User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Autoimmune diseases linked to spike in post-MI events
, in a large propensity-matched analysis.
At a median of 2 years after their MI, Medicare beneficiaries with an IMID had adjusted risks that were:
- 15% higher for all-cause death (hazard ratio, 1.15);
- 12% higher for heart failure (HR, 1.12);
- 8% higher for recurrent MI (HR, 1.08); and
- 6% higher risk for coronary reintervention (HR, 1.06; P < .05 for all).
In addition, interventions known to improve outcomes in this context, such as coronary revascularization, were less common in patients with IMID.
“This could be because they usually are sicker and have more risk factors when they present, like kidney disease, so maybe they’re not eligible for the therapy. But by itself, it was surprising they’re not offered these interventions as common[ly] as people who don’t have the disease,” Amgad Mentias, MD, a clinical cardiologist at the Cleveland Clinic, said in an interview.
The study was published Sept. 14 in the Journal of the American Heart Association, with Dr. Mentias as senior author and Heba Wassif, MD, MPH, also with Cleveland Clinic, as first author.
IMIDs, such as rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel disease, are known to be associated with significantly higher cardiovascular disease (CVD) risk due to a greater prevalence of traditional CVD risk factors and chronic systemic inflammation.
Certain disease-modifying agents may also increase patients’ cardiovascular risk. This has been a long-simmering issue for the arthritis and ulcerative colitis drug tofacitinib (Xeljanz, Xeljanz XR), resulting in an updated boxed warning in 2021.
Many of these patients also have joint disease, pain, and fatigue, which can limit physical activity, Dr. Mentias said. “So these small nuances of how to manage these patients, or balance between controlling the inflammation but also improv[ing] cardiac risk factors, is not an easy task.”
Evidence regarding post-MI events has been inconsistent and limited to smaller single-center studies, he said. After propensity-score matching, the present study included 59,820 patients with and 178,547 patients without rheumatic IMIDs followed for a maximum of 6 years.
They were drawn from a cohort of 1.6 million persons aged 65 or older in the Medicare Provider Analysis and Review (MedPAR) file who had been admitted for an MI between 2014 and 2019. Of these, 60,072 had a prior history of rheumatic IMIDs, most commonly rheumatoid arthritis (77.8%), followed by systemic lupus erythematosus (12.2%), psoriasis (5.1%), systemic sclerosis (2.8%), and myositis/dermatomyositis (1.8%).
Patients with an IMID were more often women; had a higher prevalence of valve disease, pulmonary hypertension, hypothyroidism, and anemia; and were more likely to present with non–ST-segment MI (NSTEMI).
Rates of coronary angiography (46.1% vs. 51.5%), percutaneous coronary intervention (31.6% vs. 33.6%), and coronary artery bypass grafting (7.7% vs. 10.7%) were significantly lower in patients with IMIDs who had NSTEMI, compared with patients without an IMID who had NSTEMI. Rates of these interventions were also lower in patients with IMIDs who presented with STEMI versus their peers without an IMID, at 78.2% vs. 80.7%, 70.2% vs. 71.5%, and 4.9% vs. 6.4%, respectively.
Dr. Mentias pointed out that the emerging subspecialty of cardiorheumatology is gaining traction, especially at large hospitals and academic centers, but that less than one-third of persons in the United States with an IMID are likely to be under the care of such specialists.
“It’s important before developing an MI to try and control the different risk factors and improve the risk profile for these patients as much as possible by both specialties, and then, after an unfortunate event like MI happens, it’s important to make sure we offer therapies and treatments that are known to improve outcomes,” he said.
Commenting for this article, Jon Tyler Giles, MD, a clinical researcher who focuses on cardiovascular diseases in rheumatology at Columbia University Vagelos College of Physicians and Surgeons, New York, said that “at least for rheumatoid arthritis, this is something that we already knew. People with rheumatic arthritis, when they have a heart attack, are less likely to get the standard kind of treatments and have worse outcomes. This is a little larger sample, but it’s not a surprise, not a surprise at all.”
He noted that the study could have answered questions regarding potential drivers, but “they didn’t dig down into any of the factors that were associated with the poorer outcomes in the patients with rheumatoid arthritis and lupus and scleroderma.”
Indeed, the investigators acknowledge that the study lacked information on coronary anatomy, duration and severity of the autoimmune disease, imaging data, and medications such as anti-inflammatory or immune-targeted therapies.
Dr. Giles highlighted several factors that can contribute to a poorer post-MI prognosis in patients with rheumatic diseases; these include frailty, being more hypercoaguable, increased rates of myocardial dysfunction and other heart and blood vessel diseases, and chronic treatment with steroids and nonsteroidal anti-inflammatory drugs that often interferes with anticoagulation after a MI or when putting in a stent. “So, there’s lot of moving parts, and not one single thing that is likely the answer.”
In addition, he said, “there’s always going to be a portion of these patients who, despite doing the best that we can with treatment, are going to have very severe disease. That may or may not be the subset of patients that did the worst, but likely they’re overrepresented in those patients.”
Finally, the inability to move the needle may lie with the lack of evidence-based screening and management guidelines for cardiovascular disease in any rheumatic disease, Dr. Giles observed. “There’s no guideline for us to use to decide who needs screening over and above what’s recommended for the general population, and then, even if you do screen, what do you do other than what you would normally?”
Rheumatologists are often reluctant to take up the cardiovascular screening side of things because visits are short, and a lot of that time is spent trying to manage the inflammatory components of a patient’s disease, he said. There’s also a barrier in getting some patients to add a cardiologist to the mix of physicians they already see, especially if they don’t have any symptoms.
“If someone has had an event, it’s a lot easier for people to be convinced to go see the cardiologist, obviously, but prior to having an event, the preventative side of things is something that often gets missed or goes to the wayside,” Dr. Giles said.
The study was partly funded by philanthropic gifts by the Haslam family, Bailey family, and Khouri family to the Cleveland Clinic for coauthor Dr. Milind Desai’s research. Dr. Desai is a consultant for Medtronic and Bristol Myers Squibb and serves on an executive steering committee of a BMS-sponsored trial. The remaining authors report having no relevant disclosures. Dr. Giles is a consultant on drug cardiovascular safety for Pfizer, AbbVie, and Eli Lilly.
A version of this article first appeared on Medscape.com.
, in a large propensity-matched analysis.
At a median of 2 years after their MI, Medicare beneficiaries with an IMID had adjusted risks that were:
- 15% higher for all-cause death (hazard ratio, 1.15);
- 12% higher for heart failure (HR, 1.12);
- 8% higher for recurrent MI (HR, 1.08); and
- 6% higher risk for coronary reintervention (HR, 1.06; P < .05 for all).
In addition, interventions known to improve outcomes in this context, such as coronary revascularization, were less common in patients with IMID.
“This could be because they usually are sicker and have more risk factors when they present, like kidney disease, so maybe they’re not eligible for the therapy. But by itself, it was surprising they’re not offered these interventions as common[ly] as people who don’t have the disease,” Amgad Mentias, MD, a clinical cardiologist at the Cleveland Clinic, said in an interview.
The study was published Sept. 14 in the Journal of the American Heart Association, with Dr. Mentias as senior author and Heba Wassif, MD, MPH, also with Cleveland Clinic, as first author.
IMIDs, such as rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel disease, are known to be associated with significantly higher cardiovascular disease (CVD) risk due to a greater prevalence of traditional CVD risk factors and chronic systemic inflammation.
Certain disease-modifying agents may also increase patients’ cardiovascular risk. This has been a long-simmering issue for the arthritis and ulcerative colitis drug tofacitinib (Xeljanz, Xeljanz XR), resulting in an updated boxed warning in 2021.
Many of these patients also have joint disease, pain, and fatigue, which can limit physical activity, Dr. Mentias said. “So these small nuances of how to manage these patients, or balance between controlling the inflammation but also improv[ing] cardiac risk factors, is not an easy task.”
Evidence regarding post-MI events has been inconsistent and limited to smaller single-center studies, he said. After propensity-score matching, the present study included 59,820 patients with and 178,547 patients without rheumatic IMIDs followed for a maximum of 6 years.
They were drawn from a cohort of 1.6 million persons aged 65 or older in the Medicare Provider Analysis and Review (MedPAR) file who had been admitted for an MI between 2014 and 2019. Of these, 60,072 had a prior history of rheumatic IMIDs, most commonly rheumatoid arthritis (77.8%), followed by systemic lupus erythematosus (12.2%), psoriasis (5.1%), systemic sclerosis (2.8%), and myositis/dermatomyositis (1.8%).
Patients with an IMID were more often women; had a higher prevalence of valve disease, pulmonary hypertension, hypothyroidism, and anemia; and were more likely to present with non–ST-segment MI (NSTEMI).
Rates of coronary angiography (46.1% vs. 51.5%), percutaneous coronary intervention (31.6% vs. 33.6%), and coronary artery bypass grafting (7.7% vs. 10.7%) were significantly lower in patients with IMIDs who had NSTEMI, compared with patients without an IMID who had NSTEMI. Rates of these interventions were also lower in patients with IMIDs who presented with STEMI versus their peers without an IMID, at 78.2% vs. 80.7%, 70.2% vs. 71.5%, and 4.9% vs. 6.4%, respectively.
Dr. Mentias pointed out that the emerging subspecialty of cardiorheumatology is gaining traction, especially at large hospitals and academic centers, but that less than one-third of persons in the United States with an IMID are likely to be under the care of such specialists.
“It’s important before developing an MI to try and control the different risk factors and improve the risk profile for these patients as much as possible by both specialties, and then, after an unfortunate event like MI happens, it’s important to make sure we offer therapies and treatments that are known to improve outcomes,” he said.
Commenting for this article, Jon Tyler Giles, MD, a clinical researcher who focuses on cardiovascular diseases in rheumatology at Columbia University Vagelos College of Physicians and Surgeons, New York, said that “at least for rheumatoid arthritis, this is something that we already knew. People with rheumatic arthritis, when they have a heart attack, are less likely to get the standard kind of treatments and have worse outcomes. This is a little larger sample, but it’s not a surprise, not a surprise at all.”
He noted that the study could have answered questions regarding potential drivers, but “they didn’t dig down into any of the factors that were associated with the poorer outcomes in the patients with rheumatoid arthritis and lupus and scleroderma.”
Indeed, the investigators acknowledge that the study lacked information on coronary anatomy, duration and severity of the autoimmune disease, imaging data, and medications such as anti-inflammatory or immune-targeted therapies.
Dr. Giles highlighted several factors that can contribute to a poorer post-MI prognosis in patients with rheumatic diseases; these include frailty, being more hypercoaguable, increased rates of myocardial dysfunction and other heart and blood vessel diseases, and chronic treatment with steroids and nonsteroidal anti-inflammatory drugs that often interferes with anticoagulation after a MI or when putting in a stent. “So, there’s lot of moving parts, and not one single thing that is likely the answer.”
In addition, he said, “there’s always going to be a portion of these patients who, despite doing the best that we can with treatment, are going to have very severe disease. That may or may not be the subset of patients that did the worst, but likely they’re overrepresented in those patients.”
Finally, the inability to move the needle may lie with the lack of evidence-based screening and management guidelines for cardiovascular disease in any rheumatic disease, Dr. Giles observed. “There’s no guideline for us to use to decide who needs screening over and above what’s recommended for the general population, and then, even if you do screen, what do you do other than what you would normally?”
Rheumatologists are often reluctant to take up the cardiovascular screening side of things because visits are short, and a lot of that time is spent trying to manage the inflammatory components of a patient’s disease, he said. There’s also a barrier in getting some patients to add a cardiologist to the mix of physicians they already see, especially if they don’t have any symptoms.
“If someone has had an event, it’s a lot easier for people to be convinced to go see the cardiologist, obviously, but prior to having an event, the preventative side of things is something that often gets missed or goes to the wayside,” Dr. Giles said.
The study was partly funded by philanthropic gifts by the Haslam family, Bailey family, and Khouri family to the Cleveland Clinic for coauthor Dr. Milind Desai’s research. Dr. Desai is a consultant for Medtronic and Bristol Myers Squibb and serves on an executive steering committee of a BMS-sponsored trial. The remaining authors report having no relevant disclosures. Dr. Giles is a consultant on drug cardiovascular safety for Pfizer, AbbVie, and Eli Lilly.
A version of this article first appeared on Medscape.com.
, in a large propensity-matched analysis.
At a median of 2 years after their MI, Medicare beneficiaries with an IMID had adjusted risks that were:
- 15% higher for all-cause death (hazard ratio, 1.15);
- 12% higher for heart failure (HR, 1.12);
- 8% higher for recurrent MI (HR, 1.08); and
- 6% higher risk for coronary reintervention (HR, 1.06; P < .05 for all).
In addition, interventions known to improve outcomes in this context, such as coronary revascularization, were less common in patients with IMID.
“This could be because they usually are sicker and have more risk factors when they present, like kidney disease, so maybe they’re not eligible for the therapy. But by itself, it was surprising they’re not offered these interventions as common[ly] as people who don’t have the disease,” Amgad Mentias, MD, a clinical cardiologist at the Cleveland Clinic, said in an interview.
The study was published Sept. 14 in the Journal of the American Heart Association, with Dr. Mentias as senior author and Heba Wassif, MD, MPH, also with Cleveland Clinic, as first author.
IMIDs, such as rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel disease, are known to be associated with significantly higher cardiovascular disease (CVD) risk due to a greater prevalence of traditional CVD risk factors and chronic systemic inflammation.
Certain disease-modifying agents may also increase patients’ cardiovascular risk. This has been a long-simmering issue for the arthritis and ulcerative colitis drug tofacitinib (Xeljanz, Xeljanz XR), resulting in an updated boxed warning in 2021.
Many of these patients also have joint disease, pain, and fatigue, which can limit physical activity, Dr. Mentias said. “So these small nuances of how to manage these patients, or balance between controlling the inflammation but also improv[ing] cardiac risk factors, is not an easy task.”
Evidence regarding post-MI events has been inconsistent and limited to smaller single-center studies, he said. After propensity-score matching, the present study included 59,820 patients with and 178,547 patients without rheumatic IMIDs followed for a maximum of 6 years.
They were drawn from a cohort of 1.6 million persons aged 65 or older in the Medicare Provider Analysis and Review (MedPAR) file who had been admitted for an MI between 2014 and 2019. Of these, 60,072 had a prior history of rheumatic IMIDs, most commonly rheumatoid arthritis (77.8%), followed by systemic lupus erythematosus (12.2%), psoriasis (5.1%), systemic sclerosis (2.8%), and myositis/dermatomyositis (1.8%).
Patients with an IMID were more often women; had a higher prevalence of valve disease, pulmonary hypertension, hypothyroidism, and anemia; and were more likely to present with non–ST-segment MI (NSTEMI).
Rates of coronary angiography (46.1% vs. 51.5%), percutaneous coronary intervention (31.6% vs. 33.6%), and coronary artery bypass grafting (7.7% vs. 10.7%) were significantly lower in patients with IMIDs who had NSTEMI, compared with patients without an IMID who had NSTEMI. Rates of these interventions were also lower in patients with IMIDs who presented with STEMI versus their peers without an IMID, at 78.2% vs. 80.7%, 70.2% vs. 71.5%, and 4.9% vs. 6.4%, respectively.
Dr. Mentias pointed out that the emerging subspecialty of cardiorheumatology is gaining traction, especially at large hospitals and academic centers, but that less than one-third of persons in the United States with an IMID are likely to be under the care of such specialists.
“It’s important before developing an MI to try and control the different risk factors and improve the risk profile for these patients as much as possible by both specialties, and then, after an unfortunate event like MI happens, it’s important to make sure we offer therapies and treatments that are known to improve outcomes,” he said.
Commenting for this article, Jon Tyler Giles, MD, a clinical researcher who focuses on cardiovascular diseases in rheumatology at Columbia University Vagelos College of Physicians and Surgeons, New York, said that “at least for rheumatoid arthritis, this is something that we already knew. People with rheumatic arthritis, when they have a heart attack, are less likely to get the standard kind of treatments and have worse outcomes. This is a little larger sample, but it’s not a surprise, not a surprise at all.”
He noted that the study could have answered questions regarding potential drivers, but “they didn’t dig down into any of the factors that were associated with the poorer outcomes in the patients with rheumatoid arthritis and lupus and scleroderma.”
Indeed, the investigators acknowledge that the study lacked information on coronary anatomy, duration and severity of the autoimmune disease, imaging data, and medications such as anti-inflammatory or immune-targeted therapies.
Dr. Giles highlighted several factors that can contribute to a poorer post-MI prognosis in patients with rheumatic diseases; these include frailty, being more hypercoaguable, increased rates of myocardial dysfunction and other heart and blood vessel diseases, and chronic treatment with steroids and nonsteroidal anti-inflammatory drugs that often interferes with anticoagulation after a MI or when putting in a stent. “So, there’s lot of moving parts, and not one single thing that is likely the answer.”
In addition, he said, “there’s always going to be a portion of these patients who, despite doing the best that we can with treatment, are going to have very severe disease. That may or may not be the subset of patients that did the worst, but likely they’re overrepresented in those patients.”
Finally, the inability to move the needle may lie with the lack of evidence-based screening and management guidelines for cardiovascular disease in any rheumatic disease, Dr. Giles observed. “There’s no guideline for us to use to decide who needs screening over and above what’s recommended for the general population, and then, even if you do screen, what do you do other than what you would normally?”
Rheumatologists are often reluctant to take up the cardiovascular screening side of things because visits are short, and a lot of that time is spent trying to manage the inflammatory components of a patient’s disease, he said. There’s also a barrier in getting some patients to add a cardiologist to the mix of physicians they already see, especially if they don’t have any symptoms.
“If someone has had an event, it’s a lot easier for people to be convinced to go see the cardiologist, obviously, but prior to having an event, the preventative side of things is something that often gets missed or goes to the wayside,” Dr. Giles said.
The study was partly funded by philanthropic gifts by the Haslam family, Bailey family, and Khouri family to the Cleveland Clinic for coauthor Dr. Milind Desai’s research. Dr. Desai is a consultant for Medtronic and Bristol Myers Squibb and serves on an executive steering committee of a BMS-sponsored trial. The remaining authors report having no relevant disclosures. Dr. Giles is a consultant on drug cardiovascular safety for Pfizer, AbbVie, and Eli Lilly.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
People of color bearing brunt of long COVID, doctors say
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
‘Dr. Caveman’ had a leg up on amputation
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
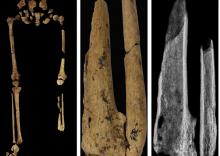
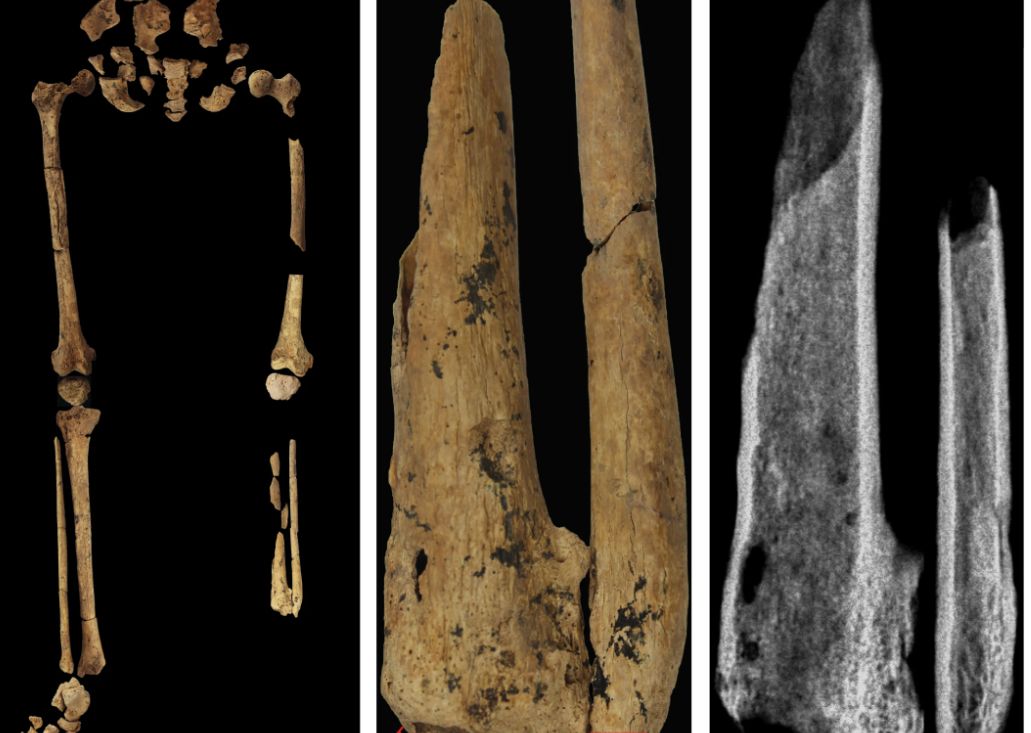
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
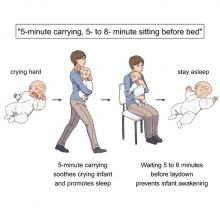
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
TBI is an unrecognized risk factor for cardiovascular disease
(CVD). More severe TBI is associated with higher risk of CVD, new research shows.
Given the relatively young age of post-9/11–era veterans with TBI, there may be an increased burden of heart disease in the future as these veterans age and develop traditional risk factors for CVD, the investigators, led by Ian J. Stewart, MD, with Uniformed Services University, Bethesda, Md., wrote.
The study was published online in JAMA Neurology.
Novel data
Since Sept. 11, 2001, 4.5 million people have served in the U.S. military, with their time in service defined by the long-running wars in Iraq and Afghanistan. Estimates suggest that up to 20% of post-9/11 veterans sustained a TBI.
While some evidence suggests that TBI increases the risk of CVD, prior reports have focused mainly on cerebrovascular outcomes. Until now, the potential association of TBI with CVD has not been comprehensively examined in post-9/11–era veterans.
The retrospective cohort study included 1,559,928 predominantly male post-9/11 veterans, including 301,169 (19.3%) with a history of TBI and 1,258,759 (81%) with no TBI history.
In fully adjusted models, compared with veterans with no TBI history, a history of mild, moderate/severe, or penetrating TBI was associated with increased risk of developing the composite CVD endpoint (coronary artery disease, stroke, peripheral artery disease, and CVD death).
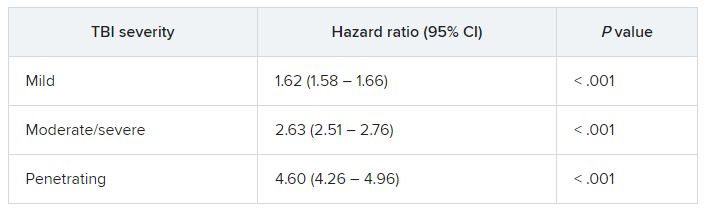
TBIs of all severities were associated with the individual components of the composite outcome, except penetrating TBI and CVD death.
“The association of TBI with subsequent CVD was not attenuated in multivariable models, suggesting that TBI may be accounting for risk that is independent from the other variables,” Dr. Stewart and colleagues wrote.
They noted that the risk was highest shortly after injury, but TBI remained significantly associated with CVD for years after the initial insult.
Why TBI may raise the risk of subsequent CVD remains unclear.
It’s possible that patients with TBI develop more traditional risk factors for CVD through time than do patients without TBI. A study in mice found that TBI led to increased rates of atherosclerosis, the researchers said.
An additional mechanism may be disruption of autonomic regulation, which has been known to occur after TBI.
Another potential pathway is through mental health diagnoses, such as posttraumatic stress disorder; a large body of work has identified associations between PTSD and CVD, including among post-9/11 veterans.
Further work is needed to determine how this risk can be modified to improve outcomes for post-9/11–era veterans, the researchers write.
Unrecognized CVD risk factor?
Reached for comment, Shaheen E. Lakhan, MD, PhD, a neurologist and researcher from Boston who wasn’t involved in the study, said the effects of TBI on heart health are “very underreported, and most clinicians would not make the link.”
“When the brain suffers a traumatic injury, it activates a cascade of neuro-inflammation that goes haywire in an attempt to protect further brain damage. Oftentimes, these inflammatory by-products leak into the body, especially in trauma, when the barriers are broken between brain and body, and can cause systemic body inflammation, which is well associated with heart disease,” Dr. Lakhan said.
In addition, Dr. Lakhan said, “TBI itself localized to just the brain can negatively affect good health habits, leading to worsening heart health, too.”
“Research like this brings light where not much exists and underscores the importance of protecting our brains from physical trauma,” he said.
The study was supported by the assistant secretary of defense for health affairs, endorsed by the Department of Defense through the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military-Relevant Brain Injury Consortium, and by the U.S. Department of Veterans Affairs. Dr. Stewart and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(CVD). More severe TBI is associated with higher risk of CVD, new research shows.
Given the relatively young age of post-9/11–era veterans with TBI, there may be an increased burden of heart disease in the future as these veterans age and develop traditional risk factors for CVD, the investigators, led by Ian J. Stewart, MD, with Uniformed Services University, Bethesda, Md., wrote.
The study was published online in JAMA Neurology.
Novel data
Since Sept. 11, 2001, 4.5 million people have served in the U.S. military, with their time in service defined by the long-running wars in Iraq and Afghanistan. Estimates suggest that up to 20% of post-9/11 veterans sustained a TBI.
While some evidence suggests that TBI increases the risk of CVD, prior reports have focused mainly on cerebrovascular outcomes. Until now, the potential association of TBI with CVD has not been comprehensively examined in post-9/11–era veterans.
The retrospective cohort study included 1,559,928 predominantly male post-9/11 veterans, including 301,169 (19.3%) with a history of TBI and 1,258,759 (81%) with no TBI history.
In fully adjusted models, compared with veterans with no TBI history, a history of mild, moderate/severe, or penetrating TBI was associated with increased risk of developing the composite CVD endpoint (coronary artery disease, stroke, peripheral artery disease, and CVD death).

TBIs of all severities were associated with the individual components of the composite outcome, except penetrating TBI and CVD death.
“The association of TBI with subsequent CVD was not attenuated in multivariable models, suggesting that TBI may be accounting for risk that is independent from the other variables,” Dr. Stewart and colleagues wrote.
They noted that the risk was highest shortly after injury, but TBI remained significantly associated with CVD for years after the initial insult.
Why TBI may raise the risk of subsequent CVD remains unclear.
It’s possible that patients with TBI develop more traditional risk factors for CVD through time than do patients without TBI. A study in mice found that TBI led to increased rates of atherosclerosis, the researchers said.
An additional mechanism may be disruption of autonomic regulation, which has been known to occur after TBI.
Another potential pathway is through mental health diagnoses, such as posttraumatic stress disorder; a large body of work has identified associations between PTSD and CVD, including among post-9/11 veterans.
Further work is needed to determine how this risk can be modified to improve outcomes for post-9/11–era veterans, the researchers write.
Unrecognized CVD risk factor?
Reached for comment, Shaheen E. Lakhan, MD, PhD, a neurologist and researcher from Boston who wasn’t involved in the study, said the effects of TBI on heart health are “very underreported, and most clinicians would not make the link.”
“When the brain suffers a traumatic injury, it activates a cascade of neuro-inflammation that goes haywire in an attempt to protect further brain damage. Oftentimes, these inflammatory by-products leak into the body, especially in trauma, when the barriers are broken between brain and body, and can cause systemic body inflammation, which is well associated with heart disease,” Dr. Lakhan said.
In addition, Dr. Lakhan said, “TBI itself localized to just the brain can negatively affect good health habits, leading to worsening heart health, too.”
“Research like this brings light where not much exists and underscores the importance of protecting our brains from physical trauma,” he said.
The study was supported by the assistant secretary of defense for health affairs, endorsed by the Department of Defense through the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military-Relevant Brain Injury Consortium, and by the U.S. Department of Veterans Affairs. Dr. Stewart and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(CVD). More severe TBI is associated with higher risk of CVD, new research shows.
Given the relatively young age of post-9/11–era veterans with TBI, there may be an increased burden of heart disease in the future as these veterans age and develop traditional risk factors for CVD, the investigators, led by Ian J. Stewart, MD, with Uniformed Services University, Bethesda, Md., wrote.
The study was published online in JAMA Neurology.
Novel data
Since Sept. 11, 2001, 4.5 million people have served in the U.S. military, with their time in service defined by the long-running wars in Iraq and Afghanistan. Estimates suggest that up to 20% of post-9/11 veterans sustained a TBI.
While some evidence suggests that TBI increases the risk of CVD, prior reports have focused mainly on cerebrovascular outcomes. Until now, the potential association of TBI with CVD has not been comprehensively examined in post-9/11–era veterans.
The retrospective cohort study included 1,559,928 predominantly male post-9/11 veterans, including 301,169 (19.3%) with a history of TBI and 1,258,759 (81%) with no TBI history.
In fully adjusted models, compared with veterans with no TBI history, a history of mild, moderate/severe, or penetrating TBI was associated with increased risk of developing the composite CVD endpoint (coronary artery disease, stroke, peripheral artery disease, and CVD death).

TBIs of all severities were associated with the individual components of the composite outcome, except penetrating TBI and CVD death.
“The association of TBI with subsequent CVD was not attenuated in multivariable models, suggesting that TBI may be accounting for risk that is independent from the other variables,” Dr. Stewart and colleagues wrote.
They noted that the risk was highest shortly after injury, but TBI remained significantly associated with CVD for years after the initial insult.
Why TBI may raise the risk of subsequent CVD remains unclear.
It’s possible that patients with TBI develop more traditional risk factors for CVD through time than do patients without TBI. A study in mice found that TBI led to increased rates of atherosclerosis, the researchers said.
An additional mechanism may be disruption of autonomic regulation, which has been known to occur after TBI.
Another potential pathway is through mental health diagnoses, such as posttraumatic stress disorder; a large body of work has identified associations between PTSD and CVD, including among post-9/11 veterans.
Further work is needed to determine how this risk can be modified to improve outcomes for post-9/11–era veterans, the researchers write.
Unrecognized CVD risk factor?
Reached for comment, Shaheen E. Lakhan, MD, PhD, a neurologist and researcher from Boston who wasn’t involved in the study, said the effects of TBI on heart health are “very underreported, and most clinicians would not make the link.”
“When the brain suffers a traumatic injury, it activates a cascade of neuro-inflammation that goes haywire in an attempt to protect further brain damage. Oftentimes, these inflammatory by-products leak into the body, especially in trauma, when the barriers are broken between brain and body, and can cause systemic body inflammation, which is well associated with heart disease,” Dr. Lakhan said.
In addition, Dr. Lakhan said, “TBI itself localized to just the brain can negatively affect good health habits, leading to worsening heart health, too.”
“Research like this brings light where not much exists and underscores the importance of protecting our brains from physical trauma,” he said.
The study was supported by the assistant secretary of defense for health affairs, endorsed by the Department of Defense through the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military-Relevant Brain Injury Consortium, and by the U.S. Department of Veterans Affairs. Dr. Stewart and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Your poop may hold the secret to long life
Lots of things can disrupt your gut health over the years. A high-sugar diet, stress, antibiotics – all are linked to bad changes in the gut microbiome, the microbes that live in your intestinal tract. And this can raise the risk of diseases.
It could be possible, scientists say, by having people take a sample of their own stool when they are young to be put back into their colons when they are older.
While the science to back this up isn’t quite there yet, some researchers are saying we shouldn’t wait. They are calling on existing stool banks to let people start banking their stool now, so it’s there for them to use if the science becomes available.
But how would that work?
First, you’d go to a stool bank and provide a fresh sample of your poop, which would be screened for diseases, washed, processed, and deposited into a long-term storage facility.
Then, down the road, if you get a condition such as inflammatory bowel disease, heart disease, or type 2 diabetes – or if you have a procedure that wipes out your microbiome, like a course of antibiotics or chemotherapy – doctors could use your preserved stool to “re-colonize” your gut, restoring it to its earlier, healthier state, said Scott Weiss, MD, professor of medicine at Harvard Medical School, Boston, and a coauthor of a recent paper on the topic. They would do that using fecal microbiota transplantation, or FMT.
Timing is everything. You’d want a sample from when you’re healthy – say, between the ages of 18 and 35, or before a chronic condition is likely, said Dr. Weiss. But if you’re still healthy into your late 30s, 40s, or even 50s, providing a sample then could still benefit you later in life.
If we could pull off a banking system like this, it could have the potential to treat autoimmune disease, inflammatory bowel disease, diabetes, obesity, and heart disease – or even reverse the effects of aging. How can we make this happen?
Stool banks of today
While stool banks do exist today, the samples inside are destined not for the original donors but rather for sick patients hoping to treat an illness. Using FMT, doctors transfer the fecal material to the patient’s colon, restoring helpful gut microbiota.
Some research shows FMT may help treat inflammatory bowel diseases, such as Crohn’s or ulcerative colitis. Animal studies suggest it could help treat obesity, lengthen lifespan, and reverse some effects of aging, such as age-related decline in brain function. Other clinical trials are looking into its potential as a cancer treatment, said Dr. Weiss.
But outside the lab, FMT is mainly used for one purpose: to treat Clostridioides difficile infection. It works even better than antibiotics, research shows.
But first you need to find a healthy donor, and that’s harder than you might think.
Finding healthy stool samples
Banking our bodily substances is nothing new. Blood banks, for example, are common throughout the United States, and cord blood banking – preserving blood from a baby’s umbilical cord to aid possible future medical needs of the child – is becoming more popular. Sperm donors are highly sought after, and doctors regularly transplant kidneys and bone marrow to patients in need.
So why are we so particular about poop?
Part of the reason may be because feces (like blood, for that matter) can harbor disease – which is why it’s so important to find healthy stool donors. Problem is, this can be surprisingly hard to do.
To donate fecal matter, people must go through a rigorous screening process, said Majdi Osman, MD, chief medical officer for OpenBiome, a nonprofit microbiome research organization.
Until recently, OpenBiome operated a stool donation program, though it has since shifted its focus to research. Potential donors were screened for diseases and mental health conditions, pathogens, and antibiotic resistance. The pass rate was less than 3%.
“We take a very cautious approach because the association between diseases and the microbiome is still being understood,” Dr. Osman said.
FMT also carries risks – though so far, they seem mild. Side effects include mild diarrhea, nausea, belly pain, and fatigue. (The reason? Even the healthiest donor stool may not mix perfectly with your own.)
That’s where the idea of using your own stool comes in, said Yang-Yu Liu, PhD, a Harvard researcher who studies the microbiome and the lead author of the paper mentioned above. It’s not just more appealing but may also be a better “match” for your body.
Should you bank your stool?
While the researchers say we have reason to be optimistic about the future, it’s important to remember that many challenges remain. FMT is early in development, and there’s a lot about the microbiome we still don’t know.
There’s no guarantee, for example, that restoring a person’s microbiome to its formerly disease-free state will keep diseases at bay forever, said Dr. Weiss. If your genes raise your odds of having Crohn’s, for instance, it’s possible the disease could come back.
We also don’t know how long stool samples can be preserved, said Dr. Liu. Stool banks currently store fecal matter for 1 or 2 years, not decades. To protect the proteins and DNA structures for that long, samples would likely need to be stashed at the liquid nitrogen storage temperature of –196° C. (Currently, samples are stored at about –80° C.) Even then, testing would be needed to confirm if the fragile microorganisms in the stool can survive.
This raises another question: Who’s going to regulate all this?
The FDA regulates the use of FMT as a drug for the treatment of C. diff, but as Dr. Liu pointed out, many gastroenterologists consider the gut microbiota an organ. In that case, human fecal matter could be regulated the same way blood, bone, or even egg cells are.
Cord blood banking may be a helpful model, Dr. Liu said.
“We don’t have to start from scratch.”
Then there’s the question of cost. Cord blood banks could be a point of reference for that too, the researchers say. They charge about $1,500 to $2,820 for the first collection and processing, plus a yearly storage fee of $185 to $370.
Despite the unknowns, one thing is for sure: The interest in fecal banking is real – and growing. At least one microbiome firm, Cordlife Group Limited, based in Singapore, announced that it has started to allow people to bank their stool for future use.
“More people should talk about it and think about it,” said Dr. Liu.
A version of this article first appeared on WebMD.com.
Lots of things can disrupt your gut health over the years. A high-sugar diet, stress, antibiotics – all are linked to bad changes in the gut microbiome, the microbes that live in your intestinal tract. And this can raise the risk of diseases.
It could be possible, scientists say, by having people take a sample of their own stool when they are young to be put back into their colons when they are older.
While the science to back this up isn’t quite there yet, some researchers are saying we shouldn’t wait. They are calling on existing stool banks to let people start banking their stool now, so it’s there for them to use if the science becomes available.
But how would that work?
First, you’d go to a stool bank and provide a fresh sample of your poop, which would be screened for diseases, washed, processed, and deposited into a long-term storage facility.
Then, down the road, if you get a condition such as inflammatory bowel disease, heart disease, or type 2 diabetes – or if you have a procedure that wipes out your microbiome, like a course of antibiotics or chemotherapy – doctors could use your preserved stool to “re-colonize” your gut, restoring it to its earlier, healthier state, said Scott Weiss, MD, professor of medicine at Harvard Medical School, Boston, and a coauthor of a recent paper on the topic. They would do that using fecal microbiota transplantation, or FMT.
Timing is everything. You’d want a sample from when you’re healthy – say, between the ages of 18 and 35, or before a chronic condition is likely, said Dr. Weiss. But if you’re still healthy into your late 30s, 40s, or even 50s, providing a sample then could still benefit you later in life.
If we could pull off a banking system like this, it could have the potential to treat autoimmune disease, inflammatory bowel disease, diabetes, obesity, and heart disease – or even reverse the effects of aging. How can we make this happen?
Stool banks of today
While stool banks do exist today, the samples inside are destined not for the original donors but rather for sick patients hoping to treat an illness. Using FMT, doctors transfer the fecal material to the patient’s colon, restoring helpful gut microbiota.
Some research shows FMT may help treat inflammatory bowel diseases, such as Crohn’s or ulcerative colitis. Animal studies suggest it could help treat obesity, lengthen lifespan, and reverse some effects of aging, such as age-related decline in brain function. Other clinical trials are looking into its potential as a cancer treatment, said Dr. Weiss.
But outside the lab, FMT is mainly used for one purpose: to treat Clostridioides difficile infection. It works even better than antibiotics, research shows.
But first you need to find a healthy donor, and that’s harder than you might think.
Finding healthy stool samples
Banking our bodily substances is nothing new. Blood banks, for example, are common throughout the United States, and cord blood banking – preserving blood from a baby’s umbilical cord to aid possible future medical needs of the child – is becoming more popular. Sperm donors are highly sought after, and doctors regularly transplant kidneys and bone marrow to patients in need.
So why are we so particular about poop?
Part of the reason may be because feces (like blood, for that matter) can harbor disease – which is why it’s so important to find healthy stool donors. Problem is, this can be surprisingly hard to do.
To donate fecal matter, people must go through a rigorous screening process, said Majdi Osman, MD, chief medical officer for OpenBiome, a nonprofit microbiome research organization.
Until recently, OpenBiome operated a stool donation program, though it has since shifted its focus to research. Potential donors were screened for diseases and mental health conditions, pathogens, and antibiotic resistance. The pass rate was less than 3%.
“We take a very cautious approach because the association between diseases and the microbiome is still being understood,” Dr. Osman said.
FMT also carries risks – though so far, they seem mild. Side effects include mild diarrhea, nausea, belly pain, and fatigue. (The reason? Even the healthiest donor stool may not mix perfectly with your own.)
That’s where the idea of using your own stool comes in, said Yang-Yu Liu, PhD, a Harvard researcher who studies the microbiome and the lead author of the paper mentioned above. It’s not just more appealing but may also be a better “match” for your body.
Should you bank your stool?
While the researchers say we have reason to be optimistic about the future, it’s important to remember that many challenges remain. FMT is early in development, and there’s a lot about the microbiome we still don’t know.
There’s no guarantee, for example, that restoring a person’s microbiome to its formerly disease-free state will keep diseases at bay forever, said Dr. Weiss. If your genes raise your odds of having Crohn’s, for instance, it’s possible the disease could come back.
We also don’t know how long stool samples can be preserved, said Dr. Liu. Stool banks currently store fecal matter for 1 or 2 years, not decades. To protect the proteins and DNA structures for that long, samples would likely need to be stashed at the liquid nitrogen storage temperature of –196° C. (Currently, samples are stored at about –80° C.) Even then, testing would be needed to confirm if the fragile microorganisms in the stool can survive.
This raises another question: Who’s going to regulate all this?
The FDA regulates the use of FMT as a drug for the treatment of C. diff, but as Dr. Liu pointed out, many gastroenterologists consider the gut microbiota an organ. In that case, human fecal matter could be regulated the same way blood, bone, or even egg cells are.
Cord blood banking may be a helpful model, Dr. Liu said.
“We don’t have to start from scratch.”
Then there’s the question of cost. Cord blood banks could be a point of reference for that too, the researchers say. They charge about $1,500 to $2,820 for the first collection and processing, plus a yearly storage fee of $185 to $370.
Despite the unknowns, one thing is for sure: The interest in fecal banking is real – and growing. At least one microbiome firm, Cordlife Group Limited, based in Singapore, announced that it has started to allow people to bank their stool for future use.
“More people should talk about it and think about it,” said Dr. Liu.
A version of this article first appeared on WebMD.com.
Lots of things can disrupt your gut health over the years. A high-sugar diet, stress, antibiotics – all are linked to bad changes in the gut microbiome, the microbes that live in your intestinal tract. And this can raise the risk of diseases.
It could be possible, scientists say, by having people take a sample of their own stool when they are young to be put back into their colons when they are older.
While the science to back this up isn’t quite there yet, some researchers are saying we shouldn’t wait. They are calling on existing stool banks to let people start banking their stool now, so it’s there for them to use if the science becomes available.
But how would that work?
First, you’d go to a stool bank and provide a fresh sample of your poop, which would be screened for diseases, washed, processed, and deposited into a long-term storage facility.
Then, down the road, if you get a condition such as inflammatory bowel disease, heart disease, or type 2 diabetes – or if you have a procedure that wipes out your microbiome, like a course of antibiotics or chemotherapy – doctors could use your preserved stool to “re-colonize” your gut, restoring it to its earlier, healthier state, said Scott Weiss, MD, professor of medicine at Harvard Medical School, Boston, and a coauthor of a recent paper on the topic. They would do that using fecal microbiota transplantation, or FMT.
Timing is everything. You’d want a sample from when you’re healthy – say, between the ages of 18 and 35, or before a chronic condition is likely, said Dr. Weiss. But if you’re still healthy into your late 30s, 40s, or even 50s, providing a sample then could still benefit you later in life.
If we could pull off a banking system like this, it could have the potential to treat autoimmune disease, inflammatory bowel disease, diabetes, obesity, and heart disease – or even reverse the effects of aging. How can we make this happen?
Stool banks of today
While stool banks do exist today, the samples inside are destined not for the original donors but rather for sick patients hoping to treat an illness. Using FMT, doctors transfer the fecal material to the patient’s colon, restoring helpful gut microbiota.
Some research shows FMT may help treat inflammatory bowel diseases, such as Crohn’s or ulcerative colitis. Animal studies suggest it could help treat obesity, lengthen lifespan, and reverse some effects of aging, such as age-related decline in brain function. Other clinical trials are looking into its potential as a cancer treatment, said Dr. Weiss.
But outside the lab, FMT is mainly used for one purpose: to treat Clostridioides difficile infection. It works even better than antibiotics, research shows.
But first you need to find a healthy donor, and that’s harder than you might think.
Finding healthy stool samples
Banking our bodily substances is nothing new. Blood banks, for example, are common throughout the United States, and cord blood banking – preserving blood from a baby’s umbilical cord to aid possible future medical needs of the child – is becoming more popular. Sperm donors are highly sought after, and doctors regularly transplant kidneys and bone marrow to patients in need.
So why are we so particular about poop?
Part of the reason may be because feces (like blood, for that matter) can harbor disease – which is why it’s so important to find healthy stool donors. Problem is, this can be surprisingly hard to do.
To donate fecal matter, people must go through a rigorous screening process, said Majdi Osman, MD, chief medical officer for OpenBiome, a nonprofit microbiome research organization.
Until recently, OpenBiome operated a stool donation program, though it has since shifted its focus to research. Potential donors were screened for diseases and mental health conditions, pathogens, and antibiotic resistance. The pass rate was less than 3%.
“We take a very cautious approach because the association between diseases and the microbiome is still being understood,” Dr. Osman said.
FMT also carries risks – though so far, they seem mild. Side effects include mild diarrhea, nausea, belly pain, and fatigue. (The reason? Even the healthiest donor stool may not mix perfectly with your own.)
That’s where the idea of using your own stool comes in, said Yang-Yu Liu, PhD, a Harvard researcher who studies the microbiome and the lead author of the paper mentioned above. It’s not just more appealing but may also be a better “match” for your body.
Should you bank your stool?
While the researchers say we have reason to be optimistic about the future, it’s important to remember that many challenges remain. FMT is early in development, and there’s a lot about the microbiome we still don’t know.
There’s no guarantee, for example, that restoring a person’s microbiome to its formerly disease-free state will keep diseases at bay forever, said Dr. Weiss. If your genes raise your odds of having Crohn’s, for instance, it’s possible the disease could come back.
We also don’t know how long stool samples can be preserved, said Dr. Liu. Stool banks currently store fecal matter for 1 or 2 years, not decades. To protect the proteins and DNA structures for that long, samples would likely need to be stashed at the liquid nitrogen storage temperature of –196° C. (Currently, samples are stored at about –80° C.) Even then, testing would be needed to confirm if the fragile microorganisms in the stool can survive.
This raises another question: Who’s going to regulate all this?
The FDA regulates the use of FMT as a drug for the treatment of C. diff, but as Dr. Liu pointed out, many gastroenterologists consider the gut microbiota an organ. In that case, human fecal matter could be regulated the same way blood, bone, or even egg cells are.
Cord blood banking may be a helpful model, Dr. Liu said.
“We don’t have to start from scratch.”
Then there’s the question of cost. Cord blood banks could be a point of reference for that too, the researchers say. They charge about $1,500 to $2,820 for the first collection and processing, plus a yearly storage fee of $185 to $370.
Despite the unknowns, one thing is for sure: The interest in fecal banking is real – and growing. At least one microbiome firm, Cordlife Group Limited, based in Singapore, announced that it has started to allow people to bank their stool for future use.
“More people should talk about it and think about it,” said Dr. Liu.
A version of this article first appeared on WebMD.com.
Heparin pretreatment may safely open arteries before STEMI cath
, suggests a large registry study.
An open infarct-related artery (IRA) at angiography on cath-lab arrival presents STEMI patients an opportunity for earlier reperfusion and a chance, in theory at least, for smaller infarcts and maybe improved clinical outcomes.
In the new analysis, which covers more than 40,000 patients with STEMI in Sweden, the 38% who received heparin before cath-lab arrival were 11% less likely to show IRA occlusion at angiography prior to direct percutaneous coronary intervention (PCI). They also showed a 13% lower 30-day mortality compared with patients who were started on heparin in the cath lab. Importantly, their risk of major bleeding in the hospital did not increase.
The “early reperfusion” associated with IRA patency at angiography “could have long-term benefit due to smaller infarct size,” potentially explaining the observed 30-day survival gain in the pretreatment group, Oskar Love Emilsson, Lund (Sweden) University, said in an interview.
Mr. Emilsson, a third-year medical student, reported the analysis at the annual congress of the European Society of Cardiology, and is lead author on its same-day publication in the journal EuroIntervention.
He mentioned a few cautions in interpreting the study, which is based primarily on data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). It included several sensitivity analyses that continued to back pretreatment heparin as a significant predictor of an unoccluded IRA but didn’t consistently support the 30-day mortality benefit seen in the primary analysis.
And, although the pretreatment group overall didn’t have more major bleeds, the risk did go up significantly for those older than 75 or those who weighed less than 60 kg (132 pounds) or underwent catheterization with an access route other than the radial artery. Extra caution should be exercised in such patients who receive heparin before cath-lab arrival for PCI, Mr. Emilsson observed.
“Our results suggest that heparin pretreatment might be a good option to improve patency of infarct related arteries in STEMI,” and potentially clinical outcomes, he said. “However, a definite answer would require a randomized controlled trial.”
Meanwhile, the current study may be the largest yet to look at clinical outcomes after pretreatment with unfractionated heparin before PCI for acute STEMI, the report states. There have been some observational studies, subanalyses of STEMI trials, and even a few limited randomized trials – including the HEAP trial published in 2000 – to weigh in on the subject. Some have supported the strategy, others have not.
“With rapid door-to-balloon times in STEMI, it can be challenging to show a significant difference between a prehospital heparin approach and heparin given in the lab,” observed Sunil V. Rao, MD, NYU Langone Health System, New York, who is not connected with the current study.
Many EDs in the United States have “a STEMI protocol that calls for an IV bolus of heparin. It would be tougher in the U.S. to give it in the ambulance but again, it’s not clear how much advantage that would really provide,” he told this news organization.
Support from randomized trials would be needed before the practice could be formally recommended. “The SCAAR registries have set the standard for how registries should be conducted,” Dr. Rao said. “This is a very well done observational study, but it is observational.”
The priority for STEMI patients, he added, “really should be to get them to the lab as fast as possible. If the ED protocol includes heparin before the cath lab, that’s great, but I don’t think we should delay getting these patients to the lab to accommodate pre–cath-lab heparin.”
The current analysis covered 41,631 patients with STEMI from 2008 through to 2016, of whom 38% were pretreated with heparin in an ambulance or the ED. The remaining 62% initiated heparin in the cath lab.
About one-third of the group had an open IRA at angiography. The adjusted risk ratio (RR) for IRA occlusion at angiography for patients pretreated vs. not pretreated with heparin was 0.89 (95% confidence interval [CI], 0.87-0.90).
The corresponding RR for death within 30 days was 0.87 (95% CI, 0.77-0.99), and for major in-hospital bleeding it was 1.01 (95% CI, 0.86-1.18).
The analysis was adjusted for other medications received before cath-lab arrival, especially a long list of antiplatelets and non-heparin antithrombins. That strengthens the case for heparin pretreatment as an independent predictor of an open IRA at initial angiography, Mr. Emilsson said.
Comparisons of propensity-score–matched subgroups of the total cohort, conducted separately for the IRA-occlusion endpoint and the endpoints of 30-day mortality and major bleeding, produced similar results.
Some observational data suggest that antiplatelet pretreatment with a P2Y12 inhibitor may promote IRA patency on angiography after cath lab arrival, Dr. Rao observed. “This indicates that there probably is a role of earlier antithrombotic therapy in STEMI patients, but the randomized trials have not shown a consistent benefit,” he said, referring in particular to the ATLANTIC trial.
Mr. Emilsson and Dr. Rao disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests a large registry study.
An open infarct-related artery (IRA) at angiography on cath-lab arrival presents STEMI patients an opportunity for earlier reperfusion and a chance, in theory at least, for smaller infarcts and maybe improved clinical outcomes.
In the new analysis, which covers more than 40,000 patients with STEMI in Sweden, the 38% who received heparin before cath-lab arrival were 11% less likely to show IRA occlusion at angiography prior to direct percutaneous coronary intervention (PCI). They also showed a 13% lower 30-day mortality compared with patients who were started on heparin in the cath lab. Importantly, their risk of major bleeding in the hospital did not increase.
The “early reperfusion” associated with IRA patency at angiography “could have long-term benefit due to smaller infarct size,” potentially explaining the observed 30-day survival gain in the pretreatment group, Oskar Love Emilsson, Lund (Sweden) University, said in an interview.
Mr. Emilsson, a third-year medical student, reported the analysis at the annual congress of the European Society of Cardiology, and is lead author on its same-day publication in the journal EuroIntervention.
He mentioned a few cautions in interpreting the study, which is based primarily on data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). It included several sensitivity analyses that continued to back pretreatment heparin as a significant predictor of an unoccluded IRA but didn’t consistently support the 30-day mortality benefit seen in the primary analysis.
And, although the pretreatment group overall didn’t have more major bleeds, the risk did go up significantly for those older than 75 or those who weighed less than 60 kg (132 pounds) or underwent catheterization with an access route other than the radial artery. Extra caution should be exercised in such patients who receive heparin before cath-lab arrival for PCI, Mr. Emilsson observed.
“Our results suggest that heparin pretreatment might be a good option to improve patency of infarct related arteries in STEMI,” and potentially clinical outcomes, he said. “However, a definite answer would require a randomized controlled trial.”
Meanwhile, the current study may be the largest yet to look at clinical outcomes after pretreatment with unfractionated heparin before PCI for acute STEMI, the report states. There have been some observational studies, subanalyses of STEMI trials, and even a few limited randomized trials – including the HEAP trial published in 2000 – to weigh in on the subject. Some have supported the strategy, others have not.
“With rapid door-to-balloon times in STEMI, it can be challenging to show a significant difference between a prehospital heparin approach and heparin given in the lab,” observed Sunil V. Rao, MD, NYU Langone Health System, New York, who is not connected with the current study.
Many EDs in the United States have “a STEMI protocol that calls for an IV bolus of heparin. It would be tougher in the U.S. to give it in the ambulance but again, it’s not clear how much advantage that would really provide,” he told this news organization.
Support from randomized trials would be needed before the practice could be formally recommended. “The SCAAR registries have set the standard for how registries should be conducted,” Dr. Rao said. “This is a very well done observational study, but it is observational.”
The priority for STEMI patients, he added, “really should be to get them to the lab as fast as possible. If the ED protocol includes heparin before the cath lab, that’s great, but I don’t think we should delay getting these patients to the lab to accommodate pre–cath-lab heparin.”
The current analysis covered 41,631 patients with STEMI from 2008 through to 2016, of whom 38% were pretreated with heparin in an ambulance or the ED. The remaining 62% initiated heparin in the cath lab.
About one-third of the group had an open IRA at angiography. The adjusted risk ratio (RR) for IRA occlusion at angiography for patients pretreated vs. not pretreated with heparin was 0.89 (95% confidence interval [CI], 0.87-0.90).
The corresponding RR for death within 30 days was 0.87 (95% CI, 0.77-0.99), and for major in-hospital bleeding it was 1.01 (95% CI, 0.86-1.18).
The analysis was adjusted for other medications received before cath-lab arrival, especially a long list of antiplatelets and non-heparin antithrombins. That strengthens the case for heparin pretreatment as an independent predictor of an open IRA at initial angiography, Mr. Emilsson said.
Comparisons of propensity-score–matched subgroups of the total cohort, conducted separately for the IRA-occlusion endpoint and the endpoints of 30-day mortality and major bleeding, produced similar results.
Some observational data suggest that antiplatelet pretreatment with a P2Y12 inhibitor may promote IRA patency on angiography after cath lab arrival, Dr. Rao observed. “This indicates that there probably is a role of earlier antithrombotic therapy in STEMI patients, but the randomized trials have not shown a consistent benefit,” he said, referring in particular to the ATLANTIC trial.
Mr. Emilsson and Dr. Rao disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests a large registry study.
An open infarct-related artery (IRA) at angiography on cath-lab arrival presents STEMI patients an opportunity for earlier reperfusion and a chance, in theory at least, for smaller infarcts and maybe improved clinical outcomes.
In the new analysis, which covers more than 40,000 patients with STEMI in Sweden, the 38% who received heparin before cath-lab arrival were 11% less likely to show IRA occlusion at angiography prior to direct percutaneous coronary intervention (PCI). They also showed a 13% lower 30-day mortality compared with patients who were started on heparin in the cath lab. Importantly, their risk of major bleeding in the hospital did not increase.
The “early reperfusion” associated with IRA patency at angiography “could have long-term benefit due to smaller infarct size,” potentially explaining the observed 30-day survival gain in the pretreatment group, Oskar Love Emilsson, Lund (Sweden) University, said in an interview.
Mr. Emilsson, a third-year medical student, reported the analysis at the annual congress of the European Society of Cardiology, and is lead author on its same-day publication in the journal EuroIntervention.
He mentioned a few cautions in interpreting the study, which is based primarily on data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). It included several sensitivity analyses that continued to back pretreatment heparin as a significant predictor of an unoccluded IRA but didn’t consistently support the 30-day mortality benefit seen in the primary analysis.
And, although the pretreatment group overall didn’t have more major bleeds, the risk did go up significantly for those older than 75 or those who weighed less than 60 kg (132 pounds) or underwent catheterization with an access route other than the radial artery. Extra caution should be exercised in such patients who receive heparin before cath-lab arrival for PCI, Mr. Emilsson observed.
“Our results suggest that heparin pretreatment might be a good option to improve patency of infarct related arteries in STEMI,” and potentially clinical outcomes, he said. “However, a definite answer would require a randomized controlled trial.”
Meanwhile, the current study may be the largest yet to look at clinical outcomes after pretreatment with unfractionated heparin before PCI for acute STEMI, the report states. There have been some observational studies, subanalyses of STEMI trials, and even a few limited randomized trials – including the HEAP trial published in 2000 – to weigh in on the subject. Some have supported the strategy, others have not.
“With rapid door-to-balloon times in STEMI, it can be challenging to show a significant difference between a prehospital heparin approach and heparin given in the lab,” observed Sunil V. Rao, MD, NYU Langone Health System, New York, who is not connected with the current study.
Many EDs in the United States have “a STEMI protocol that calls for an IV bolus of heparin. It would be tougher in the U.S. to give it in the ambulance but again, it’s not clear how much advantage that would really provide,” he told this news organization.
Support from randomized trials would be needed before the practice could be formally recommended. “The SCAAR registries have set the standard for how registries should be conducted,” Dr. Rao said. “This is a very well done observational study, but it is observational.”
The priority for STEMI patients, he added, “really should be to get them to the lab as fast as possible. If the ED protocol includes heparin before the cath lab, that’s great, but I don’t think we should delay getting these patients to the lab to accommodate pre–cath-lab heparin.”
The current analysis covered 41,631 patients with STEMI from 2008 through to 2016, of whom 38% were pretreated with heparin in an ambulance or the ED. The remaining 62% initiated heparin in the cath lab.
About one-third of the group had an open IRA at angiography. The adjusted risk ratio (RR) for IRA occlusion at angiography for patients pretreated vs. not pretreated with heparin was 0.89 (95% confidence interval [CI], 0.87-0.90).
The corresponding RR for death within 30 days was 0.87 (95% CI, 0.77-0.99), and for major in-hospital bleeding it was 1.01 (95% CI, 0.86-1.18).
The analysis was adjusted for other medications received before cath-lab arrival, especially a long list of antiplatelets and non-heparin antithrombins. That strengthens the case for heparin pretreatment as an independent predictor of an open IRA at initial angiography, Mr. Emilsson said.
Comparisons of propensity-score–matched subgroups of the total cohort, conducted separately for the IRA-occlusion endpoint and the endpoints of 30-day mortality and major bleeding, produced similar results.
Some observational data suggest that antiplatelet pretreatment with a P2Y12 inhibitor may promote IRA patency on angiography after cath lab arrival, Dr. Rao observed. “This indicates that there probably is a role of earlier antithrombotic therapy in STEMI patients, but the randomized trials have not shown a consistent benefit,” he said, referring in particular to the ATLANTIC trial.
Mr. Emilsson and Dr. Rao disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
PARADISE-MI results obscured as post hoc analysis finds flaws
A post hoc analysis of the PARADISE-MI trial, although not intended to alter the conclusions generated by the published data, suggests that clinically relevant benefits were obscured, providing the basis for recommending different analyses for future studies that are more suited to capture the most clinically significant endpoints.
“What these data show us is that we need clinical trial designs moving towards more pragmatic information that better reflect clinical practice,” reported Otavio Berwanger, MD, PhD, director of the Academic Research Organization at Hospital Israelita Albert Einstein, São Paulo, Brazil.
The reevaluation of the PARADISE-MI data, presented at the annual congress of the European Society of Cardiology in Barcelona, was based on a win ratio analysis and on the inclusion of investigator-reported endpoints, not just adjudicated events. Both appear to reveal clinically meaningful benefits not reflected in the published study, according to Dr. Berwanger.
In PARADISE-MI, which was published in the New England Journal of Medicine last year, more than 5,500 patients were randomized to the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan or the ACE inhibitor ramipril after a myocardial infarction. A reduced left ventricular ejection fraction (LVEF), pulmonary congestion, or both were required for enrollment.
For the primary composite outcomes of death from cardiovascular (CV) causes or incident heart failure, the ARNI had a 10% numerical advantage, but it did not reach statistical significance (hazard ratio [HR], 0.90; P = .17).
“PARADISE-MI was a neutral trial. This post hoc analysis will not change that result,” Dr. Berwanger emphasized. However, the post hoc analysis does provide a basis for exploring why conventional trial designs might not be providing answers that are relevant and helpful for clinical practice.
New analysis provides positive trial result
When the data from PARADISE-MI are reevaluated in a hierarchical win ratio analysis with CV death serving as the most severe and important outcome, the principal conclusion changes. Whether events are reevaluated in this format by the clinical events committee (CEC) or by investigators, there is a greater number of total wins than total losses for the ARNI. Combined, sacubitril/valsartan was associated with a win ratio of 1.17 (95% confidence interval, 1.03-1.33; P = 0.015) over ramipril.
Using a sports analogy, Dr. Berwanger explained that the win ratio analysis divides the total number of wins to the total number of losses to provide a much more clinically relevant approach to keeping score. It also used a hierarchical analysis so that the most serious and important events are considered first.
In addition to CV death, this analysis included first hospitalization for heart failure and first outpatient heart failure events. CEC-defined events and events reported by investigators were evaluated separately.
The ARNI had more wins than losses in every category for all outcomes, whether CEC adjudicated or investigator reported, but most of this benefit was generated by the endpoint of CEC-adjudicated CV deaths. This accounted for 36.9% of all events (investigator-documented CV death accounted for 0.7%). This is important because PARADISE-MI, like many standard trials, was conducted on a time-to-primary event basis.
“In this type of analysis, the first event is what counts. Usually time-to-first-event analyses are dominated by nonfatal events,” Dr. Berwanger explained. He believes that placing more weight on the most serious events results in an emphasis on what outcomes are of greatest clinical interest.
In addition, Dr. Berwanger argued that it is important to consider investigator-reported events, not just CEC-adjudicated events. While adjudicated events improve the rigor of the data, Dr. Berwanger suggested it omits outcomes with which clinicians are most concerned.
Investigator, adjudicated outcomes differ
Again, using PARADISE-MI as an example, he reevaluated the primary outcome based on investigator reports. When investigator-reported events are included, the number of events increased in both the ARNI (443 vs. 338) and ramipril (516 vs. 373) arms, but the advantage of the ARNI over the ACE inhibitors now reached statistical significance (HR, 0.85; P = .01).
“The data suggest that maybe we should find definitions for adjudication that are closer to clinical judgment in the real world and clinical practice,” Dr. Berwanger said.
One possible explanation for the neutral result in PARADISE-MI is that benefit of an ARNI over an ACE inhibitor would only be expected in those at risk for progressive left ventricular dysfunction, and it is likely that a substantial proportion of patients enrolled in this trial recovered, according to Johann Bauersachs, MD, PhD, professor and head of cardiology at Hannover (Germany) Medical School.
“You cannot predict which patients with reduced LV function following an MI will go on to chronic remodeling and which will recover,” said Dr. Bauersachs, who was an ESC-invited discussant of Dr. Berwanger’s post hoc analysis.
He agreed that Dr. Berwanger has raised several important issues in standard trial design that might have prevented PARADISE-MI from showing a benefit from an ARNI, but he pointed out that there are other potential issues, such as the low use of mineralocorticoid antagonists in PARADISE-MI, that may have skewed results.
However, he agreed generally with the premise that there is a need for trial design likely to generate more clinically useful information.
“We have now seen the win-ratio approach used in several studies,” said Dr. Bauersachs, citing in particular the EMPULSE trial presented at the 2022 meeting of the American College of Cardiology. “It is a very useful tool, and I think we will be seeing it used more in the future.”
However, he indicated that the issues raised by Dr. Berwanger are not necessarily easily resolved. Dr. Bauersachs endorsed the effort to consider trial designs that generate data that are more immediately clinically applicable but suggested that different types of designs may be required for different types of clinical questions.
Dr. Berwanger reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Pfizer, Servier, and Novartis, which provided funding for the PARADISE-MI trial. Dr. Bauersachs reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Cardior, Corvia, CVRx, Novartis, Pfizer, Vifor, and Zoll.
A post hoc analysis of the PARADISE-MI trial, although not intended to alter the conclusions generated by the published data, suggests that clinically relevant benefits were obscured, providing the basis for recommending different analyses for future studies that are more suited to capture the most clinically significant endpoints.
“What these data show us is that we need clinical trial designs moving towards more pragmatic information that better reflect clinical practice,” reported Otavio Berwanger, MD, PhD, director of the Academic Research Organization at Hospital Israelita Albert Einstein, São Paulo, Brazil.
The reevaluation of the PARADISE-MI data, presented at the annual congress of the European Society of Cardiology in Barcelona, was based on a win ratio analysis and on the inclusion of investigator-reported endpoints, not just adjudicated events. Both appear to reveal clinically meaningful benefits not reflected in the published study, according to Dr. Berwanger.
In PARADISE-MI, which was published in the New England Journal of Medicine last year, more than 5,500 patients were randomized to the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan or the ACE inhibitor ramipril after a myocardial infarction. A reduced left ventricular ejection fraction (LVEF), pulmonary congestion, or both were required for enrollment.
For the primary composite outcomes of death from cardiovascular (CV) causes or incident heart failure, the ARNI had a 10% numerical advantage, but it did not reach statistical significance (hazard ratio [HR], 0.90; P = .17).
“PARADISE-MI was a neutral trial. This post hoc analysis will not change that result,” Dr. Berwanger emphasized. However, the post hoc analysis does provide a basis for exploring why conventional trial designs might not be providing answers that are relevant and helpful for clinical practice.
New analysis provides positive trial result
When the data from PARADISE-MI are reevaluated in a hierarchical win ratio analysis with CV death serving as the most severe and important outcome, the principal conclusion changes. Whether events are reevaluated in this format by the clinical events committee (CEC) or by investigators, there is a greater number of total wins than total losses for the ARNI. Combined, sacubitril/valsartan was associated with a win ratio of 1.17 (95% confidence interval, 1.03-1.33; P = 0.015) over ramipril.
Using a sports analogy, Dr. Berwanger explained that the win ratio analysis divides the total number of wins to the total number of losses to provide a much more clinically relevant approach to keeping score. It also used a hierarchical analysis so that the most serious and important events are considered first.
In addition to CV death, this analysis included first hospitalization for heart failure and first outpatient heart failure events. CEC-defined events and events reported by investigators were evaluated separately.
The ARNI had more wins than losses in every category for all outcomes, whether CEC adjudicated or investigator reported, but most of this benefit was generated by the endpoint of CEC-adjudicated CV deaths. This accounted for 36.9% of all events (investigator-documented CV death accounted for 0.7%). This is important because PARADISE-MI, like many standard trials, was conducted on a time-to-primary event basis.
“In this type of analysis, the first event is what counts. Usually time-to-first-event analyses are dominated by nonfatal events,” Dr. Berwanger explained. He believes that placing more weight on the most serious events results in an emphasis on what outcomes are of greatest clinical interest.
In addition, Dr. Berwanger argued that it is important to consider investigator-reported events, not just CEC-adjudicated events. While adjudicated events improve the rigor of the data, Dr. Berwanger suggested it omits outcomes with which clinicians are most concerned.
Investigator, adjudicated outcomes differ
Again, using PARADISE-MI as an example, he reevaluated the primary outcome based on investigator reports. When investigator-reported events are included, the number of events increased in both the ARNI (443 vs. 338) and ramipril (516 vs. 373) arms, but the advantage of the ARNI over the ACE inhibitors now reached statistical significance (HR, 0.85; P = .01).
“The data suggest that maybe we should find definitions for adjudication that are closer to clinical judgment in the real world and clinical practice,” Dr. Berwanger said.
One possible explanation for the neutral result in PARADISE-MI is that benefit of an ARNI over an ACE inhibitor would only be expected in those at risk for progressive left ventricular dysfunction, and it is likely that a substantial proportion of patients enrolled in this trial recovered, according to Johann Bauersachs, MD, PhD, professor and head of cardiology at Hannover (Germany) Medical School.
“You cannot predict which patients with reduced LV function following an MI will go on to chronic remodeling and which will recover,” said Dr. Bauersachs, who was an ESC-invited discussant of Dr. Berwanger’s post hoc analysis.
He agreed that Dr. Berwanger has raised several important issues in standard trial design that might have prevented PARADISE-MI from showing a benefit from an ARNI, but he pointed out that there are other potential issues, such as the low use of mineralocorticoid antagonists in PARADISE-MI, that may have skewed results.
However, he agreed generally with the premise that there is a need for trial design likely to generate more clinically useful information.
“We have now seen the win-ratio approach used in several studies,” said Dr. Bauersachs, citing in particular the EMPULSE trial presented at the 2022 meeting of the American College of Cardiology. “It is a very useful tool, and I think we will be seeing it used more in the future.”
However, he indicated that the issues raised by Dr. Berwanger are not necessarily easily resolved. Dr. Bauersachs endorsed the effort to consider trial designs that generate data that are more immediately clinically applicable but suggested that different types of designs may be required for different types of clinical questions.
Dr. Berwanger reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Pfizer, Servier, and Novartis, which provided funding for the PARADISE-MI trial. Dr. Bauersachs reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Cardior, Corvia, CVRx, Novartis, Pfizer, Vifor, and Zoll.
A post hoc analysis of the PARADISE-MI trial, although not intended to alter the conclusions generated by the published data, suggests that clinically relevant benefits were obscured, providing the basis for recommending different analyses for future studies that are more suited to capture the most clinically significant endpoints.
“What these data show us is that we need clinical trial designs moving towards more pragmatic information that better reflect clinical practice,” reported Otavio Berwanger, MD, PhD, director of the Academic Research Organization at Hospital Israelita Albert Einstein, São Paulo, Brazil.
The reevaluation of the PARADISE-MI data, presented at the annual congress of the European Society of Cardiology in Barcelona, was based on a win ratio analysis and on the inclusion of investigator-reported endpoints, not just adjudicated events. Both appear to reveal clinically meaningful benefits not reflected in the published study, according to Dr. Berwanger.
In PARADISE-MI, which was published in the New England Journal of Medicine last year, more than 5,500 patients were randomized to the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan or the ACE inhibitor ramipril after a myocardial infarction. A reduced left ventricular ejection fraction (LVEF), pulmonary congestion, or both were required for enrollment.
For the primary composite outcomes of death from cardiovascular (CV) causes or incident heart failure, the ARNI had a 10% numerical advantage, but it did not reach statistical significance (hazard ratio [HR], 0.90; P = .17).
“PARADISE-MI was a neutral trial. This post hoc analysis will not change that result,” Dr. Berwanger emphasized. However, the post hoc analysis does provide a basis for exploring why conventional trial designs might not be providing answers that are relevant and helpful for clinical practice.
New analysis provides positive trial result
When the data from PARADISE-MI are reevaluated in a hierarchical win ratio analysis with CV death serving as the most severe and important outcome, the principal conclusion changes. Whether events are reevaluated in this format by the clinical events committee (CEC) or by investigators, there is a greater number of total wins than total losses for the ARNI. Combined, sacubitril/valsartan was associated with a win ratio of 1.17 (95% confidence interval, 1.03-1.33; P = 0.015) over ramipril.
Using a sports analogy, Dr. Berwanger explained that the win ratio analysis divides the total number of wins to the total number of losses to provide a much more clinically relevant approach to keeping score. It also used a hierarchical analysis so that the most serious and important events are considered first.
In addition to CV death, this analysis included first hospitalization for heart failure and first outpatient heart failure events. CEC-defined events and events reported by investigators were evaluated separately.
The ARNI had more wins than losses in every category for all outcomes, whether CEC adjudicated or investigator reported, but most of this benefit was generated by the endpoint of CEC-adjudicated CV deaths. This accounted for 36.9% of all events (investigator-documented CV death accounted for 0.7%). This is important because PARADISE-MI, like many standard trials, was conducted on a time-to-primary event basis.
“In this type of analysis, the first event is what counts. Usually time-to-first-event analyses are dominated by nonfatal events,” Dr. Berwanger explained. He believes that placing more weight on the most serious events results in an emphasis on what outcomes are of greatest clinical interest.
In addition, Dr. Berwanger argued that it is important to consider investigator-reported events, not just CEC-adjudicated events. While adjudicated events improve the rigor of the data, Dr. Berwanger suggested it omits outcomes with which clinicians are most concerned.
Investigator, adjudicated outcomes differ
Again, using PARADISE-MI as an example, he reevaluated the primary outcome based on investigator reports. When investigator-reported events are included, the number of events increased in both the ARNI (443 vs. 338) and ramipril (516 vs. 373) arms, but the advantage of the ARNI over the ACE inhibitors now reached statistical significance (HR, 0.85; P = .01).
“The data suggest that maybe we should find definitions for adjudication that are closer to clinical judgment in the real world and clinical practice,” Dr. Berwanger said.
One possible explanation for the neutral result in PARADISE-MI is that benefit of an ARNI over an ACE inhibitor would only be expected in those at risk for progressive left ventricular dysfunction, and it is likely that a substantial proportion of patients enrolled in this trial recovered, according to Johann Bauersachs, MD, PhD, professor and head of cardiology at Hannover (Germany) Medical School.
“You cannot predict which patients with reduced LV function following an MI will go on to chronic remodeling and which will recover,” said Dr. Bauersachs, who was an ESC-invited discussant of Dr. Berwanger’s post hoc analysis.
He agreed that Dr. Berwanger has raised several important issues in standard trial design that might have prevented PARADISE-MI from showing a benefit from an ARNI, but he pointed out that there are other potential issues, such as the low use of mineralocorticoid antagonists in PARADISE-MI, that may have skewed results.
However, he agreed generally with the premise that there is a need for trial design likely to generate more clinically useful information.
“We have now seen the win-ratio approach used in several studies,” said Dr. Bauersachs, citing in particular the EMPULSE trial presented at the 2022 meeting of the American College of Cardiology. “It is a very useful tool, and I think we will be seeing it used more in the future.”
However, he indicated that the issues raised by Dr. Berwanger are not necessarily easily resolved. Dr. Bauersachs endorsed the effort to consider trial designs that generate data that are more immediately clinically applicable but suggested that different types of designs may be required for different types of clinical questions.
Dr. Berwanger reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Pfizer, Servier, and Novartis, which provided funding for the PARADISE-MI trial. Dr. Bauersachs reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Cardior, Corvia, CVRx, Novartis, Pfizer, Vifor, and Zoll.
FROM ESC CONGRESS 2022
Fish oil pills do not reduce fractures in healthy seniors: VITAL
Omega-3 supplements did not reduce fractures during a median 5.3-year follow-up in the more than 25,000 generally healthy men and women (≥ age 50 and ≥ age 55, respectively) in the Vitamin D and Omega-3 Trial (VITAL).
The large randomized controlled trial tested whether omega-3 fatty acid or vitamin D supplements prevented cardiovascular disease or cancer in a representative sample of midlife and older adults from 50 U.S. states – which they did not. In a further analysis of VITAL, vitamin D supplements (cholecalciferol, 2,000 IU/day) did not lower the risk of incident total, nonvertebral, and hip fractures, compared with placebo.
Now this new analysis shows that omega-3 fatty acid supplements (1 g/day of fish oil) did not reduce the risk of such fractures in the VITAL population either. Meryl S. LeBoff, MD, presented the latest findings during an oral session at the annual meeting of the American Society for Bone and Mineral Research.
“In this, the largest randomized controlled trial in the world, we did not find an effect of omega-3 fatty acid supplements on fractures,” Dr. LeBoff, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston, told this news organization.
The current analysis did “unexpectedly” show that among participants who received the omega-3 fatty acid supplements, there was an increase in fractures in men, and fracture risk was higher in people with a normal or low body mass index and lower in people with higher BMI.
However, these subgroup findings need to be interpreted with caution and may be caused by chance, Dr. LeBoff warned. The researchers will be investigating these findings in further analyses.
Should patients take omega-3 supplements or not?
Asked whether, in the meantime, patients should start or keep taking fish oil supplements for possible health benefits, she noted that certain individuals might benefit.
For example, in VITAL, participants who ate less than 1.5 servings of fish per week and received omega-3 fatty acid supplements had a decrease in the combined cardiovascular endpoint, and Black participants who took fish oil supplements had a substantially reduced risk of the outcome, regardless of fish intake.
“I think everybody needs to review [the study findings] with clinicians and make a decision in terms of what would be best for them,” she said.
Session comoderator Bente Langdahl, MD, PhD, commented that “many people take omega-3 because they think it will help” knee, hip, or other joint pain.
Perhaps men are more prone to joint pain because of osteoarthritis and the supplements lessen the pain, so these men became more physically active and more prone to fractures, she speculated.
The current study shows that, “so far, we haven’t been able to demonstrate a reduced rate of fractures with fish oil supplements in clinical randomized trials” conducted in relatively healthy and not the oldest patients, she summarized. “We’re not talking about 80-year-olds.”
In this “well-conducted study, they were not able to see any difference” with omega-3 fatty acid supplements versus placebo, but apparently, there are no harms associated with taking these supplements, she said.
To patients who ask her about such supplements, Dr. Langdahl advised: “Try it out for 3 months. If it really helps you, if it takes away your joint pain or whatever, then that might work for you. But then remember to stop again because it might just be a temporary effect.”
Could fish oil supplements protect against fractures?
An estimated 22% of U.S. adults aged 60 and older take omega-3 fatty acid supplements, Dr. LeBoff noted.
Preclinical studies have shown that omega-3 fatty acids reduce bone resorption and have anti-inflammatory effects, but observational studies have reported conflicting findings.
The researchers conducted this ancillary study of VITAL to fill these knowledge gaps.
VITAL enrolled a national sample of 25,871 U.S. men and women, including 5,106 Black participants, with a mean age of 67 and a mean BMI of 28 kg/m2.
Importantly, participants were not recruited by low bone density, fractures, or vitamin D deficiency. Prior to entry, participants were required to stop taking omega-3 supplements and limit nonstudy vitamin D and calcium supplements.
The omega-3 fatty acid supplements used in the study contained eicosapentaenoic acid and docosahexaenoic acid in a 1.2:1 ratio.
VITAL had a 2x2 factorial design whereby 6,463 participants were randomized to receive the omega-3 fatty acid supplement and 6,474 were randomized to placebo. (Remaining participants were randomized to receive vitamin D or placebo.)
Participants in the omega-3 fatty acid and placebo groups had similar baseline characteristics. For example, about half (50.5%) were women, and on average, they ate 1.1 servings of dark-meat fish (such as salmon) per week.
Participants completed detailed questionnaires at baseline and each year.
Plasma omega-3 levels were measured at baseline and, in 1,583 participants, at 1 year of follow-up. The mean omega-3 index rose 54.7% in the omega-3 fatty acid group and changed less than 2% in the placebo group at 1 year.
Study pill adherence was 87.0% at 2 years and 85.7% at 5 years.
Fractures were self-reported on annual questionnaires and centrally adjudicated in medical record review.
No clinically meaningful effect of omega-3 fatty acids on fractures
During a median 5.3-year follow-up, researchers adjudicated 2,133 total fractures and confirmed 1,991 fractures (93%) in 1551 participants.
Incidences of total, nonvertebral, and hip fractures were similar in both groups.
Compared with placebo, omega-3 fatty acid supplements had no significant effect on risk of total fractures (hazard ratio, 1.02; 95% confidence interval, 0.92-1.13), nonvertebral fractures (HR, 1.01; 95% CI, 0.91-1.12), or hip fractures (HR, 0.89; 95% CI, 0.61-1.30), all adjusted for age, sex, and race.
The “confidence intervals were narrow, likely excluding a clinically meaningful effect,” Dr. LeBoff noted.
Among men, those who received fish oil supplements had a greater risk of fracture than those who received placebo (HR, 1.27; 95% CI, 1.07-1.51), but this result “was not corrected for multiple hypothesis testing,” Dr. LeBoff cautioned.
In the overall population, participants with a BMI less than 25 who received fish oil versus placebo had an increased risk of fracture, and those with a BMI of at least 30 who received fish oil versus placebo had a decreased risk of fracture, but the limits of the confidence intervals crossed 1.00.
After excluding digit, skull, and pathologic fractures, there was no significant reduction in total fractures (HR, 1.02; 95% CI, 0.92-1.14), nonvertebral fractures (HR, 1.02; 95% CI, 0.92-1.14), or hip fractures (HR, 0.90; 95% CI, 0.61-1.33), with omega-3 supplements versus placebo.
Similarly, there was no significant reduction in risk of major osteoporotic fractures (hip, wrist, humerus, and clinical spine fractures) or wrist fractures with omega-3 supplements versus placebo.
VITAL only studied one dose of omega-3 fatty acid supplements, and results may not be generalizable to younger adults, or older adults living in residential communities, Dr. LeBoff noted.
The study was supported by grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. VITAL was funded by the National Cancer Institute and the National Heart, Lung, and Blood Institute. Dr. LeBoff and Dr. Langdahl have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Omega-3 supplements did not reduce fractures during a median 5.3-year follow-up in the more than 25,000 generally healthy men and women (≥ age 50 and ≥ age 55, respectively) in the Vitamin D and Omega-3 Trial (VITAL).
The large randomized controlled trial tested whether omega-3 fatty acid or vitamin D supplements prevented cardiovascular disease or cancer in a representative sample of midlife and older adults from 50 U.S. states – which they did not. In a further analysis of VITAL, vitamin D supplements (cholecalciferol, 2,000 IU/day) did not lower the risk of incident total, nonvertebral, and hip fractures, compared with placebo.
Now this new analysis shows that omega-3 fatty acid supplements (1 g/day of fish oil) did not reduce the risk of such fractures in the VITAL population either. Meryl S. LeBoff, MD, presented the latest findings during an oral session at the annual meeting of the American Society for Bone and Mineral Research.
“In this, the largest randomized controlled trial in the world, we did not find an effect of omega-3 fatty acid supplements on fractures,” Dr. LeBoff, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston, told this news organization.
The current analysis did “unexpectedly” show that among participants who received the omega-3 fatty acid supplements, there was an increase in fractures in men, and fracture risk was higher in people with a normal or low body mass index and lower in people with higher BMI.
However, these subgroup findings need to be interpreted with caution and may be caused by chance, Dr. LeBoff warned. The researchers will be investigating these findings in further analyses.
Should patients take omega-3 supplements or not?
Asked whether, in the meantime, patients should start or keep taking fish oil supplements for possible health benefits, she noted that certain individuals might benefit.
For example, in VITAL, participants who ate less than 1.5 servings of fish per week and received omega-3 fatty acid supplements had a decrease in the combined cardiovascular endpoint, and Black participants who took fish oil supplements had a substantially reduced risk of the outcome, regardless of fish intake.
“I think everybody needs to review [the study findings] with clinicians and make a decision in terms of what would be best for them,” she said.
Session comoderator Bente Langdahl, MD, PhD, commented that “many people take omega-3 because they think it will help” knee, hip, or other joint pain.
Perhaps men are more prone to joint pain because of osteoarthritis and the supplements lessen the pain, so these men became more physically active and more prone to fractures, she speculated.
The current study shows that, “so far, we haven’t been able to demonstrate a reduced rate of fractures with fish oil supplements in clinical randomized trials” conducted in relatively healthy and not the oldest patients, she summarized. “We’re not talking about 80-year-olds.”
In this “well-conducted study, they were not able to see any difference” with omega-3 fatty acid supplements versus placebo, but apparently, there are no harms associated with taking these supplements, she said.
To patients who ask her about such supplements, Dr. Langdahl advised: “Try it out for 3 months. If it really helps you, if it takes away your joint pain or whatever, then that might work for you. But then remember to stop again because it might just be a temporary effect.”
Could fish oil supplements protect against fractures?
An estimated 22% of U.S. adults aged 60 and older take omega-3 fatty acid supplements, Dr. LeBoff noted.
Preclinical studies have shown that omega-3 fatty acids reduce bone resorption and have anti-inflammatory effects, but observational studies have reported conflicting findings.
The researchers conducted this ancillary study of VITAL to fill these knowledge gaps.
VITAL enrolled a national sample of 25,871 U.S. men and women, including 5,106 Black participants, with a mean age of 67 and a mean BMI of 28 kg/m2.
Importantly, participants were not recruited by low bone density, fractures, or vitamin D deficiency. Prior to entry, participants were required to stop taking omega-3 supplements and limit nonstudy vitamin D and calcium supplements.
The omega-3 fatty acid supplements used in the study contained eicosapentaenoic acid and docosahexaenoic acid in a 1.2:1 ratio.
VITAL had a 2x2 factorial design whereby 6,463 participants were randomized to receive the omega-3 fatty acid supplement and 6,474 were randomized to placebo. (Remaining participants were randomized to receive vitamin D or placebo.)
Participants in the omega-3 fatty acid and placebo groups had similar baseline characteristics. For example, about half (50.5%) were women, and on average, they ate 1.1 servings of dark-meat fish (such as salmon) per week.
Participants completed detailed questionnaires at baseline and each year.
Plasma omega-3 levels were measured at baseline and, in 1,583 participants, at 1 year of follow-up. The mean omega-3 index rose 54.7% in the omega-3 fatty acid group and changed less than 2% in the placebo group at 1 year.
Study pill adherence was 87.0% at 2 years and 85.7% at 5 years.
Fractures were self-reported on annual questionnaires and centrally adjudicated in medical record review.
No clinically meaningful effect of omega-3 fatty acids on fractures
During a median 5.3-year follow-up, researchers adjudicated 2,133 total fractures and confirmed 1,991 fractures (93%) in 1551 participants.
Incidences of total, nonvertebral, and hip fractures were similar in both groups.
Compared with placebo, omega-3 fatty acid supplements had no significant effect on risk of total fractures (hazard ratio, 1.02; 95% confidence interval, 0.92-1.13), nonvertebral fractures (HR, 1.01; 95% CI, 0.91-1.12), or hip fractures (HR, 0.89; 95% CI, 0.61-1.30), all adjusted for age, sex, and race.
The “confidence intervals were narrow, likely excluding a clinically meaningful effect,” Dr. LeBoff noted.
Among men, those who received fish oil supplements had a greater risk of fracture than those who received placebo (HR, 1.27; 95% CI, 1.07-1.51), but this result “was not corrected for multiple hypothesis testing,” Dr. LeBoff cautioned.
In the overall population, participants with a BMI less than 25 who received fish oil versus placebo had an increased risk of fracture, and those with a BMI of at least 30 who received fish oil versus placebo had a decreased risk of fracture, but the limits of the confidence intervals crossed 1.00.
After excluding digit, skull, and pathologic fractures, there was no significant reduction in total fractures (HR, 1.02; 95% CI, 0.92-1.14), nonvertebral fractures (HR, 1.02; 95% CI, 0.92-1.14), or hip fractures (HR, 0.90; 95% CI, 0.61-1.33), with omega-3 supplements versus placebo.
Similarly, there was no significant reduction in risk of major osteoporotic fractures (hip, wrist, humerus, and clinical spine fractures) or wrist fractures with omega-3 supplements versus placebo.
VITAL only studied one dose of omega-3 fatty acid supplements, and results may not be generalizable to younger adults, or older adults living in residential communities, Dr. LeBoff noted.
The study was supported by grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. VITAL was funded by the National Cancer Institute and the National Heart, Lung, and Blood Institute. Dr. LeBoff and Dr. Langdahl have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Omega-3 supplements did not reduce fractures during a median 5.3-year follow-up in the more than 25,000 generally healthy men and women (≥ age 50 and ≥ age 55, respectively) in the Vitamin D and Omega-3 Trial (VITAL).
The large randomized controlled trial tested whether omega-3 fatty acid or vitamin D supplements prevented cardiovascular disease or cancer in a representative sample of midlife and older adults from 50 U.S. states – which they did not. In a further analysis of VITAL, vitamin D supplements (cholecalciferol, 2,000 IU/day) did not lower the risk of incident total, nonvertebral, and hip fractures, compared with placebo.
Now this new analysis shows that omega-3 fatty acid supplements (1 g/day of fish oil) did not reduce the risk of such fractures in the VITAL population either. Meryl S. LeBoff, MD, presented the latest findings during an oral session at the annual meeting of the American Society for Bone and Mineral Research.
“In this, the largest randomized controlled trial in the world, we did not find an effect of omega-3 fatty acid supplements on fractures,” Dr. LeBoff, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston, told this news organization.
The current analysis did “unexpectedly” show that among participants who received the omega-3 fatty acid supplements, there was an increase in fractures in men, and fracture risk was higher in people with a normal or low body mass index and lower in people with higher BMI.
However, these subgroup findings need to be interpreted with caution and may be caused by chance, Dr. LeBoff warned. The researchers will be investigating these findings in further analyses.
Should patients take omega-3 supplements or not?
Asked whether, in the meantime, patients should start or keep taking fish oil supplements for possible health benefits, she noted that certain individuals might benefit.
For example, in VITAL, participants who ate less than 1.5 servings of fish per week and received omega-3 fatty acid supplements had a decrease in the combined cardiovascular endpoint, and Black participants who took fish oil supplements had a substantially reduced risk of the outcome, regardless of fish intake.
“I think everybody needs to review [the study findings] with clinicians and make a decision in terms of what would be best for them,” she said.
Session comoderator Bente Langdahl, MD, PhD, commented that “many people take omega-3 because they think it will help” knee, hip, or other joint pain.
Perhaps men are more prone to joint pain because of osteoarthritis and the supplements lessen the pain, so these men became more physically active and more prone to fractures, she speculated.
The current study shows that, “so far, we haven’t been able to demonstrate a reduced rate of fractures with fish oil supplements in clinical randomized trials” conducted in relatively healthy and not the oldest patients, she summarized. “We’re not talking about 80-year-olds.”
In this “well-conducted study, they were not able to see any difference” with omega-3 fatty acid supplements versus placebo, but apparently, there are no harms associated with taking these supplements, she said.
To patients who ask her about such supplements, Dr. Langdahl advised: “Try it out for 3 months. If it really helps you, if it takes away your joint pain or whatever, then that might work for you. But then remember to stop again because it might just be a temporary effect.”
Could fish oil supplements protect against fractures?
An estimated 22% of U.S. adults aged 60 and older take omega-3 fatty acid supplements, Dr. LeBoff noted.
Preclinical studies have shown that omega-3 fatty acids reduce bone resorption and have anti-inflammatory effects, but observational studies have reported conflicting findings.
The researchers conducted this ancillary study of VITAL to fill these knowledge gaps.
VITAL enrolled a national sample of 25,871 U.S. men and women, including 5,106 Black participants, with a mean age of 67 and a mean BMI of 28 kg/m2.
Importantly, participants were not recruited by low bone density, fractures, or vitamin D deficiency. Prior to entry, participants were required to stop taking omega-3 supplements and limit nonstudy vitamin D and calcium supplements.
The omega-3 fatty acid supplements used in the study contained eicosapentaenoic acid and docosahexaenoic acid in a 1.2:1 ratio.
VITAL had a 2x2 factorial design whereby 6,463 participants were randomized to receive the omega-3 fatty acid supplement and 6,474 were randomized to placebo. (Remaining participants were randomized to receive vitamin D or placebo.)
Participants in the omega-3 fatty acid and placebo groups had similar baseline characteristics. For example, about half (50.5%) were women, and on average, they ate 1.1 servings of dark-meat fish (such as salmon) per week.
Participants completed detailed questionnaires at baseline and each year.
Plasma omega-3 levels were measured at baseline and, in 1,583 participants, at 1 year of follow-up. The mean omega-3 index rose 54.7% in the omega-3 fatty acid group and changed less than 2% in the placebo group at 1 year.
Study pill adherence was 87.0% at 2 years and 85.7% at 5 years.
Fractures were self-reported on annual questionnaires and centrally adjudicated in medical record review.
No clinically meaningful effect of omega-3 fatty acids on fractures
During a median 5.3-year follow-up, researchers adjudicated 2,133 total fractures and confirmed 1,991 fractures (93%) in 1551 participants.
Incidences of total, nonvertebral, and hip fractures were similar in both groups.
Compared with placebo, omega-3 fatty acid supplements had no significant effect on risk of total fractures (hazard ratio, 1.02; 95% confidence interval, 0.92-1.13), nonvertebral fractures (HR, 1.01; 95% CI, 0.91-1.12), or hip fractures (HR, 0.89; 95% CI, 0.61-1.30), all adjusted for age, sex, and race.
The “confidence intervals were narrow, likely excluding a clinically meaningful effect,” Dr. LeBoff noted.
Among men, those who received fish oil supplements had a greater risk of fracture than those who received placebo (HR, 1.27; 95% CI, 1.07-1.51), but this result “was not corrected for multiple hypothesis testing,” Dr. LeBoff cautioned.
In the overall population, participants with a BMI less than 25 who received fish oil versus placebo had an increased risk of fracture, and those with a BMI of at least 30 who received fish oil versus placebo had a decreased risk of fracture, but the limits of the confidence intervals crossed 1.00.
After excluding digit, skull, and pathologic fractures, there was no significant reduction in total fractures (HR, 1.02; 95% CI, 0.92-1.14), nonvertebral fractures (HR, 1.02; 95% CI, 0.92-1.14), or hip fractures (HR, 0.90; 95% CI, 0.61-1.33), with omega-3 supplements versus placebo.
Similarly, there was no significant reduction in risk of major osteoporotic fractures (hip, wrist, humerus, and clinical spine fractures) or wrist fractures with omega-3 supplements versus placebo.
VITAL only studied one dose of omega-3 fatty acid supplements, and results may not be generalizable to younger adults, or older adults living in residential communities, Dr. LeBoff noted.
The study was supported by grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. VITAL was funded by the National Cancer Institute and the National Heart, Lung, and Blood Institute. Dr. LeBoff and Dr. Langdahl have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASMBR 2022
Myocardial infarction in women younger than 50: Lessons to learn
Young women (under 50) are increasingly having heart attacks without doctors really knowing why. This is where the Young Women Presenting Acute Myocardial Infarction in France (WAMIF) study comes in, the results of which were presented in an e-poster at the annual congress of the European Society of Cardiology by Stéphane Manzo-Silberman, MD, Institute of Cardiology, Pitié-Salpétrière, Paris. The results (yet to be published) fight several of the preconceived ideas on the topic, Dr. Manzo-Silberman commented in an interview.
Significantly higher hospital death rates in women
“Cardiovascular disease is the main cause of death in women, killing seven times more than breast cancer,” notes Dr. Manzo-Silberman. The hospital death rate is significantly higher in women and, despite going down, is significantly higher than in men (more than double), particularly in women under 50. What’s more, in addition to the typical risk factors, women present specific risk factors related to hormone changes, high-risk inflammatory profiles, and thrombophilia.”
The WAMIF study was designed to determine the clinical, biological, and morphological features linked to hospital mortality after 12 months in women under 50. The prospective, observational study included all women in this age range from 30 sites in France between May 2017 and June 2019.
90% with retrosternal chest pain
The age of the 314 women enrolled was 44.9 years on average. Nearly two-thirds (192) presented with ST-segment elevation myocardial infarction and the other 122 without. In terms of symptoms, 91.6% of these women presented with typical chest pain, and 59.7% had related symptoms.
“With more than 90% having retrosternal pain, the idea that myocardial infarction presents with atypical symptoms in women has been widely challenged, despite the fact that more than half present with related symptoms and it isn’t known in which order these symptoms occur, Dr. Manzo-Silberman said in an interview. But what we can say is that if at any point a young woman mentions chest pain, even when occurring as part of several other symptoms, MI must be deemed a possibility until it has been ruled out.”
The risk profile revealed that 75.5% were smokers, 35% had a family history of heart disease, 33% had pregnancy complications, and 55% had recently experienced a stressful situation. The analysis also showed that cannabis use and oral contraception were primary risk factors in women younger than 35.
“With regard to risk factors, when designing this study we expected that lots of these young women would have largely atypical autoimmune conditions, with high levels of inflammation. We looked for everything, but this was not actually the case. Instead, we found very many women to have classic risk factors; three-quarters were smokers, a modifiable risk factor, which can largely be prevented. The other aspect concerns contraception, and it’s why I insist that gynecologists must be involved insofar as they must inform their patients how to manage their risk factors and tweak their contraception.”
Coronary angiography findings showed that only 1% received a normal result, 29.3% had vessel damage, and 14.6% had aortic dissection. “We were surprised again here because we expected that with young women we would see lots of heart attacks without obstruction, [in other words] normal coronary arteries, atypical forms of MI,” commented Dr. Manzo-Silberman. “In fact, most presented with atheroma, often obstructive lesions, or even triple-vessel disease, in nearly a third of the cohort. So that’s another misconception dispelled – we can’t just think that because a woman is young, nothing will be found. Coronary catheterization should be considered, and the diagnostic process should be completed in full.”
After 1 year, there had been two cancer-related deaths and 25 patients had undergone several angioplasty procedures. Nevertheless, 90.4% had not experienced any type of CV event, and 72% had not even had any symptoms.
“The final surprise was prognosis,” he said. “Previous studies, especially some authored by Viola Vaccarino, MD, PhD, showed an excess hospital rate in women and we had expected this to be the case here, but no hospital deaths were recorded. However, not far off 10% of women attended (at least once) the emergency department in the year following for recurrent chest pain which was not ischemic – ECG normal, troponin normal – so something was missing in their education as a patient.”
“So, there are improvements to be made in terms of secondary prevention, follow-up, and in the education of these young female patients who have experienced the major event that is a myocardial infarction,” concluded Dr. Manzo-Silberman.
This content was originally published on Medscape French edition.
Young women (under 50) are increasingly having heart attacks without doctors really knowing why. This is where the Young Women Presenting Acute Myocardial Infarction in France (WAMIF) study comes in, the results of which were presented in an e-poster at the annual congress of the European Society of Cardiology by Stéphane Manzo-Silberman, MD, Institute of Cardiology, Pitié-Salpétrière, Paris. The results (yet to be published) fight several of the preconceived ideas on the topic, Dr. Manzo-Silberman commented in an interview.
Significantly higher hospital death rates in women
“Cardiovascular disease is the main cause of death in women, killing seven times more than breast cancer,” notes Dr. Manzo-Silberman. The hospital death rate is significantly higher in women and, despite going down, is significantly higher than in men (more than double), particularly in women under 50. What’s more, in addition to the typical risk factors, women present specific risk factors related to hormone changes, high-risk inflammatory profiles, and thrombophilia.”
The WAMIF study was designed to determine the clinical, biological, and morphological features linked to hospital mortality after 12 months in women under 50. The prospective, observational study included all women in this age range from 30 sites in France between May 2017 and June 2019.
90% with retrosternal chest pain
The age of the 314 women enrolled was 44.9 years on average. Nearly two-thirds (192) presented with ST-segment elevation myocardial infarction and the other 122 without. In terms of symptoms, 91.6% of these women presented with typical chest pain, and 59.7% had related symptoms.
“With more than 90% having retrosternal pain, the idea that myocardial infarction presents with atypical symptoms in women has been widely challenged, despite the fact that more than half present with related symptoms and it isn’t known in which order these symptoms occur, Dr. Manzo-Silberman said in an interview. But what we can say is that if at any point a young woman mentions chest pain, even when occurring as part of several other symptoms, MI must be deemed a possibility until it has been ruled out.”
The risk profile revealed that 75.5% were smokers, 35% had a family history of heart disease, 33% had pregnancy complications, and 55% had recently experienced a stressful situation. The analysis also showed that cannabis use and oral contraception were primary risk factors in women younger than 35.
“With regard to risk factors, when designing this study we expected that lots of these young women would have largely atypical autoimmune conditions, with high levels of inflammation. We looked for everything, but this was not actually the case. Instead, we found very many women to have classic risk factors; three-quarters were smokers, a modifiable risk factor, which can largely be prevented. The other aspect concerns contraception, and it’s why I insist that gynecologists must be involved insofar as they must inform their patients how to manage their risk factors and tweak their contraception.”
Coronary angiography findings showed that only 1% received a normal result, 29.3% had vessel damage, and 14.6% had aortic dissection. “We were surprised again here because we expected that with young women we would see lots of heart attacks without obstruction, [in other words] normal coronary arteries, atypical forms of MI,” commented Dr. Manzo-Silberman. “In fact, most presented with atheroma, often obstructive lesions, or even triple-vessel disease, in nearly a third of the cohort. So that’s another misconception dispelled – we can’t just think that because a woman is young, nothing will be found. Coronary catheterization should be considered, and the diagnostic process should be completed in full.”
After 1 year, there had been two cancer-related deaths and 25 patients had undergone several angioplasty procedures. Nevertheless, 90.4% had not experienced any type of CV event, and 72% had not even had any symptoms.
“The final surprise was prognosis,” he said. “Previous studies, especially some authored by Viola Vaccarino, MD, PhD, showed an excess hospital rate in women and we had expected this to be the case here, but no hospital deaths were recorded. However, not far off 10% of women attended (at least once) the emergency department in the year following for recurrent chest pain which was not ischemic – ECG normal, troponin normal – so something was missing in their education as a patient.”
“So, there are improvements to be made in terms of secondary prevention, follow-up, and in the education of these young female patients who have experienced the major event that is a myocardial infarction,” concluded Dr. Manzo-Silberman.
This content was originally published on Medscape French edition.
Young women (under 50) are increasingly having heart attacks without doctors really knowing why. This is where the Young Women Presenting Acute Myocardial Infarction in France (WAMIF) study comes in, the results of which were presented in an e-poster at the annual congress of the European Society of Cardiology by Stéphane Manzo-Silberman, MD, Institute of Cardiology, Pitié-Salpétrière, Paris. The results (yet to be published) fight several of the preconceived ideas on the topic, Dr. Manzo-Silberman commented in an interview.
Significantly higher hospital death rates in women
“Cardiovascular disease is the main cause of death in women, killing seven times more than breast cancer,” notes Dr. Manzo-Silberman. The hospital death rate is significantly higher in women and, despite going down, is significantly higher than in men (more than double), particularly in women under 50. What’s more, in addition to the typical risk factors, women present specific risk factors related to hormone changes, high-risk inflammatory profiles, and thrombophilia.”
The WAMIF study was designed to determine the clinical, biological, and morphological features linked to hospital mortality after 12 months in women under 50. The prospective, observational study included all women in this age range from 30 sites in France between May 2017 and June 2019.
90% with retrosternal chest pain
The age of the 314 women enrolled was 44.9 years on average. Nearly two-thirds (192) presented with ST-segment elevation myocardial infarction and the other 122 without. In terms of symptoms, 91.6% of these women presented with typical chest pain, and 59.7% had related symptoms.
“With more than 90% having retrosternal pain, the idea that myocardial infarction presents with atypical symptoms in women has been widely challenged, despite the fact that more than half present with related symptoms and it isn’t known in which order these symptoms occur, Dr. Manzo-Silberman said in an interview. But what we can say is that if at any point a young woman mentions chest pain, even when occurring as part of several other symptoms, MI must be deemed a possibility until it has been ruled out.”
The risk profile revealed that 75.5% were smokers, 35% had a family history of heart disease, 33% had pregnancy complications, and 55% had recently experienced a stressful situation. The analysis also showed that cannabis use and oral contraception were primary risk factors in women younger than 35.
“With regard to risk factors, when designing this study we expected that lots of these young women would have largely atypical autoimmune conditions, with high levels of inflammation. We looked for everything, but this was not actually the case. Instead, we found very many women to have classic risk factors; three-quarters were smokers, a modifiable risk factor, which can largely be prevented. The other aspect concerns contraception, and it’s why I insist that gynecologists must be involved insofar as they must inform their patients how to manage their risk factors and tweak their contraception.”
Coronary angiography findings showed that only 1% received a normal result, 29.3% had vessel damage, and 14.6% had aortic dissection. “We were surprised again here because we expected that with young women we would see lots of heart attacks without obstruction, [in other words] normal coronary arteries, atypical forms of MI,” commented Dr. Manzo-Silberman. “In fact, most presented with atheroma, often obstructive lesions, or even triple-vessel disease, in nearly a third of the cohort. So that’s another misconception dispelled – we can’t just think that because a woman is young, nothing will be found. Coronary catheterization should be considered, and the diagnostic process should be completed in full.”
After 1 year, there had been two cancer-related deaths and 25 patients had undergone several angioplasty procedures. Nevertheless, 90.4% had not experienced any type of CV event, and 72% had not even had any symptoms.
“The final surprise was prognosis,” he said. “Previous studies, especially some authored by Viola Vaccarino, MD, PhD, showed an excess hospital rate in women and we had expected this to be the case here, but no hospital deaths were recorded. However, not far off 10% of women attended (at least once) the emergency department in the year following for recurrent chest pain which was not ischemic – ECG normal, troponin normal – so something was missing in their education as a patient.”
“So, there are improvements to be made in terms of secondary prevention, follow-up, and in the education of these young female patients who have experienced the major event that is a myocardial infarction,” concluded Dr. Manzo-Silberman.
This content was originally published on Medscape French edition.
FROM ESC CONGRESS 2022
Dietary change tops for reducing CVD risk in stage 1 hypertension
Healthy lifestyle changes to reduce systolic blood pressure to below 130 mm Hg may prevent 26,000 heart attacks and strokes and reduce health care costs over the next 10 years, a new simulation study suggests.
Among the various lifestyle changes, adopting the Dietary Approaches to Stop Hypertension diet, known as the DASH diet, may have the greatest impact for young and middle-aged adults with stage 1 hypertension.
“This research reveals that we should look to feasible ways our food system could make healthy eating the default option,” Kendra Sims, PhD, MPH, postdoctoral fellow at University of California, San Francisco, told this news organization.
“Above all, it means collaborating with the patient about nourishing choices that fit best into their culture and lifestyle,” Dr. Sims said.
Be proactive
“What is important is that people not wait until they have hypertension to start thinking about healthful diets,” commented Taylor Wallace, PhD, department of nutrition and food studies, George Mason University, Fairfax, Va., who was not involved in the study.
“It’s all about prevention in my mind. Whether you are hypertensive or are perfectly healthy, the DASH diet or any other dietary pattern that emphasizes consumption of fruits, vegetables, whole grains, lean meats, seafood, nuts/seeds, and low/non-fat dairy and decreased intake of saturated fats, added sugars, and sodium is a good idea,” Dr. Wallace said in an interview.
The study was presented at the American Heart Association Hypertension Scientific Sessions 2022 in San Diego.
Dr. Sims and colleagues used U.S. statistics from multiple sources to simulate CVD events, mortality, and health care costs between 2018 and 2027 in adults aged 35-64 years with untreated stage 1 hypertension, defined as systolic BP of 130 to 139 mm Hg.
The researchers estimate that 8.8 million U.S. adults (5.5 million women) aged 35-64 years have untreated stage 1 hypertension and would be recommended for lifestyle change, such as physical activity, weight loss, moderating alcohol intake, and adoption of the DASH diet.
Controlling blood pressure to less than 130 mm Hg in this population could prevent 26,000 CVD events, avoid 2,900 deaths, and lead to $1.6 billion saved in associated health care costs, the researchers calculate.
The largest benefit would come from adoption of the DASH diet, with an estimated 15,000 CVD events prevented among men and 11,000 among women.
Even small changes can help
“Young and middle-aged adults with stage 1 hypertension aren’t as low risk as you – or even your doctor – might think,” Dr. Sims told this news organization.
“Millions of working-aged people are walking around with elevated blood pressure, which is symptomless but is also a leading preventable cause of disability and death. Most do not follow the recommended DASH diet,” Dr. Sims said.
“Unfortunately, the availability and affordability of healthy food sources does not easily allow people to follow the DASH diet,” Dr. Sims adds in a conference news release.
“Clinicians should consider whether their patients live in food deserts or places with limited walkability. Health counseling should include addressing these specific challenges to blood pressure control,” Dr. Sims says.
Dr. Wallace noted that diet changes don’t have to be drastic.
“Honestly, just increasing fruit and vegetable intake has been shown to displace calories from saturated fats, added sugars, and sodium,” he told this news organization.
“It’s hard for people to stick to ‘diets’ long-term, so shifting toward healthier dietary patterns without having to read a book on the DASH diet or count calories and carbs seems like a more practical solution for the general population, although I have no issues with the DASH diet and think it is a great dietary pattern for heart health,” Dr. Wallace said.
The study had no funding. Dr. Sims reports no relevant financial relationships. Dr. Wallace is principal and CEO of Think Healthy Group; chief food and nutrition scientist with Produce for Better Health Foundation; editor, Journal of Dietary Supplements; deputy editor, Journal of the American College of Nutrition; nutrition section editor, Annals of Medicine; and advisory board member with Forbes Health.
A version of this article first appeared on Medscape.com.
Healthy lifestyle changes to reduce systolic blood pressure to below 130 mm Hg may prevent 26,000 heart attacks and strokes and reduce health care costs over the next 10 years, a new simulation study suggests.
Among the various lifestyle changes, adopting the Dietary Approaches to Stop Hypertension diet, known as the DASH diet, may have the greatest impact for young and middle-aged adults with stage 1 hypertension.
“This research reveals that we should look to feasible ways our food system could make healthy eating the default option,” Kendra Sims, PhD, MPH, postdoctoral fellow at University of California, San Francisco, told this news organization.
“Above all, it means collaborating with the patient about nourishing choices that fit best into their culture and lifestyle,” Dr. Sims said.
Be proactive
“What is important is that people not wait until they have hypertension to start thinking about healthful diets,” commented Taylor Wallace, PhD, department of nutrition and food studies, George Mason University, Fairfax, Va., who was not involved in the study.
“It’s all about prevention in my mind. Whether you are hypertensive or are perfectly healthy, the DASH diet or any other dietary pattern that emphasizes consumption of fruits, vegetables, whole grains, lean meats, seafood, nuts/seeds, and low/non-fat dairy and decreased intake of saturated fats, added sugars, and sodium is a good idea,” Dr. Wallace said in an interview.
The study was presented at the American Heart Association Hypertension Scientific Sessions 2022 in San Diego.
Dr. Sims and colleagues used U.S. statistics from multiple sources to simulate CVD events, mortality, and health care costs between 2018 and 2027 in adults aged 35-64 years with untreated stage 1 hypertension, defined as systolic BP of 130 to 139 mm Hg.
The researchers estimate that 8.8 million U.S. adults (5.5 million women) aged 35-64 years have untreated stage 1 hypertension and would be recommended for lifestyle change, such as physical activity, weight loss, moderating alcohol intake, and adoption of the DASH diet.
Controlling blood pressure to less than 130 mm Hg in this population could prevent 26,000 CVD events, avoid 2,900 deaths, and lead to $1.6 billion saved in associated health care costs, the researchers calculate.
The largest benefit would come from adoption of the DASH diet, with an estimated 15,000 CVD events prevented among men and 11,000 among women.
Even small changes can help
“Young and middle-aged adults with stage 1 hypertension aren’t as low risk as you – or even your doctor – might think,” Dr. Sims told this news organization.
“Millions of working-aged people are walking around with elevated blood pressure, which is symptomless but is also a leading preventable cause of disability and death. Most do not follow the recommended DASH diet,” Dr. Sims said.
“Unfortunately, the availability and affordability of healthy food sources does not easily allow people to follow the DASH diet,” Dr. Sims adds in a conference news release.
“Clinicians should consider whether their patients live in food deserts or places with limited walkability. Health counseling should include addressing these specific challenges to blood pressure control,” Dr. Sims says.
Dr. Wallace noted that diet changes don’t have to be drastic.
“Honestly, just increasing fruit and vegetable intake has been shown to displace calories from saturated fats, added sugars, and sodium,” he told this news organization.
“It’s hard for people to stick to ‘diets’ long-term, so shifting toward healthier dietary patterns without having to read a book on the DASH diet or count calories and carbs seems like a more practical solution for the general population, although I have no issues with the DASH diet and think it is a great dietary pattern for heart health,” Dr. Wallace said.
The study had no funding. Dr. Sims reports no relevant financial relationships. Dr. Wallace is principal and CEO of Think Healthy Group; chief food and nutrition scientist with Produce for Better Health Foundation; editor, Journal of Dietary Supplements; deputy editor, Journal of the American College of Nutrition; nutrition section editor, Annals of Medicine; and advisory board member with Forbes Health.
A version of this article first appeared on Medscape.com.
Healthy lifestyle changes to reduce systolic blood pressure to below 130 mm Hg may prevent 26,000 heart attacks and strokes and reduce health care costs over the next 10 years, a new simulation study suggests.
Among the various lifestyle changes, adopting the Dietary Approaches to Stop Hypertension diet, known as the DASH diet, may have the greatest impact for young and middle-aged adults with stage 1 hypertension.
“This research reveals that we should look to feasible ways our food system could make healthy eating the default option,” Kendra Sims, PhD, MPH, postdoctoral fellow at University of California, San Francisco, told this news organization.
“Above all, it means collaborating with the patient about nourishing choices that fit best into their culture and lifestyle,” Dr. Sims said.
Be proactive
“What is important is that people not wait until they have hypertension to start thinking about healthful diets,” commented Taylor Wallace, PhD, department of nutrition and food studies, George Mason University, Fairfax, Va., who was not involved in the study.
“It’s all about prevention in my mind. Whether you are hypertensive or are perfectly healthy, the DASH diet or any other dietary pattern that emphasizes consumption of fruits, vegetables, whole grains, lean meats, seafood, nuts/seeds, and low/non-fat dairy and decreased intake of saturated fats, added sugars, and sodium is a good idea,” Dr. Wallace said in an interview.
The study was presented at the American Heart Association Hypertension Scientific Sessions 2022 in San Diego.
Dr. Sims and colleagues used U.S. statistics from multiple sources to simulate CVD events, mortality, and health care costs between 2018 and 2027 in adults aged 35-64 years with untreated stage 1 hypertension, defined as systolic BP of 130 to 139 mm Hg.
The researchers estimate that 8.8 million U.S. adults (5.5 million women) aged 35-64 years have untreated stage 1 hypertension and would be recommended for lifestyle change, such as physical activity, weight loss, moderating alcohol intake, and adoption of the DASH diet.
Controlling blood pressure to less than 130 mm Hg in this population could prevent 26,000 CVD events, avoid 2,900 deaths, and lead to $1.6 billion saved in associated health care costs, the researchers calculate.
The largest benefit would come from adoption of the DASH diet, with an estimated 15,000 CVD events prevented among men and 11,000 among women.
Even small changes can help
“Young and middle-aged adults with stage 1 hypertension aren’t as low risk as you – or even your doctor – might think,” Dr. Sims told this news organization.
“Millions of working-aged people are walking around with elevated blood pressure, which is symptomless but is also a leading preventable cause of disability and death. Most do not follow the recommended DASH diet,” Dr. Sims said.
“Unfortunately, the availability and affordability of healthy food sources does not easily allow people to follow the DASH diet,” Dr. Sims adds in a conference news release.
“Clinicians should consider whether their patients live in food deserts or places with limited walkability. Health counseling should include addressing these specific challenges to blood pressure control,” Dr. Sims says.
Dr. Wallace noted that diet changes don’t have to be drastic.
“Honestly, just increasing fruit and vegetable intake has been shown to displace calories from saturated fats, added sugars, and sodium,” he told this news organization.
“It’s hard for people to stick to ‘diets’ long-term, so shifting toward healthier dietary patterns without having to read a book on the DASH diet or count calories and carbs seems like a more practical solution for the general population, although I have no issues with the DASH diet and think it is a great dietary pattern for heart health,” Dr. Wallace said.
The study had no funding. Dr. Sims reports no relevant financial relationships. Dr. Wallace is principal and CEO of Think Healthy Group; chief food and nutrition scientist with Produce for Better Health Foundation; editor, Journal of Dietary Supplements; deputy editor, Journal of the American College of Nutrition; nutrition section editor, Annals of Medicine; and advisory board member with Forbes Health.
A version of this article first appeared on Medscape.com.