User login
Ixekizumab approved for plaque psoriasis
Ixekizumab is approved to treat moderate to severe plaque psoriasis in adults, according to an announcement from the Food and Drug Administration.
“Today’s approval provides patients suffering from plaque psoriasis with another important treatment option to help relieve the skin irritation and discomfort from the condition,” Dr. Julie Beitz, director of the FDA’s Office of Drug Evaluation III, said in a statement .
Ixekizumab is an IgG4 monoclonal antibody that selectively binds with interleukin 17A (IL-17A) cytokines, inhibiting interaction with the IL-17 receptor. It is approved for patients who are candidates for systemic therapy, phototherapy, or a combination of both.
The safety and efficacy of ixekizumab were established in three randomized, placebo-controlled clinical trials with a total of 3,866 participants with plaque psoriasis who were candidates for systemic therapy or phototherapy. Patients treated with ixekizumab achieved greater clinical response than did those who received placebo.
The therapy was approved with a medication guide to inform patients that they may have a greater risk of an infection, or an allergic or autoimmune condition, according to the FDA announcement. The agency advised that physicians should monitor patient for serious allergic reactions and development or worsening of inflammatory bowel disease.
The most common adverse events seen in clinical trials of ixekizumab were upper respiratory infections, injection site reactions, and tinea.
Ixekizumab will be marketed as Taltz by Eli Lilly and Company.
On Twitter @denisefulton
Ixekizumab is approved to treat moderate to severe plaque psoriasis in adults, according to an announcement from the Food and Drug Administration.
“Today’s approval provides patients suffering from plaque psoriasis with another important treatment option to help relieve the skin irritation and discomfort from the condition,” Dr. Julie Beitz, director of the FDA’s Office of Drug Evaluation III, said in a statement .
Ixekizumab is an IgG4 monoclonal antibody that selectively binds with interleukin 17A (IL-17A) cytokines, inhibiting interaction with the IL-17 receptor. It is approved for patients who are candidates for systemic therapy, phototherapy, or a combination of both.
The safety and efficacy of ixekizumab were established in three randomized, placebo-controlled clinical trials with a total of 3,866 participants with plaque psoriasis who were candidates for systemic therapy or phototherapy. Patients treated with ixekizumab achieved greater clinical response than did those who received placebo.
The therapy was approved with a medication guide to inform patients that they may have a greater risk of an infection, or an allergic or autoimmune condition, according to the FDA announcement. The agency advised that physicians should monitor patient for serious allergic reactions and development or worsening of inflammatory bowel disease.
The most common adverse events seen in clinical trials of ixekizumab were upper respiratory infections, injection site reactions, and tinea.
Ixekizumab will be marketed as Taltz by Eli Lilly and Company.
On Twitter @denisefulton
Ixekizumab is approved to treat moderate to severe plaque psoriasis in adults, according to an announcement from the Food and Drug Administration.
“Today’s approval provides patients suffering from plaque psoriasis with another important treatment option to help relieve the skin irritation and discomfort from the condition,” Dr. Julie Beitz, director of the FDA’s Office of Drug Evaluation III, said in a statement .
Ixekizumab is an IgG4 monoclonal antibody that selectively binds with interleukin 17A (IL-17A) cytokines, inhibiting interaction with the IL-17 receptor. It is approved for patients who are candidates for systemic therapy, phototherapy, or a combination of both.
The safety and efficacy of ixekizumab were established in three randomized, placebo-controlled clinical trials with a total of 3,866 participants with plaque psoriasis who were candidates for systemic therapy or phototherapy. Patients treated with ixekizumab achieved greater clinical response than did those who received placebo.
The therapy was approved with a medication guide to inform patients that they may have a greater risk of an infection, or an allergic or autoimmune condition, according to the FDA announcement. The agency advised that physicians should monitor patient for serious allergic reactions and development or worsening of inflammatory bowel disease.
The most common adverse events seen in clinical trials of ixekizumab were upper respiratory infections, injection site reactions, and tinea.
Ixekizumab will be marketed as Taltz by Eli Lilly and Company.
On Twitter @denisefulton
Atopic Dermatitis Treatments Moving Forward: Report From the AAD Meeting
Although psoriasis was once at the forefront of therapeutic advancements in dermatology, atopic dermatitis (AD) is now taking center stage with several new treatments in the pipeline. Dr. Emma Guttman-Yassky provides an overview of the future of AD treatment, which includes new topical and systemic agents that currently are moving forward in advanced clinical trials or are close to registration. She also discusses strategies for improving disease management in AD patients, noting that prevention and education of both patients and their caregivers are key to effective treatment.
Although psoriasis was once at the forefront of therapeutic advancements in dermatology, atopic dermatitis (AD) is now taking center stage with several new treatments in the pipeline. Dr. Emma Guttman-Yassky provides an overview of the future of AD treatment, which includes new topical and systemic agents that currently are moving forward in advanced clinical trials or are close to registration. She also discusses strategies for improving disease management in AD patients, noting that prevention and education of both patients and their caregivers are key to effective treatment.
Although psoriasis was once at the forefront of therapeutic advancements in dermatology, atopic dermatitis (AD) is now taking center stage with several new treatments in the pipeline. Dr. Emma Guttman-Yassky provides an overview of the future of AD treatment, which includes new topical and systemic agents that currently are moving forward in advanced clinical trials or are close to registration. She also discusses strategies for improving disease management in AD patients, noting that prevention and education of both patients and their caregivers are key to effective treatment.
Sparse, poor evidence supports fumarates for psoriasis
Even though fumaric acid esters are increasingly considered to be a suitable, even a first-line, systemic treatment for moderate to severe psoriasis in some parts of Europe, the evidence supporting their use is sparse and of low quality, according to a report published online in the British Journal of Dermatology.
Fumarates were introduced as anti-psoriasis agents decades ago in Germany. The agents are thought to exert immunomodulating, antiproliferative, and antiangiogenic effects, and they are frequently used off label for psoriasis in the Netherlands and the United Kingdom, said Dr. Deepak M.W. Balak of the department of dermatology, Erasmus University Medical Center, Rotterdam, the Netherlands, and his associates.
To summarize the clinical evidence for this treatment, the investigators performed a systematic review of publications, identifying 68 studies that reported the clinical effects of these agents in comparison with either placebo or other therapies. The researchers were unable to perform a meta-analysis of the data “due to considerable clinical heterogeneity among the studies” in design, patient populations, the drug formulations and dosages examined, the comparator treatments, and the outcomes measured.
Only seven randomized clinical trials were available for review. These had relatively small sample sizes and included only 449 patients in total. They assessed different drug formulations and different, short treatment durations ranging from 2.8 to 4 months. The overall quality of the evidence was rated “moderate.”
All randomized controlled trials reported statistically significant efficacy with fumaric acid ester treatment; mean Psoriasis Area Severity Index (PASI) scores decreased in 42%-65% of patients after 12-16 weeks of treatment. Adverse events were common, affecting 69%-92% of patients, and chiefly involved gastrointestinal complaints, flushing, and laboratory abnormalities such as elevated liver enzymes (up to 62%), eosinophilia (up to 46%), and lymphocytopenia (up to 38%). A total of 8%-39% of patients discontinued treatment because of adverse effects.
There also were 37 observational studies involving a total of 3,457 patients. Almost all were open-label, single-center, uncontrolled cohort studies or retrospective case series with small samples. Treatment duration ranged from 1 month to 14 years. The overall quality of the evidence was rated “very low” (Br J Dermatol. 2016. doi: 10.1111/bjd.14500).
These studies reported similar outcomes to the randomized clinical trials: significant reductions in the extent and severity of psoriasis with fumarate treatment, and frequent adverse effects, predominantly GI problems, flushing, and laboratory abnormalities. Mean reductions in PASI were 13%-86% after 3-4 months of treatment. Several immunosuppressive adverse effects were linked to the treatment, including Kaposi’s sarcoma, organizing pneumonia, tuberculous lymphadenitis, squamous cell carcinoma, melanoma, and seven cases of progressive multifocal leukoencephalopathy. In addition, several renal complications were reported, including six cases of Fanconi syndrome and nine cases of acute renal insufficiency, and there was one case of collagenous colitis.
“Fumaric acid esters have a long history as a systemic psoriasis treatment” dating back to the 1950s, “but their development was not based on high-quality evidence,” Dr. Balak and his associates said.
They added that several randomized clinical trials assessing these agents are currently underway, but their findings haven’t yet been published. And new fumarates for the treatment of psoriasis currently are in development.
No sponsor or funding source was identified for this study. Dr. Balak and his associates reported having no relevant financial disclosures.
Even though fumaric acid esters are increasingly considered to be a suitable, even a first-line, systemic treatment for moderate to severe psoriasis in some parts of Europe, the evidence supporting their use is sparse and of low quality, according to a report published online in the British Journal of Dermatology.
Fumarates were introduced as anti-psoriasis agents decades ago in Germany. The agents are thought to exert immunomodulating, antiproliferative, and antiangiogenic effects, and they are frequently used off label for psoriasis in the Netherlands and the United Kingdom, said Dr. Deepak M.W. Balak of the department of dermatology, Erasmus University Medical Center, Rotterdam, the Netherlands, and his associates.
To summarize the clinical evidence for this treatment, the investigators performed a systematic review of publications, identifying 68 studies that reported the clinical effects of these agents in comparison with either placebo or other therapies. The researchers were unable to perform a meta-analysis of the data “due to considerable clinical heterogeneity among the studies” in design, patient populations, the drug formulations and dosages examined, the comparator treatments, and the outcomes measured.
Only seven randomized clinical trials were available for review. These had relatively small sample sizes and included only 449 patients in total. They assessed different drug formulations and different, short treatment durations ranging from 2.8 to 4 months. The overall quality of the evidence was rated “moderate.”
All randomized controlled trials reported statistically significant efficacy with fumaric acid ester treatment; mean Psoriasis Area Severity Index (PASI) scores decreased in 42%-65% of patients after 12-16 weeks of treatment. Adverse events were common, affecting 69%-92% of patients, and chiefly involved gastrointestinal complaints, flushing, and laboratory abnormalities such as elevated liver enzymes (up to 62%), eosinophilia (up to 46%), and lymphocytopenia (up to 38%). A total of 8%-39% of patients discontinued treatment because of adverse effects.
There also were 37 observational studies involving a total of 3,457 patients. Almost all were open-label, single-center, uncontrolled cohort studies or retrospective case series with small samples. Treatment duration ranged from 1 month to 14 years. The overall quality of the evidence was rated “very low” (Br J Dermatol. 2016. doi: 10.1111/bjd.14500).
These studies reported similar outcomes to the randomized clinical trials: significant reductions in the extent and severity of psoriasis with fumarate treatment, and frequent adverse effects, predominantly GI problems, flushing, and laboratory abnormalities. Mean reductions in PASI were 13%-86% after 3-4 months of treatment. Several immunosuppressive adverse effects were linked to the treatment, including Kaposi’s sarcoma, organizing pneumonia, tuberculous lymphadenitis, squamous cell carcinoma, melanoma, and seven cases of progressive multifocal leukoencephalopathy. In addition, several renal complications were reported, including six cases of Fanconi syndrome and nine cases of acute renal insufficiency, and there was one case of collagenous colitis.
“Fumaric acid esters have a long history as a systemic psoriasis treatment” dating back to the 1950s, “but their development was not based on high-quality evidence,” Dr. Balak and his associates said.
They added that several randomized clinical trials assessing these agents are currently underway, but their findings haven’t yet been published. And new fumarates for the treatment of psoriasis currently are in development.
No sponsor or funding source was identified for this study. Dr. Balak and his associates reported having no relevant financial disclosures.
Even though fumaric acid esters are increasingly considered to be a suitable, even a first-line, systemic treatment for moderate to severe psoriasis in some parts of Europe, the evidence supporting their use is sparse and of low quality, according to a report published online in the British Journal of Dermatology.
Fumarates were introduced as anti-psoriasis agents decades ago in Germany. The agents are thought to exert immunomodulating, antiproliferative, and antiangiogenic effects, and they are frequently used off label for psoriasis in the Netherlands and the United Kingdom, said Dr. Deepak M.W. Balak of the department of dermatology, Erasmus University Medical Center, Rotterdam, the Netherlands, and his associates.
To summarize the clinical evidence for this treatment, the investigators performed a systematic review of publications, identifying 68 studies that reported the clinical effects of these agents in comparison with either placebo or other therapies. The researchers were unable to perform a meta-analysis of the data “due to considerable clinical heterogeneity among the studies” in design, patient populations, the drug formulations and dosages examined, the comparator treatments, and the outcomes measured.
Only seven randomized clinical trials were available for review. These had relatively small sample sizes and included only 449 patients in total. They assessed different drug formulations and different, short treatment durations ranging from 2.8 to 4 months. The overall quality of the evidence was rated “moderate.”
All randomized controlled trials reported statistically significant efficacy with fumaric acid ester treatment; mean Psoriasis Area Severity Index (PASI) scores decreased in 42%-65% of patients after 12-16 weeks of treatment. Adverse events were common, affecting 69%-92% of patients, and chiefly involved gastrointestinal complaints, flushing, and laboratory abnormalities such as elevated liver enzymes (up to 62%), eosinophilia (up to 46%), and lymphocytopenia (up to 38%). A total of 8%-39% of patients discontinued treatment because of adverse effects.
There also were 37 observational studies involving a total of 3,457 patients. Almost all were open-label, single-center, uncontrolled cohort studies or retrospective case series with small samples. Treatment duration ranged from 1 month to 14 years. The overall quality of the evidence was rated “very low” (Br J Dermatol. 2016. doi: 10.1111/bjd.14500).
These studies reported similar outcomes to the randomized clinical trials: significant reductions in the extent and severity of psoriasis with fumarate treatment, and frequent adverse effects, predominantly GI problems, flushing, and laboratory abnormalities. Mean reductions in PASI were 13%-86% after 3-4 months of treatment. Several immunosuppressive adverse effects were linked to the treatment, including Kaposi’s sarcoma, organizing pneumonia, tuberculous lymphadenitis, squamous cell carcinoma, melanoma, and seven cases of progressive multifocal leukoencephalopathy. In addition, several renal complications were reported, including six cases of Fanconi syndrome and nine cases of acute renal insufficiency, and there was one case of collagenous colitis.
“Fumaric acid esters have a long history as a systemic psoriasis treatment” dating back to the 1950s, “but their development was not based on high-quality evidence,” Dr. Balak and his associates said.
They added that several randomized clinical trials assessing these agents are currently underway, but their findings haven’t yet been published. And new fumarates for the treatment of psoriasis currently are in development.
No sponsor or funding source was identified for this study. Dr. Balak and his associates reported having no relevant financial disclosures.
FROM BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Only sparse, low-quality evidence supports using fumaric acid esters as a treatment for psoriasis.
Major finding: Mean PASI scores decreased 42%-65% in patients treated with fumaric acid esters for 12-16 weeks in randomized controlled trials, but adverse events affected 69%-92% of patients.
Data source: A systematic review of 7 randomized clinical trials and 37 observational studies, involving a total of 3,906 patients.
Disclosures: No sponsor or funding source was identified for this study. Dr. Balak and his associates reported having no relevant financial disclosures.
Fresh evidence of methotrexate efficacy in psoriatic arthritis
MAUI, HAWAII – The effectiveness of methotrexate in psoriatic arthritis is a matter of debate, but Dr. Arthur Kavanaugh is a believer based in part upon a recent subanalysis of the TICOPA study.
Moreover, his new 5-year follow-up analysis from the GO-REVEAL study of golimumab (Simponi) with or without concomitant methotrexate suggests that methotrexate plus the tumor necrosis factor inhibitor provided synergistic efficacy, he said at the 2016 Rheumatology Winter Clinical Symposium.
The 5-year analysis doesn’t provide definitive proof of synergistic benefit because it wasn’t designed or powered with that endpoint in mind (Arthritis Care Res. 2016;68[2]:267–74). No randomized trial completed to date has been. But the first-ever trial set up to test the synergistic efficacy hypothesis is underway. It’s a 52-week, double-blind, multicenter, randomized trial of etanercept (Enbrel) and methotrexate versus either alone in combination with placebo. And while the Amgen-sponsored study won’t be completed until 2018, Dr. Kavanaugh is ready to predict the outcome based in part upon the message contained in his GO-REVEAL findings.
“I’m placing my bet down now that there will be synergy for the X-ray outcome of change in SHS [Sharp/van der Heijde Score] for sure, and maybe for clinical efficacy as well, both joints and skin,” declared Dr. Kavanaugh, the conference director and professor of medicine at the University of California, San Diego.
He pointed to a new subanalysis of the Tight Control of Psoriatic Arthritis (TICOPA) study reported by rheumatologists at the University of Leeds (England) as evidence that methotrexate is effective in psoriatic arthritis. Of the 188 patients in the tight control arm who received methotrexate in the first 12 weeks of the trial, 41% had an ACR 20 response, meaning a 20% improvement in disease signs and symptoms at 12 weeks. A total of 19% had an ACR 50 response. And 27% had at least a 75% improvement in Psoriasis Area and Severity Index, or PASI 75. A 63% reduction in the proportion of patients with dactylitis and a 26% decrease in the proportion of patients with enthesitis was observed in the early methotrexate group. There was a suggestion of a dose-response effect, with better outcomes seen in the 108 participants who received a mean dose greater than 15 mg/week (J Rheumatol. 2016 Feb;43[2]:356-61).
This is a more impressive result than earlier reported from the Methotrexate In Psoriatic Arthritis (MIPA) trial, where the ACR 20 response rate was only 34% (Rheumatology [Oxford]. 2012;51[8]:1368-77). That may well be because methotrexate was given at only 15 mg/week in MIPA, in Dr. Kavanaugh’s view.
“I think methotrexate can work for the peripheral arthritis. This TICOPA analysis gives us a sense of the extent of the improvement, and also the extent of improvement in the skin,” the rheumatologist commented.
Turning to the week 256 results of GO-REVEAL, he said there was no difference in clinical response between psoriatic arthritis patients on golimumab alone or golimumab plus methotrexate at baseline. But among patients who were doing well clinically, with an assessment of minimal disease activity (MDA) on three or more consecutive clinic visits, only those on golimumab plus methotrexate at baseline showed radiologic improvement. The 57 patients on combination therapy who achieved MDA on at least three consecutive visits showed a mean 1.29-point improvement in SHS; the 48 rated as having MDA on four or more consecutive occasions similarly had a mean 1.24-point improvement.
In contrast, the 59 participants who achieved MDA on three or more consecutive visits but were on golimumab without methotrexate at baseline had a 0.25-point increase in SHS, and the 47 who had MDA on at least four consecutive visits had a 0.38-point SHS bump.
Dr. Kavanaugh reported having financial relationships with roughly a dozen pharmaceutical companies
MAUI, HAWAII – The effectiveness of methotrexate in psoriatic arthritis is a matter of debate, but Dr. Arthur Kavanaugh is a believer based in part upon a recent subanalysis of the TICOPA study.
Moreover, his new 5-year follow-up analysis from the GO-REVEAL study of golimumab (Simponi) with or without concomitant methotrexate suggests that methotrexate plus the tumor necrosis factor inhibitor provided synergistic efficacy, he said at the 2016 Rheumatology Winter Clinical Symposium.
The 5-year analysis doesn’t provide definitive proof of synergistic benefit because it wasn’t designed or powered with that endpoint in mind (Arthritis Care Res. 2016;68[2]:267–74). No randomized trial completed to date has been. But the first-ever trial set up to test the synergistic efficacy hypothesis is underway. It’s a 52-week, double-blind, multicenter, randomized trial of etanercept (Enbrel) and methotrexate versus either alone in combination with placebo. And while the Amgen-sponsored study won’t be completed until 2018, Dr. Kavanaugh is ready to predict the outcome based in part upon the message contained in his GO-REVEAL findings.
“I’m placing my bet down now that there will be synergy for the X-ray outcome of change in SHS [Sharp/van der Heijde Score] for sure, and maybe for clinical efficacy as well, both joints and skin,” declared Dr. Kavanaugh, the conference director and professor of medicine at the University of California, San Diego.
He pointed to a new subanalysis of the Tight Control of Psoriatic Arthritis (TICOPA) study reported by rheumatologists at the University of Leeds (England) as evidence that methotrexate is effective in psoriatic arthritis. Of the 188 patients in the tight control arm who received methotrexate in the first 12 weeks of the trial, 41% had an ACR 20 response, meaning a 20% improvement in disease signs and symptoms at 12 weeks. A total of 19% had an ACR 50 response. And 27% had at least a 75% improvement in Psoriasis Area and Severity Index, or PASI 75. A 63% reduction in the proportion of patients with dactylitis and a 26% decrease in the proportion of patients with enthesitis was observed in the early methotrexate group. There was a suggestion of a dose-response effect, with better outcomes seen in the 108 participants who received a mean dose greater than 15 mg/week (J Rheumatol. 2016 Feb;43[2]:356-61).
This is a more impressive result than earlier reported from the Methotrexate In Psoriatic Arthritis (MIPA) trial, where the ACR 20 response rate was only 34% (Rheumatology [Oxford]. 2012;51[8]:1368-77). That may well be because methotrexate was given at only 15 mg/week in MIPA, in Dr. Kavanaugh’s view.
“I think methotrexate can work for the peripheral arthritis. This TICOPA analysis gives us a sense of the extent of the improvement, and also the extent of improvement in the skin,” the rheumatologist commented.
Turning to the week 256 results of GO-REVEAL, he said there was no difference in clinical response between psoriatic arthritis patients on golimumab alone or golimumab plus methotrexate at baseline. But among patients who were doing well clinically, with an assessment of minimal disease activity (MDA) on three or more consecutive clinic visits, only those on golimumab plus methotrexate at baseline showed radiologic improvement. The 57 patients on combination therapy who achieved MDA on at least three consecutive visits showed a mean 1.29-point improvement in SHS; the 48 rated as having MDA on four or more consecutive occasions similarly had a mean 1.24-point improvement.
In contrast, the 59 participants who achieved MDA on three or more consecutive visits but were on golimumab without methotrexate at baseline had a 0.25-point increase in SHS, and the 47 who had MDA on at least four consecutive visits had a 0.38-point SHS bump.
Dr. Kavanaugh reported having financial relationships with roughly a dozen pharmaceutical companies
MAUI, HAWAII – The effectiveness of methotrexate in psoriatic arthritis is a matter of debate, but Dr. Arthur Kavanaugh is a believer based in part upon a recent subanalysis of the TICOPA study.
Moreover, his new 5-year follow-up analysis from the GO-REVEAL study of golimumab (Simponi) with or without concomitant methotrexate suggests that methotrexate plus the tumor necrosis factor inhibitor provided synergistic efficacy, he said at the 2016 Rheumatology Winter Clinical Symposium.
The 5-year analysis doesn’t provide definitive proof of synergistic benefit because it wasn’t designed or powered with that endpoint in mind (Arthritis Care Res. 2016;68[2]:267–74). No randomized trial completed to date has been. But the first-ever trial set up to test the synergistic efficacy hypothesis is underway. It’s a 52-week, double-blind, multicenter, randomized trial of etanercept (Enbrel) and methotrexate versus either alone in combination with placebo. And while the Amgen-sponsored study won’t be completed until 2018, Dr. Kavanaugh is ready to predict the outcome based in part upon the message contained in his GO-REVEAL findings.
“I’m placing my bet down now that there will be synergy for the X-ray outcome of change in SHS [Sharp/van der Heijde Score] for sure, and maybe for clinical efficacy as well, both joints and skin,” declared Dr. Kavanaugh, the conference director and professor of medicine at the University of California, San Diego.
He pointed to a new subanalysis of the Tight Control of Psoriatic Arthritis (TICOPA) study reported by rheumatologists at the University of Leeds (England) as evidence that methotrexate is effective in psoriatic arthritis. Of the 188 patients in the tight control arm who received methotrexate in the first 12 weeks of the trial, 41% had an ACR 20 response, meaning a 20% improvement in disease signs and symptoms at 12 weeks. A total of 19% had an ACR 50 response. And 27% had at least a 75% improvement in Psoriasis Area and Severity Index, or PASI 75. A 63% reduction in the proportion of patients with dactylitis and a 26% decrease in the proportion of patients with enthesitis was observed in the early methotrexate group. There was a suggestion of a dose-response effect, with better outcomes seen in the 108 participants who received a mean dose greater than 15 mg/week (J Rheumatol. 2016 Feb;43[2]:356-61).
This is a more impressive result than earlier reported from the Methotrexate In Psoriatic Arthritis (MIPA) trial, where the ACR 20 response rate was only 34% (Rheumatology [Oxford]. 2012;51[8]:1368-77). That may well be because methotrexate was given at only 15 mg/week in MIPA, in Dr. Kavanaugh’s view.
“I think methotrexate can work for the peripheral arthritis. This TICOPA analysis gives us a sense of the extent of the improvement, and also the extent of improvement in the skin,” the rheumatologist commented.
Turning to the week 256 results of GO-REVEAL, he said there was no difference in clinical response between psoriatic arthritis patients on golimumab alone or golimumab plus methotrexate at baseline. But among patients who were doing well clinically, with an assessment of minimal disease activity (MDA) on three or more consecutive clinic visits, only those on golimumab plus methotrexate at baseline showed radiologic improvement. The 57 patients on combination therapy who achieved MDA on at least three consecutive visits showed a mean 1.29-point improvement in SHS; the 48 rated as having MDA on four or more consecutive occasions similarly had a mean 1.24-point improvement.
In contrast, the 59 participants who achieved MDA on three or more consecutive visits but were on golimumab without methotrexate at baseline had a 0.25-point increase in SHS, and the 47 who had MDA on at least four consecutive visits had a 0.38-point SHS bump.
Dr. Kavanaugh reported having financial relationships with roughly a dozen pharmaceutical companies
EXPERT ANALYSIS FROM RWCS 2016
How to Manage Psoriasis Safely in Pregnant Women
Pregnant women should avoid biologics and other systemic medications for psoriasis management, according to Jenny Eileen Murase, MD, Assistant Clinical Professor of Dermatology, University of California, San Francisco, at the 74th Annual Meeting of the American Academy of Dermatology (March 4-8, 2016) in Washington, DC. Patients should opt to use topical treatments such as moisturizers, emollients, and low- to moderate-dose corticosteroids.
Some women may experience improvement of their psoriasis during pregnancy because of an autoimmune system shift, often to the point of not needing treatment to manage the condition. “About half of pregnant women experience a dramatic improvement that may allow them to temporarily discontinue treatment,” Dr. Murase explained.
If additional therapy is needed, phototherapy may be utilized. Narrowband UVB is the best option for pregnant women but broadband UVB may be considered. Psoralen plus UVA should be avoided, as psoralen may enter breast milk and lead to light sensitivity in babies.
If the treatment regimen for the condition is discontinued or altered during pregnancy, Dr. Murase recommends that patients restart their prepregnancy regimen as soon as possible after giving birth, as the condition may flare postpregnancy.
In a recent installment of “Practical Pearls From the Cutis Board,” Jeffrey M. Weinberg, MD, discussed first-line treatments for psoriasis in pregnant women. “Pregnant patients need to know that it is important to carefully monitor them throughout their pregnancy,” he stated. “Although many drugs are not contraindicated, it is still important for the dermatologist to consult with the patient’s obstetrician to discuss risks and benefits of different therapies.”
He also indicated that if a pregnant woman does wish to continue biologic therapy, close monitoring and enrollment in a pregnancy registry (http://www.pregnancystudies.org) would be good options. “This registry is analyzing whether medications that are used to treat autoimmune diseases are safe to take during pregnancy.”
Dermatologists need to see these patients regularly to keep the dialogue ongoing and to monitor their condition.
Pregnant women should avoid biologics and other systemic medications for psoriasis management, according to Jenny Eileen Murase, MD, Assistant Clinical Professor of Dermatology, University of California, San Francisco, at the 74th Annual Meeting of the American Academy of Dermatology (March 4-8, 2016) in Washington, DC. Patients should opt to use topical treatments such as moisturizers, emollients, and low- to moderate-dose corticosteroids.
Some women may experience improvement of their psoriasis during pregnancy because of an autoimmune system shift, often to the point of not needing treatment to manage the condition. “About half of pregnant women experience a dramatic improvement that may allow them to temporarily discontinue treatment,” Dr. Murase explained.
If additional therapy is needed, phototherapy may be utilized. Narrowband UVB is the best option for pregnant women but broadband UVB may be considered. Psoralen plus UVA should be avoided, as psoralen may enter breast milk and lead to light sensitivity in babies.
If the treatment regimen for the condition is discontinued or altered during pregnancy, Dr. Murase recommends that patients restart their prepregnancy regimen as soon as possible after giving birth, as the condition may flare postpregnancy.
In a recent installment of “Practical Pearls From the Cutis Board,” Jeffrey M. Weinberg, MD, discussed first-line treatments for psoriasis in pregnant women. “Pregnant patients need to know that it is important to carefully monitor them throughout their pregnancy,” he stated. “Although many drugs are not contraindicated, it is still important for the dermatologist to consult with the patient’s obstetrician to discuss risks and benefits of different therapies.”
He also indicated that if a pregnant woman does wish to continue biologic therapy, close monitoring and enrollment in a pregnancy registry (http://www.pregnancystudies.org) would be good options. “This registry is analyzing whether medications that are used to treat autoimmune diseases are safe to take during pregnancy.”
Dermatologists need to see these patients regularly to keep the dialogue ongoing and to monitor their condition.
Pregnant women should avoid biologics and other systemic medications for psoriasis management, according to Jenny Eileen Murase, MD, Assistant Clinical Professor of Dermatology, University of California, San Francisco, at the 74th Annual Meeting of the American Academy of Dermatology (March 4-8, 2016) in Washington, DC. Patients should opt to use topical treatments such as moisturizers, emollients, and low- to moderate-dose corticosteroids.
Some women may experience improvement of their psoriasis during pregnancy because of an autoimmune system shift, often to the point of not needing treatment to manage the condition. “About half of pregnant women experience a dramatic improvement that may allow them to temporarily discontinue treatment,” Dr. Murase explained.
If additional therapy is needed, phototherapy may be utilized. Narrowband UVB is the best option for pregnant women but broadband UVB may be considered. Psoralen plus UVA should be avoided, as psoralen may enter breast milk and lead to light sensitivity in babies.
If the treatment regimen for the condition is discontinued or altered during pregnancy, Dr. Murase recommends that patients restart their prepregnancy regimen as soon as possible after giving birth, as the condition may flare postpregnancy.
In a recent installment of “Practical Pearls From the Cutis Board,” Jeffrey M. Weinberg, MD, discussed first-line treatments for psoriasis in pregnant women. “Pregnant patients need to know that it is important to carefully monitor them throughout their pregnancy,” he stated. “Although many drugs are not contraindicated, it is still important for the dermatologist to consult with the patient’s obstetrician to discuss risks and benefits of different therapies.”
He also indicated that if a pregnant woman does wish to continue biologic therapy, close monitoring and enrollment in a pregnancy registry (http://www.pregnancystudies.org) would be good options. “This registry is analyzing whether medications that are used to treat autoimmune diseases are safe to take during pregnancy.”
Dermatologists need to see these patients regularly to keep the dialogue ongoing and to monitor their condition.
Don’t overlook topical tazarotene for psoriasis
WAIKOLOA, HAWAII – Tazarotene remains an important and effective albeit greatly underutilized topical therapy in psoriasis – but it’s on its way to becoming an even better drug, Dr. Linda Stein Gold said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The topical retinoid has been formulated together with the superpotent steroid halobetasol propionate 0.01% in an investigational fixed combination lotion known for now as IDP-118. This medication, under development by Valeant, is in an ongoing, phase III multicenter, double-blind randomized trial with the vehicle lotion serving as control in adults with moderate to severe plaque psoriasis. A year-long, open-label, phase III safety study is also in progress, according to Dr. Stein Gold of Henry Ford Hospital in Detroit.
Tazarotene (Tazorac) is approved by the Food and Drug Administration as a 0.05% and 0.1% cream or gel for psoriasis and in the 0.1% cream or gel for acne. But when Dr. Stein Gold asked her large Hawaii audience for a show of hands as to who is prescribing tazarotene for their psoriasis patients, not a hand went up.
“Tazarotene carries some baggage,” she observed. “It’s pregnancy category X, and it also is quite irritating. If you use tazarotene on psoriatic skin, you’ll get a lot of irritation. But if you do so in combination with a potent or superpotent topical steroid, you’re not only able to increase the efficacy, but you also minimize the tolerability issues.
These dual benefits are the result of the two treatments’ differing mechanisms of action. This has been known for a long time. Indeed, it was demonstrated in a randomized trial nearly 2 decades ago (J Am Acad Dermatol. 1998 Oct;39[4 Pt 2]:S139-43). But only with the recent appreciation that 80% of psoriasis patients treat their disease exclusively with topical therapies has a pharmaceutical company moved to take advantage of these synergistic effects.
Dr. Stein Gold, director of dermatology research at Detroit’s Henry Ford Health System, said that the pharmaceutical industry has finally noted the considerable unmet need for additional topical psoriasis therapies that will be more effective and/or cosmetically elegant or have a novel mechanism of action. A slew of novel topical agents are now in the developmental pipeline in phase II studies for psoriasis. Among these new molecules are a topical formulation of methotrexate in a proprietary vehicle; the Janus kinase (JAK) 1 and 2 inhibitor ruxolitinib (Jakafi) in a cream for treatment of both psoriasis and atopic dermatitis; a tyrosine kinase inhibitor cream and ointment; an integrin inhibitor cream, and a phosphodiesterase-4 inhibitor ointment.
In addition, in October 2015, the FDA approved an aerosol foam fixed combination of calcipotriene 0.005% and betamethasone dipropionate 0.064% (Enstilar) for psoriasis. It’s more effective and cosmetically elegant than the fixed-combination ointment, she noted.
“Topical therapy is still going strong. I think there is always going to be a need for topical psoriasis therapies,” the dermatologist declared.
She reported serving as a consultant to and/or scientific advisory board member for numerous pharmaceutical companies.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Tazarotene remains an important and effective albeit greatly underutilized topical therapy in psoriasis – but it’s on its way to becoming an even better drug, Dr. Linda Stein Gold said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The topical retinoid has been formulated together with the superpotent steroid halobetasol propionate 0.01% in an investigational fixed combination lotion known for now as IDP-118. This medication, under development by Valeant, is in an ongoing, phase III multicenter, double-blind randomized trial with the vehicle lotion serving as control in adults with moderate to severe plaque psoriasis. A year-long, open-label, phase III safety study is also in progress, according to Dr. Stein Gold of Henry Ford Hospital in Detroit.
Tazarotene (Tazorac) is approved by the Food and Drug Administration as a 0.05% and 0.1% cream or gel for psoriasis and in the 0.1% cream or gel for acne. But when Dr. Stein Gold asked her large Hawaii audience for a show of hands as to who is prescribing tazarotene for their psoriasis patients, not a hand went up.
“Tazarotene carries some baggage,” she observed. “It’s pregnancy category X, and it also is quite irritating. If you use tazarotene on psoriatic skin, you’ll get a lot of irritation. But if you do so in combination with a potent or superpotent topical steroid, you’re not only able to increase the efficacy, but you also minimize the tolerability issues.
These dual benefits are the result of the two treatments’ differing mechanisms of action. This has been known for a long time. Indeed, it was demonstrated in a randomized trial nearly 2 decades ago (J Am Acad Dermatol. 1998 Oct;39[4 Pt 2]:S139-43). But only with the recent appreciation that 80% of psoriasis patients treat their disease exclusively with topical therapies has a pharmaceutical company moved to take advantage of these synergistic effects.
Dr. Stein Gold, director of dermatology research at Detroit’s Henry Ford Health System, said that the pharmaceutical industry has finally noted the considerable unmet need for additional topical psoriasis therapies that will be more effective and/or cosmetically elegant or have a novel mechanism of action. A slew of novel topical agents are now in the developmental pipeline in phase II studies for psoriasis. Among these new molecules are a topical formulation of methotrexate in a proprietary vehicle; the Janus kinase (JAK) 1 and 2 inhibitor ruxolitinib (Jakafi) in a cream for treatment of both psoriasis and atopic dermatitis; a tyrosine kinase inhibitor cream and ointment; an integrin inhibitor cream, and a phosphodiesterase-4 inhibitor ointment.
In addition, in October 2015, the FDA approved an aerosol foam fixed combination of calcipotriene 0.005% and betamethasone dipropionate 0.064% (Enstilar) for psoriasis. It’s more effective and cosmetically elegant than the fixed-combination ointment, she noted.
“Topical therapy is still going strong. I think there is always going to be a need for topical psoriasis therapies,” the dermatologist declared.
She reported serving as a consultant to and/or scientific advisory board member for numerous pharmaceutical companies.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Tazarotene remains an important and effective albeit greatly underutilized topical therapy in psoriasis – but it’s on its way to becoming an even better drug, Dr. Linda Stein Gold said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The topical retinoid has been formulated together with the superpotent steroid halobetasol propionate 0.01% in an investigational fixed combination lotion known for now as IDP-118. This medication, under development by Valeant, is in an ongoing, phase III multicenter, double-blind randomized trial with the vehicle lotion serving as control in adults with moderate to severe plaque psoriasis. A year-long, open-label, phase III safety study is also in progress, according to Dr. Stein Gold of Henry Ford Hospital in Detroit.
Tazarotene (Tazorac) is approved by the Food and Drug Administration as a 0.05% and 0.1% cream or gel for psoriasis and in the 0.1% cream or gel for acne. But when Dr. Stein Gold asked her large Hawaii audience for a show of hands as to who is prescribing tazarotene for their psoriasis patients, not a hand went up.
“Tazarotene carries some baggage,” she observed. “It’s pregnancy category X, and it also is quite irritating. If you use tazarotene on psoriatic skin, you’ll get a lot of irritation. But if you do so in combination with a potent or superpotent topical steroid, you’re not only able to increase the efficacy, but you also minimize the tolerability issues.
These dual benefits are the result of the two treatments’ differing mechanisms of action. This has been known for a long time. Indeed, it was demonstrated in a randomized trial nearly 2 decades ago (J Am Acad Dermatol. 1998 Oct;39[4 Pt 2]:S139-43). But only with the recent appreciation that 80% of psoriasis patients treat their disease exclusively with topical therapies has a pharmaceutical company moved to take advantage of these synergistic effects.
Dr. Stein Gold, director of dermatology research at Detroit’s Henry Ford Health System, said that the pharmaceutical industry has finally noted the considerable unmet need for additional topical psoriasis therapies that will be more effective and/or cosmetically elegant or have a novel mechanism of action. A slew of novel topical agents are now in the developmental pipeline in phase II studies for psoriasis. Among these new molecules are a topical formulation of methotrexate in a proprietary vehicle; the Janus kinase (JAK) 1 and 2 inhibitor ruxolitinib (Jakafi) in a cream for treatment of both psoriasis and atopic dermatitis; a tyrosine kinase inhibitor cream and ointment; an integrin inhibitor cream, and a phosphodiesterase-4 inhibitor ointment.
In addition, in October 2015, the FDA approved an aerosol foam fixed combination of calcipotriene 0.005% and betamethasone dipropionate 0.064% (Enstilar) for psoriasis. It’s more effective and cosmetically elegant than the fixed-combination ointment, she noted.
“Topical therapy is still going strong. I think there is always going to be a need for topical psoriasis therapies,” the dermatologist declared.
She reported serving as a consultant to and/or scientific advisory board member for numerous pharmaceutical companies.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
60 weeks of ixekizumab effective, well tolerated in moderate-to-severe plaque psoriasis
WASHINGTON – After 60 weeks of continuous therapy with the investigational drug ixekizumab, 722 patients who initially had moderate to severe plaque psoriasis had Psoriasis Area Severity Index (PASI) 75, 90, and 100 response rates of 87%, 78%, and 57%, respectively, based on study results presented by Dr. Andrew Blauvelt at the annual meeting of the American Academy of Dermatology.
Additionally, the rate of complete clearance or mild severity of plaque psoriasis based on the Physician Global Assessment measure was 79% in the study patients. Dr. Blauvelt said “these were tremendous results over time.”
The findings come from the open-label continuation study of ixekizumab in UNCOVER-3. At week 12 of that study, 722 ixekizumab-treated patients were continued on or converted to a regimen of 80 mg ixekizumab every 4 weeks.
In UNCOVER-3, all study participants initially received induction therapy with ixekizumab and then were randomized to placebo (193 patients), etanercept 50 mg twice weekly (382 patients), 80 mg ixekizumab every 4 weeks (386 patients) after a 160 mg starting dose, or 80 mg ixekizumab every 2 weeks (385 patients) after an initial 160 mg starting dose. After 12 weeks, patients entered open-label treatment with the study drug once every 4 weeks.
The long-term safety and tolerability profile was similar to the one seen during the induction period and cited in previously published data. Dr. Blauvelt, President of the Oregon Medical Research Center, said the finding was “good news, in that there were no unexpected safety signals [not already] seen with initial therapy.”
Ixekizumab is an IgG4 monoclonal antibody that selectively binds with interleukin 17A (IL-17A) cytokines, inhibiting interaction with the IL-17 receptor.
According to a spokesperson from Eli Lilly, sponsor of the UNCOVER trial and maker of ixekizumab, a decision from the U.S. Food and Drug Administration on whether to approve ixekizumab for use in psoriasis is expected during the first quarter of 2016.
Dr. Blauvelt is an advisor or consultant for and has received research grants from a wide variety of drug companies including Eli Lilly.
On Twitter @whitneymcknight
WASHINGTON – After 60 weeks of continuous therapy with the investigational drug ixekizumab, 722 patients who initially had moderate to severe plaque psoriasis had Psoriasis Area Severity Index (PASI) 75, 90, and 100 response rates of 87%, 78%, and 57%, respectively, based on study results presented by Dr. Andrew Blauvelt at the annual meeting of the American Academy of Dermatology.
Additionally, the rate of complete clearance or mild severity of plaque psoriasis based on the Physician Global Assessment measure was 79% in the study patients. Dr. Blauvelt said “these were tremendous results over time.”
The findings come from the open-label continuation study of ixekizumab in UNCOVER-3. At week 12 of that study, 722 ixekizumab-treated patients were continued on or converted to a regimen of 80 mg ixekizumab every 4 weeks.
In UNCOVER-3, all study participants initially received induction therapy with ixekizumab and then were randomized to placebo (193 patients), etanercept 50 mg twice weekly (382 patients), 80 mg ixekizumab every 4 weeks (386 patients) after a 160 mg starting dose, or 80 mg ixekizumab every 2 weeks (385 patients) after an initial 160 mg starting dose. After 12 weeks, patients entered open-label treatment with the study drug once every 4 weeks.
The long-term safety and tolerability profile was similar to the one seen during the induction period and cited in previously published data. Dr. Blauvelt, President of the Oregon Medical Research Center, said the finding was “good news, in that there were no unexpected safety signals [not already] seen with initial therapy.”
Ixekizumab is an IgG4 monoclonal antibody that selectively binds with interleukin 17A (IL-17A) cytokines, inhibiting interaction with the IL-17 receptor.
According to a spokesperson from Eli Lilly, sponsor of the UNCOVER trial and maker of ixekizumab, a decision from the U.S. Food and Drug Administration on whether to approve ixekizumab for use in psoriasis is expected during the first quarter of 2016.
Dr. Blauvelt is an advisor or consultant for and has received research grants from a wide variety of drug companies including Eli Lilly.
On Twitter @whitneymcknight
WASHINGTON – After 60 weeks of continuous therapy with the investigational drug ixekizumab, 722 patients who initially had moderate to severe plaque psoriasis had Psoriasis Area Severity Index (PASI) 75, 90, and 100 response rates of 87%, 78%, and 57%, respectively, based on study results presented by Dr. Andrew Blauvelt at the annual meeting of the American Academy of Dermatology.
Additionally, the rate of complete clearance or mild severity of plaque psoriasis based on the Physician Global Assessment measure was 79% in the study patients. Dr. Blauvelt said “these were tremendous results over time.”
The findings come from the open-label continuation study of ixekizumab in UNCOVER-3. At week 12 of that study, 722 ixekizumab-treated patients were continued on or converted to a regimen of 80 mg ixekizumab every 4 weeks.
In UNCOVER-3, all study participants initially received induction therapy with ixekizumab and then were randomized to placebo (193 patients), etanercept 50 mg twice weekly (382 patients), 80 mg ixekizumab every 4 weeks (386 patients) after a 160 mg starting dose, or 80 mg ixekizumab every 2 weeks (385 patients) after an initial 160 mg starting dose. After 12 weeks, patients entered open-label treatment with the study drug once every 4 weeks.
The long-term safety and tolerability profile was similar to the one seen during the induction period and cited in previously published data. Dr. Blauvelt, President of the Oregon Medical Research Center, said the finding was “good news, in that there were no unexpected safety signals [not already] seen with initial therapy.”
Ixekizumab is an IgG4 monoclonal antibody that selectively binds with interleukin 17A (IL-17A) cytokines, inhibiting interaction with the IL-17 receptor.
According to a spokesperson from Eli Lilly, sponsor of the UNCOVER trial and maker of ixekizumab, a decision from the U.S. Food and Drug Administration on whether to approve ixekizumab for use in psoriasis is expected during the first quarter of 2016.
Dr. Blauvelt is an advisor or consultant for and has received research grants from a wide variety of drug companies including Eli Lilly.
On Twitter @whitneymcknight
AT AAD 2016
Key clinical point: Continuous therapy with the investigational drug ixekizumab appears to be safe and effective for long-term use.
Major finding: At Week 60, the PASI 75, 90, and 100 response rates were 87%, 78%, and 57%, respectively.
Data source: Phase 3 UNCOVER-3 trial of 1,346 patients randomized to placebo, active control, or ixekizumab at every 4 weeks or every 2 weeks after induction.
Disclosures: This trial was sponsored by Eli Lilly, maker of ixekizumab. Dr. Blauvelt is an advisor or consultant for and has received research grants from a wide variety of drug companies including Eli Lilly.
Minimal disease activity criteria in PsA fall short
The minimal disease activity criteria used to identify low disease activity in psoriatic arthritis (PsA) may not be useful as a treatment target in patients with extended skin involvement, according to the results of a small study.
Minimal disease activity (MDA) for PsA is a composite measure encompassing clinically important aspects of the disease: arthritis, psoriasis, enthesitis, pain, patient-assessed global disease activity, and physical function and provides an objective target for therapy in clinical trials, Dr. Josefina Marin of the Hospital Italiano de Buenos Aires and her associates wrote in the Journal of Rheumatology.
To evaluate components of the MDA criteria, the research team enrolled 83 consecutive patients with PsA aged over 18 years who were attending their clinic. Patients needed to fulfill five out of the seven criteria to be classified as having MDA.
An assessment by a single experienced rheumatologist revealed that 41 patients met MDA criteria, with only 7.4% of these patients not satisfying the tender/swollen joint–count criteria.
However, only 51% (n = 21) of patients fulfilling MDA fulfilled the skin criteria (Psoriasis Area and Severity Index [PASI] and body surface area [BSA]), a percentage not statistically different from the 36% of patients not in MDA (n = 15; P = .154). “This is important because MDA may therefore not be useful as a treatment target in patients with extended skin involvement,” the investigators wrote (J Rheum. 2016 Mar 1. doi: 10.3899/jrheum.151101).
The investigators noted that the selection of the target was of crucial importance when implementing a treat-to-target and tight control strategy. “In this sense our study provides reassurance that almost all patients at MDA will have no swollen/tender joints but also raises some concerns that the skin might not be well controlled if the MDA is used as the only target to treat patients with both joint and skin involvement,” they wrote. One explanation could be that the cutoff values selected in the MDA criteria for skin involvement are too stringent for therapies currently available, they suggested.
MDA seems to be a valuable tool to define low disease activity in patients with PsA, but the skin component requires further evaluation, they concluded.
The research was partially supported by the PANLAR/Abbvie Rheumatology prize 2012.
The minimal disease activity criteria used to identify low disease activity in psoriatic arthritis (PsA) may not be useful as a treatment target in patients with extended skin involvement, according to the results of a small study.
Minimal disease activity (MDA) for PsA is a composite measure encompassing clinically important aspects of the disease: arthritis, psoriasis, enthesitis, pain, patient-assessed global disease activity, and physical function and provides an objective target for therapy in clinical trials, Dr. Josefina Marin of the Hospital Italiano de Buenos Aires and her associates wrote in the Journal of Rheumatology.
To evaluate components of the MDA criteria, the research team enrolled 83 consecutive patients with PsA aged over 18 years who were attending their clinic. Patients needed to fulfill five out of the seven criteria to be classified as having MDA.
An assessment by a single experienced rheumatologist revealed that 41 patients met MDA criteria, with only 7.4% of these patients not satisfying the tender/swollen joint–count criteria.
However, only 51% (n = 21) of patients fulfilling MDA fulfilled the skin criteria (Psoriasis Area and Severity Index [PASI] and body surface area [BSA]), a percentage not statistically different from the 36% of patients not in MDA (n = 15; P = .154). “This is important because MDA may therefore not be useful as a treatment target in patients with extended skin involvement,” the investigators wrote (J Rheum. 2016 Mar 1. doi: 10.3899/jrheum.151101).
The investigators noted that the selection of the target was of crucial importance when implementing a treat-to-target and tight control strategy. “In this sense our study provides reassurance that almost all patients at MDA will have no swollen/tender joints but also raises some concerns that the skin might not be well controlled if the MDA is used as the only target to treat patients with both joint and skin involvement,” they wrote. One explanation could be that the cutoff values selected in the MDA criteria for skin involvement are too stringent for therapies currently available, they suggested.
MDA seems to be a valuable tool to define low disease activity in patients with PsA, but the skin component requires further evaluation, they concluded.
The research was partially supported by the PANLAR/Abbvie Rheumatology prize 2012.
The minimal disease activity criteria used to identify low disease activity in psoriatic arthritis (PsA) may not be useful as a treatment target in patients with extended skin involvement, according to the results of a small study.
Minimal disease activity (MDA) for PsA is a composite measure encompassing clinically important aspects of the disease: arthritis, psoriasis, enthesitis, pain, patient-assessed global disease activity, and physical function and provides an objective target for therapy in clinical trials, Dr. Josefina Marin of the Hospital Italiano de Buenos Aires and her associates wrote in the Journal of Rheumatology.
To evaluate components of the MDA criteria, the research team enrolled 83 consecutive patients with PsA aged over 18 years who were attending their clinic. Patients needed to fulfill five out of the seven criteria to be classified as having MDA.
An assessment by a single experienced rheumatologist revealed that 41 patients met MDA criteria, with only 7.4% of these patients not satisfying the tender/swollen joint–count criteria.
However, only 51% (n = 21) of patients fulfilling MDA fulfilled the skin criteria (Psoriasis Area and Severity Index [PASI] and body surface area [BSA]), a percentage not statistically different from the 36% of patients not in MDA (n = 15; P = .154). “This is important because MDA may therefore not be useful as a treatment target in patients with extended skin involvement,” the investigators wrote (J Rheum. 2016 Mar 1. doi: 10.3899/jrheum.151101).
The investigators noted that the selection of the target was of crucial importance when implementing a treat-to-target and tight control strategy. “In this sense our study provides reassurance that almost all patients at MDA will have no swollen/tender joints but also raises some concerns that the skin might not be well controlled if the MDA is used as the only target to treat patients with both joint and skin involvement,” they wrote. One explanation could be that the cutoff values selected in the MDA criteria for skin involvement are too stringent for therapies currently available, they suggested.
MDA seems to be a valuable tool to define low disease activity in patients with PsA, but the skin component requires further evaluation, they concluded.
The research was partially supported by the PANLAR/Abbvie Rheumatology prize 2012.
FROM THE JOURNAL OF RHEUMATOLOGY
Key clinical point: Minimal disease activity criteria in PsA may not be stringent enough for people with extended skin involvement.
Major finding: Only half of the PsA patients classified as having minimal disease activity met PASI and body surface area criteria.
Data source: A single-center study of 83 consecutive PsA patients aged over 18 years.
Disclosures: The research was partially supported by the PANLAR/Abbvie Rheumatology prize 2012.
Effects of Tumor Necrosis Factor α Inhibitors Extend Beyond Psoriasis: Insulin Sensitivity in Psoriasis Patients With Type 2 Diabetes Mellitus
Psoriasis is a chronic inflammatory disorder associated with increased expression of proinflammatory mediators such as tumor necrosis factor (TNF) α.1 Anti-TNF drugs (eg, etanercept, adalimumab, infliximab) were proven to be highly effective for the treatment of psoriasis over the last 2 decades.2 Interestingly, TNF inhibitors have been thought to be effective in improving insulin resistance in patients with type 2 diabetes mellitus (DM) by blocking TNF, which is involved in the inflammatory condition in DM.
Type 2 DM is a common chronic condition characterized by hyperglycemia resulting from a combination of peripheral and hepatic insulin resistance and impaired insulin secretion.3 It is characterized by defects in both insulin secretion and insulin sensitivity.4,5 Type 2 DM has been linked with a marked increase in cardiovascular disease, morbidity, and mortality.6 Evidence-based literature regarding the role of chronic inflammation as an important pathogenetic factor in type 2 DM has been growing.7-9 It also has been suggested that pharmacological strategies to reduce this underlying associated silent inflammation are useful in treating DM, which also is true for other conditions such as obesity, metabolic syndrome, and cardiovascular diseases.10
Psoriasis predisposes patients to insulin resistance and may put them at risk for developing DM.11,12 The association between psoriasis and DM suggests that systemic immunosuppression also may diminish the risk for developing DM. Several longitudinal studies have found that TNF inhibitors improve insulin resistance.13,14 Dandona et al15 reported a considerable decrease of TNF-α levels with the concurrent restoration of insulin sensitivity during weight loss.
Pereira et al16 found a notable connection between psoriasis, DM, and insulin resistance with an odds ratio of 2.63 of abnormal glucose homeostasis in patients with psoriasis compared to controls. Yazdani-Biuki et al17 proved that extended administration of anti–TNF-α antibody was able to improve insulin sensitivity in insulin-resistant patients. The same finding was established by Kiortsis et al.14
In this prospective controlled study, we evaluated the effects of anti-TNF agents on insulin resistance and sensitivity in psoriasis patients with type 2 DM treated with anti-TNF agents.
Methods
A total of 70 patients attending the dermatological outpatient clinics at Farwaniya Hospital (Kuwait City, Kuwait) between January 2012 and September 2014 were enrolled in the study and were randomly distributed into 2 equal groups (n=35 each). The study was approved by the hospital ethics committee. Patients were included in the study if they had moderate to severe psoriasis (ie, psoriasis area severity index score ≥10) with documented type 2 DM and high fasting plasma glucose (FPG) levels (ie, >10 mmol/L). Patients who were currently being treated with oral hypoglycemic agents but not insulin therapy were included in the study. Patients were excluded if they had phototherapy within the last 4 weeks, prior biologic therapy, current and prior insulin therapy, a change in oral hypoglycemic drug dosage in the last 2 months, other serious systemic illness (eg, malignancy, hepatitis B or C virus, metabolic or endocrine disease), and/or abnormal laboratory investigations (eg, liver/kidney profile, chest radiograph abnormality, positive Mantoux test). All of the patients enrolled in the study provided informed consent and underwent routine baseline investigations including complete blood cell counts, general health profile, chest radiograph, antinuclear antibody test, Mantoux test, FPG and insulin levels, glycated hemoglobin (HbA1C), homeostasis model assessment (HOMA), routine urine examination, and enzyme-linked immunosorbent assay for tuberculosis. The study group was treated with anti-TNF agents, and the control group received conventional antipsoriatic medications.
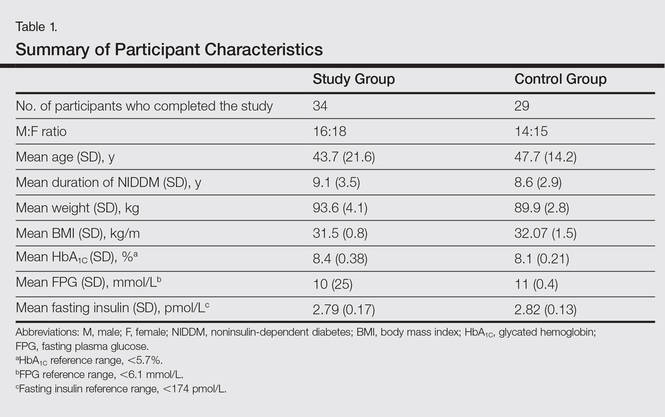
All the patients included in the study had high FPG levels (ie, >10 mmol/L) at the time of enrollment and were currently being treated with oral hypoglycemic agents. The dose of oral hypoglycemic agents was unchanged for at least 2 months before entry into the run-in period and throughout the 24-week study period. Demographic details including sex, age, medical history (eg, type of psoriasis, prior and concomitant treatments) were collected from the participants’ clinical histories. Participants from both groups were appropriately matched in terms of age, sex, body weight, body mass index, and duration of type 2 DM (Table 1). The primary end point of the study was to analyze and compare clinical and serum data collected at baseline and after 24 weeks of therapy.
A complete biochemical profile was repeated in both groups after 24 weeks of treatment. Each participant underwent a baseline short insulin sensitivity test immediately before treatment and at 4 and 24 weeks of treatment. We assessed insulin resistance via HOMA, calculated as follows: FPG [mmol/L] × fasting serum insulin [pmol/L] / 22.5.18 Oral glucose tolerance tests were performed to calculate the HOMA of insulin resistance. Serum insulin concentration was determined via enzyme-linked immunosorbent assay.
Statistical analysis was performed using SPSS software (version 12.0). Continuous patient characteristics were analyzed using mean and SD as well as discrete data as counts and proportions. Association was examined using χ2 tests for categorical variables and 2-sided t test/Wilcoxon rank sum test for continuous variables. Analysis of variance was used to compare the results in 3 different anti-TNF agents used in the study group.
Results
Of the 35 participants enrolled in the study group, 34 (97.1%) completed the study and were evaluated. The study group included 16 men and 18 women aged 19 to 63 years (mean age [SD], 43.7 [21.6] years) who were treated with TNF-α inhibitors—8 participants with etanercept, 14 with adalimumab, and 12 with infliximab—according to the standard dosage schedule for 24 weeks.
Of the 35 participants enrolled in the control group, 29 (82.9%) completed the study and were evaluated. Six patients did not follow up for the complete duration of the 24-week study period and were not evaluated. The control group included 14 men and 15 women aged 18 to 65 years (mean age [SD], 47.7 [14.2] years) who were treated with other systemic therapies—8 participants with topical corticosteroids or calcipotriol only, 7 with cyclosporine A, and 14 with methotrexate. The dose of the drug was kept stable throughout the 24-week study period.
Demographic and baseline characteristics for all participants are shown in Table 1. There were no significant differences in demographic or baseline characteristics among the study group versus the control group, and all participants were similar in age; body mass index; as well as FPG, fasting insulin, and HbA1C levels.
At baseline, both study and control participants had elevated mean (SD) FPG levels (10 [25] mmol/L and 11 [0.4] mmol/L, respectively), fasting insulin levels (2.79 [0.17] pmol/L and 2.82 [0.13] pmol/L, respectively), and HbA1C levels (8.4% [0.38%] and 8.1% [0.21%], respectively)(Table 1).
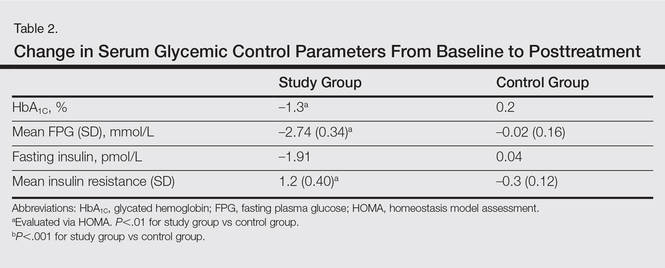
The study group showed significant improvements in glycemic control at the end of the study (Table 2). At week 24, study group participants had a mean (SD) decrease in FPG levels of 2.74 (0.34) mmol/L versus 0.02 (0.16) mmol/L in the control group. This difference between the 2 groups after 24 weeks was found to be statistically significant (P<.01). On further analysis of the study group, no statistically significant difference (P>.01) was noted in the 3 anti-TNF agents used. Compared to the control group, the study group showed a significant decrease from baseline values of FPG and HbA1C (P<.01). Fasting insulin levels decreased significantly for study group participants as compared with control (–1.91 pmol/L vs 0.04 pmol/L)(P<.001)(Table 2). However, on analysis of the 3 anti-TNF agents, no statistically significant difference was found (P>.05). Participants in the control group showed no significant change in fasting insulin and FPG levels.
To confirm that there was a change in insulin sensitivity in response to TNF-α inhibitors, we analyzed FPG and fasting insulin values using the HOMA method. There was no change in mean relative insulin resistance in the control group in response to therapy (mean [SD], 5.4 [0.31] vs 5.6 [0.15], before vs after therapy), while there was mild improvement in relative insulin resistance in the study group (5.9 [0.52] vs 4.8 [0.34], before vs after therapy). There also was a significant difference in the change in relative insulin resistance in response to treatment between the study and control groups (1.2 [0.40] vs –0.3 [0.12]; P<.01)(Table 2).
Comment
There has been an unprecedented rise in the rate of obesity and associated metabolic diseases such as type 2 DM. Following the current trend, it is estimated that the world will have approximately 592 million cases of type 2 DM by the year 2035.19 Almost two-thirds of these patients are estimated to die of cardiovascular diseases.
Although the pathophysiology of type 2 DM is not known, insulin resistance in the muscles and liver as well as failure of pancreatic β cells represent the core of the complex pathophysiology. The associated underlying silent inflammation was thought to have a key role in both insulin resistance and insulin secretory defects seen in type 2 DM. Furthermore, recent data suggest the central role of TNF-α, IL-1, and IL-6 pathways in this inflammation.10 Tumor necrosis factor α has been shown to have a dual effect on insulin resistance as well as pancreatic β cell function. It blocks the function of insulin at the receptor level and has been implicated as a causative factor in obesity-associated insulin resistance and also in the pathogenesis of type 2 DM.20,21 Furthermore, cytokines that activate nuclear factor κβ (a nuclear transcription factor closely involved in the regulation of cellular inflammatory response), such as TNF-α, are thought to be a common denominator for β-cell apoptosis in types 1 and 2 DM.22 Additionally, it has been suggested that TNF-α is a powerful regulator of adipose tissue.23 Neutralizing TNF-α in obese Zucker rats has shown increased insulin sensitivity.3 Tumor necrosis factor α and IL-6 as well as C-reactive protein and plasminogen activator inhibitor 1 are negatively associated with insulin sensitivity.24-27 These findings have led researchers to investigate the role of anti-TNF agents for the management of type 2 DM.28
Psoriasis has now come to be known as a systemic inflammatory disorder and is associated with increased expression of TNF-α. It predisposes patients to insulin resistance and places them at higher risk for developing DM.11,12 Systematic reports recommend that there is a link between psoriasis and DM featured by helper T cell (TH1) cytokines.29 This link can stimulate insulin resistance and metabolic syndrome as well as inflammatory cytokines identified to motivate psoriasis.29,30 The association between psoriasis and type 2 DM proposes a possible pathophysiologic connection between the 2 diseases. Patients with psoriasis have altered T-cell subtype 1 pathways and dysregulated oxidative and angiogenic mechanisms.31,32 Many of these immune pathways may similarly predispose psoriasis patients to impaired glucose tolerance and DM. Inflammation may cause insulin resistance and DM through numerous mechanisms. Systemic inflammation linked with psoriasis may lead to high levels of circulating IL-1, IL-6, and TNF-α that predispose patients to impaired glucose tolerance and type 2 DM.33
Several longitudinal investigations have found that TNF inhibitors improve insulin resistance.13,14,34-38 Gonzalez-Gay et al13 confirmed a rapid beneficial effect of infliximab on insulin resistance and insulin sensitivity in rheumatoid arthritis (RA) patients, which might support the long-term use of drugs that act by blocking TNF-α to diminish the mechanisms implicated in the development of atherosclerosis in patients with RA. Kiortsis et al14 performed a complete biochemical profile before and after 6 months of treatment with infliximab in 17 patients with ankylosing spondylitis and 28 patients with RA. The researchers found a significant decrease of the HOMA index in the percentile of their patients with the highest insulin resistance (P<.01).14
Stagakis et al34 found that 12 weeks of treatment with anti-TNF agents may improve insulin resistance in patients with active RA and high insulin resistance. Treatment with anti-TNF agents was shown to restore the phosphorylation status of serine phosphorylation of insulin receptor substrate 1 (Ser312-IRS-1) and AKT (protein kinase B), which are important mediators in the insulin signaling cascade. The investigators concluded that treatment with anti-TNF agents may improve insulin resistance and sensitivity in RA patients with active disease and high insulin resistance.34
Solomon et al35 studied the link between disease-modifying antirheumatic drugs and DM risk in patients with RA and psoriasis. The authors proposed that initiation of treatment with TNF inhibitors in psoriasis patients was associated with a reduced incidence of DM. The results showed a lower risk for developing DM in patients with psoriasis who were treated with a TNF inhibitor compared with numerous other drugs.35
Marra et al36 studied the effects of etanercept on insulin sensitivity in 9 patients with psoriasis. They reported a decrease in insulin resistance evaluated by HOMA after 24 weeks of etanercept treatment.36 Wambier et al37 reported severe hypoglycemia after initiation of anti-TNF therapy with etanercept in a patient with generalized pustular psoriasis and type 2 DM.
Yazdani-Biuki et al38 reported the case of a patient who demonstrated a relapse of type 2 DM after an interruption of prolonged treatment with infliximab, an anti–TNF-α antibody for psoriatic arthritis. The improvement in insulin sensitivity of this patient has been reported along with post hoc evidence that chronic administration of infliximab improves insulin resistance in a small sample of patients with inflammatory joint diseases.17
Other studies on the effects of TNF inhibitors on insulin resistance and sensitivity have yielded conflicting results. Martínez-Abundis et al39 studied the effects of etanercept on insulin resistance and sensitivity in a randomized trial of psoriatic patients at risk for developing type 2 DM. Results indicated that anti-TNF therapy had no significant influence on insulin sensitivity measured using a hyperinsulinemic clamp during 2 weeks of etanercept treatment in psoriatic patients with risk factors for type 2 DM. The explanation of this discrepancy may be due to the short duration of the study period.
It is still unknown if psoriasis treatment affects a patient’s risk for developing DM. However, Solomon et al35 evaluated the association of incidental DM among patients with prescribed TNF inhibitors or methotrexate and proposed that initiation of treatment with TNF inhibitors was associated with a diminished incidence of DM.
Our study supports and confirms that psoriasis patients treated with TNF-α inhibitors showed improved glycemic indices and insulin resistance compared with control patients treated with other common systemic drugs for psoriasis. We did not take into consideration other conventional risk factors such as hypertension and coronary artery disease. The number of participants included in the current study was not large enough to evaluate each of the anti-TNF agents in a separate group, and participants were not followed up long enough to see the impact of the biochemical changes noted in the results on long-term morbidity or mortality.
Conclusion
Our study confirms a beneficial effect of TNF-α inhibitors on insulin resistance and insulin sensitivity in psoriasis patients with type 2 DM. Treatment with TNF-α inhibitors may have beneficial effects on insulin sensitivity in even the most insulin-resistant patients with psoriasis. The study results may support the hypothesis that long-term use of TNF inhibitors may reduce the mechanisms involved in the development of DM in patients with psoriasis. The improvement in insulin sensitivity may in turn decrease the coronary artery disease risk in these patients. Additional large, prospective, multicenter studies are required to further analyze the effects of anti–TNF-α antibodies on insulin sensitivity and β cell function in insulin-resistant or diabetic psoriasis patients.
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866-873.
- Boehncke WH, Prinz J, Gottlieb AB. Biologic therapies for psoriasis. a systematic review. J Rheumatol. 2006;33:1447-1451.
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. a balanced overview. Diabetes Care. 1992;15:318-368.
- DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;4:177-269.
- Reaven GM. Banting Lecture 1988. role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607.
- Erkelens DW. Insulin resistance syndrome and type 2 diabetes mellitus. Am J Cardiol. 2001;88(7B):38J-42J.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91.
- Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271-1278.
- Van der Poll T, Romijn JA, Endert E, et al. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol. 1991;261:457-465.
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166-172.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Solomon DH, Love TJ, Canning C, et al. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69:2114-2117.
- Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83-86.
- Kiortsis DN, Mavridis AK, Vasakos S, et al. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765-766.
- Dandona P, Weinstock R, Thusu K, et al. Tumor necrosis factor-α in sera of obese patients: fall with weight loss. J Clin Endocrinol Metabol. 1998;83:2907-2910.
- Pereira RR, Amladi ST, Varthakavi PK. A study of the prevalence of diabetes, insulin resistance, lipid abnormalities, and cardiovascular risk factors in patients with chronic plaque psoriasis. Ind J Dermatol. 2011;56:520-526.
- Yazdani-Biuki B, Stelzl H, Brezinschek HP, et al. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-α antibody infliximab. Eur J Clin Invest. 2004;34:641-642.
- Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders. The Bruneck Study. Diabetes. 1998;47:1643-1649.
- Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, prediabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, prediabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035-3087.
- Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182:169-175.
- Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212-217.
- Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433-1440.
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349-356.
- Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334.
- Festa A, D’Agostino R Jr, Tracy RP, et al. Insulin Resistance Atherosclerosis Study. elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131-1137.
- Meigs JB, O’Donnell CJ, Tofler GH, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55:530-537.
- Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007;167:1676-1685.
- Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Exp Opin Invest Drugs. 2014;24:1-25.
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119.
- Karadag AS, Yavuz B, Ertugrul DT, et al. Is psoriasis a pre-atherosclerotic disease? increased insulin resistance and impaired endothelial function in patients with psoriasis. Int J Dermatol. 2010;49:642-646.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. A tale of two plaques: convergent mechanisms of T-cell–mediated inflammation in psoriasis and atherosclerosis. Exp Dermatol. 2011;20:544-549.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. Angiogenesis and oxidative stress: common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci. 2011;63:1-9.
- Boehncke S, Thaci D, Beschmann H, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157:1249-1251.
- Stagakis I, Bertsias G, Karvounaris S, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14:R141.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Marra M, Campanati A, Testa R, et al. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol. 2007;20:731-736.
- Wambier CG, Foss-Freitas MC, Paschoal RS, et al. Severe hypoglycemia after initiation of anti-tumor necrosis factor therapy with etanercept in a patient with generalized pustular psoriasis and type 2 diabetes mellitus. J Am Acad Dermatol. 2009;60:883-885.
- Yazdani-Biuki B, Mueller T, Brezinschek HP, et al. Relapse of diabetes after interruption of chronic administration of anti-tumor necrosis factor-alpha antibody infliximab: a case observation. Diabetes Care. 2006;29:1712.
- Martínez-Abundis E, Reynoso-von Drateln C, Hernández-Salazar E, et al. Effect of etanercept on insulin secretion and insulin sensitivity in a randomized trial with psoriatic patients at risk for developing type 2 diabetes mellitus. Arch Dermatol Res. 2007;299:461-465.
Psoriasis is a chronic inflammatory disorder associated with increased expression of proinflammatory mediators such as tumor necrosis factor (TNF) α.1 Anti-TNF drugs (eg, etanercept, adalimumab, infliximab) were proven to be highly effective for the treatment of psoriasis over the last 2 decades.2 Interestingly, TNF inhibitors have been thought to be effective in improving insulin resistance in patients with type 2 diabetes mellitus (DM) by blocking TNF, which is involved in the inflammatory condition in DM.
Type 2 DM is a common chronic condition characterized by hyperglycemia resulting from a combination of peripheral and hepatic insulin resistance and impaired insulin secretion.3 It is characterized by defects in both insulin secretion and insulin sensitivity.4,5 Type 2 DM has been linked with a marked increase in cardiovascular disease, morbidity, and mortality.6 Evidence-based literature regarding the role of chronic inflammation as an important pathogenetic factor in type 2 DM has been growing.7-9 It also has been suggested that pharmacological strategies to reduce this underlying associated silent inflammation are useful in treating DM, which also is true for other conditions such as obesity, metabolic syndrome, and cardiovascular diseases.10
Psoriasis predisposes patients to insulin resistance and may put them at risk for developing DM.11,12 The association between psoriasis and DM suggests that systemic immunosuppression also may diminish the risk for developing DM. Several longitudinal studies have found that TNF inhibitors improve insulin resistance.13,14 Dandona et al15 reported a considerable decrease of TNF-α levels with the concurrent restoration of insulin sensitivity during weight loss.
Pereira et al16 found a notable connection between psoriasis, DM, and insulin resistance with an odds ratio of 2.63 of abnormal glucose homeostasis in patients with psoriasis compared to controls. Yazdani-Biuki et al17 proved that extended administration of anti–TNF-α antibody was able to improve insulin sensitivity in insulin-resistant patients. The same finding was established by Kiortsis et al.14
In this prospective controlled study, we evaluated the effects of anti-TNF agents on insulin resistance and sensitivity in psoriasis patients with type 2 DM treated with anti-TNF agents.
Methods
A total of 70 patients attending the dermatological outpatient clinics at Farwaniya Hospital (Kuwait City, Kuwait) between January 2012 and September 2014 were enrolled in the study and were randomly distributed into 2 equal groups (n=35 each). The study was approved by the hospital ethics committee. Patients were included in the study if they had moderate to severe psoriasis (ie, psoriasis area severity index score ≥10) with documented type 2 DM and high fasting plasma glucose (FPG) levels (ie, >10 mmol/L). Patients who were currently being treated with oral hypoglycemic agents but not insulin therapy were included in the study. Patients were excluded if they had phototherapy within the last 4 weeks, prior biologic therapy, current and prior insulin therapy, a change in oral hypoglycemic drug dosage in the last 2 months, other serious systemic illness (eg, malignancy, hepatitis B or C virus, metabolic or endocrine disease), and/or abnormal laboratory investigations (eg, liver/kidney profile, chest radiograph abnormality, positive Mantoux test). All of the patients enrolled in the study provided informed consent and underwent routine baseline investigations including complete blood cell counts, general health profile, chest radiograph, antinuclear antibody test, Mantoux test, FPG and insulin levels, glycated hemoglobin (HbA1C), homeostasis model assessment (HOMA), routine urine examination, and enzyme-linked immunosorbent assay for tuberculosis. The study group was treated with anti-TNF agents, and the control group received conventional antipsoriatic medications.
All the patients included in the study had high FPG levels (ie, >10 mmol/L) at the time of enrollment and were currently being treated with oral hypoglycemic agents. The dose of oral hypoglycemic agents was unchanged for at least 2 months before entry into the run-in period and throughout the 24-week study period. Demographic details including sex, age, medical history (eg, type of psoriasis, prior and concomitant treatments) were collected from the participants’ clinical histories. Participants from both groups were appropriately matched in terms of age, sex, body weight, body mass index, and duration of type 2 DM (Table 1). The primary end point of the study was to analyze and compare clinical and serum data collected at baseline and after 24 weeks of therapy.

A complete biochemical profile was repeated in both groups after 24 weeks of treatment. Each participant underwent a baseline short insulin sensitivity test immediately before treatment and at 4 and 24 weeks of treatment. We assessed insulin resistance via HOMA, calculated as follows: FPG [mmol/L] × fasting serum insulin [pmol/L] / 22.5.18 Oral glucose tolerance tests were performed to calculate the HOMA of insulin resistance. Serum insulin concentration was determined via enzyme-linked immunosorbent assay.
Statistical analysis was performed using SPSS software (version 12.0). Continuous patient characteristics were analyzed using mean and SD as well as discrete data as counts and proportions. Association was examined using χ2 tests for categorical variables and 2-sided t test/Wilcoxon rank sum test for continuous variables. Analysis of variance was used to compare the results in 3 different anti-TNF agents used in the study group.
Results
Of the 35 participants enrolled in the study group, 34 (97.1%) completed the study and were evaluated. The study group included 16 men and 18 women aged 19 to 63 years (mean age [SD], 43.7 [21.6] years) who were treated with TNF-α inhibitors—8 participants with etanercept, 14 with adalimumab, and 12 with infliximab—according to the standard dosage schedule for 24 weeks.
Of the 35 participants enrolled in the control group, 29 (82.9%) completed the study and were evaluated. Six patients did not follow up for the complete duration of the 24-week study period and were not evaluated. The control group included 14 men and 15 women aged 18 to 65 years (mean age [SD], 47.7 [14.2] years) who were treated with other systemic therapies—8 participants with topical corticosteroids or calcipotriol only, 7 with cyclosporine A, and 14 with methotrexate. The dose of the drug was kept stable throughout the 24-week study period.
Demographic and baseline characteristics for all participants are shown in Table 1. There were no significant differences in demographic or baseline characteristics among the study group versus the control group, and all participants were similar in age; body mass index; as well as FPG, fasting insulin, and HbA1C levels.
At baseline, both study and control participants had elevated mean (SD) FPG levels (10 [25] mmol/L and 11 [0.4] mmol/L, respectively), fasting insulin levels (2.79 [0.17] pmol/L and 2.82 [0.13] pmol/L, respectively), and HbA1C levels (8.4% [0.38%] and 8.1% [0.21%], respectively)(Table 1).
The study group showed significant improvements in glycemic control at the end of the study (Table 2). At week 24, study group participants had a mean (SD) decrease in FPG levels of 2.74 (0.34) mmol/L versus 0.02 (0.16) mmol/L in the control group. This difference between the 2 groups after 24 weeks was found to be statistically significant (P<.01). On further analysis of the study group, no statistically significant difference (P>.01) was noted in the 3 anti-TNF agents used. Compared to the control group, the study group showed a significant decrease from baseline values of FPG and HbA1C (P<.01). Fasting insulin levels decreased significantly for study group participants as compared with control (–1.91 pmol/L vs 0.04 pmol/L)(P<.001)(Table 2). However, on analysis of the 3 anti-TNF agents, no statistically significant difference was found (P>.05). Participants in the control group showed no significant change in fasting insulin and FPG levels.
To confirm that there was a change in insulin sensitivity in response to TNF-α inhibitors, we analyzed FPG and fasting insulin values using the HOMA method. There was no change in mean relative insulin resistance in the control group in response to therapy (mean [SD], 5.4 [0.31] vs 5.6 [0.15], before vs after therapy), while there was mild improvement in relative insulin resistance in the study group (5.9 [0.52] vs 4.8 [0.34], before vs after therapy). There also was a significant difference in the change in relative insulin resistance in response to treatment between the study and control groups (1.2 [0.40] vs –0.3 [0.12]; P<.01)(Table 2).

Comment
There has been an unprecedented rise in the rate of obesity and associated metabolic diseases such as type 2 DM. Following the current trend, it is estimated that the world will have approximately 592 million cases of type 2 DM by the year 2035.19 Almost two-thirds of these patients are estimated to die of cardiovascular diseases.
Although the pathophysiology of type 2 DM is not known, insulin resistance in the muscles and liver as well as failure of pancreatic β cells represent the core of the complex pathophysiology. The associated underlying silent inflammation was thought to have a key role in both insulin resistance and insulin secretory defects seen in type 2 DM. Furthermore, recent data suggest the central role of TNF-α, IL-1, and IL-6 pathways in this inflammation.10 Tumor necrosis factor α has been shown to have a dual effect on insulin resistance as well as pancreatic β cell function. It blocks the function of insulin at the receptor level and has been implicated as a causative factor in obesity-associated insulin resistance and also in the pathogenesis of type 2 DM.20,21 Furthermore, cytokines that activate nuclear factor κβ (a nuclear transcription factor closely involved in the regulation of cellular inflammatory response), such as TNF-α, are thought to be a common denominator for β-cell apoptosis in types 1 and 2 DM.22 Additionally, it has been suggested that TNF-α is a powerful regulator of adipose tissue.23 Neutralizing TNF-α in obese Zucker rats has shown increased insulin sensitivity.3 Tumor necrosis factor α and IL-6 as well as C-reactive protein and plasminogen activator inhibitor 1 are negatively associated with insulin sensitivity.24-27 These findings have led researchers to investigate the role of anti-TNF agents for the management of type 2 DM.28
Psoriasis has now come to be known as a systemic inflammatory disorder and is associated with increased expression of TNF-α. It predisposes patients to insulin resistance and places them at higher risk for developing DM.11,12 Systematic reports recommend that there is a link between psoriasis and DM featured by helper T cell (TH1) cytokines.29 This link can stimulate insulin resistance and metabolic syndrome as well as inflammatory cytokines identified to motivate psoriasis.29,30 The association between psoriasis and type 2 DM proposes a possible pathophysiologic connection between the 2 diseases. Patients with psoriasis have altered T-cell subtype 1 pathways and dysregulated oxidative and angiogenic mechanisms.31,32 Many of these immune pathways may similarly predispose psoriasis patients to impaired glucose tolerance and DM. Inflammation may cause insulin resistance and DM through numerous mechanisms. Systemic inflammation linked with psoriasis may lead to high levels of circulating IL-1, IL-6, and TNF-α that predispose patients to impaired glucose tolerance and type 2 DM.33
Several longitudinal investigations have found that TNF inhibitors improve insulin resistance.13,14,34-38 Gonzalez-Gay et al13 confirmed a rapid beneficial effect of infliximab on insulin resistance and insulin sensitivity in rheumatoid arthritis (RA) patients, which might support the long-term use of drugs that act by blocking TNF-α to diminish the mechanisms implicated in the development of atherosclerosis in patients with RA. Kiortsis et al14 performed a complete biochemical profile before and after 6 months of treatment with infliximab in 17 patients with ankylosing spondylitis and 28 patients with RA. The researchers found a significant decrease of the HOMA index in the percentile of their patients with the highest insulin resistance (P<.01).14
Stagakis et al34 found that 12 weeks of treatment with anti-TNF agents may improve insulin resistance in patients with active RA and high insulin resistance. Treatment with anti-TNF agents was shown to restore the phosphorylation status of serine phosphorylation of insulin receptor substrate 1 (Ser312-IRS-1) and AKT (protein kinase B), which are important mediators in the insulin signaling cascade. The investigators concluded that treatment with anti-TNF agents may improve insulin resistance and sensitivity in RA patients with active disease and high insulin resistance.34
Solomon et al35 studied the link between disease-modifying antirheumatic drugs and DM risk in patients with RA and psoriasis. The authors proposed that initiation of treatment with TNF inhibitors in psoriasis patients was associated with a reduced incidence of DM. The results showed a lower risk for developing DM in patients with psoriasis who were treated with a TNF inhibitor compared with numerous other drugs.35
Marra et al36 studied the effects of etanercept on insulin sensitivity in 9 patients with psoriasis. They reported a decrease in insulin resistance evaluated by HOMA after 24 weeks of etanercept treatment.36 Wambier et al37 reported severe hypoglycemia after initiation of anti-TNF therapy with etanercept in a patient with generalized pustular psoriasis and type 2 DM.
Yazdani-Biuki et al38 reported the case of a patient who demonstrated a relapse of type 2 DM after an interruption of prolonged treatment with infliximab, an anti–TNF-α antibody for psoriatic arthritis. The improvement in insulin sensitivity of this patient has been reported along with post hoc evidence that chronic administration of infliximab improves insulin resistance in a small sample of patients with inflammatory joint diseases.17
Other studies on the effects of TNF inhibitors on insulin resistance and sensitivity have yielded conflicting results. Martínez-Abundis et al39 studied the effects of etanercept on insulin resistance and sensitivity in a randomized trial of psoriatic patients at risk for developing type 2 DM. Results indicated that anti-TNF therapy had no significant influence on insulin sensitivity measured using a hyperinsulinemic clamp during 2 weeks of etanercept treatment in psoriatic patients with risk factors for type 2 DM. The explanation of this discrepancy may be due to the short duration of the study period.
It is still unknown if psoriasis treatment affects a patient’s risk for developing DM. However, Solomon et al35 evaluated the association of incidental DM among patients with prescribed TNF inhibitors or methotrexate and proposed that initiation of treatment with TNF inhibitors was associated with a diminished incidence of DM.
Our study supports and confirms that psoriasis patients treated with TNF-α inhibitors showed improved glycemic indices and insulin resistance compared with control patients treated with other common systemic drugs for psoriasis. We did not take into consideration other conventional risk factors such as hypertension and coronary artery disease. The number of participants included in the current study was not large enough to evaluate each of the anti-TNF agents in a separate group, and participants were not followed up long enough to see the impact of the biochemical changes noted in the results on long-term morbidity or mortality.
Conclusion
Our study confirms a beneficial effect of TNF-α inhibitors on insulin resistance and insulin sensitivity in psoriasis patients with type 2 DM. Treatment with TNF-α inhibitors may have beneficial effects on insulin sensitivity in even the most insulin-resistant patients with psoriasis. The study results may support the hypothesis that long-term use of TNF inhibitors may reduce the mechanisms involved in the development of DM in patients with psoriasis. The improvement in insulin sensitivity may in turn decrease the coronary artery disease risk in these patients. Additional large, prospective, multicenter studies are required to further analyze the effects of anti–TNF-α antibodies on insulin sensitivity and β cell function in insulin-resistant or diabetic psoriasis patients.
Psoriasis is a chronic inflammatory disorder associated with increased expression of proinflammatory mediators such as tumor necrosis factor (TNF) α.1 Anti-TNF drugs (eg, etanercept, adalimumab, infliximab) were proven to be highly effective for the treatment of psoriasis over the last 2 decades.2 Interestingly, TNF inhibitors have been thought to be effective in improving insulin resistance in patients with type 2 diabetes mellitus (DM) by blocking TNF, which is involved in the inflammatory condition in DM.
Type 2 DM is a common chronic condition characterized by hyperglycemia resulting from a combination of peripheral and hepatic insulin resistance and impaired insulin secretion.3 It is characterized by defects in both insulin secretion and insulin sensitivity.4,5 Type 2 DM has been linked with a marked increase in cardiovascular disease, morbidity, and mortality.6 Evidence-based literature regarding the role of chronic inflammation as an important pathogenetic factor in type 2 DM has been growing.7-9 It also has been suggested that pharmacological strategies to reduce this underlying associated silent inflammation are useful in treating DM, which also is true for other conditions such as obesity, metabolic syndrome, and cardiovascular diseases.10
Psoriasis predisposes patients to insulin resistance and may put them at risk for developing DM.11,12 The association between psoriasis and DM suggests that systemic immunosuppression also may diminish the risk for developing DM. Several longitudinal studies have found that TNF inhibitors improve insulin resistance.13,14 Dandona et al15 reported a considerable decrease of TNF-α levels with the concurrent restoration of insulin sensitivity during weight loss.
Pereira et al16 found a notable connection between psoriasis, DM, and insulin resistance with an odds ratio of 2.63 of abnormal glucose homeostasis in patients with psoriasis compared to controls. Yazdani-Biuki et al17 proved that extended administration of anti–TNF-α antibody was able to improve insulin sensitivity in insulin-resistant patients. The same finding was established by Kiortsis et al.14
In this prospective controlled study, we evaluated the effects of anti-TNF agents on insulin resistance and sensitivity in psoriasis patients with type 2 DM treated with anti-TNF agents.
Methods
A total of 70 patients attending the dermatological outpatient clinics at Farwaniya Hospital (Kuwait City, Kuwait) between January 2012 and September 2014 were enrolled in the study and were randomly distributed into 2 equal groups (n=35 each). The study was approved by the hospital ethics committee. Patients were included in the study if they had moderate to severe psoriasis (ie, psoriasis area severity index score ≥10) with documented type 2 DM and high fasting plasma glucose (FPG) levels (ie, >10 mmol/L). Patients who were currently being treated with oral hypoglycemic agents but not insulin therapy were included in the study. Patients were excluded if they had phototherapy within the last 4 weeks, prior biologic therapy, current and prior insulin therapy, a change in oral hypoglycemic drug dosage in the last 2 months, other serious systemic illness (eg, malignancy, hepatitis B or C virus, metabolic or endocrine disease), and/or abnormal laboratory investigations (eg, liver/kidney profile, chest radiograph abnormality, positive Mantoux test). All of the patients enrolled in the study provided informed consent and underwent routine baseline investigations including complete blood cell counts, general health profile, chest radiograph, antinuclear antibody test, Mantoux test, FPG and insulin levels, glycated hemoglobin (HbA1C), homeostasis model assessment (HOMA), routine urine examination, and enzyme-linked immunosorbent assay for tuberculosis. The study group was treated with anti-TNF agents, and the control group received conventional antipsoriatic medications.
All the patients included in the study had high FPG levels (ie, >10 mmol/L) at the time of enrollment and were currently being treated with oral hypoglycemic agents. The dose of oral hypoglycemic agents was unchanged for at least 2 months before entry into the run-in period and throughout the 24-week study period. Demographic details including sex, age, medical history (eg, type of psoriasis, prior and concomitant treatments) were collected from the participants’ clinical histories. Participants from both groups were appropriately matched in terms of age, sex, body weight, body mass index, and duration of type 2 DM (Table 1). The primary end point of the study was to analyze and compare clinical and serum data collected at baseline and after 24 weeks of therapy.

A complete biochemical profile was repeated in both groups after 24 weeks of treatment. Each participant underwent a baseline short insulin sensitivity test immediately before treatment and at 4 and 24 weeks of treatment. We assessed insulin resistance via HOMA, calculated as follows: FPG [mmol/L] × fasting serum insulin [pmol/L] / 22.5.18 Oral glucose tolerance tests were performed to calculate the HOMA of insulin resistance. Serum insulin concentration was determined via enzyme-linked immunosorbent assay.
Statistical analysis was performed using SPSS software (version 12.0). Continuous patient characteristics were analyzed using mean and SD as well as discrete data as counts and proportions. Association was examined using χ2 tests for categorical variables and 2-sided t test/Wilcoxon rank sum test for continuous variables. Analysis of variance was used to compare the results in 3 different anti-TNF agents used in the study group.
Results
Of the 35 participants enrolled in the study group, 34 (97.1%) completed the study and were evaluated. The study group included 16 men and 18 women aged 19 to 63 years (mean age [SD], 43.7 [21.6] years) who were treated with TNF-α inhibitors—8 participants with etanercept, 14 with adalimumab, and 12 with infliximab—according to the standard dosage schedule for 24 weeks.
Of the 35 participants enrolled in the control group, 29 (82.9%) completed the study and were evaluated. Six patients did not follow up for the complete duration of the 24-week study period and were not evaluated. The control group included 14 men and 15 women aged 18 to 65 years (mean age [SD], 47.7 [14.2] years) who were treated with other systemic therapies—8 participants with topical corticosteroids or calcipotriol only, 7 with cyclosporine A, and 14 with methotrexate. The dose of the drug was kept stable throughout the 24-week study period.
Demographic and baseline characteristics for all participants are shown in Table 1. There were no significant differences in demographic or baseline characteristics among the study group versus the control group, and all participants were similar in age; body mass index; as well as FPG, fasting insulin, and HbA1C levels.
At baseline, both study and control participants had elevated mean (SD) FPG levels (10 [25] mmol/L and 11 [0.4] mmol/L, respectively), fasting insulin levels (2.79 [0.17] pmol/L and 2.82 [0.13] pmol/L, respectively), and HbA1C levels (8.4% [0.38%] and 8.1% [0.21%], respectively)(Table 1).
The study group showed significant improvements in glycemic control at the end of the study (Table 2). At week 24, study group participants had a mean (SD) decrease in FPG levels of 2.74 (0.34) mmol/L versus 0.02 (0.16) mmol/L in the control group. This difference between the 2 groups after 24 weeks was found to be statistically significant (P<.01). On further analysis of the study group, no statistically significant difference (P>.01) was noted in the 3 anti-TNF agents used. Compared to the control group, the study group showed a significant decrease from baseline values of FPG and HbA1C (P<.01). Fasting insulin levels decreased significantly for study group participants as compared with control (–1.91 pmol/L vs 0.04 pmol/L)(P<.001)(Table 2). However, on analysis of the 3 anti-TNF agents, no statistically significant difference was found (P>.05). Participants in the control group showed no significant change in fasting insulin and FPG levels.
To confirm that there was a change in insulin sensitivity in response to TNF-α inhibitors, we analyzed FPG and fasting insulin values using the HOMA method. There was no change in mean relative insulin resistance in the control group in response to therapy (mean [SD], 5.4 [0.31] vs 5.6 [0.15], before vs after therapy), while there was mild improvement in relative insulin resistance in the study group (5.9 [0.52] vs 4.8 [0.34], before vs after therapy). There also was a significant difference in the change in relative insulin resistance in response to treatment between the study and control groups (1.2 [0.40] vs –0.3 [0.12]; P<.01)(Table 2).

Comment
There has been an unprecedented rise in the rate of obesity and associated metabolic diseases such as type 2 DM. Following the current trend, it is estimated that the world will have approximately 592 million cases of type 2 DM by the year 2035.19 Almost two-thirds of these patients are estimated to die of cardiovascular diseases.
Although the pathophysiology of type 2 DM is not known, insulin resistance in the muscles and liver as well as failure of pancreatic β cells represent the core of the complex pathophysiology. The associated underlying silent inflammation was thought to have a key role in both insulin resistance and insulin secretory defects seen in type 2 DM. Furthermore, recent data suggest the central role of TNF-α, IL-1, and IL-6 pathways in this inflammation.10 Tumor necrosis factor α has been shown to have a dual effect on insulin resistance as well as pancreatic β cell function. It blocks the function of insulin at the receptor level and has been implicated as a causative factor in obesity-associated insulin resistance and also in the pathogenesis of type 2 DM.20,21 Furthermore, cytokines that activate nuclear factor κβ (a nuclear transcription factor closely involved in the regulation of cellular inflammatory response), such as TNF-α, are thought to be a common denominator for β-cell apoptosis in types 1 and 2 DM.22 Additionally, it has been suggested that TNF-α is a powerful regulator of adipose tissue.23 Neutralizing TNF-α in obese Zucker rats has shown increased insulin sensitivity.3 Tumor necrosis factor α and IL-6 as well as C-reactive protein and plasminogen activator inhibitor 1 are negatively associated with insulin sensitivity.24-27 These findings have led researchers to investigate the role of anti-TNF agents for the management of type 2 DM.28
Psoriasis has now come to be known as a systemic inflammatory disorder and is associated with increased expression of TNF-α. It predisposes patients to insulin resistance and places them at higher risk for developing DM.11,12 Systematic reports recommend that there is a link between psoriasis and DM featured by helper T cell (TH1) cytokines.29 This link can stimulate insulin resistance and metabolic syndrome as well as inflammatory cytokines identified to motivate psoriasis.29,30 The association between psoriasis and type 2 DM proposes a possible pathophysiologic connection between the 2 diseases. Patients with psoriasis have altered T-cell subtype 1 pathways and dysregulated oxidative and angiogenic mechanisms.31,32 Many of these immune pathways may similarly predispose psoriasis patients to impaired glucose tolerance and DM. Inflammation may cause insulin resistance and DM through numerous mechanisms. Systemic inflammation linked with psoriasis may lead to high levels of circulating IL-1, IL-6, and TNF-α that predispose patients to impaired glucose tolerance and type 2 DM.33
Several longitudinal investigations have found that TNF inhibitors improve insulin resistance.13,14,34-38 Gonzalez-Gay et al13 confirmed a rapid beneficial effect of infliximab on insulin resistance and insulin sensitivity in rheumatoid arthritis (RA) patients, which might support the long-term use of drugs that act by blocking TNF-α to diminish the mechanisms implicated in the development of atherosclerosis in patients with RA. Kiortsis et al14 performed a complete biochemical profile before and after 6 months of treatment with infliximab in 17 patients with ankylosing spondylitis and 28 patients with RA. The researchers found a significant decrease of the HOMA index in the percentile of their patients with the highest insulin resistance (P<.01).14
Stagakis et al34 found that 12 weeks of treatment with anti-TNF agents may improve insulin resistance in patients with active RA and high insulin resistance. Treatment with anti-TNF agents was shown to restore the phosphorylation status of serine phosphorylation of insulin receptor substrate 1 (Ser312-IRS-1) and AKT (protein kinase B), which are important mediators in the insulin signaling cascade. The investigators concluded that treatment with anti-TNF agents may improve insulin resistance and sensitivity in RA patients with active disease and high insulin resistance.34
Solomon et al35 studied the link between disease-modifying antirheumatic drugs and DM risk in patients with RA and psoriasis. The authors proposed that initiation of treatment with TNF inhibitors in psoriasis patients was associated with a reduced incidence of DM. The results showed a lower risk for developing DM in patients with psoriasis who were treated with a TNF inhibitor compared with numerous other drugs.35
Marra et al36 studied the effects of etanercept on insulin sensitivity in 9 patients with psoriasis. They reported a decrease in insulin resistance evaluated by HOMA after 24 weeks of etanercept treatment.36 Wambier et al37 reported severe hypoglycemia after initiation of anti-TNF therapy with etanercept in a patient with generalized pustular psoriasis and type 2 DM.
Yazdani-Biuki et al38 reported the case of a patient who demonstrated a relapse of type 2 DM after an interruption of prolonged treatment with infliximab, an anti–TNF-α antibody for psoriatic arthritis. The improvement in insulin sensitivity of this patient has been reported along with post hoc evidence that chronic administration of infliximab improves insulin resistance in a small sample of patients with inflammatory joint diseases.17
Other studies on the effects of TNF inhibitors on insulin resistance and sensitivity have yielded conflicting results. Martínez-Abundis et al39 studied the effects of etanercept on insulin resistance and sensitivity in a randomized trial of psoriatic patients at risk for developing type 2 DM. Results indicated that anti-TNF therapy had no significant influence on insulin sensitivity measured using a hyperinsulinemic clamp during 2 weeks of etanercept treatment in psoriatic patients with risk factors for type 2 DM. The explanation of this discrepancy may be due to the short duration of the study period.
It is still unknown if psoriasis treatment affects a patient’s risk for developing DM. However, Solomon et al35 evaluated the association of incidental DM among patients with prescribed TNF inhibitors or methotrexate and proposed that initiation of treatment with TNF inhibitors was associated with a diminished incidence of DM.
Our study supports and confirms that psoriasis patients treated with TNF-α inhibitors showed improved glycemic indices and insulin resistance compared with control patients treated with other common systemic drugs for psoriasis. We did not take into consideration other conventional risk factors such as hypertension and coronary artery disease. The number of participants included in the current study was not large enough to evaluate each of the anti-TNF agents in a separate group, and participants were not followed up long enough to see the impact of the biochemical changes noted in the results on long-term morbidity or mortality.
Conclusion
Our study confirms a beneficial effect of TNF-α inhibitors on insulin resistance and insulin sensitivity in psoriasis patients with type 2 DM. Treatment with TNF-α inhibitors may have beneficial effects on insulin sensitivity in even the most insulin-resistant patients with psoriasis. The study results may support the hypothesis that long-term use of TNF inhibitors may reduce the mechanisms involved in the development of DM in patients with psoriasis. The improvement in insulin sensitivity may in turn decrease the coronary artery disease risk in these patients. Additional large, prospective, multicenter studies are required to further analyze the effects of anti–TNF-α antibodies on insulin sensitivity and β cell function in insulin-resistant or diabetic psoriasis patients.
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866-873.
- Boehncke WH, Prinz J, Gottlieb AB. Biologic therapies for psoriasis. a systematic review. J Rheumatol. 2006;33:1447-1451.
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. a balanced overview. Diabetes Care. 1992;15:318-368.
- DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;4:177-269.
- Reaven GM. Banting Lecture 1988. role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607.
- Erkelens DW. Insulin resistance syndrome and type 2 diabetes mellitus. Am J Cardiol. 2001;88(7B):38J-42J.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91.
- Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271-1278.
- Van der Poll T, Romijn JA, Endert E, et al. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol. 1991;261:457-465.
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166-172.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Solomon DH, Love TJ, Canning C, et al. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69:2114-2117.
- Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83-86.
- Kiortsis DN, Mavridis AK, Vasakos S, et al. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765-766.
- Dandona P, Weinstock R, Thusu K, et al. Tumor necrosis factor-α in sera of obese patients: fall with weight loss. J Clin Endocrinol Metabol. 1998;83:2907-2910.
- Pereira RR, Amladi ST, Varthakavi PK. A study of the prevalence of diabetes, insulin resistance, lipid abnormalities, and cardiovascular risk factors in patients with chronic plaque psoriasis. Ind J Dermatol. 2011;56:520-526.
- Yazdani-Biuki B, Stelzl H, Brezinschek HP, et al. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-α antibody infliximab. Eur J Clin Invest. 2004;34:641-642.
- Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders. The Bruneck Study. Diabetes. 1998;47:1643-1649.
- Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, prediabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, prediabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035-3087.
- Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182:169-175.
- Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212-217.
- Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433-1440.
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349-356.
- Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334.
- Festa A, D’Agostino R Jr, Tracy RP, et al. Insulin Resistance Atherosclerosis Study. elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131-1137.
- Meigs JB, O’Donnell CJ, Tofler GH, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55:530-537.
- Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007;167:1676-1685.
- Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Exp Opin Invest Drugs. 2014;24:1-25.
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119.
- Karadag AS, Yavuz B, Ertugrul DT, et al. Is psoriasis a pre-atherosclerotic disease? increased insulin resistance and impaired endothelial function in patients with psoriasis. Int J Dermatol. 2010;49:642-646.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. A tale of two plaques: convergent mechanisms of T-cell–mediated inflammation in psoriasis and atherosclerosis. Exp Dermatol. 2011;20:544-549.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. Angiogenesis and oxidative stress: common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci. 2011;63:1-9.
- Boehncke S, Thaci D, Beschmann H, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157:1249-1251.
- Stagakis I, Bertsias G, Karvounaris S, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14:R141.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Marra M, Campanati A, Testa R, et al. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol. 2007;20:731-736.
- Wambier CG, Foss-Freitas MC, Paschoal RS, et al. Severe hypoglycemia after initiation of anti-tumor necrosis factor therapy with etanercept in a patient with generalized pustular psoriasis and type 2 diabetes mellitus. J Am Acad Dermatol. 2009;60:883-885.
- Yazdani-Biuki B, Mueller T, Brezinschek HP, et al. Relapse of diabetes after interruption of chronic administration of anti-tumor necrosis factor-alpha antibody infliximab: a case observation. Diabetes Care. 2006;29:1712.
- Martínez-Abundis E, Reynoso-von Drateln C, Hernández-Salazar E, et al. Effect of etanercept on insulin secretion and insulin sensitivity in a randomized trial with psoriatic patients at risk for developing type 2 diabetes mellitus. Arch Dermatol Res. 2007;299:461-465.
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866-873.
- Boehncke WH, Prinz J, Gottlieb AB. Biologic therapies for psoriasis. a systematic review. J Rheumatol. 2006;33:1447-1451.
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. a balanced overview. Diabetes Care. 1992;15:318-368.
- DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;4:177-269.
- Reaven GM. Banting Lecture 1988. role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607.
- Erkelens DW. Insulin resistance syndrome and type 2 diabetes mellitus. Am J Cardiol. 2001;88(7B):38J-42J.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91.
- Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271-1278.
- Van der Poll T, Romijn JA, Endert E, et al. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol. 1991;261:457-465.
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166-172.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Solomon DH, Love TJ, Canning C, et al. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69:2114-2117.
- Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83-86.
- Kiortsis DN, Mavridis AK, Vasakos S, et al. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765-766.
- Dandona P, Weinstock R, Thusu K, et al. Tumor necrosis factor-α in sera of obese patients: fall with weight loss. J Clin Endocrinol Metabol. 1998;83:2907-2910.
- Pereira RR, Amladi ST, Varthakavi PK. A study of the prevalence of diabetes, insulin resistance, lipid abnormalities, and cardiovascular risk factors in patients with chronic plaque psoriasis. Ind J Dermatol. 2011;56:520-526.
- Yazdani-Biuki B, Stelzl H, Brezinschek HP, et al. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-α antibody infliximab. Eur J Clin Invest. 2004;34:641-642.
- Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders. The Bruneck Study. Diabetes. 1998;47:1643-1649.
- Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, prediabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, prediabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035-3087.
- Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182:169-175.
- Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212-217.
- Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433-1440.
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349-356.
- Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334.
- Festa A, D’Agostino R Jr, Tracy RP, et al. Insulin Resistance Atherosclerosis Study. elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131-1137.
- Meigs JB, O’Donnell CJ, Tofler GH, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55:530-537.
- Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007;167:1676-1685.
- Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Exp Opin Invest Drugs. 2014;24:1-25.
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119.
- Karadag AS, Yavuz B, Ertugrul DT, et al. Is psoriasis a pre-atherosclerotic disease? increased insulin resistance and impaired endothelial function in patients with psoriasis. Int J Dermatol. 2010;49:642-646.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. A tale of two plaques: convergent mechanisms of T-cell–mediated inflammation in psoriasis and atherosclerosis. Exp Dermatol. 2011;20:544-549.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. Angiogenesis and oxidative stress: common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci. 2011;63:1-9.
- Boehncke S, Thaci D, Beschmann H, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157:1249-1251.
- Stagakis I, Bertsias G, Karvounaris S, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14:R141.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Marra M, Campanati A, Testa R, et al. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol. 2007;20:731-736.
- Wambier CG, Foss-Freitas MC, Paschoal RS, et al. Severe hypoglycemia after initiation of anti-tumor necrosis factor therapy with etanercept in a patient with generalized pustular psoriasis and type 2 diabetes mellitus. J Am Acad Dermatol. 2009;60:883-885.
- Yazdani-Biuki B, Mueller T, Brezinschek HP, et al. Relapse of diabetes after interruption of chronic administration of anti-tumor necrosis factor-alpha antibody infliximab: a case observation. Diabetes Care. 2006;29:1712.
- Martínez-Abundis E, Reynoso-von Drateln C, Hernández-Salazar E, et al. Effect of etanercept on insulin secretion and insulin sensitivity in a randomized trial with psoriatic patients at risk for developing type 2 diabetes mellitus. Arch Dermatol Res. 2007;299:461-465.
Practice Points
- Psoriasis is associated with an increased incidence of insulin resistance and type 2 diabetes mellitus (DM).
- Anti–tumor necrosis factor drugs, which are effective for the treatment of psoriasis, were found to improve insulin resistance in psoriasis patients with type 2 DM.
Brodalumab met primary endpoints, deaths called ‘unrelated’
The investigational interleukin-17 inhibitor brodalumab met both primary endpoints in a phase III, double-blind, placebo-controlled trial of 661 patients with moderate to severe plaque psoriasis, investigators reported in the British Journal of Dermatology.
By week 12, 83% of patients on 210 mg brodalumab achieved Psoriasis Area and Severity Index (PASI) 75, as did 60% of patients on 140 mg and 3% of placebo patients, said Dr. Kim Papp of Probity Medical Research in Waterloo, Ont., and associates. The coprimary endpoint, a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear skin), was achieved by 76%, 54%, and 1% of patients, respectively. “Skin clearance continues to improve beyond 12 weeks, and is sustained through 1 year of therapy,” and the safety profile “was considered to be acceptable,” the researchers said.
The AMAGINE-1 trial included a 12-week induction period followed by a withdrawal-retreatment period lasting up to 52 weeks. At the end of induction, brodalumab patients who achieved sPGA 0/1 were rerandomized to placebo or to induction dose. Four weeks later, those with sPGA scores of at least 3 received another induction dose. After 12 weeks of retreatment, patients scoring sPGA 2/3 were rescued with brodalumab 210 mg every 2 weeks (Br J Dermatol. 2016 Feb 23. doi: 10.1111/bjd.14493).
The psoriasis community has closely watched brodalumab, which handily beat ustekinumab in the AMAGINE-2 psoriasis trial before Amgen abruptly pulled out of development in the wake of two suicides by trial participants. AstraZeneca and Valeant Pharmaceuticals then partnered on the agent, and the Food and Drug Administration accepted a Biologics License Application in January 2016.
Dr. Papp and associates published details of the suicides, noting that one involved a 59-year-old man who died 2 months after his last 210-mg dose. He had no history of psychiatric disorders and normal baseline Hospital Anxiety and Depression Scale scores, which worsened on placebo but normalized by week 52. “The investigator reported that there was no reasonable possibility that the event of suicide was related to investigational product,” the researchers said. The second suicide involved a 56-year-old man who also received 210 mg brodalumab. He had a history of severe depression, but his Hospital Anxiety and Depression Scale (HADS) scores were normal when last assessed, his psoriasis was well controlled, and his death was considered unrelated to treatment. Indeed, moderate or severe baseline HADS scores were more likely to improve when patients received brodalumab instead of placebo, the researchers said. The other two deaths on study involved patients with substantial cardiovascular and hepatic comorbidities and also were considered unrelated to treatment.
Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
The investigational interleukin-17 inhibitor brodalumab met both primary endpoints in a phase III, double-blind, placebo-controlled trial of 661 patients with moderate to severe plaque psoriasis, investigators reported in the British Journal of Dermatology.
By week 12, 83% of patients on 210 mg brodalumab achieved Psoriasis Area and Severity Index (PASI) 75, as did 60% of patients on 140 mg and 3% of placebo patients, said Dr. Kim Papp of Probity Medical Research in Waterloo, Ont., and associates. The coprimary endpoint, a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear skin), was achieved by 76%, 54%, and 1% of patients, respectively. “Skin clearance continues to improve beyond 12 weeks, and is sustained through 1 year of therapy,” and the safety profile “was considered to be acceptable,” the researchers said.
The AMAGINE-1 trial included a 12-week induction period followed by a withdrawal-retreatment period lasting up to 52 weeks. At the end of induction, brodalumab patients who achieved sPGA 0/1 were rerandomized to placebo or to induction dose. Four weeks later, those with sPGA scores of at least 3 received another induction dose. After 12 weeks of retreatment, patients scoring sPGA 2/3 were rescued with brodalumab 210 mg every 2 weeks (Br J Dermatol. 2016 Feb 23. doi: 10.1111/bjd.14493).
The psoriasis community has closely watched brodalumab, which handily beat ustekinumab in the AMAGINE-2 psoriasis trial before Amgen abruptly pulled out of development in the wake of two suicides by trial participants. AstraZeneca and Valeant Pharmaceuticals then partnered on the agent, and the Food and Drug Administration accepted a Biologics License Application in January 2016.
Dr. Papp and associates published details of the suicides, noting that one involved a 59-year-old man who died 2 months after his last 210-mg dose. He had no history of psychiatric disorders and normal baseline Hospital Anxiety and Depression Scale scores, which worsened on placebo but normalized by week 52. “The investigator reported that there was no reasonable possibility that the event of suicide was related to investigational product,” the researchers said. The second suicide involved a 56-year-old man who also received 210 mg brodalumab. He had a history of severe depression, but his Hospital Anxiety and Depression Scale (HADS) scores were normal when last assessed, his psoriasis was well controlled, and his death was considered unrelated to treatment. Indeed, moderate or severe baseline HADS scores were more likely to improve when patients received brodalumab instead of placebo, the researchers said. The other two deaths on study involved patients with substantial cardiovascular and hepatic comorbidities and also were considered unrelated to treatment.
Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
The investigational interleukin-17 inhibitor brodalumab met both primary endpoints in a phase III, double-blind, placebo-controlled trial of 661 patients with moderate to severe plaque psoriasis, investigators reported in the British Journal of Dermatology.
By week 12, 83% of patients on 210 mg brodalumab achieved Psoriasis Area and Severity Index (PASI) 75, as did 60% of patients on 140 mg and 3% of placebo patients, said Dr. Kim Papp of Probity Medical Research in Waterloo, Ont., and associates. The coprimary endpoint, a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear skin), was achieved by 76%, 54%, and 1% of patients, respectively. “Skin clearance continues to improve beyond 12 weeks, and is sustained through 1 year of therapy,” and the safety profile “was considered to be acceptable,” the researchers said.
The AMAGINE-1 trial included a 12-week induction period followed by a withdrawal-retreatment period lasting up to 52 weeks. At the end of induction, brodalumab patients who achieved sPGA 0/1 were rerandomized to placebo or to induction dose. Four weeks later, those with sPGA scores of at least 3 received another induction dose. After 12 weeks of retreatment, patients scoring sPGA 2/3 were rescued with brodalumab 210 mg every 2 weeks (Br J Dermatol. 2016 Feb 23. doi: 10.1111/bjd.14493).
The psoriasis community has closely watched brodalumab, which handily beat ustekinumab in the AMAGINE-2 psoriasis trial before Amgen abruptly pulled out of development in the wake of two suicides by trial participants. AstraZeneca and Valeant Pharmaceuticals then partnered on the agent, and the Food and Drug Administration accepted a Biologics License Application in January 2016.
Dr. Papp and associates published details of the suicides, noting that one involved a 59-year-old man who died 2 months after his last 210-mg dose. He had no history of psychiatric disorders and normal baseline Hospital Anxiety and Depression Scale scores, which worsened on placebo but normalized by week 52. “The investigator reported that there was no reasonable possibility that the event of suicide was related to investigational product,” the researchers said. The second suicide involved a 56-year-old man who also received 210 mg brodalumab. He had a history of severe depression, but his Hospital Anxiety and Depression Scale (HADS) scores were normal when last assessed, his psoriasis was well controlled, and his death was considered unrelated to treatment. Indeed, moderate or severe baseline HADS scores were more likely to improve when patients received brodalumab instead of placebo, the researchers said. The other two deaths on study involved patients with substantial cardiovascular and hepatic comorbidities and also were considered unrelated to treatment.
Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
Key clinical point: The interleukin-17 inhibitor brodalumab met its coprimary endpoints in a phase III trial of patients with moderate to severe plaque psoriasis.
Major finding: At week 12, 83% of patients on 210 mg brodalumab achieved PASI 75, as did 60% of 140-mg patients and 3% of the placebo group. The coprimary endpoint, a static Physician Global Assessment score of 0/1, was met for 76% of 210-mg patients, 54% of 140-mg patients, and 1% of placebo patients. All four deaths were considered unrelated to treatment.
Data source: AMAGINE-1, a phase III, multicenter, randomized, double-blind study of brodalumab (210 or 140 mg) or placebo in 661 patients.
Disclosures: Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.