User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
A compounded, nonbenzodiazepine option for treating acute anxiety
Treating short-term or situational anxiety or anxiety attacks with benzodiazepines carries the risk of withdrawal and dependence. Other options include various antidepressants and buspirone. Although such medications decrease overall anxiety and can prevent anxiety from building, they are not effective for breakthrough anxiety. Other mainstays are antihistamines, antipsychotics, or newer antiepileptics such as gabapentin and pregabalin, but none of these have strong clinical literature support regarding their effectiveness for treating anxiety disorders.
PanX compounded medications are dual drug combinations of a beta blocker plus an antiemetic antimuscarinic agent.1 They are designed and patented for as-needed treatment of anxiety disorders without using any controlled substances. Compounded medications are not FDA-approved, but are commercially available and subject to Section 503A of the Federal Food, Drug, and Cosmetics Act of 2013.2
In PanX medications, the beta blocker is intended to address the sympathetic cardiovascular symptoms of anxiety. Beta adrenergic receptor antagonists have been prescribed off-label for decades to treat social anxiety disorder, including performance anxiety. At least 7 beta blockers—atenolol, propranolol, pindolol, timolol, nadolol, betaxolol, and oxprenolol—have been reported to have anxiolytic effects, although these are limited to cardiovascular symptoms of anxiety.1
However, there is a need to augment the limited effects of the beta blocker with another agent, such as an antimuscarinic agent, which is intended for parasympathetic noncardiovascular and CNS symptoms of anxiety. Scopolamine is a preferred antimuscarinic because it has been known for over a century to exhibit anxiolytic effects.3 Scopolamine’s mechanism of action is antagonism of acetylcholine binding to the M1 and/or M2 muscarinic receptors.4
We present a case of a patient who needed a nonbenzodiazepine treatment for acute anxiety. She received a compounded PanX combination of the beta-1 selective beta blocker atenolol, 25 mg, plus scopolamine hydrobromide, 0.2 mg, as needed for acute anxiety.
Case report
Acute anxiety, benzodiazepine abuse
Ms. L, age 30, with a family history of depression and anxiety, has had anxiety, depression, and posttraumatic stress disorder since she was in her mid-20s. She is evaluated in a 30-day rehabilitation program for alprazolam abuse. She is detoxed from alprazolam and stabilized with lurasidone, 60 mg once in the morning, gabapentin, 1,200 mg 4 times a day, and quetiapine, 125 mg as needed for sleep.
Ms. L improves significantly and is transferred to an intensive outpatient program. While there, she experiences increased periods of anxiety related to ruminative thoughts about relationship, occupational, and living stressors. She requests a medication for breakthrough anxiety and recognizes that, because of her history, a benzodiazepine is not medically indicated.
Ms. L signs a consent to a physician-sponsored trial of a PanX medication consisting of orally disintegrating tablets of atenolol, 25 mg, plus scopolamine hydrobromide, 0.2 mg, (in a polyglycol troche base plus mannitol, silica gel, and Steviol glycosides), which is prepared by a compounding pharmacy. Over 6 days, she takes the PanX combination 3 times. Immediately before she takes the medication, her symptoms are intense anxiety, nervousness, and agitation; feelings of panic; increased heart rate and palpitations; and shortness of breath. Ms. L says these symptoms developed approximately 20 minutes before she took the PanX combination. Approximately 30 minutes after taking the medication, she describes having a complete resolution of these symptoms that lasted for 4 hours. She says the medication “calmed [her] down” and had a “Klonopin or benzo-like effect.” She notes that her heart rate slowed quickly, followed by her breathing, and that she also was “more focused.” No information regarding her heart rate or blood pressure when she experienced the symptoms or after treatment is available. She denies experiencing dry mouth, dizziness, fatigue, sleepiness, blurred vision, or confusion.
Targets for future research
This case provides some preliminary clinical evidence of a rapid anxiolytic effect from a novel medication—a beta blocker plus scopolamine combination—that was beneficial in a situation where it may be likely that a benzodiazepine would have been utilized. This is our first case report documenting a trial of any PanX combination (ie, a combination of any beta blocker with any antimuscarinic agent) regarding anxiolytic efficacy and timing, tolerability, and adverse effects. With recognition that this is a report of 1 patient who took the medication 3 times, there is much that is not known.
Additional clinical studies are needed to evaluate the efficacy, tolerability, and adverse effects associated with using a beta blocker/antiemetic antimuscarinic combination to treat acute anxiety. Medication interactions also need to be considered. Whether this combination medication would be best for treating breakthrough anxiety or other acute anxiety episodes, and/or used as a regularly dosed medication is unknown. With documented risks of long-term benzodiazepine use, other novel therapeutics, such as the atenolol/scopolamine combination, may be welcome in treating acute anxiety.
1. Dooley TP. Treating anxiety with either beta blockers or antiemetic antimuscarinic drugs: a review. Mental Health Fam Med. 2015;11(1):89-99.
2. U.S. Food and Drug Administration. Guidance, compliance and regulatory information: compounding. Section 503A of the Federal Food, Drug, and Cosmetic Act. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/pharmacycompounding/ucm376733.htm. Updated December 12, 2013. Accessed October 25, 2017.
3. Houde A. Scopolamine: a physiological and clinical study. The Am J Clin Med. 1906;13:365-367.
4. Witkin JM, Overshiner C, Li X, et al. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther. 2014;351(2):448-456.
Treating short-term or situational anxiety or anxiety attacks with benzodiazepines carries the risk of withdrawal and dependence. Other options include various antidepressants and buspirone. Although such medications decrease overall anxiety and can prevent anxiety from building, they are not effective for breakthrough anxiety. Other mainstays are antihistamines, antipsychotics, or newer antiepileptics such as gabapentin and pregabalin, but none of these have strong clinical literature support regarding their effectiveness for treating anxiety disorders.
PanX compounded medications are dual drug combinations of a beta blocker plus an antiemetic antimuscarinic agent.1 They are designed and patented for as-needed treatment of anxiety disorders without using any controlled substances. Compounded medications are not FDA-approved, but are commercially available and subject to Section 503A of the Federal Food, Drug, and Cosmetics Act of 2013.2
In PanX medications, the beta blocker is intended to address the sympathetic cardiovascular symptoms of anxiety. Beta adrenergic receptor antagonists have been prescribed off-label for decades to treat social anxiety disorder, including performance anxiety. At least 7 beta blockers—atenolol, propranolol, pindolol, timolol, nadolol, betaxolol, and oxprenolol—have been reported to have anxiolytic effects, although these are limited to cardiovascular symptoms of anxiety.1
However, there is a need to augment the limited effects of the beta blocker with another agent, such as an antimuscarinic agent, which is intended for parasympathetic noncardiovascular and CNS symptoms of anxiety. Scopolamine is a preferred antimuscarinic because it has been known for over a century to exhibit anxiolytic effects.3 Scopolamine’s mechanism of action is antagonism of acetylcholine binding to the M1 and/or M2 muscarinic receptors.4
We present a case of a patient who needed a nonbenzodiazepine treatment for acute anxiety. She received a compounded PanX combination of the beta-1 selective beta blocker atenolol, 25 mg, plus scopolamine hydrobromide, 0.2 mg, as needed for acute anxiety.
Case report
Acute anxiety, benzodiazepine abuse
Ms. L, age 30, with a family history of depression and anxiety, has had anxiety, depression, and posttraumatic stress disorder since she was in her mid-20s. She is evaluated in a 30-day rehabilitation program for alprazolam abuse. She is detoxed from alprazolam and stabilized with lurasidone, 60 mg once in the morning, gabapentin, 1,200 mg 4 times a day, and quetiapine, 125 mg as needed for sleep.
Ms. L improves significantly and is transferred to an intensive outpatient program. While there, she experiences increased periods of anxiety related to ruminative thoughts about relationship, occupational, and living stressors. She requests a medication for breakthrough anxiety and recognizes that, because of her history, a benzodiazepine is not medically indicated.
Ms. L signs a consent to a physician-sponsored trial of a PanX medication consisting of orally disintegrating tablets of atenolol, 25 mg, plus scopolamine hydrobromide, 0.2 mg, (in a polyglycol troche base plus mannitol, silica gel, and Steviol glycosides), which is prepared by a compounding pharmacy. Over 6 days, she takes the PanX combination 3 times. Immediately before she takes the medication, her symptoms are intense anxiety, nervousness, and agitation; feelings of panic; increased heart rate and palpitations; and shortness of breath. Ms. L says these symptoms developed approximately 20 minutes before she took the PanX combination. Approximately 30 minutes after taking the medication, she describes having a complete resolution of these symptoms that lasted for 4 hours. She says the medication “calmed [her] down” and had a “Klonopin or benzo-like effect.” She notes that her heart rate slowed quickly, followed by her breathing, and that she also was “more focused.” No information regarding her heart rate or blood pressure when she experienced the symptoms or after treatment is available. She denies experiencing dry mouth, dizziness, fatigue, sleepiness, blurred vision, or confusion.
Targets for future research
This case provides some preliminary clinical evidence of a rapid anxiolytic effect from a novel medication—a beta blocker plus scopolamine combination—that was beneficial in a situation where it may be likely that a benzodiazepine would have been utilized. This is our first case report documenting a trial of any PanX combination (ie, a combination of any beta blocker with any antimuscarinic agent) regarding anxiolytic efficacy and timing, tolerability, and adverse effects. With recognition that this is a report of 1 patient who took the medication 3 times, there is much that is not known.
Additional clinical studies are needed to evaluate the efficacy, tolerability, and adverse effects associated with using a beta blocker/antiemetic antimuscarinic combination to treat acute anxiety. Medication interactions also need to be considered. Whether this combination medication would be best for treating breakthrough anxiety or other acute anxiety episodes, and/or used as a regularly dosed medication is unknown. With documented risks of long-term benzodiazepine use, other novel therapeutics, such as the atenolol/scopolamine combination, may be welcome in treating acute anxiety.
Treating short-term or situational anxiety or anxiety attacks with benzodiazepines carries the risk of withdrawal and dependence. Other options include various antidepressants and buspirone. Although such medications decrease overall anxiety and can prevent anxiety from building, they are not effective for breakthrough anxiety. Other mainstays are antihistamines, antipsychotics, or newer antiepileptics such as gabapentin and pregabalin, but none of these have strong clinical literature support regarding their effectiveness for treating anxiety disorders.
PanX compounded medications are dual drug combinations of a beta blocker plus an antiemetic antimuscarinic agent.1 They are designed and patented for as-needed treatment of anxiety disorders without using any controlled substances. Compounded medications are not FDA-approved, but are commercially available and subject to Section 503A of the Federal Food, Drug, and Cosmetics Act of 2013.2
In PanX medications, the beta blocker is intended to address the sympathetic cardiovascular symptoms of anxiety. Beta adrenergic receptor antagonists have been prescribed off-label for decades to treat social anxiety disorder, including performance anxiety. At least 7 beta blockers—atenolol, propranolol, pindolol, timolol, nadolol, betaxolol, and oxprenolol—have been reported to have anxiolytic effects, although these are limited to cardiovascular symptoms of anxiety.1
However, there is a need to augment the limited effects of the beta blocker with another agent, such as an antimuscarinic agent, which is intended for parasympathetic noncardiovascular and CNS symptoms of anxiety. Scopolamine is a preferred antimuscarinic because it has been known for over a century to exhibit anxiolytic effects.3 Scopolamine’s mechanism of action is antagonism of acetylcholine binding to the M1 and/or M2 muscarinic receptors.4
We present a case of a patient who needed a nonbenzodiazepine treatment for acute anxiety. She received a compounded PanX combination of the beta-1 selective beta blocker atenolol, 25 mg, plus scopolamine hydrobromide, 0.2 mg, as needed for acute anxiety.
Case report
Acute anxiety, benzodiazepine abuse
Ms. L, age 30, with a family history of depression and anxiety, has had anxiety, depression, and posttraumatic stress disorder since she was in her mid-20s. She is evaluated in a 30-day rehabilitation program for alprazolam abuse. She is detoxed from alprazolam and stabilized with lurasidone, 60 mg once in the morning, gabapentin, 1,200 mg 4 times a day, and quetiapine, 125 mg as needed for sleep.
Ms. L improves significantly and is transferred to an intensive outpatient program. While there, she experiences increased periods of anxiety related to ruminative thoughts about relationship, occupational, and living stressors. She requests a medication for breakthrough anxiety and recognizes that, because of her history, a benzodiazepine is not medically indicated.
Ms. L signs a consent to a physician-sponsored trial of a PanX medication consisting of orally disintegrating tablets of atenolol, 25 mg, plus scopolamine hydrobromide, 0.2 mg, (in a polyglycol troche base plus mannitol, silica gel, and Steviol glycosides), which is prepared by a compounding pharmacy. Over 6 days, she takes the PanX combination 3 times. Immediately before she takes the medication, her symptoms are intense anxiety, nervousness, and agitation; feelings of panic; increased heart rate and palpitations; and shortness of breath. Ms. L says these symptoms developed approximately 20 minutes before she took the PanX combination. Approximately 30 minutes after taking the medication, she describes having a complete resolution of these symptoms that lasted for 4 hours. She says the medication “calmed [her] down” and had a “Klonopin or benzo-like effect.” She notes that her heart rate slowed quickly, followed by her breathing, and that she also was “more focused.” No information regarding her heart rate or blood pressure when she experienced the symptoms or after treatment is available. She denies experiencing dry mouth, dizziness, fatigue, sleepiness, blurred vision, or confusion.
Targets for future research
This case provides some preliminary clinical evidence of a rapid anxiolytic effect from a novel medication—a beta blocker plus scopolamine combination—that was beneficial in a situation where it may be likely that a benzodiazepine would have been utilized. This is our first case report documenting a trial of any PanX combination (ie, a combination of any beta blocker with any antimuscarinic agent) regarding anxiolytic efficacy and timing, tolerability, and adverse effects. With recognition that this is a report of 1 patient who took the medication 3 times, there is much that is not known.
Additional clinical studies are needed to evaluate the efficacy, tolerability, and adverse effects associated with using a beta blocker/antiemetic antimuscarinic combination to treat acute anxiety. Medication interactions also need to be considered. Whether this combination medication would be best for treating breakthrough anxiety or other acute anxiety episodes, and/or used as a regularly dosed medication is unknown. With documented risks of long-term benzodiazepine use, other novel therapeutics, such as the atenolol/scopolamine combination, may be welcome in treating acute anxiety.
1. Dooley TP. Treating anxiety with either beta blockers or antiemetic antimuscarinic drugs: a review. Mental Health Fam Med. 2015;11(1):89-99.
2. U.S. Food and Drug Administration. Guidance, compliance and regulatory information: compounding. Section 503A of the Federal Food, Drug, and Cosmetic Act. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/pharmacycompounding/ucm376733.htm. Updated December 12, 2013. Accessed October 25, 2017.
3. Houde A. Scopolamine: a physiological and clinical study. The Am J Clin Med. 1906;13:365-367.
4. Witkin JM, Overshiner C, Li X, et al. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther. 2014;351(2):448-456.
1. Dooley TP. Treating anxiety with either beta blockers or antiemetic antimuscarinic drugs: a review. Mental Health Fam Med. 2015;11(1):89-99.
2. U.S. Food and Drug Administration. Guidance, compliance and regulatory information: compounding. Section 503A of the Federal Food, Drug, and Cosmetic Act. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/pharmacycompounding/ucm376733.htm. Updated December 12, 2013. Accessed October 25, 2017.
3. Houde A. Scopolamine: a physiological and clinical study. The Am J Clin Med. 1906;13:365-367.
4. Witkin JM, Overshiner C, Li X, et al. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther. 2014;351(2):448-456.
Scandinavian registries answer key questions about ADHD
PARIS – Huge longitudinal Scandinavian population registries constitute a unique data source that, in recent years, has provided new insights into attention-deficit/hyperactivity disorder and its associated risks of suicidal behavior, accidents, and early mortality, Henrik Larsson, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“Randomized, controlled trials have provided little information about real-world effectiveness of ADHD medications, such as their potential effects on adverse health outcomes,” said Dr. Larsson of the Karolinska Institute in Stockholm.
Suicidality
Dr. Larsson was senior author of a Swedish national registry study that identified 51,707 patients with ADHD matched by sex and birth year to 258,535 controls. The ADHD patients had significantly higher rates of both attempted and completed suicide.
After adjustment for socioeconomic status, these individuals were at 8.46-fold increased risk for attempted suicide. However, after further adjustment for comorbid psychiatric disorders, this dropped substantially to a 3.62-fold increased risk.
The same pattern pertained to completed suicide: The risk after adjustment for socioeconomic status was increased 12.3-fold in individuals with ADHD, compared with controls, but the risk dropped to 5.91-fold after further adjustment for comorbid psychiatric disorders (JAMA Psychiatry. 2014 Aug;71[8]:958-64).
The clinical take home point: “Detection and treatment of comorbid conditions probably will help reduce suicidal behavior in ADHD,” Dr. Larsson said.
This study also showed that increased familial risk also is a key factor in the increased risk of suicidal behavior in the ADHD population. Parents of individuals with ADHD were at 2.42-fold increased risk of attempted suicide, compared with controls, and full siblings were at 2.28-fold increased risk. In contrast, the risk in half-siblings, while significantly greater than in controls, was lower than in the genetically closer first-degree relatives: Maternal half-siblings were at 1.57-fold increased risk, and paternal half-siblings were at 1.57-fold greater risk. Cousins were at 1.39-fold increased risk.
The same held true for completed suicide risk. And the familial associations remained significant even after excluding relatives with ADHD.
“Regarding the shared familial factors, I’m tempted to hypothesize that this might involve pleiotropic effects reflecting genetic variants associated with impulsivity,” said Dr. Larsson. To further understand the biological mechanisms underlying ADHD and associated adverse health outcomes requires multiple disciplines to work together. For such work, Dr. Larsson collaborates with international colleagues in several consortia.
ADHD medications and suicidality
Concerns regarding this question were raised by a meta-analysis based on clinical trials data that suggested patients’ increased suicidality might be caused by the effects of ADHD medications. But the meta-analysis was seriously flawed by what epidemiologists call confounding by indication, which is the potential for bias to be introduced when a group of patients on medication is compared with another group off medication. The confounding results from the fact that ADHD patients on medication are different from those who aren’t: They are likely to be more symptomatic and have more comorbidities.
To bypass the confounding issue, Dr. Larsson and his coinvestigators turned to the Swedish registries and identified 37,936 patients with ADHD with a total of 7,019 suicide-related events during nearly 151,000 person-years of follow-up. When they compared patients on drug treatment with those who were not, they found – as in the other investigators’ meta-analysis – that drug treatment was associated with a statistically significant 1.31-fold increased risk of suicide-related events. However, when they performed a more appropriate between-individual analysis, Dr. Larsson and his colleagues found that, when patients with ADHD were using stimulant medications, they had a significant 19% lower risk of suicide-related events than when they were off medication. While on nonstimulant ADHD medications, their suicidality risk was no different from when off medication (BMJ. 2014 Jun 18;348:g3769. doi: 10.1136/bmj.g3769).
ADHD and early mortality risk
Prior studies have established that ADHD is associated with a proclivity to engage in risk-taking behaviors, including substance abuse, criminality, risky sexual behavior, and accidents, which are themselves associated with early mortality.
Sure enough, when Danish investigators turned to their national registries, identified 32,061 individuals with ADHD born during 1981-2011, and followed them through 2013, they found that, during nearly 25 million person-years of follow-up, the mortality rate was 5.85 deaths per 10,000 person-years in individuals with ADHD, compared with 2.21 deaths per 10,000 person-years in controls, resulting in a fully adjusted mortality rate ratio of 2.07. The rate ratio was 1.86 for ADHD patients under age 6 years, 1.58 in those aged 6-17 years, and 4.25 for patients aged 18 years and older (Lancet. 2015 May 30;385[9983]:2190-6).
Accidents were the most common cause of death. Could ADHD medications modify this risk of fatal accidents?
Serious motor vehicle accidents
Dr. Larsson and his coinvestigators used registry data to follow 17,408 Swedish adults with ADHD for serious transport accidents involving a trip to the emergency room or death during 2006-2009. The risk was increased by an adjusted 1.47-fold in men with ADHD and by 1.45-fold in women with the disorder. However, in the within-individual analysis, men were 58% less likely to have a serious transport accident when they were on ADHD medication than when off medication. There was no statistically significant effect of ADHD medications on the risk in women with ADHD.
The investigators estimated that 41%-49% of transport accidents in men with ADHD could have been avoided had they been on drug therapy continuously throughout the follow-up period (JAMA Psychiatry. 2014 Mar;71[3]:319-25).
Similar results – that is, data showing that being on ADHD medication reduces the elevated risk of serious accidents – have been reported in four other independent studies conducted in Denmark, Germany, Hong Kong, and most recently in a U.S. analysis by Dr. Larsson and coinvestigators of more than 2.3 million patients with ADHD in a U.S. commercial health insurance claims database (JAMA Psychiatry. 2017 Jun 1;74[6]:597-603).
These findings collectively highlight the public health importance of diagnosing and treating ADHD.
But Dr. Larsson wanted his audience to take home another key lesson: “ADHD is a disorder that can be associated with serious outcomes, including suicide and accidents. It’s nevertheless important to remember that the absolute risks here are very low for any of these outcomes, so the majority of individuals with ADHD will never suffer from any of these outcomes. It’s important to keep that in mind.”
Dr. Larsson’s research is funded by the Swedish Research Council, the National Institute of Mental Health, FORTE, Horizon 2020, and Shire.
[email protected]
PARIS – Huge longitudinal Scandinavian population registries constitute a unique data source that, in recent years, has provided new insights into attention-deficit/hyperactivity disorder and its associated risks of suicidal behavior, accidents, and early mortality, Henrik Larsson, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“Randomized, controlled trials have provided little information about real-world effectiveness of ADHD medications, such as their potential effects on adverse health outcomes,” said Dr. Larsson of the Karolinska Institute in Stockholm.
Suicidality
Dr. Larsson was senior author of a Swedish national registry study that identified 51,707 patients with ADHD matched by sex and birth year to 258,535 controls. The ADHD patients had significantly higher rates of both attempted and completed suicide.
After adjustment for socioeconomic status, these individuals were at 8.46-fold increased risk for attempted suicide. However, after further adjustment for comorbid psychiatric disorders, this dropped substantially to a 3.62-fold increased risk.
The same pattern pertained to completed suicide: The risk after adjustment for socioeconomic status was increased 12.3-fold in individuals with ADHD, compared with controls, but the risk dropped to 5.91-fold after further adjustment for comorbid psychiatric disorders (JAMA Psychiatry. 2014 Aug;71[8]:958-64).
The clinical take home point: “Detection and treatment of comorbid conditions probably will help reduce suicidal behavior in ADHD,” Dr. Larsson said.
This study also showed that increased familial risk also is a key factor in the increased risk of suicidal behavior in the ADHD population. Parents of individuals with ADHD were at 2.42-fold increased risk of attempted suicide, compared with controls, and full siblings were at 2.28-fold increased risk. In contrast, the risk in half-siblings, while significantly greater than in controls, was lower than in the genetically closer first-degree relatives: Maternal half-siblings were at 1.57-fold increased risk, and paternal half-siblings were at 1.57-fold greater risk. Cousins were at 1.39-fold increased risk.
The same held true for completed suicide risk. And the familial associations remained significant even after excluding relatives with ADHD.
“Regarding the shared familial factors, I’m tempted to hypothesize that this might involve pleiotropic effects reflecting genetic variants associated with impulsivity,” said Dr. Larsson. To further understand the biological mechanisms underlying ADHD and associated adverse health outcomes requires multiple disciplines to work together. For such work, Dr. Larsson collaborates with international colleagues in several consortia.
ADHD medications and suicidality
Concerns regarding this question were raised by a meta-analysis based on clinical trials data that suggested patients’ increased suicidality might be caused by the effects of ADHD medications. But the meta-analysis was seriously flawed by what epidemiologists call confounding by indication, which is the potential for bias to be introduced when a group of patients on medication is compared with another group off medication. The confounding results from the fact that ADHD patients on medication are different from those who aren’t: They are likely to be more symptomatic and have more comorbidities.
To bypass the confounding issue, Dr. Larsson and his coinvestigators turned to the Swedish registries and identified 37,936 patients with ADHD with a total of 7,019 suicide-related events during nearly 151,000 person-years of follow-up. When they compared patients on drug treatment with those who were not, they found – as in the other investigators’ meta-analysis – that drug treatment was associated with a statistically significant 1.31-fold increased risk of suicide-related events. However, when they performed a more appropriate between-individual analysis, Dr. Larsson and his colleagues found that, when patients with ADHD were using stimulant medications, they had a significant 19% lower risk of suicide-related events than when they were off medication. While on nonstimulant ADHD medications, their suicidality risk was no different from when off medication (BMJ. 2014 Jun 18;348:g3769. doi: 10.1136/bmj.g3769).
ADHD and early mortality risk
Prior studies have established that ADHD is associated with a proclivity to engage in risk-taking behaviors, including substance abuse, criminality, risky sexual behavior, and accidents, which are themselves associated with early mortality.
Sure enough, when Danish investigators turned to their national registries, identified 32,061 individuals with ADHD born during 1981-2011, and followed them through 2013, they found that, during nearly 25 million person-years of follow-up, the mortality rate was 5.85 deaths per 10,000 person-years in individuals with ADHD, compared with 2.21 deaths per 10,000 person-years in controls, resulting in a fully adjusted mortality rate ratio of 2.07. The rate ratio was 1.86 for ADHD patients under age 6 years, 1.58 in those aged 6-17 years, and 4.25 for patients aged 18 years and older (Lancet. 2015 May 30;385[9983]:2190-6).
Accidents were the most common cause of death. Could ADHD medications modify this risk of fatal accidents?
Serious motor vehicle accidents
Dr. Larsson and his coinvestigators used registry data to follow 17,408 Swedish adults with ADHD for serious transport accidents involving a trip to the emergency room or death during 2006-2009. The risk was increased by an adjusted 1.47-fold in men with ADHD and by 1.45-fold in women with the disorder. However, in the within-individual analysis, men were 58% less likely to have a serious transport accident when they were on ADHD medication than when off medication. There was no statistically significant effect of ADHD medications on the risk in women with ADHD.
The investigators estimated that 41%-49% of transport accidents in men with ADHD could have been avoided had they been on drug therapy continuously throughout the follow-up period (JAMA Psychiatry. 2014 Mar;71[3]:319-25).
Similar results – that is, data showing that being on ADHD medication reduces the elevated risk of serious accidents – have been reported in four other independent studies conducted in Denmark, Germany, Hong Kong, and most recently in a U.S. analysis by Dr. Larsson and coinvestigators of more than 2.3 million patients with ADHD in a U.S. commercial health insurance claims database (JAMA Psychiatry. 2017 Jun 1;74[6]:597-603).
These findings collectively highlight the public health importance of diagnosing and treating ADHD.
But Dr. Larsson wanted his audience to take home another key lesson: “ADHD is a disorder that can be associated with serious outcomes, including suicide and accidents. It’s nevertheless important to remember that the absolute risks here are very low for any of these outcomes, so the majority of individuals with ADHD will never suffer from any of these outcomes. It’s important to keep that in mind.”
Dr. Larsson’s research is funded by the Swedish Research Council, the National Institute of Mental Health, FORTE, Horizon 2020, and Shire.
[email protected]
PARIS – Huge longitudinal Scandinavian population registries constitute a unique data source that, in recent years, has provided new insights into attention-deficit/hyperactivity disorder and its associated risks of suicidal behavior, accidents, and early mortality, Henrik Larsson, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“Randomized, controlled trials have provided little information about real-world effectiveness of ADHD medications, such as their potential effects on adverse health outcomes,” said Dr. Larsson of the Karolinska Institute in Stockholm.
Suicidality
Dr. Larsson was senior author of a Swedish national registry study that identified 51,707 patients with ADHD matched by sex and birth year to 258,535 controls. The ADHD patients had significantly higher rates of both attempted and completed suicide.
After adjustment for socioeconomic status, these individuals were at 8.46-fold increased risk for attempted suicide. However, after further adjustment for comorbid psychiatric disorders, this dropped substantially to a 3.62-fold increased risk.
The same pattern pertained to completed suicide: The risk after adjustment for socioeconomic status was increased 12.3-fold in individuals with ADHD, compared with controls, but the risk dropped to 5.91-fold after further adjustment for comorbid psychiatric disorders (JAMA Psychiatry. 2014 Aug;71[8]:958-64).
The clinical take home point: “Detection and treatment of comorbid conditions probably will help reduce suicidal behavior in ADHD,” Dr. Larsson said.
This study also showed that increased familial risk also is a key factor in the increased risk of suicidal behavior in the ADHD population. Parents of individuals with ADHD were at 2.42-fold increased risk of attempted suicide, compared with controls, and full siblings were at 2.28-fold increased risk. In contrast, the risk in half-siblings, while significantly greater than in controls, was lower than in the genetically closer first-degree relatives: Maternal half-siblings were at 1.57-fold increased risk, and paternal half-siblings were at 1.57-fold greater risk. Cousins were at 1.39-fold increased risk.
The same held true for completed suicide risk. And the familial associations remained significant even after excluding relatives with ADHD.
“Regarding the shared familial factors, I’m tempted to hypothesize that this might involve pleiotropic effects reflecting genetic variants associated with impulsivity,” said Dr. Larsson. To further understand the biological mechanisms underlying ADHD and associated adverse health outcomes requires multiple disciplines to work together. For such work, Dr. Larsson collaborates with international colleagues in several consortia.
ADHD medications and suicidality
Concerns regarding this question were raised by a meta-analysis based on clinical trials data that suggested patients’ increased suicidality might be caused by the effects of ADHD medications. But the meta-analysis was seriously flawed by what epidemiologists call confounding by indication, which is the potential for bias to be introduced when a group of patients on medication is compared with another group off medication. The confounding results from the fact that ADHD patients on medication are different from those who aren’t: They are likely to be more symptomatic and have more comorbidities.
To bypass the confounding issue, Dr. Larsson and his coinvestigators turned to the Swedish registries and identified 37,936 patients with ADHD with a total of 7,019 suicide-related events during nearly 151,000 person-years of follow-up. When they compared patients on drug treatment with those who were not, they found – as in the other investigators’ meta-analysis – that drug treatment was associated with a statistically significant 1.31-fold increased risk of suicide-related events. However, when they performed a more appropriate between-individual analysis, Dr. Larsson and his colleagues found that, when patients with ADHD were using stimulant medications, they had a significant 19% lower risk of suicide-related events than when they were off medication. While on nonstimulant ADHD medications, their suicidality risk was no different from when off medication (BMJ. 2014 Jun 18;348:g3769. doi: 10.1136/bmj.g3769).
ADHD and early mortality risk
Prior studies have established that ADHD is associated with a proclivity to engage in risk-taking behaviors, including substance abuse, criminality, risky sexual behavior, and accidents, which are themselves associated with early mortality.
Sure enough, when Danish investigators turned to their national registries, identified 32,061 individuals with ADHD born during 1981-2011, and followed them through 2013, they found that, during nearly 25 million person-years of follow-up, the mortality rate was 5.85 deaths per 10,000 person-years in individuals with ADHD, compared with 2.21 deaths per 10,000 person-years in controls, resulting in a fully adjusted mortality rate ratio of 2.07. The rate ratio was 1.86 for ADHD patients under age 6 years, 1.58 in those aged 6-17 years, and 4.25 for patients aged 18 years and older (Lancet. 2015 May 30;385[9983]:2190-6).
Accidents were the most common cause of death. Could ADHD medications modify this risk of fatal accidents?
Serious motor vehicle accidents
Dr. Larsson and his coinvestigators used registry data to follow 17,408 Swedish adults with ADHD for serious transport accidents involving a trip to the emergency room or death during 2006-2009. The risk was increased by an adjusted 1.47-fold in men with ADHD and by 1.45-fold in women with the disorder. However, in the within-individual analysis, men were 58% less likely to have a serious transport accident when they were on ADHD medication than when off medication. There was no statistically significant effect of ADHD medications on the risk in women with ADHD.
The investigators estimated that 41%-49% of transport accidents in men with ADHD could have been avoided had they been on drug therapy continuously throughout the follow-up period (JAMA Psychiatry. 2014 Mar;71[3]:319-25).
Similar results – that is, data showing that being on ADHD medication reduces the elevated risk of serious accidents – have been reported in four other independent studies conducted in Denmark, Germany, Hong Kong, and most recently in a U.S. analysis by Dr. Larsson and coinvestigators of more than 2.3 million patients with ADHD in a U.S. commercial health insurance claims database (JAMA Psychiatry. 2017 Jun 1;74[6]:597-603).
These findings collectively highlight the public health importance of diagnosing and treating ADHD.
But Dr. Larsson wanted his audience to take home another key lesson: “ADHD is a disorder that can be associated with serious outcomes, including suicide and accidents. It’s nevertheless important to remember that the absolute risks here are very low for any of these outcomes, so the majority of individuals with ADHD will never suffer from any of these outcomes. It’s important to keep that in mind.”
Dr. Larsson’s research is funded by the Swedish Research Council, the National Institute of Mental Health, FORTE, Horizon 2020, and Shire.
[email protected]
expert analysis from THE ECNP CONGRESS
Methamphetamine-induced psychosis: Who says all drug use is reversible?
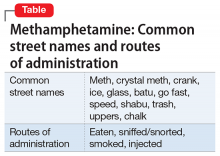
Use of methamphetamine, an N-methyl analog of amphetamine, is a serious public health problem; throughout the world an estimated 35.7 million people use the drug recreationally.1 Methamphetamine is easy to obtain because it is cheap to produce and can be synthesized anywhere. In the United States, methamphetamine is commonly manufactured in small-scale laboratories using relatively inexpensive, legally available ingredients. Large-scale manufacturing in clandestine laboratories also contributes to methamphetamine abuse. The drug, known as meth, crystal meth, ice, and other names, is available as a powder, tablet, or crystalline salt, and is used by various routes of administration (Table).
Although FDA-approved for treating attention-deficit/hyperactivity disorder, methamphetamine is taken recreationally for its euphoric effects; however, it also produces anhedonia, paranoia, and a host of cognitive deficits and other adverse effects.
Methamphetamine causes psychiatric diseases that resemble naturally occurring illnesses but are more difficult to treat. Dependence occurs over a period of escalating use (Figure). Long-term exposure to the drug has been shown to cause severe neurotoxic and neuropathological effects with consequent disturbances in several cognitive domains.4
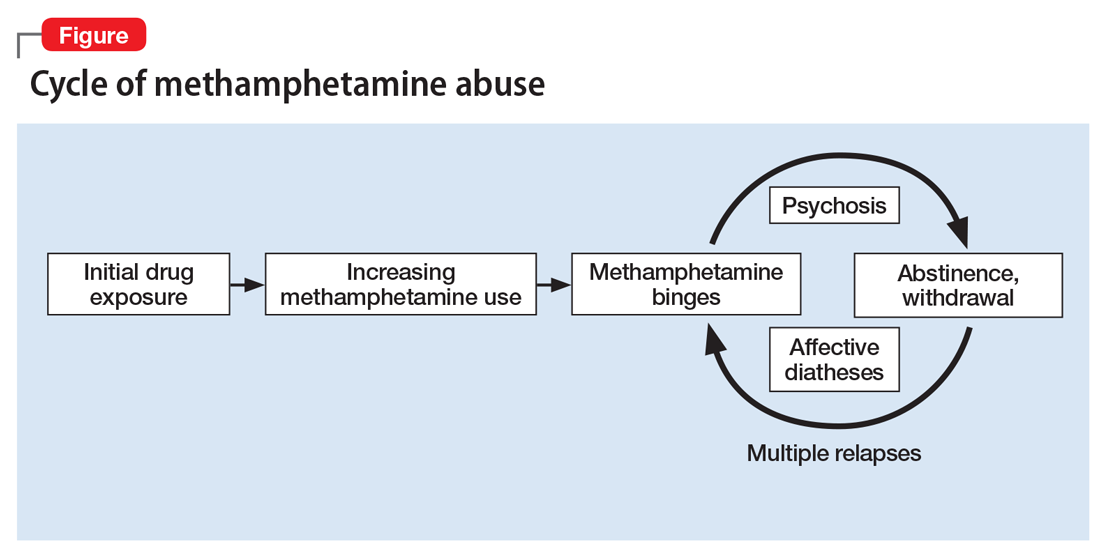
Despite advances in understanding the basic neurobiology of methamphetamine-induced effects on the brain, much remains to be done to translate this knowledge to treating patients and the complications that result from chronic abuse of this stimulant. In this review, we:
- provide a brief synopsis of the clinical presentation of patients who use methamphetamine
- describe some of the complications of methamphetamine abuse/dependence, focusing on methamphetamine-induced psychosis
- suggest ways to approach the treatment of these patients, including those with methamphetamine-induced psychosis.
Acute effects of methamphetamine use
Psychiatric symptoms. Patients under the influence of methamphetamine may present with clinical symptoms that mimic psychiatric disorders. For example, the drug can cause marked euphoria, hyperactivity, and disturbed speech patterns, thus mimicking a manic state. Patients also may present with anxiety, agitation, and irritability or aggressiveness. Although an individual may take methamphetamine for sexual enhancement, the drug can cause hypersexuality, which often is associated with unintended and unsafe sexual activities. These signs and symptoms are exacerbated during drug binges that can last for days, during which time large quantities of the drug are consumed.
Methamphetamine users may become preoccupied with their own thought patterns, and their actions can become compulsive and nonsensical. For example, a patient may become obsessed with an object of no specific value in his (her) environment, such as a doorknob or a cloud. Patients also may become suspicious of their friends and family members or think that police officers are after them. Less commonly, a patient also may suffer from poverty of speech, psychomotor retardation, and diminished social engagement similar to that reported in some patients with schizophrenia with deficit syndrome. Usually, acute symptoms will last 4 to 7 days after drug cessation, and then resolve completely with protracted abstinence from the drug.
Neurologic signs of methamphetamine use include hemorrhagic strokes in young people without any evidence of previous neurologic impairments. Studies have documented similarities between methamphetamine-induced neurotoxicity and traumatic brain injury.5 Postmortem studies have reported the presence of arteriovenous malformation in some patients with hemorrhagic strokes.
Hyperthermia is a dangerous acute effect of methamphetamine use. High body temperatures can cause both peripheral and central abnormalities, including muscular and cardiovascular dysfunction, renal failure secondary to rhabdomyolysis, heat stroke, and other heat-induced malignant syndromes. Some of the central dysfunctions may be related to heat-induced production of free radicals in various brain regions. There are no pharmacologic treatments for methamphetamine-induced thermal dysregulation.6 Therefore, clinicians need to focus on reducing body temperature by using cooling fans or cold water baths. Efforts should be made to avoid overhydrating patients because of the risk of developing the syndrome of inappropriate antidiuretic hormone secretion.
Chronic methamphetamine abuse
Psychosis is a long-term complication of chronic abuse of the drug.7 Although psychosis has been a reported complication of methamphetamine use since the 1950s,8 most of the subsequent literature is from Japan, where methamphetamine use was highly prevalent after World War II.9,10 The prevalence of methamphetamine-induced psychosis in methamphetamine-dependent patients varies from 13% (in the United States11) to 50% (in Asia12). This difference might be related to variability in the purity of methamphetamine used in different locations.
Methamphetamine users may experience a pre-psychotic state that consists of ideas of reference and delusional moods. This is followed by a psychotic state that includes hallucinations and delusions. The time it takes to develop these symptoms can vary from a few months up to >20 years after starting to use methamphetamine.10,13 Psychosis can occur in patients who do not have a history of psychiatric illness.10
The clinical presentation of methamphetamine-induced psychosis includes delusions of reference and persecutions.8-10 Paranoid delusions may be accompanied by violent behavior. Some patients may present with grandiose or jealousy delusions. Patients may experience auditory, tactile, or visual hallucinations. They may exhibit mania and logorrheic verbal outputs, symptoms consistent with a diagnosis of methamphetamine-induced mood disorder with manic features. Patients who use large daily doses of the drug also may report that there are ants or other parasites crawling under their skin (eg, formication, “meth mites”) and might present with infected excoriations of their skin as a result of attempting to remove insects. This is clinically important because penicillin-resistant bacteria are common in patients who use methamphetamine, and strains tend to be virulent.
Psychotic symptoms can last from a few days to several weeks after stopping methamphetamine use, although methamphetamine-induced psychosis can persist after long periods of abstinence.14 Psychotic symptoms may recur with re-exposure to the drug9 or repeated stressful life events.15 Patients with recurrent psychosis in the absence of a drug trigger appear to have high levels of peripheral norepinephrine.15 Patients with psychosis caused by long-term methamphetamine use will not necessarily show signs of sympathomimetic dysfunction because they may not have any methamphetamine in the body when they first present for clinical evaluation. Importantly, patients with methamphetamine-induced psychosis have been reported to have poor outcomes at follow-up.16 They have an increased risk of suicide, recurrent drug-induced psychosis, and comorbid alcohol abuse.16
Doses required to induce psychosis vary from patient to patient and may depend on the patient’s genetic background and/or environmental conditions. Methamphetamine can increase the severity of many psychiatric symptoms17 and may expedite the development of schizophrenia in first-degree relatives of patients with schizophrenia.18
The diagnosis of methamphetamine-induced psychosis should focus on differentiating it from schizophrenia. Wang et al19 found similar patterns of delusions in patients with schizophrenia and those with methamphetamine-induced psychosis. However, compared with patients with schizophrenia, patients with methamphetamine-induced psychosis have a higher prevalence of visual and tactile hallucinations, and less disorganization, blunted affect, and motor retardation. Some patients may present with depression and suicidal ideation; these features may be more prominent during withdrawal, but also may be obvious during periods of active use.16
Although these clinical features may be helpful initially, more comparative neurobiologic investigations are needed to identify potential biologic differences between schizophrenia and methamphetamine-induced psychosis because these differences will impact therapeutic approaches to these diverse population groups.
Neurologic complications. Chronic methamphetamine users may develop various neurologic disorders.20 They may present with stereotypies involving finger movements or repeated rubbing of mouth or face, orofacial dyskinesia, and choreoathetoid movements reminiscent of classical neurologic disorders. These movement disorders can persist after cessation of methamphetamine use. In some cases, these movement abnormalities may respond to dopamine receptor antagonists such as haloperidol.
Neuropsychological findings. Chronic methamphetamine users show mild signs of cognitive decline that affects a broad range of neuropsychological functions.21-23 There are deficits in several cognitive processes that are dependent on the function of frontostriatal and limbic circuits.24-26 Specifically, episodic memory, executive functions, complex information processing speed, and psychomotor functions all have been reported to be negatively impacted.
Methamphetamine use often results in psychiatric distress that impacts users’ interpersonal relationships.27 Additionally, impulsivity may exacerbate their psychosocial difficulties and promote maintenance of drug-seeking behaviors.28 Cognitive deficits lead to poor health outcomes, high-risk behaviors, employment difficulties, and repeated relapse.29,30
Partial recovery of neuropsychological functioning and improvement in affective distress can be achieved after sustained abstinence from methamphetamine, but recovery may not be complete. Because cognitive dysfunction can influence treatment outcomes, clinicians need to be fully aware of the cognitive status of those patients, and a thorough neuropsychological evaluation is necessary before initiating treatment.
Treatment
Methamphetamine abuse. Because patients who abuse methamphetamine are at high risk of developing psychosis, neurologic complications, and neuropsychological disorders, initiating treatment early in the course of their addiction is of paramount importance. Treatment of methamphetamine addiction is complicated by the fact that these patients have a high prevalence of comorbid psychiatric disorders, which clinicians need to keep in mind when selecting therapeutic interventions.
There are no FDA-approved agents for treating methamphetamine abuse.31 Several drugs have been tried with varying degrees of success, including bupropion, modafinil, and naltrexone. A study of modafinil found no clinically significant effects for treating methamphetamine abuse; however, only approximately one-half of participants in this study took modafinil as instructed.32 Certain selective serotonin reuptake inhibitors, including fluoxetine and paroxetine, have not been shown to be effective in treating these patients. Naltrexone may be a reasonable medication to consider because of the high prevalence of comorbid alcohol abuse among methamphetamine users.
Other treatments for methamphetamine addiction consist of behavioral interventions such as cognitive-behavioral therapy. Clinical experience has shown that the risk of relapse depends on how long the patient has been abstinent prior to entering a treatment program, the presence of attention and memory deficits, and findings of poor decision-making on neuropsychological tests.
The presence of cognitive abnormalities has been reported to impact methamphetamine abusers’ response to treatment.33 These findings suggest the need to develop approaches that might improve cognition in patients who are undergoing treatment for methamphetamine abuse. The monoaminergic agent modafinil and similar drugs need to be evaluated in large populations to increase the possibility of identifying characteristics of patients who might respond to cognitive enhancement.34
Methamphetamine-induced psychosis. First-generation antipsychotics, such as haloperidol or fluphenazine, need to be used sparingly in patients with methamphetamine-induced psychosis because of the risk of developing extrapyramidal symptoms (EPS) and because these patients are prone to develop motor complications as a result of methamphetamine abuse. Second-generation antipsychotics, such as risperidone and olanzapine, may be more appropriate because of the lower risks of EPS.35 The presence of high norepinephrine levels in some patients with recurrent methamphetamine psychosis suggests that drugs that block norepinephrine receptors, such as prazosin or propranolol, might be of therapeutic benefit if they are shown to be effective in controlled clinical trials.
1. United Nations Office on Drugs and Crime. World Drug Report 2016. United Nations publication, Sales No. E.16.XI.7. http://www.unodc.org/wdr2016. Published 2016. Accessed September 28, 2017.
2. Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379-407.
3. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760-773.
4. Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127(1):91-107.
5. Gold MS, Kobeissy FH, Wang KK, et al. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol Psychiatry. 2009;66(2):118-127.
6. Gold MS, Graham NA, Kobeissy FH, et al. Speed, cocaine, and other psychostimulants death rates. Am J Cardiol. 2007;100(7):1184.
7. Shelly J, Uhlmann A, Sinclair H, et al. First-rank symptoms in methamphetamine psychosis and schizophrenia. Psychopathology. 2016;49(6):429-435.
8. Connell PH. Amphetamine psychosis. In: Connell PH. Maudsley monographs. No. 5. London, United Kingdom: Oxford Press; 1958:5.
9. Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci. 1992;654(1):160-170.
10. Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci. 2004;1025(1):279-287.
11. Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, et al; Methamphetamine Treatment Project Corporate Authors. Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug Alcohol Rev. 2010;29(1):12-20.
12. Sulaiman AH, Said MA, Habil MH, et al. The risk and associated factors of methamphetamine psychosis in methamphetamine-dependent patients in Malaysia. Compr Psychiatry. 2014;55(suppl 1):S89-S94.
13. Fasihpour B, Molavi S, Shariat SV. Clinical features of inpatients with methamphetamine-induced psychosis. J Ment Health. 2013;22(4):341-349.
14. Akiyama K, Saito A, Shimoda K. Chronic methamphetamine psychosis after long-term abstinence in Japanese incarcerated patients. Am J Addict. 2011;20(3):240-249.
15. Yui K, Goto K, Ikemoto S, et al. Methamphetamine psychosis: spontaneous recurrence of paranoid-hallucinatory states and monoamine neurotransmitter function. J Clin Psychopharmacol. 1997;17(1):34-43.
16. Kittirattanapaiboon P, Mahatnirunkul S, Booncharoen H, et al. Long-term outcomes in methamphetamine psychosis patients after first hospitalisation. Drug Alcohol Rev. 2010;29(4):456-461.
17. McKetin R, Dawe S, Burns RA, et al. The profile of psychiatric symptoms exacerbated by methamphetamine use. Drug Alcohol Depend. 2016;161:104-109.
18. Li H, Lu Q, Xiao E, et al. Methamphetamine enhances the development of schizophrenia in first-degree relatives of patients with schizophrenia. Can J Psychiatry. 2014;59(2):107-113.
19. Wang LJ, Lin SK, Chen YC, et al. Differences in clinical features of methamphetamine users with persistent psychosis and patients with schizophrenia. Psychopathology. 2016;49(2):108-115.
20. Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin North Am. 2013;36(2):261-275.
21. Simon SL, Domier C, Carnell J, et al. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9(3):222-231.
22. Paulus MP, Hozack NE, Zauscher BE, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26(1):53-63.
23. Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology (Berl). 2009;203(3):609-616.
24. Monterosso JR, Ainslie G, Xu J, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28(5):383-393.
25. Nestor LJ, Ghahremani DG, Monterosso J, et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194(3):287-295.
26. Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275-297.
27. Cretzmeyer M, Sarrazin MV, Huber DL, et al. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat. 2003;24(3):267-277.
28. Semple SJ, Zians J, Grant I, et al. Impulsivity and methamphetamine use. J Subst Abuse Treat. 2005;29(2):85-93.
29. Hester R, Lee N, Pennay A, et al. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol. 2010;18(6):489-497.
30. Weber E, Blackstone K, Iudicello JE, et al; Translational Methamphetamine AIDS Research Center (TMARC) Group. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125(1-2):146-153.
31. Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol. 2017;10(3):305-314.
32. Anderson AL, Li SH, Biswas K, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120(1-3):135-141.
33. Cadet JL, Bisagno V. Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry. 2016;6:189.
34. Loland CJ, Mereu M, Okunola OM, et al. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72(5):405-413.
35. Farnia V, Shakeri J, Tatari F, et al. Randomized controlled trial of aripiprazole versus risperidone for the treatment of amphetamine-induced psychosis. Am J Drug Alcohol Abuse. 2014;40(1):10-15.
Use of methamphetamine, an N-methyl analog of amphetamine, is a serious public health problem; throughout the world an estimated 35.7 million people use the drug recreationally.1 Methamphetamine is easy to obtain because it is cheap to produce and can be synthesized anywhere. In the United States, methamphetamine is commonly manufactured in small-scale laboratories using relatively inexpensive, legally available ingredients. Large-scale manufacturing in clandestine laboratories also contributes to methamphetamine abuse. The drug, known as meth, crystal meth, ice, and other names, is available as a powder, tablet, or crystalline salt, and is used by various routes of administration (Table).
Although FDA-approved for treating attention-deficit/hyperactivity disorder, methamphetamine is taken recreationally for its euphoric effects; however, it also produces anhedonia, paranoia, and a host of cognitive deficits and other adverse effects.
Methamphetamine causes psychiatric diseases that resemble naturally occurring illnesses but are more difficult to treat. Dependence occurs over a period of escalating use (Figure). Long-term exposure to the drug has been shown to cause severe neurotoxic and neuropathological effects with consequent disturbances in several cognitive domains.4

Despite advances in understanding the basic neurobiology of methamphetamine-induced effects on the brain, much remains to be done to translate this knowledge to treating patients and the complications that result from chronic abuse of this stimulant. In this review, we:
- provide a brief synopsis of the clinical presentation of patients who use methamphetamine
- describe some of the complications of methamphetamine abuse/dependence, focusing on methamphetamine-induced psychosis
- suggest ways to approach the treatment of these patients, including those with methamphetamine-induced psychosis.
Acute effects of methamphetamine use
Psychiatric symptoms. Patients under the influence of methamphetamine may present with clinical symptoms that mimic psychiatric disorders. For example, the drug can cause marked euphoria, hyperactivity, and disturbed speech patterns, thus mimicking a manic state. Patients also may present with anxiety, agitation, and irritability or aggressiveness. Although an individual may take methamphetamine for sexual enhancement, the drug can cause hypersexuality, which often is associated with unintended and unsafe sexual activities. These signs and symptoms are exacerbated during drug binges that can last for days, during which time large quantities of the drug are consumed.
Methamphetamine users may become preoccupied with their own thought patterns, and their actions can become compulsive and nonsensical. For example, a patient may become obsessed with an object of no specific value in his (her) environment, such as a doorknob or a cloud. Patients also may become suspicious of their friends and family members or think that police officers are after them. Less commonly, a patient also may suffer from poverty of speech, psychomotor retardation, and diminished social engagement similar to that reported in some patients with schizophrenia with deficit syndrome. Usually, acute symptoms will last 4 to 7 days after drug cessation, and then resolve completely with protracted abstinence from the drug.
Neurologic signs of methamphetamine use include hemorrhagic strokes in young people without any evidence of previous neurologic impairments. Studies have documented similarities between methamphetamine-induced neurotoxicity and traumatic brain injury.5 Postmortem studies have reported the presence of arteriovenous malformation in some patients with hemorrhagic strokes.
Hyperthermia is a dangerous acute effect of methamphetamine use. High body temperatures can cause both peripheral and central abnormalities, including muscular and cardiovascular dysfunction, renal failure secondary to rhabdomyolysis, heat stroke, and other heat-induced malignant syndromes. Some of the central dysfunctions may be related to heat-induced production of free radicals in various brain regions. There are no pharmacologic treatments for methamphetamine-induced thermal dysregulation.6 Therefore, clinicians need to focus on reducing body temperature by using cooling fans or cold water baths. Efforts should be made to avoid overhydrating patients because of the risk of developing the syndrome of inappropriate antidiuretic hormone secretion.
Chronic methamphetamine abuse
Psychosis is a long-term complication of chronic abuse of the drug.7 Although psychosis has been a reported complication of methamphetamine use since the 1950s,8 most of the subsequent literature is from Japan, where methamphetamine use was highly prevalent after World War II.9,10 The prevalence of methamphetamine-induced psychosis in methamphetamine-dependent patients varies from 13% (in the United States11) to 50% (in Asia12). This difference might be related to variability in the purity of methamphetamine used in different locations.
Methamphetamine users may experience a pre-psychotic state that consists of ideas of reference and delusional moods. This is followed by a psychotic state that includes hallucinations and delusions. The time it takes to develop these symptoms can vary from a few months up to >20 years after starting to use methamphetamine.10,13 Psychosis can occur in patients who do not have a history of psychiatric illness.10
The clinical presentation of methamphetamine-induced psychosis includes delusions of reference and persecutions.8-10 Paranoid delusions may be accompanied by violent behavior. Some patients may present with grandiose or jealousy delusions. Patients may experience auditory, tactile, or visual hallucinations. They may exhibit mania and logorrheic verbal outputs, symptoms consistent with a diagnosis of methamphetamine-induced mood disorder with manic features. Patients who use large daily doses of the drug also may report that there are ants or other parasites crawling under their skin (eg, formication, “meth mites”) and might present with infected excoriations of their skin as a result of attempting to remove insects. This is clinically important because penicillin-resistant bacteria are common in patients who use methamphetamine, and strains tend to be virulent.
Psychotic symptoms can last from a few days to several weeks after stopping methamphetamine use, although methamphetamine-induced psychosis can persist after long periods of abstinence.14 Psychotic symptoms may recur with re-exposure to the drug9 or repeated stressful life events.15 Patients with recurrent psychosis in the absence of a drug trigger appear to have high levels of peripheral norepinephrine.15 Patients with psychosis caused by long-term methamphetamine use will not necessarily show signs of sympathomimetic dysfunction because they may not have any methamphetamine in the body when they first present for clinical evaluation. Importantly, patients with methamphetamine-induced psychosis have been reported to have poor outcomes at follow-up.16 They have an increased risk of suicide, recurrent drug-induced psychosis, and comorbid alcohol abuse.16
Doses required to induce psychosis vary from patient to patient and may depend on the patient’s genetic background and/or environmental conditions. Methamphetamine can increase the severity of many psychiatric symptoms17 and may expedite the development of schizophrenia in first-degree relatives of patients with schizophrenia.18
The diagnosis of methamphetamine-induced psychosis should focus on differentiating it from schizophrenia. Wang et al19 found similar patterns of delusions in patients with schizophrenia and those with methamphetamine-induced psychosis. However, compared with patients with schizophrenia, patients with methamphetamine-induced psychosis have a higher prevalence of visual and tactile hallucinations, and less disorganization, blunted affect, and motor retardation. Some patients may present with depression and suicidal ideation; these features may be more prominent during withdrawal, but also may be obvious during periods of active use.16
Although these clinical features may be helpful initially, more comparative neurobiologic investigations are needed to identify potential biologic differences between schizophrenia and methamphetamine-induced psychosis because these differences will impact therapeutic approaches to these diverse population groups.
Neurologic complications. Chronic methamphetamine users may develop various neurologic disorders.20 They may present with stereotypies involving finger movements or repeated rubbing of mouth or face, orofacial dyskinesia, and choreoathetoid movements reminiscent of classical neurologic disorders. These movement disorders can persist after cessation of methamphetamine use. In some cases, these movement abnormalities may respond to dopamine receptor antagonists such as haloperidol.
Neuropsychological findings. Chronic methamphetamine users show mild signs of cognitive decline that affects a broad range of neuropsychological functions.21-23 There are deficits in several cognitive processes that are dependent on the function of frontostriatal and limbic circuits.24-26 Specifically, episodic memory, executive functions, complex information processing speed, and psychomotor functions all have been reported to be negatively impacted.
Methamphetamine use often results in psychiatric distress that impacts users’ interpersonal relationships.27 Additionally, impulsivity may exacerbate their psychosocial difficulties and promote maintenance of drug-seeking behaviors.28 Cognitive deficits lead to poor health outcomes, high-risk behaviors, employment difficulties, and repeated relapse.29,30
Partial recovery of neuropsychological functioning and improvement in affective distress can be achieved after sustained abstinence from methamphetamine, but recovery may not be complete. Because cognitive dysfunction can influence treatment outcomes, clinicians need to be fully aware of the cognitive status of those patients, and a thorough neuropsychological evaluation is necessary before initiating treatment.
Treatment
Methamphetamine abuse. Because patients who abuse methamphetamine are at high risk of developing psychosis, neurologic complications, and neuropsychological disorders, initiating treatment early in the course of their addiction is of paramount importance. Treatment of methamphetamine addiction is complicated by the fact that these patients have a high prevalence of comorbid psychiatric disorders, which clinicians need to keep in mind when selecting therapeutic interventions.
There are no FDA-approved agents for treating methamphetamine abuse.31 Several drugs have been tried with varying degrees of success, including bupropion, modafinil, and naltrexone. A study of modafinil found no clinically significant effects for treating methamphetamine abuse; however, only approximately one-half of participants in this study took modafinil as instructed.32 Certain selective serotonin reuptake inhibitors, including fluoxetine and paroxetine, have not been shown to be effective in treating these patients. Naltrexone may be a reasonable medication to consider because of the high prevalence of comorbid alcohol abuse among methamphetamine users.
Other treatments for methamphetamine addiction consist of behavioral interventions such as cognitive-behavioral therapy. Clinical experience has shown that the risk of relapse depends on how long the patient has been abstinent prior to entering a treatment program, the presence of attention and memory deficits, and findings of poor decision-making on neuropsychological tests.
The presence of cognitive abnormalities has been reported to impact methamphetamine abusers’ response to treatment.33 These findings suggest the need to develop approaches that might improve cognition in patients who are undergoing treatment for methamphetamine abuse. The monoaminergic agent modafinil and similar drugs need to be evaluated in large populations to increase the possibility of identifying characteristics of patients who might respond to cognitive enhancement.34
Methamphetamine-induced psychosis. First-generation antipsychotics, such as haloperidol or fluphenazine, need to be used sparingly in patients with methamphetamine-induced psychosis because of the risk of developing extrapyramidal symptoms (EPS) and because these patients are prone to develop motor complications as a result of methamphetamine abuse. Second-generation antipsychotics, such as risperidone and olanzapine, may be more appropriate because of the lower risks of EPS.35 The presence of high norepinephrine levels in some patients with recurrent methamphetamine psychosis suggests that drugs that block norepinephrine receptors, such as prazosin or propranolol, might be of therapeutic benefit if they are shown to be effective in controlled clinical trials.
Use of methamphetamine, an N-methyl analog of amphetamine, is a serious public health problem; throughout the world an estimated 35.7 million people use the drug recreationally.1 Methamphetamine is easy to obtain because it is cheap to produce and can be synthesized anywhere. In the United States, methamphetamine is commonly manufactured in small-scale laboratories using relatively inexpensive, legally available ingredients. Large-scale manufacturing in clandestine laboratories also contributes to methamphetamine abuse. The drug, known as meth, crystal meth, ice, and other names, is available as a powder, tablet, or crystalline salt, and is used by various routes of administration (Table).
Although FDA-approved for treating attention-deficit/hyperactivity disorder, methamphetamine is taken recreationally for its euphoric effects; however, it also produces anhedonia, paranoia, and a host of cognitive deficits and other adverse effects.
Methamphetamine causes psychiatric diseases that resemble naturally occurring illnesses but are more difficult to treat. Dependence occurs over a period of escalating use (Figure). Long-term exposure to the drug has been shown to cause severe neurotoxic and neuropathological effects with consequent disturbances in several cognitive domains.4

Despite advances in understanding the basic neurobiology of methamphetamine-induced effects on the brain, much remains to be done to translate this knowledge to treating patients and the complications that result from chronic abuse of this stimulant. In this review, we:
- provide a brief synopsis of the clinical presentation of patients who use methamphetamine
- describe some of the complications of methamphetamine abuse/dependence, focusing on methamphetamine-induced psychosis
- suggest ways to approach the treatment of these patients, including those with methamphetamine-induced psychosis.
Acute effects of methamphetamine use
Psychiatric symptoms. Patients under the influence of methamphetamine may present with clinical symptoms that mimic psychiatric disorders. For example, the drug can cause marked euphoria, hyperactivity, and disturbed speech patterns, thus mimicking a manic state. Patients also may present with anxiety, agitation, and irritability or aggressiveness. Although an individual may take methamphetamine for sexual enhancement, the drug can cause hypersexuality, which often is associated with unintended and unsafe sexual activities. These signs and symptoms are exacerbated during drug binges that can last for days, during which time large quantities of the drug are consumed.
Methamphetamine users may become preoccupied with their own thought patterns, and their actions can become compulsive and nonsensical. For example, a patient may become obsessed with an object of no specific value in his (her) environment, such as a doorknob or a cloud. Patients also may become suspicious of their friends and family members or think that police officers are after them. Less commonly, a patient also may suffer from poverty of speech, psychomotor retardation, and diminished social engagement similar to that reported in some patients with schizophrenia with deficit syndrome. Usually, acute symptoms will last 4 to 7 days after drug cessation, and then resolve completely with protracted abstinence from the drug.
Neurologic signs of methamphetamine use include hemorrhagic strokes in young people without any evidence of previous neurologic impairments. Studies have documented similarities between methamphetamine-induced neurotoxicity and traumatic brain injury.5 Postmortem studies have reported the presence of arteriovenous malformation in some patients with hemorrhagic strokes.
Hyperthermia is a dangerous acute effect of methamphetamine use. High body temperatures can cause both peripheral and central abnormalities, including muscular and cardiovascular dysfunction, renal failure secondary to rhabdomyolysis, heat stroke, and other heat-induced malignant syndromes. Some of the central dysfunctions may be related to heat-induced production of free radicals in various brain regions. There are no pharmacologic treatments for methamphetamine-induced thermal dysregulation.6 Therefore, clinicians need to focus on reducing body temperature by using cooling fans or cold water baths. Efforts should be made to avoid overhydrating patients because of the risk of developing the syndrome of inappropriate antidiuretic hormone secretion.
Chronic methamphetamine abuse
Psychosis is a long-term complication of chronic abuse of the drug.7 Although psychosis has been a reported complication of methamphetamine use since the 1950s,8 most of the subsequent literature is from Japan, where methamphetamine use was highly prevalent after World War II.9,10 The prevalence of methamphetamine-induced psychosis in methamphetamine-dependent patients varies from 13% (in the United States11) to 50% (in Asia12). This difference might be related to variability in the purity of methamphetamine used in different locations.
Methamphetamine users may experience a pre-psychotic state that consists of ideas of reference and delusional moods. This is followed by a psychotic state that includes hallucinations and delusions. The time it takes to develop these symptoms can vary from a few months up to >20 years after starting to use methamphetamine.10,13 Psychosis can occur in patients who do not have a history of psychiatric illness.10
The clinical presentation of methamphetamine-induced psychosis includes delusions of reference and persecutions.8-10 Paranoid delusions may be accompanied by violent behavior. Some patients may present with grandiose or jealousy delusions. Patients may experience auditory, tactile, or visual hallucinations. They may exhibit mania and logorrheic verbal outputs, symptoms consistent with a diagnosis of methamphetamine-induced mood disorder with manic features. Patients who use large daily doses of the drug also may report that there are ants or other parasites crawling under their skin (eg, formication, “meth mites”) and might present with infected excoriations of their skin as a result of attempting to remove insects. This is clinically important because penicillin-resistant bacteria are common in patients who use methamphetamine, and strains tend to be virulent.
Psychotic symptoms can last from a few days to several weeks after stopping methamphetamine use, although methamphetamine-induced psychosis can persist after long periods of abstinence.14 Psychotic symptoms may recur with re-exposure to the drug9 or repeated stressful life events.15 Patients with recurrent psychosis in the absence of a drug trigger appear to have high levels of peripheral norepinephrine.15 Patients with psychosis caused by long-term methamphetamine use will not necessarily show signs of sympathomimetic dysfunction because they may not have any methamphetamine in the body when they first present for clinical evaluation. Importantly, patients with methamphetamine-induced psychosis have been reported to have poor outcomes at follow-up.16 They have an increased risk of suicide, recurrent drug-induced psychosis, and comorbid alcohol abuse.16
Doses required to induce psychosis vary from patient to patient and may depend on the patient’s genetic background and/or environmental conditions. Methamphetamine can increase the severity of many psychiatric symptoms17 and may expedite the development of schizophrenia in first-degree relatives of patients with schizophrenia.18
The diagnosis of methamphetamine-induced psychosis should focus on differentiating it from schizophrenia. Wang et al19 found similar patterns of delusions in patients with schizophrenia and those with methamphetamine-induced psychosis. However, compared with patients with schizophrenia, patients with methamphetamine-induced psychosis have a higher prevalence of visual and tactile hallucinations, and less disorganization, blunted affect, and motor retardation. Some patients may present with depression and suicidal ideation; these features may be more prominent during withdrawal, but also may be obvious during periods of active use.16
Although these clinical features may be helpful initially, more comparative neurobiologic investigations are needed to identify potential biologic differences between schizophrenia and methamphetamine-induced psychosis because these differences will impact therapeutic approaches to these diverse population groups.
Neurologic complications. Chronic methamphetamine users may develop various neurologic disorders.20 They may present with stereotypies involving finger movements or repeated rubbing of mouth or face, orofacial dyskinesia, and choreoathetoid movements reminiscent of classical neurologic disorders. These movement disorders can persist after cessation of methamphetamine use. In some cases, these movement abnormalities may respond to dopamine receptor antagonists such as haloperidol.
Neuropsychological findings. Chronic methamphetamine users show mild signs of cognitive decline that affects a broad range of neuropsychological functions.21-23 There are deficits in several cognitive processes that are dependent on the function of frontostriatal and limbic circuits.24-26 Specifically, episodic memory, executive functions, complex information processing speed, and psychomotor functions all have been reported to be negatively impacted.
Methamphetamine use often results in psychiatric distress that impacts users’ interpersonal relationships.27 Additionally, impulsivity may exacerbate their psychosocial difficulties and promote maintenance of drug-seeking behaviors.28 Cognitive deficits lead to poor health outcomes, high-risk behaviors, employment difficulties, and repeated relapse.29,30
Partial recovery of neuropsychological functioning and improvement in affective distress can be achieved after sustained abstinence from methamphetamine, but recovery may not be complete. Because cognitive dysfunction can influence treatment outcomes, clinicians need to be fully aware of the cognitive status of those patients, and a thorough neuropsychological evaluation is necessary before initiating treatment.
Treatment
Methamphetamine abuse. Because patients who abuse methamphetamine are at high risk of developing psychosis, neurologic complications, and neuropsychological disorders, initiating treatment early in the course of their addiction is of paramount importance. Treatment of methamphetamine addiction is complicated by the fact that these patients have a high prevalence of comorbid psychiatric disorders, which clinicians need to keep in mind when selecting therapeutic interventions.
There are no FDA-approved agents for treating methamphetamine abuse.31 Several drugs have been tried with varying degrees of success, including bupropion, modafinil, and naltrexone. A study of modafinil found no clinically significant effects for treating methamphetamine abuse; however, only approximately one-half of participants in this study took modafinil as instructed.32 Certain selective serotonin reuptake inhibitors, including fluoxetine and paroxetine, have not been shown to be effective in treating these patients. Naltrexone may be a reasonable medication to consider because of the high prevalence of comorbid alcohol abuse among methamphetamine users.
Other treatments for methamphetamine addiction consist of behavioral interventions such as cognitive-behavioral therapy. Clinical experience has shown that the risk of relapse depends on how long the patient has been abstinent prior to entering a treatment program, the presence of attention and memory deficits, and findings of poor decision-making on neuropsychological tests.
The presence of cognitive abnormalities has been reported to impact methamphetamine abusers’ response to treatment.33 These findings suggest the need to develop approaches that might improve cognition in patients who are undergoing treatment for methamphetamine abuse. The monoaminergic agent modafinil and similar drugs need to be evaluated in large populations to increase the possibility of identifying characteristics of patients who might respond to cognitive enhancement.34
Methamphetamine-induced psychosis. First-generation antipsychotics, such as haloperidol or fluphenazine, need to be used sparingly in patients with methamphetamine-induced psychosis because of the risk of developing extrapyramidal symptoms (EPS) and because these patients are prone to develop motor complications as a result of methamphetamine abuse. Second-generation antipsychotics, such as risperidone and olanzapine, may be more appropriate because of the lower risks of EPS.35 The presence of high norepinephrine levels in some patients with recurrent methamphetamine psychosis suggests that drugs that block norepinephrine receptors, such as prazosin or propranolol, might be of therapeutic benefit if they are shown to be effective in controlled clinical trials.
1. United Nations Office on Drugs and Crime. World Drug Report 2016. United Nations publication, Sales No. E.16.XI.7. http://www.unodc.org/wdr2016. Published 2016. Accessed September 28, 2017.
2. Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379-407.
3. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760-773.
4. Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127(1):91-107.
5. Gold MS, Kobeissy FH, Wang KK, et al. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol Psychiatry. 2009;66(2):118-127.
6. Gold MS, Graham NA, Kobeissy FH, et al. Speed, cocaine, and other psychostimulants death rates. Am J Cardiol. 2007;100(7):1184.
7. Shelly J, Uhlmann A, Sinclair H, et al. First-rank symptoms in methamphetamine psychosis and schizophrenia. Psychopathology. 2016;49(6):429-435.
8. Connell PH. Amphetamine psychosis. In: Connell PH. Maudsley monographs. No. 5. London, United Kingdom: Oxford Press; 1958:5.
9. Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci. 1992;654(1):160-170.
10. Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci. 2004;1025(1):279-287.
11. Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, et al; Methamphetamine Treatment Project Corporate Authors. Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug Alcohol Rev. 2010;29(1):12-20.
12. Sulaiman AH, Said MA, Habil MH, et al. The risk and associated factors of methamphetamine psychosis in methamphetamine-dependent patients in Malaysia. Compr Psychiatry. 2014;55(suppl 1):S89-S94.
13. Fasihpour B, Molavi S, Shariat SV. Clinical features of inpatients with methamphetamine-induced psychosis. J Ment Health. 2013;22(4):341-349.
14. Akiyama K, Saito A, Shimoda K. Chronic methamphetamine psychosis after long-term abstinence in Japanese incarcerated patients. Am J Addict. 2011;20(3):240-249.
15. Yui K, Goto K, Ikemoto S, et al. Methamphetamine psychosis: spontaneous recurrence of paranoid-hallucinatory states and monoamine neurotransmitter function. J Clin Psychopharmacol. 1997;17(1):34-43.
16. Kittirattanapaiboon P, Mahatnirunkul S, Booncharoen H, et al. Long-term outcomes in methamphetamine psychosis patients after first hospitalisation. Drug Alcohol Rev. 2010;29(4):456-461.
17. McKetin R, Dawe S, Burns RA, et al. The profile of psychiatric symptoms exacerbated by methamphetamine use. Drug Alcohol Depend. 2016;161:104-109.
18. Li H, Lu Q, Xiao E, et al. Methamphetamine enhances the development of schizophrenia in first-degree relatives of patients with schizophrenia. Can J Psychiatry. 2014;59(2):107-113.
19. Wang LJ, Lin SK, Chen YC, et al. Differences in clinical features of methamphetamine users with persistent psychosis and patients with schizophrenia. Psychopathology. 2016;49(2):108-115.
20. Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin North Am. 2013;36(2):261-275.
21. Simon SL, Domier C, Carnell J, et al. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9(3):222-231.
22. Paulus MP, Hozack NE, Zauscher BE, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26(1):53-63.
23. Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology (Berl). 2009;203(3):609-616.
24. Monterosso JR, Ainslie G, Xu J, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28(5):383-393.
25. Nestor LJ, Ghahremani DG, Monterosso J, et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194(3):287-295.
26. Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275-297.
27. Cretzmeyer M, Sarrazin MV, Huber DL, et al. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat. 2003;24(3):267-277.
28. Semple SJ, Zians J, Grant I, et al. Impulsivity and methamphetamine use. J Subst Abuse Treat. 2005;29(2):85-93.
29. Hester R, Lee N, Pennay A, et al. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol. 2010;18(6):489-497.
30. Weber E, Blackstone K, Iudicello JE, et al; Translational Methamphetamine AIDS Research Center (TMARC) Group. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125(1-2):146-153.
31. Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol. 2017;10(3):305-314.
32. Anderson AL, Li SH, Biswas K, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120(1-3):135-141.
33. Cadet JL, Bisagno V. Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry. 2016;6:189.
34. Loland CJ, Mereu M, Okunola OM, et al. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72(5):405-413.
35. Farnia V, Shakeri J, Tatari F, et al. Randomized controlled trial of aripiprazole versus risperidone for the treatment of amphetamine-induced psychosis. Am J Drug Alcohol Abuse. 2014;40(1):10-15.
1. United Nations Office on Drugs and Crime. World Drug Report 2016. United Nations publication, Sales No. E.16.XI.7. http://www.unodc.org/wdr2016. Published 2016. Accessed September 28, 2017.
2. Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379-407.
3. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760-773.
4. Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127(1):91-107.
5. Gold MS, Kobeissy FH, Wang KK, et al. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol Psychiatry. 2009;66(2):118-127.
6. Gold MS, Graham NA, Kobeissy FH, et al. Speed, cocaine, and other psychostimulants death rates. Am J Cardiol. 2007;100(7):1184.
7. Shelly J, Uhlmann A, Sinclair H, et al. First-rank symptoms in methamphetamine psychosis and schizophrenia. Psychopathology. 2016;49(6):429-435.
8. Connell PH. Amphetamine psychosis. In: Connell PH. Maudsley monographs. No. 5. London, United Kingdom: Oxford Press; 1958:5.
9. Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci. 1992;654(1):160-170.
10. Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci. 2004;1025(1):279-287.
11. Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, et al; Methamphetamine Treatment Project Corporate Authors. Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug Alcohol Rev. 2010;29(1):12-20.
12. Sulaiman AH, Said MA, Habil MH, et al. The risk and associated factors of methamphetamine psychosis in methamphetamine-dependent patients in Malaysia. Compr Psychiatry. 2014;55(suppl 1):S89-S94.
13. Fasihpour B, Molavi S, Shariat SV. Clinical features of inpatients with methamphetamine-induced psychosis. J Ment Health. 2013;22(4):341-349.
14. Akiyama K, Saito A, Shimoda K. Chronic methamphetamine psychosis after long-term abstinence in Japanese incarcerated patients. Am J Addict. 2011;20(3):240-249.
15. Yui K, Goto K, Ikemoto S, et al. Methamphetamine psychosis: spontaneous recurrence of paranoid-hallucinatory states and monoamine neurotransmitter function. J Clin Psychopharmacol. 1997;17(1):34-43.
16. Kittirattanapaiboon P, Mahatnirunkul S, Booncharoen H, et al. Long-term outcomes in methamphetamine psychosis patients after first hospitalisation. Drug Alcohol Rev. 2010;29(4):456-461.
17. McKetin R, Dawe S, Burns RA, et al. The profile of psychiatric symptoms exacerbated by methamphetamine use. Drug Alcohol Depend. 2016;161:104-109.
18. Li H, Lu Q, Xiao E, et al. Methamphetamine enhances the development of schizophrenia in first-degree relatives of patients with schizophrenia. Can J Psychiatry. 2014;59(2):107-113.
19. Wang LJ, Lin SK, Chen YC, et al. Differences in clinical features of methamphetamine users with persistent psychosis and patients with schizophrenia. Psychopathology. 2016;49(2):108-115.
20. Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin North Am. 2013;36(2):261-275.
21. Simon SL, Domier C, Carnell J, et al. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9(3):222-231.
22. Paulus MP, Hozack NE, Zauscher BE, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26(1):53-63.
23. Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology (Berl). 2009;203(3):609-616.
24. Monterosso JR, Ainslie G, Xu J, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28(5):383-393.
25. Nestor LJ, Ghahremani DG, Monterosso J, et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194(3):287-295.
26. Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275-297.
27. Cretzmeyer M, Sarrazin MV, Huber DL, et al. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat. 2003;24(3):267-277.
28. Semple SJ, Zians J, Grant I, et al. Impulsivity and methamphetamine use. J Subst Abuse Treat. 2005;29(2):85-93.
29. Hester R, Lee N, Pennay A, et al. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol. 2010;18(6):489-497.
30. Weber E, Blackstone K, Iudicello JE, et al; Translational Methamphetamine AIDS Research Center (TMARC) Group. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125(1-2):146-153.
31. Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol. 2017;10(3):305-314.
32. Anderson AL, Li SH, Biswas K, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120(1-3):135-141.
33. Cadet JL, Bisagno V. Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry. 2016;6:189.
34. Loland CJ, Mereu M, Okunola OM, et al. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72(5):405-413.
35. Farnia V, Shakeri J, Tatari F, et al. Randomized controlled trial of aripiprazole versus risperidone for the treatment of amphetamine-induced psychosis. Am J Drug Alcohol Abuse. 2014;40(1):10-15.
Prescribing antipsychotics in geriatric patients: Focus on major depressive disorder
The proportion of older adults in the world population is growing rapidly. In the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.1 As a result, there is an increased urgency in examining benefits vs risks of antipsychotics in older individuals. In a 2010 U.S. nationally representative observational study, antipsychotic use was observed to rise slowly during early and middle adulthood, peaking at approximately age 55, declining slightly between ages 55 and 65, and then rising again after age 65, with >2% of individuals ages 80 to 84 receiving an antipsychotic.2 This is likely due to the chronology of psychotic, mood, and neurocognitive disorders across the life span. In this large national study, long-term antipsychotic treatment was common, and older patients were more likely to receive their prescriptions from non-psychiatrist physicians than from psychiatrists.2 Among patients receiving an antipsychotic, the proportion of those receiving it for >120 days was 54% for individuals ages 70 to 74; 49% for individuals ages 75 to 79; and 46% for individuals ages 80 to 84.
This 3-part review summarizes findings and risk–benefit considerations when prescribing antipsychotics to older individuals. Part 1 focused on those with chronic psychotic disorders, such as schizophrenia or bipolar disorder,3 and part 3 will cover patients with dementia. This review (part 2) aims to:
- briefly summarize the results of randomized controlled trials (RCTs) of second-generation antipsychotics (SGAs) and other major studies and analyses in older patients with major depressive disorder (MDD)
- provide a summative opinion on the relative risks and benefits associated with using antipsychotics in older adults with MDD
- highlight the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
The prevalence of MDD and clinically significant depressive symptoms in communitydwelling older adults is 3% to 4% and 15%, respectively, and as high as 16% and 50%, respectively, in nursing home residents.4 Because late-life depression is associated with suffering, disability, and excessive mortality, it needs to be recognized and treated aggressively.5 Antidepressants are the mainstay of pharmacotherapy for late-life depression. Guidelines and expert opinion informed by the current evidence recommend using selective serotonin reuptake inhibitors, such as
Over the past decade, several antipsychotics have been FDA-approved for treating MDD:
Data from several rigorously conducted RCTs support using an antidepressant plus an FGA or SGA as first-line pharmacotherapy in younger and older patients with “psychotic depression.”8-12 SGAs also can be used as augmenting agents when there is only a partial response to antidepressants.13-15 In this situation, guidelines and experts favor an augmentation strategy over switching to another antidepressant.5,9,10,16 Until recently, most published pharmacologic trials for late-life treatment-resistant depression supported using lithium to augment antidepressants.14,17 However, because several antipsychotics are now FDA-approved for treating MDD, and in light of positive findings from several studies relevant to older patients,18-21 many experts now support using SGAs to augment antidepressants in older patients with nonpsychotic depression.5,15
Clinical trials
Olanzapine plus sertraline as first-line pharmacotherapy for MDD with psychotic features. Meyers et al11 reported on a double-blind randomized comparison of olanzapine plus placebo vs olanzapine plus sertraline in 259 patients with MDD with psychotic features. An unusual feature of this trial is that it included a similar number of younger and older participants (ages 18 to 93): 117 participants were age <60 (mean age [standard deviation (SD)]: 41.3 [10.8]) and 142 were age ≥60 (mean age [SD]: 71.7 [7.8]). The same dose titration schedules based on efficacy and tolerability were used in both younger and older participants. At the end of the study, the mean dose (SD) of sertraline (or placebo) did not differ significantly in younger (174.3 mg/d [34.1]) and older participants (165.7 mg/d [43.4]). However, the mean dose (SD) of olanzapine was significantly higher in younger patients (15.7 mg/d [4.7]) than in older participants (13.4 mg/d [5.1]).
In both age groups, olanzapine plus sertraline was more efficacious than olanzapine plus placebo, and there was no statistical interaction between age, time, and treatment group (ie, the trajectories of improvement were similar in older and younger patients receiving either olanzapine or olanzapine plus sertraline). Similarly, drop-out rates because of poor tolerability did not differ significantly in younger (4.3%) and older participants (5.6%). However, in a multinomial regression, older participants were more likely to discontinue treatment because of poor tolerability.22 Older participants were significantly less likely to experience weight gain (mean [SD]: +3.3 [4.9] vs +6.5 [6.6] kg) or an increase in fasting glucose and more likely to experience a fall, pedal edema, or extrapyramidal symptoms.11,22-24 Cholesterol and triglyceride increased significantly and similarly in both age groups. The incidence of symptoms of tardive dyskinesia (TD) over the 12-week trial was low (<5%) in both younger and older participants, and clinically diagnosed TD was reported in only 1 (older) participant.25
Venlafaxine plus aripiprazole for treatment-resistant MDD. In the largest double-blind randomized study of augmentation pharmacotherapy for late-life treatment-resistant depression published to date, Lenze et al21 compared venlafaxine plus aripiprazole vs venlafaxine plus placebo in 181 patients age >60 (mean age 66, with 49 participants age >70) with MDD who did not remit after 12 weeks of treatment with venlafaxine (up to 300 mg/d). After 12 weeks of augmentation, remission rates were significantly higher with aripiprazole than with placebo: 40 (44%) vs 26 (29%); odds ratio (95% confidence interval [CI]): 2.0 (1.1 to 3.7). The median final aripiprazole dose was 7 mg/d (range 2 to 15 mg/d) in remitters and 10 mg/d (range 2 to 15 mg/d) in nonremitters.
Five of 90 participants (5%) discontinued aripiprazole (1 each: suicide, jitteriness/akathisia, worsening parkinsonism; and 2 withdrew consent); 8 of 90 (9%) discontinued placebo (2 each: lack of efficacy, headache; 1: worsening parkinsonism; and 3 withdrew consent). The completed suicide occurred after 5 weeks of treatment with aripiprazole and was judged to be “neither due to emergent suicidal ideation nor to aripiprazole side-effects, but was concluded by investigators to be a result of the individual’s persisting and long-standing suicidal ideation.”21 Including the suicide, there were 4 serious adverse events (5%) in those receiving aripiprazole (1 each: suicide, congestive heart failure, mild stroke, and diverticulitis) and 2 (2%) in those receiving placebo (1 each: myocardial infarction, hospitalized for vomiting due to accidentally taking extra venlafaxine). In 86 participants receiving aripiprazole and 87 receiving placebo, the most frequently reported adverse effects were increased dream activity (aripiprazole: 23 [27%] vs placebo: 12 [14%]), weight gain (17 [20%] vs 8 [9%]), and tremor (5 [6%] vs 0). Akathisia and parkinsonism were observed more frequently with aripiprazole than with placebo (akathisia: 24 [26%] of 91 vs 11 [12%] of 90; parkinsonism: 15 [17%] of 86 vs 2 [2%] of 81). Akathisia was generally mild and resolved with dose adjustment; however, it was associated with a transient increase in suicidality in 3 (3%) participants receiving aripiprazole vs 0 receiving placebo and persisted at the end of the trial in 5 (5%) participants receiving aripiprazole vs 2 (2%) receiving placebo. Participants receiving aripiprazole had a significantly larger increase in weight (mean [SD]: +1.93 [3.00] vs +0.01 [3.15] kg), but there were no differences between aripiprazole and placebo in changes in body fat, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides, glucose, insulin concentration, or QTc.
Citalopram plus risperidone for treatment-resistant MDD. Alexopoulos et al26 reported an analysis of data from 110 patients age ≥55 years (mean age [SD]: 63.4 [4.8]), among 489 mixed-age patients with MDD. Participants (n = 110) who did not respond to 1 to 3 antidepressants (venlafaxine, sertraline, mirtazapine, fluoxetine, paroxetine, or bupropion in >90%) during their current depressive episode completed 4 to 6 weeks of treatment with citalopram up to 40 mg/d; 93 did not respond and were treated with open-label risperidone (0.25 to 1 mg/d) augmentation for 4 to 6 weeks. Sixty-three (68%) of these 93 patients remitted and were randomized to 24 weeks of double-blind continuation treatment with citalopram plus risperidone vs citalopram plus placebo. Neither the median times to relapse (105 vs 57 days) nor the relapse rates (risperidone: 18 of 32 [56%] vs placebo: 20 of 31 [65%]) differed significantly. During the open-label risperidone augmentation, the most common adverse events were dizziness and dry mouth (n = 9 each, 9.7% of 93). During the continuation phase, headache (n = 3; 9.1% of 32) was observed with risperidone but not with placebo (n = 0). There was no incident parkinsonism or abnormal movements noted, but risperidone was associated with weight gain during both the open-label risperidone augmentation phase (mean [SD]: +0.9 [2.1] kg) and the continuation phase (risperidone: +0.8 [3.5] vs placebo: −0.3 [2.8] kg).
Quetiapine XR monotherapy for MDD. Katila et al27 reported on a placebo-controlled RCT of quetiapine XR (median dose, 158.7 mg/d; range, 50 to 300 mg/d) in 338 patients age ≥66 years (mean age [SD], 71.3 [7.5]) presenting with MDD and a major depressive episode with a duration <1 year and no history of failed antidepressants trials from 2 classes (more than two-thirds of participants had not received treatment). After 9 weeks, the reduction in depressive symptoms on the Montgomery-Åsberg Depression Rating Scale was significantly larger with quetiapine XR than with placebo (mean [SD]: −16.0 [9.3] vs −9.0 [9.9]). There were congruent, significant differences between quetiapine and placebo in terms of response rate (quetiapine XR: 105 of 164 [64%] vs placebo: 52 of 171 [30.4%]) and remission rate (92 of 164 [56.1%] vs placebo: 40 of 171 [23.4%]). The drop-out rates for all causes were similar, but the drop-out rate attributed to adverse events was higher with quetiapine than placebo (16 of 166 [9.6%] vs 7 of 172 [4.1%]). Most quetiapine drop-outs were attributable to dizziness, headache, and somnolence (n = 4 each), and placebo drop-outs were because of headache (n = 2). Consistent with the profile of quetiapine, adverse events with a rate that was at least 5% higher with quetiapine than with placebo included somnolence (64 of 166 [38.6%] vs 16 of 172 [9.3%]), dry mouth (34 [20.5%] vs 18 [10.5%]), and extrapyramidal symptoms (12 [7.2%] vs 4 [2.3%]). Changes in weight and laboratory test results (eg, glucose, lipid profile) were minimal and not clinically meaningful.
Other clinical data. The efficacy and relatively good tolerability of aripiprazole in older patients with treatment-resistant depression observed in the RCT by Lenze et al21 is congruent with the earlier results of 2 small (N = 20 and 24) pilot studies.18,19 In both studies, the remission rate was 50%, and the most prevalent adverse effects were agitation/restlessness/akathisia or drowsiness/sedation. Similarly, in a post hoc pooled analysis of 409 participants ages 50 to 67 from 3 placebo-controlled randomized trials, the remission rate was significantly higher with aripiprazole than with placebo (32.5% vs 17.1%), and the most common adverse effects were akathisia or restlessness (64 of 210 [30.4%]), somnolence (18 [8.6%]), and insomnia (17 [8.1%]).20
Clinical considerations
When assessing the relative benefits and risks of antipsychotics in older patients, it is important to remember that conclusions and summative opinions are necessarily influenced by the source of the data. Because much of what we know about the use of antipsychotics in geriatric adults is from clinical trials, we know more about their acute efficacy and tolerability than their long-term effectiveness and safety.28 There are similar issues regarding the role of antipsychotics in treating MDD in late life. Based on the results of several RCTs,8,11 a combination of an antidepressant plus an antipsychotic is the recommended pharmacotherapy for the acute treatment of MDD with psychotic features (Table 2).8,11,12,19-21,23-27 However, there are no published data to guide how long the antipsychotic should be continued.29
In older patients with MDD without psychotic features, 1 relatively large placebo-controlled RCT,21 2 smaller open studies,18,19 and a post hoc analysis of a large placebo-controlled RCT in mixed-age adults20 support the efficacy and relatively good tolerability of aripiprazole augmentation of an antidepressant for treatment-resistant MDD. Similarly, 1 large placebo-controlled RCT supports the efficacy and relatively good tolerability of quetiapine for non–treatment-resistant MDD. However, there are no comparative data assessing the relative merits of using these antipsychotics vs other pharmacologic strategies (eg, switching to another antidepressant, lithium augmentation, or combination of 2 antidepressants). Because older patients are more likely to experience adverse effects that may have more serious consequences (Table 3), many prudent clinicians reserve using antipsychotics as a third-line treatment in older patients with MDD without psychotic features and limit the duration of their use to a few months.30
Although some data have accumulated in recent years, there are significant gaps in knowledge on the safety and tolerability of antipsychotics in older adults. The era of “big data” may provide important answers to questions such as the relative place of antipsychotics vs lithium in preserving brain health among people with bipolar disorder or treatment-resistant MDD31; whether there are true ethnic differences in terms of drugs response and adverse effect prevalence in antipsychotics32,33; or the role of pharmacogenetic evaluation in establishing individual risk–benefit ratios of antipsychotics.34
1. United Nations. Department of Economic and Social Affairs Population Division. World Population Ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Published 2001. Accessed September 27, 2017.
2. Olfson M, King M, Schoenbaum M. Antipsychotic treatment of adults in the United States. J Clin Psychiatry. 2015;76(10):1346-1353.
3. Sajatovic M, Kales HC, Mulsant BH. Prescribing antipsychotics in geriatric patients: focus on schizophrenia and bipolar disorder. Current Psychiatry. 2017;16(10):20-26,28.
4. Hybels CF, Blazer DG. Epidemiology of late-life mental disorders. Clin Geriatr Med. 2003;19(4):663-696,v.
5. Mulsant BH, Blumberger DM, Ismail Z, et al. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30(3):517-534.
6. Mulsant BH, Pollock BG. Psychopharmacology. In: Steffens DC, Blazer DG, Thakur ME, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
7. U.S. Food and Drug Administration. Public health advisory. deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed September 27, 2017.
8. Andreescu C, Mulsant BH, Rothschild AJ, et al. Pharmacotherapy of major depression with psychotic features: what is the evidence? Psychiatric Annals. 2006;35(1):31-38.
9. Buchanan D, Tourigny-Rivard MF, Cappeliez P, et al. National guidelines for seniors’ mental health: the assessment and treatment of depression. Canadian Journal of Geriatrics. 2006;9(suppl 2):S52-S58.
10. Canadian Coalition for Senior’s Mental Health. National guidelines for senior’s mental health. The assessment and treatment of depression 2006. http://www.ccsmh.ca/projects/depression. Accessed February 28, 2016.
11. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
12. Mulsant BH, Sweet RA, Rosen J, et al. A double-blind randomized comparison of nortriptyline plus perphenazine versus nortriptyline plus placebo in the treatment of psychotic depression in late life. J Clin Psychiatry. 2001;62(8):597-604.
13. Cakir S, Senkal Z. Atypical antipsychotics as add-on treatment in late-life depression. Clin Interv Aging. 2016;11:1193-1198.
14. Maust DT, Oslin DW, Thase ME. Going beyond antidepressant monotherapy for incomplete response in nonpsychotic late-life depression: a critical review. Am J Geriatr Psychiatry. 2013;21(10):973-986.
15. Patel K, Abdool PS, Rajji TK, et al. Pharmacotherapy of major depression in late life: what is the role of new agents? Expert Opin Pharmacother. 2017;18(6):599-609.
16. Alexopoulos GS, Katz IR, Reynolds CF 3rd, et al. Pharmacotherapy of depression in older patients: a summary of the expert consensus guidelines. J Psychiatr Pract. 2001;7(6):361-376.
17. Cooper C, Katona C, Lyketsos K, et al. A systematic review of treatments for refractory depression in older people. Am J Psychiatry. 2011;168(7):681-688.
18. Rutherford B, Sneed J, Miyazaki M, et al. An open trial of aripiprazole augmentation for SSRI non-remitters with late-life depression. Int J Geriatr Psychiatry. 2007;22(10):986-991.
19. Sheffrin M, Driscoll HC, Lenze EJ, et al. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: getting to remission. J Clin Psychiatry. 2009;70(2):208-213.
20. Steffens DC, Nelson JC, Eudicone JM, et al. Efficacy and safety of adjunctive aripiprazole in major depressive disorder in older patients: a pooled subpopulation analysis. Int J Geriatr Psychiatry. 2011;26(6):564-572.
21. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10011):2404-2412.
22. Deligiannidis KM, Rothschild AJ, Barton BA, et al. A gender analysis of the study of pharmacotherapy of psychotic depression (STOP-PD): gender and age as predictors of response and treatment-associated changes in body mass index and metabolic measures. J Clin Psychiatry. 2013;74(10):1003-1009.
23. Flint AJ, Iaboni A, Mulsant BH, et al. Effect of sertraline on risk of falling in older adults with psychotic depression on olanzapine: results of a randomized placebo-controlled trial. Am J Geriatr Psychiatry. 2014;22(4):332-336.
24. Smith E, Rothschild AJ, Heo M, et al. Weight gain during olanzapine treatment for psychotic depression: effects of dose and age. Int Clin Psychopharmacol. 2008;23(3):130-137.
25. Blumberger DM, Mulsant BH, Kanellopoulos D, et al. The incidence of tardive dyskinesia in the study of pharmacotherapy for psychotic depression. J Clin Psychopharmacol. 2013;33(3):391-397.
26. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry. 2008;16(1):21-30.
27. Katila H, Mezhebovsky I, Mulroy A, et al. Randomized, double-blind study of the efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in elderly patients with major depressive disorder. Am J Geriatr Psychiatry. 2013;21(8):769-784.
28. Sultana J, Trifiro G. Drug safety warnings: a message in a bottle. J Drug Des Res. 2014;1(1):1004.
29. Flint A, Meyers BS, Rothschild AR, et al; STOP-PD II Study Group. Sustaining remission of psychotic depression: rationale, design and methodology of STOP-PD II. BMC Psychiatry. 2013;13:38.
30. Alexopoulos GS; PROSPECT Group. Interventions for depressed elderly primary care patients. Int J Geriatr Psychiatry. 2001;16(6):553-559.
31. Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
32. Bigos KL, Bies RR, Pollock BG, et al. Genetic variation in CYP3A43 explains racial difference in olanzapine clearance. Mol Psychiatry. 2011;16(6):620-625.
33. Jin Y, Pollock BG, Coley K, et al. Population pharmacokinetics of perphenazine in schizophrenia patients from CATIE: impact of race and smoking. J Clin Pharmacol. 2010;50(1):73-80.
34. Mulsant BH. Is there a role for antidepressant and antipsychotic pharmacogenetics in clinical practice in 2014? Can J Psychiatry. 2014;59(2):59-61.
The proportion of older adults in the world population is growing rapidly. In the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.1 As a result, there is an increased urgency in examining benefits vs risks of antipsychotics in older individuals. In a 2010 U.S. nationally representative observational study, antipsychotic use was observed to rise slowly during early and middle adulthood, peaking at approximately age 55, declining slightly between ages 55 and 65, and then rising again after age 65, with >2% of individuals ages 80 to 84 receiving an antipsychotic.2 This is likely due to the chronology of psychotic, mood, and neurocognitive disorders across the life span. In this large national study, long-term antipsychotic treatment was common, and older patients were more likely to receive their prescriptions from non-psychiatrist physicians than from psychiatrists.2 Among patients receiving an antipsychotic, the proportion of those receiving it for >120 days was 54% for individuals ages 70 to 74; 49% for individuals ages 75 to 79; and 46% for individuals ages 80 to 84.
This 3-part review summarizes findings and risk–benefit considerations when prescribing antipsychotics to older individuals. Part 1 focused on those with chronic psychotic disorders, such as schizophrenia or bipolar disorder,3 and part 3 will cover patients with dementia. This review (part 2) aims to:
- briefly summarize the results of randomized controlled trials (RCTs) of second-generation antipsychotics (SGAs) and other major studies and analyses in older patients with major depressive disorder (MDD)
- provide a summative opinion on the relative risks and benefits associated with using antipsychotics in older adults with MDD
- highlight the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
The prevalence of MDD and clinically significant depressive symptoms in communitydwelling older adults is 3% to 4% and 15%, respectively, and as high as 16% and 50%, respectively, in nursing home residents.4 Because late-life depression is associated with suffering, disability, and excessive mortality, it needs to be recognized and treated aggressively.5 Antidepressants are the mainstay of pharmacotherapy for late-life depression. Guidelines and expert opinion informed by the current evidence recommend using selective serotonin reuptake inhibitors, such as
Over the past decade, several antipsychotics have been FDA-approved for treating MDD:

Data from several rigorously conducted RCTs support using an antidepressant plus an FGA or SGA as first-line pharmacotherapy in younger and older patients with “psychotic depression.”8-12 SGAs also can be used as augmenting agents when there is only a partial response to antidepressants.13-15 In this situation, guidelines and experts favor an augmentation strategy over switching to another antidepressant.5,9,10,16 Until recently, most published pharmacologic trials for late-life treatment-resistant depression supported using lithium to augment antidepressants.14,17 However, because several antipsychotics are now FDA-approved for treating MDD, and in light of positive findings from several studies relevant to older patients,18-21 many experts now support using SGAs to augment antidepressants in older patients with nonpsychotic depression.5,15
Clinical trials
Olanzapine plus sertraline as first-line pharmacotherapy for MDD with psychotic features. Meyers et al11 reported on a double-blind randomized comparison of olanzapine plus placebo vs olanzapine plus sertraline in 259 patients with MDD with psychotic features. An unusual feature of this trial is that it included a similar number of younger and older participants (ages 18 to 93): 117 participants were age <60 (mean age [standard deviation (SD)]: 41.3 [10.8]) and 142 were age ≥60 (mean age [SD]: 71.7 [7.8]). The same dose titration schedules based on efficacy and tolerability were used in both younger and older participants. At the end of the study, the mean dose (SD) of sertraline (or placebo) did not differ significantly in younger (174.3 mg/d [34.1]) and older participants (165.7 mg/d [43.4]). However, the mean dose (SD) of olanzapine was significantly higher in younger patients (15.7 mg/d [4.7]) than in older participants (13.4 mg/d [5.1]).
In both age groups, olanzapine plus sertraline was more efficacious than olanzapine plus placebo, and there was no statistical interaction between age, time, and treatment group (ie, the trajectories of improvement were similar in older and younger patients receiving either olanzapine or olanzapine plus sertraline). Similarly, drop-out rates because of poor tolerability did not differ significantly in younger (4.3%) and older participants (5.6%). However, in a multinomial regression, older participants were more likely to discontinue treatment because of poor tolerability.22 Older participants were significantly less likely to experience weight gain (mean [SD]: +3.3 [4.9] vs +6.5 [6.6] kg) or an increase in fasting glucose and more likely to experience a fall, pedal edema, or extrapyramidal symptoms.11,22-24 Cholesterol and triglyceride increased significantly and similarly in both age groups. The incidence of symptoms of tardive dyskinesia (TD) over the 12-week trial was low (<5%) in both younger and older participants, and clinically diagnosed TD was reported in only 1 (older) participant.25
Venlafaxine plus aripiprazole for treatment-resistant MDD. In the largest double-blind randomized study of augmentation pharmacotherapy for late-life treatment-resistant depression published to date, Lenze et al21 compared venlafaxine plus aripiprazole vs venlafaxine plus placebo in 181 patients age >60 (mean age 66, with 49 participants age >70) with MDD who did not remit after 12 weeks of treatment with venlafaxine (up to 300 mg/d). After 12 weeks of augmentation, remission rates were significantly higher with aripiprazole than with placebo: 40 (44%) vs 26 (29%); odds ratio (95% confidence interval [CI]): 2.0 (1.1 to 3.7). The median final aripiprazole dose was 7 mg/d (range 2 to 15 mg/d) in remitters and 10 mg/d (range 2 to 15 mg/d) in nonremitters.
Five of 90 participants (5%) discontinued aripiprazole (1 each: suicide, jitteriness/akathisia, worsening parkinsonism; and 2 withdrew consent); 8 of 90 (9%) discontinued placebo (2 each: lack of efficacy, headache; 1: worsening parkinsonism; and 3 withdrew consent). The completed suicide occurred after 5 weeks of treatment with aripiprazole and was judged to be “neither due to emergent suicidal ideation nor to aripiprazole side-effects, but was concluded by investigators to be a result of the individual’s persisting and long-standing suicidal ideation.”21 Including the suicide, there were 4 serious adverse events (5%) in those receiving aripiprazole (1 each: suicide, congestive heart failure, mild stroke, and diverticulitis) and 2 (2%) in those receiving placebo (1 each: myocardial infarction, hospitalized for vomiting due to accidentally taking extra venlafaxine). In 86 participants receiving aripiprazole and 87 receiving placebo, the most frequently reported adverse effects were increased dream activity (aripiprazole: 23 [27%] vs placebo: 12 [14%]), weight gain (17 [20%] vs 8 [9%]), and tremor (5 [6%] vs 0). Akathisia and parkinsonism were observed more frequently with aripiprazole than with placebo (akathisia: 24 [26%] of 91 vs 11 [12%] of 90; parkinsonism: 15 [17%] of 86 vs 2 [2%] of 81). Akathisia was generally mild and resolved with dose adjustment; however, it was associated with a transient increase in suicidality in 3 (3%) participants receiving aripiprazole vs 0 receiving placebo and persisted at the end of the trial in 5 (5%) participants receiving aripiprazole vs 2 (2%) receiving placebo. Participants receiving aripiprazole had a significantly larger increase in weight (mean [SD]: +1.93 [3.00] vs +0.01 [3.15] kg), but there were no differences between aripiprazole and placebo in changes in body fat, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides, glucose, insulin concentration, or QTc.
Citalopram plus risperidone for treatment-resistant MDD. Alexopoulos et al26 reported an analysis of data from 110 patients age ≥55 years (mean age [SD]: 63.4 [4.8]), among 489 mixed-age patients with MDD. Participants (n = 110) who did not respond to 1 to 3 antidepressants (venlafaxine, sertraline, mirtazapine, fluoxetine, paroxetine, or bupropion in >90%) during their current depressive episode completed 4 to 6 weeks of treatment with citalopram up to 40 mg/d; 93 did not respond and were treated with open-label risperidone (0.25 to 1 mg/d) augmentation for 4 to 6 weeks. Sixty-three (68%) of these 93 patients remitted and were randomized to 24 weeks of double-blind continuation treatment with citalopram plus risperidone vs citalopram plus placebo. Neither the median times to relapse (105 vs 57 days) nor the relapse rates (risperidone: 18 of 32 [56%] vs placebo: 20 of 31 [65%]) differed significantly. During the open-label risperidone augmentation, the most common adverse events were dizziness and dry mouth (n = 9 each, 9.7% of 93). During the continuation phase, headache (n = 3; 9.1% of 32) was observed with risperidone but not with placebo (n = 0). There was no incident parkinsonism or abnormal movements noted, but risperidone was associated with weight gain during both the open-label risperidone augmentation phase (mean [SD]: +0.9 [2.1] kg) and the continuation phase (risperidone: +0.8 [3.5] vs placebo: −0.3 [2.8] kg).
Quetiapine XR monotherapy for MDD. Katila et al27 reported on a placebo-controlled RCT of quetiapine XR (median dose, 158.7 mg/d; range, 50 to 300 mg/d) in 338 patients age ≥66 years (mean age [SD], 71.3 [7.5]) presenting with MDD and a major depressive episode with a duration <1 year and no history of failed antidepressants trials from 2 classes (more than two-thirds of participants had not received treatment). After 9 weeks, the reduction in depressive symptoms on the Montgomery-Åsberg Depression Rating Scale was significantly larger with quetiapine XR than with placebo (mean [SD]: −16.0 [9.3] vs −9.0 [9.9]). There were congruent, significant differences between quetiapine and placebo in terms of response rate (quetiapine XR: 105 of 164 [64%] vs placebo: 52 of 171 [30.4%]) and remission rate (92 of 164 [56.1%] vs placebo: 40 of 171 [23.4%]). The drop-out rates for all causes were similar, but the drop-out rate attributed to adverse events was higher with quetiapine than placebo (16 of 166 [9.6%] vs 7 of 172 [4.1%]). Most quetiapine drop-outs were attributable to dizziness, headache, and somnolence (n = 4 each), and placebo drop-outs were because of headache (n = 2). Consistent with the profile of quetiapine, adverse events with a rate that was at least 5% higher with quetiapine than with placebo included somnolence (64 of 166 [38.6%] vs 16 of 172 [9.3%]), dry mouth (34 [20.5%] vs 18 [10.5%]), and extrapyramidal symptoms (12 [7.2%] vs 4 [2.3%]). Changes in weight and laboratory test results (eg, glucose, lipid profile) were minimal and not clinically meaningful.
Other clinical data. The efficacy and relatively good tolerability of aripiprazole in older patients with treatment-resistant depression observed in the RCT by Lenze et al21 is congruent with the earlier results of 2 small (N = 20 and 24) pilot studies.18,19 In both studies, the remission rate was 50%, and the most prevalent adverse effects were agitation/restlessness/akathisia or drowsiness/sedation. Similarly, in a post hoc pooled analysis of 409 participants ages 50 to 67 from 3 placebo-controlled randomized trials, the remission rate was significantly higher with aripiprazole than with placebo (32.5% vs 17.1%), and the most common adverse effects were akathisia or restlessness (64 of 210 [30.4%]), somnolence (18 [8.6%]), and insomnia (17 [8.1%]).20
Clinical considerations
When assessing the relative benefits and risks of antipsychotics in older patients, it is important to remember that conclusions and summative opinions are necessarily influenced by the source of the data. Because much of what we know about the use of antipsychotics in geriatric adults is from clinical trials, we know more about their acute efficacy and tolerability than their long-term effectiveness and safety.28 There are similar issues regarding the role of antipsychotics in treating MDD in late life. Based on the results of several RCTs,8,11 a combination of an antidepressant plus an antipsychotic is the recommended pharmacotherapy for the acute treatment of MDD with psychotic features (Table 2).8,11,12,19-21,23-27 However, there are no published data to guide how long the antipsychotic should be continued.29

In older patients with MDD without psychotic features, 1 relatively large placebo-controlled RCT,21 2 smaller open studies,18,19 and a post hoc analysis of a large placebo-controlled RCT in mixed-age adults20 support the efficacy and relatively good tolerability of aripiprazole augmentation of an antidepressant for treatment-resistant MDD. Similarly, 1 large placebo-controlled RCT supports the efficacy and relatively good tolerability of quetiapine for non–treatment-resistant MDD. However, there are no comparative data assessing the relative merits of using these antipsychotics vs other pharmacologic strategies (eg, switching to another antidepressant, lithium augmentation, or combination of 2 antidepressants). Because older patients are more likely to experience adverse effects that may have more serious consequences (Table 3), many prudent clinicians reserve using antipsychotics as a third-line treatment in older patients with MDD without psychotic features and limit the duration of their use to a few months.30
Although some data have accumulated in recent years, there are significant gaps in knowledge on the safety and tolerability of antipsychotics in older adults. The era of “big data” may provide important answers to questions such as the relative place of antipsychotics vs lithium in preserving brain health among people with bipolar disorder or treatment-resistant MDD31; whether there are true ethnic differences in terms of drugs response and adverse effect prevalence in antipsychotics32,33; or the role of pharmacogenetic evaluation in establishing individual risk–benefit ratios of antipsychotics.34
The proportion of older adults in the world population is growing rapidly. In the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.1 As a result, there is an increased urgency in examining benefits vs risks of antipsychotics in older individuals. In a 2010 U.S. nationally representative observational study, antipsychotic use was observed to rise slowly during early and middle adulthood, peaking at approximately age 55, declining slightly between ages 55 and 65, and then rising again after age 65, with >2% of individuals ages 80 to 84 receiving an antipsychotic.2 This is likely due to the chronology of psychotic, mood, and neurocognitive disorders across the life span. In this large national study, long-term antipsychotic treatment was common, and older patients were more likely to receive their prescriptions from non-psychiatrist physicians than from psychiatrists.2 Among patients receiving an antipsychotic, the proportion of those receiving it for >120 days was 54% for individuals ages 70 to 74; 49% for individuals ages 75 to 79; and 46% for individuals ages 80 to 84.
This 3-part review summarizes findings and risk–benefit considerations when prescribing antipsychotics to older individuals. Part 1 focused on those with chronic psychotic disorders, such as schizophrenia or bipolar disorder,3 and part 3 will cover patients with dementia. This review (part 2) aims to:
- briefly summarize the results of randomized controlled trials (RCTs) of second-generation antipsychotics (SGAs) and other major studies and analyses in older patients with major depressive disorder (MDD)
- provide a summative opinion on the relative risks and benefits associated with using antipsychotics in older adults with MDD
- highlight the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
The prevalence of MDD and clinically significant depressive symptoms in communitydwelling older adults is 3% to 4% and 15%, respectively, and as high as 16% and 50%, respectively, in nursing home residents.4 Because late-life depression is associated with suffering, disability, and excessive mortality, it needs to be recognized and treated aggressively.5 Antidepressants are the mainstay of pharmacotherapy for late-life depression. Guidelines and expert opinion informed by the current evidence recommend using selective serotonin reuptake inhibitors, such as
Over the past decade, several antipsychotics have been FDA-approved for treating MDD:

Data from several rigorously conducted RCTs support using an antidepressant plus an FGA or SGA as first-line pharmacotherapy in younger and older patients with “psychotic depression.”8-12 SGAs also can be used as augmenting agents when there is only a partial response to antidepressants.13-15 In this situation, guidelines and experts favor an augmentation strategy over switching to another antidepressant.5,9,10,16 Until recently, most published pharmacologic trials for late-life treatment-resistant depression supported using lithium to augment antidepressants.14,17 However, because several antipsychotics are now FDA-approved for treating MDD, and in light of positive findings from several studies relevant to older patients,18-21 many experts now support using SGAs to augment antidepressants in older patients with nonpsychotic depression.5,15
Clinical trials
Olanzapine plus sertraline as first-line pharmacotherapy for MDD with psychotic features. Meyers et al11 reported on a double-blind randomized comparison of olanzapine plus placebo vs olanzapine plus sertraline in 259 patients with MDD with psychotic features. An unusual feature of this trial is that it included a similar number of younger and older participants (ages 18 to 93): 117 participants were age <60 (mean age [standard deviation (SD)]: 41.3 [10.8]) and 142 were age ≥60 (mean age [SD]: 71.7 [7.8]). The same dose titration schedules based on efficacy and tolerability were used in both younger and older participants. At the end of the study, the mean dose (SD) of sertraline (or placebo) did not differ significantly in younger (174.3 mg/d [34.1]) and older participants (165.7 mg/d [43.4]). However, the mean dose (SD) of olanzapine was significantly higher in younger patients (15.7 mg/d [4.7]) than in older participants (13.4 mg/d [5.1]).
In both age groups, olanzapine plus sertraline was more efficacious than olanzapine plus placebo, and there was no statistical interaction between age, time, and treatment group (ie, the trajectories of improvement were similar in older and younger patients receiving either olanzapine or olanzapine plus sertraline). Similarly, drop-out rates because of poor tolerability did not differ significantly in younger (4.3%) and older participants (5.6%). However, in a multinomial regression, older participants were more likely to discontinue treatment because of poor tolerability.22 Older participants were significantly less likely to experience weight gain (mean [SD]: +3.3 [4.9] vs +6.5 [6.6] kg) or an increase in fasting glucose and more likely to experience a fall, pedal edema, or extrapyramidal symptoms.11,22-24 Cholesterol and triglyceride increased significantly and similarly in both age groups. The incidence of symptoms of tardive dyskinesia (TD) over the 12-week trial was low (<5%) in both younger and older participants, and clinically diagnosed TD was reported in only 1 (older) participant.25
Venlafaxine plus aripiprazole for treatment-resistant MDD. In the largest double-blind randomized study of augmentation pharmacotherapy for late-life treatment-resistant depression published to date, Lenze et al21 compared venlafaxine plus aripiprazole vs venlafaxine plus placebo in 181 patients age >60 (mean age 66, with 49 participants age >70) with MDD who did not remit after 12 weeks of treatment with venlafaxine (up to 300 mg/d). After 12 weeks of augmentation, remission rates were significantly higher with aripiprazole than with placebo: 40 (44%) vs 26 (29%); odds ratio (95% confidence interval [CI]): 2.0 (1.1 to 3.7). The median final aripiprazole dose was 7 mg/d (range 2 to 15 mg/d) in remitters and 10 mg/d (range 2 to 15 mg/d) in nonremitters.
Five of 90 participants (5%) discontinued aripiprazole (1 each: suicide, jitteriness/akathisia, worsening parkinsonism; and 2 withdrew consent); 8 of 90 (9%) discontinued placebo (2 each: lack of efficacy, headache; 1: worsening parkinsonism; and 3 withdrew consent). The completed suicide occurred after 5 weeks of treatment with aripiprazole and was judged to be “neither due to emergent suicidal ideation nor to aripiprazole side-effects, but was concluded by investigators to be a result of the individual’s persisting and long-standing suicidal ideation.”21 Including the suicide, there were 4 serious adverse events (5%) in those receiving aripiprazole (1 each: suicide, congestive heart failure, mild stroke, and diverticulitis) and 2 (2%) in those receiving placebo (1 each: myocardial infarction, hospitalized for vomiting due to accidentally taking extra venlafaxine). In 86 participants receiving aripiprazole and 87 receiving placebo, the most frequently reported adverse effects were increased dream activity (aripiprazole: 23 [27%] vs placebo: 12 [14%]), weight gain (17 [20%] vs 8 [9%]), and tremor (5 [6%] vs 0). Akathisia and parkinsonism were observed more frequently with aripiprazole than with placebo (akathisia: 24 [26%] of 91 vs 11 [12%] of 90; parkinsonism: 15 [17%] of 86 vs 2 [2%] of 81). Akathisia was generally mild and resolved with dose adjustment; however, it was associated with a transient increase in suicidality in 3 (3%) participants receiving aripiprazole vs 0 receiving placebo and persisted at the end of the trial in 5 (5%) participants receiving aripiprazole vs 2 (2%) receiving placebo. Participants receiving aripiprazole had a significantly larger increase in weight (mean [SD]: +1.93 [3.00] vs +0.01 [3.15] kg), but there were no differences between aripiprazole and placebo in changes in body fat, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides, glucose, insulin concentration, or QTc.
Citalopram plus risperidone for treatment-resistant MDD. Alexopoulos et al26 reported an analysis of data from 110 patients age ≥55 years (mean age [SD]: 63.4 [4.8]), among 489 mixed-age patients with MDD. Participants (n = 110) who did not respond to 1 to 3 antidepressants (venlafaxine, sertraline, mirtazapine, fluoxetine, paroxetine, or bupropion in >90%) during their current depressive episode completed 4 to 6 weeks of treatment with citalopram up to 40 mg/d; 93 did not respond and were treated with open-label risperidone (0.25 to 1 mg/d) augmentation for 4 to 6 weeks. Sixty-three (68%) of these 93 patients remitted and were randomized to 24 weeks of double-blind continuation treatment with citalopram plus risperidone vs citalopram plus placebo. Neither the median times to relapse (105 vs 57 days) nor the relapse rates (risperidone: 18 of 32 [56%] vs placebo: 20 of 31 [65%]) differed significantly. During the open-label risperidone augmentation, the most common adverse events were dizziness and dry mouth (n = 9 each, 9.7% of 93). During the continuation phase, headache (n = 3; 9.1% of 32) was observed with risperidone but not with placebo (n = 0). There was no incident parkinsonism or abnormal movements noted, but risperidone was associated with weight gain during both the open-label risperidone augmentation phase (mean [SD]: +0.9 [2.1] kg) and the continuation phase (risperidone: +0.8 [3.5] vs placebo: −0.3 [2.8] kg).
Quetiapine XR monotherapy for MDD. Katila et al27 reported on a placebo-controlled RCT of quetiapine XR (median dose, 158.7 mg/d; range, 50 to 300 mg/d) in 338 patients age ≥66 years (mean age [SD], 71.3 [7.5]) presenting with MDD and a major depressive episode with a duration <1 year and no history of failed antidepressants trials from 2 classes (more than two-thirds of participants had not received treatment). After 9 weeks, the reduction in depressive symptoms on the Montgomery-Åsberg Depression Rating Scale was significantly larger with quetiapine XR than with placebo (mean [SD]: −16.0 [9.3] vs −9.0 [9.9]). There were congruent, significant differences between quetiapine and placebo in terms of response rate (quetiapine XR: 105 of 164 [64%] vs placebo: 52 of 171 [30.4%]) and remission rate (92 of 164 [56.1%] vs placebo: 40 of 171 [23.4%]). The drop-out rates for all causes were similar, but the drop-out rate attributed to adverse events was higher with quetiapine than placebo (16 of 166 [9.6%] vs 7 of 172 [4.1%]). Most quetiapine drop-outs were attributable to dizziness, headache, and somnolence (n = 4 each), and placebo drop-outs were because of headache (n = 2). Consistent with the profile of quetiapine, adverse events with a rate that was at least 5% higher with quetiapine than with placebo included somnolence (64 of 166 [38.6%] vs 16 of 172 [9.3%]), dry mouth (34 [20.5%] vs 18 [10.5%]), and extrapyramidal symptoms (12 [7.2%] vs 4 [2.3%]). Changes in weight and laboratory test results (eg, glucose, lipid profile) were minimal and not clinically meaningful.
Other clinical data. The efficacy and relatively good tolerability of aripiprazole in older patients with treatment-resistant depression observed in the RCT by Lenze et al21 is congruent with the earlier results of 2 small (N = 20 and 24) pilot studies.18,19 In both studies, the remission rate was 50%, and the most prevalent adverse effects were agitation/restlessness/akathisia or drowsiness/sedation. Similarly, in a post hoc pooled analysis of 409 participants ages 50 to 67 from 3 placebo-controlled randomized trials, the remission rate was significantly higher with aripiprazole than with placebo (32.5% vs 17.1%), and the most common adverse effects were akathisia or restlessness (64 of 210 [30.4%]), somnolence (18 [8.6%]), and insomnia (17 [8.1%]).20
Clinical considerations
When assessing the relative benefits and risks of antipsychotics in older patients, it is important to remember that conclusions and summative opinions are necessarily influenced by the source of the data. Because much of what we know about the use of antipsychotics in geriatric adults is from clinical trials, we know more about their acute efficacy and tolerability than their long-term effectiveness and safety.28 There are similar issues regarding the role of antipsychotics in treating MDD in late life. Based on the results of several RCTs,8,11 a combination of an antidepressant plus an antipsychotic is the recommended pharmacotherapy for the acute treatment of MDD with psychotic features (Table 2).8,11,12,19-21,23-27 However, there are no published data to guide how long the antipsychotic should be continued.29

In older patients with MDD without psychotic features, 1 relatively large placebo-controlled RCT,21 2 smaller open studies,18,19 and a post hoc analysis of a large placebo-controlled RCT in mixed-age adults20 support the efficacy and relatively good tolerability of aripiprazole augmentation of an antidepressant for treatment-resistant MDD. Similarly, 1 large placebo-controlled RCT supports the efficacy and relatively good tolerability of quetiapine for non–treatment-resistant MDD. However, there are no comparative data assessing the relative merits of using these antipsychotics vs other pharmacologic strategies (eg, switching to another antidepressant, lithium augmentation, or combination of 2 antidepressants). Because older patients are more likely to experience adverse effects that may have more serious consequences (Table 3), many prudent clinicians reserve using antipsychotics as a third-line treatment in older patients with MDD without psychotic features and limit the duration of their use to a few months.30
Although some data have accumulated in recent years, there are significant gaps in knowledge on the safety and tolerability of antipsychotics in older adults. The era of “big data” may provide important answers to questions such as the relative place of antipsychotics vs lithium in preserving brain health among people with bipolar disorder or treatment-resistant MDD31; whether there are true ethnic differences in terms of drugs response and adverse effect prevalence in antipsychotics32,33; or the role of pharmacogenetic evaluation in establishing individual risk–benefit ratios of antipsychotics.34
1. United Nations. Department of Economic and Social Affairs Population Division. World Population Ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Published 2001. Accessed September 27, 2017.
2. Olfson M, King M, Schoenbaum M. Antipsychotic treatment of adults in the United States. J Clin Psychiatry. 2015;76(10):1346-1353.
3. Sajatovic M, Kales HC, Mulsant BH. Prescribing antipsychotics in geriatric patients: focus on schizophrenia and bipolar disorder. Current Psychiatry. 2017;16(10):20-26,28.
4. Hybels CF, Blazer DG. Epidemiology of late-life mental disorders. Clin Geriatr Med. 2003;19(4):663-696,v.
5. Mulsant BH, Blumberger DM, Ismail Z, et al. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30(3):517-534.
6. Mulsant BH, Pollock BG. Psychopharmacology. In: Steffens DC, Blazer DG, Thakur ME, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
7. U.S. Food and Drug Administration. Public health advisory. deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed September 27, 2017.
8. Andreescu C, Mulsant BH, Rothschild AJ, et al. Pharmacotherapy of major depression with psychotic features: what is the evidence? Psychiatric Annals. 2006;35(1):31-38.
9. Buchanan D, Tourigny-Rivard MF, Cappeliez P, et al. National guidelines for seniors’ mental health: the assessment and treatment of depression. Canadian Journal of Geriatrics. 2006;9(suppl 2):S52-S58.
10. Canadian Coalition for Senior’s Mental Health. National guidelines for senior’s mental health. The assessment and treatment of depression 2006. http://www.ccsmh.ca/projects/depression. Accessed February 28, 2016.
11. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
12. Mulsant BH, Sweet RA, Rosen J, et al. A double-blind randomized comparison of nortriptyline plus perphenazine versus nortriptyline plus placebo in the treatment of psychotic depression in late life. J Clin Psychiatry. 2001;62(8):597-604.
13. Cakir S, Senkal Z. Atypical antipsychotics as add-on treatment in late-life depression. Clin Interv Aging. 2016;11:1193-1198.
14. Maust DT, Oslin DW, Thase ME. Going beyond antidepressant monotherapy for incomplete response in nonpsychotic late-life depression: a critical review. Am J Geriatr Psychiatry. 2013;21(10):973-986.
15. Patel K, Abdool PS, Rajji TK, et al. Pharmacotherapy of major depression in late life: what is the role of new agents? Expert Opin Pharmacother. 2017;18(6):599-609.
16. Alexopoulos GS, Katz IR, Reynolds CF 3rd, et al. Pharmacotherapy of depression in older patients: a summary of the expert consensus guidelines. J Psychiatr Pract. 2001;7(6):361-376.
17. Cooper C, Katona C, Lyketsos K, et al. A systematic review of treatments for refractory depression in older people. Am J Psychiatry. 2011;168(7):681-688.
18. Rutherford B, Sneed J, Miyazaki M, et al. An open trial of aripiprazole augmentation for SSRI non-remitters with late-life depression. Int J Geriatr Psychiatry. 2007;22(10):986-991.
19. Sheffrin M, Driscoll HC, Lenze EJ, et al. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: getting to remission. J Clin Psychiatry. 2009;70(2):208-213.
20. Steffens DC, Nelson JC, Eudicone JM, et al. Efficacy and safety of adjunctive aripiprazole in major depressive disorder in older patients: a pooled subpopulation analysis. Int J Geriatr Psychiatry. 2011;26(6):564-572.
21. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10011):2404-2412.
22. Deligiannidis KM, Rothschild AJ, Barton BA, et al. A gender analysis of the study of pharmacotherapy of psychotic depression (STOP-PD): gender and age as predictors of response and treatment-associated changes in body mass index and metabolic measures. J Clin Psychiatry. 2013;74(10):1003-1009.
23. Flint AJ, Iaboni A, Mulsant BH, et al. Effect of sertraline on risk of falling in older adults with psychotic depression on olanzapine: results of a randomized placebo-controlled trial. Am J Geriatr Psychiatry. 2014;22(4):332-336.
24. Smith E, Rothschild AJ, Heo M, et al. Weight gain during olanzapine treatment for psychotic depression: effects of dose and age. Int Clin Psychopharmacol. 2008;23(3):130-137.
25. Blumberger DM, Mulsant BH, Kanellopoulos D, et al. The incidence of tardive dyskinesia in the study of pharmacotherapy for psychotic depression. J Clin Psychopharmacol. 2013;33(3):391-397.
26. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry. 2008;16(1):21-30.
27. Katila H, Mezhebovsky I, Mulroy A, et al. Randomized, double-blind study of the efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in elderly patients with major depressive disorder. Am J Geriatr Psychiatry. 2013;21(8):769-784.
28. Sultana J, Trifiro G. Drug safety warnings: a message in a bottle. J Drug Des Res. 2014;1(1):1004.
29. Flint A, Meyers BS, Rothschild AR, et al; STOP-PD II Study Group. Sustaining remission of psychotic depression: rationale, design and methodology of STOP-PD II. BMC Psychiatry. 2013;13:38.
30. Alexopoulos GS; PROSPECT Group. Interventions for depressed elderly primary care patients. Int J Geriatr Psychiatry. 2001;16(6):553-559.
31. Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
32. Bigos KL, Bies RR, Pollock BG, et al. Genetic variation in CYP3A43 explains racial difference in olanzapine clearance. Mol Psychiatry. 2011;16(6):620-625.
33. Jin Y, Pollock BG, Coley K, et al. Population pharmacokinetics of perphenazine in schizophrenia patients from CATIE: impact of race and smoking. J Clin Pharmacol. 2010;50(1):73-80.
34. Mulsant BH. Is there a role for antidepressant and antipsychotic pharmacogenetics in clinical practice in 2014? Can J Psychiatry. 2014;59(2):59-61.
1. United Nations. Department of Economic and Social Affairs Population Division. World Population Ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Published 2001. Accessed September 27, 2017.
2. Olfson M, King M, Schoenbaum M. Antipsychotic treatment of adults in the United States. J Clin Psychiatry. 2015;76(10):1346-1353.
3. Sajatovic M, Kales HC, Mulsant BH. Prescribing antipsychotics in geriatric patients: focus on schizophrenia and bipolar disorder. Current Psychiatry. 2017;16(10):20-26,28.
4. Hybels CF, Blazer DG. Epidemiology of late-life mental disorders. Clin Geriatr Med. 2003;19(4):663-696,v.
5. Mulsant BH, Blumberger DM, Ismail Z, et al. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30(3):517-534.
6. Mulsant BH, Pollock BG. Psychopharmacology. In: Steffens DC, Blazer DG, Thakur ME, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
7. U.S. Food and Drug Administration. Public health advisory. deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed September 27, 2017.
8. Andreescu C, Mulsant BH, Rothschild AJ, et al. Pharmacotherapy of major depression with psychotic features: what is the evidence? Psychiatric Annals. 2006;35(1):31-38.
9. Buchanan D, Tourigny-Rivard MF, Cappeliez P, et al. National guidelines for seniors’ mental health: the assessment and treatment of depression. Canadian Journal of Geriatrics. 2006;9(suppl 2):S52-S58.
10. Canadian Coalition for Senior’s Mental Health. National guidelines for senior’s mental health. The assessment and treatment of depression 2006. http://www.ccsmh.ca/projects/depression. Accessed February 28, 2016.
11. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
12. Mulsant BH, Sweet RA, Rosen J, et al. A double-blind randomized comparison of nortriptyline plus perphenazine versus nortriptyline plus placebo in the treatment of psychotic depression in late life. J Clin Psychiatry. 2001;62(8):597-604.
13. Cakir S, Senkal Z. Atypical antipsychotics as add-on treatment in late-life depression. Clin Interv Aging. 2016;11:1193-1198.
14. Maust DT, Oslin DW, Thase ME. Going beyond antidepressant monotherapy for incomplete response in nonpsychotic late-life depression: a critical review. Am J Geriatr Psychiatry. 2013;21(10):973-986.
15. Patel K, Abdool PS, Rajji TK, et al. Pharmacotherapy of major depression in late life: what is the role of new agents? Expert Opin Pharmacother. 2017;18(6):599-609.
16. Alexopoulos GS, Katz IR, Reynolds CF 3rd, et al. Pharmacotherapy of depression in older patients: a summary of the expert consensus guidelines. J Psychiatr Pract. 2001;7(6):361-376.
17. Cooper C, Katona C, Lyketsos K, et al. A systematic review of treatments for refractory depression in older people. Am J Psychiatry. 2011;168(7):681-688.
18. Rutherford B, Sneed J, Miyazaki M, et al. An open trial of aripiprazole augmentation for SSRI non-remitters with late-life depression. Int J Geriatr Psychiatry. 2007;22(10):986-991.
19. Sheffrin M, Driscoll HC, Lenze EJ, et al. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: getting to remission. J Clin Psychiatry. 2009;70(2):208-213.
20. Steffens DC, Nelson JC, Eudicone JM, et al. Efficacy and safety of adjunctive aripiprazole in major depressive disorder in older patients: a pooled subpopulation analysis. Int J Geriatr Psychiatry. 2011;26(6):564-572.
21. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10011):2404-2412.
22. Deligiannidis KM, Rothschild AJ, Barton BA, et al. A gender analysis of the study of pharmacotherapy of psychotic depression (STOP-PD): gender and age as predictors of response and treatment-associated changes in body mass index and metabolic measures. J Clin Psychiatry. 2013;74(10):1003-1009.
23. Flint AJ, Iaboni A, Mulsant BH, et al. Effect of sertraline on risk of falling in older adults with psychotic depression on olanzapine: results of a randomized placebo-controlled trial. Am J Geriatr Psychiatry. 2014;22(4):332-336.
24. Smith E, Rothschild AJ, Heo M, et al. Weight gain during olanzapine treatment for psychotic depression: effects of dose and age. Int Clin Psychopharmacol. 2008;23(3):130-137.
25. Blumberger DM, Mulsant BH, Kanellopoulos D, et al. The incidence of tardive dyskinesia in the study of pharmacotherapy for psychotic depression. J Clin Psychopharmacol. 2013;33(3):391-397.
26. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry. 2008;16(1):21-30.
27. Katila H, Mezhebovsky I, Mulroy A, et al. Randomized, double-blind study of the efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in elderly patients with major depressive disorder. Am J Geriatr Psychiatry. 2013;21(8):769-784.
28. Sultana J, Trifiro G. Drug safety warnings: a message in a bottle. J Drug Des Res. 2014;1(1):1004.
29. Flint A, Meyers BS, Rothschild AR, et al; STOP-PD II Study Group. Sustaining remission of psychotic depression: rationale, design and methodology of STOP-PD II. BMC Psychiatry. 2013;13:38.
30. Alexopoulos GS; PROSPECT Group. Interventions for depressed elderly primary care patients. Int J Geriatr Psychiatry. 2001;16(6):553-559.
31. Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
32. Bigos KL, Bies RR, Pollock BG, et al. Genetic variation in CYP3A43 explains racial difference in olanzapine clearance. Mol Psychiatry. 2011;16(6):620-625.
33. Jin Y, Pollock BG, Coley K, et al. Population pharmacokinetics of perphenazine in schizophrenia patients from CATIE: impact of race and smoking. J Clin Pharmacol. 2010;50(1):73-80.
34. Mulsant BH. Is there a role for antidepressant and antipsychotic pharmacogenetics in clinical practice in 2014? Can J Psychiatry. 2014;59(2):59-61.
Errors of omission and commission in psychiatric practice
There are many rewards for full-time academic psychiatrists such as myself, including didactic teaching, clinical supervision, and 1:1 mentorship of freshly minted medical school graduates, transforming them into accomplished clinical psychiatrists. The technical and personal growth of psychiatric residents over 4 years of post-MD training can be amazing and very gratifying to witness.
But the road to clinical competence often is littered with mistakes. It is the duty of the clinical supervisor to convert every error into a learning opportunity to hone the skills of a future psychiatrist. Over time, fewer mistakes occur, not only because of maturity and seasoning, but also because psychiatric residents learn how to thoughtfully deliberate about their clinical decision-making to select the best treatment option for their patients.
Yet, even with exemplary training, the rigors and constraints of clinical practice inevitably lead to some unforced errors, mostly minor but sometimes consequential. Even experienced practitioners are not immune from making a mistake in the hustle and bustle of daily work (exacerbated by the time-consuming pressures of electronic health record documentation). No one is infallible, but everyone must avoid making the same mistake twice, even if mounting demands lead to “shortcuts” that may not necessarily put the patient at risk but could lead to suboptimal outcomes. But once in a while, a serious complication may ensue.
Here are some common errors of omission or commission that even competent practitioners may make in a busy clinical practice.
Rushing to a diagnosis. To arrive at a primary psychiatric diagnosis, all potential secondary causes must be ruled out. This includes systematic screening for possible drug-induced psychopathology related not only to drugs of abuse, but also to prescription medications, some of which can have serious iatrogenic effects, including depression, anxiety, mania, psychosis, or cognitive dulling. The other important cause to rule out is the possibility of a general medical condition triggering psychiatric symptoms, which requires targeted questioning about medical history, a review of organ systems, and ordering key laboratory tests.
Skipping a baseline cognitive assessment. Cognitive impairment, especially memory and executive function, is now well recognized as an important component of major psychiatric disorders, including schizophrenia, bipolar disorder, major depressive disorder, anxiety, and attention-deficit/hyperactivity disorder. A standardized cognitive battery can provide a valuable profile of brain functions. Knowing the patient’s cognitive strengths and weaknesses before initiating pharmacotherapy is essential to assess the positive or negative impact of the medications. It also can help with patients’ vocational rehabilitation, matching them with jobs compatible with their cognitive strengths.
Inaccurate differential diagnosis. Is it borderline personality or bipolar disorder? Is it schizophrenia or psychotic bipolar disorder? Is it unipolar or bipolar depression? Is it a conversion reaction or a genuine medical condition? The answers to such questions are critical, because inaccurate diagnosis can lead to a lack of improvement and prolonged suffering for patients or adverse effects that could be avoided.
Using a high dose of a medication immediately for a first-episode psychiatric disorder. One of the least patient-friendly medical decisions is to start a first-episode patient on a high dose of a medication on day 1. Gradual titration can circumvent intolerable adverse effects and help establish the lowest effective dose. Patient acceptance and adherence are far more likely if the patient’s brain is not “abruptly medicated.”
Using combination therapy right away. There are a few psychiatric conditions for which combination therapy is FDA-approved and regarded as “rational polypharmacy.” However, it always makes sense to start with 1 (primary) medication and assess its efficacy, tolerability, and safety before adding an adjunctive agent. Some patients may improve substantially with monotherapy, which is always preferable. Using drug combinations as the initial intervention can be problematic, especially if they are not evidence-based and off-label.
Selecting an obesogenic drug as first-line. Many psychotropics, such as antipsychotics, antidepressants, or mood stabilizers, often come as a class of several agents. Clinicians can select any member of the same class (such as selective serotonin reuptake inhibitors [SSRIs] or atypical antipsychotics) because they are all FDA-approved for efficacy. However, the major difference among what often are called “me too” drugs is the adverse effects profile. For many psychotropic medications, significant weight gain is one of the worst medical adverse effects, because it often leads to metabolic dysregulation (hyperglycemia, dyslipidemia, and hypertension) and increases the risk of cardiovascular disease. Many psychiatric patients become obese and have great difficulty losing weight, especially if they are sedentary and have poor eating habits.
Using benzodiazepines as a first-line treatment for anxiety. Although certainly efficacious, and rapidly so, benzodiazepines must be avoided as a first-line treatment for anxiety. The addiction potential is significant, and patients with anxiety will subsequently not respond adequately to standard anxiolytic pharmacotherapy, such as an SSRI, because the anxiolytic effect of these other medications is gradual and not as rapid or potent. Some primary care providers (PCPs) resort to using strong benzodiazepines (such as alprazolam) as first-line, and then refer the patient to a psychiatrist, who finds it quite challenging to steer the patient to an evidence-based option that is less harmful for long-term management. The residents and I have encountered such situations often, sometimes leading to complex interactions with patients who demand renewal of a high dose of a benzodiazepine that had been prescribed to them by a different clinician.
Low utilization of some efficacious agents. It is surprising how some useful pharmacotherapeutic strategies are not employed as often as they should be. This includes lithium for a manic episode; a long-acting injectable antipsychotic in the early phase of schizophrenia; clozapine for patients who failed to respond to a couple of antipsychotics or have chronic suicidal tendencies; lurasidone or quetiapine for bipolar depression (the only FDA-approved medications for this condition); or monoamine oxidase inhibitors for treatment-resistant depression. These drugs can be useful, although some require ongoing blood-level measurements and monitoring for efficacy and adverse effects.
Not recognizing tardive dyskinesia (TD) earlier. TD is one of the most serious neurologic complications of dopamine-receptor working agents (antipsychotics). FDA-approved treatments finally arrived in 2017, but the recognition of the abnormal oro-bucco-lingual or facial choreiform movements remain low (and the use of the Abnormal Involuntary Movement Scale to screen for TD has faded since second-generation antipsychotics were introduced). It is essential to identify this adverse effect early and treat it promptly to avoid its worsening and potential irreversibility.
Other errors of omission or commission include:
- Not collaborating actively with the patient’s PCP to integrate the medical care to improve the patient’s overall health, not just mental health. Collaborative care improves clinical outcomes for most patients.
- Not using available pharmacogenetics testing to provide the patient with “personalized medicine,” such as establishing if they are poor or rapid metabolizers of certain cytochrome hepatic enzymes or checking whether they are less likely to respond to antidepressant medications.
- “Lowering expectations” for patients with severe psychiatric disorders, giving them the message (verbally or nonverbally) that their condition is “hopeless” and that recovery is beyond their reach. Giving hope and trying hard to find better treatment options are the foundation of good medical practice, especially for the sickest patients.
Psychiatrists always aim to do the right thing for their patients, even when the pressures of clinical practice are intense and palpable. But sometimes, an inadvertent slip may occur in the form of an error of omission or commission. These unforced errors are rarely dangerous, but they have the potential to delay response, increase the disease burden, or complicate the illness course. Compassion may be in generous supply, but it’s not enough: We must strive to make our patient-centered, evidence-based clinical practice an error-free zone.
There are many rewards for full-time academic psychiatrists such as myself, including didactic teaching, clinical supervision, and 1:1 mentorship of freshly minted medical school graduates, transforming them into accomplished clinical psychiatrists. The technical and personal growth of psychiatric residents over 4 years of post-MD training can be amazing and very gratifying to witness.
But the road to clinical competence often is littered with mistakes. It is the duty of the clinical supervisor to convert every error into a learning opportunity to hone the skills of a future psychiatrist. Over time, fewer mistakes occur, not only because of maturity and seasoning, but also because psychiatric residents learn how to thoughtfully deliberate about their clinical decision-making to select the best treatment option for their patients.
Yet, even with exemplary training, the rigors and constraints of clinical practice inevitably lead to some unforced errors, mostly minor but sometimes consequential. Even experienced practitioners are not immune from making a mistake in the hustle and bustle of daily work (exacerbated by the time-consuming pressures of electronic health record documentation). No one is infallible, but everyone must avoid making the same mistake twice, even if mounting demands lead to “shortcuts” that may not necessarily put the patient at risk but could lead to suboptimal outcomes. But once in a while, a serious complication may ensue.
Here are some common errors of omission or commission that even competent practitioners may make in a busy clinical practice.
Rushing to a diagnosis. To arrive at a primary psychiatric diagnosis, all potential secondary causes must be ruled out. This includes systematic screening for possible drug-induced psychopathology related not only to drugs of abuse, but also to prescription medications, some of which can have serious iatrogenic effects, including depression, anxiety, mania, psychosis, or cognitive dulling. The other important cause to rule out is the possibility of a general medical condition triggering psychiatric symptoms, which requires targeted questioning about medical history, a review of organ systems, and ordering key laboratory tests.
Skipping a baseline cognitive assessment. Cognitive impairment, especially memory and executive function, is now well recognized as an important component of major psychiatric disorders, including schizophrenia, bipolar disorder, major depressive disorder, anxiety, and attention-deficit/hyperactivity disorder. A standardized cognitive battery can provide a valuable profile of brain functions. Knowing the patient’s cognitive strengths and weaknesses before initiating pharmacotherapy is essential to assess the positive or negative impact of the medications. It also can help with patients’ vocational rehabilitation, matching them with jobs compatible with their cognitive strengths.
Inaccurate differential diagnosis. Is it borderline personality or bipolar disorder? Is it schizophrenia or psychotic bipolar disorder? Is it unipolar or bipolar depression? Is it a conversion reaction or a genuine medical condition? The answers to such questions are critical, because inaccurate diagnosis can lead to a lack of improvement and prolonged suffering for patients or adverse effects that could be avoided.
Using a high dose of a medication immediately for a first-episode psychiatric disorder. One of the least patient-friendly medical decisions is to start a first-episode patient on a high dose of a medication on day 1. Gradual titration can circumvent intolerable adverse effects and help establish the lowest effective dose. Patient acceptance and adherence are far more likely if the patient’s brain is not “abruptly medicated.”
Using combination therapy right away. There are a few psychiatric conditions for which combination therapy is FDA-approved and regarded as “rational polypharmacy.” However, it always makes sense to start with 1 (primary) medication and assess its efficacy, tolerability, and safety before adding an adjunctive agent. Some patients may improve substantially with monotherapy, which is always preferable. Using drug combinations as the initial intervention can be problematic, especially if they are not evidence-based and off-label.
Selecting an obesogenic drug as first-line. Many psychotropics, such as antipsychotics, antidepressants, or mood stabilizers, often come as a class of several agents. Clinicians can select any member of the same class (such as selective serotonin reuptake inhibitors [SSRIs] or atypical antipsychotics) because they are all FDA-approved for efficacy. However, the major difference among what often are called “me too” drugs is the adverse effects profile. For many psychotropic medications, significant weight gain is one of the worst medical adverse effects, because it often leads to metabolic dysregulation (hyperglycemia, dyslipidemia, and hypertension) and increases the risk of cardiovascular disease. Many psychiatric patients become obese and have great difficulty losing weight, especially if they are sedentary and have poor eating habits.
Using benzodiazepines as a first-line treatment for anxiety. Although certainly efficacious, and rapidly so, benzodiazepines must be avoided as a first-line treatment for anxiety. The addiction potential is significant, and patients with anxiety will subsequently not respond adequately to standard anxiolytic pharmacotherapy, such as an SSRI, because the anxiolytic effect of these other medications is gradual and not as rapid or potent. Some primary care providers (PCPs) resort to using strong benzodiazepines (such as alprazolam) as first-line, and then refer the patient to a psychiatrist, who finds it quite challenging to steer the patient to an evidence-based option that is less harmful for long-term management. The residents and I have encountered such situations often, sometimes leading to complex interactions with patients who demand renewal of a high dose of a benzodiazepine that had been prescribed to them by a different clinician.
Low utilization of some efficacious agents. It is surprising how some useful pharmacotherapeutic strategies are not employed as often as they should be. This includes lithium for a manic episode; a long-acting injectable antipsychotic in the early phase of schizophrenia; clozapine for patients who failed to respond to a couple of antipsychotics or have chronic suicidal tendencies; lurasidone or quetiapine for bipolar depression (the only FDA-approved medications for this condition); or monoamine oxidase inhibitors for treatment-resistant depression. These drugs can be useful, although some require ongoing blood-level measurements and monitoring for efficacy and adverse effects.
Not recognizing tardive dyskinesia (TD) earlier. TD is one of the most serious neurologic complications of dopamine-receptor working agents (antipsychotics). FDA-approved treatments finally arrived in 2017, but the recognition of the abnormal oro-bucco-lingual or facial choreiform movements remain low (and the use of the Abnormal Involuntary Movement Scale to screen for TD has faded since second-generation antipsychotics were introduced). It is essential to identify this adverse effect early and treat it promptly to avoid its worsening and potential irreversibility.
Other errors of omission or commission include:
- Not collaborating actively with the patient’s PCP to integrate the medical care to improve the patient’s overall health, not just mental health. Collaborative care improves clinical outcomes for most patients.
- Not using available pharmacogenetics testing to provide the patient with “personalized medicine,” such as establishing if they are poor or rapid metabolizers of certain cytochrome hepatic enzymes or checking whether they are less likely to respond to antidepressant medications.
- “Lowering expectations” for patients with severe psychiatric disorders, giving them the message (verbally or nonverbally) that their condition is “hopeless” and that recovery is beyond their reach. Giving hope and trying hard to find better treatment options are the foundation of good medical practice, especially for the sickest patients.
Psychiatrists always aim to do the right thing for their patients, even when the pressures of clinical practice are intense and palpable. But sometimes, an inadvertent slip may occur in the form of an error of omission or commission. These unforced errors are rarely dangerous, but they have the potential to delay response, increase the disease burden, or complicate the illness course. Compassion may be in generous supply, but it’s not enough: We must strive to make our patient-centered, evidence-based clinical practice an error-free zone.
There are many rewards for full-time academic psychiatrists such as myself, including didactic teaching, clinical supervision, and 1:1 mentorship of freshly minted medical school graduates, transforming them into accomplished clinical psychiatrists. The technical and personal growth of psychiatric residents over 4 years of post-MD training can be amazing and very gratifying to witness.
But the road to clinical competence often is littered with mistakes. It is the duty of the clinical supervisor to convert every error into a learning opportunity to hone the skills of a future psychiatrist. Over time, fewer mistakes occur, not only because of maturity and seasoning, but also because psychiatric residents learn how to thoughtfully deliberate about their clinical decision-making to select the best treatment option for their patients.
Yet, even with exemplary training, the rigors and constraints of clinical practice inevitably lead to some unforced errors, mostly minor but sometimes consequential. Even experienced practitioners are not immune from making a mistake in the hustle and bustle of daily work (exacerbated by the time-consuming pressures of electronic health record documentation). No one is infallible, but everyone must avoid making the same mistake twice, even if mounting demands lead to “shortcuts” that may not necessarily put the patient at risk but could lead to suboptimal outcomes. But once in a while, a serious complication may ensue.
Here are some common errors of omission or commission that even competent practitioners may make in a busy clinical practice.
Rushing to a diagnosis. To arrive at a primary psychiatric diagnosis, all potential secondary causes must be ruled out. This includes systematic screening for possible drug-induced psychopathology related not only to drugs of abuse, but also to prescription medications, some of which can have serious iatrogenic effects, including depression, anxiety, mania, psychosis, or cognitive dulling. The other important cause to rule out is the possibility of a general medical condition triggering psychiatric symptoms, which requires targeted questioning about medical history, a review of organ systems, and ordering key laboratory tests.
Skipping a baseline cognitive assessment. Cognitive impairment, especially memory and executive function, is now well recognized as an important component of major psychiatric disorders, including schizophrenia, bipolar disorder, major depressive disorder, anxiety, and attention-deficit/hyperactivity disorder. A standardized cognitive battery can provide a valuable profile of brain functions. Knowing the patient’s cognitive strengths and weaknesses before initiating pharmacotherapy is essential to assess the positive or negative impact of the medications. It also can help with patients’ vocational rehabilitation, matching them with jobs compatible with their cognitive strengths.
Inaccurate differential diagnosis. Is it borderline personality or bipolar disorder? Is it schizophrenia or psychotic bipolar disorder? Is it unipolar or bipolar depression? Is it a conversion reaction or a genuine medical condition? The answers to such questions are critical, because inaccurate diagnosis can lead to a lack of improvement and prolonged suffering for patients or adverse effects that could be avoided.
Using a high dose of a medication immediately for a first-episode psychiatric disorder. One of the least patient-friendly medical decisions is to start a first-episode patient on a high dose of a medication on day 1. Gradual titration can circumvent intolerable adverse effects and help establish the lowest effective dose. Patient acceptance and adherence are far more likely if the patient’s brain is not “abruptly medicated.”
Using combination therapy right away. There are a few psychiatric conditions for which combination therapy is FDA-approved and regarded as “rational polypharmacy.” However, it always makes sense to start with 1 (primary) medication and assess its efficacy, tolerability, and safety before adding an adjunctive agent. Some patients may improve substantially with monotherapy, which is always preferable. Using drug combinations as the initial intervention can be problematic, especially if they are not evidence-based and off-label.
Selecting an obesogenic drug as first-line. Many psychotropics, such as antipsychotics, antidepressants, or mood stabilizers, often come as a class of several agents. Clinicians can select any member of the same class (such as selective serotonin reuptake inhibitors [SSRIs] or atypical antipsychotics) because they are all FDA-approved for efficacy. However, the major difference among what often are called “me too” drugs is the adverse effects profile. For many psychotropic medications, significant weight gain is one of the worst medical adverse effects, because it often leads to metabolic dysregulation (hyperglycemia, dyslipidemia, and hypertension) and increases the risk of cardiovascular disease. Many psychiatric patients become obese and have great difficulty losing weight, especially if they are sedentary and have poor eating habits.
Using benzodiazepines as a first-line treatment for anxiety. Although certainly efficacious, and rapidly so, benzodiazepines must be avoided as a first-line treatment for anxiety. The addiction potential is significant, and patients with anxiety will subsequently not respond adequately to standard anxiolytic pharmacotherapy, such as an SSRI, because the anxiolytic effect of these other medications is gradual and not as rapid or potent. Some primary care providers (PCPs) resort to using strong benzodiazepines (such as alprazolam) as first-line, and then refer the patient to a psychiatrist, who finds it quite challenging to steer the patient to an evidence-based option that is less harmful for long-term management. The residents and I have encountered such situations often, sometimes leading to complex interactions with patients who demand renewal of a high dose of a benzodiazepine that had been prescribed to them by a different clinician.
Low utilization of some efficacious agents. It is surprising how some useful pharmacotherapeutic strategies are not employed as often as they should be. This includes lithium for a manic episode; a long-acting injectable antipsychotic in the early phase of schizophrenia; clozapine for patients who failed to respond to a couple of antipsychotics or have chronic suicidal tendencies; lurasidone or quetiapine for bipolar depression (the only FDA-approved medications for this condition); or monoamine oxidase inhibitors for treatment-resistant depression. These drugs can be useful, although some require ongoing blood-level measurements and monitoring for efficacy and adverse effects.
Not recognizing tardive dyskinesia (TD) earlier. TD is one of the most serious neurologic complications of dopamine-receptor working agents (antipsychotics). FDA-approved treatments finally arrived in 2017, but the recognition of the abnormal oro-bucco-lingual or facial choreiform movements remain low (and the use of the Abnormal Involuntary Movement Scale to screen for TD has faded since second-generation antipsychotics were introduced). It is essential to identify this adverse effect early and treat it promptly to avoid its worsening and potential irreversibility.
Other errors of omission or commission include:
- Not collaborating actively with the patient’s PCP to integrate the medical care to improve the patient’s overall health, not just mental health. Collaborative care improves clinical outcomes for most patients.
- Not using available pharmacogenetics testing to provide the patient with “personalized medicine,” such as establishing if they are poor or rapid metabolizers of certain cytochrome hepatic enzymes or checking whether they are less likely to respond to antidepressant medications.
- “Lowering expectations” for patients with severe psychiatric disorders, giving them the message (verbally or nonverbally) that their condition is “hopeless” and that recovery is beyond their reach. Giving hope and trying hard to find better treatment options are the foundation of good medical practice, especially for the sickest patients.
Psychiatrists always aim to do the right thing for their patients, even when the pressures of clinical practice are intense and palpable. But sometimes, an inadvertent slip may occur in the form of an error of omission or commission. These unforced errors are rarely dangerous, but they have the potential to delay response, increase the disease burden, or complicate the illness course. Compassion may be in generous supply, but it’s not enough: We must strive to make our patient-centered, evidence-based clinical practice an error-free zone.
The placebo effect in psychiatric practice
“It is a mystery how a ubiquitous treatment used since antiquity was unknown, unnamed, and unidentified until recently. It is even more remarkable because this is the only treatment common to all societies and cultures.”1
The treatment discussed above is not a specific pill, surgery, plant, or herb. Rather, the authors are referring to placebo. Indeed, the history of medical treatment is largely a chronicle of placebos. When subjected to scientific scrutiny, the overwhelming majority of treatments have turned out to be devoid of intrinsic therapeutic value; they derived their benefits from the placebo effect. Despite these benefits, the term “placebo” comes with unfortunate baggage. Latin for “I shall please,” it is the first word of the Christian vespers for the dead. In the 12th century these vespers were commonly referred to as placebos. By the 1300s, the term had become secular and pejorative, suggesting a flatterer or sycophant. When the word entered medical terminology in the late 18th century, the negative connotation stuck. A placebo was defined as a medicine given to please patients rather than to benefit them. In the modern era, the lack of pharmacologic activity became part of the definition as well.
The word placebo brings with it connotations of deception, fakery, and ineffectiveness. But one of the things about placebos that contribute mightily to the health care community’s aversion toward them is, in fact, their effectiveness. They bring relief across a wide range of medical conditions.2 In doing so, placebos impugn the value of our most cherished remedies, hamper the development of new therapeutics, and threaten our livelihoods as health professionals.3
Placebos often are conceptualized as any treatment that lacks intrinsic therapeutic value, such as sugar pills. But looking at what placebo treatment actually entails, both in placebo-controlled treatment trials and in clinical settings, suggests a more comprehensive definition. Placebos encompass all the elements common to any treatment or healing situation. These include a recognized healer, evaluation, diagnosis, prognosis, plausible treatment, and most importantly, the expectation that one will recover. Along these lines, the placebo response can be thought of as the response to the common elements of the treatment or healing situation.3
Research regarding the placebo effect has mushroomed in the past 2 decades. Over this time, we have learned a good deal about both the mechanisms underlying the placebo effect and how the placebo effect can be applied to enhance the benefit of conventional treatment. Brain imaging technology has revealed that when placebo treatment alleviates pain, Parkinson’s disease, and depression, brain changes occur that are similar to those observed with active pharmacologic treatment.4,5 Recent studies also show that deliberate, open (nondeceptive) use of placebo can improve the symptoms of several conditions, including depression, pain, and irritable bowel syndrome.6 Furthermore, intermittent substitution of placebo pills for pharmacologically active treatment in a conditioning paradigm can be as effective as the “real” treatment.7 Also, research over the past decade has verified that certain common features of the treatment situation, particularly the quality of the doctor–patient encounter, contribute to the placebo response and have a demonstrable impact on the outcome of treatment.8 Clearly, the placebo effect has gone from being simply a nuisance that interferes with the evaluation of new treatments to a variable worthy of study and application in its own right. Although, for the most part, clinical practice has not kept up with these advances.
Placebos seem to have their greatest impact on the subjective symptoms of disease—pain, distress, and discouragement. It should come as no surprise, then, that placebos are particularly effective in certain psychiatric conditions. In some forms of anxiety and depressive disorders, for example, distress is the illness, and placebos reliably bring relief. Patients with panic disorder, mild to moderate depression, or generalized anxiety disorder get almost as much relief with placebo as they do with conventional treatment (about one-half improve with placebo).9-11 But <20% of those with obsessive-compulsive disorder improve with placebo, and placebo response rates are also low in patients with schizophrenia or dementia. Mania, attention-deficit/hyperactivity disorder (ADHD), and severe depression fall somewhere in the middle.3
Harnessing the placebo response
There may be a few circumstances in psychiatric practice when it makes sense to intentionally prescribe a placebo as treatment, and we discuss those below. But far more frequently, what we know about the elements that contribute to the placebo effect can be applied to enhance the benefits of any treatment. Patients might be best served if deliberate mobilization of the placebo effect was a standard adjunct to conventional clinical care.
Various components of the treatment situation, collectively referred to as placebo, are a powerful antidote for illness, and some of these healing components exert their influence without special activity on the clinician’s part:
- Simply seeking psychiatric care can bring relief by providing some sense of control over distressing symptoms. The standard trappings of the office or clinic and customary office procedures—from the presentation of one’s insurance card to taking a history—offer reassurance and evoke the expectation that improvement or recovery is around the corner.
- The comfort provided by the psychiatrist’s presence is enhanced when patients feel that they are in the hands of a recognized healer. Psychiatrists inspire confidence when they look like a psychiatrist, or more precisely, like the patient’s idea of what a psychiatrist should look like. In our culture, that means a white coat or business attire.
A thorough evaluation is one of the common treatment elements that does the most to reduce distress and inspire confidence. The quality of an evaluation bears a strong relationship to patients’ satisfaction with the medical encounter, and can influence the amount of disability they suffer.3,12-15
Although guidelines for conducting effective psychiatric interviews have been around for almost 100 years, psychiatrists vary considerably in the extent to which they elicit complete and accurate information, build rapport, give patients the sense that they are listened to, and provide a thorough assessment. The degree to which patients feel that the clinician is responsive to their concerns depends as much on the style of the interview as on the amount of time devoted to it. Nonverbal behavior can carry the message that the clinician is paying full attention. Something as simple as not answering the phone during an interview (this seems obvious, but a surprising and troubling number of mental health professionals take phone calls during interviews and treatment sessions) conveys an important message about the importance that the clinician places on the patient’s problems.3
The idea that the treatment situation itself provides reassurance and reduces distress, and in doing so, powers a good bit of the placebo effect, is enshrined in such concepts as the importance of good bedside manner. Many feel that the doctor’s thoughtful attention, positive regard, and optimism—so valued by patients—are justified on humanitarian grounds alone; actual evidence that this caring behavior contributes to healing isn’t required. To many, the healing properties of the treatment situation are self-evident. But as the costs of health care snowball and the demands for efficiency and cost-effectiveness rise, the time that psychiatrists can devote to patients has dwindled. Third-party payors demand evidence, beyond intuition and common sense, that diagnostic procedures and treatments have some usefulness, and rightly so.
Is there any evidence that the common components of the treatment situation provide benefit?3 More specifically, does the quality of the doctor–patient relationship and the patient’s feelings about a therapeutic encounter promote healing? Several studies suggest that the doctor–patient relationship has a demonstrable impact on symptom relief.16 In 1 study, oncologists were randomly assigned to receive a Communication Skills Training (CST) program or not. CST included a 1.5-day face-to-face workshop and 6 hours of monthly videoconferencing that focused on improving communication skills with patients.17 Lessons included building rapport, engaging in appropriate eye contact, and normalizing difficult experiences. One week after initially consulting with their physician, patients who saw an oncologist in the CST group experienced less anxiety and depression than those who saw an oncologist who did not receive CST. The benefit of CST for patient anxiety mostly persisted at a 3-month follow-up.
A recent meta-analysis pooled the results of 47 studies to examine the relationship between how much trust patients have for their doctors and health outcomes. There was a small to medium association: More trust was associated with greater improvement.18 It is possible that a good doctor–patient relationship enhances expectancies. However, it is also likely that a positive therapeutic relationship is inherently soothing and reduces distress or dysfunction independent of expectation. Regardless of the precise mechanism, these studies warrant attention. We all understand that it is important on ethical grounds to treat patients with respect and kindness. Research shows that this type of behavior also promotes recovery.
Patient expectations. The idea that expectation of improvement has a major impact on treatment outcome is firmly grounded in research on the placebo effect. Studies have shown that what people expect to experience as an outcome of treatment has a substantial impact on what they actually experience. In a classic study, a doctor told some patients with symptoms of minor illness that they would feel better soon and another group with the same symptoms that he didn’t know what ailed them.19 Two weeks later, 64% of patients in the “positive expectation” group were improved, compared with only 39% of patients in the “negative” group. In another study, adults were exposed to an allergen that caused a skin reaction.20 Hand lotion (ie, a therapeutically inert substance) was then spread on the skin. Patients were led to believe that the cream would either alleviate or exacerbate the itching. The experimentally-induced wheal-and-flare was measured in both groups a few minutes after the allergen and cream were applied. The wheal-and-flare were worse for participants in the group that expected exacerbation.
Not uncommonly, expectation can have more impact on clinical outcome than a drug’s pharmacologic activity. In a double-blind placebo-controlled study, patients with depression were treated with St. John’s wort, sertraline, or placebo.21 They improved to the same extent with all 3 treatments. But when patients were asked to guess the treatment to which they had been assigned, those who thought they had received placebo showed little improvement, irrespective of which intervention they actually received, and those who guessed they had been given St. John’s wort or sertraline showed uniformly large improvement, irrespective of which intervention they actually received (including placebo). The researchers concluded that “Patient beliefs regarding treatment may have a stronger association with clinical outcome than the actual medication received.”
Psychiatrists who wish to use all the therapeutic tools at their disposal must attend to and manage patient expectations. One part of channeling a patient’s expectation is to thoroughly assess the patient’s beliefs regarding the efficacy of various treatments. If a patient’s uncle said that a certain drug is a miracle cure for anxiety, and the patient believes it to be true, then that expectation must be taken into consideration. Many patients prefer alternative treatments to conventional therapies. As long as there is no reason to think an alternative treatment will cause harm, a compromise might be reasonable. For example, if a patient with schizophrenia wants to treat her symptoms with herbal tea, the psychiatrist could say, “In addition to the tea, I recommend that you also take clozapine. The combination is likely to improve your symptoms.”3 More than anything else, the words a psychiatrist uses when recommending treatment shape the patient’s expectations. “You should be feeling a lot less anxious soon after you start taking this” has a different effect than “Try this. It may help.”
Prescribing ‘open-label’ placebo
There may be some limited circumstances where an actual placebo (eg, a sugar pill) might be suitable as a treatment. These include when placebo and conventional treatment provide similar results and a patient is reluctant to take conventional medicine, or when there is no effective conventional treatment. The deceptive prescription of placebo (providing placebo and calling it a drug) has a long history and was considered ethical—and recommended by medical authorities—until the latter half of the 20th century. This practice was deemed unethical in the 1980s, because it was dishonest and violated patient autonomy. Because it was widely believed that placebos given openly would be ineffective, the end of placebo treatment seemed at hand. An intriguing body of evidence, however, suggests that placebos can be effective even when patients know they are taking a placebo. Patients given an “open-label” placebo are told something along the lines of “the pill being prescribed contains no medicine, but some people improve with it, perhaps because the pill stimulates the body’s self-healing.” Open-label placebo has been evaluated for depression,22 low back pain,23 irritable bowel syndrome,24 neurosis,25 allergic rhinitis,26 and anxiety.27 Most of these studies are small, and some were uncontrolled. Yet they consistently have shown that symptoms improve with a nondeceptive placebo, and improve to a greater extent than with no treatment.
The most recent trial is a promising example of the potential of open-label placebos. In this study, 96 patients with chronic low back pain were randomly assigned to 3 weeks of treatment as usual (TAU) or 3 weeks of TAU plus open-label placebo.23 Patients who received open-label placebo were educated about the placebo effect and shown a film clip describing promising results of a prior open-label placebo study. They were then given placebo pills to be take once daily, and clearly told the pills contained no active medication. After 3 weeks, patients in the TAU plus placebo group reported less pain and less disability than patients who received TAU without a placebo. Some patients even requested a placebo prescription at the end of the study.
The placebo response provides a rational basis for prescribing innocuous alternative therapies with no intrinsic therapeutic value. Patients who prefer and believe in the effectiveness of alternative remedies—herbal compounds, massage, magnets, homeopathic solutions, etc.—can be recommended these treatments to mobilize a placebo response.
Using a conditioning model. Prescribing a placebo to obtain a conditioned drug response has enormous but untapped clinical potential. Both animal and human research indicates that a wide range of drug responses, from immune suppression to motor stimulation, can be conditioned (a neutral stimulus, such as a pill or injection, associated with drug administration can in itself evoke the drug effect). In many conditioning or dose-extending models, a particular response to real medication (such as pain relief after analgesics) first becomes conditioned due to repeated exposure to the drug given in a particular vehicle. Then, the treatment shifts to some doses comprising of real medicine and some doses comprising of placebo. Because the drug response has been conditioned, it is thought that the response to an identically appearing placebo will mirror the drug response. The active drug often is only replaced by placebo for certain doses under a schedule of partial reinforcement, given the ubiquity of extinction (the conditioned response lessens when the conditioned stimulus is presented alone on repeated trials).
In 1 version of a conditioning study, children with ADHD were randomized to 1 of 3 groups.28 One group (full dose) took the standard dose of medication for 2 months, a second group (reduced dose) took a standard dose during 1 month followed by a half dose during the second month, and children in the third group (reduced dose with placebo) took the standard dose plus a visually distinctive placebo during the first month, followed by a half dose plus the visually distinctive placebo during the second month. Not surprisingly, ADHD symptoms were worse among children in the reduced-dose group. However, there was no difference between those in the reduced-dose with placebo group and those in the full-dose group. It appears as though the symptom reduction associated with a 100% dose was an unconditioned response that could be mimicked with the addition of a placebo pill.
In another study, patients with psoriasis were randomly assigned to receive a full dose of active medication (0.1% triamcinolone cream) twice a day, or a full dose of active medication for 25% to 50% of the doses, with a placebo (moisturizing cream) given for the other 50% to 75% of the doses.29 Relapse rates were not statistically different between groups.
1. Shapiro AK, Shapiro E. The powerful placebo: from ancient priest to modern physician. Baltimore, MD: Johns Hopkins University Press; 1997.
2. Beecher HK. The powerful placebo. J Am Med Assoc. 1955;159(17):1602-1606.
3. Brown WA. The placebo effect in clinical practice. New York, NY: Oxford University Press; 2013.
4. Mayberg HS, Silva JA, Brannan SK, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159(5):728-737.
5. de la Fuente-Fernández R, Ruth TJ, Sossi V, et al. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293(5532):1164-1166.
6. Charlesworth JEG, Petkovic G, Kelley JM, et al. Effects of placebos without deception compared with no treatment: a systematic review and meta‐analysis. J Evid Based Med. 2017;10(2):97-107.
7. Colloca L, Enck P, DeGrazia D. Relieving pain using dose-extending placebos: a scoping review. Pain. 2016;157(8):1590-1598.
8. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336(7651):999-1003.
9. Khan A, Kolts RL, Rapaport MH, et al. Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med. 2005;35(5):743-749.
10. Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840-1847.
11. Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5(2):e45.
12. Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136(5):374-383.
13. Kelley JM, Kraft-Todd G, Schapira L, et al. The influence of the patient-clinician relationship on healthcare outcomes: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(4):e94207.
14. Olsson B, Olsson B, Tibblin G. Effect of patients’ expectations on recovery from acute tonsillitis. Fam Pract. 1989;6(3):188-192.
15. Sox HC, Margulies I, Sox CH. Psychologically mediated effects of diagnostic tests. Ann Intern Med. 1981;95(6):680-685.
16. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433.
17. Girgis A, Cockburn J, Butow P, et al. Improving patient emotional functioning and psychological morbidity: evaluation of a consultation skills training program for oncologists. Patient Educ Couns. 2009;77(3):456-462.
18. Birkhäuer J, Gaab J, Kossowsky J, et al. Trust in the health care professional and health outcome: a meta-analysis. PLoS One. 2017;12(2):e0170988.
19. Thomas KB. General practice consultations: is there any point in being positive? Br Med J (Clin Res Ed). 1987;294(6581):1200-1202.
20. Howe LC, Goyer JP, Crum AJ. Harnessing the placebo effect: exploring the influence of physician characteristics on placebo response [published online May 9, 2017]. Health Psychol. doi: 10.1037/hea0000499.
21. Chen JA, Papakostas GI, Youn SJ, et al. Association between patient beliefs regarding assigned treatment and clinical response: reanalysis of data from the Hypericum Depression Trial Study Group. J Clin Psychiatry. 2011;72(12):1669-1676.
22. Kelley JM, Kaptchuk TJ, Cusin C, et al. Open-label placebo for major depressive disorder: a pilot randomized controlled trial. Psychother Psychosom. 2012;81(5):312-314.
23. Carvalho C, Caetano JM, Cunha L, et al. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain. 2016;157(12):2766-2772.
24. Kaptchuk TJ, Friedlander E, Kelley JM, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5(12):e15591.
25. Park LC, Covi L. Nonblind placebo trial: an exploration of neurotic patients’ responses to placebo when its inert content is disclosed. Arch Gen Psychiatry. 1965;12(4):336-345.
26. Schaefer M, Harke R, Denke C. Open-label placebos improve symptoms in allergic rhinitis: a randomized controlled trial. Psychother Psychosom. 2016;85(6):373-374.
27. Aulas JJ, Rosner I. Efficacy of a non blind placebo prescription [in French]. Encephale. 2003;29(1):68-71.
28. Sandler AD, Glesne CE, Bodfish JW. Conditioned placebo dose reduction: a new treatment in attention deficit hyperactivity disorder? J Dev Behav Pediatr. 2010;31(5):369-375.
29. Ader R, Mercurio MG, Walton J, et al. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom Med. 2010;72(2):192-197.
30. Weiss RD, O’Malley SS, Hosking JD, et al; COMBINE Study Research Group. Do patients with alcohol dependence respond to placebo? Results from the COMBINE Study. J Stud Alcohol Drugs. 2008;69(6):878-884.
31. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207.
“It is a mystery how a ubiquitous treatment used since antiquity was unknown, unnamed, and unidentified until recently. It is even more remarkable because this is the only treatment common to all societies and cultures.”1
The treatment discussed above is not a specific pill, surgery, plant, or herb. Rather, the authors are referring to placebo. Indeed, the history of medical treatment is largely a chronicle of placebos. When subjected to scientific scrutiny, the overwhelming majority of treatments have turned out to be devoid of intrinsic therapeutic value; they derived their benefits from the placebo effect. Despite these benefits, the term “placebo” comes with unfortunate baggage. Latin for “I shall please,” it is the first word of the Christian vespers for the dead. In the 12th century these vespers were commonly referred to as placebos. By the 1300s, the term had become secular and pejorative, suggesting a flatterer or sycophant. When the word entered medical terminology in the late 18th century, the negative connotation stuck. A placebo was defined as a medicine given to please patients rather than to benefit them. In the modern era, the lack of pharmacologic activity became part of the definition as well.
The word placebo brings with it connotations of deception, fakery, and ineffectiveness. But one of the things about placebos that contribute mightily to the health care community’s aversion toward them is, in fact, their effectiveness. They bring relief across a wide range of medical conditions.2 In doing so, placebos impugn the value of our most cherished remedies, hamper the development of new therapeutics, and threaten our livelihoods as health professionals.3
Placebos often are conceptualized as any treatment that lacks intrinsic therapeutic value, such as sugar pills. But looking at what placebo treatment actually entails, both in placebo-controlled treatment trials and in clinical settings, suggests a more comprehensive definition. Placebos encompass all the elements common to any treatment or healing situation. These include a recognized healer, evaluation, diagnosis, prognosis, plausible treatment, and most importantly, the expectation that one will recover. Along these lines, the placebo response can be thought of as the response to the common elements of the treatment or healing situation.3
Research regarding the placebo effect has mushroomed in the past 2 decades. Over this time, we have learned a good deal about both the mechanisms underlying the placebo effect and how the placebo effect can be applied to enhance the benefit of conventional treatment. Brain imaging technology has revealed that when placebo treatment alleviates pain, Parkinson’s disease, and depression, brain changes occur that are similar to those observed with active pharmacologic treatment.4,5 Recent studies also show that deliberate, open (nondeceptive) use of placebo can improve the symptoms of several conditions, including depression, pain, and irritable bowel syndrome.6 Furthermore, intermittent substitution of placebo pills for pharmacologically active treatment in a conditioning paradigm can be as effective as the “real” treatment.7 Also, research over the past decade has verified that certain common features of the treatment situation, particularly the quality of the doctor–patient encounter, contribute to the placebo response and have a demonstrable impact on the outcome of treatment.8 Clearly, the placebo effect has gone from being simply a nuisance that interferes with the evaluation of new treatments to a variable worthy of study and application in its own right. Although, for the most part, clinical practice has not kept up with these advances.
Placebos seem to have their greatest impact on the subjective symptoms of disease—pain, distress, and discouragement. It should come as no surprise, then, that placebos are particularly effective in certain psychiatric conditions. In some forms of anxiety and depressive disorders, for example, distress is the illness, and placebos reliably bring relief. Patients with panic disorder, mild to moderate depression, or generalized anxiety disorder get almost as much relief with placebo as they do with conventional treatment (about one-half improve with placebo).9-11 But <20% of those with obsessive-compulsive disorder improve with placebo, and placebo response rates are also low in patients with schizophrenia or dementia. Mania, attention-deficit/hyperactivity disorder (ADHD), and severe depression fall somewhere in the middle.3
Harnessing the placebo response
There may be a few circumstances in psychiatric practice when it makes sense to intentionally prescribe a placebo as treatment, and we discuss those below. But far more frequently, what we know about the elements that contribute to the placebo effect can be applied to enhance the benefits of any treatment. Patients might be best served if deliberate mobilization of the placebo effect was a standard adjunct to conventional clinical care.
Various components of the treatment situation, collectively referred to as placebo, are a powerful antidote for illness, and some of these healing components exert their influence without special activity on the clinician’s part:
- Simply seeking psychiatric care can bring relief by providing some sense of control over distressing symptoms. The standard trappings of the office or clinic and customary office procedures—from the presentation of one’s insurance card to taking a history—offer reassurance and evoke the expectation that improvement or recovery is around the corner.
- The comfort provided by the psychiatrist’s presence is enhanced when patients feel that they are in the hands of a recognized healer. Psychiatrists inspire confidence when they look like a psychiatrist, or more precisely, like the patient’s idea of what a psychiatrist should look like. In our culture, that means a white coat or business attire.
A thorough evaluation is one of the common treatment elements that does the most to reduce distress and inspire confidence. The quality of an evaluation bears a strong relationship to patients’ satisfaction with the medical encounter, and can influence the amount of disability they suffer.3,12-15
Although guidelines for conducting effective psychiatric interviews have been around for almost 100 years, psychiatrists vary considerably in the extent to which they elicit complete and accurate information, build rapport, give patients the sense that they are listened to, and provide a thorough assessment. The degree to which patients feel that the clinician is responsive to their concerns depends as much on the style of the interview as on the amount of time devoted to it. Nonverbal behavior can carry the message that the clinician is paying full attention. Something as simple as not answering the phone during an interview (this seems obvious, but a surprising and troubling number of mental health professionals take phone calls during interviews and treatment sessions) conveys an important message about the importance that the clinician places on the patient’s problems.3
The idea that the treatment situation itself provides reassurance and reduces distress, and in doing so, powers a good bit of the placebo effect, is enshrined in such concepts as the importance of good bedside manner. Many feel that the doctor’s thoughtful attention, positive regard, and optimism—so valued by patients—are justified on humanitarian grounds alone; actual evidence that this caring behavior contributes to healing isn’t required. To many, the healing properties of the treatment situation are self-evident. But as the costs of health care snowball and the demands for efficiency and cost-effectiveness rise, the time that psychiatrists can devote to patients has dwindled. Third-party payors demand evidence, beyond intuition and common sense, that diagnostic procedures and treatments have some usefulness, and rightly so.
Is there any evidence that the common components of the treatment situation provide benefit?3 More specifically, does the quality of the doctor–patient relationship and the patient’s feelings about a therapeutic encounter promote healing? Several studies suggest that the doctor–patient relationship has a demonstrable impact on symptom relief.16 In 1 study, oncologists were randomly assigned to receive a Communication Skills Training (CST) program or not. CST included a 1.5-day face-to-face workshop and 6 hours of monthly videoconferencing that focused on improving communication skills with patients.17 Lessons included building rapport, engaging in appropriate eye contact, and normalizing difficult experiences. One week after initially consulting with their physician, patients who saw an oncologist in the CST group experienced less anxiety and depression than those who saw an oncologist who did not receive CST. The benefit of CST for patient anxiety mostly persisted at a 3-month follow-up.
A recent meta-analysis pooled the results of 47 studies to examine the relationship between how much trust patients have for their doctors and health outcomes. There was a small to medium association: More trust was associated with greater improvement.18 It is possible that a good doctor–patient relationship enhances expectancies. However, it is also likely that a positive therapeutic relationship is inherently soothing and reduces distress or dysfunction independent of expectation. Regardless of the precise mechanism, these studies warrant attention. We all understand that it is important on ethical grounds to treat patients with respect and kindness. Research shows that this type of behavior also promotes recovery.
Patient expectations. The idea that expectation of improvement has a major impact on treatment outcome is firmly grounded in research on the placebo effect. Studies have shown that what people expect to experience as an outcome of treatment has a substantial impact on what they actually experience. In a classic study, a doctor told some patients with symptoms of minor illness that they would feel better soon and another group with the same symptoms that he didn’t know what ailed them.19 Two weeks later, 64% of patients in the “positive expectation” group were improved, compared with only 39% of patients in the “negative” group. In another study, adults were exposed to an allergen that caused a skin reaction.20 Hand lotion (ie, a therapeutically inert substance) was then spread on the skin. Patients were led to believe that the cream would either alleviate or exacerbate the itching. The experimentally-induced wheal-and-flare was measured in both groups a few minutes after the allergen and cream were applied. The wheal-and-flare were worse for participants in the group that expected exacerbation.
Not uncommonly, expectation can have more impact on clinical outcome than a drug’s pharmacologic activity. In a double-blind placebo-controlled study, patients with depression were treated with St. John’s wort, sertraline, or placebo.21 They improved to the same extent with all 3 treatments. But when patients were asked to guess the treatment to which they had been assigned, those who thought they had received placebo showed little improvement, irrespective of which intervention they actually received, and those who guessed they had been given St. John’s wort or sertraline showed uniformly large improvement, irrespective of which intervention they actually received (including placebo). The researchers concluded that “Patient beliefs regarding treatment may have a stronger association with clinical outcome than the actual medication received.”
Psychiatrists who wish to use all the therapeutic tools at their disposal must attend to and manage patient expectations. One part of channeling a patient’s expectation is to thoroughly assess the patient’s beliefs regarding the efficacy of various treatments. If a patient’s uncle said that a certain drug is a miracle cure for anxiety, and the patient believes it to be true, then that expectation must be taken into consideration. Many patients prefer alternative treatments to conventional therapies. As long as there is no reason to think an alternative treatment will cause harm, a compromise might be reasonable. For example, if a patient with schizophrenia wants to treat her symptoms with herbal tea, the psychiatrist could say, “In addition to the tea, I recommend that you also take clozapine. The combination is likely to improve your symptoms.”3 More than anything else, the words a psychiatrist uses when recommending treatment shape the patient’s expectations. “You should be feeling a lot less anxious soon after you start taking this” has a different effect than “Try this. It may help.”
Prescribing ‘open-label’ placebo
There may be some limited circumstances where an actual placebo (eg, a sugar pill) might be suitable as a treatment. These include when placebo and conventional treatment provide similar results and a patient is reluctant to take conventional medicine, or when there is no effective conventional treatment. The deceptive prescription of placebo (providing placebo and calling it a drug) has a long history and was considered ethical—and recommended by medical authorities—until the latter half of the 20th century. This practice was deemed unethical in the 1980s, because it was dishonest and violated patient autonomy. Because it was widely believed that placebos given openly would be ineffective, the end of placebo treatment seemed at hand. An intriguing body of evidence, however, suggests that placebos can be effective even when patients know they are taking a placebo. Patients given an “open-label” placebo are told something along the lines of “the pill being prescribed contains no medicine, but some people improve with it, perhaps because the pill stimulates the body’s self-healing.” Open-label placebo has been evaluated for depression,22 low back pain,23 irritable bowel syndrome,24 neurosis,25 allergic rhinitis,26 and anxiety.27 Most of these studies are small, and some were uncontrolled. Yet they consistently have shown that symptoms improve with a nondeceptive placebo, and improve to a greater extent than with no treatment.
The most recent trial is a promising example of the potential of open-label placebos. In this study, 96 patients with chronic low back pain were randomly assigned to 3 weeks of treatment as usual (TAU) or 3 weeks of TAU plus open-label placebo.23 Patients who received open-label placebo were educated about the placebo effect and shown a film clip describing promising results of a prior open-label placebo study. They were then given placebo pills to be take once daily, and clearly told the pills contained no active medication. After 3 weeks, patients in the TAU plus placebo group reported less pain and less disability than patients who received TAU without a placebo. Some patients even requested a placebo prescription at the end of the study.
The placebo response provides a rational basis for prescribing innocuous alternative therapies with no intrinsic therapeutic value. Patients who prefer and believe in the effectiveness of alternative remedies—herbal compounds, massage, magnets, homeopathic solutions, etc.—can be recommended these treatments to mobilize a placebo response.
Using a conditioning model. Prescribing a placebo to obtain a conditioned drug response has enormous but untapped clinical potential. Both animal and human research indicates that a wide range of drug responses, from immune suppression to motor stimulation, can be conditioned (a neutral stimulus, such as a pill or injection, associated with drug administration can in itself evoke the drug effect). In many conditioning or dose-extending models, a particular response to real medication (such as pain relief after analgesics) first becomes conditioned due to repeated exposure to the drug given in a particular vehicle. Then, the treatment shifts to some doses comprising of real medicine and some doses comprising of placebo. Because the drug response has been conditioned, it is thought that the response to an identically appearing placebo will mirror the drug response. The active drug often is only replaced by placebo for certain doses under a schedule of partial reinforcement, given the ubiquity of extinction (the conditioned response lessens when the conditioned stimulus is presented alone on repeated trials).
In 1 version of a conditioning study, children with ADHD were randomized to 1 of 3 groups.28 One group (full dose) took the standard dose of medication for 2 months, a second group (reduced dose) took a standard dose during 1 month followed by a half dose during the second month, and children in the third group (reduced dose with placebo) took the standard dose plus a visually distinctive placebo during the first month, followed by a half dose plus the visually distinctive placebo during the second month. Not surprisingly, ADHD symptoms were worse among children in the reduced-dose group. However, there was no difference between those in the reduced-dose with placebo group and those in the full-dose group. It appears as though the symptom reduction associated with a 100% dose was an unconditioned response that could be mimicked with the addition of a placebo pill.
In another study, patients with psoriasis were randomly assigned to receive a full dose of active medication (0.1% triamcinolone cream) twice a day, or a full dose of active medication for 25% to 50% of the doses, with a placebo (moisturizing cream) given for the other 50% to 75% of the doses.29 Relapse rates were not statistically different between groups.
“It is a mystery how a ubiquitous treatment used since antiquity was unknown, unnamed, and unidentified until recently. It is even more remarkable because this is the only treatment common to all societies and cultures.”1
The treatment discussed above is not a specific pill, surgery, plant, or herb. Rather, the authors are referring to placebo. Indeed, the history of medical treatment is largely a chronicle of placebos. When subjected to scientific scrutiny, the overwhelming majority of treatments have turned out to be devoid of intrinsic therapeutic value; they derived their benefits from the placebo effect. Despite these benefits, the term “placebo” comes with unfortunate baggage. Latin for “I shall please,” it is the first word of the Christian vespers for the dead. In the 12th century these vespers were commonly referred to as placebos. By the 1300s, the term had become secular and pejorative, suggesting a flatterer or sycophant. When the word entered medical terminology in the late 18th century, the negative connotation stuck. A placebo was defined as a medicine given to please patients rather than to benefit them. In the modern era, the lack of pharmacologic activity became part of the definition as well.
The word placebo brings with it connotations of deception, fakery, and ineffectiveness. But one of the things about placebos that contribute mightily to the health care community’s aversion toward them is, in fact, their effectiveness. They bring relief across a wide range of medical conditions.2 In doing so, placebos impugn the value of our most cherished remedies, hamper the development of new therapeutics, and threaten our livelihoods as health professionals.3
Placebos often are conceptualized as any treatment that lacks intrinsic therapeutic value, such as sugar pills. But looking at what placebo treatment actually entails, both in placebo-controlled treatment trials and in clinical settings, suggests a more comprehensive definition. Placebos encompass all the elements common to any treatment or healing situation. These include a recognized healer, evaluation, diagnosis, prognosis, plausible treatment, and most importantly, the expectation that one will recover. Along these lines, the placebo response can be thought of as the response to the common elements of the treatment or healing situation.3
Research regarding the placebo effect has mushroomed in the past 2 decades. Over this time, we have learned a good deal about both the mechanisms underlying the placebo effect and how the placebo effect can be applied to enhance the benefit of conventional treatment. Brain imaging technology has revealed that when placebo treatment alleviates pain, Parkinson’s disease, and depression, brain changes occur that are similar to those observed with active pharmacologic treatment.4,5 Recent studies also show that deliberate, open (nondeceptive) use of placebo can improve the symptoms of several conditions, including depression, pain, and irritable bowel syndrome.6 Furthermore, intermittent substitution of placebo pills for pharmacologically active treatment in a conditioning paradigm can be as effective as the “real” treatment.7 Also, research over the past decade has verified that certain common features of the treatment situation, particularly the quality of the doctor–patient encounter, contribute to the placebo response and have a demonstrable impact on the outcome of treatment.8 Clearly, the placebo effect has gone from being simply a nuisance that interferes with the evaluation of new treatments to a variable worthy of study and application in its own right. Although, for the most part, clinical practice has not kept up with these advances.
Placebos seem to have their greatest impact on the subjective symptoms of disease—pain, distress, and discouragement. It should come as no surprise, then, that placebos are particularly effective in certain psychiatric conditions. In some forms of anxiety and depressive disorders, for example, distress is the illness, and placebos reliably bring relief. Patients with panic disorder, mild to moderate depression, or generalized anxiety disorder get almost as much relief with placebo as they do with conventional treatment (about one-half improve with placebo).9-11 But <20% of those with obsessive-compulsive disorder improve with placebo, and placebo response rates are also low in patients with schizophrenia or dementia. Mania, attention-deficit/hyperactivity disorder (ADHD), and severe depression fall somewhere in the middle.3
Harnessing the placebo response
There may be a few circumstances in psychiatric practice when it makes sense to intentionally prescribe a placebo as treatment, and we discuss those below. But far more frequently, what we know about the elements that contribute to the placebo effect can be applied to enhance the benefits of any treatment. Patients might be best served if deliberate mobilization of the placebo effect was a standard adjunct to conventional clinical care.
Various components of the treatment situation, collectively referred to as placebo, are a powerful antidote for illness, and some of these healing components exert their influence without special activity on the clinician’s part:
- Simply seeking psychiatric care can bring relief by providing some sense of control over distressing symptoms. The standard trappings of the office or clinic and customary office procedures—from the presentation of one’s insurance card to taking a history—offer reassurance and evoke the expectation that improvement or recovery is around the corner.
- The comfort provided by the psychiatrist’s presence is enhanced when patients feel that they are in the hands of a recognized healer. Psychiatrists inspire confidence when they look like a psychiatrist, or more precisely, like the patient’s idea of what a psychiatrist should look like. In our culture, that means a white coat or business attire.
A thorough evaluation is one of the common treatment elements that does the most to reduce distress and inspire confidence. The quality of an evaluation bears a strong relationship to patients’ satisfaction with the medical encounter, and can influence the amount of disability they suffer.3,12-15
Although guidelines for conducting effective psychiatric interviews have been around for almost 100 years, psychiatrists vary considerably in the extent to which they elicit complete and accurate information, build rapport, give patients the sense that they are listened to, and provide a thorough assessment. The degree to which patients feel that the clinician is responsive to their concerns depends as much on the style of the interview as on the amount of time devoted to it. Nonverbal behavior can carry the message that the clinician is paying full attention. Something as simple as not answering the phone during an interview (this seems obvious, but a surprising and troubling number of mental health professionals take phone calls during interviews and treatment sessions) conveys an important message about the importance that the clinician places on the patient’s problems.3
The idea that the treatment situation itself provides reassurance and reduces distress, and in doing so, powers a good bit of the placebo effect, is enshrined in such concepts as the importance of good bedside manner. Many feel that the doctor’s thoughtful attention, positive regard, and optimism—so valued by patients—are justified on humanitarian grounds alone; actual evidence that this caring behavior contributes to healing isn’t required. To many, the healing properties of the treatment situation are self-evident. But as the costs of health care snowball and the demands for efficiency and cost-effectiveness rise, the time that psychiatrists can devote to patients has dwindled. Third-party payors demand evidence, beyond intuition and common sense, that diagnostic procedures and treatments have some usefulness, and rightly so.
Is there any evidence that the common components of the treatment situation provide benefit?3 More specifically, does the quality of the doctor–patient relationship and the patient’s feelings about a therapeutic encounter promote healing? Several studies suggest that the doctor–patient relationship has a demonstrable impact on symptom relief.16 In 1 study, oncologists were randomly assigned to receive a Communication Skills Training (CST) program or not. CST included a 1.5-day face-to-face workshop and 6 hours of monthly videoconferencing that focused on improving communication skills with patients.17 Lessons included building rapport, engaging in appropriate eye contact, and normalizing difficult experiences. One week after initially consulting with their physician, patients who saw an oncologist in the CST group experienced less anxiety and depression than those who saw an oncologist who did not receive CST. The benefit of CST for patient anxiety mostly persisted at a 3-month follow-up.
A recent meta-analysis pooled the results of 47 studies to examine the relationship between how much trust patients have for their doctors and health outcomes. There was a small to medium association: More trust was associated with greater improvement.18 It is possible that a good doctor–patient relationship enhances expectancies. However, it is also likely that a positive therapeutic relationship is inherently soothing and reduces distress or dysfunction independent of expectation. Regardless of the precise mechanism, these studies warrant attention. We all understand that it is important on ethical grounds to treat patients with respect and kindness. Research shows that this type of behavior also promotes recovery.
Patient expectations. The idea that expectation of improvement has a major impact on treatment outcome is firmly grounded in research on the placebo effect. Studies have shown that what people expect to experience as an outcome of treatment has a substantial impact on what they actually experience. In a classic study, a doctor told some patients with symptoms of minor illness that they would feel better soon and another group with the same symptoms that he didn’t know what ailed them.19 Two weeks later, 64% of patients in the “positive expectation” group were improved, compared with only 39% of patients in the “negative” group. In another study, adults were exposed to an allergen that caused a skin reaction.20 Hand lotion (ie, a therapeutically inert substance) was then spread on the skin. Patients were led to believe that the cream would either alleviate or exacerbate the itching. The experimentally-induced wheal-and-flare was measured in both groups a few minutes after the allergen and cream were applied. The wheal-and-flare were worse for participants in the group that expected exacerbation.
Not uncommonly, expectation can have more impact on clinical outcome than a drug’s pharmacologic activity. In a double-blind placebo-controlled study, patients with depression were treated with St. John’s wort, sertraline, or placebo.21 They improved to the same extent with all 3 treatments. But when patients were asked to guess the treatment to which they had been assigned, those who thought they had received placebo showed little improvement, irrespective of which intervention they actually received, and those who guessed they had been given St. John’s wort or sertraline showed uniformly large improvement, irrespective of which intervention they actually received (including placebo). The researchers concluded that “Patient beliefs regarding treatment may have a stronger association with clinical outcome than the actual medication received.”
Psychiatrists who wish to use all the therapeutic tools at their disposal must attend to and manage patient expectations. One part of channeling a patient’s expectation is to thoroughly assess the patient’s beliefs regarding the efficacy of various treatments. If a patient’s uncle said that a certain drug is a miracle cure for anxiety, and the patient believes it to be true, then that expectation must be taken into consideration. Many patients prefer alternative treatments to conventional therapies. As long as there is no reason to think an alternative treatment will cause harm, a compromise might be reasonable. For example, if a patient with schizophrenia wants to treat her symptoms with herbal tea, the psychiatrist could say, “In addition to the tea, I recommend that you also take clozapine. The combination is likely to improve your symptoms.”3 More than anything else, the words a psychiatrist uses when recommending treatment shape the patient’s expectations. “You should be feeling a lot less anxious soon after you start taking this” has a different effect than “Try this. It may help.”
Prescribing ‘open-label’ placebo
There may be some limited circumstances where an actual placebo (eg, a sugar pill) might be suitable as a treatment. These include when placebo and conventional treatment provide similar results and a patient is reluctant to take conventional medicine, or when there is no effective conventional treatment. The deceptive prescription of placebo (providing placebo and calling it a drug) has a long history and was considered ethical—and recommended by medical authorities—until the latter half of the 20th century. This practice was deemed unethical in the 1980s, because it was dishonest and violated patient autonomy. Because it was widely believed that placebos given openly would be ineffective, the end of placebo treatment seemed at hand. An intriguing body of evidence, however, suggests that placebos can be effective even when patients know they are taking a placebo. Patients given an “open-label” placebo are told something along the lines of “the pill being prescribed contains no medicine, but some people improve with it, perhaps because the pill stimulates the body’s self-healing.” Open-label placebo has been evaluated for depression,22 low back pain,23 irritable bowel syndrome,24 neurosis,25 allergic rhinitis,26 and anxiety.27 Most of these studies are small, and some were uncontrolled. Yet they consistently have shown that symptoms improve with a nondeceptive placebo, and improve to a greater extent than with no treatment.
The most recent trial is a promising example of the potential of open-label placebos. In this study, 96 patients with chronic low back pain were randomly assigned to 3 weeks of treatment as usual (TAU) or 3 weeks of TAU plus open-label placebo.23 Patients who received open-label placebo were educated about the placebo effect and shown a film clip describing promising results of a prior open-label placebo study. They were then given placebo pills to be take once daily, and clearly told the pills contained no active medication. After 3 weeks, patients in the TAU plus placebo group reported less pain and less disability than patients who received TAU without a placebo. Some patients even requested a placebo prescription at the end of the study.
The placebo response provides a rational basis for prescribing innocuous alternative therapies with no intrinsic therapeutic value. Patients who prefer and believe in the effectiveness of alternative remedies—herbal compounds, massage, magnets, homeopathic solutions, etc.—can be recommended these treatments to mobilize a placebo response.
Using a conditioning model. Prescribing a placebo to obtain a conditioned drug response has enormous but untapped clinical potential. Both animal and human research indicates that a wide range of drug responses, from immune suppression to motor stimulation, can be conditioned (a neutral stimulus, such as a pill or injection, associated with drug administration can in itself evoke the drug effect). In many conditioning or dose-extending models, a particular response to real medication (such as pain relief after analgesics) first becomes conditioned due to repeated exposure to the drug given in a particular vehicle. Then, the treatment shifts to some doses comprising of real medicine and some doses comprising of placebo. Because the drug response has been conditioned, it is thought that the response to an identically appearing placebo will mirror the drug response. The active drug often is only replaced by placebo for certain doses under a schedule of partial reinforcement, given the ubiquity of extinction (the conditioned response lessens when the conditioned stimulus is presented alone on repeated trials).
In 1 version of a conditioning study, children with ADHD were randomized to 1 of 3 groups.28 One group (full dose) took the standard dose of medication for 2 months, a second group (reduced dose) took a standard dose during 1 month followed by a half dose during the second month, and children in the third group (reduced dose with placebo) took the standard dose plus a visually distinctive placebo during the first month, followed by a half dose plus the visually distinctive placebo during the second month. Not surprisingly, ADHD symptoms were worse among children in the reduced-dose group. However, there was no difference between those in the reduced-dose with placebo group and those in the full-dose group. It appears as though the symptom reduction associated with a 100% dose was an unconditioned response that could be mimicked with the addition of a placebo pill.
In another study, patients with psoriasis were randomly assigned to receive a full dose of active medication (0.1% triamcinolone cream) twice a day, or a full dose of active medication for 25% to 50% of the doses, with a placebo (moisturizing cream) given for the other 50% to 75% of the doses.29 Relapse rates were not statistically different between groups.
1. Shapiro AK, Shapiro E. The powerful placebo: from ancient priest to modern physician. Baltimore, MD: Johns Hopkins University Press; 1997.
2. Beecher HK. The powerful placebo. J Am Med Assoc. 1955;159(17):1602-1606.
3. Brown WA. The placebo effect in clinical practice. New York, NY: Oxford University Press; 2013.
4. Mayberg HS, Silva JA, Brannan SK, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159(5):728-737.
5. de la Fuente-Fernández R, Ruth TJ, Sossi V, et al. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293(5532):1164-1166.
6. Charlesworth JEG, Petkovic G, Kelley JM, et al. Effects of placebos without deception compared with no treatment: a systematic review and meta‐analysis. J Evid Based Med. 2017;10(2):97-107.
7. Colloca L, Enck P, DeGrazia D. Relieving pain using dose-extending placebos: a scoping review. Pain. 2016;157(8):1590-1598.
8. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336(7651):999-1003.
9. Khan A, Kolts RL, Rapaport MH, et al. Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med. 2005;35(5):743-749.
10. Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840-1847.
11. Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5(2):e45.
12. Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136(5):374-383.
13. Kelley JM, Kraft-Todd G, Schapira L, et al. The influence of the patient-clinician relationship on healthcare outcomes: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(4):e94207.
14. Olsson B, Olsson B, Tibblin G. Effect of patients’ expectations on recovery from acute tonsillitis. Fam Pract. 1989;6(3):188-192.
15. Sox HC, Margulies I, Sox CH. Psychologically mediated effects of diagnostic tests. Ann Intern Med. 1981;95(6):680-685.
16. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433.
17. Girgis A, Cockburn J, Butow P, et al. Improving patient emotional functioning and psychological morbidity: evaluation of a consultation skills training program for oncologists. Patient Educ Couns. 2009;77(3):456-462.
18. Birkhäuer J, Gaab J, Kossowsky J, et al. Trust in the health care professional and health outcome: a meta-analysis. PLoS One. 2017;12(2):e0170988.
19. Thomas KB. General practice consultations: is there any point in being positive? Br Med J (Clin Res Ed). 1987;294(6581):1200-1202.
20. Howe LC, Goyer JP, Crum AJ. Harnessing the placebo effect: exploring the influence of physician characteristics on placebo response [published online May 9, 2017]. Health Psychol. doi: 10.1037/hea0000499.
21. Chen JA, Papakostas GI, Youn SJ, et al. Association between patient beliefs regarding assigned treatment and clinical response: reanalysis of data from the Hypericum Depression Trial Study Group. J Clin Psychiatry. 2011;72(12):1669-1676.
22. Kelley JM, Kaptchuk TJ, Cusin C, et al. Open-label placebo for major depressive disorder: a pilot randomized controlled trial. Psychother Psychosom. 2012;81(5):312-314.
23. Carvalho C, Caetano JM, Cunha L, et al. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain. 2016;157(12):2766-2772.
24. Kaptchuk TJ, Friedlander E, Kelley JM, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5(12):e15591.
25. Park LC, Covi L. Nonblind placebo trial: an exploration of neurotic patients’ responses to placebo when its inert content is disclosed. Arch Gen Psychiatry. 1965;12(4):336-345.
26. Schaefer M, Harke R, Denke C. Open-label placebos improve symptoms in allergic rhinitis: a randomized controlled trial. Psychother Psychosom. 2016;85(6):373-374.
27. Aulas JJ, Rosner I. Efficacy of a non blind placebo prescription [in French]. Encephale. 2003;29(1):68-71.
28. Sandler AD, Glesne CE, Bodfish JW. Conditioned placebo dose reduction: a new treatment in attention deficit hyperactivity disorder? J Dev Behav Pediatr. 2010;31(5):369-375.
29. Ader R, Mercurio MG, Walton J, et al. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom Med. 2010;72(2):192-197.
30. Weiss RD, O’Malley SS, Hosking JD, et al; COMBINE Study Research Group. Do patients with alcohol dependence respond to placebo? Results from the COMBINE Study. J Stud Alcohol Drugs. 2008;69(6):878-884.
31. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207.
1. Shapiro AK, Shapiro E. The powerful placebo: from ancient priest to modern physician. Baltimore, MD: Johns Hopkins University Press; 1997.
2. Beecher HK. The powerful placebo. J Am Med Assoc. 1955;159(17):1602-1606.
3. Brown WA. The placebo effect in clinical practice. New York, NY: Oxford University Press; 2013.
4. Mayberg HS, Silva JA, Brannan SK, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159(5):728-737.
5. de la Fuente-Fernández R, Ruth TJ, Sossi V, et al. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293(5532):1164-1166.
6. Charlesworth JEG, Petkovic G, Kelley JM, et al. Effects of placebos without deception compared with no treatment: a systematic review and meta‐analysis. J Evid Based Med. 2017;10(2):97-107.
7. Colloca L, Enck P, DeGrazia D. Relieving pain using dose-extending placebos: a scoping review. Pain. 2016;157(8):1590-1598.
8. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336(7651):999-1003.
9. Khan A, Kolts RL, Rapaport MH, et al. Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med. 2005;35(5):743-749.
10. Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840-1847.
11. Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5(2):e45.
12. Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136(5):374-383.
13. Kelley JM, Kraft-Todd G, Schapira L, et al. The influence of the patient-clinician relationship on healthcare outcomes: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(4):e94207.
14. Olsson B, Olsson B, Tibblin G. Effect of patients’ expectations on recovery from acute tonsillitis. Fam Pract. 1989;6(3):188-192.
15. Sox HC, Margulies I, Sox CH. Psychologically mediated effects of diagnostic tests. Ann Intern Med. 1981;95(6):680-685.
16. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433.
17. Girgis A, Cockburn J, Butow P, et al. Improving patient emotional functioning and psychological morbidity: evaluation of a consultation skills training program for oncologists. Patient Educ Couns. 2009;77(3):456-462.
18. Birkhäuer J, Gaab J, Kossowsky J, et al. Trust in the health care professional and health outcome: a meta-analysis. PLoS One. 2017;12(2):e0170988.
19. Thomas KB. General practice consultations: is there any point in being positive? Br Med J (Clin Res Ed). 1987;294(6581):1200-1202.
20. Howe LC, Goyer JP, Crum AJ. Harnessing the placebo effect: exploring the influence of physician characteristics on placebo response [published online May 9, 2017]. Health Psychol. doi: 10.1037/hea0000499.
21. Chen JA, Papakostas GI, Youn SJ, et al. Association between patient beliefs regarding assigned treatment and clinical response: reanalysis of data from the Hypericum Depression Trial Study Group. J Clin Psychiatry. 2011;72(12):1669-1676.
22. Kelley JM, Kaptchuk TJ, Cusin C, et al. Open-label placebo for major depressive disorder: a pilot randomized controlled trial. Psychother Psychosom. 2012;81(5):312-314.
23. Carvalho C, Caetano JM, Cunha L, et al. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain. 2016;157(12):2766-2772.
24. Kaptchuk TJ, Friedlander E, Kelley JM, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5(12):e15591.
25. Park LC, Covi L. Nonblind placebo trial: an exploration of neurotic patients’ responses to placebo when its inert content is disclosed. Arch Gen Psychiatry. 1965;12(4):336-345.
26. Schaefer M, Harke R, Denke C. Open-label placebos improve symptoms in allergic rhinitis: a randomized controlled trial. Psychother Psychosom. 2016;85(6):373-374.
27. Aulas JJ, Rosner I. Efficacy of a non blind placebo prescription [in French]. Encephale. 2003;29(1):68-71.
28. Sandler AD, Glesne CE, Bodfish JW. Conditioned placebo dose reduction: a new treatment in attention deficit hyperactivity disorder? J Dev Behav Pediatr. 2010;31(5):369-375.
29. Ader R, Mercurio MG, Walton J, et al. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom Med. 2010;72(2):192-197.
30. Weiss RD, O’Malley SS, Hosking JD, et al; COMBINE Study Research Group. Do patients with alcohol dependence respond to placebo? Results from the COMBINE Study. J Stud Alcohol Drugs. 2008;69(6):878-884.
31. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207.
The placebo effect in psychiatric practice
Are we on the verge of a cocaine epidemic?
Lithium-induced bradycardia: A rare but serious adverse effect
Mr. C, age 30, with schizoaffective disorder, bipolar type, Cannabis abuse, and nicotine dependence, has been enrolled in a Program of Assertive Community Treatment (PACT) for approximately 5 years. He presents to the PACT clinic for follow-up with his psychiatrist. Mr. C reports dizziness, lightheadedness, blurred vision, and nausea worsening over the last few days, and he appears drowsy and hypoactive. He does not report any chest pain, abdominal pain, swelling, cold extremities, shortness of breath, vomiting, diarrhea, or blood loss. Mr. C admits he has eaten only once daily for several weeks because of delusional ideation that he is responsible for others suffering from anorexia nervosa.
His medical history includes gastroesophageal reflux disease. Mr. C’s medication regimen for the past year included total daily oral doses of
Because of Mr. C’s complaints, appearance, and low HR, the psychiatrist calls emergency medical services (EMS). Although the paramedics recommend emergency transport to the hospital, Mr. C refuses. The psychiatrist instructs Mr. C to stop taking lithium because of suspected lithium-induced bradycardia and a concern that he may be more susceptible to lithium toxicity with prolonged anorexia nervosa. When nursing staff evaluate Mr. C the next day, his vitals are HR 60 bpm, respirations 20 breaths per minute, and blood pressure 124/81 mm Hg; his dizziness, blurred vision, lightheadedness, and nausea are resolved.
Bradycardia is defined as a HR <60 bpm; however, symptoms may not occur until the HR is <50 bpm. Symptoms include fatigue, dizziness, lightheadedness, chest pain, shortness of breath, and syncope. The incidence of bradycardia during lithium treatment is unknown; it is considered a rare but serious adverse effect. A literature review reveals several case reports of bradycardia with lithium treatment,2-4 including symptomatic bradycardia after a single dose of lithium.5 Other possible causes of bradycardia include anorexia nervosa, hypothermia, hypothyroidism, hypoxia, infection, stroke, acute myocardial infarction, sedative or opiate use, increased vagal tone with exercise conditioning, and other medications including fluphenazine.6
Mr. C’s symptoms may have been assumed to be secondary to several possible causes, including bradycardia, dehydration from poor oral intake, lithium toxicity, or an undiagnosed medical condition. The combination of nausea, dizziness, anorexia nervosa, blurred vision, and lightheadedness in a patient receiving lithium would certainly trigger a clinician’s concern for lithium toxicity, but he (she) may not be aware of the risk of bradycardia as an adverse effect of lithium. Because Mr. C refused hospital transportation by EMS, discontinuing lithium appears to have been the safest option. Laboratory studies from the day after Mr. C presented to the clinic appeared to lessen the probability that lithium toxicity, hypothyroidism,
Although psychiatrists may be vigilant about monitoring for signs and symptoms of toxicity with lithium use by utilizing regular laboratory studies, they may not be as vigilant with monitoring vital signs at every patient visit (Table). This case demonstrates the importance of regular vital sign measurements to be able to detect this rare but serious adverse effect.
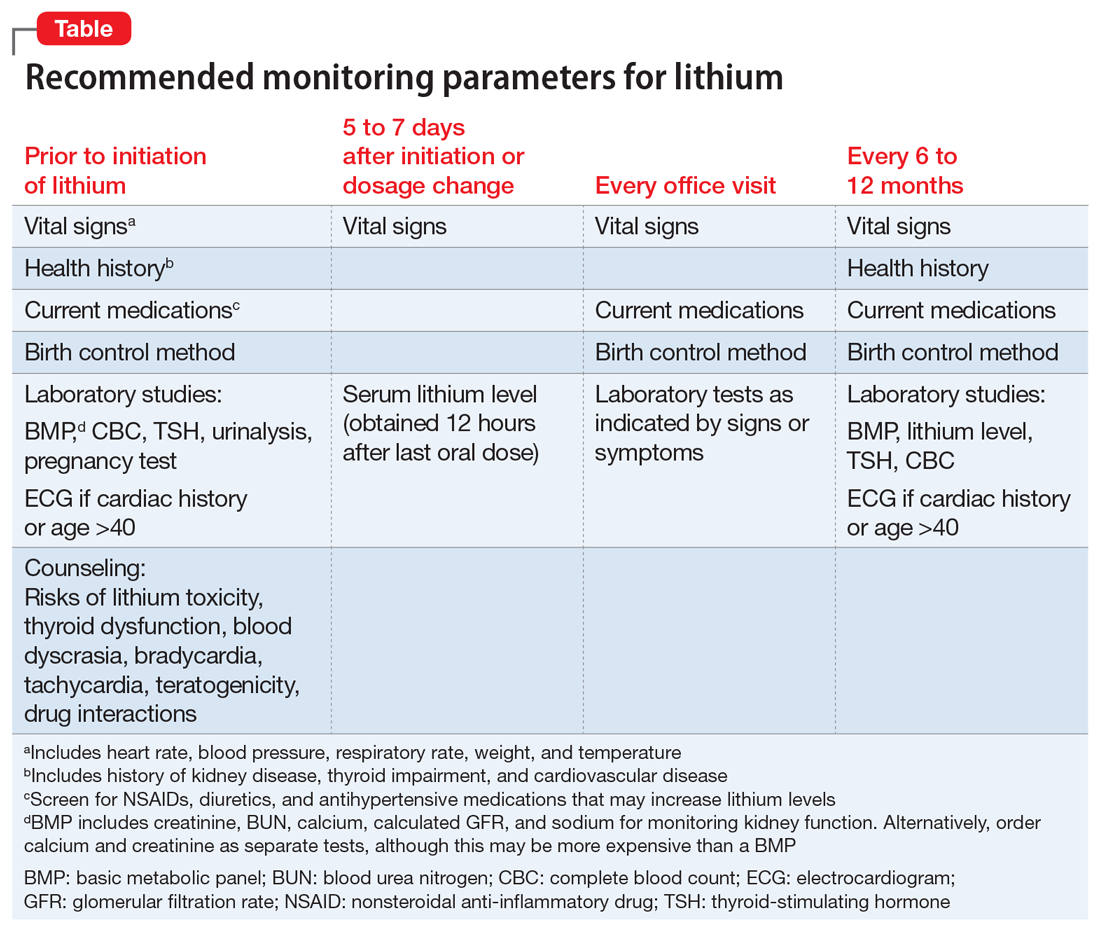
1. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
2. White B, Larry J, Kantharia BK. Protracted presyncope and profound bradycardia due to lithium toxicity. Int J Cardiol. 2008;125(3):e48-e50.
3. Palatnik A, Kates R. Bradycardia and medications: identify the dangerous pace. Nurs Manage. 2003;34(6):56A-56F.
4. La Rocca R, Foschi A, Preston NM, et al. QT interval prolongation and bradycardia in lithium-induced nephrogenic diabetes insipidus. Int J Cardiol. 2012;162(1):e1-e2.
5. Sabharwal MS, Annapureddy N, Agarwal SK, et al. Severe bradycardia caused by a single dose of lithium. Intern Med. 2013;52(7):767-769.
6. Homoud MK. Sinus bradycardia. UpToDate. www.uptodate.com/contents/sinus-bradycardia. Updated June 7, 2017. Accessed August 28, 2017.
Mr. C, age 30, with schizoaffective disorder, bipolar type, Cannabis abuse, and nicotine dependence, has been enrolled in a Program of Assertive Community Treatment (PACT) for approximately 5 years. He presents to the PACT clinic for follow-up with his psychiatrist. Mr. C reports dizziness, lightheadedness, blurred vision, and nausea worsening over the last few days, and he appears drowsy and hypoactive. He does not report any chest pain, abdominal pain, swelling, cold extremities, shortness of breath, vomiting, diarrhea, or blood loss. Mr. C admits he has eaten only once daily for several weeks because of delusional ideation that he is responsible for others suffering from anorexia nervosa.
His medical history includes gastroesophageal reflux disease. Mr. C’s medication regimen for the past year included total daily oral doses of
Because of Mr. C’s complaints, appearance, and low HR, the psychiatrist calls emergency medical services (EMS). Although the paramedics recommend emergency transport to the hospital, Mr. C refuses. The psychiatrist instructs Mr. C to stop taking lithium because of suspected lithium-induced bradycardia and a concern that he may be more susceptible to lithium toxicity with prolonged anorexia nervosa. When nursing staff evaluate Mr. C the next day, his vitals are HR 60 bpm, respirations 20 breaths per minute, and blood pressure 124/81 mm Hg; his dizziness, blurred vision, lightheadedness, and nausea are resolved.
Bradycardia is defined as a HR <60 bpm; however, symptoms may not occur until the HR is <50 bpm. Symptoms include fatigue, dizziness, lightheadedness, chest pain, shortness of breath, and syncope. The incidence of bradycardia during lithium treatment is unknown; it is considered a rare but serious adverse effect. A literature review reveals several case reports of bradycardia with lithium treatment,2-4 including symptomatic bradycardia after a single dose of lithium.5 Other possible causes of bradycardia include anorexia nervosa, hypothermia, hypothyroidism, hypoxia, infection, stroke, acute myocardial infarction, sedative or opiate use, increased vagal tone with exercise conditioning, and other medications including fluphenazine.6
Mr. C’s symptoms may have been assumed to be secondary to several possible causes, including bradycardia, dehydration from poor oral intake, lithium toxicity, or an undiagnosed medical condition. The combination of nausea, dizziness, anorexia nervosa, blurred vision, and lightheadedness in a patient receiving lithium would certainly trigger a clinician’s concern for lithium toxicity, but he (she) may not be aware of the risk of bradycardia as an adverse effect of lithium. Because Mr. C refused hospital transportation by EMS, discontinuing lithium appears to have been the safest option. Laboratory studies from the day after Mr. C presented to the clinic appeared to lessen the probability that lithium toxicity, hypothyroidism,
Although psychiatrists may be vigilant about monitoring for signs and symptoms of toxicity with lithium use by utilizing regular laboratory studies, they may not be as vigilant with monitoring vital signs at every patient visit (Table). This case demonstrates the importance of regular vital sign measurements to be able to detect this rare but serious adverse effect.

Mr. C, age 30, with schizoaffective disorder, bipolar type, Cannabis abuse, and nicotine dependence, has been enrolled in a Program of Assertive Community Treatment (PACT) for approximately 5 years. He presents to the PACT clinic for follow-up with his psychiatrist. Mr. C reports dizziness, lightheadedness, blurred vision, and nausea worsening over the last few days, and he appears drowsy and hypoactive. He does not report any chest pain, abdominal pain, swelling, cold extremities, shortness of breath, vomiting, diarrhea, or blood loss. Mr. C admits he has eaten only once daily for several weeks because of delusional ideation that he is responsible for others suffering from anorexia nervosa.
His medical history includes gastroesophageal reflux disease. Mr. C’s medication regimen for the past year included total daily oral doses of
Because of Mr. C’s complaints, appearance, and low HR, the psychiatrist calls emergency medical services (EMS). Although the paramedics recommend emergency transport to the hospital, Mr. C refuses. The psychiatrist instructs Mr. C to stop taking lithium because of suspected lithium-induced bradycardia and a concern that he may be more susceptible to lithium toxicity with prolonged anorexia nervosa. When nursing staff evaluate Mr. C the next day, his vitals are HR 60 bpm, respirations 20 breaths per minute, and blood pressure 124/81 mm Hg; his dizziness, blurred vision, lightheadedness, and nausea are resolved.
Bradycardia is defined as a HR <60 bpm; however, symptoms may not occur until the HR is <50 bpm. Symptoms include fatigue, dizziness, lightheadedness, chest pain, shortness of breath, and syncope. The incidence of bradycardia during lithium treatment is unknown; it is considered a rare but serious adverse effect. A literature review reveals several case reports of bradycardia with lithium treatment,2-4 including symptomatic bradycardia after a single dose of lithium.5 Other possible causes of bradycardia include anorexia nervosa, hypothermia, hypothyroidism, hypoxia, infection, stroke, acute myocardial infarction, sedative or opiate use, increased vagal tone with exercise conditioning, and other medications including fluphenazine.6
Mr. C’s symptoms may have been assumed to be secondary to several possible causes, including bradycardia, dehydration from poor oral intake, lithium toxicity, or an undiagnosed medical condition. The combination of nausea, dizziness, anorexia nervosa, blurred vision, and lightheadedness in a patient receiving lithium would certainly trigger a clinician’s concern for lithium toxicity, but he (she) may not be aware of the risk of bradycardia as an adverse effect of lithium. Because Mr. C refused hospital transportation by EMS, discontinuing lithium appears to have been the safest option. Laboratory studies from the day after Mr. C presented to the clinic appeared to lessen the probability that lithium toxicity, hypothyroidism,
Although psychiatrists may be vigilant about monitoring for signs and symptoms of toxicity with lithium use by utilizing regular laboratory studies, they may not be as vigilant with monitoring vital signs at every patient visit (Table). This case demonstrates the importance of regular vital sign measurements to be able to detect this rare but serious adverse effect.

1. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
2. White B, Larry J, Kantharia BK. Protracted presyncope and profound bradycardia due to lithium toxicity. Int J Cardiol. 2008;125(3):e48-e50.
3. Palatnik A, Kates R. Bradycardia and medications: identify the dangerous pace. Nurs Manage. 2003;34(6):56A-56F.
4. La Rocca R, Foschi A, Preston NM, et al. QT interval prolongation and bradycardia in lithium-induced nephrogenic diabetes insipidus. Int J Cardiol. 2012;162(1):e1-e2.
5. Sabharwal MS, Annapureddy N, Agarwal SK, et al. Severe bradycardia caused by a single dose of lithium. Intern Med. 2013;52(7):767-769.
6. Homoud MK. Sinus bradycardia. UpToDate. www.uptodate.com/contents/sinus-bradycardia. Updated June 7, 2017. Accessed August 28, 2017.
1. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
2. White B, Larry J, Kantharia BK. Protracted presyncope and profound bradycardia due to lithium toxicity. Int J Cardiol. 2008;125(3):e48-e50.
3. Palatnik A, Kates R. Bradycardia and medications: identify the dangerous pace. Nurs Manage. 2003;34(6):56A-56F.
4. La Rocca R, Foschi A, Preston NM, et al. QT interval prolongation and bradycardia in lithium-induced nephrogenic diabetes insipidus. Int J Cardiol. 2012;162(1):e1-e2.
5. Sabharwal MS, Annapureddy N, Agarwal SK, et al. Severe bradycardia caused by a single dose of lithium. Intern Med. 2013;52(7):767-769.
6. Homoud MK. Sinus bradycardia. UpToDate. www.uptodate.com/contents/sinus-bradycardia. Updated June 7, 2017. Accessed August 28, 2017.
‘Self-anesthetizing’ to cope with grief
CASE Grieving, delusional
Mr. M, age 51, is brought to the emergency department (ED) because of new-onset delusions and decreased self-care over the last 2 weeks following the sudden death of his wife. He has become expansive and grandiose, with pressured speech, increased energy, and markedly reduced sleep. Mr. M is preoccupied with the idea that he is “the first to survive a human reboot process” and says that his and his wife’s bodies and brains had been “split apart.” Mr. M has limited his food and fluid intake and lost 15 lb within the past 2 to 3 weeks.
Mr. M has no history of any affective, psychotic, or other major mental disorders or treatment. He reports that he has regularly used Cannabis over the last 10 years, and a few years ago, he started occasionally using nitrous oxide (N2O). He says that in the week following his wife’s death, he used N2O almost daily and in copious amounts. In an attempt to “self-anesthetize” himself after his wife’s funeral, he isolated himself in his bedroom and used escalating amounts of Cannabis and N2O, while continually working on a book about their life together.
At first, Mr. M shows little emotion and describes his situation as “interesting and fascinating.” He mentions that he thinks he might have been “psychotic” the week after his wife’s death, but he shows no sustained insight and immediately relapses into psychotic thinking. Over several hours in the ED, he is tearful and sad about his wife’s death. Mr. M recalls a similar experience of grief after his mother died when he was a teenager, but at that time he did not abuse substances or have psychotic symptoms. He is fully alert, fully oriented, and has no significant deficits of attention or memory.
[polldaddy:9859135]
The authors’ observations
Grief was a precipitating event, but by itself grief cannot explain psychosis. Psychotic depression is a possibility, but Mr. M’s psychotic features are incongruent with his mood. Mania would be a diagnosis of exclusion. Mr. M had no prior history of major affective illness. Mr. M was abusing Cannabis, which might independently contribute to psychosis1; however, he had been using it recreationally for 10 years without psychiatric problems. N2O, however, can cause symptoms consistent with Mr. M’s presentation.
[polldaddy:9859140]
EVALUATION Laboratory tests
Mr. M’s physical examination is notable only for an elevated blood pressure of 196/120 mm Hg. Neurologic examination is normal. Toxicology is positive for cannabinoids and negative for amphetamines, cocaine, opiates, and phencyclidine. Chemistries are normal except for a potassium of 3.4 mEq/L (reference range, 3.7 to 5.2 mEq/L) and a blood urine nitrogen of 25 mg/dL (reference range, 6 to 20 mg/dL), which are consistent with reduced food and fluid intake. Mr. M shows no signs of anemia. Hematocrit is 42% and mean corpuscular volume is 90 fL. Syphilis screen is negative; a head CT scan is unremarkable.
The authors’ observations
N2O, also known as “laughing gas,” is routinely used by dentists and pediatric anesthesiologists, and has other medical uses. Some studies have examined an adjunctive use of N2O for pain control in the ED and during colonoscopies.3,4
In the 2013 U.S. National Survey on Drug Use and Health, 16% of respondents reported lifetime illicit use of N2O.5,6 It is readily available in tanks used in medicine and industry and in small dispensers called “whippits” that can be legally purchased. Acute effects of N2O include euphoric mood, numbness, feeling of warmth, dizziness, and auditory hallucinations.7 The anesthetic effects of N2O are linked to endogenous release of opiates, and recent research links its anxiolytic activity to the facilitation of GABAergic inhibitory and N-methyl-
Beginning with a 1960 report of a series of patients with “megaloblastic madness,”17 there have been calls for increased awareness of the potential for vitamin B12 deficiency–induced psychiatric disorders, even in the absence of other hematologic or neurologic sequelae that would alert clinicians of the deficiency. In a case series of 141 patients with a broad array of neurologic and psychiatric symptoms associated with vitamin B12 deficiency, 40 (28%) patients had no anemia or macrocytosis.2
Vitamin B12-responsive psychosis has been reported as the sole manifestation of illness, without associated neurologic or hematologic symptoms, in only a few case reports. Vitamin B12 levels in these cases ranged from 75 to 236 pg/mL (reference range, 160 to 950 pg/mL).18-20 In all of these cases, the vitamin B12 deficiency was traced to dietary causes. The clinical evaluation of suspected vitamin B12 deficiency is outlined in the Figure.21 Mr. M had used Cannabis recreationally for a long time, and his Cannabis use acutely escalated with use of N2O. Long-term use of Cannabis alone is a risk factor for psychotic illness.22 Combined abuse of Cannabis and N2O has been reported to provoke psychotic illness. In a case report of a 22-year-old male who was treated for paranoid delusions, using Cannabis and 100 cartridges of N2O daily was associated with low vitamin B12 and elevated homocysteine and methylmalonic acid levels.23
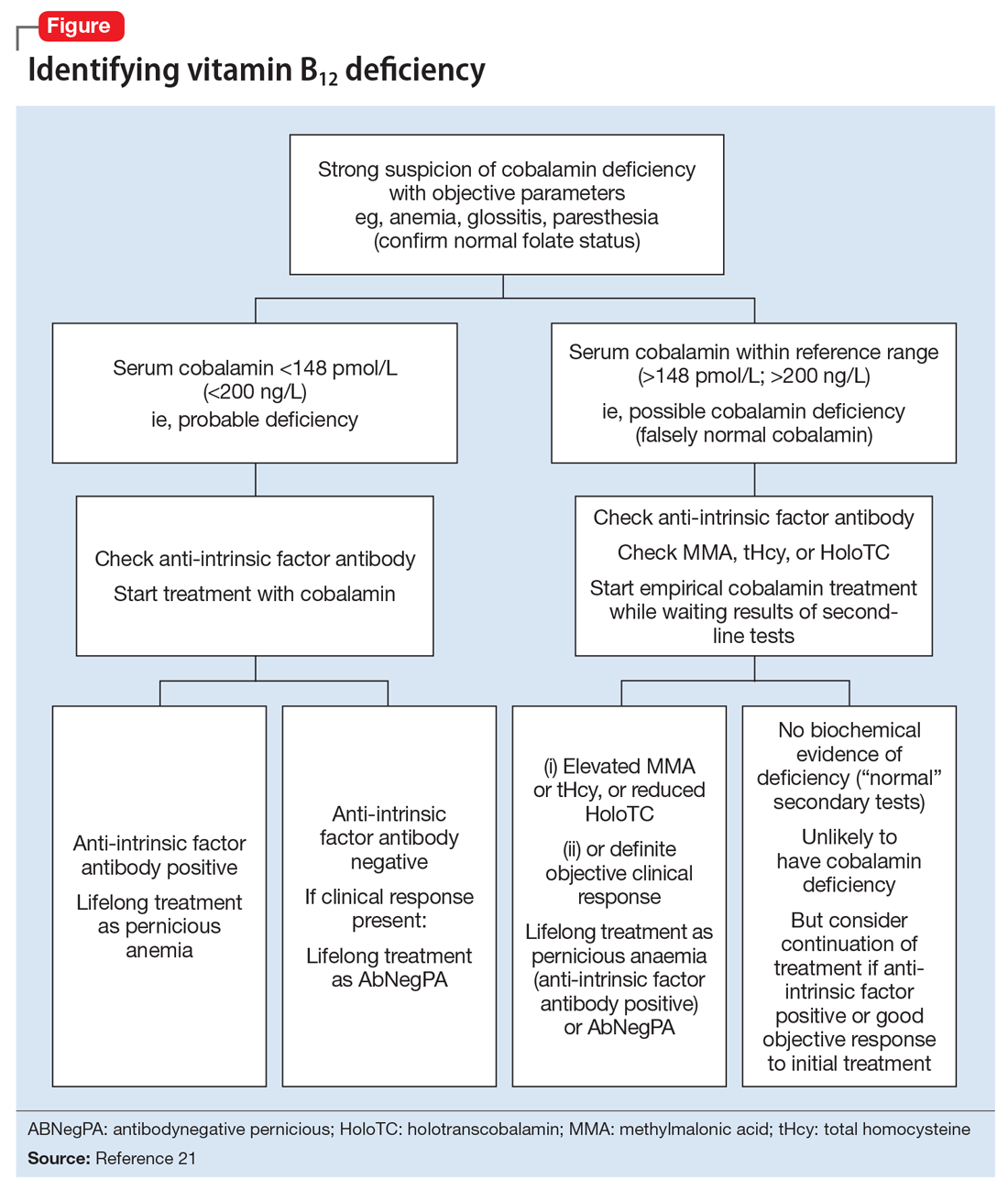
Cannabis use may have played a role in Mr. M’s escalating N2O use. In a study comparing 9 active Cannabis users with 9 non-using controls, users rated the subjective effects of N2O as more intense than non-users.24 In our patient’s case, Cannabis may have played a role in both sustaining his escalating N2O abuse and potentiating its psychotomimetic effects.
It also is possible that Mr. M may have been “self-medicating” his grief with N2O. In a recent placebo-controlled crossover trial of 20 patients with treatment-resistant depression, Nagele et al25 found a significant rapid and week-long antidepressant effect of subanesthetic N2O use. A model involving NMDA receptor activation has been proposed.25,26 Zorumski et al26 further reviewed possible antidepressant mechanisms of N2O. They compared N2O with ketamine as an NMDA receptor antagonist, but also noted its distinct effects on glutaminergic and GABAergic neurotransmitter systems as well as other receptors and channels.26 However, illicit use of N2O poses toxicity dangers and has no current indication for psychiatric treatment.
TREATMENT Supplementation
Mr. M is diagnosed with substance-induced psychotic disorder. His symptoms were precipitated by an acute increase in N2O use, which has been shown to cause vitamin B12 deficiency, which we consider was likely a primary contributor to his presentation. Other potential contributing factors are premorbid hyperthymic temperament, a possible propensity to psychotic thinking under stress, the sudden death of his wife, acute grief, the potentiating role of Cannabis, dehydration, and general malnutrition. The death of a loved one is associated with an increased risk of developing substance use disorders.27
During a 15-day psychiatric hospitalization, Mr. M is given olanzapine, increased to 15 mg/d and oral vitamin B12, 1,000 mcg/d for 4 days, then IM cyanocobalamin for 7 days. Mr. M’s symptoms steadily improve, with normalization of sleep and near-total resolution of delusions. On hospital Day 14, his vitamin B12 levels are within normal limits (844 pg/mL). At discharge, Mr. M shows residual mild grandiosity, with limited insight into his illness and what caused it, but frank delusional ideation has clearly receded. He still shows some signs of grief. Mr. M is advised to stop using Cannabis and N2O and about the potential consequences of continued use.
The authors’ observations
For patients with vitamin B12 deficiency, guidelines from the National Health Service in the United Kingdom and the British Society for Haematology recommend treatment with IM hydroxocobalamin, 1,000 IU, 3 times weekly, for 2 weeks.21,28 For patients with neurologic symptoms, the British National Foundation recommends treatment with IM hydroxocobalamin, 1,000 IU, on alternative days until there is no further improvement.21
This case is a reminder for clinicians to screen for inhalant use, specifically N2O, which can precipitate vitamin B12 deficiency with psychiatric symptoms as the only presenting concern. Clinicians should consider measuring vitamin B12 levels in psychiatric patients at risk of deficiency of this nutrient, including older adults, vegetarians, and those with alimentary disorders.29,30 Dietary sources of vitamin B12 include meat, milk, egg, fish, and shellfish.31 The body can store a total of 2 to 5 mg of vitamin B12; thus, it takes 2 to 5 years to develop vitamin B12 deficiency from malabsorption and can take as long as 20 years to develop vitamin B12 deficiency from vegetarianism.32 However, by chemically inactivating vitamin B12, N2O causes a rapid functional deficiency, as was seen in our patient.
OUTCOME Improved insight
At a 1-week follow-up appointment with a psychiatrist, Mr. M has no evident psychotic symptoms. He reports that he has not used Cannabis or N2O, and he discontinues olanzapine following this visit. Two weeks later, Mr. M shows no psychotic or affective symptoms other than grief, which is appropriately expressed. His insight has improved. He commits to not using Cannabis, N2O, or any other illicit substances. Mr. M is referred back to his long-standing primary care provider with the understanding that if any psychiatric symptoms recur he will see a psychiatrist again.
1. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187-194.
2. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26)1720-1728.
3. Herres J, Chudnofsky CR, Manur R, et al. The use of inhaled nitrous oxide for analgesia in adult ED patients: a pilot study. Am J Emerg Med. 2016;34(2):269-273.
4. Aboumarzouk OM, Agarwal T, Syed Nong Chek SA, et al. Nitrous oxide for colonoscopy. Cochrane Database Syst Rev. 2011;(8):CD008506.
5. National Institute on Drug Abuse. Drug facts: inhalants. http://www.drugabuse.gov/publications/drugfacts/inhalants. Updated February 2017. Accessed September 30, 2017.
6. SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health 2012 and 2013: Table 1.88C. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2013.pdf. Published September 4, 2017. Accessed September 30, 2017.
7. Brouette T, Anton R. Clinical review of inhalants. Am J Addict. 2001;10(1):79-94.
8. Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54(1):9-18.
9. Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016;25(5):358-369.
10. Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. 2011;301(1-2):1-8.
11. van Tonder SV, Ruck A, van der Westhuyzen J, et al. Dissociation of methionine synthetase (EC 2.1.1.13) activity and impairment of DNA synthesis in fruit bats (Rousettus aegyptiacus) with nitrous oxide-induced vitamin B12 deficiency. Br J Nutr. 1986;55(1):187-192.
12. Schrier SL, Mentzer WC, Tirnauer JS. Diagnosis and treatment of vitamin B12 and folate deficiency. UpToDate. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-vitamin-b12-and-folate-deficiency. Updated September 30, 2011. Accessed September 8, 2015.
13. Sethi NK, Mullin P, Torgovnick J, et al. Nitrous oxide “whippit” abuse presenting with cobalamin responsive psychosis. J Med Toxicol. 2006;2(2):71-74.
14. Cousaert C, Heylens G, Audenaert K. Laughing gas abuse is no joke. An overview of the implications for psychiatric practice. Clin Neurol Neurosurg. 2013;115(7):859-862.
15. Brodsky L, Zuniga J. Nitrous oxide: a psychotogenic agent. Compr Psychiatry. 1975;16(2):185-188.
16. Wong SL, Harrison R, Mattman A, et al. Nitrous oxide (N2O)-induced acute psychosis. Can J Neurol Sci. 2014;41(5):672-674.
17. Smith AD. Megaloblastic madness. Br Med J. 1960;2(5216):1840-1845.
18. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Associ J. 2001;3(9):701-703.
19. Kuo SC, Yeh SB, Yeh YW, et al. Schizophrenia-like psychotic episode precipitated by cobalamin deficiency. Gen Hosp Psychiatry. 2009;31(6):586-588.
20. Raveendranathan D, Shiva L, Venkatasubramanian G, et al. Vitamin B12 deficiency masquerading as clozapine-resistant psychotic symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25(2):E34-E35.
21. Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513.
22. Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
23. Garakani A, Welch AK, Jaffe RJ, et al. Psychosis and low cyanocobalamin in a patient abusing nitrous oxide and cannabis. Psychosomatics. 2014;55(6):715-719.
24. Yajnik S, Thapar P, Lichtor JL, et al. Effects of marijuana history on the subjective, psychomotor, and reinforcing effects of nitrous oxide in human. Drug Alcohol Depend. 1994;36(3):227-236.
25. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78(1):10-18.
26. Zorumski CF, Nagele P, Mennerick S, et al. Treatment-resistant major depression: rationale for NMDA receptors as targets and nitrous oxide as therapy. Front Psychiatry. 2015;6:172.
27. Shear MK. Clinical practice. Complicated grief. N Engl J Med. 2015;372(2):153-160.
28. Knechtli CJC, Crowe JN. Guidelines for the investigation & management of vitamin B12 deficiency. Royal United Hospital Bath, National Health Service. http://www.ruh.nhs.uk/For_Clinicians/departments_ruh/Pathology/documents/haematology/B12_-_advice_on_investigation_management.pdf. Accessed June 14, 2016.
29. Jayaram N, Rao MG, Narashima A, et al. Vitamin B12 levels and psychiatric symptomatology: a case series. J Neuropsychiatry Clin Neurosci. 2013;25(2):150-152.
30. Marks PW, Zukerberg LR. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 30-2004. A 37-year-old woman with paresthesias of the arms and legs. N Engl J Med. 2004;351(13):1333-1341.
31. Watanabe F. Vitamin B12 sources and bioavailablility. Exp Biol Med (Maywood). 2007;232(10):1266-1274.
32. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
CASE Grieving, delusional
Mr. M, age 51, is brought to the emergency department (ED) because of new-onset delusions and decreased self-care over the last 2 weeks following the sudden death of his wife. He has become expansive and grandiose, with pressured speech, increased energy, and markedly reduced sleep. Mr. M is preoccupied with the idea that he is “the first to survive a human reboot process” and says that his and his wife’s bodies and brains had been “split apart.” Mr. M has limited his food and fluid intake and lost 15 lb within the past 2 to 3 weeks.
Mr. M has no history of any affective, psychotic, or other major mental disorders or treatment. He reports that he has regularly used Cannabis over the last 10 years, and a few years ago, he started occasionally using nitrous oxide (N2O). He says that in the week following his wife’s death, he used N2O almost daily and in copious amounts. In an attempt to “self-anesthetize” himself after his wife’s funeral, he isolated himself in his bedroom and used escalating amounts of Cannabis and N2O, while continually working on a book about their life together.
At first, Mr. M shows little emotion and describes his situation as “interesting and fascinating.” He mentions that he thinks he might have been “psychotic” the week after his wife’s death, but he shows no sustained insight and immediately relapses into psychotic thinking. Over several hours in the ED, he is tearful and sad about his wife’s death. Mr. M recalls a similar experience of grief after his mother died when he was a teenager, but at that time he did not abuse substances or have psychotic symptoms. He is fully alert, fully oriented, and has no significant deficits of attention or memory.
[polldaddy:9859135]
The authors’ observations
Grief was a precipitating event, but by itself grief cannot explain psychosis. Psychotic depression is a possibility, but Mr. M’s psychotic features are incongruent with his mood. Mania would be a diagnosis of exclusion. Mr. M had no prior history of major affective illness. Mr. M was abusing Cannabis, which might independently contribute to psychosis1; however, he had been using it recreationally for 10 years without psychiatric problems. N2O, however, can cause symptoms consistent with Mr. M’s presentation.
[polldaddy:9859140]
EVALUATION Laboratory tests
Mr. M’s physical examination is notable only for an elevated blood pressure of 196/120 mm Hg. Neurologic examination is normal. Toxicology is positive for cannabinoids and negative for amphetamines, cocaine, opiates, and phencyclidine. Chemistries are normal except for a potassium of 3.4 mEq/L (reference range, 3.7 to 5.2 mEq/L) and a blood urine nitrogen of 25 mg/dL (reference range, 6 to 20 mg/dL), which are consistent with reduced food and fluid intake. Mr. M shows no signs of anemia. Hematocrit is 42% and mean corpuscular volume is 90 fL. Syphilis screen is negative; a head CT scan is unremarkable.
The authors’ observations
N2O, also known as “laughing gas,” is routinely used by dentists and pediatric anesthesiologists, and has other medical uses. Some studies have examined an adjunctive use of N2O for pain control in the ED and during colonoscopies.3,4
In the 2013 U.S. National Survey on Drug Use and Health, 16% of respondents reported lifetime illicit use of N2O.5,6 It is readily available in tanks used in medicine and industry and in small dispensers called “whippits” that can be legally purchased. Acute effects of N2O include euphoric mood, numbness, feeling of warmth, dizziness, and auditory hallucinations.7 The anesthetic effects of N2O are linked to endogenous release of opiates, and recent research links its anxiolytic activity to the facilitation of GABAergic inhibitory and N-methyl-
Beginning with a 1960 report of a series of patients with “megaloblastic madness,”17 there have been calls for increased awareness of the potential for vitamin B12 deficiency–induced psychiatric disorders, even in the absence of other hematologic or neurologic sequelae that would alert clinicians of the deficiency. In a case series of 141 patients with a broad array of neurologic and psychiatric symptoms associated with vitamin B12 deficiency, 40 (28%) patients had no anemia or macrocytosis.2
Vitamin B12-responsive psychosis has been reported as the sole manifestation of illness, without associated neurologic or hematologic symptoms, in only a few case reports. Vitamin B12 levels in these cases ranged from 75 to 236 pg/mL (reference range, 160 to 950 pg/mL).18-20 In all of these cases, the vitamin B12 deficiency was traced to dietary causes. The clinical evaluation of suspected vitamin B12 deficiency is outlined in the Figure.21 Mr. M had used Cannabis recreationally for a long time, and his Cannabis use acutely escalated with use of N2O. Long-term use of Cannabis alone is a risk factor for psychotic illness.22 Combined abuse of Cannabis and N2O has been reported to provoke psychotic illness. In a case report of a 22-year-old male who was treated for paranoid delusions, using Cannabis and 100 cartridges of N2O daily was associated with low vitamin B12 and elevated homocysteine and methylmalonic acid levels.23

Cannabis use may have played a role in Mr. M’s escalating N2O use. In a study comparing 9 active Cannabis users with 9 non-using controls, users rated the subjective effects of N2O as more intense than non-users.24 In our patient’s case, Cannabis may have played a role in both sustaining his escalating N2O abuse and potentiating its psychotomimetic effects.
It also is possible that Mr. M may have been “self-medicating” his grief with N2O. In a recent placebo-controlled crossover trial of 20 patients with treatment-resistant depression, Nagele et al25 found a significant rapid and week-long antidepressant effect of subanesthetic N2O use. A model involving NMDA receptor activation has been proposed.25,26 Zorumski et al26 further reviewed possible antidepressant mechanisms of N2O. They compared N2O with ketamine as an NMDA receptor antagonist, but also noted its distinct effects on glutaminergic and GABAergic neurotransmitter systems as well as other receptors and channels.26 However, illicit use of N2O poses toxicity dangers and has no current indication for psychiatric treatment.
TREATMENT Supplementation
Mr. M is diagnosed with substance-induced psychotic disorder. His symptoms were precipitated by an acute increase in N2O use, which has been shown to cause vitamin B12 deficiency, which we consider was likely a primary contributor to his presentation. Other potential contributing factors are premorbid hyperthymic temperament, a possible propensity to psychotic thinking under stress, the sudden death of his wife, acute grief, the potentiating role of Cannabis, dehydration, and general malnutrition. The death of a loved one is associated with an increased risk of developing substance use disorders.27
During a 15-day psychiatric hospitalization, Mr. M is given olanzapine, increased to 15 mg/d and oral vitamin B12, 1,000 mcg/d for 4 days, then IM cyanocobalamin for 7 days. Mr. M’s symptoms steadily improve, with normalization of sleep and near-total resolution of delusions. On hospital Day 14, his vitamin B12 levels are within normal limits (844 pg/mL). At discharge, Mr. M shows residual mild grandiosity, with limited insight into his illness and what caused it, but frank delusional ideation has clearly receded. He still shows some signs of grief. Mr. M is advised to stop using Cannabis and N2O and about the potential consequences of continued use.
The authors’ observations
For patients with vitamin B12 deficiency, guidelines from the National Health Service in the United Kingdom and the British Society for Haematology recommend treatment with IM hydroxocobalamin, 1,000 IU, 3 times weekly, for 2 weeks.21,28 For patients with neurologic symptoms, the British National Foundation recommends treatment with IM hydroxocobalamin, 1,000 IU, on alternative days until there is no further improvement.21
This case is a reminder for clinicians to screen for inhalant use, specifically N2O, which can precipitate vitamin B12 deficiency with psychiatric symptoms as the only presenting concern. Clinicians should consider measuring vitamin B12 levels in psychiatric patients at risk of deficiency of this nutrient, including older adults, vegetarians, and those with alimentary disorders.29,30 Dietary sources of vitamin B12 include meat, milk, egg, fish, and shellfish.31 The body can store a total of 2 to 5 mg of vitamin B12; thus, it takes 2 to 5 years to develop vitamin B12 deficiency from malabsorption and can take as long as 20 years to develop vitamin B12 deficiency from vegetarianism.32 However, by chemically inactivating vitamin B12, N2O causes a rapid functional deficiency, as was seen in our patient.
OUTCOME Improved insight
At a 1-week follow-up appointment with a psychiatrist, Mr. M has no evident psychotic symptoms. He reports that he has not used Cannabis or N2O, and he discontinues olanzapine following this visit. Two weeks later, Mr. M shows no psychotic or affective symptoms other than grief, which is appropriately expressed. His insight has improved. He commits to not using Cannabis, N2O, or any other illicit substances. Mr. M is referred back to his long-standing primary care provider with the understanding that if any psychiatric symptoms recur he will see a psychiatrist again.
CASE Grieving, delusional
Mr. M, age 51, is brought to the emergency department (ED) because of new-onset delusions and decreased self-care over the last 2 weeks following the sudden death of his wife. He has become expansive and grandiose, with pressured speech, increased energy, and markedly reduced sleep. Mr. M is preoccupied with the idea that he is “the first to survive a human reboot process” and says that his and his wife’s bodies and brains had been “split apart.” Mr. M has limited his food and fluid intake and lost 15 lb within the past 2 to 3 weeks.
Mr. M has no history of any affective, psychotic, or other major mental disorders or treatment. He reports that he has regularly used Cannabis over the last 10 years, and a few years ago, he started occasionally using nitrous oxide (N2O). He says that in the week following his wife’s death, he used N2O almost daily and in copious amounts. In an attempt to “self-anesthetize” himself after his wife’s funeral, he isolated himself in his bedroom and used escalating amounts of Cannabis and N2O, while continually working on a book about their life together.
At first, Mr. M shows little emotion and describes his situation as “interesting and fascinating.” He mentions that he thinks he might have been “psychotic” the week after his wife’s death, but he shows no sustained insight and immediately relapses into psychotic thinking. Over several hours in the ED, he is tearful and sad about his wife’s death. Mr. M recalls a similar experience of grief after his mother died when he was a teenager, but at that time he did not abuse substances or have psychotic symptoms. He is fully alert, fully oriented, and has no significant deficits of attention or memory.
[polldaddy:9859135]
The authors’ observations
Grief was a precipitating event, but by itself grief cannot explain psychosis. Psychotic depression is a possibility, but Mr. M’s psychotic features are incongruent with his mood. Mania would be a diagnosis of exclusion. Mr. M had no prior history of major affective illness. Mr. M was abusing Cannabis, which might independently contribute to psychosis1; however, he had been using it recreationally for 10 years without psychiatric problems. N2O, however, can cause symptoms consistent with Mr. M’s presentation.
[polldaddy:9859140]
EVALUATION Laboratory tests
Mr. M’s physical examination is notable only for an elevated blood pressure of 196/120 mm Hg. Neurologic examination is normal. Toxicology is positive for cannabinoids and negative for amphetamines, cocaine, opiates, and phencyclidine. Chemistries are normal except for a potassium of 3.4 mEq/L (reference range, 3.7 to 5.2 mEq/L) and a blood urine nitrogen of 25 mg/dL (reference range, 6 to 20 mg/dL), which are consistent with reduced food and fluid intake. Mr. M shows no signs of anemia. Hematocrit is 42% and mean corpuscular volume is 90 fL. Syphilis screen is negative; a head CT scan is unremarkable.
The authors’ observations
N2O, also known as “laughing gas,” is routinely used by dentists and pediatric anesthesiologists, and has other medical uses. Some studies have examined an adjunctive use of N2O for pain control in the ED and during colonoscopies.3,4
In the 2013 U.S. National Survey on Drug Use and Health, 16% of respondents reported lifetime illicit use of N2O.5,6 It is readily available in tanks used in medicine and industry and in small dispensers called “whippits” that can be legally purchased. Acute effects of N2O include euphoric mood, numbness, feeling of warmth, dizziness, and auditory hallucinations.7 The anesthetic effects of N2O are linked to endogenous release of opiates, and recent research links its anxiolytic activity to the facilitation of GABAergic inhibitory and N-methyl-
Beginning with a 1960 report of a series of patients with “megaloblastic madness,”17 there have been calls for increased awareness of the potential for vitamin B12 deficiency–induced psychiatric disorders, even in the absence of other hematologic or neurologic sequelae that would alert clinicians of the deficiency. In a case series of 141 patients with a broad array of neurologic and psychiatric symptoms associated with vitamin B12 deficiency, 40 (28%) patients had no anemia or macrocytosis.2
Vitamin B12-responsive psychosis has been reported as the sole manifestation of illness, without associated neurologic or hematologic symptoms, in only a few case reports. Vitamin B12 levels in these cases ranged from 75 to 236 pg/mL (reference range, 160 to 950 pg/mL).18-20 In all of these cases, the vitamin B12 deficiency was traced to dietary causes. The clinical evaluation of suspected vitamin B12 deficiency is outlined in the Figure.21 Mr. M had used Cannabis recreationally for a long time, and his Cannabis use acutely escalated with use of N2O. Long-term use of Cannabis alone is a risk factor for psychotic illness.22 Combined abuse of Cannabis and N2O has been reported to provoke psychotic illness. In a case report of a 22-year-old male who was treated for paranoid delusions, using Cannabis and 100 cartridges of N2O daily was associated with low vitamin B12 and elevated homocysteine and methylmalonic acid levels.23

Cannabis use may have played a role in Mr. M’s escalating N2O use. In a study comparing 9 active Cannabis users with 9 non-using controls, users rated the subjective effects of N2O as more intense than non-users.24 In our patient’s case, Cannabis may have played a role in both sustaining his escalating N2O abuse and potentiating its psychotomimetic effects.
It also is possible that Mr. M may have been “self-medicating” his grief with N2O. In a recent placebo-controlled crossover trial of 20 patients with treatment-resistant depression, Nagele et al25 found a significant rapid and week-long antidepressant effect of subanesthetic N2O use. A model involving NMDA receptor activation has been proposed.25,26 Zorumski et al26 further reviewed possible antidepressant mechanisms of N2O. They compared N2O with ketamine as an NMDA receptor antagonist, but also noted its distinct effects on glutaminergic and GABAergic neurotransmitter systems as well as other receptors and channels.26 However, illicit use of N2O poses toxicity dangers and has no current indication for psychiatric treatment.
TREATMENT Supplementation
Mr. M is diagnosed with substance-induced psychotic disorder. His symptoms were precipitated by an acute increase in N2O use, which has been shown to cause vitamin B12 deficiency, which we consider was likely a primary contributor to his presentation. Other potential contributing factors are premorbid hyperthymic temperament, a possible propensity to psychotic thinking under stress, the sudden death of his wife, acute grief, the potentiating role of Cannabis, dehydration, and general malnutrition. The death of a loved one is associated with an increased risk of developing substance use disorders.27
During a 15-day psychiatric hospitalization, Mr. M is given olanzapine, increased to 15 mg/d and oral vitamin B12, 1,000 mcg/d for 4 days, then IM cyanocobalamin for 7 days. Mr. M’s symptoms steadily improve, with normalization of sleep and near-total resolution of delusions. On hospital Day 14, his vitamin B12 levels are within normal limits (844 pg/mL). At discharge, Mr. M shows residual mild grandiosity, with limited insight into his illness and what caused it, but frank delusional ideation has clearly receded. He still shows some signs of grief. Mr. M is advised to stop using Cannabis and N2O and about the potential consequences of continued use.
The authors’ observations
For patients with vitamin B12 deficiency, guidelines from the National Health Service in the United Kingdom and the British Society for Haematology recommend treatment with IM hydroxocobalamin, 1,000 IU, 3 times weekly, for 2 weeks.21,28 For patients with neurologic symptoms, the British National Foundation recommends treatment with IM hydroxocobalamin, 1,000 IU, on alternative days until there is no further improvement.21
This case is a reminder for clinicians to screen for inhalant use, specifically N2O, which can precipitate vitamin B12 deficiency with psychiatric symptoms as the only presenting concern. Clinicians should consider measuring vitamin B12 levels in psychiatric patients at risk of deficiency of this nutrient, including older adults, vegetarians, and those with alimentary disorders.29,30 Dietary sources of vitamin B12 include meat, milk, egg, fish, and shellfish.31 The body can store a total of 2 to 5 mg of vitamin B12; thus, it takes 2 to 5 years to develop vitamin B12 deficiency from malabsorption and can take as long as 20 years to develop vitamin B12 deficiency from vegetarianism.32 However, by chemically inactivating vitamin B12, N2O causes a rapid functional deficiency, as was seen in our patient.
OUTCOME Improved insight
At a 1-week follow-up appointment with a psychiatrist, Mr. M has no evident psychotic symptoms. He reports that he has not used Cannabis or N2O, and he discontinues olanzapine following this visit. Two weeks later, Mr. M shows no psychotic or affective symptoms other than grief, which is appropriately expressed. His insight has improved. He commits to not using Cannabis, N2O, or any other illicit substances. Mr. M is referred back to his long-standing primary care provider with the understanding that if any psychiatric symptoms recur he will see a psychiatrist again.
1. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187-194.
2. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26)1720-1728.
3. Herres J, Chudnofsky CR, Manur R, et al. The use of inhaled nitrous oxide for analgesia in adult ED patients: a pilot study. Am J Emerg Med. 2016;34(2):269-273.
4. Aboumarzouk OM, Agarwal T, Syed Nong Chek SA, et al. Nitrous oxide for colonoscopy. Cochrane Database Syst Rev. 2011;(8):CD008506.
5. National Institute on Drug Abuse. Drug facts: inhalants. http://www.drugabuse.gov/publications/drugfacts/inhalants. Updated February 2017. Accessed September 30, 2017.
6. SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health 2012 and 2013: Table 1.88C. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2013.pdf. Published September 4, 2017. Accessed September 30, 2017.
7. Brouette T, Anton R. Clinical review of inhalants. Am J Addict. 2001;10(1):79-94.
8. Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54(1):9-18.
9. Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016;25(5):358-369.
10. Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. 2011;301(1-2):1-8.
11. van Tonder SV, Ruck A, van der Westhuyzen J, et al. Dissociation of methionine synthetase (EC 2.1.1.13) activity and impairment of DNA synthesis in fruit bats (Rousettus aegyptiacus) with nitrous oxide-induced vitamin B12 deficiency. Br J Nutr. 1986;55(1):187-192.
12. Schrier SL, Mentzer WC, Tirnauer JS. Diagnosis and treatment of vitamin B12 and folate deficiency. UpToDate. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-vitamin-b12-and-folate-deficiency. Updated September 30, 2011. Accessed September 8, 2015.
13. Sethi NK, Mullin P, Torgovnick J, et al. Nitrous oxide “whippit” abuse presenting with cobalamin responsive psychosis. J Med Toxicol. 2006;2(2):71-74.
14. Cousaert C, Heylens G, Audenaert K. Laughing gas abuse is no joke. An overview of the implications for psychiatric practice. Clin Neurol Neurosurg. 2013;115(7):859-862.
15. Brodsky L, Zuniga J. Nitrous oxide: a psychotogenic agent. Compr Psychiatry. 1975;16(2):185-188.
16. Wong SL, Harrison R, Mattman A, et al. Nitrous oxide (N2O)-induced acute psychosis. Can J Neurol Sci. 2014;41(5):672-674.
17. Smith AD. Megaloblastic madness. Br Med J. 1960;2(5216):1840-1845.
18. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Associ J. 2001;3(9):701-703.
19. Kuo SC, Yeh SB, Yeh YW, et al. Schizophrenia-like psychotic episode precipitated by cobalamin deficiency. Gen Hosp Psychiatry. 2009;31(6):586-588.
20. Raveendranathan D, Shiva L, Venkatasubramanian G, et al. Vitamin B12 deficiency masquerading as clozapine-resistant psychotic symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25(2):E34-E35.
21. Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513.
22. Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
23. Garakani A, Welch AK, Jaffe RJ, et al. Psychosis and low cyanocobalamin in a patient abusing nitrous oxide and cannabis. Psychosomatics. 2014;55(6):715-719.
24. Yajnik S, Thapar P, Lichtor JL, et al. Effects of marijuana history on the subjective, psychomotor, and reinforcing effects of nitrous oxide in human. Drug Alcohol Depend. 1994;36(3):227-236.
25. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78(1):10-18.
26. Zorumski CF, Nagele P, Mennerick S, et al. Treatment-resistant major depression: rationale for NMDA receptors as targets and nitrous oxide as therapy. Front Psychiatry. 2015;6:172.
27. Shear MK. Clinical practice. Complicated grief. N Engl J Med. 2015;372(2):153-160.
28. Knechtli CJC, Crowe JN. Guidelines for the investigation & management of vitamin B12 deficiency. Royal United Hospital Bath, National Health Service. http://www.ruh.nhs.uk/For_Clinicians/departments_ruh/Pathology/documents/haematology/B12_-_advice_on_investigation_management.pdf. Accessed June 14, 2016.
29. Jayaram N, Rao MG, Narashima A, et al. Vitamin B12 levels and psychiatric symptomatology: a case series. J Neuropsychiatry Clin Neurosci. 2013;25(2):150-152.
30. Marks PW, Zukerberg LR. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 30-2004. A 37-year-old woman with paresthesias of the arms and legs. N Engl J Med. 2004;351(13):1333-1341.
31. Watanabe F. Vitamin B12 sources and bioavailablility. Exp Biol Med (Maywood). 2007;232(10):1266-1274.
32. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
1. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187-194.
2. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26)1720-1728.
3. Herres J, Chudnofsky CR, Manur R, et al. The use of inhaled nitrous oxide for analgesia in adult ED patients: a pilot study. Am J Emerg Med. 2016;34(2):269-273.
4. Aboumarzouk OM, Agarwal T, Syed Nong Chek SA, et al. Nitrous oxide for colonoscopy. Cochrane Database Syst Rev. 2011;(8):CD008506.
5. National Institute on Drug Abuse. Drug facts: inhalants. http://www.drugabuse.gov/publications/drugfacts/inhalants. Updated February 2017. Accessed September 30, 2017.
6. SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health 2012 and 2013: Table 1.88C. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2013.pdf. Published September 4, 2017. Accessed September 30, 2017.
7. Brouette T, Anton R. Clinical review of inhalants. Am J Addict. 2001;10(1):79-94.
8. Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54(1):9-18.
9. Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016;25(5):358-369.
10. Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. 2011;301(1-2):1-8.
11. van Tonder SV, Ruck A, van der Westhuyzen J, et al. Dissociation of methionine synthetase (EC 2.1.1.13) activity and impairment of DNA synthesis in fruit bats (Rousettus aegyptiacus) with nitrous oxide-induced vitamin B12 deficiency. Br J Nutr. 1986;55(1):187-192.
12. Schrier SL, Mentzer WC, Tirnauer JS. Diagnosis and treatment of vitamin B12 and folate deficiency. UpToDate. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-vitamin-b12-and-folate-deficiency. Updated September 30, 2011. Accessed September 8, 2015.
13. Sethi NK, Mullin P, Torgovnick J, et al. Nitrous oxide “whippit” abuse presenting with cobalamin responsive psychosis. J Med Toxicol. 2006;2(2):71-74.
14. Cousaert C, Heylens G, Audenaert K. Laughing gas abuse is no joke. An overview of the implications for psychiatric practice. Clin Neurol Neurosurg. 2013;115(7):859-862.
15. Brodsky L, Zuniga J. Nitrous oxide: a psychotogenic agent. Compr Psychiatry. 1975;16(2):185-188.
16. Wong SL, Harrison R, Mattman A, et al. Nitrous oxide (N2O)-induced acute psychosis. Can J Neurol Sci. 2014;41(5):672-674.
17. Smith AD. Megaloblastic madness. Br Med J. 1960;2(5216):1840-1845.
18. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Associ J. 2001;3(9):701-703.
19. Kuo SC, Yeh SB, Yeh YW, et al. Schizophrenia-like psychotic episode precipitated by cobalamin deficiency. Gen Hosp Psychiatry. 2009;31(6):586-588.
20. Raveendranathan D, Shiva L, Venkatasubramanian G, et al. Vitamin B12 deficiency masquerading as clozapine-resistant psychotic symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25(2):E34-E35.
21. Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513.
22. Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
23. Garakani A, Welch AK, Jaffe RJ, et al. Psychosis and low cyanocobalamin in a patient abusing nitrous oxide and cannabis. Psychosomatics. 2014;55(6):715-719.
24. Yajnik S, Thapar P, Lichtor JL, et al. Effects of marijuana history on the subjective, psychomotor, and reinforcing effects of nitrous oxide in human. Drug Alcohol Depend. 1994;36(3):227-236.
25. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78(1):10-18.
26. Zorumski CF, Nagele P, Mennerick S, et al. Treatment-resistant major depression: rationale for NMDA receptors as targets and nitrous oxide as therapy. Front Psychiatry. 2015;6:172.
27. Shear MK. Clinical practice. Complicated grief. N Engl J Med. 2015;372(2):153-160.
28. Knechtli CJC, Crowe JN. Guidelines for the investigation & management of vitamin B12 deficiency. Royal United Hospital Bath, National Health Service. http://www.ruh.nhs.uk/For_Clinicians/departments_ruh/Pathology/documents/haematology/B12_-_advice_on_investigation_management.pdf. Accessed June 14, 2016.
29. Jayaram N, Rao MG, Narashima A, et al. Vitamin B12 levels and psychiatric symptomatology: a case series. J Neuropsychiatry Clin Neurosci. 2013;25(2):150-152.
30. Marks PW, Zukerberg LR. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 30-2004. A 37-year-old woman with paresthesias of the arms and legs. N Engl J Med. 2004;351(13):1333-1341.
31. Watanabe F. Vitamin B12 sources and bioavailablility. Exp Biol Med (Maywood). 2007;232(10):1266-1274.
32. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.