User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Using antipsychotics for dementia
Sexting: What are the clinical and legal implications?
Sexting includes sending sexually explicit (or sexually suggestive) text messages and photos, usually by cell phone. This article focuses on sexts involving photos. Cell phones are almost ubiquitous among American teens, and with technological advances, sexts are getting easier to send. Sexting may occur to initiate a relationship or sustain one. Some teenagers are coerced into sexting. Many people do not realize the potential long-term consequences of sexting—particularly because of the impulsive nature of sexting and the belief that the behavior is harmless.
Media attention has recently focused on teens who face legal charges related to sexting. Sexting photos may be considered child pornography—even though the teens made it themselves. There are also social consequences to sexting. Photos meant to be private are sometimes forwarded to others. Cyberbullying is not uncommon with teen sexting, and suicides after experiencing this behavior have been reported.
Sexting may be a form of modern flirtation, but in some cases, it may be a marker of other risk behaviors, such as substance abuse. Psychiatrists must be aware of the frequency and meaning of this potentially dangerous behavior. Clinicians should feel comfortable asking their patients about it and provide education and counseling.
CASE
Private photos get shared
K, age 14, a freshman with no psychiatric history, is referred to you by her school psychologist for evaluation of suicidal ideation. K reports depressed mood, poor sleep, inattention, loss of appetite, anhedonia, and feelings of guilt for the past month. She recently ended a relationship with her boyfriend of 1 year after she learned that he had shared with his friends naked photos of her that she had sent him. The school administration learned of the photos when a student posted them on one of the school computers.
K’s boyfriend, age 16, was suspended after the school learned that he had shared the photos without K’s consent. K, who is a good student, missed several days of school, including cheerleading practice; previously she had never missed a day of school.
On evaluation, K is tearful, stating that her life is “over.” She says that her ex-boyfriend’s friends are harassing her, calling her “slut” and making sexual comments. She also feels guilty, because she learned that the police interviewed her ex-boyfriend in connection with posting her photos on the Internet. In a text, he said he “might get charged with child pornography.” On further questioning, K confides that she had naked photos of her ex-boyfriend on her phone. She admits to sharing the pictures with her best friend, because she was “angry and wanted to get back” at her ex-boyfriend. She also reports a several-month history of sexting with her ex-boyfriend. K deleted the photos and texts after learning that her ex-boyfriend “was in trouble with the police.”
K has no prior sexual experience. She dated 1 boy her age prior to her ex-boyfriend. She had never been evaluated by a mental health clinician. She is dysphoric and reports feeling “hopeless … Unless this can be erased, I can’t go back to school.”
Sexting: What is the extent of the problem?
The true prevalence of sexting is difficult to ascertain, because different studies have used different definitions and methodologies. However, the rates are far from negligible. Sexting rates increase with age, over the teen years.1-3 Among American minors, 2.5% to 28% of middle school and high school students report that they have sent a sext (Table 11-9). Studies of American young adults (age ≥18) and university students have found 30% to 60% have sent sexts, and >40% have received a sext.4,5
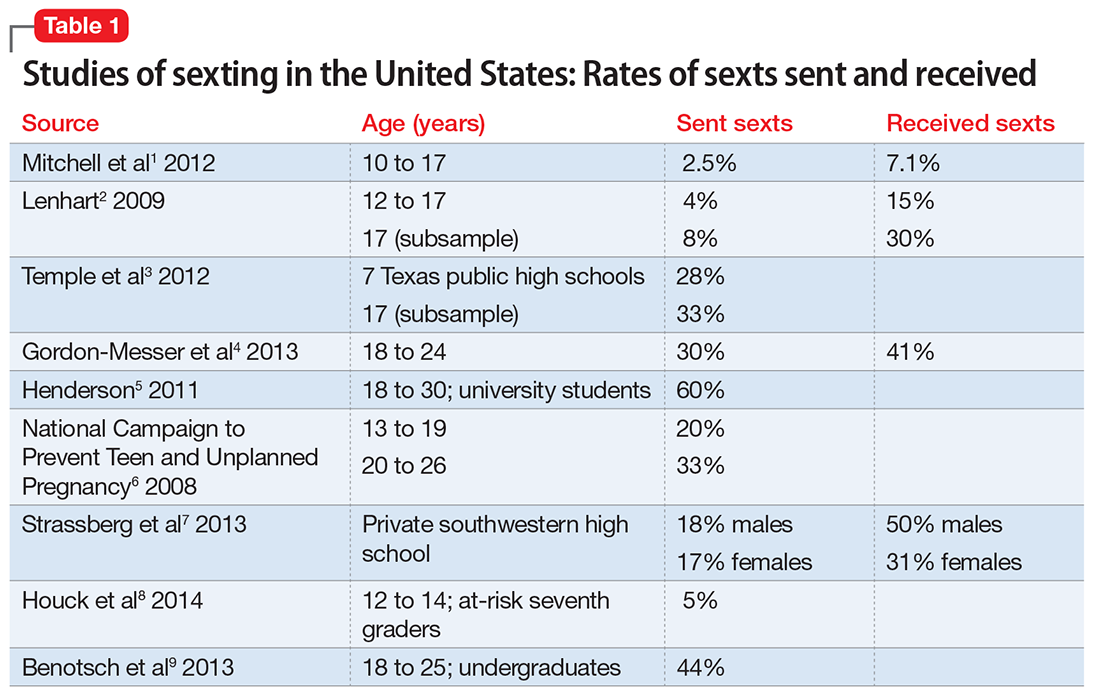
Many people receive sexts—including individuals who are not the intended recipient. In 1 study, although most teens intended to share sexts only with their boyfriend/girlfriend, 25% to 33% had received sext photos intended for other people.6 In another recent study, 25% of teens had forwarded a sext that they received.7 Moreover, 12% of teenage boys and 5% of teenage girls had sent a sexually explicit photo that they took of another teen to a third person.7 Forwarding sexts can add exponentially to the psychosocial risks of the photographed teenager.
Who sexts? Current research indicates that the likelihood of sexting is related to age, personality, and social situation. Teens are approaching the peak age of their sex drive, and often are curious and feel invincible. Teens are more impulsive than adults. When it takes less than a minute to send a sext, irreversible poor choices can be made quickly. Teens who send sexts often engage in more text messaging than other teens.7
Teens may use sexting to initiate or sustain a relationship. Sexts also may be sent because of coercion. More than one-half of girls cited pressure to sext from a boy.6 Temple et al3 found that more than one-half of their study sample had been asked to send a sext. Girls were more likely than boys to be asked to send a sext; most were troubled by this.
One study that assessed knowledge of potential legal consequences of sexting found that many teens who sent sexts were aware of the potential consequences.7 Regarding personality traits, sexting among undergraduates was predicted by neuroticism and low agreeableness.10 Conversely, sending text messages with sexually suggestive writing was predicted by extraversion and problematic cell phone use.
Comorbidities. There are mixed findings about whether sexting is simply a modern dating strategy or a marker of other risk behaviors; age may play an important discriminating role. Sexual activity appears to be correlated with sexting. According to Temple and Choi,11 “Sexting fits within the context of adolescent sexual development and may be a viable indicator of adolescent sexual activity.”11
Some authors have suggested that sexting is a contemporary risk behavior that is likely to correlate with other risk behaviors. Among young teens—seventh graders who were referred to a risk prevention trial because of behavioral/emotional difficulties—those who sexted were more likely to engage in early sexual behaviors.8 These younger at-risk teens also had less understanding of their emotions and greater difficulty in regulating their emotions.
Among the general population of high school students, teens who sext are more likely to be sexually active.3 High school girls who engaged in sexting were noted to engage in other risk behaviors, including having multiple partners in the past year and using alcohol or drugs before sex.3 Teens who had sent a sext were more likely to be sexually active 1 year later than teens who had not.11Studies of sexting among university students also have had mixed findings. One study found that among undergraduates, sexting was associated with substance use and other risk behaviors.9 Another young adult study found sexting was not related to sexual risk or psychological well-being.4
Legal issues affect psychiatrists as well as patients
As a psychiatrist evaluating K, what are your duties as a mandated reporter? Psychiatrists are legally required to report suspected maltreatment or abuse of children.12 The circumstances under which psychiatrists may have a mandate to report include when a psychiatrist:
- evaluates a child and suspects abuse
- suspects child abuse based on an adult patient’s report
- learns from a third party that a child may have been/is being abused.
Psychiatrists usually are not mandated to report other types of potentially criminal behavior. As such, reporting sexting might be considered a breach of confidentiality. Psychiatrists should be familiar with the specific reporting guidelines for the jurisdiction in which they practice. Psychiatrists who work with individuals who commit crimes should focus on changing the potentially dangerous behaviors rather than reporting them.
Does the transmission of naked photos of a minor in a sexual pose or act constitute child pornography or another criminal offense? The legal answer varies, but the role of the psychiatrist does not. Psychiatrists should educate their patients about potentially dangerous behaviors.
With regards to the legal consequences, some states classify underage sexting photos as child pornography. Others have less rigid definitions of child pornography and take into account the age of the participants and their intent. Such jurisdictions point out that sexting naked photos among adolescents is “age appropriate.” Some have enacted specific sexting laws to address the transmission of obscene material to a child through the Internet. In some jurisdictions, sexting laws are categorized to refer to behavior of individuals under or over age 18. The term “revenge porn” is used to refer to nonconsensual pornography with its dissemination motivated by spite.13 Some states have defined specific revenge porn laws to address the behavior. Currently, 20 states have sexting laws and 26 states have revenge porn laws.14 Twenty states address a minor age <18 sending the photo, while only 18 address the recipient. The law in this area can be complex and detailed, taking into account the age of the sender, the intentions of the sender, and the nature of the relationship between the sender and the recipient and the behavior of the recipient.
Laws regarding sexting vary greatly. Sexting may be a misdemeanor or a felony, depending on the state, the specific behavior, and the frequency. In the United States, 11 state laws include a diversion remedy—an option to pursue the case outside of the criminal juvenile system; 10 laws require counseling or another informal sanction; 11 states laws have the potential for misdemeanor punishment; and 4 state laws have the potential for felony punishment.14 Depending on the criminal charge, the perpetrator may have to register as a sex offender. For example, in some jurisdictions, a conviction for possession of child pornography requires sex offender registration. Thirty-eight states include juvenile sex offenders in their sex offender registries. Other states require juveniles to register if they are age ≥15 years or have been tried as an adult.15
The frequency of police involvement in sexting cases also greatly varies. A national study examining the characteristics of youth sexting cases revealed that law enforcement agencies handled approximately 3,477 cases of youth-produced sexual photos in 2008 and 2009.16 Situations that involved an adult or a minor engaged in malicious, nonconsensual, or abusive behavior comprised two-thirds of cases. Arrests occurred in 62% of the adult-involved cases and 36% of the aggravated youth-only cases. Arrests occurred in only 18% of investigated non-aggravated youth-only cases. Table 2 describes recent American sexting legal cases and their outcomes.
In K’s case, depending on the jurisdiction, K or her ex-boyfriend may be subject to arrest for child pornography, revenge pornography, or sexting.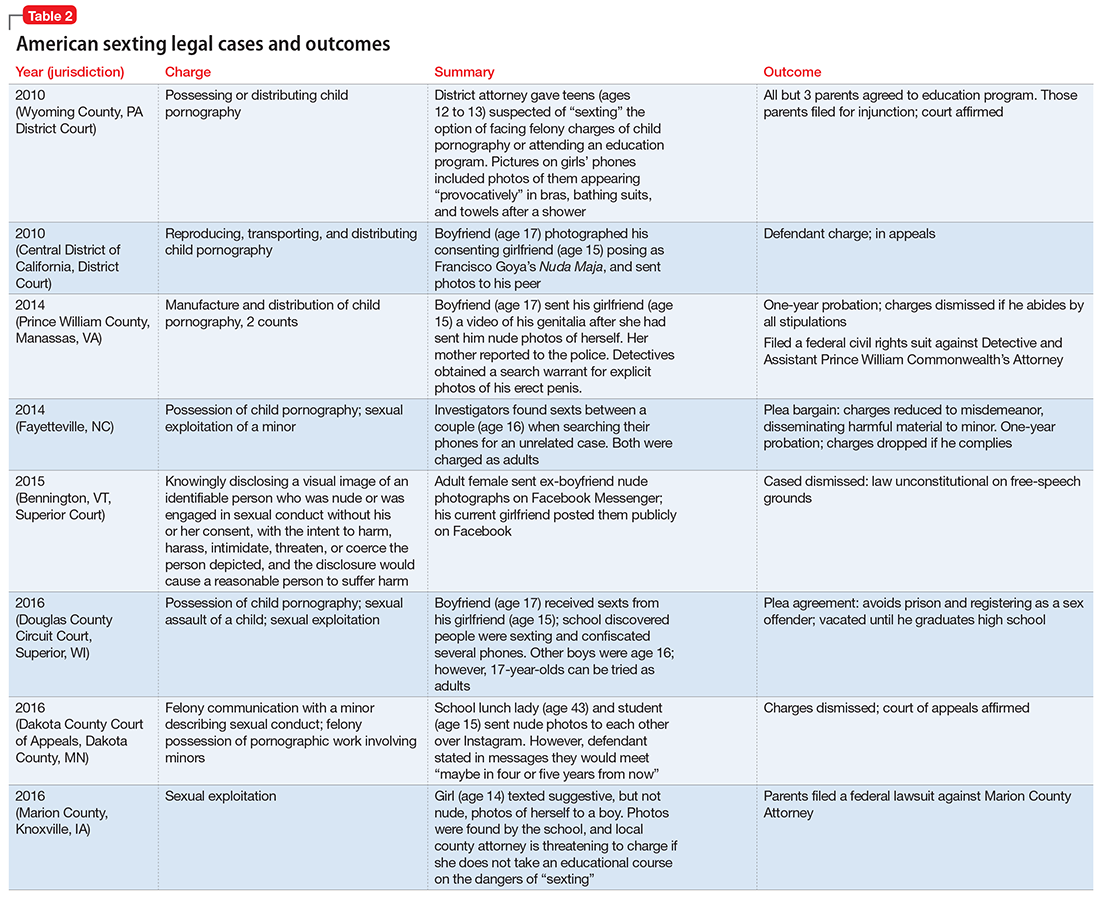
Potential social and psychiatric consequences
What are the social and psychiatric ramifications for K? Aside from potential legal consequences of sexting, K is experiencing psychological and social consequences. She has developed depressive symptoms and suicidal ideation. Her ex-boyfriend’s dissemination of her nude photos on the school computer could be interpreted as cyberbullying. (The National Center for Missing and Exploited Children defines cyberbullying as “bullying through the use of technology or electronic devices such as telephones, cell phones, computers, and the Internet.”17 All 50 states have enacted laws against bullying; 48 states have electronic harassment in their bullying laws; and 22 states have laws specifically referencing “cyberbullying.”)
Her depressive symptoms developed in response to her feelings of guilt and shame related to sexting as well as the subsequent peer harassment. She is refusing to return to school because of her concerns about bullying. A careful inquiry into suicidality should be part of the evaluation when sexting has led to psychiatric symptoms. Several cases of sexting and cyberbullying have ended in suicide (Table 3).
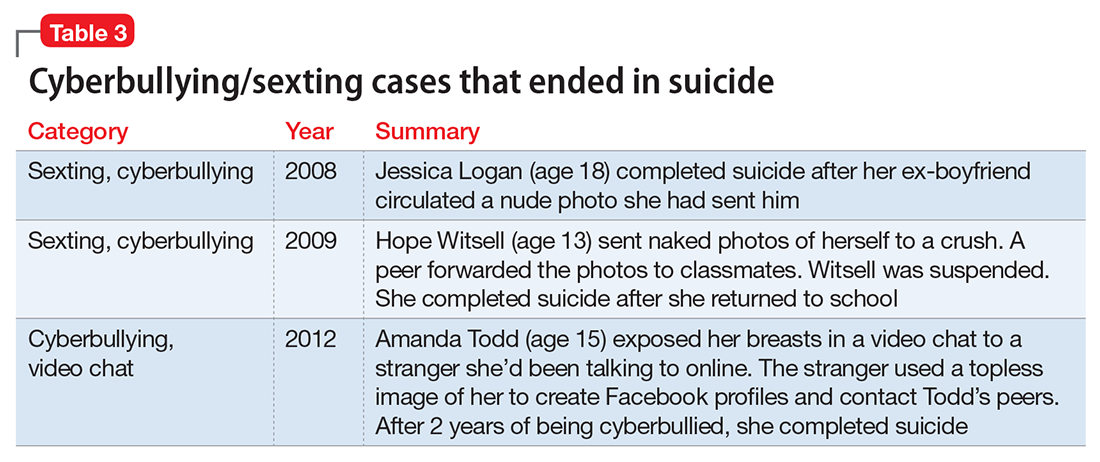
How to ask patients about sexting
To screen patients for sexting, clinicians need to develop a new skill set, which at first may be uncomfortable. However, the questions to ask are not all that different from other questions about adolescent and young adult sexuality. The importance of patients seeing that we as physicians are comfortable with the topic and approachable about their sexual health cannot be overemphasized. When discussing sexting with patients, it is essential to:
- explain that you are asking questions about their sexual health because they are important to overall health
- engage patients in discussion in a nonthreatening and nonjudgmental way
- develop rapport so patients feel comfortable disclosing behavior that may be embarrassing
- listen to their stories and build a context for understanding their experiences. As you listen, ask questions when needed to help move the story along.
Sometimes when asking about topics that are uncomfortable, clinicians revert from open-ended to closed-ended questions, but when asking about a patient’s sexual life, it is especially important to be open-ended and ask questions in a nonjudgmental way. Contextualizing sexual questions by (for example) asking them while discussing the teen’s relationships will make them seem more natural.18 To best understand, inquire explicitly about specific behaviors, but do so without appearing voyeuristic.18
Sexting may precede sexual intercourse. Keep in mind that a patient may report that she (he) is not sexually active but still may be involved in sexting. Therefore, discuss sexting even if your patient reports not being sexually active. By understanding the prevalence of sexting among teens, you can ask questions in a normalizing way. Clinicians can inquire about sexting while discussing relationships and dating or online risk behaviors.
Also consider whether any of your patient’s sexual behaviors, including sexting, are the result of coercion: “Some of my patients tell me they feel pressured or coerced into having sex. Have you ever felt this way?”19 and “Have you ever been picked on or bullied? Is that still a problem?” are suggested safety screening questions about bullying,18 and one can also ask about specific cyberbullying behaviors.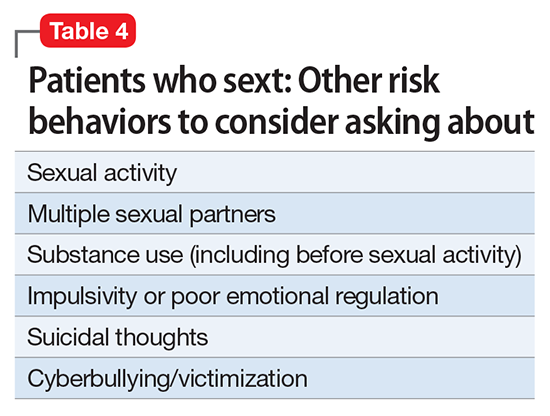
1. Mitchell KJ, Finkelhor D, Jones LM, et al. Prevalence and characteristics of youth sexting: a national study. Pediatrics. 2012;129(1):13-20.
2. Lenhart A. Teens and sexting. The Pew Research Center. http://www.pewinternet.org/2009/12/15/teens-and-sexting. Published December 15, 2009. Accessed October 31, 2017.
3. Temple JR, Paul JA, van den Berg P, et al. Teen sexting and its association with sexual behaviors. Arch Pediatr Adolesc Med. 2012;166(9):828-833.
4. Gordon-Messer D, Bauermeister JA, Grodzinski A, et al. Sexting among young adults. J Adolesc Health. 2013;52(3):301-306.
5. Henderson L. Sexting and sexual relationships among teens and young adults. McNair Scholars Research Journal. 2011;7(1):31-39.
6. The National Campaign to Prevent Teen and Unplanned Pregnancy. Sex and tech: results from a survey of teens and young adults. https://thenationalcampaign.org/sites/default/files/resource-primary-download/sex_and_tech_summary.pdf. Published December 2008. Accessed October 31, 2017.
7. Strassberg DS, McKinnon RK, Sustaíta MA, et al. Sexting by high school students: an exploratory and descriptive study. Arch Sex Behav. 2013;42(1):15-21.
8. Houck CD, Barker D, Rizzo C, et al. Sexting and sexual behavior in at-risk adolescents. Pediatrics. 2014;133(2):e276-e282.
9. Benotsch EG, Snipes DJ, Martin AM, et al. Sexting, substance use, and sexual risk behavior in young adults. J Adolesc Health. 2013;52(3):307-313.
10. Delevi R, Weisskirch RS. Personality factors as predictors of sexting. Comput Human Behav. 2013;29(6):2589-2594.
11. Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134(5):1287-1292.
12. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psych Clin North Am. 2016;39(4):691-700.
13. Citron DK, Franks MA. Criminalizing revenge porn. Wake Forest Law Review. 2014;49:345-391.
14. Hinduja S, Patchin JW. State cyberbullying laws: a brief review of state cyberbullying laws and policies. Cyberbullying Research Center. https://cyberbullying.org/Bullying-and-Cyberbullying-Laws.pdf. Updated 2016. Accessed October 31, 2017.
15. Beitsch R. States slowly scale back juvenile sex offender registries. The Pew Charitable Trusts. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2015/11/19/states-slowly-scale-back-juvenile-sex-offender-registries. Published November 19, 2015. Accessed October 31, 2017.
16. Wolak J, Finkelhor D, Mitchell KJ. How often are teens arrested for sexting? Data from a national sample of police cases. Pediatrics. 2012;129(1):4-12.
17. The Campus School at Boston College. Bullying prevention policy. https://www.bc.edu/bc-web/schools/lsoe/sites/campus-school/who-we-are/policies-and-procedures/bullying-prevention-policy.html. Accessed October 31, 2017.
18. Goldenring JM, Rosen DS. Getting into adolescent heads: an essential update. Contemporary Pediatrics. 2004;21(1):64.
19. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: the psychosocial interview for adolescents updated for a new century fueled by media. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/content/tags/adolescent-medicine/heeadsss-30-psychosocial-interview-adolesce?page=full. Published January 1, 2014. Accessed October 31, 2017.
Sexting includes sending sexually explicit (or sexually suggestive) text messages and photos, usually by cell phone. This article focuses on sexts involving photos. Cell phones are almost ubiquitous among American teens, and with technological advances, sexts are getting easier to send. Sexting may occur to initiate a relationship or sustain one. Some teenagers are coerced into sexting. Many people do not realize the potential long-term consequences of sexting—particularly because of the impulsive nature of sexting and the belief that the behavior is harmless.
Media attention has recently focused on teens who face legal charges related to sexting. Sexting photos may be considered child pornography—even though the teens made it themselves. There are also social consequences to sexting. Photos meant to be private are sometimes forwarded to others. Cyberbullying is not uncommon with teen sexting, and suicides after experiencing this behavior have been reported.
Sexting may be a form of modern flirtation, but in some cases, it may be a marker of other risk behaviors, such as substance abuse. Psychiatrists must be aware of the frequency and meaning of this potentially dangerous behavior. Clinicians should feel comfortable asking their patients about it and provide education and counseling.
CASE
Private photos get shared
K, age 14, a freshman with no psychiatric history, is referred to you by her school psychologist for evaluation of suicidal ideation. K reports depressed mood, poor sleep, inattention, loss of appetite, anhedonia, and feelings of guilt for the past month. She recently ended a relationship with her boyfriend of 1 year after she learned that he had shared with his friends naked photos of her that she had sent him. The school administration learned of the photos when a student posted them on one of the school computers.
K’s boyfriend, age 16, was suspended after the school learned that he had shared the photos without K’s consent. K, who is a good student, missed several days of school, including cheerleading practice; previously she had never missed a day of school.
On evaluation, K is tearful, stating that her life is “over.” She says that her ex-boyfriend’s friends are harassing her, calling her “slut” and making sexual comments. She also feels guilty, because she learned that the police interviewed her ex-boyfriend in connection with posting her photos on the Internet. In a text, he said he “might get charged with child pornography.” On further questioning, K confides that she had naked photos of her ex-boyfriend on her phone. She admits to sharing the pictures with her best friend, because she was “angry and wanted to get back” at her ex-boyfriend. She also reports a several-month history of sexting with her ex-boyfriend. K deleted the photos and texts after learning that her ex-boyfriend “was in trouble with the police.”
K has no prior sexual experience. She dated 1 boy her age prior to her ex-boyfriend. She had never been evaluated by a mental health clinician. She is dysphoric and reports feeling “hopeless … Unless this can be erased, I can’t go back to school.”
Sexting: What is the extent of the problem?
The true prevalence of sexting is difficult to ascertain, because different studies have used different definitions and methodologies. However, the rates are far from negligible. Sexting rates increase with age, over the teen years.1-3 Among American minors, 2.5% to 28% of middle school and high school students report that they have sent a sext (Table 11-9). Studies of American young adults (age ≥18) and university students have found 30% to 60% have sent sexts, and >40% have received a sext.4,5

Many people receive sexts—including individuals who are not the intended recipient. In 1 study, although most teens intended to share sexts only with their boyfriend/girlfriend, 25% to 33% had received sext photos intended for other people.6 In another recent study, 25% of teens had forwarded a sext that they received.7 Moreover, 12% of teenage boys and 5% of teenage girls had sent a sexually explicit photo that they took of another teen to a third person.7 Forwarding sexts can add exponentially to the psychosocial risks of the photographed teenager.
Who sexts? Current research indicates that the likelihood of sexting is related to age, personality, and social situation. Teens are approaching the peak age of their sex drive, and often are curious and feel invincible. Teens are more impulsive than adults. When it takes less than a minute to send a sext, irreversible poor choices can be made quickly. Teens who send sexts often engage in more text messaging than other teens.7
Teens may use sexting to initiate or sustain a relationship. Sexts also may be sent because of coercion. More than one-half of girls cited pressure to sext from a boy.6 Temple et al3 found that more than one-half of their study sample had been asked to send a sext. Girls were more likely than boys to be asked to send a sext; most were troubled by this.
One study that assessed knowledge of potential legal consequences of sexting found that many teens who sent sexts were aware of the potential consequences.7 Regarding personality traits, sexting among undergraduates was predicted by neuroticism and low agreeableness.10 Conversely, sending text messages with sexually suggestive writing was predicted by extraversion and problematic cell phone use.
Comorbidities. There are mixed findings about whether sexting is simply a modern dating strategy or a marker of other risk behaviors; age may play an important discriminating role. Sexual activity appears to be correlated with sexting. According to Temple and Choi,11 “Sexting fits within the context of adolescent sexual development and may be a viable indicator of adolescent sexual activity.”11
Some authors have suggested that sexting is a contemporary risk behavior that is likely to correlate with other risk behaviors. Among young teens—seventh graders who were referred to a risk prevention trial because of behavioral/emotional difficulties—those who sexted were more likely to engage in early sexual behaviors.8 These younger at-risk teens also had less understanding of their emotions and greater difficulty in regulating their emotions.
Among the general population of high school students, teens who sext are more likely to be sexually active.3 High school girls who engaged in sexting were noted to engage in other risk behaviors, including having multiple partners in the past year and using alcohol or drugs before sex.3 Teens who had sent a sext were more likely to be sexually active 1 year later than teens who had not.11Studies of sexting among university students also have had mixed findings. One study found that among undergraduates, sexting was associated with substance use and other risk behaviors.9 Another young adult study found sexting was not related to sexual risk or psychological well-being.4
Legal issues affect psychiatrists as well as patients
As a psychiatrist evaluating K, what are your duties as a mandated reporter? Psychiatrists are legally required to report suspected maltreatment or abuse of children.12 The circumstances under which psychiatrists may have a mandate to report include when a psychiatrist:
- evaluates a child and suspects abuse
- suspects child abuse based on an adult patient’s report
- learns from a third party that a child may have been/is being abused.
Psychiatrists usually are not mandated to report other types of potentially criminal behavior. As such, reporting sexting might be considered a breach of confidentiality. Psychiatrists should be familiar with the specific reporting guidelines for the jurisdiction in which they practice. Psychiatrists who work with individuals who commit crimes should focus on changing the potentially dangerous behaviors rather than reporting them.
Does the transmission of naked photos of a minor in a sexual pose or act constitute child pornography or another criminal offense? The legal answer varies, but the role of the psychiatrist does not. Psychiatrists should educate their patients about potentially dangerous behaviors.
With regards to the legal consequences, some states classify underage sexting photos as child pornography. Others have less rigid definitions of child pornography and take into account the age of the participants and their intent. Such jurisdictions point out that sexting naked photos among adolescents is “age appropriate.” Some have enacted specific sexting laws to address the transmission of obscene material to a child through the Internet. In some jurisdictions, sexting laws are categorized to refer to behavior of individuals under or over age 18. The term “revenge porn” is used to refer to nonconsensual pornography with its dissemination motivated by spite.13 Some states have defined specific revenge porn laws to address the behavior. Currently, 20 states have sexting laws and 26 states have revenge porn laws.14 Twenty states address a minor age <18 sending the photo, while only 18 address the recipient. The law in this area can be complex and detailed, taking into account the age of the sender, the intentions of the sender, and the nature of the relationship between the sender and the recipient and the behavior of the recipient.
Laws regarding sexting vary greatly. Sexting may be a misdemeanor or a felony, depending on the state, the specific behavior, and the frequency. In the United States, 11 state laws include a diversion remedy—an option to pursue the case outside of the criminal juvenile system; 10 laws require counseling or another informal sanction; 11 states laws have the potential for misdemeanor punishment; and 4 state laws have the potential for felony punishment.14 Depending on the criminal charge, the perpetrator may have to register as a sex offender. For example, in some jurisdictions, a conviction for possession of child pornography requires sex offender registration. Thirty-eight states include juvenile sex offenders in their sex offender registries. Other states require juveniles to register if they are age ≥15 years or have been tried as an adult.15
The frequency of police involvement in sexting cases also greatly varies. A national study examining the characteristics of youth sexting cases revealed that law enforcement agencies handled approximately 3,477 cases of youth-produced sexual photos in 2008 and 2009.16 Situations that involved an adult or a minor engaged in malicious, nonconsensual, or abusive behavior comprised two-thirds of cases. Arrests occurred in 62% of the adult-involved cases and 36% of the aggravated youth-only cases. Arrests occurred in only 18% of investigated non-aggravated youth-only cases. Table 2 describes recent American sexting legal cases and their outcomes.
In K’s case, depending on the jurisdiction, K or her ex-boyfriend may be subject to arrest for child pornography, revenge pornography, or sexting.
Potential social and psychiatric consequences
What are the social and psychiatric ramifications for K? Aside from potential legal consequences of sexting, K is experiencing psychological and social consequences. She has developed depressive symptoms and suicidal ideation. Her ex-boyfriend’s dissemination of her nude photos on the school computer could be interpreted as cyberbullying. (The National Center for Missing and Exploited Children defines cyberbullying as “bullying through the use of technology or electronic devices such as telephones, cell phones, computers, and the Internet.”17 All 50 states have enacted laws against bullying; 48 states have electronic harassment in their bullying laws; and 22 states have laws specifically referencing “cyberbullying.”)
Her depressive symptoms developed in response to her feelings of guilt and shame related to sexting as well as the subsequent peer harassment. She is refusing to return to school because of her concerns about bullying. A careful inquiry into suicidality should be part of the evaluation when sexting has led to psychiatric symptoms. Several cases of sexting and cyberbullying have ended in suicide (Table 3).

How to ask patients about sexting
To screen patients for sexting, clinicians need to develop a new skill set, which at first may be uncomfortable. However, the questions to ask are not all that different from other questions about adolescent and young adult sexuality. The importance of patients seeing that we as physicians are comfortable with the topic and approachable about their sexual health cannot be overemphasized. When discussing sexting with patients, it is essential to:
- explain that you are asking questions about their sexual health because they are important to overall health
- engage patients in discussion in a nonthreatening and nonjudgmental way
- develop rapport so patients feel comfortable disclosing behavior that may be embarrassing
- listen to their stories and build a context for understanding their experiences. As you listen, ask questions when needed to help move the story along.
Sometimes when asking about topics that are uncomfortable, clinicians revert from open-ended to closed-ended questions, but when asking about a patient’s sexual life, it is especially important to be open-ended and ask questions in a nonjudgmental way. Contextualizing sexual questions by (for example) asking them while discussing the teen’s relationships will make them seem more natural.18 To best understand, inquire explicitly about specific behaviors, but do so without appearing voyeuristic.18
Sexting may precede sexual intercourse. Keep in mind that a patient may report that she (he) is not sexually active but still may be involved in sexting. Therefore, discuss sexting even if your patient reports not being sexually active. By understanding the prevalence of sexting among teens, you can ask questions in a normalizing way. Clinicians can inquire about sexting while discussing relationships and dating or online risk behaviors.
Also consider whether any of your patient’s sexual behaviors, including sexting, are the result of coercion: “Some of my patients tell me they feel pressured or coerced into having sex. Have you ever felt this way?”19 and “Have you ever been picked on or bullied? Is that still a problem?” are suggested safety screening questions about bullying,18 and one can also ask about specific cyberbullying behaviors.
Sexting includes sending sexually explicit (or sexually suggestive) text messages and photos, usually by cell phone. This article focuses on sexts involving photos. Cell phones are almost ubiquitous among American teens, and with technological advances, sexts are getting easier to send. Sexting may occur to initiate a relationship or sustain one. Some teenagers are coerced into sexting. Many people do not realize the potential long-term consequences of sexting—particularly because of the impulsive nature of sexting and the belief that the behavior is harmless.
Media attention has recently focused on teens who face legal charges related to sexting. Sexting photos may be considered child pornography—even though the teens made it themselves. There are also social consequences to sexting. Photos meant to be private are sometimes forwarded to others. Cyberbullying is not uncommon with teen sexting, and suicides after experiencing this behavior have been reported.
Sexting may be a form of modern flirtation, but in some cases, it may be a marker of other risk behaviors, such as substance abuse. Psychiatrists must be aware of the frequency and meaning of this potentially dangerous behavior. Clinicians should feel comfortable asking their patients about it and provide education and counseling.
CASE
Private photos get shared
K, age 14, a freshman with no psychiatric history, is referred to you by her school psychologist for evaluation of suicidal ideation. K reports depressed mood, poor sleep, inattention, loss of appetite, anhedonia, and feelings of guilt for the past month. She recently ended a relationship with her boyfriend of 1 year after she learned that he had shared with his friends naked photos of her that she had sent him. The school administration learned of the photos when a student posted them on one of the school computers.
K’s boyfriend, age 16, was suspended after the school learned that he had shared the photos without K’s consent. K, who is a good student, missed several days of school, including cheerleading practice; previously she had never missed a day of school.
On evaluation, K is tearful, stating that her life is “over.” She says that her ex-boyfriend’s friends are harassing her, calling her “slut” and making sexual comments. She also feels guilty, because she learned that the police interviewed her ex-boyfriend in connection with posting her photos on the Internet. In a text, he said he “might get charged with child pornography.” On further questioning, K confides that she had naked photos of her ex-boyfriend on her phone. She admits to sharing the pictures with her best friend, because she was “angry and wanted to get back” at her ex-boyfriend. She also reports a several-month history of sexting with her ex-boyfriend. K deleted the photos and texts after learning that her ex-boyfriend “was in trouble with the police.”
K has no prior sexual experience. She dated 1 boy her age prior to her ex-boyfriend. She had never been evaluated by a mental health clinician. She is dysphoric and reports feeling “hopeless … Unless this can be erased, I can’t go back to school.”
Sexting: What is the extent of the problem?
The true prevalence of sexting is difficult to ascertain, because different studies have used different definitions and methodologies. However, the rates are far from negligible. Sexting rates increase with age, over the teen years.1-3 Among American minors, 2.5% to 28% of middle school and high school students report that they have sent a sext (Table 11-9). Studies of American young adults (age ≥18) and university students have found 30% to 60% have sent sexts, and >40% have received a sext.4,5

Many people receive sexts—including individuals who are not the intended recipient. In 1 study, although most teens intended to share sexts only with their boyfriend/girlfriend, 25% to 33% had received sext photos intended for other people.6 In another recent study, 25% of teens had forwarded a sext that they received.7 Moreover, 12% of teenage boys and 5% of teenage girls had sent a sexually explicit photo that they took of another teen to a third person.7 Forwarding sexts can add exponentially to the psychosocial risks of the photographed teenager.
Who sexts? Current research indicates that the likelihood of sexting is related to age, personality, and social situation. Teens are approaching the peak age of their sex drive, and often are curious and feel invincible. Teens are more impulsive than adults. When it takes less than a minute to send a sext, irreversible poor choices can be made quickly. Teens who send sexts often engage in more text messaging than other teens.7
Teens may use sexting to initiate or sustain a relationship. Sexts also may be sent because of coercion. More than one-half of girls cited pressure to sext from a boy.6 Temple et al3 found that more than one-half of their study sample had been asked to send a sext. Girls were more likely than boys to be asked to send a sext; most were troubled by this.
One study that assessed knowledge of potential legal consequences of sexting found that many teens who sent sexts were aware of the potential consequences.7 Regarding personality traits, sexting among undergraduates was predicted by neuroticism and low agreeableness.10 Conversely, sending text messages with sexually suggestive writing was predicted by extraversion and problematic cell phone use.
Comorbidities. There are mixed findings about whether sexting is simply a modern dating strategy or a marker of other risk behaviors; age may play an important discriminating role. Sexual activity appears to be correlated with sexting. According to Temple and Choi,11 “Sexting fits within the context of adolescent sexual development and may be a viable indicator of adolescent sexual activity.”11
Some authors have suggested that sexting is a contemporary risk behavior that is likely to correlate with other risk behaviors. Among young teens—seventh graders who were referred to a risk prevention trial because of behavioral/emotional difficulties—those who sexted were more likely to engage in early sexual behaviors.8 These younger at-risk teens also had less understanding of their emotions and greater difficulty in regulating their emotions.
Among the general population of high school students, teens who sext are more likely to be sexually active.3 High school girls who engaged in sexting were noted to engage in other risk behaviors, including having multiple partners in the past year and using alcohol or drugs before sex.3 Teens who had sent a sext were more likely to be sexually active 1 year later than teens who had not.11Studies of sexting among university students also have had mixed findings. One study found that among undergraduates, sexting was associated with substance use and other risk behaviors.9 Another young adult study found sexting was not related to sexual risk or psychological well-being.4
Legal issues affect psychiatrists as well as patients
As a psychiatrist evaluating K, what are your duties as a mandated reporter? Psychiatrists are legally required to report suspected maltreatment or abuse of children.12 The circumstances under which psychiatrists may have a mandate to report include when a psychiatrist:
- evaluates a child and suspects abuse
- suspects child abuse based on an adult patient’s report
- learns from a third party that a child may have been/is being abused.
Psychiatrists usually are not mandated to report other types of potentially criminal behavior. As such, reporting sexting might be considered a breach of confidentiality. Psychiatrists should be familiar with the specific reporting guidelines for the jurisdiction in which they practice. Psychiatrists who work with individuals who commit crimes should focus on changing the potentially dangerous behaviors rather than reporting them.
Does the transmission of naked photos of a minor in a sexual pose or act constitute child pornography or another criminal offense? The legal answer varies, but the role of the psychiatrist does not. Psychiatrists should educate their patients about potentially dangerous behaviors.
With regards to the legal consequences, some states classify underage sexting photos as child pornography. Others have less rigid definitions of child pornography and take into account the age of the participants and their intent. Such jurisdictions point out that sexting naked photos among adolescents is “age appropriate.” Some have enacted specific sexting laws to address the transmission of obscene material to a child through the Internet. In some jurisdictions, sexting laws are categorized to refer to behavior of individuals under or over age 18. The term “revenge porn” is used to refer to nonconsensual pornography with its dissemination motivated by spite.13 Some states have defined specific revenge porn laws to address the behavior. Currently, 20 states have sexting laws and 26 states have revenge porn laws.14 Twenty states address a minor age <18 sending the photo, while only 18 address the recipient. The law in this area can be complex and detailed, taking into account the age of the sender, the intentions of the sender, and the nature of the relationship between the sender and the recipient and the behavior of the recipient.
Laws regarding sexting vary greatly. Sexting may be a misdemeanor or a felony, depending on the state, the specific behavior, and the frequency. In the United States, 11 state laws include a diversion remedy—an option to pursue the case outside of the criminal juvenile system; 10 laws require counseling or another informal sanction; 11 states laws have the potential for misdemeanor punishment; and 4 state laws have the potential for felony punishment.14 Depending on the criminal charge, the perpetrator may have to register as a sex offender. For example, in some jurisdictions, a conviction for possession of child pornography requires sex offender registration. Thirty-eight states include juvenile sex offenders in their sex offender registries. Other states require juveniles to register if they are age ≥15 years or have been tried as an adult.15
The frequency of police involvement in sexting cases also greatly varies. A national study examining the characteristics of youth sexting cases revealed that law enforcement agencies handled approximately 3,477 cases of youth-produced sexual photos in 2008 and 2009.16 Situations that involved an adult or a minor engaged in malicious, nonconsensual, or abusive behavior comprised two-thirds of cases. Arrests occurred in 62% of the adult-involved cases and 36% of the aggravated youth-only cases. Arrests occurred in only 18% of investigated non-aggravated youth-only cases. Table 2 describes recent American sexting legal cases and their outcomes.
In K’s case, depending on the jurisdiction, K or her ex-boyfriend may be subject to arrest for child pornography, revenge pornography, or sexting.
Potential social and psychiatric consequences
What are the social and psychiatric ramifications for K? Aside from potential legal consequences of sexting, K is experiencing psychological and social consequences. She has developed depressive symptoms and suicidal ideation. Her ex-boyfriend’s dissemination of her nude photos on the school computer could be interpreted as cyberbullying. (The National Center for Missing and Exploited Children defines cyberbullying as “bullying through the use of technology or electronic devices such as telephones, cell phones, computers, and the Internet.”17 All 50 states have enacted laws against bullying; 48 states have electronic harassment in their bullying laws; and 22 states have laws specifically referencing “cyberbullying.”)
Her depressive symptoms developed in response to her feelings of guilt and shame related to sexting as well as the subsequent peer harassment. She is refusing to return to school because of her concerns about bullying. A careful inquiry into suicidality should be part of the evaluation when sexting has led to psychiatric symptoms. Several cases of sexting and cyberbullying have ended in suicide (Table 3).

How to ask patients about sexting
To screen patients for sexting, clinicians need to develop a new skill set, which at first may be uncomfortable. However, the questions to ask are not all that different from other questions about adolescent and young adult sexuality. The importance of patients seeing that we as physicians are comfortable with the topic and approachable about their sexual health cannot be overemphasized. When discussing sexting with patients, it is essential to:
- explain that you are asking questions about their sexual health because they are important to overall health
- engage patients in discussion in a nonthreatening and nonjudgmental way
- develop rapport so patients feel comfortable disclosing behavior that may be embarrassing
- listen to their stories and build a context for understanding their experiences. As you listen, ask questions when needed to help move the story along.
Sometimes when asking about topics that are uncomfortable, clinicians revert from open-ended to closed-ended questions, but when asking about a patient’s sexual life, it is especially important to be open-ended and ask questions in a nonjudgmental way. Contextualizing sexual questions by (for example) asking them while discussing the teen’s relationships will make them seem more natural.18 To best understand, inquire explicitly about specific behaviors, but do so without appearing voyeuristic.18
Sexting may precede sexual intercourse. Keep in mind that a patient may report that she (he) is not sexually active but still may be involved in sexting. Therefore, discuss sexting even if your patient reports not being sexually active. By understanding the prevalence of sexting among teens, you can ask questions in a normalizing way. Clinicians can inquire about sexting while discussing relationships and dating or online risk behaviors.
Also consider whether any of your patient’s sexual behaviors, including sexting, are the result of coercion: “Some of my patients tell me they feel pressured or coerced into having sex. Have you ever felt this way?”19 and “Have you ever been picked on or bullied? Is that still a problem?” are suggested safety screening questions about bullying,18 and one can also ask about specific cyberbullying behaviors.
1. Mitchell KJ, Finkelhor D, Jones LM, et al. Prevalence and characteristics of youth sexting: a national study. Pediatrics. 2012;129(1):13-20.
2. Lenhart A. Teens and sexting. The Pew Research Center. http://www.pewinternet.org/2009/12/15/teens-and-sexting. Published December 15, 2009. Accessed October 31, 2017.
3. Temple JR, Paul JA, van den Berg P, et al. Teen sexting and its association with sexual behaviors. Arch Pediatr Adolesc Med. 2012;166(9):828-833.
4. Gordon-Messer D, Bauermeister JA, Grodzinski A, et al. Sexting among young adults. J Adolesc Health. 2013;52(3):301-306.
5. Henderson L. Sexting and sexual relationships among teens and young adults. McNair Scholars Research Journal. 2011;7(1):31-39.
6. The National Campaign to Prevent Teen and Unplanned Pregnancy. Sex and tech: results from a survey of teens and young adults. https://thenationalcampaign.org/sites/default/files/resource-primary-download/sex_and_tech_summary.pdf. Published December 2008. Accessed October 31, 2017.
7. Strassberg DS, McKinnon RK, Sustaíta MA, et al. Sexting by high school students: an exploratory and descriptive study. Arch Sex Behav. 2013;42(1):15-21.
8. Houck CD, Barker D, Rizzo C, et al. Sexting and sexual behavior in at-risk adolescents. Pediatrics. 2014;133(2):e276-e282.
9. Benotsch EG, Snipes DJ, Martin AM, et al. Sexting, substance use, and sexual risk behavior in young adults. J Adolesc Health. 2013;52(3):307-313.
10. Delevi R, Weisskirch RS. Personality factors as predictors of sexting. Comput Human Behav. 2013;29(6):2589-2594.
11. Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134(5):1287-1292.
12. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psych Clin North Am. 2016;39(4):691-700.
13. Citron DK, Franks MA. Criminalizing revenge porn. Wake Forest Law Review. 2014;49:345-391.
14. Hinduja S, Patchin JW. State cyberbullying laws: a brief review of state cyberbullying laws and policies. Cyberbullying Research Center. https://cyberbullying.org/Bullying-and-Cyberbullying-Laws.pdf. Updated 2016. Accessed October 31, 2017.
15. Beitsch R. States slowly scale back juvenile sex offender registries. The Pew Charitable Trusts. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2015/11/19/states-slowly-scale-back-juvenile-sex-offender-registries. Published November 19, 2015. Accessed October 31, 2017.
16. Wolak J, Finkelhor D, Mitchell KJ. How often are teens arrested for sexting? Data from a national sample of police cases. Pediatrics. 2012;129(1):4-12.
17. The Campus School at Boston College. Bullying prevention policy. https://www.bc.edu/bc-web/schools/lsoe/sites/campus-school/who-we-are/policies-and-procedures/bullying-prevention-policy.html. Accessed October 31, 2017.
18. Goldenring JM, Rosen DS. Getting into adolescent heads: an essential update. Contemporary Pediatrics. 2004;21(1):64.
19. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: the psychosocial interview for adolescents updated for a new century fueled by media. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/content/tags/adolescent-medicine/heeadsss-30-psychosocial-interview-adolesce?page=full. Published January 1, 2014. Accessed October 31, 2017.
1. Mitchell KJ, Finkelhor D, Jones LM, et al. Prevalence and characteristics of youth sexting: a national study. Pediatrics. 2012;129(1):13-20.
2. Lenhart A. Teens and sexting. The Pew Research Center. http://www.pewinternet.org/2009/12/15/teens-and-sexting. Published December 15, 2009. Accessed October 31, 2017.
3. Temple JR, Paul JA, van den Berg P, et al. Teen sexting and its association with sexual behaviors. Arch Pediatr Adolesc Med. 2012;166(9):828-833.
4. Gordon-Messer D, Bauermeister JA, Grodzinski A, et al. Sexting among young adults. J Adolesc Health. 2013;52(3):301-306.
5. Henderson L. Sexting and sexual relationships among teens and young adults. McNair Scholars Research Journal. 2011;7(1):31-39.
6. The National Campaign to Prevent Teen and Unplanned Pregnancy. Sex and tech: results from a survey of teens and young adults. https://thenationalcampaign.org/sites/default/files/resource-primary-download/sex_and_tech_summary.pdf. Published December 2008. Accessed October 31, 2017.
7. Strassberg DS, McKinnon RK, Sustaíta MA, et al. Sexting by high school students: an exploratory and descriptive study. Arch Sex Behav. 2013;42(1):15-21.
8. Houck CD, Barker D, Rizzo C, et al. Sexting and sexual behavior in at-risk adolescents. Pediatrics. 2014;133(2):e276-e282.
9. Benotsch EG, Snipes DJ, Martin AM, et al. Sexting, substance use, and sexual risk behavior in young adults. J Adolesc Health. 2013;52(3):307-313.
10. Delevi R, Weisskirch RS. Personality factors as predictors of sexting. Comput Human Behav. 2013;29(6):2589-2594.
11. Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134(5):1287-1292.
12. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psych Clin North Am. 2016;39(4):691-700.
13. Citron DK, Franks MA. Criminalizing revenge porn. Wake Forest Law Review. 2014;49:345-391.
14. Hinduja S, Patchin JW. State cyberbullying laws: a brief review of state cyberbullying laws and policies. Cyberbullying Research Center. https://cyberbullying.org/Bullying-and-Cyberbullying-Laws.pdf. Updated 2016. Accessed October 31, 2017.
15. Beitsch R. States slowly scale back juvenile sex offender registries. The Pew Charitable Trusts. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2015/11/19/states-slowly-scale-back-juvenile-sex-offender-registries. Published November 19, 2015. Accessed October 31, 2017.
16. Wolak J, Finkelhor D, Mitchell KJ. How often are teens arrested for sexting? Data from a national sample of police cases. Pediatrics. 2012;129(1):4-12.
17. The Campus School at Boston College. Bullying prevention policy. https://www.bc.edu/bc-web/schools/lsoe/sites/campus-school/who-we-are/policies-and-procedures/bullying-prevention-policy.html. Accessed October 31, 2017.
18. Goldenring JM, Rosen DS. Getting into adolescent heads: an essential update. Contemporary Pediatrics. 2004;21(1):64.
19. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: the psychosocial interview for adolescents updated for a new century fueled by media. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/content/tags/adolescent-medicine/heeadsss-30-psychosocial-interview-adolesce?page=full. Published January 1, 2014. Accessed October 31, 2017.
The dawn of precision psychiatry
Imagine being able to precisely select the medication with the optimal efficacy, safety, and tolerability at the outset of treatment for every psychiatric patient who needs pharmacotherapy. Imagine how much the patient would appreciate not receiving a series of drugs and suffering multiple adverse effects and unremitting symptoms until the “right medication” is identified. Imagine how gratifying it would be for you as a psychiatrist to watch every one of your patients improve rapidly with minimal complaints or adverse effects.
Precision psychiatry is the indispensable vehicle to achieve personalized medicine for psychiatric patients. Precision psychiatry is a cherished goal, but it remains an aspirational objective. Other medical specialties, especially oncology and cardiology, have made remarkable strides in precision medicine, but the journey to precision psychiatry is still in its early stages. Yet there is every reason to believe that we are making progress toward that cherished goal.
To implement precision psychiatry, we must be able to identify the biosignature of each patient’s psychiatric brain disorder. But there is a formidable challenge to overcome: the complex, extensive heterogeneity of psychiatric disorders, which requires intense and inspired neurobiology research. So, while clinicians go on with the mundane trial-and-error approach of contemporary psychopharmacology, psychiatric neuroscientists are diligently deconstructing major psychiatric disorders into specific biotypes with unique biosignatures that will one day guide accurate and prompt clinical management.
Psychiatric practitioners may be too busy to keep tabs on the progress being made in identifying various biomarkers that are the key ingredients to decoding the biosignature of each psychiatric patient. Take schizophrenia, for example. There are myriad clinical variations that comprise this heterogeneous brain syndrome, including level of premorbid functioning; acute vs gradual onset of psychosis; the type and severity of hallucinations or delusions; the dimensional spectrum of negative symptoms and cognitive impairments; the presence and intensity of suicidal or homicidal urges; and the type of medical and psychiatric comorbidities. No wonder every patient is a unique and fascinating clinical puzzle, and yet, patients with schizophrenia are still being homogenized under a single DSM diagnostic category.
There are hundreds of biomarkers in schizophrenia,1 but none can be used clinically until the biosignatures of the many diseases within the schizophrenia syndrome are identified. That grueling research quest will take time, given that so far >340 risk genes for schizophrenia have been discovered, along with countless copy number variants representing gene deletions or duplications, plus dozens of de novo mutations that preclude coding for any protein. Add to these the numerous prenatal pregnancy adverse events, delivery complications, and early childhood abuse—all of which are associated with neurodevelopmental disruptions that set up the brain for schizophrenia spectrum disorders in adulthood—and we have a perplexing conundrum to tackle.
Precision psychiatry will ultimately enable practitioners to recognize various psychotic diseases that are more specific than the current DSM psychosis categories. Further, precision psychiatry will provide guidance as to which member within a class of so-called “me-too” drugs is the optimal match for each patient. This will stand in stark contrast to the chaotic hit-or-miss approach.
Precision psychiatry also will reveal the absurdity of current FDA clinical trials design for drug development. How can a molecule with a putative mechanism of action relevant to a specific biotype be administered to a hodgepodge of heterogeneous biotypes that have been lumped in 1 clinical category, and yet be expected to exert efficacy in most biotypes? It is a small miracle that some new drugs beat placebo despite the extensive variability in both placebo responses and drug responses. But it is well known that in all FDA placebo-controlled trials, the therapeutic response across the patient population varies from extremely high to extremely low, and worsening may even occur in a subset of patients receiving either the active drug or placebo. Perhaps drug response should be used as 1 methodology to classify biotypes of patients encompassed within a heterogeneous syndrome such as schizophrenia.
Precision psychiatry will represent a huge paradigm shift in the science and practice of our specialty. In his landmark book, Thomas Kuhn defined a paradigm as “an entire worldview in which a theory exists and all the implications that come from that view.”2 Precision psychiatry will completely disrupt the current antiquated clinical paradigm, transforming psychiatry into the clinical neuroscience it is. Many “omics,” such as genomics, epigenomics, transcriptomics, proteomics, metabolomics, lipidomics, and metagenomics, will inevitably find their way into the jargon of psychiatrists.3
A marriage of science and technology is essential for the emergence of precision psychiatry. To achieve this transformative amalgamation, we need to reconfigure our concepts, reengineer our methods, reinvent our models, and redesign our approaches to patient care.
As Peter Drucker said, “The best way to predict the future is to create it.”4 Precision psychiatry is our future. Let’s create it!
1. Nasrallah HA.
2. Kuhn TS. The structure of scientific revolutions. Chicago, IL: University of Chicago Press; 1964.
3. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
4. Cohen WA. Drucker on leadership: new lessons from the father of modern management. San Francisco, CA: Jossey-Bass; 2010.
Imagine being able to precisely select the medication with the optimal efficacy, safety, and tolerability at the outset of treatment for every psychiatric patient who needs pharmacotherapy. Imagine how much the patient would appreciate not receiving a series of drugs and suffering multiple adverse effects and unremitting symptoms until the “right medication” is identified. Imagine how gratifying it would be for you as a psychiatrist to watch every one of your patients improve rapidly with minimal complaints or adverse effects.
Precision psychiatry is the indispensable vehicle to achieve personalized medicine for psychiatric patients. Precision psychiatry is a cherished goal, but it remains an aspirational objective. Other medical specialties, especially oncology and cardiology, have made remarkable strides in precision medicine, but the journey to precision psychiatry is still in its early stages. Yet there is every reason to believe that we are making progress toward that cherished goal.
To implement precision psychiatry, we must be able to identify the biosignature of each patient’s psychiatric brain disorder. But there is a formidable challenge to overcome: the complex, extensive heterogeneity of psychiatric disorders, which requires intense and inspired neurobiology research. So, while clinicians go on with the mundane trial-and-error approach of contemporary psychopharmacology, psychiatric neuroscientists are diligently deconstructing major psychiatric disorders into specific biotypes with unique biosignatures that will one day guide accurate and prompt clinical management.
Psychiatric practitioners may be too busy to keep tabs on the progress being made in identifying various biomarkers that are the key ingredients to decoding the biosignature of each psychiatric patient. Take schizophrenia, for example. There are myriad clinical variations that comprise this heterogeneous brain syndrome, including level of premorbid functioning; acute vs gradual onset of psychosis; the type and severity of hallucinations or delusions; the dimensional spectrum of negative symptoms and cognitive impairments; the presence and intensity of suicidal or homicidal urges; and the type of medical and psychiatric comorbidities. No wonder every patient is a unique and fascinating clinical puzzle, and yet, patients with schizophrenia are still being homogenized under a single DSM diagnostic category.
There are hundreds of biomarkers in schizophrenia,1 but none can be used clinically until the biosignatures of the many diseases within the schizophrenia syndrome are identified. That grueling research quest will take time, given that so far >340 risk genes for schizophrenia have been discovered, along with countless copy number variants representing gene deletions or duplications, plus dozens of de novo mutations that preclude coding for any protein. Add to these the numerous prenatal pregnancy adverse events, delivery complications, and early childhood abuse—all of which are associated with neurodevelopmental disruptions that set up the brain for schizophrenia spectrum disorders in adulthood—and we have a perplexing conundrum to tackle.
Precision psychiatry will ultimately enable practitioners to recognize various psychotic diseases that are more specific than the current DSM psychosis categories. Further, precision psychiatry will provide guidance as to which member within a class of so-called “me-too” drugs is the optimal match for each patient. This will stand in stark contrast to the chaotic hit-or-miss approach.
Precision psychiatry also will reveal the absurdity of current FDA clinical trials design for drug development. How can a molecule with a putative mechanism of action relevant to a specific biotype be administered to a hodgepodge of heterogeneous biotypes that have been lumped in 1 clinical category, and yet be expected to exert efficacy in most biotypes? It is a small miracle that some new drugs beat placebo despite the extensive variability in both placebo responses and drug responses. But it is well known that in all FDA placebo-controlled trials, the therapeutic response across the patient population varies from extremely high to extremely low, and worsening may even occur in a subset of patients receiving either the active drug or placebo. Perhaps drug response should be used as 1 methodology to classify biotypes of patients encompassed within a heterogeneous syndrome such as schizophrenia.
Precision psychiatry will represent a huge paradigm shift in the science and practice of our specialty. In his landmark book, Thomas Kuhn defined a paradigm as “an entire worldview in which a theory exists and all the implications that come from that view.”2 Precision psychiatry will completely disrupt the current antiquated clinical paradigm, transforming psychiatry into the clinical neuroscience it is. Many “omics,” such as genomics, epigenomics, transcriptomics, proteomics, metabolomics, lipidomics, and metagenomics, will inevitably find their way into the jargon of psychiatrists.3
A marriage of science and technology is essential for the emergence of precision psychiatry. To achieve this transformative amalgamation, we need to reconfigure our concepts, reengineer our methods, reinvent our models, and redesign our approaches to patient care.
As Peter Drucker said, “The best way to predict the future is to create it.”4 Precision psychiatry is our future. Let’s create it!
Imagine being able to precisely select the medication with the optimal efficacy, safety, and tolerability at the outset of treatment for every psychiatric patient who needs pharmacotherapy. Imagine how much the patient would appreciate not receiving a series of drugs and suffering multiple adverse effects and unremitting symptoms until the “right medication” is identified. Imagine how gratifying it would be for you as a psychiatrist to watch every one of your patients improve rapidly with minimal complaints or adverse effects.
Precision psychiatry is the indispensable vehicle to achieve personalized medicine for psychiatric patients. Precision psychiatry is a cherished goal, but it remains an aspirational objective. Other medical specialties, especially oncology and cardiology, have made remarkable strides in precision medicine, but the journey to precision psychiatry is still in its early stages. Yet there is every reason to believe that we are making progress toward that cherished goal.
To implement precision psychiatry, we must be able to identify the biosignature of each patient’s psychiatric brain disorder. But there is a formidable challenge to overcome: the complex, extensive heterogeneity of psychiatric disorders, which requires intense and inspired neurobiology research. So, while clinicians go on with the mundane trial-and-error approach of contemporary psychopharmacology, psychiatric neuroscientists are diligently deconstructing major psychiatric disorders into specific biotypes with unique biosignatures that will one day guide accurate and prompt clinical management.
Psychiatric practitioners may be too busy to keep tabs on the progress being made in identifying various biomarkers that are the key ingredients to decoding the biosignature of each psychiatric patient. Take schizophrenia, for example. There are myriad clinical variations that comprise this heterogeneous brain syndrome, including level of premorbid functioning; acute vs gradual onset of psychosis; the type and severity of hallucinations or delusions; the dimensional spectrum of negative symptoms and cognitive impairments; the presence and intensity of suicidal or homicidal urges; and the type of medical and psychiatric comorbidities. No wonder every patient is a unique and fascinating clinical puzzle, and yet, patients with schizophrenia are still being homogenized under a single DSM diagnostic category.
There are hundreds of biomarkers in schizophrenia,1 but none can be used clinically until the biosignatures of the many diseases within the schizophrenia syndrome are identified. That grueling research quest will take time, given that so far >340 risk genes for schizophrenia have been discovered, along with countless copy number variants representing gene deletions or duplications, plus dozens of de novo mutations that preclude coding for any protein. Add to these the numerous prenatal pregnancy adverse events, delivery complications, and early childhood abuse—all of which are associated with neurodevelopmental disruptions that set up the brain for schizophrenia spectrum disorders in adulthood—and we have a perplexing conundrum to tackle.
Precision psychiatry will ultimately enable practitioners to recognize various psychotic diseases that are more specific than the current DSM psychosis categories. Further, precision psychiatry will provide guidance as to which member within a class of so-called “me-too” drugs is the optimal match for each patient. This will stand in stark contrast to the chaotic hit-or-miss approach.
Precision psychiatry also will reveal the absurdity of current FDA clinical trials design for drug development. How can a molecule with a putative mechanism of action relevant to a specific biotype be administered to a hodgepodge of heterogeneous biotypes that have been lumped in 1 clinical category, and yet be expected to exert efficacy in most biotypes? It is a small miracle that some new drugs beat placebo despite the extensive variability in both placebo responses and drug responses. But it is well known that in all FDA placebo-controlled trials, the therapeutic response across the patient population varies from extremely high to extremely low, and worsening may even occur in a subset of patients receiving either the active drug or placebo. Perhaps drug response should be used as 1 methodology to classify biotypes of patients encompassed within a heterogeneous syndrome such as schizophrenia.
Precision psychiatry will represent a huge paradigm shift in the science and practice of our specialty. In his landmark book, Thomas Kuhn defined a paradigm as “an entire worldview in which a theory exists and all the implications that come from that view.”2 Precision psychiatry will completely disrupt the current antiquated clinical paradigm, transforming psychiatry into the clinical neuroscience it is. Many “omics,” such as genomics, epigenomics, transcriptomics, proteomics, metabolomics, lipidomics, and metagenomics, will inevitably find their way into the jargon of psychiatrists.3
A marriage of science and technology is essential for the emergence of precision psychiatry. To achieve this transformative amalgamation, we need to reconfigure our concepts, reengineer our methods, reinvent our models, and redesign our approaches to patient care.
As Peter Drucker said, “The best way to predict the future is to create it.”4 Precision psychiatry is our future. Let’s create it!
1. Nasrallah HA.
2. Kuhn TS. The structure of scientific revolutions. Chicago, IL: University of Chicago Press; 1964.
3. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
4. Cohen WA. Drucker on leadership: new lessons from the father of modern management. San Francisco, CA: Jossey-Bass; 2010.
1. Nasrallah HA.
2. Kuhn TS. The structure of scientific revolutions. Chicago, IL: University of Chicago Press; 1964.
3. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
4. Cohen WA. Drucker on leadership: new lessons from the father of modern management. San Francisco, CA: Jossey-Bass; 2010.
The end of the line: Concluding your practice when facing serious illness
Dear Dr. Mossman,
I have a possibly fatal disease. So far, my symptoms and treatment haven’t kept me from my usual activities. But if my illness worsens, I’ll have to quit practicing psychiatry. What should I be doing now to make sure I fulfill my ethical and legal obligations to my patients?
Submitted by “Dr. F”
“Remember, with great power comes great responsibility.”
- Peter Parker, Spider-Man (2002)
Peter Parker’s movie-ending statement applies to doctors as well as Spider-Man. Although we don’t swing from building to building to save cities from heinous villains, practicing medicine is a privilege that society bestows only upon physicians who retain the knowledge, skills, and ability to treat patients competently.
Doctors retire from practice for many reasons, including when deteriorating physical health or cognitive capacity prevents them from performing clinical duties properly. Dr. F’s situation is not rare. As the physician population ages,1,2 a growing number of his colleagues will face similar circumstances,3,4 and with them, the responsibility and emotional turmoil of arranging to end their medical practices.
In many ways, concluding a psychiatric practice is similar to retiring from practice in other specialties. But because we care for patients’ minds as well as their bodies, retirement affects psychiatrists in distinctive ways that reflect our patients’ feelings toward us and our feelings toward them. To answer Dr. F’s question, this article considers having to stop practicing from 3 vantage points:
- the emotional impact on patients
- the emotional impact on the psychiatrist
- fulfilling one’s legal obligations while attending to the emotions of patients as well as oneself.
Emotional impact on patients
A content analysis study suggests that the traits patients appreciate in family physicians include the availability to listen, caring and compassion, trusted medical judgment, conveying the patient’s importance during encounters, feelings of connectedness, knowledge and understanding of the patient’s family, and relationship longevity.5 The same factors likely apply to relationships between psychiatrists and their patients, particularly if treatment encounters have extended over years and have involved conversations beyond those needed merely to write prescriptions.
Psychoanalytic publications offer many descriptions of patients’ reactions to the illness or death of their mental health professional. A 1978 study of 27 analysands whose physicians died during ongoing therapy reported reactions that ranged from a minimal impact to protracted mourning accompanied by helplessness, intense crying, and recurrent dreams about the analyst.6 Although a few patients were relieved that death had ended a difficult treatment, many were angry at their doctor for not attending to self-care and for breaking their treatment agreement, or because they had missed out on hoped-for benefits.
A 2010 study described the pain and distress that patients may experience following the death of their analyst or psychotherapist. These accounts emphasized the emotional isolation of grieving patients, who do not have the social support that bereaved persons receive after losing a loved one.7 Successful psychotherapy provides a special relationship characterized by trust, intimacy, and safety. But if the therapist suddenly dies, this relationship “is transformed into a solitude like no other.”8
Because the sudden “rupture of an analytic process is bound to be traumatic and may cause iatrogenic injury to the patient,” Traesdal9 advocates that therapists in situations similar to Dr. F’s discuss their possible death “on the reality level at least once during any analysis or psychotherapy.… It is extremely helpful to a patient to have discussed … how to handle the situation” if the therapist dies. This discussion also offers the patient an opportunity to confront a cultural taboo around death and to increase capacity to tolerate pain, illness, and aging.10,11
Most psychiatric care today is not psychoanalysis; psychiatrists provide other forms of care that create less intense doctor–patient relationships. Yet knowledge of these kinds of reactions may help Dr. F stay attuned to his patients’ concerns and to contemplate what they may experience, to greater or lesser degrees, if his health declines.
Retirement’s emotional impact on the psychiatrist
Published guidance on concluding a psychiatric practice is sparse, considering that all psychiatrists are mortal and stop practicing at some point.12Not thinking about or planning for retirement is a psychiatric tradition that started with Freud. He saw patients until shortly before his death and did not seem to have planned for ending his practice, despite suffering with jaw cancer for 16 years.13
Practicing medicine often is more than just a career; it is a core aspect of many physicians’ identity.14 Most of us spend a large fraction of our waking hours caring for patients and meeting other job requirements (eg, teaching, maintaining knowledge and skills), and many of us have scant time to pursue nonmedical interests. An intense prioritization of one’s “medical identity” makes retirement a blow to a doctor’s self-worth and sense of meaning in life.15,16
Because their work is not physically demanding, most psychiatrists continue to practice beyond the age of 65 years.12,17 More important, perhaps, is that being a psychiatrist is uniquely rewarding. As Benjamin Rush observed in an 1810 letter to Pennsylvania Hospital, successfully treating any medical disease is gratifying, but “what is this pleasure compared with that of restoring a fellow creature from the anguish and folly of madness and of reviving in him the knowledge of himself, his family, his friends, and his God!”18
Physicians in any specialty that involves repeated contact with the same patients form emotional bonds with their patients that retirement breaks.14 Psychiatrists’ interest in how patients think, feel, and cope with problems creates special attachments17 that can make some terminations “emotionally excruciating.”12
Psychiatrists with serious illness
What guidance might Dr. F find regarding whether to broach the subject of his illness with patients, and if so, how? No one has conducted controlled trials to answer these questions. Rather, published discussion of psychiatrists’ serious illness is found mainly in the psychotherapy literature. What’s available consists of individual accounts and case series that lack scientific rigor and offer little clarity about what the therapist should say, when to say it, and how to initiate the discussion.19,20 Yet Dr. F may find some of these authors’ ideas and suggestions helpful, particularly if his psychiatric practice includes providing psychotherapy.
As a rule, psychiatrists avoid talking about themselves, but having a serious illness that could affect treatment often justifies deviating from this practice. Although Dr. F (like many psychiatrists) may be concerned that discussing his health will make patients anxious or “contaminate” what they are able or willing to say,21 not providing information or avoiding discussion (especially if a patient asks about your health) may quickly undermine a patient’s trust.21,22 Even in psychoanalytic treatment, it makes little sense to encourage patients “to speak freely on the pretense that all is well, despite obvious evidence to the contrary.”19
Physicians often deny—or at least avoid thinking about—their own mortality.23 But avoiding talking about something so important (and often so obvious) as one’s illness may risk supporting patients’ denial of crucial matters in their own lives.19,21 Moreover, Dr. F’s inadvertent self-disclosure (eg, by displaying obvious signs of illness) may do more harm to therapy than a planned statement in which Dr. F has prepared what he’ll say to answer his patients’ questions.20
That Dr. F has continued working while suffering from a potentially fatal illness seems noble. Yet by doing so, he accepts not only the burdens of his illness but also the obligation to continue to serve his patients competently. This requires maintaining emotional steadiness and not using patients for emotional support, but instead obtaining and using the support of his friends, colleagues, family, consultants, and caregivers.20
Legal obligations
Retirement does not end a physician’s professional legal obligations.24 The legal rules and duties for psychiatrists who leave their practices are similar to those that apply to other physicians. Mishandling these aspects of retirement can result in various legal, licensure-related, or economic consequences, depending on your circumstances and employment arrangements.
Employment contracts in hospital or group practices often require notice of impending departures. If applicable to Dr. F’s situation, failure to comply with such conditions may lead to forfeiture of buyout payments, paying for malpractice tail coverage, or lawsuits claiming violation of contractual agreements.25
Retirement also creates practical and legal responsibilities to patients that are separate from the interpersonal and emotional issues previously discussed. How will those who need ongoing care and coverage be cared for? When withdrawing from a patient’s care (because of retirement or other reasons), a physician should give the patient enough advance notice to set up satisfactory treatment arrangements elsewhere and should facilitate transfer of the patient’s care, if appropriate.26 Failure to meet this ethical obligation may lead to a malpractice action alleging abandonment, which is defined as “the unilateral severance of the professional relationship … without reasonable notice at a time when there is still the necessity of continuing medical attention.”27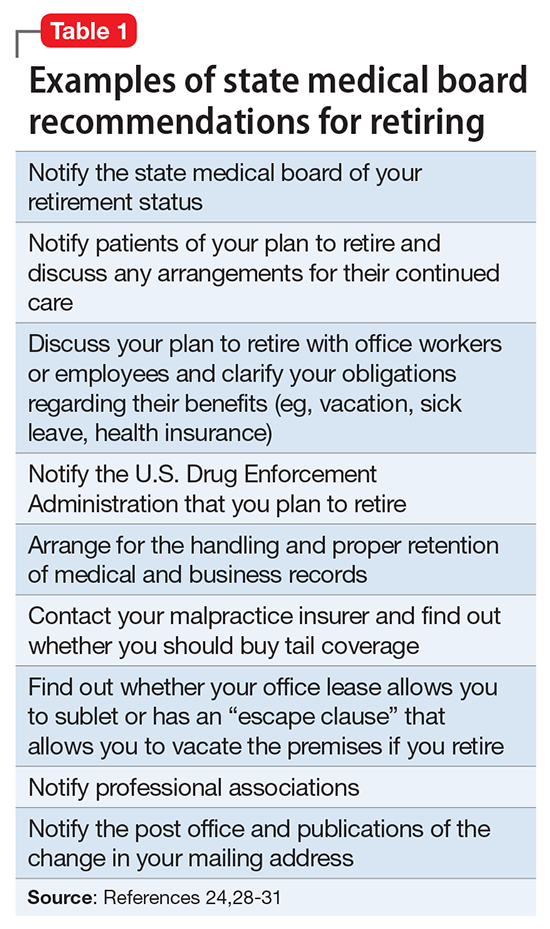
Further obligations come from medical licensing boards, which, in many states, have established time frames and specific procedures for informing patients and the public when a physician is leaving practice. Table 124,28-31 lists examples of these. If Dr. F works in a state where the board hasn’t promulgated such regulations, Table 124,28-31 may still help him think through how to discharge his ethical responsibilities to notify patients, colleagues, and business entities that he is ending his practice. References 28-30 and 32 discuss several of these matters, suggest timetables for various steps of a practice closure, and provide sample letters for notifying patients.
Physicians also must preserve their medical records for a certain period after they retire. States with rules on this matter require record preservation for 5 to 10 years or until 2 or 3 years after minor patients reach the age of majority.33 The Health Insurance Portability and Accountability Act of 1996 requires covered entities, which include most psychiatrists, to retain records for 6 years,34 and certain Medicare programs require retention for 10 years.35
Depending on Dr. F’s location and type of practice, his records should be preserved for the longest period that applies. If he is leaving a group practice that owns the records, arranging for this should be easy. If leaving an independent practice, he may need to ask another practice to perform this function.25
A ‘professional will’
Dr. F also might consider a measure that many psychotherapists recommend13,19,36 and that in some states is required by mental health licensing boards or professional codes37,38: creating a “professional will” that contains instructions for handling practice matters in case of death or disability.39

1. LoboPrabhu SM, Molinari VA, Hamilton JD, et al. The aging physician with cognitive impairment: approaches to oversight, prevention, and remediation. Am J Geriatr Psychiatry. 2009;17(6):445-454.
2. Dellinger EP, Pellegrini CA, Gallagher TH. The aging physician and the medical profession: a review. JAMA Surg. 2017;152(10):967-971.
3. Dall T, West T, Chakrabarti R, et al. The complexities of physician supply and demand: projections from 2014 to 2025. Association of American Medical Colleges. https://www.aamc.org/download/458082/data/2016_complexities_of_supply_and_demand_projections.pdf. Published 2016. Accessed September 26, 2017.
4. Draper B, Winfield S, Luscombe G. The older psychiatrist and retirement. Int J Geriatr Psychiatry. 1997;12(2):233-239.
5. Merenstein B, Merenstein J. Patient reflections: saying good-bye to a retiring family doctor. J Am Board Fam Med. 2008;21(5):461-465.
6. Lord R, Ritvo S, Solnit AJ. Patients’ reactions to the death of the psychoanalyst. Intern J Psychoanal. 1978;59(2-3):189-197.
7. Power A. Forced endings in psychotherapy and psychoanalysis: attachment and loss in retirement. New York, NY: Routledge; 2016.
8. Robutti A. When the patient loses his/her analyst. Italian Psychoanalytic Annual. 2010;4:129-145.
9. Traesdal T. When the analyst dies: dealing with the aftermath. J Am Psychoanal Assoc. 2005;53(4):1235-1255.
10. Deutsch RA. A voice lost, a voice found: after the death of the analyst. In: Deutsch RA, ed. Traumatic ruptures: abandonment and betrayal in the analytic relationship. New York, NY: Routledge; 2014:32-45.
11. Ward VP. On Yoda, trouble, and transformation: the cultural context of therapy and supervision. Contemp Fam Ther. 2009;31(3):171-176.
12. Moffic HS. Mental bootcamp: today is the first day of your retirement! Psychiatr Times. http://www.psychiatrictimes.com/blogs/couch-crisis/mental-bootcamp-today-first-day-your-retirement. Published June 25, 2012. Accessed October 31, 2017.
13. Shatsky P. Everything ends: identity and the therapist’s retirement. Clin Soc Work J. 2016;44(2):143-149.
14. Collier R.
15. Onyura B, Bohnen J, Wasylenki D, et al. Reimagining the self at late-career transitions: how identity threat influences academic physicians’ retirement considerations. Acad Med. 2015;90(6):794-801.
16. Silver MP. Critical reflection on physician retirement. Can Fam Physician. 2016;62(10):783-784.
17. Clemens NA. A psychiatrist retires: an oxymoron? J Psychiatr Pract. 2011;17(5):351-354.
18. Packard FR. The earliest hospitals. In: Packard FR. History of medicine in the United States. Philadelphia, PA: Lippincott; 1901:348.
19. Galatzer-Levy RM. The death of the analyst: patients whose previous analyst died while they were in treatment. J Amer Psychoanalytic Assoc. 2004;52(4):999-1024.
20. Fajardo B. Life-threatening illness in the analyst. J Am Psychoanal Assoc. 2001;49(2):569-586.
21. Dewald PA. Serious illness in the analyst: transference, countertransference, and reality responses. J Am Psychoanal Assoc. 1982;30(2):347-363.
22. Howe E. Should psychiatrists self disclose? Innov Clin Neurosci. 2011;8(12):14-17.
23. Rizq R, Voller D. ‘Who is the third who walks always beside you?’ On the death of a psychoanalyst. Psychodyn Pract. 2013;19(2):143-167.
24. Babitsky S, Mangraviti JJ. The biggest legal mistakes physicians make—and how to avoid them. Falmouth, MA: SEAK, Inc.; 2005.
25. Armon BD, Bayus K. Legal considerations when making a practice change. Chest. 2014;146(1):215-219.
26. American Medical Association. Opinions on patient-physician relationships: 1.1.5 terminating a patient-physician relationship. https://www.ama-assn.org/sites/default/files/media-browser/code-of-medical-ethics-chapter-1.pdf. Published 2016. Accessed September 29, 2017.
27. Lee v Dewbre, 362 S.W. 2d 900 (Tex Civ App 7th Dist 1962).
28. Medical Association of Georgia. Issues for the retiring physician. https://www.mag.org/georgia/uploadedfiles/issues-retiring-physicians.pdf. Accessed October 1, 2017.
29. Massachusetts Medical Society. Issues for the retiring physician. http://www.massmed.org/physicians/practice-management/practice-ownership-and-operations/issues-for-the-retiring-physician-(pdf). Published 2012. Accessed October 1, 2017.
30. North Carolina Medical Board. The doctor is out: a physician’s guide to closing a practice. https://www.ncmedboard.org/images/uploads/article_images/Physicians_Guide_to_Closing_a_Practice_05_12_2014.pdf. Published May 12, 2014. Accessed October 1, 2017.
31. 243 Code of Mass. Regulations §2.06(4)(a).
32. Sampson K. Physician’s guide to closing a practice. Maine Medical Association. https://www.mainemed.com/sites/default/files/content/Closing%20Practice%20Guide%20FINAL%206.2014.pdf. Published 2014. Accessed October 1, 2017.
33. HealthIT.gov. State medical record laws: minimum medical record retention periods for records held by medical doctors and hospitals. https://www.healthit.gov/sites/default/files/appa7-1.pdf. Accessed September 29, 2017.
34. 45 CFR §164.316(b)(2).
35. 42 CFR §422.504(d)(2)(iii).
36. Pope KS, Vasquez MJT. How to survive and thrive as a therapist: information, ideas, and resources for psychologists in practice. Washington, DC: American Psychological Association; 2005.
37. Becher EH, Ogasawara T, Harris SM. Death of a clinician: the personal, practical and clinical implications of therapist mortality. Contemp Fam Ther. 2012;34(3):313-321.
38. Hovey JK. Mortality practices: how clinical social workers interact with their mortality within their clinical and professional practice. Theses, Dissertations, and Projects.Paper 1081. http://scholarworks.smith.edu/cgi/viewcontent.cgi?article=2158&context=theses. Published 2014. Accessed October 1, 2017.
39. Frankel AS, Alban A. Professional wills: protecting patients, family members and colleagues. The Steve Frankel Group. https://www.sfrankelgroup.com/professional-wills.html. Accessed October 31, 2017.
Dear Dr. Mossman,
I have a possibly fatal disease. So far, my symptoms and treatment haven’t kept me from my usual activities. But if my illness worsens, I’ll have to quit practicing psychiatry. What should I be doing now to make sure I fulfill my ethical and legal obligations to my patients?
Submitted by “Dr. F”
“Remember, with great power comes great responsibility.”
- Peter Parker, Spider-Man (2002)
Peter Parker’s movie-ending statement applies to doctors as well as Spider-Man. Although we don’t swing from building to building to save cities from heinous villains, practicing medicine is a privilege that society bestows only upon physicians who retain the knowledge, skills, and ability to treat patients competently.
Doctors retire from practice for many reasons, including when deteriorating physical health or cognitive capacity prevents them from performing clinical duties properly. Dr. F’s situation is not rare. As the physician population ages,1,2 a growing number of his colleagues will face similar circumstances,3,4 and with them, the responsibility and emotional turmoil of arranging to end their medical practices.
In many ways, concluding a psychiatric practice is similar to retiring from practice in other specialties. But because we care for patients’ minds as well as their bodies, retirement affects psychiatrists in distinctive ways that reflect our patients’ feelings toward us and our feelings toward them. To answer Dr. F’s question, this article considers having to stop practicing from 3 vantage points:
- the emotional impact on patients
- the emotional impact on the psychiatrist
- fulfilling one’s legal obligations while attending to the emotions of patients as well as oneself.
Emotional impact on patients
A content analysis study suggests that the traits patients appreciate in family physicians include the availability to listen, caring and compassion, trusted medical judgment, conveying the patient’s importance during encounters, feelings of connectedness, knowledge and understanding of the patient’s family, and relationship longevity.5 The same factors likely apply to relationships between psychiatrists and their patients, particularly if treatment encounters have extended over years and have involved conversations beyond those needed merely to write prescriptions.
Psychoanalytic publications offer many descriptions of patients’ reactions to the illness or death of their mental health professional. A 1978 study of 27 analysands whose physicians died during ongoing therapy reported reactions that ranged from a minimal impact to protracted mourning accompanied by helplessness, intense crying, and recurrent dreams about the analyst.6 Although a few patients were relieved that death had ended a difficult treatment, many were angry at their doctor for not attending to self-care and for breaking their treatment agreement, or because they had missed out on hoped-for benefits.
A 2010 study described the pain and distress that patients may experience following the death of their analyst or psychotherapist. These accounts emphasized the emotional isolation of grieving patients, who do not have the social support that bereaved persons receive after losing a loved one.7 Successful psychotherapy provides a special relationship characterized by trust, intimacy, and safety. But if the therapist suddenly dies, this relationship “is transformed into a solitude like no other.”8
Because the sudden “rupture of an analytic process is bound to be traumatic and may cause iatrogenic injury to the patient,” Traesdal9 advocates that therapists in situations similar to Dr. F’s discuss their possible death “on the reality level at least once during any analysis or psychotherapy.… It is extremely helpful to a patient to have discussed … how to handle the situation” if the therapist dies. This discussion also offers the patient an opportunity to confront a cultural taboo around death and to increase capacity to tolerate pain, illness, and aging.10,11
Most psychiatric care today is not psychoanalysis; psychiatrists provide other forms of care that create less intense doctor–patient relationships. Yet knowledge of these kinds of reactions may help Dr. F stay attuned to his patients’ concerns and to contemplate what they may experience, to greater or lesser degrees, if his health declines.
Retirement’s emotional impact on the psychiatrist
Published guidance on concluding a psychiatric practice is sparse, considering that all psychiatrists are mortal and stop practicing at some point.12Not thinking about or planning for retirement is a psychiatric tradition that started with Freud. He saw patients until shortly before his death and did not seem to have planned for ending his practice, despite suffering with jaw cancer for 16 years.13
Practicing medicine often is more than just a career; it is a core aspect of many physicians’ identity.14 Most of us spend a large fraction of our waking hours caring for patients and meeting other job requirements (eg, teaching, maintaining knowledge and skills), and many of us have scant time to pursue nonmedical interests. An intense prioritization of one’s “medical identity” makes retirement a blow to a doctor’s self-worth and sense of meaning in life.15,16
Because their work is not physically demanding, most psychiatrists continue to practice beyond the age of 65 years.12,17 More important, perhaps, is that being a psychiatrist is uniquely rewarding. As Benjamin Rush observed in an 1810 letter to Pennsylvania Hospital, successfully treating any medical disease is gratifying, but “what is this pleasure compared with that of restoring a fellow creature from the anguish and folly of madness and of reviving in him the knowledge of himself, his family, his friends, and his God!”18
Physicians in any specialty that involves repeated contact with the same patients form emotional bonds with their patients that retirement breaks.14 Psychiatrists’ interest in how patients think, feel, and cope with problems creates special attachments17 that can make some terminations “emotionally excruciating.”12
Psychiatrists with serious illness
What guidance might Dr. F find regarding whether to broach the subject of his illness with patients, and if so, how? No one has conducted controlled trials to answer these questions. Rather, published discussion of psychiatrists’ serious illness is found mainly in the psychotherapy literature. What’s available consists of individual accounts and case series that lack scientific rigor and offer little clarity about what the therapist should say, when to say it, and how to initiate the discussion.19,20 Yet Dr. F may find some of these authors’ ideas and suggestions helpful, particularly if his psychiatric practice includes providing psychotherapy.
As a rule, psychiatrists avoid talking about themselves, but having a serious illness that could affect treatment often justifies deviating from this practice. Although Dr. F (like many psychiatrists) may be concerned that discussing his health will make patients anxious or “contaminate” what they are able or willing to say,21 not providing information or avoiding discussion (especially if a patient asks about your health) may quickly undermine a patient’s trust.21,22 Even in psychoanalytic treatment, it makes little sense to encourage patients “to speak freely on the pretense that all is well, despite obvious evidence to the contrary.”19
Physicians often deny—or at least avoid thinking about—their own mortality.23 But avoiding talking about something so important (and often so obvious) as one’s illness may risk supporting patients’ denial of crucial matters in their own lives.19,21 Moreover, Dr. F’s inadvertent self-disclosure (eg, by displaying obvious signs of illness) may do more harm to therapy than a planned statement in which Dr. F has prepared what he’ll say to answer his patients’ questions.20
That Dr. F has continued working while suffering from a potentially fatal illness seems noble. Yet by doing so, he accepts not only the burdens of his illness but also the obligation to continue to serve his patients competently. This requires maintaining emotional steadiness and not using patients for emotional support, but instead obtaining and using the support of his friends, colleagues, family, consultants, and caregivers.20
Legal obligations
Retirement does not end a physician’s professional legal obligations.24 The legal rules and duties for psychiatrists who leave their practices are similar to those that apply to other physicians. Mishandling these aspects of retirement can result in various legal, licensure-related, or economic consequences, depending on your circumstances and employment arrangements.
Employment contracts in hospital or group practices often require notice of impending departures. If applicable to Dr. F’s situation, failure to comply with such conditions may lead to forfeiture of buyout payments, paying for malpractice tail coverage, or lawsuits claiming violation of contractual agreements.25
Retirement also creates practical and legal responsibilities to patients that are separate from the interpersonal and emotional issues previously discussed. How will those who need ongoing care and coverage be cared for? When withdrawing from a patient’s care (because of retirement or other reasons), a physician should give the patient enough advance notice to set up satisfactory treatment arrangements elsewhere and should facilitate transfer of the patient’s care, if appropriate.26 Failure to meet this ethical obligation may lead to a malpractice action alleging abandonment, which is defined as “the unilateral severance of the professional relationship … without reasonable notice at a time when there is still the necessity of continuing medical attention.”27
Further obligations come from medical licensing boards, which, in many states, have established time frames and specific procedures for informing patients and the public when a physician is leaving practice. Table 124,28-31 lists examples of these. If Dr. F works in a state where the board hasn’t promulgated such regulations, Table 124,28-31 may still help him think through how to discharge his ethical responsibilities to notify patients, colleagues, and business entities that he is ending his practice. References 28-30 and 32 discuss several of these matters, suggest timetables for various steps of a practice closure, and provide sample letters for notifying patients.
Physicians also must preserve their medical records for a certain period after they retire. States with rules on this matter require record preservation for 5 to 10 years or until 2 or 3 years after minor patients reach the age of majority.33 The Health Insurance Portability and Accountability Act of 1996 requires covered entities, which include most psychiatrists, to retain records for 6 years,34 and certain Medicare programs require retention for 10 years.35
Depending on Dr. F’s location and type of practice, his records should be preserved for the longest period that applies. If he is leaving a group practice that owns the records, arranging for this should be easy. If leaving an independent practice, he may need to ask another practice to perform this function.25
A ‘professional will’
Dr. F also might consider a measure that many psychotherapists recommend13,19,36 and that in some states is required by mental health licensing boards or professional codes37,38: creating a “professional will” that contains instructions for handling practice matters in case of death or disability.39

Dear Dr. Mossman,
I have a possibly fatal disease. So far, my symptoms and treatment haven’t kept me from my usual activities. But if my illness worsens, I’ll have to quit practicing psychiatry. What should I be doing now to make sure I fulfill my ethical and legal obligations to my patients?
Submitted by “Dr. F”
“Remember, with great power comes great responsibility.”
- Peter Parker, Spider-Man (2002)
Peter Parker’s movie-ending statement applies to doctors as well as Spider-Man. Although we don’t swing from building to building to save cities from heinous villains, practicing medicine is a privilege that society bestows only upon physicians who retain the knowledge, skills, and ability to treat patients competently.
Doctors retire from practice for many reasons, including when deteriorating physical health or cognitive capacity prevents them from performing clinical duties properly. Dr. F’s situation is not rare. As the physician population ages,1,2 a growing number of his colleagues will face similar circumstances,3,4 and with them, the responsibility and emotional turmoil of arranging to end their medical practices.
In many ways, concluding a psychiatric practice is similar to retiring from practice in other specialties. But because we care for patients’ minds as well as their bodies, retirement affects psychiatrists in distinctive ways that reflect our patients’ feelings toward us and our feelings toward them. To answer Dr. F’s question, this article considers having to stop practicing from 3 vantage points:
- the emotional impact on patients
- the emotional impact on the psychiatrist
- fulfilling one’s legal obligations while attending to the emotions of patients as well as oneself.
Emotional impact on patients
A content analysis study suggests that the traits patients appreciate in family physicians include the availability to listen, caring and compassion, trusted medical judgment, conveying the patient’s importance during encounters, feelings of connectedness, knowledge and understanding of the patient’s family, and relationship longevity.5 The same factors likely apply to relationships between psychiatrists and their patients, particularly if treatment encounters have extended over years and have involved conversations beyond those needed merely to write prescriptions.
Psychoanalytic publications offer many descriptions of patients’ reactions to the illness or death of their mental health professional. A 1978 study of 27 analysands whose physicians died during ongoing therapy reported reactions that ranged from a minimal impact to protracted mourning accompanied by helplessness, intense crying, and recurrent dreams about the analyst.6 Although a few patients were relieved that death had ended a difficult treatment, many were angry at their doctor for not attending to self-care and for breaking their treatment agreement, or because they had missed out on hoped-for benefits.
A 2010 study described the pain and distress that patients may experience following the death of their analyst or psychotherapist. These accounts emphasized the emotional isolation of grieving patients, who do not have the social support that bereaved persons receive after losing a loved one.7 Successful psychotherapy provides a special relationship characterized by trust, intimacy, and safety. But if the therapist suddenly dies, this relationship “is transformed into a solitude like no other.”8
Because the sudden “rupture of an analytic process is bound to be traumatic and may cause iatrogenic injury to the patient,” Traesdal9 advocates that therapists in situations similar to Dr. F’s discuss their possible death “on the reality level at least once during any analysis or psychotherapy.… It is extremely helpful to a patient to have discussed … how to handle the situation” if the therapist dies. This discussion also offers the patient an opportunity to confront a cultural taboo around death and to increase capacity to tolerate pain, illness, and aging.10,11
Most psychiatric care today is not psychoanalysis; psychiatrists provide other forms of care that create less intense doctor–patient relationships. Yet knowledge of these kinds of reactions may help Dr. F stay attuned to his patients’ concerns and to contemplate what they may experience, to greater or lesser degrees, if his health declines.
Retirement’s emotional impact on the psychiatrist
Published guidance on concluding a psychiatric practice is sparse, considering that all psychiatrists are mortal and stop practicing at some point.12Not thinking about or planning for retirement is a psychiatric tradition that started with Freud. He saw patients until shortly before his death and did not seem to have planned for ending his practice, despite suffering with jaw cancer for 16 years.13
Practicing medicine often is more than just a career; it is a core aspect of many physicians’ identity.14 Most of us spend a large fraction of our waking hours caring for patients and meeting other job requirements (eg, teaching, maintaining knowledge and skills), and many of us have scant time to pursue nonmedical interests. An intense prioritization of one’s “medical identity” makes retirement a blow to a doctor’s self-worth and sense of meaning in life.15,16
Because their work is not physically demanding, most psychiatrists continue to practice beyond the age of 65 years.12,17 More important, perhaps, is that being a psychiatrist is uniquely rewarding. As Benjamin Rush observed in an 1810 letter to Pennsylvania Hospital, successfully treating any medical disease is gratifying, but “what is this pleasure compared with that of restoring a fellow creature from the anguish and folly of madness and of reviving in him the knowledge of himself, his family, his friends, and his God!”18
Physicians in any specialty that involves repeated contact with the same patients form emotional bonds with their patients that retirement breaks.14 Psychiatrists’ interest in how patients think, feel, and cope with problems creates special attachments17 that can make some terminations “emotionally excruciating.”12
Psychiatrists with serious illness
What guidance might Dr. F find regarding whether to broach the subject of his illness with patients, and if so, how? No one has conducted controlled trials to answer these questions. Rather, published discussion of psychiatrists’ serious illness is found mainly in the psychotherapy literature. What’s available consists of individual accounts and case series that lack scientific rigor and offer little clarity about what the therapist should say, when to say it, and how to initiate the discussion.19,20 Yet Dr. F may find some of these authors’ ideas and suggestions helpful, particularly if his psychiatric practice includes providing psychotherapy.
As a rule, psychiatrists avoid talking about themselves, but having a serious illness that could affect treatment often justifies deviating from this practice. Although Dr. F (like many psychiatrists) may be concerned that discussing his health will make patients anxious or “contaminate” what they are able or willing to say,21 not providing information or avoiding discussion (especially if a patient asks about your health) may quickly undermine a patient’s trust.21,22 Even in psychoanalytic treatment, it makes little sense to encourage patients “to speak freely on the pretense that all is well, despite obvious evidence to the contrary.”19
Physicians often deny—or at least avoid thinking about—their own mortality.23 But avoiding talking about something so important (and often so obvious) as one’s illness may risk supporting patients’ denial of crucial matters in their own lives.19,21 Moreover, Dr. F’s inadvertent self-disclosure (eg, by displaying obvious signs of illness) may do more harm to therapy than a planned statement in which Dr. F has prepared what he’ll say to answer his patients’ questions.20
That Dr. F has continued working while suffering from a potentially fatal illness seems noble. Yet by doing so, he accepts not only the burdens of his illness but also the obligation to continue to serve his patients competently. This requires maintaining emotional steadiness and not using patients for emotional support, but instead obtaining and using the support of his friends, colleagues, family, consultants, and caregivers.20
Legal obligations
Retirement does not end a physician’s professional legal obligations.24 The legal rules and duties for psychiatrists who leave their practices are similar to those that apply to other physicians. Mishandling these aspects of retirement can result in various legal, licensure-related, or economic consequences, depending on your circumstances and employment arrangements.
Employment contracts in hospital or group practices often require notice of impending departures. If applicable to Dr. F’s situation, failure to comply with such conditions may lead to forfeiture of buyout payments, paying for malpractice tail coverage, or lawsuits claiming violation of contractual agreements.25
Retirement also creates practical and legal responsibilities to patients that are separate from the interpersonal and emotional issues previously discussed. How will those who need ongoing care and coverage be cared for? When withdrawing from a patient’s care (because of retirement or other reasons), a physician should give the patient enough advance notice to set up satisfactory treatment arrangements elsewhere and should facilitate transfer of the patient’s care, if appropriate.26 Failure to meet this ethical obligation may lead to a malpractice action alleging abandonment, which is defined as “the unilateral severance of the professional relationship … without reasonable notice at a time when there is still the necessity of continuing medical attention.”27
Further obligations come from medical licensing boards, which, in many states, have established time frames and specific procedures for informing patients and the public when a physician is leaving practice. Table 124,28-31 lists examples of these. If Dr. F works in a state where the board hasn’t promulgated such regulations, Table 124,28-31 may still help him think through how to discharge his ethical responsibilities to notify patients, colleagues, and business entities that he is ending his practice. References 28-30 and 32 discuss several of these matters, suggest timetables for various steps of a practice closure, and provide sample letters for notifying patients.
Physicians also must preserve their medical records for a certain period after they retire. States with rules on this matter require record preservation for 5 to 10 years or until 2 or 3 years after minor patients reach the age of majority.33 The Health Insurance Portability and Accountability Act of 1996 requires covered entities, which include most psychiatrists, to retain records for 6 years,34 and certain Medicare programs require retention for 10 years.35
Depending on Dr. F’s location and type of practice, his records should be preserved for the longest period that applies. If he is leaving a group practice that owns the records, arranging for this should be easy. If leaving an independent practice, he may need to ask another practice to perform this function.25
A ‘professional will’
Dr. F also might consider a measure that many psychotherapists recommend13,19,36 and that in some states is required by mental health licensing boards or professional codes37,38: creating a “professional will” that contains instructions for handling practice matters in case of death or disability.39

1. LoboPrabhu SM, Molinari VA, Hamilton JD, et al. The aging physician with cognitive impairment: approaches to oversight, prevention, and remediation. Am J Geriatr Psychiatry. 2009;17(6):445-454.
2. Dellinger EP, Pellegrini CA, Gallagher TH. The aging physician and the medical profession: a review. JAMA Surg. 2017;152(10):967-971.
3. Dall T, West T, Chakrabarti R, et al. The complexities of physician supply and demand: projections from 2014 to 2025. Association of American Medical Colleges. https://www.aamc.org/download/458082/data/2016_complexities_of_supply_and_demand_projections.pdf. Published 2016. Accessed September 26, 2017.
4. Draper B, Winfield S, Luscombe G. The older psychiatrist and retirement. Int J Geriatr Psychiatry. 1997;12(2):233-239.
5. Merenstein B, Merenstein J. Patient reflections: saying good-bye to a retiring family doctor. J Am Board Fam Med. 2008;21(5):461-465.
6. Lord R, Ritvo S, Solnit AJ. Patients’ reactions to the death of the psychoanalyst. Intern J Psychoanal. 1978;59(2-3):189-197.
7. Power A. Forced endings in psychotherapy and psychoanalysis: attachment and loss in retirement. New York, NY: Routledge; 2016.
8. Robutti A. When the patient loses his/her analyst. Italian Psychoanalytic Annual. 2010;4:129-145.
9. Traesdal T. When the analyst dies: dealing with the aftermath. J Am Psychoanal Assoc. 2005;53(4):1235-1255.
10. Deutsch RA. A voice lost, a voice found: after the death of the analyst. In: Deutsch RA, ed. Traumatic ruptures: abandonment and betrayal in the analytic relationship. New York, NY: Routledge; 2014:32-45.
11. Ward VP. On Yoda, trouble, and transformation: the cultural context of therapy and supervision. Contemp Fam Ther. 2009;31(3):171-176.
12. Moffic HS. Mental bootcamp: today is the first day of your retirement! Psychiatr Times. http://www.psychiatrictimes.com/blogs/couch-crisis/mental-bootcamp-today-first-day-your-retirement. Published June 25, 2012. Accessed October 31, 2017.
13. Shatsky P. Everything ends: identity and the therapist’s retirement. Clin Soc Work J. 2016;44(2):143-149.
14. Collier R.
15. Onyura B, Bohnen J, Wasylenki D, et al. Reimagining the self at late-career transitions: how identity threat influences academic physicians’ retirement considerations. Acad Med. 2015;90(6):794-801.
16. Silver MP. Critical reflection on physician retirement. Can Fam Physician. 2016;62(10):783-784.
17. Clemens NA. A psychiatrist retires: an oxymoron? J Psychiatr Pract. 2011;17(5):351-354.
18. Packard FR. The earliest hospitals. In: Packard FR. History of medicine in the United States. Philadelphia, PA: Lippincott; 1901:348.
19. Galatzer-Levy RM. The death of the analyst: patients whose previous analyst died while they were in treatment. J Amer Psychoanalytic Assoc. 2004;52(4):999-1024.
20. Fajardo B. Life-threatening illness in the analyst. J Am Psychoanal Assoc. 2001;49(2):569-586.
21. Dewald PA. Serious illness in the analyst: transference, countertransference, and reality responses. J Am Psychoanal Assoc. 1982;30(2):347-363.
22. Howe E. Should psychiatrists self disclose? Innov Clin Neurosci. 2011;8(12):14-17.
23. Rizq R, Voller D. ‘Who is the third who walks always beside you?’ On the death of a psychoanalyst. Psychodyn Pract. 2013;19(2):143-167.
24. Babitsky S, Mangraviti JJ. The biggest legal mistakes physicians make—and how to avoid them. Falmouth, MA: SEAK, Inc.; 2005.
25. Armon BD, Bayus K. Legal considerations when making a practice change. Chest. 2014;146(1):215-219.
26. American Medical Association. Opinions on patient-physician relationships: 1.1.5 terminating a patient-physician relationship. https://www.ama-assn.org/sites/default/files/media-browser/code-of-medical-ethics-chapter-1.pdf. Published 2016. Accessed September 29, 2017.
27. Lee v Dewbre, 362 S.W. 2d 900 (Tex Civ App 7th Dist 1962).
28. Medical Association of Georgia. Issues for the retiring physician. https://www.mag.org/georgia/uploadedfiles/issues-retiring-physicians.pdf. Accessed October 1, 2017.
29. Massachusetts Medical Society. Issues for the retiring physician. http://www.massmed.org/physicians/practice-management/practice-ownership-and-operations/issues-for-the-retiring-physician-(pdf). Published 2012. Accessed October 1, 2017.
30. North Carolina Medical Board. The doctor is out: a physician’s guide to closing a practice. https://www.ncmedboard.org/images/uploads/article_images/Physicians_Guide_to_Closing_a_Practice_05_12_2014.pdf. Published May 12, 2014. Accessed October 1, 2017.
31. 243 Code of Mass. Regulations §2.06(4)(a).
32. Sampson K. Physician’s guide to closing a practice. Maine Medical Association. https://www.mainemed.com/sites/default/files/content/Closing%20Practice%20Guide%20FINAL%206.2014.pdf. Published 2014. Accessed October 1, 2017.
33. HealthIT.gov. State medical record laws: minimum medical record retention periods for records held by medical doctors and hospitals. https://www.healthit.gov/sites/default/files/appa7-1.pdf. Accessed September 29, 2017.
34. 45 CFR §164.316(b)(2).
35. 42 CFR §422.504(d)(2)(iii).
36. Pope KS, Vasquez MJT. How to survive and thrive as a therapist: information, ideas, and resources for psychologists in practice. Washington, DC: American Psychological Association; 2005.
37. Becher EH, Ogasawara T, Harris SM. Death of a clinician: the personal, practical and clinical implications of therapist mortality. Contemp Fam Ther. 2012;34(3):313-321.
38. Hovey JK. Mortality practices: how clinical social workers interact with their mortality within their clinical and professional practice. Theses, Dissertations, and Projects.Paper 1081. http://scholarworks.smith.edu/cgi/viewcontent.cgi?article=2158&context=theses. Published 2014. Accessed October 1, 2017.
39. Frankel AS, Alban A. Professional wills: protecting patients, family members and colleagues. The Steve Frankel Group. https://www.sfrankelgroup.com/professional-wills.html. Accessed October 31, 2017.
1. LoboPrabhu SM, Molinari VA, Hamilton JD, et al. The aging physician with cognitive impairment: approaches to oversight, prevention, and remediation. Am J Geriatr Psychiatry. 2009;17(6):445-454.
2. Dellinger EP, Pellegrini CA, Gallagher TH. The aging physician and the medical profession: a review. JAMA Surg. 2017;152(10):967-971.
3. Dall T, West T, Chakrabarti R, et al. The complexities of physician supply and demand: projections from 2014 to 2025. Association of American Medical Colleges. https://www.aamc.org/download/458082/data/2016_complexities_of_supply_and_demand_projections.pdf. Published 2016. Accessed September 26, 2017.
4. Draper B, Winfield S, Luscombe G. The older psychiatrist and retirement. Int J Geriatr Psychiatry. 1997;12(2):233-239.
5. Merenstein B, Merenstein J. Patient reflections: saying good-bye to a retiring family doctor. J Am Board Fam Med. 2008;21(5):461-465.
6. Lord R, Ritvo S, Solnit AJ. Patients’ reactions to the death of the psychoanalyst. Intern J Psychoanal. 1978;59(2-3):189-197.
7. Power A. Forced endings in psychotherapy and psychoanalysis: attachment and loss in retirement. New York, NY: Routledge; 2016.
8. Robutti A. When the patient loses his/her analyst. Italian Psychoanalytic Annual. 2010;4:129-145.
9. Traesdal T. When the analyst dies: dealing with the aftermath. J Am Psychoanal Assoc. 2005;53(4):1235-1255.
10. Deutsch RA. A voice lost, a voice found: after the death of the analyst. In: Deutsch RA, ed. Traumatic ruptures: abandonment and betrayal in the analytic relationship. New York, NY: Routledge; 2014:32-45.
11. Ward VP. On Yoda, trouble, and transformation: the cultural context of therapy and supervision. Contemp Fam Ther. 2009;31(3):171-176.
12. Moffic HS. Mental bootcamp: today is the first day of your retirement! Psychiatr Times. http://www.psychiatrictimes.com/blogs/couch-crisis/mental-bootcamp-today-first-day-your-retirement. Published June 25, 2012. Accessed October 31, 2017.
13. Shatsky P. Everything ends: identity and the therapist’s retirement. Clin Soc Work J. 2016;44(2):143-149.
14. Collier R.
15. Onyura B, Bohnen J, Wasylenki D, et al. Reimagining the self at late-career transitions: how identity threat influences academic physicians’ retirement considerations. Acad Med. 2015;90(6):794-801.
16. Silver MP. Critical reflection on physician retirement. Can Fam Physician. 2016;62(10):783-784.
17. Clemens NA. A psychiatrist retires: an oxymoron? J Psychiatr Pract. 2011;17(5):351-354.
18. Packard FR. The earliest hospitals. In: Packard FR. History of medicine in the United States. Philadelphia, PA: Lippincott; 1901:348.
19. Galatzer-Levy RM. The death of the analyst: patients whose previous analyst died while they were in treatment. J Amer Psychoanalytic Assoc. 2004;52(4):999-1024.
20. Fajardo B. Life-threatening illness in the analyst. J Am Psychoanal Assoc. 2001;49(2):569-586.
21. Dewald PA. Serious illness in the analyst: transference, countertransference, and reality responses. J Am Psychoanal Assoc. 1982;30(2):347-363.
22. Howe E. Should psychiatrists self disclose? Innov Clin Neurosci. 2011;8(12):14-17.
23. Rizq R, Voller D. ‘Who is the third who walks always beside you?’ On the death of a psychoanalyst. Psychodyn Pract. 2013;19(2):143-167.
24. Babitsky S, Mangraviti JJ. The biggest legal mistakes physicians make—and how to avoid them. Falmouth, MA: SEAK, Inc.; 2005.
25. Armon BD, Bayus K. Legal considerations when making a practice change. Chest. 2014;146(1):215-219.
26. American Medical Association. Opinions on patient-physician relationships: 1.1.5 terminating a patient-physician relationship. https://www.ama-assn.org/sites/default/files/media-browser/code-of-medical-ethics-chapter-1.pdf. Published 2016. Accessed September 29, 2017.
27. Lee v Dewbre, 362 S.W. 2d 900 (Tex Civ App 7th Dist 1962).
28. Medical Association of Georgia. Issues for the retiring physician. https://www.mag.org/georgia/uploadedfiles/issues-retiring-physicians.pdf. Accessed October 1, 2017.
29. Massachusetts Medical Society. Issues for the retiring physician. http://www.massmed.org/physicians/practice-management/practice-ownership-and-operations/issues-for-the-retiring-physician-(pdf). Published 2012. Accessed October 1, 2017.
30. North Carolina Medical Board. The doctor is out: a physician’s guide to closing a practice. https://www.ncmedboard.org/images/uploads/article_images/Physicians_Guide_to_Closing_a_Practice_05_12_2014.pdf. Published May 12, 2014. Accessed October 1, 2017.
31. 243 Code of Mass. Regulations §2.06(4)(a).
32. Sampson K. Physician’s guide to closing a practice. Maine Medical Association. https://www.mainemed.com/sites/default/files/content/Closing%20Practice%20Guide%20FINAL%206.2014.pdf. Published 2014. Accessed October 1, 2017.
33. HealthIT.gov. State medical record laws: minimum medical record retention periods for records held by medical doctors and hospitals. https://www.healthit.gov/sites/default/files/appa7-1.pdf. Accessed September 29, 2017.
34. 45 CFR §164.316(b)(2).
35. 42 CFR §422.504(d)(2)(iii).
36. Pope KS, Vasquez MJT. How to survive and thrive as a therapist: information, ideas, and resources for psychologists in practice. Washington, DC: American Psychological Association; 2005.
37. Becher EH, Ogasawara T, Harris SM. Death of a clinician: the personal, practical and clinical implications of therapist mortality. Contemp Fam Ther. 2012;34(3):313-321.
38. Hovey JK. Mortality practices: how clinical social workers interact with their mortality within their clinical and professional practice. Theses, Dissertations, and Projects.Paper 1081. http://scholarworks.smith.edu/cgi/viewcontent.cgi?article=2158&context=theses. Published 2014. Accessed October 1, 2017.
39. Frankel AS, Alban A. Professional wills: protecting patients, family members and colleagues. The Steve Frankel Group. https://www.sfrankelgroup.com/professional-wills.html. Accessed October 31, 2017.
Self-mutilation after recent-onset psychosis
CASE Bleeding, bewildered
Mr. K, age 23, a South Asian male, is discovered in the bathroom bleeding profusely. Mr. K’s parents inform emergency medical services (EMS) personnel that Mr. K is “not in his right mind” and speculate that he is depressed. EMS personnel find Mr. K sitting in a pool of blood in the bathtub, holding a cloth over his pubic area and complaining of significant pain. They estimate that Mr. K has lost approximately 1 L of blood. Cursory evaluation reveals that his penis is severed; no other injuries or lacerations are notable. Mr. K states, “I did not want it anymore.” A kitchen knife that he used to self-amputate is found nearby. He is awake, alert, and able to follow simple directives.
In the emergency room, Mr. K is in mild-to-moderate distress. He has no history of medical illness, but his parents report that he previously required psychiatric treatment. Mr. K is not able to elaborate. He reluctantly discloses an intermittent history of Cannabis use. Physical examination reveals tachycardia (heart rate: 115 to 120 beats per minute), and despite blood loss, systolic hypertension (blood pressure: 142/70 to 167/70 mm Hg). His pulse oximetry is 97% to 99%; he is afebrile. Laboratory tests are notable for anemia (hemoglobin, 7.2 g/dL [reference range, 14.0 to 17.5 g/dL]; hematocrit, 21.2% [reference range, 41% to 50%]) and serum toxicology screen is positive for benzodiazepines, which had been administered en route to allay his distress.
Mr. K continues to hold pressure on his pubic area. When pressure is released, active arterial spurting of bright red blood is notable. Genital examination reveals a cleanly amputated phallus. Emergent surgical intervention is required to stop the hemorrhage and reattach the penis. Initially, Mr. K is opposed to reattachment, but after a brief discussion with his parents, he consents to surgery. Urology and plastic surgery consultations are elicited to perform the microvascular portion of the procedure.
[polldaddy:9881368]
The authors’ observations
Self-injurious behaviors occur in approximately 1% to 4% of adults in the United States, with chronic and severe self-injury occurring among approximately 1% of the U.S. population.1,2 Intentional GSM is a relatively rare catastrophic event that is often, but not solely, associated with severe mental illness. Because many cases go unreported, the prevalence of GSM is difficult to estimate.3,4 Although GSM has been described in both men and women, the literature has predominantly focused on GSM among men.5 Genital self-injury has been described in several (ie, ethnic/racial and religious) contexts and has been legally sanctioned.6-8
Psychiatric disorders associated with, and precipitating factors underlying, GSM have long remained elusive.8 GSM has been described in case reports and small case series in both psychiatric and urologic literature. These reports provide incomplete descriptions of the diagnostic conditions and psychosocial factors underlying male GSM.
A recent systematic review of 173 cases of men who engaged in GSM published in the past 115 years (since the first case of GSM was published in the psychiatric literature9) revealed that having some form of psychopathology elevates the probability of GSM10,11; rarely the individual did not have a psychiatric condition.11-17 Nearly one-half of the men had psychosis; most had a schizophrenia spectrum disorder diagnosis. Other psychiatric conditions associated with GSM include personality disorders, substance use disorder, and gender dysphoria. GSM is rarely associated with anxiety or mood disorders.
GSM is a heterogeneous form of self-injury that ranges from superficial genital lacerations, amputation, or castration to combinations of these injuries. Compared with individuals with other psychiatric disorders, a significantly greater proportion of individuals with schizophrenia spectrum disorders engage in self-amputation (auto-penectomy). By contrast, persons with gender dysphoria tend to engage in self-castration at significantly higher rates than those with other psychiatric conditions.11 Despite these trends, clinicians should not infer a specific psychiatric diagnosis based on the severity or type of self-inflicted injury.
HISTORY Command hallucinations
Postoperatively, Mr. K is managed in the trauma intensive care unit. During psychiatric consultation, Mr. K demonstrates a blunted affect. His speech is low in volume but clear and coherent. His thoughts are generally linear for specific lines of inquiry (eg, about perceived level of pain) but otherwise are impoverished. Mr. K often digresses into repetitively mumbled prayers. He appears distracted, as if responding to internal stimuli. Although he acknowledges the GSM, he does not discuss the factors underlying his decision to proceed with auto-penectomy. Over successive evaluations, he reluctantly discloses that he had been experiencing disparaging auditory hallucinations that told him that his penis “was too small” and commanded him to “cut it off.”
Psychiatric history reveals that Mr. K required psychiatric hospitalization 7 months earlier due to new-onset auditory hallucinations, paranoia, and thought disorganization, in the context of daily Cannabis use. At the time, the differential diagnosis included new-onset schizophrenia and substance-induced psychosis. His symptoms improved quickly with risperidone, 2 mg/d, and he was discharged in a stable condition with referrals for outpatient care. Mr. K admits he had stopped taking risperidone several weeks before the GSM because he was convinced that he had been cured. At that time, Mr. K had told his parents he was no longer required to take medication or engage in outpatient psychiatric treatment, and they did not question this. Mr. K struggled to sustain part-time employment (in a family business), having taken a leave of absence from graduate school after his first hospitalization. He continued to use Cannabis regularly but denies being intoxicated at the time of the GSM. Throughout his surgical hospitalization, Mr. K’s thoughts remain disorganized. He denies that the GSM was a suicide attempt or having current suicidal thoughts, intent, or plans. He also denies having religious preoccupations, over-valued religious beliefs, or delusions.
Mr. K identifies as heterosexual, and denies experiencing distress related to sexual orientation or gender identity or guilt related to sexual impulses or actions. He also denies having a history of trauma or victimization and does not report any symptoms of posttraumatic stress disorder or body dysmorphic disorder.
The authors’ observations
Little is known about how many individuals who engage in GSM eventually complete suicide. Although suicidal ideation and intent have been infrequently associated with GSM, suicide has been most notably reported among patients with schizophrenia spectrum disorders and psychotic mood disorders.11,18,23-26 For these individuals, suicidal ideation co-occurred with delusions, hallucinations, and pathological guilt preoccupations. Significant self-inflicted injury can be harbinger of distress that could lead to suicide if not optimally treated. Other psychosocial stressors, such as disruptions in interpersonal functioning arising from changes in or loss of social support or perceived rejection, may contribute to a patient’s level of distress, complicating underlying psychiatric disturbances and increasing vulnerability toward GSM.11,27
Substance use also increases vulnerability toward GSM.11,18,24,28 As is the case with patients who engage in various non-GSM self-injurious behaviors,29,30 substance use or intoxication likely contribute to disinhibition or a dissociative state, which enables individuals to engage in self-injury.30
A lack of access to treatment is a rare precipitant for GSM, except among individuals with gender dysphoria. Studies have found that many patients with gender dysphoria who performed self-castration did so in a premeditated manner with low suicidal intent, and the behavior often was related to a lack of or refusal for gender confirmation surgery.31-34
In the hospital setting, surgical/urological interventions need to be directed at the potentially life-threatening sequelae of self-injury. Although complications vary, depending on the type of injury incurred, urgent measures are needed to manage blood loss because hemorrhage can be fatal.23,35,36 Other consequences that can arise include urinary fistulae, urethral strictures, mummification of the glans penis, and development of sensory abnormalities after repair of the injured tissues or reattachment.8 More superficial injuries may require only hemostasis and simple suturing, whereas extensive injuries, such as complete amputation, can be addressed through microvascular techniques.
The psychiatrist’s role. The psychiatrist should act as an advocate for the GSM patient to create an environment conducive to healing. A patient who is experiencing hallucinations or delusions may feel overwhelmed by medical and familial attention. Pharmacologic treatment for prevailing mental illness, such as psychosis, should be initiated in the inpatient setting. An estimated 20% to 25% of those who self-inflict genital injury may repeatedly mutilate their genitals.19,28 Patients unduly influenced by command hallucinations, delusional thought processes, mood disturbances, or suicidal ideation may attempt to complete the injury, or reinjure themselves after surgical/urological intervention, which may require safety measures, such as 1:1 observation, restraints, or physical barriers, to prevent reinjury.37
Self-injury elicits strong, emotional responses from health care professionals, including fascination, apprehension, and hopelessness. Psychiatrists who care for such patients should monitor members of the patient’s treatment team for psychological reactions. In addition, the patient’s behavior while hospitalized may stir feelings of retaliation, anger, fear, and frustration.11,24,37 Collaborative relationships with medical and surgical specialties can help staff manage emotional reactions and avoid the inadvertent expression of those feelings in their interactions with the patient; these reactions might otherwise undermine treatment.24,34 Family education can help mitigate any guilt family members may harbor for not preventing the injury.37
Although efforts to understand the intended goal(s) and precipitants of the self-injury are likely to be worthwhile, the overwhelming distress associated with GSM and its emergent treatment may preclude intensive exploration.
TREATMENT Restarting medication
While on the surgical unit, Mr. K is restarted on risperidone, 2 mg/d. He appears to tolerate the medication without adverse effects. However, because Mr. K continues to experience auditory hallucinations, and the treatment team remains concerned that he might again experience commands to harm himself, he is transferred to an acute psychiatric inpatient setting.
Urology follow-up reveals necrosis/mummification of the replanted penis and an open scrotal wound. After discussing options with the patient and family, the urologist transfers Mr. K back to the surgical unit for wound closure and removal of the replanted penis. A urethrostomy is performed to allow for bladder emptying.
[polldaddy:9881371]
The authors’ observations
Because most published case reports of GSM among men have focused on acute treatment, there is a dearth of literature available on the long-term course of GSM to inform treatment strategies. Because recovery is a non-static process and a patient’s reactions to his injury will likely evolve over time, a multifaceted approach invoking psychiatric and psychotherapeutic interventions is necessary to help patients after initial injury and surgical management37,40-43 (Table 211,20,27,41).
OUTCOME Return to school, work
Mr. K is discharged with close follow-up at a specialized clinic for new-onset psychosis. Post-discharge treatment consists of education about the course of schizophrenia and the need for medication adherence to prevent relapse. Mr. K also is educated on the relationship between Cannabis use and psychosis, and he abstains from illicit substance use. Family involvement is encouraged to help with medication compliance and monitoring for symptom reemergence.
Therapy focuses on exploring the antecedents of the auto-penectomy, Mr. K’s body image issue concerns, and his feelings related to eventual prosthesis implantation. He insists that he cannot recall any precipitating factors for his self-injury other than the command hallucinations. He does not report sexual guilt, although he had been sexually active with his girlfriend in the months prior to his GSM, which goes against his family’s religious beliefs. He reports significant regret and shame for the self-mutilation, and blames himself for not informing family members about his hallucinations. Therapy involves addressing his attribution of blame using cognitive techniques and focuses on measures that can be taken to prevent further self-harm. Efforts are directed at exploring whether cultural and religious traditions impacted the therapeutic alliance, medication adherence, self-esteem and body image, sexuality, and future goals. Over the course of 1 year, he resumes his graduate studies and part-time work, and explores prosthetic placement for cosmetic purposes.
The authors’ observations
Research suggests that major self-mutilation among patients with psychotic illness is likely to occur during the first episode or early in the course of illness and/or with suboptimal treatment.44,45 Mr. K was enlisted in an intensive outpatient treatment program involving biweekly psychotherapy sessions and psychiatric follow-up. Initial sessions focused on education regarding the importance of medication adherence and exploration of signs and symptoms that might suggest reemergence of a psychotic decompensation. The psychiatrist monitored Mr. K closely to ensure he was able to tolerate his medications to mitigate the possibility that adverse effects would undermine adherence. Mr. K’s reactions to having a psychiatric illness also were explored because of concerns that such self-appraisals might trigger shame, embarrassment, denial, and other responses that might undermine treatment adherence. His family members were apprised of treatment goals and enlisted to foster adherence with medication and follow-up appointments.
Mr. K’s Cannabis use was addressed because ongoing use likely had a negative impact on his schizophrenia (ie, a greater propensity toward relapse and rehospitalization and a poorer therapeutic response to antipsychotic medication).46,47 He was strongly encouraged to avoid Cannabis and other illicit substances.
Psychiatrists can help in examining the meaning behind the injury while helping the patient to adapt to the sequelae and cultivate skills to meet functional demands.41 Once Mr. K’s psychotic symptoms were in remission, treatment began to address the antecedents of the GSM, as well as the resultant physical consequences. It was reasonable to explore how Mr. K now viewed his actions, as well as the consequences that his actions produced in terms of his physical appearance, sexual functioning, capacity for sexual intimacy, and reproductive potential. It was also important to recognize how such highly intimate and deeply personal self-schema are framed and organized against his cultural and religious background.27,33
Body image concerns and expectations for future urologic intervention also should be explored. Although Mr. K was not averse to such exploration, he did not spontaneously address such topics in great depth. The discussion was unforced and effectively left open as an issue that could be explored in future sessions.
1. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68(4):609-620.
2. Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501-1508.
3. Krasucki C, Kemp R, David A. A case study of female genital self-mutilation in schizophrenia. Br J Med Psychol. 1995;68(pt 2):179-186.
4. Lennon S. Genital self-mutilation in acute mania. Med J Aust. 1963;50(1):79-81.
5. Schweitzer I. Genital self-amputation and the Klingsor syndrome. Aust N Z J Psychiatry. 1990;24(4):566-569.
6. Anumonye A. Self-inflicted amputation of the penis in two Nigerian males. Niger Med J. 1973;3(1):51-52.
7. Bowman KM, Crook GH. Emotional changes following castration. Psychiatr Res Rep Am Psychiatr Assoc. 1960;12:81-96.
8. Eke N. Genital self-mutilation: there is no method in this madness. BJU Int. 2000;85(3):295-298.
9. Stroch D. Self-castration. JAMA. 1901;36(4):270.
10. Veeder TA, Leo RJ. Male genital self-mutilation: a comprehensive review of psychiatric disorders. Poster presented at: Academy of Psychosomatic Medicine Meeting, Austin, Texas, November 10, 2016.
11. Veeder TA, Leo RJ. Male genital self-mutilation: a systematic review of psychiatric disorders and psychosocial factors. Gen Hosp Psychiatry. 2017;44:43-50.
12. Battle AO. The psychological appraisal of a patient who had performed self-castration. British Journal of Projective Psychology & Personality Study. 1973;18(2):5-17.
13. Bhatia MS, Arora S. Penile self-mutilation. Br J Psychiatry. 2001;178(1):86-87.
14. Gleeson MJ, Connolly J, Grainger R. Self-castration as treatment for alopecia. Br J Urol. 1993;71(5):614-615.
15. Hendershot E, Stutson AC, Adair TW. A case of extreme sexual self-mutilation. J Forensic Sci. 2010;55(1):245-247.
16. Hermann M, Thorstenson A. A rare case of male‐to‐eunuch gender dysphoria. Sex Med. 2015;3(4):331-333.
17. Nerli RB, Ravish IR, Amarkhed SS, et al. Genital self-mutilation in nonpsychotic heterosexual males: case report of two cases. Indian J Psychiatry. 2008;50(4):285-287.
18. Blacker KH, Wong N. Four cases of autocastration. Arch Gen Psychiatry. 1963;8:169-176.
19. Catalano G, Catalano MC, Carroll KM. Repetitive male genital self-mutilation: a case report and discussion of possible risk factors. J Sex Marital Ther. 2002;28(1):27-37.
20. Martin T, Gattaz WF. Psychiatric aspects of male genital self-mutilation. Psychopathology. 1991;24(3):170-178.
21. Money J. The Skoptic syndrome: castration and genital self-mutilation as an example of sexual body-image pathology. J Psychol Human Sex. 1988;1(1):113-128.
22. Nakaya M. On background factors of male genital self-mutilation. Psychopathology. 1996;29(4):242-248.
23. Borenstein A, Yaffe B, Seidman DS, et al. Successful microvascular replantation of an amputated penis. Isr J Med Sci. 1991;27(7):395-398.
24. Greilsheimer H, Groves JE. Male genital self-mutilation. Arch Gen Psychiatry. 1979;36(4):441-446.
25. Mendez R, Kiely WF, Morrow JW. Self-emasculation. J Urol. 1972;107(6):981-985.
26. Siddique RA, Deshpande S. A case of genital self-mutilation in a patient with psychosis. German J Psychiatry. 2007;10(1):25-28.
27. Qureshi NA. Male genital self-mutilation with special emphasis on the sociocultural meanings. Neurosciences (Riyadh). 2009;14(2):178-181.
28. Romilly CS, Isaac MT. Male genital self-mutilation. Br J Hosp Med. 1996;55(7):427-431.
29. Gahr M, Plener PL, Kölle MA, et al. Self-mutilation induced by psychotropic substances: a systematic review. Psychiatry Res. 2012;200(2-3):977-983.
30. Evren C, Sar V, Evren B, et al. Self-mutilation among male patients with alcohol dependency: the role of dissociation. Compr Psychiatry. 2008;49(5):489-495.
31. Brown GR. Autocastration and autopenectomy as surgical self-treatment in incarcerated persons with gender identity disorder. Int J Transgend. 2010;12(1):31-39.
32. Master VA, McAninch JW, Santucci RA. Genital self-mutilation and the Internet. J Urol. 2000;164(5):1656.
33. Premand NE, Eytan A. A case of non-psychotic autocastration: the importance of cultural factors. Psychiatry. 2005;68(2):174-178.
34. Simopoulos EF, Trinidad AC. Two cases of male genital self-mutilation: an examination of liaison dynamics. Psychosomatics. 2012;53(2):178-180.
35. Darewicz B, Galek L, Darewicz J, et al. Successful microsurgical replantation of an amputated penis. Int Urol Nephrol. 2001;33(2):385-386.
36. Raheem OA, Mirheydar HS, Patel ND, et al. Surgical management of traumatic penile amputation: a case report and review of the world literature. Sex Med. 2015;3(1):49-53.
37. Young LD, Feinsilver DL. Male genital self-mutilation: combined surgical and psychiatric care. Psychosomatics. 1986;27(7):513-517.
38. Walsh B. Clinical assessment of self-injury: a practical guide. J Clin Psychol. 2007;63(11):1057-1066.
39. Nafisi N, Stanley B. Developing and maintaining the therapeutic alliance with self-injuring patients. J Clin Psychol. 2007;63(11):1069-1079.
40. Fisch RZ. Genital self-mutilation in males: psychodynamic anatomy of a psychosis. Am J Psychother. 1987;41(3):453-458.
41. King PR. Cognitive-behavioral intervention in a case of self-mutilation. Clin Case Stud. 2014;13(2):181-189.
42. Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. J Ment Health Couns. 2006;28(2):166-185.
43. Walsh BW. Treating self-injury: a practical guide. New York, NY: The Guilford Press; 2006.
44. Large M, Babidge N, Andrews D, et al. Major self-mutilation in the first episode of psychosis. Schizophr Bull. 2009;35(5):1012-1021.
45. Large MM, Nielssen OB, Babidge N. Untreated psychosis is the main cause of major self-mutilation. Isr J Psychiatry Relat Sci. 2011;48(1):65.
46. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21.
47. Bowers MB Jr, Mazure CM, Nelson JC, et al. Psychotogenic drug use and neuroleptic response. Schizophr Bull. 1990;16(1):81-85.
CASE Bleeding, bewildered
Mr. K, age 23, a South Asian male, is discovered in the bathroom bleeding profusely. Mr. K’s parents inform emergency medical services (EMS) personnel that Mr. K is “not in his right mind” and speculate that he is depressed. EMS personnel find Mr. K sitting in a pool of blood in the bathtub, holding a cloth over his pubic area and complaining of significant pain. They estimate that Mr. K has lost approximately 1 L of blood. Cursory evaluation reveals that his penis is severed; no other injuries or lacerations are notable. Mr. K states, “I did not want it anymore.” A kitchen knife that he used to self-amputate is found nearby. He is awake, alert, and able to follow simple directives.
In the emergency room, Mr. K is in mild-to-moderate distress. He has no history of medical illness, but his parents report that he previously required psychiatric treatment. Mr. K is not able to elaborate. He reluctantly discloses an intermittent history of Cannabis use. Physical examination reveals tachycardia (heart rate: 115 to 120 beats per minute), and despite blood loss, systolic hypertension (blood pressure: 142/70 to 167/70 mm Hg). His pulse oximetry is 97% to 99%; he is afebrile. Laboratory tests are notable for anemia (hemoglobin, 7.2 g/dL [reference range, 14.0 to 17.5 g/dL]; hematocrit, 21.2% [reference range, 41% to 50%]) and serum toxicology screen is positive for benzodiazepines, which had been administered en route to allay his distress.
Mr. K continues to hold pressure on his pubic area. When pressure is released, active arterial spurting of bright red blood is notable. Genital examination reveals a cleanly amputated phallus. Emergent surgical intervention is required to stop the hemorrhage and reattach the penis. Initially, Mr. K is opposed to reattachment, but after a brief discussion with his parents, he consents to surgery. Urology and plastic surgery consultations are elicited to perform the microvascular portion of the procedure.
[polldaddy:9881368]
The authors’ observations
Self-injurious behaviors occur in approximately 1% to 4% of adults in the United States, with chronic and severe self-injury occurring among approximately 1% of the U.S. population.1,2 Intentional GSM is a relatively rare catastrophic event that is often, but not solely, associated with severe mental illness. Because many cases go unreported, the prevalence of GSM is difficult to estimate.3,4 Although GSM has been described in both men and women, the literature has predominantly focused on GSM among men.5 Genital self-injury has been described in several (ie, ethnic/racial and religious) contexts and has been legally sanctioned.6-8
Psychiatric disorders associated with, and precipitating factors underlying, GSM have long remained elusive.8 GSM has been described in case reports and small case series in both psychiatric and urologic literature. These reports provide incomplete descriptions of the diagnostic conditions and psychosocial factors underlying male GSM.
A recent systematic review of 173 cases of men who engaged in GSM published in the past 115 years (since the first case of GSM was published in the psychiatric literature9) revealed that having some form of psychopathology elevates the probability of GSM10,11; rarely the individual did not have a psychiatric condition.11-17 Nearly one-half of the men had psychosis; most had a schizophrenia spectrum disorder diagnosis. Other psychiatric conditions associated with GSM include personality disorders, substance use disorder, and gender dysphoria. GSM is rarely associated with anxiety or mood disorders.
GSM is a heterogeneous form of self-injury that ranges from superficial genital lacerations, amputation, or castration to combinations of these injuries. Compared with individuals with other psychiatric disorders, a significantly greater proportion of individuals with schizophrenia spectrum disorders engage in self-amputation (auto-penectomy). By contrast, persons with gender dysphoria tend to engage in self-castration at significantly higher rates than those with other psychiatric conditions.11 Despite these trends, clinicians should not infer a specific psychiatric diagnosis based on the severity or type of self-inflicted injury.
HISTORY Command hallucinations
Postoperatively, Mr. K is managed in the trauma intensive care unit. During psychiatric consultation, Mr. K demonstrates a blunted affect. His speech is low in volume but clear and coherent. His thoughts are generally linear for specific lines of inquiry (eg, about perceived level of pain) but otherwise are impoverished. Mr. K often digresses into repetitively mumbled prayers. He appears distracted, as if responding to internal stimuli. Although he acknowledges the GSM, he does not discuss the factors underlying his decision to proceed with auto-penectomy. Over successive evaluations, he reluctantly discloses that he had been experiencing disparaging auditory hallucinations that told him that his penis “was too small” and commanded him to “cut it off.”
Psychiatric history reveals that Mr. K required psychiatric hospitalization 7 months earlier due to new-onset auditory hallucinations, paranoia, and thought disorganization, in the context of daily Cannabis use. At the time, the differential diagnosis included new-onset schizophrenia and substance-induced psychosis. His symptoms improved quickly with risperidone, 2 mg/d, and he was discharged in a stable condition with referrals for outpatient care. Mr. K admits he had stopped taking risperidone several weeks before the GSM because he was convinced that he had been cured. At that time, Mr. K had told his parents he was no longer required to take medication or engage in outpatient psychiatric treatment, and they did not question this. Mr. K struggled to sustain part-time employment (in a family business), having taken a leave of absence from graduate school after his first hospitalization. He continued to use Cannabis regularly but denies being intoxicated at the time of the GSM. Throughout his surgical hospitalization, Mr. K’s thoughts remain disorganized. He denies that the GSM was a suicide attempt or having current suicidal thoughts, intent, or plans. He also denies having religious preoccupations, over-valued religious beliefs, or delusions.
Mr. K identifies as heterosexual, and denies experiencing distress related to sexual orientation or gender identity or guilt related to sexual impulses or actions. He also denies having a history of trauma or victimization and does not report any symptoms of posttraumatic stress disorder or body dysmorphic disorder.
The authors’ observations
Little is known about how many individuals who engage in GSM eventually complete suicide. Although suicidal ideation and intent have been infrequently associated with GSM, suicide has been most notably reported among patients with schizophrenia spectrum disorders and psychotic mood disorders.11,18,23-26 For these individuals, suicidal ideation co-occurred with delusions, hallucinations, and pathological guilt preoccupations. Significant self-inflicted injury can be harbinger of distress that could lead to suicide if not optimally treated. Other psychosocial stressors, such as disruptions in interpersonal functioning arising from changes in or loss of social support or perceived rejection, may contribute to a patient’s level of distress, complicating underlying psychiatric disturbances and increasing vulnerability toward GSM.11,27
Substance use also increases vulnerability toward GSM.11,18,24,28 As is the case with patients who engage in various non-GSM self-injurious behaviors,29,30 substance use or intoxication likely contribute to disinhibition or a dissociative state, which enables individuals to engage in self-injury.30
A lack of access to treatment is a rare precipitant for GSM, except among individuals with gender dysphoria. Studies have found that many patients with gender dysphoria who performed self-castration did so in a premeditated manner with low suicidal intent, and the behavior often was related to a lack of or refusal for gender confirmation surgery.31-34
In the hospital setting, surgical/urological interventions need to be directed at the potentially life-threatening sequelae of self-injury. Although complications vary, depending on the type of injury incurred, urgent measures are needed to manage blood loss because hemorrhage can be fatal.23,35,36 Other consequences that can arise include urinary fistulae, urethral strictures, mummification of the glans penis, and development of sensory abnormalities after repair of the injured tissues or reattachment.8 More superficial injuries may require only hemostasis and simple suturing, whereas extensive injuries, such as complete amputation, can be addressed through microvascular techniques.
The psychiatrist’s role. The psychiatrist should act as an advocate for the GSM patient to create an environment conducive to healing. A patient who is experiencing hallucinations or delusions may feel overwhelmed by medical and familial attention. Pharmacologic treatment for prevailing mental illness, such as psychosis, should be initiated in the inpatient setting. An estimated 20% to 25% of those who self-inflict genital injury may repeatedly mutilate their genitals.19,28 Patients unduly influenced by command hallucinations, delusional thought processes, mood disturbances, or suicidal ideation may attempt to complete the injury, or reinjure themselves after surgical/urological intervention, which may require safety measures, such as 1:1 observation, restraints, or physical barriers, to prevent reinjury.37
Self-injury elicits strong, emotional responses from health care professionals, including fascination, apprehension, and hopelessness. Psychiatrists who care for such patients should monitor members of the patient’s treatment team for psychological reactions. In addition, the patient’s behavior while hospitalized may stir feelings of retaliation, anger, fear, and frustration.11,24,37 Collaborative relationships with medical and surgical specialties can help staff manage emotional reactions and avoid the inadvertent expression of those feelings in their interactions with the patient; these reactions might otherwise undermine treatment.24,34 Family education can help mitigate any guilt family members may harbor for not preventing the injury.37
Although efforts to understand the intended goal(s) and precipitants of the self-injury are likely to be worthwhile, the overwhelming distress associated with GSM and its emergent treatment may preclude intensive exploration.
TREATMENT Restarting medication
While on the surgical unit, Mr. K is restarted on risperidone, 2 mg/d. He appears to tolerate the medication without adverse effects. However, because Mr. K continues to experience auditory hallucinations, and the treatment team remains concerned that he might again experience commands to harm himself, he is transferred to an acute psychiatric inpatient setting.
Urology follow-up reveals necrosis/mummification of the replanted penis and an open scrotal wound. After discussing options with the patient and family, the urologist transfers Mr. K back to the surgical unit for wound closure and removal of the replanted penis. A urethrostomy is performed to allow for bladder emptying.
[polldaddy:9881371]
The authors’ observations
Because most published case reports of GSM among men have focused on acute treatment, there is a dearth of literature available on the long-term course of GSM to inform treatment strategies. Because recovery is a non-static process and a patient’s reactions to his injury will likely evolve over time, a multifaceted approach invoking psychiatric and psychotherapeutic interventions is necessary to help patients after initial injury and surgical management37,40-43 (Table 211,20,27,41).

OUTCOME Return to school, work
Mr. K is discharged with close follow-up at a specialized clinic for new-onset psychosis. Post-discharge treatment consists of education about the course of schizophrenia and the need for medication adherence to prevent relapse. Mr. K also is educated on the relationship between Cannabis use and psychosis, and he abstains from illicit substance use. Family involvement is encouraged to help with medication compliance and monitoring for symptom reemergence.
Therapy focuses on exploring the antecedents of the auto-penectomy, Mr. K’s body image issue concerns, and his feelings related to eventual prosthesis implantation. He insists that he cannot recall any precipitating factors for his self-injury other than the command hallucinations. He does not report sexual guilt, although he had been sexually active with his girlfriend in the months prior to his GSM, which goes against his family’s religious beliefs. He reports significant regret and shame for the self-mutilation, and blames himself for not informing family members about his hallucinations. Therapy involves addressing his attribution of blame using cognitive techniques and focuses on measures that can be taken to prevent further self-harm. Efforts are directed at exploring whether cultural and religious traditions impacted the therapeutic alliance, medication adherence, self-esteem and body image, sexuality, and future goals. Over the course of 1 year, he resumes his graduate studies and part-time work, and explores prosthetic placement for cosmetic purposes.
The authors’ observations
Research suggests that major self-mutilation among patients with psychotic illness is likely to occur during the first episode or early in the course of illness and/or with suboptimal treatment.44,45 Mr. K was enlisted in an intensive outpatient treatment program involving biweekly psychotherapy sessions and psychiatric follow-up. Initial sessions focused on education regarding the importance of medication adherence and exploration of signs and symptoms that might suggest reemergence of a psychotic decompensation. The psychiatrist monitored Mr. K closely to ensure he was able to tolerate his medications to mitigate the possibility that adverse effects would undermine adherence. Mr. K’s reactions to having a psychiatric illness also were explored because of concerns that such self-appraisals might trigger shame, embarrassment, denial, and other responses that might undermine treatment adherence. His family members were apprised of treatment goals and enlisted to foster adherence with medication and follow-up appointments.
Mr. K’s Cannabis use was addressed because ongoing use likely had a negative impact on his schizophrenia (ie, a greater propensity toward relapse and rehospitalization and a poorer therapeutic response to antipsychotic medication).46,47 He was strongly encouraged to avoid Cannabis and other illicit substances.
Psychiatrists can help in examining the meaning behind the injury while helping the patient to adapt to the sequelae and cultivate skills to meet functional demands.41 Once Mr. K’s psychotic symptoms were in remission, treatment began to address the antecedents of the GSM, as well as the resultant physical consequences. It was reasonable to explore how Mr. K now viewed his actions, as well as the consequences that his actions produced in terms of his physical appearance, sexual functioning, capacity for sexual intimacy, and reproductive potential. It was also important to recognize how such highly intimate and deeply personal self-schema are framed and organized against his cultural and religious background.27,33
Body image concerns and expectations for future urologic intervention also should be explored. Although Mr. K was not averse to such exploration, he did not spontaneously address such topics in great depth. The discussion was unforced and effectively left open as an issue that could be explored in future sessions.
CASE Bleeding, bewildered
Mr. K, age 23, a South Asian male, is discovered in the bathroom bleeding profusely. Mr. K’s parents inform emergency medical services (EMS) personnel that Mr. K is “not in his right mind” and speculate that he is depressed. EMS personnel find Mr. K sitting in a pool of blood in the bathtub, holding a cloth over his pubic area and complaining of significant pain. They estimate that Mr. K has lost approximately 1 L of blood. Cursory evaluation reveals that his penis is severed; no other injuries or lacerations are notable. Mr. K states, “I did not want it anymore.” A kitchen knife that he used to self-amputate is found nearby. He is awake, alert, and able to follow simple directives.
In the emergency room, Mr. K is in mild-to-moderate distress. He has no history of medical illness, but his parents report that he previously required psychiatric treatment. Mr. K is not able to elaborate. He reluctantly discloses an intermittent history of Cannabis use. Physical examination reveals tachycardia (heart rate: 115 to 120 beats per minute), and despite blood loss, systolic hypertension (blood pressure: 142/70 to 167/70 mm Hg). His pulse oximetry is 97% to 99%; he is afebrile. Laboratory tests are notable for anemia (hemoglobin, 7.2 g/dL [reference range, 14.0 to 17.5 g/dL]; hematocrit, 21.2% [reference range, 41% to 50%]) and serum toxicology screen is positive for benzodiazepines, which had been administered en route to allay his distress.
Mr. K continues to hold pressure on his pubic area. When pressure is released, active arterial spurting of bright red blood is notable. Genital examination reveals a cleanly amputated phallus. Emergent surgical intervention is required to stop the hemorrhage and reattach the penis. Initially, Mr. K is opposed to reattachment, but after a brief discussion with his parents, he consents to surgery. Urology and plastic surgery consultations are elicited to perform the microvascular portion of the procedure.
[polldaddy:9881368]
The authors’ observations
Self-injurious behaviors occur in approximately 1% to 4% of adults in the United States, with chronic and severe self-injury occurring among approximately 1% of the U.S. population.1,2 Intentional GSM is a relatively rare catastrophic event that is often, but not solely, associated with severe mental illness. Because many cases go unreported, the prevalence of GSM is difficult to estimate.3,4 Although GSM has been described in both men and women, the literature has predominantly focused on GSM among men.5 Genital self-injury has been described in several (ie, ethnic/racial and religious) contexts and has been legally sanctioned.6-8
Psychiatric disorders associated with, and precipitating factors underlying, GSM have long remained elusive.8 GSM has been described in case reports and small case series in both psychiatric and urologic literature. These reports provide incomplete descriptions of the diagnostic conditions and psychosocial factors underlying male GSM.
A recent systematic review of 173 cases of men who engaged in GSM published in the past 115 years (since the first case of GSM was published in the psychiatric literature9) revealed that having some form of psychopathology elevates the probability of GSM10,11; rarely the individual did not have a psychiatric condition.11-17 Nearly one-half of the men had psychosis; most had a schizophrenia spectrum disorder diagnosis. Other psychiatric conditions associated with GSM include personality disorders, substance use disorder, and gender dysphoria. GSM is rarely associated with anxiety or mood disorders.
GSM is a heterogeneous form of self-injury that ranges from superficial genital lacerations, amputation, or castration to combinations of these injuries. Compared with individuals with other psychiatric disorders, a significantly greater proportion of individuals with schizophrenia spectrum disorders engage in self-amputation (auto-penectomy). By contrast, persons with gender dysphoria tend to engage in self-castration at significantly higher rates than those with other psychiatric conditions.11 Despite these trends, clinicians should not infer a specific psychiatric diagnosis based on the severity or type of self-inflicted injury.
HISTORY Command hallucinations
Postoperatively, Mr. K is managed in the trauma intensive care unit. During psychiatric consultation, Mr. K demonstrates a blunted affect. His speech is low in volume but clear and coherent. His thoughts are generally linear for specific lines of inquiry (eg, about perceived level of pain) but otherwise are impoverished. Mr. K often digresses into repetitively mumbled prayers. He appears distracted, as if responding to internal stimuli. Although he acknowledges the GSM, he does not discuss the factors underlying his decision to proceed with auto-penectomy. Over successive evaluations, he reluctantly discloses that he had been experiencing disparaging auditory hallucinations that told him that his penis “was too small” and commanded him to “cut it off.”
Psychiatric history reveals that Mr. K required psychiatric hospitalization 7 months earlier due to new-onset auditory hallucinations, paranoia, and thought disorganization, in the context of daily Cannabis use. At the time, the differential diagnosis included new-onset schizophrenia and substance-induced psychosis. His symptoms improved quickly with risperidone, 2 mg/d, and he was discharged in a stable condition with referrals for outpatient care. Mr. K admits he had stopped taking risperidone several weeks before the GSM because he was convinced that he had been cured. At that time, Mr. K had told his parents he was no longer required to take medication or engage in outpatient psychiatric treatment, and they did not question this. Mr. K struggled to sustain part-time employment (in a family business), having taken a leave of absence from graduate school after his first hospitalization. He continued to use Cannabis regularly but denies being intoxicated at the time of the GSM. Throughout his surgical hospitalization, Mr. K’s thoughts remain disorganized. He denies that the GSM was a suicide attempt or having current suicidal thoughts, intent, or plans. He also denies having religious preoccupations, over-valued religious beliefs, or delusions.
Mr. K identifies as heterosexual, and denies experiencing distress related to sexual orientation or gender identity or guilt related to sexual impulses or actions. He also denies having a history of trauma or victimization and does not report any symptoms of posttraumatic stress disorder or body dysmorphic disorder.
The authors’ observations
Little is known about how many individuals who engage in GSM eventually complete suicide. Although suicidal ideation and intent have been infrequently associated with GSM, suicide has been most notably reported among patients with schizophrenia spectrum disorders and psychotic mood disorders.11,18,23-26 For these individuals, suicidal ideation co-occurred with delusions, hallucinations, and pathological guilt preoccupations. Significant self-inflicted injury can be harbinger of distress that could lead to suicide if not optimally treated. Other psychosocial stressors, such as disruptions in interpersonal functioning arising from changes in or loss of social support or perceived rejection, may contribute to a patient’s level of distress, complicating underlying psychiatric disturbances and increasing vulnerability toward GSM.11,27
Substance use also increases vulnerability toward GSM.11,18,24,28 As is the case with patients who engage in various non-GSM self-injurious behaviors,29,30 substance use or intoxication likely contribute to disinhibition or a dissociative state, which enables individuals to engage in self-injury.30
A lack of access to treatment is a rare precipitant for GSM, except among individuals with gender dysphoria. Studies have found that many patients with gender dysphoria who performed self-castration did so in a premeditated manner with low suicidal intent, and the behavior often was related to a lack of or refusal for gender confirmation surgery.31-34
In the hospital setting, surgical/urological interventions need to be directed at the potentially life-threatening sequelae of self-injury. Although complications vary, depending on the type of injury incurred, urgent measures are needed to manage blood loss because hemorrhage can be fatal.23,35,36 Other consequences that can arise include urinary fistulae, urethral strictures, mummification of the glans penis, and development of sensory abnormalities after repair of the injured tissues or reattachment.8 More superficial injuries may require only hemostasis and simple suturing, whereas extensive injuries, such as complete amputation, can be addressed through microvascular techniques.
The psychiatrist’s role. The psychiatrist should act as an advocate for the GSM patient to create an environment conducive to healing. A patient who is experiencing hallucinations or delusions may feel overwhelmed by medical and familial attention. Pharmacologic treatment for prevailing mental illness, such as psychosis, should be initiated in the inpatient setting. An estimated 20% to 25% of those who self-inflict genital injury may repeatedly mutilate their genitals.19,28 Patients unduly influenced by command hallucinations, delusional thought processes, mood disturbances, or suicidal ideation may attempt to complete the injury, or reinjure themselves after surgical/urological intervention, which may require safety measures, such as 1:1 observation, restraints, or physical barriers, to prevent reinjury.37
Self-injury elicits strong, emotional responses from health care professionals, including fascination, apprehension, and hopelessness. Psychiatrists who care for such patients should monitor members of the patient’s treatment team for psychological reactions. In addition, the patient’s behavior while hospitalized may stir feelings of retaliation, anger, fear, and frustration.11,24,37 Collaborative relationships with medical and surgical specialties can help staff manage emotional reactions and avoid the inadvertent expression of those feelings in their interactions with the patient; these reactions might otherwise undermine treatment.24,34 Family education can help mitigate any guilt family members may harbor for not preventing the injury.37
Although efforts to understand the intended goal(s) and precipitants of the self-injury are likely to be worthwhile, the overwhelming distress associated with GSM and its emergent treatment may preclude intensive exploration.
TREATMENT Restarting medication
While on the surgical unit, Mr. K is restarted on risperidone, 2 mg/d. He appears to tolerate the medication without adverse effects. However, because Mr. K continues to experience auditory hallucinations, and the treatment team remains concerned that he might again experience commands to harm himself, he is transferred to an acute psychiatric inpatient setting.
Urology follow-up reveals necrosis/mummification of the replanted penis and an open scrotal wound. After discussing options with the patient and family, the urologist transfers Mr. K back to the surgical unit for wound closure and removal of the replanted penis. A urethrostomy is performed to allow for bladder emptying.
[polldaddy:9881371]
The authors’ observations
Because most published case reports of GSM among men have focused on acute treatment, there is a dearth of literature available on the long-term course of GSM to inform treatment strategies. Because recovery is a non-static process and a patient’s reactions to his injury will likely evolve over time, a multifaceted approach invoking psychiatric and psychotherapeutic interventions is necessary to help patients after initial injury and surgical management37,40-43 (Table 211,20,27,41).

OUTCOME Return to school, work
Mr. K is discharged with close follow-up at a specialized clinic for new-onset psychosis. Post-discharge treatment consists of education about the course of schizophrenia and the need for medication adherence to prevent relapse. Mr. K also is educated on the relationship between Cannabis use and psychosis, and he abstains from illicit substance use. Family involvement is encouraged to help with medication compliance and monitoring for symptom reemergence.
Therapy focuses on exploring the antecedents of the auto-penectomy, Mr. K’s body image issue concerns, and his feelings related to eventual prosthesis implantation. He insists that he cannot recall any precipitating factors for his self-injury other than the command hallucinations. He does not report sexual guilt, although he had been sexually active with his girlfriend in the months prior to his GSM, which goes against his family’s religious beliefs. He reports significant regret and shame for the self-mutilation, and blames himself for not informing family members about his hallucinations. Therapy involves addressing his attribution of blame using cognitive techniques and focuses on measures that can be taken to prevent further self-harm. Efforts are directed at exploring whether cultural and religious traditions impacted the therapeutic alliance, medication adherence, self-esteem and body image, sexuality, and future goals. Over the course of 1 year, he resumes his graduate studies and part-time work, and explores prosthetic placement for cosmetic purposes.
The authors’ observations
Research suggests that major self-mutilation among patients with psychotic illness is likely to occur during the first episode or early in the course of illness and/or with suboptimal treatment.44,45 Mr. K was enlisted in an intensive outpatient treatment program involving biweekly psychotherapy sessions and psychiatric follow-up. Initial sessions focused on education regarding the importance of medication adherence and exploration of signs and symptoms that might suggest reemergence of a psychotic decompensation. The psychiatrist monitored Mr. K closely to ensure he was able to tolerate his medications to mitigate the possibility that adverse effects would undermine adherence. Mr. K’s reactions to having a psychiatric illness also were explored because of concerns that such self-appraisals might trigger shame, embarrassment, denial, and other responses that might undermine treatment adherence. His family members were apprised of treatment goals and enlisted to foster adherence with medication and follow-up appointments.
Mr. K’s Cannabis use was addressed because ongoing use likely had a negative impact on his schizophrenia (ie, a greater propensity toward relapse and rehospitalization and a poorer therapeutic response to antipsychotic medication).46,47 He was strongly encouraged to avoid Cannabis and other illicit substances.
Psychiatrists can help in examining the meaning behind the injury while helping the patient to adapt to the sequelae and cultivate skills to meet functional demands.41 Once Mr. K’s psychotic symptoms were in remission, treatment began to address the antecedents of the GSM, as well as the resultant physical consequences. It was reasonable to explore how Mr. K now viewed his actions, as well as the consequences that his actions produced in terms of his physical appearance, sexual functioning, capacity for sexual intimacy, and reproductive potential. It was also important to recognize how such highly intimate and deeply personal self-schema are framed and organized against his cultural and religious background.27,33
Body image concerns and expectations for future urologic intervention also should be explored. Although Mr. K was not averse to such exploration, he did not spontaneously address such topics in great depth. The discussion was unforced and effectively left open as an issue that could be explored in future sessions.
1. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68(4):609-620.
2. Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501-1508.
3. Krasucki C, Kemp R, David A. A case study of female genital self-mutilation in schizophrenia. Br J Med Psychol. 1995;68(pt 2):179-186.
4. Lennon S. Genital self-mutilation in acute mania. Med J Aust. 1963;50(1):79-81.
5. Schweitzer I. Genital self-amputation and the Klingsor syndrome. Aust N Z J Psychiatry. 1990;24(4):566-569.
6. Anumonye A. Self-inflicted amputation of the penis in two Nigerian males. Niger Med J. 1973;3(1):51-52.
7. Bowman KM, Crook GH. Emotional changes following castration. Psychiatr Res Rep Am Psychiatr Assoc. 1960;12:81-96.
8. Eke N. Genital self-mutilation: there is no method in this madness. BJU Int. 2000;85(3):295-298.
9. Stroch D. Self-castration. JAMA. 1901;36(4):270.
10. Veeder TA, Leo RJ. Male genital self-mutilation: a comprehensive review of psychiatric disorders. Poster presented at: Academy of Psychosomatic Medicine Meeting, Austin, Texas, November 10, 2016.
11. Veeder TA, Leo RJ. Male genital self-mutilation: a systematic review of psychiatric disorders and psychosocial factors. Gen Hosp Psychiatry. 2017;44:43-50.
12. Battle AO. The psychological appraisal of a patient who had performed self-castration. British Journal of Projective Psychology & Personality Study. 1973;18(2):5-17.
13. Bhatia MS, Arora S. Penile self-mutilation. Br J Psychiatry. 2001;178(1):86-87.
14. Gleeson MJ, Connolly J, Grainger R. Self-castration as treatment for alopecia. Br J Urol. 1993;71(5):614-615.
15. Hendershot E, Stutson AC, Adair TW. A case of extreme sexual self-mutilation. J Forensic Sci. 2010;55(1):245-247.
16. Hermann M, Thorstenson A. A rare case of male‐to‐eunuch gender dysphoria. Sex Med. 2015;3(4):331-333.
17. Nerli RB, Ravish IR, Amarkhed SS, et al. Genital self-mutilation in nonpsychotic heterosexual males: case report of two cases. Indian J Psychiatry. 2008;50(4):285-287.
18. Blacker KH, Wong N. Four cases of autocastration. Arch Gen Psychiatry. 1963;8:169-176.
19. Catalano G, Catalano MC, Carroll KM. Repetitive male genital self-mutilation: a case report and discussion of possible risk factors. J Sex Marital Ther. 2002;28(1):27-37.
20. Martin T, Gattaz WF. Psychiatric aspects of male genital self-mutilation. Psychopathology. 1991;24(3):170-178.
21. Money J. The Skoptic syndrome: castration and genital self-mutilation as an example of sexual body-image pathology. J Psychol Human Sex. 1988;1(1):113-128.
22. Nakaya M. On background factors of male genital self-mutilation. Psychopathology. 1996;29(4):242-248.
23. Borenstein A, Yaffe B, Seidman DS, et al. Successful microvascular replantation of an amputated penis. Isr J Med Sci. 1991;27(7):395-398.
24. Greilsheimer H, Groves JE. Male genital self-mutilation. Arch Gen Psychiatry. 1979;36(4):441-446.
25. Mendez R, Kiely WF, Morrow JW. Self-emasculation. J Urol. 1972;107(6):981-985.
26. Siddique RA, Deshpande S. A case of genital self-mutilation in a patient with psychosis. German J Psychiatry. 2007;10(1):25-28.
27. Qureshi NA. Male genital self-mutilation with special emphasis on the sociocultural meanings. Neurosciences (Riyadh). 2009;14(2):178-181.
28. Romilly CS, Isaac MT. Male genital self-mutilation. Br J Hosp Med. 1996;55(7):427-431.
29. Gahr M, Plener PL, Kölle MA, et al. Self-mutilation induced by psychotropic substances: a systematic review. Psychiatry Res. 2012;200(2-3):977-983.
30. Evren C, Sar V, Evren B, et al. Self-mutilation among male patients with alcohol dependency: the role of dissociation. Compr Psychiatry. 2008;49(5):489-495.
31. Brown GR. Autocastration and autopenectomy as surgical self-treatment in incarcerated persons with gender identity disorder. Int J Transgend. 2010;12(1):31-39.
32. Master VA, McAninch JW, Santucci RA. Genital self-mutilation and the Internet. J Urol. 2000;164(5):1656.
33. Premand NE, Eytan A. A case of non-psychotic autocastration: the importance of cultural factors. Psychiatry. 2005;68(2):174-178.
34. Simopoulos EF, Trinidad AC. Two cases of male genital self-mutilation: an examination of liaison dynamics. Psychosomatics. 2012;53(2):178-180.
35. Darewicz B, Galek L, Darewicz J, et al. Successful microsurgical replantation of an amputated penis. Int Urol Nephrol. 2001;33(2):385-386.
36. Raheem OA, Mirheydar HS, Patel ND, et al. Surgical management of traumatic penile amputation: a case report and review of the world literature. Sex Med. 2015;3(1):49-53.
37. Young LD, Feinsilver DL. Male genital self-mutilation: combined surgical and psychiatric care. Psychosomatics. 1986;27(7):513-517.
38. Walsh B. Clinical assessment of self-injury: a practical guide. J Clin Psychol. 2007;63(11):1057-1066.
39. Nafisi N, Stanley B. Developing and maintaining the therapeutic alliance with self-injuring patients. J Clin Psychol. 2007;63(11):1069-1079.
40. Fisch RZ. Genital self-mutilation in males: psychodynamic anatomy of a psychosis. Am J Psychother. 1987;41(3):453-458.
41. King PR. Cognitive-behavioral intervention in a case of self-mutilation. Clin Case Stud. 2014;13(2):181-189.
42. Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. J Ment Health Couns. 2006;28(2):166-185.
43. Walsh BW. Treating self-injury: a practical guide. New York, NY: The Guilford Press; 2006.
44. Large M, Babidge N, Andrews D, et al. Major self-mutilation in the first episode of psychosis. Schizophr Bull. 2009;35(5):1012-1021.
45. Large MM, Nielssen OB, Babidge N. Untreated psychosis is the main cause of major self-mutilation. Isr J Psychiatry Relat Sci. 2011;48(1):65.
46. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21.
47. Bowers MB Jr, Mazure CM, Nelson JC, et al. Psychotogenic drug use and neuroleptic response. Schizophr Bull. 1990;16(1):81-85.
1. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68(4):609-620.
2. Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501-1508.
3. Krasucki C, Kemp R, David A. A case study of female genital self-mutilation in schizophrenia. Br J Med Psychol. 1995;68(pt 2):179-186.
4. Lennon S. Genital self-mutilation in acute mania. Med J Aust. 1963;50(1):79-81.
5. Schweitzer I. Genital self-amputation and the Klingsor syndrome. Aust N Z J Psychiatry. 1990;24(4):566-569.
6. Anumonye A. Self-inflicted amputation of the penis in two Nigerian males. Niger Med J. 1973;3(1):51-52.
7. Bowman KM, Crook GH. Emotional changes following castration. Psychiatr Res Rep Am Psychiatr Assoc. 1960;12:81-96.
8. Eke N. Genital self-mutilation: there is no method in this madness. BJU Int. 2000;85(3):295-298.
9. Stroch D. Self-castration. JAMA. 1901;36(4):270.
10. Veeder TA, Leo RJ. Male genital self-mutilation: a comprehensive review of psychiatric disorders. Poster presented at: Academy of Psychosomatic Medicine Meeting, Austin, Texas, November 10, 2016.
11. Veeder TA, Leo RJ. Male genital self-mutilation: a systematic review of psychiatric disorders and psychosocial factors. Gen Hosp Psychiatry. 2017;44:43-50.
12. Battle AO. The psychological appraisal of a patient who had performed self-castration. British Journal of Projective Psychology & Personality Study. 1973;18(2):5-17.
13. Bhatia MS, Arora S. Penile self-mutilation. Br J Psychiatry. 2001;178(1):86-87.
14. Gleeson MJ, Connolly J, Grainger R. Self-castration as treatment for alopecia. Br J Urol. 1993;71(5):614-615.
15. Hendershot E, Stutson AC, Adair TW. A case of extreme sexual self-mutilation. J Forensic Sci. 2010;55(1):245-247.
16. Hermann M, Thorstenson A. A rare case of male‐to‐eunuch gender dysphoria. Sex Med. 2015;3(4):331-333.
17. Nerli RB, Ravish IR, Amarkhed SS, et al. Genital self-mutilation in nonpsychotic heterosexual males: case report of two cases. Indian J Psychiatry. 2008;50(4):285-287.
18. Blacker KH, Wong N. Four cases of autocastration. Arch Gen Psychiatry. 1963;8:169-176.
19. Catalano G, Catalano MC, Carroll KM. Repetitive male genital self-mutilation: a case report and discussion of possible risk factors. J Sex Marital Ther. 2002;28(1):27-37.
20. Martin T, Gattaz WF. Psychiatric aspects of male genital self-mutilation. Psychopathology. 1991;24(3):170-178.
21. Money J. The Skoptic syndrome: castration and genital self-mutilation as an example of sexual body-image pathology. J Psychol Human Sex. 1988;1(1):113-128.
22. Nakaya M. On background factors of male genital self-mutilation. Psychopathology. 1996;29(4):242-248.
23. Borenstein A, Yaffe B, Seidman DS, et al. Successful microvascular replantation of an amputated penis. Isr J Med Sci. 1991;27(7):395-398.
24. Greilsheimer H, Groves JE. Male genital self-mutilation. Arch Gen Psychiatry. 1979;36(4):441-446.
25. Mendez R, Kiely WF, Morrow JW. Self-emasculation. J Urol. 1972;107(6):981-985.
26. Siddique RA, Deshpande S. A case of genital self-mutilation in a patient with psychosis. German J Psychiatry. 2007;10(1):25-28.
27. Qureshi NA. Male genital self-mutilation with special emphasis on the sociocultural meanings. Neurosciences (Riyadh). 2009;14(2):178-181.
28. Romilly CS, Isaac MT. Male genital self-mutilation. Br J Hosp Med. 1996;55(7):427-431.
29. Gahr M, Plener PL, Kölle MA, et al. Self-mutilation induced by psychotropic substances: a systematic review. Psychiatry Res. 2012;200(2-3):977-983.
30. Evren C, Sar V, Evren B, et al. Self-mutilation among male patients with alcohol dependency: the role of dissociation. Compr Psychiatry. 2008;49(5):489-495.
31. Brown GR. Autocastration and autopenectomy as surgical self-treatment in incarcerated persons with gender identity disorder. Int J Transgend. 2010;12(1):31-39.
32. Master VA, McAninch JW, Santucci RA. Genital self-mutilation and the Internet. J Urol. 2000;164(5):1656.
33. Premand NE, Eytan A. A case of non-psychotic autocastration: the importance of cultural factors. Psychiatry. 2005;68(2):174-178.
34. Simopoulos EF, Trinidad AC. Two cases of male genital self-mutilation: an examination of liaison dynamics. Psychosomatics. 2012;53(2):178-180.
35. Darewicz B, Galek L, Darewicz J, et al. Successful microsurgical replantation of an amputated penis. Int Urol Nephrol. 2001;33(2):385-386.
36. Raheem OA, Mirheydar HS, Patel ND, et al. Surgical management of traumatic penile amputation: a case report and review of the world literature. Sex Med. 2015;3(1):49-53.
37. Young LD, Feinsilver DL. Male genital self-mutilation: combined surgical and psychiatric care. Psychosomatics. 1986;27(7):513-517.
38. Walsh B. Clinical assessment of self-injury: a practical guide. J Clin Psychol. 2007;63(11):1057-1066.
39. Nafisi N, Stanley B. Developing and maintaining the therapeutic alliance with self-injuring patients. J Clin Psychol. 2007;63(11):1069-1079.
40. Fisch RZ. Genital self-mutilation in males: psychodynamic anatomy of a psychosis. Am J Psychother. 1987;41(3):453-458.
41. King PR. Cognitive-behavioral intervention in a case of self-mutilation. Clin Case Stud. 2014;13(2):181-189.
42. Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. J Ment Health Couns. 2006;28(2):166-185.
43. Walsh BW. Treating self-injury: a practical guide. New York, NY: The Guilford Press; 2006.
44. Large M, Babidge N, Andrews D, et al. Major self-mutilation in the first episode of psychosis. Schizophr Bull. 2009;35(5):1012-1021.
45. Large MM, Nielssen OB, Babidge N. Untreated psychosis is the main cause of major self-mutilation. Isr J Psychiatry Relat Sci. 2011;48(1):65.
46. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21.
47. Bowers MB Jr, Mazure CM, Nelson JC, et al. Psychotogenic drug use and neuroleptic response. Schizophr Bull. 1990;16(1):81-85.
6 Brief exercises for introducing mindfulness
Mindfulness is actively being aware of one’s inner and outer environments in the present moment. Core mindfulness skills include observation, description, participation, a nonjudgmental approach, focusing on 1 thing at a time, and effectiveness.1
Psychotherapeutic interventions based on each of these skills have been developed to instill a mindful state in psychiatric patients. Evidence suggests these interventions can be helpful when treating borderline personality disorder, somatization, pain, depression, and anxiety, among other conditions.2
Elements of mindfulness can be integrated into brief interventions. The following 6 simple, practical exercises can be used to help patients develop these skills.
Observation involves noticing internal and external experiences, including thoughts and sensations, without applying words or labels. Guide your patient through the following exercise:
Focus your attention on the ground beneath your feet, feeling the pressure, temperature, and texture of this sensation. Do the same with your seat, your breath, and the sounds, sights, and smells of the room. Be aware of your thoughts and watch them come and go like fish in a fishbowl.
Description entails assigning purely descriptive words to one’s observations. To help your patient develop this skill, ask him (her) to describe the sensations he (she) observed in the previous exercise.
Participation entails immersive engagement in an activity. Ask your patient to listen to a song he has never heard before, and then play it again and dance or sing along. Instruct him to engage wholly, conscious of each step or note, without being judgmental or self-conscious. If he feels embarrassed or self-critical, tell him to observe these thoughts and emotions, put them aside, and return to the activity.
A nonjudgmental approach consists of separating out the facts and recognizing emotional responses without clinging to them. To practice this skill, ask your patient to play a song that he likes and one that he dislikes. The patient should listen to each, observing and describing the way they sound without judgment. Tell the patient that if judgmental words or phrases, such as “beautiful,” “ugly,” “I love…,” or “I hate…,” appear as thoughts, he should observe them, put them aside, and then return to nonjudgmental description and observation.
Focusing on 1 thing at a time means dedicating complete attention to a single task, activity, or thought. Give your patient a short paragraph or poem to read. Instruct
Effectiveness involves focusing on what works to attain one’s goals. For this exercise, set up a task for your patient by placing several items in a location that is neither immediately obvious nor readily accessible without an intermediate step. Instruct your patient to obtain these objects. Then guide them as follows:
What do you have to do to get them? Ask permission? Borrow a key? Recruit assistance? Determine the location? Brainstorm ways to obtain the items, and then complete the task.
1. Linehan MM. DBT skills training manual. 2nd ed. New York, NY: The Guilford Press; 2015.
2. Gotink RA, Chu P, Busschbach JJ, et al. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10(4):e0124344. doi: 10.1371/journal.pone.0124344.
Mindfulness is actively being aware of one’s inner and outer environments in the present moment. Core mindfulness skills include observation, description, participation, a nonjudgmental approach, focusing on 1 thing at a time, and effectiveness.1
Psychotherapeutic interventions based on each of these skills have been developed to instill a mindful state in psychiatric patients. Evidence suggests these interventions can be helpful when treating borderline personality disorder, somatization, pain, depression, and anxiety, among other conditions.2
Elements of mindfulness can be integrated into brief interventions. The following 6 simple, practical exercises can be used to help patients develop these skills.
Observation involves noticing internal and external experiences, including thoughts and sensations, without applying words or labels. Guide your patient through the following exercise:
Focus your attention on the ground beneath your feet, feeling the pressure, temperature, and texture of this sensation. Do the same with your seat, your breath, and the sounds, sights, and smells of the room. Be aware of your thoughts and watch them come and go like fish in a fishbowl.
Description entails assigning purely descriptive words to one’s observations. To help your patient develop this skill, ask him (her) to describe the sensations he (she) observed in the previous exercise.
Participation entails immersive engagement in an activity. Ask your patient to listen to a song he has never heard before, and then play it again and dance or sing along. Instruct him to engage wholly, conscious of each step or note, without being judgmental or self-conscious. If he feels embarrassed or self-critical, tell him to observe these thoughts and emotions, put them aside, and return to the activity.
A nonjudgmental approach consists of separating out the facts and recognizing emotional responses without clinging to them. To practice this skill, ask your patient to play a song that he likes and one that he dislikes. The patient should listen to each, observing and describing the way they sound without judgment. Tell the patient that if judgmental words or phrases, such as “beautiful,” “ugly,” “I love…,” or “I hate…,” appear as thoughts, he should observe them, put them aside, and then return to nonjudgmental description and observation.
Focusing on 1 thing at a time means dedicating complete attention to a single task, activity, or thought. Give your patient a short paragraph or poem to read. Instruct
Effectiveness involves focusing on what works to attain one’s goals. For this exercise, set up a task for your patient by placing several items in a location that is neither immediately obvious nor readily accessible without an intermediate step. Instruct your patient to obtain these objects. Then guide them as follows:
What do you have to do to get them? Ask permission? Borrow a key? Recruit assistance? Determine the location? Brainstorm ways to obtain the items, and then complete the task.
Mindfulness is actively being aware of one’s inner and outer environments in the present moment. Core mindfulness skills include observation, description, participation, a nonjudgmental approach, focusing on 1 thing at a time, and effectiveness.1
Psychotherapeutic interventions based on each of these skills have been developed to instill a mindful state in psychiatric patients. Evidence suggests these interventions can be helpful when treating borderline personality disorder, somatization, pain, depression, and anxiety, among other conditions.2
Elements of mindfulness can be integrated into brief interventions. The following 6 simple, practical exercises can be used to help patients develop these skills.
Observation involves noticing internal and external experiences, including thoughts and sensations, without applying words or labels. Guide your patient through the following exercise:
Focus your attention on the ground beneath your feet, feeling the pressure, temperature, and texture of this sensation. Do the same with your seat, your breath, and the sounds, sights, and smells of the room. Be aware of your thoughts and watch them come and go like fish in a fishbowl.
Description entails assigning purely descriptive words to one’s observations. To help your patient develop this skill, ask him (her) to describe the sensations he (she) observed in the previous exercise.
Participation entails immersive engagement in an activity. Ask your patient to listen to a song he has never heard before, and then play it again and dance or sing along. Instruct him to engage wholly, conscious of each step or note, without being judgmental or self-conscious. If he feels embarrassed or self-critical, tell him to observe these thoughts and emotions, put them aside, and return to the activity.
A nonjudgmental approach consists of separating out the facts and recognizing emotional responses without clinging to them. To practice this skill, ask your patient to play a song that he likes and one that he dislikes. The patient should listen to each, observing and describing the way they sound without judgment. Tell the patient that if judgmental words or phrases, such as “beautiful,” “ugly,” “I love…,” or “I hate…,” appear as thoughts, he should observe them, put them aside, and then return to nonjudgmental description and observation.
Focusing on 1 thing at a time means dedicating complete attention to a single task, activity, or thought. Give your patient a short paragraph or poem to read. Instruct
Effectiveness involves focusing on what works to attain one’s goals. For this exercise, set up a task for your patient by placing several items in a location that is neither immediately obvious nor readily accessible without an intermediate step. Instruct your patient to obtain these objects. Then guide them as follows:
What do you have to do to get them? Ask permission? Borrow a key? Recruit assistance? Determine the location? Brainstorm ways to obtain the items, and then complete the task.
1. Linehan MM. DBT skills training manual. 2nd ed. New York, NY: The Guilford Press; 2015.
2. Gotink RA, Chu P, Busschbach JJ, et al. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10(4):e0124344. doi: 10.1371/journal.pone.0124344.
1. Linehan MM. DBT skills training manual. 2nd ed. New York, NY: The Guilford Press; 2015.
2. Gotink RA, Chu P, Busschbach JJ, et al. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10(4):e0124344. doi: 10.1371/journal.pone.0124344.
Caring for Muslim patients: Understanding cultural and religious factors
Patients who are Muslim—followers of the religion of Islam—struggle with a political climate that has demonized them and the continued fallout of terrorist attacks perpetrated by individuals who identify themselves as Muslim. These patients may experience low self-esteem, bullying, depression, anxiety, or posttraumatic stress disorder.1 Some have expressed feeling judged, labeled, attacked, and subjected to discrimination. Islamophobia and a spike in hate crimes have further marginalized this already vulnerable population.2 Thus, understanding your Muslim patients is the first step to treating their mental illness.
How Muslim culture might affect care
Muslims are not a monolithic group; they vary widely in their religious adherence, cultural background, and acculturation. Some are American-born, including a significant African American Muslim population. Others are children of immigrants or have recently immigrated, including many who came to the United States because of the ongoing war in Syria. Many can trace their heritage to >50 predominantly Muslim countries. Many Muslim patients want to find a balance between their religious and American identities.
As clinicians, we should not make assumptions based on outward appearances or our preconceived notions of our patients, especially when it comes to gender roles. Our job is to ask how highly personal, individualized decisions, such as a woman’s choice to wear a hijab as an expression of her faith and a symbol of modesty, factor into our patients’ day-to-day lives. Doing so can help build the therapeutic alliance and improve the accuracy of the diagnosis and the appropriateness of treatment.
Mental health clinicians are well aware of the dangers of the social stigma that their patients may experience.3 These dangers are no different when it comes to Muslim patients, who often may face “double discrimination” for their religion and for having a mental illness. They may seek support from religious leaders, family, and friends before seeing a mental health provider. Some may view their mental illness as a weakness of faith, a punishment by God, or an affliction caused by a supernatural spirit, and therefore may feel that following religious doctrine will resolve their psychological distress.4 They may need additional encouragement to see a therapist or take psychotropics, and they may prefer specific treatments that reflect their cultural values, such as supplements.
Because some Muslim patients may be more comfortable presenting their psychological concerns as somatic symptoms, they may first seek care from a primary care physician. Some patients may not be open or comfortable enough to address sensitive issues, such as substance use. Providing psychoeducation, comparing mental illness with medical illness, and emphasizing doctor–patient confidentiality may help these patients overcome the stigma that can act as a barrier to care.
Provide culturally competent care
Resources are available to help us provide the best possible care to our patients from various cultures and religions, including Muslim patients. A good starting point is the DSM-5’s Cultural Formulation Interview, which is a set of 16 questions psychiatrists can use to determine the impact of culture on a patient’s clinical presentation and care.5 Other resources include the American Psychiatric Association’s Assessment of Cultural Factors and the American Academy of Child and Adolescent Psychiatry’s Practice Parameter for Cultural Competence.6
When treating Muslim patients, remember to:
- Ask about what roles their culture and religion play
- Understand their explanation of their symptoms
- Work to overcome any stigma patients may perceive related to having a psychiatric disorder
- Engage your team to identify cultural and religious factors
- Connect to community resources, such as the patient’s family and friends.
1. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
2. Nadal KL, Griffin KE, Hamit S, et al. Subtle and overt forms of Islamophobia: microaggressions toward Muslim Americans. J Muslim Mental Health. 2012;6(2):15-37.
3. Ciftci A, Jones N, Corrigan PW. Mental health stigma in the Muslim community. J Muslim Mental Health. 2013;7(1):17-32.
4. Haque A. Religion and mental health: the case of American Muslims. J Relig Health. 2004;43(1):45-58.
5. American Psychiatric Association. Cultural formulation interview. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:750-757.
6. Pumariega AJ, Rothe E, Mian A, et al; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice parameter for cultural competence in child and adolescent psychiatric practice. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1101-1115.
Patients who are Muslim—followers of the religion of Islam—struggle with a political climate that has demonized them and the continued fallout of terrorist attacks perpetrated by individuals who identify themselves as Muslim. These patients may experience low self-esteem, bullying, depression, anxiety, or posttraumatic stress disorder.1 Some have expressed feeling judged, labeled, attacked, and subjected to discrimination. Islamophobia and a spike in hate crimes have further marginalized this already vulnerable population.2 Thus, understanding your Muslim patients is the first step to treating their mental illness.
How Muslim culture might affect care
Muslims are not a monolithic group; they vary widely in their religious adherence, cultural background, and acculturation. Some are American-born, including a significant African American Muslim population. Others are children of immigrants or have recently immigrated, including many who came to the United States because of the ongoing war in Syria. Many can trace their heritage to >50 predominantly Muslim countries. Many Muslim patients want to find a balance between their religious and American identities.
As clinicians, we should not make assumptions based on outward appearances or our preconceived notions of our patients, especially when it comes to gender roles. Our job is to ask how highly personal, individualized decisions, such as a woman’s choice to wear a hijab as an expression of her faith and a symbol of modesty, factor into our patients’ day-to-day lives. Doing so can help build the therapeutic alliance and improve the accuracy of the diagnosis and the appropriateness of treatment.
Mental health clinicians are well aware of the dangers of the social stigma that their patients may experience.3 These dangers are no different when it comes to Muslim patients, who often may face “double discrimination” for their religion and for having a mental illness. They may seek support from religious leaders, family, and friends before seeing a mental health provider. Some may view their mental illness as a weakness of faith, a punishment by God, or an affliction caused by a supernatural spirit, and therefore may feel that following religious doctrine will resolve their psychological distress.4 They may need additional encouragement to see a therapist or take psychotropics, and they may prefer specific treatments that reflect their cultural values, such as supplements.
Because some Muslim patients may be more comfortable presenting their psychological concerns as somatic symptoms, they may first seek care from a primary care physician. Some patients may not be open or comfortable enough to address sensitive issues, such as substance use. Providing psychoeducation, comparing mental illness with medical illness, and emphasizing doctor–patient confidentiality may help these patients overcome the stigma that can act as a barrier to care.
Provide culturally competent care
Resources are available to help us provide the best possible care to our patients from various cultures and religions, including Muslim patients. A good starting point is the DSM-5’s Cultural Formulation Interview, which is a set of 16 questions psychiatrists can use to determine the impact of culture on a patient’s clinical presentation and care.5 Other resources include the American Psychiatric Association’s Assessment of Cultural Factors and the American Academy of Child and Adolescent Psychiatry’s Practice Parameter for Cultural Competence.6
When treating Muslim patients, remember to:
- Ask about what roles their culture and religion play
- Understand their explanation of their symptoms
- Work to overcome any stigma patients may perceive related to having a psychiatric disorder
- Engage your team to identify cultural and religious factors
- Connect to community resources, such as the patient’s family and friends.
Patients who are Muslim—followers of the religion of Islam—struggle with a political climate that has demonized them and the continued fallout of terrorist attacks perpetrated by individuals who identify themselves as Muslim. These patients may experience low self-esteem, bullying, depression, anxiety, or posttraumatic stress disorder.1 Some have expressed feeling judged, labeled, attacked, and subjected to discrimination. Islamophobia and a spike in hate crimes have further marginalized this already vulnerable population.2 Thus, understanding your Muslim patients is the first step to treating their mental illness.
How Muslim culture might affect care
Muslims are not a monolithic group; they vary widely in their religious adherence, cultural background, and acculturation. Some are American-born, including a significant African American Muslim population. Others are children of immigrants or have recently immigrated, including many who came to the United States because of the ongoing war in Syria. Many can trace their heritage to >50 predominantly Muslim countries. Many Muslim patients want to find a balance between their religious and American identities.
As clinicians, we should not make assumptions based on outward appearances or our preconceived notions of our patients, especially when it comes to gender roles. Our job is to ask how highly personal, individualized decisions, such as a woman’s choice to wear a hijab as an expression of her faith and a symbol of modesty, factor into our patients’ day-to-day lives. Doing so can help build the therapeutic alliance and improve the accuracy of the diagnosis and the appropriateness of treatment.
Mental health clinicians are well aware of the dangers of the social stigma that their patients may experience.3 These dangers are no different when it comes to Muslim patients, who often may face “double discrimination” for their religion and for having a mental illness. They may seek support from religious leaders, family, and friends before seeing a mental health provider. Some may view their mental illness as a weakness of faith, a punishment by God, or an affliction caused by a supernatural spirit, and therefore may feel that following religious doctrine will resolve their psychological distress.4 They may need additional encouragement to see a therapist or take psychotropics, and they may prefer specific treatments that reflect their cultural values, such as supplements.
Because some Muslim patients may be more comfortable presenting their psychological concerns as somatic symptoms, they may first seek care from a primary care physician. Some patients may not be open or comfortable enough to address sensitive issues, such as substance use. Providing psychoeducation, comparing mental illness with medical illness, and emphasizing doctor–patient confidentiality may help these patients overcome the stigma that can act as a barrier to care.
Provide culturally competent care
Resources are available to help us provide the best possible care to our patients from various cultures and religions, including Muslim patients. A good starting point is the DSM-5’s Cultural Formulation Interview, which is a set of 16 questions psychiatrists can use to determine the impact of culture on a patient’s clinical presentation and care.5 Other resources include the American Psychiatric Association’s Assessment of Cultural Factors and the American Academy of Child and Adolescent Psychiatry’s Practice Parameter for Cultural Competence.6
When treating Muslim patients, remember to:
- Ask about what roles their culture and religion play
- Understand their explanation of their symptoms
- Work to overcome any stigma patients may perceive related to having a psychiatric disorder
- Engage your team to identify cultural and religious factors
- Connect to community resources, such as the patient’s family and friends.
1. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
2. Nadal KL, Griffin KE, Hamit S, et al. Subtle and overt forms of Islamophobia: microaggressions toward Muslim Americans. J Muslim Mental Health. 2012;6(2):15-37.
3. Ciftci A, Jones N, Corrigan PW. Mental health stigma in the Muslim community. J Muslim Mental Health. 2013;7(1):17-32.
4. Haque A. Religion and mental health: the case of American Muslims. J Relig Health. 2004;43(1):45-58.
5. American Psychiatric Association. Cultural formulation interview. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:750-757.
6. Pumariega AJ, Rothe E, Mian A, et al; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice parameter for cultural competence in child and adolescent psychiatric practice. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1101-1115.
1. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
2. Nadal KL, Griffin KE, Hamit S, et al. Subtle and overt forms of Islamophobia: microaggressions toward Muslim Americans. J Muslim Mental Health. 2012;6(2):15-37.
3. Ciftci A, Jones N, Corrigan PW. Mental health stigma in the Muslim community. J Muslim Mental Health. 2013;7(1):17-32.
4. Haque A. Religion and mental health: the case of American Muslims. J Relig Health. 2004;43(1):45-58.
5. American Psychiatric Association. Cultural formulation interview. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:750-757.
6. Pumariega AJ, Rothe E, Mian A, et al; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice parameter for cultural competence in child and adolescent psychiatric practice. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1101-1115.
ADHD and insomnia appear intertwined
PARIS – Converging evidence suggests that attention-deficit/hyperactivity disorder and sleep difficulties share a common underlying etiology involving circadian rhythm disturbance, J.J. Sandra Kooij, MD, PhD, declared at the annual congress of the European College of Neuropsychopharmacology.
“If you review the evidence, it looks more and more like ADHD and sleeplessness are two sides of the same physiological and mental coin,” said Dr. Kooij, a psychiatrist at VU University Medical Center, Amsterdam, and chair of the European Network Adult ADHD.
Since this study remains ongoing, Dr. Kooij focused instead on the evidence suggesting that ADHD and sleep problems have a shared etiology.
Multiple studies have shown that roughly 75% of children and adults with ADHD have sleep-onset insomnia. It takes them longer to fall asleep, and they have a shorter than normal sleep duration because they have to get up in the morning for school or work. Dr. Kooij and her colleagues have shown that in adult ADHD patients with delayed sleep onset syndrome, their evening dim light melatonin onset, change in core body temperature, and other physiologic harbingers of sleep are delayed by an average of 1.5 hours (J Sleep Res. 2013 Dec;22[6]:607-16).
“My ADHD patients sleep a mean of 5-6 hours per night on a chronic basis, versus 7-8 hours in normal individuals. It leads to daytime sleepiness and dysfunction due to inattentiveness and social problems,” the psychiatrist said.
Other investigators have demonstrated that the prevalence of ADHD varies across the United States and that geographic differences in solar intensity explain 34%-57% of this variance in ADHD rates. The investigators postulated that the ADHD-preventive effect of high solar irradiation might be tied to improvement in circadian clock disturbances (Biol Psychiatry. 2013 Oct 15;74[8]:585-90).
In a study of 2,090 adult participants in The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues showed that ADHD, depression, anxiety, and circadian rhythm sleep problems are fellow travelers.
The prevalence of sleep duration of fewer than 6 hours per night was 15% in subjects with high ADHD symptoms and a lifetime history of an anxiety and/or depression diagnosis, 5% in those with lifetime anxiety/depression but no ADHD, and 4% in healthy controls. Delayed sleep phase syndrome was present in 16% of individuals with ADHD and a history of depression and/or anxiety, 8% in those with a lifetime history of anxiety/depression without ADHD, and 5% of healthy controls. The take-home message: Circadian rhythm sleep disorders in patients with ADHD are not necessarily attributable to comorbid anxiety and/or depression (J Affect Disord. 2016 Aug;200:74-81).
Seasonal affective disorder is commonly comorbid with ADHD. In another analysis from The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues determined that the prevalence of probable seasonal affective disorder using the Seasonal Pattern Assessment Questionnaire was 9.9% in participants with clinically significant ADHD symptoms, compared with 3.3% in the non-ADHD subjects. Self-reported delayed sleep onset was extremely common in participants with ADHD as well as in those with probable seasonal affective disorder (J Psychiat Res. 2016 Oct;81:87-94).
Patients with ADHD have an increased prevalence of obesity. Their chronic short sleep pushes them toward an unstable eating pattern in which they skip breakfast, then engage in binge eating later in the day. The hope is that treating the circadian rhythm disruption associated with ADHD will prevent obesity in this population (J Psychosom Res. 2015 Nov;79[5]:443-50).
“If you mess up sleep, you mess up the body: bowel movements, blood pressure, body temperature, the leptin/ghrelin ratio, reaction time, coordination. That’s why I call my patients chronically jet-lagged,” Dr. Kooij said.
She reported having no financial conflicts regarding her presentation.
PARIS – Converging evidence suggests that attention-deficit/hyperactivity disorder and sleep difficulties share a common underlying etiology involving circadian rhythm disturbance, J.J. Sandra Kooij, MD, PhD, declared at the annual congress of the European College of Neuropsychopharmacology.
“If you review the evidence, it looks more and more like ADHD and sleeplessness are two sides of the same physiological and mental coin,” said Dr. Kooij, a psychiatrist at VU University Medical Center, Amsterdam, and chair of the European Network Adult ADHD.
Since this study remains ongoing, Dr. Kooij focused instead on the evidence suggesting that ADHD and sleep problems have a shared etiology.
Multiple studies have shown that roughly 75% of children and adults with ADHD have sleep-onset insomnia. It takes them longer to fall asleep, and they have a shorter than normal sleep duration because they have to get up in the morning for school or work. Dr. Kooij and her colleagues have shown that in adult ADHD patients with delayed sleep onset syndrome, their evening dim light melatonin onset, change in core body temperature, and other physiologic harbingers of sleep are delayed by an average of 1.5 hours (J Sleep Res. 2013 Dec;22[6]:607-16).
“My ADHD patients sleep a mean of 5-6 hours per night on a chronic basis, versus 7-8 hours in normal individuals. It leads to daytime sleepiness and dysfunction due to inattentiveness and social problems,” the psychiatrist said.
Other investigators have demonstrated that the prevalence of ADHD varies across the United States and that geographic differences in solar intensity explain 34%-57% of this variance in ADHD rates. The investigators postulated that the ADHD-preventive effect of high solar irradiation might be tied to improvement in circadian clock disturbances (Biol Psychiatry. 2013 Oct 15;74[8]:585-90).
In a study of 2,090 adult participants in The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues showed that ADHD, depression, anxiety, and circadian rhythm sleep problems are fellow travelers.
The prevalence of sleep duration of fewer than 6 hours per night was 15% in subjects with high ADHD symptoms and a lifetime history of an anxiety and/or depression diagnosis, 5% in those with lifetime anxiety/depression but no ADHD, and 4% in healthy controls. Delayed sleep phase syndrome was present in 16% of individuals with ADHD and a history of depression and/or anxiety, 8% in those with a lifetime history of anxiety/depression without ADHD, and 5% of healthy controls. The take-home message: Circadian rhythm sleep disorders in patients with ADHD are not necessarily attributable to comorbid anxiety and/or depression (J Affect Disord. 2016 Aug;200:74-81).
Seasonal affective disorder is commonly comorbid with ADHD. In another analysis from The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues determined that the prevalence of probable seasonal affective disorder using the Seasonal Pattern Assessment Questionnaire was 9.9% in participants with clinically significant ADHD symptoms, compared with 3.3% in the non-ADHD subjects. Self-reported delayed sleep onset was extremely common in participants with ADHD as well as in those with probable seasonal affective disorder (J Psychiat Res. 2016 Oct;81:87-94).
Patients with ADHD have an increased prevalence of obesity. Their chronic short sleep pushes them toward an unstable eating pattern in which they skip breakfast, then engage in binge eating later in the day. The hope is that treating the circadian rhythm disruption associated with ADHD will prevent obesity in this population (J Psychosom Res. 2015 Nov;79[5]:443-50).
“If you mess up sleep, you mess up the body: bowel movements, blood pressure, body temperature, the leptin/ghrelin ratio, reaction time, coordination. That’s why I call my patients chronically jet-lagged,” Dr. Kooij said.
She reported having no financial conflicts regarding her presentation.
PARIS – Converging evidence suggests that attention-deficit/hyperactivity disorder and sleep difficulties share a common underlying etiology involving circadian rhythm disturbance, J.J. Sandra Kooij, MD, PhD, declared at the annual congress of the European College of Neuropsychopharmacology.
“If you review the evidence, it looks more and more like ADHD and sleeplessness are two sides of the same physiological and mental coin,” said Dr. Kooij, a psychiatrist at VU University Medical Center, Amsterdam, and chair of the European Network Adult ADHD.
Since this study remains ongoing, Dr. Kooij focused instead on the evidence suggesting that ADHD and sleep problems have a shared etiology.
Multiple studies have shown that roughly 75% of children and adults with ADHD have sleep-onset insomnia. It takes them longer to fall asleep, and they have a shorter than normal sleep duration because they have to get up in the morning for school or work. Dr. Kooij and her colleagues have shown that in adult ADHD patients with delayed sleep onset syndrome, their evening dim light melatonin onset, change in core body temperature, and other physiologic harbingers of sleep are delayed by an average of 1.5 hours (J Sleep Res. 2013 Dec;22[6]:607-16).
“My ADHD patients sleep a mean of 5-6 hours per night on a chronic basis, versus 7-8 hours in normal individuals. It leads to daytime sleepiness and dysfunction due to inattentiveness and social problems,” the psychiatrist said.
Other investigators have demonstrated that the prevalence of ADHD varies across the United States and that geographic differences in solar intensity explain 34%-57% of this variance in ADHD rates. The investigators postulated that the ADHD-preventive effect of high solar irradiation might be tied to improvement in circadian clock disturbances (Biol Psychiatry. 2013 Oct 15;74[8]:585-90).
In a study of 2,090 adult participants in The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues showed that ADHD, depression, anxiety, and circadian rhythm sleep problems are fellow travelers.
The prevalence of sleep duration of fewer than 6 hours per night was 15% in subjects with high ADHD symptoms and a lifetime history of an anxiety and/or depression diagnosis, 5% in those with lifetime anxiety/depression but no ADHD, and 4% in healthy controls. Delayed sleep phase syndrome was present in 16% of individuals with ADHD and a history of depression and/or anxiety, 8% in those with a lifetime history of anxiety/depression without ADHD, and 5% of healthy controls. The take-home message: Circadian rhythm sleep disorders in patients with ADHD are not necessarily attributable to comorbid anxiety and/or depression (J Affect Disord. 2016 Aug;200:74-81).
Seasonal affective disorder is commonly comorbid with ADHD. In another analysis from The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues determined that the prevalence of probable seasonal affective disorder using the Seasonal Pattern Assessment Questionnaire was 9.9% in participants with clinically significant ADHD symptoms, compared with 3.3% in the non-ADHD subjects. Self-reported delayed sleep onset was extremely common in participants with ADHD as well as in those with probable seasonal affective disorder (J Psychiat Res. 2016 Oct;81:87-94).
Patients with ADHD have an increased prevalence of obesity. Their chronic short sleep pushes them toward an unstable eating pattern in which they skip breakfast, then engage in binge eating later in the day. The hope is that treating the circadian rhythm disruption associated with ADHD will prevent obesity in this population (J Psychosom Res. 2015 Nov;79[5]:443-50).
“If you mess up sleep, you mess up the body: bowel movements, blood pressure, body temperature, the leptin/ghrelin ratio, reaction time, coordination. That’s why I call my patients chronically jet-lagged,” Dr. Kooij said.
She reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
More to psychiatry than just neuroscience; The impact of childhood trauma
More to psychiatry than just neuroscience
In his editorial “Advancing clinical neuroscience literacy among psychiatric practitioners” (From the Editor,
I find that many of my patients look for more or different drugs to fix their dysfunctional patterns in life—many of which stem from their dysfunctional and traumatic childhoods. Thus, it is more than just drugs and neurochemical pathways, more than just the “dysregulated neural circuitry,” that we need to focus on in our psychiatric practice.
I finished my psychiatric residency in 1972, before we knew much about neuroscience. Since then, we have learned so much about neuroscience and the specific neuroscience mechanisms involved in the brain and mind. Those advances have done much to aid our core understanding of psychiatric disorders. However, let us not forget that there is more to the mind than just neurochemistry, and more to our practice of psychiatry than just neuroscience.
Leonard Korn, MD
Psychiatrist
Portsmouth Regional Hospital
Portsmouth, New Hampshire
Dr. Nasrallah responds
It is now widely accepted in our field that all psychological phenomena and all human behaviors are associated with neurobiological components. All life events, especially traumatic experiences, are transduced into structural and chemical changes, often within minutes. The formation of dendritic spines to encode the memory of one’s experiences throughout waking hours is well established in neuroscience, and hundreds of studies have been published about this.
Psychotherapy is a neurobiological intervention that induces neuroplasticity and leads to structural brain repair, because talking, listening, triggering memories, inducing insight, and “connecting the dots” in one’s behavior are all biological events.1,2 There is no such thing as a purely psychological process independent of the brain. The mind is the product of ongoing complex, intricate activity of brain neurocircuits whose neurobiological activity is translated into thoughts, emotions, impulses, and behaviors. The mind is perpetually tethered to its neurological roots.
Thus, reductionism actually describes a scientific fact and is not a term with pejorative connotations used to shut down scientific discourse about the biological basis of human behavior. By advancing their clinical neuroscience literacy, psychiatric practitioners will understand that they deal with a specific brain pathology in every patient that they treat and that the medications and psychotherapeutic interventions they employ are synergistic biological treatments.3
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
References
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Out-of-the-box questions about psychotherapy. Current Psychiatry. 2010;9(10):13-14.
3. Nasrallah HA. Medications with psychotherapy: a synergy to heal the brain. C urrent Psychiatry. 2006;5(10):11-12.
The impact of childhood trauma
I enjoyed Dr. Nasrallah’s article “Beyond DSM-5: Clinical and biologic features shared by major psychiatric syndromes” (From the Editor, Current Psychiatry. October 2017, p. 4,6-7), but there was only 1 mention of childhood trauma, which shares features with most of the commonalities he described, such as inflammation, smaller brain volumes, gene and environment interaction, shortened telomeres, and elevated cortisol levels. The Adverse Childhood Experiences Study1 taught us about the impact of childhood trauma on the entire organism. We need to focus on that commonality.
Susan Jones, MD
Child and Adolescent Psychiatrist
Virginia Treatment Center for Children
Assistant Professor
Virginia Commonwealth University
School of Medicine
Richmond, Virginia
Reference
1. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258
Dr. Nasrallah responds
It is worth pointing out that childhood trauma predominantly leads to psychotic and mood disorders in adulthood, and the criteria I mentioned would then hold true.
More to psychiatry than just neuroscience
In his editorial “Advancing clinical neuroscience literacy among psychiatric practitioners” (From the Editor,
I find that many of my patients look for more or different drugs to fix their dysfunctional patterns in life—many of which stem from their dysfunctional and traumatic childhoods. Thus, it is more than just drugs and neurochemical pathways, more than just the “dysregulated neural circuitry,” that we need to focus on in our psychiatric practice.
I finished my psychiatric residency in 1972, before we knew much about neuroscience. Since then, we have learned so much about neuroscience and the specific neuroscience mechanisms involved in the brain and mind. Those advances have done much to aid our core understanding of psychiatric disorders. However, let us not forget that there is more to the mind than just neurochemistry, and more to our practice of psychiatry than just neuroscience.
Leonard Korn, MD
Psychiatrist
Portsmouth Regional Hospital
Portsmouth, New Hampshire
Dr. Nasrallah responds
It is now widely accepted in our field that all psychological phenomena and all human behaviors are associated with neurobiological components. All life events, especially traumatic experiences, are transduced into structural and chemical changes, often within minutes. The formation of dendritic spines to encode the memory of one’s experiences throughout waking hours is well established in neuroscience, and hundreds of studies have been published about this.
Psychotherapy is a neurobiological intervention that induces neuroplasticity and leads to structural brain repair, because talking, listening, triggering memories, inducing insight, and “connecting the dots” in one’s behavior are all biological events.1,2 There is no such thing as a purely psychological process independent of the brain. The mind is the product of ongoing complex, intricate activity of brain neurocircuits whose neurobiological activity is translated into thoughts, emotions, impulses, and behaviors. The mind is perpetually tethered to its neurological roots.
Thus, reductionism actually describes a scientific fact and is not a term with pejorative connotations used to shut down scientific discourse about the biological basis of human behavior. By advancing their clinical neuroscience literacy, psychiatric practitioners will understand that they deal with a specific brain pathology in every patient that they treat and that the medications and psychotherapeutic interventions they employ are synergistic biological treatments.3
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
References
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Out-of-the-box questions about psychotherapy. Current Psychiatry. 2010;9(10):13-14.
3. Nasrallah HA. Medications with psychotherapy: a synergy to heal the brain. C urrent Psychiatry. 2006;5(10):11-12.
The impact of childhood trauma
I enjoyed Dr. Nasrallah’s article “Beyond DSM-5: Clinical and biologic features shared by major psychiatric syndromes” (From the Editor, Current Psychiatry. October 2017, p. 4,6-7), but there was only 1 mention of childhood trauma, which shares features with most of the commonalities he described, such as inflammation, smaller brain volumes, gene and environment interaction, shortened telomeres, and elevated cortisol levels. The Adverse Childhood Experiences Study1 taught us about the impact of childhood trauma on the entire organism. We need to focus on that commonality.
Susan Jones, MD
Child and Adolescent Psychiatrist
Virginia Treatment Center for Children
Assistant Professor
Virginia Commonwealth University
School of Medicine
Richmond, Virginia
Reference
1. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258
Dr. Nasrallah responds
It is worth pointing out that childhood trauma predominantly leads to psychotic and mood disorders in adulthood, and the criteria I mentioned would then hold true.
More to psychiatry than just neuroscience
In his editorial “Advancing clinical neuroscience literacy among psychiatric practitioners” (From the Editor,
I find that many of my patients look for more or different drugs to fix their dysfunctional patterns in life—many of which stem from their dysfunctional and traumatic childhoods. Thus, it is more than just drugs and neurochemical pathways, more than just the “dysregulated neural circuitry,” that we need to focus on in our psychiatric practice.
I finished my psychiatric residency in 1972, before we knew much about neuroscience. Since then, we have learned so much about neuroscience and the specific neuroscience mechanisms involved in the brain and mind. Those advances have done much to aid our core understanding of psychiatric disorders. However, let us not forget that there is more to the mind than just neurochemistry, and more to our practice of psychiatry than just neuroscience.
Leonard Korn, MD
Psychiatrist
Portsmouth Regional Hospital
Portsmouth, New Hampshire
Dr. Nasrallah responds
It is now widely accepted in our field that all psychological phenomena and all human behaviors are associated with neurobiological components. All life events, especially traumatic experiences, are transduced into structural and chemical changes, often within minutes. The formation of dendritic spines to encode the memory of one’s experiences throughout waking hours is well established in neuroscience, and hundreds of studies have been published about this.
Psychotherapy is a neurobiological intervention that induces neuroplasticity and leads to structural brain repair, because talking, listening, triggering memories, inducing insight, and “connecting the dots” in one’s behavior are all biological events.1,2 There is no such thing as a purely psychological process independent of the brain. The mind is the product of ongoing complex, intricate activity of brain neurocircuits whose neurobiological activity is translated into thoughts, emotions, impulses, and behaviors. The mind is perpetually tethered to its neurological roots.
Thus, reductionism actually describes a scientific fact and is not a term with pejorative connotations used to shut down scientific discourse about the biological basis of human behavior. By advancing their clinical neuroscience literacy, psychiatric practitioners will understand that they deal with a specific brain pathology in every patient that they treat and that the medications and psychotherapeutic interventions they employ are synergistic biological treatments.3
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
References
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Out-of-the-box questions about psychotherapy. Current Psychiatry. 2010;9(10):13-14.
3. Nasrallah HA. Medications with psychotherapy: a synergy to heal the brain. C urrent Psychiatry. 2006;5(10):11-12.
The impact of childhood trauma
I enjoyed Dr. Nasrallah’s article “Beyond DSM-5: Clinical and biologic features shared by major psychiatric syndromes” (From the Editor, Current Psychiatry. October 2017, p. 4,6-7), but there was only 1 mention of childhood trauma, which shares features with most of the commonalities he described, such as inflammation, smaller brain volumes, gene and environment interaction, shortened telomeres, and elevated cortisol levels. The Adverse Childhood Experiences Study1 taught us about the impact of childhood trauma on the entire organism. We need to focus on that commonality.
Susan Jones, MD
Child and Adolescent Psychiatrist
Virginia Treatment Center for Children
Assistant Professor
Virginia Commonwealth University
School of Medicine
Richmond, Virginia
Reference
1. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258
Dr. Nasrallah responds
It is worth pointing out that childhood trauma predominantly leads to psychotic and mood disorders in adulthood, and the criteria I mentioned would then hold true.






