User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Dulaglutide OK for primary, secondary CV risk reduction in U.S.
The US Food and Drug Administration (FDA) has additionally approved dulaglutide (Trulicity) for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes with and without established cardiovascular disease (CVD) or multiple CV risk factors, the company has announced.
Dulaglutide is a once-weekly injectable glucagonlike peptide-1 (GLP-1) receptor agonist first approved in the United States in 2014 for the treatment of type 2 diabetes.
It is now the first and only type 2 diabetes medicine approved to reduce the risk of CV events for both primary and secondary prevention populations. The European Medicines Agency approved a similar indication for dulaglutide last fall.
The new US indication is based on results of the CV outcomes trial for dulaglutide, known as REWIND, which was the longest-running CV outcomes trial in the GLP-1 agonist class.
Chair of the REWIND study, Hertzel Gerstein, MD, professor of medicine at McMaster University and Hamilton Health Sciences, Ontario, Canada, said in a Lilly statement that the trial included a “broad population of people living with type 2 diabetes, reflective of those in the general population. We therefore assessed the effect of Trulicity in people with established CVD as well as those with multiple CV risk factors.”
“Globally, over 415 million people have type 2 diabetes, which is itself a CV risk factor. However, only about one third have established CVD, which is why this new indication, and the supporting evidence, is important for the millions of people in the United States living with diabetes,” he added.
Other GLP-1 agonists have been granted approvals for additional reduction of CV events in patients with type 2 diabetes, but only for secondary prevention.
Most recently the FDA expanded the indication for once-weekly semaglutide to include reducing the risk for MACE, including CV death, nonfatal myocardial infarction, or nonfatal stroke, in adults with type 2 diabetes who have established CVD.
Additional approval based on REWIND trial
The REWIND trial included primarily people with type 2 diabetes without established CVD. The full study results were presented at the 2019 American Diabetes Association Scientific Sessions.
REWIND showed a significant reduction in risk of MACE – a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or CV death – which occurred in 12.0% of patients in the dulaglutide group, compared with 13.4% of patients in the placebo group, for a risk reduction of 0.88 (95% confidence interval, 0.79-0.99; P = .026), which was consistent across subgroups.
All three components of the MACE primary endpoint showed a reduction with dulaglutide, compared with placebo, including CV death (hazard ratio, 0.91; 95% CI, 0.78-1.06) and nonfatal MI (HR, 0.96; 95% CI, 0.79-1.16), with the strongest and only significant effect seen in nonfatal stroke (HR, 0.76; 95% CI, 0.61-0.95).
No difference was seen between groups in hospital admissions for heart failure.
Dulaglutide was also found to modestly reduce weight by around 1.5 kg (P = .0001) and systolic blood pressure by 1.7 mm Hg (P = .0001).
The safety profile of dulaglutide in REWIND was consistent with other members of the GLP-1 agonist class, with gastrointestinal events being the most common adverse event leading to discontinuation.
Sherry Martin, MD, Lilly’s vice president, medical affairs, noted in the company statement: “For the first time, health care providers can prescribe a diabetes medicine proven to significantly reduce the risk of experiencing a CV event for people with type 2 diabetes with and without established CVD.”
“Trulicity can help people achieve their A1C goals and protect them from experiencing a CV event with a once-weekly, easy-to-use treatment option,” added Martin.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has additionally approved dulaglutide (Trulicity) for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes with and without established cardiovascular disease (CVD) or multiple CV risk factors, the company has announced.
Dulaglutide is a once-weekly injectable glucagonlike peptide-1 (GLP-1) receptor agonist first approved in the United States in 2014 for the treatment of type 2 diabetes.
It is now the first and only type 2 diabetes medicine approved to reduce the risk of CV events for both primary and secondary prevention populations. The European Medicines Agency approved a similar indication for dulaglutide last fall.
The new US indication is based on results of the CV outcomes trial for dulaglutide, known as REWIND, which was the longest-running CV outcomes trial in the GLP-1 agonist class.
Chair of the REWIND study, Hertzel Gerstein, MD, professor of medicine at McMaster University and Hamilton Health Sciences, Ontario, Canada, said in a Lilly statement that the trial included a “broad population of people living with type 2 diabetes, reflective of those in the general population. We therefore assessed the effect of Trulicity in people with established CVD as well as those with multiple CV risk factors.”
“Globally, over 415 million people have type 2 diabetes, which is itself a CV risk factor. However, only about one third have established CVD, which is why this new indication, and the supporting evidence, is important for the millions of people in the United States living with diabetes,” he added.
Other GLP-1 agonists have been granted approvals for additional reduction of CV events in patients with type 2 diabetes, but only for secondary prevention.
Most recently the FDA expanded the indication for once-weekly semaglutide to include reducing the risk for MACE, including CV death, nonfatal myocardial infarction, or nonfatal stroke, in adults with type 2 diabetes who have established CVD.
Additional approval based on REWIND trial
The REWIND trial included primarily people with type 2 diabetes without established CVD. The full study results were presented at the 2019 American Diabetes Association Scientific Sessions.
REWIND showed a significant reduction in risk of MACE – a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or CV death – which occurred in 12.0% of patients in the dulaglutide group, compared with 13.4% of patients in the placebo group, for a risk reduction of 0.88 (95% confidence interval, 0.79-0.99; P = .026), which was consistent across subgroups.
All three components of the MACE primary endpoint showed a reduction with dulaglutide, compared with placebo, including CV death (hazard ratio, 0.91; 95% CI, 0.78-1.06) and nonfatal MI (HR, 0.96; 95% CI, 0.79-1.16), with the strongest and only significant effect seen in nonfatal stroke (HR, 0.76; 95% CI, 0.61-0.95).
No difference was seen between groups in hospital admissions for heart failure.
Dulaglutide was also found to modestly reduce weight by around 1.5 kg (P = .0001) and systolic blood pressure by 1.7 mm Hg (P = .0001).
The safety profile of dulaglutide in REWIND was consistent with other members of the GLP-1 agonist class, with gastrointestinal events being the most common adverse event leading to discontinuation.
Sherry Martin, MD, Lilly’s vice president, medical affairs, noted in the company statement: “For the first time, health care providers can prescribe a diabetes medicine proven to significantly reduce the risk of experiencing a CV event for people with type 2 diabetes with and without established CVD.”
“Trulicity can help people achieve their A1C goals and protect them from experiencing a CV event with a once-weekly, easy-to-use treatment option,” added Martin.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has additionally approved dulaglutide (Trulicity) for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes with and without established cardiovascular disease (CVD) or multiple CV risk factors, the company has announced.
Dulaglutide is a once-weekly injectable glucagonlike peptide-1 (GLP-1) receptor agonist first approved in the United States in 2014 for the treatment of type 2 diabetes.
It is now the first and only type 2 diabetes medicine approved to reduce the risk of CV events for both primary and secondary prevention populations. The European Medicines Agency approved a similar indication for dulaglutide last fall.
The new US indication is based on results of the CV outcomes trial for dulaglutide, known as REWIND, which was the longest-running CV outcomes trial in the GLP-1 agonist class.
Chair of the REWIND study, Hertzel Gerstein, MD, professor of medicine at McMaster University and Hamilton Health Sciences, Ontario, Canada, said in a Lilly statement that the trial included a “broad population of people living with type 2 diabetes, reflective of those in the general population. We therefore assessed the effect of Trulicity in people with established CVD as well as those with multiple CV risk factors.”
“Globally, over 415 million people have type 2 diabetes, which is itself a CV risk factor. However, only about one third have established CVD, which is why this new indication, and the supporting evidence, is important for the millions of people in the United States living with diabetes,” he added.
Other GLP-1 agonists have been granted approvals for additional reduction of CV events in patients with type 2 diabetes, but only for secondary prevention.
Most recently the FDA expanded the indication for once-weekly semaglutide to include reducing the risk for MACE, including CV death, nonfatal myocardial infarction, or nonfatal stroke, in adults with type 2 diabetes who have established CVD.
Additional approval based on REWIND trial
The REWIND trial included primarily people with type 2 diabetes without established CVD. The full study results were presented at the 2019 American Diabetes Association Scientific Sessions.
REWIND showed a significant reduction in risk of MACE – a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or CV death – which occurred in 12.0% of patients in the dulaglutide group, compared with 13.4% of patients in the placebo group, for a risk reduction of 0.88 (95% confidence interval, 0.79-0.99; P = .026), which was consistent across subgroups.
All three components of the MACE primary endpoint showed a reduction with dulaglutide, compared with placebo, including CV death (hazard ratio, 0.91; 95% CI, 0.78-1.06) and nonfatal MI (HR, 0.96; 95% CI, 0.79-1.16), with the strongest and only significant effect seen in nonfatal stroke (HR, 0.76; 95% CI, 0.61-0.95).
No difference was seen between groups in hospital admissions for heart failure.
Dulaglutide was also found to modestly reduce weight by around 1.5 kg (P = .0001) and systolic blood pressure by 1.7 mm Hg (P = .0001).
The safety profile of dulaglutide in REWIND was consistent with other members of the GLP-1 agonist class, with gastrointestinal events being the most common adverse event leading to discontinuation.
Sherry Martin, MD, Lilly’s vice president, medical affairs, noted in the company statement: “For the first time, health care providers can prescribe a diabetes medicine proven to significantly reduce the risk of experiencing a CV event for people with type 2 diabetes with and without established CVD.”
“Trulicity can help people achieve their A1C goals and protect them from experiencing a CV event with a once-weekly, easy-to-use treatment option,” added Martin.
This article first appeared on Medscape.com.
Supreme Court roundup: Latest health care decisions
The Trump administration can move forward with expanding a rule that makes it more difficult for immigrants to remain in the United States if they receive health care assistance, the U.S. Supreme Court ruled in a 5-4 vote.
The Feb. 21 order allows the administration to broaden the so-called “public charge rule” while legal challenges against the expanded regulation continue in the lower courts. The Supreme Court’s decision, which lifts a preliminary injunction against the expansion, applies to enforcement only in Illinois, where a district court blocked the revised rule from moving forward in October 2019. The Supreme Court’s measure follows another 5-4 order in January, in which justices lifted a nationwide injunction against the revised rule.
Under the long-standing public charge rule, immigration officials can refuse to admit immigrants into the United States or can deny them permanent legal status if they are deemed likely to become a public charge. Previously, immigration officers considered cash aid, such as Temporary Assistance for Needy Families or long-term institutionalized care, as potential public charge reasons for denial.
The revised regulation allows officials to consider previously excluded programs in their determination, including nonemergency Medicaid, the Supplemental Nutrition Assistance Program, and several housing programs. Use of these programs for more than 12 months in the aggregate during a 36-month period may result in a “public charge” designation and lead to green card denial.
Eight legal challenges were immediately filed against the rule changes, including a complaint issued by 14 states. At least five trial courts have since blocked the measure, while appeals courts have lifted some of the injunctions and upheld enforcement.
In its Jan. 27 order lifting the nationwide injunction, Associate Justice Neil M. Gorsuch wrote that nationwide injunctions are being overused by trial courts with negative consequences.
“The real problem here is the increasingly common practice of trial courts ordering relief that transcends the cases before them. Whether framed as injunctions of ‘nationwide,’ ‘universal,’ or ‘cosmic’ scope, these orders share the same basic flaw – they direct how the defendant must act toward persons who are not parties to the case,” he wrote. “It has become increasingly apparent that this court must, at some point, confront these important objections to this increasingly widespread practice. As the brief and furious history of the regulation before us illustrates, the routine issuance of universal injunctions is patently unworkable, sowing chaos for litigants, the government, courts, and all those affected by these conflicting decisions.”
In the court’s Feb. 21 order lifting the injunction in Illinois, justices gave no explanation for overturning the lower court’s injunction. However, Associate Justice Sonia Sotomayor issued a sharply-worded dissent, criticizing her fellow justices for allowing the rule to proceed.
“In sum, the government’s only claimed hardship is that it must enforce an existing interpretation of an immigration rule in one state – just as it has done for the past 20 years – while an updated version of the rule takes effect in the remaining 49,” she wrote. “The government has not quantified or explained any burdens that would arise from this state of the world.”
ACA cases still in limbo
Meanwhile, the Supreme Court still has not decided whether it will hear Texas v. United States, a case that could effectively dismantle the Affordable Care Act.
The high court was expected to announce whether it would take the high-profile case at a private Feb. 21 conference, but the justices have released no update. The case was relisted for consideration at the court’s Feb. 28 conference.
Texas v. United States stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Because the Trump administration declined to defend the law, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. The Democratic attorneys general and governors appealed the decision to the U.S. Supreme Court.
If the Supreme Court agrees to hear the challenge, the court could fast-track the case and schedule arguments for the current term or wait until its next term, which starts in October 2020. If justices decline to hear the case, the challenge will remain with the district court for more analysis about the law’s severability.
Another ACA-related case – Maine Community Health Options v. U.S. – also remains in limbo. Justices heard the case, which was consolidated with two similar challenges, on Dec. 10, 2019, but still have not issued a decision.
The consolidated challenges center on whether the federal government owes insurers billions based on an Affordable Care Act provision intended to help health plans mitigate risk under the law. The ACA’s risk corridor program required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged and they want the Supreme Court to force the government to reimburse them to the tune of $12 billion.
The Department of Justice counters that the government is not required to pay the insurers because of appropriations measures passed by Congress in 2014 and in later years that limited the funding available to compensate insurers for their losses.
The federal government and insurers have each experienced wins and losses at the lower court level. Most recently, the U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
A Supreme Court decision in the case could come as soon as Feb. 26.
Court to hear women’s health cases
Two closely watched reproductive health cases will go before the court this spring.
On March 4, justices will hear oral arguments in June Medical Services v. Russo, regarding the constitutionality of a Louisiana law that requires physicians performing abortions to have admitting privileges at a nearby hospital. Doctors who perform abortions without admitting privileges at a hospital within 30 miles face fines and imprisonment, according to the state law, originally passed in 2014. Clinics that employ such doctors can also have their licenses revoked.
June Medical Services LLC, a women’s health clinic, sued over the law. A district court ruled in favor of the plaintiff, but the 5th U.S. Circuit Court of Appeals reversed and upheld Louisiana’s law. The clinic appealed to the U.S. Supreme Court. Louisiana officials argue the challenge should be dismissed, and the law allowed to proceed, because the plaintiffs lack standing.
The Supreme Court in 2016 heard a similar case – Whole Woman’s Health v. Hellerstedt – concerning a comparable law in Texas. In that case, justices struck down the measure as unconstitutional.
And on April 29, justices will hear arguments in Little Sisters of the Poor v. Pennsylvania, a consolidated case about whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraceptive mandate. Entities that object to providing contraception on the basis of religious beliefs can opt out of complying with the mandate, according to the 2018 regulations. Additionally, nonprofit organizations and small businesses that have nonreligious moral convictions against the mandate can skip compliance. A number of states and entities sued over the new rules.
A federal appeals court temporarily barred the regulations from moving forward, ruling the plaintiffs were likely to succeed in proving the Trump administration did not follow appropriate procedures when it promulgated the new rules and that the regulations were not authorized under the ACA.
Justices will decide whether the parties have standing in the case, whether the Trump administration followed correct rule-making procedures, and if the regulations can stand.
The Trump administration can move forward with expanding a rule that makes it more difficult for immigrants to remain in the United States if they receive health care assistance, the U.S. Supreme Court ruled in a 5-4 vote.
The Feb. 21 order allows the administration to broaden the so-called “public charge rule” while legal challenges against the expanded regulation continue in the lower courts. The Supreme Court’s decision, which lifts a preliminary injunction against the expansion, applies to enforcement only in Illinois, where a district court blocked the revised rule from moving forward in October 2019. The Supreme Court’s measure follows another 5-4 order in January, in which justices lifted a nationwide injunction against the revised rule.
Under the long-standing public charge rule, immigration officials can refuse to admit immigrants into the United States or can deny them permanent legal status if they are deemed likely to become a public charge. Previously, immigration officers considered cash aid, such as Temporary Assistance for Needy Families or long-term institutionalized care, as potential public charge reasons for denial.
The revised regulation allows officials to consider previously excluded programs in their determination, including nonemergency Medicaid, the Supplemental Nutrition Assistance Program, and several housing programs. Use of these programs for more than 12 months in the aggregate during a 36-month period may result in a “public charge” designation and lead to green card denial.
Eight legal challenges were immediately filed against the rule changes, including a complaint issued by 14 states. At least five trial courts have since blocked the measure, while appeals courts have lifted some of the injunctions and upheld enforcement.
In its Jan. 27 order lifting the nationwide injunction, Associate Justice Neil M. Gorsuch wrote that nationwide injunctions are being overused by trial courts with negative consequences.
“The real problem here is the increasingly common practice of trial courts ordering relief that transcends the cases before them. Whether framed as injunctions of ‘nationwide,’ ‘universal,’ or ‘cosmic’ scope, these orders share the same basic flaw – they direct how the defendant must act toward persons who are not parties to the case,” he wrote. “It has become increasingly apparent that this court must, at some point, confront these important objections to this increasingly widespread practice. As the brief and furious history of the regulation before us illustrates, the routine issuance of universal injunctions is patently unworkable, sowing chaos for litigants, the government, courts, and all those affected by these conflicting decisions.”
In the court’s Feb. 21 order lifting the injunction in Illinois, justices gave no explanation for overturning the lower court’s injunction. However, Associate Justice Sonia Sotomayor issued a sharply-worded dissent, criticizing her fellow justices for allowing the rule to proceed.
“In sum, the government’s only claimed hardship is that it must enforce an existing interpretation of an immigration rule in one state – just as it has done for the past 20 years – while an updated version of the rule takes effect in the remaining 49,” she wrote. “The government has not quantified or explained any burdens that would arise from this state of the world.”
ACA cases still in limbo
Meanwhile, the Supreme Court still has not decided whether it will hear Texas v. United States, a case that could effectively dismantle the Affordable Care Act.
The high court was expected to announce whether it would take the high-profile case at a private Feb. 21 conference, but the justices have released no update. The case was relisted for consideration at the court’s Feb. 28 conference.
Texas v. United States stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Because the Trump administration declined to defend the law, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. The Democratic attorneys general and governors appealed the decision to the U.S. Supreme Court.
If the Supreme Court agrees to hear the challenge, the court could fast-track the case and schedule arguments for the current term or wait until its next term, which starts in October 2020. If justices decline to hear the case, the challenge will remain with the district court for more analysis about the law’s severability.
Another ACA-related case – Maine Community Health Options v. U.S. – also remains in limbo. Justices heard the case, which was consolidated with two similar challenges, on Dec. 10, 2019, but still have not issued a decision.
The consolidated challenges center on whether the federal government owes insurers billions based on an Affordable Care Act provision intended to help health plans mitigate risk under the law. The ACA’s risk corridor program required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged and they want the Supreme Court to force the government to reimburse them to the tune of $12 billion.
The Department of Justice counters that the government is not required to pay the insurers because of appropriations measures passed by Congress in 2014 and in later years that limited the funding available to compensate insurers for their losses.
The federal government and insurers have each experienced wins and losses at the lower court level. Most recently, the U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
A Supreme Court decision in the case could come as soon as Feb. 26.
Court to hear women’s health cases
Two closely watched reproductive health cases will go before the court this spring.
On March 4, justices will hear oral arguments in June Medical Services v. Russo, regarding the constitutionality of a Louisiana law that requires physicians performing abortions to have admitting privileges at a nearby hospital. Doctors who perform abortions without admitting privileges at a hospital within 30 miles face fines and imprisonment, according to the state law, originally passed in 2014. Clinics that employ such doctors can also have their licenses revoked.
June Medical Services LLC, a women’s health clinic, sued over the law. A district court ruled in favor of the plaintiff, but the 5th U.S. Circuit Court of Appeals reversed and upheld Louisiana’s law. The clinic appealed to the U.S. Supreme Court. Louisiana officials argue the challenge should be dismissed, and the law allowed to proceed, because the plaintiffs lack standing.
The Supreme Court in 2016 heard a similar case – Whole Woman’s Health v. Hellerstedt – concerning a comparable law in Texas. In that case, justices struck down the measure as unconstitutional.
And on April 29, justices will hear arguments in Little Sisters of the Poor v. Pennsylvania, a consolidated case about whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraceptive mandate. Entities that object to providing contraception on the basis of religious beliefs can opt out of complying with the mandate, according to the 2018 regulations. Additionally, nonprofit organizations and small businesses that have nonreligious moral convictions against the mandate can skip compliance. A number of states and entities sued over the new rules.
A federal appeals court temporarily barred the regulations from moving forward, ruling the plaintiffs were likely to succeed in proving the Trump administration did not follow appropriate procedures when it promulgated the new rules and that the regulations were not authorized under the ACA.
Justices will decide whether the parties have standing in the case, whether the Trump administration followed correct rule-making procedures, and if the regulations can stand.
The Trump administration can move forward with expanding a rule that makes it more difficult for immigrants to remain in the United States if they receive health care assistance, the U.S. Supreme Court ruled in a 5-4 vote.
The Feb. 21 order allows the administration to broaden the so-called “public charge rule” while legal challenges against the expanded regulation continue in the lower courts. The Supreme Court’s decision, which lifts a preliminary injunction against the expansion, applies to enforcement only in Illinois, where a district court blocked the revised rule from moving forward in October 2019. The Supreme Court’s measure follows another 5-4 order in January, in which justices lifted a nationwide injunction against the revised rule.
Under the long-standing public charge rule, immigration officials can refuse to admit immigrants into the United States or can deny them permanent legal status if they are deemed likely to become a public charge. Previously, immigration officers considered cash aid, such as Temporary Assistance for Needy Families or long-term institutionalized care, as potential public charge reasons for denial.
The revised regulation allows officials to consider previously excluded programs in their determination, including nonemergency Medicaid, the Supplemental Nutrition Assistance Program, and several housing programs. Use of these programs for more than 12 months in the aggregate during a 36-month period may result in a “public charge” designation and lead to green card denial.
Eight legal challenges were immediately filed against the rule changes, including a complaint issued by 14 states. At least five trial courts have since blocked the measure, while appeals courts have lifted some of the injunctions and upheld enforcement.
In its Jan. 27 order lifting the nationwide injunction, Associate Justice Neil M. Gorsuch wrote that nationwide injunctions are being overused by trial courts with negative consequences.
“The real problem here is the increasingly common practice of trial courts ordering relief that transcends the cases before them. Whether framed as injunctions of ‘nationwide,’ ‘universal,’ or ‘cosmic’ scope, these orders share the same basic flaw – they direct how the defendant must act toward persons who are not parties to the case,” he wrote. “It has become increasingly apparent that this court must, at some point, confront these important objections to this increasingly widespread practice. As the brief and furious history of the regulation before us illustrates, the routine issuance of universal injunctions is patently unworkable, sowing chaos for litigants, the government, courts, and all those affected by these conflicting decisions.”
In the court’s Feb. 21 order lifting the injunction in Illinois, justices gave no explanation for overturning the lower court’s injunction. However, Associate Justice Sonia Sotomayor issued a sharply-worded dissent, criticizing her fellow justices for allowing the rule to proceed.
“In sum, the government’s only claimed hardship is that it must enforce an existing interpretation of an immigration rule in one state – just as it has done for the past 20 years – while an updated version of the rule takes effect in the remaining 49,” she wrote. “The government has not quantified or explained any burdens that would arise from this state of the world.”
ACA cases still in limbo
Meanwhile, the Supreme Court still has not decided whether it will hear Texas v. United States, a case that could effectively dismantle the Affordable Care Act.
The high court was expected to announce whether it would take the high-profile case at a private Feb. 21 conference, but the justices have released no update. The case was relisted for consideration at the court’s Feb. 28 conference.
Texas v. United States stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Because the Trump administration declined to defend the law, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. The Democratic attorneys general and governors appealed the decision to the U.S. Supreme Court.
If the Supreme Court agrees to hear the challenge, the court could fast-track the case and schedule arguments for the current term or wait until its next term, which starts in October 2020. If justices decline to hear the case, the challenge will remain with the district court for more analysis about the law’s severability.
Another ACA-related case – Maine Community Health Options v. U.S. – also remains in limbo. Justices heard the case, which was consolidated with two similar challenges, on Dec. 10, 2019, but still have not issued a decision.
The consolidated challenges center on whether the federal government owes insurers billions based on an Affordable Care Act provision intended to help health plans mitigate risk under the law. The ACA’s risk corridor program required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged and they want the Supreme Court to force the government to reimburse them to the tune of $12 billion.
The Department of Justice counters that the government is not required to pay the insurers because of appropriations measures passed by Congress in 2014 and in later years that limited the funding available to compensate insurers for their losses.
The federal government and insurers have each experienced wins and losses at the lower court level. Most recently, the U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
A Supreme Court decision in the case could come as soon as Feb. 26.
Court to hear women’s health cases
Two closely watched reproductive health cases will go before the court this spring.
On March 4, justices will hear oral arguments in June Medical Services v. Russo, regarding the constitutionality of a Louisiana law that requires physicians performing abortions to have admitting privileges at a nearby hospital. Doctors who perform abortions without admitting privileges at a hospital within 30 miles face fines and imprisonment, according to the state law, originally passed in 2014. Clinics that employ such doctors can also have their licenses revoked.
June Medical Services LLC, a women’s health clinic, sued over the law. A district court ruled in favor of the plaintiff, but the 5th U.S. Circuit Court of Appeals reversed and upheld Louisiana’s law. The clinic appealed to the U.S. Supreme Court. Louisiana officials argue the challenge should be dismissed, and the law allowed to proceed, because the plaintiffs lack standing.
The Supreme Court in 2016 heard a similar case – Whole Woman’s Health v. Hellerstedt – concerning a comparable law in Texas. In that case, justices struck down the measure as unconstitutional.
And on April 29, justices will hear arguments in Little Sisters of the Poor v. Pennsylvania, a consolidated case about whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraceptive mandate. Entities that object to providing contraception on the basis of religious beliefs can opt out of complying with the mandate, according to the 2018 regulations. Additionally, nonprofit organizations and small businesses that have nonreligious moral convictions against the mandate can skip compliance. A number of states and entities sued over the new rules.
A federal appeals court temporarily barred the regulations from moving forward, ruling the plaintiffs were likely to succeed in proving the Trump administration did not follow appropriate procedures when it promulgated the new rules and that the regulations were not authorized under the ACA.
Justices will decide whether the parties have standing in the case, whether the Trump administration followed correct rule-making procedures, and if the regulations can stand.
Lipidologists welcome bempedoic acid as new lipid-lowering option
Bempedoic acid, the first agent in a new class of drugs that reduce LDL cholesterol, received Food and Drug Administration approval on Feb. 21 for treating selected hypercholesterolemic patients and is a welcome addition to the medicine cabinet, say lipid experts.
However, it is a tertiary option at least until results from a 14,000 patient clinical-outcome trial of bempedoic acid come out, likely in 2022, they agreed.
“I’m excited to have a new tool in the toolkit for treating high-risk patients, but I will always reach first for the drugs proven to reduce clinical outcomes,” said Erin D. Michos, MD, director of Women’s Cardiovascular Health and associate director of Preventive Cardiology at Johns Hopkins Medicine in Baltimore. That sentiment, shared by other experts, should for the time being relegate bempedoic acid (Nexletol) to a backup role behind statins, ezetimibe, and the PCSK9 inhibitor antibodies that are all now on the U.S. market and all buttressed with evidence of their ability to cut cardiovascular disease death and other CVD outcomes from large outcome studies.
The existing evidence base for bempedoic acid rests primarily two multicenter, randomized, placebo-controlled clinical trials of bempedoic acid in patients with LDL levels above 70 mg/dL while on maximally tolerated lipid-lowering therapy. In CLEAR Harmony, results showed that treatment with bempedoic acid cut LDL-cholesterol levels by an average of 18% more compared with placebo (N Engl J Med 2019;380:1022-32). In CLEAR Wisdom, bempedoic acid reduced LDL cholesterol levels by 17% (JAMA. 2019;322[18]:1780-8).
While those two trials proved the drug’s ability to lower levels of LDL cholesterol, they lacked the power to address whether this effect cut the incidence of CVD events, a question that the CLEAR Outcomes trial aims to answer.
“I believe in the lipid hypothesis, but the main thing we need to see is whether bempedoic acid leads to a meaningful reduction in CVD events. The window for bempedoic acid will remain narrow until we see the outcomes results,” Dr. Michos said in an interview.
Bempedoic acid is a prodrug that’s activated in liver and targets the same cholesterol synthesis pathway as statins by inhibition of ATP-citrate lyase, an enzyme that’s upstream of HMG-CoA reductase, thereby enhancing LDL cholesterol clearance via up-regulation of LDL receptors
.
“,” said Jennifer G. Robinson, MD, professor of epidemiology and medicine and director of the Prevention Intervention Center of the University of Iowa in Iowa City. That would be just a portion of the newly labeled target population. The FDA’s approved label for bempedoic acid cites the drug as an “adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of LDL-C.”
The current lack of outcomes evidence for bempedoic acid was not an issue for Robert H. Eckel, MD, an endocrinologist and lipid management specialist at the University of Colorado at Denver in Aurora. Having results from CLEAR Outcomes “may be helpful, but LDL cholesterol lowering in the range where the FDA has indicated using bempedoic acid seems all we need for now,” he said in an interview. Viewing bempedoic acid as potentially useful for both HeFH and ASCVD patients, Dr. Eckel particularly cited the possibility of using the new drug in combination with ezetimibe, another oral, once-daily agent with a moderate but additive effect for cutting LDL cholesterol.
Combined treatment with bempedoic acid and ezetimibe “may be successful in avoiding [using] a PCSK9 inhibitor in some patients, and in particular patients with HeFH or those who are statin intolerant.” But like his colleagues, Dr. Eckel agreed that, for the moment, ezetimibe has an edge over bempedoic acid because of its more extensive evidence base. “If the combination of bempedoic acid and ezetimibe is not needed, the decision [of which one of these to use] needs to depend on the outcome trial results for ezetimibe,” he said. Other factors clinicians could apply if faced with choosing between these two agents include the significant reduction in high-sensitivity C-reactive protein that bempedoic acid produces; the downside that bempedoic acid can cause in some patients an early and persistent rise in serum uric acid levels that can trigger gout flares in patients with a history of gout or at risk for gout; and cost, he said.
Cost is the room-dwelling elephant that colors many decisions about which lipid-lowering drug to use for patients, with options running the price gamut from the generic and uniformly affordable statins and ezetimibe, to the notoriously pricey PCSK9 inhibitors that remain for many patients either prohibitively expensive or hard to get covered by some insurers. Bempedoic acid seems on track to fall somewhere between these two poles, although staff members from Esperion, the company that developed and will market bempedoic acid as Nexletol starting on March 30, declared in a conference call on Feb. 24 that “cost will not be an issue,” for indicated patients prescribed the drug. Company representatives cited a program of coupons, discounts, and rebates they have planned that they anticipate will allow patients who meet the labeled indications to have an out-of-pocket cost for bempedoic acid of “as low as” $10 for a 90-pill supply. They also noted their goal of getting bempedoic acid onto the lowest tier of the Medicare formulary.
How these steps actually play out in the fun house of U.S. prescription drug pricing and preauthorizations remains to be seen. “Out-of-pocket costs are not the real drivers” of drug access, noted Dr. Robinson. “Insurers will likely start with restricted access and prior authorization requirements, just as they did with ezetimibe when it was on patent and prior to having the results from a CVD outcomes trial.” For the time being, bempedoic acid can generally be seen as “expensive ezetimibe,” summed up Dr. Robinson.
Despite that somewhat dismissive characterization, experts are intrigued by the possibility of combining two moderately potent, oral, and safe lipid-lowering drugs in selected patients as a potential alternative to the still financially challenging PCSK9 inhibitors. Combining bempedoic acid and ezetimibe “has a lot of appeal,” said Dr. Michos. “Even though preauthorization has gotten better, it’s still a challenge to get a PCSK9 inhibitor approved.”
Much of her enthusiasm stems from a study reported last year that randomized 301 patients to treatment with bempedoic acid, ezetimibe, or both. The results showed that combined treatment has a similar safety profile to treatment with either drug alone, and produced a cut in LDL cholesterol that was roughly additive for the reductions produced by each drug by itself: Ezetimibe alone cut LDL by about 23%, bempedoic acid alone by about 17%, and the two dosed together once daily resulted in an average 36% drop (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864671). The results showed that, “in patients requiring intensive LDL cholesterol lowering, who cannot afford PCSK9 inhibitors, or have statin intolerance, bempedoic acid and ezetimibe are stronger together and can serve as an alternative approach for lipid management in ASCVD prevention,” wrote Dr. Michos and a coauthor in a commentary that appeared with the study results (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864672).
The concept of combined bempedoic acid and ezetimibe treatment is so appealing that the bempedoic acid manufacturer, Esperion, has already developed a single-pill formulation of the two drugs that received FDA marketing approval on February 26. A company statement said that marketing of this combined formulation, Nexlizet, will start in July 2020.
Although interest in bempedoic acid seems running high for patients included in the new FDA indication, Dr. Michos and others see possibly greater potential for what would now be off-label use for primary prevention in high-risk patients without HeFH, patients who generally don’t qualify for insurance coverage of a PCSK9 inhibitor.
“Use in primary prevention in [non-HeFH] patients with insufficient lowering of LDL cholesterol wouldn’t surprise me,” but a big concern will be out-of-pocket cost when off-label use precludes insurance coverage or discount eligibility, noted Dr. Eckel. An Esperion spokesperson said that the undiscounted, wholesale acquisition cost for bempedoic acid is expected to be roughly $10/pill, or about $300 for a 30-day supply, positioning it more or less midway between generic statins and ezetimibe and the list price for a PCSk9 inhibitor of roughly $500/month.
“I’m most excited about bempedoic acid in the off-label space, for patients who can’t get approved for a PCSK9 inhibitor, for treating patients with subclinical ASCVD, or really high-risk patients with multiple risk factors including diabetes,” especially when these patients are intolerant of a high-intensity statin regimen, said Dr. Michos. “I have a clinic full of patients” who can’t take their full, indicated dosage of a high-intensity statin, and when those patients also can’t get on treatment with a PCSK9 inhibitor then bempedoic acid will be an important part of their alternative regimen, she explained.
Dr. Michos had no disclosures. Dr. Robinson has received research funding from Esperion and from several other companies, and she has been a consultant to Amgen, Merck, Novartis, Novo Nordisk, Pfizer, Regeneron, and Sanofi. Dr. Eckel has received honoraria from Kowa, Merck, Novo Nordisk, and Sanofi/Regeneron.
This article was updated 2/27/20.
Bempedoic acid, the first agent in a new class of drugs that reduce LDL cholesterol, received Food and Drug Administration approval on Feb. 21 for treating selected hypercholesterolemic patients and is a welcome addition to the medicine cabinet, say lipid experts.
However, it is a tertiary option at least until results from a 14,000 patient clinical-outcome trial of bempedoic acid come out, likely in 2022, they agreed.
“I’m excited to have a new tool in the toolkit for treating high-risk patients, but I will always reach first for the drugs proven to reduce clinical outcomes,” said Erin D. Michos, MD, director of Women’s Cardiovascular Health and associate director of Preventive Cardiology at Johns Hopkins Medicine in Baltimore. That sentiment, shared by other experts, should for the time being relegate bempedoic acid (Nexletol) to a backup role behind statins, ezetimibe, and the PCSK9 inhibitor antibodies that are all now on the U.S. market and all buttressed with evidence of their ability to cut cardiovascular disease death and other CVD outcomes from large outcome studies.
The existing evidence base for bempedoic acid rests primarily two multicenter, randomized, placebo-controlled clinical trials of bempedoic acid in patients with LDL levels above 70 mg/dL while on maximally tolerated lipid-lowering therapy. In CLEAR Harmony, results showed that treatment with bempedoic acid cut LDL-cholesterol levels by an average of 18% more compared with placebo (N Engl J Med 2019;380:1022-32). In CLEAR Wisdom, bempedoic acid reduced LDL cholesterol levels by 17% (JAMA. 2019;322[18]:1780-8).
While those two trials proved the drug’s ability to lower levels of LDL cholesterol, they lacked the power to address whether this effect cut the incidence of CVD events, a question that the CLEAR Outcomes trial aims to answer.
“I believe in the lipid hypothesis, but the main thing we need to see is whether bempedoic acid leads to a meaningful reduction in CVD events. The window for bempedoic acid will remain narrow until we see the outcomes results,” Dr. Michos said in an interview.
Bempedoic acid is a prodrug that’s activated in liver and targets the same cholesterol synthesis pathway as statins by inhibition of ATP-citrate lyase, an enzyme that’s upstream of HMG-CoA reductase, thereby enhancing LDL cholesterol clearance via up-regulation of LDL receptors
.
“,” said Jennifer G. Robinson, MD, professor of epidemiology and medicine and director of the Prevention Intervention Center of the University of Iowa in Iowa City. That would be just a portion of the newly labeled target population. The FDA’s approved label for bempedoic acid cites the drug as an “adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of LDL-C.”
The current lack of outcomes evidence for bempedoic acid was not an issue for Robert H. Eckel, MD, an endocrinologist and lipid management specialist at the University of Colorado at Denver in Aurora. Having results from CLEAR Outcomes “may be helpful, but LDL cholesterol lowering in the range where the FDA has indicated using bempedoic acid seems all we need for now,” he said in an interview. Viewing bempedoic acid as potentially useful for both HeFH and ASCVD patients, Dr. Eckel particularly cited the possibility of using the new drug in combination with ezetimibe, another oral, once-daily agent with a moderate but additive effect for cutting LDL cholesterol.
Combined treatment with bempedoic acid and ezetimibe “may be successful in avoiding [using] a PCSK9 inhibitor in some patients, and in particular patients with HeFH or those who are statin intolerant.” But like his colleagues, Dr. Eckel agreed that, for the moment, ezetimibe has an edge over bempedoic acid because of its more extensive evidence base. “If the combination of bempedoic acid and ezetimibe is not needed, the decision [of which one of these to use] needs to depend on the outcome trial results for ezetimibe,” he said. Other factors clinicians could apply if faced with choosing between these two agents include the significant reduction in high-sensitivity C-reactive protein that bempedoic acid produces; the downside that bempedoic acid can cause in some patients an early and persistent rise in serum uric acid levels that can trigger gout flares in patients with a history of gout or at risk for gout; and cost, he said.
Cost is the room-dwelling elephant that colors many decisions about which lipid-lowering drug to use for patients, with options running the price gamut from the generic and uniformly affordable statins and ezetimibe, to the notoriously pricey PCSK9 inhibitors that remain for many patients either prohibitively expensive or hard to get covered by some insurers. Bempedoic acid seems on track to fall somewhere between these two poles, although staff members from Esperion, the company that developed and will market bempedoic acid as Nexletol starting on March 30, declared in a conference call on Feb. 24 that “cost will not be an issue,” for indicated patients prescribed the drug. Company representatives cited a program of coupons, discounts, and rebates they have planned that they anticipate will allow patients who meet the labeled indications to have an out-of-pocket cost for bempedoic acid of “as low as” $10 for a 90-pill supply. They also noted their goal of getting bempedoic acid onto the lowest tier of the Medicare formulary.
How these steps actually play out in the fun house of U.S. prescription drug pricing and preauthorizations remains to be seen. “Out-of-pocket costs are not the real drivers” of drug access, noted Dr. Robinson. “Insurers will likely start with restricted access and prior authorization requirements, just as they did with ezetimibe when it was on patent and prior to having the results from a CVD outcomes trial.” For the time being, bempedoic acid can generally be seen as “expensive ezetimibe,” summed up Dr. Robinson.
Despite that somewhat dismissive characterization, experts are intrigued by the possibility of combining two moderately potent, oral, and safe lipid-lowering drugs in selected patients as a potential alternative to the still financially challenging PCSK9 inhibitors. Combining bempedoic acid and ezetimibe “has a lot of appeal,” said Dr. Michos. “Even though preauthorization has gotten better, it’s still a challenge to get a PCSK9 inhibitor approved.”
Much of her enthusiasm stems from a study reported last year that randomized 301 patients to treatment with bempedoic acid, ezetimibe, or both. The results showed that combined treatment has a similar safety profile to treatment with either drug alone, and produced a cut in LDL cholesterol that was roughly additive for the reductions produced by each drug by itself: Ezetimibe alone cut LDL by about 23%, bempedoic acid alone by about 17%, and the two dosed together once daily resulted in an average 36% drop (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864671). The results showed that, “in patients requiring intensive LDL cholesterol lowering, who cannot afford PCSK9 inhibitors, or have statin intolerance, bempedoic acid and ezetimibe are stronger together and can serve as an alternative approach for lipid management in ASCVD prevention,” wrote Dr. Michos and a coauthor in a commentary that appeared with the study results (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864672).
The concept of combined bempedoic acid and ezetimibe treatment is so appealing that the bempedoic acid manufacturer, Esperion, has already developed a single-pill formulation of the two drugs that received FDA marketing approval on February 26. A company statement said that marketing of this combined formulation, Nexlizet, will start in July 2020.
Although interest in bempedoic acid seems running high for patients included in the new FDA indication, Dr. Michos and others see possibly greater potential for what would now be off-label use for primary prevention in high-risk patients without HeFH, patients who generally don’t qualify for insurance coverage of a PCSK9 inhibitor.
“Use in primary prevention in [non-HeFH] patients with insufficient lowering of LDL cholesterol wouldn’t surprise me,” but a big concern will be out-of-pocket cost when off-label use precludes insurance coverage or discount eligibility, noted Dr. Eckel. An Esperion spokesperson said that the undiscounted, wholesale acquisition cost for bempedoic acid is expected to be roughly $10/pill, or about $300 for a 30-day supply, positioning it more or less midway between generic statins and ezetimibe and the list price for a PCSk9 inhibitor of roughly $500/month.
“I’m most excited about bempedoic acid in the off-label space, for patients who can’t get approved for a PCSK9 inhibitor, for treating patients with subclinical ASCVD, or really high-risk patients with multiple risk factors including diabetes,” especially when these patients are intolerant of a high-intensity statin regimen, said Dr. Michos. “I have a clinic full of patients” who can’t take their full, indicated dosage of a high-intensity statin, and when those patients also can’t get on treatment with a PCSK9 inhibitor then bempedoic acid will be an important part of their alternative regimen, she explained.
Dr. Michos had no disclosures. Dr. Robinson has received research funding from Esperion and from several other companies, and she has been a consultant to Amgen, Merck, Novartis, Novo Nordisk, Pfizer, Regeneron, and Sanofi. Dr. Eckel has received honoraria from Kowa, Merck, Novo Nordisk, and Sanofi/Regeneron.
This article was updated 2/27/20.
Bempedoic acid, the first agent in a new class of drugs that reduce LDL cholesterol, received Food and Drug Administration approval on Feb. 21 for treating selected hypercholesterolemic patients and is a welcome addition to the medicine cabinet, say lipid experts.
However, it is a tertiary option at least until results from a 14,000 patient clinical-outcome trial of bempedoic acid come out, likely in 2022, they agreed.
“I’m excited to have a new tool in the toolkit for treating high-risk patients, but I will always reach first for the drugs proven to reduce clinical outcomes,” said Erin D. Michos, MD, director of Women’s Cardiovascular Health and associate director of Preventive Cardiology at Johns Hopkins Medicine in Baltimore. That sentiment, shared by other experts, should for the time being relegate bempedoic acid (Nexletol) to a backup role behind statins, ezetimibe, and the PCSK9 inhibitor antibodies that are all now on the U.S. market and all buttressed with evidence of their ability to cut cardiovascular disease death and other CVD outcomes from large outcome studies.
The existing evidence base for bempedoic acid rests primarily two multicenter, randomized, placebo-controlled clinical trials of bempedoic acid in patients with LDL levels above 70 mg/dL while on maximally tolerated lipid-lowering therapy. In CLEAR Harmony, results showed that treatment with bempedoic acid cut LDL-cholesterol levels by an average of 18% more compared with placebo (N Engl J Med 2019;380:1022-32). In CLEAR Wisdom, bempedoic acid reduced LDL cholesterol levels by 17% (JAMA. 2019;322[18]:1780-8).
While those two trials proved the drug’s ability to lower levels of LDL cholesterol, they lacked the power to address whether this effect cut the incidence of CVD events, a question that the CLEAR Outcomes trial aims to answer.
“I believe in the lipid hypothesis, but the main thing we need to see is whether bempedoic acid leads to a meaningful reduction in CVD events. The window for bempedoic acid will remain narrow until we see the outcomes results,” Dr. Michos said in an interview.
Bempedoic acid is a prodrug that’s activated in liver and targets the same cholesterol synthesis pathway as statins by inhibition of ATP-citrate lyase, an enzyme that’s upstream of HMG-CoA reductase, thereby enhancing LDL cholesterol clearance via up-regulation of LDL receptors
.
“,” said Jennifer G. Robinson, MD, professor of epidemiology and medicine and director of the Prevention Intervention Center of the University of Iowa in Iowa City. That would be just a portion of the newly labeled target population. The FDA’s approved label for bempedoic acid cites the drug as an “adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of LDL-C.”
The current lack of outcomes evidence for bempedoic acid was not an issue for Robert H. Eckel, MD, an endocrinologist and lipid management specialist at the University of Colorado at Denver in Aurora. Having results from CLEAR Outcomes “may be helpful, but LDL cholesterol lowering in the range where the FDA has indicated using bempedoic acid seems all we need for now,” he said in an interview. Viewing bempedoic acid as potentially useful for both HeFH and ASCVD patients, Dr. Eckel particularly cited the possibility of using the new drug in combination with ezetimibe, another oral, once-daily agent with a moderate but additive effect for cutting LDL cholesterol.
Combined treatment with bempedoic acid and ezetimibe “may be successful in avoiding [using] a PCSK9 inhibitor in some patients, and in particular patients with HeFH or those who are statin intolerant.” But like his colleagues, Dr. Eckel agreed that, for the moment, ezetimibe has an edge over bempedoic acid because of its more extensive evidence base. “If the combination of bempedoic acid and ezetimibe is not needed, the decision [of which one of these to use] needs to depend on the outcome trial results for ezetimibe,” he said. Other factors clinicians could apply if faced with choosing between these two agents include the significant reduction in high-sensitivity C-reactive protein that bempedoic acid produces; the downside that bempedoic acid can cause in some patients an early and persistent rise in serum uric acid levels that can trigger gout flares in patients with a history of gout or at risk for gout; and cost, he said.
Cost is the room-dwelling elephant that colors many decisions about which lipid-lowering drug to use for patients, with options running the price gamut from the generic and uniformly affordable statins and ezetimibe, to the notoriously pricey PCSK9 inhibitors that remain for many patients either prohibitively expensive or hard to get covered by some insurers. Bempedoic acid seems on track to fall somewhere between these two poles, although staff members from Esperion, the company that developed and will market bempedoic acid as Nexletol starting on March 30, declared in a conference call on Feb. 24 that “cost will not be an issue,” for indicated patients prescribed the drug. Company representatives cited a program of coupons, discounts, and rebates they have planned that they anticipate will allow patients who meet the labeled indications to have an out-of-pocket cost for bempedoic acid of “as low as” $10 for a 90-pill supply. They also noted their goal of getting bempedoic acid onto the lowest tier of the Medicare formulary.
How these steps actually play out in the fun house of U.S. prescription drug pricing and preauthorizations remains to be seen. “Out-of-pocket costs are not the real drivers” of drug access, noted Dr. Robinson. “Insurers will likely start with restricted access and prior authorization requirements, just as they did with ezetimibe when it was on patent and prior to having the results from a CVD outcomes trial.” For the time being, bempedoic acid can generally be seen as “expensive ezetimibe,” summed up Dr. Robinson.
Despite that somewhat dismissive characterization, experts are intrigued by the possibility of combining two moderately potent, oral, and safe lipid-lowering drugs in selected patients as a potential alternative to the still financially challenging PCSK9 inhibitors. Combining bempedoic acid and ezetimibe “has a lot of appeal,” said Dr. Michos. “Even though preauthorization has gotten better, it’s still a challenge to get a PCSK9 inhibitor approved.”
Much of her enthusiasm stems from a study reported last year that randomized 301 patients to treatment with bempedoic acid, ezetimibe, or both. The results showed that combined treatment has a similar safety profile to treatment with either drug alone, and produced a cut in LDL cholesterol that was roughly additive for the reductions produced by each drug by itself: Ezetimibe alone cut LDL by about 23%, bempedoic acid alone by about 17%, and the two dosed together once daily resulted in an average 36% drop (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864671). The results showed that, “in patients requiring intensive LDL cholesterol lowering, who cannot afford PCSK9 inhibitors, or have statin intolerance, bempedoic acid and ezetimibe are stronger together and can serve as an alternative approach for lipid management in ASCVD prevention,” wrote Dr. Michos and a coauthor in a commentary that appeared with the study results (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864672).
The concept of combined bempedoic acid and ezetimibe treatment is so appealing that the bempedoic acid manufacturer, Esperion, has already developed a single-pill formulation of the two drugs that received FDA marketing approval on February 26. A company statement said that marketing of this combined formulation, Nexlizet, will start in July 2020.
Although interest in bempedoic acid seems running high for patients included in the new FDA indication, Dr. Michos and others see possibly greater potential for what would now be off-label use for primary prevention in high-risk patients without HeFH, patients who generally don’t qualify for insurance coverage of a PCSK9 inhibitor.
“Use in primary prevention in [non-HeFH] patients with insufficient lowering of LDL cholesterol wouldn’t surprise me,” but a big concern will be out-of-pocket cost when off-label use precludes insurance coverage or discount eligibility, noted Dr. Eckel. An Esperion spokesperson said that the undiscounted, wholesale acquisition cost for bempedoic acid is expected to be roughly $10/pill, or about $300 for a 30-day supply, positioning it more or less midway between generic statins and ezetimibe and the list price for a PCSk9 inhibitor of roughly $500/month.
“I’m most excited about bempedoic acid in the off-label space, for patients who can’t get approved for a PCSK9 inhibitor, for treating patients with subclinical ASCVD, or really high-risk patients with multiple risk factors including diabetes,” especially when these patients are intolerant of a high-intensity statin regimen, said Dr. Michos. “I have a clinic full of patients” who can’t take their full, indicated dosage of a high-intensity statin, and when those patients also can’t get on treatment with a PCSK9 inhibitor then bempedoic acid will be an important part of their alternative regimen, she explained.
Dr. Michos had no disclosures. Dr. Robinson has received research funding from Esperion and from several other companies, and she has been a consultant to Amgen, Merck, Novartis, Novo Nordisk, Pfizer, Regeneron, and Sanofi. Dr. Eckel has received honoraria from Kowa, Merck, Novo Nordisk, and Sanofi/Regeneron.
This article was updated 2/27/20.
Key to denervation response for hypertension may be in carotid body
NATIONAL HARBOR, MD. – Of characteristics that might predict which patients with resistant hypertension will respond to carotid body ablation, the relative activity of the carotid body organ itself might be critical for moving this technology forward, an investigator on a first-in-man study suggests.
The study, first presented in 2018 at the European Society of Cardiology congress, showed carotid body ablation resulted in significant but modest reductions in blood pressure in patients with resistant hypertension.
For many reasons, the carotid body is an attractive target for sustained or indefinite control of resistant hypertension, but median systolic blood pressure reductions following ultrasound ablation were highly variable in the first-in-man study, according to Felix Mahfoud, MD, of Saarland University Hospital in Homburg, Germany, a coinvestigator on the study who discussed the findings at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Of several strategies being pursued to separate those most likely to gain a major benefit, simply measuring carotid body activity is now emerging as particularly promising.
“Patients with a high degree of carotid body activity had a significantly larger fall in blood pressure versus all other methods of grouping patients,” Dr. Mahfoud reported.
The carotid body is a “grain-size” organ of about 2 mm in size that sits on the carotid bifurcation. It communicates directly with the brain to alter sympathetic activity in response to changing levels of such physiologic variable as oxygen, carbon dioxide, and pH, according to Dr. Mahfoud.
In a 2016 proof-of-principle study conducted in resistant hypertension patients, surgical resection of the carotid body was associated with a median 18–mm Hg reduction in systolic blood pressure (SBP) on 24-hour ambulatory monitoring that was sustained through 24 months (Narkiewicz K et al. JACC Basic Transl Sci. 2016;29:313-24).
Subsequently, ultrasound ablation of the carotid body was by way of a transcatheter approach. Dr. Mahfoud’s unpublished first-in-man study, conducted in 2018 enrolled 38 patients with resistant hypertension. The median reductions from baseline of 7-8 mm Hg at 1, 3, and 6 months were significant (P less than .01), but the benefit was disappointingly modest.
However, the variability was large. Some patients achieved SBP reductions of up to 20 mm Hg at 6 months, prompting additional analyses to understand if the best responders could be identified. When compared to the mean reduction of 7 mm Hg at 6 months, this represented a 13–mm Hg additional reduction. There are now several potential approaches being considered.
In addition to carotid body activity, which is readily measured and has substantial potential to serve as a routine selection criterion, isolated systolic hypertension (ISH) was also found to be a discriminator for response. For those with ISH, which Dr. Mahfoud noted is also characterized as “stiff arteries,” SBP reductions at 6 months were negligible, but in those without ISH, the median reduction from baseline was 11 mm Hg.
Further investigations are now planned to evaluate potential predictors of response, according to Dr. Mahfoud. He believes carotid body ablation might have advantages over alternatives, including other experimental therapies, in at least some patients.
To deliver ultrasound ablation in the first-in-man study, a propriety catheter (Cibiem transvenous system) was advanced through the jugular vein guided with intravascular ultrasound. When the carotid body was reached, two to three ultrasound ablations of 8-12 seconds each were applied. The procedure time was 20-30 minutes.
Initially, an arterial approach to the carotid body was used. However, after a transient ischemic attack early in the series, the approach was switched to the jugular vein. There have been no serious subsequent procedural-related complications since.
Although the median SBP reductions were modest, they were not insignificant in a population selected for severe resistant hypertension. The median SBP at entry was 180 mm Hg in patients taking a median of 4.5 antihypertensive drugs, according to Dr. Mahfoud.
In other words, this approach still retains promise for selected patients if larger studies demonstrate that response can be predicted, and the data continue to support tolerability.
A venous approach to the carotid body with intravascular ultrasound guidance and therapeutic ultrasound appears “to offer a safe and effective treatment option in resistant hypertension,” according to Dr. Mahfoud. “A companion diagnostic test is being developed to determine whether patients are likely to respond to this therapy.”
Dr. Mahfoud reports financial relationships with Medtronic, St. Jude, and ReCor.
This article was updated to clarify the study details and correct misspellings of the presenter's name.
SOURCE: CRT 2020.
NATIONAL HARBOR, MD. – Of characteristics that might predict which patients with resistant hypertension will respond to carotid body ablation, the relative activity of the carotid body organ itself might be critical for moving this technology forward, an investigator on a first-in-man study suggests.
The study, first presented in 2018 at the European Society of Cardiology congress, showed carotid body ablation resulted in significant but modest reductions in blood pressure in patients with resistant hypertension.
For many reasons, the carotid body is an attractive target for sustained or indefinite control of resistant hypertension, but median systolic blood pressure reductions following ultrasound ablation were highly variable in the first-in-man study, according to Felix Mahfoud, MD, of Saarland University Hospital in Homburg, Germany, a coinvestigator on the study who discussed the findings at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Of several strategies being pursued to separate those most likely to gain a major benefit, simply measuring carotid body activity is now emerging as particularly promising.
“Patients with a high degree of carotid body activity had a significantly larger fall in blood pressure versus all other methods of grouping patients,” Dr. Mahfoud reported.
The carotid body is a “grain-size” organ of about 2 mm in size that sits on the carotid bifurcation. It communicates directly with the brain to alter sympathetic activity in response to changing levels of such physiologic variable as oxygen, carbon dioxide, and pH, according to Dr. Mahfoud.
In a 2016 proof-of-principle study conducted in resistant hypertension patients, surgical resection of the carotid body was associated with a median 18–mm Hg reduction in systolic blood pressure (SBP) on 24-hour ambulatory monitoring that was sustained through 24 months (Narkiewicz K et al. JACC Basic Transl Sci. 2016;29:313-24).
Subsequently, ultrasound ablation of the carotid body was by way of a transcatheter approach. Dr. Mahfoud’s unpublished first-in-man study, conducted in 2018 enrolled 38 patients with resistant hypertension. The median reductions from baseline of 7-8 mm Hg at 1, 3, and 6 months were significant (P less than .01), but the benefit was disappointingly modest.
However, the variability was large. Some patients achieved SBP reductions of up to 20 mm Hg at 6 months, prompting additional analyses to understand if the best responders could be identified. When compared to the mean reduction of 7 mm Hg at 6 months, this represented a 13–mm Hg additional reduction. There are now several potential approaches being considered.
In addition to carotid body activity, which is readily measured and has substantial potential to serve as a routine selection criterion, isolated systolic hypertension (ISH) was also found to be a discriminator for response. For those with ISH, which Dr. Mahfoud noted is also characterized as “stiff arteries,” SBP reductions at 6 months were negligible, but in those without ISH, the median reduction from baseline was 11 mm Hg.
Further investigations are now planned to evaluate potential predictors of response, according to Dr. Mahfoud. He believes carotid body ablation might have advantages over alternatives, including other experimental therapies, in at least some patients.
To deliver ultrasound ablation in the first-in-man study, a propriety catheter (Cibiem transvenous system) was advanced through the jugular vein guided with intravascular ultrasound. When the carotid body was reached, two to three ultrasound ablations of 8-12 seconds each were applied. The procedure time was 20-30 minutes.
Initially, an arterial approach to the carotid body was used. However, after a transient ischemic attack early in the series, the approach was switched to the jugular vein. There have been no serious subsequent procedural-related complications since.
Although the median SBP reductions were modest, they were not insignificant in a population selected for severe resistant hypertension. The median SBP at entry was 180 mm Hg in patients taking a median of 4.5 antihypertensive drugs, according to Dr. Mahfoud.
In other words, this approach still retains promise for selected patients if larger studies demonstrate that response can be predicted, and the data continue to support tolerability.
A venous approach to the carotid body with intravascular ultrasound guidance and therapeutic ultrasound appears “to offer a safe and effective treatment option in resistant hypertension,” according to Dr. Mahfoud. “A companion diagnostic test is being developed to determine whether patients are likely to respond to this therapy.”
Dr. Mahfoud reports financial relationships with Medtronic, St. Jude, and ReCor.
This article was updated to clarify the study details and correct misspellings of the presenter's name.
SOURCE: CRT 2020.
NATIONAL HARBOR, MD. – Of characteristics that might predict which patients with resistant hypertension will respond to carotid body ablation, the relative activity of the carotid body organ itself might be critical for moving this technology forward, an investigator on a first-in-man study suggests.
The study, first presented in 2018 at the European Society of Cardiology congress, showed carotid body ablation resulted in significant but modest reductions in blood pressure in patients with resistant hypertension.
For many reasons, the carotid body is an attractive target for sustained or indefinite control of resistant hypertension, but median systolic blood pressure reductions following ultrasound ablation were highly variable in the first-in-man study, according to Felix Mahfoud, MD, of Saarland University Hospital in Homburg, Germany, a coinvestigator on the study who discussed the findings at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Of several strategies being pursued to separate those most likely to gain a major benefit, simply measuring carotid body activity is now emerging as particularly promising.
“Patients with a high degree of carotid body activity had a significantly larger fall in blood pressure versus all other methods of grouping patients,” Dr. Mahfoud reported.
The carotid body is a “grain-size” organ of about 2 mm in size that sits on the carotid bifurcation. It communicates directly with the brain to alter sympathetic activity in response to changing levels of such physiologic variable as oxygen, carbon dioxide, and pH, according to Dr. Mahfoud.
In a 2016 proof-of-principle study conducted in resistant hypertension patients, surgical resection of the carotid body was associated with a median 18–mm Hg reduction in systolic blood pressure (SBP) on 24-hour ambulatory monitoring that was sustained through 24 months (Narkiewicz K et al. JACC Basic Transl Sci. 2016;29:313-24).
Subsequently, ultrasound ablation of the carotid body was by way of a transcatheter approach. Dr. Mahfoud’s unpublished first-in-man study, conducted in 2018 enrolled 38 patients with resistant hypertension. The median reductions from baseline of 7-8 mm Hg at 1, 3, and 6 months were significant (P less than .01), but the benefit was disappointingly modest.
However, the variability was large. Some patients achieved SBP reductions of up to 20 mm Hg at 6 months, prompting additional analyses to understand if the best responders could be identified. When compared to the mean reduction of 7 mm Hg at 6 months, this represented a 13–mm Hg additional reduction. There are now several potential approaches being considered.
In addition to carotid body activity, which is readily measured and has substantial potential to serve as a routine selection criterion, isolated systolic hypertension (ISH) was also found to be a discriminator for response. For those with ISH, which Dr. Mahfoud noted is also characterized as “stiff arteries,” SBP reductions at 6 months were negligible, but in those without ISH, the median reduction from baseline was 11 mm Hg.
Further investigations are now planned to evaluate potential predictors of response, according to Dr. Mahfoud. He believes carotid body ablation might have advantages over alternatives, including other experimental therapies, in at least some patients.
To deliver ultrasound ablation in the first-in-man study, a propriety catheter (Cibiem transvenous system) was advanced through the jugular vein guided with intravascular ultrasound. When the carotid body was reached, two to three ultrasound ablations of 8-12 seconds each were applied. The procedure time was 20-30 minutes.
Initially, an arterial approach to the carotid body was used. However, after a transient ischemic attack early in the series, the approach was switched to the jugular vein. There have been no serious subsequent procedural-related complications since.
Although the median SBP reductions were modest, they were not insignificant in a population selected for severe resistant hypertension. The median SBP at entry was 180 mm Hg in patients taking a median of 4.5 antihypertensive drugs, according to Dr. Mahfoud.
In other words, this approach still retains promise for selected patients if larger studies demonstrate that response can be predicted, and the data continue to support tolerability.
A venous approach to the carotid body with intravascular ultrasound guidance and therapeutic ultrasound appears “to offer a safe and effective treatment option in resistant hypertension,” according to Dr. Mahfoud. “A companion diagnostic test is being developed to determine whether patients are likely to respond to this therapy.”
Dr. Mahfoud reports financial relationships with Medtronic, St. Jude, and ReCor.
This article was updated to clarify the study details and correct misspellings of the presenter's name.
SOURCE: CRT 2020.
REPORTING FROM CRT 2020
Study implicates gut bacteria in PAH
Model finds microbiota highly predictive
A unique collection of bacteria in the gut may have a strong association with pulmonary arterial hypertension and could be highly predictive of the disease in undiagnosed patients, according to a study published in the journal Hypertension.
This is the first study to show that people with PAH have a common specific gut microbiota profile, wrote lead study author Mohan Raizada, PhD, distinguished professor in the department of physiology and functional genomics at the University of Florida, Gainesville.
The findings have the potential to change how cardiologists diagnose and treat PAH, he added. “While current PAH treatments focus on the lungs, looking at the lung/gut axis could open the door to new therapies centered in the digestive system,” Dr. Raizada said.
The researchers developed a model that found the specific microbiota profile was 83% accurate in predicting the presence or absence of PAH. If a larger study can validate the findings, the researchers wrote, this could lead to a new test for diagnosing PAH that’s less invasive than cardiac catheterization. It could also lead to new treatments that target the gut microbiome.
Study investigators collected stool samples from 18 PAH patients and 12 people without a history of cardiopulmonary disease. The microbiota DNA from the stool samples were isolated and sequenced. The analysis revealed that PAH patients had reduced richness and evenness of the gut bacteria, known as alpha diversity. They had increased levels of bacteria associated with atherosclerosis, and healthy patients had increased levels of bacteria that produced short-chain fatty acids.
Although recent studies have begun to show potential associations between the gut microbiome and cardiovascular diseases, this research is in its infancy, Mariell Jessup, MD, commented. “Even though the study by Dr. Raizada and colleagues predicted pulmonary arterial hypertension based on an individual’s microbiome with some accuracy, it is an observational study, so it does not prove cause and effect. Many other factors, especially diet, affect the gut microbiome,” added Dr. Jessup, Chief Science and Medical Officer for the American Heart Association.
She stressed that, “In addition, even if studies confirm an association between the gut microbiome and cardiovascular diseases such as PAH, more research is needed to determine if improving gut microbiota could directly impact PAH or other cardiovascular diseases. The findings of this study will not impact clinical practice.”
Dr. Raizada and his coinvestigators offered two possible mechanisms through which the gut microbiome influences pulmonary physiology. One is that lower levels of bacteria that produce the short-chain fatty acid butyrate, such as Coprococcus, Butyrivibrio, Lachnospiraceae, and Eubacterium, along with Clostridia in the gut of PAH patients, may increase gut permeability. Reduced butyrate weakens gut barrier function and can induce inflammation and leakage. This can allow microbial metabolites to enter the circulatory system, disrupting metabolism and immunity and affecting pulmonary vessels.
The second potential mechanism is that increased Collinsella in the PAH cohort may be the culprit that increases gut permeability, resulting in the ensuing gut barrier dysfunction and inflammation. The study noted Collinsella contributed most of the increased genes for the biosynthesis on the amino acid proline in these patients, and that a previous study implicated Collinsella and its parent, Cariobacteriales, in trimethylamine/trimethylamine N-oxide production (TMA/TMAO) in atherosclerosis (Cell. 2015;163[7]:1585-95). The non-PAH patients had higher levels of bacteria that had a low correlation with TMA/TMAO.
“We were very surprised to see such an association within a small group of study subjects,” wrote Dr. Raizada and associates. “It usually requires hundreds of patients to achieve such significance.”
More research is needed to determine if the specific microbiota associated with PAH causes the disease or is a result of it, they concluded.
The study was funded by grants from the National Institutes of Health, the NIH National Center for Research Resources, and the U.S. Department of Defense. Dr. Raizada and coauthors reported no relevant financial relationships.
SOURCE: Raizada MK et al. Hypertension. 2020. doi: 10.1161/HYPERTENSIONAHA.119.14294.
Model finds microbiota highly predictive
Model finds microbiota highly predictive
A unique collection of bacteria in the gut may have a strong association with pulmonary arterial hypertension and could be highly predictive of the disease in undiagnosed patients, according to a study published in the journal Hypertension.
This is the first study to show that people with PAH have a common specific gut microbiota profile, wrote lead study author Mohan Raizada, PhD, distinguished professor in the department of physiology and functional genomics at the University of Florida, Gainesville.
The findings have the potential to change how cardiologists diagnose and treat PAH, he added. “While current PAH treatments focus on the lungs, looking at the lung/gut axis could open the door to new therapies centered in the digestive system,” Dr. Raizada said.
The researchers developed a model that found the specific microbiota profile was 83% accurate in predicting the presence or absence of PAH. If a larger study can validate the findings, the researchers wrote, this could lead to a new test for diagnosing PAH that’s less invasive than cardiac catheterization. It could also lead to new treatments that target the gut microbiome.
Study investigators collected stool samples from 18 PAH patients and 12 people without a history of cardiopulmonary disease. The microbiota DNA from the stool samples were isolated and sequenced. The analysis revealed that PAH patients had reduced richness and evenness of the gut bacteria, known as alpha diversity. They had increased levels of bacteria associated with atherosclerosis, and healthy patients had increased levels of bacteria that produced short-chain fatty acids.
Although recent studies have begun to show potential associations between the gut microbiome and cardiovascular diseases, this research is in its infancy, Mariell Jessup, MD, commented. “Even though the study by Dr. Raizada and colleagues predicted pulmonary arterial hypertension based on an individual’s microbiome with some accuracy, it is an observational study, so it does not prove cause and effect. Many other factors, especially diet, affect the gut microbiome,” added Dr. Jessup, Chief Science and Medical Officer for the American Heart Association.
She stressed that, “In addition, even if studies confirm an association between the gut microbiome and cardiovascular diseases such as PAH, more research is needed to determine if improving gut microbiota could directly impact PAH or other cardiovascular diseases. The findings of this study will not impact clinical practice.”
Dr. Raizada and his coinvestigators offered two possible mechanisms through which the gut microbiome influences pulmonary physiology. One is that lower levels of bacteria that produce the short-chain fatty acid butyrate, such as Coprococcus, Butyrivibrio, Lachnospiraceae, and Eubacterium, along with Clostridia in the gut of PAH patients, may increase gut permeability. Reduced butyrate weakens gut barrier function and can induce inflammation and leakage. This can allow microbial metabolites to enter the circulatory system, disrupting metabolism and immunity and affecting pulmonary vessels.
The second potential mechanism is that increased Collinsella in the PAH cohort may be the culprit that increases gut permeability, resulting in the ensuing gut barrier dysfunction and inflammation. The study noted Collinsella contributed most of the increased genes for the biosynthesis on the amino acid proline in these patients, and that a previous study implicated Collinsella and its parent, Cariobacteriales, in trimethylamine/trimethylamine N-oxide production (TMA/TMAO) in atherosclerosis (Cell. 2015;163[7]:1585-95). The non-PAH patients had higher levels of bacteria that had a low correlation with TMA/TMAO.
“We were very surprised to see such an association within a small group of study subjects,” wrote Dr. Raizada and associates. “It usually requires hundreds of patients to achieve such significance.”
More research is needed to determine if the specific microbiota associated with PAH causes the disease or is a result of it, they concluded.
The study was funded by grants from the National Institutes of Health, the NIH National Center for Research Resources, and the U.S. Department of Defense. Dr. Raizada and coauthors reported no relevant financial relationships.
SOURCE: Raizada MK et al. Hypertension. 2020. doi: 10.1161/HYPERTENSIONAHA.119.14294.
A unique collection of bacteria in the gut may have a strong association with pulmonary arterial hypertension and could be highly predictive of the disease in undiagnosed patients, according to a study published in the journal Hypertension.
This is the first study to show that people with PAH have a common specific gut microbiota profile, wrote lead study author Mohan Raizada, PhD, distinguished professor in the department of physiology and functional genomics at the University of Florida, Gainesville.
The findings have the potential to change how cardiologists diagnose and treat PAH, he added. “While current PAH treatments focus on the lungs, looking at the lung/gut axis could open the door to new therapies centered in the digestive system,” Dr. Raizada said.
The researchers developed a model that found the specific microbiota profile was 83% accurate in predicting the presence or absence of PAH. If a larger study can validate the findings, the researchers wrote, this could lead to a new test for diagnosing PAH that’s less invasive than cardiac catheterization. It could also lead to new treatments that target the gut microbiome.
Study investigators collected stool samples from 18 PAH patients and 12 people without a history of cardiopulmonary disease. The microbiota DNA from the stool samples were isolated and sequenced. The analysis revealed that PAH patients had reduced richness and evenness of the gut bacteria, known as alpha diversity. They had increased levels of bacteria associated with atherosclerosis, and healthy patients had increased levels of bacteria that produced short-chain fatty acids.
Although recent studies have begun to show potential associations between the gut microbiome and cardiovascular diseases, this research is in its infancy, Mariell Jessup, MD, commented. “Even though the study by Dr. Raizada and colleagues predicted pulmonary arterial hypertension based on an individual’s microbiome with some accuracy, it is an observational study, so it does not prove cause and effect. Many other factors, especially diet, affect the gut microbiome,” added Dr. Jessup, Chief Science and Medical Officer for the American Heart Association.
She stressed that, “In addition, even if studies confirm an association between the gut microbiome and cardiovascular diseases such as PAH, more research is needed to determine if improving gut microbiota could directly impact PAH or other cardiovascular diseases. The findings of this study will not impact clinical practice.”
Dr. Raizada and his coinvestigators offered two possible mechanisms through which the gut microbiome influences pulmonary physiology. One is that lower levels of bacteria that produce the short-chain fatty acid butyrate, such as Coprococcus, Butyrivibrio, Lachnospiraceae, and Eubacterium, along with Clostridia in the gut of PAH patients, may increase gut permeability. Reduced butyrate weakens gut barrier function and can induce inflammation and leakage. This can allow microbial metabolites to enter the circulatory system, disrupting metabolism and immunity and affecting pulmonary vessels.
The second potential mechanism is that increased Collinsella in the PAH cohort may be the culprit that increases gut permeability, resulting in the ensuing gut barrier dysfunction and inflammation. The study noted Collinsella contributed most of the increased genes for the biosynthesis on the amino acid proline in these patients, and that a previous study implicated Collinsella and its parent, Cariobacteriales, in trimethylamine/trimethylamine N-oxide production (TMA/TMAO) in atherosclerosis (Cell. 2015;163[7]:1585-95). The non-PAH patients had higher levels of bacteria that had a low correlation with TMA/TMAO.
“We were very surprised to see such an association within a small group of study subjects,” wrote Dr. Raizada and associates. “It usually requires hundreds of patients to achieve such significance.”
More research is needed to determine if the specific microbiota associated with PAH causes the disease or is a result of it, they concluded.
The study was funded by grants from the National Institutes of Health, the NIH National Center for Research Resources, and the U.S. Department of Defense. Dr. Raizada and coauthors reported no relevant financial relationships.
SOURCE: Raizada MK et al. Hypertension. 2020. doi: 10.1161/HYPERTENSIONAHA.119.14294.
FROM HYPERTENSION
First clinical evidence of neuroprotection in acute stroke?
LOS ANGELES – A new potential neuroprotectant agent has been found to be beneficial for patients with acute ischemic stroke undergoing endovascular thrombectomy in a large placebo-controlled trial, but only for those patients who did not also receive thrombolysis.
There was no difference between groups on the primary outcome in the main analysis of the trial, lead author Michael Hill, MD, reported.
However, “In our study, we found a dramatic interaction of nerinetide with alteplase. There was a large benefit of nerinetide in patients not given thrombolysis, but in patients who received alteplase, this benefit was completely obliterated,” Dr. Hill said in an interview.
“In patients not treated with thrombolysis, we found a large effect size with a 9.5% absolute improvement in patients having an independent outcome (modified Rankin Score [mRS] 0-2) and a number need to treat of 10 to 11,” he said. “We also found a mortality benefit and a reduction in the size of strokes, with all other secondary outcomes going in the right direction.
“The drug works really well in patients who do not get thrombolysis, but it doesn’t work at all in patients who have had thrombolysis. The thrombolytic appears to break the peptide down so it is inactive,” he added.
“This is the first evidence that neuroprotection is possible in human stroke. This has never been shown before,” Dr. Hill noted. “Many previous clinical trials of potential neuroprotectants have been negative. We think this is a major breakthrough. This is pretty exciting stuff with really tantalizing results.”
Dr. Hill, professor of neurology at the University of Calgary (Alta.), presented results of the ESCAPE-NA1 trial on Feb. 20 at the International Stroke Conference (ISC) 2020. The trial was also simultaneously published online (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30258-0).
Endogenous nitric oxide
The new agent – known as NA1 or nerinetide – is a 20-amino-acid peptide with a novel mechanism of action; it inhibits signaling that leads to neuronal excitotoxicity. “It reduces endogenous nitric oxide generated inside the cell during ischemia, which is one of the main biochemical processes contributing to cell death,” Dr. Hill explained. In a primate model of ischemia reperfusion that was published in Nature in 2012, it was highly protective, he added.
The drug is given just once at the time of thrombectomy. It is short lived in the blood but detectable in the brain for up to 24 hours, he said.
The trial included 1,105 patients who had experienced acute ischemic stroke due to large-vessel occlusion within a 12-hour treatment window and for whom imaging results suitable for thrombectomy were available. The patients were randomly assigned to receive either intravenous nerinetide in a single dose of 2.6 mg/kg or saline placebo at the time of thrombectomy.
Patients were stratified by intravenous alteplase treatment and by declared endovascular device choice.
The primary outcome was a favorable functional outcome 90 days after randomization, defined as an mRS score of 0-2. In the main analysis of the whole population, this favorable outcome was achieved for 61.4% of the group that received nerinetide and for 59.2% of the placebo group, a nonsignificant difference. Secondary outcomes were also similar between the two groups.
But an exploratory analysis showed evidence that nerinetide’s treatment effect was modified by alteplase treatment. Among the patients who did not receive alteplase, use of nerinetide was associated with improved outcomes, whereas no benefit was found in the alteplase stratum. The difference in absolute risk slightly but not significantly favored placebo.
In the stratum that did not receive alteplase (40% of the trial population), the favorable mRS outcome was achieved by 59.3% of patients who received nerinetide, compared with 49.8% of those given placebo – a significant difference (adjusted risk ratio, 1.18; 95% confidence interval, 1.01-1.38).
There was also a 7.5% absolute risk reduction in mortality at 90 days post treatment with nerinetide for the patients who did not receive thrombolysis. This resulted in an approximate halving of the hazard of death (adjusted hazard ratio, 0.56).
In addition, infarct size was reduced in those patients who received nerinetide but not thrombolysis.
Among the patients who received alteplase, the proportion of patients who achieved an mRS of 0-2 was similar between groups, as were median infarct volumes.
The observed treatment effect modification by alteplase was supported by reductions in peak plasma nerinetide concentrations in the alteplase stratum, the researchers reported.
They said that the combination of the clinical results in the no-thrombolytic stratum and subsequent tests documenting that nerinetide is broken down by plasmin (which is generated by alteplase) “provide evidence that the clinical observation of effect modification is not a chance finding.” But they added: “This novel observation will require additional confirmation, and we cannot draw a definitive conclusion on treatment effect in this study.”
“Shaking up the field”
There is still more work to do, Dr. Hill said. “We don’t fully understand the pharmacology, and we will certainly have to do another trial, but we believe this agent is going to shake the field up. This is a totally new drug, and we have to think carefully about where it could fit in.”
“The obvious first group is those patients who do not receive thrombolysis. This is a large group, as most patients do not present in time for thrombolysis. Then we can work on the biochemistry and see if we can develop a version of nerinetide that is resistant to breakdown by thrombolysis,” he said.
Another possibility would be to withhold thrombolysis and give nerinetide instead. “It may be that thrombolysis is not needed if patients are receiving thrombectomy – this is being suggested now in initial studies,” Hill stated.
They also chose a very select group of patients – those undergoing thrombectomy, who represent only 10% to 15% of stroke patients. “We have to work out how to expand that population,” he said.
Hill noted that there have been many examples in the past of potential neuroprotectant agents that have worked in animal models of ischemia-reperfusion but that failed in humans with acute stroke.
“Until recently, we have not had a reliable ischemia-reperfusion model in humans, but now with endovascular therapy, we have a situation where the blood flow is reliably restored, which is an ideal situation to test new neuroprotectant agents. That may be another factor that has contributed to our positive findings,” he said.
In an accompanying comment in The Lancet, Graeme J. Hankey, MD, of the University of Western Australia, Perth, noted that although endovascular thrombectomy after use of intravenous alteplase improves reperfusion and clinical outcomes for a fifth of patients with ischemic stroke caused by large-artery occlusion, half of patients do not recover an independent lifestyle. Cytoprotection aims to augment the resilience of neurons, neurovascular units, and white matter during ischemia until perfusion is restored (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30316-0).
Dr. Hankey also pointed out that numerous cytoprotection strategies have been reported to reduce brain infarction in preclinical models of ischemic stroke but have not been found to improve clinical outcomes in clinical trials involving patients with ischemic stroke.
The advent of thrombectomy provides an opportunity to reassess cytoprotection as an adjunctive therapy for patients with types of temporary brain ischemia that align more closely with successful preclinical models of ischemia, cytoprotection, and reperfusion, he added.
On the results of the current study and the benefit in the no-thrombolysis group, Dr. Hankey stated: “Although this result might be a chance finding or confounded by the indication for alteplase, complementary pharmacokinetic data in a small number of patients treated with nerinetide showed that alteplase lowered plasma concentrations of nerinetide, probably by converting plasminogen to plasmin, which cleaves peptide bonds not only in fibrin but also in the eicosapeptide nerinetide.”
He said the ESCAPE-NA1 trial “informs the study of cytoprotection as an adjunct therapy to reperfusion in acute ischemic stroke” and suggested that researchers who have reported encouraging results of other cytoprotective therapies for ischemic stroke should test their compounds for interactions with concurrent thrombolytic therapies.
The ESCAPE-NA1 trial was sponsored by NoNO, the company developing nerinetide. Dr. Hill has received grants from NoNO for the conduct of the study, is named on a U.S. patent for systems and methods for assisting in decision making and triaging for acute stroke patients, and owns stock in Calgary Scientific. Other coauthors are employees of NoNO or have stock options in the company. Dr. Hankey has received personal honoraria from the American Heart Association, AC Immune, Bayer, Bristol-Myers Squibb, and Medscape outside the area of work that he commented on.
This article first appeared on Medscape.com.
LOS ANGELES – A new potential neuroprotectant agent has been found to be beneficial for patients with acute ischemic stroke undergoing endovascular thrombectomy in a large placebo-controlled trial, but only for those patients who did not also receive thrombolysis.
There was no difference between groups on the primary outcome in the main analysis of the trial, lead author Michael Hill, MD, reported.
However, “In our study, we found a dramatic interaction of nerinetide with alteplase. There was a large benefit of nerinetide in patients not given thrombolysis, but in patients who received alteplase, this benefit was completely obliterated,” Dr. Hill said in an interview.
“In patients not treated with thrombolysis, we found a large effect size with a 9.5% absolute improvement in patients having an independent outcome (modified Rankin Score [mRS] 0-2) and a number need to treat of 10 to 11,” he said. “We also found a mortality benefit and a reduction in the size of strokes, with all other secondary outcomes going in the right direction.
“The drug works really well in patients who do not get thrombolysis, but it doesn’t work at all in patients who have had thrombolysis. The thrombolytic appears to break the peptide down so it is inactive,” he added.
“This is the first evidence that neuroprotection is possible in human stroke. This has never been shown before,” Dr. Hill noted. “Many previous clinical trials of potential neuroprotectants have been negative. We think this is a major breakthrough. This is pretty exciting stuff with really tantalizing results.”
Dr. Hill, professor of neurology at the University of Calgary (Alta.), presented results of the ESCAPE-NA1 trial on Feb. 20 at the International Stroke Conference (ISC) 2020. The trial was also simultaneously published online (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30258-0).
Endogenous nitric oxide
The new agent – known as NA1 or nerinetide – is a 20-amino-acid peptide with a novel mechanism of action; it inhibits signaling that leads to neuronal excitotoxicity. “It reduces endogenous nitric oxide generated inside the cell during ischemia, which is one of the main biochemical processes contributing to cell death,” Dr. Hill explained. In a primate model of ischemia reperfusion that was published in Nature in 2012, it was highly protective, he added.
The drug is given just once at the time of thrombectomy. It is short lived in the blood but detectable in the brain for up to 24 hours, he said.
The trial included 1,105 patients who had experienced acute ischemic stroke due to large-vessel occlusion within a 12-hour treatment window and for whom imaging results suitable for thrombectomy were available. The patients were randomly assigned to receive either intravenous nerinetide in a single dose of 2.6 mg/kg or saline placebo at the time of thrombectomy.
Patients were stratified by intravenous alteplase treatment and by declared endovascular device choice.
The primary outcome was a favorable functional outcome 90 days after randomization, defined as an mRS score of 0-2. In the main analysis of the whole population, this favorable outcome was achieved for 61.4% of the group that received nerinetide and for 59.2% of the placebo group, a nonsignificant difference. Secondary outcomes were also similar between the two groups.
But an exploratory analysis showed evidence that nerinetide’s treatment effect was modified by alteplase treatment. Among the patients who did not receive alteplase, use of nerinetide was associated with improved outcomes, whereas no benefit was found in the alteplase stratum. The difference in absolute risk slightly but not significantly favored placebo.
In the stratum that did not receive alteplase (40% of the trial population), the favorable mRS outcome was achieved by 59.3% of patients who received nerinetide, compared with 49.8% of those given placebo – a significant difference (adjusted risk ratio, 1.18; 95% confidence interval, 1.01-1.38).
There was also a 7.5% absolute risk reduction in mortality at 90 days post treatment with nerinetide for the patients who did not receive thrombolysis. This resulted in an approximate halving of the hazard of death (adjusted hazard ratio, 0.56).
In addition, infarct size was reduced in those patients who received nerinetide but not thrombolysis.
Among the patients who received alteplase, the proportion of patients who achieved an mRS of 0-2 was similar between groups, as were median infarct volumes.
The observed treatment effect modification by alteplase was supported by reductions in peak plasma nerinetide concentrations in the alteplase stratum, the researchers reported.
They said that the combination of the clinical results in the no-thrombolytic stratum and subsequent tests documenting that nerinetide is broken down by plasmin (which is generated by alteplase) “provide evidence that the clinical observation of effect modification is not a chance finding.” But they added: “This novel observation will require additional confirmation, and we cannot draw a definitive conclusion on treatment effect in this study.”
“Shaking up the field”
There is still more work to do, Dr. Hill said. “We don’t fully understand the pharmacology, and we will certainly have to do another trial, but we believe this agent is going to shake the field up. This is a totally new drug, and we have to think carefully about where it could fit in.”
“The obvious first group is those patients who do not receive thrombolysis. This is a large group, as most patients do not present in time for thrombolysis. Then we can work on the biochemistry and see if we can develop a version of nerinetide that is resistant to breakdown by thrombolysis,” he said.
Another possibility would be to withhold thrombolysis and give nerinetide instead. “It may be that thrombolysis is not needed if patients are receiving thrombectomy – this is being suggested now in initial studies,” Hill stated.
They also chose a very select group of patients – those undergoing thrombectomy, who represent only 10% to 15% of stroke patients. “We have to work out how to expand that population,” he said.
Hill noted that there have been many examples in the past of potential neuroprotectant agents that have worked in animal models of ischemia-reperfusion but that failed in humans with acute stroke.
“Until recently, we have not had a reliable ischemia-reperfusion model in humans, but now with endovascular therapy, we have a situation where the blood flow is reliably restored, which is an ideal situation to test new neuroprotectant agents. That may be another factor that has contributed to our positive findings,” he said.
In an accompanying comment in The Lancet, Graeme J. Hankey, MD, of the University of Western Australia, Perth, noted that although endovascular thrombectomy after use of intravenous alteplase improves reperfusion and clinical outcomes for a fifth of patients with ischemic stroke caused by large-artery occlusion, half of patients do not recover an independent lifestyle. Cytoprotection aims to augment the resilience of neurons, neurovascular units, and white matter during ischemia until perfusion is restored (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30316-0).
Dr. Hankey also pointed out that numerous cytoprotection strategies have been reported to reduce brain infarction in preclinical models of ischemic stroke but have not been found to improve clinical outcomes in clinical trials involving patients with ischemic stroke.
The advent of thrombectomy provides an opportunity to reassess cytoprotection as an adjunctive therapy for patients with types of temporary brain ischemia that align more closely with successful preclinical models of ischemia, cytoprotection, and reperfusion, he added.
On the results of the current study and the benefit in the no-thrombolysis group, Dr. Hankey stated: “Although this result might be a chance finding or confounded by the indication for alteplase, complementary pharmacokinetic data in a small number of patients treated with nerinetide showed that alteplase lowered plasma concentrations of nerinetide, probably by converting plasminogen to plasmin, which cleaves peptide bonds not only in fibrin but also in the eicosapeptide nerinetide.”
He said the ESCAPE-NA1 trial “informs the study of cytoprotection as an adjunct therapy to reperfusion in acute ischemic stroke” and suggested that researchers who have reported encouraging results of other cytoprotective therapies for ischemic stroke should test their compounds for interactions with concurrent thrombolytic therapies.
The ESCAPE-NA1 trial was sponsored by NoNO, the company developing nerinetide. Dr. Hill has received grants from NoNO for the conduct of the study, is named on a U.S. patent for systems and methods for assisting in decision making and triaging for acute stroke patients, and owns stock in Calgary Scientific. Other coauthors are employees of NoNO or have stock options in the company. Dr. Hankey has received personal honoraria from the American Heart Association, AC Immune, Bayer, Bristol-Myers Squibb, and Medscape outside the area of work that he commented on.
This article first appeared on Medscape.com.
LOS ANGELES – A new potential neuroprotectant agent has been found to be beneficial for patients with acute ischemic stroke undergoing endovascular thrombectomy in a large placebo-controlled trial, but only for those patients who did not also receive thrombolysis.
There was no difference between groups on the primary outcome in the main analysis of the trial, lead author Michael Hill, MD, reported.
However, “In our study, we found a dramatic interaction of nerinetide with alteplase. There was a large benefit of nerinetide in patients not given thrombolysis, but in patients who received alteplase, this benefit was completely obliterated,” Dr. Hill said in an interview.
“In patients not treated with thrombolysis, we found a large effect size with a 9.5% absolute improvement in patients having an independent outcome (modified Rankin Score [mRS] 0-2) and a number need to treat of 10 to 11,” he said. “We also found a mortality benefit and a reduction in the size of strokes, with all other secondary outcomes going in the right direction.
“The drug works really well in patients who do not get thrombolysis, but it doesn’t work at all in patients who have had thrombolysis. The thrombolytic appears to break the peptide down so it is inactive,” he added.
“This is the first evidence that neuroprotection is possible in human stroke. This has never been shown before,” Dr. Hill noted. “Many previous clinical trials of potential neuroprotectants have been negative. We think this is a major breakthrough. This is pretty exciting stuff with really tantalizing results.”
Dr. Hill, professor of neurology at the University of Calgary (Alta.), presented results of the ESCAPE-NA1 trial on Feb. 20 at the International Stroke Conference (ISC) 2020. The trial was also simultaneously published online (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30258-0).
Endogenous nitric oxide
The new agent – known as NA1 or nerinetide – is a 20-amino-acid peptide with a novel mechanism of action; it inhibits signaling that leads to neuronal excitotoxicity. “It reduces endogenous nitric oxide generated inside the cell during ischemia, which is one of the main biochemical processes contributing to cell death,” Dr. Hill explained. In a primate model of ischemia reperfusion that was published in Nature in 2012, it was highly protective, he added.
The drug is given just once at the time of thrombectomy. It is short lived in the blood but detectable in the brain for up to 24 hours, he said.
The trial included 1,105 patients who had experienced acute ischemic stroke due to large-vessel occlusion within a 12-hour treatment window and for whom imaging results suitable for thrombectomy were available. The patients were randomly assigned to receive either intravenous nerinetide in a single dose of 2.6 mg/kg or saline placebo at the time of thrombectomy.
Patients were stratified by intravenous alteplase treatment and by declared endovascular device choice.
The primary outcome was a favorable functional outcome 90 days after randomization, defined as an mRS score of 0-2. In the main analysis of the whole population, this favorable outcome was achieved for 61.4% of the group that received nerinetide and for 59.2% of the placebo group, a nonsignificant difference. Secondary outcomes were also similar between the two groups.
But an exploratory analysis showed evidence that nerinetide’s treatment effect was modified by alteplase treatment. Among the patients who did not receive alteplase, use of nerinetide was associated with improved outcomes, whereas no benefit was found in the alteplase stratum. The difference in absolute risk slightly but not significantly favored placebo.
In the stratum that did not receive alteplase (40% of the trial population), the favorable mRS outcome was achieved by 59.3% of patients who received nerinetide, compared with 49.8% of those given placebo – a significant difference (adjusted risk ratio, 1.18; 95% confidence interval, 1.01-1.38).
There was also a 7.5% absolute risk reduction in mortality at 90 days post treatment with nerinetide for the patients who did not receive thrombolysis. This resulted in an approximate halving of the hazard of death (adjusted hazard ratio, 0.56).
In addition, infarct size was reduced in those patients who received nerinetide but not thrombolysis.
Among the patients who received alteplase, the proportion of patients who achieved an mRS of 0-2 was similar between groups, as were median infarct volumes.
The observed treatment effect modification by alteplase was supported by reductions in peak plasma nerinetide concentrations in the alteplase stratum, the researchers reported.
They said that the combination of the clinical results in the no-thrombolytic stratum and subsequent tests documenting that nerinetide is broken down by plasmin (which is generated by alteplase) “provide evidence that the clinical observation of effect modification is not a chance finding.” But they added: “This novel observation will require additional confirmation, and we cannot draw a definitive conclusion on treatment effect in this study.”
“Shaking up the field”
There is still more work to do, Dr. Hill said. “We don’t fully understand the pharmacology, and we will certainly have to do another trial, but we believe this agent is going to shake the field up. This is a totally new drug, and we have to think carefully about where it could fit in.”
“The obvious first group is those patients who do not receive thrombolysis. This is a large group, as most patients do not present in time for thrombolysis. Then we can work on the biochemistry and see if we can develop a version of nerinetide that is resistant to breakdown by thrombolysis,” he said.
Another possibility would be to withhold thrombolysis and give nerinetide instead. “It may be that thrombolysis is not needed if patients are receiving thrombectomy – this is being suggested now in initial studies,” Hill stated.
They also chose a very select group of patients – those undergoing thrombectomy, who represent only 10% to 15% of stroke patients. “We have to work out how to expand that population,” he said.
Hill noted that there have been many examples in the past of potential neuroprotectant agents that have worked in animal models of ischemia-reperfusion but that failed in humans with acute stroke.
“Until recently, we have not had a reliable ischemia-reperfusion model in humans, but now with endovascular therapy, we have a situation where the blood flow is reliably restored, which is an ideal situation to test new neuroprotectant agents. That may be another factor that has contributed to our positive findings,” he said.
In an accompanying comment in The Lancet, Graeme J. Hankey, MD, of the University of Western Australia, Perth, noted that although endovascular thrombectomy after use of intravenous alteplase improves reperfusion and clinical outcomes for a fifth of patients with ischemic stroke caused by large-artery occlusion, half of patients do not recover an independent lifestyle. Cytoprotection aims to augment the resilience of neurons, neurovascular units, and white matter during ischemia until perfusion is restored (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30316-0).
Dr. Hankey also pointed out that numerous cytoprotection strategies have been reported to reduce brain infarction in preclinical models of ischemic stroke but have not been found to improve clinical outcomes in clinical trials involving patients with ischemic stroke.
The advent of thrombectomy provides an opportunity to reassess cytoprotection as an adjunctive therapy for patients with types of temporary brain ischemia that align more closely with successful preclinical models of ischemia, cytoprotection, and reperfusion, he added.
On the results of the current study and the benefit in the no-thrombolysis group, Dr. Hankey stated: “Although this result might be a chance finding or confounded by the indication for alteplase, complementary pharmacokinetic data in a small number of patients treated with nerinetide showed that alteplase lowered plasma concentrations of nerinetide, probably by converting plasminogen to plasmin, which cleaves peptide bonds not only in fibrin but also in the eicosapeptide nerinetide.”
He said the ESCAPE-NA1 trial “informs the study of cytoprotection as an adjunct therapy to reperfusion in acute ischemic stroke” and suggested that researchers who have reported encouraging results of other cytoprotective therapies for ischemic stroke should test their compounds for interactions with concurrent thrombolytic therapies.
The ESCAPE-NA1 trial was sponsored by NoNO, the company developing nerinetide. Dr. Hill has received grants from NoNO for the conduct of the study, is named on a U.S. patent for systems and methods for assisting in decision making and triaging for acute stroke patients, and owns stock in Calgary Scientific. Other coauthors are employees of NoNO or have stock options in the company. Dr. Hankey has received personal honoraria from the American Heart Association, AC Immune, Bayer, Bristol-Myers Squibb, and Medscape outside the area of work that he commented on.
This article first appeared on Medscape.com.
New lipid-lowering drug earns FDA approval
The Food and Drug Administration has approved bempedoic acid (Nexletol) for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional LDL cholesterol lowering.
The oral adenosine triphosphate–citrate lyase (ACL) inhibitor is indicated as an adjunct to diet and maximally tolerated statin therapy in these patients, and approved at the 180 mg once daily dose, the agency announced today.
The safety and efficacy of bempedoic acid were demonstrated over 52 weeks in two multicenter randomized, clinical trials involving 3,009 adults with HeFH or established ASCVD on maximally tolerated statin therapy.
The difference between bempedoic acid and placebo for the primary outcome of change in LDL cholesterol from baseline to week 12 was –18% in the first trial, CLEAR Harmony (95% confidence interval, –20% to –16%; P less than .001), and –17% in the second trial, CLEAR Wisdom (95% CI, –21% to –14%; P less than .001).
The label notes that the effect on cardiovascular morbidity and mortality has not been determined. The label also includes warnings stating that bempedoic acid may increase blood uric acid levels and is associated with an increased risk of tendon rupture or injury.
In clinical trials, 26% of bempedoic acid–treated patients with normal baseline uric acid values versus 9.5% of placebo-treated patients experienced hyperuricemia one or more times, and 3.5% of patients experienced clinically significant hyperuricemia reported as an adverse reaction versus 1.1% with placebo, according to the label. Gout was reported in 1.5% of patients treated with bempedoic acid and 0.4% of those treated with placebo.
Also in clinical trials, the risk of tendon rupture was 0.5% with bempedoic acid and 0% with placebo. Tendon rupture involved the rotator cuff, biceps tendon, or Achilles tendon, and occurred within weeks to months of starting the drug. Rupture may “occur more frequently in patients over 60 years of age, in those taking corticosteroid or fluoroquinolone drugs, in patients with renal failure, and in patients with previous tendon disorders,” the label states.
The label also advises that patients avoid concomitant use of bempedoic acid with simvastatin greater than 20 mg or pravastatin greater than 40 mg because it causes an increase in statin concentrations and may increase the risk of related myopathy.
A decision is expected shortly on a new drug application submitted by Esperion for an LDL cholesterol–lowering indication for bempedoic acid 180 mg/ezetimibe 10 mg combination tablet.
Full prescribing information is available online.
This article first appeared on Medscape.com.
The Food and Drug Administration has approved bempedoic acid (Nexletol) for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional LDL cholesterol lowering.
The oral adenosine triphosphate–citrate lyase (ACL) inhibitor is indicated as an adjunct to diet and maximally tolerated statin therapy in these patients, and approved at the 180 mg once daily dose, the agency announced today.
The safety and efficacy of bempedoic acid were demonstrated over 52 weeks in two multicenter randomized, clinical trials involving 3,009 adults with HeFH or established ASCVD on maximally tolerated statin therapy.
The difference between bempedoic acid and placebo for the primary outcome of change in LDL cholesterol from baseline to week 12 was –18% in the first trial, CLEAR Harmony (95% confidence interval, –20% to –16%; P less than .001), and –17% in the second trial, CLEAR Wisdom (95% CI, –21% to –14%; P less than .001).
The label notes that the effect on cardiovascular morbidity and mortality has not been determined. The label also includes warnings stating that bempedoic acid may increase blood uric acid levels and is associated with an increased risk of tendon rupture or injury.
In clinical trials, 26% of bempedoic acid–treated patients with normal baseline uric acid values versus 9.5% of placebo-treated patients experienced hyperuricemia one or more times, and 3.5% of patients experienced clinically significant hyperuricemia reported as an adverse reaction versus 1.1% with placebo, according to the label. Gout was reported in 1.5% of patients treated with bempedoic acid and 0.4% of those treated with placebo.
Also in clinical trials, the risk of tendon rupture was 0.5% with bempedoic acid and 0% with placebo. Tendon rupture involved the rotator cuff, biceps tendon, or Achilles tendon, and occurred within weeks to months of starting the drug. Rupture may “occur more frequently in patients over 60 years of age, in those taking corticosteroid or fluoroquinolone drugs, in patients with renal failure, and in patients with previous tendon disorders,” the label states.
The label also advises that patients avoid concomitant use of bempedoic acid with simvastatin greater than 20 mg or pravastatin greater than 40 mg because it causes an increase in statin concentrations and may increase the risk of related myopathy.
A decision is expected shortly on a new drug application submitted by Esperion for an LDL cholesterol–lowering indication for bempedoic acid 180 mg/ezetimibe 10 mg combination tablet.
Full prescribing information is available online.
This article first appeared on Medscape.com.
The Food and Drug Administration has approved bempedoic acid (Nexletol) for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional LDL cholesterol lowering.
The oral adenosine triphosphate–citrate lyase (ACL) inhibitor is indicated as an adjunct to diet and maximally tolerated statin therapy in these patients, and approved at the 180 mg once daily dose, the agency announced today.
The safety and efficacy of bempedoic acid were demonstrated over 52 weeks in two multicenter randomized, clinical trials involving 3,009 adults with HeFH or established ASCVD on maximally tolerated statin therapy.
The difference between bempedoic acid and placebo for the primary outcome of change in LDL cholesterol from baseline to week 12 was –18% in the first trial, CLEAR Harmony (95% confidence interval, –20% to –16%; P less than .001), and –17% in the second trial, CLEAR Wisdom (95% CI, –21% to –14%; P less than .001).
The label notes that the effect on cardiovascular morbidity and mortality has not been determined. The label also includes warnings stating that bempedoic acid may increase blood uric acid levels and is associated with an increased risk of tendon rupture or injury.
In clinical trials, 26% of bempedoic acid–treated patients with normal baseline uric acid values versus 9.5% of placebo-treated patients experienced hyperuricemia one or more times, and 3.5% of patients experienced clinically significant hyperuricemia reported as an adverse reaction versus 1.1% with placebo, according to the label. Gout was reported in 1.5% of patients treated with bempedoic acid and 0.4% of those treated with placebo.
Also in clinical trials, the risk of tendon rupture was 0.5% with bempedoic acid and 0% with placebo. Tendon rupture involved the rotator cuff, biceps tendon, or Achilles tendon, and occurred within weeks to months of starting the drug. Rupture may “occur more frequently in patients over 60 years of age, in those taking corticosteroid or fluoroquinolone drugs, in patients with renal failure, and in patients with previous tendon disorders,” the label states.
The label also advises that patients avoid concomitant use of bempedoic acid with simvastatin greater than 20 mg or pravastatin greater than 40 mg because it causes an increase in statin concentrations and may increase the risk of related myopathy.
A decision is expected shortly on a new drug application submitted by Esperion for an LDL cholesterol–lowering indication for bempedoic acid 180 mg/ezetimibe 10 mg combination tablet.
Full prescribing information is available online.
This article first appeared on Medscape.com.
Higher endovascular thrombectomy volumes yield better stroke outcomes
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
REPORTING FROM ISC 2020
TNK dose in large-vessel stroke: 0.25 mg/kg is sufficient
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
ARCADIA: Predicting risk of atrial cardiopathy poststroke
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.
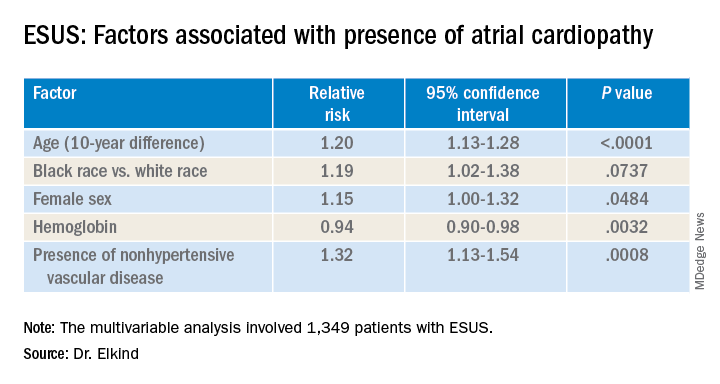
Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.

Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.

Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
REPORTING FROM ISC 2020

















