User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
‘Difficult’ patients: How to improve rapport
As psychiatrists, we all come across patients who press our buttons and engender negative feelings, such as anger, frustration, and inadequacy.1 These patients have been referred to as “hateful” or “difficult” because they disrupt the treatment alliance.1,2 We are quick to point our fingers at such patients for making our jobs harder, being noncompliant, resisting the therapeutic alliance, and in general, being “problem patients.”3 However, the physician–patient relationship is a 2-way street. Although our patients knowingly or unknowingly play a role in this dynamic, we could be overlooking our role in adversely affecting this relationship. The following factors influence the physician–patient bond.1,2
Countertransference. We may have negative feelings toward a patient based on our personalities and/or if the patient reminds us of someone we may not like, which could lead us to overprescribe or underprescribe medications, conduct unnecessary medical workups, distance ourselves from the patient, etc. Accepting our disdain for certain patients and understanding why we have these emotions will allow us to better understand them, ensure that we are not impeding the delivery of appropriate clinical care, and improve rapport.
Listening. It may seem obvious that not listening to our patients negatively impacts rapport. However, in today’s technological world, we may not be really listening to our patients even when we think we are. Answering a text message or reading the patient’s electronic medical record while they are talking to us may increase productivity, but doing so also can interfere with our ability to form a therapeutic alliance. Although we may hear what our patients are saying, such distractions can create a hurdle in listening to what they are telling us.
Empathy often is confused for sympathy. Sympathy entails expressing concern and compassion for one’s distress, whereas empathy includes recognizing and sharing the patient’s emotions. Identifying with and understanding our patients’ situations, drives, and feelings allows us to understand what they are experiencing, see why they are reacting in a negative manner, and protect them from unnecessary emotional distress. Empathy can lead us to know what needs to be said and what should be said. It also can demystify a patient’s suffering. Not providing empathy or substituting sympathy can disrupt the therapeutic alliance.
Projective identification. Patients can project intolerable and negative feelings onto us and coerce us into identifying with what has been projected, allowing them to indirectly take control of our emotions. Our subsequent reactions can unsettle the physician–patient relationship. We need to be attuned to this process and recognize what the patient is provoking within us. Once we understand the process, we can realize that this is how they deal with others under similarly stressful conditions, and then react in a more supportive and healthy manner, rather than reviling our patients and negatively impacting the therapeutic relationship.
1. Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
2. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298(16):883-887.
3. Boland R. The ‘problem patient’: modest advice for frustrated clinicians. R I Med J (2013). 2014;97(6):29-32.
As psychiatrists, we all come across patients who press our buttons and engender negative feelings, such as anger, frustration, and inadequacy.1 These patients have been referred to as “hateful” or “difficult” because they disrupt the treatment alliance.1,2 We are quick to point our fingers at such patients for making our jobs harder, being noncompliant, resisting the therapeutic alliance, and in general, being “problem patients.”3 However, the physician–patient relationship is a 2-way street. Although our patients knowingly or unknowingly play a role in this dynamic, we could be overlooking our role in adversely affecting this relationship. The following factors influence the physician–patient bond.1,2
Countertransference. We may have negative feelings toward a patient based on our personalities and/or if the patient reminds us of someone we may not like, which could lead us to overprescribe or underprescribe medications, conduct unnecessary medical workups, distance ourselves from the patient, etc. Accepting our disdain for certain patients and understanding why we have these emotions will allow us to better understand them, ensure that we are not impeding the delivery of appropriate clinical care, and improve rapport.
Listening. It may seem obvious that not listening to our patients negatively impacts rapport. However, in today’s technological world, we may not be really listening to our patients even when we think we are. Answering a text message or reading the patient’s electronic medical record while they are talking to us may increase productivity, but doing so also can interfere with our ability to form a therapeutic alliance. Although we may hear what our patients are saying, such distractions can create a hurdle in listening to what they are telling us.
Empathy often is confused for sympathy. Sympathy entails expressing concern and compassion for one’s distress, whereas empathy includes recognizing and sharing the patient’s emotions. Identifying with and understanding our patients’ situations, drives, and feelings allows us to understand what they are experiencing, see why they are reacting in a negative manner, and protect them from unnecessary emotional distress. Empathy can lead us to know what needs to be said and what should be said. It also can demystify a patient’s suffering. Not providing empathy or substituting sympathy can disrupt the therapeutic alliance.
Projective identification. Patients can project intolerable and negative feelings onto us and coerce us into identifying with what has been projected, allowing them to indirectly take control of our emotions. Our subsequent reactions can unsettle the physician–patient relationship. We need to be attuned to this process and recognize what the patient is provoking within us. Once we understand the process, we can realize that this is how they deal with others under similarly stressful conditions, and then react in a more supportive and healthy manner, rather than reviling our patients and negatively impacting the therapeutic relationship.
As psychiatrists, we all come across patients who press our buttons and engender negative feelings, such as anger, frustration, and inadequacy.1 These patients have been referred to as “hateful” or “difficult” because they disrupt the treatment alliance.1,2 We are quick to point our fingers at such patients for making our jobs harder, being noncompliant, resisting the therapeutic alliance, and in general, being “problem patients.”3 However, the physician–patient relationship is a 2-way street. Although our patients knowingly or unknowingly play a role in this dynamic, we could be overlooking our role in adversely affecting this relationship. The following factors influence the physician–patient bond.1,2
Countertransference. We may have negative feelings toward a patient based on our personalities and/or if the patient reminds us of someone we may not like, which could lead us to overprescribe or underprescribe medications, conduct unnecessary medical workups, distance ourselves from the patient, etc. Accepting our disdain for certain patients and understanding why we have these emotions will allow us to better understand them, ensure that we are not impeding the delivery of appropriate clinical care, and improve rapport.
Listening. It may seem obvious that not listening to our patients negatively impacts rapport. However, in today’s technological world, we may not be really listening to our patients even when we think we are. Answering a text message or reading the patient’s electronic medical record while they are talking to us may increase productivity, but doing so also can interfere with our ability to form a therapeutic alliance. Although we may hear what our patients are saying, such distractions can create a hurdle in listening to what they are telling us.
Empathy often is confused for sympathy. Sympathy entails expressing concern and compassion for one’s distress, whereas empathy includes recognizing and sharing the patient’s emotions. Identifying with and understanding our patients’ situations, drives, and feelings allows us to understand what they are experiencing, see why they are reacting in a negative manner, and protect them from unnecessary emotional distress. Empathy can lead us to know what needs to be said and what should be said. It also can demystify a patient’s suffering. Not providing empathy or substituting sympathy can disrupt the therapeutic alliance.
Projective identification. Patients can project intolerable and negative feelings onto us and coerce us into identifying with what has been projected, allowing them to indirectly take control of our emotions. Our subsequent reactions can unsettle the physician–patient relationship. We need to be attuned to this process and recognize what the patient is provoking within us. Once we understand the process, we can realize that this is how they deal with others under similarly stressful conditions, and then react in a more supportive and healthy manner, rather than reviling our patients and negatively impacting the therapeutic relationship.
1. Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
2. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298(16):883-887.
3. Boland R. The ‘problem patient’: modest advice for frustrated clinicians. R I Med J (2013). 2014;97(6):29-32.
1. Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
2. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298(16):883-887.
3. Boland R. The ‘problem patient’: modest advice for frustrated clinicians. R I Med J (2013). 2014;97(6):29-32.
A shot in the arm: Boost your knowledge about immunizations for psychiatric patients
Patients with chronic, severe mental illness live much shorter lives than the general population. The 25-year loss in life expectancy for people with chronic mental illness has been attributed to higher rates of cardiovascular disease driven by increased smoking, obesity, poverty, and poor nutrition.1 These individuals also face the added burden of struggling with a psychiatric condition that often interferes with their ability to make optimal preventative health decisions, including staying up to date on vaccinations.2 A recent review from Toronto, Canada, found that the influenza vaccination rates among homeless adults with mental illness—a population at high risk of respiratory illness—was only 6.7% compared with 31.1% for the general population of Ontario.3
Mental health professionals may serve as the only contacts to offer medical care to this vulnerable population, leading some psychiatric leaders to advocate that psychiatrists be considered primary care providers within accountable care organizations. Because most vaccines are easily available, mental health professionals should know about key immunizations to guide their patients accordingly.
In the United States, approximately 45,000 adults die annually from vaccine-preventable diseases, the majority from influenza.4 When combined with the most recent Adult Immunization Schedule and general recommendations adapted from the CDC,5,6 the mnemonic ARM SHOT allows for a quick assessment of risk factors to guide administration and education about most vaccinations (Table 1). ARM SHOT involves assessing the following components of an individual’s health status and living arrangements to determine one’s risk of contracting communicable diseases:
- Age
- Risk of exposure
- Medical conditions (comorbidities)
- Substance use history
- HIV status or other immunocompromised states
- Occupancy, or living arrangements
- Tobacco use.

We recommend keeping a copy of the Adult Immunization Schedule (age ≥19) and/or the immunization schedule for children and adolescents (age ≤18) close for quick reference. Here, we provide a case and then explore how each component of the ARM SHOT mnemonic applies in decision-making.
Case Evaluating risk, assess needs
Ms. W, age 24, has bipolar I disorder, most recently manic with psychotic features. She presents for follow-up in clinic after a 5-day hospitalization for mania and comorbid alcohol use disorder. Her medical comorbidities include asthma and active tobacco use. She is taking lurasidone, 20 mg/d, and lithium, 900 mg/d. Her case manager is working to place Ms. W in a residential substance use disorder treatment program. Ms. W is on a waiting list to establish care with a primary care physician and has a history of poor engagement with medical services in general; prior attempts to place her with a primary care physician failed.
In advance of Ms. W’s transfer to a residential treatment facility, you have been asked to place a Mantoux screening test for tuberculosis (purified protein derivative), which raises the important question about her susceptibility to infectious diseases in general. To protect Ms. W from preventable diseases for which vaccines are available, you review the ARM SHOT mnemonic to broadly assess her candidacy for vaccinations.
Age
Age may be the most important determinant of a patient’s need for vaccination (Table 2). The CDC immunization schedules account for age-specific risks for diseases, complications, and responses to vaccination (Figure 1).6
Influenza vaccination. Adults can have an intramuscular or intradermal inactivated influenza vaccination yearly in the fall or winter, unless they have an allergy to a vaccine component such as egg protein. Those with such an allergy can receive a recombinant influenza vaccine. Until the 2016 to 2017 flu season, an intranasal mist of live, attenuated influenza vaccine was available to healthy, non-pregnant women, ages 2 to 49, without high-risk medical conditions. However, the CDC dropped its recommendation for this vaccine because data showed it did not effectively prevent the flu.7 Individuals age ≥65 can receive either the standard- or high-dose inactivated influenza vaccination. The latter contains 4 times the amount of antigen with the intention of triggering a stronger immune response in older adults.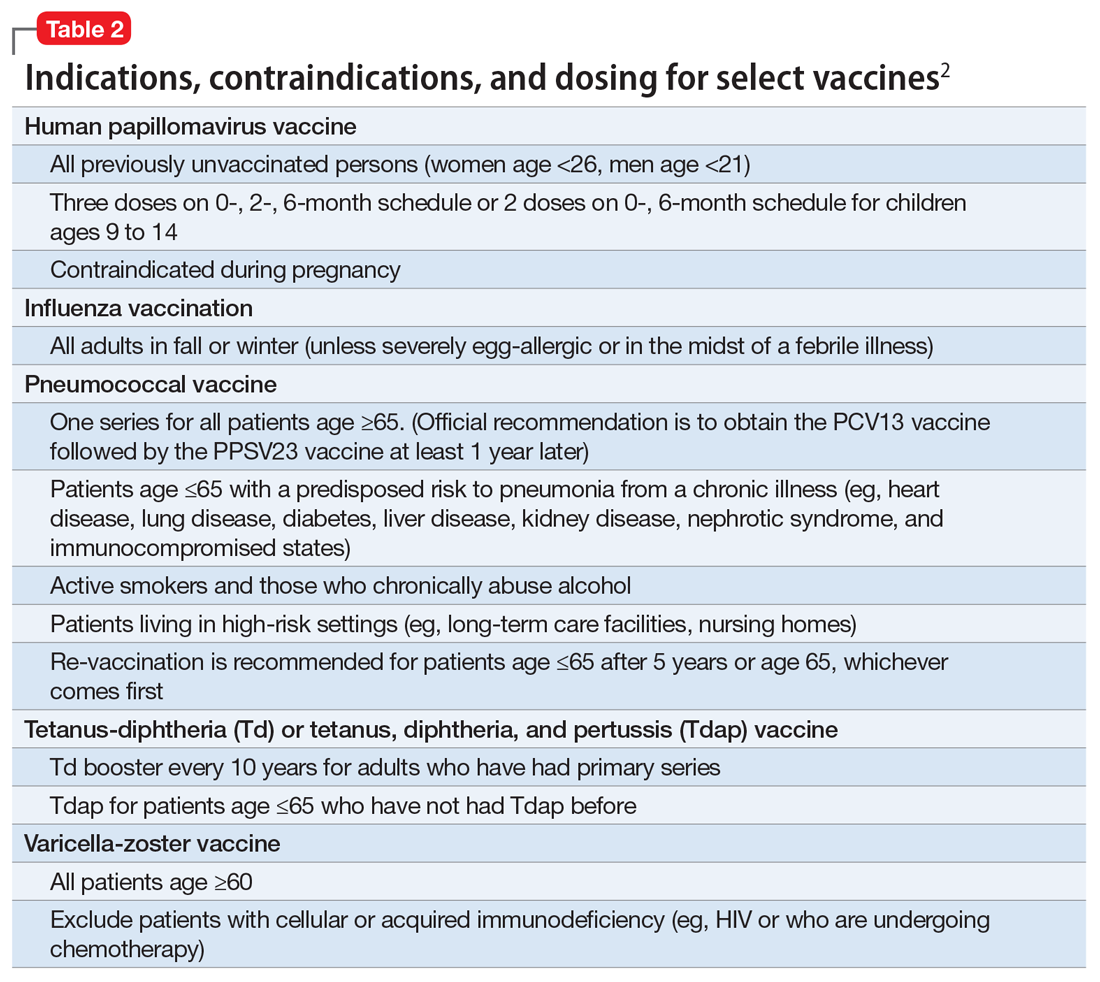
Pneumonia immunization. All patients age ≥65 should receive vaccinations for Streptococcus pneumoniae and its variants in the form of one 13-valent pneumococcal conjugate vaccine and, at least 1 year later, one 23-valent pneumococcal polysaccharide vaccine (PPSV23). Immunization reduces the morbidity and mortality from pneumococcal illness by decreasing the burden of a pneumonia, bacteremia, or meningitis infection. Adults, ages 19 to 64, with a chronic disease (referred to as “special populations” in CDC tables), such as diabetes, heart or lung disease, alcoholism, or cirrhosis, or those who smoke cigarettes, should receive PPSV23 with a second dose administered at least 5 years after the first. The CDC recommends a 1-time re-vaccination at age 65 for patients if >5 years have passed since the last PPSV23 and if the patient was younger than age 65 at the time of primary vaccine for S. pneumoniae. This can be a rather tricky clinical situation; the health care provider should verify a patient’s immunization history to ensure that she (he) is receiving only necessary vaccines. However, when the history cannot be verified, err on the side of inclusion, because risks are minimal.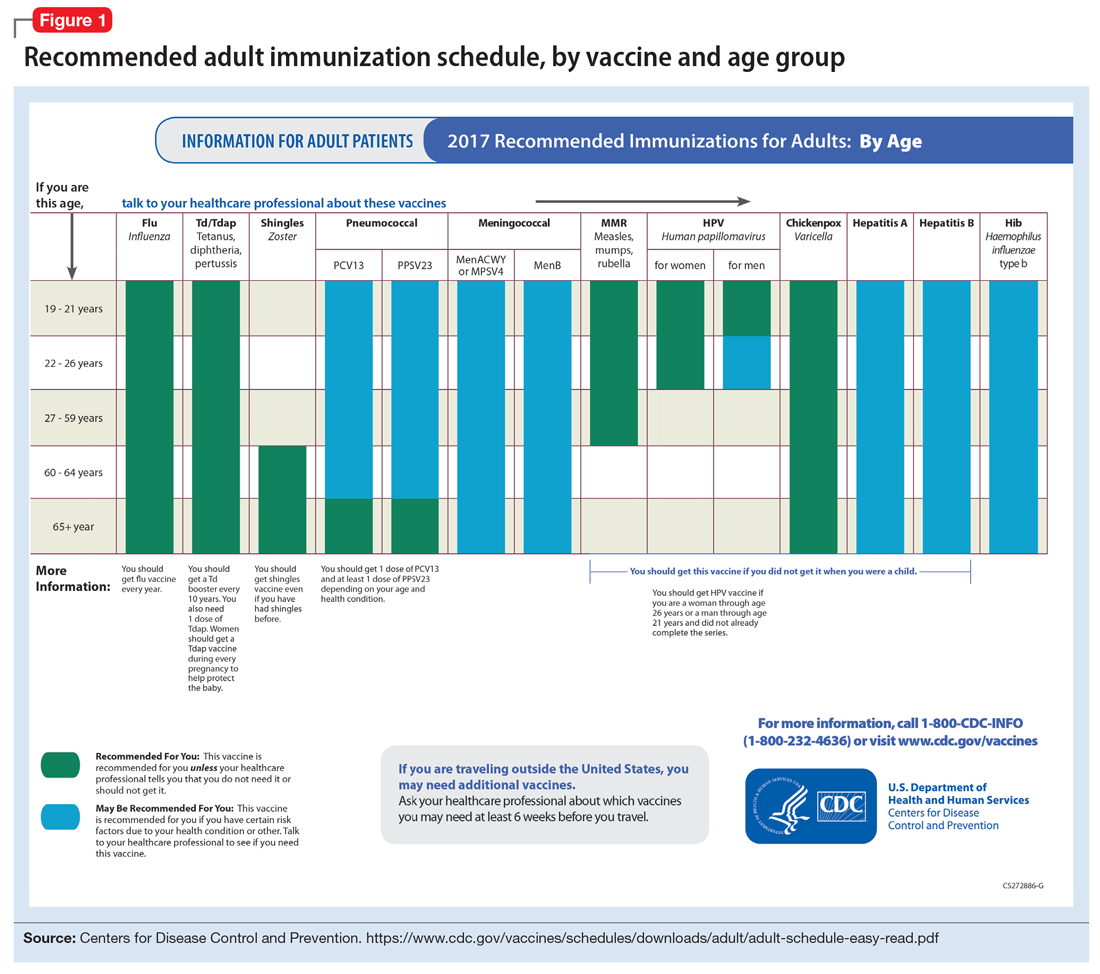
Shingles vaccination. Adults age ≥60 who are not immunocompromised should receive a single dose of live attenuated vaccine from varicella-zoster virus (VZV) to limit the risk of shingles from a prior chickenpox infection. The vaccine is approximately 66.5% effective at preventing postherpetic neuralgia for up to 4.9 years. Individuals as young as age 50 may have the vaccine because the risk of herpes zoster radically increases from then on,8 although most insurers only cover VZV vaccination after age 60.
Tetanus, diphtheria, and acellular pertussis (Tdap) vaccine. All adults should complete the 3-dose primary vaccination series for tetanus, diphtheria, and pertussis (also known as whooping cough) and this should include 1 dose of Tdap. Administration of the primary series is staged so that the second dose is given 4 weeks after the initial dose and the final dose 6 to 12 months after the first dose. After receiving the primary series, adults should receive a tetanus-diphtheria booster dose every 10 years. For adults ages 19 to 64, the Advisory Committee on Immunization Practices (ACIP) recommends 1 dose of Tdap in place of a booster vaccination to decrease the transmission risk of pertussis to vulnerable persons, especially children.
Human papillomavirus (HPV) immunization. The ACIP recommendation9 has been for children to receive routine vaccination for the 4 major strains of HPV—strains 6, 11, 16, and 18—starting at ages 11 to 12 to confer protection from HPV-associated diseases, such as genital warts, oropharyngeal cancer, and anal cancer; cancers of the cervix, vulva, and vagina in women; and penile cancer in men. Ideally, the vaccines are administered prior to HPV exposure from sexual contact. The quadrivalent HPV vaccine is safe and is administered as a 3-dose series, with the second and third doses given 2 and 6 months, respectively, after the initial dose. Adolescent girls also have the option of a bivalent HPV vaccine.
In 2016, the FDA approved a 9-valent HPV vaccine, a simpler 2-dose schedule for children ages 9 to 14 (2 doses at least 6 months apart). Leading cancer centers have endorsed this vaccine based on strong comparative data with the 3-dose regimen.10 For those not previously vaccinated, the HPV vaccine is available for women ages 13 to 26 and for men ages 13 to 21 (although men ages 22 to 26 can receive the vaccine, and it is recommended for men who have sex with men [MSM]). Women do not require Papanicolaou, serum pregnancy, HPV DNA, or HPV antibody tests prior to vaccination. If a woman becomes pregnant, remaining doses of the vaccine should be postponed until after delivery. Women still need to follow recommendations for cervical cancer screening because the HPV vaccine does not cover all genital strains of the virus. For sexually active individuals who might have HPV or genital warts, immunization has no clinical effect except to prevent other HPV strains.
Measles, mumps, and rubella (MMR) vaccine. All adults should receive, at minimum, 1 dose of MMR vaccination unless serological immunity can be verified or if contraindicated. Two doses of the vaccine are recommended for students attending post-high school institutions, health care personnel, and international travelers because they are at higher risk for exposure and transmission of measles and mumps. Individuals born before 1957 are considered immune to measles and mumps. A measles outbreak from December 2014 to February 201511 highlighted the importance of maintaining one’s immunity status for MMR.
Case continued
Based on Ms. W’s age, she should be offered vaccinations for influenza and opportunities to receive vaccinations for HPV, Tdap (the primary series, a Tdap or Td booster), and MMR, if appropriate and not completed previously.
Risk of exposure
Certain behaviors will increase the risk of exposure to and transmission of diseases communicable by blood and other bodily fluids (Table 3). These behaviors include needle injections (eg, during use of illicit drugs) and sexual activity with multiple partners, including MSM or promiscuity/impulsivity during a manic episode. A common consequence of risky behaviors is comorbid infection of HIV and viral hepatitis for those with substance use disorder or those who engage in high-risk sexual practices.12,13
Hepatitis B virus (HBV) immunization. Vaccination is one of the most effective ways to prevent HBV infection, which is why it is offered to all health care workers. HBV immunization is a 3-dose series in which the second and third doses are given 1 and 6 months after the initial doses, respectively. In addition to certain medical risk factors or conditions that indicate HBV vaccination, people should be offered the vaccine if they are in a higher risk occupation, travel, are of Asian or Pacific Islander ethnicity from an endemic area, or have any present or suspected sexually transmitted diseases.
Hepatitis A virus (HAV) vaccination. HAV is transmitted via fecal–oral routes, often from contaminated water or food, or through household or sexual contact with an infected person. Individuals should receive the HAV vaccine if they use illicit drugs by any route of administration, work with primates infected with HAV, travel to countries with unknown or high rates of HAV, or have chronic liver disease (ie, hepatitis, alcohol use disorder, or non-alcoholic fatty liver disease) or clotting deficiencies. The CDC Health Information for International Travel, commonly called the “Yellow Book,” publishes vaccination recommendations for those who plan travel to specific countries.14
Case continued
Ms. W’s history of mania (if such episodes included increased sexual activity) and substance use would make her a candidate for the HBV and HAV vaccinations and could also strengthen our previous recommendation that she receive the HPV vaccination.
Medical conditions
Patients with certain medical conditions may have difficulty fighting infections or become more susceptible to morbidity and mortality from coinfection with vaccine-preventable illnesses. Secondary effects of psychotropic medications that may carry implications for vaccine recommendations (eg, risk of agranulocytosis and impaired cell-medicated immunity with mirtazapine and clozapine or renal impairment from lithium use) are of particular concern in psychiatric patients.2
To help care for these patients, the CDC has developed a “medical conditions” schedule (Figure 2). This schedule makes vaccination recommendations for those with a weakened immune system, including patients with HIV, chronic obstructive pulmonary disease (COPD), diabetes, hepatitis, asplenia, end-stage renal disease, cardiac disease, and pregnancy.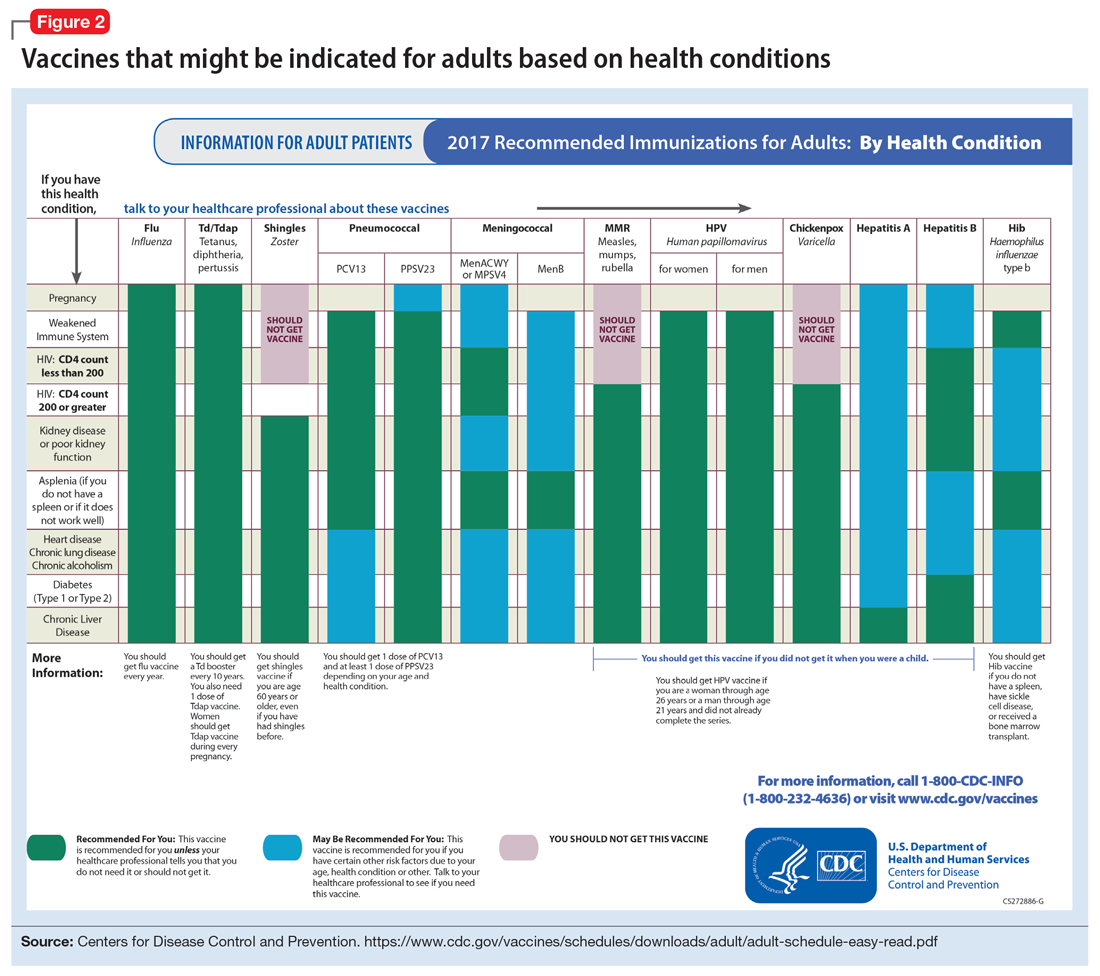
Because patients with psychiatric illness face a greater risk of heart disease and diabetes, these conditions may warrant special reference on the schedule. The increased cardiometabolic risk factors in these patients may be due in part to genetics, socioeconomic status, lifestyle behaviors, and medications to treat their mental illness (eg, antipsychotics). Patients with bipolar disorder or schizophrenia in particular tend to have higher rates of COPD (mainly from chronic bronchitis) and asthma than the general population.12 Pay special attention to the indications schedule for those with chronic lung disease, especially patients who continue to smoke cigarettes.
Case continued
Because of Ms. W’s asthma, the CDC schedule recommends ensuring she is up to date on her influenza, pneumococcal, and Tdap vaccinations.
Substance use
Patients with combined psychiatric and substance use disorders (“dual diagnosis”) have lower rates of receiving preventive care than patients with either condition alone.15 Substance use can be behaviorally disinhibiting, leading to increased risk of exposures from sexual contact or other risky activities. The use of illicit substances can provide a nidus for infection depending on the route of administration and can result in negative effects on organ systems, compromising one’s ability to ward off infection.
Patients who use any illicit drugs, regardless of the method of delivery, should be recommended for HAV vaccination. For those with alcohol use disorder and/or chronic liver disease, and/or seeking treatment for substance use, hepatitis B screening and vaccination is recommended.
Case continued
From a substance use perspective, discussion of vaccination status for both hepatitis A and B would be important for Ms. W.
HIV or immunocompromised
Persons with severe mental illness have high rates of HIV, with almost 8 times the risk of exposure, compared with the general population due to myriad reasons, including greater rates of substance abuse, higher risk sexual behavior, and lack of awareness of HIV transmission.12,13 Patients with mental illness are also at risk of leukopenia and agranulocytosis from certain drugs used to treat their conditions, such as clozapine.
Pregnancy is a challenge for women with mental illness because of the pharmacologic risk and immune-system compromise to the mother and baby. A pregnant woman who has HIV with a CD4 count <200, or has a weakened immune system from an organ transplant or a similar condition, is a candidate for certain vaccines based on the Adult Immunization Schedule (Figure 2). However, these patients should avoid live vaccines, such as the intranasal mist of live influenza, MMR, VZV, and varicella, to avoid illness from these inoculations.
Case continued
Ms. W should undergo testing for pregnancy and HIV (and preferably other sexually transmitted infections per general preventive health guidelines) before receiving any live vaccinations.
Occupancy
Aside from direct transmission of bodily fluids, infectious diseases also can spread through droplets/secretions from the throat and respiratory tract. Close quarters or lengthy contact enhances communicability by droplets, and therefore people who reside in a communal living space (eg, individuals in substance use treatment facilities or those who reside in a nursing home) are most susceptible.
The bacterial disease Neisseria meningitidis (meningococcus) can spread through droplets and can cause pneumonia, bacteremia, and meningitis. Vaccination is indicated, and in some states is mandated, for college students who live in residence halls and missed routine vaccination by age 16. Meningococcus conjugate vaccine is administered in 2 doses; each dose may be given at least 2 months apart for those with HIV, asplenia, or persistent complement-related disorders. A single dose may be recommended for travelers to areas where meningococcal disease is hyperendemic or epidemic, military recruits, or microbiologists. For those age ≥55 and older, meningococcal polysaccharide vaccine is recommended over meningococcal conjugate vaccine.
Influenza, MMR, diphtheria, pertussis, and pneumococcus also spread through droplet contact.
Case continued
If Ms. W had not previously received the meningococcus vaccine as part of adolescent immunizations, she could benefit from this vaccine because she plans to enter a residential substance use disorder treatment program.
Tobacco use
Patients with psychiatric illness are twice as likely to smoke compared with the general population.16 Adult smokers, especially those with chronic lung disease, are at higher risk for influenza and pneumococcal-related illness; they should be vaccinated against these illnesses regardless of age (as discussed in the “Age” section).
Case continued
Because she smokes, Ms. W should receive counseling on vaccinations, such as influenza and pneumonia, to lessen her risk of respiratory illnesses and downstream sepsis.
Conclusion
Ms. W’s case represents an unfortunately all-too-common scenario where her multifaceted biopsychosocial circumstances place her at high risk for vaccine-preventable conditions. Her weight is recorded and laboratory work ordered to evaluate her pregnancy status, blood counts, lipids, complete metabolic panel, lithium level, and HIV status. Fortunately, she had received her series of MMR, meningococcal, and Tdap vaccinations when she was younger. Influenza, HPV, HAV, HBV, and pneumococcal vaccinations were all recommended to her, all of which can be given on the same day (HAV and HBV often are available as a combined vaccine). Ms. W receives a renewal of her psychiatric medications and counseling on healthy living habits (eg, diet and exercise, quitting tobacco and alcohol use, and safe sex practices) and the importance of immunizations.
Vaccination is 1 of the 10 great public health achievements of the 20th century when one considers how immunization of vaccine-preventable diseases has reduced morbidity, mortality, and health-associated costs.17 As mental health professionals, we can help pass on the direct and indirect benefits of immunizations to an often underserved and undertreated population to help improve their health outcomes and quality of life.
1. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
2. Raj YP, Lloyd L. Adult immunizations. In: McCarron RM, Xiong GL, Keenan GR, et al, eds. Preventive medical care in psychiatry. Arlington, VA: American Psychiatric Publishing. 2015;215-227.
3. Young S, Dosani N, Whisler A, et al. Influenza vaccination rates among homeless adults with mental illness in Toronto. J Prim Care Community Health. 2015;6(3):211-214.
4. Kroger AT, Atkinson WL, Marcues EK, et al; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). General recommendations on immunization: recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-15):1-48.
5. Centers for Disease Control and Prevention. Recommended Adult Immunization by Vaccine and Age Group. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated February 27, 2017. Accessed February 1, 2017.
6. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(2):1-64.
7. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. https://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Updated June 22, 2016. Accessed February 1, 2017.
8. Hales CM, Harpaz, R, Ortega-Sanchez I, et al; Centers for Disease Control and Prevention. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63(33):729-731.
9. Petrosky E, Bocchini Jr JA, Hariri S, et al; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccine recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11)300-304.
10. Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411-2421.
11. Zipprich J, Winter K, Hacker J, et al; Centers for Disease Control and Prevention (CDC). Measles outbreak—California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(6):153-154.
12. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
13. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
14. Centers for Disease for Control and Prevention. CDC yellow book 2018: health information for international travel. New York, NY: Oxford University Press; 2017.
15. Druss BG, Rosenheck RA, Desai MM, et al. Quality of preventive medical care for patients with mental disorders. Med Care. 2002;40(2):129-136.
16. Lasser K, Boyd J, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606-2610.
17. Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60(19);619-623.
Patients with chronic, severe mental illness live much shorter lives than the general population. The 25-year loss in life expectancy for people with chronic mental illness has been attributed to higher rates of cardiovascular disease driven by increased smoking, obesity, poverty, and poor nutrition.1 These individuals also face the added burden of struggling with a psychiatric condition that often interferes with their ability to make optimal preventative health decisions, including staying up to date on vaccinations.2 A recent review from Toronto, Canada, found that the influenza vaccination rates among homeless adults with mental illness—a population at high risk of respiratory illness—was only 6.7% compared with 31.1% for the general population of Ontario.3
Mental health professionals may serve as the only contacts to offer medical care to this vulnerable population, leading some psychiatric leaders to advocate that psychiatrists be considered primary care providers within accountable care organizations. Because most vaccines are easily available, mental health professionals should know about key immunizations to guide their patients accordingly.
In the United States, approximately 45,000 adults die annually from vaccine-preventable diseases, the majority from influenza.4 When combined with the most recent Adult Immunization Schedule and general recommendations adapted from the CDC,5,6 the mnemonic ARM SHOT allows for a quick assessment of risk factors to guide administration and education about most vaccinations (Table 1). ARM SHOT involves assessing the following components of an individual’s health status and living arrangements to determine one’s risk of contracting communicable diseases:
- Age
- Risk of exposure
- Medical conditions (comorbidities)
- Substance use history
- HIV status or other immunocompromised states
- Occupancy, or living arrangements
- Tobacco use.

We recommend keeping a copy of the Adult Immunization Schedule (age ≥19) and/or the immunization schedule for children and adolescents (age ≤18) close for quick reference. Here, we provide a case and then explore how each component of the ARM SHOT mnemonic applies in decision-making.
Case Evaluating risk, assess needs
Ms. W, age 24, has bipolar I disorder, most recently manic with psychotic features. She presents for follow-up in clinic after a 5-day hospitalization for mania and comorbid alcohol use disorder. Her medical comorbidities include asthma and active tobacco use. She is taking lurasidone, 20 mg/d, and lithium, 900 mg/d. Her case manager is working to place Ms. W in a residential substance use disorder treatment program. Ms. W is on a waiting list to establish care with a primary care physician and has a history of poor engagement with medical services in general; prior attempts to place her with a primary care physician failed.
In advance of Ms. W’s transfer to a residential treatment facility, you have been asked to place a Mantoux screening test for tuberculosis (purified protein derivative), which raises the important question about her susceptibility to infectious diseases in general. To protect Ms. W from preventable diseases for which vaccines are available, you review the ARM SHOT mnemonic to broadly assess her candidacy for vaccinations.
Age
Age may be the most important determinant of a patient’s need for vaccination (Table 2). The CDC immunization schedules account for age-specific risks for diseases, complications, and responses to vaccination (Figure 1).6
Influenza vaccination. Adults can have an intramuscular or intradermal inactivated influenza vaccination yearly in the fall or winter, unless they have an allergy to a vaccine component such as egg protein. Those with such an allergy can receive a recombinant influenza vaccine. Until the 2016 to 2017 flu season, an intranasal mist of live, attenuated influenza vaccine was available to healthy, non-pregnant women, ages 2 to 49, without high-risk medical conditions. However, the CDC dropped its recommendation for this vaccine because data showed it did not effectively prevent the flu.7 Individuals age ≥65 can receive either the standard- or high-dose inactivated influenza vaccination. The latter contains 4 times the amount of antigen with the intention of triggering a stronger immune response in older adults.
Pneumonia immunization. All patients age ≥65 should receive vaccinations for Streptococcus pneumoniae and its variants in the form of one 13-valent pneumococcal conjugate vaccine and, at least 1 year later, one 23-valent pneumococcal polysaccharide vaccine (PPSV23). Immunization reduces the morbidity and mortality from pneumococcal illness by decreasing the burden of a pneumonia, bacteremia, or meningitis infection. Adults, ages 19 to 64, with a chronic disease (referred to as “special populations” in CDC tables), such as diabetes, heart or lung disease, alcoholism, or cirrhosis, or those who smoke cigarettes, should receive PPSV23 with a second dose administered at least 5 years after the first. The CDC recommends a 1-time re-vaccination at age 65 for patients if >5 years have passed since the last PPSV23 and if the patient was younger than age 65 at the time of primary vaccine for S. pneumoniae. This can be a rather tricky clinical situation; the health care provider should verify a patient’s immunization history to ensure that she (he) is receiving only necessary vaccines. However, when the history cannot be verified, err on the side of inclusion, because risks are minimal.
Shingles vaccination. Adults age ≥60 who are not immunocompromised should receive a single dose of live attenuated vaccine from varicella-zoster virus (VZV) to limit the risk of shingles from a prior chickenpox infection. The vaccine is approximately 66.5% effective at preventing postherpetic neuralgia for up to 4.9 years. Individuals as young as age 50 may have the vaccine because the risk of herpes zoster radically increases from then on,8 although most insurers only cover VZV vaccination after age 60.
Tetanus, diphtheria, and acellular pertussis (Tdap) vaccine. All adults should complete the 3-dose primary vaccination series for tetanus, diphtheria, and pertussis (also known as whooping cough) and this should include 1 dose of Tdap. Administration of the primary series is staged so that the second dose is given 4 weeks after the initial dose and the final dose 6 to 12 months after the first dose. After receiving the primary series, adults should receive a tetanus-diphtheria booster dose every 10 years. For adults ages 19 to 64, the Advisory Committee on Immunization Practices (ACIP) recommends 1 dose of Tdap in place of a booster vaccination to decrease the transmission risk of pertussis to vulnerable persons, especially children.
Human papillomavirus (HPV) immunization. The ACIP recommendation9 has been for children to receive routine vaccination for the 4 major strains of HPV—strains 6, 11, 16, and 18—starting at ages 11 to 12 to confer protection from HPV-associated diseases, such as genital warts, oropharyngeal cancer, and anal cancer; cancers of the cervix, vulva, and vagina in women; and penile cancer in men. Ideally, the vaccines are administered prior to HPV exposure from sexual contact. The quadrivalent HPV vaccine is safe and is administered as a 3-dose series, with the second and third doses given 2 and 6 months, respectively, after the initial dose. Adolescent girls also have the option of a bivalent HPV vaccine.
In 2016, the FDA approved a 9-valent HPV vaccine, a simpler 2-dose schedule for children ages 9 to 14 (2 doses at least 6 months apart). Leading cancer centers have endorsed this vaccine based on strong comparative data with the 3-dose regimen.10 For those not previously vaccinated, the HPV vaccine is available for women ages 13 to 26 and for men ages 13 to 21 (although men ages 22 to 26 can receive the vaccine, and it is recommended for men who have sex with men [MSM]). Women do not require Papanicolaou, serum pregnancy, HPV DNA, or HPV antibody tests prior to vaccination. If a woman becomes pregnant, remaining doses of the vaccine should be postponed until after delivery. Women still need to follow recommendations for cervical cancer screening because the HPV vaccine does not cover all genital strains of the virus. For sexually active individuals who might have HPV or genital warts, immunization has no clinical effect except to prevent other HPV strains.
Measles, mumps, and rubella (MMR) vaccine. All adults should receive, at minimum, 1 dose of MMR vaccination unless serological immunity can be verified or if contraindicated. Two doses of the vaccine are recommended for students attending post-high school institutions, health care personnel, and international travelers because they are at higher risk for exposure and transmission of measles and mumps. Individuals born before 1957 are considered immune to measles and mumps. A measles outbreak from December 2014 to February 201511 highlighted the importance of maintaining one’s immunity status for MMR.
Case continued
Based on Ms. W’s age, she should be offered vaccinations for influenza and opportunities to receive vaccinations for HPV, Tdap (the primary series, a Tdap or Td booster), and MMR, if appropriate and not completed previously.
Risk of exposure
Certain behaviors will increase the risk of exposure to and transmission of diseases communicable by blood and other bodily fluids (Table 3). These behaviors include needle injections (eg, during use of illicit drugs) and sexual activity with multiple partners, including MSM or promiscuity/impulsivity during a manic episode. A common consequence of risky behaviors is comorbid infection of HIV and viral hepatitis for those with substance use disorder or those who engage in high-risk sexual practices.12,13
Hepatitis B virus (HBV) immunization. Vaccination is one of the most effective ways to prevent HBV infection, which is why it is offered to all health care workers. HBV immunization is a 3-dose series in which the second and third doses are given 1 and 6 months after the initial doses, respectively. In addition to certain medical risk factors or conditions that indicate HBV vaccination, people should be offered the vaccine if they are in a higher risk occupation, travel, are of Asian or Pacific Islander ethnicity from an endemic area, or have any present or suspected sexually transmitted diseases.
Hepatitis A virus (HAV) vaccination. HAV is transmitted via fecal–oral routes, often from contaminated water or food, or through household or sexual contact with an infected person. Individuals should receive the HAV vaccine if they use illicit drugs by any route of administration, work with primates infected with HAV, travel to countries with unknown or high rates of HAV, or have chronic liver disease (ie, hepatitis, alcohol use disorder, or non-alcoholic fatty liver disease) or clotting deficiencies. The CDC Health Information for International Travel, commonly called the “Yellow Book,” publishes vaccination recommendations for those who plan travel to specific countries.14
Case continued
Ms. W’s history of mania (if such episodes included increased sexual activity) and substance use would make her a candidate for the HBV and HAV vaccinations and could also strengthen our previous recommendation that she receive the HPV vaccination.
Medical conditions
Patients with certain medical conditions may have difficulty fighting infections or become more susceptible to morbidity and mortality from coinfection with vaccine-preventable illnesses. Secondary effects of psychotropic medications that may carry implications for vaccine recommendations (eg, risk of agranulocytosis and impaired cell-medicated immunity with mirtazapine and clozapine or renal impairment from lithium use) are of particular concern in psychiatric patients.2
To help care for these patients, the CDC has developed a “medical conditions” schedule (Figure 2). This schedule makes vaccination recommendations for those with a weakened immune system, including patients with HIV, chronic obstructive pulmonary disease (COPD), diabetes, hepatitis, asplenia, end-stage renal disease, cardiac disease, and pregnancy.
Because patients with psychiatric illness face a greater risk of heart disease and diabetes, these conditions may warrant special reference on the schedule. The increased cardiometabolic risk factors in these patients may be due in part to genetics, socioeconomic status, lifestyle behaviors, and medications to treat their mental illness (eg, antipsychotics). Patients with bipolar disorder or schizophrenia in particular tend to have higher rates of COPD (mainly from chronic bronchitis) and asthma than the general population.12 Pay special attention to the indications schedule for those with chronic lung disease, especially patients who continue to smoke cigarettes.
Case continued
Because of Ms. W’s asthma, the CDC schedule recommends ensuring she is up to date on her influenza, pneumococcal, and Tdap vaccinations.
Substance use
Patients with combined psychiatric and substance use disorders (“dual diagnosis”) have lower rates of receiving preventive care than patients with either condition alone.15 Substance use can be behaviorally disinhibiting, leading to increased risk of exposures from sexual contact or other risky activities. The use of illicit substances can provide a nidus for infection depending on the route of administration and can result in negative effects on organ systems, compromising one’s ability to ward off infection.
Patients who use any illicit drugs, regardless of the method of delivery, should be recommended for HAV vaccination. For those with alcohol use disorder and/or chronic liver disease, and/or seeking treatment for substance use, hepatitis B screening and vaccination is recommended.
Case continued
From a substance use perspective, discussion of vaccination status for both hepatitis A and B would be important for Ms. W.
HIV or immunocompromised
Persons with severe mental illness have high rates of HIV, with almost 8 times the risk of exposure, compared with the general population due to myriad reasons, including greater rates of substance abuse, higher risk sexual behavior, and lack of awareness of HIV transmission.12,13 Patients with mental illness are also at risk of leukopenia and agranulocytosis from certain drugs used to treat their conditions, such as clozapine.
Pregnancy is a challenge for women with mental illness because of the pharmacologic risk and immune-system compromise to the mother and baby. A pregnant woman who has HIV with a CD4 count <200, or has a weakened immune system from an organ transplant or a similar condition, is a candidate for certain vaccines based on the Adult Immunization Schedule (Figure 2). However, these patients should avoid live vaccines, such as the intranasal mist of live influenza, MMR, VZV, and varicella, to avoid illness from these inoculations.
Case continued
Ms. W should undergo testing for pregnancy and HIV (and preferably other sexually transmitted infections per general preventive health guidelines) before receiving any live vaccinations.
Occupancy
Aside from direct transmission of bodily fluids, infectious diseases also can spread through droplets/secretions from the throat and respiratory tract. Close quarters or lengthy contact enhances communicability by droplets, and therefore people who reside in a communal living space (eg, individuals in substance use treatment facilities or those who reside in a nursing home) are most susceptible.
The bacterial disease Neisseria meningitidis (meningococcus) can spread through droplets and can cause pneumonia, bacteremia, and meningitis. Vaccination is indicated, and in some states is mandated, for college students who live in residence halls and missed routine vaccination by age 16. Meningococcus conjugate vaccine is administered in 2 doses; each dose may be given at least 2 months apart for those with HIV, asplenia, or persistent complement-related disorders. A single dose may be recommended for travelers to areas where meningococcal disease is hyperendemic or epidemic, military recruits, or microbiologists. For those age ≥55 and older, meningococcal polysaccharide vaccine is recommended over meningococcal conjugate vaccine.
Influenza, MMR, diphtheria, pertussis, and pneumococcus also spread through droplet contact.
Case continued
If Ms. W had not previously received the meningococcus vaccine as part of adolescent immunizations, she could benefit from this vaccine because she plans to enter a residential substance use disorder treatment program.
Tobacco use
Patients with psychiatric illness are twice as likely to smoke compared with the general population.16 Adult smokers, especially those with chronic lung disease, are at higher risk for influenza and pneumococcal-related illness; they should be vaccinated against these illnesses regardless of age (as discussed in the “Age” section).
Case continued
Because she smokes, Ms. W should receive counseling on vaccinations, such as influenza and pneumonia, to lessen her risk of respiratory illnesses and downstream sepsis.
Conclusion
Ms. W’s case represents an unfortunately all-too-common scenario where her multifaceted biopsychosocial circumstances place her at high risk for vaccine-preventable conditions. Her weight is recorded and laboratory work ordered to evaluate her pregnancy status, blood counts, lipids, complete metabolic panel, lithium level, and HIV status. Fortunately, she had received her series of MMR, meningococcal, and Tdap vaccinations when she was younger. Influenza, HPV, HAV, HBV, and pneumococcal vaccinations were all recommended to her, all of which can be given on the same day (HAV and HBV often are available as a combined vaccine). Ms. W receives a renewal of her psychiatric medications and counseling on healthy living habits (eg, diet and exercise, quitting tobacco and alcohol use, and safe sex practices) and the importance of immunizations.
Vaccination is 1 of the 10 great public health achievements of the 20th century when one considers how immunization of vaccine-preventable diseases has reduced morbidity, mortality, and health-associated costs.17 As mental health professionals, we can help pass on the direct and indirect benefits of immunizations to an often underserved and undertreated population to help improve their health outcomes and quality of life.
Patients with chronic, severe mental illness live much shorter lives than the general population. The 25-year loss in life expectancy for people with chronic mental illness has been attributed to higher rates of cardiovascular disease driven by increased smoking, obesity, poverty, and poor nutrition.1 These individuals also face the added burden of struggling with a psychiatric condition that often interferes with their ability to make optimal preventative health decisions, including staying up to date on vaccinations.2 A recent review from Toronto, Canada, found that the influenza vaccination rates among homeless adults with mental illness—a population at high risk of respiratory illness—was only 6.7% compared with 31.1% for the general population of Ontario.3
Mental health professionals may serve as the only contacts to offer medical care to this vulnerable population, leading some psychiatric leaders to advocate that psychiatrists be considered primary care providers within accountable care organizations. Because most vaccines are easily available, mental health professionals should know about key immunizations to guide their patients accordingly.
In the United States, approximately 45,000 adults die annually from vaccine-preventable diseases, the majority from influenza.4 When combined with the most recent Adult Immunization Schedule and general recommendations adapted from the CDC,5,6 the mnemonic ARM SHOT allows for a quick assessment of risk factors to guide administration and education about most vaccinations (Table 1). ARM SHOT involves assessing the following components of an individual’s health status and living arrangements to determine one’s risk of contracting communicable diseases:
- Age
- Risk of exposure
- Medical conditions (comorbidities)
- Substance use history
- HIV status or other immunocompromised states
- Occupancy, or living arrangements
- Tobacco use.

We recommend keeping a copy of the Adult Immunization Schedule (age ≥19) and/or the immunization schedule for children and adolescents (age ≤18) close for quick reference. Here, we provide a case and then explore how each component of the ARM SHOT mnemonic applies in decision-making.
Case Evaluating risk, assess needs
Ms. W, age 24, has bipolar I disorder, most recently manic with psychotic features. She presents for follow-up in clinic after a 5-day hospitalization for mania and comorbid alcohol use disorder. Her medical comorbidities include asthma and active tobacco use. She is taking lurasidone, 20 mg/d, and lithium, 900 mg/d. Her case manager is working to place Ms. W in a residential substance use disorder treatment program. Ms. W is on a waiting list to establish care with a primary care physician and has a history of poor engagement with medical services in general; prior attempts to place her with a primary care physician failed.
In advance of Ms. W’s transfer to a residential treatment facility, you have been asked to place a Mantoux screening test for tuberculosis (purified protein derivative), which raises the important question about her susceptibility to infectious diseases in general. To protect Ms. W from preventable diseases for which vaccines are available, you review the ARM SHOT mnemonic to broadly assess her candidacy for vaccinations.
Age
Age may be the most important determinant of a patient’s need for vaccination (Table 2). The CDC immunization schedules account for age-specific risks for diseases, complications, and responses to vaccination (Figure 1).6
Influenza vaccination. Adults can have an intramuscular or intradermal inactivated influenza vaccination yearly in the fall or winter, unless they have an allergy to a vaccine component such as egg protein. Those with such an allergy can receive a recombinant influenza vaccine. Until the 2016 to 2017 flu season, an intranasal mist of live, attenuated influenza vaccine was available to healthy, non-pregnant women, ages 2 to 49, without high-risk medical conditions. However, the CDC dropped its recommendation for this vaccine because data showed it did not effectively prevent the flu.7 Individuals age ≥65 can receive either the standard- or high-dose inactivated influenza vaccination. The latter contains 4 times the amount of antigen with the intention of triggering a stronger immune response in older adults.
Pneumonia immunization. All patients age ≥65 should receive vaccinations for Streptococcus pneumoniae and its variants in the form of one 13-valent pneumococcal conjugate vaccine and, at least 1 year later, one 23-valent pneumococcal polysaccharide vaccine (PPSV23). Immunization reduces the morbidity and mortality from pneumococcal illness by decreasing the burden of a pneumonia, bacteremia, or meningitis infection. Adults, ages 19 to 64, with a chronic disease (referred to as “special populations” in CDC tables), such as diabetes, heart or lung disease, alcoholism, or cirrhosis, or those who smoke cigarettes, should receive PPSV23 with a second dose administered at least 5 years after the first. The CDC recommends a 1-time re-vaccination at age 65 for patients if >5 years have passed since the last PPSV23 and if the patient was younger than age 65 at the time of primary vaccine for S. pneumoniae. This can be a rather tricky clinical situation; the health care provider should verify a patient’s immunization history to ensure that she (he) is receiving only necessary vaccines. However, when the history cannot be verified, err on the side of inclusion, because risks are minimal.
Shingles vaccination. Adults age ≥60 who are not immunocompromised should receive a single dose of live attenuated vaccine from varicella-zoster virus (VZV) to limit the risk of shingles from a prior chickenpox infection. The vaccine is approximately 66.5% effective at preventing postherpetic neuralgia for up to 4.9 years. Individuals as young as age 50 may have the vaccine because the risk of herpes zoster radically increases from then on,8 although most insurers only cover VZV vaccination after age 60.
Tetanus, diphtheria, and acellular pertussis (Tdap) vaccine. All adults should complete the 3-dose primary vaccination series for tetanus, diphtheria, and pertussis (also known as whooping cough) and this should include 1 dose of Tdap. Administration of the primary series is staged so that the second dose is given 4 weeks after the initial dose and the final dose 6 to 12 months after the first dose. After receiving the primary series, adults should receive a tetanus-diphtheria booster dose every 10 years. For adults ages 19 to 64, the Advisory Committee on Immunization Practices (ACIP) recommends 1 dose of Tdap in place of a booster vaccination to decrease the transmission risk of pertussis to vulnerable persons, especially children.
Human papillomavirus (HPV) immunization. The ACIP recommendation9 has been for children to receive routine vaccination for the 4 major strains of HPV—strains 6, 11, 16, and 18—starting at ages 11 to 12 to confer protection from HPV-associated diseases, such as genital warts, oropharyngeal cancer, and anal cancer; cancers of the cervix, vulva, and vagina in women; and penile cancer in men. Ideally, the vaccines are administered prior to HPV exposure from sexual contact. The quadrivalent HPV vaccine is safe and is administered as a 3-dose series, with the second and third doses given 2 and 6 months, respectively, after the initial dose. Adolescent girls also have the option of a bivalent HPV vaccine.
In 2016, the FDA approved a 9-valent HPV vaccine, a simpler 2-dose schedule for children ages 9 to 14 (2 doses at least 6 months apart). Leading cancer centers have endorsed this vaccine based on strong comparative data with the 3-dose regimen.10 For those not previously vaccinated, the HPV vaccine is available for women ages 13 to 26 and for men ages 13 to 21 (although men ages 22 to 26 can receive the vaccine, and it is recommended for men who have sex with men [MSM]). Women do not require Papanicolaou, serum pregnancy, HPV DNA, or HPV antibody tests prior to vaccination. If a woman becomes pregnant, remaining doses of the vaccine should be postponed until after delivery. Women still need to follow recommendations for cervical cancer screening because the HPV vaccine does not cover all genital strains of the virus. For sexually active individuals who might have HPV or genital warts, immunization has no clinical effect except to prevent other HPV strains.
Measles, mumps, and rubella (MMR) vaccine. All adults should receive, at minimum, 1 dose of MMR vaccination unless serological immunity can be verified or if contraindicated. Two doses of the vaccine are recommended for students attending post-high school institutions, health care personnel, and international travelers because they are at higher risk for exposure and transmission of measles and mumps. Individuals born before 1957 are considered immune to measles and mumps. A measles outbreak from December 2014 to February 201511 highlighted the importance of maintaining one’s immunity status for MMR.
Case continued
Based on Ms. W’s age, she should be offered vaccinations for influenza and opportunities to receive vaccinations for HPV, Tdap (the primary series, a Tdap or Td booster), and MMR, if appropriate and not completed previously.
Risk of exposure
Certain behaviors will increase the risk of exposure to and transmission of diseases communicable by blood and other bodily fluids (Table 3). These behaviors include needle injections (eg, during use of illicit drugs) and sexual activity with multiple partners, including MSM or promiscuity/impulsivity during a manic episode. A common consequence of risky behaviors is comorbid infection of HIV and viral hepatitis for those with substance use disorder or those who engage in high-risk sexual practices.12,13
Hepatitis B virus (HBV) immunization. Vaccination is one of the most effective ways to prevent HBV infection, which is why it is offered to all health care workers. HBV immunization is a 3-dose series in which the second and third doses are given 1 and 6 months after the initial doses, respectively. In addition to certain medical risk factors or conditions that indicate HBV vaccination, people should be offered the vaccine if they are in a higher risk occupation, travel, are of Asian or Pacific Islander ethnicity from an endemic area, or have any present or suspected sexually transmitted diseases.
Hepatitis A virus (HAV) vaccination. HAV is transmitted via fecal–oral routes, often from contaminated water or food, or through household or sexual contact with an infected person. Individuals should receive the HAV vaccine if they use illicit drugs by any route of administration, work with primates infected with HAV, travel to countries with unknown or high rates of HAV, or have chronic liver disease (ie, hepatitis, alcohol use disorder, or non-alcoholic fatty liver disease) or clotting deficiencies. The CDC Health Information for International Travel, commonly called the “Yellow Book,” publishes vaccination recommendations for those who plan travel to specific countries.14
Case continued
Ms. W’s history of mania (if such episodes included increased sexual activity) and substance use would make her a candidate for the HBV and HAV vaccinations and could also strengthen our previous recommendation that she receive the HPV vaccination.
Medical conditions
Patients with certain medical conditions may have difficulty fighting infections or become more susceptible to morbidity and mortality from coinfection with vaccine-preventable illnesses. Secondary effects of psychotropic medications that may carry implications for vaccine recommendations (eg, risk of agranulocytosis and impaired cell-medicated immunity with mirtazapine and clozapine or renal impairment from lithium use) are of particular concern in psychiatric patients.2
To help care for these patients, the CDC has developed a “medical conditions” schedule (Figure 2). This schedule makes vaccination recommendations for those with a weakened immune system, including patients with HIV, chronic obstructive pulmonary disease (COPD), diabetes, hepatitis, asplenia, end-stage renal disease, cardiac disease, and pregnancy.
Because patients with psychiatric illness face a greater risk of heart disease and diabetes, these conditions may warrant special reference on the schedule. The increased cardiometabolic risk factors in these patients may be due in part to genetics, socioeconomic status, lifestyle behaviors, and medications to treat their mental illness (eg, antipsychotics). Patients with bipolar disorder or schizophrenia in particular tend to have higher rates of COPD (mainly from chronic bronchitis) and asthma than the general population.12 Pay special attention to the indications schedule for those with chronic lung disease, especially patients who continue to smoke cigarettes.
Case continued
Because of Ms. W’s asthma, the CDC schedule recommends ensuring she is up to date on her influenza, pneumococcal, and Tdap vaccinations.
Substance use
Patients with combined psychiatric and substance use disorders (“dual diagnosis”) have lower rates of receiving preventive care than patients with either condition alone.15 Substance use can be behaviorally disinhibiting, leading to increased risk of exposures from sexual contact or other risky activities. The use of illicit substances can provide a nidus for infection depending on the route of administration and can result in negative effects on organ systems, compromising one’s ability to ward off infection.
Patients who use any illicit drugs, regardless of the method of delivery, should be recommended for HAV vaccination. For those with alcohol use disorder and/or chronic liver disease, and/or seeking treatment for substance use, hepatitis B screening and vaccination is recommended.
Case continued
From a substance use perspective, discussion of vaccination status for both hepatitis A and B would be important for Ms. W.
HIV or immunocompromised
Persons with severe mental illness have high rates of HIV, with almost 8 times the risk of exposure, compared with the general population due to myriad reasons, including greater rates of substance abuse, higher risk sexual behavior, and lack of awareness of HIV transmission.12,13 Patients with mental illness are also at risk of leukopenia and agranulocytosis from certain drugs used to treat their conditions, such as clozapine.
Pregnancy is a challenge for women with mental illness because of the pharmacologic risk and immune-system compromise to the mother and baby. A pregnant woman who has HIV with a CD4 count <200, or has a weakened immune system from an organ transplant or a similar condition, is a candidate for certain vaccines based on the Adult Immunization Schedule (Figure 2). However, these patients should avoid live vaccines, such as the intranasal mist of live influenza, MMR, VZV, and varicella, to avoid illness from these inoculations.
Case continued
Ms. W should undergo testing for pregnancy and HIV (and preferably other sexually transmitted infections per general preventive health guidelines) before receiving any live vaccinations.
Occupancy
Aside from direct transmission of bodily fluids, infectious diseases also can spread through droplets/secretions from the throat and respiratory tract. Close quarters or lengthy contact enhances communicability by droplets, and therefore people who reside in a communal living space (eg, individuals in substance use treatment facilities or those who reside in a nursing home) are most susceptible.
The bacterial disease Neisseria meningitidis (meningococcus) can spread through droplets and can cause pneumonia, bacteremia, and meningitis. Vaccination is indicated, and in some states is mandated, for college students who live in residence halls and missed routine vaccination by age 16. Meningococcus conjugate vaccine is administered in 2 doses; each dose may be given at least 2 months apart for those with HIV, asplenia, or persistent complement-related disorders. A single dose may be recommended for travelers to areas where meningococcal disease is hyperendemic or epidemic, military recruits, or microbiologists. For those age ≥55 and older, meningococcal polysaccharide vaccine is recommended over meningococcal conjugate vaccine.
Influenza, MMR, diphtheria, pertussis, and pneumococcus also spread through droplet contact.
Case continued
If Ms. W had not previously received the meningococcus vaccine as part of adolescent immunizations, she could benefit from this vaccine because she plans to enter a residential substance use disorder treatment program.
Tobacco use
Patients with psychiatric illness are twice as likely to smoke compared with the general population.16 Adult smokers, especially those with chronic lung disease, are at higher risk for influenza and pneumococcal-related illness; they should be vaccinated against these illnesses regardless of age (as discussed in the “Age” section).
Case continued
Because she smokes, Ms. W should receive counseling on vaccinations, such as influenza and pneumonia, to lessen her risk of respiratory illnesses and downstream sepsis.
Conclusion
Ms. W’s case represents an unfortunately all-too-common scenario where her multifaceted biopsychosocial circumstances place her at high risk for vaccine-preventable conditions. Her weight is recorded and laboratory work ordered to evaluate her pregnancy status, blood counts, lipids, complete metabolic panel, lithium level, and HIV status. Fortunately, she had received her series of MMR, meningococcal, and Tdap vaccinations when she was younger. Influenza, HPV, HAV, HBV, and pneumococcal vaccinations were all recommended to her, all of which can be given on the same day (HAV and HBV often are available as a combined vaccine). Ms. W receives a renewal of her psychiatric medications and counseling on healthy living habits (eg, diet and exercise, quitting tobacco and alcohol use, and safe sex practices) and the importance of immunizations.
Vaccination is 1 of the 10 great public health achievements of the 20th century when one considers how immunization of vaccine-preventable diseases has reduced morbidity, mortality, and health-associated costs.17 As mental health professionals, we can help pass on the direct and indirect benefits of immunizations to an often underserved and undertreated population to help improve their health outcomes and quality of life.
1. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
2. Raj YP, Lloyd L. Adult immunizations. In: McCarron RM, Xiong GL, Keenan GR, et al, eds. Preventive medical care in psychiatry. Arlington, VA: American Psychiatric Publishing. 2015;215-227.
3. Young S, Dosani N, Whisler A, et al. Influenza vaccination rates among homeless adults with mental illness in Toronto. J Prim Care Community Health. 2015;6(3):211-214.
4. Kroger AT, Atkinson WL, Marcues EK, et al; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). General recommendations on immunization: recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-15):1-48.
5. Centers for Disease Control and Prevention. Recommended Adult Immunization by Vaccine and Age Group. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated February 27, 2017. Accessed February 1, 2017.
6. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(2):1-64.
7. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. https://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Updated June 22, 2016. Accessed February 1, 2017.
8. Hales CM, Harpaz, R, Ortega-Sanchez I, et al; Centers for Disease Control and Prevention. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63(33):729-731.
9. Petrosky E, Bocchini Jr JA, Hariri S, et al; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccine recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11)300-304.
10. Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411-2421.
11. Zipprich J, Winter K, Hacker J, et al; Centers for Disease Control and Prevention (CDC). Measles outbreak—California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(6):153-154.
12. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
13. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
14. Centers for Disease for Control and Prevention. CDC yellow book 2018: health information for international travel. New York, NY: Oxford University Press; 2017.
15. Druss BG, Rosenheck RA, Desai MM, et al. Quality of preventive medical care for patients with mental disorders. Med Care. 2002;40(2):129-136.
16. Lasser K, Boyd J, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606-2610.
17. Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60(19);619-623.
1. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
2. Raj YP, Lloyd L. Adult immunizations. In: McCarron RM, Xiong GL, Keenan GR, et al, eds. Preventive medical care in psychiatry. Arlington, VA: American Psychiatric Publishing. 2015;215-227.
3. Young S, Dosani N, Whisler A, et al. Influenza vaccination rates among homeless adults with mental illness in Toronto. J Prim Care Community Health. 2015;6(3):211-214.
4. Kroger AT, Atkinson WL, Marcues EK, et al; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). General recommendations on immunization: recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-15):1-48.
5. Centers for Disease Control and Prevention. Recommended Adult Immunization by Vaccine and Age Group. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated February 27, 2017. Accessed February 1, 2017.
6. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(2):1-64.
7. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. https://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Updated June 22, 2016. Accessed February 1, 2017.
8. Hales CM, Harpaz, R, Ortega-Sanchez I, et al; Centers for Disease Control and Prevention. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63(33):729-731.
9. Petrosky E, Bocchini Jr JA, Hariri S, et al; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccine recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11)300-304.
10. Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411-2421.
11. Zipprich J, Winter K, Hacker J, et al; Centers for Disease Control and Prevention (CDC). Measles outbreak—California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(6):153-154.
12. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
13. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
14. Centers for Disease for Control and Prevention. CDC yellow book 2018: health information for international travel. New York, NY: Oxford University Press; 2017.
15. Druss BG, Rosenheck RA, Desai MM, et al. Quality of preventive medical care for patients with mental disorders. Med Care. 2002;40(2):129-136.
16. Lasser K, Boyd J, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606-2610.
17. Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60(19);619-623.
Dozing off: Examining excessive daytime sleepiness in psychiatric patients
Excessive daytime sleepiness (EDS) is “the inability to maintain wakefulness and alertness during the major waking periods of the day, with sleep occurring unintentionally or at inappropriate times, almost daily for at least 3 months,” according to the American Academy of Sleep Medicine.1 EDS is common, with a prevalence up to 25% to 30% in the general population.1-4 The prevalence rate varies in different studies, primarily because of inconsistent definitions of EDS, and therefore differences in diagnosis and assessment.1,2,4 In a study of 300 psychiatric outpatients, 34% had EDS.3 However, studies and evidence reviewing EDS in psychiatric patients are limited.
The causes of EDS are many and varied,1,8 including medical and psychiatric etiologies. A thorough history, screening at-risk patients, and timely sleep center referral are vital to detect and appropriately manage the cause of EDS.5
This article reviews the literature on EDS, with a focus on the risks of untreated EDS, common etiologies of the condition, as well as a brief description of screening and treatment strategies.
EDS vs fatigue
Many patients describe EDS as “fatigue”1; however, a patient’s report of fatigue could be mistaken for EDS.4 Although there is overlap, it is important for physicians to distinguish between these 2 entities for accurate identification and treatment.1,4
Risk of inadequate screening
A study of 117 patients with symptomatic coronary artery disease showed that EDS is associated with significantly greater incidence of cardiovascular adverse events at 16-month follow up.2 This study had limitations such as small sample size; therefore, more studies are needed. Because of these risks, timely and accurate diagnosis not only improves the patient’s quality of life and reduces polypharmacy but also can be life-saving.
Common causes of EDS in psychiatric patients
Because of the high prevalence and severity of impairments caused by EDS, it is essential for psychiatrists to be informed about causes of EDS and thoroughly assess for the potential underlying etiology before concluding that the sleep problem is a manifestation of the psychiatric disorder and prescribing psychotropic medication for it.
Some common causes of EDS in psychiatric patients include:
Sleep-disordered breathing.8 Obstructive sleep apnea (OSA) is often underdiagnosed,6,7 and considering how common it is,6 psychiatrists likely will see many patients with OSA in their practice.5 OSA has a higher prevalence among patients with psychiatric disorders such as depression6,9 and schizophrenia. Additionally, there is evidence suggesting that patients with OSA are more likely to suffer from depression and EDS than healthy controls6,9,10; some of the proposed mechanisms are sleep fragmentation and hypoxemia.6,9-11 OSA is the most common form of sleep-disordered breathing and is a common cause of EDS.1,2,12 Also, undiagnosed and untreated OSA in patients with depression could cause refractoriness to pharmacological treatment of depression.6,9,10
When unrecognized and untreated, OSA can be life-threatening. Despite this, OSA is not regularly screened for in clinical psychiatric practice.6,10 Therefore, it is imperative that psychiatrists be well-acquainted with measures to identify at-risk patients and refer to a sleep specialist when appropriate.
OSA is accompanied by irritability, cognitive difficulties, and poor sleep, creating an overlap with symptoms of depressive disorders.6,10 Use of sedative hypnotic medications, such as benzodiazepines, which further reduces muscle tone in the airway and suppresses respiratory effort, can worsen OSA symptoms5,6,10 and pose cerebrovascular, cardiovascular, and potentially life-threatening risks, and therefore is not indicated in this population.9,13
Obesity is a risk factor for OSA.6 Patients with mood disorders or schizophrenia or other psychotic disorders are at higher risk of obesity because of psychotropic-induced weight gain, stress-induced mechanisms, and/or lower levels of self-care. When these patients have unrecognized or untreated OSA and are prescribed sedative medications at night or stimulant medications during the day, they could be at increased cardiac or respiratory risks without resolving their underlying condition. A diligent psychiatrist can dramatically reduce the risks by referring a patient for nocturnal polysomnography,1 helping the patient implement lifestyle modifications (eg, exercise, weight loss, and healthy nutrition), prescribing judiciously, and monitoring closely for such risks. An accurate diagnosis of and treatment for OSA can improve sleep6 dramatically and help depressive symptoms through better sleep, more daytime energy and concentration, and adequate oxygenation of the brain while sleeping.
Psychiatrists can screen for OSA using the STOP-Bang (Snoring, Tired, Observed apnea, Pressure, Body mass index, Age, Neck circumference, Gender) Questionnaire, which is a quick, 8-item screening scale that helps to categorize OSA risk as mild, moderate, or severe.12 Hypertension, snoring, and/or gasping for breath (“observed apnea”)—a history which often is provided by spouses or significant others—daytime dozing and/or tiredness, having a large neck circumference or volume, body mass index, male sex, and age are items on the STOP-Bang Questionnaire and also are features that should raise high clinical suspicion of OSA.12 Referral for nocturnal polysomnography in at-risk patients should be the next step1,5 in any sleep-related breathing disorder.
Treatment for OSA involves continuous positive airway pressure (CPAP) therapy, which has been shown to relieve OSA and decrease related EDS.5,6 Other treatment modalities, such as oral appliances and surgery, may be used5 in some cases, but more studies are needed for conclusive results.
Several studies have shown improved depression, mood, and cognition after administering treatment such as CPAP6,9,14 in patients with OSA and depression. Considering the significant risks of cardiovascular,8 cerebrovascular,8 and overall morbidity and mortality associated with untreated OSA,12 it is important to routinely screen for sleep-disordered breathing in patients with depression9 or other psychiatric disorders and refer for specialized sleep evaluation and treatment, when indicated.
Medications. EDS can result from some prescription and over-the-counter medications.1,2,5,7 Sedating antidepressants, antihistamines, antipsychotics, anticonvulsants,1,8 and beta blockers2 could cause sedation, which can persist during daytime, although a few studies did not find an association between antipsychotic use and EDS.3 Benzodiazepines and other sedative-hypnotics,1,7 especially long-acting agents or higher dosages,5 can lead to EDS and decreased alertness. Non-psychotropics, such as opioid pain medications,1,7 antitussives, and skeletal muscle relaxants, also can contribute to or cause daytime sedation.7 When using these agents, psychiatrists should monitor and routinely assess patients while aiming for the lowest effective dosage when feasible.
This strategy creates a framework for psychiatrists to routinely educate patients about these commonly encountered side effects, reduce polypharmacy when possible, and help patients effectively manage or prevent these adverse effects.
Depression.1 Some studies found >45% patients with depression had EDS.3,13,15 Besides an association between depression and EDS,13,16 Chellappa and Araújo13 also found a significant association between EDS and suicidal ideation. The causes of EDS in patients with depression may be varied, ranging from restless legs syndrome, residual depressive symptoms,15 to OSA. Depression is often comorbid with OSA,6 with up to 20% of patients with depression suffering from OSA,10 creating higher risk for EDS. Depressive disorders are routinely assessed during an evaluation of OSA at sleep centers, but OSA often is not screened in psychiatric practice.10
There is a strong need for regular screening for OSA in patients with depression, particularly because most studies show a link between the 2 conditions.10 Both depression and OSA have some common risk factors, such as obesity, hypertension, and metabolic syndrome.10 Patients with these conditions are at greater risk for OSA, and therefore a psychiatrist should proactively screen and refer such patients for nocturnal polysomnography when they suspect OSA. Patients with OSA and depression often present to the psychiatrist with depressive symptoms that appear to be resistant to pharmacological treatment,10 therefore underscoring the importance of screening and ruling out OSA in patients with depression.
Circadian rhythm disorders, restless legs syndrome, alcohol and other substance use, and use of prescription sedative-hypnotics are more common in patients with depression; therefore, this population is at high risk for EDS.
Circadian rhythm disorders and insufficient sleep syndrome. Insufficient sleep syndrome1,2,8 frequently causes EDS and occurs more commonly in busy people who try to get by with less sleep.8 Over time, the effect of sleep loss is cumulative and can be accompanied by mood symptoms, such as irritability, fatigue, and problems with concentration.8 Shift workers1,8 commonly experience insufficient sleep as well as circadian rhythm disorders and EDS. Modafinil is FDA-approved for EDS in shift work sleep disorder.
Geriatric patients may experience advanced sleep phase syndrome involving early awakenings.8 Adolescents, on the other hand, often suffer from delayed sleep phase syndrome, which is a type of circadian rhythm disorder, related to increasing academic and social pressures, natural pubertal shift to later sleep onset, pervading technology use, and often nebulous bedtime routines. This can be a cause of sleep persisting into daytime.8 Taking a careful history and a sleep diary may be useful because this disorder might be confused for insomnia. Treatment involves gradual shifting of the time of sleep onset through bright light exposure and other modalities.8
Adolescents might not be forthcoming about the severity of their sleep problems; therefore, psychiatrists should screen proactively through clinical interviews of patients and parents and consider this possibility when encountering an adolescent with recent-onset attention or cognitive difficulties.
Treatment for circadian rhythm disorders usually includes planned or prescribed sleep scheduling, timed light exposure,8 and occasional use of melatonin or other sedative agents.17
Hypersomnia of central origin, which includes narcolepsy, idiopathic hypersomnia, and recurrent hypersomnia, can present with EDS.1,18,19 Narcolepsy is a rare, debilitating sleep disorder that manifests as EDS or sleep attacks, with or without cataplexy, and sleep paralysis.5,8,18,19 The Multiple Sleep Latency Test and polysomnography are used for diagnosis.1,5 Shortened REM latency is a classic finding often noted on polysomnography. Treatment involves pharmacologic and behavioral strategies and education.5,8 Modafinil is FDA-approved for EDS associated with narcolepsy. Stimulant medications have been used for narcolepsy in the past; further studies are needed to establish benefit–risk ratio of use in this population.18
Kleine-Levin syndrome is a form of recurrent hypersomnia, a less common sleep disorder, characterized by episodes of excessive sleepiness accompanied by hyperphagia and hypersexuality.5,18,19
Other medical conditions,1 such as the rare familial fatal insomnia, neurological conditions1 such as encephalitis,8 epilepsy,8 Alzheimer’s disease or other types of dementia,8 Parkinson’s disease,1 or multiple sclerosis,1,18 can cause excessive daytime fatigue by causing secondary insomnia or hypersomnia.
Treating the underlying disorder is an important first step in these cases. In addition, coordinating with neurologists or other specialists involved in caring for patients with these conditions is important. Regularly reviewing and simplifying the often complex medication regimen, when possible, can go a long way in mitigating EDS in this population.
Other disorders affecting sleep. Restless legs syndrome and periodic limb movement disorder are other causes of EDS.3 Treatment involves lifestyle modifications, iron supplementation in certain patients, and use of dopaminergic agents such as ropinirole, pramipexole, and other medications, depending on severity of the condition, comorbidities, and other factors.20
Alcohol or substance use. Substance use or withdrawal can be associated with sleep disorders, such as hypersomnia,19 insomnia,19 and related EDS.5 For example, alcohol use disorder affects REM sleep, and can cause EDS. Secondary central apnea can be the result of long-standing opioid use19 and can present like EDS.
Insomnia. Primary insomnia rarely causes EDS.5 Insomnia due to a medical or psychiatric condition may be an indirect cause of EDS by causing sleep deprivation.
Steps for timely and accurate diagnosis
Utilize the following steps for facilitating timely diagnosis and treatment of EDS:
Thorough history. Patients often describe “tiredness” instead of sleepiness.8 Therefore, the astute psychiatrist should explore further when patients are presenting with this concern, especially by asking more specific questions such as the tendency to doze off during daytime.8
Family members can be vital sources for obtaining a complete history,5 especially because patients might deny,8 minimize, or not be fully aware1 of the extent of their symptoms. Asking family members about patient’s snoring, irregular breathing, or gasping at night can be particularly valuable.5 Obtaining a family history of sleep disorders can be particularly important, especially in conditions such as OSA and narcolepsy.
Asking about any history of safety issues,8 including sleepiness during driving, cooking, or other activities, is also important.
Use of scales and other screening measures. Psychiatrists can use initial screening measures in the office setting. Epworth Sleepiness Scale15,21 is a validated,2 short, self-administered measure to assess the level of daytime sleepiness; however, it has some limitations such as not being able to measure changes in sleepiness from hour to hour or day to day. Because of its limitations, the Epworth Sleepiness Scale should not be used by itself as a diagnostic tool.3 It has been commonly used for detecting OSA2 and narcolepsy. The Stanford Sleepiness Scale is a self-rating scale that measures the subjective degree of sleepiness and alertness; it has limitations as well, such as having little correlation with chronic sleep loss.8 Other tools such as visual analogue scales also could be helpful.8 For more specialized testing, such as Multiple Sleep Latency Test or polysomnography, referral to a sleep specialist is ideal.8
Education. The assessment is an opportunity for the psychiatrist to educate patients about sleep hygiene, the importance of regular bedtimes, and getting adequate sleep to avoid accumulating a sleep deficit.
Urgent referral of at-risk populations. Prompt or urgent referral of at-risk populations, such as geriatric patients or those with a history of dozing off during driving, is invaluable in preventing morbidity and mortality from untreated sleep disorders.
Patients with severe daytime sleepiness should be advised to not drive or operate heavy machinery until this condition is adequately controlled.18
Bottom Line
1. Chervin RD. Approach to the patient with excessive daytime sleepiness. http://www.uptodate.com/contents/approach-to-the-patient-with-excessive-daytime-sleepiness. Updated January 2016. Accessed June 5, 2017.
2. Lee CH, Ng WY, Hau W, et al. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med. 2013;9(12):1267-1272.
3. Hawley CJ, Gale TM, Sivakumaran T, et al. Excessive daytime sleepiness in psychiatric disorders: prevalence, correlates and clinical significance. Psychiatry Res. 2010;175(1-2):138-141.
4. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54(1):61-69.
5. Krahn LE. Excessive daytime sleepiness: diagnosing the causes. Current Psychiatry. 2002;1(1):49-57.
6. Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17-25.
7. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
8. Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124(pt 8):1482-1491.
9. Cheng P, Casement M, Chen CF, et al. Sleep disordered breathing in major depressive disorder. J Sleep Res. 2013;22(4):459-462.
10. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.
11. Bardwell WA, Berry CC, Ancoli-Israel S, et al. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47(6):583-596.
12. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631-638.
13. Chellappa SL, Araújo JF. Excessive daytime sleepiness in patients with depressive disorder. Rev Bras Psiquiatr. 2006;28(2):126-129.
14. Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11(6):552-557.
15. Lundt L. Use of the Epworth Sleepiness Scale to evaluate the symptom of excessive sleepiness in major depressive disorder. Gen Hosp Psychiatry. 2005;27(2):146-148.
16. Hawley CJ. Excessive daytime sleepiness in psychiatry: a relevant focus for clinical attention and treatment? Int J Psychiatry Clin Pract. 2006;10(2):117-123.
17. Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701-715.
18. Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705-1711.
19. Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012;9(4):687-701.
20. National Institute of Neurological Disorders and Stroke. Restless legs syndrome information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Restless-Legs-Syndrome-Information-Page. Accessed June 2, 2017.
21. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376-381.
Excessive daytime sleepiness (EDS) is “the inability to maintain wakefulness and alertness during the major waking periods of the day, with sleep occurring unintentionally or at inappropriate times, almost daily for at least 3 months,” according to the American Academy of Sleep Medicine.1 EDS is common, with a prevalence up to 25% to 30% in the general population.1-4 The prevalence rate varies in different studies, primarily because of inconsistent definitions of EDS, and therefore differences in diagnosis and assessment.1,2,4 In a study of 300 psychiatric outpatients, 34% had EDS.3 However, studies and evidence reviewing EDS in psychiatric patients are limited.
The causes of EDS are many and varied,1,8 including medical and psychiatric etiologies. A thorough history, screening at-risk patients, and timely sleep center referral are vital to detect and appropriately manage the cause of EDS.5
This article reviews the literature on EDS, with a focus on the risks of untreated EDS, common etiologies of the condition, as well as a brief description of screening and treatment strategies.
EDS vs fatigue
Many patients describe EDS as “fatigue”1; however, a patient’s report of fatigue could be mistaken for EDS.4 Although there is overlap, it is important for physicians to distinguish between these 2 entities for accurate identification and treatment.1,4
Risk of inadequate screening
A study of 117 patients with symptomatic coronary artery disease showed that EDS is associated with significantly greater incidence of cardiovascular adverse events at 16-month follow up.2 This study had limitations such as small sample size; therefore, more studies are needed. Because of these risks, timely and accurate diagnosis not only improves the patient’s quality of life and reduces polypharmacy but also can be life-saving.
Common causes of EDS in psychiatric patients
Because of the high prevalence and severity of impairments caused by EDS, it is essential for psychiatrists to be informed about causes of EDS and thoroughly assess for the potential underlying etiology before concluding that the sleep problem is a manifestation of the psychiatric disorder and prescribing psychotropic medication for it.
Some common causes of EDS in psychiatric patients include:
Sleep-disordered breathing.8 Obstructive sleep apnea (OSA) is often underdiagnosed,6,7 and considering how common it is,6 psychiatrists likely will see many patients with OSA in their practice.5 OSA has a higher prevalence among patients with psychiatric disorders such as depression6,9 and schizophrenia. Additionally, there is evidence suggesting that patients with OSA are more likely to suffer from depression and EDS than healthy controls6,9,10; some of the proposed mechanisms are sleep fragmentation and hypoxemia.6,9-11 OSA is the most common form of sleep-disordered breathing and is a common cause of EDS.1,2,12 Also, undiagnosed and untreated OSA in patients with depression could cause refractoriness to pharmacological treatment of depression.6,9,10
When unrecognized and untreated, OSA can be life-threatening. Despite this, OSA is not regularly screened for in clinical psychiatric practice.6,10 Therefore, it is imperative that psychiatrists be well-acquainted with measures to identify at-risk patients and refer to a sleep specialist when appropriate.
OSA is accompanied by irritability, cognitive difficulties, and poor sleep, creating an overlap with symptoms of depressive disorders.6,10 Use of sedative hypnotic medications, such as benzodiazepines, which further reduces muscle tone in the airway and suppresses respiratory effort, can worsen OSA symptoms5,6,10 and pose cerebrovascular, cardiovascular, and potentially life-threatening risks, and therefore is not indicated in this population.9,13
Obesity is a risk factor for OSA.6 Patients with mood disorders or schizophrenia or other psychotic disorders are at higher risk of obesity because of psychotropic-induced weight gain, stress-induced mechanisms, and/or lower levels of self-care. When these patients have unrecognized or untreated OSA and are prescribed sedative medications at night or stimulant medications during the day, they could be at increased cardiac or respiratory risks without resolving their underlying condition. A diligent psychiatrist can dramatically reduce the risks by referring a patient for nocturnal polysomnography,1 helping the patient implement lifestyle modifications (eg, exercise, weight loss, and healthy nutrition), prescribing judiciously, and monitoring closely for such risks. An accurate diagnosis of and treatment for OSA can improve sleep6 dramatically and help depressive symptoms through better sleep, more daytime energy and concentration, and adequate oxygenation of the brain while sleeping.
Psychiatrists can screen for OSA using the STOP-Bang (Snoring, Tired, Observed apnea, Pressure, Body mass index, Age, Neck circumference, Gender) Questionnaire, which is a quick, 8-item screening scale that helps to categorize OSA risk as mild, moderate, or severe.12 Hypertension, snoring, and/or gasping for breath (“observed apnea”)—a history which often is provided by spouses or significant others—daytime dozing and/or tiredness, having a large neck circumference or volume, body mass index, male sex, and age are items on the STOP-Bang Questionnaire and also are features that should raise high clinical suspicion of OSA.12 Referral for nocturnal polysomnography in at-risk patients should be the next step1,5 in any sleep-related breathing disorder.
Treatment for OSA involves continuous positive airway pressure (CPAP) therapy, which has been shown to relieve OSA and decrease related EDS.5,6 Other treatment modalities, such as oral appliances and surgery, may be used5 in some cases, but more studies are needed for conclusive results.
Several studies have shown improved depression, mood, and cognition after administering treatment such as CPAP6,9,14 in patients with OSA and depression. Considering the significant risks of cardiovascular,8 cerebrovascular,8 and overall morbidity and mortality associated with untreated OSA,12 it is important to routinely screen for sleep-disordered breathing in patients with depression9 or other psychiatric disorders and refer for specialized sleep evaluation and treatment, when indicated.
Medications. EDS can result from some prescription and over-the-counter medications.1,2,5,7 Sedating antidepressants, antihistamines, antipsychotics, anticonvulsants,1,8 and beta blockers2 could cause sedation, which can persist during daytime, although a few studies did not find an association between antipsychotic use and EDS.3 Benzodiazepines and other sedative-hypnotics,1,7 especially long-acting agents or higher dosages,5 can lead to EDS and decreased alertness. Non-psychotropics, such as opioid pain medications,1,7 antitussives, and skeletal muscle relaxants, also can contribute to or cause daytime sedation.7 When using these agents, psychiatrists should monitor and routinely assess patients while aiming for the lowest effective dosage when feasible.
This strategy creates a framework for psychiatrists to routinely educate patients about these commonly encountered side effects, reduce polypharmacy when possible, and help patients effectively manage or prevent these adverse effects.
Depression.1 Some studies found >45% patients with depression had EDS.3,13,15 Besides an association between depression and EDS,13,16 Chellappa and Araújo13 also found a significant association between EDS and suicidal ideation. The causes of EDS in patients with depression may be varied, ranging from restless legs syndrome, residual depressive symptoms,15 to OSA. Depression is often comorbid with OSA,6 with up to 20% of patients with depression suffering from OSA,10 creating higher risk for EDS. Depressive disorders are routinely assessed during an evaluation of OSA at sleep centers, but OSA often is not screened in psychiatric practice.10
There is a strong need for regular screening for OSA in patients with depression, particularly because most studies show a link between the 2 conditions.10 Both depression and OSA have some common risk factors, such as obesity, hypertension, and metabolic syndrome.10 Patients with these conditions are at greater risk for OSA, and therefore a psychiatrist should proactively screen and refer such patients for nocturnal polysomnography when they suspect OSA. Patients with OSA and depression often present to the psychiatrist with depressive symptoms that appear to be resistant to pharmacological treatment,10 therefore underscoring the importance of screening and ruling out OSA in patients with depression.
Circadian rhythm disorders, restless legs syndrome, alcohol and other substance use, and use of prescription sedative-hypnotics are more common in patients with depression; therefore, this population is at high risk for EDS.
Circadian rhythm disorders and insufficient sleep syndrome. Insufficient sleep syndrome1,2,8 frequently causes EDS and occurs more commonly in busy people who try to get by with less sleep.8 Over time, the effect of sleep loss is cumulative and can be accompanied by mood symptoms, such as irritability, fatigue, and problems with concentration.8 Shift workers1,8 commonly experience insufficient sleep as well as circadian rhythm disorders and EDS. Modafinil is FDA-approved for EDS in shift work sleep disorder.
Geriatric patients may experience advanced sleep phase syndrome involving early awakenings.8 Adolescents, on the other hand, often suffer from delayed sleep phase syndrome, which is a type of circadian rhythm disorder, related to increasing academic and social pressures, natural pubertal shift to later sleep onset, pervading technology use, and often nebulous bedtime routines. This can be a cause of sleep persisting into daytime.8 Taking a careful history and a sleep diary may be useful because this disorder might be confused for insomnia. Treatment involves gradual shifting of the time of sleep onset through bright light exposure and other modalities.8
Adolescents might not be forthcoming about the severity of their sleep problems; therefore, psychiatrists should screen proactively through clinical interviews of patients and parents and consider this possibility when encountering an adolescent with recent-onset attention or cognitive difficulties.
Treatment for circadian rhythm disorders usually includes planned or prescribed sleep scheduling, timed light exposure,8 and occasional use of melatonin or other sedative agents.17
Hypersomnia of central origin, which includes narcolepsy, idiopathic hypersomnia, and recurrent hypersomnia, can present with EDS.1,18,19 Narcolepsy is a rare, debilitating sleep disorder that manifests as EDS or sleep attacks, with or without cataplexy, and sleep paralysis.5,8,18,19 The Multiple Sleep Latency Test and polysomnography are used for diagnosis.1,5 Shortened REM latency is a classic finding often noted on polysomnography. Treatment involves pharmacologic and behavioral strategies and education.5,8 Modafinil is FDA-approved for EDS associated with narcolepsy. Stimulant medications have been used for narcolepsy in the past; further studies are needed to establish benefit–risk ratio of use in this population.18
Kleine-Levin syndrome is a form of recurrent hypersomnia, a less common sleep disorder, characterized by episodes of excessive sleepiness accompanied by hyperphagia and hypersexuality.5,18,19
Other medical conditions,1 such as the rare familial fatal insomnia, neurological conditions1 such as encephalitis,8 epilepsy,8 Alzheimer’s disease or other types of dementia,8 Parkinson’s disease,1 or multiple sclerosis,1,18 can cause excessive daytime fatigue by causing secondary insomnia or hypersomnia.
Treating the underlying disorder is an important first step in these cases. In addition, coordinating with neurologists or other specialists involved in caring for patients with these conditions is important. Regularly reviewing and simplifying the often complex medication regimen, when possible, can go a long way in mitigating EDS in this population.
Other disorders affecting sleep. Restless legs syndrome and periodic limb movement disorder are other causes of EDS.3 Treatment involves lifestyle modifications, iron supplementation in certain patients, and use of dopaminergic agents such as ropinirole, pramipexole, and other medications, depending on severity of the condition, comorbidities, and other factors.20
Alcohol or substance use. Substance use or withdrawal can be associated with sleep disorders, such as hypersomnia,19 insomnia,19 and related EDS.5 For example, alcohol use disorder affects REM sleep, and can cause EDS. Secondary central apnea can be the result of long-standing opioid use19 and can present like EDS.
Insomnia. Primary insomnia rarely causes EDS.5 Insomnia due to a medical or psychiatric condition may be an indirect cause of EDS by causing sleep deprivation.
Steps for timely and accurate diagnosis
Utilize the following steps for facilitating timely diagnosis and treatment of EDS:
Thorough history. Patients often describe “tiredness” instead of sleepiness.8 Therefore, the astute psychiatrist should explore further when patients are presenting with this concern, especially by asking more specific questions such as the tendency to doze off during daytime.8
Family members can be vital sources for obtaining a complete history,5 especially because patients might deny,8 minimize, or not be fully aware1 of the extent of their symptoms. Asking family members about patient’s snoring, irregular breathing, or gasping at night can be particularly valuable.5 Obtaining a family history of sleep disorders can be particularly important, especially in conditions such as OSA and narcolepsy.
Asking about any history of safety issues,8 including sleepiness during driving, cooking, or other activities, is also important.
Use of scales and other screening measures. Psychiatrists can use initial screening measures in the office setting. Epworth Sleepiness Scale15,21 is a validated,2 short, self-administered measure to assess the level of daytime sleepiness; however, it has some limitations such as not being able to measure changes in sleepiness from hour to hour or day to day. Because of its limitations, the Epworth Sleepiness Scale should not be used by itself as a diagnostic tool.3 It has been commonly used for detecting OSA2 and narcolepsy. The Stanford Sleepiness Scale is a self-rating scale that measures the subjective degree of sleepiness and alertness; it has limitations as well, such as having little correlation with chronic sleep loss.8 Other tools such as visual analogue scales also could be helpful.8 For more specialized testing, such as Multiple Sleep Latency Test or polysomnography, referral to a sleep specialist is ideal.8
Education. The assessment is an opportunity for the psychiatrist to educate patients about sleep hygiene, the importance of regular bedtimes, and getting adequate sleep to avoid accumulating a sleep deficit.
Urgent referral of at-risk populations. Prompt or urgent referral of at-risk populations, such as geriatric patients or those with a history of dozing off during driving, is invaluable in preventing morbidity and mortality from untreated sleep disorders.
Patients with severe daytime sleepiness should be advised to not drive or operate heavy machinery until this condition is adequately controlled.18
Bottom Line
Excessive daytime sleepiness (EDS) is “the inability to maintain wakefulness and alertness during the major waking periods of the day, with sleep occurring unintentionally or at inappropriate times, almost daily for at least 3 months,” according to the American Academy of Sleep Medicine.1 EDS is common, with a prevalence up to 25% to 30% in the general population.1-4 The prevalence rate varies in different studies, primarily because of inconsistent definitions of EDS, and therefore differences in diagnosis and assessment.1,2,4 In a study of 300 psychiatric outpatients, 34% had EDS.3 However, studies and evidence reviewing EDS in psychiatric patients are limited.
The causes of EDS are many and varied,1,8 including medical and psychiatric etiologies. A thorough history, screening at-risk patients, and timely sleep center referral are vital to detect and appropriately manage the cause of EDS.5
This article reviews the literature on EDS, with a focus on the risks of untreated EDS, common etiologies of the condition, as well as a brief description of screening and treatment strategies.
EDS vs fatigue
Many patients describe EDS as “fatigue”1; however, a patient’s report of fatigue could be mistaken for EDS.4 Although there is overlap, it is important for physicians to distinguish between these 2 entities for accurate identification and treatment.1,4
Risk of inadequate screening
A study of 117 patients with symptomatic coronary artery disease showed that EDS is associated with significantly greater incidence of cardiovascular adverse events at 16-month follow up.2 This study had limitations such as small sample size; therefore, more studies are needed. Because of these risks, timely and accurate diagnosis not only improves the patient’s quality of life and reduces polypharmacy but also can be life-saving.
Common causes of EDS in psychiatric patients
Because of the high prevalence and severity of impairments caused by EDS, it is essential for psychiatrists to be informed about causes of EDS and thoroughly assess for the potential underlying etiology before concluding that the sleep problem is a manifestation of the psychiatric disorder and prescribing psychotropic medication for it.
Some common causes of EDS in psychiatric patients include:
Sleep-disordered breathing.8 Obstructive sleep apnea (OSA) is often underdiagnosed,6,7 and considering how common it is,6 psychiatrists likely will see many patients with OSA in their practice.5 OSA has a higher prevalence among patients with psychiatric disorders such as depression6,9 and schizophrenia. Additionally, there is evidence suggesting that patients with OSA are more likely to suffer from depression and EDS than healthy controls6,9,10; some of the proposed mechanisms are sleep fragmentation and hypoxemia.6,9-11 OSA is the most common form of sleep-disordered breathing and is a common cause of EDS.1,2,12 Also, undiagnosed and untreated OSA in patients with depression could cause refractoriness to pharmacological treatment of depression.6,9,10
When unrecognized and untreated, OSA can be life-threatening. Despite this, OSA is not regularly screened for in clinical psychiatric practice.6,10 Therefore, it is imperative that psychiatrists be well-acquainted with measures to identify at-risk patients and refer to a sleep specialist when appropriate.
OSA is accompanied by irritability, cognitive difficulties, and poor sleep, creating an overlap with symptoms of depressive disorders.6,10 Use of sedative hypnotic medications, such as benzodiazepines, which further reduces muscle tone in the airway and suppresses respiratory effort, can worsen OSA symptoms5,6,10 and pose cerebrovascular, cardiovascular, and potentially life-threatening risks, and therefore is not indicated in this population.9,13
Obesity is a risk factor for OSA.6 Patients with mood disorders or schizophrenia or other psychotic disorders are at higher risk of obesity because of psychotropic-induced weight gain, stress-induced mechanisms, and/or lower levels of self-care. When these patients have unrecognized or untreated OSA and are prescribed sedative medications at night or stimulant medications during the day, they could be at increased cardiac or respiratory risks without resolving their underlying condition. A diligent psychiatrist can dramatically reduce the risks by referring a patient for nocturnal polysomnography,1 helping the patient implement lifestyle modifications (eg, exercise, weight loss, and healthy nutrition), prescribing judiciously, and monitoring closely for such risks. An accurate diagnosis of and treatment for OSA can improve sleep6 dramatically and help depressive symptoms through better sleep, more daytime energy and concentration, and adequate oxygenation of the brain while sleeping.
Psychiatrists can screen for OSA using the STOP-Bang (Snoring, Tired, Observed apnea, Pressure, Body mass index, Age, Neck circumference, Gender) Questionnaire, which is a quick, 8-item screening scale that helps to categorize OSA risk as mild, moderate, or severe.12 Hypertension, snoring, and/or gasping for breath (“observed apnea”)—a history which often is provided by spouses or significant others—daytime dozing and/or tiredness, having a large neck circumference or volume, body mass index, male sex, and age are items on the STOP-Bang Questionnaire and also are features that should raise high clinical suspicion of OSA.12 Referral for nocturnal polysomnography in at-risk patients should be the next step1,5 in any sleep-related breathing disorder.
Treatment for OSA involves continuous positive airway pressure (CPAP) therapy, which has been shown to relieve OSA and decrease related EDS.5,6 Other treatment modalities, such as oral appliances and surgery, may be used5 in some cases, but more studies are needed for conclusive results.
Several studies have shown improved depression, mood, and cognition after administering treatment such as CPAP6,9,14 in patients with OSA and depression. Considering the significant risks of cardiovascular,8 cerebrovascular,8 and overall morbidity and mortality associated with untreated OSA,12 it is important to routinely screen for sleep-disordered breathing in patients with depression9 or other psychiatric disorders and refer for specialized sleep evaluation and treatment, when indicated.
Medications. EDS can result from some prescription and over-the-counter medications.1,2,5,7 Sedating antidepressants, antihistamines, antipsychotics, anticonvulsants,1,8 and beta blockers2 could cause sedation, which can persist during daytime, although a few studies did not find an association between antipsychotic use and EDS.3 Benzodiazepines and other sedative-hypnotics,1,7 especially long-acting agents or higher dosages,5 can lead to EDS and decreased alertness. Non-psychotropics, such as opioid pain medications,1,7 antitussives, and skeletal muscle relaxants, also can contribute to or cause daytime sedation.7 When using these agents, psychiatrists should monitor and routinely assess patients while aiming for the lowest effective dosage when feasible.
This strategy creates a framework for psychiatrists to routinely educate patients about these commonly encountered side effects, reduce polypharmacy when possible, and help patients effectively manage or prevent these adverse effects.
Depression.1 Some studies found >45% patients with depression had EDS.3,13,15 Besides an association between depression and EDS,13,16 Chellappa and Araújo13 also found a significant association between EDS and suicidal ideation. The causes of EDS in patients with depression may be varied, ranging from restless legs syndrome, residual depressive symptoms,15 to OSA. Depression is often comorbid with OSA,6 with up to 20% of patients with depression suffering from OSA,10 creating higher risk for EDS. Depressive disorders are routinely assessed during an evaluation of OSA at sleep centers, but OSA often is not screened in psychiatric practice.10
There is a strong need for regular screening for OSA in patients with depression, particularly because most studies show a link between the 2 conditions.10 Both depression and OSA have some common risk factors, such as obesity, hypertension, and metabolic syndrome.10 Patients with these conditions are at greater risk for OSA, and therefore a psychiatrist should proactively screen and refer such patients for nocturnal polysomnography when they suspect OSA. Patients with OSA and depression often present to the psychiatrist with depressive symptoms that appear to be resistant to pharmacological treatment,10 therefore underscoring the importance of screening and ruling out OSA in patients with depression.
Circadian rhythm disorders, restless legs syndrome, alcohol and other substance use, and use of prescription sedative-hypnotics are more common in patients with depression; therefore, this population is at high risk for EDS.
Circadian rhythm disorders and insufficient sleep syndrome. Insufficient sleep syndrome1,2,8 frequently causes EDS and occurs more commonly in busy people who try to get by with less sleep.8 Over time, the effect of sleep loss is cumulative and can be accompanied by mood symptoms, such as irritability, fatigue, and problems with concentration.8 Shift workers1,8 commonly experience insufficient sleep as well as circadian rhythm disorders and EDS. Modafinil is FDA-approved for EDS in shift work sleep disorder.
Geriatric patients may experience advanced sleep phase syndrome involving early awakenings.8 Adolescents, on the other hand, often suffer from delayed sleep phase syndrome, which is a type of circadian rhythm disorder, related to increasing academic and social pressures, natural pubertal shift to later sleep onset, pervading technology use, and often nebulous bedtime routines. This can be a cause of sleep persisting into daytime.8 Taking a careful history and a sleep diary may be useful because this disorder might be confused for insomnia. Treatment involves gradual shifting of the time of sleep onset through bright light exposure and other modalities.8
Adolescents might not be forthcoming about the severity of their sleep problems; therefore, psychiatrists should screen proactively through clinical interviews of patients and parents and consider this possibility when encountering an adolescent with recent-onset attention or cognitive difficulties.
Treatment for circadian rhythm disorders usually includes planned or prescribed sleep scheduling, timed light exposure,8 and occasional use of melatonin or other sedative agents.17
Hypersomnia of central origin, which includes narcolepsy, idiopathic hypersomnia, and recurrent hypersomnia, can present with EDS.1,18,19 Narcolepsy is a rare, debilitating sleep disorder that manifests as EDS or sleep attacks, with or without cataplexy, and sleep paralysis.5,8,18,19 The Multiple Sleep Latency Test and polysomnography are used for diagnosis.1,5 Shortened REM latency is a classic finding often noted on polysomnography. Treatment involves pharmacologic and behavioral strategies and education.5,8 Modafinil is FDA-approved for EDS associated with narcolepsy. Stimulant medications have been used for narcolepsy in the past; further studies are needed to establish benefit–risk ratio of use in this population.18
Kleine-Levin syndrome is a form of recurrent hypersomnia, a less common sleep disorder, characterized by episodes of excessive sleepiness accompanied by hyperphagia and hypersexuality.5,18,19
Other medical conditions,1 such as the rare familial fatal insomnia, neurological conditions1 such as encephalitis,8 epilepsy,8 Alzheimer’s disease or other types of dementia,8 Parkinson’s disease,1 or multiple sclerosis,1,18 can cause excessive daytime fatigue by causing secondary insomnia or hypersomnia.
Treating the underlying disorder is an important first step in these cases. In addition, coordinating with neurologists or other specialists involved in caring for patients with these conditions is important. Regularly reviewing and simplifying the often complex medication regimen, when possible, can go a long way in mitigating EDS in this population.
Other disorders affecting sleep. Restless legs syndrome and periodic limb movement disorder are other causes of EDS.3 Treatment involves lifestyle modifications, iron supplementation in certain patients, and use of dopaminergic agents such as ropinirole, pramipexole, and other medications, depending on severity of the condition, comorbidities, and other factors.20
Alcohol or substance use. Substance use or withdrawal can be associated with sleep disorders, such as hypersomnia,19 insomnia,19 and related EDS.5 For example, alcohol use disorder affects REM sleep, and can cause EDS. Secondary central apnea can be the result of long-standing opioid use19 and can present like EDS.
Insomnia. Primary insomnia rarely causes EDS.5 Insomnia due to a medical or psychiatric condition may be an indirect cause of EDS by causing sleep deprivation.
Steps for timely and accurate diagnosis
Utilize the following steps for facilitating timely diagnosis and treatment of EDS:
Thorough history. Patients often describe “tiredness” instead of sleepiness.8 Therefore, the astute psychiatrist should explore further when patients are presenting with this concern, especially by asking more specific questions such as the tendency to doze off during daytime.8
Family members can be vital sources for obtaining a complete history,5 especially because patients might deny,8 minimize, or not be fully aware1 of the extent of their symptoms. Asking family members about patient’s snoring, irregular breathing, or gasping at night can be particularly valuable.5 Obtaining a family history of sleep disorders can be particularly important, especially in conditions such as OSA and narcolepsy.
Asking about any history of safety issues,8 including sleepiness during driving, cooking, or other activities, is also important.
Use of scales and other screening measures. Psychiatrists can use initial screening measures in the office setting. Epworth Sleepiness Scale15,21 is a validated,2 short, self-administered measure to assess the level of daytime sleepiness; however, it has some limitations such as not being able to measure changes in sleepiness from hour to hour or day to day. Because of its limitations, the Epworth Sleepiness Scale should not be used by itself as a diagnostic tool.3 It has been commonly used for detecting OSA2 and narcolepsy. The Stanford Sleepiness Scale is a self-rating scale that measures the subjective degree of sleepiness and alertness; it has limitations as well, such as having little correlation with chronic sleep loss.8 Other tools such as visual analogue scales also could be helpful.8 For more specialized testing, such as Multiple Sleep Latency Test or polysomnography, referral to a sleep specialist is ideal.8
Education. The assessment is an opportunity for the psychiatrist to educate patients about sleep hygiene, the importance of regular bedtimes, and getting adequate sleep to avoid accumulating a sleep deficit.
Urgent referral of at-risk populations. Prompt or urgent referral of at-risk populations, such as geriatric patients or those with a history of dozing off during driving, is invaluable in preventing morbidity and mortality from untreated sleep disorders.
Patients with severe daytime sleepiness should be advised to not drive or operate heavy machinery until this condition is adequately controlled.18
Bottom Line
1. Chervin RD. Approach to the patient with excessive daytime sleepiness. http://www.uptodate.com/contents/approach-to-the-patient-with-excessive-daytime-sleepiness. Updated January 2016. Accessed June 5, 2017.
2. Lee CH, Ng WY, Hau W, et al. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med. 2013;9(12):1267-1272.
3. Hawley CJ, Gale TM, Sivakumaran T, et al. Excessive daytime sleepiness in psychiatric disorders: prevalence, correlates and clinical significance. Psychiatry Res. 2010;175(1-2):138-141.
4. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54(1):61-69.
5. Krahn LE. Excessive daytime sleepiness: diagnosing the causes. Current Psychiatry. 2002;1(1):49-57.
6. Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17-25.
7. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
8. Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124(pt 8):1482-1491.
9. Cheng P, Casement M, Chen CF, et al. Sleep disordered breathing in major depressive disorder. J Sleep Res. 2013;22(4):459-462.
10. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.
11. Bardwell WA, Berry CC, Ancoli-Israel S, et al. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47(6):583-596.
12. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631-638.
13. Chellappa SL, Araújo JF. Excessive daytime sleepiness in patients with depressive disorder. Rev Bras Psiquiatr. 2006;28(2):126-129.
14. Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11(6):552-557.
15. Lundt L. Use of the Epworth Sleepiness Scale to evaluate the symptom of excessive sleepiness in major depressive disorder. Gen Hosp Psychiatry. 2005;27(2):146-148.
16. Hawley CJ. Excessive daytime sleepiness in psychiatry: a relevant focus for clinical attention and treatment? Int J Psychiatry Clin Pract. 2006;10(2):117-123.
17. Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701-715.
18. Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705-1711.
19. Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012;9(4):687-701.
20. National Institute of Neurological Disorders and Stroke. Restless legs syndrome information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Restless-Legs-Syndrome-Information-Page. Accessed June 2, 2017.
21. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376-381.
1. Chervin RD. Approach to the patient with excessive daytime sleepiness. http://www.uptodate.com/contents/approach-to-the-patient-with-excessive-daytime-sleepiness. Updated January 2016. Accessed June 5, 2017.
2. Lee CH, Ng WY, Hau W, et al. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med. 2013;9(12):1267-1272.
3. Hawley CJ, Gale TM, Sivakumaran T, et al. Excessive daytime sleepiness in psychiatric disorders: prevalence, correlates and clinical significance. Psychiatry Res. 2010;175(1-2):138-141.
4. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54(1):61-69.
5. Krahn LE. Excessive daytime sleepiness: diagnosing the causes. Current Psychiatry. 2002;1(1):49-57.
6. Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17-25.
7. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
8. Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124(pt 8):1482-1491.
9. Cheng P, Casement M, Chen CF, et al. Sleep disordered breathing in major depressive disorder. J Sleep Res. 2013;22(4):459-462.
10. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.
11. Bardwell WA, Berry CC, Ancoli-Israel S, et al. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47(6):583-596.
12. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631-638.
13. Chellappa SL, Araújo JF. Excessive daytime sleepiness in patients with depressive disorder. Rev Bras Psiquiatr. 2006;28(2):126-129.
14. Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11(6):552-557.
15. Lundt L. Use of the Epworth Sleepiness Scale to evaluate the symptom of excessive sleepiness in major depressive disorder. Gen Hosp Psychiatry. 2005;27(2):146-148.
16. Hawley CJ. Excessive daytime sleepiness in psychiatry: a relevant focus for clinical attention and treatment? Int J Psychiatry Clin Pract. 2006;10(2):117-123.
17. Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701-715.
18. Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705-1711.
19. Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012;9(4):687-701.
20. National Institute of Neurological Disorders and Stroke. Restless legs syndrome information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Restless-Legs-Syndrome-Information-Page. Accessed June 2, 2017.
21. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376-381.
Accelerated aging in schizophrenia
Excessive daytime sleepiness
Nutraceuticals for traumatic brain injury: Should you recommend their use?
Traumatic brain injury (TBI) affects more than 2 million people in the United States each year.1 TBI can trigger a cascade of secondary injury mechanisms, such as inflammation, hypoxic/ischemic injury, excitotoxicity, and oxidative stress,2 that could contribute to cognitive and behavioral changes. Although neuropsychiatric symptoms might not be obvious after a TBI, they have a high prevalence in these patients, can last long term, and may be difficult to treat.3 Despite research advances in understanding the biological basis of TBI and identifying potential therapeutic targets, treatment options for individuals with TBI remain limited.
As a result, clinicians have turned to alternative treatments for TBI, including nutraceuticals. In this article, we will:
- provide an overview of nutraceuticals used in treating TBI, first exploring outcomes soon after TBI, then concentrating on neuropsychiatric outcomes
- evaluate the existing evidence, including recommended dietary allowances (Table 1)4,5 and side effects (Table 2)
- review recommendations for their clinical use.
Pharmacologic approaches are limited
Nutraceuticals have gained attention for managing TBI-associated neuropsychiatric disorders because of the limited evidence supporting current approaches. Existing strategies encompass pharmacologic and non-pharmacologic interventions, psychoeducation, supportive and behavioral psychotherapies, and cognitive rehabilitation.6
Many pharmacologic options exist for specific neurobehavioral symptoms, but the evidence for their use is based on small studies, case reports, and knowledge extrapolated from their use in idiopathic psychiatric disorders.7,8 No FDA-approved drugs have been effective for treating neuropsychiatric disturbances after a TBI. Off-label use of antidepressants, anticonvulsants, dopaminergic agents, and cholinesterase inhibitors in TBI has been associated with inadequate clinical response and/or intolerable side effects.9,10
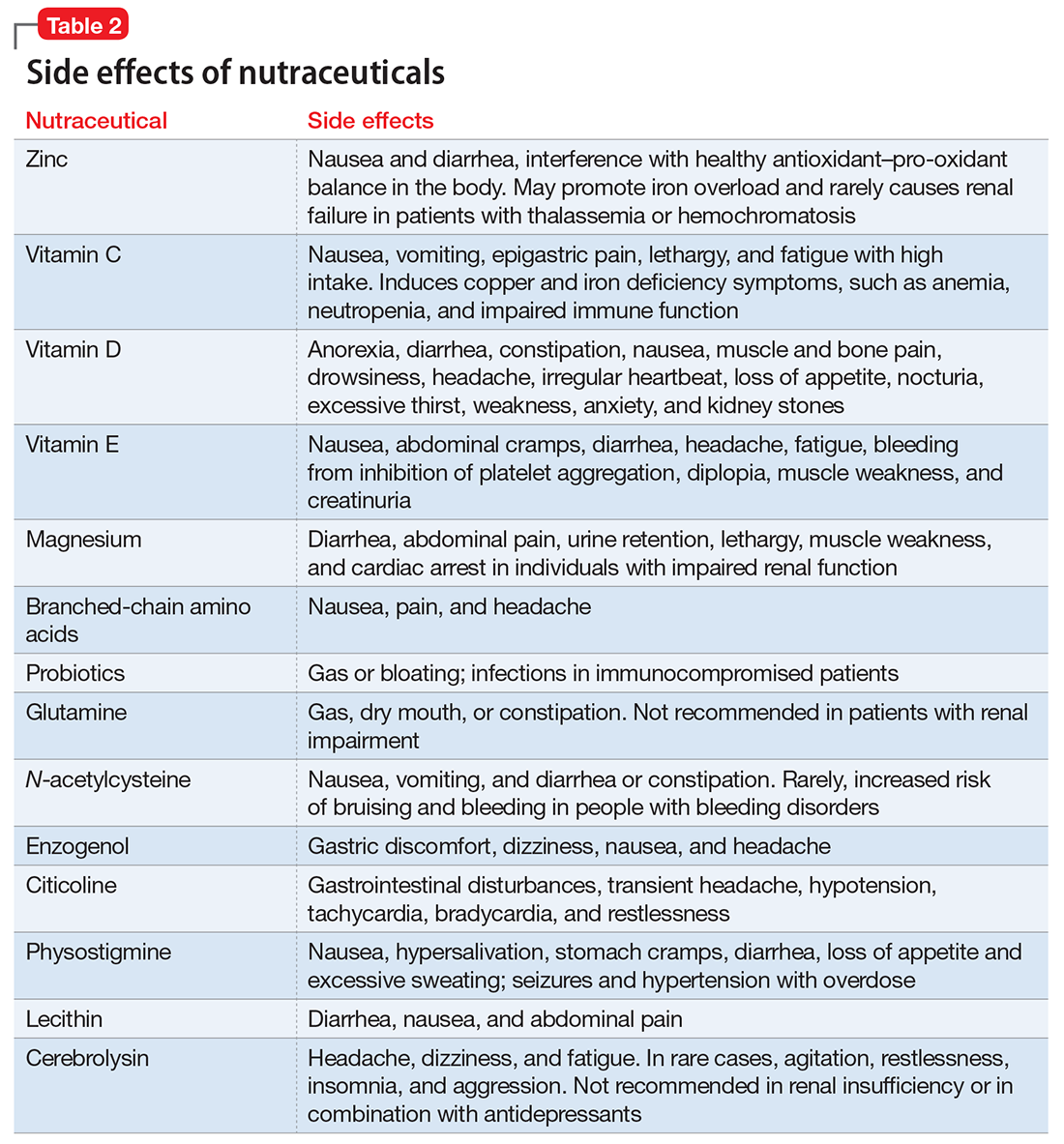
What are nutraceuticals?
DeFelice11 introduced the term “nutraceutical” to refer to “any substance that is a food or part of a food and provides medical or health benefits, including the prevention and treatment of disease.” The term has been expanded to include dietary supplements, such as vitamins, minerals, amino acids, herbal or other botanicals, and food products that provide health benefits beyond what they normally provide in food form. The FDA does not regulate the marketing or manufacturing of nutraceuticals; therefore, their bioavailability and metabolism can vary.
Despite their widespread use, the evidence supporting the efficacy of nutraceuticals for patients with TBI is limited. Their effects might vary by population and depend on dose, timing, TBI severity, and whether taken alone or in combination with other nutraceutical or pharmaceutical agents. Fourteen randomized controlled trials (RCTs) have addressed the use of nutraceuticals in TBI (Table 3), but further research is needed to clarify for which conditions they provide maximum benefit.
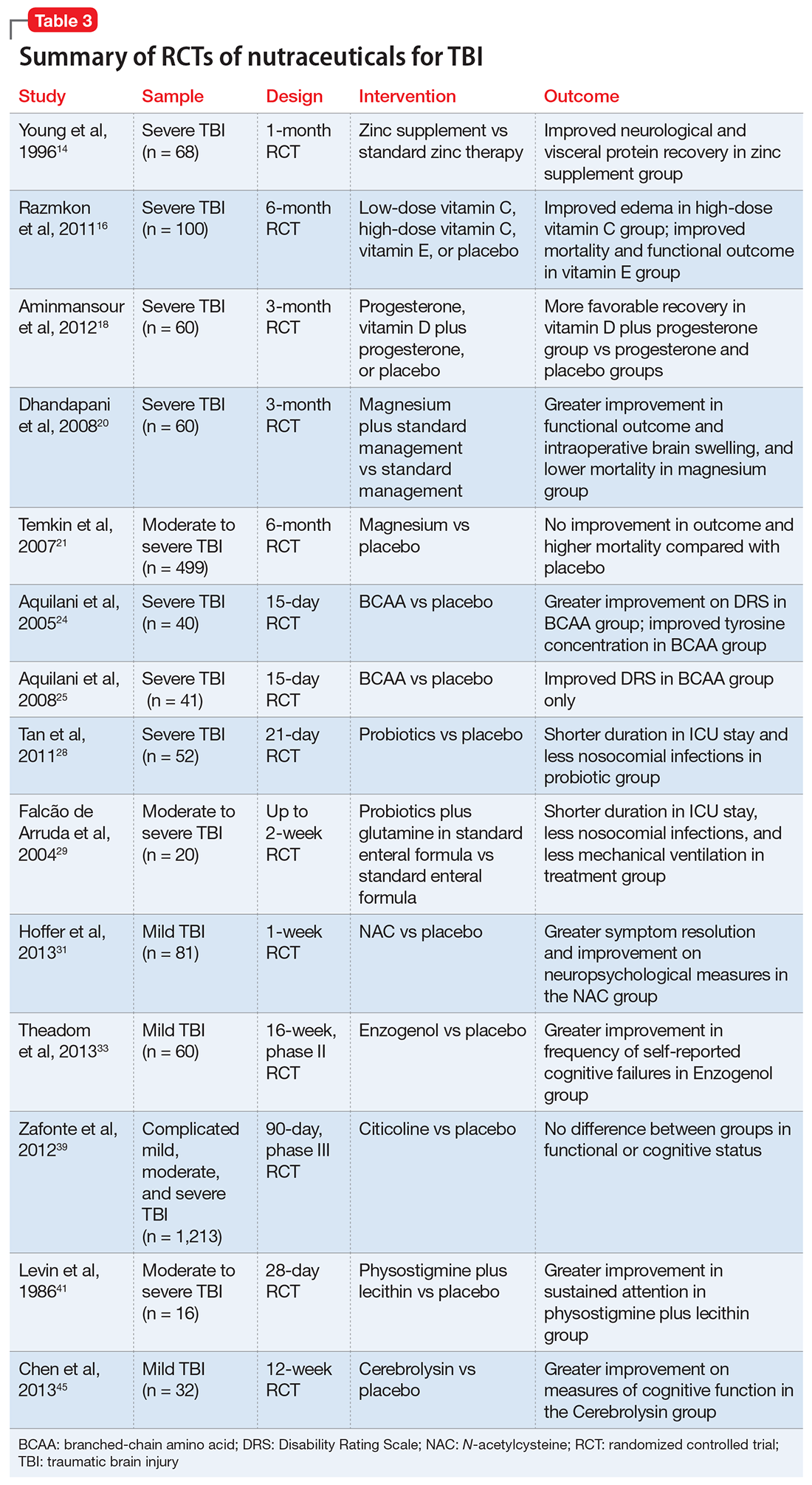
Nutraceuticals and their potential use in TBI
Zinc is considered essential for optimal CNS functioning. Patients with TBI might be at risk for zinc deficiency, which has been associated with increased cell death and behavioral deficits.12,13 A randomized, prospective, double-blinded controlled trial examined the effects of supplemental zinc administration (12 mg for 15 days) compared with standard zinc therapy (2.5 mg for 15 days) over 1 month in 68 adults with acute severe closed head injury.14 The supplemental zinc group showed improved visceral protein levels, lower mortality, and more favorable neurologic recovery based on higher adjusted mean Glasgow Coma Scale score on day 28 and mean motor score on days 15 and 21.
Rodent studies have shown that zinc supplementation could reduce deficits in spatial learning and memory and depression-like behaviors and help decrease stress and anxiety,12 although no human clinical trials have been conducted. Despite the potential neuroprotective effects of zinc supplementation, evidence exists that endogenous zinc release and accumulation following TBI can trigger cellular changes that result in neuronal death.13
Vitamins C and E. Oxidative damage is believed to play a significant role in secondary injury in TBI, so research has focused on the role of antioxidants, such as vitamins C and E, to promote post-TBI recovery.15 One RCT16 of 100 adults with acute severe head injury reported that vitamin E administration was associated with reduced mortality and lower Glasgow Outcome Scale (GOS) scores, and vitamin C was associated with stabilized or reduced perilesional edema/infarct on CT scan.
Vitamin D. An animal study reported that vitamin D supplementation can help reduce inflammation, oxidative stress, and cell death in TBI, and that vitamin D deficiency has been associated with increased inflammation and behavioral deficits.17 Further evidence suggests that vitamin D may have a synergistic effect when used in combination with the hormone progesterone. A RCT of 60 patients with severe TBI reported that 60% of those who received progesterone plus vitamin D had GOS scores of 4 (good recovery) or 5 (moderate disability) vs 45% receiving progesterone alone or 25% receiving placebo.18
Magnesium, one of the most widely used nutraceuticals, is considered essential for CNS functioning, including the regulation of N-methyl-
A RCT evaluated the safety and efficacy of magnesium supplementation in 60 patients with severe closed TBI, with one-half randomized to standard care and the other also receiving magnesium sulfate (MgSO4; initiation dose of 4 g IV and 10 g IM, continuation dose of 5 g IM every 4 hours for 24 hours).20 After 3 months, more patients in the MgSO4 group had higher GOS scores than controls (73.3% vs 40%), lower 1-month mortality rates (13.3% vs 43.3%), and lower rates of intraoperative brain swelling (29.4% vs 73.3%).
However, a larger RCT of 499 patients with moderate or severe TBI randomized to high-dose (1.25 to 2.5 mmol/L) or low-dose (1.0 to 1.85 mmol/L) IV MgSO4 or placebo provided conflicting results.21 Participants received MgSO4 8 hours after injury and continued for 5 days. After 6 months, patients in the high-dose MgSO4 and placebo groups had similar composite primary outcome measures (eg, seizures, neuropsychological measures, functional status measures), although the high-dose group had a higher mortality rate than the placebo group. Patients who received low-dose MgSO4 showed worse outcomes than those assigned to placebo.
Amino acids. Branched-chain amino acids (BCAAs), including valine, isoleucine, and leucine, are essential in protein and neurotransmitter synthesis. Reduced levels of endogenous BCAAs have been reported in patients with mild or severe TBI.22 Preclinical studies suggest that BCAAs can improve hippocampal-dependent cognitive functioning following TBI.23
Two RCTs of BCAAs have been conducted in humans. One study randomized 40 men with severe TBI to IV BCAAs or placebo.24 After 15 days, the BCAA group showed greater improvement in Disability Rating Scale scores. The study also found that supplementation increased total BCAA levels without negatively affecting plasma levels of neurotransmitter precursors tyrosine and tryptophan. A second study found that 41 patients in a vegetative or minimally conscious state who received BCAA supplementation for 15 days had higher Disability Rating Scale scores than those receiving placebo.25
Probiotics and glutamine. Probiotics are non-pathogenic microorganisms that have been shown to modulate the host’s immune system.26 TBI is associated with immunological changes, including a shift from T-helper type 1 (TH1) cells to T-helper type 2 (TH2) cells that increase susceptibility to infection.27
A RCT of 52 patients with severe TBI suggested a correlation between probiotic administration-modulated cytokine levels and TH1/TH2 balance.28 A 3-times daily probiotic mix of Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus for 21 days led to shorter average ICU stays (6.8 vs 10.7 days, P = .034) and a decrease in nosocomial infections (34.6% vs 57.7%, P = .095) vs placebo, although the latter difference was not statistically significant.28
A prospective RCT of 20 patients with brain injury29 found a similar impact of early enteral nutrition supplemented with Lactobacillus johnsonii and glutamine, 30 g, vs a standard enteral nutrition formula. The treatment group experienced fewer nosocomial infections (50% vs 100%, P = .03), shorter ICU stays (10 vs 22 days, P < .01), and fewer days on mechanical ventilation (7 vs 14, P = .04). Despite these studies, evidence for the use of glutamine in patients with TBI is scarce and inconclusive.
N-acetylcysteine (NAC) comes from the amino acid L-cysteine. NAC is an effective scavenger of free radicals and improves cerebral microcirculatory blood flow and tissue oxygenation.30 A randomized, double-blind, placebo-controlled study of oral NAC supplementation in 81 active duty service members with mild TBI found NAC had a significant effect on outcomes.31 Oral NAC supplementation led to improved neuropsychological test results, number of mild TBI symptoms, complete symptom resolution by day 7 of treatment compared with placebo, and NAC was well tolerated. Lack of replication studies and generalizability of findings to civilian, moderate, or chronic TBI populations are key limitations of this study.
Proposed mechanisms for the neuroprotective benefit of NAC include its antioxidant and inflammatory activation of cysteine/glutamate exchange, metabotropic glutamate receptor modulation, and glutathione synthesis.32 NAC has poor blood–brain permeability, but the vascular disruption seen in acute TBI might facilitate its delivery to affected neural sites.31 As such, the benefits of NAC in subacute or chronic TBI are questionable.
Neuropsychiatric outcomes of nutraceuticals
Enzogenol. This flavonoid-rich extract from the bark of the Monterey pine tree (Pinus radiata), known by the trade name Enzogenol, reportedly has antioxidant and anti-inflammatory properties that may counter oxidative damage and neuroinflammation following TBI. A phase II trial randomized participants to Enzogenol, 1,000 mg/d, or placebo for 6 weeks, then all participants received Enzogenol for 6 weeks followed by placebo for 4 weeks.33 Enzogenol was well tolerated with few side effects.
Compared with placebo, participants receiving Enzogenol showed no significant change in mood, as measured by the Hospital Anxiety and Depression Scale, and greater improvement in overall cognition as assessed by the Cognitive Failures Questionnaire. However, measures of working memory (digit span, arithmetic, and letter–number sequencing subtests of the Wechsler Adult Intelligence Scale) and episodic memory (California Verbal Learning Test) showed no benefit from Enzogenol.
Citicoline (CDP-choline) is an endogenous compound widely available as a nutraceutical that has been approved for TBI therapy in 59 countries.34 Animal studies indicate that it could possess neuroprotective properties. Proposed mechanisms for such effects have included stabilizing cell membranes, reducing inflammation, reducing the presence of free radicals, or stimulating production of acetylcholine.35,36 A study in rats found that CDP-choline was associated with increased levels of acetylcholine in the hippocampus and neocortex, which may help reduce neurobehavioral deficits.37
A study of 14 adults with mild to moderate closed head injury38 found that patients who received CDP-choline showed a greater reduction in post-concussion symptoms and improvement in recognition memory than controls who received placebo. However, the Citicoline Brain Injury Treatment Trial, a large randomized trial of 1,213 adults with complicated mild, moderate, or severe TBI, reported that CDP-choline did not improve functional and cognitive status.39
Physostigmine and lecithin. The cholinergic system is a key modulatory neurotransmitter system of the brain that mediates conscious awareness, attention, learning, and working memory.40 A double-blind, placebo-controlled study of 16 patients with moderate to severe closed head injury provided inconsistent evidence for the efficacy of physostigmine and lecithin in the treatment of memory and attention disturbances.41The results showed no differences between the physostigmine–lecithin combination vs lecithin alone, although sustained attention on the Continuous Performance Test was more efficient with physostigmine than placebo when the drug condition occurred first in the crossover design. The lack of encouraging data and concerns about its cardiovascular and proconvulsant properties in patients with TBI may explain the dearth of studies with physostigmine.
Cerebrolysin. A peptide preparation produced from purified pig brain proteins, known by the trade name Cerebrolysin, is popular in Asia and Europe for its nootropic properties. Cerebrolysin may activate cerebral mechanisms related to attention and memory processes,42 and some data have shown efficacy in improving cognitive symptoms and daily activities in patients with Alzheimer’s disease43 and TBI.44
A blinded 12-week study of 32 participants with acute mild TBI reported that those randomized to Cerebrolysin showed improvement in cognitive functioning vs the placebo group.45 The authors concluded that Cerebrolysin provides an advantage for patients with mild TBI and brain contusion if treatment starts within 24 hours of mild TBI onset. Cerebrolysin was well tolerated. Major limitations of this study were small sample size, lack of information regarding comorbid neuropsychiatric conditions and treatments, and short treatment duration.
A recent Cochrane review of 6 RCTs with 1,501 participants found no clinical benefit of Cerebrolysin for treating acute ischemic stroke, and found moderate-quality evidence of an increase with non-fatal serious adverse events but not in total serious adverse events.46 We do not recommend Cerebrolysin use in patients with TBI at this time until additional efficacy and safety data are available.
Nutraceuticals used in other populations
Other nutraceuticals with preclinical evidence of possible benefit in TBI but lacking evidence from human clinical trials include omega-3 fatty acids,47 curcumin,48 and resveratrol,49 providing further proof that results from experimental studies do not necessarily extend to clinical trials.50
Studies of nutraceuticals in other neurological and psychiatric populations have yielded some promising results. Significant interest has focused on the association between vitamin D deficiency, dementia, and neurodegenerative conditions such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease.51 The role of vitamin D in regulation of calcium-mediated neuronal excitotoxicity and oxidative stress and in the induction of synaptic structural proteins, neurotrophic factors, and deficient neurotransmitters makes it an attractive candidate as a neuroprotective agent.52
RCTs of nutraceuticals also have reported positive findings for a variety of mood and anxiety disorders, such as St. John’s wort, S-adenosylmethionine, omega-3 fatty acids for major depression53 and bipolar depression,54 and kava for generalized anxiety disorder.55 More research, however, is needed in these areas.
The use of nonpharmacologic agents in TBI often relies on similar neuropsychiatric symptom profiles of idiopathic psychiatric disorders. Attention-deficit/hyperactivity disorder (ADHD) closely resembles TBI, but systemic reviews of studies of zinc, magnesium, and polyunsaturated fatty acids supplementation in ADHD provide no evidence of therapeutic benefit.56-58
Educate patients in role of nutraceuticals
Despite lack of FDA oversight and limited empirical support, nutraceuticals continue to be widely marketed and used for their putative health benefits59 and have gained increased attention among clinicians.60 Because nutritional deficiency may make the brain less able than other organs to recover from injury,61 supplementation is an option, especially in individuals who could be at greater risk of TBI (eg, athletes and military personnel).
Lacking robust scientific evidence to support the use of nutraceuticals either for enhancing TBI recovery or treating neuropsychiatric disturbances, clinicians must educate patients that these agents are not completely benign and can have significant side effects and drug interactions.62,63 Nutraceuticals may contain multiple ingredients, some of which can be toxic, particularly at higher doses. Many patients may not volunteer information about their nutraceutical use to their health care providers,64 so we must ask them about that and inform them of the potential for adverse events and drug interactions.
1. Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html. Updated January 22, 2016. Accessed June 5, 2017.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9.
3. Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50(3):198-205.
4. National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheets. https://ods.od.nih.gov/factsheets/list-all. Accessed June 5, 2017.
5. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy of Sciences; 2002.
6. Rao V, Koliatsos V, Ahmed F, et al. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol. 2015;35(1):64-82.
7. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
8. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533; discussion 1533.
9. Bengtsson M, Godbolt AK. Effects of acetylcholinesterase inhibitors on cognitive function in patients with chronic traumatic brain injury: a systematic review. J Rehabil Med. 2016;48(1):1-5.
10. Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
11. DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol. 1995;6(2):59-61.
12. Cope EC, Morris DR, Levenson CW. Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury. Nutr Rev. 2012;70(7):410-413.
13. Morris DR, Levenson CW. Zinc in traumatic brain injury: from neuroprotection to neurotoxicity. Curr Opin Clin Nutr Metab Care. 2013;16(6):708-711.
14. Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13(1):25-34.
15. Fernández-Gajardo R, Matamala JM, Carrasco R, et al. Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs. 2014;28(3):229-248.
16. Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133-137.
17. Cekic M, Cutler SM, VanLandingham JW, et al. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864-874.
18. Aminmansour B, Nikbakht H, Ghorbani A, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo group. Adv Biomed Res. 2012;1:58.
19. Cernak I, Savic VJ, Kotur J, et al. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma. 2000;17(1):53-68.
20. Dhandapani SS, Gupta A, Vivekanandhan S, et al. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. The Indian Journal of Neurotrauma. 2008;5(1):27-33.
21. Temkin NR, Anderson GD, Winn HR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29-38.
22. Jeter CB, Hergenroeder GW, Ward NH 3rd, et al. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30(8):671-679.
23. Cole JT, Mitala CM, Kundu S, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366-371.
24. Aquilani R, Iadarola P, Contardi A, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86(9):1729-1735.
25. Aquilani R, Boselli M, Boschi F, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008;89(9):1642-1647.
26. Kang HJ, Im SH. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo). 2015;61(suppl):S103-S105.
27. DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130(11):1159-1162; discussion 1162-1163.
28. Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290.
29. Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106(3):287-292.
30. Cuzzocrea S, Mazzon E, Costantino G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol. 2000;130(6):1219-1226.
31. Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163.
32. Eakin K, Baratz-Goldstein R, Pick CG, et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617.
33. Theadom A, Mahon S, Barker-Collo S, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20(8):1135-1144.
34. Arenth PM, Russell KC, Ricker JH, et al. CDP-choline as a biological supplement during neurorecovery: a focused review. PM R. 2011;3(6 suppl 1):S123-S131.
35. Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10(5):839-846.
36. Guseva MV, Hopkins DM, Scheff SW, et al. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25(8):975-983.
37. Dixon CE, Ma X, Marion DW. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma. 1997;14(3):161-169.
38. Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(suppl):S39-S42.
39. Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA. 2012;308(19):1993-2000.
40. Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273-280.
41. Levin HS, Peters BH, Kalisky Z, et al. Effects of oral physostigmine and lecithin on memory and attention in closed head-injured patients. Cent Nerv Syst Trauma. 1986;3(4):333-342.
42. Alvarez XA, Lombardi VR, Corzo L, et al. Oral cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. J Neural Transm Suppl. 2000;59:315-328.
43. Ruether E, Husmann R, Kinzler E, et al. A 28-week, double-blind, placebo-controlled study with cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol. 2001;16(5):253-263.
44. Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochir Suppl. 2005;95:59-60.
45. Chen CC, Wei ST, Tsaia SC, et al. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. Br J Neurosurg. 2013;27(6):803-807.
46. Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2016;12:CD007026.
47. Barrett EC, McBurney MI, Ciappio ED. ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv Nutr. 2014;5(3):268-277.
48. Sharma S, Zhuang Y, Ying Z, et al. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161(4):1037-1044.
49. Gatson JW, Liu MM, Abdelfattah K, et al. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):470-475; discussion 474-475.
50. Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 2014;174(3):460-462.
51. Mpandzou G, Aït Ben Haddou E, Regragui W, et al. Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris). 2016;172(2):109-122.
52. Karakis I, Pase MP, Beiser A, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J Alzheimers Dis. 2016;51(2):451-461.
53. Sarris J, Papakostas GI, Vitolo O, et al. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. 2014;164:76-81.
54. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
55. Sarris J, Stough C, Bousman C, et al. Kava in the treatment of generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2013;33(5):643-648.
56. Hariri M, Azadbakht L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med. 2015;6:83.
57. Gillies D, Sinn JKh, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986.
58. Ghanizadeh A, Berk M. Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: a systematic review of randomized controlled clinical trials. Eur J Clin Nutr. 2013;67(1):122-124.
59. U.S. Food and Drug Administration. Can a dietary supplement treat a concussion? No! http://www.fda.gov/forconsumers/consumerupdates/ucm378845.htm. Updated February 13, 2015. Accessed June 5, 2017.
60. Sarris J, Logan AC, Akbaraly TN, et al; International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274.
61. Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. 2014;9(1):e86472.
62. Edie CF, Dewan N. Which psychotropics interact with four common supplements. Current Psychiatry. 2005;4(1):16-30.
63. Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578-592.
64. National Center for Complementary and Integrative Health. Complementary and alternative medicine: what people aged 50 and older discuss with their health care providers. https://nccih.nih.gov/research/statistics/2010. Published 2011. Accessed June 5, 2017.
Traumatic brain injury (TBI) affects more than 2 million people in the United States each year.1 TBI can trigger a cascade of secondary injury mechanisms, such as inflammation, hypoxic/ischemic injury, excitotoxicity, and oxidative stress,2 that could contribute to cognitive and behavioral changes. Although neuropsychiatric symptoms might not be obvious after a TBI, they have a high prevalence in these patients, can last long term, and may be difficult to treat.3 Despite research advances in understanding the biological basis of TBI and identifying potential therapeutic targets, treatment options for individuals with TBI remain limited.
As a result, clinicians have turned to alternative treatments for TBI, including nutraceuticals. In this article, we will:
- provide an overview of nutraceuticals used in treating TBI, first exploring outcomes soon after TBI, then concentrating on neuropsychiatric outcomes
- evaluate the existing evidence, including recommended dietary allowances (Table 1)4,5 and side effects (Table 2)
- review recommendations for their clinical use.

Pharmacologic approaches are limited
Nutraceuticals have gained attention for managing TBI-associated neuropsychiatric disorders because of the limited evidence supporting current approaches. Existing strategies encompass pharmacologic and non-pharmacologic interventions, psychoeducation, supportive and behavioral psychotherapies, and cognitive rehabilitation.6
Many pharmacologic options exist for specific neurobehavioral symptoms, but the evidence for their use is based on small studies, case reports, and knowledge extrapolated from their use in idiopathic psychiatric disorders.7,8 No FDA-approved drugs have been effective for treating neuropsychiatric disturbances after a TBI. Off-label use of antidepressants, anticonvulsants, dopaminergic agents, and cholinesterase inhibitors in TBI has been associated with inadequate clinical response and/or intolerable side effects.9,10

What are nutraceuticals?
DeFelice11 introduced the term “nutraceutical” to refer to “any substance that is a food or part of a food and provides medical or health benefits, including the prevention and treatment of disease.” The term has been expanded to include dietary supplements, such as vitamins, minerals, amino acids, herbal or other botanicals, and food products that provide health benefits beyond what they normally provide in food form. The FDA does not regulate the marketing or manufacturing of nutraceuticals; therefore, their bioavailability and metabolism can vary.
Despite their widespread use, the evidence supporting the efficacy of nutraceuticals for patients with TBI is limited. Their effects might vary by population and depend on dose, timing, TBI severity, and whether taken alone or in combination with other nutraceutical or pharmaceutical agents. Fourteen randomized controlled trials (RCTs) have addressed the use of nutraceuticals in TBI (Table 3), but further research is needed to clarify for which conditions they provide maximum benefit.

Nutraceuticals and their potential use in TBI
Zinc is considered essential for optimal CNS functioning. Patients with TBI might be at risk for zinc deficiency, which has been associated with increased cell death and behavioral deficits.12,13 A randomized, prospective, double-blinded controlled trial examined the effects of supplemental zinc administration (12 mg for 15 days) compared with standard zinc therapy (2.5 mg for 15 days) over 1 month in 68 adults with acute severe closed head injury.14 The supplemental zinc group showed improved visceral protein levels, lower mortality, and more favorable neurologic recovery based on higher adjusted mean Glasgow Coma Scale score on day 28 and mean motor score on days 15 and 21.
Rodent studies have shown that zinc supplementation could reduce deficits in spatial learning and memory and depression-like behaviors and help decrease stress and anxiety,12 although no human clinical trials have been conducted. Despite the potential neuroprotective effects of zinc supplementation, evidence exists that endogenous zinc release and accumulation following TBI can trigger cellular changes that result in neuronal death.13
Vitamins C and E. Oxidative damage is believed to play a significant role in secondary injury in TBI, so research has focused on the role of antioxidants, such as vitamins C and E, to promote post-TBI recovery.15 One RCT16 of 100 adults with acute severe head injury reported that vitamin E administration was associated with reduced mortality and lower Glasgow Outcome Scale (GOS) scores, and vitamin C was associated with stabilized or reduced perilesional edema/infarct on CT scan.
Vitamin D. An animal study reported that vitamin D supplementation can help reduce inflammation, oxidative stress, and cell death in TBI, and that vitamin D deficiency has been associated with increased inflammation and behavioral deficits.17 Further evidence suggests that vitamin D may have a synergistic effect when used in combination with the hormone progesterone. A RCT of 60 patients with severe TBI reported that 60% of those who received progesterone plus vitamin D had GOS scores of 4 (good recovery) or 5 (moderate disability) vs 45% receiving progesterone alone or 25% receiving placebo.18
Magnesium, one of the most widely used nutraceuticals, is considered essential for CNS functioning, including the regulation of N-methyl-
A RCT evaluated the safety and efficacy of magnesium supplementation in 60 patients with severe closed TBI, with one-half randomized to standard care and the other also receiving magnesium sulfate (MgSO4; initiation dose of 4 g IV and 10 g IM, continuation dose of 5 g IM every 4 hours for 24 hours).20 After 3 months, more patients in the MgSO4 group had higher GOS scores than controls (73.3% vs 40%), lower 1-month mortality rates (13.3% vs 43.3%), and lower rates of intraoperative brain swelling (29.4% vs 73.3%).
However, a larger RCT of 499 patients with moderate or severe TBI randomized to high-dose (1.25 to 2.5 mmol/L) or low-dose (1.0 to 1.85 mmol/L) IV MgSO4 or placebo provided conflicting results.21 Participants received MgSO4 8 hours after injury and continued for 5 days. After 6 months, patients in the high-dose MgSO4 and placebo groups had similar composite primary outcome measures (eg, seizures, neuropsychological measures, functional status measures), although the high-dose group had a higher mortality rate than the placebo group. Patients who received low-dose MgSO4 showed worse outcomes than those assigned to placebo.
Amino acids. Branched-chain amino acids (BCAAs), including valine, isoleucine, and leucine, are essential in protein and neurotransmitter synthesis. Reduced levels of endogenous BCAAs have been reported in patients with mild or severe TBI.22 Preclinical studies suggest that BCAAs can improve hippocampal-dependent cognitive functioning following TBI.23
Two RCTs of BCAAs have been conducted in humans. One study randomized 40 men with severe TBI to IV BCAAs or placebo.24 After 15 days, the BCAA group showed greater improvement in Disability Rating Scale scores. The study also found that supplementation increased total BCAA levels without negatively affecting plasma levels of neurotransmitter precursors tyrosine and tryptophan. A second study found that 41 patients in a vegetative or minimally conscious state who received BCAA supplementation for 15 days had higher Disability Rating Scale scores than those receiving placebo.25
Probiotics and glutamine. Probiotics are non-pathogenic microorganisms that have been shown to modulate the host’s immune system.26 TBI is associated with immunological changes, including a shift from T-helper type 1 (TH1) cells to T-helper type 2 (TH2) cells that increase susceptibility to infection.27
A RCT of 52 patients with severe TBI suggested a correlation between probiotic administration-modulated cytokine levels and TH1/TH2 balance.28 A 3-times daily probiotic mix of Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus for 21 days led to shorter average ICU stays (6.8 vs 10.7 days, P = .034) and a decrease in nosocomial infections (34.6% vs 57.7%, P = .095) vs placebo, although the latter difference was not statistically significant.28
A prospective RCT of 20 patients with brain injury29 found a similar impact of early enteral nutrition supplemented with Lactobacillus johnsonii and glutamine, 30 g, vs a standard enteral nutrition formula. The treatment group experienced fewer nosocomial infections (50% vs 100%, P = .03), shorter ICU stays (10 vs 22 days, P < .01), and fewer days on mechanical ventilation (7 vs 14, P = .04). Despite these studies, evidence for the use of glutamine in patients with TBI is scarce and inconclusive.
N-acetylcysteine (NAC) comes from the amino acid L-cysteine. NAC is an effective scavenger of free radicals and improves cerebral microcirculatory blood flow and tissue oxygenation.30 A randomized, double-blind, placebo-controlled study of oral NAC supplementation in 81 active duty service members with mild TBI found NAC had a significant effect on outcomes.31 Oral NAC supplementation led to improved neuropsychological test results, number of mild TBI symptoms, complete symptom resolution by day 7 of treatment compared with placebo, and NAC was well tolerated. Lack of replication studies and generalizability of findings to civilian, moderate, or chronic TBI populations are key limitations of this study.
Proposed mechanisms for the neuroprotective benefit of NAC include its antioxidant and inflammatory activation of cysteine/glutamate exchange, metabotropic glutamate receptor modulation, and glutathione synthesis.32 NAC has poor blood–brain permeability, but the vascular disruption seen in acute TBI might facilitate its delivery to affected neural sites.31 As such, the benefits of NAC in subacute or chronic TBI are questionable.
Neuropsychiatric outcomes of nutraceuticals
Enzogenol. This flavonoid-rich extract from the bark of the Monterey pine tree (Pinus radiata), known by the trade name Enzogenol, reportedly has antioxidant and anti-inflammatory properties that may counter oxidative damage and neuroinflammation following TBI. A phase II trial randomized participants to Enzogenol, 1,000 mg/d, or placebo for 6 weeks, then all participants received Enzogenol for 6 weeks followed by placebo for 4 weeks.33 Enzogenol was well tolerated with few side effects.
Compared with placebo, participants receiving Enzogenol showed no significant change in mood, as measured by the Hospital Anxiety and Depression Scale, and greater improvement in overall cognition as assessed by the Cognitive Failures Questionnaire. However, measures of working memory (digit span, arithmetic, and letter–number sequencing subtests of the Wechsler Adult Intelligence Scale) and episodic memory (California Verbal Learning Test) showed no benefit from Enzogenol.
Citicoline (CDP-choline) is an endogenous compound widely available as a nutraceutical that has been approved for TBI therapy in 59 countries.34 Animal studies indicate that it could possess neuroprotective properties. Proposed mechanisms for such effects have included stabilizing cell membranes, reducing inflammation, reducing the presence of free radicals, or stimulating production of acetylcholine.35,36 A study in rats found that CDP-choline was associated with increased levels of acetylcholine in the hippocampus and neocortex, which may help reduce neurobehavioral deficits.37
A study of 14 adults with mild to moderate closed head injury38 found that patients who received CDP-choline showed a greater reduction in post-concussion symptoms and improvement in recognition memory than controls who received placebo. However, the Citicoline Brain Injury Treatment Trial, a large randomized trial of 1,213 adults with complicated mild, moderate, or severe TBI, reported that CDP-choline did not improve functional and cognitive status.39
Physostigmine and lecithin. The cholinergic system is a key modulatory neurotransmitter system of the brain that mediates conscious awareness, attention, learning, and working memory.40 A double-blind, placebo-controlled study of 16 patients with moderate to severe closed head injury provided inconsistent evidence for the efficacy of physostigmine and lecithin in the treatment of memory and attention disturbances.41The results showed no differences between the physostigmine–lecithin combination vs lecithin alone, although sustained attention on the Continuous Performance Test was more efficient with physostigmine than placebo when the drug condition occurred first in the crossover design. The lack of encouraging data and concerns about its cardiovascular and proconvulsant properties in patients with TBI may explain the dearth of studies with physostigmine.
Cerebrolysin. A peptide preparation produced from purified pig brain proteins, known by the trade name Cerebrolysin, is popular in Asia and Europe for its nootropic properties. Cerebrolysin may activate cerebral mechanisms related to attention and memory processes,42 and some data have shown efficacy in improving cognitive symptoms and daily activities in patients with Alzheimer’s disease43 and TBI.44
A blinded 12-week study of 32 participants with acute mild TBI reported that those randomized to Cerebrolysin showed improvement in cognitive functioning vs the placebo group.45 The authors concluded that Cerebrolysin provides an advantage for patients with mild TBI and brain contusion if treatment starts within 24 hours of mild TBI onset. Cerebrolysin was well tolerated. Major limitations of this study were small sample size, lack of information regarding comorbid neuropsychiatric conditions and treatments, and short treatment duration.
A recent Cochrane review of 6 RCTs with 1,501 participants found no clinical benefit of Cerebrolysin for treating acute ischemic stroke, and found moderate-quality evidence of an increase with non-fatal serious adverse events but not in total serious adverse events.46 We do not recommend Cerebrolysin use in patients with TBI at this time until additional efficacy and safety data are available.
Nutraceuticals used in other populations
Other nutraceuticals with preclinical evidence of possible benefit in TBI but lacking evidence from human clinical trials include omega-3 fatty acids,47 curcumin,48 and resveratrol,49 providing further proof that results from experimental studies do not necessarily extend to clinical trials.50
Studies of nutraceuticals in other neurological and psychiatric populations have yielded some promising results. Significant interest has focused on the association between vitamin D deficiency, dementia, and neurodegenerative conditions such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease.51 The role of vitamin D in regulation of calcium-mediated neuronal excitotoxicity and oxidative stress and in the induction of synaptic structural proteins, neurotrophic factors, and deficient neurotransmitters makes it an attractive candidate as a neuroprotective agent.52
RCTs of nutraceuticals also have reported positive findings for a variety of mood and anxiety disorders, such as St. John’s wort, S-adenosylmethionine, omega-3 fatty acids for major depression53 and bipolar depression,54 and kava for generalized anxiety disorder.55 More research, however, is needed in these areas.
The use of nonpharmacologic agents in TBI often relies on similar neuropsychiatric symptom profiles of idiopathic psychiatric disorders. Attention-deficit/hyperactivity disorder (ADHD) closely resembles TBI, but systemic reviews of studies of zinc, magnesium, and polyunsaturated fatty acids supplementation in ADHD provide no evidence of therapeutic benefit.56-58
Educate patients in role of nutraceuticals
Despite lack of FDA oversight and limited empirical support, nutraceuticals continue to be widely marketed and used for their putative health benefits59 and have gained increased attention among clinicians.60 Because nutritional deficiency may make the brain less able than other organs to recover from injury,61 supplementation is an option, especially in individuals who could be at greater risk of TBI (eg, athletes and military personnel).
Lacking robust scientific evidence to support the use of nutraceuticals either for enhancing TBI recovery or treating neuropsychiatric disturbances, clinicians must educate patients that these agents are not completely benign and can have significant side effects and drug interactions.62,63 Nutraceuticals may contain multiple ingredients, some of which can be toxic, particularly at higher doses. Many patients may not volunteer information about their nutraceutical use to their health care providers,64 so we must ask them about that and inform them of the potential for adverse events and drug interactions.
Traumatic brain injury (TBI) affects more than 2 million people in the United States each year.1 TBI can trigger a cascade of secondary injury mechanisms, such as inflammation, hypoxic/ischemic injury, excitotoxicity, and oxidative stress,2 that could contribute to cognitive and behavioral changes. Although neuropsychiatric symptoms might not be obvious after a TBI, they have a high prevalence in these patients, can last long term, and may be difficult to treat.3 Despite research advances in understanding the biological basis of TBI and identifying potential therapeutic targets, treatment options for individuals with TBI remain limited.
As a result, clinicians have turned to alternative treatments for TBI, including nutraceuticals. In this article, we will:
- provide an overview of nutraceuticals used in treating TBI, first exploring outcomes soon after TBI, then concentrating on neuropsychiatric outcomes
- evaluate the existing evidence, including recommended dietary allowances (Table 1)4,5 and side effects (Table 2)
- review recommendations for their clinical use.

Pharmacologic approaches are limited
Nutraceuticals have gained attention for managing TBI-associated neuropsychiatric disorders because of the limited evidence supporting current approaches. Existing strategies encompass pharmacologic and non-pharmacologic interventions, psychoeducation, supportive and behavioral psychotherapies, and cognitive rehabilitation.6
Many pharmacologic options exist for specific neurobehavioral symptoms, but the evidence for their use is based on small studies, case reports, and knowledge extrapolated from their use in idiopathic psychiatric disorders.7,8 No FDA-approved drugs have been effective for treating neuropsychiatric disturbances after a TBI. Off-label use of antidepressants, anticonvulsants, dopaminergic agents, and cholinesterase inhibitors in TBI has been associated with inadequate clinical response and/or intolerable side effects.9,10

What are nutraceuticals?
DeFelice11 introduced the term “nutraceutical” to refer to “any substance that is a food or part of a food and provides medical or health benefits, including the prevention and treatment of disease.” The term has been expanded to include dietary supplements, such as vitamins, minerals, amino acids, herbal or other botanicals, and food products that provide health benefits beyond what they normally provide in food form. The FDA does not regulate the marketing or manufacturing of nutraceuticals; therefore, their bioavailability and metabolism can vary.
Despite their widespread use, the evidence supporting the efficacy of nutraceuticals for patients with TBI is limited. Their effects might vary by population and depend on dose, timing, TBI severity, and whether taken alone or in combination with other nutraceutical or pharmaceutical agents. Fourteen randomized controlled trials (RCTs) have addressed the use of nutraceuticals in TBI (Table 3), but further research is needed to clarify for which conditions they provide maximum benefit.

Nutraceuticals and their potential use in TBI
Zinc is considered essential for optimal CNS functioning. Patients with TBI might be at risk for zinc deficiency, which has been associated with increased cell death and behavioral deficits.12,13 A randomized, prospective, double-blinded controlled trial examined the effects of supplemental zinc administration (12 mg for 15 days) compared with standard zinc therapy (2.5 mg for 15 days) over 1 month in 68 adults with acute severe closed head injury.14 The supplemental zinc group showed improved visceral protein levels, lower mortality, and more favorable neurologic recovery based on higher adjusted mean Glasgow Coma Scale score on day 28 and mean motor score on days 15 and 21.
Rodent studies have shown that zinc supplementation could reduce deficits in spatial learning and memory and depression-like behaviors and help decrease stress and anxiety,12 although no human clinical trials have been conducted. Despite the potential neuroprotective effects of zinc supplementation, evidence exists that endogenous zinc release and accumulation following TBI can trigger cellular changes that result in neuronal death.13
Vitamins C and E. Oxidative damage is believed to play a significant role in secondary injury in TBI, so research has focused on the role of antioxidants, such as vitamins C and E, to promote post-TBI recovery.15 One RCT16 of 100 adults with acute severe head injury reported that vitamin E administration was associated with reduced mortality and lower Glasgow Outcome Scale (GOS) scores, and vitamin C was associated with stabilized or reduced perilesional edema/infarct on CT scan.
Vitamin D. An animal study reported that vitamin D supplementation can help reduce inflammation, oxidative stress, and cell death in TBI, and that vitamin D deficiency has been associated with increased inflammation and behavioral deficits.17 Further evidence suggests that vitamin D may have a synergistic effect when used in combination with the hormone progesterone. A RCT of 60 patients with severe TBI reported that 60% of those who received progesterone plus vitamin D had GOS scores of 4 (good recovery) or 5 (moderate disability) vs 45% receiving progesterone alone or 25% receiving placebo.18
Magnesium, one of the most widely used nutraceuticals, is considered essential for CNS functioning, including the regulation of N-methyl-
A RCT evaluated the safety and efficacy of magnesium supplementation in 60 patients with severe closed TBI, with one-half randomized to standard care and the other also receiving magnesium sulfate (MgSO4; initiation dose of 4 g IV and 10 g IM, continuation dose of 5 g IM every 4 hours for 24 hours).20 After 3 months, more patients in the MgSO4 group had higher GOS scores than controls (73.3% vs 40%), lower 1-month mortality rates (13.3% vs 43.3%), and lower rates of intraoperative brain swelling (29.4% vs 73.3%).
However, a larger RCT of 499 patients with moderate or severe TBI randomized to high-dose (1.25 to 2.5 mmol/L) or low-dose (1.0 to 1.85 mmol/L) IV MgSO4 or placebo provided conflicting results.21 Participants received MgSO4 8 hours after injury and continued for 5 days. After 6 months, patients in the high-dose MgSO4 and placebo groups had similar composite primary outcome measures (eg, seizures, neuropsychological measures, functional status measures), although the high-dose group had a higher mortality rate than the placebo group. Patients who received low-dose MgSO4 showed worse outcomes than those assigned to placebo.
Amino acids. Branched-chain amino acids (BCAAs), including valine, isoleucine, and leucine, are essential in protein and neurotransmitter synthesis. Reduced levels of endogenous BCAAs have been reported in patients with mild or severe TBI.22 Preclinical studies suggest that BCAAs can improve hippocampal-dependent cognitive functioning following TBI.23
Two RCTs of BCAAs have been conducted in humans. One study randomized 40 men with severe TBI to IV BCAAs or placebo.24 After 15 days, the BCAA group showed greater improvement in Disability Rating Scale scores. The study also found that supplementation increased total BCAA levels without negatively affecting plasma levels of neurotransmitter precursors tyrosine and tryptophan. A second study found that 41 patients in a vegetative or minimally conscious state who received BCAA supplementation for 15 days had higher Disability Rating Scale scores than those receiving placebo.25
Probiotics and glutamine. Probiotics are non-pathogenic microorganisms that have been shown to modulate the host’s immune system.26 TBI is associated with immunological changes, including a shift from T-helper type 1 (TH1) cells to T-helper type 2 (TH2) cells that increase susceptibility to infection.27
A RCT of 52 patients with severe TBI suggested a correlation between probiotic administration-modulated cytokine levels and TH1/TH2 balance.28 A 3-times daily probiotic mix of Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus for 21 days led to shorter average ICU stays (6.8 vs 10.7 days, P = .034) and a decrease in nosocomial infections (34.6% vs 57.7%, P = .095) vs placebo, although the latter difference was not statistically significant.28
A prospective RCT of 20 patients with brain injury29 found a similar impact of early enteral nutrition supplemented with Lactobacillus johnsonii and glutamine, 30 g, vs a standard enteral nutrition formula. The treatment group experienced fewer nosocomial infections (50% vs 100%, P = .03), shorter ICU stays (10 vs 22 days, P < .01), and fewer days on mechanical ventilation (7 vs 14, P = .04). Despite these studies, evidence for the use of glutamine in patients with TBI is scarce and inconclusive.
N-acetylcysteine (NAC) comes from the amino acid L-cysteine. NAC is an effective scavenger of free radicals and improves cerebral microcirculatory blood flow and tissue oxygenation.30 A randomized, double-blind, placebo-controlled study of oral NAC supplementation in 81 active duty service members with mild TBI found NAC had a significant effect on outcomes.31 Oral NAC supplementation led to improved neuropsychological test results, number of mild TBI symptoms, complete symptom resolution by day 7 of treatment compared with placebo, and NAC was well tolerated. Lack of replication studies and generalizability of findings to civilian, moderate, or chronic TBI populations are key limitations of this study.
Proposed mechanisms for the neuroprotective benefit of NAC include its antioxidant and inflammatory activation of cysteine/glutamate exchange, metabotropic glutamate receptor modulation, and glutathione synthesis.32 NAC has poor blood–brain permeability, but the vascular disruption seen in acute TBI might facilitate its delivery to affected neural sites.31 As such, the benefits of NAC in subacute or chronic TBI are questionable.
Neuropsychiatric outcomes of nutraceuticals
Enzogenol. This flavonoid-rich extract from the bark of the Monterey pine tree (Pinus radiata), known by the trade name Enzogenol, reportedly has antioxidant and anti-inflammatory properties that may counter oxidative damage and neuroinflammation following TBI. A phase II trial randomized participants to Enzogenol, 1,000 mg/d, or placebo for 6 weeks, then all participants received Enzogenol for 6 weeks followed by placebo for 4 weeks.33 Enzogenol was well tolerated with few side effects.
Compared with placebo, participants receiving Enzogenol showed no significant change in mood, as measured by the Hospital Anxiety and Depression Scale, and greater improvement in overall cognition as assessed by the Cognitive Failures Questionnaire. However, measures of working memory (digit span, arithmetic, and letter–number sequencing subtests of the Wechsler Adult Intelligence Scale) and episodic memory (California Verbal Learning Test) showed no benefit from Enzogenol.
Citicoline (CDP-choline) is an endogenous compound widely available as a nutraceutical that has been approved for TBI therapy in 59 countries.34 Animal studies indicate that it could possess neuroprotective properties. Proposed mechanisms for such effects have included stabilizing cell membranes, reducing inflammation, reducing the presence of free radicals, or stimulating production of acetylcholine.35,36 A study in rats found that CDP-choline was associated with increased levels of acetylcholine in the hippocampus and neocortex, which may help reduce neurobehavioral deficits.37
A study of 14 adults with mild to moderate closed head injury38 found that patients who received CDP-choline showed a greater reduction in post-concussion symptoms and improvement in recognition memory than controls who received placebo. However, the Citicoline Brain Injury Treatment Trial, a large randomized trial of 1,213 adults with complicated mild, moderate, or severe TBI, reported that CDP-choline did not improve functional and cognitive status.39
Physostigmine and lecithin. The cholinergic system is a key modulatory neurotransmitter system of the brain that mediates conscious awareness, attention, learning, and working memory.40 A double-blind, placebo-controlled study of 16 patients with moderate to severe closed head injury provided inconsistent evidence for the efficacy of physostigmine and lecithin in the treatment of memory and attention disturbances.41The results showed no differences between the physostigmine–lecithin combination vs lecithin alone, although sustained attention on the Continuous Performance Test was more efficient with physostigmine than placebo when the drug condition occurred first in the crossover design. The lack of encouraging data and concerns about its cardiovascular and proconvulsant properties in patients with TBI may explain the dearth of studies with physostigmine.
Cerebrolysin. A peptide preparation produced from purified pig brain proteins, known by the trade name Cerebrolysin, is popular in Asia and Europe for its nootropic properties. Cerebrolysin may activate cerebral mechanisms related to attention and memory processes,42 and some data have shown efficacy in improving cognitive symptoms and daily activities in patients with Alzheimer’s disease43 and TBI.44
A blinded 12-week study of 32 participants with acute mild TBI reported that those randomized to Cerebrolysin showed improvement in cognitive functioning vs the placebo group.45 The authors concluded that Cerebrolysin provides an advantage for patients with mild TBI and brain contusion if treatment starts within 24 hours of mild TBI onset. Cerebrolysin was well tolerated. Major limitations of this study were small sample size, lack of information regarding comorbid neuropsychiatric conditions and treatments, and short treatment duration.
A recent Cochrane review of 6 RCTs with 1,501 participants found no clinical benefit of Cerebrolysin for treating acute ischemic stroke, and found moderate-quality evidence of an increase with non-fatal serious adverse events but not in total serious adverse events.46 We do not recommend Cerebrolysin use in patients with TBI at this time until additional efficacy and safety data are available.
Nutraceuticals used in other populations
Other nutraceuticals with preclinical evidence of possible benefit in TBI but lacking evidence from human clinical trials include omega-3 fatty acids,47 curcumin,48 and resveratrol,49 providing further proof that results from experimental studies do not necessarily extend to clinical trials.50
Studies of nutraceuticals in other neurological and psychiatric populations have yielded some promising results. Significant interest has focused on the association between vitamin D deficiency, dementia, and neurodegenerative conditions such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease.51 The role of vitamin D in regulation of calcium-mediated neuronal excitotoxicity and oxidative stress and in the induction of synaptic structural proteins, neurotrophic factors, and deficient neurotransmitters makes it an attractive candidate as a neuroprotective agent.52
RCTs of nutraceuticals also have reported positive findings for a variety of mood and anxiety disorders, such as St. John’s wort, S-adenosylmethionine, omega-3 fatty acids for major depression53 and bipolar depression,54 and kava for generalized anxiety disorder.55 More research, however, is needed in these areas.
The use of nonpharmacologic agents in TBI often relies on similar neuropsychiatric symptom profiles of idiopathic psychiatric disorders. Attention-deficit/hyperactivity disorder (ADHD) closely resembles TBI, but systemic reviews of studies of zinc, magnesium, and polyunsaturated fatty acids supplementation in ADHD provide no evidence of therapeutic benefit.56-58
Educate patients in role of nutraceuticals
Despite lack of FDA oversight and limited empirical support, nutraceuticals continue to be widely marketed and used for their putative health benefits59 and have gained increased attention among clinicians.60 Because nutritional deficiency may make the brain less able than other organs to recover from injury,61 supplementation is an option, especially in individuals who could be at greater risk of TBI (eg, athletes and military personnel).
Lacking robust scientific evidence to support the use of nutraceuticals either for enhancing TBI recovery or treating neuropsychiatric disturbances, clinicians must educate patients that these agents are not completely benign and can have significant side effects and drug interactions.62,63 Nutraceuticals may contain multiple ingredients, some of which can be toxic, particularly at higher doses. Many patients may not volunteer information about their nutraceutical use to their health care providers,64 so we must ask them about that and inform them of the potential for adverse events and drug interactions.
1. Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html. Updated January 22, 2016. Accessed June 5, 2017.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9.
3. Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50(3):198-205.
4. National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheets. https://ods.od.nih.gov/factsheets/list-all. Accessed June 5, 2017.
5. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy of Sciences; 2002.
6. Rao V, Koliatsos V, Ahmed F, et al. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol. 2015;35(1):64-82.
7. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
8. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533; discussion 1533.
9. Bengtsson M, Godbolt AK. Effects of acetylcholinesterase inhibitors on cognitive function in patients with chronic traumatic brain injury: a systematic review. J Rehabil Med. 2016;48(1):1-5.
10. Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
11. DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol. 1995;6(2):59-61.
12. Cope EC, Morris DR, Levenson CW. Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury. Nutr Rev. 2012;70(7):410-413.
13. Morris DR, Levenson CW. Zinc in traumatic brain injury: from neuroprotection to neurotoxicity. Curr Opin Clin Nutr Metab Care. 2013;16(6):708-711.
14. Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13(1):25-34.
15. Fernández-Gajardo R, Matamala JM, Carrasco R, et al. Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs. 2014;28(3):229-248.
16. Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133-137.
17. Cekic M, Cutler SM, VanLandingham JW, et al. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864-874.
18. Aminmansour B, Nikbakht H, Ghorbani A, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo group. Adv Biomed Res. 2012;1:58.
19. Cernak I, Savic VJ, Kotur J, et al. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma. 2000;17(1):53-68.
20. Dhandapani SS, Gupta A, Vivekanandhan S, et al. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. The Indian Journal of Neurotrauma. 2008;5(1):27-33.
21. Temkin NR, Anderson GD, Winn HR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29-38.
22. Jeter CB, Hergenroeder GW, Ward NH 3rd, et al. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30(8):671-679.
23. Cole JT, Mitala CM, Kundu S, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366-371.
24. Aquilani R, Iadarola P, Contardi A, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86(9):1729-1735.
25. Aquilani R, Boselli M, Boschi F, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008;89(9):1642-1647.
26. Kang HJ, Im SH. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo). 2015;61(suppl):S103-S105.
27. DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130(11):1159-1162; discussion 1162-1163.
28. Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290.
29. Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106(3):287-292.
30. Cuzzocrea S, Mazzon E, Costantino G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol. 2000;130(6):1219-1226.
31. Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163.
32. Eakin K, Baratz-Goldstein R, Pick CG, et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617.
33. Theadom A, Mahon S, Barker-Collo S, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20(8):1135-1144.
34. Arenth PM, Russell KC, Ricker JH, et al. CDP-choline as a biological supplement during neurorecovery: a focused review. PM R. 2011;3(6 suppl 1):S123-S131.
35. Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10(5):839-846.
36. Guseva MV, Hopkins DM, Scheff SW, et al. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25(8):975-983.
37. Dixon CE, Ma X, Marion DW. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma. 1997;14(3):161-169.
38. Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(suppl):S39-S42.
39. Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA. 2012;308(19):1993-2000.
40. Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273-280.
41. Levin HS, Peters BH, Kalisky Z, et al. Effects of oral physostigmine and lecithin on memory and attention in closed head-injured patients. Cent Nerv Syst Trauma. 1986;3(4):333-342.
42. Alvarez XA, Lombardi VR, Corzo L, et al. Oral cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. J Neural Transm Suppl. 2000;59:315-328.
43. Ruether E, Husmann R, Kinzler E, et al. A 28-week, double-blind, placebo-controlled study with cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol. 2001;16(5):253-263.
44. Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochir Suppl. 2005;95:59-60.
45. Chen CC, Wei ST, Tsaia SC, et al. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. Br J Neurosurg. 2013;27(6):803-807.
46. Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2016;12:CD007026.
47. Barrett EC, McBurney MI, Ciappio ED. ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv Nutr. 2014;5(3):268-277.
48. Sharma S, Zhuang Y, Ying Z, et al. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161(4):1037-1044.
49. Gatson JW, Liu MM, Abdelfattah K, et al. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):470-475; discussion 474-475.
50. Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 2014;174(3):460-462.
51. Mpandzou G, Aït Ben Haddou E, Regragui W, et al. Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris). 2016;172(2):109-122.
52. Karakis I, Pase MP, Beiser A, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J Alzheimers Dis. 2016;51(2):451-461.
53. Sarris J, Papakostas GI, Vitolo O, et al. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. 2014;164:76-81.
54. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
55. Sarris J, Stough C, Bousman C, et al. Kava in the treatment of generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2013;33(5):643-648.
56. Hariri M, Azadbakht L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med. 2015;6:83.
57. Gillies D, Sinn JKh, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986.
58. Ghanizadeh A, Berk M. Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: a systematic review of randomized controlled clinical trials. Eur J Clin Nutr. 2013;67(1):122-124.
59. U.S. Food and Drug Administration. Can a dietary supplement treat a concussion? No! http://www.fda.gov/forconsumers/consumerupdates/ucm378845.htm. Updated February 13, 2015. Accessed June 5, 2017.
60. Sarris J, Logan AC, Akbaraly TN, et al; International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274.
61. Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. 2014;9(1):e86472.
62. Edie CF, Dewan N. Which psychotropics interact with four common supplements. Current Psychiatry. 2005;4(1):16-30.
63. Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578-592.
64. National Center for Complementary and Integrative Health. Complementary and alternative medicine: what people aged 50 and older discuss with their health care providers. https://nccih.nih.gov/research/statistics/2010. Published 2011. Accessed June 5, 2017.
1. Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html. Updated January 22, 2016. Accessed June 5, 2017.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9.
3. Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50(3):198-205.
4. National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheets. https://ods.od.nih.gov/factsheets/list-all. Accessed June 5, 2017.
5. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy of Sciences; 2002.
6. Rao V, Koliatsos V, Ahmed F, et al. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol. 2015;35(1):64-82.
7. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
8. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533; discussion 1533.
9. Bengtsson M, Godbolt AK. Effects of acetylcholinesterase inhibitors on cognitive function in patients with chronic traumatic brain injury: a systematic review. J Rehabil Med. 2016;48(1):1-5.
10. Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
11. DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol. 1995;6(2):59-61.
12. Cope EC, Morris DR, Levenson CW. Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury. Nutr Rev. 2012;70(7):410-413.
13. Morris DR, Levenson CW. Zinc in traumatic brain injury: from neuroprotection to neurotoxicity. Curr Opin Clin Nutr Metab Care. 2013;16(6):708-711.
14. Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13(1):25-34.
15. Fernández-Gajardo R, Matamala JM, Carrasco R, et al. Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs. 2014;28(3):229-248.
16. Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133-137.
17. Cekic M, Cutler SM, VanLandingham JW, et al. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864-874.
18. Aminmansour B, Nikbakht H, Ghorbani A, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo group. Adv Biomed Res. 2012;1:58.
19. Cernak I, Savic VJ, Kotur J, et al. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma. 2000;17(1):53-68.
20. Dhandapani SS, Gupta A, Vivekanandhan S, et al. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. The Indian Journal of Neurotrauma. 2008;5(1):27-33.
21. Temkin NR, Anderson GD, Winn HR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29-38.
22. Jeter CB, Hergenroeder GW, Ward NH 3rd, et al. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30(8):671-679.
23. Cole JT, Mitala CM, Kundu S, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366-371.
24. Aquilani R, Iadarola P, Contardi A, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86(9):1729-1735.
25. Aquilani R, Boselli M, Boschi F, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008;89(9):1642-1647.
26. Kang HJ, Im SH. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo). 2015;61(suppl):S103-S105.
27. DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130(11):1159-1162; discussion 1162-1163.
28. Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290.
29. Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106(3):287-292.
30. Cuzzocrea S, Mazzon E, Costantino G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol. 2000;130(6):1219-1226.
31. Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163.
32. Eakin K, Baratz-Goldstein R, Pick CG, et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617.
33. Theadom A, Mahon S, Barker-Collo S, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20(8):1135-1144.
34. Arenth PM, Russell KC, Ricker JH, et al. CDP-choline as a biological supplement during neurorecovery: a focused review. PM R. 2011;3(6 suppl 1):S123-S131.
35. Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10(5):839-846.
36. Guseva MV, Hopkins DM, Scheff SW, et al. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25(8):975-983.
37. Dixon CE, Ma X, Marion DW. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma. 1997;14(3):161-169.
38. Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(suppl):S39-S42.
39. Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA. 2012;308(19):1993-2000.
40. Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273-280.
41. Levin HS, Peters BH, Kalisky Z, et al. Effects of oral physostigmine and lecithin on memory and attention in closed head-injured patients. Cent Nerv Syst Trauma. 1986;3(4):333-342.
42. Alvarez XA, Lombardi VR, Corzo L, et al. Oral cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. J Neural Transm Suppl. 2000;59:315-328.
43. Ruether E, Husmann R, Kinzler E, et al. A 28-week, double-blind, placebo-controlled study with cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol. 2001;16(5):253-263.
44. Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochir Suppl. 2005;95:59-60.
45. Chen CC, Wei ST, Tsaia SC, et al. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. Br J Neurosurg. 2013;27(6):803-807.
46. Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2016;12:CD007026.
47. Barrett EC, McBurney MI, Ciappio ED. ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv Nutr. 2014;5(3):268-277.
48. Sharma S, Zhuang Y, Ying Z, et al. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161(4):1037-1044.
49. Gatson JW, Liu MM, Abdelfattah K, et al. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):470-475; discussion 474-475.
50. Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 2014;174(3):460-462.
51. Mpandzou G, Aït Ben Haddou E, Regragui W, et al. Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris). 2016;172(2):109-122.
52. Karakis I, Pase MP, Beiser A, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J Alzheimers Dis. 2016;51(2):451-461.
53. Sarris J, Papakostas GI, Vitolo O, et al. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. 2014;164:76-81.
54. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
55. Sarris J, Stough C, Bousman C, et al. Kava in the treatment of generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2013;33(5):643-648.
56. Hariri M, Azadbakht L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med. 2015;6:83.
57. Gillies D, Sinn JKh, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986.
58. Ghanizadeh A, Berk M. Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: a systematic review of randomized controlled clinical trials. Eur J Clin Nutr. 2013;67(1):122-124.
59. U.S. Food and Drug Administration. Can a dietary supplement treat a concussion? No! http://www.fda.gov/forconsumers/consumerupdates/ucm378845.htm. Updated February 13, 2015. Accessed June 5, 2017.
60. Sarris J, Logan AC, Akbaraly TN, et al; International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274.
61. Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. 2014;9(1):e86472.
62. Edie CF, Dewan N. Which psychotropics interact with four common supplements. Current Psychiatry. 2005;4(1):16-30.
63. Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578-592.
64. National Center for Complementary and Integrative Health. Complementary and alternative medicine: what people aged 50 and older discuss with their health care providers. https://nccih.nih.gov/research/statistics/2010. Published 2011. Accessed June 5, 2017.
Glutamate’s exciting roles in body, brain, and mind: A fertile future pharmacotherapy target
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.
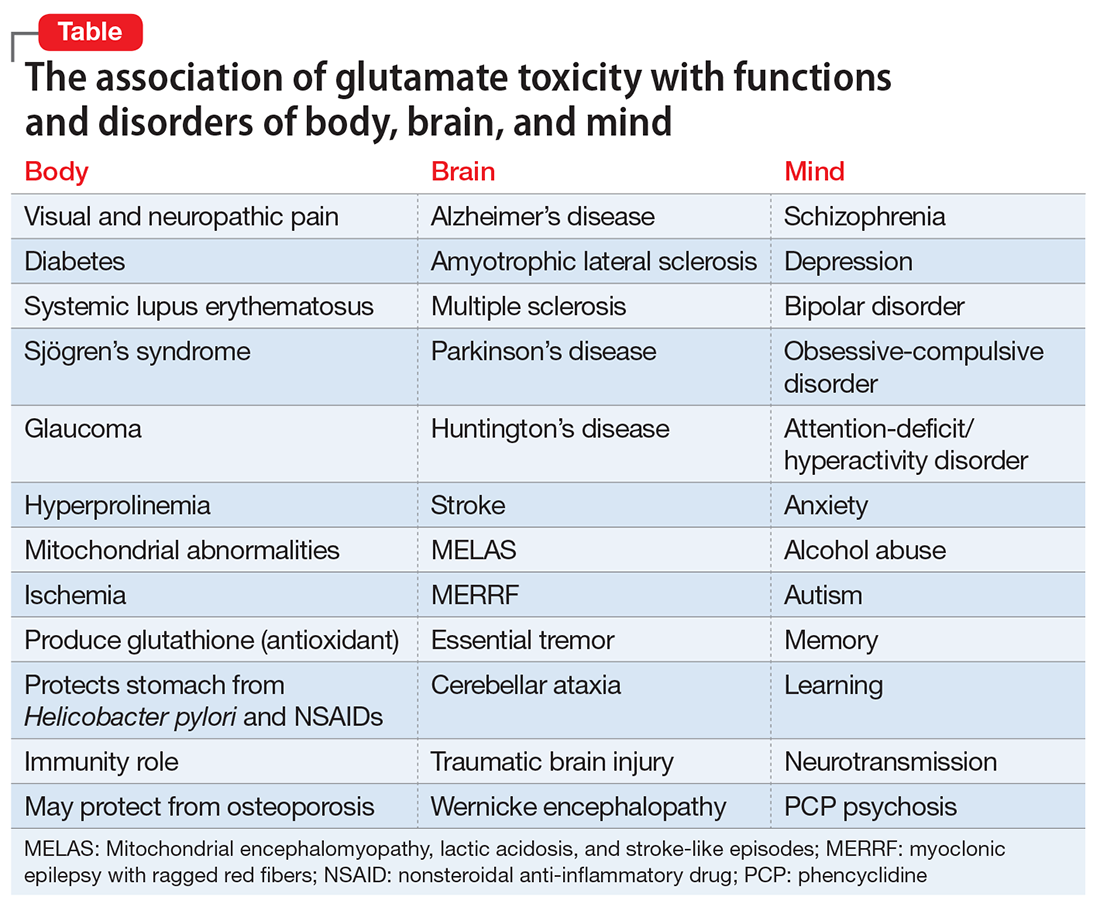
GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.

GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.

GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
Suicidal and paranoid thoughts after starting hepatitis C virus treatment
CASE Suicidal and paranoid
Ms. B, age 53, has a 30-year history of bipolar disorder, a 1-year history of hepatitis C virus (HCV), and previous inpatient psychiatric hospitalizations secondary to acute mania. She presents to our hospital describing her symptoms as the “worst depression ever” and reports suicidal ideation and paranoid thoughts of people watching and following her. Ms. B describes significant neurovegetative symptoms of depression, including poor sleep, poor appetite, low energy and concentration, and chronic feelings of hopelessness with thoughts of “ending it all.” Ms. B reports that her symptoms started 3 weeks ago, a few days after she started taking sofosbuvir and ribavirin for refractory HCV.
Ms. B’s medication regimen consisted of quetiapine, 400 mg at bedtime, fluoxetine, 40 mg/d, and lamotrigine, 150 mg/d, for bipolar disorder, when she started taking sofosbuvir and ribavirin. Ms. B admits she stopped taking her psychotropic and antiviral medications after she noticed progressively worsening depression with intrusive suicidal thoughts, including ruminative thoughts of overdosing on them.
At evaluation, Ms. B is casually dressed, pleasant, with fair hygiene and poor eye contact. Her speech is decreased in rate, volume, and tone; mood is “devastated and depressed”; affect is labile and tearful. Her thought process reveals occasional thought blocking and her thought content includes suicidal ideations and paranoid thoughts. Her cognition is intact; insight and judgment are poor. During evaluation, Ms. B reveals a history of alcohol and marijuana use, but reports that she has not used either for the past 15 years. She further states that she had agreed to a trial of medication first for her liver disease and had deferred any discussion of liver transplant at the time of her diagnosis with HCV.
Laboratory tests reveal a normal complete blood count, creatinine, and electrolytes. However, liver functions were elevated, including aspartate aminotransferase (AST) of 107 U/L (reference range, 8 to 48 U/L) and alanine aminotransferase of 117 U/L (reference range, 7 to 55 U/L). Although increased, the levels of AST and ALT were slightly less than her levels pre-sofosbuvir–ribavirin trial, indicating some response to the medication.
[polldaddy:9777325]
The authors’ observations
Approximately 170 million people worldwide suffer from chronic HCV infection, affecting 2.7 to 5.2 million people in the United States, with 350,000 deaths attributed to liver disease caused by HCV.1
The standard treatment of HCV genotype 1, which represents 70% of all cases of chronic HCV in the United States, is 12 to 32 weeks of an oral protease inhibitor combined with 24 to 48 weeks of peg-interferon (IFN)–alpha-2a plus ribavirin, with the duration of therapy guided by the on-treatment response and the stage of hepatic fibrosis.1
In 2013, the FDA approved sofosbuvir, a direct-acting antiviral drug for chronic HCV. It is a nucleotide analogue HCV NS5B polymerase inhibitor with similar in vitro activity against all HCV genotypes.1 This medication is efficient when used with an antiviral regimen in adults with HCV with liver disease, cirrhosis, HIV coinfection, and hepatocellular carcinoma awaiting liver transplant.2
TREATMENT Medication restarted
Ms. B is admitted to the psychiatric unit for management of severe depression and suicidal thoughts, and quetiapine, 400 mg at bedtime, fluoxetine, 40 mg/d, and lamotrigine, 150 mg/d, are restarted. The hepatology team is consulted for further evaluation and management of her liver disease.
She receives supportive psychotherapy, art therapy, and group therapy to develop better coping skills for her depression and suicidal thoughts and psychoeducation about her medical and psychiatric illness to understand the importance of treatment adherence for symptom improvement. Over the course of her hospital stay, Ms. B has subjective and objective improvements of her depressive symptoms.
The authors’ observations
Psychiatric adverse effects associated with IFN-α therapy in chronic HCV patients are the main cause of antiviral treatment discontinuation, resulting in a decreased rate of sustained viral response.3 Chronic HCV is a major health burden; therefore there is a need for treatment options that are more efficient, safer, simpler, more convenient, and preferably IFN-free.
Sofosbuvir has met many of these criteria and has been found to be safe and well tolerated when administered alone or with ribavirin. Sofosbuvir represents a major breakthrough in HCV care to achieve cures and prevent IFN-associated morbidity and mortality.4,5
Our case report highlights, however, that significant depressive symptoms may be associated with sofosbuvir. Hepatologists should be cautious when prescribing sofosbuvir in patients with comorbid psychiatric illness to avoid exacerbating depressive symptoms and increasing the risk of suicidality.
[polldaddy:9777328]
OUTCOME Refuses treatment
Ms. B is seen by the hepatology team who discuss the best treatment options for HCV, including ledipasvir/sofosbuvir, daclatasvir and ribavirin, and ombitasvir/paritaprevir/ritonavir plus dasabuvir. However, she refuses treatment for HCV stating, “I would rather have no depression with hepatitis C than feel depressed and suicidal while getting treatment for hepatitis C.”
Ms. B is discharged with referral to the outpatient psychiatry clinic and hepatology clinic for monitoring her liver function and restarting sofosbuvir and ribavirin for HCV once her mood symptoms improved.
The authors’ observations
A robust psychiatric evaluation is required before initiating the previously mentioned antiviral therapy to identify high-risk patients to prevent emergence or exacerbation of new psychiatric symptoms, including depression and mania, when treating with IFN-free or IFN-containing regimens. Collaborative care involving a hepatologist and psychiatrist is necessary for comprehensive monitoring of a patient’s psychiatric symptoms and management with medication and psychotherapy. This will limit psychiatric morbidity in patients receiving antiviral treatment with sofosbuvir and ribavirin.
It’s imperative to improve medication adherence for patients by adopting strategies, such as:
- identifying factors leading to noncompliance
- establishing a strong rapport with the patients
- providing psychoeducation about the illness, discussing the benefits and risks of medications and the importance of maintenance treatment
- simplifying medication regimen.6
More research on medication management of HCV in patients with comorbid psychiatric illness should be encouraged and focused on initiating and monitoring non-IFN treatment regimens for patients with HCV and preexisting bipolar disorder or other mood disorders.
1. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
2. Centers for Disease Control and Prevention. Hepatitis C FAQ for health professionals. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm#section4. Updated January 27, 2017. Accessed June 2, 2017.
3. Lucaciu LA, Dumitrascu DL. Depression and suicide ideation in chronic hepatitis C patients untreated and treated with interferon: prevalence, prevention, and treatment. Ann Gastroenterol. 2015;28(4):440-447.
4. Lam B, Henry L, Younossi Z. Sofosbuvir (Sovaldi) for the treatment of hepatitis C. Expert Rev Clin Pharmacol. 2014;7(5):555-566.
5. Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomized, phase 2 trial. Lancet 2014;383(9916):515-523.
6. Balon R. Managing compliance. Psychiatric Times. www.psychiatrictimes.com/articles/managing-compliance. Published May 1, 2002. Accessed June 14, 2017.
CASE Suicidal and paranoid
Ms. B, age 53, has a 30-year history of bipolar disorder, a 1-year history of hepatitis C virus (HCV), and previous inpatient psychiatric hospitalizations secondary to acute mania. She presents to our hospital describing her symptoms as the “worst depression ever” and reports suicidal ideation and paranoid thoughts of people watching and following her. Ms. B describes significant neurovegetative symptoms of depression, including poor sleep, poor appetite, low energy and concentration, and chronic feelings of hopelessness with thoughts of “ending it all.” Ms. B reports that her symptoms started 3 weeks ago, a few days after she started taking sofosbuvir and ribavirin for refractory HCV.
Ms. B’s medication regimen consisted of quetiapine, 400 mg at bedtime, fluoxetine, 40 mg/d, and lamotrigine, 150 mg/d, for bipolar disorder, when she started taking sofosbuvir and ribavirin. Ms. B admits she stopped taking her psychotropic and antiviral medications after she noticed progressively worsening depression with intrusive suicidal thoughts, including ruminative thoughts of overdosing on them.
At evaluation, Ms. B is casually dressed, pleasant, with fair hygiene and poor eye contact. Her speech is decreased in rate, volume, and tone; mood is “devastated and depressed”; affect is labile and tearful. Her thought process reveals occasional thought blocking and her thought content includes suicidal ideations and paranoid thoughts. Her cognition is intact; insight and judgment are poor. During evaluation, Ms. B reveals a history of alcohol and marijuana use, but reports that she has not used either for the past 15 years. She further states that she had agreed to a trial of medication first for her liver disease and had deferred any discussion of liver transplant at the time of her diagnosis with HCV.
Laboratory tests reveal a normal complete blood count, creatinine, and electrolytes. However, liver functions were elevated, including aspartate aminotransferase (AST) of 107 U/L (reference range, 8 to 48 U/L) and alanine aminotransferase of 117 U/L (reference range, 7 to 55 U/L). Although increased, the levels of AST and ALT were slightly less than her levels pre-sofosbuvir–ribavirin trial, indicating some response to the medication.
[polldaddy:9777325]
The authors’ observations
Approximately 170 million people worldwide suffer from chronic HCV infection, affecting 2.7 to 5.2 million people in the United States, with 350,000 deaths attributed to liver disease caused by HCV.1
The standard treatment of HCV genotype 1, which represents 70% of all cases of chronic HCV in the United States, is 12 to 32 weeks of an oral protease inhibitor combined with 24 to 48 weeks of peg-interferon (IFN)–alpha-2a plus ribavirin, with the duration of therapy guided by the on-treatment response and the stage of hepatic fibrosis.1
In 2013, the FDA approved sofosbuvir, a direct-acting antiviral drug for chronic HCV. It is a nucleotide analogue HCV NS5B polymerase inhibitor with similar in vitro activity against all HCV genotypes.1 This medication is efficient when used with an antiviral regimen in adults with HCV with liver disease, cirrhosis, HIV coinfection, and hepatocellular carcinoma awaiting liver transplant.2
TREATMENT Medication restarted
Ms. B is admitted to the psychiatric unit for management of severe depression and suicidal thoughts, and quetiapine, 400 mg at bedtime, fluoxetine, 40 mg/d, and lamotrigine, 150 mg/d, are restarted. The hepatology team is consulted for further evaluation and management of her liver disease.
She receives supportive psychotherapy, art therapy, and group therapy to develop better coping skills for her depression and suicidal thoughts and psychoeducation about her medical and psychiatric illness to understand the importance of treatment adherence for symptom improvement. Over the course of her hospital stay, Ms. B has subjective and objective improvements of her depressive symptoms.
The authors’ observations
Psychiatric adverse effects associated with IFN-α therapy in chronic HCV patients are the main cause of antiviral treatment discontinuation, resulting in a decreased rate of sustained viral response.3 Chronic HCV is a major health burden; therefore there is a need for treatment options that are more efficient, safer, simpler, more convenient, and preferably IFN-free.
Sofosbuvir has met many of these criteria and has been found to be safe and well tolerated when administered alone or with ribavirin. Sofosbuvir represents a major breakthrough in HCV care to achieve cures and prevent IFN-associated morbidity and mortality.4,5
Our case report highlights, however, that significant depressive symptoms may be associated with sofosbuvir. Hepatologists should be cautious when prescribing sofosbuvir in patients with comorbid psychiatric illness to avoid exacerbating depressive symptoms and increasing the risk of suicidality.
[polldaddy:9777328]
OUTCOME Refuses treatment
Ms. B is seen by the hepatology team who discuss the best treatment options for HCV, including ledipasvir/sofosbuvir, daclatasvir and ribavirin, and ombitasvir/paritaprevir/ritonavir plus dasabuvir. However, she refuses treatment for HCV stating, “I would rather have no depression with hepatitis C than feel depressed and suicidal while getting treatment for hepatitis C.”
Ms. B is discharged with referral to the outpatient psychiatry clinic and hepatology clinic for monitoring her liver function and restarting sofosbuvir and ribavirin for HCV once her mood symptoms improved.
The authors’ observations
A robust psychiatric evaluation is required before initiating the previously mentioned antiviral therapy to identify high-risk patients to prevent emergence or exacerbation of new psychiatric symptoms, including depression and mania, when treating with IFN-free or IFN-containing regimens. Collaborative care involving a hepatologist and psychiatrist is necessary for comprehensive monitoring of a patient’s psychiatric symptoms and management with medication and psychotherapy. This will limit psychiatric morbidity in patients receiving antiviral treatment with sofosbuvir and ribavirin.
It’s imperative to improve medication adherence for patients by adopting strategies, such as:
- identifying factors leading to noncompliance
- establishing a strong rapport with the patients
- providing psychoeducation about the illness, discussing the benefits and risks of medications and the importance of maintenance treatment
- simplifying medication regimen.6
More research on medication management of HCV in patients with comorbid psychiatric illness should be encouraged and focused on initiating and monitoring non-IFN treatment regimens for patients with HCV and preexisting bipolar disorder or other mood disorders.
CASE Suicidal and paranoid
Ms. B, age 53, has a 30-year history of bipolar disorder, a 1-year history of hepatitis C virus (HCV), and previous inpatient psychiatric hospitalizations secondary to acute mania. She presents to our hospital describing her symptoms as the “worst depression ever” and reports suicidal ideation and paranoid thoughts of people watching and following her. Ms. B describes significant neurovegetative symptoms of depression, including poor sleep, poor appetite, low energy and concentration, and chronic feelings of hopelessness with thoughts of “ending it all.” Ms. B reports that her symptoms started 3 weeks ago, a few days after she started taking sofosbuvir and ribavirin for refractory HCV.
Ms. B’s medication regimen consisted of quetiapine, 400 mg at bedtime, fluoxetine, 40 mg/d, and lamotrigine, 150 mg/d, for bipolar disorder, when she started taking sofosbuvir and ribavirin. Ms. B admits she stopped taking her psychotropic and antiviral medications after she noticed progressively worsening depression with intrusive suicidal thoughts, including ruminative thoughts of overdosing on them.
At evaluation, Ms. B is casually dressed, pleasant, with fair hygiene and poor eye contact. Her speech is decreased in rate, volume, and tone; mood is “devastated and depressed”; affect is labile and tearful. Her thought process reveals occasional thought blocking and her thought content includes suicidal ideations and paranoid thoughts. Her cognition is intact; insight and judgment are poor. During evaluation, Ms. B reveals a history of alcohol and marijuana use, but reports that she has not used either for the past 15 years. She further states that she had agreed to a trial of medication first for her liver disease and had deferred any discussion of liver transplant at the time of her diagnosis with HCV.
Laboratory tests reveal a normal complete blood count, creatinine, and electrolytes. However, liver functions were elevated, including aspartate aminotransferase (AST) of 107 U/L (reference range, 8 to 48 U/L) and alanine aminotransferase of 117 U/L (reference range, 7 to 55 U/L). Although increased, the levels of AST and ALT were slightly less than her levels pre-sofosbuvir–ribavirin trial, indicating some response to the medication.
[polldaddy:9777325]
The authors’ observations
Approximately 170 million people worldwide suffer from chronic HCV infection, affecting 2.7 to 5.2 million people in the United States, with 350,000 deaths attributed to liver disease caused by HCV.1
The standard treatment of HCV genotype 1, which represents 70% of all cases of chronic HCV in the United States, is 12 to 32 weeks of an oral protease inhibitor combined with 24 to 48 weeks of peg-interferon (IFN)–alpha-2a plus ribavirin, with the duration of therapy guided by the on-treatment response and the stage of hepatic fibrosis.1
In 2013, the FDA approved sofosbuvir, a direct-acting antiviral drug for chronic HCV. It is a nucleotide analogue HCV NS5B polymerase inhibitor with similar in vitro activity against all HCV genotypes.1 This medication is efficient when used with an antiviral regimen in adults with HCV with liver disease, cirrhosis, HIV coinfection, and hepatocellular carcinoma awaiting liver transplant.2
TREATMENT Medication restarted
Ms. B is admitted to the psychiatric unit for management of severe depression and suicidal thoughts, and quetiapine, 400 mg at bedtime, fluoxetine, 40 mg/d, and lamotrigine, 150 mg/d, are restarted. The hepatology team is consulted for further evaluation and management of her liver disease.
She receives supportive psychotherapy, art therapy, and group therapy to develop better coping skills for her depression and suicidal thoughts and psychoeducation about her medical and psychiatric illness to understand the importance of treatment adherence for symptom improvement. Over the course of her hospital stay, Ms. B has subjective and objective improvements of her depressive symptoms.
The authors’ observations
Psychiatric adverse effects associated with IFN-α therapy in chronic HCV patients are the main cause of antiviral treatment discontinuation, resulting in a decreased rate of sustained viral response.3 Chronic HCV is a major health burden; therefore there is a need for treatment options that are more efficient, safer, simpler, more convenient, and preferably IFN-free.
Sofosbuvir has met many of these criteria and has been found to be safe and well tolerated when administered alone or with ribavirin. Sofosbuvir represents a major breakthrough in HCV care to achieve cures and prevent IFN-associated morbidity and mortality.4,5
Our case report highlights, however, that significant depressive symptoms may be associated with sofosbuvir. Hepatologists should be cautious when prescribing sofosbuvir in patients with comorbid psychiatric illness to avoid exacerbating depressive symptoms and increasing the risk of suicidality.
[polldaddy:9777328]
OUTCOME Refuses treatment
Ms. B is seen by the hepatology team who discuss the best treatment options for HCV, including ledipasvir/sofosbuvir, daclatasvir and ribavirin, and ombitasvir/paritaprevir/ritonavir plus dasabuvir. However, she refuses treatment for HCV stating, “I would rather have no depression with hepatitis C than feel depressed and suicidal while getting treatment for hepatitis C.”
Ms. B is discharged with referral to the outpatient psychiatry clinic and hepatology clinic for monitoring her liver function and restarting sofosbuvir and ribavirin for HCV once her mood symptoms improved.
The authors’ observations
A robust psychiatric evaluation is required before initiating the previously mentioned antiviral therapy to identify high-risk patients to prevent emergence or exacerbation of new psychiatric symptoms, including depression and mania, when treating with IFN-free or IFN-containing regimens. Collaborative care involving a hepatologist and psychiatrist is necessary for comprehensive monitoring of a patient’s psychiatric symptoms and management with medication and psychotherapy. This will limit psychiatric morbidity in patients receiving antiviral treatment with sofosbuvir and ribavirin.
It’s imperative to improve medication adherence for patients by adopting strategies, such as:
- identifying factors leading to noncompliance
- establishing a strong rapport with the patients
- providing psychoeducation about the illness, discussing the benefits and risks of medications and the importance of maintenance treatment
- simplifying medication regimen.6
More research on medication management of HCV in patients with comorbid psychiatric illness should be encouraged and focused on initiating and monitoring non-IFN treatment regimens for patients with HCV and preexisting bipolar disorder or other mood disorders.
1. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
2. Centers for Disease Control and Prevention. Hepatitis C FAQ for health professionals. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm#section4. Updated January 27, 2017. Accessed June 2, 2017.
3. Lucaciu LA, Dumitrascu DL. Depression and suicide ideation in chronic hepatitis C patients untreated and treated with interferon: prevalence, prevention, and treatment. Ann Gastroenterol. 2015;28(4):440-447.
4. Lam B, Henry L, Younossi Z. Sofosbuvir (Sovaldi) for the treatment of hepatitis C. Expert Rev Clin Pharmacol. 2014;7(5):555-566.
5. Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomized, phase 2 trial. Lancet 2014;383(9916):515-523.
6. Balon R. Managing compliance. Psychiatric Times. www.psychiatrictimes.com/articles/managing-compliance. Published May 1, 2002. Accessed June 14, 2017.
1. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
2. Centers for Disease Control and Prevention. Hepatitis C FAQ for health professionals. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm#section4. Updated January 27, 2017. Accessed June 2, 2017.
3. Lucaciu LA, Dumitrascu DL. Depression and suicide ideation in chronic hepatitis C patients untreated and treated with interferon: prevalence, prevention, and treatment. Ann Gastroenterol. 2015;28(4):440-447.
4. Lam B, Henry L, Younossi Z. Sofosbuvir (Sovaldi) for the treatment of hepatitis C. Expert Rev Clin Pharmacol. 2014;7(5):555-566.
5. Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomized, phase 2 trial. Lancet 2014;383(9916):515-523.
6. Balon R. Managing compliance. Psychiatric Times. www.psychiatrictimes.com/articles/managing-compliance. Published May 1, 2002. Accessed June 14, 2017.
Eating disorders: Are they age-restricted?
Eating disorders are thought to affect only the young. Although the mean age of presentation is 17 years for anorexia nervosa and 18 to 25 years for bulimia nervosa, many women >65 years suffer from these disorders.1 Often, geriatric patients with a history of eating disorders during their youth that partially remitted have the same disorders re-emerge during their golden years. Because many practitioners think of eating disorders as a younger person’s illness, we could miss an opportunity to help these individuals when screening our geriatric patients.
DSM-52 categorizes feeding and eating disorders as:
- binge eating disorder
- anorexia nervosa
- bulimia nervosa
- other specified feeding and eating disorders
- pica
- avoidant/restrictive food intake disorder.
Binge eating disorder’s main feature is recurrent binge eating, which is the sense that one has lost control when consuming a larger amount of food within a discrete time period than what most people might eat in the same time period. Binge eating may include eating rapidly, feeling uncomfortably
Anorexia nervosa is defined as the restriction of energy intake relative to necessary energy requirements, leading to significantly low body weight in the context of age, sex, developmental trajectory, and physical health, as well as an intense fear of gaining weight or persistent behaviors interfering with weight gain.
Bulimia nervosa is repetitive loss of control when eating large amounts of food (more than most would eat in a period), with compensatory behaviors to prevent weight gain. It is possible that the value attached to youthful slenderness leads to dissatisfaction among older women as their bodies change; binging might provide a sense of control during a time of uncertainty.
Body mass index typically is highest at middle age and slowly declines. In part, this decline is caused by a reduction in energy intake because of modifications in eating habits and lowered appetite often seen during aging. Older women eat 30% fewer calories than younger women.3,4 Social isolation, chronic disease, and depression also contribute to diminished food intake. It is important to remember that distorted body image can occur in older individuals as well. Anorexia nervosa has the highest fatality rate among psychiatric conditions,5 and geriatric patients could be at particularly high risk.
Assessment
Assess for eating disorders in a geriatric patient by exploring the patient’s perception of body image and ruling out underlying causes of weight loss and medical comorbidities. Take a detailed history, including:
- body image and disordered thinking about food
- abnormal behaviors or rituals surrounding food
- history of eating disorders, psychiatric illness, or hospitalization
- medical history
- current and past medications
- illicit drug use or addiction to prescription medications.
Collateral informants, such as partners and adult children of the patient, may yield important information. Because geriatric patients often take several medications, contacting the primary care physician is important in the integrated care of the patient.
A thorough physical and mental status examination will provide information about the patient’s physical appearance. For example, if the patient appears emaciated or weak, the content and process of thoughts related to food will help rule out other etiologies, such as psychosis, depressive disorders, or anxiety. Vital signs and a full physical examination are needed when caring for patients with an eating disorder, regardless of age, but particularly in medically fragile geriatric patients. Because osteoporosis and osteopenia are concerns for many older patients, it’s important to collaborate with the primary care physician early to help minimize bone loss.
Treatment
While ensuring medical stability of the patient, psychotherapy is the treatment of choice for eating disorders in geriatric patients. Moderate to severe binge eating disorder can be treated with lisdexamfetamine. For bulimia nervosa, consider a combination of SSRI and psychotherapy. There is no FDA-approved medication for treating anorexia nervosa; therefore identifying and treating underlying medical causes and/or psychiatric comorbidities can help improve prognosis. Despite this, 1 study showed 20% of geriatric patients with an eating disorder die of complications from eating disorders.6
1. Currin L, Schmidt U, Treasure J, et al. Time trends in eating disorder incidence. Br J Psychiatry. 2005;186(2):132-135.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Morley JE, Thomas DR. Anorexia and aging: pathophysiology. Nutrition. 1999;15(6):499-503.
4. Morley JE. Peptides and aging: their role in anorexia and memory. Peptides. 2015;72(10):112-118.
5. Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders. Arch Gen Psychiatry. 2011;68(7):724-731.
6. Lapid MI, Prom MC, Burton MC, et al. Eating disorders in the elderly. Int Psychogeriatr. 2010;22(4):523-536.
Eating disorders are thought to affect only the young. Although the mean age of presentation is 17 years for anorexia nervosa and 18 to 25 years for bulimia nervosa, many women >65 years suffer from these disorders.1 Often, geriatric patients with a history of eating disorders during their youth that partially remitted have the same disorders re-emerge during their golden years. Because many practitioners think of eating disorders as a younger person’s illness, we could miss an opportunity to help these individuals when screening our geriatric patients.
DSM-52 categorizes feeding and eating disorders as:
- binge eating disorder
- anorexia nervosa
- bulimia nervosa
- other specified feeding and eating disorders
- pica
- avoidant/restrictive food intake disorder.
Binge eating disorder’s main feature is recurrent binge eating, which is the sense that one has lost control when consuming a larger amount of food within a discrete time period than what most people might eat in the same time period. Binge eating may include eating rapidly, feeling uncomfortably
Anorexia nervosa is defined as the restriction of energy intake relative to necessary energy requirements, leading to significantly low body weight in the context of age, sex, developmental trajectory, and physical health, as well as an intense fear of gaining weight or persistent behaviors interfering with weight gain.
Bulimia nervosa is repetitive loss of control when eating large amounts of food (more than most would eat in a period), with compensatory behaviors to prevent weight gain. It is possible that the value attached to youthful slenderness leads to dissatisfaction among older women as their bodies change; binging might provide a sense of control during a time of uncertainty.
Body mass index typically is highest at middle age and slowly declines. In part, this decline is caused by a reduction in energy intake because of modifications in eating habits and lowered appetite often seen during aging. Older women eat 30% fewer calories than younger women.3,4 Social isolation, chronic disease, and depression also contribute to diminished food intake. It is important to remember that distorted body image can occur in older individuals as well. Anorexia nervosa has the highest fatality rate among psychiatric conditions,5 and geriatric patients could be at particularly high risk.
Assessment
Assess for eating disorders in a geriatric patient by exploring the patient’s perception of body image and ruling out underlying causes of weight loss and medical comorbidities. Take a detailed history, including:
- body image and disordered thinking about food
- abnormal behaviors or rituals surrounding food
- history of eating disorders, psychiatric illness, or hospitalization
- medical history
- current and past medications
- illicit drug use or addiction to prescription medications.
Collateral informants, such as partners and adult children of the patient, may yield important information. Because geriatric patients often take several medications, contacting the primary care physician is important in the integrated care of the patient.
A thorough physical and mental status examination will provide information about the patient’s physical appearance. For example, if the patient appears emaciated or weak, the content and process of thoughts related to food will help rule out other etiologies, such as psychosis, depressive disorders, or anxiety. Vital signs and a full physical examination are needed when caring for patients with an eating disorder, regardless of age, but particularly in medically fragile geriatric patients. Because osteoporosis and osteopenia are concerns for many older patients, it’s important to collaborate with the primary care physician early to help minimize bone loss.
Treatment
While ensuring medical stability of the patient, psychotherapy is the treatment of choice for eating disorders in geriatric patients. Moderate to severe binge eating disorder can be treated with lisdexamfetamine. For bulimia nervosa, consider a combination of SSRI and psychotherapy. There is no FDA-approved medication for treating anorexia nervosa; therefore identifying and treating underlying medical causes and/or psychiatric comorbidities can help improve prognosis. Despite this, 1 study showed 20% of geriatric patients with an eating disorder die of complications from eating disorders.6
Eating disorders are thought to affect only the young. Although the mean age of presentation is 17 years for anorexia nervosa and 18 to 25 years for bulimia nervosa, many women >65 years suffer from these disorders.1 Often, geriatric patients with a history of eating disorders during their youth that partially remitted have the same disorders re-emerge during their golden years. Because many practitioners think of eating disorders as a younger person’s illness, we could miss an opportunity to help these individuals when screening our geriatric patients.
DSM-52 categorizes feeding and eating disorders as:
- binge eating disorder
- anorexia nervosa
- bulimia nervosa
- other specified feeding and eating disorders
- pica
- avoidant/restrictive food intake disorder.
Binge eating disorder’s main feature is recurrent binge eating, which is the sense that one has lost control when consuming a larger amount of food within a discrete time period than what most people might eat in the same time period. Binge eating may include eating rapidly, feeling uncomfortably
Anorexia nervosa is defined as the restriction of energy intake relative to necessary energy requirements, leading to significantly low body weight in the context of age, sex, developmental trajectory, and physical health, as well as an intense fear of gaining weight or persistent behaviors interfering with weight gain.
Bulimia nervosa is repetitive loss of control when eating large amounts of food (more than most would eat in a period), with compensatory behaviors to prevent weight gain. It is possible that the value attached to youthful slenderness leads to dissatisfaction among older women as their bodies change; binging might provide a sense of control during a time of uncertainty.
Body mass index typically is highest at middle age and slowly declines. In part, this decline is caused by a reduction in energy intake because of modifications in eating habits and lowered appetite often seen during aging. Older women eat 30% fewer calories than younger women.3,4 Social isolation, chronic disease, and depression also contribute to diminished food intake. It is important to remember that distorted body image can occur in older individuals as well. Anorexia nervosa has the highest fatality rate among psychiatric conditions,5 and geriatric patients could be at particularly high risk.
Assessment
Assess for eating disorders in a geriatric patient by exploring the patient’s perception of body image and ruling out underlying causes of weight loss and medical comorbidities. Take a detailed history, including:
- body image and disordered thinking about food
- abnormal behaviors or rituals surrounding food
- history of eating disorders, psychiatric illness, or hospitalization
- medical history
- current and past medications
- illicit drug use or addiction to prescription medications.
Collateral informants, such as partners and adult children of the patient, may yield important information. Because geriatric patients often take several medications, contacting the primary care physician is important in the integrated care of the patient.
A thorough physical and mental status examination will provide information about the patient’s physical appearance. For example, if the patient appears emaciated or weak, the content and process of thoughts related to food will help rule out other etiologies, such as psychosis, depressive disorders, or anxiety. Vital signs and a full physical examination are needed when caring for patients with an eating disorder, regardless of age, but particularly in medically fragile geriatric patients. Because osteoporosis and osteopenia are concerns for many older patients, it’s important to collaborate with the primary care physician early to help minimize bone loss.
Treatment
While ensuring medical stability of the patient, psychotherapy is the treatment of choice for eating disorders in geriatric patients. Moderate to severe binge eating disorder can be treated with lisdexamfetamine. For bulimia nervosa, consider a combination of SSRI and psychotherapy. There is no FDA-approved medication for treating anorexia nervosa; therefore identifying and treating underlying medical causes and/or psychiatric comorbidities can help improve prognosis. Despite this, 1 study showed 20% of geriatric patients with an eating disorder die of complications from eating disorders.6
1. Currin L, Schmidt U, Treasure J, et al. Time trends in eating disorder incidence. Br J Psychiatry. 2005;186(2):132-135.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Morley JE, Thomas DR. Anorexia and aging: pathophysiology. Nutrition. 1999;15(6):499-503.
4. Morley JE. Peptides and aging: their role in anorexia and memory. Peptides. 2015;72(10):112-118.
5. Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders. Arch Gen Psychiatry. 2011;68(7):724-731.
6. Lapid MI, Prom MC, Burton MC, et al. Eating disorders in the elderly. Int Psychogeriatr. 2010;22(4):523-536.
1. Currin L, Schmidt U, Treasure J, et al. Time trends in eating disorder incidence. Br J Psychiatry. 2005;186(2):132-135.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Morley JE, Thomas DR. Anorexia and aging: pathophysiology. Nutrition. 1999;15(6):499-503.
4. Morley JE. Peptides and aging: their role in anorexia and memory. Peptides. 2015;72(10):112-118.
5. Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders. Arch Gen Psychiatry. 2011;68(7):724-731.
6. Lapid MI, Prom MC, Burton MC, et al. Eating disorders in the elderly. Int Psychogeriatr. 2010;22(4):523-536.