User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
NAMS affirms value of hormone therapy for menopausal women
Hormone therapy remains a topic for debate, but a constant in the 2 decades since the Women’s Health Initiative has been the demonstrated effectiveness for relief of vasomotor symptoms and reduction of fracture risk in menopausal women, according to the latest hormone therapy position statement of the North American Menopause Society.
“Healthcare professionals caring for menopausal women should understand the basic concepts of relative risk and absolute risk,” wrote Stephanie S. Faubion, MD, director of the Mayo Clinic Center for Women’s Health and medical director of NAMS, and members of the NAMS 2022 Hormone Therapy Position Statement Advisory Panel in Menopause.
The authors noted that the risks of hormone therapy vary considerably based on type, dose, duration, route of administration, timing of the start of therapy, and whether or not a progestogen is included.
The 2022 statement was commissioned to review new literature and identify the strength of recommendations and quality of evidence since the previous statement in 2017.
The current statement represents not so much a practice-changing update, “but rather that the literature has filled out in some areas,” Dr. Faubion said in an interview. “The recommendations overall haven’t changed,” she said. “The position statement reiterates that hormone therapy, which is significantly underutilized, remains a safe and effective treatment for menopause symptoms, which remain undertreated, with the benefits outweighing the risks for most healthy women who are within 10 years of menopause onset and under the age of 60 years,” she emphasized. “Individualizing therapy is key to maximizing benefits and minimizing risks,” she added.
Overall, the authors confirmed that hormone therapy remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause (GSM), and has been shown to prevent bone loss and fracture. The risks of hormone therapy differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progestogen is used.
Risks and benefits should be stratified by age and time since the start of menopause, according to the statement.
For women younger than 60 years or within 10 years of the onset of menopause who have no contraindications, the potential benefits outweigh the risks in most cases for use of hormone therapy to manage vasomotor symptoms and to help prevent bone loss and reduce fracture risk.
For women who begin hormone therapy more than 10 or 20 years from the start of menopause, or who are aged 60 years and older, the risk-benefit ratio may be less favorable because of the increased absolute risk of coronary heart disease, stroke, venous thromboembolism, and dementia. However, strategies such as lower doses and transdermal administration may reduce this risk, according to the statement.
The authors continue to recommend that longer durations of hormone therapy be for documented indications, such as VMS relief, and that patients on longer duration of therapy be reassessed periodically as part of a shared decision-making process. Women with persistent VMS or quality of life issues, or those at risk for osteoporosis, may continue hormone therapy beyond age 60 or 65 years after appropriate evaluation and risk-benefit counseling.
Women with ongoing GSM without indications for systemic therapy whose GSM persists after over-the-counter therapies may try low-dose vaginal estrogen or other nonestrogen therapies regardless of age and for an extended duration if needed, according to the statement.
Challenges, research gaps, and goals
“Barriers to the use of hormone therapy include lack of access to high quality care,” Dr. Faubion said in an interview. The NAMS website, menopause.org, features an option to search for a NAMS-certified provider by ZIP code, she noted.
“Coverage of hormone therapy is highly variable and depends on the insurance company, but most women have access to one form or another with insurance coverage,” she said. “We need to continue to advocate for adequate coverage of menopause symptom treatments, including hormone therapy, so that women’s symptoms – which can significantly affect quality of life – are adequately managed.
“Additional research is needed on the thrombotic risk (venous thromboembolism, pulmonary embolism, and stroke) of oral versus transdermal therapies (including different formulations, doses, and durations of therapy),” Dr. Faubion told this news organization. “More clinical trial data are needed to confirm or refute the potential beneficial effects of hormone therapy on coronary heart disease and all-cause mortality when initiated in perimenopause or early postmenopause,” she said.
Other areas for research include “the breast effects of different estrogen preparations, including the role for selective estrogen receptor modulator (SERM) and tissue selective estrogen complex therapies, optimal progestogen or SERM regimens to prevent endometrial hyperplasia, the relationship between vasomotor symptoms and the risk for heart disease and cognitive changes, and the risks of premature ovarian insufficiency,” Dr. Faubion emphasized.
Looking ahead, “Studies are needed on the effects of longer use of low-dose vaginal estrogen therapy after breast or endometrial cancer, extended use of hormone therapy in women who are early initiators, improved tools to personalize or individualize benefits and risks of hormone therapy, and the role of aging and genetics,” said Dr. Faubion. Other areas for further research include “the long-term benefits and risks on women’s health of lifestyle modification or complementary or nonhormone therapies, if chosen in addition to or over hormone therapy for vasomotor symptoms, bone health, and cardiovascular disease risk reduction,” she added.
The complete statement was published in Menopause: The Journal of the North American Menopause Society.
The position statement received no outside funding. The authors had no financial conflicts to disclose.
Hormone therapy remains a topic for debate, but a constant in the 2 decades since the Women’s Health Initiative has been the demonstrated effectiveness for relief of vasomotor symptoms and reduction of fracture risk in menopausal women, according to the latest hormone therapy position statement of the North American Menopause Society.
“Healthcare professionals caring for menopausal women should understand the basic concepts of relative risk and absolute risk,” wrote Stephanie S. Faubion, MD, director of the Mayo Clinic Center for Women’s Health and medical director of NAMS, and members of the NAMS 2022 Hormone Therapy Position Statement Advisory Panel in Menopause.
The authors noted that the risks of hormone therapy vary considerably based on type, dose, duration, route of administration, timing of the start of therapy, and whether or not a progestogen is included.
The 2022 statement was commissioned to review new literature and identify the strength of recommendations and quality of evidence since the previous statement in 2017.
The current statement represents not so much a practice-changing update, “but rather that the literature has filled out in some areas,” Dr. Faubion said in an interview. “The recommendations overall haven’t changed,” she said. “The position statement reiterates that hormone therapy, which is significantly underutilized, remains a safe and effective treatment for menopause symptoms, which remain undertreated, with the benefits outweighing the risks for most healthy women who are within 10 years of menopause onset and under the age of 60 years,” she emphasized. “Individualizing therapy is key to maximizing benefits and minimizing risks,” she added.
Overall, the authors confirmed that hormone therapy remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause (GSM), and has been shown to prevent bone loss and fracture. The risks of hormone therapy differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progestogen is used.
Risks and benefits should be stratified by age and time since the start of menopause, according to the statement.
For women younger than 60 years or within 10 years of the onset of menopause who have no contraindications, the potential benefits outweigh the risks in most cases for use of hormone therapy to manage vasomotor symptoms and to help prevent bone loss and reduce fracture risk.
For women who begin hormone therapy more than 10 or 20 years from the start of menopause, or who are aged 60 years and older, the risk-benefit ratio may be less favorable because of the increased absolute risk of coronary heart disease, stroke, venous thromboembolism, and dementia. However, strategies such as lower doses and transdermal administration may reduce this risk, according to the statement.
The authors continue to recommend that longer durations of hormone therapy be for documented indications, such as VMS relief, and that patients on longer duration of therapy be reassessed periodically as part of a shared decision-making process. Women with persistent VMS or quality of life issues, or those at risk for osteoporosis, may continue hormone therapy beyond age 60 or 65 years after appropriate evaluation and risk-benefit counseling.
Women with ongoing GSM without indications for systemic therapy whose GSM persists after over-the-counter therapies may try low-dose vaginal estrogen or other nonestrogen therapies regardless of age and for an extended duration if needed, according to the statement.
Challenges, research gaps, and goals
“Barriers to the use of hormone therapy include lack of access to high quality care,” Dr. Faubion said in an interview. The NAMS website, menopause.org, features an option to search for a NAMS-certified provider by ZIP code, she noted.
“Coverage of hormone therapy is highly variable and depends on the insurance company, but most women have access to one form or another with insurance coverage,” she said. “We need to continue to advocate for adequate coverage of menopause symptom treatments, including hormone therapy, so that women’s symptoms – which can significantly affect quality of life – are adequately managed.
“Additional research is needed on the thrombotic risk (venous thromboembolism, pulmonary embolism, and stroke) of oral versus transdermal therapies (including different formulations, doses, and durations of therapy),” Dr. Faubion told this news organization. “More clinical trial data are needed to confirm or refute the potential beneficial effects of hormone therapy on coronary heart disease and all-cause mortality when initiated in perimenopause or early postmenopause,” she said.
Other areas for research include “the breast effects of different estrogen preparations, including the role for selective estrogen receptor modulator (SERM) and tissue selective estrogen complex therapies, optimal progestogen or SERM regimens to prevent endometrial hyperplasia, the relationship between vasomotor symptoms and the risk for heart disease and cognitive changes, and the risks of premature ovarian insufficiency,” Dr. Faubion emphasized.
Looking ahead, “Studies are needed on the effects of longer use of low-dose vaginal estrogen therapy after breast or endometrial cancer, extended use of hormone therapy in women who are early initiators, improved tools to personalize or individualize benefits and risks of hormone therapy, and the role of aging and genetics,” said Dr. Faubion. Other areas for further research include “the long-term benefits and risks on women’s health of lifestyle modification or complementary or nonhormone therapies, if chosen in addition to or over hormone therapy for vasomotor symptoms, bone health, and cardiovascular disease risk reduction,” she added.
The complete statement was published in Menopause: The Journal of the North American Menopause Society.
The position statement received no outside funding. The authors had no financial conflicts to disclose.
Hormone therapy remains a topic for debate, but a constant in the 2 decades since the Women’s Health Initiative has been the demonstrated effectiveness for relief of vasomotor symptoms and reduction of fracture risk in menopausal women, according to the latest hormone therapy position statement of the North American Menopause Society.
“Healthcare professionals caring for menopausal women should understand the basic concepts of relative risk and absolute risk,” wrote Stephanie S. Faubion, MD, director of the Mayo Clinic Center for Women’s Health and medical director of NAMS, and members of the NAMS 2022 Hormone Therapy Position Statement Advisory Panel in Menopause.
The authors noted that the risks of hormone therapy vary considerably based on type, dose, duration, route of administration, timing of the start of therapy, and whether or not a progestogen is included.
The 2022 statement was commissioned to review new literature and identify the strength of recommendations and quality of evidence since the previous statement in 2017.
The current statement represents not so much a practice-changing update, “but rather that the literature has filled out in some areas,” Dr. Faubion said in an interview. “The recommendations overall haven’t changed,” she said. “The position statement reiterates that hormone therapy, which is significantly underutilized, remains a safe and effective treatment for menopause symptoms, which remain undertreated, with the benefits outweighing the risks for most healthy women who are within 10 years of menopause onset and under the age of 60 years,” she emphasized. “Individualizing therapy is key to maximizing benefits and minimizing risks,” she added.
Overall, the authors confirmed that hormone therapy remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause (GSM), and has been shown to prevent bone loss and fracture. The risks of hormone therapy differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progestogen is used.
Risks and benefits should be stratified by age and time since the start of menopause, according to the statement.
For women younger than 60 years or within 10 years of the onset of menopause who have no contraindications, the potential benefits outweigh the risks in most cases for use of hormone therapy to manage vasomotor symptoms and to help prevent bone loss and reduce fracture risk.
For women who begin hormone therapy more than 10 or 20 years from the start of menopause, or who are aged 60 years and older, the risk-benefit ratio may be less favorable because of the increased absolute risk of coronary heart disease, stroke, venous thromboembolism, and dementia. However, strategies such as lower doses and transdermal administration may reduce this risk, according to the statement.
The authors continue to recommend that longer durations of hormone therapy be for documented indications, such as VMS relief, and that patients on longer duration of therapy be reassessed periodically as part of a shared decision-making process. Women with persistent VMS or quality of life issues, or those at risk for osteoporosis, may continue hormone therapy beyond age 60 or 65 years after appropriate evaluation and risk-benefit counseling.
Women with ongoing GSM without indications for systemic therapy whose GSM persists after over-the-counter therapies may try low-dose vaginal estrogen or other nonestrogen therapies regardless of age and for an extended duration if needed, according to the statement.
Challenges, research gaps, and goals
“Barriers to the use of hormone therapy include lack of access to high quality care,” Dr. Faubion said in an interview. The NAMS website, menopause.org, features an option to search for a NAMS-certified provider by ZIP code, she noted.
“Coverage of hormone therapy is highly variable and depends on the insurance company, but most women have access to one form or another with insurance coverage,” she said. “We need to continue to advocate for adequate coverage of menopause symptom treatments, including hormone therapy, so that women’s symptoms – which can significantly affect quality of life – are adequately managed.
“Additional research is needed on the thrombotic risk (venous thromboembolism, pulmonary embolism, and stroke) of oral versus transdermal therapies (including different formulations, doses, and durations of therapy),” Dr. Faubion told this news organization. “More clinical trial data are needed to confirm or refute the potential beneficial effects of hormone therapy on coronary heart disease and all-cause mortality when initiated in perimenopause or early postmenopause,” she said.
Other areas for research include “the breast effects of different estrogen preparations, including the role for selective estrogen receptor modulator (SERM) and tissue selective estrogen complex therapies, optimal progestogen or SERM regimens to prevent endometrial hyperplasia, the relationship between vasomotor symptoms and the risk for heart disease and cognitive changes, and the risks of premature ovarian insufficiency,” Dr. Faubion emphasized.
Looking ahead, “Studies are needed on the effects of longer use of low-dose vaginal estrogen therapy after breast or endometrial cancer, extended use of hormone therapy in women who are early initiators, improved tools to personalize or individualize benefits and risks of hormone therapy, and the role of aging and genetics,” said Dr. Faubion. Other areas for further research include “the long-term benefits and risks on women’s health of lifestyle modification or complementary or nonhormone therapies, if chosen in addition to or over hormone therapy for vasomotor symptoms, bone health, and cardiovascular disease risk reduction,” she added.
The complete statement was published in Menopause: The Journal of the North American Menopause Society.
The position statement received no outside funding. The authors had no financial conflicts to disclose.
FROM MENOPAUSE
Concerns that low LDL-C alters cognitive function challenged in novel analysis
PCSK9 inhibitors, which are among the most effective therapies for reducing LDL cholesterol (LDL-C), are associated with a neutral effect on cognitive function, according to a genetics-based Mendelian randomization study intended to sort out through the complexity of confounders.
The same study linked HMG-Co A reductase inhibitors (statins) with the potential for modest adverse neurocognitive effects, although these are likely to be outweighed by cardiovascular benefits, according to a collaborating team of investigators from the U.S. National Institutes of Health and the University of Oxford (England).
For clinicians and patients who continue to harbor concerns that cognitive function is threatened by very low LDL-C, this novel approach to evaluating risk is “reassuring,” according to the authors.
Early in clinical testing of PCSK9 inhibitors, a potential signal for adverse effects on cognitive function was reported but unconfirmed. This signal raised concern that extremely low levels of LDL-C, such as < 25 mg/dL, achieved with PCSK9 inhibitors might pose a risk to neurocognitive function.
Of several factors that provided a basis for concern, the PCSK9 enzyme is known to participate in brain development, according to the authors of this newly published study.
Mendelian randomization addresses complex issue
The objective of this Mendelian randomization analysis was to evaluate the relationship of PCSK9 inhibitors and statins on long-term neurocognitive function. Used previously to address other clinical issues, a drug-effect Mendelian randomization analysis evaluates genetic variants to determine whether there is a causal relationship between a risk, which in this case was lipid-lowering drugs, to a specific outcome, which was cognitive performance.
By looking directly at genetic variants that simulate the pharmacological inhibition of drug gene targets, the bias of confounders of clinical effects, such as baseline cognitive function, are avoided, according to the authors.
The message from this drug-effect Mendelian analysis was simple, according to the senior author of the study, Falk W. Lohoff, MD, chief of the section on clinical genomics and experimental therapeutics, National Institute of Alcohol Abuse and Alcoholism.
“Based on our data, we do not see a significant cognitive risk profile with PCSK9 inhibition associated with low LDL-C,” Dr. Lohoff said in an interview. He cautioned that “future long-term clinical studies are needed to confirm the absence of this effect,” but he and his coauthors noted that these data concur with the clinical studies.
From genome-wide association studies, single-nucleotide polymorphisms in PCSK9 and HMG-Co A reductase were extracted from a sample of more than 700,000 individuals of predominantly European ancestry. In the analysis, the investigators evaluated whether inhibition of PCSK9 or HMG-Co A reductase had an effect on seven clinical outcomes that relate to neurocognitive function, including memory, verbal intelligence, and reaction time, as well as biomarkers of cognitive function, such as cortical surface area.
The genetic effect of PCSK9 inhibition was “null for every cognitive-related outcome evaluated,” the investigators reported. The genetic effect of HMG-Co A reductase inhibition had a statistically significant but modest effect on cognitive performance (P = .03) and cortical surface area (P = .03). While the impact of HMG-Co A reductase inhibition on reaction time was stronger on a statistical basis (P = .0002), the investigators reported that it translated into a decrease of only 0.067 milliseconds per 38.7 mg/dL. They characterized this as a “small impact” unlikely to outweigh clinical benefits.
In an editorial that accompanied publication of this study, Brian A. Ference, MD, MPhil, provided context for the suitability of a Mendelian randomization analysis to address this or other questions regarding the impact of lipid-lowering therapies on clinical outcomes, and he ultimately concurred with the major conclusions
Ultimately, this analysis is consistent with other evidence that PCSK9 inhibition does not pose a risk of impaired cognitive function, he wrote. For statins, he concluded that this study “does not provide compelling evidence” to challenge their current clinical use.
Data do not support low LDL-C as cognitive risk factor
Moreover, this study – as well as other evidence – argues strongly against very low levels of LDL-C, regardless of how they are achieved, as a risk factor for diminished cognitive function, Dr. Ference, director of research in the division of translational therapeutics, University of Cambridge (England), said in an interview.
“There is no evidence from Mendelian randomization studies that lifelong exposure to lower LDL-C increases the risk of cognitive impairment,” he said. “This is true when evaluating lifelong exposure to lower LDL-C due to genetic variants in a wide variety of different genes or the genes that encode the target PCKS9 inhibitors, statins, or other lipid-lowering therapies.”
In other words, this study “adds to the accumulating evidence” that LDL-C lowering by itself does not contribute to an adverse impact on cognitive function despite persistent concern. This should not be surprising. Dr. Ference emphasized that there has never been strong evidence for an association.
“As I point out in the editorial, there is no biologically plausible mechanism by which reducing peripheral LDL-C should impact neurological function in any way, because the therapies do not cross the blood brain barrier, and because the nervous system produces its own cholesterol to maintain the integrity of membranes in nervous system cells,” he explained.
Dr. Lohoff reports no potential conflicts of interest. Dr. Ference has financial relationships with numerous pharmaceutical companies including those that make lipid-lowering therapies.
PCSK9 inhibitors, which are among the most effective therapies for reducing LDL cholesterol (LDL-C), are associated with a neutral effect on cognitive function, according to a genetics-based Mendelian randomization study intended to sort out through the complexity of confounders.
The same study linked HMG-Co A reductase inhibitors (statins) with the potential for modest adverse neurocognitive effects, although these are likely to be outweighed by cardiovascular benefits, according to a collaborating team of investigators from the U.S. National Institutes of Health and the University of Oxford (England).
For clinicians and patients who continue to harbor concerns that cognitive function is threatened by very low LDL-C, this novel approach to evaluating risk is “reassuring,” according to the authors.
Early in clinical testing of PCSK9 inhibitors, a potential signal for adverse effects on cognitive function was reported but unconfirmed. This signal raised concern that extremely low levels of LDL-C, such as < 25 mg/dL, achieved with PCSK9 inhibitors might pose a risk to neurocognitive function.
Of several factors that provided a basis for concern, the PCSK9 enzyme is known to participate in brain development, according to the authors of this newly published study.
Mendelian randomization addresses complex issue
The objective of this Mendelian randomization analysis was to evaluate the relationship of PCSK9 inhibitors and statins on long-term neurocognitive function. Used previously to address other clinical issues, a drug-effect Mendelian randomization analysis evaluates genetic variants to determine whether there is a causal relationship between a risk, which in this case was lipid-lowering drugs, to a specific outcome, which was cognitive performance.
By looking directly at genetic variants that simulate the pharmacological inhibition of drug gene targets, the bias of confounders of clinical effects, such as baseline cognitive function, are avoided, according to the authors.
The message from this drug-effect Mendelian analysis was simple, according to the senior author of the study, Falk W. Lohoff, MD, chief of the section on clinical genomics and experimental therapeutics, National Institute of Alcohol Abuse and Alcoholism.
“Based on our data, we do not see a significant cognitive risk profile with PCSK9 inhibition associated with low LDL-C,” Dr. Lohoff said in an interview. He cautioned that “future long-term clinical studies are needed to confirm the absence of this effect,” but he and his coauthors noted that these data concur with the clinical studies.
From genome-wide association studies, single-nucleotide polymorphisms in PCSK9 and HMG-Co A reductase were extracted from a sample of more than 700,000 individuals of predominantly European ancestry. In the analysis, the investigators evaluated whether inhibition of PCSK9 or HMG-Co A reductase had an effect on seven clinical outcomes that relate to neurocognitive function, including memory, verbal intelligence, and reaction time, as well as biomarkers of cognitive function, such as cortical surface area.
The genetic effect of PCSK9 inhibition was “null for every cognitive-related outcome evaluated,” the investigators reported. The genetic effect of HMG-Co A reductase inhibition had a statistically significant but modest effect on cognitive performance (P = .03) and cortical surface area (P = .03). While the impact of HMG-Co A reductase inhibition on reaction time was stronger on a statistical basis (P = .0002), the investigators reported that it translated into a decrease of only 0.067 milliseconds per 38.7 mg/dL. They characterized this as a “small impact” unlikely to outweigh clinical benefits.
In an editorial that accompanied publication of this study, Brian A. Ference, MD, MPhil, provided context for the suitability of a Mendelian randomization analysis to address this or other questions regarding the impact of lipid-lowering therapies on clinical outcomes, and he ultimately concurred with the major conclusions
Ultimately, this analysis is consistent with other evidence that PCSK9 inhibition does not pose a risk of impaired cognitive function, he wrote. For statins, he concluded that this study “does not provide compelling evidence” to challenge their current clinical use.
Data do not support low LDL-C as cognitive risk factor
Moreover, this study – as well as other evidence – argues strongly against very low levels of LDL-C, regardless of how they are achieved, as a risk factor for diminished cognitive function, Dr. Ference, director of research in the division of translational therapeutics, University of Cambridge (England), said in an interview.
“There is no evidence from Mendelian randomization studies that lifelong exposure to lower LDL-C increases the risk of cognitive impairment,” he said. “This is true when evaluating lifelong exposure to lower LDL-C due to genetic variants in a wide variety of different genes or the genes that encode the target PCKS9 inhibitors, statins, or other lipid-lowering therapies.”
In other words, this study “adds to the accumulating evidence” that LDL-C lowering by itself does not contribute to an adverse impact on cognitive function despite persistent concern. This should not be surprising. Dr. Ference emphasized that there has never been strong evidence for an association.
“As I point out in the editorial, there is no biologically plausible mechanism by which reducing peripheral LDL-C should impact neurological function in any way, because the therapies do not cross the blood brain barrier, and because the nervous system produces its own cholesterol to maintain the integrity of membranes in nervous system cells,” he explained.
Dr. Lohoff reports no potential conflicts of interest. Dr. Ference has financial relationships with numerous pharmaceutical companies including those that make lipid-lowering therapies.
PCSK9 inhibitors, which are among the most effective therapies for reducing LDL cholesterol (LDL-C), are associated with a neutral effect on cognitive function, according to a genetics-based Mendelian randomization study intended to sort out through the complexity of confounders.
The same study linked HMG-Co A reductase inhibitors (statins) with the potential for modest adverse neurocognitive effects, although these are likely to be outweighed by cardiovascular benefits, according to a collaborating team of investigators from the U.S. National Institutes of Health and the University of Oxford (England).
For clinicians and patients who continue to harbor concerns that cognitive function is threatened by very low LDL-C, this novel approach to evaluating risk is “reassuring,” according to the authors.
Early in clinical testing of PCSK9 inhibitors, a potential signal for adverse effects on cognitive function was reported but unconfirmed. This signal raised concern that extremely low levels of LDL-C, such as < 25 mg/dL, achieved with PCSK9 inhibitors might pose a risk to neurocognitive function.
Of several factors that provided a basis for concern, the PCSK9 enzyme is known to participate in brain development, according to the authors of this newly published study.
Mendelian randomization addresses complex issue
The objective of this Mendelian randomization analysis was to evaluate the relationship of PCSK9 inhibitors and statins on long-term neurocognitive function. Used previously to address other clinical issues, a drug-effect Mendelian randomization analysis evaluates genetic variants to determine whether there is a causal relationship between a risk, which in this case was lipid-lowering drugs, to a specific outcome, which was cognitive performance.
By looking directly at genetic variants that simulate the pharmacological inhibition of drug gene targets, the bias of confounders of clinical effects, such as baseline cognitive function, are avoided, according to the authors.
The message from this drug-effect Mendelian analysis was simple, according to the senior author of the study, Falk W. Lohoff, MD, chief of the section on clinical genomics and experimental therapeutics, National Institute of Alcohol Abuse and Alcoholism.
“Based on our data, we do not see a significant cognitive risk profile with PCSK9 inhibition associated with low LDL-C,” Dr. Lohoff said in an interview. He cautioned that “future long-term clinical studies are needed to confirm the absence of this effect,” but he and his coauthors noted that these data concur with the clinical studies.
From genome-wide association studies, single-nucleotide polymorphisms in PCSK9 and HMG-Co A reductase were extracted from a sample of more than 700,000 individuals of predominantly European ancestry. In the analysis, the investigators evaluated whether inhibition of PCSK9 or HMG-Co A reductase had an effect on seven clinical outcomes that relate to neurocognitive function, including memory, verbal intelligence, and reaction time, as well as biomarkers of cognitive function, such as cortical surface area.
The genetic effect of PCSK9 inhibition was “null for every cognitive-related outcome evaluated,” the investigators reported. The genetic effect of HMG-Co A reductase inhibition had a statistically significant but modest effect on cognitive performance (P = .03) and cortical surface area (P = .03). While the impact of HMG-Co A reductase inhibition on reaction time was stronger on a statistical basis (P = .0002), the investigators reported that it translated into a decrease of only 0.067 milliseconds per 38.7 mg/dL. They characterized this as a “small impact” unlikely to outweigh clinical benefits.
In an editorial that accompanied publication of this study, Brian A. Ference, MD, MPhil, provided context for the suitability of a Mendelian randomization analysis to address this or other questions regarding the impact of lipid-lowering therapies on clinical outcomes, and he ultimately concurred with the major conclusions
Ultimately, this analysis is consistent with other evidence that PCSK9 inhibition does not pose a risk of impaired cognitive function, he wrote. For statins, he concluded that this study “does not provide compelling evidence” to challenge their current clinical use.
Data do not support low LDL-C as cognitive risk factor
Moreover, this study – as well as other evidence – argues strongly against very low levels of LDL-C, regardless of how they are achieved, as a risk factor for diminished cognitive function, Dr. Ference, director of research in the division of translational therapeutics, University of Cambridge (England), said in an interview.
“There is no evidence from Mendelian randomization studies that lifelong exposure to lower LDL-C increases the risk of cognitive impairment,” he said. “This is true when evaluating lifelong exposure to lower LDL-C due to genetic variants in a wide variety of different genes or the genes that encode the target PCKS9 inhibitors, statins, or other lipid-lowering therapies.”
In other words, this study “adds to the accumulating evidence” that LDL-C lowering by itself does not contribute to an adverse impact on cognitive function despite persistent concern. This should not be surprising. Dr. Ference emphasized that there has never been strong evidence for an association.
“As I point out in the editorial, there is no biologically plausible mechanism by which reducing peripheral LDL-C should impact neurological function in any way, because the therapies do not cross the blood brain barrier, and because the nervous system produces its own cholesterol to maintain the integrity of membranes in nervous system cells,” he explained.
Dr. Lohoff reports no potential conflicts of interest. Dr. Ference has financial relationships with numerous pharmaceutical companies including those that make lipid-lowering therapies.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Is prostasin a clue to diabetes/cancer link?
People with elevated levels of protein prostasin seem to have a higher risk of developing diabetes and dying from cancer, according to a large, prospective, population-based study. The finding may provide new insights into why people with diabetes have an increased risk of cancer.
The study claims to be the first to investigate the link between plasma prostasin levels and cancer mortality, the study authors wrote in Diabetologia. The study analyzed plasma prostasin samples from 4,297 older adults (average age, 57.5 years) from the Malmö (Sweden) Diet and Cancer Study Cardiovascular Cohort.
“This study from the general population shows that prostasin, a protein that could be measured in blood, is associated with increased risk of developing diabetes,” senior author Gunnar Engström, MD, PhD, professor of epidemiology at Lund University in Malmö, Sweden, said in a comment. “Furthermore, it was associated with increased risk of death from cancer, especially in individuals with elevated glucose levels in the prediabetic range.
“The relationship between diabetes and cancer is poorly understood,” Dr. Engström said. “To our knowledge, this is the first big population study of prostasin and risk of diabetes.”
He noted previous studies have found a relationship between prostasin and cancer outcomes. “Prostasin could be a possible shared link between the two diseases and the results could help us understand why individuals with diabetes have increased risk of cancer.”
Patients in the study were assigned to quartiles based on prostasin levels. Those in the highest quartile had almost twice the risk of prevalent diabetes than did those in the lowest quartile (adjusted odds ratio, 1.95; 95% confidence interval, 1.39-2.76; P < .0001).
During the follow-up periods of 21.9 years for diabetes and 23.5 years for cancer, on average, 702 participants developed diabetes and 651 died from cancer. Again, the analysis found a significantly higher adjusted hazard ratio for participants in the fourth quartile: about 75% higher for diabetes (HR, 1.76; 95% CI, 1.41-2.19; P < .0001), and, after multivariable analysis, about 40% higher for death from cancer (HR, 1.43; 95% CI, 1.14-1.8; P = .0008).
Potential diabetes-cancer ‘interaction’
The study also identified what it called “a significant interaction” between prostasin and fasting blood glucose for cancer mortality risk (P = .022). In patients with impaired fasting blood glucose levels at baseline, the risk for cancer mortality was about 50% greater with each standard deviation increase in prostasin (HR, 1.52; 95% CI, 1.07-2.16; P = .019). Those with normal fasting blood glucose at baseline had a significantly lower risk with each SD increase in prostasin (HR, 1.11; 95% CI, 1.01-1.21; P = .025).
Further research is needed to validate the potential of prostasin as a biomarker for diabetes and cancer risks, Dr. Engström said. “The results need to be replicated in other studies. A study of cancer mortality in a big cohort of diabetes patients would be of great interest. We also need to examine whether prostasin is causally related to cancer and/or diabetes, or whether prostasin could act as a valuable risk marker in clinical settings. If causal, there could a possible molecular target for treatment.”
He added: “Biomarkers of diabetes and cancer are of great interest in the era of personalized medicine, both for disease prevention and for treatment of those with established disease.”
Li-Mei Chen, MD, PhD, a research associate professor at the University of Central Florida, Orlando, has studied the role of prostasin in epidemiology. She noted that one of the challenges of using prostasin in clinical or research settings is the lack of a standardized assay, which the Malmö study acknowledged. Dr. Engström and colleagues wrote that “prostasin levels were measured in arbitrary units (NPX values), and thus could not be compared directly with absolute values.”
Dr. Chen pointed out that the study reported a lower range of 0.24 pg/mL and an upper range of 7,800 pg/mL.
This means that, “in different groups that measure prostasin, the absolute quantity could have a difference in the thousands or tens of thousands,” she said. “That makes the judgment difficult of whether for this person you have a high level of prostasin in the blood and the other one you don’t if the difference is over a thousandfold.”
The Malmö study used the Proseek Multiplex Oncology I panel to determine plasma prostasin concentration, but Dr. Chen noted that she couldn’t find any data validating the panel for measuring prostasin. “It’s really hard for me to say whether this is of value or not because if the method that generated the data is not verified by another method, you don’t really know what you’re measuring.
“If the data are questionable, it’s really hard to say whether it means whether it’s a marker for cancer or diabetes,” Dr. Chen added. “That’s the biggest question I have, but actually the authors realize that.”
Dr. Engström confirmed that, “if prostasin is used to identify patients with increased risk of diabetes and cancer mortality, we also need to develop standardized assays for clinical use.”
Dr. Engström and coauthors had no disclosures. The study received funding from the Swedish Heart Lung Foundation, the National Natural Science Foundation of China, and the Natural Science Foundation of Jiangsu Province. The Malmö Diet and Cancer study received grants from the Swedish Cancer Society, the Swedish Medical Research Council, AFA Insurance, the Albert Påhlsson and Gunnar Nilsson Foundations, Malmö City Council, and Lund University. Dr. Chen had no relevant disclosures.
People with elevated levels of protein prostasin seem to have a higher risk of developing diabetes and dying from cancer, according to a large, prospective, population-based study. The finding may provide new insights into why people with diabetes have an increased risk of cancer.
The study claims to be the first to investigate the link between plasma prostasin levels and cancer mortality, the study authors wrote in Diabetologia. The study analyzed plasma prostasin samples from 4,297 older adults (average age, 57.5 years) from the Malmö (Sweden) Diet and Cancer Study Cardiovascular Cohort.
“This study from the general population shows that prostasin, a protein that could be measured in blood, is associated with increased risk of developing diabetes,” senior author Gunnar Engström, MD, PhD, professor of epidemiology at Lund University in Malmö, Sweden, said in a comment. “Furthermore, it was associated with increased risk of death from cancer, especially in individuals with elevated glucose levels in the prediabetic range.
“The relationship between diabetes and cancer is poorly understood,” Dr. Engström said. “To our knowledge, this is the first big population study of prostasin and risk of diabetes.”
He noted previous studies have found a relationship between prostasin and cancer outcomes. “Prostasin could be a possible shared link between the two diseases and the results could help us understand why individuals with diabetes have increased risk of cancer.”
Patients in the study were assigned to quartiles based on prostasin levels. Those in the highest quartile had almost twice the risk of prevalent diabetes than did those in the lowest quartile (adjusted odds ratio, 1.95; 95% confidence interval, 1.39-2.76; P < .0001).
During the follow-up periods of 21.9 years for diabetes and 23.5 years for cancer, on average, 702 participants developed diabetes and 651 died from cancer. Again, the analysis found a significantly higher adjusted hazard ratio for participants in the fourth quartile: about 75% higher for diabetes (HR, 1.76; 95% CI, 1.41-2.19; P < .0001), and, after multivariable analysis, about 40% higher for death from cancer (HR, 1.43; 95% CI, 1.14-1.8; P = .0008).
Potential diabetes-cancer ‘interaction’
The study also identified what it called “a significant interaction” between prostasin and fasting blood glucose for cancer mortality risk (P = .022). In patients with impaired fasting blood glucose levels at baseline, the risk for cancer mortality was about 50% greater with each standard deviation increase in prostasin (HR, 1.52; 95% CI, 1.07-2.16; P = .019). Those with normal fasting blood glucose at baseline had a significantly lower risk with each SD increase in prostasin (HR, 1.11; 95% CI, 1.01-1.21; P = .025).
Further research is needed to validate the potential of prostasin as a biomarker for diabetes and cancer risks, Dr. Engström said. “The results need to be replicated in other studies. A study of cancer mortality in a big cohort of diabetes patients would be of great interest. We also need to examine whether prostasin is causally related to cancer and/or diabetes, or whether prostasin could act as a valuable risk marker in clinical settings. If causal, there could a possible molecular target for treatment.”
He added: “Biomarkers of diabetes and cancer are of great interest in the era of personalized medicine, both for disease prevention and for treatment of those with established disease.”
Li-Mei Chen, MD, PhD, a research associate professor at the University of Central Florida, Orlando, has studied the role of prostasin in epidemiology. She noted that one of the challenges of using prostasin in clinical or research settings is the lack of a standardized assay, which the Malmö study acknowledged. Dr. Engström and colleagues wrote that “prostasin levels were measured in arbitrary units (NPX values), and thus could not be compared directly with absolute values.”
Dr. Chen pointed out that the study reported a lower range of 0.24 pg/mL and an upper range of 7,800 pg/mL.
This means that, “in different groups that measure prostasin, the absolute quantity could have a difference in the thousands or tens of thousands,” she said. “That makes the judgment difficult of whether for this person you have a high level of prostasin in the blood and the other one you don’t if the difference is over a thousandfold.”
The Malmö study used the Proseek Multiplex Oncology I panel to determine plasma prostasin concentration, but Dr. Chen noted that she couldn’t find any data validating the panel for measuring prostasin. “It’s really hard for me to say whether this is of value or not because if the method that generated the data is not verified by another method, you don’t really know what you’re measuring.
“If the data are questionable, it’s really hard to say whether it means whether it’s a marker for cancer or diabetes,” Dr. Chen added. “That’s the biggest question I have, but actually the authors realize that.”
Dr. Engström confirmed that, “if prostasin is used to identify patients with increased risk of diabetes and cancer mortality, we also need to develop standardized assays for clinical use.”
Dr. Engström and coauthors had no disclosures. The study received funding from the Swedish Heart Lung Foundation, the National Natural Science Foundation of China, and the Natural Science Foundation of Jiangsu Province. The Malmö Diet and Cancer study received grants from the Swedish Cancer Society, the Swedish Medical Research Council, AFA Insurance, the Albert Påhlsson and Gunnar Nilsson Foundations, Malmö City Council, and Lund University. Dr. Chen had no relevant disclosures.
People with elevated levels of protein prostasin seem to have a higher risk of developing diabetes and dying from cancer, according to a large, prospective, population-based study. The finding may provide new insights into why people with diabetes have an increased risk of cancer.
The study claims to be the first to investigate the link between plasma prostasin levels and cancer mortality, the study authors wrote in Diabetologia. The study analyzed plasma prostasin samples from 4,297 older adults (average age, 57.5 years) from the Malmö (Sweden) Diet and Cancer Study Cardiovascular Cohort.
“This study from the general population shows that prostasin, a protein that could be measured in blood, is associated with increased risk of developing diabetes,” senior author Gunnar Engström, MD, PhD, professor of epidemiology at Lund University in Malmö, Sweden, said in a comment. “Furthermore, it was associated with increased risk of death from cancer, especially in individuals with elevated glucose levels in the prediabetic range.
“The relationship between diabetes and cancer is poorly understood,” Dr. Engström said. “To our knowledge, this is the first big population study of prostasin and risk of diabetes.”
He noted previous studies have found a relationship between prostasin and cancer outcomes. “Prostasin could be a possible shared link between the two diseases and the results could help us understand why individuals with diabetes have increased risk of cancer.”
Patients in the study were assigned to quartiles based on prostasin levels. Those in the highest quartile had almost twice the risk of prevalent diabetes than did those in the lowest quartile (adjusted odds ratio, 1.95; 95% confidence interval, 1.39-2.76; P < .0001).
During the follow-up periods of 21.9 years for diabetes and 23.5 years for cancer, on average, 702 participants developed diabetes and 651 died from cancer. Again, the analysis found a significantly higher adjusted hazard ratio for participants in the fourth quartile: about 75% higher for diabetes (HR, 1.76; 95% CI, 1.41-2.19; P < .0001), and, after multivariable analysis, about 40% higher for death from cancer (HR, 1.43; 95% CI, 1.14-1.8; P = .0008).
Potential diabetes-cancer ‘interaction’
The study also identified what it called “a significant interaction” between prostasin and fasting blood glucose for cancer mortality risk (P = .022). In patients with impaired fasting blood glucose levels at baseline, the risk for cancer mortality was about 50% greater with each standard deviation increase in prostasin (HR, 1.52; 95% CI, 1.07-2.16; P = .019). Those with normal fasting blood glucose at baseline had a significantly lower risk with each SD increase in prostasin (HR, 1.11; 95% CI, 1.01-1.21; P = .025).
Further research is needed to validate the potential of prostasin as a biomarker for diabetes and cancer risks, Dr. Engström said. “The results need to be replicated in other studies. A study of cancer mortality in a big cohort of diabetes patients would be of great interest. We also need to examine whether prostasin is causally related to cancer and/or diabetes, or whether prostasin could act as a valuable risk marker in clinical settings. If causal, there could a possible molecular target for treatment.”
He added: “Biomarkers of diabetes and cancer are of great interest in the era of personalized medicine, both for disease prevention and for treatment of those with established disease.”
Li-Mei Chen, MD, PhD, a research associate professor at the University of Central Florida, Orlando, has studied the role of prostasin in epidemiology. She noted that one of the challenges of using prostasin in clinical or research settings is the lack of a standardized assay, which the Malmö study acknowledged. Dr. Engström and colleagues wrote that “prostasin levels were measured in arbitrary units (NPX values), and thus could not be compared directly with absolute values.”
Dr. Chen pointed out that the study reported a lower range of 0.24 pg/mL and an upper range of 7,800 pg/mL.
This means that, “in different groups that measure prostasin, the absolute quantity could have a difference in the thousands or tens of thousands,” she said. “That makes the judgment difficult of whether for this person you have a high level of prostasin in the blood and the other one you don’t if the difference is over a thousandfold.”
The Malmö study used the Proseek Multiplex Oncology I panel to determine plasma prostasin concentration, but Dr. Chen noted that she couldn’t find any data validating the panel for measuring prostasin. “It’s really hard for me to say whether this is of value or not because if the method that generated the data is not verified by another method, you don’t really know what you’re measuring.
“If the data are questionable, it’s really hard to say whether it means whether it’s a marker for cancer or diabetes,” Dr. Chen added. “That’s the biggest question I have, but actually the authors realize that.”
Dr. Engström confirmed that, “if prostasin is used to identify patients with increased risk of diabetes and cancer mortality, we also need to develop standardized assays for clinical use.”
Dr. Engström and coauthors had no disclosures. The study received funding from the Swedish Heart Lung Foundation, the National Natural Science Foundation of China, and the Natural Science Foundation of Jiangsu Province. The Malmö Diet and Cancer study received grants from the Swedish Cancer Society, the Swedish Medical Research Council, AFA Insurance, the Albert Påhlsson and Gunnar Nilsson Foundations, Malmö City Council, and Lund University. Dr. Chen had no relevant disclosures.
FROM DIABETOLOGIA
Social isolation, loneliness tied to death, MI, stroke: AHA
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Hot weather risk for nonfatal MI hinted for antiplatelets, beta-blockers
Patients who take beta-blockers or antiplatelet agents are lowering their risk for cardiovascular events, but the protection may fall short for those who spend time outdoors on hot summer days, hints a limited analysis published as a letter in Nature Cardiovascular Research.
Patients taking either a beta-blocker or antiplatelet, or both medications together, appeared at elevated risk for nonfatal acute MI specifically on days when the weather turned hot, suggests the registry cohort study that covered 14 years of clinical and meteorologic data.
“The take-away message is not that patients should stop using these two medications, by no means. We’re raising cautions for patients taking them, to watch out for themselves during high-heat days,” lead author Kai Chen, PhD, Yale University, New Haven, Conn., said in an interview.
“We’re not giving the message that these drugs have harmful effects” because the nature of the links between the medications and MI in the study, with its potential for confounding, remain unknown, said Dr. Chen, from the department of environmental health sciences and Yale Center on Climate Change and Health.
For example, patients who take beta-blockers or antiplatelets tend to be sicker than patients not on the drugs, which could make heat-related MI more likely, and the drugs wrongly appear to be culprits, he observed. The analysis contained signals that could support either scenario.
The study is based on cases of nonfatal MI in Augsburg, Germany, that are part of the MONICA-KORA MI registry. The odds of a heat-related nonfatal MI, it suggests, were increased 63% among patients taking antiplatelets and by 65% among those on beta-blockers, compared with those not on these drugs. The odds went up by 75% among those on both drug classes, but the risks weren’t raised in patients not taking them.
Rising heat-related MI
Chen said analysis was inspired by a 2019 report – also based on MONICA-KORA, from many of the same authors and using similar methods to track events by daily air temperature – that showed a rising trend for heat-related MI and declining rate for MI related to cold weather from 1987 to 2014. A next step, he figured, would be to determine whether the MI risk trends were associated with any cardiovascular medications.
The current study’s signal of risk related to antiplatelets and beta-blockers did not emerge for ACE inhibitors, calcium-channel blockers, or diuretics. Statins showed a link to increased nonfatal MI risk, but solely among participants aged younger than 60 years, who were also far less likely to have pre-existing coronary heart disease (CHD). He and his colleagues chose not to highlight that finding, Dr. Chen said, because the age subgroup analysis was grossly underpowered.
The overall analysis involved 2,494 cases of nonfatal MI that occurred during the warmer months – May to September – from 2001 to 2014. It was limited to nonfatal cases – those with at least a month of survival after hospital admission – because of insufficient data on medication use associated with fatal MIs, the report states.
Nonfatal MIs were defined as heat-related if they struck on days reaching the 95th percentile for temperature across the 14 years, in this case 24.2 °C (about 75.6 °F), relative to the average temperature of lowest nonfatal MI risk across the cohort, 7.5 °C (about 45.5 °F).
Patients served as both cases and their own controls, in that air temperature exposures on the day of their MI (case day) were compared with the remaining same days of the week in the same calendar month (control days). That approach, the report stated, “automatically controls for long-term time trends, seasonality, day of the week, and time-invariant confounders (for example, pre-existing cardiovascular disease).”
The odds ratio for heat-related MI for patients on antiplatelets was 1.63 (95% confidence interval, 1.07-2.46), and for antiplatelet nonusers was 0.94 (95% CI, 0.68-1.29). The difference between the two ratios was significant (P = .04).
The corresponding OR for patients taking beta-blockers was 1.65 (95% CI, 1.11-2.45), and for nonusers of beta-blockers was 0.90 (95% CI, 0.64-1.26). Again, the OR difference was significant (P = .02).
The ORs for users of both medication classes and nonusers of either med class, respectively, were 1.75 (95% CI, 1.12-2.73) and 0.84 (95% CI, 0.59-1.19). The latter OR was significantly lower than former (P = .01).
In a sign that antiplatelet and beta-blocker use might have been just a marker for sicker patients who were more vulnerable to heat-related MI, Chen said, the nonfatal MI risk was significantly elevated (OR, 2.17; 95% CI, 1.40-3.38) among patients with pre-existing CHD, but not among those free of pre-existing CHD (OR, 0.88; 95% CI, 0.65-1.20); the odds difference was P < .01.
That signal of confounding by indication is somewhat countered, the report states, by variations in nonfatal MI risk by age group. The increased chances of an event seen overall in relation to beta-blockers and antiplatelets were more pronounced among the 39% of patients aged 25-59 years (P < .01). That’s in spite that group’s lower CHD prevalence. The risk elevation solely among the older patients was attenuated and rendered nonsignificant, even with their greater CHD burden, the report noted.
The report speculates on a potential mechanism by which beta-blockers, at least, might conceivably raise the risk for heat-related MI. “Beta-receptor blockers inhibit skin vasodilation, resulting in reduced heat dissipation through convection and, at the same time, could intensify the blood-pressure-lowering effect of other antihypertensive drugs, which then could lead to syncope.”
Beta-blockers, Dr. Chen said, “can mechanistically make people more vulnerable to heat. That’s one potential explanation. Or it could be that these people taking the medications are just sicker. Whatever the reasons, the phenomenon we observed is that these patients taking these two medications are at higher risk during high-temperature days.”
Dr. Chen and the other authors declare no competing interests.
A version of this article first appeared on Medscape.com.
Patients who take beta-blockers or antiplatelet agents are lowering their risk for cardiovascular events, but the protection may fall short for those who spend time outdoors on hot summer days, hints a limited analysis published as a letter in Nature Cardiovascular Research.
Patients taking either a beta-blocker or antiplatelet, or both medications together, appeared at elevated risk for nonfatal acute MI specifically on days when the weather turned hot, suggests the registry cohort study that covered 14 years of clinical and meteorologic data.
“The take-away message is not that patients should stop using these two medications, by no means. We’re raising cautions for patients taking them, to watch out for themselves during high-heat days,” lead author Kai Chen, PhD, Yale University, New Haven, Conn., said in an interview.
“We’re not giving the message that these drugs have harmful effects” because the nature of the links between the medications and MI in the study, with its potential for confounding, remain unknown, said Dr. Chen, from the department of environmental health sciences and Yale Center on Climate Change and Health.
For example, patients who take beta-blockers or antiplatelets tend to be sicker than patients not on the drugs, which could make heat-related MI more likely, and the drugs wrongly appear to be culprits, he observed. The analysis contained signals that could support either scenario.
The study is based on cases of nonfatal MI in Augsburg, Germany, that are part of the MONICA-KORA MI registry. The odds of a heat-related nonfatal MI, it suggests, were increased 63% among patients taking antiplatelets and by 65% among those on beta-blockers, compared with those not on these drugs. The odds went up by 75% among those on both drug classes, but the risks weren’t raised in patients not taking them.
Rising heat-related MI
Chen said analysis was inspired by a 2019 report – also based on MONICA-KORA, from many of the same authors and using similar methods to track events by daily air temperature – that showed a rising trend for heat-related MI and declining rate for MI related to cold weather from 1987 to 2014. A next step, he figured, would be to determine whether the MI risk trends were associated with any cardiovascular medications.
The current study’s signal of risk related to antiplatelets and beta-blockers did not emerge for ACE inhibitors, calcium-channel blockers, or diuretics. Statins showed a link to increased nonfatal MI risk, but solely among participants aged younger than 60 years, who were also far less likely to have pre-existing coronary heart disease (CHD). He and his colleagues chose not to highlight that finding, Dr. Chen said, because the age subgroup analysis was grossly underpowered.
The overall analysis involved 2,494 cases of nonfatal MI that occurred during the warmer months – May to September – from 2001 to 2014. It was limited to nonfatal cases – those with at least a month of survival after hospital admission – because of insufficient data on medication use associated with fatal MIs, the report states.
Nonfatal MIs were defined as heat-related if they struck on days reaching the 95th percentile for temperature across the 14 years, in this case 24.2 °C (about 75.6 °F), relative to the average temperature of lowest nonfatal MI risk across the cohort, 7.5 °C (about 45.5 °F).
Patients served as both cases and their own controls, in that air temperature exposures on the day of their MI (case day) were compared with the remaining same days of the week in the same calendar month (control days). That approach, the report stated, “automatically controls for long-term time trends, seasonality, day of the week, and time-invariant confounders (for example, pre-existing cardiovascular disease).”
The odds ratio for heat-related MI for patients on antiplatelets was 1.63 (95% confidence interval, 1.07-2.46), and for antiplatelet nonusers was 0.94 (95% CI, 0.68-1.29). The difference between the two ratios was significant (P = .04).
The corresponding OR for patients taking beta-blockers was 1.65 (95% CI, 1.11-2.45), and for nonusers of beta-blockers was 0.90 (95% CI, 0.64-1.26). Again, the OR difference was significant (P = .02).
The ORs for users of both medication classes and nonusers of either med class, respectively, were 1.75 (95% CI, 1.12-2.73) and 0.84 (95% CI, 0.59-1.19). The latter OR was significantly lower than former (P = .01).
In a sign that antiplatelet and beta-blocker use might have been just a marker for sicker patients who were more vulnerable to heat-related MI, Chen said, the nonfatal MI risk was significantly elevated (OR, 2.17; 95% CI, 1.40-3.38) among patients with pre-existing CHD, but not among those free of pre-existing CHD (OR, 0.88; 95% CI, 0.65-1.20); the odds difference was P < .01.
That signal of confounding by indication is somewhat countered, the report states, by variations in nonfatal MI risk by age group. The increased chances of an event seen overall in relation to beta-blockers and antiplatelets were more pronounced among the 39% of patients aged 25-59 years (P < .01). That’s in spite that group’s lower CHD prevalence. The risk elevation solely among the older patients was attenuated and rendered nonsignificant, even with their greater CHD burden, the report noted.
The report speculates on a potential mechanism by which beta-blockers, at least, might conceivably raise the risk for heat-related MI. “Beta-receptor blockers inhibit skin vasodilation, resulting in reduced heat dissipation through convection and, at the same time, could intensify the blood-pressure-lowering effect of other antihypertensive drugs, which then could lead to syncope.”
Beta-blockers, Dr. Chen said, “can mechanistically make people more vulnerable to heat. That’s one potential explanation. Or it could be that these people taking the medications are just sicker. Whatever the reasons, the phenomenon we observed is that these patients taking these two medications are at higher risk during high-temperature days.”
Dr. Chen and the other authors declare no competing interests.
A version of this article first appeared on Medscape.com.
Patients who take beta-blockers or antiplatelet agents are lowering their risk for cardiovascular events, but the protection may fall short for those who spend time outdoors on hot summer days, hints a limited analysis published as a letter in Nature Cardiovascular Research.
Patients taking either a beta-blocker or antiplatelet, or both medications together, appeared at elevated risk for nonfatal acute MI specifically on days when the weather turned hot, suggests the registry cohort study that covered 14 years of clinical and meteorologic data.
“The take-away message is not that patients should stop using these two medications, by no means. We’re raising cautions for patients taking them, to watch out for themselves during high-heat days,” lead author Kai Chen, PhD, Yale University, New Haven, Conn., said in an interview.
“We’re not giving the message that these drugs have harmful effects” because the nature of the links between the medications and MI in the study, with its potential for confounding, remain unknown, said Dr. Chen, from the department of environmental health sciences and Yale Center on Climate Change and Health.
For example, patients who take beta-blockers or antiplatelets tend to be sicker than patients not on the drugs, which could make heat-related MI more likely, and the drugs wrongly appear to be culprits, he observed. The analysis contained signals that could support either scenario.
The study is based on cases of nonfatal MI in Augsburg, Germany, that are part of the MONICA-KORA MI registry. The odds of a heat-related nonfatal MI, it suggests, were increased 63% among patients taking antiplatelets and by 65% among those on beta-blockers, compared with those not on these drugs. The odds went up by 75% among those on both drug classes, but the risks weren’t raised in patients not taking them.
Rising heat-related MI
Chen said analysis was inspired by a 2019 report – also based on MONICA-KORA, from many of the same authors and using similar methods to track events by daily air temperature – that showed a rising trend for heat-related MI and declining rate for MI related to cold weather from 1987 to 2014. A next step, he figured, would be to determine whether the MI risk trends were associated with any cardiovascular medications.
The current study’s signal of risk related to antiplatelets and beta-blockers did not emerge for ACE inhibitors, calcium-channel blockers, or diuretics. Statins showed a link to increased nonfatal MI risk, but solely among participants aged younger than 60 years, who were also far less likely to have pre-existing coronary heart disease (CHD). He and his colleagues chose not to highlight that finding, Dr. Chen said, because the age subgroup analysis was grossly underpowered.
The overall analysis involved 2,494 cases of nonfatal MI that occurred during the warmer months – May to September – from 2001 to 2014. It was limited to nonfatal cases – those with at least a month of survival after hospital admission – because of insufficient data on medication use associated with fatal MIs, the report states.
Nonfatal MIs were defined as heat-related if they struck on days reaching the 95th percentile for temperature across the 14 years, in this case 24.2 °C (about 75.6 °F), relative to the average temperature of lowest nonfatal MI risk across the cohort, 7.5 °C (about 45.5 °F).
Patients served as both cases and their own controls, in that air temperature exposures on the day of their MI (case day) were compared with the remaining same days of the week in the same calendar month (control days). That approach, the report stated, “automatically controls for long-term time trends, seasonality, day of the week, and time-invariant confounders (for example, pre-existing cardiovascular disease).”
The odds ratio for heat-related MI for patients on antiplatelets was 1.63 (95% confidence interval, 1.07-2.46), and for antiplatelet nonusers was 0.94 (95% CI, 0.68-1.29). The difference between the two ratios was significant (P = .04).
The corresponding OR for patients taking beta-blockers was 1.65 (95% CI, 1.11-2.45), and for nonusers of beta-blockers was 0.90 (95% CI, 0.64-1.26). Again, the OR difference was significant (P = .02).
The ORs for users of both medication classes and nonusers of either med class, respectively, were 1.75 (95% CI, 1.12-2.73) and 0.84 (95% CI, 0.59-1.19). The latter OR was significantly lower than former (P = .01).
In a sign that antiplatelet and beta-blocker use might have been just a marker for sicker patients who were more vulnerable to heat-related MI, Chen said, the nonfatal MI risk was significantly elevated (OR, 2.17; 95% CI, 1.40-3.38) among patients with pre-existing CHD, but not among those free of pre-existing CHD (OR, 0.88; 95% CI, 0.65-1.20); the odds difference was P < .01.
That signal of confounding by indication is somewhat countered, the report states, by variations in nonfatal MI risk by age group. The increased chances of an event seen overall in relation to beta-blockers and antiplatelets were more pronounced among the 39% of patients aged 25-59 years (P < .01). That’s in spite that group’s lower CHD prevalence. The risk elevation solely among the older patients was attenuated and rendered nonsignificant, even with their greater CHD burden, the report noted.
The report speculates on a potential mechanism by which beta-blockers, at least, might conceivably raise the risk for heat-related MI. “Beta-receptor blockers inhibit skin vasodilation, resulting in reduced heat dissipation through convection and, at the same time, could intensify the blood-pressure-lowering effect of other antihypertensive drugs, which then could lead to syncope.”
Beta-blockers, Dr. Chen said, “can mechanistically make people more vulnerable to heat. That’s one potential explanation. Or it could be that these people taking the medications are just sicker. Whatever the reasons, the phenomenon we observed is that these patients taking these two medications are at higher risk during high-temperature days.”
Dr. Chen and the other authors declare no competing interests.
A version of this article first appeared on Medscape.com.
FROM NATURE CARDIOVASCULAR RESEARCH
Neuropathy drives hypoglycemia cluelessness in T1D
Researchers published the study covered in this summary on researchsquare.com as a preprint that has not yet been peer reviewed.
Key takeaways
- In Japanese adults with type 1 diabetes insulin-pump treatment (continuous subcutaneous insulin infusion) and higher problem-solving perception appear protective against impaired awareness of hypoglycemia (IAH), while diabetic peripheral neuropathy (DPN) is associated with increased risk.
- Diabetes distress and fear of hypoglycemia are common in people with IAH.
Why this matters
- Adults with type 1 diabetes and IAH have a reduced ability to perceive hypoglycemic symptoms and are at risk of severe hypoglycemic events because they are unable to take immediate corrective action.
- This is the first study to identify protective factors and risk factors of IAH in Japanese adults with type 1 diabetes.
- People with IAH may plan to loosen tight glucose management and intentionally omit insulin injection to prevent severe hypoglycemia.
- The information in this report may help improve the management of people with problematic hypoglycemia, the authors suggested. Treatment with an insulin pump and structured education aimed at improving problem-solving skills may be useful interventions for adults with type 1 diabetes and IAH, they suggested.
Study design
- The study involved a cross-sectional analysis of 288 Japanese adults with type 1 diabetes who averaged 50 years old, had diabetes for an average of about 18 years, had an average hemoglobin A1c at baseline of 7.7%, and included about 37% men and 63% women.
- The cohort included 55 people with IAH (19%) and 233 with no impairment of their hypoglycemia awareness, based on their score on the .
Key results
- DPN was significantly more prevalent in the IAH group than in the control group (12.0% vs. 26.5%). A logistic regression analysis showed that the odds ratio for DPN was 2.63-fold higher among people with IAH, compared with those without IAH, but there were no differences in other complications or by HbA1c levels.
- Treatment with continuous subcutaneous insulin therapy (an insulin pump) was significantly less prevalent in the IAH group, compared with those without IAH (23.6% vs 39.5%), with an adjusted odds ratio of 0.48. The two subgroups showed no differences in use of continuous glucose monitoring, used by 56% of the people in each of the two subgroups.
- The two subgroups showed no differences in their healthy lifestyle score, sleep debt, or rates of excessive drinking.
- Mean autonomic symptom scores for both sweating and shaking were significantly reduced in the IAH group, but no between-group differences appeared for palpations or hunger.
- All mean neuroglycopenic symptom scores were significantly lower in those without IAH, including confusion and speech difficulty.
- Scores for measures of diabetes distress and for the worry component of the fear of hypoglycemia were significantly higher in the IAH group, but there were no differences in other psychological measures.
- Higher were significantly associated with decreased IAH risk with a calculated odds ratio of 0.54, but other aspects of hypoglycemia problem-solving such as detection control, goal setting, and strategy evaluation showed no significant links.
Limitations
- The study used a cross-sectional design, which is not suited to making causal inferences.
- The authors characterized DPN as either present or absent. They did not evaluate or analyze the severity of peripheral neuropathy.
- The authors evaluated diabetic cardiac autonomic neuropathy (DCAN) by a person’s coefficient of variation of R-R intervals, and definitive diagnosis of DCAN required at least two positive results on a cardiac autonomic test. More vigorous evaluation using a more definitive assessment of DCAN is needed to relate DCAN and IAH status.
Disclosures
- The study received no commercial funding.
- The authors have disclosed no relevant financial relationships.
This is a summary of a preprint research study, “Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross- sectional analysis of baseline data from the PR-IAH study,” written by researchers at several hospitals in Japan, all affiliated with the National Hospital Organization, on Research Square. The study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on researchsquare.com as a preprint that has not yet been peer reviewed.
Key takeaways
- In Japanese adults with type 1 diabetes insulin-pump treatment (continuous subcutaneous insulin infusion) and higher problem-solving perception appear protective against impaired awareness of hypoglycemia (IAH), while diabetic peripheral neuropathy (DPN) is associated with increased risk.
- Diabetes distress and fear of hypoglycemia are common in people with IAH.
Why this matters
- Adults with type 1 diabetes and IAH have a reduced ability to perceive hypoglycemic symptoms and are at risk of severe hypoglycemic events because they are unable to take immediate corrective action.
- This is the first study to identify protective factors and risk factors of IAH in Japanese adults with type 1 diabetes.
- People with IAH may plan to loosen tight glucose management and intentionally omit insulin injection to prevent severe hypoglycemia.
- The information in this report may help improve the management of people with problematic hypoglycemia, the authors suggested. Treatment with an insulin pump and structured education aimed at improving problem-solving skills may be useful interventions for adults with type 1 diabetes and IAH, they suggested.
Study design
- The study involved a cross-sectional analysis of 288 Japanese adults with type 1 diabetes who averaged 50 years old, had diabetes for an average of about 18 years, had an average hemoglobin A1c at baseline of 7.7%, and included about 37% men and 63% women.
- The cohort included 55 people with IAH (19%) and 233 with no impairment of their hypoglycemia awareness, based on their score on the .
Key results
- DPN was significantly more prevalent in the IAH group than in the control group (12.0% vs. 26.5%). A logistic regression analysis showed that the odds ratio for DPN was 2.63-fold higher among people with IAH, compared with those without IAH, but there were no differences in other complications or by HbA1c levels.
- Treatment with continuous subcutaneous insulin therapy (an insulin pump) was significantly less prevalent in the IAH group, compared with those without IAH (23.6% vs 39.5%), with an adjusted odds ratio of 0.48. The two subgroups showed no differences in use of continuous glucose monitoring, used by 56% of the people in each of the two subgroups.
- The two subgroups showed no differences in their healthy lifestyle score, sleep debt, or rates of excessive drinking.
- Mean autonomic symptom scores for both sweating and shaking were significantly reduced in the IAH group, but no between-group differences appeared for palpations or hunger.
- All mean neuroglycopenic symptom scores were significantly lower in those without IAH, including confusion and speech difficulty.
- Scores for measures of diabetes distress and for the worry component of the fear of hypoglycemia were significantly higher in the IAH group, but there were no differences in other psychological measures.
- Higher were significantly associated with decreased IAH risk with a calculated odds ratio of 0.54, but other aspects of hypoglycemia problem-solving such as detection control, goal setting, and strategy evaluation showed no significant links.
Limitations
- The study used a cross-sectional design, which is not suited to making causal inferences.
- The authors characterized DPN as either present or absent. They did not evaluate or analyze the severity of peripheral neuropathy.
- The authors evaluated diabetic cardiac autonomic neuropathy (DCAN) by a person’s coefficient of variation of R-R intervals, and definitive diagnosis of DCAN required at least two positive results on a cardiac autonomic test. More vigorous evaluation using a more definitive assessment of DCAN is needed to relate DCAN and IAH status.
Disclosures
- The study received no commercial funding.
- The authors have disclosed no relevant financial relationships.
This is a summary of a preprint research study, “Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross- sectional analysis of baseline data from the PR-IAH study,” written by researchers at several hospitals in Japan, all affiliated with the National Hospital Organization, on Research Square. The study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on researchsquare.com as a preprint that has not yet been peer reviewed.
Key takeaways
- In Japanese adults with type 1 diabetes insulin-pump treatment (continuous subcutaneous insulin infusion) and higher problem-solving perception appear protective against impaired awareness of hypoglycemia (IAH), while diabetic peripheral neuropathy (DPN) is associated with increased risk.
- Diabetes distress and fear of hypoglycemia are common in people with IAH.
Why this matters
- Adults with type 1 diabetes and IAH have a reduced ability to perceive hypoglycemic symptoms and are at risk of severe hypoglycemic events because they are unable to take immediate corrective action.
- This is the first study to identify protective factors and risk factors of IAH in Japanese adults with type 1 diabetes.
- People with IAH may plan to loosen tight glucose management and intentionally omit insulin injection to prevent severe hypoglycemia.
- The information in this report may help improve the management of people with problematic hypoglycemia, the authors suggested. Treatment with an insulin pump and structured education aimed at improving problem-solving skills may be useful interventions for adults with type 1 diabetes and IAH, they suggested.
Study design
- The study involved a cross-sectional analysis of 288 Japanese adults with type 1 diabetes who averaged 50 years old, had diabetes for an average of about 18 years, had an average hemoglobin A1c at baseline of 7.7%, and included about 37% men and 63% women.
- The cohort included 55 people with IAH (19%) and 233 with no impairment of their hypoglycemia awareness, based on their score on the .
Key results
- DPN was significantly more prevalent in the IAH group than in the control group (12.0% vs. 26.5%). A logistic regression analysis showed that the odds ratio for DPN was 2.63-fold higher among people with IAH, compared with those without IAH, but there were no differences in other complications or by HbA1c levels.
- Treatment with continuous subcutaneous insulin therapy (an insulin pump) was significantly less prevalent in the IAH group, compared with those without IAH (23.6% vs 39.5%), with an adjusted odds ratio of 0.48. The two subgroups showed no differences in use of continuous glucose monitoring, used by 56% of the people in each of the two subgroups.
- The two subgroups showed no differences in their healthy lifestyle score, sleep debt, or rates of excessive drinking.
- Mean autonomic symptom scores for both sweating and shaking were significantly reduced in the IAH group, but no between-group differences appeared for palpations or hunger.
- All mean neuroglycopenic symptom scores were significantly lower in those without IAH, including confusion and speech difficulty.
- Scores for measures of diabetes distress and for the worry component of the fear of hypoglycemia were significantly higher in the IAH group, but there were no differences in other psychological measures.
- Higher were significantly associated with decreased IAH risk with a calculated odds ratio of 0.54, but other aspects of hypoglycemia problem-solving such as detection control, goal setting, and strategy evaluation showed no significant links.
Limitations
- The study used a cross-sectional design, which is not suited to making causal inferences.
- The authors characterized DPN as either present or absent. They did not evaluate or analyze the severity of peripheral neuropathy.
- The authors evaluated diabetic cardiac autonomic neuropathy (DCAN) by a person’s coefficient of variation of R-R intervals, and definitive diagnosis of DCAN required at least two positive results on a cardiac autonomic test. More vigorous evaluation using a more definitive assessment of DCAN is needed to relate DCAN and IAH status.
Disclosures
- The study received no commercial funding.
- The authors have disclosed no relevant financial relationships.
This is a summary of a preprint research study, “Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross- sectional analysis of baseline data from the PR-IAH study,” written by researchers at several hospitals in Japan, all affiliated with the National Hospital Organization, on Research Square. The study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com.
A version of this article first appeared on Medscape.com.
Omecamtiv mecarbil fails to improve exercise capacity in HFrEF
Treatment with the novel agent omecamtiv mecarbil did not improve exercise capacity in people with chronic heart failure with reduced ejection fraction (HFrEF), in the METEORIC-HF trial.
The double-blind, phase 3 study failed to achieve its primary endpoint of change in peak oxygen uptake (VO2) after 20 weeks of treatment with omecamtiv mecarbil, compared with placebo.
There also was no benefit on secondary measures of total workload, ventilatory efficiency, and daily physical activity, according to results presented earlier this year at ACC 2022 and formally published this month in JAMA.
“These findings do not support the use of omecamtiv mecarbil for treatment of HFrEF for improvement of exercise capacity,” lead author Gregory D. Lewis, MD, Massachusetts General Hospital, Boston, and colleagues conclude in the paper.
Researchers had hoped that the oral selective myosin activator would prove useful in this subset of patients, having previously shown in the GALACTIC-HF trial to provide a significant improvement in heart failure (HF) events and cardiovascular death.
A prespecified subgroup analysis from that trial also found that HF patients with the lowest ejection fraction derived the greatest relative benefit from omecamtiv mecarbil.
“The lack of effect of omecamtiv mecarbil on exercise performance is inconsistent with its known mechanism of action of directly enhancing ventricular performance and reducing the risk of cardiovascular events,” Dr. Lewis and colleagues observe.
The drug’s novel mechanism of action, direct activation of myosin, contrasts with that of currently available inotropic agents, such as dobutamine or milrinone. It is not yet approved by the U.S. Food and Drug Administration but is scheduled for an advisory committee meeting on Dec. 13, 2022, and has been assigned a Prescription Drug User Fee Act date of Feb. 28, 2023.
METEORIC-HF randomly assigned 276 patients with New York Heart Association class II or III symptoms and a left ventricular ejection fraction of 35% or less to omecamtiv mecarbil (n = 185) or placebo (n = 91), given orally twice daily at a dose of 25 mg, 37.5 mg, or 50 mg based on target plasma levels for 20 weeks, on top of guideline-directed medical therapy.
The patients’ median age was 64 years and 15% were women. The median ejection fraction was 28% and median baseline peak VO2 was 14.2 mL/kg per minute in the omecamtiv mecarbil group and 15.0 mL/kg per minute in the control group.
At 20 weeks, the mean change in peak VO2 in the omecamtiv mecarbil group was –0.24 mL/kg per minute and 0.21 mL/kg per minute in the placebo group (95% confidence interval, –1.02-0.13; P = .13).
For the secondary outcomes, the change in workload achieved on stress testing declined in the omecamtiv mecarbil group (–3.8 vs. 1.6). The drug had a neutral effect on minute ventilation relative to carbon dioxide production throughout exercise (0.28 vs. –0.14 VE/VCO2 slope) and average total daily activity units, measured over 2 weeks by accelerometer (–0.2 vs. –0.5).
The authors suggest that “one possible explanation for discordance between clinical events in a long-term follow-up study and exercise capacity improvement is that cardiac performance was not exclusively responsible for limiting exercise capacity in trial participants with HFrEF who were stable and very well treated with both pharmacologic and device HFrEF therapy.”
In an accompanying editorial, Mark H. Drazner, MD, MSc, University of Texas Southwestern Medical Center, Dallas, writes that another possible explanation is that participants in METEORIC-HF had less severe heart failure, compared with participants in GALACTIC-HF, and so were less likely to benefit from omecamtiv mecarbil.
METEORIC-HF excluded participants who had a HF hospitalization that required intravenous diuretics in the preceding 3 months, whereas 25% of participants in GALACTIC-HF were inpatients for decompensated HF and 36% had a HF hospitalization within the preceding 3 months.
Another plausible explanation for the differing results is that a therapy that improves long-term clinical outcomes may not improve exercise capacity, Dr. Drazner writes. “The available data are persuasive to suggest this may be the case.”
Some pharmacologic therapies, such as flosequinan, improved exercise capacity in patients with HF yet increased long-term mortality, he noted. Several medications that have a class I recommendation in the 2022 Heart Failure Guideline for the treatment of HFrEF also have not been shown to improve exercise capacity, as measured by peak VO2 or by 6-minute walk distance.
In this context, Dr. Drazner said he doesn’t anticipate the METEORIC-HF findings to derail FDA approval. However, should the drug be approved, clinicians will have increasingly complex decisions to make about which therapies should be prescribed to which patients.
“Some clinicians may contemplate using omecamtiv mecarbil either in the subgroup of patients with very low ejection fractions or more severe disease, believing this strategy will maximize the benefits of this therapy, but those approaches should be pursued with caution given they are predicated on subgroup and post hoc analyses, respectively,” he wrote.
Dr. Drazner concludes that medications known to improve survival in patients with HFrEF are used at “disappointingly low rates and suboptimal doses in the United States. Implementation strategies to improve use of such therapies are needed, and those efforts should be prioritized before adoption of therapies that reduce morbidity but not cardiovascular mortality.”
The study was sponsored by Amgen and Cytokinetics. Dr. Lewis reports financial relationships with the National Institutes of Health, American Heart Association, Amgen, Cytokinetics, Applied Therapeutics, AstraZeneca, SoniVie, Pfizer, Merck, Boehringer Ingelheim, Novartis, American Regent, Cyclerion, MyoKardia, Novo Nordisk, and UpToDate. Dr. Drazner reports being a member of the writing committee of the 2022 Heart Failure guidelines; and that he is supported by the James M. Wooten Chair in Cardiology at the University of Texas Southwestern Medical Center, which was a clinical site in METEORIC-HF. However, Dr. Drazner was not a study investigator in the trial.
A version of this article first appeared on Medscape.com.
Treatment with the novel agent omecamtiv mecarbil did not improve exercise capacity in people with chronic heart failure with reduced ejection fraction (HFrEF), in the METEORIC-HF trial.
The double-blind, phase 3 study failed to achieve its primary endpoint of change in peak oxygen uptake (VO2) after 20 weeks of treatment with omecamtiv mecarbil, compared with placebo.
There also was no benefit on secondary measures of total workload, ventilatory efficiency, and daily physical activity, according to results presented earlier this year at ACC 2022 and formally published this month in JAMA.
“These findings do not support the use of omecamtiv mecarbil for treatment of HFrEF for improvement of exercise capacity,” lead author Gregory D. Lewis, MD, Massachusetts General Hospital, Boston, and colleagues conclude in the paper.
Researchers had hoped that the oral selective myosin activator would prove useful in this subset of patients, having previously shown in the GALACTIC-HF trial to provide a significant improvement in heart failure (HF) events and cardiovascular death.
A prespecified subgroup analysis from that trial also found that HF patients with the lowest ejection fraction derived the greatest relative benefit from omecamtiv mecarbil.
“The lack of effect of omecamtiv mecarbil on exercise performance is inconsistent with its known mechanism of action of directly enhancing ventricular performance and reducing the risk of cardiovascular events,” Dr. Lewis and colleagues observe.
The drug’s novel mechanism of action, direct activation of myosin, contrasts with that of currently available inotropic agents, such as dobutamine or milrinone. It is not yet approved by the U.S. Food and Drug Administration but is scheduled for an advisory committee meeting on Dec. 13, 2022, and has been assigned a Prescription Drug User Fee Act date of Feb. 28, 2023.
METEORIC-HF randomly assigned 276 patients with New York Heart Association class II or III symptoms and a left ventricular ejection fraction of 35% or less to omecamtiv mecarbil (n = 185) or placebo (n = 91), given orally twice daily at a dose of 25 mg, 37.5 mg, or 50 mg based on target plasma levels for 20 weeks, on top of guideline-directed medical therapy.
The patients’ median age was 64 years and 15% were women. The median ejection fraction was 28% and median baseline peak VO2 was 14.2 mL/kg per minute in the omecamtiv mecarbil group and 15.0 mL/kg per minute in the control group.
At 20 weeks, the mean change in peak VO2 in the omecamtiv mecarbil group was –0.24 mL/kg per minute and 0.21 mL/kg per minute in the placebo group (95% confidence interval, –1.02-0.13; P = .13).
For the secondary outcomes, the change in workload achieved on stress testing declined in the omecamtiv mecarbil group (–3.8 vs. 1.6). The drug had a neutral effect on minute ventilation relative to carbon dioxide production throughout exercise (0.28 vs. –0.14 VE/VCO2 slope) and average total daily activity units, measured over 2 weeks by accelerometer (–0.2 vs. –0.5).
The authors suggest that “one possible explanation for discordance between clinical events in a long-term follow-up study and exercise capacity improvement is that cardiac performance was not exclusively responsible for limiting exercise capacity in trial participants with HFrEF who were stable and very well treated with both pharmacologic and device HFrEF therapy.”
In an accompanying editorial, Mark H. Drazner, MD, MSc, University of Texas Southwestern Medical Center, Dallas, writes that another possible explanation is that participants in METEORIC-HF had less severe heart failure, compared with participants in GALACTIC-HF, and so were less likely to benefit from omecamtiv mecarbil.
METEORIC-HF excluded participants who had a HF hospitalization that required intravenous diuretics in the preceding 3 months, whereas 25% of participants in GALACTIC-HF were inpatients for decompensated HF and 36% had a HF hospitalization within the preceding 3 months.
Another plausible explanation for the differing results is that a therapy that improves long-term clinical outcomes may not improve exercise capacity, Dr. Drazner writes. “The available data are persuasive to suggest this may be the case.”
Some pharmacologic therapies, such as flosequinan, improved exercise capacity in patients with HF yet increased long-term mortality, he noted. Several medications that have a class I recommendation in the 2022 Heart Failure Guideline for the treatment of HFrEF also have not been shown to improve exercise capacity, as measured by peak VO2 or by 6-minute walk distance.
In this context, Dr. Drazner said he doesn’t anticipate the METEORIC-HF findings to derail FDA approval. However, should the drug be approved, clinicians will have increasingly complex decisions to make about which therapies should be prescribed to which patients.
“Some clinicians may contemplate using omecamtiv mecarbil either in the subgroup of patients with very low ejection fractions or more severe disease, believing this strategy will maximize the benefits of this therapy, but those approaches should be pursued with caution given they are predicated on subgroup and post hoc analyses, respectively,” he wrote.
Dr. Drazner concludes that medications known to improve survival in patients with HFrEF are used at “disappointingly low rates and suboptimal doses in the United States. Implementation strategies to improve use of such therapies are needed, and those efforts should be prioritized before adoption of therapies that reduce morbidity but not cardiovascular mortality.”
The study was sponsored by Amgen and Cytokinetics. Dr. Lewis reports financial relationships with the National Institutes of Health, American Heart Association, Amgen, Cytokinetics, Applied Therapeutics, AstraZeneca, SoniVie, Pfizer, Merck, Boehringer Ingelheim, Novartis, American Regent, Cyclerion, MyoKardia, Novo Nordisk, and UpToDate. Dr. Drazner reports being a member of the writing committee of the 2022 Heart Failure guidelines; and that he is supported by the James M. Wooten Chair in Cardiology at the University of Texas Southwestern Medical Center, which was a clinical site in METEORIC-HF. However, Dr. Drazner was not a study investigator in the trial.
A version of this article first appeared on Medscape.com.
Treatment with the novel agent omecamtiv mecarbil did not improve exercise capacity in people with chronic heart failure with reduced ejection fraction (HFrEF), in the METEORIC-HF trial.
The double-blind, phase 3 study failed to achieve its primary endpoint of change in peak oxygen uptake (VO2) after 20 weeks of treatment with omecamtiv mecarbil, compared with placebo.
There also was no benefit on secondary measures of total workload, ventilatory efficiency, and daily physical activity, according to results presented earlier this year at ACC 2022 and formally published this month in JAMA.
“These findings do not support the use of omecamtiv mecarbil for treatment of HFrEF for improvement of exercise capacity,” lead author Gregory D. Lewis, MD, Massachusetts General Hospital, Boston, and colleagues conclude in the paper.
Researchers had hoped that the oral selective myosin activator would prove useful in this subset of patients, having previously shown in the GALACTIC-HF trial to provide a significant improvement in heart failure (HF) events and cardiovascular death.
A prespecified subgroup analysis from that trial also found that HF patients with the lowest ejection fraction derived the greatest relative benefit from omecamtiv mecarbil.
“The lack of effect of omecamtiv mecarbil on exercise performance is inconsistent with its known mechanism of action of directly enhancing ventricular performance and reducing the risk of cardiovascular events,” Dr. Lewis and colleagues observe.
The drug’s novel mechanism of action, direct activation of myosin, contrasts with that of currently available inotropic agents, such as dobutamine or milrinone. It is not yet approved by the U.S. Food and Drug Administration but is scheduled for an advisory committee meeting on Dec. 13, 2022, and has been assigned a Prescription Drug User Fee Act date of Feb. 28, 2023.
METEORIC-HF randomly assigned 276 patients with New York Heart Association class II or III symptoms and a left ventricular ejection fraction of 35% or less to omecamtiv mecarbil (n = 185) or placebo (n = 91), given orally twice daily at a dose of 25 mg, 37.5 mg, or 50 mg based on target plasma levels for 20 weeks, on top of guideline-directed medical therapy.
The patients’ median age was 64 years and 15% were women. The median ejection fraction was 28% and median baseline peak VO2 was 14.2 mL/kg per minute in the omecamtiv mecarbil group and 15.0 mL/kg per minute in the control group.
At 20 weeks, the mean change in peak VO2 in the omecamtiv mecarbil group was –0.24 mL/kg per minute and 0.21 mL/kg per minute in the placebo group (95% confidence interval, –1.02-0.13; P = .13).
For the secondary outcomes, the change in workload achieved on stress testing declined in the omecamtiv mecarbil group (–3.8 vs. 1.6). The drug had a neutral effect on minute ventilation relative to carbon dioxide production throughout exercise (0.28 vs. –0.14 VE/VCO2 slope) and average total daily activity units, measured over 2 weeks by accelerometer (–0.2 vs. –0.5).
The authors suggest that “one possible explanation for discordance between clinical events in a long-term follow-up study and exercise capacity improvement is that cardiac performance was not exclusively responsible for limiting exercise capacity in trial participants with HFrEF who were stable and very well treated with both pharmacologic and device HFrEF therapy.”
In an accompanying editorial, Mark H. Drazner, MD, MSc, University of Texas Southwestern Medical Center, Dallas, writes that another possible explanation is that participants in METEORIC-HF had less severe heart failure, compared with participants in GALACTIC-HF, and so were less likely to benefit from omecamtiv mecarbil.
METEORIC-HF excluded participants who had a HF hospitalization that required intravenous diuretics in the preceding 3 months, whereas 25% of participants in GALACTIC-HF were inpatients for decompensated HF and 36% had a HF hospitalization within the preceding 3 months.
Another plausible explanation for the differing results is that a therapy that improves long-term clinical outcomes may not improve exercise capacity, Dr. Drazner writes. “The available data are persuasive to suggest this may be the case.”
Some pharmacologic therapies, such as flosequinan, improved exercise capacity in patients with HF yet increased long-term mortality, he noted. Several medications that have a class I recommendation in the 2022 Heart Failure Guideline for the treatment of HFrEF also have not been shown to improve exercise capacity, as measured by peak VO2 or by 6-minute walk distance.
In this context, Dr. Drazner said he doesn’t anticipate the METEORIC-HF findings to derail FDA approval. However, should the drug be approved, clinicians will have increasingly complex decisions to make about which therapies should be prescribed to which patients.
“Some clinicians may contemplate using omecamtiv mecarbil either in the subgroup of patients with very low ejection fractions or more severe disease, believing this strategy will maximize the benefits of this therapy, but those approaches should be pursued with caution given they are predicated on subgroup and post hoc analyses, respectively,” he wrote.
Dr. Drazner concludes that medications known to improve survival in patients with HFrEF are used at “disappointingly low rates and suboptimal doses in the United States. Implementation strategies to improve use of such therapies are needed, and those efforts should be prioritized before adoption of therapies that reduce morbidity but not cardiovascular mortality.”
The study was sponsored by Amgen and Cytokinetics. Dr. Lewis reports financial relationships with the National Institutes of Health, American Heart Association, Amgen, Cytokinetics, Applied Therapeutics, AstraZeneca, SoniVie, Pfizer, Merck, Boehringer Ingelheim, Novartis, American Regent, Cyclerion, MyoKardia, Novo Nordisk, and UpToDate. Dr. Drazner reports being a member of the writing committee of the 2022 Heart Failure guidelines; and that he is supported by the James M. Wooten Chair in Cardiology at the University of Texas Southwestern Medical Center, which was a clinical site in METEORIC-HF. However, Dr. Drazner was not a study investigator in the trial.
A version of this article first appeared on Medscape.com.
Onset and awareness of hypertension varies by race, ethnicity
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.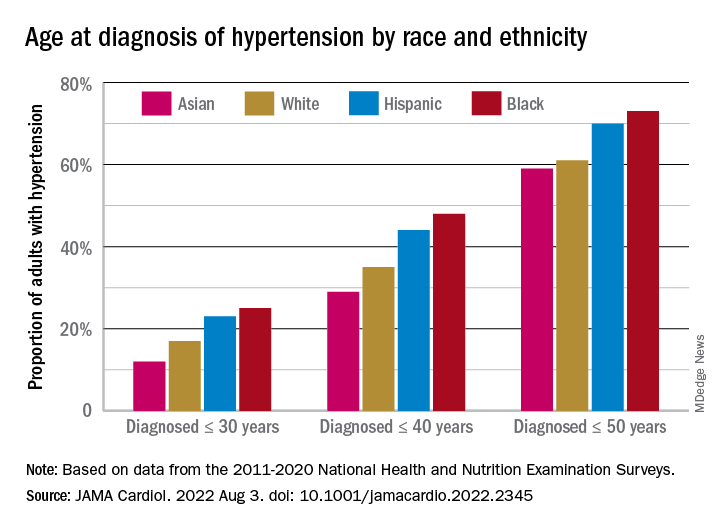
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
FROM JAMA CARDIOLOGY
One in eight COVID patients likely to develop long COVID: Large study
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
Long COVID doubles risk of some serious outcomes in children, teens
Researchers from the Centers for Disease Control and Prevention report that
Heart inflammation; a blood clot in the lung; or a blood clot in the lower leg, thigh, or pelvis were the most common bad outcomes in a new study. Even though the risk was higher for these and some other serious events, the overall numbers were small.
“Many of these conditions were rare or uncommon among children in this analysis, but even a small increase in these conditions is notable,” a CDC new release stated.
The investigators said their findings stress the importance of COVID-19 vaccination in Americans under the age of 18.
The study was published online in the CDC’s Morbidity and Mortality Weekly Report.
Less is known about long COVID in children
Lyudmyla Kompaniyets, PhD, and colleagues noted that most research on long COVID to date has been done in adults, so little information is available about the risks to Americans ages 17 and younger.
To learn more, they compared post–COVID-19 symptoms and conditions between 781,419 children and teenagers with confirmed COVID-19 to another 2,344,257 without COVID-19. They looked at medical claims and laboratory data for these children and teenagers from March 1, 2020, through Jan. 31, 2022, to see who got any of 15 specific outcomes linked to long COVID-19.
Long COVID was defined as a condition where symptoms that last for or begin at least 4 weeks after a COVID-19 diagnosis.
Compared to children with no history of a COVID-19 diagnosis, the long COVID-19 group was 101% more likely to have an acute pulmonary embolism, 99% more likely to have myocarditis or cardiomyopathy, 87% more likely to have a venous thromboembolic event, 32% more likely to have acute and unspecified renal failure, and 23% more likely to have type 1 diabetes.
“This report points to the fact that the risks of COVID infection itself, both in terms of the acute effects, MIS-C [multisystem inflammatory syndrome in children], as well as the long-term effects, are real, are concerning, and are potentially very serious,” said Stuart Berger, MD, chair of the American Academy of Pediatrics Section on Cardiology and Cardiac Surgery.
“The message that we should take away from this is that we should be very keen on all the methods of prevention for COVID, especially the vaccine,” said Dr. Berger, chief of cardiology in the department of pediatrics at Northwestern University in Chicago.
A ‘wake-up call’
The study findings are “sobering” and are “a reminder of the seriousness of COVID infection,” says Gregory Poland, MD, an infectious disease expert at the Mayo Clinic in Rochester, Minn.
“When you look in particular at the more serious complications from COVID in this young age group, those are life-altering complications that will have consequences and ramifications throughout their lives,” he said.
“I would take this as a serious wake-up call to parents [at a time when] the immunization rates in younger children are so pitifully low,” Dr. Poland said.
Still early days
The study is suggestive but not definitive, said Peter Katona, MD, professor of medicine and infectious diseases expert at the UCLA Fielding School of Public Health.
It’s still too early to draw conclusions about long COVID, including in children, because many questions remain, he said: Should long COVID be defined as symptoms at 1 month or 3 months after infection? How do you define brain fog?
Dr. Katona and colleagues are studying long COVID intervention among students at UCLA to answer some of these questions, including the incidence and effect of early intervention.
The study had “at least seven limitations,” the researchers noted. Among them was the use of medical claims data that noted long COVID outcomes but not how severe they were; some people in the no COVID group might have had the illness but not been diagnosed; and the researchers did not adjust for vaccination status.
Dr. Poland noted that the study was done during surges in COVID variants including Delta and Omicron. In other words, any long COVID effects linked to more recent variants such as BA.5 or BA.2.75 are unknown.
A version of this article first appeared on WebMD.com.
Researchers from the Centers for Disease Control and Prevention report that
Heart inflammation; a blood clot in the lung; or a blood clot in the lower leg, thigh, or pelvis were the most common bad outcomes in a new study. Even though the risk was higher for these and some other serious events, the overall numbers were small.
“Many of these conditions were rare or uncommon among children in this analysis, but even a small increase in these conditions is notable,” a CDC new release stated.
The investigators said their findings stress the importance of COVID-19 vaccination in Americans under the age of 18.
The study was published online in the CDC’s Morbidity and Mortality Weekly Report.
Less is known about long COVID in children
Lyudmyla Kompaniyets, PhD, and colleagues noted that most research on long COVID to date has been done in adults, so little information is available about the risks to Americans ages 17 and younger.
To learn more, they compared post–COVID-19 symptoms and conditions between 781,419 children and teenagers with confirmed COVID-19 to another 2,344,257 without COVID-19. They looked at medical claims and laboratory data for these children and teenagers from March 1, 2020, through Jan. 31, 2022, to see who got any of 15 specific outcomes linked to long COVID-19.
Long COVID was defined as a condition where symptoms that last for or begin at least 4 weeks after a COVID-19 diagnosis.
Compared to children with no history of a COVID-19 diagnosis, the long COVID-19 group was 101% more likely to have an acute pulmonary embolism, 99% more likely to have myocarditis or cardiomyopathy, 87% more likely to have a venous thromboembolic event, 32% more likely to have acute and unspecified renal failure, and 23% more likely to have type 1 diabetes.
“This report points to the fact that the risks of COVID infection itself, both in terms of the acute effects, MIS-C [multisystem inflammatory syndrome in children], as well as the long-term effects, are real, are concerning, and are potentially very serious,” said Stuart Berger, MD, chair of the American Academy of Pediatrics Section on Cardiology and Cardiac Surgery.
“The message that we should take away from this is that we should be very keen on all the methods of prevention for COVID, especially the vaccine,” said Dr. Berger, chief of cardiology in the department of pediatrics at Northwestern University in Chicago.
A ‘wake-up call’
The study findings are “sobering” and are “a reminder of the seriousness of COVID infection,” says Gregory Poland, MD, an infectious disease expert at the Mayo Clinic in Rochester, Minn.
“When you look in particular at the more serious complications from COVID in this young age group, those are life-altering complications that will have consequences and ramifications throughout their lives,” he said.
“I would take this as a serious wake-up call to parents [at a time when] the immunization rates in younger children are so pitifully low,” Dr. Poland said.
Still early days
The study is suggestive but not definitive, said Peter Katona, MD, professor of medicine and infectious diseases expert at the UCLA Fielding School of Public Health.
It’s still too early to draw conclusions about long COVID, including in children, because many questions remain, he said: Should long COVID be defined as symptoms at 1 month or 3 months after infection? How do you define brain fog?
Dr. Katona and colleagues are studying long COVID intervention among students at UCLA to answer some of these questions, including the incidence and effect of early intervention.
The study had “at least seven limitations,” the researchers noted. Among them was the use of medical claims data that noted long COVID outcomes but not how severe they were; some people in the no COVID group might have had the illness but not been diagnosed; and the researchers did not adjust for vaccination status.
Dr. Poland noted that the study was done during surges in COVID variants including Delta and Omicron. In other words, any long COVID effects linked to more recent variants such as BA.5 or BA.2.75 are unknown.
A version of this article first appeared on WebMD.com.
Researchers from the Centers for Disease Control and Prevention report that
Heart inflammation; a blood clot in the lung; or a blood clot in the lower leg, thigh, or pelvis were the most common bad outcomes in a new study. Even though the risk was higher for these and some other serious events, the overall numbers were small.
“Many of these conditions were rare or uncommon among children in this analysis, but even a small increase in these conditions is notable,” a CDC new release stated.
The investigators said their findings stress the importance of COVID-19 vaccination in Americans under the age of 18.
The study was published online in the CDC’s Morbidity and Mortality Weekly Report.
Less is known about long COVID in children
Lyudmyla Kompaniyets, PhD, and colleagues noted that most research on long COVID to date has been done in adults, so little information is available about the risks to Americans ages 17 and younger.
To learn more, they compared post–COVID-19 symptoms and conditions between 781,419 children and teenagers with confirmed COVID-19 to another 2,344,257 without COVID-19. They looked at medical claims and laboratory data for these children and teenagers from March 1, 2020, through Jan. 31, 2022, to see who got any of 15 specific outcomes linked to long COVID-19.
Long COVID was defined as a condition where symptoms that last for or begin at least 4 weeks after a COVID-19 diagnosis.
Compared to children with no history of a COVID-19 diagnosis, the long COVID-19 group was 101% more likely to have an acute pulmonary embolism, 99% more likely to have myocarditis or cardiomyopathy, 87% more likely to have a venous thromboembolic event, 32% more likely to have acute and unspecified renal failure, and 23% more likely to have type 1 diabetes.
“This report points to the fact that the risks of COVID infection itself, both in terms of the acute effects, MIS-C [multisystem inflammatory syndrome in children], as well as the long-term effects, are real, are concerning, and are potentially very serious,” said Stuart Berger, MD, chair of the American Academy of Pediatrics Section on Cardiology and Cardiac Surgery.
“The message that we should take away from this is that we should be very keen on all the methods of prevention for COVID, especially the vaccine,” said Dr. Berger, chief of cardiology in the department of pediatrics at Northwestern University in Chicago.
A ‘wake-up call’
The study findings are “sobering” and are “a reminder of the seriousness of COVID infection,” says Gregory Poland, MD, an infectious disease expert at the Mayo Clinic in Rochester, Minn.
“When you look in particular at the more serious complications from COVID in this young age group, those are life-altering complications that will have consequences and ramifications throughout their lives,” he said.
“I would take this as a serious wake-up call to parents [at a time when] the immunization rates in younger children are so pitifully low,” Dr. Poland said.
Still early days
The study is suggestive but not definitive, said Peter Katona, MD, professor of medicine and infectious diseases expert at the UCLA Fielding School of Public Health.
It’s still too early to draw conclusions about long COVID, including in children, because many questions remain, he said: Should long COVID be defined as symptoms at 1 month or 3 months after infection? How do you define brain fog?
Dr. Katona and colleagues are studying long COVID intervention among students at UCLA to answer some of these questions, including the incidence and effect of early intervention.
The study had “at least seven limitations,” the researchers noted. Among them was the use of medical claims data that noted long COVID outcomes but not how severe they were; some people in the no COVID group might have had the illness but not been diagnosed; and the researchers did not adjust for vaccination status.
Dr. Poland noted that the study was done during surges in COVID variants including Delta and Omicron. In other words, any long COVID effects linked to more recent variants such as BA.5 or BA.2.75 are unknown.
A version of this article first appeared on WebMD.com.
FROM THE MMWR








