User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
One-third of outpatients with COVID-19 are unwell weeks later
, according to survey results in Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention.
Mark W. Tenforde, MD, PhD, for the CDC-COVID-19 Response Team, and colleagues conducted a multistate telephone survey of symptomatic adults who tested positive for SARS-CoV-2. The researchers found that 35% had not returned to their usual state of wellness when they were interviewed 2-3 weeks after testing.
Among the 270 of 274 people interviewed for whom there were data on return to health, 175 (65%) reported that they had returned to baseline health an average of 7 days from the date of testing.
Among the 274 symptomatic outpatients, the median number of symptoms was seven. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms.
Prolonged illness is well described in adults hospitalized with severe COVID-19, especially among the older adult population, but little is known about other groups.
The proportion who had not returned to health differed by age: 26% of interviewees aged 18-34 years, 32% of those aged 35-49 years, and 47% of those at least 50 years old reported not having returned to their usual health (P = .010) within 14-21 days after receiving positive test results.
Among respondents aged 18-34 years who had no chronic medical condition, 19% (9 of 48) reported not having returned to their usual state of health during that time.
Public health messaging targeting younger adults, a group who might not be expected to be sick for weeks with mild disease, is particularly important, the authors wrote.
Kyle Annen, DO, medical director of transfusion services and patient blood management at Children’s Hospital Colorado and assistant professor of pathology at the University of Colorado, Denver, said in an interview that an important message is that delayed recovery (symptoms of fatigue, cough, and shortness of breath) was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization.
“This should impact the perception of this being a mild illness in the young adult population and encourage them to comply with recommendations of social distancing, masking, and hand washing,” she said.
Recovery time of more than 2 weeks will affect work and school performance, especially prolonged fatigue, she noted. This was one of the prominent symptoms that were reported to be slow to dissipate.
“I think the most interesting point in this study is that of underlying conditions; psychiatric conditions were significantly correlated with prolonged recovery. I don’t think that many people think of depression and anxiety as an underlying medical condition in regards to COVID-19 risk. This could potentially have an impact, as depression and anxiety rates will likely increase as COVID-19 continues,” she said.
Buddy Creech, MD, MPH, said in an interview that it is “important to realize that the spectrum of disease with COVID is wide, including mild disease, severe disease, and prolonged disease. This report helps us understand some of the risk factors for those with prolonged symptoms and may allow us to refine even more clearly how we prioritize treatment and vaccine administration, once available.
“It also highlights the challenge of dealing with this virus. Not only do the symptoms vary widely, but so do the incubation period, the duration of symptoms, and the residual symptoms that sometimes occur. Clearly, there is much we still need to understand about this virus,” he said.
The interviews were conducted from April 15 to June 25 with a random sample of adults at least 18 years old who had received a first positive test result for SARS-CoV-2 at an outpatient visit at one of 14 US academic healthcare systems in 13 states.
A version of this article originally appeared on Medscape.com.
, according to survey results in Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention.
Mark W. Tenforde, MD, PhD, for the CDC-COVID-19 Response Team, and colleagues conducted a multistate telephone survey of symptomatic adults who tested positive for SARS-CoV-2. The researchers found that 35% had not returned to their usual state of wellness when they were interviewed 2-3 weeks after testing.
Among the 270 of 274 people interviewed for whom there were data on return to health, 175 (65%) reported that they had returned to baseline health an average of 7 days from the date of testing.
Among the 274 symptomatic outpatients, the median number of symptoms was seven. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms.
Prolonged illness is well described in adults hospitalized with severe COVID-19, especially among the older adult population, but little is known about other groups.
The proportion who had not returned to health differed by age: 26% of interviewees aged 18-34 years, 32% of those aged 35-49 years, and 47% of those at least 50 years old reported not having returned to their usual health (P = .010) within 14-21 days after receiving positive test results.
Among respondents aged 18-34 years who had no chronic medical condition, 19% (9 of 48) reported not having returned to their usual state of health during that time.
Public health messaging targeting younger adults, a group who might not be expected to be sick for weeks with mild disease, is particularly important, the authors wrote.
Kyle Annen, DO, medical director of transfusion services and patient blood management at Children’s Hospital Colorado and assistant professor of pathology at the University of Colorado, Denver, said in an interview that an important message is that delayed recovery (symptoms of fatigue, cough, and shortness of breath) was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization.
“This should impact the perception of this being a mild illness in the young adult population and encourage them to comply with recommendations of social distancing, masking, and hand washing,” she said.
Recovery time of more than 2 weeks will affect work and school performance, especially prolonged fatigue, she noted. This was one of the prominent symptoms that were reported to be slow to dissipate.
“I think the most interesting point in this study is that of underlying conditions; psychiatric conditions were significantly correlated with prolonged recovery. I don’t think that many people think of depression and anxiety as an underlying medical condition in regards to COVID-19 risk. This could potentially have an impact, as depression and anxiety rates will likely increase as COVID-19 continues,” she said.
Buddy Creech, MD, MPH, said in an interview that it is “important to realize that the spectrum of disease with COVID is wide, including mild disease, severe disease, and prolonged disease. This report helps us understand some of the risk factors for those with prolonged symptoms and may allow us to refine even more clearly how we prioritize treatment and vaccine administration, once available.
“It also highlights the challenge of dealing with this virus. Not only do the symptoms vary widely, but so do the incubation period, the duration of symptoms, and the residual symptoms that sometimes occur. Clearly, there is much we still need to understand about this virus,” he said.
The interviews were conducted from April 15 to June 25 with a random sample of adults at least 18 years old who had received a first positive test result for SARS-CoV-2 at an outpatient visit at one of 14 US academic healthcare systems in 13 states.
A version of this article originally appeared on Medscape.com.
, according to survey results in Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention.
Mark W. Tenforde, MD, PhD, for the CDC-COVID-19 Response Team, and colleagues conducted a multistate telephone survey of symptomatic adults who tested positive for SARS-CoV-2. The researchers found that 35% had not returned to their usual state of wellness when they were interviewed 2-3 weeks after testing.
Among the 270 of 274 people interviewed for whom there were data on return to health, 175 (65%) reported that they had returned to baseline health an average of 7 days from the date of testing.
Among the 274 symptomatic outpatients, the median number of symptoms was seven. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms.
Prolonged illness is well described in adults hospitalized with severe COVID-19, especially among the older adult population, but little is known about other groups.
The proportion who had not returned to health differed by age: 26% of interviewees aged 18-34 years, 32% of those aged 35-49 years, and 47% of those at least 50 years old reported not having returned to their usual health (P = .010) within 14-21 days after receiving positive test results.
Among respondents aged 18-34 years who had no chronic medical condition, 19% (9 of 48) reported not having returned to their usual state of health during that time.
Public health messaging targeting younger adults, a group who might not be expected to be sick for weeks with mild disease, is particularly important, the authors wrote.
Kyle Annen, DO, medical director of transfusion services and patient blood management at Children’s Hospital Colorado and assistant professor of pathology at the University of Colorado, Denver, said in an interview that an important message is that delayed recovery (symptoms of fatigue, cough, and shortness of breath) was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization.
“This should impact the perception of this being a mild illness in the young adult population and encourage them to comply with recommendations of social distancing, masking, and hand washing,” she said.
Recovery time of more than 2 weeks will affect work and school performance, especially prolonged fatigue, she noted. This was one of the prominent symptoms that were reported to be slow to dissipate.
“I think the most interesting point in this study is that of underlying conditions; psychiatric conditions were significantly correlated with prolonged recovery. I don’t think that many people think of depression and anxiety as an underlying medical condition in regards to COVID-19 risk. This could potentially have an impact, as depression and anxiety rates will likely increase as COVID-19 continues,” she said.
Buddy Creech, MD, MPH, said in an interview that it is “important to realize that the spectrum of disease with COVID is wide, including mild disease, severe disease, and prolonged disease. This report helps us understand some of the risk factors for those with prolonged symptoms and may allow us to refine even more clearly how we prioritize treatment and vaccine administration, once available.
“It also highlights the challenge of dealing with this virus. Not only do the symptoms vary widely, but so do the incubation period, the duration of symptoms, and the residual symptoms that sometimes occur. Clearly, there is much we still need to understand about this virus,” he said.
The interviews were conducted from April 15 to June 25 with a random sample of adults at least 18 years old who had received a first positive test result for SARS-CoV-2 at an outpatient visit at one of 14 US academic healthcare systems in 13 states.
A version of this article originally appeared on Medscape.com.
Small NY study: Mother-baby transmission of COVID-19 not seen
according to a study out of New York-Presbyterian Hospital.
“It is suggested in the cumulative data that the virus does not confer additional risk to the fetus during labor or during the early postnatal period in both preterm and term infants,” concluded Jeffrey Perlman, MB ChB, and colleagues in Pediatrics.
But other experts suggest substantial gaps remain in our understanding of maternal transmission of SARS-CoV-2.
“Much more needs to be known,” Munish Gupta, MD, and colleagues from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, said in an accompanying editorial.
The prospective study is the first to describe a cohort of U.S. COVID-19–related deliveries, with the prior neonatal impact of COVID-19 “almost exclusively” reported from China, noted the authors. They included a cohort of 326 women who were tested for SARS-CoV-2 on admission to labor and delivery at New York-Presbyterian Hospital between March 22 and April 15th, 2020. Of the 31 (10%) mothers who tested positive, 15 (48%) were asymptomatic and 16 (52%) were symptomatic.
Two babies were born prematurely (one by Cesarean) and were isolated in negative pressure rooms with continuous positive airway pressure. Both were moved out of isolation after two negative test results and “have exhibited an unremarkable clinical course,” the authors reported.
The other 29 term babies were cared for in their mothers’ rooms, with breastfeeding allowed, if desired. These babies and their mothers were discharged from the hospital between 24 and 48 hours after delivery.
“Visitor restriction for mothers who were positive for COVID-19 included 14 days of no visitation from the start of symptoms,” noted the team.
They added “since the prepublication release there have been a total of 47 mothers positive for COVID-19, resulting in 47 infants; 4 have been admitted to neonatal intensive care. In addition, 32 other infants have been tested for a variety of indications within the unit. All infants test results have been negative.”
The brief report outlined the institution’s checklist for delivery preparedness in either the operating room or labor delivery room, including personal protective equipment, resuscitation, transportation to the neonatal intensive care unit, and early postresuscitation care. “Suspected or confirmed COVID-19 alone in an otherwise uncomplicated pregnancy is not an indication for the resuscitation team or the neonatal fellow,” they noted, adding delivery room preparation and management should include contact precautions. “With scrupulous attention to infectious precautions, horizontal viral transmission should be minimized,” they advised.
Dr. Perlman and associates emphasized that rapid turnaround SARSCoV-2 testing is “crucial to minimize the likelihood of a provider becoming infected and/or infecting the infant.”
Although the findings are “clearly reassuring,” Dr. Gupta and colleagues have reservations. “To what extent does this report address concerns for infection risk with a rooming-in approach to care?” they asked in their accompanying editorial. “The answer is likely some, but not much.”
Many questions remain, they said, including: “What precautions were used to minimize infection risk during the postbirth hospital course? What was the approach to skin-to-skin care and direct mother-newborn contact? Were restrictions placed on family members? Were changes made to routine interventions such as hearing screens or circumcisions? What practices were in place around environmental cleaning? Most important, how did the newborns do after discharge?”
The current uncertainty around neonatal COVID-19 infection risk has led to “disparate” variations in care recommendations, they pointed out. Whereas China’s consensus guidelines recommend a 14-day separation of COVID-19–positive mothers from their healthy infants, a practice supported by the American Academy of Pediatrics “when possible,” the Italian Society of Neonatology, the Royal College of Paediatrics and Child Health, and the Canadian Paediatric Society advise “rooming-in and breastfeeding with appropriate infection prevention measures.”
Dr. Gupta and colleagues pointed to the following as at least three “critical and time-sensitive needs for research around neonatal care and outcomes related to COVID-19”:
- Studies need to have much larger sample sizes and include diverse populations. This will allow for reliable measurement of outcomes.
- Descriptions of care practices must be in detail, especially about infection prevention; these should be presented in a way to compare the efficacy of different approaches.
- There needs to be follow-up information on outcomes of both the mother and the neonate after the birth hospitalization.
Asked to comment, Lillian Beard, MD, of George Washington University in Washington welcomed the data as “good news.”
“Although small, the study was done during a 3-week peak period at the hottest spot of the pandemic in the United States during that period. It illustrates how delivery room preparedness, adequate personal protective equipment, and carefully planned infection control precautions can positively impact outcomes even during a seemingly impossible period,” she said.
“Although there are many uncertainties about maternal COVID-19 transmission and neonatal infection risks ... in my opinion, during the after birth hospitalization, the inherent benefits of rooming in for breast feeding and the opportunities for the demonstration and teaching of infection prevention practices for the family home, far outweigh the risks of disease transmission,” said Dr. Beard, who was not involved with the study.
The study and the commentary emphasize the likely low risk of vertical transmission of the virus, with horizontal transmission being the greater risk. However, cases of transplacental transmission have been reported, and the lead investigator of one recent placental study cautions against complacency.
“Neonates can get infected in both ways. The majority of cases seem to be horizontal, but those who have been infected or highly suspected to be vertically infected are not a small percentage either,” said Daniele de Luca, MD, PhD, president-elect of the European Society for Pediatric and Neonatal Intensive Care (ESPNIC) and a neonatologist at Antoine Béclère Hospital in Clamart, France.
“Perlman’s data are interesting and consistent with other reports around the world. However, two things must be remembered,” he said in an interview. “First, newborn infants are at relatively low risk from SARS-CoV-2 infections, but this is very far from zero risk. Neonatal SARS-CoV-2 infections do exist and have been described around the world. While they have a mild course in the majority of cases, neonatologists should not forget them and should be prepared to offer the best care to these babies.”
“Second, how this can be balanced with the need to promote breastfeeding and avoid overtreatment or separation from the mother is a question far from being answered. Gupta et al. in their commentary are right in saying that we have more questions than answers. While waiting for the results of large initiatives (such as the ESPNIC EPICENTRE Registry that they cite) to answer these open points, the best we can do is to provide a personalised case by case approach, transparent information to parents, and an open counselling informing clinical decisions.”
The study received no external funding. Dr. Perlman and associates had no financial disclosures. Dr. Gupta and colleagues had no relevant financial disclosures. Neither Dr. Beard nor Dr. de Luca had any relevant financial disclosures.
SOURCE: Perlman J et al. Pediatrics. 2020;146(2):e20201567.
according to a study out of New York-Presbyterian Hospital.
“It is suggested in the cumulative data that the virus does not confer additional risk to the fetus during labor or during the early postnatal period in both preterm and term infants,” concluded Jeffrey Perlman, MB ChB, and colleagues in Pediatrics.
But other experts suggest substantial gaps remain in our understanding of maternal transmission of SARS-CoV-2.
“Much more needs to be known,” Munish Gupta, MD, and colleagues from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, said in an accompanying editorial.
The prospective study is the first to describe a cohort of U.S. COVID-19–related deliveries, with the prior neonatal impact of COVID-19 “almost exclusively” reported from China, noted the authors. They included a cohort of 326 women who were tested for SARS-CoV-2 on admission to labor and delivery at New York-Presbyterian Hospital between March 22 and April 15th, 2020. Of the 31 (10%) mothers who tested positive, 15 (48%) were asymptomatic and 16 (52%) were symptomatic.
Two babies were born prematurely (one by Cesarean) and were isolated in negative pressure rooms with continuous positive airway pressure. Both were moved out of isolation after two negative test results and “have exhibited an unremarkable clinical course,” the authors reported.
The other 29 term babies were cared for in their mothers’ rooms, with breastfeeding allowed, if desired. These babies and their mothers were discharged from the hospital between 24 and 48 hours after delivery.
“Visitor restriction for mothers who were positive for COVID-19 included 14 days of no visitation from the start of symptoms,” noted the team.
They added “since the prepublication release there have been a total of 47 mothers positive for COVID-19, resulting in 47 infants; 4 have been admitted to neonatal intensive care. In addition, 32 other infants have been tested for a variety of indications within the unit. All infants test results have been negative.”
The brief report outlined the institution’s checklist for delivery preparedness in either the operating room or labor delivery room, including personal protective equipment, resuscitation, transportation to the neonatal intensive care unit, and early postresuscitation care. “Suspected or confirmed COVID-19 alone in an otherwise uncomplicated pregnancy is not an indication for the resuscitation team or the neonatal fellow,” they noted, adding delivery room preparation and management should include contact precautions. “With scrupulous attention to infectious precautions, horizontal viral transmission should be minimized,” they advised.
Dr. Perlman and associates emphasized that rapid turnaround SARSCoV-2 testing is “crucial to minimize the likelihood of a provider becoming infected and/or infecting the infant.”
Although the findings are “clearly reassuring,” Dr. Gupta and colleagues have reservations. “To what extent does this report address concerns for infection risk with a rooming-in approach to care?” they asked in their accompanying editorial. “The answer is likely some, but not much.”
Many questions remain, they said, including: “What precautions were used to minimize infection risk during the postbirth hospital course? What was the approach to skin-to-skin care and direct mother-newborn contact? Were restrictions placed on family members? Were changes made to routine interventions such as hearing screens or circumcisions? What practices were in place around environmental cleaning? Most important, how did the newborns do after discharge?”
The current uncertainty around neonatal COVID-19 infection risk has led to “disparate” variations in care recommendations, they pointed out. Whereas China’s consensus guidelines recommend a 14-day separation of COVID-19–positive mothers from their healthy infants, a practice supported by the American Academy of Pediatrics “when possible,” the Italian Society of Neonatology, the Royal College of Paediatrics and Child Health, and the Canadian Paediatric Society advise “rooming-in and breastfeeding with appropriate infection prevention measures.”
Dr. Gupta and colleagues pointed to the following as at least three “critical and time-sensitive needs for research around neonatal care and outcomes related to COVID-19”:
- Studies need to have much larger sample sizes and include diverse populations. This will allow for reliable measurement of outcomes.
- Descriptions of care practices must be in detail, especially about infection prevention; these should be presented in a way to compare the efficacy of different approaches.
- There needs to be follow-up information on outcomes of both the mother and the neonate after the birth hospitalization.
Asked to comment, Lillian Beard, MD, of George Washington University in Washington welcomed the data as “good news.”
“Although small, the study was done during a 3-week peak period at the hottest spot of the pandemic in the United States during that period. It illustrates how delivery room preparedness, adequate personal protective equipment, and carefully planned infection control precautions can positively impact outcomes even during a seemingly impossible period,” she said.
“Although there are many uncertainties about maternal COVID-19 transmission and neonatal infection risks ... in my opinion, during the after birth hospitalization, the inherent benefits of rooming in for breast feeding and the opportunities for the demonstration and teaching of infection prevention practices for the family home, far outweigh the risks of disease transmission,” said Dr. Beard, who was not involved with the study.
The study and the commentary emphasize the likely low risk of vertical transmission of the virus, with horizontal transmission being the greater risk. However, cases of transplacental transmission have been reported, and the lead investigator of one recent placental study cautions against complacency.
“Neonates can get infected in both ways. The majority of cases seem to be horizontal, but those who have been infected or highly suspected to be vertically infected are not a small percentage either,” said Daniele de Luca, MD, PhD, president-elect of the European Society for Pediatric and Neonatal Intensive Care (ESPNIC) and a neonatologist at Antoine Béclère Hospital in Clamart, France.
“Perlman’s data are interesting and consistent with other reports around the world. However, two things must be remembered,” he said in an interview. “First, newborn infants are at relatively low risk from SARS-CoV-2 infections, but this is very far from zero risk. Neonatal SARS-CoV-2 infections do exist and have been described around the world. While they have a mild course in the majority of cases, neonatologists should not forget them and should be prepared to offer the best care to these babies.”
“Second, how this can be balanced with the need to promote breastfeeding and avoid overtreatment or separation from the mother is a question far from being answered. Gupta et al. in their commentary are right in saying that we have more questions than answers. While waiting for the results of large initiatives (such as the ESPNIC EPICENTRE Registry that they cite) to answer these open points, the best we can do is to provide a personalised case by case approach, transparent information to parents, and an open counselling informing clinical decisions.”
The study received no external funding. Dr. Perlman and associates had no financial disclosures. Dr. Gupta and colleagues had no relevant financial disclosures. Neither Dr. Beard nor Dr. de Luca had any relevant financial disclosures.
SOURCE: Perlman J et al. Pediatrics. 2020;146(2):e20201567.
according to a study out of New York-Presbyterian Hospital.
“It is suggested in the cumulative data that the virus does not confer additional risk to the fetus during labor or during the early postnatal period in both preterm and term infants,” concluded Jeffrey Perlman, MB ChB, and colleagues in Pediatrics.
But other experts suggest substantial gaps remain in our understanding of maternal transmission of SARS-CoV-2.
“Much more needs to be known,” Munish Gupta, MD, and colleagues from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, said in an accompanying editorial.
The prospective study is the first to describe a cohort of U.S. COVID-19–related deliveries, with the prior neonatal impact of COVID-19 “almost exclusively” reported from China, noted the authors. They included a cohort of 326 women who were tested for SARS-CoV-2 on admission to labor and delivery at New York-Presbyterian Hospital between March 22 and April 15th, 2020. Of the 31 (10%) mothers who tested positive, 15 (48%) were asymptomatic and 16 (52%) were symptomatic.
Two babies were born prematurely (one by Cesarean) and were isolated in negative pressure rooms with continuous positive airway pressure. Both were moved out of isolation after two negative test results and “have exhibited an unremarkable clinical course,” the authors reported.
The other 29 term babies were cared for in their mothers’ rooms, with breastfeeding allowed, if desired. These babies and their mothers were discharged from the hospital between 24 and 48 hours after delivery.
“Visitor restriction for mothers who were positive for COVID-19 included 14 days of no visitation from the start of symptoms,” noted the team.
They added “since the prepublication release there have been a total of 47 mothers positive for COVID-19, resulting in 47 infants; 4 have been admitted to neonatal intensive care. In addition, 32 other infants have been tested for a variety of indications within the unit. All infants test results have been negative.”
The brief report outlined the institution’s checklist for delivery preparedness in either the operating room or labor delivery room, including personal protective equipment, resuscitation, transportation to the neonatal intensive care unit, and early postresuscitation care. “Suspected or confirmed COVID-19 alone in an otherwise uncomplicated pregnancy is not an indication for the resuscitation team or the neonatal fellow,” they noted, adding delivery room preparation and management should include contact precautions. “With scrupulous attention to infectious precautions, horizontal viral transmission should be minimized,” they advised.
Dr. Perlman and associates emphasized that rapid turnaround SARSCoV-2 testing is “crucial to minimize the likelihood of a provider becoming infected and/or infecting the infant.”
Although the findings are “clearly reassuring,” Dr. Gupta and colleagues have reservations. “To what extent does this report address concerns for infection risk with a rooming-in approach to care?” they asked in their accompanying editorial. “The answer is likely some, but not much.”
Many questions remain, they said, including: “What precautions were used to minimize infection risk during the postbirth hospital course? What was the approach to skin-to-skin care and direct mother-newborn contact? Were restrictions placed on family members? Were changes made to routine interventions such as hearing screens or circumcisions? What practices were in place around environmental cleaning? Most important, how did the newborns do after discharge?”
The current uncertainty around neonatal COVID-19 infection risk has led to “disparate” variations in care recommendations, they pointed out. Whereas China’s consensus guidelines recommend a 14-day separation of COVID-19–positive mothers from their healthy infants, a practice supported by the American Academy of Pediatrics “when possible,” the Italian Society of Neonatology, the Royal College of Paediatrics and Child Health, and the Canadian Paediatric Society advise “rooming-in and breastfeeding with appropriate infection prevention measures.”
Dr. Gupta and colleagues pointed to the following as at least three “critical and time-sensitive needs for research around neonatal care and outcomes related to COVID-19”:
- Studies need to have much larger sample sizes and include diverse populations. This will allow for reliable measurement of outcomes.
- Descriptions of care practices must be in detail, especially about infection prevention; these should be presented in a way to compare the efficacy of different approaches.
- There needs to be follow-up information on outcomes of both the mother and the neonate after the birth hospitalization.
Asked to comment, Lillian Beard, MD, of George Washington University in Washington welcomed the data as “good news.”
“Although small, the study was done during a 3-week peak period at the hottest spot of the pandemic in the United States during that period. It illustrates how delivery room preparedness, adequate personal protective equipment, and carefully planned infection control precautions can positively impact outcomes even during a seemingly impossible period,” she said.
“Although there are many uncertainties about maternal COVID-19 transmission and neonatal infection risks ... in my opinion, during the after birth hospitalization, the inherent benefits of rooming in for breast feeding and the opportunities for the demonstration and teaching of infection prevention practices for the family home, far outweigh the risks of disease transmission,” said Dr. Beard, who was not involved with the study.
The study and the commentary emphasize the likely low risk of vertical transmission of the virus, with horizontal transmission being the greater risk. However, cases of transplacental transmission have been reported, and the lead investigator of one recent placental study cautions against complacency.
“Neonates can get infected in both ways. The majority of cases seem to be horizontal, but those who have been infected or highly suspected to be vertically infected are not a small percentage either,” said Daniele de Luca, MD, PhD, president-elect of the European Society for Pediatric and Neonatal Intensive Care (ESPNIC) and a neonatologist at Antoine Béclère Hospital in Clamart, France.
“Perlman’s data are interesting and consistent with other reports around the world. However, two things must be remembered,” he said in an interview. “First, newborn infants are at relatively low risk from SARS-CoV-2 infections, but this is very far from zero risk. Neonatal SARS-CoV-2 infections do exist and have been described around the world. While they have a mild course in the majority of cases, neonatologists should not forget them and should be prepared to offer the best care to these babies.”
“Second, how this can be balanced with the need to promote breastfeeding and avoid overtreatment or separation from the mother is a question far from being answered. Gupta et al. in their commentary are right in saying that we have more questions than answers. While waiting for the results of large initiatives (such as the ESPNIC EPICENTRE Registry that they cite) to answer these open points, the best we can do is to provide a personalised case by case approach, transparent information to parents, and an open counselling informing clinical decisions.”
The study received no external funding. Dr. Perlman and associates had no financial disclosures. Dr. Gupta and colleagues had no relevant financial disclosures. Neither Dr. Beard nor Dr. de Luca had any relevant financial disclosures.
SOURCE: Perlman J et al. Pediatrics. 2020;146(2):e20201567.
FROM PEDIATRICS
Men occupy most leadership roles in medicine
Since the early 2000s, approximately half of medical students in the United States – and in many years, more than half – have been women, but according to an update provided at the virtual Pediatric Hospital Medicine.
In pediatrics, a specialty in which approximately 70% of physicians are now women, there has been progress, but still less than 30% of pediatric department chairs are female, said Vincent Chiang, MD, chief medical officer of Boston Children’s Hospital, during a presentation at the virtual meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Citing published data and a survey he personally conducted of the top children’s hospitals identified by the U.S. News and World Report, Dr. Chiang said a minority of division chiefs, chief medical officers, chief financial officers, and other leaders are female. At his institution, only 2 of 16 division chiefs are female.
“No matter how you slice it, women are underrepresented in leadership positions,” he noted.
The problem is certainly not confined to medicine. Dr. Chiang cited data showing that women and men have reached “near parity” in workforce participation in the United States even though the 20% earnings gap has changed little over time.
According to 2020 data from the World Economic Forum, the United States ranked 51 for the gender gap calculated on the basis of economic, political, educational, and health attainment. Even if this places the United States in the top third of the rankings, it is far behind Iceland and the Scandinavian countries that lead the list.
Efforts to reduce structural biases are part of the fix, but Dr. Chiang cautioned that fundamental changes might never occur if the plan is to wait for an approach based on meritocracy. He said that existing structural biases are “slanted away from women,” who are not necessarily granted the opportunities that are readily available to men.
“A meritocracy only works if the initial playing field was level. Otherwise, it just perpetuates the inequalities,” he said.
The problem is not a shortage of women with the skills to lead. In a study by Zenger/Folkman, a consulting company that works on leadership skill development, women performed better than men in 16 of 18 leadership categories, according to Dr. Chiang.
“There is certainly no shortage of capable women,” he noted.
Of the many issues, Dr. Chiang highlighted two. The first is the challenge of placing women on leadership pathways. This is likely to require proactive strategies, such as fast-track advancement programs that guide female candidates toward leadership roles.
The second is more nuanced. According to Dr. Chiang, women who want to assume a leadership role should think more actively about how and who is making decisions at their institution so they can position themselves appropriately. This is nuanced because “there is a certain amount of gamesmanship,” he said. The rise to leadership “has never been a pure meritocracy.”
Importantly, many of the key decisions in any institution involve money, according to Dr. Chiang. As a result, he advised those seeking leadership roles to join audit committees or otherwise take on responsibility for profit-and-loss management. Even in a nonprofit institution, “you need to make the numbers work,” he said, citing the common catchphrase: “No margin, no mission.”
However, Dr. Chiang acknowledged the many obstacles that prevent women from working their way into positions of leadership. For example, networking is important, but women are not necessarily attracted or invited to some of the social engagements, such as golf outings, where strong relationships are created.
In a survey of 100,000 people working at Fortune 500 companies, “82% of women say they feel excluded at work and much of that comes from that informal networking,” Dr. Chiang said. “Whereas 92% of men think they are not excluding women in their daily work.”
There is no single solution, but Dr. Chiang believes that concrete structural changes are needed. Female doctors remain grossly underrepresented in leadership roles even as they now represent more than half of the workforce for many specialties. Based on the need for proactive approaches outlined by Dr. Chiang, it appears unlikely that gender inequality will ever resolve itself.
Lisa S. Rotenstein, MD, who has written on fixing the gender imbalance in health care, including for the Harvard Business Review, said she agreed during an interview that structural changes are critical.
“In order to address current disparities, leaders should be thinking about how to remove both the formal and informal obstacles that prevent women and minorities from getting into the rooms where these decisions are being made,” said Dr. Rotenstein, who is an instructor in medicine at Brigham and Women’s Hospital, Harvard Medical School in Boston.
“This will need to involve sponsorship that gets women invited to the right committees or in positions with responsibility for profit-and-loss management,” she added.
Dr. Rotenstein spoke about improving “access to the pipeline” that leads to leadership roles. The ways in which women are excluded from opportunities is often subtle and difficult to penetrate without fundamental changes, she explained.
“Institutions need to understand the processes that lead to leadership roles and make the changes that allow women and minorities to participate,” she said. It is not enough to recognize the problem, according to Dr. Rotenstein.
Like Dr. Chiang, she noted that changes are needed in the methods that move underrepresented groups into leadership roles.
Dr. Chiang reported no potential conflicts of interest relevant to this study.
Since the early 2000s, approximately half of medical students in the United States – and in many years, more than half – have been women, but according to an update provided at the virtual Pediatric Hospital Medicine.
In pediatrics, a specialty in which approximately 70% of physicians are now women, there has been progress, but still less than 30% of pediatric department chairs are female, said Vincent Chiang, MD, chief medical officer of Boston Children’s Hospital, during a presentation at the virtual meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Citing published data and a survey he personally conducted of the top children’s hospitals identified by the U.S. News and World Report, Dr. Chiang said a minority of division chiefs, chief medical officers, chief financial officers, and other leaders are female. At his institution, only 2 of 16 division chiefs are female.
“No matter how you slice it, women are underrepresented in leadership positions,” he noted.
The problem is certainly not confined to medicine. Dr. Chiang cited data showing that women and men have reached “near parity” in workforce participation in the United States even though the 20% earnings gap has changed little over time.
According to 2020 data from the World Economic Forum, the United States ranked 51 for the gender gap calculated on the basis of economic, political, educational, and health attainment. Even if this places the United States in the top third of the rankings, it is far behind Iceland and the Scandinavian countries that lead the list.
Efforts to reduce structural biases are part of the fix, but Dr. Chiang cautioned that fundamental changes might never occur if the plan is to wait for an approach based on meritocracy. He said that existing structural biases are “slanted away from women,” who are not necessarily granted the opportunities that are readily available to men.
“A meritocracy only works if the initial playing field was level. Otherwise, it just perpetuates the inequalities,” he said.
The problem is not a shortage of women with the skills to lead. In a study by Zenger/Folkman, a consulting company that works on leadership skill development, women performed better than men in 16 of 18 leadership categories, according to Dr. Chiang.
“There is certainly no shortage of capable women,” he noted.
Of the many issues, Dr. Chiang highlighted two. The first is the challenge of placing women on leadership pathways. This is likely to require proactive strategies, such as fast-track advancement programs that guide female candidates toward leadership roles.
The second is more nuanced. According to Dr. Chiang, women who want to assume a leadership role should think more actively about how and who is making decisions at their institution so they can position themselves appropriately. This is nuanced because “there is a certain amount of gamesmanship,” he said. The rise to leadership “has never been a pure meritocracy.”
Importantly, many of the key decisions in any institution involve money, according to Dr. Chiang. As a result, he advised those seeking leadership roles to join audit committees or otherwise take on responsibility for profit-and-loss management. Even in a nonprofit institution, “you need to make the numbers work,” he said, citing the common catchphrase: “No margin, no mission.”
However, Dr. Chiang acknowledged the many obstacles that prevent women from working their way into positions of leadership. For example, networking is important, but women are not necessarily attracted or invited to some of the social engagements, such as golf outings, where strong relationships are created.
In a survey of 100,000 people working at Fortune 500 companies, “82% of women say they feel excluded at work and much of that comes from that informal networking,” Dr. Chiang said. “Whereas 92% of men think they are not excluding women in their daily work.”
There is no single solution, but Dr. Chiang believes that concrete structural changes are needed. Female doctors remain grossly underrepresented in leadership roles even as they now represent more than half of the workforce for many specialties. Based on the need for proactive approaches outlined by Dr. Chiang, it appears unlikely that gender inequality will ever resolve itself.
Lisa S. Rotenstein, MD, who has written on fixing the gender imbalance in health care, including for the Harvard Business Review, said she agreed during an interview that structural changes are critical.
“In order to address current disparities, leaders should be thinking about how to remove both the formal and informal obstacles that prevent women and minorities from getting into the rooms where these decisions are being made,” said Dr. Rotenstein, who is an instructor in medicine at Brigham and Women’s Hospital, Harvard Medical School in Boston.
“This will need to involve sponsorship that gets women invited to the right committees or in positions with responsibility for profit-and-loss management,” she added.
Dr. Rotenstein spoke about improving “access to the pipeline” that leads to leadership roles. The ways in which women are excluded from opportunities is often subtle and difficult to penetrate without fundamental changes, she explained.
“Institutions need to understand the processes that lead to leadership roles and make the changes that allow women and minorities to participate,” she said. It is not enough to recognize the problem, according to Dr. Rotenstein.
Like Dr. Chiang, she noted that changes are needed in the methods that move underrepresented groups into leadership roles.
Dr. Chiang reported no potential conflicts of interest relevant to this study.
Since the early 2000s, approximately half of medical students in the United States – and in many years, more than half – have been women, but according to an update provided at the virtual Pediatric Hospital Medicine.
In pediatrics, a specialty in which approximately 70% of physicians are now women, there has been progress, but still less than 30% of pediatric department chairs are female, said Vincent Chiang, MD, chief medical officer of Boston Children’s Hospital, during a presentation at the virtual meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Citing published data and a survey he personally conducted of the top children’s hospitals identified by the U.S. News and World Report, Dr. Chiang said a minority of division chiefs, chief medical officers, chief financial officers, and other leaders are female. At his institution, only 2 of 16 division chiefs are female.
“No matter how you slice it, women are underrepresented in leadership positions,” he noted.
The problem is certainly not confined to medicine. Dr. Chiang cited data showing that women and men have reached “near parity” in workforce participation in the United States even though the 20% earnings gap has changed little over time.
According to 2020 data from the World Economic Forum, the United States ranked 51 for the gender gap calculated on the basis of economic, political, educational, and health attainment. Even if this places the United States in the top third of the rankings, it is far behind Iceland and the Scandinavian countries that lead the list.
Efforts to reduce structural biases are part of the fix, but Dr. Chiang cautioned that fundamental changes might never occur if the plan is to wait for an approach based on meritocracy. He said that existing structural biases are “slanted away from women,” who are not necessarily granted the opportunities that are readily available to men.
“A meritocracy only works if the initial playing field was level. Otherwise, it just perpetuates the inequalities,” he said.
The problem is not a shortage of women with the skills to lead. In a study by Zenger/Folkman, a consulting company that works on leadership skill development, women performed better than men in 16 of 18 leadership categories, according to Dr. Chiang.
“There is certainly no shortage of capable women,” he noted.
Of the many issues, Dr. Chiang highlighted two. The first is the challenge of placing women on leadership pathways. This is likely to require proactive strategies, such as fast-track advancement programs that guide female candidates toward leadership roles.
The second is more nuanced. According to Dr. Chiang, women who want to assume a leadership role should think more actively about how and who is making decisions at their institution so they can position themselves appropriately. This is nuanced because “there is a certain amount of gamesmanship,” he said. The rise to leadership “has never been a pure meritocracy.”
Importantly, many of the key decisions in any institution involve money, according to Dr. Chiang. As a result, he advised those seeking leadership roles to join audit committees or otherwise take on responsibility for profit-and-loss management. Even in a nonprofit institution, “you need to make the numbers work,” he said, citing the common catchphrase: “No margin, no mission.”
However, Dr. Chiang acknowledged the many obstacles that prevent women from working their way into positions of leadership. For example, networking is important, but women are not necessarily attracted or invited to some of the social engagements, such as golf outings, where strong relationships are created.
In a survey of 100,000 people working at Fortune 500 companies, “82% of women say they feel excluded at work and much of that comes from that informal networking,” Dr. Chiang said. “Whereas 92% of men think they are not excluding women in their daily work.”
There is no single solution, but Dr. Chiang believes that concrete structural changes are needed. Female doctors remain grossly underrepresented in leadership roles even as they now represent more than half of the workforce for many specialties. Based on the need for proactive approaches outlined by Dr. Chiang, it appears unlikely that gender inequality will ever resolve itself.
Lisa S. Rotenstein, MD, who has written on fixing the gender imbalance in health care, including for the Harvard Business Review, said she agreed during an interview that structural changes are critical.
“In order to address current disparities, leaders should be thinking about how to remove both the formal and informal obstacles that prevent women and minorities from getting into the rooms where these decisions are being made,” said Dr. Rotenstein, who is an instructor in medicine at Brigham and Women’s Hospital, Harvard Medical School in Boston.
“This will need to involve sponsorship that gets women invited to the right committees or in positions with responsibility for profit-and-loss management,” she added.
Dr. Rotenstein spoke about improving “access to the pipeline” that leads to leadership roles. The ways in which women are excluded from opportunities is often subtle and difficult to penetrate without fundamental changes, she explained.
“Institutions need to understand the processes that lead to leadership roles and make the changes that allow women and minorities to participate,” she said. It is not enough to recognize the problem, according to Dr. Rotenstein.
Like Dr. Chiang, she noted that changes are needed in the methods that move underrepresented groups into leadership roles.
Dr. Chiang reported no potential conflicts of interest relevant to this study.
FROM PHM20
New CDC guidance for health care personnel exposed to HCV
The new guidance was developed in part as a result of an increase in the incidence of acute HCV infection in the United States, which increases the risk for occupational exposure among HCP. “[I]n certain health care settings, HCP might be exposed to source patients with early HCV infection before those patients develop serologic evidence of infection or symptoms indicative of viral hepatitis,” wrote the authors of the report, published online July 24 in the CDC’s Morbidity and Mortality Weekly Report.
The guidelines, which no longer recommend waiting for spontaneous resolution upon initial diagnosis, include recommendations and algorithms for baseline and follow-up testing, appropriate test type, and recommendations for clinical management. The recommendations were developed on the basis of a current literature review, expert opinion from subject matter experts, and recent guidance from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.
Baseline testing ASAP
Baseline testing of the source patient and the HCP should be performed as soon as possible, preferably within 48 hours of exposure. The source patient should be tested for HCV RNA using a nucleic acid test. Alternatively, screening anti-HCV serology can be performed in patients at low risk for HCV and a nucleic acid test performed if serology is positive.
Baseline testing for the HCP should include anti-HCV testing and, if positive, HCV RNA testing is recommended. HCPs who test positive for HCV RNA at baseline are considered to have a preexisting HCV infection and should be referred for treatment.
Follow-up testing
For HCPs with exposure to blood or body fluids from a patient who is anti-HCV positive but HCV RNA negative, follow-up testing is not required.
If the source patient is HCV RNA positive, or if status of the source patient is unknown, the authors recommend that exposed HCPs have HCV RNA follow-up testing at 3-6 weeks post exposure, in addition to baseline testing. A final anti-HCV test is recommended at 4-6 months post exposure as there can be potential periods of aviremia during acute HCV infection.
Exposed HCPs who develop signs of illness indicative of HCV infection at any time should be tested for HCV RNA.
HCPs with positive HCV RNA test results should be referred for care and curative antiviral therapy.
Postexposure prophylaxis is not recommended
Recent data have shown that the risk for HCV infection from percutaneous exposure is 0.2% and from mucocutaneous exposure is 0%. On the basis of this information, the CDC guidelines no longer recommend routine postexposure prophylaxis for HCPs with occupational exposure to HCV. Rather, curative antiviral regimens should be reserved for instances of documented HCV transmission.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The new guidance was developed in part as a result of an increase in the incidence of acute HCV infection in the United States, which increases the risk for occupational exposure among HCP. “[I]n certain health care settings, HCP might be exposed to source patients with early HCV infection before those patients develop serologic evidence of infection or symptoms indicative of viral hepatitis,” wrote the authors of the report, published online July 24 in the CDC’s Morbidity and Mortality Weekly Report.
The guidelines, which no longer recommend waiting for spontaneous resolution upon initial diagnosis, include recommendations and algorithms for baseline and follow-up testing, appropriate test type, and recommendations for clinical management. The recommendations were developed on the basis of a current literature review, expert opinion from subject matter experts, and recent guidance from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.
Baseline testing ASAP
Baseline testing of the source patient and the HCP should be performed as soon as possible, preferably within 48 hours of exposure. The source patient should be tested for HCV RNA using a nucleic acid test. Alternatively, screening anti-HCV serology can be performed in patients at low risk for HCV and a nucleic acid test performed if serology is positive.
Baseline testing for the HCP should include anti-HCV testing and, if positive, HCV RNA testing is recommended. HCPs who test positive for HCV RNA at baseline are considered to have a preexisting HCV infection and should be referred for treatment.
Follow-up testing
For HCPs with exposure to blood or body fluids from a patient who is anti-HCV positive but HCV RNA negative, follow-up testing is not required.
If the source patient is HCV RNA positive, or if status of the source patient is unknown, the authors recommend that exposed HCPs have HCV RNA follow-up testing at 3-6 weeks post exposure, in addition to baseline testing. A final anti-HCV test is recommended at 4-6 months post exposure as there can be potential periods of aviremia during acute HCV infection.
Exposed HCPs who develop signs of illness indicative of HCV infection at any time should be tested for HCV RNA.
HCPs with positive HCV RNA test results should be referred for care and curative antiviral therapy.
Postexposure prophylaxis is not recommended
Recent data have shown that the risk for HCV infection from percutaneous exposure is 0.2% and from mucocutaneous exposure is 0%. On the basis of this information, the CDC guidelines no longer recommend routine postexposure prophylaxis for HCPs with occupational exposure to HCV. Rather, curative antiviral regimens should be reserved for instances of documented HCV transmission.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The new guidance was developed in part as a result of an increase in the incidence of acute HCV infection in the United States, which increases the risk for occupational exposure among HCP. “[I]n certain health care settings, HCP might be exposed to source patients with early HCV infection before those patients develop serologic evidence of infection or symptoms indicative of viral hepatitis,” wrote the authors of the report, published online July 24 in the CDC’s Morbidity and Mortality Weekly Report.
The guidelines, which no longer recommend waiting for spontaneous resolution upon initial diagnosis, include recommendations and algorithms for baseline and follow-up testing, appropriate test type, and recommendations for clinical management. The recommendations were developed on the basis of a current literature review, expert opinion from subject matter experts, and recent guidance from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.
Baseline testing ASAP
Baseline testing of the source patient and the HCP should be performed as soon as possible, preferably within 48 hours of exposure. The source patient should be tested for HCV RNA using a nucleic acid test. Alternatively, screening anti-HCV serology can be performed in patients at low risk for HCV and a nucleic acid test performed if serology is positive.
Baseline testing for the HCP should include anti-HCV testing and, if positive, HCV RNA testing is recommended. HCPs who test positive for HCV RNA at baseline are considered to have a preexisting HCV infection and should be referred for treatment.
Follow-up testing
For HCPs with exposure to blood or body fluids from a patient who is anti-HCV positive but HCV RNA negative, follow-up testing is not required.
If the source patient is HCV RNA positive, or if status of the source patient is unknown, the authors recommend that exposed HCPs have HCV RNA follow-up testing at 3-6 weeks post exposure, in addition to baseline testing. A final anti-HCV test is recommended at 4-6 months post exposure as there can be potential periods of aviremia during acute HCV infection.
Exposed HCPs who develop signs of illness indicative of HCV infection at any time should be tested for HCV RNA.
HCPs with positive HCV RNA test results should be referred for care and curative antiviral therapy.
Postexposure prophylaxis is not recommended
Recent data have shown that the risk for HCV infection from percutaneous exposure is 0.2% and from mucocutaneous exposure is 0%. On the basis of this information, the CDC guidelines no longer recommend routine postexposure prophylaxis for HCPs with occupational exposure to HCV. Rather, curative antiviral regimens should be reserved for instances of documented HCV transmission.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Rapid drop of antibodies seen in those with mild COVID-19
The research was conducted by F. Javier Ibarrondo, PhD, and colleagues and was published online on July 21 in a letter to the editor of the New England Journal of Medicine. Ibarrondo is associate researcher at the University of California, Los Angeles. (The original letter incorrectly calculated the half-life at 73 days.)
Coauthor Otto Yang, MD, professor of medicine in the division of infectious diseases at UCLA, told Medscape Medical News that the rapidity in the antibody drop at 5 weeks “is striking compared to other infections.”
The phenomenon has been suspected and has been observed before but had not been quantified.
“Our paper is the first to put firm numbers on the dropping of antibodies after early infection,” he said.
The researchers evaluated 34 people (average age, 43 years) who had recovered from mild COVID-19 and had referred themselves to UCLA for observational research.
Previous report also found a quick fade
As Medscape Medical News reported, a previous study from China that was published in Nature Medicine also found that the antibodies fade quickly.
Interpreting the meaning of the current research comes with a few caveats, Dr. Yang said.
“One is that we don’t know for sure that antibodies are what protect people from getting infected,” he said. Although it’s a reasonable assumption, he said, that’s not always the case.
Another caveat is that even if antibodies do protect, the tests being used to measure them – including the test that was used in this study – may not measure them the right way, and it is not yet known how many antibodies are needed for protection, he explained.
The UCLA researchers used an enzyme-linked immunosorbent assay to detect anti–SARS-CoV-2 spike receptor–binding domain immunoglobulin G concentrations.
“No reason for anybody to be getting an antibody test medically”
The study provides further proof that “[t]here’s no reason for anybody to be getting an antibody test medically right now,” Dr. Yang said.
Additionally, “FDA-approved tests are not approved for quantitative measures, only qualitative,” he continued. He noted that the findings may have implications with respect to herd immunity.
“Herd immunity depends on a lot of people having immunity to the infection all at the same time. If infection is followed by only brief protection from infection, the natural infection is not going to reach herd immunity,” he explained.
Buddy Creech, MD, MPH, associate professor of pediatrics and director of the Vanderbilt Vaccine Research Program in Nashville, Tenn., pointed out that antibodies “are just part of the story.”
“When we make an immune response to any germ,” he said, “we not only make an immune response for the time being but for the future. The next time we’re exposed, we can call into action B cells and T cells who have been there and done that.”
So even though the antibodies fade over time, other arms of the immune system are being trained for future action, he said.
Herd immunity does not require that populations have a huge level of antibodies that remains forever, he explained.
“It requires that in general, we’re not going to get infected as easily, and we’re not going to have disease as easily, and we’re not going to transmit the virus for as long,” he said.
Dr. Creech said he and others researching COVID-19 find that studies that show that antibodies fade quickly provide more proof “that this coronavirus is going to be here to stay unless we can take care of it through very effective treatments to take it from potentially fatal disease to one that is nothing more than a cold” or until a vaccine is developed.
He noted there are four other coronaviruses in widespread circulation every year that “amount to about 25% of the common cold.”
This study may help narrow the window as to when convalescent plasma – plasma that is taken from people who have recovered from COVID-19 and that is used to help people who are acutely ill with the disease – will be most effective, Dr. Creech explained. He said the results suggest that it is important that plasma be collected within the first couple of months after recovery so as to capture the most antibodies.
This study is important as another snapshot “so we understand the differences between severe and mild disease, so we can study it over time, so we have all the tools we need as we start these pivotal vaccine studies to make sure we’re making the right immune response for the right duration of time so we can put an end to this pandemic,” Dr. Creech concluded.
The study was supported by grants from the AIDS Healthcare Foundation, the Doris Duke Charitable Foundation, the National Institutes of Health, the James B. Pendleton Charitable Trust, and the McCarthy Family Foundation. A coauthor reports receiving grants from Gilead outside the submitted work. Dr. Creech has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The research was conducted by F. Javier Ibarrondo, PhD, and colleagues and was published online on July 21 in a letter to the editor of the New England Journal of Medicine. Ibarrondo is associate researcher at the University of California, Los Angeles. (The original letter incorrectly calculated the half-life at 73 days.)
Coauthor Otto Yang, MD, professor of medicine in the division of infectious diseases at UCLA, told Medscape Medical News that the rapidity in the antibody drop at 5 weeks “is striking compared to other infections.”
The phenomenon has been suspected and has been observed before but had not been quantified.
“Our paper is the first to put firm numbers on the dropping of antibodies after early infection,” he said.
The researchers evaluated 34 people (average age, 43 years) who had recovered from mild COVID-19 and had referred themselves to UCLA for observational research.
Previous report also found a quick fade
As Medscape Medical News reported, a previous study from China that was published in Nature Medicine also found that the antibodies fade quickly.
Interpreting the meaning of the current research comes with a few caveats, Dr. Yang said.
“One is that we don’t know for sure that antibodies are what protect people from getting infected,” he said. Although it’s a reasonable assumption, he said, that’s not always the case.
Another caveat is that even if antibodies do protect, the tests being used to measure them – including the test that was used in this study – may not measure them the right way, and it is not yet known how many antibodies are needed for protection, he explained.
The UCLA researchers used an enzyme-linked immunosorbent assay to detect anti–SARS-CoV-2 spike receptor–binding domain immunoglobulin G concentrations.
“No reason for anybody to be getting an antibody test medically”
The study provides further proof that “[t]here’s no reason for anybody to be getting an antibody test medically right now,” Dr. Yang said.
Additionally, “FDA-approved tests are not approved for quantitative measures, only qualitative,” he continued. He noted that the findings may have implications with respect to herd immunity.
“Herd immunity depends on a lot of people having immunity to the infection all at the same time. If infection is followed by only brief protection from infection, the natural infection is not going to reach herd immunity,” he explained.
Buddy Creech, MD, MPH, associate professor of pediatrics and director of the Vanderbilt Vaccine Research Program in Nashville, Tenn., pointed out that antibodies “are just part of the story.”
“When we make an immune response to any germ,” he said, “we not only make an immune response for the time being but for the future. The next time we’re exposed, we can call into action B cells and T cells who have been there and done that.”
So even though the antibodies fade over time, other arms of the immune system are being trained for future action, he said.
Herd immunity does not require that populations have a huge level of antibodies that remains forever, he explained.
“It requires that in general, we’re not going to get infected as easily, and we’re not going to have disease as easily, and we’re not going to transmit the virus for as long,” he said.
Dr. Creech said he and others researching COVID-19 find that studies that show that antibodies fade quickly provide more proof “that this coronavirus is going to be here to stay unless we can take care of it through very effective treatments to take it from potentially fatal disease to one that is nothing more than a cold” or until a vaccine is developed.
He noted there are four other coronaviruses in widespread circulation every year that “amount to about 25% of the common cold.”
This study may help narrow the window as to when convalescent plasma – plasma that is taken from people who have recovered from COVID-19 and that is used to help people who are acutely ill with the disease – will be most effective, Dr. Creech explained. He said the results suggest that it is important that plasma be collected within the first couple of months after recovery so as to capture the most antibodies.
This study is important as another snapshot “so we understand the differences between severe and mild disease, so we can study it over time, so we have all the tools we need as we start these pivotal vaccine studies to make sure we’re making the right immune response for the right duration of time so we can put an end to this pandemic,” Dr. Creech concluded.
The study was supported by grants from the AIDS Healthcare Foundation, the Doris Duke Charitable Foundation, the National Institutes of Health, the James B. Pendleton Charitable Trust, and the McCarthy Family Foundation. A coauthor reports receiving grants from Gilead outside the submitted work. Dr. Creech has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The research was conducted by F. Javier Ibarrondo, PhD, and colleagues and was published online on July 21 in a letter to the editor of the New England Journal of Medicine. Ibarrondo is associate researcher at the University of California, Los Angeles. (The original letter incorrectly calculated the half-life at 73 days.)
Coauthor Otto Yang, MD, professor of medicine in the division of infectious diseases at UCLA, told Medscape Medical News that the rapidity in the antibody drop at 5 weeks “is striking compared to other infections.”
The phenomenon has been suspected and has been observed before but had not been quantified.
“Our paper is the first to put firm numbers on the dropping of antibodies after early infection,” he said.
The researchers evaluated 34 people (average age, 43 years) who had recovered from mild COVID-19 and had referred themselves to UCLA for observational research.
Previous report also found a quick fade
As Medscape Medical News reported, a previous study from China that was published in Nature Medicine also found that the antibodies fade quickly.
Interpreting the meaning of the current research comes with a few caveats, Dr. Yang said.
“One is that we don’t know for sure that antibodies are what protect people from getting infected,” he said. Although it’s a reasonable assumption, he said, that’s not always the case.
Another caveat is that even if antibodies do protect, the tests being used to measure them – including the test that was used in this study – may not measure them the right way, and it is not yet known how many antibodies are needed for protection, he explained.
The UCLA researchers used an enzyme-linked immunosorbent assay to detect anti–SARS-CoV-2 spike receptor–binding domain immunoglobulin G concentrations.
“No reason for anybody to be getting an antibody test medically”
The study provides further proof that “[t]here’s no reason for anybody to be getting an antibody test medically right now,” Dr. Yang said.
Additionally, “FDA-approved tests are not approved for quantitative measures, only qualitative,” he continued. He noted that the findings may have implications with respect to herd immunity.
“Herd immunity depends on a lot of people having immunity to the infection all at the same time. If infection is followed by only brief protection from infection, the natural infection is not going to reach herd immunity,” he explained.
Buddy Creech, MD, MPH, associate professor of pediatrics and director of the Vanderbilt Vaccine Research Program in Nashville, Tenn., pointed out that antibodies “are just part of the story.”
“When we make an immune response to any germ,” he said, “we not only make an immune response for the time being but for the future. The next time we’re exposed, we can call into action B cells and T cells who have been there and done that.”
So even though the antibodies fade over time, other arms of the immune system are being trained for future action, he said.
Herd immunity does not require that populations have a huge level of antibodies that remains forever, he explained.
“It requires that in general, we’re not going to get infected as easily, and we’re not going to have disease as easily, and we’re not going to transmit the virus for as long,” he said.
Dr. Creech said he and others researching COVID-19 find that studies that show that antibodies fade quickly provide more proof “that this coronavirus is going to be here to stay unless we can take care of it through very effective treatments to take it from potentially fatal disease to one that is nothing more than a cold” or until a vaccine is developed.
He noted there are four other coronaviruses in widespread circulation every year that “amount to about 25% of the common cold.”
This study may help narrow the window as to when convalescent plasma – plasma that is taken from people who have recovered from COVID-19 and that is used to help people who are acutely ill with the disease – will be most effective, Dr. Creech explained. He said the results suggest that it is important that plasma be collected within the first couple of months after recovery so as to capture the most antibodies.
This study is important as another snapshot “so we understand the differences between severe and mild disease, so we can study it over time, so we have all the tools we need as we start these pivotal vaccine studies to make sure we’re making the right immune response for the right duration of time so we can put an end to this pandemic,” Dr. Creech concluded.
The study was supported by grants from the AIDS Healthcare Foundation, the Doris Duke Charitable Foundation, the National Institutes of Health, the James B. Pendleton Charitable Trust, and the McCarthy Family Foundation. A coauthor reports receiving grants from Gilead outside the submitted work. Dr. Creech has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Is the presence of enanthem a clue for COVID-19?
Larger studies should explore and confirm this association, the study’s authors and other experts suggested.
Dermatologists are already aware of the connection between enanthem and viral etiology. “As seen with other viral infections, we wondered if COVID-19 could produce enanthem in addition to skin rash exanthem,” one of the study author’s, Juan Jiménez-Cauhe, MD, a dermatologist with Hospital Universitario Ramon y Cajal, Madrid, said in an interview. He and his colleagues summarized their findings in a research letter in JAMA Dermatology.
They examined the oral cavity of 21 COVID-19 patients at a tertiary care hospital who also had a skin rash from March 30 to April 8. They classified enanthems into four categories: petechial, macular, macular with petechiae, or erythematovesicular. Six of the patients presented with oral lesions, all of them located in the palate; in one patient, the enanthem was macular, it was petechial in two patients and was macular with petechiae in three patients. The six patients ranged between the ages of 40 and 69 years; four were women.
Petechial or vesicular patterns are often associated with viral infections. In this particular study, the investigators did not observe vesicular lesions.
On average, mucocutaneous lesions appeared about 12 days after the onset of COVID-19 symptoms. “Interestingly, this latency was shorter in patients with petechial enanthem, compared with those with a macular lesion with petechiae appearance,” the authors wrote.
This shorter time might suggest an association for SARS-CoV-2, said Dr. Jiménez-Cauhe. Strong cough may have also caused petechial lesions on the palate, but it’s unlikely, as they appeared close in time to COVID-19 symptoms. It’s also unlikely that any drugs caused the lesions, as drug rashes can take 2-3 weeks to appear.
This fits in line with other evidence of broader skin manifestations appearing at the same time or after COVID-19, Esther Freeman, MD, said in an interview. Dr. Freeman, director of global health dermatology at Massachusetts General Hospital, Boston, is the principal investigator of the COVID-19 Dermatology Registry, a collaboration of the American Academy of Dermatology and International League of Dermatological Societies.
The study’s small cohort made it difficult to establish a solid association between the oral lesions and SARS-CoV-2. “However, the presence of enanthem in a patient with a skin rash is a useful finding that suggests a viral etiology rather than a drug reaction. This is particularly useful in COVID-19 patients, who were receiving many drugs as part of the treatment,” Dr. Jimenez-Cauhe said. Future studies should assess whether the presence of enanthem and exanthem lead physicians to consider SARS-CoV-2 as possible agents, ruling out infection with a blood or nasopharyngeal test.
This study adds to the growing body of knowledge on cutaneous and mucocutaneous findings associated with SARS-CoV-2 infection, Jules Lipoff, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, said in an interview. “One challenge in evaluating these findings is that these findings are nonspecific, and medication reactions can often cause similar rashes, such as morbilliform eruptions that can be associated with both viruses and medications.”
Enanthems, as the study authors noted, are more specific to viral infections and are less commonly associated with medication reactions. “So, even though this is a small case series with significant limitations, it does add more evidence that COVID-19 is directly responsible for findings in the skin and mucous membranes,” said Dr. Lipoff.
Dr. Freeman noted that the study may also encourage clinicians to look in a patient’s mouth when assessing for SARS-CoV-2. Additional research should examine these data in a larger population.
Several studies by Dr. Freeman, Dr. Lipoff, and others strongly suggest that SARS-CoV-2 has a spectrum of associated dermatologic manifestations. One evaluated perniolike skin lesions (J Am Acad Dermatol. 2020 Aug; 83[2]:486-92). The other was a case series from the COVID-19 registry that examined 716 cases of new-onset dermatologic symptoms in patients from 31 countries with confirmed/suspected SARS-CoV-2 (J Am Acad Dermatol. 2020 Jul 2;S0190-9622[20]32126-5.).
The authors of the report had no disclosures.
SOURCE: Jimenez-Cauhe J et al. JAMA Dermatol. 2020 Jul 15. doi: 10.1001/jamadermatol.2020.2550.
Larger studies should explore and confirm this association, the study’s authors and other experts suggested.
Dermatologists are already aware of the connection between enanthem and viral etiology. “As seen with other viral infections, we wondered if COVID-19 could produce enanthem in addition to skin rash exanthem,” one of the study author’s, Juan Jiménez-Cauhe, MD, a dermatologist with Hospital Universitario Ramon y Cajal, Madrid, said in an interview. He and his colleagues summarized their findings in a research letter in JAMA Dermatology.
They examined the oral cavity of 21 COVID-19 patients at a tertiary care hospital who also had a skin rash from March 30 to April 8. They classified enanthems into four categories: petechial, macular, macular with petechiae, or erythematovesicular. Six of the patients presented with oral lesions, all of them located in the palate; in one patient, the enanthem was macular, it was petechial in two patients and was macular with petechiae in three patients. The six patients ranged between the ages of 40 and 69 years; four were women.
Petechial or vesicular patterns are often associated with viral infections. In this particular study, the investigators did not observe vesicular lesions.
On average, mucocutaneous lesions appeared about 12 days after the onset of COVID-19 symptoms. “Interestingly, this latency was shorter in patients with petechial enanthem, compared with those with a macular lesion with petechiae appearance,” the authors wrote.
This shorter time might suggest an association for SARS-CoV-2, said Dr. Jiménez-Cauhe. Strong cough may have also caused petechial lesions on the palate, but it’s unlikely, as they appeared close in time to COVID-19 symptoms. It’s also unlikely that any drugs caused the lesions, as drug rashes can take 2-3 weeks to appear.
This fits in line with other evidence of broader skin manifestations appearing at the same time or after COVID-19, Esther Freeman, MD, said in an interview. Dr. Freeman, director of global health dermatology at Massachusetts General Hospital, Boston, is the principal investigator of the COVID-19 Dermatology Registry, a collaboration of the American Academy of Dermatology and International League of Dermatological Societies.
The study’s small cohort made it difficult to establish a solid association between the oral lesions and SARS-CoV-2. “However, the presence of enanthem in a patient with a skin rash is a useful finding that suggests a viral etiology rather than a drug reaction. This is particularly useful in COVID-19 patients, who were receiving many drugs as part of the treatment,” Dr. Jimenez-Cauhe said. Future studies should assess whether the presence of enanthem and exanthem lead physicians to consider SARS-CoV-2 as possible agents, ruling out infection with a blood or nasopharyngeal test.
This study adds to the growing body of knowledge on cutaneous and mucocutaneous findings associated with SARS-CoV-2 infection, Jules Lipoff, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, said in an interview. “One challenge in evaluating these findings is that these findings are nonspecific, and medication reactions can often cause similar rashes, such as morbilliform eruptions that can be associated with both viruses and medications.”
Enanthems, as the study authors noted, are more specific to viral infections and are less commonly associated with medication reactions. “So, even though this is a small case series with significant limitations, it does add more evidence that COVID-19 is directly responsible for findings in the skin and mucous membranes,” said Dr. Lipoff.
Dr. Freeman noted that the study may also encourage clinicians to look in a patient’s mouth when assessing for SARS-CoV-2. Additional research should examine these data in a larger population.
Several studies by Dr. Freeman, Dr. Lipoff, and others strongly suggest that SARS-CoV-2 has a spectrum of associated dermatologic manifestations. One evaluated perniolike skin lesions (J Am Acad Dermatol. 2020 Aug; 83[2]:486-92). The other was a case series from the COVID-19 registry that examined 716 cases of new-onset dermatologic symptoms in patients from 31 countries with confirmed/suspected SARS-CoV-2 (J Am Acad Dermatol. 2020 Jul 2;S0190-9622[20]32126-5.).
The authors of the report had no disclosures.
SOURCE: Jimenez-Cauhe J et al. JAMA Dermatol. 2020 Jul 15. doi: 10.1001/jamadermatol.2020.2550.
Larger studies should explore and confirm this association, the study’s authors and other experts suggested.
Dermatologists are already aware of the connection between enanthem and viral etiology. “As seen with other viral infections, we wondered if COVID-19 could produce enanthem in addition to skin rash exanthem,” one of the study author’s, Juan Jiménez-Cauhe, MD, a dermatologist with Hospital Universitario Ramon y Cajal, Madrid, said in an interview. He and his colleagues summarized their findings in a research letter in JAMA Dermatology.
They examined the oral cavity of 21 COVID-19 patients at a tertiary care hospital who also had a skin rash from March 30 to April 8. They classified enanthems into four categories: petechial, macular, macular with petechiae, or erythematovesicular. Six of the patients presented with oral lesions, all of them located in the palate; in one patient, the enanthem was macular, it was petechial in two patients and was macular with petechiae in three patients. The six patients ranged between the ages of 40 and 69 years; four were women.
Petechial or vesicular patterns are often associated with viral infections. In this particular study, the investigators did not observe vesicular lesions.
On average, mucocutaneous lesions appeared about 12 days after the onset of COVID-19 symptoms. “Interestingly, this latency was shorter in patients with petechial enanthem, compared with those with a macular lesion with petechiae appearance,” the authors wrote.
This shorter time might suggest an association for SARS-CoV-2, said Dr. Jiménez-Cauhe. Strong cough may have also caused petechial lesions on the palate, but it’s unlikely, as they appeared close in time to COVID-19 symptoms. It’s also unlikely that any drugs caused the lesions, as drug rashes can take 2-3 weeks to appear.
This fits in line with other evidence of broader skin manifestations appearing at the same time or after COVID-19, Esther Freeman, MD, said in an interview. Dr. Freeman, director of global health dermatology at Massachusetts General Hospital, Boston, is the principal investigator of the COVID-19 Dermatology Registry, a collaboration of the American Academy of Dermatology and International League of Dermatological Societies.
The study’s small cohort made it difficult to establish a solid association between the oral lesions and SARS-CoV-2. “However, the presence of enanthem in a patient with a skin rash is a useful finding that suggests a viral etiology rather than a drug reaction. This is particularly useful in COVID-19 patients, who were receiving many drugs as part of the treatment,” Dr. Jimenez-Cauhe said. Future studies should assess whether the presence of enanthem and exanthem lead physicians to consider SARS-CoV-2 as possible agents, ruling out infection with a blood or nasopharyngeal test.
This study adds to the growing body of knowledge on cutaneous and mucocutaneous findings associated with SARS-CoV-2 infection, Jules Lipoff, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, said in an interview. “One challenge in evaluating these findings is that these findings are nonspecific, and medication reactions can often cause similar rashes, such as morbilliform eruptions that can be associated with both viruses and medications.”
Enanthems, as the study authors noted, are more specific to viral infections and are less commonly associated with medication reactions. “So, even though this is a small case series with significant limitations, it does add more evidence that COVID-19 is directly responsible for findings in the skin and mucous membranes,” said Dr. Lipoff.
Dr. Freeman noted that the study may also encourage clinicians to look in a patient’s mouth when assessing for SARS-CoV-2. Additional research should examine these data in a larger population.
Several studies by Dr. Freeman, Dr. Lipoff, and others strongly suggest that SARS-CoV-2 has a spectrum of associated dermatologic manifestations. One evaluated perniolike skin lesions (J Am Acad Dermatol. 2020 Aug; 83[2]:486-92). The other was a case series from the COVID-19 registry that examined 716 cases of new-onset dermatologic symptoms in patients from 31 countries with confirmed/suspected SARS-CoV-2 (J Am Acad Dermatol. 2020 Jul 2;S0190-9622[20]32126-5.).
The authors of the report had no disclosures.
SOURCE: Jimenez-Cauhe J et al. JAMA Dermatol. 2020 Jul 15. doi: 10.1001/jamadermatol.2020.2550.
FROM JAMA DERMATOLOGY
Low-dose prasugrel preserves efficacy but lowers bleeding in elderly
In elderly or low-weight patients with acute coronary syndrome (ACS), a reduced dose of prasugrel relative to a full-dose of ticagrelor is associated with lower numerical rates of ischemic events and bleeding events, according to a prespecified substudy of the ISAR-REACT 5 trial.
“The present study provides the strongest support for reduced-dose prasugrel as the standard for elderly and low-weight patients with ACS undergoing an invasive treatment strategy,” according to the senior author, Adnan Kastrati, MD, professor of cardiology and head of the Catheterization Laboratory at Deutsches Herzzentrum, Technical University of Munich.
The main results of ISAR-REACT 5, an open-label, head-to-head comparison of prasugrel and ticagrelor in patients with ACS, showed that the risk of the composite primary endpoint of death, myocardial infarction, or stroke 1 year after randomization was significantly higher for those on ticagrelor than prasugrel (hazard ratio, 1.39; P = .006). The bleeding risk on ticagrelor was also higher but not significantly different (5.4% vs. 4.8%; P = .46) (Schüpke S et al. N Engl J Med. 2019 Oct;381:1524-34).
In this substudy newly published in Annals of Internal Medicine, outcomes were compared in the 1,099 patients who were 75 years or older or weighed less than 60 kg. In this group, unlike those younger or weighing more, patients were randomized to receive a reduced maintenance dose of 5 mg of once-daily prasugrel (rather than 10 mg) or full dose ticagrelor (90 mg twice daily).
At 1 year, the low-dose prasugrel strategy relative to ticagrelor was associated with a lower rate of events (12.7% vs. 14.6%) and a lower rate of bleeding (8.1% vs. 10.6%), defined as Bleeding Academic Research Consortium (BARC) type 3-5 events.
Neither the 18% reduction for the efficacy endpoint (HR, 0.82; 95% CI 0.60-1.14) nor the 28% reduction in the bleeding endpoint (HR, 0.72; 95% CI 0.46-1.12) reached significance, but Dr. Kastrati reported that there was a significant “treatment effect-by-study-group interaction” for BARC 1-5 bleeding (P = .004) favoring prasugrel. This supports low-dose prasugrel as a strategy to prevent the excess bleeding risk previously observed with the standard 10-mg dose of prasugrel.
In other words, a reduced dose of prasugrel, compared with the standard dose of ticagrelor, in low-weight and elderly patients “is associated with maintained anti-ischemic efficacy while protecting these patients against the excess risk of bleeding,” he and his coinvestigators concluded.
Low-weight and older patients represented 27% of those enrolled in ISAR-REACT 5. When compared to the study population as a whole, the risk for both ischemic and bleeding events was at least twice as high, the authors of an accompanying editorial observed. They praised this effort to refine the optimal antiplatelet regimen in a very-high-risk ACS population.
“The current analysis suggests that the prasugrel dose reduction regimen for elderly or underweight patients with ACS is effective and safe,” according to the editorial coauthors, David Conen, MD, and P.J. Devereaux, MD, PhD, who are affiliated with the Population Health Research Institute, Hamilton, Ontario.
This substudy was underpowered to show superiority for the efficacy and safety outcomes in elderly and low-weight ACS patients, which makes these results “hypothesis generating,” but the authors believe that they provide the best available evidence for selecting antiplatelet therapy in this challenging subgroup. Although the exclusion of patients at very high risk of bleeding from ISAR-REACT 5 suggest findings might not be relevant to all elderly and low-weight individuals, the investigators believe the data do inform clinical practice.
“Our study is the first head-to-head randomized comparison of the reduced dose of prasugrel against standard dose of ticagrelor in elderly and low-weight patients,” said Dr. Kastrati in an interview. “Specifically designed studies for this subset of patients are very unlikely to be conducted in the future.”
Dr. Kastrati reported no potential conflicts of interest relevant to this study.
SOURCE: Menichelli M et al. Ann Intern Med. 2020 Jul 21. doi: 10.7326/M20-1806.
In elderly or low-weight patients with acute coronary syndrome (ACS), a reduced dose of prasugrel relative to a full-dose of ticagrelor is associated with lower numerical rates of ischemic events and bleeding events, according to a prespecified substudy of the ISAR-REACT 5 trial.
“The present study provides the strongest support for reduced-dose prasugrel as the standard for elderly and low-weight patients with ACS undergoing an invasive treatment strategy,” according to the senior author, Adnan Kastrati, MD, professor of cardiology and head of the Catheterization Laboratory at Deutsches Herzzentrum, Technical University of Munich.
The main results of ISAR-REACT 5, an open-label, head-to-head comparison of prasugrel and ticagrelor in patients with ACS, showed that the risk of the composite primary endpoint of death, myocardial infarction, or stroke 1 year after randomization was significantly higher for those on ticagrelor than prasugrel (hazard ratio, 1.39; P = .006). The bleeding risk on ticagrelor was also higher but not significantly different (5.4% vs. 4.8%; P = .46) (Schüpke S et al. N Engl J Med. 2019 Oct;381:1524-34).
In this substudy newly published in Annals of Internal Medicine, outcomes were compared in the 1,099 patients who were 75 years or older or weighed less than 60 kg. In this group, unlike those younger or weighing more, patients were randomized to receive a reduced maintenance dose of 5 mg of once-daily prasugrel (rather than 10 mg) or full dose ticagrelor (90 mg twice daily).
At 1 year, the low-dose prasugrel strategy relative to ticagrelor was associated with a lower rate of events (12.7% vs. 14.6%) and a lower rate of bleeding (8.1% vs. 10.6%), defined as Bleeding Academic Research Consortium (BARC) type 3-5 events.
Neither the 18% reduction for the efficacy endpoint (HR, 0.82; 95% CI 0.60-1.14) nor the 28% reduction in the bleeding endpoint (HR, 0.72; 95% CI 0.46-1.12) reached significance, but Dr. Kastrati reported that there was a significant “treatment effect-by-study-group interaction” for BARC 1-5 bleeding (P = .004) favoring prasugrel. This supports low-dose prasugrel as a strategy to prevent the excess bleeding risk previously observed with the standard 10-mg dose of prasugrel.
In other words, a reduced dose of prasugrel, compared with the standard dose of ticagrelor, in low-weight and elderly patients “is associated with maintained anti-ischemic efficacy while protecting these patients against the excess risk of bleeding,” he and his coinvestigators concluded.
Low-weight and older patients represented 27% of those enrolled in ISAR-REACT 5. When compared to the study population as a whole, the risk for both ischemic and bleeding events was at least twice as high, the authors of an accompanying editorial observed. They praised this effort to refine the optimal antiplatelet regimen in a very-high-risk ACS population.
“The current analysis suggests that the prasugrel dose reduction regimen for elderly or underweight patients with ACS is effective and safe,” according to the editorial coauthors, David Conen, MD, and P.J. Devereaux, MD, PhD, who are affiliated with the Population Health Research Institute, Hamilton, Ontario.
This substudy was underpowered to show superiority for the efficacy and safety outcomes in elderly and low-weight ACS patients, which makes these results “hypothesis generating,” but the authors believe that they provide the best available evidence for selecting antiplatelet therapy in this challenging subgroup. Although the exclusion of patients at very high risk of bleeding from ISAR-REACT 5 suggest findings might not be relevant to all elderly and low-weight individuals, the investigators believe the data do inform clinical practice.
“Our study is the first head-to-head randomized comparison of the reduced dose of prasugrel against standard dose of ticagrelor in elderly and low-weight patients,” said Dr. Kastrati in an interview. “Specifically designed studies for this subset of patients are very unlikely to be conducted in the future.”
Dr. Kastrati reported no potential conflicts of interest relevant to this study.
SOURCE: Menichelli M et al. Ann Intern Med. 2020 Jul 21. doi: 10.7326/M20-1806.
In elderly or low-weight patients with acute coronary syndrome (ACS), a reduced dose of prasugrel relative to a full-dose of ticagrelor is associated with lower numerical rates of ischemic events and bleeding events, according to a prespecified substudy of the ISAR-REACT 5 trial.
“The present study provides the strongest support for reduced-dose prasugrel as the standard for elderly and low-weight patients with ACS undergoing an invasive treatment strategy,” according to the senior author, Adnan Kastrati, MD, professor of cardiology and head of the Catheterization Laboratory at Deutsches Herzzentrum, Technical University of Munich.
The main results of ISAR-REACT 5, an open-label, head-to-head comparison of prasugrel and ticagrelor in patients with ACS, showed that the risk of the composite primary endpoint of death, myocardial infarction, or stroke 1 year after randomization was significantly higher for those on ticagrelor than prasugrel (hazard ratio, 1.39; P = .006). The bleeding risk on ticagrelor was also higher but not significantly different (5.4% vs. 4.8%; P = .46) (Schüpke S et al. N Engl J Med. 2019 Oct;381:1524-34).
In this substudy newly published in Annals of Internal Medicine, outcomes were compared in the 1,099 patients who were 75 years or older or weighed less than 60 kg. In this group, unlike those younger or weighing more, patients were randomized to receive a reduced maintenance dose of 5 mg of once-daily prasugrel (rather than 10 mg) or full dose ticagrelor (90 mg twice daily).
At 1 year, the low-dose prasugrel strategy relative to ticagrelor was associated with a lower rate of events (12.7% vs. 14.6%) and a lower rate of bleeding (8.1% vs. 10.6%), defined as Bleeding Academic Research Consortium (BARC) type 3-5 events.
Neither the 18% reduction for the efficacy endpoint (HR, 0.82; 95% CI 0.60-1.14) nor the 28% reduction in the bleeding endpoint (HR, 0.72; 95% CI 0.46-1.12) reached significance, but Dr. Kastrati reported that there was a significant “treatment effect-by-study-group interaction” for BARC 1-5 bleeding (P = .004) favoring prasugrel. This supports low-dose prasugrel as a strategy to prevent the excess bleeding risk previously observed with the standard 10-mg dose of prasugrel.
In other words, a reduced dose of prasugrel, compared with the standard dose of ticagrelor, in low-weight and elderly patients “is associated with maintained anti-ischemic efficacy while protecting these patients against the excess risk of bleeding,” he and his coinvestigators concluded.
Low-weight and older patients represented 27% of those enrolled in ISAR-REACT 5. When compared to the study population as a whole, the risk for both ischemic and bleeding events was at least twice as high, the authors of an accompanying editorial observed. They praised this effort to refine the optimal antiplatelet regimen in a very-high-risk ACS population.
“The current analysis suggests that the prasugrel dose reduction regimen for elderly or underweight patients with ACS is effective and safe,” according to the editorial coauthors, David Conen, MD, and P.J. Devereaux, MD, PhD, who are affiliated with the Population Health Research Institute, Hamilton, Ontario.
This substudy was underpowered to show superiority for the efficacy and safety outcomes in elderly and low-weight ACS patients, which makes these results “hypothesis generating,” but the authors believe that they provide the best available evidence for selecting antiplatelet therapy in this challenging subgroup. Although the exclusion of patients at very high risk of bleeding from ISAR-REACT 5 suggest findings might not be relevant to all elderly and low-weight individuals, the investigators believe the data do inform clinical practice.
“Our study is the first head-to-head randomized comparison of the reduced dose of prasugrel against standard dose of ticagrelor in elderly and low-weight patients,” said Dr. Kastrati in an interview. “Specifically designed studies for this subset of patients are very unlikely to be conducted in the future.”
Dr. Kastrati reported no potential conflicts of interest relevant to this study.
SOURCE: Menichelli M et al. Ann Intern Med. 2020 Jul 21. doi: 10.7326/M20-1806.
FROM ANNALS OF INTERNAL MEDICINE
Internists’ use of ultrasound can reduce radiology referrals
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.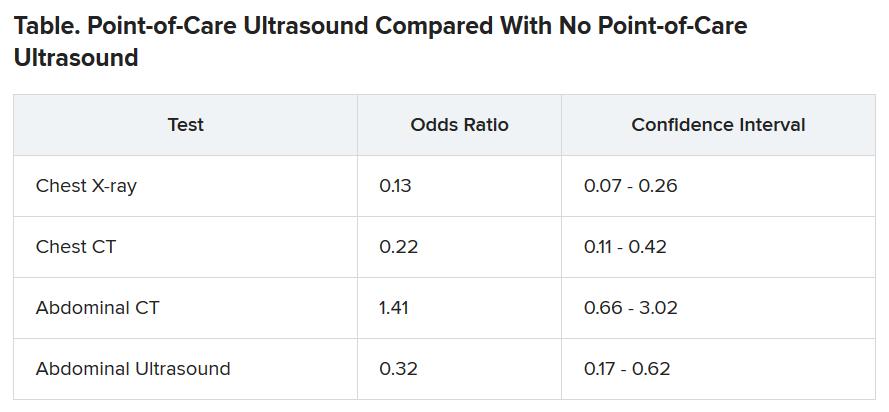
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Ob.gyns. struggle to keep pace with changing COVID-19 knowledge
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
In early April, Maura Quinlan, MD, was working nights on the labor and delivery unit at Northwestern Medicine Prentice Women’s Hospital in Chicago. At the time, hospital policy was to test only patients with known COVID-19 symptoms for SARS-CoV-2. Women in labor wore N95 masks, but only while pushing – and practitioners didn’t always don proper protection in time.
Babies came and families rejoiced. But Dr. Quinlan looks back on those weeks with a degree of horror. “We were laboring a bunch of patients that probably had COVID,” she said, and they were doing so without proper protection.
She’s probably right. According to one study in the New England Journal of Medicine, 13.7% of 211 women who came into the labor and delivery unit at one New York City hospital between March 22 and April 2 were asymptomatic but infected, potentially putting staff and doctors at risk.
Dr. Quinlan already knew she and her fellow ob.gyns. had been walking a thin line and, upon seeing that research, her heart sank. In the middle of a pandemic, they had been racing to keep up with the reality of delivering babies. But despite their efforts to protect both practitioners and patients, some aspects slipped through the cracks. Today, every laboring patient admitted to Northwestern is now tested for the novel coronavirus.
Across the country, hospital labor and delivery wards have been working to find a careful and informed balance among multiple competing interests: the safety of their health care workers, the health of tiny and vulnerable new humans, and the stability of a birthing mother. Each hospital has been making the best decisions it can based on available data. The result is a patchwork of policies, but all of them center around rapid testing and appropriate protection.
Shifting recommendations
One case study of women in a New York City hospital during the height of the city’s surge found that, of seven confirmed COVID-19–positive patients, two were asymptomatic upon admission to the obstetrical service, and these same two patients ultimately required unplanned ICU admission. The women’s care prior to their positive diagnosis had exposed multiple health care workers, all of whom lacked appropriate personal protective equipment (PPE), the study authors wrote. “Further, five of seven confirmed COVID-19–positive women were afebrile on initial screen, and four did not first report a cough. In some locations where testing availability remains limited, the minimal symptoms reported for some of these cases might have been insufficient to prompt COVID-19 testing.”
As studies like this pour in, societies continue to update their recommendations accordingly. The latest guidance from the American College of Obstetricians and Gynecologists came on July 1. The group suggests testing all labor and delivery patients, particularly in high-prevalence areas. If tests are in short supply, it recommends prioritizing testing pregnant women with suspected COVID-19 and those who develop symptoms during admission.
At Northwestern, the hospital requests patients stay home and quarantine for the weeks leading up to their delivery date. Then, they rapidly test every patient who comes in for delivery and aim to have results available within a few hours.
The hospital’s 30-room labor and delivery wing remains reserved for patients who test negative. Those with positive COVID-19 results are sent to a 6-bed COVID labor and delivery unit elsewhere in the hospital. “We were lucky we had the space to do that, because smaller community hospitals wouldn’t have a separate unused unit where they could put these women,” Dr. Quinlan said.
In the COVID unit, women deliver without a support person – no partner, doula, or family member can join. Doctors and nurses wear full PPE and work only in that ward. And because some research shows that pregnant women who are asymptomatic or presymptomatic may develop symptoms quickly after starting labor with no measurable illness, Dr. Quinlan must decide on a case-by-case basis what to do, if anything at all.
Delaying an induction could allow the infection to resolve or it could result in her patient moving from presymptomatic disease to full-blown pneumonia. Accelerating labor could bring on symptoms or it could allow a mother to deliver safely and get out of the hospital as quickly as possible. “There is an advantage to having the baby now if you feel okay – even if it’s alone – and getting home,” Dr. Quinlan said.
The hospital also tests the partners of women who are COVID-19 positive. Those with negative results can take the newborn home and try to maintain distance until the mother is no longer symptomatic.
In different parts of the country, hospitals have developed different approaches. Southern California is experiencing its own surge, but at the Ronald Reagan University of California, Los Angeles, Medical Center there still haven’t been enough COVID-19 patients to warrant a separate labor and delivery unit.
At UCLA, staff swab patients when they enter the labor and delivery ward — those who test positive have specific room designations. For both COVID-19–positive patients and women who progress faster than test results can be returned, the goals are the same, said Rashmi Rao, MD, an ob.gyn. at UCLA: Deliver in the safest way possible for both mother and baby.
All women, positive or negative, must wear masks during labor – as much as they can tolerate, at least. For patients who are only mildly ill or asymptomatic, the only difference is that everyone wears protective gear. But if a patient’s oxygen levels dip, or her baby is in distress, the team moves more quickly to a cesarean delivery than they’d do with a healthy patient.
Just as hospital policies have been evolving, rules for visitors have been constantly changing too. Initially, UCLA allowed a support person to be present during delivery but had to leave immediately following. Now, each new mother is allowed one visitor for the duration of their stay. And the hospital suggests that patients who are COVID-19 positive recover in separate rooms from their babies and encourages them to maintain distance from their infants, except when breastfeeding.
“We respect and understand that this is a joyous occasion and we’re trying to keep families together as much as possible,” Dr. Rao said.
Care conundrums
How hospitals protect their smallest charges keeps changing too. Reports have been circulating about newborns being taken away from COVID-19-positive mothers, especially in marginalized communities. The stories have led many to worry they’d be forcibly separated from their babies. Most hospitals, however, leave it up to the woman and her doctors to decide how much separation is needed. “After delivery, it depends on how someone is feeling,” Dr. Rao said.
The American Academy of Pediatrics recommends that mothers who are COVID-19–positive pump breast milk and have a healthy caregiver use that milk, or formula, to bottle-feed the baby, with the new mother remaining 6 feet away from the child as much as she can. If that’s not possible, she should wear gloves and a mask while breastfeeding until she has been naturally afebrile for 72 hours and at least 1 week removed from the first appearance of her symptoms.
“It’s tragically hard,” said Dr. Quinlan, to keep a COVID-19–positive mother even 6 feet away from her newborn baby. “If a mother declines separation, we ask the acting pediatric team to discuss the theoretical risks and paucity of data.”
Until recently, research indicated that SARS-CoV-2 wasn’t being transmitted through the uterus from mothers to their babies. And despite a recent case study reporting transplacental transmission between a mother and her fetus in France, researchers still say that the risk of transference is low. To ensure newborn risk remains as low as possible, UCLA’s policy is to swab the baby when he/she is 24 hours old and keep watch for signs of infection: increased lethargy, difficulty waking, or gastrointestinal symptoms like vomiting.
Transmission via breast milk has also, to date, proven relatively unlikely. One study in The Lancet detected the novel coronavirus in breast milk, although it’s not clear that the virus can be passed on in the fluid, says Christina Chambers, PhD, a professor of pediatrics at the University of California, San Diego. Dr. Chambers is studying breast milk to see if the virus or antibodies to it are present. She is also investigating how infection with SARS-CoV-2 impacts women at different times in pregnancy, something that’s still an open question.
“[In] pregnant women with a deteriorating infection, the decisions are the same you would make with any delivery: Save the mom and save the baby,” Dr. Chambers said. “Beyond that, I am encouraged to see that pregnant women are prioritized to being tested,” something that will help researchers understand prevalence of disease in order to better understand whether some symptoms are more dangerous than others.
The situation is evolving so quickly that hospitals and providers are simply trying to stay abreast of the flood of new research. In the absence of definitive answers, they are using the information available and adjusting on the fly. “We are cautiously waiting for more data,” said Dr. Rao. “With the information we have we are doing the best we can to keep our patients safe. And we’re just going to keep at it.”
A version of this article originally appeared on Medscape.com.
OSHA in the COVID-19 era
As more and more reopened medical practices ramp up toward normal activity, the safety of patients and health care workers alike remains paramount. As always,
Most of the modified guidelines are already familiar: wear masks (and other personal protective equipment as necessary); maintain social distancing; have hand cleaner, soap, and water readily available; and sanitize between patient examinations.
It is also important to remember that COVID-19 is now a reportable disease; check with your local health authorities as to where and how. Also remember that, if you decide to screen employees and/or patients for fevers and other symptoms of COVID-19, those data are subject to HIPAA rules and must be kept confidential.
Now might be a good time to confirm that you remain in compliance with both the new and old regulations. Even if you hold regular safety meetings – which often is not the case – it is always a good idea to occasionally conduct a comprehensive review, which could save you a lot in fines.
So get your OSHA logs out, and walk through your office. Start by making sure you have an official OSHA poster, which enumerates employee rights and explains how to file complaints. Every office must have one posted in plain site, and is what an OSHA inspector will look for first. They are available for free at OSHA’s website or you can order one by calling 800-321-OSHA.
How long have you had your written exposure control plan for blood-borne pathogens? This plan should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, as well as your implementation of universal precautions. It should be updated annually to reflect changes in technology – and new threats, such as COVID-19.
Review your list of hazardous substances, which all employees have a right to know about. OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are classified as “hazardous.” Also remember that you’re probably using new disinfectants, which may need to be added to your list. For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
It is not necessary to adopt every new safety device as it comes on the market, but you should document which ones you are using and which ones you decide not to use – and why. For example, if you and your employees decide against buying a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it is worth, you still should document how you made that decision and why you believe that your current protocol is as good or better.
All at-risk employees should be provided with hepatitis B vaccine at no cost to them. And after any exposure to dangerous pathogens – which now include COVID-19 – you also must provide and pay for appropriate medical treatment and follow-up.
Another important consideration in your review: Electrical devices and their power sources in the office. All electrically powered equipment – medical or clerical – must operate safely and should all be examined. It is particularly important to check how wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Medical and dental offices are not required to keep an injury and illness log under federal OSHA regulations, which other businesses must. However, your state may have a requirement that supersedes the federal law so you should check with your state, or with your local OSHA office, regarding any such requirements.
It is important to take OSHA regulations seriously because failure to comply with them can result in stiff penalties running into many thousands of dollars.
To be certain you are complying with all the rules, you can call your local OSHA office and request an inspection. This is the easiest and cheapest way because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they discover.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
As more and more reopened medical practices ramp up toward normal activity, the safety of patients and health care workers alike remains paramount. As always,
Most of the modified guidelines are already familiar: wear masks (and other personal protective equipment as necessary); maintain social distancing; have hand cleaner, soap, and water readily available; and sanitize between patient examinations.
It is also important to remember that COVID-19 is now a reportable disease; check with your local health authorities as to where and how. Also remember that, if you decide to screen employees and/or patients for fevers and other symptoms of COVID-19, those data are subject to HIPAA rules and must be kept confidential.
Now might be a good time to confirm that you remain in compliance with both the new and old regulations. Even if you hold regular safety meetings – which often is not the case – it is always a good idea to occasionally conduct a comprehensive review, which could save you a lot in fines.
So get your OSHA logs out, and walk through your office. Start by making sure you have an official OSHA poster, which enumerates employee rights and explains how to file complaints. Every office must have one posted in plain site, and is what an OSHA inspector will look for first. They are available for free at OSHA’s website or you can order one by calling 800-321-OSHA.
How long have you had your written exposure control plan for blood-borne pathogens? This plan should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, as well as your implementation of universal precautions. It should be updated annually to reflect changes in technology – and new threats, such as COVID-19.
Review your list of hazardous substances, which all employees have a right to know about. OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are classified as “hazardous.” Also remember that you’re probably using new disinfectants, which may need to be added to your list. For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
It is not necessary to adopt every new safety device as it comes on the market, but you should document which ones you are using and which ones you decide not to use – and why. For example, if you and your employees decide against buying a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it is worth, you still should document how you made that decision and why you believe that your current protocol is as good or better.
All at-risk employees should be provided with hepatitis B vaccine at no cost to them. And after any exposure to dangerous pathogens – which now include COVID-19 – you also must provide and pay for appropriate medical treatment and follow-up.
Another important consideration in your review: Electrical devices and their power sources in the office. All electrically powered equipment – medical or clerical – must operate safely and should all be examined. It is particularly important to check how wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Medical and dental offices are not required to keep an injury and illness log under federal OSHA regulations, which other businesses must. However, your state may have a requirement that supersedes the federal law so you should check with your state, or with your local OSHA office, regarding any such requirements.
It is important to take OSHA regulations seriously because failure to comply with them can result in stiff penalties running into many thousands of dollars.
To be certain you are complying with all the rules, you can call your local OSHA office and request an inspection. This is the easiest and cheapest way because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they discover.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
As more and more reopened medical practices ramp up toward normal activity, the safety of patients and health care workers alike remains paramount. As always,
Most of the modified guidelines are already familiar: wear masks (and other personal protective equipment as necessary); maintain social distancing; have hand cleaner, soap, and water readily available; and sanitize between patient examinations.
It is also important to remember that COVID-19 is now a reportable disease; check with your local health authorities as to where and how. Also remember that, if you decide to screen employees and/or patients for fevers and other symptoms of COVID-19, those data are subject to HIPAA rules and must be kept confidential.
Now might be a good time to confirm that you remain in compliance with both the new and old regulations. Even if you hold regular safety meetings – which often is not the case – it is always a good idea to occasionally conduct a comprehensive review, which could save you a lot in fines.
So get your OSHA logs out, and walk through your office. Start by making sure you have an official OSHA poster, which enumerates employee rights and explains how to file complaints. Every office must have one posted in plain site, and is what an OSHA inspector will look for first. They are available for free at OSHA’s website or you can order one by calling 800-321-OSHA.
How long have you had your written exposure control plan for blood-borne pathogens? This plan should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, as well as your implementation of universal precautions. It should be updated annually to reflect changes in technology – and new threats, such as COVID-19.
Review your list of hazardous substances, which all employees have a right to know about. OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are classified as “hazardous.” Also remember that you’re probably using new disinfectants, which may need to be added to your list. For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
It is not necessary to adopt every new safety device as it comes on the market, but you should document which ones you are using and which ones you decide not to use – and why. For example, if you and your employees decide against buying a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it is worth, you still should document how you made that decision and why you believe that your current protocol is as good or better.
All at-risk employees should be provided with hepatitis B vaccine at no cost to them. And after any exposure to dangerous pathogens – which now include COVID-19 – you also must provide and pay for appropriate medical treatment and follow-up.
Another important consideration in your review: Electrical devices and their power sources in the office. All electrically powered equipment – medical or clerical – must operate safely and should all be examined. It is particularly important to check how wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Medical and dental offices are not required to keep an injury and illness log under federal OSHA regulations, which other businesses must. However, your state may have a requirement that supersedes the federal law so you should check with your state, or with your local OSHA office, regarding any such requirements.
It is important to take OSHA regulations seriously because failure to comply with them can result in stiff penalties running into many thousands of dollars.
To be certain you are complying with all the rules, you can call your local OSHA office and request an inspection. This is the easiest and cheapest way because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they discover.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].









