User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Belimumab for pregnant women with lupus: B-cell concerns remain
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
FROM ANNALS OF THE RHEUMATIC DISEASES
AAD unveils updated guidelines for topical AD treatment in adults
, and topical phosphodiesterase-4 (PDE-4) and Janus kinase (JAK) inhibitors. The guidelines also conditionally recommend the use of bathing and wet wrap therapy but recommend against the use of topical antimicrobials, antiseptics, and antihistamines.
The development updates the AAD’s 2014 recommendations for managing AD with topical therapies, published almost 9 years ago. “At that time, the only U.S. FDA–approved systemic medication for atopic dermatitis was prednisone – universally felt amongst dermatologists to be the least appropriate systemic medication for this condition, at least chronically,” Robert Sidbury, MD, MPH, who cochaired a 14-member multidisciplinary work group that assembled the updated guidelines, told this news organization in an interview.
“Since 2017, there have been two different biologic medications approved for moderate to severe AD (dupilumab and tralokinumab) with certainly a third or more right around the corner. There have been two new oral agents approved for moderate to severe AD – upadacitinib and abrocitinib – with others on the way,” he noted. While these are not topical therapies, the purview of the newly released guidelines, he said, “there have also been new topical medications approved since that time (crisaborole and ruxolitinib). It was high time for an update.”
For the new guidelines, which were published online in the Journal of the American Academy of Dermatology, Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital, guidelines cochair Dawn M. R. Davis, MD, a dermatologist at Mayo Clinic, Rochester, Minn., and colleagues conducted a systematic review of evidence regarding the use of nonprescription topical agents such as moisturizers, bathing practices, and wet wraps, as well as topical pharmacologic modalities such as corticosteroids, calcineurin inhibitors, JAK inhibitors, PDE-4 inhibitors, antimicrobials, and antihistamines.
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
12 recommendations
Of the 12 recommendations made for adults with AD, the work group ranked 7 as “strong” based on the evidence reviewed, and the rest as “conditional.” The “strong” recommendations include the use of moisturizers; the use of tacrolimus 0.03% or 0.1%; the use of pimecrolimus 1% cream for mild to moderate AD; use of topical steroids; intermittent use of medium-potency topical corticosteroids as maintenance therapy to reduce flares and relapse; the use of the topical PDE-4 inhibitor crisaborole, and the use of the topical JAK inhibitor ruxolitinib.
Regarding ruxolitinib cream 1.5%, the work group advised that the treatment area “should not exceed 20% body surface area, and a maximum of 60 grams should be applied per week; these stipulations are aimed at reducing systemic absorption, as black box warnings include serious infections, mortality, malignancies (for example, lymphoma), major adverse cardiovascular events, and thrombosis.”
Conditional recommendations in the guidelines include those for bathing for treatment and maintenance and the use of wet dressings, and those against the use of topical antimicrobials, topical antihistamines, and topical antiseptics.
According to Dr. Sidbury, the topic of bathing generated robust discussion among the work group members. “Though [each group member] has strong opinions and individual practice styles, they were also able to recognize that the evidence is all that matters in a project like this, which led to a ‘conditional’ recommendation regarding bathing frequency backed by ‘low’ evidence,” he said. “While this may seem like ‘guidance’ that doesn’t ‘guide,’ I would argue it informs the guideline consumer exactly where we are in terms of this question and allows them to use their best judgment and experience as their true north here.”
In the realm of topical steroids, Dr. Sidbury said that topical steroid addiction (TSA) and topical steroid withdrawal (TSW) have been a “controversial but persistent concern” from some patients and providers. “Two systematic reviews of this topic were mentioned, and it was made clear that the evidence base [for the concepts] is weak,” he said. “With that important caveat ,the guideline committee delineated both a definition of TSW/TSA and potential risk factors.”
Dr. Sidbury marveled at the potential impact of newer medicines such as crisaborole and ruxolitinib on younger AD patients as well. Crisaborole is now Food and Drug Administration approved down to 3 months of age for mild to moderate AD. “This is extraordinary and expands treatment options for all providers at an age when parents and providers are most conservative in their practice,” he said. “Ruxolitinib, also nonsteroidal, is FDA approved for mild to moderate AD down to 12 years of age. Having spent a good percentage of my practice years either being able to offer only topical steroids, or later topical steroids and topical calcineurin inhibitors like tacrolimus or pimecrolimus, having additional options is wonderful.”
In the guidelines, the work group noted that “significant gaps remain” in current understanding of various topical AD therapies. “Studies are needed which examine quality of life and other patient-important outcomes, changes to the cutaneous microbiome, as well as long term follow-up, and use in special and diverse populations (e.g., pregnancy, lactation, immunosuppression, multiple comorbidities, skin of color, pediatric),” they wrote. “Furthermore, increased use of new systemic AD treatment options (dupilumab, tralokinumab, abrocitinib, upadacitinib) in patients with moderate to severe disease may result in a selection bias toward milder disease in current and future AD topical therapy studies.”
Use of topical therapies to manage AD in pediatric patients will be covered in a forthcoming AAD guideline. The first updated AD guideline, on comorbidities associated with AD in adults, was released in January 2022.
Dr. Sidbury reported that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, an investigator for Brickell Biotech and Galderma USA, and a consultant for Galderma Global and Microes. Other work group members reported having financial disclosures with many pharmaceutical companies.
, and topical phosphodiesterase-4 (PDE-4) and Janus kinase (JAK) inhibitors. The guidelines also conditionally recommend the use of bathing and wet wrap therapy but recommend against the use of topical antimicrobials, antiseptics, and antihistamines.
The development updates the AAD’s 2014 recommendations for managing AD with topical therapies, published almost 9 years ago. “At that time, the only U.S. FDA–approved systemic medication for atopic dermatitis was prednisone – universally felt amongst dermatologists to be the least appropriate systemic medication for this condition, at least chronically,” Robert Sidbury, MD, MPH, who cochaired a 14-member multidisciplinary work group that assembled the updated guidelines, told this news organization in an interview.
“Since 2017, there have been two different biologic medications approved for moderate to severe AD (dupilumab and tralokinumab) with certainly a third or more right around the corner. There have been two new oral agents approved for moderate to severe AD – upadacitinib and abrocitinib – with others on the way,” he noted. While these are not topical therapies, the purview of the newly released guidelines, he said, “there have also been new topical medications approved since that time (crisaborole and ruxolitinib). It was high time for an update.”
For the new guidelines, which were published online in the Journal of the American Academy of Dermatology, Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital, guidelines cochair Dawn M. R. Davis, MD, a dermatologist at Mayo Clinic, Rochester, Minn., and colleagues conducted a systematic review of evidence regarding the use of nonprescription topical agents such as moisturizers, bathing practices, and wet wraps, as well as topical pharmacologic modalities such as corticosteroids, calcineurin inhibitors, JAK inhibitors, PDE-4 inhibitors, antimicrobials, and antihistamines.
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
12 recommendations
Of the 12 recommendations made for adults with AD, the work group ranked 7 as “strong” based on the evidence reviewed, and the rest as “conditional.” The “strong” recommendations include the use of moisturizers; the use of tacrolimus 0.03% or 0.1%; the use of pimecrolimus 1% cream for mild to moderate AD; use of topical steroids; intermittent use of medium-potency topical corticosteroids as maintenance therapy to reduce flares and relapse; the use of the topical PDE-4 inhibitor crisaborole, and the use of the topical JAK inhibitor ruxolitinib.
Regarding ruxolitinib cream 1.5%, the work group advised that the treatment area “should not exceed 20% body surface area, and a maximum of 60 grams should be applied per week; these stipulations are aimed at reducing systemic absorption, as black box warnings include serious infections, mortality, malignancies (for example, lymphoma), major adverse cardiovascular events, and thrombosis.”
Conditional recommendations in the guidelines include those for bathing for treatment and maintenance and the use of wet dressings, and those against the use of topical antimicrobials, topical antihistamines, and topical antiseptics.
According to Dr. Sidbury, the topic of bathing generated robust discussion among the work group members. “Though [each group member] has strong opinions and individual practice styles, they were also able to recognize that the evidence is all that matters in a project like this, which led to a ‘conditional’ recommendation regarding bathing frequency backed by ‘low’ evidence,” he said. “While this may seem like ‘guidance’ that doesn’t ‘guide,’ I would argue it informs the guideline consumer exactly where we are in terms of this question and allows them to use their best judgment and experience as their true north here.”
In the realm of topical steroids, Dr. Sidbury said that topical steroid addiction (TSA) and topical steroid withdrawal (TSW) have been a “controversial but persistent concern” from some patients and providers. “Two systematic reviews of this topic were mentioned, and it was made clear that the evidence base [for the concepts] is weak,” he said. “With that important caveat ,the guideline committee delineated both a definition of TSW/TSA and potential risk factors.”
Dr. Sidbury marveled at the potential impact of newer medicines such as crisaborole and ruxolitinib on younger AD patients as well. Crisaborole is now Food and Drug Administration approved down to 3 months of age for mild to moderate AD. “This is extraordinary and expands treatment options for all providers at an age when parents and providers are most conservative in their practice,” he said. “Ruxolitinib, also nonsteroidal, is FDA approved for mild to moderate AD down to 12 years of age. Having spent a good percentage of my practice years either being able to offer only topical steroids, or later topical steroids and topical calcineurin inhibitors like tacrolimus or pimecrolimus, having additional options is wonderful.”
In the guidelines, the work group noted that “significant gaps remain” in current understanding of various topical AD therapies. “Studies are needed which examine quality of life and other patient-important outcomes, changes to the cutaneous microbiome, as well as long term follow-up, and use in special and diverse populations (e.g., pregnancy, lactation, immunosuppression, multiple comorbidities, skin of color, pediatric),” they wrote. “Furthermore, increased use of new systemic AD treatment options (dupilumab, tralokinumab, abrocitinib, upadacitinib) in patients with moderate to severe disease may result in a selection bias toward milder disease in current and future AD topical therapy studies.”
Use of topical therapies to manage AD in pediatric patients will be covered in a forthcoming AAD guideline. The first updated AD guideline, on comorbidities associated with AD in adults, was released in January 2022.
Dr. Sidbury reported that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, an investigator for Brickell Biotech and Galderma USA, and a consultant for Galderma Global and Microes. Other work group members reported having financial disclosures with many pharmaceutical companies.
, and topical phosphodiesterase-4 (PDE-4) and Janus kinase (JAK) inhibitors. The guidelines also conditionally recommend the use of bathing and wet wrap therapy but recommend against the use of topical antimicrobials, antiseptics, and antihistamines.
The development updates the AAD’s 2014 recommendations for managing AD with topical therapies, published almost 9 years ago. “At that time, the only U.S. FDA–approved systemic medication for atopic dermatitis was prednisone – universally felt amongst dermatologists to be the least appropriate systemic medication for this condition, at least chronically,” Robert Sidbury, MD, MPH, who cochaired a 14-member multidisciplinary work group that assembled the updated guidelines, told this news organization in an interview.
“Since 2017, there have been two different biologic medications approved for moderate to severe AD (dupilumab and tralokinumab) with certainly a third or more right around the corner. There have been two new oral agents approved for moderate to severe AD – upadacitinib and abrocitinib – with others on the way,” he noted. While these are not topical therapies, the purview of the newly released guidelines, he said, “there have also been new topical medications approved since that time (crisaborole and ruxolitinib). It was high time for an update.”
For the new guidelines, which were published online in the Journal of the American Academy of Dermatology, Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital, guidelines cochair Dawn M. R. Davis, MD, a dermatologist at Mayo Clinic, Rochester, Minn., and colleagues conducted a systematic review of evidence regarding the use of nonprescription topical agents such as moisturizers, bathing practices, and wet wraps, as well as topical pharmacologic modalities such as corticosteroids, calcineurin inhibitors, JAK inhibitors, PDE-4 inhibitors, antimicrobials, and antihistamines.
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
12 recommendations
Of the 12 recommendations made for adults with AD, the work group ranked 7 as “strong” based on the evidence reviewed, and the rest as “conditional.” The “strong” recommendations include the use of moisturizers; the use of tacrolimus 0.03% or 0.1%; the use of pimecrolimus 1% cream for mild to moderate AD; use of topical steroids; intermittent use of medium-potency topical corticosteroids as maintenance therapy to reduce flares and relapse; the use of the topical PDE-4 inhibitor crisaborole, and the use of the topical JAK inhibitor ruxolitinib.
Regarding ruxolitinib cream 1.5%, the work group advised that the treatment area “should not exceed 20% body surface area, and a maximum of 60 grams should be applied per week; these stipulations are aimed at reducing systemic absorption, as black box warnings include serious infections, mortality, malignancies (for example, lymphoma), major adverse cardiovascular events, and thrombosis.”
Conditional recommendations in the guidelines include those for bathing for treatment and maintenance and the use of wet dressings, and those against the use of topical antimicrobials, topical antihistamines, and topical antiseptics.
According to Dr. Sidbury, the topic of bathing generated robust discussion among the work group members. “Though [each group member] has strong opinions and individual practice styles, they were also able to recognize that the evidence is all that matters in a project like this, which led to a ‘conditional’ recommendation regarding bathing frequency backed by ‘low’ evidence,” he said. “While this may seem like ‘guidance’ that doesn’t ‘guide,’ I would argue it informs the guideline consumer exactly where we are in terms of this question and allows them to use their best judgment and experience as their true north here.”
In the realm of topical steroids, Dr. Sidbury said that topical steroid addiction (TSA) and topical steroid withdrawal (TSW) have been a “controversial but persistent concern” from some patients and providers. “Two systematic reviews of this topic were mentioned, and it was made clear that the evidence base [for the concepts] is weak,” he said. “With that important caveat ,the guideline committee delineated both a definition of TSW/TSA and potential risk factors.”
Dr. Sidbury marveled at the potential impact of newer medicines such as crisaborole and ruxolitinib on younger AD patients as well. Crisaborole is now Food and Drug Administration approved down to 3 months of age for mild to moderate AD. “This is extraordinary and expands treatment options for all providers at an age when parents and providers are most conservative in their practice,” he said. “Ruxolitinib, also nonsteroidal, is FDA approved for mild to moderate AD down to 12 years of age. Having spent a good percentage of my practice years either being able to offer only topical steroids, or later topical steroids and topical calcineurin inhibitors like tacrolimus or pimecrolimus, having additional options is wonderful.”
In the guidelines, the work group noted that “significant gaps remain” in current understanding of various topical AD therapies. “Studies are needed which examine quality of life and other patient-important outcomes, changes to the cutaneous microbiome, as well as long term follow-up, and use in special and diverse populations (e.g., pregnancy, lactation, immunosuppression, multiple comorbidities, skin of color, pediatric),” they wrote. “Furthermore, increased use of new systemic AD treatment options (dupilumab, tralokinumab, abrocitinib, upadacitinib) in patients with moderate to severe disease may result in a selection bias toward milder disease in current and future AD topical therapy studies.”
Use of topical therapies to manage AD in pediatric patients will be covered in a forthcoming AAD guideline. The first updated AD guideline, on comorbidities associated with AD in adults, was released in January 2022.
Dr. Sidbury reported that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, an investigator for Brickell Biotech and Galderma USA, and a consultant for Galderma Global and Microes. Other work group members reported having financial disclosures with many pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
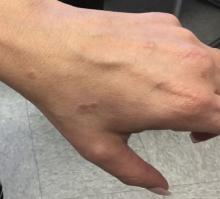
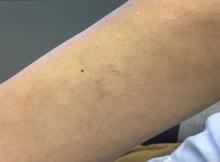
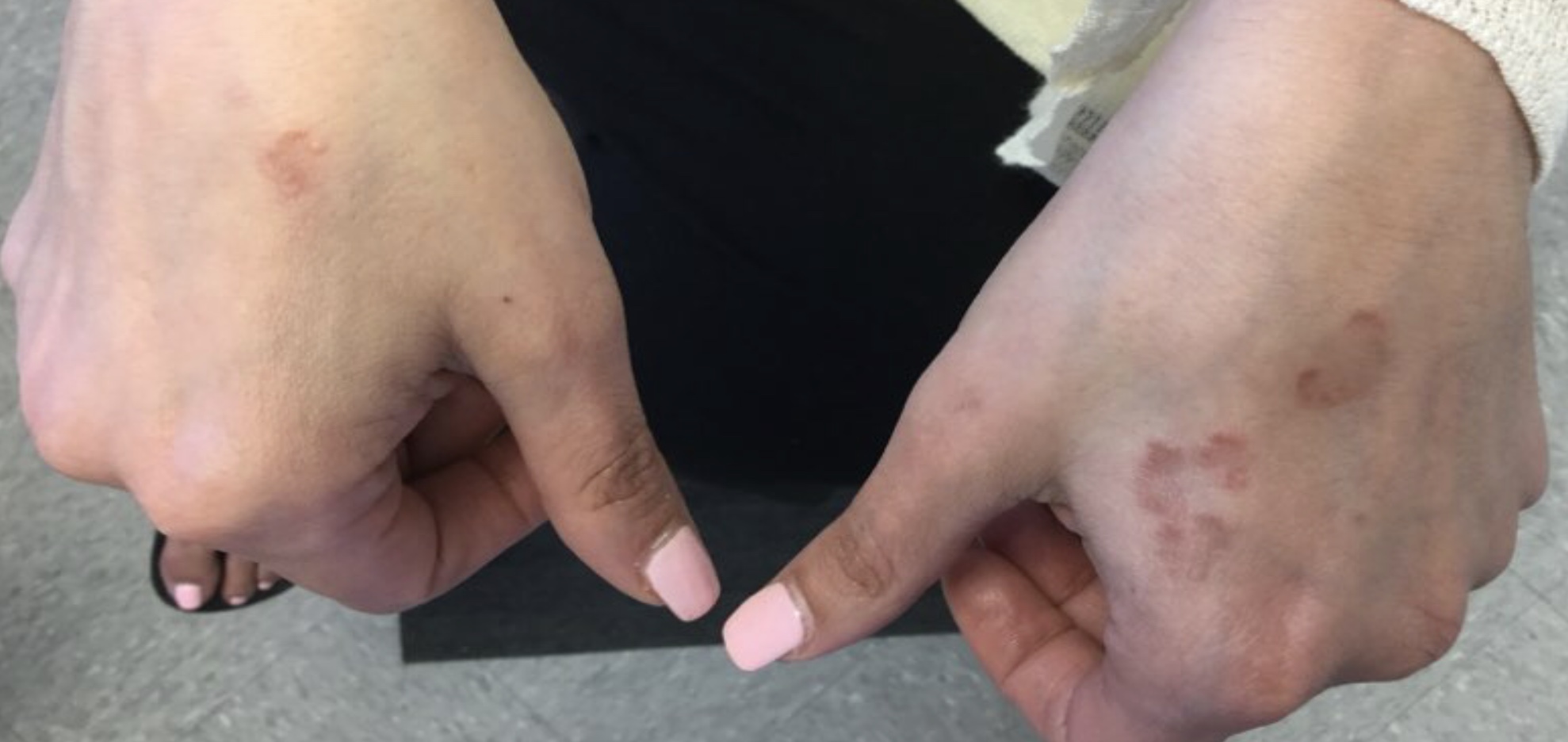
A 50-year-old woman with no significant history presented with erythematous, annular plaques, and papules on the dorsal hands and arms
. The prevalence and incidence is approximately 0.1%-0.4%. Although the condition is benign, it may be associated with more serious conditions such as HIV and malignancy. GA affects women more frequently than men but can affect any age group, although it most commonly presents in those ages 30 years and younger. While the exact etiology is unknown, GA has been most strongly associated with diabetes mellitus, hyperlipidemia, and autoimmune diseases.
The disease presents as localized, annular erythematous plaques and papules on the dorsal hands and feet in approximately 75% of cases. However, eruptions may appear on the trunk and extremities and can be categorized into patchy, generalized, interstitial, subcutaneous, or perforating subtypes. The lesions are often asymptomatic and typically not associated with any other symptoms.
The pathogenesis of GA is still under investigation, but recent studies suggest that a Th1-mediated dysregulation of the JAK-STAT pathway may contribute to the disease. Other hypotheses include a delayed hypersensitivity reaction or cell mediated immune response. The mechanism may be multifaceted, and epidemiologic research suggests a genetic predisposition in White individuals, but these findings may be associated with socioeconomic factors and disparities in health care.
GA presents on histology with palisading histiocytes surrounding focal collagen necrobiosis with mucin deposition. Tissue samples also display leukocytic infiltration of the dermis featuring multinucleated giant cells. There are defining features of the different subtypes, but focal collagen necrosis, the presence of histiocytes, and mucin deposition are consistent findings across all presentations.
GA lesions commonly regress on their own, but they tend to recur and can be functionally and visually unappealing to patients. The most common treatments for GA include topical corticosteroids, intralesional corticosteroid injections, and other anti-inflammatory drugs. These interventions can be administered in a variety of ways as the inflammation caused by GA exists on a spectrum, and less severe cases can be managed with topical or intralesional treatment. Systemic therapy may be necessary for severe and recalcitrant cases. Other interventions that have shown promise in smaller studies include phototherapy, hydroxychloroquine, and TNF-alpha inhibitors.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa Bay Regional Campus, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Joshi TP and Duvic M. Am J Clin Dermatol. 2022 Jan;23(1):37-50. doi: 10.1007/s40257-021-00636-1.
Muse M et al. Dermatol Online J. 2021 Apr 15;27(4):13030/qt0m50398n.
Schmieder SJ et al. Granuloma Annulare. NIH National Center for Biotechnology Information [Updated 2022 Nov 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. 7.
. The prevalence and incidence is approximately 0.1%-0.4%. Although the condition is benign, it may be associated with more serious conditions such as HIV and malignancy. GA affects women more frequently than men but can affect any age group, although it most commonly presents in those ages 30 years and younger. While the exact etiology is unknown, GA has been most strongly associated with diabetes mellitus, hyperlipidemia, and autoimmune diseases.
The disease presents as localized, annular erythematous plaques and papules on the dorsal hands and feet in approximately 75% of cases. However, eruptions may appear on the trunk and extremities and can be categorized into patchy, generalized, interstitial, subcutaneous, or perforating subtypes. The lesions are often asymptomatic and typically not associated with any other symptoms.
The pathogenesis of GA is still under investigation, but recent studies suggest that a Th1-mediated dysregulation of the JAK-STAT pathway may contribute to the disease. Other hypotheses include a delayed hypersensitivity reaction or cell mediated immune response. The mechanism may be multifaceted, and epidemiologic research suggests a genetic predisposition in White individuals, but these findings may be associated with socioeconomic factors and disparities in health care.
GA presents on histology with palisading histiocytes surrounding focal collagen necrobiosis with mucin deposition. Tissue samples also display leukocytic infiltration of the dermis featuring multinucleated giant cells. There are defining features of the different subtypes, but focal collagen necrosis, the presence of histiocytes, and mucin deposition are consistent findings across all presentations.
GA lesions commonly regress on their own, but they tend to recur and can be functionally and visually unappealing to patients. The most common treatments for GA include topical corticosteroids, intralesional corticosteroid injections, and other anti-inflammatory drugs. These interventions can be administered in a variety of ways as the inflammation caused by GA exists on a spectrum, and less severe cases can be managed with topical or intralesional treatment. Systemic therapy may be necessary for severe and recalcitrant cases. Other interventions that have shown promise in smaller studies include phototherapy, hydroxychloroquine, and TNF-alpha inhibitors.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa Bay Regional Campus, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Joshi TP and Duvic M. Am J Clin Dermatol. 2022 Jan;23(1):37-50. doi: 10.1007/s40257-021-00636-1.
Muse M et al. Dermatol Online J. 2021 Apr 15;27(4):13030/qt0m50398n.
Schmieder SJ et al. Granuloma Annulare. NIH National Center for Biotechnology Information [Updated 2022 Nov 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. 7.
. The prevalence and incidence is approximately 0.1%-0.4%. Although the condition is benign, it may be associated with more serious conditions such as HIV and malignancy. GA affects women more frequently than men but can affect any age group, although it most commonly presents in those ages 30 years and younger. While the exact etiology is unknown, GA has been most strongly associated with diabetes mellitus, hyperlipidemia, and autoimmune diseases.
The disease presents as localized, annular erythematous plaques and papules on the dorsal hands and feet in approximately 75% of cases. However, eruptions may appear on the trunk and extremities and can be categorized into patchy, generalized, interstitial, subcutaneous, or perforating subtypes. The lesions are often asymptomatic and typically not associated with any other symptoms.
The pathogenesis of GA is still under investigation, but recent studies suggest that a Th1-mediated dysregulation of the JAK-STAT pathway may contribute to the disease. Other hypotheses include a delayed hypersensitivity reaction or cell mediated immune response. The mechanism may be multifaceted, and epidemiologic research suggests a genetic predisposition in White individuals, but these findings may be associated with socioeconomic factors and disparities in health care.
GA presents on histology with palisading histiocytes surrounding focal collagen necrobiosis with mucin deposition. Tissue samples also display leukocytic infiltration of the dermis featuring multinucleated giant cells. There are defining features of the different subtypes, but focal collagen necrosis, the presence of histiocytes, and mucin deposition are consistent findings across all presentations.
GA lesions commonly regress on their own, but they tend to recur and can be functionally and visually unappealing to patients. The most common treatments for GA include topical corticosteroids, intralesional corticosteroid injections, and other anti-inflammatory drugs. These interventions can be administered in a variety of ways as the inflammation caused by GA exists on a spectrum, and less severe cases can be managed with topical or intralesional treatment. Systemic therapy may be necessary for severe and recalcitrant cases. Other interventions that have shown promise in smaller studies include phototherapy, hydroxychloroquine, and TNF-alpha inhibitors.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa Bay Regional Campus, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Joshi TP and Duvic M. Am J Clin Dermatol. 2022 Jan;23(1):37-50. doi: 10.1007/s40257-021-00636-1.
Muse M et al. Dermatol Online J. 2021 Apr 15;27(4):13030/qt0m50398n.
Schmieder SJ et al. Granuloma Annulare. NIH National Center for Biotechnology Information [Updated 2022 Nov 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. 7.
Regular vitamin D supplements may lower melanoma risk
. They also found a trend for benefit with occasional use.
The study, published in Melanoma Research, involved almost 500 individuals attending a dermatology clinic who reported on their use of vitamin D supplements.
Regular users had a significant 55% reduction in the odds of having a past or present melanoma diagnosis, while occasional use was associated with a nonsignificant 46% reduction. The reduction was similar for all skin cancer types.
However, senior author Ilkka T. Harvima, MD, PhD, department of dermatology, University of Eastern Finland and Kuopio (Finland) University Hospital, warned there are limitations to the study.
Despite adjustment for several possible confounding factors, “it is still possible that some other, yet unidentified or untested, factors can still confound the present result,” he said.
Consequently, “the causal link between vitamin D and melanoma cannot be confirmed by the present results,” Dr. Harvima said in a statement.
Even if the link were to be proven, “the question about the optimal dose of oral vitamin D in order to for it to have beneficial effects remains to be answered,” he said.
“Until we know more, national intake recommendations should be followed.”
The incidence of cutaneous malignant melanoma and other skin cancers has been increasing steadily in Western populations, particularly in immunosuppressed individuals, the authors pointed out, and they attributed the rise to an increased exposure to ultraviolet radiation.
While ultraviolet radiation exposure is a well-known risk factor, “the other side of the coin is that public sun protection campaigns have led to alerts that insufficient sun exposure is a significant public health problem, resulting in insufficient vitamin D status.”
For their study, the team reviewed the records of 498 patients aged 21-79 years at a dermatology outpatient clinic who were deemed by an experienced dermatologist to be at risk of any type of skin cancer.
Among these patients, 295 individuals had a history of past or present cutaneous malignancy, with 100 diagnosed with melanoma, 213 with basal cell carcinoma, and 41 with squamous cell carcinoma. A further 70 subjects had cancer elsewhere, including breast, prostate, kidney, bladder, intestine, and blood cancers.
A subgroup of 96 patients were immunocompromised and were considered separately.
The 402 remaining patients were categorized, based on their self-reported use of oral vitamin D preparations, as nonusers (n = 99), occasional users (n = 126), and regular users (n = 177).
Regular use of vitamin D was associated with being more educated (P = .032), less frequent outdoor working (P = .003), lower tobacco pack years (P = .001), and more frequent solarium exposure (P = .002).
There was no significant association between vitamin D use and photoaging, actinic keratoses, nevi, basal or squamous cell carcinoma, body mass index, or self-estimated lifetime exposure to sunlight or sunburns.
However, there were significant associations between regular use of vitamin D and a lower incidence of melanoma and other cancer types.
There were significantly fewer individuals in the regular vitamin D use group with a past or present history of melanoma when compared with the nonuse group, at 18.1% vs. 32.3% (P = .021), or any type of skin cancer, at 62.1% vs. 74.7% (P = .027).
Multivariate logistic regression analysis revealed that regular vitamin D use was significantly associated with a reduced melanoma risk, at an odds ratio vs. nonuse of 0.447 (P = .016).
Occasional use was associated with a reduced, albeit nonsignificant, risk, with an odds ratio versus nonuse of 0.540 (P = .08).
For any type of skin cancers, regular vitamin D use was associated with an odds ratio vs. nonuse of 0.478 (P = .032), while that for occasional vitamin D use was 0.543 (P = .061).
“Somewhat similar” results were obtained when the investigators looked at the subgroup of immunocompromised individuals, although they note that “the number of subjects was low.”
The study was supported by the Cancer Center of Eastern Finland of the University of Eastern Finland, the Finnish Cancer Research Foundation, and the VTR-funding of Kuopio University Hospital. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
. They also found a trend for benefit with occasional use.
The study, published in Melanoma Research, involved almost 500 individuals attending a dermatology clinic who reported on their use of vitamin D supplements.
Regular users had a significant 55% reduction in the odds of having a past or present melanoma diagnosis, while occasional use was associated with a nonsignificant 46% reduction. The reduction was similar for all skin cancer types.
However, senior author Ilkka T. Harvima, MD, PhD, department of dermatology, University of Eastern Finland and Kuopio (Finland) University Hospital, warned there are limitations to the study.
Despite adjustment for several possible confounding factors, “it is still possible that some other, yet unidentified or untested, factors can still confound the present result,” he said.
Consequently, “the causal link between vitamin D and melanoma cannot be confirmed by the present results,” Dr. Harvima said in a statement.
Even if the link were to be proven, “the question about the optimal dose of oral vitamin D in order to for it to have beneficial effects remains to be answered,” he said.
“Until we know more, national intake recommendations should be followed.”
The incidence of cutaneous malignant melanoma and other skin cancers has been increasing steadily in Western populations, particularly in immunosuppressed individuals, the authors pointed out, and they attributed the rise to an increased exposure to ultraviolet radiation.
While ultraviolet radiation exposure is a well-known risk factor, “the other side of the coin is that public sun protection campaigns have led to alerts that insufficient sun exposure is a significant public health problem, resulting in insufficient vitamin D status.”
For their study, the team reviewed the records of 498 patients aged 21-79 years at a dermatology outpatient clinic who were deemed by an experienced dermatologist to be at risk of any type of skin cancer.
Among these patients, 295 individuals had a history of past or present cutaneous malignancy, with 100 diagnosed with melanoma, 213 with basal cell carcinoma, and 41 with squamous cell carcinoma. A further 70 subjects had cancer elsewhere, including breast, prostate, kidney, bladder, intestine, and blood cancers.
A subgroup of 96 patients were immunocompromised and were considered separately.
The 402 remaining patients were categorized, based on their self-reported use of oral vitamin D preparations, as nonusers (n = 99), occasional users (n = 126), and regular users (n = 177).
Regular use of vitamin D was associated with being more educated (P = .032), less frequent outdoor working (P = .003), lower tobacco pack years (P = .001), and more frequent solarium exposure (P = .002).
There was no significant association between vitamin D use and photoaging, actinic keratoses, nevi, basal or squamous cell carcinoma, body mass index, or self-estimated lifetime exposure to sunlight or sunburns.
However, there were significant associations between regular use of vitamin D and a lower incidence of melanoma and other cancer types.
There were significantly fewer individuals in the regular vitamin D use group with a past or present history of melanoma when compared with the nonuse group, at 18.1% vs. 32.3% (P = .021), or any type of skin cancer, at 62.1% vs. 74.7% (P = .027).
Multivariate logistic regression analysis revealed that regular vitamin D use was significantly associated with a reduced melanoma risk, at an odds ratio vs. nonuse of 0.447 (P = .016).
Occasional use was associated with a reduced, albeit nonsignificant, risk, with an odds ratio versus nonuse of 0.540 (P = .08).
For any type of skin cancers, regular vitamin D use was associated with an odds ratio vs. nonuse of 0.478 (P = .032), while that for occasional vitamin D use was 0.543 (P = .061).
“Somewhat similar” results were obtained when the investigators looked at the subgroup of immunocompromised individuals, although they note that “the number of subjects was low.”
The study was supported by the Cancer Center of Eastern Finland of the University of Eastern Finland, the Finnish Cancer Research Foundation, and the VTR-funding of Kuopio University Hospital. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
. They also found a trend for benefit with occasional use.
The study, published in Melanoma Research, involved almost 500 individuals attending a dermatology clinic who reported on their use of vitamin D supplements.
Regular users had a significant 55% reduction in the odds of having a past or present melanoma diagnosis, while occasional use was associated with a nonsignificant 46% reduction. The reduction was similar for all skin cancer types.
However, senior author Ilkka T. Harvima, MD, PhD, department of dermatology, University of Eastern Finland and Kuopio (Finland) University Hospital, warned there are limitations to the study.
Despite adjustment for several possible confounding factors, “it is still possible that some other, yet unidentified or untested, factors can still confound the present result,” he said.
Consequently, “the causal link between vitamin D and melanoma cannot be confirmed by the present results,” Dr. Harvima said in a statement.
Even if the link were to be proven, “the question about the optimal dose of oral vitamin D in order to for it to have beneficial effects remains to be answered,” he said.
“Until we know more, national intake recommendations should be followed.”
The incidence of cutaneous malignant melanoma and other skin cancers has been increasing steadily in Western populations, particularly in immunosuppressed individuals, the authors pointed out, and they attributed the rise to an increased exposure to ultraviolet radiation.
While ultraviolet radiation exposure is a well-known risk factor, “the other side of the coin is that public sun protection campaigns have led to alerts that insufficient sun exposure is a significant public health problem, resulting in insufficient vitamin D status.”
For their study, the team reviewed the records of 498 patients aged 21-79 years at a dermatology outpatient clinic who were deemed by an experienced dermatologist to be at risk of any type of skin cancer.
Among these patients, 295 individuals had a history of past or present cutaneous malignancy, with 100 diagnosed with melanoma, 213 with basal cell carcinoma, and 41 with squamous cell carcinoma. A further 70 subjects had cancer elsewhere, including breast, prostate, kidney, bladder, intestine, and blood cancers.
A subgroup of 96 patients were immunocompromised and were considered separately.
The 402 remaining patients were categorized, based on their self-reported use of oral vitamin D preparations, as nonusers (n = 99), occasional users (n = 126), and regular users (n = 177).
Regular use of vitamin D was associated with being more educated (P = .032), less frequent outdoor working (P = .003), lower tobacco pack years (P = .001), and more frequent solarium exposure (P = .002).
There was no significant association between vitamin D use and photoaging, actinic keratoses, nevi, basal or squamous cell carcinoma, body mass index, or self-estimated lifetime exposure to sunlight or sunburns.
However, there were significant associations between regular use of vitamin D and a lower incidence of melanoma and other cancer types.
There were significantly fewer individuals in the regular vitamin D use group with a past or present history of melanoma when compared with the nonuse group, at 18.1% vs. 32.3% (P = .021), or any type of skin cancer, at 62.1% vs. 74.7% (P = .027).
Multivariate logistic regression analysis revealed that regular vitamin D use was significantly associated with a reduced melanoma risk, at an odds ratio vs. nonuse of 0.447 (P = .016).
Occasional use was associated with a reduced, albeit nonsignificant, risk, with an odds ratio versus nonuse of 0.540 (P = .08).
For any type of skin cancers, regular vitamin D use was associated with an odds ratio vs. nonuse of 0.478 (P = .032), while that for occasional vitamin D use was 0.543 (P = .061).
“Somewhat similar” results were obtained when the investigators looked at the subgroup of immunocompromised individuals, although they note that “the number of subjects was low.”
The study was supported by the Cancer Center of Eastern Finland of the University of Eastern Finland, the Finnish Cancer Research Foundation, and the VTR-funding of Kuopio University Hospital. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MELANOMA RESEARCH
Picosecond lasers for tattoo removal could benefit from enhancements, expert says
SAN DIEGO – When picosecond lasers hit the market about 10 years ago, they became a game-changer for tattoo removal, boasting the delivery of energy that is about threefold faster than with nanosecond lasers.
However, picosecond lasers are far from perfect even in the hands of the most experienced clinicians, according to Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford. “They have been very difficult to build from an engineering perspective,” he said at the annual Masters of Aesthetics Symposium. It took a long time for these lasers to come to the market, and they are still fairly expensive and require a lot of maintenance, he noted. In addition, “they are also not quite as ‘picosecond’ as they need to be. I think there is definitely room to improve, but this is the gold standard.”
Today, . Tattoo ink particles average about 0.1 mcm in size, and the thermal relaxation size works out to be less than 10 nanoseconds, with shorter pulses better at capturing the ink particles that are smaller than average.
Black is the most common tattoo color dermatologists treat. “For that, you can typically use a 1064, which has the highest absorption, but you can also use many of the other wavelengths,” he said. “Other colors are less common, followed by red, for which you would use a 532-nm wavelength.”
Dr. Ibrahimi underscored the importance of setting realistic expectations during consults with patients seeking options for tattoo removal. Even with picosecond laser technology, many treatments are typically required and “a good patient consultation is key to setting proper expectations,” he said. “If you promise someone results in 4 to 5 treatments like many of the device companies will say you can achieve, you’re going to have a large group of patients who are disappointed.”
The clinical endpoint to strive for during tattoo removal is whitening of the ink, which typically fades about 20 minutes after treatment. That whitening corresponds to cavitation, or the production of gas vacuoles in the cells that were holding the ink. This discovery led to a technique intended to enhance tattoo removal. In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and colleagues published results of a study that compared a single Q-switched laser treatment pass with four treatment passes separated by 20 minutes. After treating 18 tattoos in 12 adults, they found that the technique, known as the “R20” method, was more effective than a single-pass treatment (P < .01).
“Subsequent to this, there has been conflicting data on whether this is truly effective or not,” said Dr. Ibrahimi, who is also on the board of directors for the American Society for Dermatologic Surgery and the American Society for Laser Medicine and Surgery. “Most of us agree that one additional pass would be helpful, but when you’re doing this in the private practice setting, it’s often challenging because patients aren’t necessarily willing to pay more for more than just one pass for their tattoo removal.”
Another recent advance is use of a topical square silicone patch infused with perfluorodecalin (PFD) for use during tattoo removal, which has been shown to reduce epidermal whitening. The patch contains a fluorocarbon “that is very good at dissolving gas, and it is already widely used in medicine,” he said. When applied, “it almost instantaneously takes the whitening away; you don’t have to wait the 20 minutes to do your second pass.”
A different technology designed to speed up tattoo removal is the Resonic Rapid Acoustic Pulse device (marketed as Resonic, from Allergan Aesthetics), which is cleared by the FDA for use as an accessory to the 1064 nm Q-switched laser for black tattoo removal in patients with skin types I-III. “This uses acoustic pulses of sound waves; they’re rapid and powerful,” Dr. Ibrahimi said. “They can clear those cavitation bubbles much like the PFD patches do. It’s also thought that they further disperse the tattoo ink particles by supplementing the laser energy as well. It is also purported to alter the body’s healing response, or immune response, which is important in tattoo clearing.”
Dr. Ibrahimi disclosed that he is a member of the Advisory Board for Accure Acne, AbbVie (which owns Allergan), Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
SAN DIEGO – When picosecond lasers hit the market about 10 years ago, they became a game-changer for tattoo removal, boasting the delivery of energy that is about threefold faster than with nanosecond lasers.
However, picosecond lasers are far from perfect even in the hands of the most experienced clinicians, according to Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford. “They have been very difficult to build from an engineering perspective,” he said at the annual Masters of Aesthetics Symposium. It took a long time for these lasers to come to the market, and they are still fairly expensive and require a lot of maintenance, he noted. In addition, “they are also not quite as ‘picosecond’ as they need to be. I think there is definitely room to improve, but this is the gold standard.”
Today, . Tattoo ink particles average about 0.1 mcm in size, and the thermal relaxation size works out to be less than 10 nanoseconds, with shorter pulses better at capturing the ink particles that are smaller than average.
Black is the most common tattoo color dermatologists treat. “For that, you can typically use a 1064, which has the highest absorption, but you can also use many of the other wavelengths,” he said. “Other colors are less common, followed by red, for which you would use a 532-nm wavelength.”
Dr. Ibrahimi underscored the importance of setting realistic expectations during consults with patients seeking options for tattoo removal. Even with picosecond laser technology, many treatments are typically required and “a good patient consultation is key to setting proper expectations,” he said. “If you promise someone results in 4 to 5 treatments like many of the device companies will say you can achieve, you’re going to have a large group of patients who are disappointed.”
The clinical endpoint to strive for during tattoo removal is whitening of the ink, which typically fades about 20 minutes after treatment. That whitening corresponds to cavitation, or the production of gas vacuoles in the cells that were holding the ink. This discovery led to a technique intended to enhance tattoo removal. In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and colleagues published results of a study that compared a single Q-switched laser treatment pass with four treatment passes separated by 20 minutes. After treating 18 tattoos in 12 adults, they found that the technique, known as the “R20” method, was more effective than a single-pass treatment (P < .01).
“Subsequent to this, there has been conflicting data on whether this is truly effective or not,” said Dr. Ibrahimi, who is also on the board of directors for the American Society for Dermatologic Surgery and the American Society for Laser Medicine and Surgery. “Most of us agree that one additional pass would be helpful, but when you’re doing this in the private practice setting, it’s often challenging because patients aren’t necessarily willing to pay more for more than just one pass for their tattoo removal.”
Another recent advance is use of a topical square silicone patch infused with perfluorodecalin (PFD) for use during tattoo removal, which has been shown to reduce epidermal whitening. The patch contains a fluorocarbon “that is very good at dissolving gas, and it is already widely used in medicine,” he said. When applied, “it almost instantaneously takes the whitening away; you don’t have to wait the 20 minutes to do your second pass.”
A different technology designed to speed up tattoo removal is the Resonic Rapid Acoustic Pulse device (marketed as Resonic, from Allergan Aesthetics), which is cleared by the FDA for use as an accessory to the 1064 nm Q-switched laser for black tattoo removal in patients with skin types I-III. “This uses acoustic pulses of sound waves; they’re rapid and powerful,” Dr. Ibrahimi said. “They can clear those cavitation bubbles much like the PFD patches do. It’s also thought that they further disperse the tattoo ink particles by supplementing the laser energy as well. It is also purported to alter the body’s healing response, or immune response, which is important in tattoo clearing.”
Dr. Ibrahimi disclosed that he is a member of the Advisory Board for Accure Acne, AbbVie (which owns Allergan), Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
SAN DIEGO – When picosecond lasers hit the market about 10 years ago, they became a game-changer for tattoo removal, boasting the delivery of energy that is about threefold faster than with nanosecond lasers.
However, picosecond lasers are far from perfect even in the hands of the most experienced clinicians, according to Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford. “They have been very difficult to build from an engineering perspective,” he said at the annual Masters of Aesthetics Symposium. It took a long time for these lasers to come to the market, and they are still fairly expensive and require a lot of maintenance, he noted. In addition, “they are also not quite as ‘picosecond’ as they need to be. I think there is definitely room to improve, but this is the gold standard.”
Today, . Tattoo ink particles average about 0.1 mcm in size, and the thermal relaxation size works out to be less than 10 nanoseconds, with shorter pulses better at capturing the ink particles that are smaller than average.
Black is the most common tattoo color dermatologists treat. “For that, you can typically use a 1064, which has the highest absorption, but you can also use many of the other wavelengths,” he said. “Other colors are less common, followed by red, for which you would use a 532-nm wavelength.”
Dr. Ibrahimi underscored the importance of setting realistic expectations during consults with patients seeking options for tattoo removal. Even with picosecond laser technology, many treatments are typically required and “a good patient consultation is key to setting proper expectations,” he said. “If you promise someone results in 4 to 5 treatments like many of the device companies will say you can achieve, you’re going to have a large group of patients who are disappointed.”
The clinical endpoint to strive for during tattoo removal is whitening of the ink, which typically fades about 20 minutes after treatment. That whitening corresponds to cavitation, or the production of gas vacuoles in the cells that were holding the ink. This discovery led to a technique intended to enhance tattoo removal. In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and colleagues published results of a study that compared a single Q-switched laser treatment pass with four treatment passes separated by 20 minutes. After treating 18 tattoos in 12 adults, they found that the technique, known as the “R20” method, was more effective than a single-pass treatment (P < .01).
“Subsequent to this, there has been conflicting data on whether this is truly effective or not,” said Dr. Ibrahimi, who is also on the board of directors for the American Society for Dermatologic Surgery and the American Society for Laser Medicine and Surgery. “Most of us agree that one additional pass would be helpful, but when you’re doing this in the private practice setting, it’s often challenging because patients aren’t necessarily willing to pay more for more than just one pass for their tattoo removal.”
Another recent advance is use of a topical square silicone patch infused with perfluorodecalin (PFD) for use during tattoo removal, which has been shown to reduce epidermal whitening. The patch contains a fluorocarbon “that is very good at dissolving gas, and it is already widely used in medicine,” he said. When applied, “it almost instantaneously takes the whitening away; you don’t have to wait the 20 minutes to do your second pass.”
A different technology designed to speed up tattoo removal is the Resonic Rapid Acoustic Pulse device (marketed as Resonic, from Allergan Aesthetics), which is cleared by the FDA for use as an accessory to the 1064 nm Q-switched laser for black tattoo removal in patients with skin types I-III. “This uses acoustic pulses of sound waves; they’re rapid and powerful,” Dr. Ibrahimi said. “They can clear those cavitation bubbles much like the PFD patches do. It’s also thought that they further disperse the tattoo ink particles by supplementing the laser energy as well. It is also purported to alter the body’s healing response, or immune response, which is important in tattoo clearing.”
Dr. Ibrahimi disclosed that he is a member of the Advisory Board for Accure Acne, AbbVie (which owns Allergan), Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
AT MOAS 2022
Early retirement and the terrible, horrible, no good, very bad cognitive decline
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
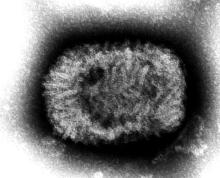
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
What to do when patients don’t listen
The term “nonadherent” has gradually replaced “noncompliant” in the physician lexicon as a nod to the evolving doctor-patient relationship. Noncompliance implies that a patient isn’t following their doctor’s orders. Adherence, on the other hand, is a measure of how closely your patient’s behavior matches the recommendations you’ve made. It’s a subtle difference but an important distinction in approaching care.
“Noncompliance is inherently negative feedback to the patient, whereas there’s a reason for nonadherence, and it’s usually external,” said Sharon Rabinovitz, MD, president of the Georgia Academy of Family Physicians.
Why won’t patients listen?
The reasons behind a patient’s nonadherence are multifaceted, but they are often driven by social determinants of health, such as transportation, poor health literacy, finances, and lack of access to pharmacies.
Other times, patients don’t want to take medicine, don’t prioritize their health, or they find the dietary and lifestyle modifications doctors suggest too hard to make or they struggle at losing weight, eating more healthfully, or cutting back on alcohol, for instance.
“When you come down to it, the big hindrance of it all is cost and the ability for the patient to be able to afford some of the things that we think they should be able to do,” said Teresa Lovins, MD, a physician in private practice Columbus, Ind., and a member of the board of directors of the American Academy of Family Physicians.
Another common deterrent to treatment is undesired side effects that a patient may not want to mention.
“For example, a lot of patients who are taking antidepressants have sexual dysfunction associated with those medications,” said Dr. Rabinovitz. “If you don’t ask the right questions, you’re not going to be able to fully assess the experience the patient is having and a reason why they might not take it [the medication].”
Much nonadherence is intentional and is based on experience, belief systems, and knowledge. For example, the American Medical Association finds that patients may not understand why they need a certain treatment (and therefore dismiss it), or they may be overloaded with multiple medications, fear dependency on a drug, have a mistrust of pharmaceutical companies or the medical system as a whole, or have symptoms of depression that make taking healthy actions more difficult. In addition, patients may be unable to afford their medication, or their lack of symptoms may lead them to believe they don’t really need the prescription, as occurs with disorders such as hypertension or high cholesterol.
“In my training, we did something called Balint training, where we would get together as a group with attendings and discuss cases that were difficult from a biopsychosocial perspective and consider all the factors in the patient perspective, including family dynamics, social systems, and economic realities,” said Russell Blackwelder, MD, director of geriatric education and associate professor of family medicine at the Medical University of South Carolina, Charleston.
“That training was, for me, very helpful for opening up and being more empathetic and really examining the patient’s point of view and everything that impacts them.”
Dr. Lovins agreed that it’s crucial to establish a good rapport and build mutual trust.
“If you don’t know the patient, you have a harder time asking the right questions to get to the meat of why they’re not taking their medicine or what they’re not doing to help their health,” she said. “It takes a little bit of trust on both parts to get to that question that really gets to the heart of why they’re not doing what you’re asking them to do.”
How to encourage adherence
Although there may not be a one-size-fits-all approach for achieving general adherence or adherence to a medication regimen, some methods may increase success.
Kenneth Zweig, MD, an internist at Northern Virginia Family Practice Associates, Alexandria, said that convincing patients to make one small change that they can sustain can get the ball rolling.
“I had one patient who was very overweight and had high blood pressure, high cholesterol, back pain, insomnia, and depression, who was also drinking three to four beers a night,” Dr. Zweig said. “After a long discussion, I challenged him to stop all alcohol for 1 week. At the end of the week, he noticed that he slept better, lost some weight, had lower blood pressure, and had more energy. Once he saw the benefits of this one change, he was motivated to improve other aspects of his health as well. He improved his diet, started exercising, and lost over 50 pounds. He has persisted with these lifestyle changes ever since.”
A team-based approach may also increase treatment understanding and adherence. In one older study, patients who were assigned to team-based care, including care by pharmacists, were significantly more adherent to medication regimens. Patients were more comfortable asking questions and raising concerns when they felt their treatment plan was a collaboration between several providers and themselves.
Dr. Lovins said to always approach the patient with a positive. “Say, what can we do together to make this work? What are your questions about this medication? And try and focus on the positive things that you can change instead of leaving the patient with a negative feeling or that you’re angry with them or that you’re unhappy with their choices. Patients respond better when they are treated as part of the team.”
Fear of judgment can also be a barrier to honesty between patients and their doctors. Shame creates a reluctance to admit nonadherence. Dr. Lovins said in an interview that it’s the physician’s responsibility to create a blame-free space for patients to speak openly about their struggles with treatment and reasons for nonadherence.
When should you redirect care?
Ultimately, the goal is good care and treatment of disease. However, if you and your patient are at an impasse and progress is stalling or failing, it may be appropriate to encourage the patient to seek care elsewhere.
“Just like any relationship, some physician-patient relationships are just not a good fit,” said Dr. Blackwelder. And this may be the reason why the patient is nonadherent — something between the two of you doesn’t click.
While there are ethical considerations for this decision, most medical boards have guidelines on how to go about it, Dr. Blackwelder said in an interview. “In the state of South Carolina, we have to be available to provide urgent coverage for at least 30 days and notify the patient in writing that they need to find somebody else and to help them find somebody else if we can.”
Just as with care, a clear conversation is the best practice if you’re proposing a potential shift away from a physician-patient relationship. You might say: We’re not making the kind of progress I’d like to see, and I’m wondering if you think working with another doctor may help you.
“The most important thing is being very honest and transparent with the patient that you’re concerned you’re not making the appropriate strides forward,” said Dr. Rabinovitz. Then you can ask, ‘Am I the right doctor to help you reach your goals? And if not, how can I help you get to where you need to be?’ ”
A version of this article first appeared on Medscape.com.
The term “nonadherent” has gradually replaced “noncompliant” in the physician lexicon as a nod to the evolving doctor-patient relationship. Noncompliance implies that a patient isn’t following their doctor’s orders. Adherence, on the other hand, is a measure of how closely your patient’s behavior matches the recommendations you’ve made. It’s a subtle difference but an important distinction in approaching care.
“Noncompliance is inherently negative feedback to the patient, whereas there’s a reason for nonadherence, and it’s usually external,” said Sharon Rabinovitz, MD, president of the Georgia Academy of Family Physicians.
Why won’t patients listen?
The reasons behind a patient’s nonadherence are multifaceted, but they are often driven by social determinants of health, such as transportation, poor health literacy, finances, and lack of access to pharmacies.
Other times, patients don’t want to take medicine, don’t prioritize their health, or they find the dietary and lifestyle modifications doctors suggest too hard to make or they struggle at losing weight, eating more healthfully, or cutting back on alcohol, for instance.
“When you come down to it, the big hindrance of it all is cost and the ability for the patient to be able to afford some of the things that we think they should be able to do,” said Teresa Lovins, MD, a physician in private practice Columbus, Ind., and a member of the board of directors of the American Academy of Family Physicians.
Another common deterrent to treatment is undesired side effects that a patient may not want to mention.
“For example, a lot of patients who are taking antidepressants have sexual dysfunction associated with those medications,” said Dr. Rabinovitz. “If you don’t ask the right questions, you’re not going to be able to fully assess the experience the patient is having and a reason why they might not take it [the medication].”
Much nonadherence is intentional and is based on experience, belief systems, and knowledge. For example, the American Medical Association finds that patients may not understand why they need a certain treatment (and therefore dismiss it), or they may be overloaded with multiple medications, fear dependency on a drug, have a mistrust of pharmaceutical companies or the medical system as a whole, or have symptoms of depression that make taking healthy actions more difficult. In addition, patients may be unable to afford their medication, or their lack of symptoms may lead them to believe they don’t really need the prescription, as occurs with disorders such as hypertension or high cholesterol.
“In my training, we did something called Balint training, where we would get together as a group with attendings and discuss cases that were difficult from a biopsychosocial perspective and consider all the factors in the patient perspective, including family dynamics, social systems, and economic realities,” said Russell Blackwelder, MD, director of geriatric education and associate professor of family medicine at the Medical University of South Carolina, Charleston.
“That training was, for me, very helpful for opening up and being more empathetic and really examining the patient’s point of view and everything that impacts them.”
Dr. Lovins agreed that it’s crucial to establish a good rapport and build mutual trust.
“If you don’t know the patient, you have a harder time asking the right questions to get to the meat of why they’re not taking their medicine or what they’re not doing to help their health,” she said. “It takes a little bit of trust on both parts to get to that question that really gets to the heart of why they’re not doing what you’re asking them to do.”
How to encourage adherence
Although there may not be a one-size-fits-all approach for achieving general adherence or adherence to a medication regimen, some methods may increase success.
Kenneth Zweig, MD, an internist at Northern Virginia Family Practice Associates, Alexandria, said that convincing patients to make one small change that they can sustain can get the ball rolling.
“I had one patient who was very overweight and had high blood pressure, high cholesterol, back pain, insomnia, and depression, who was also drinking three to four beers a night,” Dr. Zweig said. “After a long discussion, I challenged him to stop all alcohol for 1 week. At the end of the week, he noticed that he slept better, lost some weight, had lower blood pressure, and had more energy. Once he saw the benefits of this one change, he was motivated to improve other aspects of his health as well. He improved his diet, started exercising, and lost over 50 pounds. He has persisted with these lifestyle changes ever since.”
A team-based approach may also increase treatment understanding and adherence. In one older study, patients who were assigned to team-based care, including care by pharmacists, were significantly more adherent to medication regimens. Patients were more comfortable asking questions and raising concerns when they felt their treatment plan was a collaboration between several providers and themselves.
Dr. Lovins said to always approach the patient with a positive. “Say, what can we do together to make this work? What are your questions about this medication? And try and focus on the positive things that you can change instead of leaving the patient with a negative feeling or that you’re angry with them or that you’re unhappy with their choices. Patients respond better when they are treated as part of the team.”
Fear of judgment can also be a barrier to honesty between patients and their doctors. Shame creates a reluctance to admit nonadherence. Dr. Lovins said in an interview that it’s the physician’s responsibility to create a blame-free space for patients to speak openly about their struggles with treatment and reasons for nonadherence.
When should you redirect care?
Ultimately, the goal is good care and treatment of disease. However, if you and your patient are at an impasse and progress is stalling or failing, it may be appropriate to encourage the patient to seek care elsewhere.
“Just like any relationship, some physician-patient relationships are just not a good fit,” said Dr. Blackwelder. And this may be the reason why the patient is nonadherent — something between the two of you doesn’t click.
While there are ethical considerations for this decision, most medical boards have guidelines on how to go about it, Dr. Blackwelder said in an interview. “In the state of South Carolina, we have to be available to provide urgent coverage for at least 30 days and notify the patient in writing that they need to find somebody else and to help them find somebody else if we can.”
Just as with care, a clear conversation is the best practice if you’re proposing a potential shift away from a physician-patient relationship. You might say: We’re not making the kind of progress I’d like to see, and I’m wondering if you think working with another doctor may help you.
“The most important thing is being very honest and transparent with the patient that you’re concerned you’re not making the appropriate strides forward,” said Dr. Rabinovitz. Then you can ask, ‘Am I the right doctor to help you reach your goals? And if not, how can I help you get to where you need to be?’ ”
A version of this article first appeared on Medscape.com.
The term “nonadherent” has gradually replaced “noncompliant” in the physician lexicon as a nod to the evolving doctor-patient relationship. Noncompliance implies that a patient isn’t following their doctor’s orders. Adherence, on the other hand, is a measure of how closely your patient’s behavior matches the recommendations you’ve made. It’s a subtle difference but an important distinction in approaching care.
“Noncompliance is inherently negative feedback to the patient, whereas there’s a reason for nonadherence, and it’s usually external,” said Sharon Rabinovitz, MD, president of the Georgia Academy of Family Physicians.
Why won’t patients listen?
The reasons behind a patient’s nonadherence are multifaceted, but they are often driven by social determinants of health, such as transportation, poor health literacy, finances, and lack of access to pharmacies.
Other times, patients don’t want to take medicine, don’t prioritize their health, or they find the dietary and lifestyle modifications doctors suggest too hard to make or they struggle at losing weight, eating more healthfully, or cutting back on alcohol, for instance.
“When you come down to it, the big hindrance of it all is cost and the ability for the patient to be able to afford some of the things that we think they should be able to do,” said Teresa Lovins, MD, a physician in private practice Columbus, Ind., and a member of the board of directors of the American Academy of Family Physicians.
Another common deterrent to treatment is undesired side effects that a patient may not want to mention.
“For example, a lot of patients who are taking antidepressants have sexual dysfunction associated with those medications,” said Dr. Rabinovitz. “If you don’t ask the right questions, you’re not going to be able to fully assess the experience the patient is having and a reason why they might not take it [the medication].”
Much nonadherence is intentional and is based on experience, belief systems, and knowledge. For example, the American Medical Association finds that patients may not understand why they need a certain treatment (and therefore dismiss it), or they may be overloaded with multiple medications, fear dependency on a drug, have a mistrust of pharmaceutical companies or the medical system as a whole, or have symptoms of depression that make taking healthy actions more difficult. In addition, patients may be unable to afford their medication, or their lack of symptoms may lead them to believe they don’t really need the prescription, as occurs with disorders such as hypertension or high cholesterol.
“In my training, we did something called Balint training, where we would get together as a group with attendings and discuss cases that were difficult from a biopsychosocial perspective and consider all the factors in the patient perspective, including family dynamics, social systems, and economic realities,” said Russell Blackwelder, MD, director of geriatric education and associate professor of family medicine at the Medical University of South Carolina, Charleston.
“That training was, for me, very helpful for opening up and being more empathetic and really examining the patient’s point of view and everything that impacts them.”
Dr. Lovins agreed that it’s crucial to establish a good rapport and build mutual trust.
“If you don’t know the patient, you have a harder time asking the right questions to get to the meat of why they’re not taking their medicine or what they’re not doing to help their health,” she said. “It takes a little bit of trust on both parts to get to that question that really gets to the heart of why they’re not doing what you’re asking them to do.”
How to encourage adherence
Although there may not be a one-size-fits-all approach for achieving general adherence or adherence to a medication regimen, some methods may increase success.
Kenneth Zweig, MD, an internist at Northern Virginia Family Practice Associates, Alexandria, said that convincing patients to make one small change that they can sustain can get the ball rolling.
“I had one patient who was very overweight and had high blood pressure, high cholesterol, back pain, insomnia, and depression, who was also drinking three to four beers a night,” Dr. Zweig said. “After a long discussion, I challenged him to stop all alcohol for 1 week. At the end of the week, he noticed that he slept better, lost some weight, had lower blood pressure, and had more energy. Once he saw the benefits of this one change, he was motivated to improve other aspects of his health as well. He improved his diet, started exercising, and lost over 50 pounds. He has persisted with these lifestyle changes ever since.”
A team-based approach may also increase treatment understanding and adherence. In one older study, patients who were assigned to team-based care, including care by pharmacists, were significantly more adherent to medication regimens. Patients were more comfortable asking questions and raising concerns when they felt their treatment plan was a collaboration between several providers and themselves.
Dr. Lovins said to always approach the patient with a positive. “Say, what can we do together to make this work? What are your questions about this medication? And try and focus on the positive things that you can change instead of leaving the patient with a negative feeling or that you’re angry with them or that you’re unhappy with their choices. Patients respond better when they are treated as part of the team.”
Fear of judgment can also be a barrier to honesty between patients and their doctors. Shame creates a reluctance to admit nonadherence. Dr. Lovins said in an interview that it’s the physician’s responsibility to create a blame-free space for patients to speak openly about their struggles with treatment and reasons for nonadherence.
When should you redirect care?
Ultimately, the goal is good care and treatment of disease. However, if you and your patient are at an impasse and progress is stalling or failing, it may be appropriate to encourage the patient to seek care elsewhere.
“Just like any relationship, some physician-patient relationships are just not a good fit,” said Dr. Blackwelder. And this may be the reason why the patient is nonadherent — something between the two of you doesn’t click.
While there are ethical considerations for this decision, most medical boards have guidelines on how to go about it, Dr. Blackwelder said in an interview. “In the state of South Carolina, we have to be available to provide urgent coverage for at least 30 days and notify the patient in writing that they need to find somebody else and to help them find somebody else if we can.”
Just as with care, a clear conversation is the best practice if you’re proposing a potential shift away from a physician-patient relationship. You might say: We’re not making the kind of progress I’d like to see, and I’m wondering if you think working with another doctor may help you.
“The most important thing is being very honest and transparent with the patient that you’re concerned you’re not making the appropriate strides forward,” said Dr. Rabinovitz. Then you can ask, ‘Am I the right doctor to help you reach your goals? And if not, how can I help you get to where you need to be?’ ”
A version of this article first appeared on Medscape.com.
Age competency exams for physicians – yes or no?
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Sandeep Jauhar, a practicing cardiologist and professor of medicine at Northwell Health, a frequent New York Times op-ed contributor, and highly regarded author of the upcoming book “My Father’s Brain: Life in the Shadow of Alzheimer’s.”
Sandeep Jauhar, MD: Thanks for having me.
Dr. Glatter: Your recent op-ed piece in the New York Times caught my eye. In your piece, you refer to a 2020 survey in which almost one-third of licensed doctors in the United States were 60 years of age or older, up from a quarter in 2010. You also state that, due to a 20% prevalence of mild cognitive impairment in persons older than 65, practicing physicians above this age should probably be screened by a battery of tests to ensure that their reasoning and cognitive abilities are intact. The title of the article is “How Would You Feel About a 100-Year-Old Doctor?”
How would you envision such a process? What aspects of day-to-day functioning would the exams truly be evaluating?
Dr. Jauhar: A significant number of people over 65 have measurable cognitive impairment. By cognitive impairment, we’re not talking about dementia. The best estimates are that 1 in 10 people over age 65 have dementia, and roughly 1 in 5 have what’s called MCI, or mild cognitive impairment, which is cognitive impairment out of proportion to what you’d expect from normal aging. It’s a significant issue.
The argument that I made in the op-ed is that neurocognitive assessment is important. That’s not to say that everyone over age 65 has significant cognitive impairment or that older doctors can’t practice medicine safely and effectively. They absolutely can. The question is, do we leave neurocognitive assessment to physicians who may possibly be suffering from impairment?
In dementia, people very often have impaired self-awareness, a condition called anosognosia, which is a neurological term for not being aware of your own impairment because of your impairment.
I would argue that, instead of having voluntary neurocognitive screening, it should be mandated. The question is how to do that effectively, fairly, and transparently.
One could argue a gerontocracy in medicine today, where there are so many older physicians. What do we do about that? That really is something that I think needs to be debated.
Dr. Glatter: The question I have is, if we (that is, physicians and the health care profession) don’t take care of this, someone’s going to do it for us. We need to jump on this now while we have the opportunity. The AMA has been opposed to this, except when you have reason to suspect cognitive decline or are concerned about patient safety. A mandatory age of retirement is certainly something they’re not for, and we know this.
Your argument in your op-ed piece is very well thought out, and you lay the groundwork for testing (looking at someone’s memory, coordination, processing speed, and other executive functions). Certainly, for a psychiatrist, hearing is important, and for a dermatologist, vision is important. For a surgeon, there are other issues. Based on the specialty, we must be careful to see the important aspects of functioning. I am sure you would agree with this.
Dr. Jauhar: Obviously, the hand skills that are important for ophthalmological surgery certainly aren’t required for office-based psychological counseling, for example. We have to be smart about how we assess impairment.
You describe the spectrum of actions. On the one hand, there’s mandatory retirement at the age of 65 or 70 years. We know that commercial pilots are mandated to essentially retire at 65, and air-traffic controllers must retire in their late 50s.
We know that there’s a large amount of variability in competence. There are internists in their 80s with whom I’ve worked, and I’m absolutely wowed by their experience and judgment. There are new medical resident graduates who don’t really seem to have the requisite level of competence that would make me feel comfortable to have them as my doctor or a doctor for a member of my family.
To mandate retirement, I think the AMA is absolutely right. To not call for any kind of competency testing, to me, seems equally unwise. Because at the end of the day, you have to balance individual physician needs or wants to continue practicing with patient safety. I haven’t really come across too many physicians who say, “There’s absolutely no need for a competency testing.”
We have to meet somewhere in the middle. The middle is either voluntary cognitive competency testing or mandatory. I would argue that, because we know that as the brain changes we have cognitive impairment, but we’re not always aware that we need help, mandatory testing is the way.
One other thing that you mentioned was about having the solution imposed on us. You and I are doctors. We deal with bureaucracy. We deal with poorly thought-out solutions to issues in health care that make our lives that much more difficult. I don’t want that solution imposed on us by some outside agency. I think we need to figure this out within medicine and figure out the right way of doing it.
The AMA is on board with this. They haven’t called for mandatory testing, but they have said that if testing were to occur, these are the guidelines. The guidelines are fair and equitable, not too time-consuming, transparent, and not punitive. If someone comes out and doesn’t test well, we shouldn’t force them out of the profession. We can find ways to use their experience to help train younger doctors, for example.
Dr. Glatter: I wanted to segue to an area where there has been some challenge to the legality of these mandatory types of age restrictions and imposing the exams as well. There’s been a lawsuit as well by the EEOC [Equal Employment Opportunity Commission], on behalf of Yale. Basically, there’s been a concern that ageism is part of what’s going on. Yale now screens their providers beginning at age 70, and they have a program. UCSD [University of California, San Diego] has a program in place. Obviously, these institutions are looking at it. This is a very small part of the overall picture.
Health care systems overall, we’re talking about a fraction of them in the country are really addressing the issue of competency exams. The question is, where do we go from here? How do we get engagement or adoption and get physicians as a whole to embrace this concept?
Dr. Jauhar: The EEOC filed a lawsuit on behalf of the Yale medical staff that argued that Yale’s plan to do vision testing and neurocognitive screening – there may be a physical exam also – constitutes age discrimination because it’s reserved for doctors over the age of 70. Those are the physicians who are most likely to have cognitive impairment.
We have rules already for impaired physicians who are, for example, addicted to illicit drugs or have alcohol abuse. We already have some of those measures in place. This is focused on cognitive impairment in aging physicians because cognitive impairment is an issue that arises with aging. We have to be clear about that.
Most younger physicians will not have measurable cognitive impairment that would impair their ability to practice. To force young physicians (for example, physicians in their forties) to undergo such screening, all in the name of preventing age discrimination, doesn’t strike me as being a good use of resources. They’re more likely to be false positives, as you know from Bayesian statistics. When you have low pretest probability, you’re more likely to get false positives.
How are we going to screen hundreds of thousands of physicians? We have to make a choice about the group that really is more likely to benefit from such screening. Very few hospitals are addressing this issue and it’s going to become more important.
Dr. Glatter: Surgeons have been particularly active in pushing for age-based screening. In 2016, the American College of Surgeons started making surgeons at age 65-70 undergo voluntary health and neurocognitive assessments, and encouraged physicians to disclose any concerning findings as part of their professional obligation, which is pretty impressive in my mind.
Surgeons’ skill set is quite demanding physically and technically. That the Society of Surgical Chairs took it upon themselves to institute this is pretty telling.
Dr. Jauhar: The overall society called for screening, but then in a separate survey of surgical chairs, the idea was advanced that we should have mandatory retirement. Now, I don’t particularly agree with that.
I’ve seen it, where you have the aging surgeon who was a star in their day, and no one wants to say anything when their skills have visibly degraded, and no one wants to carry that torch and tell them that they need to retire. What happens is people whisper, and unfortunately, bad outcomes have to occur before people tend to get involved, and that’s what I’m trying to prevent.
Dr. Glatter: The question is whether older physicians have worse patient outcomes. The evidence is inconclusive, but studies have shown higher mortality rates for cardiovascular surgeons in terms of the procedures that they do. On the flip side, there are also higher mortality rates for GI surgery performed by younger surgeons. It’s a mixed bag.
Dr. Jauhar: For specialized surgery, you need the accrual of a certain amount of experience. The optimal age is about 60, because they’ve seen many things and they’ve seen complications. They don’t have a hand tremor yet so they’re still functioning well, and they’ve accrued a lot of experience. We have to be smart about who we screen.
There’s a learning curve in surgery. By no means am I arguing that younger surgeons are better surgeons. I would say that there’s probably a tipping point where once you get past a certain age and physical deterioration starts to take effect, that can overshadow the accrual of cognitive and surgical experience. We have to balance those things.
I would say neurocognitive screening and vision testing are important, but exactly what do you measure? How much of a hand tremor would constitute a risk? These things have to be figured out. I just want doctors to be leading the charge here and not have this imposed by bureaucrats.
Dr. Glatter: I was reading that some doctors have had these exams administered and they can really pass cognitive aspects of the exam, but there have been nuances in the actual practicing of medicine, day-to-day functioning, which they’re not good at.
Someone made a comment that the only way to know if a doctor can do well in practice is to observe their practice and observe them taking care of patients. In other words, you can game the system and pass the cognitive exam in some form but then have a problem practicing medicine.
Dr. Jauhar: Ultimately, outcomes have to be measured. We can’t adopt such a granular approach for every aging physician. There has to be some sort of screening that maybe raises a red flag and then hospitals and department chairs need to investigate further. What are the outcomes? What are people saying in the operating room? I think the screening is just that; it’s a way of opening the door to further investigation, but it’s not a witch hunt.
I have the highest respect for older physicians, and I learn from them every day, honestly, especially in my field (cardiology), because some of the older physicians can hear and see things on physical exam that I didn’t even know existed. There’s much to be learned from them.
This is not intended to be a witch hunt or to try to get rid of older physicians – by any means. We want to avoid some of the outcomes that I read about in the New York Times comments section. It’s not fair to our patients not to do at least some sort of screening to prevent those kinds of mistakes.
Dr. Glatter: I wanted to go back to data from Yale between October 2016 and January 2019, where 141 Yale clinicians who ranged in age from 69 to 92 years completed cognitive assessments. Of those, 18 clinicians, or about 13% of those tested, demonstrated cognitive deficits that were “deemed likely to impair their ability to practice medicine independently.” That’s telling. These are subtleties, but they’re important to identify. I would love to get your comment on that.
Dr. Jauhar: It’s in keeping with what we know about the proportion of our older citizens who have cognitive impairment. About 10% have dementia and about 20% have at least mild cognitive impairment. That’s in keeping with what we know, and this was a general screening.
There are certain programs, like in San Diego, for example, where physicians are referred, and so there’s a selection bias. But this was just general screening. It’s worrisome. I’m an aging physician myself. I want fairness in this process because I’m going to be assessed as well.
I just don’t really understand yet why there’s so much circling of the wagons and so much resistance. It seems like it would be good for physicians also to be removed from situations where they might get into potential litigation because of mistakes and physical or visual impairment. It seems like it’d be good for patients and physicians alike.
Dr. Glatter: It’s difficult to give up your profession, change fields, or become administrative at some point, and [decide] when to make that transition. As we all get older, we’re not going to have the ability to do what we did in our 20s, 30s, and so forth.
Dr. Jauhar: Much of the resistance is coming from doctors who are used to high levels of autonomy. I’m certainly sympathetic to that because I don’t want anyone telling me how to practice. The reason this is coming up and hasn’t come up in the past is not because of loss of autonomy but because of an actual demographic change. Many physicians were trained in the 1960s, ’70s, or ’80s. They’re getting to retirement age but they’re not retiring, and we can speculate as to why that is.
In America’s educational system, doctors incur a huge amount of debt. I know physicians who are still paying off their debt and they’re in their 50s and 60s, so I’m very sympathetic to that. I’m not trying to force doctors out of practicing. I just want whoever is practicing to be competent and to practice safely. We have to figure out how to do that.
Dr. Glatter: The fact that there is a shortage of physicians forecast in the next 10-15 years makes many physicians reluctant to retire. They feel like they want to be part of that support network and we don’t want to have a dire situation, especially in the rural areas. We’re not immune from aging. We’re human beings. We all have to realize that.
Dr. Jauhar: I know that the ACC is starting to debate this issue, in part because of my op-ed. My hope is that it will start a conversation and we will institute a plan that comes from physicians and serves our patients, and doesn’t serve some cottage industry of testing or serve the needs of insurers or bureaucrats. It has to serve the doctor-patient relationship.
Dr. Glatter: In some random surveys that I’ve read, up to 30%-40% of physicians do support some type of age-based screening or competency assessment. The needle’s moving. It’s just not there yet. I think that wider adoption is coming.
Dr. Jauhar: Data are coming as more hospitals start to adopt these late practitioner programs. Some of the data that came out of Yale, for example, are very important. We’re going to see more published data in this area, and it will clarify what we need to do and how big the problem is.
Dr. Glatter: I want to thank you again for your time and for writing the op-ed because it certainly was well read and opened the eyes of not only physicians, but also the public at large. It’s a conversation that has to be had. Thank you for doing this.
Dr. Jauhar: Thanks for inviting me, Robert. It was a pleasure to talk to you.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, at Hofstra University, Hempstead, N.Y. Dr. Jauhar is director of the heart failure program, Long Island Jewish Medical Center, New Hyde Park, N.Y. Neither Dr. Glatter nor Dr. Jauhar reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Sandeep Jauhar, a practicing cardiologist and professor of medicine at Northwell Health, a frequent New York Times op-ed contributor, and highly regarded author of the upcoming book “My Father’s Brain: Life in the Shadow of Alzheimer’s.”
Sandeep Jauhar, MD: Thanks for having me.
Dr. Glatter: Your recent op-ed piece in the New York Times caught my eye. In your piece, you refer to a 2020 survey in which almost one-third of licensed doctors in the United States were 60 years of age or older, up from a quarter in 2010. You also state that, due to a 20% prevalence of mild cognitive impairment in persons older than 65, practicing physicians above this age should probably be screened by a battery of tests to ensure that their reasoning and cognitive abilities are intact. The title of the article is “How Would You Feel About a 100-Year-Old Doctor?”
How would you envision such a process? What aspects of day-to-day functioning would the exams truly be evaluating?
Dr. Jauhar: A significant number of people over 65 have measurable cognitive impairment. By cognitive impairment, we’re not talking about dementia. The best estimates are that 1 in 10 people over age 65 have dementia, and roughly 1 in 5 have what’s called MCI, or mild cognitive impairment, which is cognitive impairment out of proportion to what you’d expect from normal aging. It’s a significant issue.
The argument that I made in the op-ed is that neurocognitive assessment is important. That’s not to say that everyone over age 65 has significant cognitive impairment or that older doctors can’t practice medicine safely and effectively. They absolutely can. The question is, do we leave neurocognitive assessment to physicians who may possibly be suffering from impairment?
In dementia, people very often have impaired self-awareness, a condition called anosognosia, which is a neurological term for not being aware of your own impairment because of your impairment.
I would argue that, instead of having voluntary neurocognitive screening, it should be mandated. The question is how to do that effectively, fairly, and transparently.
One could argue a gerontocracy in medicine today, where there are so many older physicians. What do we do about that? That really is something that I think needs to be debated.
Dr. Glatter: The question I have is, if we (that is, physicians and the health care profession) don’t take care of this, someone’s going to do it for us. We need to jump on this now while we have the opportunity. The AMA has been opposed to this, except when you have reason to suspect cognitive decline or are concerned about patient safety. A mandatory age of retirement is certainly something they’re not for, and we know this.
Your argument in your op-ed piece is very well thought out, and you lay the groundwork for testing (looking at someone’s memory, coordination, processing speed, and other executive functions). Certainly, for a psychiatrist, hearing is important, and for a dermatologist, vision is important. For a surgeon, there are other issues. Based on the specialty, we must be careful to see the important aspects of functioning. I am sure you would agree with this.
Dr. Jauhar: Obviously, the hand skills that are important for ophthalmological surgery certainly aren’t required for office-based psychological counseling, for example. We have to be smart about how we assess impairment.
You describe the spectrum of actions. On the one hand, there’s mandatory retirement at the age of 65 or 70 years. We know that commercial pilots are mandated to essentially retire at 65, and air-traffic controllers must retire in their late 50s.
We know that there’s a large amount of variability in competence. There are internists in their 80s with whom I’ve worked, and I’m absolutely wowed by their experience and judgment. There are new medical resident graduates who don’t really seem to have the requisite level of competence that would make me feel comfortable to have them as my doctor or a doctor for a member of my family.
To mandate retirement, I think the AMA is absolutely right. To not call for any kind of competency testing, to me, seems equally unwise. Because at the end of the day, you have to balance individual physician needs or wants to continue practicing with patient safety. I haven’t really come across too many physicians who say, “There’s absolutely no need for a competency testing.”
We have to meet somewhere in the middle. The middle is either voluntary cognitive competency testing or mandatory. I would argue that, because we know that as the brain changes we have cognitive impairment, but we’re not always aware that we need help, mandatory testing is the way.
One other thing that you mentioned was about having the solution imposed on us. You and I are doctors. We deal with bureaucracy. We deal with poorly thought-out solutions to issues in health care that make our lives that much more difficult. I don’t want that solution imposed on us by some outside agency. I think we need to figure this out within medicine and figure out the right way of doing it.
The AMA is on board with this. They haven’t called for mandatory testing, but they have said that if testing were to occur, these are the guidelines. The guidelines are fair and equitable, not too time-consuming, transparent, and not punitive. If someone comes out and doesn’t test well, we shouldn’t force them out of the profession. We can find ways to use their experience to help train younger doctors, for example.
Dr. Glatter: I wanted to segue to an area where there has been some challenge to the legality of these mandatory types of age restrictions and imposing the exams as well. There’s been a lawsuit as well by the EEOC [Equal Employment Opportunity Commission], on behalf of Yale. Basically, there’s been a concern that ageism is part of what’s going on. Yale now screens their providers beginning at age 70, and they have a program. UCSD [University of California, San Diego] has a program in place. Obviously, these institutions are looking at it. This is a very small part of the overall picture.
Health care systems overall, we’re talking about a fraction of them in the country are really addressing the issue of competency exams. The question is, where do we go from here? How do we get engagement or adoption and get physicians as a whole to embrace this concept?
Dr. Jauhar: The EEOC filed a lawsuit on behalf of the Yale medical staff that argued that Yale’s plan to do vision testing and neurocognitive screening – there may be a physical exam also – constitutes age discrimination because it’s reserved for doctors over the age of 70. Those are the physicians who are most likely to have cognitive impairment.
We have rules already for impaired physicians who are, for example, addicted to illicit drugs or have alcohol abuse. We already have some of those measures in place. This is focused on cognitive impairment in aging physicians because cognitive impairment is an issue that arises with aging. We have to be clear about that.
Most younger physicians will not have measurable cognitive impairment that would impair their ability to practice. To force young physicians (for example, physicians in their forties) to undergo such screening, all in the name of preventing age discrimination, doesn’t strike me as being a good use of resources. They’re more likely to be false positives, as you know from Bayesian statistics. When you have low pretest probability, you’re more likely to get false positives.
How are we going to screen hundreds of thousands of physicians? We have to make a choice about the group that really is more likely to benefit from such screening. Very few hospitals are addressing this issue and it’s going to become more important.
Dr. Glatter: Surgeons have been particularly active in pushing for age-based screening. In 2016, the American College of Surgeons started making surgeons at age 65-70 undergo voluntary health and neurocognitive assessments, and encouraged physicians to disclose any concerning findings as part of their professional obligation, which is pretty impressive in my mind.
Surgeons’ skill set is quite demanding physically and technically. That the Society of Surgical Chairs took it upon themselves to institute this is pretty telling.
Dr. Jauhar: The overall society called for screening, but then in a separate survey of surgical chairs, the idea was advanced that we should have mandatory retirement. Now, I don’t particularly agree with that.
I’ve seen it, where you have the aging surgeon who was a star in their day, and no one wants to say anything when their skills have visibly degraded, and no one wants to carry that torch and tell them that they need to retire. What happens is people whisper, and unfortunately, bad outcomes have to occur before people tend to get involved, and that’s what I’m trying to prevent.
Dr. Glatter: The question is whether older physicians have worse patient outcomes. The evidence is inconclusive, but studies have shown higher mortality rates for cardiovascular surgeons in terms of the procedures that they do. On the flip side, there are also higher mortality rates for GI surgery performed by younger surgeons. It’s a mixed bag.
Dr. Jauhar: For specialized surgery, you need the accrual of a certain amount of experience. The optimal age is about 60, because they’ve seen many things and they’ve seen complications. They don’t have a hand tremor yet so they’re still functioning well, and they’ve accrued a lot of experience. We have to be smart about who we screen.
There’s a learning curve in surgery. By no means am I arguing that younger surgeons are better surgeons. I would say that there’s probably a tipping point where once you get past a certain age and physical deterioration starts to take effect, that can overshadow the accrual of cognitive and surgical experience. We have to balance those things.
I would say neurocognitive screening and vision testing are important, but exactly what do you measure? How much of a hand tremor would constitute a risk? These things have to be figured out. I just want doctors to be leading the charge here and not have this imposed by bureaucrats.
Dr. Glatter: I was reading that some doctors have had these exams administered and they can really pass cognitive aspects of the exam, but there have been nuances in the actual practicing of medicine, day-to-day functioning, which they’re not good at.
Someone made a comment that the only way to know if a doctor can do well in practice is to observe their practice and observe them taking care of patients. In other words, you can game the system and pass the cognitive exam in some form but then have a problem practicing medicine.
Dr. Jauhar: Ultimately, outcomes have to be measured. We can’t adopt such a granular approach for every aging physician. There has to be some sort of screening that maybe raises a red flag and then hospitals and department chairs need to investigate further. What are the outcomes? What are people saying in the operating room? I think the screening is just that; it’s a way of opening the door to further investigation, but it’s not a witch hunt.
I have the highest respect for older physicians, and I learn from them every day, honestly, especially in my field (cardiology), because some of the older physicians can hear and see things on physical exam that I didn’t even know existed. There’s much to be learned from them.
This is not intended to be a witch hunt or to try to get rid of older physicians – by any means. We want to avoid some of the outcomes that I read about in the New York Times comments section. It’s not fair to our patients not to do at least some sort of screening to prevent those kinds of mistakes.
Dr. Glatter: I wanted to go back to data from Yale between October 2016 and January 2019, where 141 Yale clinicians who ranged in age from 69 to 92 years completed cognitive assessments. Of those, 18 clinicians, or about 13% of those tested, demonstrated cognitive deficits that were “deemed likely to impair their ability to practice medicine independently.” That’s telling. These are subtleties, but they’re important to identify. I would love to get your comment on that.
Dr. Jauhar: It’s in keeping with what we know about the proportion of our older citizens who have cognitive impairment. About 10% have dementia and about 20% have at least mild cognitive impairment. That’s in keeping with what we know, and this was a general screening.
There are certain programs, like in San Diego, for example, where physicians are referred, and so there’s a selection bias. But this was just general screening. It’s worrisome. I’m an aging physician myself. I want fairness in this process because I’m going to be assessed as well.
I just don’t really understand yet why there’s so much circling of the wagons and so much resistance. It seems like it would be good for physicians also to be removed from situations where they might get into potential litigation because of mistakes and physical or visual impairment. It seems like it’d be good for patients and physicians alike.
Dr. Glatter: It’s difficult to give up your profession, change fields, or become administrative at some point, and [decide] when to make that transition. As we all get older, we’re not going to have the ability to do what we did in our 20s, 30s, and so forth.
Dr. Jauhar: Much of the resistance is coming from doctors who are used to high levels of autonomy. I’m certainly sympathetic to that because I don’t want anyone telling me how to practice. The reason this is coming up and hasn’t come up in the past is not because of loss of autonomy but because of an actual demographic change. Many physicians were trained in the 1960s, ’70s, or ’80s. They’re getting to retirement age but they’re not retiring, and we can speculate as to why that is.
In America’s educational system, doctors incur a huge amount of debt. I know physicians who are still paying off their debt and they’re in their 50s and 60s, so I’m very sympathetic to that. I’m not trying to force doctors out of practicing. I just want whoever is practicing to be competent and to practice safely. We have to figure out how to do that.
Dr. Glatter: The fact that there is a shortage of physicians forecast in the next 10-15 years makes many physicians reluctant to retire. They feel like they want to be part of that support network and we don’t want to have a dire situation, especially in the rural areas. We’re not immune from aging. We’re human beings. We all have to realize that.
Dr. Jauhar: I know that the ACC is starting to debate this issue, in part because of my op-ed. My hope is that it will start a conversation and we will institute a plan that comes from physicians and serves our patients, and doesn’t serve some cottage industry of testing or serve the needs of insurers or bureaucrats. It has to serve the doctor-patient relationship.
Dr. Glatter: In some random surveys that I’ve read, up to 30%-40% of physicians do support some type of age-based screening or competency assessment. The needle’s moving. It’s just not there yet. I think that wider adoption is coming.
Dr. Jauhar: Data are coming as more hospitals start to adopt these late practitioner programs. Some of the data that came out of Yale, for example, are very important. We’re going to see more published data in this area, and it will clarify what we need to do and how big the problem is.
Dr. Glatter: I want to thank you again for your time and for writing the op-ed because it certainly was well read and opened the eyes of not only physicians, but also the public at large. It’s a conversation that has to be had. Thank you for doing this.
Dr. Jauhar: Thanks for inviting me, Robert. It was a pleasure to talk to you.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, at Hofstra University, Hempstead, N.Y. Dr. Jauhar is director of the heart failure program, Long Island Jewish Medical Center, New Hyde Park, N.Y. Neither Dr. Glatter nor Dr. Jauhar reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Sandeep Jauhar, a practicing cardiologist and professor of medicine at Northwell Health, a frequent New York Times op-ed contributor, and highly regarded author of the upcoming book “My Father’s Brain: Life in the Shadow of Alzheimer’s.”
Sandeep Jauhar, MD: Thanks for having me.
Dr. Glatter: Your recent op-ed piece in the New York Times caught my eye. In your piece, you refer to a 2020 survey in which almost one-third of licensed doctors in the United States were 60 years of age or older, up from a quarter in 2010. You also state that, due to a 20% prevalence of mild cognitive impairment in persons older than 65, practicing physicians above this age should probably be screened by a battery of tests to ensure that their reasoning and cognitive abilities are intact. The title of the article is “How Would You Feel About a 100-Year-Old Doctor?”
How would you envision such a process? What aspects of day-to-day functioning would the exams truly be evaluating?
Dr. Jauhar: A significant number of people over 65 have measurable cognitive impairment. By cognitive impairment, we’re not talking about dementia. The best estimates are that 1 in 10 people over age 65 have dementia, and roughly 1 in 5 have what’s called MCI, or mild cognitive impairment, which is cognitive impairment out of proportion to what you’d expect from normal aging. It’s a significant issue.
The argument that I made in the op-ed is that neurocognitive assessment is important. That’s not to say that everyone over age 65 has significant cognitive impairment or that older doctors can’t practice medicine safely and effectively. They absolutely can. The question is, do we leave neurocognitive assessment to physicians who may possibly be suffering from impairment?
In dementia, people very often have impaired self-awareness, a condition called anosognosia, which is a neurological term for not being aware of your own impairment because of your impairment.
I would argue that, instead of having voluntary neurocognitive screening, it should be mandated. The question is how to do that effectively, fairly, and transparently.
One could argue a gerontocracy in medicine today, where there are so many older physicians. What do we do about that? That really is something that I think needs to be debated.
Dr. Glatter: The question I have is, if we (that is, physicians and the health care profession) don’t take care of this, someone’s going to do it for us. We need to jump on this now while we have the opportunity. The AMA has been opposed to this, except when you have reason to suspect cognitive decline or are concerned about patient safety. A mandatory age of retirement is certainly something they’re not for, and we know this.
Your argument in your op-ed piece is very well thought out, and you lay the groundwork for testing (looking at someone’s memory, coordination, processing speed, and other executive functions). Certainly, for a psychiatrist, hearing is important, and for a dermatologist, vision is important. For a surgeon, there are other issues. Based on the specialty, we must be careful to see the important aspects of functioning. I am sure you would agree with this.
Dr. Jauhar: Obviously, the hand skills that are important for ophthalmological surgery certainly aren’t required for office-based psychological counseling, for example. We have to be smart about how we assess impairment.
You describe the spectrum of actions. On the one hand, there’s mandatory retirement at the age of 65 or 70 years. We know that commercial pilots are mandated to essentially retire at 65, and air-traffic controllers must retire in their late 50s.
We know that there’s a large amount of variability in competence. There are internists in their 80s with whom I’ve worked, and I’m absolutely wowed by their experience and judgment. There are new medical resident graduates who don’t really seem to have the requisite level of competence that would make me feel comfortable to have them as my doctor or a doctor for a member of my family.
To mandate retirement, I think the AMA is absolutely right. To not call for any kind of competency testing, to me, seems equally unwise. Because at the end of the day, you have to balance individual physician needs or wants to continue practicing with patient safety. I haven’t really come across too many physicians who say, “There’s absolutely no need for a competency testing.”
We have to meet somewhere in the middle. The middle is either voluntary cognitive competency testing or mandatory. I would argue that, because we know that as the brain changes we have cognitive impairment, but we’re not always aware that we need help, mandatory testing is the way.
One other thing that you mentioned was about having the solution imposed on us. You and I are doctors. We deal with bureaucracy. We deal with poorly thought-out solutions to issues in health care that make our lives that much more difficult. I don’t want that solution imposed on us by some outside agency. I think we need to figure this out within medicine and figure out the right way of doing it.
The AMA is on board with this. They haven’t called for mandatory testing, but they have said that if testing were to occur, these are the guidelines. The guidelines are fair and equitable, not too time-consuming, transparent, and not punitive. If someone comes out and doesn’t test well, we shouldn’t force them out of the profession. We can find ways to use their experience to help train younger doctors, for example.
Dr. Glatter: I wanted to segue to an area where there has been some challenge to the legality of these mandatory types of age restrictions and imposing the exams as well. There’s been a lawsuit as well by the EEOC [Equal Employment Opportunity Commission], on behalf of Yale. Basically, there’s been a concern that ageism is part of what’s going on. Yale now screens their providers beginning at age 70, and they have a program. UCSD [University of California, San Diego] has a program in place. Obviously, these institutions are looking at it. This is a very small part of the overall picture.
Health care systems overall, we’re talking about a fraction of them in the country are really addressing the issue of competency exams. The question is, where do we go from here? How do we get engagement or adoption and get physicians as a whole to embrace this concept?
Dr. Jauhar: The EEOC filed a lawsuit on behalf of the Yale medical staff that argued that Yale’s plan to do vision testing and neurocognitive screening – there may be a physical exam also – constitutes age discrimination because it’s reserved for doctors over the age of 70. Those are the physicians who are most likely to have cognitive impairment.
We have rules already for impaired physicians who are, for example, addicted to illicit drugs or have alcohol abuse. We already have some of those measures in place. This is focused on cognitive impairment in aging physicians because cognitive impairment is an issue that arises with aging. We have to be clear about that.
Most younger physicians will not have measurable cognitive impairment that would impair their ability to practice. To force young physicians (for example, physicians in their forties) to undergo such screening, all in the name of preventing age discrimination, doesn’t strike me as being a good use of resources. They’re more likely to be false positives, as you know from Bayesian statistics. When you have low pretest probability, you’re more likely to get false positives.
How are we going to screen hundreds of thousands of physicians? We have to make a choice about the group that really is more likely to benefit from such screening. Very few hospitals are addressing this issue and it’s going to become more important.
Dr. Glatter: Surgeons have been particularly active in pushing for age-based screening. In 2016, the American College of Surgeons started making surgeons at age 65-70 undergo voluntary health and neurocognitive assessments, and encouraged physicians to disclose any concerning findings as part of their professional obligation, which is pretty impressive in my mind.
Surgeons’ skill set is quite demanding physically and technically. That the Society of Surgical Chairs took it upon themselves to institute this is pretty telling.
Dr. Jauhar: The overall society called for screening, but then in a separate survey of surgical chairs, the idea was advanced that we should have mandatory retirement. Now, I don’t particularly agree with that.
I’ve seen it, where you have the aging surgeon who was a star in their day, and no one wants to say anything when their skills have visibly degraded, and no one wants to carry that torch and tell them that they need to retire. What happens is people whisper, and unfortunately, bad outcomes have to occur before people tend to get involved, and that’s what I’m trying to prevent.
Dr. Glatter: The question is whether older physicians have worse patient outcomes. The evidence is inconclusive, but studies have shown higher mortality rates for cardiovascular surgeons in terms of the procedures that they do. On the flip side, there are also higher mortality rates for GI surgery performed by younger surgeons. It’s a mixed bag.
Dr. Jauhar: For specialized surgery, you need the accrual of a certain amount of experience. The optimal age is about 60, because they’ve seen many things and they’ve seen complications. They don’t have a hand tremor yet so they’re still functioning well, and they’ve accrued a lot of experience. We have to be smart about who we screen.
There’s a learning curve in surgery. By no means am I arguing that younger surgeons are better surgeons. I would say that there’s probably a tipping point where once you get past a certain age and physical deterioration starts to take effect, that can overshadow the accrual of cognitive and surgical experience. We have to balance those things.
I would say neurocognitive screening and vision testing are important, but exactly what do you measure? How much of a hand tremor would constitute a risk? These things have to be figured out. I just want doctors to be leading the charge here and not have this imposed by bureaucrats.
Dr. Glatter: I was reading that some doctors have had these exams administered and they can really pass cognitive aspects of the exam, but there have been nuances in the actual practicing of medicine, day-to-day functioning, which they’re not good at.
Someone made a comment that the only way to know if a doctor can do well in practice is to observe their practice and observe them taking care of patients. In other words, you can game the system and pass the cognitive exam in some form but then have a problem practicing medicine.
Dr. Jauhar: Ultimately, outcomes have to be measured. We can’t adopt such a granular approach for every aging physician. There has to be some sort of screening that maybe raises a red flag and then hospitals and department chairs need to investigate further. What are the outcomes? What are people saying in the operating room? I think the screening is just that; it’s a way of opening the door to further investigation, but it’s not a witch hunt.
I have the highest respect for older physicians, and I learn from them every day, honestly, especially in my field (cardiology), because some of the older physicians can hear and see things on physical exam that I didn’t even know existed. There’s much to be learned from them.
This is not intended to be a witch hunt or to try to get rid of older physicians – by any means. We want to avoid some of the outcomes that I read about in the New York Times comments section. It’s not fair to our patients not to do at least some sort of screening to prevent those kinds of mistakes.
Dr. Glatter: I wanted to go back to data from Yale between October 2016 and January 2019, where 141 Yale clinicians who ranged in age from 69 to 92 years completed cognitive assessments. Of those, 18 clinicians, or about 13% of those tested, demonstrated cognitive deficits that were “deemed likely to impair their ability to practice medicine independently.” That’s telling. These are subtleties, but they’re important to identify. I would love to get your comment on that.
Dr. Jauhar: It’s in keeping with what we know about the proportion of our older citizens who have cognitive impairment. About 10% have dementia and about 20% have at least mild cognitive impairment. That’s in keeping with what we know, and this was a general screening.
There are certain programs, like in San Diego, for example, where physicians are referred, and so there’s a selection bias. But this was just general screening. It’s worrisome. I’m an aging physician myself. I want fairness in this process because I’m going to be assessed as well.
I just don’t really understand yet why there’s so much circling of the wagons and so much resistance. It seems like it would be good for physicians also to be removed from situations where they might get into potential litigation because of mistakes and physical or visual impairment. It seems like it’d be good for patients and physicians alike.
Dr. Glatter: It’s difficult to give up your profession, change fields, or become administrative at some point, and [decide] when to make that transition. As we all get older, we’re not going to have the ability to do what we did in our 20s, 30s, and so forth.
Dr. Jauhar: Much of the resistance is coming from doctors who are used to high levels of autonomy. I’m certainly sympathetic to that because I don’t want anyone telling me how to practice. The reason this is coming up and hasn’t come up in the past is not because of loss of autonomy but because of an actual demographic change. Many physicians were trained in the 1960s, ’70s, or ’80s. They’re getting to retirement age but they’re not retiring, and we can speculate as to why that is.
In America’s educational system, doctors incur a huge amount of debt. I know physicians who are still paying off their debt and they’re in their 50s and 60s, so I’m very sympathetic to that. I’m not trying to force doctors out of practicing. I just want whoever is practicing to be competent and to practice safely. We have to figure out how to do that.
Dr. Glatter: The fact that there is a shortage of physicians forecast in the next 10-15 years makes many physicians reluctant to retire. They feel like they want to be part of that support network and we don’t want to have a dire situation, especially in the rural areas. We’re not immune from aging. We’re human beings. We all have to realize that.
Dr. Jauhar: I know that the ACC is starting to debate this issue, in part because of my op-ed. My hope is that it will start a conversation and we will institute a plan that comes from physicians and serves our patients, and doesn’t serve some cottage industry of testing or serve the needs of insurers or bureaucrats. It has to serve the doctor-patient relationship.
Dr. Glatter: In some random surveys that I’ve read, up to 30%-40% of physicians do support some type of age-based screening or competency assessment. The needle’s moving. It’s just not there yet. I think that wider adoption is coming.
Dr. Jauhar: Data are coming as more hospitals start to adopt these late practitioner programs. Some of the data that came out of Yale, for example, are very important. We’re going to see more published data in this area, and it will clarify what we need to do and how big the problem is.
Dr. Glatter: I want to thank you again for your time and for writing the op-ed because it certainly was well read and opened the eyes of not only physicians, but also the public at large. It’s a conversation that has to be had. Thank you for doing this.
Dr. Jauhar: Thanks for inviting me, Robert. It was a pleasure to talk to you.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, at Hofstra University, Hempstead, N.Y. Dr. Jauhar is director of the heart failure program, Long Island Jewish Medical Center, New Hyde Park, N.Y. Neither Dr. Glatter nor Dr. Jauhar reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Kaposi’s sarcoma: Antiretroviral-related improvements in survival measured
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Study spotlights clinicopathologic features, survival outcomes of pediatric melanoma
.
“Cutaneous melanomas are rare in children and much less common in adolescents than in later life,” researchers led by Mary-Ann El Sharouni, PhD, wrote in the study, which was published online in the Journal of the American Academy of Dermatology. “Management of these young patients currently follows guidelines developed for adults. Better understanding of melanoma occurring in the first 2 decades of life is, therefore, warranted.”
Drawing from two datasets – one from the Netherlands and the other from Melanoma Institute Australia (MIA) at the University of Sydney – Dr. El Sharouni of the MIA and of the department of dermatology at University Medical Center Utrecht in the Netherlands, and colleagues, evaluated all patients younger than 20 years of age who were diagnosed with invasive melanoma between January 2000 and December 2014. The pooled cohort included 397 Dutch and 117 Australian individuals. Of these, 62 were children and 452 were adolescents. To determine melanoma subtypes, the researchers reevaluated pathology reports and used multivariate Cox models to calculate recurrence-free survival (RFS) and overall survival (OS).
The median Breslow thickness was 2.7 mm in children and 1.0 mm in adolescents. Most patients (83%) had conventional melanoma, which consisted of superficial spreading, nodular, desmoplastic, and acral lentiginous forms, while 78 had spitzoid melanoma and 8 had melanoma associated with a congenital nevus. The 10-year RFS was 91.5% in children and 86.4% in adolescents (P =.32), while the 10-year OS was 100% in children and 92.7% in adolescents (P = .09).
On multivariable analysis, which was possible only for the adolescent cohort because of the small number of children, ulceration status and anatomic site were associated with RFS and OS, whereas age, sex, mitotic index, sentinel node status, and melanoma subtype were not. Breslow thickness > 4 mm was associated with worse RFS. As for affected anatomic site, those with melanomas located on the upper and lower limbs had a better overall RFS and OS compared with those who had head or neck melanomas.
The authors acknowledged certain limitation of the analysis, including its retrospective design and the small number of children. “Our data suggest that adolescent melanomas are often similar to adult-type melanomas, whilst those which occur in young children frequently occur via different molecular mechanisms,” they concluded. “In the future it is likely that further understanding of these molecular mechanisms and ability to classify melanomas based on their molecular characteristics will assist in further refining prognostic estimates and possible guiding treatment for young patients with melanoma.”
Rebecca M. Thiede, MD, assistant program director of the division of dermatology at the University of Arizona, Tucson, who was asked to comment on the study, said that the analysis “greatly contributes to dermatology, as we are still learning the differences between melanoma in children and adolescents versus adults.
This study found that adolescents with melanoma had worse survival if mitosis were present and/or located on head/neck, which could aid in aggressiveness of treatment.”
A key strength of analysis, she continued, is the large sample size of 514 patients, “given that melanoma in this population is very rare. A limitation which [the researchers] brought up is the discrepancy of diagnosis via histopathology of melanoma in children versus adults. The study relied on the pathology report given the retrospective nature of this [analysis, and it] was based on Australian and Dutch populations, which may limit its scope in other countries.”
Dr. El Sharouni was supported by a research fellowship grant from the European Academy of Dermatology and Venereology (EADV), while two of her coauthors, Richard A. Scolyer, MD, and John F. Thompson, MD, were recipients of an Australian National Health and Medical Research Council Program Grant. The study was also supported by a research program grant from Cancer Institute New South Wales. Dr. Thiede reported having no financial disclosures.
.
“Cutaneous melanomas are rare in children and much less common in adolescents than in later life,” researchers led by Mary-Ann El Sharouni, PhD, wrote in the study, which was published online in the Journal of the American Academy of Dermatology. “Management of these young patients currently follows guidelines developed for adults. Better understanding of melanoma occurring in the first 2 decades of life is, therefore, warranted.”
Drawing from two datasets – one from the Netherlands and the other from Melanoma Institute Australia (MIA) at the University of Sydney – Dr. El Sharouni of the MIA and of the department of dermatology at University Medical Center Utrecht in the Netherlands, and colleagues, evaluated all patients younger than 20 years of age who were diagnosed with invasive melanoma between January 2000 and December 2014. The pooled cohort included 397 Dutch and 117 Australian individuals. Of these, 62 were children and 452 were adolescents. To determine melanoma subtypes, the researchers reevaluated pathology reports and used multivariate Cox models to calculate recurrence-free survival (RFS) and overall survival (OS).
The median Breslow thickness was 2.7 mm in children and 1.0 mm in adolescents. Most patients (83%) had conventional melanoma, which consisted of superficial spreading, nodular, desmoplastic, and acral lentiginous forms, while 78 had spitzoid melanoma and 8 had melanoma associated with a congenital nevus. The 10-year RFS was 91.5% in children and 86.4% in adolescents (P =.32), while the 10-year OS was 100% in children and 92.7% in adolescents (P = .09).
On multivariable analysis, which was possible only for the adolescent cohort because of the small number of children, ulceration status and anatomic site were associated with RFS and OS, whereas age, sex, mitotic index, sentinel node status, and melanoma subtype were not. Breslow thickness > 4 mm was associated with worse RFS. As for affected anatomic site, those with melanomas located on the upper and lower limbs had a better overall RFS and OS compared with those who had head or neck melanomas.
The authors acknowledged certain limitation of the analysis, including its retrospective design and the small number of children. “Our data suggest that adolescent melanomas are often similar to adult-type melanomas, whilst those which occur in young children frequently occur via different molecular mechanisms,” they concluded. “In the future it is likely that further understanding of these molecular mechanisms and ability to classify melanomas based on their molecular characteristics will assist in further refining prognostic estimates and possible guiding treatment for young patients with melanoma.”
Rebecca M. Thiede, MD, assistant program director of the division of dermatology at the University of Arizona, Tucson, who was asked to comment on the study, said that the analysis “greatly contributes to dermatology, as we are still learning the differences between melanoma in children and adolescents versus adults.
This study found that adolescents with melanoma had worse survival if mitosis were present and/or located on head/neck, which could aid in aggressiveness of treatment.”
A key strength of analysis, she continued, is the large sample size of 514 patients, “given that melanoma in this population is very rare. A limitation which [the researchers] brought up is the discrepancy of diagnosis via histopathology of melanoma in children versus adults. The study relied on the pathology report given the retrospective nature of this [analysis, and it] was based on Australian and Dutch populations, which may limit its scope in other countries.”
Dr. El Sharouni was supported by a research fellowship grant from the European Academy of Dermatology and Venereology (EADV), while two of her coauthors, Richard A. Scolyer, MD, and John F. Thompson, MD, were recipients of an Australian National Health and Medical Research Council Program Grant. The study was also supported by a research program grant from Cancer Institute New South Wales. Dr. Thiede reported having no financial disclosures.
.
“Cutaneous melanomas are rare in children and much less common in adolescents than in later life,” researchers led by Mary-Ann El Sharouni, PhD, wrote in the study, which was published online in the Journal of the American Academy of Dermatology. “Management of these young patients currently follows guidelines developed for adults. Better understanding of melanoma occurring in the first 2 decades of life is, therefore, warranted.”
Drawing from two datasets – one from the Netherlands and the other from Melanoma Institute Australia (MIA) at the University of Sydney – Dr. El Sharouni of the MIA and of the department of dermatology at University Medical Center Utrecht in the Netherlands, and colleagues, evaluated all patients younger than 20 years of age who were diagnosed with invasive melanoma between January 2000 and December 2014. The pooled cohort included 397 Dutch and 117 Australian individuals. Of these, 62 were children and 452 were adolescents. To determine melanoma subtypes, the researchers reevaluated pathology reports and used multivariate Cox models to calculate recurrence-free survival (RFS) and overall survival (OS).
The median Breslow thickness was 2.7 mm in children and 1.0 mm in adolescents. Most patients (83%) had conventional melanoma, which consisted of superficial spreading, nodular, desmoplastic, and acral lentiginous forms, while 78 had spitzoid melanoma and 8 had melanoma associated with a congenital nevus. The 10-year RFS was 91.5% in children and 86.4% in adolescents (P =.32), while the 10-year OS was 100% in children and 92.7% in adolescents (P = .09).
On multivariable analysis, which was possible only for the adolescent cohort because of the small number of children, ulceration status and anatomic site were associated with RFS and OS, whereas age, sex, mitotic index, sentinel node status, and melanoma subtype were not. Breslow thickness > 4 mm was associated with worse RFS. As for affected anatomic site, those with melanomas located on the upper and lower limbs had a better overall RFS and OS compared with those who had head or neck melanomas.
The authors acknowledged certain limitation of the analysis, including its retrospective design and the small number of children. “Our data suggest that adolescent melanomas are often similar to adult-type melanomas, whilst those which occur in young children frequently occur via different molecular mechanisms,” they concluded. “In the future it is likely that further understanding of these molecular mechanisms and ability to classify melanomas based on their molecular characteristics will assist in further refining prognostic estimates and possible guiding treatment for young patients with melanoma.”
Rebecca M. Thiede, MD, assistant program director of the division of dermatology at the University of Arizona, Tucson, who was asked to comment on the study, said that the analysis “greatly contributes to dermatology, as we are still learning the differences between melanoma in children and adolescents versus adults.
This study found that adolescents with melanoma had worse survival if mitosis were present and/or located on head/neck, which could aid in aggressiveness of treatment.”
A key strength of analysis, she continued, is the large sample size of 514 patients, “given that melanoma in this population is very rare. A limitation which [the researchers] brought up is the discrepancy of diagnosis via histopathology of melanoma in children versus adults. The study relied on the pathology report given the retrospective nature of this [analysis, and it] was based on Australian and Dutch populations, which may limit its scope in other countries.”
Dr. El Sharouni was supported by a research fellowship grant from the European Academy of Dermatology and Venereology (EADV), while two of her coauthors, Richard A. Scolyer, MD, and John F. Thompson, MD, were recipients of an Australian National Health and Medical Research Council Program Grant. The study was also supported by a research program grant from Cancer Institute New South Wales. Dr. Thiede reported having no financial disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY