User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
When your patient is a physician: Overcoming the challenges
Physicians’ physical and mental well-being has become a major concern in health care. In the United States, an estimated 300 to 400 physicians die from suicide each year.1 Compared with the general population, the suicide rates for male and female physicians are 1.41 and 2.27 times higher, respectively.2 As psychiatrists, we can play an instrumental role in preserving our colleagues’ mental health. While treating a fellow physician can be rewarding, these situations also can be challenging. Here we describe a few of the challenges of treating physicians, and solutions we can employ to minimize potential pitfalls.
Challenges: How our relationship can affect care
We may view physician-patients as “VIPs” because of their profession, which might lead us to assume they are more knowledgeable than the average patient.1,3 This mindset could result in taking an inadequate history, having an incomplete informed-consent discussion, avoiding or limiting educational discussions, performing an inadequate suicide risk assessment, or underestimating the need for higher levels of care (eg, psychiatric hospitalization).1
We may have difficulty maintaining appropriate professional boundaries due to the relationship (eg, friend, colleague, or mentor) we have established with a physician-patient.3 It may be difficult to establish the usual roles of patient and physician, particularly if we have a professional relationship with a physician-patient that requires routine contact at work. The issue of boundaries can become compounded if there is an emotional component to the relationship, which may make it difficult to discuss sensitive topics.3 A physician-patient may be reluctant to discuss sensitive information due to concerns about the confidentiality of their medical record.3 They also might obtain our personal contact information through work-related networks and use it to contact us about their care.
Solutions: Treat them as you would any other patient
Although physician-patients may have more medical knowledge than other patients, we should avoid showing deference and making assumptions about their knowledge of psychiatric illnesses and treatment. As we would with other patients, we should always1:
- conduct a thorough evaluation
- develop a comprehensive treatment plan
- provide appropriate informed consent
- adequately assess suicide risk.
We should also maintain boundaries as best we can, while understanding that our professional relationships might complicate this.
We should ask our physician-patients if they have been self-prescribing and/or self-treating.1 We shouldn’t shy away from considering inpatient treatment for physician-patients (when clinically indicated) because of our concern that such treatment might jeopardize their ability to practice medicine. Also, to help decrease barriers to and enhance engagement in treatment, consider recommending treatment options that can take place outside of the physician-patient’s work environment.3
Continue to: We should provide...
We should provide the same confidentiality considerations to physician-patients as we do to other patients. However, at times, we may need to break confidentiality for safety concerns or reporting that is required by law. We may have to contact a state licensing board if a physician-patient continues to practice while impaired despite engaging in treatment.1 We should understand the procedures for reporting; have referral resources available for these patients, such as recovering physician programs; and know whom to contact for further counsel, such as risk management or legal teams.1
The best way to provide optimal psychiatric care to a physician colleague is to acknowledge the potential challenges at the onset of treatment, and work collaboratively to avoid the potential pitfalls during the course of treatment.
1. Fischer-Sanchez D. Risk management considerations when treating fellow physicians. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.7a21. Published July 3, 2018. Accessed May 9, 2019.
Physicians’ physical and mental well-being has become a major concern in health care. In the United States, an estimated 300 to 400 physicians die from suicide each year.1 Compared with the general population, the suicide rates for male and female physicians are 1.41 and 2.27 times higher, respectively.2 As psychiatrists, we can play an instrumental role in preserving our colleagues’ mental health. While treating a fellow physician can be rewarding, these situations also can be challenging. Here we describe a few of the challenges of treating physicians, and solutions we can employ to minimize potential pitfalls.
Challenges: How our relationship can affect care
We may view physician-patients as “VIPs” because of their profession, which might lead us to assume they are more knowledgeable than the average patient.1,3 This mindset could result in taking an inadequate history, having an incomplete informed-consent discussion, avoiding or limiting educational discussions, performing an inadequate suicide risk assessment, or underestimating the need for higher levels of care (eg, psychiatric hospitalization).1
We may have difficulty maintaining appropriate professional boundaries due to the relationship (eg, friend, colleague, or mentor) we have established with a physician-patient.3 It may be difficult to establish the usual roles of patient and physician, particularly if we have a professional relationship with a physician-patient that requires routine contact at work. The issue of boundaries can become compounded if there is an emotional component to the relationship, which may make it difficult to discuss sensitive topics.3 A physician-patient may be reluctant to discuss sensitive information due to concerns about the confidentiality of their medical record.3 They also might obtain our personal contact information through work-related networks and use it to contact us about their care.
Solutions: Treat them as you would any other patient
Although physician-patients may have more medical knowledge than other patients, we should avoid showing deference and making assumptions about their knowledge of psychiatric illnesses and treatment. As we would with other patients, we should always1:
- conduct a thorough evaluation
- develop a comprehensive treatment plan
- provide appropriate informed consent
- adequately assess suicide risk.
We should also maintain boundaries as best we can, while understanding that our professional relationships might complicate this.
We should ask our physician-patients if they have been self-prescribing and/or self-treating.1 We shouldn’t shy away from considering inpatient treatment for physician-patients (when clinically indicated) because of our concern that such treatment might jeopardize their ability to practice medicine. Also, to help decrease barriers to and enhance engagement in treatment, consider recommending treatment options that can take place outside of the physician-patient’s work environment.3
Continue to: We should provide...
We should provide the same confidentiality considerations to physician-patients as we do to other patients. However, at times, we may need to break confidentiality for safety concerns or reporting that is required by law. We may have to contact a state licensing board if a physician-patient continues to practice while impaired despite engaging in treatment.1 We should understand the procedures for reporting; have referral resources available for these patients, such as recovering physician programs; and know whom to contact for further counsel, such as risk management or legal teams.1
The best way to provide optimal psychiatric care to a physician colleague is to acknowledge the potential challenges at the onset of treatment, and work collaboratively to avoid the potential pitfalls during the course of treatment.
Physicians’ physical and mental well-being has become a major concern in health care. In the United States, an estimated 300 to 400 physicians die from suicide each year.1 Compared with the general population, the suicide rates for male and female physicians are 1.41 and 2.27 times higher, respectively.2 As psychiatrists, we can play an instrumental role in preserving our colleagues’ mental health. While treating a fellow physician can be rewarding, these situations also can be challenging. Here we describe a few of the challenges of treating physicians, and solutions we can employ to minimize potential pitfalls.
Challenges: How our relationship can affect care
We may view physician-patients as “VIPs” because of their profession, which might lead us to assume they are more knowledgeable than the average patient.1,3 This mindset could result in taking an inadequate history, having an incomplete informed-consent discussion, avoiding or limiting educational discussions, performing an inadequate suicide risk assessment, or underestimating the need for higher levels of care (eg, psychiatric hospitalization).1
We may have difficulty maintaining appropriate professional boundaries due to the relationship (eg, friend, colleague, or mentor) we have established with a physician-patient.3 It may be difficult to establish the usual roles of patient and physician, particularly if we have a professional relationship with a physician-patient that requires routine contact at work. The issue of boundaries can become compounded if there is an emotional component to the relationship, which may make it difficult to discuss sensitive topics.3 A physician-patient may be reluctant to discuss sensitive information due to concerns about the confidentiality of their medical record.3 They also might obtain our personal contact information through work-related networks and use it to contact us about their care.
Solutions: Treat them as you would any other patient
Although physician-patients may have more medical knowledge than other patients, we should avoid showing deference and making assumptions about their knowledge of psychiatric illnesses and treatment. As we would with other patients, we should always1:
- conduct a thorough evaluation
- develop a comprehensive treatment plan
- provide appropriate informed consent
- adequately assess suicide risk.
We should also maintain boundaries as best we can, while understanding that our professional relationships might complicate this.
We should ask our physician-patients if they have been self-prescribing and/or self-treating.1 We shouldn’t shy away from considering inpatient treatment for physician-patients (when clinically indicated) because of our concern that such treatment might jeopardize their ability to practice medicine. Also, to help decrease barriers to and enhance engagement in treatment, consider recommending treatment options that can take place outside of the physician-patient’s work environment.3
Continue to: We should provide...
We should provide the same confidentiality considerations to physician-patients as we do to other patients. However, at times, we may need to break confidentiality for safety concerns or reporting that is required by law. We may have to contact a state licensing board if a physician-patient continues to practice while impaired despite engaging in treatment.1 We should understand the procedures for reporting; have referral resources available for these patients, such as recovering physician programs; and know whom to contact for further counsel, such as risk management or legal teams.1
The best way to provide optimal psychiatric care to a physician colleague is to acknowledge the potential challenges at the onset of treatment, and work collaboratively to avoid the potential pitfalls during the course of treatment.
1. Fischer-Sanchez D. Risk management considerations when treating fellow physicians. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.7a21. Published July 3, 2018. Accessed May 9, 2019.
1. Fischer-Sanchez D. Risk management considerations when treating fellow physicians. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.7a21. Published July 3, 2018. Accessed May 9, 2019.
Mobile apps and mental health: Using technology to quantify real-time clinical risk
In today’s global society, smartphones are ubiquitous, used by >2.5 billion people.1 They provide limitless availability of on-demand services and resources, unparalleled computing power by size, and the ability to connect with anyone in the world.
Digital applications and new mobile technologies can be used to change the nature of the psychiatrist–patient relationship. The future of clinical practice is changing with the help of smartphones and apps. Diagnosis, follow-up, and treatment will never look the same as we come to better understand and apply emerging technologies.2
Both Android and iOS—the 2 largest mobile operating systems by market share3—provide outlets for the dissemination of mobile applications. There are currently >10,000 mental health–related apps available for download.4 One particular use case of mental health–related apps is digital phenotyping.
In this article, we aim to:
- define digital phenotyping
- explore the potential advances in patient care afforded by emerging technology
- discuss the ethical dilemmas and future of mental health apps.
The possibilities of digital phenotyping
Digital phenotyping is capturing a patient’s real-time clinical state using digital technology to better understand the patient’s state outside of the clinic. While digital phenotyping may seem new, the concepts behind it are grounded in good clinical care.
For example, it is important to assess sleep and physical activity for nearly all patients, regardless of diagnosis. However, the patient’s retrospective recollection of sleep, mood, and other clinically relevant metrics is often unreliable, especially when visits are months apart. With smartphones, it is possible to automatically collect metrics for sleep, activity, mood, and much more in real time from the convenience of our patients’ personal devices (Figure 1).
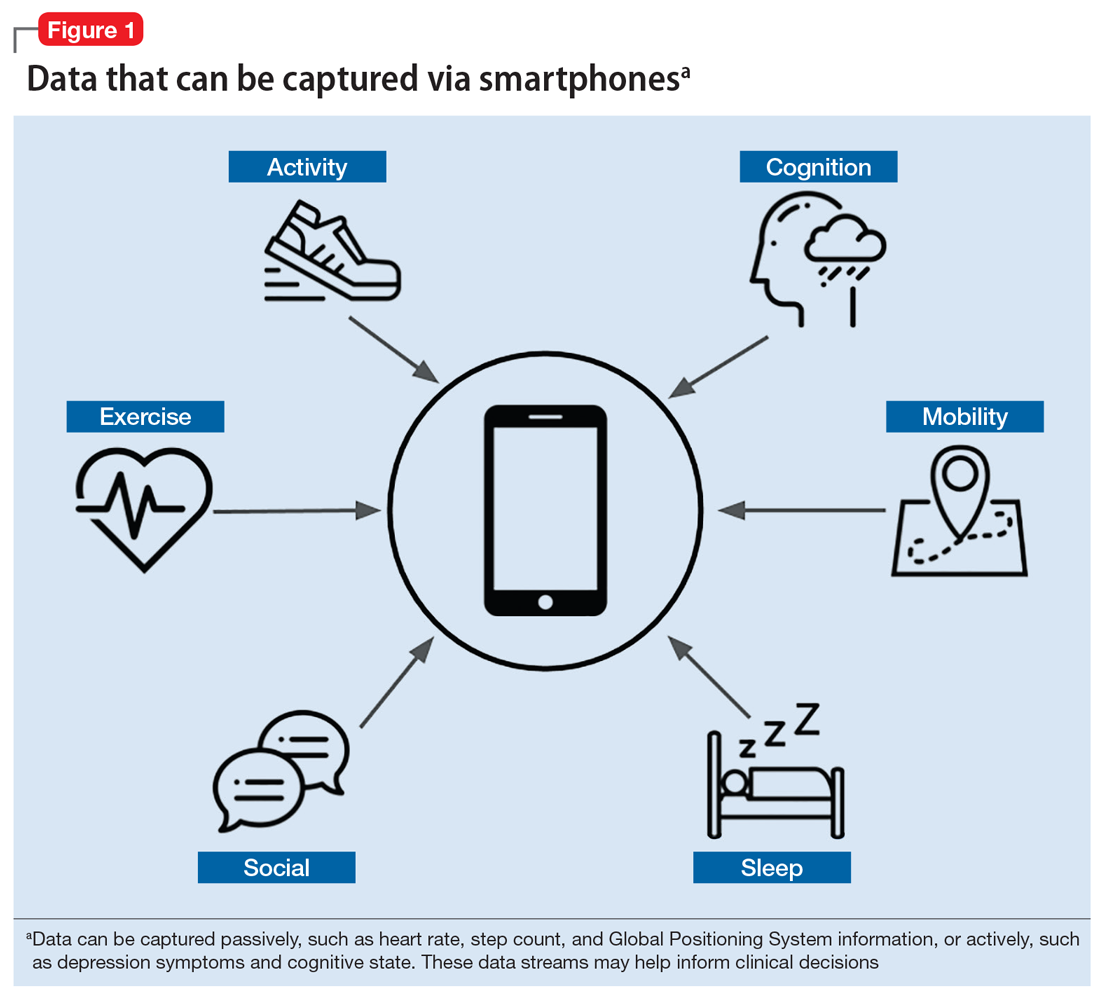
Smartphones can capture a seemingly endless number of data streams, from patient-interfacing active data, such as journal entries, messaging, and games, to data that is captured passively, such as screen time, Global Positioning System information, and step count. Clinicians can work with patients to customize which digital phenotyping data they would like to capture. In one study, researchers worked with 17 patients with schizophrenia by capturing self-reported surveys, anonymized phone call logs, and location data to see if they could predict relapse by observing variations in how patients interact with their smartphones.5 They observed that the rate of behavioral anomalies was 71% higher in the 2 weeks prior to relapse than during other periods. The data captured by the smartphone will depend on the patient and the clinical needs. Some clinicians may only want to collect data on step count and screen time to learn if a patient is overusing his or her smartphone, which might be related to becoming less physically active.
Continue to: One novel data stream...
One novel data stream offered by smartphone digital phenotyping is cognition. While we know that impaired cognition is a core symptom of schizophrenia, and that cognition is affected by depression and anxiety, cognitive symptoms are clinically challenging to quantify. Thus, the cognitive burden of mental illness and the cognitive effects of treatment are often overlooked. However, smartphones are beginning to offer a novel means of capturing a patient’s cognitive state through the use of common clinical tests. For example, the Trail Making Test measures visual attention and executive function by having participants connect dots that differ in number, color, or shape in an ascending pattern.6 By having patients perform this test on a smartphone, clinicians can utilize the touchscreen to capture the user’s discrete actions, such as time to completion and misclicks. These data can be used to build novel measures of cognitive performance that can account for learning bias and other confounding variables.7 While these digital cognitive biomarkers are still in active research, it is likely that they will quickly be developed for broad clinical use.
In addition to the novel data offered by digital phenotyping, another benefit is the low cost and ease of use. Unlike wearable devices such as smartwatches, which can also offer data on steps and sleep, smartphone-based digital phenotyping does not require patients to purchase or use additional devices. Running on patients’ smartphones, digital phenotyping offers the ability to capture rich and continuous health data without added effort or cost. Given that the average person interacts with their phone more than 2,600 times per day,8 smartphones are well suited for capturing large amounts of information that may provide insights into patients’ mental health.
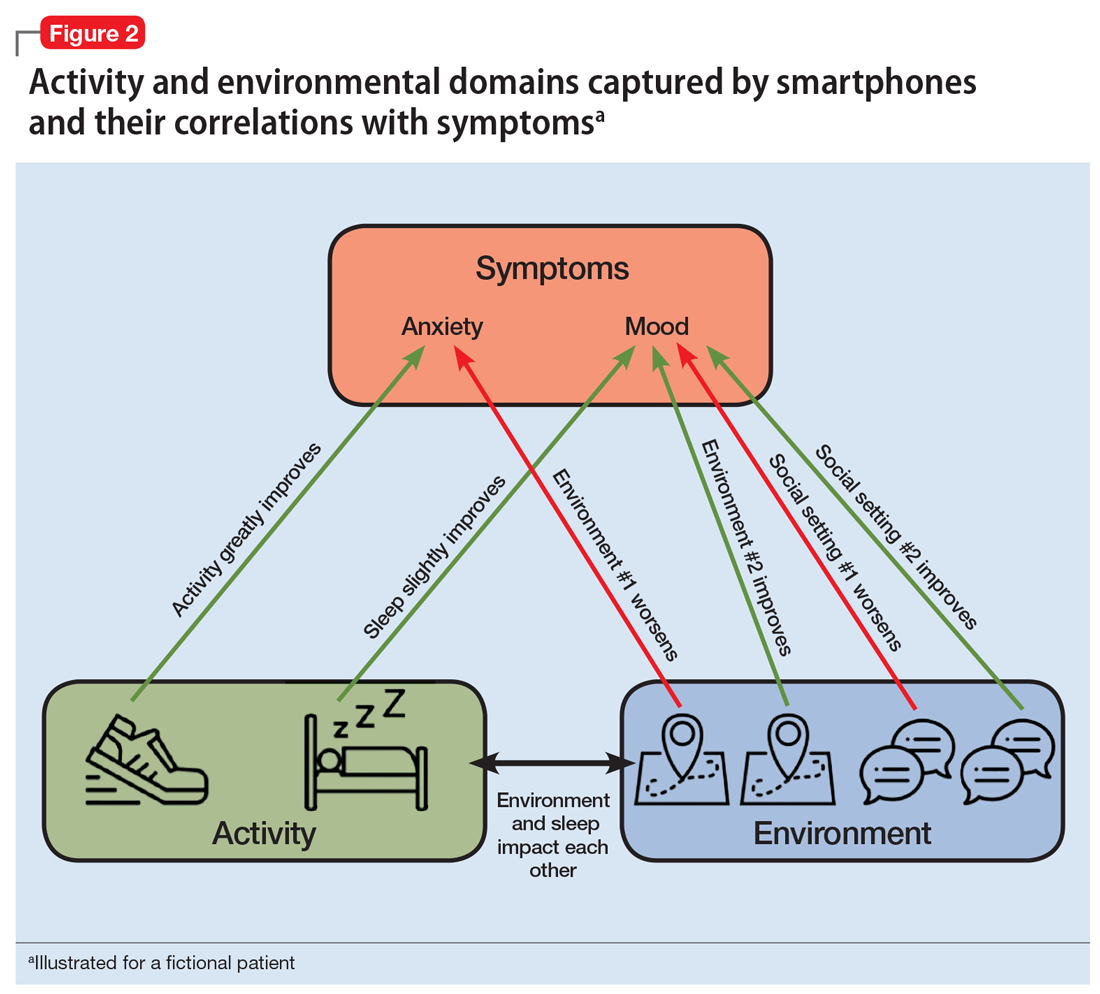
For illnesses such as depression and anxiety, the clinical relevance of digital phenotyping is in the ability to capture symptoms as they occur in context. Figure 2 provides a simplified example of how we can learn that for this fictitious patient, exercise greatly improves anxiety, whereas being in a certain environment worsens it. Other insights about sleep and social settings could also provide further information about the context of the patient’s symptoms. While these correlations alone will not lead to better clinical outcomes, it is easy to imagine how such data could help a patient and clinician start a conversation about making impactful changes.
Continue to: Case report...
Case report: Digital phenotyping
To illustrate how digital phenotyping could be put to clinical use, we created the following case report of a fictional patient who agrees to be monitored via her smartphone.
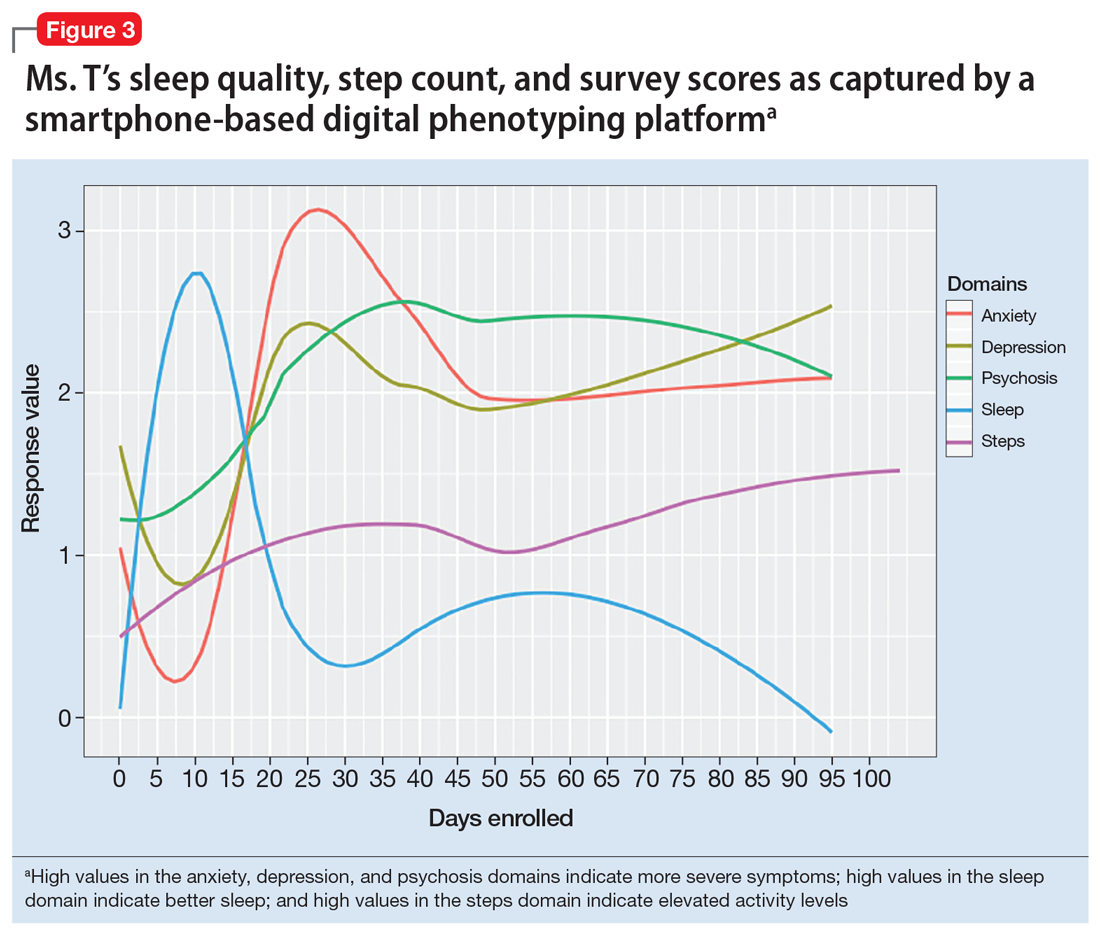
Consider a hypothetical patient we will call Ms. T who is in her mid-20s and has been diagnosed with schizophrenia. On a follow-up visit, she says she has insomnia. She also reports having a recent loss of appetite and higher levels of anxiety. After reviewing her smartphone data (Figure 3), the clinician sees an inversely proportional relationship between her sleep quality and symptoms of anxiety, psychosis, and depression, which suggests that these symptoms might be due to poor sleep. Her step count has been fairly stable, indicating that there is no significant correlation between physical activity and her other symptoms.
Continue to: The clinician shows...
The clinician shows Ms. T the data to help her understand why a trial of cognitive-behavioral therapy for insomnia, or at least improving sleep hygiene, may offer several benefits. The clinician advises her to continue to use the app to help assess her response to these interventions and monitor her progress in real time.
Dilemma: The ethics of continuous observation
The rich data captured by digital phenotyping afford many clinical opportunities, but also raise concerns. Among these are 3 significant ethical implications.
Firstly, the same data that may help a clinician learn about what environments are associated with less anxiety for the patient may also reveal personal details about where that patient has been or with whom they have interacted. In the wrong hands, such personal data could cause harm. And even in the hands of a trusted clinician, a breach in the patient’s privacy begs the question: “Should such information be anyone’s business at all?”
Secondly, many apps that offer digital phenotyping could also store patient data—something that currently pervades social media and causes reasonable discomfort for many people. You might have personally encountered this with social media platforms such as Facebook. When it comes to mobile mental health apps, clinicians should carefully understand the data usage agreement of any digital phenotyping app they wish to use and then share this information with their patients.
Finally, while it is possible to collect the types of data outlined in this article, less is known about how to use it directly in clinical care. Understanding for each patient which data streams are most meaningful and which data streams are noise that should be ignored is an area of ongoing research. A good first step may be to begin with data streams that are known to be clinically relevant and valuable, such as sleep and physical activity.9-11
Continue to: Discussion...
Discussion: Genomic sequencing and digital phenotyping
Although smartphones can gather a wide range of active and passive data, other data streams hold potential for predicting relapse and performing other clinically relevant actions. One data stream that could be of clinical use is genomic sequencing.12 The genotyping of patients provides a wealth of information about the underlying biology, and genomic sequencing has never been cheaper.13
Combining the data gathered via digital phenotyping with that of genotyping could help elucidate the mechanisms by which specific diseases and symptoms occur. This could be very promising to better understand and treat our patients. However, as is the case with genomics, digital phenotyping has important ethical implications. If used in the proper way to benefit our patients, the future for this new method is bright.
1. Statista. Number of smartphone users worldwide from 2014 to 2020 (in billions). https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/. Accessed April 29, 2019.
2. Thibaut F. Digital applications: the future in psychiatry? Dialogues Clin Neurosci. 2016;18(2):123.
3. Statista. Global market share held by the leading smartphone operating systems in sales to end users from 1st quarter 2009 to 2nd quarter 2018. https://www.statista.com/statistics/266136/global-market-share-held-by-smartphone-operating-systems/. Accessed April 19, 2019.
4. Torous J, Roberts L. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
5. Barnett I, Torous J, Staples P, et al. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology. 2018;43(8):1660-1666.
6. Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychological Assessment. 1995;7(2):220-221.
7. Brouillette RM, Foil H, Fontenot S, et al. Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PloS One. 2013;8(6):e65925. doi: 10.1371/journal.pone.0065925.
8. Winnick W. Putting a finger on our phone obsession. dscout. https://blog.dscout.com/mobile-touches. Published June 16, 2016. Accessed April 29, 2019.
9. Waite F, Myers E, Harvey AG, et al. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273-287.
10. Mcgurk SR, Mueser KT, Xie H, et al. (2015). Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852-861.
11. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546-556.
12. Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258-267.
13. National Human Genome Research Institute. The cost of sequencing a human genome. https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/. Updated July 6, 2016. Accessed April 29, 2019.
In today’s global society, smartphones are ubiquitous, used by >2.5 billion people.1 They provide limitless availability of on-demand services and resources, unparalleled computing power by size, and the ability to connect with anyone in the world.
Digital applications and new mobile technologies can be used to change the nature of the psychiatrist–patient relationship. The future of clinical practice is changing with the help of smartphones and apps. Diagnosis, follow-up, and treatment will never look the same as we come to better understand and apply emerging technologies.2
Both Android and iOS—the 2 largest mobile operating systems by market share3—provide outlets for the dissemination of mobile applications. There are currently >10,000 mental health–related apps available for download.4 One particular use case of mental health–related apps is digital phenotyping.
In this article, we aim to:
- define digital phenotyping
- explore the potential advances in patient care afforded by emerging technology
- discuss the ethical dilemmas and future of mental health apps.
The possibilities of digital phenotyping
Digital phenotyping is capturing a patient’s real-time clinical state using digital technology to better understand the patient’s state outside of the clinic. While digital phenotyping may seem new, the concepts behind it are grounded in good clinical care.
For example, it is important to assess sleep and physical activity for nearly all patients, regardless of diagnosis. However, the patient’s retrospective recollection of sleep, mood, and other clinically relevant metrics is often unreliable, especially when visits are months apart. With smartphones, it is possible to automatically collect metrics for sleep, activity, mood, and much more in real time from the convenience of our patients’ personal devices (Figure 1).

Smartphones can capture a seemingly endless number of data streams, from patient-interfacing active data, such as journal entries, messaging, and games, to data that is captured passively, such as screen time, Global Positioning System information, and step count. Clinicians can work with patients to customize which digital phenotyping data they would like to capture. In one study, researchers worked with 17 patients with schizophrenia by capturing self-reported surveys, anonymized phone call logs, and location data to see if they could predict relapse by observing variations in how patients interact with their smartphones.5 They observed that the rate of behavioral anomalies was 71% higher in the 2 weeks prior to relapse than during other periods. The data captured by the smartphone will depend on the patient and the clinical needs. Some clinicians may only want to collect data on step count and screen time to learn if a patient is overusing his or her smartphone, which might be related to becoming less physically active.
Continue to: One novel data stream...
One novel data stream offered by smartphone digital phenotyping is cognition. While we know that impaired cognition is a core symptom of schizophrenia, and that cognition is affected by depression and anxiety, cognitive symptoms are clinically challenging to quantify. Thus, the cognitive burden of mental illness and the cognitive effects of treatment are often overlooked. However, smartphones are beginning to offer a novel means of capturing a patient’s cognitive state through the use of common clinical tests. For example, the Trail Making Test measures visual attention and executive function by having participants connect dots that differ in number, color, or shape in an ascending pattern.6 By having patients perform this test on a smartphone, clinicians can utilize the touchscreen to capture the user’s discrete actions, such as time to completion and misclicks. These data can be used to build novel measures of cognitive performance that can account for learning bias and other confounding variables.7 While these digital cognitive biomarkers are still in active research, it is likely that they will quickly be developed for broad clinical use.
In addition to the novel data offered by digital phenotyping, another benefit is the low cost and ease of use. Unlike wearable devices such as smartwatches, which can also offer data on steps and sleep, smartphone-based digital phenotyping does not require patients to purchase or use additional devices. Running on patients’ smartphones, digital phenotyping offers the ability to capture rich and continuous health data without added effort or cost. Given that the average person interacts with their phone more than 2,600 times per day,8 smartphones are well suited for capturing large amounts of information that may provide insights into patients’ mental health.
For illnesses such as depression and anxiety, the clinical relevance of digital phenotyping is in the ability to capture symptoms as they occur in context. Figure 2 provides a simplified example of how we can learn that for this fictitious patient, exercise greatly improves anxiety, whereas being in a certain environment worsens it. Other insights about sleep and social settings could also provide further information about the context of the patient’s symptoms. While these correlations alone will not lead to better clinical outcomes, it is easy to imagine how such data could help a patient and clinician start a conversation about making impactful changes.

Continue to: Case report...
Case report: Digital phenotyping
To illustrate how digital phenotyping could be put to clinical use, we created the following case report of a fictional patient who agrees to be monitored via her smartphone.
Consider a hypothetical patient we will call Ms. T who is in her mid-20s and has been diagnosed with schizophrenia. On a follow-up visit, she says she has insomnia. She also reports having a recent loss of appetite and higher levels of anxiety. After reviewing her smartphone data (Figure 3), the clinician sees an inversely proportional relationship between her sleep quality and symptoms of anxiety, psychosis, and depression, which suggests that these symptoms might be due to poor sleep. Her step count has been fairly stable, indicating that there is no significant correlation between physical activity and her other symptoms.

Continue to: The clinician shows...
The clinician shows Ms. T the data to help her understand why a trial of cognitive-behavioral therapy for insomnia, or at least improving sleep hygiene, may offer several benefits. The clinician advises her to continue to use the app to help assess her response to these interventions and monitor her progress in real time.
Dilemma: The ethics of continuous observation
The rich data captured by digital phenotyping afford many clinical opportunities, but also raise concerns. Among these are 3 significant ethical implications.
Firstly, the same data that may help a clinician learn about what environments are associated with less anxiety for the patient may also reveal personal details about where that patient has been or with whom they have interacted. In the wrong hands, such personal data could cause harm. And even in the hands of a trusted clinician, a breach in the patient’s privacy begs the question: “Should such information be anyone’s business at all?”
Secondly, many apps that offer digital phenotyping could also store patient data—something that currently pervades social media and causes reasonable discomfort for many people. You might have personally encountered this with social media platforms such as Facebook. When it comes to mobile mental health apps, clinicians should carefully understand the data usage agreement of any digital phenotyping app they wish to use and then share this information with their patients.
Finally, while it is possible to collect the types of data outlined in this article, less is known about how to use it directly in clinical care. Understanding for each patient which data streams are most meaningful and which data streams are noise that should be ignored is an area of ongoing research. A good first step may be to begin with data streams that are known to be clinically relevant and valuable, such as sleep and physical activity.9-11
Continue to: Discussion...
Discussion: Genomic sequencing and digital phenotyping
Although smartphones can gather a wide range of active and passive data, other data streams hold potential for predicting relapse and performing other clinically relevant actions. One data stream that could be of clinical use is genomic sequencing.12 The genotyping of patients provides a wealth of information about the underlying biology, and genomic sequencing has never been cheaper.13
Combining the data gathered via digital phenotyping with that of genotyping could help elucidate the mechanisms by which specific diseases and symptoms occur. This could be very promising to better understand and treat our patients. However, as is the case with genomics, digital phenotyping has important ethical implications. If used in the proper way to benefit our patients, the future for this new method is bright.
In today’s global society, smartphones are ubiquitous, used by >2.5 billion people.1 They provide limitless availability of on-demand services and resources, unparalleled computing power by size, and the ability to connect with anyone in the world.
Digital applications and new mobile technologies can be used to change the nature of the psychiatrist–patient relationship. The future of clinical practice is changing with the help of smartphones and apps. Diagnosis, follow-up, and treatment will never look the same as we come to better understand and apply emerging technologies.2
Both Android and iOS—the 2 largest mobile operating systems by market share3—provide outlets for the dissemination of mobile applications. There are currently >10,000 mental health–related apps available for download.4 One particular use case of mental health–related apps is digital phenotyping.
In this article, we aim to:
- define digital phenotyping
- explore the potential advances in patient care afforded by emerging technology
- discuss the ethical dilemmas and future of mental health apps.
The possibilities of digital phenotyping
Digital phenotyping is capturing a patient’s real-time clinical state using digital technology to better understand the patient’s state outside of the clinic. While digital phenotyping may seem new, the concepts behind it are grounded in good clinical care.
For example, it is important to assess sleep and physical activity for nearly all patients, regardless of diagnosis. However, the patient’s retrospective recollection of sleep, mood, and other clinically relevant metrics is often unreliable, especially when visits are months apart. With smartphones, it is possible to automatically collect metrics for sleep, activity, mood, and much more in real time from the convenience of our patients’ personal devices (Figure 1).

Smartphones can capture a seemingly endless number of data streams, from patient-interfacing active data, such as journal entries, messaging, and games, to data that is captured passively, such as screen time, Global Positioning System information, and step count. Clinicians can work with patients to customize which digital phenotyping data they would like to capture. In one study, researchers worked with 17 patients with schizophrenia by capturing self-reported surveys, anonymized phone call logs, and location data to see if they could predict relapse by observing variations in how patients interact with their smartphones.5 They observed that the rate of behavioral anomalies was 71% higher in the 2 weeks prior to relapse than during other periods. The data captured by the smartphone will depend on the patient and the clinical needs. Some clinicians may only want to collect data on step count and screen time to learn if a patient is overusing his or her smartphone, which might be related to becoming less physically active.
Continue to: One novel data stream...
One novel data stream offered by smartphone digital phenotyping is cognition. While we know that impaired cognition is a core symptom of schizophrenia, and that cognition is affected by depression and anxiety, cognitive symptoms are clinically challenging to quantify. Thus, the cognitive burden of mental illness and the cognitive effects of treatment are often overlooked. However, smartphones are beginning to offer a novel means of capturing a patient’s cognitive state through the use of common clinical tests. For example, the Trail Making Test measures visual attention and executive function by having participants connect dots that differ in number, color, or shape in an ascending pattern.6 By having patients perform this test on a smartphone, clinicians can utilize the touchscreen to capture the user’s discrete actions, such as time to completion and misclicks. These data can be used to build novel measures of cognitive performance that can account for learning bias and other confounding variables.7 While these digital cognitive biomarkers are still in active research, it is likely that they will quickly be developed for broad clinical use.
In addition to the novel data offered by digital phenotyping, another benefit is the low cost and ease of use. Unlike wearable devices such as smartwatches, which can also offer data on steps and sleep, smartphone-based digital phenotyping does not require patients to purchase or use additional devices. Running on patients’ smartphones, digital phenotyping offers the ability to capture rich and continuous health data without added effort or cost. Given that the average person interacts with their phone more than 2,600 times per day,8 smartphones are well suited for capturing large amounts of information that may provide insights into patients’ mental health.
For illnesses such as depression and anxiety, the clinical relevance of digital phenotyping is in the ability to capture symptoms as they occur in context. Figure 2 provides a simplified example of how we can learn that for this fictitious patient, exercise greatly improves anxiety, whereas being in a certain environment worsens it. Other insights about sleep and social settings could also provide further information about the context of the patient’s symptoms. While these correlations alone will not lead to better clinical outcomes, it is easy to imagine how such data could help a patient and clinician start a conversation about making impactful changes.

Continue to: Case report...
Case report: Digital phenotyping
To illustrate how digital phenotyping could be put to clinical use, we created the following case report of a fictional patient who agrees to be monitored via her smartphone.
Consider a hypothetical patient we will call Ms. T who is in her mid-20s and has been diagnosed with schizophrenia. On a follow-up visit, she says she has insomnia. She also reports having a recent loss of appetite and higher levels of anxiety. After reviewing her smartphone data (Figure 3), the clinician sees an inversely proportional relationship between her sleep quality and symptoms of anxiety, psychosis, and depression, which suggests that these symptoms might be due to poor sleep. Her step count has been fairly stable, indicating that there is no significant correlation between physical activity and her other symptoms.

Continue to: The clinician shows...
The clinician shows Ms. T the data to help her understand why a trial of cognitive-behavioral therapy for insomnia, or at least improving sleep hygiene, may offer several benefits. The clinician advises her to continue to use the app to help assess her response to these interventions and monitor her progress in real time.
Dilemma: The ethics of continuous observation
The rich data captured by digital phenotyping afford many clinical opportunities, but also raise concerns. Among these are 3 significant ethical implications.
Firstly, the same data that may help a clinician learn about what environments are associated with less anxiety for the patient may also reveal personal details about where that patient has been or with whom they have interacted. In the wrong hands, such personal data could cause harm. And even in the hands of a trusted clinician, a breach in the patient’s privacy begs the question: “Should such information be anyone’s business at all?”
Secondly, many apps that offer digital phenotyping could also store patient data—something that currently pervades social media and causes reasonable discomfort for many people. You might have personally encountered this with social media platforms such as Facebook. When it comes to mobile mental health apps, clinicians should carefully understand the data usage agreement of any digital phenotyping app they wish to use and then share this information with their patients.
Finally, while it is possible to collect the types of data outlined in this article, less is known about how to use it directly in clinical care. Understanding for each patient which data streams are most meaningful and which data streams are noise that should be ignored is an area of ongoing research. A good first step may be to begin with data streams that are known to be clinically relevant and valuable, such as sleep and physical activity.9-11
Continue to: Discussion...
Discussion: Genomic sequencing and digital phenotyping
Although smartphones can gather a wide range of active and passive data, other data streams hold potential for predicting relapse and performing other clinically relevant actions. One data stream that could be of clinical use is genomic sequencing.12 The genotyping of patients provides a wealth of information about the underlying biology, and genomic sequencing has never been cheaper.13
Combining the data gathered via digital phenotyping with that of genotyping could help elucidate the mechanisms by which specific diseases and symptoms occur. This could be very promising to better understand and treat our patients. However, as is the case with genomics, digital phenotyping has important ethical implications. If used in the proper way to benefit our patients, the future for this new method is bright.
1. Statista. Number of smartphone users worldwide from 2014 to 2020 (in billions). https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/. Accessed April 29, 2019.
2. Thibaut F. Digital applications: the future in psychiatry? Dialogues Clin Neurosci. 2016;18(2):123.
3. Statista. Global market share held by the leading smartphone operating systems in sales to end users from 1st quarter 2009 to 2nd quarter 2018. https://www.statista.com/statistics/266136/global-market-share-held-by-smartphone-operating-systems/. Accessed April 19, 2019.
4. Torous J, Roberts L. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
5. Barnett I, Torous J, Staples P, et al. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology. 2018;43(8):1660-1666.
6. Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychological Assessment. 1995;7(2):220-221.
7. Brouillette RM, Foil H, Fontenot S, et al. Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PloS One. 2013;8(6):e65925. doi: 10.1371/journal.pone.0065925.
8. Winnick W. Putting a finger on our phone obsession. dscout. https://blog.dscout.com/mobile-touches. Published June 16, 2016. Accessed April 29, 2019.
9. Waite F, Myers E, Harvey AG, et al. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273-287.
10. Mcgurk SR, Mueser KT, Xie H, et al. (2015). Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852-861.
11. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546-556.
12. Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258-267.
13. National Human Genome Research Institute. The cost of sequencing a human genome. https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/. Updated July 6, 2016. Accessed April 29, 2019.
1. Statista. Number of smartphone users worldwide from 2014 to 2020 (in billions). https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/. Accessed April 29, 2019.
2. Thibaut F. Digital applications: the future in psychiatry? Dialogues Clin Neurosci. 2016;18(2):123.
3. Statista. Global market share held by the leading smartphone operating systems in sales to end users from 1st quarter 2009 to 2nd quarter 2018. https://www.statista.com/statistics/266136/global-market-share-held-by-smartphone-operating-systems/. Accessed April 19, 2019.
4. Torous J, Roberts L. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
5. Barnett I, Torous J, Staples P, et al. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology. 2018;43(8):1660-1666.
6. Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychological Assessment. 1995;7(2):220-221.
7. Brouillette RM, Foil H, Fontenot S, et al. Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PloS One. 2013;8(6):e65925. doi: 10.1371/journal.pone.0065925.
8. Winnick W. Putting a finger on our phone obsession. dscout. https://blog.dscout.com/mobile-touches. Published June 16, 2016. Accessed April 29, 2019.
9. Waite F, Myers E, Harvey AG, et al. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273-287.
10. Mcgurk SR, Mueser KT, Xie H, et al. (2015). Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852-861.
11. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546-556.
12. Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258-267.
13. National Human Genome Research Institute. The cost of sequencing a human genome. https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/. Updated July 6, 2016. Accessed April 29, 2019.
It’s time to implement measurement-based care in psychiatric practice
In an editorial published in Current Psychiatry 10 years ago, I cited a stunning fact based on a readers’ survey: 98% of psychiatrists did not use any of the 4 clinical rating scales that are routinely used in the clinical trials required for FDA approval of medications for psychotic, mood, and anxiety disorders.1
As a follow-up, Ahmed Aboraya, MD, DrPH, and I would like to report on the state of measurement-based care (MBC), a term coined by Trivedi in 2006 and defined by Fortney as “the systematic administration of symptom rating scales and use of the results to drive clinical decision making at the level of the individual patient.”2
We will start with the creator of modern rating scales, Father Thomas Verner Moore (1877-1969), who is considered one of the most underrecognized legends in the history of modern psychiatry. Moore was a psychologist and psychiatrist who can lay claim to 3 major achievements in psychiatry: the creation of rating scales in psychiatry, the use of factor analysis to deconstruct psychosis, and the formulation of specific definitions for symptoms and signs of psychopathology. Moore’s 1933 book described the rating scales used in his research.3
Since that time, researchers have continued to invent clinician-rated scales, self-report scales, and other measures in psychiatry. The Handbook of Psychiatric Measures, which was published in 2000 by the American Psychiatric Association Task Force chaired by AJ Rush Jr., includes >240 measures covering adult and child psychiatric disorders.4
Recent research has shown the superiority of MBC compared with usual standard care (USC) in improving patient outcomes.2,5-7 A recent well-designed, blind-rater, randomized trial by Guo et al8 showed that MBC is more effective than USC both in achieving response and remission, and reducing the time to response and remission. Given the evidence of the benefits of MBC in improving patient outcomes, and the plethora of reliable and validated rating scales, an important question arises: Why has MBC not yet been established as the standard of care in psychiatric clinical practice? There are many barriers to implementing MBC,9 including:
- time constraints (most commonly cited reason by psychiatrists)
- mismatch between clinical needs and the content of the measure (ie, rating scales are designed for research and not for clinicians’ use)
- measurements produced by rating scales may not always be clinically relevant
- administering rating scales may interfere with establishing rapport with patients
- some measures, such as standardized diagnostic interviews, can be cumbersome, unwieldy, and complicated
- the lack of formal training for most clinicians (among the top barriers for residents and faculty)
- lack of availability of training manuals and protocols.
Clinician researchers have started to adapt and invent instruments that can be used in clinical settings. For more than 20 years, Mark Zimmerman, MD, has been the principal investigator of the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project, aimed at integrating the assessment methods of researchers into routine clinical practice.10 Zimmerman has developed self-report scales and outcome measures such as the Psychiatric Diagnostic Screening Questionnaire (PDSQ), the Clinically Useful Depression Outcome Scale (CUDOS), the Standardized Clinical Outcome Rating for Depression (SCOR-D), the Clinically Useful Anxiety Outcome Scale (CUXOS), the Remission from Depression Questionnaire (RDQ), and the Clinically Useful Patient Satisfaction Scale (CUPSS).11-18
We have been critical of the utility of the existing diagnostic interviews and rating scales. I (AA) developed the Standard for Clinicians’ Interview in Psychiatry (SCIP) as a MBC tool that addresses the most common barriers that clinicians face.9,19-23 The SCIP includes 18 clinician-rated scales for the following symptom domains: generalized anxiety, obsessions, compulsions, posttraumatic stress, depression, mania, delusions, hallucinations, disorganized thoughts, aggression, negative symptoms, alcohol use, drug use, attention deficit, hyperactivity, anorexia, binge-eating, and bulimia. The SCIP rating scales meet the criteria for MBC because they are efficient, reliable, and valid. They reflect how clinicians assess psychiatric disorders, and are relevant to decision-making. Both self-report and clinician-rated scales are important MBC tools and complementary to each other. The choice to use self-report scales, clinician-rated scales, or both depends on several factors, including the clinical setting (inpatient or outpatient), psychiatric diagnoses, and patient characteristics. No measure or scale will ever replace a seasoned and experienced clinician who has been evaluating and treating real-world patients for years. Just as thermometers, stethoscopes, and laboratories help other types of physicians to reach accurate diagnoses and provide appropriate management, the use of MBC by psychiatrists will enhance the accuracy of diagnoses and improve the outcomes of care.
Continue to: On a positive note...
On a positive note, I (AA) have completed a MBC curriculum for training psychiatry residents that includes 11 videotaped interviews with actual patients covering the major adult psychiatric disorders: generalized anxiety, panic, depressive, posttraumatic stress, bipolar, psychotic, eating, and attention-deficit/hyperactivity. The interviews show and teach how to rate psychopathology items, how to score the dimensions, and how to evaluate the severity of the disorder(s). All of the SCIP’s 18 scales have been uploaded into the Epic electronic health record (EHR) system at West Virginia University hospitals. A pilot project for implementing MBC in the treatment of adult psychiatric disorders at the West Virginia University residency program and other programs is underway. If we instruct residents in MBC during their psychiatric training, they will likely practice it for the rest of their clinical careers. Except for a minority of clinicians who are involved in clinical trials and who use rating scales in practice, most practicing clinicians were never trained to use scales. For more information about the MBC curriculum and videotapes, contact Dr. Aboraya at [email protected] or visit www.scip-psychiatry.com.
Today, some of the barriers that impede the implementation of MBC in psychiatric practice have been resolved, but much more work remains. Now is the time to implement MBC and provide an answer to AJ Rush, who asked, “Isn’t it about time to employ measurement-based care in practice?”24 The 3 main ingredients for MBC implementation—useful measures, integration of EHR, and health information technologies—exist today. We strongly encourage psychiatrists, nurse practitioners, and other mental health professionals to adopt MBC in their daily practice.
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
2. Fortney JC, Unutzer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2016;68(2):179-188.
3. Moore TV. The essential psychoses and their fundamental syndromes. Baltimore, MD: Williams & Wilkins; 1933.
4. Rush AJ. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000.
5. Scott K, Lewis CC. Using measurement-based care to enhance any treatment. Cogn Behav Pract. 2015;22(1):49-59.
6. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61-S71.
7. Harding KJ, Rush AJ, Arbuckle M, et al. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136-1143.
8. Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004-1013.
9. Aboraya A, Nasrallah HA, Elswick D, et al. Measurement-based care in psychiatry: past, present and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
10. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
11. Zimmerman M, Mattia JI. The reliability and validity of a screening questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60(10):677-683.
12. Zimmerman M, Mattia JI. The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Compr Psychiatry. 2001;42(3):175-189.
13. Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131-140.
14. Zimmerman M, Posternak MA, Chelminski I, et al. Standardized clinical outcome rating scale for depression for use in clinical practice. Depress Anxiety. 2005;22(1):36-40.
15. Zimmerman M, Chelminski I, Young D, et al. A clinically useful anxiety outcome scale. J Clin Psychiatry. 2010;71(5):534-542.
16. Zimmerman M, Galione JN, Attiullah N, et al. Depressed patients’ perspectives of 2 measures of outcome: the Quick Inventory of Depressive Symptomatology (QIDS) and the Remission from Depression Questionnaire (RDQ). Ann Clin Psychiatry. 2011;23(3):208-212.
17. Zimmerman M, Martinez JH, Attiullah N, et al. The remission from depression questionnaire as an outcome measure in the treatment of depression. Depress Anxiety. 2014;31(6):533-538.
18. Zimmerman M, Gazarian D, Multach M, et al. A clinically useful self-report measure of psychiatric patients’ satisfaction with the initial evaluation. Psychiatry Res. 2017;252:38-44.
19. Aboraya A. The validity results of the Standard for Clinicians’ Interview in Psychiatry (SCIP). Schizophrenia Bulletin. 2015;41(Suppl 1):S103-S104.
20. Aboraya A. Instruction manual for the Standard for Clinicians’ Interview in Psychiatry (SCIP). http://innovationscns.com/wp-content/uploads/SCIP_Instruction_Manual.pdf. Accessed April 29, 2019.
21. Aboraya A, El-Missiry A, Barlowe J, et al. The reliability of the Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional and numeric output. Schizophr Res. 2014;156(2-3):174-183.
22. Aboraya A, Nasrallah HA, Muvvala S, et al. The Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional, and numeric output-conceptual development, design, and description of the SCIP. Innov Clin Neurosci. 2016;13(5-6):31-77.
23. Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, drawbacks, and a new alternative for schizophrenia research. Ann Clin Psychiatry. 2016;28(2):125-131.
24. Rush AJ. Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934-936.
In an editorial published in Current Psychiatry 10 years ago, I cited a stunning fact based on a readers’ survey: 98% of psychiatrists did not use any of the 4 clinical rating scales that are routinely used in the clinical trials required for FDA approval of medications for psychotic, mood, and anxiety disorders.1
As a follow-up, Ahmed Aboraya, MD, DrPH, and I would like to report on the state of measurement-based care (MBC), a term coined by Trivedi in 2006 and defined by Fortney as “the systematic administration of symptom rating scales and use of the results to drive clinical decision making at the level of the individual patient.”2
We will start with the creator of modern rating scales, Father Thomas Verner Moore (1877-1969), who is considered one of the most underrecognized legends in the history of modern psychiatry. Moore was a psychologist and psychiatrist who can lay claim to 3 major achievements in psychiatry: the creation of rating scales in psychiatry, the use of factor analysis to deconstruct psychosis, and the formulation of specific definitions for symptoms and signs of psychopathology. Moore’s 1933 book described the rating scales used in his research.3
Since that time, researchers have continued to invent clinician-rated scales, self-report scales, and other measures in psychiatry. The Handbook of Psychiatric Measures, which was published in 2000 by the American Psychiatric Association Task Force chaired by AJ Rush Jr., includes >240 measures covering adult and child psychiatric disorders.4
Recent research has shown the superiority of MBC compared with usual standard care (USC) in improving patient outcomes.2,5-7 A recent well-designed, blind-rater, randomized trial by Guo et al8 showed that MBC is more effective than USC both in achieving response and remission, and reducing the time to response and remission. Given the evidence of the benefits of MBC in improving patient outcomes, and the plethora of reliable and validated rating scales, an important question arises: Why has MBC not yet been established as the standard of care in psychiatric clinical practice? There are many barriers to implementing MBC,9 including:
- time constraints (most commonly cited reason by psychiatrists)
- mismatch between clinical needs and the content of the measure (ie, rating scales are designed for research and not for clinicians’ use)
- measurements produced by rating scales may not always be clinically relevant
- administering rating scales may interfere with establishing rapport with patients
- some measures, such as standardized diagnostic interviews, can be cumbersome, unwieldy, and complicated
- the lack of formal training for most clinicians (among the top barriers for residents and faculty)
- lack of availability of training manuals and protocols.
Clinician researchers have started to adapt and invent instruments that can be used in clinical settings. For more than 20 years, Mark Zimmerman, MD, has been the principal investigator of the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project, aimed at integrating the assessment methods of researchers into routine clinical practice.10 Zimmerman has developed self-report scales and outcome measures such as the Psychiatric Diagnostic Screening Questionnaire (PDSQ), the Clinically Useful Depression Outcome Scale (CUDOS), the Standardized Clinical Outcome Rating for Depression (SCOR-D), the Clinically Useful Anxiety Outcome Scale (CUXOS), the Remission from Depression Questionnaire (RDQ), and the Clinically Useful Patient Satisfaction Scale (CUPSS).11-18
We have been critical of the utility of the existing diagnostic interviews and rating scales. I (AA) developed the Standard for Clinicians’ Interview in Psychiatry (SCIP) as a MBC tool that addresses the most common barriers that clinicians face.9,19-23 The SCIP includes 18 clinician-rated scales for the following symptom domains: generalized anxiety, obsessions, compulsions, posttraumatic stress, depression, mania, delusions, hallucinations, disorganized thoughts, aggression, negative symptoms, alcohol use, drug use, attention deficit, hyperactivity, anorexia, binge-eating, and bulimia. The SCIP rating scales meet the criteria for MBC because they are efficient, reliable, and valid. They reflect how clinicians assess psychiatric disorders, and are relevant to decision-making. Both self-report and clinician-rated scales are important MBC tools and complementary to each other. The choice to use self-report scales, clinician-rated scales, or both depends on several factors, including the clinical setting (inpatient or outpatient), psychiatric diagnoses, and patient characteristics. No measure or scale will ever replace a seasoned and experienced clinician who has been evaluating and treating real-world patients for years. Just as thermometers, stethoscopes, and laboratories help other types of physicians to reach accurate diagnoses and provide appropriate management, the use of MBC by psychiatrists will enhance the accuracy of diagnoses and improve the outcomes of care.
Continue to: On a positive note...
On a positive note, I (AA) have completed a MBC curriculum for training psychiatry residents that includes 11 videotaped interviews with actual patients covering the major adult psychiatric disorders: generalized anxiety, panic, depressive, posttraumatic stress, bipolar, psychotic, eating, and attention-deficit/hyperactivity. The interviews show and teach how to rate psychopathology items, how to score the dimensions, and how to evaluate the severity of the disorder(s). All of the SCIP’s 18 scales have been uploaded into the Epic electronic health record (EHR) system at West Virginia University hospitals. A pilot project for implementing MBC in the treatment of adult psychiatric disorders at the West Virginia University residency program and other programs is underway. If we instruct residents in MBC during their psychiatric training, they will likely practice it for the rest of their clinical careers. Except for a minority of clinicians who are involved in clinical trials and who use rating scales in practice, most practicing clinicians were never trained to use scales. For more information about the MBC curriculum and videotapes, contact Dr. Aboraya at [email protected] or visit www.scip-psychiatry.com.
Today, some of the barriers that impede the implementation of MBC in psychiatric practice have been resolved, but much more work remains. Now is the time to implement MBC and provide an answer to AJ Rush, who asked, “Isn’t it about time to employ measurement-based care in practice?”24 The 3 main ingredients for MBC implementation—useful measures, integration of EHR, and health information technologies—exist today. We strongly encourage psychiatrists, nurse practitioners, and other mental health professionals to adopt MBC in their daily practice.
To comment on this editorial or other topics of interest: [email protected].
In an editorial published in Current Psychiatry 10 years ago, I cited a stunning fact based on a readers’ survey: 98% of psychiatrists did not use any of the 4 clinical rating scales that are routinely used in the clinical trials required for FDA approval of medications for psychotic, mood, and anxiety disorders.1
As a follow-up, Ahmed Aboraya, MD, DrPH, and I would like to report on the state of measurement-based care (MBC), a term coined by Trivedi in 2006 and defined by Fortney as “the systematic administration of symptom rating scales and use of the results to drive clinical decision making at the level of the individual patient.”2
We will start with the creator of modern rating scales, Father Thomas Verner Moore (1877-1969), who is considered one of the most underrecognized legends in the history of modern psychiatry. Moore was a psychologist and psychiatrist who can lay claim to 3 major achievements in psychiatry: the creation of rating scales in psychiatry, the use of factor analysis to deconstruct psychosis, and the formulation of specific definitions for symptoms and signs of psychopathology. Moore’s 1933 book described the rating scales used in his research.3
Since that time, researchers have continued to invent clinician-rated scales, self-report scales, and other measures in psychiatry. The Handbook of Psychiatric Measures, which was published in 2000 by the American Psychiatric Association Task Force chaired by AJ Rush Jr., includes >240 measures covering adult and child psychiatric disorders.4
Recent research has shown the superiority of MBC compared with usual standard care (USC) in improving patient outcomes.2,5-7 A recent well-designed, blind-rater, randomized trial by Guo et al8 showed that MBC is more effective than USC both in achieving response and remission, and reducing the time to response and remission. Given the evidence of the benefits of MBC in improving patient outcomes, and the plethora of reliable and validated rating scales, an important question arises: Why has MBC not yet been established as the standard of care in psychiatric clinical practice? There are many barriers to implementing MBC,9 including:
- time constraints (most commonly cited reason by psychiatrists)
- mismatch between clinical needs and the content of the measure (ie, rating scales are designed for research and not for clinicians’ use)
- measurements produced by rating scales may not always be clinically relevant
- administering rating scales may interfere with establishing rapport with patients
- some measures, such as standardized diagnostic interviews, can be cumbersome, unwieldy, and complicated
- the lack of formal training for most clinicians (among the top barriers for residents and faculty)
- lack of availability of training manuals and protocols.
Clinician researchers have started to adapt and invent instruments that can be used in clinical settings. For more than 20 years, Mark Zimmerman, MD, has been the principal investigator of the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project, aimed at integrating the assessment methods of researchers into routine clinical practice.10 Zimmerman has developed self-report scales and outcome measures such as the Psychiatric Diagnostic Screening Questionnaire (PDSQ), the Clinically Useful Depression Outcome Scale (CUDOS), the Standardized Clinical Outcome Rating for Depression (SCOR-D), the Clinically Useful Anxiety Outcome Scale (CUXOS), the Remission from Depression Questionnaire (RDQ), and the Clinically Useful Patient Satisfaction Scale (CUPSS).11-18
We have been critical of the utility of the existing diagnostic interviews and rating scales. I (AA) developed the Standard for Clinicians’ Interview in Psychiatry (SCIP) as a MBC tool that addresses the most common barriers that clinicians face.9,19-23 The SCIP includes 18 clinician-rated scales for the following symptom domains: generalized anxiety, obsessions, compulsions, posttraumatic stress, depression, mania, delusions, hallucinations, disorganized thoughts, aggression, negative symptoms, alcohol use, drug use, attention deficit, hyperactivity, anorexia, binge-eating, and bulimia. The SCIP rating scales meet the criteria for MBC because they are efficient, reliable, and valid. They reflect how clinicians assess psychiatric disorders, and are relevant to decision-making. Both self-report and clinician-rated scales are important MBC tools and complementary to each other. The choice to use self-report scales, clinician-rated scales, or both depends on several factors, including the clinical setting (inpatient or outpatient), psychiatric diagnoses, and patient characteristics. No measure or scale will ever replace a seasoned and experienced clinician who has been evaluating and treating real-world patients for years. Just as thermometers, stethoscopes, and laboratories help other types of physicians to reach accurate diagnoses and provide appropriate management, the use of MBC by psychiatrists will enhance the accuracy of diagnoses and improve the outcomes of care.
Continue to: On a positive note...
On a positive note, I (AA) have completed a MBC curriculum for training psychiatry residents that includes 11 videotaped interviews with actual patients covering the major adult psychiatric disorders: generalized anxiety, panic, depressive, posttraumatic stress, bipolar, psychotic, eating, and attention-deficit/hyperactivity. The interviews show and teach how to rate psychopathology items, how to score the dimensions, and how to evaluate the severity of the disorder(s). All of the SCIP’s 18 scales have been uploaded into the Epic electronic health record (EHR) system at West Virginia University hospitals. A pilot project for implementing MBC in the treatment of adult psychiatric disorders at the West Virginia University residency program and other programs is underway. If we instruct residents in MBC during their psychiatric training, they will likely practice it for the rest of their clinical careers. Except for a minority of clinicians who are involved in clinical trials and who use rating scales in practice, most practicing clinicians were never trained to use scales. For more information about the MBC curriculum and videotapes, contact Dr. Aboraya at [email protected] or visit www.scip-psychiatry.com.
Today, some of the barriers that impede the implementation of MBC in psychiatric practice have been resolved, but much more work remains. Now is the time to implement MBC and provide an answer to AJ Rush, who asked, “Isn’t it about time to employ measurement-based care in practice?”24 The 3 main ingredients for MBC implementation—useful measures, integration of EHR, and health information technologies—exist today. We strongly encourage psychiatrists, nurse practitioners, and other mental health professionals to adopt MBC in their daily practice.
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
2. Fortney JC, Unutzer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2016;68(2):179-188.
3. Moore TV. The essential psychoses and their fundamental syndromes. Baltimore, MD: Williams & Wilkins; 1933.
4. Rush AJ. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000.
5. Scott K, Lewis CC. Using measurement-based care to enhance any treatment. Cogn Behav Pract. 2015;22(1):49-59.
6. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61-S71.
7. Harding KJ, Rush AJ, Arbuckle M, et al. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136-1143.
8. Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004-1013.
9. Aboraya A, Nasrallah HA, Elswick D, et al. Measurement-based care in psychiatry: past, present and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
10. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
11. Zimmerman M, Mattia JI. The reliability and validity of a screening questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60(10):677-683.
12. Zimmerman M, Mattia JI. The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Compr Psychiatry. 2001;42(3):175-189.
13. Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131-140.
14. Zimmerman M, Posternak MA, Chelminski I, et al. Standardized clinical outcome rating scale for depression for use in clinical practice. Depress Anxiety. 2005;22(1):36-40.
15. Zimmerman M, Chelminski I, Young D, et al. A clinically useful anxiety outcome scale. J Clin Psychiatry. 2010;71(5):534-542.
16. Zimmerman M, Galione JN, Attiullah N, et al. Depressed patients’ perspectives of 2 measures of outcome: the Quick Inventory of Depressive Symptomatology (QIDS) and the Remission from Depression Questionnaire (RDQ). Ann Clin Psychiatry. 2011;23(3):208-212.
17. Zimmerman M, Martinez JH, Attiullah N, et al. The remission from depression questionnaire as an outcome measure in the treatment of depression. Depress Anxiety. 2014;31(6):533-538.
18. Zimmerman M, Gazarian D, Multach M, et al. A clinically useful self-report measure of psychiatric patients’ satisfaction with the initial evaluation. Psychiatry Res. 2017;252:38-44.
19. Aboraya A. The validity results of the Standard for Clinicians’ Interview in Psychiatry (SCIP). Schizophrenia Bulletin. 2015;41(Suppl 1):S103-S104.
20. Aboraya A. Instruction manual for the Standard for Clinicians’ Interview in Psychiatry (SCIP). http://innovationscns.com/wp-content/uploads/SCIP_Instruction_Manual.pdf. Accessed April 29, 2019.
21. Aboraya A, El-Missiry A, Barlowe J, et al. The reliability of the Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional and numeric output. Schizophr Res. 2014;156(2-3):174-183.
22. Aboraya A, Nasrallah HA, Muvvala S, et al. The Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional, and numeric output-conceptual development, design, and description of the SCIP. Innov Clin Neurosci. 2016;13(5-6):31-77.
23. Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, drawbacks, and a new alternative for schizophrenia research. Ann Clin Psychiatry. 2016;28(2):125-131.
24. Rush AJ. Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934-936.
1. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
2. Fortney JC, Unutzer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2016;68(2):179-188.
3. Moore TV. The essential psychoses and their fundamental syndromes. Baltimore, MD: Williams & Wilkins; 1933.
4. Rush AJ. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000.
5. Scott K, Lewis CC. Using measurement-based care to enhance any treatment. Cogn Behav Pract. 2015;22(1):49-59.
6. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61-S71.
7. Harding KJ, Rush AJ, Arbuckle M, et al. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136-1143.
8. Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004-1013.
9. Aboraya A, Nasrallah HA, Elswick D, et al. Measurement-based care in psychiatry: past, present and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
10. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
11. Zimmerman M, Mattia JI. The reliability and validity of a screening questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60(10):677-683.
12. Zimmerman M, Mattia JI. The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Compr Psychiatry. 2001;42(3):175-189.
13. Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131-140.
14. Zimmerman M, Posternak MA, Chelminski I, et al. Standardized clinical outcome rating scale for depression for use in clinical practice. Depress Anxiety. 2005;22(1):36-40.
15. Zimmerman M, Chelminski I, Young D, et al. A clinically useful anxiety outcome scale. J Clin Psychiatry. 2010;71(5):534-542.
16. Zimmerman M, Galione JN, Attiullah N, et al. Depressed patients’ perspectives of 2 measures of outcome: the Quick Inventory of Depressive Symptomatology (QIDS) and the Remission from Depression Questionnaire (RDQ). Ann Clin Psychiatry. 2011;23(3):208-212.
17. Zimmerman M, Martinez JH, Attiullah N, et al. The remission from depression questionnaire as an outcome measure in the treatment of depression. Depress Anxiety. 2014;31(6):533-538.
18. Zimmerman M, Gazarian D, Multach M, et al. A clinically useful self-report measure of psychiatric patients’ satisfaction with the initial evaluation. Psychiatry Res. 2017;252:38-44.
19. Aboraya A. The validity results of the Standard for Clinicians’ Interview in Psychiatry (SCIP). Schizophrenia Bulletin. 2015;41(Suppl 1):S103-S104.
20. Aboraya A. Instruction manual for the Standard for Clinicians’ Interview in Psychiatry (SCIP). http://innovationscns.com/wp-content/uploads/SCIP_Instruction_Manual.pdf. Accessed April 29, 2019.
21. Aboraya A, El-Missiry A, Barlowe J, et al. The reliability of the Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional and numeric output. Schizophr Res. 2014;156(2-3):174-183.
22. Aboraya A, Nasrallah HA, Muvvala S, et al. The Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional, and numeric output-conceptual development, design, and description of the SCIP. Innov Clin Neurosci. 2016;13(5-6):31-77.
23. Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, drawbacks, and a new alternative for schizophrenia research. Ann Clin Psychiatry. 2016;28(2):125-131.
24. Rush AJ. Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934-936.
From sweet to belligerent in the blink of an eye
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7

A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.
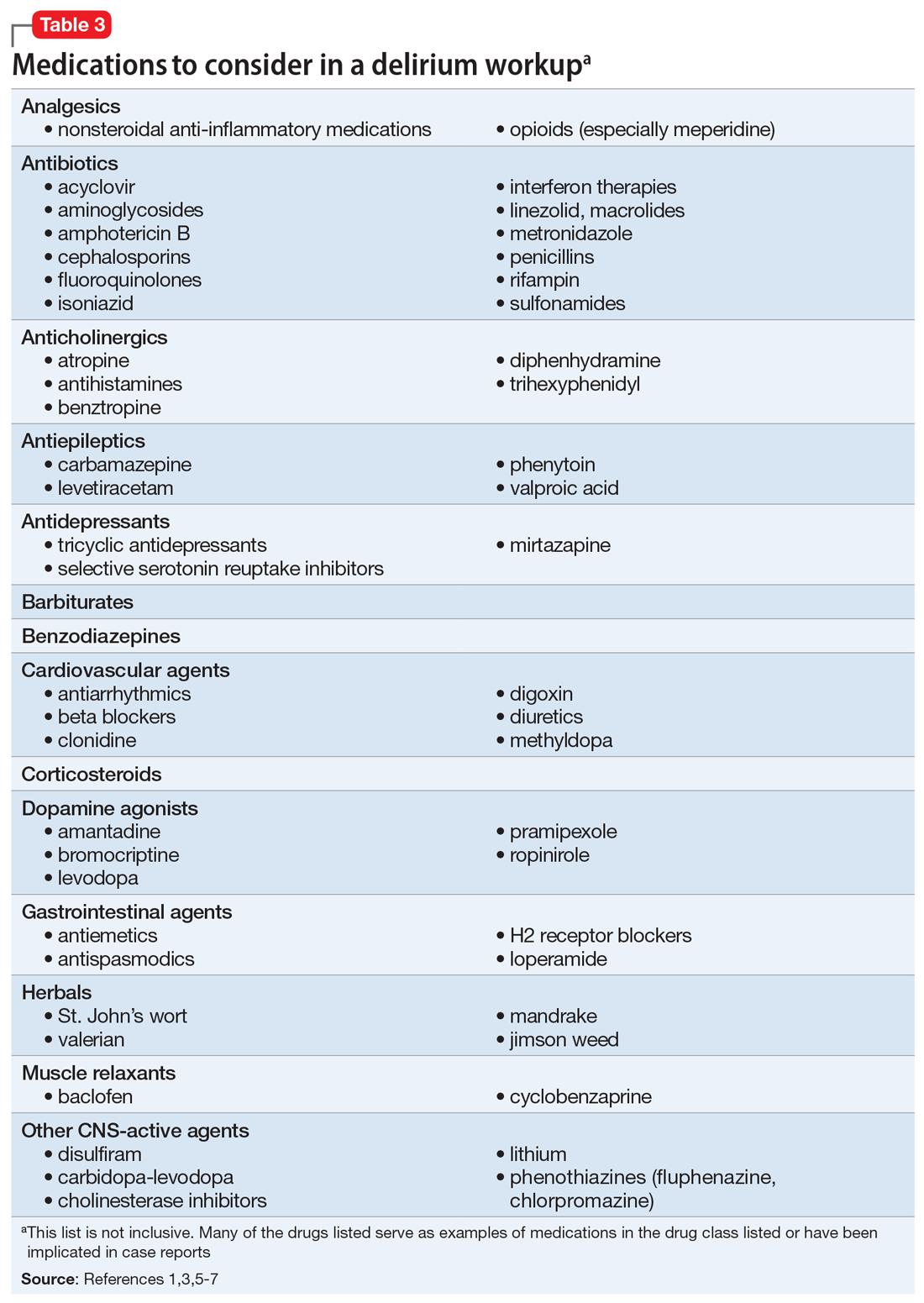
[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7

A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.

[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7

A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.

[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
Caring for patients on probation or parole
Mr. A, age 35, presents to your outpatient community mental health practice. He has a history of psychosis that began in his late teens. Since then, his symptoms have included derogatory auditory hallucinations, a recurrent persecutory delusion that governmental agencies are tracking his movements, and intermittent disorganized speech. At age 30, Mr. A assaulted a stranger out of fear that the individual was a government agent. He was arrested and experienced a severe psychotic decompensation while awaiting trial. He was found incompetent to stand trial and sent to a state hospital for restoration.
After 6 months of treatment and observation, Mr. A was deemed competent to proceed and returned to jail. He was subsequently convicted of assault and sentenced to 7 years in prison. While in prison, he received regular mental health care with infrequent recurrence of minor psychotic symptoms. He was released on parole due to his good behavior, but as part of his conditions of parole, he was mandated to follow up with an outpatient mental health clinician.
After telling you the story of how he ended up in your office, Mr. A says he needs you to speak regularly with his parole officer to verify his attendance at appointments and to discuss any mental health concerns you may have. Since you have not worked with a patient on parole before, your mind is full of questions: What are the expectations regarding your communication with his parole officer? Could Mr. A return to prison if you express concerns about his mental health? What can you do to improve his chances of success in the community?
Given the high rates of mental illness among individuals incarcerated in the United States, it shouldn’t be surprising that there are similarly high rates of mental illness among those on supervised release from jails and prisons. Clinicians who work with patients on community release need to understand basic concepts related to probation and parole, and how to promote patients’ stability in the community to reduce recidivism and re-incarceration. The court may require individuals on probation or parole to adhere to certain conditions of release, which could include seeing a psychiatrist or psychotherapist, participating in substance abuse treatment, and/or taking psychotropic medication. The court usually closely monitors the probationer or parolee’s adherence, and noncompliance can be grounds for probation or parole violation and revocation.
This article reviews the concepts of probation and parole (Box1,2), describes the prevalence of mental illness among probationers and parolees, and discusses the unique challenges and opportunities psychiatrists and other mental health professionals face when working with individuals on community supervision.
Box
The US Bureau of Justice Statistics (BJS) defines probation as a “court-ordered period of correctional supervision in the community, generally as an alternative to incarceration.” Probation allows individuals to be released from jail to community supervision, with the potential for dismissal or lowering of charges if they adhere to the conditions of probation. Conditions of probation may include participating in substance abuse or mental health treatment programs, abstaining from drugs and alcohol, and avoiding contact with known felons. Failure to comply with conditions of probation can lead to re-incarceration and probation revocation.1 If probation is revoked, a probationer may be sentenced, potentially to prison, depending on the severity of the original offense.2
The BJS defines parole as “a period of conditional supervised release in the community following a term in state or federal prison.”2 Parole allows for the community supervision of individuals who have already been convicted of and sentenced to prison for a crime. Individuals may be released on parole if they demonstrate good behavior while incarcerated. Similar to probationers, parolees must adhere to the conditions of parole, and violation of these may lead to re-incarceration.1
As of December 31, 2016, there were more than 4.5 million adults on community supervision in the United States, representing 1 out of every 55 adults in the US population. Individuals on probation accounted for 81% of adults on community supervision. The number of people on community supervision has dropped continuously over the last decade, a trend driven by 2% annual decreases in the probation population. In contrast, the parolee population has continued to grow over time and was approximately 900,000 individuals at the end of 2016.2
Mental illness among probationers and parolees
Research on mental illness in people involved in the criminal justice system has largely focused on those who are incarcerated. Studies have documented high rates of severe mental illness (SMI), such as schizophrenia and bipolar disorder, among those who are incarcerated; some estimate the rates to be 3 times as high as those of community samples.3,4 In addition to SMI, substance use disorders and personality disorders (in particular, antisocial personality disorder) are common among people who are incarcerated.5,6
Comparatively little is known about mental illness among probationers and parolees, although presumably there would be a similarly high prevalence of SMI, substance use disorders, and other psychiatric disorders among this population. A 1997 Bureau of Justice Statistics (BJS) survey of approximately 3.4 million probationers found that 13.8% self-reported a mental or emotional condition and 8.2% self-reported a history of an “overnight stay in a mental hospital.”7 The BJS estimated that there were approximately 550,000 probationers with mental illness in the United States. The study’s author noted that probationers with mental illness were more likely to have a history of prior offenses and more likely to be violent recidivists. In terms of substance use, compared with other probationers, those with mental illness were more likely to report using drugs in the month before their most recent offense and at the time of the offense.7
Continue to: More recent research...
More recent research, although limited, has shed some light on the role of mental health services for individuals on probation and parole. In 2009, Crilly et al8 reported that 23% of probationers reported accessing mental health services within the past year. Other studies have found that probationer and parolee engagement in mental health care reduces the risk of recidivism.9,10 A 2011 study evaluated 100 individuals on probation and parole in 2 counties in a southeastern state. The authors found that 75% of participants reported that they needed counseling for a mental health concern in the past year, but that only approximately 30% of them actually sought help. Individuals reporting higher levels of posttraumatic stress disorder symptomatology or greater drug use before being on probation or parole were more likely to seek counseling in the past year.11
An alternative: Problem-solving courts
Problem-solving courts (PSCs) offer an alternative to standard probation and/or sentencing. Problem-solving courts are founded on the concept of therapeutic jurisprudence, which seeks to change “the behavior of litigants and [ensure] the future well-being of communities.”12 Types of PSCs include drug court (the most common type in the United States), domestic violence court, veterans court, and mental health court (MHC), among others.
An individual may choose a PSC over standard probation because participants usually receive more assistance in obtaining treatment and closer supervision with an emphasis on rehabilitation rather than incapacitation or retribution. The success of PSCs relies heavily on the judge, as he/she plays a pivotal role in developing relationships with the participants, considering therapeutic alternatives to “bad” behaviors, determining sanctions, and relying on community mental health partners to assist participants in complying with conditions of the court.13-15
Psychiatrists and other mental health clinicians should be aware of MHCs, which are a type of PSC that provides for the community supervision of individuals with mental illness. Mental health courts vary in terms of eligibility criteria. Some accept individuals who merely report a history of mental illness, whereas others have specific diagnostic requirements.16 Some accept individuals accused of minor violations such as ordinance violations or misdemeanor offenses, while others accept individuals accused of felonies. Like other PSCs, participation in an MHC is voluntary, and most require a participant to enter a guilty plea upon entry.17 Participants may choose to enter an MHC to avoid prison time or to reduce or expunge charges after completing the program. Many MHCs also assign a probation officer to follow the participant in the community, similar to a standard probation model. Participants are usually expected to engage in psychiatric treatment, including psychotherapy, substance abuse counseling, medication management, and other services. If they do not comply with these conditions, they face sanctions that could include jail “shock” time, enhanced supervision, or an increase in psychiatric services.
Outpatient mental health professionals play an integral role in MHCs. Depending on the model, he/she may be asked to communicate treatment recommendations, attend weekly meetings at the court, and provide suggestions for interventions when the participant relapses, recidivates, and/or decompensates psychiatrically. This collaborative model can work well and allow the clinician unique opportunities to educate the court and advocate for his/her patient. However, clinicians who participate in an MHC need to remain aware of the potential to become a de facto probation officer, and need to maintain appropriate boundaries and roles. They should ensure that the patient provides initial and ongoing consent for them to communicate with the court, and share their programmatic recommendations with the patient to preserve the therapeutic alliance.
Continue to: Challenges upon re-entering the community
Challenges upon re-entering the community
Individuals recently released from jail or prison face unique challenges when re-entering the community. An individual who has been incarcerated, particularly for months to years, has likely lost his/her job, housing, health insurance, and access to primary supports. People with mental illness with a history of incarceration have higher rates of homelessness, substance use disorders, and unemployment than those with no history of incarceration.7,18 For individuals with mental illness, these additional stressors lead to further psychiatric decompensation, recidivism, and overutilization of emergency and crisis services upon release from prison or jail. The loss of health insurance presents great challenges: when someone is incarcerated, his/her Medicaid is suspended or terminated.19 This can happen at any point during incarceration. In states that terminate rather than suspend Medicaid, former prisoners face even longer waits to re-establish access to needed health care.
The period immediately after release is a critical time for individuals to be linked with substance and mental health treatment. Binswanger et al20 found former prisoners were at highest risk of mortality in the 2 weeks following release from prison; the highest rates of death were from drug overdose, cardiovascular disease, homicide, and suicide. A subsequent study found that women were at increased risk of drug overdose and opioid-related deaths.21 One explanation for the increase in drug-related deaths is the loss of physiologic tolerance while incarcerated; however, a lack of treatment while incarcerated, high levels of stress upon re-entry, and poor linkage to aftercare also may be contributing factors. Among prisoners recently released from New York City jails, Lim et al22 found that those with a history of homelessness and previous incarceration had the highest rates of drug-related deaths and homicides in the first 2 weeks after release. Non-Hispanic white men had the highest risk of drug-related deaths and suicides. While the risk of death is greatest immediately after release, former prisoners face increased mortality from multiple causes for multiple years after release.20-22
Clinicians who work with recently released prisoners should be aware of these individuals’ risks and actively work with them and other members of the mental health team to ensure these patients have access to social services, employment training, housing, and substance use resources, including medication-assisted treatment. Patients with SMI should be considered for more intensive services, such as assertive community treatment (ACT) or even forensic ACT (FACT) services, given that FACTs have a modest impact in reducing recidivism.23
Knowing whether the patient is on probation or parole and the terms of his/her supervision can also be useful in creating and executing a collaborative treatment plan. The clinician can assist the patient in meeting conditions of probation/parole such as:
- creating a stable home plan with a permanent address
- planning routine check-ins with probation/parole officers, and
- keeping documentation of ongoing mental health and substance use treatment.
Being aware of other terms of supervision, such as abstaining from alcohol and drugs, or remaining in one’s jurisdiction, also can help the patient avoid technical violations and a return to jail or prison.
Continue to: How to best help patients on community supervision
How to best help patients on community supervision
There are some clinical recommendations when working with patients on community supervision. First, do not assume that someone who has been incarcerated has antisocial personality disorder. Behaviors primarily related to seeking or using drugs or survival-type crimes should not be considered “antisocial” without additional evidence of pervasive and persistent conduct demonstrating impulsivity, lack of empathy, dishonesty, or repeated disregard for social norms and others’ rights. To meet criteria for antisocial personality disorder, these behaviors must have begun during childhood or adolescence.
If a patient does meet criteria for antisocial personality disorder, remember that he/she may also have a psychotic, mood, substance use, or other disorder that could lead to a greater likelihood of violence, recidivism, or other poor outcomes if left untreated. Treating any co-occurring disorders could enhance the patient’s engagement with treatment. There is some evidence that certain psychotropic medications, su
In addition to promoting patients’ mental health, such efforts can prevent re-arrest and re-incarceration and make a lasting positive impact on patients’ lives.
CASE CONTINUED
Mr. A signs a release-of-information form and you call his parole officer. His parole officer states that he would like to speak with you every few months to check on Mr. A’s treatment adherence. Within a few months, you transition Mr. A from an oral antipsychotic medication to a long-acting injectable antipsychotic medication to manage his psychotic disorder. He presents on time each month to your clinic to receive the injection.
Five months later, Mr. A receives 2 weeks of “shock time” at the local county jail for “dropping a dirty urine” that was positive for cannabinoids at a meeting with his parole officer. During his time in jail, he receives no treatment and he misses his monthly long-acting injectable dose.
Continue to: Upon release...
Upon release, he demonstrates the recurrence of some mild persecutory fears and hallucinations, but you resume him on his prior treatment regimen, and he recovers.
You encourage the parole officer to notify you if Mr. A violates parole and is incarcerated so that you can speak with clinicians in the jail to ensure that Mr. A remains adequately treated while incarcerated.
In the coming years, you continue to work with Mr. A and his parole officer to manage his mental health condition and to navigate his parole requirements in order to reduce his risk of relapse and recidivism. After Mr. A completes his time on parole, you continue to see him for outpatient follow-up.
Bottom Line
Clinicians may provide psychiatric care to probationers and parolees in traditional outpatient settings or in collaboration with a mental health court (MHC) or forensic assertive community treatment team. It is crucial to be aware of the legal expectations of individuals on community supervision, as well as the unique mental health risks and challenges they face. You can help reduce probationers’ and parolees’ risk of relapse and recidivism and support their recovery in the community by engaging in collaborative treatment planning involving the patient, the court, and/or MHCs.
Related Resources
- Lamb HR. Weinberger LE. Understanding and treating offenders with serious mental illness in public sector mental health. Behav Sci Law. 2017;35(4):303-318.
- Worcester S. Mental illness and the criminal justice system: Reducing the risks. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/173208/schizophrenia-other-psychotic-disorders/mental-illness-and-criminal. Published August 22, 2018.
1. Bureau of Justice Statistics. FAQ detail: What is the difference between probation and parole? U.S. Department of Justice. https://www.bjs.gov/index.cfm?ty=qa&iid=324. Accessed November 17, 2018.
2. Kaeble D. Probation and parole in the United States, 2016. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/ppus16.pdf. Published April 2018. Accessed April 23, 2019.
3. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Diamond, P.M., et al., The prevalence of mental illness in prison. Adm Policy Ment Health. 2001;29(1):21-40.
5. MacDonald R, Kaba F, Rosner Z, et al. The Rikers Island hot spotters: defining the needs of the most frequently incarcerated. Am J Public Health. 2015;105(11):2262-2268.
6. Trestman RL, Ford J, Zhang W, et al. Current and lifetime psychiatric illness among inmates not identified as acutely mentally ill at intake in Connecticut’s jails. J Am Acad Psychiatry Law. 2007;35(4):490-500.
7. Ditton PM. Bureau of Justice Statistics special report: mental health and treatment of inmates and probationers. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/mhtip.pdf. Published July 1999. Accessed April 24, 2019.
8. Crilly JF, Caine ED, Lamberti JS, et al. Mental health services use and symptom prevalence in a cohort of adults on probation. Psychiatr Serv. 2009;60(4):542-544.
9. Herinckx HA, Swart SC, Ama SM, et al. Rearrest and linkage to mental health services among clients of the Clark County mental health court program. Psychiatr Serv. 2005;56(7):853-857.
10. Solomon P, Draine J, Marcus SC. Predicting incarceration of clients of a psychiatric probation and parole service. Psychiatr Serv. 2002;53(1):50-56.
11. Owens GP, Rogers SM, Whitesell AA. Use of mental health services and barriers to care for individuals on probation or parole. J Offender Rehabil. 2011;50(1):35-47.
12. Berman G, Feinblatt J. Problem‐solving courts: a brief primer. Law and Policy. 2001;23(2):126.
13. The Council of State Governments Justice Center. Mental health courts: a guide to research-informed policy and practice. U.S. Department of Justice. https://www.bja.gov/Publications/CSG_MHC_Research.pdf. Published 2009. Accessed November 22, 2018.
14. Landess J, Holoyda B. Mental health courts and forensic assertive community treatment teams as correctional diversion programs. Behav Sci Law. 2017;35(5-6):501-511.
15. Sammon KC. Therapeutic jurisprudence: an examination of problem‐solving justice in New York. Journal of Civil Rights and Economic Development. 2008;23:923.
16. Sarteschi CM, Vaughn MG, Kim, K. Assessing the effectiveness of mental health courts: a quantitative review. Journal of Criminal Justice. 2011;39(1):12-20.
17. Strong SM, Rantala RR. Census of problem-solving courts, 2012. U.S. Department of Justice, Bureau of Justice Assistance. http://www.bjs.gov/content/pub/pdf/cpsc12.pdf. Revised October 12, 2016. Accessed April 24, 2019.
18. McGuire JF, Rosenheck RA. Criminal history as a prognostic indicator in the treatment of homeless people with severe mental illness. Psychiatr Serv. 2004;55(1):42-48.
19. Families USA. Medicaid suspension policies for incarcerated people: a 50-state map. Families USA. https://familiesusa.org/product/medicaid-suspension-policies-incarcerated-people-50-state-map. Published July 2016. Accessed December 7, 2018.
20. Binswanger IA, Stern MF, Deyo RA, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356(2):157-165.
21. Binswanger IA, Blatchford PJ, Mueller SR, et al. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592-600.
22. Lim S, Seligson AL, Parvez FM, et al. Risks of drug-related death, suicide, and homicide during the immediate post-release period among people released from New York City Jails, 2001-2005. Am J Epidemiol. 2012;175(6):519-526.
23. Cusack KJ, Morrissey JP, Cuddeback GS, et al. Criminal justice involvement, behavioral health service use, and costs of forensic assertive community treatment: a randomized trial. Community Ment Health J. 2010;46(4):356-363.
24. Felthous AR, Stanford MS. A proposed algorithm for the pharmacotherapy of impulsive aggression. J Am Acad Psychiatry Law. 2015:43(4);456-467.
Mr. A, age 35, presents to your outpatient community mental health practice. He has a history of psychosis that began in his late teens. Since then, his symptoms have included derogatory auditory hallucinations, a recurrent persecutory delusion that governmental agencies are tracking his movements, and intermittent disorganized speech. At age 30, Mr. A assaulted a stranger out of fear that the individual was a government agent. He was arrested and experienced a severe psychotic decompensation while awaiting trial. He was found incompetent to stand trial and sent to a state hospital for restoration.
After 6 months of treatment and observation, Mr. A was deemed competent to proceed and returned to jail. He was subsequently convicted of assault and sentenced to 7 years in prison. While in prison, he received regular mental health care with infrequent recurrence of minor psychotic symptoms. He was released on parole due to his good behavior, but as part of his conditions of parole, he was mandated to follow up with an outpatient mental health clinician.
After telling you the story of how he ended up in your office, Mr. A says he needs you to speak regularly with his parole officer to verify his attendance at appointments and to discuss any mental health concerns you may have. Since you have not worked with a patient on parole before, your mind is full of questions: What are the expectations regarding your communication with his parole officer? Could Mr. A return to prison if you express concerns about his mental health? What can you do to improve his chances of success in the community?
Given the high rates of mental illness among individuals incarcerated in the United States, it shouldn’t be surprising that there are similarly high rates of mental illness among those on supervised release from jails and prisons. Clinicians who work with patients on community release need to understand basic concepts related to probation and parole, and how to promote patients’ stability in the community to reduce recidivism and re-incarceration. The court may require individuals on probation or parole to adhere to certain conditions of release, which could include seeing a psychiatrist or psychotherapist, participating in substance abuse treatment, and/or taking psychotropic medication. The court usually closely monitors the probationer or parolee’s adherence, and noncompliance can be grounds for probation or parole violation and revocation.
This article reviews the concepts of probation and parole (Box1,2), describes the prevalence of mental illness among probationers and parolees, and discusses the unique challenges and opportunities psychiatrists and other mental health professionals face when working with individuals on community supervision.
Box
The US Bureau of Justice Statistics (BJS) defines probation as a “court-ordered period of correctional supervision in the community, generally as an alternative to incarceration.” Probation allows individuals to be released from jail to community supervision, with the potential for dismissal or lowering of charges if they adhere to the conditions of probation. Conditions of probation may include participating in substance abuse or mental health treatment programs, abstaining from drugs and alcohol, and avoiding contact with known felons. Failure to comply with conditions of probation can lead to re-incarceration and probation revocation.1 If probation is revoked, a probationer may be sentenced, potentially to prison, depending on the severity of the original offense.2
The BJS defines parole as “a period of conditional supervised release in the community following a term in state or federal prison.”2 Parole allows for the community supervision of individuals who have already been convicted of and sentenced to prison for a crime. Individuals may be released on parole if they demonstrate good behavior while incarcerated. Similar to probationers, parolees must adhere to the conditions of parole, and violation of these may lead to re-incarceration.1
As of December 31, 2016, there were more than 4.5 million adults on community supervision in the United States, representing 1 out of every 55 adults in the US population. Individuals on probation accounted for 81% of adults on community supervision. The number of people on community supervision has dropped continuously over the last decade, a trend driven by 2% annual decreases in the probation population. In contrast, the parolee population has continued to grow over time and was approximately 900,000 individuals at the end of 2016.2
Mental illness among probationers and parolees
Research on mental illness in people involved in the criminal justice system has largely focused on those who are incarcerated. Studies have documented high rates of severe mental illness (SMI), such as schizophrenia and bipolar disorder, among those who are incarcerated; some estimate the rates to be 3 times as high as those of community samples.3,4 In addition to SMI, substance use disorders and personality disorders (in particular, antisocial personality disorder) are common among people who are incarcerated.5,6
Comparatively little is known about mental illness among probationers and parolees, although presumably there would be a similarly high prevalence of SMI, substance use disorders, and other psychiatric disorders among this population. A 1997 Bureau of Justice Statistics (BJS) survey of approximately 3.4 million probationers found that 13.8% self-reported a mental or emotional condition and 8.2% self-reported a history of an “overnight stay in a mental hospital.”7 The BJS estimated that there were approximately 550,000 probationers with mental illness in the United States. The study’s author noted that probationers with mental illness were more likely to have a history of prior offenses and more likely to be violent recidivists. In terms of substance use, compared with other probationers, those with mental illness were more likely to report using drugs in the month before their most recent offense and at the time of the offense.7
Continue to: More recent research...
More recent research, although limited, has shed some light on the role of mental health services for individuals on probation and parole. In 2009, Crilly et al8 reported that 23% of probationers reported accessing mental health services within the past year. Other studies have found that probationer and parolee engagement in mental health care reduces the risk of recidivism.9,10 A 2011 study evaluated 100 individuals on probation and parole in 2 counties in a southeastern state. The authors found that 75% of participants reported that they needed counseling for a mental health concern in the past year, but that only approximately 30% of them actually sought help. Individuals reporting higher levels of posttraumatic stress disorder symptomatology or greater drug use before being on probation or parole were more likely to seek counseling in the past year.11
An alternative: Problem-solving courts
Problem-solving courts (PSCs) offer an alternative to standard probation and/or sentencing. Problem-solving courts are founded on the concept of therapeutic jurisprudence, which seeks to change “the behavior of litigants and [ensure] the future well-being of communities.”12 Types of PSCs include drug court (the most common type in the United States), domestic violence court, veterans court, and mental health court (MHC), among others.
An individual may choose a PSC over standard probation because participants usually receive more assistance in obtaining treatment and closer supervision with an emphasis on rehabilitation rather than incapacitation or retribution. The success of PSCs relies heavily on the judge, as he/she plays a pivotal role in developing relationships with the participants, considering therapeutic alternatives to “bad” behaviors, determining sanctions, and relying on community mental health partners to assist participants in complying with conditions of the court.13-15
Psychiatrists and other mental health clinicians should be aware of MHCs, which are a type of PSC that provides for the community supervision of individuals with mental illness. Mental health courts vary in terms of eligibility criteria. Some accept individuals who merely report a history of mental illness, whereas others have specific diagnostic requirements.16 Some accept individuals accused of minor violations such as ordinance violations or misdemeanor offenses, while others accept individuals accused of felonies. Like other PSCs, participation in an MHC is voluntary, and most require a participant to enter a guilty plea upon entry.17 Participants may choose to enter an MHC to avoid prison time or to reduce or expunge charges after completing the program. Many MHCs also assign a probation officer to follow the participant in the community, similar to a standard probation model. Participants are usually expected to engage in psychiatric treatment, including psychotherapy, substance abuse counseling, medication management, and other services. If they do not comply with these conditions, they face sanctions that could include jail “shock” time, enhanced supervision, or an increase in psychiatric services.
Outpatient mental health professionals play an integral role in MHCs. Depending on the model, he/she may be asked to communicate treatment recommendations, attend weekly meetings at the court, and provide suggestions for interventions when the participant relapses, recidivates, and/or decompensates psychiatrically. This collaborative model can work well and allow the clinician unique opportunities to educate the court and advocate for his/her patient. However, clinicians who participate in an MHC need to remain aware of the potential to become a de facto probation officer, and need to maintain appropriate boundaries and roles. They should ensure that the patient provides initial and ongoing consent for them to communicate with the court, and share their programmatic recommendations with the patient to preserve the therapeutic alliance.
Continue to: Challenges upon re-entering the community
Challenges upon re-entering the community
Individuals recently released from jail or prison face unique challenges when re-entering the community. An individual who has been incarcerated, particularly for months to years, has likely lost his/her job, housing, health insurance, and access to primary supports. People with mental illness with a history of incarceration have higher rates of homelessness, substance use disorders, and unemployment than those with no history of incarceration.7,18 For individuals with mental illness, these additional stressors lead to further psychiatric decompensation, recidivism, and overutilization of emergency and crisis services upon release from prison or jail. The loss of health insurance presents great challenges: when someone is incarcerated, his/her Medicaid is suspended or terminated.19 This can happen at any point during incarceration. In states that terminate rather than suspend Medicaid, former prisoners face even longer waits to re-establish access to needed health care.
The period immediately after release is a critical time for individuals to be linked with substance and mental health treatment. Binswanger et al20 found former prisoners were at highest risk of mortality in the 2 weeks following release from prison; the highest rates of death were from drug overdose, cardiovascular disease, homicide, and suicide. A subsequent study found that women were at increased risk of drug overdose and opioid-related deaths.21 One explanation for the increase in drug-related deaths is the loss of physiologic tolerance while incarcerated; however, a lack of treatment while incarcerated, high levels of stress upon re-entry, and poor linkage to aftercare also may be contributing factors. Among prisoners recently released from New York City jails, Lim et al22 found that those with a history of homelessness and previous incarceration had the highest rates of drug-related deaths and homicides in the first 2 weeks after release. Non-Hispanic white men had the highest risk of drug-related deaths and suicides. While the risk of death is greatest immediately after release, former prisoners face increased mortality from multiple causes for multiple years after release.20-22
Clinicians who work with recently released prisoners should be aware of these individuals’ risks and actively work with them and other members of the mental health team to ensure these patients have access to social services, employment training, housing, and substance use resources, including medication-assisted treatment. Patients with SMI should be considered for more intensive services, such as assertive community treatment (ACT) or even forensic ACT (FACT) services, given that FACTs have a modest impact in reducing recidivism.23
Knowing whether the patient is on probation or parole and the terms of his/her supervision can also be useful in creating and executing a collaborative treatment plan. The clinician can assist the patient in meeting conditions of probation/parole such as:
- creating a stable home plan with a permanent address
- planning routine check-ins with probation/parole officers, and
- keeping documentation of ongoing mental health and substance use treatment.
Being aware of other terms of supervision, such as abstaining from alcohol and drugs, or remaining in one’s jurisdiction, also can help the patient avoid technical violations and a return to jail or prison.
Continue to: How to best help patients on community supervision
How to best help patients on community supervision
There are some clinical recommendations when working with patients on community supervision. First, do not assume that someone who has been incarcerated has antisocial personality disorder. Behaviors primarily related to seeking or using drugs or survival-type crimes should not be considered “antisocial” without additional evidence of pervasive and persistent conduct demonstrating impulsivity, lack of empathy, dishonesty, or repeated disregard for social norms and others’ rights. To meet criteria for antisocial personality disorder, these behaviors must have begun during childhood or adolescence.
If a patient does meet criteria for antisocial personality disorder, remember that he/she may also have a psychotic, mood, substance use, or other disorder that could lead to a greater likelihood of violence, recidivism, or other poor outcomes if left untreated. Treating any co-occurring disorders could enhance the patient’s engagement with treatment. There is some evidence that certain psychotropic medications, su
In addition to promoting patients’ mental health, such efforts can prevent re-arrest and re-incarceration and make a lasting positive impact on patients’ lives.
CASE CONTINUED
Mr. A signs a release-of-information form and you call his parole officer. His parole officer states that he would like to speak with you every few months to check on Mr. A’s treatment adherence. Within a few months, you transition Mr. A from an oral antipsychotic medication to a long-acting injectable antipsychotic medication to manage his psychotic disorder. He presents on time each month to your clinic to receive the injection.
Five months later, Mr. A receives 2 weeks of “shock time” at the local county jail for “dropping a dirty urine” that was positive for cannabinoids at a meeting with his parole officer. During his time in jail, he receives no treatment and he misses his monthly long-acting injectable dose.
Continue to: Upon release...
Upon release, he demonstrates the recurrence of some mild persecutory fears and hallucinations, but you resume him on his prior treatment regimen, and he recovers.
You encourage the parole officer to notify you if Mr. A violates parole and is incarcerated so that you can speak with clinicians in the jail to ensure that Mr. A remains adequately treated while incarcerated.
In the coming years, you continue to work with Mr. A and his parole officer to manage his mental health condition and to navigate his parole requirements in order to reduce his risk of relapse and recidivism. After Mr. A completes his time on parole, you continue to see him for outpatient follow-up.
Bottom Line
Clinicians may provide psychiatric care to probationers and parolees in traditional outpatient settings or in collaboration with a mental health court (MHC) or forensic assertive community treatment team. It is crucial to be aware of the legal expectations of individuals on community supervision, as well as the unique mental health risks and challenges they face. You can help reduce probationers’ and parolees’ risk of relapse and recidivism and support their recovery in the community by engaging in collaborative treatment planning involving the patient, the court, and/or MHCs.
Related Resources
- Lamb HR. Weinberger LE. Understanding and treating offenders with serious mental illness in public sector mental health. Behav Sci Law. 2017;35(4):303-318.
- Worcester S. Mental illness and the criminal justice system: Reducing the risks. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/173208/schizophrenia-other-psychotic-disorders/mental-illness-and-criminal. Published August 22, 2018.
Mr. A, age 35, presents to your outpatient community mental health practice. He has a history of psychosis that began in his late teens. Since then, his symptoms have included derogatory auditory hallucinations, a recurrent persecutory delusion that governmental agencies are tracking his movements, and intermittent disorganized speech. At age 30, Mr. A assaulted a stranger out of fear that the individual was a government agent. He was arrested and experienced a severe psychotic decompensation while awaiting trial. He was found incompetent to stand trial and sent to a state hospital for restoration.
After 6 months of treatment and observation, Mr. A was deemed competent to proceed and returned to jail. He was subsequently convicted of assault and sentenced to 7 years in prison. While in prison, he received regular mental health care with infrequent recurrence of minor psychotic symptoms. He was released on parole due to his good behavior, but as part of his conditions of parole, he was mandated to follow up with an outpatient mental health clinician.
After telling you the story of how he ended up in your office, Mr. A says he needs you to speak regularly with his parole officer to verify his attendance at appointments and to discuss any mental health concerns you may have. Since you have not worked with a patient on parole before, your mind is full of questions: What are the expectations regarding your communication with his parole officer? Could Mr. A return to prison if you express concerns about his mental health? What can you do to improve his chances of success in the community?
Given the high rates of mental illness among individuals incarcerated in the United States, it shouldn’t be surprising that there are similarly high rates of mental illness among those on supervised release from jails and prisons. Clinicians who work with patients on community release need to understand basic concepts related to probation and parole, and how to promote patients’ stability in the community to reduce recidivism and re-incarceration. The court may require individuals on probation or parole to adhere to certain conditions of release, which could include seeing a psychiatrist or psychotherapist, participating in substance abuse treatment, and/or taking psychotropic medication. The court usually closely monitors the probationer or parolee’s adherence, and noncompliance can be grounds for probation or parole violation and revocation.
This article reviews the concepts of probation and parole (Box1,2), describes the prevalence of mental illness among probationers and parolees, and discusses the unique challenges and opportunities psychiatrists and other mental health professionals face when working with individuals on community supervision.
Box
The US Bureau of Justice Statistics (BJS) defines probation as a “court-ordered period of correctional supervision in the community, generally as an alternative to incarceration.” Probation allows individuals to be released from jail to community supervision, with the potential for dismissal or lowering of charges if they adhere to the conditions of probation. Conditions of probation may include participating in substance abuse or mental health treatment programs, abstaining from drugs and alcohol, and avoiding contact with known felons. Failure to comply with conditions of probation can lead to re-incarceration and probation revocation.1 If probation is revoked, a probationer may be sentenced, potentially to prison, depending on the severity of the original offense.2
The BJS defines parole as “a period of conditional supervised release in the community following a term in state or federal prison.”2 Parole allows for the community supervision of individuals who have already been convicted of and sentenced to prison for a crime. Individuals may be released on parole if they demonstrate good behavior while incarcerated. Similar to probationers, parolees must adhere to the conditions of parole, and violation of these may lead to re-incarceration.1
As of December 31, 2016, there were more than 4.5 million adults on community supervision in the United States, representing 1 out of every 55 adults in the US population. Individuals on probation accounted for 81% of adults on community supervision. The number of people on community supervision has dropped continuously over the last decade, a trend driven by 2% annual decreases in the probation population. In contrast, the parolee population has continued to grow over time and was approximately 900,000 individuals at the end of 2016.2
Mental illness among probationers and parolees
Research on mental illness in people involved in the criminal justice system has largely focused on those who are incarcerated. Studies have documented high rates of severe mental illness (SMI), such as schizophrenia and bipolar disorder, among those who are incarcerated; some estimate the rates to be 3 times as high as those of community samples.3,4 In addition to SMI, substance use disorders and personality disorders (in particular, antisocial personality disorder) are common among people who are incarcerated.5,6
Comparatively little is known about mental illness among probationers and parolees, although presumably there would be a similarly high prevalence of SMI, substance use disorders, and other psychiatric disorders among this population. A 1997 Bureau of Justice Statistics (BJS) survey of approximately 3.4 million probationers found that 13.8% self-reported a mental or emotional condition and 8.2% self-reported a history of an “overnight stay in a mental hospital.”7 The BJS estimated that there were approximately 550,000 probationers with mental illness in the United States. The study’s author noted that probationers with mental illness were more likely to have a history of prior offenses and more likely to be violent recidivists. In terms of substance use, compared with other probationers, those with mental illness were more likely to report using drugs in the month before their most recent offense and at the time of the offense.7
Continue to: More recent research...
More recent research, although limited, has shed some light on the role of mental health services for individuals on probation and parole. In 2009, Crilly et al8 reported that 23% of probationers reported accessing mental health services within the past year. Other studies have found that probationer and parolee engagement in mental health care reduces the risk of recidivism.9,10 A 2011 study evaluated 100 individuals on probation and parole in 2 counties in a southeastern state. The authors found that 75% of participants reported that they needed counseling for a mental health concern in the past year, but that only approximately 30% of them actually sought help. Individuals reporting higher levels of posttraumatic stress disorder symptomatology or greater drug use before being on probation or parole were more likely to seek counseling in the past year.11
An alternative: Problem-solving courts
Problem-solving courts (PSCs) offer an alternative to standard probation and/or sentencing. Problem-solving courts are founded on the concept of therapeutic jurisprudence, which seeks to change “the behavior of litigants and [ensure] the future well-being of communities.”12 Types of PSCs include drug court (the most common type in the United States), domestic violence court, veterans court, and mental health court (MHC), among others.
An individual may choose a PSC over standard probation because participants usually receive more assistance in obtaining treatment and closer supervision with an emphasis on rehabilitation rather than incapacitation or retribution. The success of PSCs relies heavily on the judge, as he/she plays a pivotal role in developing relationships with the participants, considering therapeutic alternatives to “bad” behaviors, determining sanctions, and relying on community mental health partners to assist participants in complying with conditions of the court.13-15
Psychiatrists and other mental health clinicians should be aware of MHCs, which are a type of PSC that provides for the community supervision of individuals with mental illness. Mental health courts vary in terms of eligibility criteria. Some accept individuals who merely report a history of mental illness, whereas others have specific diagnostic requirements.16 Some accept individuals accused of minor violations such as ordinance violations or misdemeanor offenses, while others accept individuals accused of felonies. Like other PSCs, participation in an MHC is voluntary, and most require a participant to enter a guilty plea upon entry.17 Participants may choose to enter an MHC to avoid prison time or to reduce or expunge charges after completing the program. Many MHCs also assign a probation officer to follow the participant in the community, similar to a standard probation model. Participants are usually expected to engage in psychiatric treatment, including psychotherapy, substance abuse counseling, medication management, and other services. If they do not comply with these conditions, they face sanctions that could include jail “shock” time, enhanced supervision, or an increase in psychiatric services.
Outpatient mental health professionals play an integral role in MHCs. Depending on the model, he/she may be asked to communicate treatment recommendations, attend weekly meetings at the court, and provide suggestions for interventions when the participant relapses, recidivates, and/or decompensates psychiatrically. This collaborative model can work well and allow the clinician unique opportunities to educate the court and advocate for his/her patient. However, clinicians who participate in an MHC need to remain aware of the potential to become a de facto probation officer, and need to maintain appropriate boundaries and roles. They should ensure that the patient provides initial and ongoing consent for them to communicate with the court, and share their programmatic recommendations with the patient to preserve the therapeutic alliance.
Continue to: Challenges upon re-entering the community
Challenges upon re-entering the community
Individuals recently released from jail or prison face unique challenges when re-entering the community. An individual who has been incarcerated, particularly for months to years, has likely lost his/her job, housing, health insurance, and access to primary supports. People with mental illness with a history of incarceration have higher rates of homelessness, substance use disorders, and unemployment than those with no history of incarceration.7,18 For individuals with mental illness, these additional stressors lead to further psychiatric decompensation, recidivism, and overutilization of emergency and crisis services upon release from prison or jail. The loss of health insurance presents great challenges: when someone is incarcerated, his/her Medicaid is suspended or terminated.19 This can happen at any point during incarceration. In states that terminate rather than suspend Medicaid, former prisoners face even longer waits to re-establish access to needed health care.
The period immediately after release is a critical time for individuals to be linked with substance and mental health treatment. Binswanger et al20 found former prisoners were at highest risk of mortality in the 2 weeks following release from prison; the highest rates of death were from drug overdose, cardiovascular disease, homicide, and suicide. A subsequent study found that women were at increased risk of drug overdose and opioid-related deaths.21 One explanation for the increase in drug-related deaths is the loss of physiologic tolerance while incarcerated; however, a lack of treatment while incarcerated, high levels of stress upon re-entry, and poor linkage to aftercare also may be contributing factors. Among prisoners recently released from New York City jails, Lim et al22 found that those with a history of homelessness and previous incarceration had the highest rates of drug-related deaths and homicides in the first 2 weeks after release. Non-Hispanic white men had the highest risk of drug-related deaths and suicides. While the risk of death is greatest immediately after release, former prisoners face increased mortality from multiple causes for multiple years after release.20-22
Clinicians who work with recently released prisoners should be aware of these individuals’ risks and actively work with them and other members of the mental health team to ensure these patients have access to social services, employment training, housing, and substance use resources, including medication-assisted treatment. Patients with SMI should be considered for more intensive services, such as assertive community treatment (ACT) or even forensic ACT (FACT) services, given that FACTs have a modest impact in reducing recidivism.23
Knowing whether the patient is on probation or parole and the terms of his/her supervision can also be useful in creating and executing a collaborative treatment plan. The clinician can assist the patient in meeting conditions of probation/parole such as:
- creating a stable home plan with a permanent address
- planning routine check-ins with probation/parole officers, and
- keeping documentation of ongoing mental health and substance use treatment.
Being aware of other terms of supervision, such as abstaining from alcohol and drugs, or remaining in one’s jurisdiction, also can help the patient avoid technical violations and a return to jail or prison.
Continue to: How to best help patients on community supervision
How to best help patients on community supervision
There are some clinical recommendations when working with patients on community supervision. First, do not assume that someone who has been incarcerated has antisocial personality disorder. Behaviors primarily related to seeking or using drugs or survival-type crimes should not be considered “antisocial” without additional evidence of pervasive and persistent conduct demonstrating impulsivity, lack of empathy, dishonesty, or repeated disregard for social norms and others’ rights. To meet criteria for antisocial personality disorder, these behaviors must have begun during childhood or adolescence.
If a patient does meet criteria for antisocial personality disorder, remember that he/she may also have a psychotic, mood, substance use, or other disorder that could lead to a greater likelihood of violence, recidivism, or other poor outcomes if left untreated. Treating any co-occurring disorders could enhance the patient’s engagement with treatment. There is some evidence that certain psychotropic medications, su
In addition to promoting patients’ mental health, such efforts can prevent re-arrest and re-incarceration and make a lasting positive impact on patients’ lives.
CASE CONTINUED
Mr. A signs a release-of-information form and you call his parole officer. His parole officer states that he would like to speak with you every few months to check on Mr. A’s treatment adherence. Within a few months, you transition Mr. A from an oral antipsychotic medication to a long-acting injectable antipsychotic medication to manage his psychotic disorder. He presents on time each month to your clinic to receive the injection.
Five months later, Mr. A receives 2 weeks of “shock time” at the local county jail for “dropping a dirty urine” that was positive for cannabinoids at a meeting with his parole officer. During his time in jail, he receives no treatment and he misses his monthly long-acting injectable dose.
Continue to: Upon release...
Upon release, he demonstrates the recurrence of some mild persecutory fears and hallucinations, but you resume him on his prior treatment regimen, and he recovers.
You encourage the parole officer to notify you if Mr. A violates parole and is incarcerated so that you can speak with clinicians in the jail to ensure that Mr. A remains adequately treated while incarcerated.
In the coming years, you continue to work with Mr. A and his parole officer to manage his mental health condition and to navigate his parole requirements in order to reduce his risk of relapse and recidivism. After Mr. A completes his time on parole, you continue to see him for outpatient follow-up.
Bottom Line
Clinicians may provide psychiatric care to probationers and parolees in traditional outpatient settings or in collaboration with a mental health court (MHC) or forensic assertive community treatment team. It is crucial to be aware of the legal expectations of individuals on community supervision, as well as the unique mental health risks and challenges they face. You can help reduce probationers’ and parolees’ risk of relapse and recidivism and support their recovery in the community by engaging in collaborative treatment planning involving the patient, the court, and/or MHCs.
Related Resources
- Lamb HR. Weinberger LE. Understanding and treating offenders with serious mental illness in public sector mental health. Behav Sci Law. 2017;35(4):303-318.
- Worcester S. Mental illness and the criminal justice system: Reducing the risks. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/173208/schizophrenia-other-psychotic-disorders/mental-illness-and-criminal. Published August 22, 2018.
1. Bureau of Justice Statistics. FAQ detail: What is the difference between probation and parole? U.S. Department of Justice. https://www.bjs.gov/index.cfm?ty=qa&iid=324. Accessed November 17, 2018.
2. Kaeble D. Probation and parole in the United States, 2016. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/ppus16.pdf. Published April 2018. Accessed April 23, 2019.
3. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Diamond, P.M., et al., The prevalence of mental illness in prison. Adm Policy Ment Health. 2001;29(1):21-40.
5. MacDonald R, Kaba F, Rosner Z, et al. The Rikers Island hot spotters: defining the needs of the most frequently incarcerated. Am J Public Health. 2015;105(11):2262-2268.
6. Trestman RL, Ford J, Zhang W, et al. Current and lifetime psychiatric illness among inmates not identified as acutely mentally ill at intake in Connecticut’s jails. J Am Acad Psychiatry Law. 2007;35(4):490-500.
7. Ditton PM. Bureau of Justice Statistics special report: mental health and treatment of inmates and probationers. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/mhtip.pdf. Published July 1999. Accessed April 24, 2019.
8. Crilly JF, Caine ED, Lamberti JS, et al. Mental health services use and symptom prevalence in a cohort of adults on probation. Psychiatr Serv. 2009;60(4):542-544.
9. Herinckx HA, Swart SC, Ama SM, et al. Rearrest and linkage to mental health services among clients of the Clark County mental health court program. Psychiatr Serv. 2005;56(7):853-857.
10. Solomon P, Draine J, Marcus SC. Predicting incarceration of clients of a psychiatric probation and parole service. Psychiatr Serv. 2002;53(1):50-56.
11. Owens GP, Rogers SM, Whitesell AA. Use of mental health services and barriers to care for individuals on probation or parole. J Offender Rehabil. 2011;50(1):35-47.
12. Berman G, Feinblatt J. Problem‐solving courts: a brief primer. Law and Policy. 2001;23(2):126.
13. The Council of State Governments Justice Center. Mental health courts: a guide to research-informed policy and practice. U.S. Department of Justice. https://www.bja.gov/Publications/CSG_MHC_Research.pdf. Published 2009. Accessed November 22, 2018.
14. Landess J, Holoyda B. Mental health courts and forensic assertive community treatment teams as correctional diversion programs. Behav Sci Law. 2017;35(5-6):501-511.
15. Sammon KC. Therapeutic jurisprudence: an examination of problem‐solving justice in New York. Journal of Civil Rights and Economic Development. 2008;23:923.
16. Sarteschi CM, Vaughn MG, Kim, K. Assessing the effectiveness of mental health courts: a quantitative review. Journal of Criminal Justice. 2011;39(1):12-20.
17. Strong SM, Rantala RR. Census of problem-solving courts, 2012. U.S. Department of Justice, Bureau of Justice Assistance. http://www.bjs.gov/content/pub/pdf/cpsc12.pdf. Revised October 12, 2016. Accessed April 24, 2019.
18. McGuire JF, Rosenheck RA. Criminal history as a prognostic indicator in the treatment of homeless people with severe mental illness. Psychiatr Serv. 2004;55(1):42-48.
19. Families USA. Medicaid suspension policies for incarcerated people: a 50-state map. Families USA. https://familiesusa.org/product/medicaid-suspension-policies-incarcerated-people-50-state-map. Published July 2016. Accessed December 7, 2018.
20. Binswanger IA, Stern MF, Deyo RA, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356(2):157-165.
21. Binswanger IA, Blatchford PJ, Mueller SR, et al. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592-600.
22. Lim S, Seligson AL, Parvez FM, et al. Risks of drug-related death, suicide, and homicide during the immediate post-release period among people released from New York City Jails, 2001-2005. Am J Epidemiol. 2012;175(6):519-526.
23. Cusack KJ, Morrissey JP, Cuddeback GS, et al. Criminal justice involvement, behavioral health service use, and costs of forensic assertive community treatment: a randomized trial. Community Ment Health J. 2010;46(4):356-363.
24. Felthous AR, Stanford MS. A proposed algorithm for the pharmacotherapy of impulsive aggression. J Am Acad Psychiatry Law. 2015:43(4);456-467.
1. Bureau of Justice Statistics. FAQ detail: What is the difference between probation and parole? U.S. Department of Justice. https://www.bjs.gov/index.cfm?ty=qa&iid=324. Accessed November 17, 2018.
2. Kaeble D. Probation and parole in the United States, 2016. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/ppus16.pdf. Published April 2018. Accessed April 23, 2019.
3. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Diamond, P.M., et al., The prevalence of mental illness in prison. Adm Policy Ment Health. 2001;29(1):21-40.
5. MacDonald R, Kaba F, Rosner Z, et al. The Rikers Island hot spotters: defining the needs of the most frequently incarcerated. Am J Public Health. 2015;105(11):2262-2268.
6. Trestman RL, Ford J, Zhang W, et al. Current and lifetime psychiatric illness among inmates not identified as acutely mentally ill at intake in Connecticut’s jails. J Am Acad Psychiatry Law. 2007;35(4):490-500.
7. Ditton PM. Bureau of Justice Statistics special report: mental health and treatment of inmates and probationers. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/mhtip.pdf. Published July 1999. Accessed April 24, 2019.
8. Crilly JF, Caine ED, Lamberti JS, et al. Mental health services use and symptom prevalence in a cohort of adults on probation. Psychiatr Serv. 2009;60(4):542-544.
9. Herinckx HA, Swart SC, Ama SM, et al. Rearrest and linkage to mental health services among clients of the Clark County mental health court program. Psychiatr Serv. 2005;56(7):853-857.
10. Solomon P, Draine J, Marcus SC. Predicting incarceration of clients of a psychiatric probation and parole service. Psychiatr Serv. 2002;53(1):50-56.
11. Owens GP, Rogers SM, Whitesell AA. Use of mental health services and barriers to care for individuals on probation or parole. J Offender Rehabil. 2011;50(1):35-47.
12. Berman G, Feinblatt J. Problem‐solving courts: a brief primer. Law and Policy. 2001;23(2):126.
13. The Council of State Governments Justice Center. Mental health courts: a guide to research-informed policy and practice. U.S. Department of Justice. https://www.bja.gov/Publications/CSG_MHC_Research.pdf. Published 2009. Accessed November 22, 2018.
14. Landess J, Holoyda B. Mental health courts and forensic assertive community treatment teams as correctional diversion programs. Behav Sci Law. 2017;35(5-6):501-511.
15. Sammon KC. Therapeutic jurisprudence: an examination of problem‐solving justice in New York. Journal of Civil Rights and Economic Development. 2008;23:923.
16. Sarteschi CM, Vaughn MG, Kim, K. Assessing the effectiveness of mental health courts: a quantitative review. Journal of Criminal Justice. 2011;39(1):12-20.
17. Strong SM, Rantala RR. Census of problem-solving courts, 2012. U.S. Department of Justice, Bureau of Justice Assistance. http://www.bjs.gov/content/pub/pdf/cpsc12.pdf. Revised October 12, 2016. Accessed April 24, 2019.
18. McGuire JF, Rosenheck RA. Criminal history as a prognostic indicator in the treatment of homeless people with severe mental illness. Psychiatr Serv. 2004;55(1):42-48.
19. Families USA. Medicaid suspension policies for incarcerated people: a 50-state map. Families USA. https://familiesusa.org/product/medicaid-suspension-policies-incarcerated-people-50-state-map. Published July 2016. Accessed December 7, 2018.
20. Binswanger IA, Stern MF, Deyo RA, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356(2):157-165.
21. Binswanger IA, Blatchford PJ, Mueller SR, et al. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592-600.
22. Lim S, Seligson AL, Parvez FM, et al. Risks of drug-related death, suicide, and homicide during the immediate post-release period among people released from New York City Jails, 2001-2005. Am J Epidemiol. 2012;175(6):519-526.
23. Cusack KJ, Morrissey JP, Cuddeback GS, et al. Criminal justice involvement, behavioral health service use, and costs of forensic assertive community treatment: a randomized trial. Community Ment Health J. 2010;46(4):356-363.
24. Felthous AR, Stanford MS. A proposed algorithm for the pharmacotherapy of impulsive aggression. J Am Acad Psychiatry Law. 2015:43(4);456-467.
Agitation in children and adolescents: Diagnostic and treatment considerations
Managing agitation—verbal and/or motor restlessness that often is accompanied by irritability and a predisposition to aggression or violence—can be challenging in any patient, but particularly so in children and adolescents. In the United States, the prevalence of children and adolescents presenting to an emergency department (ED) for treatment of psychiatric symptoms, including agitation, has been on the rise.1,2
Similar to the multitude of causes of fever, agitation among children and adolescents has many possible causes.3 Because agitation can pose a risk for harm to others and/or self, it is important to manage it proactively. Other than studies that focus on agitation in pediatric anesthesia, there is a dearth of studies examining agitation and its treatment in children and adolescents. There is also a scarcity of training in the management of acute agitation in children and adolescents. In a 2017 survey of pediatric hospitalists and consultation-liaison psychiatrists at 38 academic children’s hospitals in North America, approximately 60% of respondents indicated that they had received no training in the evaluation or management of pediatric acute agitation.4 In addition, approximately 54% of participants said they did not screen for risk factors for pediatric agitation, even though 84% encountered the condition at least once a month, and as often as weekly.4
This article reviews evidence on the causes and treatments of agitation in children and adolescents. For the purposes of this review, child refers to a patient age 6 to 12, and adolescent refers to a patient age 13 to 17.
Identifying the cause
Addressing the underlying cause of agitation is essential. It’s also important to manage acute agitation while the underlying cause is being investigated in a way that does not jeopardize the patient’s emotional or physical safety.
Agitation in children or teens can be due to psychiatric causes such as autism, attention-deficit/hyperactivity disorder (ADHD), or posttraumatic stress disorder (PTSD), or due to medical conditions such as delirium, traumatic brain injury, or other conditions (Table 1).
In a 2005 study of 194 children with agitation in a pediatric post-anesthesia care unit, pain (27%) and anxiety (25%) were found to be the most common causes of agitation.3 Anesthesia-related agitation was a less common cause (11%). Physiologic anomalies were found to be the underlying cause of agitation in only 3 children in this study, but were undiagnosed for a prolonged period in 2 of these 3 children, which highlights the importance of a thorough differential diagnosis in the management of agitation in children.3
Assessment of an agitated child should include a comprehensive history, physical exam, and laboratory testing as indicated. When a pediatric patient comes to the ED with a chief presentation of agitation, a thorough medical and psychiatric assessment should be performed. For patients with a history of psychiatric diagnoses, do not assume that the cause of agitation is psychiatric.
Continue to: Psychiatric causes
Psychiatric causes
Autism spectrum disorder. Children and teens with autism often feel overwhelmed due to transitions, changes, and/or sensory overload. This sensory overload may be in response to relatively subtle sensory stimuli, so it may not always be apparent to parents or others around them.
Research suggests that in general, the ability to cope effectively with emotions is difficult without optimal language development. Due to cognitive/language delays and a related lack of emotional attunement and limited skills in recognizing, expressing, or coping with emotions, difficult emotions in children and adolescents with autism can manifest as agitation.
Attention-deficit/hyperactivity disorder. Children with ADHD may be at a higher risk for agitation, in part due to poor impulse control and limited coping skills. In addition, chronic negative feedback (from parents, teachers, or both) may contribute to low self-esteem, mood symptoms, defiance, and/or other behavioral difficulties. In addition to standard pharmacotherapy for ADHD, treatment involves parent behavior modification training. Setting firm yet empathic limits, “picking battles,” and implementing a developmentally appropriate behavioral plan to manage disruptive behavior in children or adolescents with ADHD can go a long way in helping to prevent the emergence of agitation.
Posttraumatic stress disorder. In some young children, new-onset, unexplained agitation may be the only sign of abuse or trauma. Children who have undergone trauma tend to experience confusion and distress. This may manifest as agitation or aggression, or other symptoms such as increased anxiety or nightmares.5 Trauma may be in the form of witnessing violence (domestic or other); experiencing physical, sexual, and/or emotional abuse; or witnessing/experiencing other significant threats to the safety of self and/or loved ones. Re-establishing (or establishing) a sense of psychological and physical safety is paramount in such patients.6 Psychotherapy is the first-line modality of treatment in children and adolescents with PTSD.6 In general, there is a scarcity of research on medication treatments for PTSD symptoms among children and adolescents.6
Oppositional defiant disorder/conduct disorder. Oppositional defiant disorder (ODD) can be comorbid with ADHD. The diagnosis of ODD requires a pervasive pattern of anger, defiance, vindictiveness, and hostility, particularly towards authority figures. However, these symptoms need to be differentiated from the normal range of childhood behavior. Occasionally, children learn to cope maladaptively through disruptive behavior or agitation. Although a parent or caregiver may see this behavior as intentionally malevolent, in a child with limited coping skills (whether due to young age, developmental/cognitive/language/learning delays, or social communication deficits) or one who has witnessed frequent agitation or aggression in the family environment, agitation and disruptive behavior may be a maladaptive form of coping. Thus, diligence needs to be exercised in the diagnosis of ODD and in understanding the psychosocial factors affecting the child, particularly because impulsiveness and uncooperativeness on their own have been found to be linked to greater likelihood of prescription of psychotropic medications from multiple classes.7 Family-based interventions, particularly parent training, family therapy, and age-appropriate child skills training, are of prime importance in managing this condition.8 Research shows that a shortage of resources, system issues, and cultural roadblocks in implementing family-based psychosocial interventions also can contribute to the increased use of psychotropic medications for aggression in children and teens with ODD, conduct disorder, or ADHD.8 The astute clinician needs to be cognizant of this before prescribing.
Continue to: Hallucinations/psychosis
Hallucinations/psychosis. Hallucinations (whether from psychiatric or medical causes) are significantly associated with agitation.9 In particular, auditory command hallucinations have been linked to agitation. Command hallucinations in children and adolescents may be secondary to early-onset schizophrenia; however, this diagnosis is rare.10 Hallucinations can also be an adverse effect of amphetamine-based stimulant medications in children and adolescents. Visual hallucinations are most often a sign of an underlying medical disorder such as delirium, occipital lobe mass/infection, or drug intoxication or withdrawal. Hallucinations need to be distinguished from the normal, imaginative play of a young child.10
Bipolar mania. In adults, bipolar disorder is a primary psychiatric cause of agitation. In children and adolescents, the diagnosis of bipolar disorder can be complex and requires careful and nuanced history-taking. The risks of agitation are greater with bipolar disorder than with unipolar depression.11,12
Disruptive mood dysregulation disorder. Prior to DSM-5, many children and adolescents with chronic, non-episodic irritability and severe outbursts out of proportion to the situation or stimuli were given a diagnosis of bipolar disorder. These symptoms, in combination with other symptoms, are now considered part of disruptive mood dysregulation disorder when severe outbursts in a child or adolescent occur 3 to 4 times a week consistently, for at least 1 year. The diagnosis of disruptive mood dysregulation disorder requires ruling out other psychiatric and medical conditions, particularly ADHD.13
Substance intoxication/withdrawal. Intoxication or withdrawal from substances such as alcohol, stimulant medications, opioids, methamphetamines, and other agents can lead to agitation. This is more likely to occur among adolescents than children.14
Adjustment disorder. Parental divorce, especially if it is conflictual, or other life stressors, such as experiencing a move or frequent moves, may contribute to the development of agitation in children and adolescents.
Continue to: Depression
Depression. In children and adolescents, depression can manifest as anger or irritability, and occasionally as agitation.
Medical causes
Delirium. Refractory agitation is often a manifestation of delirium in children and adolescents.15 If unrecognized and untreated, delirium can be fatal.16 Therefore, it is imperative that clinicians routinely assess for delirium in any patient who presents with agitation.
Because a patient with delirium often presents with agitation and visual or auditory hallucinations, the medical team may tend to assume these symptoms are secondary to a psychiatric disorder. In this case, the role of the consultation-liaison psychiatrist is critical for guiding the medical team, particularly to continue a thorough exploration of underlying causes while avoiding polypharmacy. Noise, bright lights, frequent changes in nursing staff or caregivers, anticholinergic or benzodiazepine medications, and frequent changes in schedules should be avoided to prevent delirium from occurring or getting worse.17 A multidisciplinary team approach is key in identifying the underlying cause and managing delirium in pediatric patients.
Traumatic brain injury. Agitation may be a presenting symptom in youth with traumatic brain injury (TBI).18 Agitation may present often in the acute recovery phase.19 There is limited evidence on the efficacy and safety of pharmacotherapy for agitation in pediatric patients with TBI.18
Autoimmune conditions. In a study of 27 patients with
Continue to: Medication-induced/iatrogenic
Medication-induced/iatrogenic. Agitation can be an adverse effect of medications such as amantadine (often used for TBI),18 atypical antipsychotics,21 selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors.
Infection. Agitation can be a result of encephalitis, meningitis, or other infectious processes.22
Metabolic conditions. Hepatic or renal failure, diabetic ketoacidosis, and thyroid toxicosis may cause agitation in children or adolescents.22
Start with nonpharmacologic interventions
Few studies have examined de-escalation techniques in agitated children and adolescents. However, verbal de-escalation is generally viewed as the first-line technique for managing agitation in children and adolescents. When feasible, teaching and modeling developmentally appropriate stress management skills for children and teens can be a beneficial preventative strategy to reduce the incidence and worsening of agitation.23
Clinicians should refrain from using coercion.24 Coercion could harm the therapeutic alliance, thereby impeding assessment of the underlying causes of agitation, and can be particularly harmful for patients who have a history of trauma or abuse. Even in pediatric patients with no such history, coercion is discouraged due to its punitive connotations and potential to adversely impact a vulnerable child or teen.
Continue to: Establishing a therapeutic rapport...
Establishing a therapeutic rapport with the patient, when feasible, can facilitate smoother de-escalation by offering the patient an outlet to air his/her frustrations and emotions, and by helping the patient feel understood.24 To facilitate this, ensure that the patient’s basic comforts and needs are met, such as access to a warm bed, food, and safety.25
The psychiatrist’s role is to help uncover and address the underlying reason for the patient’s agony or distress. Once the child or adolescent has calmed, explore potential triggers or causes of the agitation.
There has been a significant move away from the use of restraints for managing agitation in children and adolescents.26 Restraints have a psychologically traumatizing effect,27 and have been linked to life-threatening injuries and death in children.24
Pharmacotherapy: Proceed with caution
There are no FDA-approved medications for the treatment of agitation in the general pediatric population, and any medication use in this population is off-label. There is also a dearth of research examining the safety and efficacy of using psychotropic medications for agitation in pediatric patients. Because children and adolescents are more susceptible to adverse effects and risks associated with the use of psychotropic medications, special caution is warranted. In general, pharmacologic interventions are not recommended without the use of psychotherapy-based modalities.
In the past, the aim of using medications to treat patients with agitation was to put the patient to sleep.25 This practice did not help clinicians to assess for underlying causes, and was often accompanied by a greater risk of adverse effects and reactions.24 Therefore, the goal of medication treatment for agitation is to help calm the patient instead of inducing sleep.25
Continue to: Pharmacotherapy should...
Pharmacotherapy should be used only when behavioral interventions have been unsuccessful. Key considerations for using psychotropic medications to address agitation in children and adolescents are summarized in Table 2.25
Antipsychotics, particularly second-generation antipsychotics (SGAs), have been commonly used to manage acute agitation in children and adolescents, and there has been an upswing in the use of these medications in the United States in the last several years.28 Research indicates that males, children and adolescents in foster care, and those with Medicaid have been the more frequent youth recipients of SGAs.29 Of particular concern is the prevalence of antipsychotic use among children younger than age 6. In the last few decades, there has been an increase in the prescription of antipsychotics for children younger than age 6, particularly for disruptive behavior and aggression.30 In a study of preschool-age Medicaid patients in Kentucky, 70,777 prescriptions for SGAs were given to 6,915 children <6 years of age; 73% of these prescriptions were for male patients.30 Because there is a lack of controlled studies examining the safety and efficacy of SGAs among children and adolescents, especially with long-term use, further research is needed and caution is warranted.28
The FDA has approved
Externalizing disorders among children and adolescents tend to get treated with antipsychotics.28 A Canadian study examining records of 6,916 children found that most children who had been prescribed risperidone received it for ADHD or conduct disorder, and most patients had not received laboratory testing for monitoring the antipsychotic medication they were taking.31 In a 2018 study examining medical records of 120 pediatric patients who presented to an ED in British Columbia with agitation, antipsychotics were the most commonly used medications for patients with autism spectrum disorder; most patients received at least 1 dose.14
For children and adolescents with agitation or aggression who were admitted to inpatient units, IM
Continue to: In case reports...
In case reports, a combination of olanzapine with CNS-suppressing agents has resulted in death. Therefore, do not combine olanzapine with agents such as benzodiazepines.25 In a patient with a likely medical source of agitation, insufficient evidence exists to support the use of olanzapine, and additional research is needed.25
Low-dose haloperidol has been found to be effective for delirium-related agitation in pediatric studies.15 Before initiating an antipsychotic for any child or adolescent, review the patient’s family history for reports of early cardiac death and the patient’s own history of cardiac symptoms, palpitations, syncope, or prolonged QT interval. Monitor for QT prolongation. Among commonly used antipsychotics, the risk of QT prolongation is higher with IV administration of haloperidol and with ziprasidone. Studies show that compared with oral or IM
A few studies have found risperidone to be efficacious for treating ODD and conduct disorder; however, this use is off-label, and its considerable adverse effect and risk profile needs to be weighed against the potential benefit.8
Antipsychotic polypharmacy should be avoided because of the higher risk of adverse effects and interactions, and a lack of robust, controlled studies evaluating the safety of using antipsychotics for non-FDA-approved indications in children and adolescents.7 All patients who receive antipsychotics require monitoring for extrapyramidal symptoms, tardive dyskinesia, neuroleptic malignant syndrome, orthostatic hypotension, sedation, metabolic syndrome, and other potential adverse effects. Patients receiving risperidone need to have their prolactin levels monitored periodically, and their parents should be made aware of the potential for hyperprolactinemia and other adverse effects. Aripiprazole and quetiapine may increase the risk of suicidality.
Antiepileptics. A meta-analysis of 7 randomized controlled trials examining the use of antiepileptic medications (
Continue to: In a retrospective case series...
In a retrospective case series of 30 pediatric patients with autism spectrum disorder who were given oxcarbazepine, Douglas et al35 found that 47% of participants experienced significant improvement in irritability/agitation. However, 23% of patients reported significant adverse effects leading to discontinuation. Insufficient evidence exists for the safety and efficacy of oxcarbazepine in this population.35
Benzodiazepines. The use of benzodiazepines in pediatric patients has been associated with paradoxical disinhibition reactions, particularly in children with autism and other developmental or cognitive disabilities or delays.21 There is a lack of data on the safety and efficacy of long-term use of benzodiazepines in children, especially in light of these patients’ developing brains, the risk of cognitive impairment, and the potential for dependence with long-term use. Despite this, some studies show that the use of benzodiazepines is fairly common among pediatric patients who present to the ED with agitation.14 In a recent retrospective study, Kendrick et al14 found that among pediatric patients with agitation who were brought to the ED, benzodiazepines were the most commonly prescribed medications.
Other medications. Clonidine and
Diphenhydramine, in both oral and IM forms, has been used to treat agitation in children,32 but has also been associated with a paradoxical disinhibition reaction in pediatric patients21 and therefore should be used only sparingly and with caution. Diphenhydramine has anticholinergic properties, and may worsen delirium.15 Stimulant medications can help aggressive behavior in children and adolescents with ADHD.37
Bottom Line
Agitation among children and adolescents has many possible causes. A combination of a comprehensive assessment and evidence-based, judicious treatment interventions can help prevent and manage agitation in this vulnerable population.
Related Resources
- Baker M, Carlson GA. What do we really know about PRN use in agitated children with mental health conditions: a clinical review. Evid Based Ment Health. 2018;21(4):166-170.
- Gerson R, Malas N, Mroczkowski MM. Crisis in the emergency department: the evaluation and management of acute agitation in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2018;27(3):367-386.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Clonidine • Catapres
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra, Spritam
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Quetiapine • Seroquel
Topiramate • Topamax
Risperidone • Risperdal
Valproate • Depakene
Ziprasidone • Geodon
1. Frosch E, Kelly P. Issues in pediatric psychiatric emergency care. In: Emergency psychiatry. Cambridge, UK: Cambridge University Press; 2011:185-199.
2. American College of Emergency Physicians. Pediatric mental health emergencies in the emergency department. https://www.acep.org/patient-care/policy-statements/pediatric-mental-health-emergencies-in-the-emergency-medical-services-system/. Revised September 2018. Accessed February 23, 2019.
3. Voepel-Lewis, T, Burke C, Hadden S, et al. Nurses’ diagnoses and treatment decisions regarding care of the agitated child. J Perianesth Nurs. 2005;20(4):239-248.
4. Malas N, Spital L, Fischer J, et al. National survey on pediatric acute agitation and behavioral escalation in academic inpatient pediatric care settings. Psychosomatics. 2017;58(3):299-306.
5. Famularo R, Kinscherff R, Fenton T. Symptom differences in acute and chronic presentation of childhood post-traumatic stress disorder. Child Abuse Negl. 1990;14(3):439-444.
6. Kaminer D, Seedat S, Stein DJ. Post-traumatic stress disorder in children. World Psychiatry. 2005;4(2):121-125.
7. Ninan A, Stewart SL, Theall LA, et al. Adverse effects of psychotropic medications in children: predictive factors. J Can Acad Child Adolesc Psychiatry. 2014;23(3):218-225.
8. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 2: antipsychotics and traditional mood stabilizers. Can J Psychiatry. 2015;60(2):52-61.
9. Vareilles D, Bréhin C, Cortey C, et al. Hallucinations: Etiological analysis of children admitted to a pediatric emergency department. Arch Pediatr. 2017;24(5):445-452.
10. Bartlett J. Childhood-onset schizophrenia: what do we really know? Health Psychol Behav Med. 2014;2(1):735-747.
11. Diler RS, Goldstein TR, Hafeman D, et al. Distinguishing bipolar depression from unipolar depression in youth: Preliminary findings. J Child Adolesc Psychopharmacol. 2017;27(4):310-319.
12. Dervic K, Garcia-Amador M, Sudol K, et al. Bipolar I and II versus unipolar depression: clinical differences and impulsivity/aggression traits. Eur Psychiatry. 2015;30(1):106-113.
13. Masi L, Gignac M ADHD and DMDD comorbidities, similarities and distinctions. J Child Adolesc Behav2016;4:325.
14. Kendrick JG, Goldman RD, Carr RR. Pharmacologic management of agitation and aggression in a pediatric emergency department - a retrospective cohort study. J Pediatr Pharmacol Ther. 2018;23(6):455-459.
15. Schieveld JN, Staal M, Voogd L, et al. Refractory agitation as a marker for pediatric delirium in very young infants at a pediatric intensive care unit. Intensive Care Med. 2010;36(11):1982-1983.
16. Traube C, Silver G, Gerber LM, et al. Delirium and mortality in critically ill children: epidemiology and outcomes of pediatric delirium. Crit Care Med. 2017;45(5):891-898.
17. Bettencourt A, Mullen JE. Delirium in children: identification, prevention, and management. Crit Care Nurse. 2017;37(3):e9-e18.
18. Suskauer SJ, Trovato MK. Update on pharmaceutical intervention for disorders of consciousness and agitation after traumatic brain injury in children. PM R. 2013;5(2):142-147.
19. Nowicki M, Pearlman L, Campbell C, et al. Agitated behavior scale in pediatric traumatic brain injury. Brain Inj. 2019. doi: 10.1080/02699052.2019.1565893.
20. Mohammad SS, Jones H, Hong M, et al. Symptomatic treatment of children with anti-NMDAR encephalitis. Dev Med Child Neurol. 2016;58(4):376-384.
21. Sonnier L, Barzman D. Pharmacologic management of acutely agitated pediatric patients. Pediatr Drugs. 2011;13(1):1-10.
22. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the american association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3-10.
23. Masters KJ, Bellonci C, Bernet W, et al; American Academy of Child and Adolescent Psychiatry. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):4S-25S.
24. Croce ND, Mantovani C. Using de-escalation techniques to prevent violent behavior in pediatric psychiatric emergencies: It is possible. Pediatric Dimensions, 2017;2(1):1-2.
25. Marzullo LR. Pharmacologic management of the agitated child. Pediatr Emerg Care. 2014;30(4):269-275.
26. Caldwell B, Albert C, Azeem MW, et al. Successful seclusion and restraint prevention effort in child and adolescent programs. J Psychosoc Nurs Ment Health Serv. 2014;52(11):30-38.
27. De Hert M, Dirix N, Demunter H, et al. Prevalence and correlates of seclusion and restraint use in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry. 2011;20(5):221-230.
28. Crystal S, Olfson M, Huang C, et al. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770-w781.
29. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of atypical antipsychotic medication in children and adolescents. https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_Antipsychotic_Medications_Web.pdf. Accessed March 4, 2019.
30. Lohr WD, Chowning RT, Stevenson MD, et al. Trends in atypical antipsychotics prescribed to children six years of age or less on Medicaid in Kentucky. J Child Adolesc Psychopharmacol. 2015;25(5):440-443.
31. Chen W, Cepoiu-Martin M, Stang A, et al. Antipsychotic prescribing and safety monitoring practices in children and youth: a population-based study in Alberta, Canada. Clin Drug Investig. 2018;38(5):449-455.
32. Deshmukh P, Kulkarni G, Barzman D. Recommendations for pharmacological management of inpatient aggression in children and adolescents. Psychiatry (Edgmont). 2010;7(2):32-40.
33. Haldol [package insert]. Beerse, Belgium: Janssen Pharmaceutica NV; 2005.
34. Hirota T, Veenstra-Vanderweele J, Hollander E, et al. Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
35. Douglas JF, Sanders KB, Benneyworth MH, et al. Brief report: retrospective case series of oxcarbazepine for irritability/agitation symptoms in autism spectrum disorder. J Autism Dev Disord. 2013;43(5):1243-1247.
36. Harmon RJ, Riggs PD. Clonidine for posttraumatic stress disorder in preschool children. J Am Acad
37. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 1: Psychostimulants, alpha-2 Agonists, and atomoxetine. Can J Psychiatry. 2015;60(2):42-51
Managing agitation—verbal and/or motor restlessness that often is accompanied by irritability and a predisposition to aggression or violence—can be challenging in any patient, but particularly so in children and adolescents. In the United States, the prevalence of children and adolescents presenting to an emergency department (ED) for treatment of psychiatric symptoms, including agitation, has been on the rise.1,2
Similar to the multitude of causes of fever, agitation among children and adolescents has many possible causes.3 Because agitation can pose a risk for harm to others and/or self, it is important to manage it proactively. Other than studies that focus on agitation in pediatric anesthesia, there is a dearth of studies examining agitation and its treatment in children and adolescents. There is also a scarcity of training in the management of acute agitation in children and adolescents. In a 2017 survey of pediatric hospitalists and consultation-liaison psychiatrists at 38 academic children’s hospitals in North America, approximately 60% of respondents indicated that they had received no training in the evaluation or management of pediatric acute agitation.4 In addition, approximately 54% of participants said they did not screen for risk factors for pediatric agitation, even though 84% encountered the condition at least once a month, and as often as weekly.4
This article reviews evidence on the causes and treatments of agitation in children and adolescents. For the purposes of this review, child refers to a patient age 6 to 12, and adolescent refers to a patient age 13 to 17.
Identifying the cause
Addressing the underlying cause of agitation is essential. It’s also important to manage acute agitation while the underlying cause is being investigated in a way that does not jeopardize the patient’s emotional or physical safety.
Agitation in children or teens can be due to psychiatric causes such as autism, attention-deficit/hyperactivity disorder (ADHD), or posttraumatic stress disorder (PTSD), or due to medical conditions such as delirium, traumatic brain injury, or other conditions (Table 1).
In a 2005 study of 194 children with agitation in a pediatric post-anesthesia care unit, pain (27%) and anxiety (25%) were found to be the most common causes of agitation.3 Anesthesia-related agitation was a less common cause (11%). Physiologic anomalies were found to be the underlying cause of agitation in only 3 children in this study, but were undiagnosed for a prolonged period in 2 of these 3 children, which highlights the importance of a thorough differential diagnosis in the management of agitation in children.3
Assessment of an agitated child should include a comprehensive history, physical exam, and laboratory testing as indicated. When a pediatric patient comes to the ED with a chief presentation of agitation, a thorough medical and psychiatric assessment should be performed. For patients with a history of psychiatric diagnoses, do not assume that the cause of agitation is psychiatric.
Continue to: Psychiatric causes
Psychiatric causes
Autism spectrum disorder. Children and teens with autism often feel overwhelmed due to transitions, changes, and/or sensory overload. This sensory overload may be in response to relatively subtle sensory stimuli, so it may not always be apparent to parents or others around them.
Research suggests that in general, the ability to cope effectively with emotions is difficult without optimal language development. Due to cognitive/language delays and a related lack of emotional attunement and limited skills in recognizing, expressing, or coping with emotions, difficult emotions in children and adolescents with autism can manifest as agitation.
Attention-deficit/hyperactivity disorder. Children with ADHD may be at a higher risk for agitation, in part due to poor impulse control and limited coping skills. In addition, chronic negative feedback (from parents, teachers, or both) may contribute to low self-esteem, mood symptoms, defiance, and/or other behavioral difficulties. In addition to standard pharmacotherapy for ADHD, treatment involves parent behavior modification training. Setting firm yet empathic limits, “picking battles,” and implementing a developmentally appropriate behavioral plan to manage disruptive behavior in children or adolescents with ADHD can go a long way in helping to prevent the emergence of agitation.
Posttraumatic stress disorder. In some young children, new-onset, unexplained agitation may be the only sign of abuse or trauma. Children who have undergone trauma tend to experience confusion and distress. This may manifest as agitation or aggression, or other symptoms such as increased anxiety or nightmares.5 Trauma may be in the form of witnessing violence (domestic or other); experiencing physical, sexual, and/or emotional abuse; or witnessing/experiencing other significant threats to the safety of self and/or loved ones. Re-establishing (or establishing) a sense of psychological and physical safety is paramount in such patients.6 Psychotherapy is the first-line modality of treatment in children and adolescents with PTSD.6 In general, there is a scarcity of research on medication treatments for PTSD symptoms among children and adolescents.6
Oppositional defiant disorder/conduct disorder. Oppositional defiant disorder (ODD) can be comorbid with ADHD. The diagnosis of ODD requires a pervasive pattern of anger, defiance, vindictiveness, and hostility, particularly towards authority figures. However, these symptoms need to be differentiated from the normal range of childhood behavior. Occasionally, children learn to cope maladaptively through disruptive behavior or agitation. Although a parent or caregiver may see this behavior as intentionally malevolent, in a child with limited coping skills (whether due to young age, developmental/cognitive/language/learning delays, or social communication deficits) or one who has witnessed frequent agitation or aggression in the family environment, agitation and disruptive behavior may be a maladaptive form of coping. Thus, diligence needs to be exercised in the diagnosis of ODD and in understanding the psychosocial factors affecting the child, particularly because impulsiveness and uncooperativeness on their own have been found to be linked to greater likelihood of prescription of psychotropic medications from multiple classes.7 Family-based interventions, particularly parent training, family therapy, and age-appropriate child skills training, are of prime importance in managing this condition.8 Research shows that a shortage of resources, system issues, and cultural roadblocks in implementing family-based psychosocial interventions also can contribute to the increased use of psychotropic medications for aggression in children and teens with ODD, conduct disorder, or ADHD.8 The astute clinician needs to be cognizant of this before prescribing.
Continue to: Hallucinations/psychosis
Hallucinations/psychosis. Hallucinations (whether from psychiatric or medical causes) are significantly associated with agitation.9 In particular, auditory command hallucinations have been linked to agitation. Command hallucinations in children and adolescents may be secondary to early-onset schizophrenia; however, this diagnosis is rare.10 Hallucinations can also be an adverse effect of amphetamine-based stimulant medications in children and adolescents. Visual hallucinations are most often a sign of an underlying medical disorder such as delirium, occipital lobe mass/infection, or drug intoxication or withdrawal. Hallucinations need to be distinguished from the normal, imaginative play of a young child.10
Bipolar mania. In adults, bipolar disorder is a primary psychiatric cause of agitation. In children and adolescents, the diagnosis of bipolar disorder can be complex and requires careful and nuanced history-taking. The risks of agitation are greater with bipolar disorder than with unipolar depression.11,12
Disruptive mood dysregulation disorder. Prior to DSM-5, many children and adolescents with chronic, non-episodic irritability and severe outbursts out of proportion to the situation or stimuli were given a diagnosis of bipolar disorder. These symptoms, in combination with other symptoms, are now considered part of disruptive mood dysregulation disorder when severe outbursts in a child or adolescent occur 3 to 4 times a week consistently, for at least 1 year. The diagnosis of disruptive mood dysregulation disorder requires ruling out other psychiatric and medical conditions, particularly ADHD.13
Substance intoxication/withdrawal. Intoxication or withdrawal from substances such as alcohol, stimulant medications, opioids, methamphetamines, and other agents can lead to agitation. This is more likely to occur among adolescents than children.14
Adjustment disorder. Parental divorce, especially if it is conflictual, or other life stressors, such as experiencing a move or frequent moves, may contribute to the development of agitation in children and adolescents.
Continue to: Depression
Depression. In children and adolescents, depression can manifest as anger or irritability, and occasionally as agitation.
Medical causes
Delirium. Refractory agitation is often a manifestation of delirium in children and adolescents.15 If unrecognized and untreated, delirium can be fatal.16 Therefore, it is imperative that clinicians routinely assess for delirium in any patient who presents with agitation.
Because a patient with delirium often presents with agitation and visual or auditory hallucinations, the medical team may tend to assume these symptoms are secondary to a psychiatric disorder. In this case, the role of the consultation-liaison psychiatrist is critical for guiding the medical team, particularly to continue a thorough exploration of underlying causes while avoiding polypharmacy. Noise, bright lights, frequent changes in nursing staff or caregivers, anticholinergic or benzodiazepine medications, and frequent changes in schedules should be avoided to prevent delirium from occurring or getting worse.17 A multidisciplinary team approach is key in identifying the underlying cause and managing delirium in pediatric patients.
Traumatic brain injury. Agitation may be a presenting symptom in youth with traumatic brain injury (TBI).18 Agitation may present often in the acute recovery phase.19 There is limited evidence on the efficacy and safety of pharmacotherapy for agitation in pediatric patients with TBI.18
Autoimmune conditions. In a study of 27 patients with
Continue to: Medication-induced/iatrogenic
Medication-induced/iatrogenic. Agitation can be an adverse effect of medications such as amantadine (often used for TBI),18 atypical antipsychotics,21 selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors.
Infection. Agitation can be a result of encephalitis, meningitis, or other infectious processes.22
Metabolic conditions. Hepatic or renal failure, diabetic ketoacidosis, and thyroid toxicosis may cause agitation in children or adolescents.22
Start with nonpharmacologic interventions
Few studies have examined de-escalation techniques in agitated children and adolescents. However, verbal de-escalation is generally viewed as the first-line technique for managing agitation in children and adolescents. When feasible, teaching and modeling developmentally appropriate stress management skills for children and teens can be a beneficial preventative strategy to reduce the incidence and worsening of agitation.23
Clinicians should refrain from using coercion.24 Coercion could harm the therapeutic alliance, thereby impeding assessment of the underlying causes of agitation, and can be particularly harmful for patients who have a history of trauma or abuse. Even in pediatric patients with no such history, coercion is discouraged due to its punitive connotations and potential to adversely impact a vulnerable child or teen.
Continue to: Establishing a therapeutic rapport...
Establishing a therapeutic rapport with the patient, when feasible, can facilitate smoother de-escalation by offering the patient an outlet to air his/her frustrations and emotions, and by helping the patient feel understood.24 To facilitate this, ensure that the patient’s basic comforts and needs are met, such as access to a warm bed, food, and safety.25
The psychiatrist’s role is to help uncover and address the underlying reason for the patient’s agony or distress. Once the child or adolescent has calmed, explore potential triggers or causes of the agitation.
There has been a significant move away from the use of restraints for managing agitation in children and adolescents.26 Restraints have a psychologically traumatizing effect,27 and have been linked to life-threatening injuries and death in children.24
Pharmacotherapy: Proceed with caution
There are no FDA-approved medications for the treatment of agitation in the general pediatric population, and any medication use in this population is off-label. There is also a dearth of research examining the safety and efficacy of using psychotropic medications for agitation in pediatric patients. Because children and adolescents are more susceptible to adverse effects and risks associated with the use of psychotropic medications, special caution is warranted. In general, pharmacologic interventions are not recommended without the use of psychotherapy-based modalities.
In the past, the aim of using medications to treat patients with agitation was to put the patient to sleep.25 This practice did not help clinicians to assess for underlying causes, and was often accompanied by a greater risk of adverse effects and reactions.24 Therefore, the goal of medication treatment for agitation is to help calm the patient instead of inducing sleep.25
Continue to: Pharmacotherapy should...
Pharmacotherapy should be used only when behavioral interventions have been unsuccessful. Key considerations for using psychotropic medications to address agitation in children and adolescents are summarized in Table 2.25
Antipsychotics, particularly second-generation antipsychotics (SGAs), have been commonly used to manage acute agitation in children and adolescents, and there has been an upswing in the use of these medications in the United States in the last several years.28 Research indicates that males, children and adolescents in foster care, and those with Medicaid have been the more frequent youth recipients of SGAs.29 Of particular concern is the prevalence of antipsychotic use among children younger than age 6. In the last few decades, there has been an increase in the prescription of antipsychotics for children younger than age 6, particularly for disruptive behavior and aggression.30 In a study of preschool-age Medicaid patients in Kentucky, 70,777 prescriptions for SGAs were given to 6,915 children <6 years of age; 73% of these prescriptions were for male patients.30 Because there is a lack of controlled studies examining the safety and efficacy of SGAs among children and adolescents, especially with long-term use, further research is needed and caution is warranted.28
The FDA has approved
Externalizing disorders among children and adolescents tend to get treated with antipsychotics.28 A Canadian study examining records of 6,916 children found that most children who had been prescribed risperidone received it for ADHD or conduct disorder, and most patients had not received laboratory testing for monitoring the antipsychotic medication they were taking.31 In a 2018 study examining medical records of 120 pediatric patients who presented to an ED in British Columbia with agitation, antipsychotics were the most commonly used medications for patients with autism spectrum disorder; most patients received at least 1 dose.14
For children and adolescents with agitation or aggression who were admitted to inpatient units, IM
Continue to: In case reports...
In case reports, a combination of olanzapine with CNS-suppressing agents has resulted in death. Therefore, do not combine olanzapine with agents such as benzodiazepines.25 In a patient with a likely medical source of agitation, insufficient evidence exists to support the use of olanzapine, and additional research is needed.25
Low-dose haloperidol has been found to be effective for delirium-related agitation in pediatric studies.15 Before initiating an antipsychotic for any child or adolescent, review the patient’s family history for reports of early cardiac death and the patient’s own history of cardiac symptoms, palpitations, syncope, or prolonged QT interval. Monitor for QT prolongation. Among commonly used antipsychotics, the risk of QT prolongation is higher with IV administration of haloperidol and with ziprasidone. Studies show that compared with oral or IM
A few studies have found risperidone to be efficacious for treating ODD and conduct disorder; however, this use is off-label, and its considerable adverse effect and risk profile needs to be weighed against the potential benefit.8
Antipsychotic polypharmacy should be avoided because of the higher risk of adverse effects and interactions, and a lack of robust, controlled studies evaluating the safety of using antipsychotics for non-FDA-approved indications in children and adolescents.7 All patients who receive antipsychotics require monitoring for extrapyramidal symptoms, tardive dyskinesia, neuroleptic malignant syndrome, orthostatic hypotension, sedation, metabolic syndrome, and other potential adverse effects. Patients receiving risperidone need to have their prolactin levels monitored periodically, and their parents should be made aware of the potential for hyperprolactinemia and other adverse effects. Aripiprazole and quetiapine may increase the risk of suicidality.
Antiepileptics. A meta-analysis of 7 randomized controlled trials examining the use of antiepileptic medications (
Continue to: In a retrospective case series...
In a retrospective case series of 30 pediatric patients with autism spectrum disorder who were given oxcarbazepine, Douglas et al35 found that 47% of participants experienced significant improvement in irritability/agitation. However, 23% of patients reported significant adverse effects leading to discontinuation. Insufficient evidence exists for the safety and efficacy of oxcarbazepine in this population.35
Benzodiazepines. The use of benzodiazepines in pediatric patients has been associated with paradoxical disinhibition reactions, particularly in children with autism and other developmental or cognitive disabilities or delays.21 There is a lack of data on the safety and efficacy of long-term use of benzodiazepines in children, especially in light of these patients’ developing brains, the risk of cognitive impairment, and the potential for dependence with long-term use. Despite this, some studies show that the use of benzodiazepines is fairly common among pediatric patients who present to the ED with agitation.14 In a recent retrospective study, Kendrick et al14 found that among pediatric patients with agitation who were brought to the ED, benzodiazepines were the most commonly prescribed medications.
Other medications. Clonidine and
Diphenhydramine, in both oral and IM forms, has been used to treat agitation in children,32 but has also been associated with a paradoxical disinhibition reaction in pediatric patients21 and therefore should be used only sparingly and with caution. Diphenhydramine has anticholinergic properties, and may worsen delirium.15 Stimulant medications can help aggressive behavior in children and adolescents with ADHD.37
Bottom Line
Agitation among children and adolescents has many possible causes. A combination of a comprehensive assessment and evidence-based, judicious treatment interventions can help prevent and manage agitation in this vulnerable population.
Related Resources
- Baker M, Carlson GA. What do we really know about PRN use in agitated children with mental health conditions: a clinical review. Evid Based Ment Health. 2018;21(4):166-170.
- Gerson R, Malas N, Mroczkowski MM. Crisis in the emergency department: the evaluation and management of acute agitation in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2018;27(3):367-386.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Clonidine • Catapres
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra, Spritam
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Quetiapine • Seroquel
Topiramate • Topamax
Risperidone • Risperdal
Valproate • Depakene
Ziprasidone • Geodon
Managing agitation—verbal and/or motor restlessness that often is accompanied by irritability and a predisposition to aggression or violence—can be challenging in any patient, but particularly so in children and adolescents. In the United States, the prevalence of children and adolescents presenting to an emergency department (ED) for treatment of psychiatric symptoms, including agitation, has been on the rise.1,2
Similar to the multitude of causes of fever, agitation among children and adolescents has many possible causes.3 Because agitation can pose a risk for harm to others and/or self, it is important to manage it proactively. Other than studies that focus on agitation in pediatric anesthesia, there is a dearth of studies examining agitation and its treatment in children and adolescents. There is also a scarcity of training in the management of acute agitation in children and adolescents. In a 2017 survey of pediatric hospitalists and consultation-liaison psychiatrists at 38 academic children’s hospitals in North America, approximately 60% of respondents indicated that they had received no training in the evaluation or management of pediatric acute agitation.4 In addition, approximately 54% of participants said they did not screen for risk factors for pediatric agitation, even though 84% encountered the condition at least once a month, and as often as weekly.4
This article reviews evidence on the causes and treatments of agitation in children and adolescents. For the purposes of this review, child refers to a patient age 6 to 12, and adolescent refers to a patient age 13 to 17.
Identifying the cause
Addressing the underlying cause of agitation is essential. It’s also important to manage acute agitation while the underlying cause is being investigated in a way that does not jeopardize the patient’s emotional or physical safety.
Agitation in children or teens can be due to psychiatric causes such as autism, attention-deficit/hyperactivity disorder (ADHD), or posttraumatic stress disorder (PTSD), or due to medical conditions such as delirium, traumatic brain injury, or other conditions (Table 1).
In a 2005 study of 194 children with agitation in a pediatric post-anesthesia care unit, pain (27%) and anxiety (25%) were found to be the most common causes of agitation.3 Anesthesia-related agitation was a less common cause (11%). Physiologic anomalies were found to be the underlying cause of agitation in only 3 children in this study, but were undiagnosed for a prolonged period in 2 of these 3 children, which highlights the importance of a thorough differential diagnosis in the management of agitation in children.3
Assessment of an agitated child should include a comprehensive history, physical exam, and laboratory testing as indicated. When a pediatric patient comes to the ED with a chief presentation of agitation, a thorough medical and psychiatric assessment should be performed. For patients with a history of psychiatric diagnoses, do not assume that the cause of agitation is psychiatric.
Continue to: Psychiatric causes
Psychiatric causes
Autism spectrum disorder. Children and teens with autism often feel overwhelmed due to transitions, changes, and/or sensory overload. This sensory overload may be in response to relatively subtle sensory stimuli, so it may not always be apparent to parents or others around them.
Research suggests that in general, the ability to cope effectively with emotions is difficult without optimal language development. Due to cognitive/language delays and a related lack of emotional attunement and limited skills in recognizing, expressing, or coping with emotions, difficult emotions in children and adolescents with autism can manifest as agitation.
Attention-deficit/hyperactivity disorder. Children with ADHD may be at a higher risk for agitation, in part due to poor impulse control and limited coping skills. In addition, chronic negative feedback (from parents, teachers, or both) may contribute to low self-esteem, mood symptoms, defiance, and/or other behavioral difficulties. In addition to standard pharmacotherapy for ADHD, treatment involves parent behavior modification training. Setting firm yet empathic limits, “picking battles,” and implementing a developmentally appropriate behavioral plan to manage disruptive behavior in children or adolescents with ADHD can go a long way in helping to prevent the emergence of agitation.
Posttraumatic stress disorder. In some young children, new-onset, unexplained agitation may be the only sign of abuse or trauma. Children who have undergone trauma tend to experience confusion and distress. This may manifest as agitation or aggression, or other symptoms such as increased anxiety or nightmares.5 Trauma may be in the form of witnessing violence (domestic or other); experiencing physical, sexual, and/or emotional abuse; or witnessing/experiencing other significant threats to the safety of self and/or loved ones. Re-establishing (or establishing) a sense of psychological and physical safety is paramount in such patients.6 Psychotherapy is the first-line modality of treatment in children and adolescents with PTSD.6 In general, there is a scarcity of research on medication treatments for PTSD symptoms among children and adolescents.6
Oppositional defiant disorder/conduct disorder. Oppositional defiant disorder (ODD) can be comorbid with ADHD. The diagnosis of ODD requires a pervasive pattern of anger, defiance, vindictiveness, and hostility, particularly towards authority figures. However, these symptoms need to be differentiated from the normal range of childhood behavior. Occasionally, children learn to cope maladaptively through disruptive behavior or agitation. Although a parent or caregiver may see this behavior as intentionally malevolent, in a child with limited coping skills (whether due to young age, developmental/cognitive/language/learning delays, or social communication deficits) or one who has witnessed frequent agitation or aggression in the family environment, agitation and disruptive behavior may be a maladaptive form of coping. Thus, diligence needs to be exercised in the diagnosis of ODD and in understanding the psychosocial factors affecting the child, particularly because impulsiveness and uncooperativeness on their own have been found to be linked to greater likelihood of prescription of psychotropic medications from multiple classes.7 Family-based interventions, particularly parent training, family therapy, and age-appropriate child skills training, are of prime importance in managing this condition.8 Research shows that a shortage of resources, system issues, and cultural roadblocks in implementing family-based psychosocial interventions also can contribute to the increased use of psychotropic medications for aggression in children and teens with ODD, conduct disorder, or ADHD.8 The astute clinician needs to be cognizant of this before prescribing.
Continue to: Hallucinations/psychosis
Hallucinations/psychosis. Hallucinations (whether from psychiatric or medical causes) are significantly associated with agitation.9 In particular, auditory command hallucinations have been linked to agitation. Command hallucinations in children and adolescents may be secondary to early-onset schizophrenia; however, this diagnosis is rare.10 Hallucinations can also be an adverse effect of amphetamine-based stimulant medications in children and adolescents. Visual hallucinations are most often a sign of an underlying medical disorder such as delirium, occipital lobe mass/infection, or drug intoxication or withdrawal. Hallucinations need to be distinguished from the normal, imaginative play of a young child.10
Bipolar mania. In adults, bipolar disorder is a primary psychiatric cause of agitation. In children and adolescents, the diagnosis of bipolar disorder can be complex and requires careful and nuanced history-taking. The risks of agitation are greater with bipolar disorder than with unipolar depression.11,12
Disruptive mood dysregulation disorder. Prior to DSM-5, many children and adolescents with chronic, non-episodic irritability and severe outbursts out of proportion to the situation or stimuli were given a diagnosis of bipolar disorder. These symptoms, in combination with other symptoms, are now considered part of disruptive mood dysregulation disorder when severe outbursts in a child or adolescent occur 3 to 4 times a week consistently, for at least 1 year. The diagnosis of disruptive mood dysregulation disorder requires ruling out other psychiatric and medical conditions, particularly ADHD.13
Substance intoxication/withdrawal. Intoxication or withdrawal from substances such as alcohol, stimulant medications, opioids, methamphetamines, and other agents can lead to agitation. This is more likely to occur among adolescents than children.14
Adjustment disorder. Parental divorce, especially if it is conflictual, or other life stressors, such as experiencing a move or frequent moves, may contribute to the development of agitation in children and adolescents.
Continue to: Depression
Depression. In children and adolescents, depression can manifest as anger or irritability, and occasionally as agitation.
Medical causes
Delirium. Refractory agitation is often a manifestation of delirium in children and adolescents.15 If unrecognized and untreated, delirium can be fatal.16 Therefore, it is imperative that clinicians routinely assess for delirium in any patient who presents with agitation.
Because a patient with delirium often presents with agitation and visual or auditory hallucinations, the medical team may tend to assume these symptoms are secondary to a psychiatric disorder. In this case, the role of the consultation-liaison psychiatrist is critical for guiding the medical team, particularly to continue a thorough exploration of underlying causes while avoiding polypharmacy. Noise, bright lights, frequent changes in nursing staff or caregivers, anticholinergic or benzodiazepine medications, and frequent changes in schedules should be avoided to prevent delirium from occurring or getting worse.17 A multidisciplinary team approach is key in identifying the underlying cause and managing delirium in pediatric patients.
Traumatic brain injury. Agitation may be a presenting symptom in youth with traumatic brain injury (TBI).18 Agitation may present often in the acute recovery phase.19 There is limited evidence on the efficacy and safety of pharmacotherapy for agitation in pediatric patients with TBI.18
Autoimmune conditions. In a study of 27 patients with
Continue to: Medication-induced/iatrogenic
Medication-induced/iatrogenic. Agitation can be an adverse effect of medications such as amantadine (often used for TBI),18 atypical antipsychotics,21 selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors.
Infection. Agitation can be a result of encephalitis, meningitis, or other infectious processes.22
Metabolic conditions. Hepatic or renal failure, diabetic ketoacidosis, and thyroid toxicosis may cause agitation in children or adolescents.22
Start with nonpharmacologic interventions
Few studies have examined de-escalation techniques in agitated children and adolescents. However, verbal de-escalation is generally viewed as the first-line technique for managing agitation in children and adolescents. When feasible, teaching and modeling developmentally appropriate stress management skills for children and teens can be a beneficial preventative strategy to reduce the incidence and worsening of agitation.23
Clinicians should refrain from using coercion.24 Coercion could harm the therapeutic alliance, thereby impeding assessment of the underlying causes of agitation, and can be particularly harmful for patients who have a history of trauma or abuse. Even in pediatric patients with no such history, coercion is discouraged due to its punitive connotations and potential to adversely impact a vulnerable child or teen.
Continue to: Establishing a therapeutic rapport...
Establishing a therapeutic rapport with the patient, when feasible, can facilitate smoother de-escalation by offering the patient an outlet to air his/her frustrations and emotions, and by helping the patient feel understood.24 To facilitate this, ensure that the patient’s basic comforts and needs are met, such as access to a warm bed, food, and safety.25
The psychiatrist’s role is to help uncover and address the underlying reason for the patient’s agony or distress. Once the child or adolescent has calmed, explore potential triggers or causes of the agitation.
There has been a significant move away from the use of restraints for managing agitation in children and adolescents.26 Restraints have a psychologically traumatizing effect,27 and have been linked to life-threatening injuries and death in children.24
Pharmacotherapy: Proceed with caution
There are no FDA-approved medications for the treatment of agitation in the general pediatric population, and any medication use in this population is off-label. There is also a dearth of research examining the safety and efficacy of using psychotropic medications for agitation in pediatric patients. Because children and adolescents are more susceptible to adverse effects and risks associated with the use of psychotropic medications, special caution is warranted. In general, pharmacologic interventions are not recommended without the use of psychotherapy-based modalities.
In the past, the aim of using medications to treat patients with agitation was to put the patient to sleep.25 This practice did not help clinicians to assess for underlying causes, and was often accompanied by a greater risk of adverse effects and reactions.24 Therefore, the goal of medication treatment for agitation is to help calm the patient instead of inducing sleep.25
Continue to: Pharmacotherapy should...
Pharmacotherapy should be used only when behavioral interventions have been unsuccessful. Key considerations for using psychotropic medications to address agitation in children and adolescents are summarized in Table 2.25
Antipsychotics, particularly second-generation antipsychotics (SGAs), have been commonly used to manage acute agitation in children and adolescents, and there has been an upswing in the use of these medications in the United States in the last several years.28 Research indicates that males, children and adolescents in foster care, and those with Medicaid have been the more frequent youth recipients of SGAs.29 Of particular concern is the prevalence of antipsychotic use among children younger than age 6. In the last few decades, there has been an increase in the prescription of antipsychotics for children younger than age 6, particularly for disruptive behavior and aggression.30 In a study of preschool-age Medicaid patients in Kentucky, 70,777 prescriptions for SGAs were given to 6,915 children <6 years of age; 73% of these prescriptions were for male patients.30 Because there is a lack of controlled studies examining the safety and efficacy of SGAs among children and adolescents, especially with long-term use, further research is needed and caution is warranted.28
The FDA has approved
Externalizing disorders among children and adolescents tend to get treated with antipsychotics.28 A Canadian study examining records of 6,916 children found that most children who had been prescribed risperidone received it for ADHD or conduct disorder, and most patients had not received laboratory testing for monitoring the antipsychotic medication they were taking.31 In a 2018 study examining medical records of 120 pediatric patients who presented to an ED in British Columbia with agitation, antipsychotics were the most commonly used medications for patients with autism spectrum disorder; most patients received at least 1 dose.14
For children and adolescents with agitation or aggression who were admitted to inpatient units, IM
Continue to: In case reports...
In case reports, a combination of olanzapine with CNS-suppressing agents has resulted in death. Therefore, do not combine olanzapine with agents such as benzodiazepines.25 In a patient with a likely medical source of agitation, insufficient evidence exists to support the use of olanzapine, and additional research is needed.25
Low-dose haloperidol has been found to be effective for delirium-related agitation in pediatric studies.15 Before initiating an antipsychotic for any child or adolescent, review the patient’s family history for reports of early cardiac death and the patient’s own history of cardiac symptoms, palpitations, syncope, or prolonged QT interval. Monitor for QT prolongation. Among commonly used antipsychotics, the risk of QT prolongation is higher with IV administration of haloperidol and with ziprasidone. Studies show that compared with oral or IM
A few studies have found risperidone to be efficacious for treating ODD and conduct disorder; however, this use is off-label, and its considerable adverse effect and risk profile needs to be weighed against the potential benefit.8
Antipsychotic polypharmacy should be avoided because of the higher risk of adverse effects and interactions, and a lack of robust, controlled studies evaluating the safety of using antipsychotics for non-FDA-approved indications in children and adolescents.7 All patients who receive antipsychotics require monitoring for extrapyramidal symptoms, tardive dyskinesia, neuroleptic malignant syndrome, orthostatic hypotension, sedation, metabolic syndrome, and other potential adverse effects. Patients receiving risperidone need to have their prolactin levels monitored periodically, and their parents should be made aware of the potential for hyperprolactinemia and other adverse effects. Aripiprazole and quetiapine may increase the risk of suicidality.
Antiepileptics. A meta-analysis of 7 randomized controlled trials examining the use of antiepileptic medications (
Continue to: In a retrospective case series...
In a retrospective case series of 30 pediatric patients with autism spectrum disorder who were given oxcarbazepine, Douglas et al35 found that 47% of participants experienced significant improvement in irritability/agitation. However, 23% of patients reported significant adverse effects leading to discontinuation. Insufficient evidence exists for the safety and efficacy of oxcarbazepine in this population.35
Benzodiazepines. The use of benzodiazepines in pediatric patients has been associated with paradoxical disinhibition reactions, particularly in children with autism and other developmental or cognitive disabilities or delays.21 There is a lack of data on the safety and efficacy of long-term use of benzodiazepines in children, especially in light of these patients’ developing brains, the risk of cognitive impairment, and the potential for dependence with long-term use. Despite this, some studies show that the use of benzodiazepines is fairly common among pediatric patients who present to the ED with agitation.14 In a recent retrospective study, Kendrick et al14 found that among pediatric patients with agitation who were brought to the ED, benzodiazepines were the most commonly prescribed medications.
Other medications. Clonidine and
Diphenhydramine, in both oral and IM forms, has been used to treat agitation in children,32 but has also been associated with a paradoxical disinhibition reaction in pediatric patients21 and therefore should be used only sparingly and with caution. Diphenhydramine has anticholinergic properties, and may worsen delirium.15 Stimulant medications can help aggressive behavior in children and adolescents with ADHD.37
Bottom Line
Agitation among children and adolescents has many possible causes. A combination of a comprehensive assessment and evidence-based, judicious treatment interventions can help prevent and manage agitation in this vulnerable population.
Related Resources
- Baker M, Carlson GA. What do we really know about PRN use in agitated children with mental health conditions: a clinical review. Evid Based Ment Health. 2018;21(4):166-170.
- Gerson R, Malas N, Mroczkowski MM. Crisis in the emergency department: the evaluation and management of acute agitation in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2018;27(3):367-386.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Clonidine • Catapres
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra, Spritam
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Quetiapine • Seroquel
Topiramate • Topamax
Risperidone • Risperdal
Valproate • Depakene
Ziprasidone • Geodon
1. Frosch E, Kelly P. Issues in pediatric psychiatric emergency care. In: Emergency psychiatry. Cambridge, UK: Cambridge University Press; 2011:185-199.
2. American College of Emergency Physicians. Pediatric mental health emergencies in the emergency department. https://www.acep.org/patient-care/policy-statements/pediatric-mental-health-emergencies-in-the-emergency-medical-services-system/. Revised September 2018. Accessed February 23, 2019.
3. Voepel-Lewis, T, Burke C, Hadden S, et al. Nurses’ diagnoses and treatment decisions regarding care of the agitated child. J Perianesth Nurs. 2005;20(4):239-248.
4. Malas N, Spital L, Fischer J, et al. National survey on pediatric acute agitation and behavioral escalation in academic inpatient pediatric care settings. Psychosomatics. 2017;58(3):299-306.
5. Famularo R, Kinscherff R, Fenton T. Symptom differences in acute and chronic presentation of childhood post-traumatic stress disorder. Child Abuse Negl. 1990;14(3):439-444.
6. Kaminer D, Seedat S, Stein DJ. Post-traumatic stress disorder in children. World Psychiatry. 2005;4(2):121-125.
7. Ninan A, Stewart SL, Theall LA, et al. Adverse effects of psychotropic medications in children: predictive factors. J Can Acad Child Adolesc Psychiatry. 2014;23(3):218-225.
8. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 2: antipsychotics and traditional mood stabilizers. Can J Psychiatry. 2015;60(2):52-61.
9. Vareilles D, Bréhin C, Cortey C, et al. Hallucinations: Etiological analysis of children admitted to a pediatric emergency department. Arch Pediatr. 2017;24(5):445-452.
10. Bartlett J. Childhood-onset schizophrenia: what do we really know? Health Psychol Behav Med. 2014;2(1):735-747.
11. Diler RS, Goldstein TR, Hafeman D, et al. Distinguishing bipolar depression from unipolar depression in youth: Preliminary findings. J Child Adolesc Psychopharmacol. 2017;27(4):310-319.
12. Dervic K, Garcia-Amador M, Sudol K, et al. Bipolar I and II versus unipolar depression: clinical differences and impulsivity/aggression traits. Eur Psychiatry. 2015;30(1):106-113.
13. Masi L, Gignac M ADHD and DMDD comorbidities, similarities and distinctions. J Child Adolesc Behav2016;4:325.
14. Kendrick JG, Goldman RD, Carr RR. Pharmacologic management of agitation and aggression in a pediatric emergency department - a retrospective cohort study. J Pediatr Pharmacol Ther. 2018;23(6):455-459.
15. Schieveld JN, Staal M, Voogd L, et al. Refractory agitation as a marker for pediatric delirium in very young infants at a pediatric intensive care unit. Intensive Care Med. 2010;36(11):1982-1983.
16. Traube C, Silver G, Gerber LM, et al. Delirium and mortality in critically ill children: epidemiology and outcomes of pediatric delirium. Crit Care Med. 2017;45(5):891-898.
17. Bettencourt A, Mullen JE. Delirium in children: identification, prevention, and management. Crit Care Nurse. 2017;37(3):e9-e18.
18. Suskauer SJ, Trovato MK. Update on pharmaceutical intervention for disorders of consciousness and agitation after traumatic brain injury in children. PM R. 2013;5(2):142-147.
19. Nowicki M, Pearlman L, Campbell C, et al. Agitated behavior scale in pediatric traumatic brain injury. Brain Inj. 2019. doi: 10.1080/02699052.2019.1565893.
20. Mohammad SS, Jones H, Hong M, et al. Symptomatic treatment of children with anti-NMDAR encephalitis. Dev Med Child Neurol. 2016;58(4):376-384.
21. Sonnier L, Barzman D. Pharmacologic management of acutely agitated pediatric patients. Pediatr Drugs. 2011;13(1):1-10.
22. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the american association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3-10.
23. Masters KJ, Bellonci C, Bernet W, et al; American Academy of Child and Adolescent Psychiatry. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):4S-25S.
24. Croce ND, Mantovani C. Using de-escalation techniques to prevent violent behavior in pediatric psychiatric emergencies: It is possible. Pediatric Dimensions, 2017;2(1):1-2.
25. Marzullo LR. Pharmacologic management of the agitated child. Pediatr Emerg Care. 2014;30(4):269-275.
26. Caldwell B, Albert C, Azeem MW, et al. Successful seclusion and restraint prevention effort in child and adolescent programs. J Psychosoc Nurs Ment Health Serv. 2014;52(11):30-38.
27. De Hert M, Dirix N, Demunter H, et al. Prevalence and correlates of seclusion and restraint use in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry. 2011;20(5):221-230.
28. Crystal S, Olfson M, Huang C, et al. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770-w781.
29. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of atypical antipsychotic medication in children and adolescents. https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_Antipsychotic_Medications_Web.pdf. Accessed March 4, 2019.
30. Lohr WD, Chowning RT, Stevenson MD, et al. Trends in atypical antipsychotics prescribed to children six years of age or less on Medicaid in Kentucky. J Child Adolesc Psychopharmacol. 2015;25(5):440-443.
31. Chen W, Cepoiu-Martin M, Stang A, et al. Antipsychotic prescribing and safety monitoring practices in children and youth: a population-based study in Alberta, Canada. Clin Drug Investig. 2018;38(5):449-455.
32. Deshmukh P, Kulkarni G, Barzman D. Recommendations for pharmacological management of inpatient aggression in children and adolescents. Psychiatry (Edgmont). 2010;7(2):32-40.
33. Haldol [package insert]. Beerse, Belgium: Janssen Pharmaceutica NV; 2005.
34. Hirota T, Veenstra-Vanderweele J, Hollander E, et al. Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
35. Douglas JF, Sanders KB, Benneyworth MH, et al. Brief report: retrospective case series of oxcarbazepine for irritability/agitation symptoms in autism spectrum disorder. J Autism Dev Disord. 2013;43(5):1243-1247.
36. Harmon RJ, Riggs PD. Clonidine for posttraumatic stress disorder in preschool children. J Am Acad
37. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 1: Psychostimulants, alpha-2 Agonists, and atomoxetine. Can J Psychiatry. 2015;60(2):42-51
1. Frosch E, Kelly P. Issues in pediatric psychiatric emergency care. In: Emergency psychiatry. Cambridge, UK: Cambridge University Press; 2011:185-199.
2. American College of Emergency Physicians. Pediatric mental health emergencies in the emergency department. https://www.acep.org/patient-care/policy-statements/pediatric-mental-health-emergencies-in-the-emergency-medical-services-system/. Revised September 2018. Accessed February 23, 2019.
3. Voepel-Lewis, T, Burke C, Hadden S, et al. Nurses’ diagnoses and treatment decisions regarding care of the agitated child. J Perianesth Nurs. 2005;20(4):239-248.
4. Malas N, Spital L, Fischer J, et al. National survey on pediatric acute agitation and behavioral escalation in academic inpatient pediatric care settings. Psychosomatics. 2017;58(3):299-306.
5. Famularo R, Kinscherff R, Fenton T. Symptom differences in acute and chronic presentation of childhood post-traumatic stress disorder. Child Abuse Negl. 1990;14(3):439-444.
6. Kaminer D, Seedat S, Stein DJ. Post-traumatic stress disorder in children. World Psychiatry. 2005;4(2):121-125.
7. Ninan A, Stewart SL, Theall LA, et al. Adverse effects of psychotropic medications in children: predictive factors. J Can Acad Child Adolesc Psychiatry. 2014;23(3):218-225.
8. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 2: antipsychotics and traditional mood stabilizers. Can J Psychiatry. 2015;60(2):52-61.
9. Vareilles D, Bréhin C, Cortey C, et al. Hallucinations: Etiological analysis of children admitted to a pediatric emergency department. Arch Pediatr. 2017;24(5):445-452.
10. Bartlett J. Childhood-onset schizophrenia: what do we really know? Health Psychol Behav Med. 2014;2(1):735-747.
11. Diler RS, Goldstein TR, Hafeman D, et al. Distinguishing bipolar depression from unipolar depression in youth: Preliminary findings. J Child Adolesc Psychopharmacol. 2017;27(4):310-319.
12. Dervic K, Garcia-Amador M, Sudol K, et al. Bipolar I and II versus unipolar depression: clinical differences and impulsivity/aggression traits. Eur Psychiatry. 2015;30(1):106-113.
13. Masi L, Gignac M ADHD and DMDD comorbidities, similarities and distinctions. J Child Adolesc Behav2016;4:325.
14. Kendrick JG, Goldman RD, Carr RR. Pharmacologic management of agitation and aggression in a pediatric emergency department - a retrospective cohort study. J Pediatr Pharmacol Ther. 2018;23(6):455-459.
15. Schieveld JN, Staal M, Voogd L, et al. Refractory agitation as a marker for pediatric delirium in very young infants at a pediatric intensive care unit. Intensive Care Med. 2010;36(11):1982-1983.
16. Traube C, Silver G, Gerber LM, et al. Delirium and mortality in critically ill children: epidemiology and outcomes of pediatric delirium. Crit Care Med. 2017;45(5):891-898.
17. Bettencourt A, Mullen JE. Delirium in children: identification, prevention, and management. Crit Care Nurse. 2017;37(3):e9-e18.
18. Suskauer SJ, Trovato MK. Update on pharmaceutical intervention for disorders of consciousness and agitation after traumatic brain injury in children. PM R. 2013;5(2):142-147.
19. Nowicki M, Pearlman L, Campbell C, et al. Agitated behavior scale in pediatric traumatic brain injury. Brain Inj. 2019. doi: 10.1080/02699052.2019.1565893.
20. Mohammad SS, Jones H, Hong M, et al. Symptomatic treatment of children with anti-NMDAR encephalitis. Dev Med Child Neurol. 2016;58(4):376-384.
21. Sonnier L, Barzman D. Pharmacologic management of acutely agitated pediatric patients. Pediatr Drugs. 2011;13(1):1-10.
22. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the american association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3-10.
23. Masters KJ, Bellonci C, Bernet W, et al; American Academy of Child and Adolescent Psychiatry. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):4S-25S.
24. Croce ND, Mantovani C. Using de-escalation techniques to prevent violent behavior in pediatric psychiatric emergencies: It is possible. Pediatric Dimensions, 2017;2(1):1-2.
25. Marzullo LR. Pharmacologic management of the agitated child. Pediatr Emerg Care. 2014;30(4):269-275.
26. Caldwell B, Albert C, Azeem MW, et al. Successful seclusion and restraint prevention effort in child and adolescent programs. J Psychosoc Nurs Ment Health Serv. 2014;52(11):30-38.
27. De Hert M, Dirix N, Demunter H, et al. Prevalence and correlates of seclusion and restraint use in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry. 2011;20(5):221-230.
28. Crystal S, Olfson M, Huang C, et al. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770-w781.
29. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of atypical antipsychotic medication in children and adolescents. https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_Antipsychotic_Medications_Web.pdf. Accessed March 4, 2019.
30. Lohr WD, Chowning RT, Stevenson MD, et al. Trends in atypical antipsychotics prescribed to children six years of age or less on Medicaid in Kentucky. J Child Adolesc Psychopharmacol. 2015;25(5):440-443.
31. Chen W, Cepoiu-Martin M, Stang A, et al. Antipsychotic prescribing and safety monitoring practices in children and youth: a population-based study in Alberta, Canada. Clin Drug Investig. 2018;38(5):449-455.
32. Deshmukh P, Kulkarni G, Barzman D. Recommendations for pharmacological management of inpatient aggression in children and adolescents. Psychiatry (Edgmont). 2010;7(2):32-40.
33. Haldol [package insert]. Beerse, Belgium: Janssen Pharmaceutica NV; 2005.
34. Hirota T, Veenstra-Vanderweele J, Hollander E, et al. Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
35. Douglas JF, Sanders KB, Benneyworth MH, et al. Brief report: retrospective case series of oxcarbazepine for irritability/agitation symptoms in autism spectrum disorder. J Autism Dev Disord. 2013;43(5):1243-1247.
36. Harmon RJ, Riggs PD. Clonidine for posttraumatic stress disorder in preschool children. J Am Acad
37. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 1: Psychostimulants, alpha-2 Agonists, and atomoxetine. Can J Psychiatry. 2015;60(2):42-51
Unipolar vs bipolar depression: A clinician’s perspective
Mrs. W, age 36, who is married, has a history of military service, and is currently employed as a paralegal, is referred to our practice by her family physician. She complains of severe depression that impairs her ability to function at work. She had seen several other psychiatrists in both military and civilian settings, and had been treated with multiple antidepressants, including fluoxetine, sertraline, bupropion, and paroxetine.
At the time of her initial psychiatric evaluation, she is taking duloxetine, 90 mg/d, but still is experiencing depressive symptoms. She is tearful, sad, lacks energy, spends too much time in bed, and is experiencing thoughts of hopelessness, despair, and escape, verging on thoughts of suicide. As a result, she needs to scale back her work schedule to part-time. When asked about how long she had been suffering from depression, she responds “I’ve been depressed all my life.” She had been briefly hospitalized at age 16, when she made a suicide attempt by overdose. There had been no subsequent suicide attempts or psychiatric hospitalizations, although she acknowledges having intermittent suicidal thoughts.
Mrs. W’s clinical presentation is similar to that of many patients entering our practice—patients who have recurrent depression that began in early life and a history of failure to respond to multiple antidepressants. She and other patients with similar presentations are not suffering from treatment-resistant depression and in need of a trial of electroconvulsive therapy, transcranial magnetic stimulation, direct current stimulation, vagus nerve stimulation, or intranasal esketamine. She has bipolar disorder, and had been repeatedly misdiagnosed and treated inappropriately with antidepressant monotherapy.
In a previous article1 (“Controversies in bipolar disorder: Trust evidence or experience?,”
In this article, based on our more than 25 years of experience in diagnosing and treating psychiatric disorders in patients of all ages, we expand on those observations.
Misdiagnosis is common
Bipolar depression is frequently misdiagnosed as unipolar depression in outpatient2-8 and inpatient9 settings, and in children and adolescents.10 Mrs. W is typical of patients who have what we consider a bipolar spectrum disorder and receive an inaccurate diagnosis and treatment that is ineffective or may worsen the course of their illness.
Reliance on DSM-511 and its predecessor, DSM-IV, is a part of the problem of misdiagnosis because the diagnostic criteria for bipolar disorder fail to capture the clinical features of many patients with “softer” (less obvious manic and hypomanic) variants of the disorder.12,13 For example, DSM-5 criteria for a hypomanic episode (the mild high experienced by patients with a soft bipolar disorder) require that the episode lasts “at least 4 consecutive days” and is “present most of the day, nearly every day.” In our experience, the majority of hypomanic episodes are shorter—ranging from a half-day to 2 days, averaging perhaps 1.5 days.
Continue to: DSM-5 also requires...
DSM-5 also requires severity criteria for hypomania that patients with unequivocal hypomanic episodes often do not meet. For example, they may fail to experience flight of ideas or racing thoughts, or engage in activities such as “unrestrained buying sprees, sexual indiscretions, or foolish business investments.” These patients usually describe these mild highs as feeling normal and report a happier mood, more smiles and laughter, increased energy, less sleepiness, increased talkativeness, increased socialization, and improved motivation to complete tasks left undone and projects left unfinished because of the previous depressive episode. These softer (subthreshold) hypomanic episodes are authentic and, if clinicians do not identify them, may lead to misdiagnosis and inappropriate treatment.
Patients who present with depression often fail to report these brief, subthreshold hypomanic episodes or consider them to be irrelevant to their diagnosis and treatment.12,13 Probing questions can often elicit these unreported highs. For example, a patient with depression should be asked, “Have you had a single good day during the last month?” and “Where were you and what did you do during that day?” Eliciting a history of brief periods of improved mood is the key to differentiating between unipolar and bipolar depression. Screening instruments such as the Mood Disorders Questionnaire14 and the Bipolar Spectrum Diagnostic Scale15 may be helpful in distinguishing between unipolar and bipolar depression. However, we offer our thoughts on making that crucial distinction.
Distinguishing between these 2 types of depression
Although it may be difficult to distinguish between unipolar and bipolar depression, especially in the absence of a history of distinct manic or hypomanic episodes, we find the following criteria to be useful in making that determination.
Age of onset. Bipolar spectrum disorders typically begin earlier in life than unipolar depression.10,16-19 A typical presentation of bipolar disorder in children and adolescents is depression or agitated mixed states with features of both mania and depression, often accompanied by rapid mood cycling.20,21 Unipolar depression usually begins later in life, and patients do not have a history of significant depressive episodes or mood swings in childhood or adolescence. An important question to ask a patient with a chief complaint of depression is, “How old were you when you first experienced an episode of depression?”
Gender differences. Bipolar spectrum disorders with more subtle (softer) presentations, such as subthreshold highs, occur more often in women than men.22 However, overall rates of bipolar disorder may be slightly higher in men than in women.23 Unipolar melancholic depression occurs at approximately the same frequency in men and women.24
Continue to: Rapidity of onset
Rapidity of onset. Bipolar depressive episodes develop more rapidly than unipolar episodes. It is common for a patient with a bipolar spectrum disorder to transition from normal to very depressed virtually overnight, whereas in our clinical experience, unipolar episodes progress more slowly, often over several months.
Deliberate self-harm. Adolescents and young adults with a bipolar spectrum disorder frequently engage in self-injurious behavior, usually cutting with a knife, razor, or even sharp fingernails.25 Although these patients may also have thoughts of suicide and make suicide attempts, the individual usually perceives cutting as a means of gaining relief from tension and distress. These behaviors are often associated with a diagnosis of a personality disorder; in our opinion, however, they are hallmarks of a bipolar spectrum disorder.
ADHD. Bipolar disorder frequently co-occurs with attention-deficit/hyperactivity disorder (ADHD).26,27 Adults with bipolar disorder often have ADHD symptoms, which can complicate their treatment and cause functional impairment even after their mood disorder has been stabilized.28
Substance use disorders. Excessive use of alcohol and drugs is common among people with a wide range of psychiatric disorders, but patients with bipolar disorder have an unusually high rate of co-occurring substance use disorders—40% to 50%.29,30
Appetite and weight differences. Patients with unipolar depression usually experience loss of appetite and weight loss, whereas in our clinical experience, patients with bipolar depression often overeat, crave carbohydrates, and gain weight.
Continue to: Sleep problems
Sleep problems. Patients with bipolar depression have an increased need for sleep (the opposite of what they experience during highs), are sleepy during the day regardless of how many hours they sleep, and have difficulty getting up in the morning. Patients with unipolar depression also have a sleep disturbance: they may fall asleep easily, sleep for a few hours, and then awaken but are unable to fall back to sleep.31 Yet these patients usually do not complain of sleepiness during the day.
Diurnal variation of mood. Patients with unipolar depression often report that their depressive symptoms fluctuate in a circadian manner. For example, they may report that their depression is worse in the morning but improves toward evening.31 This regular alteration of circadian rhythm usually is not evident in patients with bipolar depression, whose mood may vary unpredictably or in response to stressors. Some patients with bipolar disorder, however, exhibit ultradian (ultra-rapid) mood cycling, which may be confused with the diurnal mood variation seen in patients with unipolar depression.
Tendency to recur. Although both unipolar and bipolar depressive episodes recur, a pattern of multiple recurring episodes beginning in early life is characteristic of bipolar spectrum disorders.
Behavioral history. Patients with bipolar depression are more likely than patients with unipolar depression to have a history of multiple marriages, multiple romantic relationships, episodes of promiscuity, legal problems, or financial extravagance.
Response to antidepressants. Patients with bipolar depression exhibit atypical responses to antidepressant monotherapy, such as worsening of depressive symptoms, initial improvement of mood with subsequent loss of effectiveness, premature response to an antidepressant (eg, improvement of mood within 1 to 2 days of beginning the antidepressant), fluctuation of depressive symptoms (mood cycling), or precipitation of a hypomanic or manic episode. We believe that a history of multiple failed antidepressant trials is compelling evidence of misdiagnosis of a bipolar spectrum disorder as unipolar depression.
Continue to: Genetics
Genetics. Bipolar disorder is one of the most heritable of illnesses.32 Family history is important, but affected relatives may have been misdiagnosed with unipolar depression or schizophrenia, or said to have experienced “nervous breakdowns.”
Consequences of misdiagnosis
Misdiagnosis of patients with bipolar disorder is not benign. We see patients who have suffered needlessly for years with severe depression and mood instability. After trying antidepressant after antidepressant without benefit, they begin to feel hopeless, believing they have tried everything and that nothing works for them. Often, these patients have dropped out of high school or college, or lost jobs, friends, and spouses due to their disabling but misdiagnosed psychiatric disorder. Patients with misdiagnosed bipolar disorder have an increased risk of suicide attempts and psychiatric hospitalization.5,8
Misdiagnosis of patients with bipolar disorder is not limited to nonpsychiatric physicians. The majority of patients with bipolar spectrum disorders are misdiagnosed by outpatient psychiatrists as having unipolar depression.2-7 At least 45% of patients hospitalized for depression have bipolar disorder—and most of these patients are treated inappropriately with antidepressants.9 The STAR*D study,33,34 a large randomized clinical trial of antidepressants, concluded that more than one-third of patients had not remitted from their depression after treatment with 3 different antidepressants. In our opinion, many of the nonresponding patients may have undiagnosed bipolar depression, which predictably leads to a failure to respond adequately to antidepressants. We believe that the customary inclusion and exclusion criteria used to select participants for these research studies miss subtle (subthreshold) hypomanic episodes that fall short of meeting DSM criteria for duration and severity. This phenomenon may account for the results of studies that conclude that antidepressants are, at best, minimally more effective than placebo.35
When a patient with a bipolar spectrum disorder is misdiagnosed and treated with an antidepressant, the usual result is mood destabilization. Reports of mood swings, increased crying, and suicidal thoughts and suicidal gestures in children, adolescents, and young adults treated with antidepressants led the FDA to issue a “black-box” warning.36 Because bipolar depression typically begins in youth,10,18,19 the behaviors cited in the warning may reflect misdiagnosis of bipolar depression as unipolar depression, and consequent mood destabilization as a result of treatment with an antidepressant in the absence of a mood stabilizer.
Depression and life stressors
Since many patients who are depressed present with a history of significant stressors, clinicians often face the problem of distinguishing between clinical depression and stress-induced depression. We believe that one typical symptom of depression—increased sensitivity to stressors—may help in making that distinction. A patient who is depressed will often attribute depression to stressors such as marital conflict, divorce, problems with a teenage child, work pressures, financial pressures, or the illness or death of a family member or pet. If clinical depression (unipolar or bipolar) is present, the symptoms are persistent, sometimes antedate the stressor by days or weeks, often outlast the stressor, increase in severity over time, and are disproportional to the stressor. Clinical depression can also cause the patient to become obsessed with traumatic events or losses that occurred many years earlier.
Continue to: Our approach to treatment
Our approach to treatment
Patients with mood disorders often benefit from a combination of pharmacologic management and psychotherapy. Psychotherapy is particularly important in addressing the functional impairment, diminished self-worth, and interpersonal conflicts that often accompany clinical depression. Several styles or systems of psychotherapy have been developed to benefit patients with mood disorders. Their effectiveness may depend on the patient’s ability to gain insight,37 but in our opinion, the most important attribute of helpful psychotherapy is the rapport established between the patient and the therapist, and the therapist’s ability to empathize with the patient and instill in the patient a sense of optimism and hope. We often recommend that patients attend meetings of the Depression and Bipolar Support Alliance (DBSA), a national support group with chapters throughout the country. Patients often find that attending these meetings is both educational and emotionally rewarding.
The foundational pharmacologic treatment for bipolar disorder is a mood stabilizer. The medications we consider to be effective mood stabilizers (some with an FDA indication for bipolar maintenance, some without) are lithium carbonate, divalproex sodium, carbamazepine, oxcarbazepine, and lamotrigine.
Each of these mood stabilizers has its advantages, disadvantages, risks, and adverse effects. For example, although divalproex is a reliable mood stabilizer, it has a significant risk of causing birth defects if taken during pregnancy and can cause increased appetite and weight gain. Carbamazepine has significant drug interactions and the potential to cause neurologic adverse effects, while oxcarbazepine, a derivative of carbamazepine, has fewer drug interactions but is more likely to cause hyponatremia. Lamotrigine must be titrated very slowly to reduce the risk of a potentially fatal skin rash (ie, Stevens-Johnson syndrome or toxic epidermal necrolysis). Lithium is effective but has a significant adverse-effect burden: impairment of renal function with long-term use, nephrogenic diabetes insipidus, hypothyroidism, hyperparathyroidism, acne, and weight gain. Lithium also has potential interactions with multiple commonly prescribed medications, including antihypertensives and diuretics, as well as over-the-counter pain relievers such as ibuprofen and naproxen.
Second-generation antipsychotics (SGAs) have mood stabilizing, antidepressant, and anti-manic properties and are often useful in managing bipolar disorder. In our experience, for patients with bipolar disorder, SGAs are best used in combination with a mood stabilizer. Although virtually all SGAs have demonstrated effectiveness in the treatment of psychosis and some phases of bipolar disorder, the newer agents (aripiprazole, brexpiprazole, lurasidone, and cariprazine) are relatively free of metabolic adverse effects such as weight gain, abnormal cholesterol levels, increased prolactin levels, insulin resistance, and increased risk of diabetes.
Antidepressants may be effective in treating unipolar depression, but when treating bipolar depression, they should be used cautiously and only in combination with a mood stabilizer.
Continue to: As we observed...
As we observed in our previous article,1 thyroid laboratory monitoring and supplementation are critical components of managing mood disorders (Box 138-41).
Box 1
Conventional laboratory reference ranges often indicate that thyroid-stimulating hormone (TSH) levels as high as 4.0, 4.5, or 5.0 mU/L are normal. A recent meta- analysis determined that treatment of subclinical hypothyroidism (elevated TSH with normal free thyroxine) does not benefit patients’ quality of life.38 Patients with mood disorders, however, often fail to respond to mood stabilizers and other psychiatric medications unless their TSH is <3.0 or even <2.5 mU/L.39,40 We typically augment with liothyronine because, unlike levothyroxine, it works quickly, does not require deiodination to be activated, and, contrary to some reports, its elimination and biologic half-life are sufficient for single daily dosing.41
Moving towards better diagnoses
The emergence of a criteria-based psychiatric system in 1980 with the publication of DSM-III, and its subsequent revisions and updates, constituted a major advance in psychiatric diagnosis. As we learn more about the pathophysiology, genetics, and epigenetics of psychiatric symptoms and syndromes, future diagnostic systems will improve problems of validity that have yet to be resolved. While we believe that, for the most part, DSM-5 was an advance over the previous diagnostic iteration, we have 2 issues with DSM-5 in terms of the diagnosis of bipolar disorder (Box 210,12,13,18,19,42).
Box 2
Based on our clinical experience treating thousands of patients over 25 years, we have 2 issues with DSM-5 regarding bipolar disorder:
1. The DSM-5 criteria for hypomania fail to reflect the features of clinical presentations commonly seen in our practice. The majority of patients with authentic bipolar syndromes do not have hypomanias that last for at least 4 days or reach the level of severity required for a DSM-5 diagnosis of hypomania. This results in misdiagnosis of patients with bipolar depression as suffering from unipolar depression, which leads to inappropriate treatment with antidepressant monotherapy.
2. Bipolar disorder frequently makes its first appearance in childhood and adolescence,10,18,19 and increasing numbers of young patients have been receiving this diagnosis.42 In our opinion, this increase reflects clinicians’ improved diagnostic skills. Perhaps alarmed by the increase in young people receiving a diagnosis of bipolar disorder, the authors of DSM-5 created a new diagnosis for children: disruptive mood dysregulation disorder. This diagnostic addition is based on the finding that children with these mood symptoms may not subsequently exhibit classic DSM-5 manic or hypomanic episodes. But the lack of such episodes does not preclude a diagnosis of bipolar disorder, because many adults with unequivocal bipolar spectrum disorders have subthreshold hypomanias and thus fail to exhibit classic manic or hypomanic episodes.12,13
A rose by any other name would smell as sweet. Children who exhibit symptoms of disruptive mood dysregulation disorder— chronic irritability and protracted temper outbursts—usually suffer from depression and mood instability. In our opinion, it is irrational and confusing to clinicians to separate out with a new diagnosis an arbitrarily defined group of children who exhibit substantially the same symptoms as those who receive a diagnosis of bipolar disorder.
Patients with a chief complaint of depression are often given a diagnosis of “major depression, rule out bipolar disorder.” We believe that this formula should be turned on its head. In our opinion, based on our clinical experience, we think that most patients who present to a clinician’s office or psychiatric hospital with depression have bipolar depression, not unipolar depression. We hope that our experience and observations derived from treating thousands of patients over more than 25 years may be helpful to clinicians who sometimes struggle to bring relief to their patients with mood disorders.
CASE CONTINUED
Return to work
Mrs. W is now doing well. She is taking a lower dosage of duloxetine, 60 mg/d, in combination with the mood stabilizer lamotrigine, 200 mg/d. She returns to work full-time as a paralegal and no longer is experiencing depressive episodes.
Bottom Line
Patients with bipolar depression are often misdiagnosed with unipolar depression and treated inappropriately with antidepressant monotherapy, which often results in mood destabilization. Based on our clinical experience, a careful assessment of select criteria, including age of onset, rapidity of onset, comorbidities, diurnal mood variations, and more, can be useful for distinguishing between unipolar and bipolar depression.
Related Resources
- Nasrallah HA. Misdiagnosing bipolar depression as major depressive disorder. Current Psychiatry. 2013;12(10):20-21,A.
- Ghaemi SN. Bipolar spectrum: a review of the concept and a vision for the future. Psychiatry Investig. 2013;10(3):218-224.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol, Equetro
Cariprazine • Vraylar
Divalproex • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Lamotrigine • Lamictal
Levothyroxine • Synthroid, Levoxyl
Liothyronine • Cytomel
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oxcarbazepine • Oxtellar XR, Trileptal
Paroxetine • Paxil
Sertraline • Zoloft
1. Miller GE, Noel RL. Controversies in bipolar disorder: trust evidence or experience? Current Psychiatry. 2009;8(2):27-28,31-33,39.
2. Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry. 2004;6(1):27-33.
3. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52(1-3):135-144.
4. Blanco C, Laje G, Olfson M, et al. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159(6):1005-1010.
5. Shi L, Thiebaud P, McCombs JS. The impact of unrecognized bipolar disorders for patients treated with antidepressants in the fee-for-services California Medicaid (Medi-Cal) program. J Affect Disord. 2004;82(3):373-383.
6. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
7. Hughes T, Cardno A, West R, et al. Unrecognized bipolar disorder among UK primary care patients prescribed antidepressants: an observational study. Br J Gen Pract. 2016;66(643):e71-e77.
8. Keck PE Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatric Pract. 2008;14(Suppl 2):31-38.
9. Goldberg JF, Harrow M, Whiteside JF. Risk for bipolar illness in inpatients initially hospitalized for unipolar depression. Am J Psychiatry. 2001:158(8):1265-1270.
10. Chilakamarri JK, Filkowski MM, Ghaemi SN. Misdiagnosis of bipolar disorder in children and adolescents: a comparison with ADHD and major depressive disorder. Ann Clin Psychiatry. 2011;23(1):25-29.
11. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
12. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84(2-3):117-125.
13. Baldassano C. Distinctions between bipolar I and bipolar II depression. Current Psychiatry. 2017;16(8):S7-S16.
14. Hirschfeld MA, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: The Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873-1875.
15. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity a new bipolar spectrum diagnostic scale. J Affect Disorder. 2005;84(2-3):273-277
16. Suppes T, Leverich G, Keck P, et al. The Stanley Foundation Continuing Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disorder. 2001;67(1-3):45-49.
17. Perlis RH, Miyahara S, Marangell LB. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP–BD). Biol Psychiatry. 2004;55(9):875-881.
18. Baldessarini RJ, Bolzani L, Kruz N, et al. Onset age of bipolar disorders at six international sites. J Affect Disord. 2010;121(1-2):143-146.
19. Post RM, Altshuler LL, Kupka R, et al. More childhood onset bipolar disorder in the United States than Canada or Europe: implications for treatment and prevention. Neurosci Biobehav Rev. 2017;74(Pt A):204-213.
20. Geller B, Luby J. Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1168-1176.
21. Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
22. Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26(3):595-620.
23. Deflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22(5):437-452.
24. Bogren M, Brådvik L, Holmstrand C, et al. Gender differences in subtypes of depression by first incidence and age of onset: a follow-up of the Lunby population. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):179-189.
25. Singhal A, Ross J, Seminog O, et al. Risks of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194-204.
26. Joshi G, Wilens T. Comorbidity in pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Amer. 2009;18(2):291-319.
27. Youngtrom EA, Arnold LE, Frazier TW. Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clin Psychol (New York). 2010;17(4):350-359.
28. McIntyre RS, Kennedy SH, Soczynska JK, et al. Attention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the International Mood Disorders Collaborative Project. Prime Care Companion J Clin Psychiatry. 2010;12(3). doi:10.4088/PCC.09m00861gry.
29. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiological Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
30. Hunt GE, Malhi GS, Cleary M, et al. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349.
31. Agargun MY, Besiroglu L, Cilli AS, et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord. 2007;98(3):267-270.
32. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66(3):287-296.
33. Rush AJ, Trivedi MH, Wisniewski SR, et al. Buproprion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
34. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
35. Kirsch I. Antidepressants and the placebo effect. Z Psychol. 2014;222(3):128-134.
36. U.S. Food and Drug Administration. Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm161679.htm. Published February 5, 2018. Accessed May 10, 2019.
37. Jennissen S, Huber J, Ehrenthal JC, et al. Association between insight and outcome of psychotherapy; systematic review and meta-analysis. Am J Psychiatry. 2018;175(10):961-969.
38. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism. JAMA. 2018;320(13):1349-1359.
39. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry. 2002;159(1):116-121.
40. Parmentier T, Sienaert P. The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord. 2018;229:410-414.
41. Koda-Kimbe MA, Alldredge BK. Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs (10th ed). Baltimore, MD: Walters Klower Health/Lippincott Williams & Wilkins; 2012.
42. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
Mrs. W, age 36, who is married, has a history of military service, and is currently employed as a paralegal, is referred to our practice by her family physician. She complains of severe depression that impairs her ability to function at work. She had seen several other psychiatrists in both military and civilian settings, and had been treated with multiple antidepressants, including fluoxetine, sertraline, bupropion, and paroxetine.
At the time of her initial psychiatric evaluation, she is taking duloxetine, 90 mg/d, but still is experiencing depressive symptoms. She is tearful, sad, lacks energy, spends too much time in bed, and is experiencing thoughts of hopelessness, despair, and escape, verging on thoughts of suicide. As a result, she needs to scale back her work schedule to part-time. When asked about how long she had been suffering from depression, she responds “I’ve been depressed all my life.” She had been briefly hospitalized at age 16, when she made a suicide attempt by overdose. There had been no subsequent suicide attempts or psychiatric hospitalizations, although she acknowledges having intermittent suicidal thoughts.
Mrs. W’s clinical presentation is similar to that of many patients entering our practice—patients who have recurrent depression that began in early life and a history of failure to respond to multiple antidepressants. She and other patients with similar presentations are not suffering from treatment-resistant depression and in need of a trial of electroconvulsive therapy, transcranial magnetic stimulation, direct current stimulation, vagus nerve stimulation, or intranasal esketamine. She has bipolar disorder, and had been repeatedly misdiagnosed and treated inappropriately with antidepressant monotherapy.
In a previous article1 (“Controversies in bipolar disorder: Trust evidence or experience?,”
In this article, based on our more than 25 years of experience in diagnosing and treating psychiatric disorders in patients of all ages, we expand on those observations.
Misdiagnosis is common
Bipolar depression is frequently misdiagnosed as unipolar depression in outpatient2-8 and inpatient9 settings, and in children and adolescents.10 Mrs. W is typical of patients who have what we consider a bipolar spectrum disorder and receive an inaccurate diagnosis and treatment that is ineffective or may worsen the course of their illness.
Reliance on DSM-511 and its predecessor, DSM-IV, is a part of the problem of misdiagnosis because the diagnostic criteria for bipolar disorder fail to capture the clinical features of many patients with “softer” (less obvious manic and hypomanic) variants of the disorder.12,13 For example, DSM-5 criteria for a hypomanic episode (the mild high experienced by patients with a soft bipolar disorder) require that the episode lasts “at least 4 consecutive days” and is “present most of the day, nearly every day.” In our experience, the majority of hypomanic episodes are shorter—ranging from a half-day to 2 days, averaging perhaps 1.5 days.
Continue to: DSM-5 also requires...
DSM-5 also requires severity criteria for hypomania that patients with unequivocal hypomanic episodes often do not meet. For example, they may fail to experience flight of ideas or racing thoughts, or engage in activities such as “unrestrained buying sprees, sexual indiscretions, or foolish business investments.” These patients usually describe these mild highs as feeling normal and report a happier mood, more smiles and laughter, increased energy, less sleepiness, increased talkativeness, increased socialization, and improved motivation to complete tasks left undone and projects left unfinished because of the previous depressive episode. These softer (subthreshold) hypomanic episodes are authentic and, if clinicians do not identify them, may lead to misdiagnosis and inappropriate treatment.
Patients who present with depression often fail to report these brief, subthreshold hypomanic episodes or consider them to be irrelevant to their diagnosis and treatment.12,13 Probing questions can often elicit these unreported highs. For example, a patient with depression should be asked, “Have you had a single good day during the last month?” and “Where were you and what did you do during that day?” Eliciting a history of brief periods of improved mood is the key to differentiating between unipolar and bipolar depression. Screening instruments such as the Mood Disorders Questionnaire14 and the Bipolar Spectrum Diagnostic Scale15 may be helpful in distinguishing between unipolar and bipolar depression. However, we offer our thoughts on making that crucial distinction.
Distinguishing between these 2 types of depression
Although it may be difficult to distinguish between unipolar and bipolar depression, especially in the absence of a history of distinct manic or hypomanic episodes, we find the following criteria to be useful in making that determination.
Age of onset. Bipolar spectrum disorders typically begin earlier in life than unipolar depression.10,16-19 A typical presentation of bipolar disorder in children and adolescents is depression or agitated mixed states with features of both mania and depression, often accompanied by rapid mood cycling.20,21 Unipolar depression usually begins later in life, and patients do not have a history of significant depressive episodes or mood swings in childhood or adolescence. An important question to ask a patient with a chief complaint of depression is, “How old were you when you first experienced an episode of depression?”
Gender differences. Bipolar spectrum disorders with more subtle (softer) presentations, such as subthreshold highs, occur more often in women than men.22 However, overall rates of bipolar disorder may be slightly higher in men than in women.23 Unipolar melancholic depression occurs at approximately the same frequency in men and women.24
Continue to: Rapidity of onset
Rapidity of onset. Bipolar depressive episodes develop more rapidly than unipolar episodes. It is common for a patient with a bipolar spectrum disorder to transition from normal to very depressed virtually overnight, whereas in our clinical experience, unipolar episodes progress more slowly, often over several months.
Deliberate self-harm. Adolescents and young adults with a bipolar spectrum disorder frequently engage in self-injurious behavior, usually cutting with a knife, razor, or even sharp fingernails.25 Although these patients may also have thoughts of suicide and make suicide attempts, the individual usually perceives cutting as a means of gaining relief from tension and distress. These behaviors are often associated with a diagnosis of a personality disorder; in our opinion, however, they are hallmarks of a bipolar spectrum disorder.
ADHD. Bipolar disorder frequently co-occurs with attention-deficit/hyperactivity disorder (ADHD).26,27 Adults with bipolar disorder often have ADHD symptoms, which can complicate their treatment and cause functional impairment even after their mood disorder has been stabilized.28
Substance use disorders. Excessive use of alcohol and drugs is common among people with a wide range of psychiatric disorders, but patients with bipolar disorder have an unusually high rate of co-occurring substance use disorders—40% to 50%.29,30
Appetite and weight differences. Patients with unipolar depression usually experience loss of appetite and weight loss, whereas in our clinical experience, patients with bipolar depression often overeat, crave carbohydrates, and gain weight.
Continue to: Sleep problems
Sleep problems. Patients with bipolar depression have an increased need for sleep (the opposite of what they experience during highs), are sleepy during the day regardless of how many hours they sleep, and have difficulty getting up in the morning. Patients with unipolar depression also have a sleep disturbance: they may fall asleep easily, sleep for a few hours, and then awaken but are unable to fall back to sleep.31 Yet these patients usually do not complain of sleepiness during the day.
Diurnal variation of mood. Patients with unipolar depression often report that their depressive symptoms fluctuate in a circadian manner. For example, they may report that their depression is worse in the morning but improves toward evening.31 This regular alteration of circadian rhythm usually is not evident in patients with bipolar depression, whose mood may vary unpredictably or in response to stressors. Some patients with bipolar disorder, however, exhibit ultradian (ultra-rapid) mood cycling, which may be confused with the diurnal mood variation seen in patients with unipolar depression.
Tendency to recur. Although both unipolar and bipolar depressive episodes recur, a pattern of multiple recurring episodes beginning in early life is characteristic of bipolar spectrum disorders.
Behavioral history. Patients with bipolar depression are more likely than patients with unipolar depression to have a history of multiple marriages, multiple romantic relationships, episodes of promiscuity, legal problems, or financial extravagance.
Response to antidepressants. Patients with bipolar depression exhibit atypical responses to antidepressant monotherapy, such as worsening of depressive symptoms, initial improvement of mood with subsequent loss of effectiveness, premature response to an antidepressant (eg, improvement of mood within 1 to 2 days of beginning the antidepressant), fluctuation of depressive symptoms (mood cycling), or precipitation of a hypomanic or manic episode. We believe that a history of multiple failed antidepressant trials is compelling evidence of misdiagnosis of a bipolar spectrum disorder as unipolar depression.
Continue to: Genetics
Genetics. Bipolar disorder is one of the most heritable of illnesses.32 Family history is important, but affected relatives may have been misdiagnosed with unipolar depression or schizophrenia, or said to have experienced “nervous breakdowns.”
Consequences of misdiagnosis
Misdiagnosis of patients with bipolar disorder is not benign. We see patients who have suffered needlessly for years with severe depression and mood instability. After trying antidepressant after antidepressant without benefit, they begin to feel hopeless, believing they have tried everything and that nothing works for them. Often, these patients have dropped out of high school or college, or lost jobs, friends, and spouses due to their disabling but misdiagnosed psychiatric disorder. Patients with misdiagnosed bipolar disorder have an increased risk of suicide attempts and psychiatric hospitalization.5,8
Misdiagnosis of patients with bipolar disorder is not limited to nonpsychiatric physicians. The majority of patients with bipolar spectrum disorders are misdiagnosed by outpatient psychiatrists as having unipolar depression.2-7 At least 45% of patients hospitalized for depression have bipolar disorder—and most of these patients are treated inappropriately with antidepressants.9 The STAR*D study,33,34 a large randomized clinical trial of antidepressants, concluded that more than one-third of patients had not remitted from their depression after treatment with 3 different antidepressants. In our opinion, many of the nonresponding patients may have undiagnosed bipolar depression, which predictably leads to a failure to respond adequately to antidepressants. We believe that the customary inclusion and exclusion criteria used to select participants for these research studies miss subtle (subthreshold) hypomanic episodes that fall short of meeting DSM criteria for duration and severity. This phenomenon may account for the results of studies that conclude that antidepressants are, at best, minimally more effective than placebo.35
When a patient with a bipolar spectrum disorder is misdiagnosed and treated with an antidepressant, the usual result is mood destabilization. Reports of mood swings, increased crying, and suicidal thoughts and suicidal gestures in children, adolescents, and young adults treated with antidepressants led the FDA to issue a “black-box” warning.36 Because bipolar depression typically begins in youth,10,18,19 the behaviors cited in the warning may reflect misdiagnosis of bipolar depression as unipolar depression, and consequent mood destabilization as a result of treatment with an antidepressant in the absence of a mood stabilizer.
Depression and life stressors
Since many patients who are depressed present with a history of significant stressors, clinicians often face the problem of distinguishing between clinical depression and stress-induced depression. We believe that one typical symptom of depression—increased sensitivity to stressors—may help in making that distinction. A patient who is depressed will often attribute depression to stressors such as marital conflict, divorce, problems with a teenage child, work pressures, financial pressures, or the illness or death of a family member or pet. If clinical depression (unipolar or bipolar) is present, the symptoms are persistent, sometimes antedate the stressor by days or weeks, often outlast the stressor, increase in severity over time, and are disproportional to the stressor. Clinical depression can also cause the patient to become obsessed with traumatic events or losses that occurred many years earlier.
Continue to: Our approach to treatment
Our approach to treatment
Patients with mood disorders often benefit from a combination of pharmacologic management and psychotherapy. Psychotherapy is particularly important in addressing the functional impairment, diminished self-worth, and interpersonal conflicts that often accompany clinical depression. Several styles or systems of psychotherapy have been developed to benefit patients with mood disorders. Their effectiveness may depend on the patient’s ability to gain insight,37 but in our opinion, the most important attribute of helpful psychotherapy is the rapport established between the patient and the therapist, and the therapist’s ability to empathize with the patient and instill in the patient a sense of optimism and hope. We often recommend that patients attend meetings of the Depression and Bipolar Support Alliance (DBSA), a national support group with chapters throughout the country. Patients often find that attending these meetings is both educational and emotionally rewarding.
The foundational pharmacologic treatment for bipolar disorder is a mood stabilizer. The medications we consider to be effective mood stabilizers (some with an FDA indication for bipolar maintenance, some without) are lithium carbonate, divalproex sodium, carbamazepine, oxcarbazepine, and lamotrigine.
Each of these mood stabilizers has its advantages, disadvantages, risks, and adverse effects. For example, although divalproex is a reliable mood stabilizer, it has a significant risk of causing birth defects if taken during pregnancy and can cause increased appetite and weight gain. Carbamazepine has significant drug interactions and the potential to cause neurologic adverse effects, while oxcarbazepine, a derivative of carbamazepine, has fewer drug interactions but is more likely to cause hyponatremia. Lamotrigine must be titrated very slowly to reduce the risk of a potentially fatal skin rash (ie, Stevens-Johnson syndrome or toxic epidermal necrolysis). Lithium is effective but has a significant adverse-effect burden: impairment of renal function with long-term use, nephrogenic diabetes insipidus, hypothyroidism, hyperparathyroidism, acne, and weight gain. Lithium also has potential interactions with multiple commonly prescribed medications, including antihypertensives and diuretics, as well as over-the-counter pain relievers such as ibuprofen and naproxen.
Second-generation antipsychotics (SGAs) have mood stabilizing, antidepressant, and anti-manic properties and are often useful in managing bipolar disorder. In our experience, for patients with bipolar disorder, SGAs are best used in combination with a mood stabilizer. Although virtually all SGAs have demonstrated effectiveness in the treatment of psychosis and some phases of bipolar disorder, the newer agents (aripiprazole, brexpiprazole, lurasidone, and cariprazine) are relatively free of metabolic adverse effects such as weight gain, abnormal cholesterol levels, increased prolactin levels, insulin resistance, and increased risk of diabetes.
Antidepressants may be effective in treating unipolar depression, but when treating bipolar depression, they should be used cautiously and only in combination with a mood stabilizer.
Continue to: As we observed...
As we observed in our previous article,1 thyroid laboratory monitoring and supplementation are critical components of managing mood disorders (Box 138-41).
Box 1
Conventional laboratory reference ranges often indicate that thyroid-stimulating hormone (TSH) levels as high as 4.0, 4.5, or 5.0 mU/L are normal. A recent meta- analysis determined that treatment of subclinical hypothyroidism (elevated TSH with normal free thyroxine) does not benefit patients’ quality of life.38 Patients with mood disorders, however, often fail to respond to mood stabilizers and other psychiatric medications unless their TSH is <3.0 or even <2.5 mU/L.39,40 We typically augment with liothyronine because, unlike levothyroxine, it works quickly, does not require deiodination to be activated, and, contrary to some reports, its elimination and biologic half-life are sufficient for single daily dosing.41
Moving towards better diagnoses
The emergence of a criteria-based psychiatric system in 1980 with the publication of DSM-III, and its subsequent revisions and updates, constituted a major advance in psychiatric diagnosis. As we learn more about the pathophysiology, genetics, and epigenetics of psychiatric symptoms and syndromes, future diagnostic systems will improve problems of validity that have yet to be resolved. While we believe that, for the most part, DSM-5 was an advance over the previous diagnostic iteration, we have 2 issues with DSM-5 in terms of the diagnosis of bipolar disorder (Box 210,12,13,18,19,42).
Box 2
Based on our clinical experience treating thousands of patients over 25 years, we have 2 issues with DSM-5 regarding bipolar disorder:
1. The DSM-5 criteria for hypomania fail to reflect the features of clinical presentations commonly seen in our practice. The majority of patients with authentic bipolar syndromes do not have hypomanias that last for at least 4 days or reach the level of severity required for a DSM-5 diagnosis of hypomania. This results in misdiagnosis of patients with bipolar depression as suffering from unipolar depression, which leads to inappropriate treatment with antidepressant monotherapy.
2. Bipolar disorder frequently makes its first appearance in childhood and adolescence,10,18,19 and increasing numbers of young patients have been receiving this diagnosis.42 In our opinion, this increase reflects clinicians’ improved diagnostic skills. Perhaps alarmed by the increase in young people receiving a diagnosis of bipolar disorder, the authors of DSM-5 created a new diagnosis for children: disruptive mood dysregulation disorder. This diagnostic addition is based on the finding that children with these mood symptoms may not subsequently exhibit classic DSM-5 manic or hypomanic episodes. But the lack of such episodes does not preclude a diagnosis of bipolar disorder, because many adults with unequivocal bipolar spectrum disorders have subthreshold hypomanias and thus fail to exhibit classic manic or hypomanic episodes.12,13
A rose by any other name would smell as sweet. Children who exhibit symptoms of disruptive mood dysregulation disorder— chronic irritability and protracted temper outbursts—usually suffer from depression and mood instability. In our opinion, it is irrational and confusing to clinicians to separate out with a new diagnosis an arbitrarily defined group of children who exhibit substantially the same symptoms as those who receive a diagnosis of bipolar disorder.
Patients with a chief complaint of depression are often given a diagnosis of “major depression, rule out bipolar disorder.” We believe that this formula should be turned on its head. In our opinion, based on our clinical experience, we think that most patients who present to a clinician’s office or psychiatric hospital with depression have bipolar depression, not unipolar depression. We hope that our experience and observations derived from treating thousands of patients over more than 25 years may be helpful to clinicians who sometimes struggle to bring relief to their patients with mood disorders.
CASE CONTINUED
Return to work
Mrs. W is now doing well. She is taking a lower dosage of duloxetine, 60 mg/d, in combination with the mood stabilizer lamotrigine, 200 mg/d. She returns to work full-time as a paralegal and no longer is experiencing depressive episodes.
Bottom Line
Patients with bipolar depression are often misdiagnosed with unipolar depression and treated inappropriately with antidepressant monotherapy, which often results in mood destabilization. Based on our clinical experience, a careful assessment of select criteria, including age of onset, rapidity of onset, comorbidities, diurnal mood variations, and more, can be useful for distinguishing between unipolar and bipolar depression.
Related Resources
- Nasrallah HA. Misdiagnosing bipolar depression as major depressive disorder. Current Psychiatry. 2013;12(10):20-21,A.
- Ghaemi SN. Bipolar spectrum: a review of the concept and a vision for the future. Psychiatry Investig. 2013;10(3):218-224.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol, Equetro
Cariprazine • Vraylar
Divalproex • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Lamotrigine • Lamictal
Levothyroxine • Synthroid, Levoxyl
Liothyronine • Cytomel
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oxcarbazepine • Oxtellar XR, Trileptal
Paroxetine • Paxil
Sertraline • Zoloft
Mrs. W, age 36, who is married, has a history of military service, and is currently employed as a paralegal, is referred to our practice by her family physician. She complains of severe depression that impairs her ability to function at work. She had seen several other psychiatrists in both military and civilian settings, and had been treated with multiple antidepressants, including fluoxetine, sertraline, bupropion, and paroxetine.
At the time of her initial psychiatric evaluation, she is taking duloxetine, 90 mg/d, but still is experiencing depressive symptoms. She is tearful, sad, lacks energy, spends too much time in bed, and is experiencing thoughts of hopelessness, despair, and escape, verging on thoughts of suicide. As a result, she needs to scale back her work schedule to part-time. When asked about how long she had been suffering from depression, she responds “I’ve been depressed all my life.” She had been briefly hospitalized at age 16, when she made a suicide attempt by overdose. There had been no subsequent suicide attempts or psychiatric hospitalizations, although she acknowledges having intermittent suicidal thoughts.
Mrs. W’s clinical presentation is similar to that of many patients entering our practice—patients who have recurrent depression that began in early life and a history of failure to respond to multiple antidepressants. She and other patients with similar presentations are not suffering from treatment-resistant depression and in need of a trial of electroconvulsive therapy, transcranial magnetic stimulation, direct current stimulation, vagus nerve stimulation, or intranasal esketamine. She has bipolar disorder, and had been repeatedly misdiagnosed and treated inappropriately with antidepressant monotherapy.
In a previous article1 (“Controversies in bipolar disorder: Trust evidence or experience?,”
In this article, based on our more than 25 years of experience in diagnosing and treating psychiatric disorders in patients of all ages, we expand on those observations.
Misdiagnosis is common
Bipolar depression is frequently misdiagnosed as unipolar depression in outpatient2-8 and inpatient9 settings, and in children and adolescents.10 Mrs. W is typical of patients who have what we consider a bipolar spectrum disorder and receive an inaccurate diagnosis and treatment that is ineffective or may worsen the course of their illness.
Reliance on DSM-511 and its predecessor, DSM-IV, is a part of the problem of misdiagnosis because the diagnostic criteria for bipolar disorder fail to capture the clinical features of many patients with “softer” (less obvious manic and hypomanic) variants of the disorder.12,13 For example, DSM-5 criteria for a hypomanic episode (the mild high experienced by patients with a soft bipolar disorder) require that the episode lasts “at least 4 consecutive days” and is “present most of the day, nearly every day.” In our experience, the majority of hypomanic episodes are shorter—ranging from a half-day to 2 days, averaging perhaps 1.5 days.
Continue to: DSM-5 also requires...
DSM-5 also requires severity criteria for hypomania that patients with unequivocal hypomanic episodes often do not meet. For example, they may fail to experience flight of ideas or racing thoughts, or engage in activities such as “unrestrained buying sprees, sexual indiscretions, or foolish business investments.” These patients usually describe these mild highs as feeling normal and report a happier mood, more smiles and laughter, increased energy, less sleepiness, increased talkativeness, increased socialization, and improved motivation to complete tasks left undone and projects left unfinished because of the previous depressive episode. These softer (subthreshold) hypomanic episodes are authentic and, if clinicians do not identify them, may lead to misdiagnosis and inappropriate treatment.
Patients who present with depression often fail to report these brief, subthreshold hypomanic episodes or consider them to be irrelevant to their diagnosis and treatment.12,13 Probing questions can often elicit these unreported highs. For example, a patient with depression should be asked, “Have you had a single good day during the last month?” and “Where were you and what did you do during that day?” Eliciting a history of brief periods of improved mood is the key to differentiating between unipolar and bipolar depression. Screening instruments such as the Mood Disorders Questionnaire14 and the Bipolar Spectrum Diagnostic Scale15 may be helpful in distinguishing between unipolar and bipolar depression. However, we offer our thoughts on making that crucial distinction.
Distinguishing between these 2 types of depression
Although it may be difficult to distinguish between unipolar and bipolar depression, especially in the absence of a history of distinct manic or hypomanic episodes, we find the following criteria to be useful in making that determination.
Age of onset. Bipolar spectrum disorders typically begin earlier in life than unipolar depression.10,16-19 A typical presentation of bipolar disorder in children and adolescents is depression or agitated mixed states with features of both mania and depression, often accompanied by rapid mood cycling.20,21 Unipolar depression usually begins later in life, and patients do not have a history of significant depressive episodes or mood swings in childhood or adolescence. An important question to ask a patient with a chief complaint of depression is, “How old were you when you first experienced an episode of depression?”
Gender differences. Bipolar spectrum disorders with more subtle (softer) presentations, such as subthreshold highs, occur more often in women than men.22 However, overall rates of bipolar disorder may be slightly higher in men than in women.23 Unipolar melancholic depression occurs at approximately the same frequency in men and women.24
Continue to: Rapidity of onset
Rapidity of onset. Bipolar depressive episodes develop more rapidly than unipolar episodes. It is common for a patient with a bipolar spectrum disorder to transition from normal to very depressed virtually overnight, whereas in our clinical experience, unipolar episodes progress more slowly, often over several months.
Deliberate self-harm. Adolescents and young adults with a bipolar spectrum disorder frequently engage in self-injurious behavior, usually cutting with a knife, razor, or even sharp fingernails.25 Although these patients may also have thoughts of suicide and make suicide attempts, the individual usually perceives cutting as a means of gaining relief from tension and distress. These behaviors are often associated with a diagnosis of a personality disorder; in our opinion, however, they are hallmarks of a bipolar spectrum disorder.
ADHD. Bipolar disorder frequently co-occurs with attention-deficit/hyperactivity disorder (ADHD).26,27 Adults with bipolar disorder often have ADHD symptoms, which can complicate their treatment and cause functional impairment even after their mood disorder has been stabilized.28
Substance use disorders. Excessive use of alcohol and drugs is common among people with a wide range of psychiatric disorders, but patients with bipolar disorder have an unusually high rate of co-occurring substance use disorders—40% to 50%.29,30
Appetite and weight differences. Patients with unipolar depression usually experience loss of appetite and weight loss, whereas in our clinical experience, patients with bipolar depression often overeat, crave carbohydrates, and gain weight.
Continue to: Sleep problems
Sleep problems. Patients with bipolar depression have an increased need for sleep (the opposite of what they experience during highs), are sleepy during the day regardless of how many hours they sleep, and have difficulty getting up in the morning. Patients with unipolar depression also have a sleep disturbance: they may fall asleep easily, sleep for a few hours, and then awaken but are unable to fall back to sleep.31 Yet these patients usually do not complain of sleepiness during the day.
Diurnal variation of mood. Patients with unipolar depression often report that their depressive symptoms fluctuate in a circadian manner. For example, they may report that their depression is worse in the morning but improves toward evening.31 This regular alteration of circadian rhythm usually is not evident in patients with bipolar depression, whose mood may vary unpredictably or in response to stressors. Some patients with bipolar disorder, however, exhibit ultradian (ultra-rapid) mood cycling, which may be confused with the diurnal mood variation seen in patients with unipolar depression.
Tendency to recur. Although both unipolar and bipolar depressive episodes recur, a pattern of multiple recurring episodes beginning in early life is characteristic of bipolar spectrum disorders.
Behavioral history. Patients with bipolar depression are more likely than patients with unipolar depression to have a history of multiple marriages, multiple romantic relationships, episodes of promiscuity, legal problems, or financial extravagance.
Response to antidepressants. Patients with bipolar depression exhibit atypical responses to antidepressant monotherapy, such as worsening of depressive symptoms, initial improvement of mood with subsequent loss of effectiveness, premature response to an antidepressant (eg, improvement of mood within 1 to 2 days of beginning the antidepressant), fluctuation of depressive symptoms (mood cycling), or precipitation of a hypomanic or manic episode. We believe that a history of multiple failed antidepressant trials is compelling evidence of misdiagnosis of a bipolar spectrum disorder as unipolar depression.
Continue to: Genetics
Genetics. Bipolar disorder is one of the most heritable of illnesses.32 Family history is important, but affected relatives may have been misdiagnosed with unipolar depression or schizophrenia, or said to have experienced “nervous breakdowns.”
Consequences of misdiagnosis
Misdiagnosis of patients with bipolar disorder is not benign. We see patients who have suffered needlessly for years with severe depression and mood instability. After trying antidepressant after antidepressant without benefit, they begin to feel hopeless, believing they have tried everything and that nothing works for them. Often, these patients have dropped out of high school or college, or lost jobs, friends, and spouses due to their disabling but misdiagnosed psychiatric disorder. Patients with misdiagnosed bipolar disorder have an increased risk of suicide attempts and psychiatric hospitalization.5,8
Misdiagnosis of patients with bipolar disorder is not limited to nonpsychiatric physicians. The majority of patients with bipolar spectrum disorders are misdiagnosed by outpatient psychiatrists as having unipolar depression.2-7 At least 45% of patients hospitalized for depression have bipolar disorder—and most of these patients are treated inappropriately with antidepressants.9 The STAR*D study,33,34 a large randomized clinical trial of antidepressants, concluded that more than one-third of patients had not remitted from their depression after treatment with 3 different antidepressants. In our opinion, many of the nonresponding patients may have undiagnosed bipolar depression, which predictably leads to a failure to respond adequately to antidepressants. We believe that the customary inclusion and exclusion criteria used to select participants for these research studies miss subtle (subthreshold) hypomanic episodes that fall short of meeting DSM criteria for duration and severity. This phenomenon may account for the results of studies that conclude that antidepressants are, at best, minimally more effective than placebo.35
When a patient with a bipolar spectrum disorder is misdiagnosed and treated with an antidepressant, the usual result is mood destabilization. Reports of mood swings, increased crying, and suicidal thoughts and suicidal gestures in children, adolescents, and young adults treated with antidepressants led the FDA to issue a “black-box” warning.36 Because bipolar depression typically begins in youth,10,18,19 the behaviors cited in the warning may reflect misdiagnosis of bipolar depression as unipolar depression, and consequent mood destabilization as a result of treatment with an antidepressant in the absence of a mood stabilizer.
Depression and life stressors
Since many patients who are depressed present with a history of significant stressors, clinicians often face the problem of distinguishing between clinical depression and stress-induced depression. We believe that one typical symptom of depression—increased sensitivity to stressors—may help in making that distinction. A patient who is depressed will often attribute depression to stressors such as marital conflict, divorce, problems with a teenage child, work pressures, financial pressures, or the illness or death of a family member or pet. If clinical depression (unipolar or bipolar) is present, the symptoms are persistent, sometimes antedate the stressor by days or weeks, often outlast the stressor, increase in severity over time, and are disproportional to the stressor. Clinical depression can also cause the patient to become obsessed with traumatic events or losses that occurred many years earlier.
Continue to: Our approach to treatment
Our approach to treatment
Patients with mood disorders often benefit from a combination of pharmacologic management and psychotherapy. Psychotherapy is particularly important in addressing the functional impairment, diminished self-worth, and interpersonal conflicts that often accompany clinical depression. Several styles or systems of psychotherapy have been developed to benefit patients with mood disorders. Their effectiveness may depend on the patient’s ability to gain insight,37 but in our opinion, the most important attribute of helpful psychotherapy is the rapport established between the patient and the therapist, and the therapist’s ability to empathize with the patient and instill in the patient a sense of optimism and hope. We often recommend that patients attend meetings of the Depression and Bipolar Support Alliance (DBSA), a national support group with chapters throughout the country. Patients often find that attending these meetings is both educational and emotionally rewarding.
The foundational pharmacologic treatment for bipolar disorder is a mood stabilizer. The medications we consider to be effective mood stabilizers (some with an FDA indication for bipolar maintenance, some without) are lithium carbonate, divalproex sodium, carbamazepine, oxcarbazepine, and lamotrigine.
Each of these mood stabilizers has its advantages, disadvantages, risks, and adverse effects. For example, although divalproex is a reliable mood stabilizer, it has a significant risk of causing birth defects if taken during pregnancy and can cause increased appetite and weight gain. Carbamazepine has significant drug interactions and the potential to cause neurologic adverse effects, while oxcarbazepine, a derivative of carbamazepine, has fewer drug interactions but is more likely to cause hyponatremia. Lamotrigine must be titrated very slowly to reduce the risk of a potentially fatal skin rash (ie, Stevens-Johnson syndrome or toxic epidermal necrolysis). Lithium is effective but has a significant adverse-effect burden: impairment of renal function with long-term use, nephrogenic diabetes insipidus, hypothyroidism, hyperparathyroidism, acne, and weight gain. Lithium also has potential interactions with multiple commonly prescribed medications, including antihypertensives and diuretics, as well as over-the-counter pain relievers such as ibuprofen and naproxen.
Second-generation antipsychotics (SGAs) have mood stabilizing, antidepressant, and anti-manic properties and are often useful in managing bipolar disorder. In our experience, for patients with bipolar disorder, SGAs are best used in combination with a mood stabilizer. Although virtually all SGAs have demonstrated effectiveness in the treatment of psychosis and some phases of bipolar disorder, the newer agents (aripiprazole, brexpiprazole, lurasidone, and cariprazine) are relatively free of metabolic adverse effects such as weight gain, abnormal cholesterol levels, increased prolactin levels, insulin resistance, and increased risk of diabetes.
Antidepressants may be effective in treating unipolar depression, but when treating bipolar depression, they should be used cautiously and only in combination with a mood stabilizer.
Continue to: As we observed...
As we observed in our previous article,1 thyroid laboratory monitoring and supplementation are critical components of managing mood disorders (Box 138-41).
Box 1
Conventional laboratory reference ranges often indicate that thyroid-stimulating hormone (TSH) levels as high as 4.0, 4.5, or 5.0 mU/L are normal. A recent meta- analysis determined that treatment of subclinical hypothyroidism (elevated TSH with normal free thyroxine) does not benefit patients’ quality of life.38 Patients with mood disorders, however, often fail to respond to mood stabilizers and other psychiatric medications unless their TSH is <3.0 or even <2.5 mU/L.39,40 We typically augment with liothyronine because, unlike levothyroxine, it works quickly, does not require deiodination to be activated, and, contrary to some reports, its elimination and biologic half-life are sufficient for single daily dosing.41
Moving towards better diagnoses
The emergence of a criteria-based psychiatric system in 1980 with the publication of DSM-III, and its subsequent revisions and updates, constituted a major advance in psychiatric diagnosis. As we learn more about the pathophysiology, genetics, and epigenetics of psychiatric symptoms and syndromes, future diagnostic systems will improve problems of validity that have yet to be resolved. While we believe that, for the most part, DSM-5 was an advance over the previous diagnostic iteration, we have 2 issues with DSM-5 in terms of the diagnosis of bipolar disorder (Box 210,12,13,18,19,42).
Box 2
Based on our clinical experience treating thousands of patients over 25 years, we have 2 issues with DSM-5 regarding bipolar disorder:
1. The DSM-5 criteria for hypomania fail to reflect the features of clinical presentations commonly seen in our practice. The majority of patients with authentic bipolar syndromes do not have hypomanias that last for at least 4 days or reach the level of severity required for a DSM-5 diagnosis of hypomania. This results in misdiagnosis of patients with bipolar depression as suffering from unipolar depression, which leads to inappropriate treatment with antidepressant monotherapy.
2. Bipolar disorder frequently makes its first appearance in childhood and adolescence,10,18,19 and increasing numbers of young patients have been receiving this diagnosis.42 In our opinion, this increase reflects clinicians’ improved diagnostic skills. Perhaps alarmed by the increase in young people receiving a diagnosis of bipolar disorder, the authors of DSM-5 created a new diagnosis for children: disruptive mood dysregulation disorder. This diagnostic addition is based on the finding that children with these mood symptoms may not subsequently exhibit classic DSM-5 manic or hypomanic episodes. But the lack of such episodes does not preclude a diagnosis of bipolar disorder, because many adults with unequivocal bipolar spectrum disorders have subthreshold hypomanias and thus fail to exhibit classic manic or hypomanic episodes.12,13
A rose by any other name would smell as sweet. Children who exhibit symptoms of disruptive mood dysregulation disorder— chronic irritability and protracted temper outbursts—usually suffer from depression and mood instability. In our opinion, it is irrational and confusing to clinicians to separate out with a new diagnosis an arbitrarily defined group of children who exhibit substantially the same symptoms as those who receive a diagnosis of bipolar disorder.
Patients with a chief complaint of depression are often given a diagnosis of “major depression, rule out bipolar disorder.” We believe that this formula should be turned on its head. In our opinion, based on our clinical experience, we think that most patients who present to a clinician’s office or psychiatric hospital with depression have bipolar depression, not unipolar depression. We hope that our experience and observations derived from treating thousands of patients over more than 25 years may be helpful to clinicians who sometimes struggle to bring relief to their patients with mood disorders.
CASE CONTINUED
Return to work
Mrs. W is now doing well. She is taking a lower dosage of duloxetine, 60 mg/d, in combination with the mood stabilizer lamotrigine, 200 mg/d. She returns to work full-time as a paralegal and no longer is experiencing depressive episodes.
Bottom Line
Patients with bipolar depression are often misdiagnosed with unipolar depression and treated inappropriately with antidepressant monotherapy, which often results in mood destabilization. Based on our clinical experience, a careful assessment of select criteria, including age of onset, rapidity of onset, comorbidities, diurnal mood variations, and more, can be useful for distinguishing between unipolar and bipolar depression.
Related Resources
- Nasrallah HA. Misdiagnosing bipolar depression as major depressive disorder. Current Psychiatry. 2013;12(10):20-21,A.
- Ghaemi SN. Bipolar spectrum: a review of the concept and a vision for the future. Psychiatry Investig. 2013;10(3):218-224.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol, Equetro
Cariprazine • Vraylar
Divalproex • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Lamotrigine • Lamictal
Levothyroxine • Synthroid, Levoxyl
Liothyronine • Cytomel
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oxcarbazepine • Oxtellar XR, Trileptal
Paroxetine • Paxil
Sertraline • Zoloft
1. Miller GE, Noel RL. Controversies in bipolar disorder: trust evidence or experience? Current Psychiatry. 2009;8(2):27-28,31-33,39.
2. Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry. 2004;6(1):27-33.
3. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52(1-3):135-144.
4. Blanco C, Laje G, Olfson M, et al. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159(6):1005-1010.
5. Shi L, Thiebaud P, McCombs JS. The impact of unrecognized bipolar disorders for patients treated with antidepressants in the fee-for-services California Medicaid (Medi-Cal) program. J Affect Disord. 2004;82(3):373-383.
6. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
7. Hughes T, Cardno A, West R, et al. Unrecognized bipolar disorder among UK primary care patients prescribed antidepressants: an observational study. Br J Gen Pract. 2016;66(643):e71-e77.
8. Keck PE Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatric Pract. 2008;14(Suppl 2):31-38.
9. Goldberg JF, Harrow M, Whiteside JF. Risk for bipolar illness in inpatients initially hospitalized for unipolar depression. Am J Psychiatry. 2001:158(8):1265-1270.
10. Chilakamarri JK, Filkowski MM, Ghaemi SN. Misdiagnosis of bipolar disorder in children and adolescents: a comparison with ADHD and major depressive disorder. Ann Clin Psychiatry. 2011;23(1):25-29.
11. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
12. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84(2-3):117-125.
13. Baldassano C. Distinctions between bipolar I and bipolar II depression. Current Psychiatry. 2017;16(8):S7-S16.
14. Hirschfeld MA, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: The Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873-1875.
15. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity a new bipolar spectrum diagnostic scale. J Affect Disorder. 2005;84(2-3):273-277
16. Suppes T, Leverich G, Keck P, et al. The Stanley Foundation Continuing Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disorder. 2001;67(1-3):45-49.
17. Perlis RH, Miyahara S, Marangell LB. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP–BD). Biol Psychiatry. 2004;55(9):875-881.
18. Baldessarini RJ, Bolzani L, Kruz N, et al. Onset age of bipolar disorders at six international sites. J Affect Disord. 2010;121(1-2):143-146.
19. Post RM, Altshuler LL, Kupka R, et al. More childhood onset bipolar disorder in the United States than Canada or Europe: implications for treatment and prevention. Neurosci Biobehav Rev. 2017;74(Pt A):204-213.
20. Geller B, Luby J. Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1168-1176.
21. Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
22. Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26(3):595-620.
23. Deflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22(5):437-452.
24. Bogren M, Brådvik L, Holmstrand C, et al. Gender differences in subtypes of depression by first incidence and age of onset: a follow-up of the Lunby population. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):179-189.
25. Singhal A, Ross J, Seminog O, et al. Risks of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194-204.
26. Joshi G, Wilens T. Comorbidity in pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Amer. 2009;18(2):291-319.
27. Youngtrom EA, Arnold LE, Frazier TW. Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clin Psychol (New York). 2010;17(4):350-359.
28. McIntyre RS, Kennedy SH, Soczynska JK, et al. Attention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the International Mood Disorders Collaborative Project. Prime Care Companion J Clin Psychiatry. 2010;12(3). doi:10.4088/PCC.09m00861gry.
29. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiological Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
30. Hunt GE, Malhi GS, Cleary M, et al. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349.
31. Agargun MY, Besiroglu L, Cilli AS, et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord. 2007;98(3):267-270.
32. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66(3):287-296.
33. Rush AJ, Trivedi MH, Wisniewski SR, et al. Buproprion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
34. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
35. Kirsch I. Antidepressants and the placebo effect. Z Psychol. 2014;222(3):128-134.
36. U.S. Food and Drug Administration. Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm161679.htm. Published February 5, 2018. Accessed May 10, 2019.
37. Jennissen S, Huber J, Ehrenthal JC, et al. Association between insight and outcome of psychotherapy; systematic review and meta-analysis. Am J Psychiatry. 2018;175(10):961-969.
38. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism. JAMA. 2018;320(13):1349-1359.
39. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry. 2002;159(1):116-121.
40. Parmentier T, Sienaert P. The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord. 2018;229:410-414.
41. Koda-Kimbe MA, Alldredge BK. Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs (10th ed). Baltimore, MD: Walters Klower Health/Lippincott Williams & Wilkins; 2012.
42. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
1. Miller GE, Noel RL. Controversies in bipolar disorder: trust evidence or experience? Current Psychiatry. 2009;8(2):27-28,31-33,39.
2. Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry. 2004;6(1):27-33.
3. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52(1-3):135-144.
4. Blanco C, Laje G, Olfson M, et al. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159(6):1005-1010.
5. Shi L, Thiebaud P, McCombs JS. The impact of unrecognized bipolar disorders for patients treated with antidepressants in the fee-for-services California Medicaid (Medi-Cal) program. J Affect Disord. 2004;82(3):373-383.
6. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
7. Hughes T, Cardno A, West R, et al. Unrecognized bipolar disorder among UK primary care patients prescribed antidepressants: an observational study. Br J Gen Pract. 2016;66(643):e71-e77.
8. Keck PE Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatric Pract. 2008;14(Suppl 2):31-38.
9. Goldberg JF, Harrow M, Whiteside JF. Risk for bipolar illness in inpatients initially hospitalized for unipolar depression. Am J Psychiatry. 2001:158(8):1265-1270.
10. Chilakamarri JK, Filkowski MM, Ghaemi SN. Misdiagnosis of bipolar disorder in children and adolescents: a comparison with ADHD and major depressive disorder. Ann Clin Psychiatry. 2011;23(1):25-29.
11. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
12. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84(2-3):117-125.
13. Baldassano C. Distinctions between bipolar I and bipolar II depression. Current Psychiatry. 2017;16(8):S7-S16.
14. Hirschfeld MA, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: The Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873-1875.
15. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity a new bipolar spectrum diagnostic scale. J Affect Disorder. 2005;84(2-3):273-277
16. Suppes T, Leverich G, Keck P, et al. The Stanley Foundation Continuing Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disorder. 2001;67(1-3):45-49.
17. Perlis RH, Miyahara S, Marangell LB. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP–BD). Biol Psychiatry. 2004;55(9):875-881.
18. Baldessarini RJ, Bolzani L, Kruz N, et al. Onset age of bipolar disorders at six international sites. J Affect Disord. 2010;121(1-2):143-146.
19. Post RM, Altshuler LL, Kupka R, et al. More childhood onset bipolar disorder in the United States than Canada or Europe: implications for treatment and prevention. Neurosci Biobehav Rev. 2017;74(Pt A):204-213.
20. Geller B, Luby J. Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1168-1176.
21. Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
22. Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26(3):595-620.
23. Deflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22(5):437-452.
24. Bogren M, Brådvik L, Holmstrand C, et al. Gender differences in subtypes of depression by first incidence and age of onset: a follow-up of the Lunby population. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):179-189.
25. Singhal A, Ross J, Seminog O, et al. Risks of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194-204.
26. Joshi G, Wilens T. Comorbidity in pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Amer. 2009;18(2):291-319.
27. Youngtrom EA, Arnold LE, Frazier TW. Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clin Psychol (New York). 2010;17(4):350-359.
28. McIntyre RS, Kennedy SH, Soczynska JK, et al. Attention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the International Mood Disorders Collaborative Project. Prime Care Companion J Clin Psychiatry. 2010;12(3). doi:10.4088/PCC.09m00861gry.
29. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiological Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
30. Hunt GE, Malhi GS, Cleary M, et al. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349.
31. Agargun MY, Besiroglu L, Cilli AS, et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord. 2007;98(3):267-270.
32. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66(3):287-296.
33. Rush AJ, Trivedi MH, Wisniewski SR, et al. Buproprion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
34. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
35. Kirsch I. Antidepressants and the placebo effect. Z Psychol. 2014;222(3):128-134.
36. U.S. Food and Drug Administration. Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm161679.htm. Published February 5, 2018. Accessed May 10, 2019.
37. Jennissen S, Huber J, Ehrenthal JC, et al. Association between insight and outcome of psychotherapy; systematic review and meta-analysis. Am J Psychiatry. 2018;175(10):961-969.
38. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism. JAMA. 2018;320(13):1349-1359.
39. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry. 2002;159(1):116-121.
40. Parmentier T, Sienaert P. The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord. 2018;229:410-414.
41. Koda-Kimbe MA, Alldredge BK. Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs (10th ed). Baltimore, MD: Walters Klower Health/Lippincott Williams & Wilkins; 2012.
42. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
An insidious onset of symptoms
CASE Tremors, increasing anxiety
Ms. S, age 56, has a history of depression and anxiety.
The authors’ observations
The incidence of serotonin syndrome has increased because of increasing use of serotonergic agents.1-3 Although the severity could range from benign to life-threatening, the potential lethality combined with difficulty of diagnosis makes this condition of continued interest. Stimulation of the 5-hydroxytryptamine (5-HT) receptor subtypes, specifically 5-HT1A and 5-HT2, are implicated in this syndrome.4,5
Serotonin syndrome is a clinical diagnosis with a triad of symptoms that includes mental status changes, autonomic hyperactivity, and neuromuscular abnormalities.1,2 However, because of the varied presentation and similarity to other syndromes such as NMS, serotonin syndrome often is undiagnosed.5
TREATMENT Discontinue fluoxetine
Several months after the fluoxetine increase, Ms. S's physical symptoms emerge. Several weeks later, she notices significant hypertension of 162/102 mm Hg by checking her blood pressure at home. She had no history of hypertension before taking fluoxetine. She then tells her psychiatrist she has been experiencing confusion, shakiness, loss of balance, forgetfulness, joint pain, sweating, and fatigue, along with worsening anxiety. The treatment team makes a diagnosis of serotonin syndrome and recommends discontinuing fluoxetine and starting cyproheptadine, 4 mg initially, and then repeating the cyproheptadine dose in several hours if her symptoms do not resolve. Approximately, 2.5 months after the serotonin syndrome reaction, Ms. S receives hydroxyzine, 10 mg every 8 hours, as needed for anxiety.
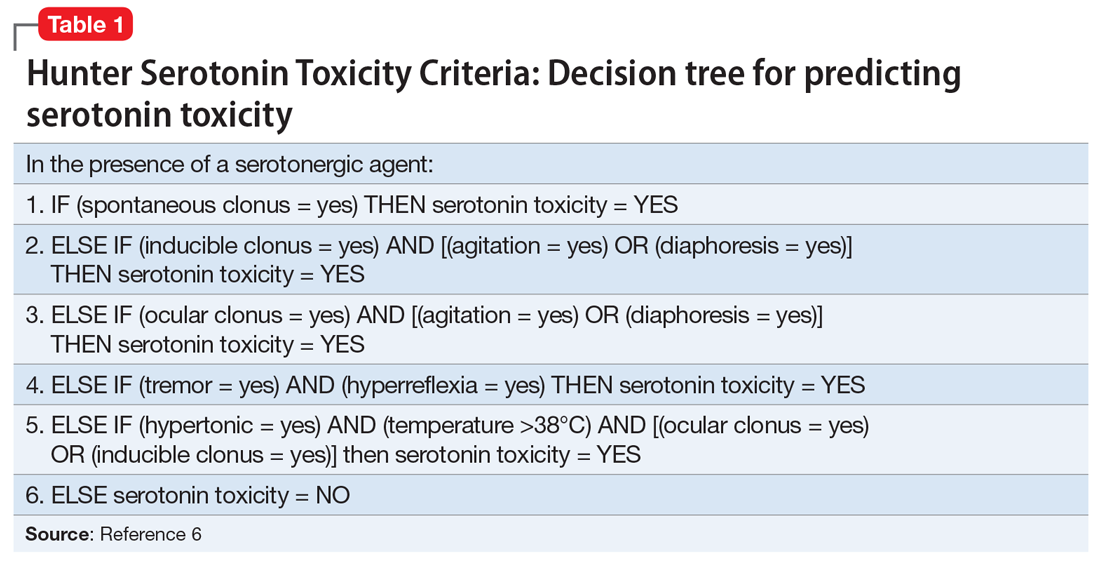
The authors’ observations
The diagnosis of serotonin syndrome is most accurately made using Hunter Serotonin Toxicity Criteria (Table 16). Because Ms. S had an insidious onset of symptoms, and treatment was initiated before full evaluation, it is unknown if she met Hunter criteria. To meet these criteria, a patient must have ≥1 of the following6:
- spontaneous clonus
- inducible clonus plus agitation or diaphoresis
- ocular clonus plus agitation or diaphoresis
- tremor plus hyperreflexia
- hypertonia plus temperature >38°C plus ocular or inducible clonus.
The Sternbach diagnostic criteria for serotonin syndrome (Table 23) is another commonly used tool.3 These criteria include the addition or increase of a serotonin agent and absence of substances or metabolic derangements that could account for symptoms and at least 3 of the following 10 symptoms3:
- mental status changes (confusion, hypomania)
- agitation
- myoclonus
- hyperreflexia
- diaphoresis
- shivering
- tremor
- diarrhea
- incoordination
- fever.
Continue to: Ms. S met the Sternbach diagnostic criteria...
Ms. S met the Sternbach diagnostic criteria for serotonin syndrome.
Ms. S was taking a single serotonin agent and initially had mild symptoms. More commonly, a patient who presents with serotonin syndrome has been receiving ≥2 serotonergic agents or toxic levels of a single agent, and these agents usually include a psychotropic medication such as a monoamine oxidase inhibitor, tricyclic antidepressant, or SSRI, as well as a medication from a different class, such as dextromethorphan, linezolid, tramadol, methylene blue, and/or St. John’s wort.1,7-13 However, in this case, Ms. S also was taking bupropion, a known inhibitor of cytochrome P450 2D6. Bupropion might have increased Ms. S’s fluoxetine levels.
Ms. S was a healthy, middle-age patient who took no medications other than those listed, had no medical comorbidities, and had a straightforward psychiatric history, which makes the diagnosis of serotonin syndrome clearer. However, other potential differential diagnoses, such as NMS, delirium tremens, and anticholinergic toxicity, might cloud the clinical picture. When differentiating NMS and serotonin syndrome, it is helpful to note whether a patient shows tremor, diarrhea, and myoclonus present in the absence of muscular, “lead-pipe” rigidity, which suggests a diagnosis of serotonin syndrome.2,3,5
[polldaddy:10279173]
The authors’ observations
Treating serotonin syndrome includes supportive care, discontinuing offending agents, administering benzodiazepines, and using a serotonin antagonist as an antidote for patients with moderate-to-severe cases. Cyproheptadine is an antihistaminergic medication with non-specific 5-HT1A and 5-HT2 antagonism. It is FDA-approved for specific allergic reactions, urticaria, and anaphylaxis adjunctive therapy, but not for serotonin syndrome. Case series support the use of cyproheptadine for acute management of serotonin syndrome, with rapid symptom improvement.4,7,14-18 We observed a similar outcome with Ms. S. Her significant autonomic symptoms resolved rapidly, although she experienced some residual, mild symptoms that took weeks to resolve.
Continue to: Because serotonergic agents...
Because serotonergic agents are used frequently and readily by primary care clinicians as well as psychiatrists, the ability to properly diagnose this syndrome is vital, particularly because severe cases can rapidly deteriorate.1,9,16,17 This presentation of a single serotonergic agent causing significant symptoms that worsened over months is not typical, but important to recognize as a patient begins to experience autonomic instability. As was the case with Ms. S, it is important to remain vigilant when changing dosages or adding medications. Symptoms of serotonin syndrome might be vague and difficult to diagnose, especially if the clinician is not aware of the variability of presentation of this syndrome. Cyproheptadine can be used safely and rapidly and should be considered a treatment option for serotonin syndrome.
OUTCOME Hypertension resolves
After her first dose of cyproheptadine, Ms. S’s blood pressure drops to 146/86 mm Hg. Three hours later, she repeats the cyproheptadine dose and her blood pressure drops to 106/60 mm Hg. She reports that her anxiety has lessened, although she is still tremulous. Overall, she says she feels better. She experiences improvement of her condition with a pharmacologic regimen of bupropion, gabapentin, and hydroxyzine.
Several weeks later, her health returns to baseline, with complete resolution of hypertension.
Bottom Line
Although serotonin syndrome is most commonly associated with co-administered serotonergic medications, symptoms can emerge with a single, moderately dosed agent. Treatment includes withdrawing the offending agent, and administering a serotonin antagonist. Mild cases of serotonin syndrome usually resolve.
Related Resources
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
- Iqbal MM, Basil MJ, Kaplan J, et al. Overview of serotonin syndrome. Ann Clin Psychiatry. 2012;24(4):310-318.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cyproheptadine • Periactin
Dextromethorphan • Benylin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Neurontin
Hydroxyzine • Atarax
Linezolid • Zyvox
Tramadol • Ultram, Ryzolt
1. Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome: presentation of 2 cases and review of literature. Medicine (Baltimore). 2000;79(4):201-209.
2. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
3. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
4. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619.
5. Mills KC. Serotonin syndrome a clinical update. Crit Care Clin. 1997;13(4):763-783.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635-642.
7. Horowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care. 1999;15(5):325-327.
8. Kolecki P. Isolated venlafaxine-induced serotonin syndrome. J Emerg Med. 1996;15:491-493.
9. Pan J, Shen W. Serotonin syndrome induced by low-dose venlafaxine. Ann Pharmacother. 2003;37(2):209-211.
10. Hernández JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother. 2002;36(4):641-643.
11. Patel DD, Galarneau D. Serotonin syndrome with fluoxetine: two case reports. Ochsner J. 2016;16(4):554-557.
12. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
13. Zuschlag ZD, Warren MW, K Schultz S. Serotonin toxicity and urinary analgesics: a case report and systematic literature review of methylene blue-induced serotonin syndrome. Psychosomatics. 2018;59(6):539-546.
14. Kapur S, Zipursky RB, Jones C, et al. Cyproheptadine: a potent in vivo serotonin antagonist. Am J Psychiatry. 1997;154(6):884.
15. Baigel GD. Cyproheptadine and the treatment of an unconscious patient with the serotonin syndrome. Eur J Anaesthesiol. 2003;20(7):586-588.
16. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331:1021-1022.
17. McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Pharmacother. 2001;35(7-8):870-873.
18. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. J Toxicol Clin Toxicol. 1997;35(2):211-212.
CASE Tremors, increasing anxiety
Ms. S, age 56, has a history of depression and anxiety.
The authors’ observations
The incidence of serotonin syndrome has increased because of increasing use of serotonergic agents.1-3 Although the severity could range from benign to life-threatening, the potential lethality combined with difficulty of diagnosis makes this condition of continued interest. Stimulation of the 5-hydroxytryptamine (5-HT) receptor subtypes, specifically 5-HT1A and 5-HT2, are implicated in this syndrome.4,5
Serotonin syndrome is a clinical diagnosis with a triad of symptoms that includes mental status changes, autonomic hyperactivity, and neuromuscular abnormalities.1,2 However, because of the varied presentation and similarity to other syndromes such as NMS, serotonin syndrome often is undiagnosed.5
TREATMENT Discontinue fluoxetine
Several months after the fluoxetine increase, Ms. S's physical symptoms emerge. Several weeks later, she notices significant hypertension of 162/102 mm Hg by checking her blood pressure at home. She had no history of hypertension before taking fluoxetine. She then tells her psychiatrist she has been experiencing confusion, shakiness, loss of balance, forgetfulness, joint pain, sweating, and fatigue, along with worsening anxiety. The treatment team makes a diagnosis of serotonin syndrome and recommends discontinuing fluoxetine and starting cyproheptadine, 4 mg initially, and then repeating the cyproheptadine dose in several hours if her symptoms do not resolve. Approximately, 2.5 months after the serotonin syndrome reaction, Ms. S receives hydroxyzine, 10 mg every 8 hours, as needed for anxiety.

The authors’ observations
The diagnosis of serotonin syndrome is most accurately made using Hunter Serotonin Toxicity Criteria (Table 16). Because Ms. S had an insidious onset of symptoms, and treatment was initiated before full evaluation, it is unknown if she met Hunter criteria. To meet these criteria, a patient must have ≥1 of the following6:
- spontaneous clonus
- inducible clonus plus agitation or diaphoresis
- ocular clonus plus agitation or diaphoresis
- tremor plus hyperreflexia
- hypertonia plus temperature >38°C plus ocular or inducible clonus.
The Sternbach diagnostic criteria for serotonin syndrome (Table 23) is another commonly used tool.3 These criteria include the addition or increase of a serotonin agent and absence of substances or metabolic derangements that could account for symptoms and at least 3 of the following 10 symptoms3:
- mental status changes (confusion, hypomania)
- agitation
- myoclonus
- hyperreflexia
- diaphoresis
- shivering
- tremor
- diarrhea
- incoordination
- fever.
Continue to: Ms. S met the Sternbach diagnostic criteria...
Ms. S met the Sternbach diagnostic criteria for serotonin syndrome.
Ms. S was taking a single serotonin agent and initially had mild symptoms. More commonly, a patient who presents with serotonin syndrome has been receiving ≥2 serotonergic agents or toxic levels of a single agent, and these agents usually include a psychotropic medication such as a monoamine oxidase inhibitor, tricyclic antidepressant, or SSRI, as well as a medication from a different class, such as dextromethorphan, linezolid, tramadol, methylene blue, and/or St. John’s wort.1,7-13 However, in this case, Ms. S also was taking bupropion, a known inhibitor of cytochrome P450 2D6. Bupropion might have increased Ms. S’s fluoxetine levels.
Ms. S was a healthy, middle-age patient who took no medications other than those listed, had no medical comorbidities, and had a straightforward psychiatric history, which makes the diagnosis of serotonin syndrome clearer. However, other potential differential diagnoses, such as NMS, delirium tremens, and anticholinergic toxicity, might cloud the clinical picture. When differentiating NMS and serotonin syndrome, it is helpful to note whether a patient shows tremor, diarrhea, and myoclonus present in the absence of muscular, “lead-pipe” rigidity, which suggests a diagnosis of serotonin syndrome.2,3,5
[polldaddy:10279173]
The authors’ observations
Treating serotonin syndrome includes supportive care, discontinuing offending agents, administering benzodiazepines, and using a serotonin antagonist as an antidote for patients with moderate-to-severe cases. Cyproheptadine is an antihistaminergic medication with non-specific 5-HT1A and 5-HT2 antagonism. It is FDA-approved for specific allergic reactions, urticaria, and anaphylaxis adjunctive therapy, but not for serotonin syndrome. Case series support the use of cyproheptadine for acute management of serotonin syndrome, with rapid symptom improvement.4,7,14-18 We observed a similar outcome with Ms. S. Her significant autonomic symptoms resolved rapidly, although she experienced some residual, mild symptoms that took weeks to resolve.
Continue to: Because serotonergic agents...
Because serotonergic agents are used frequently and readily by primary care clinicians as well as psychiatrists, the ability to properly diagnose this syndrome is vital, particularly because severe cases can rapidly deteriorate.1,9,16,17 This presentation of a single serotonergic agent causing significant symptoms that worsened over months is not typical, but important to recognize as a patient begins to experience autonomic instability. As was the case with Ms. S, it is important to remain vigilant when changing dosages or adding medications. Symptoms of serotonin syndrome might be vague and difficult to diagnose, especially if the clinician is not aware of the variability of presentation of this syndrome. Cyproheptadine can be used safely and rapidly and should be considered a treatment option for serotonin syndrome.
OUTCOME Hypertension resolves
After her first dose of cyproheptadine, Ms. S’s blood pressure drops to 146/86 mm Hg. Three hours later, she repeats the cyproheptadine dose and her blood pressure drops to 106/60 mm Hg. She reports that her anxiety has lessened, although she is still tremulous. Overall, she says she feels better. She experiences improvement of her condition with a pharmacologic regimen of bupropion, gabapentin, and hydroxyzine.
Several weeks later, her health returns to baseline, with complete resolution of hypertension.
Bottom Line
Although serotonin syndrome is most commonly associated with co-administered serotonergic medications, symptoms can emerge with a single, moderately dosed agent. Treatment includes withdrawing the offending agent, and administering a serotonin antagonist. Mild cases of serotonin syndrome usually resolve.
Related Resources
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
- Iqbal MM, Basil MJ, Kaplan J, et al. Overview of serotonin syndrome. Ann Clin Psychiatry. 2012;24(4):310-318.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cyproheptadine • Periactin
Dextromethorphan • Benylin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Neurontin
Hydroxyzine • Atarax
Linezolid • Zyvox
Tramadol • Ultram, Ryzolt
CASE Tremors, increasing anxiety
Ms. S, age 56, has a history of depression and anxiety.
The authors’ observations
The incidence of serotonin syndrome has increased because of increasing use of serotonergic agents.1-3 Although the severity could range from benign to life-threatening, the potential lethality combined with difficulty of diagnosis makes this condition of continued interest. Stimulation of the 5-hydroxytryptamine (5-HT) receptor subtypes, specifically 5-HT1A and 5-HT2, are implicated in this syndrome.4,5
Serotonin syndrome is a clinical diagnosis with a triad of symptoms that includes mental status changes, autonomic hyperactivity, and neuromuscular abnormalities.1,2 However, because of the varied presentation and similarity to other syndromes such as NMS, serotonin syndrome often is undiagnosed.5
TREATMENT Discontinue fluoxetine
Several months after the fluoxetine increase, Ms. S's physical symptoms emerge. Several weeks later, she notices significant hypertension of 162/102 mm Hg by checking her blood pressure at home. She had no history of hypertension before taking fluoxetine. She then tells her psychiatrist she has been experiencing confusion, shakiness, loss of balance, forgetfulness, joint pain, sweating, and fatigue, along with worsening anxiety. The treatment team makes a diagnosis of serotonin syndrome and recommends discontinuing fluoxetine and starting cyproheptadine, 4 mg initially, and then repeating the cyproheptadine dose in several hours if her symptoms do not resolve. Approximately, 2.5 months after the serotonin syndrome reaction, Ms. S receives hydroxyzine, 10 mg every 8 hours, as needed for anxiety.

The authors’ observations
The diagnosis of serotonin syndrome is most accurately made using Hunter Serotonin Toxicity Criteria (Table 16). Because Ms. S had an insidious onset of symptoms, and treatment was initiated before full evaluation, it is unknown if she met Hunter criteria. To meet these criteria, a patient must have ≥1 of the following6:
- spontaneous clonus
- inducible clonus plus agitation or diaphoresis
- ocular clonus plus agitation or diaphoresis
- tremor plus hyperreflexia
- hypertonia plus temperature >38°C plus ocular or inducible clonus.
The Sternbach diagnostic criteria for serotonin syndrome (Table 23) is another commonly used tool.3 These criteria include the addition or increase of a serotonin agent and absence of substances or metabolic derangements that could account for symptoms and at least 3 of the following 10 symptoms3:
- mental status changes (confusion, hypomania)
- agitation
- myoclonus
- hyperreflexia
- diaphoresis
- shivering
- tremor
- diarrhea
- incoordination
- fever.
Continue to: Ms. S met the Sternbach diagnostic criteria...
Ms. S met the Sternbach diagnostic criteria for serotonin syndrome.
Ms. S was taking a single serotonin agent and initially had mild symptoms. More commonly, a patient who presents with serotonin syndrome has been receiving ≥2 serotonergic agents or toxic levels of a single agent, and these agents usually include a psychotropic medication such as a monoamine oxidase inhibitor, tricyclic antidepressant, or SSRI, as well as a medication from a different class, such as dextromethorphan, linezolid, tramadol, methylene blue, and/or St. John’s wort.1,7-13 However, in this case, Ms. S also was taking bupropion, a known inhibitor of cytochrome P450 2D6. Bupropion might have increased Ms. S’s fluoxetine levels.
Ms. S was a healthy, middle-age patient who took no medications other than those listed, had no medical comorbidities, and had a straightforward psychiatric history, which makes the diagnosis of serotonin syndrome clearer. However, other potential differential diagnoses, such as NMS, delirium tremens, and anticholinergic toxicity, might cloud the clinical picture. When differentiating NMS and serotonin syndrome, it is helpful to note whether a patient shows tremor, diarrhea, and myoclonus present in the absence of muscular, “lead-pipe” rigidity, which suggests a diagnosis of serotonin syndrome.2,3,5
[polldaddy:10279173]
The authors’ observations
Treating serotonin syndrome includes supportive care, discontinuing offending agents, administering benzodiazepines, and using a serotonin antagonist as an antidote for patients with moderate-to-severe cases. Cyproheptadine is an antihistaminergic medication with non-specific 5-HT1A and 5-HT2 antagonism. It is FDA-approved for specific allergic reactions, urticaria, and anaphylaxis adjunctive therapy, but not for serotonin syndrome. Case series support the use of cyproheptadine for acute management of serotonin syndrome, with rapid symptom improvement.4,7,14-18 We observed a similar outcome with Ms. S. Her significant autonomic symptoms resolved rapidly, although she experienced some residual, mild symptoms that took weeks to resolve.
Continue to: Because serotonergic agents...
Because serotonergic agents are used frequently and readily by primary care clinicians as well as psychiatrists, the ability to properly diagnose this syndrome is vital, particularly because severe cases can rapidly deteriorate.1,9,16,17 This presentation of a single serotonergic agent causing significant symptoms that worsened over months is not typical, but important to recognize as a patient begins to experience autonomic instability. As was the case with Ms. S, it is important to remain vigilant when changing dosages or adding medications. Symptoms of serotonin syndrome might be vague and difficult to diagnose, especially if the clinician is not aware of the variability of presentation of this syndrome. Cyproheptadine can be used safely and rapidly and should be considered a treatment option for serotonin syndrome.
OUTCOME Hypertension resolves
After her first dose of cyproheptadine, Ms. S’s blood pressure drops to 146/86 mm Hg. Three hours later, she repeats the cyproheptadine dose and her blood pressure drops to 106/60 mm Hg. She reports that her anxiety has lessened, although she is still tremulous. Overall, she says she feels better. She experiences improvement of her condition with a pharmacologic regimen of bupropion, gabapentin, and hydroxyzine.
Several weeks later, her health returns to baseline, with complete resolution of hypertension.
Bottom Line
Although serotonin syndrome is most commonly associated with co-administered serotonergic medications, symptoms can emerge with a single, moderately dosed agent. Treatment includes withdrawing the offending agent, and administering a serotonin antagonist. Mild cases of serotonin syndrome usually resolve.
Related Resources
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
- Iqbal MM, Basil MJ, Kaplan J, et al. Overview of serotonin syndrome. Ann Clin Psychiatry. 2012;24(4):310-318.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cyproheptadine • Periactin
Dextromethorphan • Benylin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Neurontin
Hydroxyzine • Atarax
Linezolid • Zyvox
Tramadol • Ultram, Ryzolt
1. Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome: presentation of 2 cases and review of literature. Medicine (Baltimore). 2000;79(4):201-209.
2. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
3. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
4. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619.
5. Mills KC. Serotonin syndrome a clinical update. Crit Care Clin. 1997;13(4):763-783.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635-642.
7. Horowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care. 1999;15(5):325-327.
8. Kolecki P. Isolated venlafaxine-induced serotonin syndrome. J Emerg Med. 1996;15:491-493.
9. Pan J, Shen W. Serotonin syndrome induced by low-dose venlafaxine. Ann Pharmacother. 2003;37(2):209-211.
10. Hernández JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother. 2002;36(4):641-643.
11. Patel DD, Galarneau D. Serotonin syndrome with fluoxetine: two case reports. Ochsner J. 2016;16(4):554-557.
12. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
13. Zuschlag ZD, Warren MW, K Schultz S. Serotonin toxicity and urinary analgesics: a case report and systematic literature review of methylene blue-induced serotonin syndrome. Psychosomatics. 2018;59(6):539-546.
14. Kapur S, Zipursky RB, Jones C, et al. Cyproheptadine: a potent in vivo serotonin antagonist. Am J Psychiatry. 1997;154(6):884.
15. Baigel GD. Cyproheptadine and the treatment of an unconscious patient with the serotonin syndrome. Eur J Anaesthesiol. 2003;20(7):586-588.
16. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331:1021-1022.
17. McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Pharmacother. 2001;35(7-8):870-873.
18. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. J Toxicol Clin Toxicol. 1997;35(2):211-212.
1. Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome: presentation of 2 cases and review of literature. Medicine (Baltimore). 2000;79(4):201-209.
2. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
3. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
4. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619.
5. Mills KC. Serotonin syndrome a clinical update. Crit Care Clin. 1997;13(4):763-783.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635-642.
7. Horowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care. 1999;15(5):325-327.
8. Kolecki P. Isolated venlafaxine-induced serotonin syndrome. J Emerg Med. 1996;15:491-493.
9. Pan J, Shen W. Serotonin syndrome induced by low-dose venlafaxine. Ann Pharmacother. 2003;37(2):209-211.
10. Hernández JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother. 2002;36(4):641-643.
11. Patel DD, Galarneau D. Serotonin syndrome with fluoxetine: two case reports. Ochsner J. 2016;16(4):554-557.
12. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
13. Zuschlag ZD, Warren MW, K Schultz S. Serotonin toxicity and urinary analgesics: a case report and systematic literature review of methylene blue-induced serotonin syndrome. Psychosomatics. 2018;59(6):539-546.
14. Kapur S, Zipursky RB, Jones C, et al. Cyproheptadine: a potent in vivo serotonin antagonist. Am J Psychiatry. 1997;154(6):884.
15. Baigel GD. Cyproheptadine and the treatment of an unconscious patient with the serotonin syndrome. Eur J Anaesthesiol. 2003;20(7):586-588.
16. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331:1021-1022.
17. McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Pharmacother. 2001;35(7-8):870-873.
18. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. J Toxicol Clin Toxicol. 1997;35(2):211-212.
Racing thoughts: What to consider
Have you ever had times in your life when you had a tremendous amount of energy, like too much energy, with racing thoughts? I initially ask patients this question when evaluating for bipolar disorder. Some patients insist that they have racing thoughts—thoughts occurring at a rate faster than they can be expressed through speech1—but not episodes of hyperactivity. This response suggests that some patients can have racing thoughts without a diagnosis of bipolar disorder.
Among the patients I treat, racing thoughts vary in severity, duration, and treatment. When untreated, a patient’s racing thoughts may range from a mild disturbance lasting a few days to a more severe disturbance occurring daily. In this article, I suggest treatments that may help ameliorate racing thoughts, and describe possible causes that include, but are not limited to, mood disorders.
Major depressive disorder
Many patients with major depressive disorder (MDD) have racing thoughts that often go unrecognized, and this symptom is associated with more severe depression.2 Those with a DSM-5 diagnosis of MDD with mixed features could experience prolonged racing thoughts during a major depressive episode.1 Untreated racing thoughts may explain why many patients with MDD do not improve with an antidepressant alone.3 These patients might benefit from augmentation with a mood stabilizer such as lithium4 or a second-generation antipsychotic.5
Other potential causes
Racing thoughts are a symptom, not a diagnosis. Apprehension and anxiety could cause racing thoughts that do not require treatment with a mood stabilizer or antipsychotic. Patients who often worry about having panic attacks or experience severe chronic stress may have racing thoughts. Also, some patients may be taking medications or illicit drugs or have a medical disorder that could cause symptoms of mania or hypomania that include racing thoughts (Table1).
In summary, when caring for a patient who reports having racing thoughts, consider:
- whether that patient actually does have racing thoughts
- the potential causes, severity, duration, and treatment of the racing thoughts
- the possibility that for a patient with MDD, augmenting an antidepressant with a mood stabilizer or antipsychotic could decrease racing thoughts, thereby helping to alleviate many cases of MDD.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59:570-575.
3. Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012;37(4):851-864.
4. Bauer M, Adli M, Bschor T, et al. Lithium’s emerging role in the treatment of refractory major depressive episodes: augmentation of antidepressants. Neuropsychobiology. 2010;62(1):36-42.
5. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980-991.
Have you ever had times in your life when you had a tremendous amount of energy, like too much energy, with racing thoughts? I initially ask patients this question when evaluating for bipolar disorder. Some patients insist that they have racing thoughts—thoughts occurring at a rate faster than they can be expressed through speech1—but not episodes of hyperactivity. This response suggests that some patients can have racing thoughts without a diagnosis of bipolar disorder.
Among the patients I treat, racing thoughts vary in severity, duration, and treatment. When untreated, a patient’s racing thoughts may range from a mild disturbance lasting a few days to a more severe disturbance occurring daily. In this article, I suggest treatments that may help ameliorate racing thoughts, and describe possible causes that include, but are not limited to, mood disorders.
Major depressive disorder
Many patients with major depressive disorder (MDD) have racing thoughts that often go unrecognized, and this symptom is associated with more severe depression.2 Those with a DSM-5 diagnosis of MDD with mixed features could experience prolonged racing thoughts during a major depressive episode.1 Untreated racing thoughts may explain why many patients with MDD do not improve with an antidepressant alone.3 These patients might benefit from augmentation with a mood stabilizer such as lithium4 or a second-generation antipsychotic.5
Other potential causes
Racing thoughts are a symptom, not a diagnosis. Apprehension and anxiety could cause racing thoughts that do not require treatment with a mood stabilizer or antipsychotic. Patients who often worry about having panic attacks or experience severe chronic stress may have racing thoughts. Also, some patients may be taking medications or illicit drugs or have a medical disorder that could cause symptoms of mania or hypomania that include racing thoughts (Table1).
In summary, when caring for a patient who reports having racing thoughts, consider:
- whether that patient actually does have racing thoughts
- the potential causes, severity, duration, and treatment of the racing thoughts
- the possibility that for a patient with MDD, augmenting an antidepressant with a mood stabilizer or antipsychotic could decrease racing thoughts, thereby helping to alleviate many cases of MDD.
Have you ever had times in your life when you had a tremendous amount of energy, like too much energy, with racing thoughts? I initially ask patients this question when evaluating for bipolar disorder. Some patients insist that they have racing thoughts—thoughts occurring at a rate faster than they can be expressed through speech1—but not episodes of hyperactivity. This response suggests that some patients can have racing thoughts without a diagnosis of bipolar disorder.
Among the patients I treat, racing thoughts vary in severity, duration, and treatment. When untreated, a patient’s racing thoughts may range from a mild disturbance lasting a few days to a more severe disturbance occurring daily. In this article, I suggest treatments that may help ameliorate racing thoughts, and describe possible causes that include, but are not limited to, mood disorders.
Major depressive disorder
Many patients with major depressive disorder (MDD) have racing thoughts that often go unrecognized, and this symptom is associated with more severe depression.2 Those with a DSM-5 diagnosis of MDD with mixed features could experience prolonged racing thoughts during a major depressive episode.1 Untreated racing thoughts may explain why many patients with MDD do not improve with an antidepressant alone.3 These patients might benefit from augmentation with a mood stabilizer such as lithium4 or a second-generation antipsychotic.5
Other potential causes
Racing thoughts are a symptom, not a diagnosis. Apprehension and anxiety could cause racing thoughts that do not require treatment with a mood stabilizer or antipsychotic. Patients who often worry about having panic attacks or experience severe chronic stress may have racing thoughts. Also, some patients may be taking medications or illicit drugs or have a medical disorder that could cause symptoms of mania or hypomania that include racing thoughts (Table1).
In summary, when caring for a patient who reports having racing thoughts, consider:
- whether that patient actually does have racing thoughts
- the potential causes, severity, duration, and treatment of the racing thoughts
- the possibility that for a patient with MDD, augmenting an antidepressant with a mood stabilizer or antipsychotic could decrease racing thoughts, thereby helping to alleviate many cases of MDD.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59:570-575.
3. Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012;37(4):851-864.
4. Bauer M, Adli M, Bschor T, et al. Lithium’s emerging role in the treatment of refractory major depressive episodes: augmentation of antidepressants. Neuropsychobiology. 2010;62(1):36-42.
5. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980-991.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59:570-575.
3. Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012;37(4):851-864.
4. Bauer M, Adli M, Bschor T, et al. Lithium’s emerging role in the treatment of refractory major depressive episodes: augmentation of antidepressants. Neuropsychobiology. 2010;62(1):36-42.
5. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980-991.