User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
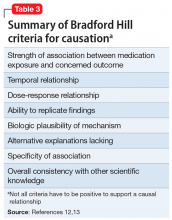
Needed: More studies of CSF molecular biomarkers in psychiatric disorders
Psychiatry and neurology are the brain’s twin medical disciplines. Unlike neurologic brain disorders, where localizing the “lesion” is a primary objective, psychiatric brain disorders are much more subtle, with no “gross” lesions but numerous cellular and molecular pathologies within neural circuits.
Measuring the molecular components of the cerebrospinal fluid (CSF), the glorious “sewage system” of the brain, may help reveal granular clues to the neurobiology of psychiatric disorders.
Mental illnesses involve the disruption of brain structures and functions in a diffuse manner across the cortex. Abnormal neuroplasticity has been implicated in several major psychiatric disorders. Examples include hypoplasia of the hippocampus in major depressive disorder and cortical thinning/dysplasia in schizophrenia. Reductions of neurotropic factors such as nerve growth factor or brain-derived neurotropic factor have been reported in mood and psychotic disorders, and appear to correlate with neuroplasticity changes.
Recent advances in psychiatric neuroscience have provided many clues to the pathophysiology of psychopathological conditions, including neuroinflammation, oxidative stress, apoptosis, impaired energy metabolism, abnormal metabolomics and lipidomics, and hypo- and hyperfunction of various neurotransmitters systems (especially glutamate N-methyl-
Thus, psychiatric research should focus on exploring and detecting molecular signatures (ie, biomarkers) of psychiatric disorders, including biomarkers of axonal and synaptic damage, glial activation, and oxidative stress. This is especially critical given the extensive heterogeneity of schizophrenia and mood and anxiety disorders. The CSF is a vastly unexploited substrate for discovering molecular biomarkers that will pave the way to precision psychiatry, and possibly open the door for completely new therapeutic strategies to tackle the most challenging neuropsychiatric disorders.
A role for CSF analysis
It’s quite puzzling why acute psychiatric episodes of schizophrenia, bipolar disorder, major depressive disorder, or panic attacks are not routinely assessed with a spinal tap, in conjunction with other brain measures such as neuroimaging (morphology, spectroscopy, cerebral blood flow, and diffusion tensor imaging) as well as a comprehensive neurocognitive examination and neurophysiological tests such as pre-pulse inhibition, mismatch negativity, and P-50, N-10, and P-300 evoked potentials. Combining CSF analysis with all those measures may help us stratify the spectra of psychosis, depression, and anxiety, as well as posttraumatic stress disorder and obsessive-compulsive disorder, into unique biotypes with overlapping clinical phenotypes and specific treatment approaches.
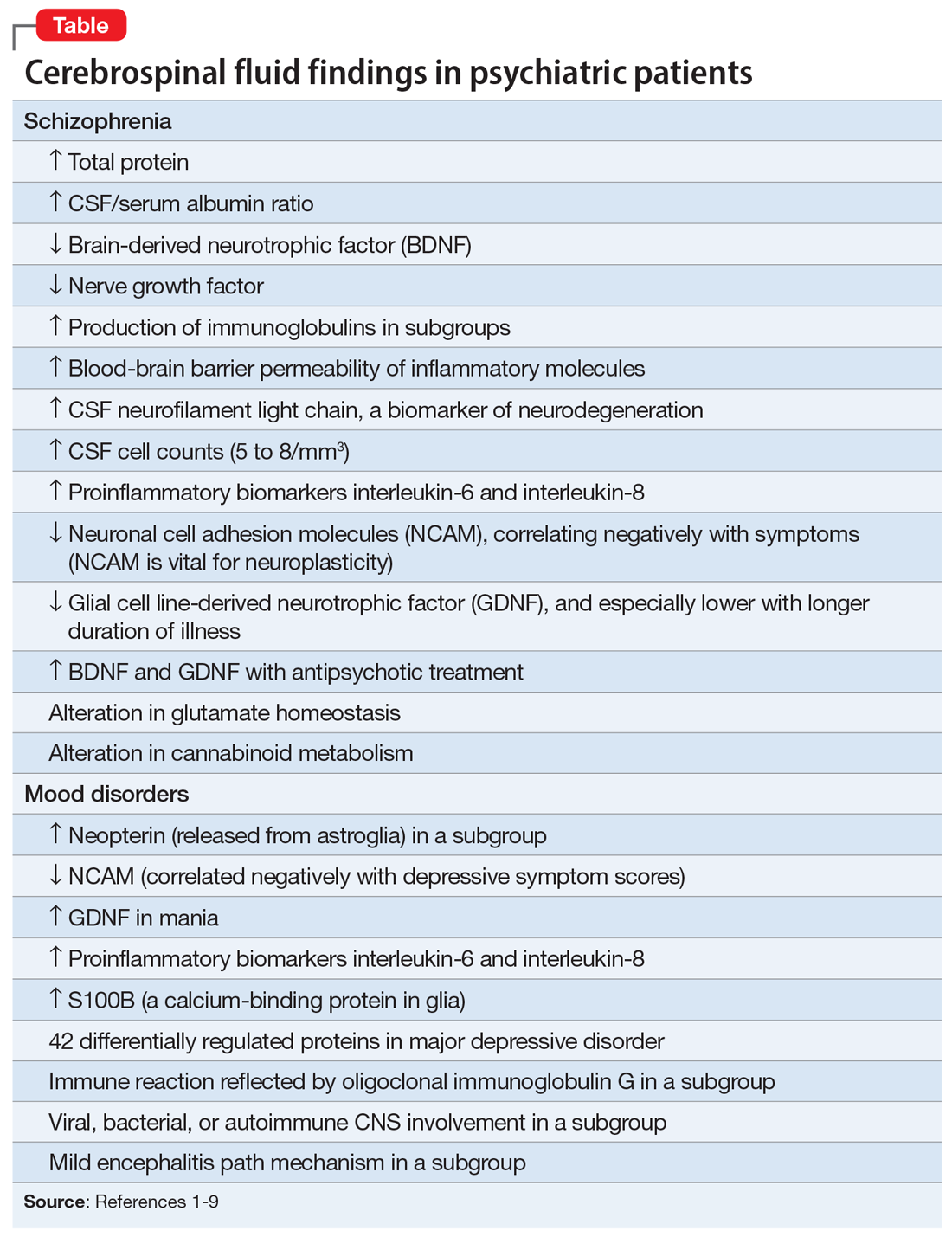
There are relatively few published CSF studies in psychiatric patients (mostly schizophrenia and bipolar and depressive disorders). The Table1-9 shows some of those findings. More than 365 biomarkers have been reported in schizophrenia, most of them in serum and tissue.10 However, none of them can be used for diagnostic purposes because schizophrenia is a syndrome comprised of several hundred different diseases (biotypes) that have similar clinical symptoms. Many of the serum and tissue biomarkers have not been studied in CSF, and they must if advances in the neurobiology and treatment of the psychotic and mood spectra are to be achieved. And adapting the CSF biomarkers described in neurologic disorders such as multiple sclerosis11 to schizophrenia and bipolar disorder (which also have well-established myelin pathologies) may yield a trove of neurobiologic findings.
If CSF studies eventually prove to be very useful for identifying subtypes for diagnosis and treatment, psychiatrists do not have to do the lumbar puncture themselves, but may refer patients to a “spinal tap” laboratory, just as they refer patients to a phlebotomy laboratory for routine blood tests. The adoption of CSF assessment in psychiatry will solidify its status as a clinical neuroscience, like its sister, neurology.
1. Vasic N, Connemann BJ, Wolf RC, et al. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262(5):375-391.
2. Pollak TA, Drndarski S, Stone JM, et al. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79-92.
3. Katisko K, Cajanus A, Jääskeläinen O, et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol. 2020;267(1):162-167.
4. Bechter K, Reiber H, Herzog S, et al. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321-330.
5. Hidese S, Hattori K, Sasayama D, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:12-18.
6. Tunca Z, Kıvırcık Akdede B, Özerdem A, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia: a comparative study in four major psychiatric disorders. Eur Psychiatry. 2015;30(2):198-204.
7. Al Shweiki MR, Oeckl P, Steinacker P, et al. Major depressive disorder: insight into candidate cerebrospinal fluid protein biomarkers from proteomics studies. Expert Rev Proteomics. 2017;14(6):499-514.
8. Kroksmark H, Vinberg M. Does S100B have a potential role in affective disorders? A literature review. Nord J Psychiatry. 2018;72(7):462-470.
9. Orlovska-Waast S, Köhler-Forsberg O, Brix SW, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(6):869-887.
10. Nasrallah HA. Lab tests for psychiatric disorders: few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
11. Porter L, Shoushtarizadeh A, Jelinek GA, et al. Metabolomic biomarkers of multiple sclerosis: a systematic review. Front Mol Biosci. 2020;7:574133. doi: 10.3389/fmolb.2020.574133
Psychiatry and neurology are the brain’s twin medical disciplines. Unlike neurologic brain disorders, where localizing the “lesion” is a primary objective, psychiatric brain disorders are much more subtle, with no “gross” lesions but numerous cellular and molecular pathologies within neural circuits.
Measuring the molecular components of the cerebrospinal fluid (CSF), the glorious “sewage system” of the brain, may help reveal granular clues to the neurobiology of psychiatric disorders.
Mental illnesses involve the disruption of brain structures and functions in a diffuse manner across the cortex. Abnormal neuroplasticity has been implicated in several major psychiatric disorders. Examples include hypoplasia of the hippocampus in major depressive disorder and cortical thinning/dysplasia in schizophrenia. Reductions of neurotropic factors such as nerve growth factor or brain-derived neurotropic factor have been reported in mood and psychotic disorders, and appear to correlate with neuroplasticity changes.
Recent advances in psychiatric neuroscience have provided many clues to the pathophysiology of psychopathological conditions, including neuroinflammation, oxidative stress, apoptosis, impaired energy metabolism, abnormal metabolomics and lipidomics, and hypo- and hyperfunction of various neurotransmitters systems (especially glutamate N-methyl-
Thus, psychiatric research should focus on exploring and detecting molecular signatures (ie, biomarkers) of psychiatric disorders, including biomarkers of axonal and synaptic damage, glial activation, and oxidative stress. This is especially critical given the extensive heterogeneity of schizophrenia and mood and anxiety disorders. The CSF is a vastly unexploited substrate for discovering molecular biomarkers that will pave the way to precision psychiatry, and possibly open the door for completely new therapeutic strategies to tackle the most challenging neuropsychiatric disorders.
A role for CSF analysis
It’s quite puzzling why acute psychiatric episodes of schizophrenia, bipolar disorder, major depressive disorder, or panic attacks are not routinely assessed with a spinal tap, in conjunction with other brain measures such as neuroimaging (morphology, spectroscopy, cerebral blood flow, and diffusion tensor imaging) as well as a comprehensive neurocognitive examination and neurophysiological tests such as pre-pulse inhibition, mismatch negativity, and P-50, N-10, and P-300 evoked potentials. Combining CSF analysis with all those measures may help us stratify the spectra of psychosis, depression, and anxiety, as well as posttraumatic stress disorder and obsessive-compulsive disorder, into unique biotypes with overlapping clinical phenotypes and specific treatment approaches.
There are relatively few published CSF studies in psychiatric patients (mostly schizophrenia and bipolar and depressive disorders). The Table1-9 shows some of those findings. More than 365 biomarkers have been reported in schizophrenia, most of them in serum and tissue.10 However, none of them can be used for diagnostic purposes because schizophrenia is a syndrome comprised of several hundred different diseases (biotypes) that have similar clinical symptoms. Many of the serum and tissue biomarkers have not been studied in CSF, and they must if advances in the neurobiology and treatment of the psychotic and mood spectra are to be achieved. And adapting the CSF biomarkers described in neurologic disorders such as multiple sclerosis11 to schizophrenia and bipolar disorder (which also have well-established myelin pathologies) may yield a trove of neurobiologic findings.

If CSF studies eventually prove to be very useful for identifying subtypes for diagnosis and treatment, psychiatrists do not have to do the lumbar puncture themselves, but may refer patients to a “spinal tap” laboratory, just as they refer patients to a phlebotomy laboratory for routine blood tests. The adoption of CSF assessment in psychiatry will solidify its status as a clinical neuroscience, like its sister, neurology.
Psychiatry and neurology are the brain’s twin medical disciplines. Unlike neurologic brain disorders, where localizing the “lesion” is a primary objective, psychiatric brain disorders are much more subtle, with no “gross” lesions but numerous cellular and molecular pathologies within neural circuits.
Measuring the molecular components of the cerebrospinal fluid (CSF), the glorious “sewage system” of the brain, may help reveal granular clues to the neurobiology of psychiatric disorders.
Mental illnesses involve the disruption of brain structures and functions in a diffuse manner across the cortex. Abnormal neuroplasticity has been implicated in several major psychiatric disorders. Examples include hypoplasia of the hippocampus in major depressive disorder and cortical thinning/dysplasia in schizophrenia. Reductions of neurotropic factors such as nerve growth factor or brain-derived neurotropic factor have been reported in mood and psychotic disorders, and appear to correlate with neuroplasticity changes.
Recent advances in psychiatric neuroscience have provided many clues to the pathophysiology of psychopathological conditions, including neuroinflammation, oxidative stress, apoptosis, impaired energy metabolism, abnormal metabolomics and lipidomics, and hypo- and hyperfunction of various neurotransmitters systems (especially glutamate N-methyl-
Thus, psychiatric research should focus on exploring and detecting molecular signatures (ie, biomarkers) of psychiatric disorders, including biomarkers of axonal and synaptic damage, glial activation, and oxidative stress. This is especially critical given the extensive heterogeneity of schizophrenia and mood and anxiety disorders. The CSF is a vastly unexploited substrate for discovering molecular biomarkers that will pave the way to precision psychiatry, and possibly open the door for completely new therapeutic strategies to tackle the most challenging neuropsychiatric disorders.
A role for CSF analysis
It’s quite puzzling why acute psychiatric episodes of schizophrenia, bipolar disorder, major depressive disorder, or panic attacks are not routinely assessed with a spinal tap, in conjunction with other brain measures such as neuroimaging (morphology, spectroscopy, cerebral blood flow, and diffusion tensor imaging) as well as a comprehensive neurocognitive examination and neurophysiological tests such as pre-pulse inhibition, mismatch negativity, and P-50, N-10, and P-300 evoked potentials. Combining CSF analysis with all those measures may help us stratify the spectra of psychosis, depression, and anxiety, as well as posttraumatic stress disorder and obsessive-compulsive disorder, into unique biotypes with overlapping clinical phenotypes and specific treatment approaches.
There are relatively few published CSF studies in psychiatric patients (mostly schizophrenia and bipolar and depressive disorders). The Table1-9 shows some of those findings. More than 365 biomarkers have been reported in schizophrenia, most of them in serum and tissue.10 However, none of them can be used for diagnostic purposes because schizophrenia is a syndrome comprised of several hundred different diseases (biotypes) that have similar clinical symptoms. Many of the serum and tissue biomarkers have not been studied in CSF, and they must if advances in the neurobiology and treatment of the psychotic and mood spectra are to be achieved. And adapting the CSF biomarkers described in neurologic disorders such as multiple sclerosis11 to schizophrenia and bipolar disorder (which also have well-established myelin pathologies) may yield a trove of neurobiologic findings.

If CSF studies eventually prove to be very useful for identifying subtypes for diagnosis and treatment, psychiatrists do not have to do the lumbar puncture themselves, but may refer patients to a “spinal tap” laboratory, just as they refer patients to a phlebotomy laboratory for routine blood tests. The adoption of CSF assessment in psychiatry will solidify its status as a clinical neuroscience, like its sister, neurology.
1. Vasic N, Connemann BJ, Wolf RC, et al. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262(5):375-391.
2. Pollak TA, Drndarski S, Stone JM, et al. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79-92.
3. Katisko K, Cajanus A, Jääskeläinen O, et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol. 2020;267(1):162-167.
4. Bechter K, Reiber H, Herzog S, et al. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321-330.
5. Hidese S, Hattori K, Sasayama D, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:12-18.
6. Tunca Z, Kıvırcık Akdede B, Özerdem A, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia: a comparative study in four major psychiatric disorders. Eur Psychiatry. 2015;30(2):198-204.
7. Al Shweiki MR, Oeckl P, Steinacker P, et al. Major depressive disorder: insight into candidate cerebrospinal fluid protein biomarkers from proteomics studies. Expert Rev Proteomics. 2017;14(6):499-514.
8. Kroksmark H, Vinberg M. Does S100B have a potential role in affective disorders? A literature review. Nord J Psychiatry. 2018;72(7):462-470.
9. Orlovska-Waast S, Köhler-Forsberg O, Brix SW, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(6):869-887.
10. Nasrallah HA. Lab tests for psychiatric disorders: few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
11. Porter L, Shoushtarizadeh A, Jelinek GA, et al. Metabolomic biomarkers of multiple sclerosis: a systematic review. Front Mol Biosci. 2020;7:574133. doi: 10.3389/fmolb.2020.574133
1. Vasic N, Connemann BJ, Wolf RC, et al. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262(5):375-391.
2. Pollak TA, Drndarski S, Stone JM, et al. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79-92.
3. Katisko K, Cajanus A, Jääskeläinen O, et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol. 2020;267(1):162-167.
4. Bechter K, Reiber H, Herzog S, et al. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321-330.
5. Hidese S, Hattori K, Sasayama D, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:12-18.
6. Tunca Z, Kıvırcık Akdede B, Özerdem A, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia: a comparative study in four major psychiatric disorders. Eur Psychiatry. 2015;30(2):198-204.
7. Al Shweiki MR, Oeckl P, Steinacker P, et al. Major depressive disorder: insight into candidate cerebrospinal fluid protein biomarkers from proteomics studies. Expert Rev Proteomics. 2017;14(6):499-514.
8. Kroksmark H, Vinberg M. Does S100B have a potential role in affective disorders? A literature review. Nord J Psychiatry. 2018;72(7):462-470.
9. Orlovska-Waast S, Köhler-Forsberg O, Brix SW, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(6):869-887.
10. Nasrallah HA. Lab tests for psychiatric disorders: few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
11. Porter L, Shoushtarizadeh A, Jelinek GA, et al. Metabolomic biomarkers of multiple sclerosis: a systematic review. Front Mol Biosci. 2020;7:574133. doi: 10.3389/fmolb.2020.574133
Late-onset, treatment-resistant anxiety and depression
CASE Anxious and can’t sleep
Mr. A, age 41, presents to his primary care physician (PCP) with anxiety and insomnia. He describes having generalized anxiety with initial and middle insomnia, and says he is sleeping an average of 2 hours per night. He denies any other psychiatric symptoms. Mr. A has no significant psychiatric or medical history.
Mr. A is initiated on zolpidem tartrate, 12.5 mg every night at bedtime, and paroxetine, 20 mg every night at bedtime, for anxiety and insomnia, but these medications result in little to no improvement.
During a 4-month period, he is treated with trials of alprazolam, 0.5 mg every 8 hours as needed; diazepam 5 mg twice a day as needed; diphenhydramine, 50 mg at bedtime; and eszopiclone, 3 mg at bedtime. Despite these treatments, he experiences increased anxiety and insomnia, and develops depressive symptoms, including depressed mood, poor concentration, general malaise, extreme fatigue, a 15-pound unintentional weight loss, erectile dysfunction, and decreased libido. Mr. A denies having suicidal or homicidal ideations. Additionally, he typically goes to the gym approximately 3 times per week, and has noticed that the amount of weight he is able to lift has decreased, which is distressing. Previously, he had been able to lift 300 pounds, but now he can only lift 200 pounds.
[polldaddy:10891920]
The authors’ observations
Insomnia, anxiety, and depression are common chief complaints in medical settings. However, some psychiatric presentations may have an underlying medical etiology.
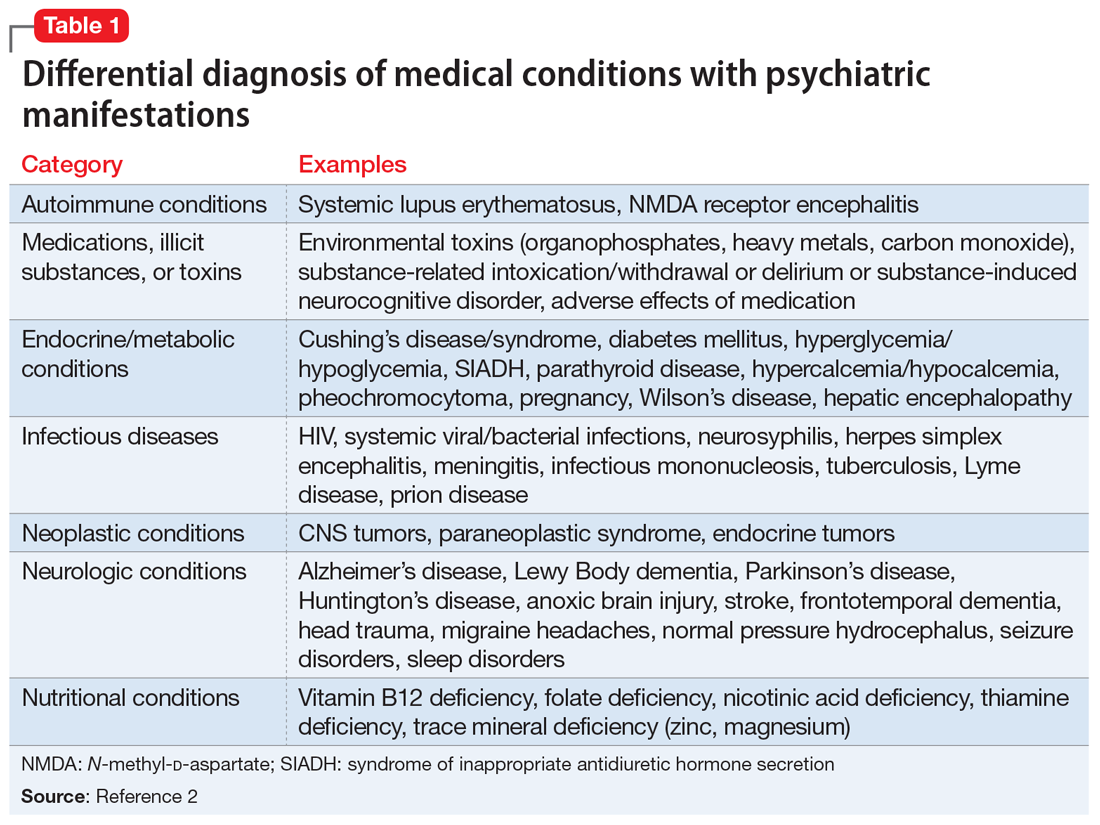
DSM-5 requires that medical conditions be ruled out in order for a patient to meet criteria for a psychiatric diagnosis.1 Medical differential diagnoses for patients with psychiatric symptoms can include autoimmune, drug/toxin, metabolic, infectious, neoplastic, neurologic, and nutritional etiologies (Table 12). To rule out the possibility of an underlying medical etiology, general screening guidelines include complete blood count, complete metabolic panel, urinalysis, and urine drug screen with alcohol. Human immunodeficiency virus testing and thyroid hormone testing are also commonly ordered.3 Further laboratory testing and imaging is typically not warranted in the absence of historical or physical findings because they are not advocated as cost-effective, so health care professionals must use their clinical judgment to determine appropriate further evaluation. The onset of anxiety most commonly occurs in late adolescence early and adulthood, but Mr. A experienced his first symptoms of anxiety at age 41.2 Mr. A’s age, lack of psychiatric or family history of mental illness, acute onset of symptoms, and failure of symptoms to abate with standard psychiatric treatments warrant a more extensive workup.
EVALUATION Imaging reveals an important finding
Because Mr. A’s symptoms do not improve with standard psychiatric treatments, his PCP orders standard laboratory bloodwork to investigate a possible medical etiology; however, his results are all within normal range.
After the PCP’s niece is coincidentally diagnosed with a pituitary macroadenoma, the PCP orders brain imaging for Mr. A. Results of an MRI show that Mr. A has a 1.6-cm macroadenoma of the pituitary. He is referred to an endocrinologist, who orders additional laboratory tests that show an elevated 24-hour free urine cortisol level of 73 μg/24 h (normal range: 3.5 to 45 μg/24 h), suggesting that Mr. A’s anxiety may be due to Cushing’s disease or that his anxiety caused falsely elevated urinary cortisol levels. Four weeks later, bloodwork is repeated and shows an abnormal dexamethasone suppression test, and 2 more elevated 24-hour free urine cortisol levels of 76 μg/24 h and 150 μg/24 h. A repeat MRI shows a 1.8-cm, mostly cystic sellar mass, indicating the need for surgical intervention. Although the tumor is large and shows optic nerve compression, Mr. A does not complain of headaches or changes in vision.
Continue to: Two months later...
Two months later, Mr. A undergoes a transsphenoidal tumor resection of the pituitary adenoma, and biopsy results confirm an adrenocorticotropic hormone (ACTH)-secreting pituitary macroadenoma, which is consistent with Cushing’s disease. Following surgery, steroid treatment with dexamethasone is discontinued due to a persistently elevated
[polldaddy:10891923]
The authors’ observations
Chronic excess glucocorticoid production is the underlying pathophysiology of Cushing’s disease, which is most commonly caused by an ACTH-producing adenoma.4,5 When these hormones become dysregulated, the result can be over- or underproduction of cortisol, which can lead to physical and psychiatric manifestations.6
Cushing’s disease most commonly manifests with the physical symptoms of centripetal fat deposition, abdominal striae, facial plethora, muscle atrophy, bone density loss, immunosuppression, and cardiovascular complications.5
Hypercortisolism can precipitate anxiety (12% to 79%), mood disorders (50% to 70%), and (less commonly) psychotic disorders; however, in a clinical setting, if a patient presented with one of these as a chief complaint, they would likely first be treated psychiatrically rather than worked up medically for a rare medical condition.5,7-13
Mr. A’s initial bloodwork was unremarkable, but cortisol levels were not obtained at that time because testing for cortisol levels to rule out an underlying medical condition is not routine in patients with depression and anxiety. In Mr. A’s case, a neuroendocrine workup was only ordered once his PCP’s niece coincidentally was diagnosed with a pituitary adenoma.
Continue to: For Mr. A...
For Mr. A, Cushing’s disease presented as a psychiatric disorder with anxiety and insomnia that were resistant to numerous psychiatric medications during an 8-month period. If Mr. A’s PCP had not ordered a brain MRI, he may have continued to receive ineffective psychiatric treatment for some time. Many of Mr. A’s physical symptoms were consistent with Cushing’s disease and mental illness, including erectile dysfunction, fatigue, and muscle weakness; however, his 15-pound weight loss pointed more toward psychiatric illness and further disguised his underlying medical diagnosis, because sudden weight gain is commonly seen in Cushing’s disease (Table 24,5,7,9).
TREATMENT Persistent psychiatric symptoms, then finally relief
Four weeks after surgery, Mr. A’s psychiatric symptoms gradually intensify, which prompts him to see a psychiatrist. A mental status examination (MSE) shows that he is well-nourished, with normal activity, appropriate behavior, and coherent thought process, but depressed mood and flat affect. He denies suicidal or homicidal ideation. He reports that despite being advised to have realistic expectations, he had high hopes that the surgery would lead to remission of all his symptoms, and expresses disappointment that he does not feel “back to normal.”
Six days later, Mr. A’s wife takes him to the hospital. His MSE shows that he has a tense appearance, fidgety activity, depressed and anxious mood, restricted affect, circumstantial thought process, and paranoid delusions that his wife was plotting against him. He says he still is experiencing insomnia. He also discloses having suicidal ideations with a plan and intent to overdose on medication, as well as homicidal ideations about killing his wife and children. Mr. A provides reasons for why he would want to hurt his family, and does not appear to be bothered by these thoughts.
Mr. A is admitted to the inpatient psychiatric unit and is prescribed quetiapine, 100 mg every night at bedtime. During the next 2 days, quetiapine is titrated to 300 mg every night at bedtime. On hospital Day 3, Mr. A says he is feeling worse than the previous days. He is still having vague suicidal thoughts and feels agitated, guilty, and depressed. To treat these persistent symptoms, quetiapine is further increased to 400 mg every night at bedtime, and he is initiated on bupropion XL, 150 mg, to treat persistent symptoms.
After 1 week of hospitalization, the treatment team meets with Mr. A and his wife, who has been supportive throughout her husband’s hospitalization. During the meeting, they both agree that Mr. A has experienced some improvement because he is no longer having suicidal or homicidal thoughts, but he is still feeling depressed and frustrated by his continued insomnia. Following the meeting, Mr. A’s quetiapine is further increased to 450 mg every night at bedtime to address continued insomnia, and bupropion XL is increased to 300 mg/d to address continued depressive symptoms. During the next few days, his affective symptoms improve; however, his initial insomnia continues, and quetiapine is further increased to 500 mg every night at bedtime.
Continue to: On hospital Day 20...
On hospital Day 20, Mr. A is discharged back to his outpatient psychiatrist and receives quetiapine, 500 mg every night at bedtime, and bupropion XL, 300 mg/d. Although Mr. A’s depression and anxiety continue to be well controlled, his insomnia persists. Sleep hygiene is addressed, and alprazolam, 0.5 mg every night at bedtime, is added to his regimen, which proves to be effective.
OUTCOME A slow remission
After a year of treatment, Mr. A is slowly tapered off of all medications. Two years later, he is in complete remission of all psychiatric symptoms and no longer requires any psychotropic medications.
The authors’ observations
Treatment for hypercortisolism in patients with psychiatric symptoms triggered by glucocorticoid imbalance has typically resulted in a decrease in the severity of their psychiatric symptoms.9,11 A prospective longitudinal study examining 33 patients found that correction of hypercortisolism in patients with Cushing’s syndrome often led to resolution of their psychiatric symptoms, with 87.9% of patients back to baseline within 1 year.14 However, to our knowledge, few reports have described the management of patients whose symptoms are resistant to treatment of hypercortisolism.
In our case, after transsphenoidal resection of an adenoma, Mr. A became suicidal and paranoid, and his anxiety and insomnia also persisted. A possible explanation for the worsening of Mr. A’s symptoms after surgery could be the slow recovery of the hypothalamic-pituitary-adrenal (HPA) axis and therefore a temporary deficiency in glucocorticoid, which caused an increase in catecholamines, leading to an increase in stress.14 This concept of a “slow recovery” is supported by the fact that Mr. A was successfully weaned off all medication after 1 year of treatment, and achieved complete remission of psychiatric symptoms for >2 years. Furthermore, the severity of Mr. A’s symptoms appeared to correlate with his 24-hour urine cortisol and
Future research should evaluate the utility of screening all patients with treatment-resistant anxiety and/or insomnia for hypercortisolism. Even without other clues to endocrinopathies, serum cortisol levels can be used as a screening tool for diagnosing underlying medical causes in patients with anxiety and depression.2 A greater understanding of the relationship between medical and psychiatric manifestations will allow clinicians to better care for patients. Further research is needed to elucidate the quantitative relationship between cortisol levels and anxiety to evaluate severity, guide treatment planning, and follow treatment response for patients with anxiety. It may be useful to determine the threshold between elevated cortisol levels due to anxiety vs elevated cortisol due to an underlying medical pathology such as Cushing’s disease. Additionally, little research has been conducted to compare how psychiatric symptoms respond to pituitary macroadenoma resection alone, pharmaceutical intervention alone, or a combination of these approaches. It would be beneficial to evaluate these treatment strategies to elucidate the most effective method to reduce psychiatric symptoms in patients with hypercortisolism, and perhaps to reduce the incidence of post-resection worsening of psychiatric symptoms.
Continue to: This case was challenging...
This case was challenging because Mr. A did not initially respond to psychiatric intervention, his psychiatric symptoms worsened after transsphenoidal resection of the pituitary adenoma, and his symptoms were alleviated only after psychiatric medications were re-initiated following surgery. This case highlights the importance of considering an underlying medically diagnosable and treatable cause of psychiatric illness, and illustrates the complex ongoing management that may be necessary to help a patient with this condition achieve their baseline. Further, Mr. A’s case shows that the absence of response to standard psychiatric therapies should warrant earlier laboratory and/or imaging evaluation prior to or in conjunction with psychiatric referral. Additionally, testing for cortisol levels is not typically done for a patient with treatment-resistant anxiety, and this case highlights the importance of considering hypercortisolism in such circumstances.
Bottom Line
Consider testing cortisol levels in patients with treatment-resistant anxiety and insomnia, because cortisol plays a role in Cushing’s disease and anxiety. The severity of psychiatric manifestations of Cushing’s disease may correlate with cortisol levels. Treatment should focus on symptomatic management and underlying etiology.
Related Resources
- Roberts LW, Hales RE, Yudofsky SC, ed. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019.
- Rotham J. Cushing’s syndrome: a tale of frequent misdiagnosis. National Center for Health Research. 2020. www.center4research.org/cushings-syndrome-frequent-misdiagnosis/
- Middleman D. Psychiatric issues of Cushing’s patients: coping with Cushing’s. Cushing’s Support and Research Foundation. www.csrf.net/coping-with-cushings/psychiatric-issues-of-cushings-patients/
Drug Brand Names
Alprazolam • Xanax
Bupropion • Wellbutrin
Dexamethasone • Decadron
Diazepam • Valium
Eszopiclone • Lunesta
Paroxetine • Paxil
Quetiapine • Seroquel
Zolpidem tartrate • Ambien CR
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Sadock BJ, Sadock VA, Ruiz P, et al. Neural sciences. In: Sadock BJ, Sadock VA, Ruiz P, et al. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Wolters Kluwer; 2015.
3. Anfinson TJ, Kathol RG. Screening laboratory evaluation in psychiatric patients: a review. Gen Hosp Psychiatry. 1992;14(4):248-257.
4. Fehm HL, Voigt KH. Pathophysiology of Cushing’s disease. Pathobiol Annu. 1979;9:225-255.
5. Fujii Y, Mizoguchi Y, Masuoka J, et al. Cushing’s syndrome and psychosis: a case report and literature review. Prim Care Companion CNS Disord. 2018;20(5):18.
6. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2011;4(2):739-769.
7. Santos A, Resimini E, Pascual JC, et al. Psychiatric symptoms in patients with Cushing’s syndrome: prevalence diagnosis, and management. Drugs. 2017;77(8):829-842.
8. Arnaldi G, Angeli A, Atkinson B, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593-5602.
9. Sonino N, Fava GA. Psychosomatic aspects of Cushing’s disease. Psychother Psychosom. 1998;67(3):140-146.
10. Loosen PT, Chambliss B, DeBold CR, et al. Psychiatric phenomenology in Cushing’s disease. Pharmacopsychiatry. 1992;25(4):192-198.
11. Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol. 1996;45(6):715-720.
12. Katho RG, Delahunt JW, Hannah L. Transition from bipolar affective disorder to intermittent Cushing’s syndrome: case report. J Clin Psychiatry. 1985;46(5):194-196.
13. Hirsh D, Orr G, Kantarovich V, et al. Cushing’s syndrome presenting as a schizophrenia-like psychotic state. Isr J Psychiatry Relat Sci. 2000;37(1):46-50.
14. Dorn LD, Burgess ES, Friedman TC, et al. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82(3):912-919.
15. Starkman MN, Schteingart DE, Schork MA. Cushing’s syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19(3):177-178.
CASE Anxious and can’t sleep
Mr. A, age 41, presents to his primary care physician (PCP) with anxiety and insomnia. He describes having generalized anxiety with initial and middle insomnia, and says he is sleeping an average of 2 hours per night. He denies any other psychiatric symptoms. Mr. A has no significant psychiatric or medical history.
Mr. A is initiated on zolpidem tartrate, 12.5 mg every night at bedtime, and paroxetine, 20 mg every night at bedtime, for anxiety and insomnia, but these medications result in little to no improvement.
During a 4-month period, he is treated with trials of alprazolam, 0.5 mg every 8 hours as needed; diazepam 5 mg twice a day as needed; diphenhydramine, 50 mg at bedtime; and eszopiclone, 3 mg at bedtime. Despite these treatments, he experiences increased anxiety and insomnia, and develops depressive symptoms, including depressed mood, poor concentration, general malaise, extreme fatigue, a 15-pound unintentional weight loss, erectile dysfunction, and decreased libido. Mr. A denies having suicidal or homicidal ideations. Additionally, he typically goes to the gym approximately 3 times per week, and has noticed that the amount of weight he is able to lift has decreased, which is distressing. Previously, he had been able to lift 300 pounds, but now he can only lift 200 pounds.
[polldaddy:10891920]
The authors’ observations
Insomnia, anxiety, and depression are common chief complaints in medical settings. However, some psychiatric presentations may have an underlying medical etiology.
DSM-5 requires that medical conditions be ruled out in order for a patient to meet criteria for a psychiatric diagnosis.1 Medical differential diagnoses for patients with psychiatric symptoms can include autoimmune, drug/toxin, metabolic, infectious, neoplastic, neurologic, and nutritional etiologies (Table 12). To rule out the possibility of an underlying medical etiology, general screening guidelines include complete blood count, complete metabolic panel, urinalysis, and urine drug screen with alcohol. Human immunodeficiency virus testing and thyroid hormone testing are also commonly ordered.3 Further laboratory testing and imaging is typically not warranted in the absence of historical or physical findings because they are not advocated as cost-effective, so health care professionals must use their clinical judgment to determine appropriate further evaluation. The onset of anxiety most commonly occurs in late adolescence early and adulthood, but Mr. A experienced his first symptoms of anxiety at age 41.2 Mr. A’s age, lack of psychiatric or family history of mental illness, acute onset of symptoms, and failure of symptoms to abate with standard psychiatric treatments warrant a more extensive workup.

EVALUATION Imaging reveals an important finding
Because Mr. A’s symptoms do not improve with standard psychiatric treatments, his PCP orders standard laboratory bloodwork to investigate a possible medical etiology; however, his results are all within normal range.
After the PCP’s niece is coincidentally diagnosed with a pituitary macroadenoma, the PCP orders brain imaging for Mr. A. Results of an MRI show that Mr. A has a 1.6-cm macroadenoma of the pituitary. He is referred to an endocrinologist, who orders additional laboratory tests that show an elevated 24-hour free urine cortisol level of 73 μg/24 h (normal range: 3.5 to 45 μg/24 h), suggesting that Mr. A’s anxiety may be due to Cushing’s disease or that his anxiety caused falsely elevated urinary cortisol levels. Four weeks later, bloodwork is repeated and shows an abnormal dexamethasone suppression test, and 2 more elevated 24-hour free urine cortisol levels of 76 μg/24 h and 150 μg/24 h. A repeat MRI shows a 1.8-cm, mostly cystic sellar mass, indicating the need for surgical intervention. Although the tumor is large and shows optic nerve compression, Mr. A does not complain of headaches or changes in vision.
Continue to: Two months later...
Two months later, Mr. A undergoes a transsphenoidal tumor resection of the pituitary adenoma, and biopsy results confirm an adrenocorticotropic hormone (ACTH)-secreting pituitary macroadenoma, which is consistent with Cushing’s disease. Following surgery, steroid treatment with dexamethasone is discontinued due to a persistently elevated
[polldaddy:10891923]
The authors’ observations
Chronic excess glucocorticoid production is the underlying pathophysiology of Cushing’s disease, which is most commonly caused by an ACTH-producing adenoma.4,5 When these hormones become dysregulated, the result can be over- or underproduction of cortisol, which can lead to physical and psychiatric manifestations.6
Cushing’s disease most commonly manifests with the physical symptoms of centripetal fat deposition, abdominal striae, facial plethora, muscle atrophy, bone density loss, immunosuppression, and cardiovascular complications.5
Hypercortisolism can precipitate anxiety (12% to 79%), mood disorders (50% to 70%), and (less commonly) psychotic disorders; however, in a clinical setting, if a patient presented with one of these as a chief complaint, they would likely first be treated psychiatrically rather than worked up medically for a rare medical condition.5,7-13
Mr. A’s initial bloodwork was unremarkable, but cortisol levels were not obtained at that time because testing for cortisol levels to rule out an underlying medical condition is not routine in patients with depression and anxiety. In Mr. A’s case, a neuroendocrine workup was only ordered once his PCP’s niece coincidentally was diagnosed with a pituitary adenoma.
Continue to: For Mr. A...
For Mr. A, Cushing’s disease presented as a psychiatric disorder with anxiety and insomnia that were resistant to numerous psychiatric medications during an 8-month period. If Mr. A’s PCP had not ordered a brain MRI, he may have continued to receive ineffective psychiatric treatment for some time. Many of Mr. A’s physical symptoms were consistent with Cushing’s disease and mental illness, including erectile dysfunction, fatigue, and muscle weakness; however, his 15-pound weight loss pointed more toward psychiatric illness and further disguised his underlying medical diagnosis, because sudden weight gain is commonly seen in Cushing’s disease (Table 24,5,7,9).
TREATMENT Persistent psychiatric symptoms, then finally relief
Four weeks after surgery, Mr. A’s psychiatric symptoms gradually intensify, which prompts him to see a psychiatrist. A mental status examination (MSE) shows that he is well-nourished, with normal activity, appropriate behavior, and coherent thought process, but depressed mood and flat affect. He denies suicidal or homicidal ideation. He reports that despite being advised to have realistic expectations, he had high hopes that the surgery would lead to remission of all his symptoms, and expresses disappointment that he does not feel “back to normal.”
Six days later, Mr. A’s wife takes him to the hospital. His MSE shows that he has a tense appearance, fidgety activity, depressed and anxious mood, restricted affect, circumstantial thought process, and paranoid delusions that his wife was plotting against him. He says he still is experiencing insomnia. He also discloses having suicidal ideations with a plan and intent to overdose on medication, as well as homicidal ideations about killing his wife and children. Mr. A provides reasons for why he would want to hurt his family, and does not appear to be bothered by these thoughts.
Mr. A is admitted to the inpatient psychiatric unit and is prescribed quetiapine, 100 mg every night at bedtime. During the next 2 days, quetiapine is titrated to 300 mg every night at bedtime. On hospital Day 3, Mr. A says he is feeling worse than the previous days. He is still having vague suicidal thoughts and feels agitated, guilty, and depressed. To treat these persistent symptoms, quetiapine is further increased to 400 mg every night at bedtime, and he is initiated on bupropion XL, 150 mg, to treat persistent symptoms.
After 1 week of hospitalization, the treatment team meets with Mr. A and his wife, who has been supportive throughout her husband’s hospitalization. During the meeting, they both agree that Mr. A has experienced some improvement because he is no longer having suicidal or homicidal thoughts, but he is still feeling depressed and frustrated by his continued insomnia. Following the meeting, Mr. A’s quetiapine is further increased to 450 mg every night at bedtime to address continued insomnia, and bupropion XL is increased to 300 mg/d to address continued depressive symptoms. During the next few days, his affective symptoms improve; however, his initial insomnia continues, and quetiapine is further increased to 500 mg every night at bedtime.
Continue to: On hospital Day 20...
On hospital Day 20, Mr. A is discharged back to his outpatient psychiatrist and receives quetiapine, 500 mg every night at bedtime, and bupropion XL, 300 mg/d. Although Mr. A’s depression and anxiety continue to be well controlled, his insomnia persists. Sleep hygiene is addressed, and alprazolam, 0.5 mg every night at bedtime, is added to his regimen, which proves to be effective.
OUTCOME A slow remission
After a year of treatment, Mr. A is slowly tapered off of all medications. Two years later, he is in complete remission of all psychiatric symptoms and no longer requires any psychotropic medications.
The authors’ observations
Treatment for hypercortisolism in patients with psychiatric symptoms triggered by glucocorticoid imbalance has typically resulted in a decrease in the severity of their psychiatric symptoms.9,11 A prospective longitudinal study examining 33 patients found that correction of hypercortisolism in patients with Cushing’s syndrome often led to resolution of their psychiatric symptoms, with 87.9% of patients back to baseline within 1 year.14 However, to our knowledge, few reports have described the management of patients whose symptoms are resistant to treatment of hypercortisolism.
In our case, after transsphenoidal resection of an adenoma, Mr. A became suicidal and paranoid, and his anxiety and insomnia also persisted. A possible explanation for the worsening of Mr. A’s symptoms after surgery could be the slow recovery of the hypothalamic-pituitary-adrenal (HPA) axis and therefore a temporary deficiency in glucocorticoid, which caused an increase in catecholamines, leading to an increase in stress.14 This concept of a “slow recovery” is supported by the fact that Mr. A was successfully weaned off all medication after 1 year of treatment, and achieved complete remission of psychiatric symptoms for >2 years. Furthermore, the severity of Mr. A’s symptoms appeared to correlate with his 24-hour urine cortisol and
Future research should evaluate the utility of screening all patients with treatment-resistant anxiety and/or insomnia for hypercortisolism. Even without other clues to endocrinopathies, serum cortisol levels can be used as a screening tool for diagnosing underlying medical causes in patients with anxiety and depression.2 A greater understanding of the relationship between medical and psychiatric manifestations will allow clinicians to better care for patients. Further research is needed to elucidate the quantitative relationship between cortisol levels and anxiety to evaluate severity, guide treatment planning, and follow treatment response for patients with anxiety. It may be useful to determine the threshold between elevated cortisol levels due to anxiety vs elevated cortisol due to an underlying medical pathology such as Cushing’s disease. Additionally, little research has been conducted to compare how psychiatric symptoms respond to pituitary macroadenoma resection alone, pharmaceutical intervention alone, or a combination of these approaches. It would be beneficial to evaluate these treatment strategies to elucidate the most effective method to reduce psychiatric symptoms in patients with hypercortisolism, and perhaps to reduce the incidence of post-resection worsening of psychiatric symptoms.
Continue to: This case was challenging...
This case was challenging because Mr. A did not initially respond to psychiatric intervention, his psychiatric symptoms worsened after transsphenoidal resection of the pituitary adenoma, and his symptoms were alleviated only after psychiatric medications were re-initiated following surgery. This case highlights the importance of considering an underlying medically diagnosable and treatable cause of psychiatric illness, and illustrates the complex ongoing management that may be necessary to help a patient with this condition achieve their baseline. Further, Mr. A’s case shows that the absence of response to standard psychiatric therapies should warrant earlier laboratory and/or imaging evaluation prior to or in conjunction with psychiatric referral. Additionally, testing for cortisol levels is not typically done for a patient with treatment-resistant anxiety, and this case highlights the importance of considering hypercortisolism in such circumstances.
Bottom Line
Consider testing cortisol levels in patients with treatment-resistant anxiety and insomnia, because cortisol plays a role in Cushing’s disease and anxiety. The severity of psychiatric manifestations of Cushing’s disease may correlate with cortisol levels. Treatment should focus on symptomatic management and underlying etiology.
Related Resources
- Roberts LW, Hales RE, Yudofsky SC, ed. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019.
- Rotham J. Cushing’s syndrome: a tale of frequent misdiagnosis. National Center for Health Research. 2020. www.center4research.org/cushings-syndrome-frequent-misdiagnosis/
- Middleman D. Psychiatric issues of Cushing’s patients: coping with Cushing’s. Cushing’s Support and Research Foundation. www.csrf.net/coping-with-cushings/psychiatric-issues-of-cushings-patients/
Drug Brand Names
Alprazolam • Xanax
Bupropion • Wellbutrin
Dexamethasone • Decadron
Diazepam • Valium
Eszopiclone • Lunesta
Paroxetine • Paxil
Quetiapine • Seroquel
Zolpidem tartrate • Ambien CR
CASE Anxious and can’t sleep
Mr. A, age 41, presents to his primary care physician (PCP) with anxiety and insomnia. He describes having generalized anxiety with initial and middle insomnia, and says he is sleeping an average of 2 hours per night. He denies any other psychiatric symptoms. Mr. A has no significant psychiatric or medical history.
Mr. A is initiated on zolpidem tartrate, 12.5 mg every night at bedtime, and paroxetine, 20 mg every night at bedtime, for anxiety and insomnia, but these medications result in little to no improvement.
During a 4-month period, he is treated with trials of alprazolam, 0.5 mg every 8 hours as needed; diazepam 5 mg twice a day as needed; diphenhydramine, 50 mg at bedtime; and eszopiclone, 3 mg at bedtime. Despite these treatments, he experiences increased anxiety and insomnia, and develops depressive symptoms, including depressed mood, poor concentration, general malaise, extreme fatigue, a 15-pound unintentional weight loss, erectile dysfunction, and decreased libido. Mr. A denies having suicidal or homicidal ideations. Additionally, he typically goes to the gym approximately 3 times per week, and has noticed that the amount of weight he is able to lift has decreased, which is distressing. Previously, he had been able to lift 300 pounds, but now he can only lift 200 pounds.
[polldaddy:10891920]
The authors’ observations
Insomnia, anxiety, and depression are common chief complaints in medical settings. However, some psychiatric presentations may have an underlying medical etiology.
DSM-5 requires that medical conditions be ruled out in order for a patient to meet criteria for a psychiatric diagnosis.1 Medical differential diagnoses for patients with psychiatric symptoms can include autoimmune, drug/toxin, metabolic, infectious, neoplastic, neurologic, and nutritional etiologies (Table 12). To rule out the possibility of an underlying medical etiology, general screening guidelines include complete blood count, complete metabolic panel, urinalysis, and urine drug screen with alcohol. Human immunodeficiency virus testing and thyroid hormone testing are also commonly ordered.3 Further laboratory testing and imaging is typically not warranted in the absence of historical or physical findings because they are not advocated as cost-effective, so health care professionals must use their clinical judgment to determine appropriate further evaluation. The onset of anxiety most commonly occurs in late adolescence early and adulthood, but Mr. A experienced his first symptoms of anxiety at age 41.2 Mr. A’s age, lack of psychiatric or family history of mental illness, acute onset of symptoms, and failure of symptoms to abate with standard psychiatric treatments warrant a more extensive workup.

EVALUATION Imaging reveals an important finding
Because Mr. A’s symptoms do not improve with standard psychiatric treatments, his PCP orders standard laboratory bloodwork to investigate a possible medical etiology; however, his results are all within normal range.
After the PCP’s niece is coincidentally diagnosed with a pituitary macroadenoma, the PCP orders brain imaging for Mr. A. Results of an MRI show that Mr. A has a 1.6-cm macroadenoma of the pituitary. He is referred to an endocrinologist, who orders additional laboratory tests that show an elevated 24-hour free urine cortisol level of 73 μg/24 h (normal range: 3.5 to 45 μg/24 h), suggesting that Mr. A’s anxiety may be due to Cushing’s disease or that his anxiety caused falsely elevated urinary cortisol levels. Four weeks later, bloodwork is repeated and shows an abnormal dexamethasone suppression test, and 2 more elevated 24-hour free urine cortisol levels of 76 μg/24 h and 150 μg/24 h. A repeat MRI shows a 1.8-cm, mostly cystic sellar mass, indicating the need for surgical intervention. Although the tumor is large and shows optic nerve compression, Mr. A does not complain of headaches or changes in vision.
Continue to: Two months later...
Two months later, Mr. A undergoes a transsphenoidal tumor resection of the pituitary adenoma, and biopsy results confirm an adrenocorticotropic hormone (ACTH)-secreting pituitary macroadenoma, which is consistent with Cushing’s disease. Following surgery, steroid treatment with dexamethasone is discontinued due to a persistently elevated
[polldaddy:10891923]
The authors’ observations
Chronic excess glucocorticoid production is the underlying pathophysiology of Cushing’s disease, which is most commonly caused by an ACTH-producing adenoma.4,5 When these hormones become dysregulated, the result can be over- or underproduction of cortisol, which can lead to physical and psychiatric manifestations.6
Cushing’s disease most commonly manifests with the physical symptoms of centripetal fat deposition, abdominal striae, facial plethora, muscle atrophy, bone density loss, immunosuppression, and cardiovascular complications.5
Hypercortisolism can precipitate anxiety (12% to 79%), mood disorders (50% to 70%), and (less commonly) psychotic disorders; however, in a clinical setting, if a patient presented with one of these as a chief complaint, they would likely first be treated psychiatrically rather than worked up medically for a rare medical condition.5,7-13
Mr. A’s initial bloodwork was unremarkable, but cortisol levels were not obtained at that time because testing for cortisol levels to rule out an underlying medical condition is not routine in patients with depression and anxiety. In Mr. A’s case, a neuroendocrine workup was only ordered once his PCP’s niece coincidentally was diagnosed with a pituitary adenoma.
Continue to: For Mr. A...
For Mr. A, Cushing’s disease presented as a psychiatric disorder with anxiety and insomnia that were resistant to numerous psychiatric medications during an 8-month period. If Mr. A’s PCP had not ordered a brain MRI, he may have continued to receive ineffective psychiatric treatment for some time. Many of Mr. A’s physical symptoms were consistent with Cushing’s disease and mental illness, including erectile dysfunction, fatigue, and muscle weakness; however, his 15-pound weight loss pointed more toward psychiatric illness and further disguised his underlying medical diagnosis, because sudden weight gain is commonly seen in Cushing’s disease (Table 24,5,7,9).
TREATMENT Persistent psychiatric symptoms, then finally relief
Four weeks after surgery, Mr. A’s psychiatric symptoms gradually intensify, which prompts him to see a psychiatrist. A mental status examination (MSE) shows that he is well-nourished, with normal activity, appropriate behavior, and coherent thought process, but depressed mood and flat affect. He denies suicidal or homicidal ideation. He reports that despite being advised to have realistic expectations, he had high hopes that the surgery would lead to remission of all his symptoms, and expresses disappointment that he does not feel “back to normal.”
Six days later, Mr. A’s wife takes him to the hospital. His MSE shows that he has a tense appearance, fidgety activity, depressed and anxious mood, restricted affect, circumstantial thought process, and paranoid delusions that his wife was plotting against him. He says he still is experiencing insomnia. He also discloses having suicidal ideations with a plan and intent to overdose on medication, as well as homicidal ideations about killing his wife and children. Mr. A provides reasons for why he would want to hurt his family, and does not appear to be bothered by these thoughts.
Mr. A is admitted to the inpatient psychiatric unit and is prescribed quetiapine, 100 mg every night at bedtime. During the next 2 days, quetiapine is titrated to 300 mg every night at bedtime. On hospital Day 3, Mr. A says he is feeling worse than the previous days. He is still having vague suicidal thoughts and feels agitated, guilty, and depressed. To treat these persistent symptoms, quetiapine is further increased to 400 mg every night at bedtime, and he is initiated on bupropion XL, 150 mg, to treat persistent symptoms.
After 1 week of hospitalization, the treatment team meets with Mr. A and his wife, who has been supportive throughout her husband’s hospitalization. During the meeting, they both agree that Mr. A has experienced some improvement because he is no longer having suicidal or homicidal thoughts, but he is still feeling depressed and frustrated by his continued insomnia. Following the meeting, Mr. A’s quetiapine is further increased to 450 mg every night at bedtime to address continued insomnia, and bupropion XL is increased to 300 mg/d to address continued depressive symptoms. During the next few days, his affective symptoms improve; however, his initial insomnia continues, and quetiapine is further increased to 500 mg every night at bedtime.
Continue to: On hospital Day 20...
On hospital Day 20, Mr. A is discharged back to his outpatient psychiatrist and receives quetiapine, 500 mg every night at bedtime, and bupropion XL, 300 mg/d. Although Mr. A’s depression and anxiety continue to be well controlled, his insomnia persists. Sleep hygiene is addressed, and alprazolam, 0.5 mg every night at bedtime, is added to his regimen, which proves to be effective.
OUTCOME A slow remission
After a year of treatment, Mr. A is slowly tapered off of all medications. Two years later, he is in complete remission of all psychiatric symptoms and no longer requires any psychotropic medications.
The authors’ observations
Treatment for hypercortisolism in patients with psychiatric symptoms triggered by glucocorticoid imbalance has typically resulted in a decrease in the severity of their psychiatric symptoms.9,11 A prospective longitudinal study examining 33 patients found that correction of hypercortisolism in patients with Cushing’s syndrome often led to resolution of their psychiatric symptoms, with 87.9% of patients back to baseline within 1 year.14 However, to our knowledge, few reports have described the management of patients whose symptoms are resistant to treatment of hypercortisolism.
In our case, after transsphenoidal resection of an adenoma, Mr. A became suicidal and paranoid, and his anxiety and insomnia also persisted. A possible explanation for the worsening of Mr. A’s symptoms after surgery could be the slow recovery of the hypothalamic-pituitary-adrenal (HPA) axis and therefore a temporary deficiency in glucocorticoid, which caused an increase in catecholamines, leading to an increase in stress.14 This concept of a “slow recovery” is supported by the fact that Mr. A was successfully weaned off all medication after 1 year of treatment, and achieved complete remission of psychiatric symptoms for >2 years. Furthermore, the severity of Mr. A’s symptoms appeared to correlate with his 24-hour urine cortisol and
Future research should evaluate the utility of screening all patients with treatment-resistant anxiety and/or insomnia for hypercortisolism. Even without other clues to endocrinopathies, serum cortisol levels can be used as a screening tool for diagnosing underlying medical causes in patients with anxiety and depression.2 A greater understanding of the relationship between medical and psychiatric manifestations will allow clinicians to better care for patients. Further research is needed to elucidate the quantitative relationship between cortisol levels and anxiety to evaluate severity, guide treatment planning, and follow treatment response for patients with anxiety. It may be useful to determine the threshold between elevated cortisol levels due to anxiety vs elevated cortisol due to an underlying medical pathology such as Cushing’s disease. Additionally, little research has been conducted to compare how psychiatric symptoms respond to pituitary macroadenoma resection alone, pharmaceutical intervention alone, or a combination of these approaches. It would be beneficial to evaluate these treatment strategies to elucidate the most effective method to reduce psychiatric symptoms in patients with hypercortisolism, and perhaps to reduce the incidence of post-resection worsening of psychiatric symptoms.
Continue to: This case was challenging...
This case was challenging because Mr. A did not initially respond to psychiatric intervention, his psychiatric symptoms worsened after transsphenoidal resection of the pituitary adenoma, and his symptoms were alleviated only after psychiatric medications were re-initiated following surgery. This case highlights the importance of considering an underlying medically diagnosable and treatable cause of psychiatric illness, and illustrates the complex ongoing management that may be necessary to help a patient with this condition achieve their baseline. Further, Mr. A’s case shows that the absence of response to standard psychiatric therapies should warrant earlier laboratory and/or imaging evaluation prior to or in conjunction with psychiatric referral. Additionally, testing for cortisol levels is not typically done for a patient with treatment-resistant anxiety, and this case highlights the importance of considering hypercortisolism in such circumstances.
Bottom Line
Consider testing cortisol levels in patients with treatment-resistant anxiety and insomnia, because cortisol plays a role in Cushing’s disease and anxiety. The severity of psychiatric manifestations of Cushing’s disease may correlate with cortisol levels. Treatment should focus on symptomatic management and underlying etiology.
Related Resources
- Roberts LW, Hales RE, Yudofsky SC, ed. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019.
- Rotham J. Cushing’s syndrome: a tale of frequent misdiagnosis. National Center for Health Research. 2020. www.center4research.org/cushings-syndrome-frequent-misdiagnosis/
- Middleman D. Psychiatric issues of Cushing’s patients: coping with Cushing’s. Cushing’s Support and Research Foundation. www.csrf.net/coping-with-cushings/psychiatric-issues-of-cushings-patients/
Drug Brand Names
Alprazolam • Xanax
Bupropion • Wellbutrin
Dexamethasone • Decadron
Diazepam • Valium
Eszopiclone • Lunesta
Paroxetine • Paxil
Quetiapine • Seroquel
Zolpidem tartrate • Ambien CR
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Sadock BJ, Sadock VA, Ruiz P, et al. Neural sciences. In: Sadock BJ, Sadock VA, Ruiz P, et al. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Wolters Kluwer; 2015.
3. Anfinson TJ, Kathol RG. Screening laboratory evaluation in psychiatric patients: a review. Gen Hosp Psychiatry. 1992;14(4):248-257.
4. Fehm HL, Voigt KH. Pathophysiology of Cushing’s disease. Pathobiol Annu. 1979;9:225-255.
5. Fujii Y, Mizoguchi Y, Masuoka J, et al. Cushing’s syndrome and psychosis: a case report and literature review. Prim Care Companion CNS Disord. 2018;20(5):18.
6. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2011;4(2):739-769.
7. Santos A, Resimini E, Pascual JC, et al. Psychiatric symptoms in patients with Cushing’s syndrome: prevalence diagnosis, and management. Drugs. 2017;77(8):829-842.
8. Arnaldi G, Angeli A, Atkinson B, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593-5602.
9. Sonino N, Fava GA. Psychosomatic aspects of Cushing’s disease. Psychother Psychosom. 1998;67(3):140-146.
10. Loosen PT, Chambliss B, DeBold CR, et al. Psychiatric phenomenology in Cushing’s disease. Pharmacopsychiatry. 1992;25(4):192-198.
11. Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol. 1996;45(6):715-720.
12. Katho RG, Delahunt JW, Hannah L. Transition from bipolar affective disorder to intermittent Cushing’s syndrome: case report. J Clin Psychiatry. 1985;46(5):194-196.
13. Hirsh D, Orr G, Kantarovich V, et al. Cushing’s syndrome presenting as a schizophrenia-like psychotic state. Isr J Psychiatry Relat Sci. 2000;37(1):46-50.
14. Dorn LD, Burgess ES, Friedman TC, et al. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82(3):912-919.
15. Starkman MN, Schteingart DE, Schork MA. Cushing’s syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19(3):177-178.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Sadock BJ, Sadock VA, Ruiz P, et al. Neural sciences. In: Sadock BJ, Sadock VA, Ruiz P, et al. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Wolters Kluwer; 2015.
3. Anfinson TJ, Kathol RG. Screening laboratory evaluation in psychiatric patients: a review. Gen Hosp Psychiatry. 1992;14(4):248-257.
4. Fehm HL, Voigt KH. Pathophysiology of Cushing’s disease. Pathobiol Annu. 1979;9:225-255.
5. Fujii Y, Mizoguchi Y, Masuoka J, et al. Cushing’s syndrome and psychosis: a case report and literature review. Prim Care Companion CNS Disord. 2018;20(5):18.
6. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2011;4(2):739-769.
7. Santos A, Resimini E, Pascual JC, et al. Psychiatric symptoms in patients with Cushing’s syndrome: prevalence diagnosis, and management. Drugs. 2017;77(8):829-842.
8. Arnaldi G, Angeli A, Atkinson B, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593-5602.
9. Sonino N, Fava GA. Psychosomatic aspects of Cushing’s disease. Psychother Psychosom. 1998;67(3):140-146.
10. Loosen PT, Chambliss B, DeBold CR, et al. Psychiatric phenomenology in Cushing’s disease. Pharmacopsychiatry. 1992;25(4):192-198.
11. Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol. 1996;45(6):715-720.
12. Katho RG, Delahunt JW, Hannah L. Transition from bipolar affective disorder to intermittent Cushing’s syndrome: case report. J Clin Psychiatry. 1985;46(5):194-196.
13. Hirsh D, Orr G, Kantarovich V, et al. Cushing’s syndrome presenting as a schizophrenia-like psychotic state. Isr J Psychiatry Relat Sci. 2000;37(1):46-50.
14. Dorn LD, Burgess ES, Friedman TC, et al. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82(3):912-919.
15. Starkman MN, Schteingart DE, Schork MA. Cushing’s syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19(3):177-178.
Medicine’s ‘Big Lie’
While today “The Big Lie” mainly refers to the actions of the prior President, an older and bigger lie that has a real effect on every American is one perpetrated by our very own health care conglomerate. Americans pay the highest rates for health care on the planet; health care consumes about 17% of our gross domestic product.1 If we got higher-quality care, faster services, longer lives, or even greater consumer happiness, paying those rates might be worth it. But we don’t.
Worse yet is the idea that “board certification” assures the public that the doctor from whom they receive/purchase care is of a higher quality than one who is not so credentialed. That is our “Big Lie!” For decades, the public has been told that they should seek out board-certified doctors. Doctors in training have been told they must get board-certified. Hospitals brag about employing only board-certified doctors, insurers sometimes mandate board certification for a doctor to get paid, and employers use board certification as a benchmark for hiring and as a factor in compensation.
The sacred secret is that board certification makes no difference. There is no substantial evidence in any branch of medicine that doctors who are board-certified are better. There is no evidence that board-certified doctors get their patients healthier with more frequency, faster, less expensively, or with fewer medical errors than other doctors. The reality is that board certification is a sham. It’s a certificate granted after taking a very expensive test, and it is now part of an industry that is misleading the public and harming the trust the medical profession had once earned. Board certification is the equivalent of a diploma mill or an online certificate in any other field.
Why has this been kept under wraps for so long? Follow the money. The American Board of Medical Specialties (ABMS) oversees 24 specialty boards and reported revenue of $22.2M and expenses of $19.3M on its 2019 IRS Form 990.2 They make profit every year. But, looking further, these “not-for-profit” educational entities are sitting on hundreds of millions of dollars in their “foundations.” Take the American Board of Psychiatry and Neurology, for instance. They had more than $140M in assets in 2019.3 How is this possible? Easy. They have misled the American public and been remarkably successful convincing other organizations, such as the Joint Commission, the Accreditation Council for Graduate Medical Education, and the National Committee for Quality Assurance, that board certification is an assurance of quality. They charge high fees to “candidates” for taking the computer-based test and have developed a system called maintenance of certification (MOC) that is onerous, expensive, and serves as an annuity that forces doctors to pay annually to keep their board certification.
Medicine is a science. In the practice of our discipline, we are expected to follow the science and to adhere to scientific principles. Yet there is neither scientific proof nor good evidence that board certification means anything in terms of competence, safety to the public, or quality of care. Doctors favor life-long learning, and continuing education has long been the standard and should remain so, not board certification or MOC. The mandatory continuing education required in every state to maintain a medical license is sufficient to prove doctors are current in their field of practice and to protect the public.
It is time for the medical community to admit that the emperor wears no clothes, and demand that the money grab of the ABMS and its affiliates be halted. This would result in greater access to care for patients and would reduce the cost of medical care, as the hundreds of millions being “stolen” from doctors today—costs that get passed on to patients—could be recouped and used for treating patients who clearly are in need and are being forgotten as the medical-industrial complex continues to flex its muscles and ensnare more of our national budget in its tentacles.
Neil S. Kaye, MD, DLFAPA
Hockessin, Delaware
1. The World Bank. Current health expenditure (% of GDP). Accessed July 12, 2021. https://data.worldbank.org/indicator/SH.XPD.CHEX.GD.ZS
2. American Board of Medical Specialties. 2019 Form 990. Return of Organization Exempt From Income Tax. Accessed July 12, 2021. https://www.abms.org/wp-content/uploads/2021/01/2019-american-board-of-medical-specialties-form-990.pdf
3. ProPublica. American Board of Psychiatry and Neurology. Accessed July 13, 2021. https://projects.propublica.org/nonprofits/organizations/410654864
While today “The Big Lie” mainly refers to the actions of the prior President, an older and bigger lie that has a real effect on every American is one perpetrated by our very own health care conglomerate. Americans pay the highest rates for health care on the planet; health care consumes about 17% of our gross domestic product.1 If we got higher-quality care, faster services, longer lives, or even greater consumer happiness, paying those rates might be worth it. But we don’t.
Worse yet is the idea that “board certification” assures the public that the doctor from whom they receive/purchase care is of a higher quality than one who is not so credentialed. That is our “Big Lie!” For decades, the public has been told that they should seek out board-certified doctors. Doctors in training have been told they must get board-certified. Hospitals brag about employing only board-certified doctors, insurers sometimes mandate board certification for a doctor to get paid, and employers use board certification as a benchmark for hiring and as a factor in compensation.
The sacred secret is that board certification makes no difference. There is no substantial evidence in any branch of medicine that doctors who are board-certified are better. There is no evidence that board-certified doctors get their patients healthier with more frequency, faster, less expensively, or with fewer medical errors than other doctors. The reality is that board certification is a sham. It’s a certificate granted after taking a very expensive test, and it is now part of an industry that is misleading the public and harming the trust the medical profession had once earned. Board certification is the equivalent of a diploma mill or an online certificate in any other field.
Why has this been kept under wraps for so long? Follow the money. The American Board of Medical Specialties (ABMS) oversees 24 specialty boards and reported revenue of $22.2M and expenses of $19.3M on its 2019 IRS Form 990.2 They make profit every year. But, looking further, these “not-for-profit” educational entities are sitting on hundreds of millions of dollars in their “foundations.” Take the American Board of Psychiatry and Neurology, for instance. They had more than $140M in assets in 2019.3 How is this possible? Easy. They have misled the American public and been remarkably successful convincing other organizations, such as the Joint Commission, the Accreditation Council for Graduate Medical Education, and the National Committee for Quality Assurance, that board certification is an assurance of quality. They charge high fees to “candidates” for taking the computer-based test and have developed a system called maintenance of certification (MOC) that is onerous, expensive, and serves as an annuity that forces doctors to pay annually to keep their board certification.
Medicine is a science. In the practice of our discipline, we are expected to follow the science and to adhere to scientific principles. Yet there is neither scientific proof nor good evidence that board certification means anything in terms of competence, safety to the public, or quality of care. Doctors favor life-long learning, and continuing education has long been the standard and should remain so, not board certification or MOC. The mandatory continuing education required in every state to maintain a medical license is sufficient to prove doctors are current in their field of practice and to protect the public.
It is time for the medical community to admit that the emperor wears no clothes, and demand that the money grab of the ABMS and its affiliates be halted. This would result in greater access to care for patients and would reduce the cost of medical care, as the hundreds of millions being “stolen” from doctors today—costs that get passed on to patients—could be recouped and used for treating patients who clearly are in need and are being forgotten as the medical-industrial complex continues to flex its muscles and ensnare more of our national budget in its tentacles.
Neil S. Kaye, MD, DLFAPA
Hockessin, Delaware
While today “The Big Lie” mainly refers to the actions of the prior President, an older and bigger lie that has a real effect on every American is one perpetrated by our very own health care conglomerate. Americans pay the highest rates for health care on the planet; health care consumes about 17% of our gross domestic product.1 If we got higher-quality care, faster services, longer lives, or even greater consumer happiness, paying those rates might be worth it. But we don’t.
Worse yet is the idea that “board certification” assures the public that the doctor from whom they receive/purchase care is of a higher quality than one who is not so credentialed. That is our “Big Lie!” For decades, the public has been told that they should seek out board-certified doctors. Doctors in training have been told they must get board-certified. Hospitals brag about employing only board-certified doctors, insurers sometimes mandate board certification for a doctor to get paid, and employers use board certification as a benchmark for hiring and as a factor in compensation.
The sacred secret is that board certification makes no difference. There is no substantial evidence in any branch of medicine that doctors who are board-certified are better. There is no evidence that board-certified doctors get their patients healthier with more frequency, faster, less expensively, or with fewer medical errors than other doctors. The reality is that board certification is a sham. It’s a certificate granted after taking a very expensive test, and it is now part of an industry that is misleading the public and harming the trust the medical profession had once earned. Board certification is the equivalent of a diploma mill or an online certificate in any other field.
Why has this been kept under wraps for so long? Follow the money. The American Board of Medical Specialties (ABMS) oversees 24 specialty boards and reported revenue of $22.2M and expenses of $19.3M on its 2019 IRS Form 990.2 They make profit every year. But, looking further, these “not-for-profit” educational entities are sitting on hundreds of millions of dollars in their “foundations.” Take the American Board of Psychiatry and Neurology, for instance. They had more than $140M in assets in 2019.3 How is this possible? Easy. They have misled the American public and been remarkably successful convincing other organizations, such as the Joint Commission, the Accreditation Council for Graduate Medical Education, and the National Committee for Quality Assurance, that board certification is an assurance of quality. They charge high fees to “candidates” for taking the computer-based test and have developed a system called maintenance of certification (MOC) that is onerous, expensive, and serves as an annuity that forces doctors to pay annually to keep their board certification.
Medicine is a science. In the practice of our discipline, we are expected to follow the science and to adhere to scientific principles. Yet there is neither scientific proof nor good evidence that board certification means anything in terms of competence, safety to the public, or quality of care. Doctors favor life-long learning, and continuing education has long been the standard and should remain so, not board certification or MOC. The mandatory continuing education required in every state to maintain a medical license is sufficient to prove doctors are current in their field of practice and to protect the public.
It is time for the medical community to admit that the emperor wears no clothes, and demand that the money grab of the ABMS and its affiliates be halted. This would result in greater access to care for patients and would reduce the cost of medical care, as the hundreds of millions being “stolen” from doctors today—costs that get passed on to patients—could be recouped and used for treating patients who clearly are in need and are being forgotten as the medical-industrial complex continues to flex its muscles and ensnare more of our national budget in its tentacles.
Neil S. Kaye, MD, DLFAPA
Hockessin, Delaware
1. The World Bank. Current health expenditure (% of GDP). Accessed July 12, 2021. https://data.worldbank.org/indicator/SH.XPD.CHEX.GD.ZS
2. American Board of Medical Specialties. 2019 Form 990. Return of Organization Exempt From Income Tax. Accessed July 12, 2021. https://www.abms.org/wp-content/uploads/2021/01/2019-american-board-of-medical-specialties-form-990.pdf
3. ProPublica. American Board of Psychiatry and Neurology. Accessed July 13, 2021. https://projects.propublica.org/nonprofits/organizations/410654864
1. The World Bank. Current health expenditure (% of GDP). Accessed July 12, 2021. https://data.worldbank.org/indicator/SH.XPD.CHEX.GD.ZS
2. American Board of Medical Specialties. 2019 Form 990. Return of Organization Exempt From Income Tax. Accessed July 12, 2021. https://www.abms.org/wp-content/uploads/2021/01/2019-american-board-of-medical-specialties-form-990.pdf
3. ProPublica. American Board of Psychiatry and Neurology. Accessed July 13, 2021. https://projects.propublica.org/nonprofits/organizations/410654864
Workplace violence: Enhance your safety in outpatient settings
In the health care setting, workplace violence directed by patients against clinicians or other staff (eg, verbal or physical assaults) is common.1-3 Factors that contribute to violent incidents within mental health settings include communication problems, substance use, patients’ noncompliance with medications, procedural failures (administrative and legal), and a lack of resources.4
Being verbally or physically assaulted, stalked, or threatened by a patient is a reality for mental health professionals, especially in outpatient settings with limited resources and a lack of onsite security.5 Addressing the concerns outlined in this article can enhance your safety in outpatient settings. These steps should be customized for your practice with the possible assistance of legal counsel, risk management, and/or law enforcement.5
Plans and policies to mitigate the risk of violence. Assess for hazards within and around the workplace.5 Learn to assess your patient’s violence risk level in pre-screening interviews before their first appointment. Create a violence prevention and response plan, which may involve calling law enforcement if you fear for your safety or the safety of others.5 The confidentiality clauses of the Health Insurance Portability and Accountability Act make an exception to allow for disclosure to prevent or reduce a serious and substantial threat to the health or safety of an individual or society (you should limit your disclosure to pertinent nonclinical information).5,6 Develop policies and procedures to identify, communicate, track, and document patients’ concerning behaviors as well as policies and procedures to terminate care of patients who display these concerning behaviors.5 These plans and policies should include informing patients that neither violence nor threats of any kind will be tolerated. Frequently review these plans and policies with clinic personnel; these documents should be easily accessible to everyone (eg, posted on a board).
Communication and education. Keep open lines of communication with all clinic personnel, and encourage them to promptly report incidents and any concerning patient behaviors. Frequently check in with them about any safety concerns they have, and encourage them to suggest ways to reduce risks.7 Include discussions about safety during clinic meetings. Educate clinic personnel about the nonverbal warning signs of behavior escalation, and provide de-escalation and response training.5 Hold simulation drills so clinic personnel can become more familiar with the violence prevention and response plan.
Office safety. Install a security barrier between the waiting room and office spaces so that patients cannot easily barge into the office spaces. Ensure access to the office areas is restricted to clinic personnel using access card readers, electronic locks, locks with deadbolts, etc.5 Escort patients within the office and ensure that individuals who are not associated with the clinic are not permitted to enter any area of the office alone.5 Install video surveillance cameras at entrances, exits, and other strategic locations and post signs signaling their presence.5 Post signs stating that concealed weapons are not allowed on the premises. Install panic buttons in each office, at the reception desk, and other areas (eg, restrooms).5 Develop a code word or phrase that will allow front desk staff to know that you are in trouble when they call your office. Have a designated room in which staff can gather and lock themselves if they are not able to escape.5 Provide law enforcement with floor plans of the clinic to help expedite their response.2
Personal safety. During patient visits, position yourself so you can exit a room quickly if needed, and avoid having your back to the exit.5,7 Ensure the patient is not blocking the exit. Avoid wearing attire that can be used as a weapon against you, such as a tie or necklace, or can impede your escape, such as high heels.7 Avoid wearing valuable accessories that can be damaged or destroyed during a “take down.”7 Wear an audible alarm.5 Avoid posting personal information that is publicly accessible (eg, in the office or online) and may reveal your habits.5 Insist upon a “buddy system” in which no one works alone, including outside normal business hours, or goes to their car alone.5
1. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374(17):1661-1669.
2. Workplace violence: issues in response. Rugala EA, Issacs AR (eds). Critical Incident Response Group, National Center for Analysis of Violent Crime, FBI Academy. 2003. Accessed November 27, 2020. https://www.fbi.gov/file-repository/stats-services-publications-workplace-violence-workplace-violence/view
3. Velani KH. 2019 Healthcare Crime Survey. International Association for Healthcare Security and Safety – Foundation (IAHSS – Foundation). Accessed November 27, 2020. https://iahssf.org/crime-surveys/2019-healthcare-crime-survey/3/
4. O’Rourke M, Wrigley C, Hammond S. Violence within mental health services: how to enhance risk management. Risk Manag Healthc Policy. 2018;11:159-167.
5. Neal D. Seven actions to ensure safety in psychiatric office settings. Psychiatric News. 2020;55(7):15.
6. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
7. Xiong GL, Newman WJ. Take CAUTION in emergency and inpatient psychiatric settings. Current Psychiatry. 2013;12(7):9-10.
In the health care setting, workplace violence directed by patients against clinicians or other staff (eg, verbal or physical assaults) is common.1-3 Factors that contribute to violent incidents within mental health settings include communication problems, substance use, patients’ noncompliance with medications, procedural failures (administrative and legal), and a lack of resources.4
Being verbally or physically assaulted, stalked, or threatened by a patient is a reality for mental health professionals, especially in outpatient settings with limited resources and a lack of onsite security.5 Addressing the concerns outlined in this article can enhance your safety in outpatient settings. These steps should be customized for your practice with the possible assistance of legal counsel, risk management, and/or law enforcement.5
Plans and policies to mitigate the risk of violence. Assess for hazards within and around the workplace.5 Learn to assess your patient’s violence risk level in pre-screening interviews before their first appointment. Create a violence prevention and response plan, which may involve calling law enforcement if you fear for your safety or the safety of others.5 The confidentiality clauses of the Health Insurance Portability and Accountability Act make an exception to allow for disclosure to prevent or reduce a serious and substantial threat to the health or safety of an individual or society (you should limit your disclosure to pertinent nonclinical information).5,6 Develop policies and procedures to identify, communicate, track, and document patients’ concerning behaviors as well as policies and procedures to terminate care of patients who display these concerning behaviors.5 These plans and policies should include informing patients that neither violence nor threats of any kind will be tolerated. Frequently review these plans and policies with clinic personnel; these documents should be easily accessible to everyone (eg, posted on a board).
Communication and education. Keep open lines of communication with all clinic personnel, and encourage them to promptly report incidents and any concerning patient behaviors. Frequently check in with them about any safety concerns they have, and encourage them to suggest ways to reduce risks.7 Include discussions about safety during clinic meetings. Educate clinic personnel about the nonverbal warning signs of behavior escalation, and provide de-escalation and response training.5 Hold simulation drills so clinic personnel can become more familiar with the violence prevention and response plan.
Office safety. Install a security barrier between the waiting room and office spaces so that patients cannot easily barge into the office spaces. Ensure access to the office areas is restricted to clinic personnel using access card readers, electronic locks, locks with deadbolts, etc.5 Escort patients within the office and ensure that individuals who are not associated with the clinic are not permitted to enter any area of the office alone.5 Install video surveillance cameras at entrances, exits, and other strategic locations and post signs signaling their presence.5 Post signs stating that concealed weapons are not allowed on the premises. Install panic buttons in each office, at the reception desk, and other areas (eg, restrooms).5 Develop a code word or phrase that will allow front desk staff to know that you are in trouble when they call your office. Have a designated room in which staff can gather and lock themselves if they are not able to escape.5 Provide law enforcement with floor plans of the clinic to help expedite their response.2
Personal safety. During patient visits, position yourself so you can exit a room quickly if needed, and avoid having your back to the exit.5,7 Ensure the patient is not blocking the exit. Avoid wearing attire that can be used as a weapon against you, such as a tie or necklace, or can impede your escape, such as high heels.7 Avoid wearing valuable accessories that can be damaged or destroyed during a “take down.”7 Wear an audible alarm.5 Avoid posting personal information that is publicly accessible (eg, in the office or online) and may reveal your habits.5 Insist upon a “buddy system” in which no one works alone, including outside normal business hours, or goes to their car alone.5
In the health care setting, workplace violence directed by patients against clinicians or other staff (eg, verbal or physical assaults) is common.1-3 Factors that contribute to violent incidents within mental health settings include communication problems, substance use, patients’ noncompliance with medications, procedural failures (administrative and legal), and a lack of resources.4
Being verbally or physically assaulted, stalked, or threatened by a patient is a reality for mental health professionals, especially in outpatient settings with limited resources and a lack of onsite security.5 Addressing the concerns outlined in this article can enhance your safety in outpatient settings. These steps should be customized for your practice with the possible assistance of legal counsel, risk management, and/or law enforcement.5
Plans and policies to mitigate the risk of violence. Assess for hazards within and around the workplace.5 Learn to assess your patient’s violence risk level in pre-screening interviews before their first appointment. Create a violence prevention and response plan, which may involve calling law enforcement if you fear for your safety or the safety of others.5 The confidentiality clauses of the Health Insurance Portability and Accountability Act make an exception to allow for disclosure to prevent or reduce a serious and substantial threat to the health or safety of an individual or society (you should limit your disclosure to pertinent nonclinical information).5,6 Develop policies and procedures to identify, communicate, track, and document patients’ concerning behaviors as well as policies and procedures to terminate care of patients who display these concerning behaviors.5 These plans and policies should include informing patients that neither violence nor threats of any kind will be tolerated. Frequently review these plans and policies with clinic personnel; these documents should be easily accessible to everyone (eg, posted on a board).
Communication and education. Keep open lines of communication with all clinic personnel, and encourage them to promptly report incidents and any concerning patient behaviors. Frequently check in with them about any safety concerns they have, and encourage them to suggest ways to reduce risks.7 Include discussions about safety during clinic meetings. Educate clinic personnel about the nonverbal warning signs of behavior escalation, and provide de-escalation and response training.5 Hold simulation drills so clinic personnel can become more familiar with the violence prevention and response plan.
Office safety. Install a security barrier between the waiting room and office spaces so that patients cannot easily barge into the office spaces. Ensure access to the office areas is restricted to clinic personnel using access card readers, electronic locks, locks with deadbolts, etc.5 Escort patients within the office and ensure that individuals who are not associated with the clinic are not permitted to enter any area of the office alone.5 Install video surveillance cameras at entrances, exits, and other strategic locations and post signs signaling their presence.5 Post signs stating that concealed weapons are not allowed on the premises. Install panic buttons in each office, at the reception desk, and other areas (eg, restrooms).5 Develop a code word or phrase that will allow front desk staff to know that you are in trouble when they call your office. Have a designated room in which staff can gather and lock themselves if they are not able to escape.5 Provide law enforcement with floor plans of the clinic to help expedite their response.2
Personal safety. During patient visits, position yourself so you can exit a room quickly if needed, and avoid having your back to the exit.5,7 Ensure the patient is not blocking the exit. Avoid wearing attire that can be used as a weapon against you, such as a tie or necklace, or can impede your escape, such as high heels.7 Avoid wearing valuable accessories that can be damaged or destroyed during a “take down.”7 Wear an audible alarm.5 Avoid posting personal information that is publicly accessible (eg, in the office or online) and may reveal your habits.5 Insist upon a “buddy system” in which no one works alone, including outside normal business hours, or goes to their car alone.5
1. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374(17):1661-1669.
2. Workplace violence: issues in response. Rugala EA, Issacs AR (eds). Critical Incident Response Group, National Center for Analysis of Violent Crime, FBI Academy. 2003. Accessed November 27, 2020. https://www.fbi.gov/file-repository/stats-services-publications-workplace-violence-workplace-violence/view
3. Velani KH. 2019 Healthcare Crime Survey. International Association for Healthcare Security and Safety – Foundation (IAHSS – Foundation). Accessed November 27, 2020. https://iahssf.org/crime-surveys/2019-healthcare-crime-survey/3/
4. O’Rourke M, Wrigley C, Hammond S. Violence within mental health services: how to enhance risk management. Risk Manag Healthc Policy. 2018;11:159-167.
5. Neal D. Seven actions to ensure safety in psychiatric office settings. Psychiatric News. 2020;55(7):15.
6. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
7. Xiong GL, Newman WJ. Take CAUTION in emergency and inpatient psychiatric settings. Current Psychiatry. 2013;12(7):9-10.
1. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374(17):1661-1669.
2. Workplace violence: issues in response. Rugala EA, Issacs AR (eds). Critical Incident Response Group, National Center for Analysis of Violent Crime, FBI Academy. 2003. Accessed November 27, 2020. https://www.fbi.gov/file-repository/stats-services-publications-workplace-violence-workplace-violence/view
3. Velani KH. 2019 Healthcare Crime Survey. International Association for Healthcare Security and Safety – Foundation (IAHSS – Foundation). Accessed November 27, 2020. https://iahssf.org/crime-surveys/2019-healthcare-crime-survey/3/
4. O’Rourke M, Wrigley C, Hammond S. Violence within mental health services: how to enhance risk management. Risk Manag Healthc Policy. 2018;11:159-167.
5. Neal D. Seven actions to ensure safety in psychiatric office settings. Psychiatric News. 2020;55(7):15.
6. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
7. Xiong GL, Newman WJ. Take CAUTION in emergency and inpatient psychiatric settings. Current Psychiatry. 2013;12(7):9-10.
Writing letters for transgender patients undergoing medical transition
Transgender and nonconforming people are estimated to make up 0.3% to 1.4% of the population, and these estimates are likely undercounts.1 Knowingly or unknowingly, psychiatric and mental health clinicians are caring for transgender patients, and need to become familiar with ways to provide proper clinical care for this often-marginalized population. Being knowledgeable about the requirements for letter writing for patients who are transgender and desire to transition medically is one way that we can assist and affirm these individuals.
The World Professional Association for Transgender Health (WPATH) publishes standards of care (SOC) that discuss the role of mental health professionals during a patient’s gender transition.2 The initial mental health evaluation should establish if gender dysphoria exists, and not just assume that it does. It is also important to assess whether the patient has had past negative experiences in the treatment setting or a history of trauma, and to evaluate for stressors in social life. Some transgender people may present to mental health professionals solely for the purpose of pursuing gender-related services, and others do not. The transgender person may or may not choose to undergo hormone replacement therapy or surgical transition.
Within the United States, a small percentage of clinicians use the informed consent model and, instead of requiring letters for medical intervention, will conduct an assessment to determine if the patient can provide informed consent about the procedures. But because the WPATH SOC are considered the primary standards and insurance companies will not cover the surgeries without these letters, most surgeons will not accept a patient without this documentation.3
Letters for hormone therapy and upper body surgery
Adults need 1 letter of recommendation from a qualified mental health professional, and the following WPATH criteria must be met: 1) persistent, well-documented gender dysphoria, 2) capacity to make a fully informed decision to consent for treatment, 3) age of majority, and 4) if significant medical or mental health conditions are present, they must be reasonably well-controlled.4
The letter should contain identifying characteristics; diagnoses and psychosocial assessment; duration of clinical relationship; type of evaluation or therapy; an explanation that the criteria for hormone therapy have been met/clinical rationale (gender dysphoria, capacity to consent, age of majority, that other mental health conditions are reasonably well-controlled); a statement that informed consent had been obtained; and a statement that the referring clinician is available for coordination of care.
Letters for lower body surgery
WPATH recommends letters from 2 mental health clinicians who evaluated the patient. In addition to the criteria set for hormone therapy described above, the SOC recommend 12 months of continuous living in the gender role that is congruent with a patient’s gender identity before genital surgery. It is also suggested that the patient undergoes 12 months of hormone therapy before hysterectomy/oophorectomy in transgender men or before orchiectomy in transgender women.4
The letter should contain identifying characteristics; diagnoses and psychosocial assessment; duration of clinical relationship; type of evaluation or therapy; criteria for surgery/clinical rationale (gender dysphoria, capacity to consent, age of majority, other health concerns are well-controlled, hormone therapy, real-life experience), informed consent; and availability for coordination of care.
Transgender individuals need clinicians who can provide competent, sensitive health care, and gender affirmation can enhance psychological health.
1. Winter S, Diamond M, Green J, et al. Transgender people: health at the margins of society. Lancet. 2016;388(10042):390-400.
2. World Professional Association for Transgender Health. Standards of care for the health of transsexual, transgender, and gender nonconforming people. 7th version. Published 2012. Accessed July 14, 2021. https://www.wpath.org/publications/soc
3. Budge SL, Dickey LM. Barriers, challenges, and decision-making in the letter writing process for gender transition. Psychiatr Clin N Am. 2016;40(1):65-78.
4. Coleman E, Bocking W, Botzer M, et al. Standards of care for the health of transsexual, transgender and gender-nonconforming people. Version 7. International Journal of Transgenderism. 2011;13:165-232.
Transgender and nonconforming people are estimated to make up 0.3% to 1.4% of the population, and these estimates are likely undercounts.1 Knowingly or unknowingly, psychiatric and mental health clinicians are caring for transgender patients, and need to become familiar with ways to provide proper clinical care for this often-marginalized population. Being knowledgeable about the requirements for letter writing for patients who are transgender and desire to transition medically is one way that we can assist and affirm these individuals.
The World Professional Association for Transgender Health (WPATH) publishes standards of care (SOC) that discuss the role of mental health professionals during a patient’s gender transition.2 The initial mental health evaluation should establish if gender dysphoria exists, and not just assume that it does. It is also important to assess whether the patient has had past negative experiences in the treatment setting or a history of trauma, and to evaluate for stressors in social life. Some transgender people may present to mental health professionals solely for the purpose of pursuing gender-related services, and others do not. The transgender person may or may not choose to undergo hormone replacement therapy or surgical transition.
Within the United States, a small percentage of clinicians use the informed consent model and, instead of requiring letters for medical intervention, will conduct an assessment to determine if the patient can provide informed consent about the procedures. But because the WPATH SOC are considered the primary standards and insurance companies will not cover the surgeries without these letters, most surgeons will not accept a patient without this documentation.3
Letters for hormone therapy and upper body surgery
Adults need 1 letter of recommendation from a qualified mental health professional, and the following WPATH criteria must be met: 1) persistent, well-documented gender dysphoria, 2) capacity to make a fully informed decision to consent for treatment, 3) age of majority, and 4) if significant medical or mental health conditions are present, they must be reasonably well-controlled.4
The letter should contain identifying characteristics; diagnoses and psychosocial assessment; duration of clinical relationship; type of evaluation or therapy; an explanation that the criteria for hormone therapy have been met/clinical rationale (gender dysphoria, capacity to consent, age of majority, that other mental health conditions are reasonably well-controlled); a statement that informed consent had been obtained; and a statement that the referring clinician is available for coordination of care.
Letters for lower body surgery
WPATH recommends letters from 2 mental health clinicians who evaluated the patient. In addition to the criteria set for hormone therapy described above, the SOC recommend 12 months of continuous living in the gender role that is congruent with a patient’s gender identity before genital surgery. It is also suggested that the patient undergoes 12 months of hormone therapy before hysterectomy/oophorectomy in transgender men or before orchiectomy in transgender women.4
The letter should contain identifying characteristics; diagnoses and psychosocial assessment; duration of clinical relationship; type of evaluation or therapy; criteria for surgery/clinical rationale (gender dysphoria, capacity to consent, age of majority, other health concerns are well-controlled, hormone therapy, real-life experience), informed consent; and availability for coordination of care.
Transgender individuals need clinicians who can provide competent, sensitive health care, and gender affirmation can enhance psychological health.
Transgender and nonconforming people are estimated to make up 0.3% to 1.4% of the population, and these estimates are likely undercounts.1 Knowingly or unknowingly, psychiatric and mental health clinicians are caring for transgender patients, and need to become familiar with ways to provide proper clinical care for this often-marginalized population. Being knowledgeable about the requirements for letter writing for patients who are transgender and desire to transition medically is one way that we can assist and affirm these individuals.
The World Professional Association for Transgender Health (WPATH) publishes standards of care (SOC) that discuss the role of mental health professionals during a patient’s gender transition.2 The initial mental health evaluation should establish if gender dysphoria exists, and not just assume that it does. It is also important to assess whether the patient has had past negative experiences in the treatment setting or a history of trauma, and to evaluate for stressors in social life. Some transgender people may present to mental health professionals solely for the purpose of pursuing gender-related services, and others do not. The transgender person may or may not choose to undergo hormone replacement therapy or surgical transition.
Within the United States, a small percentage of clinicians use the informed consent model and, instead of requiring letters for medical intervention, will conduct an assessment to determine if the patient can provide informed consent about the procedures. But because the WPATH SOC are considered the primary standards and insurance companies will not cover the surgeries without these letters, most surgeons will not accept a patient without this documentation.3
Letters for hormone therapy and upper body surgery
Adults need 1 letter of recommendation from a qualified mental health professional, and the following WPATH criteria must be met: 1) persistent, well-documented gender dysphoria, 2) capacity to make a fully informed decision to consent for treatment, 3) age of majority, and 4) if significant medical or mental health conditions are present, they must be reasonably well-controlled.4
The letter should contain identifying characteristics; diagnoses and psychosocial assessment; duration of clinical relationship; type of evaluation or therapy; an explanation that the criteria for hormone therapy have been met/clinical rationale (gender dysphoria, capacity to consent, age of majority, that other mental health conditions are reasonably well-controlled); a statement that informed consent had been obtained; and a statement that the referring clinician is available for coordination of care.
Letters for lower body surgery
WPATH recommends letters from 2 mental health clinicians who evaluated the patient. In addition to the criteria set for hormone therapy described above, the SOC recommend 12 months of continuous living in the gender role that is congruent with a patient’s gender identity before genital surgery. It is also suggested that the patient undergoes 12 months of hormone therapy before hysterectomy/oophorectomy in transgender men or before orchiectomy in transgender women.4
The letter should contain identifying characteristics; diagnoses and psychosocial assessment; duration of clinical relationship; type of evaluation or therapy; criteria for surgery/clinical rationale (gender dysphoria, capacity to consent, age of majority, other health concerns are well-controlled, hormone therapy, real-life experience), informed consent; and availability for coordination of care.
Transgender individuals need clinicians who can provide competent, sensitive health care, and gender affirmation can enhance psychological health.
1. Winter S, Diamond M, Green J, et al. Transgender people: health at the margins of society. Lancet. 2016;388(10042):390-400.
2. World Professional Association for Transgender Health. Standards of care for the health of transsexual, transgender, and gender nonconforming people. 7th version. Published 2012. Accessed July 14, 2021. https://www.wpath.org/publications/soc
3. Budge SL, Dickey LM. Barriers, challenges, and decision-making in the letter writing process for gender transition. Psychiatr Clin N Am. 2016;40(1):65-78.
4. Coleman E, Bocking W, Botzer M, et al. Standards of care for the health of transsexual, transgender and gender-nonconforming people. Version 7. International Journal of Transgenderism. 2011;13:165-232.
1. Winter S, Diamond M, Green J, et al. Transgender people: health at the margins of society. Lancet. 2016;388(10042):390-400.
2. World Professional Association for Transgender Health. Standards of care for the health of transsexual, transgender, and gender nonconforming people. 7th version. Published 2012. Accessed July 14, 2021. https://www.wpath.org/publications/soc
3. Budge SL, Dickey LM. Barriers, challenges, and decision-making in the letter writing process for gender transition. Psychiatr Clin N Am. 2016;40(1):65-78.
4. Coleman E, Bocking W, Botzer M, et al. Standards of care for the health of transsexual, transgender and gender-nonconforming people. Version 7. International Journal of Transgenderism. 2011;13:165-232.
Building a better work/life balance
Physician burnout is a common and serious problem. In a 2017 survey of >14,000 US physicians across 27 specialties, 42% reported burnout,1 which typically is defined as a long-term stress reaction marked by emotional exhaustion, depersonalization, and a lack of sense of personal accomplishment.2
Creating a focused, yet comfortable professional life is essential for preventing burnout. For our patients’ sake and for our own personal fulfillment, there is much we can do to maintain a healthy professional and home life balance. This article describes the factors that contribute to physician burnout, and outlines steps you can take to improve your work/life balance.
The multifactorial roots of stress
Many physicians frequently blend their professional and personal lives. Most are absorbed in their practices, which leaves limited time for family interactions, daily life, or wellness.
Work hours are often long, and schedules are filled with obligations. In recent years, changes to medical practice have resulted in many additional responsibilities for physicians, such as administrative tasks and adapting to electronic health care, particularly to the use of electronic health records (EHRs). This has escalated workload and worries while diminishing patient interaction, creating more distant clinical relationships and providing less financial remuneration. Monetary pressures to see more patients limit the quality of care. Overload often forces physicians to stay at work late and/or labor excessive hours at home with less family interaction. The introduction of EHRs escalated this trend, while detracting from a healthy family, personal, and professional life. Addressing cumbersome documentation requirements while striving to maintain contact with patients is frustrating.3
Physician job dissatisfaction has worsened over time. Burnout escalates errors, diminishes patient rapport and safety, and produces suboptimal outcomes, all resulting in declining professional satisfaction.
Improving your work/life balance
The following strategies can help you make changes to better balance your professional and personal lives.
Assess priorities and goals. Before taking steps to achieve an optimal work-home balance, first review medical, spousal, and parental expectations. Social support is key.
Continue to: Identify stressors
Identify stressors. Use self-report questionnaires and collegial discussions to assess for the presence and/or severity of burnout. Prevention and/or intervention at personal and organizational levels can positively impact physician well-being.4
Focus on self-care. Prioritize your personal health care, sleep hygiene, exercise routines, quality of diet, and recreational activities. Do not self-prescribe medications, and avoid excessive alcohol use.5
Make changes to your practice. In your office, make efforts to maximize social connectiveness. Consider assigning routine tasks to other staff members. Upgrading your typing skills, employing medical records scribes, and/or using voice recording systems can reduce your workload.5
Advocate for better legislation. Both through professional medical organizations and at government levels, work to modify regulations that require physicians to spend their time on nonclinical tasks. This might include advocating to simplify EHRs and insurance company reimbursement requirements to decrease paperwork and reduce barriers to prescribing. Stress management seminars, which typically are offered at state and national conferences, can foster interpersonal and professional competencies throughout one’s medical career.6 Medical licensure boards should make efforts to reduce the stigma of reporting mental health issues; they should assure confidentiality protection and help for those who seek assistance.5
1. Peckham C. Medscape psychiatrist lifestyle report: race and ethnicity, bias and burnout. Medscape. Published January 11, 2017. Accessed July 12, 2021. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1
2. Agency for Healthcare Research and Quality. Physician burnout. Published July 2017. Accessed July 13, 2021. https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
3. Lippmann S. Can shrinks “shrink” the electronic health record? Internet and Psychiatry. December 19, 2019. Accessed on August 15, 2020. https://www.internetandpsychiatry.com/wp/editorials/can-shrinks-shrink-the-electronic-health-record/
4. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to prevent and reduce physician burnout: a systematic review and meta-analysis. Lancet. 2016;388(10057):2272-2281.
5. Mohanty D, Prabhu A, Lippmann S. Physician burnout: signs and solutions. J Fam Pract. 2019;68(8):442-446.
6. McCue JD, Sachs CL. A stress management workshop improves residents’ coping skills. Arch Intern Med. 1991;151(11):2273-2277.
Physician burnout is a common and serious problem. In a 2017 survey of >14,000 US physicians across 27 specialties, 42% reported burnout,1 which typically is defined as a long-term stress reaction marked by emotional exhaustion, depersonalization, and a lack of sense of personal accomplishment.2
Creating a focused, yet comfortable professional life is essential for preventing burnout. For our patients’ sake and for our own personal fulfillment, there is much we can do to maintain a healthy professional and home life balance. This article describes the factors that contribute to physician burnout, and outlines steps you can take to improve your work/life balance.
The multifactorial roots of stress
Many physicians frequently blend their professional and personal lives. Most are absorbed in their practices, which leaves limited time for family interactions, daily life, or wellness.
Work hours are often long, and schedules are filled with obligations. In recent years, changes to medical practice have resulted in many additional responsibilities for physicians, such as administrative tasks and adapting to electronic health care, particularly to the use of electronic health records (EHRs). This has escalated workload and worries while diminishing patient interaction, creating more distant clinical relationships and providing less financial remuneration. Monetary pressures to see more patients limit the quality of care. Overload often forces physicians to stay at work late and/or labor excessive hours at home with less family interaction. The introduction of EHRs escalated this trend, while detracting from a healthy family, personal, and professional life. Addressing cumbersome documentation requirements while striving to maintain contact with patients is frustrating.3
Physician job dissatisfaction has worsened over time. Burnout escalates errors, diminishes patient rapport and safety, and produces suboptimal outcomes, all resulting in declining professional satisfaction.
Improving your work/life balance
The following strategies can help you make changes to better balance your professional and personal lives.
Assess priorities and goals. Before taking steps to achieve an optimal work-home balance, first review medical, spousal, and parental expectations. Social support is key.
Continue to: Identify stressors
Identify stressors. Use self-report questionnaires and collegial discussions to assess for the presence and/or severity of burnout. Prevention and/or intervention at personal and organizational levels can positively impact physician well-being.4
Focus on self-care. Prioritize your personal health care, sleep hygiene, exercise routines, quality of diet, and recreational activities. Do not self-prescribe medications, and avoid excessive alcohol use.5
Make changes to your practice. In your office, make efforts to maximize social connectiveness. Consider assigning routine tasks to other staff members. Upgrading your typing skills, employing medical records scribes, and/or using voice recording systems can reduce your workload.5
Advocate for better legislation. Both through professional medical organizations and at government levels, work to modify regulations that require physicians to spend their time on nonclinical tasks. This might include advocating to simplify EHRs and insurance company reimbursement requirements to decrease paperwork and reduce barriers to prescribing. Stress management seminars, which typically are offered at state and national conferences, can foster interpersonal and professional competencies throughout one’s medical career.6 Medical licensure boards should make efforts to reduce the stigma of reporting mental health issues; they should assure confidentiality protection and help for those who seek assistance.5
Physician burnout is a common and serious problem. In a 2017 survey of >14,000 US physicians across 27 specialties, 42% reported burnout,1 which typically is defined as a long-term stress reaction marked by emotional exhaustion, depersonalization, and a lack of sense of personal accomplishment.2
Creating a focused, yet comfortable professional life is essential for preventing burnout. For our patients’ sake and for our own personal fulfillment, there is much we can do to maintain a healthy professional and home life balance. This article describes the factors that contribute to physician burnout, and outlines steps you can take to improve your work/life balance.
The multifactorial roots of stress
Many physicians frequently blend their professional and personal lives. Most are absorbed in their practices, which leaves limited time for family interactions, daily life, or wellness.
Work hours are often long, and schedules are filled with obligations. In recent years, changes to medical practice have resulted in many additional responsibilities for physicians, such as administrative tasks and adapting to electronic health care, particularly to the use of electronic health records (EHRs). This has escalated workload and worries while diminishing patient interaction, creating more distant clinical relationships and providing less financial remuneration. Monetary pressures to see more patients limit the quality of care. Overload often forces physicians to stay at work late and/or labor excessive hours at home with less family interaction. The introduction of EHRs escalated this trend, while detracting from a healthy family, personal, and professional life. Addressing cumbersome documentation requirements while striving to maintain contact with patients is frustrating.3
Physician job dissatisfaction has worsened over time. Burnout escalates errors, diminishes patient rapport and safety, and produces suboptimal outcomes, all resulting in declining professional satisfaction.
Improving your work/life balance
The following strategies can help you make changes to better balance your professional and personal lives.
Assess priorities and goals. Before taking steps to achieve an optimal work-home balance, first review medical, spousal, and parental expectations. Social support is key.
Continue to: Identify stressors
Identify stressors. Use self-report questionnaires and collegial discussions to assess for the presence and/or severity of burnout. Prevention and/or intervention at personal and organizational levels can positively impact physician well-being.4
Focus on self-care. Prioritize your personal health care, sleep hygiene, exercise routines, quality of diet, and recreational activities. Do not self-prescribe medications, and avoid excessive alcohol use.5
Make changes to your practice. In your office, make efforts to maximize social connectiveness. Consider assigning routine tasks to other staff members. Upgrading your typing skills, employing medical records scribes, and/or using voice recording systems can reduce your workload.5
Advocate for better legislation. Both through professional medical organizations and at government levels, work to modify regulations that require physicians to spend their time on nonclinical tasks. This might include advocating to simplify EHRs and insurance company reimbursement requirements to decrease paperwork and reduce barriers to prescribing. Stress management seminars, which typically are offered at state and national conferences, can foster interpersonal and professional competencies throughout one’s medical career.6 Medical licensure boards should make efforts to reduce the stigma of reporting mental health issues; they should assure confidentiality protection and help for those who seek assistance.5
1. Peckham C. Medscape psychiatrist lifestyle report: race and ethnicity, bias and burnout. Medscape. Published January 11, 2017. Accessed July 12, 2021. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1
2. Agency for Healthcare Research and Quality. Physician burnout. Published July 2017. Accessed July 13, 2021. https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
3. Lippmann S. Can shrinks “shrink” the electronic health record? Internet and Psychiatry. December 19, 2019. Accessed on August 15, 2020. https://www.internetandpsychiatry.com/wp/editorials/can-shrinks-shrink-the-electronic-health-record/
4. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to prevent and reduce physician burnout: a systematic review and meta-analysis. Lancet. 2016;388(10057):2272-2281.
5. Mohanty D, Prabhu A, Lippmann S. Physician burnout: signs and solutions. J Fam Pract. 2019;68(8):442-446.
6. McCue JD, Sachs CL. A stress management workshop improves residents’ coping skills. Arch Intern Med. 1991;151(11):2273-2277.
1. Peckham C. Medscape psychiatrist lifestyle report: race and ethnicity, bias and burnout. Medscape. Published January 11, 2017. Accessed July 12, 2021. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1
2. Agency for Healthcare Research and Quality. Physician burnout. Published July 2017. Accessed July 13, 2021. https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
3. Lippmann S. Can shrinks “shrink” the electronic health record? Internet and Psychiatry. December 19, 2019. Accessed on August 15, 2020. https://www.internetandpsychiatry.com/wp/editorials/can-shrinks-shrink-the-electronic-health-record/
4. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to prevent and reduce physician burnout: a systematic review and meta-analysis. Lancet. 2016;388(10057):2272-2281.
5. Mohanty D, Prabhu A, Lippmann S. Physician burnout: signs and solutions. J Fam Pract. 2019;68(8):442-446.
6. McCue JD, Sachs CL. A stress management workshop improves residents’ coping skills. Arch Intern Med. 1991;151(11):2273-2277.
Avoiding malpractice while treating depression in pregnant women
Many physicians have seen advertisements that encourage women who took an antidepressant while they were pregnant and had a negative outcome to contact a law firm. These ads could make patients more reluctant to take prescribed antidepressants, and psychiatrists more hesitant to prescribe necessary medications during pregnancy—which is a disservice to the mother and child.
More recently, several headline-grabbing studies appeared to suggest that there is an increased risk to infants who are exposed to antidepressants prenatally. Unfortunately, many patients do not understand that replication of these studies is often lacking, and methodological and confounding issues abound. All of this makes it difficult for patients and their families to know if they should take an antidepressant during pregnancy, and for psychiatrists to know what to discuss about the risks and benefits of various antidepressants during pregnancy. This article reviews the rationale for treatment of depression in pregnancy; the risks of untreated depression in pregnancy, as well as the potential risks of medication; ethical issues in the treatment of depression in pregnancy; the limitations of available research; and best approaches for practice.
Risks of untreated depression in pregnancy
Pregnant women may have misconceptions about treatment during pregnancy, and psychiatrists often are hesitant to treat pregnant women. However, the risks of untreated depression during pregnancy are even greater than the risks of untreated depression at other points in a woman’s life. In addition to general psychiatric risks seen in depression, pregnant women may experience other issues, such as preeclampsia and liver metabolism changes.1-2 Risks to the fetus related to untreated or partially treated mental health concerns include poor prenatal care related to poor self-care, an increased risk of exposure to illicit substances or alcohol related to “self-medication,” preterm delivery, and low birthweight (Table 13-8). Further risks for an infant of a mother with untreated depression include decreased cognitive performance and poor bonding with poor stress adaptation.5,6 Thus, appropriate treatment of depression is even more important during pregnancy than at other times of life.
Potential risks of treating depression in pregnancy
When prescribing psychotropic medications to a pregnant woman, there are several naturally occurring adverse outcomes to consider. For example, miscarriages, stillbirths, and congenital malformations can occur without explanation in the general population. In addition, also consider the specific health history of the mother and the available research literature regarding the specific psychotropic agent (keeping in mind that there are ethical issues associated with conducting prospective research in pregnant women, such as it being unethical to withhold treatment to pregnant women who are depressed in order to have a control group, and that retrospective research is often confounded by recall bias). Potential risks to be aware of include miscarriage (spontaneous abortion), malformation (teratogenesis, birth defects), preterm delivery, neonatal adaptation syndrome, and behavioral teratogenesis (Table 13-8).
Selective serotonin reuptake inhibitors (SSRIs), the usual medication treatment of choice for depression, have at times been implicated in adverse pregnancy outcomes, but no strong evidence suggests they increase the miscarriage rate. Overall data are reassuring regarding the risk of malformation associated with SSRI use. Of note, the FDA had switched paroxetine from a Class C drug to a Class D drug after early reports of a potential 1.5% to 2% risk of fetal cardiac malformations compared with a 1% baseline risk in the general population (these FDA pregnancy risk letter categories have since been phased out).9,10 Nevertheless, the absolute risk remains small. Another large study found that there was no substantial increased risk of cardiac malformations attributable to antidepressant use during the first trimester.11
Lessons from a class action suit
Since we last reviewed pregnancy and antidepressants in 2013,8 several class action lawsuits against the manufacturers of psychotropic medications have been heard. Product liability actions brought against manufacturers are different from medical malpractice suits brought against individual physicians, which may result from lack of informed consent, suicide, or homicide.
One of the largest class action suits was against Zoloft (specifically Zoloft and Pfizer, since the brand manufacturer is responsible for the product insert information.)12,13 At the time, sertraline was already commonly prescribed due to the relatively safe reproductive profile.
Continue to: Many of the more than 300...
Many of the more than 300 federal claims were united in a multi-district litigation (MDL) suit under the United States District Court of Eastern Pennsylvania (MDL 2342). Pfizer issued Daubert challenges (efforts to exclude the introduction of “junk science” into the courtroom) against the plaintiffs’ experts’ scientific methods and results.12,13 The plaintiffs (those suing Pfizer) had to prove that the medications caused the negative outcome, not that they were merely temporally associated. Subsequently, 2 plaintiff experts—a PharmD and a biostatistician—were removed. Pfizer successfully challenged the methodological soundness of the plaintiffs’ experts’ testimony (Table 212,13), and the case was dismissed. In general, the courts identified the Bradford Hill criteria as often being important (though not definitive) methodology for determining causation (Table 312,13).
A concept raised in prior psychotropic lawsuits was the “learned intermediary doctrine,” in which pharmaceutical companies stated that once a risk is known, it is the responsibility of the prescribing physician to assess risks vs benefits and inform the patient.8 Many aspects of the larger class action lawsuits related to failure of the company to do adequate research to identify risks and appropriately inform the public and the medical community of these risks.14
Challenges in interpreting the literature
Some of the difficulties in interpreting the literature on the association of antidepressants and birth defects can be seen in a 2020 study by Anderson et al.15 This study was published in JAMA Psychiatry, received widespread coverage in the media, and was discussed on the CDC’s website.16 Anderson et al15 compared a large cohort of 30,630 infants with birth defects from the multicenter case-control National Birth Defects Prevention Study with 11,478 randomly selected controls with no defects. Three primary study groups were women whose pregnancies resulted in:
- birth defects with no antidepressant exposure (n = 28,719)
- birth defects with exposure to an antidepressant (n = 1,911)
- no birth defect control group (n = 10,886 no antidepressant exposure, n = 592 antidepressant exposure).
This study reported there were “some associations between maternal antidepressant use and specific birth defects” and “Venlafaxine was associated with more birth defects than other antidepressants, which needs confirmation.”15 However, in an accompanying editorial, Wisner et al17 discussed potential problems and limitations with this study and research of this nature in general (Table 417). In addition, Anderson et al15 used certain “controversial” statistical practices.18 For example, “[T]o align with American Statistical Association guidelines to consider effect sizes when interpreting results instead of statistical significance, we noted associations as meaningfully elevated if [adjusted odds ratios] were 2.0 or greater and lower confidence interval bounds were 0.8 or greater.”15
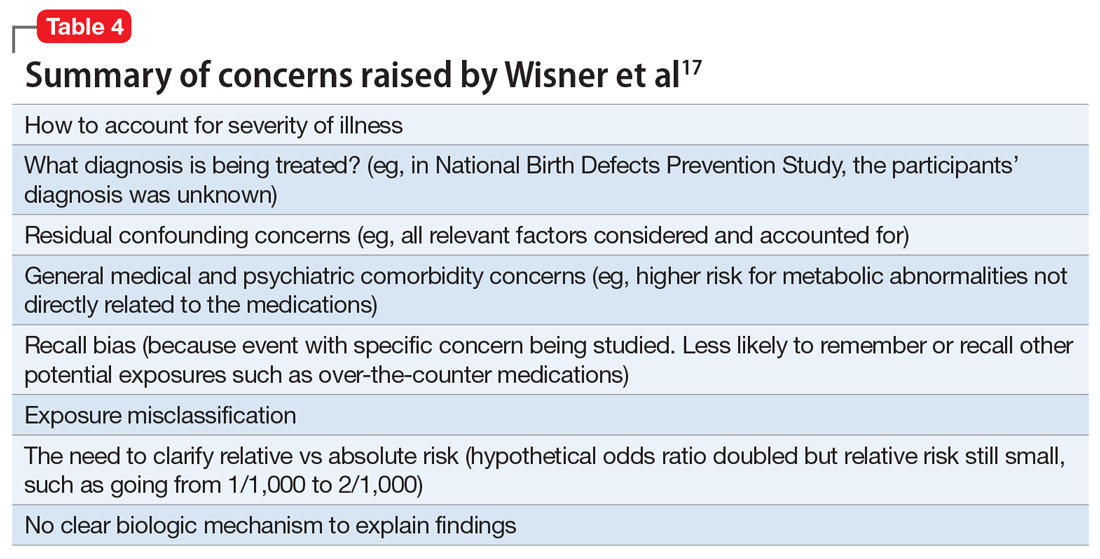
Those who read only abstracts or news stories may believe this study of >40,000 participants included a large number of women who were receiving venlafaxine. However, the number of pregnant women who were prescribed venlafaxine was actually very small—112 who took venlafaxine experienced a birth defect. In addition, the authors noted “Venlafaxine was associated with many of the same defects across the samples (data not shown).”15 As discussed above, historically one of the areas the courts have considered was whether or not appropriate methodology was applied, and whether the results could be replicated with the data provided.
Continue to: Further, new studies...
Further, new studies need to be considered in context of the literature as a whole and collective clinical experience. A recent systematic review found that among 3,186 infants exposed to venlafaxine during the first trimester, there were 107 major malformations.19 This indicated a relative risk estimate of 1.12, with a 95% CI of 0.92 to 1.35. The authors concluded that venlafaxine exposure in the first trimester was not associated with an increased risk of malformations.
Expectant parents may come across a headline that implies a specific antidepressant causes problems, but have not read the study or know how to interpret it. Often it is best for a physician to find out what the basis of the concern is, and if possible, review the study with the patient to make sure it is in the right context, and if it applies to the individual patient’s situation.
Consider the ethical issues
In addition to preventive ethics, other critical ethical issues in pregnancy include omission bias, beneficence, and autonomy.4,20-24 Omission bias occurs when physicians are more concerned about acts of commission (in which treatment leads to a negative outcome) than they are about acts of omission, which involve not treating the patient’s illness. To address this, it is important to discuss with the patient both the risks of treating and the risks of not treating maternal depression, so that the mother can make the best decision for her own specific set of circumstances.
Regarding beneficence (promoting the patient’s best interest), consider both the mother’s and the infant’s best interest, which usually are quite closely related. Women may feel guilty about taking a medication that they perceive is harmful for the fetus but good for their own mental health. Physicians can help with this by providing education about the benefits of treating depression for the fetus’ benefit as well. The fetus is completely dependent on the environment that the mother places them in, not merely the medication effects (eg, psychologic/physiologic stress effects, poor diet, lack of exercise, risk of “self-medication”).
Regarding autonomy (a woman’s own decision-making), Coverdale et al21 discussed strategies that can enhance a pregnant patient’s autonomy—including discussing treatment options and counselling about the effects of depression itself in pregnancy, as well as considering the effects of depression on the process of decision-making. For example, a woman with depression may see the world through a negative lens or may have difficulty concentrating. Patients may also require education about the concept of relative risk in comparison to absolute risk—especially in light of attention-grabbing headlines.
Continue to: Finally, as part of...
Finally, as part of preventative ethics, anticipate the ethical dilemmas before the common situation of pregnancy. Almost one-half of pregnancies are unplanned.25 Many women thus expose their fetus to medication during the critical early period of organogenesis, before noticing they were pregnant. Therefore, even if a patient of childbearing age insists that she is not sexually active, the prudent psychiatrist should still begin discussions about medications in pregnancy.
An outline of best practices
Best practice includes preventive ethics, and when treating any woman of childbearing age, psychiatrists should consider prescribing medications that are known to be relatively safe in pregnancy rather than risky in pregnancy. Therefore, any psychiatrist whose practice includes women of childbearing age should have a working knowledge of which agents are relatively safe in pregnancy. After a woman is pregnant, careful decision-making about medication should continue. Consult with reproductive psychiatry colleagues where necessary.
A patient with depression would usually merit closer follow-up during the pregnancy. In some cases, psychotherapy alone can be effective in depression. However, approximately 6% to 13% of women are prescribed antidepressants during pregnancy, and this has been increasing.26 Women who discontinue their antidepressant while pregnant are more likely to relapse than those who continue their medication,27 thus exposing their fetus to negative effects of depression as well as medication (prior to discontinuation).
When possible, monotherapy (one agent) in the lowest effective dose is often the judicious approach to treatment. For a patient prescribed pre-existing polypharmacy at time of pregnancy, a risk-benefit analysis of which medications should remain, which should be stopped, and a plan for taper, if needed, should be discussed and documented. Using too little of an antidepressant dose would expose the fetus to both depression and medication, whereas using a maximum dose when not needed would expose the fetus to more medication than is necessary to treat the mother’s symptoms. This discussion with the mother (and her partner, if available) should be documented in the chart. The mother should understand both the risk of untreated illness and the potential risks of medications, as well as the benefits of medications and alternatives. It is important for the mother to realize that there is no risk-free option, and that malformations can occur in the general population as well as in individuals with untreated depression, separate from any medication exposure. In fact, most malformations do not have a known cause, and overall approximately 3% of pregnancies result in a birth defect.28
If possible, discuss the treatment plan with the patient’s obstetrician, or ask the mother to discuss the plan with her obstetrician, so that everyone is on the same page. This discussion can help attenuate patient anxiety that results from hearing different things from different clinicians. Communication with other treating professionals (eg, OB/GYNs, pediatricians) can be beneficial and reduce liability if multiple physicians have agreed on a treatment plan—even if there is a negative outcome. With malpractice, a clinician is not necessarily at fault for a bad outcome or adverse effect, but is at fault for lack of informed consent or negligence (deviation from standard of care), which is harder for an attorney to demonstrate if there is deliberation, communication, and a plan that multiple doctors agree upon.
Continue to: Be aware that informed consent...
Be aware that informed consent is an ongoing process, and a woman may need to be reminded or informed of potential risks at varying stages of her life (eg, when starting a new relationship, getting married, etc.). Documentation can include that the clinician has discussed the risks, benefits, adverse effects, and alternatives of various medications, and a description of any patient-specific or medication-specific issues. In addition to verbal discussions, giving patients printed information can be helpful, as can directing them to appropriate websites (see Related Resources). Some physicians require patients to sign a form to indicate that they are aware of known risks.
Similar to being proactive before your patient becomes pregnant, think proactively regarding the postpartum period. Is your patient planning to breastfeed? Is the medication compatible with breastfeeding, or is bottle feeding the best option considering the mother’s specific circumstances? For example, developing severe symptoms, experiencing insomnia, needing to take a contraindicated medication, or having a vulnerable infant might sway a mother towards not breastfeeding. The expectant mother (and her partner, where possible) should be educated about postpartum risks and the importance of sleep in preventing postpartum depression.
Bottom Line
Concerns about being sued should not prevent appropriate care of depression in a woman who is pregnant. Discuss with your patient both the risk of untreated mental illness and the risk of medications to ensure she understands that avoiding antidepressants does not guarantee a safe or healthy pregnancy.
Related Resources
- MotherToBaby. www.mothertobaby.org/
- Centers for Disease Control and Prevention. Treating for two: medicine and pregnancy. www.cdc.gov/pregnancy/meds/treatingfortwo/index.html
- MGH Center for Women’s Mental Health. Reproductive psychiatry resource and information center. www.womensmentalhealth.org/
Drug Brand Names
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Palmsten K, Setoguchi S, Margulis AV, et al. Elevated risk of preeclampsia in pregnant women with depression: depression or antidepressants? Am J Epidemiol. 2012;175(10):988-997.
2. Sit DK, Perel JM, Helsel JC, et al. Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry. 2008;69(4):652-658.
3. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012-1024.
4. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
5. Friedman SH, Resnick PJ. Postpartum depression: an update. Women’s Health. 2009;5(3):287-295.
6. Liu Y, Kaaya S, Chai J, et al. Maternal depressive symptoms and early childhood cognitive development: a meta-analysis. Psychol Med. 2017;47(4):680-689.
7. Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009; 166(5):557-566.
8. Friedman SH, Hall RCW. Antidepressant use during pregnancy: How to avoid clinical and legal pitfalls. Current Psychiatry. 2013;12(2):21-25.
9. Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29(5):918-926.
10. Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749-752.
11. Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370(25):2397-2407.
12. In re: Zoloft (sertraline hydrochloride) products liability litigation. MDL No. 2342. No. 12-md-2342. United States District Court, E.D. Pennsylvania. June 27, 2014.
13. In re: Zoloft (sertraline hydrocloride) products liability litigation. MDL No. 2342. United States District Court, E.D. Pennsylvania. December 2, 2015.
14. Kirsch N, Pacheco LD, Hossain A, et al. Medicolegal review: perinatal Effexor lawsuits and legal strategies adverse to prescribing obstetric providers. AJP Rep. 2019;9(1):e88-e91.
15. Anderson KN, Lind JN, Simeone RM, et al. Maternal use of specific antidepressant medications during early pregnancy and the risk of selected birth defects. JAMA Psychiatry. 2020;77(12):1246-1255.
16. Centers for Disease Control and Prevention. Use of the antidepressant venlafaxine during early pregnancy may be linked to specific birth defects. Published October 28, 2020. Accessed October 29, 2020. https://www.cdc.gov/ncbddd/birthdefects/features/venlafaxine-during-pregnancy.html
17. Wisner KL, Oberlander TF, Huybrechts KF. The association between antidepressant exposure and birth defects--are we there yet? JAMA Psychiatry. 2020;77(12):1215-1216.
18. Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. American Statistician. 2016;70(2):129-133.
19. Lassen D, Ennis ZN, Damkier P. First-trimester pregnancy exposure to venlafaxine or duloxetine and risk of major congenital malformations: a systematic review. Basic Clin Pharmacol Toxicol. 2016;118(1):32-36.
20. Miller LJ. Ethical issues in perinatal mental health. Psychiatr Clin North Am. 2009;32(2):259-270.
21. Coverdale JH, McCullough JB, Chervenak FA. Enhancing decision-making by depressed pregnant patients. J Perinat Med. 2002;30(4):349-351.
22. Coverdale JH, McCullough LB, Chervenak FA, et al. Clinical implications of respect for autonomy in the psychiatric treatment of pregnant patients with depression. Psychiatr Serv. 1997;48:209-212.
23. Coverdale JH, Chervenak FA, McCullough LB, et al. Ethically justified clinically comprehensive guidelines for the management of the depressed pregnant patient. Am J Obstet Gynecol. 1996;174(1):169-173.
24. Wisner KL, Zarin DA, Holmboe ES, et al. Risk-benefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157(12):1933-1940.
25. Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84(5):478-485.
26. Cooper WO, Willy ME, Pont SJ, et al. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544.e1-5.
27. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
28. Centers for Disease Control and Prevention. Update on overall prevalence of major birth defects--Atlanta, Georgia, 1978-2005. MMWR Morb Mortal Wkly Rep. 2008;57(1):1-5.
Many physicians have seen advertisements that encourage women who took an antidepressant while they were pregnant and had a negative outcome to contact a law firm. These ads could make patients more reluctant to take prescribed antidepressants, and psychiatrists more hesitant to prescribe necessary medications during pregnancy—which is a disservice to the mother and child.
More recently, several headline-grabbing studies appeared to suggest that there is an increased risk to infants who are exposed to antidepressants prenatally. Unfortunately, many patients do not understand that replication of these studies is often lacking, and methodological and confounding issues abound. All of this makes it difficult for patients and their families to know if they should take an antidepressant during pregnancy, and for psychiatrists to know what to discuss about the risks and benefits of various antidepressants during pregnancy. This article reviews the rationale for treatment of depression in pregnancy; the risks of untreated depression in pregnancy, as well as the potential risks of medication; ethical issues in the treatment of depression in pregnancy; the limitations of available research; and best approaches for practice.
Risks of untreated depression in pregnancy
Pregnant women may have misconceptions about treatment during pregnancy, and psychiatrists often are hesitant to treat pregnant women. However, the risks of untreated depression during pregnancy are even greater than the risks of untreated depression at other points in a woman’s life. In addition to general psychiatric risks seen in depression, pregnant women may experience other issues, such as preeclampsia and liver metabolism changes.1-2 Risks to the fetus related to untreated or partially treated mental health concerns include poor prenatal care related to poor self-care, an increased risk of exposure to illicit substances or alcohol related to “self-medication,” preterm delivery, and low birthweight (Table 13-8). Further risks for an infant of a mother with untreated depression include decreased cognitive performance and poor bonding with poor stress adaptation.5,6 Thus, appropriate treatment of depression is even more important during pregnancy than at other times of life.
Potential risks of treating depression in pregnancy
When prescribing psychotropic medications to a pregnant woman, there are several naturally occurring adverse outcomes to consider. For example, miscarriages, stillbirths, and congenital malformations can occur without explanation in the general population. In addition, also consider the specific health history of the mother and the available research literature regarding the specific psychotropic agent (keeping in mind that there are ethical issues associated with conducting prospective research in pregnant women, such as it being unethical to withhold treatment to pregnant women who are depressed in order to have a control group, and that retrospective research is often confounded by recall bias). Potential risks to be aware of include miscarriage (spontaneous abortion), malformation (teratogenesis, birth defects), preterm delivery, neonatal adaptation syndrome, and behavioral teratogenesis (Table 13-8).
Selective serotonin reuptake inhibitors (SSRIs), the usual medication treatment of choice for depression, have at times been implicated in adverse pregnancy outcomes, but no strong evidence suggests they increase the miscarriage rate. Overall data are reassuring regarding the risk of malformation associated with SSRI use. Of note, the FDA had switched paroxetine from a Class C drug to a Class D drug after early reports of a potential 1.5% to 2% risk of fetal cardiac malformations compared with a 1% baseline risk in the general population (these FDA pregnancy risk letter categories have since been phased out).9,10 Nevertheless, the absolute risk remains small. Another large study found that there was no substantial increased risk of cardiac malformations attributable to antidepressant use during the first trimester.11
Lessons from a class action suit
Since we last reviewed pregnancy and antidepressants in 2013,8 several class action lawsuits against the manufacturers of psychotropic medications have been heard. Product liability actions brought against manufacturers are different from medical malpractice suits brought against individual physicians, which may result from lack of informed consent, suicide, or homicide.
One of the largest class action suits was against Zoloft (specifically Zoloft and Pfizer, since the brand manufacturer is responsible for the product insert information.)12,13 At the time, sertraline was already commonly prescribed due to the relatively safe reproductive profile.
Continue to: Many of the more than 300...
Many of the more than 300 federal claims were united in a multi-district litigation (MDL) suit under the United States District Court of Eastern Pennsylvania (MDL 2342). Pfizer issued Daubert challenges (efforts to exclude the introduction of “junk science” into the courtroom) against the plaintiffs’ experts’ scientific methods and results.12,13 The plaintiffs (those suing Pfizer) had to prove that the medications caused the negative outcome, not that they were merely temporally associated. Subsequently, 2 plaintiff experts—a PharmD and a biostatistician—were removed. Pfizer successfully challenged the methodological soundness of the plaintiffs’ experts’ testimony (Table 212,13), and the case was dismissed. In general, the courts identified the Bradford Hill criteria as often being important (though not definitive) methodology for determining causation (Table 312,13).

A concept raised in prior psychotropic lawsuits was the “learned intermediary doctrine,” in which pharmaceutical companies stated that once a risk is known, it is the responsibility of the prescribing physician to assess risks vs benefits and inform the patient.8 Many aspects of the larger class action lawsuits related to failure of the company to do adequate research to identify risks and appropriately inform the public and the medical community of these risks.14
Challenges in interpreting the literature
Some of the difficulties in interpreting the literature on the association of antidepressants and birth defects can be seen in a 2020 study by Anderson et al.15 This study was published in JAMA Psychiatry, received widespread coverage in the media, and was discussed on the CDC’s website.16 Anderson et al15 compared a large cohort of 30,630 infants with birth defects from the multicenter case-control National Birth Defects Prevention Study with 11,478 randomly selected controls with no defects. Three primary study groups were women whose pregnancies resulted in:
- birth defects with no antidepressant exposure (n = 28,719)
- birth defects with exposure to an antidepressant (n = 1,911)
- no birth defect control group (n = 10,886 no antidepressant exposure, n = 592 antidepressant exposure).
This study reported there were “some associations between maternal antidepressant use and specific birth defects” and “Venlafaxine was associated with more birth defects than other antidepressants, which needs confirmation.”15 However, in an accompanying editorial, Wisner et al17 discussed potential problems and limitations with this study and research of this nature in general (Table 417). In addition, Anderson et al15 used certain “controversial” statistical practices.18 For example, “[T]o align with American Statistical Association guidelines to consider effect sizes when interpreting results instead of statistical significance, we noted associations as meaningfully elevated if [adjusted odds ratios] were 2.0 or greater and lower confidence interval bounds were 0.8 or greater.”15

Those who read only abstracts or news stories may believe this study of >40,000 participants included a large number of women who were receiving venlafaxine. However, the number of pregnant women who were prescribed venlafaxine was actually very small—112 who took venlafaxine experienced a birth defect. In addition, the authors noted “Venlafaxine was associated with many of the same defects across the samples (data not shown).”15 As discussed above, historically one of the areas the courts have considered was whether or not appropriate methodology was applied, and whether the results could be replicated with the data provided.
Continue to: Further, new studies...
Further, new studies need to be considered in context of the literature as a whole and collective clinical experience. A recent systematic review found that among 3,186 infants exposed to venlafaxine during the first trimester, there were 107 major malformations.19 This indicated a relative risk estimate of 1.12, with a 95% CI of 0.92 to 1.35. The authors concluded that venlafaxine exposure in the first trimester was not associated with an increased risk of malformations.
Expectant parents may come across a headline that implies a specific antidepressant causes problems, but have not read the study or know how to interpret it. Often it is best for a physician to find out what the basis of the concern is, and if possible, review the study with the patient to make sure it is in the right context, and if it applies to the individual patient’s situation.
Consider the ethical issues
In addition to preventive ethics, other critical ethical issues in pregnancy include omission bias, beneficence, and autonomy.4,20-24 Omission bias occurs when physicians are more concerned about acts of commission (in which treatment leads to a negative outcome) than they are about acts of omission, which involve not treating the patient’s illness. To address this, it is important to discuss with the patient both the risks of treating and the risks of not treating maternal depression, so that the mother can make the best decision for her own specific set of circumstances.
Regarding beneficence (promoting the patient’s best interest), consider both the mother’s and the infant’s best interest, which usually are quite closely related. Women may feel guilty about taking a medication that they perceive is harmful for the fetus but good for their own mental health. Physicians can help with this by providing education about the benefits of treating depression for the fetus’ benefit as well. The fetus is completely dependent on the environment that the mother places them in, not merely the medication effects (eg, psychologic/physiologic stress effects, poor diet, lack of exercise, risk of “self-medication”).
Regarding autonomy (a woman’s own decision-making), Coverdale et al21 discussed strategies that can enhance a pregnant patient’s autonomy—including discussing treatment options and counselling about the effects of depression itself in pregnancy, as well as considering the effects of depression on the process of decision-making. For example, a woman with depression may see the world through a negative lens or may have difficulty concentrating. Patients may also require education about the concept of relative risk in comparison to absolute risk—especially in light of attention-grabbing headlines.
Continue to: Finally, as part of...
Finally, as part of preventative ethics, anticipate the ethical dilemmas before the common situation of pregnancy. Almost one-half of pregnancies are unplanned.25 Many women thus expose their fetus to medication during the critical early period of organogenesis, before noticing they were pregnant. Therefore, even if a patient of childbearing age insists that she is not sexually active, the prudent psychiatrist should still begin discussions about medications in pregnancy.
An outline of best practices
Best practice includes preventive ethics, and when treating any woman of childbearing age, psychiatrists should consider prescribing medications that are known to be relatively safe in pregnancy rather than risky in pregnancy. Therefore, any psychiatrist whose practice includes women of childbearing age should have a working knowledge of which agents are relatively safe in pregnancy. After a woman is pregnant, careful decision-making about medication should continue. Consult with reproductive psychiatry colleagues where necessary.
A patient with depression would usually merit closer follow-up during the pregnancy. In some cases, psychotherapy alone can be effective in depression. However, approximately 6% to 13% of women are prescribed antidepressants during pregnancy, and this has been increasing.26 Women who discontinue their antidepressant while pregnant are more likely to relapse than those who continue their medication,27 thus exposing their fetus to negative effects of depression as well as medication (prior to discontinuation).
When possible, monotherapy (one agent) in the lowest effective dose is often the judicious approach to treatment. For a patient prescribed pre-existing polypharmacy at time of pregnancy, a risk-benefit analysis of which medications should remain, which should be stopped, and a plan for taper, if needed, should be discussed and documented. Using too little of an antidepressant dose would expose the fetus to both depression and medication, whereas using a maximum dose when not needed would expose the fetus to more medication than is necessary to treat the mother’s symptoms. This discussion with the mother (and her partner, if available) should be documented in the chart. The mother should understand both the risk of untreated illness and the potential risks of medications, as well as the benefits of medications and alternatives. It is important for the mother to realize that there is no risk-free option, and that malformations can occur in the general population as well as in individuals with untreated depression, separate from any medication exposure. In fact, most malformations do not have a known cause, and overall approximately 3% of pregnancies result in a birth defect.28
If possible, discuss the treatment plan with the patient’s obstetrician, or ask the mother to discuss the plan with her obstetrician, so that everyone is on the same page. This discussion can help attenuate patient anxiety that results from hearing different things from different clinicians. Communication with other treating professionals (eg, OB/GYNs, pediatricians) can be beneficial and reduce liability if multiple physicians have agreed on a treatment plan—even if there is a negative outcome. With malpractice, a clinician is not necessarily at fault for a bad outcome or adverse effect, but is at fault for lack of informed consent or negligence (deviation from standard of care), which is harder for an attorney to demonstrate if there is deliberation, communication, and a plan that multiple doctors agree upon.
Continue to: Be aware that informed consent...
Be aware that informed consent is an ongoing process, and a woman may need to be reminded or informed of potential risks at varying stages of her life (eg, when starting a new relationship, getting married, etc.). Documentation can include that the clinician has discussed the risks, benefits, adverse effects, and alternatives of various medications, and a description of any patient-specific or medication-specific issues. In addition to verbal discussions, giving patients printed information can be helpful, as can directing them to appropriate websites (see Related Resources). Some physicians require patients to sign a form to indicate that they are aware of known risks.
Similar to being proactive before your patient becomes pregnant, think proactively regarding the postpartum period. Is your patient planning to breastfeed? Is the medication compatible with breastfeeding, or is bottle feeding the best option considering the mother’s specific circumstances? For example, developing severe symptoms, experiencing insomnia, needing to take a contraindicated medication, or having a vulnerable infant might sway a mother towards not breastfeeding. The expectant mother (and her partner, where possible) should be educated about postpartum risks and the importance of sleep in preventing postpartum depression.
Bottom Line
Concerns about being sued should not prevent appropriate care of depression in a woman who is pregnant. Discuss with your patient both the risk of untreated mental illness and the risk of medications to ensure she understands that avoiding antidepressants does not guarantee a safe or healthy pregnancy.
Related Resources
- MotherToBaby. www.mothertobaby.org/
- Centers for Disease Control and Prevention. Treating for two: medicine and pregnancy. www.cdc.gov/pregnancy/meds/treatingfortwo/index.html
- MGH Center for Women’s Mental Health. Reproductive psychiatry resource and information center. www.womensmentalhealth.org/
Drug Brand Names
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Many physicians have seen advertisements that encourage women who took an antidepressant while they were pregnant and had a negative outcome to contact a law firm. These ads could make patients more reluctant to take prescribed antidepressants, and psychiatrists more hesitant to prescribe necessary medications during pregnancy—which is a disservice to the mother and child.
More recently, several headline-grabbing studies appeared to suggest that there is an increased risk to infants who are exposed to antidepressants prenatally. Unfortunately, many patients do not understand that replication of these studies is often lacking, and methodological and confounding issues abound. All of this makes it difficult for patients and their families to know if they should take an antidepressant during pregnancy, and for psychiatrists to know what to discuss about the risks and benefits of various antidepressants during pregnancy. This article reviews the rationale for treatment of depression in pregnancy; the risks of untreated depression in pregnancy, as well as the potential risks of medication; ethical issues in the treatment of depression in pregnancy; the limitations of available research; and best approaches for practice.
Risks of untreated depression in pregnancy
Pregnant women may have misconceptions about treatment during pregnancy, and psychiatrists often are hesitant to treat pregnant women. However, the risks of untreated depression during pregnancy are even greater than the risks of untreated depression at other points in a woman’s life. In addition to general psychiatric risks seen in depression, pregnant women may experience other issues, such as preeclampsia and liver metabolism changes.1-2 Risks to the fetus related to untreated or partially treated mental health concerns include poor prenatal care related to poor self-care, an increased risk of exposure to illicit substances or alcohol related to “self-medication,” preterm delivery, and low birthweight (Table 13-8). Further risks for an infant of a mother with untreated depression include decreased cognitive performance and poor bonding with poor stress adaptation.5,6 Thus, appropriate treatment of depression is even more important during pregnancy than at other times of life.
Potential risks of treating depression in pregnancy
When prescribing psychotropic medications to a pregnant woman, there are several naturally occurring adverse outcomes to consider. For example, miscarriages, stillbirths, and congenital malformations can occur without explanation in the general population. In addition, also consider the specific health history of the mother and the available research literature regarding the specific psychotropic agent (keeping in mind that there are ethical issues associated with conducting prospective research in pregnant women, such as it being unethical to withhold treatment to pregnant women who are depressed in order to have a control group, and that retrospective research is often confounded by recall bias). Potential risks to be aware of include miscarriage (spontaneous abortion), malformation (teratogenesis, birth defects), preterm delivery, neonatal adaptation syndrome, and behavioral teratogenesis (Table 13-8).
Selective serotonin reuptake inhibitors (SSRIs), the usual medication treatment of choice for depression, have at times been implicated in adverse pregnancy outcomes, but no strong evidence suggests they increase the miscarriage rate. Overall data are reassuring regarding the risk of malformation associated with SSRI use. Of note, the FDA had switched paroxetine from a Class C drug to a Class D drug after early reports of a potential 1.5% to 2% risk of fetal cardiac malformations compared with a 1% baseline risk in the general population (these FDA pregnancy risk letter categories have since been phased out).9,10 Nevertheless, the absolute risk remains small. Another large study found that there was no substantial increased risk of cardiac malformations attributable to antidepressant use during the first trimester.11
Lessons from a class action suit
Since we last reviewed pregnancy and antidepressants in 2013,8 several class action lawsuits against the manufacturers of psychotropic medications have been heard. Product liability actions brought against manufacturers are different from medical malpractice suits brought against individual physicians, which may result from lack of informed consent, suicide, or homicide.
One of the largest class action suits was against Zoloft (specifically Zoloft and Pfizer, since the brand manufacturer is responsible for the product insert information.)12,13 At the time, sertraline was already commonly prescribed due to the relatively safe reproductive profile.
Continue to: Many of the more than 300...
Many of the more than 300 federal claims were united in a multi-district litigation (MDL) suit under the United States District Court of Eastern Pennsylvania (MDL 2342). Pfizer issued Daubert challenges (efforts to exclude the introduction of “junk science” into the courtroom) against the plaintiffs’ experts’ scientific methods and results.12,13 The plaintiffs (those suing Pfizer) had to prove that the medications caused the negative outcome, not that they were merely temporally associated. Subsequently, 2 plaintiff experts—a PharmD and a biostatistician—were removed. Pfizer successfully challenged the methodological soundness of the plaintiffs’ experts’ testimony (Table 212,13), and the case was dismissed. In general, the courts identified the Bradford Hill criteria as often being important (though not definitive) methodology for determining causation (Table 312,13).

A concept raised in prior psychotropic lawsuits was the “learned intermediary doctrine,” in which pharmaceutical companies stated that once a risk is known, it is the responsibility of the prescribing physician to assess risks vs benefits and inform the patient.8 Many aspects of the larger class action lawsuits related to failure of the company to do adequate research to identify risks and appropriately inform the public and the medical community of these risks.14
Challenges in interpreting the literature
Some of the difficulties in interpreting the literature on the association of antidepressants and birth defects can be seen in a 2020 study by Anderson et al.15 This study was published in JAMA Psychiatry, received widespread coverage in the media, and was discussed on the CDC’s website.16 Anderson et al15 compared a large cohort of 30,630 infants with birth defects from the multicenter case-control National Birth Defects Prevention Study with 11,478 randomly selected controls with no defects. Three primary study groups were women whose pregnancies resulted in:
- birth defects with no antidepressant exposure (n = 28,719)
- birth defects with exposure to an antidepressant (n = 1,911)
- no birth defect control group (n = 10,886 no antidepressant exposure, n = 592 antidepressant exposure).
This study reported there were “some associations between maternal antidepressant use and specific birth defects” and “Venlafaxine was associated with more birth defects than other antidepressants, which needs confirmation.”15 However, in an accompanying editorial, Wisner et al17 discussed potential problems and limitations with this study and research of this nature in general (Table 417). In addition, Anderson et al15 used certain “controversial” statistical practices.18 For example, “[T]o align with American Statistical Association guidelines to consider effect sizes when interpreting results instead of statistical significance, we noted associations as meaningfully elevated if [adjusted odds ratios] were 2.0 or greater and lower confidence interval bounds were 0.8 or greater.”15

Those who read only abstracts or news stories may believe this study of >40,000 participants included a large number of women who were receiving venlafaxine. However, the number of pregnant women who were prescribed venlafaxine was actually very small—112 who took venlafaxine experienced a birth defect. In addition, the authors noted “Venlafaxine was associated with many of the same defects across the samples (data not shown).”15 As discussed above, historically one of the areas the courts have considered was whether or not appropriate methodology was applied, and whether the results could be replicated with the data provided.
Continue to: Further, new studies...
Further, new studies need to be considered in context of the literature as a whole and collective clinical experience. A recent systematic review found that among 3,186 infants exposed to venlafaxine during the first trimester, there were 107 major malformations.19 This indicated a relative risk estimate of 1.12, with a 95% CI of 0.92 to 1.35. The authors concluded that venlafaxine exposure in the first trimester was not associated with an increased risk of malformations.
Expectant parents may come across a headline that implies a specific antidepressant causes problems, but have not read the study or know how to interpret it. Often it is best for a physician to find out what the basis of the concern is, and if possible, review the study with the patient to make sure it is in the right context, and if it applies to the individual patient’s situation.
Consider the ethical issues
In addition to preventive ethics, other critical ethical issues in pregnancy include omission bias, beneficence, and autonomy.4,20-24 Omission bias occurs when physicians are more concerned about acts of commission (in which treatment leads to a negative outcome) than they are about acts of omission, which involve not treating the patient’s illness. To address this, it is important to discuss with the patient both the risks of treating and the risks of not treating maternal depression, so that the mother can make the best decision for her own specific set of circumstances.
Regarding beneficence (promoting the patient’s best interest), consider both the mother’s and the infant’s best interest, which usually are quite closely related. Women may feel guilty about taking a medication that they perceive is harmful for the fetus but good for their own mental health. Physicians can help with this by providing education about the benefits of treating depression for the fetus’ benefit as well. The fetus is completely dependent on the environment that the mother places them in, not merely the medication effects (eg, psychologic/physiologic stress effects, poor diet, lack of exercise, risk of “self-medication”).
Regarding autonomy (a woman’s own decision-making), Coverdale et al21 discussed strategies that can enhance a pregnant patient’s autonomy—including discussing treatment options and counselling about the effects of depression itself in pregnancy, as well as considering the effects of depression on the process of decision-making. For example, a woman with depression may see the world through a negative lens or may have difficulty concentrating. Patients may also require education about the concept of relative risk in comparison to absolute risk—especially in light of attention-grabbing headlines.
Continue to: Finally, as part of...
Finally, as part of preventative ethics, anticipate the ethical dilemmas before the common situation of pregnancy. Almost one-half of pregnancies are unplanned.25 Many women thus expose their fetus to medication during the critical early period of organogenesis, before noticing they were pregnant. Therefore, even if a patient of childbearing age insists that she is not sexually active, the prudent psychiatrist should still begin discussions about medications in pregnancy.
An outline of best practices
Best practice includes preventive ethics, and when treating any woman of childbearing age, psychiatrists should consider prescribing medications that are known to be relatively safe in pregnancy rather than risky in pregnancy. Therefore, any psychiatrist whose practice includes women of childbearing age should have a working knowledge of which agents are relatively safe in pregnancy. After a woman is pregnant, careful decision-making about medication should continue. Consult with reproductive psychiatry colleagues where necessary.
A patient with depression would usually merit closer follow-up during the pregnancy. In some cases, psychotherapy alone can be effective in depression. However, approximately 6% to 13% of women are prescribed antidepressants during pregnancy, and this has been increasing.26 Women who discontinue their antidepressant while pregnant are more likely to relapse than those who continue their medication,27 thus exposing their fetus to negative effects of depression as well as medication (prior to discontinuation).
When possible, monotherapy (one agent) in the lowest effective dose is often the judicious approach to treatment. For a patient prescribed pre-existing polypharmacy at time of pregnancy, a risk-benefit analysis of which medications should remain, which should be stopped, and a plan for taper, if needed, should be discussed and documented. Using too little of an antidepressant dose would expose the fetus to both depression and medication, whereas using a maximum dose when not needed would expose the fetus to more medication than is necessary to treat the mother’s symptoms. This discussion with the mother (and her partner, if available) should be documented in the chart. The mother should understand both the risk of untreated illness and the potential risks of medications, as well as the benefits of medications and alternatives. It is important for the mother to realize that there is no risk-free option, and that malformations can occur in the general population as well as in individuals with untreated depression, separate from any medication exposure. In fact, most malformations do not have a known cause, and overall approximately 3% of pregnancies result in a birth defect.28
If possible, discuss the treatment plan with the patient’s obstetrician, or ask the mother to discuss the plan with her obstetrician, so that everyone is on the same page. This discussion can help attenuate patient anxiety that results from hearing different things from different clinicians. Communication with other treating professionals (eg, OB/GYNs, pediatricians) can be beneficial and reduce liability if multiple physicians have agreed on a treatment plan—even if there is a negative outcome. With malpractice, a clinician is not necessarily at fault for a bad outcome or adverse effect, but is at fault for lack of informed consent or negligence (deviation from standard of care), which is harder for an attorney to demonstrate if there is deliberation, communication, and a plan that multiple doctors agree upon.
Continue to: Be aware that informed consent...
Be aware that informed consent is an ongoing process, and a woman may need to be reminded or informed of potential risks at varying stages of her life (eg, when starting a new relationship, getting married, etc.). Documentation can include that the clinician has discussed the risks, benefits, adverse effects, and alternatives of various medications, and a description of any patient-specific or medication-specific issues. In addition to verbal discussions, giving patients printed information can be helpful, as can directing them to appropriate websites (see Related Resources). Some physicians require patients to sign a form to indicate that they are aware of known risks.
Similar to being proactive before your patient becomes pregnant, think proactively regarding the postpartum period. Is your patient planning to breastfeed? Is the medication compatible with breastfeeding, or is bottle feeding the best option considering the mother’s specific circumstances? For example, developing severe symptoms, experiencing insomnia, needing to take a contraindicated medication, or having a vulnerable infant might sway a mother towards not breastfeeding. The expectant mother (and her partner, where possible) should be educated about postpartum risks and the importance of sleep in preventing postpartum depression.
Bottom Line
Concerns about being sued should not prevent appropriate care of depression in a woman who is pregnant. Discuss with your patient both the risk of untreated mental illness and the risk of medications to ensure she understands that avoiding antidepressants does not guarantee a safe or healthy pregnancy.
Related Resources
- MotherToBaby. www.mothertobaby.org/
- Centers for Disease Control and Prevention. Treating for two: medicine and pregnancy. www.cdc.gov/pregnancy/meds/treatingfortwo/index.html
- MGH Center for Women’s Mental Health. Reproductive psychiatry resource and information center. www.womensmentalhealth.org/
Drug Brand Names
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Palmsten K, Setoguchi S, Margulis AV, et al. Elevated risk of preeclampsia in pregnant women with depression: depression or antidepressants? Am J Epidemiol. 2012;175(10):988-997.
2. Sit DK, Perel JM, Helsel JC, et al. Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry. 2008;69(4):652-658.
3. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012-1024.
4. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
5. Friedman SH, Resnick PJ. Postpartum depression: an update. Women’s Health. 2009;5(3):287-295.
6. Liu Y, Kaaya S, Chai J, et al. Maternal depressive symptoms and early childhood cognitive development: a meta-analysis. Psychol Med. 2017;47(4):680-689.
7. Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009; 166(5):557-566.
8. Friedman SH, Hall RCW. Antidepressant use during pregnancy: How to avoid clinical and legal pitfalls. Current Psychiatry. 2013;12(2):21-25.
9. Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29(5):918-926.
10. Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749-752.
11. Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370(25):2397-2407.
12. In re: Zoloft (sertraline hydrochloride) products liability litigation. MDL No. 2342. No. 12-md-2342. United States District Court, E.D. Pennsylvania. June 27, 2014.
13. In re: Zoloft (sertraline hydrocloride) products liability litigation. MDL No. 2342. United States District Court, E.D. Pennsylvania. December 2, 2015.
14. Kirsch N, Pacheco LD, Hossain A, et al. Medicolegal review: perinatal Effexor lawsuits and legal strategies adverse to prescribing obstetric providers. AJP Rep. 2019;9(1):e88-e91.
15. Anderson KN, Lind JN, Simeone RM, et al. Maternal use of specific antidepressant medications during early pregnancy and the risk of selected birth defects. JAMA Psychiatry. 2020;77(12):1246-1255.
16. Centers for Disease Control and Prevention. Use of the antidepressant venlafaxine during early pregnancy may be linked to specific birth defects. Published October 28, 2020. Accessed October 29, 2020. https://www.cdc.gov/ncbddd/birthdefects/features/venlafaxine-during-pregnancy.html
17. Wisner KL, Oberlander TF, Huybrechts KF. The association between antidepressant exposure and birth defects--are we there yet? JAMA Psychiatry. 2020;77(12):1215-1216.
18. Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. American Statistician. 2016;70(2):129-133.
19. Lassen D, Ennis ZN, Damkier P. First-trimester pregnancy exposure to venlafaxine or duloxetine and risk of major congenital malformations: a systematic review. Basic Clin Pharmacol Toxicol. 2016;118(1):32-36.
20. Miller LJ. Ethical issues in perinatal mental health. Psychiatr Clin North Am. 2009;32(2):259-270.
21. Coverdale JH, McCullough JB, Chervenak FA. Enhancing decision-making by depressed pregnant patients. J Perinat Med. 2002;30(4):349-351.
22. Coverdale JH, McCullough LB, Chervenak FA, et al. Clinical implications of respect for autonomy in the psychiatric treatment of pregnant patients with depression. Psychiatr Serv. 1997;48:209-212.
23. Coverdale JH, Chervenak FA, McCullough LB, et al. Ethically justified clinically comprehensive guidelines for the management of the depressed pregnant patient. Am J Obstet Gynecol. 1996;174(1):169-173.
24. Wisner KL, Zarin DA, Holmboe ES, et al. Risk-benefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157(12):1933-1940.
25. Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84(5):478-485.
26. Cooper WO, Willy ME, Pont SJ, et al. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544.e1-5.
27. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
28. Centers for Disease Control and Prevention. Update on overall prevalence of major birth defects--Atlanta, Georgia, 1978-2005. MMWR Morb Mortal Wkly Rep. 2008;57(1):1-5.
1. Palmsten K, Setoguchi S, Margulis AV, et al. Elevated risk of preeclampsia in pregnant women with depression: depression or antidepressants? Am J Epidemiol. 2012;175(10):988-997.
2. Sit DK, Perel JM, Helsel JC, et al. Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry. 2008;69(4):652-658.
3. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012-1024.
4. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
5. Friedman SH, Resnick PJ. Postpartum depression: an update. Women’s Health. 2009;5(3):287-295.
6. Liu Y, Kaaya S, Chai J, et al. Maternal depressive symptoms and early childhood cognitive development: a meta-analysis. Psychol Med. 2017;47(4):680-689.
7. Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009; 166(5):557-566.
8. Friedman SH, Hall RCW. Antidepressant use during pregnancy: How to avoid clinical and legal pitfalls. Current Psychiatry. 2013;12(2):21-25.
9. Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29(5):918-926.
10. Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749-752.
11. Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370(25):2397-2407.
12. In re: Zoloft (sertraline hydrochloride) products liability litigation. MDL No. 2342. No. 12-md-2342. United States District Court, E.D. Pennsylvania. June 27, 2014.
13. In re: Zoloft (sertraline hydrocloride) products liability litigation. MDL No. 2342. United States District Court, E.D. Pennsylvania. December 2, 2015.
14. Kirsch N, Pacheco LD, Hossain A, et al. Medicolegal review: perinatal Effexor lawsuits and legal strategies adverse to prescribing obstetric providers. AJP Rep. 2019;9(1):e88-e91.
15. Anderson KN, Lind JN, Simeone RM, et al. Maternal use of specific antidepressant medications during early pregnancy and the risk of selected birth defects. JAMA Psychiatry. 2020;77(12):1246-1255.
16. Centers for Disease Control and Prevention. Use of the antidepressant venlafaxine during early pregnancy may be linked to specific birth defects. Published October 28, 2020. Accessed October 29, 2020. https://www.cdc.gov/ncbddd/birthdefects/features/venlafaxine-during-pregnancy.html
17. Wisner KL, Oberlander TF, Huybrechts KF. The association between antidepressant exposure and birth defects--are we there yet? JAMA Psychiatry. 2020;77(12):1215-1216.
18. Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. American Statistician. 2016;70(2):129-133.
19. Lassen D, Ennis ZN, Damkier P. First-trimester pregnancy exposure to venlafaxine or duloxetine and risk of major congenital malformations: a systematic review. Basic Clin Pharmacol Toxicol. 2016;118(1):32-36.
20. Miller LJ. Ethical issues in perinatal mental health. Psychiatr Clin North Am. 2009;32(2):259-270.
21. Coverdale JH, McCullough JB, Chervenak FA. Enhancing decision-making by depressed pregnant patients. J Perinat Med. 2002;30(4):349-351.
22. Coverdale JH, McCullough LB, Chervenak FA, et al. Clinical implications of respect for autonomy in the psychiatric treatment of pregnant patients with depression. Psychiatr Serv. 1997;48:209-212.
23. Coverdale JH, Chervenak FA, McCullough LB, et al. Ethically justified clinically comprehensive guidelines for the management of the depressed pregnant patient. Am J Obstet Gynecol. 1996;174(1):169-173.
24. Wisner KL, Zarin DA, Holmboe ES, et al. Risk-benefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157(12):1933-1940.
25. Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84(5):478-485.
26. Cooper WO, Willy ME, Pont SJ, et al. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544.e1-5.
27. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
28. Centers for Disease Control and Prevention. Update on overall prevalence of major birth defects--Atlanta, Georgia, 1978-2005. MMWR Morb Mortal Wkly Rep. 2008;57(1):1-5.
My palliative care rotation: Lessons of gratitude, mindfulness, and kindness
As a psychiatry resident and as a part of consultation-liaison service, I have visited many palliative care patients to assist other physicians in managing psychiatric issues such as depression, anxiety, or delirium. But recently, as the first resident from our Department of Psychiatry who was sent to a palliative care rotation, I followed these patients as a part of a primary palliative care team. Doing so allowed me to see patients from the other side of the bridge.
Palliative care focuses on providing relief from the suffering and stress of a patient’s illness, with the primary goal of improving the quality of life of the patient and their families. The palliative care team works in collaboration with the patient’s other clinicians to provide an extra layer of support. They provide biopsychosociocultural interventions that are in harmony with the needs of the patient rather than the prognosis of the illness. To do so, they first must evaluate the needs of the patient and their family. This is a time-consuming, energy-consuming, emotionally draining job.
During my palliative care rotation, I attended table rounds, bedside rounds, family meetings, long counseling sessions, and disposition planning meetings. This rotation also gave me the opportunity to place my feet in the shoes of a palliative care team and to reflect on how it feels to be the physician of a patient who is dying, which as a psychiatric resident I had seldom experienced. I learned that although working with patients who are dying can cause stress, burnout, and compassion fatigue, it also helps physicians appreciate the little things in life. To appreciate all the blessings we have that we usually take for granted. To practice gratitude. To be kind.
Upon reflection, I learned that the rounds of palliative care are actually mindfulness-based discussions that provide cushions of supportive work, facilitate feelings of being in control, tend to alleviate physical as well as mental suffering, foster clear-sighted hope, and assist in establishing small, subjectively significant, realistic goals for the patient’s immediate future, and to help the patient achieve these goals.
A valuable lesson from a patient
I want to highlight a case of a 65-year-old woman I first visited while I was shadowing my attending, who had been providing palliative care to the patient and her family for several months. The patient was admitted to a tertiary care hospital because cancer had invaded her small bowel and caused mechanical obstruction, resulting in intractable vomiting, abdominal distension, and anorexia. She underwent open laparotomy and ileostomy for symptomatic relief. A nasogastric tube was placed, and she was put on total parenteral nutrition. The day I met her was her third postoperative day. She had been improving significantly, and she wanted to eat. She was missing food. Most of the discussion in the round among my attending, the patient, and her family was centered around how to get to the point where she would be able to eat again and appreciate the taste of biryani.
What my attending did was incredible. After assessing the patient’s needs, he instilled a realistic hope: the hope of tasting food again. The attending, while acknowledging the patient’s apprehensions, respectfully and supportively kept her from wandering into the future, made every possible attempt to bring her attention back to the present moment, and helped her establish goals for the present and her immediate future. My attending was not toxic-positive, forcing his patient to uselessly revisit her current trauma. Instead, he was kind, empathic, and considerate. His primary focus was to understand rather than to be understood, to help her find meaning, and to improve her quality of life—a quality she defined for herself, which was to taste the food of her choice.
That day, when I returned to my working station in the psychiatry ward and had lunch in the break room, I thought, “When I eat, how often do I think about eating?” Mostly I either think about work, tasks, and presentations, or I scroll on social media.
Our taste buds indeed get adapted to repetitive stimulation, but the experience of eating our favorite dish is the naked truth of being alive, and is something that I have been taking for granted for a long time. These are little things in life that I need to appreciate, and learn to cultivate their power.
As a psychiatry resident and as a part of consultation-liaison service, I have visited many palliative care patients to assist other physicians in managing psychiatric issues such as depression, anxiety, or delirium. But recently, as the first resident from our Department of Psychiatry who was sent to a palliative care rotation, I followed these patients as a part of a primary palliative care team. Doing so allowed me to see patients from the other side of the bridge.
Palliative care focuses on providing relief from the suffering and stress of a patient’s illness, with the primary goal of improving the quality of life of the patient and their families. The palliative care team works in collaboration with the patient’s other clinicians to provide an extra layer of support. They provide biopsychosociocultural interventions that are in harmony with the needs of the patient rather than the prognosis of the illness. To do so, they first must evaluate the needs of the patient and their family. This is a time-consuming, energy-consuming, emotionally draining job.
During my palliative care rotation, I attended table rounds, bedside rounds, family meetings, long counseling sessions, and disposition planning meetings. This rotation also gave me the opportunity to place my feet in the shoes of a palliative care team and to reflect on how it feels to be the physician of a patient who is dying, which as a psychiatric resident I had seldom experienced. I learned that although working with patients who are dying can cause stress, burnout, and compassion fatigue, it also helps physicians appreciate the little things in life. To appreciate all the blessings we have that we usually take for granted. To practice gratitude. To be kind.
Upon reflection, I learned that the rounds of palliative care are actually mindfulness-based discussions that provide cushions of supportive work, facilitate feelings of being in control, tend to alleviate physical as well as mental suffering, foster clear-sighted hope, and assist in establishing small, subjectively significant, realistic goals for the patient’s immediate future, and to help the patient achieve these goals.
A valuable lesson from a patient
I want to highlight a case of a 65-year-old woman I first visited while I was shadowing my attending, who had been providing palliative care to the patient and her family for several months. The patient was admitted to a tertiary care hospital because cancer had invaded her small bowel and caused mechanical obstruction, resulting in intractable vomiting, abdominal distension, and anorexia. She underwent open laparotomy and ileostomy for symptomatic relief. A nasogastric tube was placed, and she was put on total parenteral nutrition. The day I met her was her third postoperative day. She had been improving significantly, and she wanted to eat. She was missing food. Most of the discussion in the round among my attending, the patient, and her family was centered around how to get to the point where she would be able to eat again and appreciate the taste of biryani.
What my attending did was incredible. After assessing the patient’s needs, he instilled a realistic hope: the hope of tasting food again. The attending, while acknowledging the patient’s apprehensions, respectfully and supportively kept her from wandering into the future, made every possible attempt to bring her attention back to the present moment, and helped her establish goals for the present and her immediate future. My attending was not toxic-positive, forcing his patient to uselessly revisit her current trauma. Instead, he was kind, empathic, and considerate. His primary focus was to understand rather than to be understood, to help her find meaning, and to improve her quality of life—a quality she defined for herself, which was to taste the food of her choice.
That day, when I returned to my working station in the psychiatry ward and had lunch in the break room, I thought, “When I eat, how often do I think about eating?” Mostly I either think about work, tasks, and presentations, or I scroll on social media.
Our taste buds indeed get adapted to repetitive stimulation, but the experience of eating our favorite dish is the naked truth of being alive, and is something that I have been taking for granted for a long time. These are little things in life that I need to appreciate, and learn to cultivate their power.
As a psychiatry resident and as a part of consultation-liaison service, I have visited many palliative care patients to assist other physicians in managing psychiatric issues such as depression, anxiety, or delirium. But recently, as the first resident from our Department of Psychiatry who was sent to a palliative care rotation, I followed these patients as a part of a primary palliative care team. Doing so allowed me to see patients from the other side of the bridge.
Palliative care focuses on providing relief from the suffering and stress of a patient’s illness, with the primary goal of improving the quality of life of the patient and their families. The palliative care team works in collaboration with the patient’s other clinicians to provide an extra layer of support. They provide biopsychosociocultural interventions that are in harmony with the needs of the patient rather than the prognosis of the illness. To do so, they first must evaluate the needs of the patient and their family. This is a time-consuming, energy-consuming, emotionally draining job.
During my palliative care rotation, I attended table rounds, bedside rounds, family meetings, long counseling sessions, and disposition planning meetings. This rotation also gave me the opportunity to place my feet in the shoes of a palliative care team and to reflect on how it feels to be the physician of a patient who is dying, which as a psychiatric resident I had seldom experienced. I learned that although working with patients who are dying can cause stress, burnout, and compassion fatigue, it also helps physicians appreciate the little things in life. To appreciate all the blessings we have that we usually take for granted. To practice gratitude. To be kind.
Upon reflection, I learned that the rounds of palliative care are actually mindfulness-based discussions that provide cushions of supportive work, facilitate feelings of being in control, tend to alleviate physical as well as mental suffering, foster clear-sighted hope, and assist in establishing small, subjectively significant, realistic goals for the patient’s immediate future, and to help the patient achieve these goals.
A valuable lesson from a patient
I want to highlight a case of a 65-year-old woman I first visited while I was shadowing my attending, who had been providing palliative care to the patient and her family for several months. The patient was admitted to a tertiary care hospital because cancer had invaded her small bowel and caused mechanical obstruction, resulting in intractable vomiting, abdominal distension, and anorexia. She underwent open laparotomy and ileostomy for symptomatic relief. A nasogastric tube was placed, and she was put on total parenteral nutrition. The day I met her was her third postoperative day. She had been improving significantly, and she wanted to eat. She was missing food. Most of the discussion in the round among my attending, the patient, and her family was centered around how to get to the point where she would be able to eat again and appreciate the taste of biryani.
What my attending did was incredible. After assessing the patient’s needs, he instilled a realistic hope: the hope of tasting food again. The attending, while acknowledging the patient’s apprehensions, respectfully and supportively kept her from wandering into the future, made every possible attempt to bring her attention back to the present moment, and helped her establish goals for the present and her immediate future. My attending was not toxic-positive, forcing his patient to uselessly revisit her current trauma. Instead, he was kind, empathic, and considerate. His primary focus was to understand rather than to be understood, to help her find meaning, and to improve her quality of life—a quality she defined for herself, which was to taste the food of her choice.
That day, when I returned to my working station in the psychiatry ward and had lunch in the break room, I thought, “When I eat, how often do I think about eating?” Mostly I either think about work, tasks, and presentations, or I scroll on social media.
Our taste buds indeed get adapted to repetitive stimulation, but the experience of eating our favorite dish is the naked truth of being alive, and is something that I have been taking for granted for a long time. These are little things in life that I need to appreciate, and learn to cultivate their power.
Becoming vaccine ambassadors: A new role for psychiatrists
After more than 600,000 deaths in the United States from the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), several safe and effective vaccines against the virus have become available. Vaccines are the most effective preventive measure against COVID-19 and the most promising way to achieve herd immunity to end the current pandemic. However, obstacles to reaching this goal include vaccine skepticism, structural barriers, or simple inertia to get vaccinated. These challenges provide opportunities for psychiatrists to use their medical knowledge and expertise, applying behavior management techniques such as motivational interviewing and nudging to encourage their patients to get vaccinated. In particular, marginalized patients with serious mental illness (SMI), who are subject to disproportionately high rates of COVID-19 infection and more severe outcomes,1 have much to gain if psychiatrists become involved in the COVID-19 vaccination campaign.
In this article, we define vaccine hesitancy and highlight what makes psychiatrists ideal vaccine ambassadors, given their unique skill set and longitudinal, trust-based connection with their patients. We expand on the particular vulnerabilities of patients with SMI, including structural barriers to vaccination that lead to health disparities and inequity. Finally, building on “The ABCs of successful vaccinations” framework published in
What is vaccine hesitancy?
The World Health Organization (WHO) defines vaccine hesitancy as a “delay in acceptance or refusal of vaccines despite availability of vaccine services.”3,4 Vaccine hesitancy occurs on a continuum ranging from uncertainty about accepting a vaccine to absolute refusal.4,5 It involves a complex decision-making process driven by contextual, individual, and social influences, and vaccine-specific issues.4 In the “3C” model developed by the WHO Strategic Advisory Group of Experts (SAGE) Working Group, vaccine hesitancy is influenced by confidence (trust in vaccines, in the health care system, and in policy makers), complacency (lower perceived risk), and convenience (availability, affordability, accessibility, language and health literacy, appeal of vaccination program).4
In 2019, the WHO named vaccine hesitancy as one of the top 10 global health threats.3 Hesitancy to receive COVID-19 vaccines may be particularly high because of their rapid development. In addition, the tumultuous political environment that often featured inconsistent messaging about the virus, its dangers, and its transmission since the early days of the pandemic created widespread public confusion and doubt as scientific understandings evolved. “Anti-vaxxer” movements that completely rejected vaccine efficacy disseminated misinformation online. Followers of these movements may have such extreme overvalued ideas that any effort to persuade them otherwise with scientific evidence will accomplish very little.6,7 Therefore, focusing on individuals who are “sitting on the fence” about getting vaccinated can be more productive because they represent a much larger group than those who adamantly refuse vaccines, and they may be more amenable to changing beliefs and behaviors.8
The US Census Bureau’s Household Pulse Survey asked, “How likely are you to accept the vaccine?”9 As of late June 2021, 11.4% of US adults reported they would “definitely not get a vaccine” or “probably not get a vaccine,” and that number increases to 16.9% when including those who are “unsure,” although there is wide geographical variability.10
A recent study in Denmark showed that willingness to receive the COVID-19 vaccine was slightly lower among patients with mental illness (84.8%) compared with the general population (89.5%).11 Given the small difference, vaccine hesitancy was not considered to be a major barrier for vaccination among patients with mental illness in Denmark. This is similar to the findings of a pre-pandemic study at a community mental health clinic in the United States involving other vaccinations, which suggested that 84% of patients with SMI perceived vaccinations as safe, effective, and important.12 In this clinic, identified barriers to vaccinations in general among patients with SMI included lack of awareness and knowledge (42.2%), accessibility (16.3%), personal cost (13.3%), fears about immunization (10.4%), and lack of recommendations by primary care providers (PCPs) (1.5%).12
It is critical to distinguish attitude-driven vaccine hesitancy from a lack of education and opportunity to receive a vaccine. Particularly disadvantaged communities may be mislabeled as “vaccine hesitant” when in fact they may not have the ability to be as proactive as other population groups (eg, difficulty scheduling appointments over the Internet).
Continue to: What makes psychiatrists ideal vaccine ambassadors?
What makes psychiatrists ideal vaccine ambassadors?
There are several reasons psychiatrists can be well-positioned to contribute to the success of vaccination campaigns (Table 1). These include their frequent contact with patients and their care teams, the high trust those patients have in them, and their medical expertise and skills in applied behavioral and social science techniques, including motivational interviewing and nudging. Vaccination efforts and outreach are more effective when led by the clinician with whom the patient has the most contact because resolving vaccine hesitancy is not a one-time discussion but requires ongoing communication, persistence, and consistency.13 Patients may contact their psychiatrists more frequently than their other clinicians, including PCPs. For this reason, psychiatrists can serve as the gateway to health care, particularly for patients with SMI.14 In addition, interruptions in nonemergency services caused by the COVID-19 pandemic may affect vaccine delivery because patients may have been unable to see their PCPs regularly during the pandemic.15
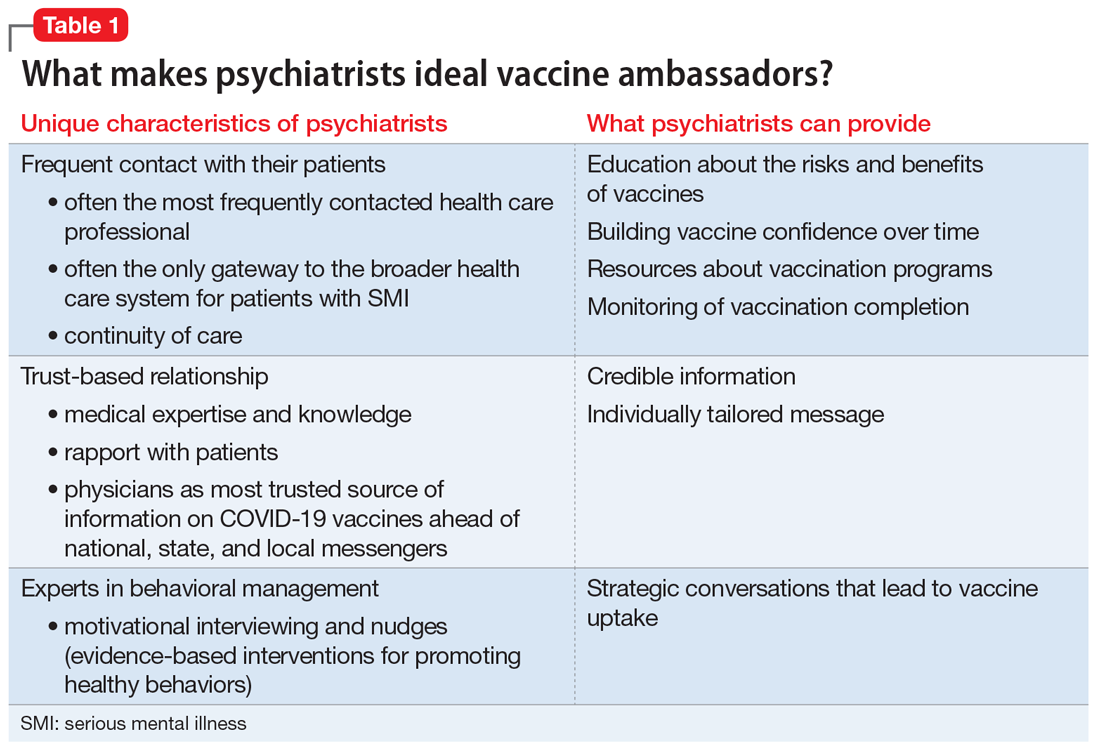
Psychiatrists’ medical expertise and their ability to develop rapport with their patients promote trust-building. Receiving credible information from a trusted source such as a patient’s psychiatrist can be impactful. A recent poll suggested that individual health care clinicians have been consistently identified as the most trusted sources for vaccine information, including for the COVID-19 vaccines.16 There is also higher trust when there is greater continuity of care both in terms of length of time the patient has known the clinician and the number of consultations,17 an inherent part of psychiatric practice. In addition, research has shown that patients trust their psychiatrists as much as they trust their general practitioners.18
Psychiatrists are experts in behavior change, promoting healthy behaviors through motivational interviewing and nudging. They also have experience with managing patients who hold overvalued ideas as well as dealing with uncertainty, given their scientific and medical training.
Motivational interviewing is a patient-centered, collaborative approach widely used by psychiatrists to treat unhealthy behaviors such as substance use. Clinicians elicit and strengthen the patient’s desire and motivation for change while respecting their autonomy. Instead of presenting persuasive facts, the clinician creates a welcoming, nonthreatening, safe environment by engaging patients in open dialogue, reflecting back the patients’ concerns with empathy, helping them realize contradictions in behavior, and supporting self-sufficiency.19 In a nonpsychiatric setting, studies have shown the effectiveness of motivational interviewing in increasing uptake of human papillomavirus vaccines and of pediatric vaccines.20
Nudging, which comes from behavioral economics and psychology, underscores the importance of structuring a choice architecture in changing the way people make their everyday decisions.21 Nudging still gives people a choice and respects autonomy, but it leads patients to more efficient and productive decision-making. Many nudges are based around giving good “default options” because people often do not make efforts to deviate from default options. In addition, social nudges are powerful, giving people a social reference point and normalizing certain behaviors.21 Psychiatrists have become skilled in nudging from working with patients with varying levels of insight and cognitive capabilities. That is, they give simple choices, prompts, and frequent feedback to reinforce “good” decisions and to discourage “bad” decisions.
Continue to: Managing overvalued ideas
Managing overvalued ideas. Psychiatrists are also well-versed in having discussions with patients who hold irrational beliefs (psychosis) or overvalued ideas. For example, psychiatrists frequently manage anorexia nervosa and hypochondria, which are rooted in overvalued ideas.7 While psychiatrists may not be able to directly confront the overvalued ideas, they can work around such ideas while waiting for more flexible moments. Similarly, managing patients with intense emotional commitment7 to commonly held anti-vaccination ideas may not be much different. Psychiatrists can work around resistance until patients may be less strongly attached to those overvalued ideas in instances when other techniques, such as motivational interviewing and nudging, may be more effective.
Managing uncertainty. Psychiatrists are experts in managing “not knowing” and uncertainty. Due to their medical scientific training, they are familiar with the process of science, and how understanding changes through trial and error. In contrast, most patients usually only see the end product (ie, a drug comes to market). Discussions with patients that acknowledge uncertainty and emphasize that changes in what is known are expected and appropriate as scientific knowledge evolves could help preempt skepticism when messages are updated.
Why do patients with SMI need more help?
SMI as a high-risk group. Patients with SMI are part of a “tragic” epidemiologic triad of agent-host-environment15 that places them at remarkably elevated risk for COVID-19 infection and more serious complications and death when infected.1 After age, a diagnosis of a schizophrenia spectrum disorder is the second largest predictor of mortality from COVID-19, with a 2.7-fold increase in mortality.22 This is how the elements of the triad come together: SARS-Cov-2 is a highly infectious agent affecting individuals who are vulnerable hosts because of their high frequency of medical comorbidities, including cardiovascular disease, type 2 diabetes, and respiratory tract diseases, which are all risk factors for worse outcomes due to COVID-19.23 In addition, SMI is associated with socioeconomic risk factors for SARS-Cov-2 infection, including poverty, homelessness, and crowded settings such as jails, group homes, hospitals, and shelters, which constitute ideal environments for high transmission of the virus.
Structural barriers to vaccination. Studies have suggested lower rates of vaccination among people with SMI for various other infectious diseases compared with the general population.12 For example, in 1 outpatient mental health setting, influenza vaccination rates were 24% to 28%, which was lower than the national vaccination rate of 40.9% for the same influenza season (2010 to 2011).24 More recently, a study in Israel examining the COVID-19 vaccination rate among >25,000 patients with schizophrenia suggested under-vaccination of this cohort. The results showed that the odds of getting the COVID-19 vaccination were significantly lower in the schizophrenia group compared with the general population (odds ratio = 0.80, 95% CI: 0.77 to 0.83).25
Patients with SMI encounter considerable system-level barriers to vaccinations in general, such as reduced access to health care due to cost and a lack of transportation,12 the digital divide given their reduced access to the internet and computers for information and scheduling,26 and lack of vaccination recommendations from their PCPs.12 Studies have also shown that patients with SMI often receive suboptimal medical care because of stigmatization and discrimination.27 They also have lower rates of preventive care utilization, seeking medical services only in times of crisis and seeking mental health services more often than physical health care.28-30
Continue to: Patients with SMI face...
Patients with SMI face additional individual challenges that impede vaccine uptake, such as lack of knowledge and awareness about the virus and vaccinations, general cognitive impairment, low digital literacy skills,31 low language literacy and educational attainment, baseline delusions, and negative symptoms such as apathy, avolition, and anhedonia.1 Thus, even if they overcome the external barriers and obtain vaccine-related information, these patients may experience difficulty in understanding the content and applying this information to their personal circumstances as a result of low health literacy.
How psychiatrists can help
The concept of using mental health care sites and trained clinicians to increase medical disease prevention is not new. The rigorously tested intervention model STIRR (Screen, Test, Immunize, Reduce risk, and Refer) uses co-located nurse practitioners in community mental health centers to provide risk assessment, counseling, and blood testing for hepatitis and HIV, as well as on-site vaccinations for hepatitis to patients dually diagnosed with SMI and substance use disorders.32
Prioritization of patients with SMI for vaccine eligibility does not directly lead to vaccine uptake. Patients with SMI need extra support from their primary point of health care contact, namely their psychiatrists. Psychiatrists may bring a set of specialized skills uniquely suited to this moment to address vaccine hesitancy and overall lack of vaccine resources and awareness. Freudenreich et al2 recently proposed “The ABCs of Successful Vaccinations” framework that psychiatrists can use in their interactions with patients to encourage vaccination by focusing on:
- attitudes towards vaccination
- barriers to vaccination
- completed vaccination series.
Understand attitudes toward vaccination. Decision-making may be an emotional and psychological experience that is informed by thoughts and feelings,34 and psychiatrists are uniquely positioned to tailor messages to individual patients by using motivational interviewing and applying nudging techniques.8 Given the large role of the pandemic in everyday life, it would be natural to address vaccine-related concerns in the course of routine rapport-building. Table 219,34-38 shows example phrases of COVID-19 vaccine messages that are based on communication strategies that have demonstrated success in health behavior domains (including vaccinations).39

Continue to: First, a strong recommendation...
First, a strong recommendation should be made using the presumptive approach.40 If vaccine hesitancy is detected, psychiatrists should next attempt to understand patients’ reasoning with open-ended questions to probe vaccine-related concerns. Motivational interviewing can then be used to target the fence sitters (rather than anti-vaxxers).6 Psychiatrists can also communicate with therapists about the need for further follow up on patients’ hesitancies.
When assuring patients of vaccine safety and efficacy, it is helpful to explain the vaccine development process, including FDA approval, extensive clinical trials, monitoring, and the distribution process. Providing clear, transparent, accurate information about the risks and benefits of the vaccines is important, as well as monitoring misinformation and developing convincing counter messages that elicit positive emotions toward the vaccines.41 Examples of messages to counter common vaccine-related concerns and misinformation are shown in Table 3.42-44

Know the barriers to vaccination. The role of the psychiatrist is to help patients, particularly those with SMIs, overcome logistical barriers and address hesitancy, which are both essential for vaccine uptake. Psychiatrists can help identify actual barriers (eg, transportation, digital access for information and scheduling) and perceived barriers, improve information access, and help patients obtain self-efficacy to take the actions needed to get vaccinated, particularly by collaborating with and communicating these concerns to other social services (Table 4).41
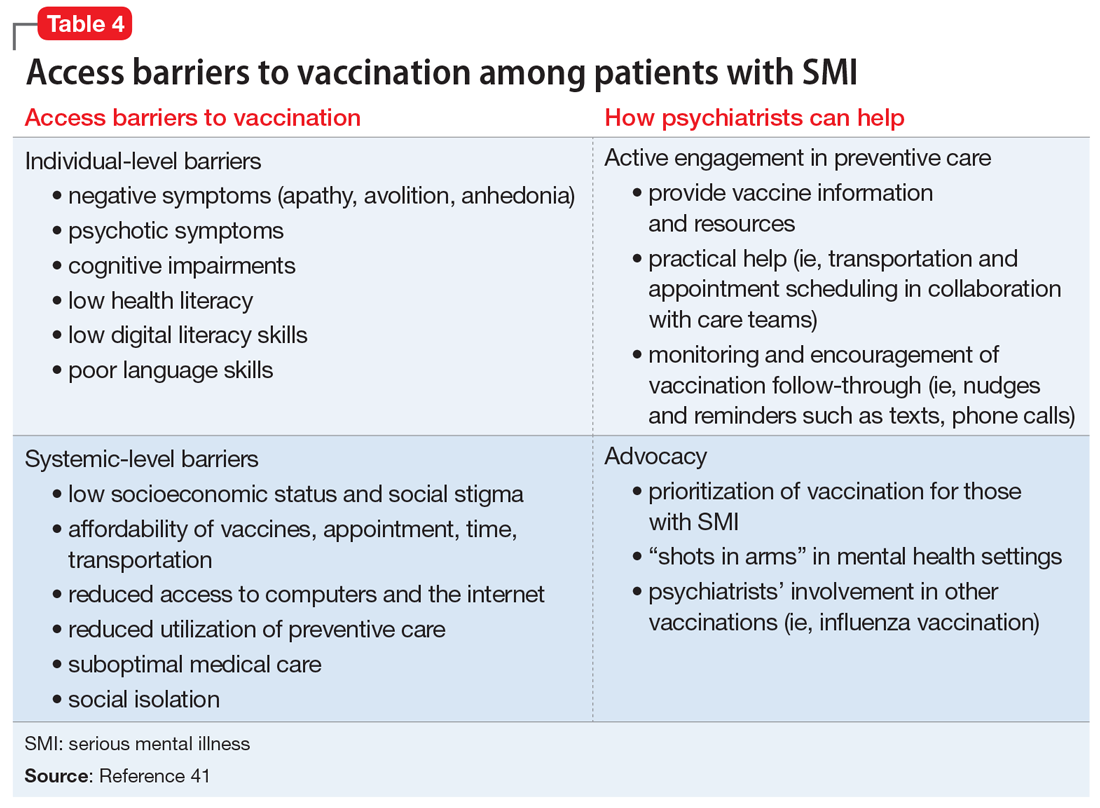
Monitor for vaccination series completion. Especially for vaccines that require more than a single dose over time, patients need more reminders, nudges, practical support, and encouragement to complete vaccination. A surprising degree of confusion regarding the timing of protection and benefit from the second COVID-19 injection (for the 2-injection vaccines) was uncovered in a recent survey of >1,000 US adults who had received their vaccinations in February 2021.45 Attentive monitoring of vaccination series completion by psychiatrists can thus increase the likelihood that a patient will follow through (Table 4).41 This can be as simple as asking about completion of the series during appointments, but further aided by communicating to the larger care team (social workers, care managers, care coordinators) when identifying that the patient may need further assistance.
The Figure2,6,7,19,40 summarizes the steps that psychiatrists can take to help patients get vaccinated by assessing attitudes towards vaccination (vaccine hesitancy), helping to remove barriers to vaccination, and ensuring via patient follow-up that a vaccine series is completed.
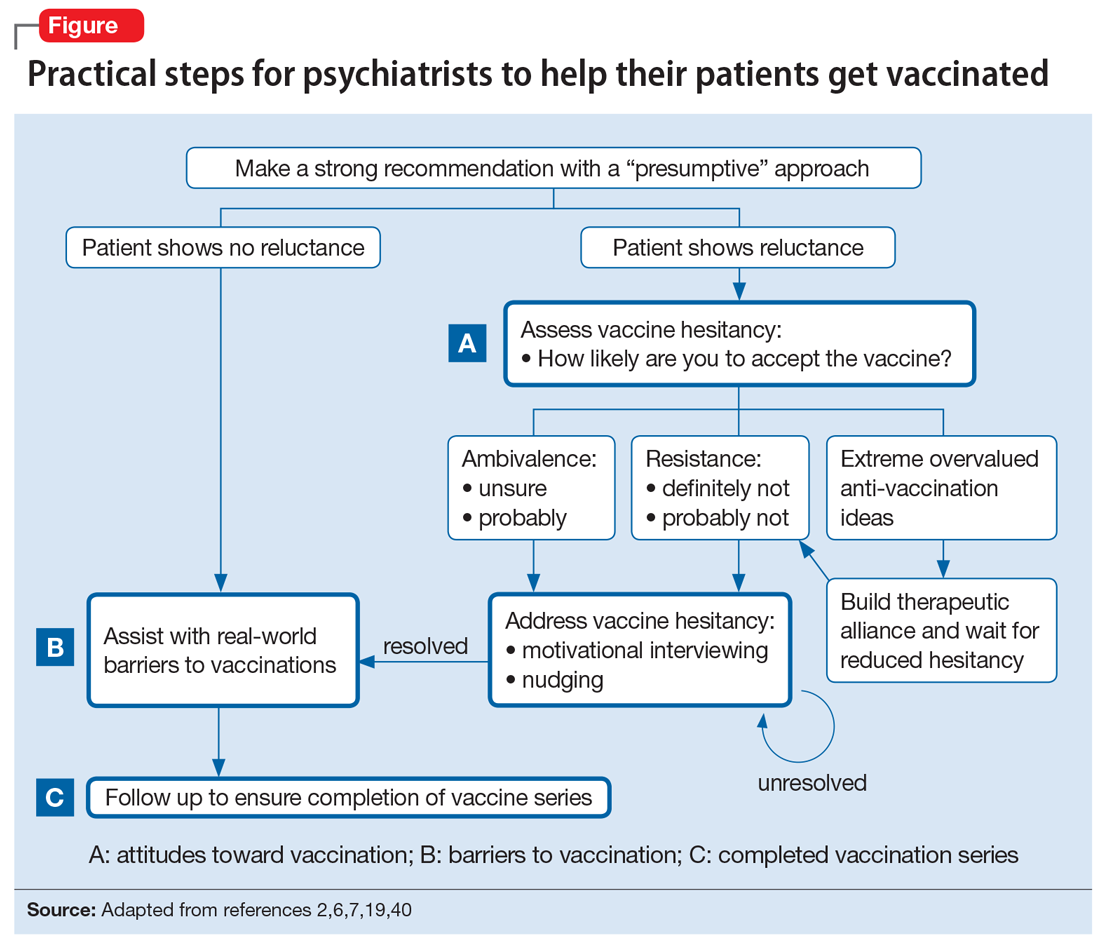
Continue to: Active involvement is key
Active involvement is key
The active involvement of psychiatrists in COVID-19 vaccination efforts can protect patients from the virus, reduce health disparities among patients with SMI, and promote herd immunity, helping to end the pandemic. Psychiatry practices can serve as ideal platforms to deliver evidence-based COVID-19 vaccine information and encourage vaccine uptake, particularly for marginalized populations.
Vaccination programs in mental health practices can even be conceptualized as a moral mandate in the spirit of addressing distributive injustice. The population management challenges of individual-level barriers and follow-through could be dramatically reduced—if not nearly eliminated—through policy-level changes that allow vaccinations to be administered in places where patients with SMI are already engaged: that is, “shots in arms” in mental health settings. As noted, some studies have shown that mental health settings can play a key role in other preventive care campaigns, such as the annual influenza and hepatitis vaccinations, and thus the incorporation of preventive care need not be limited to just COVID-19 vaccination efforts.
The COVID-19 pandemic is an opportunity to rethink the role of psychiatrists and psychiatric offices and clinics in preventive health care. The health risks and disparities of patients with SMI require the proactive involvement of psychiatrists at both the level of their individual patients and at the federal and state levels to advocate for policy changes that can benefit these populations. Overall, psychiatrists occupy a special role within the medical establishment that enables them to uniquely advocate for patients with SMI and ensure they are not forgotten during the COVID-19 pandemic.
Bottom Line
Psychiatrists could apply behavior management techniques such as motivational interviewing and nudging to address vaccine hesitancy in their patients and move them to accepting the COVID-19 vaccination. This could be particularly valuable for patients with serious mental illness, who face increased risks from COVID-19 and additional barriers to getting vaccinated.
Related Resources
- American Psychiatric Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-Coronavirus
- Baddeley M. Behavioural economics: a very short introduction. Oxford University Press; 2017.
- Centers for Disease Control and Prevention. Vaccines for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
- Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
- Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
1. Mazereel V, Van Assche K, Detraux J, et al. COVID-19 vaccination for people with severe mental illness: why, what, and how? Lancet Psychiatry. 2021;8(5):444-450.
2. Freudenreich O, Van Alphen MU, Lim C. The ABCs of successful vaccinations: a role for psychiatry. Current Psychiatry. 2021;20(3):48-50.
3. World Health Organization (WHO). Ten threats to global health in 2019. Accessed July 2, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
4. MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161-4164.
5. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
6. Betsch C, Korn L, Holtmann C. Don’t try to convert the antivaccinators, instead target the fence-sitters. Proc Natl Acad Sci. 2015;112(49):E6725-E6726.
7. Rahman T, Hartz SM, Xiong W, et al. Extreme overvalued beliefs. J Am Acad Psychiatry Law. 2020;48(3):319-326.
8. Leask J. Target the fence-sitters. Nature. 2011;473(7348):443-445.
9. United States Census Bureau. Household Pulse Survey COVID-19 Vaccination Tracker. Updated June 30, 2021. Accessed July 2, 2021. https://www.census.gov/library/visualizations/interactive/household-pulse-survey-covid-19-vaccination-tracker.html
10. United States Census Bureau. Measuring household experiences during the coronavirus pandemic. Updated May 5, 2021. Accessed July 2, 2021. https://www.census.gov/data/experimental-data-products/household-pulse-survey.html
11. Jefsen OH, Kølbæk P, Gil Y, et al. COVID-19 vaccine willingness among patients with mental illness compared with the general population. Acta Neuropsychiatrica. 2021:1-24. doi:10.1017/neu.2021.15
12. Miles LW, Williams N, Luthy KE, et al. Adult vaccination rates in the mentally ill population: an outpatient improvement project. J Am Psychiatr Nurses Assoc. 2020;26(2):172-180.
13. Lewandowsky S, Ecker UK, Seifert CM, et al. Misinformation and its correction: continued influence and successful debiasing. Psychol Sci Public Interest. 2012;13(3):106-131.
14. Druss BG, Rosenheck RA. Locus of mental health treatment in an integrated service system. Psychiatr Serv. 2000;51(7):890-892.
15. Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. Current Psychiatry. 2020;19(9):24-35.
16. Hamel L, Kirzinger A, Muñana C, et al. KFF COVID-19 vaccine monitor: December 2020. Accessed July 2, 2021. https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
17. Kai J, Crosland A. Perspectives of people with enduring mental ill health from a community-based qualitative study. Br J Gen Pract. 2001;51(470):730-736.
18. Mather G, Baker D, Laugharne R. Patient trust in psychiatrists. Psychosis. 2012;4(2):161-167.
19. Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
20. Reno JE, O’Leary S, Garrett K, et al. Improving provider communication about HPV vaccines for vaccine-hesitant parents through the use of motivational interviewing. J Health Commun. 2018;23(4):313-320.
21. Baddeley M. Behavioural economics: a very short introduction. Volume 505. Oxford University Press; 2017.
22. Nemani K, Li C, Olfson M, et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry. 2021;78(4):380-386.
23. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52.
24. Lorenz RA, Norris MM, Norton LC, et al. Factors associated with influenza vaccination decisions among patients with mental illness. Int J Psychiatry Med. 2013;46(1):1-13.
25. Bitan DT. Patients with schizophrenia are under‐vaccinated for COVID‐19: a report from Israel. World Psychiatry. 2021;20(2):300.
26. Robotham D, Satkunanathan S, Doughty L, et al. Do we still have a digital divide in mental health? A five-year survey follow-up. J Med Internet Res. 2016;18(11):e309.
27. De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138.
28. Carrà G, Bartoli F, Carretta D, et al. The prevalence of metabolic syndrome in people with severe mental illness: a mediation analysis. Soc Psychiatry Psychiatr Epidemiol. 2014;49(11):1739-1746.
29. Lin MT, Burgess JF, Carey K. The association between serious psychological distress and emergency department utilization among young adults in the USA. Soc Psychiatry Psychiatr Epidemiol. 2012;47(6):939-947.
30. DeCoux M. Acute versus primary care: the health care decision making process for individuals with severe mental illness. Issues Ment Health Nurs. 2005;26(9):935-951.
31. Hoffman L, Wisniewski H, Hays R, et al. Digital opportunities for outcomes in recovery services (DOORS): a pragmatic hands-on group approach toward increasing digital health and smartphone competencies, autonomy, relatedness, and alliance for those with serious mental illness. J Psychiatr Pract. 2020;26(2):80-88.
32. Rosenberg SD, Goldberg RW, Dixon LB, et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr Serv. 2010;61(9):885-891.
33. Slade EP, Rosenberg S, Dixon LB, et al. Costs of a public health model to increase receipt of hepatitis-related services for persons with mental illness. Psychiatr Serv. 2013;64(2):127-133.
34. Brewer NT, Chapman GB, Rothman AJ, et al. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149-207.
35. Nabet B, Gable J, Eder J, et al. PolicyLab evidence to action brief: addressing vaccine hesitancy to protect children & communities against preventable diseases. Children’s Hospital of Philadelphia. Published Spring 2017. Accessed July 2, 2021. https://policylab.chop.edu/sites/default/files/pdf/publications/Addressing_Vaccine_Hesitancy.pdf
36. Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037-1046.
37. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1(3):1-6.
38. Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44(2):105-113.
39. Parkerson N, Leader A. Vaccine hesitancy in the era of COVID. Population Health Leadership Series: PopTalk webinars. Paper 26. Published February 10, 2021. https://jdc.jefferson.edu/phlspoptalk/26/
40. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18(2):S23-S27.
41. Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
42. International Society for Vaccines and the MJH Life Sciences COVID-19 coalition. Building confidence in COVID-19 vaccination: a toolbox of talks from leaders in the field. March 9, 2021. https://globalmeet.webcasts.com/starthere.jsp?ei=1435659&tp_key=59ed660099
43. Centers for Disease Control and Prevention. Frequently asked questions about COVID-19 vaccination. Accessed July 2, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
44. Singh BR, Gandharava S, Gandharva R. Covid-19 vaccines and community immunity. Infectious Diseases Research. 2021;2(1):5.
45. Goldfarb JL, Kreps S, Brownstein JS, et al. Beyond the first dose - Covid-19 vaccine follow-through and continued protective measures. N Engl J Med. 2021;85(2):101-103.
After more than 600,000 deaths in the United States from the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), several safe and effective vaccines against the virus have become available. Vaccines are the most effective preventive measure against COVID-19 and the most promising way to achieve herd immunity to end the current pandemic. However, obstacles to reaching this goal include vaccine skepticism, structural barriers, or simple inertia to get vaccinated. These challenges provide opportunities for psychiatrists to use their medical knowledge and expertise, applying behavior management techniques such as motivational interviewing and nudging to encourage their patients to get vaccinated. In particular, marginalized patients with serious mental illness (SMI), who are subject to disproportionately high rates of COVID-19 infection and more severe outcomes,1 have much to gain if psychiatrists become involved in the COVID-19 vaccination campaign.
In this article, we define vaccine hesitancy and highlight what makes psychiatrists ideal vaccine ambassadors, given their unique skill set and longitudinal, trust-based connection with their patients. We expand on the particular vulnerabilities of patients with SMI, including structural barriers to vaccination that lead to health disparities and inequity. Finally, building on “The ABCs of successful vaccinations” framework published in
What is vaccine hesitancy?
The World Health Organization (WHO) defines vaccine hesitancy as a “delay in acceptance or refusal of vaccines despite availability of vaccine services.”3,4 Vaccine hesitancy occurs on a continuum ranging from uncertainty about accepting a vaccine to absolute refusal.4,5 It involves a complex decision-making process driven by contextual, individual, and social influences, and vaccine-specific issues.4 In the “3C” model developed by the WHO Strategic Advisory Group of Experts (SAGE) Working Group, vaccine hesitancy is influenced by confidence (trust in vaccines, in the health care system, and in policy makers), complacency (lower perceived risk), and convenience (availability, affordability, accessibility, language and health literacy, appeal of vaccination program).4
In 2019, the WHO named vaccine hesitancy as one of the top 10 global health threats.3 Hesitancy to receive COVID-19 vaccines may be particularly high because of their rapid development. In addition, the tumultuous political environment that often featured inconsistent messaging about the virus, its dangers, and its transmission since the early days of the pandemic created widespread public confusion and doubt as scientific understandings evolved. “Anti-vaxxer” movements that completely rejected vaccine efficacy disseminated misinformation online. Followers of these movements may have such extreme overvalued ideas that any effort to persuade them otherwise with scientific evidence will accomplish very little.6,7 Therefore, focusing on individuals who are “sitting on the fence” about getting vaccinated can be more productive because they represent a much larger group than those who adamantly refuse vaccines, and they may be more amenable to changing beliefs and behaviors.8
The US Census Bureau’s Household Pulse Survey asked, “How likely are you to accept the vaccine?”9 As of late June 2021, 11.4% of US adults reported they would “definitely not get a vaccine” or “probably not get a vaccine,” and that number increases to 16.9% when including those who are “unsure,” although there is wide geographical variability.10
A recent study in Denmark showed that willingness to receive the COVID-19 vaccine was slightly lower among patients with mental illness (84.8%) compared with the general population (89.5%).11 Given the small difference, vaccine hesitancy was not considered to be a major barrier for vaccination among patients with mental illness in Denmark. This is similar to the findings of a pre-pandemic study at a community mental health clinic in the United States involving other vaccinations, which suggested that 84% of patients with SMI perceived vaccinations as safe, effective, and important.12 In this clinic, identified barriers to vaccinations in general among patients with SMI included lack of awareness and knowledge (42.2%), accessibility (16.3%), personal cost (13.3%), fears about immunization (10.4%), and lack of recommendations by primary care providers (PCPs) (1.5%).12
It is critical to distinguish attitude-driven vaccine hesitancy from a lack of education and opportunity to receive a vaccine. Particularly disadvantaged communities may be mislabeled as “vaccine hesitant” when in fact they may not have the ability to be as proactive as other population groups (eg, difficulty scheduling appointments over the Internet).
Continue to: What makes psychiatrists ideal vaccine ambassadors?
What makes psychiatrists ideal vaccine ambassadors?
There are several reasons psychiatrists can be well-positioned to contribute to the success of vaccination campaigns (Table 1). These include their frequent contact with patients and their care teams, the high trust those patients have in them, and their medical expertise and skills in applied behavioral and social science techniques, including motivational interviewing and nudging. Vaccination efforts and outreach are more effective when led by the clinician with whom the patient has the most contact because resolving vaccine hesitancy is not a one-time discussion but requires ongoing communication, persistence, and consistency.13 Patients may contact their psychiatrists more frequently than their other clinicians, including PCPs. For this reason, psychiatrists can serve as the gateway to health care, particularly for patients with SMI.14 In addition, interruptions in nonemergency services caused by the COVID-19 pandemic may affect vaccine delivery because patients may have been unable to see their PCPs regularly during the pandemic.15

Psychiatrists’ medical expertise and their ability to develop rapport with their patients promote trust-building. Receiving credible information from a trusted source such as a patient’s psychiatrist can be impactful. A recent poll suggested that individual health care clinicians have been consistently identified as the most trusted sources for vaccine information, including for the COVID-19 vaccines.16 There is also higher trust when there is greater continuity of care both in terms of length of time the patient has known the clinician and the number of consultations,17 an inherent part of psychiatric practice. In addition, research has shown that patients trust their psychiatrists as much as they trust their general practitioners.18
Psychiatrists are experts in behavior change, promoting healthy behaviors through motivational interviewing and nudging. They also have experience with managing patients who hold overvalued ideas as well as dealing with uncertainty, given their scientific and medical training.
Motivational interviewing is a patient-centered, collaborative approach widely used by psychiatrists to treat unhealthy behaviors such as substance use. Clinicians elicit and strengthen the patient’s desire and motivation for change while respecting their autonomy. Instead of presenting persuasive facts, the clinician creates a welcoming, nonthreatening, safe environment by engaging patients in open dialogue, reflecting back the patients’ concerns with empathy, helping them realize contradictions in behavior, and supporting self-sufficiency.19 In a nonpsychiatric setting, studies have shown the effectiveness of motivational interviewing in increasing uptake of human papillomavirus vaccines and of pediatric vaccines.20
Nudging, which comes from behavioral economics and psychology, underscores the importance of structuring a choice architecture in changing the way people make their everyday decisions.21 Nudging still gives people a choice and respects autonomy, but it leads patients to more efficient and productive decision-making. Many nudges are based around giving good “default options” because people often do not make efforts to deviate from default options. In addition, social nudges are powerful, giving people a social reference point and normalizing certain behaviors.21 Psychiatrists have become skilled in nudging from working with patients with varying levels of insight and cognitive capabilities. That is, they give simple choices, prompts, and frequent feedback to reinforce “good” decisions and to discourage “bad” decisions.
Continue to: Managing overvalued ideas
Managing overvalued ideas. Psychiatrists are also well-versed in having discussions with patients who hold irrational beliefs (psychosis) or overvalued ideas. For example, psychiatrists frequently manage anorexia nervosa and hypochondria, which are rooted in overvalued ideas.7 While psychiatrists may not be able to directly confront the overvalued ideas, they can work around such ideas while waiting for more flexible moments. Similarly, managing patients with intense emotional commitment7 to commonly held anti-vaccination ideas may not be much different. Psychiatrists can work around resistance until patients may be less strongly attached to those overvalued ideas in instances when other techniques, such as motivational interviewing and nudging, may be more effective.
Managing uncertainty. Psychiatrists are experts in managing “not knowing” and uncertainty. Due to their medical scientific training, they are familiar with the process of science, and how understanding changes through trial and error. In contrast, most patients usually only see the end product (ie, a drug comes to market). Discussions with patients that acknowledge uncertainty and emphasize that changes in what is known are expected and appropriate as scientific knowledge evolves could help preempt skepticism when messages are updated.
Why do patients with SMI need more help?
SMI as a high-risk group. Patients with SMI are part of a “tragic” epidemiologic triad of agent-host-environment15 that places them at remarkably elevated risk for COVID-19 infection and more serious complications and death when infected.1 After age, a diagnosis of a schizophrenia spectrum disorder is the second largest predictor of mortality from COVID-19, with a 2.7-fold increase in mortality.22 This is how the elements of the triad come together: SARS-Cov-2 is a highly infectious agent affecting individuals who are vulnerable hosts because of their high frequency of medical comorbidities, including cardiovascular disease, type 2 diabetes, and respiratory tract diseases, which are all risk factors for worse outcomes due to COVID-19.23 In addition, SMI is associated with socioeconomic risk factors for SARS-Cov-2 infection, including poverty, homelessness, and crowded settings such as jails, group homes, hospitals, and shelters, which constitute ideal environments for high transmission of the virus.
Structural barriers to vaccination. Studies have suggested lower rates of vaccination among people with SMI for various other infectious diseases compared with the general population.12 For example, in 1 outpatient mental health setting, influenza vaccination rates were 24% to 28%, which was lower than the national vaccination rate of 40.9% for the same influenza season (2010 to 2011).24 More recently, a study in Israel examining the COVID-19 vaccination rate among >25,000 patients with schizophrenia suggested under-vaccination of this cohort. The results showed that the odds of getting the COVID-19 vaccination were significantly lower in the schizophrenia group compared with the general population (odds ratio = 0.80, 95% CI: 0.77 to 0.83).25
Patients with SMI encounter considerable system-level barriers to vaccinations in general, such as reduced access to health care due to cost and a lack of transportation,12 the digital divide given their reduced access to the internet and computers for information and scheduling,26 and lack of vaccination recommendations from their PCPs.12 Studies have also shown that patients with SMI often receive suboptimal medical care because of stigmatization and discrimination.27 They also have lower rates of preventive care utilization, seeking medical services only in times of crisis and seeking mental health services more often than physical health care.28-30
Continue to: Patients with SMI face...
Patients with SMI face additional individual challenges that impede vaccine uptake, such as lack of knowledge and awareness about the virus and vaccinations, general cognitive impairment, low digital literacy skills,31 low language literacy and educational attainment, baseline delusions, and negative symptoms such as apathy, avolition, and anhedonia.1 Thus, even if they overcome the external barriers and obtain vaccine-related information, these patients may experience difficulty in understanding the content and applying this information to their personal circumstances as a result of low health literacy.
How psychiatrists can help
The concept of using mental health care sites and trained clinicians to increase medical disease prevention is not new. The rigorously tested intervention model STIRR (Screen, Test, Immunize, Reduce risk, and Refer) uses co-located nurse practitioners in community mental health centers to provide risk assessment, counseling, and blood testing for hepatitis and HIV, as well as on-site vaccinations for hepatitis to patients dually diagnosed with SMI and substance use disorders.32
Prioritization of patients with SMI for vaccine eligibility does not directly lead to vaccine uptake. Patients with SMI need extra support from their primary point of health care contact, namely their psychiatrists. Psychiatrists may bring a set of specialized skills uniquely suited to this moment to address vaccine hesitancy and overall lack of vaccine resources and awareness. Freudenreich et al2 recently proposed “The ABCs of Successful Vaccinations” framework that psychiatrists can use in their interactions with patients to encourage vaccination by focusing on:
- attitudes towards vaccination
- barriers to vaccination
- completed vaccination series.
Understand attitudes toward vaccination. Decision-making may be an emotional and psychological experience that is informed by thoughts and feelings,34 and psychiatrists are uniquely positioned to tailor messages to individual patients by using motivational interviewing and applying nudging techniques.8 Given the large role of the pandemic in everyday life, it would be natural to address vaccine-related concerns in the course of routine rapport-building. Table 219,34-38 shows example phrases of COVID-19 vaccine messages that are based on communication strategies that have demonstrated success in health behavior domains (including vaccinations).39

Continue to: First, a strong recommendation...
First, a strong recommendation should be made using the presumptive approach.40 If vaccine hesitancy is detected, psychiatrists should next attempt to understand patients’ reasoning with open-ended questions to probe vaccine-related concerns. Motivational interviewing can then be used to target the fence sitters (rather than anti-vaxxers).6 Psychiatrists can also communicate with therapists about the need for further follow up on patients’ hesitancies.
When assuring patients of vaccine safety and efficacy, it is helpful to explain the vaccine development process, including FDA approval, extensive clinical trials, monitoring, and the distribution process. Providing clear, transparent, accurate information about the risks and benefits of the vaccines is important, as well as monitoring misinformation and developing convincing counter messages that elicit positive emotions toward the vaccines.41 Examples of messages to counter common vaccine-related concerns and misinformation are shown in Table 3.42-44

Know the barriers to vaccination. The role of the psychiatrist is to help patients, particularly those with SMIs, overcome logistical barriers and address hesitancy, which are both essential for vaccine uptake. Psychiatrists can help identify actual barriers (eg, transportation, digital access for information and scheduling) and perceived barriers, improve information access, and help patients obtain self-efficacy to take the actions needed to get vaccinated, particularly by collaborating with and communicating these concerns to other social services (Table 4).41

Monitor for vaccination series completion. Especially for vaccines that require more than a single dose over time, patients need more reminders, nudges, practical support, and encouragement to complete vaccination. A surprising degree of confusion regarding the timing of protection and benefit from the second COVID-19 injection (for the 2-injection vaccines) was uncovered in a recent survey of >1,000 US adults who had received their vaccinations in February 2021.45 Attentive monitoring of vaccination series completion by psychiatrists can thus increase the likelihood that a patient will follow through (Table 4).41 This can be as simple as asking about completion of the series during appointments, but further aided by communicating to the larger care team (social workers, care managers, care coordinators) when identifying that the patient may need further assistance.
The Figure2,6,7,19,40 summarizes the steps that psychiatrists can take to help patients get vaccinated by assessing attitudes towards vaccination (vaccine hesitancy), helping to remove barriers to vaccination, and ensuring via patient follow-up that a vaccine series is completed.

Continue to: Active involvement is key
Active involvement is key
The active involvement of psychiatrists in COVID-19 vaccination efforts can protect patients from the virus, reduce health disparities among patients with SMI, and promote herd immunity, helping to end the pandemic. Psychiatry practices can serve as ideal platforms to deliver evidence-based COVID-19 vaccine information and encourage vaccine uptake, particularly for marginalized populations.
Vaccination programs in mental health practices can even be conceptualized as a moral mandate in the spirit of addressing distributive injustice. The population management challenges of individual-level barriers and follow-through could be dramatically reduced—if not nearly eliminated—through policy-level changes that allow vaccinations to be administered in places where patients with SMI are already engaged: that is, “shots in arms” in mental health settings. As noted, some studies have shown that mental health settings can play a key role in other preventive care campaigns, such as the annual influenza and hepatitis vaccinations, and thus the incorporation of preventive care need not be limited to just COVID-19 vaccination efforts.
The COVID-19 pandemic is an opportunity to rethink the role of psychiatrists and psychiatric offices and clinics in preventive health care. The health risks and disparities of patients with SMI require the proactive involvement of psychiatrists at both the level of their individual patients and at the federal and state levels to advocate for policy changes that can benefit these populations. Overall, psychiatrists occupy a special role within the medical establishment that enables them to uniquely advocate for patients with SMI and ensure they are not forgotten during the COVID-19 pandemic.
Bottom Line
Psychiatrists could apply behavior management techniques such as motivational interviewing and nudging to address vaccine hesitancy in their patients and move them to accepting the COVID-19 vaccination. This could be particularly valuable for patients with serious mental illness, who face increased risks from COVID-19 and additional barriers to getting vaccinated.
Related Resources
- American Psychiatric Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-Coronavirus
- Baddeley M. Behavioural economics: a very short introduction. Oxford University Press; 2017.
- Centers for Disease Control and Prevention. Vaccines for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
- Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
- Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
After more than 600,000 deaths in the United States from the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), several safe and effective vaccines against the virus have become available. Vaccines are the most effective preventive measure against COVID-19 and the most promising way to achieve herd immunity to end the current pandemic. However, obstacles to reaching this goal include vaccine skepticism, structural barriers, or simple inertia to get vaccinated. These challenges provide opportunities for psychiatrists to use their medical knowledge and expertise, applying behavior management techniques such as motivational interviewing and nudging to encourage their patients to get vaccinated. In particular, marginalized patients with serious mental illness (SMI), who are subject to disproportionately high rates of COVID-19 infection and more severe outcomes,1 have much to gain if psychiatrists become involved in the COVID-19 vaccination campaign.
In this article, we define vaccine hesitancy and highlight what makes psychiatrists ideal vaccine ambassadors, given their unique skill set and longitudinal, trust-based connection with their patients. We expand on the particular vulnerabilities of patients with SMI, including structural barriers to vaccination that lead to health disparities and inequity. Finally, building on “The ABCs of successful vaccinations” framework published in
What is vaccine hesitancy?
The World Health Organization (WHO) defines vaccine hesitancy as a “delay in acceptance or refusal of vaccines despite availability of vaccine services.”3,4 Vaccine hesitancy occurs on a continuum ranging from uncertainty about accepting a vaccine to absolute refusal.4,5 It involves a complex decision-making process driven by contextual, individual, and social influences, and vaccine-specific issues.4 In the “3C” model developed by the WHO Strategic Advisory Group of Experts (SAGE) Working Group, vaccine hesitancy is influenced by confidence (trust in vaccines, in the health care system, and in policy makers), complacency (lower perceived risk), and convenience (availability, affordability, accessibility, language and health literacy, appeal of vaccination program).4
In 2019, the WHO named vaccine hesitancy as one of the top 10 global health threats.3 Hesitancy to receive COVID-19 vaccines may be particularly high because of their rapid development. In addition, the tumultuous political environment that often featured inconsistent messaging about the virus, its dangers, and its transmission since the early days of the pandemic created widespread public confusion and doubt as scientific understandings evolved. “Anti-vaxxer” movements that completely rejected vaccine efficacy disseminated misinformation online. Followers of these movements may have such extreme overvalued ideas that any effort to persuade them otherwise with scientific evidence will accomplish very little.6,7 Therefore, focusing on individuals who are “sitting on the fence” about getting vaccinated can be more productive because they represent a much larger group than those who adamantly refuse vaccines, and they may be more amenable to changing beliefs and behaviors.8
The US Census Bureau’s Household Pulse Survey asked, “How likely are you to accept the vaccine?”9 As of late June 2021, 11.4% of US adults reported they would “definitely not get a vaccine” or “probably not get a vaccine,” and that number increases to 16.9% when including those who are “unsure,” although there is wide geographical variability.10
A recent study in Denmark showed that willingness to receive the COVID-19 vaccine was slightly lower among patients with mental illness (84.8%) compared with the general population (89.5%).11 Given the small difference, vaccine hesitancy was not considered to be a major barrier for vaccination among patients with mental illness in Denmark. This is similar to the findings of a pre-pandemic study at a community mental health clinic in the United States involving other vaccinations, which suggested that 84% of patients with SMI perceived vaccinations as safe, effective, and important.12 In this clinic, identified barriers to vaccinations in general among patients with SMI included lack of awareness and knowledge (42.2%), accessibility (16.3%), personal cost (13.3%), fears about immunization (10.4%), and lack of recommendations by primary care providers (PCPs) (1.5%).12
It is critical to distinguish attitude-driven vaccine hesitancy from a lack of education and opportunity to receive a vaccine. Particularly disadvantaged communities may be mislabeled as “vaccine hesitant” when in fact they may not have the ability to be as proactive as other population groups (eg, difficulty scheduling appointments over the Internet).
Continue to: What makes psychiatrists ideal vaccine ambassadors?
What makes psychiatrists ideal vaccine ambassadors?
There are several reasons psychiatrists can be well-positioned to contribute to the success of vaccination campaigns (Table 1). These include their frequent contact with patients and their care teams, the high trust those patients have in them, and their medical expertise and skills in applied behavioral and social science techniques, including motivational interviewing and nudging. Vaccination efforts and outreach are more effective when led by the clinician with whom the patient has the most contact because resolving vaccine hesitancy is not a one-time discussion but requires ongoing communication, persistence, and consistency.13 Patients may contact their psychiatrists more frequently than their other clinicians, including PCPs. For this reason, psychiatrists can serve as the gateway to health care, particularly for patients with SMI.14 In addition, interruptions in nonemergency services caused by the COVID-19 pandemic may affect vaccine delivery because patients may have been unable to see their PCPs regularly during the pandemic.15

Psychiatrists’ medical expertise and their ability to develop rapport with their patients promote trust-building. Receiving credible information from a trusted source such as a patient’s psychiatrist can be impactful. A recent poll suggested that individual health care clinicians have been consistently identified as the most trusted sources for vaccine information, including for the COVID-19 vaccines.16 There is also higher trust when there is greater continuity of care both in terms of length of time the patient has known the clinician and the number of consultations,17 an inherent part of psychiatric practice. In addition, research has shown that patients trust their psychiatrists as much as they trust their general practitioners.18
Psychiatrists are experts in behavior change, promoting healthy behaviors through motivational interviewing and nudging. They also have experience with managing patients who hold overvalued ideas as well as dealing with uncertainty, given their scientific and medical training.
Motivational interviewing is a patient-centered, collaborative approach widely used by psychiatrists to treat unhealthy behaviors such as substance use. Clinicians elicit and strengthen the patient’s desire and motivation for change while respecting their autonomy. Instead of presenting persuasive facts, the clinician creates a welcoming, nonthreatening, safe environment by engaging patients in open dialogue, reflecting back the patients’ concerns with empathy, helping them realize contradictions in behavior, and supporting self-sufficiency.19 In a nonpsychiatric setting, studies have shown the effectiveness of motivational interviewing in increasing uptake of human papillomavirus vaccines and of pediatric vaccines.20
Nudging, which comes from behavioral economics and psychology, underscores the importance of structuring a choice architecture in changing the way people make their everyday decisions.21 Nudging still gives people a choice and respects autonomy, but it leads patients to more efficient and productive decision-making. Many nudges are based around giving good “default options” because people often do not make efforts to deviate from default options. In addition, social nudges are powerful, giving people a social reference point and normalizing certain behaviors.21 Psychiatrists have become skilled in nudging from working with patients with varying levels of insight and cognitive capabilities. That is, they give simple choices, prompts, and frequent feedback to reinforce “good” decisions and to discourage “bad” decisions.
Continue to: Managing overvalued ideas
Managing overvalued ideas. Psychiatrists are also well-versed in having discussions with patients who hold irrational beliefs (psychosis) or overvalued ideas. For example, psychiatrists frequently manage anorexia nervosa and hypochondria, which are rooted in overvalued ideas.7 While psychiatrists may not be able to directly confront the overvalued ideas, they can work around such ideas while waiting for more flexible moments. Similarly, managing patients with intense emotional commitment7 to commonly held anti-vaccination ideas may not be much different. Psychiatrists can work around resistance until patients may be less strongly attached to those overvalued ideas in instances when other techniques, such as motivational interviewing and nudging, may be more effective.
Managing uncertainty. Psychiatrists are experts in managing “not knowing” and uncertainty. Due to their medical scientific training, they are familiar with the process of science, and how understanding changes through trial and error. In contrast, most patients usually only see the end product (ie, a drug comes to market). Discussions with patients that acknowledge uncertainty and emphasize that changes in what is known are expected and appropriate as scientific knowledge evolves could help preempt skepticism when messages are updated.
Why do patients with SMI need more help?
SMI as a high-risk group. Patients with SMI are part of a “tragic” epidemiologic triad of agent-host-environment15 that places them at remarkably elevated risk for COVID-19 infection and more serious complications and death when infected.1 After age, a diagnosis of a schizophrenia spectrum disorder is the second largest predictor of mortality from COVID-19, with a 2.7-fold increase in mortality.22 This is how the elements of the triad come together: SARS-Cov-2 is a highly infectious agent affecting individuals who are vulnerable hosts because of their high frequency of medical comorbidities, including cardiovascular disease, type 2 diabetes, and respiratory tract diseases, which are all risk factors for worse outcomes due to COVID-19.23 In addition, SMI is associated with socioeconomic risk factors for SARS-Cov-2 infection, including poverty, homelessness, and crowded settings such as jails, group homes, hospitals, and shelters, which constitute ideal environments for high transmission of the virus.
Structural barriers to vaccination. Studies have suggested lower rates of vaccination among people with SMI for various other infectious diseases compared with the general population.12 For example, in 1 outpatient mental health setting, influenza vaccination rates were 24% to 28%, which was lower than the national vaccination rate of 40.9% for the same influenza season (2010 to 2011).24 More recently, a study in Israel examining the COVID-19 vaccination rate among >25,000 patients with schizophrenia suggested under-vaccination of this cohort. The results showed that the odds of getting the COVID-19 vaccination were significantly lower in the schizophrenia group compared with the general population (odds ratio = 0.80, 95% CI: 0.77 to 0.83).25
Patients with SMI encounter considerable system-level barriers to vaccinations in general, such as reduced access to health care due to cost and a lack of transportation,12 the digital divide given their reduced access to the internet and computers for information and scheduling,26 and lack of vaccination recommendations from their PCPs.12 Studies have also shown that patients with SMI often receive suboptimal medical care because of stigmatization and discrimination.27 They also have lower rates of preventive care utilization, seeking medical services only in times of crisis and seeking mental health services more often than physical health care.28-30
Continue to: Patients with SMI face...
Patients with SMI face additional individual challenges that impede vaccine uptake, such as lack of knowledge and awareness about the virus and vaccinations, general cognitive impairment, low digital literacy skills,31 low language literacy and educational attainment, baseline delusions, and negative symptoms such as apathy, avolition, and anhedonia.1 Thus, even if they overcome the external barriers and obtain vaccine-related information, these patients may experience difficulty in understanding the content and applying this information to their personal circumstances as a result of low health literacy.
How psychiatrists can help
The concept of using mental health care sites and trained clinicians to increase medical disease prevention is not new. The rigorously tested intervention model STIRR (Screen, Test, Immunize, Reduce risk, and Refer) uses co-located nurse practitioners in community mental health centers to provide risk assessment, counseling, and blood testing for hepatitis and HIV, as well as on-site vaccinations for hepatitis to patients dually diagnosed with SMI and substance use disorders.32
Prioritization of patients with SMI for vaccine eligibility does not directly lead to vaccine uptake. Patients with SMI need extra support from their primary point of health care contact, namely their psychiatrists. Psychiatrists may bring a set of specialized skills uniquely suited to this moment to address vaccine hesitancy and overall lack of vaccine resources and awareness. Freudenreich et al2 recently proposed “The ABCs of Successful Vaccinations” framework that psychiatrists can use in their interactions with patients to encourage vaccination by focusing on:
- attitudes towards vaccination
- barriers to vaccination
- completed vaccination series.
Understand attitudes toward vaccination. Decision-making may be an emotional and psychological experience that is informed by thoughts and feelings,34 and psychiatrists are uniquely positioned to tailor messages to individual patients by using motivational interviewing and applying nudging techniques.8 Given the large role of the pandemic in everyday life, it would be natural to address vaccine-related concerns in the course of routine rapport-building. Table 219,34-38 shows example phrases of COVID-19 vaccine messages that are based on communication strategies that have demonstrated success in health behavior domains (including vaccinations).39

Continue to: First, a strong recommendation...
First, a strong recommendation should be made using the presumptive approach.40 If vaccine hesitancy is detected, psychiatrists should next attempt to understand patients’ reasoning with open-ended questions to probe vaccine-related concerns. Motivational interviewing can then be used to target the fence sitters (rather than anti-vaxxers).6 Psychiatrists can also communicate with therapists about the need for further follow up on patients’ hesitancies.
When assuring patients of vaccine safety and efficacy, it is helpful to explain the vaccine development process, including FDA approval, extensive clinical trials, monitoring, and the distribution process. Providing clear, transparent, accurate information about the risks and benefits of the vaccines is important, as well as monitoring misinformation and developing convincing counter messages that elicit positive emotions toward the vaccines.41 Examples of messages to counter common vaccine-related concerns and misinformation are shown in Table 3.42-44

Know the barriers to vaccination. The role of the psychiatrist is to help patients, particularly those with SMIs, overcome logistical barriers and address hesitancy, which are both essential for vaccine uptake. Psychiatrists can help identify actual barriers (eg, transportation, digital access for information and scheduling) and perceived barriers, improve information access, and help patients obtain self-efficacy to take the actions needed to get vaccinated, particularly by collaborating with and communicating these concerns to other social services (Table 4).41

Monitor for vaccination series completion. Especially for vaccines that require more than a single dose over time, patients need more reminders, nudges, practical support, and encouragement to complete vaccination. A surprising degree of confusion regarding the timing of protection and benefit from the second COVID-19 injection (for the 2-injection vaccines) was uncovered in a recent survey of >1,000 US adults who had received their vaccinations in February 2021.45 Attentive monitoring of vaccination series completion by psychiatrists can thus increase the likelihood that a patient will follow through (Table 4).41 This can be as simple as asking about completion of the series during appointments, but further aided by communicating to the larger care team (social workers, care managers, care coordinators) when identifying that the patient may need further assistance.
The Figure2,6,7,19,40 summarizes the steps that psychiatrists can take to help patients get vaccinated by assessing attitudes towards vaccination (vaccine hesitancy), helping to remove barriers to vaccination, and ensuring via patient follow-up that a vaccine series is completed.

Continue to: Active involvement is key
Active involvement is key
The active involvement of psychiatrists in COVID-19 vaccination efforts can protect patients from the virus, reduce health disparities among patients with SMI, and promote herd immunity, helping to end the pandemic. Psychiatry practices can serve as ideal platforms to deliver evidence-based COVID-19 vaccine information and encourage vaccine uptake, particularly for marginalized populations.
Vaccination programs in mental health practices can even be conceptualized as a moral mandate in the spirit of addressing distributive injustice. The population management challenges of individual-level barriers and follow-through could be dramatically reduced—if not nearly eliminated—through policy-level changes that allow vaccinations to be administered in places where patients with SMI are already engaged: that is, “shots in arms” in mental health settings. As noted, some studies have shown that mental health settings can play a key role in other preventive care campaigns, such as the annual influenza and hepatitis vaccinations, and thus the incorporation of preventive care need not be limited to just COVID-19 vaccination efforts.
The COVID-19 pandemic is an opportunity to rethink the role of psychiatrists and psychiatric offices and clinics in preventive health care. The health risks and disparities of patients with SMI require the proactive involvement of psychiatrists at both the level of their individual patients and at the federal and state levels to advocate for policy changes that can benefit these populations. Overall, psychiatrists occupy a special role within the medical establishment that enables them to uniquely advocate for patients with SMI and ensure they are not forgotten during the COVID-19 pandemic.
Bottom Line
Psychiatrists could apply behavior management techniques such as motivational interviewing and nudging to address vaccine hesitancy in their patients and move them to accepting the COVID-19 vaccination. This could be particularly valuable for patients with serious mental illness, who face increased risks from COVID-19 and additional barriers to getting vaccinated.
Related Resources
- American Psychiatric Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-Coronavirus
- Baddeley M. Behavioural economics: a very short introduction. Oxford University Press; 2017.
- Centers for Disease Control and Prevention. Vaccines for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
- Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
- Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
1. Mazereel V, Van Assche K, Detraux J, et al. COVID-19 vaccination for people with severe mental illness: why, what, and how? Lancet Psychiatry. 2021;8(5):444-450.
2. Freudenreich O, Van Alphen MU, Lim C. The ABCs of successful vaccinations: a role for psychiatry. Current Psychiatry. 2021;20(3):48-50.
3. World Health Organization (WHO). Ten threats to global health in 2019. Accessed July 2, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
4. MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161-4164.
5. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
6. Betsch C, Korn L, Holtmann C. Don’t try to convert the antivaccinators, instead target the fence-sitters. Proc Natl Acad Sci. 2015;112(49):E6725-E6726.
7. Rahman T, Hartz SM, Xiong W, et al. Extreme overvalued beliefs. J Am Acad Psychiatry Law. 2020;48(3):319-326.
8. Leask J. Target the fence-sitters. Nature. 2011;473(7348):443-445.
9. United States Census Bureau. Household Pulse Survey COVID-19 Vaccination Tracker. Updated June 30, 2021. Accessed July 2, 2021. https://www.census.gov/library/visualizations/interactive/household-pulse-survey-covid-19-vaccination-tracker.html
10. United States Census Bureau. Measuring household experiences during the coronavirus pandemic. Updated May 5, 2021. Accessed July 2, 2021. https://www.census.gov/data/experimental-data-products/household-pulse-survey.html
11. Jefsen OH, Kølbæk P, Gil Y, et al. COVID-19 vaccine willingness among patients with mental illness compared with the general population. Acta Neuropsychiatrica. 2021:1-24. doi:10.1017/neu.2021.15
12. Miles LW, Williams N, Luthy KE, et al. Adult vaccination rates in the mentally ill population: an outpatient improvement project. J Am Psychiatr Nurses Assoc. 2020;26(2):172-180.
13. Lewandowsky S, Ecker UK, Seifert CM, et al. Misinformation and its correction: continued influence and successful debiasing. Psychol Sci Public Interest. 2012;13(3):106-131.
14. Druss BG, Rosenheck RA. Locus of mental health treatment in an integrated service system. Psychiatr Serv. 2000;51(7):890-892.
15. Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. Current Psychiatry. 2020;19(9):24-35.
16. Hamel L, Kirzinger A, Muñana C, et al. KFF COVID-19 vaccine monitor: December 2020. Accessed July 2, 2021. https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
17. Kai J, Crosland A. Perspectives of people with enduring mental ill health from a community-based qualitative study. Br J Gen Pract. 2001;51(470):730-736.
18. Mather G, Baker D, Laugharne R. Patient trust in psychiatrists. Psychosis. 2012;4(2):161-167.
19. Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
20. Reno JE, O’Leary S, Garrett K, et al. Improving provider communication about HPV vaccines for vaccine-hesitant parents through the use of motivational interviewing. J Health Commun. 2018;23(4):313-320.
21. Baddeley M. Behavioural economics: a very short introduction. Volume 505. Oxford University Press; 2017.
22. Nemani K, Li C, Olfson M, et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry. 2021;78(4):380-386.
23. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52.
24. Lorenz RA, Norris MM, Norton LC, et al. Factors associated with influenza vaccination decisions among patients with mental illness. Int J Psychiatry Med. 2013;46(1):1-13.
25. Bitan DT. Patients with schizophrenia are under‐vaccinated for COVID‐19: a report from Israel. World Psychiatry. 2021;20(2):300.
26. Robotham D, Satkunanathan S, Doughty L, et al. Do we still have a digital divide in mental health? A five-year survey follow-up. J Med Internet Res. 2016;18(11):e309.
27. De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138.
28. Carrà G, Bartoli F, Carretta D, et al. The prevalence of metabolic syndrome in people with severe mental illness: a mediation analysis. Soc Psychiatry Psychiatr Epidemiol. 2014;49(11):1739-1746.
29. Lin MT, Burgess JF, Carey K. The association between serious psychological distress and emergency department utilization among young adults in the USA. Soc Psychiatry Psychiatr Epidemiol. 2012;47(6):939-947.
30. DeCoux M. Acute versus primary care: the health care decision making process for individuals with severe mental illness. Issues Ment Health Nurs. 2005;26(9):935-951.
31. Hoffman L, Wisniewski H, Hays R, et al. Digital opportunities for outcomes in recovery services (DOORS): a pragmatic hands-on group approach toward increasing digital health and smartphone competencies, autonomy, relatedness, and alliance for those with serious mental illness. J Psychiatr Pract. 2020;26(2):80-88.
32. Rosenberg SD, Goldberg RW, Dixon LB, et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr Serv. 2010;61(9):885-891.
33. Slade EP, Rosenberg S, Dixon LB, et al. Costs of a public health model to increase receipt of hepatitis-related services for persons with mental illness. Psychiatr Serv. 2013;64(2):127-133.
34. Brewer NT, Chapman GB, Rothman AJ, et al. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149-207.
35. Nabet B, Gable J, Eder J, et al. PolicyLab evidence to action brief: addressing vaccine hesitancy to protect children & communities against preventable diseases. Children’s Hospital of Philadelphia. Published Spring 2017. Accessed July 2, 2021. https://policylab.chop.edu/sites/default/files/pdf/publications/Addressing_Vaccine_Hesitancy.pdf
36. Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037-1046.
37. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1(3):1-6.
38. Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44(2):105-113.
39. Parkerson N, Leader A. Vaccine hesitancy in the era of COVID. Population Health Leadership Series: PopTalk webinars. Paper 26. Published February 10, 2021. https://jdc.jefferson.edu/phlspoptalk/26/
40. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18(2):S23-S27.
41. Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
42. International Society for Vaccines and the MJH Life Sciences COVID-19 coalition. Building confidence in COVID-19 vaccination: a toolbox of talks from leaders in the field. March 9, 2021. https://globalmeet.webcasts.com/starthere.jsp?ei=1435659&tp_key=59ed660099
43. Centers for Disease Control and Prevention. Frequently asked questions about COVID-19 vaccination. Accessed July 2, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
44. Singh BR, Gandharava S, Gandharva R. Covid-19 vaccines and community immunity. Infectious Diseases Research. 2021;2(1):5.
45. Goldfarb JL, Kreps S, Brownstein JS, et al. Beyond the first dose - Covid-19 vaccine follow-through and continued protective measures. N Engl J Med. 2021;85(2):101-103.
1. Mazereel V, Van Assche K, Detraux J, et al. COVID-19 vaccination for people with severe mental illness: why, what, and how? Lancet Psychiatry. 2021;8(5):444-450.
2. Freudenreich O, Van Alphen MU, Lim C. The ABCs of successful vaccinations: a role for psychiatry. Current Psychiatry. 2021;20(3):48-50.
3. World Health Organization (WHO). Ten threats to global health in 2019. Accessed July 2, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
4. MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161-4164.
5. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
6. Betsch C, Korn L, Holtmann C. Don’t try to convert the antivaccinators, instead target the fence-sitters. Proc Natl Acad Sci. 2015;112(49):E6725-E6726.
7. Rahman T, Hartz SM, Xiong W, et al. Extreme overvalued beliefs. J Am Acad Psychiatry Law. 2020;48(3):319-326.
8. Leask J. Target the fence-sitters. Nature. 2011;473(7348):443-445.
9. United States Census Bureau. Household Pulse Survey COVID-19 Vaccination Tracker. Updated June 30, 2021. Accessed July 2, 2021. https://www.census.gov/library/visualizations/interactive/household-pulse-survey-covid-19-vaccination-tracker.html
10. United States Census Bureau. Measuring household experiences during the coronavirus pandemic. Updated May 5, 2021. Accessed July 2, 2021. https://www.census.gov/data/experimental-data-products/household-pulse-survey.html
11. Jefsen OH, Kølbæk P, Gil Y, et al. COVID-19 vaccine willingness among patients with mental illness compared with the general population. Acta Neuropsychiatrica. 2021:1-24. doi:10.1017/neu.2021.15
12. Miles LW, Williams N, Luthy KE, et al. Adult vaccination rates in the mentally ill population: an outpatient improvement project. J Am Psychiatr Nurses Assoc. 2020;26(2):172-180.
13. Lewandowsky S, Ecker UK, Seifert CM, et al. Misinformation and its correction: continued influence and successful debiasing. Psychol Sci Public Interest. 2012;13(3):106-131.
14. Druss BG, Rosenheck RA. Locus of mental health treatment in an integrated service system. Psychiatr Serv. 2000;51(7):890-892.
15. Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. Current Psychiatry. 2020;19(9):24-35.
16. Hamel L, Kirzinger A, Muñana C, et al. KFF COVID-19 vaccine monitor: December 2020. Accessed July 2, 2021. https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
17. Kai J, Crosland A. Perspectives of people with enduring mental ill health from a community-based qualitative study. Br J Gen Pract. 2001;51(470):730-736.
18. Mather G, Baker D, Laugharne R. Patient trust in psychiatrists. Psychosis. 2012;4(2):161-167.
19. Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
20. Reno JE, O’Leary S, Garrett K, et al. Improving provider communication about HPV vaccines for vaccine-hesitant parents through the use of motivational interviewing. J Health Commun. 2018;23(4):313-320.
21. Baddeley M. Behavioural economics: a very short introduction. Volume 505. Oxford University Press; 2017.
22. Nemani K, Li C, Olfson M, et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry. 2021;78(4):380-386.
23. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52.
24. Lorenz RA, Norris MM, Norton LC, et al. Factors associated with influenza vaccination decisions among patients with mental illness. Int J Psychiatry Med. 2013;46(1):1-13.
25. Bitan DT. Patients with schizophrenia are under‐vaccinated for COVID‐19: a report from Israel. World Psychiatry. 2021;20(2):300.
26. Robotham D, Satkunanathan S, Doughty L, et al. Do we still have a digital divide in mental health? A five-year survey follow-up. J Med Internet Res. 2016;18(11):e309.
27. De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138.
28. Carrà G, Bartoli F, Carretta D, et al. The prevalence of metabolic syndrome in people with severe mental illness: a mediation analysis. Soc Psychiatry Psychiatr Epidemiol. 2014;49(11):1739-1746.
29. Lin MT, Burgess JF, Carey K. The association between serious psychological distress and emergency department utilization among young adults in the USA. Soc Psychiatry Psychiatr Epidemiol. 2012;47(6):939-947.
30. DeCoux M. Acute versus primary care: the health care decision making process for individuals with severe mental illness. Issues Ment Health Nurs. 2005;26(9):935-951.
31. Hoffman L, Wisniewski H, Hays R, et al. Digital opportunities for outcomes in recovery services (DOORS): a pragmatic hands-on group approach toward increasing digital health and smartphone competencies, autonomy, relatedness, and alliance for those with serious mental illness. J Psychiatr Pract. 2020;26(2):80-88.
32. Rosenberg SD, Goldberg RW, Dixon LB, et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr Serv. 2010;61(9):885-891.
33. Slade EP, Rosenberg S, Dixon LB, et al. Costs of a public health model to increase receipt of hepatitis-related services for persons with mental illness. Psychiatr Serv. 2013;64(2):127-133.
34. Brewer NT, Chapman GB, Rothman AJ, et al. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149-207.
35. Nabet B, Gable J, Eder J, et al. PolicyLab evidence to action brief: addressing vaccine hesitancy to protect children & communities against preventable diseases. Children’s Hospital of Philadelphia. Published Spring 2017. Accessed July 2, 2021. https://policylab.chop.edu/sites/default/files/pdf/publications/Addressing_Vaccine_Hesitancy.pdf
36. Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037-1046.
37. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1(3):1-6.
38. Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44(2):105-113.
39. Parkerson N, Leader A. Vaccine hesitancy in the era of COVID. Population Health Leadership Series: PopTalk webinars. Paper 26. Published February 10, 2021. https://jdc.jefferson.edu/phlspoptalk/26/
40. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18(2):S23-S27.
41. Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
42. International Society for Vaccines and the MJH Life Sciences COVID-19 coalition. Building confidence in COVID-19 vaccination: a toolbox of talks from leaders in the field. March 9, 2021. https://globalmeet.webcasts.com/starthere.jsp?ei=1435659&tp_key=59ed660099
43. Centers for Disease Control and Prevention. Frequently asked questions about COVID-19 vaccination. Accessed July 2, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
44. Singh BR, Gandharava S, Gandharva R. Covid-19 vaccines and community immunity. Infectious Diseases Research. 2021;2(1):5.
45. Goldfarb JL, Kreps S, Brownstein JS, et al. Beyond the first dose - Covid-19 vaccine follow-through and continued protective measures. N Engl J Med. 2021;85(2):101-103.