User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
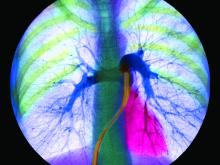
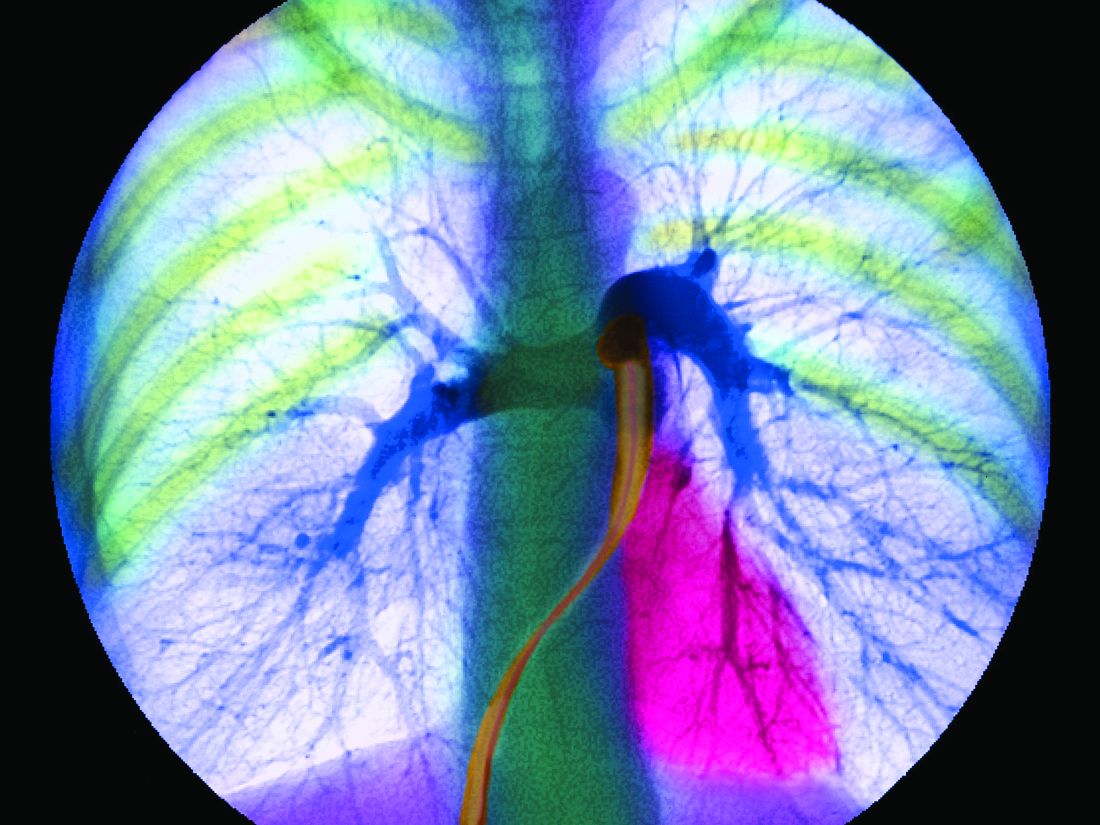
Exercise PH poised for comeback as new definition takes hold
Patients with a pulmonary artery pressure/cardiac output slope greater than 3 mm Hg/L/min on cardiopulmonary exercise tests have more than double the risk of cardiovascular hospitalization and all-cause mortality, according to a prospective study of 714 subjects with exertional dyspnea but preserved ejection fractions.
The findings “suggest that across a wide range of individuals with chronic dyspnea, exercise can unmask abnormal pulmonary vascular responses that in turn bear significant clinical implications. These findings, coupled with a growing body of work ... suggest that reintroduction of an exercise based definition of [pulmonary hypertension (PH)] in PH guidelines” – using the pulmonary artery pressure/cardiac output slope – “merits consideration,” wrote Jennifer Ho, MD, a heart failure and transplantation cardiologist at Massachusetts General Hospital, Boston, and colleagues (J Am Coll Cardiol. 2020 Jan 7;75[1]:17-26. doi: 10.1016/j.jacc.2019.10.048).
A new definition takes hold
The slope captures the steepness of pulmonary artery pressure increase as cardiac output goes up, giving a measure of overall pulmonary resistance. A value above 3 mm Hg/L/min means that pulmonary artery pressure (PAP) is too high for a given cardiac output (CO). The slope “is preferable to using a single absolute cut point value for exercise PAP” to define exercise pulmonary hypertension.“ Indeed, we confirm that in the absence of elevated PAP/CO, an absolute exercise PAP [above] 30 mm Hg” – the definition of exercise-induced pulmonary hypertension in years past – “does not portend worse outcomes,” Dr. Ho and her team noted.
In an accompanying editorial titled, “Exercise Pulmonary Hypertension Is Back,” Marius Hoeper, MD, a senior physician in the department of respiratory medicine at Hannover (Germany) Medical School, explained that the findings likely signal the revival of exercise pulmonary hypertension as a useful clinical concept (J Am Coll Cardiol. 2020 Jan 7;75[1]:27-8. doi: 10.1016/j.jacc.2019.11.010).
The standalone 30 mm Hg cut point was largely abandoned about a decade ago when it was realized that pressures above that mark were “not necessarily abnormal in certain subjects, for instance in athletes or elderly individuals,” he said.
But it’s become clear in recent years, and now confirmed by Dr. Ho and her team, that what matters is not the stand-alone measurement, but it’s relationship to cardiac output. “There is now sufficient evidence to define exercise PH by an abnormal [mean]PAP/CO slope [above] 3 mm Hg/L/min,” Dr. Hoeper said.
Abnormal slopes in over 40%
Each subject in the Massachusetts General study had an average of 10 paired PAP and CO measurements taken by invasive hemodynamic monitoring, including pulmonary artery catheterization via the internal jugular vein, while they road a stationary bicycle. The measurements were used to calculate the PAP/CO slope. A slope greater than 3 mm Hg/L/min was defined as abnormal based on previous research.
Results of the one-time assessment were correlated with the study’s primary outcome – cardiovascular hospitalization or all-cause death – over a mean follow up of 3.7 years. Subjects were 57 years old, on average, and 59% were women; just 2% had a previous diagnosis of pulmonary hypertension. Overall, 41% of the subjects had abnormal PAP/CO slopes, 26% had abnormal slopes without resting pulmonary hypertension, and 208 subjects (29%) met the primary outcome.
After adjustments for age, sex, and cardiopulmonary comorbidities, abnormal slopes more than doubled the risk of the primary outcome (hazard ratio [HR] 2.03; 95% confidence interval [CI]: 1.48-2.78; P less than .001). The risk remained elevated even in the absence of resting pulmonary hypertension (HR 1.75, 95% CI 1.21-2.54, P = .003), and in people with only mildly elevated resting PAPs of 21-29 mm Hg.
Older people were more likely to have abnormally elevated slopes, as well as were those with cardiopulmonary comorbidities, lower exercise tolerance, lower peak oxygen uptake, and more severely impaired right ventricular function. Diabetes, prior heart failure, chronic obstructive pulmonary disease, and interstitial lung disease were more prevalent in the elevated slope group, and their median N-terminal pro–B type natriuretic peptide level was 154 pg/mL, versus 52 pg/mL among people with normal slopes.
A simpler test is needed
In his editorial, Dr. Hoeper noted that diagnosing exercise PH by elevated slope “will occasionally help physicians and patients to better understand exertional dyspnea and to detect early pulmonary vascular disease in patients at risk,” but for the most part, the new definition “will have little immediate [effect] on clinical practice, as evidence-based treatments for this condition are not yet available.”
Even so, “having a globally accepted gold standard” for exercise PH based on the PAP/CO slope might well spur development of “simpler, noninvasive” ways to measure it so it can be used outside of specialty settings.
Dr. Ho and her team agreed. “These findings should prompt additional work using less invasive measurement modalities such as exercise echocardiography to evaluate” exercise PAP/CO slopes, they said.
The work was funded by the National Institutes of Health, Gilead Sciences, the American Heart Association, and the Massachusetts General Hospital Heart Failure Research Innovation Fund. The investigators had no relevant disclosures. Dr. Hoeper reported lecture and consultation fees from Actelion, Bayer, Merck Sharp and Dohme, and Pfizer.
SOURCE: Ho JE et al., J Am Coll Cardiol. 2020 Jan 7;75(1):17-26. doi: 10.1016/j.jacc.2019.10.048.
Patients with a pulmonary artery pressure/cardiac output slope greater than 3 mm Hg/L/min on cardiopulmonary exercise tests have more than double the risk of cardiovascular hospitalization and all-cause mortality, according to a prospective study of 714 subjects with exertional dyspnea but preserved ejection fractions.
The findings “suggest that across a wide range of individuals with chronic dyspnea, exercise can unmask abnormal pulmonary vascular responses that in turn bear significant clinical implications. These findings, coupled with a growing body of work ... suggest that reintroduction of an exercise based definition of [pulmonary hypertension (PH)] in PH guidelines” – using the pulmonary artery pressure/cardiac output slope – “merits consideration,” wrote Jennifer Ho, MD, a heart failure and transplantation cardiologist at Massachusetts General Hospital, Boston, and colleagues (J Am Coll Cardiol. 2020 Jan 7;75[1]:17-26. doi: 10.1016/j.jacc.2019.10.048).
A new definition takes hold
The slope captures the steepness of pulmonary artery pressure increase as cardiac output goes up, giving a measure of overall pulmonary resistance. A value above 3 mm Hg/L/min means that pulmonary artery pressure (PAP) is too high for a given cardiac output (CO). The slope “is preferable to using a single absolute cut point value for exercise PAP” to define exercise pulmonary hypertension.“ Indeed, we confirm that in the absence of elevated PAP/CO, an absolute exercise PAP [above] 30 mm Hg” – the definition of exercise-induced pulmonary hypertension in years past – “does not portend worse outcomes,” Dr. Ho and her team noted.
In an accompanying editorial titled, “Exercise Pulmonary Hypertension Is Back,” Marius Hoeper, MD, a senior physician in the department of respiratory medicine at Hannover (Germany) Medical School, explained that the findings likely signal the revival of exercise pulmonary hypertension as a useful clinical concept (J Am Coll Cardiol. 2020 Jan 7;75[1]:27-8. doi: 10.1016/j.jacc.2019.11.010).
The standalone 30 mm Hg cut point was largely abandoned about a decade ago when it was realized that pressures above that mark were “not necessarily abnormal in certain subjects, for instance in athletes or elderly individuals,” he said.
But it’s become clear in recent years, and now confirmed by Dr. Ho and her team, that what matters is not the stand-alone measurement, but it’s relationship to cardiac output. “There is now sufficient evidence to define exercise PH by an abnormal [mean]PAP/CO slope [above] 3 mm Hg/L/min,” Dr. Hoeper said.
Abnormal slopes in over 40%
Each subject in the Massachusetts General study had an average of 10 paired PAP and CO measurements taken by invasive hemodynamic monitoring, including pulmonary artery catheterization via the internal jugular vein, while they road a stationary bicycle. The measurements were used to calculate the PAP/CO slope. A slope greater than 3 mm Hg/L/min was defined as abnormal based on previous research.
Results of the one-time assessment were correlated with the study’s primary outcome – cardiovascular hospitalization or all-cause death – over a mean follow up of 3.7 years. Subjects were 57 years old, on average, and 59% were women; just 2% had a previous diagnosis of pulmonary hypertension. Overall, 41% of the subjects had abnormal PAP/CO slopes, 26% had abnormal slopes without resting pulmonary hypertension, and 208 subjects (29%) met the primary outcome.
After adjustments for age, sex, and cardiopulmonary comorbidities, abnormal slopes more than doubled the risk of the primary outcome (hazard ratio [HR] 2.03; 95% confidence interval [CI]: 1.48-2.78; P less than .001). The risk remained elevated even in the absence of resting pulmonary hypertension (HR 1.75, 95% CI 1.21-2.54, P = .003), and in people with only mildly elevated resting PAPs of 21-29 mm Hg.
Older people were more likely to have abnormally elevated slopes, as well as were those with cardiopulmonary comorbidities, lower exercise tolerance, lower peak oxygen uptake, and more severely impaired right ventricular function. Diabetes, prior heart failure, chronic obstructive pulmonary disease, and interstitial lung disease were more prevalent in the elevated slope group, and their median N-terminal pro–B type natriuretic peptide level was 154 pg/mL, versus 52 pg/mL among people with normal slopes.
A simpler test is needed
In his editorial, Dr. Hoeper noted that diagnosing exercise PH by elevated slope “will occasionally help physicians and patients to better understand exertional dyspnea and to detect early pulmonary vascular disease in patients at risk,” but for the most part, the new definition “will have little immediate [effect] on clinical practice, as evidence-based treatments for this condition are not yet available.”
Even so, “having a globally accepted gold standard” for exercise PH based on the PAP/CO slope might well spur development of “simpler, noninvasive” ways to measure it so it can be used outside of specialty settings.
Dr. Ho and her team agreed. “These findings should prompt additional work using less invasive measurement modalities such as exercise echocardiography to evaluate” exercise PAP/CO slopes, they said.
The work was funded by the National Institutes of Health, Gilead Sciences, the American Heart Association, and the Massachusetts General Hospital Heart Failure Research Innovation Fund. The investigators had no relevant disclosures. Dr. Hoeper reported lecture and consultation fees from Actelion, Bayer, Merck Sharp and Dohme, and Pfizer.
SOURCE: Ho JE et al., J Am Coll Cardiol. 2020 Jan 7;75(1):17-26. doi: 10.1016/j.jacc.2019.10.048.
Patients with a pulmonary artery pressure/cardiac output slope greater than 3 mm Hg/L/min on cardiopulmonary exercise tests have more than double the risk of cardiovascular hospitalization and all-cause mortality, according to a prospective study of 714 subjects with exertional dyspnea but preserved ejection fractions.
The findings “suggest that across a wide range of individuals with chronic dyspnea, exercise can unmask abnormal pulmonary vascular responses that in turn bear significant clinical implications. These findings, coupled with a growing body of work ... suggest that reintroduction of an exercise based definition of [pulmonary hypertension (PH)] in PH guidelines” – using the pulmonary artery pressure/cardiac output slope – “merits consideration,” wrote Jennifer Ho, MD, a heart failure and transplantation cardiologist at Massachusetts General Hospital, Boston, and colleagues (J Am Coll Cardiol. 2020 Jan 7;75[1]:17-26. doi: 10.1016/j.jacc.2019.10.048).
A new definition takes hold
The slope captures the steepness of pulmonary artery pressure increase as cardiac output goes up, giving a measure of overall pulmonary resistance. A value above 3 mm Hg/L/min means that pulmonary artery pressure (PAP) is too high for a given cardiac output (CO). The slope “is preferable to using a single absolute cut point value for exercise PAP” to define exercise pulmonary hypertension.“ Indeed, we confirm that in the absence of elevated PAP/CO, an absolute exercise PAP [above] 30 mm Hg” – the definition of exercise-induced pulmonary hypertension in years past – “does not portend worse outcomes,” Dr. Ho and her team noted.
In an accompanying editorial titled, “Exercise Pulmonary Hypertension Is Back,” Marius Hoeper, MD, a senior physician in the department of respiratory medicine at Hannover (Germany) Medical School, explained that the findings likely signal the revival of exercise pulmonary hypertension as a useful clinical concept (J Am Coll Cardiol. 2020 Jan 7;75[1]:27-8. doi: 10.1016/j.jacc.2019.11.010).
The standalone 30 mm Hg cut point was largely abandoned about a decade ago when it was realized that pressures above that mark were “not necessarily abnormal in certain subjects, for instance in athletes or elderly individuals,” he said.
But it’s become clear in recent years, and now confirmed by Dr. Ho and her team, that what matters is not the stand-alone measurement, but it’s relationship to cardiac output. “There is now sufficient evidence to define exercise PH by an abnormal [mean]PAP/CO slope [above] 3 mm Hg/L/min,” Dr. Hoeper said.
Abnormal slopes in over 40%
Each subject in the Massachusetts General study had an average of 10 paired PAP and CO measurements taken by invasive hemodynamic monitoring, including pulmonary artery catheterization via the internal jugular vein, while they road a stationary bicycle. The measurements were used to calculate the PAP/CO slope. A slope greater than 3 mm Hg/L/min was defined as abnormal based on previous research.
Results of the one-time assessment were correlated with the study’s primary outcome – cardiovascular hospitalization or all-cause death – over a mean follow up of 3.7 years. Subjects were 57 years old, on average, and 59% were women; just 2% had a previous diagnosis of pulmonary hypertension. Overall, 41% of the subjects had abnormal PAP/CO slopes, 26% had abnormal slopes without resting pulmonary hypertension, and 208 subjects (29%) met the primary outcome.
After adjustments for age, sex, and cardiopulmonary comorbidities, abnormal slopes more than doubled the risk of the primary outcome (hazard ratio [HR] 2.03; 95% confidence interval [CI]: 1.48-2.78; P less than .001). The risk remained elevated even in the absence of resting pulmonary hypertension (HR 1.75, 95% CI 1.21-2.54, P = .003), and in people with only mildly elevated resting PAPs of 21-29 mm Hg.
Older people were more likely to have abnormally elevated slopes, as well as were those with cardiopulmonary comorbidities, lower exercise tolerance, lower peak oxygen uptake, and more severely impaired right ventricular function. Diabetes, prior heart failure, chronic obstructive pulmonary disease, and interstitial lung disease were more prevalent in the elevated slope group, and their median N-terminal pro–B type natriuretic peptide level was 154 pg/mL, versus 52 pg/mL among people with normal slopes.
A simpler test is needed
In his editorial, Dr. Hoeper noted that diagnosing exercise PH by elevated slope “will occasionally help physicians and patients to better understand exertional dyspnea and to detect early pulmonary vascular disease in patients at risk,” but for the most part, the new definition “will have little immediate [effect] on clinical practice, as evidence-based treatments for this condition are not yet available.”
Even so, “having a globally accepted gold standard” for exercise PH based on the PAP/CO slope might well spur development of “simpler, noninvasive” ways to measure it so it can be used outside of specialty settings.
Dr. Ho and her team agreed. “These findings should prompt additional work using less invasive measurement modalities such as exercise echocardiography to evaluate” exercise PAP/CO slopes, they said.
The work was funded by the National Institutes of Health, Gilead Sciences, the American Heart Association, and the Massachusetts General Hospital Heart Failure Research Innovation Fund. The investigators had no relevant disclosures. Dr. Hoeper reported lecture and consultation fees from Actelion, Bayer, Merck Sharp and Dohme, and Pfizer.
SOURCE: Ho JE et al., J Am Coll Cardiol. 2020 Jan 7;75(1):17-26. doi: 10.1016/j.jacc.2019.10.048.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Cardiologists’ happiness average both in and outside the office
Compared with their colleagues, cardiologists are in the middle when it comes to happiness both in and outside the workplace, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

About 28% of cardiologists reported that they were very happy in the workplace, according to the Medscape report, with dermatologists taking the top spot at 41%; 51% of cardiologists said that they were very happy outside of work, 9 percentage points behind rheumatologists at 60%.
The burnout rate for cardiologists was 29%, well below the 41% average for all physicians. About 15% of cardiologists reported that they were both burned out and depressed. An overabundance of bureaucratic tasks was the most commonly reported contributing factor to burnout at 64%, followed by spending too many hours at work at 39% and increased usage of EHRs at 33%.
Cardiologists most commonly dealt with burnout by exercising (45%), talking with friends/family (43%), and isolating themselves from others (42%). In addition, 47% of cardiologists reported taking 3-4 weeks of vacation, slightly more than the 44% average for all physicians; only 29% said they had taken less than 3 weeks’ vacation.
About 10% of cardiologists said that they’d contemplated suicide and 1% reported that they’d attempted it; 83% reported that they’d never thought about suicide. Only 10% of cardiologists reported that they were either currently seeking help or were planning to seek professional help for symptoms of burnout and/or depression, while 76% said they had no plans to consult help and had not done so in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
Compared with their colleagues, cardiologists are in the middle when it comes to happiness both in and outside the workplace, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

About 28% of cardiologists reported that they were very happy in the workplace, according to the Medscape report, with dermatologists taking the top spot at 41%; 51% of cardiologists said that they were very happy outside of work, 9 percentage points behind rheumatologists at 60%.
The burnout rate for cardiologists was 29%, well below the 41% average for all physicians. About 15% of cardiologists reported that they were both burned out and depressed. An overabundance of bureaucratic tasks was the most commonly reported contributing factor to burnout at 64%, followed by spending too many hours at work at 39% and increased usage of EHRs at 33%.
Cardiologists most commonly dealt with burnout by exercising (45%), talking with friends/family (43%), and isolating themselves from others (42%). In addition, 47% of cardiologists reported taking 3-4 weeks of vacation, slightly more than the 44% average for all physicians; only 29% said they had taken less than 3 weeks’ vacation.
About 10% of cardiologists said that they’d contemplated suicide and 1% reported that they’d attempted it; 83% reported that they’d never thought about suicide. Only 10% of cardiologists reported that they were either currently seeking help or were planning to seek professional help for symptoms of burnout and/or depression, while 76% said they had no plans to consult help and had not done so in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
Compared with their colleagues, cardiologists are in the middle when it comes to happiness both in and outside the workplace, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

About 28% of cardiologists reported that they were very happy in the workplace, according to the Medscape report, with dermatologists taking the top spot at 41%; 51% of cardiologists said that they were very happy outside of work, 9 percentage points behind rheumatologists at 60%.
The burnout rate for cardiologists was 29%, well below the 41% average for all physicians. About 15% of cardiologists reported that they were both burned out and depressed. An overabundance of bureaucratic tasks was the most commonly reported contributing factor to burnout at 64%, followed by spending too many hours at work at 39% and increased usage of EHRs at 33%.
Cardiologists most commonly dealt with burnout by exercising (45%), talking with friends/family (43%), and isolating themselves from others (42%). In addition, 47% of cardiologists reported taking 3-4 weeks of vacation, slightly more than the 44% average for all physicians; only 29% said they had taken less than 3 weeks’ vacation.
About 10% of cardiologists said that they’d contemplated suicide and 1% reported that they’d attempted it; 83% reported that they’d never thought about suicide. Only 10% of cardiologists reported that they were either currently seeking help or were planning to seek professional help for symptoms of burnout and/or depression, while 76% said they had no plans to consult help and had not done so in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
Tools for preventing heart failure
SNOWMASS, COLO. – If ever there was a major chronic disease that’s teed up and ready to be stamped into submission through diligent application of preventive medicine, it’s the epidemic of heart failure.
“The best way to treat heart failure is to prevent it in the first place. There will be more than 1 million new cases of heart failure this year, and the vast majority of them could have been prevented,” Gregg C. Fonarow, MD, asserted at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Using firmly evidence-based, guideline-directed therapies, it’s often possible to prevent patients at high risk for developing heart failure (HF) from actually doing so. Or, in the terminology of the ACC/American Heart Association heart failure guidelines coauthored by Dr. Fonarow, the goal is to keep patients who are stage A – that is, pre-HF but at high risk because of hypertension, coronary artery disease, diabetes, family history of cardiomyopathy, or other reasons – from progressing to stage B, marked by asymptomatic left ventricular dysfunction, a prior MI, or asymptomatic valvular disease; and blocking those who are stage B from then moving on to stage C, the classic symptomatic form of HF; and thence to end-stage stage D disease.
Heart failure is an enormous public health problem, and one of the most expensive of all diseases. The prognostic impact of newly diagnosed HF is profound, with 10-15 years of life lost, compared with the general population. Even today, roughly one in five newly diagnosed patients won’t survive for a year, and the 5-year mortality is about 50%, said Dr. Fonarow, who is professor of cardiovascular medicine and chief of the division of cardiology at the University of California, Los Angeles, and director of the Ahmanson-UCLA Cardiomyopathy Center, also in Los Angeles.
Symptomatic stage C is “the tip of the iceberg,” the cardiologist stressed. Vastly more patients are in stages A and B. In order to keep them from progressing to stage C, it’s first necessary to identify them. That’s why the 2013 guidelines give a class IC recommendation for periodic evaluation for signs and symptoms of HF in patients who are at high risk, and for a noninvasive assessment of left ventricular ejection fraction in those with a strong family history of cardiomyopathy or who are on cardiotoxic drugs (J Am Coll Cardiol. 2013 Oct 15;62[16]:e147-239).
The two biggest risk factors for the development of symptomatic stage C HF are hypertension and atherosclerotic cardiovascular disease. Close to 80% of patients presenting with heart failure have prevalent hypertension, and a history of ischemic heart disease is nearly as common.
Other major modifiable risk factors are diabetes, overweight and obesity, metabolic syndrome, dyslipidemia, smoking, valvular heart disease, and chronic kidney disease.
Hypertension
Most patients with high blood pressure believe they’re on antihypertensive medication to prevent MI and stroke, but in reality the largest benefit is what Dr. Fonarow termed the “phenomenal” reduction in the risk of developing HF, which amounted to a 52% relative risk reduction in one meta-analysis of older randomized trials. In the contemporary era, the landmark SPRINT trial of close to 10,000 randomized hypertensive patients showed that more-intensive blood pressure lowering to a target systolic BP of less than 120 mm Hg resulted in a 38% reduction in the risk of new-onset HF, compared with standard treatment to a target of less than 140 mm Hg. That’s why the 2017 focused update of the HF guidelines gives a strong class IB recommendation for a target blood pressure of less than 130/80 mm Hg in hypertensive patients with stage A HF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803).
Atherosclerotic cardiovascular disease
Within 6 years after diagnosis of an MI, 22% of men and 46% of women will develop symptomatic heart failure. Intensive statin therapy gets a strong recommendation post MI in the guidelines, not only because in a meta-analysis of four major randomized trials it resulted in a further 64% reduction in the risk of coronary death or recurrent MI, compared with moderate statin therapy, but also because of the 27% relative risk reduction in new-onset HF. ACE inhibitors get a class IA recommendation for prevention of symptomatic HF in patients who are stage A with a history of atherosclerotic disease, diabetes, or hypertension. Angiotensin receptor blockers get a class IC recommendation.
Diabetes
Diabetes markedly increases the risk of developing HF: by two to four times overall and by four to eight times in younger diabetes patients. The two chronic diseases are highly comorbid, with roughly 45% of patients with HF also having diabetes. Moreover, diabetes in HF patients is associated with a substantially worse prognosis, even when standard HF therapies are applied.
Choices regarding glycemic management can markedly affect HF risk and outcomes. Randomized trials show that the peroxisome proliferator-activated receptor agonists double the risk of HF. The glucagonlike peptide–1 receptor agonists are absolutely neutral with regard to HF outcomes. Similarly, the dipeptidyl peptidase–4 inhibitors have no impact on the risks of major adverse cardiovascular events or HF. Intensive glycemic control has no impact on the risk of new-onset HF. Insulin therapy, too, is neutral on this score.
“Depressingly, even lifestyle modification with weight loss, once you have type 2 diabetes, does not lower the risk,” Dr. Fonarow continued.
In contrast, the sodium-glucose transporter 2 (SGLT2) inhibitors have impressive cardiovascular and renal protective benefits in patients with type 2 diabetes, as demonstrated in a meta-analysis of more than 34,000 participants in the randomized trials of empagliflozin (Jardiance) in EMPA-REG OUTCOME, canagliflozin (Invokana) in CANVAS/CANVAS-R, and dapagliflozin (Farxiga) in DECLARE-TIMI 58. The SGLT2 inhibitors collectively reduced the risk of HF hospitalization by 21% in participants with no baseline history of the disease and by 29% in those with a history of HF. Moreover, the risk of progression of renal disease was reduced by 45% (Lancet. 2019 Jan 5;393[10166]:31-9).
More recently, the landmark DAPA-HF trial established SGLT2 inhibitor therapy as part of standard-of-care, guideline-directed medical therapy for patients with HF with reduced ejection fraction regardless of whether they have comorbid type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
These are remarkable medications, generally very well tolerated, and it’s critical that cardiologists get on board in prescribing them, Dr. Fonarow emphasized. He alerted his colleagues to what he called an “incredibly helpful” review article that provides practical guidance for cardiologists in how to start using the SGLT2 inhibitors (JACC Heart Fail. 2019 Feb;7[2]:169-72).
“It’s pretty straightforward,” according to Dr. Fonarow. “If you’re comfortable enough in using ACE inhibitors, angiotensin receptor blockers, and beta-blockers, I think you’ll find these medications fit similarly when you actually get experience in utilizing them.”
He reported serving as a consultant to 10 pharmaceutical or medical device companies.
SNOWMASS, COLO. – If ever there was a major chronic disease that’s teed up and ready to be stamped into submission through diligent application of preventive medicine, it’s the epidemic of heart failure.
“The best way to treat heart failure is to prevent it in the first place. There will be more than 1 million new cases of heart failure this year, and the vast majority of them could have been prevented,” Gregg C. Fonarow, MD, asserted at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Using firmly evidence-based, guideline-directed therapies, it’s often possible to prevent patients at high risk for developing heart failure (HF) from actually doing so. Or, in the terminology of the ACC/American Heart Association heart failure guidelines coauthored by Dr. Fonarow, the goal is to keep patients who are stage A – that is, pre-HF but at high risk because of hypertension, coronary artery disease, diabetes, family history of cardiomyopathy, or other reasons – from progressing to stage B, marked by asymptomatic left ventricular dysfunction, a prior MI, or asymptomatic valvular disease; and blocking those who are stage B from then moving on to stage C, the classic symptomatic form of HF; and thence to end-stage stage D disease.
Heart failure is an enormous public health problem, and one of the most expensive of all diseases. The prognostic impact of newly diagnosed HF is profound, with 10-15 years of life lost, compared with the general population. Even today, roughly one in five newly diagnosed patients won’t survive for a year, and the 5-year mortality is about 50%, said Dr. Fonarow, who is professor of cardiovascular medicine and chief of the division of cardiology at the University of California, Los Angeles, and director of the Ahmanson-UCLA Cardiomyopathy Center, also in Los Angeles.
Symptomatic stage C is “the tip of the iceberg,” the cardiologist stressed. Vastly more patients are in stages A and B. In order to keep them from progressing to stage C, it’s first necessary to identify them. That’s why the 2013 guidelines give a class IC recommendation for periodic evaluation for signs and symptoms of HF in patients who are at high risk, and for a noninvasive assessment of left ventricular ejection fraction in those with a strong family history of cardiomyopathy or who are on cardiotoxic drugs (J Am Coll Cardiol. 2013 Oct 15;62[16]:e147-239).
The two biggest risk factors for the development of symptomatic stage C HF are hypertension and atherosclerotic cardiovascular disease. Close to 80% of patients presenting with heart failure have prevalent hypertension, and a history of ischemic heart disease is nearly as common.
Other major modifiable risk factors are diabetes, overweight and obesity, metabolic syndrome, dyslipidemia, smoking, valvular heart disease, and chronic kidney disease.
Hypertension
Most patients with high blood pressure believe they’re on antihypertensive medication to prevent MI and stroke, but in reality the largest benefit is what Dr. Fonarow termed the “phenomenal” reduction in the risk of developing HF, which amounted to a 52% relative risk reduction in one meta-analysis of older randomized trials. In the contemporary era, the landmark SPRINT trial of close to 10,000 randomized hypertensive patients showed that more-intensive blood pressure lowering to a target systolic BP of less than 120 mm Hg resulted in a 38% reduction in the risk of new-onset HF, compared with standard treatment to a target of less than 140 mm Hg. That’s why the 2017 focused update of the HF guidelines gives a strong class IB recommendation for a target blood pressure of less than 130/80 mm Hg in hypertensive patients with stage A HF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803).
Atherosclerotic cardiovascular disease
Within 6 years after diagnosis of an MI, 22% of men and 46% of women will develop symptomatic heart failure. Intensive statin therapy gets a strong recommendation post MI in the guidelines, not only because in a meta-analysis of four major randomized trials it resulted in a further 64% reduction in the risk of coronary death or recurrent MI, compared with moderate statin therapy, but also because of the 27% relative risk reduction in new-onset HF. ACE inhibitors get a class IA recommendation for prevention of symptomatic HF in patients who are stage A with a history of atherosclerotic disease, diabetes, or hypertension. Angiotensin receptor blockers get a class IC recommendation.
Diabetes
Diabetes markedly increases the risk of developing HF: by two to four times overall and by four to eight times in younger diabetes patients. The two chronic diseases are highly comorbid, with roughly 45% of patients with HF also having diabetes. Moreover, diabetes in HF patients is associated with a substantially worse prognosis, even when standard HF therapies are applied.
Choices regarding glycemic management can markedly affect HF risk and outcomes. Randomized trials show that the peroxisome proliferator-activated receptor agonists double the risk of HF. The glucagonlike peptide–1 receptor agonists are absolutely neutral with regard to HF outcomes. Similarly, the dipeptidyl peptidase–4 inhibitors have no impact on the risks of major adverse cardiovascular events or HF. Intensive glycemic control has no impact on the risk of new-onset HF. Insulin therapy, too, is neutral on this score.
“Depressingly, even lifestyle modification with weight loss, once you have type 2 diabetes, does not lower the risk,” Dr. Fonarow continued.
In contrast, the sodium-glucose transporter 2 (SGLT2) inhibitors have impressive cardiovascular and renal protective benefits in patients with type 2 diabetes, as demonstrated in a meta-analysis of more than 34,000 participants in the randomized trials of empagliflozin (Jardiance) in EMPA-REG OUTCOME, canagliflozin (Invokana) in CANVAS/CANVAS-R, and dapagliflozin (Farxiga) in DECLARE-TIMI 58. The SGLT2 inhibitors collectively reduced the risk of HF hospitalization by 21% in participants with no baseline history of the disease and by 29% in those with a history of HF. Moreover, the risk of progression of renal disease was reduced by 45% (Lancet. 2019 Jan 5;393[10166]:31-9).
More recently, the landmark DAPA-HF trial established SGLT2 inhibitor therapy as part of standard-of-care, guideline-directed medical therapy for patients with HF with reduced ejection fraction regardless of whether they have comorbid type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
These are remarkable medications, generally very well tolerated, and it’s critical that cardiologists get on board in prescribing them, Dr. Fonarow emphasized. He alerted his colleagues to what he called an “incredibly helpful” review article that provides practical guidance for cardiologists in how to start using the SGLT2 inhibitors (JACC Heart Fail. 2019 Feb;7[2]:169-72).
“It’s pretty straightforward,” according to Dr. Fonarow. “If you’re comfortable enough in using ACE inhibitors, angiotensin receptor blockers, and beta-blockers, I think you’ll find these medications fit similarly when you actually get experience in utilizing them.”
He reported serving as a consultant to 10 pharmaceutical or medical device companies.
SNOWMASS, COLO. – If ever there was a major chronic disease that’s teed up and ready to be stamped into submission through diligent application of preventive medicine, it’s the epidemic of heart failure.
“The best way to treat heart failure is to prevent it in the first place. There will be more than 1 million new cases of heart failure this year, and the vast majority of them could have been prevented,” Gregg C. Fonarow, MD, asserted at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Using firmly evidence-based, guideline-directed therapies, it’s often possible to prevent patients at high risk for developing heart failure (HF) from actually doing so. Or, in the terminology of the ACC/American Heart Association heart failure guidelines coauthored by Dr. Fonarow, the goal is to keep patients who are stage A – that is, pre-HF but at high risk because of hypertension, coronary artery disease, diabetes, family history of cardiomyopathy, or other reasons – from progressing to stage B, marked by asymptomatic left ventricular dysfunction, a prior MI, or asymptomatic valvular disease; and blocking those who are stage B from then moving on to stage C, the classic symptomatic form of HF; and thence to end-stage stage D disease.
Heart failure is an enormous public health problem, and one of the most expensive of all diseases. The prognostic impact of newly diagnosed HF is profound, with 10-15 years of life lost, compared with the general population. Even today, roughly one in five newly diagnosed patients won’t survive for a year, and the 5-year mortality is about 50%, said Dr. Fonarow, who is professor of cardiovascular medicine and chief of the division of cardiology at the University of California, Los Angeles, and director of the Ahmanson-UCLA Cardiomyopathy Center, also in Los Angeles.
Symptomatic stage C is “the tip of the iceberg,” the cardiologist stressed. Vastly more patients are in stages A and B. In order to keep them from progressing to stage C, it’s first necessary to identify them. That’s why the 2013 guidelines give a class IC recommendation for periodic evaluation for signs and symptoms of HF in patients who are at high risk, and for a noninvasive assessment of left ventricular ejection fraction in those with a strong family history of cardiomyopathy or who are on cardiotoxic drugs (J Am Coll Cardiol. 2013 Oct 15;62[16]:e147-239).
The two biggest risk factors for the development of symptomatic stage C HF are hypertension and atherosclerotic cardiovascular disease. Close to 80% of patients presenting with heart failure have prevalent hypertension, and a history of ischemic heart disease is nearly as common.
Other major modifiable risk factors are diabetes, overweight and obesity, metabolic syndrome, dyslipidemia, smoking, valvular heart disease, and chronic kidney disease.
Hypertension
Most patients with high blood pressure believe they’re on antihypertensive medication to prevent MI and stroke, but in reality the largest benefit is what Dr. Fonarow termed the “phenomenal” reduction in the risk of developing HF, which amounted to a 52% relative risk reduction in one meta-analysis of older randomized trials. In the contemporary era, the landmark SPRINT trial of close to 10,000 randomized hypertensive patients showed that more-intensive blood pressure lowering to a target systolic BP of less than 120 mm Hg resulted in a 38% reduction in the risk of new-onset HF, compared with standard treatment to a target of less than 140 mm Hg. That’s why the 2017 focused update of the HF guidelines gives a strong class IB recommendation for a target blood pressure of less than 130/80 mm Hg in hypertensive patients with stage A HF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803).
Atherosclerotic cardiovascular disease
Within 6 years after diagnosis of an MI, 22% of men and 46% of women will develop symptomatic heart failure. Intensive statin therapy gets a strong recommendation post MI in the guidelines, not only because in a meta-analysis of four major randomized trials it resulted in a further 64% reduction in the risk of coronary death or recurrent MI, compared with moderate statin therapy, but also because of the 27% relative risk reduction in new-onset HF. ACE inhibitors get a class IA recommendation for prevention of symptomatic HF in patients who are stage A with a history of atherosclerotic disease, diabetes, or hypertension. Angiotensin receptor blockers get a class IC recommendation.
Diabetes
Diabetes markedly increases the risk of developing HF: by two to four times overall and by four to eight times in younger diabetes patients. The two chronic diseases are highly comorbid, with roughly 45% of patients with HF also having diabetes. Moreover, diabetes in HF patients is associated with a substantially worse prognosis, even when standard HF therapies are applied.
Choices regarding glycemic management can markedly affect HF risk and outcomes. Randomized trials show that the peroxisome proliferator-activated receptor agonists double the risk of HF. The glucagonlike peptide–1 receptor agonists are absolutely neutral with regard to HF outcomes. Similarly, the dipeptidyl peptidase–4 inhibitors have no impact on the risks of major adverse cardiovascular events or HF. Intensive glycemic control has no impact on the risk of new-onset HF. Insulin therapy, too, is neutral on this score.
“Depressingly, even lifestyle modification with weight loss, once you have type 2 diabetes, does not lower the risk,” Dr. Fonarow continued.
In contrast, the sodium-glucose transporter 2 (SGLT2) inhibitors have impressive cardiovascular and renal protective benefits in patients with type 2 diabetes, as demonstrated in a meta-analysis of more than 34,000 participants in the randomized trials of empagliflozin (Jardiance) in EMPA-REG OUTCOME, canagliflozin (Invokana) in CANVAS/CANVAS-R, and dapagliflozin (Farxiga) in DECLARE-TIMI 58. The SGLT2 inhibitors collectively reduced the risk of HF hospitalization by 21% in participants with no baseline history of the disease and by 29% in those with a history of HF. Moreover, the risk of progression of renal disease was reduced by 45% (Lancet. 2019 Jan 5;393[10166]:31-9).
More recently, the landmark DAPA-HF trial established SGLT2 inhibitor therapy as part of standard-of-care, guideline-directed medical therapy for patients with HF with reduced ejection fraction regardless of whether they have comorbid type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
These are remarkable medications, generally very well tolerated, and it’s critical that cardiologists get on board in prescribing them, Dr. Fonarow emphasized. He alerted his colleagues to what he called an “incredibly helpful” review article that provides practical guidance for cardiologists in how to start using the SGLT2 inhibitors (JACC Heart Fail. 2019 Feb;7[2]:169-72).
“It’s pretty straightforward,” according to Dr. Fonarow. “If you’re comfortable enough in using ACE inhibitors, angiotensin receptor blockers, and beta-blockers, I think you’ll find these medications fit similarly when you actually get experience in utilizing them.”
He reported serving as a consultant to 10 pharmaceutical or medical device companies.
EXPERT ANALYSIS FROM ACC SNOWMASS 2020
Thrombectomy access lags for U.S. stroke patients
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
FROM STROKE
More conflicting evidence on paclitaxel devices in PAD
The controversy regarding the safety of treating peripheral artery disease (PAD) with paclitaxel-coated devices has only deepened in the new year, with two recent studies suggesting opposite safety findings.
The debate began with a 2018 meta-analysis showing a late mortality signal associated with paclitaxel drug-coated balloons (DCBs) that sent reverberations through the interventional cardiology community (J Am Heart Assoc. 2018 Dec 18;7[24]:e011245).
Now, in a new meta-analysis involving eight randomized controlled trials (RCTs) and more than 1,400 patients with critical limb ischemia (CLI), the same researchers found significantly more early amputations and deaths in those treated with DCB below the knee, compared with conventional balloon angioplasty.
“The findings of our latest report add to previous evidence underpinning major safety concerns around use of paclitaxel in lower limb angioplasties – increased long-term patient mortality in cases of intermittent claudication,” lead author Konstantinos Katsanos MD, MSc, PhD, Patras University Hospital, Greece, said in an interview.
By contrast, a retrospective study of insurance claims in Germany showed no heightened mortality with paclitaxel-coated balloons and stents, compared with uncoated devices, in close to 38,000 patients with PAD.
On the contrary, use of paclitaxel-coated devices was associated with higher long-term survival, better amputation-free survival (AFS), and lower rates of major cardiovascular events in the treatment of chronic limb-threatening ischemia (CLTI).
These findings “emphasize the difference between population-based evidence and randomized trials,” lead author Christian-Alexander Behrendt, MD, University Medical Center Hamburg-Eppendorf, Germany, said in an interview.
Downstream “showers”
In the new meta-analysis led by Dr. Katsanos, published online Jan. 15, the 1,420 patients were treated with five different DCBs and 97% had CLI (J Vasc Intervent Radiol 2020 Feb;31[2]:202-12).
In up to 1-year follow-up, the paclitaxel DCB group had fewer target lesion revascularizations (TLR) than those of the uncoated device group (11.8% vs. 25.6%; risk ratio, 0.53; 95% confidence interval, 0.35-0.81) but worse AFS (13.7% vs. 9.4%; hazard ratio [HR], 1.52; 95% CI, 1.12-2.07).
The latter finding was driven by nonsignificant increased risks for all-cause death (odds ratio [OR], 1.39; 95% CI, 0.94-2.07) and major amputations (OR, 1.63; 95% CI, 0.92-2.90).
In dose-subgroup analyses, AFS was significantly worse in cases with high-dose (3.0-3.5 mcg/mm2) devices, but not in the single trial with a low-dose DCB (2.0 mcg/mm2).
“Considering the well-described downstream ‘showers’ of paclitaxel particles with current drug-coated balloons, we hypothesize that nontarget paclitaxel embolization is a plausible mechanism for distal foot and systemic toxicity,” Dr. Katsanos said.
Short time frame
Eric Secemsky, MD, of Harvard Medical School, and director of vascular intervention at Beth Israel Deaconess Medical Center, Boston, suggested in an interview that this theorized mechanism of harm in below-the-knee procedures could potentially shed light on a similar mechanism at play in above-the-knee procedures.
“We didn’t understand why people could potentially be dying in above-the-knee [procedures], and the suggestion here is that these devices might perhaps be causing particular embolization or maybe delayed wound healing,” Dr. Secemsky speculated.
However, “I don’t know that this is true, so I am cautious to say this is true,” he emphasized.
Dr. Secemsky said a strength of the Katsanos analysis is that the RCTs included more than 1,000 patients, but noted that it is hard to vet the quality and rigor of the data, as some of the studies have not yet been published. He also noted that paclitaxel-coated devices are not approved by the Food and Drug Administration in the United States for below-the-knee procedures.
Moreover, he continued, “two studies were driving the signal of harm: the IN.PACT DEEP, which included an iteration of their DCB that is no longer being tested; and the unpublished SINGA-PACLI trial. Those studies contributed most of the adverse events seen in this meta-analysis.”
In addition, the trials had different lengths of follow-up (6-12 months), he said. “Thus, the five trials with data available to 12 months are driving the 1-year findings, whereas three RCTs, including the primary RCT showing safety [Lutonix-BTK trial], only contribute data to 6 months.”
For this reason, “we are not too excited about this meta-analysis as of now, [because] all it tells us is that we need more data to support the safety of drug-coated devices in this population,” Dr. Secemsky said.
Dr. Katsanos explained that, “to address the differences in follow-up period and number of cases lost to follow-up, the primary endpoint was calculated on the log-hazard scale and expressed as a hazard ratio, as recommended for time-to-event outcomes.”
He highlighted that a short-term time frame of 6 months to 1 year was chosen “because it is clinically relevant to limb-threatening CLI.”
Sensitivity tests also “showed consistent direction and magnitude of the summary treatment effects in case of both AFS and freedom from TLR,” Dr. Katsanos emphasized.
Lower mortality, fewer amputations
The second study, published online Jan. 8, drew on health insurance claims in the German BARMER database to analyze 37,914 patients (mean age, 73.3 years, 49% female) and 21,546 propensity-score-matched patients with symptomatic CLTI or intermittent claudication (IC) with an index revascularization during 2010-2018 (Eur J Vasc Endovasc Surg. 2020 Jan 8. doi: 10.1016/j.ejvs.2019.12.034).
Patients were first stratified by CLTI or IC, and then by balloon vs. stent use. Paclitaxel-coated devices were then compared with uncoated devices within each stratum. The primary outcome was all-cause mortality at the end of follow-up.
From 2010 to 2018, the annual use of paclitaxel-coated devices increased dramatically from 3% to 39% in the CLTI group and from 4% to 48% in the IC group (P less than .001 for both).
A total of 2,454 deaths occurred within 5 years of follow-up (median, 2.7 years; longest, 8 years).
A Cox proportional hazards model (based on propensity-score-matched cohorts at 5 years) showed that, compared with uncoated devices, use of paclitaxel-coated devices in the CLTI group was associated with several improvements:
- Overall survival: HR, 0.83; 95% CI, 0.77-0.90.
- Amputation-free survival: HR, 0.85; 95% CI, 0.78-0.91.
- Major cardiovascular events: HR, 0.82; 95% CI, 0.77-0.88.
In the IC group, mortality was significantly better with DCB (HR, 0.87; 95% CI, 0.76-0.99) or a combination of DCB and drug-eluting stents (HR, 0.88; 95% CI, 0.80-0.98) than with uncoated devices, but similar for DES alone (HR, 0.91; 95% CI, 0.77-1.08).
No benefit was found for paclitaxel-coated devices in the IC group for AFS (HR, 0.91; 95% CI, 0.82-1.00) or major cardiovascular events (HR, 0.93; 95% CI, 0.87-1.00).
The authors acknowledge that “unmeasured confounding” may partly explain the results. It may be that patients revascularized with DCB or DES “are more likely to be treated in highly specialized trial centers with clear follow-up protocol.”
Moreover, these patients may have received “the best treatment,” including statin therapy, added Dr. Behrendt.
More evidence needed
Dr. Secemsky, who was not involved with either study, said the German investigators “did a wonderful job with this analysis in a large population of several thousand patients, showing nicely that after accounting for differences in comorbidities, the patients had no evidence of harm with [paclitaxel-coated] devices through 5 years.”
However, he cautioned, median follow-up time was just over 2 years. “Although the investigators had data all the way out to 5 years, over time, the number of patients contributing data became smaller, which results in more uncertainty with these longer-term findings,” he said. “As such, we still need to look at additional long-term data in this patient population to confirm the safety of these devices.”
At present, the “major consideration we want to address is whether it’s safe to use these devices, and we’re undertaking these analyses to examine safety, not to see if they improve mortality,” although the present study “has a suggestion of mortality benefit,” Dr. Secemsky said.
Dr. Katsanos added that paclitaxel-coated balloons “remain under investigation for below-knee arteries and critical limb ischemia,” with “a few randomized controlled trials on the way.”
“We need definitive evidence from high-quality multicenter controlled trials that these devices may improve wound healing and limb salvage without any systemic mortality risk,” he said.
Dr. Katsanos receives personal fees from Boston Scientific and Philips Healthcare. The study by Dr. Behrendt was part of the IDOMENEO project funded by the German Joint Federal Committee. Dr. Behrendt reports no relevant financial relationships. Dr. Secemsky reports institutional grants from Cook Medical, BD Bard, Medtronic, Beth Israel Deaconess Medical Center, and Boston Scientific, and reports consultancy for Cook Medical, BD Bard, and Medtronic.
This article first appeared on Medscape.com.
The controversy regarding the safety of treating peripheral artery disease (PAD) with paclitaxel-coated devices has only deepened in the new year, with two recent studies suggesting opposite safety findings.
The debate began with a 2018 meta-analysis showing a late mortality signal associated with paclitaxel drug-coated balloons (DCBs) that sent reverberations through the interventional cardiology community (J Am Heart Assoc. 2018 Dec 18;7[24]:e011245).
Now, in a new meta-analysis involving eight randomized controlled trials (RCTs) and more than 1,400 patients with critical limb ischemia (CLI), the same researchers found significantly more early amputations and deaths in those treated with DCB below the knee, compared with conventional balloon angioplasty.
“The findings of our latest report add to previous evidence underpinning major safety concerns around use of paclitaxel in lower limb angioplasties – increased long-term patient mortality in cases of intermittent claudication,” lead author Konstantinos Katsanos MD, MSc, PhD, Patras University Hospital, Greece, said in an interview.
By contrast, a retrospective study of insurance claims in Germany showed no heightened mortality with paclitaxel-coated balloons and stents, compared with uncoated devices, in close to 38,000 patients with PAD.
On the contrary, use of paclitaxel-coated devices was associated with higher long-term survival, better amputation-free survival (AFS), and lower rates of major cardiovascular events in the treatment of chronic limb-threatening ischemia (CLTI).
These findings “emphasize the difference between population-based evidence and randomized trials,” lead author Christian-Alexander Behrendt, MD, University Medical Center Hamburg-Eppendorf, Germany, said in an interview.
Downstream “showers”
In the new meta-analysis led by Dr. Katsanos, published online Jan. 15, the 1,420 patients were treated with five different DCBs and 97% had CLI (J Vasc Intervent Radiol 2020 Feb;31[2]:202-12).
In up to 1-year follow-up, the paclitaxel DCB group had fewer target lesion revascularizations (TLR) than those of the uncoated device group (11.8% vs. 25.6%; risk ratio, 0.53; 95% confidence interval, 0.35-0.81) but worse AFS (13.7% vs. 9.4%; hazard ratio [HR], 1.52; 95% CI, 1.12-2.07).
The latter finding was driven by nonsignificant increased risks for all-cause death (odds ratio [OR], 1.39; 95% CI, 0.94-2.07) and major amputations (OR, 1.63; 95% CI, 0.92-2.90).
In dose-subgroup analyses, AFS was significantly worse in cases with high-dose (3.0-3.5 mcg/mm2) devices, but not in the single trial with a low-dose DCB (2.0 mcg/mm2).
“Considering the well-described downstream ‘showers’ of paclitaxel particles with current drug-coated balloons, we hypothesize that nontarget paclitaxel embolization is a plausible mechanism for distal foot and systemic toxicity,” Dr. Katsanos said.
Short time frame
Eric Secemsky, MD, of Harvard Medical School, and director of vascular intervention at Beth Israel Deaconess Medical Center, Boston, suggested in an interview that this theorized mechanism of harm in below-the-knee procedures could potentially shed light on a similar mechanism at play in above-the-knee procedures.
“We didn’t understand why people could potentially be dying in above-the-knee [procedures], and the suggestion here is that these devices might perhaps be causing particular embolization or maybe delayed wound healing,” Dr. Secemsky speculated.
However, “I don’t know that this is true, so I am cautious to say this is true,” he emphasized.
Dr. Secemsky said a strength of the Katsanos analysis is that the RCTs included more than 1,000 patients, but noted that it is hard to vet the quality and rigor of the data, as some of the studies have not yet been published. He also noted that paclitaxel-coated devices are not approved by the Food and Drug Administration in the United States for below-the-knee procedures.
Moreover, he continued, “two studies were driving the signal of harm: the IN.PACT DEEP, which included an iteration of their DCB that is no longer being tested; and the unpublished SINGA-PACLI trial. Those studies contributed most of the adverse events seen in this meta-analysis.”
In addition, the trials had different lengths of follow-up (6-12 months), he said. “Thus, the five trials with data available to 12 months are driving the 1-year findings, whereas three RCTs, including the primary RCT showing safety [Lutonix-BTK trial], only contribute data to 6 months.”
For this reason, “we are not too excited about this meta-analysis as of now, [because] all it tells us is that we need more data to support the safety of drug-coated devices in this population,” Dr. Secemsky said.
Dr. Katsanos explained that, “to address the differences in follow-up period and number of cases lost to follow-up, the primary endpoint was calculated on the log-hazard scale and expressed as a hazard ratio, as recommended for time-to-event outcomes.”
He highlighted that a short-term time frame of 6 months to 1 year was chosen “because it is clinically relevant to limb-threatening CLI.”
Sensitivity tests also “showed consistent direction and magnitude of the summary treatment effects in case of both AFS and freedom from TLR,” Dr. Katsanos emphasized.
Lower mortality, fewer amputations
The second study, published online Jan. 8, drew on health insurance claims in the German BARMER database to analyze 37,914 patients (mean age, 73.3 years, 49% female) and 21,546 propensity-score-matched patients with symptomatic CLTI or intermittent claudication (IC) with an index revascularization during 2010-2018 (Eur J Vasc Endovasc Surg. 2020 Jan 8. doi: 10.1016/j.ejvs.2019.12.034).
Patients were first stratified by CLTI or IC, and then by balloon vs. stent use. Paclitaxel-coated devices were then compared with uncoated devices within each stratum. The primary outcome was all-cause mortality at the end of follow-up.
From 2010 to 2018, the annual use of paclitaxel-coated devices increased dramatically from 3% to 39% in the CLTI group and from 4% to 48% in the IC group (P less than .001 for both).
A total of 2,454 deaths occurred within 5 years of follow-up (median, 2.7 years; longest, 8 years).
A Cox proportional hazards model (based on propensity-score-matched cohorts at 5 years) showed that, compared with uncoated devices, use of paclitaxel-coated devices in the CLTI group was associated with several improvements:
- Overall survival: HR, 0.83; 95% CI, 0.77-0.90.
- Amputation-free survival: HR, 0.85; 95% CI, 0.78-0.91.
- Major cardiovascular events: HR, 0.82; 95% CI, 0.77-0.88.
In the IC group, mortality was significantly better with DCB (HR, 0.87; 95% CI, 0.76-0.99) or a combination of DCB and drug-eluting stents (HR, 0.88; 95% CI, 0.80-0.98) than with uncoated devices, but similar for DES alone (HR, 0.91; 95% CI, 0.77-1.08).
No benefit was found for paclitaxel-coated devices in the IC group for AFS (HR, 0.91; 95% CI, 0.82-1.00) or major cardiovascular events (HR, 0.93; 95% CI, 0.87-1.00).
The authors acknowledge that “unmeasured confounding” may partly explain the results. It may be that patients revascularized with DCB or DES “are more likely to be treated in highly specialized trial centers with clear follow-up protocol.”
Moreover, these patients may have received “the best treatment,” including statin therapy, added Dr. Behrendt.
More evidence needed
Dr. Secemsky, who was not involved with either study, said the German investigators “did a wonderful job with this analysis in a large population of several thousand patients, showing nicely that after accounting for differences in comorbidities, the patients had no evidence of harm with [paclitaxel-coated] devices through 5 years.”
However, he cautioned, median follow-up time was just over 2 years. “Although the investigators had data all the way out to 5 years, over time, the number of patients contributing data became smaller, which results in more uncertainty with these longer-term findings,” he said. “As such, we still need to look at additional long-term data in this patient population to confirm the safety of these devices.”
At present, the “major consideration we want to address is whether it’s safe to use these devices, and we’re undertaking these analyses to examine safety, not to see if they improve mortality,” although the present study “has a suggestion of mortality benefit,” Dr. Secemsky said.
Dr. Katsanos added that paclitaxel-coated balloons “remain under investigation for below-knee arteries and critical limb ischemia,” with “a few randomized controlled trials on the way.”
“We need definitive evidence from high-quality multicenter controlled trials that these devices may improve wound healing and limb salvage without any systemic mortality risk,” he said.
Dr. Katsanos receives personal fees from Boston Scientific and Philips Healthcare. The study by Dr. Behrendt was part of the IDOMENEO project funded by the German Joint Federal Committee. Dr. Behrendt reports no relevant financial relationships. Dr. Secemsky reports institutional grants from Cook Medical, BD Bard, Medtronic, Beth Israel Deaconess Medical Center, and Boston Scientific, and reports consultancy for Cook Medical, BD Bard, and Medtronic.
This article first appeared on Medscape.com.
The controversy regarding the safety of treating peripheral artery disease (PAD) with paclitaxel-coated devices has only deepened in the new year, with two recent studies suggesting opposite safety findings.
The debate began with a 2018 meta-analysis showing a late mortality signal associated with paclitaxel drug-coated balloons (DCBs) that sent reverberations through the interventional cardiology community (J Am Heart Assoc. 2018 Dec 18;7[24]:e011245).
Now, in a new meta-analysis involving eight randomized controlled trials (RCTs) and more than 1,400 patients with critical limb ischemia (CLI), the same researchers found significantly more early amputations and deaths in those treated with DCB below the knee, compared with conventional balloon angioplasty.
“The findings of our latest report add to previous evidence underpinning major safety concerns around use of paclitaxel in lower limb angioplasties – increased long-term patient mortality in cases of intermittent claudication,” lead author Konstantinos Katsanos MD, MSc, PhD, Patras University Hospital, Greece, said in an interview.
By contrast, a retrospective study of insurance claims in Germany showed no heightened mortality with paclitaxel-coated balloons and stents, compared with uncoated devices, in close to 38,000 patients with PAD.
On the contrary, use of paclitaxel-coated devices was associated with higher long-term survival, better amputation-free survival (AFS), and lower rates of major cardiovascular events in the treatment of chronic limb-threatening ischemia (CLTI).
These findings “emphasize the difference between population-based evidence and randomized trials,” lead author Christian-Alexander Behrendt, MD, University Medical Center Hamburg-Eppendorf, Germany, said in an interview.
Downstream “showers”
In the new meta-analysis led by Dr. Katsanos, published online Jan. 15, the 1,420 patients were treated with five different DCBs and 97% had CLI (J Vasc Intervent Radiol 2020 Feb;31[2]:202-12).
In up to 1-year follow-up, the paclitaxel DCB group had fewer target lesion revascularizations (TLR) than those of the uncoated device group (11.8% vs. 25.6%; risk ratio, 0.53; 95% confidence interval, 0.35-0.81) but worse AFS (13.7% vs. 9.4%; hazard ratio [HR], 1.52; 95% CI, 1.12-2.07).
The latter finding was driven by nonsignificant increased risks for all-cause death (odds ratio [OR], 1.39; 95% CI, 0.94-2.07) and major amputations (OR, 1.63; 95% CI, 0.92-2.90).
In dose-subgroup analyses, AFS was significantly worse in cases with high-dose (3.0-3.5 mcg/mm2) devices, but not in the single trial with a low-dose DCB (2.0 mcg/mm2).
“Considering the well-described downstream ‘showers’ of paclitaxel particles with current drug-coated balloons, we hypothesize that nontarget paclitaxel embolization is a plausible mechanism for distal foot and systemic toxicity,” Dr. Katsanos said.
Short time frame
Eric Secemsky, MD, of Harvard Medical School, and director of vascular intervention at Beth Israel Deaconess Medical Center, Boston, suggested in an interview that this theorized mechanism of harm in below-the-knee procedures could potentially shed light on a similar mechanism at play in above-the-knee procedures.
“We didn’t understand why people could potentially be dying in above-the-knee [procedures], and the suggestion here is that these devices might perhaps be causing particular embolization or maybe delayed wound healing,” Dr. Secemsky speculated.
However, “I don’t know that this is true, so I am cautious to say this is true,” he emphasized.
Dr. Secemsky said a strength of the Katsanos analysis is that the RCTs included more than 1,000 patients, but noted that it is hard to vet the quality and rigor of the data, as some of the studies have not yet been published. He also noted that paclitaxel-coated devices are not approved by the Food and Drug Administration in the United States for below-the-knee procedures.
Moreover, he continued, “two studies were driving the signal of harm: the IN.PACT DEEP, which included an iteration of their DCB that is no longer being tested; and the unpublished SINGA-PACLI trial. Those studies contributed most of the adverse events seen in this meta-analysis.”
In addition, the trials had different lengths of follow-up (6-12 months), he said. “Thus, the five trials with data available to 12 months are driving the 1-year findings, whereas three RCTs, including the primary RCT showing safety [Lutonix-BTK trial], only contribute data to 6 months.”
For this reason, “we are not too excited about this meta-analysis as of now, [because] all it tells us is that we need more data to support the safety of drug-coated devices in this population,” Dr. Secemsky said.
Dr. Katsanos explained that, “to address the differences in follow-up period and number of cases lost to follow-up, the primary endpoint was calculated on the log-hazard scale and expressed as a hazard ratio, as recommended for time-to-event outcomes.”
He highlighted that a short-term time frame of 6 months to 1 year was chosen “because it is clinically relevant to limb-threatening CLI.”
Sensitivity tests also “showed consistent direction and magnitude of the summary treatment effects in case of both AFS and freedom from TLR,” Dr. Katsanos emphasized.
Lower mortality, fewer amputations
The second study, published online Jan. 8, drew on health insurance claims in the German BARMER database to analyze 37,914 patients (mean age, 73.3 years, 49% female) and 21,546 propensity-score-matched patients with symptomatic CLTI or intermittent claudication (IC) with an index revascularization during 2010-2018 (Eur J Vasc Endovasc Surg. 2020 Jan 8. doi: 10.1016/j.ejvs.2019.12.034).
Patients were first stratified by CLTI or IC, and then by balloon vs. stent use. Paclitaxel-coated devices were then compared with uncoated devices within each stratum. The primary outcome was all-cause mortality at the end of follow-up.
From 2010 to 2018, the annual use of paclitaxel-coated devices increased dramatically from 3% to 39% in the CLTI group and from 4% to 48% in the IC group (P less than .001 for both).
A total of 2,454 deaths occurred within 5 years of follow-up (median, 2.7 years; longest, 8 years).
A Cox proportional hazards model (based on propensity-score-matched cohorts at 5 years) showed that, compared with uncoated devices, use of paclitaxel-coated devices in the CLTI group was associated with several improvements:
- Overall survival: HR, 0.83; 95% CI, 0.77-0.90.
- Amputation-free survival: HR, 0.85; 95% CI, 0.78-0.91.
- Major cardiovascular events: HR, 0.82; 95% CI, 0.77-0.88.
In the IC group, mortality was significantly better with DCB (HR, 0.87; 95% CI, 0.76-0.99) or a combination of DCB and drug-eluting stents (HR, 0.88; 95% CI, 0.80-0.98) than with uncoated devices, but similar for DES alone (HR, 0.91; 95% CI, 0.77-1.08).
No benefit was found for paclitaxel-coated devices in the IC group for AFS (HR, 0.91; 95% CI, 0.82-1.00) or major cardiovascular events (HR, 0.93; 95% CI, 0.87-1.00).
The authors acknowledge that “unmeasured confounding” may partly explain the results. It may be that patients revascularized with DCB or DES “are more likely to be treated in highly specialized trial centers with clear follow-up protocol.”
Moreover, these patients may have received “the best treatment,” including statin therapy, added Dr. Behrendt.
More evidence needed
Dr. Secemsky, who was not involved with either study, said the German investigators “did a wonderful job with this analysis in a large population of several thousand patients, showing nicely that after accounting for differences in comorbidities, the patients had no evidence of harm with [paclitaxel-coated] devices through 5 years.”
However, he cautioned, median follow-up time was just over 2 years. “Although the investigators had data all the way out to 5 years, over time, the number of patients contributing data became smaller, which results in more uncertainty with these longer-term findings,” he said. “As such, we still need to look at additional long-term data in this patient population to confirm the safety of these devices.”
At present, the “major consideration we want to address is whether it’s safe to use these devices, and we’re undertaking these analyses to examine safety, not to see if they improve mortality,” although the present study “has a suggestion of mortality benefit,” Dr. Secemsky said.
Dr. Katsanos added that paclitaxel-coated balloons “remain under investigation for below-knee arteries and critical limb ischemia,” with “a few randomized controlled trials on the way.”
“We need definitive evidence from high-quality multicenter controlled trials that these devices may improve wound healing and limb salvage without any systemic mortality risk,” he said.
Dr. Katsanos receives personal fees from Boston Scientific and Philips Healthcare. The study by Dr. Behrendt was part of the IDOMENEO project funded by the German Joint Federal Committee. Dr. Behrendt reports no relevant financial relationships. Dr. Secemsky reports institutional grants from Cook Medical, BD Bard, Medtronic, Beth Israel Deaconess Medical Center, and Boston Scientific, and reports consultancy for Cook Medical, BD Bard, and Medtronic.
This article first appeared on Medscape.com.
Trump seeks to cut NIH, CDC budgets, some Medicare spending
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
Glaring gap in CV event reporting in pivotal cancer trials
Clinical trials supporting Food and Drug Adminstration approval of contemporary cancer therapies frequently failed to capture major adverse cardiovascular events (MACE) and, when they did, reported rates 2.6-fold lower than noncancer trials, new research shows.
Overall, 51.3% of trials did not report MACE, with that number reaching 57.6% in trials enrolling patients with baseline cardiovascular disease (CVD).
Nearly 40% of trials did not report any CVD events in follow-up, the authors reported online Feb. 10, 2020, in the Journal of the American College of Cardiology (2020;75:620-8).
“Even in drug classes where there were established or emerging associations with cardiotoxic events, often there were no reported heart events or cardiovascular events across years of follow-up in trials that examined hundreds or even thousands of patients. That was actually pretty surprising,” senior author Daniel Addison, MD, codirector of the cardio-oncology program at the Ohio State University Medical Center, Columbus, said in an interview.
The study was prompted by a series of events that crescendoed when his team was called to the ICU to determine whether a novel targeted agent played a role in the heart decline of a patient with acute myeloid leukemia. “I had a resident ask me a very important question: ‘How do we really know for sure that the trial actually reflects the true risk of heart events?’ to which I told him, ‘it’s difficult to know,’ ” he said.
“I think many of us rely heavily on what we see in the trials, particularly when they make it to the top journals, and quite frankly, we generally take it at face value,” Dr. Addison observed.
Lower Rate of Reported Events
The investigators reviewed CV events reported in 97,365 patients (median age, 61 years; 46% female) enrolled in 189 phase 2 and 3 trials supporting FDA approval of 123 anticancer drugs from 1998 to 2018. Biologic, targeted, or immune-based therapies accounted for 72.5% of drug approvals.
Over 148,138 person-years of follow-up (median trial duration, 30 months), there were 1,148 incidents of MACE (375 heart failure, 253 MIs, 180 strokes, 65 atrial fibrillation, 29 coronary revascularizations, and 246 CVD deaths). MACE rates were higher in the intervention group than in the control group (792 vs. 356; P less than .01). Among the 64 trials that excluded patients with baseline CVD, there were 269 incidents of MACE.
To put this finding in context, the researchers examined the reported incidence of MACE among some 6,000 similarly aged participants in the Multi-Ethnic Study of Atherosclerosis (MESA). The overall weighted-average incidence rate was 1,408 per 100,000 person-years among MESA participants, compared with 542 events per 100,000 person-years among oncology trial participants (716 per 100,000 in the intervention arm). This represents a reported-to-expected ratio of 0.38 – a 2.6-fold lower rate of reported events (P less than .001) – and a risk difference of 866.
Further, MACE reporting was lower by a factor of 1.7 among all cancer trial participants irrespective of baseline CVD status (reported-to-expected ratio, 0.56; risk difference, 613; P less than .001).
There was no significant difference in MACE reporting between independent or industry-sponsored trials, the authors report.
No malicious intent
“There are likely some that might lean toward not wanting to attribute blame to a new drug when the drug is in a study, but I really think that the leading factor is lack of awareness,” Dr. Addison said. “I’ve talked with several cancer collaborators around the country who run large clinical trials, and I think often, when an event may be brought to someone’s attention, there is a tendency to just write it off as kind of a generic expected event due to age, or just something that’s not really pertinent to the study. So they don’t really focus on it as much.”
“Closer collaboration between cardiologists and cancer physicians is needed to better determine true cardiac risks among patients treated with these drugs.”
Breast cancer oncologist Marc E. Lippman, MD, of Georgetown University Medical Center and Georgetown Lombardi Comprehensive Cancer Center, Washington, D.C., isn’t convinced a lack of awareness is the culprit.
“I don’t agree with that at all,” he said in an interview. “I think there are very, very clear rules and guidelines these days for adverse-event reporting. I think that’s not a very likely explanation – that it’s not on the radar.”
Part of the problem may be that some of the toxicities, particularly cardiovascular, may not emerge for years, he said. Participant screening for the trials also likely removed patients with high cardiovascular risk. “It’s very understandable to me – I’m not saying it’s good particularly – but I think it’s very understandable that, if you’re trying to develop a drug, the last thing you’d want to have is a lot of toxicity that you might have avoided by just being restrictive in who you let into the study,” Dr. Lippman said.
The underreported CVD events may also reflect the rapidly changing profile of cardiovascular toxicities associated with novel anticancer therapies.
“Providers, both cancer and noncancer, generally put cardiotoxicity in the box of anthracyclines and radiation, but particularly over the last decade, we’ve begun to understand it’s well beyond any one class of drugs,” Dr. Addison said.
“I agree completely,” Dr. Lippman said. For example, “the checkpoint inhibitors are so unbelievably different in terms of their toxicities that many people simply didn’t even know what they were getting into at first.”
One size does not fit all
Javid Moslehi, MD, director of the cardio-oncology program at Vanderbilt University, Nashville, Tenn., said echocardiography – recommended to detect changes in left ventricular function in patients exposed to anthracyclines or targeted agents like trastuzumab (Herceptin) – isn’t enough to address today’s cancer therapy–related CVD events.
“Initial drugs like anthracyclines or Herceptin in cardio-oncology were associated with systolic cardiac dysfunction, whereas the majority of issues we see in the cardio-oncology clinics today are vascular, metabolic, arrhythmogenic, and inflammatory,” he said in an interview. “Echocardiography misses the big and increasingly complex picture.”
His group, for example, has been studying myocarditis associated with immunotherapies, but none of the clinical trials require screening or surveillance for myocarditis with a cardiac biomarker like troponin.
The group also recently identified 303 deaths in patients exposed to ibrutinib, a drug that revolutionized the treatment of several B-cell malignancies but is associated with higher rates of atrial fibrillation, which is also associated with increased bleeding risk. “So there’s a little bit of a double whammy there, given that we often treat atrial fibrillation with anticoagulation and where we can cause complications in patients,” Dr. Moslehi noted.
Although there needs to be closer collaboration between cardiologists and oncologists on individual trials, cardiologists also have to realize that oncology care has become very personalized, he suggested.
“What’s probably relevant for the breast cancer patient may not be relevant for the prostate cancer patient and their respective treatments,” Dr. Moslehi said. “So if we were to say, ‘every person should get an echo,’ that may be less relevant to the prostate cancer patient where treatments can cause vascular and metabolic perturbations or to the patient treated with immunotherapy who may have myocarditis, where many of the echos can be normal. There’s no one-size-fits-all for these things.”
Wearable technologies like smartwatches could play a role in improving the reporting of CVD events with novel therapies but a lot more research needs to be done to validate these tools, Dr. Addison said. “But as we continue on into the 21st century, this is going to expand and may potentially help us,” he added.
In the interim, better standardization is needed of the cardiovascular events reported in oncology trials, particularly the Common Terminology Criteria for Adverse Events (CTCAE), said Dr. Moslehi, who also serves as chair of the American Heart Association’s subcommittee on cardio-oncology.
“Cardiovascular definitions are not exactly uniform and are not consistent with what we in cardiology consider to be important or relevant,” he said. “So I think there needs to be better standardization of these definitions, specifically within the CTCAE, which is what the oncologists use to identify adverse events.”
In a linked editorial (J Am Coll Cardiol. 2020;75:629-31), Dr. Lippman and cardiologist Nanette Bishopric, MD, of the Medstar Heart and Vascular Institute in Washington, D.C., suggested it may also be time to organize a consortium that can carry out “rigorous multicenter clinical investigations to evaluate the cardiotoxicity of emerging cancer treatments,” similar to the Thrombosis in Myocardial Infarction Study Group.
“The success of this consortium in pioneering and targeting multiple generations of drugs for the treatment of MI, involving tens of thousands of patients and thousands of collaborations across multiple national borders, is a model for how to move forward in providing the new hope of cancer cure without the trade-off of years lost to heart disease,” the editorialists concluded.
The study was supported in part by National Institutes of Health grants, including a K12-CA133250 grant to Dr. Addison. Dr. Bishopric reported being on the scientific board of C&C Biopharma. Dr. Lippman reports being on the board of directors of and holding stock in Seattle Genetics. Dr. Moslehi reported having served on advisory boards for Pfizer, Novartis, Bristol-Myers Squibb, Deciphera, Audentes Pharmaceuticals, Nektar, Takeda, Ipsen, Myokardia, AstraZeneca, GlaxoSmithKline, Intrexon, and Regeneron.
This article first appeared on Medscape.com.
Clinical trials supporting Food and Drug Adminstration approval of contemporary cancer therapies frequently failed to capture major adverse cardiovascular events (MACE) and, when they did, reported rates 2.6-fold lower than noncancer trials, new research shows.
Overall, 51.3% of trials did not report MACE, with that number reaching 57.6% in trials enrolling patients with baseline cardiovascular disease (CVD).
Nearly 40% of trials did not report any CVD events in follow-up, the authors reported online Feb. 10, 2020, in the Journal of the American College of Cardiology (2020;75:620-8).
“Even in drug classes where there were established or emerging associations with cardiotoxic events, often there were no reported heart events or cardiovascular events across years of follow-up in trials that examined hundreds or even thousands of patients. That was actually pretty surprising,” senior author Daniel Addison, MD, codirector of the cardio-oncology program at the Ohio State University Medical Center, Columbus, said in an interview.
The study was prompted by a series of events that crescendoed when his team was called to the ICU to determine whether a novel targeted agent played a role in the heart decline of a patient with acute myeloid leukemia. “I had a resident ask me a very important question: ‘How do we really know for sure that the trial actually reflects the true risk of heart events?’ to which I told him, ‘it’s difficult to know,’ ” he said.
“I think many of us rely heavily on what we see in the trials, particularly when they make it to the top journals, and quite frankly, we generally take it at face value,” Dr. Addison observed.
Lower Rate of Reported Events
The investigators reviewed CV events reported in 97,365 patients (median age, 61 years; 46% female) enrolled in 189 phase 2 and 3 trials supporting FDA approval of 123 anticancer drugs from 1998 to 2018. Biologic, targeted, or immune-based therapies accounted for 72.5% of drug approvals.
Over 148,138 person-years of follow-up (median trial duration, 30 months), there were 1,148 incidents of MACE (375 heart failure, 253 MIs, 180 strokes, 65 atrial fibrillation, 29 coronary revascularizations, and 246 CVD deaths). MACE rates were higher in the intervention group than in the control group (792 vs. 356; P less than .01). Among the 64 trials that excluded patients with baseline CVD, there were 269 incidents of MACE.
To put this finding in context, the researchers examined the reported incidence of MACE among some 6,000 similarly aged participants in the Multi-Ethnic Study of Atherosclerosis (MESA). The overall weighted-average incidence rate was 1,408 per 100,000 person-years among MESA participants, compared with 542 events per 100,000 person-years among oncology trial participants (716 per 100,000 in the intervention arm). This represents a reported-to-expected ratio of 0.38 – a 2.6-fold lower rate of reported events (P less than .001) – and a risk difference of 866.
Further, MACE reporting was lower by a factor of 1.7 among all cancer trial participants irrespective of baseline CVD status (reported-to-expected ratio, 0.56; risk difference, 613; P less than .001).
There was no significant difference in MACE reporting between independent or industry-sponsored trials, the authors report.
No malicious intent
“There are likely some that might lean toward not wanting to attribute blame to a new drug when the drug is in a study, but I really think that the leading factor is lack of awareness,” Dr. Addison said. “I’ve talked with several cancer collaborators around the country who run large clinical trials, and I think often, when an event may be brought to someone’s attention, there is a tendency to just write it off as kind of a generic expected event due to age, or just something that’s not really pertinent to the study. So they don’t really focus on it as much.”
“Closer collaboration between cardiologists and cancer physicians is needed to better determine true cardiac risks among patients treated with these drugs.”
Breast cancer oncologist Marc E. Lippman, MD, of Georgetown University Medical Center and Georgetown Lombardi Comprehensive Cancer Center, Washington, D.C., isn’t convinced a lack of awareness is the culprit.
“I don’t agree with that at all,” he said in an interview. “I think there are very, very clear rules and guidelines these days for adverse-event reporting. I think that’s not a very likely explanation – that it’s not on the radar.”
Part of the problem may be that some of the toxicities, particularly cardiovascular, may not emerge for years, he said. Participant screening for the trials also likely removed patients with high cardiovascular risk. “It’s very understandable to me – I’m not saying it’s good particularly – but I think it’s very understandable that, if you’re trying to develop a drug, the last thing you’d want to have is a lot of toxicity that you might have avoided by just being restrictive in who you let into the study,” Dr. Lippman said.
The underreported CVD events may also reflect the rapidly changing profile of cardiovascular toxicities associated with novel anticancer therapies.
“Providers, both cancer and noncancer, generally put cardiotoxicity in the box of anthracyclines and radiation, but particularly over the last decade, we’ve begun to understand it’s well beyond any one class of drugs,” Dr. Addison said.
“I agree completely,” Dr. Lippman said. For example, “the checkpoint inhibitors are so unbelievably different in terms of their toxicities that many people simply didn’t even know what they were getting into at first.”
One size does not fit all
Javid Moslehi, MD, director of the cardio-oncology program at Vanderbilt University, Nashville, Tenn., said echocardiography – recommended to detect changes in left ventricular function in patients exposed to anthracyclines or targeted agents like trastuzumab (Herceptin) – isn’t enough to address today’s cancer therapy–related CVD events.
“Initial drugs like anthracyclines or Herceptin in cardio-oncology were associated with systolic cardiac dysfunction, whereas the majority of issues we see in the cardio-oncology clinics today are vascular, metabolic, arrhythmogenic, and inflammatory,” he said in an interview. “Echocardiography misses the big and increasingly complex picture.”
His group, for example, has been studying myocarditis associated with immunotherapies, but none of the clinical trials require screening or surveillance for myocarditis with a cardiac biomarker like troponin.
The group also recently identified 303 deaths in patients exposed to ibrutinib, a drug that revolutionized the treatment of several B-cell malignancies but is associated with higher rates of atrial fibrillation, which is also associated with increased bleeding risk. “So there’s a little bit of a double whammy there, given that we often treat atrial fibrillation with anticoagulation and where we can cause complications in patients,” Dr. Moslehi noted.
Although there needs to be closer collaboration between cardiologists and oncologists on individual trials, cardiologists also have to realize that oncology care has become very personalized, he suggested.
“What’s probably relevant for the breast cancer patient may not be relevant for the prostate cancer patient and their respective treatments,” Dr. Moslehi said. “So if we were to say, ‘every person should get an echo,’ that may be less relevant to the prostate cancer patient where treatments can cause vascular and metabolic perturbations or to the patient treated with immunotherapy who may have myocarditis, where many of the echos can be normal. There’s no one-size-fits-all for these things.”
Wearable technologies like smartwatches could play a role in improving the reporting of CVD events with novel therapies but a lot more research needs to be done to validate these tools, Dr. Addison said. “But as we continue on into the 21st century, this is going to expand and may potentially help us,” he added.
In the interim, better standardization is needed of the cardiovascular events reported in oncology trials, particularly the Common Terminology Criteria for Adverse Events (CTCAE), said Dr. Moslehi, who also serves as chair of the American Heart Association’s subcommittee on cardio-oncology.
“Cardiovascular definitions are not exactly uniform and are not consistent with what we in cardiology consider to be important or relevant,” he said. “So I think there needs to be better standardization of these definitions, specifically within the CTCAE, which is what the oncologists use to identify adverse events.”
In a linked editorial (J Am Coll Cardiol. 2020;75:629-31), Dr. Lippman and cardiologist Nanette Bishopric, MD, of the Medstar Heart and Vascular Institute in Washington, D.C., suggested it may also be time to organize a consortium that can carry out “rigorous multicenter clinical investigations to evaluate the cardiotoxicity of emerging cancer treatments,” similar to the Thrombosis in Myocardial Infarction Study Group.
“The success of this consortium in pioneering and targeting multiple generations of drugs for the treatment of MI, involving tens of thousands of patients and thousands of collaborations across multiple national borders, is a model for how to move forward in providing the new hope of cancer cure without the trade-off of years lost to heart disease,” the editorialists concluded.
The study was supported in part by National Institutes of Health grants, including a K12-CA133250 grant to Dr. Addison. Dr. Bishopric reported being on the scientific board of C&C Biopharma. Dr. Lippman reports being on the board of directors of and holding stock in Seattle Genetics. Dr. Moslehi reported having served on advisory boards for Pfizer, Novartis, Bristol-Myers Squibb, Deciphera, Audentes Pharmaceuticals, Nektar, Takeda, Ipsen, Myokardia, AstraZeneca, GlaxoSmithKline, Intrexon, and Regeneron.
This article first appeared on Medscape.com.
Clinical trials supporting Food and Drug Adminstration approval of contemporary cancer therapies frequently failed to capture major adverse cardiovascular events (MACE) and, when they did, reported rates 2.6-fold lower than noncancer trials, new research shows.
Overall, 51.3% of trials did not report MACE, with that number reaching 57.6% in trials enrolling patients with baseline cardiovascular disease (CVD).
Nearly 40% of trials did not report any CVD events in follow-up, the authors reported online Feb. 10, 2020, in the Journal of the American College of Cardiology (2020;75:620-8).
“Even in drug classes where there were established or emerging associations with cardiotoxic events, often there were no reported heart events or cardiovascular events across years of follow-up in trials that examined hundreds or even thousands of patients. That was actually pretty surprising,” senior author Daniel Addison, MD, codirector of the cardio-oncology program at the Ohio State University Medical Center, Columbus, said in an interview.
The study was prompted by a series of events that crescendoed when his team was called to the ICU to determine whether a novel targeted agent played a role in the heart decline of a patient with acute myeloid leukemia. “I had a resident ask me a very important question: ‘How do we really know for sure that the trial actually reflects the true risk of heart events?’ to which I told him, ‘it’s difficult to know,’ ” he said.
“I think many of us rely heavily on what we see in the trials, particularly when they make it to the top journals, and quite frankly, we generally take it at face value,” Dr. Addison observed.
Lower Rate of Reported Events
The investigators reviewed CV events reported in 97,365 patients (median age, 61 years; 46% female) enrolled in 189 phase 2 and 3 trials supporting FDA approval of 123 anticancer drugs from 1998 to 2018. Biologic, targeted, or immune-based therapies accounted for 72.5% of drug approvals.
Over 148,138 person-years of follow-up (median trial duration, 30 months), there were 1,148 incidents of MACE (375 heart failure, 253 MIs, 180 strokes, 65 atrial fibrillation, 29 coronary revascularizations, and 246 CVD deaths). MACE rates were higher in the intervention group than in the control group (792 vs. 356; P less than .01). Among the 64 trials that excluded patients with baseline CVD, there were 269 incidents of MACE.
To put this finding in context, the researchers examined the reported incidence of MACE among some 6,000 similarly aged participants in the Multi-Ethnic Study of Atherosclerosis (MESA). The overall weighted-average incidence rate was 1,408 per 100,000 person-years among MESA participants, compared with 542 events per 100,000 person-years among oncology trial participants (716 per 100,000 in the intervention arm). This represents a reported-to-expected ratio of 0.38 – a 2.6-fold lower rate of reported events (P less than .001) – and a risk difference of 866.
Further, MACE reporting was lower by a factor of 1.7 among all cancer trial participants irrespective of baseline CVD status (reported-to-expected ratio, 0.56; risk difference, 613; P less than .001).
There was no significant difference in MACE reporting between independent or industry-sponsored trials, the authors report.
No malicious intent
“There are likely some that might lean toward not wanting to attribute blame to a new drug when the drug is in a study, but I really think that the leading factor is lack of awareness,” Dr. Addison said. “I’ve talked with several cancer collaborators around the country who run large clinical trials, and I think often, when an event may be brought to someone’s attention, there is a tendency to just write it off as kind of a generic expected event due to age, or just something that’s not really pertinent to the study. So they don’t really focus on it as much.”
“Closer collaboration between cardiologists and cancer physicians is needed to better determine true cardiac risks among patients treated with these drugs.”
Breast cancer oncologist Marc E. Lippman, MD, of Georgetown University Medical Center and Georgetown Lombardi Comprehensive Cancer Center, Washington, D.C., isn’t convinced a lack of awareness is the culprit.
“I don’t agree with that at all,” he said in an interview. “I think there are very, very clear rules and guidelines these days for adverse-event reporting. I think that’s not a very likely explanation – that it’s not on the radar.”
Part of the problem may be that some of the toxicities, particularly cardiovascular, may not emerge for years, he said. Participant screening for the trials also likely removed patients with high cardiovascular risk. “It’s very understandable to me – I’m not saying it’s good particularly – but I think it’s very understandable that, if you’re trying to develop a drug, the last thing you’d want to have is a lot of toxicity that you might have avoided by just being restrictive in who you let into the study,” Dr. Lippman said.
The underreported CVD events may also reflect the rapidly changing profile of cardiovascular toxicities associated with novel anticancer therapies.
“Providers, both cancer and noncancer, generally put cardiotoxicity in the box of anthracyclines and radiation, but particularly over the last decade, we’ve begun to understand it’s well beyond any one class of drugs,” Dr. Addison said.
“I agree completely,” Dr. Lippman said. For example, “the checkpoint inhibitors are so unbelievably different in terms of their toxicities that many people simply didn’t even know what they were getting into at first.”
One size does not fit all
Javid Moslehi, MD, director of the cardio-oncology program at Vanderbilt University, Nashville, Tenn., said echocardiography – recommended to detect changes in left ventricular function in patients exposed to anthracyclines or targeted agents like trastuzumab (Herceptin) – isn’t enough to address today’s cancer therapy–related CVD events.
“Initial drugs like anthracyclines or Herceptin in cardio-oncology were associated with systolic cardiac dysfunction, whereas the majority of issues we see in the cardio-oncology clinics today are vascular, metabolic, arrhythmogenic, and inflammatory,” he said in an interview. “Echocardiography misses the big and increasingly complex picture.”
His group, for example, has been studying myocarditis associated with immunotherapies, but none of the clinical trials require screening or surveillance for myocarditis with a cardiac biomarker like troponin.
The group also recently identified 303 deaths in patients exposed to ibrutinib, a drug that revolutionized the treatment of several B-cell malignancies but is associated with higher rates of atrial fibrillation, which is also associated with increased bleeding risk. “So there’s a little bit of a double whammy there, given that we often treat atrial fibrillation with anticoagulation and where we can cause complications in patients,” Dr. Moslehi noted.
Although there needs to be closer collaboration between cardiologists and oncologists on individual trials, cardiologists also have to realize that oncology care has become very personalized, he suggested.
“What’s probably relevant for the breast cancer patient may not be relevant for the prostate cancer patient and their respective treatments,” Dr. Moslehi said. “So if we were to say, ‘every person should get an echo,’ that may be less relevant to the prostate cancer patient where treatments can cause vascular and metabolic perturbations or to the patient treated with immunotherapy who may have myocarditis, where many of the echos can be normal. There’s no one-size-fits-all for these things.”
Wearable technologies like smartwatches could play a role in improving the reporting of CVD events with novel therapies but a lot more research needs to be done to validate these tools, Dr. Addison said. “But as we continue on into the 21st century, this is going to expand and may potentially help us,” he added.
In the interim, better standardization is needed of the cardiovascular events reported in oncology trials, particularly the Common Terminology Criteria for Adverse Events (CTCAE), said Dr. Moslehi, who also serves as chair of the American Heart Association’s subcommittee on cardio-oncology.
“Cardiovascular definitions are not exactly uniform and are not consistent with what we in cardiology consider to be important or relevant,” he said. “So I think there needs to be better standardization of these definitions, specifically within the CTCAE, which is what the oncologists use to identify adverse events.”
In a linked editorial (J Am Coll Cardiol. 2020;75:629-31), Dr. Lippman and cardiologist Nanette Bishopric, MD, of the Medstar Heart and Vascular Institute in Washington, D.C., suggested it may also be time to organize a consortium that can carry out “rigorous multicenter clinical investigations to evaluate the cardiotoxicity of emerging cancer treatments,” similar to the Thrombosis in Myocardial Infarction Study Group.
“The success of this consortium in pioneering and targeting multiple generations of drugs for the treatment of MI, involving tens of thousands of patients and thousands of collaborations across multiple national borders, is a model for how to move forward in providing the new hope of cancer cure without the trade-off of years lost to heart disease,” the editorialists concluded.
The study was supported in part by National Institutes of Health grants, including a K12-CA133250 grant to Dr. Addison. Dr. Bishopric reported being on the scientific board of C&C Biopharma. Dr. Lippman reports being on the board of directors of and holding stock in Seattle Genetics. Dr. Moslehi reported having served on advisory boards for Pfizer, Novartis, Bristol-Myers Squibb, Deciphera, Audentes Pharmaceuticals, Nektar, Takeda, Ipsen, Myokardia, AstraZeneca, GlaxoSmithKline, Intrexon, and Regeneron.
This article first appeared on Medscape.com.
Mobile stroke unit had clinical impact on EVT
In its first year of operation, a mobile stroke unit in Melbourne demonstrated substantial savings in time to commencement of both thrombolysis and endovascular thrombectomy (EVT), results from a prospective study showed.
“While previously published data from MSU [mobile stroke unit] services in Europe and North America show substantial reductions in time to thrombolysis of approximately 30-45 minutes, little is known about the clinical impact on EVT,” first author Henry Zhao, MBBS, and colleagues wrote in a study published in Stroke.
Launched in November 2017, the Melbourne MSU is based at a large comprehensive stroke center and operates with a 20-km radius, servicing about 1.7 million people within the city of Melbourne. It is staffed with an onboard neurologist or senior stroke fellow who provides primary assessment and treatment decisions, a stroke advanced practice nurse who provides clinical support and treatment administration, a clinician who provides CT imaging, and advanced life support and mobile intensive care paramedics who provide transport logistics and paramedicine support. For the current analysis, MSU patients who received reperfusion therapy were compared with control patients presenting to metropolitan Melbourne stroke units via standard ambulance within MSU operating hours. The primary outcome was median time difference in first ambulance dispatch to treatment, which the researchers used quantile regression analysis to determine. Time savings were subsequently converted to disability-adjusted life years (DALY) avoiding using published estimates.
Dr. Zhao of the Melbourne Brain Centre and department of neurology at Royal Melbourne Hospital and his colleagues reported that, in its first year of operation, the Melbourne MSU administered prehospital thrombolysis to 100 patients with a mean age of nearly 74 years. More than half of the patients (62%) were male. Compared with controls, the median time savings per MSU patient was 26 minutes for dispatch to hospital arrival and 15 minutes for hospital arrival to thrombolysis (P less than .0010 for both associations). The calculated overall time saving from dispatch to thrombolysis was 42.5 minutes.
Over the same time period, 41 MSU patients with a mean age of 76 years received EVT dispatch-to-treatment time saving of 51 minutes (P less than 0.001). This included a median time saving of 17 minutes for EVT hospital arrival to arterial puncture for MSU patients (P = .001). Overall estimated median DALYs saved through earlier provision of reperfusion therapies were 20.9 for thrombolysis and 24.6 for EVT.
“The benefit in EVT patients was primarily driven by prehospital MSU diagnosis of large vessel occlusion, which enabled bypass of a local non-EVT center directly to a comprehensive stroke center in almost 50% of patients with large vessel occlusion,” the researchers wrote. “Even when patients were located close to an EVT center, MSU pre-notification and facilitated workflows achieved a reduction in hospital arrival to arterial puncture by one-third. Furthermore, the time saving was seen despite the majority of EVT patients receiving repeat imaging in hospital to visualize the extracranial circulation.”
The study is scheduled to be presented at the International Stroke Conference on Feb. 20.
The Melbourne MSU received funding from the Australian Commonwealth Government, Victorian State Government, Royal Melbourne Hospital Neurosciences Foundation, Stroke Foundation, the Florey Institute of Neurosciences and Mental Health, the University of Melbourne, Boehringer Ingelheim, and private donation. Dr. Zhao disclosed that he has received grants from the Australian Commonwealth Government and the University of Melbourne and personal fees from Boehringer Ingelheim.
SOURCE: Zhao H et al. Stroke. 2020 Feb 12. doi: 10.1161/strokeaha.119.027843.
In its first year of operation, a mobile stroke unit in Melbourne demonstrated substantial savings in time to commencement of both thrombolysis and endovascular thrombectomy (EVT), results from a prospective study showed.
“While previously published data from MSU [mobile stroke unit] services in Europe and North America show substantial reductions in time to thrombolysis of approximately 30-45 minutes, little is known about the clinical impact on EVT,” first author Henry Zhao, MBBS, and colleagues wrote in a study published in Stroke.
Launched in November 2017, the Melbourne MSU is based at a large comprehensive stroke center and operates with a 20-km radius, servicing about 1.7 million people within the city of Melbourne. It is staffed with an onboard neurologist or senior stroke fellow who provides primary assessment and treatment decisions, a stroke advanced practice nurse who provides clinical support and treatment administration, a clinician who provides CT imaging, and advanced life support and mobile intensive care paramedics who provide transport logistics and paramedicine support. For the current analysis, MSU patients who received reperfusion therapy were compared with control patients presenting to metropolitan Melbourne stroke units via standard ambulance within MSU operating hours. The primary outcome was median time difference in first ambulance dispatch to treatment, which the researchers used quantile regression analysis to determine. Time savings were subsequently converted to disability-adjusted life years (DALY) avoiding using published estimates.
Dr. Zhao of the Melbourne Brain Centre and department of neurology at Royal Melbourne Hospital and his colleagues reported that, in its first year of operation, the Melbourne MSU administered prehospital thrombolysis to 100 patients with a mean age of nearly 74 years. More than half of the patients (62%) were male. Compared with controls, the median time savings per MSU patient was 26 minutes for dispatch to hospital arrival and 15 minutes for hospital arrival to thrombolysis (P less than .0010 for both associations). The calculated overall time saving from dispatch to thrombolysis was 42.5 minutes.
Over the same time period, 41 MSU patients with a mean age of 76 years received EVT dispatch-to-treatment time saving of 51 minutes (P less than 0.001). This included a median time saving of 17 minutes for EVT hospital arrival to arterial puncture for MSU patients (P = .001). Overall estimated median DALYs saved through earlier provision of reperfusion therapies were 20.9 for thrombolysis and 24.6 for EVT.
“The benefit in EVT patients was primarily driven by prehospital MSU diagnosis of large vessel occlusion, which enabled bypass of a local non-EVT center directly to a comprehensive stroke center in almost 50% of patients with large vessel occlusion,” the researchers wrote. “Even when patients were located close to an EVT center, MSU pre-notification and facilitated workflows achieved a reduction in hospital arrival to arterial puncture by one-third. Furthermore, the time saving was seen despite the majority of EVT patients receiving repeat imaging in hospital to visualize the extracranial circulation.”
The study is scheduled to be presented at the International Stroke Conference on Feb. 20.
The Melbourne MSU received funding from the Australian Commonwealth Government, Victorian State Government, Royal Melbourne Hospital Neurosciences Foundation, Stroke Foundation, the Florey Institute of Neurosciences and Mental Health, the University of Melbourne, Boehringer Ingelheim, and private donation. Dr. Zhao disclosed that he has received grants from the Australian Commonwealth Government and the University of Melbourne and personal fees from Boehringer Ingelheim.
SOURCE: Zhao H et al. Stroke. 2020 Feb 12. doi: 10.1161/strokeaha.119.027843.
In its first year of operation, a mobile stroke unit in Melbourne demonstrated substantial savings in time to commencement of both thrombolysis and endovascular thrombectomy (EVT), results from a prospective study showed.
“While previously published data from MSU [mobile stroke unit] services in Europe and North America show substantial reductions in time to thrombolysis of approximately 30-45 minutes, little is known about the clinical impact on EVT,” first author Henry Zhao, MBBS, and colleagues wrote in a study published in Stroke.
Launched in November 2017, the Melbourne MSU is based at a large comprehensive stroke center and operates with a 20-km radius, servicing about 1.7 million people within the city of Melbourne. It is staffed with an onboard neurologist or senior stroke fellow who provides primary assessment and treatment decisions, a stroke advanced practice nurse who provides clinical support and treatment administration, a clinician who provides CT imaging, and advanced life support and mobile intensive care paramedics who provide transport logistics and paramedicine support. For the current analysis, MSU patients who received reperfusion therapy were compared with control patients presenting to metropolitan Melbourne stroke units via standard ambulance within MSU operating hours. The primary outcome was median time difference in first ambulance dispatch to treatment, which the researchers used quantile regression analysis to determine. Time savings were subsequently converted to disability-adjusted life years (DALY) avoiding using published estimates.
Dr. Zhao of the Melbourne Brain Centre and department of neurology at Royal Melbourne Hospital and his colleagues reported that, in its first year of operation, the Melbourne MSU administered prehospital thrombolysis to 100 patients with a mean age of nearly 74 years. More than half of the patients (62%) were male. Compared with controls, the median time savings per MSU patient was 26 minutes for dispatch to hospital arrival and 15 minutes for hospital arrival to thrombolysis (P less than .0010 for both associations). The calculated overall time saving from dispatch to thrombolysis was 42.5 minutes.
Over the same time period, 41 MSU patients with a mean age of 76 years received EVT dispatch-to-treatment time saving of 51 minutes (P less than 0.001). This included a median time saving of 17 minutes for EVT hospital arrival to arterial puncture for MSU patients (P = .001). Overall estimated median DALYs saved through earlier provision of reperfusion therapies were 20.9 for thrombolysis and 24.6 for EVT.
“The benefit in EVT patients was primarily driven by prehospital MSU diagnosis of large vessel occlusion, which enabled bypass of a local non-EVT center directly to a comprehensive stroke center in almost 50% of patients with large vessel occlusion,” the researchers wrote. “Even when patients were located close to an EVT center, MSU pre-notification and facilitated workflows achieved a reduction in hospital arrival to arterial puncture by one-third. Furthermore, the time saving was seen despite the majority of EVT patients receiving repeat imaging in hospital to visualize the extracranial circulation.”
The study is scheduled to be presented at the International Stroke Conference on Feb. 20.
The Melbourne MSU received funding from the Australian Commonwealth Government, Victorian State Government, Royal Melbourne Hospital Neurosciences Foundation, Stroke Foundation, the Florey Institute of Neurosciences and Mental Health, the University of Melbourne, Boehringer Ingelheim, and private donation. Dr. Zhao disclosed that he has received grants from the Australian Commonwealth Government and the University of Melbourne and personal fees from Boehringer Ingelheim.
SOURCE: Zhao H et al. Stroke. 2020 Feb 12. doi: 10.1161/strokeaha.119.027843.
FROM STROKE
Key clinical point: A mobile stroke unit (MSU) substantially reduced time to reperfusion therapies.
Major finding: Compared with controls, the median time savings per MSU patient was 26 minutes for dispatch to hospital arrival and 15 minutes for hospital arrival to thrombolysis (P less than .0010 for both associations).
Study details: A prospective study of 100 stroke patients.
Disclosures: The Melbourne MSU received funding from the Australian Commonwealth Government, Victorian State Government, Royal Melbourne Hospital Neurosciences Foundation, Stroke Foundation, the Florey Institute of Neurosciences and Mental Health, the University of Melbourne, Boehringer Ingelheim, and private donation. Dr. Zhao disclosed that he has received grants from the Australian Commonwealth Government and the University of Melbourne and personal fees from Boehringer Ingelheim.
Source: Zhao H et al. Stroke. 2020 Feb 12. doi: 10.1161/strokeaha.119.027843.
Be alert for embezzlement
With myriad complex, high-tech problems facing private practice in this modern era, I am periodically reminded by long-time readers to revisit some of the low-tech issues that will always require our attention.
Few are lower tech (in most cases) and more easily overlooked than theft from within. Embezzlement remains far more common in medical offices than generally assumed – and it often occurs in full view of physicians who think everything is fine. Most embezzlers are not skillful or discreet; their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
- Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: The person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
- Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
- Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Last year, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
- Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.” Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
- Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
- Consider computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and there should be safeguards built into your system. Ask about them. If they aren’t there, ask why.
- Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information web sites. My columns on hiring are available on the MDedge Dermatology website.
- Look for “red flags.” Examples include employees who refuse to take vacations, because someone else will have do their work or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
- Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and provide assurance of some measure of recovery if your safeguards fail. Also, just knowing that your staff is bonded will scare off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
With myriad complex, high-tech problems facing private practice in this modern era, I am periodically reminded by long-time readers to revisit some of the low-tech issues that will always require our attention.
Few are lower tech (in most cases) and more easily overlooked than theft from within. Embezzlement remains far more common in medical offices than generally assumed – and it often occurs in full view of physicians who think everything is fine. Most embezzlers are not skillful or discreet; their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
- Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: The person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
- Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
- Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Last year, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
- Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.” Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
- Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
- Consider computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and there should be safeguards built into your system. Ask about them. If they aren’t there, ask why.
- Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information web sites. My columns on hiring are available on the MDedge Dermatology website.
- Look for “red flags.” Examples include employees who refuse to take vacations, because someone else will have do their work or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
- Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and provide assurance of some measure of recovery if your safeguards fail. Also, just knowing that your staff is bonded will scare off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
With myriad complex, high-tech problems facing private practice in this modern era, I am periodically reminded by long-time readers to revisit some of the low-tech issues that will always require our attention.
Few are lower tech (in most cases) and more easily overlooked than theft from within. Embezzlement remains far more common in medical offices than generally assumed – and it often occurs in full view of physicians who think everything is fine. Most embezzlers are not skillful or discreet; their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
- Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: The person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
- Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
- Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Last year, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
- Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.” Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
- Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
- Consider computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and there should be safeguards built into your system. Ask about them. If they aren’t there, ask why.
- Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information web sites. My columns on hiring are available on the MDedge Dermatology website.
- Look for “red flags.” Examples include employees who refuse to take vacations, because someone else will have do their work or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
- Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and provide assurance of some measure of recovery if your safeguards fail. Also, just knowing that your staff is bonded will scare off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
What you absolutely need to know about tail coverage
A 28-year-old pediatrician working in a large group practice in California found a new job in Pennsylvania. The job would allow her to live with her husband, who was a nonphysician.
On her last day of work at the California job, the practice’s office manager asked her, “Do you know about the tail coverage?”
He explained that it is malpractice insurance for any cases filed against her after leaving the job. Without it, he said, she would not be covered for those claims.
The physician (who asked not to be identified) had very little savings and suddenly had to pay a five-figure bill for tail coverage. To provide the extra malpractice coverage, she and her husband had to use savings they’d set aside to buy a house.
Getting tail coverage, known formally as an extended reporting endorsement, often comes as a complete and costly surprise for new doctors, says Dennis Hursh, Esq, a health care attorney based in Middletown, Penn., who deals with physicians’ employment contracts.
“Having to pay for a tail can disrupt lives,” Hursh said. “A tail can cost about one third of a young doctor’s salary. If you don’t feel you can afford to pay that, you may be forced to stay with a job you don’t like.”
Most medical residents don’t think about tail coverage until they apply for their first job, but last year, residents at Hahnemann University Hospital in Philadelphia got a painful early lesson.
In the summer, the hospital went out of business because of financial problems. Hundreds of medical residents and fellows not only were forced to find new programs but also had to prepare to buy tail coverage for their training years at Hahnemann.
“All the guarantees have been yanked out from under us,” said Tom Sibert, MD, a former internal medicine resident at the hospital, who is now finishing his training in California. “Residents don’t have that kind of money.”
Hahnemann trainees have asked the judge in the bankruptcy proceedings to put them ahead of other creditors and to ensure their tail coverage is paid. As of early February, the issue had not been resolved.
Meanwhile, Sibert and many other former trainees were trying to get quotes for purchasing tail coverage. They have been shocked by the amounts they would have to pay.
How tail coverage works
Medical malpractice tail coverage protects from incidents that took place when doctors were at their previous jobs but that later resulted in malpractice claims after they had left that employer.
One type of malpractice insurance, an occurrence policy, does not need tail coverage. Occurrence policies cover any incident that occurred when the policy was in force, no matter when a claim was filed – even if it is filed many years after the claims-filing period of the policy ends.
However, most malpractice policies – as many as 85%, according to one estimate – are claims-made policies. Claims-made policies are more much common because they’re significantly less expensive than occurrence policies.
Under a claims-made policy, coverage for malpractice claims completely stops when the policy ends. It does not cover incidents that occurred when the policy was in force but for which the patients later filed claims, as the occurrence policy does. So a tail is needed to cover these claims.
Physicians in all stages of their career may need tail coverage when they leave a job, change malpractice carriers, or retire.
But young physicians often have greater problems with tail coverage, for several reasons. They tend to be employed, and as such, they cannot choose the coverage they want. As a result, they most likely get claims-made coverage. In addition, the job turnover tends to be higher for these doctors. When leaving a job, the tail comes into play. More than half of new physicians leave their first job within 5 years, and of those, more than half leave after only 1 or 2 years.
Young physicians have no experience with tails and may not even know what they are. “In training, malpractice coverage is not a problem because the program handles it,” Mr. Hursh said. Accreditation standards require that teaching hospitals buy coverage, including a tail when residents leave.
So when young physicians are offered their first job and are handed an employment contract to sign, they may not even look for tail coverage, says Mr. Hursh, who wrote The Final Hurdle, a Physician’s Guide to Negotiating a Fair Employment Agreement. Instead, “young physicians tend to focus on issues like salary, benefits, and signing bonuses,” he said.
Mr. Hursh says the tail is usually the most expensive potential cost in the contract.
There’s no easy way to get out of paying the tail coverage once it is enshrined in the contract. The full tail can cost five or even six figures, depending on the physicians’ specialty, the local malpractice premium, and the physician’s own claims history.
Can you negotiate your tail coverage?
Negotiating tail coverage in the employment contract involves some familiarity with medical malpractice insurance and a close reading of the contract. First, you have to determine that the employer is providing claims-made coverage, which would require a tail if you leave. Then you have to determine whether the employer will pay for the tail coverage.
Often, the contract does not even mention tail coverage. “It could merely state that the practice will be responsible for malpractice coverage while you are working there,” Mr. Hursh said. Although it never specifies the tail, this language indicates that you will be paying for it, he says.
Therefore, it’s wise to have a conversation with your prospective employer about the tail. “Some new doctors never ask the question ‘What happens if I leave? Do I get tail coverage?’ ” said Israel Teitelbaum, an attorney who is chairman of Contemporary Insurance Services, an insurance broker in Silver Spring, Md.
Talking about the tail, however, can be a touchy subject for many young doctors applying for their first job. The tail matters only if you leave the job, and you may not want to imply that you would ever want to leave. Too much money, however, is on the line for you not to ask, Mr. Teitelbaum said.
Even if the employer verbally agrees to pay for the tail coverage, experts advise that you try to get the employer’s commitment in writing and have it put it into the contract.
Getting the employer to cover the tail in the initial contract is crucial because once you have agreed to work there, “it’s much more difficult to get it changed,” Mr. Teitelbaum said. However, even if tail coverage is not in the first contract, you shouldn’t give up, he says. You should try again in the next contract a few years later.
“It’s never too late to bring it up,” Mr. Teitelbaum said. After a few years of employment, you have a track record at the job. “A doctor who is very desirable to the employer may be able to get tail coverage on contract renewal.”
Coverage: Large employers vs. small employers
Willingness to pay for an employee’s tail coverage varies depending on the size of the employer. Large employers – systems, hospitals, and large practices – are much more likely to cover the tail than small and medium-sized practices.
Large employers tend to pay for at least part of the tail because they realize that it is in their interest to do so. Since they have the deepest pockets, they’re often the first to be named in a lawsuit. They might have to pay the whole claim if the physician did not have tail coverage.
However, many large employers want to use tail coverage as a bargaining chip to make sure doctors stay for a while at least. One typical arrangement, Mr. Hursh says, is to pay only one-fifth of the tail if the physician leaves in the first year of employment and then to pay one fifth more in each succeeding year until year five, when the employer assumes the entire cost of the tail.
Smaller practices, on the other hand, are usually close-fisted about tail coverage. “They tend to view the tail as an unnecessary expense,” Mr. Hursh said. “They don’t want to pay for a doctor who is not generating revenue for them any more.”
Traditionally, when physicians become partners, practices are more generous and agree to pay their tails if they leave, Mr. Hursh says. But he thinks this is changing, too – recent partnership contracts he has reviewed did not provide for tail coverage.
Times you don’t need to pay for tail coverage
Even if you’re responsible for the tail coverage, your insurance arrangement may be such that you don’t have to pay for it, says Michelle Perron, a malpractice insurance broker in North Hampton, N.H.
For example, if the carrier at your new job is the same as the one at your old job, your coverage would continue with no break, and you would not need a tail, she says. Even if you move to another state, your old carrier might also sell policies there, and you would then likely have seamless coverage, Ms. Perron says. This would be handy if you could choose your new carrier.
Even when you change carriers, Ms. Perron says, the new one might agree to pick up the old carrier’s coverage in return for getting your business, assuming you are an independent physician buying your own coverage. The new carrier would issue prior acts coverage, also known as nose coverage.
Older doctors going into retirement also have a potential tail coverage problem, but their tail coverage premium is often waived, Ms. Perron says. The need for a tail has to do with claims arising post retirement, after your coverage has ended. Typically, if you have been with the carrier for at least 5 years and you are age 55 years or older, your carrier will waive the tail coverage premium, she says.
However, if the retired doctor starts practicing again, even part time, the carrier may want to take back the free tail, she says. Some retired doctors get around this by buying a lower-priced tail from another company, but the former carrier may still want its money back, Ms. Perron says.
Can you just go without tail coverage?
What happens if physicians with a tail commitment choose to wing it and not pay for the tail? If a claim was never made against them, they may believe that the expense is unnecessary. The situation, however, is not so simple.
Some states require having tail coverage. Malpractice coverage is required in seven states, and at least some of those states explicitly extend this requirement to tails. They are Colorado, Connecticut, Kansas, Massachusetts, New Jersey, Rhode Island, and Wisconsin. Eleven more states tie malpractice coverage, perhaps including tails, to some benefit for the doctor, such as tort reform. These states include Indiana, Nebraska, New Mexico, New York, and Pennsylvania.
Many hospitals require tail coverage for privileges, and some insurers do as well. In addition, Ms. Perron says a missing tail reduces your prospects when looking for a job. “For the employer, having to pay coverage for a new hire will cost more than starting fresh with someone else,” she said.
Still, it’s important to remember the risk of being sued. “If you don’t buy the tail coverage, you are at risk for a lawsuit for many years to come,” Mr. Teitelbaum said.
Doctors should consider their potential lifetime risk, not just their current risk. Although only 8% of doctors younger than age 40 have been sued for malpractice, that figure climbs to almost half by the time doctors reach age 55.
The risks are higher in some specialties. About 63% of general surgeons and ob.gyns. have been sued.
Many of these claims are without merit, and doctors pay only the legal expenses of defending the case. Some doctors may think they could risk frivolous suits and cover legal expenses out of pocket. An American Medical Association survey showed that 68% of closed claims against doctors were dropped, dismissed, or withdrawn. It said these claims cost an average of more than $30,000 to defend.
However, Mr. Teitelbaum puts the defense costs for so-called frivolous suits much higher than the AMA, at $250,000 or more. “Even if you’re sure you won’t have to pay a claim, you still have to defend yourself against frivolous suits,” he said. “You won’t recover those expenses.”
How to lower your tail coverage cost
Physicians typically have 60 days to buy tail coverage after their regular coverage has ended. Specialized brokers such as Mr. Teitelbaum and Ms. Perron help physicians look for the best tails to buy.
The cost of the tail depends on how long you’ve been at your job when you leave it, Ms. Perron says. If you leave in the first 1 or 2 years of the policy, she says, the tail price will be lower because the coverage period is shorter.
Usually the most expensive tail available is from the carrier that issued the original policy. Why is this? “Carriers rarely sell a tail that undercuts their retail price,” Mr. Teitelbaum said. “They don’t want to compete with themselves, and in fact doing so could pose regulatory problems for them.”
Instead of buying from their own carrier, doctors can purchase stand-alone tails from competitors, which Mr. Teitelbaum says are 10%-30% less expensive than the policy the original carrier issues. However, stand-alone tails are not always easy to find, especially for high-cost specialties such as neurosurgery and ob.gyn., he says.
Some physicians try to bring down the cost of the tail by limiting the duration of the tail. You can buy tails that only cover claims filed 1-5 years after the incident took place, rather than indefinitely. These limits mirror the typical statute of limitations – the time limit to file a claim in each state. This limit is as little as 2 years in some states, though it can be as long as 6 years in others.
However, some states make exceptions to the statute of limitations. The 2- to 6-year clock doesn’t start ticking until the mistake is discovered or, in the case of children, when they reach adulthood. “This means that with a limited tail, you always have risk,” Perron said.
And yet some doctors insist on these time-limited tails. “If a doctor opts for 3 years’ coverage, that’s better than no years,” Mr. Teitelbaum said. “But I would advise them to take at least 5 years because that gives you coverage for the basic statute of limitations in most states. Three-year tails do yield savings, but often they’re not enough to warrant the risk.”
Another way to reduce costs is to lower the coverage limits of the tail. The standard coverage limit is $1 million per case and $3 million per year, so doctors might be able to save money on the premium by buying limits of $200,000/$600,000. But Mr. Teitelbaum says most companies would refuse to sell a policy with a limit lower than that of the expiring policy.
Further ways to reduce the cost of the tail include buying tail coverage that doesn’t give the physician the right to approve a settlement or that doesn’t include legal fees in the coverage limits. But these options, too, raise the physician’s risks. Whichever option you choose, the important thing is to protect yourself against costly lawsuits.
This article first appeared on Medscape.com.
A 28-year-old pediatrician working in a large group practice in California found a new job in Pennsylvania. The job would allow her to live with her husband, who was a nonphysician.
On her last day of work at the California job, the practice’s office manager asked her, “Do you know about the tail coverage?”
He explained that it is malpractice insurance for any cases filed against her after leaving the job. Without it, he said, she would not be covered for those claims.
The physician (who asked not to be identified) had very little savings and suddenly had to pay a five-figure bill for tail coverage. To provide the extra malpractice coverage, she and her husband had to use savings they’d set aside to buy a house.
Getting tail coverage, known formally as an extended reporting endorsement, often comes as a complete and costly surprise for new doctors, says Dennis Hursh, Esq, a health care attorney based in Middletown, Penn., who deals with physicians’ employment contracts.
“Having to pay for a tail can disrupt lives,” Hursh said. “A tail can cost about one third of a young doctor’s salary. If you don’t feel you can afford to pay that, you may be forced to stay with a job you don’t like.”
Most medical residents don’t think about tail coverage until they apply for their first job, but last year, residents at Hahnemann University Hospital in Philadelphia got a painful early lesson.
In the summer, the hospital went out of business because of financial problems. Hundreds of medical residents and fellows not only were forced to find new programs but also had to prepare to buy tail coverage for their training years at Hahnemann.
“All the guarantees have been yanked out from under us,” said Tom Sibert, MD, a former internal medicine resident at the hospital, who is now finishing his training in California. “Residents don’t have that kind of money.”
Hahnemann trainees have asked the judge in the bankruptcy proceedings to put them ahead of other creditors and to ensure their tail coverage is paid. As of early February, the issue had not been resolved.
Meanwhile, Sibert and many other former trainees were trying to get quotes for purchasing tail coverage. They have been shocked by the amounts they would have to pay.
How tail coverage works
Medical malpractice tail coverage protects from incidents that took place when doctors were at their previous jobs but that later resulted in malpractice claims after they had left that employer.
One type of malpractice insurance, an occurrence policy, does not need tail coverage. Occurrence policies cover any incident that occurred when the policy was in force, no matter when a claim was filed – even if it is filed many years after the claims-filing period of the policy ends.
However, most malpractice policies – as many as 85%, according to one estimate – are claims-made policies. Claims-made policies are more much common because they’re significantly less expensive than occurrence policies.
Under a claims-made policy, coverage for malpractice claims completely stops when the policy ends. It does not cover incidents that occurred when the policy was in force but for which the patients later filed claims, as the occurrence policy does. So a tail is needed to cover these claims.
Physicians in all stages of their career may need tail coverage when they leave a job, change malpractice carriers, or retire.
But young physicians often have greater problems with tail coverage, for several reasons. They tend to be employed, and as such, they cannot choose the coverage they want. As a result, they most likely get claims-made coverage. In addition, the job turnover tends to be higher for these doctors. When leaving a job, the tail comes into play. More than half of new physicians leave their first job within 5 years, and of those, more than half leave after only 1 or 2 years.
Young physicians have no experience with tails and may not even know what they are. “In training, malpractice coverage is not a problem because the program handles it,” Mr. Hursh said. Accreditation standards require that teaching hospitals buy coverage, including a tail when residents leave.
So when young physicians are offered their first job and are handed an employment contract to sign, they may not even look for tail coverage, says Mr. Hursh, who wrote The Final Hurdle, a Physician’s Guide to Negotiating a Fair Employment Agreement. Instead, “young physicians tend to focus on issues like salary, benefits, and signing bonuses,” he said.
Mr. Hursh says the tail is usually the most expensive potential cost in the contract.
There’s no easy way to get out of paying the tail coverage once it is enshrined in the contract. The full tail can cost five or even six figures, depending on the physicians’ specialty, the local malpractice premium, and the physician’s own claims history.
Can you negotiate your tail coverage?
Negotiating tail coverage in the employment contract involves some familiarity with medical malpractice insurance and a close reading of the contract. First, you have to determine that the employer is providing claims-made coverage, which would require a tail if you leave. Then you have to determine whether the employer will pay for the tail coverage.
Often, the contract does not even mention tail coverage. “It could merely state that the practice will be responsible for malpractice coverage while you are working there,” Mr. Hursh said. Although it never specifies the tail, this language indicates that you will be paying for it, he says.
Therefore, it’s wise to have a conversation with your prospective employer about the tail. “Some new doctors never ask the question ‘What happens if I leave? Do I get tail coverage?’ ” said Israel Teitelbaum, an attorney who is chairman of Contemporary Insurance Services, an insurance broker in Silver Spring, Md.
Talking about the tail, however, can be a touchy subject for many young doctors applying for their first job. The tail matters only if you leave the job, and you may not want to imply that you would ever want to leave. Too much money, however, is on the line for you not to ask, Mr. Teitelbaum said.
Even if the employer verbally agrees to pay for the tail coverage, experts advise that you try to get the employer’s commitment in writing and have it put it into the contract.
Getting the employer to cover the tail in the initial contract is crucial because once you have agreed to work there, “it’s much more difficult to get it changed,” Mr. Teitelbaum said. However, even if tail coverage is not in the first contract, you shouldn’t give up, he says. You should try again in the next contract a few years later.
“It’s never too late to bring it up,” Mr. Teitelbaum said. After a few years of employment, you have a track record at the job. “A doctor who is very desirable to the employer may be able to get tail coverage on contract renewal.”
Coverage: Large employers vs. small employers
Willingness to pay for an employee’s tail coverage varies depending on the size of the employer. Large employers – systems, hospitals, and large practices – are much more likely to cover the tail than small and medium-sized practices.
Large employers tend to pay for at least part of the tail because they realize that it is in their interest to do so. Since they have the deepest pockets, they’re often the first to be named in a lawsuit. They might have to pay the whole claim if the physician did not have tail coverage.
However, many large employers want to use tail coverage as a bargaining chip to make sure doctors stay for a while at least. One typical arrangement, Mr. Hursh says, is to pay only one-fifth of the tail if the physician leaves in the first year of employment and then to pay one fifth more in each succeeding year until year five, when the employer assumes the entire cost of the tail.
Smaller practices, on the other hand, are usually close-fisted about tail coverage. “They tend to view the tail as an unnecessary expense,” Mr. Hursh said. “They don’t want to pay for a doctor who is not generating revenue for them any more.”
Traditionally, when physicians become partners, practices are more generous and agree to pay their tails if they leave, Mr. Hursh says. But he thinks this is changing, too – recent partnership contracts he has reviewed did not provide for tail coverage.
Times you don’t need to pay for tail coverage
Even if you’re responsible for the tail coverage, your insurance arrangement may be such that you don’t have to pay for it, says Michelle Perron, a malpractice insurance broker in North Hampton, N.H.
For example, if the carrier at your new job is the same as the one at your old job, your coverage would continue with no break, and you would not need a tail, she says. Even if you move to another state, your old carrier might also sell policies there, and you would then likely have seamless coverage, Ms. Perron says. This would be handy if you could choose your new carrier.
Even when you change carriers, Ms. Perron says, the new one might agree to pick up the old carrier’s coverage in return for getting your business, assuming you are an independent physician buying your own coverage. The new carrier would issue prior acts coverage, also known as nose coverage.
Older doctors going into retirement also have a potential tail coverage problem, but their tail coverage premium is often waived, Ms. Perron says. The need for a tail has to do with claims arising post retirement, after your coverage has ended. Typically, if you have been with the carrier for at least 5 years and you are age 55 years or older, your carrier will waive the tail coverage premium, she says.
However, if the retired doctor starts practicing again, even part time, the carrier may want to take back the free tail, she says. Some retired doctors get around this by buying a lower-priced tail from another company, but the former carrier may still want its money back, Ms. Perron says.
Can you just go without tail coverage?
What happens if physicians with a tail commitment choose to wing it and not pay for the tail? If a claim was never made against them, they may believe that the expense is unnecessary. The situation, however, is not so simple.
Some states require having tail coverage. Malpractice coverage is required in seven states, and at least some of those states explicitly extend this requirement to tails. They are Colorado, Connecticut, Kansas, Massachusetts, New Jersey, Rhode Island, and Wisconsin. Eleven more states tie malpractice coverage, perhaps including tails, to some benefit for the doctor, such as tort reform. These states include Indiana, Nebraska, New Mexico, New York, and Pennsylvania.
Many hospitals require tail coverage for privileges, and some insurers do as well. In addition, Ms. Perron says a missing tail reduces your prospects when looking for a job. “For the employer, having to pay coverage for a new hire will cost more than starting fresh with someone else,” she said.
Still, it’s important to remember the risk of being sued. “If you don’t buy the tail coverage, you are at risk for a lawsuit for many years to come,” Mr. Teitelbaum said.
Doctors should consider their potential lifetime risk, not just their current risk. Although only 8% of doctors younger than age 40 have been sued for malpractice, that figure climbs to almost half by the time doctors reach age 55.
The risks are higher in some specialties. About 63% of general surgeons and ob.gyns. have been sued.
Many of these claims are without merit, and doctors pay only the legal expenses of defending the case. Some doctors may think they could risk frivolous suits and cover legal expenses out of pocket. An American Medical Association survey showed that 68% of closed claims against doctors were dropped, dismissed, or withdrawn. It said these claims cost an average of more than $30,000 to defend.
However, Mr. Teitelbaum puts the defense costs for so-called frivolous suits much higher than the AMA, at $250,000 or more. “Even if you’re sure you won’t have to pay a claim, you still have to defend yourself against frivolous suits,” he said. “You won’t recover those expenses.”
How to lower your tail coverage cost
Physicians typically have 60 days to buy tail coverage after their regular coverage has ended. Specialized brokers such as Mr. Teitelbaum and Ms. Perron help physicians look for the best tails to buy.
The cost of the tail depends on how long you’ve been at your job when you leave it, Ms. Perron says. If you leave in the first 1 or 2 years of the policy, she says, the tail price will be lower because the coverage period is shorter.
Usually the most expensive tail available is from the carrier that issued the original policy. Why is this? “Carriers rarely sell a tail that undercuts their retail price,” Mr. Teitelbaum said. “They don’t want to compete with themselves, and in fact doing so could pose regulatory problems for them.”
Instead of buying from their own carrier, doctors can purchase stand-alone tails from competitors, which Mr. Teitelbaum says are 10%-30% less expensive than the policy the original carrier issues. However, stand-alone tails are not always easy to find, especially for high-cost specialties such as neurosurgery and ob.gyn., he says.
Some physicians try to bring down the cost of the tail by limiting the duration of the tail. You can buy tails that only cover claims filed 1-5 years after the incident took place, rather than indefinitely. These limits mirror the typical statute of limitations – the time limit to file a claim in each state. This limit is as little as 2 years in some states, though it can be as long as 6 years in others.
However, some states make exceptions to the statute of limitations. The 2- to 6-year clock doesn’t start ticking until the mistake is discovered or, in the case of children, when they reach adulthood. “This means that with a limited tail, you always have risk,” Perron said.
And yet some doctors insist on these time-limited tails. “If a doctor opts for 3 years’ coverage, that’s better than no years,” Mr. Teitelbaum said. “But I would advise them to take at least 5 years because that gives you coverage for the basic statute of limitations in most states. Three-year tails do yield savings, but often they’re not enough to warrant the risk.”
Another way to reduce costs is to lower the coverage limits of the tail. The standard coverage limit is $1 million per case and $3 million per year, so doctors might be able to save money on the premium by buying limits of $200,000/$600,000. But Mr. Teitelbaum says most companies would refuse to sell a policy with a limit lower than that of the expiring policy.
Further ways to reduce the cost of the tail include buying tail coverage that doesn’t give the physician the right to approve a settlement or that doesn’t include legal fees in the coverage limits. But these options, too, raise the physician’s risks. Whichever option you choose, the important thing is to protect yourself against costly lawsuits.
This article first appeared on Medscape.com.
A 28-year-old pediatrician working in a large group practice in California found a new job in Pennsylvania. The job would allow her to live with her husband, who was a nonphysician.
On her last day of work at the California job, the practice’s office manager asked her, “Do you know about the tail coverage?”
He explained that it is malpractice insurance for any cases filed against her after leaving the job. Without it, he said, she would not be covered for those claims.
The physician (who asked not to be identified) had very little savings and suddenly had to pay a five-figure bill for tail coverage. To provide the extra malpractice coverage, she and her husband had to use savings they’d set aside to buy a house.
Getting tail coverage, known formally as an extended reporting endorsement, often comes as a complete and costly surprise for new doctors, says Dennis Hursh, Esq, a health care attorney based in Middletown, Penn., who deals with physicians’ employment contracts.
“Having to pay for a tail can disrupt lives,” Hursh said. “A tail can cost about one third of a young doctor’s salary. If you don’t feel you can afford to pay that, you may be forced to stay with a job you don’t like.”
Most medical residents don’t think about tail coverage until they apply for their first job, but last year, residents at Hahnemann University Hospital in Philadelphia got a painful early lesson.
In the summer, the hospital went out of business because of financial problems. Hundreds of medical residents and fellows not only were forced to find new programs but also had to prepare to buy tail coverage for their training years at Hahnemann.
“All the guarantees have been yanked out from under us,” said Tom Sibert, MD, a former internal medicine resident at the hospital, who is now finishing his training in California. “Residents don’t have that kind of money.”
Hahnemann trainees have asked the judge in the bankruptcy proceedings to put them ahead of other creditors and to ensure their tail coverage is paid. As of early February, the issue had not been resolved.
Meanwhile, Sibert and many other former trainees were trying to get quotes for purchasing tail coverage. They have been shocked by the amounts they would have to pay.
How tail coverage works
Medical malpractice tail coverage protects from incidents that took place when doctors were at their previous jobs but that later resulted in malpractice claims after they had left that employer.
One type of malpractice insurance, an occurrence policy, does not need tail coverage. Occurrence policies cover any incident that occurred when the policy was in force, no matter when a claim was filed – even if it is filed many years after the claims-filing period of the policy ends.
However, most malpractice policies – as many as 85%, according to one estimate – are claims-made policies. Claims-made policies are more much common because they’re significantly less expensive than occurrence policies.
Under a claims-made policy, coverage for malpractice claims completely stops when the policy ends. It does not cover incidents that occurred when the policy was in force but for which the patients later filed claims, as the occurrence policy does. So a tail is needed to cover these claims.
Physicians in all stages of their career may need tail coverage when they leave a job, change malpractice carriers, or retire.
But young physicians often have greater problems with tail coverage, for several reasons. They tend to be employed, and as such, they cannot choose the coverage they want. As a result, they most likely get claims-made coverage. In addition, the job turnover tends to be higher for these doctors. When leaving a job, the tail comes into play. More than half of new physicians leave their first job within 5 years, and of those, more than half leave after only 1 or 2 years.
Young physicians have no experience with tails and may not even know what they are. “In training, malpractice coverage is not a problem because the program handles it,” Mr. Hursh said. Accreditation standards require that teaching hospitals buy coverage, including a tail when residents leave.
So when young physicians are offered their first job and are handed an employment contract to sign, they may not even look for tail coverage, says Mr. Hursh, who wrote The Final Hurdle, a Physician’s Guide to Negotiating a Fair Employment Agreement. Instead, “young physicians tend to focus on issues like salary, benefits, and signing bonuses,” he said.
Mr. Hursh says the tail is usually the most expensive potential cost in the contract.
There’s no easy way to get out of paying the tail coverage once it is enshrined in the contract. The full tail can cost five or even six figures, depending on the physicians’ specialty, the local malpractice premium, and the physician’s own claims history.
Can you negotiate your tail coverage?
Negotiating tail coverage in the employment contract involves some familiarity with medical malpractice insurance and a close reading of the contract. First, you have to determine that the employer is providing claims-made coverage, which would require a tail if you leave. Then you have to determine whether the employer will pay for the tail coverage.
Often, the contract does not even mention tail coverage. “It could merely state that the practice will be responsible for malpractice coverage while you are working there,” Mr. Hursh said. Although it never specifies the tail, this language indicates that you will be paying for it, he says.
Therefore, it’s wise to have a conversation with your prospective employer about the tail. “Some new doctors never ask the question ‘What happens if I leave? Do I get tail coverage?’ ” said Israel Teitelbaum, an attorney who is chairman of Contemporary Insurance Services, an insurance broker in Silver Spring, Md.
Talking about the tail, however, can be a touchy subject for many young doctors applying for their first job. The tail matters only if you leave the job, and you may not want to imply that you would ever want to leave. Too much money, however, is on the line for you not to ask, Mr. Teitelbaum said.
Even if the employer verbally agrees to pay for the tail coverage, experts advise that you try to get the employer’s commitment in writing and have it put it into the contract.
Getting the employer to cover the tail in the initial contract is crucial because once you have agreed to work there, “it’s much more difficult to get it changed,” Mr. Teitelbaum said. However, even if tail coverage is not in the first contract, you shouldn’t give up, he says. You should try again in the next contract a few years later.
“It’s never too late to bring it up,” Mr. Teitelbaum said. After a few years of employment, you have a track record at the job. “A doctor who is very desirable to the employer may be able to get tail coverage on contract renewal.”
Coverage: Large employers vs. small employers
Willingness to pay for an employee’s tail coverage varies depending on the size of the employer. Large employers – systems, hospitals, and large practices – are much more likely to cover the tail than small and medium-sized practices.
Large employers tend to pay for at least part of the tail because they realize that it is in their interest to do so. Since they have the deepest pockets, they’re often the first to be named in a lawsuit. They might have to pay the whole claim if the physician did not have tail coverage.
However, many large employers want to use tail coverage as a bargaining chip to make sure doctors stay for a while at least. One typical arrangement, Mr. Hursh says, is to pay only one-fifth of the tail if the physician leaves in the first year of employment and then to pay one fifth more in each succeeding year until year five, when the employer assumes the entire cost of the tail.
Smaller practices, on the other hand, are usually close-fisted about tail coverage. “They tend to view the tail as an unnecessary expense,” Mr. Hursh said. “They don’t want to pay for a doctor who is not generating revenue for them any more.”
Traditionally, when physicians become partners, practices are more generous and agree to pay their tails if they leave, Mr. Hursh says. But he thinks this is changing, too – recent partnership contracts he has reviewed did not provide for tail coverage.
Times you don’t need to pay for tail coverage
Even if you’re responsible for the tail coverage, your insurance arrangement may be such that you don’t have to pay for it, says Michelle Perron, a malpractice insurance broker in North Hampton, N.H.
For example, if the carrier at your new job is the same as the one at your old job, your coverage would continue with no break, and you would not need a tail, she says. Even if you move to another state, your old carrier might also sell policies there, and you would then likely have seamless coverage, Ms. Perron says. This would be handy if you could choose your new carrier.
Even when you change carriers, Ms. Perron says, the new one might agree to pick up the old carrier’s coverage in return for getting your business, assuming you are an independent physician buying your own coverage. The new carrier would issue prior acts coverage, also known as nose coverage.
Older doctors going into retirement also have a potential tail coverage problem, but their tail coverage premium is often waived, Ms. Perron says. The need for a tail has to do with claims arising post retirement, after your coverage has ended. Typically, if you have been with the carrier for at least 5 years and you are age 55 years or older, your carrier will waive the tail coverage premium, she says.
However, if the retired doctor starts practicing again, even part time, the carrier may want to take back the free tail, she says. Some retired doctors get around this by buying a lower-priced tail from another company, but the former carrier may still want its money back, Ms. Perron says.
Can you just go without tail coverage?
What happens if physicians with a tail commitment choose to wing it and not pay for the tail? If a claim was never made against them, they may believe that the expense is unnecessary. The situation, however, is not so simple.
Some states require having tail coverage. Malpractice coverage is required in seven states, and at least some of those states explicitly extend this requirement to tails. They are Colorado, Connecticut, Kansas, Massachusetts, New Jersey, Rhode Island, and Wisconsin. Eleven more states tie malpractice coverage, perhaps including tails, to some benefit for the doctor, such as tort reform. These states include Indiana, Nebraska, New Mexico, New York, and Pennsylvania.
Many hospitals require tail coverage for privileges, and some insurers do as well. In addition, Ms. Perron says a missing tail reduces your prospects when looking for a job. “For the employer, having to pay coverage for a new hire will cost more than starting fresh with someone else,” she said.
Still, it’s important to remember the risk of being sued. “If you don’t buy the tail coverage, you are at risk for a lawsuit for many years to come,” Mr. Teitelbaum said.
Doctors should consider their potential lifetime risk, not just their current risk. Although only 8% of doctors younger than age 40 have been sued for malpractice, that figure climbs to almost half by the time doctors reach age 55.
The risks are higher in some specialties. About 63% of general surgeons and ob.gyns. have been sued.
Many of these claims are without merit, and doctors pay only the legal expenses of defending the case. Some doctors may think they could risk frivolous suits and cover legal expenses out of pocket. An American Medical Association survey showed that 68% of closed claims against doctors were dropped, dismissed, or withdrawn. It said these claims cost an average of more than $30,000 to defend.
However, Mr. Teitelbaum puts the defense costs for so-called frivolous suits much higher than the AMA, at $250,000 or more. “Even if you’re sure you won’t have to pay a claim, you still have to defend yourself against frivolous suits,” he said. “You won’t recover those expenses.”
How to lower your tail coverage cost
Physicians typically have 60 days to buy tail coverage after their regular coverage has ended. Specialized brokers such as Mr. Teitelbaum and Ms. Perron help physicians look for the best tails to buy.
The cost of the tail depends on how long you’ve been at your job when you leave it, Ms. Perron says. If you leave in the first 1 or 2 years of the policy, she says, the tail price will be lower because the coverage period is shorter.
Usually the most expensive tail available is from the carrier that issued the original policy. Why is this? “Carriers rarely sell a tail that undercuts their retail price,” Mr. Teitelbaum said. “They don’t want to compete with themselves, and in fact doing so could pose regulatory problems for them.”
Instead of buying from their own carrier, doctors can purchase stand-alone tails from competitors, which Mr. Teitelbaum says are 10%-30% less expensive than the policy the original carrier issues. However, stand-alone tails are not always easy to find, especially for high-cost specialties such as neurosurgery and ob.gyn., he says.
Some physicians try to bring down the cost of the tail by limiting the duration of the tail. You can buy tails that only cover claims filed 1-5 years after the incident took place, rather than indefinitely. These limits mirror the typical statute of limitations – the time limit to file a claim in each state. This limit is as little as 2 years in some states, though it can be as long as 6 years in others.
However, some states make exceptions to the statute of limitations. The 2- to 6-year clock doesn’t start ticking until the mistake is discovered or, in the case of children, when they reach adulthood. “This means that with a limited tail, you always have risk,” Perron said.
And yet some doctors insist on these time-limited tails. “If a doctor opts for 3 years’ coverage, that’s better than no years,” Mr. Teitelbaum said. “But I would advise them to take at least 5 years because that gives you coverage for the basic statute of limitations in most states. Three-year tails do yield savings, but often they’re not enough to warrant the risk.”
Another way to reduce costs is to lower the coverage limits of the tail. The standard coverage limit is $1 million per case and $3 million per year, so doctors might be able to save money on the premium by buying limits of $200,000/$600,000. But Mr. Teitelbaum says most companies would refuse to sell a policy with a limit lower than that of the expiring policy.
Further ways to reduce the cost of the tail include buying tail coverage that doesn’t give the physician the right to approve a settlement or that doesn’t include legal fees in the coverage limits. But these options, too, raise the physician’s risks. Whichever option you choose, the important thing is to protect yourself against costly lawsuits.
This article first appeared on Medscape.com.