User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
New recommendations for hyperglycemia management
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today we’re going to talk about the consensus report by the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia.
After lifestyle modifications, metformin is no longer the go-to drug for every patient in the management of hyperglycemia. It is recommended that we assess each patient’s personal characteristics in deciding what medication to prescribe. For patients at high cardiorenal risk, refer to the left side of the algorithm and to the right side for all other patients.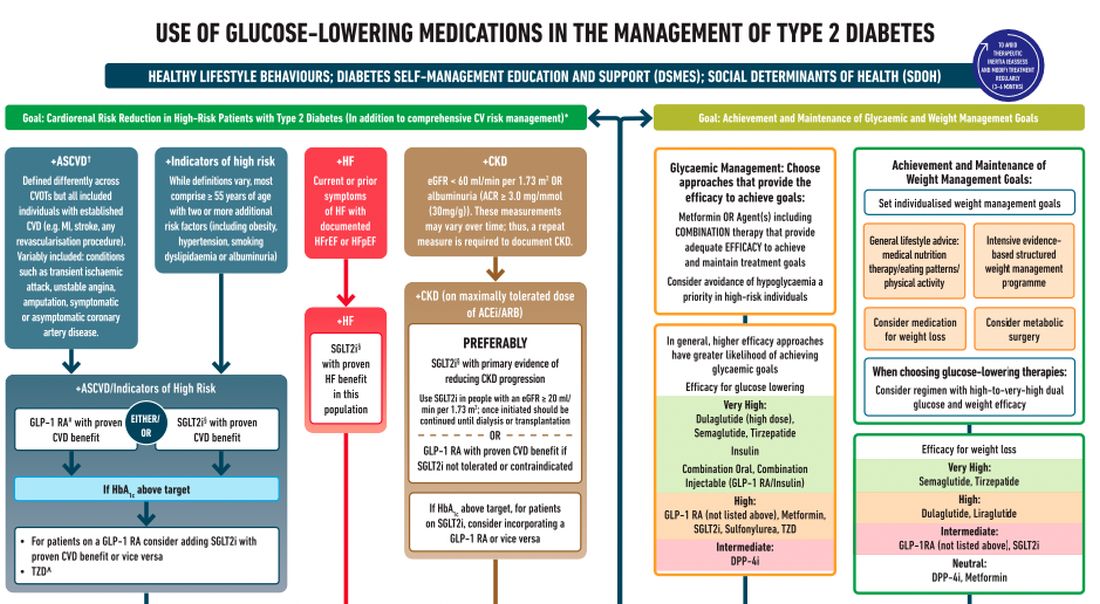
Cardiovascular disease. First, assess whether the patient is at high risk for atherosclerotic cardiovascular disease (ASCVD) or already has ASCVD. How is ASCVD defined? Either coronary artery disease (a history of a myocardial infarction [MI] or coronary disease), peripheral vascular disease, stroke, or transient ischemic attack.
What is high risk for ASCVD? Diabetes in someone older than 55 years with two or more additional risk factors. If the patient is at high risk for or has existing ASCVD then it is recommended to prescribe a glucagon-like peptide 1 (GLP-1) agonist with proven CVD benefit or an sodium-glucose cotransporter 2 (SGLT-2) inhibitor with proven CVD benefit.
For patients at very high risk for ASCVD, it might be reasonable to combine both agents. The recommendation to use these agents holds true whether the patients are at their A1c goals or not. The patient doesn’t need to be on metformin to benefit from these agents. The patient with reduced or preserved ejection fraction heart failure should be taking an SGLT-2 inhibitor.
Chronic kidney disease. Next up, chronic kidney disease (CKD). CKD is defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine albumin to creatinine ratio > 30. In that case, the patient should be preferentially on an SGLT-2 inhibitor. Patients not able to take an SGLT-2 for some reason should be prescribed a GLP-1 receptor agonist.
If someone doesn’t fit into that high cardiorenal risk category, then we go to the right side of the algorithm. The goal then is achievement and maintenance of glycemic and weight management goals.
Glycemic management. In choosing medicine for glycemic management, metformin is a reasonable choice. You may need to add another agent to metformin to reach the patient’s glycemic goal. If the patient is far away from goal, then a medication with higher efficacy at lowering glucose might be chosen.
Efficacy is listed as:
- Very high efficacy for glucose lowering: dulaglutide at a high dose, semaglutide, tirzepatide, insulin, or combination injectable agents (GLP-1 receptor agonist/insulin combinations).
- High glucose-lowering efficacy: a GLP-1 receptor agonist not already mentioned, metformin, SGLT-2 inhibitors, sulfonylureas, thiazolidinediones.
- Intermediate glucose lowering efficacy: dipeptidyl peptidase 4 (DPP-4) inhibitors.
Weight management. For weight management, lifestyle modification (diet and exercise) is important. If lifestyle modification alone is insufficient, consider either a medication that specifically helps with weight management or metabolic surgery.
We particularly want to focus on weight management in patients who have complications from obesity. What would those complications be? Sleep apnea, hip or knee pain from arthritis, back pain – that is, biomechanical complications of obesity or nonalcoholic fatty liver disease. Medications for weight loss are listed by degree of efficacy:
- Very high efficacy for weight loss: semaglutide, tirzepatide.
- High efficacy for weight loss: dulaglutide and liraglutide.
- Intermediate for weight loss: GLP-1 receptor agonist (not listed above), SGLT-2 inhibitor.
- Neutral for weight loss: DPP-4 inhibitors and metformin.
Where does insulin fit in? If patients present with a very high A1c, if they are on other medications and their A1c is still not to goal, or if they are catabolic and losing weight because of their diabetes, then insulin has an important place in management.
These are incredibly important guidelines that provide a clear algorithm for a personalized approach to diabetes management.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He reported conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today we’re going to talk about the consensus report by the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia.
After lifestyle modifications, metformin is no longer the go-to drug for every patient in the management of hyperglycemia. It is recommended that we assess each patient’s personal characteristics in deciding what medication to prescribe. For patients at high cardiorenal risk, refer to the left side of the algorithm and to the right side for all other patients.
Cardiovascular disease. First, assess whether the patient is at high risk for atherosclerotic cardiovascular disease (ASCVD) or already has ASCVD. How is ASCVD defined? Either coronary artery disease (a history of a myocardial infarction [MI] or coronary disease), peripheral vascular disease, stroke, or transient ischemic attack.
What is high risk for ASCVD? Diabetes in someone older than 55 years with two or more additional risk factors. If the patient is at high risk for or has existing ASCVD then it is recommended to prescribe a glucagon-like peptide 1 (GLP-1) agonist with proven CVD benefit or an sodium-glucose cotransporter 2 (SGLT-2) inhibitor with proven CVD benefit.
For patients at very high risk for ASCVD, it might be reasonable to combine both agents. The recommendation to use these agents holds true whether the patients are at their A1c goals or not. The patient doesn’t need to be on metformin to benefit from these agents. The patient with reduced or preserved ejection fraction heart failure should be taking an SGLT-2 inhibitor.
Chronic kidney disease. Next up, chronic kidney disease (CKD). CKD is defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine albumin to creatinine ratio > 30. In that case, the patient should be preferentially on an SGLT-2 inhibitor. Patients not able to take an SGLT-2 for some reason should be prescribed a GLP-1 receptor agonist.
If someone doesn’t fit into that high cardiorenal risk category, then we go to the right side of the algorithm. The goal then is achievement and maintenance of glycemic and weight management goals.
Glycemic management. In choosing medicine for glycemic management, metformin is a reasonable choice. You may need to add another agent to metformin to reach the patient’s glycemic goal. If the patient is far away from goal, then a medication with higher efficacy at lowering glucose might be chosen.
Efficacy is listed as:
- Very high efficacy for glucose lowering: dulaglutide at a high dose, semaglutide, tirzepatide, insulin, or combination injectable agents (GLP-1 receptor agonist/insulin combinations).
- High glucose-lowering efficacy: a GLP-1 receptor agonist not already mentioned, metformin, SGLT-2 inhibitors, sulfonylureas, thiazolidinediones.
- Intermediate glucose lowering efficacy: dipeptidyl peptidase 4 (DPP-4) inhibitors.
Weight management. For weight management, lifestyle modification (diet and exercise) is important. If lifestyle modification alone is insufficient, consider either a medication that specifically helps with weight management or metabolic surgery.
We particularly want to focus on weight management in patients who have complications from obesity. What would those complications be? Sleep apnea, hip or knee pain from arthritis, back pain – that is, biomechanical complications of obesity or nonalcoholic fatty liver disease. Medications for weight loss are listed by degree of efficacy:
- Very high efficacy for weight loss: semaglutide, tirzepatide.
- High efficacy for weight loss: dulaglutide and liraglutide.
- Intermediate for weight loss: GLP-1 receptor agonist (not listed above), SGLT-2 inhibitor.
- Neutral for weight loss: DPP-4 inhibitors and metformin.
Where does insulin fit in? If patients present with a very high A1c, if they are on other medications and their A1c is still not to goal, or if they are catabolic and losing weight because of their diabetes, then insulin has an important place in management.
These are incredibly important guidelines that provide a clear algorithm for a personalized approach to diabetes management.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He reported conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today we’re going to talk about the consensus report by the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia.
After lifestyle modifications, metformin is no longer the go-to drug for every patient in the management of hyperglycemia. It is recommended that we assess each patient’s personal characteristics in deciding what medication to prescribe. For patients at high cardiorenal risk, refer to the left side of the algorithm and to the right side for all other patients.
Cardiovascular disease. First, assess whether the patient is at high risk for atherosclerotic cardiovascular disease (ASCVD) or already has ASCVD. How is ASCVD defined? Either coronary artery disease (a history of a myocardial infarction [MI] or coronary disease), peripheral vascular disease, stroke, or transient ischemic attack.
What is high risk for ASCVD? Diabetes in someone older than 55 years with two or more additional risk factors. If the patient is at high risk for or has existing ASCVD then it is recommended to prescribe a glucagon-like peptide 1 (GLP-1) agonist with proven CVD benefit or an sodium-glucose cotransporter 2 (SGLT-2) inhibitor with proven CVD benefit.
For patients at very high risk for ASCVD, it might be reasonable to combine both agents. The recommendation to use these agents holds true whether the patients are at their A1c goals or not. The patient doesn’t need to be on metformin to benefit from these agents. The patient with reduced or preserved ejection fraction heart failure should be taking an SGLT-2 inhibitor.
Chronic kidney disease. Next up, chronic kidney disease (CKD). CKD is defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine albumin to creatinine ratio > 30. In that case, the patient should be preferentially on an SGLT-2 inhibitor. Patients not able to take an SGLT-2 for some reason should be prescribed a GLP-1 receptor agonist.
If someone doesn’t fit into that high cardiorenal risk category, then we go to the right side of the algorithm. The goal then is achievement and maintenance of glycemic and weight management goals.
Glycemic management. In choosing medicine for glycemic management, metformin is a reasonable choice. You may need to add another agent to metformin to reach the patient’s glycemic goal. If the patient is far away from goal, then a medication with higher efficacy at lowering glucose might be chosen.
Efficacy is listed as:
- Very high efficacy for glucose lowering: dulaglutide at a high dose, semaglutide, tirzepatide, insulin, or combination injectable agents (GLP-1 receptor agonist/insulin combinations).
- High glucose-lowering efficacy: a GLP-1 receptor agonist not already mentioned, metformin, SGLT-2 inhibitors, sulfonylureas, thiazolidinediones.
- Intermediate glucose lowering efficacy: dipeptidyl peptidase 4 (DPP-4) inhibitors.
Weight management. For weight management, lifestyle modification (diet and exercise) is important. If lifestyle modification alone is insufficient, consider either a medication that specifically helps with weight management or metabolic surgery.
We particularly want to focus on weight management in patients who have complications from obesity. What would those complications be? Sleep apnea, hip or knee pain from arthritis, back pain – that is, biomechanical complications of obesity or nonalcoholic fatty liver disease. Medications for weight loss are listed by degree of efficacy:
- Very high efficacy for weight loss: semaglutide, tirzepatide.
- High efficacy for weight loss: dulaglutide and liraglutide.
- Intermediate for weight loss: GLP-1 receptor agonist (not listed above), SGLT-2 inhibitor.
- Neutral for weight loss: DPP-4 inhibitors and metformin.
Where does insulin fit in? If patients present with a very high A1c, if they are on other medications and their A1c is still not to goal, or if they are catabolic and losing weight because of their diabetes, then insulin has an important place in management.
These are incredibly important guidelines that provide a clear algorithm for a personalized approach to diabetes management.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He reported conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer. A version of this article first appeared on Medscape.com.
Patients complain some obesity care startups offer pills, and not much else
Many Americans turn to the latest big idea to lose weight – fad diets, fitness crazes, dodgy herbs and pills, bariatric surgery, just to name a few. They’re rarely the magic solution people dream of.
Now a wave of startups offer access to a new category of drugs coupled with intensive behavioral coaching online. But already concerns are emerging.
These startups, spurred by hundreds of millions of dollars in funding from blue-chip venture capital firms, have signed up well over 100,000 patients and could reach millions more. These patients pay hundreds, if not thousands, of dollars to access new drugs, called glucagonlike peptide–1 (GLP-1) agonists, along with online coaching to encourage healthy habits.
The startups initially positioned themselves in lofty terms. “This is the last weight-loss program you’ll try,” said a 2020 marketing analysis by startup Calibrate Health, in messaging designed to reach one of its target demographics, the “working mom.” (Company spokesperson Michelle Wellington said the document does not reflect Calibrate’s current marketing strategy.)
But while doctors and patients are intrigued by the new model, some customers complain online that reality is short of the buildup: They say they got canned advice and unresponsive clinicians – and some report they couldn’t get the newest drugs.
Calibrate Health, a New York City–based startup, reported earlier in 2022 it had served 20,000 people. Another startup, Found, headquartered in San Francisco, has served 135,000 patients since July 2020, CEO Sarah Jones Simmer said in an interview. Calibrate costs patients nearly $1,600 a year, not counting the price of drugs, which can hit nearly $1,500 monthly without insurance, according to drug price savings site GoodRx. (Insurers reimburse for GLP-1agonists in limited circumstances, patients said.) Found offers a 6-month plan for nearly $600, a company spokesperson said. (That price includes generic drugs, but not the newer GLP-1 agonists, like Wegovy.)
The two companies are beneficiaries of over $200 million in combined venture funding, according to tracking by Crunchbase, a repository of venture capital investments. The firms say they’re on the vanguard of weight care, both citing the influence of biology and other scientific factors as key ingredients to their approaches.
There’s potentially a big market for these startups. Just over 4 in 10 Americans are obese, according to the Centers for Disease Control and Prevention, driving up their risk for cardiovascular conditions and type 2 diabetes. Effective medical treatments are elusive and hard to access.
Centers that provide this specialty care “are overwhelmed,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General in Boston, a teaching hospital affiliated with Harvard. Her own clinic has a wait list of 3,000.
Dr. Stanford, who said she has advised several of these telemedicine startups, is bullish on their potential.
Scott Butsch, MD, director of obesity medicine at the Cleveland Clinic, said the startups can offer care with less judgment and stigma than in-person peers. They’re also more convenient.
Dr. Butsch, who learned about the model through consultancies, patients, and colleagues, wonders whether the startups are operating “to strategically find which patients respond to which drug.” He said they should coordinate well with behavioral specialists, as antidepressants or other medications may be driving weight gain. “Obesity is a complex disease and requires treatments that match its complexity. I think programs that do not have a multidisciplinary team are less comprehensive and, in the long term, less effective.”
The startups market a two-pronged product: first, the new class of GLP-1 agonists. While these medications are effective at provoking weight loss, Wegovy, one of two in this class specifically approved for this purpose, is in short supply because of manufacturing difficulties, according to its maker, Novo Nordisk. Others in the category can be prescribed off label. But doctors generally aren’t familiar with the medications, Stanford said. In theory, the startups can bridge some of those gaps: They offer more specialized, knowledgeable clinicians.
Then there’s the other prong: behavioral changes. The companies use televisits and online messaging with nutritionists or coaches to help patients incorporate new diet and exercise habits. The weight loss figures achieved by participants in clinical trials for the new drugs – up to 15% of body mass – were tied to such changes, according to Novo Nordisk.
Social media sites are bursting with these startups’ ads, everywhere from podcasts to Instagram. A search of Meta’s ad library finds 40,000 ads on Facebook and Instagram between the two firms.
The ads complement people’s own postings on social media: Numerous Facebook groups are devoted to the new type of drugs – some even focused on helping patients manage side effects, like changes in their bowel movements. The buzz is quantifiable: On TikTok, mentions of the new GLP-1 agonists tripled from last June to this June, according to an analysis by investment bankers at Morgan Stanley.
There’s now a feverish, expectant appetite for these medications among the startups’ clientele. Patients often complained that their friends had obtained a drug they weren’t offered, recalled Alexandra Coults, a former pharmacist consultant for Found. Ms. Coults said patients may have perceived some sort of bait-and-switch when in reality clinical reasons – like drug contraindications – guide prescribing decisions.
Patient expectations influence care, Ms. Coults said. Customers came in with ideas shaped by the culture of fad diets and New Year’s resolutions. “Quite a few people would sign up for 1 month and not continue.”
In interviews with KHN and in online complaints, patients also questioned the quality of care they received. Some said intake – which began by filling out a form and proceeded to an online visit with a doctor – was perfunctory. Once medication began, they said, requests for counseling about side effects were slow to be answered.
Jess Garrant, a Found patient, recalled that after she was prescribed zonisamide, a generic anticonvulsant that has shown some ability to help with weight loss, she felt “absolutely weird.”
“I was up all night and my thoughts were racing,” she wrote in a blog post. She developed sores in her mouth.
She sought advice and help from Found physicians, but their replies “weren’t quick.” Nonemergency communications are routed through the company’s portal.
It took a week to complete a switch of medications and have a new prescription arrive at her home, she said. Meanwhile, she said, she went to an urgent care clinic for the mouth sores.
Found frequently prescribes generic medications – often off label – rather than just the new GLP-1 agonists, company executives said in an interview. Found said older generics like zonisamide are more accessible than the GLP-1 agonists advertised on social media and their own website. Both Dr. Butsch and Dr. Stanford said they’ve prescribed zonisamide successfully. Dr. Butsch said ramping up dosage rapidly can increase the risk of side effects.
But Kim Boyd, MD, chief medical officer of competitor Calibrate, said the older drugs “just haven’t worked.”
Patients of both companies have critiqued online and in interviews the startups’ behavioral care – which experts across the board maintain is integral to successful weight loss treatment. But some patients felt they simply had canned advice.
Other patients said they had ups and downs with their coaches. Dana Crom, an attorney, said she had gone through many coaches with Calibrate. Some were good, effective cheerleaders; others, not so good. But when kinks in the program arose, she said, the coach wasn’t able to help her navigate them. While the coach can report trouble with medications or the app, it appears those reports are no more effective than messages sent through the portal, Ms. Crom said.
And what about when her yearlong subscription ends? Ms. Crom said she’d consider continuing with Calibrate.
Relationships with coaches, given the need to change behavior, are a critical element of the business models. Patients’ results depend “on how adherent they are to lifestyle changes,” said Found’s chief medical officer, Rehka Kumar, MD.
While the startups offer care to a larger geographic footprint, it’s not clear whether the demographics of their patient populations are different from those of the traditional bricks-and-mortar model. Calibrate’s patients are overwhelmingly White; over 8 in 10 have at least an undergraduate degree; and over 8 in 10 are women, according to the company.
And its earlier marketing strategies reflected that. The September 2020 “segmentation” document laid out three types of customers the company could hope to attract: perimenopausal or menopausal women, with income ranging from $75,000 to $150,000 a year; working mothers, with a similar income; and “men.”
Isabelle Kenyon, Calibrate’s CEO, said the company now hopes to expand its reach to partner with large employers, and that will help diversify its patients.
Patients will need to be convinced that the model – more affordable, more accessible – works for them. For her part, Ms. Garrant, who no longer is using Found, reflected on her experience, writing in her blog post that she was hoping for more follow-up and a more personal approach. “I don’t think it’s a helpful way to lose weight,” she said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Many Americans turn to the latest big idea to lose weight – fad diets, fitness crazes, dodgy herbs and pills, bariatric surgery, just to name a few. They’re rarely the magic solution people dream of.
Now a wave of startups offer access to a new category of drugs coupled with intensive behavioral coaching online. But already concerns are emerging.
These startups, spurred by hundreds of millions of dollars in funding from blue-chip venture capital firms, have signed up well over 100,000 patients and could reach millions more. These patients pay hundreds, if not thousands, of dollars to access new drugs, called glucagonlike peptide–1 (GLP-1) agonists, along with online coaching to encourage healthy habits.
The startups initially positioned themselves in lofty terms. “This is the last weight-loss program you’ll try,” said a 2020 marketing analysis by startup Calibrate Health, in messaging designed to reach one of its target demographics, the “working mom.” (Company spokesperson Michelle Wellington said the document does not reflect Calibrate’s current marketing strategy.)
But while doctors and patients are intrigued by the new model, some customers complain online that reality is short of the buildup: They say they got canned advice and unresponsive clinicians – and some report they couldn’t get the newest drugs.
Calibrate Health, a New York City–based startup, reported earlier in 2022 it had served 20,000 people. Another startup, Found, headquartered in San Francisco, has served 135,000 patients since July 2020, CEO Sarah Jones Simmer said in an interview. Calibrate costs patients nearly $1,600 a year, not counting the price of drugs, which can hit nearly $1,500 monthly without insurance, according to drug price savings site GoodRx. (Insurers reimburse for GLP-1agonists in limited circumstances, patients said.) Found offers a 6-month plan for nearly $600, a company spokesperson said. (That price includes generic drugs, but not the newer GLP-1 agonists, like Wegovy.)
The two companies are beneficiaries of over $200 million in combined venture funding, according to tracking by Crunchbase, a repository of venture capital investments. The firms say they’re on the vanguard of weight care, both citing the influence of biology and other scientific factors as key ingredients to their approaches.
There’s potentially a big market for these startups. Just over 4 in 10 Americans are obese, according to the Centers for Disease Control and Prevention, driving up their risk for cardiovascular conditions and type 2 diabetes. Effective medical treatments are elusive and hard to access.
Centers that provide this specialty care “are overwhelmed,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General in Boston, a teaching hospital affiliated with Harvard. Her own clinic has a wait list of 3,000.
Dr. Stanford, who said she has advised several of these telemedicine startups, is bullish on their potential.
Scott Butsch, MD, director of obesity medicine at the Cleveland Clinic, said the startups can offer care with less judgment and stigma than in-person peers. They’re also more convenient.
Dr. Butsch, who learned about the model through consultancies, patients, and colleagues, wonders whether the startups are operating “to strategically find which patients respond to which drug.” He said they should coordinate well with behavioral specialists, as antidepressants or other medications may be driving weight gain. “Obesity is a complex disease and requires treatments that match its complexity. I think programs that do not have a multidisciplinary team are less comprehensive and, in the long term, less effective.”
The startups market a two-pronged product: first, the new class of GLP-1 agonists. While these medications are effective at provoking weight loss, Wegovy, one of two in this class specifically approved for this purpose, is in short supply because of manufacturing difficulties, according to its maker, Novo Nordisk. Others in the category can be prescribed off label. But doctors generally aren’t familiar with the medications, Stanford said. In theory, the startups can bridge some of those gaps: They offer more specialized, knowledgeable clinicians.
Then there’s the other prong: behavioral changes. The companies use televisits and online messaging with nutritionists or coaches to help patients incorporate new diet and exercise habits. The weight loss figures achieved by participants in clinical trials for the new drugs – up to 15% of body mass – were tied to such changes, according to Novo Nordisk.
Social media sites are bursting with these startups’ ads, everywhere from podcasts to Instagram. A search of Meta’s ad library finds 40,000 ads on Facebook and Instagram between the two firms.
The ads complement people’s own postings on social media: Numerous Facebook groups are devoted to the new type of drugs – some even focused on helping patients manage side effects, like changes in their bowel movements. The buzz is quantifiable: On TikTok, mentions of the new GLP-1 agonists tripled from last June to this June, according to an analysis by investment bankers at Morgan Stanley.
There’s now a feverish, expectant appetite for these medications among the startups’ clientele. Patients often complained that their friends had obtained a drug they weren’t offered, recalled Alexandra Coults, a former pharmacist consultant for Found. Ms. Coults said patients may have perceived some sort of bait-and-switch when in reality clinical reasons – like drug contraindications – guide prescribing decisions.
Patient expectations influence care, Ms. Coults said. Customers came in with ideas shaped by the culture of fad diets and New Year’s resolutions. “Quite a few people would sign up for 1 month and not continue.”
In interviews with KHN and in online complaints, patients also questioned the quality of care they received. Some said intake – which began by filling out a form and proceeded to an online visit with a doctor – was perfunctory. Once medication began, they said, requests for counseling about side effects were slow to be answered.
Jess Garrant, a Found patient, recalled that after she was prescribed zonisamide, a generic anticonvulsant that has shown some ability to help with weight loss, she felt “absolutely weird.”
“I was up all night and my thoughts were racing,” she wrote in a blog post. She developed sores in her mouth.
She sought advice and help from Found physicians, but their replies “weren’t quick.” Nonemergency communications are routed through the company’s portal.
It took a week to complete a switch of medications and have a new prescription arrive at her home, she said. Meanwhile, she said, she went to an urgent care clinic for the mouth sores.
Found frequently prescribes generic medications – often off label – rather than just the new GLP-1 agonists, company executives said in an interview. Found said older generics like zonisamide are more accessible than the GLP-1 agonists advertised on social media and their own website. Both Dr. Butsch and Dr. Stanford said they’ve prescribed zonisamide successfully. Dr. Butsch said ramping up dosage rapidly can increase the risk of side effects.
But Kim Boyd, MD, chief medical officer of competitor Calibrate, said the older drugs “just haven’t worked.”
Patients of both companies have critiqued online and in interviews the startups’ behavioral care – which experts across the board maintain is integral to successful weight loss treatment. But some patients felt they simply had canned advice.
Other patients said they had ups and downs with their coaches. Dana Crom, an attorney, said she had gone through many coaches with Calibrate. Some were good, effective cheerleaders; others, not so good. But when kinks in the program arose, she said, the coach wasn’t able to help her navigate them. While the coach can report trouble with medications or the app, it appears those reports are no more effective than messages sent through the portal, Ms. Crom said.
And what about when her yearlong subscription ends? Ms. Crom said she’d consider continuing with Calibrate.
Relationships with coaches, given the need to change behavior, are a critical element of the business models. Patients’ results depend “on how adherent they are to lifestyle changes,” said Found’s chief medical officer, Rehka Kumar, MD.
While the startups offer care to a larger geographic footprint, it’s not clear whether the demographics of their patient populations are different from those of the traditional bricks-and-mortar model. Calibrate’s patients are overwhelmingly White; over 8 in 10 have at least an undergraduate degree; and over 8 in 10 are women, according to the company.
And its earlier marketing strategies reflected that. The September 2020 “segmentation” document laid out three types of customers the company could hope to attract: perimenopausal or menopausal women, with income ranging from $75,000 to $150,000 a year; working mothers, with a similar income; and “men.”
Isabelle Kenyon, Calibrate’s CEO, said the company now hopes to expand its reach to partner with large employers, and that will help diversify its patients.
Patients will need to be convinced that the model – more affordable, more accessible – works for them. For her part, Ms. Garrant, who no longer is using Found, reflected on her experience, writing in her blog post that she was hoping for more follow-up and a more personal approach. “I don’t think it’s a helpful way to lose weight,” she said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Many Americans turn to the latest big idea to lose weight – fad diets, fitness crazes, dodgy herbs and pills, bariatric surgery, just to name a few. They’re rarely the magic solution people dream of.
Now a wave of startups offer access to a new category of drugs coupled with intensive behavioral coaching online. But already concerns are emerging.
These startups, spurred by hundreds of millions of dollars in funding from blue-chip venture capital firms, have signed up well over 100,000 patients and could reach millions more. These patients pay hundreds, if not thousands, of dollars to access new drugs, called glucagonlike peptide–1 (GLP-1) agonists, along with online coaching to encourage healthy habits.
The startups initially positioned themselves in lofty terms. “This is the last weight-loss program you’ll try,” said a 2020 marketing analysis by startup Calibrate Health, in messaging designed to reach one of its target demographics, the “working mom.” (Company spokesperson Michelle Wellington said the document does not reflect Calibrate’s current marketing strategy.)
But while doctors and patients are intrigued by the new model, some customers complain online that reality is short of the buildup: They say they got canned advice and unresponsive clinicians – and some report they couldn’t get the newest drugs.
Calibrate Health, a New York City–based startup, reported earlier in 2022 it had served 20,000 people. Another startup, Found, headquartered in San Francisco, has served 135,000 patients since July 2020, CEO Sarah Jones Simmer said in an interview. Calibrate costs patients nearly $1,600 a year, not counting the price of drugs, which can hit nearly $1,500 monthly without insurance, according to drug price savings site GoodRx. (Insurers reimburse for GLP-1agonists in limited circumstances, patients said.) Found offers a 6-month plan for nearly $600, a company spokesperson said. (That price includes generic drugs, but not the newer GLP-1 agonists, like Wegovy.)
The two companies are beneficiaries of over $200 million in combined venture funding, according to tracking by Crunchbase, a repository of venture capital investments. The firms say they’re on the vanguard of weight care, both citing the influence of biology and other scientific factors as key ingredients to their approaches.
There’s potentially a big market for these startups. Just over 4 in 10 Americans are obese, according to the Centers for Disease Control and Prevention, driving up their risk for cardiovascular conditions and type 2 diabetes. Effective medical treatments are elusive and hard to access.
Centers that provide this specialty care “are overwhelmed,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General in Boston, a teaching hospital affiliated with Harvard. Her own clinic has a wait list of 3,000.
Dr. Stanford, who said she has advised several of these telemedicine startups, is bullish on their potential.
Scott Butsch, MD, director of obesity medicine at the Cleveland Clinic, said the startups can offer care with less judgment and stigma than in-person peers. They’re also more convenient.
Dr. Butsch, who learned about the model through consultancies, patients, and colleagues, wonders whether the startups are operating “to strategically find which patients respond to which drug.” He said they should coordinate well with behavioral specialists, as antidepressants or other medications may be driving weight gain. “Obesity is a complex disease and requires treatments that match its complexity. I think programs that do not have a multidisciplinary team are less comprehensive and, in the long term, less effective.”
The startups market a two-pronged product: first, the new class of GLP-1 agonists. While these medications are effective at provoking weight loss, Wegovy, one of two in this class specifically approved for this purpose, is in short supply because of manufacturing difficulties, according to its maker, Novo Nordisk. Others in the category can be prescribed off label. But doctors generally aren’t familiar with the medications, Stanford said. In theory, the startups can bridge some of those gaps: They offer more specialized, knowledgeable clinicians.
Then there’s the other prong: behavioral changes. The companies use televisits and online messaging with nutritionists or coaches to help patients incorporate new diet and exercise habits. The weight loss figures achieved by participants in clinical trials for the new drugs – up to 15% of body mass – were tied to such changes, according to Novo Nordisk.
Social media sites are bursting with these startups’ ads, everywhere from podcasts to Instagram. A search of Meta’s ad library finds 40,000 ads on Facebook and Instagram between the two firms.
The ads complement people’s own postings on social media: Numerous Facebook groups are devoted to the new type of drugs – some even focused on helping patients manage side effects, like changes in their bowel movements. The buzz is quantifiable: On TikTok, mentions of the new GLP-1 agonists tripled from last June to this June, according to an analysis by investment bankers at Morgan Stanley.
There’s now a feverish, expectant appetite for these medications among the startups’ clientele. Patients often complained that their friends had obtained a drug they weren’t offered, recalled Alexandra Coults, a former pharmacist consultant for Found. Ms. Coults said patients may have perceived some sort of bait-and-switch when in reality clinical reasons – like drug contraindications – guide prescribing decisions.
Patient expectations influence care, Ms. Coults said. Customers came in with ideas shaped by the culture of fad diets and New Year’s resolutions. “Quite a few people would sign up for 1 month and not continue.”
In interviews with KHN and in online complaints, patients also questioned the quality of care they received. Some said intake – which began by filling out a form and proceeded to an online visit with a doctor – was perfunctory. Once medication began, they said, requests for counseling about side effects were slow to be answered.
Jess Garrant, a Found patient, recalled that after she was prescribed zonisamide, a generic anticonvulsant that has shown some ability to help with weight loss, she felt “absolutely weird.”
“I was up all night and my thoughts were racing,” she wrote in a blog post. She developed sores in her mouth.
She sought advice and help from Found physicians, but their replies “weren’t quick.” Nonemergency communications are routed through the company’s portal.
It took a week to complete a switch of medications and have a new prescription arrive at her home, she said. Meanwhile, she said, she went to an urgent care clinic for the mouth sores.
Found frequently prescribes generic medications – often off label – rather than just the new GLP-1 agonists, company executives said in an interview. Found said older generics like zonisamide are more accessible than the GLP-1 agonists advertised on social media and their own website. Both Dr. Butsch and Dr. Stanford said they’ve prescribed zonisamide successfully. Dr. Butsch said ramping up dosage rapidly can increase the risk of side effects.
But Kim Boyd, MD, chief medical officer of competitor Calibrate, said the older drugs “just haven’t worked.”
Patients of both companies have critiqued online and in interviews the startups’ behavioral care – which experts across the board maintain is integral to successful weight loss treatment. But some patients felt they simply had canned advice.
Other patients said they had ups and downs with their coaches. Dana Crom, an attorney, said she had gone through many coaches with Calibrate. Some were good, effective cheerleaders; others, not so good. But when kinks in the program arose, she said, the coach wasn’t able to help her navigate them. While the coach can report trouble with medications or the app, it appears those reports are no more effective than messages sent through the portal, Ms. Crom said.
And what about when her yearlong subscription ends? Ms. Crom said she’d consider continuing with Calibrate.
Relationships with coaches, given the need to change behavior, are a critical element of the business models. Patients’ results depend “on how adherent they are to lifestyle changes,” said Found’s chief medical officer, Rehka Kumar, MD.
While the startups offer care to a larger geographic footprint, it’s not clear whether the demographics of their patient populations are different from those of the traditional bricks-and-mortar model. Calibrate’s patients are overwhelmingly White; over 8 in 10 have at least an undergraduate degree; and over 8 in 10 are women, according to the company.
And its earlier marketing strategies reflected that. The September 2020 “segmentation” document laid out three types of customers the company could hope to attract: perimenopausal or menopausal women, with income ranging from $75,000 to $150,000 a year; working mothers, with a similar income; and “men.”
Isabelle Kenyon, Calibrate’s CEO, said the company now hopes to expand its reach to partner with large employers, and that will help diversify its patients.
Patients will need to be convinced that the model – more affordable, more accessible – works for them. For her part, Ms. Garrant, who no longer is using Found, reflected on her experience, writing in her blog post that she was hoping for more follow-up and a more personal approach. “I don’t think it’s a helpful way to lose weight,” she said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Sham-controlled renal denervation trial for hypertension is a near miss
SPYRAL HTN–ON MED hits headwinds
CHICAGO – Renal denervation, relative to a sham procedure, was linked with statistically significant reductions in blood pressure in the newly completed SPYRAL HTN–ON MED trial, but several factors are likely to have worked in concert to prevent the study from meeting its primary endpoint.
Of these differences, probably none was more important than the substantially higher proportion of patients in the sham group that received additional BP-lowering medications over the course of the study, David E. Kandzari, MD, reported at the American Heart Association scientific sessions.
The SPYRAL HTN–ON MED pivotal trial followed the previously completed SPYRAL HTN–ON MED pilot study, which did show a significant BP-lowering effect on antihypertensive medications followed radiofrequency denervation. In a recent update of the pilot study, the effect was persistent out to 3 years.
In the SPYRAL HTN–ON MED program, patients on their second screening visit were required to have a systolic pressure of between 140 and 170 mm Hg on 24-hour ambulatory BP monitoring (ABPM) while taking up to three antihypertensive medications. Patients who entered the study were randomized to renal denervation or sham control while maintaining their baseline antihypertensive therapies.
The previously reported pilot study comprised 80 patients. The expansion pivotal trial added 257 more patients for a total cohort of 337 patients. The primary efficacy endpoint was based on a Bayesian analysis of change in 24-hour systolic ABPM at 6 months for those in the experimental arm versus those on medications alone. Participants from both the pilot and pivotal trials were included.
The prespecified definition of success for renal denervation was a 97.5% threshold for probability of superiority on the basis of this Bayesian analysis. However, the Bayesian analysis was distorted by differences in the pilot and expansion cohorts, which complicated the superiority calculation. As a result, the analysis only yielded a 51% probability of superiority, a level substantially below the predefined threshold.
Despite differences seen in BP control in favor of renal denervation, several factors were identified that likely contributed to the missed primary endpoint. One stood out.
“Significant differences in medication prescriptions were disproportionate in favor of the sham group,” reported Dr. Kandzari, chief of Piedmont Heart Institute, Atlanta. He said these differences, which were a violation of the protocol mandate, led to a “bias toward the null” for the primary outcome.
The failure to meet the primary outcome was particularly disappointing in the wake of the favorable pilot study and the SPYRAL HTN–OFF MED pivotal trial, which were both positive.
In the pilot study, which did not have a medication imbalance, a 7.3–mm Hg reduction (P = .004) in 24-hour ABPM was seen at 6 months. Relative reductions in office-based systolic pressure reductions for renal denervation versus sham were 6.6 mm Hg (P = .03) and 4.0 mm Hg (P = .03) for the pilot and expansions groups, respectively.
On the basis of a Win ratio derived from a hierarchical analysis of ABMP and medication burden reduction, the 1.50 advantage (P = .005) for the renal denervation arm in the newly completed SPYRAL HTN–ON MED trial was also compelling.
At study entry, the median number of medications was 1.9 in both the renal denervation and sham arms. At the end of 6 months, the median number of medications was unchanged in the experimental arm but rose to 2.1 (P = .01) in the sham group. Similarly, there was little change in the medication burden from the start to the end of the trial in the denervation group (2.8 vs. 3.0), but a statistically significant change in the sham group (2.9 vs. 3.5; P = .04).
Furthermore, the net percentage change of patients receiving medications favoring BP reduction over the course of the study did not differ between the experimental and control arms of the pilot cohort, but was more than 10 times higher among controls in the expansion group (1.9% vs. 21.8%; P < .0001).
Medication changes over the course of the SPYRAL HTN–ON MED trial were even greater in some specific subgroups. Among Black participants, for example, 14.2% of those randomized to renal denervation and 54.6% of those randomized to the sham group increased their antihypertensive therapies over the course of the study.
The COVID-19 epidemic is suspected of playing another role in the negative results, according to Dr. Kandzari. After a brief pause in enrollment, the SPYRAL HTN–ON MED trial was resumed, but approximately 80% of the expansion cohort data were collected during this period. When compared, variances in office and 24-hour ABPM were observed for participants who were or were not evaluated during COVID.
“Significant differences in 24-hour ABPM patterns pre- and during COVID may reflect changes in patient behavior and lifestyle,” Dr. Kandzari speculated.
The data from this study differ from essentially all of the other studies in the SPYRAL HTN program as well as several other sham-controlled studies with renal denervation, according to Dr. Kandzari.
The AHA-invited discussant, Ajay J. Kirtane, MD, director of the Cardiac Catheterization Laboratories at Columbia University, New York, largely agreed that several variables appeared to conspire against a positive result in this trial, but he zeroed in on the imbalance of antihypertensive medications.
“Any trial that attempts to show a difference between renal denervation and a sham procedure must insure that antihypertensive medications are the same in the two arms. They cannot be different,” he said.
As an active investigator in the field of renal denervation, Dr. Kirtane thinks the evidence does support a benefit from renal denervation, but he believes data are still needed to determine which patients are candidates.
“Renal denervation is not going to be a replacement for previous established therapies, but it will be an adjunct,” he predicted. The preponderance of evidence supports clinically meaningful reductions in BP with this approach, “but we need to determine who to consider [for this therapy] and to have realistic expectations about the degree of benefit.”
Dr. Kandzari reported financial relationships with Abbott Vascular, Ablative Solutions, Biotronik, Boston Scientific, CSI, Medtronic Cardiovascular, OrbusNeich, and Teleflex. Dr. Kirtane reported financial relationships with Abbott Vascular, Abiomed, Boston Scientific, Cardiovascular Systems, Cathworks, Chiesi, Medtronic, Opens, Philipps, Regeneron, ReCor Medical, Siemens, Spectranetics, and Zoll.
SPYRAL HTN–ON MED hits headwinds
SPYRAL HTN–ON MED hits headwinds
CHICAGO – Renal denervation, relative to a sham procedure, was linked with statistically significant reductions in blood pressure in the newly completed SPYRAL HTN–ON MED trial, but several factors are likely to have worked in concert to prevent the study from meeting its primary endpoint.
Of these differences, probably none was more important than the substantially higher proportion of patients in the sham group that received additional BP-lowering medications over the course of the study, David E. Kandzari, MD, reported at the American Heart Association scientific sessions.
The SPYRAL HTN–ON MED pivotal trial followed the previously completed SPYRAL HTN–ON MED pilot study, which did show a significant BP-lowering effect on antihypertensive medications followed radiofrequency denervation. In a recent update of the pilot study, the effect was persistent out to 3 years.
In the SPYRAL HTN–ON MED program, patients on their second screening visit were required to have a systolic pressure of between 140 and 170 mm Hg on 24-hour ambulatory BP monitoring (ABPM) while taking up to three antihypertensive medications. Patients who entered the study were randomized to renal denervation or sham control while maintaining their baseline antihypertensive therapies.
The previously reported pilot study comprised 80 patients. The expansion pivotal trial added 257 more patients for a total cohort of 337 patients. The primary efficacy endpoint was based on a Bayesian analysis of change in 24-hour systolic ABPM at 6 months for those in the experimental arm versus those on medications alone. Participants from both the pilot and pivotal trials were included.
The prespecified definition of success for renal denervation was a 97.5% threshold for probability of superiority on the basis of this Bayesian analysis. However, the Bayesian analysis was distorted by differences in the pilot and expansion cohorts, which complicated the superiority calculation. As a result, the analysis only yielded a 51% probability of superiority, a level substantially below the predefined threshold.
Despite differences seen in BP control in favor of renal denervation, several factors were identified that likely contributed to the missed primary endpoint. One stood out.
“Significant differences in medication prescriptions were disproportionate in favor of the sham group,” reported Dr. Kandzari, chief of Piedmont Heart Institute, Atlanta. He said these differences, which were a violation of the protocol mandate, led to a “bias toward the null” for the primary outcome.
The failure to meet the primary outcome was particularly disappointing in the wake of the favorable pilot study and the SPYRAL HTN–OFF MED pivotal trial, which were both positive.
In the pilot study, which did not have a medication imbalance, a 7.3–mm Hg reduction (P = .004) in 24-hour ABPM was seen at 6 months. Relative reductions in office-based systolic pressure reductions for renal denervation versus sham were 6.6 mm Hg (P = .03) and 4.0 mm Hg (P = .03) for the pilot and expansions groups, respectively.
On the basis of a Win ratio derived from a hierarchical analysis of ABMP and medication burden reduction, the 1.50 advantage (P = .005) for the renal denervation arm in the newly completed SPYRAL HTN–ON MED trial was also compelling.
At study entry, the median number of medications was 1.9 in both the renal denervation and sham arms. At the end of 6 months, the median number of medications was unchanged in the experimental arm but rose to 2.1 (P = .01) in the sham group. Similarly, there was little change in the medication burden from the start to the end of the trial in the denervation group (2.8 vs. 3.0), but a statistically significant change in the sham group (2.9 vs. 3.5; P = .04).
Furthermore, the net percentage change of patients receiving medications favoring BP reduction over the course of the study did not differ between the experimental and control arms of the pilot cohort, but was more than 10 times higher among controls in the expansion group (1.9% vs. 21.8%; P < .0001).
Medication changes over the course of the SPYRAL HTN–ON MED trial were even greater in some specific subgroups. Among Black participants, for example, 14.2% of those randomized to renal denervation and 54.6% of those randomized to the sham group increased their antihypertensive therapies over the course of the study.
The COVID-19 epidemic is suspected of playing another role in the negative results, according to Dr. Kandzari. After a brief pause in enrollment, the SPYRAL HTN–ON MED trial was resumed, but approximately 80% of the expansion cohort data were collected during this period. When compared, variances in office and 24-hour ABPM were observed for participants who were or were not evaluated during COVID.
“Significant differences in 24-hour ABPM patterns pre- and during COVID may reflect changes in patient behavior and lifestyle,” Dr. Kandzari speculated.
The data from this study differ from essentially all of the other studies in the SPYRAL HTN program as well as several other sham-controlled studies with renal denervation, according to Dr. Kandzari.
The AHA-invited discussant, Ajay J. Kirtane, MD, director of the Cardiac Catheterization Laboratories at Columbia University, New York, largely agreed that several variables appeared to conspire against a positive result in this trial, but he zeroed in on the imbalance of antihypertensive medications.
“Any trial that attempts to show a difference between renal denervation and a sham procedure must insure that antihypertensive medications are the same in the two arms. They cannot be different,” he said.
As an active investigator in the field of renal denervation, Dr. Kirtane thinks the evidence does support a benefit from renal denervation, but he believes data are still needed to determine which patients are candidates.
“Renal denervation is not going to be a replacement for previous established therapies, but it will be an adjunct,” he predicted. The preponderance of evidence supports clinically meaningful reductions in BP with this approach, “but we need to determine who to consider [for this therapy] and to have realistic expectations about the degree of benefit.”
Dr. Kandzari reported financial relationships with Abbott Vascular, Ablative Solutions, Biotronik, Boston Scientific, CSI, Medtronic Cardiovascular, OrbusNeich, and Teleflex. Dr. Kirtane reported financial relationships with Abbott Vascular, Abiomed, Boston Scientific, Cardiovascular Systems, Cathworks, Chiesi, Medtronic, Opens, Philipps, Regeneron, ReCor Medical, Siemens, Spectranetics, and Zoll.
CHICAGO – Renal denervation, relative to a sham procedure, was linked with statistically significant reductions in blood pressure in the newly completed SPYRAL HTN–ON MED trial, but several factors are likely to have worked in concert to prevent the study from meeting its primary endpoint.
Of these differences, probably none was more important than the substantially higher proportion of patients in the sham group that received additional BP-lowering medications over the course of the study, David E. Kandzari, MD, reported at the American Heart Association scientific sessions.
The SPYRAL HTN–ON MED pivotal trial followed the previously completed SPYRAL HTN–ON MED pilot study, which did show a significant BP-lowering effect on antihypertensive medications followed radiofrequency denervation. In a recent update of the pilot study, the effect was persistent out to 3 years.
In the SPYRAL HTN–ON MED program, patients on their second screening visit were required to have a systolic pressure of between 140 and 170 mm Hg on 24-hour ambulatory BP monitoring (ABPM) while taking up to three antihypertensive medications. Patients who entered the study were randomized to renal denervation or sham control while maintaining their baseline antihypertensive therapies.
The previously reported pilot study comprised 80 patients. The expansion pivotal trial added 257 more patients for a total cohort of 337 patients. The primary efficacy endpoint was based on a Bayesian analysis of change in 24-hour systolic ABPM at 6 months for those in the experimental arm versus those on medications alone. Participants from both the pilot and pivotal trials were included.
The prespecified definition of success for renal denervation was a 97.5% threshold for probability of superiority on the basis of this Bayesian analysis. However, the Bayesian analysis was distorted by differences in the pilot and expansion cohorts, which complicated the superiority calculation. As a result, the analysis only yielded a 51% probability of superiority, a level substantially below the predefined threshold.
Despite differences seen in BP control in favor of renal denervation, several factors were identified that likely contributed to the missed primary endpoint. One stood out.
“Significant differences in medication prescriptions were disproportionate in favor of the sham group,” reported Dr. Kandzari, chief of Piedmont Heart Institute, Atlanta. He said these differences, which were a violation of the protocol mandate, led to a “bias toward the null” for the primary outcome.
The failure to meet the primary outcome was particularly disappointing in the wake of the favorable pilot study and the SPYRAL HTN–OFF MED pivotal trial, which were both positive.
In the pilot study, which did not have a medication imbalance, a 7.3–mm Hg reduction (P = .004) in 24-hour ABPM was seen at 6 months. Relative reductions in office-based systolic pressure reductions for renal denervation versus sham were 6.6 mm Hg (P = .03) and 4.0 mm Hg (P = .03) for the pilot and expansions groups, respectively.
On the basis of a Win ratio derived from a hierarchical analysis of ABMP and medication burden reduction, the 1.50 advantage (P = .005) for the renal denervation arm in the newly completed SPYRAL HTN–ON MED trial was also compelling.
At study entry, the median number of medications was 1.9 in both the renal denervation and sham arms. At the end of 6 months, the median number of medications was unchanged in the experimental arm but rose to 2.1 (P = .01) in the sham group. Similarly, there was little change in the medication burden from the start to the end of the trial in the denervation group (2.8 vs. 3.0), but a statistically significant change in the sham group (2.9 vs. 3.5; P = .04).
Furthermore, the net percentage change of patients receiving medications favoring BP reduction over the course of the study did not differ between the experimental and control arms of the pilot cohort, but was more than 10 times higher among controls in the expansion group (1.9% vs. 21.8%; P < .0001).
Medication changes over the course of the SPYRAL HTN–ON MED trial were even greater in some specific subgroups. Among Black participants, for example, 14.2% of those randomized to renal denervation and 54.6% of those randomized to the sham group increased their antihypertensive therapies over the course of the study.
The COVID-19 epidemic is suspected of playing another role in the negative results, according to Dr. Kandzari. After a brief pause in enrollment, the SPYRAL HTN–ON MED trial was resumed, but approximately 80% of the expansion cohort data were collected during this period. When compared, variances in office and 24-hour ABPM were observed for participants who were or were not evaluated during COVID.
“Significant differences in 24-hour ABPM patterns pre- and during COVID may reflect changes in patient behavior and lifestyle,” Dr. Kandzari speculated.
The data from this study differ from essentially all of the other studies in the SPYRAL HTN program as well as several other sham-controlled studies with renal denervation, according to Dr. Kandzari.
The AHA-invited discussant, Ajay J. Kirtane, MD, director of the Cardiac Catheterization Laboratories at Columbia University, New York, largely agreed that several variables appeared to conspire against a positive result in this trial, but he zeroed in on the imbalance of antihypertensive medications.
“Any trial that attempts to show a difference between renal denervation and a sham procedure must insure that antihypertensive medications are the same in the two arms. They cannot be different,” he said.
As an active investigator in the field of renal denervation, Dr. Kirtane thinks the evidence does support a benefit from renal denervation, but he believes data are still needed to determine which patients are candidates.
“Renal denervation is not going to be a replacement for previous established therapies, but it will be an adjunct,” he predicted. The preponderance of evidence supports clinically meaningful reductions in BP with this approach, “but we need to determine who to consider [for this therapy] and to have realistic expectations about the degree of benefit.”
Dr. Kandzari reported financial relationships with Abbott Vascular, Ablative Solutions, Biotronik, Boston Scientific, CSI, Medtronic Cardiovascular, OrbusNeich, and Teleflex. Dr. Kirtane reported financial relationships with Abbott Vascular, Abiomed, Boston Scientific, Cardiovascular Systems, Cathworks, Chiesi, Medtronic, Opens, Philipps, Regeneron, ReCor Medical, Siemens, Spectranetics, and Zoll.
AT AHA 2022
Starting a podcast
In my last column, I discussed . At this writing (November 2022), more than 600 million blogs are online, compared with about 2 million podcasts, and relatively few of them are run by physicians. With podcasts, you have a better chance of standing out in a crowded online world.
Starting a podcast is not difficult, but there are several steps you need to go through before launching one.
As with blogging, start by outlining a long-range plan. Your general topic will probably be your specialty, but you will need to narrow your focus to a few specific subjects, such as the problems you see most often, or a subspecialty that you concentrate on. You can always expand your topic later, as you get more popular. Choose a name for your podcast, and purchase a domain name that accurately describes it.
You will also need to choose a hosting service. Numerous inexpensive hosting platforms are available, and a simple Google search will find them for you. Many of them provide free learning materials, helpful creative tools, and customer support to get you through the confusing technical aspects. They can also help you choose a music introduction (to add a bit of polish), and help you piece together your audio segments. Buzzsprout, RSS.com, and Podbean get good reviews on many sites. (As always, I have no financial interest in any company or service mentioned herein.)
Hosting services can assist you in creating a template – a framework that you can reuse each time you record an episode – containing your intro and exit music, tracks for your conversations, etc. This will make your podcasts instantly recognizable each time your listeners tune in.
Many podcasting experts recommend recruiting a co-host. This can be an associate within your practice, a friend who practices elsewhere, or perhaps a resident in an academic setting. You will be able to spread the workload of creating, editing, and promoting. Plus, it is much easier to generate interesting content when two people are having a conversation, rather than one person lecturing from a prepared script. You might also consider having multiple co-hosts, either to expand episodes into group discussions, or to take turns working with you in covering different subjects.
How long you make your podcast is entirely up to you. Some consultants recommend specific time frames, such as 5 minutes (because that’s an average attention span), or 28 minutes (because that’s the average driving commute time). There are short podcasts and long ones; whatever works for you is fine, as long as you don’t drift off the topic. Furthermore, no one says they must all be the same length; when you are finished talking, you are done. And no one says you must stick with one subject throughout. Combining several short segments might hold more listeners’ interest and will make it easier to share small clips on social media.
Content guidelines are similar to those for blogs. Give people content that will be of interest or benefit to them. Talk about subjects – medical and otherwise – that are relevant to your practice or are prominent in the news.
As with blogs, try to avoid polarizing political discussions, and while it’s fine to discuss treatments and procedures that you offer, aggressive solicitation tends to make viewers look elsewhere. Keep any medical advice in general terms; don’t portray any specific patients as examples.
When your podcast is ready, your hosting platform will show you how to submit it to iTunes, and how to submit your podcast RSS feed to other podcast directories. As you upload new episodes, your host will automatically update your RSS feed, so that any directory you are listed on will receive the new episode.
Once you are uploaded, you can use your host’s social sharing tools to spread the word. As with blogs, use social media, such as your practice’s Facebook page, to push podcast updates into patients’ feeds and track relevant Twitter hashtags to find online communities that might be interested in your subject matter. You should also find your episode embed code (which your host will have) and place it in a prominent place on your website so patients can listen directly from there.
Transcriptions are another excellent promotional tool. Search engines will “read” your podcasts and list them in searches. Some podcast hosts will do transcribing for a fee, but there are independent transcription services as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In my last column, I discussed . At this writing (November 2022), more than 600 million blogs are online, compared with about 2 million podcasts, and relatively few of them are run by physicians. With podcasts, you have a better chance of standing out in a crowded online world.
Starting a podcast is not difficult, but there are several steps you need to go through before launching one.
As with blogging, start by outlining a long-range plan. Your general topic will probably be your specialty, but you will need to narrow your focus to a few specific subjects, such as the problems you see most often, or a subspecialty that you concentrate on. You can always expand your topic later, as you get more popular. Choose a name for your podcast, and purchase a domain name that accurately describes it.
You will also need to choose a hosting service. Numerous inexpensive hosting platforms are available, and a simple Google search will find them for you. Many of them provide free learning materials, helpful creative tools, and customer support to get you through the confusing technical aspects. They can also help you choose a music introduction (to add a bit of polish), and help you piece together your audio segments. Buzzsprout, RSS.com, and Podbean get good reviews on many sites. (As always, I have no financial interest in any company or service mentioned herein.)
Hosting services can assist you in creating a template – a framework that you can reuse each time you record an episode – containing your intro and exit music, tracks for your conversations, etc. This will make your podcasts instantly recognizable each time your listeners tune in.
Many podcasting experts recommend recruiting a co-host. This can be an associate within your practice, a friend who practices elsewhere, or perhaps a resident in an academic setting. You will be able to spread the workload of creating, editing, and promoting. Plus, it is much easier to generate interesting content when two people are having a conversation, rather than one person lecturing from a prepared script. You might also consider having multiple co-hosts, either to expand episodes into group discussions, or to take turns working with you in covering different subjects.
How long you make your podcast is entirely up to you. Some consultants recommend specific time frames, such as 5 minutes (because that’s an average attention span), or 28 minutes (because that’s the average driving commute time). There are short podcasts and long ones; whatever works for you is fine, as long as you don’t drift off the topic. Furthermore, no one says they must all be the same length; when you are finished talking, you are done. And no one says you must stick with one subject throughout. Combining several short segments might hold more listeners’ interest and will make it easier to share small clips on social media.
Content guidelines are similar to those for blogs. Give people content that will be of interest or benefit to them. Talk about subjects – medical and otherwise – that are relevant to your practice or are prominent in the news.
As with blogs, try to avoid polarizing political discussions, and while it’s fine to discuss treatments and procedures that you offer, aggressive solicitation tends to make viewers look elsewhere. Keep any medical advice in general terms; don’t portray any specific patients as examples.
When your podcast is ready, your hosting platform will show you how to submit it to iTunes, and how to submit your podcast RSS feed to other podcast directories. As you upload new episodes, your host will automatically update your RSS feed, so that any directory you are listed on will receive the new episode.
Once you are uploaded, you can use your host’s social sharing tools to spread the word. As with blogs, use social media, such as your practice’s Facebook page, to push podcast updates into patients’ feeds and track relevant Twitter hashtags to find online communities that might be interested in your subject matter. You should also find your episode embed code (which your host will have) and place it in a prominent place on your website so patients can listen directly from there.
Transcriptions are another excellent promotional tool. Search engines will “read” your podcasts and list them in searches. Some podcast hosts will do transcribing for a fee, but there are independent transcription services as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In my last column, I discussed . At this writing (November 2022), more than 600 million blogs are online, compared with about 2 million podcasts, and relatively few of them are run by physicians. With podcasts, you have a better chance of standing out in a crowded online world.
Starting a podcast is not difficult, but there are several steps you need to go through before launching one.
As with blogging, start by outlining a long-range plan. Your general topic will probably be your specialty, but you will need to narrow your focus to a few specific subjects, such as the problems you see most often, or a subspecialty that you concentrate on. You can always expand your topic later, as you get more popular. Choose a name for your podcast, and purchase a domain name that accurately describes it.
You will also need to choose a hosting service. Numerous inexpensive hosting platforms are available, and a simple Google search will find them for you. Many of them provide free learning materials, helpful creative tools, and customer support to get you through the confusing technical aspects. They can also help you choose a music introduction (to add a bit of polish), and help you piece together your audio segments. Buzzsprout, RSS.com, and Podbean get good reviews on many sites. (As always, I have no financial interest in any company or service mentioned herein.)
Hosting services can assist you in creating a template – a framework that you can reuse each time you record an episode – containing your intro and exit music, tracks for your conversations, etc. This will make your podcasts instantly recognizable each time your listeners tune in.
Many podcasting experts recommend recruiting a co-host. This can be an associate within your practice, a friend who practices elsewhere, or perhaps a resident in an academic setting. You will be able to spread the workload of creating, editing, and promoting. Plus, it is much easier to generate interesting content when two people are having a conversation, rather than one person lecturing from a prepared script. You might also consider having multiple co-hosts, either to expand episodes into group discussions, or to take turns working with you in covering different subjects.
How long you make your podcast is entirely up to you. Some consultants recommend specific time frames, such as 5 minutes (because that’s an average attention span), or 28 minutes (because that’s the average driving commute time). There are short podcasts and long ones; whatever works for you is fine, as long as you don’t drift off the topic. Furthermore, no one says they must all be the same length; when you are finished talking, you are done. And no one says you must stick with one subject throughout. Combining several short segments might hold more listeners’ interest and will make it easier to share small clips on social media.
Content guidelines are similar to those for blogs. Give people content that will be of interest or benefit to them. Talk about subjects – medical and otherwise – that are relevant to your practice or are prominent in the news.
As with blogs, try to avoid polarizing political discussions, and while it’s fine to discuss treatments and procedures that you offer, aggressive solicitation tends to make viewers look elsewhere. Keep any medical advice in general terms; don’t portray any specific patients as examples.
When your podcast is ready, your hosting platform will show you how to submit it to iTunes, and how to submit your podcast RSS feed to other podcast directories. As you upload new episodes, your host will automatically update your RSS feed, so that any directory you are listed on will receive the new episode.
Once you are uploaded, you can use your host’s social sharing tools to spread the word. As with blogs, use social media, such as your practice’s Facebook page, to push podcast updates into patients’ feeds and track relevant Twitter hashtags to find online communities that might be interested in your subject matter. You should also find your episode embed code (which your host will have) and place it in a prominent place on your website so patients can listen directly from there.
Transcriptions are another excellent promotional tool. Search engines will “read” your podcasts and list them in searches. Some podcast hosts will do transcribing for a fee, but there are independent transcription services as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
‘Key cause’ of type 2 diabetes identified
Understanding of the key mechanisms underlying the progression of type 2 diabetes has been advanced by new research from Oxford (England) University suggesting potential ways to “slow the seemingly inexorable decline in beta-cell function in T2D”.
The study in mice elucidated a “key cause” of T2D by showing that
Scientists already knew that chronic hyperglycemia leads to a progressive decline in beta-cell function and, conversely, that the failure of pancreatic beta-cells to produce insulin results in chronically elevated blood glucose. However, the exact cause of beta-cell failure in T2D has remained unclear. T2D typically presents in later adult life, and by the time of diagnosis as much as 50% of beta-cell function has been lost.
In the United Kingdom there are nearly 5 million people diagnosed with T2D, which costs the National Health Service some £10 billion annually.
Glucose metabolites, rather than glucose itself, drives failure of cells to release insulin
The new study, published in Nature Communications, used both an animal model of diabetes and in vitro culture of beta-cells in a high glucose medium. In both cases the researchers showed, for the first time, that it is glucose metabolites, rather than glucose itself, that drives the failure of beta-cells to release insulin and is key to the progression of type 2 diabetes.
Senior researcher Frances Ashcroft, PhD, of the department of physiology, anatomy and genetics at the University of Oxford said: “This suggests a potential way in which the decline in beta-cell function in T2D might be slowed or prevented.”
Blood glucose concentration is controlled within narrow limits, the team explained. When it is too low for more than few minutes, consciousness is rapidly lost because the brain is starved of fuel. However chronic elevation of blood glucose leads to the serious complications found in poorly controlled diabetes, such as retinopathy, nephropathy, peripheral neuropathy, and cardiac disease. Insulin, released from pancreatic beta-cells when blood glucose levels rise, is the only hormone that can lower the blood glucose concentration, and insufficient secretion results in diabetes. In T2D, the beta-cells are still present (unlike in T1D), but they have a reduced insulin content and the coupling between glucose and insulin release is impaired.
Vicious spiral of hyperglycemia and beta-cell damage
Previous work by the same team had shown that chronic hyperglycemia damages the ability of the beta-cell to produce insulin and to release it when blood glucose levels rise. This suggested that “prolonged hyperglycemia sets off a vicious spiral in which an increase in blood glucose leads to beta-cell damage and less insulin secretion - which causes an even greater increase in blood glucose and a further decline in beta-cell function,” the team explained.
Lead researcher Elizabeth Haythorne, PhD, said: “We realized that we next needed to understand how glucose damages beta-cell function, so we can think about how we might stop it and so slow the seemingly inexorable decline in beta-cell function in T2D.”
In the new study, they showed that altered glycolysis in T2D occurs, in part, through marked up-regulation of mammalian target of rapamycin complex 1 (mTORC1), a protein complex involved in control of cell growth, dysregulation of which underlies a variety of human diseases, including diabetes. Up-regulation of mTORC1 led to changes in metabolic gene expression, oxidative phosphorylation and insulin secretion. Furthermore, they demonstrated that reducing the rate at which glucose is metabolized and at which its metabolites build up could prevent the effects of chronic hyperglycemia and the ensuing beta-cell failure.
“High blood glucose levels cause an increased rate of glucose metabolism in the beta-cell, which leads to a metabolic bottleneck and the pooling of upstream metabolites,” the team said. “These metabolites switch off the insulin gene, so less insulin is made, as well as switching off numerous genes involved in metabolism and stimulus-secretion coupling. Consequently, the beta-cells become glucose blind and no longer respond to changes in blood glucose with insulin secretion.”
Blocking metabolic enzyme could maintain insulin secretion
The team attempted to block the first step in glucose metabolism, and therefore prevent the gene changes from taking place, by blocking the enzyme glucokinase, which regulates the process. They found that this could maintain glucose-stimulated insulin secretion even in the presence of chronic hyperglycemia.
“Our results support the idea that progressive impairment of beta-cell metabolism, induced by increasing hyperglycemia, speeds T2D development, and suggest that reducing glycolysis at the level of glucokinase may slow this progression,” they said.
Dr. Ashcroft said: “This is potentially a useful way to try to prevent beta-cell decline in diabetes. Because glucose metabolism normally stimulates insulin secretion, it was previously hypothesized that increasing glucose metabolism would enhance insulin secretion in T2D and glucokinase activators were trialled, with varying results.
“Our data suggests that glucokinase activators could have an adverse effect and, somewhat counter-intuitively, that a glucokinase inhibitor might be a better strategy to treat T2D. Of course, it would be important to reduce glucose flux in T2D to that found in people without diabetes – and no further. But there is a very long way to go before we can tell if this approach would be useful for treating beta-cell decline in T2D.
“In the meantime, the key message from our study if you have type 2 diabetes is that it is important to keep your blood glucose well controlled.”
This study was funded by the UK Medical Research Council, the Biotechnology and Biological Sciences Research Council, the John Fell Fund, and the Nuffield Benefaction for Medicine/Wellcome Institutional Strategic Support Fund. The authors declared no competing interests.
A version of this article first appeared on Medscape UK.
Understanding of the key mechanisms underlying the progression of type 2 diabetes has been advanced by new research from Oxford (England) University suggesting potential ways to “slow the seemingly inexorable decline in beta-cell function in T2D”.
The study in mice elucidated a “key cause” of T2D by showing that
Scientists already knew that chronic hyperglycemia leads to a progressive decline in beta-cell function and, conversely, that the failure of pancreatic beta-cells to produce insulin results in chronically elevated blood glucose. However, the exact cause of beta-cell failure in T2D has remained unclear. T2D typically presents in later adult life, and by the time of diagnosis as much as 50% of beta-cell function has been lost.
In the United Kingdom there are nearly 5 million people diagnosed with T2D, which costs the National Health Service some £10 billion annually.
Glucose metabolites, rather than glucose itself, drives failure of cells to release insulin
The new study, published in Nature Communications, used both an animal model of diabetes and in vitro culture of beta-cells in a high glucose medium. In both cases the researchers showed, for the first time, that it is glucose metabolites, rather than glucose itself, that drives the failure of beta-cells to release insulin and is key to the progression of type 2 diabetes.
Senior researcher Frances Ashcroft, PhD, of the department of physiology, anatomy and genetics at the University of Oxford said: “This suggests a potential way in which the decline in beta-cell function in T2D might be slowed or prevented.”
Blood glucose concentration is controlled within narrow limits, the team explained. When it is too low for more than few minutes, consciousness is rapidly lost because the brain is starved of fuel. However chronic elevation of blood glucose leads to the serious complications found in poorly controlled diabetes, such as retinopathy, nephropathy, peripheral neuropathy, and cardiac disease. Insulin, released from pancreatic beta-cells when blood glucose levels rise, is the only hormone that can lower the blood glucose concentration, and insufficient secretion results in diabetes. In T2D, the beta-cells are still present (unlike in T1D), but they have a reduced insulin content and the coupling between glucose and insulin release is impaired.
Vicious spiral of hyperglycemia and beta-cell damage
Previous work by the same team had shown that chronic hyperglycemia damages the ability of the beta-cell to produce insulin and to release it when blood glucose levels rise. This suggested that “prolonged hyperglycemia sets off a vicious spiral in which an increase in blood glucose leads to beta-cell damage and less insulin secretion - which causes an even greater increase in blood glucose and a further decline in beta-cell function,” the team explained.
Lead researcher Elizabeth Haythorne, PhD, said: “We realized that we next needed to understand how glucose damages beta-cell function, so we can think about how we might stop it and so slow the seemingly inexorable decline in beta-cell function in T2D.”
In the new study, they showed that altered glycolysis in T2D occurs, in part, through marked up-regulation of mammalian target of rapamycin complex 1 (mTORC1), a protein complex involved in control of cell growth, dysregulation of which underlies a variety of human diseases, including diabetes. Up-regulation of mTORC1 led to changes in metabolic gene expression, oxidative phosphorylation and insulin secretion. Furthermore, they demonstrated that reducing the rate at which glucose is metabolized and at which its metabolites build up could prevent the effects of chronic hyperglycemia and the ensuing beta-cell failure.
“High blood glucose levels cause an increased rate of glucose metabolism in the beta-cell, which leads to a metabolic bottleneck and the pooling of upstream metabolites,” the team said. “These metabolites switch off the insulin gene, so less insulin is made, as well as switching off numerous genes involved in metabolism and stimulus-secretion coupling. Consequently, the beta-cells become glucose blind and no longer respond to changes in blood glucose with insulin secretion.”
Blocking metabolic enzyme could maintain insulin secretion
The team attempted to block the first step in glucose metabolism, and therefore prevent the gene changes from taking place, by blocking the enzyme glucokinase, which regulates the process. They found that this could maintain glucose-stimulated insulin secretion even in the presence of chronic hyperglycemia.
“Our results support the idea that progressive impairment of beta-cell metabolism, induced by increasing hyperglycemia, speeds T2D development, and suggest that reducing glycolysis at the level of glucokinase may slow this progression,” they said.
Dr. Ashcroft said: “This is potentially a useful way to try to prevent beta-cell decline in diabetes. Because glucose metabolism normally stimulates insulin secretion, it was previously hypothesized that increasing glucose metabolism would enhance insulin secretion in T2D and glucokinase activators were trialled, with varying results.
“Our data suggests that glucokinase activators could have an adverse effect and, somewhat counter-intuitively, that a glucokinase inhibitor might be a better strategy to treat T2D. Of course, it would be important to reduce glucose flux in T2D to that found in people without diabetes – and no further. But there is a very long way to go before we can tell if this approach would be useful for treating beta-cell decline in T2D.
“In the meantime, the key message from our study if you have type 2 diabetes is that it is important to keep your blood glucose well controlled.”
This study was funded by the UK Medical Research Council, the Biotechnology and Biological Sciences Research Council, the John Fell Fund, and the Nuffield Benefaction for Medicine/Wellcome Institutional Strategic Support Fund. The authors declared no competing interests.
A version of this article first appeared on Medscape UK.
Understanding of the key mechanisms underlying the progression of type 2 diabetes has been advanced by new research from Oxford (England) University suggesting potential ways to “slow the seemingly inexorable decline in beta-cell function in T2D”.
The study in mice elucidated a “key cause” of T2D by showing that
Scientists already knew that chronic hyperglycemia leads to a progressive decline in beta-cell function and, conversely, that the failure of pancreatic beta-cells to produce insulin results in chronically elevated blood glucose. However, the exact cause of beta-cell failure in T2D has remained unclear. T2D typically presents in later adult life, and by the time of diagnosis as much as 50% of beta-cell function has been lost.
In the United Kingdom there are nearly 5 million people diagnosed with T2D, which costs the National Health Service some £10 billion annually.
Glucose metabolites, rather than glucose itself, drives failure of cells to release insulin
The new study, published in Nature Communications, used both an animal model of diabetes and in vitro culture of beta-cells in a high glucose medium. In both cases the researchers showed, for the first time, that it is glucose metabolites, rather than glucose itself, that drives the failure of beta-cells to release insulin and is key to the progression of type 2 diabetes.
Senior researcher Frances Ashcroft, PhD, of the department of physiology, anatomy and genetics at the University of Oxford said: “This suggests a potential way in which the decline in beta-cell function in T2D might be slowed or prevented.”
Blood glucose concentration is controlled within narrow limits, the team explained. When it is too low for more than few minutes, consciousness is rapidly lost because the brain is starved of fuel. However chronic elevation of blood glucose leads to the serious complications found in poorly controlled diabetes, such as retinopathy, nephropathy, peripheral neuropathy, and cardiac disease. Insulin, released from pancreatic beta-cells when blood glucose levels rise, is the only hormone that can lower the blood glucose concentration, and insufficient secretion results in diabetes. In T2D, the beta-cells are still present (unlike in T1D), but they have a reduced insulin content and the coupling between glucose and insulin release is impaired.
Vicious spiral of hyperglycemia and beta-cell damage
Previous work by the same team had shown that chronic hyperglycemia damages the ability of the beta-cell to produce insulin and to release it when blood glucose levels rise. This suggested that “prolonged hyperglycemia sets off a vicious spiral in which an increase in blood glucose leads to beta-cell damage and less insulin secretion - which causes an even greater increase in blood glucose and a further decline in beta-cell function,” the team explained.
Lead researcher Elizabeth Haythorne, PhD, said: “We realized that we next needed to understand how glucose damages beta-cell function, so we can think about how we might stop it and so slow the seemingly inexorable decline in beta-cell function in T2D.”
In the new study, they showed that altered glycolysis in T2D occurs, in part, through marked up-regulation of mammalian target of rapamycin complex 1 (mTORC1), a protein complex involved in control of cell growth, dysregulation of which underlies a variety of human diseases, including diabetes. Up-regulation of mTORC1 led to changes in metabolic gene expression, oxidative phosphorylation and insulin secretion. Furthermore, they demonstrated that reducing the rate at which glucose is metabolized and at which its metabolites build up could prevent the effects of chronic hyperglycemia and the ensuing beta-cell failure.
“High blood glucose levels cause an increased rate of glucose metabolism in the beta-cell, which leads to a metabolic bottleneck and the pooling of upstream metabolites,” the team said. “These metabolites switch off the insulin gene, so less insulin is made, as well as switching off numerous genes involved in metabolism and stimulus-secretion coupling. Consequently, the beta-cells become glucose blind and no longer respond to changes in blood glucose with insulin secretion.”
Blocking metabolic enzyme could maintain insulin secretion
The team attempted to block the first step in glucose metabolism, and therefore prevent the gene changes from taking place, by blocking the enzyme glucokinase, which regulates the process. They found that this could maintain glucose-stimulated insulin secretion even in the presence of chronic hyperglycemia.
“Our results support the idea that progressive impairment of beta-cell metabolism, induced by increasing hyperglycemia, speeds T2D development, and suggest that reducing glycolysis at the level of glucokinase may slow this progression,” they said.
Dr. Ashcroft said: “This is potentially a useful way to try to prevent beta-cell decline in diabetes. Because glucose metabolism normally stimulates insulin secretion, it was previously hypothesized that increasing glucose metabolism would enhance insulin secretion in T2D and glucokinase activators were trialled, with varying results.
“Our data suggests that glucokinase activators could have an adverse effect and, somewhat counter-intuitively, that a glucokinase inhibitor might be a better strategy to treat T2D. Of course, it would be important to reduce glucose flux in T2D to that found in people without diabetes – and no further. But there is a very long way to go before we can tell if this approach would be useful for treating beta-cell decline in T2D.
“In the meantime, the key message from our study if you have type 2 diabetes is that it is important to keep your blood glucose well controlled.”
This study was funded by the UK Medical Research Council, the Biotechnology and Biological Sciences Research Council, the John Fell Fund, and the Nuffield Benefaction for Medicine/Wellcome Institutional Strategic Support Fund. The authors declared no competing interests.
A version of this article first appeared on Medscape UK.
FROM NATURE COMMUNICATIONS
Tirzepatide cuts BP during obesity treatment
CHICAGO – compared with placebo, while causing modest increases in heart rate, in a prespecified substudy of the SURMOUNT-1 trial.
“The large effects on ambulatory 24-hour blood pressure raise the possibility that there may be important long-term benefits of [tirzepatide] on the complications of obesity,” said James A. de Lemos, MD, during a presentation at the American Heart Association scientific sessions.
“The findings are concordant with the [previously reported] office-based measurements, and the blood pressure reductions provide further evidence for the potential benefits of tirzepatide on cardiovascular health and outcomes,” said Dr. de Lemos, a cardiologist and professor at the University of Texas Southwestern Medical Center, Dallas.
The substudy included 600 of the 2,539 people enrolled in SURMOUNT-1, the first of two pivotal trials for tirzepatide (Mounjaro) in people without diabetes but with obesity or overweight (body mass index of 27-29 kg/m2) plus at least one weight-related complication. The primary endpoints of SURMOUNT-1 were the percent change in weight from baseline to 72 weeks on treatment with either of three different weekly injected doses of tirzepatide, compared with control subjects who received placebo, and the percentage of enrolled subjects achieving at least 5% loss in baseline weight, compared with the controls.
Tirzepatide treatment led to significant increases in both results, compared with controls, with the highest dose tested, 15 mg/week, resulting in an average 20.9% drop in weight from baseline after 72 weeks of treatment, and 91% of enrolled subjects on that dose achieving the 5% weight-loss threshold during the same time frame, in results published in 2022 in the New England Journal of Medicine.
24-hour ambulatory pressures from 494 people
The substudy enrolled 600 of the SURMOUNT-1 participants and involved 24-hour ambulatory BP and heart rate measurements at entry and after 36 weeks on treatment. Full results were available for 494 of these people. The substudy included only study participants who entered with a BP of less than 140/90 mm Hg. Enrollment in SURMOUNT-1 overall excluded people with a BP of 160/100 mm Hg or higher. The average BP among all enrolled participants was about 123/80 mm Hg, while heart rates averaged about 73 beats per minute.
Systolic BP measured with the ambulatory monitor fell from baseline by an average of 5.6, 8.8, and 6.2 mm Hg in the people who received tirzepatide in weekly doses of 5, 10, or 15 mg, respectively, and rose by an average 1.8 mm Hg among the controls, Dr. de Lemos reported. Diastolic BP dropped among the tirzepatide recipients by an average of 1.5, 2.4, and 0.0 mm Hg in the three ascending tirzepatide treatment arms, and rose by an average 0.5 mm Hg among the controls. All of the differences between the intervention groups and the controls were significant except for the change in diastolic BP among participants who received 15 mg of tirzepatide weekly.
The results showed that 36 weeks on tirzepatide treatment was associated with “arguably clinically meaningful” reductions in systolic and diastolic BPs, Dr. de Lemos said. “There is a lot of optimism that this will translate into clinical benefits.” He also noted that, “within the limits of cross-study comparisons, the blood pressure changes look favorable, compared with the single-incretin mechanism GLP-1 [glucagonlike peptide–1] receptor agonists.”
Heart rate fell by an average 1.8 bpm in the controls, and rose by an average 0.3, 0.5, and 3.6 bpm among the three groups receiving ascending weekly tirzepatide doses, effects that were “consistent with what’s been seen with the GLP-1 receptor agonists,” noted Dr. de Lemos.
Tirzepatide is known as a “twincretin” because it shares this GLP-1 receptor agonism and also has a second incretin agonist activity, to the receptor for the glucose-dependent insulinotropic polypeptide.
Lowering of blood pressure plateaus
Changes in BP over time during the 72 weeks on treatment, data first presented in the original report, showed that average systolic pressure in the people who received tirzepatide fell sharply during the first 24 weeks on treatment, and then leveled out with little further change over time. Furthermore, all three tirzepatide doses produced roughly similar systolic BP reductions. Changes in diastolic pressure over time showed a mostly similar pattern of reduction, although a modest ongoing decrease in average diastolic pressure continued beyond 24 weeks.
This pattern of a plateau in BP reduction has been seen before in studies using other treatments to produce weight loss, including bariatric surgery, said Naveed Sattar, MBChB, PhD, professor of metabolic medicine at the University of Glasgow, who was not involved in SURMOUNT-1. He attributed the plateau in BP reduction among tirzepatide-treated people to them hitting a wall in their BP nadir based on homeostatic limits. Dr. Sattar noted that most enrolled participants had normal BPs at entry based on the reported study averages.
“It’s hard to go lower, but the blood pressure reduction may be larger in people who start at higher pressure levels,” Dr. Sattar said in an interview.
Another inferred cap on BP reductions in the trial hypothesizes that the individual clinicians who managed the enrolled patients may have cut back on other BP-lowering agents as the pressures of the tirzepatide recipients fell to relatively low levels, suggested Darren McGuire, MD, a cardiologist and professor at UT Southwestern Medical Center, who also was not involved in the SURMOUNT-1 study.
Incretin agonists as antihypertensive drugs
The substantial BP-lowering seen with tirzepatide, as well as with other incretin agonist agents, suggests a new way to think about BP control in people with overweight or obesity, Dr. Sattar said.
“Until now, we haven’t had tools where people lose so much weight. Now that we have these tools [incretin agonists as well as bariatric surgery], we see substantial blood pressure reductions. It makes you think we should use weight-loss agents to lower blood pressure rather than a beta-blocker or angiotensin-converting enzyme inhibitor; then we’d also produce all the other benefits from weight loss,” Dr. Sattar suggested.
Dr. de Lemos said he sees signals that the BP reductions caused by tirzepatide and the GLP-1 receptor agonists may go beyond just weight-loss effects.
“There appears to be a larger blood pressure reduction than anticipated based on the change in weight,” he said during his presentation. “GLP-1 is active in most vascular tissues, so these [receptor agonist] agents likely have vascular or cardiac effects, or even effects on other tissues that may affect blood pressure.”
Heart rate increases were usually modest
The experiences with GLP-1 receptor agonists also suggest that the heart rate increases seen with tirzepatide treatment in SURMOUNT-1 will not have long-term effects. “The [Food and Drug Administration] mandated this heart rate substudy to make sure that the increase in heart rate was not larger than what would be anticipated” with a GLP-1 receptor agonist, Dr. de Lemos explained.
SURMOUNT-1 had a treatment-stopping rule to prevent a person’s heart rate from rising beyond 10 bpm from baseline. “Trivial numbers” of patients experienced a heart rate increase of this magnitude, he said. If used in routine practice, Dr. de Lemos said that he would closely investigate a patient with a heart rate increase greater than 10 mm Hg. The average increase seen with the highest dose, about 4 bpm above baseline, would generally not be concerning.
Tirzepatide received U.S. marketing approval from the FDA in May 2022 for treating people with type 2 diabetes. In October 2022, the FDA gave tirzepatide “Fast Track” designation for the pending application for approval of an indication to treat people with overweight or obesity who match the entry criteria for SURMOUNT-1 and for the second pivotal trial for this indication, SURMOUNT-2. According to a statement from Eli Lilly, the company that is developing and markets tirzepatide (Mounjaro), the FDA’s decision on the obesity indication will remain pending until the SURMOUNT-2 results are available, which the company expects will occur in 2023.
SURMOUNT-1 and SURMOUNT-2 were sponsored by Lilly, the company that markets tirzepatide. Dr. de Lemos has been a consultant to Lilly as well as to Amgen, AstraZeneca, Janssen, Novo Nordisk, Ortho, Quidel Cardiovascular, and Regeneron. Dr. Sattar has financial ties to Lilly, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Hammi, Merck Sharpe & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, and Sanofi-Aventis. Dr. McGuire has ties to Lilly as well as to Altimmune, Applied Therapeutics, Bayer, Boehringer Ingelheim, CSL Behring, Lexicon, Merck, Metavant, Novo Nordisk, and Sanofi.
CHICAGO – compared with placebo, while causing modest increases in heart rate, in a prespecified substudy of the SURMOUNT-1 trial.
“The large effects on ambulatory 24-hour blood pressure raise the possibility that there may be important long-term benefits of [tirzepatide] on the complications of obesity,” said James A. de Lemos, MD, during a presentation at the American Heart Association scientific sessions.
“The findings are concordant with the [previously reported] office-based measurements, and the blood pressure reductions provide further evidence for the potential benefits of tirzepatide on cardiovascular health and outcomes,” said Dr. de Lemos, a cardiologist and professor at the University of Texas Southwestern Medical Center, Dallas.
The substudy included 600 of the 2,539 people enrolled in SURMOUNT-1, the first of two pivotal trials for tirzepatide (Mounjaro) in people without diabetes but with obesity or overweight (body mass index of 27-29 kg/m2) plus at least one weight-related complication. The primary endpoints of SURMOUNT-1 were the percent change in weight from baseline to 72 weeks on treatment with either of three different weekly injected doses of tirzepatide, compared with control subjects who received placebo, and the percentage of enrolled subjects achieving at least 5% loss in baseline weight, compared with the controls.
Tirzepatide treatment led to significant increases in both results, compared with controls, with the highest dose tested, 15 mg/week, resulting in an average 20.9% drop in weight from baseline after 72 weeks of treatment, and 91% of enrolled subjects on that dose achieving the 5% weight-loss threshold during the same time frame, in results published in 2022 in the New England Journal of Medicine.
24-hour ambulatory pressures from 494 people
The substudy enrolled 600 of the SURMOUNT-1 participants and involved 24-hour ambulatory BP and heart rate measurements at entry and after 36 weeks on treatment. Full results were available for 494 of these people. The substudy included only study participants who entered with a BP of less than 140/90 mm Hg. Enrollment in SURMOUNT-1 overall excluded people with a BP of 160/100 mm Hg or higher. The average BP among all enrolled participants was about 123/80 mm Hg, while heart rates averaged about 73 beats per minute.
Systolic BP measured with the ambulatory monitor fell from baseline by an average of 5.6, 8.8, and 6.2 mm Hg in the people who received tirzepatide in weekly doses of 5, 10, or 15 mg, respectively, and rose by an average 1.8 mm Hg among the controls, Dr. de Lemos reported. Diastolic BP dropped among the tirzepatide recipients by an average of 1.5, 2.4, and 0.0 mm Hg in the three ascending tirzepatide treatment arms, and rose by an average 0.5 mm Hg among the controls. All of the differences between the intervention groups and the controls were significant except for the change in diastolic BP among participants who received 15 mg of tirzepatide weekly.
The results showed that 36 weeks on tirzepatide treatment was associated with “arguably clinically meaningful” reductions in systolic and diastolic BPs, Dr. de Lemos said. “There is a lot of optimism that this will translate into clinical benefits.” He also noted that, “within the limits of cross-study comparisons, the blood pressure changes look favorable, compared with the single-incretin mechanism GLP-1 [glucagonlike peptide–1] receptor agonists.”
Heart rate fell by an average 1.8 bpm in the controls, and rose by an average 0.3, 0.5, and 3.6 bpm among the three groups receiving ascending weekly tirzepatide doses, effects that were “consistent with what’s been seen with the GLP-1 receptor agonists,” noted Dr. de Lemos.
Tirzepatide is known as a “twincretin” because it shares this GLP-1 receptor agonism and also has a second incretin agonist activity, to the receptor for the glucose-dependent insulinotropic polypeptide.
Lowering of blood pressure plateaus
Changes in BP over time during the 72 weeks on treatment, data first presented in the original report, showed that average systolic pressure in the people who received tirzepatide fell sharply during the first 24 weeks on treatment, and then leveled out with little further change over time. Furthermore, all three tirzepatide doses produced roughly similar systolic BP reductions. Changes in diastolic pressure over time showed a mostly similar pattern of reduction, although a modest ongoing decrease in average diastolic pressure continued beyond 24 weeks.
This pattern of a plateau in BP reduction has been seen before in studies using other treatments to produce weight loss, including bariatric surgery, said Naveed Sattar, MBChB, PhD, professor of metabolic medicine at the University of Glasgow, who was not involved in SURMOUNT-1. He attributed the plateau in BP reduction among tirzepatide-treated people to them hitting a wall in their BP nadir based on homeostatic limits. Dr. Sattar noted that most enrolled participants had normal BPs at entry based on the reported study averages.
“It’s hard to go lower, but the blood pressure reduction may be larger in people who start at higher pressure levels,” Dr. Sattar said in an interview.
Another inferred cap on BP reductions in the trial hypothesizes that the individual clinicians who managed the enrolled patients may have cut back on other BP-lowering agents as the pressures of the tirzepatide recipients fell to relatively low levels, suggested Darren McGuire, MD, a cardiologist and professor at UT Southwestern Medical Center, who also was not involved in the SURMOUNT-1 study.
Incretin agonists as antihypertensive drugs
The substantial BP-lowering seen with tirzepatide, as well as with other incretin agonist agents, suggests a new way to think about BP control in people with overweight or obesity, Dr. Sattar said.
“Until now, we haven’t had tools where people lose so much weight. Now that we have these tools [incretin agonists as well as bariatric surgery], we see substantial blood pressure reductions. It makes you think we should use weight-loss agents to lower blood pressure rather than a beta-blocker or angiotensin-converting enzyme inhibitor; then we’d also produce all the other benefits from weight loss,” Dr. Sattar suggested.
Dr. de Lemos said he sees signals that the BP reductions caused by tirzepatide and the GLP-1 receptor agonists may go beyond just weight-loss effects.
“There appears to be a larger blood pressure reduction than anticipated based on the change in weight,” he said during his presentation. “GLP-1 is active in most vascular tissues, so these [receptor agonist] agents likely have vascular or cardiac effects, or even effects on other tissues that may affect blood pressure.”
Heart rate increases were usually modest
The experiences with GLP-1 receptor agonists also suggest that the heart rate increases seen with tirzepatide treatment in SURMOUNT-1 will not have long-term effects. “The [Food and Drug Administration] mandated this heart rate substudy to make sure that the increase in heart rate was not larger than what would be anticipated” with a GLP-1 receptor agonist, Dr. de Lemos explained.
SURMOUNT-1 had a treatment-stopping rule to prevent a person’s heart rate from rising beyond 10 bpm from baseline. “Trivial numbers” of patients experienced a heart rate increase of this magnitude, he said. If used in routine practice, Dr. de Lemos said that he would closely investigate a patient with a heart rate increase greater than 10 mm Hg. The average increase seen with the highest dose, about 4 bpm above baseline, would generally not be concerning.
Tirzepatide received U.S. marketing approval from the FDA in May 2022 for treating people with type 2 diabetes. In October 2022, the FDA gave tirzepatide “Fast Track” designation for the pending application for approval of an indication to treat people with overweight or obesity who match the entry criteria for SURMOUNT-1 and for the second pivotal trial for this indication, SURMOUNT-2. According to a statement from Eli Lilly, the company that is developing and markets tirzepatide (Mounjaro), the FDA’s decision on the obesity indication will remain pending until the SURMOUNT-2 results are available, which the company expects will occur in 2023.
SURMOUNT-1 and SURMOUNT-2 were sponsored by Lilly, the company that markets tirzepatide. Dr. de Lemos has been a consultant to Lilly as well as to Amgen, AstraZeneca, Janssen, Novo Nordisk, Ortho, Quidel Cardiovascular, and Regeneron. Dr. Sattar has financial ties to Lilly, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Hammi, Merck Sharpe & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, and Sanofi-Aventis. Dr. McGuire has ties to Lilly as well as to Altimmune, Applied Therapeutics, Bayer, Boehringer Ingelheim, CSL Behring, Lexicon, Merck, Metavant, Novo Nordisk, and Sanofi.
CHICAGO – compared with placebo, while causing modest increases in heart rate, in a prespecified substudy of the SURMOUNT-1 trial.
“The large effects on ambulatory 24-hour blood pressure raise the possibility that there may be important long-term benefits of [tirzepatide] on the complications of obesity,” said James A. de Lemos, MD, during a presentation at the American Heart Association scientific sessions.
“The findings are concordant with the [previously reported] office-based measurements, and the blood pressure reductions provide further evidence for the potential benefits of tirzepatide on cardiovascular health and outcomes,” said Dr. de Lemos, a cardiologist and professor at the University of Texas Southwestern Medical Center, Dallas.
The substudy included 600 of the 2,539 people enrolled in SURMOUNT-1, the first of two pivotal trials for tirzepatide (Mounjaro) in people without diabetes but with obesity or overweight (body mass index of 27-29 kg/m2) plus at least one weight-related complication. The primary endpoints of SURMOUNT-1 were the percent change in weight from baseline to 72 weeks on treatment with either of three different weekly injected doses of tirzepatide, compared with control subjects who received placebo, and the percentage of enrolled subjects achieving at least 5% loss in baseline weight, compared with the controls.
Tirzepatide treatment led to significant increases in both results, compared with controls, with the highest dose tested, 15 mg/week, resulting in an average 20.9% drop in weight from baseline after 72 weeks of treatment, and 91% of enrolled subjects on that dose achieving the 5% weight-loss threshold during the same time frame, in results published in 2022 in the New England Journal of Medicine.
24-hour ambulatory pressures from 494 people
The substudy enrolled 600 of the SURMOUNT-1 participants and involved 24-hour ambulatory BP and heart rate measurements at entry and after 36 weeks on treatment. Full results were available for 494 of these people. The substudy included only study participants who entered with a BP of less than 140/90 mm Hg. Enrollment in SURMOUNT-1 overall excluded people with a BP of 160/100 mm Hg or higher. The average BP among all enrolled participants was about 123/80 mm Hg, while heart rates averaged about 73 beats per minute.
Systolic BP measured with the ambulatory monitor fell from baseline by an average of 5.6, 8.8, and 6.2 mm Hg in the people who received tirzepatide in weekly doses of 5, 10, or 15 mg, respectively, and rose by an average 1.8 mm Hg among the controls, Dr. de Lemos reported. Diastolic BP dropped among the tirzepatide recipients by an average of 1.5, 2.4, and 0.0 mm Hg in the three ascending tirzepatide treatment arms, and rose by an average 0.5 mm Hg among the controls. All of the differences between the intervention groups and the controls were significant except for the change in diastolic BP among participants who received 15 mg of tirzepatide weekly.
The results showed that 36 weeks on tirzepatide treatment was associated with “arguably clinically meaningful” reductions in systolic and diastolic BPs, Dr. de Lemos said. “There is a lot of optimism that this will translate into clinical benefits.” He also noted that, “within the limits of cross-study comparisons, the blood pressure changes look favorable, compared with the single-incretin mechanism GLP-1 [glucagonlike peptide–1] receptor agonists.”
Heart rate fell by an average 1.8 bpm in the controls, and rose by an average 0.3, 0.5, and 3.6 bpm among the three groups receiving ascending weekly tirzepatide doses, effects that were “consistent with what’s been seen with the GLP-1 receptor agonists,” noted Dr. de Lemos.
Tirzepatide is known as a “twincretin” because it shares this GLP-1 receptor agonism and also has a second incretin agonist activity, to the receptor for the glucose-dependent insulinotropic polypeptide.
Lowering of blood pressure plateaus
Changes in BP over time during the 72 weeks on treatment, data first presented in the original report, showed that average systolic pressure in the people who received tirzepatide fell sharply during the first 24 weeks on treatment, and then leveled out with little further change over time. Furthermore, all three tirzepatide doses produced roughly similar systolic BP reductions. Changes in diastolic pressure over time showed a mostly similar pattern of reduction, although a modest ongoing decrease in average diastolic pressure continued beyond 24 weeks.
This pattern of a plateau in BP reduction has been seen before in studies using other treatments to produce weight loss, including bariatric surgery, said Naveed Sattar, MBChB, PhD, professor of metabolic medicine at the University of Glasgow, who was not involved in SURMOUNT-1. He attributed the plateau in BP reduction among tirzepatide-treated people to them hitting a wall in their BP nadir based on homeostatic limits. Dr. Sattar noted that most enrolled participants had normal BPs at entry based on the reported study averages.
“It’s hard to go lower, but the blood pressure reduction may be larger in people who start at higher pressure levels,” Dr. Sattar said in an interview.
Another inferred cap on BP reductions in the trial hypothesizes that the individual clinicians who managed the enrolled patients may have cut back on other BP-lowering agents as the pressures of the tirzepatide recipients fell to relatively low levels, suggested Darren McGuire, MD, a cardiologist and professor at UT Southwestern Medical Center, who also was not involved in the SURMOUNT-1 study.
Incretin agonists as antihypertensive drugs
The substantial BP-lowering seen with tirzepatide, as well as with other incretin agonist agents, suggests a new way to think about BP control in people with overweight or obesity, Dr. Sattar said.
“Until now, we haven’t had tools where people lose so much weight. Now that we have these tools [incretin agonists as well as bariatric surgery], we see substantial blood pressure reductions. It makes you think we should use weight-loss agents to lower blood pressure rather than a beta-blocker or angiotensin-converting enzyme inhibitor; then we’d also produce all the other benefits from weight loss,” Dr. Sattar suggested.
Dr. de Lemos said he sees signals that the BP reductions caused by tirzepatide and the GLP-1 receptor agonists may go beyond just weight-loss effects.
“There appears to be a larger blood pressure reduction than anticipated based on the change in weight,” he said during his presentation. “GLP-1 is active in most vascular tissues, so these [receptor agonist] agents likely have vascular or cardiac effects, or even effects on other tissues that may affect blood pressure.”
Heart rate increases were usually modest
The experiences with GLP-1 receptor agonists also suggest that the heart rate increases seen with tirzepatide treatment in SURMOUNT-1 will not have long-term effects. “The [Food and Drug Administration] mandated this heart rate substudy to make sure that the increase in heart rate was not larger than what would be anticipated” with a GLP-1 receptor agonist, Dr. de Lemos explained.
SURMOUNT-1 had a treatment-stopping rule to prevent a person’s heart rate from rising beyond 10 bpm from baseline. “Trivial numbers” of patients experienced a heart rate increase of this magnitude, he said. If used in routine practice, Dr. de Lemos said that he would closely investigate a patient with a heart rate increase greater than 10 mm Hg. The average increase seen with the highest dose, about 4 bpm above baseline, would generally not be concerning.
Tirzepatide received U.S. marketing approval from the FDA in May 2022 for treating people with type 2 diabetes. In October 2022, the FDA gave tirzepatide “Fast Track” designation for the pending application for approval of an indication to treat people with overweight or obesity who match the entry criteria for SURMOUNT-1 and for the second pivotal trial for this indication, SURMOUNT-2. According to a statement from Eli Lilly, the company that is developing and markets tirzepatide (Mounjaro), the FDA’s decision on the obesity indication will remain pending until the SURMOUNT-2 results are available, which the company expects will occur in 2023.
SURMOUNT-1 and SURMOUNT-2 were sponsored by Lilly, the company that markets tirzepatide. Dr. de Lemos has been a consultant to Lilly as well as to Amgen, AstraZeneca, Janssen, Novo Nordisk, Ortho, Quidel Cardiovascular, and Regeneron. Dr. Sattar has financial ties to Lilly, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Hammi, Merck Sharpe & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, and Sanofi-Aventis. Dr. McGuire has ties to Lilly as well as to Altimmune, Applied Therapeutics, Bayer, Boehringer Ingelheim, CSL Behring, Lexicon, Merck, Metavant, Novo Nordisk, and Sanofi.
AT AHA 2022
Statins boost glycemia slightly, but CVD benefits prevail
CHICAGO – A new, expanded meta-analysis confirmed the long-known effect that statin treatment has on raising blood glucose levels and causing incident diabetes, but it also documented that these effects are small and any risk they pose to statin users is dwarfed by the cholesterol-lowering effect of statins and their ability to reduce risk for atherosclerotic cardiovascular disease (ASCVD).
This meta-analysis of 23 trials with a total of more than 150,000 participants showed that statin therapy significantly increased the risk for new-onset diabetes and worsening glycemia, driven by a “very small but generalized increase in glucose,” with a greater effect from high-intensity statin regimens and a similar but somewhat more muted effect from low- and moderate-intensity statin treatment, David Preiss, MBChB, PhD, reported at the American Heart Association scientific sessions.
Dr. Preiss also stressed that despite this, “the cardiovascular benefits of statin therapy remain substantial and profound” in people regardless of whether they have diabetes, prediabetes, or normoglycemia when they start statin treatment, noting that the impact of even high-intensity statin treatment is “absolutely tiny” increases in hemoglobin A1c and blood glucose.
“This does not detract from the substantial benefit of statin treatment,” declared Dr. Preiss, a metabolic medicine specialist and endocrinologist at Oxford (England) University.
Small glycemia increases ‘nudge’ some into diabetes
The data Dr. Preiss reported showed that high-intensity statin treatment (atorvastatin at a daily dose of at least 40 mg, or rosuvastatin at a daily dose of at least 20 mg) led to an average increase in A1c levels of 0.08 percentage points among people without diabetes when their treatment began and 0.24 percentage points among people already diagnosed with diabetes. Blood glucose levels rose by an average of 0.04 mmol/L (less than 1 mg/d) in those without diabetes, and by an average 0.22 mmol/L (about 4 mg/dL) in those with diabetes. People who received low- or moderate-intensity statin regimens had significant but smaller increases.
“We’re not talking about people going from no diabetes to frank diabetes. We’re talking about [statins] nudging a very small number of people across a diabetes threshold,” an A1c of 6.5% that is set somewhat arbitrarily based on an increased risk for developing retinopathy, Dr. Preiss said. ”A person just needs to lose a [daily] can of Coke’s worth of weight to eliminate any apparent diabetes risk,” he noted.
Benefit outweighs risks by three- to sevenfold
Dr. Preiss presented two other examples of what his findings showed to illustrate the relatively small risk posed by statin therapy compared with its potential benefits. Treating 10,000 people for 5 years with a high-intensity statin regimen in those with established ASCVD (secondary prevention) would result in an increment of 150 extra people developing diabetes because of the hyperglycemic effect of statins, compared with an expected prevention of 1,000 ASCVD events. Among 10,000 people at high ASCVD risk and taking a high-intensity statin regimen for primary prevention 5 years of treatment would result in roughly 130 extra cases of incident diabetes while preventing about 500 ASCVD events.
In addition, applying the new risk estimates to the people included in the UK Biobank database, whose median A1c is 5.5%, showed that a high-intensity statin regimen could be expected to raise the prevalence of those with an A1c of 6.5% or greater from 4.5% to 5.7%.
Several preventive cardiologists who heard the report and were not involved with the analysis agreed with Dr. Preiss that the benefits of statin treatment substantially offset this confirmed hyperglycemic effect.
Risk ‘more than counterbalanced by benefit’
“He clearly showed that the small hyperglycemia risk posed by statin use is more than counterbalanced by its benefit for reducing ASCVD events,” commented Neil J. Stone, MD, a cardiologist and professor of medicine at Northwestern University, Chicago. “I agree that, for those with prediabetes who are on the road to diabetes with or without a statin, the small increase in glucose with a statin should not dissuade statin usage because the benefit is so large. Rather, it should focus efforts to improve diet, increase physical activity, and keep weight controlled.”
Dr. Stone also noted in an interview that in the JUPITER trial, which examined the effects of a daily 20-mg dose of rosuvastatin (Crestor), a high-intensity regimen, study participants with diabetes risk factors who were assigned to rosuvastatin had an onset of diabetes that was earlier than people assigned to placebo by only about 5.4 weeks, yet this group had evidence of significant benefit.
“I agree with Dr. Preiss that the benefits of statins in reducing heart attack, stroke, and cardiovascular death far outweigh their modest effects on glycemia,” commented Brendan M. Everett, MD, a cardiologist and preventive medicine specialist at Brigham and Women’s Hospital in Boston. “This is particularly true for those with preexisting prediabetes or diabetes, who have an elevated risk of atherosclerotic events and thus stand to derive more significant benefit from statins. The benefits of lowering LDL cholesterol with a statin for preventing seriously morbid, and potentially fatal, cardiovascular events far outweigh the extremely modest, or even negligible, increases in the risk of diabetes that could be seen with the extremely small increases in A1c,” Dr. Everett said in an interview.
The new findings “reaffirm that there is a increased risk [from statins] but the most important point is that it is a very, very tiny difference in A1c,” commented Marc S. Sabatine, MD, a cardiologist and professor at Harvard Medical School, Boston. “These data have been known for quite some time, but this analysis was done in a more rigorous way.” The finding of “a small increase in risk for diabetes is really because diabetes has a biochemical threshold and statin treatment nudges some people a little past a line that is semi-arbitrary. It’s important to be cognizant of this, but it in no way dissuades me from treating patients aggressively with statins to reduce their ASCVD risk. I would monitor their A1c levels, and if they go higher and can’t be controlled with lifestyle we have plenty of medications that can control it,” he said in an interview.
No difference by statin type
The meta-analysis used data from 13 placebo-controlled statin trials that together involved 123,940 participants and had an average 4.3 years of follow-up, and four trials that compared one statin with another and collectively involved 30,734 participants with an average 4.9 years of follow-up.
The analyses showed that high-intensity statin treatment increased the rate of incident diabetes by a significant 36% relative to controls and increased the rate of worsening glycemia by a significant 24% compared with controls. Low- or moderate-intensity statin regimens increased incident diabetes by a significant 10% and raised the incidence of worsening glycemia by a significant 10% compared with controls, Dr. Preiss reported.
These effects did not significantly differ by type of statin (the study included people treated with atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin), nor across a variety of subgroups based on age, sex, race, body mass index, diabetes risk, renal function, cholesterol levels, or cardiovascular disease. The effect was also consistent regardless of the duration of treatment.
Dr. Preiss also downplayed the magnitude of the apparent difference in risk posed by high-intensity and less intense statin regimens. “I suspect the apparent heterogeneity is true, but not quite as big as what we see,” he said.
The mechanisms by which statins have this effect remain unclear, but evidence suggests that it may be a direct effect of the main action of statins, inhibition of the HMG-CoA reductase enzyme.
The study received no commercial funding. Dr. Preiss and Dr. Stone had no disclosures. Dr. Everett has been a consultant to Eli Lilly, Gilead, Ipsen, Janssen, and Provention. Dr. Sabatine has been a consultant to Althera, Amgen, Anthos Therapeutics, AstraZeneca, Beren Therapeutics, Bristol-Myers Squibb, DalCor, Dr Reddy’s Laboratories, Fibrogen, Intarcia, Merck, Moderna, Novo Nordisk, and Silence Therapeutics.
CHICAGO – A new, expanded meta-analysis confirmed the long-known effect that statin treatment has on raising blood glucose levels and causing incident diabetes, but it also documented that these effects are small and any risk they pose to statin users is dwarfed by the cholesterol-lowering effect of statins and their ability to reduce risk for atherosclerotic cardiovascular disease (ASCVD).
This meta-analysis of 23 trials with a total of more than 150,000 participants showed that statin therapy significantly increased the risk for new-onset diabetes and worsening glycemia, driven by a “very small but generalized increase in glucose,” with a greater effect from high-intensity statin regimens and a similar but somewhat more muted effect from low- and moderate-intensity statin treatment, David Preiss, MBChB, PhD, reported at the American Heart Association scientific sessions.
Dr. Preiss also stressed that despite this, “the cardiovascular benefits of statin therapy remain substantial and profound” in people regardless of whether they have diabetes, prediabetes, or normoglycemia when they start statin treatment, noting that the impact of even high-intensity statin treatment is “absolutely tiny” increases in hemoglobin A1c and blood glucose.
“This does not detract from the substantial benefit of statin treatment,” declared Dr. Preiss, a metabolic medicine specialist and endocrinologist at Oxford (England) University.
Small glycemia increases ‘nudge’ some into diabetes
The data Dr. Preiss reported showed that high-intensity statin treatment (atorvastatin at a daily dose of at least 40 mg, or rosuvastatin at a daily dose of at least 20 mg) led to an average increase in A1c levels of 0.08 percentage points among people without diabetes when their treatment began and 0.24 percentage points among people already diagnosed with diabetes. Blood glucose levels rose by an average of 0.04 mmol/L (less than 1 mg/d) in those without diabetes, and by an average 0.22 mmol/L (about 4 mg/dL) in those with diabetes. People who received low- or moderate-intensity statin regimens had significant but smaller increases.
“We’re not talking about people going from no diabetes to frank diabetes. We’re talking about [statins] nudging a very small number of people across a diabetes threshold,” an A1c of 6.5% that is set somewhat arbitrarily based on an increased risk for developing retinopathy, Dr. Preiss said. ”A person just needs to lose a [daily] can of Coke’s worth of weight to eliminate any apparent diabetes risk,” he noted.
Benefit outweighs risks by three- to sevenfold
Dr. Preiss presented two other examples of what his findings showed to illustrate the relatively small risk posed by statin therapy compared with its potential benefits. Treating 10,000 people for 5 years with a high-intensity statin regimen in those with established ASCVD (secondary prevention) would result in an increment of 150 extra people developing diabetes because of the hyperglycemic effect of statins, compared with an expected prevention of 1,000 ASCVD events. Among 10,000 people at high ASCVD risk and taking a high-intensity statin regimen for primary prevention 5 years of treatment would result in roughly 130 extra cases of incident diabetes while preventing about 500 ASCVD events.
In addition, applying the new risk estimates to the people included in the UK Biobank database, whose median A1c is 5.5%, showed that a high-intensity statin regimen could be expected to raise the prevalence of those with an A1c of 6.5% or greater from 4.5% to 5.7%.
Several preventive cardiologists who heard the report and were not involved with the analysis agreed with Dr. Preiss that the benefits of statin treatment substantially offset this confirmed hyperglycemic effect.
Risk ‘more than counterbalanced by benefit’
“He clearly showed that the small hyperglycemia risk posed by statin use is more than counterbalanced by its benefit for reducing ASCVD events,” commented Neil J. Stone, MD, a cardiologist and professor of medicine at Northwestern University, Chicago. “I agree that, for those with prediabetes who are on the road to diabetes with or without a statin, the small increase in glucose with a statin should not dissuade statin usage because the benefit is so large. Rather, it should focus efforts to improve diet, increase physical activity, and keep weight controlled.”
Dr. Stone also noted in an interview that in the JUPITER trial, which examined the effects of a daily 20-mg dose of rosuvastatin (Crestor), a high-intensity regimen, study participants with diabetes risk factors who were assigned to rosuvastatin had an onset of diabetes that was earlier than people assigned to placebo by only about 5.4 weeks, yet this group had evidence of significant benefit.
“I agree with Dr. Preiss that the benefits of statins in reducing heart attack, stroke, and cardiovascular death far outweigh their modest effects on glycemia,” commented Brendan M. Everett, MD, a cardiologist and preventive medicine specialist at Brigham and Women’s Hospital in Boston. “This is particularly true for those with preexisting prediabetes or diabetes, who have an elevated risk of atherosclerotic events and thus stand to derive more significant benefit from statins. The benefits of lowering LDL cholesterol with a statin for preventing seriously morbid, and potentially fatal, cardiovascular events far outweigh the extremely modest, or even negligible, increases in the risk of diabetes that could be seen with the extremely small increases in A1c,” Dr. Everett said in an interview.
The new findings “reaffirm that there is a increased risk [from statins] but the most important point is that it is a very, very tiny difference in A1c,” commented Marc S. Sabatine, MD, a cardiologist and professor at Harvard Medical School, Boston. “These data have been known for quite some time, but this analysis was done in a more rigorous way.” The finding of “a small increase in risk for diabetes is really because diabetes has a biochemical threshold and statin treatment nudges some people a little past a line that is semi-arbitrary. It’s important to be cognizant of this, but it in no way dissuades me from treating patients aggressively with statins to reduce their ASCVD risk. I would monitor their A1c levels, and if they go higher and can’t be controlled with lifestyle we have plenty of medications that can control it,” he said in an interview.
No difference by statin type
The meta-analysis used data from 13 placebo-controlled statin trials that together involved 123,940 participants and had an average 4.3 years of follow-up, and four trials that compared one statin with another and collectively involved 30,734 participants with an average 4.9 years of follow-up.
The analyses showed that high-intensity statin treatment increased the rate of incident diabetes by a significant 36% relative to controls and increased the rate of worsening glycemia by a significant 24% compared with controls. Low- or moderate-intensity statin regimens increased incident diabetes by a significant 10% and raised the incidence of worsening glycemia by a significant 10% compared with controls, Dr. Preiss reported.
These effects did not significantly differ by type of statin (the study included people treated with atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin), nor across a variety of subgroups based on age, sex, race, body mass index, diabetes risk, renal function, cholesterol levels, or cardiovascular disease. The effect was also consistent regardless of the duration of treatment.
Dr. Preiss also downplayed the magnitude of the apparent difference in risk posed by high-intensity and less intense statin regimens. “I suspect the apparent heterogeneity is true, but not quite as big as what we see,” he said.
The mechanisms by which statins have this effect remain unclear, but evidence suggests that it may be a direct effect of the main action of statins, inhibition of the HMG-CoA reductase enzyme.
The study received no commercial funding. Dr. Preiss and Dr. Stone had no disclosures. Dr. Everett has been a consultant to Eli Lilly, Gilead, Ipsen, Janssen, and Provention. Dr. Sabatine has been a consultant to Althera, Amgen, Anthos Therapeutics, AstraZeneca, Beren Therapeutics, Bristol-Myers Squibb, DalCor, Dr Reddy’s Laboratories, Fibrogen, Intarcia, Merck, Moderna, Novo Nordisk, and Silence Therapeutics.
CHICAGO – A new, expanded meta-analysis confirmed the long-known effect that statin treatment has on raising blood glucose levels and causing incident diabetes, but it also documented that these effects are small and any risk they pose to statin users is dwarfed by the cholesterol-lowering effect of statins and their ability to reduce risk for atherosclerotic cardiovascular disease (ASCVD).
This meta-analysis of 23 trials with a total of more than 150,000 participants showed that statin therapy significantly increased the risk for new-onset diabetes and worsening glycemia, driven by a “very small but generalized increase in glucose,” with a greater effect from high-intensity statin regimens and a similar but somewhat more muted effect from low- and moderate-intensity statin treatment, David Preiss, MBChB, PhD, reported at the American Heart Association scientific sessions.
Dr. Preiss also stressed that despite this, “the cardiovascular benefits of statin therapy remain substantial and profound” in people regardless of whether they have diabetes, prediabetes, or normoglycemia when they start statin treatment, noting that the impact of even high-intensity statin treatment is “absolutely tiny” increases in hemoglobin A1c and blood glucose.
“This does not detract from the substantial benefit of statin treatment,” declared Dr. Preiss, a metabolic medicine specialist and endocrinologist at Oxford (England) University.
Small glycemia increases ‘nudge’ some into diabetes
The data Dr. Preiss reported showed that high-intensity statin treatment (atorvastatin at a daily dose of at least 40 mg, or rosuvastatin at a daily dose of at least 20 mg) led to an average increase in A1c levels of 0.08 percentage points among people without diabetes when their treatment began and 0.24 percentage points among people already diagnosed with diabetes. Blood glucose levels rose by an average of 0.04 mmol/L (less than 1 mg/d) in those without diabetes, and by an average 0.22 mmol/L (about 4 mg/dL) in those with diabetes. People who received low- or moderate-intensity statin regimens had significant but smaller increases.
“We’re not talking about people going from no diabetes to frank diabetes. We’re talking about [statins] nudging a very small number of people across a diabetes threshold,” an A1c of 6.5% that is set somewhat arbitrarily based on an increased risk for developing retinopathy, Dr. Preiss said. ”A person just needs to lose a [daily] can of Coke’s worth of weight to eliminate any apparent diabetes risk,” he noted.
Benefit outweighs risks by three- to sevenfold
Dr. Preiss presented two other examples of what his findings showed to illustrate the relatively small risk posed by statin therapy compared with its potential benefits. Treating 10,000 people for 5 years with a high-intensity statin regimen in those with established ASCVD (secondary prevention) would result in an increment of 150 extra people developing diabetes because of the hyperglycemic effect of statins, compared with an expected prevention of 1,000 ASCVD events. Among 10,000 people at high ASCVD risk and taking a high-intensity statin regimen for primary prevention 5 years of treatment would result in roughly 130 extra cases of incident diabetes while preventing about 500 ASCVD events.
In addition, applying the new risk estimates to the people included in the UK Biobank database, whose median A1c is 5.5%, showed that a high-intensity statin regimen could be expected to raise the prevalence of those with an A1c of 6.5% or greater from 4.5% to 5.7%.
Several preventive cardiologists who heard the report and were not involved with the analysis agreed with Dr. Preiss that the benefits of statin treatment substantially offset this confirmed hyperglycemic effect.
Risk ‘more than counterbalanced by benefit’
“He clearly showed that the small hyperglycemia risk posed by statin use is more than counterbalanced by its benefit for reducing ASCVD events,” commented Neil J. Stone, MD, a cardiologist and professor of medicine at Northwestern University, Chicago. “I agree that, for those with prediabetes who are on the road to diabetes with or without a statin, the small increase in glucose with a statin should not dissuade statin usage because the benefit is so large. Rather, it should focus efforts to improve diet, increase physical activity, and keep weight controlled.”
Dr. Stone also noted in an interview that in the JUPITER trial, which examined the effects of a daily 20-mg dose of rosuvastatin (Crestor), a high-intensity regimen, study participants with diabetes risk factors who were assigned to rosuvastatin had an onset of diabetes that was earlier than people assigned to placebo by only about 5.4 weeks, yet this group had evidence of significant benefit.
“I agree with Dr. Preiss that the benefits of statins in reducing heart attack, stroke, and cardiovascular death far outweigh their modest effects on glycemia,” commented Brendan M. Everett, MD, a cardiologist and preventive medicine specialist at Brigham and Women’s Hospital in Boston. “This is particularly true for those with preexisting prediabetes or diabetes, who have an elevated risk of atherosclerotic events and thus stand to derive more significant benefit from statins. The benefits of lowering LDL cholesterol with a statin for preventing seriously morbid, and potentially fatal, cardiovascular events far outweigh the extremely modest, or even negligible, increases in the risk of diabetes that could be seen with the extremely small increases in A1c,” Dr. Everett said in an interview.
The new findings “reaffirm that there is a increased risk [from statins] but the most important point is that it is a very, very tiny difference in A1c,” commented Marc S. Sabatine, MD, a cardiologist and professor at Harvard Medical School, Boston. “These data have been known for quite some time, but this analysis was done in a more rigorous way.” The finding of “a small increase in risk for diabetes is really because diabetes has a biochemical threshold and statin treatment nudges some people a little past a line that is semi-arbitrary. It’s important to be cognizant of this, but it in no way dissuades me from treating patients aggressively with statins to reduce their ASCVD risk. I would monitor their A1c levels, and if they go higher and can’t be controlled with lifestyle we have plenty of medications that can control it,” he said in an interview.
No difference by statin type
The meta-analysis used data from 13 placebo-controlled statin trials that together involved 123,940 participants and had an average 4.3 years of follow-up, and four trials that compared one statin with another and collectively involved 30,734 participants with an average 4.9 years of follow-up.
The analyses showed that high-intensity statin treatment increased the rate of incident diabetes by a significant 36% relative to controls and increased the rate of worsening glycemia by a significant 24% compared with controls. Low- or moderate-intensity statin regimens increased incident diabetes by a significant 10% and raised the incidence of worsening glycemia by a significant 10% compared with controls, Dr. Preiss reported.
These effects did not significantly differ by type of statin (the study included people treated with atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin), nor across a variety of subgroups based on age, sex, race, body mass index, diabetes risk, renal function, cholesterol levels, or cardiovascular disease. The effect was also consistent regardless of the duration of treatment.
Dr. Preiss also downplayed the magnitude of the apparent difference in risk posed by high-intensity and less intense statin regimens. “I suspect the apparent heterogeneity is true, but not quite as big as what we see,” he said.
The mechanisms by which statins have this effect remain unclear, but evidence suggests that it may be a direct effect of the main action of statins, inhibition of the HMG-CoA reductase enzyme.
The study received no commercial funding. Dr. Preiss and Dr. Stone had no disclosures. Dr. Everett has been a consultant to Eli Lilly, Gilead, Ipsen, Janssen, and Provention. Dr. Sabatine has been a consultant to Althera, Amgen, Anthos Therapeutics, AstraZeneca, Beren Therapeutics, Bristol-Myers Squibb, DalCor, Dr Reddy’s Laboratories, Fibrogen, Intarcia, Merck, Moderna, Novo Nordisk, and Silence Therapeutics.
AT AHA 2022
Repeat COVID infection doubles mortality risk
Getting COVID-19 a second time doubles a person’s chance of dying and triples the likelihood of being hospitalized in the next 6 months, a new study found.
Vaccination and booster status did not improve survival or hospitalization rates among people who were infected more than once.
“Reinfection with COVID-19 increases the risk of both acute outcomes and long COVID,” study author Ziyad Al-Aly, MD, told Reuters. “This was evident in unvaccinated, vaccinated and boosted people.”
The study was published in the journal Nature Medicine.
Researchers analyzed U.S. Department of Veterans Affairs data, including 443,588 people with a first infection of SARS-CoV-2, 40,947 people who were infected two or more times, and 5.3 million people who had not been infected with coronavirus, whose data served as the control group.
“During the past few months, there’s been an air of invincibility among people who have had COVID-19 or their vaccinations and boosters, and especially among people who have had an infection and also received vaccines; some people started to [refer] to these individuals as having a sort of superimmunity to the virus,” Dr. Al-Aly said in a press release from Washington University in St. Louis. “Without ambiguity, our research showed that getting an infection a second, third or fourth time contributes to additional health risks in the acute phase, meaning the first 30 days after infection, and in the months beyond, meaning the long COVID phase.”
Being infected with COVID-19 more than once also dramatically increased the risk of developing lung problems, heart conditions, or brain conditions. The heightened risks persisted for 6 months.
Researchers said a limitation of their study was that data primarily came from White males.
An expert not involved in the study told Reuters that the Veterans Affairs population does not reflect the general population. Patients at VA health facilities are generally older with more than normal health complications, said John Moore, PhD, a professor of microbiology and immunology at Weill Cornell Medicine, New York.
Dr. Al-Aly encouraged people to be vigilant as they plan for the holiday season, Reuters reported.
“We had started seeing a lot of patients coming to the clinic with an air of invincibility,” he told Reuters. “They wondered, ‘Does getting a reinfection really matter?’ The answer is yes, it absolutely does.”
A version of this article first appeared on WebMD.com.
Getting COVID-19 a second time doubles a person’s chance of dying and triples the likelihood of being hospitalized in the next 6 months, a new study found.
Vaccination and booster status did not improve survival or hospitalization rates among people who were infected more than once.
“Reinfection with COVID-19 increases the risk of both acute outcomes and long COVID,” study author Ziyad Al-Aly, MD, told Reuters. “This was evident in unvaccinated, vaccinated and boosted people.”
The study was published in the journal Nature Medicine.
Researchers analyzed U.S. Department of Veterans Affairs data, including 443,588 people with a first infection of SARS-CoV-2, 40,947 people who were infected two or more times, and 5.3 million people who had not been infected with coronavirus, whose data served as the control group.
“During the past few months, there’s been an air of invincibility among people who have had COVID-19 or their vaccinations and boosters, and especially among people who have had an infection and also received vaccines; some people started to [refer] to these individuals as having a sort of superimmunity to the virus,” Dr. Al-Aly said in a press release from Washington University in St. Louis. “Without ambiguity, our research showed that getting an infection a second, third or fourth time contributes to additional health risks in the acute phase, meaning the first 30 days after infection, and in the months beyond, meaning the long COVID phase.”
Being infected with COVID-19 more than once also dramatically increased the risk of developing lung problems, heart conditions, or brain conditions. The heightened risks persisted for 6 months.
Researchers said a limitation of their study was that data primarily came from White males.
An expert not involved in the study told Reuters that the Veterans Affairs population does not reflect the general population. Patients at VA health facilities are generally older with more than normal health complications, said John Moore, PhD, a professor of microbiology and immunology at Weill Cornell Medicine, New York.
Dr. Al-Aly encouraged people to be vigilant as they plan for the holiday season, Reuters reported.
“We had started seeing a lot of patients coming to the clinic with an air of invincibility,” he told Reuters. “They wondered, ‘Does getting a reinfection really matter?’ The answer is yes, it absolutely does.”
A version of this article first appeared on WebMD.com.
Getting COVID-19 a second time doubles a person’s chance of dying and triples the likelihood of being hospitalized in the next 6 months, a new study found.
Vaccination and booster status did not improve survival or hospitalization rates among people who were infected more than once.
“Reinfection with COVID-19 increases the risk of both acute outcomes and long COVID,” study author Ziyad Al-Aly, MD, told Reuters. “This was evident in unvaccinated, vaccinated and boosted people.”
The study was published in the journal Nature Medicine.
Researchers analyzed U.S. Department of Veterans Affairs data, including 443,588 people with a first infection of SARS-CoV-2, 40,947 people who were infected two or more times, and 5.3 million people who had not been infected with coronavirus, whose data served as the control group.
“During the past few months, there’s been an air of invincibility among people who have had COVID-19 or their vaccinations and boosters, and especially among people who have had an infection and also received vaccines; some people started to [refer] to these individuals as having a sort of superimmunity to the virus,” Dr. Al-Aly said in a press release from Washington University in St. Louis. “Without ambiguity, our research showed that getting an infection a second, third or fourth time contributes to additional health risks in the acute phase, meaning the first 30 days after infection, and in the months beyond, meaning the long COVID phase.”
Being infected with COVID-19 more than once also dramatically increased the risk of developing lung problems, heart conditions, or brain conditions. The heightened risks persisted for 6 months.
Researchers said a limitation of their study was that data primarily came from White males.
An expert not involved in the study told Reuters that the Veterans Affairs population does not reflect the general population. Patients at VA health facilities are generally older with more than normal health complications, said John Moore, PhD, a professor of microbiology and immunology at Weill Cornell Medicine, New York.
Dr. Al-Aly encouraged people to be vigilant as they plan for the holiday season, Reuters reported.
“We had started seeing a lot of patients coming to the clinic with an air of invincibility,” he told Reuters. “They wondered, ‘Does getting a reinfection really matter?’ The answer is yes, it absolutely does.”
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE
Has the time come for glucose monitors for people without diabetes?
Use of continuous glucose monitoring (CGM) by people without diabetes is becoming increasingly popular despite little evidence of benefit thus far, prompting discussion in the diabetes technology community about best practices.
Emerging uses for CGM outside of diabetes include improving glucose patterns to avoid diabetes, improving mental or physical performance, and promoting motivation for healthy behavior change. Such uses are not approved by the Food and Drug Administration and not covered by health insurance, yet a growing number of people are paying digital health companies for the devices as part of wellness packages.
In a related issue that highlights a limitation in this area, new data suggest that the “glucose management indicator (GMI)” feature of CGMs used for diabetes management – a percentage derived from people with diabetes and elevated A1c – may overestimate the actual A1c level in people without diabetes or those with diabetes who maintain A1c less than 6.5%.
“This is an evolving space ... CGM in people with prediabetes may be beneficial, but we need more data and evidence to recommend it. CGM metrics such as time-in-range and GMI are designed for people with type 1 and type 2 diabetes, and therefore, they are not applicable for people without diabetes,” Viral Shah, MD, said in an interview.
During the recent virtual Diabetes Technology Society meeting, Dr. Shah presented results from a soon-to-be published study finding that on average, GMI was 0.59% higher in people with A1c less than 5.7% and 0.49% higher for A1c 5.7%-6.4%, both significant (P < .0001). Dr. Shah, of the Barbara Davis Center for Diabetes, Adult Clinic, Aurora, Colorado, also presented those data in June at the annual scientific sessions of the American Diabetes Association.
Juan Espinoza, MD, of Children’s Hospital Los Angeles, told this news organization that there are data showing that CGM can be a “powerful biofeedback tool” in people with obesity who don’t have diabetes. “Since they don’t have diabetes the time in range or GMI is meaningless. What’s useful for them is seeing the glucose changes in real time and then using that as a trigger for behavioral change.”
‘An idea whose time has come?’
Dr. Espinoza was a co-author on a review published online in the Journal of Diabetes Science and Technology, entitled, “Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come?”
The review examines several aspects of the issue, beginning with studies that used CGM to investigate glucose concentrations in people with normal fasting glucose and glucose tolerance tests. Nearly all those individuals – from populations around the world – fell in the blood glucose range of 70-140 mg/dL.
Also reviewed are studies using CGM to study effects of diet, exercise, and stress on glucose levels in people without diabetes. Subsequent sections summarize the limited data that are available suggesting potential benefit for use of CGM in metabolic disease including prediabetes and obesity, non-metabolic conditions such as steroid treatment or parenteral nutrition, health and wellness, and among elite athletes. In that last group, glucose levels in both the hypoglycemic and hyperglycemic ranges during intensive activity have been documented.
Currently, there are four CGM devices that are FDA-approved for use in people with diabetes: FreeStyle Libre (Abbott), the implantable Eversense (Senseonics), and devices from Dexcom and Medtronic.
As Dr. Espinoza and colleagues explain in their review, most of the commercial health and wellness CGM programs, such as Nutrisense, Signos, and Supersapiens, actually use sensors made by those same manufacturers. Nutrisense and Supersapiens use the Libre, and Signos uses the Dexcom.
But, rather than the manufacturer’s apps meant for use by people with diabetes, the wellness companies pair the sensors with their own specially designed apps and typically offer additional services such as health coaching or nutrition counseling “to improve general health.”
Subscribers pay a monthly fee. Signos, for example, charges $399 for 1 month, $199/month for 3 months, or $159/month for 6 months. A prescription is required, but the company’s website says, “rest assured, an independent physician will handle the prescription for you, so you won’t need to arrange for a doctor visit. It is included in the cost of membership.”
Several consumer health product companies are now developing non-invasive glucose monitors, most often as a wristwatch, for people without diabetes to measure glucose optically from the skin in the wrist.
“It remains to be determined how accurate these new devices will be and how they will be regulated,” the researchers write.
What to do with the data?
The dedicated health and wellness apps typically provide average glucose and trend data but not the GMI. However, in theory users could access that metric by downloading the manufacturers’ viewing apps – for example, Clarity for Dexcom or LibreView for Libre.
Moreover, a person without diabetes could always obtain an off-label prescription from their physician for a FreeStyle Libre and purchase it at a pharmacy. At Walmart, for example, the cost for two boxes of two glucose meters with 14 days of wear each is $136.77. In that situation as well, users could download the viewing app that contains the summary data including the GMI that could potentially mislead in the setting of consistent normoglycemia.
Dr. Espinoza said: “I think there’s certainly value in glucose levels. We know the summary metrics are useful in type 1 diabetes. We don’t know which summary metrics are going to be useful in any other disease states. We may need brand new summary metrics for other disease states where it’s not about time in range. Maybe the thing that matters is the frequency or height of spikes. We don’t have a measure for that.”
He added that despite the availability of normative data, “even people without diabetes are a fairly heterogenous group. They can still have insulin resistance, so it’s tricky. From a science standpoint, we probably need studies with hundreds of patients with well-established A1c and [insulin resistance measures], weight, and body mass index. Then and only then will we be able to give an accurate glucose profile.”
In the meantime, “more data is always a good thing, but the hard thing is figuring out what do we do with it. Maybe it’s biofeedback for behavioral modification. We don’t know yet. But these are powerful tools and maybe we should learn how to use them better.”
Dr. Shah has reported receiving research grants and participating in advisory boards for Dexcom and Sanofi US. Dr. Espinoza has reported receiving research funding from the National Institutes of Health and FDA.
A version of this article first appeared on Medscape.com.
Use of continuous glucose monitoring (CGM) by people without diabetes is becoming increasingly popular despite little evidence of benefit thus far, prompting discussion in the diabetes technology community about best practices.
Emerging uses for CGM outside of diabetes include improving glucose patterns to avoid diabetes, improving mental or physical performance, and promoting motivation for healthy behavior change. Such uses are not approved by the Food and Drug Administration and not covered by health insurance, yet a growing number of people are paying digital health companies for the devices as part of wellness packages.
In a related issue that highlights a limitation in this area, new data suggest that the “glucose management indicator (GMI)” feature of CGMs used for diabetes management – a percentage derived from people with diabetes and elevated A1c – may overestimate the actual A1c level in people without diabetes or those with diabetes who maintain A1c less than 6.5%.
“This is an evolving space ... CGM in people with prediabetes may be beneficial, but we need more data and evidence to recommend it. CGM metrics such as time-in-range and GMI are designed for people with type 1 and type 2 diabetes, and therefore, they are not applicable for people without diabetes,” Viral Shah, MD, said in an interview.
During the recent virtual Diabetes Technology Society meeting, Dr. Shah presented results from a soon-to-be published study finding that on average, GMI was 0.59% higher in people with A1c less than 5.7% and 0.49% higher for A1c 5.7%-6.4%, both significant (P < .0001). Dr. Shah, of the Barbara Davis Center for Diabetes, Adult Clinic, Aurora, Colorado, also presented those data in June at the annual scientific sessions of the American Diabetes Association.
Juan Espinoza, MD, of Children’s Hospital Los Angeles, told this news organization that there are data showing that CGM can be a “powerful biofeedback tool” in people with obesity who don’t have diabetes. “Since they don’t have diabetes the time in range or GMI is meaningless. What’s useful for them is seeing the glucose changes in real time and then using that as a trigger for behavioral change.”
‘An idea whose time has come?’
Dr. Espinoza was a co-author on a review published online in the Journal of Diabetes Science and Technology, entitled, “Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come?”
The review examines several aspects of the issue, beginning with studies that used CGM to investigate glucose concentrations in people with normal fasting glucose and glucose tolerance tests. Nearly all those individuals – from populations around the world – fell in the blood glucose range of 70-140 mg/dL.
Also reviewed are studies using CGM to study effects of diet, exercise, and stress on glucose levels in people without diabetes. Subsequent sections summarize the limited data that are available suggesting potential benefit for use of CGM in metabolic disease including prediabetes and obesity, non-metabolic conditions such as steroid treatment or parenteral nutrition, health and wellness, and among elite athletes. In that last group, glucose levels in both the hypoglycemic and hyperglycemic ranges during intensive activity have been documented.
Currently, there are four CGM devices that are FDA-approved for use in people with diabetes: FreeStyle Libre (Abbott), the implantable Eversense (Senseonics), and devices from Dexcom and Medtronic.
As Dr. Espinoza and colleagues explain in their review, most of the commercial health and wellness CGM programs, such as Nutrisense, Signos, and Supersapiens, actually use sensors made by those same manufacturers. Nutrisense and Supersapiens use the Libre, and Signos uses the Dexcom.
But, rather than the manufacturer’s apps meant for use by people with diabetes, the wellness companies pair the sensors with their own specially designed apps and typically offer additional services such as health coaching or nutrition counseling “to improve general health.”
Subscribers pay a monthly fee. Signos, for example, charges $399 for 1 month, $199/month for 3 months, or $159/month for 6 months. A prescription is required, but the company’s website says, “rest assured, an independent physician will handle the prescription for you, so you won’t need to arrange for a doctor visit. It is included in the cost of membership.”
Several consumer health product companies are now developing non-invasive glucose monitors, most often as a wristwatch, for people without diabetes to measure glucose optically from the skin in the wrist.
“It remains to be determined how accurate these new devices will be and how they will be regulated,” the researchers write.
What to do with the data?
The dedicated health and wellness apps typically provide average glucose and trend data but not the GMI. However, in theory users could access that metric by downloading the manufacturers’ viewing apps – for example, Clarity for Dexcom or LibreView for Libre.
Moreover, a person without diabetes could always obtain an off-label prescription from their physician for a FreeStyle Libre and purchase it at a pharmacy. At Walmart, for example, the cost for two boxes of two glucose meters with 14 days of wear each is $136.77. In that situation as well, users could download the viewing app that contains the summary data including the GMI that could potentially mislead in the setting of consistent normoglycemia.
Dr. Espinoza said: “I think there’s certainly value in glucose levels. We know the summary metrics are useful in type 1 diabetes. We don’t know which summary metrics are going to be useful in any other disease states. We may need brand new summary metrics for other disease states where it’s not about time in range. Maybe the thing that matters is the frequency or height of spikes. We don’t have a measure for that.”
He added that despite the availability of normative data, “even people without diabetes are a fairly heterogenous group. They can still have insulin resistance, so it’s tricky. From a science standpoint, we probably need studies with hundreds of patients with well-established A1c and [insulin resistance measures], weight, and body mass index. Then and only then will we be able to give an accurate glucose profile.”
In the meantime, “more data is always a good thing, but the hard thing is figuring out what do we do with it. Maybe it’s biofeedback for behavioral modification. We don’t know yet. But these are powerful tools and maybe we should learn how to use them better.”
Dr. Shah has reported receiving research grants and participating in advisory boards for Dexcom and Sanofi US. Dr. Espinoza has reported receiving research funding from the National Institutes of Health and FDA.
A version of this article first appeared on Medscape.com.
Use of continuous glucose monitoring (CGM) by people without diabetes is becoming increasingly popular despite little evidence of benefit thus far, prompting discussion in the diabetes technology community about best practices.
Emerging uses for CGM outside of diabetes include improving glucose patterns to avoid diabetes, improving mental or physical performance, and promoting motivation for healthy behavior change. Such uses are not approved by the Food and Drug Administration and not covered by health insurance, yet a growing number of people are paying digital health companies for the devices as part of wellness packages.
In a related issue that highlights a limitation in this area, new data suggest that the “glucose management indicator (GMI)” feature of CGMs used for diabetes management – a percentage derived from people with diabetes and elevated A1c – may overestimate the actual A1c level in people without diabetes or those with diabetes who maintain A1c less than 6.5%.
“This is an evolving space ... CGM in people with prediabetes may be beneficial, but we need more data and evidence to recommend it. CGM metrics such as time-in-range and GMI are designed for people with type 1 and type 2 diabetes, and therefore, they are not applicable for people without diabetes,” Viral Shah, MD, said in an interview.
During the recent virtual Diabetes Technology Society meeting, Dr. Shah presented results from a soon-to-be published study finding that on average, GMI was 0.59% higher in people with A1c less than 5.7% and 0.49% higher for A1c 5.7%-6.4%, both significant (P < .0001). Dr. Shah, of the Barbara Davis Center for Diabetes, Adult Clinic, Aurora, Colorado, also presented those data in June at the annual scientific sessions of the American Diabetes Association.
Juan Espinoza, MD, of Children’s Hospital Los Angeles, told this news organization that there are data showing that CGM can be a “powerful biofeedback tool” in people with obesity who don’t have diabetes. “Since they don’t have diabetes the time in range or GMI is meaningless. What’s useful for them is seeing the glucose changes in real time and then using that as a trigger for behavioral change.”
‘An idea whose time has come?’
Dr. Espinoza was a co-author on a review published online in the Journal of Diabetes Science and Technology, entitled, “Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come?”
The review examines several aspects of the issue, beginning with studies that used CGM to investigate glucose concentrations in people with normal fasting glucose and glucose tolerance tests. Nearly all those individuals – from populations around the world – fell in the blood glucose range of 70-140 mg/dL.
Also reviewed are studies using CGM to study effects of diet, exercise, and stress on glucose levels in people without diabetes. Subsequent sections summarize the limited data that are available suggesting potential benefit for use of CGM in metabolic disease including prediabetes and obesity, non-metabolic conditions such as steroid treatment or parenteral nutrition, health and wellness, and among elite athletes. In that last group, glucose levels in both the hypoglycemic and hyperglycemic ranges during intensive activity have been documented.
Currently, there are four CGM devices that are FDA-approved for use in people with diabetes: FreeStyle Libre (Abbott), the implantable Eversense (Senseonics), and devices from Dexcom and Medtronic.
As Dr. Espinoza and colleagues explain in their review, most of the commercial health and wellness CGM programs, such as Nutrisense, Signos, and Supersapiens, actually use sensors made by those same manufacturers. Nutrisense and Supersapiens use the Libre, and Signos uses the Dexcom.
But, rather than the manufacturer’s apps meant for use by people with diabetes, the wellness companies pair the sensors with their own specially designed apps and typically offer additional services such as health coaching or nutrition counseling “to improve general health.”
Subscribers pay a monthly fee. Signos, for example, charges $399 for 1 month, $199/month for 3 months, or $159/month for 6 months. A prescription is required, but the company’s website says, “rest assured, an independent physician will handle the prescription for you, so you won’t need to arrange for a doctor visit. It is included in the cost of membership.”
Several consumer health product companies are now developing non-invasive glucose monitors, most often as a wristwatch, for people without diabetes to measure glucose optically from the skin in the wrist.
“It remains to be determined how accurate these new devices will be and how they will be regulated,” the researchers write.
What to do with the data?
The dedicated health and wellness apps typically provide average glucose and trend data but not the GMI. However, in theory users could access that metric by downloading the manufacturers’ viewing apps – for example, Clarity for Dexcom or LibreView for Libre.
Moreover, a person without diabetes could always obtain an off-label prescription from their physician for a FreeStyle Libre and purchase it at a pharmacy. At Walmart, for example, the cost for two boxes of two glucose meters with 14 days of wear each is $136.77. In that situation as well, users could download the viewing app that contains the summary data including the GMI that could potentially mislead in the setting of consistent normoglycemia.
Dr. Espinoza said: “I think there’s certainly value in glucose levels. We know the summary metrics are useful in type 1 diabetes. We don’t know which summary metrics are going to be useful in any other disease states. We may need brand new summary metrics for other disease states where it’s not about time in range. Maybe the thing that matters is the frequency or height of spikes. We don’t have a measure for that.”
He added that despite the availability of normative data, “even people without diabetes are a fairly heterogenous group. They can still have insulin resistance, so it’s tricky. From a science standpoint, we probably need studies with hundreds of patients with well-established A1c and [insulin resistance measures], weight, and body mass index. Then and only then will we be able to give an accurate glucose profile.”
In the meantime, “more data is always a good thing, but the hard thing is figuring out what do we do with it. Maybe it’s biofeedback for behavioral modification. We don’t know yet. But these are powerful tools and maybe we should learn how to use them better.”
Dr. Shah has reported receiving research grants and participating in advisory boards for Dexcom and Sanofi US. Dr. Espinoza has reported receiving research funding from the National Institutes of Health and FDA.
A version of this article first appeared on Medscape.com.
AT ADA 2022
No benefit of rivaroxaban in COVID outpatients: PREVENT-HD
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
FROM AHA 2022