User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Statin intolerance ‘overestimated and overdiagnosed’
Statin intolerance is far less common than previously reported, according to a new meta-analysis, with data on more than 4 million adults from around the world, looking at reported statin adverse effects.
The study puts the prevalence of statin intolerance at 6% to 10%, meaning that statin intolerance is “overestimated and overdiagnosed” in most cases, Maciej Banach, MD, PhD, from the Medical University of Lodz and the University of Zielona Góra, Poland, said in a news release.
It also means that “around 93% of patients on statin therapy can be treated effectively, with very good tolerability and without any safety issues,” Dr. Banach added.
The study, conducted on behalf of the Lipid and Blood Pressure Meta-Analysis Collaboration and the International Lipid Expert Panel, was published online Feb. 16 in the European Heart Journal.
Reassuring data
In a statement from the British nonprofit Science Media Center, Sir Nilesh J. Samani, MBChB, MD, medical director of the British Heart Foundation, said: “Decades of evidence have proven that statins save lives. This latest analysis, showing that the risk of side effects from statins are less than previously thought, should provide reassurance to those who are recommended this medicine to reduce their risk of a heart attack or stroke.”
The reported prevalence of statin intolerance varies widely, from 2% to 3% to as high as 50%, chiefly because “there is still a lack of a clear and easy way to apply the definition of statin intolerance,” Dr. Banach told this news organization.
“The ones we use in lipid clinics – by National Lipid Association (NLA), European Atherosclerosis Society (EAS), and International Lipid Expert Panel (ILEP) – are not used or are rarely used in everyday clinical practice by GPs and other specialists,” Dr. Banach explained.
He also blames “physician inertia: When they listen to a patient complain of muscle pain, or see elevated alanine aminotransferase (ALT), in most of the cases, they will immediately discontinue statins, without any further investigations. One should remember that there are many secondary causes of statin intolerance,” Dr. Banach said.
To get a better handle on the true prevalence of statin intolerance, the study team did a meta-analysis of 4,143,517 patients worldwide from 176 studies: 112 randomized controlled trials and 64 cohort studies.
The overall prevalence of statin intolerance was 9.1% (95% confidence interval, 8.0%-10.0%).
The prevalence of statin intolerance was even lower when assessed with diagnostic criteria from the NLA (7.0%; 95% CI, 6.0%-8.0%), the ILEP (6.7%; 95% CI, 5.0%-8.0%), and the EAS (5.9%; 95% CI, 4.0%-7.0%).
The main factors associated with an increased risk for statin intolerance are female gender, hypothyroidism, high statin dose, advanced age, concomitant use of anti-arrhythmic drugs, and obesity. Other factors include race (being Asian or African American), type 2 diabetes, alcohol use, and chronic liver and renal diseases.
“Our findings mean that we should evaluate patients’ symptoms very carefully, firstly to see whether symptoms are indeed caused by statins, and secondly to evaluate whether it might be patients’ perceptions that statins are harmful – so called nocebo or drucebo effect – which could be responsible for more than 50% of all symptoms, rather than the drug itself,” Dr. Banach said.
He encourages use of the Statin-Associated Muscle Symptom Clinical Index (SAMS-CI) to assess the likelihood that a patient’s muscle symptoms are caused or worsened by statin use.
Substantial analysis, valid results
“This is a substantial analysis [and], based on what we know about statin side effects to date, the results are likely to be broadly valid and indicate that we should not overestimate statin side effects or be too quick to stop statins without due consideration,” Riyaz Patel, MBBS, professor of cardiology, University College London, told the Science Media Center.
“Some patients do experience real side effects, and we do our best to help them with alternative therapies, as with any other medicine. However, for the vast majority of people experiencing statin side effects, we can usually work with the patient to understand the symptoms, use proven strategies to manage these, and ensure they do not miss out on the well-established benefits of statins,” Mr. Patel said.
“This is especially important for people who have already had a heart attack or stroke, where statin therapy is really important in preventing further events,” Mr. Patel added.
Also weighing in on the results, Peter Sever, MB BChir, professor of clinical pharmacology and therapeutics, Imperial College London, said: “The importance for clinicians and patients is to realize that commonly reported symptoms, such as muscle aches and pains and lethargy, are not due to the chemistry of the drug.”
“These ‘nocebo’ symptoms may be psychological in origin, but they are no less real than pharmacological symptoms in how they affect quality of life,” Mr. Sever told the Science Media Center.
“However, it’s important to note that as they are not directly caused by the drug, they should not override the decision to prescribe and take statins on account of their proven benefit in reducing death and disability from heart attacks, strokes, and other cardiovascular conditions,” he added.
This meta-analysis was conducted independently; no company or institution supported it financially. Dr. Banach is on the speakers bureau for Amgen, Herbapol, Kogen, KRKA, Polpharma, Mylan/Viatris, Novartis, Novo Nordisk, Sanofi-Aventis, Teva, and Zentiva; is a consultant to Abbott Vascular, Amgen, Daichii Sankyo, Esperion, FreiaPharmaceuticals, Novartis, Polfarmex, and Sanofi-Aventis; has received grants from Amgen, Mylan/Viatris, Sanofi, and Valeant; and serves as CMO for Nomi Biotech Corporation. Dr. Samani has no relevant disclosures. Mr. Patel has received past honoraria and consulting fees from drug companies manufacturing new cholesterol-lowering drugs and currently works with NICE as a topic advisor for CVD prevention. Mr. Sever has received research grants and consultancy from Pfizer and Amgen.
A version of this article first appeared on Medscape.com.
Statin intolerance is far less common than previously reported, according to a new meta-analysis, with data on more than 4 million adults from around the world, looking at reported statin adverse effects.
The study puts the prevalence of statin intolerance at 6% to 10%, meaning that statin intolerance is “overestimated and overdiagnosed” in most cases, Maciej Banach, MD, PhD, from the Medical University of Lodz and the University of Zielona Góra, Poland, said in a news release.
It also means that “around 93% of patients on statin therapy can be treated effectively, with very good tolerability and without any safety issues,” Dr. Banach added.
The study, conducted on behalf of the Lipid and Blood Pressure Meta-Analysis Collaboration and the International Lipid Expert Panel, was published online Feb. 16 in the European Heart Journal.
Reassuring data
In a statement from the British nonprofit Science Media Center, Sir Nilesh J. Samani, MBChB, MD, medical director of the British Heart Foundation, said: “Decades of evidence have proven that statins save lives. This latest analysis, showing that the risk of side effects from statins are less than previously thought, should provide reassurance to those who are recommended this medicine to reduce their risk of a heart attack or stroke.”
The reported prevalence of statin intolerance varies widely, from 2% to 3% to as high as 50%, chiefly because “there is still a lack of a clear and easy way to apply the definition of statin intolerance,” Dr. Banach told this news organization.
“The ones we use in lipid clinics – by National Lipid Association (NLA), European Atherosclerosis Society (EAS), and International Lipid Expert Panel (ILEP) – are not used or are rarely used in everyday clinical practice by GPs and other specialists,” Dr. Banach explained.
He also blames “physician inertia: When they listen to a patient complain of muscle pain, or see elevated alanine aminotransferase (ALT), in most of the cases, they will immediately discontinue statins, without any further investigations. One should remember that there are many secondary causes of statin intolerance,” Dr. Banach said.
To get a better handle on the true prevalence of statin intolerance, the study team did a meta-analysis of 4,143,517 patients worldwide from 176 studies: 112 randomized controlled trials and 64 cohort studies.
The overall prevalence of statin intolerance was 9.1% (95% confidence interval, 8.0%-10.0%).
The prevalence of statin intolerance was even lower when assessed with diagnostic criteria from the NLA (7.0%; 95% CI, 6.0%-8.0%), the ILEP (6.7%; 95% CI, 5.0%-8.0%), and the EAS (5.9%; 95% CI, 4.0%-7.0%).
The main factors associated with an increased risk for statin intolerance are female gender, hypothyroidism, high statin dose, advanced age, concomitant use of anti-arrhythmic drugs, and obesity. Other factors include race (being Asian or African American), type 2 diabetes, alcohol use, and chronic liver and renal diseases.
“Our findings mean that we should evaluate patients’ symptoms very carefully, firstly to see whether symptoms are indeed caused by statins, and secondly to evaluate whether it might be patients’ perceptions that statins are harmful – so called nocebo or drucebo effect – which could be responsible for more than 50% of all symptoms, rather than the drug itself,” Dr. Banach said.
He encourages use of the Statin-Associated Muscle Symptom Clinical Index (SAMS-CI) to assess the likelihood that a patient’s muscle symptoms are caused or worsened by statin use.
Substantial analysis, valid results
“This is a substantial analysis [and], based on what we know about statin side effects to date, the results are likely to be broadly valid and indicate that we should not overestimate statin side effects or be too quick to stop statins without due consideration,” Riyaz Patel, MBBS, professor of cardiology, University College London, told the Science Media Center.
“Some patients do experience real side effects, and we do our best to help them with alternative therapies, as with any other medicine. However, for the vast majority of people experiencing statin side effects, we can usually work with the patient to understand the symptoms, use proven strategies to manage these, and ensure they do not miss out on the well-established benefits of statins,” Mr. Patel said.
“This is especially important for people who have already had a heart attack or stroke, where statin therapy is really important in preventing further events,” Mr. Patel added.
Also weighing in on the results, Peter Sever, MB BChir, professor of clinical pharmacology and therapeutics, Imperial College London, said: “The importance for clinicians and patients is to realize that commonly reported symptoms, such as muscle aches and pains and lethargy, are not due to the chemistry of the drug.”
“These ‘nocebo’ symptoms may be psychological in origin, but they are no less real than pharmacological symptoms in how they affect quality of life,” Mr. Sever told the Science Media Center.
“However, it’s important to note that as they are not directly caused by the drug, they should not override the decision to prescribe and take statins on account of their proven benefit in reducing death and disability from heart attacks, strokes, and other cardiovascular conditions,” he added.
This meta-analysis was conducted independently; no company or institution supported it financially. Dr. Banach is on the speakers bureau for Amgen, Herbapol, Kogen, KRKA, Polpharma, Mylan/Viatris, Novartis, Novo Nordisk, Sanofi-Aventis, Teva, and Zentiva; is a consultant to Abbott Vascular, Amgen, Daichii Sankyo, Esperion, FreiaPharmaceuticals, Novartis, Polfarmex, and Sanofi-Aventis; has received grants from Amgen, Mylan/Viatris, Sanofi, and Valeant; and serves as CMO for Nomi Biotech Corporation. Dr. Samani has no relevant disclosures. Mr. Patel has received past honoraria and consulting fees from drug companies manufacturing new cholesterol-lowering drugs and currently works with NICE as a topic advisor for CVD prevention. Mr. Sever has received research grants and consultancy from Pfizer and Amgen.
A version of this article first appeared on Medscape.com.
Statin intolerance is far less common than previously reported, according to a new meta-analysis, with data on more than 4 million adults from around the world, looking at reported statin adverse effects.
The study puts the prevalence of statin intolerance at 6% to 10%, meaning that statin intolerance is “overestimated and overdiagnosed” in most cases, Maciej Banach, MD, PhD, from the Medical University of Lodz and the University of Zielona Góra, Poland, said in a news release.
It also means that “around 93% of patients on statin therapy can be treated effectively, with very good tolerability and without any safety issues,” Dr. Banach added.
The study, conducted on behalf of the Lipid and Blood Pressure Meta-Analysis Collaboration and the International Lipid Expert Panel, was published online Feb. 16 in the European Heart Journal.
Reassuring data
In a statement from the British nonprofit Science Media Center, Sir Nilesh J. Samani, MBChB, MD, medical director of the British Heart Foundation, said: “Decades of evidence have proven that statins save lives. This latest analysis, showing that the risk of side effects from statins are less than previously thought, should provide reassurance to those who are recommended this medicine to reduce their risk of a heart attack or stroke.”
The reported prevalence of statin intolerance varies widely, from 2% to 3% to as high as 50%, chiefly because “there is still a lack of a clear and easy way to apply the definition of statin intolerance,” Dr. Banach told this news organization.
“The ones we use in lipid clinics – by National Lipid Association (NLA), European Atherosclerosis Society (EAS), and International Lipid Expert Panel (ILEP) – are not used or are rarely used in everyday clinical practice by GPs and other specialists,” Dr. Banach explained.
He also blames “physician inertia: When they listen to a patient complain of muscle pain, or see elevated alanine aminotransferase (ALT), in most of the cases, they will immediately discontinue statins, without any further investigations. One should remember that there are many secondary causes of statin intolerance,” Dr. Banach said.
To get a better handle on the true prevalence of statin intolerance, the study team did a meta-analysis of 4,143,517 patients worldwide from 176 studies: 112 randomized controlled trials and 64 cohort studies.
The overall prevalence of statin intolerance was 9.1% (95% confidence interval, 8.0%-10.0%).
The prevalence of statin intolerance was even lower when assessed with diagnostic criteria from the NLA (7.0%; 95% CI, 6.0%-8.0%), the ILEP (6.7%; 95% CI, 5.0%-8.0%), and the EAS (5.9%; 95% CI, 4.0%-7.0%).
The main factors associated with an increased risk for statin intolerance are female gender, hypothyroidism, high statin dose, advanced age, concomitant use of anti-arrhythmic drugs, and obesity. Other factors include race (being Asian or African American), type 2 diabetes, alcohol use, and chronic liver and renal diseases.
“Our findings mean that we should evaluate patients’ symptoms very carefully, firstly to see whether symptoms are indeed caused by statins, and secondly to evaluate whether it might be patients’ perceptions that statins are harmful – so called nocebo or drucebo effect – which could be responsible for more than 50% of all symptoms, rather than the drug itself,” Dr. Banach said.
He encourages use of the Statin-Associated Muscle Symptom Clinical Index (SAMS-CI) to assess the likelihood that a patient’s muscle symptoms are caused or worsened by statin use.
Substantial analysis, valid results
“This is a substantial analysis [and], based on what we know about statin side effects to date, the results are likely to be broadly valid and indicate that we should not overestimate statin side effects or be too quick to stop statins without due consideration,” Riyaz Patel, MBBS, professor of cardiology, University College London, told the Science Media Center.
“Some patients do experience real side effects, and we do our best to help them with alternative therapies, as with any other medicine. However, for the vast majority of people experiencing statin side effects, we can usually work with the patient to understand the symptoms, use proven strategies to manage these, and ensure they do not miss out on the well-established benefits of statins,” Mr. Patel said.
“This is especially important for people who have already had a heart attack or stroke, where statin therapy is really important in preventing further events,” Mr. Patel added.
Also weighing in on the results, Peter Sever, MB BChir, professor of clinical pharmacology and therapeutics, Imperial College London, said: “The importance for clinicians and patients is to realize that commonly reported symptoms, such as muscle aches and pains and lethargy, are not due to the chemistry of the drug.”
“These ‘nocebo’ symptoms may be psychological in origin, but they are no less real than pharmacological symptoms in how they affect quality of life,” Mr. Sever told the Science Media Center.
“However, it’s important to note that as they are not directly caused by the drug, they should not override the decision to prescribe and take statins on account of their proven benefit in reducing death and disability from heart attacks, strokes, and other cardiovascular conditions,” he added.
This meta-analysis was conducted independently; no company or institution supported it financially. Dr. Banach is on the speakers bureau for Amgen, Herbapol, Kogen, KRKA, Polpharma, Mylan/Viatris, Novartis, Novo Nordisk, Sanofi-Aventis, Teva, and Zentiva; is a consultant to Abbott Vascular, Amgen, Daichii Sankyo, Esperion, FreiaPharmaceuticals, Novartis, Polfarmex, and Sanofi-Aventis; has received grants from Amgen, Mylan/Viatris, Sanofi, and Valeant; and serves as CMO for Nomi Biotech Corporation. Dr. Samani has no relevant disclosures. Mr. Patel has received past honoraria and consulting fees from drug companies manufacturing new cholesterol-lowering drugs and currently works with NICE as a topic advisor for CVD prevention. Mr. Sever has received research grants and consultancy from Pfizer and Amgen.
A version of this article first appeared on Medscape.com.
Tiny hitchhikers like to ride in the trunk
Junk (germs) in the trunk
It’s been a long drive, and you’ve got a long way to go. You pull into a rest stop to use the bathroom and get some food. Quick, which order do you do those things in?
If you’re not a crazy person, you’d use the bathroom and then get your food. Who would bring food into a dirty bathroom? That’s kind of gross. Most people would take care of business, grab food, then get back in the car, eating along the way. Unfortunately, if you’re searching for a sanitary eating environment, your car may not actually be much better than that bathroom, according to new research from Aston University in Birmingham, England.
Let’s start off with the good news. The steering wheels of the five used cars that were swabbed for bacteria were pretty clean. Definitely cleaner than either of the toilet seats analyzed, likely thanks to increased usage of sanitizer, courtesy of the current pandemic. It’s easy to wipe down the steering wheel. Things break down, though, once we look elsewhere. The interiors of the five cars all contained just as much, if not more, bacteria than the toilet seats, with fecal matter commonly appearing on the driver’s seat.
The car interiors were less than sanitary, but they paled in comparison with the real winner here: the trunk. In each of the five cars, bacteria levels there far exceeded those in the toilets, and included everyone’s favorites – Escherichia coli and Staphylococcus aureus.
So, snacking on a bag of chips as you drive along is probably okay, but the food that popped out of its bag and spent the last 5 minutes rolling around the back? Perhaps less okay. You may want to wash it. Or burn it. Or torch the entire car for good measure like we’re about to do. Next time we’ll buy a car without poop in it.
Shut the lid when you flush
Maybe you’ve never thought about this, but it’s actually extremely important to shut the toilet lid when you flush. Just think of all those germs flying around from the force of the flush. Is your toothbrush anywhere near the toilet? Ew. Those pesky little bacteria and viruses are everywhere, and we know we can’t really escape them, but we should really do our best once we’re made aware of where to find them.
It seems like a no-brainer these days since we’ve all been really focused on cleanliness during the pandemic, but according to a poll in the United Kingdom, 55% of the 2,000 participants said they don’t put the lid down while flushing.
The OnePoll survey commissioned by Harpic, a company that makes toilet-cleaning products, also advised that toilet water isn’t even completely clean after flushed several times and can still be contaminated with many germs. Company researchers took specialized pictures of flushing toilets and they looked like tiny little Fourth of July fireworks shows, minus the sparklers. The pictures proved that droplets can go all over the place, including on bathroom users.
“There has never been a more important time to take extra care around our homes, although the risks associated with germ spread in unhygienic bathrooms are high, the solution to keeping them clean is simple,” a Harpic researcher said. Since other studies have shown that coronavirus can be found in feces, it’s become increasingly important to keep ourselves and others safe. Fireworks are pretty, but not when they come out of your toilet.
The latest in MRI fashion
Do you see that photo just below? Looks like something you could buy at the Lego store, right? Well, it’s not. Nor is it the proverbial thinking cap come to life.
(Did someone just say “come to life”? That reminds us of our favorite scene from Frosty the Snowman.)
Anywaaay, about the photo. That funny-looking chapeau is what we in the science business call a metamaterial.
Nope, metamaterials have nothing to do with Facebook parent company Meta. We checked. According to a statement from Boston University, they are engineered structures “created from small unit cells that might be unspectacular alone, but when grouped together in a precise way, get new superpowers not found in nature.”
Superpowers, eh? Who doesn’t want superpowers? Even if they come with a funny hat.
The unit cells, known as resonators, are just plastic tubes wrapped in copper wiring, but when they are grouped in an array and precisely arranged into a helmet, they can channel the magnetic field of the MRI machine during a scan. In theory, that would create “crisper images that can be captured at twice the normal speed,” Xin Zhang, PhD, and her team at BU’s Photonics Center explained in the university statement.
In the future, the metamaterial device could “be used in conjunction with cheaper low-field MRI machines to make the technology more widely available, particularly in the developing world,” they suggested. Or, like so many other superpowers, it could fall into the wrong hands. Like those of Lex Luthor. Or Mark Zuckerberg. Or Frosty the Snowman.
The highway of the mind
How fast can you think on your feet? Well, according to a recently published study, it could be a legitimate measure of intelligence. Here’s the science.
Researchers from the University of Würzburg in Germany and Indiana University have suggested that a person’s intelligence score measures the ability, based on certain neuronal networks and their communication structures, to switch between resting state and different task states.
The investigators set up a study to observe almost 800 people while they completed seven tasks. By monitoring brain activity with functional magnetic resonance imaging, the teams found that subjects who had higher intelligence scores required “less adjustment when switching between different cognitive states,” they said in a separate statement.
It comes down to the network architecture of their brains.
Kirsten Hilger, PhD, head of the German group, described it in terms of highways. The resting state of the brain is normal traffic. It’s always moving. Holiday traffic is the task. The ability to handle the increased flow of commuters is a function of the highway infrastructure. The better the infrastructure, the higher the intelligence.
So the next time you’re stuck in traffic, think how efficient your brain would be with such a task. The quicker, the better.
Junk (germs) in the trunk
It’s been a long drive, and you’ve got a long way to go. You pull into a rest stop to use the bathroom and get some food. Quick, which order do you do those things in?
If you’re not a crazy person, you’d use the bathroom and then get your food. Who would bring food into a dirty bathroom? That’s kind of gross. Most people would take care of business, grab food, then get back in the car, eating along the way. Unfortunately, if you’re searching for a sanitary eating environment, your car may not actually be much better than that bathroom, according to new research from Aston University in Birmingham, England.
Let’s start off with the good news. The steering wheels of the five used cars that were swabbed for bacteria were pretty clean. Definitely cleaner than either of the toilet seats analyzed, likely thanks to increased usage of sanitizer, courtesy of the current pandemic. It’s easy to wipe down the steering wheel. Things break down, though, once we look elsewhere. The interiors of the five cars all contained just as much, if not more, bacteria than the toilet seats, with fecal matter commonly appearing on the driver’s seat.
The car interiors were less than sanitary, but they paled in comparison with the real winner here: the trunk. In each of the five cars, bacteria levels there far exceeded those in the toilets, and included everyone’s favorites – Escherichia coli and Staphylococcus aureus.
So, snacking on a bag of chips as you drive along is probably okay, but the food that popped out of its bag and spent the last 5 minutes rolling around the back? Perhaps less okay. You may want to wash it. Or burn it. Or torch the entire car for good measure like we’re about to do. Next time we’ll buy a car without poop in it.
Shut the lid when you flush
Maybe you’ve never thought about this, but it’s actually extremely important to shut the toilet lid when you flush. Just think of all those germs flying around from the force of the flush. Is your toothbrush anywhere near the toilet? Ew. Those pesky little bacteria and viruses are everywhere, and we know we can’t really escape them, but we should really do our best once we’re made aware of where to find them.
It seems like a no-brainer these days since we’ve all been really focused on cleanliness during the pandemic, but according to a poll in the United Kingdom, 55% of the 2,000 participants said they don’t put the lid down while flushing.
The OnePoll survey commissioned by Harpic, a company that makes toilet-cleaning products, also advised that toilet water isn’t even completely clean after flushed several times and can still be contaminated with many germs. Company researchers took specialized pictures of flushing toilets and they looked like tiny little Fourth of July fireworks shows, minus the sparklers. The pictures proved that droplets can go all over the place, including on bathroom users.
“There has never been a more important time to take extra care around our homes, although the risks associated with germ spread in unhygienic bathrooms are high, the solution to keeping them clean is simple,” a Harpic researcher said. Since other studies have shown that coronavirus can be found in feces, it’s become increasingly important to keep ourselves and others safe. Fireworks are pretty, but not when they come out of your toilet.
The latest in MRI fashion
Do you see that photo just below? Looks like something you could buy at the Lego store, right? Well, it’s not. Nor is it the proverbial thinking cap come to life.
(Did someone just say “come to life”? That reminds us of our favorite scene from Frosty the Snowman.)
Anywaaay, about the photo. That funny-looking chapeau is what we in the science business call a metamaterial.
Nope, metamaterials have nothing to do with Facebook parent company Meta. We checked. According to a statement from Boston University, they are engineered structures “created from small unit cells that might be unspectacular alone, but when grouped together in a precise way, get new superpowers not found in nature.”
Superpowers, eh? Who doesn’t want superpowers? Even if they come with a funny hat.
The unit cells, known as resonators, are just plastic tubes wrapped in copper wiring, but when they are grouped in an array and precisely arranged into a helmet, they can channel the magnetic field of the MRI machine during a scan. In theory, that would create “crisper images that can be captured at twice the normal speed,” Xin Zhang, PhD, and her team at BU’s Photonics Center explained in the university statement.
In the future, the metamaterial device could “be used in conjunction with cheaper low-field MRI machines to make the technology more widely available, particularly in the developing world,” they suggested. Or, like so many other superpowers, it could fall into the wrong hands. Like those of Lex Luthor. Or Mark Zuckerberg. Or Frosty the Snowman.
The highway of the mind
How fast can you think on your feet? Well, according to a recently published study, it could be a legitimate measure of intelligence. Here’s the science.
Researchers from the University of Würzburg in Germany and Indiana University have suggested that a person’s intelligence score measures the ability, based on certain neuronal networks and their communication structures, to switch between resting state and different task states.
The investigators set up a study to observe almost 800 people while they completed seven tasks. By monitoring brain activity with functional magnetic resonance imaging, the teams found that subjects who had higher intelligence scores required “less adjustment when switching between different cognitive states,” they said in a separate statement.
It comes down to the network architecture of their brains.
Kirsten Hilger, PhD, head of the German group, described it in terms of highways. The resting state of the brain is normal traffic. It’s always moving. Holiday traffic is the task. The ability to handle the increased flow of commuters is a function of the highway infrastructure. The better the infrastructure, the higher the intelligence.
So the next time you’re stuck in traffic, think how efficient your brain would be with such a task. The quicker, the better.
Junk (germs) in the trunk
It’s been a long drive, and you’ve got a long way to go. You pull into a rest stop to use the bathroom and get some food. Quick, which order do you do those things in?
If you’re not a crazy person, you’d use the bathroom and then get your food. Who would bring food into a dirty bathroom? That’s kind of gross. Most people would take care of business, grab food, then get back in the car, eating along the way. Unfortunately, if you’re searching for a sanitary eating environment, your car may not actually be much better than that bathroom, according to new research from Aston University in Birmingham, England.
Let’s start off with the good news. The steering wheels of the five used cars that were swabbed for bacteria were pretty clean. Definitely cleaner than either of the toilet seats analyzed, likely thanks to increased usage of sanitizer, courtesy of the current pandemic. It’s easy to wipe down the steering wheel. Things break down, though, once we look elsewhere. The interiors of the five cars all contained just as much, if not more, bacteria than the toilet seats, with fecal matter commonly appearing on the driver’s seat.
The car interiors were less than sanitary, but they paled in comparison with the real winner here: the trunk. In each of the five cars, bacteria levels there far exceeded those in the toilets, and included everyone’s favorites – Escherichia coli and Staphylococcus aureus.
So, snacking on a bag of chips as you drive along is probably okay, but the food that popped out of its bag and spent the last 5 minutes rolling around the back? Perhaps less okay. You may want to wash it. Or burn it. Or torch the entire car for good measure like we’re about to do. Next time we’ll buy a car without poop in it.
Shut the lid when you flush
Maybe you’ve never thought about this, but it’s actually extremely important to shut the toilet lid when you flush. Just think of all those germs flying around from the force of the flush. Is your toothbrush anywhere near the toilet? Ew. Those pesky little bacteria and viruses are everywhere, and we know we can’t really escape them, but we should really do our best once we’re made aware of where to find them.
It seems like a no-brainer these days since we’ve all been really focused on cleanliness during the pandemic, but according to a poll in the United Kingdom, 55% of the 2,000 participants said they don’t put the lid down while flushing.
The OnePoll survey commissioned by Harpic, a company that makes toilet-cleaning products, also advised that toilet water isn’t even completely clean after flushed several times and can still be contaminated with many germs. Company researchers took specialized pictures of flushing toilets and they looked like tiny little Fourth of July fireworks shows, minus the sparklers. The pictures proved that droplets can go all over the place, including on bathroom users.
“There has never been a more important time to take extra care around our homes, although the risks associated with germ spread in unhygienic bathrooms are high, the solution to keeping them clean is simple,” a Harpic researcher said. Since other studies have shown that coronavirus can be found in feces, it’s become increasingly important to keep ourselves and others safe. Fireworks are pretty, but not when they come out of your toilet.
The latest in MRI fashion
Do you see that photo just below? Looks like something you could buy at the Lego store, right? Well, it’s not. Nor is it the proverbial thinking cap come to life.
(Did someone just say “come to life”? That reminds us of our favorite scene from Frosty the Snowman.)
Anywaaay, about the photo. That funny-looking chapeau is what we in the science business call a metamaterial.
Nope, metamaterials have nothing to do with Facebook parent company Meta. We checked. According to a statement from Boston University, they are engineered structures “created from small unit cells that might be unspectacular alone, but when grouped together in a precise way, get new superpowers not found in nature.”
Superpowers, eh? Who doesn’t want superpowers? Even if they come with a funny hat.
The unit cells, known as resonators, are just plastic tubes wrapped in copper wiring, but when they are grouped in an array and precisely arranged into a helmet, they can channel the magnetic field of the MRI machine during a scan. In theory, that would create “crisper images that can be captured at twice the normal speed,” Xin Zhang, PhD, and her team at BU’s Photonics Center explained in the university statement.
In the future, the metamaterial device could “be used in conjunction with cheaper low-field MRI machines to make the technology more widely available, particularly in the developing world,” they suggested. Or, like so many other superpowers, it could fall into the wrong hands. Like those of Lex Luthor. Or Mark Zuckerberg. Or Frosty the Snowman.
The highway of the mind
How fast can you think on your feet? Well, according to a recently published study, it could be a legitimate measure of intelligence. Here’s the science.
Researchers from the University of Würzburg in Germany and Indiana University have suggested that a person’s intelligence score measures the ability, based on certain neuronal networks and their communication structures, to switch between resting state and different task states.
The investigators set up a study to observe almost 800 people while they completed seven tasks. By monitoring brain activity with functional magnetic resonance imaging, the teams found that subjects who had higher intelligence scores required “less adjustment when switching between different cognitive states,” they said in a separate statement.
It comes down to the network architecture of their brains.
Kirsten Hilger, PhD, head of the German group, described it in terms of highways. The resting state of the brain is normal traffic. It’s always moving. Holiday traffic is the task. The ability to handle the increased flow of commuters is a function of the highway infrastructure. The better the infrastructure, the higher the intelligence.
So the next time you’re stuck in traffic, think how efficient your brain would be with such a task. The quicker, the better.
Stroke risk is highest right after COVID infection
, new research shows.
The study among Medicare beneficiaries with COVID-19 also showed that stroke risk is higher for relatively young older adults, those aged 65 to 74 years, and those without a history of stroke.
The study highlights the impact COVID-19 has on the cardiovascular system, said study author Quanhe Yang, PhD, senior scientist, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta.
“Clinicians and patients should understand that stroke might be one of the very important clinical consequences of COVID-19.”
The study was presented during the hybrid International Stroke Conference held in New Orleans and online. The meeting was presented by the American Stroke Association, a division of the American Heart Association.
Stroke is the fifth leading cause of death in the U.S. As an increasing number of people become infected with COVID-19, “it’s important to determine if there’s a relationship between COVID and the risk of stroke,” said Dr. Yang.
Findings from prior research examining the link between stroke and COVID-19 have been inconsistent, he noted. Some studies found an association while others did not, and in still others, the association was not as strong as expected.
Many factors may contribute to these inconsistent findings, said Dr. Yang, including differences in study design, inclusion criteria, comparison groups, sample sizes, and countries where the research was carried out. Dr. Yang pointed out that many of these studies were done in the early stages of the pandemic or didn’t include older adults, the population most at risk for stroke.
The current study included 19,553 Medicare beneficiaries aged 65 years and older diagnosed with COVID-19 and hospitalized with acute ischemic stroke. The median age at diagnosis of COVID-19 was 80.5 years, 57.5% were women, and more than 75% were non-Hispanic Whites.
To ensure the stroke occurred after a COVID infection, researchers used a self-controlled case series study design, a “within person” comparison between the risk period and the control period.
They divided the study period (Jan. 1, 2019 to Feb. 28, 2021) into the exposure or stroke risk periods after the COVID diagnosis (0-3 days; 4-7 days; 8-15 days; and 15-28 days) and control periods.
Strokes that occurred 7 days before or 28 days after a COVID diagnosis served as a control period. “Any stroke that occurred outside the risk window is in the control period,” explained Dr. Yang.
He added that the control period provides a baseline. “Without COVID-19, this is what I would expect” in terms of the number of strokes.
To estimate the incidence rate ratio (IRR), investigators compared the incidence of acute ischemic stroke in the various risk periods with control periods.
The IRR was 10.97 (95% confidence interval, 10.30-11.68) at 0-3 days. The risk then quickly declined but stayed higher than the control period. The IRRs were: 1.59 (95% CI, 1.35-1.87) at 4-7 days; 1.23 (95% CI, 1.07-1.41) at 8-14 days; and 1.06 (95% CI, 0.95-1.18) at 15-28 days.
The temporary increase in stroke risk early after an infection isn’t novel; the pattern has been observed with influenza, respiratory infections, and shingles, said Dr. Yang. “But COVID-19 appears to be particularly risky.”
Although the mechanism driving the early increased stroke risk isn’t fully understood, it’s likely tied to an “exaggerated inflammatory response,” said Dr. Yang. This can trigger the cascade of events setting the stage for a stroke – a hypercoagulation state leading to the formation of blood clots that then block arteries to the brain, he said.
It’s also possible the infection directly affects endothelial cells, leading to rupture of plaque, again blocking arteries and raising stroke risks, added Dr. Yang.
The association was stronger among younger beneficiaries, aged 65 to 74 years, compared with those 85 years and older, a finding Dr. Yang said was somewhat surprising. But he noted other studies have found stroke patients with COVID are younger than stroke patients without COVID – by some 5 to 6 years.
“If COVID-19 disproportionately affects younger patients, that may explain the stronger association,” said Dr. Yang. “Stroke risk increases tremendously with age, so if you’re a younger age, your baseline stroke risk is lower.”
The association was also stronger among beneficiaries without a history of stroke. Again, this could be related to the stronger association among younger patients who are less likely to have suffered a stroke. The association was largely consistent across sex and race/ethnicities.
Dr. Yang stressed that the findings need to be confirmed with further studies.
The study was carried out before widespread use of vaccinations in the U.S. Once those data are available, Dr. Yang and his colleagues plan to determine if vaccinations modify the association between COVID-19 and stroke risk.
The new results contribute to the mounting evidence that a COVID-19 infection “can actually affect multiple human organs structurally or functionally in addition to the impact on [the] respiratory system,” said Dr. Yang.
Some dates of COVID-19 diagnoses may be incorrect due to limited test availability, particularly early in the pandemic. Another limitation of the study was possible misclassification from the use of Medicare real-time preliminary claims.
In a provided statement, Louise D. McCullough, MD, PhD, chair of the ISC 2022 and professor and chair of neurology, McGovern Medical School, University of Texas Health Science Center at Houston, noted that the study focused on older adults because it was examining Medicare beneficiaries.
“But everyone is likely at risk for stroke after COVID,” she said. “Any infection is linked to stroke risk, probably because any infection will cause inflammation, and inflammation can cause clots or thrombus, which is the cause of stroke.”
There was no outside funding for the study. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
, new research shows.
The study among Medicare beneficiaries with COVID-19 also showed that stroke risk is higher for relatively young older adults, those aged 65 to 74 years, and those without a history of stroke.
The study highlights the impact COVID-19 has on the cardiovascular system, said study author Quanhe Yang, PhD, senior scientist, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta.
“Clinicians and patients should understand that stroke might be one of the very important clinical consequences of COVID-19.”
The study was presented during the hybrid International Stroke Conference held in New Orleans and online. The meeting was presented by the American Stroke Association, a division of the American Heart Association.
Stroke is the fifth leading cause of death in the U.S. As an increasing number of people become infected with COVID-19, “it’s important to determine if there’s a relationship between COVID and the risk of stroke,” said Dr. Yang.
Findings from prior research examining the link between stroke and COVID-19 have been inconsistent, he noted. Some studies found an association while others did not, and in still others, the association was not as strong as expected.
Many factors may contribute to these inconsistent findings, said Dr. Yang, including differences in study design, inclusion criteria, comparison groups, sample sizes, and countries where the research was carried out. Dr. Yang pointed out that many of these studies were done in the early stages of the pandemic or didn’t include older adults, the population most at risk for stroke.
The current study included 19,553 Medicare beneficiaries aged 65 years and older diagnosed with COVID-19 and hospitalized with acute ischemic stroke. The median age at diagnosis of COVID-19 was 80.5 years, 57.5% were women, and more than 75% were non-Hispanic Whites.
To ensure the stroke occurred after a COVID infection, researchers used a self-controlled case series study design, a “within person” comparison between the risk period and the control period.
They divided the study period (Jan. 1, 2019 to Feb. 28, 2021) into the exposure or stroke risk periods after the COVID diagnosis (0-3 days; 4-7 days; 8-15 days; and 15-28 days) and control periods.
Strokes that occurred 7 days before or 28 days after a COVID diagnosis served as a control period. “Any stroke that occurred outside the risk window is in the control period,” explained Dr. Yang.
He added that the control period provides a baseline. “Without COVID-19, this is what I would expect” in terms of the number of strokes.
To estimate the incidence rate ratio (IRR), investigators compared the incidence of acute ischemic stroke in the various risk periods with control periods.
The IRR was 10.97 (95% confidence interval, 10.30-11.68) at 0-3 days. The risk then quickly declined but stayed higher than the control period. The IRRs were: 1.59 (95% CI, 1.35-1.87) at 4-7 days; 1.23 (95% CI, 1.07-1.41) at 8-14 days; and 1.06 (95% CI, 0.95-1.18) at 15-28 days.
The temporary increase in stroke risk early after an infection isn’t novel; the pattern has been observed with influenza, respiratory infections, and shingles, said Dr. Yang. “But COVID-19 appears to be particularly risky.”
Although the mechanism driving the early increased stroke risk isn’t fully understood, it’s likely tied to an “exaggerated inflammatory response,” said Dr. Yang. This can trigger the cascade of events setting the stage for a stroke – a hypercoagulation state leading to the formation of blood clots that then block arteries to the brain, he said.
It’s also possible the infection directly affects endothelial cells, leading to rupture of plaque, again blocking arteries and raising stroke risks, added Dr. Yang.
The association was stronger among younger beneficiaries, aged 65 to 74 years, compared with those 85 years and older, a finding Dr. Yang said was somewhat surprising. But he noted other studies have found stroke patients with COVID are younger than stroke patients without COVID – by some 5 to 6 years.
“If COVID-19 disproportionately affects younger patients, that may explain the stronger association,” said Dr. Yang. “Stroke risk increases tremendously with age, so if you’re a younger age, your baseline stroke risk is lower.”
The association was also stronger among beneficiaries without a history of stroke. Again, this could be related to the stronger association among younger patients who are less likely to have suffered a stroke. The association was largely consistent across sex and race/ethnicities.
Dr. Yang stressed that the findings need to be confirmed with further studies.
The study was carried out before widespread use of vaccinations in the U.S. Once those data are available, Dr. Yang and his colleagues plan to determine if vaccinations modify the association between COVID-19 and stroke risk.
The new results contribute to the mounting evidence that a COVID-19 infection “can actually affect multiple human organs structurally or functionally in addition to the impact on [the] respiratory system,” said Dr. Yang.
Some dates of COVID-19 diagnoses may be incorrect due to limited test availability, particularly early in the pandemic. Another limitation of the study was possible misclassification from the use of Medicare real-time preliminary claims.
In a provided statement, Louise D. McCullough, MD, PhD, chair of the ISC 2022 and professor and chair of neurology, McGovern Medical School, University of Texas Health Science Center at Houston, noted that the study focused on older adults because it was examining Medicare beneficiaries.
“But everyone is likely at risk for stroke after COVID,” she said. “Any infection is linked to stroke risk, probably because any infection will cause inflammation, and inflammation can cause clots or thrombus, which is the cause of stroke.”
There was no outside funding for the study. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
, new research shows.
The study among Medicare beneficiaries with COVID-19 also showed that stroke risk is higher for relatively young older adults, those aged 65 to 74 years, and those without a history of stroke.
The study highlights the impact COVID-19 has on the cardiovascular system, said study author Quanhe Yang, PhD, senior scientist, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta.
“Clinicians and patients should understand that stroke might be one of the very important clinical consequences of COVID-19.”
The study was presented during the hybrid International Stroke Conference held in New Orleans and online. The meeting was presented by the American Stroke Association, a division of the American Heart Association.
Stroke is the fifth leading cause of death in the U.S. As an increasing number of people become infected with COVID-19, “it’s important to determine if there’s a relationship between COVID and the risk of stroke,” said Dr. Yang.
Findings from prior research examining the link between stroke and COVID-19 have been inconsistent, he noted. Some studies found an association while others did not, and in still others, the association was not as strong as expected.
Many factors may contribute to these inconsistent findings, said Dr. Yang, including differences in study design, inclusion criteria, comparison groups, sample sizes, and countries where the research was carried out. Dr. Yang pointed out that many of these studies were done in the early stages of the pandemic or didn’t include older adults, the population most at risk for stroke.
The current study included 19,553 Medicare beneficiaries aged 65 years and older diagnosed with COVID-19 and hospitalized with acute ischemic stroke. The median age at diagnosis of COVID-19 was 80.5 years, 57.5% were women, and more than 75% were non-Hispanic Whites.
To ensure the stroke occurred after a COVID infection, researchers used a self-controlled case series study design, a “within person” comparison between the risk period and the control period.
They divided the study period (Jan. 1, 2019 to Feb. 28, 2021) into the exposure or stroke risk periods after the COVID diagnosis (0-3 days; 4-7 days; 8-15 days; and 15-28 days) and control periods.
Strokes that occurred 7 days before or 28 days after a COVID diagnosis served as a control period. “Any stroke that occurred outside the risk window is in the control period,” explained Dr. Yang.
He added that the control period provides a baseline. “Without COVID-19, this is what I would expect” in terms of the number of strokes.
To estimate the incidence rate ratio (IRR), investigators compared the incidence of acute ischemic stroke in the various risk periods with control periods.
The IRR was 10.97 (95% confidence interval, 10.30-11.68) at 0-3 days. The risk then quickly declined but stayed higher than the control period. The IRRs were: 1.59 (95% CI, 1.35-1.87) at 4-7 days; 1.23 (95% CI, 1.07-1.41) at 8-14 days; and 1.06 (95% CI, 0.95-1.18) at 15-28 days.
The temporary increase in stroke risk early after an infection isn’t novel; the pattern has been observed with influenza, respiratory infections, and shingles, said Dr. Yang. “But COVID-19 appears to be particularly risky.”
Although the mechanism driving the early increased stroke risk isn’t fully understood, it’s likely tied to an “exaggerated inflammatory response,” said Dr. Yang. This can trigger the cascade of events setting the stage for a stroke – a hypercoagulation state leading to the formation of blood clots that then block arteries to the brain, he said.
It’s also possible the infection directly affects endothelial cells, leading to rupture of plaque, again blocking arteries and raising stroke risks, added Dr. Yang.
The association was stronger among younger beneficiaries, aged 65 to 74 years, compared with those 85 years and older, a finding Dr. Yang said was somewhat surprising. But he noted other studies have found stroke patients with COVID are younger than stroke patients without COVID – by some 5 to 6 years.
“If COVID-19 disproportionately affects younger patients, that may explain the stronger association,” said Dr. Yang. “Stroke risk increases tremendously with age, so if you’re a younger age, your baseline stroke risk is lower.”
The association was also stronger among beneficiaries without a history of stroke. Again, this could be related to the stronger association among younger patients who are less likely to have suffered a stroke. The association was largely consistent across sex and race/ethnicities.
Dr. Yang stressed that the findings need to be confirmed with further studies.
The study was carried out before widespread use of vaccinations in the U.S. Once those data are available, Dr. Yang and his colleagues plan to determine if vaccinations modify the association between COVID-19 and stroke risk.
The new results contribute to the mounting evidence that a COVID-19 infection “can actually affect multiple human organs structurally or functionally in addition to the impact on [the] respiratory system,” said Dr. Yang.
Some dates of COVID-19 diagnoses may be incorrect due to limited test availability, particularly early in the pandemic. Another limitation of the study was possible misclassification from the use of Medicare real-time preliminary claims.
In a provided statement, Louise D. McCullough, MD, PhD, chair of the ISC 2022 and professor and chair of neurology, McGovern Medical School, University of Texas Health Science Center at Houston, noted that the study focused on older adults because it was examining Medicare beneficiaries.
“But everyone is likely at risk for stroke after COVID,” she said. “Any infection is linked to stroke risk, probably because any infection will cause inflammation, and inflammation can cause clots or thrombus, which is the cause of stroke.”
There was no outside funding for the study. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
FROM ISC 2022
Medical boards pressured to let it slide when doctors spread COVID misinformation
Tennessee’s Board of Medical Examiners unanimously adopted in September 2021 a statement that said doctors spreading COVID misinformation – such as suggesting that vaccines contain microchips – could jeopardize their license to practice.
“I’m very glad that we’re taking this step,” Dr. Stephen Loyd, MD, the panel’s vice president, said at the time. “If you’re spreading this willful misinformation, for me it’s going to be really hard to do anything other than put you on probation or take your license for a year. There has to be a message sent for this. It’s not okay.”
The board’s statement was posted on a government website.
The growing tension in Tennessee between conservative lawmakers and the state’s medical board may be the most prominent example in the country. But the Federation of State Medical Boards, which created the language adopted by at least 15 state boards, is tracking legislation introduced by Republicans in at least 14 states that would restrict a medical board’s authority to discipline doctors for their advice on COVID.
Humayun Chaudhry, DO, the federation’s CEO, called it “an unwelcome trend.” The nonprofit association, based in Euless, Tex., said the statement is merely a COVID-specific restatement of an existing rule: that doctors who engage in behavior that puts patients at risk could face disciplinary action.
Although doctors have leeway to decide which treatments to provide, the medical boards that oversee them have broad authority over licensing. Often, doctors are investigated for violating guidelines on prescribing high-powered drugs. But physicians are sometimes punished for other “unprofessional conduct.” In 2013, Tennessee’s board fined U.S. Rep. Scott DesJarlais for separately having sexual relations with two female patients more than a decade earlier.
Still, stopping doctors from sharing unsound medical advice has proved challenging. Even defining misinformation has been difficult. And during the pandemic, resistance from some state legislatures is complicating the effort.
A relatively small group of physicians peddle COVID misinformation, but many of them associate with America’s Frontline Doctors. Its founder, Simone Gold, MD, has claimed patients are dying from COVID treatments, not the virus itself. Sherri Tenpenny, DO, said in a legislative hearing in Ohio that the COVID vaccine could magnetize patients. Stella Immanuel, MD, has pushed hydroxychloroquine as a COVID cure in Texas, although clinical trials showed that it had no benefit. None of them agreed to requests for comment.
The Texas Medical Board fined Dr. Immanuel $500 for not informing a patient of the risks associated with using hydroxychloroquine as an off-label COVID treatment.
In Tennessee, state lawmakers called a special legislative session in October to address COVID restrictions, and Republican Gov. Bill Lee signed a sweeping package of bills that push back against pandemic rules. One included language directed at the medical board’s recent COVID policy statement, making it more difficult for the panel to investigate complaints about physicians’ advice on COVID vaccines or treatments.
In November, Republican state Rep. John Ragan sent the medical board a letter demanding that the statement be deleted from the state’s website. Rep. Ragan leads a legislative panel that had raised the prospect of defunding the state’s health department over its promotion of COVID vaccines to teens.
Among his demands, Rep. Ragan listed 20 questions he wanted the medical board to answer in writing, including why the misinformation “policy” was proposed nearly two years into the pandemic, which scholars would determine what constitutes misinformation, and how was the “policy” not an infringement on the doctor-patient relationship.
“If you fail to act promptly, your organization will be required to appear before the Joint Government Operations Committee to explain your inaction,” Rep. Ragan wrote in the letter, obtained by Kaiser Health News and Nashville Public Radio.
In response to a request for comment, Rep. Ragan said that “any executive agency, including Board of Medical Examiners, that refuses to follow the law is subject to dissolution.”
He set a deadline of Dec. 7.
In Florida, a Republican-sponsored bill making its way through the state legislature proposes to ban medical boards from revoking or threatening to revoke doctors’ licenses for what they say unless “direct physical harm” of a patient occurred. If the publicized complaint can’t be proved, the board could owe a doctor up to $1.5 million in damages.
Although Florida’s medical board has not adopted the Federation of State Medical Boards’ COVID misinformation statement, the panel has considered misinformation complaints against physicians, including the state’s surgeon general, Joseph Ladapo, MD, PhD.
Dr. Chaudhry said he’s surprised just how many COVID-related complaints are being filed across the country. Often, boards do not publicize investigations before a violation of ethics or standards is confirmed. But in response to a survey by the federation in late 2021, two-thirds of state boards reported an increase in misinformation complaints. And the federation said 12 boards had taken action against a licensed physician.
“At the end of the day, if a physician who is licensed engages in activity that causes harm, the state medical boards are the ones that historically have been set up to look into the situation and make a judgment about what happened or didn’t happen,” Dr. Chaudhry said. “And if you start to chip away at that, it becomes a slippery slope.”
The Georgia Composite Medical Board adopted a version of the federation’s misinformation guidance in early November and has been receiving 10-20 complaints each month, said Debi Dalton, MD, the chairperson. Two months in, no one had been sanctioned.
Dr. Dalton said that even putting out a misinformation policy leaves some “gray” area. Generally, physicians are expected to follow the “consensus,” rather than “the newest information that pops up on social media,” she said.
“We expect physicians to think ethically, professionally, and with the safety of patients in mind,” Dr. Dalton said.
A few physician groups are resisting attempts to root out misinformation, including the Association of American Physicians and Surgeons, known for its stands against government regulation.
Some medical boards have opted against taking a public stand against misinformation.
The Alabama Board of Medical Examiners discussed signing on to the federation’s statement, according to the minutes from an October meeting. But after debating the potential legal ramifications in a private executive session, the board opted not to act.
In Tennessee, the Board of Medical Examiners met on the day Rep. Ragan had set as the deadline and voted to remove the misinformation statement from its website to avoid being called into a legislative hearing. But then, in late January, the board decided to stick with the policy – although it did not republish the statement online immediately – and more specifically defined misinformation, calling it “content that is false, inaccurate or misleading, even if spread unintentionally.”
Board members acknowledged they would likely get more pushback from lawmakers but said they wanted to protect their profession from interference.
“Doctors who are putting forth good evidence-based medicine deserve the protection of this board so they can actually say: ‘Hey, I’m in line with this guideline, and this is a source of truth,’” said Melanie Blake, MD, the board’s president. “We should be a source of truth.”
The medical board was looking into nearly 30 open complaints related to COVID when its misinformation statement came down from its website. As of early February, no Tennessee physician had faced disciplinary action.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Nashville Public Radio, NPR, and KHN.
Tennessee’s Board of Medical Examiners unanimously adopted in September 2021 a statement that said doctors spreading COVID misinformation – such as suggesting that vaccines contain microchips – could jeopardize their license to practice.
“I’m very glad that we’re taking this step,” Dr. Stephen Loyd, MD, the panel’s vice president, said at the time. “If you’re spreading this willful misinformation, for me it’s going to be really hard to do anything other than put you on probation or take your license for a year. There has to be a message sent for this. It’s not okay.”
The board’s statement was posted on a government website.
The growing tension in Tennessee between conservative lawmakers and the state’s medical board may be the most prominent example in the country. But the Federation of State Medical Boards, which created the language adopted by at least 15 state boards, is tracking legislation introduced by Republicans in at least 14 states that would restrict a medical board’s authority to discipline doctors for their advice on COVID.
Humayun Chaudhry, DO, the federation’s CEO, called it “an unwelcome trend.” The nonprofit association, based in Euless, Tex., said the statement is merely a COVID-specific restatement of an existing rule: that doctors who engage in behavior that puts patients at risk could face disciplinary action.
Although doctors have leeway to decide which treatments to provide, the medical boards that oversee them have broad authority over licensing. Often, doctors are investigated for violating guidelines on prescribing high-powered drugs. But physicians are sometimes punished for other “unprofessional conduct.” In 2013, Tennessee’s board fined U.S. Rep. Scott DesJarlais for separately having sexual relations with two female patients more than a decade earlier.
Still, stopping doctors from sharing unsound medical advice has proved challenging. Even defining misinformation has been difficult. And during the pandemic, resistance from some state legislatures is complicating the effort.
A relatively small group of physicians peddle COVID misinformation, but many of them associate with America’s Frontline Doctors. Its founder, Simone Gold, MD, has claimed patients are dying from COVID treatments, not the virus itself. Sherri Tenpenny, DO, said in a legislative hearing in Ohio that the COVID vaccine could magnetize patients. Stella Immanuel, MD, has pushed hydroxychloroquine as a COVID cure in Texas, although clinical trials showed that it had no benefit. None of them agreed to requests for comment.
The Texas Medical Board fined Dr. Immanuel $500 for not informing a patient of the risks associated with using hydroxychloroquine as an off-label COVID treatment.
In Tennessee, state lawmakers called a special legislative session in October to address COVID restrictions, and Republican Gov. Bill Lee signed a sweeping package of bills that push back against pandemic rules. One included language directed at the medical board’s recent COVID policy statement, making it more difficult for the panel to investigate complaints about physicians’ advice on COVID vaccines or treatments.
In November, Republican state Rep. John Ragan sent the medical board a letter demanding that the statement be deleted from the state’s website. Rep. Ragan leads a legislative panel that had raised the prospect of defunding the state’s health department over its promotion of COVID vaccines to teens.
Among his demands, Rep. Ragan listed 20 questions he wanted the medical board to answer in writing, including why the misinformation “policy” was proposed nearly two years into the pandemic, which scholars would determine what constitutes misinformation, and how was the “policy” not an infringement on the doctor-patient relationship.
“If you fail to act promptly, your organization will be required to appear before the Joint Government Operations Committee to explain your inaction,” Rep. Ragan wrote in the letter, obtained by Kaiser Health News and Nashville Public Radio.
In response to a request for comment, Rep. Ragan said that “any executive agency, including Board of Medical Examiners, that refuses to follow the law is subject to dissolution.”
He set a deadline of Dec. 7.
In Florida, a Republican-sponsored bill making its way through the state legislature proposes to ban medical boards from revoking or threatening to revoke doctors’ licenses for what they say unless “direct physical harm” of a patient occurred. If the publicized complaint can’t be proved, the board could owe a doctor up to $1.5 million in damages.
Although Florida’s medical board has not adopted the Federation of State Medical Boards’ COVID misinformation statement, the panel has considered misinformation complaints against physicians, including the state’s surgeon general, Joseph Ladapo, MD, PhD.
Dr. Chaudhry said he’s surprised just how many COVID-related complaints are being filed across the country. Often, boards do not publicize investigations before a violation of ethics or standards is confirmed. But in response to a survey by the federation in late 2021, two-thirds of state boards reported an increase in misinformation complaints. And the federation said 12 boards had taken action against a licensed physician.
“At the end of the day, if a physician who is licensed engages in activity that causes harm, the state medical boards are the ones that historically have been set up to look into the situation and make a judgment about what happened or didn’t happen,” Dr. Chaudhry said. “And if you start to chip away at that, it becomes a slippery slope.”
The Georgia Composite Medical Board adopted a version of the federation’s misinformation guidance in early November and has been receiving 10-20 complaints each month, said Debi Dalton, MD, the chairperson. Two months in, no one had been sanctioned.
Dr. Dalton said that even putting out a misinformation policy leaves some “gray” area. Generally, physicians are expected to follow the “consensus,” rather than “the newest information that pops up on social media,” she said.
“We expect physicians to think ethically, professionally, and with the safety of patients in mind,” Dr. Dalton said.
A few physician groups are resisting attempts to root out misinformation, including the Association of American Physicians and Surgeons, known for its stands against government regulation.
Some medical boards have opted against taking a public stand against misinformation.
The Alabama Board of Medical Examiners discussed signing on to the federation’s statement, according to the minutes from an October meeting. But after debating the potential legal ramifications in a private executive session, the board opted not to act.
In Tennessee, the Board of Medical Examiners met on the day Rep. Ragan had set as the deadline and voted to remove the misinformation statement from its website to avoid being called into a legislative hearing. But then, in late January, the board decided to stick with the policy – although it did not republish the statement online immediately – and more specifically defined misinformation, calling it “content that is false, inaccurate or misleading, even if spread unintentionally.”
Board members acknowledged they would likely get more pushback from lawmakers but said they wanted to protect their profession from interference.
“Doctors who are putting forth good evidence-based medicine deserve the protection of this board so they can actually say: ‘Hey, I’m in line with this guideline, and this is a source of truth,’” said Melanie Blake, MD, the board’s president. “We should be a source of truth.”
The medical board was looking into nearly 30 open complaints related to COVID when its misinformation statement came down from its website. As of early February, no Tennessee physician had faced disciplinary action.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Nashville Public Radio, NPR, and KHN.
Tennessee’s Board of Medical Examiners unanimously adopted in September 2021 a statement that said doctors spreading COVID misinformation – such as suggesting that vaccines contain microchips – could jeopardize their license to practice.
“I’m very glad that we’re taking this step,” Dr. Stephen Loyd, MD, the panel’s vice president, said at the time. “If you’re spreading this willful misinformation, for me it’s going to be really hard to do anything other than put you on probation or take your license for a year. There has to be a message sent for this. It’s not okay.”
The board’s statement was posted on a government website.
The growing tension in Tennessee between conservative lawmakers and the state’s medical board may be the most prominent example in the country. But the Federation of State Medical Boards, which created the language adopted by at least 15 state boards, is tracking legislation introduced by Republicans in at least 14 states that would restrict a medical board’s authority to discipline doctors for their advice on COVID.
Humayun Chaudhry, DO, the federation’s CEO, called it “an unwelcome trend.” The nonprofit association, based in Euless, Tex., said the statement is merely a COVID-specific restatement of an existing rule: that doctors who engage in behavior that puts patients at risk could face disciplinary action.
Although doctors have leeway to decide which treatments to provide, the medical boards that oversee them have broad authority over licensing. Often, doctors are investigated for violating guidelines on prescribing high-powered drugs. But physicians are sometimes punished for other “unprofessional conduct.” In 2013, Tennessee’s board fined U.S. Rep. Scott DesJarlais for separately having sexual relations with two female patients more than a decade earlier.
Still, stopping doctors from sharing unsound medical advice has proved challenging. Even defining misinformation has been difficult. And during the pandemic, resistance from some state legislatures is complicating the effort.
A relatively small group of physicians peddle COVID misinformation, but many of them associate with America’s Frontline Doctors. Its founder, Simone Gold, MD, has claimed patients are dying from COVID treatments, not the virus itself. Sherri Tenpenny, DO, said in a legislative hearing in Ohio that the COVID vaccine could magnetize patients. Stella Immanuel, MD, has pushed hydroxychloroquine as a COVID cure in Texas, although clinical trials showed that it had no benefit. None of them agreed to requests for comment.
The Texas Medical Board fined Dr. Immanuel $500 for not informing a patient of the risks associated with using hydroxychloroquine as an off-label COVID treatment.
In Tennessee, state lawmakers called a special legislative session in October to address COVID restrictions, and Republican Gov. Bill Lee signed a sweeping package of bills that push back against pandemic rules. One included language directed at the medical board’s recent COVID policy statement, making it more difficult for the panel to investigate complaints about physicians’ advice on COVID vaccines or treatments.
In November, Republican state Rep. John Ragan sent the medical board a letter demanding that the statement be deleted from the state’s website. Rep. Ragan leads a legislative panel that had raised the prospect of defunding the state’s health department over its promotion of COVID vaccines to teens.
Among his demands, Rep. Ragan listed 20 questions he wanted the medical board to answer in writing, including why the misinformation “policy” was proposed nearly two years into the pandemic, which scholars would determine what constitutes misinformation, and how was the “policy” not an infringement on the doctor-patient relationship.
“If you fail to act promptly, your organization will be required to appear before the Joint Government Operations Committee to explain your inaction,” Rep. Ragan wrote in the letter, obtained by Kaiser Health News and Nashville Public Radio.
In response to a request for comment, Rep. Ragan said that “any executive agency, including Board of Medical Examiners, that refuses to follow the law is subject to dissolution.”
He set a deadline of Dec. 7.
In Florida, a Republican-sponsored bill making its way through the state legislature proposes to ban medical boards from revoking or threatening to revoke doctors’ licenses for what they say unless “direct physical harm” of a patient occurred. If the publicized complaint can’t be proved, the board could owe a doctor up to $1.5 million in damages.
Although Florida’s medical board has not adopted the Federation of State Medical Boards’ COVID misinformation statement, the panel has considered misinformation complaints against physicians, including the state’s surgeon general, Joseph Ladapo, MD, PhD.
Dr. Chaudhry said he’s surprised just how many COVID-related complaints are being filed across the country. Often, boards do not publicize investigations before a violation of ethics or standards is confirmed. But in response to a survey by the federation in late 2021, two-thirds of state boards reported an increase in misinformation complaints. And the federation said 12 boards had taken action against a licensed physician.
“At the end of the day, if a physician who is licensed engages in activity that causes harm, the state medical boards are the ones that historically have been set up to look into the situation and make a judgment about what happened or didn’t happen,” Dr. Chaudhry said. “And if you start to chip away at that, it becomes a slippery slope.”
The Georgia Composite Medical Board adopted a version of the federation’s misinformation guidance in early November and has been receiving 10-20 complaints each month, said Debi Dalton, MD, the chairperson. Two months in, no one had been sanctioned.
Dr. Dalton said that even putting out a misinformation policy leaves some “gray” area. Generally, physicians are expected to follow the “consensus,” rather than “the newest information that pops up on social media,” she said.
“We expect physicians to think ethically, professionally, and with the safety of patients in mind,” Dr. Dalton said.
A few physician groups are resisting attempts to root out misinformation, including the Association of American Physicians and Surgeons, known for its stands against government regulation.
Some medical boards have opted against taking a public stand against misinformation.
The Alabama Board of Medical Examiners discussed signing on to the federation’s statement, according to the minutes from an October meeting. But after debating the potential legal ramifications in a private executive session, the board opted not to act.
In Tennessee, the Board of Medical Examiners met on the day Rep. Ragan had set as the deadline and voted to remove the misinformation statement from its website to avoid being called into a legislative hearing. But then, in late January, the board decided to stick with the policy – although it did not republish the statement online immediately – and more specifically defined misinformation, calling it “content that is false, inaccurate or misleading, even if spread unintentionally.”
Board members acknowledged they would likely get more pushback from lawmakers but said they wanted to protect their profession from interference.
“Doctors who are putting forth good evidence-based medicine deserve the protection of this board so they can actually say: ‘Hey, I’m in line with this guideline, and this is a source of truth,’” said Melanie Blake, MD, the board’s president. “We should be a source of truth.”
The medical board was looking into nearly 30 open complaints related to COVID when its misinformation statement came down from its website. As of early February, no Tennessee physician had faced disciplinary action.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Nashville Public Radio, NPR, and KHN.
New study shows natural immunity to COVID has enduring strength
It’s a matter of quality, not quantity. That’s the gist of a new Israeli study that shows that unvaccinated people with a prior SARS-CoV-2 infection create antibodies that are more effective in the long run compared with others who were vaccinated but never infected.
“While the quantity of antibodies decreases with time in both COVID-19 recovered patients and vaccinated individuals, ” lead author Carmit Cohen, PhD, said in an interview.
This difference could explain why previously infected patients appear to be better protected against a new infection than those who have only been vaccinated, according to a news release attached to the research.
One key caveat: This research does not include people from the later part of the pandemic.
This means there is a catch in terms of timing, William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tenn., said when asked to comment on the study: “The study involved only the early COVID strains – it has no information on either the Delta or Omicron variants. Thus, the results primarily are of scientific or historical interest but are not immediately relevant to the current situation.”
The findings come from an early release of a study to be presented at the European Congress of Clinical Microbiology & Infectious Diseases in April.
An unexpected finding of the study showed that obese people had better protection – a higher and more sustained immune response – compared with overweight and normal-weight individuals.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” Dr. Schaffner said. “Obesity does predispose to more severe disease.”
A focus on earlier strains
Dr. Cohen – a senior research assistant in infectious disease prevention at the Sheba Medical Center in Ramat Gan, Israel – and her colleagues recruited participants between March 25, 2020 and Nov. 25, 2020 and completed analysis in April 2021. This means they assessed people with a history of infection from the original, the Alpha, and some Beta strains of SARS-CoV-2.
Dr. Cohen indicated that the next phase of their research will examine innate and acquired immune responses to the more recent Delta and Omicron variants.
The investigators analyzed the antibody-induced immune response up to 1 year in 130 COVID-19 recovered but unvaccinated individuals versus up to 8 months among 402 others matched by age and body mass index (BMI) and without previous infection who received two doses of the Pfizer vaccine.
The numbers of antibodies a month after vaccination were higher than those in the COVID-19 recovered patients. However, these numbers also declined more steeply in the vaccinated group, they note.
To assess the antibody performance, the investigators used the avidity index. This assay measures antibody function based on the strength of the interactions between the antibody and the viral antigen.
They found that the avidity index was higher in vaccinated individuals than in recovered patients initially but changes over time. At up to 6 months, the index did not significantly change in vaccinated individuals, whereas it gradually increased in recovered patients. This increase would potentially protect them from reinfection, the authors note.
These findings stand in stark contrast to an Oct. 29, 2021, Centers for Disease Control and Prevention study that found that COVID-19 vaccines provided five times the protection of natural immunity.
Those results, published in the organization’s Morbidity and Mortality Weekly Report, suggest that vaccination helps people mount a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least 6 months.
Protection linked to obesity
Another finding that ran against the scientific grain was the data about obesity.
There was a higher and more persistent antibody performance among people with a BMI of 30 kg/m2.
This could relate to greater disease severity and/or a more pronounced initial response to infection among the obese group.
“Our hypothesis is that patients with obesity begin with a more pronounced response – reflected also by the disease manifestation – and the trend of decline is similar, therefore the kinetics of immune response remain higher throughout the study,” Dr. Cohen said.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” said Dr. Schaffner, who is also the current medical director of the National Foundation for Infectious Diseases. “Obesity does predispose to more severe disease.”
Before the boosters
Along with using participants from only the earlier part of the pandemic, another limitation of the study was that the vaccinated group had only two doses of vaccine; boosters were not given during the time of the study, Dr. Schaffner said.
“Again, not the current situation.”
“That said, the strength and duration of natural immunity provided by the early variants was solid for up to a year, confirming previous reports,” he said.
A version of this article first appeared on Medscape.com.
It’s a matter of quality, not quantity. That’s the gist of a new Israeli study that shows that unvaccinated people with a prior SARS-CoV-2 infection create antibodies that are more effective in the long run compared with others who were vaccinated but never infected.
“While the quantity of antibodies decreases with time in both COVID-19 recovered patients and vaccinated individuals, ” lead author Carmit Cohen, PhD, said in an interview.
This difference could explain why previously infected patients appear to be better protected against a new infection than those who have only been vaccinated, according to a news release attached to the research.
One key caveat: This research does not include people from the later part of the pandemic.
This means there is a catch in terms of timing, William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tenn., said when asked to comment on the study: “The study involved only the early COVID strains – it has no information on either the Delta or Omicron variants. Thus, the results primarily are of scientific or historical interest but are not immediately relevant to the current situation.”
The findings come from an early release of a study to be presented at the European Congress of Clinical Microbiology & Infectious Diseases in April.
An unexpected finding of the study showed that obese people had better protection – a higher and more sustained immune response – compared with overweight and normal-weight individuals.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” Dr. Schaffner said. “Obesity does predispose to more severe disease.”
A focus on earlier strains
Dr. Cohen – a senior research assistant in infectious disease prevention at the Sheba Medical Center in Ramat Gan, Israel – and her colleagues recruited participants between March 25, 2020 and Nov. 25, 2020 and completed analysis in April 2021. This means they assessed people with a history of infection from the original, the Alpha, and some Beta strains of SARS-CoV-2.
Dr. Cohen indicated that the next phase of their research will examine innate and acquired immune responses to the more recent Delta and Omicron variants.
The investigators analyzed the antibody-induced immune response up to 1 year in 130 COVID-19 recovered but unvaccinated individuals versus up to 8 months among 402 others matched by age and body mass index (BMI) and without previous infection who received two doses of the Pfizer vaccine.
The numbers of antibodies a month after vaccination were higher than those in the COVID-19 recovered patients. However, these numbers also declined more steeply in the vaccinated group, they note.
To assess the antibody performance, the investigators used the avidity index. This assay measures antibody function based on the strength of the interactions between the antibody and the viral antigen.
They found that the avidity index was higher in vaccinated individuals than in recovered patients initially but changes over time. At up to 6 months, the index did not significantly change in vaccinated individuals, whereas it gradually increased in recovered patients. This increase would potentially protect them from reinfection, the authors note.
These findings stand in stark contrast to an Oct. 29, 2021, Centers for Disease Control and Prevention study that found that COVID-19 vaccines provided five times the protection of natural immunity.
Those results, published in the organization’s Morbidity and Mortality Weekly Report, suggest that vaccination helps people mount a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least 6 months.
Protection linked to obesity
Another finding that ran against the scientific grain was the data about obesity.
There was a higher and more persistent antibody performance among people with a BMI of 30 kg/m2.
This could relate to greater disease severity and/or a more pronounced initial response to infection among the obese group.
“Our hypothesis is that patients with obesity begin with a more pronounced response – reflected also by the disease manifestation – and the trend of decline is similar, therefore the kinetics of immune response remain higher throughout the study,” Dr. Cohen said.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” said Dr. Schaffner, who is also the current medical director of the National Foundation for Infectious Diseases. “Obesity does predispose to more severe disease.”
Before the boosters
Along with using participants from only the earlier part of the pandemic, another limitation of the study was that the vaccinated group had only two doses of vaccine; boosters were not given during the time of the study, Dr. Schaffner said.
“Again, not the current situation.”
“That said, the strength and duration of natural immunity provided by the early variants was solid for up to a year, confirming previous reports,” he said.
A version of this article first appeared on Medscape.com.
It’s a matter of quality, not quantity. That’s the gist of a new Israeli study that shows that unvaccinated people with a prior SARS-CoV-2 infection create antibodies that are more effective in the long run compared with others who were vaccinated but never infected.
“While the quantity of antibodies decreases with time in both COVID-19 recovered patients and vaccinated individuals, ” lead author Carmit Cohen, PhD, said in an interview.
This difference could explain why previously infected patients appear to be better protected against a new infection than those who have only been vaccinated, according to a news release attached to the research.
One key caveat: This research does not include people from the later part of the pandemic.
This means there is a catch in terms of timing, William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tenn., said when asked to comment on the study: “The study involved only the early COVID strains – it has no information on either the Delta or Omicron variants. Thus, the results primarily are of scientific or historical interest but are not immediately relevant to the current situation.”
The findings come from an early release of a study to be presented at the European Congress of Clinical Microbiology & Infectious Diseases in April.
An unexpected finding of the study showed that obese people had better protection – a higher and more sustained immune response – compared with overweight and normal-weight individuals.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” Dr. Schaffner said. “Obesity does predispose to more severe disease.”
A focus on earlier strains
Dr. Cohen – a senior research assistant in infectious disease prevention at the Sheba Medical Center in Ramat Gan, Israel – and her colleagues recruited participants between March 25, 2020 and Nov. 25, 2020 and completed analysis in April 2021. This means they assessed people with a history of infection from the original, the Alpha, and some Beta strains of SARS-CoV-2.
Dr. Cohen indicated that the next phase of their research will examine innate and acquired immune responses to the more recent Delta and Omicron variants.
The investigators analyzed the antibody-induced immune response up to 1 year in 130 COVID-19 recovered but unvaccinated individuals versus up to 8 months among 402 others matched by age and body mass index (BMI) and without previous infection who received two doses of the Pfizer vaccine.
The numbers of antibodies a month after vaccination were higher than those in the COVID-19 recovered patients. However, these numbers also declined more steeply in the vaccinated group, they note.
To assess the antibody performance, the investigators used the avidity index. This assay measures antibody function based on the strength of the interactions between the antibody and the viral antigen.
They found that the avidity index was higher in vaccinated individuals than in recovered patients initially but changes over time. At up to 6 months, the index did not significantly change in vaccinated individuals, whereas it gradually increased in recovered patients. This increase would potentially protect them from reinfection, the authors note.
These findings stand in stark contrast to an Oct. 29, 2021, Centers for Disease Control and Prevention study that found that COVID-19 vaccines provided five times the protection of natural immunity.
Those results, published in the organization’s Morbidity and Mortality Weekly Report, suggest that vaccination helps people mount a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least 6 months.
Protection linked to obesity
Another finding that ran against the scientific grain was the data about obesity.
There was a higher and more persistent antibody performance among people with a BMI of 30 kg/m2.
This could relate to greater disease severity and/or a more pronounced initial response to infection among the obese group.
“Our hypothesis is that patients with obesity begin with a more pronounced response – reflected also by the disease manifestation – and the trend of decline is similar, therefore the kinetics of immune response remain higher throughout the study,” Dr. Cohen said.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” said Dr. Schaffner, who is also the current medical director of the National Foundation for Infectious Diseases. “Obesity does predispose to more severe disease.”
Before the boosters
Along with using participants from only the earlier part of the pandemic, another limitation of the study was that the vaccinated group had only two doses of vaccine; boosters were not given during the time of the study, Dr. Schaffner said.
“Again, not the current situation.”
“That said, the strength and duration of natural immunity provided by the early variants was solid for up to a year, confirming previous reports,” he said.
A version of this article first appeared on Medscape.com.
Too much marijuana can make you unpleasantly, dangerously sick
At the center of the emerging science on the unintended consequences of daily long-term use of marijuana lies a paradox.
For years, medical marijuana has been used to ease nausea from cancer chemotherapy and GI conditions. Now, with greater legalization comes growing awareness that chronic use of marijuana – also known as cannabis – can trigger a condition where, ironically, a person has hard-to-control vomiting and nausea.
Some people with the disorder, known as “cannabinoid hyperemesis syndrome,” also report crippling belly pain.
Linda can relate. The 33-year-old Oregon resident, who asked to remain anonymous to protect her privacy, refers to a medieval spiky metal ball on a chain when describing the pain.
“Picture a mace inside your stomach, pushing up inside your chest and, at the same time, exploding out,” she said.
To seek relief, she gets down on her knees, adopts a child’s yoga pose, and runs hot water in the bathroom for hours on end, a trick many with the disorder says has provided relief. She also occasionally goes outside and tries walking it off.
“I would just wander around my neighborhood, a lot of times at like 4 or 5 in the morning,” she said. “The fresh air helps a little bit. I just keep walking down the street, take about 10 steps, stop, vomit – walk a little bit more, stop, vomit.”
Her first experience with the disorder began in the middle of one night in 2017 while she was at a conference in Las Vegas.
“We went out to eat the night before, and I woke up about 4 in the morning with just the most intense pain I’ve ever had,” she said. “I found myself in a really hot shower in between throwing up everything and trying to say get some water down. I was sharing an Airbnb with my colleagues, so it was less than ideal.”
Many people with cannabinoid hyperemesis syndrome find relief from hot baths or showers. Researchers believe that hot water helps because temperature sensors in the skin send signals to the brain that can help ease the symptoms, at least for a while.
The problem is that people with this syndrome “can’t live in the water,” said emergency doctor and medical cannabis expert Leigh Vinocur, MD.
Fast-forward 6 months to another event in Boulder, Colo. Again, Linda woke up and could not stop vomiting.
“I was not feeling any better. Showering wasn’t helping. I ended up in the hospital,” she said.
She received opioids for her pain. But neither she nor the ED staff were quite sure what was happening. Her discharge paperwork read “cannabis allergy.”
Cannabinoid hyperemesis syndrome “shatters that image of cannabis only being a good thing. It’s a bold statement, but, you know, once you start to think about it, it’s like a little too much of anything isn’t good,” Linda said.
Experts suggest greater awareness is needed to identify this syndrome earlier, by both cannabinoid users and doctors. The bouts of vomiting, in particular, can get so severe that people can end up hospitalized with dehydration, electrolyte disorders, and weight loss.
The severe electrolyte imbalances “can really be life-threatening,” said David Johnson, MD, a professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk.
“By the time they come into emergency care, they’re in bad shape,” Dr. Vinocur agreed. “Many try to ignore it, but they continue to vomit.”
Genetic risk factors?
One mystery is why some regular marijuana users get this syndrome while others do not.
“I can say that not everybody gets this, thank goodness,” said Ethan Russo, MD. “But there has to be a reason that certain people are susceptible and others are not.”
Interestingly, a new study from Dr. Russo and colleagues suggests that genes play a role. They identified five genetic changes that could make a chronic marijuana user more likely to have cannabinoid hyperemesis syndrome in a study published July 5, 2021, in the journal Cannabis and Cannabinoid Research.
They compared 28 people with the disorder with 12 other high-frequency marijuana users without these symptoms.
The results are not final but could help guide future research, Dr. Russo said.
“What we’ve discovered – and it was far more than we expected – is that there’s a lot more to this than a hypersensitivity to cannabis,” said Dr. Russo, a neurologist and founder/CEO of CReDO Science, a firm that promotes cannabis research and develops commercial products.
Also, he said, those affected by cannabinoid hyperemesis syndrome could be at higher risk for other conditions, such as addiction to alcohol or other substances, dementia, diabetes, and heart disease.
“Most people with [cannabinoid hyperemesis syndrome] are going to be younger,” he said. “What we’ve demonstrated is there is a risk for more serious problems for decades to come. So someone who has these symptoms really deserves a look at this genetic screening.”
Battling disbelief
Getting back to the paradox, many users don’t believe marijuana can trigger serious vomiting and nausea because of its reputation for doing the opposite.
“Folks that have this are just uniquely resistant to the concept that cannabis is actually the problem and not the solution,” Dr. Russo said.
“It’s kind of counterintuitive because people think: ‘Oh, cannabis helps with nausea,’ so they use more of it,” said Dr. Vinocur, who is also a spokesperson for the American College of Emergency Physicians and runs a medical cannabis practice.
Most kinds of marijuana act in this way – doing opposite things at different doses. Once a certain threshold is passed, people with cannabinoid hyperemesis syndrome are “just uniquely susceptible and really can’t tolerate any significant amount of THC,” Dr. Russo said, referring to tetrahydrocannabinol, the substance that gets marijuana users high.
Once diagnosed, quitting is the most effective strategy. But it can be tough to persuade someone to stop using marijuana.
“You do have to try and convince them ... to try abstinence and to watch and see what happens,” Dr. Vinocur said.
People should “realize the root cause of this is its cannabinoid ingestion, and the treatment is really best directed at absolute avoidance,” Dr. Johnson said.
Unfortunately, evidence also shows that once a person stops using marijuana and gets relief, going back to marijuana or other forms of cannabinoids can cause the syndrome to start all over again.
“We’ve had people that quit for a month, a year, 2 years and upon resumption, almost invariably, they’re back into bouts of the hyperemesis along with all the other [symptoms],” Dr. Russo said.
Marijuana and cannabinoids can cause digestive problems, Dr. Johnson said, which may cause more problems.
What recent research reveals
Cannabinoid hyperemesis syndrome is a relatively young disorder – first described in 2004 – and early reports and case studies are giving way now to studies looking into potential treatments.
So far, the strongest evidence suggests a role for an over-the-counter cream called capsaicin to help manage symptoms, but more studies are needed.
Similar to hot showers, this ingredient from chili peppers can warm the skin and trigger the temperature-sensitive skin sensors to lessen the symptoms, Dr. Johnson said.
An October 2021 study in Spain looked at 54 ED visits among 29 people with cannabinoid hyperemesis syndrome. For the 75% treated with capsaicin, vomiting stopped after an average of 18 minutes.
Lead author Guillermo Burillo-Putze, MD, PhD, said he is most surprised by the growing number of new cases of the disorder.
“This should be of concern given the increase in cannabis use due to its legalization and permissiveness,” said Dr. Burillo-Putze, an emergency doctor at Hospital Universitario de Canarias, Santa Cruz de Tenerife, Spain.
Cannabinoid hyperemesis syndrome appears not to discriminate across racial and ethic groups. Although most studies to date include White participants, a July 2021 study of 29 people, 90% of whom were Black, found repeat visits to the ED were common.
The study found that 16 people returned 42 times to the ED and accounted for 10 hospital admissions, for example.
Cannabis conspiracy theories
“Unfortunately, this condition has become the subject of great speculation hinging on conspiracy theories as its true cause,” Dr. Russo noted in a September 2021 letter to the editor in the American Journal of Emergency Medicine.
Some “myth busting” is in order, he said.
For example, cannabinoid hyperemesis syndrome does not happen because of exposure to products from a tree called neem or from pesticides applied to marijuana plants during cultivation, Dr. Russo said. It can also occur with high-dose synthetic cannabinoids.
The state of recreational and medical marijuana
Recreational marijuana is legal in 18 states, Washington, D.C., and Guam as of January 2022, according to a report in U.S. News. More states permit medical marijuana use – 37 in total, plus Washington, D.C., according to Britannica ProCon.
One of the states where only medicinal use is legal is Maryland, which is where Dr. Vinocur practices.
“We are seeing increasing numbers of cases” of cannabinoid hyperemesis syndrome, she said.
In addition to chronic use or higher doses, it’s likely that the higher potency levels of THC in the legal marijuana industry trigger the syndrome in some people as well.
Linda estimates she ended up in emergency rooms at least a half-dozen times in the last 5 years. In April 2021, she had a “pretty serious event.” She blames it on traveling a lot for work, not eating right, and not getting enough sleep. She broke her 2-year abstinence with alcohol.
“I basically didn’t listen to my body and paid a pretty significant price for it,” she said.
Linda did not stop altogether but said she “drastically changed the types and form of the cannabis I was using.”
“I can tell you on the record that I would be a hundred percent dead without this plant,” she said.
“The prospect of living without it was more detrimental to me than all of those things I just described to you, because addiction runs in my family and I had opiate problems myself that I overcame with cannabis.”
A version of this article first appeared on Medscape.com.
At the center of the emerging science on the unintended consequences of daily long-term use of marijuana lies a paradox.
For years, medical marijuana has been used to ease nausea from cancer chemotherapy and GI conditions. Now, with greater legalization comes growing awareness that chronic use of marijuana – also known as cannabis – can trigger a condition where, ironically, a person has hard-to-control vomiting and nausea.
Some people with the disorder, known as “cannabinoid hyperemesis syndrome,” also report crippling belly pain.
Linda can relate. The 33-year-old Oregon resident, who asked to remain anonymous to protect her privacy, refers to a medieval spiky metal ball on a chain when describing the pain.
“Picture a mace inside your stomach, pushing up inside your chest and, at the same time, exploding out,” she said.
To seek relief, she gets down on her knees, adopts a child’s yoga pose, and runs hot water in the bathroom for hours on end, a trick many with the disorder says has provided relief. She also occasionally goes outside and tries walking it off.
“I would just wander around my neighborhood, a lot of times at like 4 or 5 in the morning,” she said. “The fresh air helps a little bit. I just keep walking down the street, take about 10 steps, stop, vomit – walk a little bit more, stop, vomit.”
Her first experience with the disorder began in the middle of one night in 2017 while she was at a conference in Las Vegas.
“We went out to eat the night before, and I woke up about 4 in the morning with just the most intense pain I’ve ever had,” she said. “I found myself in a really hot shower in between throwing up everything and trying to say get some water down. I was sharing an Airbnb with my colleagues, so it was less than ideal.”
Many people with cannabinoid hyperemesis syndrome find relief from hot baths or showers. Researchers believe that hot water helps because temperature sensors in the skin send signals to the brain that can help ease the symptoms, at least for a while.
The problem is that people with this syndrome “can’t live in the water,” said emergency doctor and medical cannabis expert Leigh Vinocur, MD.
Fast-forward 6 months to another event in Boulder, Colo. Again, Linda woke up and could not stop vomiting.
“I was not feeling any better. Showering wasn’t helping. I ended up in the hospital,” she said.
She received opioids for her pain. But neither she nor the ED staff were quite sure what was happening. Her discharge paperwork read “cannabis allergy.”
Cannabinoid hyperemesis syndrome “shatters that image of cannabis only being a good thing. It’s a bold statement, but, you know, once you start to think about it, it’s like a little too much of anything isn’t good,” Linda said.
Experts suggest greater awareness is needed to identify this syndrome earlier, by both cannabinoid users and doctors. The bouts of vomiting, in particular, can get so severe that people can end up hospitalized with dehydration, electrolyte disorders, and weight loss.
The severe electrolyte imbalances “can really be life-threatening,” said David Johnson, MD, a professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk.
“By the time they come into emergency care, they’re in bad shape,” Dr. Vinocur agreed. “Many try to ignore it, but they continue to vomit.”
Genetic risk factors?
One mystery is why some regular marijuana users get this syndrome while others do not.
“I can say that not everybody gets this, thank goodness,” said Ethan Russo, MD. “But there has to be a reason that certain people are susceptible and others are not.”
Interestingly, a new study from Dr. Russo and colleagues suggests that genes play a role. They identified five genetic changes that could make a chronic marijuana user more likely to have cannabinoid hyperemesis syndrome in a study published July 5, 2021, in the journal Cannabis and Cannabinoid Research.
They compared 28 people with the disorder with 12 other high-frequency marijuana users without these symptoms.
The results are not final but could help guide future research, Dr. Russo said.
“What we’ve discovered – and it was far more than we expected – is that there’s a lot more to this than a hypersensitivity to cannabis,” said Dr. Russo, a neurologist and founder/CEO of CReDO Science, a firm that promotes cannabis research and develops commercial products.
Also, he said, those affected by cannabinoid hyperemesis syndrome could be at higher risk for other conditions, such as addiction to alcohol or other substances, dementia, diabetes, and heart disease.
“Most people with [cannabinoid hyperemesis syndrome] are going to be younger,” he said. “What we’ve demonstrated is there is a risk for more serious problems for decades to come. So someone who has these symptoms really deserves a look at this genetic screening.”
Battling disbelief
Getting back to the paradox, many users don’t believe marijuana can trigger serious vomiting and nausea because of its reputation for doing the opposite.
“Folks that have this are just uniquely resistant to the concept that cannabis is actually the problem and not the solution,” Dr. Russo said.
“It’s kind of counterintuitive because people think: ‘Oh, cannabis helps with nausea,’ so they use more of it,” said Dr. Vinocur, who is also a spokesperson for the American College of Emergency Physicians and runs a medical cannabis practice.
Most kinds of marijuana act in this way – doing opposite things at different doses. Once a certain threshold is passed, people with cannabinoid hyperemesis syndrome are “just uniquely susceptible and really can’t tolerate any significant amount of THC,” Dr. Russo said, referring to tetrahydrocannabinol, the substance that gets marijuana users high.
Once diagnosed, quitting is the most effective strategy. But it can be tough to persuade someone to stop using marijuana.
“You do have to try and convince them ... to try abstinence and to watch and see what happens,” Dr. Vinocur said.
People should “realize the root cause of this is its cannabinoid ingestion, and the treatment is really best directed at absolute avoidance,” Dr. Johnson said.
Unfortunately, evidence also shows that once a person stops using marijuana and gets relief, going back to marijuana or other forms of cannabinoids can cause the syndrome to start all over again.
“We’ve had people that quit for a month, a year, 2 years and upon resumption, almost invariably, they’re back into bouts of the hyperemesis along with all the other [symptoms],” Dr. Russo said.
Marijuana and cannabinoids can cause digestive problems, Dr. Johnson said, which may cause more problems.
What recent research reveals
Cannabinoid hyperemesis syndrome is a relatively young disorder – first described in 2004 – and early reports and case studies are giving way now to studies looking into potential treatments.
So far, the strongest evidence suggests a role for an over-the-counter cream called capsaicin to help manage symptoms, but more studies are needed.
Similar to hot showers, this ingredient from chili peppers can warm the skin and trigger the temperature-sensitive skin sensors to lessen the symptoms, Dr. Johnson said.
An October 2021 study in Spain looked at 54 ED visits among 29 people with cannabinoid hyperemesis syndrome. For the 75% treated with capsaicin, vomiting stopped after an average of 18 minutes.
Lead author Guillermo Burillo-Putze, MD, PhD, said he is most surprised by the growing number of new cases of the disorder.
“This should be of concern given the increase in cannabis use due to its legalization and permissiveness,” said Dr. Burillo-Putze, an emergency doctor at Hospital Universitario de Canarias, Santa Cruz de Tenerife, Spain.
Cannabinoid hyperemesis syndrome appears not to discriminate across racial and ethic groups. Although most studies to date include White participants, a July 2021 study of 29 people, 90% of whom were Black, found repeat visits to the ED were common.
The study found that 16 people returned 42 times to the ED and accounted for 10 hospital admissions, for example.
Cannabis conspiracy theories
“Unfortunately, this condition has become the subject of great speculation hinging on conspiracy theories as its true cause,” Dr. Russo noted in a September 2021 letter to the editor in the American Journal of Emergency Medicine.
Some “myth busting” is in order, he said.
For example, cannabinoid hyperemesis syndrome does not happen because of exposure to products from a tree called neem or from pesticides applied to marijuana plants during cultivation, Dr. Russo said. It can also occur with high-dose synthetic cannabinoids.
The state of recreational and medical marijuana
Recreational marijuana is legal in 18 states, Washington, D.C., and Guam as of January 2022, according to a report in U.S. News. More states permit medical marijuana use – 37 in total, plus Washington, D.C., according to Britannica ProCon.
One of the states where only medicinal use is legal is Maryland, which is where Dr. Vinocur practices.
“We are seeing increasing numbers of cases” of cannabinoid hyperemesis syndrome, she said.
In addition to chronic use or higher doses, it’s likely that the higher potency levels of THC in the legal marijuana industry trigger the syndrome in some people as well.
Linda estimates she ended up in emergency rooms at least a half-dozen times in the last 5 years. In April 2021, she had a “pretty serious event.” She blames it on traveling a lot for work, not eating right, and not getting enough sleep. She broke her 2-year abstinence with alcohol.
“I basically didn’t listen to my body and paid a pretty significant price for it,” she said.
Linda did not stop altogether but said she “drastically changed the types and form of the cannabis I was using.”
“I can tell you on the record that I would be a hundred percent dead without this plant,” she said.
“The prospect of living without it was more detrimental to me than all of those things I just described to you, because addiction runs in my family and I had opiate problems myself that I overcame with cannabis.”
A version of this article first appeared on Medscape.com.
At the center of the emerging science on the unintended consequences of daily long-term use of marijuana lies a paradox.
For years, medical marijuana has been used to ease nausea from cancer chemotherapy and GI conditions. Now, with greater legalization comes growing awareness that chronic use of marijuana – also known as cannabis – can trigger a condition where, ironically, a person has hard-to-control vomiting and nausea.
Some people with the disorder, known as “cannabinoid hyperemesis syndrome,” also report crippling belly pain.
Linda can relate. The 33-year-old Oregon resident, who asked to remain anonymous to protect her privacy, refers to a medieval spiky metal ball on a chain when describing the pain.
“Picture a mace inside your stomach, pushing up inside your chest and, at the same time, exploding out,” she said.
To seek relief, she gets down on her knees, adopts a child’s yoga pose, and runs hot water in the bathroom for hours on end, a trick many with the disorder says has provided relief. She also occasionally goes outside and tries walking it off.
“I would just wander around my neighborhood, a lot of times at like 4 or 5 in the morning,” she said. “The fresh air helps a little bit. I just keep walking down the street, take about 10 steps, stop, vomit – walk a little bit more, stop, vomit.”
Her first experience with the disorder began in the middle of one night in 2017 while she was at a conference in Las Vegas.
“We went out to eat the night before, and I woke up about 4 in the morning with just the most intense pain I’ve ever had,” she said. “I found myself in a really hot shower in between throwing up everything and trying to say get some water down. I was sharing an Airbnb with my colleagues, so it was less than ideal.”
Many people with cannabinoid hyperemesis syndrome find relief from hot baths or showers. Researchers believe that hot water helps because temperature sensors in the skin send signals to the brain that can help ease the symptoms, at least for a while.
The problem is that people with this syndrome “can’t live in the water,” said emergency doctor and medical cannabis expert Leigh Vinocur, MD.
Fast-forward 6 months to another event in Boulder, Colo. Again, Linda woke up and could not stop vomiting.
“I was not feeling any better. Showering wasn’t helping. I ended up in the hospital,” she said.
She received opioids for her pain. But neither she nor the ED staff were quite sure what was happening. Her discharge paperwork read “cannabis allergy.”
Cannabinoid hyperemesis syndrome “shatters that image of cannabis only being a good thing. It’s a bold statement, but, you know, once you start to think about it, it’s like a little too much of anything isn’t good,” Linda said.
Experts suggest greater awareness is needed to identify this syndrome earlier, by both cannabinoid users and doctors. The bouts of vomiting, in particular, can get so severe that people can end up hospitalized with dehydration, electrolyte disorders, and weight loss.
The severe electrolyte imbalances “can really be life-threatening,” said David Johnson, MD, a professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk.
“By the time they come into emergency care, they’re in bad shape,” Dr. Vinocur agreed. “Many try to ignore it, but they continue to vomit.”
Genetic risk factors?
One mystery is why some regular marijuana users get this syndrome while others do not.
“I can say that not everybody gets this, thank goodness,” said Ethan Russo, MD. “But there has to be a reason that certain people are susceptible and others are not.”
Interestingly, a new study from Dr. Russo and colleagues suggests that genes play a role. They identified five genetic changes that could make a chronic marijuana user more likely to have cannabinoid hyperemesis syndrome in a study published July 5, 2021, in the journal Cannabis and Cannabinoid Research.
They compared 28 people with the disorder with 12 other high-frequency marijuana users without these symptoms.
The results are not final but could help guide future research, Dr. Russo said.
“What we’ve discovered – and it was far more than we expected – is that there’s a lot more to this than a hypersensitivity to cannabis,” said Dr. Russo, a neurologist and founder/CEO of CReDO Science, a firm that promotes cannabis research and develops commercial products.
Also, he said, those affected by cannabinoid hyperemesis syndrome could be at higher risk for other conditions, such as addiction to alcohol or other substances, dementia, diabetes, and heart disease.
“Most people with [cannabinoid hyperemesis syndrome] are going to be younger,” he said. “What we’ve demonstrated is there is a risk for more serious problems for decades to come. So someone who has these symptoms really deserves a look at this genetic screening.”
Battling disbelief
Getting back to the paradox, many users don’t believe marijuana can trigger serious vomiting and nausea because of its reputation for doing the opposite.
“Folks that have this are just uniquely resistant to the concept that cannabis is actually the problem and not the solution,” Dr. Russo said.
“It’s kind of counterintuitive because people think: ‘Oh, cannabis helps with nausea,’ so they use more of it,” said Dr. Vinocur, who is also a spokesperson for the American College of Emergency Physicians and runs a medical cannabis practice.
Most kinds of marijuana act in this way – doing opposite things at different doses. Once a certain threshold is passed, people with cannabinoid hyperemesis syndrome are “just uniquely susceptible and really can’t tolerate any significant amount of THC,” Dr. Russo said, referring to tetrahydrocannabinol, the substance that gets marijuana users high.
Once diagnosed, quitting is the most effective strategy. But it can be tough to persuade someone to stop using marijuana.
“You do have to try and convince them ... to try abstinence and to watch and see what happens,” Dr. Vinocur said.
People should “realize the root cause of this is its cannabinoid ingestion, and the treatment is really best directed at absolute avoidance,” Dr. Johnson said.
Unfortunately, evidence also shows that once a person stops using marijuana and gets relief, going back to marijuana or other forms of cannabinoids can cause the syndrome to start all over again.
“We’ve had people that quit for a month, a year, 2 years and upon resumption, almost invariably, they’re back into bouts of the hyperemesis along with all the other [symptoms],” Dr. Russo said.
Marijuana and cannabinoids can cause digestive problems, Dr. Johnson said, which may cause more problems.
What recent research reveals
Cannabinoid hyperemesis syndrome is a relatively young disorder – first described in 2004 – and early reports and case studies are giving way now to studies looking into potential treatments.
So far, the strongest evidence suggests a role for an over-the-counter cream called capsaicin to help manage symptoms, but more studies are needed.
Similar to hot showers, this ingredient from chili peppers can warm the skin and trigger the temperature-sensitive skin sensors to lessen the symptoms, Dr. Johnson said.
An October 2021 study in Spain looked at 54 ED visits among 29 people with cannabinoid hyperemesis syndrome. For the 75% treated with capsaicin, vomiting stopped after an average of 18 minutes.
Lead author Guillermo Burillo-Putze, MD, PhD, said he is most surprised by the growing number of new cases of the disorder.
“This should be of concern given the increase in cannabis use due to its legalization and permissiveness,” said Dr. Burillo-Putze, an emergency doctor at Hospital Universitario de Canarias, Santa Cruz de Tenerife, Spain.
Cannabinoid hyperemesis syndrome appears not to discriminate across racial and ethic groups. Although most studies to date include White participants, a July 2021 study of 29 people, 90% of whom were Black, found repeat visits to the ED were common.
The study found that 16 people returned 42 times to the ED and accounted for 10 hospital admissions, for example.
Cannabis conspiracy theories
“Unfortunately, this condition has become the subject of great speculation hinging on conspiracy theories as its true cause,” Dr. Russo noted in a September 2021 letter to the editor in the American Journal of Emergency Medicine.
Some “myth busting” is in order, he said.
For example, cannabinoid hyperemesis syndrome does not happen because of exposure to products from a tree called neem or from pesticides applied to marijuana plants during cultivation, Dr. Russo said. It can also occur with high-dose synthetic cannabinoids.
The state of recreational and medical marijuana
Recreational marijuana is legal in 18 states, Washington, D.C., and Guam as of January 2022, according to a report in U.S. News. More states permit medical marijuana use – 37 in total, plus Washington, D.C., according to Britannica ProCon.
One of the states where only medicinal use is legal is Maryland, which is where Dr. Vinocur practices.
“We are seeing increasing numbers of cases” of cannabinoid hyperemesis syndrome, she said.
In addition to chronic use or higher doses, it’s likely that the higher potency levels of THC in the legal marijuana industry trigger the syndrome in some people as well.
Linda estimates she ended up in emergency rooms at least a half-dozen times in the last 5 years. In April 2021, she had a “pretty serious event.” She blames it on traveling a lot for work, not eating right, and not getting enough sleep. She broke her 2-year abstinence with alcohol.
“I basically didn’t listen to my body and paid a pretty significant price for it,” she said.
Linda did not stop altogether but said she “drastically changed the types and form of the cannabis I was using.”
“I can tell you on the record that I would be a hundred percent dead without this plant,” she said.
“The prospect of living without it was more detrimental to me than all of those things I just described to you, because addiction runs in my family and I had opiate problems myself that I overcame with cannabis.”
A version of this article first appeared on Medscape.com.
Children and COVID: Weekly cases down by more than half
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.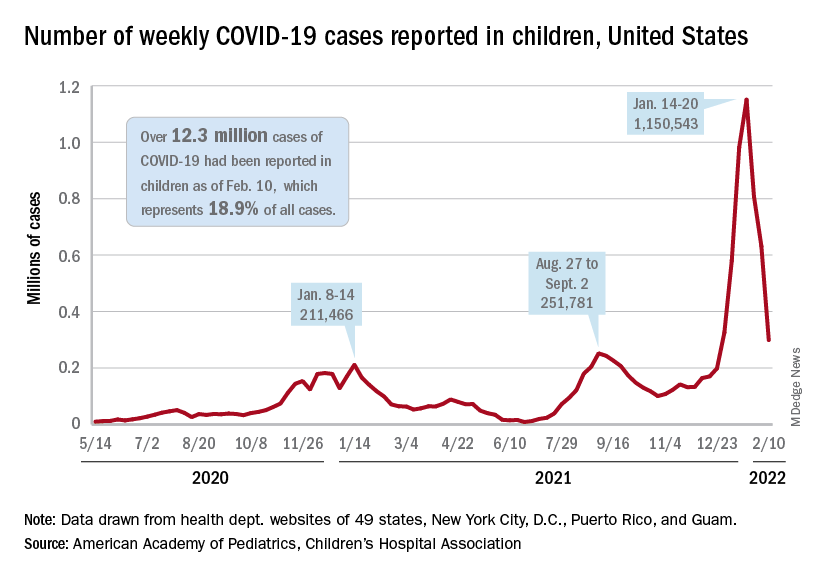
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
Long COVID symptoms linked to effects on vagus nerve
Several long COVID symptoms could be linked to the effects of the coronavirus on a vital central nerve, according to new research being released in the spring.
The vagus nerve, which runs from the brain into the body, connects to the heart, lungs, intestines, and several muscles involved with swallowing. It plays a role in several body functions that control heart rate, speech, the gag reflex, sweating, and digestion.
Those with long COVID and vagus nerve problems could face long-term issues with their voice, a hard time swallowing, dizziness, a high heart rate, low blood pressure, and diarrhea, the study authors found.
Their findings will be presented at the 2022 European Congress of Clinical Microbiology and Infectious Diseases in late April.
“Most long COVID subjects with vagus nerve dysfunction symptoms had a range of significant, clinically relevant, structural and/or functional alterations in their vagus nerve, including nerve thickening, trouble swallowing, and symptoms of impaired breathing,” the study authors wrote. “Our findings so far thus point at vagus nerve dysfunction as a central pathophysiological feature of long COVID.”
Researchers from the University Hospital Germans Trias i Pujol in Barcelona performed a study to look at vagus nerve functioning in long COVID patients. Among 348 patients, about 66% had at least one symptom that suggested vagus nerve dysfunction. The researchers did a broad evaluation with imaging and functional tests for 22 patients in the university’s Long COVID Clinic from March to June 2021.
Of the 22 patients, 20 were women, and the median age was 44. The most frequent symptoms related to vagus nerve dysfunction were diarrhea (73%), high heart rates (59%), dizziness (45%), swallowing problems (45%), voice problems (45%), and low blood pressure (14%).
Almost all (19 of 22 patients) had three or more symptoms related to vagus nerve dysfunction. The average length of symptoms was 14 months.
Of 22 patients, 6 had a change in the vagus nerve in the neck, which the researchers observed by ultrasound. They had a thickening of the vagus nerve and increased “echogenicity,” which suggests inflammation.
What’s more, 10 of 22 patients had flattened “diaphragmatic curves” during a thoracic ultrasound, which means the diaphragm doesn’t move as well as it should during breathing, and abnormal breathing. In another assessment, 10 of 16 patients had lower maximum inspiration pressures, suggesting a weakness in breathing muscles.
Eating and digestion were also impaired in some patients, with 13 reporting trouble with swallowing. During a gastric and bowel function assessment, eight patients couldn’t move food from the esophagus to the stomach as well as they should, while nine patients had acid reflux. Three patients had a hiatal hernia, which happens when the upper part of the stomach bulges through the diaphragm into the chest cavity.
The voices of some patients changed as well. Eight patients had an abnormal voice handicap index 30 test, which is a standard way to measure voice function. Among those, seven patients had dysphonia, or persistent voice problems.
The study is ongoing, and the research team is continuing to recruit patients to study the links between long COVID and the vagus nerve. The full paper isn’t yet available, and the research hasn’t yet been peer reviewed.
“The study appears to add to a growing collection of data suggesting at least some of the symptoms of long COVID is mediated through a direct impact on the nervous system,” David Strain, MD, a clinical senior lecturer at the University of Exeter (England), told the Science Media Centre.
“Establishing vagal nerve damage is useful information, as there are recognized, albeit not perfect, treatments for other causes of vagal nerve dysfunction that may be extrapolated to be beneficial for people with this type of long COVID,” he said.
A version of this article first appeared on WebMD.com.
Several long COVID symptoms could be linked to the effects of the coronavirus on a vital central nerve, according to new research being released in the spring.
The vagus nerve, which runs from the brain into the body, connects to the heart, lungs, intestines, and several muscles involved with swallowing. It plays a role in several body functions that control heart rate, speech, the gag reflex, sweating, and digestion.
Those with long COVID and vagus nerve problems could face long-term issues with their voice, a hard time swallowing, dizziness, a high heart rate, low blood pressure, and diarrhea, the study authors found.
Their findings will be presented at the 2022 European Congress of Clinical Microbiology and Infectious Diseases in late April.
“Most long COVID subjects with vagus nerve dysfunction symptoms had a range of significant, clinically relevant, structural and/or functional alterations in their vagus nerve, including nerve thickening, trouble swallowing, and symptoms of impaired breathing,” the study authors wrote. “Our findings so far thus point at vagus nerve dysfunction as a central pathophysiological feature of long COVID.”
Researchers from the University Hospital Germans Trias i Pujol in Barcelona performed a study to look at vagus nerve functioning in long COVID patients. Among 348 patients, about 66% had at least one symptom that suggested vagus nerve dysfunction. The researchers did a broad evaluation with imaging and functional tests for 22 patients in the university’s Long COVID Clinic from March to June 2021.
Of the 22 patients, 20 were women, and the median age was 44. The most frequent symptoms related to vagus nerve dysfunction were diarrhea (73%), high heart rates (59%), dizziness (45%), swallowing problems (45%), voice problems (45%), and low blood pressure (14%).
Almost all (19 of 22 patients) had three or more symptoms related to vagus nerve dysfunction. The average length of symptoms was 14 months.
Of 22 patients, 6 had a change in the vagus nerve in the neck, which the researchers observed by ultrasound. They had a thickening of the vagus nerve and increased “echogenicity,” which suggests inflammation.
What’s more, 10 of 22 patients had flattened “diaphragmatic curves” during a thoracic ultrasound, which means the diaphragm doesn’t move as well as it should during breathing, and abnormal breathing. In another assessment, 10 of 16 patients had lower maximum inspiration pressures, suggesting a weakness in breathing muscles.
Eating and digestion were also impaired in some patients, with 13 reporting trouble with swallowing. During a gastric and bowel function assessment, eight patients couldn’t move food from the esophagus to the stomach as well as they should, while nine patients had acid reflux. Three patients had a hiatal hernia, which happens when the upper part of the stomach bulges through the diaphragm into the chest cavity.
The voices of some patients changed as well. Eight patients had an abnormal voice handicap index 30 test, which is a standard way to measure voice function. Among those, seven patients had dysphonia, or persistent voice problems.
The study is ongoing, and the research team is continuing to recruit patients to study the links between long COVID and the vagus nerve. The full paper isn’t yet available, and the research hasn’t yet been peer reviewed.
“The study appears to add to a growing collection of data suggesting at least some of the symptoms of long COVID is mediated through a direct impact on the nervous system,” David Strain, MD, a clinical senior lecturer at the University of Exeter (England), told the Science Media Centre.
“Establishing vagal nerve damage is useful information, as there are recognized, albeit not perfect, treatments for other causes of vagal nerve dysfunction that may be extrapolated to be beneficial for people with this type of long COVID,” he said.
A version of this article first appeared on WebMD.com.
Several long COVID symptoms could be linked to the effects of the coronavirus on a vital central nerve, according to new research being released in the spring.
The vagus nerve, which runs from the brain into the body, connects to the heart, lungs, intestines, and several muscles involved with swallowing. It plays a role in several body functions that control heart rate, speech, the gag reflex, sweating, and digestion.
Those with long COVID and vagus nerve problems could face long-term issues with their voice, a hard time swallowing, dizziness, a high heart rate, low blood pressure, and diarrhea, the study authors found.
Their findings will be presented at the 2022 European Congress of Clinical Microbiology and Infectious Diseases in late April.
“Most long COVID subjects with vagus nerve dysfunction symptoms had a range of significant, clinically relevant, structural and/or functional alterations in their vagus nerve, including nerve thickening, trouble swallowing, and symptoms of impaired breathing,” the study authors wrote. “Our findings so far thus point at vagus nerve dysfunction as a central pathophysiological feature of long COVID.”
Researchers from the University Hospital Germans Trias i Pujol in Barcelona performed a study to look at vagus nerve functioning in long COVID patients. Among 348 patients, about 66% had at least one symptom that suggested vagus nerve dysfunction. The researchers did a broad evaluation with imaging and functional tests for 22 patients in the university’s Long COVID Clinic from March to June 2021.
Of the 22 patients, 20 were women, and the median age was 44. The most frequent symptoms related to vagus nerve dysfunction were diarrhea (73%), high heart rates (59%), dizziness (45%), swallowing problems (45%), voice problems (45%), and low blood pressure (14%).
Almost all (19 of 22 patients) had three or more symptoms related to vagus nerve dysfunction. The average length of symptoms was 14 months.
Of 22 patients, 6 had a change in the vagus nerve in the neck, which the researchers observed by ultrasound. They had a thickening of the vagus nerve and increased “echogenicity,” which suggests inflammation.
What’s more, 10 of 22 patients had flattened “diaphragmatic curves” during a thoracic ultrasound, which means the diaphragm doesn’t move as well as it should during breathing, and abnormal breathing. In another assessment, 10 of 16 patients had lower maximum inspiration pressures, suggesting a weakness in breathing muscles.
Eating and digestion were also impaired in some patients, with 13 reporting trouble with swallowing. During a gastric and bowel function assessment, eight patients couldn’t move food from the esophagus to the stomach as well as they should, while nine patients had acid reflux. Three patients had a hiatal hernia, which happens when the upper part of the stomach bulges through the diaphragm into the chest cavity.
The voices of some patients changed as well. Eight patients had an abnormal voice handicap index 30 test, which is a standard way to measure voice function. Among those, seven patients had dysphonia, or persistent voice problems.
The study is ongoing, and the research team is continuing to recruit patients to study the links between long COVID and the vagus nerve. The full paper isn’t yet available, and the research hasn’t yet been peer reviewed.
“The study appears to add to a growing collection of data suggesting at least some of the symptoms of long COVID is mediated through a direct impact on the nervous system,” David Strain, MD, a clinical senior lecturer at the University of Exeter (England), told the Science Media Centre.
“Establishing vagal nerve damage is useful information, as there are recognized, albeit not perfect, treatments for other causes of vagal nerve dysfunction that may be extrapolated to be beneficial for people with this type of long COVID,” he said.
A version of this article first appeared on WebMD.com.
Tenecteplase for stroke linked to reduced ICH risk
preliminary results from a large, multicenter registry study suggest.
“In clinical practice where centers are using tenecteplase, we’re seeing that the rate of symptomatic hemorrhage after getting a thrombolytic is half that with tenecteplase than with alteplase,” said lead author Steven J. Warach, MD, PhD, professor of neurology at Dell Medical School, University of Texas, Austin.
“For clinicians who have switched or are considering switching to tenecteplase, I think these results are very reassuring,” he said at the International Stroke Conference, presented by the American Stroke Association, a division of the American Heart Association.
Tenecteplase is a relatively new agent that is approved by the U.S. Food and Drug Administration to treat myocardial infarction but not ischemic stroke, although clinicians sometimes use it off-label for this purpose. American Heart Association guidelines recommend tenecteplase might be reasonable to consider for ischemic stroke in select patients.
The current standard of care for stroke is alteplase, which has been approved for this indication since 1996.
Five randomized clinical trials comparing the two thrombolytics weren’t large enough to make definitive conclusions about differences, said Dr. Warach. “The event rate for serious bleeding into the brain was thankfully low in both groups.”
Results from a meta-analysis that combined data from those five trials were also not definitive. “Numerically, it looked like the rate was lower for tenecteplase, but the sample size was just too low to make any statistically confident statement.”
However, tenecteplase has practical advantages over alteplase. Tenecteplase is a single bolus injection lasting 5 seconds while alteplase is administered by injection followed by an hour-long infusion.
Given these potential advantages, some centers have changed their practice and started using the newer drug beginning in July 2018.
The current study used an ongoing large registry to compare rates of symptomatic intracranial hemorrhage in patients treated with either of these drugs. The registry includes data collected July 2018 to June 2021 from various hospitals and programs in New Zealand, Australia, and the U.S.
Symptomatic intracranial hemorrhage was defined as a severe bleed causing pressure on the brain, extensive swelling, and worsening by at least four points on the National Institutes of Health Stroke Scale (NIHSS).
Researchers abstracted data from the various registries. As not all centers record data in the same format, statisticians then “cleaned” or harmonized the data to make it more standardized, said Dr. Warach.
They controlled for factors known to put a patient at higher risk for symptomatic hemorrhage, including age, sex, baseline NIHSS, and time to treatment.
Dr. Warach noted that at baseline, the tenecteplase group had higher values on most of these factors “that would predict intracranial hemorrhage.”
In an earlier analysis of 7,891 patients, the tenecteplase group was older (73 vs. 70 years; P < .001), less likely to be female (44.1% vs. 48.7%; P = .001), and had higher NIHSS scores (9 vs. 7; P < .001).
Also, a greater percentage of those in the tenecteplase group underwent mechanical thrombectomy (36.7% vs. 18.0%; P < .001). Dr. Warach explained that some centers would opt for tenecteplase if they knew the patient was a candidate for thrombectomy “because that was where the data was clearly strong and positive.”
An updated analysis included 9,238 patients – 7,313 who received alteplase and 1,925 tenecteplase. In the updated unadjusted analysis, the symptomatic intracranial hemorrhage rate was 3.6% for alteplase and 1.8% for tenecteplase (odds ratio, 0.49; P < .001). The adjusted OR was 0.42 (P < .001.)
The difference was even greater in those who underwent thrombectomy. For patients undergoing this procedure after a thrombolytic, the symptomatic intracranial hemorrhage rate was 5.9% for alteplase and 2.4% for tenecteplase.
“That even in those higher-risk patients we’re seeing an even greater difference is promising,” said Dr. Warach.
He and his colleagues plan to assess other potential benefits of tenecteplase, for example, the time it takes for patients to recover, “once we have all the data standardized and cleaned.”
Results of three large phase 3 trials comparing the two thrombolytics are expected within the next year or two, said Dr. Warach.
Joseph Broderick, MD, professor and director of the UC Gardner Neuroscience Institute, director of the National Coordinating Center for NIH’s StrokeNet, and professor of medicine at the University of Cincinnati College of Medicine, Cincinnati, stressed that for both drugs, speed is of the utmost importance to protect the brain.
“No matter which of these drugs is going to be used, the key thing is that they have to be used as quickly as possible,” he said.
Also important is imaging the brain before administering either of these medications to ensure the issue is an ischemic stroke and not an intracerebral hemorrhage, said Dr. Broderick. “If you have a broken blood vessel, you want to seal the leak, not break up the clot and make the bleeding worse.”
Dr. Warach receives payment as chair of the safety committee of another Genentech study comparing tenecteplase versus placebo in patients with large vessel occlusion whose stroke began more than 4.5 hours before treatment.
A version of this article first appeared on Medscape.com.
preliminary results from a large, multicenter registry study suggest.
“In clinical practice where centers are using tenecteplase, we’re seeing that the rate of symptomatic hemorrhage after getting a thrombolytic is half that with tenecteplase than with alteplase,” said lead author Steven J. Warach, MD, PhD, professor of neurology at Dell Medical School, University of Texas, Austin.
“For clinicians who have switched or are considering switching to tenecteplase, I think these results are very reassuring,” he said at the International Stroke Conference, presented by the American Stroke Association, a division of the American Heart Association.
Tenecteplase is a relatively new agent that is approved by the U.S. Food and Drug Administration to treat myocardial infarction but not ischemic stroke, although clinicians sometimes use it off-label for this purpose. American Heart Association guidelines recommend tenecteplase might be reasonable to consider for ischemic stroke in select patients.
The current standard of care for stroke is alteplase, which has been approved for this indication since 1996.
Five randomized clinical trials comparing the two thrombolytics weren’t large enough to make definitive conclusions about differences, said Dr. Warach. “The event rate for serious bleeding into the brain was thankfully low in both groups.”
Results from a meta-analysis that combined data from those five trials were also not definitive. “Numerically, it looked like the rate was lower for tenecteplase, but the sample size was just too low to make any statistically confident statement.”
However, tenecteplase has practical advantages over alteplase. Tenecteplase is a single bolus injection lasting 5 seconds while alteplase is administered by injection followed by an hour-long infusion.
Given these potential advantages, some centers have changed their practice and started using the newer drug beginning in July 2018.
The current study used an ongoing large registry to compare rates of symptomatic intracranial hemorrhage in patients treated with either of these drugs. The registry includes data collected July 2018 to June 2021 from various hospitals and programs in New Zealand, Australia, and the U.S.
Symptomatic intracranial hemorrhage was defined as a severe bleed causing pressure on the brain, extensive swelling, and worsening by at least four points on the National Institutes of Health Stroke Scale (NIHSS).
Researchers abstracted data from the various registries. As not all centers record data in the same format, statisticians then “cleaned” or harmonized the data to make it more standardized, said Dr. Warach.
They controlled for factors known to put a patient at higher risk for symptomatic hemorrhage, including age, sex, baseline NIHSS, and time to treatment.
Dr. Warach noted that at baseline, the tenecteplase group had higher values on most of these factors “that would predict intracranial hemorrhage.”
In an earlier analysis of 7,891 patients, the tenecteplase group was older (73 vs. 70 years; P < .001), less likely to be female (44.1% vs. 48.7%; P = .001), and had higher NIHSS scores (9 vs. 7; P < .001).
Also, a greater percentage of those in the tenecteplase group underwent mechanical thrombectomy (36.7% vs. 18.0%; P < .001). Dr. Warach explained that some centers would opt for tenecteplase if they knew the patient was a candidate for thrombectomy “because that was where the data was clearly strong and positive.”
An updated analysis included 9,238 patients – 7,313 who received alteplase and 1,925 tenecteplase. In the updated unadjusted analysis, the symptomatic intracranial hemorrhage rate was 3.6% for alteplase and 1.8% for tenecteplase (odds ratio, 0.49; P < .001). The adjusted OR was 0.42 (P < .001.)
The difference was even greater in those who underwent thrombectomy. For patients undergoing this procedure after a thrombolytic, the symptomatic intracranial hemorrhage rate was 5.9% for alteplase and 2.4% for tenecteplase.
“That even in those higher-risk patients we’re seeing an even greater difference is promising,” said Dr. Warach.
He and his colleagues plan to assess other potential benefits of tenecteplase, for example, the time it takes for patients to recover, “once we have all the data standardized and cleaned.”
Results of three large phase 3 trials comparing the two thrombolytics are expected within the next year or two, said Dr. Warach.
Joseph Broderick, MD, professor and director of the UC Gardner Neuroscience Institute, director of the National Coordinating Center for NIH’s StrokeNet, and professor of medicine at the University of Cincinnati College of Medicine, Cincinnati, stressed that for both drugs, speed is of the utmost importance to protect the brain.
“No matter which of these drugs is going to be used, the key thing is that they have to be used as quickly as possible,” he said.
Also important is imaging the brain before administering either of these medications to ensure the issue is an ischemic stroke and not an intracerebral hemorrhage, said Dr. Broderick. “If you have a broken blood vessel, you want to seal the leak, not break up the clot and make the bleeding worse.”
Dr. Warach receives payment as chair of the safety committee of another Genentech study comparing tenecteplase versus placebo in patients with large vessel occlusion whose stroke began more than 4.5 hours before treatment.
A version of this article first appeared on Medscape.com.
preliminary results from a large, multicenter registry study suggest.
“In clinical practice where centers are using tenecteplase, we’re seeing that the rate of symptomatic hemorrhage after getting a thrombolytic is half that with tenecteplase than with alteplase,” said lead author Steven J. Warach, MD, PhD, professor of neurology at Dell Medical School, University of Texas, Austin.
“For clinicians who have switched or are considering switching to tenecteplase, I think these results are very reassuring,” he said at the International Stroke Conference, presented by the American Stroke Association, a division of the American Heart Association.
Tenecteplase is a relatively new agent that is approved by the U.S. Food and Drug Administration to treat myocardial infarction but not ischemic stroke, although clinicians sometimes use it off-label for this purpose. American Heart Association guidelines recommend tenecteplase might be reasonable to consider for ischemic stroke in select patients.
The current standard of care for stroke is alteplase, which has been approved for this indication since 1996.
Five randomized clinical trials comparing the two thrombolytics weren’t large enough to make definitive conclusions about differences, said Dr. Warach. “The event rate for serious bleeding into the brain was thankfully low in both groups.”
Results from a meta-analysis that combined data from those five trials were also not definitive. “Numerically, it looked like the rate was lower for tenecteplase, but the sample size was just too low to make any statistically confident statement.”
However, tenecteplase has practical advantages over alteplase. Tenecteplase is a single bolus injection lasting 5 seconds while alteplase is administered by injection followed by an hour-long infusion.
Given these potential advantages, some centers have changed their practice and started using the newer drug beginning in July 2018.
The current study used an ongoing large registry to compare rates of symptomatic intracranial hemorrhage in patients treated with either of these drugs. The registry includes data collected July 2018 to June 2021 from various hospitals and programs in New Zealand, Australia, and the U.S.
Symptomatic intracranial hemorrhage was defined as a severe bleed causing pressure on the brain, extensive swelling, and worsening by at least four points on the National Institutes of Health Stroke Scale (NIHSS).
Researchers abstracted data from the various registries. As not all centers record data in the same format, statisticians then “cleaned” or harmonized the data to make it more standardized, said Dr. Warach.
They controlled for factors known to put a patient at higher risk for symptomatic hemorrhage, including age, sex, baseline NIHSS, and time to treatment.
Dr. Warach noted that at baseline, the tenecteplase group had higher values on most of these factors “that would predict intracranial hemorrhage.”
In an earlier analysis of 7,891 patients, the tenecteplase group was older (73 vs. 70 years; P < .001), less likely to be female (44.1% vs. 48.7%; P = .001), and had higher NIHSS scores (9 vs. 7; P < .001).
Also, a greater percentage of those in the tenecteplase group underwent mechanical thrombectomy (36.7% vs. 18.0%; P < .001). Dr. Warach explained that some centers would opt for tenecteplase if they knew the patient was a candidate for thrombectomy “because that was where the data was clearly strong and positive.”
An updated analysis included 9,238 patients – 7,313 who received alteplase and 1,925 tenecteplase. In the updated unadjusted analysis, the symptomatic intracranial hemorrhage rate was 3.6% for alteplase and 1.8% for tenecteplase (odds ratio, 0.49; P < .001). The adjusted OR was 0.42 (P < .001.)
The difference was even greater in those who underwent thrombectomy. For patients undergoing this procedure after a thrombolytic, the symptomatic intracranial hemorrhage rate was 5.9% for alteplase and 2.4% for tenecteplase.
“That even in those higher-risk patients we’re seeing an even greater difference is promising,” said Dr. Warach.
He and his colleagues plan to assess other potential benefits of tenecteplase, for example, the time it takes for patients to recover, “once we have all the data standardized and cleaned.”
Results of three large phase 3 trials comparing the two thrombolytics are expected within the next year or two, said Dr. Warach.
Joseph Broderick, MD, professor and director of the UC Gardner Neuroscience Institute, director of the National Coordinating Center for NIH’s StrokeNet, and professor of medicine at the University of Cincinnati College of Medicine, Cincinnati, stressed that for both drugs, speed is of the utmost importance to protect the brain.
“No matter which of these drugs is going to be used, the key thing is that they have to be used as quickly as possible,” he said.
Also important is imaging the brain before administering either of these medications to ensure the issue is an ischemic stroke and not an intracerebral hemorrhage, said Dr. Broderick. “If you have a broken blood vessel, you want to seal the leak, not break up the clot and make the bleeding worse.”
Dr. Warach receives payment as chair of the safety committee of another Genentech study comparing tenecteplase versus placebo in patients with large vessel occlusion whose stroke began more than 4.5 hours before treatment.
A version of this article first appeared on Medscape.com.
FROM ISC 2022
‘Remarkable’ benefit with intra-arterial tPA after stroke thrombectomy: CHOICE
in a new study.
The phase 2b CHOICE study was presented at the International Stroke Conference by Ángel Chamorro, MD, University of Barcelona, who received a round of applause as the results were revealed.
The study was also published online in JAMA to coincide with the presentation at the ISC.
The main results showed a remarkable and significant 18.4% absolute increase in the number of patients achieving an excellent neurologic outcome, defined as modified Rankin Scale (mRS) score of 0-1, after treatment with intra-arterial alteplase immediately following thrombectomy. This was despite the fact that the study was stopped early because of difficulty obtaining placebo supplies during the pandemic, having only enrolled 121 of the planned 200 patients.
This benefit was achieved without any increase in intracranial hemorrhage, which Dr. Chamorro described as “reassuring.”
He explained that although mechanical thrombectomy gives a high rate of successful reperfusion, only about 27% of patients achieve complete freedom of disability (mRS 0-1) at 3 months. He suggested that this may be the result of impaired reperfusion of the microcirculation despite complete recanalization of the occluded vessel.
The researchers postulated that thrombi could persist within the microcirculation in patients with normal or nearly normal cerebral angiograms at the end of thrombectomy and that these smaller thrombi may be dissolved by a dose of intra-arterial thrombolysis.
‘Dramatic and exciting results’
The CHOICE study was greeted with enthusiasm from commentators at the ISC meeting, which was presented by the American Stroke Association, a division of the American Heart Association. Louise McCullough, MD, chair of the late-breaking science session at which the study was presented and ISC program chair, described the results as “very dramatic and very exciting.”
“The CHOICE trial is going to be a highlight of the meeting because it could change care now,” Dr. McCullough said. “By just giving a little adjunctive tPA after the main clot is out, everybody seems to benefit, and there was no increased risk in bleeding. I think that’s the one that people are going to take back to their practice. But it was a very small trial, so you have to be cautious.”
And Peter Panagos, MD, professor of emergency medicine and neurology at Washington University School of Medicine, St. Louis, said: “It’s great to see this study. The 18% treatment effect is very impressive.”
Dr. Panagos added: “This study addresses a well-described finding from many of the interventional trials, that despite excellent outcomes in recanalization, patients don’t do as well as predicted. The thought is that either re-stenosis or propagation of smaller clots downstream from the original clot in small-caliber vessels [is what] causes additional, unintended damage. The use of intra-arterial thrombolysis after recanalization may assist in dissolving those smaller, downstream clots and debris and improve outcomes.”
But he pointed out that enthusiasm over these results must be matched with some concerns, including the small study size and wide confidence intervals – so larger, randomized studies will be required to confirm and change current clinical practice.
An abbreviated phase 2b trial
The CHOICE trial was conducted in seven centers in Catalonia, Spain.
For the study, patients with large vessel occlusion acute ischemic stroke treated with thrombectomy within 24 hours after stroke onset and who had achieved successful reperfusion (an expanded TICI angiographic score of 2b50 to 3) were randomly assigned to receive intra-arterial alteplase (0.225 mg/kg; maximum dose, 22.5 mg) infused over 15 to 30 minutes or placebo.
Because of the lack of continued availability of placebo supplies, the study had to be stopped early after 121 patients were enrolled (65 alteplase; 56 placebo), and after a few dropouts who did not receive treatment, the analysis was performed on 61 patients who received alteplase and 52 given placebo.
Results showed that the proportion of patients with an mRS score of 0 or 1 at 90 days was 59% (36/61) with alteplase and 40.4% (21/52) with placebo (adjusted risk difference, 18.4%; 95% confidence interval, 0.3%-36.4%; P = .047).
The proportion of patients with symptomatic intracranial hemorrhage within 24 hours was 0% with alteplase and 3.8% with placebo (risk difference, −3.8%; 95% CI, −13.2% to 2.5%).
Mortality at 90 days was 8% with alteplase and 15% with placebo (risk difference, −7.2%; 95% CI, −19.2% to 4.8%).
The improved clinical outcomes in the alteplase group were seen despite only minor differences between the treatment groups in angiographic scores or in other surrogate imaging, Dr. Chamorro pointed out, suggesting that the improved functional outcome may be explained by an amelioration in the microcirculatory reperfusion.
He said the study also supported the safety of intra-arterial alteplase infusion for 15-30 minutes at the dose used. Of note, 60% of the study population had also received IV alteplase before thrombectomy.
In the JAMA study, the authors report that current guidelines recommend that all eligible patients receive intravenous alteplase before thrombectomy, and the results of this trial do not contradict this recommendation.
“The study results support the safety of adjunct intra-arterial alteplase in patients with successful reperfusion at the end of thrombectomy, including in patients treated previously with intravenous alteplase, although the findings on effectiveness should be interpreted as preliminary, requiring replication before any recommendations for practice change,” they concluded.
Dr. Chamorro said that his group was now planning a second larger trial, CHOICE-2.
In an accompanying editorial in JAMA, Pooja Khatri, MD, MSc, University of Cincinnati, said “the 18% treatment effect observed in this 113-patient trial is remarkable.”
However, she cautions that consideration of its clinical implications must be tempered because of the lack of precision of the effect estimate, given wide 95% confidence intervals, the small sample size, and the observation that trials with early termination are well known to overestimate treatment effect.
But she acknowledges that the results suggest “that additional reperfusion therapy may be warranted after relatively successful mechanical thrombectomy of large vessel occlusions, whether to treat the residual primary thrombus, more distal arterial occlusions, or perhaps even microthromboses.”
Dr. Khatri noted that this approach runs counter to the recent movement to consider bypass of intravenous alteplase altogether in thrombectomy-eligible patients and suggests that additional or perhaps more targeted thrombolysis will be the most beneficial approach.
Further studies testing current thrombolytic agents, novel clot-dissolving agents, and other adjunctive antithrombotic and anti-inflammatory agents are needed, she concluded.
A version of this article first appeared on Medscape.com.
in a new study.
The phase 2b CHOICE study was presented at the International Stroke Conference by Ángel Chamorro, MD, University of Barcelona, who received a round of applause as the results were revealed.
The study was also published online in JAMA to coincide with the presentation at the ISC.
The main results showed a remarkable and significant 18.4% absolute increase in the number of patients achieving an excellent neurologic outcome, defined as modified Rankin Scale (mRS) score of 0-1, after treatment with intra-arterial alteplase immediately following thrombectomy. This was despite the fact that the study was stopped early because of difficulty obtaining placebo supplies during the pandemic, having only enrolled 121 of the planned 200 patients.
This benefit was achieved without any increase in intracranial hemorrhage, which Dr. Chamorro described as “reassuring.”
He explained that although mechanical thrombectomy gives a high rate of successful reperfusion, only about 27% of patients achieve complete freedom of disability (mRS 0-1) at 3 months. He suggested that this may be the result of impaired reperfusion of the microcirculation despite complete recanalization of the occluded vessel.
The researchers postulated that thrombi could persist within the microcirculation in patients with normal or nearly normal cerebral angiograms at the end of thrombectomy and that these smaller thrombi may be dissolved by a dose of intra-arterial thrombolysis.
‘Dramatic and exciting results’
The CHOICE study was greeted with enthusiasm from commentators at the ISC meeting, which was presented by the American Stroke Association, a division of the American Heart Association. Louise McCullough, MD, chair of the late-breaking science session at which the study was presented and ISC program chair, described the results as “very dramatic and very exciting.”
“The CHOICE trial is going to be a highlight of the meeting because it could change care now,” Dr. McCullough said. “By just giving a little adjunctive tPA after the main clot is out, everybody seems to benefit, and there was no increased risk in bleeding. I think that’s the one that people are going to take back to their practice. But it was a very small trial, so you have to be cautious.”
And Peter Panagos, MD, professor of emergency medicine and neurology at Washington University School of Medicine, St. Louis, said: “It’s great to see this study. The 18% treatment effect is very impressive.”
Dr. Panagos added: “This study addresses a well-described finding from many of the interventional trials, that despite excellent outcomes in recanalization, patients don’t do as well as predicted. The thought is that either re-stenosis or propagation of smaller clots downstream from the original clot in small-caliber vessels [is what] causes additional, unintended damage. The use of intra-arterial thrombolysis after recanalization may assist in dissolving those smaller, downstream clots and debris and improve outcomes.”
But he pointed out that enthusiasm over these results must be matched with some concerns, including the small study size and wide confidence intervals – so larger, randomized studies will be required to confirm and change current clinical practice.
An abbreviated phase 2b trial
The CHOICE trial was conducted in seven centers in Catalonia, Spain.
For the study, patients with large vessel occlusion acute ischemic stroke treated with thrombectomy within 24 hours after stroke onset and who had achieved successful reperfusion (an expanded TICI angiographic score of 2b50 to 3) were randomly assigned to receive intra-arterial alteplase (0.225 mg/kg; maximum dose, 22.5 mg) infused over 15 to 30 minutes or placebo.
Because of the lack of continued availability of placebo supplies, the study had to be stopped early after 121 patients were enrolled (65 alteplase; 56 placebo), and after a few dropouts who did not receive treatment, the analysis was performed on 61 patients who received alteplase and 52 given placebo.
Results showed that the proportion of patients with an mRS score of 0 or 1 at 90 days was 59% (36/61) with alteplase and 40.4% (21/52) with placebo (adjusted risk difference, 18.4%; 95% confidence interval, 0.3%-36.4%; P = .047).
The proportion of patients with symptomatic intracranial hemorrhage within 24 hours was 0% with alteplase and 3.8% with placebo (risk difference, −3.8%; 95% CI, −13.2% to 2.5%).
Mortality at 90 days was 8% with alteplase and 15% with placebo (risk difference, −7.2%; 95% CI, −19.2% to 4.8%).
The improved clinical outcomes in the alteplase group were seen despite only minor differences between the treatment groups in angiographic scores or in other surrogate imaging, Dr. Chamorro pointed out, suggesting that the improved functional outcome may be explained by an amelioration in the microcirculatory reperfusion.
He said the study also supported the safety of intra-arterial alteplase infusion for 15-30 minutes at the dose used. Of note, 60% of the study population had also received IV alteplase before thrombectomy.
In the JAMA study, the authors report that current guidelines recommend that all eligible patients receive intravenous alteplase before thrombectomy, and the results of this trial do not contradict this recommendation.
“The study results support the safety of adjunct intra-arterial alteplase in patients with successful reperfusion at the end of thrombectomy, including in patients treated previously with intravenous alteplase, although the findings on effectiveness should be interpreted as preliminary, requiring replication before any recommendations for practice change,” they concluded.
Dr. Chamorro said that his group was now planning a second larger trial, CHOICE-2.
In an accompanying editorial in JAMA, Pooja Khatri, MD, MSc, University of Cincinnati, said “the 18% treatment effect observed in this 113-patient trial is remarkable.”
However, she cautions that consideration of its clinical implications must be tempered because of the lack of precision of the effect estimate, given wide 95% confidence intervals, the small sample size, and the observation that trials with early termination are well known to overestimate treatment effect.
But she acknowledges that the results suggest “that additional reperfusion therapy may be warranted after relatively successful mechanical thrombectomy of large vessel occlusions, whether to treat the residual primary thrombus, more distal arterial occlusions, or perhaps even microthromboses.”
Dr. Khatri noted that this approach runs counter to the recent movement to consider bypass of intravenous alteplase altogether in thrombectomy-eligible patients and suggests that additional or perhaps more targeted thrombolysis will be the most beneficial approach.
Further studies testing current thrombolytic agents, novel clot-dissolving agents, and other adjunctive antithrombotic and anti-inflammatory agents are needed, she concluded.
A version of this article first appeared on Medscape.com.
in a new study.
The phase 2b CHOICE study was presented at the International Stroke Conference by Ángel Chamorro, MD, University of Barcelona, who received a round of applause as the results were revealed.
The study was also published online in JAMA to coincide with the presentation at the ISC.
The main results showed a remarkable and significant 18.4% absolute increase in the number of patients achieving an excellent neurologic outcome, defined as modified Rankin Scale (mRS) score of 0-1, after treatment with intra-arterial alteplase immediately following thrombectomy. This was despite the fact that the study was stopped early because of difficulty obtaining placebo supplies during the pandemic, having only enrolled 121 of the planned 200 patients.
This benefit was achieved without any increase in intracranial hemorrhage, which Dr. Chamorro described as “reassuring.”
He explained that although mechanical thrombectomy gives a high rate of successful reperfusion, only about 27% of patients achieve complete freedom of disability (mRS 0-1) at 3 months. He suggested that this may be the result of impaired reperfusion of the microcirculation despite complete recanalization of the occluded vessel.
The researchers postulated that thrombi could persist within the microcirculation in patients with normal or nearly normal cerebral angiograms at the end of thrombectomy and that these smaller thrombi may be dissolved by a dose of intra-arterial thrombolysis.
‘Dramatic and exciting results’
The CHOICE study was greeted with enthusiasm from commentators at the ISC meeting, which was presented by the American Stroke Association, a division of the American Heart Association. Louise McCullough, MD, chair of the late-breaking science session at which the study was presented and ISC program chair, described the results as “very dramatic and very exciting.”
“The CHOICE trial is going to be a highlight of the meeting because it could change care now,” Dr. McCullough said. “By just giving a little adjunctive tPA after the main clot is out, everybody seems to benefit, and there was no increased risk in bleeding. I think that’s the one that people are going to take back to their practice. But it was a very small trial, so you have to be cautious.”
And Peter Panagos, MD, professor of emergency medicine and neurology at Washington University School of Medicine, St. Louis, said: “It’s great to see this study. The 18% treatment effect is very impressive.”
Dr. Panagos added: “This study addresses a well-described finding from many of the interventional trials, that despite excellent outcomes in recanalization, patients don’t do as well as predicted. The thought is that either re-stenosis or propagation of smaller clots downstream from the original clot in small-caliber vessels [is what] causes additional, unintended damage. The use of intra-arterial thrombolysis after recanalization may assist in dissolving those smaller, downstream clots and debris and improve outcomes.”
But he pointed out that enthusiasm over these results must be matched with some concerns, including the small study size and wide confidence intervals – so larger, randomized studies will be required to confirm and change current clinical practice.
An abbreviated phase 2b trial
The CHOICE trial was conducted in seven centers in Catalonia, Spain.
For the study, patients with large vessel occlusion acute ischemic stroke treated with thrombectomy within 24 hours after stroke onset and who had achieved successful reperfusion (an expanded TICI angiographic score of 2b50 to 3) were randomly assigned to receive intra-arterial alteplase (0.225 mg/kg; maximum dose, 22.5 mg) infused over 15 to 30 minutes or placebo.
Because of the lack of continued availability of placebo supplies, the study had to be stopped early after 121 patients were enrolled (65 alteplase; 56 placebo), and after a few dropouts who did not receive treatment, the analysis was performed on 61 patients who received alteplase and 52 given placebo.
Results showed that the proportion of patients with an mRS score of 0 or 1 at 90 days was 59% (36/61) with alteplase and 40.4% (21/52) with placebo (adjusted risk difference, 18.4%; 95% confidence interval, 0.3%-36.4%; P = .047).
The proportion of patients with symptomatic intracranial hemorrhage within 24 hours was 0% with alteplase and 3.8% with placebo (risk difference, −3.8%; 95% CI, −13.2% to 2.5%).
Mortality at 90 days was 8% with alteplase and 15% with placebo (risk difference, −7.2%; 95% CI, −19.2% to 4.8%).
The improved clinical outcomes in the alteplase group were seen despite only minor differences between the treatment groups in angiographic scores or in other surrogate imaging, Dr. Chamorro pointed out, suggesting that the improved functional outcome may be explained by an amelioration in the microcirculatory reperfusion.
He said the study also supported the safety of intra-arterial alteplase infusion for 15-30 minutes at the dose used. Of note, 60% of the study population had also received IV alteplase before thrombectomy.
In the JAMA study, the authors report that current guidelines recommend that all eligible patients receive intravenous alteplase before thrombectomy, and the results of this trial do not contradict this recommendation.
“The study results support the safety of adjunct intra-arterial alteplase in patients with successful reperfusion at the end of thrombectomy, including in patients treated previously with intravenous alteplase, although the findings on effectiveness should be interpreted as preliminary, requiring replication before any recommendations for practice change,” they concluded.
Dr. Chamorro said that his group was now planning a second larger trial, CHOICE-2.
In an accompanying editorial in JAMA, Pooja Khatri, MD, MSc, University of Cincinnati, said “the 18% treatment effect observed in this 113-patient trial is remarkable.”
However, she cautions that consideration of its clinical implications must be tempered because of the lack of precision of the effect estimate, given wide 95% confidence intervals, the small sample size, and the observation that trials with early termination are well known to overestimate treatment effect.
But she acknowledges that the results suggest “that additional reperfusion therapy may be warranted after relatively successful mechanical thrombectomy of large vessel occlusions, whether to treat the residual primary thrombus, more distal arterial occlusions, or perhaps even microthromboses.”
Dr. Khatri noted that this approach runs counter to the recent movement to consider bypass of intravenous alteplase altogether in thrombectomy-eligible patients and suggests that additional or perhaps more targeted thrombolysis will be the most beneficial approach.
Further studies testing current thrombolytic agents, novel clot-dissolving agents, and other adjunctive antithrombotic and anti-inflammatory agents are needed, she concluded.
A version of this article first appeared on Medscape.com.
FROM ISC 2022