User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Neurodegenerative aspects of psychiatric disorders
Obesity, psychiatric disorders, and pregnancy
Rapid weight loss, irritability, and nausea after restarting ADHD treatment
CASE Medication management
Mr. L, age 58, presents to the outpatient psychiatric clinic seeking treatment for attention-deficit/hyperactivity disorder (ADHD), which was first diagnosed 11 years ago. Since discontinuing his ADHD medication, lisdexamfetamine 60 mg/d, 8 months ago, he has not been completing tasks and has been distracted in his job as a limousine driver. Mr. L says that when he was taking the medication, “I could focus and prioritize.” He reports that he has trouble retaining information and is easily distracted. He says he generally is organized with appointments and keeping track of things but is messy, forgetful, tardy, and impatient. Procrastination is an ongoing problem. He denies misplacing things or being impulsive. Mr. L reports that as a child he was frequently reprimanded for talking in class. He states, “I get in trouble even now for talking too much.”
Mr. L is cooperative and polite, maintains good eye contact, and is alert. No psychomotor abnormalities are noted. His speech is spontaneous and coherent, with normal rate, rhythm, and volume. He reports that his mood is “all right,” and denies suicidal or homicidal ideation. His insight is full, judgment is intact, and thought is linear and logical. Mr. L sleeps 5 hours at night and takes a nap during the day, but his energy varies.
His psychiatric history is negative for suicide attempts or hospitalizations. Mr. L denies a history of major depressive episodes, manic symptoms, hallucinations, or delusions. Anxiety history is negative for excessive worrying, obsessions and compulsions, and panic attacks. Mr. L has no family history of mental illness or substance abuse, and he denies any personal history of drug use. He stopped using tobacco 14 years ago. Mr. L says he drinks 3 caffeinated drinks a day and 2 glasses of wine once a week. Previous medications included
A review of systems is negative. Vital signs are unremarkable. A recent electrocardiogram (EKG) showed normal sinus rhythm. Thyroid-stimulating hormone, comprehensive metabolic panel (CMP), lipids, iron, vitamin B12, folate, complete blood count (CBC), hemoglobin A1c, and urine analysis are normal, except for mildly elevated low-density lipoprotein. Testing for hepatitis C is negative.
The previous diagnosis of ADHD is confirmed, and Mr. L is started on
[polldaddy:9928295]
The author’s observations
Anxiety, irritability, agitation, and palpitations can all be symptoms of stimulant medications.1,2 There are numerous other iatrogenic causes, including steroid-based asthma treatments, thyroid medications, antidepressants in bipolar patients, and caffeine-based migraine treatments. Mr. L’s theory that his 15-lb weight loss was the result of his methylphenidate ER dose being too high was a reasonable one. Often, medication doses need to be adjusted with weight changes. His decrease in energy during the day could be explained by the methylphenidate ER controlling his hyperactive symptoms, which include high energy. At night, when the medication wears off, his hyperactivity symptoms could be returning, which would account for the increase in energy when he gets home from work. Although longer-acting stimulants tend to have a more benign adverse effects profile, they can cause insomnia if they are still in the patient’s system at bedtime. Shorter-acting stimulants wear off quickly but can be advantageous for patients who want to target concentration during certain times of day, such as for school and homework.
TREATMENT A surprising cause
The next month, Mr. L presents to the emergency room complaining of jitteriness, headache, and tingling in his fingers, and is evaluated for suspected carbon monoxide (CO) poisoning. Three months earlier, he had noted the odor of exhaust fumes in the limousine he drives 7 days a week. He took it to the mechanic twice for evaluation, but no cause was found. Despite his concerns, he continued to drive the car until an older client, in frail health, suddenly became short of breath and developed chest pain shortly after entering his vehicle, on a day when the odor was particularly bad. Before that, a family of passengers had complained of headaches upon entering his vehicle. The third time he brought his car to be checked, the mechanic identified an exhaust system leak.
[polldaddy:9928298]
The author’s observations
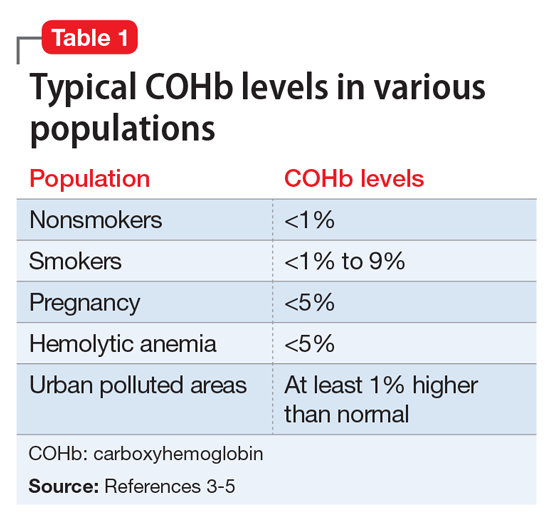
Work-up for suspected CO poisoning includes ABG, COHb level, CBC, basic metabolic panel, EKG, cardiac enzymes, and chest radiography, as well as other laboratory tests as deemed appropriate. Treatment includes oxygen by mask for low-level poisoning.
High levels of poisoning may require hyperbaric oxygen, which should be considered for patients who are unconscious or have an abnormal score on the Carbon Monoxide Neuropsychological Screening Battery, COHb of >40%, signs of cardiac ischemia or arrhythmia, history of ischemic heart disease with COHb level >20%, recurrent symptoms for up to 3 weeks, or symptoms that have not resolved with normobaric oxygen after 4 to 6 hours.9 Any pregnant woman with CO poisoning should receive hyperbaric therapy.10
OUTCOME Lasting improvement
Mr. L presents for follow-up in the psychiatric clinic 3 weeks after his emergency room visit. After his limousine was repaired, his symptoms resolved. He no longer experiences fatigue during the day with higher energy at night, palpitations, jitteriness, headache, or tingling. His concentration has improved, so he opts to stick with the 18-mg dose of methylphenidate ER rather than increase it to the initial dose. He places a CO detector in his vehicle, which proves to be a good decision when it gives him a warning that the exhaust leak had not been properly repaired.
[polldaddy:9928299]
The author’s observations
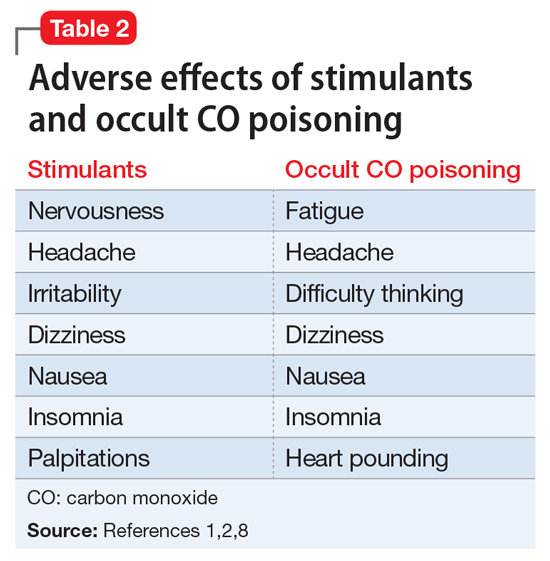
Although the correct cause of Mr. L’s symptoms was found incidentally, this case is an important reminder to always consider medical causes in the differential diagnosis. We are taught in medical school to look first for horses (more likely causes), not zebras (less likely causes), but sometimes zebras do occur. Be mindful that medical causes should be considered not only for symptoms of primary illnesses, but also for symptoms thought to be caused by adverse effects of medications. The differential diagnosis for Mr. L’s symptoms (palpitations, agitation, anxiety, irritability, weight loss, fatigue, nausea, and headache) included metabolic and endocrine abnormalities (thyroid disease, pheochromocytoma, hypoglycemia); psychiatric conditions (panic, bipolar disorder, depression); substance abuse (caffeine, cocaine, amphetamines); immune disorders; cardiac disorders; malignancy; toxic exposure; infectious sources; and nutritional deficiencies. CO poisoning can cause many of these symptoms (Table 2).1,2,8
Intentional CO poisoning should be considered in an obtunded or unconscious patient with depression. Patients may consider CO poisoning a more peaceful way to complete suicide than shooting, cutting, or hanging. As for unintentional poisoning, clinical suspicion can be increased by time of year, occupation, locale, and smoking status. Winter months increase risk because of the high use of heating devices, cars warming up in the garage, closed fireplace flues, and vehicle tailpipes blocked by snow. As in Mr. L’s case, occupation also may increase suspicion; drivers, mechanics, tollbooth operators, parking attendants, miners, and firefighters are all at increased risk for CO poisoning. Regarding locale, polluted urban environments as well as cold climates requiring heating sources cause higher risks for CO exposure. Rarely, excessive smoking can result in CO poisoning. The author once had a patient with schizophrenia who was admitted to the hospital with delirium. It was determined that he had CO poisoning from his 5-pack-a-day smoking habit.
Psychiatric patients often have the frustrating experience of their physical symptoms being attributed to psychiatric causes, which results in major medical issues being overlooked. We psychiatrists can fall into the same trap of overlooking medical illnesses, as indicated in this case, where Mr. L’s CO poisoning initially was attributed to adverse effects of his psychiatric medication.
1. Drugs.com. Amphetamine side effects. https://www.drugs.com/sfx/amphetamine-side-effects.html. Accessed December 7, 2017.
2. Golmirzaei J, Mahboobi H, Yazdanparast M, et al. Psychopharmacology of attention-deficit hyperactivity disorder: effects and side effects. Curr Pharm Des. 2016;22(5):590-594.
3. Bleecker ML. Carbon monoxide intoxication. Handb Clin Neurol. 2015;131(3):191-203.
4. Carter D. Carbon monoxide: the forgotten killer. http://scot.nhs.uk/sehd/cmo/CMO(1998)19.pdf. Published September 7, 1998. Accessed January 10, 2018.
5. Stewart RD, Baretta ED, Platte LR, et al. Carboxyhemoglobin levels in American blood donors. JAMA. 1974;229(9):1187-1195.
6. AA1Car. Troubleshoot odors & smells inside your car. http://www.aa1car.com/library/troubleshoot_odors.htm. Accessed December 7, 2017.
7. Rodkey FL, O’Neal JD, Collison HA, et al. Relative affinity of hemoglobin S and hemoglobin A for carbon monoxide and oxygen. Clin Chem. 1974;20(1):83-84.
8. Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med. 1987;146(1):52-56.
9. Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339(22):1603-1608.
10. Guzman JA. Carbon monoxide poisoning. Critical Care Clin. 2012;28(4):537-548.
CASE Medication management
Mr. L, age 58, presents to the outpatient psychiatric clinic seeking treatment for attention-deficit/hyperactivity disorder (ADHD), which was first diagnosed 11 years ago. Since discontinuing his ADHD medication, lisdexamfetamine 60 mg/d, 8 months ago, he has not been completing tasks and has been distracted in his job as a limousine driver. Mr. L says that when he was taking the medication, “I could focus and prioritize.” He reports that he has trouble retaining information and is easily distracted. He says he generally is organized with appointments and keeping track of things but is messy, forgetful, tardy, and impatient. Procrastination is an ongoing problem. He denies misplacing things or being impulsive. Mr. L reports that as a child he was frequently reprimanded for talking in class. He states, “I get in trouble even now for talking too much.”
Mr. L is cooperative and polite, maintains good eye contact, and is alert. No psychomotor abnormalities are noted. His speech is spontaneous and coherent, with normal rate, rhythm, and volume. He reports that his mood is “all right,” and denies suicidal or homicidal ideation. His insight is full, judgment is intact, and thought is linear and logical. Mr. L sleeps 5 hours at night and takes a nap during the day, but his energy varies.
His psychiatric history is negative for suicide attempts or hospitalizations. Mr. L denies a history of major depressive episodes, manic symptoms, hallucinations, or delusions. Anxiety history is negative for excessive worrying, obsessions and compulsions, and panic attacks. Mr. L has no family history of mental illness or substance abuse, and he denies any personal history of drug use. He stopped using tobacco 14 years ago. Mr. L says he drinks 3 caffeinated drinks a day and 2 glasses of wine once a week. Previous medications included
A review of systems is negative. Vital signs are unremarkable. A recent electrocardiogram (EKG) showed normal sinus rhythm. Thyroid-stimulating hormone, comprehensive metabolic panel (CMP), lipids, iron, vitamin B12, folate, complete blood count (CBC), hemoglobin A1c, and urine analysis are normal, except for mildly elevated low-density lipoprotein. Testing for hepatitis C is negative.
The previous diagnosis of ADHD is confirmed, and Mr. L is started on
[polldaddy:9928295]
The author’s observations
Anxiety, irritability, agitation, and palpitations can all be symptoms of stimulant medications.1,2 There are numerous other iatrogenic causes, including steroid-based asthma treatments, thyroid medications, antidepressants in bipolar patients, and caffeine-based migraine treatments. Mr. L’s theory that his 15-lb weight loss was the result of his methylphenidate ER dose being too high was a reasonable one. Often, medication doses need to be adjusted with weight changes. His decrease in energy during the day could be explained by the methylphenidate ER controlling his hyperactive symptoms, which include high energy. At night, when the medication wears off, his hyperactivity symptoms could be returning, which would account for the increase in energy when he gets home from work. Although longer-acting stimulants tend to have a more benign adverse effects profile, they can cause insomnia if they are still in the patient’s system at bedtime. Shorter-acting stimulants wear off quickly but can be advantageous for patients who want to target concentration during certain times of day, such as for school and homework.
TREATMENT A surprising cause
The next month, Mr. L presents to the emergency room complaining of jitteriness, headache, and tingling in his fingers, and is evaluated for suspected carbon monoxide (CO) poisoning. Three months earlier, he had noted the odor of exhaust fumes in the limousine he drives 7 days a week. He took it to the mechanic twice for evaluation, but no cause was found. Despite his concerns, he continued to drive the car until an older client, in frail health, suddenly became short of breath and developed chest pain shortly after entering his vehicle, on a day when the odor was particularly bad. Before that, a family of passengers had complained of headaches upon entering his vehicle. The third time he brought his car to be checked, the mechanic identified an exhaust system leak.

[polldaddy:9928298]
The author’s observations

Work-up for suspected CO poisoning includes ABG, COHb level, CBC, basic metabolic panel, EKG, cardiac enzymes, and chest radiography, as well as other laboratory tests as deemed appropriate. Treatment includes oxygen by mask for low-level poisoning.
High levels of poisoning may require hyperbaric oxygen, which should be considered for patients who are unconscious or have an abnormal score on the Carbon Monoxide Neuropsychological Screening Battery, COHb of >40%, signs of cardiac ischemia or arrhythmia, history of ischemic heart disease with COHb level >20%, recurrent symptoms for up to 3 weeks, or symptoms that have not resolved with normobaric oxygen after 4 to 6 hours.9 Any pregnant woman with CO poisoning should receive hyperbaric therapy.10
OUTCOME Lasting improvement
Mr. L presents for follow-up in the psychiatric clinic 3 weeks after his emergency room visit. After his limousine was repaired, his symptoms resolved. He no longer experiences fatigue during the day with higher energy at night, palpitations, jitteriness, headache, or tingling. His concentration has improved, so he opts to stick with the 18-mg dose of methylphenidate ER rather than increase it to the initial dose. He places a CO detector in his vehicle, which proves to be a good decision when it gives him a warning that the exhaust leak had not been properly repaired.
[polldaddy:9928299]
The author’s observations
Although the correct cause of Mr. L’s symptoms was found incidentally, this case is an important reminder to always consider medical causes in the differential diagnosis. We are taught in medical school to look first for horses (more likely causes), not zebras (less likely causes), but sometimes zebras do occur. Be mindful that medical causes should be considered not only for symptoms of primary illnesses, but also for symptoms thought to be caused by adverse effects of medications. The differential diagnosis for Mr. L’s symptoms (palpitations, agitation, anxiety, irritability, weight loss, fatigue, nausea, and headache) included metabolic and endocrine abnormalities (thyroid disease, pheochromocytoma, hypoglycemia); psychiatric conditions (panic, bipolar disorder, depression); substance abuse (caffeine, cocaine, amphetamines); immune disorders; cardiac disorders; malignancy; toxic exposure; infectious sources; and nutritional deficiencies. CO poisoning can cause many of these symptoms (Table 2).1,2,8
Intentional CO poisoning should be considered in an obtunded or unconscious patient with depression. Patients may consider CO poisoning a more peaceful way to complete suicide than shooting, cutting, or hanging. As for unintentional poisoning, clinical suspicion can be increased by time of year, occupation, locale, and smoking status. Winter months increase risk because of the high use of heating devices, cars warming up in the garage, closed fireplace flues, and vehicle tailpipes blocked by snow. As in Mr. L’s case, occupation also may increase suspicion; drivers, mechanics, tollbooth operators, parking attendants, miners, and firefighters are all at increased risk for CO poisoning. Regarding locale, polluted urban environments as well as cold climates requiring heating sources cause higher risks for CO exposure. Rarely, excessive smoking can result in CO poisoning. The author once had a patient with schizophrenia who was admitted to the hospital with delirium. It was determined that he had CO poisoning from his 5-pack-a-day smoking habit.
Psychiatric patients often have the frustrating experience of their physical symptoms being attributed to psychiatric causes, which results in major medical issues being overlooked. We psychiatrists can fall into the same trap of overlooking medical illnesses, as indicated in this case, where Mr. L’s CO poisoning initially was attributed to adverse effects of his psychiatric medication.
CASE Medication management
Mr. L, age 58, presents to the outpatient psychiatric clinic seeking treatment for attention-deficit/hyperactivity disorder (ADHD), which was first diagnosed 11 years ago. Since discontinuing his ADHD medication, lisdexamfetamine 60 mg/d, 8 months ago, he has not been completing tasks and has been distracted in his job as a limousine driver. Mr. L says that when he was taking the medication, “I could focus and prioritize.” He reports that he has trouble retaining information and is easily distracted. He says he generally is organized with appointments and keeping track of things but is messy, forgetful, tardy, and impatient. Procrastination is an ongoing problem. He denies misplacing things or being impulsive. Mr. L reports that as a child he was frequently reprimanded for talking in class. He states, “I get in trouble even now for talking too much.”
Mr. L is cooperative and polite, maintains good eye contact, and is alert. No psychomotor abnormalities are noted. His speech is spontaneous and coherent, with normal rate, rhythm, and volume. He reports that his mood is “all right,” and denies suicidal or homicidal ideation. His insight is full, judgment is intact, and thought is linear and logical. Mr. L sleeps 5 hours at night and takes a nap during the day, but his energy varies.
His psychiatric history is negative for suicide attempts or hospitalizations. Mr. L denies a history of major depressive episodes, manic symptoms, hallucinations, or delusions. Anxiety history is negative for excessive worrying, obsessions and compulsions, and panic attacks. Mr. L has no family history of mental illness or substance abuse, and he denies any personal history of drug use. He stopped using tobacco 14 years ago. Mr. L says he drinks 3 caffeinated drinks a day and 2 glasses of wine once a week. Previous medications included
A review of systems is negative. Vital signs are unremarkable. A recent electrocardiogram (EKG) showed normal sinus rhythm. Thyroid-stimulating hormone, comprehensive metabolic panel (CMP), lipids, iron, vitamin B12, folate, complete blood count (CBC), hemoglobin A1c, and urine analysis are normal, except for mildly elevated low-density lipoprotein. Testing for hepatitis C is negative.
The previous diagnosis of ADHD is confirmed, and Mr. L is started on
[polldaddy:9928295]
The author’s observations
Anxiety, irritability, agitation, and palpitations can all be symptoms of stimulant medications.1,2 There are numerous other iatrogenic causes, including steroid-based asthma treatments, thyroid medications, antidepressants in bipolar patients, and caffeine-based migraine treatments. Mr. L’s theory that his 15-lb weight loss was the result of his methylphenidate ER dose being too high was a reasonable one. Often, medication doses need to be adjusted with weight changes. His decrease in energy during the day could be explained by the methylphenidate ER controlling his hyperactive symptoms, which include high energy. At night, when the medication wears off, his hyperactivity symptoms could be returning, which would account for the increase in energy when he gets home from work. Although longer-acting stimulants tend to have a more benign adverse effects profile, they can cause insomnia if they are still in the patient’s system at bedtime. Shorter-acting stimulants wear off quickly but can be advantageous for patients who want to target concentration during certain times of day, such as for school and homework.
TREATMENT A surprising cause
The next month, Mr. L presents to the emergency room complaining of jitteriness, headache, and tingling in his fingers, and is evaluated for suspected carbon monoxide (CO) poisoning. Three months earlier, he had noted the odor of exhaust fumes in the limousine he drives 7 days a week. He took it to the mechanic twice for evaluation, but no cause was found. Despite his concerns, he continued to drive the car until an older client, in frail health, suddenly became short of breath and developed chest pain shortly after entering his vehicle, on a day when the odor was particularly bad. Before that, a family of passengers had complained of headaches upon entering his vehicle. The third time he brought his car to be checked, the mechanic identified an exhaust system leak.

[polldaddy:9928298]
The author’s observations

Work-up for suspected CO poisoning includes ABG, COHb level, CBC, basic metabolic panel, EKG, cardiac enzymes, and chest radiography, as well as other laboratory tests as deemed appropriate. Treatment includes oxygen by mask for low-level poisoning.
High levels of poisoning may require hyperbaric oxygen, which should be considered for patients who are unconscious or have an abnormal score on the Carbon Monoxide Neuropsychological Screening Battery, COHb of >40%, signs of cardiac ischemia or arrhythmia, history of ischemic heart disease with COHb level >20%, recurrent symptoms for up to 3 weeks, or symptoms that have not resolved with normobaric oxygen after 4 to 6 hours.9 Any pregnant woman with CO poisoning should receive hyperbaric therapy.10
OUTCOME Lasting improvement
Mr. L presents for follow-up in the psychiatric clinic 3 weeks after his emergency room visit. After his limousine was repaired, his symptoms resolved. He no longer experiences fatigue during the day with higher energy at night, palpitations, jitteriness, headache, or tingling. His concentration has improved, so he opts to stick with the 18-mg dose of methylphenidate ER rather than increase it to the initial dose. He places a CO detector in his vehicle, which proves to be a good decision when it gives him a warning that the exhaust leak had not been properly repaired.
[polldaddy:9928299]
The author’s observations
Although the correct cause of Mr. L’s symptoms was found incidentally, this case is an important reminder to always consider medical causes in the differential diagnosis. We are taught in medical school to look first for horses (more likely causes), not zebras (less likely causes), but sometimes zebras do occur. Be mindful that medical causes should be considered not only for symptoms of primary illnesses, but also for symptoms thought to be caused by adverse effects of medications. The differential diagnosis for Mr. L’s symptoms (palpitations, agitation, anxiety, irritability, weight loss, fatigue, nausea, and headache) included metabolic and endocrine abnormalities (thyroid disease, pheochromocytoma, hypoglycemia); psychiatric conditions (panic, bipolar disorder, depression); substance abuse (caffeine, cocaine, amphetamines); immune disorders; cardiac disorders; malignancy; toxic exposure; infectious sources; and nutritional deficiencies. CO poisoning can cause many of these symptoms (Table 2).1,2,8
Intentional CO poisoning should be considered in an obtunded or unconscious patient with depression. Patients may consider CO poisoning a more peaceful way to complete suicide than shooting, cutting, or hanging. As for unintentional poisoning, clinical suspicion can be increased by time of year, occupation, locale, and smoking status. Winter months increase risk because of the high use of heating devices, cars warming up in the garage, closed fireplace flues, and vehicle tailpipes blocked by snow. As in Mr. L’s case, occupation also may increase suspicion; drivers, mechanics, tollbooth operators, parking attendants, miners, and firefighters are all at increased risk for CO poisoning. Regarding locale, polluted urban environments as well as cold climates requiring heating sources cause higher risks for CO exposure. Rarely, excessive smoking can result in CO poisoning. The author once had a patient with schizophrenia who was admitted to the hospital with delirium. It was determined that he had CO poisoning from his 5-pack-a-day smoking habit.
Psychiatric patients often have the frustrating experience of their physical symptoms being attributed to psychiatric causes, which results in major medical issues being overlooked. We psychiatrists can fall into the same trap of overlooking medical illnesses, as indicated in this case, where Mr. L’s CO poisoning initially was attributed to adverse effects of his psychiatric medication.
1. Drugs.com. Amphetamine side effects. https://www.drugs.com/sfx/amphetamine-side-effects.html. Accessed December 7, 2017.
2. Golmirzaei J, Mahboobi H, Yazdanparast M, et al. Psychopharmacology of attention-deficit hyperactivity disorder: effects and side effects. Curr Pharm Des. 2016;22(5):590-594.
3. Bleecker ML. Carbon monoxide intoxication. Handb Clin Neurol. 2015;131(3):191-203.
4. Carter D. Carbon monoxide: the forgotten killer. http://scot.nhs.uk/sehd/cmo/CMO(1998)19.pdf. Published September 7, 1998. Accessed January 10, 2018.
5. Stewart RD, Baretta ED, Platte LR, et al. Carboxyhemoglobin levels in American blood donors. JAMA. 1974;229(9):1187-1195.
6. AA1Car. Troubleshoot odors & smells inside your car. http://www.aa1car.com/library/troubleshoot_odors.htm. Accessed December 7, 2017.
7. Rodkey FL, O’Neal JD, Collison HA, et al. Relative affinity of hemoglobin S and hemoglobin A for carbon monoxide and oxygen. Clin Chem. 1974;20(1):83-84.
8. Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med. 1987;146(1):52-56.
9. Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339(22):1603-1608.
10. Guzman JA. Carbon monoxide poisoning. Critical Care Clin. 2012;28(4):537-548.
1. Drugs.com. Amphetamine side effects. https://www.drugs.com/sfx/amphetamine-side-effects.html. Accessed December 7, 2017.
2. Golmirzaei J, Mahboobi H, Yazdanparast M, et al. Psychopharmacology of attention-deficit hyperactivity disorder: effects and side effects. Curr Pharm Des. 2016;22(5):590-594.
3. Bleecker ML. Carbon monoxide intoxication. Handb Clin Neurol. 2015;131(3):191-203.
4. Carter D. Carbon monoxide: the forgotten killer. http://scot.nhs.uk/sehd/cmo/CMO(1998)19.pdf. Published September 7, 1998. Accessed January 10, 2018.
5. Stewart RD, Baretta ED, Platte LR, et al. Carboxyhemoglobin levels in American blood donors. JAMA. 1974;229(9):1187-1195.
6. AA1Car. Troubleshoot odors & smells inside your car. http://www.aa1car.com/library/troubleshoot_odors.htm. Accessed December 7, 2017.
7. Rodkey FL, O’Neal JD, Collison HA, et al. Relative affinity of hemoglobin S and hemoglobin A for carbon monoxide and oxygen. Clin Chem. 1974;20(1):83-84.
8. Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med. 1987;146(1):52-56.
9. Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339(22):1603-1608.
10. Guzman JA. Carbon monoxide poisoning. Critical Care Clin. 2012;28(4):537-548.
4 Ways to help your patients with schizophrenia quit smoking
Tobacco-related cardiovascular disease is the primary reason adults with schizophrenia die on average 28 years earlier than their peers in the U.S. general population.1 To address this, clinicians need to prioritize smoking cessation and emphasize to patients with schizophrenia that quitting is the most important change they can make to improve their health. Here are 4 ways to help patients with schizophrenia quit smoking.
Provide hope, but be realistic. Most patients with schizophrenia who smoke want to quit; however, patients and clinicians alike have been discouraged by low quit rates and high relapse rates. Smoking often is viewed as one of the few remaining personal freedoms, as a lower priority than active psychiatric symptoms, or even as neuroprotective. By perpetuating these falsehoods and avoiding addressing smoking cessation, we are failing our patients.
With persistent engagement and use of effective pharmacotherapeutic interventions, smoking cessation is attainable and does not worsen psychiatric symptoms. Additionally, smoking cessation could save patients >$4,000 a year. It is crucial to make smoking cessation a priority at every appointment, and to offer patients hope and practical guidance through repeated attempts to quit.
Offer varenicline. For patients with schizophrenia, cessation counseling or behavioral interventions alone have a poor efficacy rate of approximately 5% (compared with 15% to 20% in the general population).2 Varenicline is the most effective smoking cessation treatment; it increases cessation rates 5-fold among patients with schizophrenia.3 As demonstrated by the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES),4 varenicline does not lead to an increased risk of suicidality or serious neuropsychiatric adverse effects.
When starting a patient on varenicline, set a quit date 4 weeks from medication initiation. Individuals with schizophrenia often have a greater smoking burden and experience more intense symptoms of nicotine withdrawal. A 4-week period between medication initiation and the quit date will allow these patients to gradually experience reduced cravings and separate minor adverse effects of the medication from those of nicotine withdrawal. Concurrent prescription of nicotine replacement therapy (eg, patch, gum, lozenge, inhaler) also is safe and can assist in quit attempts.
Consider varenicline maintenance therapy. After a successful quit attempt, increase the likelihood of sustained cessation by continuing varenicline beyond 12 weeks. Varenicline can be used as a maintenance medication to prevent smoking relapse in patients with schizophrenia; when prescribed to these patients for an additional 3 months, it can reduce the relapse rate similarly to that seen in smokers in the general population.5
Adjust antipsychotic dosages. Tobacco smoke increases the activity of cytochrome P450 1A2, which metabolizes several antipsychotics. Thus, after successful smoking cessation, concentrations of clozapine, fluphenazine, haloperidol, and olanzapine may increase, and dose reduction may be warranted. Conversely, if a patient resumes smoking, dosages of these medications may need to be increased.
Acknowledgments
The authors thank Anne Eden Evins, MD, MPH, and Corinne Cather, PhD, for their input on this article.
1. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
2. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2(2):CD007253.
3. Evins AE, Benowitz N, West R, et al. Neuropsychiatric safety and efficacy of varenicline and bupropion vs. nicotine patch and placebo in the psychiatric cohort of the EAGLES trial. Paper presented at: Society for Research on Nicotine and Tobacco, 22nd Annual Meeting; March 2-5, 2016; Chicago, IL.
4. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520.
5. Evins AE, Hoeppner SS, Schoenfeld DA, et al. Maintenance pharmacotherapy normalizes the relapse curve in recently abstinent tobacco smokers with schizophrenia and bipolar disorder. Schizophr Res. 2017;183:124-129.
Tobacco-related cardiovascular disease is the primary reason adults with schizophrenia die on average 28 years earlier than their peers in the U.S. general population.1 To address this, clinicians need to prioritize smoking cessation and emphasize to patients with schizophrenia that quitting is the most important change they can make to improve their health. Here are 4 ways to help patients with schizophrenia quit smoking.
Provide hope, but be realistic. Most patients with schizophrenia who smoke want to quit; however, patients and clinicians alike have been discouraged by low quit rates and high relapse rates. Smoking often is viewed as one of the few remaining personal freedoms, as a lower priority than active psychiatric symptoms, or even as neuroprotective. By perpetuating these falsehoods and avoiding addressing smoking cessation, we are failing our patients.
With persistent engagement and use of effective pharmacotherapeutic interventions, smoking cessation is attainable and does not worsen psychiatric symptoms. Additionally, smoking cessation could save patients >$4,000 a year. It is crucial to make smoking cessation a priority at every appointment, and to offer patients hope and practical guidance through repeated attempts to quit.
Offer varenicline. For patients with schizophrenia, cessation counseling or behavioral interventions alone have a poor efficacy rate of approximately 5% (compared with 15% to 20% in the general population).2 Varenicline is the most effective smoking cessation treatment; it increases cessation rates 5-fold among patients with schizophrenia.3 As demonstrated by the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES),4 varenicline does not lead to an increased risk of suicidality or serious neuropsychiatric adverse effects.
When starting a patient on varenicline, set a quit date 4 weeks from medication initiation. Individuals with schizophrenia often have a greater smoking burden and experience more intense symptoms of nicotine withdrawal. A 4-week period between medication initiation and the quit date will allow these patients to gradually experience reduced cravings and separate minor adverse effects of the medication from those of nicotine withdrawal. Concurrent prescription of nicotine replacement therapy (eg, patch, gum, lozenge, inhaler) also is safe and can assist in quit attempts.
Consider varenicline maintenance therapy. After a successful quit attempt, increase the likelihood of sustained cessation by continuing varenicline beyond 12 weeks. Varenicline can be used as a maintenance medication to prevent smoking relapse in patients with schizophrenia; when prescribed to these patients for an additional 3 months, it can reduce the relapse rate similarly to that seen in smokers in the general population.5
Adjust antipsychotic dosages. Tobacco smoke increases the activity of cytochrome P450 1A2, which metabolizes several antipsychotics. Thus, after successful smoking cessation, concentrations of clozapine, fluphenazine, haloperidol, and olanzapine may increase, and dose reduction may be warranted. Conversely, if a patient resumes smoking, dosages of these medications may need to be increased.
Acknowledgments
The authors thank Anne Eden Evins, MD, MPH, and Corinne Cather, PhD, for their input on this article.
Tobacco-related cardiovascular disease is the primary reason adults with schizophrenia die on average 28 years earlier than their peers in the U.S. general population.1 To address this, clinicians need to prioritize smoking cessation and emphasize to patients with schizophrenia that quitting is the most important change they can make to improve their health. Here are 4 ways to help patients with schizophrenia quit smoking.
Provide hope, but be realistic. Most patients with schizophrenia who smoke want to quit; however, patients and clinicians alike have been discouraged by low quit rates and high relapse rates. Smoking often is viewed as one of the few remaining personal freedoms, as a lower priority than active psychiatric symptoms, or even as neuroprotective. By perpetuating these falsehoods and avoiding addressing smoking cessation, we are failing our patients.
With persistent engagement and use of effective pharmacotherapeutic interventions, smoking cessation is attainable and does not worsen psychiatric symptoms. Additionally, smoking cessation could save patients >$4,000 a year. It is crucial to make smoking cessation a priority at every appointment, and to offer patients hope and practical guidance through repeated attempts to quit.
Offer varenicline. For patients with schizophrenia, cessation counseling or behavioral interventions alone have a poor efficacy rate of approximately 5% (compared with 15% to 20% in the general population).2 Varenicline is the most effective smoking cessation treatment; it increases cessation rates 5-fold among patients with schizophrenia.3 As demonstrated by the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES),4 varenicline does not lead to an increased risk of suicidality or serious neuropsychiatric adverse effects.
When starting a patient on varenicline, set a quit date 4 weeks from medication initiation. Individuals with schizophrenia often have a greater smoking burden and experience more intense symptoms of nicotine withdrawal. A 4-week period between medication initiation and the quit date will allow these patients to gradually experience reduced cravings and separate minor adverse effects of the medication from those of nicotine withdrawal. Concurrent prescription of nicotine replacement therapy (eg, patch, gum, lozenge, inhaler) also is safe and can assist in quit attempts.
Consider varenicline maintenance therapy. After a successful quit attempt, increase the likelihood of sustained cessation by continuing varenicline beyond 12 weeks. Varenicline can be used as a maintenance medication to prevent smoking relapse in patients with schizophrenia; when prescribed to these patients for an additional 3 months, it can reduce the relapse rate similarly to that seen in smokers in the general population.5
Adjust antipsychotic dosages. Tobacco smoke increases the activity of cytochrome P450 1A2, which metabolizes several antipsychotics. Thus, after successful smoking cessation, concentrations of clozapine, fluphenazine, haloperidol, and olanzapine may increase, and dose reduction may be warranted. Conversely, if a patient resumes smoking, dosages of these medications may need to be increased.
Acknowledgments
The authors thank Anne Eden Evins, MD, MPH, and Corinne Cather, PhD, for their input on this article.
1. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
2. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2(2):CD007253.
3. Evins AE, Benowitz N, West R, et al. Neuropsychiatric safety and efficacy of varenicline and bupropion vs. nicotine patch and placebo in the psychiatric cohort of the EAGLES trial. Paper presented at: Society for Research on Nicotine and Tobacco, 22nd Annual Meeting; March 2-5, 2016; Chicago, IL.
4. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520.
5. Evins AE, Hoeppner SS, Schoenfeld DA, et al. Maintenance pharmacotherapy normalizes the relapse curve in recently abstinent tobacco smokers with schizophrenia and bipolar disorder. Schizophr Res. 2017;183:124-129.
1. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
2. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2(2):CD007253.
3. Evins AE, Benowitz N, West R, et al. Neuropsychiatric safety and efficacy of varenicline and bupropion vs. nicotine patch and placebo in the psychiatric cohort of the EAGLES trial. Paper presented at: Society for Research on Nicotine and Tobacco, 22nd Annual Meeting; March 2-5, 2016; Chicago, IL.
4. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520.
5. Evins AE, Hoeppner SS, Schoenfeld DA, et al. Maintenance pharmacotherapy normalizes the relapse curve in recently abstinent tobacco smokers with schizophrenia and bipolar disorder. Schizophr Res. 2017;183:124-129.
Decreasing suicide risk with math
Suicide is a common reality, accounting for approximately 800,000 deaths per year worldwide.1 Properly assessing and minimizing suicide risk can be challenging. We are taught that lithium and clozapine can decrease suicidality, and many psychiatrists prescribe these medications with the firm, “evidence-based” belief that doing so reduces suicide risk. Paradoxically, what they in fact might be doing is the exact opposite; they may be giving high-risk patients the opportunity and the means to attempt suicide with a lethal amount of medication.
One patient diagnosed with a mood disorder who attempted suicide had a surprising point of view. After taking a large qu
Operations research is a subfield of mathematics that tries to optimize one or more variables when multiple variables are in play. One example would be to maximize profit while minimizing cost. During World War II, operations research was used to decrease the number of munitions used to shoot down airplanes, and to sink submarines more efficiently.
Focusing on the patient who attempted suicide by overdose, the question was: If she was discharged from the psychiatry unit with a 30-day supply of medication, how lethal would that prescription be if deliberately taken all at once? And what can be done to minimize this suicide risk? Psychiatrists know that some medications are more dangerous than others, but few have performed quantitative analysis to determine the potential lethality of these medications. The math analysis did not involve multivariable calculus or differential equations, only multiplication and division. The results were eye-opening.
Calculating relative lethality
The lethal dose 50 (LD50) is the dose of a medication expressed in mg/kg that results in the death of 50% of the animals (usually rats) used in a controlled experiment. Open-source data for the LD50 of medications is provided by the manufacturers.
I tabulated this data for a wide range of psychiatric medications, including antipsychotics, mood stabilizers, and selective serotonin reuptake inhibitors, in a spreadsheet with columns for maximum daily dose, 30-day supply of the medication, LD50 in mg/kg, LD50 for a 60-kg subject, and percentage of the 30-day supply compared with LD50. I then sorted this data by relative lethality (for my complete data, see Figure 1 and the Table).
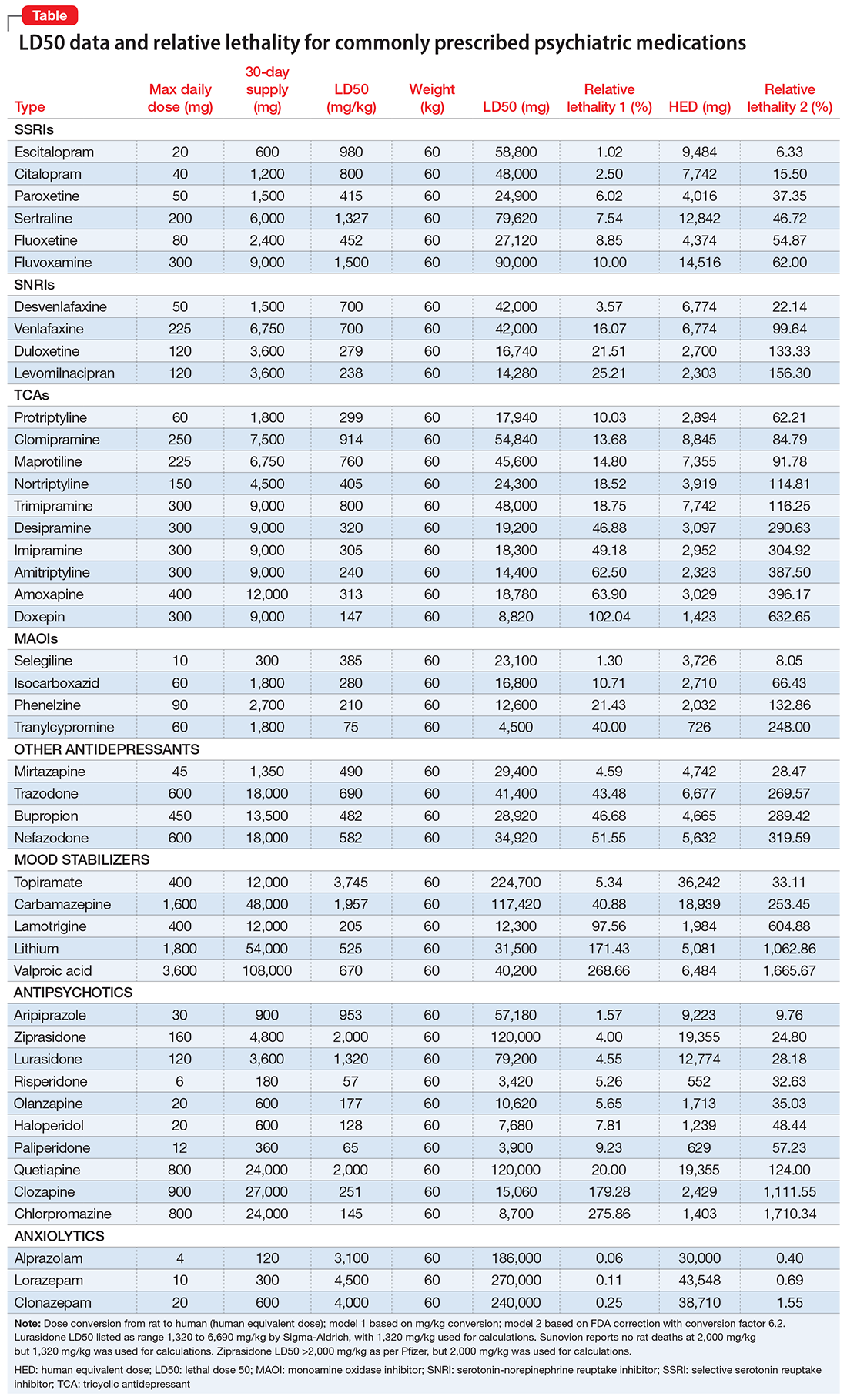
The rat dose in mg/kg was extrapolated to the human equivalent dose (HED) in mg/kg using a conversion factor of 6.2 (for a person who weighs 60 kg, the HED = LD50/6.2) as suggested by the FDA.2 The dose for the first fatality is smaller than the HED, and toxicity occurs at even smaller doses. After simplifying all the terms, the formula for the HED-relative lethality is f(x) = 310x/LD50, where x is the daily dose of a medication prescribed for 30 days. This is the equation of a straight line with a slope inversely proportional to the LD50 of each medication and a y-axis intercept of 0. Each medication line shows that any dose rising above 100% on the y-axis is a quantum higher than the lethal dose.
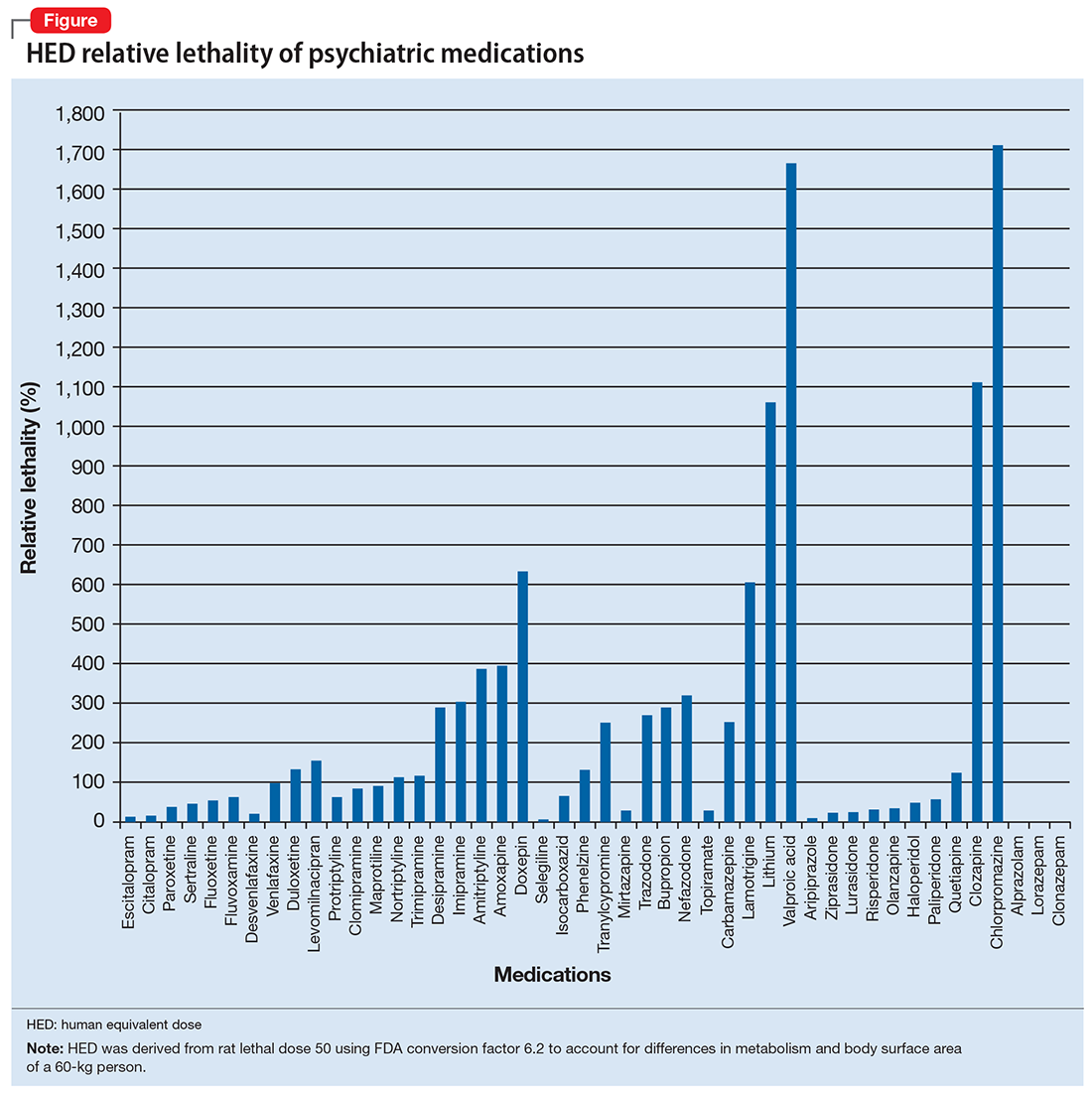
Some commonly prescribed psychotropics are highly lethal
The relative lethality of many commonly prescribed psychiatric medications, including those frequently used to reduce suicidality, varies tremendously. For example, it is widely known that the first-line mood stabilizer lithium has a narrow therapeutic window and can rapidly become toxic. If a patient becomes dehydrated, even a normal lithium dose can be toxic or lethal. Lithium has a relative lethality of 1,063% (Figure 2). Clozapine has a relative lethality of 1,112%. Valproic acid has an even higher relative lethality of 1,666%. By contrast, aripiprazole and olanzapine have a relative lethality of 10% and 35%, respectively. For preventing suicide, prescribing a second-generation antipsychotic with a lower relative lethality may be preferable over prescribing a medication with a higher relative lethality.
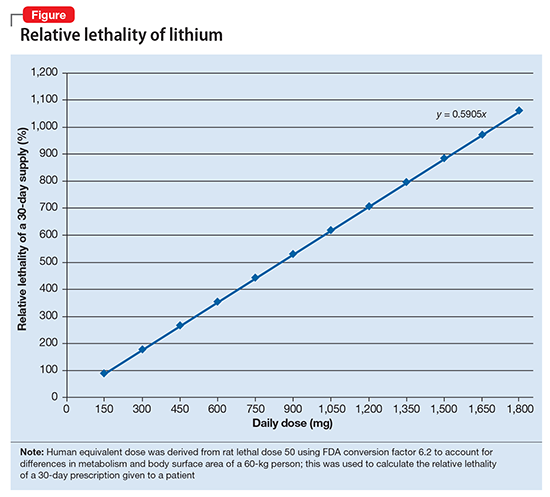
According to U.S. poison control centers,3 from 2000 to 2014, there were 15,036 serious outcomes, including 61 deaths, associated with lithium use, and 6,109 serious outcomes, including 37 deaths, associated with valproic acid. In contrast, there were only 1,446 serious outcomes and no deaths associated with aripiprazole use.3 These outcomes may be underreported, but they are consistent with the mathematical model predicting that medications with a higher relative lethality will have higher morbidity and mortality outcomes, regardless of a patient’s intent to overdose.
Many psychiatrists have a preferred antidepressant, mood stabilizer, or antipsychotic, and may prescribe this medication to many of their patients based on familiarity with the agent or other factors. However, simple math can give the decision process of selecting a specific medication for a given patient a more quantitative basis.
Even a small reduction in suicide would save many lives
Ultimately, the math problem comes down to 4 minutes, which is approximately how long the brain can survive without oxygen. By prescribing medications with a lower relative lethality, or by prescribing a less-than-30-day supply of the most lethal medications, it may be possible to decrease overdose morbidity and mortality, and also buy enough time for emergency personnel to save a life. If simple math can put even a 1% dent in the rate of death from suicide, approximately 8,000 lives might be saved every year.
1. World Health Organization. Suicide. Fact sheet. http://www.who.int/mediacentre/factsheets/fs398/en. Updated August 2017. Accessed January 3, 2018.
2. U.S. Food and Drug Administration. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. https://www.fda.gov/downloads/drugs/guidances/ucm078932.pdf. Published July 6, 2005. Accessed January 8, 2018.
3. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
Suicide is a common reality, accounting for approximately 800,000 deaths per year worldwide.1 Properly assessing and minimizing suicide risk can be challenging. We are taught that lithium and clozapine can decrease suicidality, and many psychiatrists prescribe these medications with the firm, “evidence-based” belief that doing so reduces suicide risk. Paradoxically, what they in fact might be doing is the exact opposite; they may be giving high-risk patients the opportunity and the means to attempt suicide with a lethal amount of medication.
One patient diagnosed with a mood disorder who attempted suicide had a surprising point of view. After taking a large qu
Operations research is a subfield of mathematics that tries to optimize one or more variables when multiple variables are in play. One example would be to maximize profit while minimizing cost. During World War II, operations research was used to decrease the number of munitions used to shoot down airplanes, and to sink submarines more efficiently.
Focusing on the patient who attempted suicide by overdose, the question was: If she was discharged from the psychiatry unit with a 30-day supply of medication, how lethal would that prescription be if deliberately taken all at once? And what can be done to minimize this suicide risk? Psychiatrists know that some medications are more dangerous than others, but few have performed quantitative analysis to determine the potential lethality of these medications. The math analysis did not involve multivariable calculus or differential equations, only multiplication and division. The results were eye-opening.
Calculating relative lethality
The lethal dose 50 (LD50) is the dose of a medication expressed in mg/kg that results in the death of 50% of the animals (usually rats) used in a controlled experiment. Open-source data for the LD50 of medications is provided by the manufacturers.
I tabulated this data for a wide range of psychiatric medications, including antipsychotics, mood stabilizers, and selective serotonin reuptake inhibitors, in a spreadsheet with columns for maximum daily dose, 30-day supply of the medication, LD50 in mg/kg, LD50 for a 60-kg subject, and percentage of the 30-day supply compared with LD50. I then sorted this data by relative lethality (for my complete data, see Figure 1 and the Table).

The rat dose in mg/kg was extrapolated to the human equivalent dose (HED) in mg/kg using a conversion factor of 6.2 (for a person who weighs 60 kg, the HED = LD50/6.2) as suggested by the FDA.2 The dose for the first fatality is smaller than the HED, and toxicity occurs at even smaller doses. After simplifying all the terms, the formula for the HED-relative lethality is f(x) = 310x/LD50, where x is the daily dose of a medication prescribed for 30 days. This is the equation of a straight line with a slope inversely proportional to the LD50 of each medication and a y-axis intercept of 0. Each medication line shows that any dose rising above 100% on the y-axis is a quantum higher than the lethal dose.

Some commonly prescribed psychotropics are highly lethal
The relative lethality of many commonly prescribed psychiatric medications, including those frequently used to reduce suicidality, varies tremendously. For example, it is widely known that the first-line mood stabilizer lithium has a narrow therapeutic window and can rapidly become toxic. If a patient becomes dehydrated, even a normal lithium dose can be toxic or lethal. Lithium has a relative lethality of 1,063% (Figure 2). Clozapine has a relative lethality of 1,112%. Valproic acid has an even higher relative lethality of 1,666%. By contrast, aripiprazole and olanzapine have a relative lethality of 10% and 35%, respectively. For preventing suicide, prescribing a second-generation antipsychotic with a lower relative lethality may be preferable over prescribing a medication with a higher relative lethality.

According to U.S. poison control centers,3 from 2000 to 2014, there were 15,036 serious outcomes, including 61 deaths, associated with lithium use, and 6,109 serious outcomes, including 37 deaths, associated with valproic acid. In contrast, there were only 1,446 serious outcomes and no deaths associated with aripiprazole use.3 These outcomes may be underreported, but they are consistent with the mathematical model predicting that medications with a higher relative lethality will have higher morbidity and mortality outcomes, regardless of a patient’s intent to overdose.
Many psychiatrists have a preferred antidepressant, mood stabilizer, or antipsychotic, and may prescribe this medication to many of their patients based on familiarity with the agent or other factors. However, simple math can give the decision process of selecting a specific medication for a given patient a more quantitative basis.
Even a small reduction in suicide would save many lives
Ultimately, the math problem comes down to 4 minutes, which is approximately how long the brain can survive without oxygen. By prescribing medications with a lower relative lethality, or by prescribing a less-than-30-day supply of the most lethal medications, it may be possible to decrease overdose morbidity and mortality, and also buy enough time for emergency personnel to save a life. If simple math can put even a 1% dent in the rate of death from suicide, approximately 8,000 lives might be saved every year.
Suicide is a common reality, accounting for approximately 800,000 deaths per year worldwide.1 Properly assessing and minimizing suicide risk can be challenging. We are taught that lithium and clozapine can decrease suicidality, and many psychiatrists prescribe these medications with the firm, “evidence-based” belief that doing so reduces suicide risk. Paradoxically, what they in fact might be doing is the exact opposite; they may be giving high-risk patients the opportunity and the means to attempt suicide with a lethal amount of medication.
One patient diagnosed with a mood disorder who attempted suicide had a surprising point of view. After taking a large qu
Operations research is a subfield of mathematics that tries to optimize one or more variables when multiple variables are in play. One example would be to maximize profit while minimizing cost. During World War II, operations research was used to decrease the number of munitions used to shoot down airplanes, and to sink submarines more efficiently.
Focusing on the patient who attempted suicide by overdose, the question was: If she was discharged from the psychiatry unit with a 30-day supply of medication, how lethal would that prescription be if deliberately taken all at once? And what can be done to minimize this suicide risk? Psychiatrists know that some medications are more dangerous than others, but few have performed quantitative analysis to determine the potential lethality of these medications. The math analysis did not involve multivariable calculus or differential equations, only multiplication and division. The results were eye-opening.
Calculating relative lethality
The lethal dose 50 (LD50) is the dose of a medication expressed in mg/kg that results in the death of 50% of the animals (usually rats) used in a controlled experiment. Open-source data for the LD50 of medications is provided by the manufacturers.
I tabulated this data for a wide range of psychiatric medications, including antipsychotics, mood stabilizers, and selective serotonin reuptake inhibitors, in a spreadsheet with columns for maximum daily dose, 30-day supply of the medication, LD50 in mg/kg, LD50 for a 60-kg subject, and percentage of the 30-day supply compared with LD50. I then sorted this data by relative lethality (for my complete data, see Figure 1 and the Table).

The rat dose in mg/kg was extrapolated to the human equivalent dose (HED) in mg/kg using a conversion factor of 6.2 (for a person who weighs 60 kg, the HED = LD50/6.2) as suggested by the FDA.2 The dose for the first fatality is smaller than the HED, and toxicity occurs at even smaller doses. After simplifying all the terms, the formula for the HED-relative lethality is f(x) = 310x/LD50, where x is the daily dose of a medication prescribed for 30 days. This is the equation of a straight line with a slope inversely proportional to the LD50 of each medication and a y-axis intercept of 0. Each medication line shows that any dose rising above 100% on the y-axis is a quantum higher than the lethal dose.

Some commonly prescribed psychotropics are highly lethal
The relative lethality of many commonly prescribed psychiatric medications, including those frequently used to reduce suicidality, varies tremendously. For example, it is widely known that the first-line mood stabilizer lithium has a narrow therapeutic window and can rapidly become toxic. If a patient becomes dehydrated, even a normal lithium dose can be toxic or lethal. Lithium has a relative lethality of 1,063% (Figure 2). Clozapine has a relative lethality of 1,112%. Valproic acid has an even higher relative lethality of 1,666%. By contrast, aripiprazole and olanzapine have a relative lethality of 10% and 35%, respectively. For preventing suicide, prescribing a second-generation antipsychotic with a lower relative lethality may be preferable over prescribing a medication with a higher relative lethality.

According to U.S. poison control centers,3 from 2000 to 2014, there were 15,036 serious outcomes, including 61 deaths, associated with lithium use, and 6,109 serious outcomes, including 37 deaths, associated with valproic acid. In contrast, there were only 1,446 serious outcomes and no deaths associated with aripiprazole use.3 These outcomes may be underreported, but they are consistent with the mathematical model predicting that medications with a higher relative lethality will have higher morbidity and mortality outcomes, regardless of a patient’s intent to overdose.
Many psychiatrists have a preferred antidepressant, mood stabilizer, or antipsychotic, and may prescribe this medication to many of their patients based on familiarity with the agent or other factors. However, simple math can give the decision process of selecting a specific medication for a given patient a more quantitative basis.
Even a small reduction in suicide would save many lives
Ultimately, the math problem comes down to 4 minutes, which is approximately how long the brain can survive without oxygen. By prescribing medications with a lower relative lethality, or by prescribing a less-than-30-day supply of the most lethal medications, it may be possible to decrease overdose morbidity and mortality, and also buy enough time for emergency personnel to save a life. If simple math can put even a 1% dent in the rate of death from suicide, approximately 8,000 lives might be saved every year.
1. World Health Organization. Suicide. Fact sheet. http://www.who.int/mediacentre/factsheets/fs398/en. Updated August 2017. Accessed January 3, 2018.
2. U.S. Food and Drug Administration. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. https://www.fda.gov/downloads/drugs/guidances/ucm078932.pdf. Published July 6, 2005. Accessed January 8, 2018.
3. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
1. World Health Organization. Suicide. Fact sheet. http://www.who.int/mediacentre/factsheets/fs398/en. Updated August 2017. Accessed January 3, 2018.
2. U.S. Food and Drug Administration. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. https://www.fda.gov/downloads/drugs/guidances/ucm078932.pdf. Published July 6, 2005. Accessed January 8, 2018.
3. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
The stigma toward BPD
In response to Dr. Mark Zimmerman’s article, “Improving the recognition of borderline personality disorder” (
Why all the stigma? Because mental health professionals don’t have complete information. The assumption used to be that BPD was “intractable” with no treatment. Even if this were true, it still would not be a reason to fail to disclose a diagnosis, because in other fields of medicine, the concept of “therapeutic privilege” fell by the wayside long ago. However, we now know that in many individuals with BPD, symptoms improve over time, and there are several effective treatments.
In DSM-II, published in 1968, obsessive-compulsive disorder (OCD) was characterized as an “obsessive compulsive neurosis.” It was not reclassified as the current OCD diagnosis until DSM-III-R was published in 1987, after the FDA approved clomipramine. Why is this important? Because once people realized that there was a treatment, they started acknowledging OCD more often.
The first step in addressing the stigma toward BPD is that mental health professionals must recognize their own bias toward this diagnosis. We must be re-educated that this diagnosis carries hope, symptoms improve, and that there are effective treatments. This is how professionals will increase the recognition of BPD.
Assistant Professor and Compliance Officer
Department of Psychiatry
University of Florida
Clinic Director
UF Child and Adolescent Psychiatry Clinic at Springhill Health Center
Gainesville, Florida
References
1. Unruh BT, Gunderson JG. “Good enough” psychiatric residency training in borderline personality disorder: challenges, choice points, and a model generalist curriculum. Harv Rev Psychiatry. 2016;24(5):367-377.
2. Sheehan L, Nieweglowski K, Corrigan P. The stigma of personality disorders. Curr Psychiatry Rep. 2016;18(1):11.
Continue to: The author responds
The author responds
I agree with Dr. Shapiro that stigma by mental health clinicians contributes to the underdiagnosis of BPD. Mental health professions often hold a negative view of patients with personality disorders, particularly those with BPD, and see these patients as being more difficult to treat.1-3 They are the patients that some clinicians are reluctant to treat.3,4 Clinicians perceive patients with personality disorders as less mentally ill, more manipulative, and more able to control their behavior than patients with other psychiatric disorders.3,5 Consistent with this, clinicians have less sympathetic attitudes and behave less empathically toward patients with BPD.5,6 The term “borderline” also is sometimes used pejoratively to describe patients.1
As I described in my article, there are several possible reasons BPD is underdiagnosed. Foremost is that mood disorders, anxiety disorders, and substance use disorders are common in patients with BPD, and the symptoms of these other disorders are typically patients’ chief concerns when they present for treatment. Patients with BPD do not usually report the features of BPD—such as abandonment fears, chronic feelings of emptiness, or an identity disturbance—as their chief concerns. If they did, BPD would likely be easier to recognize. On a related note, clinicians do not have the time, or do not take the time, to conduct a thorough enough evaluation to diagnose BPD when it occurs in a patient who presents for treatment of a mood disorder, anxiety disorder, or substance use disorder. Our clinical research group found that when psychiatrists are presented with the results of a semi-structured interview, BPD is much more frequently diagnosed.7 Such a finding would not be expected if stigma was the primary or sole reason for underdiagnosis.
Dr. Shapiro highlights the clinical consequence of underrecognition and underdiagnosis: the underutilization of empirically supported psychotherapies for BPD. A corollary of underdiagnosing BPD is overdiagnosis of bipolar disorder and overprescription of medication.8
There are other consequences of bias and stigma toward BPD. Despite the high levels of psychosocial morbidity, reduced health-related quality of life, high utilization of services, and excess mortality associated with BPD, this disorder is not included in the Global Burden of Disease Study. Thus, the public health significance of BPD is less fully appreciated. Finally, there is evidence that the level of funding for research from the National Institutes of Health is not commensurate with the level of psychosocial morbidity, mortality, and health expenditures associated with the disorder.9 Thus, the stigma toward BPD exists in both clinical and research communities.
Mark Zimmerman, MD
Professor of Psychiatry and Human Behavior
Warren Alpert Medical School of Brown University
Rhode Island Hospital
Providence, Rhode Island
References
1. Cleary M, Siegfried N, Walter G. Experience, knowledge and attitudes of mental health staff regarding clients with a borderline personality disorder. Int J Ment Health Nurs. 2002;11(3):186-191.
2. Gallop R, Lancee WJ, Garfinkel P. How nursing staff respond to the label “borderline personality disorder.” Hosp Community Psychiatry. 1989;40(8):815-819.
3. Lewis G, Appleby L. Personality disorder: the patients psychiatrists dislike. Br J Psychiatry. 1988;153:44-49.
4. Black DW, Pfohl B, Blum N, et al. Attitudes toward borderline personality disorder: a survey of 706 mental health clinicians. CNS Spectr. 2011;16(3):67-74.
5. Markham D, Trower P. The effects of the psychiatric label ‘borderline personality disorder’ on nursing staff’s perceptions and causal attributions for challenging behaviours. Br J Clin Psychol. 2003;42(pt 3):243-256.
6. Fraser K, Gallop R. Nurses’ confirming/disconfirming responses to patients diagnosed with borderline personality disorder. Arch Psychiatr Nurs. 1993;7(6):336-341.
7. Zimmerman M, Mattia JI. Differences between clinical and research practices in diagnosing borderline personality disorder. Am J Psychiatry. 1999;156(10):1570-1574.
8. Zimmerman M, Ruggero CJ, Chelminski I, et al. Is bipolar disorder overdiagnosed? J Clin Psychiatry. 2008;69(6):935-940.
9. Zimmerman M, Gazarian D. Is research on borderline personality disorder underfunded by the National Institute of Health? Psychiatry Res. 2014;220(3):941-944.
In response to Dr. Mark Zimmerman’s article, “Improving the recognition of borderline personality disorder” (
Why all the stigma? Because mental health professionals don’t have complete information. The assumption used to be that BPD was “intractable” with no treatment. Even if this were true, it still would not be a reason to fail to disclose a diagnosis, because in other fields of medicine, the concept of “therapeutic privilege” fell by the wayside long ago. However, we now know that in many individuals with BPD, symptoms improve over time, and there are several effective treatments.
In DSM-II, published in 1968, obsessive-compulsive disorder (OCD) was characterized as an “obsessive compulsive neurosis.” It was not reclassified as the current OCD diagnosis until DSM-III-R was published in 1987, after the FDA approved clomipramine. Why is this important? Because once people realized that there was a treatment, they started acknowledging OCD more often.
The first step in addressing the stigma toward BPD is that mental health professionals must recognize their own bias toward this diagnosis. We must be re-educated that this diagnosis carries hope, symptoms improve, and that there are effective treatments. This is how professionals will increase the recognition of BPD.
Assistant Professor and Compliance Officer
Department of Psychiatry
University of Florida
Clinic Director
UF Child and Adolescent Psychiatry Clinic at Springhill Health Center
Gainesville, Florida
References
1. Unruh BT, Gunderson JG. “Good enough” psychiatric residency training in borderline personality disorder: challenges, choice points, and a model generalist curriculum. Harv Rev Psychiatry. 2016;24(5):367-377.
2. Sheehan L, Nieweglowski K, Corrigan P. The stigma of personality disorders. Curr Psychiatry Rep. 2016;18(1):11.
Continue to: The author responds
The author responds
I agree with Dr. Shapiro that stigma by mental health clinicians contributes to the underdiagnosis of BPD. Mental health professions often hold a negative view of patients with personality disorders, particularly those with BPD, and see these patients as being more difficult to treat.1-3 They are the patients that some clinicians are reluctant to treat.3,4 Clinicians perceive patients with personality disorders as less mentally ill, more manipulative, and more able to control their behavior than patients with other psychiatric disorders.3,5 Consistent with this, clinicians have less sympathetic attitudes and behave less empathically toward patients with BPD.5,6 The term “borderline” also is sometimes used pejoratively to describe patients.1
As I described in my article, there are several possible reasons BPD is underdiagnosed. Foremost is that mood disorders, anxiety disorders, and substance use disorders are common in patients with BPD, and the symptoms of these other disorders are typically patients’ chief concerns when they present for treatment. Patients with BPD do not usually report the features of BPD—such as abandonment fears, chronic feelings of emptiness, or an identity disturbance—as their chief concerns. If they did, BPD would likely be easier to recognize. On a related note, clinicians do not have the time, or do not take the time, to conduct a thorough enough evaluation to diagnose BPD when it occurs in a patient who presents for treatment of a mood disorder, anxiety disorder, or substance use disorder. Our clinical research group found that when psychiatrists are presented with the results of a semi-structured interview, BPD is much more frequently diagnosed.7 Such a finding would not be expected if stigma was the primary or sole reason for underdiagnosis.
Dr. Shapiro highlights the clinical consequence of underrecognition and underdiagnosis: the underutilization of empirically supported psychotherapies for BPD. A corollary of underdiagnosing BPD is overdiagnosis of bipolar disorder and overprescription of medication.8
There are other consequences of bias and stigma toward BPD. Despite the high levels of psychosocial morbidity, reduced health-related quality of life, high utilization of services, and excess mortality associated with BPD, this disorder is not included in the Global Burden of Disease Study. Thus, the public health significance of BPD is less fully appreciated. Finally, there is evidence that the level of funding for research from the National Institutes of Health is not commensurate with the level of psychosocial morbidity, mortality, and health expenditures associated with the disorder.9 Thus, the stigma toward BPD exists in both clinical and research communities.
Mark Zimmerman, MD
Professor of Psychiatry and Human Behavior
Warren Alpert Medical School of Brown University
Rhode Island Hospital
Providence, Rhode Island
References
1. Cleary M, Siegfried N, Walter G. Experience, knowledge and attitudes of mental health staff regarding clients with a borderline personality disorder. Int J Ment Health Nurs. 2002;11(3):186-191.
2. Gallop R, Lancee WJ, Garfinkel P. How nursing staff respond to the label “borderline personality disorder.” Hosp Community Psychiatry. 1989;40(8):815-819.
3. Lewis G, Appleby L. Personality disorder: the patients psychiatrists dislike. Br J Psychiatry. 1988;153:44-49.
4. Black DW, Pfohl B, Blum N, et al. Attitudes toward borderline personality disorder: a survey of 706 mental health clinicians. CNS Spectr. 2011;16(3):67-74.
5. Markham D, Trower P. The effects of the psychiatric label ‘borderline personality disorder’ on nursing staff’s perceptions and causal attributions for challenging behaviours. Br J Clin Psychol. 2003;42(pt 3):243-256.
6. Fraser K, Gallop R. Nurses’ confirming/disconfirming responses to patients diagnosed with borderline personality disorder. Arch Psychiatr Nurs. 1993;7(6):336-341.
7. Zimmerman M, Mattia JI. Differences between clinical and research practices in diagnosing borderline personality disorder. Am J Psychiatry. 1999;156(10):1570-1574.
8. Zimmerman M, Ruggero CJ, Chelminski I, et al. Is bipolar disorder overdiagnosed? J Clin Psychiatry. 2008;69(6):935-940.
9. Zimmerman M, Gazarian D. Is research on borderline personality disorder underfunded by the National Institute of Health? Psychiatry Res. 2014;220(3):941-944.
In response to Dr. Mark Zimmerman’s article, “Improving the recognition of borderline personality disorder” (
Why all the stigma? Because mental health professionals don’t have complete information. The assumption used to be that BPD was “intractable” with no treatment. Even if this were true, it still would not be a reason to fail to disclose a diagnosis, because in other fields of medicine, the concept of “therapeutic privilege” fell by the wayside long ago. However, we now know that in many individuals with BPD, symptoms improve over time, and there are several effective treatments.
In DSM-II, published in 1968, obsessive-compulsive disorder (OCD) was characterized as an “obsessive compulsive neurosis.” It was not reclassified as the current OCD diagnosis until DSM-III-R was published in 1987, after the FDA approved clomipramine. Why is this important? Because once people realized that there was a treatment, they started acknowledging OCD more often.
The first step in addressing the stigma toward BPD is that mental health professionals must recognize their own bias toward this diagnosis. We must be re-educated that this diagnosis carries hope, symptoms improve, and that there are effective treatments. This is how professionals will increase the recognition of BPD.
Assistant Professor and Compliance Officer
Department of Psychiatry
University of Florida
Clinic Director
UF Child and Adolescent Psychiatry Clinic at Springhill Health Center
Gainesville, Florida
References
1. Unruh BT, Gunderson JG. “Good enough” psychiatric residency training in borderline personality disorder: challenges, choice points, and a model generalist curriculum. Harv Rev Psychiatry. 2016;24(5):367-377.
2. Sheehan L, Nieweglowski K, Corrigan P. The stigma of personality disorders. Curr Psychiatry Rep. 2016;18(1):11.
Continue to: The author responds
The author responds
I agree with Dr. Shapiro that stigma by mental health clinicians contributes to the underdiagnosis of BPD. Mental health professions often hold a negative view of patients with personality disorders, particularly those with BPD, and see these patients as being more difficult to treat.1-3 They are the patients that some clinicians are reluctant to treat.3,4 Clinicians perceive patients with personality disorders as less mentally ill, more manipulative, and more able to control their behavior than patients with other psychiatric disorders.3,5 Consistent with this, clinicians have less sympathetic attitudes and behave less empathically toward patients with BPD.5,6 The term “borderline” also is sometimes used pejoratively to describe patients.1
As I described in my article, there are several possible reasons BPD is underdiagnosed. Foremost is that mood disorders, anxiety disorders, and substance use disorders are common in patients with BPD, and the symptoms of these other disorders are typically patients’ chief concerns when they present for treatment. Patients with BPD do not usually report the features of BPD—such as abandonment fears, chronic feelings of emptiness, or an identity disturbance—as their chief concerns. If they did, BPD would likely be easier to recognize. On a related note, clinicians do not have the time, or do not take the time, to conduct a thorough enough evaluation to diagnose BPD when it occurs in a patient who presents for treatment of a mood disorder, anxiety disorder, or substance use disorder. Our clinical research group found that when psychiatrists are presented with the results of a semi-structured interview, BPD is much more frequently diagnosed.7 Such a finding would not be expected if stigma was the primary or sole reason for underdiagnosis.
Dr. Shapiro highlights the clinical consequence of underrecognition and underdiagnosis: the underutilization of empirically supported psychotherapies for BPD. A corollary of underdiagnosing BPD is overdiagnosis of bipolar disorder and overprescription of medication.8
There are other consequences of bias and stigma toward BPD. Despite the high levels of psychosocial morbidity, reduced health-related quality of life, high utilization of services, and excess mortality associated with BPD, this disorder is not included in the Global Burden of Disease Study. Thus, the public health significance of BPD is less fully appreciated. Finally, there is evidence that the level of funding for research from the National Institutes of Health is not commensurate with the level of psychosocial morbidity, mortality, and health expenditures associated with the disorder.9 Thus, the stigma toward BPD exists in both clinical and research communities.
Mark Zimmerman, MD
Professor of Psychiatry and Human Behavior
Warren Alpert Medical School of Brown University
Rhode Island Hospital
Providence, Rhode Island
References
1. Cleary M, Siegfried N, Walter G. Experience, knowledge and attitudes of mental health staff regarding clients with a borderline personality disorder. Int J Ment Health Nurs. 2002;11(3):186-191.
2. Gallop R, Lancee WJ, Garfinkel P. How nursing staff respond to the label “borderline personality disorder.” Hosp Community Psychiatry. 1989;40(8):815-819.
3. Lewis G, Appleby L. Personality disorder: the patients psychiatrists dislike. Br J Psychiatry. 1988;153:44-49.
4. Black DW, Pfohl B, Blum N, et al. Attitudes toward borderline personality disorder: a survey of 706 mental health clinicians. CNS Spectr. 2011;16(3):67-74.
5. Markham D, Trower P. The effects of the psychiatric label ‘borderline personality disorder’ on nursing staff’s perceptions and causal attributions for challenging behaviours. Br J Clin Psychol. 2003;42(pt 3):243-256.
6. Fraser K, Gallop R. Nurses’ confirming/disconfirming responses to patients diagnosed with borderline personality disorder. Arch Psychiatr Nurs. 1993;7(6):336-341.
7. Zimmerman M, Mattia JI. Differences between clinical and research practices in diagnosing borderline personality disorder. Am J Psychiatry. 1999;156(10):1570-1574.
8. Zimmerman M, Ruggero CJ, Chelminski I, et al. Is bipolar disorder overdiagnosed? J Clin Psychiatry. 2008;69(6):935-940.
9. Zimmerman M, Gazarian D. Is research on borderline personality disorder underfunded by the National Institute of Health? Psychiatry Res. 2014;220(3):941-944.
Antipsychotics for obsessive-compulsive disorder: Weighing risks vs benefits
Mr. E, age 37, has a 20-year history of obsessive-compulsive disorder (OCD), with comorbid generalized anxiety disorder and hypertension. His medication regimen consists of
Box
Antipsychotics for OCD: What the guidelines recommend
The 2013 American Psychiatric Association (APA) obsessive-compulsive disorder (OCD) treatment guidelines include recommendations regarding the use of antipsychotics in patients who do not respond to first-line treatment with selective serotonin reuptake inhibitors (SSRIs) and/or cognitive-behavioral therapy (CBT). The APA recommends evaluating contributing factors, including comorbidities, family support, and ability to tolerate psychotherapy or maximum recommended drug doses, before augmenting or switching therapies.1
In patients with a partial response to SSRIs and/or CBT, the APA suggests that augmentation may be preferable to switching treatments. Augmentation strategies for SSRIs include antipsychotics or CBT with Exposure Response Prevention (ERP); augmentation strategies for CBT include SSRIs. Combining SSRIs and CBT may decrease the chance of relapse when medication is discontinued. If the patient has a partial response to ERP, intensification of therapy also can be considered based on patient-specific factors. In non-responders, switching therapies may be necessary. Alternative treatments including a different SSRI; an antidepressant from a difference class, such as clomipramine or mirtazapine; an antipsychotic; or CBT.
The 2006 National Institute for Health and Clinical Excellence guidelines for OCD recommend additional high-intensity CBT, adding an antipsychotic to an SSRI or clomipramine, or combining clomipramine with citalopram in non-responders. There is no guidance regarding the order in which these treatments should be trialed. Antipsychotics are recommended as an entire class, and there are no recommendations regarding dosing or long-term risks. These guidelines are based on limited evidence, including only 1 trial of quetiapine and 1 trial of olanzapine.2,3
Efficacy
The 2013 National Institute for Health Care and Excellence Evidence Update included a 2010 Cochrane Review of 11 randomized controlled trials (RCTs) of antipsychotics as adjunctive treatment to SSRIs.5 All trials were <6 months, and most were limited regarding quality aspects. Two trials found no statistically significant difference with olanzapine in efficacy measures (Y-BOCS mean difference [MD] −2.96; 95% confidence interval [CI] −7.41 to 1.22; effect size d = −2.96 [−7.14, 1.22]). Among patients with no clinically significant change (defined as ≤35% reduction in Y-BOCS), there was no significant difference between groups (n = 44, 1 RCT, odds ratio [OR] 0.76; 95% CI 0.17 to 3.29; effect size d = 0.76 [0.17, 3.29]). Studies found increased weight gain with olanzapine compared with antidepressant monotherapy.
Statistically significant differences were demonstrated with the addition of quetiapine to antidepressant monotherapy as shown in Y-BOCS score at endpoint (Y-BOCS MD −2.28; 95% CI −4.05 to −0.52; effect size d −2.28 [−4.05, −0.52]). Quetiapine also demonstrated benefit for depressive and anxiety symptoms. Among patients with no clinically significant change (defined as ≤35% reduction in Y-BOCS), there was a significant difference between groups (n = 80, 2 RCTs, OR 0.27; 95% CI 0.09 to 0.87; effect size d = 0.27 [0.09, 0.87]).
Adjunctive treatment with risperidone was superior to antidepressant monotherapy for participants without a significant response in OCD symptom severity of at least 25% with validated measures (OR 0.17; 95% CI 0.04 to 0.66; effect size d = 0.17 [0.04, 0.66]), and in depressive and anxiety symptoms. Mean reduction in Y-BOCS scores was not statistically significant with risperidone (MD −3.35; 95% CI −8.25 to 1.55; effect size d = −3.35 [−8.25, 1.55]).5
A 2014 meta-analysis by Veale et al3 included double-blind, randomized trials that examined atypical antipsychotics compared with placebo for adults with OCD that used an intention-to-treat analysis. Unlike the Cochrane Review, these studies used the Y-BOCS as a primary outcome measure. Participants had a Y-BOCS score of ≥16; had at least 1 appropriate trial of an SSRI or clomipramine (defined as the maximum dose tolerated for at least 8 weeks); and had to continue taking the SSRI or
Fourteen articles were included in the meta-analysis, but all had small sample sizes and no long-term follow-up data.3 Antipsychotics in the meta-analysis included risperidone (4 studies), quetiapine (5 studies), olanzapine (2 studies),
The overall difference in Y-BOCS score change between drug and placebo groups was 2.34 points, which had an overall effect size of d = 0.40. Those taking antipsychotics had approximately a 10% reduction in Y-BOCS score over time. The overall difference was statistically significant with risperidone (overall mean reduction of 3.89 points on the Y-BOCS; 95% CI 1.43 to 5.48; effect size of d = 0.53) and aripiprazole (difference in Y-BOCS outcome 0.1 scores of 6.29 points; effect size of d = 1.11). One trial of risperidone used a low dose (0.5 mg) and had a larger effect size than the studies that used moderate doses. The overall difference was not statistically significant for quetiapine (difference of Y-BOCS outcome scores of 0.81 points) or olanzapine (difference in Y-BOCS outcome scores of −0.19; indicating <1 point difference on the Y-BOCS).3
Studies included in the meta-analysis ranged in durations from 6 to 16 weeks; duration of ≥4 weeks did not make a difference in response. One study demonstrated a worsening of symptoms in the quetiapine group between weeks 4 and 12. Only 4 studies included most patients that had a previous trial of CBT. One study with an additional treatment arm evaluating CBT found that adding CBT was superior to adjunctive risperidone or placebo. Another study found that adding clomipramine or placebo to
Two studies included in the meta-analysis classified OCD symptoms by subtype, such as by dimensions of checking; symmetry, ordering, counting, and repeating; contamination and cleaning; and hoarding. Currently, no clinically significant predictor of outcome of antipsychotic therapy has been identified. Two studies included in the meta-analysis assessed patients with comorbid tic disorders and found no difference by treatment. One study demonstrated benefit of haloperidol in patients with comorbid tic disorders compared with those without comorbid tic disorders. Of note, none of the studies included in the meta-analysis excluded patients with hoarding characteristics, which generally indicate a worse prognosis with treatment.3
In 2015, Dold et al6 provided an update to a 2013 meta-analysis7 assessing antipsychotic augmentation of SSRIs in treatment-resistant OCD. This update included 2 new RCTs. The 2013 analysis7 concluded that risperidone should be considered first-line and is preferred over olanzapine and quetiapine. However, the update found the highest effect size for aripiprazole (d = −1.35), followed by haloperidol (d = −0.82), risperidone (d = −0.59), quetiapine (d = −0.50), olanzapine (d = −0.49), and paliperidone (d = −0.21).6,7
The 2015 update6 concluded that the antipsychotic doses used in trials were moderate and that there was no association between dose and treatment response, indicating that high doses of antipsychotics may not be more effective. Dold et al6 postulated that the antipsychotic doses required for treating OCD are similar to those used in treating major depressive disorder and lower than doses used in treating schizophrenia. The 2013 meta-analysis demonstrated that moderate doses of antipsychotics resulted in statistically significant efficacy (relative risk [RR] = 3.99, 95% CI 1.92 to 8.27), while low doses did not demonstrate statistical significance (RR = 1.06, 95% CI 0.45 to 2.53).6,7
The 2015 subgroup analysis update evaluated the duration of SSRI treatment prior to the antipsychotic augmentation phase, but did not demonstrate statistically significant efficacy for studies with <8 weeks’ duration of SSRI treatment, further highlighting the need for extended duration of treatment with an SSRI prior to augmentation.6
The 2013 meta-analysis discussed populations with comorbid tic disorders, including a study that found that patients with OCD and comorbid tic disorders benefit more from adjunctive antipsychotic therapy than those without the comorbidity. The 2015 update excluded trials that included patients with comorbid tic disorders to reduce bias, which did not affect the overall effect sizes of the data.6,7
In summary, efficacy has been demonstrated for risperidone and aripiprazole. There has been no benefit demonstrated with olanzapine and limited benefit with quetiapine. One study suggested worsening of symptoms with quetiapine the longer that treatment persisted.3,5-7
Safety
Assessing potential harms related to the use of antipsychotics in treating OCD is complicated, because this information is not always assessed in trials. Instead, researchers often focus on exploring potential benefits because long-term effects of antipsychotics, including sedation, weight gain, metabolic syndrome, and extrapyramidal side effects, are well documented.3
Trials included in the meta-analysis by Veale et al3 had a maximum duration of 16 weeks, so it is likely that many of the potential harms of antipsychotic use would not yet have been measurable. The authors cautioned that, although aripiprazole and risperidone demonstrated benefit, their benefit must be weighed against the potential physical risks of long-term antipsychotic use.3One study that was not included in the meta-analysis by Veale et al3 evaluated individuals who did not respond to a SSRI, and randomly assigned them to quetiapine, olanzapine, or risperidone plus CBT. At 1-year follow-up, 50% of participants receiving an antipsychotic had an increase of >10% in body mass index (BMI) and had higher fasting blood sugars compared with only 15.2% of participants with increased BMI in the comparison group (SSRI responders).3
Foa et al8 investigated long-term outcomes (ie, 6 months) of SSRI augmentation with ERP or risperidone in patients with OCD. Forty patients were randomized to receive risperidone, and 9 were considered responders. Only 8 chose to enter the maintenance phase, and of those participants, 5 did not complete the study. Two withdrew due to worsening depression, 2 withdrew due to intolerable adverse effects, and 1 was lost to follow-up. Unfortunately, there was no further discussion of what the intolerable adverse effects were.8
Patients with comorbid schizophrenia and OCD face additional risks. Lifetime prevalence rates of OCD are greater in persons with schizophrenia compared with the general population (26% vs 8%, respectively). Most studies have demonstrated poor prognosis and medication adherence among patients with comorbid schizophrenia and OCD. Fonseka et al9 assessed the risk of antipsychotic induction and exacerbation of OCD symptoms in patients with schizophrenia. Induction and exacerbation of OCD symptoms
Evidence of olanzapine induction and exacerbation of OCD symptoms is also limited to case reports and retrospective studies. However, some studies have estimated induction of OCD symptoms with olanzapine in 11% to 20% of patients.9 There is insufficient evidence to form conclusions regarding other antipsychotics. Fonseka et al9 recommends switching to an antipsychotic with lower 5HT-2 binding affinity or adding an SSRI, such as fluvoxamine, if induction or exacerbation of OCD symptoms occurs.
Consider long-term risks
The evidence for benefits with antipsychotics in treatment-resistant OCD is limited by different populations recruited, small sample sizes, and lack of long-term follow-up. Most evidence supports using ERP over antipsychotics for treating OCD symptoms that have not responded to SSRIs. However, ERP poses its own challenges that may limit clinical utility, such as economic and time restraints. Therefore, benefits with antipsychotics, such as risperidone and aripiprazole, must be weighed against potential long-term risks of treatment, including sedation, weight gain, metabolic syndrome, and extrapyramidal side effects.
Regarding Mr. E’s case, because he had been maximized on SSRI therapy for an adequate duration (escitalopram, 40 mg/d, for 12 weeks) and completed CBT with ERP with a partial response, adding risperidone, 0.5 mg at bedtime, was an appropriate treatment option that is supported by the available guidelines and evidence. The risperidone dose is reflective of the initial dosing strategies used in clinical trials. It is recommended to assess efficacy of treatment at 8 weeks with a validated measure, such as the Y-BOCS. A dose increase may be needed to achieve clinically significant symptom improvement, because moderate doses of risperidone have demonstrated efficacy in trials; however, high doses of risperidone are unlikely to provide additional benefit and increase the risk of adverse effects. If risperidone does not provide a clinically favorable risk–benefit ratio for Mr. E, aripiprazole is a potential alternative.
1. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed December 11, 2017.
2. National Institute for Health and Care Excellence (NICE). Obsessive compulsive disorder. http://arms.evidence.nhs.uk/resources/hub/1028833/attachment. Updated September 18, 2013. Accessed December 11, 2017.
3. Veale D, Miles S, Smallcombe N, et al. Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
5. Komossa K, Depping AM, Meyer M, et al. Second-generation antipsychotics for obsessive compulsive disorder. Cochrane Database Syst Rev. 2010;12:1-44.
6. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: an update meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2015;18(9). doi: 10.1093/ijnp/pyv047.
7. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2013;16(3):557-574.
8. Foa EB, Simpson HB, Rosenfield D, et al. Six-month outcomes from a randomized trial augmenting serotonin reuptake inhibitors with exposure and response prevention or risperidone in adults with obsessive-compulsive disorder. J Clin Psychiatry. 2015;76(4):440-446.
9. Fonseka TM, Richter MA, Muller DJ. Second generation antipsychotic-induced obsessive-compulsive symptoms in schizophrenia: a review of the experimental literature. Curr Psychiatry Rep. 2014;16(11):510.
Mr. E, age 37, has a 20-year history of obsessive-compulsive disorder (OCD), with comorbid generalized anxiety disorder and hypertension. His medication regimen consists of

Box
Antipsychotics for OCD: What the guidelines recommend
The 2013 American Psychiatric Association (APA) obsessive-compulsive disorder (OCD) treatment guidelines include recommendations regarding the use of antipsychotics in patients who do not respond to first-line treatment with selective serotonin reuptake inhibitors (SSRIs) and/or cognitive-behavioral therapy (CBT). The APA recommends evaluating contributing factors, including comorbidities, family support, and ability to tolerate psychotherapy or maximum recommended drug doses, before augmenting or switching therapies.1
In patients with a partial response to SSRIs and/or CBT, the APA suggests that augmentation may be preferable to switching treatments. Augmentation strategies for SSRIs include antipsychotics or CBT with Exposure Response Prevention (ERP); augmentation strategies for CBT include SSRIs. Combining SSRIs and CBT may decrease the chance of relapse when medication is discontinued. If the patient has a partial response to ERP, intensification of therapy also can be considered based on patient-specific factors. In non-responders, switching therapies may be necessary. Alternative treatments including a different SSRI; an antidepressant from a difference class, such as clomipramine or mirtazapine; an antipsychotic; or CBT.
The 2006 National Institute for Health and Clinical Excellence guidelines for OCD recommend additional high-intensity CBT, adding an antipsychotic to an SSRI or clomipramine, or combining clomipramine with citalopram in non-responders. There is no guidance regarding the order in which these treatments should be trialed. Antipsychotics are recommended as an entire class, and there are no recommendations regarding dosing or long-term risks. These guidelines are based on limited evidence, including only 1 trial of quetiapine and 1 trial of olanzapine.2,3
Efficacy
The 2013 National Institute for Health Care and Excellence Evidence Update included a 2010 Cochrane Review of 11 randomized controlled trials (RCTs) of antipsychotics as adjunctive treatment to SSRIs.5 All trials were <6 months, and most were limited regarding quality aspects. Two trials found no statistically significant difference with olanzapine in efficacy measures (Y-BOCS mean difference [MD] −2.96; 95% confidence interval [CI] −7.41 to 1.22; effect size d = −2.96 [−7.14, 1.22]). Among patients with no clinically significant change (defined as ≤35% reduction in Y-BOCS), there was no significant difference between groups (n = 44, 1 RCT, odds ratio [OR] 0.76; 95% CI 0.17 to 3.29; effect size d = 0.76 [0.17, 3.29]). Studies found increased weight gain with olanzapine compared with antidepressant monotherapy.
Statistically significant differences were demonstrated with the addition of quetiapine to antidepressant monotherapy as shown in Y-BOCS score at endpoint (Y-BOCS MD −2.28; 95% CI −4.05 to −0.52; effect size d −2.28 [−4.05, −0.52]). Quetiapine also demonstrated benefit for depressive and anxiety symptoms. Among patients with no clinically significant change (defined as ≤35% reduction in Y-BOCS), there was a significant difference between groups (n = 80, 2 RCTs, OR 0.27; 95% CI 0.09 to 0.87; effect size d = 0.27 [0.09, 0.87]).
Adjunctive treatment with risperidone was superior to antidepressant monotherapy for participants without a significant response in OCD symptom severity of at least 25% with validated measures (OR 0.17; 95% CI 0.04 to 0.66; effect size d = 0.17 [0.04, 0.66]), and in depressive and anxiety symptoms. Mean reduction in Y-BOCS scores was not statistically significant with risperidone (MD −3.35; 95% CI −8.25 to 1.55; effect size d = −3.35 [−8.25, 1.55]).5
A 2014 meta-analysis by Veale et al3 included double-blind, randomized trials that examined atypical antipsychotics compared with placebo for adults with OCD that used an intention-to-treat analysis. Unlike the Cochrane Review, these studies used the Y-BOCS as a primary outcome measure. Participants had a Y-BOCS score of ≥16; had at least 1 appropriate trial of an SSRI or clomipramine (defined as the maximum dose tolerated for at least 8 weeks); and had to continue taking the SSRI or
Fourteen articles were included in the meta-analysis, but all had small sample sizes and no long-term follow-up data.3 Antipsychotics in the meta-analysis included risperidone (4 studies), quetiapine (5 studies), olanzapine (2 studies),
The overall difference in Y-BOCS score change between drug and placebo groups was 2.34 points, which had an overall effect size of d = 0.40. Those taking antipsychotics had approximately a 10% reduction in Y-BOCS score over time. The overall difference was statistically significant with risperidone (overall mean reduction of 3.89 points on the Y-BOCS; 95% CI 1.43 to 5.48; effect size of d = 0.53) and aripiprazole (difference in Y-BOCS outcome 0.1 scores of 6.29 points; effect size of d = 1.11). One trial of risperidone used a low dose (0.5 mg) and had a larger effect size than the studies that used moderate doses. The overall difference was not statistically significant for quetiapine (difference of Y-BOCS outcome scores of 0.81 points) or olanzapine (difference in Y-BOCS outcome scores of −0.19; indicating <1 point difference on the Y-BOCS).3
Studies included in the meta-analysis ranged in durations from 6 to 16 weeks; duration of ≥4 weeks did not make a difference in response. One study demonstrated a worsening of symptoms in the quetiapine group between weeks 4 and 12. Only 4 studies included most patients that had a previous trial of CBT. One study with an additional treatment arm evaluating CBT found that adding CBT was superior to adjunctive risperidone or placebo. Another study found that adding clomipramine or placebo to
Two studies included in the meta-analysis classified OCD symptoms by subtype, such as by dimensions of checking; symmetry, ordering, counting, and repeating; contamination and cleaning; and hoarding. Currently, no clinically significant predictor of outcome of antipsychotic therapy has been identified. Two studies included in the meta-analysis assessed patients with comorbid tic disorders and found no difference by treatment. One study demonstrated benefit of haloperidol in patients with comorbid tic disorders compared with those without comorbid tic disorders. Of note, none of the studies included in the meta-analysis excluded patients with hoarding characteristics, which generally indicate a worse prognosis with treatment.3
In 2015, Dold et al6 provided an update to a 2013 meta-analysis7 assessing antipsychotic augmentation of SSRIs in treatment-resistant OCD. This update included 2 new RCTs. The 2013 analysis7 concluded that risperidone should be considered first-line and is preferred over olanzapine and quetiapine. However, the update found the highest effect size for aripiprazole (d = −1.35), followed by haloperidol (d = −0.82), risperidone (d = −0.59), quetiapine (d = −0.50), olanzapine (d = −0.49), and paliperidone (d = −0.21).6,7
The 2015 update6 concluded that the antipsychotic doses used in trials were moderate and that there was no association between dose and treatment response, indicating that high doses of antipsychotics may not be more effective. Dold et al6 postulated that the antipsychotic doses required for treating OCD are similar to those used in treating major depressive disorder and lower than doses used in treating schizophrenia. The 2013 meta-analysis demonstrated that moderate doses of antipsychotics resulted in statistically significant efficacy (relative risk [RR] = 3.99, 95% CI 1.92 to 8.27), while low doses did not demonstrate statistical significance (RR = 1.06, 95% CI 0.45 to 2.53).6,7
The 2015 subgroup analysis update evaluated the duration of SSRI treatment prior to the antipsychotic augmentation phase, but did not demonstrate statistically significant efficacy for studies with <8 weeks’ duration of SSRI treatment, further highlighting the need for extended duration of treatment with an SSRI prior to augmentation.6
The 2013 meta-analysis discussed populations with comorbid tic disorders, including a study that found that patients with OCD and comorbid tic disorders benefit more from adjunctive antipsychotic therapy than those without the comorbidity. The 2015 update excluded trials that included patients with comorbid tic disorders to reduce bias, which did not affect the overall effect sizes of the data.6,7
In summary, efficacy has been demonstrated for risperidone and aripiprazole. There has been no benefit demonstrated with olanzapine and limited benefit with quetiapine. One study suggested worsening of symptoms with quetiapine the longer that treatment persisted.3,5-7
Safety
Assessing potential harms related to the use of antipsychotics in treating OCD is complicated, because this information is not always assessed in trials. Instead, researchers often focus on exploring potential benefits because long-term effects of antipsychotics, including sedation, weight gain, metabolic syndrome, and extrapyramidal side effects, are well documented.3
Trials included in the meta-analysis by Veale et al3 had a maximum duration of 16 weeks, so it is likely that many of the potential harms of antipsychotic use would not yet have been measurable. The authors cautioned that, although aripiprazole and risperidone demonstrated benefit, their benefit must be weighed against the potential physical risks of long-term antipsychotic use.3One study that was not included in the meta-analysis by Veale et al3 evaluated individuals who did not respond to a SSRI, and randomly assigned them to quetiapine, olanzapine, or risperidone plus CBT. At 1-year follow-up, 50% of participants receiving an antipsychotic had an increase of >10% in body mass index (BMI) and had higher fasting blood sugars compared with only 15.2% of participants with increased BMI in the comparison group (SSRI responders).3
Foa et al8 investigated long-term outcomes (ie, 6 months) of SSRI augmentation with ERP or risperidone in patients with OCD. Forty patients were randomized to receive risperidone, and 9 were considered responders. Only 8 chose to enter the maintenance phase, and of those participants, 5 did not complete the study. Two withdrew due to worsening depression, 2 withdrew due to intolerable adverse effects, and 1 was lost to follow-up. Unfortunately, there was no further discussion of what the intolerable adverse effects were.8
Patients with comorbid schizophrenia and OCD face additional risks. Lifetime prevalence rates of OCD are greater in persons with schizophrenia compared with the general population (26% vs 8%, respectively). Most studies have demonstrated poor prognosis and medication adherence among patients with comorbid schizophrenia and OCD. Fonseka et al9 assessed the risk of antipsychotic induction and exacerbation of OCD symptoms in patients with schizophrenia. Induction and exacerbation of OCD symptoms
Evidence of olanzapine induction and exacerbation of OCD symptoms is also limited to case reports and retrospective studies. However, some studies have estimated induction of OCD symptoms with olanzapine in 11% to 20% of patients.9 There is insufficient evidence to form conclusions regarding other antipsychotics. Fonseka et al9 recommends switching to an antipsychotic with lower 5HT-2 binding affinity or adding an SSRI, such as fluvoxamine, if induction or exacerbation of OCD symptoms occurs.
Consider long-term risks
The evidence for benefits with antipsychotics in treatment-resistant OCD is limited by different populations recruited, small sample sizes, and lack of long-term follow-up. Most evidence supports using ERP over antipsychotics for treating OCD symptoms that have not responded to SSRIs. However, ERP poses its own challenges that may limit clinical utility, such as economic and time restraints. Therefore, benefits with antipsychotics, such as risperidone and aripiprazole, must be weighed against potential long-term risks of treatment, including sedation, weight gain, metabolic syndrome, and extrapyramidal side effects.
Regarding Mr. E’s case, because he had been maximized on SSRI therapy for an adequate duration (escitalopram, 40 mg/d, for 12 weeks) and completed CBT with ERP with a partial response, adding risperidone, 0.5 mg at bedtime, was an appropriate treatment option that is supported by the available guidelines and evidence. The risperidone dose is reflective of the initial dosing strategies used in clinical trials. It is recommended to assess efficacy of treatment at 8 weeks with a validated measure, such as the Y-BOCS. A dose increase may be needed to achieve clinically significant symptom improvement, because moderate doses of risperidone have demonstrated efficacy in trials; however, high doses of risperidone are unlikely to provide additional benefit and increase the risk of adverse effects. If risperidone does not provide a clinically favorable risk–benefit ratio for Mr. E, aripiprazole is a potential alternative.
Mr. E, age 37, has a 20-year history of obsessive-compulsive disorder (OCD), with comorbid generalized anxiety disorder and hypertension. His medication regimen consists of

Box
Antipsychotics for OCD: What the guidelines recommend
The 2013 American Psychiatric Association (APA) obsessive-compulsive disorder (OCD) treatment guidelines include recommendations regarding the use of antipsychotics in patients who do not respond to first-line treatment with selective serotonin reuptake inhibitors (SSRIs) and/or cognitive-behavioral therapy (CBT). The APA recommends evaluating contributing factors, including comorbidities, family support, and ability to tolerate psychotherapy or maximum recommended drug doses, before augmenting or switching therapies.1
In patients with a partial response to SSRIs and/or CBT, the APA suggests that augmentation may be preferable to switching treatments. Augmentation strategies for SSRIs include antipsychotics or CBT with Exposure Response Prevention (ERP); augmentation strategies for CBT include SSRIs. Combining SSRIs and CBT may decrease the chance of relapse when medication is discontinued. If the patient has a partial response to ERP, intensification of therapy also can be considered based on patient-specific factors. In non-responders, switching therapies may be necessary. Alternative treatments including a different SSRI; an antidepressant from a difference class, such as clomipramine or mirtazapine; an antipsychotic; or CBT.
The 2006 National Institute for Health and Clinical Excellence guidelines for OCD recommend additional high-intensity CBT, adding an antipsychotic to an SSRI or clomipramine, or combining clomipramine with citalopram in non-responders. There is no guidance regarding the order in which these treatments should be trialed. Antipsychotics are recommended as an entire class, and there are no recommendations regarding dosing or long-term risks. These guidelines are based on limited evidence, including only 1 trial of quetiapine and 1 trial of olanzapine.2,3
Efficacy
The 2013 National Institute for Health Care and Excellence Evidence Update included a 2010 Cochrane Review of 11 randomized controlled trials (RCTs) of antipsychotics as adjunctive treatment to SSRIs.5 All trials were <6 months, and most were limited regarding quality aspects. Two trials found no statistically significant difference with olanzapine in efficacy measures (Y-BOCS mean difference [MD] −2.96; 95% confidence interval [CI] −7.41 to 1.22; effect size d = −2.96 [−7.14, 1.22]). Among patients with no clinically significant change (defined as ≤35% reduction in Y-BOCS), there was no significant difference between groups (n = 44, 1 RCT, odds ratio [OR] 0.76; 95% CI 0.17 to 3.29; effect size d = 0.76 [0.17, 3.29]). Studies found increased weight gain with olanzapine compared with antidepressant monotherapy.
Statistically significant differences were demonstrated with the addition of quetiapine to antidepressant monotherapy as shown in Y-BOCS score at endpoint (Y-BOCS MD −2.28; 95% CI −4.05 to −0.52; effect size d −2.28 [−4.05, −0.52]). Quetiapine also demonstrated benefit for depressive and anxiety symptoms. Among patients with no clinically significant change (defined as ≤35% reduction in Y-BOCS), there was a significant difference between groups (n = 80, 2 RCTs, OR 0.27; 95% CI 0.09 to 0.87; effect size d = 0.27 [0.09, 0.87]).
Adjunctive treatment with risperidone was superior to antidepressant monotherapy for participants without a significant response in OCD symptom severity of at least 25% with validated measures (OR 0.17; 95% CI 0.04 to 0.66; effect size d = 0.17 [0.04, 0.66]), and in depressive and anxiety symptoms. Mean reduction in Y-BOCS scores was not statistically significant with risperidone (MD −3.35; 95% CI −8.25 to 1.55; effect size d = −3.35 [−8.25, 1.55]).5
A 2014 meta-analysis by Veale et al3 included double-blind, randomized trials that examined atypical antipsychotics compared with placebo for adults with OCD that used an intention-to-treat analysis. Unlike the Cochrane Review, these studies used the Y-BOCS as a primary outcome measure. Participants had a Y-BOCS score of ≥16; had at least 1 appropriate trial of an SSRI or clomipramine (defined as the maximum dose tolerated for at least 8 weeks); and had to continue taking the SSRI or
Fourteen articles were included in the meta-analysis, but all had small sample sizes and no long-term follow-up data.3 Antipsychotics in the meta-analysis included risperidone (4 studies), quetiapine (5 studies), olanzapine (2 studies),
The overall difference in Y-BOCS score change between drug and placebo groups was 2.34 points, which had an overall effect size of d = 0.40. Those taking antipsychotics had approximately a 10% reduction in Y-BOCS score over time. The overall difference was statistically significant with risperidone (overall mean reduction of 3.89 points on the Y-BOCS; 95% CI 1.43 to 5.48; effect size of d = 0.53) and aripiprazole (difference in Y-BOCS outcome 0.1 scores of 6.29 points; effect size of d = 1.11). One trial of risperidone used a low dose (0.5 mg) and had a larger effect size than the studies that used moderate doses. The overall difference was not statistically significant for quetiapine (difference of Y-BOCS outcome scores of 0.81 points) or olanzapine (difference in Y-BOCS outcome scores of −0.19; indicating <1 point difference on the Y-BOCS).3
Studies included in the meta-analysis ranged in durations from 6 to 16 weeks; duration of ≥4 weeks did not make a difference in response. One study demonstrated a worsening of symptoms in the quetiapine group between weeks 4 and 12. Only 4 studies included most patients that had a previous trial of CBT. One study with an additional treatment arm evaluating CBT found that adding CBT was superior to adjunctive risperidone or placebo. Another study found that adding clomipramine or placebo to
Two studies included in the meta-analysis classified OCD symptoms by subtype, such as by dimensions of checking; symmetry, ordering, counting, and repeating; contamination and cleaning; and hoarding. Currently, no clinically significant predictor of outcome of antipsychotic therapy has been identified. Two studies included in the meta-analysis assessed patients with comorbid tic disorders and found no difference by treatment. One study demonstrated benefit of haloperidol in patients with comorbid tic disorders compared with those without comorbid tic disorders. Of note, none of the studies included in the meta-analysis excluded patients with hoarding characteristics, which generally indicate a worse prognosis with treatment.3
In 2015, Dold et al6 provided an update to a 2013 meta-analysis7 assessing antipsychotic augmentation of SSRIs in treatment-resistant OCD. This update included 2 new RCTs. The 2013 analysis7 concluded that risperidone should be considered first-line and is preferred over olanzapine and quetiapine. However, the update found the highest effect size for aripiprazole (d = −1.35), followed by haloperidol (d = −0.82), risperidone (d = −0.59), quetiapine (d = −0.50), olanzapine (d = −0.49), and paliperidone (d = −0.21).6,7
The 2015 update6 concluded that the antipsychotic doses used in trials were moderate and that there was no association between dose and treatment response, indicating that high doses of antipsychotics may not be more effective. Dold et al6 postulated that the antipsychotic doses required for treating OCD are similar to those used in treating major depressive disorder and lower than doses used in treating schizophrenia. The 2013 meta-analysis demonstrated that moderate doses of antipsychotics resulted in statistically significant efficacy (relative risk [RR] = 3.99, 95% CI 1.92 to 8.27), while low doses did not demonstrate statistical significance (RR = 1.06, 95% CI 0.45 to 2.53).6,7
The 2015 subgroup analysis update evaluated the duration of SSRI treatment prior to the antipsychotic augmentation phase, but did not demonstrate statistically significant efficacy for studies with <8 weeks’ duration of SSRI treatment, further highlighting the need for extended duration of treatment with an SSRI prior to augmentation.6
The 2013 meta-analysis discussed populations with comorbid tic disorders, including a study that found that patients with OCD and comorbid tic disorders benefit more from adjunctive antipsychotic therapy than those without the comorbidity. The 2015 update excluded trials that included patients with comorbid tic disorders to reduce bias, which did not affect the overall effect sizes of the data.6,7
In summary, efficacy has been demonstrated for risperidone and aripiprazole. There has been no benefit demonstrated with olanzapine and limited benefit with quetiapine. One study suggested worsening of symptoms with quetiapine the longer that treatment persisted.3,5-7
Safety
Assessing potential harms related to the use of antipsychotics in treating OCD is complicated, because this information is not always assessed in trials. Instead, researchers often focus on exploring potential benefits because long-term effects of antipsychotics, including sedation, weight gain, metabolic syndrome, and extrapyramidal side effects, are well documented.3
Trials included in the meta-analysis by Veale et al3 had a maximum duration of 16 weeks, so it is likely that many of the potential harms of antipsychotic use would not yet have been measurable. The authors cautioned that, although aripiprazole and risperidone demonstrated benefit, their benefit must be weighed against the potential physical risks of long-term antipsychotic use.3One study that was not included in the meta-analysis by Veale et al3 evaluated individuals who did not respond to a SSRI, and randomly assigned them to quetiapine, olanzapine, or risperidone plus CBT. At 1-year follow-up, 50% of participants receiving an antipsychotic had an increase of >10% in body mass index (BMI) and had higher fasting blood sugars compared with only 15.2% of participants with increased BMI in the comparison group (SSRI responders).3
Foa et al8 investigated long-term outcomes (ie, 6 months) of SSRI augmentation with ERP or risperidone in patients with OCD. Forty patients were randomized to receive risperidone, and 9 were considered responders. Only 8 chose to enter the maintenance phase, and of those participants, 5 did not complete the study. Two withdrew due to worsening depression, 2 withdrew due to intolerable adverse effects, and 1 was lost to follow-up. Unfortunately, there was no further discussion of what the intolerable adverse effects were.8
Patients with comorbid schizophrenia and OCD face additional risks. Lifetime prevalence rates of OCD are greater in persons with schizophrenia compared with the general population (26% vs 8%, respectively). Most studies have demonstrated poor prognosis and medication adherence among patients with comorbid schizophrenia and OCD. Fonseka et al9 assessed the risk of antipsychotic induction and exacerbation of OCD symptoms in patients with schizophrenia. Induction and exacerbation of OCD symptoms
Evidence of olanzapine induction and exacerbation of OCD symptoms is also limited to case reports and retrospective studies. However, some studies have estimated induction of OCD symptoms with olanzapine in 11% to 20% of patients.9 There is insufficient evidence to form conclusions regarding other antipsychotics. Fonseka et al9 recommends switching to an antipsychotic with lower 5HT-2 binding affinity or adding an SSRI, such as fluvoxamine, if induction or exacerbation of OCD symptoms occurs.
Consider long-term risks
The evidence for benefits with antipsychotics in treatment-resistant OCD is limited by different populations recruited, small sample sizes, and lack of long-term follow-up. Most evidence supports using ERP over antipsychotics for treating OCD symptoms that have not responded to SSRIs. However, ERP poses its own challenges that may limit clinical utility, such as economic and time restraints. Therefore, benefits with antipsychotics, such as risperidone and aripiprazole, must be weighed against potential long-term risks of treatment, including sedation, weight gain, metabolic syndrome, and extrapyramidal side effects.
Regarding Mr. E’s case, because he had been maximized on SSRI therapy for an adequate duration (escitalopram, 40 mg/d, for 12 weeks) and completed CBT with ERP with a partial response, adding risperidone, 0.5 mg at bedtime, was an appropriate treatment option that is supported by the available guidelines and evidence. The risperidone dose is reflective of the initial dosing strategies used in clinical trials. It is recommended to assess efficacy of treatment at 8 weeks with a validated measure, such as the Y-BOCS. A dose increase may be needed to achieve clinically significant symptom improvement, because moderate doses of risperidone have demonstrated efficacy in trials; however, high doses of risperidone are unlikely to provide additional benefit and increase the risk of adverse effects. If risperidone does not provide a clinically favorable risk–benefit ratio for Mr. E, aripiprazole is a potential alternative.
1. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed December 11, 2017.
2. National Institute for Health and Care Excellence (NICE). Obsessive compulsive disorder. http://arms.evidence.nhs.uk/resources/hub/1028833/attachment. Updated September 18, 2013. Accessed December 11, 2017.
3. Veale D, Miles S, Smallcombe N, et al. Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
5. Komossa K, Depping AM, Meyer M, et al. Second-generation antipsychotics for obsessive compulsive disorder. Cochrane Database Syst Rev. 2010;12:1-44.
6. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: an update meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2015;18(9). doi: 10.1093/ijnp/pyv047.
7. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2013;16(3):557-574.
8. Foa EB, Simpson HB, Rosenfield D, et al. Six-month outcomes from a randomized trial augmenting serotonin reuptake inhibitors with exposure and response prevention or risperidone in adults with obsessive-compulsive disorder. J Clin Psychiatry. 2015;76(4):440-446.
9. Fonseka TM, Richter MA, Muller DJ. Second generation antipsychotic-induced obsessive-compulsive symptoms in schizophrenia: a review of the experimental literature. Curr Psychiatry Rep. 2014;16(11):510.
1. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed December 11, 2017.
2. National Institute for Health and Care Excellence (NICE). Obsessive compulsive disorder. http://arms.evidence.nhs.uk/resources/hub/1028833/attachment. Updated September 18, 2013. Accessed December 11, 2017.
3. Veale D, Miles S, Smallcombe N, et al. Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
5. Komossa K, Depping AM, Meyer M, et al. Second-generation antipsychotics for obsessive compulsive disorder. Cochrane Database Syst Rev. 2010;12:1-44.
6. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: an update meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2015;18(9). doi: 10.1093/ijnp/pyv047.
7. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2013;16(3):557-574.
8. Foa EB, Simpson HB, Rosenfield D, et al. Six-month outcomes from a randomized trial augmenting serotonin reuptake inhibitors with exposure and response prevention or risperidone in adults with obsessive-compulsive disorder. J Clin Psychiatry. 2015;76(4):440-446.
9. Fonseka TM, Richter MA, Muller DJ. Second generation antipsychotic-induced obsessive-compulsive symptoms in schizophrenia: a review of the experimental literature. Curr Psychiatry Rep. 2014;16(11):510.
Integrate brief CBT interventions into medication management visits
Patients who are treated with psychotropics may experience better recovery from their symptoms and improved quality of life when they receive targeted treatment with cognitive-behavioral therapy (CBT). Clinicians can use certain CBT techniques to “jump-start” recovery in patients before prescribed medications produce their intended therapeutic effects. When practitioners are familiar with their use, techniques such as behavioral activation and tools that enhance adherence can be employed during a brief medication management (“med check”) visit.
Take these steps to implement brief CBT interventions into your patient’s routine visits:
- develop a clear, formulation-driven treatment target
- design an intervention that can be explained during a brief visit
- have handouts and worksheets available for patients to use
- provide written explanations and reminders for patients to use in out-of-session practice.
We present a case report that illustrates incorporating brief CBT interventions in a patient with major depressive disorder (MDD).
CASE REPORT
Using CBT to help a patient with MDD
Mr. L, age 52, presents with moderate MDD, and is started on fluoxetine, 20 mg/d. Mr. L has significant anhedonia and poor energy, and has been avoiding going to work and seeing friends. The psychiatrist explains to him how individuals with depression often want to refrain from activity and “shut down,” but that doing so will not improve his quality of life, and his mood will worsen.
The psychiatrist asks Mr. L to identify a pleasurable or important activity to complete before his next appointment. Mr. L decides that he would like to call a friend, because he has been isolated and his friends have been calling him. The psychiatrist encourages him to call one of his golf buddies. She instructs Mr. L to set reminders, such as cell phone alarms and notes on the refrigerator, to prompt him to “Call Phil Saturday at 10
To increase the likelihood that Mr. L will make this call, he and his psychiatrist discuss anticipated obstacles and potential facilitators of this behavior.
The psychiatrist also encourages Mr. L to complete a Behavioral Activation Worksheet (for examples, see http://www.cci.health.wa.gov.au/docs/ACF3B92.pdf or https://www.therapistaid.com/worksheets/behavioral-activation.pdf) to track his depression, pleasure, and sense of achievement before and after completing this activity.
As illustrated by this case, collaborating with the patient is critical to developing a realistic treatment plan that incorporates CBT techniques. With your help and encouragement, patients can use these tools to reach their goals and target the symptoms of their illnesses.
1. Wright JH, McCray LW. Restoring energy and enjoying life. In: Wright JH, McCray LW. Breaking free from depression: pathways to wellness. New York, NY: The Guilford Press; 2012:97-129.
2. Wright JH, Basco MR, Thase ME. Working with automatic thoughts. In: Wright JH, Basco MR, Thase ME. Learning cognitive-behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing, Inc.; 2005:118-121.
Patients who are treated with psychotropics may experience better recovery from their symptoms and improved quality of life when they receive targeted treatment with cognitive-behavioral therapy (CBT). Clinicians can use certain CBT techniques to “jump-start” recovery in patients before prescribed medications produce their intended therapeutic effects. When practitioners are familiar with their use, techniques such as behavioral activation and tools that enhance adherence can be employed during a brief medication management (“med check”) visit.
Take these steps to implement brief CBT interventions into your patient’s routine visits:
- develop a clear, formulation-driven treatment target
- design an intervention that can be explained during a brief visit
- have handouts and worksheets available for patients to use
- provide written explanations and reminders for patients to use in out-of-session practice.
We present a case report that illustrates incorporating brief CBT interventions in a patient with major depressive disorder (MDD).
CASE REPORT
Using CBT to help a patient with MDD
Mr. L, age 52, presents with moderate MDD, and is started on fluoxetine, 20 mg/d. Mr. L has significant anhedonia and poor energy, and has been avoiding going to work and seeing friends. The psychiatrist explains to him how individuals with depression often want to refrain from activity and “shut down,” but that doing so will not improve his quality of life, and his mood will worsen.
The psychiatrist asks Mr. L to identify a pleasurable or important activity to complete before his next appointment. Mr. L decides that he would like to call a friend, because he has been isolated and his friends have been calling him. The psychiatrist encourages him to call one of his golf buddies. She instructs Mr. L to set reminders, such as cell phone alarms and notes on the refrigerator, to prompt him to “Call Phil Saturday at 10
To increase the likelihood that Mr. L will make this call, he and his psychiatrist discuss anticipated obstacles and potential facilitators of this behavior.
The psychiatrist also encourages Mr. L to complete a Behavioral Activation Worksheet (for examples, see http://www.cci.health.wa.gov.au/docs/ACF3B92.pdf or https://www.therapistaid.com/worksheets/behavioral-activation.pdf) to track his depression, pleasure, and sense of achievement before and after completing this activity.
As illustrated by this case, collaborating with the patient is critical to developing a realistic treatment plan that incorporates CBT techniques. With your help and encouragement, patients can use these tools to reach their goals and target the symptoms of their illnesses.
Patients who are treated with psychotropics may experience better recovery from their symptoms and improved quality of life when they receive targeted treatment with cognitive-behavioral therapy (CBT). Clinicians can use certain CBT techniques to “jump-start” recovery in patients before prescribed medications produce their intended therapeutic effects. When practitioners are familiar with their use, techniques such as behavioral activation and tools that enhance adherence can be employed during a brief medication management (“med check”) visit.
Take these steps to implement brief CBT interventions into your patient’s routine visits:
- develop a clear, formulation-driven treatment target
- design an intervention that can be explained during a brief visit
- have handouts and worksheets available for patients to use
- provide written explanations and reminders for patients to use in out-of-session practice.
We present a case report that illustrates incorporating brief CBT interventions in a patient with major depressive disorder (MDD).
CASE REPORT
Using CBT to help a patient with MDD
Mr. L, age 52, presents with moderate MDD, and is started on fluoxetine, 20 mg/d. Mr. L has significant anhedonia and poor energy, and has been avoiding going to work and seeing friends. The psychiatrist explains to him how individuals with depression often want to refrain from activity and “shut down,” but that doing so will not improve his quality of life, and his mood will worsen.
The psychiatrist asks Mr. L to identify a pleasurable or important activity to complete before his next appointment. Mr. L decides that he would like to call a friend, because he has been isolated and his friends have been calling him. The psychiatrist encourages him to call one of his golf buddies. She instructs Mr. L to set reminders, such as cell phone alarms and notes on the refrigerator, to prompt him to “Call Phil Saturday at 10
To increase the likelihood that Mr. L will make this call, he and his psychiatrist discuss anticipated obstacles and potential facilitators of this behavior.
The psychiatrist also encourages Mr. L to complete a Behavioral Activation Worksheet (for examples, see http://www.cci.health.wa.gov.au/docs/ACF3B92.pdf or https://www.therapistaid.com/worksheets/behavioral-activation.pdf) to track his depression, pleasure, and sense of achievement before and after completing this activity.
As illustrated by this case, collaborating with the patient is critical to developing a realistic treatment plan that incorporates CBT techniques. With your help and encouragement, patients can use these tools to reach their goals and target the symptoms of their illnesses.
1. Wright JH, McCray LW. Restoring energy and enjoying life. In: Wright JH, McCray LW. Breaking free from depression: pathways to wellness. New York, NY: The Guilford Press; 2012:97-129.
2. Wright JH, Basco MR, Thase ME. Working with automatic thoughts. In: Wright JH, Basco MR, Thase ME. Learning cognitive-behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing, Inc.; 2005:118-121.
1. Wright JH, McCray LW. Restoring energy and enjoying life. In: Wright JH, McCray LW. Breaking free from depression: pathways to wellness. New York, NY: The Guilford Press; 2012:97-129.
2. Wright JH, Basco MR, Thase ME. Working with automatic thoughts. In: Wright JH, Basco MR, Thase ME. Learning cognitive-behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing, Inc.; 2005:118-121.
Career Choices: Consultation-liaison psychiatry
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he (she) has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Cornel Stanciu, MD, talked with Peter Ganpat, MD, a consultation-liaison (C-L) psychiatrist at Florida Hospital, where he provides guidance to various medical specialties on managing acute and chronic mental illness and substance use disorders. In addition, he also is the medical director for the repetitive transcranial magnetic stimulation service and staffs the inpatient unit.
Dr. Stanciu: What made you choose to become a C-L psychiatrist?
Dr. Ganpat: In my opinion, C-L is the most challenging area of psychiatry because not only are you thinking along the realms of a psychiatrist, but you’re also considering the viewpoint of the other subspecialties at the same time. For me, it brings together my medical background with my passion for psychiatry, and the patients I see daily allow for this incorporation.
Dr. Stanciu: How did your career path prepare you to become a C-L psychiatrist?
Dr. Ganpat: My career path was unique in that I completed a family medicine residency, and then immediately pursued training in psychiatry. Some may consider this as “overkill” for C-L, but as I’ve come to learn, this background grants me a level of understanding and confidence to step in when dealing with a complex case and lend a hand to the consulting physician beyond psychiatry. I do not feel a fellowship is required to practice C-L psychiatry. However, a psychosomatic fellowship will definitely provide the experience needed for this career path, and also will enable one to get a second American Board of Psychiatry and Neurology board certification.
Dr. Stanciu: What types of clinical conditions are you asked to provide input on managing, and how do you find working alongside other specialties?
Dr. Ganpat: I have been managing the full breadth of psychiatry, and in some cases I also provide medical management. Practicing in a metropolitan area with a high influx of tourists also brings in unique cultural cases. The level of respect that the other specialties give is impressive, because they have now seen what a C-L psychiatrist can do. Their performance scores also have improved as a result of my involvement. They greatly appreciate my efforts to shed light on cases or assist with the ever-challenging patient whose psychiatric complexity impedes care.
Dr. Stanciu: How would you describe a physician who is well-suited for such a setting?
Dr. Ganpat: The perfect candidate for this role should be capable of abstract as well as objective thinking. Having a good understanding of the other medical specialties and being able to solve problems is essential, because often it isn’t a clear-cut picture. It is imperative for the C-L psychiatrist to have sound teaching abilities and to be able to educate and communicate his (her) reasoning to the consulting team. It also is important to be well-versed in the psychiatric manifestations of various medical disorders and the psychiatric iatrogenesis of widely used prescription medications.
Dr. Stanciu: What challenges and surprises did you encounter when you first began to practice in this setting?
Dr. Ganpat: I think the largest challenge that I have encountered is the lack of resources. Substance abuse is a major problem here, especially opioids, and there are limited community resources for these patients, so they wind up in the hospital.
Dr. Stanciu: What are the disadvantages of C-L compared with other branches of psychiatry?
Dr. Ganpat: There isn’t much continuity of care with C-L psychiatry over the long run, but you do get to see patients improve during the duration of their hospitalization, which is very rewarding.
Dr. Stanciu: What is the typical reimbursement model for a C-L psychiatrist, and have you run into difficulties with insurance providers in this setting?
Dr. Ganpat: The reimbursement model varies from one system to the next. The common model is to bill just as any other hospital service would, based on the time or level of complexity. Obviously, the more consults you have, the more billing is generated. Most insurance carriers recognize this and so I haven’t had much of an issue with reimbursement, although some unexpected problems may arise.
Dr. Stanciu: What advice do you have for early career psychiatrists and trainees who are contemplating a C-L career?
Dr. Ganpat: If you enjoy working in the hospital and interfacing with other specialties, then consider C-L psychiatry. It is challenging but intellectually stimulating. Make sure you request a C-L rotation during your training, because the Accreditation Council for Graduate Medical Education requires it during a psychiatric residency.
Dr. Stanciu: What is the future outlook of C-L?
Dr. Ganpat: There is a shortage of C-L psychiatrists because >50% of practicing psychiatrists are in private practice in an outpatient setting. Because access to psychiatric care outside of a hospital setting is an issue, and much care is being driven to hospitals, there will be an increasing need for C-L psychiatrists.
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he (she) has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Cornel Stanciu, MD, talked with Peter Ganpat, MD, a consultation-liaison (C-L) psychiatrist at Florida Hospital, where he provides guidance to various medical specialties on managing acute and chronic mental illness and substance use disorders. In addition, he also is the medical director for the repetitive transcranial magnetic stimulation service and staffs the inpatient unit.
Dr. Stanciu: What made you choose to become a C-L psychiatrist?
Dr. Ganpat: In my opinion, C-L is the most challenging area of psychiatry because not only are you thinking along the realms of a psychiatrist, but you’re also considering the viewpoint of the other subspecialties at the same time. For me, it brings together my medical background with my passion for psychiatry, and the patients I see daily allow for this incorporation.
Dr. Stanciu: How did your career path prepare you to become a C-L psychiatrist?
Dr. Ganpat: My career path was unique in that I completed a family medicine residency, and then immediately pursued training in psychiatry. Some may consider this as “overkill” for C-L, but as I’ve come to learn, this background grants me a level of understanding and confidence to step in when dealing with a complex case and lend a hand to the consulting physician beyond psychiatry. I do not feel a fellowship is required to practice C-L psychiatry. However, a psychosomatic fellowship will definitely provide the experience needed for this career path, and also will enable one to get a second American Board of Psychiatry and Neurology board certification.
Dr. Stanciu: What types of clinical conditions are you asked to provide input on managing, and how do you find working alongside other specialties?
Dr. Ganpat: I have been managing the full breadth of psychiatry, and in some cases I also provide medical management. Practicing in a metropolitan area with a high influx of tourists also brings in unique cultural cases. The level of respect that the other specialties give is impressive, because they have now seen what a C-L psychiatrist can do. Their performance scores also have improved as a result of my involvement. They greatly appreciate my efforts to shed light on cases or assist with the ever-challenging patient whose psychiatric complexity impedes care.
Dr. Stanciu: How would you describe a physician who is well-suited for such a setting?
Dr. Ganpat: The perfect candidate for this role should be capable of abstract as well as objective thinking. Having a good understanding of the other medical specialties and being able to solve problems is essential, because often it isn’t a clear-cut picture. It is imperative for the C-L psychiatrist to have sound teaching abilities and to be able to educate and communicate his (her) reasoning to the consulting team. It also is important to be well-versed in the psychiatric manifestations of various medical disorders and the psychiatric iatrogenesis of widely used prescription medications.
Dr. Stanciu: What challenges and surprises did you encounter when you first began to practice in this setting?
Dr. Ganpat: I think the largest challenge that I have encountered is the lack of resources. Substance abuse is a major problem here, especially opioids, and there are limited community resources for these patients, so they wind up in the hospital.
Dr. Stanciu: What are the disadvantages of C-L compared with other branches of psychiatry?
Dr. Ganpat: There isn’t much continuity of care with C-L psychiatry over the long run, but you do get to see patients improve during the duration of their hospitalization, which is very rewarding.
Dr. Stanciu: What is the typical reimbursement model for a C-L psychiatrist, and have you run into difficulties with insurance providers in this setting?
Dr. Ganpat: The reimbursement model varies from one system to the next. The common model is to bill just as any other hospital service would, based on the time or level of complexity. Obviously, the more consults you have, the more billing is generated. Most insurance carriers recognize this and so I haven’t had much of an issue with reimbursement, although some unexpected problems may arise.
Dr. Stanciu: What advice do you have for early career psychiatrists and trainees who are contemplating a C-L career?
Dr. Ganpat: If you enjoy working in the hospital and interfacing with other specialties, then consider C-L psychiatry. It is challenging but intellectually stimulating. Make sure you request a C-L rotation during your training, because the Accreditation Council for Graduate Medical Education requires it during a psychiatric residency.
Dr. Stanciu: What is the future outlook of C-L?
Dr. Ganpat: There is a shortage of C-L psychiatrists because >50% of practicing psychiatrists are in private practice in an outpatient setting. Because access to psychiatric care outside of a hospital setting is an issue, and much care is being driven to hospitals, there will be an increasing need for C-L psychiatrists.
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he (she) has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Cornel Stanciu, MD, talked with Peter Ganpat, MD, a consultation-liaison (C-L) psychiatrist at Florida Hospital, where he provides guidance to various medical specialties on managing acute and chronic mental illness and substance use disorders. In addition, he also is the medical director for the repetitive transcranial magnetic stimulation service and staffs the inpatient unit.
Dr. Stanciu: What made you choose to become a C-L psychiatrist?
Dr. Ganpat: In my opinion, C-L is the most challenging area of psychiatry because not only are you thinking along the realms of a psychiatrist, but you’re also considering the viewpoint of the other subspecialties at the same time. For me, it brings together my medical background with my passion for psychiatry, and the patients I see daily allow for this incorporation.
Dr. Stanciu: How did your career path prepare you to become a C-L psychiatrist?
Dr. Ganpat: My career path was unique in that I completed a family medicine residency, and then immediately pursued training in psychiatry. Some may consider this as “overkill” for C-L, but as I’ve come to learn, this background grants me a level of understanding and confidence to step in when dealing with a complex case and lend a hand to the consulting physician beyond psychiatry. I do not feel a fellowship is required to practice C-L psychiatry. However, a psychosomatic fellowship will definitely provide the experience needed for this career path, and also will enable one to get a second American Board of Psychiatry and Neurology board certification.
Dr. Stanciu: What types of clinical conditions are you asked to provide input on managing, and how do you find working alongside other specialties?
Dr. Ganpat: I have been managing the full breadth of psychiatry, and in some cases I also provide medical management. Practicing in a metropolitan area with a high influx of tourists also brings in unique cultural cases. The level of respect that the other specialties give is impressive, because they have now seen what a C-L psychiatrist can do. Their performance scores also have improved as a result of my involvement. They greatly appreciate my efforts to shed light on cases or assist with the ever-challenging patient whose psychiatric complexity impedes care.
Dr. Stanciu: How would you describe a physician who is well-suited for such a setting?
Dr. Ganpat: The perfect candidate for this role should be capable of abstract as well as objective thinking. Having a good understanding of the other medical specialties and being able to solve problems is essential, because often it isn’t a clear-cut picture. It is imperative for the C-L psychiatrist to have sound teaching abilities and to be able to educate and communicate his (her) reasoning to the consulting team. It also is important to be well-versed in the psychiatric manifestations of various medical disorders and the psychiatric iatrogenesis of widely used prescription medications.
Dr. Stanciu: What challenges and surprises did you encounter when you first began to practice in this setting?
Dr. Ganpat: I think the largest challenge that I have encountered is the lack of resources. Substance abuse is a major problem here, especially opioids, and there are limited community resources for these patients, so they wind up in the hospital.
Dr. Stanciu: What are the disadvantages of C-L compared with other branches of psychiatry?
Dr. Ganpat: There isn’t much continuity of care with C-L psychiatry over the long run, but you do get to see patients improve during the duration of their hospitalization, which is very rewarding.
Dr. Stanciu: What is the typical reimbursement model for a C-L psychiatrist, and have you run into difficulties with insurance providers in this setting?
Dr. Ganpat: The reimbursement model varies from one system to the next. The common model is to bill just as any other hospital service would, based on the time or level of complexity. Obviously, the more consults you have, the more billing is generated. Most insurance carriers recognize this and so I haven’t had much of an issue with reimbursement, although some unexpected problems may arise.
Dr. Stanciu: What advice do you have for early career psychiatrists and trainees who are contemplating a C-L career?
Dr. Ganpat: If you enjoy working in the hospital and interfacing with other specialties, then consider C-L psychiatry. It is challenging but intellectually stimulating. Make sure you request a C-L rotation during your training, because the Accreditation Council for Graduate Medical Education requires it during a psychiatric residency.
Dr. Stanciu: What is the future outlook of C-L?
Dr. Ganpat: There is a shortage of C-L psychiatrists because >50% of practicing psychiatrists are in private practice in an outpatient setting. Because access to psychiatric care outside of a hospital setting is an issue, and much care is being driven to hospitals, there will be an increasing need for C-L psychiatrists.



