User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Sleep disturbance not linked to age or IQ in early ASD
Sleep disturbance is not associated with age and IQ in young children with autism spectrum disorder (ASD) and disruptive behaviors, according to Cynthia R. Johnson, PhD, and her associates.
They assessed 177 children aged 3-7 who were participating in the Research Units on Behavioral Intervention study, a 24-week trial. All of the children had a diagnosis of autism spectrum disorder, based on DSM-IV criteria. The diagnoses were corroborated by the Autism Diagnostic Observation Schedule and the Autism Diagnostic Interview–Revised.
The children were randomized into a parent training group or a parent education group. After getting parents to complete several forms, including the Children’s Sleep Habits Questionnaire, Dr. Johnson and her associates found no age differences between children who fell into the category of “good sleepers” (n = 52), and those characterized as “poor sleepers” (n = 46) (P = .57). In both sleep groups, more than 70% of the children had an IQ of 70 or above, and no significant difference was found in good sleepers, compared with poor sleepers, in IQ variable (P = .87) (Sleep Med. 2018. 44:61-6).
In addition, the researchers found that poor sleepers had significantly higher scores on the Aberrant Behavior Checklist subscales of irritability, hyperactivity, stereotypic behavior, and social withdrawal/lethargy, compared with good sleepers. All subscales of the parenting stress index and the PSI total score also were significantly higher in the poor sleepers group, compared with children in the good sleepers group, reported Dr. Johnson of the University of Florida, Gainesville, and her associates.
Additional studies are needed within a “comprehensive biopsychosocial model” to advance the understanding of why some children with autism experience disrupted sleep patterns and others do not. “, regardless of age and cognitive level,” Dr. Johnson and her associates concluded. “With improved sleep, better outcomes for children with ASD could be expected.”
Read the full study in Sleep Medicine
Sleep disturbance is not associated with age and IQ in young children with autism spectrum disorder (ASD) and disruptive behaviors, according to Cynthia R. Johnson, PhD, and her associates.
They assessed 177 children aged 3-7 who were participating in the Research Units on Behavioral Intervention study, a 24-week trial. All of the children had a diagnosis of autism spectrum disorder, based on DSM-IV criteria. The diagnoses were corroborated by the Autism Diagnostic Observation Schedule and the Autism Diagnostic Interview–Revised.
The children were randomized into a parent training group or a parent education group. After getting parents to complete several forms, including the Children’s Sleep Habits Questionnaire, Dr. Johnson and her associates found no age differences between children who fell into the category of “good sleepers” (n = 52), and those characterized as “poor sleepers” (n = 46) (P = .57). In both sleep groups, more than 70% of the children had an IQ of 70 or above, and no significant difference was found in good sleepers, compared with poor sleepers, in IQ variable (P = .87) (Sleep Med. 2018. 44:61-6).
In addition, the researchers found that poor sleepers had significantly higher scores on the Aberrant Behavior Checklist subscales of irritability, hyperactivity, stereotypic behavior, and social withdrawal/lethargy, compared with good sleepers. All subscales of the parenting stress index and the PSI total score also were significantly higher in the poor sleepers group, compared with children in the good sleepers group, reported Dr. Johnson of the University of Florida, Gainesville, and her associates.
Additional studies are needed within a “comprehensive biopsychosocial model” to advance the understanding of why some children with autism experience disrupted sleep patterns and others do not. “, regardless of age and cognitive level,” Dr. Johnson and her associates concluded. “With improved sleep, better outcomes for children with ASD could be expected.”
Read the full study in Sleep Medicine
Sleep disturbance is not associated with age and IQ in young children with autism spectrum disorder (ASD) and disruptive behaviors, according to Cynthia R. Johnson, PhD, and her associates.
They assessed 177 children aged 3-7 who were participating in the Research Units on Behavioral Intervention study, a 24-week trial. All of the children had a diagnosis of autism spectrum disorder, based on DSM-IV criteria. The diagnoses were corroborated by the Autism Diagnostic Observation Schedule and the Autism Diagnostic Interview–Revised.
The children were randomized into a parent training group or a parent education group. After getting parents to complete several forms, including the Children’s Sleep Habits Questionnaire, Dr. Johnson and her associates found no age differences between children who fell into the category of “good sleepers” (n = 52), and those characterized as “poor sleepers” (n = 46) (P = .57). In both sleep groups, more than 70% of the children had an IQ of 70 or above, and no significant difference was found in good sleepers, compared with poor sleepers, in IQ variable (P = .87) (Sleep Med. 2018. 44:61-6).
In addition, the researchers found that poor sleepers had significantly higher scores on the Aberrant Behavior Checklist subscales of irritability, hyperactivity, stereotypic behavior, and social withdrawal/lethargy, compared with good sleepers. All subscales of the parenting stress index and the PSI total score also were significantly higher in the poor sleepers group, compared with children in the good sleepers group, reported Dr. Johnson of the University of Florida, Gainesville, and her associates.
Additional studies are needed within a “comprehensive biopsychosocial model” to advance the understanding of why some children with autism experience disrupted sleep patterns and others do not. “, regardless of age and cognitive level,” Dr. Johnson and her associates concluded. “With improved sleep, better outcomes for children with ASD could be expected.”
Read the full study in Sleep Medicine
FROM SLEEP MEDICINE
Poor sleep, suicide risk linked in college students
Suicidal behaviors are associated with poor sleep in college students, even after controlling for depression, a cross-sectional analysis shows. Specifically, students were 6.54 times as likely to be classified with suicide risk if they were depressed and 2.70 times as likely if they had poor quality of sleep.
“Our findings add to a growing body of literature pointing to sleep as an important component to include in screening and intervention efforts to prevent suicidal ideation and attempts on college campuses,” wrote Stephen P. Becker, PhD, a pediatric psychologist affiliated with Cincinnati Children’s Hospital Medical Center, and his colleagues.
Of the 1,700 college students included in this study (J Psychiatr Res. 2018 Jan 12:99:122-8), 82.7% of those classified with suicide risk had poor sleep quality, but only 31.3% of those with poor sleep quality were classified with suicide risk.
Most previous studies had looked only at insomnia or bad dreams rather than other aspects of poor sleep quality, and because this study used the Pittsburgh Sleep Quality Index, it was able to evaluate additional well-validated components of sleep. so that screening and intervention based on sleep quality can be more effective. This is important, because suicide is one of the leading causes of death among young adults.
The researchers declared no conflicts of interest.
Read more about this study in the Journal of Psychiatric Research.
Suicidal behaviors are associated with poor sleep in college students, even after controlling for depression, a cross-sectional analysis shows. Specifically, students were 6.54 times as likely to be classified with suicide risk if they were depressed and 2.70 times as likely if they had poor quality of sleep.
“Our findings add to a growing body of literature pointing to sleep as an important component to include in screening and intervention efforts to prevent suicidal ideation and attempts on college campuses,” wrote Stephen P. Becker, PhD, a pediatric psychologist affiliated with Cincinnati Children’s Hospital Medical Center, and his colleagues.
Of the 1,700 college students included in this study (J Psychiatr Res. 2018 Jan 12:99:122-8), 82.7% of those classified with suicide risk had poor sleep quality, but only 31.3% of those with poor sleep quality were classified with suicide risk.
Most previous studies had looked only at insomnia or bad dreams rather than other aspects of poor sleep quality, and because this study used the Pittsburgh Sleep Quality Index, it was able to evaluate additional well-validated components of sleep. so that screening and intervention based on sleep quality can be more effective. This is important, because suicide is one of the leading causes of death among young adults.
The researchers declared no conflicts of interest.
Read more about this study in the Journal of Psychiatric Research.
Suicidal behaviors are associated with poor sleep in college students, even after controlling for depression, a cross-sectional analysis shows. Specifically, students were 6.54 times as likely to be classified with suicide risk if they were depressed and 2.70 times as likely if they had poor quality of sleep.
“Our findings add to a growing body of literature pointing to sleep as an important component to include in screening and intervention efforts to prevent suicidal ideation and attempts on college campuses,” wrote Stephen P. Becker, PhD, a pediatric psychologist affiliated with Cincinnati Children’s Hospital Medical Center, and his colleagues.
Of the 1,700 college students included in this study (J Psychiatr Res. 2018 Jan 12:99:122-8), 82.7% of those classified with suicide risk had poor sleep quality, but only 31.3% of those with poor sleep quality were classified with suicide risk.
Most previous studies had looked only at insomnia or bad dreams rather than other aspects of poor sleep quality, and because this study used the Pittsburgh Sleep Quality Index, it was able to evaluate additional well-validated components of sleep. so that screening and intervention based on sleep quality can be more effective. This is important, because suicide is one of the leading causes of death among young adults.
The researchers declared no conflicts of interest.
Read more about this study in the Journal of Psychiatric Research.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Delayed treatment for psychosis can have ‘deleterious’ effects
The longer that patients with schizophrenia go without treatment for a psychotic episode, the more their hippocampus atrophies, suggests a study published Feb. 21 in JAMA Psychiatry.
To conduct the study, researchers studied 71 patients between March 5, 2013, and Oct. 8, 2014, who were experiencing their first psychotic episode and were antipsychotic naive. The research team matched those patients with 73 healthy controls, treated the patients with antipsychotics, and performed MRIs on members of both groups at baseline and 8 weeks later. The primary outcome measure was change in left and right hippocampal volume integrity, which is inversely associated with hippocampal atrophy.
Results showed that many patients with psychosis had lower median hippocampal volume integrity. Furthermore, those . The duration of untreated psychosis was correlated with both decreases, but this association was only significant with left hippocampal volume integrity.
This relationship is “consistent with a persistent, possibly deleterious, effect of untreated psychosis on brain structure,” wrote Donald C. Goff, MD, of the psychiatry department at New York University, and his associates. “Larger longitudinal studies of longer duration are needed to examine the association between [duration of untreated psychosis], hippocampal volume, and clinical outcomes.”
Dr. Goff reported receiving research support from the National Institute of Mental Health, the Stanley Medical Research Institute, and Avanir Pharmaceuticals. Another author reported receiving support from numerous entities and honoraria for serving on an advisory board for Allergan. No other disclosures were reported.
Read more at JAMA Psychiatry.
The longer that patients with schizophrenia go without treatment for a psychotic episode, the more their hippocampus atrophies, suggests a study published Feb. 21 in JAMA Psychiatry.
To conduct the study, researchers studied 71 patients between March 5, 2013, and Oct. 8, 2014, who were experiencing their first psychotic episode and were antipsychotic naive. The research team matched those patients with 73 healthy controls, treated the patients with antipsychotics, and performed MRIs on members of both groups at baseline and 8 weeks later. The primary outcome measure was change in left and right hippocampal volume integrity, which is inversely associated with hippocampal atrophy.
Results showed that many patients with psychosis had lower median hippocampal volume integrity. Furthermore, those . The duration of untreated psychosis was correlated with both decreases, but this association was only significant with left hippocampal volume integrity.
This relationship is “consistent with a persistent, possibly deleterious, effect of untreated psychosis on brain structure,” wrote Donald C. Goff, MD, of the psychiatry department at New York University, and his associates. “Larger longitudinal studies of longer duration are needed to examine the association between [duration of untreated psychosis], hippocampal volume, and clinical outcomes.”
Dr. Goff reported receiving research support from the National Institute of Mental Health, the Stanley Medical Research Institute, and Avanir Pharmaceuticals. Another author reported receiving support from numerous entities and honoraria for serving on an advisory board for Allergan. No other disclosures were reported.
Read more at JAMA Psychiatry.
The longer that patients with schizophrenia go without treatment for a psychotic episode, the more their hippocampus atrophies, suggests a study published Feb. 21 in JAMA Psychiatry.
To conduct the study, researchers studied 71 patients between March 5, 2013, and Oct. 8, 2014, who were experiencing their first psychotic episode and were antipsychotic naive. The research team matched those patients with 73 healthy controls, treated the patients with antipsychotics, and performed MRIs on members of both groups at baseline and 8 weeks later. The primary outcome measure was change in left and right hippocampal volume integrity, which is inversely associated with hippocampal atrophy.
Results showed that many patients with psychosis had lower median hippocampal volume integrity. Furthermore, those . The duration of untreated psychosis was correlated with both decreases, but this association was only significant with left hippocampal volume integrity.
This relationship is “consistent with a persistent, possibly deleterious, effect of untreated psychosis on brain structure,” wrote Donald C. Goff, MD, of the psychiatry department at New York University, and his associates. “Larger longitudinal studies of longer duration are needed to examine the association between [duration of untreated psychosis], hippocampal volume, and clinical outcomes.”
Dr. Goff reported receiving research support from the National Institute of Mental Health, the Stanley Medical Research Institute, and Avanir Pharmaceuticals. Another author reported receiving support from numerous entities and honoraria for serving on an advisory board for Allergan. No other disclosures were reported.
Read more at JAMA Psychiatry.
FROM JAMA PSYCHIATRY
Virtual reality–based CBT may improve social participation in psychosis
Virtual reality–based cognitive-behavioral therapy could help reduce momentary paranoia and anxiety, and improve social cognition in individuals with psychotic disorders.
Researchers reported the results of a randomized controlled trial of personalized virtual reality-based cognitive-behavioral therapy in 116 patients with a DSM IV–diagnosed psychotic disorder and paranoid ideation in an article published online Feb. 8 in Lancet Psychiatry.
Similarly, the group that received virtual reality therapy showed significantly larger decreases in momentary anxiety, compared with those in the control group. Those decreases remained significant at follow-up.
Researchers also observed a significant drop in safety behaviors – such as lack of eye contact – in the group who received the virtual reality therapy. At follow-up, this group showed less paranoid ideation in the form of lower levels of ideas of persecution and social reference.
The treatment also was associated with a small increase in time spent with others at the 6-month follow-up; a decrease was seen in the control group. Patients who underwent virtual reality therapy also showed improvements in self-stigmatization and social functioning.
The authors noted that the benefits for social functioning might take some time to emerge after therapy, as patients in symptomatic remission do not immediately start spending more time with other people.
“When patients increasingly feel more comfortable in social situations and learn that other people are less threatening than anticipated, they might try and succeed to make and maintain social contacts and find hobbies and jobs,” the authors wrote.
However, no significant differences were found between the two groups in terms of depression and anxiety, or in quality of life measurements posttreatment and at follow-up.
Virtual reality–based CBT is intended to get around some of the limitations of exposure-based therapeutic exercises for paranoid ideation. In virtual reality settings, the environment and characters can be completely controlled by the therapist, and the therapy is real time rather than retrospective and therefore not as vulnerable to patient bias.
“Finally, many patients are reluctant or unable to undergo exposure because of strong paranoid fears or negative symptoms,” the authors wrote.
The therapy took place in four virtual social environments – a street, bus, café, and supermarket. The therapist was able to control the characteristics and responses of up to 40 human avatars, enabling personalized treatment exercises for each patient.
“Patients and therapists communicated during virtual reality sessions to explore and challenge suspicious thoughts during social situations, drop safety behaviors during social situations (such as avoiding eye contact with, keeping distance from, and refraining from communication with avatars), and test harm expectancies,” they wrote.
The sessions also were designed to target safety behaviors, such as avoiding eye contact, because such behavior prevents individuals from receiving social information that can improve social cognition and reduce the chance of incorrect paranoid appraisals.
Several limitations were cited. For example, because follow-up was restricted to 6 months, it was not possible to access the long-term effects of virtual reality-based CBT. Also, some of the patients opted not to participate in the study because traveling to the therapy location proved too frightening. “Thus our sample might have been biased, because some of the most paranoid and avoidant patients could not participate,” they wrote.
The study was supported by Fonds NutsOhra and Stichting tot Steun VCVGZ. No conflicts of interest were declared.
SOURCE: Lancet Psychiatry. 2018 Feb 8. doi: 10.1016/S2215-0366(18)30053-1.
Virtual reality–based cognitive-behavioral therapy could help reduce momentary paranoia and anxiety, and improve social cognition in individuals with psychotic disorders.
Researchers reported the results of a randomized controlled trial of personalized virtual reality-based cognitive-behavioral therapy in 116 patients with a DSM IV–diagnosed psychotic disorder and paranoid ideation in an article published online Feb. 8 in Lancet Psychiatry.
Similarly, the group that received virtual reality therapy showed significantly larger decreases in momentary anxiety, compared with those in the control group. Those decreases remained significant at follow-up.
Researchers also observed a significant drop in safety behaviors – such as lack of eye contact – in the group who received the virtual reality therapy. At follow-up, this group showed less paranoid ideation in the form of lower levels of ideas of persecution and social reference.
The treatment also was associated with a small increase in time spent with others at the 6-month follow-up; a decrease was seen in the control group. Patients who underwent virtual reality therapy also showed improvements in self-stigmatization and social functioning.
The authors noted that the benefits for social functioning might take some time to emerge after therapy, as patients in symptomatic remission do not immediately start spending more time with other people.
“When patients increasingly feel more comfortable in social situations and learn that other people are less threatening than anticipated, they might try and succeed to make and maintain social contacts and find hobbies and jobs,” the authors wrote.
However, no significant differences were found between the two groups in terms of depression and anxiety, or in quality of life measurements posttreatment and at follow-up.
Virtual reality–based CBT is intended to get around some of the limitations of exposure-based therapeutic exercises for paranoid ideation. In virtual reality settings, the environment and characters can be completely controlled by the therapist, and the therapy is real time rather than retrospective and therefore not as vulnerable to patient bias.
“Finally, many patients are reluctant or unable to undergo exposure because of strong paranoid fears or negative symptoms,” the authors wrote.
The therapy took place in four virtual social environments – a street, bus, café, and supermarket. The therapist was able to control the characteristics and responses of up to 40 human avatars, enabling personalized treatment exercises for each patient.
“Patients and therapists communicated during virtual reality sessions to explore and challenge suspicious thoughts during social situations, drop safety behaviors during social situations (such as avoiding eye contact with, keeping distance from, and refraining from communication with avatars), and test harm expectancies,” they wrote.
The sessions also were designed to target safety behaviors, such as avoiding eye contact, because such behavior prevents individuals from receiving social information that can improve social cognition and reduce the chance of incorrect paranoid appraisals.
Several limitations were cited. For example, because follow-up was restricted to 6 months, it was not possible to access the long-term effects of virtual reality-based CBT. Also, some of the patients opted not to participate in the study because traveling to the therapy location proved too frightening. “Thus our sample might have been biased, because some of the most paranoid and avoidant patients could not participate,” they wrote.
The study was supported by Fonds NutsOhra and Stichting tot Steun VCVGZ. No conflicts of interest were declared.
SOURCE: Lancet Psychiatry. 2018 Feb 8. doi: 10.1016/S2215-0366(18)30053-1.
Virtual reality–based cognitive-behavioral therapy could help reduce momentary paranoia and anxiety, and improve social cognition in individuals with psychotic disorders.
Researchers reported the results of a randomized controlled trial of personalized virtual reality-based cognitive-behavioral therapy in 116 patients with a DSM IV–diagnosed psychotic disorder and paranoid ideation in an article published online Feb. 8 in Lancet Psychiatry.
Similarly, the group that received virtual reality therapy showed significantly larger decreases in momentary anxiety, compared with those in the control group. Those decreases remained significant at follow-up.
Researchers also observed a significant drop in safety behaviors – such as lack of eye contact – in the group who received the virtual reality therapy. At follow-up, this group showed less paranoid ideation in the form of lower levels of ideas of persecution and social reference.
The treatment also was associated with a small increase in time spent with others at the 6-month follow-up; a decrease was seen in the control group. Patients who underwent virtual reality therapy also showed improvements in self-stigmatization and social functioning.
The authors noted that the benefits for social functioning might take some time to emerge after therapy, as patients in symptomatic remission do not immediately start spending more time with other people.
“When patients increasingly feel more comfortable in social situations and learn that other people are less threatening than anticipated, they might try and succeed to make and maintain social contacts and find hobbies and jobs,” the authors wrote.
However, no significant differences were found between the two groups in terms of depression and anxiety, or in quality of life measurements posttreatment and at follow-up.
Virtual reality–based CBT is intended to get around some of the limitations of exposure-based therapeutic exercises for paranoid ideation. In virtual reality settings, the environment and characters can be completely controlled by the therapist, and the therapy is real time rather than retrospective and therefore not as vulnerable to patient bias.
“Finally, many patients are reluctant or unable to undergo exposure because of strong paranoid fears or negative symptoms,” the authors wrote.
The therapy took place in four virtual social environments – a street, bus, café, and supermarket. The therapist was able to control the characteristics and responses of up to 40 human avatars, enabling personalized treatment exercises for each patient.
“Patients and therapists communicated during virtual reality sessions to explore and challenge suspicious thoughts during social situations, drop safety behaviors during social situations (such as avoiding eye contact with, keeping distance from, and refraining from communication with avatars), and test harm expectancies,” they wrote.
The sessions also were designed to target safety behaviors, such as avoiding eye contact, because such behavior prevents individuals from receiving social information that can improve social cognition and reduce the chance of incorrect paranoid appraisals.
Several limitations were cited. For example, because follow-up was restricted to 6 months, it was not possible to access the long-term effects of virtual reality-based CBT. Also, some of the patients opted not to participate in the study because traveling to the therapy location proved too frightening. “Thus our sample might have been biased, because some of the most paranoid and avoidant patients could not participate,” they wrote.
The study was supported by Fonds NutsOhra and Stichting tot Steun VCVGZ. No conflicts of interest were declared.
SOURCE: Lancet Psychiatry. 2018 Feb 8. doi: 10.1016/S2215-0366(18)30053-1.
FROM LANCET PSYCHIATRY
Key clinical point:
Major finding: Patients who received virtual reality–based CBT showed significantly less momentary paranoia and momentary anxiety, and less paranoid ideation, than controls.
Data source: Randomized controlled trial in 116 patients with psychotic disorders.
Disclosures: The study was supported by Fonds NutsOhra and Stichting tot Steun VCVGZ. No conflicts of interest were declared.
Source: Pot-Kolder RMCA et al. Lancet Psychiatry. 2018 Feb 8. doi: 10.1016/S2215-0366(18)30053-1.
Physicians often bypass cognition, depression screening in MS
SAN DIEGO – A new study finds that physicians at two . Physicians who did perform screening hardly ever used validated tools and often didn’t refer appropriate patients for higher-level care.
In addition, researchers interviewed 13 leading MS specialists from coast to coast and “found that about half reported not using formal screening tools to assess cognitive impairment and depression,” said study coauthor Tamar Sapir, PhD, chief scientific officer with Prime Education, a firm based in Fort Lauderdale, Fla., that provides a variety of health-related services such as training and research.
The study findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Three of the study authors spoke in interviews.
The researchers sought to understand how frequently MS patients are screened for cognitive problems and depression.
“Cognitive impairment is experienced by approximately half of patients with multiple sclerosis, yet many are never screened or treated, which can impact their daily activities, their ability to work, and overall quality of life,” Dr. Sapir said.
Depression, meanwhile, is believed to be much more common in patients with MS than in the general population, with one recent meta-analysis of 58 studies finding that the average prevalence was 31%. Other research suggests depression is underdiagnosed and undertreated in this population (J Neurol Sci. 2017 Jan 15;372:331-41; ISRN Neurology. 2012, Article ID 427102. doi: 10.5402/2012/427102).
For the current study, researchers tracked 300 patients at two unidentified MS clinics via their charts over a 2-year period from 2014 to 2016. Their median age was 52 years, 76% were women, and 15 had experienced at least one relapse within the previous 24 months.
“Screening for cognitive impairment and depression was documented for only 52% and 63% of MS patients, respectively, and only about a quarter of patients diagnosed with these conditions were referred to a higher level of care,” said lead author Guy J. Buckle, MD, MPH, of the Andrew C. Carlos MS Institute at Shepherd Center in Atlanta.
Among all 300 patients, just 2% and 4% were screened using a validated tool for cognitive impairment and depression, respectively.
The screening often turned up evidence of the conditions: Physicians saw signs of cognitive impairment in 69% and 78% of those screened aged under 65 years and aged 65 and older, respectively, and they detected depression in 71% and 54% of those screened in those two age groups, respectively.
Researchers also noted several disparities. “Cognitive screening was conducted more frequently in older, employed, or white patients, while the presence of cognitive impairment was documented more often in black, nonworking, and those on Medicare or Medicaid,” Dr. Buckle said. “Depression screening was performed most frequently in older or white patients, yet depression was more common in younger, nonworking patients and those on Medicare/Medicaid.”
In another part of their study, researchers surveyed 13 unidentified “national leaders” in MS research and treatment. Just seven said they used validated tools to screen for cognitive impairment and six said they used them to screen for depression.
“We hear from MS specialists that they want to be measuring for cognition but don’t know how to efficiently work it into their routine, how to approach the patient, and what tools to use,” said study coauthor Derrick S. Robertson, MD, of the University of South Florida, Tampa. “In addition, there is no one tool that is accepted in the MS treatment community.”
MS specialists who didn’t use the screening tests also pointed to factors like lack of reimbursement and lack of integration into electronic medical records. “Doubt very much that neurologists have time to use any of these tests,” one respondent said, referring to cognitive impairment screening.
What’s next? “There are several new exciting developments in clinical trials demonstrating efficacy of disease-modifying therapies in maintaining or improving cognition in patients with relapsing MS,” Dr. Robertson said. “This highlights the urgent need to overcome barriers to use of formal cognitive screening tools in clinical practice to identify patients who need a higher level of care, and perhaps even a change in treatment with the ultimate goal to improve quality of life and overall outcomes.”
Genentech funded the study through an educational grant. Dr. Sapir and three other study authors reported no relevant disclosures. Dr. Buckle and Dr. Robertson reported multiple disclosures, including principle investigator and advisory board/panel member work.
SOURCE: Buckle GJ et al. ACTRIMS Forum 2018, abstract No. P161.
SAN DIEGO – A new study finds that physicians at two . Physicians who did perform screening hardly ever used validated tools and often didn’t refer appropriate patients for higher-level care.
In addition, researchers interviewed 13 leading MS specialists from coast to coast and “found that about half reported not using formal screening tools to assess cognitive impairment and depression,” said study coauthor Tamar Sapir, PhD, chief scientific officer with Prime Education, a firm based in Fort Lauderdale, Fla., that provides a variety of health-related services such as training and research.
The study findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Three of the study authors spoke in interviews.
The researchers sought to understand how frequently MS patients are screened for cognitive problems and depression.
“Cognitive impairment is experienced by approximately half of patients with multiple sclerosis, yet many are never screened or treated, which can impact their daily activities, their ability to work, and overall quality of life,” Dr. Sapir said.
Depression, meanwhile, is believed to be much more common in patients with MS than in the general population, with one recent meta-analysis of 58 studies finding that the average prevalence was 31%. Other research suggests depression is underdiagnosed and undertreated in this population (J Neurol Sci. 2017 Jan 15;372:331-41; ISRN Neurology. 2012, Article ID 427102. doi: 10.5402/2012/427102).
For the current study, researchers tracked 300 patients at two unidentified MS clinics via their charts over a 2-year period from 2014 to 2016. Their median age was 52 years, 76% were women, and 15 had experienced at least one relapse within the previous 24 months.
“Screening for cognitive impairment and depression was documented for only 52% and 63% of MS patients, respectively, and only about a quarter of patients diagnosed with these conditions were referred to a higher level of care,” said lead author Guy J. Buckle, MD, MPH, of the Andrew C. Carlos MS Institute at Shepherd Center in Atlanta.
Among all 300 patients, just 2% and 4% were screened using a validated tool for cognitive impairment and depression, respectively.
The screening often turned up evidence of the conditions: Physicians saw signs of cognitive impairment in 69% and 78% of those screened aged under 65 years and aged 65 and older, respectively, and they detected depression in 71% and 54% of those screened in those two age groups, respectively.
Researchers also noted several disparities. “Cognitive screening was conducted more frequently in older, employed, or white patients, while the presence of cognitive impairment was documented more often in black, nonworking, and those on Medicare or Medicaid,” Dr. Buckle said. “Depression screening was performed most frequently in older or white patients, yet depression was more common in younger, nonworking patients and those on Medicare/Medicaid.”
In another part of their study, researchers surveyed 13 unidentified “national leaders” in MS research and treatment. Just seven said they used validated tools to screen for cognitive impairment and six said they used them to screen for depression.
“We hear from MS specialists that they want to be measuring for cognition but don’t know how to efficiently work it into their routine, how to approach the patient, and what tools to use,” said study coauthor Derrick S. Robertson, MD, of the University of South Florida, Tampa. “In addition, there is no one tool that is accepted in the MS treatment community.”
MS specialists who didn’t use the screening tests also pointed to factors like lack of reimbursement and lack of integration into electronic medical records. “Doubt very much that neurologists have time to use any of these tests,” one respondent said, referring to cognitive impairment screening.
What’s next? “There are several new exciting developments in clinical trials demonstrating efficacy of disease-modifying therapies in maintaining or improving cognition in patients with relapsing MS,” Dr. Robertson said. “This highlights the urgent need to overcome barriers to use of formal cognitive screening tools in clinical practice to identify patients who need a higher level of care, and perhaps even a change in treatment with the ultimate goal to improve quality of life and overall outcomes.”
Genentech funded the study through an educational grant. Dr. Sapir and three other study authors reported no relevant disclosures. Dr. Buckle and Dr. Robertson reported multiple disclosures, including principle investigator and advisory board/panel member work.
SOURCE: Buckle GJ et al. ACTRIMS Forum 2018, abstract No. P161.
SAN DIEGO – A new study finds that physicians at two . Physicians who did perform screening hardly ever used validated tools and often didn’t refer appropriate patients for higher-level care.
In addition, researchers interviewed 13 leading MS specialists from coast to coast and “found that about half reported not using formal screening tools to assess cognitive impairment and depression,” said study coauthor Tamar Sapir, PhD, chief scientific officer with Prime Education, a firm based in Fort Lauderdale, Fla., that provides a variety of health-related services such as training and research.
The study findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Three of the study authors spoke in interviews.
The researchers sought to understand how frequently MS patients are screened for cognitive problems and depression.
“Cognitive impairment is experienced by approximately half of patients with multiple sclerosis, yet many are never screened or treated, which can impact their daily activities, their ability to work, and overall quality of life,” Dr. Sapir said.
Depression, meanwhile, is believed to be much more common in patients with MS than in the general population, with one recent meta-analysis of 58 studies finding that the average prevalence was 31%. Other research suggests depression is underdiagnosed and undertreated in this population (J Neurol Sci. 2017 Jan 15;372:331-41; ISRN Neurology. 2012, Article ID 427102. doi: 10.5402/2012/427102).
For the current study, researchers tracked 300 patients at two unidentified MS clinics via their charts over a 2-year period from 2014 to 2016. Their median age was 52 years, 76% were women, and 15 had experienced at least one relapse within the previous 24 months.
“Screening for cognitive impairment and depression was documented for only 52% and 63% of MS patients, respectively, and only about a quarter of patients diagnosed with these conditions were referred to a higher level of care,” said lead author Guy J. Buckle, MD, MPH, of the Andrew C. Carlos MS Institute at Shepherd Center in Atlanta.
Among all 300 patients, just 2% and 4% were screened using a validated tool for cognitive impairment and depression, respectively.
The screening often turned up evidence of the conditions: Physicians saw signs of cognitive impairment in 69% and 78% of those screened aged under 65 years and aged 65 and older, respectively, and they detected depression in 71% and 54% of those screened in those two age groups, respectively.
Researchers also noted several disparities. “Cognitive screening was conducted more frequently in older, employed, or white patients, while the presence of cognitive impairment was documented more often in black, nonworking, and those on Medicare or Medicaid,” Dr. Buckle said. “Depression screening was performed most frequently in older or white patients, yet depression was more common in younger, nonworking patients and those on Medicare/Medicaid.”
In another part of their study, researchers surveyed 13 unidentified “national leaders” in MS research and treatment. Just seven said they used validated tools to screen for cognitive impairment and six said they used them to screen for depression.
“We hear from MS specialists that they want to be measuring for cognition but don’t know how to efficiently work it into their routine, how to approach the patient, and what tools to use,” said study coauthor Derrick S. Robertson, MD, of the University of South Florida, Tampa. “In addition, there is no one tool that is accepted in the MS treatment community.”
MS specialists who didn’t use the screening tests also pointed to factors like lack of reimbursement and lack of integration into electronic medical records. “Doubt very much that neurologists have time to use any of these tests,” one respondent said, referring to cognitive impairment screening.
What’s next? “There are several new exciting developments in clinical trials demonstrating efficacy of disease-modifying therapies in maintaining or improving cognition in patients with relapsing MS,” Dr. Robertson said. “This highlights the urgent need to overcome barriers to use of formal cognitive screening tools in clinical practice to identify patients who need a higher level of care, and perhaps even a change in treatment with the ultimate goal to improve quality of life and overall outcomes.”
Genentech funded the study through an educational grant. Dr. Sapir and three other study authors reported no relevant disclosures. Dr. Buckle and Dr. Robertson reported multiple disclosures, including principle investigator and advisory board/panel member work.
SOURCE: Buckle GJ et al. ACTRIMS Forum 2018, abstract No. P161.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point: Screening often turns up signs of trouble, but many MS patients are not screened annually for depression and cognitive impairment.
Major finding: 52% and 63% of patients with MS were screened for cognitive impairment and depression, respectively, over a 1-year period. Study details: 2-year analysis of medical records from two MS clinics in the Southeast.
Disclosures: Genentech funded the study through an educational grant. Some of the study authors reported various disclosures.
Source: Buckle GJ et al. ACTRIMS Forum 2018, abstract No. P161.
Prazosin falls short for veterans’ PTSD-related sleep problems
The alpha-1 adrenergic receptor prazosin failed to improve recurring nightmares or sleep quality compared with placebo in veterans with PTSD in a 26-week randomized trial of 304 adult veterans.
In several previous randomized trials lasting fewer than 15 weeks, veterans with PTSD and recurring nightmares who received prazosin showed benefits, including improved sleep quality and PTSD symptoms, compared with placebo patients, wrote Murray A. Raskind, MD, of the Department of Veterans Affairs Puget Sound Health Care System, Seattle, and his colleagues.
In a study published in the New England Journal of Medicine, the researchers randomized 152 veterans with sleep problems and PTSD to prazosin and 152 to a placebo. The participants were recruited from 12 VA medical centers. The average age of the participants was 52 years, more than 96% were male, and about two-thirds were white. Demographics were similar between the two groups.
After 10 weeks and after 26 weeks, there were no significant differences between the two groups in changes from baseline measures of recurring nightmares, using the mean change from baseline in Clinician-Administered PTSD Score item B2 (recurrent distressing dreams). Similarly, no significant differences appeared between the two groups based on Pittsburgh Sleep Quality Index scores.
“A possible explanation for these negative results is selection bias resulting from recruitment of patients who were mainly in clinically stable condition, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment,” reported Dr. Raskind and his colleagues.
The average maintenance dose of prazosin was 14.8 mg, compared with 16.4 mg in the placebo group; 187 male study participants reached the maximum dose of 20 mg/day (54% of the prazosin group and 70% of the placebo group).
After 10 weeks, no significant differences were found between the two groups in changes from baseline measures of “recurring distressing dreams,” using the mean change from baseline in Clinician-Administered PTSD Score item B2 (recurrent distressing dreams). The between group difference was 0.2. In addition, no significant differences were found at 10 weeks in the average change from baseline Pittsburgh Sleep Quality Index scores.
Similarly, no significant differences appeared between the two groups at 26 weeks. “ since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment,” the researchers said.
On average, patients in the prazosin group had significantly greater decreases in blood pressure, compared with the placebo group. In addition, they had fewer reports of new or worsening suicidal ideation, compared with the placebo group (8% vs.15%).
“Given the concern about suicide among veterans, it is noteworthy that the specifically solicited adverse event of new or worsening suicidal ideation was less common in the prazosin group than in the placebo group, but the absolute number of events was small; this issue warrants further study,” the researchers said.
The study was limited by several factors, including the absence of screening for sleep apnea or sleep-disordered breathing, Dr. Raskind and his colleagues noted. However, the results suggest that “further studies with more refined characterization of autonomic nervous system activity and nocturnal behaviors are needed to determine whether there might be subgroups of veterans with PTSD who can benefit from prazosin.”
Dr. Raskind had no financial conflicts to disclose. The study was supported by the Department of Veterans Affairs Cooperative Studies Program.
SOURCE: Raskind MA et al. N Engl J Med. 2018 Feb 8;378:507-17. doi: 10.1056/NEJMoa1507598.
The alpha-1 adrenergic receptor prazosin failed to improve recurring nightmares or sleep quality compared with placebo in veterans with PTSD in a 26-week randomized trial of 304 adult veterans.
In several previous randomized trials lasting fewer than 15 weeks, veterans with PTSD and recurring nightmares who received prazosin showed benefits, including improved sleep quality and PTSD symptoms, compared with placebo patients, wrote Murray A. Raskind, MD, of the Department of Veterans Affairs Puget Sound Health Care System, Seattle, and his colleagues.
In a study published in the New England Journal of Medicine, the researchers randomized 152 veterans with sleep problems and PTSD to prazosin and 152 to a placebo. The participants were recruited from 12 VA medical centers. The average age of the participants was 52 years, more than 96% were male, and about two-thirds were white. Demographics were similar between the two groups.
After 10 weeks and after 26 weeks, there were no significant differences between the two groups in changes from baseline measures of recurring nightmares, using the mean change from baseline in Clinician-Administered PTSD Score item B2 (recurrent distressing dreams). Similarly, no significant differences appeared between the two groups based on Pittsburgh Sleep Quality Index scores.
“A possible explanation for these negative results is selection bias resulting from recruitment of patients who were mainly in clinically stable condition, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment,” reported Dr. Raskind and his colleagues.
The average maintenance dose of prazosin was 14.8 mg, compared with 16.4 mg in the placebo group; 187 male study participants reached the maximum dose of 20 mg/day (54% of the prazosin group and 70% of the placebo group).
After 10 weeks, no significant differences were found between the two groups in changes from baseline measures of “recurring distressing dreams,” using the mean change from baseline in Clinician-Administered PTSD Score item B2 (recurrent distressing dreams). The between group difference was 0.2. In addition, no significant differences were found at 10 weeks in the average change from baseline Pittsburgh Sleep Quality Index scores.
Similarly, no significant differences appeared between the two groups at 26 weeks. “ since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment,” the researchers said.
On average, patients in the prazosin group had significantly greater decreases in blood pressure, compared with the placebo group. In addition, they had fewer reports of new or worsening suicidal ideation, compared with the placebo group (8% vs.15%).
“Given the concern about suicide among veterans, it is noteworthy that the specifically solicited adverse event of new or worsening suicidal ideation was less common in the prazosin group than in the placebo group, but the absolute number of events was small; this issue warrants further study,” the researchers said.
The study was limited by several factors, including the absence of screening for sleep apnea or sleep-disordered breathing, Dr. Raskind and his colleagues noted. However, the results suggest that “further studies with more refined characterization of autonomic nervous system activity and nocturnal behaviors are needed to determine whether there might be subgroups of veterans with PTSD who can benefit from prazosin.”
Dr. Raskind had no financial conflicts to disclose. The study was supported by the Department of Veterans Affairs Cooperative Studies Program.
SOURCE: Raskind MA et al. N Engl J Med. 2018 Feb 8;378:507-17. doi: 10.1056/NEJMoa1507598.
The alpha-1 adrenergic receptor prazosin failed to improve recurring nightmares or sleep quality compared with placebo in veterans with PTSD in a 26-week randomized trial of 304 adult veterans.
In several previous randomized trials lasting fewer than 15 weeks, veterans with PTSD and recurring nightmares who received prazosin showed benefits, including improved sleep quality and PTSD symptoms, compared with placebo patients, wrote Murray A. Raskind, MD, of the Department of Veterans Affairs Puget Sound Health Care System, Seattle, and his colleagues.
In a study published in the New England Journal of Medicine, the researchers randomized 152 veterans with sleep problems and PTSD to prazosin and 152 to a placebo. The participants were recruited from 12 VA medical centers. The average age of the participants was 52 years, more than 96% were male, and about two-thirds were white. Demographics were similar between the two groups.
After 10 weeks and after 26 weeks, there were no significant differences between the two groups in changes from baseline measures of recurring nightmares, using the mean change from baseline in Clinician-Administered PTSD Score item B2 (recurrent distressing dreams). Similarly, no significant differences appeared between the two groups based on Pittsburgh Sleep Quality Index scores.
“A possible explanation for these negative results is selection bias resulting from recruitment of patients who were mainly in clinically stable condition, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment,” reported Dr. Raskind and his colleagues.
The average maintenance dose of prazosin was 14.8 mg, compared with 16.4 mg in the placebo group; 187 male study participants reached the maximum dose of 20 mg/day (54% of the prazosin group and 70% of the placebo group).
After 10 weeks, no significant differences were found between the two groups in changes from baseline measures of “recurring distressing dreams,” using the mean change from baseline in Clinician-Administered PTSD Score item B2 (recurrent distressing dreams). The between group difference was 0.2. In addition, no significant differences were found at 10 weeks in the average change from baseline Pittsburgh Sleep Quality Index scores.
Similarly, no significant differences appeared between the two groups at 26 weeks. “ since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment,” the researchers said.
On average, patients in the prazosin group had significantly greater decreases in blood pressure, compared with the placebo group. In addition, they had fewer reports of new or worsening suicidal ideation, compared with the placebo group (8% vs.15%).
“Given the concern about suicide among veterans, it is noteworthy that the specifically solicited adverse event of new or worsening suicidal ideation was less common in the prazosin group than in the placebo group, but the absolute number of events was small; this issue warrants further study,” the researchers said.
The study was limited by several factors, including the absence of screening for sleep apnea or sleep-disordered breathing, Dr. Raskind and his colleagues noted. However, the results suggest that “further studies with more refined characterization of autonomic nervous system activity and nocturnal behaviors are needed to determine whether there might be subgroups of veterans with PTSD who can benefit from prazosin.”
Dr. Raskind had no financial conflicts to disclose. The study was supported by the Department of Veterans Affairs Cooperative Studies Program.
SOURCE: Raskind MA et al. N Engl J Med. 2018 Feb 8;378:507-17. doi: 10.1056/NEJMoa1507598.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Prazosin had no apparent effect on recurrent distressing dreams or sleep quality in veterans with PTSD.
Major finding: The between-group difference in scores on a measure of “recurrent distressing dreams” between the prazosin and placebo groups was a nonsignificant 0.2.
Study details: The data come from a randomized trial of 304 military veterans with PTSD who reported frequent nightmares.
Disclosures: Dr. Raskind had no financial conflicts to disclose. The study was supported by the Department of Veterans Affairs Cooperative Studies Program.
Source: Raskind MA et al. N Engl J Med. 2018;378:507-17.
Schizophrenia and gender: Do neurosteroids account for differences?
Neurosteroids may be tied to the gender differences found in the susceptibility to schizophrenia, a cross-sectional, case control study showed.
“These findings suggest that neurosteroids are involved in the pathophysiology of schizophrenia in male patients but not so much in female patients,” reported Yu-Chi Huang, MD, of the department of psychiatry at Kaohsiung Chang Gung Memorial Hospital, Kaohsiung City, Taiwan, and associates.
To conduct the study, the researchers recruited 65 patients with schizophrenia from an outpatient department and psychiatry ward of the hospital. Eligible patients were aged 18-65 years, diagnosed with schizophrenia as defined by the DSM-IV-TR, and taking a stable dose of antipsychotics for at least 1 month before the start of the study. In addition, the participants could have no history of major physical illnesses and had to be of ethnic Han Chinese origin. Thirty-six of the patients were men.
The control group was made up of 103 healthy hospital staff and community members who were within the same age range as the patients. The controls could have no history of illicit drug use, physical illnesses, or psychiatric disorders and had to be ethnic Han Chinese. Forty-seven members of the control group were males (Psychoneuroendocrinology. 2017 Oct;84:87-93).
Participants fasted and blood samples were obtained. Dehydroepiandrosterone (DHEA) levels were measured using the DHEA ELISA [enyme-linked immunosorbent assay] – ADKI-900-093, dehydroepiandrosterone sulfate (DHEAS) levels were measured with the Architect DHEA-S reagent kit, and pregnenolone levels were measured using the pregnenolone ELISA kit. Psychiatric diagnoses were assessed for both groups using a psychiatric interview based on the Mini-International Neuropsychiatric Interview, the Positive and Negative Syndrome Scale (PANSS), and the 17-item Hamilton Depression Rating Scale. Several factors tied to schizophrenia were evaluated, including the age of onset, illness duration, and use of antipsychotics. The numbers were placed into a database and analyzed.
After controlling for age and body mass index, the researchers found that in male patients with schizophrenia, DHEA and DHEAS serum levels were positively associated with the age of onset of schizophrenia (P less than .05) and negatively associated with the duration of illness (P less than .05). (P less than .05 ). Furthermore, the levels of DHEA, DHEAS, and pregnenolone were lower among the male schizophrenia patients, compared with the serum levels of the healthy male controls. No differences were found in serum levels among the female patients with schizophrenia and the healthy controls.
The findings suggest that DHEA, DHEAS, and pregnenolone could be markers of the duration of illness and the severity of general symptoms among male patients with schizophrenia. To read the entire study, click here.
Neurosteroids may be tied to the gender differences found in the susceptibility to schizophrenia, a cross-sectional, case control study showed.
“These findings suggest that neurosteroids are involved in the pathophysiology of schizophrenia in male patients but not so much in female patients,” reported Yu-Chi Huang, MD, of the department of psychiatry at Kaohsiung Chang Gung Memorial Hospital, Kaohsiung City, Taiwan, and associates.
To conduct the study, the researchers recruited 65 patients with schizophrenia from an outpatient department and psychiatry ward of the hospital. Eligible patients were aged 18-65 years, diagnosed with schizophrenia as defined by the DSM-IV-TR, and taking a stable dose of antipsychotics for at least 1 month before the start of the study. In addition, the participants could have no history of major physical illnesses and had to be of ethnic Han Chinese origin. Thirty-six of the patients were men.
The control group was made up of 103 healthy hospital staff and community members who were within the same age range as the patients. The controls could have no history of illicit drug use, physical illnesses, or psychiatric disorders and had to be ethnic Han Chinese. Forty-seven members of the control group were males (Psychoneuroendocrinology. 2017 Oct;84:87-93).
Participants fasted and blood samples were obtained. Dehydroepiandrosterone (DHEA) levels were measured using the DHEA ELISA [enyme-linked immunosorbent assay] – ADKI-900-093, dehydroepiandrosterone sulfate (DHEAS) levels were measured with the Architect DHEA-S reagent kit, and pregnenolone levels were measured using the pregnenolone ELISA kit. Psychiatric diagnoses were assessed for both groups using a psychiatric interview based on the Mini-International Neuropsychiatric Interview, the Positive and Negative Syndrome Scale (PANSS), and the 17-item Hamilton Depression Rating Scale. Several factors tied to schizophrenia were evaluated, including the age of onset, illness duration, and use of antipsychotics. The numbers were placed into a database and analyzed.
After controlling for age and body mass index, the researchers found that in male patients with schizophrenia, DHEA and DHEAS serum levels were positively associated with the age of onset of schizophrenia (P less than .05) and negatively associated with the duration of illness (P less than .05). (P less than .05 ). Furthermore, the levels of DHEA, DHEAS, and pregnenolone were lower among the male schizophrenia patients, compared with the serum levels of the healthy male controls. No differences were found in serum levels among the female patients with schizophrenia and the healthy controls.
The findings suggest that DHEA, DHEAS, and pregnenolone could be markers of the duration of illness and the severity of general symptoms among male patients with schizophrenia. To read the entire study, click here.
Neurosteroids may be tied to the gender differences found in the susceptibility to schizophrenia, a cross-sectional, case control study showed.
“These findings suggest that neurosteroids are involved in the pathophysiology of schizophrenia in male patients but not so much in female patients,” reported Yu-Chi Huang, MD, of the department of psychiatry at Kaohsiung Chang Gung Memorial Hospital, Kaohsiung City, Taiwan, and associates.
To conduct the study, the researchers recruited 65 patients with schizophrenia from an outpatient department and psychiatry ward of the hospital. Eligible patients were aged 18-65 years, diagnosed with schizophrenia as defined by the DSM-IV-TR, and taking a stable dose of antipsychotics for at least 1 month before the start of the study. In addition, the participants could have no history of major physical illnesses and had to be of ethnic Han Chinese origin. Thirty-six of the patients were men.
The control group was made up of 103 healthy hospital staff and community members who were within the same age range as the patients. The controls could have no history of illicit drug use, physical illnesses, or psychiatric disorders and had to be ethnic Han Chinese. Forty-seven members of the control group were males (Psychoneuroendocrinology. 2017 Oct;84:87-93).
Participants fasted and blood samples were obtained. Dehydroepiandrosterone (DHEA) levels were measured using the DHEA ELISA [enyme-linked immunosorbent assay] – ADKI-900-093, dehydroepiandrosterone sulfate (DHEAS) levels were measured with the Architect DHEA-S reagent kit, and pregnenolone levels were measured using the pregnenolone ELISA kit. Psychiatric diagnoses were assessed for both groups using a psychiatric interview based on the Mini-International Neuropsychiatric Interview, the Positive and Negative Syndrome Scale (PANSS), and the 17-item Hamilton Depression Rating Scale. Several factors tied to schizophrenia were evaluated, including the age of onset, illness duration, and use of antipsychotics. The numbers were placed into a database and analyzed.
After controlling for age and body mass index, the researchers found that in male patients with schizophrenia, DHEA and DHEAS serum levels were positively associated with the age of onset of schizophrenia (P less than .05) and negatively associated with the duration of illness (P less than .05). (P less than .05 ). Furthermore, the levels of DHEA, DHEAS, and pregnenolone were lower among the male schizophrenia patients, compared with the serum levels of the healthy male controls. No differences were found in serum levels among the female patients with schizophrenia and the healthy controls.
The findings suggest that DHEA, DHEAS, and pregnenolone could be markers of the duration of illness and the severity of general symptoms among male patients with schizophrenia. To read the entire study, click here.
FROM PSYCHONEUROENDOCRINOLOGY
Benzodiazepines: Sensible prescribing in light of the risks
As a group, anxiety disorders are the most common mental illness in the Unites States, affecting 40 million adults. There is a nearly 30% lifetime prevalence of anxiety disorders in the general population.1 DSM-5 anxiety disorders include generalized anxiety disorder, social anxiety disorder (social phobia), panic disorder, specific phobia, and separation anxiety disorder. Although DSM-IV-TR also classified obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) as anxiety disorders, these diagnoses were reclassified in DSM-5. Anxiety also is a frequent symptom of many other psychiatric disorders, especially major depressive disorder.
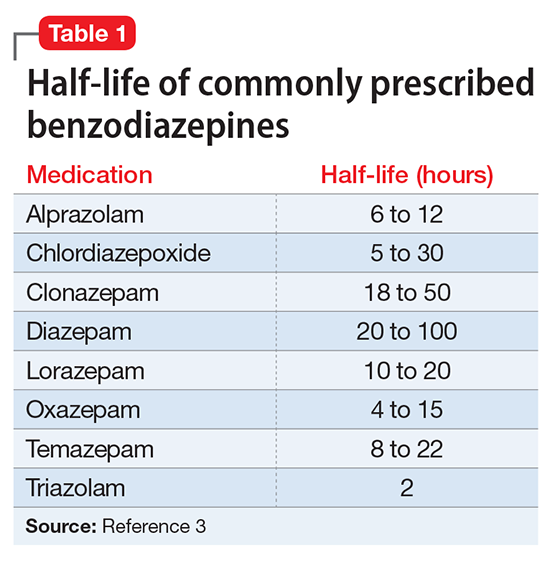
Although benzodiazepines have many potential uses, they also carry risks that prescribers should recognize. This article reviews some of the risks of benzodiazepine use, identifies patients with higher risks of adverse effects, and presents a practical approach to prescribing these medications.
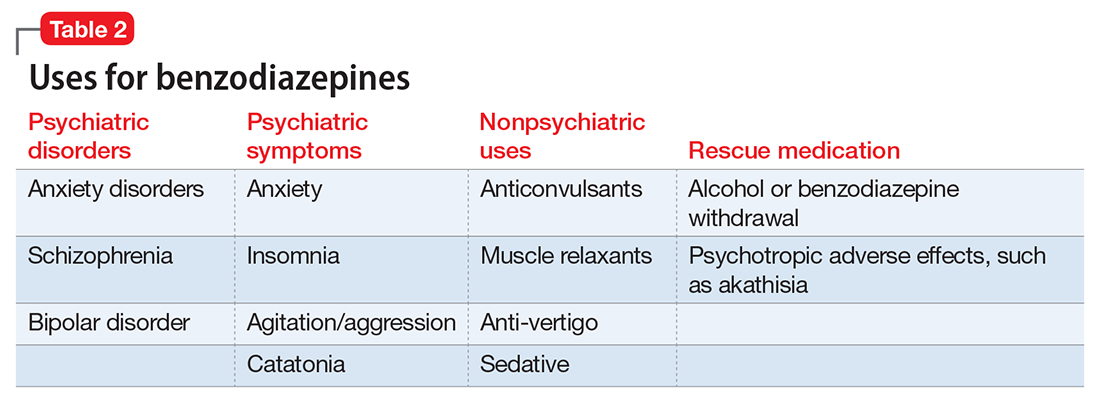
A wide range of risks
Abuse and addiction. Perhaps the most commonly recognized risk associated with benzodiazepine use is the potential for abuse and addiction.4 Prolonged benzodiazepine use typically results in physiologic tolerance, requiring higher dosing to achieve the same initial effect.5 American Psychiatric Association practice guidelines recognize the potential for benzodiazepine use to result in symptoms of dependence, including cravings and withdrawal, stating that “with ongoing use, all benzodiazepines will produce physiological dependence in most patients.”6 High-potency, short-acting compounds such as alprazolam have a higher risk for dependence, toxicity, and abuse.7 However, long-acting benzodiazepines (such as clonazepam) also can be habit-forming.8 Because of these properties, it is generally advisable to avoid prescribing benzodiazepines (and short-acting compounds in particular) when treating patients with current or past substance use disorders, except when treating withdrawal.9
Limited efficacy for other disorders. Although benzodiazepines can help reduce anxiety in patients with anxiety disorders, they have shown less promise in treating other disorders in which anxiety is a common symptom. Treating PTSD with benzodiazepines does not appear to offer any advantage over placebo, and may even result in increased symptoms over time.10,11 There is limited evidence supporting the use of benzodiazepines to treat OCD.12,13 Patients with borderline personality disorder who are treated with benzodiazepines may experience an increase in behavioral dysregulation.14
Physical ailments. Benzodiazepines can affect comorbid physical ailments. One study found that long-term benzodiazepine use among patients with comorbid pain disorders was correlated with high utilization of medical services and high disability levels.15 Benzodiazepine use also has been associated with an increased risk of exacerbating respiratory conditions, such as chronic obstructive pulmonary disease,16 and increased risk of pneumonia.17,18
Pregnancy and breastfeeding. Benzodiazepines carry risks for women who are pregnant or breastfeeding. Benzodiazepine use during pregnancy may increase the relative risk of major malformations and oral clefts. It also may result in neonatal lethargy, sedation, and weight loss. Benzodiazepine withdrawal symptoms can occur in the neonate.19 Benzodiazepines are secreted in breast milk and can result in sedation among breastfed infants.20
Geriatric patients. Older adults may be particularly vulnerable to the adverse effects of benzodiazepines. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends against prescribing benzodiazepines to geriatric patients.21 Benzodiazepine use has been associated with an increased risk for falls among older adults,22,23 with an increased risk of fractures24 that can be fatal.25 Benzodiazepines also have been associated with an increased risk of cognitive dysfunction and dementia.26,27 Despite the documented risks of using benzodiazepines in geriatric patients, benzodiazepines continue to be frequently prescribed to this age group.28,29 One study found that the rate of prescribing benzodiazepines by primary care physicians increased from 2003 to 2012, primarily among older adults with no diagnosis of pain or a psychiatric disorder.30
Mortality. Benzodiazepine use also carries an increased risk of mortality. Benzodiazepine users are at increased risk of motor vehicle accidents because of difficulty maintaining road position.31 Some research has shown that patients with schizophrenia treated with benzodiazepines have an increased risk of death compared with those who are prescribed antipsychotics or antidepressants.32 Another study showed that patients with schizophrenia who were prescribed benzodiazepines had a greater risk of death by suicide and accidental poisoning.33 Benzodiazepine use has been associated with suicidal ideation and an increased risk of suicide.34 Prescription opioids and benzodiazepines are the top 2 causes of overdose-related deaths (benzodiazepines are involved in approximately 31% of fatal overdoses35), and from 2002 to 2015 there was a 4.3-fold increase in deaths from benzodiazepine overdose in the United States.36 CDC guidelines recommend against co-prescribing opioids and benzodiazepines because of the risk of death by respiratory depression.37 As of August 2016, the FDA required black-box warnings for opioids and benzodiazepines regarding the risk of respiratory depression and death when these agents are used in combination, noting that “If these medicines are prescribed together, limit the dosages and duration of each drug to the minimum possible while achieving the desired clinical effect.”38,39
A sensible approach to prescribing
Given the risks posed by benzodiazepines, what would constitute a sensible approach to their use? Clearly, there are some patients for whom benzodiazepine use should be minimized or avoided (Table 3). In a patient who is deemed a good candidate for benzodiazepines, a long-acting agent may be preferable because of the increased risk of dependence associated with short-acting compounds. Start with a low dose, and use the lowest dose that adequately treats the patient’s symptoms.40 Using scheduled rather than “as-needed” dosing may help reduce behavioral escape patterns that reinforce anxiety and dependence in the long term.
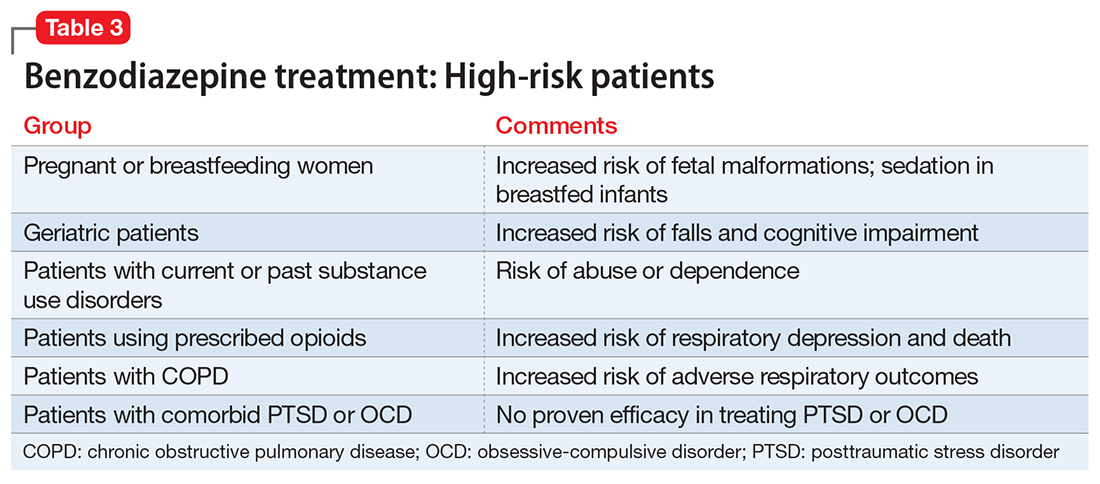
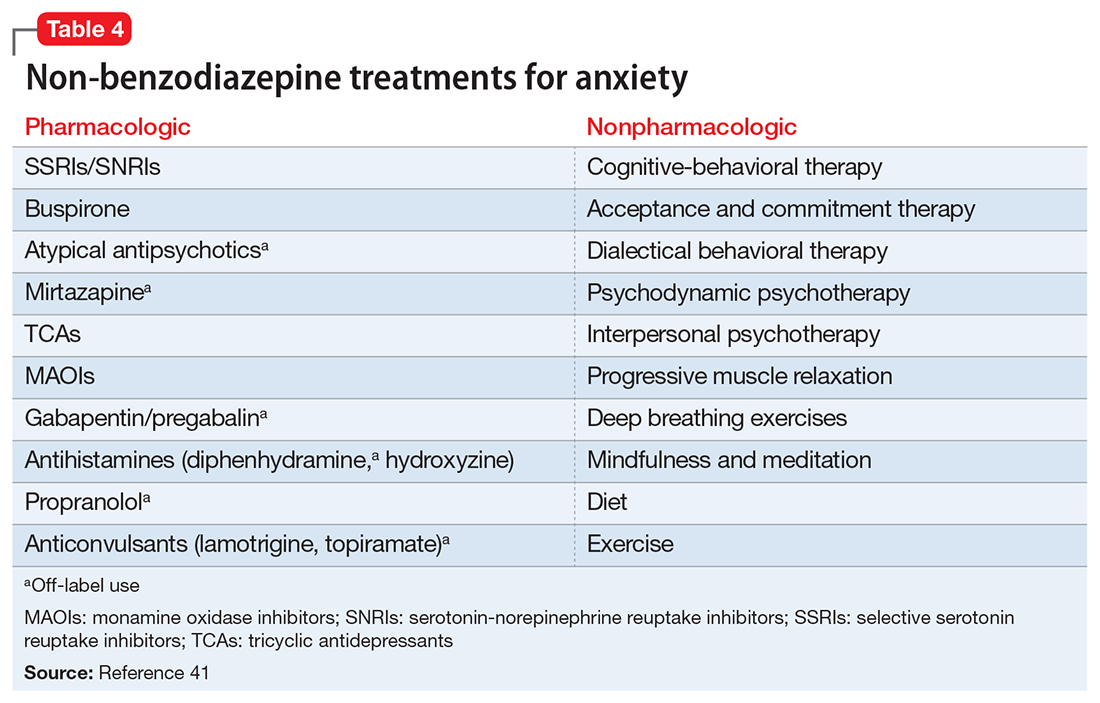
Before starting a patient on a benzodiazepine, discuss with him (her) the risks of use and an exit plan to discontinue the medication. For example, a benzodiazepine may be prescribed at the same time as a selective serotonin reuptake inhibitor (SSRI), with the goal of weaning off the benzodiazepine once the SSRI has achieved efficacy.6 Inform the patient that prescribing or treatment may be terminated if it is discovered that the patient is abusing or diverting the medication (regularly reviewing the state prescription monitoring program database can help determine if this has occurred). Strongly consider using non-benzodiazepine treatments for anxiety with (or eventually in place of) benzodiazepines (Table 441).
Reducing or stopping benzodiazepines can be challenging.42 Patients often are reluctant to stop such medications, and abrupt cessation can cause severe withdrawal. Benzodiazepine withdrawal symptoms can be severe or even fatal. Therefore, a safe and collaborative approach to reducing or stopping benzodiazepines is necessary. A starting point might be to review the risks associated with benzodiazepine use with the patient and ask about the frequency of use. Discuss with the patient a slow taper, perhaps reducing the dose by 10% to 25% increments weekly to biweekly.43,44 Less motivated patients may require a slower taper, more time, or repeated discussions. When starting a dose reduction, notify the patient that some rebound anxiety or insomnia are to be expected. With any progress the patient makes toward reducing his usage, congratulate him on such progress.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Balon R, Fava GA, Rickels K. Need for a realistic appraisal of benzodiazepines. World Psychiatry. 2015;14(2):243-244.
3. Ashton CH. Benzodiazepine equivalence table. http://www.benzo.org.uk/bzequiv.htm. Revised April 2007. Accessed May 3, 2017.
4. National Institute on Drug Abuse. Commonly abused drugs. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/commonly_abused_drugs_3.pdf. Revised January 2016. Accessed January 9, 2018.
5. Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74-89.
6. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed May 3, 2017.
7. Salzman C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148(2):151-152.
8. Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry. 2017;74(7):747-755.
9. O’Brien PL, Karnell LH, Gokhale M, et al. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223-230.
10. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry. 2002;63(12):1183-1184.
11. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57(9):390-394.
12. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed May 3, 2017.
13. Abdel-Ahad P, Kazour F. Non-antidepressant pharmacological treatment of obsessive compulsive disorder: a comprehensive review. Curr Clin Pharmacol. 2015;10(2):97-111.
14. Gardner DL, Cowdry RW. Alprazolam-induced dyscontrol in borderline personality disorder. Am J Psychiatry. 1985;142(1):98-100.
15. Ciccone DS, Just N, Bandilla EB, et al. Psychological correlates of opioid use in patients with chronic nonmalignant pain: a preliminary test of the downhill spiral hypothesis. J Pain Symptom Manage. 2000;20(3):180-192.
16. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332-340.
17. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-170.
18. Taipale H, Tolppanen AM, Koponen M, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519-E529.
19. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatric Serv. 2002;53:39-49.
20. U.S. National Library of Medicine, TOXNET Toxicology Data Network. Lactmed: alprazolam. http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+335. Accessed May 3, 2017.
21. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
22. Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48(6):682-685.
23. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952-1960.
24. Bolton JM, Morin SN, Majumdar SR, et al. Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatry. 2017;74(6):641-648.
25. Pariente A, Dartiques JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61-70.
26. Lagnaoui R, Tournier M, Moride Y, et al. The risk of cognitive impairment in older community-dwelling women after benzodiazepine use. Age Ageing. 2009;38(2):226-228.
27. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231.
28. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
29. Maust DT, Kales HC, Wiechers IR, et al. No end in sight: benzodiazepine use in older adults in the United States. J Am Geriatr Soc. 2016;64(12):2546-2553.
30. Maust DT, Blow FC, Wiechers IR, et al. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363-e371.
31. Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663-673.
32. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
33. Fontanella CA, Campo JV, Phillips GS, et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry. 2016;77(5):661-667.
34. McCall WV, Benca RM, Rosenguist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25.
35. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688.
36. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Updated September 2017. Accessed January 8, 2018.
37. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1-49.
38. U.S. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Published August 31, 2016. Accessed May 3, 2017.
39. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety/ucm518473.htm. Published August 31, 2016. Accessed May 3, 2017.
40. National Institute for Health and Care Excellence. Controlled drugs: safe use and management. https://www.nice.org.uk/guidance/ng46/evidence/full-guideline-pdf-2427186353. Published April 2016. Accessed July 25, 2017.
41. Stahl SM. Anxiety disorders and anxiolytics. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:721-772.
42. Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919-934.
43. Nardi AE, Freire RC, Valença AM, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. 2010;30(3):290-293.
44. Tampi R. How to wean geriatric patients off benzodiazepines. Psychiatric News. http://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2016.PP3b6. Published March 18, 2016. Accessed May 3, 2017.
As a group, anxiety disorders are the most common mental illness in the Unites States, affecting 40 million adults. There is a nearly 30% lifetime prevalence of anxiety disorders in the general population.1 DSM-5 anxiety disorders include generalized anxiety disorder, social anxiety disorder (social phobia), panic disorder, specific phobia, and separation anxiety disorder. Although DSM-IV-TR also classified obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) as anxiety disorders, these diagnoses were reclassified in DSM-5. Anxiety also is a frequent symptom of many other psychiatric disorders, especially major depressive disorder.

Although benzodiazepines have many potential uses, they also carry risks that prescribers should recognize. This article reviews some of the risks of benzodiazepine use, identifies patients with higher risks of adverse effects, and presents a practical approach to prescribing these medications.

A wide range of risks
Abuse and addiction. Perhaps the most commonly recognized risk associated with benzodiazepine use is the potential for abuse and addiction.4 Prolonged benzodiazepine use typically results in physiologic tolerance, requiring higher dosing to achieve the same initial effect.5 American Psychiatric Association practice guidelines recognize the potential for benzodiazepine use to result in symptoms of dependence, including cravings and withdrawal, stating that “with ongoing use, all benzodiazepines will produce physiological dependence in most patients.”6 High-potency, short-acting compounds such as alprazolam have a higher risk for dependence, toxicity, and abuse.7 However, long-acting benzodiazepines (such as clonazepam) also can be habit-forming.8 Because of these properties, it is generally advisable to avoid prescribing benzodiazepines (and short-acting compounds in particular) when treating patients with current or past substance use disorders, except when treating withdrawal.9
Limited efficacy for other disorders. Although benzodiazepines can help reduce anxiety in patients with anxiety disorders, they have shown less promise in treating other disorders in which anxiety is a common symptom. Treating PTSD with benzodiazepines does not appear to offer any advantage over placebo, and may even result in increased symptoms over time.10,11 There is limited evidence supporting the use of benzodiazepines to treat OCD.12,13 Patients with borderline personality disorder who are treated with benzodiazepines may experience an increase in behavioral dysregulation.14
Physical ailments. Benzodiazepines can affect comorbid physical ailments. One study found that long-term benzodiazepine use among patients with comorbid pain disorders was correlated with high utilization of medical services and high disability levels.15 Benzodiazepine use also has been associated with an increased risk of exacerbating respiratory conditions, such as chronic obstructive pulmonary disease,16 and increased risk of pneumonia.17,18
Pregnancy and breastfeeding. Benzodiazepines carry risks for women who are pregnant or breastfeeding. Benzodiazepine use during pregnancy may increase the relative risk of major malformations and oral clefts. It also may result in neonatal lethargy, sedation, and weight loss. Benzodiazepine withdrawal symptoms can occur in the neonate.19 Benzodiazepines are secreted in breast milk and can result in sedation among breastfed infants.20
Geriatric patients. Older adults may be particularly vulnerable to the adverse effects of benzodiazepines. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends against prescribing benzodiazepines to geriatric patients.21 Benzodiazepine use has been associated with an increased risk for falls among older adults,22,23 with an increased risk of fractures24 that can be fatal.25 Benzodiazepines also have been associated with an increased risk of cognitive dysfunction and dementia.26,27 Despite the documented risks of using benzodiazepines in geriatric patients, benzodiazepines continue to be frequently prescribed to this age group.28,29 One study found that the rate of prescribing benzodiazepines by primary care physicians increased from 2003 to 2012, primarily among older adults with no diagnosis of pain or a psychiatric disorder.30
Mortality. Benzodiazepine use also carries an increased risk of mortality. Benzodiazepine users are at increased risk of motor vehicle accidents because of difficulty maintaining road position.31 Some research has shown that patients with schizophrenia treated with benzodiazepines have an increased risk of death compared with those who are prescribed antipsychotics or antidepressants.32 Another study showed that patients with schizophrenia who were prescribed benzodiazepines had a greater risk of death by suicide and accidental poisoning.33 Benzodiazepine use has been associated with suicidal ideation and an increased risk of suicide.34 Prescription opioids and benzodiazepines are the top 2 causes of overdose-related deaths (benzodiazepines are involved in approximately 31% of fatal overdoses35), and from 2002 to 2015 there was a 4.3-fold increase in deaths from benzodiazepine overdose in the United States.36 CDC guidelines recommend against co-prescribing opioids and benzodiazepines because of the risk of death by respiratory depression.37 As of August 2016, the FDA required black-box warnings for opioids and benzodiazepines regarding the risk of respiratory depression and death when these agents are used in combination, noting that “If these medicines are prescribed together, limit the dosages and duration of each drug to the minimum possible while achieving the desired clinical effect.”38,39
A sensible approach to prescribing
Given the risks posed by benzodiazepines, what would constitute a sensible approach to their use? Clearly, there are some patients for whom benzodiazepine use should be minimized or avoided (Table 3). In a patient who is deemed a good candidate for benzodiazepines, a long-acting agent may be preferable because of the increased risk of dependence associated with short-acting compounds. Start with a low dose, and use the lowest dose that adequately treats the patient’s symptoms.40 Using scheduled rather than “as-needed” dosing may help reduce behavioral escape patterns that reinforce anxiety and dependence in the long term.

Before starting a patient on a benzodiazepine, discuss with him (her) the risks of use and an exit plan to discontinue the medication. For example, a benzodiazepine may be prescribed at the same time as a selective serotonin reuptake inhibitor (SSRI), with the goal of weaning off the benzodiazepine once the SSRI has achieved efficacy.6 Inform the patient that prescribing or treatment may be terminated if it is discovered that the patient is abusing or diverting the medication (regularly reviewing the state prescription monitoring program database can help determine if this has occurred). Strongly consider using non-benzodiazepine treatments for anxiety with (or eventually in place of) benzodiazepines (Table 441).

Reducing or stopping benzodiazepines can be challenging.42 Patients often are reluctant to stop such medications, and abrupt cessation can cause severe withdrawal. Benzodiazepine withdrawal symptoms can be severe or even fatal. Therefore, a safe and collaborative approach to reducing or stopping benzodiazepines is necessary. A starting point might be to review the risks associated with benzodiazepine use with the patient and ask about the frequency of use. Discuss with the patient a slow taper, perhaps reducing the dose by 10% to 25% increments weekly to biweekly.43,44 Less motivated patients may require a slower taper, more time, or repeated discussions. When starting a dose reduction, notify the patient that some rebound anxiety or insomnia are to be expected. With any progress the patient makes toward reducing his usage, congratulate him on such progress.
As a group, anxiety disorders are the most common mental illness in the Unites States, affecting 40 million adults. There is a nearly 30% lifetime prevalence of anxiety disorders in the general population.1 DSM-5 anxiety disorders include generalized anxiety disorder, social anxiety disorder (social phobia), panic disorder, specific phobia, and separation anxiety disorder. Although DSM-IV-TR also classified obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) as anxiety disorders, these diagnoses were reclassified in DSM-5. Anxiety also is a frequent symptom of many other psychiatric disorders, especially major depressive disorder.

Although benzodiazepines have many potential uses, they also carry risks that prescribers should recognize. This article reviews some of the risks of benzodiazepine use, identifies patients with higher risks of adverse effects, and presents a practical approach to prescribing these medications.

A wide range of risks
Abuse and addiction. Perhaps the most commonly recognized risk associated with benzodiazepine use is the potential for abuse and addiction.4 Prolonged benzodiazepine use typically results in physiologic tolerance, requiring higher dosing to achieve the same initial effect.5 American Psychiatric Association practice guidelines recognize the potential for benzodiazepine use to result in symptoms of dependence, including cravings and withdrawal, stating that “with ongoing use, all benzodiazepines will produce physiological dependence in most patients.”6 High-potency, short-acting compounds such as alprazolam have a higher risk for dependence, toxicity, and abuse.7 However, long-acting benzodiazepines (such as clonazepam) also can be habit-forming.8 Because of these properties, it is generally advisable to avoid prescribing benzodiazepines (and short-acting compounds in particular) when treating patients with current or past substance use disorders, except when treating withdrawal.9
Limited efficacy for other disorders. Although benzodiazepines can help reduce anxiety in patients with anxiety disorders, they have shown less promise in treating other disorders in which anxiety is a common symptom. Treating PTSD with benzodiazepines does not appear to offer any advantage over placebo, and may even result in increased symptoms over time.10,11 There is limited evidence supporting the use of benzodiazepines to treat OCD.12,13 Patients with borderline personality disorder who are treated with benzodiazepines may experience an increase in behavioral dysregulation.14
Physical ailments. Benzodiazepines can affect comorbid physical ailments. One study found that long-term benzodiazepine use among patients with comorbid pain disorders was correlated with high utilization of medical services and high disability levels.15 Benzodiazepine use also has been associated with an increased risk of exacerbating respiratory conditions, such as chronic obstructive pulmonary disease,16 and increased risk of pneumonia.17,18
Pregnancy and breastfeeding. Benzodiazepines carry risks for women who are pregnant or breastfeeding. Benzodiazepine use during pregnancy may increase the relative risk of major malformations and oral clefts. It also may result in neonatal lethargy, sedation, and weight loss. Benzodiazepine withdrawal symptoms can occur in the neonate.19 Benzodiazepines are secreted in breast milk and can result in sedation among breastfed infants.20
Geriatric patients. Older adults may be particularly vulnerable to the adverse effects of benzodiazepines. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends against prescribing benzodiazepines to geriatric patients.21 Benzodiazepine use has been associated with an increased risk for falls among older adults,22,23 with an increased risk of fractures24 that can be fatal.25 Benzodiazepines also have been associated with an increased risk of cognitive dysfunction and dementia.26,27 Despite the documented risks of using benzodiazepines in geriatric patients, benzodiazepines continue to be frequently prescribed to this age group.28,29 One study found that the rate of prescribing benzodiazepines by primary care physicians increased from 2003 to 2012, primarily among older adults with no diagnosis of pain or a psychiatric disorder.30
Mortality. Benzodiazepine use also carries an increased risk of mortality. Benzodiazepine users are at increased risk of motor vehicle accidents because of difficulty maintaining road position.31 Some research has shown that patients with schizophrenia treated with benzodiazepines have an increased risk of death compared with those who are prescribed antipsychotics or antidepressants.32 Another study showed that patients with schizophrenia who were prescribed benzodiazepines had a greater risk of death by suicide and accidental poisoning.33 Benzodiazepine use has been associated with suicidal ideation and an increased risk of suicide.34 Prescription opioids and benzodiazepines are the top 2 causes of overdose-related deaths (benzodiazepines are involved in approximately 31% of fatal overdoses35), and from 2002 to 2015 there was a 4.3-fold increase in deaths from benzodiazepine overdose in the United States.36 CDC guidelines recommend against co-prescribing opioids and benzodiazepines because of the risk of death by respiratory depression.37 As of August 2016, the FDA required black-box warnings for opioids and benzodiazepines regarding the risk of respiratory depression and death when these agents are used in combination, noting that “If these medicines are prescribed together, limit the dosages and duration of each drug to the minimum possible while achieving the desired clinical effect.”38,39
A sensible approach to prescribing
Given the risks posed by benzodiazepines, what would constitute a sensible approach to their use? Clearly, there are some patients for whom benzodiazepine use should be minimized or avoided (Table 3). In a patient who is deemed a good candidate for benzodiazepines, a long-acting agent may be preferable because of the increased risk of dependence associated with short-acting compounds. Start with a low dose, and use the lowest dose that adequately treats the patient’s symptoms.40 Using scheduled rather than “as-needed” dosing may help reduce behavioral escape patterns that reinforce anxiety and dependence in the long term.

Before starting a patient on a benzodiazepine, discuss with him (her) the risks of use and an exit plan to discontinue the medication. For example, a benzodiazepine may be prescribed at the same time as a selective serotonin reuptake inhibitor (SSRI), with the goal of weaning off the benzodiazepine once the SSRI has achieved efficacy.6 Inform the patient that prescribing or treatment may be terminated if it is discovered that the patient is abusing or diverting the medication (regularly reviewing the state prescription monitoring program database can help determine if this has occurred). Strongly consider using non-benzodiazepine treatments for anxiety with (or eventually in place of) benzodiazepines (Table 441).

Reducing or stopping benzodiazepines can be challenging.42 Patients often are reluctant to stop such medications, and abrupt cessation can cause severe withdrawal. Benzodiazepine withdrawal symptoms can be severe or even fatal. Therefore, a safe and collaborative approach to reducing or stopping benzodiazepines is necessary. A starting point might be to review the risks associated with benzodiazepine use with the patient and ask about the frequency of use. Discuss with the patient a slow taper, perhaps reducing the dose by 10% to 25% increments weekly to biweekly.43,44 Less motivated patients may require a slower taper, more time, or repeated discussions. When starting a dose reduction, notify the patient that some rebound anxiety or insomnia are to be expected. With any progress the patient makes toward reducing his usage, congratulate him on such progress.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Balon R, Fava GA, Rickels K. Need for a realistic appraisal of benzodiazepines. World Psychiatry. 2015;14(2):243-244.
3. Ashton CH. Benzodiazepine equivalence table. http://www.benzo.org.uk/bzequiv.htm. Revised April 2007. Accessed May 3, 2017.
4. National Institute on Drug Abuse. Commonly abused drugs. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/commonly_abused_drugs_3.pdf. Revised January 2016. Accessed January 9, 2018.
5. Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74-89.
6. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed May 3, 2017.
7. Salzman C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148(2):151-152.
8. Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry. 2017;74(7):747-755.
9. O’Brien PL, Karnell LH, Gokhale M, et al. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223-230.
10. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry. 2002;63(12):1183-1184.
11. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57(9):390-394.
12. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed May 3, 2017.
13. Abdel-Ahad P, Kazour F. Non-antidepressant pharmacological treatment of obsessive compulsive disorder: a comprehensive review. Curr Clin Pharmacol. 2015;10(2):97-111.
14. Gardner DL, Cowdry RW. Alprazolam-induced dyscontrol in borderline personality disorder. Am J Psychiatry. 1985;142(1):98-100.
15. Ciccone DS, Just N, Bandilla EB, et al. Psychological correlates of opioid use in patients with chronic nonmalignant pain: a preliminary test of the downhill spiral hypothesis. J Pain Symptom Manage. 2000;20(3):180-192.
16. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332-340.
17. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-170.
18. Taipale H, Tolppanen AM, Koponen M, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519-E529.
19. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatric Serv. 2002;53:39-49.
20. U.S. National Library of Medicine, TOXNET Toxicology Data Network. Lactmed: alprazolam. http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+335. Accessed May 3, 2017.
21. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
22. Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48(6):682-685.
23. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952-1960.
24. Bolton JM, Morin SN, Majumdar SR, et al. Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatry. 2017;74(6):641-648.
25. Pariente A, Dartiques JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61-70.
26. Lagnaoui R, Tournier M, Moride Y, et al. The risk of cognitive impairment in older community-dwelling women after benzodiazepine use. Age Ageing. 2009;38(2):226-228.
27. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231.
28. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
29. Maust DT, Kales HC, Wiechers IR, et al. No end in sight: benzodiazepine use in older adults in the United States. J Am Geriatr Soc. 2016;64(12):2546-2553.
30. Maust DT, Blow FC, Wiechers IR, et al. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363-e371.
31. Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663-673.
32. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
33. Fontanella CA, Campo JV, Phillips GS, et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry. 2016;77(5):661-667.
34. McCall WV, Benca RM, Rosenguist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25.
35. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688.
36. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Updated September 2017. Accessed January 8, 2018.
37. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1-49.
38. U.S. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Published August 31, 2016. Accessed May 3, 2017.
39. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety/ucm518473.htm. Published August 31, 2016. Accessed May 3, 2017.
40. National Institute for Health and Care Excellence. Controlled drugs: safe use and management. https://www.nice.org.uk/guidance/ng46/evidence/full-guideline-pdf-2427186353. Published April 2016. Accessed July 25, 2017.
41. Stahl SM. Anxiety disorders and anxiolytics. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:721-772.
42. Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919-934.
43. Nardi AE, Freire RC, Valença AM, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. 2010;30(3):290-293.
44. Tampi R. How to wean geriatric patients off benzodiazepines. Psychiatric News. http://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2016.PP3b6. Published March 18, 2016. Accessed May 3, 2017.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Balon R, Fava GA, Rickels K. Need for a realistic appraisal of benzodiazepines. World Psychiatry. 2015;14(2):243-244.
3. Ashton CH. Benzodiazepine equivalence table. http://www.benzo.org.uk/bzequiv.htm. Revised April 2007. Accessed May 3, 2017.
4. National Institute on Drug Abuse. Commonly abused drugs. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/commonly_abused_drugs_3.pdf. Revised January 2016. Accessed January 9, 2018.
5. Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74-89.
6. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed May 3, 2017.
7. Salzman C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148(2):151-152.
8. Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry. 2017;74(7):747-755.
9. O’Brien PL, Karnell LH, Gokhale M, et al. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223-230.
10. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry. 2002;63(12):1183-1184.
11. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57(9):390-394.
12. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed May 3, 2017.
13. Abdel-Ahad P, Kazour F. Non-antidepressant pharmacological treatment of obsessive compulsive disorder: a comprehensive review. Curr Clin Pharmacol. 2015;10(2):97-111.
14. Gardner DL, Cowdry RW. Alprazolam-induced dyscontrol in borderline personality disorder. Am J Psychiatry. 1985;142(1):98-100.
15. Ciccone DS, Just N, Bandilla EB, et al. Psychological correlates of opioid use in patients with chronic nonmalignant pain: a preliminary test of the downhill spiral hypothesis. J Pain Symptom Manage. 2000;20(3):180-192.
16. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332-340.
17. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-170.
18. Taipale H, Tolppanen AM, Koponen M, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519-E529.
19. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatric Serv. 2002;53:39-49.
20. U.S. National Library of Medicine, TOXNET Toxicology Data Network. Lactmed: alprazolam. http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+335. Accessed May 3, 2017.
21. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
22. Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48(6):682-685.
23. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952-1960.
24. Bolton JM, Morin SN, Majumdar SR, et al. Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatry. 2017;74(6):641-648.
25. Pariente A, Dartiques JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61-70.
26. Lagnaoui R, Tournier M, Moride Y, et al. The risk of cognitive impairment in older community-dwelling women after benzodiazepine use. Age Ageing. 2009;38(2):226-228.
27. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231.
28. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
29. Maust DT, Kales HC, Wiechers IR, et al. No end in sight: benzodiazepine use in older adults in the United States. J Am Geriatr Soc. 2016;64(12):2546-2553.
30. Maust DT, Blow FC, Wiechers IR, et al. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363-e371.
31. Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663-673.
32. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
33. Fontanella CA, Campo JV, Phillips GS, et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry. 2016;77(5):661-667.
34. McCall WV, Benca RM, Rosenguist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25.
35. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688.
36. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Updated September 2017. Accessed January 8, 2018.
37. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1-49.
38. U.S. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Published August 31, 2016. Accessed May 3, 2017.
39. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety/ucm518473.htm. Published August 31, 2016. Accessed May 3, 2017.
40. National Institute for Health and Care Excellence. Controlled drugs: safe use and management. https://www.nice.org.uk/guidance/ng46/evidence/full-guideline-pdf-2427186353. Published April 2016. Accessed July 25, 2017.
41. Stahl SM. Anxiety disorders and anxiolytics. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:721-772.
42. Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919-934.
43. Nardi AE, Freire RC, Valença AM, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. 2010;30(3):290-293.
44. Tampi R. How to wean geriatric patients off benzodiazepines. Psychiatric News. http://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2016.PP3b6. Published March 18, 2016. Accessed May 3, 2017.
Compulsive sexual behavior: A nonjudgmental approach
Compulsive sexual behavior (CSB), also referred to as sexual addiction or hypersexuality, is characterized by repetitive and intense preoccupations with sexual fantasies, urges, and behaviors that are distressing to the individual and/or result in psychosocial impairment. Individuals with CSB often perceive their sexual behavior to be excessive but are unable to control it. CSB can involve fantasies and urges in addition to or in place of the behavior but must cause clinically significant distress and interference in daily life to qualify as a disorder.
Because of the lack of large-scale, population-based epidemiological studies assessing CSB, its true prevalence among adults is unknown. A study of 204 psychiatric inpatients found a current prevalence of 4.4%,1 while a university-based survey estimated the prevalence of CSB at approximately 2%.2 Others have estimated that the prevalence is between 3% to 6% of adults in the United States,3,4 with males comprising the majority (≥80%) of affected individuals.5
CSB usually develops during late adolescence/early adulthood, and most who present for treatment are male.5 Mood states, including depression, happiness, and loneliness, may trigger CSB.6 Many individuals report feelings of dissociation while engaging in CSB-related behaviors, whereas others report feeling important, powerful, excited, or gratified.
Why CSB is difficult to diagnose
Although CSB may be common, it usually goes undiagnosed. This potentially problematic behavior often is not diagnosed because of:
- Shame and secrecy. Embarrassment and shame, which are fundamental to CSB, appear to explain, in part, why few patients volunteer information regarding this behavior unless specifically asked.1
- Patient lack of knowledge. Patients often do not know that their behavior can be successfully treated.
- Clinician lack of knowledge. Few health care professionals have education or training in CSB. A lack of recognition of CSB also may be due to our limited understanding regarding the limits of sexual normality. In addition, the classification of CSB is unclear and not agreed upon (Box7-9), and moral judgments often are involved in understanding sexual behaviors.10
Box
Classifying compulsive sexual behavior
No consensus on diagnostic criteria
Accurately diagnosing CSB is difficult because of a lack of consensus about the diagnostic criteria for the disorder. Christenson et al11 developed an early set of criteria for CSB as part of a larger survey of impulse control disorders. They used the following 2 criteria to diagnose CSB: (1) excessive or uncontrolled sexual behavior(s) or sexual thoughts/urges to engage in behavior, and (2) these behaviors or thoughts/urges lead to significant distress, social or occupational impairment, or legal and financial consequences.11,12
During the DSM-5 revision process, a second approach to the diagnostic criteria was proposed for hypersexuality disorder. Under the proposed criteria for hypersexuality, a person would meet the diagnosis if ≥3 of the following were endorsed over a 6-month period: (a) time consumed by sexual fantasies, urges, or behaviors repetitively interferes with other important (non-sexual) goals, activities, and obligations; (b) repetitively engaging in sexual fantasies, urges, or behaviors in response to dysphoric mood states; (c) repetitively engaging in sexual fantasies, urges, or behaviors in response to stressful life events; (d) repetitive but unsuccessful efforts to control or significantly reduce these sexual fantasies, urges, or behaviors; and (e) repetitively engaging in sexual behaviors while disregarding the risk for physical or emotional harm to self or others.9
These 2 proposed approaches to diagnosis are somewhat similar. Both suggest that the core underlying issues involve sexual urges or behaviors that are difficult to control and that lead to psychosocial dysfunction. Differences in the criteria, however, could result in different rates of CSB diagnosis; therefore, further research will need to determine which diagnostic approach reflects the neurobiology underlying CSB.
Avoid misdiagnosis
Before making a diagnosis of CSB, it is important for clinicians to consider whether they are stigmatizing “negative consequences,” distress, or social impairment based on unconscious bias toward certain sexual behaviors. In addition, we need to ensure that we are not holding sex to different standards than other behaviors (for example, there are many things in life we do that result in negative consequences and yet do not classify as a mental disorder, such as indulging in less healthy food choices). Furthermore, excessive sexual behaviors might be associated with the normal coming out process for LGBTQ individuals, partner relationship problems, or sexual/gender identity. Therefore, the behavior needs to be assessed in the context of these psychosocial environmental factors.
Differential diagnosis
Various psychiatric disorders also may include excessive sexual behavior as part of their clinical presentation, and it is important to differentiate that behavior from CSB.
Bipolar disorder. Excessive sexual behavior can occur as part of a manic episode in bipolar disorder. If the problematic sexual behavior also occurs when the person’s mood is stable, the individual may have CSB and bipolar disorder. This distinction is important because the treatment for bipolar disorder is often different for CSB, because anticonvulsants have only case reports attesting to their use in CSB.
Substance abuse. Excessive sexual behavior can occur when a person is abusing substances, particularly stimulants such as cocaine and amphetamines.13 If the sexual behavior does not occur when the person is not using drugs, then the appropriate diagnosis would not likely be CSB.
Obsessive-compulsive disorder (OCD). Individuals with OCD often are preoccupied with sexual themes and feel that they think about sex excessively.14 Although patients with OCD may be preoccupied with thoughts of sex, the key difference is that persons with CSB report feeling excited by these thoughts and derive pleasure from the behavior, whereas the sexual thoughts of OCD are perceived as unpleasant.
Other disorders that may give rise to hypersexual behavior include neurocognitive disorders, attention-deficit/hyperactivity disorder, autism spectrum disorders, and depressive disorders.
Adverse effects of medication. It is important to ask the patient whether he (she)developed CSB after starting a medication. Certain medications (eg, medications for Parkinson’s disease or restless leg syndrome, or aripiprazole to treat depression or psychosis) may cause patients to engage in problematic sexual behavior.15,16 If the sexual behavior decreases or stops when the medication dosage is reduced or the medication is stopped, a diagnosis of CSB would not be appropriate.
Comorbidity is common
Research suggests that approximately one-half of adults with CSB meet criteria for at least 1 other psychiatric disorder, such as mood, anxiety, substance use, impulse control, or personality disorders. A study of men with CSB (N = 103) found that 71% met criteria for a mood disorder, 40% for an anxiety disorder, 41% for a substance use disorder, and 24% for an impulse control disorder such as gambling disorder.17 Therefore, to successfully treat CSB, clinicians also may need to focus on how and to what extent these co-occurring disorders drive the sexual behavior.
Co-occurring medical conditions also are common among individuals with CSB. Medical concerns may include unwanted pregnancy, sexually transmitted infections, and HIV/AIDS. Thus, treating psychiatric comorbidities and providing education about sexual health, with referrals to primary care specialists, often are part of CSB treatment.
Neuroimaging and cognition
One imaging study that compared participants with and without CSB found that participants with CSB had higher activity in the ventral striatum, anterior cingulate cortex, and amygdala relative to controls during a cue-reactivity functional MRI task.18 These findings show notable similarities to the patterns of activation seen in patients addicted to drugs when assessed using drug-craving paradigms. An additional neuroimaging study assessing patients with hypersexuality using diffusion tensor imaging noted that diffusivity in a prefrontal white matter tract within a superior frontal region was greater in patients with CSB.18 This study also indicated that there was a negative correlation between observed diffusion in the noted location and overall severity score for CSB symptoms such as frequency of urges or behaviors.
In terms of cognition, a preliminary assessment of young adults with CSB compared with healthy controls did not find any differences between groups across several tasks, although the previously mentioned diffusion tensor imaging study reported elevated impulsivity in CSB.18
Approaches to treatment
Most people with CSB are reluctant to mention it to their health care providers, and most physicians are generally uncomfortable talking about sex with their patients, in part, because of a lack of training.19 Patients are more likely to bring up the topic when they are receiving treatment for anxiety, depression, or substance abuse. Therefore, clinicians must consider that sexual behavior might be associated with a coping mechanism, distressing outcome, or comorbid condition in these patients.
Pharmacologic treatment
Evidence for the pharmacologic treatment of CSB consists primarily of small, open-label studies, case series, or retrospective analyses, except for 1 double-blind, placebo-controlled study. Based on this evidence, there may be several pharmacologic treatment options for patients with CSB; however, there are no FDA-approved medications for CSB.
Antidepressants. One of the most thoroughly documented categories of pharmacologic treatment for CSB is selective serotonin reuptake inhibitors (SSRIs). Several retrospective analyses and case series have reported on the general efficacy of SSRIs in reducing symptoms of CSB.20-23 Citalopram, the only treatment for CSB that has been assessed using a double-blind, placebo-controlled methodology, was associated with significant decreases in CSB symptoms, including sexual desire/drive, frequency of masturbation, and pornography use.24
In addition to SSRIs, several additional case reports have suggested that other classes of antidepressants, such as serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants, or stimulants may be beneficial when treating CSB.25 Several case reports have indicated significant improvement of CSB symptoms using clomipramine.22 A retrospective study of nefazodone also has suggested that it may be an option for treating CSB. Patients reported notable reductions in the frequency of sexual obsessions/compulsions while taking nefazodone and reported no notable sexual adverse effects.26 One branded version of nefazodone, Serzone, was associated with rare but severe liver problems and was withdrawn from the U.S. market in 2004.
Although some initial evidence regarding antidepressant use, particularly SSRIs, to treat CSB has suggested that these medications may be potentially beneficial, the findings are far from conclusive, with only 1 controlled trial and only single-subject case reports for many of the medications studied.
Naltrexone, an opioid antagonist, has received support from available cases, open-label studies, and retrospective analyses.17,27 Although evidence for the use of naltrexone in CSB is limited to case reports and retrospective analyses, results have been positive. Naltrexone has shown notable decreases in CSB symptom severity when used as monotherapy and when used in combination with other treatments.
Anticonvulsants. Several case reports have suggested that certain anticonvulsants may be beneficial for treating CSB.
Psychotherapy
Evidence supporting specific types of psychotherapy for CSB is limited and largely drawn from uncontrolled studies and case reports.
Cognitive-behavioral therapy (CBT) is one of the more common psychotherapeutic options used for CSB. Several uncontrolled studies and case reports have found that CBT is beneficial for CSB, although methodologies have varied.
Several cases found that combining CBT with motivational interviewing was associated with significant reductions in sexual behaviors, such as frequency of sexual partners and amount of time spent online during work hours.29,30 Group CBT also has been shown to be effective for CSB.31
Acceptance and commitment therapy (ACT) has received some initial support, with 1 uncontrolled study and 1 controlled study.32,33 The controlled study used 12 sessions of individual ACT compared with a wait-list condition.32 Improvements in CSB symptoms were maintained for 3 months. The overall reduction in problematic Internet pornography use was reported as 92% immediately after the study ended, and 86% after 3 months.
Marital/relationship therapy has been used successfully in several case series and case reports, although no studies have assessed its efficacy in treating CSB using a randomized protocol. In 1 case report, the researcher found that participation in marital sex therapy elicited notable improvements over the course of 1 year and 20 sessions.34
1. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
2. Odlaug BL, Lust K, Schreiber LR, et al. Compulsive sexual behavior in young adults. Ann Clin Psychiatry. 2013;25(3):193-200.
3. Black DW. Compulsive sexual behavior: a review. J Psychiatr Pract. 1998;4(4):219-229.
4. Coleman E. Is your patient suffering from compulsive sexual behavior? Psychiatr Ann. 1992;22(6):320-325.
5. Kaplan MS, Krueger RB. Diagnosis, assessment, and treatment of hypersexuality. J Sex Res. 2010;47(2):181-198.
6. Black DW, Kehrberg LL, Flumerfelt DL, et al. Characteristics of 36 subjects reporting compulsive sexual behavior. Am J Psychiatry. 1997;154(2):243-249.
7. McElroy SL, Phillips KA, Keck PE Jr. Obsessive compulsive spectrum disorder. J Clin Psychiatry. 1994;(suppl 55):33-51; discussion 52-53.
8. McElroy SL, Pope HG Jr, Keck PE Jr, et al. Are impulse-control disorders related to bipolar disorder? Compr Psychiatry. 1996;37(4):229-240.
9. Kafka MP. Hypersexual disorder: a proposed diagnosis for DSM-V. Arch Sex Behav. 2010;39(2):377-400.
10. Levine SB. What is sexual addiction? J Sex Marital Ther. 2010;36(3):261-275.
11. Christenson GA, Faber RJ, de Zwaan M, et al. Compulsive buying: descriptive characteristics and psychiatric comorbidity. J Clin Psychiatry. 1994;55(1):5-11.
12. Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W.W. Norton & Company, Inc.; 2008.
13. Frohmader KS, Lehman MN, Laviolette SR, et al. Concurrent exposure to methamphetamine and sexual behavior enhances subsequent drug reward and causes compulsive sexual behavior in male rats. J Neurosci. 2011;31(45):16473-16482.
14. Grant JE, Pinto A, Gunnip M, et al. Sexual obsessions and clinical correlates in adults with obsessive-compulsive disorder. Compr Psychiatry. 2006;47(5):325-329.
15. Mété D, Dafreville C, Paitel V, et al. Aripiprazole, gambling disorder and compulsive sexuality [in French]. Encephale. 2016;42(3):281-283.
16. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
17. Kraus SW, Meshberg-Cohen S, Martino S, et al. Treatment of compulsive pornography use with naltrexone: a case report. Am J Psychiatry. 2015;172(12):1260-1261.
18. Derbyshire KL, Grant JE. Compulsive sexual behavior: a review of the literature. J Behav Addict. 2015;4(2):37-43.
19. Levine SB, Scott DL. Sexual education for psychiatric residents. Acad Psychiatry. 2010;34(5):349-352.
20. Alsughier N. Compulsive masturbation treated with selective serotonin reuptake inhibitors. African J Psychiatry (Johannesbg). 2015;18:299.
21. Elmore JL. SSRI reduction of nonparaphilic sexual addiction. CNS Spectr. 2000;5(11);53-56.
22. Stein DJ, Hollander E, Anthony DT, et al. Serotonergic medications for sexual obsessions, sexual addictions, and paraphilias. J Clinical Psychiatry. 1992;53(8):267-271.
23. Kafka M. Psychopharmacologic treatments for nonparaphilic compulsive sexual behaviors. CNS Spectr. 200;5(1):49-59.
24. Wainberg ML, Muench F, Morgenstern J, et al. A double-blind study of citalopram versus placebo in the treatment of compulsive sexual behaviors in gay and bisexual men. J Clin Psychiatry. 2006;67(12):1968-1973.
25. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
26. Coleman E, Raymond N, McBean A. Assessment and treatment of compulsive sexual behavior. Minn Med. 2003;86(7):42-47.
27. Raymond NC, Grant JE, Coleman E. Augmentation with naltrexone to treat compulsive sexual behavior: a case series. Ann Clin Psychiatry. 2010;22(1):56-62.
28. Fong TW, De La Garza R 2nd, Newton TF. A case report of topiramate in the treatment of nonparaphilic sexual addiction. J Clin Psychopharmacol. 2005;25(5):512-514.
29. Del Giudice MJ, Kutinsky J. Applying motivational interviewing to the treatment of sexual compulsivity and addiction. Sex Addict Comp. 2007;14(4):303-319.
30. Shepherd L. Cognitive behavior therapy for sexually addictive behavior. Clin Case Stud. 2010;9(1):18-27.
31. Sadiza J, Varma R, Jena SPK, et al. Group cognitive behaviour therapy in the management of compulsive sex behaviour. International Journal of Criminal Justice Sciences. 2011;6(1-2):309-325.
32. Crosby JM, Twohig MP. Acceptance and commitment therapy for problematic Internet pornography use: a randomized trial. Behav Ther. 2016;47(3):355-366.
33. Twohig MP, Crosby JM. Acceptance and commitment therapy as a treatment for problematic internet pornography viewing. Behav Ther. 2010;41(3):285-295.
34. Sprenkle DH. Treating a sex addict through marital sex therapy. Fam Relat. 1987;36(1):11-14.
Compulsive sexual behavior (CSB), also referred to as sexual addiction or hypersexuality, is characterized by repetitive and intense preoccupations with sexual fantasies, urges, and behaviors that are distressing to the individual and/or result in psychosocial impairment. Individuals with CSB often perceive their sexual behavior to be excessive but are unable to control it. CSB can involve fantasies and urges in addition to or in place of the behavior but must cause clinically significant distress and interference in daily life to qualify as a disorder.
Because of the lack of large-scale, population-based epidemiological studies assessing CSB, its true prevalence among adults is unknown. A study of 204 psychiatric inpatients found a current prevalence of 4.4%,1 while a university-based survey estimated the prevalence of CSB at approximately 2%.2 Others have estimated that the prevalence is between 3% to 6% of adults in the United States,3,4 with males comprising the majority (≥80%) of affected individuals.5
CSB usually develops during late adolescence/early adulthood, and most who present for treatment are male.5 Mood states, including depression, happiness, and loneliness, may trigger CSB.6 Many individuals report feelings of dissociation while engaging in CSB-related behaviors, whereas others report feeling important, powerful, excited, or gratified.
Why CSB is difficult to diagnose
Although CSB may be common, it usually goes undiagnosed. This potentially problematic behavior often is not diagnosed because of:
- Shame and secrecy. Embarrassment and shame, which are fundamental to CSB, appear to explain, in part, why few patients volunteer information regarding this behavior unless specifically asked.1
- Patient lack of knowledge. Patients often do not know that their behavior can be successfully treated.
- Clinician lack of knowledge. Few health care professionals have education or training in CSB. A lack of recognition of CSB also may be due to our limited understanding regarding the limits of sexual normality. In addition, the classification of CSB is unclear and not agreed upon (Box7-9), and moral judgments often are involved in understanding sexual behaviors.10
Box
Classifying compulsive sexual behavior
No consensus on diagnostic criteria
Accurately diagnosing CSB is difficult because of a lack of consensus about the diagnostic criteria for the disorder. Christenson et al11 developed an early set of criteria for CSB as part of a larger survey of impulse control disorders. They used the following 2 criteria to diagnose CSB: (1) excessive or uncontrolled sexual behavior(s) or sexual thoughts/urges to engage in behavior, and (2) these behaviors or thoughts/urges lead to significant distress, social or occupational impairment, or legal and financial consequences.11,12
During the DSM-5 revision process, a second approach to the diagnostic criteria was proposed for hypersexuality disorder. Under the proposed criteria for hypersexuality, a person would meet the diagnosis if ≥3 of the following were endorsed over a 6-month period: (a) time consumed by sexual fantasies, urges, or behaviors repetitively interferes with other important (non-sexual) goals, activities, and obligations; (b) repetitively engaging in sexual fantasies, urges, or behaviors in response to dysphoric mood states; (c) repetitively engaging in sexual fantasies, urges, or behaviors in response to stressful life events; (d) repetitive but unsuccessful efforts to control or significantly reduce these sexual fantasies, urges, or behaviors; and (e) repetitively engaging in sexual behaviors while disregarding the risk for physical or emotional harm to self or others.9
These 2 proposed approaches to diagnosis are somewhat similar. Both suggest that the core underlying issues involve sexual urges or behaviors that are difficult to control and that lead to psychosocial dysfunction. Differences in the criteria, however, could result in different rates of CSB diagnosis; therefore, further research will need to determine which diagnostic approach reflects the neurobiology underlying CSB.
Avoid misdiagnosis
Before making a diagnosis of CSB, it is important for clinicians to consider whether they are stigmatizing “negative consequences,” distress, or social impairment based on unconscious bias toward certain sexual behaviors. In addition, we need to ensure that we are not holding sex to different standards than other behaviors (for example, there are many things in life we do that result in negative consequences and yet do not classify as a mental disorder, such as indulging in less healthy food choices). Furthermore, excessive sexual behaviors might be associated with the normal coming out process for LGBTQ individuals, partner relationship problems, or sexual/gender identity. Therefore, the behavior needs to be assessed in the context of these psychosocial environmental factors.
Differential diagnosis
Various psychiatric disorders also may include excessive sexual behavior as part of their clinical presentation, and it is important to differentiate that behavior from CSB.
Bipolar disorder. Excessive sexual behavior can occur as part of a manic episode in bipolar disorder. If the problematic sexual behavior also occurs when the person’s mood is stable, the individual may have CSB and bipolar disorder. This distinction is important because the treatment for bipolar disorder is often different for CSB, because anticonvulsants have only case reports attesting to their use in CSB.
Substance abuse. Excessive sexual behavior can occur when a person is abusing substances, particularly stimulants such as cocaine and amphetamines.13 If the sexual behavior does not occur when the person is not using drugs, then the appropriate diagnosis would not likely be CSB.
Obsessive-compulsive disorder (OCD). Individuals with OCD often are preoccupied with sexual themes and feel that they think about sex excessively.14 Although patients with OCD may be preoccupied with thoughts of sex, the key difference is that persons with CSB report feeling excited by these thoughts and derive pleasure from the behavior, whereas the sexual thoughts of OCD are perceived as unpleasant.
Other disorders that may give rise to hypersexual behavior include neurocognitive disorders, attention-deficit/hyperactivity disorder, autism spectrum disorders, and depressive disorders.
Adverse effects of medication. It is important to ask the patient whether he (she)developed CSB after starting a medication. Certain medications (eg, medications for Parkinson’s disease or restless leg syndrome, or aripiprazole to treat depression or psychosis) may cause patients to engage in problematic sexual behavior.15,16 If the sexual behavior decreases or stops when the medication dosage is reduced or the medication is stopped, a diagnosis of CSB would not be appropriate.
Comorbidity is common
Research suggests that approximately one-half of adults with CSB meet criteria for at least 1 other psychiatric disorder, such as mood, anxiety, substance use, impulse control, or personality disorders. A study of men with CSB (N = 103) found that 71% met criteria for a mood disorder, 40% for an anxiety disorder, 41% for a substance use disorder, and 24% for an impulse control disorder such as gambling disorder.17 Therefore, to successfully treat CSB, clinicians also may need to focus on how and to what extent these co-occurring disorders drive the sexual behavior.
Co-occurring medical conditions also are common among individuals with CSB. Medical concerns may include unwanted pregnancy, sexually transmitted infections, and HIV/AIDS. Thus, treating psychiatric comorbidities and providing education about sexual health, with referrals to primary care specialists, often are part of CSB treatment.
Neuroimaging and cognition
One imaging study that compared participants with and without CSB found that participants with CSB had higher activity in the ventral striatum, anterior cingulate cortex, and amygdala relative to controls during a cue-reactivity functional MRI task.18 These findings show notable similarities to the patterns of activation seen in patients addicted to drugs when assessed using drug-craving paradigms. An additional neuroimaging study assessing patients with hypersexuality using diffusion tensor imaging noted that diffusivity in a prefrontal white matter tract within a superior frontal region was greater in patients with CSB.18 This study also indicated that there was a negative correlation between observed diffusion in the noted location and overall severity score for CSB symptoms such as frequency of urges or behaviors.
In terms of cognition, a preliminary assessment of young adults with CSB compared with healthy controls did not find any differences between groups across several tasks, although the previously mentioned diffusion tensor imaging study reported elevated impulsivity in CSB.18
Approaches to treatment
Most people with CSB are reluctant to mention it to their health care providers, and most physicians are generally uncomfortable talking about sex with their patients, in part, because of a lack of training.19 Patients are more likely to bring up the topic when they are receiving treatment for anxiety, depression, or substance abuse. Therefore, clinicians must consider that sexual behavior might be associated with a coping mechanism, distressing outcome, or comorbid condition in these patients.
Pharmacologic treatment
Evidence for the pharmacologic treatment of CSB consists primarily of small, open-label studies, case series, or retrospective analyses, except for 1 double-blind, placebo-controlled study. Based on this evidence, there may be several pharmacologic treatment options for patients with CSB; however, there are no FDA-approved medications for CSB.
Antidepressants. One of the most thoroughly documented categories of pharmacologic treatment for CSB is selective serotonin reuptake inhibitors (SSRIs). Several retrospective analyses and case series have reported on the general efficacy of SSRIs in reducing symptoms of CSB.20-23 Citalopram, the only treatment for CSB that has been assessed using a double-blind, placebo-controlled methodology, was associated with significant decreases in CSB symptoms, including sexual desire/drive, frequency of masturbation, and pornography use.24
In addition to SSRIs, several additional case reports have suggested that other classes of antidepressants, such as serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants, or stimulants may be beneficial when treating CSB.25 Several case reports have indicated significant improvement of CSB symptoms using clomipramine.22 A retrospective study of nefazodone also has suggested that it may be an option for treating CSB. Patients reported notable reductions in the frequency of sexual obsessions/compulsions while taking nefazodone and reported no notable sexual adverse effects.26 One branded version of nefazodone, Serzone, was associated with rare but severe liver problems and was withdrawn from the U.S. market in 2004.
Although some initial evidence regarding antidepressant use, particularly SSRIs, to treat CSB has suggested that these medications may be potentially beneficial, the findings are far from conclusive, with only 1 controlled trial and only single-subject case reports for many of the medications studied.
Naltrexone, an opioid antagonist, has received support from available cases, open-label studies, and retrospective analyses.17,27 Although evidence for the use of naltrexone in CSB is limited to case reports and retrospective analyses, results have been positive. Naltrexone has shown notable decreases in CSB symptom severity when used as monotherapy and when used in combination with other treatments.
Anticonvulsants. Several case reports have suggested that certain anticonvulsants may be beneficial for treating CSB.
Psychotherapy
Evidence supporting specific types of psychotherapy for CSB is limited and largely drawn from uncontrolled studies and case reports.
Cognitive-behavioral therapy (CBT) is one of the more common psychotherapeutic options used for CSB. Several uncontrolled studies and case reports have found that CBT is beneficial for CSB, although methodologies have varied.
Several cases found that combining CBT with motivational interviewing was associated with significant reductions in sexual behaviors, such as frequency of sexual partners and amount of time spent online during work hours.29,30 Group CBT also has been shown to be effective for CSB.31
Acceptance and commitment therapy (ACT) has received some initial support, with 1 uncontrolled study and 1 controlled study.32,33 The controlled study used 12 sessions of individual ACT compared with a wait-list condition.32 Improvements in CSB symptoms were maintained for 3 months. The overall reduction in problematic Internet pornography use was reported as 92% immediately after the study ended, and 86% after 3 months.
Marital/relationship therapy has been used successfully in several case series and case reports, although no studies have assessed its efficacy in treating CSB using a randomized protocol. In 1 case report, the researcher found that participation in marital sex therapy elicited notable improvements over the course of 1 year and 20 sessions.34
Compulsive sexual behavior (CSB), also referred to as sexual addiction or hypersexuality, is characterized by repetitive and intense preoccupations with sexual fantasies, urges, and behaviors that are distressing to the individual and/or result in psychosocial impairment. Individuals with CSB often perceive their sexual behavior to be excessive but are unable to control it. CSB can involve fantasies and urges in addition to or in place of the behavior but must cause clinically significant distress and interference in daily life to qualify as a disorder.
Because of the lack of large-scale, population-based epidemiological studies assessing CSB, its true prevalence among adults is unknown. A study of 204 psychiatric inpatients found a current prevalence of 4.4%,1 while a university-based survey estimated the prevalence of CSB at approximately 2%.2 Others have estimated that the prevalence is between 3% to 6% of adults in the United States,3,4 with males comprising the majority (≥80%) of affected individuals.5
CSB usually develops during late adolescence/early adulthood, and most who present for treatment are male.5 Mood states, including depression, happiness, and loneliness, may trigger CSB.6 Many individuals report feelings of dissociation while engaging in CSB-related behaviors, whereas others report feeling important, powerful, excited, or gratified.
Why CSB is difficult to diagnose
Although CSB may be common, it usually goes undiagnosed. This potentially problematic behavior often is not diagnosed because of:
- Shame and secrecy. Embarrassment and shame, which are fundamental to CSB, appear to explain, in part, why few patients volunteer information regarding this behavior unless specifically asked.1
- Patient lack of knowledge. Patients often do not know that their behavior can be successfully treated.
- Clinician lack of knowledge. Few health care professionals have education or training in CSB. A lack of recognition of CSB also may be due to our limited understanding regarding the limits of sexual normality. In addition, the classification of CSB is unclear and not agreed upon (Box7-9), and moral judgments often are involved in understanding sexual behaviors.10
Box
Classifying compulsive sexual behavior
No consensus on diagnostic criteria
Accurately diagnosing CSB is difficult because of a lack of consensus about the diagnostic criteria for the disorder. Christenson et al11 developed an early set of criteria for CSB as part of a larger survey of impulse control disorders. They used the following 2 criteria to diagnose CSB: (1) excessive or uncontrolled sexual behavior(s) or sexual thoughts/urges to engage in behavior, and (2) these behaviors or thoughts/urges lead to significant distress, social or occupational impairment, or legal and financial consequences.11,12
During the DSM-5 revision process, a second approach to the diagnostic criteria was proposed for hypersexuality disorder. Under the proposed criteria for hypersexuality, a person would meet the diagnosis if ≥3 of the following were endorsed over a 6-month period: (a) time consumed by sexual fantasies, urges, or behaviors repetitively interferes with other important (non-sexual) goals, activities, and obligations; (b) repetitively engaging in sexual fantasies, urges, or behaviors in response to dysphoric mood states; (c) repetitively engaging in sexual fantasies, urges, or behaviors in response to stressful life events; (d) repetitive but unsuccessful efforts to control or significantly reduce these sexual fantasies, urges, or behaviors; and (e) repetitively engaging in sexual behaviors while disregarding the risk for physical or emotional harm to self or others.9
These 2 proposed approaches to diagnosis are somewhat similar. Both suggest that the core underlying issues involve sexual urges or behaviors that are difficult to control and that lead to psychosocial dysfunction. Differences in the criteria, however, could result in different rates of CSB diagnosis; therefore, further research will need to determine which diagnostic approach reflects the neurobiology underlying CSB.
Avoid misdiagnosis
Before making a diagnosis of CSB, it is important for clinicians to consider whether they are stigmatizing “negative consequences,” distress, or social impairment based on unconscious bias toward certain sexual behaviors. In addition, we need to ensure that we are not holding sex to different standards than other behaviors (for example, there are many things in life we do that result in negative consequences and yet do not classify as a mental disorder, such as indulging in less healthy food choices). Furthermore, excessive sexual behaviors might be associated with the normal coming out process for LGBTQ individuals, partner relationship problems, or sexual/gender identity. Therefore, the behavior needs to be assessed in the context of these psychosocial environmental factors.
Differential diagnosis
Various psychiatric disorders also may include excessive sexual behavior as part of their clinical presentation, and it is important to differentiate that behavior from CSB.
Bipolar disorder. Excessive sexual behavior can occur as part of a manic episode in bipolar disorder. If the problematic sexual behavior also occurs when the person’s mood is stable, the individual may have CSB and bipolar disorder. This distinction is important because the treatment for bipolar disorder is often different for CSB, because anticonvulsants have only case reports attesting to their use in CSB.
Substance abuse. Excessive sexual behavior can occur when a person is abusing substances, particularly stimulants such as cocaine and amphetamines.13 If the sexual behavior does not occur when the person is not using drugs, then the appropriate diagnosis would not likely be CSB.
Obsessive-compulsive disorder (OCD). Individuals with OCD often are preoccupied with sexual themes and feel that they think about sex excessively.14 Although patients with OCD may be preoccupied with thoughts of sex, the key difference is that persons with CSB report feeling excited by these thoughts and derive pleasure from the behavior, whereas the sexual thoughts of OCD are perceived as unpleasant.
Other disorders that may give rise to hypersexual behavior include neurocognitive disorders, attention-deficit/hyperactivity disorder, autism spectrum disorders, and depressive disorders.
Adverse effects of medication. It is important to ask the patient whether he (she)developed CSB after starting a medication. Certain medications (eg, medications for Parkinson’s disease or restless leg syndrome, or aripiprazole to treat depression or psychosis) may cause patients to engage in problematic sexual behavior.15,16 If the sexual behavior decreases or stops when the medication dosage is reduced or the medication is stopped, a diagnosis of CSB would not be appropriate.
Comorbidity is common
Research suggests that approximately one-half of adults with CSB meet criteria for at least 1 other psychiatric disorder, such as mood, anxiety, substance use, impulse control, or personality disorders. A study of men with CSB (N = 103) found that 71% met criteria for a mood disorder, 40% for an anxiety disorder, 41% for a substance use disorder, and 24% for an impulse control disorder such as gambling disorder.17 Therefore, to successfully treat CSB, clinicians also may need to focus on how and to what extent these co-occurring disorders drive the sexual behavior.
Co-occurring medical conditions also are common among individuals with CSB. Medical concerns may include unwanted pregnancy, sexually transmitted infections, and HIV/AIDS. Thus, treating psychiatric comorbidities and providing education about sexual health, with referrals to primary care specialists, often are part of CSB treatment.
Neuroimaging and cognition
One imaging study that compared participants with and without CSB found that participants with CSB had higher activity in the ventral striatum, anterior cingulate cortex, and amygdala relative to controls during a cue-reactivity functional MRI task.18 These findings show notable similarities to the patterns of activation seen in patients addicted to drugs when assessed using drug-craving paradigms. An additional neuroimaging study assessing patients with hypersexuality using diffusion tensor imaging noted that diffusivity in a prefrontal white matter tract within a superior frontal region was greater in patients with CSB.18 This study also indicated that there was a negative correlation between observed diffusion in the noted location and overall severity score for CSB symptoms such as frequency of urges or behaviors.
In terms of cognition, a preliminary assessment of young adults with CSB compared with healthy controls did not find any differences between groups across several tasks, although the previously mentioned diffusion tensor imaging study reported elevated impulsivity in CSB.18
Approaches to treatment
Most people with CSB are reluctant to mention it to their health care providers, and most physicians are generally uncomfortable talking about sex with their patients, in part, because of a lack of training.19 Patients are more likely to bring up the topic when they are receiving treatment for anxiety, depression, or substance abuse. Therefore, clinicians must consider that sexual behavior might be associated with a coping mechanism, distressing outcome, or comorbid condition in these patients.
Pharmacologic treatment
Evidence for the pharmacologic treatment of CSB consists primarily of small, open-label studies, case series, or retrospective analyses, except for 1 double-blind, placebo-controlled study. Based on this evidence, there may be several pharmacologic treatment options for patients with CSB; however, there are no FDA-approved medications for CSB.
Antidepressants. One of the most thoroughly documented categories of pharmacologic treatment for CSB is selective serotonin reuptake inhibitors (SSRIs). Several retrospective analyses and case series have reported on the general efficacy of SSRIs in reducing symptoms of CSB.20-23 Citalopram, the only treatment for CSB that has been assessed using a double-blind, placebo-controlled methodology, was associated with significant decreases in CSB symptoms, including sexual desire/drive, frequency of masturbation, and pornography use.24
In addition to SSRIs, several additional case reports have suggested that other classes of antidepressants, such as serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants, or stimulants may be beneficial when treating CSB.25 Several case reports have indicated significant improvement of CSB symptoms using clomipramine.22 A retrospective study of nefazodone also has suggested that it may be an option for treating CSB. Patients reported notable reductions in the frequency of sexual obsessions/compulsions while taking nefazodone and reported no notable sexual adverse effects.26 One branded version of nefazodone, Serzone, was associated with rare but severe liver problems and was withdrawn from the U.S. market in 2004.
Although some initial evidence regarding antidepressant use, particularly SSRIs, to treat CSB has suggested that these medications may be potentially beneficial, the findings are far from conclusive, with only 1 controlled trial and only single-subject case reports for many of the medications studied.
Naltrexone, an opioid antagonist, has received support from available cases, open-label studies, and retrospective analyses.17,27 Although evidence for the use of naltrexone in CSB is limited to case reports and retrospective analyses, results have been positive. Naltrexone has shown notable decreases in CSB symptom severity when used as monotherapy and when used in combination with other treatments.
Anticonvulsants. Several case reports have suggested that certain anticonvulsants may be beneficial for treating CSB.
Psychotherapy
Evidence supporting specific types of psychotherapy for CSB is limited and largely drawn from uncontrolled studies and case reports.
Cognitive-behavioral therapy (CBT) is one of the more common psychotherapeutic options used for CSB. Several uncontrolled studies and case reports have found that CBT is beneficial for CSB, although methodologies have varied.
Several cases found that combining CBT with motivational interviewing was associated with significant reductions in sexual behaviors, such as frequency of sexual partners and amount of time spent online during work hours.29,30 Group CBT also has been shown to be effective for CSB.31
Acceptance and commitment therapy (ACT) has received some initial support, with 1 uncontrolled study and 1 controlled study.32,33 The controlled study used 12 sessions of individual ACT compared with a wait-list condition.32 Improvements in CSB symptoms were maintained for 3 months. The overall reduction in problematic Internet pornography use was reported as 92% immediately after the study ended, and 86% after 3 months.
Marital/relationship therapy has been used successfully in several case series and case reports, although no studies have assessed its efficacy in treating CSB using a randomized protocol. In 1 case report, the researcher found that participation in marital sex therapy elicited notable improvements over the course of 1 year and 20 sessions.34
1. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
2. Odlaug BL, Lust K, Schreiber LR, et al. Compulsive sexual behavior in young adults. Ann Clin Psychiatry. 2013;25(3):193-200.
3. Black DW. Compulsive sexual behavior: a review. J Psychiatr Pract. 1998;4(4):219-229.
4. Coleman E. Is your patient suffering from compulsive sexual behavior? Psychiatr Ann. 1992;22(6):320-325.
5. Kaplan MS, Krueger RB. Diagnosis, assessment, and treatment of hypersexuality. J Sex Res. 2010;47(2):181-198.
6. Black DW, Kehrberg LL, Flumerfelt DL, et al. Characteristics of 36 subjects reporting compulsive sexual behavior. Am J Psychiatry. 1997;154(2):243-249.
7. McElroy SL, Phillips KA, Keck PE Jr. Obsessive compulsive spectrum disorder. J Clin Psychiatry. 1994;(suppl 55):33-51; discussion 52-53.
8. McElroy SL, Pope HG Jr, Keck PE Jr, et al. Are impulse-control disorders related to bipolar disorder? Compr Psychiatry. 1996;37(4):229-240.
9. Kafka MP. Hypersexual disorder: a proposed diagnosis for DSM-V. Arch Sex Behav. 2010;39(2):377-400.
10. Levine SB. What is sexual addiction? J Sex Marital Ther. 2010;36(3):261-275.
11. Christenson GA, Faber RJ, de Zwaan M, et al. Compulsive buying: descriptive characteristics and psychiatric comorbidity. J Clin Psychiatry. 1994;55(1):5-11.
12. Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W.W. Norton & Company, Inc.; 2008.
13. Frohmader KS, Lehman MN, Laviolette SR, et al. Concurrent exposure to methamphetamine and sexual behavior enhances subsequent drug reward and causes compulsive sexual behavior in male rats. J Neurosci. 2011;31(45):16473-16482.
14. Grant JE, Pinto A, Gunnip M, et al. Sexual obsessions and clinical correlates in adults with obsessive-compulsive disorder. Compr Psychiatry. 2006;47(5):325-329.
15. Mété D, Dafreville C, Paitel V, et al. Aripiprazole, gambling disorder and compulsive sexuality [in French]. Encephale. 2016;42(3):281-283.
16. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
17. Kraus SW, Meshberg-Cohen S, Martino S, et al. Treatment of compulsive pornography use with naltrexone: a case report. Am J Psychiatry. 2015;172(12):1260-1261.
18. Derbyshire KL, Grant JE. Compulsive sexual behavior: a review of the literature. J Behav Addict. 2015;4(2):37-43.
19. Levine SB, Scott DL. Sexual education for psychiatric residents. Acad Psychiatry. 2010;34(5):349-352.
20. Alsughier N. Compulsive masturbation treated with selective serotonin reuptake inhibitors. African J Psychiatry (Johannesbg). 2015;18:299.
21. Elmore JL. SSRI reduction of nonparaphilic sexual addiction. CNS Spectr. 2000;5(11);53-56.
22. Stein DJ, Hollander E, Anthony DT, et al. Serotonergic medications for sexual obsessions, sexual addictions, and paraphilias. J Clinical Psychiatry. 1992;53(8):267-271.
23. Kafka M. Psychopharmacologic treatments for nonparaphilic compulsive sexual behaviors. CNS Spectr. 200;5(1):49-59.
24. Wainberg ML, Muench F, Morgenstern J, et al. A double-blind study of citalopram versus placebo in the treatment of compulsive sexual behaviors in gay and bisexual men. J Clin Psychiatry. 2006;67(12):1968-1973.
25. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
26. Coleman E, Raymond N, McBean A. Assessment and treatment of compulsive sexual behavior. Minn Med. 2003;86(7):42-47.
27. Raymond NC, Grant JE, Coleman E. Augmentation with naltrexone to treat compulsive sexual behavior: a case series. Ann Clin Psychiatry. 2010;22(1):56-62.
28. Fong TW, De La Garza R 2nd, Newton TF. A case report of topiramate in the treatment of nonparaphilic sexual addiction. J Clin Psychopharmacol. 2005;25(5):512-514.
29. Del Giudice MJ, Kutinsky J. Applying motivational interviewing to the treatment of sexual compulsivity and addiction. Sex Addict Comp. 2007;14(4):303-319.
30. Shepherd L. Cognitive behavior therapy for sexually addictive behavior. Clin Case Stud. 2010;9(1):18-27.
31. Sadiza J, Varma R, Jena SPK, et al. Group cognitive behaviour therapy in the management of compulsive sex behaviour. International Journal of Criminal Justice Sciences. 2011;6(1-2):309-325.
32. Crosby JM, Twohig MP. Acceptance and commitment therapy for problematic Internet pornography use: a randomized trial. Behav Ther. 2016;47(3):355-366.
33. Twohig MP, Crosby JM. Acceptance and commitment therapy as a treatment for problematic internet pornography viewing. Behav Ther. 2010;41(3):285-295.
34. Sprenkle DH. Treating a sex addict through marital sex therapy. Fam Relat. 1987;36(1):11-14.
1. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
2. Odlaug BL, Lust K, Schreiber LR, et al. Compulsive sexual behavior in young adults. Ann Clin Psychiatry. 2013;25(3):193-200.
3. Black DW. Compulsive sexual behavior: a review. J Psychiatr Pract. 1998;4(4):219-229.
4. Coleman E. Is your patient suffering from compulsive sexual behavior? Psychiatr Ann. 1992;22(6):320-325.
5. Kaplan MS, Krueger RB. Diagnosis, assessment, and treatment of hypersexuality. J Sex Res. 2010;47(2):181-198.
6. Black DW, Kehrberg LL, Flumerfelt DL, et al. Characteristics of 36 subjects reporting compulsive sexual behavior. Am J Psychiatry. 1997;154(2):243-249.
7. McElroy SL, Phillips KA, Keck PE Jr. Obsessive compulsive spectrum disorder. J Clin Psychiatry. 1994;(suppl 55):33-51; discussion 52-53.
8. McElroy SL, Pope HG Jr, Keck PE Jr, et al. Are impulse-control disorders related to bipolar disorder? Compr Psychiatry. 1996;37(4):229-240.
9. Kafka MP. Hypersexual disorder: a proposed diagnosis for DSM-V. Arch Sex Behav. 2010;39(2):377-400.
10. Levine SB. What is sexual addiction? J Sex Marital Ther. 2010;36(3):261-275.
11. Christenson GA, Faber RJ, de Zwaan M, et al. Compulsive buying: descriptive characteristics and psychiatric comorbidity. J Clin Psychiatry. 1994;55(1):5-11.
12. Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W.W. Norton & Company, Inc.; 2008.
13. Frohmader KS, Lehman MN, Laviolette SR, et al. Concurrent exposure to methamphetamine and sexual behavior enhances subsequent drug reward and causes compulsive sexual behavior in male rats. J Neurosci. 2011;31(45):16473-16482.
14. Grant JE, Pinto A, Gunnip M, et al. Sexual obsessions and clinical correlates in adults with obsessive-compulsive disorder. Compr Psychiatry. 2006;47(5):325-329.
15. Mété D, Dafreville C, Paitel V, et al. Aripiprazole, gambling disorder and compulsive sexuality [in French]. Encephale. 2016;42(3):281-283.
16. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
17. Kraus SW, Meshberg-Cohen S, Martino S, et al. Treatment of compulsive pornography use with naltrexone: a case report. Am J Psychiatry. 2015;172(12):1260-1261.
18. Derbyshire KL, Grant JE. Compulsive sexual behavior: a review of the literature. J Behav Addict. 2015;4(2):37-43.
19. Levine SB, Scott DL. Sexual education for psychiatric residents. Acad Psychiatry. 2010;34(5):349-352.
20. Alsughier N. Compulsive masturbation treated with selective serotonin reuptake inhibitors. African J Psychiatry (Johannesbg). 2015;18:299.
21. Elmore JL. SSRI reduction of nonparaphilic sexual addiction. CNS Spectr. 2000;5(11);53-56.
22. Stein DJ, Hollander E, Anthony DT, et al. Serotonergic medications for sexual obsessions, sexual addictions, and paraphilias. J Clinical Psychiatry. 1992;53(8):267-271.
23. Kafka M. Psychopharmacologic treatments for nonparaphilic compulsive sexual behaviors. CNS Spectr. 200;5(1):49-59.
24. Wainberg ML, Muench F, Morgenstern J, et al. A double-blind study of citalopram versus placebo in the treatment of compulsive sexual behaviors in gay and bisexual men. J Clin Psychiatry. 2006;67(12):1968-1973.
25. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
26. Coleman E, Raymond N, McBean A. Assessment and treatment of compulsive sexual behavior. Minn Med. 2003;86(7):42-47.
27. Raymond NC, Grant JE, Coleman E. Augmentation with naltrexone to treat compulsive sexual behavior: a case series. Ann Clin Psychiatry. 2010;22(1):56-62.
28. Fong TW, De La Garza R 2nd, Newton TF. A case report of topiramate in the treatment of nonparaphilic sexual addiction. J Clin Psychopharmacol. 2005;25(5):512-514.
29. Del Giudice MJ, Kutinsky J. Applying motivational interviewing to the treatment of sexual compulsivity and addiction. Sex Addict Comp. 2007;14(4):303-319.
30. Shepherd L. Cognitive behavior therapy for sexually addictive behavior. Clin Case Stud. 2010;9(1):18-27.
31. Sadiza J, Varma R, Jena SPK, et al. Group cognitive behaviour therapy in the management of compulsive sex behaviour. International Journal of Criminal Justice Sciences. 2011;6(1-2):309-325.
32. Crosby JM, Twohig MP. Acceptance and commitment therapy for problematic Internet pornography use: a randomized trial. Behav Ther. 2016;47(3):355-366.
33. Twohig MP, Crosby JM. Acceptance and commitment therapy as a treatment for problematic internet pornography viewing. Behav Ther. 2010;41(3):285-295.
34. Sprenkle DH. Treating a sex addict through marital sex therapy. Fam Relat. 1987;36(1):11-14.
The toxic zeitgeist of hyper-partisanship: A psychiatric perspective
It is always judicious to avoid discussing religious or political issues because inevitably someone will be offended. As a lifetime member of the American Psychiatric Association, I adhere to its "Goldwater Rule," which proscribes the gratuitous diagnosis of any president absent of a formal face-to-face psychiatric evaluation. But it is perfectly permissible to express a psychiatric opinion about the contemporary national political scene.
Frankly, the status of the political arena has become ugly. This should not be surprising, given that at its core, politics is an unquenchable thirst for power, and Machiavelli is its anointed godfather. The current political zeitgeist of the country is becoming downright grotesque and spiteful. Although fierce political rivalry is widely accepted as a tradition to achieve the national goals promulgated by each party, what we are witnessing today is a veritable blood sport fueled by “hyper-partisanship,” where drawing blood, not promoting the public good, has become an undisguised intent.
The intensity of hyper-partisanship has engulfed the collective national psyche and is bordering on the “religification” of politics. What used to be reasonable political views have been transformed into irrefutable articles of faith that do not lend themselves to rational debate or productive compromise. The metastasis of social media into our daily lives over the past decade is catalyzing the venomous crossfire across the political divide that used to be passionate and civil, but recently has degenerated into a raucous cacophony of hateful speech. Thoughtful debate of issues that promote the public good is becoming scarce. Instead of effectively defending the validity of their arguments, extremists focus on spewing accusations and ad hominem insults. It is worrisome that both fringe groups tenaciously uphold fixed and extreme political positions, the tenets of which can never be challenged.
Psychiatrically, those extreme ideological positions appear to be consistent with Jasper’s criteria for a delusion (a belief with an unparalleled degree of subjective feeling of certainty that cannot be influenced by experience or arguments) or McHugh’s definition of an overvalued idea, which resembles an egosyntonic obsession that is relished, amplified, and defended. Given that extremism is not just a “folie à deux” shared by 2 individuals but by many individuals, it may qualify as a “folie en masse.”
Having a political orientation is perfectly normal, a healthy evidence of absence of indolent apathy. However, the unconstrained fervor of political extremism can be as psychologically unhealthy as lethargic passivity. A significant segment of the population may see some merit on both sides of the gaping political chasm, but they are appalled by the intransigence of political extremism, which has become an impediment to the constructive compromise that is vital for progress in politics and in all human interactions.
Beliefs are a transcendent human trait. Homo sapiens represent the only animal species endowed by evolution with a large prefrontal cortex that enables each of its members to harbor a belief system. It prompts me to propose that Descartes’ famous dictum “I think, therefore I am” be revised to “I believe, therefore I am human.” But while many beliefs are reasonable and anchored in reality, irrational beliefs are odd and ambiguous, ranging from superstitions and overvalued ideas to conspiracy theories and cults, which I wrote about a decade ago.1 In fact, epidemiologic research studies have confirmed a high prevalence of subthreshold and pre-psychotic beliefs in the general population.2-5 Thus, radical political partisanship falls on the extreme end of that continuum.
The zeitgeist generated by extreme partisanship is intellectually stunting and emotionally numbing. Psychiatrists may wonder what consequences the intense anger and antipathy and scarcity of compromise between the opposing parties will have for the country’s citizens. Although psychiatrists cannot repair the dysfunctional political fragmentation at the national level, we can help patients who may be negatively affected by the conflicts permeating the national scene when we read or watch the daily news.
Just as it is disturbing for children to watch their parents undermine each other by arguing ferociously and hurling insults, so it is for a populace aghast at how frenzied and intolerant their leaders and their extremist followers have become, failing to work together for the common good and adversely impacting the mental health zeitgeist.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Kelleher I, Wigman JT, Harley M, et al. Psychotic experiences in the population: association with functioning and mental distress. Schizophr Res. 2015;165(1):9-14.
3. Landin-Romero R, McKenna PJ, Romaguera A, et al. Examining the continuum of psychosis: frequency and characteristics of psychotic-like symptoms in relatives and non-relatives of patients with schizophrenia. Schizophr Res. 2016;178(1-3):6-11.
4. Hanssen M, Bak M, Bijl R, et al. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44(pt 2):181-191.
5. Nelson B, Fusar-Poli P, Yung AR. Can we detect psychotic-like experiences in the general population? Curr Pharm Des. 2012;18(4):376-385.
It is always judicious to avoid discussing religious or political issues because inevitably someone will be offended. As a lifetime member of the American Psychiatric Association, I adhere to its "Goldwater Rule," which proscribes the gratuitous diagnosis of any president absent of a formal face-to-face psychiatric evaluation. But it is perfectly permissible to express a psychiatric opinion about the contemporary national political scene.
Frankly, the status of the political arena has become ugly. This should not be surprising, given that at its core, politics is an unquenchable thirst for power, and Machiavelli is its anointed godfather. The current political zeitgeist of the country is becoming downright grotesque and spiteful. Although fierce political rivalry is widely accepted as a tradition to achieve the national goals promulgated by each party, what we are witnessing today is a veritable blood sport fueled by “hyper-partisanship,” where drawing blood, not promoting the public good, has become an undisguised intent.
The intensity of hyper-partisanship has engulfed the collective national psyche and is bordering on the “religification” of politics. What used to be reasonable political views have been transformed into irrefutable articles of faith that do not lend themselves to rational debate or productive compromise. The metastasis of social media into our daily lives over the past decade is catalyzing the venomous crossfire across the political divide that used to be passionate and civil, but recently has degenerated into a raucous cacophony of hateful speech. Thoughtful debate of issues that promote the public good is becoming scarce. Instead of effectively defending the validity of their arguments, extremists focus on spewing accusations and ad hominem insults. It is worrisome that both fringe groups tenaciously uphold fixed and extreme political positions, the tenets of which can never be challenged.
Psychiatrically, those extreme ideological positions appear to be consistent with Jasper’s criteria for a delusion (a belief with an unparalleled degree of subjective feeling of certainty that cannot be influenced by experience or arguments) or McHugh’s definition of an overvalued idea, which resembles an egosyntonic obsession that is relished, amplified, and defended. Given that extremism is not just a “folie à deux” shared by 2 individuals but by many individuals, it may qualify as a “folie en masse.”
Having a political orientation is perfectly normal, a healthy evidence of absence of indolent apathy. However, the unconstrained fervor of political extremism can be as psychologically unhealthy as lethargic passivity. A significant segment of the population may see some merit on both sides of the gaping political chasm, but they are appalled by the intransigence of political extremism, which has become an impediment to the constructive compromise that is vital for progress in politics and in all human interactions.
Beliefs are a transcendent human trait. Homo sapiens represent the only animal species endowed by evolution with a large prefrontal cortex that enables each of its members to harbor a belief system. It prompts me to propose that Descartes’ famous dictum “I think, therefore I am” be revised to “I believe, therefore I am human.” But while many beliefs are reasonable and anchored in reality, irrational beliefs are odd and ambiguous, ranging from superstitions and overvalued ideas to conspiracy theories and cults, which I wrote about a decade ago.1 In fact, epidemiologic research studies have confirmed a high prevalence of subthreshold and pre-psychotic beliefs in the general population.2-5 Thus, radical political partisanship falls on the extreme end of that continuum.
The zeitgeist generated by extreme partisanship is intellectually stunting and emotionally numbing. Psychiatrists may wonder what consequences the intense anger and antipathy and scarcity of compromise between the opposing parties will have for the country’s citizens. Although psychiatrists cannot repair the dysfunctional political fragmentation at the national level, we can help patients who may be negatively affected by the conflicts permeating the national scene when we read or watch the daily news.
Just as it is disturbing for children to watch their parents undermine each other by arguing ferociously and hurling insults, so it is for a populace aghast at how frenzied and intolerant their leaders and their extremist followers have become, failing to work together for the common good and adversely impacting the mental health zeitgeist.
It is always judicious to avoid discussing religious or political issues because inevitably someone will be offended. As a lifetime member of the American Psychiatric Association, I adhere to its "Goldwater Rule," which proscribes the gratuitous diagnosis of any president absent of a formal face-to-face psychiatric evaluation. But it is perfectly permissible to express a psychiatric opinion about the contemporary national political scene.
Frankly, the status of the political arena has become ugly. This should not be surprising, given that at its core, politics is an unquenchable thirst for power, and Machiavelli is its anointed godfather. The current political zeitgeist of the country is becoming downright grotesque and spiteful. Although fierce political rivalry is widely accepted as a tradition to achieve the national goals promulgated by each party, what we are witnessing today is a veritable blood sport fueled by “hyper-partisanship,” where drawing blood, not promoting the public good, has become an undisguised intent.
The intensity of hyper-partisanship has engulfed the collective national psyche and is bordering on the “religification” of politics. What used to be reasonable political views have been transformed into irrefutable articles of faith that do not lend themselves to rational debate or productive compromise. The metastasis of social media into our daily lives over the past decade is catalyzing the venomous crossfire across the political divide that used to be passionate and civil, but recently has degenerated into a raucous cacophony of hateful speech. Thoughtful debate of issues that promote the public good is becoming scarce. Instead of effectively defending the validity of their arguments, extremists focus on spewing accusations and ad hominem insults. It is worrisome that both fringe groups tenaciously uphold fixed and extreme political positions, the tenets of which can never be challenged.
Psychiatrically, those extreme ideological positions appear to be consistent with Jasper’s criteria for a delusion (a belief with an unparalleled degree of subjective feeling of certainty that cannot be influenced by experience or arguments) or McHugh’s definition of an overvalued idea, which resembles an egosyntonic obsession that is relished, amplified, and defended. Given that extremism is not just a “folie à deux” shared by 2 individuals but by many individuals, it may qualify as a “folie en masse.”
Having a political orientation is perfectly normal, a healthy evidence of absence of indolent apathy. However, the unconstrained fervor of political extremism can be as psychologically unhealthy as lethargic passivity. A significant segment of the population may see some merit on both sides of the gaping political chasm, but they are appalled by the intransigence of political extremism, which has become an impediment to the constructive compromise that is vital for progress in politics and in all human interactions.
Beliefs are a transcendent human trait. Homo sapiens represent the only animal species endowed by evolution with a large prefrontal cortex that enables each of its members to harbor a belief system. It prompts me to propose that Descartes’ famous dictum “I think, therefore I am” be revised to “I believe, therefore I am human.” But while many beliefs are reasonable and anchored in reality, irrational beliefs are odd and ambiguous, ranging from superstitions and overvalued ideas to conspiracy theories and cults, which I wrote about a decade ago.1 In fact, epidemiologic research studies have confirmed a high prevalence of subthreshold and pre-psychotic beliefs in the general population.2-5 Thus, radical political partisanship falls on the extreme end of that continuum.
The zeitgeist generated by extreme partisanship is intellectually stunting and emotionally numbing. Psychiatrists may wonder what consequences the intense anger and antipathy and scarcity of compromise between the opposing parties will have for the country’s citizens. Although psychiatrists cannot repair the dysfunctional political fragmentation at the national level, we can help patients who may be negatively affected by the conflicts permeating the national scene when we read or watch the daily news.
Just as it is disturbing for children to watch their parents undermine each other by arguing ferociously and hurling insults, so it is for a populace aghast at how frenzied and intolerant their leaders and their extremist followers have become, failing to work together for the common good and adversely impacting the mental health zeitgeist.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Kelleher I, Wigman JT, Harley M, et al. Psychotic experiences in the population: association with functioning and mental distress. Schizophr Res. 2015;165(1):9-14.
3. Landin-Romero R, McKenna PJ, Romaguera A, et al. Examining the continuum of psychosis: frequency and characteristics of psychotic-like symptoms in relatives and non-relatives of patients with schizophrenia. Schizophr Res. 2016;178(1-3):6-11.
4. Hanssen M, Bak M, Bijl R, et al. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44(pt 2):181-191.
5. Nelson B, Fusar-Poli P, Yung AR. Can we detect psychotic-like experiences in the general population? Curr Pharm Des. 2012;18(4):376-385.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Kelleher I, Wigman JT, Harley M, et al. Psychotic experiences in the population: association with functioning and mental distress. Schizophr Res. 2015;165(1):9-14.
3. Landin-Romero R, McKenna PJ, Romaguera A, et al. Examining the continuum of psychosis: frequency and characteristics of psychotic-like symptoms in relatives and non-relatives of patients with schizophrenia. Schizophr Res. 2016;178(1-3):6-11.
4. Hanssen M, Bak M, Bijl R, et al. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44(pt 2):181-191.
5. Nelson B, Fusar-Poli P, Yung AR. Can we detect psychotic-like experiences in the general population? Curr Pharm Des. 2012;18(4):376-385.










