20 from March through November 2020. The monthly rate in the CPSP study was closer to 30 for first-time hospitalizations.
Dr. Katzman said that the findings about anorexia nervosa didn’t surprise her. “There was so much disruption and [so many] restrictions to young peoples’ daily routines – closures of schools and recreational activities – they lost regular connection with their peers, and they lost extracurricular and social activities,” she said. “That led to heightened anxiety and depression and really a lack of control.”
Adolescents and teens were also spending more time on social media than they were before the pandemic, she noted. “They were looking at themselves all the time, so they were getting preoccupied with their body image. There was a heightened focus on appearance, and I think that things like public-health mitigation strategies – things like hand washing, social distancing, mask wearing – may have impacted the psychological well-being of young people.”
The closure of outpatient facilities, long waiting lists to get into facilities that were opened, and “coronaphobia” about going to physicians’ offices and emergency departments compounded the problem, Dr. Katzman added.
The long-term effects of COVID and eating disorders in children are unknown, Dr. Katzman said. “This is sort of a wake-up call for the health care system that during times of stress or pandemics or crises, these kinds of things can happen, and we need to be prepared to provide the resources for vulnerable populations moving forward,” she said.
Heightened anxiety
Commenting on the data, Margaret Thew, APNP, director of the eating disorders program at Children’s Wisconsin in Milwaukee, said that isolation due to school closures and negative social media messages created the “perfect storm” for eating disorders in adolescents and teenagers because of higher rates of anxiety and depression. Ms. Thew was not involved in the research.
The storm is not over yet, she said. “What everyone needs to keep in mind is that we still have this very heightened state of anxiety and depression ... for adolescents, teenagers, and preteens alike,” Ms. Thew said in an interview, “and we know that many of them are not coping with their anxiety very well.”
In her experience, since the start of the pandemic, the average age of pediatric patients with eating disorders declined from 16 to 15 years, and the youngest age declined from 12 to 11 years.
Overall, the CPSP results show that children are affected by mental health issues at an earlier age than before the pandemic, said Ms. Thew. “Years ago, we wouldn’t have thought that an 8-year-old needed to be screened for some of these risk factors, but now we’re definitely getting more younger children who are struggling, and I think it’s taking too long for them to get the care they need because it’s being overlooked,” she said.
The report was funded by the Public Health Agency of Canada, Health Canada, Alberta Children’s Hospital Research Institute, Bethanys Hope Foundation, CHEO Research Institute, and Children’s Hospital Research Institute of Manitoba. Dr. Katzman and Ms. Thew have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FDA approves dupilumab for treatment of prurigo nodularis
The according to a press release from the manufacturers.
Recent studies of dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, show significant improvements in both itchiness and lesion counts, compared with placebo, in adults with prurigo nodularis (PN).
Approval was based on data from two randomized, controlled trials, PRIME and PRIME2, comparing dupilumab with placebo in 311 adults with uncontrolled PN, according to the release issued by Regeneron and Sanofi. Dupilumab is administered via a 300 mg subcutaneous injection every 2 weeks after a loading dose.
The primary endpoint in PRIME and PRIME 2 was a clinically meaningful improvement in itch from baseline as measured by at least a 4-point reduction in the Worst Itch Numeric Rating Scale, a 0-10 scale, at 24 and 12 weeks, respectively. In the studies, 60% and 58% of patients treated with dupilumab met the primary endpoint at 24 weeks, compared with 18% and 20% of those on placebo. At 24 weeks, 48% and 45% of patients on dupilumab achieved clear or almost clear skin, another study endpoint, compared with 18% and 16% among those on placebo.*
In PRIME and PRIME2, 44% and 37% of patients on dupilumab met the primary endpoint at 12 weeks versus16% and 22% among those on placebo.
Safety profiles were similar to those seen in other dupilumab studies, according to the release. The most common adverse events in the two studies combined were nasopharyngitis, reported in 5% of those on dupilumab versus 2% of those on placebo; conjunctivitis in 4% versus 1%; herpes infection in 3% versus 0; dizziness in 3% vs. 1%; muscle pain in 3% versus 1%; and diarrhea in 3% versus 1%.
Phase 3 data on dupilumab for PN were recently presented at the annual congress of the European Academy of Dermatology and Venereology.
A regulatory submission for dupilumab for treating PN is in progress at the European Medicines Agency, and submissions are planned to regulatory agencies in additional countries later in 2022, according to the company press release.
Dupilumab is currently approved in the United States for atopic dermatitis in children aged 6 months and older and adults with moderate to severe atopic dermatitis and in children and adults aged 6 years and older with moderate to severe eosinophilic or oral steroid-dependent asthma, as well as for the treatment of chronic rhinosinusitis with nasal polyposis in adults, and for the treatment of eosinophilic esophagitis in adults and children aged 12 years and older, weighing at least 40 kg. Dupilumab is under clinical development for the treatment of chronic spontaneous urticaria and bullous pemphigoid, according to the manufacturers.
The studies were supported by Regeneron and Sanofi.
A version of this article first appeared on Medscape.com.
*Correction, 9/30/22: An earlier version of this article misstated results of one endpoint.
The according to a press release from the manufacturers.
Recent studies of dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, show significant improvements in both itchiness and lesion counts, compared with placebo, in adults with prurigo nodularis (PN).
Approval was based on data from two randomized, controlled trials, PRIME and PRIME2, comparing dupilumab with placebo in 311 adults with uncontrolled PN, according to the release issued by Regeneron and Sanofi. Dupilumab is administered via a 300 mg subcutaneous injection every 2 weeks after a loading dose.
The primary endpoint in PRIME and PRIME 2 was a clinically meaningful improvement in itch from baseline as measured by at least a 4-point reduction in the Worst Itch Numeric Rating Scale, a 0-10 scale, at 24 and 12 weeks, respectively. In the studies, 60% and 58% of patients treated with dupilumab met the primary endpoint at 24 weeks, compared with 18% and 20% of those on placebo. At 24 weeks, 48% and 45% of patients on dupilumab achieved clear or almost clear skin, another study endpoint, compared with 18% and 16% among those on placebo.*
In PRIME and PRIME2, 44% and 37% of patients on dupilumab met the primary endpoint at 12 weeks versus16% and 22% among those on placebo.
Safety profiles were similar to those seen in other dupilumab studies, according to the release. The most common adverse events in the two studies combined were nasopharyngitis, reported in 5% of those on dupilumab versus 2% of those on placebo; conjunctivitis in 4% versus 1%; herpes infection in 3% versus 0; dizziness in 3% vs. 1%; muscle pain in 3% versus 1%; and diarrhea in 3% versus 1%.
Phase 3 data on dupilumab for PN were recently presented at the annual congress of the European Academy of Dermatology and Venereology.
A regulatory submission for dupilumab for treating PN is in progress at the European Medicines Agency, and submissions are planned to regulatory agencies in additional countries later in 2022, according to the company press release.
Dupilumab is currently approved in the United States for atopic dermatitis in children aged 6 months and older and adults with moderate to severe atopic dermatitis and in children and adults aged 6 years and older with moderate to severe eosinophilic or oral steroid-dependent asthma, as well as for the treatment of chronic rhinosinusitis with nasal polyposis in adults, and for the treatment of eosinophilic esophagitis in adults and children aged 12 years and older, weighing at least 40 kg. Dupilumab is under clinical development for the treatment of chronic spontaneous urticaria and bullous pemphigoid, according to the manufacturers.
The studies were supported by Regeneron and Sanofi.
A version of this article first appeared on Medscape.com.
*Correction, 9/30/22: An earlier version of this article misstated results of one endpoint.
The according to a press release from the manufacturers.
Recent studies of dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, show significant improvements in both itchiness and lesion counts, compared with placebo, in adults with prurigo nodularis (PN).
Approval was based on data from two randomized, controlled trials, PRIME and PRIME2, comparing dupilumab with placebo in 311 adults with uncontrolled PN, according to the release issued by Regeneron and Sanofi. Dupilumab is administered via a 300 mg subcutaneous injection every 2 weeks after a loading dose.
The primary endpoint in PRIME and PRIME 2 was a clinically meaningful improvement in itch from baseline as measured by at least a 4-point reduction in the Worst Itch Numeric Rating Scale, a 0-10 scale, at 24 and 12 weeks, respectively. In the studies, 60% and 58% of patients treated with dupilumab met the primary endpoint at 24 weeks, compared with 18% and 20% of those on placebo. At 24 weeks, 48% and 45% of patients on dupilumab achieved clear or almost clear skin, another study endpoint, compared with 18% and 16% among those on placebo.*
In PRIME and PRIME2, 44% and 37% of patients on dupilumab met the primary endpoint at 12 weeks versus16% and 22% among those on placebo.
Safety profiles were similar to those seen in other dupilumab studies, according to the release. The most common adverse events in the two studies combined were nasopharyngitis, reported in 5% of those on dupilumab versus 2% of those on placebo; conjunctivitis in 4% versus 1%; herpes infection in 3% versus 0; dizziness in 3% vs. 1%; muscle pain in 3% versus 1%; and diarrhea in 3% versus 1%.
Phase 3 data on dupilumab for PN were recently presented at the annual congress of the European Academy of Dermatology and Venereology.
A regulatory submission for dupilumab for treating PN is in progress at the European Medicines Agency, and submissions are planned to regulatory agencies in additional countries later in 2022, according to the company press release.
Dupilumab is currently approved in the United States for atopic dermatitis in children aged 6 months and older and adults with moderate to severe atopic dermatitis and in children and adults aged 6 years and older with moderate to severe eosinophilic or oral steroid-dependent asthma, as well as for the treatment of chronic rhinosinusitis with nasal polyposis in adults, and for the treatment of eosinophilic esophagitis in adults and children aged 12 years and older, weighing at least 40 kg. Dupilumab is under clinical development for the treatment of chronic spontaneous urticaria and bullous pemphigoid, according to the manufacturers.
The studies were supported by Regeneron and Sanofi.
A version of this article first appeared on Medscape.com.
*Correction, 9/30/22: An earlier version of this article misstated results of one endpoint.
USPSTF: Screen at-risk, nonpregnant people for syphilis
People at increased risk for syphilis – including asymptomatic, nonpregnant adolescents and adults who have ever been sexually active and are at high risk for the disease – should be screened for it, according to a reaffirmation by the United States Preventive Services Task Force of its 2016 recommendation of syphilis screening for people at increased risk for infection.
“Using a reaffirmation process, the authors, led by Carol M. Mangione, MD, MSPH, of the University of California, Los Angeles, wrote in JAMA.
Reported cases in the United States of primary and secondary syphilis – a sexually transmitted infection caused by the bacterium Treponema pallidum that can damage the brain, nerves, eyes, and cardiovascular system if left untreated – increased from a low of 2.1 cases per 100,000 people in 2000 and 2001 to 11.9 cases per 100,000 in 2019, the authors reported. In 2019, men accounted for 83% of all primary and secondary syphilis cases, and men who have sex with men (MSM) accounted for 57% of all primary and secondary syphilis cases in men. Screening and follow-up treatment can cure syphilis and prevent complications.
To help them evaluate the effectiveness and safety of screening, the USPSTF authors reviewed the literature and visually displayed key questions and linkages to interventions and outcomes, Michelle L. Henninger, PhD, Sarah I. Bean, MPH, and Jennifer S. Lin, MD, MCR, of the Kaiser Permanente Evidence-based Practice Center in Portland, Ore., noted in a related evidence report of the post-2016 recommendation data.
Reaffirming its 2016 recommendation, the USPSTF now advises clinicians to:
Assess risk:
- Clinicians should know how common syphilis is in their community and assess their patient’s individual risk.
- Risk for syphilis is higher in MSM, people with HIV infection or other STIs, and those who use illicit drugs or have a history of incarceration, sex work, or military service.
Screen and confirm by testing:
- Traditional screening algorithm: Start with a nontreponemal test such as Venereal Disease Research Laborator or rapid plasma reagin. If positive, confirm result with a treponemal antibody detection test, such as T. pallidum particle agglutination.
- Reverse sequence algorithm: Screen with an initial automated treponemal test such as enzyme-linked or chemiluminescence immunoassay. If positive, confirm result with a nontreponemal test.
Consider screening interval:
- Evidence on optimal screening intervals is limited for the general population, but MSM and people with HIV may benefit from screening yearly or every 3-6 months if they remain at high risk.
The authors acknowledged that primary and secondary syphilis rates are higher in Blacks, Hispanics, Native Americans/Alaska Native, and Native Hawaiians/Pacific Islanders, and that the disparities are primarily driven by social determinants of health including differences in income, education, and access to coverage and care.
They added that differences in sexual networks also play a role in disparities and that sexually active people in communities with higher STI rates may be more likely to become infected.
More testing, treatment, and research are needed
Four experts welcomed the reaffirmation.
“It is important and necessary that the task force has chosen to reaffirm their syphilis screening recommendations, given the continued increase in sexually transmitted infections in the U.S. since the 2016 published recommendations,” Judith A. O’Donnell, MD, director of the department of infection prevention and control at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
“Awareness of the ongoing incidence, understanding of the importance of screening in interrupting transmission, and getting people diagnosed and treated before serious complications are key,” she added.
Heidi Gullettt, MD, MPH, associate director of the Center for Community Health Integration at Case Western Reserve University, Cleveland, said: “The reaffirmation document authors demonstrated a comprehensive review of high-quality studies and epidemiologic data.
“Primary care clinicians rely on USPSTF recommendations to help prioritize evidence-based prevention in practice, so this reaffirmation is a critical step to remind us of the importance of regularly assessing risk and screening with a readily available screening test in the office,” she added.
Testing during office visits is not easy, Dr. Gullettt said, because of competing priorities, stigma associated with STIs, and testing and treatment costs.
“Under the Affordable Care Act, USPSTF screening recommendations are supposed to be covered without cost sharing by patients. This should be the case for syphilis screening,” Dr. Gullett pointed out. “Patients are often reluctant to do screening because of cost.”
Michael Anthony Moody, MD, director of the Collaborative Influenza Vaccine Innovation Center at Duke University, Durham, N.C., said that the true incidence and prevalence of syphilis is unknown.
“The more we test, the more accurate our data will be,” he said. “Syphilis can hide in plain sight, has symptoms that mimic many other diseases, and is usually not diagnosed. Reaffirming that testing for syphilis is important reminds providers that this is a key test for their patient’s health.”
Aniruddha Hazra, MD, medical director of the University of Chicago Medicine Sexual Wellness Clinic, noted that the United States is in a syphilis epidemic.
“Screening asymptomatic people at risk for syphilis is important, but without comprehensive education and training of primary care providers on how to address STIs and sexual health, these recommendations fall flat,” he said.
In an accompanying editorial, Susan Tuddenham, MD, MPH; and Khalil G. Ghanem, MD, PhD, of Johns Hopkins University, Baltimore, urged that funding to develop novel syphilis diagnostics be prioritized, “just as there has been for development of syphilis vaccines, which are still many years from becoming a reality.”
“Relying on emerging biomedical prevention interventions that hold promise, such as doxycycline postexposure prophylaxis, without concomitant robust screening strategies will not lead to syphilis control. Failure to modernize screening strategies for syphilis will also mean failure to control this infection,” they cautioned.
The authors of the recommendation statement and the evidence report, as well as Dr. O’Donnell, Dr. Gullettt, Dr. Moody, and Dr. Hazra, who were not involved in the study, reported no relevant financial relationships. Dr. Tuddenham reported financial relationships with the pharmaceutical and publishing industries. Dr. Ghanem reported financial relationships with the publishing industry. The research was federally funded.
A version of this article first appeared on Medscape.com.
People at increased risk for syphilis – including asymptomatic, nonpregnant adolescents and adults who have ever been sexually active and are at high risk for the disease – should be screened for it, according to a reaffirmation by the United States Preventive Services Task Force of its 2016 recommendation of syphilis screening for people at increased risk for infection.
“Using a reaffirmation process, the authors, led by Carol M. Mangione, MD, MSPH, of the University of California, Los Angeles, wrote in JAMA.
Reported cases in the United States of primary and secondary syphilis – a sexually transmitted infection caused by the bacterium Treponema pallidum that can damage the brain, nerves, eyes, and cardiovascular system if left untreated – increased from a low of 2.1 cases per 100,000 people in 2000 and 2001 to 11.9 cases per 100,000 in 2019, the authors reported. In 2019, men accounted for 83% of all primary and secondary syphilis cases, and men who have sex with men (MSM) accounted for 57% of all primary and secondary syphilis cases in men. Screening and follow-up treatment can cure syphilis and prevent complications.
To help them evaluate the effectiveness and safety of screening, the USPSTF authors reviewed the literature and visually displayed key questions and linkages to interventions and outcomes, Michelle L. Henninger, PhD, Sarah I. Bean, MPH, and Jennifer S. Lin, MD, MCR, of the Kaiser Permanente Evidence-based Practice Center in Portland, Ore., noted in a related evidence report of the post-2016 recommendation data.
Reaffirming its 2016 recommendation, the USPSTF now advises clinicians to:
Assess risk:
- Clinicians should know how common syphilis is in their community and assess their patient’s individual risk.
- Risk for syphilis is higher in MSM, people with HIV infection or other STIs, and those who use illicit drugs or have a history of incarceration, sex work, or military service.
Screen and confirm by testing:
- Traditional screening algorithm: Start with a nontreponemal test such as Venereal Disease Research Laborator or rapid plasma reagin. If positive, confirm result with a treponemal antibody detection test, such as T. pallidum particle agglutination.
- Reverse sequence algorithm: Screen with an initial automated treponemal test such as enzyme-linked or chemiluminescence immunoassay. If positive, confirm result with a nontreponemal test.
Consider screening interval:
- Evidence on optimal screening intervals is limited for the general population, but MSM and people with HIV may benefit from screening yearly or every 3-6 months if they remain at high risk.
The authors acknowledged that primary and secondary syphilis rates are higher in Blacks, Hispanics, Native Americans/Alaska Native, and Native Hawaiians/Pacific Islanders, and that the disparities are primarily driven by social determinants of health including differences in income, education, and access to coverage and care.
They added that differences in sexual networks also play a role in disparities and that sexually active people in communities with higher STI rates may be more likely to become infected.
More testing, treatment, and research are needed
Four experts welcomed the reaffirmation.
“It is important and necessary that the task force has chosen to reaffirm their syphilis screening recommendations, given the continued increase in sexually transmitted infections in the U.S. since the 2016 published recommendations,” Judith A. O’Donnell, MD, director of the department of infection prevention and control at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
“Awareness of the ongoing incidence, understanding of the importance of screening in interrupting transmission, and getting people diagnosed and treated before serious complications are key,” she added.
Heidi Gullettt, MD, MPH, associate director of the Center for Community Health Integration at Case Western Reserve University, Cleveland, said: “The reaffirmation document authors demonstrated a comprehensive review of high-quality studies and epidemiologic data.
“Primary care clinicians rely on USPSTF recommendations to help prioritize evidence-based prevention in practice, so this reaffirmation is a critical step to remind us of the importance of regularly assessing risk and screening with a readily available screening test in the office,” she added.
Testing during office visits is not easy, Dr. Gullettt said, because of competing priorities, stigma associated with STIs, and testing and treatment costs.
“Under the Affordable Care Act, USPSTF screening recommendations are supposed to be covered without cost sharing by patients. This should be the case for syphilis screening,” Dr. Gullett pointed out. “Patients are often reluctant to do screening because of cost.”
Michael Anthony Moody, MD, director of the Collaborative Influenza Vaccine Innovation Center at Duke University, Durham, N.C., said that the true incidence and prevalence of syphilis is unknown.
“The more we test, the more accurate our data will be,” he said. “Syphilis can hide in plain sight, has symptoms that mimic many other diseases, and is usually not diagnosed. Reaffirming that testing for syphilis is important reminds providers that this is a key test for their patient’s health.”
Aniruddha Hazra, MD, medical director of the University of Chicago Medicine Sexual Wellness Clinic, noted that the United States is in a syphilis epidemic.
“Screening asymptomatic people at risk for syphilis is important, but without comprehensive education and training of primary care providers on how to address STIs and sexual health, these recommendations fall flat,” he said.
In an accompanying editorial, Susan Tuddenham, MD, MPH; and Khalil G. Ghanem, MD, PhD, of Johns Hopkins University, Baltimore, urged that funding to develop novel syphilis diagnostics be prioritized, “just as there has been for development of syphilis vaccines, which are still many years from becoming a reality.”
“Relying on emerging biomedical prevention interventions that hold promise, such as doxycycline postexposure prophylaxis, without concomitant robust screening strategies will not lead to syphilis control. Failure to modernize screening strategies for syphilis will also mean failure to control this infection,” they cautioned.
The authors of the recommendation statement and the evidence report, as well as Dr. O’Donnell, Dr. Gullettt, Dr. Moody, and Dr. Hazra, who were not involved in the study, reported no relevant financial relationships. Dr. Tuddenham reported financial relationships with the pharmaceutical and publishing industries. Dr. Ghanem reported financial relationships with the publishing industry. The research was federally funded.
A version of this article first appeared on Medscape.com.
People at increased risk for syphilis – including asymptomatic, nonpregnant adolescents and adults who have ever been sexually active and are at high risk for the disease – should be screened for it, according to a reaffirmation by the United States Preventive Services Task Force of its 2016 recommendation of syphilis screening for people at increased risk for infection.
“Using a reaffirmation process, the authors, led by Carol M. Mangione, MD, MSPH, of the University of California, Los Angeles, wrote in JAMA.
Reported cases in the United States of primary and secondary syphilis – a sexually transmitted infection caused by the bacterium Treponema pallidum that can damage the brain, nerves, eyes, and cardiovascular system if left untreated – increased from a low of 2.1 cases per 100,000 people in 2000 and 2001 to 11.9 cases per 100,000 in 2019, the authors reported. In 2019, men accounted for 83% of all primary and secondary syphilis cases, and men who have sex with men (MSM) accounted for 57% of all primary and secondary syphilis cases in men. Screening and follow-up treatment can cure syphilis and prevent complications.
To help them evaluate the effectiveness and safety of screening, the USPSTF authors reviewed the literature and visually displayed key questions and linkages to interventions and outcomes, Michelle L. Henninger, PhD, Sarah I. Bean, MPH, and Jennifer S. Lin, MD, MCR, of the Kaiser Permanente Evidence-based Practice Center in Portland, Ore., noted in a related evidence report of the post-2016 recommendation data.
Reaffirming its 2016 recommendation, the USPSTF now advises clinicians to:
Assess risk:
- Clinicians should know how common syphilis is in their community and assess their patient’s individual risk.
- Risk for syphilis is higher in MSM, people with HIV infection or other STIs, and those who use illicit drugs or have a history of incarceration, sex work, or military service.
Screen and confirm by testing:
- Traditional screening algorithm: Start with a nontreponemal test such as Venereal Disease Research Laborator or rapid plasma reagin. If positive, confirm result with a treponemal antibody detection test, such as T. pallidum particle agglutination.
- Reverse sequence algorithm: Screen with an initial automated treponemal test such as enzyme-linked or chemiluminescence immunoassay. If positive, confirm result with a nontreponemal test.
Consider screening interval:
- Evidence on optimal screening intervals is limited for the general population, but MSM and people with HIV may benefit from screening yearly or every 3-6 months if they remain at high risk.
The authors acknowledged that primary and secondary syphilis rates are higher in Blacks, Hispanics, Native Americans/Alaska Native, and Native Hawaiians/Pacific Islanders, and that the disparities are primarily driven by social determinants of health including differences in income, education, and access to coverage and care.
They added that differences in sexual networks also play a role in disparities and that sexually active people in communities with higher STI rates may be more likely to become infected.
More testing, treatment, and research are needed
Four experts welcomed the reaffirmation.
“It is important and necessary that the task force has chosen to reaffirm their syphilis screening recommendations, given the continued increase in sexually transmitted infections in the U.S. since the 2016 published recommendations,” Judith A. O’Donnell, MD, director of the department of infection prevention and control at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
“Awareness of the ongoing incidence, understanding of the importance of screening in interrupting transmission, and getting people diagnosed and treated before serious complications are key,” she added.
Heidi Gullettt, MD, MPH, associate director of the Center for Community Health Integration at Case Western Reserve University, Cleveland, said: “The reaffirmation document authors demonstrated a comprehensive review of high-quality studies and epidemiologic data.
“Primary care clinicians rely on USPSTF recommendations to help prioritize evidence-based prevention in practice, so this reaffirmation is a critical step to remind us of the importance of regularly assessing risk and screening with a readily available screening test in the office,” she added.
Testing during office visits is not easy, Dr. Gullettt said, because of competing priorities, stigma associated with STIs, and testing and treatment costs.
“Under the Affordable Care Act, USPSTF screening recommendations are supposed to be covered without cost sharing by patients. This should be the case for syphilis screening,” Dr. Gullett pointed out. “Patients are often reluctant to do screening because of cost.”
Michael Anthony Moody, MD, director of the Collaborative Influenza Vaccine Innovation Center at Duke University, Durham, N.C., said that the true incidence and prevalence of syphilis is unknown.
“The more we test, the more accurate our data will be,” he said. “Syphilis can hide in plain sight, has symptoms that mimic many other diseases, and is usually not diagnosed. Reaffirming that testing for syphilis is important reminds providers that this is a key test for their patient’s health.”
Aniruddha Hazra, MD, medical director of the University of Chicago Medicine Sexual Wellness Clinic, noted that the United States is in a syphilis epidemic.
“Screening asymptomatic people at risk for syphilis is important, but without comprehensive education and training of primary care providers on how to address STIs and sexual health, these recommendations fall flat,” he said.
In an accompanying editorial, Susan Tuddenham, MD, MPH; and Khalil G. Ghanem, MD, PhD, of Johns Hopkins University, Baltimore, urged that funding to develop novel syphilis diagnostics be prioritized, “just as there has been for development of syphilis vaccines, which are still many years from becoming a reality.”
“Relying on emerging biomedical prevention interventions that hold promise, such as doxycycline postexposure prophylaxis, without concomitant robust screening strategies will not lead to syphilis control. Failure to modernize screening strategies for syphilis will also mean failure to control this infection,” they cautioned.
The authors of the recommendation statement and the evidence report, as well as Dr. O’Donnell, Dr. Gullettt, Dr. Moody, and Dr. Hazra, who were not involved in the study, reported no relevant financial relationships. Dr. Tuddenham reported financial relationships with the pharmaceutical and publishing industries. Dr. Ghanem reported financial relationships with the publishing industry. The research was federally funded.
A version of this article first appeared on Medscape.com.
FROM JAMA
Meet our newest genetically engineered frenemy, herpes
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Safer opioid supply program in Canada helps those who face overdose risks
An analysis indicates that the program is associated with a reduction in emergency department visits, hospitalizations, and overall health care costs. In addition, there were no opioid-related deaths among participants who were at high risk of overdose.
“Not only did hospital engagements decline immediately after starting SOS programs, but also the risk of overdose did not change, and there were no opioid-related deaths in the 1-year follow-up,” study author Tara Gomes, PhD, an assistant professor of health policy, management, and evaluation at the University of Toronto and a scientist at the Li Ka Shing Knowledge Institute of St. Michael’s Hospital in Toronto, said in an interview.
Dr. Gomes is the lead principal investigator of the Ontario Drug Policy Research Network, a collaboration between researchers and drug policy decision-makers in the province.
“These changes were not seen in a group of similar individuals who lived in the same city – so were exposed to the same illicit drug supply – but who were not part of this program, helping to reinforce that these changes are specific to SOS participation,” she said.
The study was published in the Canadian Medical Association Journal.
Hospital admissions declined
More than 29,000 opioid-related toxicity deaths occurred in Canada between 2016 and 2021, often as a result of high levels of fentanyl in the drug supply, according to the investigators. In response, SOS programs have been launched in several provinces, including the first formal SOS program at the London (Ont.) InterCommunity Health Centre. As part of the program, clients are prescribed pharmaceutical opioids as an alternative to the fentanyl-adulterated drug supply and are given health and social supports.
Dr. Gomes and colleagues conducted an interrupted time series analysis of residents in London, Ont., who had received a diagnosis of opioid use disorder and had had a health care encounter related to the diagnosis between January 2016 and March 2019. They followed 82 participants who entered the SOS program, as well as a comparison group of 303 people who were matched on the basis of demographic and clinical characteristics but who did not participate in the program.
The research team focused on the population’s numbers of emergency department visits, hospital admissions, infection rates, and health care costs. They used autoregressive integrated moving average models to evaluate the effect of starting the SOS program and to compare the population’s outcome rates in the year before and after entering the program.
For participants who entered the program, the rate of emergency department visits declined by about 14 visits per 100 people. In addition, hospital admissions declined by about 5 admissions per 100 people. Health care costs that weren’t related to primary care or outpatient medications declined by about $922 per person. The rate of hospital admission for infections remained about the same; the investigators observed a decline of about 1.6 infections per 100 people.
In the year after entry into the program, emergency department visits, hospital admissions, infection-related admissions, and total health care costs declined significantly among SOS clients, compared with the year before.
Conversely, there were no significant changes in any of the measured outcomes among the 303 people who didn’t participate in the program.
Medication costs increased
DR. Gomes and colleagues noted that the findings provide preliminary evidence that SOS programs can play a role in the harm-reduction options available to those who are at high risk of drug poisoning and overdose. At the same time, many questions remain.
For instance, although total health care costs declined among those enrolled in the program, the medication-related costs increased. About 34% of participants had HIV, 69.5% had hepatitis C virus infection, and 28% had infectious complications in the year before entering the program. This finding may indicate that the participants had serious medical complications resulting from their drug use and were able to seek health care services.
“We interpret that to be a positive finding, because of the very high prevalence of HIV and hepatitis C in the SOS clients. Treatments for HIV and hepatitis C are lifesaving but expensive,” said Dr. Gomes. “Therefore, these higher medication costs are likely reflective of improved access to treatments for these infections, which can greatly improve people’s health and quality of life but also save the health care system money over the longer term.”
DR. Gomes and colleagues are now beginning to evaluate other SOS programs across Ontario. They hope to better understand the various approaches that are available and determine which models can best support people who face high risks because of drug use.
A limited solution?
Commenting on the study, Andrew Ivsins, PhD, a postdoctoral fellow in social medicine at the University of British Columbia in Vancouver and a research scientist at the British Columbia Centre on Substance Abuse, said, “This is an important study and one of the first to show how safe supply can help by building connections to the health care system that didn’t exist previously.”
Dr. Ivsins, who wasn’t involved with this study, has researched safe supply programs around Vancouver. He and colleagues found that among participants in these programs, the use of illicit street-purchased drugs decreased, which led to improved health and wellness.
“Safe supply is fundamentally, at the most basic level, a response to the highly toxic drug supply and out-of-control poisoning crisis in North America,” he said. “It’s a contentious issue, but it makes so much sense that, if what’s killing people is highly toxic drugs, we need to find a way to provide an option that doesn’t kill them.”
“Up to now, safer supply has mostly been used to reduce harms, including mortality and morbidity, in persons using illicit opioids. But if we really want to lower the risk linked to heavy contamination of the unregulated drug supply, safer supply programs will have to be extended to all substances potentially sold illegally,” Marie-Eve Goyer, MD, an assistant professor of family medicine at the University of Montreal, said in an interview.
Dr. Goyer, who wasn’t involved with this study, has conducted research about substance replacement therapy in Quebec. She found that many provinces are now reporting on new potent designer benzodiazepines that are being used or that are contaminating fentanyl, which calls for a broader approach to address the drug overdose crisis.
“Let’s realize that safer supply prescription is a very medicalized (and limited) solution to an epidemic that is made of stigma, criminalization, and repressive public policies,” she said. “Without true changes in the law, we will continue to see our people dying every day.”
The study was funded by grants from the Ontario Ministry of Health and the Canadian Institutes of Health Research. Dr. Gomes has received grants to support the research of both groups, and other authors have received support or fees related to the London InterCommunity Health Centre. Dr. Ivsins and Dr. Goyer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An analysis indicates that the program is associated with a reduction in emergency department visits, hospitalizations, and overall health care costs. In addition, there were no opioid-related deaths among participants who were at high risk of overdose.
“Not only did hospital engagements decline immediately after starting SOS programs, but also the risk of overdose did not change, and there were no opioid-related deaths in the 1-year follow-up,” study author Tara Gomes, PhD, an assistant professor of health policy, management, and evaluation at the University of Toronto and a scientist at the Li Ka Shing Knowledge Institute of St. Michael’s Hospital in Toronto, said in an interview.
Dr. Gomes is the lead principal investigator of the Ontario Drug Policy Research Network, a collaboration between researchers and drug policy decision-makers in the province.
“These changes were not seen in a group of similar individuals who lived in the same city – so were exposed to the same illicit drug supply – but who were not part of this program, helping to reinforce that these changes are specific to SOS participation,” she said.
The study was published in the Canadian Medical Association Journal.
Hospital admissions declined
More than 29,000 opioid-related toxicity deaths occurred in Canada between 2016 and 2021, often as a result of high levels of fentanyl in the drug supply, according to the investigators. In response, SOS programs have been launched in several provinces, including the first formal SOS program at the London (Ont.) InterCommunity Health Centre. As part of the program, clients are prescribed pharmaceutical opioids as an alternative to the fentanyl-adulterated drug supply and are given health and social supports.
Dr. Gomes and colleagues conducted an interrupted time series analysis of residents in London, Ont., who had received a diagnosis of opioid use disorder and had had a health care encounter related to the diagnosis between January 2016 and March 2019. They followed 82 participants who entered the SOS program, as well as a comparison group of 303 people who were matched on the basis of demographic and clinical characteristics but who did not participate in the program.
The research team focused on the population’s numbers of emergency department visits, hospital admissions, infection rates, and health care costs. They used autoregressive integrated moving average models to evaluate the effect of starting the SOS program and to compare the population’s outcome rates in the year before and after entering the program.
For participants who entered the program, the rate of emergency department visits declined by about 14 visits per 100 people. In addition, hospital admissions declined by about 5 admissions per 100 people. Health care costs that weren’t related to primary care or outpatient medications declined by about $922 per person. The rate of hospital admission for infections remained about the same; the investigators observed a decline of about 1.6 infections per 100 people.
In the year after entry into the program, emergency department visits, hospital admissions, infection-related admissions, and total health care costs declined significantly among SOS clients, compared with the year before.
Conversely, there were no significant changes in any of the measured outcomes among the 303 people who didn’t participate in the program.
Medication costs increased
DR. Gomes and colleagues noted that the findings provide preliminary evidence that SOS programs can play a role in the harm-reduction options available to those who are at high risk of drug poisoning and overdose. At the same time, many questions remain.
For instance, although total health care costs declined among those enrolled in the program, the medication-related costs increased. About 34% of participants had HIV, 69.5% had hepatitis C virus infection, and 28% had infectious complications in the year before entering the program. This finding may indicate that the participants had serious medical complications resulting from their drug use and were able to seek health care services.
“We interpret that to be a positive finding, because of the very high prevalence of HIV and hepatitis C in the SOS clients. Treatments for HIV and hepatitis C are lifesaving but expensive,” said Dr. Gomes. “Therefore, these higher medication costs are likely reflective of improved access to treatments for these infections, which can greatly improve people’s health and quality of life but also save the health care system money over the longer term.”
DR. Gomes and colleagues are now beginning to evaluate other SOS programs across Ontario. They hope to better understand the various approaches that are available and determine which models can best support people who face high risks because of drug use.
A limited solution?
Commenting on the study, Andrew Ivsins, PhD, a postdoctoral fellow in social medicine at the University of British Columbia in Vancouver and a research scientist at the British Columbia Centre on Substance Abuse, said, “This is an important study and one of the first to show how safe supply can help by building connections to the health care system that didn’t exist previously.”
Dr. Ivsins, who wasn’t involved with this study, has researched safe supply programs around Vancouver. He and colleagues found that among participants in these programs, the use of illicit street-purchased drugs decreased, which led to improved health and wellness.
“Safe supply is fundamentally, at the most basic level, a response to the highly toxic drug supply and out-of-control poisoning crisis in North America,” he said. “It’s a contentious issue, but it makes so much sense that, if what’s killing people is highly toxic drugs, we need to find a way to provide an option that doesn’t kill them.”
“Up to now, safer supply has mostly been used to reduce harms, including mortality and morbidity, in persons using illicit opioids. But if we really want to lower the risk linked to heavy contamination of the unregulated drug supply, safer supply programs will have to be extended to all substances potentially sold illegally,” Marie-Eve Goyer, MD, an assistant professor of family medicine at the University of Montreal, said in an interview.
Dr. Goyer, who wasn’t involved with this study, has conducted research about substance replacement therapy in Quebec. She found that many provinces are now reporting on new potent designer benzodiazepines that are being used or that are contaminating fentanyl, which calls for a broader approach to address the drug overdose crisis.
“Let’s realize that safer supply prescription is a very medicalized (and limited) solution to an epidemic that is made of stigma, criminalization, and repressive public policies,” she said. “Without true changes in the law, we will continue to see our people dying every day.”
The study was funded by grants from the Ontario Ministry of Health and the Canadian Institutes of Health Research. Dr. Gomes has received grants to support the research of both groups, and other authors have received support or fees related to the London InterCommunity Health Centre. Dr. Ivsins and Dr. Goyer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An analysis indicates that the program is associated with a reduction in emergency department visits, hospitalizations, and overall health care costs. In addition, there were no opioid-related deaths among participants who were at high risk of overdose.
“Not only did hospital engagements decline immediately after starting SOS programs, but also the risk of overdose did not change, and there were no opioid-related deaths in the 1-year follow-up,” study author Tara Gomes, PhD, an assistant professor of health policy, management, and evaluation at the University of Toronto and a scientist at the Li Ka Shing Knowledge Institute of St. Michael’s Hospital in Toronto, said in an interview.
Dr. Gomes is the lead principal investigator of the Ontario Drug Policy Research Network, a collaboration between researchers and drug policy decision-makers in the province.
“These changes were not seen in a group of similar individuals who lived in the same city – so were exposed to the same illicit drug supply – but who were not part of this program, helping to reinforce that these changes are specific to SOS participation,” she said.
The study was published in the Canadian Medical Association Journal.
Hospital admissions declined
More than 29,000 opioid-related toxicity deaths occurred in Canada between 2016 and 2021, often as a result of high levels of fentanyl in the drug supply, according to the investigators. In response, SOS programs have been launched in several provinces, including the first formal SOS program at the London (Ont.) InterCommunity Health Centre. As part of the program, clients are prescribed pharmaceutical opioids as an alternative to the fentanyl-adulterated drug supply and are given health and social supports.
Dr. Gomes and colleagues conducted an interrupted time series analysis of residents in London, Ont., who had received a diagnosis of opioid use disorder and had had a health care encounter related to the diagnosis between January 2016 and March 2019. They followed 82 participants who entered the SOS program, as well as a comparison group of 303 people who were matched on the basis of demographic and clinical characteristics but who did not participate in the program.
The research team focused on the population’s numbers of emergency department visits, hospital admissions, infection rates, and health care costs. They used autoregressive integrated moving average models to evaluate the effect of starting the SOS program and to compare the population’s outcome rates in the year before and after entering the program.
For participants who entered the program, the rate of emergency department visits declined by about 14 visits per 100 people. In addition, hospital admissions declined by about 5 admissions per 100 people. Health care costs that weren’t related to primary care or outpatient medications declined by about $922 per person. The rate of hospital admission for infections remained about the same; the investigators observed a decline of about 1.6 infections per 100 people.
In the year after entry into the program, emergency department visits, hospital admissions, infection-related admissions, and total health care costs declined significantly among SOS clients, compared with the year before.
Conversely, there were no significant changes in any of the measured outcomes among the 303 people who didn’t participate in the program.
Medication costs increased
DR. Gomes and colleagues noted that the findings provide preliminary evidence that SOS programs can play a role in the harm-reduction options available to those who are at high risk of drug poisoning and overdose. At the same time, many questions remain.
For instance, although total health care costs declined among those enrolled in the program, the medication-related costs increased. About 34% of participants had HIV, 69.5% had hepatitis C virus infection, and 28% had infectious complications in the year before entering the program. This finding may indicate that the participants had serious medical complications resulting from their drug use and were able to seek health care services.
“We interpret that to be a positive finding, because of the very high prevalence of HIV and hepatitis C in the SOS clients. Treatments for HIV and hepatitis C are lifesaving but expensive,” said Dr. Gomes. “Therefore, these higher medication costs are likely reflective of improved access to treatments for these infections, which can greatly improve people’s health and quality of life but also save the health care system money over the longer term.”
DR. Gomes and colleagues are now beginning to evaluate other SOS programs across Ontario. They hope to better understand the various approaches that are available and determine which models can best support people who face high risks because of drug use.
A limited solution?
Commenting on the study, Andrew Ivsins, PhD, a postdoctoral fellow in social medicine at the University of British Columbia in Vancouver and a research scientist at the British Columbia Centre on Substance Abuse, said, “This is an important study and one of the first to show how safe supply can help by building connections to the health care system that didn’t exist previously.”
Dr. Ivsins, who wasn’t involved with this study, has researched safe supply programs around Vancouver. He and colleagues found that among participants in these programs, the use of illicit street-purchased drugs decreased, which led to improved health and wellness.
“Safe supply is fundamentally, at the most basic level, a response to the highly toxic drug supply and out-of-control poisoning crisis in North America,” he said. “It’s a contentious issue, but it makes so much sense that, if what’s killing people is highly toxic drugs, we need to find a way to provide an option that doesn’t kill them.”
“Up to now, safer supply has mostly been used to reduce harms, including mortality and morbidity, in persons using illicit opioids. But if we really want to lower the risk linked to heavy contamination of the unregulated drug supply, safer supply programs will have to be extended to all substances potentially sold illegally,” Marie-Eve Goyer, MD, an assistant professor of family medicine at the University of Montreal, said in an interview.
Dr. Goyer, who wasn’t involved with this study, has conducted research about substance replacement therapy in Quebec. She found that many provinces are now reporting on new potent designer benzodiazepines that are being used or that are contaminating fentanyl, which calls for a broader approach to address the drug overdose crisis.
“Let’s realize that safer supply prescription is a very medicalized (and limited) solution to an epidemic that is made of stigma, criminalization, and repressive public policies,” she said. “Without true changes in the law, we will continue to see our people dying every day.”
The study was funded by grants from the Ontario Ministry of Health and the Canadian Institutes of Health Research. Dr. Gomes has received grants to support the research of both groups, and other authors have received support or fees related to the London InterCommunity Health Centre. Dr. Ivsins and Dr. Goyer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANADIAN MEDICAL ASSOCIATION JOURNAL
Continued monkeypox spread can lead to viral mutations
Monkeypox cases are declining in the United States and the United Kingdom, but experts are urging the public to continue efforts to stanch the spread of the virus. Continued transmission of monkeypox provides more opportunities for the virus to mutate, according to Philip Johnson, PhD, assistant professor of biology at the University of Maryland, College Park, and colleagues.
the authors wrote in a correspondence published in The Lancet.
When case numbers are lower – and therefore less of a public health concern – viral transmission chains can be longer without causing alarm, Dr. Johnson explained. “The more generations of transmission, the more opportunities there are for mutations to occur,” he told this news organization. While it is difficult to anticipate how mutations can affect a virus, these changes in genetic code could be advantageous to the virus, making it more transmissible from human to human and therefore much more difficult to control.
This applies to any virus. The large Ebola outbreak from 2013 to 2016 is an example; a retrospective analysis found that specific amino acid changes in the Ebola virus increased growth in human cells and may have made the virus more infectious. More recently, the Delta and Omicron variants of SARS-CoV-2 each contained mutations that were associated with higher transmissibility. A recent study suggested that monkeypox appears to be mutating faster than expected, though it is not clear if these genetic mutations have changed the virus’ behavior.
Zoonotic infections, or viruses that originate from nonhuman animals, at first are expected to be less adapted to people, but that can change over time. When a virus continues to jump from animals to humans – as monkeypox has done since it was first identified in humans in 1970 – chances are it will gain a mutation that allows it to spread more effectively between people, said Rachel Roper, PhD, a professor of microbiology and immunology at East Carolina University, Greenville, N.C. She was not involved with The Lancet article.
“We discounted monkeypox; we didn’t pay much attention to it because it had not been that big of a problem,” she said in an interview. “We think this virus has been circulating now since 2017 and we really just realized it in May.”
Although monkeypox received global attention this past summer, the outbreak is now receiving less news coverage, and the public’s attention may be waning. Furthermore, the U.S. Congress just dropped billions of dollars from a short-term spending bill that would have provided additional COVID-19 and monkeypox funding.
Although new cases are trending downward, now is not the time to take our foot off the gas, Dr. Johnson and colleagues warned. “The epidemic is far from over, and continued drive toward elimination is essential,” the authors wrote. Because the virus exists in rodent populations in areas of central and west Africa, it is not possible to eradicate monkeypox as we did smallpox; however, “we could, through vaccination, eliminate any significant human to human transmission,” Dr. Johnson said.
Dr. Johnson also urges a more proactive approach to combating emerging infectious diseases in the future. “We wrote this article to raise awareness about the importance of dedicating resources to controlling these diseases all the way down to ideally elimination in the countries where they develop, and not just waiting until [these diseases] reach wealthier countries,” he said.
Dr. Roper agrees that a more global perspective is needed in monitoring and controlling zoonotic disease, but resources are limited. “The problem is there are a whole bunch of virus groups and a whole bunch of viruses jumping into humans all the time,” she said. “We can’t predict which virus group is going to be the next one with a big hit. I worked on SARS-CoV-1 back in 2003 to 2009, and I would have predicted that a virus from some other group would have jumped into humans next, before COVID hit,” she added.
Dr. Johnson acknowledged that it is hard to know where to focus public health resources, considering the hundreds of thousands of zoonotic viruses that may exist. He thought the best approach was to target emerging diseases that already appear to have extended transmission chains, “not just things that are hopping from animals to humans and sputtering out and disappearing, but diseases that appear to have any sustained human to human transmission.”
Dr. Johnson and Dr. Roper report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox cases are declining in the United States and the United Kingdom, but experts are urging the public to continue efforts to stanch the spread of the virus. Continued transmission of monkeypox provides more opportunities for the virus to mutate, according to Philip Johnson, PhD, assistant professor of biology at the University of Maryland, College Park, and colleagues.
the authors wrote in a correspondence published in The Lancet.
When case numbers are lower – and therefore less of a public health concern – viral transmission chains can be longer without causing alarm, Dr. Johnson explained. “The more generations of transmission, the more opportunities there are for mutations to occur,” he told this news organization. While it is difficult to anticipate how mutations can affect a virus, these changes in genetic code could be advantageous to the virus, making it more transmissible from human to human and therefore much more difficult to control.
This applies to any virus. The large Ebola outbreak from 2013 to 2016 is an example; a retrospective analysis found that specific amino acid changes in the Ebola virus increased growth in human cells and may have made the virus more infectious. More recently, the Delta and Omicron variants of SARS-CoV-2 each contained mutations that were associated with higher transmissibility. A recent study suggested that monkeypox appears to be mutating faster than expected, though it is not clear if these genetic mutations have changed the virus’ behavior.
Zoonotic infections, or viruses that originate from nonhuman animals, at first are expected to be less adapted to people, but that can change over time. When a virus continues to jump from animals to humans – as monkeypox has done since it was first identified in humans in 1970 – chances are it will gain a mutation that allows it to spread more effectively between people, said Rachel Roper, PhD, a professor of microbiology and immunology at East Carolina University, Greenville, N.C. She was not involved with The Lancet article.
“We discounted monkeypox; we didn’t pay much attention to it because it had not been that big of a problem,” she said in an interview. “We think this virus has been circulating now since 2017 and we really just realized it in May.”
Although monkeypox received global attention this past summer, the outbreak is now receiving less news coverage, and the public’s attention may be waning. Furthermore, the U.S. Congress just dropped billions of dollars from a short-term spending bill that would have provided additional COVID-19 and monkeypox funding.
Although new cases are trending downward, now is not the time to take our foot off the gas, Dr. Johnson and colleagues warned. “The epidemic is far from over, and continued drive toward elimination is essential,” the authors wrote. Because the virus exists in rodent populations in areas of central and west Africa, it is not possible to eradicate monkeypox as we did smallpox; however, “we could, through vaccination, eliminate any significant human to human transmission,” Dr. Johnson said.
Dr. Johnson also urges a more proactive approach to combating emerging infectious diseases in the future. “We wrote this article to raise awareness about the importance of dedicating resources to controlling these diseases all the way down to ideally elimination in the countries where they develop, and not just waiting until [these diseases] reach wealthier countries,” he said.
Dr. Roper agrees that a more global perspective is needed in monitoring and controlling zoonotic disease, but resources are limited. “The problem is there are a whole bunch of virus groups and a whole bunch of viruses jumping into humans all the time,” she said. “We can’t predict which virus group is going to be the next one with a big hit. I worked on SARS-CoV-1 back in 2003 to 2009, and I would have predicted that a virus from some other group would have jumped into humans next, before COVID hit,” she added.
Dr. Johnson acknowledged that it is hard to know where to focus public health resources, considering the hundreds of thousands of zoonotic viruses that may exist. He thought the best approach was to target emerging diseases that already appear to have extended transmission chains, “not just things that are hopping from animals to humans and sputtering out and disappearing, but diseases that appear to have any sustained human to human transmission.”
Dr. Johnson and Dr. Roper report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox cases are declining in the United States and the United Kingdom, but experts are urging the public to continue efforts to stanch the spread of the virus. Continued transmission of monkeypox provides more opportunities for the virus to mutate, according to Philip Johnson, PhD, assistant professor of biology at the University of Maryland, College Park, and colleagues.
the authors wrote in a correspondence published in The Lancet.
When case numbers are lower – and therefore less of a public health concern – viral transmission chains can be longer without causing alarm, Dr. Johnson explained. “The more generations of transmission, the more opportunities there are for mutations to occur,” he told this news organization. While it is difficult to anticipate how mutations can affect a virus, these changes in genetic code could be advantageous to the virus, making it more transmissible from human to human and therefore much more difficult to control.
This applies to any virus. The large Ebola outbreak from 2013 to 2016 is an example; a retrospective analysis found that specific amino acid changes in the Ebola virus increased growth in human cells and may have made the virus more infectious. More recently, the Delta and Omicron variants of SARS-CoV-2 each contained mutations that were associated with higher transmissibility. A recent study suggested that monkeypox appears to be mutating faster than expected, though it is not clear if these genetic mutations have changed the virus’ behavior.
Zoonotic infections, or viruses that originate from nonhuman animals, at first are expected to be less adapted to people, but that can change over time. When a virus continues to jump from animals to humans – as monkeypox has done since it was first identified in humans in 1970 – chances are it will gain a mutation that allows it to spread more effectively between people, said Rachel Roper, PhD, a professor of microbiology and immunology at East Carolina University, Greenville, N.C. She was not involved with The Lancet article.
“We discounted monkeypox; we didn’t pay much attention to it because it had not been that big of a problem,” she said in an interview. “We think this virus has been circulating now since 2017 and we really just realized it in May.”
Although monkeypox received global attention this past summer, the outbreak is now receiving less news coverage, and the public’s attention may be waning. Furthermore, the U.S. Congress just dropped billions of dollars from a short-term spending bill that would have provided additional COVID-19 and monkeypox funding.
Although new cases are trending downward, now is not the time to take our foot off the gas, Dr. Johnson and colleagues warned. “The epidemic is far from over, and continued drive toward elimination is essential,” the authors wrote. Because the virus exists in rodent populations in areas of central and west Africa, it is not possible to eradicate monkeypox as we did smallpox; however, “we could, through vaccination, eliminate any significant human to human transmission,” Dr. Johnson said.
Dr. Johnson also urges a more proactive approach to combating emerging infectious diseases in the future. “We wrote this article to raise awareness about the importance of dedicating resources to controlling these diseases all the way down to ideally elimination in the countries where they develop, and not just waiting until [these diseases] reach wealthier countries,” he said.
Dr. Roper agrees that a more global perspective is needed in monitoring and controlling zoonotic disease, but resources are limited. “The problem is there are a whole bunch of virus groups and a whole bunch of viruses jumping into humans all the time,” she said. “We can’t predict which virus group is going to be the next one with a big hit. I worked on SARS-CoV-1 back in 2003 to 2009, and I would have predicted that a virus from some other group would have jumped into humans next, before COVID hit,” she added.
Dr. Johnson acknowledged that it is hard to know where to focus public health resources, considering the hundreds of thousands of zoonotic viruses that may exist. He thought the best approach was to target emerging diseases that already appear to have extended transmission chains, “not just things that are hopping from animals to humans and sputtering out and disappearing, but diseases that appear to have any sustained human to human transmission.”
Dr. Johnson and Dr. Roper report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Could a vaccine (and more) fix the fentanyl crisis?
This discussion was recorded on Aug. 31, 2022. This transcript has been edited for clarity.
Robert Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have Dr. Paul Christo, a pain specialist in the Division of Pain Medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland, and host of the national radio show Aches and Gains on SiriusXM Radio, joining us to discuss the ongoing and worsening fentanyl crisis in the U.S.
Welcome, Dr Christo.
Paul J. Christo, MD, MBA: Thanks so much for having me.
Dr. Glatter: I want to begin with a sobering statistic regarding overdoses. , based on recent data from the CDC.
Let’s start by having you explain how deadly fentanyl is in terms of its potency compared with morphine and heroin.
Dr. Christo: Fentanyl is considered a synthetic opioid. It’s not a naturally occurring opioid like morphine, for example, or codeine. We use this drug, fentanyl, often in the anesthesia well. We’ve used it for many years as an anesthetic for surgery very safely. In the chronic pain world, we’ve used it to help reduce chronic pain in the form of a patch.
What we’re seeing now, though, is something entirely different, which is the use of synthetic fentanyl as a mind- and mood-altering substance for those who don’t have pain, and essentially those who are buying this off the street. Fentanyl is about 80-100 times more potent than morphine, so you can put that in perspective in terms of its danger.
Dr. Glatter: Let me have you take us through an evolution of the opioid crisis from the 1990s, from long-acting opioid OxyContin, which was approved in 1995, to where we are now. There are different phases. If you could, educate our audience on how we got to where fentanyl is now the most common opiate involved in drug overdoses.
Dr. Christo: It really stems from the epidemic related to chronic pain. We have over 100 million people in the United States alone who suffer from chronic pain. Most chronic pain, sadly, is undertreated or untreated. In the ‘90s, in the quest to reduce chronic pain to a better extent, we saw more and more literature and studies related to the use of opioids for noncancer pain (e.g., for lower back pain).
There were many primary care doctors and pain specialists who started using opioids, probably for patients who didn’t really need it. I think it was done out of good conscience in the sense that they were trying to reduce pain. We have other methods of pain relief, but we needed more. At that time, in the ‘90s, we had a greater use of opioids to treat noncancer pain.
Then from that point, we transitioned to the use of heroin. Again, this isn’t among the chronic pain population, but it was the nonchronic pain population that starting using heroin. Today we see synthetic fentanyl.
Addressing the synthetic opioid crisis
Dr. Glatter: With fentanyl being the most common opiate we’re seeing, we’re having problems trying to save patients. We’re trying to use naloxone, but obviously in increasing amounts, and sometimes it’s not adequate and we have to intubate patients.
In terms of addressing this issue of supply, the fentanyl is coming from Mexico, China, and it’s manufactured here in the United States. How do we address this crisis? What are the steps that you would recommend we take?
Dr. Christo: I think that we need to better support law enforcement to crack down on those who are manufacturing fentanyl in the United States, and also to crack down on those who are transporting it from, say, Mexico – I think it’s primarily coming from Mexico – but from outside the United States to the United States. I feel like that’s important to do.
Two, we need to better educate those who are using these mind- and mood-altering substances. We’re seeing more and more that it’s the young-adult population, those between the ages of 13 and 25, who are starting to use these substances, and they’re very dangerous.
Dr. Glatter: Are these teens seeking out heroin and it happens to be laced with fentanyl, or are they actually seeking pure fentanyl? Are they trying to buy the colorful pills that we know about? What’s your experience in terms of the population you’re treating and what you could tell us?
Dr. Christo: I think it’s both. We’re seeing young adults who are interested in the use of fentanyl as a mind- and mood-altering substance. We’re also seeing young and older adults use other drugs, like cocaine and heroin, that are laced with fentanyl, and they don’t know it. That’s exponentially more dangerous.
Fentanyl test strips
Dr. Glatter: People are unaware that there is fentanyl in what they’re using, and it is certainly leading to overdoses and deaths. I think that parents really need to be aware of this.
Dr. Christo: Yes, for sure. I think we need better educational methods in the schools to educate that population that we’re talking about (between the ages of 13 and 25). Let them know the dangers, because I don’t think they’re aware of the danger, and how potent fentanyl is in terms of its lethality, and that you don’t need very much to take in a form of a pill or to inhale or to inject intravenously to kill yourself. That is key – education at that level – and to let those who are going to use these substances (specifically, synthetic fentanyl) know that they should consider the use of fentanyl test strips.
Fentanyl test strips would be primarily used for those who are thinking that they’re using heroin but there may be fentanyl in there, or methamphetamine and there may be fentanyl, and they don’t know. The test strip gives them that knowledge.
The other harm reduction strategies would be the use of naloxone, known as Narcan. That’s a lifesaver. You just have to spritz it into the nostril. You don’t do it yourself if you’re using the substance, but you’ve got others who can do it for you. No question, that’s a lifesaver. We need to make sure that there’s greater availability of that throughout the entire country, and we’re seeing some of that in certain states. In certain states, you don’t need a prescription to get naloxone from the pharmacy.
Dr. Glatter: I think it’s so important that it should be widely available. Certainly, the COVID-19 pandemic exacerbated the number of overdoses we saw. Are overdoses coming down or are we still at a level that’s close to 2020?
Dr. Christo: Unfortunately, we’re still seeing the same level, if not seeing it escalate. Certainly, the pandemic, because of the economic cost associated with the pandemic – loss of employment, underemployment – as well as the emotional stress of the pandemic led many people to use substances on the street in order to cope. They’re coping mechanisms, and we really haven’t seen it abate quite yet.
Dr. Glatter: Do you have a message for the lawmakers on Capitol Hill as to what we can do regarding the illegal manufacturing and distribution, how we can really crack down? Are there other approaches that we could implement that might be more tangible?
Dr. Christo: Yes. No. 1 would be to support law enforcement. No. 2 would be to create and make available more overdose prevention centers. The first was in New York City. If you look at the data on overdose prevention centers, in Canada, for example, they’ve seen a 35% reduction in overdose deaths. These are places where people who are using can go to get clean needles and clean syringes. This is where people basically oversee the use of the drug and intervene if necessary.
It seems sort of antithetical. It seems like, “Boy, why would you fund a center for people to use drugs?” The data from Canada and outside Canada are such that it can be very helpful. That would be one of my messages to lawmakers as well.
Vaccines to combat the synthetic opioid crisis
Dr. Glatter: Do you think that the legislators could approach some of these factories as a way to crack down, and have law enforcement be more aggressive? Is that another possible solution?
Dr. Christo: It is. Law enforcement needs to be supported by the government, by the Biden administration, so that we can prevent the influx of fentanyl and other drugs into the United States, and also to crack down on those in the United States who are manufacturing these drugs – synthetic fentanyl, first and foremost – because we’re seeing a lot of deaths related to synthetic fentanyl.
Also, we’re seeing — and this is pretty intriguing and interesting – the use of vaccines to help prevent overdose. The first human trial is underway right now for a vaccine against oxycodone. Not only that, but there are other vaccines that are in animal trials now against heroin, cocaine, or fentanyl. There’s hope there that we can use vaccines to also help reduce deaths related to overdose from fentanyl and other opioids.
Dr. Glatter: Do you think this would be given widely to the population or only to those at higher risk?
Dr. Christo: It would probably be targeting those who are at higher risk and have a history of drug abuse. I don’t think it would be something that would be given to the entire population, but it certainly could be effective, and we’re seeing encouraging results from the human trial right now.
Dr. Glatter: That’s very intriguing. That’s something that certainly could be quite helpful in the future.
One thing I did want to address is law enforcement and first responders who have been exposed to dust, or inhaled dust possibly, or had fentanyl on their skin. There has been lots of controversy. The recent literature has dispelled the controversy that people who had supposedly passed out and required Narcan after exposure to intact skin, or even compromised skin, had an overdose of fentanyl. Maybe you could speak to that and dispel that myth.
Dr. Christo: Yes, I’ve been asked this question a couple of times in the past. It’s not sufficient just to have contact with fentanyl on the skin to lead to an overdose. You really need to ingest it. That is, take it by mouth in the form of a pill, inhale it, or inject it intravenously. Skin contact is very unlikely going to lead to an overdose and death.
Dr. Glatter: I want to thank you for a very informative interview. Do you have one or two pearls you’d like to give our audience as a takeaway?
Dr. Christo: I would say two things. One is, don’t give up if you have chronic pain because there is hope. We have nonopioid treatments that can be effective. Two, don’t give up if you have a substance use disorder. Talk to your primary care doctor or talk to emergency room physicians if you’re in the emergency room. The Substance Abuse and Mental Health Services Administration is a good resource, too. SAMHSA has an 800 number for support and a website. Take the opportunity to use the resources that are available.
Dr. Glatter is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Hofstra University, Hempstead, N.Y. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Christo is an associate professor and a pain specialist in the department of anesthesiology and critical care medicine at Johns Hopkins University, Baltimore. He also serves as director of the multidisciplinary pain fellowship program at Johns Hopkins Hospital. Christo is the author of Aches and Gains, A Comprehensive Guide to Overcoming Your Pain, and hosts an award-winning, nationally syndicated SiriusXM radio talk show on overcoming pain, called Aches and Gains.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Aug. 31, 2022. This transcript has been edited for clarity.
Robert Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have Dr. Paul Christo, a pain specialist in the Division of Pain Medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland, and host of the national radio show Aches and Gains on SiriusXM Radio, joining us to discuss the ongoing and worsening fentanyl crisis in the U.S.
Welcome, Dr Christo.
Paul J. Christo, MD, MBA: Thanks so much for having me.
Dr. Glatter: I want to begin with a sobering statistic regarding overdoses. , based on recent data from the CDC.
Let’s start by having you explain how deadly fentanyl is in terms of its potency compared with morphine and heroin.
Dr. Christo: Fentanyl is considered a synthetic opioid. It’s not a naturally occurring opioid like morphine, for example, or codeine. We use this drug, fentanyl, often in the anesthesia well. We’ve used it for many years as an anesthetic for surgery very safely. In the chronic pain world, we’ve used it to help reduce chronic pain in the form of a patch.
What we’re seeing now, though, is something entirely different, which is the use of synthetic fentanyl as a mind- and mood-altering substance for those who don’t have pain, and essentially those who are buying this off the street. Fentanyl is about 80-100 times more potent than morphine, so you can put that in perspective in terms of its danger.
Dr. Glatter: Let me have you take us through an evolution of the opioid crisis from the 1990s, from long-acting opioid OxyContin, which was approved in 1995, to where we are now. There are different phases. If you could, educate our audience on how we got to where fentanyl is now the most common opiate involved in drug overdoses.
Dr. Christo: It really stems from the epidemic related to chronic pain. We have over 100 million people in the United States alone who suffer from chronic pain. Most chronic pain, sadly, is undertreated or untreated. In the ‘90s, in the quest to reduce chronic pain to a better extent, we saw more and more literature and studies related to the use of opioids for noncancer pain (e.g., for lower back pain).
There were many primary care doctors and pain specialists who started using opioids, probably for patients who didn’t really need it. I think it was done out of good conscience in the sense that they were trying to reduce pain. We have other methods of pain relief, but we needed more. At that time, in the ‘90s, we had a greater use of opioids to treat noncancer pain.
Then from that point, we transitioned to the use of heroin. Again, this isn’t among the chronic pain population, but it was the nonchronic pain population that starting using heroin. Today we see synthetic fentanyl.
Addressing the synthetic opioid crisis
Dr. Glatter: With fentanyl being the most common opiate we’re seeing, we’re having problems trying to save patients. We’re trying to use naloxone, but obviously in increasing amounts, and sometimes it’s not adequate and we have to intubate patients.
In terms of addressing this issue of supply, the fentanyl is coming from Mexico, China, and it’s manufactured here in the United States. How do we address this crisis? What are the steps that you would recommend we take?
Dr. Christo: I think that we need to better support law enforcement to crack down on those who are manufacturing fentanyl in the United States, and also to crack down on those who are transporting it from, say, Mexico – I think it’s primarily coming from Mexico – but from outside the United States to the United States. I feel like that’s important to do.
Two, we need to better educate those who are using these mind- and mood-altering substances. We’re seeing more and more that it’s the young-adult population, those between the ages of 13 and 25, who are starting to use these substances, and they’re very dangerous.
Dr. Glatter: Are these teens seeking out heroin and it happens to be laced with fentanyl, or are they actually seeking pure fentanyl? Are they trying to buy the colorful pills that we know about? What’s your experience in terms of the population you’re treating and what you could tell us?
Dr. Christo: I think it’s both. We’re seeing young adults who are interested in the use of fentanyl as a mind- and mood-altering substance. We’re also seeing young and older adults use other drugs, like cocaine and heroin, that are laced with fentanyl, and they don’t know it. That’s exponentially more dangerous.
Fentanyl test strips
Dr. Glatter: People are unaware that there is fentanyl in what they’re using, and it is certainly leading to overdoses and deaths. I think that parents really need to be aware of this.
Dr. Christo: Yes, for sure. I think we need better educational methods in the schools to educate that population that we’re talking about (between the ages of 13 and 25). Let them know the dangers, because I don’t think they’re aware of the danger, and how potent fentanyl is in terms of its lethality, and that you don’t need very much to take in a form of a pill or to inhale or to inject intravenously to kill yourself. That is key – education at that level – and to let those who are going to use these substances (specifically, synthetic fentanyl) know that they should consider the use of fentanyl test strips.
Fentanyl test strips would be primarily used for those who are thinking that they’re using heroin but there may be fentanyl in there, or methamphetamine and there may be fentanyl, and they don’t know. The test strip gives them that knowledge.
The other harm reduction strategies would be the use of naloxone, known as Narcan. That’s a lifesaver. You just have to spritz it into the nostril. You don’t do it yourself if you’re using the substance, but you’ve got others who can do it for you. No question, that’s a lifesaver. We need to make sure that there’s greater availability of that throughout the entire country, and we’re seeing some of that in certain states. In certain states, you don’t need a prescription to get naloxone from the pharmacy.
Dr. Glatter: I think it’s so important that it should be widely available. Certainly, the COVID-19 pandemic exacerbated the number of overdoses we saw. Are overdoses coming down or are we still at a level that’s close to 2020?
Dr. Christo: Unfortunately, we’re still seeing the same level, if not seeing it escalate. Certainly, the pandemic, because of the economic cost associated with the pandemic – loss of employment, underemployment – as well as the emotional stress of the pandemic led many people to use substances on the street in order to cope. They’re coping mechanisms, and we really haven’t seen it abate quite yet.
Dr. Glatter: Do you have a message for the lawmakers on Capitol Hill as to what we can do regarding the illegal manufacturing and distribution, how we can really crack down? Are there other approaches that we could implement that might be more tangible?
Dr. Christo: Yes. No. 1 would be to support law enforcement. No. 2 would be to create and make available more overdose prevention centers. The first was in New York City. If you look at the data on overdose prevention centers, in Canada, for example, they’ve seen a 35% reduction in overdose deaths. These are places where people who are using can go to get clean needles and clean syringes. This is where people basically oversee the use of the drug and intervene if necessary.
It seems sort of antithetical. It seems like, “Boy, why would you fund a center for people to use drugs?” The data from Canada and outside Canada are such that it can be very helpful. That would be one of my messages to lawmakers as well.
Vaccines to combat the synthetic opioid crisis
Dr. Glatter: Do you think that the legislators could approach some of these factories as a way to crack down, and have law enforcement be more aggressive? Is that another possible solution?
Dr. Christo: It is. Law enforcement needs to be supported by the government, by the Biden administration, so that we can prevent the influx of fentanyl and other drugs into the United States, and also to crack down on those in the United States who are manufacturing these drugs – synthetic fentanyl, first and foremost – because we’re seeing a lot of deaths related to synthetic fentanyl.
Also, we’re seeing — and this is pretty intriguing and interesting – the use of vaccines to help prevent overdose. The first human trial is underway right now for a vaccine against oxycodone. Not only that, but there are other vaccines that are in animal trials now against heroin, cocaine, or fentanyl. There’s hope there that we can use vaccines to also help reduce deaths related to overdose from fentanyl and other opioids.
Dr. Glatter: Do you think this would be given widely to the population or only to those at higher risk?
Dr. Christo: It would probably be targeting those who are at higher risk and have a history of drug abuse. I don’t think it would be something that would be given to the entire population, but it certainly could be effective, and we’re seeing encouraging results from the human trial right now.
Dr. Glatter: That’s very intriguing. That’s something that certainly could be quite helpful in the future.
One thing I did want to address is law enforcement and first responders who have been exposed to dust, or inhaled dust possibly, or had fentanyl on their skin. There has been lots of controversy. The recent literature has dispelled the controversy that people who had supposedly passed out and required Narcan after exposure to intact skin, or even compromised skin, had an overdose of fentanyl. Maybe you could speak to that and dispel that myth.
Dr. Christo: Yes, I’ve been asked this question a couple of times in the past. It’s not sufficient just to have contact with fentanyl on the skin to lead to an overdose. You really need to ingest it. That is, take it by mouth in the form of a pill, inhale it, or inject it intravenously. Skin contact is very unlikely going to lead to an overdose and death.
Dr. Glatter: I want to thank you for a very informative interview. Do you have one or two pearls you’d like to give our audience as a takeaway?
Dr. Christo: I would say two things. One is, don’t give up if you have chronic pain because there is hope. We have nonopioid treatments that can be effective. Two, don’t give up if you have a substance use disorder. Talk to your primary care doctor or talk to emergency room physicians if you’re in the emergency room. The Substance Abuse and Mental Health Services Administration is a good resource, too. SAMHSA has an 800 number for support and a website. Take the opportunity to use the resources that are available.
Dr. Glatter is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Hofstra University, Hempstead, N.Y. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Christo is an associate professor and a pain specialist in the department of anesthesiology and critical care medicine at Johns Hopkins University, Baltimore. He also serves as director of the multidisciplinary pain fellowship program at Johns Hopkins Hospital. Christo is the author of Aches and Gains, A Comprehensive Guide to Overcoming Your Pain, and hosts an award-winning, nationally syndicated SiriusXM radio talk show on overcoming pain, called Aches and Gains.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Aug. 31, 2022. This transcript has been edited for clarity.
Robert Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have Dr. Paul Christo, a pain specialist in the Division of Pain Medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland, and host of the national radio show Aches and Gains on SiriusXM Radio, joining us to discuss the ongoing and worsening fentanyl crisis in the U.S.
Welcome, Dr Christo.
Paul J. Christo, MD, MBA: Thanks so much for having me.
Dr. Glatter: I want to begin with a sobering statistic regarding overdoses. , based on recent data from the CDC.
Let’s start by having you explain how deadly fentanyl is in terms of its potency compared with morphine and heroin.
Dr. Christo: Fentanyl is considered a synthetic opioid. It’s not a naturally occurring opioid like morphine, for example, or codeine. We use this drug, fentanyl, often in the anesthesia well. We’ve used it for many years as an anesthetic for surgery very safely. In the chronic pain world, we’ve used it to help reduce chronic pain in the form of a patch.
What we’re seeing now, though, is something entirely different, which is the use of synthetic fentanyl as a mind- and mood-altering substance for those who don’t have pain, and essentially those who are buying this off the street. Fentanyl is about 80-100 times more potent than morphine, so you can put that in perspective in terms of its danger.
Dr. Glatter: Let me have you take us through an evolution of the opioid crisis from the 1990s, from long-acting opioid OxyContin, which was approved in 1995, to where we are now. There are different phases. If you could, educate our audience on how we got to where fentanyl is now the most common opiate involved in drug overdoses.
Dr. Christo: It really stems from the epidemic related to chronic pain. We have over 100 million people in the United States alone who suffer from chronic pain. Most chronic pain, sadly, is undertreated or untreated. In the ‘90s, in the quest to reduce chronic pain to a better extent, we saw more and more literature and studies related to the use of opioids for noncancer pain (e.g., for lower back pain).
There were many primary care doctors and pain specialists who started using opioids, probably for patients who didn’t really need it. I think it was done out of good conscience in the sense that they were trying to reduce pain. We have other methods of pain relief, but we needed more. At that time, in the ‘90s, we had a greater use of opioids to treat noncancer pain.
Then from that point, we transitioned to the use of heroin. Again, this isn’t among the chronic pain population, but it was the nonchronic pain population that starting using heroin. Today we see synthetic fentanyl.
Addressing the synthetic opioid crisis
Dr. Glatter: With fentanyl being the most common opiate we’re seeing, we’re having problems trying to save patients. We’re trying to use naloxone, but obviously in increasing amounts, and sometimes it’s not adequate and we have to intubate patients.
In terms of addressing this issue of supply, the fentanyl is coming from Mexico, China, and it’s manufactured here in the United States. How do we address this crisis? What are the steps that you would recommend we take?
Dr. Christo: I think that we need to better support law enforcement to crack down on those who are manufacturing fentanyl in the United States, and also to crack down on those who are transporting it from, say, Mexico – I think it’s primarily coming from Mexico – but from outside the United States to the United States. I feel like that’s important to do.
Two, we need to better educate those who are using these mind- and mood-altering substances. We’re seeing more and more that it’s the young-adult population, those between the ages of 13 and 25, who are starting to use these substances, and they’re very dangerous.
Dr. Glatter: Are these teens seeking out heroin and it happens to be laced with fentanyl, or are they actually seeking pure fentanyl? Are they trying to buy the colorful pills that we know about? What’s your experience in terms of the population you’re treating and what you could tell us?
Dr. Christo: I think it’s both. We’re seeing young adults who are interested in the use of fentanyl as a mind- and mood-altering substance. We’re also seeing young and older adults use other drugs, like cocaine and heroin, that are laced with fentanyl, and they don’t know it. That’s exponentially more dangerous.
Fentanyl test strips
Dr. Glatter: People are unaware that there is fentanyl in what they’re using, and it is certainly leading to overdoses and deaths. I think that parents really need to be aware of this.
Dr. Christo: Yes, for sure. I think we need better educational methods in the schools to educate that population that we’re talking about (between the ages of 13 and 25). Let them know the dangers, because I don’t think they’re aware of the danger, and how potent fentanyl is in terms of its lethality, and that you don’t need very much to take in a form of a pill or to inhale or to inject intravenously to kill yourself. That is key – education at that level – and to let those who are going to use these substances (specifically, synthetic fentanyl) know that they should consider the use of fentanyl test strips.
Fentanyl test strips would be primarily used for those who are thinking that they’re using heroin but there may be fentanyl in there, or methamphetamine and there may be fentanyl, and they don’t know. The test strip gives them that knowledge.
The other harm reduction strategies would be the use of naloxone, known as Narcan. That’s a lifesaver. You just have to spritz it into the nostril. You don’t do it yourself if you’re using the substance, but you’ve got others who can do it for you. No question, that’s a lifesaver. We need to make sure that there’s greater availability of that throughout the entire country, and we’re seeing some of that in certain states. In certain states, you don’t need a prescription to get naloxone from the pharmacy.
Dr. Glatter: I think it’s so important that it should be widely available. Certainly, the COVID-19 pandemic exacerbated the number of overdoses we saw. Are overdoses coming down or are we still at a level that’s close to 2020?
Dr. Christo: Unfortunately, we’re still seeing the same level, if not seeing it escalate. Certainly, the pandemic, because of the economic cost associated with the pandemic – loss of employment, underemployment – as well as the emotional stress of the pandemic led many people to use substances on the street in order to cope. They’re coping mechanisms, and we really haven’t seen it abate quite yet.
Dr. Glatter: Do you have a message for the lawmakers on Capitol Hill as to what we can do regarding the illegal manufacturing and distribution, how we can really crack down? Are there other approaches that we could implement that might be more tangible?
Dr. Christo: Yes. No. 1 would be to support law enforcement. No. 2 would be to create and make available more overdose prevention centers. The first was in New York City. If you look at the data on overdose prevention centers, in Canada, for example, they’ve seen a 35% reduction in overdose deaths. These are places where people who are using can go to get clean needles and clean syringes. This is where people basically oversee the use of the drug and intervene if necessary.
It seems sort of antithetical. It seems like, “Boy, why would you fund a center for people to use drugs?” The data from Canada and outside Canada are such that it can be very helpful. That would be one of my messages to lawmakers as well.
Vaccines to combat the synthetic opioid crisis
Dr. Glatter: Do you think that the legislators could approach some of these factories as a way to crack down, and have law enforcement be more aggressive? Is that another possible solution?
Dr. Christo: It is. Law enforcement needs to be supported by the government, by the Biden administration, so that we can prevent the influx of fentanyl and other drugs into the United States, and also to crack down on those in the United States who are manufacturing these drugs – synthetic fentanyl, first and foremost – because we’re seeing a lot of deaths related to synthetic fentanyl.
Also, we’re seeing — and this is pretty intriguing and interesting – the use of vaccines to help prevent overdose. The first human trial is underway right now for a vaccine against oxycodone. Not only that, but there are other vaccines that are in animal trials now against heroin, cocaine, or fentanyl. There’s hope there that we can use vaccines to also help reduce deaths related to overdose from fentanyl and other opioids.
Dr. Glatter: Do you think this would be given widely to the population or only to those at higher risk?
Dr. Christo: It would probably be targeting those who are at higher risk and have a history of drug abuse. I don’t think it would be something that would be given to the entire population, but it certainly could be effective, and we’re seeing encouraging results from the human trial right now.
Dr. Glatter: That’s very intriguing. That’s something that certainly could be quite helpful in the future.
One thing I did want to address is law enforcement and first responders who have been exposed to dust, or inhaled dust possibly, or had fentanyl on their skin. There has been lots of controversy. The recent literature has dispelled the controversy that people who had supposedly passed out and required Narcan after exposure to intact skin, or even compromised skin, had an overdose of fentanyl. Maybe you could speak to that and dispel that myth.
Dr. Christo: Yes, I’ve been asked this question a couple of times in the past. It’s not sufficient just to have contact with fentanyl on the skin to lead to an overdose. You really need to ingest it. That is, take it by mouth in the form of a pill, inhale it, or inject it intravenously. Skin contact is very unlikely going to lead to an overdose and death.
Dr. Glatter: I want to thank you for a very informative interview. Do you have one or two pearls you’d like to give our audience as a takeaway?
Dr. Christo: I would say two things. One is, don’t give up if you have chronic pain because there is hope. We have nonopioid treatments that can be effective. Two, don’t give up if you have a substance use disorder. Talk to your primary care doctor or talk to emergency room physicians if you’re in the emergency room. The Substance Abuse and Mental Health Services Administration is a good resource, too. SAMHSA has an 800 number for support and a website. Take the opportunity to use the resources that are available.
Dr. Glatter is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Hofstra University, Hempstead, N.Y. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Christo is an associate professor and a pain specialist in the department of anesthesiology and critical care medicine at Johns Hopkins University, Baltimore. He also serves as director of the multidisciplinary pain fellowship program at Johns Hopkins Hospital. Christo is the author of Aches and Gains, A Comprehensive Guide to Overcoming Your Pain, and hosts an award-winning, nationally syndicated SiriusXM radio talk show on overcoming pain, called Aches and Gains.
A version of this article first appeared on Medscape.com.
Heart failure drug a new treatment option for alcoholism?
(AUD), new research suggests.
Researchers at the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and Yale University, New Haven, Conn., investigated the impact of spironolactone on AUD.
Initially, they studied rodents and found that spironolactone reduced binge drinking in mice and reduced self-administration of alcohol in rats without adversely affecting food or water intake or causing motor or coordination problems.
They also analyzed electronic health records of patients drawn from the United States Veterans Affairs health care system to explore potential changes in alcohol use after spironolactone treatment was initiated for other conditions and found a significant link between spironolactone treatment and reduction in self-reported alcohol consumption, with the largest effects observed among those who reported hazardous/heavy episodic alcohol use prior to starting spironolactone treatment.
“Combining findings across three species and different types of research studies, and then seeing similarities in these data, gives us confidence that we are onto something potentially important scientifically and clinically,” senior coauthor Lorenzo Leggio, MD, PhD, senior investigator in the Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, a joint NIDA and NIAAA laboratory, said in a news release.
The study was published online in Molecular Psychiatry.
There is a “critical need to increase the armamentarium of pharmacotherapies to treat individuals with AUD,” the authors note, adding that neuroendocrine systems involved in alcohol craving and drinking “offer promising pharmacologic targets in this regard.”
“Both our team and others have observed that patients with AUD often present with changes in peripheral hormones, including aldosterone, which plays a key role in regulating blood pressure and electrolytes,” Dr. Leggio said in an interview.
Spironolactone is a nonselective mineralocorticoid receptor (MT) antagonist. In studies in animal models, investigators said they found “an inverse correlation between alcohol drinking and the expression of the MR in the amygdala, a key brain region in the development and maintenance of AUD and addiction in general.”
Taken together, this led them to hypothesize that blocking the MR, which is the mechanism of action of spironolactone, “could be a novel pharmacotherapeutic approach for AUD,” he said.
Previous research by the same group of researchers suggested spironolactone “may be a potential new medication to treat patients with AUD.” The present study expanded on those findings and consisted of a three-part investigation.
In the current study, the investigators tested different dosages of spironolactone on binge-like alcohol consumption in male and female mice and assessed food and water intake, blood alcohol levels, motor coordination, and spontaneous locomotion.
They then tested the effects of different dosages of spironolactone injections on operant alcohol self-administration in alcohol-dependent and nondependent male and female rats, also testing blood alcohol levels and motor coordination.
Finally, they analyzed health records of veterans to examine the association between at least 60 continuous days of spironolactone treatment and self-reported alcohol consumption (measured by the Alcohol Use Disorders Identification Test-Consumption [AUDIT-C]).
Each of the spironolactone-exposed patients was matched using propensity scores with up to five unexposed patients who had reported alcohol consumption in the 2 years prior to the index date.
The final analysis included a matched cohort of 10,726 spironolactone-exposed individuals who were matched to 34,461 unexposed individuals.
New targets
Spironolactone reduced alcohol intake in mice drinking a sweetened alcohol solution; a 2-way ANOVA revealed a main effect of dose (F 4,52 = 9.09; P < .0001) and sex, with female mice drinking more alcohol, compared to male mice (F 1,13 = 6.05; P = .02).
Post hoc comparisons showed that spironolactone at doses of 50, 100, and 200 mg/kg significantly reduced alcohol intake (P values = .007, .002, and .0001, respectively).
In mice drinking an unsweetened alcohol solution, the 2-way repeated measures ANOVA similarly found a main effect of dose (F 4,52 = 5.77; P = .0006), but not of sex (F 1,13 = 1.41; P = .25).
Spironolactone had no effect on the mice’s intake of a sweet solution without alcohol and had no impact on the consumption of food and water or on locomotion and coordination.
In rats, a 2-way ANOVA revealed a significant spironolactone effect of dose (F 3,66 = 43.95; P < .001), with a post hoc test indicating that spironolactone at 25, 50, and 75 mg/kg reduced alcohol self-administration in alcohol-dependent and nondependent rats (all P values = .0001).
In humans, among the exposed individuals in the matched cohort, 25%, 57%, and 18% received daily doses of spironolactone of less than 25 mg/day, 25-49 mg/day, and 50 mg/day or higher, respectively, with a median follow-up time of 542 (interquartile range, 337-730) days.
The AUDIT-C scores decreased during the study period in both treatment groups, with a larger decrease in average AUDIT-C scores among the exposed vs. unexposed individuals.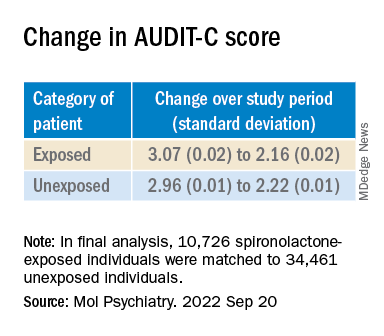
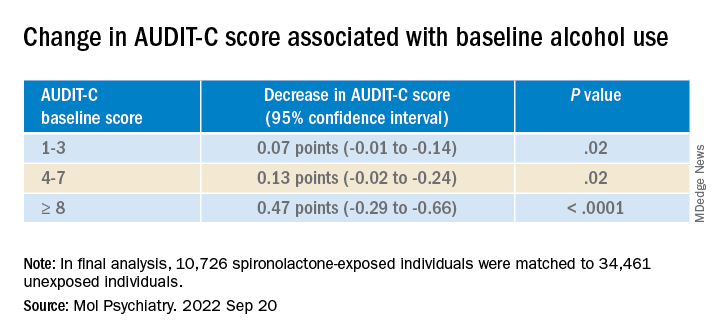
“These are very exciting times because, thanks to the progress in the addiction biomedical research field, we are increasing our understanding of the mechanisms how some people develop AUD; hence we can use this knowledge to identify new targets.” The current study “is an example of these ongoing efforts,” said Dr. Leggio.
“It is important to note that [these results] are important but preliminary.” At this juncture, “it would be too premature to think about prescribing spironolactone to treat AUD,” he added.
Exciting findings
Commenting on the study, Joyce Besheer, PhD, professor, department of psychiatry and Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, called the study an “elegant demonstration of translational science.”
“While clinical trials will be needed to determine whether this medication is effective at reducing drinking in patients with AUD, these findings are exciting as they suggest that spironolactone may be a promising compound and new treatment options for AUD are much needed,” said Dr. Besheer, who was not involved with the current study.
Dr. Leggio agreed. “We now need prospective, placebo-controlled studies to assess the potential safety and efficacy of spironolactone in people with AUD,” he said.
This work was supported by the National Institutes of Health and the NIAAA. Dr. Leggio, study coauthors, and Dr. Besheer declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AUD), new research suggests.
Researchers at the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and Yale University, New Haven, Conn., investigated the impact of spironolactone on AUD.
Initially, they studied rodents and found that spironolactone reduced binge drinking in mice and reduced self-administration of alcohol in rats without adversely affecting food or water intake or causing motor or coordination problems.
They also analyzed electronic health records of patients drawn from the United States Veterans Affairs health care system to explore potential changes in alcohol use after spironolactone treatment was initiated for other conditions and found a significant link between spironolactone treatment and reduction in self-reported alcohol consumption, with the largest effects observed among those who reported hazardous/heavy episodic alcohol use prior to starting spironolactone treatment.
“Combining findings across three species and different types of research studies, and then seeing similarities in these data, gives us confidence that we are onto something potentially important scientifically and clinically,” senior coauthor Lorenzo Leggio, MD, PhD, senior investigator in the Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, a joint NIDA and NIAAA laboratory, said in a news release.
The study was published online in Molecular Psychiatry.
There is a “critical need to increase the armamentarium of pharmacotherapies to treat individuals with AUD,” the authors note, adding that neuroendocrine systems involved in alcohol craving and drinking “offer promising pharmacologic targets in this regard.”
“Both our team and others have observed that patients with AUD often present with changes in peripheral hormones, including aldosterone, which plays a key role in regulating blood pressure and electrolytes,” Dr. Leggio said in an interview.
Spironolactone is a nonselective mineralocorticoid receptor (MT) antagonist. In studies in animal models, investigators said they found “an inverse correlation between alcohol drinking and the expression of the MR in the amygdala, a key brain region in the development and maintenance of AUD and addiction in general.”
Taken together, this led them to hypothesize that blocking the MR, which is the mechanism of action of spironolactone, “could be a novel pharmacotherapeutic approach for AUD,” he said.
Previous research by the same group of researchers suggested spironolactone “may be a potential new medication to treat patients with AUD.” The present study expanded on those findings and consisted of a three-part investigation.
In the current study, the investigators tested different dosages of spironolactone on binge-like alcohol consumption in male and female mice and assessed food and water intake, blood alcohol levels, motor coordination, and spontaneous locomotion.
They then tested the effects of different dosages of spironolactone injections on operant alcohol self-administration in alcohol-dependent and nondependent male and female rats, also testing blood alcohol levels and motor coordination.
Finally, they analyzed health records of veterans to examine the association between at least 60 continuous days of spironolactone treatment and self-reported alcohol consumption (measured by the Alcohol Use Disorders Identification Test-Consumption [AUDIT-C]).
Each of the spironolactone-exposed patients was matched using propensity scores with up to five unexposed patients who had reported alcohol consumption in the 2 years prior to the index date.
The final analysis included a matched cohort of 10,726 spironolactone-exposed individuals who were matched to 34,461 unexposed individuals.
New targets
Spironolactone reduced alcohol intake in mice drinking a sweetened alcohol solution; a 2-way ANOVA revealed a main effect of dose (F 4,52 = 9.09; P < .0001) and sex, with female mice drinking more alcohol, compared to male mice (F 1,13 = 6.05; P = .02).
Post hoc comparisons showed that spironolactone at doses of 50, 100, and 200 mg/kg significantly reduced alcohol intake (P values = .007, .002, and .0001, respectively).
In mice drinking an unsweetened alcohol solution, the 2-way repeated measures ANOVA similarly found a main effect of dose (F 4,52 = 5.77; P = .0006), but not of sex (F 1,13 = 1.41; P = .25).
Spironolactone had no effect on the mice’s intake of a sweet solution without alcohol and had no impact on the consumption of food and water or on locomotion and coordination.
In rats, a 2-way ANOVA revealed a significant spironolactone effect of dose (F 3,66 = 43.95; P < .001), with a post hoc test indicating that spironolactone at 25, 50, and 75 mg/kg reduced alcohol self-administration in alcohol-dependent and nondependent rats (all P values = .0001).
In humans, among the exposed individuals in the matched cohort, 25%, 57%, and 18% received daily doses of spironolactone of less than 25 mg/day, 25-49 mg/day, and 50 mg/day or higher, respectively, with a median follow-up time of 542 (interquartile range, 337-730) days.
The AUDIT-C scores decreased during the study period in both treatment groups, with a larger decrease in average AUDIT-C scores among the exposed vs. unexposed individuals.

“These are very exciting times because, thanks to the progress in the addiction biomedical research field, we are increasing our understanding of the mechanisms how some people develop AUD; hence we can use this knowledge to identify new targets.” The current study “is an example of these ongoing efforts,” said Dr. Leggio.
“It is important to note that [these results] are important but preliminary.” At this juncture, “it would be too premature to think about prescribing spironolactone to treat AUD,” he added.
Exciting findings
Commenting on the study, Joyce Besheer, PhD, professor, department of psychiatry and Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, called the study an “elegant demonstration of translational science.”
“While clinical trials will be needed to determine whether this medication is effective at reducing drinking in patients with AUD, these findings are exciting as they suggest that spironolactone may be a promising compound and new treatment options for AUD are much needed,” said Dr. Besheer, who was not involved with the current study.
Dr. Leggio agreed. “We now need prospective, placebo-controlled studies to assess the potential safety and efficacy of spironolactone in people with AUD,” he said.
This work was supported by the National Institutes of Health and the NIAAA. Dr. Leggio, study coauthors, and Dr. Besheer declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AUD), new research suggests.
Researchers at the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and Yale University, New Haven, Conn., investigated the impact of spironolactone on AUD.
Initially, they studied rodents and found that spironolactone reduced binge drinking in mice and reduced self-administration of alcohol in rats without adversely affecting food or water intake or causing motor or coordination problems.
They also analyzed electronic health records of patients drawn from the United States Veterans Affairs health care system to explore potential changes in alcohol use after spironolactone treatment was initiated for other conditions and found a significant link between spironolactone treatment and reduction in self-reported alcohol consumption, with the largest effects observed among those who reported hazardous/heavy episodic alcohol use prior to starting spironolactone treatment.
“Combining findings across three species and different types of research studies, and then seeing similarities in these data, gives us confidence that we are onto something potentially important scientifically and clinically,” senior coauthor Lorenzo Leggio, MD, PhD, senior investigator in the Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, a joint NIDA and NIAAA laboratory, said in a news release.
The study was published online in Molecular Psychiatry.
There is a “critical need to increase the armamentarium of pharmacotherapies to treat individuals with AUD,” the authors note, adding that neuroendocrine systems involved in alcohol craving and drinking “offer promising pharmacologic targets in this regard.”
“Both our team and others have observed that patients with AUD often present with changes in peripheral hormones, including aldosterone, which plays a key role in regulating blood pressure and electrolytes,” Dr. Leggio said in an interview.
Spironolactone is a nonselective mineralocorticoid receptor (MT) antagonist. In studies in animal models, investigators said they found “an inverse correlation between alcohol drinking and the expression of the MR in the amygdala, a key brain region in the development and maintenance of AUD and addiction in general.”
Taken together, this led them to hypothesize that blocking the MR, which is the mechanism of action of spironolactone, “could be a novel pharmacotherapeutic approach for AUD,” he said.
Previous research by the same group of researchers suggested spironolactone “may be a potential new medication to treat patients with AUD.” The present study expanded on those findings and consisted of a three-part investigation.
In the current study, the investigators tested different dosages of spironolactone on binge-like alcohol consumption in male and female mice and assessed food and water intake, blood alcohol levels, motor coordination, and spontaneous locomotion.
They then tested the effects of different dosages of spironolactone injections on operant alcohol self-administration in alcohol-dependent and nondependent male and female rats, also testing blood alcohol levels and motor coordination.
Finally, they analyzed health records of veterans to examine the association between at least 60 continuous days of spironolactone treatment and self-reported alcohol consumption (measured by the Alcohol Use Disorders Identification Test-Consumption [AUDIT-C]).
Each of the spironolactone-exposed patients was matched using propensity scores with up to five unexposed patients who had reported alcohol consumption in the 2 years prior to the index date.
The final analysis included a matched cohort of 10,726 spironolactone-exposed individuals who were matched to 34,461 unexposed individuals.
New targets
Spironolactone reduced alcohol intake in mice drinking a sweetened alcohol solution; a 2-way ANOVA revealed a main effect of dose (F 4,52 = 9.09; P < .0001) and sex, with female mice drinking more alcohol, compared to male mice (F 1,13 = 6.05; P = .02).
Post hoc comparisons showed that spironolactone at doses of 50, 100, and 200 mg/kg significantly reduced alcohol intake (P values = .007, .002, and .0001, respectively).
In mice drinking an unsweetened alcohol solution, the 2-way repeated measures ANOVA similarly found a main effect of dose (F 4,52 = 5.77; P = .0006), but not of sex (F 1,13 = 1.41; P = .25).
Spironolactone had no effect on the mice’s intake of a sweet solution without alcohol and had no impact on the consumption of food and water or on locomotion and coordination.
In rats, a 2-way ANOVA revealed a significant spironolactone effect of dose (F 3,66 = 43.95; P < .001), with a post hoc test indicating that spironolactone at 25, 50, and 75 mg/kg reduced alcohol self-administration in alcohol-dependent and nondependent rats (all P values = .0001).
In humans, among the exposed individuals in the matched cohort, 25%, 57%, and 18% received daily doses of spironolactone of less than 25 mg/day, 25-49 mg/day, and 50 mg/day or higher, respectively, with a median follow-up time of 542 (interquartile range, 337-730) days.
The AUDIT-C scores decreased during the study period in both treatment groups, with a larger decrease in average AUDIT-C scores among the exposed vs. unexposed individuals.

“These are very exciting times because, thanks to the progress in the addiction biomedical research field, we are increasing our understanding of the mechanisms how some people develop AUD; hence we can use this knowledge to identify new targets.” The current study “is an example of these ongoing efforts,” said Dr. Leggio.
“It is important to note that [these results] are important but preliminary.” At this juncture, “it would be too premature to think about prescribing spironolactone to treat AUD,” he added.
Exciting findings
Commenting on the study, Joyce Besheer, PhD, professor, department of psychiatry and Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, called the study an “elegant demonstration of translational science.”
“While clinical trials will be needed to determine whether this medication is effective at reducing drinking in patients with AUD, these findings are exciting as they suggest that spironolactone may be a promising compound and new treatment options for AUD are much needed,” said Dr. Besheer, who was not involved with the current study.
Dr. Leggio agreed. “We now need prospective, placebo-controlled studies to assess the potential safety and efficacy of spironolactone in people with AUD,” he said.
This work was supported by the National Institutes of Health and the NIAAA. Dr. Leggio, study coauthors, and Dr. Besheer declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MOLECULAR PSYCHIATRY