User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
FDA panel slams Endologix response to stent-graft safety issues
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
Texas interventional cardiologist subject of anticompetitive lawsuit
Doctors Hospital of Laredo, Texas, and the Laredo Physicians Group have filed a lawsuit against interventional cardiologist Ricardo Cigarroa, MD, alleging that he engaged in anticompetitive conduct over the availability of cardiologists in Laredo.
Also named in the lawsuit are Cigarroa Heart and Vascular Institute, Laredo Texas Hospital Company (doing business as Laredo Medical Center) and Laredo Physician Associates (LPA).
According to the complaint, in August 2020, Doctors Hospital and Laredo Physicians Group began actively recruiting cardiologists to the city of Laredo.
The complaint states that, with more than 260,000 residents, the city should have a minimum of 20 cardiologists. However, Laredo currently has only eight cardiologists and only six are interventional cardiologists.
The lawsuit alleges that when Dr. Cigarroa got wind of these recruitment efforts, he entered into a conspiracy with the Cigarroa Institute (a cardiology outpatient clinic) and Laredo Medical Center, the largest acute-care hospital in the city, to engage in “anticompetitive and tortious behavior.
“Their conspiracy had a simple but pernicious goal: deprive Doctors Hospital and Physicians Group of the doctors and employees needed to compete and provide interventional cardiology services to the Laredo market,” the complaint reads.
The alleged conspiracy unfolded in multiple steps, it notes, with Dr. Cigarroa issuing threats to Doctors Hospital, Laredo Physicians Group, and prospective interventional cardiologists being recruited.
Through threats and coercion, multiple qualified interventional cardiologists who were interested in joining Laredo Physicians Group, and to whom the group extended employment offers, decided not to join, the complaint states.
It further claims that Dr. Cigarroa, his son, and his nephew – who represent more than half of the interventional cardiologists in Laredo – informed Doctors Hospital that they would “no longer respond” to emergency calls at Doctors Hospital.
The complaint further alleges that after “scaring off competitors and further cementing their dominant market power and position,” the defendants targeted Arthur Santos, MD, Laredo’s only cardiovascular surgeon who was employed by Laredo Physicians Group.
“Defendants successfully induced Dr. Santos to agree to join Defendant Laredo Physicians Associates (LPA), breaching his enforceable noncompete contractual provision,” the complaint states.
The defendants then allegedly induced the cardiothoracic surgery technicians at Doctors Hospital to join Laredo Medical Center (LMC) and work with Dr. Cigarroa and Dr. Santos, the complaint says.
“The conspiracy to monopolize Laredo’s interventional cardiology market is a win-win-win for Defendants. Dr Cigarroa and the clinic avoid competition for interventional cardiological services, while LMC is left as the only provider of acute cardiology services in Laredo, gaining additional patients and corresponding increased revenue,” the complaint reads.
“Meanwhile, Doctors Hospital’s acute-care cardiology program will be threatened with extinction and, critically, Laredo patients are left with higher health care costs and greater health risks and without competitive market alternatives,” it states.
Dr. Cigarroa responds
According to the Laredo Times, in a statement responding to the anticompetitive conduct lawsuit, Dr. Cigarroa said: “This lawsuit is a dispute between a for-profit corporation and a physician who has demonstrated over 30 years of commitment to his patients and patient care in this community.
“It’s unfortunate that the executives [at] Doctors Hospital have chosen to put profit above the well-being of their patients and employees. Their actions confirm that they care more about their bottom line than they do about our residents and reaffirms how disconnected they are from our community,” Dr. Cigarroa said.
“My top priority continues to be the health of all Laredo residents. I will never stop caring for the patients that I love, and I will continue to help save lives,” Dr. Cigarroa said, according to the Times article.
“I am humbled to have received numerous calls of support from many Doctors Hospital employees. Their words of encouragement are a true testament to the strong relationships I have within the medical community,” Dr. Cigarroa added.
A version of this article first appeared on Medscape.com.
Doctors Hospital of Laredo, Texas, and the Laredo Physicians Group have filed a lawsuit against interventional cardiologist Ricardo Cigarroa, MD, alleging that he engaged in anticompetitive conduct over the availability of cardiologists in Laredo.
Also named in the lawsuit are Cigarroa Heart and Vascular Institute, Laredo Texas Hospital Company (doing business as Laredo Medical Center) and Laredo Physician Associates (LPA).
According to the complaint, in August 2020, Doctors Hospital and Laredo Physicians Group began actively recruiting cardiologists to the city of Laredo.
The complaint states that, with more than 260,000 residents, the city should have a minimum of 20 cardiologists. However, Laredo currently has only eight cardiologists and only six are interventional cardiologists.
The lawsuit alleges that when Dr. Cigarroa got wind of these recruitment efforts, he entered into a conspiracy with the Cigarroa Institute (a cardiology outpatient clinic) and Laredo Medical Center, the largest acute-care hospital in the city, to engage in “anticompetitive and tortious behavior.
“Their conspiracy had a simple but pernicious goal: deprive Doctors Hospital and Physicians Group of the doctors and employees needed to compete and provide interventional cardiology services to the Laredo market,” the complaint reads.
The alleged conspiracy unfolded in multiple steps, it notes, with Dr. Cigarroa issuing threats to Doctors Hospital, Laredo Physicians Group, and prospective interventional cardiologists being recruited.
Through threats and coercion, multiple qualified interventional cardiologists who were interested in joining Laredo Physicians Group, and to whom the group extended employment offers, decided not to join, the complaint states.
It further claims that Dr. Cigarroa, his son, and his nephew – who represent more than half of the interventional cardiologists in Laredo – informed Doctors Hospital that they would “no longer respond” to emergency calls at Doctors Hospital.
The complaint further alleges that after “scaring off competitors and further cementing their dominant market power and position,” the defendants targeted Arthur Santos, MD, Laredo’s only cardiovascular surgeon who was employed by Laredo Physicians Group.
“Defendants successfully induced Dr. Santos to agree to join Defendant Laredo Physicians Associates (LPA), breaching his enforceable noncompete contractual provision,” the complaint states.
The defendants then allegedly induced the cardiothoracic surgery technicians at Doctors Hospital to join Laredo Medical Center (LMC) and work with Dr. Cigarroa and Dr. Santos, the complaint says.
“The conspiracy to monopolize Laredo’s interventional cardiology market is a win-win-win for Defendants. Dr Cigarroa and the clinic avoid competition for interventional cardiological services, while LMC is left as the only provider of acute cardiology services in Laredo, gaining additional patients and corresponding increased revenue,” the complaint reads.
“Meanwhile, Doctors Hospital’s acute-care cardiology program will be threatened with extinction and, critically, Laredo patients are left with higher health care costs and greater health risks and without competitive market alternatives,” it states.
Dr. Cigarroa responds
According to the Laredo Times, in a statement responding to the anticompetitive conduct lawsuit, Dr. Cigarroa said: “This lawsuit is a dispute between a for-profit corporation and a physician who has demonstrated over 30 years of commitment to his patients and patient care in this community.
“It’s unfortunate that the executives [at] Doctors Hospital have chosen to put profit above the well-being of their patients and employees. Their actions confirm that they care more about their bottom line than they do about our residents and reaffirms how disconnected they are from our community,” Dr. Cigarroa said.
“My top priority continues to be the health of all Laredo residents. I will never stop caring for the patients that I love, and I will continue to help save lives,” Dr. Cigarroa said, according to the Times article.
“I am humbled to have received numerous calls of support from many Doctors Hospital employees. Their words of encouragement are a true testament to the strong relationships I have within the medical community,” Dr. Cigarroa added.
A version of this article first appeared on Medscape.com.
Doctors Hospital of Laredo, Texas, and the Laredo Physicians Group have filed a lawsuit against interventional cardiologist Ricardo Cigarroa, MD, alleging that he engaged in anticompetitive conduct over the availability of cardiologists in Laredo.
Also named in the lawsuit are Cigarroa Heart and Vascular Institute, Laredo Texas Hospital Company (doing business as Laredo Medical Center) and Laredo Physician Associates (LPA).
According to the complaint, in August 2020, Doctors Hospital and Laredo Physicians Group began actively recruiting cardiologists to the city of Laredo.
The complaint states that, with more than 260,000 residents, the city should have a minimum of 20 cardiologists. However, Laredo currently has only eight cardiologists and only six are interventional cardiologists.
The lawsuit alleges that when Dr. Cigarroa got wind of these recruitment efforts, he entered into a conspiracy with the Cigarroa Institute (a cardiology outpatient clinic) and Laredo Medical Center, the largest acute-care hospital in the city, to engage in “anticompetitive and tortious behavior.
“Their conspiracy had a simple but pernicious goal: deprive Doctors Hospital and Physicians Group of the doctors and employees needed to compete and provide interventional cardiology services to the Laredo market,” the complaint reads.
The alleged conspiracy unfolded in multiple steps, it notes, with Dr. Cigarroa issuing threats to Doctors Hospital, Laredo Physicians Group, and prospective interventional cardiologists being recruited.
Through threats and coercion, multiple qualified interventional cardiologists who were interested in joining Laredo Physicians Group, and to whom the group extended employment offers, decided not to join, the complaint states.
It further claims that Dr. Cigarroa, his son, and his nephew – who represent more than half of the interventional cardiologists in Laredo – informed Doctors Hospital that they would “no longer respond” to emergency calls at Doctors Hospital.
The complaint further alleges that after “scaring off competitors and further cementing their dominant market power and position,” the defendants targeted Arthur Santos, MD, Laredo’s only cardiovascular surgeon who was employed by Laredo Physicians Group.
“Defendants successfully induced Dr. Santos to agree to join Defendant Laredo Physicians Associates (LPA), breaching his enforceable noncompete contractual provision,” the complaint states.
The defendants then allegedly induced the cardiothoracic surgery technicians at Doctors Hospital to join Laredo Medical Center (LMC) and work with Dr. Cigarroa and Dr. Santos, the complaint says.
“The conspiracy to monopolize Laredo’s interventional cardiology market is a win-win-win for Defendants. Dr Cigarroa and the clinic avoid competition for interventional cardiological services, while LMC is left as the only provider of acute cardiology services in Laredo, gaining additional patients and corresponding increased revenue,” the complaint reads.
“Meanwhile, Doctors Hospital’s acute-care cardiology program will be threatened with extinction and, critically, Laredo patients are left with higher health care costs and greater health risks and without competitive market alternatives,” it states.
Dr. Cigarroa responds
According to the Laredo Times, in a statement responding to the anticompetitive conduct lawsuit, Dr. Cigarroa said: “This lawsuit is a dispute between a for-profit corporation and a physician who has demonstrated over 30 years of commitment to his patients and patient care in this community.
“It’s unfortunate that the executives [at] Doctors Hospital have chosen to put profit above the well-being of their patients and employees. Their actions confirm that they care more about their bottom line than they do about our residents and reaffirms how disconnected they are from our community,” Dr. Cigarroa said.
“My top priority continues to be the health of all Laredo residents. I will never stop caring for the patients that I love, and I will continue to help save lives,” Dr. Cigarroa said, according to the Times article.
“I am humbled to have received numerous calls of support from many Doctors Hospital employees. Their words of encouragement are a true testament to the strong relationships I have within the medical community,” Dr. Cigarroa added.
A version of this article first appeared on Medscape.com.
COVID-19 vaccine mandates are working, public health experts say
Some organizations have reported vaccination rates that jumped from less than 50% to more than 90%, according to ABC News. Workplace mandates have especially encouraged employees who were on the fence to get a shot.
“In general, vaccine mandates work,” James Colgrove, a public health professor at Columbia University’s Mailman School of Public Health, told ABC News.
For decades, the United States has monitored the effectiveness of vaccine mandates in schools, he noted, which have successfully required shots against measles, mumps, and other illnesses that used to be widespread. Certain employees, such as hospital workers, must take vaccines for their jobs, he said, and those requirements have also been effective over the years.
“The more normalized it becomes, the more people [know] someone else who is vaccinated, the more people will comply,” he said. “With any vaccine, the longer it’s been around, the more people get with it.”
With the widespread and contagious nature of COVID-19, workplaces have been forced to consider vaccine mandates to protect their employees and prevent worker shortages, Dr. Colgrove said.
Some companies began to issue vaccine rules this summer as the Delta variant caused a jump in cases, hospitalizations, and deaths. Major companies, including Google, Tyson Foods, United Airlines, and the Walt Disney Company, required in-person employees to get a shot. So far, the results from those mandates have been strong, ABC News reported.
For instance, Tyson announced a mandate in August, when less than half of its 140,000 employees were vaccinated. When the deadline came at the end of October, more than 60,000 additional employees had been vaccinated, and the vaccination rate was 96%.
“Has this made a difference in the health and safety of our team members? Absolutely. We’ve seen a significant decline in the number of active cases companywide,” Donnie King, CEO and president of Tyson Foods, said in a statement.
United Airlines has also shared that 99.7% of its 67,000 employees are vaccinated. Within 48 hours of announcing its mandate, the number of unvaccinated staffers fell from 593 to 320 people, ABC News reported.
Vaccine mandates appear to be working in the public sector as well. State health department officials in Washington told ABC News that the percentage of public employees who were vaccinated jumped from 49% in September to 96% by the vaccine mandate deadline in October.
Vaccination rates have also increased in New York City, where some employees in the fire, police, and sanitation departments protested the mandate. By the deadline, vaccination rates shifted from less than 75% to 82% in the fire department, 86% in the police department, and 91% of EMS personnel, ABC News reported.
Overall, vaccine mandates tend to reach groups who aren’t completely against the vaccine, medical experts told the news outlet. A small percentage of the population truly opposes the shot, and in most cases, unvaccinated people are on the fence or haven’t seen good enough messaging for it.
“When you look at vaccine resistance, the people who are the most opposed often make a very large amount of noise that is at odds with the actual numbers who are against vaccination,” Dr. Colgrove said.
A version of this article first appeared on WebMD.com.
Some organizations have reported vaccination rates that jumped from less than 50% to more than 90%, according to ABC News. Workplace mandates have especially encouraged employees who were on the fence to get a shot.
“In general, vaccine mandates work,” James Colgrove, a public health professor at Columbia University’s Mailman School of Public Health, told ABC News.
For decades, the United States has monitored the effectiveness of vaccine mandates in schools, he noted, which have successfully required shots against measles, mumps, and other illnesses that used to be widespread. Certain employees, such as hospital workers, must take vaccines for their jobs, he said, and those requirements have also been effective over the years.
“The more normalized it becomes, the more people [know] someone else who is vaccinated, the more people will comply,” he said. “With any vaccine, the longer it’s been around, the more people get with it.”
With the widespread and contagious nature of COVID-19, workplaces have been forced to consider vaccine mandates to protect their employees and prevent worker shortages, Dr. Colgrove said.
Some companies began to issue vaccine rules this summer as the Delta variant caused a jump in cases, hospitalizations, and deaths. Major companies, including Google, Tyson Foods, United Airlines, and the Walt Disney Company, required in-person employees to get a shot. So far, the results from those mandates have been strong, ABC News reported.
For instance, Tyson announced a mandate in August, when less than half of its 140,000 employees were vaccinated. When the deadline came at the end of October, more than 60,000 additional employees had been vaccinated, and the vaccination rate was 96%.
“Has this made a difference in the health and safety of our team members? Absolutely. We’ve seen a significant decline in the number of active cases companywide,” Donnie King, CEO and president of Tyson Foods, said in a statement.
United Airlines has also shared that 99.7% of its 67,000 employees are vaccinated. Within 48 hours of announcing its mandate, the number of unvaccinated staffers fell from 593 to 320 people, ABC News reported.
Vaccine mandates appear to be working in the public sector as well. State health department officials in Washington told ABC News that the percentage of public employees who were vaccinated jumped from 49% in September to 96% by the vaccine mandate deadline in October.
Vaccination rates have also increased in New York City, where some employees in the fire, police, and sanitation departments protested the mandate. By the deadline, vaccination rates shifted from less than 75% to 82% in the fire department, 86% in the police department, and 91% of EMS personnel, ABC News reported.
Overall, vaccine mandates tend to reach groups who aren’t completely against the vaccine, medical experts told the news outlet. A small percentage of the population truly opposes the shot, and in most cases, unvaccinated people are on the fence or haven’t seen good enough messaging for it.
“When you look at vaccine resistance, the people who are the most opposed often make a very large amount of noise that is at odds with the actual numbers who are against vaccination,” Dr. Colgrove said.
A version of this article first appeared on WebMD.com.
Some organizations have reported vaccination rates that jumped from less than 50% to more than 90%, according to ABC News. Workplace mandates have especially encouraged employees who were on the fence to get a shot.
“In general, vaccine mandates work,” James Colgrove, a public health professor at Columbia University’s Mailman School of Public Health, told ABC News.
For decades, the United States has monitored the effectiveness of vaccine mandates in schools, he noted, which have successfully required shots against measles, mumps, and other illnesses that used to be widespread. Certain employees, such as hospital workers, must take vaccines for their jobs, he said, and those requirements have also been effective over the years.
“The more normalized it becomes, the more people [know] someone else who is vaccinated, the more people will comply,” he said. “With any vaccine, the longer it’s been around, the more people get with it.”
With the widespread and contagious nature of COVID-19, workplaces have been forced to consider vaccine mandates to protect their employees and prevent worker shortages, Dr. Colgrove said.
Some companies began to issue vaccine rules this summer as the Delta variant caused a jump in cases, hospitalizations, and deaths. Major companies, including Google, Tyson Foods, United Airlines, and the Walt Disney Company, required in-person employees to get a shot. So far, the results from those mandates have been strong, ABC News reported.
For instance, Tyson announced a mandate in August, when less than half of its 140,000 employees were vaccinated. When the deadline came at the end of October, more than 60,000 additional employees had been vaccinated, and the vaccination rate was 96%.
“Has this made a difference in the health and safety of our team members? Absolutely. We’ve seen a significant decline in the number of active cases companywide,” Donnie King, CEO and president of Tyson Foods, said in a statement.
United Airlines has also shared that 99.7% of its 67,000 employees are vaccinated. Within 48 hours of announcing its mandate, the number of unvaccinated staffers fell from 593 to 320 people, ABC News reported.
Vaccine mandates appear to be working in the public sector as well. State health department officials in Washington told ABC News that the percentage of public employees who were vaccinated jumped from 49% in September to 96% by the vaccine mandate deadline in October.
Vaccination rates have also increased in New York City, where some employees in the fire, police, and sanitation departments protested the mandate. By the deadline, vaccination rates shifted from less than 75% to 82% in the fire department, 86% in the police department, and 91% of EMS personnel, ABC News reported.
Overall, vaccine mandates tend to reach groups who aren’t completely against the vaccine, medical experts told the news outlet. A small percentage of the population truly opposes the shot, and in most cases, unvaccinated people are on the fence or haven’t seen good enough messaging for it.
“When you look at vaccine resistance, the people who are the most opposed often make a very large amount of noise that is at odds with the actual numbers who are against vaccination,” Dr. Colgrove said.
A version of this article first appeared on WebMD.com.
Treating young adults with high LDL may be cost-effective
Treating elevated low-density lipoprotein cholesterol (LDL-C) in adults younger than 40 with statins is highly cost-effective in men, and intermediately cost-effective in women, a new report suggests.
In a simulated model based on data from the U.S. National Health and Nutrition Examination Survey (NHANES), lipid lowering with statins or lifestyle interventions in this age group would prevent or reduce the risk of atherosclerotic cardiovascular disease (ASCVD) and improve quality of life in later years.
The findings were published online Nov. 8 in the Journal of the American College of Cardiology.
“My group does epidemiologic analyses with cohort studies as well as health economic analyses like this one, and if you have long-term longitudinal observation, you see that the early exposures are important for what happens later,” senior author Andrew E. Moran, MD, Columbia University Irving Medical Center, New York, told this news organization.
“But when it comes to treatment studies that a lot of the treatment guidelines are based on, those are usually short-term, and they usually enroll older people. We saw the gap in the evidence that this paper tries to fill,” Dr. Moran said.
His group used a computer simulation model to synthesize evidence from observational cohort studies and clinical trials of statin treatment, as well as health services data on the costs of medicines and treatments.
Combining information from these sources, the investigators made their best estimates of the potential health benefits and costs of treating high cholesterol earlier in life, compared with standard care, which was statin treatment at age 40, or if LDL-C was 190 mg/dL or greater.
Lipid lowering incremental to standard care with moderate-intensity statins or intensive lifestyle interventions was simulated starting when young adult LDL-C was either ≥160 mg/dL or ≥130 mg/dL.
They found that approximately 27% of young adults who are free of ASCVD have LDL-C ≥130 mg/dL, and 9% have LDL-C of ≥160 mg/dL.
Their model projected that treating adults younger than 40 with statins or lifestyle interventions would prevent lifetime ASCVD events and increase quality-adjusted life years (QALYs) compared with standard care, which would begin treatment at age 40.
Incremental cost-effectiveness ratios (ICERs) were $31,000/QALY for statin treatment in young adult men with LDL-C ≥130 mg/dL, and $106,000/QALY for statin treatment in young women with LDL-C ≥130 mg/dL.
Intensive lifestyle intervention was more costly and less effective than statin therapy.
“We are straining to find these young adults with very high cholesterol,” Dr. Moran noted. “A lot of young adults don’t even see a doctor. This is an argument for engaging them in their health care and getting them involved in some basic screening. Atherosclerosis is a long-term process that starts in childhood for a lot of people.”
More innovative approaches may be needed, because the traditional health care system is not doing a good job of reaching young adults, he added. “Many of them may not have adequate health insurance. They need health care in nontraditional ways; convenience is really important for them. Perhaps part of the solution here is to think about ways of reaching this particular group that is not engaged with health care generally.”
Time to relax the age 40 threshold
The U.S. Preventive Services Task Force and the American College of Cardiology/American Heart Association should emphasize lifetime risk of elevated cholesterol, Paul A. Heidenreich, MD, MS, Stanford University School of Medicine, California, and colleagues write in an accompanying editorial.
“In addition to calculating 10-year risk, we should calculate years of life lost (or QALYs lost) from unhealthy LDL-C levels, and both lifestyle and pharmacologic treatment should be considered to treat high LDL-C in adults regardless of age. We also need to communicate that the mantra ‘lower is better’ applies not only to a single measurement but to lifetime exposure to LDL-C,” the editorialists write.
“I think treatment should be earlier than age 40,” Dr. Heidenreich said in an interview.
“Part of the reason that 40 was chosen as a threshold was because everyone looked at 10-year, or even 20-year risk, and thought there was no reason to worry until you get older. It’s interesting that we never accepted that with high blood pressure. But more and more, we are learning that it is a lifelong process,” he said.
“Statins are getting less and less expensive, and their safety is more and more established with every decade that goes by. I definitely agree with this paper that it would actually make sense to be starting much earlier for those with elevated CVD risk from their high cholesterol.”
The study was supported by the U.S. National Heart, Lung, and Blood Institute (NHLBI), the Medical Research Council, Swindon, U.K. Dr. Moran and Dr. Heidenreich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treating elevated low-density lipoprotein cholesterol (LDL-C) in adults younger than 40 with statins is highly cost-effective in men, and intermediately cost-effective in women, a new report suggests.
In a simulated model based on data from the U.S. National Health and Nutrition Examination Survey (NHANES), lipid lowering with statins or lifestyle interventions in this age group would prevent or reduce the risk of atherosclerotic cardiovascular disease (ASCVD) and improve quality of life in later years.
The findings were published online Nov. 8 in the Journal of the American College of Cardiology.
“My group does epidemiologic analyses with cohort studies as well as health economic analyses like this one, and if you have long-term longitudinal observation, you see that the early exposures are important for what happens later,” senior author Andrew E. Moran, MD, Columbia University Irving Medical Center, New York, told this news organization.
“But when it comes to treatment studies that a lot of the treatment guidelines are based on, those are usually short-term, and they usually enroll older people. We saw the gap in the evidence that this paper tries to fill,” Dr. Moran said.
His group used a computer simulation model to synthesize evidence from observational cohort studies and clinical trials of statin treatment, as well as health services data on the costs of medicines and treatments.
Combining information from these sources, the investigators made their best estimates of the potential health benefits and costs of treating high cholesterol earlier in life, compared with standard care, which was statin treatment at age 40, or if LDL-C was 190 mg/dL or greater.
Lipid lowering incremental to standard care with moderate-intensity statins or intensive lifestyle interventions was simulated starting when young adult LDL-C was either ≥160 mg/dL or ≥130 mg/dL.
They found that approximately 27% of young adults who are free of ASCVD have LDL-C ≥130 mg/dL, and 9% have LDL-C of ≥160 mg/dL.
Their model projected that treating adults younger than 40 with statins or lifestyle interventions would prevent lifetime ASCVD events and increase quality-adjusted life years (QALYs) compared with standard care, which would begin treatment at age 40.
Incremental cost-effectiveness ratios (ICERs) were $31,000/QALY for statin treatment in young adult men with LDL-C ≥130 mg/dL, and $106,000/QALY for statin treatment in young women with LDL-C ≥130 mg/dL.
Intensive lifestyle intervention was more costly and less effective than statin therapy.
“We are straining to find these young adults with very high cholesterol,” Dr. Moran noted. “A lot of young adults don’t even see a doctor. This is an argument for engaging them in their health care and getting them involved in some basic screening. Atherosclerosis is a long-term process that starts in childhood for a lot of people.”
More innovative approaches may be needed, because the traditional health care system is not doing a good job of reaching young adults, he added. “Many of them may not have adequate health insurance. They need health care in nontraditional ways; convenience is really important for them. Perhaps part of the solution here is to think about ways of reaching this particular group that is not engaged with health care generally.”
Time to relax the age 40 threshold
The U.S. Preventive Services Task Force and the American College of Cardiology/American Heart Association should emphasize lifetime risk of elevated cholesterol, Paul A. Heidenreich, MD, MS, Stanford University School of Medicine, California, and colleagues write in an accompanying editorial.
“In addition to calculating 10-year risk, we should calculate years of life lost (or QALYs lost) from unhealthy LDL-C levels, and both lifestyle and pharmacologic treatment should be considered to treat high LDL-C in adults regardless of age. We also need to communicate that the mantra ‘lower is better’ applies not only to a single measurement but to lifetime exposure to LDL-C,” the editorialists write.
“I think treatment should be earlier than age 40,” Dr. Heidenreich said in an interview.
“Part of the reason that 40 was chosen as a threshold was because everyone looked at 10-year, or even 20-year risk, and thought there was no reason to worry until you get older. It’s interesting that we never accepted that with high blood pressure. But more and more, we are learning that it is a lifelong process,” he said.
“Statins are getting less and less expensive, and their safety is more and more established with every decade that goes by. I definitely agree with this paper that it would actually make sense to be starting much earlier for those with elevated CVD risk from their high cholesterol.”
The study was supported by the U.S. National Heart, Lung, and Blood Institute (NHLBI), the Medical Research Council, Swindon, U.K. Dr. Moran and Dr. Heidenreich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treating elevated low-density lipoprotein cholesterol (LDL-C) in adults younger than 40 with statins is highly cost-effective in men, and intermediately cost-effective in women, a new report suggests.
In a simulated model based on data from the U.S. National Health and Nutrition Examination Survey (NHANES), lipid lowering with statins or lifestyle interventions in this age group would prevent or reduce the risk of atherosclerotic cardiovascular disease (ASCVD) and improve quality of life in later years.
The findings were published online Nov. 8 in the Journal of the American College of Cardiology.
“My group does epidemiologic analyses with cohort studies as well as health economic analyses like this one, and if you have long-term longitudinal observation, you see that the early exposures are important for what happens later,” senior author Andrew E. Moran, MD, Columbia University Irving Medical Center, New York, told this news organization.
“But when it comes to treatment studies that a lot of the treatment guidelines are based on, those are usually short-term, and they usually enroll older people. We saw the gap in the evidence that this paper tries to fill,” Dr. Moran said.
His group used a computer simulation model to synthesize evidence from observational cohort studies and clinical trials of statin treatment, as well as health services data on the costs of medicines and treatments.
Combining information from these sources, the investigators made their best estimates of the potential health benefits and costs of treating high cholesterol earlier in life, compared with standard care, which was statin treatment at age 40, or if LDL-C was 190 mg/dL or greater.
Lipid lowering incremental to standard care with moderate-intensity statins or intensive lifestyle interventions was simulated starting when young adult LDL-C was either ≥160 mg/dL or ≥130 mg/dL.
They found that approximately 27% of young adults who are free of ASCVD have LDL-C ≥130 mg/dL, and 9% have LDL-C of ≥160 mg/dL.
Their model projected that treating adults younger than 40 with statins or lifestyle interventions would prevent lifetime ASCVD events and increase quality-adjusted life years (QALYs) compared with standard care, which would begin treatment at age 40.
Incremental cost-effectiveness ratios (ICERs) were $31,000/QALY for statin treatment in young adult men with LDL-C ≥130 mg/dL, and $106,000/QALY for statin treatment in young women with LDL-C ≥130 mg/dL.
Intensive lifestyle intervention was more costly and less effective than statin therapy.
“We are straining to find these young adults with very high cholesterol,” Dr. Moran noted. “A lot of young adults don’t even see a doctor. This is an argument for engaging them in their health care and getting them involved in some basic screening. Atherosclerosis is a long-term process that starts in childhood for a lot of people.”
More innovative approaches may be needed, because the traditional health care system is not doing a good job of reaching young adults, he added. “Many of them may not have adequate health insurance. They need health care in nontraditional ways; convenience is really important for them. Perhaps part of the solution here is to think about ways of reaching this particular group that is not engaged with health care generally.”
Time to relax the age 40 threshold
The U.S. Preventive Services Task Force and the American College of Cardiology/American Heart Association should emphasize lifetime risk of elevated cholesterol, Paul A. Heidenreich, MD, MS, Stanford University School of Medicine, California, and colleagues write in an accompanying editorial.
“In addition to calculating 10-year risk, we should calculate years of life lost (or QALYs lost) from unhealthy LDL-C levels, and both lifestyle and pharmacologic treatment should be considered to treat high LDL-C in adults regardless of age. We also need to communicate that the mantra ‘lower is better’ applies not only to a single measurement but to lifetime exposure to LDL-C,” the editorialists write.
“I think treatment should be earlier than age 40,” Dr. Heidenreich said in an interview.
“Part of the reason that 40 was chosen as a threshold was because everyone looked at 10-year, or even 20-year risk, and thought there was no reason to worry until you get older. It’s interesting that we never accepted that with high blood pressure. But more and more, we are learning that it is a lifelong process,” he said.
“Statins are getting less and less expensive, and their safety is more and more established with every decade that goes by. I definitely agree with this paper that it would actually make sense to be starting much earlier for those with elevated CVD risk from their high cholesterol.”
The study was supported by the U.S. National Heart, Lung, and Blood Institute (NHLBI), the Medical Research Council, Swindon, U.K. Dr. Moran and Dr. Heidenreich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
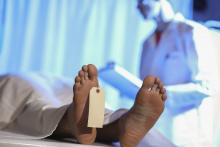
Step right up, folks, for a public dissection
The greatest autopsy on Earth?
The LOTME staff would like to apologize in advance. The following item contains historical facts.
P.T. Barnum is a rather controversial figure in American history. The greatest show on Earth was certainly popular in its day. However, Barnum got his start in 1835 by leasing a slave named Joyce Heth, an elderly Black woman who told vivid stories of caring for a young George Washington. He toured her around the country, advertising her as a 160-year-old woman who served as George Washington’s nanny. When Ms. Heth died the next year, Barnum sold tickets to the autopsy, charging the equivalent of $30 in today’s money.
When a doctor announced that Ms. Heth was actually 75-80 when she died, it caused great controversy in the press and ruined Barnum’s career. Wait, no, that’s not right. The opposite, actually. He weathered the storm, built his famous circus, and never again committed a hoax.
It’s difficult to quantify how wrong publicly dissecting a person and charging people to see said dissection is, but that was almost 200 years ago. At the very least, we can say that such terrible behavior is firmly in the distant past.
Oh wait.
David Saunders, a 98-year-old veteran of World War II and the Korean War, donated his body to science. His body, however, was purchased by DeathScience.org from a medical lab – with the buyer supposedly misleading the medical lab about its intentions, which was for use at the traveling Oddities and Curiosities Expo. Tickets went for up to $500 each to witness the public autopsy of Mr. Saunders’ body, which took place at a Marriott in Portland, Ore. It promised to be an exciting, all-day event from 9 a.m. to 4 p.m., with a break for lunch, of course. You can’t have an autopsy without a catered lunch.
Another public autopsy event was scheduled in Seattle but canceled after news of the first event broke. Oh, and for that extra little kick, Mr. Saunders died from COVID-19, meaning that all those paying customers were exposed.
P.T. Barnum is probably rolling over in his grave right now. His autopsy tickets were a bargain.
Go ahead, have that soda before math
We should all know by now that sugary drinks are bad, even artificially sweetened ones. It might not always stop us from drinking them, but we know the deal. But what if sugary drinks like soda could be helpful for girls in school?
You read that right. We said girls. A soda before class might have boys bouncing off the walls, but not girls. A recent study showed that not only was girls’ behavior unaffected by having a sugary drink, their math skills even improved.
Researchers analyzed the behavior of 4- to 6-year-old children before and after having a sugary drink. The sugar rush was actually calming for girls and helped them perform better with numerical skills, but the opposite was true for boys. “Our study is the first to provide large-scale experimental evidence on the impact of sugary drinks on preschool children. The results clearly indicate a causal impact of sugary drinks on children’s behavior and test scores,” Fritz Schiltz, PhD, said in a written statement.
This probably isn’t the green light to have as many sugary drinks as you want, but it might be interesting to see how your work is affected after a soda.
Chicken nuggets and the meat paradox
Two young children are fighting over the last chicken nugget when an adult comes in to see what’s going on.
Liam: Vegetable!
Olivia: Meat!
Liam: Chicken nuggets are vegetables!
Olivia: No, dorkface! They’re meat.
Caregiver: Good news, kids. You’re both right.
Olivia: How can we both be right?
At this point, a woman enters the room. She’s wearing a white lab coat, so she must be a scientist.
Dr. Scientist: You can’t both be right, Olivia. You are being fed a serving of the meat paradox. That’s why Liam here doesn’t know that chicken nuggets are made of chicken, which is a form of meat. Sadly, he’s not the only one.
In a recent study, scientists from Furman University in Greenville, S.C., found that 38% of 176 children aged 4-7 years thought that chicken nuggets were vegetables and more than 46% identified French fries as animal based.
Olivia: Did our caregiver lie to us, Dr. Scientist?
Dr. Scientist: Yes, Olivia. The researchers I mentioned explained that “many people experience unease while eating meat. Omnivores eat foods that entail animal suffering and death while at the same time endorsing the compassionate treatment of animals.” That’s the meat paradox.
Liam: What else did they say, Dr. Scientist?
Dr. Scientist: Over 70% of those children said that cows and pigs were not edible and 5% thought that cats and horses were. The investigators wrote “that children and youth should be viewed as agents of environmental change” in the future, but suggested that parents need to bring honesty to the table.
Caregiver: How did you get in here anyway? And how do you know their names?
Dr. Scientist: I’ve been rooting through your garbage for years. All in the name of science, of course.
Bedtimes aren’t just for children
There are multiple ways to prevent heart disease, but what if it could be as easy as switching your bedtime? A recent study in European Heart Journal–Digital Health suggests that there’s a sweet spot when it comes to sleep timing.
Through smartwatch-like devices, researchers measured the sleep-onset and wake-up times for 7 days in 88,026 participants aged 43-79 years. After 5.7 years of follow-up to see if anyone had a heart attack, stroke, or any other cardiovascular event, 3.6% developed some kind of cardiovascular disease.
Those who went to bed between 10 p.m. and 11 p.m. had a lower risk of developing heart disease. The risk was 25% higher for subjects who went to bed at midnight or later, 24% higher for bedtimes before 10 p.m., and 12% higher for bedtimes between 11 p.m. and midnight.
So, why can you go to bed before “The Tonight Show” and lower your cardiovascular risk but not before the nightly news? Well, it has something to do with your body’s natural clock.
“The optimum time to go to sleep is at a specific point in the body’s 24-hour cycle and deviations may be detrimental to health. The riskiest time was after midnight, potentially because it may reduce the likelihood of seeing morning light, which resets the body clock,” said study author Dr. David Plans of the University of Exeter, England.
Although a sleep schedule is preferred, it isn’t realistic all the time for those in certain occupations who might have to resort to other methods to keep their circadian clocks ticking optimally for their health. But if all it takes is prescribing a sleep time to reduce heart disease on a massive scale it would make a great “low-cost public health target.”
So bedtimes aren’t just for children.
The greatest autopsy on Earth?
The LOTME staff would like to apologize in advance. The following item contains historical facts.
P.T. Barnum is a rather controversial figure in American history. The greatest show on Earth was certainly popular in its day. However, Barnum got his start in 1835 by leasing a slave named Joyce Heth, an elderly Black woman who told vivid stories of caring for a young George Washington. He toured her around the country, advertising her as a 160-year-old woman who served as George Washington’s nanny. When Ms. Heth died the next year, Barnum sold tickets to the autopsy, charging the equivalent of $30 in today’s money.
When a doctor announced that Ms. Heth was actually 75-80 when she died, it caused great controversy in the press and ruined Barnum’s career. Wait, no, that’s not right. The opposite, actually. He weathered the storm, built his famous circus, and never again committed a hoax.
It’s difficult to quantify how wrong publicly dissecting a person and charging people to see said dissection is, but that was almost 200 years ago. At the very least, we can say that such terrible behavior is firmly in the distant past.
Oh wait.
David Saunders, a 98-year-old veteran of World War II and the Korean War, donated his body to science. His body, however, was purchased by DeathScience.org from a medical lab – with the buyer supposedly misleading the medical lab about its intentions, which was for use at the traveling Oddities and Curiosities Expo. Tickets went for up to $500 each to witness the public autopsy of Mr. Saunders’ body, which took place at a Marriott in Portland, Ore. It promised to be an exciting, all-day event from 9 a.m. to 4 p.m., with a break for lunch, of course. You can’t have an autopsy without a catered lunch.
Another public autopsy event was scheduled in Seattle but canceled after news of the first event broke. Oh, and for that extra little kick, Mr. Saunders died from COVID-19, meaning that all those paying customers were exposed.
P.T. Barnum is probably rolling over in his grave right now. His autopsy tickets were a bargain.
Go ahead, have that soda before math
We should all know by now that sugary drinks are bad, even artificially sweetened ones. It might not always stop us from drinking them, but we know the deal. But what if sugary drinks like soda could be helpful for girls in school?
You read that right. We said girls. A soda before class might have boys bouncing off the walls, but not girls. A recent study showed that not only was girls’ behavior unaffected by having a sugary drink, their math skills even improved.
Researchers analyzed the behavior of 4- to 6-year-old children before and after having a sugary drink. The sugar rush was actually calming for girls and helped them perform better with numerical skills, but the opposite was true for boys. “Our study is the first to provide large-scale experimental evidence on the impact of sugary drinks on preschool children. The results clearly indicate a causal impact of sugary drinks on children’s behavior and test scores,” Fritz Schiltz, PhD, said in a written statement.
This probably isn’t the green light to have as many sugary drinks as you want, but it might be interesting to see how your work is affected after a soda.
Chicken nuggets and the meat paradox
Two young children are fighting over the last chicken nugget when an adult comes in to see what’s going on.
Liam: Vegetable!
Olivia: Meat!
Liam: Chicken nuggets are vegetables!
Olivia: No, dorkface! They’re meat.
Caregiver: Good news, kids. You’re both right.
Olivia: How can we both be right?
At this point, a woman enters the room. She’s wearing a white lab coat, so she must be a scientist.
Dr. Scientist: You can’t both be right, Olivia. You are being fed a serving of the meat paradox. That’s why Liam here doesn’t know that chicken nuggets are made of chicken, which is a form of meat. Sadly, he’s not the only one.
In a recent study, scientists from Furman University in Greenville, S.C., found that 38% of 176 children aged 4-7 years thought that chicken nuggets were vegetables and more than 46% identified French fries as animal based.
Olivia: Did our caregiver lie to us, Dr. Scientist?
Dr. Scientist: Yes, Olivia. The researchers I mentioned explained that “many people experience unease while eating meat. Omnivores eat foods that entail animal suffering and death while at the same time endorsing the compassionate treatment of animals.” That’s the meat paradox.
Liam: What else did they say, Dr. Scientist?
Dr. Scientist: Over 70% of those children said that cows and pigs were not edible and 5% thought that cats and horses were. The investigators wrote “that children and youth should be viewed as agents of environmental change” in the future, but suggested that parents need to bring honesty to the table.
Caregiver: How did you get in here anyway? And how do you know their names?
Dr. Scientist: I’ve been rooting through your garbage for years. All in the name of science, of course.
Bedtimes aren’t just for children
There are multiple ways to prevent heart disease, but what if it could be as easy as switching your bedtime? A recent study in European Heart Journal–Digital Health suggests that there’s a sweet spot when it comes to sleep timing.
Through smartwatch-like devices, researchers measured the sleep-onset and wake-up times for 7 days in 88,026 participants aged 43-79 years. After 5.7 years of follow-up to see if anyone had a heart attack, stroke, or any other cardiovascular event, 3.6% developed some kind of cardiovascular disease.
Those who went to bed between 10 p.m. and 11 p.m. had a lower risk of developing heart disease. The risk was 25% higher for subjects who went to bed at midnight or later, 24% higher for bedtimes before 10 p.m., and 12% higher for bedtimes between 11 p.m. and midnight.
So, why can you go to bed before “The Tonight Show” and lower your cardiovascular risk but not before the nightly news? Well, it has something to do with your body’s natural clock.
“The optimum time to go to sleep is at a specific point in the body’s 24-hour cycle and deviations may be detrimental to health. The riskiest time was after midnight, potentially because it may reduce the likelihood of seeing morning light, which resets the body clock,” said study author Dr. David Plans of the University of Exeter, England.
Although a sleep schedule is preferred, it isn’t realistic all the time for those in certain occupations who might have to resort to other methods to keep their circadian clocks ticking optimally for their health. But if all it takes is prescribing a sleep time to reduce heart disease on a massive scale it would make a great “low-cost public health target.”
So bedtimes aren’t just for children.
The greatest autopsy on Earth?
The LOTME staff would like to apologize in advance. The following item contains historical facts.
P.T. Barnum is a rather controversial figure in American history. The greatest show on Earth was certainly popular in its day. However, Barnum got his start in 1835 by leasing a slave named Joyce Heth, an elderly Black woman who told vivid stories of caring for a young George Washington. He toured her around the country, advertising her as a 160-year-old woman who served as George Washington’s nanny. When Ms. Heth died the next year, Barnum sold tickets to the autopsy, charging the equivalent of $30 in today’s money.
When a doctor announced that Ms. Heth was actually 75-80 when she died, it caused great controversy in the press and ruined Barnum’s career. Wait, no, that’s not right. The opposite, actually. He weathered the storm, built his famous circus, and never again committed a hoax.
It’s difficult to quantify how wrong publicly dissecting a person and charging people to see said dissection is, but that was almost 200 years ago. At the very least, we can say that such terrible behavior is firmly in the distant past.
Oh wait.
David Saunders, a 98-year-old veteran of World War II and the Korean War, donated his body to science. His body, however, was purchased by DeathScience.org from a medical lab – with the buyer supposedly misleading the medical lab about its intentions, which was for use at the traveling Oddities and Curiosities Expo. Tickets went for up to $500 each to witness the public autopsy of Mr. Saunders’ body, which took place at a Marriott in Portland, Ore. It promised to be an exciting, all-day event from 9 a.m. to 4 p.m., with a break for lunch, of course. You can’t have an autopsy without a catered lunch.
Another public autopsy event was scheduled in Seattle but canceled after news of the first event broke. Oh, and for that extra little kick, Mr. Saunders died from COVID-19, meaning that all those paying customers were exposed.
P.T. Barnum is probably rolling over in his grave right now. His autopsy tickets were a bargain.
Go ahead, have that soda before math
We should all know by now that sugary drinks are bad, even artificially sweetened ones. It might not always stop us from drinking them, but we know the deal. But what if sugary drinks like soda could be helpful for girls in school?
You read that right. We said girls. A soda before class might have boys bouncing off the walls, but not girls. A recent study showed that not only was girls’ behavior unaffected by having a sugary drink, their math skills even improved.
Researchers analyzed the behavior of 4- to 6-year-old children before and after having a sugary drink. The sugar rush was actually calming for girls and helped them perform better with numerical skills, but the opposite was true for boys. “Our study is the first to provide large-scale experimental evidence on the impact of sugary drinks on preschool children. The results clearly indicate a causal impact of sugary drinks on children’s behavior and test scores,” Fritz Schiltz, PhD, said in a written statement.
This probably isn’t the green light to have as many sugary drinks as you want, but it might be interesting to see how your work is affected after a soda.
Chicken nuggets and the meat paradox
Two young children are fighting over the last chicken nugget when an adult comes in to see what’s going on.
Liam: Vegetable!
Olivia: Meat!
Liam: Chicken nuggets are vegetables!
Olivia: No, dorkface! They’re meat.
Caregiver: Good news, kids. You’re both right.
Olivia: How can we both be right?
At this point, a woman enters the room. She’s wearing a white lab coat, so she must be a scientist.
Dr. Scientist: You can’t both be right, Olivia. You are being fed a serving of the meat paradox. That’s why Liam here doesn’t know that chicken nuggets are made of chicken, which is a form of meat. Sadly, he’s not the only one.
In a recent study, scientists from Furman University in Greenville, S.C., found that 38% of 176 children aged 4-7 years thought that chicken nuggets were vegetables and more than 46% identified French fries as animal based.
Olivia: Did our caregiver lie to us, Dr. Scientist?
Dr. Scientist: Yes, Olivia. The researchers I mentioned explained that “many people experience unease while eating meat. Omnivores eat foods that entail animal suffering and death while at the same time endorsing the compassionate treatment of animals.” That’s the meat paradox.
Liam: What else did they say, Dr. Scientist?
Dr. Scientist: Over 70% of those children said that cows and pigs were not edible and 5% thought that cats and horses were. The investigators wrote “that children and youth should be viewed as agents of environmental change” in the future, but suggested that parents need to bring honesty to the table.
Caregiver: How did you get in here anyway? And how do you know their names?
Dr. Scientist: I’ve been rooting through your garbage for years. All in the name of science, of course.
Bedtimes aren’t just for children
There are multiple ways to prevent heart disease, but what if it could be as easy as switching your bedtime? A recent study in European Heart Journal–Digital Health suggests that there’s a sweet spot when it comes to sleep timing.
Through smartwatch-like devices, researchers measured the sleep-onset and wake-up times for 7 days in 88,026 participants aged 43-79 years. After 5.7 years of follow-up to see if anyone had a heart attack, stroke, or any other cardiovascular event, 3.6% developed some kind of cardiovascular disease.
Those who went to bed between 10 p.m. and 11 p.m. had a lower risk of developing heart disease. The risk was 25% higher for subjects who went to bed at midnight or later, 24% higher for bedtimes before 10 p.m., and 12% higher for bedtimes between 11 p.m. and midnight.
So, why can you go to bed before “The Tonight Show” and lower your cardiovascular risk but not before the nightly news? Well, it has something to do with your body’s natural clock.
“The optimum time to go to sleep is at a specific point in the body’s 24-hour cycle and deviations may be detrimental to health. The riskiest time was after midnight, potentially because it may reduce the likelihood of seeing morning light, which resets the body clock,” said study author Dr. David Plans of the University of Exeter, England.
Although a sleep schedule is preferred, it isn’t realistic all the time for those in certain occupations who might have to resort to other methods to keep their circadian clocks ticking optimally for their health. But if all it takes is prescribing a sleep time to reduce heart disease on a massive scale it would make a great “low-cost public health target.”
So bedtimes aren’t just for children.
AHA 2021 puts scientific dialogue, health equity center stage
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Pfizer seeks EUA expansion for COVID-19 booster
Pfizer and its European partner BioNTech on Nov. 9 asked the U.S. government to expand emergency use authorization (EUA) to allow everybody over 18 to receive their COVID-19 booster shots.
If the request is approved, He announced the goal last August but backed off after some scientists said younger people may not need boosters, especially with large parts of the world unvaccinated.
Pfizer is submitting a study of booster effects on 10,000 people to make its case, according to The Associated Press.
This would be Pfizer’s second attempt. In September, a Food and Drug Administration advisory panel turned down Pfizer’s idea of booster shots for everybody over 18.
However, the committee recommended Pfizer booster shots for people 65 and over, essential workers, and people with underlying health conditions.
The FDA and the Centers for Disease Control and Prevention authorized the Pfizer booster for those other groups and later authorization was granted for the same groups with Moderna and Johnson & Johnson boosters. People who got the two-shot Pfizer or Moderna vaccines should get a booster 6 months after the second dose and people who got the one-dose J&J vaccine should get a booster 2 months later.
The pro-booster argument has strengthened because new data have come in from Israel that confirm boosters provide protection as vaccine effectiveness wanes over time, The Washington Post reported. Also, health officials are worried about a post-holiday surge and because COVID-19 case counts and deaths are not dropping in every part of the country, though they are declining overall, according to the The Post report.
The regulatory path for a booster-for-all application is unclear. The Post, citing two unnamed officials, said the FDA probably won’t send the Pfizer application to the FDA advisory committee this time because the committee has already had extensive discussions about boosters. If the FDA gives the green light, CDC Director Rochelle Walensky, MD, would have to make updated recommendations on boosters, The Post article noted.
A version of this article first appeared on WebMD.com.
Pfizer and its European partner BioNTech on Nov. 9 asked the U.S. government to expand emergency use authorization (EUA) to allow everybody over 18 to receive their COVID-19 booster shots.
If the request is approved, He announced the goal last August but backed off after some scientists said younger people may not need boosters, especially with large parts of the world unvaccinated.
Pfizer is submitting a study of booster effects on 10,000 people to make its case, according to The Associated Press.
This would be Pfizer’s second attempt. In September, a Food and Drug Administration advisory panel turned down Pfizer’s idea of booster shots for everybody over 18.
However, the committee recommended Pfizer booster shots for people 65 and over, essential workers, and people with underlying health conditions.
The FDA and the Centers for Disease Control and Prevention authorized the Pfizer booster for those other groups and later authorization was granted for the same groups with Moderna and Johnson & Johnson boosters. People who got the two-shot Pfizer or Moderna vaccines should get a booster 6 months after the second dose and people who got the one-dose J&J vaccine should get a booster 2 months later.
The pro-booster argument has strengthened because new data have come in from Israel that confirm boosters provide protection as vaccine effectiveness wanes over time, The Washington Post reported. Also, health officials are worried about a post-holiday surge and because COVID-19 case counts and deaths are not dropping in every part of the country, though they are declining overall, according to the The Post report.
The regulatory path for a booster-for-all application is unclear. The Post, citing two unnamed officials, said the FDA probably won’t send the Pfizer application to the FDA advisory committee this time because the committee has already had extensive discussions about boosters. If the FDA gives the green light, CDC Director Rochelle Walensky, MD, would have to make updated recommendations on boosters, The Post article noted.
A version of this article first appeared on WebMD.com.
Pfizer and its European partner BioNTech on Nov. 9 asked the U.S. government to expand emergency use authorization (EUA) to allow everybody over 18 to receive their COVID-19 booster shots.
If the request is approved, He announced the goal last August but backed off after some scientists said younger people may not need boosters, especially with large parts of the world unvaccinated.
Pfizer is submitting a study of booster effects on 10,000 people to make its case, according to The Associated Press.
This would be Pfizer’s second attempt. In September, a Food and Drug Administration advisory panel turned down Pfizer’s idea of booster shots for everybody over 18.
However, the committee recommended Pfizer booster shots for people 65 and over, essential workers, and people with underlying health conditions.
The FDA and the Centers for Disease Control and Prevention authorized the Pfizer booster for those other groups and later authorization was granted for the same groups with Moderna and Johnson & Johnson boosters. People who got the two-shot Pfizer or Moderna vaccines should get a booster 6 months after the second dose and people who got the one-dose J&J vaccine should get a booster 2 months later.
The pro-booster argument has strengthened because new data have come in from Israel that confirm boosters provide protection as vaccine effectiveness wanes over time, The Washington Post reported. Also, health officials are worried about a post-holiday surge and because COVID-19 case counts and deaths are not dropping in every part of the country, though they are declining overall, according to the The Post report.
The regulatory path for a booster-for-all application is unclear. The Post, citing two unnamed officials, said the FDA probably won’t send the Pfizer application to the FDA advisory committee this time because the committee has already had extensive discussions about boosters. If the FDA gives the green light, CDC Director Rochelle Walensky, MD, would have to make updated recommendations on boosters, The Post article noted.
A version of this article first appeared on WebMD.com.
Should you tell your doctor that you’re a doctor?
The question drew spirited debate when urologist Ashley Winter, MD, made a simple, straightforward request on Twitter: “If you are a doctor & you come to an appointment please tell me you are a doctor, not because I will treat you differently but because it’s easier to speak in jargon.”
She later added, “This doesn’t’ mean I would be less patient-focused or emotional with a physician or other [healthcare worker]. Just means that, instead of saying ‘you will have a catheter draining your urine to a bag,’ I can say, ‘you will have a Foley.’ ”
The Tweet followed an encounter with a patient who told Dr. Winter that he was a doctor only after she had gone to some length explaining a surgical procedure in lay terms.
“I explained the surgery, obviously assuming he was an intelligent adult, but using fully layman’s terms,” she said in an interview. The patient then told her that he was a doctor. “I guess I felt this embarrassment — I wouldn’t have treated him differently, but I just could have discussed the procedure with him in more professional terms.”
“To some extent, it was my own fault,” she commented in an interview. “I didn’t take the time to ask [about his work] at the beginning of the consultation, but that’s a fine line, also,” added Dr. Winter, a urologist and sexual medicine physician in Portland, Ore.
“You know that patient is there because they want care from you and it’s not necessarily always at the forefront of importance to be asking them what they do for their work, but alternatively, if you don’t ask then you put them in this position where they have to find a way to go ahead and tell you.”
Several people chimed in on the thread to voice their thoughts on the matter. Some commiserated with Dr. Winter’s experience:
“I took care of a retired cardiologist in the hospital as a second-year resident and honest to god he let me ramble on ‘explaining’ his echo result and never told me. I found out a couple days later and wanted to die,” posted @MaddyAndrewsMD.
Another recalled a similarly embarrassing experience when she “went on and on” discussing headaches with a patient whose husband “was in the corner smirking.”
“They told my attending later [that the] husband was a retired FM doc who practiced medicine longer than I’ve been alive. I wanted to die,” posted @JSinghDO.
Many on the thread, though, were doctors and other healthcare professionals speaking as patients. Some said they didn’t want to disclose their status as a healthcare provider because they felt it affected the care they received.
For example, @drhelenrainford commented: “In my experience my care is less ‘caring’ when they know I am a [doctor]. I get spoken to like they are discussing a patient with me — no empathy just facts and difficult results just blurted out without consideration. Awful awful time as an inpatient …but that’s another story!”
@Dr_B_Ring said: “Nope – You and I speak different jargon – I would want you to speak to me like a human that doesn’t know your jargon. My ego would get in the way of asking about the acronyms I don’t know if you knew I was a fellow physician.”
Conversely, @lozzlemcfozzle said: “Honestly I prefer not to tell my Doctors — I’ve found people skip explanations assuming I ‘know,’ or seem a little nervous when I tell them!”
Others said they felt uncomfortable — pretentious, even — in announcing their status, or worried that they might come across as expecting special care.
“It’s such a tough needle to thread. Want to tell people early but not come off as demanding special treatment, but don’t want to wait too long and it seems like a trap,” said @MDaware.
Twitter user @MsBabyCatcher wrote: “I have a hard time doing this because I don’t want people to think I’m being pretentious or going to micromanage/dictate care.”
Replying to @MsBabyCatcher, @RedStethoscope wrote: “I used to think this too until I got [very poor] care a few times, and was advised by other doctor moms to ‘play the doctor card.’ I have gotten better/more compassionate care by making sure it’s clear that I’m a physician (which is junk, but here we are).”
Several of those responding used the words “tricky” and “awkward,” suggesting a common theme for doctors presenting as patients.
“I struggle with this. My 5-year-old broke her arm this weekend, we spent hours in the ED, of my own hospital, I never mentioned it because I didn’t want to get preferential care. But as they were explaining her type of fracture, it felt awkward and inefficient,” said @lindsay_petty.
To avoid the awkwardness, a number of respondents said they purposefully use medical jargon to open up a conversation rather than just offering up the information that they are a doctor.
Still others offered suggestions on how to broach the subject more directly when presenting as a patient:
‘”Just FYI I’m a X doc but I’m here because I really want your help and advice!” That’s what I usually do,” wrote @drcakefm.
@BeeSting14618 Tweeted: “I usually say ‘I know some of this but I’m here because I want YOUR guidance. Also I may ask dumb questions, and I’ll tell you if a question is asking your opinion or making a request.’”
A few others injected a bit of humor: “I just do the 14-part handshake that only doctors know. Is that not customary?” quipped @Branmiz25.
“Ah yes, that transmits the entire [history of present illness],” replied Dr. Winter.
Jokes aside, the topic is obviously one that touched on a shared experience among healthcare providers, Dr. Winter commented. The Twitter thread she started just “blew up.”
That’s typically a sign that the Tweet is relatable for a lot of people, she said.
“It’s definitely something that all of us as care providers and as patients understand. It’s a funny, awkward thing that can really change an interaction, so we probably all feel pretty strongly about our experiences related to that,” she added.
The debate begs the question: Is there a duty or ethical reason to disclose?
“I definitely think it is very reasonable to disclose that one is a medical professional to another doctor,” medical ethicist Charlotte Blease, PhD, said in an interview. “There are good reasons to believe doing so might make a difference to the quality of communication and transparency.”
If the ability to use medical terminology or jargon more freely improves patient understanding, autonomy, and shared decision-making, then it may be of benefit, said Dr. Blease, a Keane OpenNotes Scholar at Beth Israel Deaconess Medical Center in Boston.
“Since doctors should strive to communicate effectively with every patient and to respect their unique needs and level of understanding, then I see no reason to deny that one is a medic,” she added.”
Knowing how to share the information is another story.
“This is something that affects all of us as physicians — we’re going to be patients at some point, right?” Dr. Winter commented. “But I don’t think how to disclose that is something that was ever brought up in my medical training.”
“Maybe there should just be a discussion of this one day when people are in medical school — maybe in a professionalism course — to broach this topic or look at if there’s any literature on outcomes related to disclosure of status or what are best practices,” she suggested.
A version of this article first appeared on Medscape.com.
The question drew spirited debate when urologist Ashley Winter, MD, made a simple, straightforward request on Twitter: “If you are a doctor & you come to an appointment please tell me you are a doctor, not because I will treat you differently but because it’s easier to speak in jargon.”
She later added, “This doesn’t’ mean I would be less patient-focused or emotional with a physician or other [healthcare worker]. Just means that, instead of saying ‘you will have a catheter draining your urine to a bag,’ I can say, ‘you will have a Foley.’ ”
The Tweet followed an encounter with a patient who told Dr. Winter that he was a doctor only after she had gone to some length explaining a surgical procedure in lay terms.
“I explained the surgery, obviously assuming he was an intelligent adult, but using fully layman’s terms,” she said in an interview. The patient then told her that he was a doctor. “I guess I felt this embarrassment — I wouldn’t have treated him differently, but I just could have discussed the procedure with him in more professional terms.”
“To some extent, it was my own fault,” she commented in an interview. “I didn’t take the time to ask [about his work] at the beginning of the consultation, but that’s a fine line, also,” added Dr. Winter, a urologist and sexual medicine physician in Portland, Ore.
“You know that patient is there because they want care from you and it’s not necessarily always at the forefront of importance to be asking them what they do for their work, but alternatively, if you don’t ask then you put them in this position where they have to find a way to go ahead and tell you.”
Several people chimed in on the thread to voice their thoughts on the matter. Some commiserated with Dr. Winter’s experience:
“I took care of a retired cardiologist in the hospital as a second-year resident and honest to god he let me ramble on ‘explaining’ his echo result and never told me. I found out a couple days later and wanted to die,” posted @MaddyAndrewsMD.
Another recalled a similarly embarrassing experience when she “went on and on” discussing headaches with a patient whose husband “was in the corner smirking.”
“They told my attending later [that the] husband was a retired FM doc who practiced medicine longer than I’ve been alive. I wanted to die,” posted @JSinghDO.
Many on the thread, though, were doctors and other healthcare professionals speaking as patients. Some said they didn’t want to disclose their status as a healthcare provider because they felt it affected the care they received.
For example, @drhelenrainford commented: “In my experience my care is less ‘caring’ when they know I am a [doctor]. I get spoken to like they are discussing a patient with me — no empathy just facts and difficult results just blurted out without consideration. Awful awful time as an inpatient …but that’s another story!”
@Dr_B_Ring said: “Nope – You and I speak different jargon – I would want you to speak to me like a human that doesn’t know your jargon. My ego would get in the way of asking about the acronyms I don’t know if you knew I was a fellow physician.”
Conversely, @lozzlemcfozzle said: “Honestly I prefer not to tell my Doctors — I’ve found people skip explanations assuming I ‘know,’ or seem a little nervous when I tell them!”
Others said they felt uncomfortable — pretentious, even — in announcing their status, or worried that they might come across as expecting special care.
“It’s such a tough needle to thread. Want to tell people early but not come off as demanding special treatment, but don’t want to wait too long and it seems like a trap,” said @MDaware.
Twitter user @MsBabyCatcher wrote: “I have a hard time doing this because I don’t want people to think I’m being pretentious or going to micromanage/dictate care.”
Replying to @MsBabyCatcher, @RedStethoscope wrote: “I used to think this too until I got [very poor] care a few times, and was advised by other doctor moms to ‘play the doctor card.’ I have gotten better/more compassionate care by making sure it’s clear that I’m a physician (which is junk, but here we are).”
Several of those responding used the words “tricky” and “awkward,” suggesting a common theme for doctors presenting as patients.
“I struggle with this. My 5-year-old broke her arm this weekend, we spent hours in the ED, of my own hospital, I never mentioned it because I didn’t want to get preferential care. But as they were explaining her type of fracture, it felt awkward and inefficient,” said @lindsay_petty.
To avoid the awkwardness, a number of respondents said they purposefully use medical jargon to open up a conversation rather than just offering up the information that they are a doctor.
Still others offered suggestions on how to broach the subject more directly when presenting as a patient:
‘”Just FYI I’m a X doc but I’m here because I really want your help and advice!” That’s what I usually do,” wrote @drcakefm.
@BeeSting14618 Tweeted: “I usually say ‘I know some of this but I’m here because I want YOUR guidance. Also I may ask dumb questions, and I’ll tell you if a question is asking your opinion or making a request.’”
A few others injected a bit of humor: “I just do the 14-part handshake that only doctors know. Is that not customary?” quipped @Branmiz25.
“Ah yes, that transmits the entire [history of present illness],” replied Dr. Winter.
Jokes aside, the topic is obviously one that touched on a shared experience among healthcare providers, Dr. Winter commented. The Twitter thread she started just “blew up.”
That’s typically a sign that the Tweet is relatable for a lot of people, she said.
“It’s definitely something that all of us as care providers and as patients understand. It’s a funny, awkward thing that can really change an interaction, so we probably all feel pretty strongly about our experiences related to that,” she added.
The debate begs the question: Is there a duty or ethical reason to disclose?
“I definitely think it is very reasonable to disclose that one is a medical professional to another doctor,” medical ethicist Charlotte Blease, PhD, said in an interview. “There are good reasons to believe doing so might make a difference to the quality of communication and transparency.”
If the ability to use medical terminology or jargon more freely improves patient understanding, autonomy, and shared decision-making, then it may be of benefit, said Dr. Blease, a Keane OpenNotes Scholar at Beth Israel Deaconess Medical Center in Boston.
“Since doctors should strive to communicate effectively with every patient and to respect their unique needs and level of understanding, then I see no reason to deny that one is a medic,” she added.”
Knowing how to share the information is another story.
“This is something that affects all of us as physicians — we’re going to be patients at some point, right?” Dr. Winter commented. “But I don’t think how to disclose that is something that was ever brought up in my medical training.”
“Maybe there should just be a discussion of this one day when people are in medical school — maybe in a professionalism course — to broach this topic or look at if there’s any literature on outcomes related to disclosure of status or what are best practices,” she suggested.
A version of this article first appeared on Medscape.com.
The question drew spirited debate when urologist Ashley Winter, MD, made a simple, straightforward request on Twitter: “If you are a doctor & you come to an appointment please tell me you are a doctor, not because I will treat you differently but because it’s easier to speak in jargon.”
She later added, “This doesn’t’ mean I would be less patient-focused or emotional with a physician or other [healthcare worker]. Just means that, instead of saying ‘you will have a catheter draining your urine to a bag,’ I can say, ‘you will have a Foley.’ ”
The Tweet followed an encounter with a patient who told Dr. Winter that he was a doctor only after she had gone to some length explaining a surgical procedure in lay terms.
“I explained the surgery, obviously assuming he was an intelligent adult, but using fully layman’s terms,” she said in an interview. The patient then told her that he was a doctor. “I guess I felt this embarrassment — I wouldn’t have treated him differently, but I just could have discussed the procedure with him in more professional terms.”
“To some extent, it was my own fault,” she commented in an interview. “I didn’t take the time to ask [about his work] at the beginning of the consultation, but that’s a fine line, also,” added Dr. Winter, a urologist and sexual medicine physician in Portland, Ore.
“You know that patient is there because they want care from you and it’s not necessarily always at the forefront of importance to be asking them what they do for their work, but alternatively, if you don’t ask then you put them in this position where they have to find a way to go ahead and tell you.”
Several people chimed in on the thread to voice their thoughts on the matter. Some commiserated with Dr. Winter’s experience:
“I took care of a retired cardiologist in the hospital as a second-year resident and honest to god he let me ramble on ‘explaining’ his echo result and never told me. I found out a couple days later and wanted to die,” posted @MaddyAndrewsMD.
Another recalled a similarly embarrassing experience when she “went on and on” discussing headaches with a patient whose husband “was in the corner smirking.”
“They told my attending later [that the] husband was a retired FM doc who practiced medicine longer than I’ve been alive. I wanted to die,” posted @JSinghDO.
Many on the thread, though, were doctors and other healthcare professionals speaking as patients. Some said they didn’t want to disclose their status as a healthcare provider because they felt it affected the care they received.
For example, @drhelenrainford commented: “In my experience my care is less ‘caring’ when they know I am a [doctor]. I get spoken to like they are discussing a patient with me — no empathy just facts and difficult results just blurted out without consideration. Awful awful time as an inpatient …but that’s another story!”
@Dr_B_Ring said: “Nope – You and I speak different jargon – I would want you to speak to me like a human that doesn’t know your jargon. My ego would get in the way of asking about the acronyms I don’t know if you knew I was a fellow physician.”
Conversely, @lozzlemcfozzle said: “Honestly I prefer not to tell my Doctors — I’ve found people skip explanations assuming I ‘know,’ or seem a little nervous when I tell them!”
Others said they felt uncomfortable — pretentious, even — in announcing their status, or worried that they might come across as expecting special care.
“It’s such a tough needle to thread. Want to tell people early but not come off as demanding special treatment, but don’t want to wait too long and it seems like a trap,” said @MDaware.
Twitter user @MsBabyCatcher wrote: “I have a hard time doing this because I don’t want people to think I’m being pretentious or going to micromanage/dictate care.”
Replying to @MsBabyCatcher, @RedStethoscope wrote: “I used to think this too until I got [very poor] care a few times, and was advised by other doctor moms to ‘play the doctor card.’ I have gotten better/more compassionate care by making sure it’s clear that I’m a physician (which is junk, but here we are).”
Several of those responding used the words “tricky” and “awkward,” suggesting a common theme for doctors presenting as patients.
“I struggle with this. My 5-year-old broke her arm this weekend, we spent hours in the ED, of my own hospital, I never mentioned it because I didn’t want to get preferential care. But as they were explaining her type of fracture, it felt awkward and inefficient,” said @lindsay_petty.
To avoid the awkwardness, a number of respondents said they purposefully use medical jargon to open up a conversation rather than just offering up the information that they are a doctor.
Still others offered suggestions on how to broach the subject more directly when presenting as a patient:
‘”Just FYI I’m a X doc but I’m here because I really want your help and advice!” That’s what I usually do,” wrote @drcakefm.
@BeeSting14618 Tweeted: “I usually say ‘I know some of this but I’m here because I want YOUR guidance. Also I may ask dumb questions, and I’ll tell you if a question is asking your opinion or making a request.’”
A few others injected a bit of humor: “I just do the 14-part handshake that only doctors know. Is that not customary?” quipped @Branmiz25.
“Ah yes, that transmits the entire [history of present illness],” replied Dr. Winter.
Jokes aside, the topic is obviously one that touched on a shared experience among healthcare providers, Dr. Winter commented. The Twitter thread she started just “blew up.”
That’s typically a sign that the Tweet is relatable for a lot of people, she said.
“It’s definitely something that all of us as care providers and as patients understand. It’s a funny, awkward thing that can really change an interaction, so we probably all feel pretty strongly about our experiences related to that,” she added.
The debate begs the question: Is there a duty or ethical reason to disclose?
“I definitely think it is very reasonable to disclose that one is a medical professional to another doctor,” medical ethicist Charlotte Blease, PhD, said in an interview. “There are good reasons to believe doing so might make a difference to the quality of communication and transparency.”
If the ability to use medical terminology or jargon more freely improves patient understanding, autonomy, and shared decision-making, then it may be of benefit, said Dr. Blease, a Keane OpenNotes Scholar at Beth Israel Deaconess Medical Center in Boston.
“Since doctors should strive to communicate effectively with every patient and to respect their unique needs and level of understanding, then I see no reason to deny that one is a medic,” she added.”
Knowing how to share the information is another story.
“This is something that affects all of us as physicians — we’re going to be patients at some point, right?” Dr. Winter commented. “But I don’t think how to disclose that is something that was ever brought up in my medical training.”
“Maybe there should just be a discussion of this one day when people are in medical school — maybe in a professionalism course — to broach this topic or look at if there’s any literature on outcomes related to disclosure of status or what are best practices,” she suggested.
A version of this article first appeared on Medscape.com.
Unvaccinated people 20 times more likely to die from COVID: Texas study
During the month of September, , according to a new study from the Texas Department of State Health Services.
The data also showed that unvaccinated people were 13 times more likely to test positive for COVID-19 than people who were fully vaccinated.
“This analysis quantifies what we’ve known for months,” Jennifer Shuford, MD, the state’s chief epidemiologist, told The Dallas Morning News.
“The COVID-19 vaccines are doing an excellent job of protecting people from getting sick and from dying from COVID-19,” she said. “Vaccination remains the best way to keep yourself and the people close to you safe from this deadly disease.”
As part of the study, researchers analyzed electronic lab reports, death certificates, and state immunization records, with a particular focus on September when the contagious Delta variant surged across Texas. The research marks the state’s first statistical analysis of COVID-19 vaccinations in Texas and the effects, the newspaper reported.
The protective effect of vaccination was most noticeable among younger groups. During September, the risk of COVID-19 death was 23 times higher in unvaccinated people in their 30s and 55 times higher for unvaccinated people in their 40s.
In addition, there were fewer than 10 COVID-19 deaths in September among fully vaccinated people between ages 18-29, as compared with 339 deaths among unvaccinated people in the same age group.
Then, looking at a longer time period -- from Jan. 15 to Oct. 1 -- the researchers found that unvaccinated people were 45 times more likely to contract COVID-19 than fully vaccinated people. The protective effect of vaccination against infection was strong across all adult age groups but greatest among ages 12-17.
“All authorized COVID-19 vaccines in the United States are highly effective at protecting people from getting sick or severely ill with COVID-19, including those infected with Delta and other known variants,” the study authors wrote. “Real world data from Texas clearly shows these benefits.”
About 15.6 million people in Texas have been fully vaccinated against COVID-19 in a state of about 29 million residents, according to state data. About 66% of the population has received at least one dose, while 58% is fully vaccinated.
A version of this article first appeared on WebMD.com.
During the month of September, , according to a new study from the Texas Department of State Health Services.
The data also showed that unvaccinated people were 13 times more likely to test positive for COVID-19 than people who were fully vaccinated.
“This analysis quantifies what we’ve known for months,” Jennifer Shuford, MD, the state’s chief epidemiologist, told The Dallas Morning News.
“The COVID-19 vaccines are doing an excellent job of protecting people from getting sick and from dying from COVID-19,” she said. “Vaccination remains the best way to keep yourself and the people close to you safe from this deadly disease.”
As part of the study, researchers analyzed electronic lab reports, death certificates, and state immunization records, with a particular focus on September when the contagious Delta variant surged across Texas. The research marks the state’s first statistical analysis of COVID-19 vaccinations in Texas and the effects, the newspaper reported.
The protective effect of vaccination was most noticeable among younger groups. During September, the risk of COVID-19 death was 23 times higher in unvaccinated people in their 30s and 55 times higher for unvaccinated people in their 40s.
In addition, there were fewer than 10 COVID-19 deaths in September among fully vaccinated people between ages 18-29, as compared with 339 deaths among unvaccinated people in the same age group.
Then, looking at a longer time period -- from Jan. 15 to Oct. 1 -- the researchers found that unvaccinated people were 45 times more likely to contract COVID-19 than fully vaccinated people. The protective effect of vaccination against infection was strong across all adult age groups but greatest among ages 12-17.
“All authorized COVID-19 vaccines in the United States are highly effective at protecting people from getting sick or severely ill with COVID-19, including those infected with Delta and other known variants,” the study authors wrote. “Real world data from Texas clearly shows these benefits.”
About 15.6 million people in Texas have been fully vaccinated against COVID-19 in a state of about 29 million residents, according to state data. About 66% of the population has received at least one dose, while 58% is fully vaccinated.
A version of this article first appeared on WebMD.com.
During the month of September, , according to a new study from the Texas Department of State Health Services.
The data also showed that unvaccinated people were 13 times more likely to test positive for COVID-19 than people who were fully vaccinated.
“This analysis quantifies what we’ve known for months,” Jennifer Shuford, MD, the state’s chief epidemiologist, told The Dallas Morning News.
“The COVID-19 vaccines are doing an excellent job of protecting people from getting sick and from dying from COVID-19,” she said. “Vaccination remains the best way to keep yourself and the people close to you safe from this deadly disease.”
As part of the study, researchers analyzed electronic lab reports, death certificates, and state immunization records, with a particular focus on September when the contagious Delta variant surged across Texas. The research marks the state’s first statistical analysis of COVID-19 vaccinations in Texas and the effects, the newspaper reported.
The protective effect of vaccination was most noticeable among younger groups. During September, the risk of COVID-19 death was 23 times higher in unvaccinated people in their 30s and 55 times higher for unvaccinated people in their 40s.
In addition, there were fewer than 10 COVID-19 deaths in September among fully vaccinated people between ages 18-29, as compared with 339 deaths among unvaccinated people in the same age group.
Then, looking at a longer time period -- from Jan. 15 to Oct. 1 -- the researchers found that unvaccinated people were 45 times more likely to contract COVID-19 than fully vaccinated people. The protective effect of vaccination against infection was strong across all adult age groups but greatest among ages 12-17.
“All authorized COVID-19 vaccines in the United States are highly effective at protecting people from getting sick or severely ill with COVID-19, including those infected with Delta and other known variants,” the study authors wrote. “Real world data from Texas clearly shows these benefits.”
About 15.6 million people in Texas have been fully vaccinated against COVID-19 in a state of about 29 million residents, according to state data. About 66% of the population has received at least one dose, while 58% is fully vaccinated.
A version of this article first appeared on WebMD.com.
At 5 years, iFR found as effective and safe as FFR for guiding PCI intervention
The rate of major adverse cardiac events (MACE) over 5 years is similar whether revascularization is guided by instantaneous wave-free ratio (iFR) or fractional flow reserve (FFR), according to long-term results of the iFR-SWEDEHEART study.
“The results are about the same as reported at 12 months. There were no significant differences in any outcome we evaluated,” according to Matthias Götberg, MD, PhD.
When the initial results of the noninferiority iFR-SWEDEHEART trial were published after 1 year of follow-up, the primary MACE endpoint of death from any-cause nonfatal myocardial infarction, or unplanned revascularization, was met by 6.7% and 6.1% of those randomized to iFR or FFR, respectively.
These outcomes were not significantly different and placed iFR well within the predefined boundaries of noninferiority (P = .007).
In this new and final follow-up of iFR-SWEDEHEART, which evaluated the same 2,019 patients who were alive at 1 year (none were lost to follow-up), the MACE endpoint was met by 21.5% and 19.9% of those managed with iFR and FFR, respectively. The hazard ratio (1.09) had a wide 95% confidence interval (0.90-1.31) that did not approach statistical significance.
No differences seen across outcomes
When broken down into the MACE components, there were no differences between iFR and FFR, respectively, for all-cause death (9.4% vs. 7.9%), MI (5.8% vs. 5.7%) or unplanned revascularization (11.6% vs. 11.3%).
Across predefined subgroups, such as those defined by age, gender, stable versus unstable angina, and presence of risk factors such as diabetes, hypertension, hyperlipidemia, and smoking, there were also no significant differences in outcome.
At the time iFR-SWEDEHART was initiated, FFR had already been accepted as more effective than angiographic assessment to identify lesion ischemia and the need for percutaneous intervention (PCI). The iFR-SWEDEHEART trial tested iFR, a relatively new technology at the time, as a noninferior alternative. Unlike FFR, which requires adenosine to dilate the vessel, adding cost and patient discomfort, iFR measures the resting pressure gradient across the coronary lesion, and it is generally easier to perform.
“The advantage of iFR is that it provides an instantaneous lesion assessment without the need for adenosine,” Dr. Götberg explained in presenting the results at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
When the procedural results were compared in the published study at 1 year, it was noted that the mean number of lesions evaluated per patient was higher (1.55 vs. 1.43; P = .002), but the proportion of lesions found functionally significant was lower (29.2% vs. 36.8%; P < .0001) among those randomized to iFR than in the FFR group.
While most other procedural characteristics, such as PCI access route, fluoroscopy time, and contrast use did not differ significantly, fewer stents were placed in patients managed with iFR (1.58 vs. 1.73; P = .048), and a reduction in the average procedural time of a few minutes approached significance (P = .09).
Patient discomfort is greater with FFR
Patient discomfort measured during the procedure did differ, according to Dr. Götberg, an interventional cardiologist at Skåne University Hospital, Lund, Sweden.
Only about 30% in the FFR group reported no discomfort. Most of the others reported mild or moderate discomfort, but nearly 10% characterized the discomfort as severe. In the iFR group, more than 95% reported no discomfort. All of the remaining patients reported discomfort level as mild.
Because differences in MACE would be most likely to occur in the first year after revascularization, the similarity of the 1- and 5-year results were expected, according to Dr. Götberg. However, a 5-year follow-up was considered prudent given the relatively limited experience with iFR when the study was designed. This technique is now well established and widely used.
The study supports the premise that quicker and easier-to-obtain results with iFR are obtained without sacrificing greater relative risk of failing to identify a vulnerable lesion, according to Dr. Götberg.
Nevertheless, iFR and FFR “are not an exact match,” according to Jennifer A. Rymer, MD, an interventional cardiologist and assistant professor of medicine at Duke University, Durham, N.C. Although she called this trial an “excellent” demonstration of comparable utility in distinguishing lesions that do not require intervention from those that do, she implied that some clinicians might still prefer FFR for other reasons.
For example, FFR provides information about coronary flow reserve and microvascular resistance that are relevant to the underlying pathophysiology in a diseased vessel, according to Shmuel Banai, MD, head of interventional cardiology, Tel Aviv Medical Center. Recognizing that this information is not as readily generated by iFR, he is among those who plan to continue to use FFR despite these results.
However, for those who are now routinely performing iFR for the purposes of guiding revascularization, “these data are reassuring,” said David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta. The 5-year data essentially eliminate the likelihood that iFR relative to FFR increases the risk of missing functionally significant lesions for revascularization procedures.
Dr. Götberg reports financial relationships with Abbott, Boston Scientific, Medtronic, and Phillips Healthcare. Dr. Rymer reports no potential financial conflicts of interest. Dr. Banai has a financial relationship with Neovasc. Dr. Kandzari reports financial relationships with Ablative Solutions and Medtronic.
The rate of major adverse cardiac events (MACE) over 5 years is similar whether revascularization is guided by instantaneous wave-free ratio (iFR) or fractional flow reserve (FFR), according to long-term results of the iFR-SWEDEHEART study.
“The results are about the same as reported at 12 months. There were no significant differences in any outcome we evaluated,” according to Matthias Götberg, MD, PhD.
When the initial results of the noninferiority iFR-SWEDEHEART trial were published after 1 year of follow-up, the primary MACE endpoint of death from any-cause nonfatal myocardial infarction, or unplanned revascularization, was met by 6.7% and 6.1% of those randomized to iFR or FFR, respectively.
These outcomes were not significantly different and placed iFR well within the predefined boundaries of noninferiority (P = .007).
In this new and final follow-up of iFR-SWEDEHEART, which evaluated the same 2,019 patients who were alive at 1 year (none were lost to follow-up), the MACE endpoint was met by 21.5% and 19.9% of those managed with iFR and FFR, respectively. The hazard ratio (1.09) had a wide 95% confidence interval (0.90-1.31) that did not approach statistical significance.
No differences seen across outcomes
When broken down into the MACE components, there were no differences between iFR and FFR, respectively, for all-cause death (9.4% vs. 7.9%), MI (5.8% vs. 5.7%) or unplanned revascularization (11.6% vs. 11.3%).
Across predefined subgroups, such as those defined by age, gender, stable versus unstable angina, and presence of risk factors such as diabetes, hypertension, hyperlipidemia, and smoking, there were also no significant differences in outcome.
At the time iFR-SWEDEHART was initiated, FFR had already been accepted as more effective than angiographic assessment to identify lesion ischemia and the need for percutaneous intervention (PCI). The iFR-SWEDEHEART trial tested iFR, a relatively new technology at the time, as a noninferior alternative. Unlike FFR, which requires adenosine to dilate the vessel, adding cost and patient discomfort, iFR measures the resting pressure gradient across the coronary lesion, and it is generally easier to perform.
“The advantage of iFR is that it provides an instantaneous lesion assessment without the need for adenosine,” Dr. Götberg explained in presenting the results at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
When the procedural results were compared in the published study at 1 year, it was noted that the mean number of lesions evaluated per patient was higher (1.55 vs. 1.43; P = .002), but the proportion of lesions found functionally significant was lower (29.2% vs. 36.8%; P < .0001) among those randomized to iFR than in the FFR group.
While most other procedural characteristics, such as PCI access route, fluoroscopy time, and contrast use did not differ significantly, fewer stents were placed in patients managed with iFR (1.58 vs. 1.73; P = .048), and a reduction in the average procedural time of a few minutes approached significance (P = .09).
Patient discomfort is greater with FFR
Patient discomfort measured during the procedure did differ, according to Dr. Götberg, an interventional cardiologist at Skåne University Hospital, Lund, Sweden.
Only about 30% in the FFR group reported no discomfort. Most of the others reported mild or moderate discomfort, but nearly 10% characterized the discomfort as severe. In the iFR group, more than 95% reported no discomfort. All of the remaining patients reported discomfort level as mild.
Because differences in MACE would be most likely to occur in the first year after revascularization, the similarity of the 1- and 5-year results were expected, according to Dr. Götberg. However, a 5-year follow-up was considered prudent given the relatively limited experience with iFR when the study was designed. This technique is now well established and widely used.
The study supports the premise that quicker and easier-to-obtain results with iFR are obtained without sacrificing greater relative risk of failing to identify a vulnerable lesion, according to Dr. Götberg.
Nevertheless, iFR and FFR “are not an exact match,” according to Jennifer A. Rymer, MD, an interventional cardiologist and assistant professor of medicine at Duke University, Durham, N.C. Although she called this trial an “excellent” demonstration of comparable utility in distinguishing lesions that do not require intervention from those that do, she implied that some clinicians might still prefer FFR for other reasons.
For example, FFR provides information about coronary flow reserve and microvascular resistance that are relevant to the underlying pathophysiology in a diseased vessel, according to Shmuel Banai, MD, head of interventional cardiology, Tel Aviv Medical Center. Recognizing that this information is not as readily generated by iFR, he is among those who plan to continue to use FFR despite these results.
However, for those who are now routinely performing iFR for the purposes of guiding revascularization, “these data are reassuring,” said David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta. The 5-year data essentially eliminate the likelihood that iFR relative to FFR increases the risk of missing functionally significant lesions for revascularization procedures.
Dr. Götberg reports financial relationships with Abbott, Boston Scientific, Medtronic, and Phillips Healthcare. Dr. Rymer reports no potential financial conflicts of interest. Dr. Banai has a financial relationship with Neovasc. Dr. Kandzari reports financial relationships with Ablative Solutions and Medtronic.
The rate of major adverse cardiac events (MACE) over 5 years is similar whether revascularization is guided by instantaneous wave-free ratio (iFR) or fractional flow reserve (FFR), according to long-term results of the iFR-SWEDEHEART study.
“The results are about the same as reported at 12 months. There were no significant differences in any outcome we evaluated,” according to Matthias Götberg, MD, PhD.
When the initial results of the noninferiority iFR-SWEDEHEART trial were published after 1 year of follow-up, the primary MACE endpoint of death from any-cause nonfatal myocardial infarction, or unplanned revascularization, was met by 6.7% and 6.1% of those randomized to iFR or FFR, respectively.
These outcomes were not significantly different and placed iFR well within the predefined boundaries of noninferiority (P = .007).
In this new and final follow-up of iFR-SWEDEHEART, which evaluated the same 2,019 patients who were alive at 1 year (none were lost to follow-up), the MACE endpoint was met by 21.5% and 19.9% of those managed with iFR and FFR, respectively. The hazard ratio (1.09) had a wide 95% confidence interval (0.90-1.31) that did not approach statistical significance.
No differences seen across outcomes
When broken down into the MACE components, there were no differences between iFR and FFR, respectively, for all-cause death (9.4% vs. 7.9%), MI (5.8% vs. 5.7%) or unplanned revascularization (11.6% vs. 11.3%).
Across predefined subgroups, such as those defined by age, gender, stable versus unstable angina, and presence of risk factors such as diabetes, hypertension, hyperlipidemia, and smoking, there were also no significant differences in outcome.
At the time iFR-SWEDEHART was initiated, FFR had already been accepted as more effective than angiographic assessment to identify lesion ischemia and the need for percutaneous intervention (PCI). The iFR-SWEDEHEART trial tested iFR, a relatively new technology at the time, as a noninferior alternative. Unlike FFR, which requires adenosine to dilate the vessel, adding cost and patient discomfort, iFR measures the resting pressure gradient across the coronary lesion, and it is generally easier to perform.
“The advantage of iFR is that it provides an instantaneous lesion assessment without the need for adenosine,” Dr. Götberg explained in presenting the results at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
When the procedural results were compared in the published study at 1 year, it was noted that the mean number of lesions evaluated per patient was higher (1.55 vs. 1.43; P = .002), but the proportion of lesions found functionally significant was lower (29.2% vs. 36.8%; P < .0001) among those randomized to iFR than in the FFR group.
While most other procedural characteristics, such as PCI access route, fluoroscopy time, and contrast use did not differ significantly, fewer stents were placed in patients managed with iFR (1.58 vs. 1.73; P = .048), and a reduction in the average procedural time of a few minutes approached significance (P = .09).
Patient discomfort is greater with FFR
Patient discomfort measured during the procedure did differ, according to Dr. Götberg, an interventional cardiologist at Skåne University Hospital, Lund, Sweden.
Only about 30% in the FFR group reported no discomfort. Most of the others reported mild or moderate discomfort, but nearly 10% characterized the discomfort as severe. In the iFR group, more than 95% reported no discomfort. All of the remaining patients reported discomfort level as mild.
Because differences in MACE would be most likely to occur in the first year after revascularization, the similarity of the 1- and 5-year results were expected, according to Dr. Götberg. However, a 5-year follow-up was considered prudent given the relatively limited experience with iFR when the study was designed. This technique is now well established and widely used.
The study supports the premise that quicker and easier-to-obtain results with iFR are obtained without sacrificing greater relative risk of failing to identify a vulnerable lesion, according to Dr. Götberg.
Nevertheless, iFR and FFR “are not an exact match,” according to Jennifer A. Rymer, MD, an interventional cardiologist and assistant professor of medicine at Duke University, Durham, N.C. Although she called this trial an “excellent” demonstration of comparable utility in distinguishing lesions that do not require intervention from those that do, she implied that some clinicians might still prefer FFR for other reasons.
For example, FFR provides information about coronary flow reserve and microvascular resistance that are relevant to the underlying pathophysiology in a diseased vessel, according to Shmuel Banai, MD, head of interventional cardiology, Tel Aviv Medical Center. Recognizing that this information is not as readily generated by iFR, he is among those who plan to continue to use FFR despite these results.
However, for those who are now routinely performing iFR for the purposes of guiding revascularization, “these data are reassuring,” said David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta. The 5-year data essentially eliminate the likelihood that iFR relative to FFR increases the risk of missing functionally significant lesions for revascularization procedures.
Dr. Götberg reports financial relationships with Abbott, Boston Scientific, Medtronic, and Phillips Healthcare. Dr. Rymer reports no potential financial conflicts of interest. Dr. Banai has a financial relationship with Neovasc. Dr. Kandzari reports financial relationships with Ablative Solutions and Medtronic.
FROM TCT 2021