User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
The art of psychopharmacology: Avoiding medication changes and slowing down
As physicians, we are cognizant of the importance of patient-centered care, active listening, empathy, and patience—the so-called “hidden curriculum of medicine.”1 However, our attempts to centralize these concepts may be overshadowed by the deeply rooted drive to treat and fix. At times, we are simply treating uncertainty, whether it be diagnostic uncertainty or the uncertainty arising from clinical responses and outcomes that are far from binary. Definitive actions, such as adding medications or altering dosages, may appear to both patients and physicians to be a step closer to a “cure.” However, watchful waiting, re-evaluation, and accepting uncertainty are the true skills of effective care.
Be savvy about psychopharmacology
Psychotropics can take weeks to months to reach their full potential, and have varying responses and adverse effects. Beware of changing regimens prematurely, and keep in mind basic, yet crucial, pharmacokinetic concepts (eg, 4 to 5 half-lives to reach steady state, variations in metabolism). Receptor binding and dosing heuristics are notably common in psychiatry. Although such concepts are important to grasp, there is no one-size-fits-all rule. The brain simply does not possess the heart’s machine-like, linear functioning. Therefore, targeting individual parts (ie, receptors) will not equate to fixing the whole organ systematically or predictably.
Is the patient truly treatment-resistant?
Even the best treatment regimen has no clinical benefit if the patient cannot afford the prescription or does not take the medication. If cost is an impediment, switch from brand name drugs to generic formulations or to older medications in the same class. Before declaring the patient “treatment-resistant” and making medication changes, assess for compliance. This may require assistance from collateral informants. Ask family members to count the number of pills remaining in the bottle, and call the pharmacy to find out the last refill dates. If the patient exhibits a partial response to what should be a therapeutic dose, consider obtaining drug plasma levels to rule out rapid metabolism before deeming the medication trial a failure.2
Medications as liabilities
Overreliance on medications can result in the medications becoming liabilities. The polypharmacy problem is not unique to psychiatry.3 However, psychiatric patients may be more likely to inadvertently use medications in a maladaptive manner and disrupt the fundamental goals of long-term care. Avoid making medication adjustments in response to a patient’s life stressors and normative situational reactions. Doing so is a disservice to patients, because we are robbing them of chances to develop necessary coping skills and defenses. This can be overtly damaging in certain patient populations, such as those with borderline personality disorder, who may use medication adjustments as a crutch during crises.4
Treat the patient, not yourself
We physicians mean well in prescribing evidence-based treatments; however, if the symptoms or adverse effects are not bothersome or cause functional impairment, we risk losing sight of the patient’s goals in treatment and imposing our own instead. Displacing the treatment focus can alienate the patient, harm the therapeutic alliance, and result in “pill fatigue.” For example, we may be tempted to treat antipsychotic-induced tardive dyskinesia, even if the patient is not concerned about abnormal movements. Although we see this adverse effect
Change does not happen overnight
Picking a treatment option out of a lineup of choices, à la UWorld questions, does not always translate into patients agreeing with the suggested treatment, let alone the idea of receiving treatment at all. Motivational interviewing is our chance to shine in such situations and the reason why we are physicians, rather than answer-picking bots. Patients cannot change if they are not ready. However, we should be ready to roll with resistance while looking for signs of readiness to change. We must accept that it may take a week, a month, a year, or even longer for patients to align with our plan of action. The only futile decision is deeming our efforts as futile while discounting the benefits of incremental care.
1. Hafferty FW, Gaufberg EH, O’Donnell JF. The role of the hidden curriculum in “on doctoring” courses. AMA J Ethics. 2015;17(2):130-139.
2. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
3. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
4. Gunderson JG. The emergence of a generalist model to meet public health needs for patients with borderline personality disorder. Am J Psychiatry. 2016;173(5):452-458.
5. Kikkert MJ, Schene AH, Koeter MW, et al. Medication adherence in schizophrenia: exploring patients’, carers’ and professionals’ views. Schizophr Bull. 2005;32(4):786-794.
As physicians, we are cognizant of the importance of patient-centered care, active listening, empathy, and patience—the so-called “hidden curriculum of medicine.”1 However, our attempts to centralize these concepts may be overshadowed by the deeply rooted drive to treat and fix. At times, we are simply treating uncertainty, whether it be diagnostic uncertainty or the uncertainty arising from clinical responses and outcomes that are far from binary. Definitive actions, such as adding medications or altering dosages, may appear to both patients and physicians to be a step closer to a “cure.” However, watchful waiting, re-evaluation, and accepting uncertainty are the true skills of effective care.
Be savvy about psychopharmacology
Psychotropics can take weeks to months to reach their full potential, and have varying responses and adverse effects. Beware of changing regimens prematurely, and keep in mind basic, yet crucial, pharmacokinetic concepts (eg, 4 to 5 half-lives to reach steady state, variations in metabolism). Receptor binding and dosing heuristics are notably common in psychiatry. Although such concepts are important to grasp, there is no one-size-fits-all rule. The brain simply does not possess the heart’s machine-like, linear functioning. Therefore, targeting individual parts (ie, receptors) will not equate to fixing the whole organ systematically or predictably.
Is the patient truly treatment-resistant?
Even the best treatment regimen has no clinical benefit if the patient cannot afford the prescription or does not take the medication. If cost is an impediment, switch from brand name drugs to generic formulations or to older medications in the same class. Before declaring the patient “treatment-resistant” and making medication changes, assess for compliance. This may require assistance from collateral informants. Ask family members to count the number of pills remaining in the bottle, and call the pharmacy to find out the last refill dates. If the patient exhibits a partial response to what should be a therapeutic dose, consider obtaining drug plasma levels to rule out rapid metabolism before deeming the medication trial a failure.2
Medications as liabilities
Overreliance on medications can result in the medications becoming liabilities. The polypharmacy problem is not unique to psychiatry.3 However, psychiatric patients may be more likely to inadvertently use medications in a maladaptive manner and disrupt the fundamental goals of long-term care. Avoid making medication adjustments in response to a patient’s life stressors and normative situational reactions. Doing so is a disservice to patients, because we are robbing them of chances to develop necessary coping skills and defenses. This can be overtly damaging in certain patient populations, such as those with borderline personality disorder, who may use medication adjustments as a crutch during crises.4
Treat the patient, not yourself
We physicians mean well in prescribing evidence-based treatments; however, if the symptoms or adverse effects are not bothersome or cause functional impairment, we risk losing sight of the patient’s goals in treatment and imposing our own instead. Displacing the treatment focus can alienate the patient, harm the therapeutic alliance, and result in “pill fatigue.” For example, we may be tempted to treat antipsychotic-induced tardive dyskinesia, even if the patient is not concerned about abnormal movements. Although we see this adverse effect
Change does not happen overnight
Picking a treatment option out of a lineup of choices, à la UWorld questions, does not always translate into patients agreeing with the suggested treatment, let alone the idea of receiving treatment at all. Motivational interviewing is our chance to shine in such situations and the reason why we are physicians, rather than answer-picking bots. Patients cannot change if they are not ready. However, we should be ready to roll with resistance while looking for signs of readiness to change. We must accept that it may take a week, a month, a year, or even longer for patients to align with our plan of action. The only futile decision is deeming our efforts as futile while discounting the benefits of incremental care.
As physicians, we are cognizant of the importance of patient-centered care, active listening, empathy, and patience—the so-called “hidden curriculum of medicine.”1 However, our attempts to centralize these concepts may be overshadowed by the deeply rooted drive to treat and fix. At times, we are simply treating uncertainty, whether it be diagnostic uncertainty or the uncertainty arising from clinical responses and outcomes that are far from binary. Definitive actions, such as adding medications or altering dosages, may appear to both patients and physicians to be a step closer to a “cure.” However, watchful waiting, re-evaluation, and accepting uncertainty are the true skills of effective care.
Be savvy about psychopharmacology
Psychotropics can take weeks to months to reach their full potential, and have varying responses and adverse effects. Beware of changing regimens prematurely, and keep in mind basic, yet crucial, pharmacokinetic concepts (eg, 4 to 5 half-lives to reach steady state, variations in metabolism). Receptor binding and dosing heuristics are notably common in psychiatry. Although such concepts are important to grasp, there is no one-size-fits-all rule. The brain simply does not possess the heart’s machine-like, linear functioning. Therefore, targeting individual parts (ie, receptors) will not equate to fixing the whole organ systematically or predictably.
Is the patient truly treatment-resistant?
Even the best treatment regimen has no clinical benefit if the patient cannot afford the prescription or does not take the medication. If cost is an impediment, switch from brand name drugs to generic formulations or to older medications in the same class. Before declaring the patient “treatment-resistant” and making medication changes, assess for compliance. This may require assistance from collateral informants. Ask family members to count the number of pills remaining in the bottle, and call the pharmacy to find out the last refill dates. If the patient exhibits a partial response to what should be a therapeutic dose, consider obtaining drug plasma levels to rule out rapid metabolism before deeming the medication trial a failure.2
Medications as liabilities
Overreliance on medications can result in the medications becoming liabilities. The polypharmacy problem is not unique to psychiatry.3 However, psychiatric patients may be more likely to inadvertently use medications in a maladaptive manner and disrupt the fundamental goals of long-term care. Avoid making medication adjustments in response to a patient’s life stressors and normative situational reactions. Doing so is a disservice to patients, because we are robbing them of chances to develop necessary coping skills and defenses. This can be overtly damaging in certain patient populations, such as those with borderline personality disorder, who may use medication adjustments as a crutch during crises.4
Treat the patient, not yourself
We physicians mean well in prescribing evidence-based treatments; however, if the symptoms or adverse effects are not bothersome or cause functional impairment, we risk losing sight of the patient’s goals in treatment and imposing our own instead. Displacing the treatment focus can alienate the patient, harm the therapeutic alliance, and result in “pill fatigue.” For example, we may be tempted to treat antipsychotic-induced tardive dyskinesia, even if the patient is not concerned about abnormal movements. Although we see this adverse effect
Change does not happen overnight
Picking a treatment option out of a lineup of choices, à la UWorld questions, does not always translate into patients agreeing with the suggested treatment, let alone the idea of receiving treatment at all. Motivational interviewing is our chance to shine in such situations and the reason why we are physicians, rather than answer-picking bots. Patients cannot change if they are not ready. However, we should be ready to roll with resistance while looking for signs of readiness to change. We must accept that it may take a week, a month, a year, or even longer for patients to align with our plan of action. The only futile decision is deeming our efforts as futile while discounting the benefits of incremental care.
1. Hafferty FW, Gaufberg EH, O’Donnell JF. The role of the hidden curriculum in “on doctoring” courses. AMA J Ethics. 2015;17(2):130-139.
2. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
3. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
4. Gunderson JG. The emergence of a generalist model to meet public health needs for patients with borderline personality disorder. Am J Psychiatry. 2016;173(5):452-458.
5. Kikkert MJ, Schene AH, Koeter MW, et al. Medication adherence in schizophrenia: exploring patients’, carers’ and professionals’ views. Schizophr Bull. 2005;32(4):786-794.
1. Hafferty FW, Gaufberg EH, O’Donnell JF. The role of the hidden curriculum in “on doctoring” courses. AMA J Ethics. 2015;17(2):130-139.
2. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
3. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
4. Gunderson JG. The emergence of a generalist model to meet public health needs for patients with borderline personality disorder. Am J Psychiatry. 2016;173(5):452-458.
5. Kikkert MJ, Schene AH, Koeter MW, et al. Medication adherence in schizophrenia: exploring patients’, carers’ and professionals’ views. Schizophr Bull. 2005;32(4):786-794.
Providing psychotherapy? Keep these principles in mind
Although the biological aspects of psychiatry are crucial, psychotherapy is an integral part of psychiatry. Unfortunately, the emphasis on psychotherapy training in psychiatry residency programs has declined compared with a decade or more ago. In an era of dwindling psychotherapy training and resources, the quality and type of psychotherapy training has become more variable. In addition to helping maintain the therapeutic alliance, nuanced psychotherapy by a trained professional can be transformational by helping patients to:
- process complex life events and emotions
- feel understood
- overcome psychological barriers to recovery
- enhance self-esteem.
When providing psychotherapy for adult patients, consider these basic, but salient points that are often overlooked.
Refrain from making life decisions for patients, except in exceptional circumstances, such as in situations of abuse and other crises.1 Telling an adult patient what to do about life decisions that he finds challenging fits more under life coaching than psychotherapy. Through therapy, patients should be helped in processing the pros and cons of certain decisions and in navigating the decision-making process to arrive at a decision that makes the most sense to them. Also, it’s not uncommon for therapeutic relationships to rupture when therapists give advice such as suggesting that a patient divorce his spouse, date a certain individual, or have children.
There are many reasons why giving advice in psychotherapy is not recommended. Giving advice can be an impediment to the therapeutic process.2 What is good advice for one patient may not be good for another. Therapists who give advice often do so from their own lens and perspective. This perspective may not only be different from the patient’s priorities and life circumstances, but the therapist also may have inadequate information about the patient’s situation,1,2 which could lead to providing advice that could even harm the patient. In addition, providing advice might prevent a patient from gaining adequate agency or self-directedness while promoting an unhealthy dependence on the therapist and reinforcing the patient’s self-doubt or lack of confidence. In these cases, the patient may later resent the therapist for the advice.
Address the ‘here and now.’1 Pay attention to immediate issues or themes that emerge, and address them with the patient gently and thoughtfully, as appropriate. Ignoring these may create risks of missing vital, underlying material that could reveal more of the patient’s inner world, as these themes can sometimes reflect other themes of the patient’s life outside of treatment.
Acknowledging and empathizing, when appropriate, are key initial steps that help decrease resistance and facilitate the therapeutic process.
Explore the affect. Paying attention to the patient’s emotional state is critical.3 This holds true for all types of psychotherapy. For example, if a patient suddenly becomes tearful when telling his story or describing recent events, this is usually a sign that the subject matter affects or holds value to the patient in a significant or meaningful way and should be further explored.
‘Meet the patient where they are.’ This doesn’t mean you should yield to the patient or give in to his demands. It implies that you should assess the patient’s readiness for a particular intervention and devise interventions from that standpoint, exploring the patient’s ambivalence, noticing resistance, and continuing to acknowledge and empathize with where the patient is in life or treatment. When utilized judiciously, this technique can help the therapist align with the patient, and help the patient move forward through resistance and ambivalence.
Be nonjudgmental and empathetic. Patients place trust in their therapists when they disclose thoughts or emotions that are sensitive, meaningful, or close to the heart. A nonjudgmental response helps the patient accept his experiences and emotions. Being empathetic requires putting oneself in another’s shoes; it does not mean agreeing with the patient. Of course, if you learn that your patient abused a child or an older adult, you are required to report it to the appropriate state agency. In addition, follow the duty to warn and protect in case of any other safety issues, as appropriate.
Do not assume. Open-ended questions and exploration are key. For example, a patient told her resident therapist that her father recently passed away. The therapist expressed to the patient how hard this must be for her. However, the patient said she was relieved by her father’s death, because he had been abusive to her for years. Because of the therapist’s comment, the patient doubted her own reaction and felt guilty for not being more upset about her father’s death.
Avoid over-identifying with your patient. If you find yourself over-identifying with a patient because you have a common background or life events, seek supervision. Over-identification not only can pose barriers to objectively identifying patterns and trends in the patient’s behavior or presentation but also can increase the risk of crossing boundaries or even minimizing the patient’s experience. Exercise caution if you find yourself wanting to be liked by your patient; this is a common mistake among beginning therapists.4
Seek supervision. If you are feeling angry, frustrated, indifferent, or overly attached toward a patient, recognize this countertransference and seek consultation or supervision from an experienced colleague or supervisor. These emotions can be valuable tools that shed light not only on the patient’s life and the session itself, but also help you identify any other factors, such as your own feelings or experiences, that might be contributing to these reactions.
1. Yalom ID. The gift of therapy: an open letter to a new generation of therapists and their patients. New York, NY: HarperCollins Publishers; 2002:46-73,142-145.
2. Bender S, Messner E. Management of impasses. In: Bender S, Messner E. Becoming a therapist: what do I say, and why? New York, NY: The Guilford Press; 2003:235-258.
3.
4. Buckley P, Karasu TB, Charles E. Common mistakes in psychotherapy. Am J Psychiatry. 1979;136(12):1578-1580.
Although the biological aspects of psychiatry are crucial, psychotherapy is an integral part of psychiatry. Unfortunately, the emphasis on psychotherapy training in psychiatry residency programs has declined compared with a decade or more ago. In an era of dwindling psychotherapy training and resources, the quality and type of psychotherapy training has become more variable. In addition to helping maintain the therapeutic alliance, nuanced psychotherapy by a trained professional can be transformational by helping patients to:
- process complex life events and emotions
- feel understood
- overcome psychological barriers to recovery
- enhance self-esteem.
When providing psychotherapy for adult patients, consider these basic, but salient points that are often overlooked.
Refrain from making life decisions for patients, except in exceptional circumstances, such as in situations of abuse and other crises.1 Telling an adult patient what to do about life decisions that he finds challenging fits more under life coaching than psychotherapy. Through therapy, patients should be helped in processing the pros and cons of certain decisions and in navigating the decision-making process to arrive at a decision that makes the most sense to them. Also, it’s not uncommon for therapeutic relationships to rupture when therapists give advice such as suggesting that a patient divorce his spouse, date a certain individual, or have children.
There are many reasons why giving advice in psychotherapy is not recommended. Giving advice can be an impediment to the therapeutic process.2 What is good advice for one patient may not be good for another. Therapists who give advice often do so from their own lens and perspective. This perspective may not only be different from the patient’s priorities and life circumstances, but the therapist also may have inadequate information about the patient’s situation,1,2 which could lead to providing advice that could even harm the patient. In addition, providing advice might prevent a patient from gaining adequate agency or self-directedness while promoting an unhealthy dependence on the therapist and reinforcing the patient’s self-doubt or lack of confidence. In these cases, the patient may later resent the therapist for the advice.
Address the ‘here and now.’1 Pay attention to immediate issues or themes that emerge, and address them with the patient gently and thoughtfully, as appropriate. Ignoring these may create risks of missing vital, underlying material that could reveal more of the patient’s inner world, as these themes can sometimes reflect other themes of the patient’s life outside of treatment.
Acknowledging and empathizing, when appropriate, are key initial steps that help decrease resistance and facilitate the therapeutic process.
Explore the affect. Paying attention to the patient’s emotional state is critical.3 This holds true for all types of psychotherapy. For example, if a patient suddenly becomes tearful when telling his story or describing recent events, this is usually a sign that the subject matter affects or holds value to the patient in a significant or meaningful way and should be further explored.
‘Meet the patient where they are.’ This doesn’t mean you should yield to the patient or give in to his demands. It implies that you should assess the patient’s readiness for a particular intervention and devise interventions from that standpoint, exploring the patient’s ambivalence, noticing resistance, and continuing to acknowledge and empathize with where the patient is in life or treatment. When utilized judiciously, this technique can help the therapist align with the patient, and help the patient move forward through resistance and ambivalence.
Be nonjudgmental and empathetic. Patients place trust in their therapists when they disclose thoughts or emotions that are sensitive, meaningful, or close to the heart. A nonjudgmental response helps the patient accept his experiences and emotions. Being empathetic requires putting oneself in another’s shoes; it does not mean agreeing with the patient. Of course, if you learn that your patient abused a child or an older adult, you are required to report it to the appropriate state agency. In addition, follow the duty to warn and protect in case of any other safety issues, as appropriate.
Do not assume. Open-ended questions and exploration are key. For example, a patient told her resident therapist that her father recently passed away. The therapist expressed to the patient how hard this must be for her. However, the patient said she was relieved by her father’s death, because he had been abusive to her for years. Because of the therapist’s comment, the patient doubted her own reaction and felt guilty for not being more upset about her father’s death.
Avoid over-identifying with your patient. If you find yourself over-identifying with a patient because you have a common background or life events, seek supervision. Over-identification not only can pose barriers to objectively identifying patterns and trends in the patient’s behavior or presentation but also can increase the risk of crossing boundaries or even minimizing the patient’s experience. Exercise caution if you find yourself wanting to be liked by your patient; this is a common mistake among beginning therapists.4
Seek supervision. If you are feeling angry, frustrated, indifferent, or overly attached toward a patient, recognize this countertransference and seek consultation or supervision from an experienced colleague or supervisor. These emotions can be valuable tools that shed light not only on the patient’s life and the session itself, but also help you identify any other factors, such as your own feelings or experiences, that might be contributing to these reactions.
Although the biological aspects of psychiatry are crucial, psychotherapy is an integral part of psychiatry. Unfortunately, the emphasis on psychotherapy training in psychiatry residency programs has declined compared with a decade or more ago. In an era of dwindling psychotherapy training and resources, the quality and type of psychotherapy training has become more variable. In addition to helping maintain the therapeutic alliance, nuanced psychotherapy by a trained professional can be transformational by helping patients to:
- process complex life events and emotions
- feel understood
- overcome psychological barriers to recovery
- enhance self-esteem.
When providing psychotherapy for adult patients, consider these basic, but salient points that are often overlooked.
Refrain from making life decisions for patients, except in exceptional circumstances, such as in situations of abuse and other crises.1 Telling an adult patient what to do about life decisions that he finds challenging fits more under life coaching than psychotherapy. Through therapy, patients should be helped in processing the pros and cons of certain decisions and in navigating the decision-making process to arrive at a decision that makes the most sense to them. Also, it’s not uncommon for therapeutic relationships to rupture when therapists give advice such as suggesting that a patient divorce his spouse, date a certain individual, or have children.
There are many reasons why giving advice in psychotherapy is not recommended. Giving advice can be an impediment to the therapeutic process.2 What is good advice for one patient may not be good for another. Therapists who give advice often do so from their own lens and perspective. This perspective may not only be different from the patient’s priorities and life circumstances, but the therapist also may have inadequate information about the patient’s situation,1,2 which could lead to providing advice that could even harm the patient. In addition, providing advice might prevent a patient from gaining adequate agency or self-directedness while promoting an unhealthy dependence on the therapist and reinforcing the patient’s self-doubt or lack of confidence. In these cases, the patient may later resent the therapist for the advice.
Address the ‘here and now.’1 Pay attention to immediate issues or themes that emerge, and address them with the patient gently and thoughtfully, as appropriate. Ignoring these may create risks of missing vital, underlying material that could reveal more of the patient’s inner world, as these themes can sometimes reflect other themes of the patient’s life outside of treatment.
Acknowledging and empathizing, when appropriate, are key initial steps that help decrease resistance and facilitate the therapeutic process.
Explore the affect. Paying attention to the patient’s emotional state is critical.3 This holds true for all types of psychotherapy. For example, if a patient suddenly becomes tearful when telling his story or describing recent events, this is usually a sign that the subject matter affects or holds value to the patient in a significant or meaningful way and should be further explored.
‘Meet the patient where they are.’ This doesn’t mean you should yield to the patient or give in to his demands. It implies that you should assess the patient’s readiness for a particular intervention and devise interventions from that standpoint, exploring the patient’s ambivalence, noticing resistance, and continuing to acknowledge and empathize with where the patient is in life or treatment. When utilized judiciously, this technique can help the therapist align with the patient, and help the patient move forward through resistance and ambivalence.
Be nonjudgmental and empathetic. Patients place trust in their therapists when they disclose thoughts or emotions that are sensitive, meaningful, or close to the heart. A nonjudgmental response helps the patient accept his experiences and emotions. Being empathetic requires putting oneself in another’s shoes; it does not mean agreeing with the patient. Of course, if you learn that your patient abused a child or an older adult, you are required to report it to the appropriate state agency. In addition, follow the duty to warn and protect in case of any other safety issues, as appropriate.
Do not assume. Open-ended questions and exploration are key. For example, a patient told her resident therapist that her father recently passed away. The therapist expressed to the patient how hard this must be for her. However, the patient said she was relieved by her father’s death, because he had been abusive to her for years. Because of the therapist’s comment, the patient doubted her own reaction and felt guilty for not being more upset about her father’s death.
Avoid over-identifying with your patient. If you find yourself over-identifying with a patient because you have a common background or life events, seek supervision. Over-identification not only can pose barriers to objectively identifying patterns and trends in the patient’s behavior or presentation but also can increase the risk of crossing boundaries or even minimizing the patient’s experience. Exercise caution if you find yourself wanting to be liked by your patient; this is a common mistake among beginning therapists.4
Seek supervision. If you are feeling angry, frustrated, indifferent, or overly attached toward a patient, recognize this countertransference and seek consultation or supervision from an experienced colleague or supervisor. These emotions can be valuable tools that shed light not only on the patient’s life and the session itself, but also help you identify any other factors, such as your own feelings or experiences, that might be contributing to these reactions.
1. Yalom ID. The gift of therapy: an open letter to a new generation of therapists and their patients. New York, NY: HarperCollins Publishers; 2002:46-73,142-145.
2. Bender S, Messner E. Management of impasses. In: Bender S, Messner E. Becoming a therapist: what do I say, and why? New York, NY: The Guilford Press; 2003:235-258.
3.
4. Buckley P, Karasu TB, Charles E. Common mistakes in psychotherapy. Am J Psychiatry. 1979;136(12):1578-1580.
1. Yalom ID. The gift of therapy: an open letter to a new generation of therapists and their patients. New York, NY: HarperCollins Publishers; 2002:46-73,142-145.
2. Bender S, Messner E. Management of impasses. In: Bender S, Messner E. Becoming a therapist: what do I say, and why? New York, NY: The Guilford Press; 2003:235-258.
3.
4. Buckley P, Karasu TB, Charles E. Common mistakes in psychotherapy. Am J Psychiatry. 1979;136(12):1578-1580.
Use the ABCs when managing problem behaviors in autism
Despite a lack of evidence, polypharmacy often is used to treat autism spectrum disorder (ASD),1 while educational techniques are underutilized. Compared with the general population, children with ASD may be more prone to the adverse effects of the medications used to treat symptoms, such as antipsychotics and antidepressants.2 Therefore, when addressing problem behaviors, such as tantrums, aggressiveness, or self-injury, in a patient with ASD, before prescribing a medication, consider the ABCs of these behaviors.3
Antecedents. What happened before the behavior occurred? Where and when did the behavior occur? Was the individual unable to get a desired tangible item, such as a preferred food, toy, or another object? Was the individual told complete a task that he (she) did not want to do? Did the individual see someone else getting attention?
Behaviors. What behavior(s) occurred after each antecedent?
Consequences. What happened after the behavior occurred? Did the caregiver give the individual the item he wanted? Was the individual able to get out of doing work that he did not want to do or become the center of attention?
Having parents document the ABCs is useful not only for finding out why a behavior occurred, but also for objectively determining if and how a medication is affecting the frequency of a behavior. Charts that parents can use to document ABC data are available online (eg, http://www.positivelyautism.com/downloads/datasheet_abc.pdf). Once this data is collected, it can be used to implement appropriate interventions, which I describe as DEFG.
Differential reinforcement of other behaviors is a procedure that provides positive reinforcement for not engaging in a problem behavior or for staying on task. For example, use a token board to reward positive behaviors, with physical tokens or written marks. However, some patients require immediate reinforcement. I suggest that parents or caregivers carry small pieces of preferred food to give to the patient to reinforce positive behavior.
Exercise. A review of 18 studies reported that physical exercise, such as jogging, weight training, and bike riding, can help reduce problem behaviors in individuals with ASD.4 Among 64 participants with ASD, there was a decrease in aggression, stereotypy, off-task behavior, and elopement, and improvements in on-task and motor behavior such as playing catch.
Function. Refer to the ABCs to determine why a specific problem behavior is occurring. Each behavior can have 1 or multiple functions; therefore, develop a plan specific to the reason the patient engages in the behavior. For example, if the individual engages in a behavior to avoid a task, the parent or caregiver can give individual tokens that the individual can later exchange for a break, instead of engaging in the problem behavior to avoid the task. If a behavior appears to be done for attention, instruct the caregivers to provide frequent periods of attention when the individual engages in positive behaviors.
Go to the appropriate placement. By law, persons age ≤21 have the right to an education and to make meaningful progress. If a patient with ASD exhibits behaviors that interfere with learning, he is entitled to a placement that can provide intensive applied behavior analysis. If you feel that the child needs a different school, write an evaluation for the parent or guardian to submit to the school district and clearly outline the patient’s needs and requirements.
1. Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833-840.
2. Azeem MW, Imran N, Khawaja IS. Autism spectrum disorder: an update. Psychiatr Ann. 2016;46(1):58-62.
3. Pratt C, Dubie M. Observing behavior using A-B-C data. Indiana Resource Center for Autism. https://www.iidc.indiana.edu/pages/observing-behavior-using-a-b-c-data. Accessed October 4, 2017.
4. Lang R, Kern Koegel LK, Ashbaugh K, et al. Physical exercise and individuals with autism spectrum disorders: a systematic review. Res Autism Spectr Dis. 2010;4(4):565-576.
Despite a lack of evidence, polypharmacy often is used to treat autism spectrum disorder (ASD),1 while educational techniques are underutilized. Compared with the general population, children with ASD may be more prone to the adverse effects of the medications used to treat symptoms, such as antipsychotics and antidepressants.2 Therefore, when addressing problem behaviors, such as tantrums, aggressiveness, or self-injury, in a patient with ASD, before prescribing a medication, consider the ABCs of these behaviors.3
Antecedents. What happened before the behavior occurred? Where and when did the behavior occur? Was the individual unable to get a desired tangible item, such as a preferred food, toy, or another object? Was the individual told complete a task that he (she) did not want to do? Did the individual see someone else getting attention?
Behaviors. What behavior(s) occurred after each antecedent?
Consequences. What happened after the behavior occurred? Did the caregiver give the individual the item he wanted? Was the individual able to get out of doing work that he did not want to do or become the center of attention?
Having parents document the ABCs is useful not only for finding out why a behavior occurred, but also for objectively determining if and how a medication is affecting the frequency of a behavior. Charts that parents can use to document ABC data are available online (eg, http://www.positivelyautism.com/downloads/datasheet_abc.pdf). Once this data is collected, it can be used to implement appropriate interventions, which I describe as DEFG.
Differential reinforcement of other behaviors is a procedure that provides positive reinforcement for not engaging in a problem behavior or for staying on task. For example, use a token board to reward positive behaviors, with physical tokens or written marks. However, some patients require immediate reinforcement. I suggest that parents or caregivers carry small pieces of preferred food to give to the patient to reinforce positive behavior.
Exercise. A review of 18 studies reported that physical exercise, such as jogging, weight training, and bike riding, can help reduce problem behaviors in individuals with ASD.4 Among 64 participants with ASD, there was a decrease in aggression, stereotypy, off-task behavior, and elopement, and improvements in on-task and motor behavior such as playing catch.
Function. Refer to the ABCs to determine why a specific problem behavior is occurring. Each behavior can have 1 or multiple functions; therefore, develop a plan specific to the reason the patient engages in the behavior. For example, if the individual engages in a behavior to avoid a task, the parent or caregiver can give individual tokens that the individual can later exchange for a break, instead of engaging in the problem behavior to avoid the task. If a behavior appears to be done for attention, instruct the caregivers to provide frequent periods of attention when the individual engages in positive behaviors.
Go to the appropriate placement. By law, persons age ≤21 have the right to an education and to make meaningful progress. If a patient with ASD exhibits behaviors that interfere with learning, he is entitled to a placement that can provide intensive applied behavior analysis. If you feel that the child needs a different school, write an evaluation for the parent or guardian to submit to the school district and clearly outline the patient’s needs and requirements.
Despite a lack of evidence, polypharmacy often is used to treat autism spectrum disorder (ASD),1 while educational techniques are underutilized. Compared with the general population, children with ASD may be more prone to the adverse effects of the medications used to treat symptoms, such as antipsychotics and antidepressants.2 Therefore, when addressing problem behaviors, such as tantrums, aggressiveness, or self-injury, in a patient with ASD, before prescribing a medication, consider the ABCs of these behaviors.3
Antecedents. What happened before the behavior occurred? Where and when did the behavior occur? Was the individual unable to get a desired tangible item, such as a preferred food, toy, or another object? Was the individual told complete a task that he (she) did not want to do? Did the individual see someone else getting attention?
Behaviors. What behavior(s) occurred after each antecedent?
Consequences. What happened after the behavior occurred? Did the caregiver give the individual the item he wanted? Was the individual able to get out of doing work that he did not want to do or become the center of attention?
Having parents document the ABCs is useful not only for finding out why a behavior occurred, but also for objectively determining if and how a medication is affecting the frequency of a behavior. Charts that parents can use to document ABC data are available online (eg, http://www.positivelyautism.com/downloads/datasheet_abc.pdf). Once this data is collected, it can be used to implement appropriate interventions, which I describe as DEFG.
Differential reinforcement of other behaviors is a procedure that provides positive reinforcement for not engaging in a problem behavior or for staying on task. For example, use a token board to reward positive behaviors, with physical tokens or written marks. However, some patients require immediate reinforcement. I suggest that parents or caregivers carry small pieces of preferred food to give to the patient to reinforce positive behavior.
Exercise. A review of 18 studies reported that physical exercise, such as jogging, weight training, and bike riding, can help reduce problem behaviors in individuals with ASD.4 Among 64 participants with ASD, there was a decrease in aggression, stereotypy, off-task behavior, and elopement, and improvements in on-task and motor behavior such as playing catch.
Function. Refer to the ABCs to determine why a specific problem behavior is occurring. Each behavior can have 1 or multiple functions; therefore, develop a plan specific to the reason the patient engages in the behavior. For example, if the individual engages in a behavior to avoid a task, the parent or caregiver can give individual tokens that the individual can later exchange for a break, instead of engaging in the problem behavior to avoid the task. If a behavior appears to be done for attention, instruct the caregivers to provide frequent periods of attention when the individual engages in positive behaviors.
Go to the appropriate placement. By law, persons age ≤21 have the right to an education and to make meaningful progress. If a patient with ASD exhibits behaviors that interfere with learning, he is entitled to a placement that can provide intensive applied behavior analysis. If you feel that the child needs a different school, write an evaluation for the parent or guardian to submit to the school district and clearly outline the patient’s needs and requirements.
1. Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833-840.
2. Azeem MW, Imran N, Khawaja IS. Autism spectrum disorder: an update. Psychiatr Ann. 2016;46(1):58-62.
3. Pratt C, Dubie M. Observing behavior using A-B-C data. Indiana Resource Center for Autism. https://www.iidc.indiana.edu/pages/observing-behavior-using-a-b-c-data. Accessed October 4, 2017.
4. Lang R, Kern Koegel LK, Ashbaugh K, et al. Physical exercise and individuals with autism spectrum disorders: a systematic review. Res Autism Spectr Dis. 2010;4(4):565-576.
1. Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833-840.
2. Azeem MW, Imran N, Khawaja IS. Autism spectrum disorder: an update. Psychiatr Ann. 2016;46(1):58-62.
3. Pratt C, Dubie M. Observing behavior using A-B-C data. Indiana Resource Center for Autism. https://www.iidc.indiana.edu/pages/observing-behavior-using-a-b-c-data. Accessed October 4, 2017.
4. Lang R, Kern Koegel LK, Ashbaugh K, et al. Physical exercise and individuals with autism spectrum disorders: a systematic review. Res Autism Spectr Dis. 2010;4(4):565-576.
Employment contracts: What to check before you sign
Most psychiatrists are required to sign an employment contract before taking a job, but few of us have received any training on reviewing such contracts. We often rely on coworkers and attorneys to navigate this process for us. However, the contract is crucial, because it outlines your employer’s clinical and administrative expectations for the position, and it gives you the opportunity to lay out what you want.1 Because an employment contract is legally binding, you should thoroughly read it and look for clauses that may not work in your best interest. Although not a complete list, the following items should be reviewed before signing a contract.1,2
Benefits. Make sure you are offered a reasonable salary, but balance the dollar amount with benefits such as:
- continuing medical education allowances
- educational loan forgiveness
- health/malpractice/disability insurance
- retirement benefits
- compensation for call schedule.
In some cases, there may be a delay before you are eligible to obtain certain benefits.
Work expectations. Many contracts state that the position is “full-time” or have other nonspecific parameters for work expectations. You should inquire about objective work parameters, such as duty hours, the average frequency of the current call schedule, timeframe for completing medical documentation, and penalties for not meeting clinical or administrative requirements, so you are not surprised by:
- working longer-than-planned shifts
- performing on-call duties
- working on days that you were not expecting
- having your credentialing status placed in jeopardy.
Some group practices allow for a half-day of no scheduled appointments with patients, so you can complete paperwork and return phone calls.
Noncompete clause. This restricts you from working within a certain geographic area or for a competing employer for a finite time period after the contract terminates or expires. A noncompete clause could restrict you from practicing within a large geographical area, especially if the job is located in a densely populated area. Some noncompete clauses do not include a temporal or geographic restriction, but can limit your ability to bring patients with you to a new practice or facility when the contract expires.
Malpractice insurance. Two types of malpractice insurance are occurrence and claims-made:
- Occurrence insurance protects you whenever an action is brought against you, even if the action is brought after the contract terminates or expires.
- Claims-made insurance provides coverage if the policy with the same insurer was in effect when the malpractice was committed and when the actual action was commenced.
Although claims-made insurance is less expensive, it can leave you without coverage should you leave your employer and no longer maintain the same insurance policy. Claims-made can be converted into occurrence through the purchase of a tail endorsement. If the employer does not offer you tail coverage, then it is your responsibility to pay for this insurance, which can be expensive.
Termination language. Every contract features a termination section that lists potential causes for terminating your employment. This list is usually not exhaustive, but it sets the framework for a realistic view of reasonable causes. Contracts also commonly contain provisions that permit termination “without cause” after notice of termination is provided. Although you could negotiate for more notice time, “without cause” clauses are unlikely to be removed from the contract.
1. Claussen K. Eight physician employment contract items you need to know about. The Doctor Weighs In. https://thedoctorweighsin.com/8-physician-employment-contract-items-you-need-to-know-about. Published March 8, 2017. Accessed October 11, 2017.
2. Blustein AE, Keller LB. Physician employment contracts: what you need to know before you sign. J Am Acad Dermatol. https://www.aad.org/members/publications/directions-in-residency/archiveyment-contracts-what-you-need-to-know-before-you-sign. Accessed October 11, 2017.
Most psychiatrists are required to sign an employment contract before taking a job, but few of us have received any training on reviewing such contracts. We often rely on coworkers and attorneys to navigate this process for us. However, the contract is crucial, because it outlines your employer’s clinical and administrative expectations for the position, and it gives you the opportunity to lay out what you want.1 Because an employment contract is legally binding, you should thoroughly read it and look for clauses that may not work in your best interest. Although not a complete list, the following items should be reviewed before signing a contract.1,2
Benefits. Make sure you are offered a reasonable salary, but balance the dollar amount with benefits such as:
- continuing medical education allowances
- educational loan forgiveness
- health/malpractice/disability insurance
- retirement benefits
- compensation for call schedule.
In some cases, there may be a delay before you are eligible to obtain certain benefits.
Work expectations. Many contracts state that the position is “full-time” or have other nonspecific parameters for work expectations. You should inquire about objective work parameters, such as duty hours, the average frequency of the current call schedule, timeframe for completing medical documentation, and penalties for not meeting clinical or administrative requirements, so you are not surprised by:
- working longer-than-planned shifts
- performing on-call duties
- working on days that you were not expecting
- having your credentialing status placed in jeopardy.
Some group practices allow for a half-day of no scheduled appointments with patients, so you can complete paperwork and return phone calls.
Noncompete clause. This restricts you from working within a certain geographic area or for a competing employer for a finite time period after the contract terminates or expires. A noncompete clause could restrict you from practicing within a large geographical area, especially if the job is located in a densely populated area. Some noncompete clauses do not include a temporal or geographic restriction, but can limit your ability to bring patients with you to a new practice or facility when the contract expires.
Malpractice insurance. Two types of malpractice insurance are occurrence and claims-made:
- Occurrence insurance protects you whenever an action is brought against you, even if the action is brought after the contract terminates or expires.
- Claims-made insurance provides coverage if the policy with the same insurer was in effect when the malpractice was committed and when the actual action was commenced.
Although claims-made insurance is less expensive, it can leave you without coverage should you leave your employer and no longer maintain the same insurance policy. Claims-made can be converted into occurrence through the purchase of a tail endorsement. If the employer does not offer you tail coverage, then it is your responsibility to pay for this insurance, which can be expensive.
Termination language. Every contract features a termination section that lists potential causes for terminating your employment. This list is usually not exhaustive, but it sets the framework for a realistic view of reasonable causes. Contracts also commonly contain provisions that permit termination “without cause” after notice of termination is provided. Although you could negotiate for more notice time, “without cause” clauses are unlikely to be removed from the contract.
Most psychiatrists are required to sign an employment contract before taking a job, but few of us have received any training on reviewing such contracts. We often rely on coworkers and attorneys to navigate this process for us. However, the contract is crucial, because it outlines your employer’s clinical and administrative expectations for the position, and it gives you the opportunity to lay out what you want.1 Because an employment contract is legally binding, you should thoroughly read it and look for clauses that may not work in your best interest. Although not a complete list, the following items should be reviewed before signing a contract.1,2
Benefits. Make sure you are offered a reasonable salary, but balance the dollar amount with benefits such as:
- continuing medical education allowances
- educational loan forgiveness
- health/malpractice/disability insurance
- retirement benefits
- compensation for call schedule.
In some cases, there may be a delay before you are eligible to obtain certain benefits.
Work expectations. Many contracts state that the position is “full-time” or have other nonspecific parameters for work expectations. You should inquire about objective work parameters, such as duty hours, the average frequency of the current call schedule, timeframe for completing medical documentation, and penalties for not meeting clinical or administrative requirements, so you are not surprised by:
- working longer-than-planned shifts
- performing on-call duties
- working on days that you were not expecting
- having your credentialing status placed in jeopardy.
Some group practices allow for a half-day of no scheduled appointments with patients, so you can complete paperwork and return phone calls.
Noncompete clause. This restricts you from working within a certain geographic area or for a competing employer for a finite time period after the contract terminates or expires. A noncompete clause could restrict you from practicing within a large geographical area, especially if the job is located in a densely populated area. Some noncompete clauses do not include a temporal or geographic restriction, but can limit your ability to bring patients with you to a new practice or facility when the contract expires.
Malpractice insurance. Two types of malpractice insurance are occurrence and claims-made:
- Occurrence insurance protects you whenever an action is brought against you, even if the action is brought after the contract terminates or expires.
- Claims-made insurance provides coverage if the policy with the same insurer was in effect when the malpractice was committed and when the actual action was commenced.
Although claims-made insurance is less expensive, it can leave you without coverage should you leave your employer and no longer maintain the same insurance policy. Claims-made can be converted into occurrence through the purchase of a tail endorsement. If the employer does not offer you tail coverage, then it is your responsibility to pay for this insurance, which can be expensive.
Termination language. Every contract features a termination section that lists potential causes for terminating your employment. This list is usually not exhaustive, but it sets the framework for a realistic view of reasonable causes. Contracts also commonly contain provisions that permit termination “without cause” after notice of termination is provided. Although you could negotiate for more notice time, “without cause” clauses are unlikely to be removed from the contract.
1. Claussen K. Eight physician employment contract items you need to know about. The Doctor Weighs In. https://thedoctorweighsin.com/8-physician-employment-contract-items-you-need-to-know-about. Published March 8, 2017. Accessed October 11, 2017.
2. Blustein AE, Keller LB. Physician employment contracts: what you need to know before you sign. J Am Acad Dermatol. https://www.aad.org/members/publications/directions-in-residency/archiveyment-contracts-what-you-need-to-know-before-you-sign. Accessed October 11, 2017.
1. Claussen K. Eight physician employment contract items you need to know about. The Doctor Weighs In. https://thedoctorweighsin.com/8-physician-employment-contract-items-you-need-to-know-about. Published March 8, 2017. Accessed October 11, 2017.
2. Blustein AE, Keller LB. Physician employment contracts: what you need to know before you sign. J Am Acad Dermatol. https://www.aad.org/members/publications/directions-in-residency/archiveyment-contracts-what-you-need-to-know-before-you-sign. Accessed October 11, 2017.
3 Approaches to PMS
Throughout my 40 years in private psychiatric practice, I have found some treatments for premenstrual syndrome (PMS) that were not mentioned in “Etiology of premenstrual dysphoric disorder: 5 interwoven pieces” (Evidence-Based Reviews, Current Psychiatry. September 2017, p. 20-28).
This started in 1972 when I was serving in the Army in Oklahoma. A 28-year-old woman with severe PMS had been treated by internal medicine, an OB/GYN, and endocrinology, all to no avail. Three days before her menses began, she would start driving north. When menses commenced, she would find herself in Nebraska and have to call her husband so he could wire her money to come back.
Through my evaluation, I found that she would gain 10 lb before her menses. I prescribed a diuretic and instructed her to start taking it when she began swelling and to stop taking it after her menses began. This alleviated all of her symptoms. If a woman gains more than 3 to 5 lb, her brain also will swell, along with everything else. Because the brain is encapsulated in the skull, the swelling puts pressure on the brain, which might have been the cause of these brief psychotic episodes.
If a woman who develops PMS does not experience significant weight gain, the first thing I try is vitamin B6, 100 mg/d, prior to menses. Vitamin B6 is a cofactor in the production of numerous neurotransmitters. I found that prescribing vitamin B6 would alleviate about 20% of PMS symptoms. If the patient has a personal or family history of affective disorder, I often try antidepressants prior to menses, which alleviate approximately another 20% of her symptoms. If none of the previous 3 factors are present, I often add a low dose of progesterone, which appears to help. If all else fails, I will try a low dose of lithium, 300 mg/d, before menses. This also seems to have some positive effect.
I have not written an article about these approaches to PMS, although I have discussed them with OB/GYNs, who never seem to follow these recommendations. Because I am not university-based, I have not been able to put thes
Throughout my 40 years in private psychiatric practice, I have found some treatments for premenstrual syndrome (PMS) that were not mentioned in “Etiology of premenstrual dysphoric disorder: 5 interwoven pieces” (Evidence-Based Reviews, Current Psychiatry. September 2017, p. 20-28).
This started in 1972 when I was serving in the Army in Oklahoma. A 28-year-old woman with severe PMS had been treated by internal medicine, an OB/GYN, and endocrinology, all to no avail. Three days before her menses began, she would start driving north. When menses commenced, she would find herself in Nebraska and have to call her husband so he could wire her money to come back.
Through my evaluation, I found that she would gain 10 lb before her menses. I prescribed a diuretic and instructed her to start taking it when she began swelling and to stop taking it after her menses began. This alleviated all of her symptoms. If a woman gains more than 3 to 5 lb, her brain also will swell, along with everything else. Because the brain is encapsulated in the skull, the swelling puts pressure on the brain, which might have been the cause of these brief psychotic episodes.
If a woman who develops PMS does not experience significant weight gain, the first thing I try is vitamin B6, 100 mg/d, prior to menses. Vitamin B6 is a cofactor in the production of numerous neurotransmitters. I found that prescribing vitamin B6 would alleviate about 20% of PMS symptoms. If the patient has a personal or family history of affective disorder, I often try antidepressants prior to menses, which alleviate approximately another 20% of her symptoms. If none of the previous 3 factors are present, I often add a low dose of progesterone, which appears to help. If all else fails, I will try a low dose of lithium, 300 mg/d, before menses. This also seems to have some positive effect.
I have not written an article about these approaches to PMS, although I have discussed them with OB/GYNs, who never seem to follow these recommendations. Because I am not university-based, I have not been able to put thes
Throughout my 40 years in private psychiatric practice, I have found some treatments for premenstrual syndrome (PMS) that were not mentioned in “Etiology of premenstrual dysphoric disorder: 5 interwoven pieces” (Evidence-Based Reviews, Current Psychiatry. September 2017, p. 20-28).
This started in 1972 when I was serving in the Army in Oklahoma. A 28-year-old woman with severe PMS had been treated by internal medicine, an OB/GYN, and endocrinology, all to no avail. Three days before her menses began, she would start driving north. When menses commenced, she would find herself in Nebraska and have to call her husband so he could wire her money to come back.
Through my evaluation, I found that she would gain 10 lb before her menses. I prescribed a diuretic and instructed her to start taking it when she began swelling and to stop taking it after her menses began. This alleviated all of her symptoms. If a woman gains more than 3 to 5 lb, her brain also will swell, along with everything else. Because the brain is encapsulated in the skull, the swelling puts pressure on the brain, which might have been the cause of these brief psychotic episodes.
If a woman who develops PMS does not experience significant weight gain, the first thing I try is vitamin B6, 100 mg/d, prior to menses. Vitamin B6 is a cofactor in the production of numerous neurotransmitters. I found that prescribing vitamin B6 would alleviate about 20% of PMS symptoms. If the patient has a personal or family history of affective disorder, I often try antidepressants prior to menses, which alleviate approximately another 20% of her symptoms. If none of the previous 3 factors are present, I often add a low dose of progesterone, which appears to help. If all else fails, I will try a low dose of lithium, 300 mg/d, before menses. This also seems to have some positive effect.
I have not written an article about these approaches to PMS, although I have discussed them with OB/GYNs, who never seem to follow these recommendations. Because I am not university-based, I have not been able to put thes
Prescribing antipsychotics in geriatric patients: Focus on schizophrenia and bipolar disorder
Antipsychotics are FDA-approved as a primary treatment for schizophrenia and bipolar disorder and as adjunctive therapy for major depressive disorder. In the United States, approximately 26% of antipsychotic prescriptions written for these indications are for individuals age >65.1 Additionally, antipsychotics are widely used to treat behavioral symptoms associated with dementia.1 The rapid expansion of the use of second-generation antipsychotics (SGAs), in particular, has been driven in part by their lower risk for extrapyramidal symptoms (EPS) compared with first-generation antipsychotics (FGAs).1 However, a growing body of data indicates that all antipsychotics have a range of adverse effects in older patients. This focus is critical in light of demographic trends—in the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.2
In this context, psychiatrists need information on the relative risks of antipsychotics for older patients. This 3-part series summarizes findings and recommendations on safety and tolerability when prescribing antipsychotics in older individuals with chronic psychotic disorders, such as schizophrenia, bipolar disorder, depression, and dementia. This review aims to:
- briefly summarize the major studies and analyses relevant to older patients with these diagnoses
- provide a summative opinion on safety and tolerability issues in these older adults
- highlight the gaps in the evidence base and areas that need additional research.
Part 1 focuses on older adults with schizophrenia or bipolar disorder. Subsequent articles will focus on prescribing antipsychotics to older adults with depression and those with dementia.
Schizophrenia
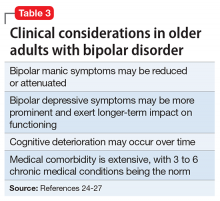
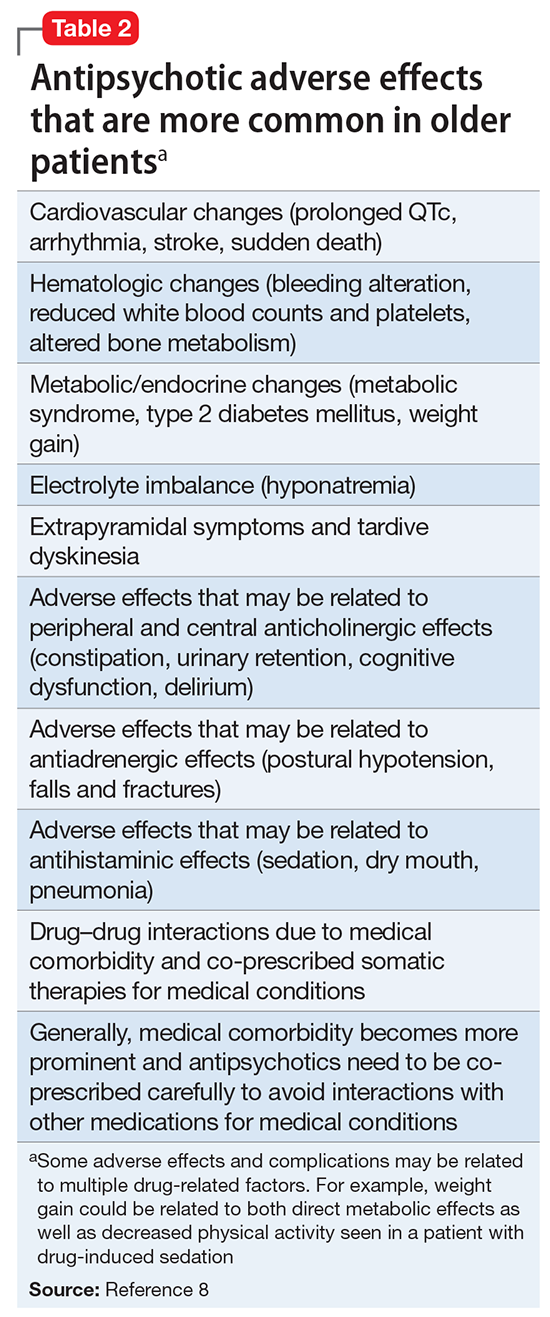
Summary of benefits, place in treatment armamentarium. Individuals with schizophrenia have a shorter life expectancy than that of the general population mostly as a result of suicide and comorbid physical illnesses,3 but the number of patients with schizophrenia age >55 will double over the next 2 decades.4 With aging, both positive and negative symptoms may be a focus of treatment (Table 1).5,6 Antipsychotics are a first-line treatment for older patients with schizophrenia with few medication alternatives.7 Safety risks associated with antipsychotics in older people span a broad spectrum (Table 2).8
A 6-week prospective RCT evaluated paliperidone extended-release vs placebo in 114 older adults (age ≥65 years; mean age, 70 years) with schizophrenia.14 There was an optional 24-week extension of open-label treatment with paliperidone. Mean daily dose of paliperidone was 8.4 mg. Efficacy measures did not show consistent statistically significant differences between treatment groups. Discontinuation rates were similar between paliperidone (7%) vs placebo (8%). Serious adverse events occurred in 3% of paliperidone-treated vs 8% of placebo-treated patients. Elevated prolactin levels occurred in one-half of paliperidone-treated patients. There were no prolactin or glucose treatment-related adverse events or significant mean changes in body weight for either paliperidone-treated or placebo-treated patients. Safety findings in the 24-week, open-label extension group were consistent with the RCT results.
Howanitz et al15 conducted a 12-week, prospective RCT that compared clozapine (mean dose, 300 mg/d) with chlorpromazine (mean dose, 600 mg/d) in 42 older adults (mean age, 67 years) with schizophrenia. Drop-out rate prior to 5 weeks was 19% and similar between groups. Common adverse effects included sialorrhea, hematologic abnormalities, sedation, tachycardia, EPS, and weight gain. Although both drugs were effective, more patients taking clozapine had tachycardia and weight gain, while more chlorpromazine patients reported sedation.
There have been other, less rigorous studies.7,8 Most of these studies evaluated risperidone and olanzapine, and most were conducted in “younger” geriatric patients (age <75 years). Although patients who participate in clinical trials may be healthier than “typical” patients, adverse effects such as EPS, sedation, and weight gain were still relatively common in these studies.
Other clinical data. A major consideration in treating older adults with schizophrenia is balancing the need to administer an antipsychotic dose high enough to alleviate psychotic symptoms while minimizing dose-dependent adverse effects. There is a U-shaped relationship between age and vulnerability to antipsychotic adverse effects,16,17 wherein adverse effects are highest at younger and older ages. Evidence supports using the lowest effective antipsychotic dose for geriatric patients with schizophrenia. Positive emission tomography (PET) studies suggest that older patients develop EPS with lower doses despite lower receptor occupancy.17,18 A recent study of 35 older patients (mean age, 60.1 years) with schizophrenia obtained PET, clinical measures, and blood pharmacokinetic measures before and after reduction of risperidone or olanzapine doses.18 A ≥40% reduction in dose was associated with reduced adverse effects, particularly EPS and elevation of prolactin levels. Moreover, the therapeutic window of striatal D2/D3 receptor occupancy appeared to be 50% to 60% in these older patients, compared with 65% to 80% in younger patients.
Long-term risks of antipsychotic treatment across the lifespan are less clear, with evidence suggesting both lower and higher mortality risk.19,20 It is difficult to fully disentangle the long-term risks of antipsychotics from the cumulative effects of lifestyle and comorbidity among individuals who have lived with schizophrenia for decades. Large naturalistic studies that include substantial numbers of older people with schizophrenia might be a way to elicit more information on long-term safety. The Schizophrenia Outpatient Health Outcome (SOHO) study was a large naturalistic trial that recruited >10,000 individuals with schizophrenia in 10 European countries.21 Although the SOHO study found differences between antipsychotics and adverse effects, such as EPS, weight gain, and sexual dysfunction, because the mean age of these patients was approximately 40 years and the follow-up period was only 3 years, it is difficult to draw conclusions that could be relevant to older individuals who have had schizophrenia for decades.
Bipolar Disorder
Clinical trials: Bipolar depression. A post hoc, secondary analysis of two 8-week, double-blind, randomized, placebo-controlled studies in bipolar depression compared 2 dosages of quetiapine (300 mg/d and 600 mg/d) with placebo in mixed-age patients.31 In a subgroup of 72 patients, ages 55 to 65, remission occurred more often with quetiapine than with placebo. Study discontinuation rates were similar between older people and younger people (age <55 years): quetiapine, 300 mg/d, 29.2%; quetiapine, 600 mg/d, 48.1%; and placebo, 29.6% in older adults, compared with 37.1%, 45.8%, and 38.1%, respectively, in younger adults. In all patients, the most common reason for discontinuation was adverse events with quetiapine and lack of efficacy for placebo. Adverse event rates were similar in older and younger adults. Dry mouth and dizziness were more common in older adults. Proportions of adults experiencing clinically significant weight gain (≥7% of body weight) were 5.3%, 8.3%, and 0% in older adults receiving quetiapine, 300 mg/d, quetiapine, 600 mg/d, and placebo, respectively, compared with 7.2%, 10.1%, and 2.6% in younger adults. EPS and treatment-emergent mania were minimal.
A secondary analysis of mixed-age, RCTs examined response in older adults (age ≥55 years) with bipolar I depression who received lurasidone as monotherapy or adjunctive therapy.32 In the monotherapy study, these patients were randomized to 6 weeks of lurasidone 20 to 60 mg/d, lurasidone 80 to 120 mg/d, or placebo. In the adjunctive therapy study, they were randomized to lurasidone 20 to 120 mg/d or placebo with either lithium or valproate. There were 83 older adults (17.1% of the sample) in the monotherapy study and 53 (15.6%) in the adjunctive therapy study. Mean improvement in depression was significantly higher for both doses of lurasidone monotherapy than placebo. Adjunctive lurasidone was not associated with statistically significant improvement vs placebo. The most frequent adverse events in older patients on lurasidone monotherapy 20 to 60 mg/d or 80 to 120 mg/d were nausea (18.5% and 9.7%, respectively) and somnolence (11.1% and 0%, respectively). Akathisia (9.7%) and insomnia (9.7%) were the most common adverse events in the group receiving 80 to 120 mg/d, with the rate of akathisia exhibiting a dose-related increase. Weight change with lurasidone was similar to placebo, and there were no clinically meaningful group changes in vital signs, electrocardiography, or laboratory parameters.
A small (N = 20) open study found improvement in older adults with bipolar depression with aripiprazole (mean dose, 10.3 mg/d).33 Adverse effects included restlessness and weight gain (n = 3, 9% each), sedation (n = 2, 10%), and drooling and diarrhea/loose stools (n = 1, 5% each). In another small study (N = 15) using asenapine (mean dose, 11.2 mg/d) in mainly older bipolar patients with depression, the most common adverse effects were gastrointestinal (GI) discomfort (n = 5, 33%) and restlessness, tremors, cognitive difficulties, and sluggishness (n = 2, 13% each).34
Clinical trials: Bipolar mania. Researchers conducted a pooled analysis of two 12-week randomized trials comparing quetiapine with placebo in a mixed-age sample with bipolar mania.35 In a subgroup of 59 older patients (mean age, 62.9 years), manic symptoms improved significantly more with quetiapine (modal dose, 550 mg/d) than with placebo. Adverse effects reported by >10% of older patients were dry mouth, somnolence, postural hypotension, insomnia, weight gain, and dizziness. Insomnia was reported by >10% of patients receiving placebo.
In a case series of 11 elderly patients with mania receiving asenapine, Baruch et al36 reported a 63% remission rate. One patient discontinued the study because of a new rash, 1 discontinued after developing peripheral edema, and 3 patients reported mild sedation.
Beyer et al37 reported on a post hoc analysis of 94 older adults (mean age, 57.1 years; range, 50.1 to 74.8 years) with acute bipolar mania receiving olanzapine (n = 47), divalproex (n = 31), or placebo (n = 16) in a pooled olanzapine clinical trials database. Patients receiving olanzapine or divalproex had improvement in mania; those receiving placebo did not improve. Safety findings were comparable with reports in younger patients with mania.
Other clinical data. Adverse effects found in mixed-age samples using secondary analyses of clinical trials need to be interpreted with caution because these types of studies usually exclude individuals with significant medical comorbidity. Medical burden, cognitive impairment, or concomitant medications generally necessitate slower drug titration and lower total daily dosing. For example, a secondary analysis of the U.S. National Institute of Health-funded Systematic Treatment Enhancement Program for Bipolar Disorder study, which had broader inclusion criteria than most clinical trials, reported that, although recovery rates in older adults with bipolar disorder were fairly good (78.5%), lower doses of risperidone were used in older vs younger patients.38
Clinical considerations
Interpretation of the relative risks of antipsychotics in older people must be tempered by the caveat that there is limited high-quality data (Table 4). Antipsychotics are the first-line therapy for older patients with schizophrenia, although their use is supported by a small number of prospective RCTs. SGAs are preferred because of their lower propensity to cause EPS and other motor adverse effects. Older persons with schizophrenia have an EPS threshold lower than younger patients and determining the lowest effective dosage may minimize EPS and cognitive adverse effects. As individuals with long-standing schizophrenia get older, their antipsychotic dosages may need to be reduced, and clinicians need to monitor for adverse effects that are more common among older people, such as tardive dyskinesia and metabolic abnormalities. In healthy, “younger” geriatric patients, monitoring for adverse effects may be similar to monitoring of younger patients. Patients who are older or frail may need more frequent assessment.
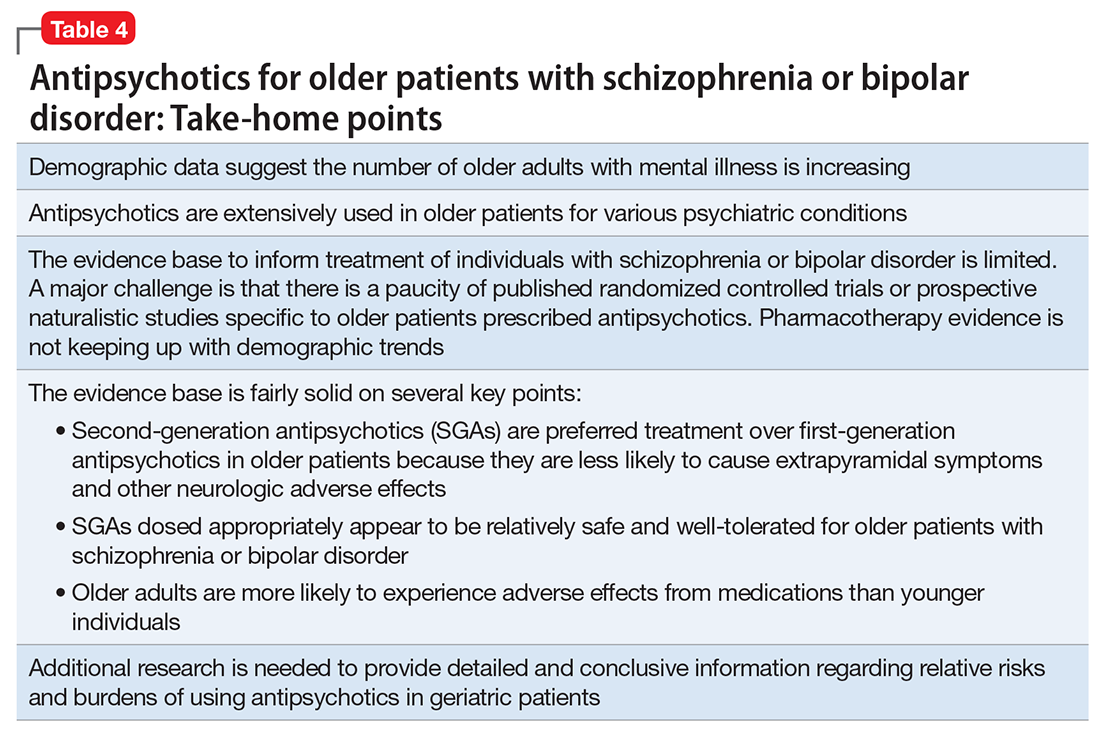
Like older adults with schizophrenia, geriatric patients with bipolar disorder have reduced drug tolerability and experience more adverse effects than younger patients. There are no prospective controlled studies that evaluated using antipsychotics in older patients with bipolar disorder. In older bipolar patients, the most problematic adverse effects of antipsychotics are akathisia, parkinsonism, other EPS, sedation and dizziness (which may increase fall risk), and GI discomfort. A key tolerability and safety consideration when treating older adults with bipolar disorder is the role of antipsychotics in relation to the use of lithium and mood stabilizers. Some studies have suggested that lithium has neuroprotective effects when used long-term; however, at least 1 report suggested that long-term antipsychotic treatment may be associated with neurodegeneration.39
The literature does not provide strong evidence on the many clinical variations that we see in routine practice settings, such as combinations of drug treatments or drugs prescribed to patients with specific comorbid conditions. There is a need for large cohort studies that monitor treatment course, medical comorbidity, and prognosis. Additionally, well-designed clinical trials such as the DART-AD, which investigated longer-term trajectories of people with dementia taking antipsychotics, should serve as a model for the type of research that is needed to better understand outcome variability among older people with chronic psychotic or bipolar disorders.40
1. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
2. United Nations, Department of Economic and Social Affairs, Population Division. World population ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Accessed September 1, 2017.
3. Lawrence D, Kisely S, Pais J. The epidemiology of excess mortality in people with mental illness. Can J Psychiatry. 2010;55(12):752-760.
4. Cohen CI, Vahia I, Reyes P, et al. Focus on geriatric psychiatry: schizophrenia in later life: clinical symptoms and social well-being. Psychiatr Serv. 2008;59(3):232-234.
5. Jeste DV, Barak Y, Madhusoodanan S, et al. International multisite double-blind trial of the atypical antipsychotics risperidone and olanzapine in 175 elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2003;11(6):638-647.
6. Kalache SM, Mulsant BH, Davies SJ, et al. The impact of aging, cognition, and symptoms on functional competence in individuals with schizophrenia across the lifespan. Schizophr Bull. 2015;41(2):374-381.
7. Suzuki T, Remington G, Uchida H, et al. Management of schizophrenia in late life with antipsychotic medications: a qualitative review. Drugs Aging. 2011;28(12):961-980.
8. Mulsant BH, Pollock BG. Psychopharmacology. In: David C. Steffens DC, Blazer DG, Thakur ME (eds). The American Psychiatric Publishing Textbook of Geriatric Psychiatry, 5th Edition. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
9. Cohen CI, Meesters PD, Zhao J. New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry. 2015;2(4):340-350.
10. Marriott RG, Neil W, Waddingham S. Antipsychotic medication for elderly people with schizophrenia. Cochrane Database Syst Rev. 2006;(1):CD005580.
11. Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev. 2012(2):CD004162.
12. Scott J, Greenwald BS, Kramer E, et al. Atypical (second generation) antipsychotic treatment response in very late-onset schizophrenia-like psychosis. Int Psychogeriatr. 2011;23(5):742-748.
13. Rado J, Janicak PG. Pharmacological and clinical profile of recently approved second-generation antipsychotics: implications for treatment of schizophrenia in older patients. Drugs Aging. 2012;29(10):783-791.
14. Tzimos A, Samokhvalov V, Kramer M, et al. Safety and tolerability of oral paliperidone extended-release tablets in elderly patients with schizophrenia: a double-blind, placebo-controlled study with six-month open-label extension. Am J Geriatr Psychiatry. 2008;16(1):31-43.
15. Howanitz E, Pardo M, Smelson DA, et al. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia. J Clin Psychiatry. 1999;60(1):41-44.
16. Sproule BA, Lake J, Mamo DC, et al. Are antipsychotic prescribing patterns different in older and younger adults?: a survey of 1357 psychiatric inpatients in Toronto. Can J Psychiatry. 2010;55(4):248-254.
17. Uchida H, Suzuki T, Mamo DC, et al. Effects of age and age of onset on prescribed antipsychotic dose in schizophrenia spectrum disorders: a survey of 1,418 patients in Japan. Am J Geriatr Psychiatry. 2008;16(7):584-593.
18. Graff-Guerrero A, Rajji TK, Mulsant BH, et al. Evaluation of antipsychotic dose reduction in late-life schizophrenia: a prospective dopamine D2/3 occupancy study. JAMA Psychiatry. 2015;72(9):927-934.
19. Khan A, Schwartz K, Stern C, et al. Mortality risk in patients with schizophrenia participating in premarketing atypical antipsychotic clinical trials. J Clin Psychiatry. 2007;68(12):1828-1833.
20. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: a systematic review. Schizophr Res. 2009;113(1):1-11.
21. Novick D, Haro JM, Perrin E, et al. Tolerability of outpatient antipsychotic treatment: 36-month results from the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Eur Neuropsychopharmacol. 2009;19(8):542-550.
22. Sajatovic M, Blow FC, Ignacio RV, et al. Age-related modifiers of clinical presentation and health service use among veterans with bipolar disorder. Psychiatr Serv. 2004;55(9):1014-1021.
23. Jeste DV, Alexopoulos GS, Bartels SJ, et al. Consensus statement on the upcoming crisis in geriatric mental health: research agenda for the next 2 decades. Arch Gen Psychiatry. 1999;56(9):848-853.
24. Sajatovic M, Chen P. Geriatric bipolar disorder. Psychiatr Clin North Am. 2011;34(2):319-333,vii.
25. Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older-age bipolar disorder from the International Society for Bipolar Disorders Task Force. Bipolar Disord. 2015;17(7):689-704.
26. Lala SV, Sajatovic M. Medical and psychiatric comorbidities among elderly individuals with bipolar disorder: a literature review. J Geriatr Psychiatry Neurol. 2012;25(1):20-25.
27. Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
28. Pillarella J, Higashi A, Alexander GC, et al. Trends in use of second-generation antipsychotics for treatment of bipolar disorder in the United States, 1998-2009. Psychiatr Serv. 2012;63(1):83-86.
29. De Fruyt J, Deschepper E, Audenaert K, et al. Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis. J Psychopharmacol. 2012;26(5):603-617.
30. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
31. Sajatovic M, Paulsson B. Quetiapine for the treatment of depressive episodes in adults aged 55 to 65 years with bipolar disorder. Paper presented at: American Association of Geriatric Psychiatry Annual Meeting; 2007; New Orleans, LA.
32. Sajatovic M, Forester B, Tsai J, et al. Efficacy and safety of lurasidone in older adults with bipolar depression: analysis of two double-blind, placebo-controlled studies. Paper presented at: American College of Neuropsychopharmacology (ACNP) 53rd Annual Meeting; 2014; Phoenix, AZ.
33. Sajatovic M, Coconcea N, Ignacio RV, et al. Aripiprazole therapy in 20 older adults with bipolar disorder: a 12-week, open-label trial. J Clin Psychiatry. 2008;69(1):41-46.
34. Sajatovic M, Dines P, Fuentes-Casiano E, et al. Asenapine in the treatment of older adults with bipolar disorder. Int J Geriatr Psychiatry. 2015;30(7):710-719.
35. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
36. Baruch Y, Tadger S, Plopski I, et al. Asenapine for elderly bipolar manic patients. J Affect Disord. 2013;145(1):130-132.
37. Beyer JL, Siegal A, Kennedy JS. Olanzapine, divalproex and placebo treatment, non-head to head comparisons of older adults acute mania. Paper presented at: 10th Congress of the International Psychogeriatric Association; 2001; Nice, France.
38. Al Jurdi RK, Marangell LB, Petersen NJ, et al. Prescription patterns of psychotropic medications in elderly compared with younger participants who achieved a “recovered” status in the systematic treatment enhancement program for bipolar disorder. Am J Geriatr Psychiatry. 2008;16(11):922-933.
39. Gildengers AG, Chung KH, Huang SH, et al. Neuroprogressive effects of lifetime illness duration in older adults with bipolar disorder. Bipolar Disord. 2014;16(6):617-623.
40. Ballard C, Lana MM, Theodoulou M, et al. A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial). PLoS Med. 2008;5(4):e76.
Antipsychotics are FDA-approved as a primary treatment for schizophrenia and bipolar disorder and as adjunctive therapy for major depressive disorder. In the United States, approximately 26% of antipsychotic prescriptions written for these indications are for individuals age >65.1 Additionally, antipsychotics are widely used to treat behavioral symptoms associated with dementia.1 The rapid expansion of the use of second-generation antipsychotics (SGAs), in particular, has been driven in part by their lower risk for extrapyramidal symptoms (EPS) compared with first-generation antipsychotics (FGAs).1 However, a growing body of data indicates that all antipsychotics have a range of adverse effects in older patients. This focus is critical in light of demographic trends—in the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.2
In this context, psychiatrists need information on the relative risks of antipsychotics for older patients. This 3-part series summarizes findings and recommendations on safety and tolerability when prescribing antipsychotics in older individuals with chronic psychotic disorders, such as schizophrenia, bipolar disorder, depression, and dementia. This review aims to:
- briefly summarize the major studies and analyses relevant to older patients with these diagnoses
- provide a summative opinion on safety and tolerability issues in these older adults
- highlight the gaps in the evidence base and areas that need additional research.
Part 1 focuses on older adults with schizophrenia or bipolar disorder. Subsequent articles will focus on prescribing antipsychotics to older adults with depression and those with dementia.
Schizophrenia
Summary of benefits, place in treatment armamentarium. Individuals with schizophrenia have a shorter life expectancy than that of the general population mostly as a result of suicide and comorbid physical illnesses,3 but the number of patients with schizophrenia age >55 will double over the next 2 decades.4 With aging, both positive and negative symptoms may be a focus of treatment (Table 1).5,6 Antipsychotics are a first-line treatment for older patients with schizophrenia with few medication alternatives.7 Safety risks associated with antipsychotics in older people span a broad spectrum (Table 2).8

A 6-week prospective RCT evaluated paliperidone extended-release vs placebo in 114 older adults (age ≥65 years; mean age, 70 years) with schizophrenia.14 There was an optional 24-week extension of open-label treatment with paliperidone. Mean daily dose of paliperidone was 8.4 mg. Efficacy measures did not show consistent statistically significant differences between treatment groups. Discontinuation rates were similar between paliperidone (7%) vs placebo (8%). Serious adverse events occurred in 3% of paliperidone-treated vs 8% of placebo-treated patients. Elevated prolactin levels occurred in one-half of paliperidone-treated patients. There were no prolactin or glucose treatment-related adverse events or significant mean changes in body weight for either paliperidone-treated or placebo-treated patients. Safety findings in the 24-week, open-label extension group were consistent with the RCT results.
Howanitz et al15 conducted a 12-week, prospective RCT that compared clozapine (mean dose, 300 mg/d) with chlorpromazine (mean dose, 600 mg/d) in 42 older adults (mean age, 67 years) with schizophrenia. Drop-out rate prior to 5 weeks was 19% and similar between groups. Common adverse effects included sialorrhea, hematologic abnormalities, sedation, tachycardia, EPS, and weight gain. Although both drugs were effective, more patients taking clozapine had tachycardia and weight gain, while more chlorpromazine patients reported sedation.
There have been other, less rigorous studies.7,8 Most of these studies evaluated risperidone and olanzapine, and most were conducted in “younger” geriatric patients (age <75 years). Although patients who participate in clinical trials may be healthier than “typical” patients, adverse effects such as EPS, sedation, and weight gain were still relatively common in these studies.
Other clinical data. A major consideration in treating older adults with schizophrenia is balancing the need to administer an antipsychotic dose high enough to alleviate psychotic symptoms while minimizing dose-dependent adverse effects. There is a U-shaped relationship between age and vulnerability to antipsychotic adverse effects,16,17 wherein adverse effects are highest at younger and older ages. Evidence supports using the lowest effective antipsychotic dose for geriatric patients with schizophrenia. Positive emission tomography (PET) studies suggest that older patients develop EPS with lower doses despite lower receptor occupancy.17,18 A recent study of 35 older patients (mean age, 60.1 years) with schizophrenia obtained PET, clinical measures, and blood pharmacokinetic measures before and after reduction of risperidone or olanzapine doses.18 A ≥40% reduction in dose was associated with reduced adverse effects, particularly EPS and elevation of prolactin levels. Moreover, the therapeutic window of striatal D2/D3 receptor occupancy appeared to be 50% to 60% in these older patients, compared with 65% to 80% in younger patients.
Long-term risks of antipsychotic treatment across the lifespan are less clear, with evidence suggesting both lower and higher mortality risk.19,20 It is difficult to fully disentangle the long-term risks of antipsychotics from the cumulative effects of lifestyle and comorbidity among individuals who have lived with schizophrenia for decades. Large naturalistic studies that include substantial numbers of older people with schizophrenia might be a way to elicit more information on long-term safety. The Schizophrenia Outpatient Health Outcome (SOHO) study was a large naturalistic trial that recruited >10,000 individuals with schizophrenia in 10 European countries.21 Although the SOHO study found differences between antipsychotics and adverse effects, such as EPS, weight gain, and sexual dysfunction, because the mean age of these patients was approximately 40 years and the follow-up period was only 3 years, it is difficult to draw conclusions that could be relevant to older individuals who have had schizophrenia for decades.
Bipolar Disorder
Clinical trials: Bipolar depression. A post hoc, secondary analysis of two 8-week, double-blind, randomized, placebo-controlled studies in bipolar depression compared 2 dosages of quetiapine (300 mg/d and 600 mg/d) with placebo in mixed-age patients.31 In a subgroup of 72 patients, ages 55 to 65, remission occurred more often with quetiapine than with placebo. Study discontinuation rates were similar between older people and younger people (age <55 years): quetiapine, 300 mg/d, 29.2%; quetiapine, 600 mg/d, 48.1%; and placebo, 29.6% in older adults, compared with 37.1%, 45.8%, and 38.1%, respectively, in younger adults. In all patients, the most common reason for discontinuation was adverse events with quetiapine and lack of efficacy for placebo. Adverse event rates were similar in older and younger adults. Dry mouth and dizziness were more common in older adults. Proportions of adults experiencing clinically significant weight gain (≥7% of body weight) were 5.3%, 8.3%, and 0% in older adults receiving quetiapine, 300 mg/d, quetiapine, 600 mg/d, and placebo, respectively, compared with 7.2%, 10.1%, and 2.6% in younger adults. EPS and treatment-emergent mania were minimal.
A secondary analysis of mixed-age, RCTs examined response in older adults (age ≥55 years) with bipolar I depression who received lurasidone as monotherapy or adjunctive therapy.32 In the monotherapy study, these patients were randomized to 6 weeks of lurasidone 20 to 60 mg/d, lurasidone 80 to 120 mg/d, or placebo. In the adjunctive therapy study, they were randomized to lurasidone 20 to 120 mg/d or placebo with either lithium or valproate. There were 83 older adults (17.1% of the sample) in the monotherapy study and 53 (15.6%) in the adjunctive therapy study. Mean improvement in depression was significantly higher for both doses of lurasidone monotherapy than placebo. Adjunctive lurasidone was not associated with statistically significant improvement vs placebo. The most frequent adverse events in older patients on lurasidone monotherapy 20 to 60 mg/d or 80 to 120 mg/d were nausea (18.5% and 9.7%, respectively) and somnolence (11.1% and 0%, respectively). Akathisia (9.7%) and insomnia (9.7%) were the most common adverse events in the group receiving 80 to 120 mg/d, with the rate of akathisia exhibiting a dose-related increase. Weight change with lurasidone was similar to placebo, and there were no clinically meaningful group changes in vital signs, electrocardiography, or laboratory parameters.
A small (N = 20) open study found improvement in older adults with bipolar depression with aripiprazole (mean dose, 10.3 mg/d).33 Adverse effects included restlessness and weight gain (n = 3, 9% each), sedation (n = 2, 10%), and drooling and diarrhea/loose stools (n = 1, 5% each). In another small study (N = 15) using asenapine (mean dose, 11.2 mg/d) in mainly older bipolar patients with depression, the most common adverse effects were gastrointestinal (GI) discomfort (n = 5, 33%) and restlessness, tremors, cognitive difficulties, and sluggishness (n = 2, 13% each).34
Clinical trials: Bipolar mania. Researchers conducted a pooled analysis of two 12-week randomized trials comparing quetiapine with placebo in a mixed-age sample with bipolar mania.35 In a subgroup of 59 older patients (mean age, 62.9 years), manic symptoms improved significantly more with quetiapine (modal dose, 550 mg/d) than with placebo. Adverse effects reported by >10% of older patients were dry mouth, somnolence, postural hypotension, insomnia, weight gain, and dizziness. Insomnia was reported by >10% of patients receiving placebo.
In a case series of 11 elderly patients with mania receiving asenapine, Baruch et al36 reported a 63% remission rate. One patient discontinued the study because of a new rash, 1 discontinued after developing peripheral edema, and 3 patients reported mild sedation.
Beyer et al37 reported on a post hoc analysis of 94 older adults (mean age, 57.1 years; range, 50.1 to 74.8 years) with acute bipolar mania receiving olanzapine (n = 47), divalproex (n = 31), or placebo (n = 16) in a pooled olanzapine clinical trials database. Patients receiving olanzapine or divalproex had improvement in mania; those receiving placebo did not improve. Safety findings were comparable with reports in younger patients with mania.
Other clinical data. Adverse effects found in mixed-age samples using secondary analyses of clinical trials need to be interpreted with caution because these types of studies usually exclude individuals with significant medical comorbidity. Medical burden, cognitive impairment, or concomitant medications generally necessitate slower drug titration and lower total daily dosing. For example, a secondary analysis of the U.S. National Institute of Health-funded Systematic Treatment Enhancement Program for Bipolar Disorder study, which had broader inclusion criteria than most clinical trials, reported that, although recovery rates in older adults with bipolar disorder were fairly good (78.5%), lower doses of risperidone were used in older vs younger patients.38
Clinical considerations
Interpretation of the relative risks of antipsychotics in older people must be tempered by the caveat that there is limited high-quality data (Table 4). Antipsychotics are the first-line therapy for older patients with schizophrenia, although their use is supported by a small number of prospective RCTs. SGAs are preferred because of their lower propensity to cause EPS and other motor adverse effects. Older persons with schizophrenia have an EPS threshold lower than younger patients and determining the lowest effective dosage may minimize EPS and cognitive adverse effects. As individuals with long-standing schizophrenia get older, their antipsychotic dosages may need to be reduced, and clinicians need to monitor for adverse effects that are more common among older people, such as tardive dyskinesia and metabolic abnormalities. In healthy, “younger” geriatric patients, monitoring for adverse effects may be similar to monitoring of younger patients. Patients who are older or frail may need more frequent assessment.

Like older adults with schizophrenia, geriatric patients with bipolar disorder have reduced drug tolerability and experience more adverse effects than younger patients. There are no prospective controlled studies that evaluated using antipsychotics in older patients with bipolar disorder. In older bipolar patients, the most problematic adverse effects of antipsychotics are akathisia, parkinsonism, other EPS, sedation and dizziness (which may increase fall risk), and GI discomfort. A key tolerability and safety consideration when treating older adults with bipolar disorder is the role of antipsychotics in relation to the use of lithium and mood stabilizers. Some studies have suggested that lithium has neuroprotective effects when used long-term; however, at least 1 report suggested that long-term antipsychotic treatment may be associated with neurodegeneration.39
The literature does not provide strong evidence on the many clinical variations that we see in routine practice settings, such as combinations of drug treatments or drugs prescribed to patients with specific comorbid conditions. There is a need for large cohort studies that monitor treatment course, medical comorbidity, and prognosis. Additionally, well-designed clinical trials such as the DART-AD, which investigated longer-term trajectories of people with dementia taking antipsychotics, should serve as a model for the type of research that is needed to better understand outcome variability among older people with chronic psychotic or bipolar disorders.40
Antipsychotics are FDA-approved as a primary treatment for schizophrenia and bipolar disorder and as adjunctive therapy for major depressive disorder. In the United States, approximately 26% of antipsychotic prescriptions written for these indications are for individuals age >65.1 Additionally, antipsychotics are widely used to treat behavioral symptoms associated with dementia.1 The rapid expansion of the use of second-generation antipsychotics (SGAs), in particular, has been driven in part by their lower risk for extrapyramidal symptoms (EPS) compared with first-generation antipsychotics (FGAs).1 However, a growing body of data indicates that all antipsychotics have a range of adverse effects in older patients. This focus is critical in light of demographic trends—in the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.2
In this context, psychiatrists need information on the relative risks of antipsychotics for older patients. This 3-part series summarizes findings and recommendations on safety and tolerability when prescribing antipsychotics in older individuals with chronic psychotic disorders, such as schizophrenia, bipolar disorder, depression, and dementia. This review aims to:
- briefly summarize the major studies and analyses relevant to older patients with these diagnoses
- provide a summative opinion on safety and tolerability issues in these older adults
- highlight the gaps in the evidence base and areas that need additional research.
Part 1 focuses on older adults with schizophrenia or bipolar disorder. Subsequent articles will focus on prescribing antipsychotics to older adults with depression and those with dementia.
Schizophrenia
Summary of benefits, place in treatment armamentarium. Individuals with schizophrenia have a shorter life expectancy than that of the general population mostly as a result of suicide and comorbid physical illnesses,3 but the number of patients with schizophrenia age >55 will double over the next 2 decades.4 With aging, both positive and negative symptoms may be a focus of treatment (Table 1).5,6 Antipsychotics are a first-line treatment for older patients with schizophrenia with few medication alternatives.7 Safety risks associated with antipsychotics in older people span a broad spectrum (Table 2).8

A 6-week prospective RCT evaluated paliperidone extended-release vs placebo in 114 older adults (age ≥65 years; mean age, 70 years) with schizophrenia.14 There was an optional 24-week extension of open-label treatment with paliperidone. Mean daily dose of paliperidone was 8.4 mg. Efficacy measures did not show consistent statistically significant differences between treatment groups. Discontinuation rates were similar between paliperidone (7%) vs placebo (8%). Serious adverse events occurred in 3% of paliperidone-treated vs 8% of placebo-treated patients. Elevated prolactin levels occurred in one-half of paliperidone-treated patients. There were no prolactin or glucose treatment-related adverse events or significant mean changes in body weight for either paliperidone-treated or placebo-treated patients. Safety findings in the 24-week, open-label extension group were consistent with the RCT results.
Howanitz et al15 conducted a 12-week, prospective RCT that compared clozapine (mean dose, 300 mg/d) with chlorpromazine (mean dose, 600 mg/d) in 42 older adults (mean age, 67 years) with schizophrenia. Drop-out rate prior to 5 weeks was 19% and similar between groups. Common adverse effects included sialorrhea, hematologic abnormalities, sedation, tachycardia, EPS, and weight gain. Although both drugs were effective, more patients taking clozapine had tachycardia and weight gain, while more chlorpromazine patients reported sedation.
There have been other, less rigorous studies.7,8 Most of these studies evaluated risperidone and olanzapine, and most were conducted in “younger” geriatric patients (age <75 years). Although patients who participate in clinical trials may be healthier than “typical” patients, adverse effects such as EPS, sedation, and weight gain were still relatively common in these studies.
Other clinical data. A major consideration in treating older adults with schizophrenia is balancing the need to administer an antipsychotic dose high enough to alleviate psychotic symptoms while minimizing dose-dependent adverse effects. There is a U-shaped relationship between age and vulnerability to antipsychotic adverse effects,16,17 wherein adverse effects are highest at younger and older ages. Evidence supports using the lowest effective antipsychotic dose for geriatric patients with schizophrenia. Positive emission tomography (PET) studies suggest that older patients develop EPS with lower doses despite lower receptor occupancy.17,18 A recent study of 35 older patients (mean age, 60.1 years) with schizophrenia obtained PET, clinical measures, and blood pharmacokinetic measures before and after reduction of risperidone or olanzapine doses.18 A ≥40% reduction in dose was associated with reduced adverse effects, particularly EPS and elevation of prolactin levels. Moreover, the therapeutic window of striatal D2/D3 receptor occupancy appeared to be 50% to 60% in these older patients, compared with 65% to 80% in younger patients.
Long-term risks of antipsychotic treatment across the lifespan are less clear, with evidence suggesting both lower and higher mortality risk.19,20 It is difficult to fully disentangle the long-term risks of antipsychotics from the cumulative effects of lifestyle and comorbidity among individuals who have lived with schizophrenia for decades. Large naturalistic studies that include substantial numbers of older people with schizophrenia might be a way to elicit more information on long-term safety. The Schizophrenia Outpatient Health Outcome (SOHO) study was a large naturalistic trial that recruited >10,000 individuals with schizophrenia in 10 European countries.21 Although the SOHO study found differences between antipsychotics and adverse effects, such as EPS, weight gain, and sexual dysfunction, because the mean age of these patients was approximately 40 years and the follow-up period was only 3 years, it is difficult to draw conclusions that could be relevant to older individuals who have had schizophrenia for decades.
Bipolar Disorder
Clinical trials: Bipolar depression. A post hoc, secondary analysis of two 8-week, double-blind, randomized, placebo-controlled studies in bipolar depression compared 2 dosages of quetiapine (300 mg/d and 600 mg/d) with placebo in mixed-age patients.31 In a subgroup of 72 patients, ages 55 to 65, remission occurred more often with quetiapine than with placebo. Study discontinuation rates were similar between older people and younger people (age <55 years): quetiapine, 300 mg/d, 29.2%; quetiapine, 600 mg/d, 48.1%; and placebo, 29.6% in older adults, compared with 37.1%, 45.8%, and 38.1%, respectively, in younger adults. In all patients, the most common reason for discontinuation was adverse events with quetiapine and lack of efficacy for placebo. Adverse event rates were similar in older and younger adults. Dry mouth and dizziness were more common in older adults. Proportions of adults experiencing clinically significant weight gain (≥7% of body weight) were 5.3%, 8.3%, and 0% in older adults receiving quetiapine, 300 mg/d, quetiapine, 600 mg/d, and placebo, respectively, compared with 7.2%, 10.1%, and 2.6% in younger adults. EPS and treatment-emergent mania were minimal.
A secondary analysis of mixed-age, RCTs examined response in older adults (age ≥55 years) with bipolar I depression who received lurasidone as monotherapy or adjunctive therapy.32 In the monotherapy study, these patients were randomized to 6 weeks of lurasidone 20 to 60 mg/d, lurasidone 80 to 120 mg/d, or placebo. In the adjunctive therapy study, they were randomized to lurasidone 20 to 120 mg/d or placebo with either lithium or valproate. There were 83 older adults (17.1% of the sample) in the monotherapy study and 53 (15.6%) in the adjunctive therapy study. Mean improvement in depression was significantly higher for both doses of lurasidone monotherapy than placebo. Adjunctive lurasidone was not associated with statistically significant improvement vs placebo. The most frequent adverse events in older patients on lurasidone monotherapy 20 to 60 mg/d or 80 to 120 mg/d were nausea (18.5% and 9.7%, respectively) and somnolence (11.1% and 0%, respectively). Akathisia (9.7%) and insomnia (9.7%) were the most common adverse events in the group receiving 80 to 120 mg/d, with the rate of akathisia exhibiting a dose-related increase. Weight change with lurasidone was similar to placebo, and there were no clinically meaningful group changes in vital signs, electrocardiography, or laboratory parameters.
A small (N = 20) open study found improvement in older adults with bipolar depression with aripiprazole (mean dose, 10.3 mg/d).33 Adverse effects included restlessness and weight gain (n = 3, 9% each), sedation (n = 2, 10%), and drooling and diarrhea/loose stools (n = 1, 5% each). In another small study (N = 15) using asenapine (mean dose, 11.2 mg/d) in mainly older bipolar patients with depression, the most common adverse effects were gastrointestinal (GI) discomfort (n = 5, 33%) and restlessness, tremors, cognitive difficulties, and sluggishness (n = 2, 13% each).34
Clinical trials: Bipolar mania. Researchers conducted a pooled analysis of two 12-week randomized trials comparing quetiapine with placebo in a mixed-age sample with bipolar mania.35 In a subgroup of 59 older patients (mean age, 62.9 years), manic symptoms improved significantly more with quetiapine (modal dose, 550 mg/d) than with placebo. Adverse effects reported by >10% of older patients were dry mouth, somnolence, postural hypotension, insomnia, weight gain, and dizziness. Insomnia was reported by >10% of patients receiving placebo.
In a case series of 11 elderly patients with mania receiving asenapine, Baruch et al36 reported a 63% remission rate. One patient discontinued the study because of a new rash, 1 discontinued after developing peripheral edema, and 3 patients reported mild sedation.
Beyer et al37 reported on a post hoc analysis of 94 older adults (mean age, 57.1 years; range, 50.1 to 74.8 years) with acute bipolar mania receiving olanzapine (n = 47), divalproex (n = 31), or placebo (n = 16) in a pooled olanzapine clinical trials database. Patients receiving olanzapine or divalproex had improvement in mania; those receiving placebo did not improve. Safety findings were comparable with reports in younger patients with mania.
Other clinical data. Adverse effects found in mixed-age samples using secondary analyses of clinical trials need to be interpreted with caution because these types of studies usually exclude individuals with significant medical comorbidity. Medical burden, cognitive impairment, or concomitant medications generally necessitate slower drug titration and lower total daily dosing. For example, a secondary analysis of the U.S. National Institute of Health-funded Systematic Treatment Enhancement Program for Bipolar Disorder study, which had broader inclusion criteria than most clinical trials, reported that, although recovery rates in older adults with bipolar disorder were fairly good (78.5%), lower doses of risperidone were used in older vs younger patients.38
Clinical considerations
Interpretation of the relative risks of antipsychotics in older people must be tempered by the caveat that there is limited high-quality data (Table 4). Antipsychotics are the first-line therapy for older patients with schizophrenia, although their use is supported by a small number of prospective RCTs. SGAs are preferred because of their lower propensity to cause EPS and other motor adverse effects. Older persons with schizophrenia have an EPS threshold lower than younger patients and determining the lowest effective dosage may minimize EPS and cognitive adverse effects. As individuals with long-standing schizophrenia get older, their antipsychotic dosages may need to be reduced, and clinicians need to monitor for adverse effects that are more common among older people, such as tardive dyskinesia and metabolic abnormalities. In healthy, “younger” geriatric patients, monitoring for adverse effects may be similar to monitoring of younger patients. Patients who are older or frail may need more frequent assessment.

Like older adults with schizophrenia, geriatric patients with bipolar disorder have reduced drug tolerability and experience more adverse effects than younger patients. There are no prospective controlled studies that evaluated using antipsychotics in older patients with bipolar disorder. In older bipolar patients, the most problematic adverse effects of antipsychotics are akathisia, parkinsonism, other EPS, sedation and dizziness (which may increase fall risk), and GI discomfort. A key tolerability and safety consideration when treating older adults with bipolar disorder is the role of antipsychotics in relation to the use of lithium and mood stabilizers. Some studies have suggested that lithium has neuroprotective effects when used long-term; however, at least 1 report suggested that long-term antipsychotic treatment may be associated with neurodegeneration.39
The literature does not provide strong evidence on the many clinical variations that we see in routine practice settings, such as combinations of drug treatments or drugs prescribed to patients with specific comorbid conditions. There is a need for large cohort studies that monitor treatment course, medical comorbidity, and prognosis. Additionally, well-designed clinical trials such as the DART-AD, which investigated longer-term trajectories of people with dementia taking antipsychotics, should serve as a model for the type of research that is needed to better understand outcome variability among older people with chronic psychotic or bipolar disorders.40
1. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
2. United Nations, Department of Economic and Social Affairs, Population Division. World population ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Accessed September 1, 2017.
3. Lawrence D, Kisely S, Pais J. The epidemiology of excess mortality in people with mental illness. Can J Psychiatry. 2010;55(12):752-760.
4. Cohen CI, Vahia I, Reyes P, et al. Focus on geriatric psychiatry: schizophrenia in later life: clinical symptoms and social well-being. Psychiatr Serv. 2008;59(3):232-234.
5. Jeste DV, Barak Y, Madhusoodanan S, et al. International multisite double-blind trial of the atypical antipsychotics risperidone and olanzapine in 175 elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2003;11(6):638-647.
6. Kalache SM, Mulsant BH, Davies SJ, et al. The impact of aging, cognition, and symptoms on functional competence in individuals with schizophrenia across the lifespan. Schizophr Bull. 2015;41(2):374-381.
7. Suzuki T, Remington G, Uchida H, et al. Management of schizophrenia in late life with antipsychotic medications: a qualitative review. Drugs Aging. 2011;28(12):961-980.
8. Mulsant BH, Pollock BG. Psychopharmacology. In: David C. Steffens DC, Blazer DG, Thakur ME (eds). The American Psychiatric Publishing Textbook of Geriatric Psychiatry, 5th Edition. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
9. Cohen CI, Meesters PD, Zhao J. New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry. 2015;2(4):340-350.
10. Marriott RG, Neil W, Waddingham S. Antipsychotic medication for elderly people with schizophrenia. Cochrane Database Syst Rev. 2006;(1):CD005580.
11. Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev. 2012(2):CD004162.
12. Scott J, Greenwald BS, Kramer E, et al. Atypical (second generation) antipsychotic treatment response in very late-onset schizophrenia-like psychosis. Int Psychogeriatr. 2011;23(5):742-748.
13. Rado J, Janicak PG. Pharmacological and clinical profile of recently approved second-generation antipsychotics: implications for treatment of schizophrenia in older patients. Drugs Aging. 2012;29(10):783-791.
14. Tzimos A, Samokhvalov V, Kramer M, et al. Safety and tolerability of oral paliperidone extended-release tablets in elderly patients with schizophrenia: a double-blind, placebo-controlled study with six-month open-label extension. Am J Geriatr Psychiatry. 2008;16(1):31-43.
15. Howanitz E, Pardo M, Smelson DA, et al. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia. J Clin Psychiatry. 1999;60(1):41-44.
16. Sproule BA, Lake J, Mamo DC, et al. Are antipsychotic prescribing patterns different in older and younger adults?: a survey of 1357 psychiatric inpatients in Toronto. Can J Psychiatry. 2010;55(4):248-254.
17. Uchida H, Suzuki T, Mamo DC, et al. Effects of age and age of onset on prescribed antipsychotic dose in schizophrenia spectrum disorders: a survey of 1,418 patients in Japan. Am J Geriatr Psychiatry. 2008;16(7):584-593.
18. Graff-Guerrero A, Rajji TK, Mulsant BH, et al. Evaluation of antipsychotic dose reduction in late-life schizophrenia: a prospective dopamine D2/3 occupancy study. JAMA Psychiatry. 2015;72(9):927-934.
19. Khan A, Schwartz K, Stern C, et al. Mortality risk in patients with schizophrenia participating in premarketing atypical antipsychotic clinical trials. J Clin Psychiatry. 2007;68(12):1828-1833.
20. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: a systematic review. Schizophr Res. 2009;113(1):1-11.
21. Novick D, Haro JM, Perrin E, et al. Tolerability of outpatient antipsychotic treatment: 36-month results from the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Eur Neuropsychopharmacol. 2009;19(8):542-550.
22. Sajatovic M, Blow FC, Ignacio RV, et al. Age-related modifiers of clinical presentation and health service use among veterans with bipolar disorder. Psychiatr Serv. 2004;55(9):1014-1021.
23. Jeste DV, Alexopoulos GS, Bartels SJ, et al. Consensus statement on the upcoming crisis in geriatric mental health: research agenda for the next 2 decades. Arch Gen Psychiatry. 1999;56(9):848-853.
24. Sajatovic M, Chen P. Geriatric bipolar disorder. Psychiatr Clin North Am. 2011;34(2):319-333,vii.
25. Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older-age bipolar disorder from the International Society for Bipolar Disorders Task Force. Bipolar Disord. 2015;17(7):689-704.
26. Lala SV, Sajatovic M. Medical and psychiatric comorbidities among elderly individuals with bipolar disorder: a literature review. J Geriatr Psychiatry Neurol. 2012;25(1):20-25.
27. Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
28. Pillarella J, Higashi A, Alexander GC, et al. Trends in use of second-generation antipsychotics for treatment of bipolar disorder in the United States, 1998-2009. Psychiatr Serv. 2012;63(1):83-86.
29. De Fruyt J, Deschepper E, Audenaert K, et al. Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis. J Psychopharmacol. 2012;26(5):603-617.
30. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
31. Sajatovic M, Paulsson B. Quetiapine for the treatment of depressive episodes in adults aged 55 to 65 years with bipolar disorder. Paper presented at: American Association of Geriatric Psychiatry Annual Meeting; 2007; New Orleans, LA.
32. Sajatovic M, Forester B, Tsai J, et al. Efficacy and safety of lurasidone in older adults with bipolar depression: analysis of two double-blind, placebo-controlled studies. Paper presented at: American College of Neuropsychopharmacology (ACNP) 53rd Annual Meeting; 2014; Phoenix, AZ.
33. Sajatovic M, Coconcea N, Ignacio RV, et al. Aripiprazole therapy in 20 older adults with bipolar disorder: a 12-week, open-label trial. J Clin Psychiatry. 2008;69(1):41-46.
34. Sajatovic M, Dines P, Fuentes-Casiano E, et al. Asenapine in the treatment of older adults with bipolar disorder. Int J Geriatr Psychiatry. 2015;30(7):710-719.
35. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
36. Baruch Y, Tadger S, Plopski I, et al. Asenapine for elderly bipolar manic patients. J Affect Disord. 2013;145(1):130-132.
37. Beyer JL, Siegal A, Kennedy JS. Olanzapine, divalproex and placebo treatment, non-head to head comparisons of older adults acute mania. Paper presented at: 10th Congress of the International Psychogeriatric Association; 2001; Nice, France.
38. Al Jurdi RK, Marangell LB, Petersen NJ, et al. Prescription patterns of psychotropic medications in elderly compared with younger participants who achieved a “recovered” status in the systematic treatment enhancement program for bipolar disorder. Am J Geriatr Psychiatry. 2008;16(11):922-933.
39. Gildengers AG, Chung KH, Huang SH, et al. Neuroprogressive effects of lifetime illness duration in older adults with bipolar disorder. Bipolar Disord. 2014;16(6):617-623.
40. Ballard C, Lana MM, Theodoulou M, et al. A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial). PLoS Med. 2008;5(4):e76.
1. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
2. United Nations, Department of Economic and Social Affairs, Population Division. World population ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Accessed September 1, 2017.
3. Lawrence D, Kisely S, Pais J. The epidemiology of excess mortality in people with mental illness. Can J Psychiatry. 2010;55(12):752-760.
4. Cohen CI, Vahia I, Reyes P, et al. Focus on geriatric psychiatry: schizophrenia in later life: clinical symptoms and social well-being. Psychiatr Serv. 2008;59(3):232-234.
5. Jeste DV, Barak Y, Madhusoodanan S, et al. International multisite double-blind trial of the atypical antipsychotics risperidone and olanzapine in 175 elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2003;11(6):638-647.
6. Kalache SM, Mulsant BH, Davies SJ, et al. The impact of aging, cognition, and symptoms on functional competence in individuals with schizophrenia across the lifespan. Schizophr Bull. 2015;41(2):374-381.
7. Suzuki T, Remington G, Uchida H, et al. Management of schizophrenia in late life with antipsychotic medications: a qualitative review. Drugs Aging. 2011;28(12):961-980.
8. Mulsant BH, Pollock BG. Psychopharmacology. In: David C. Steffens DC, Blazer DG, Thakur ME (eds). The American Psychiatric Publishing Textbook of Geriatric Psychiatry, 5th Edition. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
9. Cohen CI, Meesters PD, Zhao J. New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry. 2015;2(4):340-350.
10. Marriott RG, Neil W, Waddingham S. Antipsychotic medication for elderly people with schizophrenia. Cochrane Database Syst Rev. 2006;(1):CD005580.
11. Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev. 2012(2):CD004162.
12. Scott J, Greenwald BS, Kramer E, et al. Atypical (second generation) antipsychotic treatment response in very late-onset schizophrenia-like psychosis. Int Psychogeriatr. 2011;23(5):742-748.
13. Rado J, Janicak PG. Pharmacological and clinical profile of recently approved second-generation antipsychotics: implications for treatment of schizophrenia in older patients. Drugs Aging. 2012;29(10):783-791.
14. Tzimos A, Samokhvalov V, Kramer M, et al. Safety and tolerability of oral paliperidone extended-release tablets in elderly patients with schizophrenia: a double-blind, placebo-controlled study with six-month open-label extension. Am J Geriatr Psychiatry. 2008;16(1):31-43.
15. Howanitz E, Pardo M, Smelson DA, et al. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia. J Clin Psychiatry. 1999;60(1):41-44.
16. Sproule BA, Lake J, Mamo DC, et al. Are antipsychotic prescribing patterns different in older and younger adults?: a survey of 1357 psychiatric inpatients in Toronto. Can J Psychiatry. 2010;55(4):248-254.
17. Uchida H, Suzuki T, Mamo DC, et al. Effects of age and age of onset on prescribed antipsychotic dose in schizophrenia spectrum disorders: a survey of 1,418 patients in Japan. Am J Geriatr Psychiatry. 2008;16(7):584-593.
18. Graff-Guerrero A, Rajji TK, Mulsant BH, et al. Evaluation of antipsychotic dose reduction in late-life schizophrenia: a prospective dopamine D2/3 occupancy study. JAMA Psychiatry. 2015;72(9):927-934.
19. Khan A, Schwartz K, Stern C, et al. Mortality risk in patients with schizophrenia participating in premarketing atypical antipsychotic clinical trials. J Clin Psychiatry. 2007;68(12):1828-1833.
20. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: a systematic review. Schizophr Res. 2009;113(1):1-11.
21. Novick D, Haro JM, Perrin E, et al. Tolerability of outpatient antipsychotic treatment: 36-month results from the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Eur Neuropsychopharmacol. 2009;19(8):542-550.
22. Sajatovic M, Blow FC, Ignacio RV, et al. Age-related modifiers of clinical presentation and health service use among veterans with bipolar disorder. Psychiatr Serv. 2004;55(9):1014-1021.
23. Jeste DV, Alexopoulos GS, Bartels SJ, et al. Consensus statement on the upcoming crisis in geriatric mental health: research agenda for the next 2 decades. Arch Gen Psychiatry. 1999;56(9):848-853.
24. Sajatovic M, Chen P. Geriatric bipolar disorder. Psychiatr Clin North Am. 2011;34(2):319-333,vii.
25. Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older-age bipolar disorder from the International Society for Bipolar Disorders Task Force. Bipolar Disord. 2015;17(7):689-704.
26. Lala SV, Sajatovic M. Medical and psychiatric comorbidities among elderly individuals with bipolar disorder: a literature review. J Geriatr Psychiatry Neurol. 2012;25(1):20-25.
27. Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
28. Pillarella J, Higashi A, Alexander GC, et al. Trends in use of second-generation antipsychotics for treatment of bipolar disorder in the United States, 1998-2009. Psychiatr Serv. 2012;63(1):83-86.
29. De Fruyt J, Deschepper E, Audenaert K, et al. Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis. J Psychopharmacol. 2012;26(5):603-617.
30. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
31. Sajatovic M, Paulsson B. Quetiapine for the treatment of depressive episodes in adults aged 55 to 65 years with bipolar disorder. Paper presented at: American Association of Geriatric Psychiatry Annual Meeting; 2007; New Orleans, LA.
32. Sajatovic M, Forester B, Tsai J, et al. Efficacy and safety of lurasidone in older adults with bipolar depression: analysis of two double-blind, placebo-controlled studies. Paper presented at: American College of Neuropsychopharmacology (ACNP) 53rd Annual Meeting; 2014; Phoenix, AZ.
33. Sajatovic M, Coconcea N, Ignacio RV, et al. Aripiprazole therapy in 20 older adults with bipolar disorder: a 12-week, open-label trial. J Clin Psychiatry. 2008;69(1):41-46.
34. Sajatovic M, Dines P, Fuentes-Casiano E, et al. Asenapine in the treatment of older adults with bipolar disorder. Int J Geriatr Psychiatry. 2015;30(7):710-719.
35. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
36. Baruch Y, Tadger S, Plopski I, et al. Asenapine for elderly bipolar manic patients. J Affect Disord. 2013;145(1):130-132.
37. Beyer JL, Siegal A, Kennedy JS. Olanzapine, divalproex and placebo treatment, non-head to head comparisons of older adults acute mania. Paper presented at: 10th Congress of the International Psychogeriatric Association; 2001; Nice, France.
38. Al Jurdi RK, Marangell LB, Petersen NJ, et al. Prescription patterns of psychotropic medications in elderly compared with younger participants who achieved a “recovered” status in the systematic treatment enhancement program for bipolar disorder. Am J Geriatr Psychiatry. 2008;16(11):922-933.
39. Gildengers AG, Chung KH, Huang SH, et al. Neuroprogressive effects of lifetime illness duration in older adults with bipolar disorder. Bipolar Disord. 2014;16(6):617-623.
40. Ballard C, Lana MM, Theodoulou M, et al. A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial). PLoS Med. 2008;5(4):e76.
Improving the recognition of borderline personality disorder
Borderline personality disorder (BPD) is associated with impaired psychosocial functioning,1-4 reduced health-related quality of life,5 high utilization of services,6,7 and excess mortality.8-10 Although BPD occurs in up to 40% of psychiatric inpatients11 and 10% of outpatients,12 it is underrecognized.13-15 Often, patients with BPD do not receive an accurate diagnosis until ≥10 years after initially seeking treatment.16 The treatment and clinical implications of failing to recognize BPD include overprescribing medication and underutilizing empirically effective psychotherapies.14
This review summarizes studies of the underdiagnosis of BPD in routine clinical practice, describes which patients should be screened, and reviews alternative approaches to screening.
Underrecognition of BPD
The Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project is an ongoing clinical research study involving the integration of research assessment methods into routine clinical practice.17 In an early report from the MIDAS project, BPD diagnoses derived from structured and unstructured clinical interviews were compared between 2 groups of psychiatric outpatients in the same practice.15 Individuals in the structured interview cohort were 35 times more often diagnosed with BPD than individuals evaluated with an unstructured clinical interview. Importantly, when the information from the structured interview was presented to the clinicians, BPD was more likely to be diagnosed clinically.
Other studies13,16 also found that the rate of diagnosing BPD was higher when the diagnosis was based on a semi-structured diagnostic interview compared with an unstructured clinical interview, and that clinicians were reluctant to diagnose BPD during their routine intake diagnostic evaluation.
Clinicians, however, do not use semi-structured interviews in their practice, and they also do not tend to diagnose personality disorders (PDs) based on direct questioning, as they typically would when assessing a symptom-based disorder such as depression or anxiety. Rather, clinicians report that they rely on longitudinal observations to diagnose PDs.18 However, the results from the MIDAS project were inconsistent with clinicians’ reports. When clinicians were presented with the results of the semi-structured interview, they usually would diagnose BPD, even though it was the initial evaluation. If clinicians actually relied on longitudinal observations and considered information based on the direct question approach of research interviews to be irrelevant or invalid, then the results from the semi-structured interview should not have influenced the rate at which they diagnosed BPD. This suggests that the primary issue in diagnosing PDs is not the need for longitudinal observation but rather the need for more information, and that there is a role for screening questionnaires.
One potential criticism of studies demonstrating underrecognition of BPD in clinical practice is that patients typically were interviewed when they presented for treatment, when most were depressed or anxious. The possible pathologizing effects of psychiatric state on personality have been known for years.19 However, a large body of literature examining the treatment, prognostic, familial, and biological correlates of PDs supports the validity of diagnosing PDs in this manner. Moreover, from a clinical perspective, the sooner a clinician is aware of the presence of BPD, the more likely this information can be used for treatment planning.
Who should be screened for BPD?
BPD is underrecognized and underdiagnosed because patients with BPD often also have comorbid mood, anxiety, or substance use disorders.20,21 The symptoms associated with these disorders are typically the chief concern of patients with undiagnosed BPD who present for treatment. Patients with BPD rarely present for an intake evaluation and state that they are struggling with abandonment fears, chronic feelings of emptiness, or an identity disturbance. If patients identified these problems as their chief concerns, BPD would be easier to recognize.
Although several studies have documented the frequency of BPD in patients with a specific psychiatric diagnosis such as major depressive disorder (MDD) or attention-deficit/hyperactivity disorder,22-26 the MIDAS project examined the frequency of BPD in patients with various diagnoses and evaluated which disorders were associated with a significantly increased rate of BPD.27 The highest rate of BPD was found in patients with bipolar disorder. Approximately 25% of patients with bipolar II disorder and one-third of those with bipolar I disorder were diagnosed with BPD; these rates were significantly higher than the rate of BPD in patients without these disorders (Table 127). The rate of BPD was second highest in patients with a principal diagnosis of posttraumatic stress disorder (PTSD) and MDD; however, the rate of BPD in these patients was not significantly elevated compared with patients who did not have these principal diagnoses. Three disorders were associated with a significantly lower rate of BPD: adjustment disorder, dysthymic disorder, and generalized anxiety disorder.
It would be easy to recommend screening for BPD in all psychiatric patients. However, that is not feasible or practical. In making screening recommendations, absolute risk should be considered more important than relative risk. Clinicians should screen for BPD in patients presenting to a general psychiatric outpatient practice with a principal diagnosis of MDD, bipolar disorder, PTSD, or panic disorder with agoraphobia. That is, I recommend screening for BPD in patients with a principal diagnosis in which the prevalence of BPD is ≥10% (Table 127).
A brief review of screening statistics
Screening tests for most psychiatric disorders are based on multi-item scales in which a total score is computed from a sum of item scores, and a cutoff point is established to determine who does and does not screen positive on the test. However, sensitivity, specificity, and positive and negative predictive values are not invariant properties of a screening test with a continuous score distribution. Rather, the performance statistics of a scale can be altered by changing the threshold score to distinguish cases from non-cases. When the screening threshold is lowered, sensitivity increases and specificity decreases.
For screening, a broad net needs to be cast so that all (or almost all) cases are included. Therefore, the cutoff score should be set low to prioritize the sensitivity of the instrument. A screening scale also should have high negative predictive value so that the clinician can be confident that patients who screen negative on the test do not have the disorder.
Screening questionnaires for BPD
Several questionnaires have been developed to screen for PDs (Table 228-35). Some screen for each of the DSM PDs,28,36-42 and some screen more broadly for the presence or absence of any PD.29,43,44 The most commonly studied self-report scale for BPD is the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD),30 a 10-item self-report scale derived from a subset of questions from the BPD module of a semi-structured diagnostic interview.
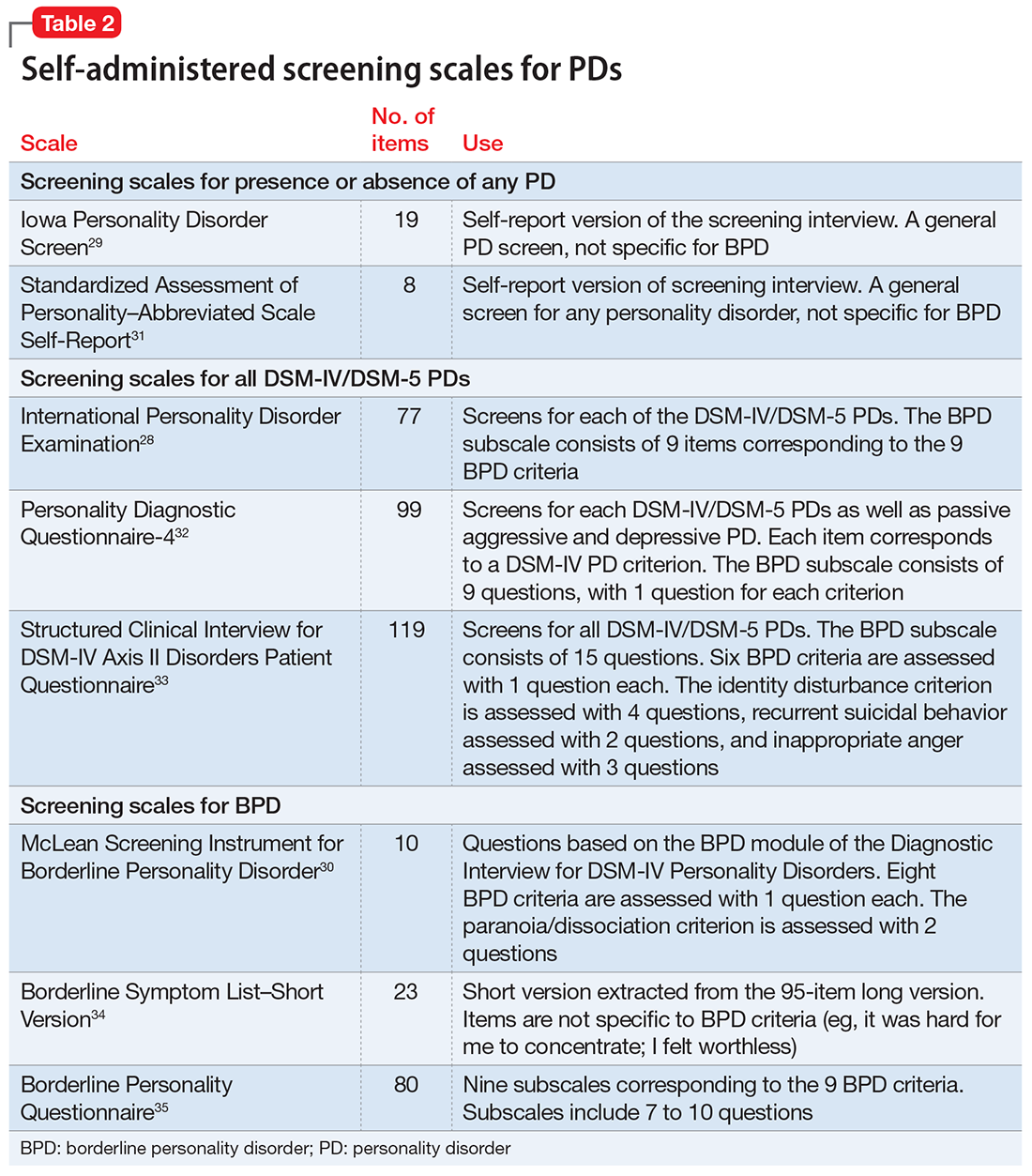
The initial validation study30 found that the optimal cutoff score was 7, which resulted in a sensitivity of 81% and specificity of 89%. Three studies have evaluated the scale in adolescents and young adults,45-47 and 3 studies examined the scale in adult outpatients.48-50 Across all 6 studies, at the optimal cutoff scores determined in each study, the sensitivity of the MSI-BPD ranged from 68% to 94% (mean, 80%) and the specificity ranged from 66% to 80% (mean, 72%).
Problems with screening questionnaires. Although screening scales have been developed for many psychiatric disorders, they have not been widely used in mental health settings. In a previous commentary, I argued that the conceptual justification for using self-report screening scales for single disorders in psychiatric settings is questionable.51 Another problem with screening scales is their potential misuse as case-finding instruments. In the literature on bipolar disorder screening, several researchers misconstrued a positive screen to indicate caseness.51 Although this is not a problem with the screening measures or the selection of a cutoff score, caution must be taken to not confuse screening with diagnosis.52
Screening for BPD as part of your diagnostic interview
An alternative approach to using self-administered questionnaires for screening is for clinicians to include questions in their evaluation as part of a psychiatric review of systems. When conducting a diagnostic interview, clinicians typically screen for disorders that are comorbid with the principal diagnosis by asking about the comorbid disorders’ necessary features or “gate criteria.” For example, in a patient with a principal diagnosis of MDD, the clinician would inquire about the presence of panic attacks, excessive worry, or substance use to screen for the presence of panic disorder, generalized anxiety disorder, or a substance use disorder. In contrast, for polythetically defined disorders such as BPD, there is no single gate criterion, because the disorder is diagnosed based on the presence of at least 5 of 9 criteria and no single one of these criteria is required to be present to establish the diagnosis.
As part of the MIDAS project, the psychometric properties of the BPD criteria were examined to determine if it was possible to identify 1 or 2 criteria that could serve as gate criteria to screen for the disorder. If the sensitivity of 1 criterion or a combination of 2 BPD criteria was sufficiently high (ie, >90%), then the assessment of this criterion (or these criteria) could be included in a psychiatric review of systems, thus potentially improving the detection of BPD. Researchers hypothesized that affective instability, considered first by Linehan53 and later by other theorists54 to be of central importance to the clinical manifestations of BPD, could function as a gate criterion. In the sample of 3,674 psychiatric outpatients who were evaluated with a semi-structured interview, the sensitivity of the affective instability criterion was 92.8%, and the negative predictive value of the criterion was 99%.
Identifying a single BPD criterion that is present in the vast majority of patients diagnosed with BPD will allow clinicians to follow their usual clinical practice when conducting a psychiatric review of systems and inquire about the gate criteria of various disorders. Several studies have found that >90% of patients with BPD report affective instability. However, this does not mean that the diagnosis of BPD can be abbreviated to an assessment of the presence or absence of affective instability. Many patients who screen positive will not have BPD when a more definitive diagnostic evaluation is conducted. In the case of BPD, the more costly definitive diagnostic procedure simply entails inquiry of the other diagnostic criteria.
1. Bellino S, Patria L, Paradiso E, et al. Major depression in patients with borderline personality disorder: a clinical investigation. Can J Psychiatry. 2005;50(4):234-238.
2. Skodol AE, Gunderson JG, McGlashan TH, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-283.
3. Gunderson JG, Stout RL, McGlashan TH, et al. Ten-year course of borderline personality disorder: psychopathology and function from the Collaborative Longitudinal Personality Disorders study. Arch Gen Psychiatry. 2011;68(8):827-837.
4. Zanarini MC, Jacoby RJ, Frankenburg FR, et al. The 10-year course of social security disability income reported by patients with borderline personality disorder and axis II comparison subjects. J Pers Disord. 2009;23(4):346-356.
5. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(4):533-545.
6. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
7. Zanarini MC, Frankenburg FR, Hennen J, et al. Mental health service utilization by borderline personality disorder patients and Axis II comparison subjects followed prospectively for 6 years. J Clin Psychiatry. 2004;65(1):28-36.
8. Pompili M, Girardi P, Ruberto A, et al. Suicide in borderline personality disorder: a meta-analysis. Nord J Psychiatry. 2005;59(5):319-324.
9. Oldham JM. Borderline personality disorder and suicidality. Am J Psychiatry. 2006;163(1):20-26.
10. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
11. Marinangeli M, Butti G, Scinto A, et al. Patterns of comorbidity among DSM-III-R personality disorders. Psychopathology. 2000;33(2):69-74.
12. Zimmerman M, Rothschild L, Chelminski I. The frequency of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162(10):1911-1918.
13. Comtois KA, Carmel A. Borderline personality disorder and high utilization of inpatient psychiatric hospitalization: concordance between research and clinical diagnosis. J Behav Health Servi Res. 2016;43(2):272-280.
14. Paris J, Black DW. Borderline personality disorder and bipolar disorder: what is the difference and why does it matter? J Nerv Ment Dis. 2015;203(1):3-7.
15. Zimmerman M, Mattia JI. Differences between clinical and research practice in diagnosing borderline personality disorder. Am J Psychiatry. 1999;156(10):1570-1574.
16. Magnavita JJ, Levy KN, Critchfield KL, et al. Ethical considerations in treatment of personality dysfunction: using evidence, principles, and clinical judgment. Professional Psychology: Research and Practice. 2010;41(1):64-74.
17. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
18. Westen D. Divergences between clinical and research methods for assessing personality disorders: implications for research and the evolution of axis II. Am J Psychiatry. 1997;154(7):895-903.
19. Zimmerman M. Diagnosing personality disorders: a review of issues and research methods. Arch Gen Psychiatry. 1994;51(3):225-245.
20. Zanarini MC, Gunderson JG, Frankenberg FR. Axis I phenomenology of borderline personality disorder. Compr Psychiatry. 1989;30(2):149-156.
21. Zimmerman M, Mattia JI. Axis I diagnostic comorbidity and borderline personality disorder. Compr Psychiatry. 1999;40(4):245-252.
22. Gunderson JG, Morey LC, Stout RL, et al. Major depressive disorder and borderline personality disorder revisited: longitudinal interactions. J Clin Psychiatry. 2004;65(8):1049-1056.
23. Bayes AJ, Parker GB. Clinical vs. DSM diagnosis of bipolar disorder, borderline personality disorder and their co-occurrence. Acta Psychiatr Scand. 2016;135(3):259-265.
24. Carpenter RW, Wood PK, Trull TJ. Comorbidity of borderline personality disorder and lifetime substance use disorders in a nationally representative sample. J Pers Disord. 2016;30(3):336-350.
25. Trull TJ, Sher KJ, Minks-Brown C, et al. Borderline personality disorder and substance use disorders: a review and integration. Clin Psychol Rev. 2000;20(2):235-253.
26. Matthies SD, Philipsen A. Common ground in attention deficit hyperactivity disorder (ADHD) and borderline personality disorder (BPD)-review of recent findings. Borderline Personal Disord Emot Dysregul. 2014;1:3.
27. Zimmerman M, Chelminski I, Dalrymple K, et al. Principal diagnoses in psychiatric outpatients with borderline personality disorder: implications for screening recommendations. Ann Clin Psychiatry. 2017;29(1):54-60.
28. Magallón-Neri EM, Forns M, Canalda G, et al. Usefulness of the International Personality Disorder Examination Screening Questionnaire for borderline and impulsive personality pathology in adolescents. Compr Psychiatry. 2013;54(3):301-308.
29. Germans S, Van Heck GL, Langbehn DR, et al. The Iowa Personality Disorder Screen. Eur J Psychol Assess. 2010;26(1):11-18.
30. Zanarini MC, Vujanovic AA, Parachini EA, et al. A screening measure for BPD: the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). J Pers Disord. 2003;17(6):568-573.
31. Germans S, Van Heck GL, Hodiamont PP. Results of the search for personality disorder screening tools: clinical implications. J Clin Psychiatry. 2012;73(2):165-173.
32. Hyler SE. Personality diagnostic questionnaire-4. New York, NY: New York State Psychiatric Institute; 1994.
33. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis II Disorders - Patient edition (SCID-I/P, version 2.0). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995.
34. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
35. Poreh AM, Rawlings D, Claridge G, et al. The BPQ: a scale for the assessment of boderline personality based on DSM-IV criteria. J Pers Disord. 2006;20(3):247-260.
36. Ekselius L, Lindstrom E, von Knorring L, et al. SCID II interviews and the SCID Screen questionnaire as diagnostic tools for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1994;90(2):120-123.
37. Hyler SE, Skodol AE, Oldham JM, et al. Validity of the Personality Diagnostic Questionnaire-Revised: a replication in an outpatient sample. Compr Psychiatry. 1992;33(2):73-77.
38. Davison S, Leese M, Taylor PJ. Examination of the screening properties of the personality diagnostic questionnaire 4+ (PDQ-4+) in a prison population. J Pers Disord. 2001;15(2):180-194.
39. Jacobsberg L, Perry S, Frances A. Diagnostic agreement between the SCID-II screening questionnaire and the Personality Disorder Examination. J Pers Assess. 1995;65(3):428-433.
40. Germans S, Van Heck GL, Masthoff ED, et al. Diagnostic efficiency among psychiatric outpatients of a self-report version of a subset of screen items of the Structured Clinical Interview for DSM-IV-TR Personality Disorders (SCID-II). Psychol Assess. 2010;22(4):945-952.
41. Lloyd C, Overall JE, Click M Jr. Screening for borderline personality disorders with the MMPI-168. J Clin Psychol. 1983;39(5):722-726.
42. Neal LA, Fox C, Carroll N, et al. Development and validation of a computerized screening test for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1997;95(4):351-356.
43. Germans S, Van Heck GL, Moran P, et al. The Self-Report Standardized Assessment of Personality-abbreviated Scale: preliminary results of a brief screening test for personality disorders. Pers Ment Health. 2008;2(2):70-76.
44. Moran P, Leese M, Lee T, et al. Standardized Assessment of Personality - Abbreviated Scale (SAPAS): preliminary validation of a brief screen for personality disorder. Br J Psychiatry. 2003;183:228-232.
45. Chanen AM, Jovev M, Djaja D, et al. Screening for borderline personality disorder in outpatient youth. J Pers Disord. 2008;22(4):353-364.
46. van Alebeek A, van der Heijden PT, Hessels C, et al. Comparison of three questionnaires to screen for borderline personality disorder in adolescents and young adults. Eur J Psychol Assess. 2017:33;123-128.
47. Noblin JL, Venta A, Sharp C. The validity of the MSI-BPD among inpatient adolescents. Assessment. 2014;21(2):210-217.
48. Kröger C, Vonau M, Kliem S, et al. Emotion dysregulation as a core feature of borderline personality disorder: comparison of the discriminatory ability of two self-rating measures. Psychopathology. 2011;44(4):253-260.
49. Soler J, Domínguez-Clav E, García-Rizo C, et al. Validation of the Spanish version of the McLean Screening Instrument for Borderline Personality Disorder. Rev Psiquiatr Salud Ment. 2016;9(4):195-202.
50. Melartin T, Häkkinen M, Koivisto M, et al. Screening of psychiatric outpatients for borderline personality disorder with the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). Nord J Psychiatry. 2009;63(6):475-479.
51. Zimmerman M. Misuse of the Mood Disorders Questionnaire as a case-finding measure and a critique of the concept of using a screening scale for bipolar disorder in psychiatric practice. Bipolar Disord. 2012;14(2):127-134.
52. Zimmerman M. Screening for bipolar disorder: confusion between case-finding and screening. Psychother Psychosom. 2014;83(5):259-262.
53. Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York, NY: Guilford Press; 1993.
54. Koenigsberg HW, Harvey PD, Mitropoulou V, et al. Are the interpersonal and identity disturbances in the borderline personality disorder criteria linked to the traits of affective instability and impulsivity? J Pers Disord. 2001;15(4):358-370.
55. Grilo CM, Becker DF, Anez LM, et al. Diagnostic efficiency of DSM-IV criteria for borderline personality disorder: an evaluation in Hispanic men and women with substance use disorders. J Consult Clin Psychol. 2004;72(1):126-131.
56. Korfine L, Hooley JM. Detecting individuals with borderline personality disorder in the community: an ascertainment strategy and comparison with a hospital sample. J Pers Disord. 2009;23(1):62-75.
57. Leppänen V, Lindeman S, Arntz A, et al. Preliminary evaluation of psychometric properties of the Finnish Borderline Personality Disorder Severity Index: Oulu-BPD-Study. Nord J Psychiatry. 2013;67(5):312-319.
58. Pfohl B, Coryell W, Zimmerman M, et al. DSM-III personality disorders: diagnostic overlap and internal consistency of individual DSM-III criteria. Compr Psychiatry. 1986;27(1):22-34.
59. Reich J. Criteria for diagnosing DSM-III borderline personality disorder. Ann Clin Psychiatry. 1990;2(3):189-197.
60. Nurnberg HG, Raskin M, Levine PE, et al. Hierarchy of DSM-III-R criteria efficiency for the diagnosis of borderline personality disorder. J Pers Disord. 1991;5(3):211-224.
61. Farmer RF, Chapman AL. Evaluation of DSM-IV personality disorder criteria as assessed by the structured clinical interview for DSM-IV personality disorders. Compr Psychiatry. 2002;43(4):285-300.
62. Grilo CM, McGlashan TH, Morey LC, et al. Internal consistency, intercriterion overlap and diagnostic efficiency of criteria sets for DSM-IV schizotypal, borderline, avoidant and obsessive-compulsive personality disorders. Acta Psychiatr Scand. 2001;104(4):264-272.
63. Grilo CM, Sanislow CA, Skodol AE, et al. Longitudinal diagnostic efficiency of DSM-IV criteria for borderline personality disorder: a 2-year prospective study. Can J Psychiatry. 2007;52(6):357-362.
Borderline personality disorder (BPD) is associated with impaired psychosocial functioning,1-4 reduced health-related quality of life,5 high utilization of services,6,7 and excess mortality.8-10 Although BPD occurs in up to 40% of psychiatric inpatients11 and 10% of outpatients,12 it is underrecognized.13-15 Often, patients with BPD do not receive an accurate diagnosis until ≥10 years after initially seeking treatment.16 The treatment and clinical implications of failing to recognize BPD include overprescribing medication and underutilizing empirically effective psychotherapies.14
This review summarizes studies of the underdiagnosis of BPD in routine clinical practice, describes which patients should be screened, and reviews alternative approaches to screening.
Underrecognition of BPD
The Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project is an ongoing clinical research study involving the integration of research assessment methods into routine clinical practice.17 In an early report from the MIDAS project, BPD diagnoses derived from structured and unstructured clinical interviews were compared between 2 groups of psychiatric outpatients in the same practice.15 Individuals in the structured interview cohort were 35 times more often diagnosed with BPD than individuals evaluated with an unstructured clinical interview. Importantly, when the information from the structured interview was presented to the clinicians, BPD was more likely to be diagnosed clinically.
Other studies13,16 also found that the rate of diagnosing BPD was higher when the diagnosis was based on a semi-structured diagnostic interview compared with an unstructured clinical interview, and that clinicians were reluctant to diagnose BPD during their routine intake diagnostic evaluation.
Clinicians, however, do not use semi-structured interviews in their practice, and they also do not tend to diagnose personality disorders (PDs) based on direct questioning, as they typically would when assessing a symptom-based disorder such as depression or anxiety. Rather, clinicians report that they rely on longitudinal observations to diagnose PDs.18 However, the results from the MIDAS project were inconsistent with clinicians’ reports. When clinicians were presented with the results of the semi-structured interview, they usually would diagnose BPD, even though it was the initial evaluation. If clinicians actually relied on longitudinal observations and considered information based on the direct question approach of research interviews to be irrelevant or invalid, then the results from the semi-structured interview should not have influenced the rate at which they diagnosed BPD. This suggests that the primary issue in diagnosing PDs is not the need for longitudinal observation but rather the need for more information, and that there is a role for screening questionnaires.
One potential criticism of studies demonstrating underrecognition of BPD in clinical practice is that patients typically were interviewed when they presented for treatment, when most were depressed or anxious. The possible pathologizing effects of psychiatric state on personality have been known for years.19 However, a large body of literature examining the treatment, prognostic, familial, and biological correlates of PDs supports the validity of diagnosing PDs in this manner. Moreover, from a clinical perspective, the sooner a clinician is aware of the presence of BPD, the more likely this information can be used for treatment planning.
Who should be screened for BPD?
BPD is underrecognized and underdiagnosed because patients with BPD often also have comorbid mood, anxiety, or substance use disorders.20,21 The symptoms associated with these disorders are typically the chief concern of patients with undiagnosed BPD who present for treatment. Patients with BPD rarely present for an intake evaluation and state that they are struggling with abandonment fears, chronic feelings of emptiness, or an identity disturbance. If patients identified these problems as their chief concerns, BPD would be easier to recognize.
Although several studies have documented the frequency of BPD in patients with a specific psychiatric diagnosis such as major depressive disorder (MDD) or attention-deficit/hyperactivity disorder,22-26 the MIDAS project examined the frequency of BPD in patients with various diagnoses and evaluated which disorders were associated with a significantly increased rate of BPD.27 The highest rate of BPD was found in patients with bipolar disorder. Approximately 25% of patients with bipolar II disorder and one-third of those with bipolar I disorder were diagnosed with BPD; these rates were significantly higher than the rate of BPD in patients without these disorders (Table 127). The rate of BPD was second highest in patients with a principal diagnosis of posttraumatic stress disorder (PTSD) and MDD; however, the rate of BPD in these patients was not significantly elevated compared with patients who did not have these principal diagnoses. Three disorders were associated with a significantly lower rate of BPD: adjustment disorder, dysthymic disorder, and generalized anxiety disorder.
It would be easy to recommend screening for BPD in all psychiatric patients. However, that is not feasible or practical. In making screening recommendations, absolute risk should be considered more important than relative risk. Clinicians should screen for BPD in patients presenting to a general psychiatric outpatient practice with a principal diagnosis of MDD, bipolar disorder, PTSD, or panic disorder with agoraphobia. That is, I recommend screening for BPD in patients with a principal diagnosis in which the prevalence of BPD is ≥10% (Table 127).
A brief review of screening statistics
Screening tests for most psychiatric disorders are based on multi-item scales in which a total score is computed from a sum of item scores, and a cutoff point is established to determine who does and does not screen positive on the test. However, sensitivity, specificity, and positive and negative predictive values are not invariant properties of a screening test with a continuous score distribution. Rather, the performance statistics of a scale can be altered by changing the threshold score to distinguish cases from non-cases. When the screening threshold is lowered, sensitivity increases and specificity decreases.
For screening, a broad net needs to be cast so that all (or almost all) cases are included. Therefore, the cutoff score should be set low to prioritize the sensitivity of the instrument. A screening scale also should have high negative predictive value so that the clinician can be confident that patients who screen negative on the test do not have the disorder.
Screening questionnaires for BPD
Several questionnaires have been developed to screen for PDs (Table 228-35). Some screen for each of the DSM PDs,28,36-42 and some screen more broadly for the presence or absence of any PD.29,43,44 The most commonly studied self-report scale for BPD is the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD),30 a 10-item self-report scale derived from a subset of questions from the BPD module of a semi-structured diagnostic interview.

The initial validation study30 found that the optimal cutoff score was 7, which resulted in a sensitivity of 81% and specificity of 89%. Three studies have evaluated the scale in adolescents and young adults,45-47 and 3 studies examined the scale in adult outpatients.48-50 Across all 6 studies, at the optimal cutoff scores determined in each study, the sensitivity of the MSI-BPD ranged from 68% to 94% (mean, 80%) and the specificity ranged from 66% to 80% (mean, 72%).
Problems with screening questionnaires. Although screening scales have been developed for many psychiatric disorders, they have not been widely used in mental health settings. In a previous commentary, I argued that the conceptual justification for using self-report screening scales for single disorders in psychiatric settings is questionable.51 Another problem with screening scales is their potential misuse as case-finding instruments. In the literature on bipolar disorder screening, several researchers misconstrued a positive screen to indicate caseness.51 Although this is not a problem with the screening measures or the selection of a cutoff score, caution must be taken to not confuse screening with diagnosis.52
Screening for BPD as part of your diagnostic interview
An alternative approach to using self-administered questionnaires for screening is for clinicians to include questions in their evaluation as part of a psychiatric review of systems. When conducting a diagnostic interview, clinicians typically screen for disorders that are comorbid with the principal diagnosis by asking about the comorbid disorders’ necessary features or “gate criteria.” For example, in a patient with a principal diagnosis of MDD, the clinician would inquire about the presence of panic attacks, excessive worry, or substance use to screen for the presence of panic disorder, generalized anxiety disorder, or a substance use disorder. In contrast, for polythetically defined disorders such as BPD, there is no single gate criterion, because the disorder is diagnosed based on the presence of at least 5 of 9 criteria and no single one of these criteria is required to be present to establish the diagnosis.
As part of the MIDAS project, the psychometric properties of the BPD criteria were examined to determine if it was possible to identify 1 or 2 criteria that could serve as gate criteria to screen for the disorder. If the sensitivity of 1 criterion or a combination of 2 BPD criteria was sufficiently high (ie, >90%), then the assessment of this criterion (or these criteria) could be included in a psychiatric review of systems, thus potentially improving the detection of BPD. Researchers hypothesized that affective instability, considered first by Linehan53 and later by other theorists54 to be of central importance to the clinical manifestations of BPD, could function as a gate criterion. In the sample of 3,674 psychiatric outpatients who were evaluated with a semi-structured interview, the sensitivity of the affective instability criterion was 92.8%, and the negative predictive value of the criterion was 99%.
Identifying a single BPD criterion that is present in the vast majority of patients diagnosed with BPD will allow clinicians to follow their usual clinical practice when conducting a psychiatric review of systems and inquire about the gate criteria of various disorders. Several studies have found that >90% of patients with BPD report affective instability. However, this does not mean that the diagnosis of BPD can be abbreviated to an assessment of the presence or absence of affective instability. Many patients who screen positive will not have BPD when a more definitive diagnostic evaluation is conducted. In the case of BPD, the more costly definitive diagnostic procedure simply entails inquiry of the other diagnostic criteria.
Borderline personality disorder (BPD) is associated with impaired psychosocial functioning,1-4 reduced health-related quality of life,5 high utilization of services,6,7 and excess mortality.8-10 Although BPD occurs in up to 40% of psychiatric inpatients11 and 10% of outpatients,12 it is underrecognized.13-15 Often, patients with BPD do not receive an accurate diagnosis until ≥10 years after initially seeking treatment.16 The treatment and clinical implications of failing to recognize BPD include overprescribing medication and underutilizing empirically effective psychotherapies.14
This review summarizes studies of the underdiagnosis of BPD in routine clinical practice, describes which patients should be screened, and reviews alternative approaches to screening.
Underrecognition of BPD
The Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project is an ongoing clinical research study involving the integration of research assessment methods into routine clinical practice.17 In an early report from the MIDAS project, BPD diagnoses derived from structured and unstructured clinical interviews were compared between 2 groups of psychiatric outpatients in the same practice.15 Individuals in the structured interview cohort were 35 times more often diagnosed with BPD than individuals evaluated with an unstructured clinical interview. Importantly, when the information from the structured interview was presented to the clinicians, BPD was more likely to be diagnosed clinically.
Other studies13,16 also found that the rate of diagnosing BPD was higher when the diagnosis was based on a semi-structured diagnostic interview compared with an unstructured clinical interview, and that clinicians were reluctant to diagnose BPD during their routine intake diagnostic evaluation.
Clinicians, however, do not use semi-structured interviews in their practice, and they also do not tend to diagnose personality disorders (PDs) based on direct questioning, as they typically would when assessing a symptom-based disorder such as depression or anxiety. Rather, clinicians report that they rely on longitudinal observations to diagnose PDs.18 However, the results from the MIDAS project were inconsistent with clinicians’ reports. When clinicians were presented with the results of the semi-structured interview, they usually would diagnose BPD, even though it was the initial evaluation. If clinicians actually relied on longitudinal observations and considered information based on the direct question approach of research interviews to be irrelevant or invalid, then the results from the semi-structured interview should not have influenced the rate at which they diagnosed BPD. This suggests that the primary issue in diagnosing PDs is not the need for longitudinal observation but rather the need for more information, and that there is a role for screening questionnaires.
One potential criticism of studies demonstrating underrecognition of BPD in clinical practice is that patients typically were interviewed when they presented for treatment, when most were depressed or anxious. The possible pathologizing effects of psychiatric state on personality have been known for years.19 However, a large body of literature examining the treatment, prognostic, familial, and biological correlates of PDs supports the validity of diagnosing PDs in this manner. Moreover, from a clinical perspective, the sooner a clinician is aware of the presence of BPD, the more likely this information can be used for treatment planning.
Who should be screened for BPD?
BPD is underrecognized and underdiagnosed because patients with BPD often also have comorbid mood, anxiety, or substance use disorders.20,21 The symptoms associated with these disorders are typically the chief concern of patients with undiagnosed BPD who present for treatment. Patients with BPD rarely present for an intake evaluation and state that they are struggling with abandonment fears, chronic feelings of emptiness, or an identity disturbance. If patients identified these problems as their chief concerns, BPD would be easier to recognize.
Although several studies have documented the frequency of BPD in patients with a specific psychiatric diagnosis such as major depressive disorder (MDD) or attention-deficit/hyperactivity disorder,22-26 the MIDAS project examined the frequency of BPD in patients with various diagnoses and evaluated which disorders were associated with a significantly increased rate of BPD.27 The highest rate of BPD was found in patients with bipolar disorder. Approximately 25% of patients with bipolar II disorder and one-third of those with bipolar I disorder were diagnosed with BPD; these rates were significantly higher than the rate of BPD in patients without these disorders (Table 127). The rate of BPD was second highest in patients with a principal diagnosis of posttraumatic stress disorder (PTSD) and MDD; however, the rate of BPD in these patients was not significantly elevated compared with patients who did not have these principal diagnoses. Three disorders were associated with a significantly lower rate of BPD: adjustment disorder, dysthymic disorder, and generalized anxiety disorder.
It would be easy to recommend screening for BPD in all psychiatric patients. However, that is not feasible or practical. In making screening recommendations, absolute risk should be considered more important than relative risk. Clinicians should screen for BPD in patients presenting to a general psychiatric outpatient practice with a principal diagnosis of MDD, bipolar disorder, PTSD, or panic disorder with agoraphobia. That is, I recommend screening for BPD in patients with a principal diagnosis in which the prevalence of BPD is ≥10% (Table 127).
A brief review of screening statistics
Screening tests for most psychiatric disorders are based on multi-item scales in which a total score is computed from a sum of item scores, and a cutoff point is established to determine who does and does not screen positive on the test. However, sensitivity, specificity, and positive and negative predictive values are not invariant properties of a screening test with a continuous score distribution. Rather, the performance statistics of a scale can be altered by changing the threshold score to distinguish cases from non-cases. When the screening threshold is lowered, sensitivity increases and specificity decreases.
For screening, a broad net needs to be cast so that all (or almost all) cases are included. Therefore, the cutoff score should be set low to prioritize the sensitivity of the instrument. A screening scale also should have high negative predictive value so that the clinician can be confident that patients who screen negative on the test do not have the disorder.
Screening questionnaires for BPD
Several questionnaires have been developed to screen for PDs (Table 228-35). Some screen for each of the DSM PDs,28,36-42 and some screen more broadly for the presence or absence of any PD.29,43,44 The most commonly studied self-report scale for BPD is the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD),30 a 10-item self-report scale derived from a subset of questions from the BPD module of a semi-structured diagnostic interview.

The initial validation study30 found that the optimal cutoff score was 7, which resulted in a sensitivity of 81% and specificity of 89%. Three studies have evaluated the scale in adolescents and young adults,45-47 and 3 studies examined the scale in adult outpatients.48-50 Across all 6 studies, at the optimal cutoff scores determined in each study, the sensitivity of the MSI-BPD ranged from 68% to 94% (mean, 80%) and the specificity ranged from 66% to 80% (mean, 72%).
Problems with screening questionnaires. Although screening scales have been developed for many psychiatric disorders, they have not been widely used in mental health settings. In a previous commentary, I argued that the conceptual justification for using self-report screening scales for single disorders in psychiatric settings is questionable.51 Another problem with screening scales is their potential misuse as case-finding instruments. In the literature on bipolar disorder screening, several researchers misconstrued a positive screen to indicate caseness.51 Although this is not a problem with the screening measures or the selection of a cutoff score, caution must be taken to not confuse screening with diagnosis.52
Screening for BPD as part of your diagnostic interview
An alternative approach to using self-administered questionnaires for screening is for clinicians to include questions in their evaluation as part of a psychiatric review of systems. When conducting a diagnostic interview, clinicians typically screen for disorders that are comorbid with the principal diagnosis by asking about the comorbid disorders’ necessary features or “gate criteria.” For example, in a patient with a principal diagnosis of MDD, the clinician would inquire about the presence of panic attacks, excessive worry, or substance use to screen for the presence of panic disorder, generalized anxiety disorder, or a substance use disorder. In contrast, for polythetically defined disorders such as BPD, there is no single gate criterion, because the disorder is diagnosed based on the presence of at least 5 of 9 criteria and no single one of these criteria is required to be present to establish the diagnosis.
As part of the MIDAS project, the psychometric properties of the BPD criteria were examined to determine if it was possible to identify 1 or 2 criteria that could serve as gate criteria to screen for the disorder. If the sensitivity of 1 criterion or a combination of 2 BPD criteria was sufficiently high (ie, >90%), then the assessment of this criterion (or these criteria) could be included in a psychiatric review of systems, thus potentially improving the detection of BPD. Researchers hypothesized that affective instability, considered first by Linehan53 and later by other theorists54 to be of central importance to the clinical manifestations of BPD, could function as a gate criterion. In the sample of 3,674 psychiatric outpatients who were evaluated with a semi-structured interview, the sensitivity of the affective instability criterion was 92.8%, and the negative predictive value of the criterion was 99%.
Identifying a single BPD criterion that is present in the vast majority of patients diagnosed with BPD will allow clinicians to follow their usual clinical practice when conducting a psychiatric review of systems and inquire about the gate criteria of various disorders. Several studies have found that >90% of patients with BPD report affective instability. However, this does not mean that the diagnosis of BPD can be abbreviated to an assessment of the presence or absence of affective instability. Many patients who screen positive will not have BPD when a more definitive diagnostic evaluation is conducted. In the case of BPD, the more costly definitive diagnostic procedure simply entails inquiry of the other diagnostic criteria.
1. Bellino S, Patria L, Paradiso E, et al. Major depression in patients with borderline personality disorder: a clinical investigation. Can J Psychiatry. 2005;50(4):234-238.
2. Skodol AE, Gunderson JG, McGlashan TH, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-283.
3. Gunderson JG, Stout RL, McGlashan TH, et al. Ten-year course of borderline personality disorder: psychopathology and function from the Collaborative Longitudinal Personality Disorders study. Arch Gen Psychiatry. 2011;68(8):827-837.
4. Zanarini MC, Jacoby RJ, Frankenburg FR, et al. The 10-year course of social security disability income reported by patients with borderline personality disorder and axis II comparison subjects. J Pers Disord. 2009;23(4):346-356.
5. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(4):533-545.
6. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
7. Zanarini MC, Frankenburg FR, Hennen J, et al. Mental health service utilization by borderline personality disorder patients and Axis II comparison subjects followed prospectively for 6 years. J Clin Psychiatry. 2004;65(1):28-36.
8. Pompili M, Girardi P, Ruberto A, et al. Suicide in borderline personality disorder: a meta-analysis. Nord J Psychiatry. 2005;59(5):319-324.
9. Oldham JM. Borderline personality disorder and suicidality. Am J Psychiatry. 2006;163(1):20-26.
10. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
11. Marinangeli M, Butti G, Scinto A, et al. Patterns of comorbidity among DSM-III-R personality disorders. Psychopathology. 2000;33(2):69-74.
12. Zimmerman M, Rothschild L, Chelminski I. The frequency of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162(10):1911-1918.
13. Comtois KA, Carmel A. Borderline personality disorder and high utilization of inpatient psychiatric hospitalization: concordance between research and clinical diagnosis. J Behav Health Servi Res. 2016;43(2):272-280.
14. Paris J, Black DW. Borderline personality disorder and bipolar disorder: what is the difference and why does it matter? J Nerv Ment Dis. 2015;203(1):3-7.
15. Zimmerman M, Mattia JI. Differences between clinical and research practice in diagnosing borderline personality disorder. Am J Psychiatry. 1999;156(10):1570-1574.
16. Magnavita JJ, Levy KN, Critchfield KL, et al. Ethical considerations in treatment of personality dysfunction: using evidence, principles, and clinical judgment. Professional Psychology: Research and Practice. 2010;41(1):64-74.
17. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
18. Westen D. Divergences between clinical and research methods for assessing personality disorders: implications for research and the evolution of axis II. Am J Psychiatry. 1997;154(7):895-903.
19. Zimmerman M. Diagnosing personality disorders: a review of issues and research methods. Arch Gen Psychiatry. 1994;51(3):225-245.
20. Zanarini MC, Gunderson JG, Frankenberg FR. Axis I phenomenology of borderline personality disorder. Compr Psychiatry. 1989;30(2):149-156.
21. Zimmerman M, Mattia JI. Axis I diagnostic comorbidity and borderline personality disorder. Compr Psychiatry. 1999;40(4):245-252.
22. Gunderson JG, Morey LC, Stout RL, et al. Major depressive disorder and borderline personality disorder revisited: longitudinal interactions. J Clin Psychiatry. 2004;65(8):1049-1056.
23. Bayes AJ, Parker GB. Clinical vs. DSM diagnosis of bipolar disorder, borderline personality disorder and their co-occurrence. Acta Psychiatr Scand. 2016;135(3):259-265.
24. Carpenter RW, Wood PK, Trull TJ. Comorbidity of borderline personality disorder and lifetime substance use disorders in a nationally representative sample. J Pers Disord. 2016;30(3):336-350.
25. Trull TJ, Sher KJ, Minks-Brown C, et al. Borderline personality disorder and substance use disorders: a review and integration. Clin Psychol Rev. 2000;20(2):235-253.
26. Matthies SD, Philipsen A. Common ground in attention deficit hyperactivity disorder (ADHD) and borderline personality disorder (BPD)-review of recent findings. Borderline Personal Disord Emot Dysregul. 2014;1:3.
27. Zimmerman M, Chelminski I, Dalrymple K, et al. Principal diagnoses in psychiatric outpatients with borderline personality disorder: implications for screening recommendations. Ann Clin Psychiatry. 2017;29(1):54-60.
28. Magallón-Neri EM, Forns M, Canalda G, et al. Usefulness of the International Personality Disorder Examination Screening Questionnaire for borderline and impulsive personality pathology in adolescents. Compr Psychiatry. 2013;54(3):301-308.
29. Germans S, Van Heck GL, Langbehn DR, et al. The Iowa Personality Disorder Screen. Eur J Psychol Assess. 2010;26(1):11-18.
30. Zanarini MC, Vujanovic AA, Parachini EA, et al. A screening measure for BPD: the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). J Pers Disord. 2003;17(6):568-573.
31. Germans S, Van Heck GL, Hodiamont PP. Results of the search for personality disorder screening tools: clinical implications. J Clin Psychiatry. 2012;73(2):165-173.
32. Hyler SE. Personality diagnostic questionnaire-4. New York, NY: New York State Psychiatric Institute; 1994.
33. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis II Disorders - Patient edition (SCID-I/P, version 2.0). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995.
34. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
35. Poreh AM, Rawlings D, Claridge G, et al. The BPQ: a scale for the assessment of boderline personality based on DSM-IV criteria. J Pers Disord. 2006;20(3):247-260.
36. Ekselius L, Lindstrom E, von Knorring L, et al. SCID II interviews and the SCID Screen questionnaire as diagnostic tools for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1994;90(2):120-123.
37. Hyler SE, Skodol AE, Oldham JM, et al. Validity of the Personality Diagnostic Questionnaire-Revised: a replication in an outpatient sample. Compr Psychiatry. 1992;33(2):73-77.
38. Davison S, Leese M, Taylor PJ. Examination of the screening properties of the personality diagnostic questionnaire 4+ (PDQ-4+) in a prison population. J Pers Disord. 2001;15(2):180-194.
39. Jacobsberg L, Perry S, Frances A. Diagnostic agreement between the SCID-II screening questionnaire and the Personality Disorder Examination. J Pers Assess. 1995;65(3):428-433.
40. Germans S, Van Heck GL, Masthoff ED, et al. Diagnostic efficiency among psychiatric outpatients of a self-report version of a subset of screen items of the Structured Clinical Interview for DSM-IV-TR Personality Disorders (SCID-II). Psychol Assess. 2010;22(4):945-952.
41. Lloyd C, Overall JE, Click M Jr. Screening for borderline personality disorders with the MMPI-168. J Clin Psychol. 1983;39(5):722-726.
42. Neal LA, Fox C, Carroll N, et al. Development and validation of a computerized screening test for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1997;95(4):351-356.
43. Germans S, Van Heck GL, Moran P, et al. The Self-Report Standardized Assessment of Personality-abbreviated Scale: preliminary results of a brief screening test for personality disorders. Pers Ment Health. 2008;2(2):70-76.
44. Moran P, Leese M, Lee T, et al. Standardized Assessment of Personality - Abbreviated Scale (SAPAS): preliminary validation of a brief screen for personality disorder. Br J Psychiatry. 2003;183:228-232.
45. Chanen AM, Jovev M, Djaja D, et al. Screening for borderline personality disorder in outpatient youth. J Pers Disord. 2008;22(4):353-364.
46. van Alebeek A, van der Heijden PT, Hessels C, et al. Comparison of three questionnaires to screen for borderline personality disorder in adolescents and young adults. Eur J Psychol Assess. 2017:33;123-128.
47. Noblin JL, Venta A, Sharp C. The validity of the MSI-BPD among inpatient adolescents. Assessment. 2014;21(2):210-217.
48. Kröger C, Vonau M, Kliem S, et al. Emotion dysregulation as a core feature of borderline personality disorder: comparison of the discriminatory ability of two self-rating measures. Psychopathology. 2011;44(4):253-260.
49. Soler J, Domínguez-Clav E, García-Rizo C, et al. Validation of the Spanish version of the McLean Screening Instrument for Borderline Personality Disorder. Rev Psiquiatr Salud Ment. 2016;9(4):195-202.
50. Melartin T, Häkkinen M, Koivisto M, et al. Screening of psychiatric outpatients for borderline personality disorder with the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). Nord J Psychiatry. 2009;63(6):475-479.
51. Zimmerman M. Misuse of the Mood Disorders Questionnaire as a case-finding measure and a critique of the concept of using a screening scale for bipolar disorder in psychiatric practice. Bipolar Disord. 2012;14(2):127-134.
52. Zimmerman M. Screening for bipolar disorder: confusion between case-finding and screening. Psychother Psychosom. 2014;83(5):259-262.
53. Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York, NY: Guilford Press; 1993.
54. Koenigsberg HW, Harvey PD, Mitropoulou V, et al. Are the interpersonal and identity disturbances in the borderline personality disorder criteria linked to the traits of affective instability and impulsivity? J Pers Disord. 2001;15(4):358-370.
55. Grilo CM, Becker DF, Anez LM, et al. Diagnostic efficiency of DSM-IV criteria for borderline personality disorder: an evaluation in Hispanic men and women with substance use disorders. J Consult Clin Psychol. 2004;72(1):126-131.
56. Korfine L, Hooley JM. Detecting individuals with borderline personality disorder in the community: an ascertainment strategy and comparison with a hospital sample. J Pers Disord. 2009;23(1):62-75.
57. Leppänen V, Lindeman S, Arntz A, et al. Preliminary evaluation of psychometric properties of the Finnish Borderline Personality Disorder Severity Index: Oulu-BPD-Study. Nord J Psychiatry. 2013;67(5):312-319.
58. Pfohl B, Coryell W, Zimmerman M, et al. DSM-III personality disorders: diagnostic overlap and internal consistency of individual DSM-III criteria. Compr Psychiatry. 1986;27(1):22-34.
59. Reich J. Criteria for diagnosing DSM-III borderline personality disorder. Ann Clin Psychiatry. 1990;2(3):189-197.
60. Nurnberg HG, Raskin M, Levine PE, et al. Hierarchy of DSM-III-R criteria efficiency for the diagnosis of borderline personality disorder. J Pers Disord. 1991;5(3):211-224.
61. Farmer RF, Chapman AL. Evaluation of DSM-IV personality disorder criteria as assessed by the structured clinical interview for DSM-IV personality disorders. Compr Psychiatry. 2002;43(4):285-300.
62. Grilo CM, McGlashan TH, Morey LC, et al. Internal consistency, intercriterion overlap and diagnostic efficiency of criteria sets for DSM-IV schizotypal, borderline, avoidant and obsessive-compulsive personality disorders. Acta Psychiatr Scand. 2001;104(4):264-272.
63. Grilo CM, Sanislow CA, Skodol AE, et al. Longitudinal diagnostic efficiency of DSM-IV criteria for borderline personality disorder: a 2-year prospective study. Can J Psychiatry. 2007;52(6):357-362.
1. Bellino S, Patria L, Paradiso E, et al. Major depression in patients with borderline personality disorder: a clinical investigation. Can J Psychiatry. 2005;50(4):234-238.
2. Skodol AE, Gunderson JG, McGlashan TH, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-283.
3. Gunderson JG, Stout RL, McGlashan TH, et al. Ten-year course of borderline personality disorder: psychopathology and function from the Collaborative Longitudinal Personality Disorders study. Arch Gen Psychiatry. 2011;68(8):827-837.
4. Zanarini MC, Jacoby RJ, Frankenburg FR, et al. The 10-year course of social security disability income reported by patients with borderline personality disorder and axis II comparison subjects. J Pers Disord. 2009;23(4):346-356.
5. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(4):533-545.
6. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
7. Zanarini MC, Frankenburg FR, Hennen J, et al. Mental health service utilization by borderline personality disorder patients and Axis II comparison subjects followed prospectively for 6 years. J Clin Psychiatry. 2004;65(1):28-36.
8. Pompili M, Girardi P, Ruberto A, et al. Suicide in borderline personality disorder: a meta-analysis. Nord J Psychiatry. 2005;59(5):319-324.
9. Oldham JM. Borderline personality disorder and suicidality. Am J Psychiatry. 2006;163(1):20-26.
10. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
11. Marinangeli M, Butti G, Scinto A, et al. Patterns of comorbidity among DSM-III-R personality disorders. Psychopathology. 2000;33(2):69-74.
12. Zimmerman M, Rothschild L, Chelminski I. The frequency of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162(10):1911-1918.
13. Comtois KA, Carmel A. Borderline personality disorder and high utilization of inpatient psychiatric hospitalization: concordance between research and clinical diagnosis. J Behav Health Servi Res. 2016;43(2):272-280.
14. Paris J, Black DW. Borderline personality disorder and bipolar disorder: what is the difference and why does it matter? J Nerv Ment Dis. 2015;203(1):3-7.
15. Zimmerman M, Mattia JI. Differences between clinical and research practice in diagnosing borderline personality disorder. Am J Psychiatry. 1999;156(10):1570-1574.
16. Magnavita JJ, Levy KN, Critchfield KL, et al. Ethical considerations in treatment of personality dysfunction: using evidence, principles, and clinical judgment. Professional Psychology: Research and Practice. 2010;41(1):64-74.
17. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
18. Westen D. Divergences between clinical and research methods for assessing personality disorders: implications for research and the evolution of axis II. Am J Psychiatry. 1997;154(7):895-903.
19. Zimmerman M. Diagnosing personality disorders: a review of issues and research methods. Arch Gen Psychiatry. 1994;51(3):225-245.
20. Zanarini MC, Gunderson JG, Frankenberg FR. Axis I phenomenology of borderline personality disorder. Compr Psychiatry. 1989;30(2):149-156.
21. Zimmerman M, Mattia JI. Axis I diagnostic comorbidity and borderline personality disorder. Compr Psychiatry. 1999;40(4):245-252.
22. Gunderson JG, Morey LC, Stout RL, et al. Major depressive disorder and borderline personality disorder revisited: longitudinal interactions. J Clin Psychiatry. 2004;65(8):1049-1056.
23. Bayes AJ, Parker GB. Clinical vs. DSM diagnosis of bipolar disorder, borderline personality disorder and their co-occurrence. Acta Psychiatr Scand. 2016;135(3):259-265.
24. Carpenter RW, Wood PK, Trull TJ. Comorbidity of borderline personality disorder and lifetime substance use disorders in a nationally representative sample. J Pers Disord. 2016;30(3):336-350.
25. Trull TJ, Sher KJ, Minks-Brown C, et al. Borderline personality disorder and substance use disorders: a review and integration. Clin Psychol Rev. 2000;20(2):235-253.
26. Matthies SD, Philipsen A. Common ground in attention deficit hyperactivity disorder (ADHD) and borderline personality disorder (BPD)-review of recent findings. Borderline Personal Disord Emot Dysregul. 2014;1:3.
27. Zimmerman M, Chelminski I, Dalrymple K, et al. Principal diagnoses in psychiatric outpatients with borderline personality disorder: implications for screening recommendations. Ann Clin Psychiatry. 2017;29(1):54-60.
28. Magallón-Neri EM, Forns M, Canalda G, et al. Usefulness of the International Personality Disorder Examination Screening Questionnaire for borderline and impulsive personality pathology in adolescents. Compr Psychiatry. 2013;54(3):301-308.
29. Germans S, Van Heck GL, Langbehn DR, et al. The Iowa Personality Disorder Screen. Eur J Psychol Assess. 2010;26(1):11-18.
30. Zanarini MC, Vujanovic AA, Parachini EA, et al. A screening measure for BPD: the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). J Pers Disord. 2003;17(6):568-573.
31. Germans S, Van Heck GL, Hodiamont PP. Results of the search for personality disorder screening tools: clinical implications. J Clin Psychiatry. 2012;73(2):165-173.
32. Hyler SE. Personality diagnostic questionnaire-4. New York, NY: New York State Psychiatric Institute; 1994.
33. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis II Disorders - Patient edition (SCID-I/P, version 2.0). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995.
34. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
35. Poreh AM, Rawlings D, Claridge G, et al. The BPQ: a scale for the assessment of boderline personality based on DSM-IV criteria. J Pers Disord. 2006;20(3):247-260.
36. Ekselius L, Lindstrom E, von Knorring L, et al. SCID II interviews and the SCID Screen questionnaire as diagnostic tools for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1994;90(2):120-123.
37. Hyler SE, Skodol AE, Oldham JM, et al. Validity of the Personality Diagnostic Questionnaire-Revised: a replication in an outpatient sample. Compr Psychiatry. 1992;33(2):73-77.
38. Davison S, Leese M, Taylor PJ. Examination of the screening properties of the personality diagnostic questionnaire 4+ (PDQ-4+) in a prison population. J Pers Disord. 2001;15(2):180-194.
39. Jacobsberg L, Perry S, Frances A. Diagnostic agreement between the SCID-II screening questionnaire and the Personality Disorder Examination. J Pers Assess. 1995;65(3):428-433.
40. Germans S, Van Heck GL, Masthoff ED, et al. Diagnostic efficiency among psychiatric outpatients of a self-report version of a subset of screen items of the Structured Clinical Interview for DSM-IV-TR Personality Disorders (SCID-II). Psychol Assess. 2010;22(4):945-952.
41. Lloyd C, Overall JE, Click M Jr. Screening for borderline personality disorders with the MMPI-168. J Clin Psychol. 1983;39(5):722-726.
42. Neal LA, Fox C, Carroll N, et al. Development and validation of a computerized screening test for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1997;95(4):351-356.
43. Germans S, Van Heck GL, Moran P, et al. The Self-Report Standardized Assessment of Personality-abbreviated Scale: preliminary results of a brief screening test for personality disorders. Pers Ment Health. 2008;2(2):70-76.
44. Moran P, Leese M, Lee T, et al. Standardized Assessment of Personality - Abbreviated Scale (SAPAS): preliminary validation of a brief screen for personality disorder. Br J Psychiatry. 2003;183:228-232.
45. Chanen AM, Jovev M, Djaja D, et al. Screening for borderline personality disorder in outpatient youth. J Pers Disord. 2008;22(4):353-364.
46. van Alebeek A, van der Heijden PT, Hessels C, et al. Comparison of three questionnaires to screen for borderline personality disorder in adolescents and young adults. Eur J Psychol Assess. 2017:33;123-128.
47. Noblin JL, Venta A, Sharp C. The validity of the MSI-BPD among inpatient adolescents. Assessment. 2014;21(2):210-217.
48. Kröger C, Vonau M, Kliem S, et al. Emotion dysregulation as a core feature of borderline personality disorder: comparison of the discriminatory ability of two self-rating measures. Psychopathology. 2011;44(4):253-260.
49. Soler J, Domínguez-Clav E, García-Rizo C, et al. Validation of the Spanish version of the McLean Screening Instrument for Borderline Personality Disorder. Rev Psiquiatr Salud Ment. 2016;9(4):195-202.
50. Melartin T, Häkkinen M, Koivisto M, et al. Screening of psychiatric outpatients for borderline personality disorder with the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). Nord J Psychiatry. 2009;63(6):475-479.
51. Zimmerman M. Misuse of the Mood Disorders Questionnaire as a case-finding measure and a critique of the concept of using a screening scale for bipolar disorder in psychiatric practice. Bipolar Disord. 2012;14(2):127-134.
52. Zimmerman M. Screening for bipolar disorder: confusion between case-finding and screening. Psychother Psychosom. 2014;83(5):259-262.
53. Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York, NY: Guilford Press; 1993.
54. Koenigsberg HW, Harvey PD, Mitropoulou V, et al. Are the interpersonal and identity disturbances in the borderline personality disorder criteria linked to the traits of affective instability and impulsivity? J Pers Disord. 2001;15(4):358-370.
55. Grilo CM, Becker DF, Anez LM, et al. Diagnostic efficiency of DSM-IV criteria for borderline personality disorder: an evaluation in Hispanic men and women with substance use disorders. J Consult Clin Psychol. 2004;72(1):126-131.
56. Korfine L, Hooley JM. Detecting individuals with borderline personality disorder in the community: an ascertainment strategy and comparison with a hospital sample. J Pers Disord. 2009;23(1):62-75.
57. Leppänen V, Lindeman S, Arntz A, et al. Preliminary evaluation of psychometric properties of the Finnish Borderline Personality Disorder Severity Index: Oulu-BPD-Study. Nord J Psychiatry. 2013;67(5):312-319.
58. Pfohl B, Coryell W, Zimmerman M, et al. DSM-III personality disorders: diagnostic overlap and internal consistency of individual DSM-III criteria. Compr Psychiatry. 1986;27(1):22-34.
59. Reich J. Criteria for diagnosing DSM-III borderline personality disorder. Ann Clin Psychiatry. 1990;2(3):189-197.
60. Nurnberg HG, Raskin M, Levine PE, et al. Hierarchy of DSM-III-R criteria efficiency for the diagnosis of borderline personality disorder. J Pers Disord. 1991;5(3):211-224.
61. Farmer RF, Chapman AL. Evaluation of DSM-IV personality disorder criteria as assessed by the structured clinical interview for DSM-IV personality disorders. Compr Psychiatry. 2002;43(4):285-300.
62. Grilo CM, McGlashan TH, Morey LC, et al. Internal consistency, intercriterion overlap and diagnostic efficiency of criteria sets for DSM-IV schizotypal, borderline, avoidant and obsessive-compulsive personality disorders. Acta Psychiatr Scand. 2001;104(4):264-272.
63. Grilo CM, Sanislow CA, Skodol AE, et al. Longitudinal diagnostic efficiency of DSM-IV criteria for borderline personality disorder: a 2-year prospective study. Can J Psychiatry. 2007;52(6):357-362.