User login
FDA advisory panel unanimously backs biosimilars for Humira, Enbrel
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
On Twitter @mitchelzoler
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
On Twitter @mitchelzoler
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
On Twitter @mitchelzoler
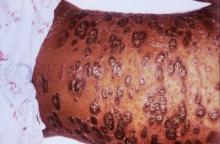
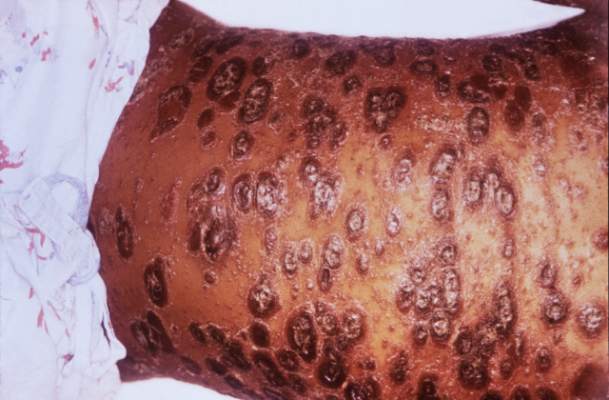
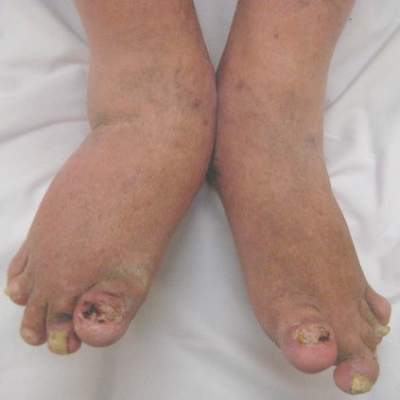
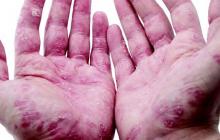
Debunking Psoriasis Myths: Is Psoriasis Contagious?
Myth: Psoriasis is contagious
One of the challenges patients with psoriasis face is misinformation among their peers, especially about the cause of psoriasis. Results from a survey conducted in France (N=1005) regarding knowledge of psoriasis and perceptions toward psoriasis patients indicated that approximately 16.5% of respondents believed that psoriasis is contagious; 6.8% believed that the disease is related to personal hygiene and 3.2% believed that it affects more people with low personal hygiene (Halioua et al). Moreover, approximately 62.4% of respondents recognized a lack of information about psoriasis and 19.7% noted that they have misconceptions about the disease.
Another study from Poland on the feelings of stigmatization among psoriasis patients (N=102) found that 72 psoriasis patients reported that they felt other people think their skin disease is contagious; only 30 psoriasis patients indicated that they did not feel this way (Hrehorów et al).
These findings underscore the need for more information on psoriasis in the general public worldwide. It is important to spread the message that psoriasis is a chronic inflammatory disease of the immune system that affects approximately 7.5 million individuals in the United States. One cannot "catch" psoriasis; psoriasis starts or worsens because of a variety of triggers (eg, infections, injury to the skin, stress, cold weather, smoking, heavy alcohol consumption, certain medications). The National Institute of Arthritis and Musculoskeletal and Skin Diseases offers a simple but revealing explanation for the cause of psoriasis: "Normally, T cells help protect the body against infection and disease. In the case of psoriasis, T cells are put into action by mistake and become so active that they trigger other immune responses, which lead to inflammation and to rapid turnover of skin cells." Genetics may play a role in the development of psoriasis, along with environmental factors.
With several new therapies available, psoriasis has gained media attention. Dermatologists can circulate more information about the causes of psoriasis, which may work toward helping patients with psoriasis cope with the social impact of the condition.
Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
Hrehorów E, Salomon J, Matusiak L, et al. Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92:67-72.
Psoriasis. American Academy of Dermatology website. https://www.aad.org/media/stats/conditions/psoriasis. Accessed July 7, 2016.
Psoriasis causes. Mayo Clinic website. http://www.mayoclinic.org/diseases-conditions/psoriasis/basics/causes/con-20030838. Published June 17, 2015. Accessed July 7, 2016.
Questions and answers about psoriasis. National Institute of Arthritis and Musculoskeletal and Skin Diseases website. http://www.niams.nih.gov/Health_Info/Psoriasis/default.asp. Published October 2013. Accessed July 7, 2016.
Myth: Psoriasis is contagious
One of the challenges patients with psoriasis face is misinformation among their peers, especially about the cause of psoriasis. Results from a survey conducted in France (N=1005) regarding knowledge of psoriasis and perceptions toward psoriasis patients indicated that approximately 16.5% of respondents believed that psoriasis is contagious; 6.8% believed that the disease is related to personal hygiene and 3.2% believed that it affects more people with low personal hygiene (Halioua et al). Moreover, approximately 62.4% of respondents recognized a lack of information about psoriasis and 19.7% noted that they have misconceptions about the disease.
Another study from Poland on the feelings of stigmatization among psoriasis patients (N=102) found that 72 psoriasis patients reported that they felt other people think their skin disease is contagious; only 30 psoriasis patients indicated that they did not feel this way (Hrehorów et al).
These findings underscore the need for more information on psoriasis in the general public worldwide. It is important to spread the message that psoriasis is a chronic inflammatory disease of the immune system that affects approximately 7.5 million individuals in the United States. One cannot "catch" psoriasis; psoriasis starts or worsens because of a variety of triggers (eg, infections, injury to the skin, stress, cold weather, smoking, heavy alcohol consumption, certain medications). The National Institute of Arthritis and Musculoskeletal and Skin Diseases offers a simple but revealing explanation for the cause of psoriasis: "Normally, T cells help protect the body against infection and disease. In the case of psoriasis, T cells are put into action by mistake and become so active that they trigger other immune responses, which lead to inflammation and to rapid turnover of skin cells." Genetics may play a role in the development of psoriasis, along with environmental factors.
With several new therapies available, psoriasis has gained media attention. Dermatologists can circulate more information about the causes of psoriasis, which may work toward helping patients with psoriasis cope with the social impact of the condition.
Myth: Psoriasis is contagious
One of the challenges patients with psoriasis face is misinformation among their peers, especially about the cause of psoriasis. Results from a survey conducted in France (N=1005) regarding knowledge of psoriasis and perceptions toward psoriasis patients indicated that approximately 16.5% of respondents believed that psoriasis is contagious; 6.8% believed that the disease is related to personal hygiene and 3.2% believed that it affects more people with low personal hygiene (Halioua et al). Moreover, approximately 62.4% of respondents recognized a lack of information about psoriasis and 19.7% noted that they have misconceptions about the disease.
Another study from Poland on the feelings of stigmatization among psoriasis patients (N=102) found that 72 psoriasis patients reported that they felt other people think their skin disease is contagious; only 30 psoriasis patients indicated that they did not feel this way (Hrehorów et al).
These findings underscore the need for more information on psoriasis in the general public worldwide. It is important to spread the message that psoriasis is a chronic inflammatory disease of the immune system that affects approximately 7.5 million individuals in the United States. One cannot "catch" psoriasis; psoriasis starts or worsens because of a variety of triggers (eg, infections, injury to the skin, stress, cold weather, smoking, heavy alcohol consumption, certain medications). The National Institute of Arthritis and Musculoskeletal and Skin Diseases offers a simple but revealing explanation for the cause of psoriasis: "Normally, T cells help protect the body against infection and disease. In the case of psoriasis, T cells are put into action by mistake and become so active that they trigger other immune responses, which lead to inflammation and to rapid turnover of skin cells." Genetics may play a role in the development of psoriasis, along with environmental factors.
With several new therapies available, psoriasis has gained media attention. Dermatologists can circulate more information about the causes of psoriasis, which may work toward helping patients with psoriasis cope with the social impact of the condition.
Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
Hrehorów E, Salomon J, Matusiak L, et al. Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92:67-72.
Psoriasis. American Academy of Dermatology website. https://www.aad.org/media/stats/conditions/psoriasis. Accessed July 7, 2016.
Psoriasis causes. Mayo Clinic website. http://www.mayoclinic.org/diseases-conditions/psoriasis/basics/causes/con-20030838. Published June 17, 2015. Accessed July 7, 2016.
Questions and answers about psoriasis. National Institute of Arthritis and Musculoskeletal and Skin Diseases website. http://www.niams.nih.gov/Health_Info/Psoriasis/default.asp. Published October 2013. Accessed July 7, 2016.
Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
Hrehorów E, Salomon J, Matusiak L, et al. Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92:67-72.
Psoriasis. American Academy of Dermatology website. https://www.aad.org/media/stats/conditions/psoriasis. Accessed July 7, 2016.
Psoriasis causes. Mayo Clinic website. http://www.mayoclinic.org/diseases-conditions/psoriasis/basics/causes/con-20030838. Published June 17, 2015. Accessed July 7, 2016.
Questions and answers about psoriasis. National Institute of Arthritis and Musculoskeletal and Skin Diseases website. http://www.niams.nih.gov/Health_Info/Psoriasis/default.asp. Published October 2013. Accessed July 7, 2016.
Severe psoriasis upped lymphoma risk in large cohort study
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Psoriasis was identified as an independent risk factor for lymphoma, with the risk of lymphoma increasing with disease severity.
Major finding: The strongest association was between severe psoriasis and cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4).
Data source: A population-based longitudinal cohort study of 12,198 patients with severe psoriasis, 184,870 patients with mild psoriasis, and 965,730 nonpsoriatic controls.
Disclosures: The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research support from Pfizer outside the submitted work.
Subclinical Joint Disease
We are aware of the clinical importance of diagnosing psoriatic arthritis (PsA) as early as possible to initiate appropriate therapy. Because psoriasis precedes PsA in the majority of cases, it is incumbent on clinicians to seek any evidence of joint involvement at each clinical encounter.
In a study published online on February 25 in Annals of the Rheumatic Diseases. Faustini et al reported that patients with psoriasis but without PsA experience structural joint changes at the entheses. Therefore, evidence for structural joint alterations may already exist at the time of apparently exclusive skin involvement in psoriatic disease.
In the analysis, 85 participants without arthritis, including 55 with psoriasis and 30 healthy controls, received high-field magnetic resonance imaging (MRI) of the hand. These scans were scored for synovitis, osteitis, tenosynovitis, and periarticular inflammation. Participants with psoriasis also received complete clinical investigation as well as high-resolution peripheral quantitative computed tomography for detecting erosions and enthesiophytes. All participants were followed for at least 1 year to evaluate for the development of PsA.
Magnetic resonance imaging evaluation showed that 47% (26/55) of participants with psoriasis possessed at least 1 inflammatory lesion. Synovitis was the most prevalent inflammatory lesion (38% [21/55]), while osteitis (11% [6/55]), tenosynovitis (4% [2/55]), and periarticular inflammation (4% [2/55]) were less frequent.
The incidence of enthesiophytes and bone erosions did not differ between patients with psoriasis, with or without inflammatory changes on MRI. The risk for developing PsA was as high as 60% in those with subclinical synovitis and symptoms related to arthralgia. However, the risk was only 13% if the patients had normal MRIs and did not report arthralgia. Faustini et al concluded that the prevalence of subclinical inflammatory lesions is high in patients with cutaneous psoriasis. Specifically, arthralgia in conjunction with MRI synovitis constitutes a high-risk constellation for the development of PsA.
What’s the issue?
These findings are critical, as they indicate the nature of the potential genesis of PsA in many patients. If the data are confirmed in future investigations, it may change the way we evaluate or treat early PsA. How will these findings affect your workup for early PsA?
We are aware of the clinical importance of diagnosing psoriatic arthritis (PsA) as early as possible to initiate appropriate therapy. Because psoriasis precedes PsA in the majority of cases, it is incumbent on clinicians to seek any evidence of joint involvement at each clinical encounter.
In a study published online on February 25 in Annals of the Rheumatic Diseases. Faustini et al reported that patients with psoriasis but without PsA experience structural joint changes at the entheses. Therefore, evidence for structural joint alterations may already exist at the time of apparently exclusive skin involvement in psoriatic disease.
In the analysis, 85 participants without arthritis, including 55 with psoriasis and 30 healthy controls, received high-field magnetic resonance imaging (MRI) of the hand. These scans were scored for synovitis, osteitis, tenosynovitis, and periarticular inflammation. Participants with psoriasis also received complete clinical investigation as well as high-resolution peripheral quantitative computed tomography for detecting erosions and enthesiophytes. All participants were followed for at least 1 year to evaluate for the development of PsA.
Magnetic resonance imaging evaluation showed that 47% (26/55) of participants with psoriasis possessed at least 1 inflammatory lesion. Synovitis was the most prevalent inflammatory lesion (38% [21/55]), while osteitis (11% [6/55]), tenosynovitis (4% [2/55]), and periarticular inflammation (4% [2/55]) were less frequent.
The incidence of enthesiophytes and bone erosions did not differ between patients with psoriasis, with or without inflammatory changes on MRI. The risk for developing PsA was as high as 60% in those with subclinical synovitis and symptoms related to arthralgia. However, the risk was only 13% if the patients had normal MRIs and did not report arthralgia. Faustini et al concluded that the prevalence of subclinical inflammatory lesions is high in patients with cutaneous psoriasis. Specifically, arthralgia in conjunction with MRI synovitis constitutes a high-risk constellation for the development of PsA.
What’s the issue?
These findings are critical, as they indicate the nature of the potential genesis of PsA in many patients. If the data are confirmed in future investigations, it may change the way we evaluate or treat early PsA. How will these findings affect your workup for early PsA?
We are aware of the clinical importance of diagnosing psoriatic arthritis (PsA) as early as possible to initiate appropriate therapy. Because psoriasis precedes PsA in the majority of cases, it is incumbent on clinicians to seek any evidence of joint involvement at each clinical encounter.
In a study published online on February 25 in Annals of the Rheumatic Diseases. Faustini et al reported that patients with psoriasis but without PsA experience structural joint changes at the entheses. Therefore, evidence for structural joint alterations may already exist at the time of apparently exclusive skin involvement in psoriatic disease.
In the analysis, 85 participants without arthritis, including 55 with psoriasis and 30 healthy controls, received high-field magnetic resonance imaging (MRI) of the hand. These scans were scored for synovitis, osteitis, tenosynovitis, and periarticular inflammation. Participants with psoriasis also received complete clinical investigation as well as high-resolution peripheral quantitative computed tomography for detecting erosions and enthesiophytes. All participants were followed for at least 1 year to evaluate for the development of PsA.
Magnetic resonance imaging evaluation showed that 47% (26/55) of participants with psoriasis possessed at least 1 inflammatory lesion. Synovitis was the most prevalent inflammatory lesion (38% [21/55]), while osteitis (11% [6/55]), tenosynovitis (4% [2/55]), and periarticular inflammation (4% [2/55]) were less frequent.
The incidence of enthesiophytes and bone erosions did not differ between patients with psoriasis, with or without inflammatory changes on MRI. The risk for developing PsA was as high as 60% in those with subclinical synovitis and symptoms related to arthralgia. However, the risk was only 13% if the patients had normal MRIs and did not report arthralgia. Faustini et al concluded that the prevalence of subclinical inflammatory lesions is high in patients with cutaneous psoriasis. Specifically, arthralgia in conjunction with MRI synovitis constitutes a high-risk constellation for the development of PsA.
What’s the issue?
These findings are critical, as they indicate the nature of the potential genesis of PsA in many patients. If the data are confirmed in future investigations, it may change the way we evaluate or treat early PsA. How will these findings affect your workup for early PsA?
Analysis supports daily folate for children with psoriasis on methotrexate
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Consider daily folate to reduce the likelihood of gastrointestinal side effects of methotrexate in children with psoriasis.
Major finding: The odds of gastrointestinal adverse effects were about 75% lower with daily folate, compared with weekly dosing or 6 days per week dosing that spared the methotrexate day (odds ratio, 0.25; P less than .001).
Data source: An international retrospective study of 446 children receiving phototherapy or systemic therapy for psoriasis.
Disclosures: The International Psoriasis Council funded the study. The investigators did not list disclosures.
Significant race and health care disparities exist among hospitalized psoriasis patients
Significant racial and health care disparities exist among hospitalized psoriasis patients, according to a recent study by Derek Y. Hsu of the Department of Dermatology at Northwestern University, Chicago, and his coauthors.
Researchers looked at a sample representative of 20% of all U.S. hospitalizations from 2002 to 2012, which showed racial and health care disparities for hospitalization of psoriasis cases. Hospitalization was associated with nonwhite race (Asian odds ratio, 2.08; 95% confidence interval, 1.55-2.78; black OR, 1.65; 95% CI, 1.43-1.89; and multiracial/other OR, 1.54; 95% CI, 1.13-2.11) and insurance status (Medicare OR, 1.33; 95% CI, 1.26-1.40; Medicaid OR, 1.32; 95% CI, 1.25-1.40; and uninsured OR, 1.94; 95% CI, 1.64-2.30). Additionally, length of stay was significantly prolonged for psoriasis patients, the investigators found.
“Patients who were admitted for psoriasis were significantly more likely to be Hispanic, Asian, or multiracial/other compared with Caucasians, less likely to be female and more likely to have Medicare, Medicaid, or be uninsured compared with private insurance,” the authors said in the report. “These patients also had higher odds of multiple chronic conditions.”
The results indicate a need for improved access to regular dermatological care for all patients with psoriasis, the authors concluded.
Read the full article in the Journal of the American Academy of Dermatology.
Significant racial and health care disparities exist among hospitalized psoriasis patients, according to a recent study by Derek Y. Hsu of the Department of Dermatology at Northwestern University, Chicago, and his coauthors.
Researchers looked at a sample representative of 20% of all U.S. hospitalizations from 2002 to 2012, which showed racial and health care disparities for hospitalization of psoriasis cases. Hospitalization was associated with nonwhite race (Asian odds ratio, 2.08; 95% confidence interval, 1.55-2.78; black OR, 1.65; 95% CI, 1.43-1.89; and multiracial/other OR, 1.54; 95% CI, 1.13-2.11) and insurance status (Medicare OR, 1.33; 95% CI, 1.26-1.40; Medicaid OR, 1.32; 95% CI, 1.25-1.40; and uninsured OR, 1.94; 95% CI, 1.64-2.30). Additionally, length of stay was significantly prolonged for psoriasis patients, the investigators found.
“Patients who were admitted for psoriasis were significantly more likely to be Hispanic, Asian, or multiracial/other compared with Caucasians, less likely to be female and more likely to have Medicare, Medicaid, or be uninsured compared with private insurance,” the authors said in the report. “These patients also had higher odds of multiple chronic conditions.”
The results indicate a need for improved access to regular dermatological care for all patients with psoriasis, the authors concluded.
Read the full article in the Journal of the American Academy of Dermatology.
Significant racial and health care disparities exist among hospitalized psoriasis patients, according to a recent study by Derek Y. Hsu of the Department of Dermatology at Northwestern University, Chicago, and his coauthors.
Researchers looked at a sample representative of 20% of all U.S. hospitalizations from 2002 to 2012, which showed racial and health care disparities for hospitalization of psoriasis cases. Hospitalization was associated with nonwhite race (Asian odds ratio, 2.08; 95% confidence interval, 1.55-2.78; black OR, 1.65; 95% CI, 1.43-1.89; and multiracial/other OR, 1.54; 95% CI, 1.13-2.11) and insurance status (Medicare OR, 1.33; 95% CI, 1.26-1.40; Medicaid OR, 1.32; 95% CI, 1.25-1.40; and uninsured OR, 1.94; 95% CI, 1.64-2.30). Additionally, length of stay was significantly prolonged for psoriasis patients, the investigators found.
“Patients who were admitted for psoriasis were significantly more likely to be Hispanic, Asian, or multiracial/other compared with Caucasians, less likely to be female and more likely to have Medicare, Medicaid, or be uninsured compared with private insurance,” the authors said in the report. “These patients also had higher odds of multiple chronic conditions.”
The results indicate a need for improved access to regular dermatological care for all patients with psoriasis, the authors concluded.
Read the full article in the Journal of the American Academy of Dermatology.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Obesity may attenuate anti-TNF response in psoriatic arthritis
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
Key clinical point: Obesity may reduce response and adherence to tumor necrosis factor–inhibitor therapy in patients with psoriatic arthritis.
Major finding: The EULAR good or moderate response rate at 6 months was 55% in obese vs. 65% in nonobese patients (P = .02).
Data source: Observational cohort study based on two Nordic registries of 1,943 patients with PsA prescribed their first TNFi.
Disclosures: DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
Debunking Psoriasis Myths: Can Diet Clear Psoriasis?
Myth: Psoriasis Can Be Treated By Eating or Avoiding Certain Foods
Patients who Google “diet and psoriasis” are flooded with search results of diets claiming to cure psoriasis. This misinformation is dangerous for patients, as there is no scientific evidence that any specific psoriasis diet can treat the condition. Patients may wish to improve their diet to prevent comorbidities such as cardiovascular disease and metabolic syndrome. Even though it may not be a cure, encouraging patients to eat healthy is never a bad thing.
In a 2014 analysis of psoriasis, obesity, body mass index (BMI), and diet literature, an increased risk for psoriasis development in the setting of obesity was discussed. There is evidence suggesting that a BMI greater than 30 kg/m2 may potentially play a role in the ability to achieve a full therapeutic effect of psoriasis therapy. “This could be for two possible reasons,” Debbaneh et al reported. “It may be a consequence of decreased drug distribution into the body due to increased body mass, or it may be a consequence of increased pro-inflammatory cytokine release as a result of increased adipocyte count.” However, this finding may be treatment specific. For example, higher body weight was an independent predictor of response to ustekinumab, providing the rationale for offering 2 weight-based dosing regimens of the drug. Overweight and obese patients also were less likely to experience clearance with adalimumab. However, studies have found no association between BMI and biologic treatment.
Weight loss through a low-calorie diet has been reported to achieve a greater reduction in psoriasis severity and a slower rebound of disease. “Interestingly, studies have shown that caloric restriction in obese subjects lowers the level of circulating inflammatory cytokines,” reported Debbaneh et al. “This may contribute to the observed beneficial effect in psoriatic disease.”
In patients whose disease has had a significant impact on quality of life, it is important that they are consulting resources online that will help them maintain a healthy lifestyle. The National Psoriasis Foundation provides useful information on diet and psoriasis, emphasizing that diet is not going to cure psoriatic disease but eating healthier can only help.
Expert Commentary
Many of my psoriasis patients ask me what should they avoid eating to prevent the psoriasis from worsening, or what did they eat to cause psoriasis to occur in the first place. I stress to patients that what they eat is not likely the cause of their psoriasis nor will avoiding certain foods prevent a flare. However, alcohol use may induce a psoriasis flare.
—Jashin J. Wu, MD (Los Angeles, California)
Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis: part I. impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
National Psoriasis Foundation. Diet and psoriasis. https://www.psoriasis.org/about-psoriasis/treatments/alternative/diet-supplements. Accessed June 20, 2016.
Myth: Psoriasis Can Be Treated By Eating or Avoiding Certain Foods
Patients who Google “diet and psoriasis” are flooded with search results of diets claiming to cure psoriasis. This misinformation is dangerous for patients, as there is no scientific evidence that any specific psoriasis diet can treat the condition. Patients may wish to improve their diet to prevent comorbidities such as cardiovascular disease and metabolic syndrome. Even though it may not be a cure, encouraging patients to eat healthy is never a bad thing.
In a 2014 analysis of psoriasis, obesity, body mass index (BMI), and diet literature, an increased risk for psoriasis development in the setting of obesity was discussed. There is evidence suggesting that a BMI greater than 30 kg/m2 may potentially play a role in the ability to achieve a full therapeutic effect of psoriasis therapy. “This could be for two possible reasons,” Debbaneh et al reported. “It may be a consequence of decreased drug distribution into the body due to increased body mass, or it may be a consequence of increased pro-inflammatory cytokine release as a result of increased adipocyte count.” However, this finding may be treatment specific. For example, higher body weight was an independent predictor of response to ustekinumab, providing the rationale for offering 2 weight-based dosing regimens of the drug. Overweight and obese patients also were less likely to experience clearance with adalimumab. However, studies have found no association between BMI and biologic treatment.
Weight loss through a low-calorie diet has been reported to achieve a greater reduction in psoriasis severity and a slower rebound of disease. “Interestingly, studies have shown that caloric restriction in obese subjects lowers the level of circulating inflammatory cytokines,” reported Debbaneh et al. “This may contribute to the observed beneficial effect in psoriatic disease.”
In patients whose disease has had a significant impact on quality of life, it is important that they are consulting resources online that will help them maintain a healthy lifestyle. The National Psoriasis Foundation provides useful information on diet and psoriasis, emphasizing that diet is not going to cure psoriatic disease but eating healthier can only help.
Expert Commentary
Many of my psoriasis patients ask me what should they avoid eating to prevent the psoriasis from worsening, or what did they eat to cause psoriasis to occur in the first place. I stress to patients that what they eat is not likely the cause of their psoriasis nor will avoiding certain foods prevent a flare. However, alcohol use may induce a psoriasis flare.
—Jashin J. Wu, MD (Los Angeles, California)
Myth: Psoriasis Can Be Treated By Eating or Avoiding Certain Foods
Patients who Google “diet and psoriasis” are flooded with search results of diets claiming to cure psoriasis. This misinformation is dangerous for patients, as there is no scientific evidence that any specific psoriasis diet can treat the condition. Patients may wish to improve their diet to prevent comorbidities such as cardiovascular disease and metabolic syndrome. Even though it may not be a cure, encouraging patients to eat healthy is never a bad thing.
In a 2014 analysis of psoriasis, obesity, body mass index (BMI), and diet literature, an increased risk for psoriasis development in the setting of obesity was discussed. There is evidence suggesting that a BMI greater than 30 kg/m2 may potentially play a role in the ability to achieve a full therapeutic effect of psoriasis therapy. “This could be for two possible reasons,” Debbaneh et al reported. “It may be a consequence of decreased drug distribution into the body due to increased body mass, or it may be a consequence of increased pro-inflammatory cytokine release as a result of increased adipocyte count.” However, this finding may be treatment specific. For example, higher body weight was an independent predictor of response to ustekinumab, providing the rationale for offering 2 weight-based dosing regimens of the drug. Overweight and obese patients also were less likely to experience clearance with adalimumab. However, studies have found no association between BMI and biologic treatment.
Weight loss through a low-calorie diet has been reported to achieve a greater reduction in psoriasis severity and a slower rebound of disease. “Interestingly, studies have shown that caloric restriction in obese subjects lowers the level of circulating inflammatory cytokines,” reported Debbaneh et al. “This may contribute to the observed beneficial effect in psoriatic disease.”
In patients whose disease has had a significant impact on quality of life, it is important that they are consulting resources online that will help them maintain a healthy lifestyle. The National Psoriasis Foundation provides useful information on diet and psoriasis, emphasizing that diet is not going to cure psoriatic disease but eating healthier can only help.
Expert Commentary
Many of my psoriasis patients ask me what should they avoid eating to prevent the psoriasis from worsening, or what did they eat to cause psoriasis to occur in the first place. I stress to patients that what they eat is not likely the cause of their psoriasis nor will avoiding certain foods prevent a flare. However, alcohol use may induce a psoriasis flare.
—Jashin J. Wu, MD (Los Angeles, California)
Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis: part I. impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
National Psoriasis Foundation. Diet and psoriasis. https://www.psoriasis.org/about-psoriasis/treatments/alternative/diet-supplements. Accessed June 20, 2016.
Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis: part I. impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
National Psoriasis Foundation. Diet and psoriasis. https://www.psoriasis.org/about-psoriasis/treatments/alternative/diet-supplements. Accessed June 20, 2016.
Debunking Psoriasis Myths: Do Biologics Cause Cancer?
Myth: Biologics Cause Cancer
Biologics generally are safe and well-tolerated therapies; however, due to their immunosuppressive properties, the risk for lymphoma has been of potential concern, leading patients to believe that biologics cause cancer. The risk of some cancers, including some solid cancers, hematologic cancers, and skin cancers, appears to be increased in patients with psoriasis, possibly associated with chronic inflammation. Some psoriasis therapies may increase the risk for malignancy, including phototherapy with psoralen plus UVA, cyclosporine, and methotrexate.
Many studies have supported a favorable safety profile for biologics in terms of the risk for developing malignancy. In a 2015 analysis of 12,093 patients enrolled in PSOLAR (Psoriasis Longitudinal Assessment and Registry), none of the biologics were found to be associated with increased risk for malignancy.
In psoriasis patients with existing or prior malignancies, the benefits of biologic therapy to improve quality of life often outweigh the negligible risks for malignancy. However, coordinated care with oncology is recommended for psoriasis patients with a history of prior malignancy.
General recommendations from the American Academy of Dermatology indicate one should carefully consider the decision to use a tumor necrosis factor (TNF) antagonist in patients with a history of malignancy, particularly lymphoma. Short-term treatment with biologics (up to 4 years) appears to be safe with respect to lymphoma risk, especially with TNF-α inhibitors. The potential risk for melanoma, cutaneous T-cell lymphoma, and nonmelanoma skin cancer in patients treated with TNF inhibitors also has been raised.
Expert Commentary
OBSERVE-5 was a 5-year phase 4, prospective, multicenter surveillance registry of 2510 psoriasis patients with at least a baseline dose of etanercept (Kimball et al, 2015). There was no increased risk for cancer when compared to the Truven Health MarketScan database, which is a proxy for the general population.
ESPRIT is an ongoing, 10-year, international, prospective, observational registry of 6059 psoriasis patients with at least a baseline dose of adalimumab (Menter et al). There are no signals of increased risk for cancer.
We do not have enough numbers of psoriasis patients on secukinumab or ixekizumab yet, but their phase 3 trials also do not seem to indicate an increased risk for cancer.
—Jashin J. Wu, MD (Los Angeles, California)
American Academy of Dermatology. Psoriasis: TNF inhibitors general recommendations. https://www.aad.org/practice-tools/quality-care/clinical-guidelines/psoriasis/biologics/tnf-inhibitors-recommendations. Accessed June 14, 2016.
Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
Kimball AB, Rothman KJ, Kricorian G, et al. OBSERVE-5: Observational postmarketing safety surveillance registry of etanercept for the treatment of psoriasis final 5-year results. J Am Acad Dermatol. 2015;72:115-122.
Kimball AB, Schenfeld J, Accortt NA, et al. 5-year incidence rates of malignancies in psoriasis patients compared with the general US population. Poster presented at: Summer Meeting of the American Academy of Dermatology; August 6-10, 2014; Chicago, IL.
Menter A, Thaçi D, Papp KA, et al. Five-year analysis from the ESPRIT 10-year postmarketing surveillance registry of adalimumab treatment for moderate to severe psoriasis. J Am Acad Dermatol. 2015;73:410-419.e6.
Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
Patel S, Patel T, Kerdel FA. The risk of malignancy or progression of existing malignancy in patients with psoriasis treated with biologics: case report and review of the literature. Int J Dermatol. 2016;55:487-493.
Myth: Biologics Cause Cancer
Biologics generally are safe and well-tolerated therapies; however, due to their immunosuppressive properties, the risk for lymphoma has been of potential concern, leading patients to believe that biologics cause cancer. The risk of some cancers, including some solid cancers, hematologic cancers, and skin cancers, appears to be increased in patients with psoriasis, possibly associated with chronic inflammation. Some psoriasis therapies may increase the risk for malignancy, including phototherapy with psoralen plus UVA, cyclosporine, and methotrexate.
Many studies have supported a favorable safety profile for biologics in terms of the risk for developing malignancy. In a 2015 analysis of 12,093 patients enrolled in PSOLAR (Psoriasis Longitudinal Assessment and Registry), none of the biologics were found to be associated with increased risk for malignancy.
In psoriasis patients with existing or prior malignancies, the benefits of biologic therapy to improve quality of life often outweigh the negligible risks for malignancy. However, coordinated care with oncology is recommended for psoriasis patients with a history of prior malignancy.
General recommendations from the American Academy of Dermatology indicate one should carefully consider the decision to use a tumor necrosis factor (TNF) antagonist in patients with a history of malignancy, particularly lymphoma. Short-term treatment with biologics (up to 4 years) appears to be safe with respect to lymphoma risk, especially with TNF-α inhibitors. The potential risk for melanoma, cutaneous T-cell lymphoma, and nonmelanoma skin cancer in patients treated with TNF inhibitors also has been raised.
Expert Commentary
OBSERVE-5 was a 5-year phase 4, prospective, multicenter surveillance registry of 2510 psoriasis patients with at least a baseline dose of etanercept (Kimball et al, 2015). There was no increased risk for cancer when compared to the Truven Health MarketScan database, which is a proxy for the general population.
ESPRIT is an ongoing, 10-year, international, prospective, observational registry of 6059 psoriasis patients with at least a baseline dose of adalimumab (Menter et al). There are no signals of increased risk for cancer.
We do not have enough numbers of psoriasis patients on secukinumab or ixekizumab yet, but their phase 3 trials also do not seem to indicate an increased risk for cancer.
—Jashin J. Wu, MD (Los Angeles, California)
Myth: Biologics Cause Cancer
Biologics generally are safe and well-tolerated therapies; however, due to their immunosuppressive properties, the risk for lymphoma has been of potential concern, leading patients to believe that biologics cause cancer. The risk of some cancers, including some solid cancers, hematologic cancers, and skin cancers, appears to be increased in patients with psoriasis, possibly associated with chronic inflammation. Some psoriasis therapies may increase the risk for malignancy, including phototherapy with psoralen plus UVA, cyclosporine, and methotrexate.
Many studies have supported a favorable safety profile for biologics in terms of the risk for developing malignancy. In a 2015 analysis of 12,093 patients enrolled in PSOLAR (Psoriasis Longitudinal Assessment and Registry), none of the biologics were found to be associated with increased risk for malignancy.
In psoriasis patients with existing or prior malignancies, the benefits of biologic therapy to improve quality of life often outweigh the negligible risks for malignancy. However, coordinated care with oncology is recommended for psoriasis patients with a history of prior malignancy.
General recommendations from the American Academy of Dermatology indicate one should carefully consider the decision to use a tumor necrosis factor (TNF) antagonist in patients with a history of malignancy, particularly lymphoma. Short-term treatment with biologics (up to 4 years) appears to be safe with respect to lymphoma risk, especially with TNF-α inhibitors. The potential risk for melanoma, cutaneous T-cell lymphoma, and nonmelanoma skin cancer in patients treated with TNF inhibitors also has been raised.
Expert Commentary
OBSERVE-5 was a 5-year phase 4, prospective, multicenter surveillance registry of 2510 psoriasis patients with at least a baseline dose of etanercept (Kimball et al, 2015). There was no increased risk for cancer when compared to the Truven Health MarketScan database, which is a proxy for the general population.
ESPRIT is an ongoing, 10-year, international, prospective, observational registry of 6059 psoriasis patients with at least a baseline dose of adalimumab (Menter et al). There are no signals of increased risk for cancer.
We do not have enough numbers of psoriasis patients on secukinumab or ixekizumab yet, but their phase 3 trials also do not seem to indicate an increased risk for cancer.
—Jashin J. Wu, MD (Los Angeles, California)
American Academy of Dermatology. Psoriasis: TNF inhibitors general recommendations. https://www.aad.org/practice-tools/quality-care/clinical-guidelines/psoriasis/biologics/tnf-inhibitors-recommendations. Accessed June 14, 2016.
Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
Kimball AB, Rothman KJ, Kricorian G, et al. OBSERVE-5: Observational postmarketing safety surveillance registry of etanercept for the treatment of psoriasis final 5-year results. J Am Acad Dermatol. 2015;72:115-122.
Kimball AB, Schenfeld J, Accortt NA, et al. 5-year incidence rates of malignancies in psoriasis patients compared with the general US population. Poster presented at: Summer Meeting of the American Academy of Dermatology; August 6-10, 2014; Chicago, IL.
Menter A, Thaçi D, Papp KA, et al. Five-year analysis from the ESPRIT 10-year postmarketing surveillance registry of adalimumab treatment for moderate to severe psoriasis. J Am Acad Dermatol. 2015;73:410-419.e6.
Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
Patel S, Patel T, Kerdel FA. The risk of malignancy or progression of existing malignancy in patients with psoriasis treated with biologics: case report and review of the literature. Int J Dermatol. 2016;55:487-493.
American Academy of Dermatology. Psoriasis: TNF inhibitors general recommendations. https://www.aad.org/practice-tools/quality-care/clinical-guidelines/psoriasis/biologics/tnf-inhibitors-recommendations. Accessed June 14, 2016.
Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
Kimball AB, Rothman KJ, Kricorian G, et al. OBSERVE-5: Observational postmarketing safety surveillance registry of etanercept for the treatment of psoriasis final 5-year results. J Am Acad Dermatol. 2015;72:115-122.
Kimball AB, Schenfeld J, Accortt NA, et al. 5-year incidence rates of malignancies in psoriasis patients compared with the general US population. Poster presented at: Summer Meeting of the American Academy of Dermatology; August 6-10, 2014; Chicago, IL.
Menter A, Thaçi D, Papp KA, et al. Five-year analysis from the ESPRIT 10-year postmarketing surveillance registry of adalimumab treatment for moderate to severe psoriasis. J Am Acad Dermatol. 2015;73:410-419.e6.
Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
Patel S, Patel T, Kerdel FA. The risk of malignancy or progression of existing malignancy in patients with psoriasis treated with biologics: case report and review of the literature. Int J Dermatol. 2016;55:487-493.
Study: TNF inhibitors improve extraintestinal IBD manifestations
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
AT DDW® 2016
Key clinical point: Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease among more than half of affected patients.
Major finding: About 55% of patients who received infliximab, adalimumab, or certolizumab had a clinical response.
Data source: A study of 1,249 patients with IBD from a national cohort.
Disclosures: A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.