User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Ticagrelor: Modest benefit, bigger bleed risk in diabetes plus stable CAD
PARIS – , though they also had more major bleeding events than patients receiving placebo plus aspirin.
The subset of patients who had received prior percutaneous coronary intervention (PCI) stood to benefit more from extended dual antiplatelet therapy (DAPT), according to clinical trial results presented to an overflow crowd at the annual congress of the European Society of Cardiology.
Findings from the full study, named The Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS), and from the PCI subgroup analysis were published concurrently with the presentation (N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2).
“This strategy of long-term dual antiplatelet therapy may be beneficial in selected patients at low risk of bleeding, but at high risk of ischemic events,” said the study’s co-principal investigator Deepak Bhatt, MD, professor of medicine at Harvard Medical School, Boston, and executive director of interventional cardiology programs at Boston’s Brigham and Women’s Hospital. In a video interview, he hypothesized that “prior PCI may serve as a sort of ‘stress test’ for bleeding,” thus identifying a subset of patients who might benefit from long-term DAPT.
Ischemic events, the primary efficacy outcome of THEMIS, occurred in 7.7% of patients taking the P2Y12 receptor antagonist ticagrelor and 8.5% of those receiving placebo, for a hazard ratio of 0.90 favoring ticagrelor (P = .04). Ischemic events included cardiovascular deaths, myocardial infarctions (MIs), and stroke.
Looking at secondary endpoints, Dr. Bhatt said that there was no difference in cardiovascular deaths between study arms, but that ischemic strokes, all MIs, and ST segment elevation MIs were all less common for patients taking ticagrelor. All-cause mortality was similar between study groups.
Though ischemic events dropped, “This benefit was achieved at the expense of more bleeding,” said Dr. Bhatt. Major bleeding, the primary safety outcome, was seen in 2.2% of those taking ticagrelor and 1.0% of the placebo group, for a hazard ratio of 2.32 (P less than .001). Dr. Bhatt and his collaborators used the Thrombolysis in Myocardial Infarction (TIMI) criteria for major bleeding for ascertainment of this outcome.
Intracranial hemorrhage was also more common for patients on ticagrelor, though incidence was low and the absolute difference was small between groups. This complication occurred in 0.7% of ticagrelor patients and 0.5% of placebo patients, yielding a hazard ratio of 1.71 (P = .0005). “This excess wasn’t in spontaneous or procedural intracranial bleeding, but rather in traumatic intracranial hemorrhage,” said Dr. Bhatt.
Fatal bleeds affected just 0.2% of those on ticagrelor and 0.1% of those receiving placebo; this difference wasn’t statistically significant.
THEMIS was an international multisite double-blind, placebo-controlled study randomizing 19,220 patients 1:1 to receive aspirin, plus placebo (N = 9,601) or ticagrelor (N = 9,619). Patients were followed for a median of 39.9 months; those with previous myocardial infarction or stroke were excluded. Patients had to be at least 50 years old and on anti-hyperglycemic medications for at least 6 months to participate. Patients in the overall study had a baseline age of 66 years, and 31% were female. Most patients were white (71%).
Stable coronary artery disease (CAD) was defined by having any of a previous history of PCI, coronary artery bypass grafting, or angiographically documented stenosis of at least 50% in at least one coronary artery.
During the study period, Dr. Bhatt explained, ticagrelor dosage was reduced from 90 to 60 mg daily as other studies yielded data about improved safety and tolerability without compromise in efficacy at the lower ticagrelor dose.
Permanent treatment discontinuation was common, but more common in patients taking ticagrelor, compared with placebo (34.5% vs. 25.4%). The most frequent reasons for ticagrelor discontinuation were dyspnea and bleeding. All patients who were randomized, save those at a study site that was closed before unblinding, were included in the modified intention-to-treat population for calculation of efficacy outcomes for both THEMIS and THEMIS-PCI.
Given the large number of patients who discontinued the study drug, an estimation was made of the number of events that would have occurred had patients remained in the trial, and outcomes were calculated using these estimations to account for missing data.
Safety outcomes were calculated by including all patients who received at least one dose of a study drug.
An exploratory composite outcome of “net irreversible harm” included all-cause death, myocardial infarction, and stroke, but also fatal bleeding and intracranial hemorrhage. In the full study population, this outcome was seen in 10.1% of the placebo group and 10.8% of the placebo group, for a nonsignificant hazard ratio of 0.93, said Dr. Bhatt.
An additional composite pre-specified exploratory outcome included acute limb ischemia or major amputation; here, the HR of 0.45 favored ticagrelor.
Dr. Bhatt made the point that these pragmatic, patient-centered outcomes are valuable tools when weighing the potential risks and benefits of therapy for a particular patient, and provide a discussion point for individualized, shared decision making.
Results of a pre-specified subgroup analysis of the 58% of THEMIS participants (n = 5,558) with prior PCI were presented by THEMIS’ co-principal investigator, Philippe Gabriel Steg, MD, of the University of Paris and the French National Institute of Health and Medical Research.
“In the history of PCI subgroup, 92% of patients had a history of receiving a stent, and 61% had received at least one drug-eluting stent,” said Dr. Steg.
Patients with PCI saw a slightly greater reduction in relative risk for ischemic events when they received ticagrelor, compared with placebo; the PCI group had a HR of 0.85 for ischemic events (P = .013), compared with a HR of 0.98 for those with no PCI history (P = .76). This meant that ticagrelor DAPT’s efficacy as measured by the primary endpoint of ischemic events lost significance when the non-PCI group was evaluated (P = .76, with P for interaction between the groups of .16).
Some secondary endpoints showed statistical significance for the interaction between PCI status and study drug status. These included the composite outcome of all-cause death, MI, or stroke (P for interaction, .021), and another “mega-composite ischemia” outcome that folded in major amputation of vascular etiology along with all-cause death, MI, and stroke (P = .023).
Looking at bleeding endpoints, there was no significant difference between the groups for TIMI major bleeding, the primary safety endpoint. Patients in the full study cohort as well as the PCI subgroup had significantly more TIMI major bleeding on ticagrelor.
Bleeding measured by Bleeding Academic Research Consortium (BARC) criteria was a secondary endpoint, and the P for interaction just reached statistical significance for the aggregate of all levels of BARC bleeding.
“But the two observations I would draw your attention to are the fact that in patients with a history of PCI, fatal bleeding occurred in the same number of patients in each group – 6 patients in each group,” added Dr. Steg. “And even more importantly, intracranial hemorrhage occurred in 33 patients in the ticagrelor group and 31 patients in the placebo group for patients with a history of PCI, whereas it was 37 and 15 for patients without a history of PCI.” This yielded a significant P value for the interaction of .036.
The exploratory net clinical benefit score favored the PCI group, for a P for interaction of .012. Dr. Steg also shared an analysis showing a net benefit for ticagrelor vs. placebo as a function of the time elapsed between PCI and trial randomization, showing patient benefit to 6 years post drug initiation for the PCI group.
“The subgroup analysis of THEMIS PCI was pre-specified, from a large, clinically meaningful population; it’s plausible and it can be easily explained from the action of dual antiplatelet therapy, and it shows a net benefit,” Dr. Steg said.
The discussant for the presentations was Colin Baigent, , and he wasn’t convinced by the THEMIS-PCI data. He pointed out that looking at the absolute numbers overall for THEMIS yields an absolute benefit of about 8 per 1,000 participants, and an absolute risk of about 12 per 1,000 participants.
“The natural instinct is to then go to the subgroups and try to find people who will see a net benefit,” he said. “Why pick out ‘history of PCI?’” among the 18 pre-specified subgroups, he asked, noting that there was not significant evidence of heterogeneity of hazard ratios among the subgroups.
Overall, “The main results of THEMIS are consistent” with previous investigations into the benefits of ticagrelor DAPT, showing modest efficacy at the expense of a two-fold rise in major bleeding events, said Dr. Baigent, professor of epidemiology at the University of Oxford (England).
The THEMIS study and the subpopulation analysis were funded by AstraZeneca, which markets ticagrelor. Dr. Bhatt reported financial relationships with AstraZeneca and multiple other pharmaceutical companies. In addition to reporting a financial relationship with AstraZeneca, Dr. Steg also reported relationships with multiple pharmaceutical companies. Dr. Baigent reported a financial relationship with Boehringer Engelheim.
Source: Steg PG et al. N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Bhatt DL et al.Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2)
PARIS – , though they also had more major bleeding events than patients receiving placebo plus aspirin.
The subset of patients who had received prior percutaneous coronary intervention (PCI) stood to benefit more from extended dual antiplatelet therapy (DAPT), according to clinical trial results presented to an overflow crowd at the annual congress of the European Society of Cardiology.
Findings from the full study, named The Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS), and from the PCI subgroup analysis were published concurrently with the presentation (N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2).
“This strategy of long-term dual antiplatelet therapy may be beneficial in selected patients at low risk of bleeding, but at high risk of ischemic events,” said the study’s co-principal investigator Deepak Bhatt, MD, professor of medicine at Harvard Medical School, Boston, and executive director of interventional cardiology programs at Boston’s Brigham and Women’s Hospital. In a video interview, he hypothesized that “prior PCI may serve as a sort of ‘stress test’ for bleeding,” thus identifying a subset of patients who might benefit from long-term DAPT.
Ischemic events, the primary efficacy outcome of THEMIS, occurred in 7.7% of patients taking the P2Y12 receptor antagonist ticagrelor and 8.5% of those receiving placebo, for a hazard ratio of 0.90 favoring ticagrelor (P = .04). Ischemic events included cardiovascular deaths, myocardial infarctions (MIs), and stroke.
Looking at secondary endpoints, Dr. Bhatt said that there was no difference in cardiovascular deaths between study arms, but that ischemic strokes, all MIs, and ST segment elevation MIs were all less common for patients taking ticagrelor. All-cause mortality was similar between study groups.
Though ischemic events dropped, “This benefit was achieved at the expense of more bleeding,” said Dr. Bhatt. Major bleeding, the primary safety outcome, was seen in 2.2% of those taking ticagrelor and 1.0% of the placebo group, for a hazard ratio of 2.32 (P less than .001). Dr. Bhatt and his collaborators used the Thrombolysis in Myocardial Infarction (TIMI) criteria for major bleeding for ascertainment of this outcome.
Intracranial hemorrhage was also more common for patients on ticagrelor, though incidence was low and the absolute difference was small between groups. This complication occurred in 0.7% of ticagrelor patients and 0.5% of placebo patients, yielding a hazard ratio of 1.71 (P = .0005). “This excess wasn’t in spontaneous or procedural intracranial bleeding, but rather in traumatic intracranial hemorrhage,” said Dr. Bhatt.
Fatal bleeds affected just 0.2% of those on ticagrelor and 0.1% of those receiving placebo; this difference wasn’t statistically significant.
THEMIS was an international multisite double-blind, placebo-controlled study randomizing 19,220 patients 1:1 to receive aspirin, plus placebo (N = 9,601) or ticagrelor (N = 9,619). Patients were followed for a median of 39.9 months; those with previous myocardial infarction or stroke were excluded. Patients had to be at least 50 years old and on anti-hyperglycemic medications for at least 6 months to participate. Patients in the overall study had a baseline age of 66 years, and 31% were female. Most patients were white (71%).
Stable coronary artery disease (CAD) was defined by having any of a previous history of PCI, coronary artery bypass grafting, or angiographically documented stenosis of at least 50% in at least one coronary artery.
During the study period, Dr. Bhatt explained, ticagrelor dosage was reduced from 90 to 60 mg daily as other studies yielded data about improved safety and tolerability without compromise in efficacy at the lower ticagrelor dose.
Permanent treatment discontinuation was common, but more common in patients taking ticagrelor, compared with placebo (34.5% vs. 25.4%). The most frequent reasons for ticagrelor discontinuation were dyspnea and bleeding. All patients who were randomized, save those at a study site that was closed before unblinding, were included in the modified intention-to-treat population for calculation of efficacy outcomes for both THEMIS and THEMIS-PCI.
Given the large number of patients who discontinued the study drug, an estimation was made of the number of events that would have occurred had patients remained in the trial, and outcomes were calculated using these estimations to account for missing data.
Safety outcomes were calculated by including all patients who received at least one dose of a study drug.
An exploratory composite outcome of “net irreversible harm” included all-cause death, myocardial infarction, and stroke, but also fatal bleeding and intracranial hemorrhage. In the full study population, this outcome was seen in 10.1% of the placebo group and 10.8% of the placebo group, for a nonsignificant hazard ratio of 0.93, said Dr. Bhatt.
An additional composite pre-specified exploratory outcome included acute limb ischemia or major amputation; here, the HR of 0.45 favored ticagrelor.
Dr. Bhatt made the point that these pragmatic, patient-centered outcomes are valuable tools when weighing the potential risks and benefits of therapy for a particular patient, and provide a discussion point for individualized, shared decision making.
Results of a pre-specified subgroup analysis of the 58% of THEMIS participants (n = 5,558) with prior PCI were presented by THEMIS’ co-principal investigator, Philippe Gabriel Steg, MD, of the University of Paris and the French National Institute of Health and Medical Research.
“In the history of PCI subgroup, 92% of patients had a history of receiving a stent, and 61% had received at least one drug-eluting stent,” said Dr. Steg.
Patients with PCI saw a slightly greater reduction in relative risk for ischemic events when they received ticagrelor, compared with placebo; the PCI group had a HR of 0.85 for ischemic events (P = .013), compared with a HR of 0.98 for those with no PCI history (P = .76). This meant that ticagrelor DAPT’s efficacy as measured by the primary endpoint of ischemic events lost significance when the non-PCI group was evaluated (P = .76, with P for interaction between the groups of .16).
Some secondary endpoints showed statistical significance for the interaction between PCI status and study drug status. These included the composite outcome of all-cause death, MI, or stroke (P for interaction, .021), and another “mega-composite ischemia” outcome that folded in major amputation of vascular etiology along with all-cause death, MI, and stroke (P = .023).
Looking at bleeding endpoints, there was no significant difference between the groups for TIMI major bleeding, the primary safety endpoint. Patients in the full study cohort as well as the PCI subgroup had significantly more TIMI major bleeding on ticagrelor.
Bleeding measured by Bleeding Academic Research Consortium (BARC) criteria was a secondary endpoint, and the P for interaction just reached statistical significance for the aggregate of all levels of BARC bleeding.
“But the two observations I would draw your attention to are the fact that in patients with a history of PCI, fatal bleeding occurred in the same number of patients in each group – 6 patients in each group,” added Dr. Steg. “And even more importantly, intracranial hemorrhage occurred in 33 patients in the ticagrelor group and 31 patients in the placebo group for patients with a history of PCI, whereas it was 37 and 15 for patients without a history of PCI.” This yielded a significant P value for the interaction of .036.
The exploratory net clinical benefit score favored the PCI group, for a P for interaction of .012. Dr. Steg also shared an analysis showing a net benefit for ticagrelor vs. placebo as a function of the time elapsed between PCI and trial randomization, showing patient benefit to 6 years post drug initiation for the PCI group.
“The subgroup analysis of THEMIS PCI was pre-specified, from a large, clinically meaningful population; it’s plausible and it can be easily explained from the action of dual antiplatelet therapy, and it shows a net benefit,” Dr. Steg said.
The discussant for the presentations was Colin Baigent, , and he wasn’t convinced by the THEMIS-PCI data. He pointed out that looking at the absolute numbers overall for THEMIS yields an absolute benefit of about 8 per 1,000 participants, and an absolute risk of about 12 per 1,000 participants.
“The natural instinct is to then go to the subgroups and try to find people who will see a net benefit,” he said. “Why pick out ‘history of PCI?’” among the 18 pre-specified subgroups, he asked, noting that there was not significant evidence of heterogeneity of hazard ratios among the subgroups.
Overall, “The main results of THEMIS are consistent” with previous investigations into the benefits of ticagrelor DAPT, showing modest efficacy at the expense of a two-fold rise in major bleeding events, said Dr. Baigent, professor of epidemiology at the University of Oxford (England).
The THEMIS study and the subpopulation analysis were funded by AstraZeneca, which markets ticagrelor. Dr. Bhatt reported financial relationships with AstraZeneca and multiple other pharmaceutical companies. In addition to reporting a financial relationship with AstraZeneca, Dr. Steg also reported relationships with multiple pharmaceutical companies. Dr. Baigent reported a financial relationship with Boehringer Engelheim.
Source: Steg PG et al. N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Bhatt DL et al.Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2)
PARIS – , though they also had more major bleeding events than patients receiving placebo plus aspirin.
The subset of patients who had received prior percutaneous coronary intervention (PCI) stood to benefit more from extended dual antiplatelet therapy (DAPT), according to clinical trial results presented to an overflow crowd at the annual congress of the European Society of Cardiology.
Findings from the full study, named The Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS), and from the PCI subgroup analysis were published concurrently with the presentation (N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2).
“This strategy of long-term dual antiplatelet therapy may be beneficial in selected patients at low risk of bleeding, but at high risk of ischemic events,” said the study’s co-principal investigator Deepak Bhatt, MD, professor of medicine at Harvard Medical School, Boston, and executive director of interventional cardiology programs at Boston’s Brigham and Women’s Hospital. In a video interview, he hypothesized that “prior PCI may serve as a sort of ‘stress test’ for bleeding,” thus identifying a subset of patients who might benefit from long-term DAPT.
Ischemic events, the primary efficacy outcome of THEMIS, occurred in 7.7% of patients taking the P2Y12 receptor antagonist ticagrelor and 8.5% of those receiving placebo, for a hazard ratio of 0.90 favoring ticagrelor (P = .04). Ischemic events included cardiovascular deaths, myocardial infarctions (MIs), and stroke.
Looking at secondary endpoints, Dr. Bhatt said that there was no difference in cardiovascular deaths between study arms, but that ischemic strokes, all MIs, and ST segment elevation MIs were all less common for patients taking ticagrelor. All-cause mortality was similar between study groups.
Though ischemic events dropped, “This benefit was achieved at the expense of more bleeding,” said Dr. Bhatt. Major bleeding, the primary safety outcome, was seen in 2.2% of those taking ticagrelor and 1.0% of the placebo group, for a hazard ratio of 2.32 (P less than .001). Dr. Bhatt and his collaborators used the Thrombolysis in Myocardial Infarction (TIMI) criteria for major bleeding for ascertainment of this outcome.
Intracranial hemorrhage was also more common for patients on ticagrelor, though incidence was low and the absolute difference was small between groups. This complication occurred in 0.7% of ticagrelor patients and 0.5% of placebo patients, yielding a hazard ratio of 1.71 (P = .0005). “This excess wasn’t in spontaneous or procedural intracranial bleeding, but rather in traumatic intracranial hemorrhage,” said Dr. Bhatt.
Fatal bleeds affected just 0.2% of those on ticagrelor and 0.1% of those receiving placebo; this difference wasn’t statistically significant.
THEMIS was an international multisite double-blind, placebo-controlled study randomizing 19,220 patients 1:1 to receive aspirin, plus placebo (N = 9,601) or ticagrelor (N = 9,619). Patients were followed for a median of 39.9 months; those with previous myocardial infarction or stroke were excluded. Patients had to be at least 50 years old and on anti-hyperglycemic medications for at least 6 months to participate. Patients in the overall study had a baseline age of 66 years, and 31% were female. Most patients were white (71%).
Stable coronary artery disease (CAD) was defined by having any of a previous history of PCI, coronary artery bypass grafting, or angiographically documented stenosis of at least 50% in at least one coronary artery.
During the study period, Dr. Bhatt explained, ticagrelor dosage was reduced from 90 to 60 mg daily as other studies yielded data about improved safety and tolerability without compromise in efficacy at the lower ticagrelor dose.
Permanent treatment discontinuation was common, but more common in patients taking ticagrelor, compared with placebo (34.5% vs. 25.4%). The most frequent reasons for ticagrelor discontinuation were dyspnea and bleeding. All patients who were randomized, save those at a study site that was closed before unblinding, were included in the modified intention-to-treat population for calculation of efficacy outcomes for both THEMIS and THEMIS-PCI.
Given the large number of patients who discontinued the study drug, an estimation was made of the number of events that would have occurred had patients remained in the trial, and outcomes were calculated using these estimations to account for missing data.
Safety outcomes were calculated by including all patients who received at least one dose of a study drug.
An exploratory composite outcome of “net irreversible harm” included all-cause death, myocardial infarction, and stroke, but also fatal bleeding and intracranial hemorrhage. In the full study population, this outcome was seen in 10.1% of the placebo group and 10.8% of the placebo group, for a nonsignificant hazard ratio of 0.93, said Dr. Bhatt.
An additional composite pre-specified exploratory outcome included acute limb ischemia or major amputation; here, the HR of 0.45 favored ticagrelor.
Dr. Bhatt made the point that these pragmatic, patient-centered outcomes are valuable tools when weighing the potential risks and benefits of therapy for a particular patient, and provide a discussion point for individualized, shared decision making.
Results of a pre-specified subgroup analysis of the 58% of THEMIS participants (n = 5,558) with prior PCI were presented by THEMIS’ co-principal investigator, Philippe Gabriel Steg, MD, of the University of Paris and the French National Institute of Health and Medical Research.
“In the history of PCI subgroup, 92% of patients had a history of receiving a stent, and 61% had received at least one drug-eluting stent,” said Dr. Steg.
Patients with PCI saw a slightly greater reduction in relative risk for ischemic events when they received ticagrelor, compared with placebo; the PCI group had a HR of 0.85 for ischemic events (P = .013), compared with a HR of 0.98 for those with no PCI history (P = .76). This meant that ticagrelor DAPT’s efficacy as measured by the primary endpoint of ischemic events lost significance when the non-PCI group was evaluated (P = .76, with P for interaction between the groups of .16).
Some secondary endpoints showed statistical significance for the interaction between PCI status and study drug status. These included the composite outcome of all-cause death, MI, or stroke (P for interaction, .021), and another “mega-composite ischemia” outcome that folded in major amputation of vascular etiology along with all-cause death, MI, and stroke (P = .023).
Looking at bleeding endpoints, there was no significant difference between the groups for TIMI major bleeding, the primary safety endpoint. Patients in the full study cohort as well as the PCI subgroup had significantly more TIMI major bleeding on ticagrelor.
Bleeding measured by Bleeding Academic Research Consortium (BARC) criteria was a secondary endpoint, and the P for interaction just reached statistical significance for the aggregate of all levels of BARC bleeding.
“But the two observations I would draw your attention to are the fact that in patients with a history of PCI, fatal bleeding occurred in the same number of patients in each group – 6 patients in each group,” added Dr. Steg. “And even more importantly, intracranial hemorrhage occurred in 33 patients in the ticagrelor group and 31 patients in the placebo group for patients with a history of PCI, whereas it was 37 and 15 for patients without a history of PCI.” This yielded a significant P value for the interaction of .036.
The exploratory net clinical benefit score favored the PCI group, for a P for interaction of .012. Dr. Steg also shared an analysis showing a net benefit for ticagrelor vs. placebo as a function of the time elapsed between PCI and trial randomization, showing patient benefit to 6 years post drug initiation for the PCI group.
“The subgroup analysis of THEMIS PCI was pre-specified, from a large, clinically meaningful population; it’s plausible and it can be easily explained from the action of dual antiplatelet therapy, and it shows a net benefit,” Dr. Steg said.
The discussant for the presentations was Colin Baigent, , and he wasn’t convinced by the THEMIS-PCI data. He pointed out that looking at the absolute numbers overall for THEMIS yields an absolute benefit of about 8 per 1,000 participants, and an absolute risk of about 12 per 1,000 participants.
“The natural instinct is to then go to the subgroups and try to find people who will see a net benefit,” he said. “Why pick out ‘history of PCI?’” among the 18 pre-specified subgroups, he asked, noting that there was not significant evidence of heterogeneity of hazard ratios among the subgroups.
Overall, “The main results of THEMIS are consistent” with previous investigations into the benefits of ticagrelor DAPT, showing modest efficacy at the expense of a two-fold rise in major bleeding events, said Dr. Baigent, professor of epidemiology at the University of Oxford (England).
The THEMIS study and the subpopulation analysis were funded by AstraZeneca, which markets ticagrelor. Dr. Bhatt reported financial relationships with AstraZeneca and multiple other pharmaceutical companies. In addition to reporting a financial relationship with AstraZeneca, Dr. Steg also reported relationships with multiple pharmaceutical companies. Dr. Baigent reported a financial relationship with Boehringer Engelheim.
Source: Steg PG et al. N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Bhatt DL et al.Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2)
AT THE ESC CONGRESS 2019
Diabetes targets remain elusive for patients
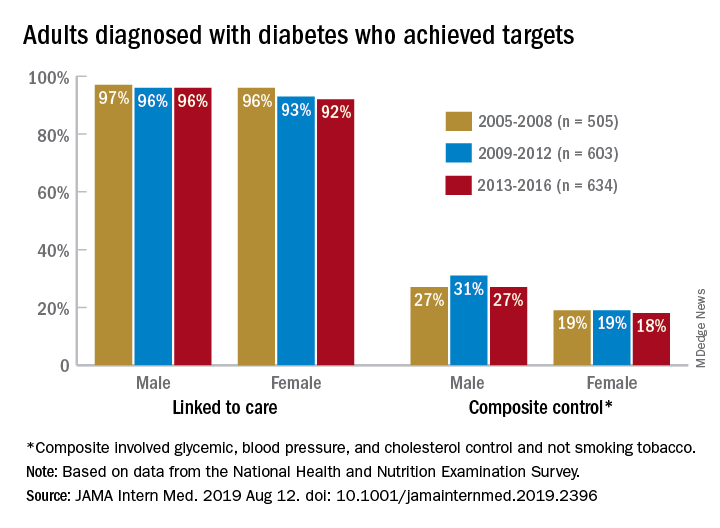
Some things never change: In 2005, most adults with diabetes missed their treatment targets. In 2016, most adults with diabetes missed their treatment targets. And during that time, from 2005 to 2016, around 96% of men and 94% of women were linked to care.
“Fewer than one in four American adults with diagnosed diabetes achieve a controlled level of blood sugar, blood pressure, and cholesterol, and do not smoke tobacco. Our results suggest that, despite major advances in diabetes drug discovery and movement to develop innovative care delivery models over the past two decades, achievement of diabetes care targets has not improved in the United States since 2005,” Pooyan Kazemian, PhD, of Massachusetts General Hospital, Boston, said in a written statement.
During 2013-2016, only 23% of adults with diabetes met a combined composite target of glycemic (HbA1c below a liberal personalized level), blood pressure (less than 140/90 mm Hg), and cholesterol (LDL cholesterol level less than 100 mg/dL) control, as well as not smoking tobacco, Dr. Kazemian and associates reported in JAMA Internal Medicine. The corresponding figures were 25% (2009-2012) and 23% (2005-2008) for the two earlier time periods covered in the study,
The investigators used data for 1,742 nonpregnant adults from the National Health and Nutrition Examination Survey to evaluate the diabetes care cascade, which they defined as “diagnosis, linkage to care, achievement of individual treatment targets, and a composite of all individual targets.”
In 2013-2016, 94% of those diagnosed were linked to care, 64% met their HbA1c target, 70% achieved blood pressure control, 57% met the cholesterol target, and 85% were nonsmokers. When targets were combined, 41% achieved blood pressure and cholesterol control, and 25% met the glycemic, blood pressure, and cholesterol targets, they said.
“We found that none of the U.S. diabetes care variables improved from 2005 to 2016,” Dr. Kazemian and associates noted. Women were less likely than men to meet their treatment goals (see graph) over the course of the study, as were adults aged 18-44 years and black and Hispanic individuals.
“Recent advances in [treatments for diabetes] have not effectively reached the populations at risk and may indicate an immediate need for better approaches to the delivery of diabetes care, including a continued focus on reaching underserved populations with persistent disparities in care,” they wrote.
The study was supported by the Boston Area Diabetes Endocrinology Research Center and Massachusetts General Hospital. One investigator reported that her husband has equity in Apolo1bio. No other disclosures were reported.
SOURCE: Kazemian P et al. JAMA Intern Med. 2019 Aug 12. doi: 10.1001/jamainternmed.2019.2396.
Some things never change: In 2005, most adults with diabetes missed their treatment targets. In 2016, most adults with diabetes missed their treatment targets. And during that time, from 2005 to 2016, around 96% of men and 94% of women were linked to care.

“Fewer than one in four American adults with diagnosed diabetes achieve a controlled level of blood sugar, blood pressure, and cholesterol, and do not smoke tobacco. Our results suggest that, despite major advances in diabetes drug discovery and movement to develop innovative care delivery models over the past two decades, achievement of diabetes care targets has not improved in the United States since 2005,” Pooyan Kazemian, PhD, of Massachusetts General Hospital, Boston, said in a written statement.
During 2013-2016, only 23% of adults with diabetes met a combined composite target of glycemic (HbA1c below a liberal personalized level), blood pressure (less than 140/90 mm Hg), and cholesterol (LDL cholesterol level less than 100 mg/dL) control, as well as not smoking tobacco, Dr. Kazemian and associates reported in JAMA Internal Medicine. The corresponding figures were 25% (2009-2012) and 23% (2005-2008) for the two earlier time periods covered in the study,
The investigators used data for 1,742 nonpregnant adults from the National Health and Nutrition Examination Survey to evaluate the diabetes care cascade, which they defined as “diagnosis, linkage to care, achievement of individual treatment targets, and a composite of all individual targets.”
In 2013-2016, 94% of those diagnosed were linked to care, 64% met their HbA1c target, 70% achieved blood pressure control, 57% met the cholesterol target, and 85% were nonsmokers. When targets were combined, 41% achieved blood pressure and cholesterol control, and 25% met the glycemic, blood pressure, and cholesterol targets, they said.
“We found that none of the U.S. diabetes care variables improved from 2005 to 2016,” Dr. Kazemian and associates noted. Women were less likely than men to meet their treatment goals (see graph) over the course of the study, as were adults aged 18-44 years and black and Hispanic individuals.
“Recent advances in [treatments for diabetes] have not effectively reached the populations at risk and may indicate an immediate need for better approaches to the delivery of diabetes care, including a continued focus on reaching underserved populations with persistent disparities in care,” they wrote.
The study was supported by the Boston Area Diabetes Endocrinology Research Center and Massachusetts General Hospital. One investigator reported that her husband has equity in Apolo1bio. No other disclosures were reported.
SOURCE: Kazemian P et al. JAMA Intern Med. 2019 Aug 12. doi: 10.1001/jamainternmed.2019.2396.
Some things never change: In 2005, most adults with diabetes missed their treatment targets. In 2016, most adults with diabetes missed their treatment targets. And during that time, from 2005 to 2016, around 96% of men and 94% of women were linked to care.

“Fewer than one in four American adults with diagnosed diabetes achieve a controlled level of blood sugar, blood pressure, and cholesterol, and do not smoke tobacco. Our results suggest that, despite major advances in diabetes drug discovery and movement to develop innovative care delivery models over the past two decades, achievement of diabetes care targets has not improved in the United States since 2005,” Pooyan Kazemian, PhD, of Massachusetts General Hospital, Boston, said in a written statement.
During 2013-2016, only 23% of adults with diabetes met a combined composite target of glycemic (HbA1c below a liberal personalized level), blood pressure (less than 140/90 mm Hg), and cholesterol (LDL cholesterol level less than 100 mg/dL) control, as well as not smoking tobacco, Dr. Kazemian and associates reported in JAMA Internal Medicine. The corresponding figures were 25% (2009-2012) and 23% (2005-2008) for the two earlier time periods covered in the study,
The investigators used data for 1,742 nonpregnant adults from the National Health and Nutrition Examination Survey to evaluate the diabetes care cascade, which they defined as “diagnosis, linkage to care, achievement of individual treatment targets, and a composite of all individual targets.”
In 2013-2016, 94% of those diagnosed were linked to care, 64% met their HbA1c target, 70% achieved blood pressure control, 57% met the cholesterol target, and 85% were nonsmokers. When targets were combined, 41% achieved blood pressure and cholesterol control, and 25% met the glycemic, blood pressure, and cholesterol targets, they said.
“We found that none of the U.S. diabetes care variables improved from 2005 to 2016,” Dr. Kazemian and associates noted. Women were less likely than men to meet their treatment goals (see graph) over the course of the study, as were adults aged 18-44 years and black and Hispanic individuals.
“Recent advances in [treatments for diabetes] have not effectively reached the populations at risk and may indicate an immediate need for better approaches to the delivery of diabetes care, including a continued focus on reaching underserved populations with persistent disparities in care,” they wrote.
The study was supported by the Boston Area Diabetes Endocrinology Research Center and Massachusetts General Hospital. One investigator reported that her husband has equity in Apolo1bio. No other disclosures were reported.
SOURCE: Kazemian P et al. JAMA Intern Med. 2019 Aug 12. doi: 10.1001/jamainternmed.2019.2396.
FROM JAMA INTERNAL MEDICINE
FDA approves Baqsimi nasal powder for emergency hypoglycemia treatment
in patients aged 4 years and older.
Injectable glucagon has been approved in the United States for several decades.
The safety and efficacy of the Baqsimi powder was assessed in two studies with adults with diabetes and one with pediatric patients. In all three studies, a single dose of Baqsimi was compared with a single dose of glucagon injection, and Baqsimi adequately raised blood sugar levels in response to insulin-induced hypoglycemia.
The most common adverse events associated with Baqsimi include nausea, vomiting, headache, upper respiratory tract irritation, watery eyes, redness of eyes, and itchiness. The safety profile is similar to that of injectable glucagon, with the addition of nasal- and eye-related symptoms because of the method of delivery.
“There are many products on the market for those who need insulin, but until now, people suffering from a severe hypoglycemic episode had to be treated with a glucagon injection that first had to be mixed in a several-step process. This new way to administer glucagon may simplify the process, which can be critical during an episode, especially since the patient may have lost consciousness or may be having a seizure. In those situations, we want the process to treat the suffering person to be as simple as possible,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
in patients aged 4 years and older.
Injectable glucagon has been approved in the United States for several decades.
The safety and efficacy of the Baqsimi powder was assessed in two studies with adults with diabetes and one with pediatric patients. In all three studies, a single dose of Baqsimi was compared with a single dose of glucagon injection, and Baqsimi adequately raised blood sugar levels in response to insulin-induced hypoglycemia.
The most common adverse events associated with Baqsimi include nausea, vomiting, headache, upper respiratory tract irritation, watery eyes, redness of eyes, and itchiness. The safety profile is similar to that of injectable glucagon, with the addition of nasal- and eye-related symptoms because of the method of delivery.
“There are many products on the market for those who need insulin, but until now, people suffering from a severe hypoglycemic episode had to be treated with a glucagon injection that first had to be mixed in a several-step process. This new way to administer glucagon may simplify the process, which can be critical during an episode, especially since the patient may have lost consciousness or may be having a seizure. In those situations, we want the process to treat the suffering person to be as simple as possible,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
in patients aged 4 years and older.
Injectable glucagon has been approved in the United States for several decades.
The safety and efficacy of the Baqsimi powder was assessed in two studies with adults with diabetes and one with pediatric patients. In all three studies, a single dose of Baqsimi was compared with a single dose of glucagon injection, and Baqsimi adequately raised blood sugar levels in response to insulin-induced hypoglycemia.
The most common adverse events associated with Baqsimi include nausea, vomiting, headache, upper respiratory tract irritation, watery eyes, redness of eyes, and itchiness. The safety profile is similar to that of injectable glucagon, with the addition of nasal- and eye-related symptoms because of the method of delivery.
“There are many products on the market for those who need insulin, but until now, people suffering from a severe hypoglycemic episode had to be treated with a glucagon injection that first had to be mixed in a several-step process. This new way to administer glucagon may simplify the process, which can be critical during an episode, especially since the patient may have lost consciousness or may be having a seizure. In those situations, we want the process to treat the suffering person to be as simple as possible,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
Inadequate glycemic control in type 1 diabetes leads to increased fracture risk
A single percentage increase in the level of hemoglobin A1c (HbA1c) in patients with newly diagnosed type 1 diabetes is significantly associated with an increase in fracture risk, according to findings in a study published in Diabetic Medicine.
To determine the effect of glycemic control on fracture risk, Rasiah Thayakaran, PhD, of the University of Birmingham (England) and colleagues analyzed data from 5,368 patients with newly diagnosed type 1 diabetes in the United Kingdom. HbA1c measurements were collected until either fracture or the end of the study, and were then converted from percentages to mmol/mol. Patient age ranged between 1 and 60 years, and the mean age was 22 years.
During 37,830 person‐years of follow‐up, 525 fractures were observed, with an incidence rate of 14 per 1,000 person‐years. The rate among men was 15 per 1,000 person‐years, compared with 12 per 1,000 person‐years among women. There was a significant association between hemoglobin level and risk of fractures (adjusted hazard ratio, 1.007 mmol/mol; 95% confidence interval, 1.002-1.011 mmol/mol), representing an increase of 7% in risk for fracture for each percentage increase in hemoglobin level.
“When assessing an individual with newly diagnosed type 1 diabetes and high HbA1c, increased clinical awareness about the fracture risk may be incorporated in decision‐making regarding the clinical management and even in prompting early antiosteoporotic intervention,” Dr. Thayakaran and coauthors wrote.
The researchers acknowledged the study’s limitations, including a possibility of residual confounding because of their use of observational data. In addition, they could not confirm whether the increase in fracture risk should be attributed to bone fragility or to increased risk of falls. Finally, though they noted using a comprehensive list of codes to identify fractures, they could not verify “completeness of recording ... and therefore reported overall fracture incidence should be interpreted with caution.”
The study was not funded. The authors reported no conflicts of interest.
SOURCE: Thayakaran R et al. Diab Med. 2019 Mar 8. doi: 10.1111/dme.13945.
A single percentage increase in the level of hemoglobin A1c (HbA1c) in patients with newly diagnosed type 1 diabetes is significantly associated with an increase in fracture risk, according to findings in a study published in Diabetic Medicine.
To determine the effect of glycemic control on fracture risk, Rasiah Thayakaran, PhD, of the University of Birmingham (England) and colleagues analyzed data from 5,368 patients with newly diagnosed type 1 diabetes in the United Kingdom. HbA1c measurements were collected until either fracture or the end of the study, and were then converted from percentages to mmol/mol. Patient age ranged between 1 and 60 years, and the mean age was 22 years.
During 37,830 person‐years of follow‐up, 525 fractures were observed, with an incidence rate of 14 per 1,000 person‐years. The rate among men was 15 per 1,000 person‐years, compared with 12 per 1,000 person‐years among women. There was a significant association between hemoglobin level and risk of fractures (adjusted hazard ratio, 1.007 mmol/mol; 95% confidence interval, 1.002-1.011 mmol/mol), representing an increase of 7% in risk for fracture for each percentage increase in hemoglobin level.
“When assessing an individual with newly diagnosed type 1 diabetes and high HbA1c, increased clinical awareness about the fracture risk may be incorporated in decision‐making regarding the clinical management and even in prompting early antiosteoporotic intervention,” Dr. Thayakaran and coauthors wrote.
The researchers acknowledged the study’s limitations, including a possibility of residual confounding because of their use of observational data. In addition, they could not confirm whether the increase in fracture risk should be attributed to bone fragility or to increased risk of falls. Finally, though they noted using a comprehensive list of codes to identify fractures, they could not verify “completeness of recording ... and therefore reported overall fracture incidence should be interpreted with caution.”
The study was not funded. The authors reported no conflicts of interest.
SOURCE: Thayakaran R et al. Diab Med. 2019 Mar 8. doi: 10.1111/dme.13945.
A single percentage increase in the level of hemoglobin A1c (HbA1c) in patients with newly diagnosed type 1 diabetes is significantly associated with an increase in fracture risk, according to findings in a study published in Diabetic Medicine.
To determine the effect of glycemic control on fracture risk, Rasiah Thayakaran, PhD, of the University of Birmingham (England) and colleagues analyzed data from 5,368 patients with newly diagnosed type 1 diabetes in the United Kingdom. HbA1c measurements were collected until either fracture or the end of the study, and were then converted from percentages to mmol/mol. Patient age ranged between 1 and 60 years, and the mean age was 22 years.
During 37,830 person‐years of follow‐up, 525 fractures were observed, with an incidence rate of 14 per 1,000 person‐years. The rate among men was 15 per 1,000 person‐years, compared with 12 per 1,000 person‐years among women. There was a significant association between hemoglobin level and risk of fractures (adjusted hazard ratio, 1.007 mmol/mol; 95% confidence interval, 1.002-1.011 mmol/mol), representing an increase of 7% in risk for fracture for each percentage increase in hemoglobin level.
“When assessing an individual with newly diagnosed type 1 diabetes and high HbA1c, increased clinical awareness about the fracture risk may be incorporated in decision‐making regarding the clinical management and even in prompting early antiosteoporotic intervention,” Dr. Thayakaran and coauthors wrote.
The researchers acknowledged the study’s limitations, including a possibility of residual confounding because of their use of observational data. In addition, they could not confirm whether the increase in fracture risk should be attributed to bone fragility or to increased risk of falls. Finally, though they noted using a comprehensive list of codes to identify fractures, they could not verify “completeness of recording ... and therefore reported overall fracture incidence should be interpreted with caution.”
The study was not funded. The authors reported no conflicts of interest.
SOURCE: Thayakaran R et al. Diab Med. 2019 Mar 8. doi: 10.1111/dme.13945.
FROM DIABETIC MEDICINE
The costs and benefits of SGLT2 inhibitors & GLP-1 RAs
The options for treating type 2 diabetes without insulin have grown beyond metformin to include a long list of sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists that can be taken with or without metformin. These new drugs have cardiovascular and kidney benefits and help with weight loss, but they also carry risks and, according to some experts, their costs can be prohibitively expensive.
Given the medical community’s long-term experience with treating patients with metformin, and metformin’s lower cost, most of the physicians interviewed for this article advise using SGLT2 inhibitors and GLP-1 receptor agonists as second-line treatments. Others said that they would prefer to use the newer drugs as first-line therapies in select high-risk patients, but prior authorization hurdles created by insurance companies make that approach too burdensome.
“The economics of U.S. health care is stacked against many of our patients with diabetes in the current era,” Robert H. Hopkins Jr., MD, said in an interview.
Even when their insurance approves the drugs, patients still may not be able to afford the copay, explained Dr. Hopkins, professor of internal medicine and pediatrics and director of the division of general internal medicine at the University of Arkansas for Medical Sciences, Little Rock. “Sometimes patients can purchase drugs at a lower cost than the copay to purchase with the ‘drug coverage’ in their insurance plan – unfortunately, this is not the case with the newer diabetes medications we are discussing here.”
“SGLT2 inhibitors and GLP-1 agonists can cost several hundred dollars a month, and insurers often balk at paying for them. They’ll say, ‘Have you tried metformin?’ ” explained endocrinologist Victor Lawrence Roberts, MD, in a interview. “We have to work with insurance companies the best we can in a stepwise fashion.”
According to Dr. Roberts, 80% of his patients with diabetes struggle with the cost of medicine in general. “They’re either underinsured or not insured or their formulary is limited.
Douglas S. Paauw, MD, agreed in an interview that the newer drugs can be problematic on the insurance front.
“For some patients they aren’t affordable, especially for the uninsured if you can’t get them on an assistance program,” said Dr. Paauw, who is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the university.
Dr. Hopkins, who is on the Internal Medicine News board, noted that “unfortunately, the treatment of type 2 diabetes in patients who cannot achieve control with metformin, diet, weight control, and exercise is a story of the ‘haves’ and the ‘have nots.’ The ‘haves’ are those who have pharmacy benefits which make access to newer agents like SGLT2 inhibitors and GLP-1 agonists a possibility.”
“I have had very few of the ‘have nots’ who have been able to even consider these newer agents, which carry price tags of $600-$1,300 a month even with the availability of discounting coupons in the marketplace,” he added. “Most of these patients end up requiring a sulfonylurea or TZD [thiazolidinedione] as a second agent to achieve glycemic control. This makes it very difficult to achieve sufficient weight and metabolic control to avoid an eventual switch to insulin.”
Fatima Z. Syed, MD, an endocrine-trained general internist at DukeHealth in Durham, N.C., said she prescribes SGLT2 inhibitors and GLP-1 receptor agonists in combination with metformin. “I prescribe them frequently, but they are not first-line treatments,” she explained.
“Nothing replaces diet and exercise” as therapy for patients with type 2 diabetes, she added.
Neil S. Skolnik, MD, said that insurance companies were not preventing patients from using these drugs in his experience. He also provided an optimistic take on the accessibility of these drugs in the near future.
“Most insurance companies are now covering select SGLT2 inhibitors and GLP-1 receptor agonists for appropriate patients and those companies that currently do not will soon have to,” said Dr. Skolnik, who is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
“The outcomes [associated with use of the new drugs] are robust, the benefits are large, and are well worth the cost,” he added.
The side effects
While others praised these drugs for their beneficial effects, they also noted that the side effects of these drugs are serious and must be discussed with patients.
GLP-1 receptor agonists are linked to gastrointestinal symptoms, especially nausea, while SGLT2 inhibitors have been linked to kidney failure, ketoacidosis, and more. The Food and Drug Administration warned in 2018 that the SGLT2 inhibitors can cause a rare serious infection known as Fournier’s gangrene – necrotizing fasciitis of the perineum.
“We have to tell our patients to let us know right away if they get pain or swelling in the genital area,” Dr. Paauw, who is on the Internal Medicine News board, noted. “The chance that an infection could explode quickly is higher in those who take these drugs.”
Amputation risks also are associated with taking the SGLT2 inhibitor canagliflozin (Invokana). The FDA requires the manufacturer of this drug to include a black-box warning about the risk of “lower-limb amputations, most frequently of the toe and midfoot,” but also the leg. In approval trials, the risk doubled versus placebo.
These amputation risks “put a damper on some of the enthusiasm on behalf of physicians and patients ... for taking this drug,” noted Dr. Roberts, who is a professor of internal medicine at the University of Central Florida, Orlando.
While a manufacturer-funded study released last year found no link to amputations, the results weren’t powerful enough to rule out a moderately increased risk.
“[If] you are at high risk for having an amputation, we really have to take this risk very seriously,” said John B. Buse, MD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill, in a presentation about the study at the 2018 annual scientific sessions of the American Diabetes Association.
The benefits
Despite these risks of adverse events, most interviewed agreed that the many benefits observed in those taking SGLT2 inhibitors or GLP-1 receptor agonists make them worth prescribing, at least to those who are able to afford them.
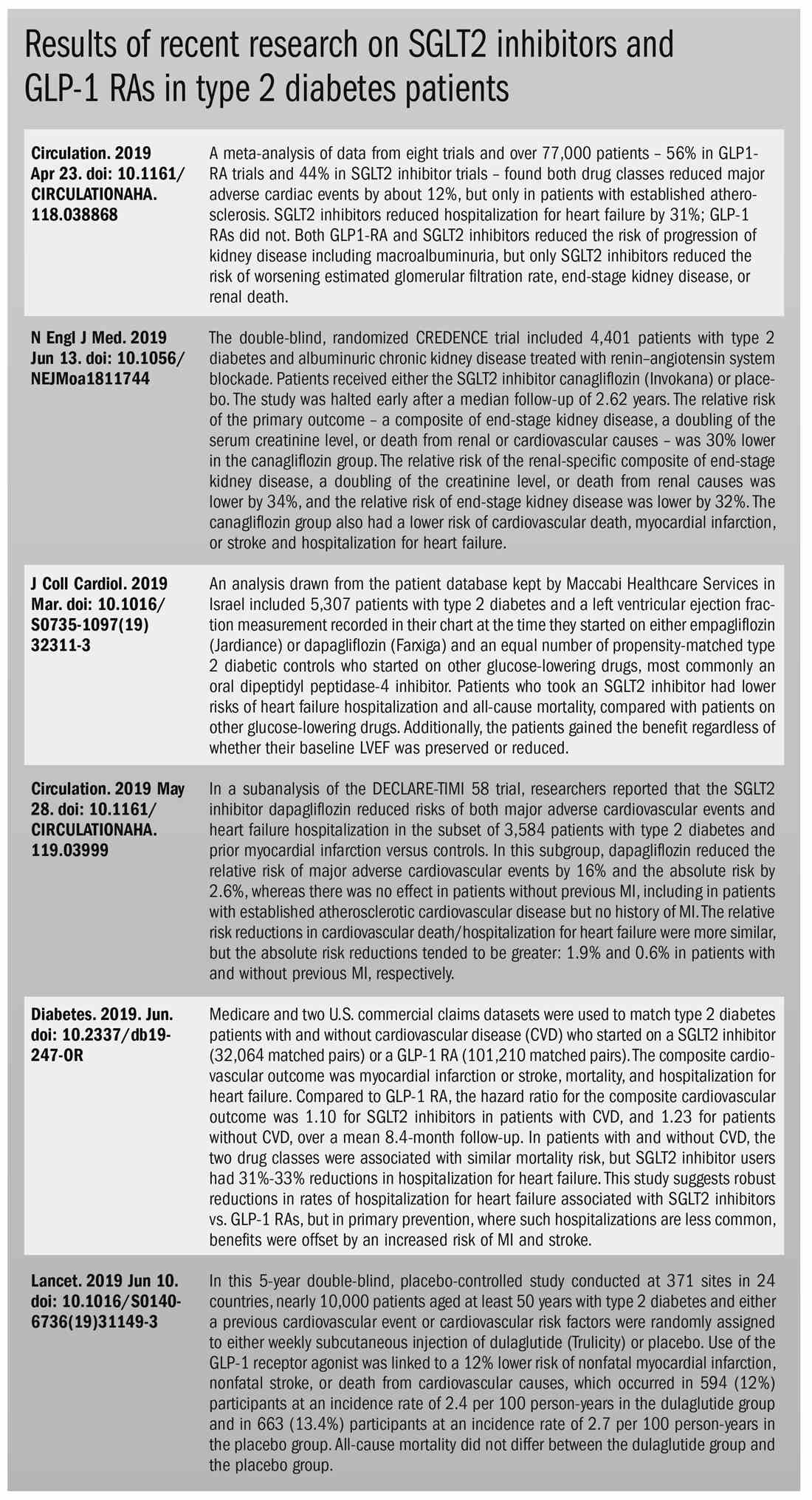
Both SGLT2 inhibitors and GLP-1 receptor agonists appear to have significant cardiovascular benefits. A 2019 meta-analysis and systematic review found that both drugs reduced major adverse cardiac events by about 12% (Circulation. 2019 Apr 23;139[17]:2022-31).
“They don’t cause hypoglycemia, they lower blood pressure, they don’t cause weight gain, and they might promote weight loss,” noted Dr. Paauw.
SGLT2 inhibitors also have shown signs of kidney benefits. The CREDENCE trial linked canagliflozin to a lowering of kidney disorders versus placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). “The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34% (hazard ratio, 0.66; 95% confidence interval, 0.53-0.81; P less than .001), and the relative risk of end-stage kidney disease was lower by 32% (HR, 0.68; 95% CI, 0.54-0.86; P = .002),” the trial investigators wrote.
“They showed very nicely that the drug improved the kidney function of those patients and reduced the kidney deterioration,” said Yehuda Handelsman, MD, an endocrinologist in Tarzana, Calif., who chaired the 2011 and 2015 American Association of Clinical Endocrinologists’ Comprehensive Diabetes Guidelines. The study was especially impressive, he added, because it included patients with low kidney function.
SGLT2 inhibitors’ “diuretic mechanism explains why there is a substantial reduction in heart failure hospitalizations in patients who take these drugs,” said cardiologist Marc E. Goldschmidt, MD, director of the Heart Success Program at Atlantic Health System’s Morristown (N.J.) Medical Center, in an interview. “Both the EMPA-REG Outcome and the CREDENCE trials demonstrated substantial benefit of this class of medications by showing a lower risk of cardiovascular death as well as death from any cause and a lower risk of hospitalization for heart failure."
Overall, the SGLT2 trial data have been very consistent with a benefit for cardiovascular risk reduction, particularly in regard to heart failure hospitalizations and even in potentially preventing heart failure in diabetics,” he added.
Dr. Skolnik, a columnist for Family Practice News, cited SGLT2 inhibitors and GLP-1 receptor agonists’ ability to slow renal disease progression, promote weight loss, and prevent poor cardiac outcomes.“These drugs should be used, in addition to metformin, in all patients with diabetes and vascular disease. These proven outcomes are far better than we ever were able to achieve previously and the strength of the evidence at this point is very strong,” said Dr. Skolnik. “In addition to the benefits of decreasing the development of cardiovascular disease, serious heart failure, and slowing progression of renal disease, these two classes of medication have additional benefits. Both classes help patients lose weight, which is very different from what was found with either sulfonylureas or insulin, which cause patients to gain weight. Also both the SGLT2 inhibitors and the GLP-1 RAs [receptor agonists] have a low incidence of hypoglycemia. For all these reasons, these have become important medications for us to use in primary care.”
Other recent trials offer “very powerful data” about SGLT2 inhibitors, Dr. Roberts said. That’s good news, since “our approach needs to be toward cardiovascular protection and preservation as well as managing blood sugar.”An Israeli trial, whose results were released in May 2019 at the annual meeting of the American College of Cardiology, found that, compared with other glucose-lowering drugs, taking an SGLT2 inhibitor was associated with lower risks of heart failure hospitalization and all-cause mortality (HR, 0.54; 95% CI, 0.44-0.65; P less than .001). This trial also offered a new detail: The patients gained the benefit regardless of whether their baseline left ventricular ejection fraction was preserved or reduced (J Coll Cardiol. 2019 Mar;73[9]:suppl 1). The SGLT2 inhibitors used in this trial included dapagliflozin (Farxiga) and empagliflozin (Jardiance).
In another study released this year, a subanalysis of the DECLARE-TIMI 58 trial, researchers reported that the SGLT2 inhibitor dapagliflozin reduced risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes and prior myocardial infarction versus controls (Circulation. 2019 May 28;139[22]:2516-27). The absolute risk reduction for major adverse cardiovascular events was 1.9% (HR, 0.81; 95% CI, 0.65-1.00; P = .046), while it was 0.6% for heart failure hospitalization (HR, 0.85; 95% CI, 0.72-1.00; P = .055).
These and other studies “speak volumes about the efficacy of managing blood sugar and addressing our biggest nemesis, which is cardiovascular disease,” Dr. Roberts said. “It’s irrefutable. The data [are] very good.”
Dr. Paauw said an SGLT2 inhibitor or GLP-1 receptor agonist is best reserved for use in select patients with cardiovascular risks and type 2 diabetes that need management beyond metformin.
For example, they might fit a 70-year-old with persistent hypertension who’s already taking a couple of blood pressure medications. “If they have another cardiovascular risk factor, the cardiovascular protection piece will be a bigger deal,” he said. Also, “it will probably help lower their blood pressure so they can avoid taking another blood pressure medicine.”
Trials of both GLP-1 receptor agonists and SGLT2 inhibitors have shown benefits “in improving [major adverse cardiac events], with the SGLT2 class showing substantial benefit in improving both heart failure and renal outcomes as well,” noted Dr. Skolnik. “It is in this context that one must address the question of whether the price of the medications are worthwhile. With such substantial benefit, there is no question in my mind that – for patients who have underlying cardiovascular illness, which includes patients with existent coronary disease, history of stroke, transient ischemic attack, or peripheral vascular disease – it is far and away worth it to prescribe these classes of medications.”
Indeed, the American Diabetes Association and the European Association for the Study of Diabetes’ most recent guidelines now call for a GLP-1 receptor agonist – instead of insulin – to be the first injectable used to treat type 2 diabetes (Diabetes Care 2018 Dec; 41[12]:2669-701).
“For the relatively small number of my patients who have been able to access and use these medications for months or longer, more have tolerated the GLP-1 agonists than SGLT2 inhibitors primarily due to urinary issues,” noted Dr. Hopkins.
Dipeptidyl peptidase–4 inhibitors are another option in patients with type 2 diabetes, but research suggests they may not be a top option for patients with cardiovascular risk. A 2018 review noted that cardiovascular outcome trials for alogliptin (Nesina), saxagliptin (Onglyza), and sitagliptin (Januvia) showed noninferiority but failed to demonstrate any superiority, compared with placebo in patients with type 2 diabetes mellitus and high cardiovascular risk (Circ Res. 2018 May 11;122[10]:1439-59).
The combination therapies
Many of the newer drugs are available as combinations with other types of diabetes drugs. In some cases, physicians create their own form of combination therapy by separately prescribing two or more diabetes drugs. Earlier this year, a study suggested the benefits of this kind of add-on therapy: Diabetes outcomes improved in patients who took the GLP-1 receptor agonist semaglutide and an SGLT2 inhibitor (Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587[19]30066-X).
Dr. Roberts suggested caution, however, when prescribing combination therapies. “My recommendation is always to begin with the individual medications to see if the patient tolerates the drugs and then decide which component needs to be titrated. It’s hard to titrate a combination drug, and it doesn’t leave a lot of flexibility. You never know which drug is doing what.
Dr. Handelsman said some patients may need to take three medications such as metformin, an SGLT2 inhibitor, and a GLP-1 receptor agonist.
“I don’t recommend using the combinations if you’re not familiar with the drugs ... These are relatively new pharmaceuticals, and most of us are on a learning curve as to how they fit into the armamentarium. If a drug is tolerated with a good response, you can certainly consider going to the combination tablets,” he added.
There is at least one drug that combines these three classes: The newly FDA-approved Qternmet XR, which combines dapagliflozin (an SGLT2 inhibitor), saxagliptin (a GLP-1 receptor agonist), and metformin. As of mid-June 2019, it was not yet available in the United States. Its sister drug Qtern, which combines dapagliflozin and saxagliptin, costs more than $500 a month with a free coupon, according to goodrx.com. In contrast, metformin is extremely inexpensive, costing just a few dollars a month for a common starting dose.
What about adding insulin?
“Both [SGLT2 inhibitors and GLP-1 receptor agonists] work very well with insulin,” Dr. Handelsman said. “There is a nice additive effect on the reduction of [hemoglobin] A1c. The only caution is that, although neither SGLT2 inhibitors nor GLP-1 receptor agonists cause hypoglycemia, in combination with insulin they do increase the risk of hypoglycemia. You may have to adjust the dose of insulin.”
Dr. Hopkins warned that cost becomes an even bigger issue when you add insulin into the mix.
“When insulin comes into the discussion, we are again stuck with astronomical costs which many struggle to afford,” he explained.
Indeed, the price tag on these drugs seems to be the biggest problem physicians have with them.
“The challenges in managing patients with diabetes aren’t the risks associated with the drugs. It’s dealing with their insurers,” noted Dr. Roberts.
Dr. Hopkins, Dr. Paauw, Dr. Roberts, and Dr. Syed reported no disclosures. Dr. Buse is an investigator for Johnson and Johnson. Dr. Goldschmidt is paid to speak by Novartis. Dr. Handelsman reported research grants, consulting work, and speaker honoraria from Amgen, Gilead, Lilly, Merck, Novo Nordisk, and others. Dr Skolnik reported nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi, and GlaxoSmithKline and personal fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly. He also serves on the advisory boards of AstraZeneca, Boehringer Ingelheim, Teva Pharmaceutical, Eli Lilly, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline.
Dr. Paauw and Dr. Skolnik are columnists for Family Practice News and Internal Medicine News.
M. Alexander Otto contributed to this report.
The options for treating type 2 diabetes without insulin have grown beyond metformin to include a long list of sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists that can be taken with or without metformin. These new drugs have cardiovascular and kidney benefits and help with weight loss, but they also carry risks and, according to some experts, their costs can be prohibitively expensive.
Given the medical community’s long-term experience with treating patients with metformin, and metformin’s lower cost, most of the physicians interviewed for this article advise using SGLT2 inhibitors and GLP-1 receptor agonists as second-line treatments. Others said that they would prefer to use the newer drugs as first-line therapies in select high-risk patients, but prior authorization hurdles created by insurance companies make that approach too burdensome.
“The economics of U.S. health care is stacked against many of our patients with diabetes in the current era,” Robert H. Hopkins Jr., MD, said in an interview.
Even when their insurance approves the drugs, patients still may not be able to afford the copay, explained Dr. Hopkins, professor of internal medicine and pediatrics and director of the division of general internal medicine at the University of Arkansas for Medical Sciences, Little Rock. “Sometimes patients can purchase drugs at a lower cost than the copay to purchase with the ‘drug coverage’ in their insurance plan – unfortunately, this is not the case with the newer diabetes medications we are discussing here.”
“SGLT2 inhibitors and GLP-1 agonists can cost several hundred dollars a month, and insurers often balk at paying for them. They’ll say, ‘Have you tried metformin?’ ” explained endocrinologist Victor Lawrence Roberts, MD, in a interview. “We have to work with insurance companies the best we can in a stepwise fashion.”
According to Dr. Roberts, 80% of his patients with diabetes struggle with the cost of medicine in general. “They’re either underinsured or not insured or their formulary is limited.
Douglas S. Paauw, MD, agreed in an interview that the newer drugs can be problematic on the insurance front.
“For some patients they aren’t affordable, especially for the uninsured if you can’t get them on an assistance program,” said Dr. Paauw, who is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the university.
Dr. Hopkins, who is on the Internal Medicine News board, noted that “unfortunately, the treatment of type 2 diabetes in patients who cannot achieve control with metformin, diet, weight control, and exercise is a story of the ‘haves’ and the ‘have nots.’ The ‘haves’ are those who have pharmacy benefits which make access to newer agents like SGLT2 inhibitors and GLP-1 agonists a possibility.”
“I have had very few of the ‘have nots’ who have been able to even consider these newer agents, which carry price tags of $600-$1,300 a month even with the availability of discounting coupons in the marketplace,” he added. “Most of these patients end up requiring a sulfonylurea or TZD [thiazolidinedione] as a second agent to achieve glycemic control. This makes it very difficult to achieve sufficient weight and metabolic control to avoid an eventual switch to insulin.”
Fatima Z. Syed, MD, an endocrine-trained general internist at DukeHealth in Durham, N.C., said she prescribes SGLT2 inhibitors and GLP-1 receptor agonists in combination with metformin. “I prescribe them frequently, but they are not first-line treatments,” she explained.
“Nothing replaces diet and exercise” as therapy for patients with type 2 diabetes, she added.
Neil S. Skolnik, MD, said that insurance companies were not preventing patients from using these drugs in his experience. He also provided an optimistic take on the accessibility of these drugs in the near future.
“Most insurance companies are now covering select SGLT2 inhibitors and GLP-1 receptor agonists for appropriate patients and those companies that currently do not will soon have to,” said Dr. Skolnik, who is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
“The outcomes [associated with use of the new drugs] are robust, the benefits are large, and are well worth the cost,” he added.
The side effects
While others praised these drugs for their beneficial effects, they also noted that the side effects of these drugs are serious and must be discussed with patients.
GLP-1 receptor agonists are linked to gastrointestinal symptoms, especially nausea, while SGLT2 inhibitors have been linked to kidney failure, ketoacidosis, and more. The Food and Drug Administration warned in 2018 that the SGLT2 inhibitors can cause a rare serious infection known as Fournier’s gangrene – necrotizing fasciitis of the perineum.
“We have to tell our patients to let us know right away if they get pain or swelling in the genital area,” Dr. Paauw, who is on the Internal Medicine News board, noted. “The chance that an infection could explode quickly is higher in those who take these drugs.”
Amputation risks also are associated with taking the SGLT2 inhibitor canagliflozin (Invokana). The FDA requires the manufacturer of this drug to include a black-box warning about the risk of “lower-limb amputations, most frequently of the toe and midfoot,” but also the leg. In approval trials, the risk doubled versus placebo.
These amputation risks “put a damper on some of the enthusiasm on behalf of physicians and patients ... for taking this drug,” noted Dr. Roberts, who is a professor of internal medicine at the University of Central Florida, Orlando.
While a manufacturer-funded study released last year found no link to amputations, the results weren’t powerful enough to rule out a moderately increased risk.
“[If] you are at high risk for having an amputation, we really have to take this risk very seriously,” said John B. Buse, MD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill, in a presentation about the study at the 2018 annual scientific sessions of the American Diabetes Association.
The benefits
Despite these risks of adverse events, most interviewed agreed that the many benefits observed in those taking SGLT2 inhibitors or GLP-1 receptor agonists make them worth prescribing, at least to those who are able to afford them.
Both SGLT2 inhibitors and GLP-1 receptor agonists appear to have significant cardiovascular benefits. A 2019 meta-analysis and systematic review found that both drugs reduced major adverse cardiac events by about 12% (Circulation. 2019 Apr 23;139[17]:2022-31).
“They don’t cause hypoglycemia, they lower blood pressure, they don’t cause weight gain, and they might promote weight loss,” noted Dr. Paauw.
SGLT2 inhibitors also have shown signs of kidney benefits. The CREDENCE trial linked canagliflozin to a lowering of kidney disorders versus placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). “The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34% (hazard ratio, 0.66; 95% confidence interval, 0.53-0.81; P less than .001), and the relative risk of end-stage kidney disease was lower by 32% (HR, 0.68; 95% CI, 0.54-0.86; P = .002),” the trial investigators wrote.
“They showed very nicely that the drug improved the kidney function of those patients and reduced the kidney deterioration,” said Yehuda Handelsman, MD, an endocrinologist in Tarzana, Calif., who chaired the 2011 and 2015 American Association of Clinical Endocrinologists’ Comprehensive Diabetes Guidelines. The study was especially impressive, he added, because it included patients with low kidney function.
SGLT2 inhibitors’ “diuretic mechanism explains why there is a substantial reduction in heart failure hospitalizations in patients who take these drugs,” said cardiologist Marc E. Goldschmidt, MD, director of the Heart Success Program at Atlantic Health System’s Morristown (N.J.) Medical Center, in an interview. “Both the EMPA-REG Outcome and the CREDENCE trials demonstrated substantial benefit of this class of medications by showing a lower risk of cardiovascular death as well as death from any cause and a lower risk of hospitalization for heart failure."
Overall, the SGLT2 trial data have been very consistent with a benefit for cardiovascular risk reduction, particularly in regard to heart failure hospitalizations and even in potentially preventing heart failure in diabetics,” he added.
Dr. Skolnik, a columnist for Family Practice News, cited SGLT2 inhibitors and GLP-1 receptor agonists’ ability to slow renal disease progression, promote weight loss, and prevent poor cardiac outcomes.“These drugs should be used, in addition to metformin, in all patients with diabetes and vascular disease. These proven outcomes are far better than we ever were able to achieve previously and the strength of the evidence at this point is very strong,” said Dr. Skolnik. “In addition to the benefits of decreasing the development of cardiovascular disease, serious heart failure, and slowing progression of renal disease, these two classes of medication have additional benefits. Both classes help patients lose weight, which is very different from what was found with either sulfonylureas or insulin, which cause patients to gain weight. Also both the SGLT2 inhibitors and the GLP-1 RAs [receptor agonists] have a low incidence of hypoglycemia. For all these reasons, these have become important medications for us to use in primary care.”
Other recent trials offer “very powerful data” about SGLT2 inhibitors, Dr. Roberts said. That’s good news, since “our approach needs to be toward cardiovascular protection and preservation as well as managing blood sugar.”An Israeli trial, whose results were released in May 2019 at the annual meeting of the American College of Cardiology, found that, compared with other glucose-lowering drugs, taking an SGLT2 inhibitor was associated with lower risks of heart failure hospitalization and all-cause mortality (HR, 0.54; 95% CI, 0.44-0.65; P less than .001). This trial also offered a new detail: The patients gained the benefit regardless of whether their baseline left ventricular ejection fraction was preserved or reduced (J Coll Cardiol. 2019 Mar;73[9]:suppl 1). The SGLT2 inhibitors used in this trial included dapagliflozin (Farxiga) and empagliflozin (Jardiance).
In another study released this year, a subanalysis of the DECLARE-TIMI 58 trial, researchers reported that the SGLT2 inhibitor dapagliflozin reduced risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes and prior myocardial infarction versus controls (Circulation. 2019 May 28;139[22]:2516-27). The absolute risk reduction for major adverse cardiovascular events was 1.9% (HR, 0.81; 95% CI, 0.65-1.00; P = .046), while it was 0.6% for heart failure hospitalization (HR, 0.85; 95% CI, 0.72-1.00; P = .055).
These and other studies “speak volumes about the efficacy of managing blood sugar and addressing our biggest nemesis, which is cardiovascular disease,” Dr. Roberts said. “It’s irrefutable. The data [are] very good.”
Dr. Paauw said an SGLT2 inhibitor or GLP-1 receptor agonist is best reserved for use in select patients with cardiovascular risks and type 2 diabetes that need management beyond metformin.
For example, they might fit a 70-year-old with persistent hypertension who’s already taking a couple of blood pressure medications. “If they have another cardiovascular risk factor, the cardiovascular protection piece will be a bigger deal,” he said. Also, “it will probably help lower their blood pressure so they can avoid taking another blood pressure medicine.”
Trials of both GLP-1 receptor agonists and SGLT2 inhibitors have shown benefits “in improving [major adverse cardiac events], with the SGLT2 class showing substantial benefit in improving both heart failure and renal outcomes as well,” noted Dr. Skolnik. “It is in this context that one must address the question of whether the price of the medications are worthwhile. With such substantial benefit, there is no question in my mind that – for patients who have underlying cardiovascular illness, which includes patients with existent coronary disease, history of stroke, transient ischemic attack, or peripheral vascular disease – it is far and away worth it to prescribe these classes of medications.”
Indeed, the American Diabetes Association and the European Association for the Study of Diabetes’ most recent guidelines now call for a GLP-1 receptor agonist – instead of insulin – to be the first injectable used to treat type 2 diabetes (Diabetes Care 2018 Dec; 41[12]:2669-701).
“For the relatively small number of my patients who have been able to access and use these medications for months or longer, more have tolerated the GLP-1 agonists than SGLT2 inhibitors primarily due to urinary issues,” noted Dr. Hopkins.
Dipeptidyl peptidase–4 inhibitors are another option in patients with type 2 diabetes, but research suggests they may not be a top option for patients with cardiovascular risk. A 2018 review noted that cardiovascular outcome trials for alogliptin (Nesina), saxagliptin (Onglyza), and sitagliptin (Januvia) showed noninferiority but failed to demonstrate any superiority, compared with placebo in patients with type 2 diabetes mellitus and high cardiovascular risk (Circ Res. 2018 May 11;122[10]:1439-59).
The combination therapies
Many of the newer drugs are available as combinations with other types of diabetes drugs. In some cases, physicians create their own form of combination therapy by separately prescribing two or more diabetes drugs. Earlier this year, a study suggested the benefits of this kind of add-on therapy: Diabetes outcomes improved in patients who took the GLP-1 receptor agonist semaglutide and an SGLT2 inhibitor (Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587[19]30066-X).
Dr. Roberts suggested caution, however, when prescribing combination therapies. “My recommendation is always to begin with the individual medications to see if the patient tolerates the drugs and then decide which component needs to be titrated. It’s hard to titrate a combination drug, and it doesn’t leave a lot of flexibility. You never know which drug is doing what.
Dr. Handelsman said some patients may need to take three medications such as metformin, an SGLT2 inhibitor, and a GLP-1 receptor agonist.
“I don’t recommend using the combinations if you’re not familiar with the drugs ... These are relatively new pharmaceuticals, and most of us are on a learning curve as to how they fit into the armamentarium. If a drug is tolerated with a good response, you can certainly consider going to the combination tablets,” he added.
There is at least one drug that combines these three classes: The newly FDA-approved Qternmet XR, which combines dapagliflozin (an SGLT2 inhibitor), saxagliptin (a GLP-1 receptor agonist), and metformin. As of mid-June 2019, it was not yet available in the United States. Its sister drug Qtern, which combines dapagliflozin and saxagliptin, costs more than $500 a month with a free coupon, according to goodrx.com. In contrast, metformin is extremely inexpensive, costing just a few dollars a month for a common starting dose.
What about adding insulin?
“Both [SGLT2 inhibitors and GLP-1 receptor agonists] work very well with insulin,” Dr. Handelsman said. “There is a nice additive effect on the reduction of [hemoglobin] A1c. The only caution is that, although neither SGLT2 inhibitors nor GLP-1 receptor agonists cause hypoglycemia, in combination with insulin they do increase the risk of hypoglycemia. You may have to adjust the dose of insulin.”
Dr. Hopkins warned that cost becomes an even bigger issue when you add insulin into the mix.
“When insulin comes into the discussion, we are again stuck with astronomical costs which many struggle to afford,” he explained.
Indeed, the price tag on these drugs seems to be the biggest problem physicians have with them.
“The challenges in managing patients with diabetes aren’t the risks associated with the drugs. It’s dealing with their insurers,” noted Dr. Roberts.
Dr. Hopkins, Dr. Paauw, Dr. Roberts, and Dr. Syed reported no disclosures. Dr. Buse is an investigator for Johnson and Johnson. Dr. Goldschmidt is paid to speak by Novartis. Dr. Handelsman reported research grants, consulting work, and speaker honoraria from Amgen, Gilead, Lilly, Merck, Novo Nordisk, and others. Dr Skolnik reported nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi, and GlaxoSmithKline and personal fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly. He also serves on the advisory boards of AstraZeneca, Boehringer Ingelheim, Teva Pharmaceutical, Eli Lilly, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline.
Dr. Paauw and Dr. Skolnik are columnists for Family Practice News and Internal Medicine News.
M. Alexander Otto contributed to this report.

The options for treating type 2 diabetes without insulin have grown beyond metformin to include a long list of sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists that can be taken with or without metformin. These new drugs have cardiovascular and kidney benefits and help with weight loss, but they also carry risks and, according to some experts, their costs can be prohibitively expensive.
Given the medical community’s long-term experience with treating patients with metformin, and metformin’s lower cost, most of the physicians interviewed for this article advise using SGLT2 inhibitors and GLP-1 receptor agonists as second-line treatments. Others said that they would prefer to use the newer drugs as first-line therapies in select high-risk patients, but prior authorization hurdles created by insurance companies make that approach too burdensome.
“The economics of U.S. health care is stacked against many of our patients with diabetes in the current era,” Robert H. Hopkins Jr., MD, said in an interview.
Even when their insurance approves the drugs, patients still may not be able to afford the copay, explained Dr. Hopkins, professor of internal medicine and pediatrics and director of the division of general internal medicine at the University of Arkansas for Medical Sciences, Little Rock. “Sometimes patients can purchase drugs at a lower cost than the copay to purchase with the ‘drug coverage’ in their insurance plan – unfortunately, this is not the case with the newer diabetes medications we are discussing here.”
“SGLT2 inhibitors and GLP-1 agonists can cost several hundred dollars a month, and insurers often balk at paying for them. They’ll say, ‘Have you tried metformin?’ ” explained endocrinologist Victor Lawrence Roberts, MD, in a interview. “We have to work with insurance companies the best we can in a stepwise fashion.”
According to Dr. Roberts, 80% of his patients with diabetes struggle with the cost of medicine in general. “They’re either underinsured or not insured or their formulary is limited.
Douglas S. Paauw, MD, agreed in an interview that the newer drugs can be problematic on the insurance front.
“For some patients they aren’t affordable, especially for the uninsured if you can’t get them on an assistance program,” said Dr. Paauw, who is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the university.
Dr. Hopkins, who is on the Internal Medicine News board, noted that “unfortunately, the treatment of type 2 diabetes in patients who cannot achieve control with metformin, diet, weight control, and exercise is a story of the ‘haves’ and the ‘have nots.’ The ‘haves’ are those who have pharmacy benefits which make access to newer agents like SGLT2 inhibitors and GLP-1 agonists a possibility.”
“I have had very few of the ‘have nots’ who have been able to even consider these newer agents, which carry price tags of $600-$1,300 a month even with the availability of discounting coupons in the marketplace,” he added. “Most of these patients end up requiring a sulfonylurea or TZD [thiazolidinedione] as a second agent to achieve glycemic control. This makes it very difficult to achieve sufficient weight and metabolic control to avoid an eventual switch to insulin.”
Fatima Z. Syed, MD, an endocrine-trained general internist at DukeHealth in Durham, N.C., said she prescribes SGLT2 inhibitors and GLP-1 receptor agonists in combination with metformin. “I prescribe them frequently, but they are not first-line treatments,” she explained.
“Nothing replaces diet and exercise” as therapy for patients with type 2 diabetes, she added.
Neil S. Skolnik, MD, said that insurance companies were not preventing patients from using these drugs in his experience. He also provided an optimistic take on the accessibility of these drugs in the near future.
“Most insurance companies are now covering select SGLT2 inhibitors and GLP-1 receptor agonists for appropriate patients and those companies that currently do not will soon have to,” said Dr. Skolnik, who is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
“The outcomes [associated with use of the new drugs] are robust, the benefits are large, and are well worth the cost,” he added.
The side effects
While others praised these drugs for their beneficial effects, they also noted that the side effects of these drugs are serious and must be discussed with patients.
GLP-1 receptor agonists are linked to gastrointestinal symptoms, especially nausea, while SGLT2 inhibitors have been linked to kidney failure, ketoacidosis, and more. The Food and Drug Administration warned in 2018 that the SGLT2 inhibitors can cause a rare serious infection known as Fournier’s gangrene – necrotizing fasciitis of the perineum.
“We have to tell our patients to let us know right away if they get pain or swelling in the genital area,” Dr. Paauw, who is on the Internal Medicine News board, noted. “The chance that an infection could explode quickly is higher in those who take these drugs.”
Amputation risks also are associated with taking the SGLT2 inhibitor canagliflozin (Invokana). The FDA requires the manufacturer of this drug to include a black-box warning about the risk of “lower-limb amputations, most frequently of the toe and midfoot,” but also the leg. In approval trials, the risk doubled versus placebo.
These amputation risks “put a damper on some of the enthusiasm on behalf of physicians and patients ... for taking this drug,” noted Dr. Roberts, who is a professor of internal medicine at the University of Central Florida, Orlando.
While a manufacturer-funded study released last year found no link to amputations, the results weren’t powerful enough to rule out a moderately increased risk.
“[If] you are at high risk for having an amputation, we really have to take this risk very seriously,” said John B. Buse, MD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill, in a presentation about the study at the 2018 annual scientific sessions of the American Diabetes Association.
The benefits
Despite these risks of adverse events, most interviewed agreed that the many benefits observed in those taking SGLT2 inhibitors or GLP-1 receptor agonists make them worth prescribing, at least to those who are able to afford them.
Both SGLT2 inhibitors and GLP-1 receptor agonists appear to have significant cardiovascular benefits. A 2019 meta-analysis and systematic review found that both drugs reduced major adverse cardiac events by about 12% (Circulation. 2019 Apr 23;139[17]:2022-31).
“They don’t cause hypoglycemia, they lower blood pressure, they don’t cause weight gain, and they might promote weight loss,” noted Dr. Paauw.
SGLT2 inhibitors also have shown signs of kidney benefits. The CREDENCE trial linked canagliflozin to a lowering of kidney disorders versus placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). “The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34% (hazard ratio, 0.66; 95% confidence interval, 0.53-0.81; P less than .001), and the relative risk of end-stage kidney disease was lower by 32% (HR, 0.68; 95% CI, 0.54-0.86; P = .002),” the trial investigators wrote.
“They showed very nicely that the drug improved the kidney function of those patients and reduced the kidney deterioration,” said Yehuda Handelsman, MD, an endocrinologist in Tarzana, Calif., who chaired the 2011 and 2015 American Association of Clinical Endocrinologists’ Comprehensive Diabetes Guidelines. The study was especially impressive, he added, because it included patients with low kidney function.
SGLT2 inhibitors’ “diuretic mechanism explains why there is a substantial reduction in heart failure hospitalizations in patients who take these drugs,” said cardiologist Marc E. Goldschmidt, MD, director of the Heart Success Program at Atlantic Health System’s Morristown (N.J.) Medical Center, in an interview. “Both the EMPA-REG Outcome and the CREDENCE trials demonstrated substantial benefit of this class of medications by showing a lower risk of cardiovascular death as well as death from any cause and a lower risk of hospitalization for heart failure."
Overall, the SGLT2 trial data have been very consistent with a benefit for cardiovascular risk reduction, particularly in regard to heart failure hospitalizations and even in potentially preventing heart failure in diabetics,” he added.
Dr. Skolnik, a columnist for Family Practice News, cited SGLT2 inhibitors and GLP-1 receptor agonists’ ability to slow renal disease progression, promote weight loss, and prevent poor cardiac outcomes.“These drugs should be used, in addition to metformin, in all patients with diabetes and vascular disease. These proven outcomes are far better than we ever were able to achieve previously and the strength of the evidence at this point is very strong,” said Dr. Skolnik. “In addition to the benefits of decreasing the development of cardiovascular disease, serious heart failure, and slowing progression of renal disease, these two classes of medication have additional benefits. Both classes help patients lose weight, which is very different from what was found with either sulfonylureas or insulin, which cause patients to gain weight. Also both the SGLT2 inhibitors and the GLP-1 RAs [receptor agonists] have a low incidence of hypoglycemia. For all these reasons, these have become important medications for us to use in primary care.”
Other recent trials offer “very powerful data” about SGLT2 inhibitors, Dr. Roberts said. That’s good news, since “our approach needs to be toward cardiovascular protection and preservation as well as managing blood sugar.”An Israeli trial, whose results were released in May 2019 at the annual meeting of the American College of Cardiology, found that, compared with other glucose-lowering drugs, taking an SGLT2 inhibitor was associated with lower risks of heart failure hospitalization and all-cause mortality (HR, 0.54; 95% CI, 0.44-0.65; P less than .001). This trial also offered a new detail: The patients gained the benefit regardless of whether their baseline left ventricular ejection fraction was preserved or reduced (J Coll Cardiol. 2019 Mar;73[9]:suppl 1). The SGLT2 inhibitors used in this trial included dapagliflozin (Farxiga) and empagliflozin (Jardiance).
In another study released this year, a subanalysis of the DECLARE-TIMI 58 trial, researchers reported that the SGLT2 inhibitor dapagliflozin reduced risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes and prior myocardial infarction versus controls (Circulation. 2019 May 28;139[22]:2516-27). The absolute risk reduction for major adverse cardiovascular events was 1.9% (HR, 0.81; 95% CI, 0.65-1.00; P = .046), while it was 0.6% for heart failure hospitalization (HR, 0.85; 95% CI, 0.72-1.00; P = .055).
These and other studies “speak volumes about the efficacy of managing blood sugar and addressing our biggest nemesis, which is cardiovascular disease,” Dr. Roberts said. “It’s irrefutable. The data [are] very good.”
Dr. Paauw said an SGLT2 inhibitor or GLP-1 receptor agonist is best reserved for use in select patients with cardiovascular risks and type 2 diabetes that need management beyond metformin.
For example, they might fit a 70-year-old with persistent hypertension who’s already taking a couple of blood pressure medications. “If they have another cardiovascular risk factor, the cardiovascular protection piece will be a bigger deal,” he said. Also, “it will probably help lower their blood pressure so they can avoid taking another blood pressure medicine.”
Trials of both GLP-1 receptor agonists and SGLT2 inhibitors have shown benefits “in improving [major adverse cardiac events], with the SGLT2 class showing substantial benefit in improving both heart failure and renal outcomes as well,” noted Dr. Skolnik. “It is in this context that one must address the question of whether the price of the medications are worthwhile. With such substantial benefit, there is no question in my mind that – for patients who have underlying cardiovascular illness, which includes patients with existent coronary disease, history of stroke, transient ischemic attack, or peripheral vascular disease – it is far and away worth it to prescribe these classes of medications.”
Indeed, the American Diabetes Association and the European Association for the Study of Diabetes’ most recent guidelines now call for a GLP-1 receptor agonist – instead of insulin – to be the first injectable used to treat type 2 diabetes (Diabetes Care 2018 Dec; 41[12]:2669-701).
“For the relatively small number of my patients who have been able to access and use these medications for months or longer, more have tolerated the GLP-1 agonists than SGLT2 inhibitors primarily due to urinary issues,” noted Dr. Hopkins.
Dipeptidyl peptidase–4 inhibitors are another option in patients with type 2 diabetes, but research suggests they may not be a top option for patients with cardiovascular risk. A 2018 review noted that cardiovascular outcome trials for alogliptin (Nesina), saxagliptin (Onglyza), and sitagliptin (Januvia) showed noninferiority but failed to demonstrate any superiority, compared with placebo in patients with type 2 diabetes mellitus and high cardiovascular risk (Circ Res. 2018 May 11;122[10]:1439-59).
The combination therapies
Many of the newer drugs are available as combinations with other types of diabetes drugs. In some cases, physicians create their own form of combination therapy by separately prescribing two or more diabetes drugs. Earlier this year, a study suggested the benefits of this kind of add-on therapy: Diabetes outcomes improved in patients who took the GLP-1 receptor agonist semaglutide and an SGLT2 inhibitor (Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587[19]30066-X).
Dr. Roberts suggested caution, however, when prescribing combination therapies. “My recommendation is always to begin with the individual medications to see if the patient tolerates the drugs and then decide which component needs to be titrated. It’s hard to titrate a combination drug, and it doesn’t leave a lot of flexibility. You never know which drug is doing what.
Dr. Handelsman said some patients may need to take three medications such as metformin, an SGLT2 inhibitor, and a GLP-1 receptor agonist.
“I don’t recommend using the combinations if you’re not familiar with the drugs ... These are relatively new pharmaceuticals, and most of us are on a learning curve as to how they fit into the armamentarium. If a drug is tolerated with a good response, you can certainly consider going to the combination tablets,” he added.
There is at least one drug that combines these three classes: The newly FDA-approved Qternmet XR, which combines dapagliflozin (an SGLT2 inhibitor), saxagliptin (a GLP-1 receptor agonist), and metformin. As of mid-June 2019, it was not yet available in the United States. Its sister drug Qtern, which combines dapagliflozin and saxagliptin, costs more than $500 a month with a free coupon, according to goodrx.com. In contrast, metformin is extremely inexpensive, costing just a few dollars a month for a common starting dose.
What about adding insulin?
“Both [SGLT2 inhibitors and GLP-1 receptor agonists] work very well with insulin,” Dr. Handelsman said. “There is a nice additive effect on the reduction of [hemoglobin] A1c. The only caution is that, although neither SGLT2 inhibitors nor GLP-1 receptor agonists cause hypoglycemia, in combination with insulin they do increase the risk of hypoglycemia. You may have to adjust the dose of insulin.”
Dr. Hopkins warned that cost becomes an even bigger issue when you add insulin into the mix.
“When insulin comes into the discussion, we are again stuck with astronomical costs which many struggle to afford,” he explained.
Indeed, the price tag on these drugs seems to be the biggest problem physicians have with them.
“The challenges in managing patients with diabetes aren’t the risks associated with the drugs. It’s dealing with their insurers,” noted Dr. Roberts.
Dr. Hopkins, Dr. Paauw, Dr. Roberts, and Dr. Syed reported no disclosures. Dr. Buse is an investigator for Johnson and Johnson. Dr. Goldschmidt is paid to speak by Novartis. Dr. Handelsman reported research grants, consulting work, and speaker honoraria from Amgen, Gilead, Lilly, Merck, Novo Nordisk, and others. Dr Skolnik reported nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi, and GlaxoSmithKline and personal fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly. He also serves on the advisory boards of AstraZeneca, Boehringer Ingelheim, Teva Pharmaceutical, Eli Lilly, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline.
Dr. Paauw and Dr. Skolnik are columnists for Family Practice News and Internal Medicine News.
M. Alexander Otto contributed to this report.

Metabolic and work productivity gains follow telemonitored exercise
while also improving productivity and mental health, research suggests.
In the June 13 online edition of Lancet Public Health, Dr. Sven Haufe of the Institute of Sports Medicine at Hannover Medical School in Germany and coauthors report the outcomes of a prospective, parallel-group, assessor-blind study of a telemonitoring-supported exercise intervention in 314 workers at a car factory. Of the participants, 162 did office work, 114 did manual work, and 30 did work that was not classified as falling under the office or manual work categories.
The participants, who had all been diagnosed with metabolic syndrome, were randomized to a 6-month exercise program involving personal counseling, use of a telemonitored activity monitor and regular feedback and consultation with an exercise scientist, or to continue their current lifestyle.
The participants were told to aim to complete 150 minutes of moderately intense physical activity per week for 6 months and to maintain a high level of daily activity. They were asked to maintain an individual heart rate range of 65%-75% relative to measured maximum heart rate when performing activities, such as walking, running, cycling, and using an elliptical trainer.
Participants wore their activity monitor, the Forerunner 35 (Garmin, Germany), on the wrist of their nondominant hand throughout the intervention period and were trained on how to use the device. Wearing time and steps were continuously recorded and recording of time, distance, and heart rate while performing cardiovascular exercise like riding a bicycle could be stopped and started by the participant. “Both continuous and self-started activity data were saved and directly forwarded via an interface from the Garmin server to a server at Hanover Medical school.” Participants also downloaded an application on their smartphones called Rebirth Active, which was specially designed for the study. The purposes of this app were to facilitate a close relationship between the participant and that participant’s supervising exercise scientist and to provide general information on the study, individual training goals, recommended heart rates during activities, tips for increasing physical activity, and the supervisor’s contact information.
Nearly half of the participants in the exercise program achieved at least the scheduled activity target of 150 minutes of physical activity per week, while the mean overall was 147 minutes per week. The researchers observed a significant reduction in mean metabolic syndrome z score in the intervention group – from 0.93 before the start of the study to 0.63 at the end of the program (P less than .0001). The control group’s mean z score level of improvement did not reach statistical significance (P = .167). The intervention was also associated with significant improvements in the metabolic syndrome components waist circumference, fasting glucose concentration, systolic blood pressure, and triglycerides, but not in HDL cholesterol levels. Additionally, the exercise group experienced greater reductions in mean body weight and mean percentage of body fat than the control group.
Workers who took part in the exercise program showed significant improvements in their performance on three subscales of the work ability index, including their current work ability, work ability in relation to demands, and mental resources. The control group showed no significant gains in these areas.
The intervention group also achieved gains in the physical and mental component scores of the quality of life questionnaires, which were significantly greater than those of the control group. Both the intervention and control groups had decreases in severity scores for anxiety and depression, but participants in the exercise program showed greater improvements.
“The observation that improvements in exercise capacity and mental health are associated with changes in work ability shows the need to offer similar interventions broadly across the working population,” the authors wrote, “not only to reduce individual risk of disease, but also to possibly ease the health care burden and economic costs arising from metabolic syndrome conditions in an aging population, an issue that should be addressed in further studies.”
The study was supported by Audi BKK health insurance and the German Research Foundation. No conflicts of interest were declared.
SOURCE: Haufe S et al. Lancet Public Health 2019. Jun 13. doi.: 10.1016/2468-2667(19)30075-1.
while also improving productivity and mental health, research suggests.
In the June 13 online edition of Lancet Public Health, Dr. Sven Haufe of the Institute of Sports Medicine at Hannover Medical School in Germany and coauthors report the outcomes of a prospective, parallel-group, assessor-blind study of a telemonitoring-supported exercise intervention in 314 workers at a car factory. Of the participants, 162 did office work, 114 did manual work, and 30 did work that was not classified as falling under the office or manual work categories.
The participants, who had all been diagnosed with metabolic syndrome, were randomized to a 6-month exercise program involving personal counseling, use of a telemonitored activity monitor and regular feedback and consultation with an exercise scientist, or to continue their current lifestyle.
The participants were told to aim to complete 150 minutes of moderately intense physical activity per week for 6 months and to maintain a high level of daily activity. They were asked to maintain an individual heart rate range of 65%-75% relative to measured maximum heart rate when performing activities, such as walking, running, cycling, and using an elliptical trainer.
Participants wore their activity monitor, the Forerunner 35 (Garmin, Germany), on the wrist of their nondominant hand throughout the intervention period and were trained on how to use the device. Wearing time and steps were continuously recorded and recording of time, distance, and heart rate while performing cardiovascular exercise like riding a bicycle could be stopped and started by the participant. “Both continuous and self-started activity data were saved and directly forwarded via an interface from the Garmin server to a server at Hanover Medical school.” Participants also downloaded an application on their smartphones called Rebirth Active, which was specially designed for the study. The purposes of this app were to facilitate a close relationship between the participant and that participant’s supervising exercise scientist and to provide general information on the study, individual training goals, recommended heart rates during activities, tips for increasing physical activity, and the supervisor’s contact information.
Nearly half of the participants in the exercise program achieved at least the scheduled activity target of 150 minutes of physical activity per week, while the mean overall was 147 minutes per week. The researchers observed a significant reduction in mean metabolic syndrome z score in the intervention group – from 0.93 before the start of the study to 0.63 at the end of the program (P less than .0001). The control group’s mean z score level of improvement did not reach statistical significance (P = .167). The intervention was also associated with significant improvements in the metabolic syndrome components waist circumference, fasting glucose concentration, systolic blood pressure, and triglycerides, but not in HDL cholesterol levels. Additionally, the exercise group experienced greater reductions in mean body weight and mean percentage of body fat than the control group.
Workers who took part in the exercise program showed significant improvements in their performance on three subscales of the work ability index, including their current work ability, work ability in relation to demands, and mental resources. The control group showed no significant gains in these areas.
The intervention group also achieved gains in the physical and mental component scores of the quality of life questionnaires, which were significantly greater than those of the control group. Both the intervention and control groups had decreases in severity scores for anxiety and depression, but participants in the exercise program showed greater improvements.
“The observation that improvements in exercise capacity and mental health are associated with changes in work ability shows the need to offer similar interventions broadly across the working population,” the authors wrote, “not only to reduce individual risk of disease, but also to possibly ease the health care burden and economic costs arising from metabolic syndrome conditions in an aging population, an issue that should be addressed in further studies.”
The study was supported by Audi BKK health insurance and the German Research Foundation. No conflicts of interest were declared.
SOURCE: Haufe S et al. Lancet Public Health 2019. Jun 13. doi.: 10.1016/2468-2667(19)30075-1.
while also improving productivity and mental health, research suggests.
In the June 13 online edition of Lancet Public Health, Dr. Sven Haufe of the Institute of Sports Medicine at Hannover Medical School in Germany and coauthors report the outcomes of a prospective, parallel-group, assessor-blind study of a telemonitoring-supported exercise intervention in 314 workers at a car factory. Of the participants, 162 did office work, 114 did manual work, and 30 did work that was not classified as falling under the office or manual work categories.
The participants, who had all been diagnosed with metabolic syndrome, were randomized to a 6-month exercise program involving personal counseling, use of a telemonitored activity monitor and regular feedback and consultation with an exercise scientist, or to continue their current lifestyle.
The participants were told to aim to complete 150 minutes of moderately intense physical activity per week for 6 months and to maintain a high level of daily activity. They were asked to maintain an individual heart rate range of 65%-75% relative to measured maximum heart rate when performing activities, such as walking, running, cycling, and using an elliptical trainer.
Participants wore their activity monitor, the Forerunner 35 (Garmin, Germany), on the wrist of their nondominant hand throughout the intervention period and were trained on how to use the device. Wearing time and steps were continuously recorded and recording of time, distance, and heart rate while performing cardiovascular exercise like riding a bicycle could be stopped and started by the participant. “Both continuous and self-started activity data were saved and directly forwarded via an interface from the Garmin server to a server at Hanover Medical school.” Participants also downloaded an application on their smartphones called Rebirth Active, which was specially designed for the study. The purposes of this app were to facilitate a close relationship between the participant and that participant’s supervising exercise scientist and to provide general information on the study, individual training goals, recommended heart rates during activities, tips for increasing physical activity, and the supervisor’s contact information.
Nearly half of the participants in the exercise program achieved at least the scheduled activity target of 150 minutes of physical activity per week, while the mean overall was 147 minutes per week. The researchers observed a significant reduction in mean metabolic syndrome z score in the intervention group – from 0.93 before the start of the study to 0.63 at the end of the program (P less than .0001). The control group’s mean z score level of improvement did not reach statistical significance (P = .167). The intervention was also associated with significant improvements in the metabolic syndrome components waist circumference, fasting glucose concentration, systolic blood pressure, and triglycerides, but not in HDL cholesterol levels. Additionally, the exercise group experienced greater reductions in mean body weight and mean percentage of body fat than the control group.
Workers who took part in the exercise program showed significant improvements in their performance on three subscales of the work ability index, including their current work ability, work ability in relation to demands, and mental resources. The control group showed no significant gains in these areas.
The intervention group also achieved gains in the physical and mental component scores of the quality of life questionnaires, which were significantly greater than those of the control group. Both the intervention and control groups had decreases in severity scores for anxiety and depression, but participants in the exercise program showed greater improvements.
“The observation that improvements in exercise capacity and mental health are associated with changes in work ability shows the need to offer similar interventions broadly across the working population,” the authors wrote, “not only to reduce individual risk of disease, but also to possibly ease the health care burden and economic costs arising from metabolic syndrome conditions in an aging population, an issue that should be addressed in further studies.”
The study was supported by Audi BKK health insurance and the German Research Foundation. No conflicts of interest were declared.
SOURCE: Haufe S et al. Lancet Public Health 2019. Jun 13. doi.: 10.1016/2468-2667(19)30075-1.
FROM LANCET PUBLIC HEALTH
Vitamin D did not reduce progression to type 2 diabetes in D2d trial
SAN FRANCISCO – Vitamin D supplementation did not significantly reduce the risk of progression from prediabetes to type 2 diabetes, according to the landmark D2d study.
A possible reason why the observed reduction was not statistically significant was that most participants already had acceptable levels of vitamin D. Still, the intervention “did not significantly reduce the risk [of diabetes],” Anastassios G. Pittas, MD, professor of medicine at Tufts Medical Center, Boston, said at the annual scientific sessions of the American Diabetes Association.
Vitamin D supplementation has been a hot topic on a range of medical fronts. As a 2016 report noted, “low vitamin D levels are also associated with hypertension, cancer, and cardiovascular disease. In addition, [diabetes] and chronic kidney disease (CKD) are also related to vitamin D levels. Vitamin D deficiency has been linked to onset and progression of [diabetes],” (World J Diabetes. 2016;7[5]:89-100).
However, as the report noted, “evidence regarding vitamin D levels and [diabetes] is contradictory, and well-controlled studies are needed.”
For the D2d study, which was coordinated out of the division of endocrinology at Tufts Medical Center, Dr. Pittas and associates recruited 2,423 patients who were considered to have prediabetes, with at least 2 of 3 ADA criteria: fasting plasma glucose level of 100-125 mg/dL; plasma glucose level 2 hours after a 75-g oral glucose load of 140-199 mg/dL; and hemoglobin A1c (HbA1c) level of 5.7%-6.4%.
All of the patients were at least 30 years old with the exception of American Indians, Alaska Natives, Native Hawaiians and other Pacific Islanders who were allowed to be aged 25-30 years. About 22% had low vitamin D levels.
The mean age of the patients was 60 years, mean body mass index was 32, 45% were women, and 33% were non-white. The trial was powered to show a reduction of 25% or more in diabetes risk with vitamin D.
The researchers randomly assigned 1,211 patients to take a once-daily capsule of vitamin D3 (cholecalciferol; 4,000 IU per day); 1,212 received a placebo.
Patients in the vitamin D group greatly boosted their mean serum 25-hydroxyvitamin D levels, from 27.7 ng/mL at baseline to 54.3 ng/mL at 24 months. In contrast, those in the placebo group saw little change, going from 28.2 ng/mL at baseline to 28.8 ng/mL at 24 months.
At a median follow-up of 2.5 years, with 99% of the study participants remaining in the trial, 616 patients developed diabetes (293 in the vitamin D group, 323 in the placebo group).
The risk was lower in the vitamin D group although the difference was not statistically significant. An analysis revealed no clear differences in any of the subgroups (race, age, body mass index, latitude-based geographic location, calcium supplement intake, and others).
However, a post hoc analysis of patients with vitamin D deficiency, which is defined by the National Academy of Medicine as having a 25-hydroxyvitamin D level of less than 12 ng/mL, showed that the vitamin D group had a 62% reduction in risk of diabetes, compared with placebo.
“Response to a nutritional intervention depends on nutritional status at baseline. Thus, if vitamin D has an effect on diabetes prevention, then people with lower levels of serum 25-hydroxyvitamin D would be expected to have a larger effect from supplementation than would those with higher baseline levels,” Dr. Pittas said.
He noted that two recent, similar trials (one in Norway and one in Japan) reported nearly identical, statistically significant risk reductions in the vitamin D group.
There was also some good news in the findings: Vitamin D supplementation “did not lead to significantly more kidney stones, high serum calcium, or low glomerular filtration rate,” Dr. Pittas said.
Although the study findings are disappointing, vitamin D supplementation is still crucial in patients who have low levels, Victor Lawrence Roberts, MD, an endocrinologist in private practice in Orlando, Fla., said in an interview.
“I diagnose at least three or four people a day with vitamin D deficiency,” Dr. Roberts said. “I’ve found if you replace vitamin D in diabetes – get it to 30 ng/ml or better – their diabetes may improve in some cases, although it may be that they’re paying more attention to their health.”
In the big picture, he said, “if people are vitamin D deficient, the vitamin should be replaced no matter what it does to their blood sugar.”
The study was published simultaneously in the New England Journal of Medicine (N Engl J Med. 2019 Jun 7. doi: 10.1056/NEJMoa1900906).
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the NIH Office of Dietary Supplements, and American Diabetes Association. Dr. Pittas reports grants from the same institutions during the conduct of the study. Many coauthors disclosed relationships with multiple drug companies, but none relevant to the topic under study.
SOURCE: Pittas AG et al. ADA 2019
This article was updated on 6/18/2019.
SAN FRANCISCO – Vitamin D supplementation did not significantly reduce the risk of progression from prediabetes to type 2 diabetes, according to the landmark D2d study.
A possible reason why the observed reduction was not statistically significant was that most participants already had acceptable levels of vitamin D. Still, the intervention “did not significantly reduce the risk [of diabetes],” Anastassios G. Pittas, MD, professor of medicine at Tufts Medical Center, Boston, said at the annual scientific sessions of the American Diabetes Association.
Vitamin D supplementation has been a hot topic on a range of medical fronts. As a 2016 report noted, “low vitamin D levels are also associated with hypertension, cancer, and cardiovascular disease. In addition, [diabetes] and chronic kidney disease (CKD) are also related to vitamin D levels. Vitamin D deficiency has been linked to onset and progression of [diabetes],” (World J Diabetes. 2016;7[5]:89-100).
However, as the report noted, “evidence regarding vitamin D levels and [diabetes] is contradictory, and well-controlled studies are needed.”
For the D2d study, which was coordinated out of the division of endocrinology at Tufts Medical Center, Dr. Pittas and associates recruited 2,423 patients who were considered to have prediabetes, with at least 2 of 3 ADA criteria: fasting plasma glucose level of 100-125 mg/dL; plasma glucose level 2 hours after a 75-g oral glucose load of 140-199 mg/dL; and hemoglobin A1c (HbA1c) level of 5.7%-6.4%.
All of the patients were at least 30 years old with the exception of American Indians, Alaska Natives, Native Hawaiians and other Pacific Islanders who were allowed to be aged 25-30 years. About 22% had low vitamin D levels.
The mean age of the patients was 60 years, mean body mass index was 32, 45% were women, and 33% were non-white. The trial was powered to show a reduction of 25% or more in diabetes risk with vitamin D.
The researchers randomly assigned 1,211 patients to take a once-daily capsule of vitamin D3 (cholecalciferol; 4,000 IU per day); 1,212 received a placebo.
Patients in the vitamin D group greatly boosted their mean serum 25-hydroxyvitamin D levels, from 27.7 ng/mL at baseline to 54.3 ng/mL at 24 months. In contrast, those in the placebo group saw little change, going from 28.2 ng/mL at baseline to 28.8 ng/mL at 24 months.
At a median follow-up of 2.5 years, with 99% of the study participants remaining in the trial, 616 patients developed diabetes (293 in the vitamin D group, 323 in the placebo group).
The risk was lower in the vitamin D group although the difference was not statistically significant. An analysis revealed no clear differences in any of the subgroups (race, age, body mass index, latitude-based geographic location, calcium supplement intake, and others).
However, a post hoc analysis of patients with vitamin D deficiency, which is defined by the National Academy of Medicine as having a 25-hydroxyvitamin D level of less than 12 ng/mL, showed that the vitamin D group had a 62% reduction in risk of diabetes, compared with placebo.
“Response to a nutritional intervention depends on nutritional status at baseline. Thus, if vitamin D has an effect on diabetes prevention, then people with lower levels of serum 25-hydroxyvitamin D would be expected to have a larger effect from supplementation than would those with higher baseline levels,” Dr. Pittas said.
He noted that two recent, similar trials (one in Norway and one in Japan) reported nearly identical, statistically significant risk reductions in the vitamin D group.
There was also some good news in the findings: Vitamin D supplementation “did not lead to significantly more kidney stones, high serum calcium, or low glomerular filtration rate,” Dr. Pittas said.
Although the study findings are disappointing, vitamin D supplementation is still crucial in patients who have low levels, Victor Lawrence Roberts, MD, an endocrinologist in private practice in Orlando, Fla., said in an interview.
“I diagnose at least three or four people a day with vitamin D deficiency,” Dr. Roberts said. “I’ve found if you replace vitamin D in diabetes – get it to 30 ng/ml or better – their diabetes may improve in some cases, although it may be that they’re paying more attention to their health.”
In the big picture, he said, “if people are vitamin D deficient, the vitamin should be replaced no matter what it does to their blood sugar.”
The study was published simultaneously in the New England Journal of Medicine (N Engl J Med. 2019 Jun 7. doi: 10.1056/NEJMoa1900906).
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the NIH Office of Dietary Supplements, and American Diabetes Association. Dr. Pittas reports grants from the same institutions during the conduct of the study. Many coauthors disclosed relationships with multiple drug companies, but none relevant to the topic under study.
SOURCE: Pittas AG et al. ADA 2019
This article was updated on 6/18/2019.
SAN FRANCISCO – Vitamin D supplementation did not significantly reduce the risk of progression from prediabetes to type 2 diabetes, according to the landmark D2d study.
A possible reason why the observed reduction was not statistically significant was that most participants already had acceptable levels of vitamin D. Still, the intervention “did not significantly reduce the risk [of diabetes],” Anastassios G. Pittas, MD, professor of medicine at Tufts Medical Center, Boston, said at the annual scientific sessions of the American Diabetes Association.
Vitamin D supplementation has been a hot topic on a range of medical fronts. As a 2016 report noted, “low vitamin D levels are also associated with hypertension, cancer, and cardiovascular disease. In addition, [diabetes] and chronic kidney disease (CKD) are also related to vitamin D levels. Vitamin D deficiency has been linked to onset and progression of [diabetes],” (World J Diabetes. 2016;7[5]:89-100).
However, as the report noted, “evidence regarding vitamin D levels and [diabetes] is contradictory, and well-controlled studies are needed.”
For the D2d study, which was coordinated out of the division of endocrinology at Tufts Medical Center, Dr. Pittas and associates recruited 2,423 patients who were considered to have prediabetes, with at least 2 of 3 ADA criteria: fasting plasma glucose level of 100-125 mg/dL; plasma glucose level 2 hours after a 75-g oral glucose load of 140-199 mg/dL; and hemoglobin A1c (HbA1c) level of 5.7%-6.4%.
All of the patients were at least 30 years old with the exception of American Indians, Alaska Natives, Native Hawaiians and other Pacific Islanders who were allowed to be aged 25-30 years. About 22% had low vitamin D levels.
The mean age of the patients was 60 years, mean body mass index was 32, 45% were women, and 33% were non-white. The trial was powered to show a reduction of 25% or more in diabetes risk with vitamin D.
The researchers randomly assigned 1,211 patients to take a once-daily capsule of vitamin D3 (cholecalciferol; 4,000 IU per day); 1,212 received a placebo.
Patients in the vitamin D group greatly boosted their mean serum 25-hydroxyvitamin D levels, from 27.7 ng/mL at baseline to 54.3 ng/mL at 24 months. In contrast, those in the placebo group saw little change, going from 28.2 ng/mL at baseline to 28.8 ng/mL at 24 months.
At a median follow-up of 2.5 years, with 99% of the study participants remaining in the trial, 616 patients developed diabetes (293 in the vitamin D group, 323 in the placebo group).
The risk was lower in the vitamin D group although the difference was not statistically significant. An analysis revealed no clear differences in any of the subgroups (race, age, body mass index, latitude-based geographic location, calcium supplement intake, and others).
However, a post hoc analysis of patients with vitamin D deficiency, which is defined by the National Academy of Medicine as having a 25-hydroxyvitamin D level of less than 12 ng/mL, showed that the vitamin D group had a 62% reduction in risk of diabetes, compared with placebo.
“Response to a nutritional intervention depends on nutritional status at baseline. Thus, if vitamin D has an effect on diabetes prevention, then people with lower levels of serum 25-hydroxyvitamin D would be expected to have a larger effect from supplementation than would those with higher baseline levels,” Dr. Pittas said.
He noted that two recent, similar trials (one in Norway and one in Japan) reported nearly identical, statistically significant risk reductions in the vitamin D group.
There was also some good news in the findings: Vitamin D supplementation “did not lead to significantly more kidney stones, high serum calcium, or low glomerular filtration rate,” Dr. Pittas said.
Although the study findings are disappointing, vitamin D supplementation is still crucial in patients who have low levels, Victor Lawrence Roberts, MD, an endocrinologist in private practice in Orlando, Fla., said in an interview.
“I diagnose at least three or four people a day with vitamin D deficiency,” Dr. Roberts said. “I’ve found if you replace vitamin D in diabetes – get it to 30 ng/ml or better – their diabetes may improve in some cases, although it may be that they’re paying more attention to their health.”
In the big picture, he said, “if people are vitamin D deficient, the vitamin should be replaced no matter what it does to their blood sugar.”
The study was published simultaneously in the New England Journal of Medicine (N Engl J Med. 2019 Jun 7. doi: 10.1056/NEJMoa1900906).
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the NIH Office of Dietary Supplements, and American Diabetes Association. Dr. Pittas reports grants from the same institutions during the conduct of the study. Many coauthors disclosed relationships with multiple drug companies, but none relevant to the topic under study.
SOURCE: Pittas AG et al. ADA 2019
This article was updated on 6/18/2019.
REPORTING FROM ADA 2019
Key clinical point: Vitamin D supplementation did significantly lower the risk of diabetes.
Major finding: Progression to diabetes occurred in 293 on vitamin D and 323 on placebo.
Study details: Randomized placebo controlled trial of 2,423 patients with prediabetes.
Disclosures: The study was funded by the NIDDK, NIH Office of Dietary Supplements, and American Diabetes Association. Dr. Pittas reports grants from the same institutions during the conduct of the study. Many coauthors disclosed relationships with multiple drug companies.
Source: Pittas AG et al. ADA 2019
AACE 2019: Top takeaways from Yehuda Handelsman and Paul Jellinger
in a video interview at the meeting.
Dr. Handelsman, medical director of the Metabolic Institute of America, in Tarzana, Calif., summarized the top take-home messages from a premeeting symposium he chaired on diabetes, cardiovascular disease (CVD), and lipid management in high-risk patients. Dr. Jellinger, professor of clinical medicine on the voluntary faculty at the University of Miami Miller School of Medicine, looked at management aspects and therapy goals based on a comparison of the lipid guideline from the American College of Cardiology and the American Heart Association with that from the AACE. Other highlights from the symposium included expert analysis of the CREDENCE trial results on canagliflozin for improving renal outcomes in patients with type 2 diabetes, advice on the management of heart failure in diabetes, and recommendations on managing hyperglycemia.
Dr. Jellinger and Dr. Handelsman, who are members of the editorial advisory board of Clinical Endocrinology News, highlighted the emergence of anabolic treatments for osteoporosis, in particular the sclerostin-neutralizing monoclonal antibody, romosozumab. The therapy was recently approved for the treatment of postmenopausal osteoporosis and is unique in that it both promotes bone formation and reduces resorption. They also noted the switch away from previous practice to now using an anabolic drug first, then going to an antiresorptive therapy, rather than the other way around.
They discussed the keynote address by social media guru, Kevin Pho, MD; a debate that centered on the merits of the American Diabetes Association’s guideline for treating diabetes versus that from the AACE; a presentation on sustained remission of type 2 diabetes with a very low calorie diet; and a report on encouraging findings with an experimental drug for Graves eye disease.
in a video interview at the meeting.
Dr. Handelsman, medical director of the Metabolic Institute of America, in Tarzana, Calif., summarized the top take-home messages from a premeeting symposium he chaired on diabetes, cardiovascular disease (CVD), and lipid management in high-risk patients. Dr. Jellinger, professor of clinical medicine on the voluntary faculty at the University of Miami Miller School of Medicine, looked at management aspects and therapy goals based on a comparison of the lipid guideline from the American College of Cardiology and the American Heart Association with that from the AACE. Other highlights from the symposium included expert analysis of the CREDENCE trial results on canagliflozin for improving renal outcomes in patients with type 2 diabetes, advice on the management of heart failure in diabetes, and recommendations on managing hyperglycemia.
Dr. Jellinger and Dr. Handelsman, who are members of the editorial advisory board of Clinical Endocrinology News, highlighted the emergence of anabolic treatments for osteoporosis, in particular the sclerostin-neutralizing monoclonal antibody, romosozumab. The therapy was recently approved for the treatment of postmenopausal osteoporosis and is unique in that it both promotes bone formation and reduces resorption. They also noted the switch away from previous practice to now using an anabolic drug first, then going to an antiresorptive therapy, rather than the other way around.
They discussed the keynote address by social media guru, Kevin Pho, MD; a debate that centered on the merits of the American Diabetes Association’s guideline for treating diabetes versus that from the AACE; a presentation on sustained remission of type 2 diabetes with a very low calorie diet; and a report on encouraging findings with an experimental drug for Graves eye disease.
in a video interview at the meeting.
Dr. Handelsman, medical director of the Metabolic Institute of America, in Tarzana, Calif., summarized the top take-home messages from a premeeting symposium he chaired on diabetes, cardiovascular disease (CVD), and lipid management in high-risk patients. Dr. Jellinger, professor of clinical medicine on the voluntary faculty at the University of Miami Miller School of Medicine, looked at management aspects and therapy goals based on a comparison of the lipid guideline from the American College of Cardiology and the American Heart Association with that from the AACE. Other highlights from the symposium included expert analysis of the CREDENCE trial results on canagliflozin for improving renal outcomes in patients with type 2 diabetes, advice on the management of heart failure in diabetes, and recommendations on managing hyperglycemia.
Dr. Jellinger and Dr. Handelsman, who are members of the editorial advisory board of Clinical Endocrinology News, highlighted the emergence of anabolic treatments for osteoporosis, in particular the sclerostin-neutralizing monoclonal antibody, romosozumab. The therapy was recently approved for the treatment of postmenopausal osteoporosis and is unique in that it both promotes bone formation and reduces resorption. They also noted the switch away from previous practice to now using an anabolic drug first, then going to an antiresorptive therapy, rather than the other way around.
They discussed the keynote address by social media guru, Kevin Pho, MD; a debate that centered on the merits of the American Diabetes Association’s guideline for treating diabetes versus that from the AACE; a presentation on sustained remission of type 2 diabetes with a very low calorie diet; and a report on encouraging findings with an experimental drug for Graves eye disease.
REPORTING FROM AACE 2019
Fournier gangrene cases surge in patients using SGLT2 inhibitors
since the US Food and Drug Administration (FDA) issued a 2018 warning about this rare but serious infection, researchers say.
Health care providers prescribing SGLT2 inhibitors to patients with diabetes should have a high index of suspicion for the signs and symptoms of Fournier gangrene, given its substantial morbidity and mortality, according to Susan J. Bersoff-Matcha, MD, and her colleagues at the FDA.
“Although the risk for [Fournier gangrene] is low, serious infection should be considered and weighed against the benefits of SGLT2 inhibitor therapy,” said Dr. Bersoff-Matcha and co-authors in their recent report published in the Annals of Internal Medicine (2019 May 6. doi: 10.7326/M19-0085).
In the previous warning, FDA officials said 12 cases of Fournier gangrene in patients taking an SGLT2 inhibitor had been reported to the agency or in medical literature from March 2013, when the first such inhibitor was approved, and May 2018.
In this latest report, a total of 55 Fournier gangrene cases had been reported in patients receiving SGLT2 inhibitors from March 2, 2013 through January 31, 2019.
The influx of reports may have been prompted by growing awareness of the safety issue, investigators said, but could also reflect the increasing prevalence of diabetes combined with SGLT2 inhibitor use. The researchers also noted that diabetes is a comorbidity in 32% to 66% of cases of Fournier gangrene.
But the likliehood that diabetes mellitus alone causes Fournier gangrene seems unlikley, given that Dr. Bersoff-Matcha and co-authors only found 19 Fournier gangrene cases associated with other classes of antiglycemic agents reported to the FDA or in the literature over a 35-year time frame.
“If Fournier gangrene were associated only with diabetes mellitus and not SGLT2 inhibitors, we would expect far more cases reported with the other antiglycemic agents, considering the 35-year timeframe and the large number of agents,” they said in their report.
Cases were reported for all FDA-approved SGLT2 inhibitors besides ertugliflozin, an agent approved for use in the U.S. in December 2017. The lack of cases reported for this drug could be related to its limited time on the market, the investigators said.
Fournier gangrene, marked by rapidly progressing necrotizing infection of the genitalia, perineum, and perianal region, requires antibiotics and immediate surgery, according to Dr. Bersoff-Matcha and colleagues.
“Serious complications and death are likely if Fournier gangrene is not recognized immediately and surgical intervention is not carried out within the first few hours of diagnosis,” they said in the report.
Of the 55 cases reported in patients receiving SGLT2 inhibitors, 39 were men and 16 were women, with an average of 9 months from the start of treatment to the event, investigators said.
At least 25 patients required multiple surgeries, including one patient who had 17 trips to the operating room, they said. A total of 8 patients had a fecal diversion procedure, and 4 patients had skin grafting.
Six patients had multiple encounters with a provider before being diagnosed, suggesting that the provider may have not recognized the infection due to its nonspecific symptoms, which include fatigue, fever, and malaise.
“Pain that seems out of proportion to findings on physical examination is a strong clinical indicator of necrotizing fasciitis and may be the most important diagnostic clue,” Dr. Bersoff-Matcha and co-authors said in their report.
The incidence of Fournier gangrene in patients taking SGLT2 inhibitors can’t be established by these cases reported to the FDA, which are spontaneously provided by health care providers and patients, investigators said.
“We suspect that our numbers underestimate the true burden,” they said in their report.
Dr. Bersoff-Matcha and co-authors disclosed no conflicts of interest related to their report.
SOURCE: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6. Doi: doi:10.7326/M19-0085.
This article was updated May 9, 2019.
since the US Food and Drug Administration (FDA) issued a 2018 warning about this rare but serious infection, researchers say.
Health care providers prescribing SGLT2 inhibitors to patients with diabetes should have a high index of suspicion for the signs and symptoms of Fournier gangrene, given its substantial morbidity and mortality, according to Susan J. Bersoff-Matcha, MD, and her colleagues at the FDA.
“Although the risk for [Fournier gangrene] is low, serious infection should be considered and weighed against the benefits of SGLT2 inhibitor therapy,” said Dr. Bersoff-Matcha and co-authors in their recent report published in the Annals of Internal Medicine (2019 May 6. doi: 10.7326/M19-0085).
In the previous warning, FDA officials said 12 cases of Fournier gangrene in patients taking an SGLT2 inhibitor had been reported to the agency or in medical literature from March 2013, when the first such inhibitor was approved, and May 2018.
In this latest report, a total of 55 Fournier gangrene cases had been reported in patients receiving SGLT2 inhibitors from March 2, 2013 through January 31, 2019.
The influx of reports may have been prompted by growing awareness of the safety issue, investigators said, but could also reflect the increasing prevalence of diabetes combined with SGLT2 inhibitor use. The researchers also noted that diabetes is a comorbidity in 32% to 66% of cases of Fournier gangrene.
But the likliehood that diabetes mellitus alone causes Fournier gangrene seems unlikley, given that Dr. Bersoff-Matcha and co-authors only found 19 Fournier gangrene cases associated with other classes of antiglycemic agents reported to the FDA or in the literature over a 35-year time frame.
“If Fournier gangrene were associated only with diabetes mellitus and not SGLT2 inhibitors, we would expect far more cases reported with the other antiglycemic agents, considering the 35-year timeframe and the large number of agents,” they said in their report.
Cases were reported for all FDA-approved SGLT2 inhibitors besides ertugliflozin, an agent approved for use in the U.S. in December 2017. The lack of cases reported for this drug could be related to its limited time on the market, the investigators said.
Fournier gangrene, marked by rapidly progressing necrotizing infection of the genitalia, perineum, and perianal region, requires antibiotics and immediate surgery, according to Dr. Bersoff-Matcha and colleagues.
“Serious complications and death are likely if Fournier gangrene is not recognized immediately and surgical intervention is not carried out within the first few hours of diagnosis,” they said in the report.
Of the 55 cases reported in patients receiving SGLT2 inhibitors, 39 were men and 16 were women, with an average of 9 months from the start of treatment to the event, investigators said.
At least 25 patients required multiple surgeries, including one patient who had 17 trips to the operating room, they said. A total of 8 patients had a fecal diversion procedure, and 4 patients had skin grafting.
Six patients had multiple encounters with a provider before being diagnosed, suggesting that the provider may have not recognized the infection due to its nonspecific symptoms, which include fatigue, fever, and malaise.
“Pain that seems out of proportion to findings on physical examination is a strong clinical indicator of necrotizing fasciitis and may be the most important diagnostic clue,” Dr. Bersoff-Matcha and co-authors said in their report.
The incidence of Fournier gangrene in patients taking SGLT2 inhibitors can’t be established by these cases reported to the FDA, which are spontaneously provided by health care providers and patients, investigators said.
“We suspect that our numbers underestimate the true burden,” they said in their report.
Dr. Bersoff-Matcha and co-authors disclosed no conflicts of interest related to their report.
SOURCE: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6. Doi: doi:10.7326/M19-0085.
This article was updated May 9, 2019.
since the US Food and Drug Administration (FDA) issued a 2018 warning about this rare but serious infection, researchers say.
Health care providers prescribing SGLT2 inhibitors to patients with diabetes should have a high index of suspicion for the signs and symptoms of Fournier gangrene, given its substantial morbidity and mortality, according to Susan J. Bersoff-Matcha, MD, and her colleagues at the FDA.
“Although the risk for [Fournier gangrene] is low, serious infection should be considered and weighed against the benefits of SGLT2 inhibitor therapy,” said Dr. Bersoff-Matcha and co-authors in their recent report published in the Annals of Internal Medicine (2019 May 6. doi: 10.7326/M19-0085).
In the previous warning, FDA officials said 12 cases of Fournier gangrene in patients taking an SGLT2 inhibitor had been reported to the agency or in medical literature from March 2013, when the first such inhibitor was approved, and May 2018.
In this latest report, a total of 55 Fournier gangrene cases had been reported in patients receiving SGLT2 inhibitors from March 2, 2013 through January 31, 2019.
The influx of reports may have been prompted by growing awareness of the safety issue, investigators said, but could also reflect the increasing prevalence of diabetes combined with SGLT2 inhibitor use. The researchers also noted that diabetes is a comorbidity in 32% to 66% of cases of Fournier gangrene.
But the likliehood that diabetes mellitus alone causes Fournier gangrene seems unlikley, given that Dr. Bersoff-Matcha and co-authors only found 19 Fournier gangrene cases associated with other classes of antiglycemic agents reported to the FDA or in the literature over a 35-year time frame.
“If Fournier gangrene were associated only with diabetes mellitus and not SGLT2 inhibitors, we would expect far more cases reported with the other antiglycemic agents, considering the 35-year timeframe and the large number of agents,” they said in their report.
Cases were reported for all FDA-approved SGLT2 inhibitors besides ertugliflozin, an agent approved for use in the U.S. in December 2017. The lack of cases reported for this drug could be related to its limited time on the market, the investigators said.
Fournier gangrene, marked by rapidly progressing necrotizing infection of the genitalia, perineum, and perianal region, requires antibiotics and immediate surgery, according to Dr. Bersoff-Matcha and colleagues.
“Serious complications and death are likely if Fournier gangrene is not recognized immediately and surgical intervention is not carried out within the first few hours of diagnosis,” they said in the report.
Of the 55 cases reported in patients receiving SGLT2 inhibitors, 39 were men and 16 were women, with an average of 9 months from the start of treatment to the event, investigators said.
At least 25 patients required multiple surgeries, including one patient who had 17 trips to the operating room, they said. A total of 8 patients had a fecal diversion procedure, and 4 patients had skin grafting.
Six patients had multiple encounters with a provider before being diagnosed, suggesting that the provider may have not recognized the infection due to its nonspecific symptoms, which include fatigue, fever, and malaise.
“Pain that seems out of proportion to findings on physical examination is a strong clinical indicator of necrotizing fasciitis and may be the most important diagnostic clue,” Dr. Bersoff-Matcha and co-authors said in their report.
The incidence of Fournier gangrene in patients taking SGLT2 inhibitors can’t be established by these cases reported to the FDA, which are spontaneously provided by health care providers and patients, investigators said.
“We suspect that our numbers underestimate the true burden,” they said in their report.
Dr. Bersoff-Matcha and co-authors disclosed no conflicts of interest related to their report.
SOURCE: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6. Doi: doi:10.7326/M19-0085.
This article was updated May 9, 2019.
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point: The number of Fournier gangrene cases reported in patients receiving sodium-glucose cotransporter-2 (SGLT2) inhibitors has increased in the time since an FDA warning was issued about this rare but potentially serious infection.
Major finding: The previous FDA warning noted 12 reported cases from March 1, 2013 through March 1, 2018. This latest report included a total of 55 cases reported through January 31, 2019.
Study details: A review of spontaneous postmarketing cases of Fournier gangrene reported to the FDA or in the medical literature.
Disclosures: Authors disclosed no conflicts of interest related to the study.
Source: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6.
Type 2 diabetes bumps up short-term risk for bone fracture
LOS ANGELES – results from a large community-based study have shown.
“Osteoporotic fractures are a significant public health burden, causing high morbidity, mortality, and associated health care costs,” Elizabeth J. Samelson, PhD, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “Risk of fractures are higher in patients with type 2 diabetes. Further, outcomes are worse in type 2 diabetes patients, with greater frequency of complications following a fracture.”
Given the projected increase in type 2 diabetes in the U.S. population, Dr. Samelson, an associate scientist at the Marcus Institute for Aging Research at Hebrew SeniorLife and Harvard Medical School, Boston, and colleagues set out to evaluate the short- and long-term risks of bone fractures associated with the disease. They drew from 2,105 women and 1,130 men who participated in the Framingham Original and Offspring Cohorts and whose baseline osteoporosis visit was around 1990. Type 2 diabetes was defined as having a fasting plasma glucose of greater than 125 mg/dL or being on treatment for the disease. Incident fractures excluded finger, toe, skull, face, and pathologic fractures, and the researchers used repeated measures analyses to estimate hazard ratios for the association between type 2 diabetes, type 2 diabetes medication use, and type 2 diabetes duration and incident fracture, adjusted for age, sex, height, and weight.
The mean age of the study participants was 67 years, and the mean follow-up was 9 years. The prevalence of type 2 diabetes in women and men was 7% and 13%, respectively, and 63% and 51% of those were on medication for the disease. The mean duration of diabetes was 8 years.
Dr. Samelson and colleagues found that the cumulative incidence of fracture was 37% in women with type 2 diabetes and 30% in those without the disease. Meanwhile, the cumulative incidence of fracture was 11% in men with type 2 diabetes and 16% in those without the disease. The researchers also found that type 2 diabetes was associated with 1-year fracture risk in women (hazard ratio, 2.23), but not in men.
In the entire study population, longer duration of type 2 diabetes increased the 2-year fracture risk (HR, 1.28), as did the use of any type 2 diabetes medication (HR, 1.70). The researchers observed no statistically significant differences between type 2 diabetes and long-term incidence of fracture.
“Previous studies have contributed to understanding the higher incidence of fractures and worse outcomes in type 2 diabetes, [but] the current study demonstrated that patients [with type 2 diabetes] have 50% to 100% higher short-term [1- to 2-year] risk of fracture independent of clinical risk factors, whereas long-term [10-year] risk of fracture was similar in [patients with] type 2 diabetes and those who do not have [the disease],” Dr. Samelson said. “The current study has some inherent limitations of observational studies, including a lack of definitive determination of causality and that the results are not generalizable to patients with similar demographics. The study, however, is robust in the availability of detailed clinical information, which allows for control of multiple confounding variables.”
Dr. Samelson reported having no financial disclosures. Coauthors Setareh Williams, PhD, and Rich Weiss, MD, are employees and shareholders of Radius Health Inc.
LOS ANGELES – results from a large community-based study have shown.
“Osteoporotic fractures are a significant public health burden, causing high morbidity, mortality, and associated health care costs,” Elizabeth J. Samelson, PhD, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “Risk of fractures are higher in patients with type 2 diabetes. Further, outcomes are worse in type 2 diabetes patients, with greater frequency of complications following a fracture.”
Given the projected increase in type 2 diabetes in the U.S. population, Dr. Samelson, an associate scientist at the Marcus Institute for Aging Research at Hebrew SeniorLife and Harvard Medical School, Boston, and colleagues set out to evaluate the short- and long-term risks of bone fractures associated with the disease. They drew from 2,105 women and 1,130 men who participated in the Framingham Original and Offspring Cohorts and whose baseline osteoporosis visit was around 1990. Type 2 diabetes was defined as having a fasting plasma glucose of greater than 125 mg/dL or being on treatment for the disease. Incident fractures excluded finger, toe, skull, face, and pathologic fractures, and the researchers used repeated measures analyses to estimate hazard ratios for the association between type 2 diabetes, type 2 diabetes medication use, and type 2 diabetes duration and incident fracture, adjusted for age, sex, height, and weight.
The mean age of the study participants was 67 years, and the mean follow-up was 9 years. The prevalence of type 2 diabetes in women and men was 7% and 13%, respectively, and 63% and 51% of those were on medication for the disease. The mean duration of diabetes was 8 years.
Dr. Samelson and colleagues found that the cumulative incidence of fracture was 37% in women with type 2 diabetes and 30% in those without the disease. Meanwhile, the cumulative incidence of fracture was 11% in men with type 2 diabetes and 16% in those without the disease. The researchers also found that type 2 diabetes was associated with 1-year fracture risk in women (hazard ratio, 2.23), but not in men.
In the entire study population, longer duration of type 2 diabetes increased the 2-year fracture risk (HR, 1.28), as did the use of any type 2 diabetes medication (HR, 1.70). The researchers observed no statistically significant differences between type 2 diabetes and long-term incidence of fracture.
“Previous studies have contributed to understanding the higher incidence of fractures and worse outcomes in type 2 diabetes, [but] the current study demonstrated that patients [with type 2 diabetes] have 50% to 100% higher short-term [1- to 2-year] risk of fracture independent of clinical risk factors, whereas long-term [10-year] risk of fracture was similar in [patients with] type 2 diabetes and those who do not have [the disease],” Dr. Samelson said. “The current study has some inherent limitations of observational studies, including a lack of definitive determination of causality and that the results are not generalizable to patients with similar demographics. The study, however, is robust in the availability of detailed clinical information, which allows for control of multiple confounding variables.”
Dr. Samelson reported having no financial disclosures. Coauthors Setareh Williams, PhD, and Rich Weiss, MD, are employees and shareholders of Radius Health Inc.
LOS ANGELES – results from a large community-based study have shown.
“Osteoporotic fractures are a significant public health burden, causing high morbidity, mortality, and associated health care costs,” Elizabeth J. Samelson, PhD, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “Risk of fractures are higher in patients with type 2 diabetes. Further, outcomes are worse in type 2 diabetes patients, with greater frequency of complications following a fracture.”
Given the projected increase in type 2 diabetes in the U.S. population, Dr. Samelson, an associate scientist at the Marcus Institute for Aging Research at Hebrew SeniorLife and Harvard Medical School, Boston, and colleagues set out to evaluate the short- and long-term risks of bone fractures associated with the disease. They drew from 2,105 women and 1,130 men who participated in the Framingham Original and Offspring Cohorts and whose baseline osteoporosis visit was around 1990. Type 2 diabetes was defined as having a fasting plasma glucose of greater than 125 mg/dL or being on treatment for the disease. Incident fractures excluded finger, toe, skull, face, and pathologic fractures, and the researchers used repeated measures analyses to estimate hazard ratios for the association between type 2 diabetes, type 2 diabetes medication use, and type 2 diabetes duration and incident fracture, adjusted for age, sex, height, and weight.
The mean age of the study participants was 67 years, and the mean follow-up was 9 years. The prevalence of type 2 diabetes in women and men was 7% and 13%, respectively, and 63% and 51% of those were on medication for the disease. The mean duration of diabetes was 8 years.
Dr. Samelson and colleagues found that the cumulative incidence of fracture was 37% in women with type 2 diabetes and 30% in those without the disease. Meanwhile, the cumulative incidence of fracture was 11% in men with type 2 diabetes and 16% in those without the disease. The researchers also found that type 2 diabetes was associated with 1-year fracture risk in women (hazard ratio, 2.23), but not in men.
In the entire study population, longer duration of type 2 diabetes increased the 2-year fracture risk (HR, 1.28), as did the use of any type 2 diabetes medication (HR, 1.70). The researchers observed no statistically significant differences between type 2 diabetes and long-term incidence of fracture.
“Previous studies have contributed to understanding the higher incidence of fractures and worse outcomes in type 2 diabetes, [but] the current study demonstrated that patients [with type 2 diabetes] have 50% to 100% higher short-term [1- to 2-year] risk of fracture independent of clinical risk factors, whereas long-term [10-year] risk of fracture was similar in [patients with] type 2 diabetes and those who do not have [the disease],” Dr. Samelson said. “The current study has some inherent limitations of observational studies, including a lack of definitive determination of causality and that the results are not generalizable to patients with similar demographics. The study, however, is robust in the availability of detailed clinical information, which allows for control of multiple confounding variables.”
Dr. Samelson reported having no financial disclosures. Coauthors Setareh Williams, PhD, and Rich Weiss, MD, are employees and shareholders of Radius Health Inc.
REPORTING FROM AACE 2019















