User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
A PSYCHIATRIC MANIFESTO: Stigma is hate speech and a hate crime
Having witnessed the devastating impact of stigma on patients with mental illness throughout my psychiatric career, I am fed up and disgusted with this malevolent scourge.
I regard the stigma that engulfs neuropsychiatric disorders as a malignancy that mutilates patients’ souls and hastens their mortality.
Stigma is hate speech
How would you feel if you had a serious medical illness, a disabling brain disorder such as schizophrenia, depression, or anxiety, and people refer to you with pejorative and insulting terms such as crazy, deranged, lunatic, unhinged, nutty, insane, wacky, berserk, cuckoo, bonkers, flaky, screwball, or unglued? This is hate speech generated by stigma against people with mental illness. Individuals with heart disease, cancer, or diabetes never get called such disgraceful and stigmatizing terms that shame, stain, besmirch, and scar them, which happens daily to persons with psychiatric brain disorders.
The damage and harm of the discriminatory stigma on our patients is multifaceted. It is painful, detrimental, pernicious, and deleterious. It is corrosive to their spirits, crippling to their self-image, and subversive to their self-confidence. Hate speech is not simply words, but a menacing weapon that assaults the core humanity of medically ill psychiatric patients.
Although hate speech is punishable by law, there are rarely any legal actions against those who hurl hate speech at psychiatric patients every day. Society has institutionalized the stigma of mental illness and takes it in stride instead of recognizing it as an illegal, harmful act.
Long before the stresses of the COVID-19 pandemic, 43% of the population had been shown to experience a diagnosable psychiatric disorder over the course of their life.1 Thus, tens of millions of people are burdened by stigma and the hate speech associated with it. This is directly related to massive ignorance about mental illness being the result of a neurobiological condition due to either genetic or intrauterine adverse events that disrupt brain development. Delusions and hallucinations are symptoms of a malfunctioning brain, depression is not a sign of personal weakness, anxiety is the most prevalent mental disorder in the world, and obsessive-compulsive disorder (OCD) is not odd behavior but the result of dysfunction of neural circuits. Correcting public misperceptions about psychiatric brain disorders can mitigate stigma, but it has yet to happen.
Stigma is a hate crime
Stigma can accelerate physical death and premature mortality. Many studies have confirmed that persons with schizophrenia do not receive basic primary care treatments for the life-shortening medical conditions that often afflict them, such as diabetes, dyslipidemia, and hypertension.2 Stigma is responsible for a significant disparity of medical3-5 and intensive care6 among individuals with mental illness compared to the general population. It’s no wonder most psychiatric disorders are associated with accelerated mortality.7 A recent study during the pandemic by Balasuriya et al8 reported that patients with depression had poor access to care. Stigma interferes with or delays necessary medical care, leading to clinical deterioration and unnecessary, preventable death. Stigma shortens life and is a hate crime.
Continue to: The extremely high suicide rates...
The extremely high suicide rates among individuals with serious mental illness, who live under the oppressiveness of stigma, is another example of how stigma is a hate crime that can cause patients with psychiatric disorders to give up and end their lives. Zaheer et al9 found that young patients with schizophrenia had an astronomical suicide rate compared to the general population (1 in 52 in individuals with schizophrenia, compared to 12 in 100,000 in the general population, roughly a 200-fold increase!). This is clearly a consequence of stigma and discrimination,10 which leads to demoralization, shame, loneliness, distress, and hopelessness. Stigma can be fatal, and that makes it a hate crime.
Stigma also limits vocational opportunities for individuals with mental illness. They are either not hired, or quickly fired. Even highly educated professionals such as physicians, nurses, lawyers, or teachers can lose their jobs if they divulge a history of a psychiatric disorder or alcohol or substance abuse, regardless of whether they are receiving treatment and are medically in remission. Even highly qualified politicians have been deemed “ineligible” for higher office if they disclose a history of psychiatric treatment. Stigma is loaded with outrageous discrimination that deprives our patients of “the pursuit of happiness,” a fundamental constitutional right.
Stigma surrounding the mental health professions
Stigma also engulfs mental health professionals, simply because they deal with psychiatric patients every day. In a classic article titled “The Enigma of Stigma,”11 Dr. Paul Fink, past president of the American Psychiatric Association (1988-1989), described how psychiatrists are perceived as “different” from other physicians by the public and by the media. He said psychiatrists are tarred by the same brush as their patients as “undesirables” in society. And movies such as Psycho and One Flew Over the Cuckoo’s Nest reinforce the stigma against both psychiatric patients and the psychiatrists and nurses who treat them. The health care system that carves out “behavioral health” from the umbrella of “medical care” further accentuates the stigma by portraying the “separateness” of psychiatry, a genuine medical specialty, from its fellow medical disciplines. This becomes fodder for the antipsychiatry movement at every turn and can even lead to questioning the existence of mental illness, as Thomas Szasz12 did by declaring that mental illness is a myth and describing psychiatry as “the science of lies.” No other medical specialty endures abuse and insults like psychiatry, and that’s a direct result of stigma.
Extinguishing stigma is a societal imperative
So what can be done to squelch stigma and defeat it once and for all, so that psychiatric patients can be treated with dignity and compassion, like people with cancer, heart attacks, diabetes, or brain tumors? The pandemic, terrible as it has been for the entire world, did have the silver lining of raising awareness about the ubiquity of psychiatric symptoms, such as anxiety and depression, across all ages, genders, educational and religious backgrounds, and socioeconomic classes. But there should also be a robust legal battle against the damaging effects of stigma. There are laws to sanction and penalize hate speech and hate crimes that must be implemented when stigma is documented. There are also parity laws, but they have no teeth and have not ameliorated the insurance discrepancies and economic burden of psychiatric disorders. A bold step would be to reclassify serious psychiatric brain disorders (schizophrenia, bipolar disorder, major depressive disorder, OCD, attention-deficit/hyperactivity disorder, generalized anxiety disorder/panic attacks, and borderline personality disorder) as neurologic disorders, which would automatically give patients with these disorders broad access to medical care, which happened when autism was reclassified as a neurologic disorder. Finally, a much more intensive public education must be disseminated about the neurobiological etiologies, brain structure, and function in psychiatric disorders, and the psychiatric symptoms associated with all neurologic disorders. Regrettably, empathy can be difficult to teach.
Stigma is hate speech and a hate crime. It must be permanently eliminated by effective laws and by erasing the widespread ignorance about the medical and neurologic roots of mental disorders, and by emphasizing the fact that they are as treatable as other general medical conditions.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
3. Druss BG, Rosenheck RA. Use of medical services by veterans with mental disorders. Psychosomatics. 1997;38(5):451-458.
4. Druss BG, Rosenheck RA. Mental disorders and access to medical care in the United States. Am J Psychiatry. 1998;155(12):1775-1777.
5. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
6. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
7. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
8. Balasuriya L, Quinton JK, Canavan ME, et al. The association between history of depression and access to care among Medicare beneficiaries during the COVID-19 pandemic. J Gen Intern Med. 2021;36(12):3778-3785.
9. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
10. Brohan E, Thornicroft G, Rüsch N, et al. Measuring discrimination experienced by people with a mental illness: replication of the short-form DISCUS in six world regions. Psychol Med. 2022:1-11. doi:10.1017/S0033291722000630
11. Fink P. The enigma of stigma and its relation to psychiatric education. Psychiatric Annals. 1983;13(9):669-690.
12. Szasz T. The Myth of Mental Illness. Harper Collins; 1960.
Having witnessed the devastating impact of stigma on patients with mental illness throughout my psychiatric career, I am fed up and disgusted with this malevolent scourge.
I regard the stigma that engulfs neuropsychiatric disorders as a malignancy that mutilates patients’ souls and hastens their mortality.
Stigma is hate speech
How would you feel if you had a serious medical illness, a disabling brain disorder such as schizophrenia, depression, or anxiety, and people refer to you with pejorative and insulting terms such as crazy, deranged, lunatic, unhinged, nutty, insane, wacky, berserk, cuckoo, bonkers, flaky, screwball, or unglued? This is hate speech generated by stigma against people with mental illness. Individuals with heart disease, cancer, or diabetes never get called such disgraceful and stigmatizing terms that shame, stain, besmirch, and scar them, which happens daily to persons with psychiatric brain disorders.
The damage and harm of the discriminatory stigma on our patients is multifaceted. It is painful, detrimental, pernicious, and deleterious. It is corrosive to their spirits, crippling to their self-image, and subversive to their self-confidence. Hate speech is not simply words, but a menacing weapon that assaults the core humanity of medically ill psychiatric patients.
Although hate speech is punishable by law, there are rarely any legal actions against those who hurl hate speech at psychiatric patients every day. Society has institutionalized the stigma of mental illness and takes it in stride instead of recognizing it as an illegal, harmful act.
Long before the stresses of the COVID-19 pandemic, 43% of the population had been shown to experience a diagnosable psychiatric disorder over the course of their life.1 Thus, tens of millions of people are burdened by stigma and the hate speech associated with it. This is directly related to massive ignorance about mental illness being the result of a neurobiological condition due to either genetic or intrauterine adverse events that disrupt brain development. Delusions and hallucinations are symptoms of a malfunctioning brain, depression is not a sign of personal weakness, anxiety is the most prevalent mental disorder in the world, and obsessive-compulsive disorder (OCD) is not odd behavior but the result of dysfunction of neural circuits. Correcting public misperceptions about psychiatric brain disorders can mitigate stigma, but it has yet to happen.
Stigma is a hate crime
Stigma can accelerate physical death and premature mortality. Many studies have confirmed that persons with schizophrenia do not receive basic primary care treatments for the life-shortening medical conditions that often afflict them, such as diabetes, dyslipidemia, and hypertension.2 Stigma is responsible for a significant disparity of medical3-5 and intensive care6 among individuals with mental illness compared to the general population. It’s no wonder most psychiatric disorders are associated with accelerated mortality.7 A recent study during the pandemic by Balasuriya et al8 reported that patients with depression had poor access to care. Stigma interferes with or delays necessary medical care, leading to clinical deterioration and unnecessary, preventable death. Stigma shortens life and is a hate crime.
Continue to: The extremely high suicide rates...
The extremely high suicide rates among individuals with serious mental illness, who live under the oppressiveness of stigma, is another example of how stigma is a hate crime that can cause patients with psychiatric disorders to give up and end their lives. Zaheer et al9 found that young patients with schizophrenia had an astronomical suicide rate compared to the general population (1 in 52 in individuals with schizophrenia, compared to 12 in 100,000 in the general population, roughly a 200-fold increase!). This is clearly a consequence of stigma and discrimination,10 which leads to demoralization, shame, loneliness, distress, and hopelessness. Stigma can be fatal, and that makes it a hate crime.
Stigma also limits vocational opportunities for individuals with mental illness. They are either not hired, or quickly fired. Even highly educated professionals such as physicians, nurses, lawyers, or teachers can lose their jobs if they divulge a history of a psychiatric disorder or alcohol or substance abuse, regardless of whether they are receiving treatment and are medically in remission. Even highly qualified politicians have been deemed “ineligible” for higher office if they disclose a history of psychiatric treatment. Stigma is loaded with outrageous discrimination that deprives our patients of “the pursuit of happiness,” a fundamental constitutional right.
Stigma surrounding the mental health professions
Stigma also engulfs mental health professionals, simply because they deal with psychiatric patients every day. In a classic article titled “The Enigma of Stigma,”11 Dr. Paul Fink, past president of the American Psychiatric Association (1988-1989), described how psychiatrists are perceived as “different” from other physicians by the public and by the media. He said psychiatrists are tarred by the same brush as their patients as “undesirables” in society. And movies such as Psycho and One Flew Over the Cuckoo’s Nest reinforce the stigma against both psychiatric patients and the psychiatrists and nurses who treat them. The health care system that carves out “behavioral health” from the umbrella of “medical care” further accentuates the stigma by portraying the “separateness” of psychiatry, a genuine medical specialty, from its fellow medical disciplines. This becomes fodder for the antipsychiatry movement at every turn and can even lead to questioning the existence of mental illness, as Thomas Szasz12 did by declaring that mental illness is a myth and describing psychiatry as “the science of lies.” No other medical specialty endures abuse and insults like psychiatry, and that’s a direct result of stigma.
Extinguishing stigma is a societal imperative
So what can be done to squelch stigma and defeat it once and for all, so that psychiatric patients can be treated with dignity and compassion, like people with cancer, heart attacks, diabetes, or brain tumors? The pandemic, terrible as it has been for the entire world, did have the silver lining of raising awareness about the ubiquity of psychiatric symptoms, such as anxiety and depression, across all ages, genders, educational and religious backgrounds, and socioeconomic classes. But there should also be a robust legal battle against the damaging effects of stigma. There are laws to sanction and penalize hate speech and hate crimes that must be implemented when stigma is documented. There are also parity laws, but they have no teeth and have not ameliorated the insurance discrepancies and economic burden of psychiatric disorders. A bold step would be to reclassify serious psychiatric brain disorders (schizophrenia, bipolar disorder, major depressive disorder, OCD, attention-deficit/hyperactivity disorder, generalized anxiety disorder/panic attacks, and borderline personality disorder) as neurologic disorders, which would automatically give patients with these disorders broad access to medical care, which happened when autism was reclassified as a neurologic disorder. Finally, a much more intensive public education must be disseminated about the neurobiological etiologies, brain structure, and function in psychiatric disorders, and the psychiatric symptoms associated with all neurologic disorders. Regrettably, empathy can be difficult to teach.
Stigma is hate speech and a hate crime. It must be permanently eliminated by effective laws and by erasing the widespread ignorance about the medical and neurologic roots of mental disorders, and by emphasizing the fact that they are as treatable as other general medical conditions.
Having witnessed the devastating impact of stigma on patients with mental illness throughout my psychiatric career, I am fed up and disgusted with this malevolent scourge.
I regard the stigma that engulfs neuropsychiatric disorders as a malignancy that mutilates patients’ souls and hastens their mortality.
Stigma is hate speech
How would you feel if you had a serious medical illness, a disabling brain disorder such as schizophrenia, depression, or anxiety, and people refer to you with pejorative and insulting terms such as crazy, deranged, lunatic, unhinged, nutty, insane, wacky, berserk, cuckoo, bonkers, flaky, screwball, or unglued? This is hate speech generated by stigma against people with mental illness. Individuals with heart disease, cancer, or diabetes never get called such disgraceful and stigmatizing terms that shame, stain, besmirch, and scar them, which happens daily to persons with psychiatric brain disorders.
The damage and harm of the discriminatory stigma on our patients is multifaceted. It is painful, detrimental, pernicious, and deleterious. It is corrosive to their spirits, crippling to their self-image, and subversive to their self-confidence. Hate speech is not simply words, but a menacing weapon that assaults the core humanity of medically ill psychiatric patients.
Although hate speech is punishable by law, there are rarely any legal actions against those who hurl hate speech at psychiatric patients every day. Society has institutionalized the stigma of mental illness and takes it in stride instead of recognizing it as an illegal, harmful act.
Long before the stresses of the COVID-19 pandemic, 43% of the population had been shown to experience a diagnosable psychiatric disorder over the course of their life.1 Thus, tens of millions of people are burdened by stigma and the hate speech associated with it. This is directly related to massive ignorance about mental illness being the result of a neurobiological condition due to either genetic or intrauterine adverse events that disrupt brain development. Delusions and hallucinations are symptoms of a malfunctioning brain, depression is not a sign of personal weakness, anxiety is the most prevalent mental disorder in the world, and obsessive-compulsive disorder (OCD) is not odd behavior but the result of dysfunction of neural circuits. Correcting public misperceptions about psychiatric brain disorders can mitigate stigma, but it has yet to happen.
Stigma is a hate crime
Stigma can accelerate physical death and premature mortality. Many studies have confirmed that persons with schizophrenia do not receive basic primary care treatments for the life-shortening medical conditions that often afflict them, such as diabetes, dyslipidemia, and hypertension.2 Stigma is responsible for a significant disparity of medical3-5 and intensive care6 among individuals with mental illness compared to the general population. It’s no wonder most psychiatric disorders are associated with accelerated mortality.7 A recent study during the pandemic by Balasuriya et al8 reported that patients with depression had poor access to care. Stigma interferes with or delays necessary medical care, leading to clinical deterioration and unnecessary, preventable death. Stigma shortens life and is a hate crime.
Continue to: The extremely high suicide rates...
The extremely high suicide rates among individuals with serious mental illness, who live under the oppressiveness of stigma, is another example of how stigma is a hate crime that can cause patients with psychiatric disorders to give up and end their lives. Zaheer et al9 found that young patients with schizophrenia had an astronomical suicide rate compared to the general population (1 in 52 in individuals with schizophrenia, compared to 12 in 100,000 in the general population, roughly a 200-fold increase!). This is clearly a consequence of stigma and discrimination,10 which leads to demoralization, shame, loneliness, distress, and hopelessness. Stigma can be fatal, and that makes it a hate crime.
Stigma also limits vocational opportunities for individuals with mental illness. They are either not hired, or quickly fired. Even highly educated professionals such as physicians, nurses, lawyers, or teachers can lose their jobs if they divulge a history of a psychiatric disorder or alcohol or substance abuse, regardless of whether they are receiving treatment and are medically in remission. Even highly qualified politicians have been deemed “ineligible” for higher office if they disclose a history of psychiatric treatment. Stigma is loaded with outrageous discrimination that deprives our patients of “the pursuit of happiness,” a fundamental constitutional right.
Stigma surrounding the mental health professions
Stigma also engulfs mental health professionals, simply because they deal with psychiatric patients every day. In a classic article titled “The Enigma of Stigma,”11 Dr. Paul Fink, past president of the American Psychiatric Association (1988-1989), described how psychiatrists are perceived as “different” from other physicians by the public and by the media. He said psychiatrists are tarred by the same brush as their patients as “undesirables” in society. And movies such as Psycho and One Flew Over the Cuckoo’s Nest reinforce the stigma against both psychiatric patients and the psychiatrists and nurses who treat them. The health care system that carves out “behavioral health” from the umbrella of “medical care” further accentuates the stigma by portraying the “separateness” of psychiatry, a genuine medical specialty, from its fellow medical disciplines. This becomes fodder for the antipsychiatry movement at every turn and can even lead to questioning the existence of mental illness, as Thomas Szasz12 did by declaring that mental illness is a myth and describing psychiatry as “the science of lies.” No other medical specialty endures abuse and insults like psychiatry, and that’s a direct result of stigma.
Extinguishing stigma is a societal imperative
So what can be done to squelch stigma and defeat it once and for all, so that psychiatric patients can be treated with dignity and compassion, like people with cancer, heart attacks, diabetes, or brain tumors? The pandemic, terrible as it has been for the entire world, did have the silver lining of raising awareness about the ubiquity of psychiatric symptoms, such as anxiety and depression, across all ages, genders, educational and religious backgrounds, and socioeconomic classes. But there should also be a robust legal battle against the damaging effects of stigma. There are laws to sanction and penalize hate speech and hate crimes that must be implemented when stigma is documented. There are also parity laws, but they have no teeth and have not ameliorated the insurance discrepancies and economic burden of psychiatric disorders. A bold step would be to reclassify serious psychiatric brain disorders (schizophrenia, bipolar disorder, major depressive disorder, OCD, attention-deficit/hyperactivity disorder, generalized anxiety disorder/panic attacks, and borderline personality disorder) as neurologic disorders, which would automatically give patients with these disorders broad access to medical care, which happened when autism was reclassified as a neurologic disorder. Finally, a much more intensive public education must be disseminated about the neurobiological etiologies, brain structure, and function in psychiatric disorders, and the psychiatric symptoms associated with all neurologic disorders. Regrettably, empathy can be difficult to teach.
Stigma is hate speech and a hate crime. It must be permanently eliminated by effective laws and by erasing the widespread ignorance about the medical and neurologic roots of mental disorders, and by emphasizing the fact that they are as treatable as other general medical conditions.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
3. Druss BG, Rosenheck RA. Use of medical services by veterans with mental disorders. Psychosomatics. 1997;38(5):451-458.
4. Druss BG, Rosenheck RA. Mental disorders and access to medical care in the United States. Am J Psychiatry. 1998;155(12):1775-1777.
5. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
6. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
7. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
8. Balasuriya L, Quinton JK, Canavan ME, et al. The association between history of depression and access to care among Medicare beneficiaries during the COVID-19 pandemic. J Gen Intern Med. 2021;36(12):3778-3785.
9. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
10. Brohan E, Thornicroft G, Rüsch N, et al. Measuring discrimination experienced by people with a mental illness: replication of the short-form DISCUS in six world regions. Psychol Med. 2022:1-11. doi:10.1017/S0033291722000630
11. Fink P. The enigma of stigma and its relation to psychiatric education. Psychiatric Annals. 1983;13(9):669-690.
12. Szasz T. The Myth of Mental Illness. Harper Collins; 1960.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
3. Druss BG, Rosenheck RA. Use of medical services by veterans with mental disorders. Psychosomatics. 1997;38(5):451-458.
4. Druss BG, Rosenheck RA. Mental disorders and access to medical care in the United States. Am J Psychiatry. 1998;155(12):1775-1777.
5. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
6. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
7. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
8. Balasuriya L, Quinton JK, Canavan ME, et al. The association between history of depression and access to care among Medicare beneficiaries during the COVID-19 pandemic. J Gen Intern Med. 2021;36(12):3778-3785.
9. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
10. Brohan E, Thornicroft G, Rüsch N, et al. Measuring discrimination experienced by people with a mental illness: replication of the short-form DISCUS in six world regions. Psychol Med. 2022:1-11. doi:10.1017/S0033291722000630
11. Fink P. The enigma of stigma and its relation to psychiatric education. Psychiatric Annals. 1983;13(9):669-690.
12. Szasz T. The Myth of Mental Illness. Harper Collins; 1960.
The brain’s Twitter system: Neuronal extracellular vesicles
Twitter, a microblogging and social networking service, has become a “go-to’” for conversations, updates, breaking news, and sharing the more mundane aspects of our lives. Tweets, which were lengthened from 140 to 280 characters in 2017, rapidly communicate and disseminate information to a wide audience. Generally, tweets are visible to everyone, though users can mute and block other users from viewing their tweets. Spikes in tweets and tweeting frequency reflect hyper-current events: the last minutes of the Super Bowl, certification of an election, or a new movie release. In fact, social scientists have analyzed tweet frequencies to examine the impact of local and national events. However, few are aware that like celebrities, politicians, influencers, and ordinary citizens, the human brain also tweets.
In this article, we describe the components of the brain’s “Twitter” system, how it works, and how it might someday be used to improve the diagnosis and treatment of psychiatric disorders.
Brain tweets
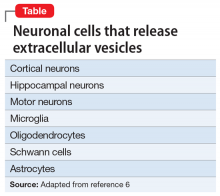
The brain’s Twitter system involves extracellular vesicles (EVs), tiny (<1 µm) membrane-bound vesicles that are released from neurons, glia, and other neuronal cells (Table). These EVs cross the blood-brain barrier and facilitate cell-to-cell communication within and among tissues (Figure 1).
First described in the 1980s,1 EVs are secreted by a diverse array of cells: mast cells reticulocytes, epithelial cells, immune cells, neurons, glia, and oligodendrocytes. Like tweets, EVs rapidly disseminate packets of information throughout the brain and body and direct the molecular activity of recipient cells in both health and disease. These “brain tweets” contain short, circumscribed messages, and the characters are the EV cargos: RNAs, proteins, lipids, and metabolites. Like a Twitter feed, EVs cast a wide communication net across the body, much of which finds its way to the blood. As neuroscientists, we can follow these tweets by isolating tissue-derived EVs in plasma and examining their surface molecules and cargo. By following this Twitter feed, we can tap into important molecular communications and identify “trending” (evolving) pathological processes, and perhaps use the brain Twitter feed to improve diagnosis and treatments. We can pinpoint, in the blood, signals from CNS processes, down to the level of identifying EV cargos from specific brain cell types.
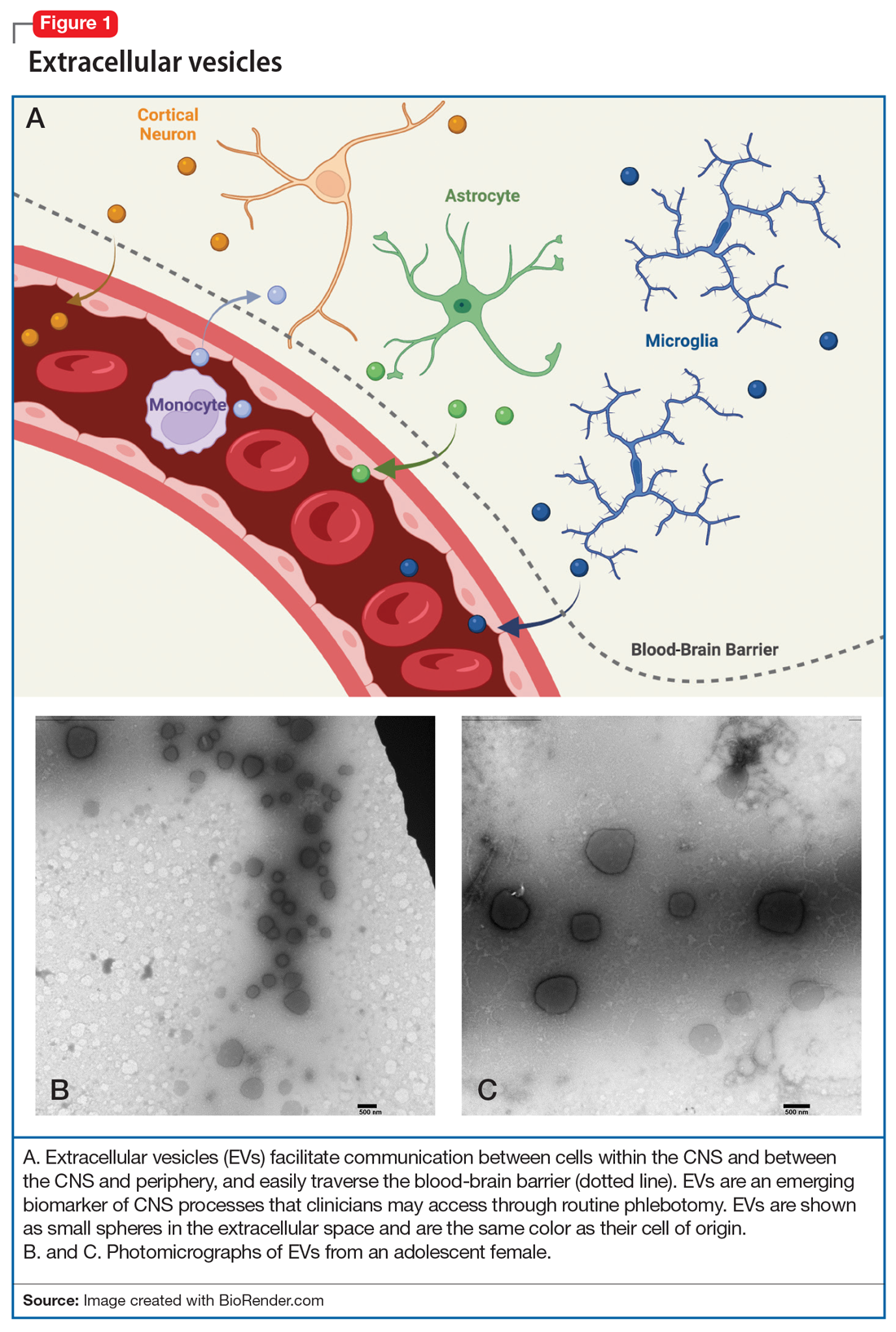
Within the CNS, EVs are secreted by neurons, where they may modulate synaptic plasticity and transfer molecular cargo among neurons. EVs also facilitate communication between neurons and glia, maintain homeostasis, trigger neuroprotective processes, and even regulate synaptic transmission.2
What’s in a brain tweet?
To discuss what’s in a brain tweet, we must first understand how a brain tweet is composed. EVs are pinched off from membranes of intercellular structures (eg, golgi or endoplasmic reticulum) or pinched off directly from cell membranes, where upon release they become EVs. There is a complex cellular machinery that transports what ultimately becomes an EV to the cell membrane.3 EVs contain unique mixtures of lipids, proteins, and nucleic acids (eg, microRNA [miRNA], mRNA, and noncoding RNA).4 To date, nearly 10,000 proteins, 11,000 lipids, 3,500 mRNAs, and 3,000 miRNAs have been identified as cargos in extracellular vesicles (Figure 1). Similar to how the release of EVs is dependent on complex intracellular machinery, the packing of these contents into what will become the EV involves a parallel set of complex machinery that is largely directed by endosomal sorting complexes required for transport (ESCRT) proteins.5 Of interest, when viruses attack cells, they hijack this EV packaging system to package and release new viruses. EVs vary in size, shape, and density; this variation is related to the cell origin, among other things. EVs also differ in their membrane lipid composition and in terms of transmembrane proteins as well as the proteins that facilitate EV binding to target cells (Figure 2).6 Ultimately, these exosomes are taken up by the recipient cells.
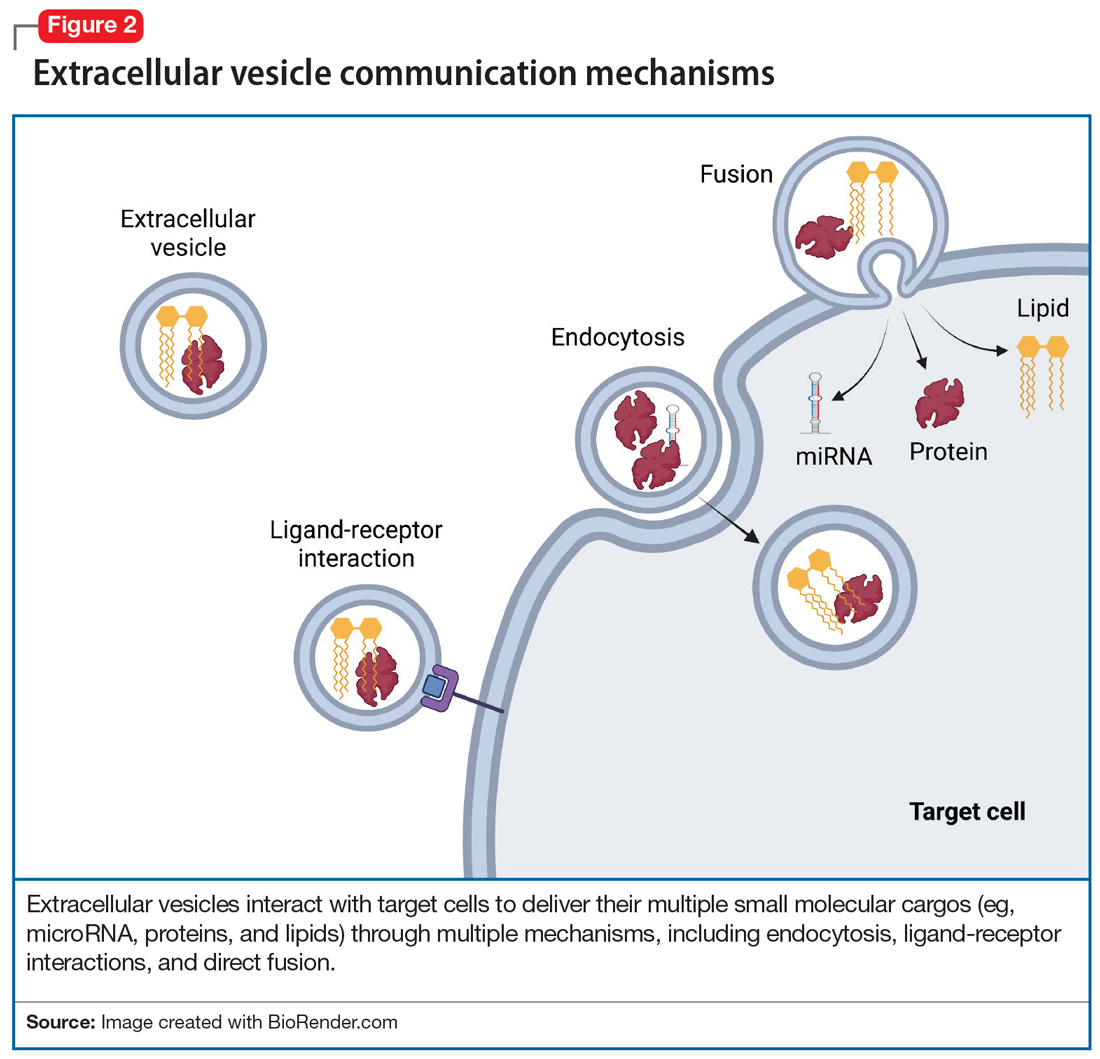
EV-facilitated neuron-to-neuron tweets have been implicated in neuronal growth and differentiation.7 EV-driven communication between cells also can decrease dendrite growth and can trigger microglia to prune synapses.8 EVs from glial cells may promote neuronal integrity, directly boost presynaptic glutamate release,9 or even, through miRNAs, change the expression of glutamate receptors.10 EVs from astrocytes transport proteins that enable neuronal repair, while EVs from microglia regulate neuronal homeostasis. EV cargos—lipids, proteins, and miRNAs—from neurons modify signal transduction and protein expression in recipient cells. Taken together, data suggest that EVs facilitate anterograde and retrograde transfer of signals across synapses,7,11 a putative mechanism for driving synaptic plasticity,12 which is a process implicated in the therapeutic efficacy of psychotropic medications and psychotherapies.
Continue to: #Targets and #neuron
#Targets and #neuron
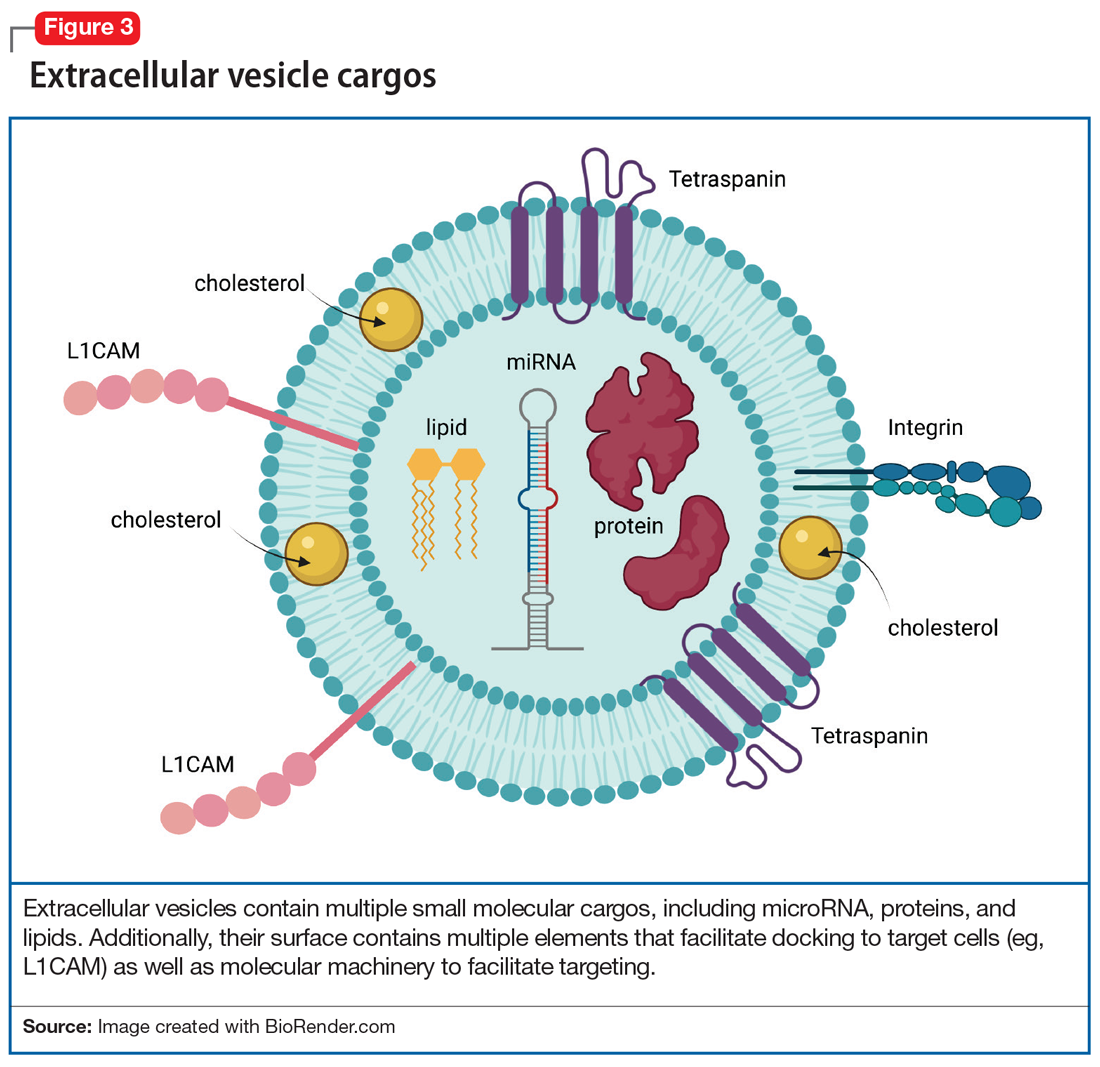
Adding a hashtag to a tweet links it to other tweets, just as membrane features of EVs direct how EVs link to target cells. When these EVs bind to target cells, they fuse and release their cargo into the target cell (Figure 2). These directed cargo—whether mRNA, proteins, or other molecules—can direct the recipient cell to modify its firing rate (in the case of neurons), alter transmitter release, and increase or decrease expression of various genes. The targeting process is complex, and our understanding of this process is evolving. Briefly, integrin, lipid composition, glycans (eg, polysaccharides), and tetraspanin components of EVs influence their affinity for specific target cells.13 Recently, we have been able to read these hashtags and isolate cell-specific, neuron-derived EVs. Immunoadsorption techniques that leverage antibodies against L1 cell adhesion molecule protein (L1CAM(+)), primarily expressed in neurons, can identify neuronally-derived EVs (Figure 3). The specific EVs contain cargos of neuronal origin and provide a “window” into molecular processes in the brain by way of the blood (or other peripheral fluids). In following the neuronal tweets, we can follow molecular measures of important brain molecules in biofluids outside the CNS, including saliva and potentially urine (Figure 1B and 1C). In following these specific neuronal Twitter feeds, we can gain critical insights into specific brain processes.
EVs in psychiatric disorders
EVs are implicated in neuroinflammation,14 neurogenesis, synaptic plasticity, and epigenetic regulation—all processes that are involved in the pathophysiology of psychiatric disorders. Postmortem research suggests that EVs in the brain carry proinflammatory molecules from microglia, as well as secretions of regulatory miRNA that are responsible for synaptic plasticity and dendritic growth in depression, bipolar disorder, schizophrenia, and addiction. In addition, second-generation antipsychotics change the composition of EV cargos in the brain, altering their RNA, protein, and lipid content, often reflecting profound changes in gene expression in various cells in the CNS. In our lab, we have identified several molecules in plasma EVs, both lipids and miRNA, that can potentially predict the response to treatment of pediatric anxiety with selective serotonin reuptake inhibitors as well as opiate addiction.15
Further, given our increasing understanding of the way in which EV cargo reflects neuronal physiology as well as the potential pathophysiologic states of cells (including neurons), studying EVs’ molecular content can identify molecular messages—in blood—that are derived from the neurons in the brain. Having the tools to examine molecular brain regulators or other markers of disease progression (eg, beta amyloid) or brain health (eg, brain-derived neurotrophic factor) may advance our understanding and treatment of psychiatric disorders and create opportunities for precision medicine driven by biological rather than ethnologic and phenomenological markers. Whereas in the not-too-distant past molecular processes in the brain were only accessible through invasive measures—such as brain biopsy or through a lumbar puncture—studying CNS-derived EVs in blood offers us an opportunity to gain access to brain molecular signatures with relative ease. Often, these molecular signatures predate clinical changes by years or months, allowing us the prospects of potentially identifying and treating CNS disorders early on, possibly even before the onset of symptoms.
Therapeutic use of the Twitter feed
EV may be used to alter brain receptor structures in a targeted way to facilitate treatment of various psychiatric disorders. One example is a proof-of-concept study in mice in which administration of artificially manufactured EVs led to a decrease of opioid receptor mu.16 This was done by constructing EVs that carry neuron-specific rabies viral glycoprotein (RVG) peptide on the membrane surface to deliver mu opioid receptor small interfering RNA into the brain. This resulted in downregulation of mu opioid receptor and a decrease in morphine relapse.16
Additional ways in which EVs can be used therapeutically is via targeted drug delivery CNS methods. EVs may represent the next generation of treatment by allowing not only medication transport into the CNS,17 but also by facilitating directed CNS transport. What if we could use a molecular hashtag to send a dopaminergic agent to the substantia nigra of a patient with Parkinson disease but avoid sending that same treatment to the limbic cortex, where it might produce perceptual disturbances or hallucinations? In the future, EVs may help clinicians access the CNS, which is traditionally restricted by the blood brain barrier, and make it easier to achieve CNS concentrations of medications13 while decreasing medication exposure in other parts of the body. The therapeutic potential of EVs for medication delivery and regenerative medicine is awe-inspiring. Several studies have modified EVs to improve their therapeutic properties and to target delivery to specific cells13 by leveraging EV surface markers.18
Future directions for EVs
A better understanding of neuron-derived EVs may eventually help us abandon nosology-based diagnostic criteria and adopt molecular-based diagnostic approaches in psychiatry. It may allow us to consider a molecular synaptic etiology of psychiatric disorders, and diagnose patients based on synaptic pathology utilizing “neuron-derived EV liquid biopsies.” Such a shift would align psychiatry with other medical fields in which diagnosis and treatment are often based on biopsies and blood tests. Because proteins in EVs often exist in their native states, intact with their posttranslational modifications, they provide a window into testing their actual in vivo functioning. EVs have an immense potential to revolutionize psychiatric diagnosis, facilitate precision treatment, predict response, and discover much-needed novel therapeutics.
Bottom Line
Much like a tweet, extracellular vesicles (EVs) encode short messages that are transmit ted efficiently throughout the CNS and body. They may represent a reservoir for CNS-specific biomarkers that can be is olated from plasma to guide psychiatric diagnosis and treatment. EVs represent a new frontier in the molecular study of psychiatric illness.
Related Resources
Vesiclepedia. www.microvesicles.org/
1. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329-339. doi:10.1083/jcb.97.2.329
2. Huo L, Du X, Li X, et al. The emerging role of neural cell-derived exosomes in intercellular communication in health and neurodegenerative diseases. Front Neurosci. 2021;15:738442. doi:10.3389/fnins.2021
3. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-83. doi: 10.1083/jcb.201211138
4. Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688-692. doi:10.1016/j.jmb.2015.09.019
5. Babst M. A protein’s final ESCRT. Traffic. 2005;6(1):2-9. doi:10.1111/j.1600-0854.2004.00246.x
6. Anakor E, Le Gall L, Dumonceaux J, et al. Exosomes in ageing and motor neurone disease: biogenesis, uptake mechanisms, modifications in disease and uses in the development of biomarkers and therapeutics. Cells. 2021;10(11)29-30. doi:10.3390/cells10112930
7. Chivet M, Javalet C, Hemming F, et al. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013;41(1):241-244. doi:10.1042/BST20120266
8. Liu HY, Huang CM, Hung YF, et al. The microRNAs Let7c and miR21 are recognized by neuronal Toll-like receptor 7 to restrict dendritic growth of neurons. Exp Neurol. 2015;269:202-212. doi:10.1016/j.expneurol.2015.04.011
9. Antonucci F, Turola E, Riganti L, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31(5):1231-1240. doi:10.1038/emboj.2011.489
10. Goncalves MB, Malmqvist T, Clarke E, et al. Neuronal RARβ signaling modulates PTEN activity directly in neurons and via exosome transfer in astrocytes to prevent glial scar formation and induce spinal cord regeneration. J Neurosci. 2015;35(47):15731-15745. doi:10.1523/JNEUROSCI.1339-15.2015
11. Korkut C, Li Y, Koles K, et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77(6):1039-1046. doi:10.1016/j.neuron.2013.01.013
12. Chivet M, Javalet C, Laulagnier K, et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3(1):24722. doi:10.3402/jev.v3
13. Dickens AM, Tovar-Y-Romo LB, Yoo SW, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10(473). doi:10.1126/scisignal.aai7696
14. Strawn J, Levine A. Treatment response biomarkers in anxiety disorders: from neuroimaging to neuronally-derived extracellular vesicles and beyond. Biomark Neuropsychiatry. 2020;3:100024.
15. Liu Y, Li D, Liu Z, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543. doi:10.1038/srep17543
16. Shahjin F, Chand S, Yelamanchili S V. Extracellular vesicles as drug delivery vehicles to the central nervous system. J Neuroimmune Pharmacol. 2020;15(3):443-458. doi:10.1007/s11481-019-09875-w
17. Murphy DE, de Jong OG, Brouwer M, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):1-12. doi:10.1038/s12276-019-0223-5
18. Meng W, He C, Hao Y, et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585-598. doi:10.1080/10717544.2020.1748758
Twitter, a microblogging and social networking service, has become a “go-to’” for conversations, updates, breaking news, and sharing the more mundane aspects of our lives. Tweets, which were lengthened from 140 to 280 characters in 2017, rapidly communicate and disseminate information to a wide audience. Generally, tweets are visible to everyone, though users can mute and block other users from viewing their tweets. Spikes in tweets and tweeting frequency reflect hyper-current events: the last minutes of the Super Bowl, certification of an election, or a new movie release. In fact, social scientists have analyzed tweet frequencies to examine the impact of local and national events. However, few are aware that like celebrities, politicians, influencers, and ordinary citizens, the human brain also tweets.
In this article, we describe the components of the brain’s “Twitter” system, how it works, and how it might someday be used to improve the diagnosis and treatment of psychiatric disorders.
Brain tweets
The brain’s Twitter system involves extracellular vesicles (EVs), tiny (<1 µm) membrane-bound vesicles that are released from neurons, glia, and other neuronal cells (Table). These EVs cross the blood-brain barrier and facilitate cell-to-cell communication within and among tissues (Figure 1).
First described in the 1980s,1 EVs are secreted by a diverse array of cells: mast cells reticulocytes, epithelial cells, immune cells, neurons, glia, and oligodendrocytes. Like tweets, EVs rapidly disseminate packets of information throughout the brain and body and direct the molecular activity of recipient cells in both health and disease. These “brain tweets” contain short, circumscribed messages, and the characters are the EV cargos: RNAs, proteins, lipids, and metabolites. Like a Twitter feed, EVs cast a wide communication net across the body, much of which finds its way to the blood. As neuroscientists, we can follow these tweets by isolating tissue-derived EVs in plasma and examining their surface molecules and cargo. By following this Twitter feed, we can tap into important molecular communications and identify “trending” (evolving) pathological processes, and perhaps use the brain Twitter feed to improve diagnosis and treatments. We can pinpoint, in the blood, signals from CNS processes, down to the level of identifying EV cargos from specific brain cell types.

Within the CNS, EVs are secreted by neurons, where they may modulate synaptic plasticity and transfer molecular cargo among neurons. EVs also facilitate communication between neurons and glia, maintain homeostasis, trigger neuroprotective processes, and even regulate synaptic transmission.2
What’s in a brain tweet?
To discuss what’s in a brain tweet, we must first understand how a brain tweet is composed. EVs are pinched off from membranes of intercellular structures (eg, golgi or endoplasmic reticulum) or pinched off directly from cell membranes, where upon release they become EVs. There is a complex cellular machinery that transports what ultimately becomes an EV to the cell membrane.3 EVs contain unique mixtures of lipids, proteins, and nucleic acids (eg, microRNA [miRNA], mRNA, and noncoding RNA).4 To date, nearly 10,000 proteins, 11,000 lipids, 3,500 mRNAs, and 3,000 miRNAs have been identified as cargos in extracellular vesicles (Figure 1). Similar to how the release of EVs is dependent on complex intracellular machinery, the packing of these contents into what will become the EV involves a parallel set of complex machinery that is largely directed by endosomal sorting complexes required for transport (ESCRT) proteins.5 Of interest, when viruses attack cells, they hijack this EV packaging system to package and release new viruses. EVs vary in size, shape, and density; this variation is related to the cell origin, among other things. EVs also differ in their membrane lipid composition and in terms of transmembrane proteins as well as the proteins that facilitate EV binding to target cells (Figure 2).6 Ultimately, these exosomes are taken up by the recipient cells.

EV-facilitated neuron-to-neuron tweets have been implicated in neuronal growth and differentiation.7 EV-driven communication between cells also can decrease dendrite growth and can trigger microglia to prune synapses.8 EVs from glial cells may promote neuronal integrity, directly boost presynaptic glutamate release,9 or even, through miRNAs, change the expression of glutamate receptors.10 EVs from astrocytes transport proteins that enable neuronal repair, while EVs from microglia regulate neuronal homeostasis. EV cargos—lipids, proteins, and miRNAs—from neurons modify signal transduction and protein expression in recipient cells. Taken together, data suggest that EVs facilitate anterograde and retrograde transfer of signals across synapses,7,11 a putative mechanism for driving synaptic plasticity,12 which is a process implicated in the therapeutic efficacy of psychotropic medications and psychotherapies.
Continue to: #Targets and #neuron
#Targets and #neuron
Adding a hashtag to a tweet links it to other tweets, just as membrane features of EVs direct how EVs link to target cells. When these EVs bind to target cells, they fuse and release their cargo into the target cell (Figure 2). These directed cargo—whether mRNA, proteins, or other molecules—can direct the recipient cell to modify its firing rate (in the case of neurons), alter transmitter release, and increase or decrease expression of various genes. The targeting process is complex, and our understanding of this process is evolving. Briefly, integrin, lipid composition, glycans (eg, polysaccharides), and tetraspanin components of EVs influence their affinity for specific target cells.13 Recently, we have been able to read these hashtags and isolate cell-specific, neuron-derived EVs. Immunoadsorption techniques that leverage antibodies against L1 cell adhesion molecule protein (L1CAM(+)), primarily expressed in neurons, can identify neuronally-derived EVs (Figure 3). The specific EVs contain cargos of neuronal origin and provide a “window” into molecular processes in the brain by way of the blood (or other peripheral fluids). In following the neuronal tweets, we can follow molecular measures of important brain molecules in biofluids outside the CNS, including saliva and potentially urine (Figure 1B and 1C). In following these specific neuronal Twitter feeds, we can gain critical insights into specific brain processes.

EVs in psychiatric disorders
EVs are implicated in neuroinflammation,14 neurogenesis, synaptic plasticity, and epigenetic regulation—all processes that are involved in the pathophysiology of psychiatric disorders. Postmortem research suggests that EVs in the brain carry proinflammatory molecules from microglia, as well as secretions of regulatory miRNA that are responsible for synaptic plasticity and dendritic growth in depression, bipolar disorder, schizophrenia, and addiction. In addition, second-generation antipsychotics change the composition of EV cargos in the brain, altering their RNA, protein, and lipid content, often reflecting profound changes in gene expression in various cells in the CNS. In our lab, we have identified several molecules in plasma EVs, both lipids and miRNA, that can potentially predict the response to treatment of pediatric anxiety with selective serotonin reuptake inhibitors as well as opiate addiction.15
Further, given our increasing understanding of the way in which EV cargo reflects neuronal physiology as well as the potential pathophysiologic states of cells (including neurons), studying EVs’ molecular content can identify molecular messages—in blood—that are derived from the neurons in the brain. Having the tools to examine molecular brain regulators or other markers of disease progression (eg, beta amyloid) or brain health (eg, brain-derived neurotrophic factor) may advance our understanding and treatment of psychiatric disorders and create opportunities for precision medicine driven by biological rather than ethnologic and phenomenological markers. Whereas in the not-too-distant past molecular processes in the brain were only accessible through invasive measures—such as brain biopsy or through a lumbar puncture—studying CNS-derived EVs in blood offers us an opportunity to gain access to brain molecular signatures with relative ease. Often, these molecular signatures predate clinical changes by years or months, allowing us the prospects of potentially identifying and treating CNS disorders early on, possibly even before the onset of symptoms.
Therapeutic use of the Twitter feed
EV may be used to alter brain receptor structures in a targeted way to facilitate treatment of various psychiatric disorders. One example is a proof-of-concept study in mice in which administration of artificially manufactured EVs led to a decrease of opioid receptor mu.16 This was done by constructing EVs that carry neuron-specific rabies viral glycoprotein (RVG) peptide on the membrane surface to deliver mu opioid receptor small interfering RNA into the brain. This resulted in downregulation of mu opioid receptor and a decrease in morphine relapse.16
Additional ways in which EVs can be used therapeutically is via targeted drug delivery CNS methods. EVs may represent the next generation of treatment by allowing not only medication transport into the CNS,17 but also by facilitating directed CNS transport. What if we could use a molecular hashtag to send a dopaminergic agent to the substantia nigra of a patient with Parkinson disease but avoid sending that same treatment to the limbic cortex, where it might produce perceptual disturbances or hallucinations? In the future, EVs may help clinicians access the CNS, which is traditionally restricted by the blood brain barrier, and make it easier to achieve CNS concentrations of medications13 while decreasing medication exposure in other parts of the body. The therapeutic potential of EVs for medication delivery and regenerative medicine is awe-inspiring. Several studies have modified EVs to improve their therapeutic properties and to target delivery to specific cells13 by leveraging EV surface markers.18
Future directions for EVs
A better understanding of neuron-derived EVs may eventually help us abandon nosology-based diagnostic criteria and adopt molecular-based diagnostic approaches in psychiatry. It may allow us to consider a molecular synaptic etiology of psychiatric disorders, and diagnose patients based on synaptic pathology utilizing “neuron-derived EV liquid biopsies.” Such a shift would align psychiatry with other medical fields in which diagnosis and treatment are often based on biopsies and blood tests. Because proteins in EVs often exist in their native states, intact with their posttranslational modifications, they provide a window into testing their actual in vivo functioning. EVs have an immense potential to revolutionize psychiatric diagnosis, facilitate precision treatment, predict response, and discover much-needed novel therapeutics.
Bottom Line
Much like a tweet, extracellular vesicles (EVs) encode short messages that are transmit ted efficiently throughout the CNS and body. They may represent a reservoir for CNS-specific biomarkers that can be is olated from plasma to guide psychiatric diagnosis and treatment. EVs represent a new frontier in the molecular study of psychiatric illness.
Related Resources
Vesiclepedia. www.microvesicles.org/
Twitter, a microblogging and social networking service, has become a “go-to’” for conversations, updates, breaking news, and sharing the more mundane aspects of our lives. Tweets, which were lengthened from 140 to 280 characters in 2017, rapidly communicate and disseminate information to a wide audience. Generally, tweets are visible to everyone, though users can mute and block other users from viewing their tweets. Spikes in tweets and tweeting frequency reflect hyper-current events: the last minutes of the Super Bowl, certification of an election, or a new movie release. In fact, social scientists have analyzed tweet frequencies to examine the impact of local and national events. However, few are aware that like celebrities, politicians, influencers, and ordinary citizens, the human brain also tweets.
In this article, we describe the components of the brain’s “Twitter” system, how it works, and how it might someday be used to improve the diagnosis and treatment of psychiatric disorders.
Brain tweets
The brain’s Twitter system involves extracellular vesicles (EVs), tiny (<1 µm) membrane-bound vesicles that are released from neurons, glia, and other neuronal cells (Table). These EVs cross the blood-brain barrier and facilitate cell-to-cell communication within and among tissues (Figure 1).
First described in the 1980s,1 EVs are secreted by a diverse array of cells: mast cells reticulocytes, epithelial cells, immune cells, neurons, glia, and oligodendrocytes. Like tweets, EVs rapidly disseminate packets of information throughout the brain and body and direct the molecular activity of recipient cells in both health and disease. These “brain tweets” contain short, circumscribed messages, and the characters are the EV cargos: RNAs, proteins, lipids, and metabolites. Like a Twitter feed, EVs cast a wide communication net across the body, much of which finds its way to the blood. As neuroscientists, we can follow these tweets by isolating tissue-derived EVs in plasma and examining their surface molecules and cargo. By following this Twitter feed, we can tap into important molecular communications and identify “trending” (evolving) pathological processes, and perhaps use the brain Twitter feed to improve diagnosis and treatments. We can pinpoint, in the blood, signals from CNS processes, down to the level of identifying EV cargos from specific brain cell types.

Within the CNS, EVs are secreted by neurons, where they may modulate synaptic plasticity and transfer molecular cargo among neurons. EVs also facilitate communication between neurons and glia, maintain homeostasis, trigger neuroprotective processes, and even regulate synaptic transmission.2
What’s in a brain tweet?
To discuss what’s in a brain tweet, we must first understand how a brain tweet is composed. EVs are pinched off from membranes of intercellular structures (eg, golgi or endoplasmic reticulum) or pinched off directly from cell membranes, where upon release they become EVs. There is a complex cellular machinery that transports what ultimately becomes an EV to the cell membrane.3 EVs contain unique mixtures of lipids, proteins, and nucleic acids (eg, microRNA [miRNA], mRNA, and noncoding RNA).4 To date, nearly 10,000 proteins, 11,000 lipids, 3,500 mRNAs, and 3,000 miRNAs have been identified as cargos in extracellular vesicles (Figure 1). Similar to how the release of EVs is dependent on complex intracellular machinery, the packing of these contents into what will become the EV involves a parallel set of complex machinery that is largely directed by endosomal sorting complexes required for transport (ESCRT) proteins.5 Of interest, when viruses attack cells, they hijack this EV packaging system to package and release new viruses. EVs vary in size, shape, and density; this variation is related to the cell origin, among other things. EVs also differ in their membrane lipid composition and in terms of transmembrane proteins as well as the proteins that facilitate EV binding to target cells (Figure 2).6 Ultimately, these exosomes are taken up by the recipient cells.

EV-facilitated neuron-to-neuron tweets have been implicated in neuronal growth and differentiation.7 EV-driven communication between cells also can decrease dendrite growth and can trigger microglia to prune synapses.8 EVs from glial cells may promote neuronal integrity, directly boost presynaptic glutamate release,9 or even, through miRNAs, change the expression of glutamate receptors.10 EVs from astrocytes transport proteins that enable neuronal repair, while EVs from microglia regulate neuronal homeostasis. EV cargos—lipids, proteins, and miRNAs—from neurons modify signal transduction and protein expression in recipient cells. Taken together, data suggest that EVs facilitate anterograde and retrograde transfer of signals across synapses,7,11 a putative mechanism for driving synaptic plasticity,12 which is a process implicated in the therapeutic efficacy of psychotropic medications and psychotherapies.
Continue to: #Targets and #neuron
#Targets and #neuron
Adding a hashtag to a tweet links it to other tweets, just as membrane features of EVs direct how EVs link to target cells. When these EVs bind to target cells, they fuse and release their cargo into the target cell (Figure 2). These directed cargo—whether mRNA, proteins, or other molecules—can direct the recipient cell to modify its firing rate (in the case of neurons), alter transmitter release, and increase or decrease expression of various genes. The targeting process is complex, and our understanding of this process is evolving. Briefly, integrin, lipid composition, glycans (eg, polysaccharides), and tetraspanin components of EVs influence their affinity for specific target cells.13 Recently, we have been able to read these hashtags and isolate cell-specific, neuron-derived EVs. Immunoadsorption techniques that leverage antibodies against L1 cell adhesion molecule protein (L1CAM(+)), primarily expressed in neurons, can identify neuronally-derived EVs (Figure 3). The specific EVs contain cargos of neuronal origin and provide a “window” into molecular processes in the brain by way of the blood (or other peripheral fluids). In following the neuronal tweets, we can follow molecular measures of important brain molecules in biofluids outside the CNS, including saliva and potentially urine (Figure 1B and 1C). In following these specific neuronal Twitter feeds, we can gain critical insights into specific brain processes.

EVs in psychiatric disorders
EVs are implicated in neuroinflammation,14 neurogenesis, synaptic plasticity, and epigenetic regulation—all processes that are involved in the pathophysiology of psychiatric disorders. Postmortem research suggests that EVs in the brain carry proinflammatory molecules from microglia, as well as secretions of regulatory miRNA that are responsible for synaptic plasticity and dendritic growth in depression, bipolar disorder, schizophrenia, and addiction. In addition, second-generation antipsychotics change the composition of EV cargos in the brain, altering their RNA, protein, and lipid content, often reflecting profound changes in gene expression in various cells in the CNS. In our lab, we have identified several molecules in plasma EVs, both lipids and miRNA, that can potentially predict the response to treatment of pediatric anxiety with selective serotonin reuptake inhibitors as well as opiate addiction.15
Further, given our increasing understanding of the way in which EV cargo reflects neuronal physiology as well as the potential pathophysiologic states of cells (including neurons), studying EVs’ molecular content can identify molecular messages—in blood—that are derived from the neurons in the brain. Having the tools to examine molecular brain regulators or other markers of disease progression (eg, beta amyloid) or brain health (eg, brain-derived neurotrophic factor) may advance our understanding and treatment of psychiatric disorders and create opportunities for precision medicine driven by biological rather than ethnologic and phenomenological markers. Whereas in the not-too-distant past molecular processes in the brain were only accessible through invasive measures—such as brain biopsy or through a lumbar puncture—studying CNS-derived EVs in blood offers us an opportunity to gain access to brain molecular signatures with relative ease. Often, these molecular signatures predate clinical changes by years or months, allowing us the prospects of potentially identifying and treating CNS disorders early on, possibly even before the onset of symptoms.
Therapeutic use of the Twitter feed
EV may be used to alter brain receptor structures in a targeted way to facilitate treatment of various psychiatric disorders. One example is a proof-of-concept study in mice in which administration of artificially manufactured EVs led to a decrease of opioid receptor mu.16 This was done by constructing EVs that carry neuron-specific rabies viral glycoprotein (RVG) peptide on the membrane surface to deliver mu opioid receptor small interfering RNA into the brain. This resulted in downregulation of mu opioid receptor and a decrease in morphine relapse.16
Additional ways in which EVs can be used therapeutically is via targeted drug delivery CNS methods. EVs may represent the next generation of treatment by allowing not only medication transport into the CNS,17 but also by facilitating directed CNS transport. What if we could use a molecular hashtag to send a dopaminergic agent to the substantia nigra of a patient with Parkinson disease but avoid sending that same treatment to the limbic cortex, where it might produce perceptual disturbances or hallucinations? In the future, EVs may help clinicians access the CNS, which is traditionally restricted by the blood brain barrier, and make it easier to achieve CNS concentrations of medications13 while decreasing medication exposure in other parts of the body. The therapeutic potential of EVs for medication delivery and regenerative medicine is awe-inspiring. Several studies have modified EVs to improve their therapeutic properties and to target delivery to specific cells13 by leveraging EV surface markers.18
Future directions for EVs
A better understanding of neuron-derived EVs may eventually help us abandon nosology-based diagnostic criteria and adopt molecular-based diagnostic approaches in psychiatry. It may allow us to consider a molecular synaptic etiology of psychiatric disorders, and diagnose patients based on synaptic pathology utilizing “neuron-derived EV liquid biopsies.” Such a shift would align psychiatry with other medical fields in which diagnosis and treatment are often based on biopsies and blood tests. Because proteins in EVs often exist in their native states, intact with their posttranslational modifications, they provide a window into testing their actual in vivo functioning. EVs have an immense potential to revolutionize psychiatric diagnosis, facilitate precision treatment, predict response, and discover much-needed novel therapeutics.
Bottom Line
Much like a tweet, extracellular vesicles (EVs) encode short messages that are transmit ted efficiently throughout the CNS and body. They may represent a reservoir for CNS-specific biomarkers that can be is olated from plasma to guide psychiatric diagnosis and treatment. EVs represent a new frontier in the molecular study of psychiatric illness.
Related Resources
Vesiclepedia. www.microvesicles.org/
1. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329-339. doi:10.1083/jcb.97.2.329
2. Huo L, Du X, Li X, et al. The emerging role of neural cell-derived exosomes in intercellular communication in health and neurodegenerative diseases. Front Neurosci. 2021;15:738442. doi:10.3389/fnins.2021
3. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-83. doi: 10.1083/jcb.201211138
4. Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688-692. doi:10.1016/j.jmb.2015.09.019
5. Babst M. A protein’s final ESCRT. Traffic. 2005;6(1):2-9. doi:10.1111/j.1600-0854.2004.00246.x
6. Anakor E, Le Gall L, Dumonceaux J, et al. Exosomes in ageing and motor neurone disease: biogenesis, uptake mechanisms, modifications in disease and uses in the development of biomarkers and therapeutics. Cells. 2021;10(11)29-30. doi:10.3390/cells10112930
7. Chivet M, Javalet C, Hemming F, et al. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013;41(1):241-244. doi:10.1042/BST20120266
8. Liu HY, Huang CM, Hung YF, et al. The microRNAs Let7c and miR21 are recognized by neuronal Toll-like receptor 7 to restrict dendritic growth of neurons. Exp Neurol. 2015;269:202-212. doi:10.1016/j.expneurol.2015.04.011
9. Antonucci F, Turola E, Riganti L, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31(5):1231-1240. doi:10.1038/emboj.2011.489
10. Goncalves MB, Malmqvist T, Clarke E, et al. Neuronal RARβ signaling modulates PTEN activity directly in neurons and via exosome transfer in astrocytes to prevent glial scar formation and induce spinal cord regeneration. J Neurosci. 2015;35(47):15731-15745. doi:10.1523/JNEUROSCI.1339-15.2015
11. Korkut C, Li Y, Koles K, et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77(6):1039-1046. doi:10.1016/j.neuron.2013.01.013
12. Chivet M, Javalet C, Laulagnier K, et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3(1):24722. doi:10.3402/jev.v3
13. Dickens AM, Tovar-Y-Romo LB, Yoo SW, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10(473). doi:10.1126/scisignal.aai7696
14. Strawn J, Levine A. Treatment response biomarkers in anxiety disorders: from neuroimaging to neuronally-derived extracellular vesicles and beyond. Biomark Neuropsychiatry. 2020;3:100024.
15. Liu Y, Li D, Liu Z, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543. doi:10.1038/srep17543
16. Shahjin F, Chand S, Yelamanchili S V. Extracellular vesicles as drug delivery vehicles to the central nervous system. J Neuroimmune Pharmacol. 2020;15(3):443-458. doi:10.1007/s11481-019-09875-w
17. Murphy DE, de Jong OG, Brouwer M, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):1-12. doi:10.1038/s12276-019-0223-5
18. Meng W, He C, Hao Y, et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585-598. doi:10.1080/10717544.2020.1748758
1. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329-339. doi:10.1083/jcb.97.2.329
2. Huo L, Du X, Li X, et al. The emerging role of neural cell-derived exosomes in intercellular communication in health and neurodegenerative diseases. Front Neurosci. 2021;15:738442. doi:10.3389/fnins.2021
3. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-83. doi: 10.1083/jcb.201211138
4. Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688-692. doi:10.1016/j.jmb.2015.09.019
5. Babst M. A protein’s final ESCRT. Traffic. 2005;6(1):2-9. doi:10.1111/j.1600-0854.2004.00246.x
6. Anakor E, Le Gall L, Dumonceaux J, et al. Exosomes in ageing and motor neurone disease: biogenesis, uptake mechanisms, modifications in disease and uses in the development of biomarkers and therapeutics. Cells. 2021;10(11)29-30. doi:10.3390/cells10112930
7. Chivet M, Javalet C, Hemming F, et al. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013;41(1):241-244. doi:10.1042/BST20120266
8. Liu HY, Huang CM, Hung YF, et al. The microRNAs Let7c and miR21 are recognized by neuronal Toll-like receptor 7 to restrict dendritic growth of neurons. Exp Neurol. 2015;269:202-212. doi:10.1016/j.expneurol.2015.04.011
9. Antonucci F, Turola E, Riganti L, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31(5):1231-1240. doi:10.1038/emboj.2011.489
10. Goncalves MB, Malmqvist T, Clarke E, et al. Neuronal RARβ signaling modulates PTEN activity directly in neurons and via exosome transfer in astrocytes to prevent glial scar formation and induce spinal cord regeneration. J Neurosci. 2015;35(47):15731-15745. doi:10.1523/JNEUROSCI.1339-15.2015
11. Korkut C, Li Y, Koles K, et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77(6):1039-1046. doi:10.1016/j.neuron.2013.01.013
12. Chivet M, Javalet C, Laulagnier K, et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3(1):24722. doi:10.3402/jev.v3
13. Dickens AM, Tovar-Y-Romo LB, Yoo SW, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10(473). doi:10.1126/scisignal.aai7696
14. Strawn J, Levine A. Treatment response biomarkers in anxiety disorders: from neuroimaging to neuronally-derived extracellular vesicles and beyond. Biomark Neuropsychiatry. 2020;3:100024.
15. Liu Y, Li D, Liu Z, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543. doi:10.1038/srep17543
16. Shahjin F, Chand S, Yelamanchili S V. Extracellular vesicles as drug delivery vehicles to the central nervous system. J Neuroimmune Pharmacol. 2020;15(3):443-458. doi:10.1007/s11481-019-09875-w
17. Murphy DE, de Jong OG, Brouwer M, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):1-12. doi:10.1038/s12276-019-0223-5
18. Meng W, He C, Hao Y, et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585-598. doi:10.1080/10717544.2020.1748758
Dexmedetomidine sublingual film for agitation
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2
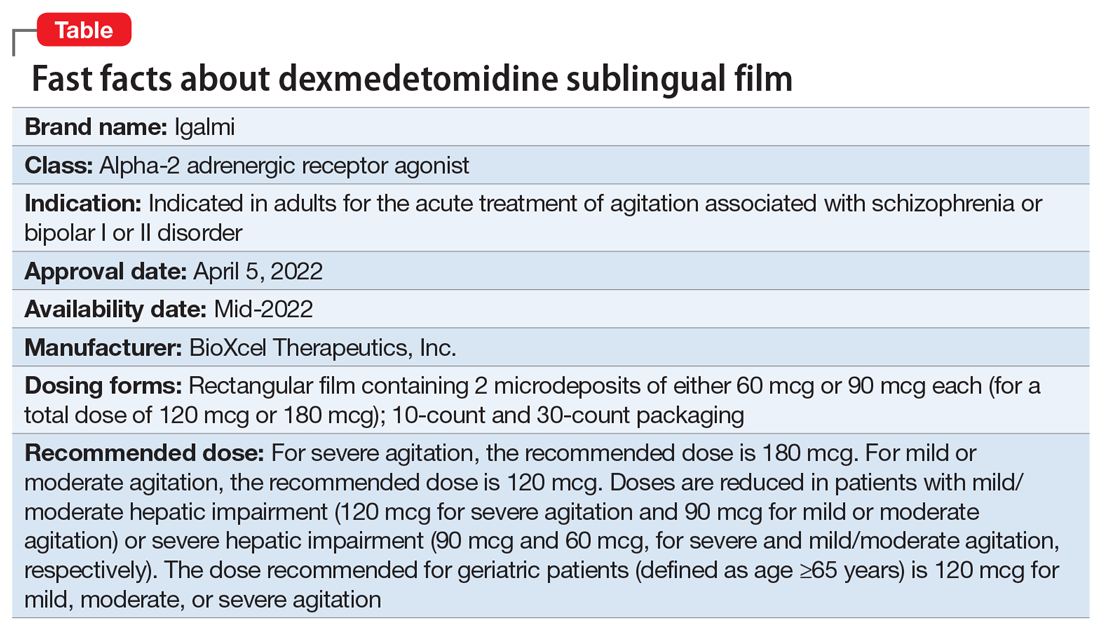
Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2

Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2

Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
Sublingual buprenorphine plus buprenorphine XR for opioid use disorder
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4
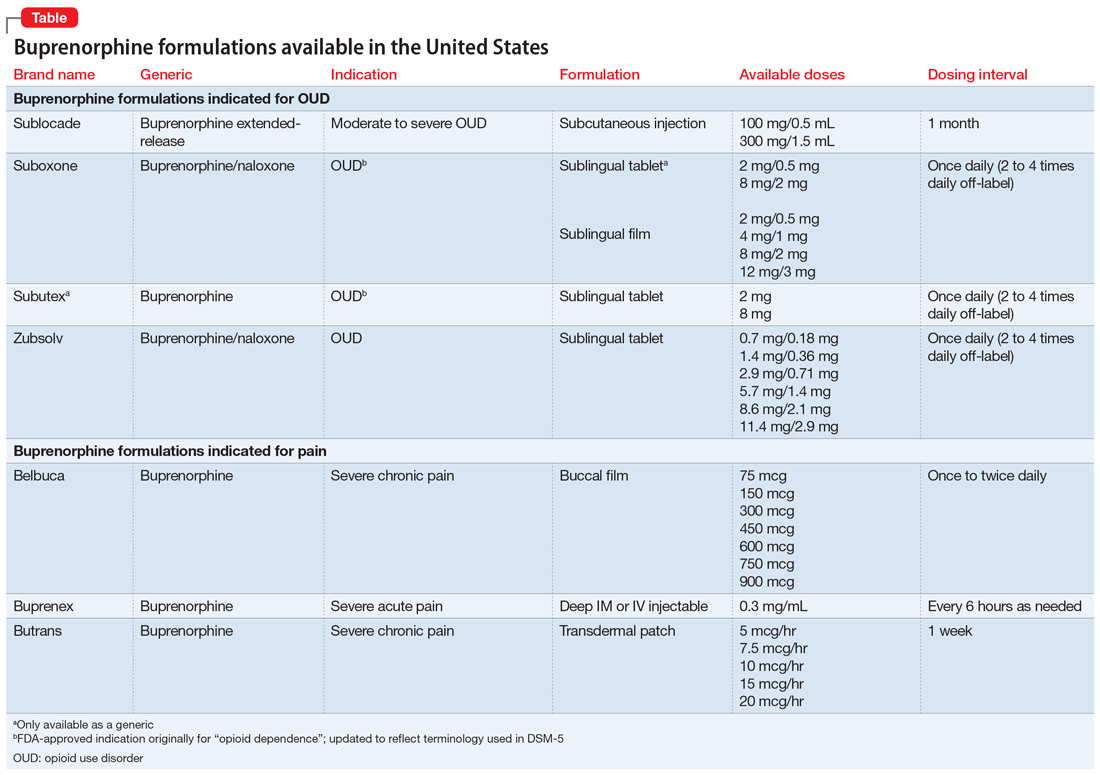
However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4

However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4

However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281
Should clozapine be discontinued in a patient receiving chemotherapy?
CASE Schizophrenia, leukemia, and chemotherapy
Mr. A, age 30, has schizophrenia but has been stable on clozapine 600 mg/d. He presents to the emergency department with generalized pain that started in his right scapula, arm, elbow, and back. Laboratory tests and a diagnostic examination reveal severe leukocytosis, thrombocytopenia, and anemia, and clinicians diagnose Mr. A with B-cell acute lymphocytic leukemia (B-ALL). Upon admission, Mr. A is neutropenic with an absolute neutrophil count (ANC) of 1,420 µL (reference range 2,500 to 6,000 µL). The hematology team recommends chemotherapy. The treating clinicians also consult the psychiatry team for recommendations on how to best manage Mr. A’s schizophrenia during chemotherapy, including whether clozapine should be discontinued.
HISTORY Stable on clozapine for >10 years
Mr. A was diagnosed with schizophrenia at age 15 after developing paranoia and auditory hallucinations of people talking to him and to each other. He had been hospitalized multiple times for worsened auditory hallucinations and paranoia that led to significant agitation and violence. Previous treatment with multiple antipsychotics, including haloperidol, quetiapine, aripiprazole, olanzapine, risperidone, and ziprasidone, was not successful. Mr. A began clozapine >10 years ago, and his symptoms have been stable since, without any further psychiatric hospitalizations. Mr. A takes clozapine 600 mg/d and divalproex sodium 1,500 mg/d, which he tolerates well and without significant adverse effects. Though he continues to have intermittent auditory hallucinations, they are mild and manageable. Mr. A lives with his mother, who reports he occasionally talks to himself but when he does not take clozapine, the auditory hallucinations worsen and cause him to become paranoid and aggressive. His ANC is monitored monthly and had been normal for several years until he was diagnosed with B-ALL.
[polldaddy:11125941]
The authors’ observations
The decision to continue clozapine during chemotherapy is challenging and should weigh the risk of agranulocytosis against that of psychiatric destabilization. Because clozapine and chemotherapy are both associated with agranulocytosis, there is concern that concurrent treatment could increase this risk in an additive or synergistic manner. To the best of our knowledge, there are currently no controlled studies investigating the interactions between clozapine and chemotherapeutic agents. Evidence on the hematopoietic consequences of concurrent clozapine and chemotherapy treatment has been limited to case reports because the topic does not lend itself well to randomized controlled trials.
A recent systematic review found no adverse outcomes among the 27 published cases in which clozapine was continued during myelosuppressive chemotherapy.1 The most notable finding was an association between clozapine discontinuation and psychiatric decompensation, which was reported in 12 of 13 cases in which clozapine was prophylactically discontinued to minimize the risk of agranulocytosis.
Patient-specific factors must also be considered, such as the likelihood that psychotic symptoms will recur or worsen if clozapine is discontinued, as well as the extent to which symptom recurrence would interfere with cancer treatment. Clinicians should evaluate the feasibility of switching to another antipsychotic by obtaining a thorough history of the patient’s previous antipsychotics, doses, treatment duration, and response. However, many patients are treated with clozapine because their psychotic symptoms did not improve with other treatments. The character and severity of the patient’s psychotic symptoms when untreated or prior to clozapine treatment can provide a clearer understanding of how a recurrence of symptoms may interfere with cancer treatment. To formulate an accurate assessment of risks and benefits, it is necessary to consider both available evidence and patient-specific factors. The significant agitation and paranoia that Mr. A experienced when not taking clozapine was likely to disrupt chemotherapy. Thus, the adverse consequences of discontinuing clozapine were both severe and likely.
TREATMENT Continuing clozapine
After an extensive discussion of risks, benefits, and alternative treatments with the hematology and psychiatry teams, Mr. A and his family decide to continue clozapine with increased ANC monitoring during chemotherapy. Concurrent treatment was pursued with close collaboration among the patient, the patient’s family, and the hematology and pharmacy teams, and in careful consideration of the clozapine risk evaluation and mitigation strategy. Mr. A’s ANC was monitored daily during chemotherapy treatments and weekly in the intervals between treatments.
As expected, chemotherapy resulted in bone marrow suppression and pancytopenia. Mr. A’s ANC steadily decreased during the next 10 days until it reached 0 µL. This was consistent with the predicted ANC nadir between Day 10 and Day 14, after which recovery was expected. However, Mr. A’s ANC remained at 0 µL on Day 15.
[polldaddy:11125947]
Continue to: The authors' observations
The authors’ observations
Temporary decreases in ANC are expected during chemotherapy, and the timing of onset and recovery is often well characterized. Prior to Day 15, the observed progressive marrow suppression was solely due to chemotherapy. However, because Mr. A’s ANC remained 0 µL longer than anticipated, reevaluation of clozapine’s effects was warranted.
Timing, clinical course, and comprehensive hematologic monitoring can provide important clues as to whether clozapine may be responsible for prolonged neutropenia. Though a prolonged ANC of 0 µL raised concern for clozapine-induced agranulocytosis (CIAG), comprehensive monitoring of hematologic cell lines was reassuring because CIAG selectively targets granulocytic cells (neutrophils).2 In contrast, chemotherapy can affect other cell lineages, including lymphocytes, red blood cells, and platelets, which causes pancytopenia.3 For Mr. A, though the clinical presentation of pancytopenia was significant and concerning, it was inconsistent with CIAG.
Additionally, the patient’s baseline risk of CIAG should be considered. After 18 weeks of clozapine treatment, the risk of CIAG decreases to a level similar to that associated with other antipsychotics.4,5 Therefore, CIAG would be unlikely in a patient treated with clozapine for more than 1 year and who did not have a history of neutropenia, as was the case with Mr. A.
While bone marrow biopsy can help differentiate between the causes of agranulocytosis,6 it is highly invasive and may not be necessary if laboratory evidence is sufficient. However, if a treatment team is strongly considering discontinuing clozapine and there are no suitable alternatives, a biopsy may provide additional clarification.
TREATMENT CAR T-cell therapy and cancer remission
Clozapine is continued with daily monitoring. On Day 19, Mr. A’s ANC increases, reaching 2,600 µL by discharge on Day 40. Mr. A remains psychiatrically stable throughout his hospitalization and does not experience any complications associated with neutropenia, despite its prolonged duration.
Continue to: Unfortunately, multiple cycles of...
Unfortunately, multiple cycles of chemotherapy fail to induce remission. Mr. A is referred for CD19/CD22 chimeric antigen receptor (CAR) T-cell therapy, which helps achieve remission. Allogeneic hematopoietic stem cell transplant (HSCT) is recommended to maximize the likelihood of sustained remission.7 As with chemotherapy, Mr. A and his family agree with the multidisciplinary treatment recommendation to continue clozapine during both CAR T-cell therapy and HSCT, because the risks associated with psychiatric decompensation were greater than a potential increased risk of agranulocytosis. Clozapine treatment is continued throughout both therapies without issue.
Four months after HSCT, Mr. A is admitted for neutropenic fever and left face cellulitis. Upon admission, his ANC is 30 µL and subsequently decreases to 0 µL. In addition to neutropenia, Mr. A is also anemic and thrombocytopenic. He undergoes a bone marrow biopsy.
[polldaddy:11125950]
The authors’ observations
While no published cases have examined the bone marrow of patients experiencing CIAG, 2 retrospective studies have characterized 2 classes of bone marrow findings associated with drug-induced agranulocytosis resulting from nonchemotherapeutic agents (Table).8,9 Type I marrow appears hypercellular with adequate neutrophil precursors but an arrested neutrophil maturation, with few or no mature forms of neutrophils beyond myelocytes.8,9 Type II demonstrates a severe reduction or complete absence of granulocytic precursors with normal or increased erythropoiesis and megakaryocytes.8,9 These findings have been used to accurately differentiate between chemotherapy and nonchemotherapy drug-induced agranulocytosis,6 resulting in successful identification and discontinuation of the responsible agent.
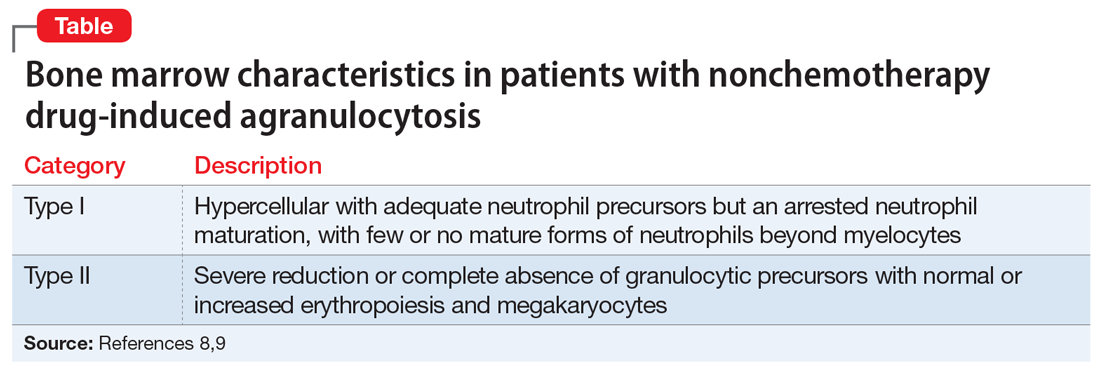
Mr. A’s bone marrow biopsy showed severe pancytopenia with profound neutropenia and normocytic anemia, without evidence of residual leukemia, inconsistent with Type I or Type II. Findings were suggestive of a myelodysplastic syndrome, consistent with secondary graft failure. Symptoms resolved after treatment with antibiotics, granulocyte colony-stimulating factor, epoetin alfa, and thrombopoietin. Mr. A’s ANC remained 0 µL for 22 days before returning to normal (>1,500 µL) by Day 29. He had no secondary complications resulting from neutropenia. As the clinical evidence suggested, Mr. A’s neutropenia was unlikely to be due to clozapine. Clozapine was continued throughout his cancer treatment, and he remained psychiatrically stable.
Clozapine, cancer treatments, and agranulocytosis
This case demonstrates that clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even with the development of agranulocytosis and prolonged neutropenia. Evidence to guide psychiatric clinicians to evaluate the likelihood that agranulocytosis is clozapine-induced is limited.
Continue to: We offer an algorithm...
We offer an algorithm to assist clinicians faced with this challenging clinical dilemma (Figure). Based on our experience and limited current evidence, we recommend continuing clozapine during cancer treatment unless there is clear evidence to suggest otherwise. Presently, no evidence in published literature suggests worsened outcomes in patients treated concurrently with clozapine and cancer therapies.
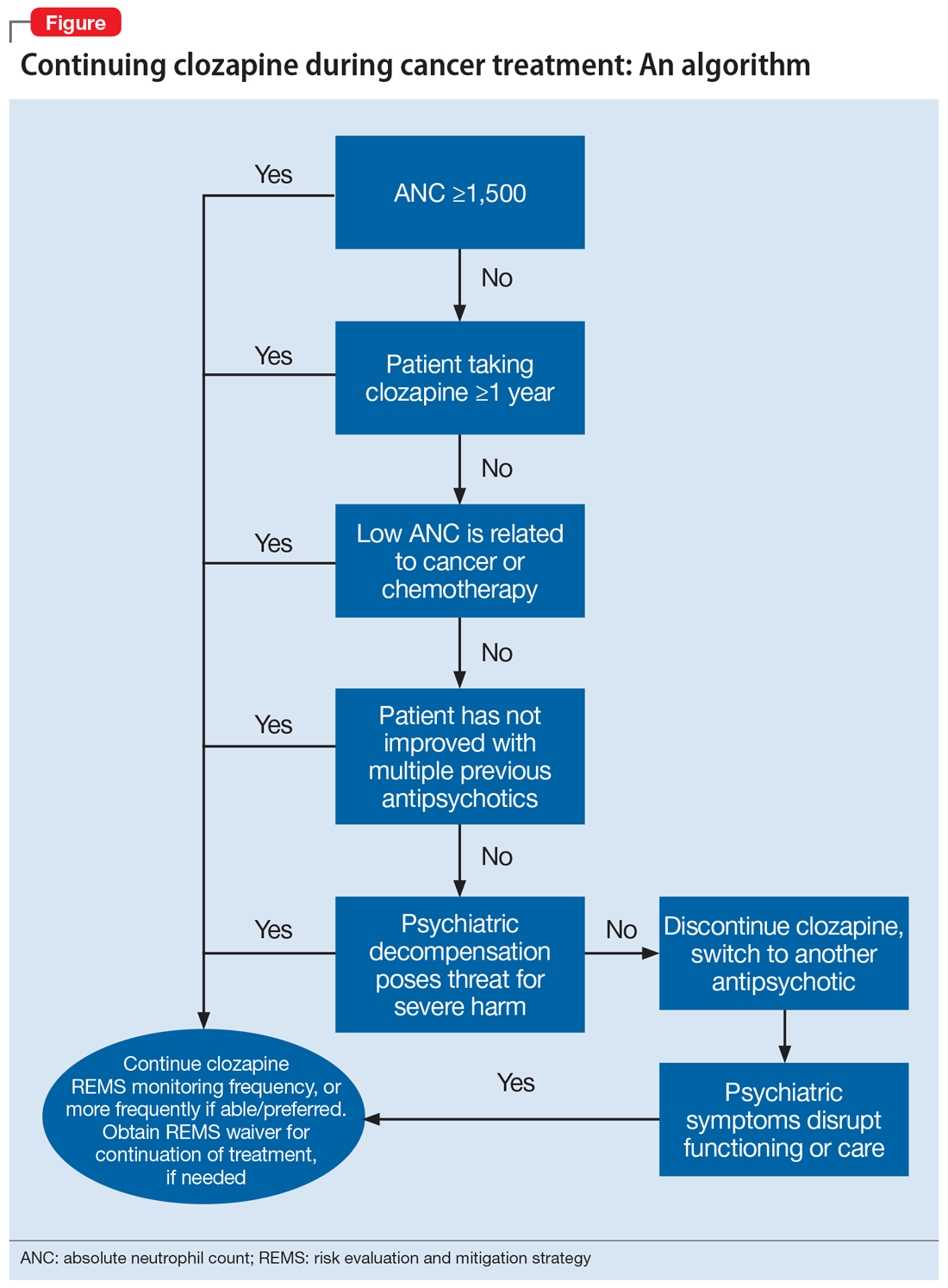
OUTCOME Cancer-free and psychiatrically stable
Mr. A continues clozapine therapy throughout all phases of treatment, without interruption. No adverse effects are determined to be secondary to clozapine. He remains psychiatrically stable throughout treatment, and able to participate and engage in his oncologic therapy. Mr. A is now more than 1 year in remission with no recurrence of graft failure, and his psychiatric symptoms continue to be well controlled with clozapine.
Bottom Line
Clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even in patients who develop agranulocytosis and prolonged neutropenia. Based on our experience and limited evidence, we offer an algorithm to assist clinicians faced with this challenging clinical dilemma.
Related Resources
- Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286. doi:10.1111/ejh.13285
- Daniel JS, Gross T. Managing clozapine-induced neutropenia and agranulocytosis. Current Psychiatry. 2016;15(12):51-53.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Divalproex sodium • Depakote
Epoetin alfa • Epogen
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
1. Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286.
2. Pick AM, Nystrom KK. Nonchemotherapy drug-induced neutropenia and agranulocytosis: could medications be the culprit? J Pharm Pract. 2014:27(5):447-452.
3. Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606-3618.
4. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
5. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483-488.
6. Azadeh N, Kelemen K, Fonseca R. Amitriptyline-induced agranulocytosis with bone marrow confirmation. Clin Lymphoma Myeloma Leuk. 2014;14(5):e183-e185.
7. Liu J, Zhang X, Zhong JF, et al. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9(13):1115-1125.
8. Apinantriyo B, Lekhakula A, Rujirojindakul P. Incidence, etiology and bone marrow characteristics of non-chemotherapy-induced agranulocytosis. Hematology. 2011;16(1):50-53.
9. Yang J, Zhong J, Xiao XH, et al. The relationship between bone marrow characteristics and the clinical prognosis of antithyroid drug-induced agranulocytosis. Endocr J. 2013;60(2):185-189.
CASE Schizophrenia, leukemia, and chemotherapy
Mr. A, age 30, has schizophrenia but has been stable on clozapine 600 mg/d. He presents to the emergency department with generalized pain that started in his right scapula, arm, elbow, and back. Laboratory tests and a diagnostic examination reveal severe leukocytosis, thrombocytopenia, and anemia, and clinicians diagnose Mr. A with B-cell acute lymphocytic leukemia (B-ALL). Upon admission, Mr. A is neutropenic with an absolute neutrophil count (ANC) of 1,420 µL (reference range 2,500 to 6,000 µL). The hematology team recommends chemotherapy. The treating clinicians also consult the psychiatry team for recommendations on how to best manage Mr. A’s schizophrenia during chemotherapy, including whether clozapine should be discontinued.
HISTORY Stable on clozapine for >10 years
Mr. A was diagnosed with schizophrenia at age 15 after developing paranoia and auditory hallucinations of people talking to him and to each other. He had been hospitalized multiple times for worsened auditory hallucinations and paranoia that led to significant agitation and violence. Previous treatment with multiple antipsychotics, including haloperidol, quetiapine, aripiprazole, olanzapine, risperidone, and ziprasidone, was not successful. Mr. A began clozapine >10 years ago, and his symptoms have been stable since, without any further psychiatric hospitalizations. Mr. A takes clozapine 600 mg/d and divalproex sodium 1,500 mg/d, which he tolerates well and without significant adverse effects. Though he continues to have intermittent auditory hallucinations, they are mild and manageable. Mr. A lives with his mother, who reports he occasionally talks to himself but when he does not take clozapine, the auditory hallucinations worsen and cause him to become paranoid and aggressive. His ANC is monitored monthly and had been normal for several years until he was diagnosed with B-ALL.
[polldaddy:11125941]
The authors’ observations
The decision to continue clozapine during chemotherapy is challenging and should weigh the risk of agranulocytosis against that of psychiatric destabilization. Because clozapine and chemotherapy are both associated with agranulocytosis, there is concern that concurrent treatment could increase this risk in an additive or synergistic manner. To the best of our knowledge, there are currently no controlled studies investigating the interactions between clozapine and chemotherapeutic agents. Evidence on the hematopoietic consequences of concurrent clozapine and chemotherapy treatment has been limited to case reports because the topic does not lend itself well to randomized controlled trials.
A recent systematic review found no adverse outcomes among the 27 published cases in which clozapine was continued during myelosuppressive chemotherapy.1 The most notable finding was an association between clozapine discontinuation and psychiatric decompensation, which was reported in 12 of 13 cases in which clozapine was prophylactically discontinued to minimize the risk of agranulocytosis.
Patient-specific factors must also be considered, such as the likelihood that psychotic symptoms will recur or worsen if clozapine is discontinued, as well as the extent to which symptom recurrence would interfere with cancer treatment. Clinicians should evaluate the feasibility of switching to another antipsychotic by obtaining a thorough history of the patient’s previous antipsychotics, doses, treatment duration, and response. However, many patients are treated with clozapine because their psychotic symptoms did not improve with other treatments. The character and severity of the patient’s psychotic symptoms when untreated or prior to clozapine treatment can provide a clearer understanding of how a recurrence of symptoms may interfere with cancer treatment. To formulate an accurate assessment of risks and benefits, it is necessary to consider both available evidence and patient-specific factors. The significant agitation and paranoia that Mr. A experienced when not taking clozapine was likely to disrupt chemotherapy. Thus, the adverse consequences of discontinuing clozapine were both severe and likely.
TREATMENT Continuing clozapine
After an extensive discussion of risks, benefits, and alternative treatments with the hematology and psychiatry teams, Mr. A and his family decide to continue clozapine with increased ANC monitoring during chemotherapy. Concurrent treatment was pursued with close collaboration among the patient, the patient’s family, and the hematology and pharmacy teams, and in careful consideration of the clozapine risk evaluation and mitigation strategy. Mr. A’s ANC was monitored daily during chemotherapy treatments and weekly in the intervals between treatments.
As expected, chemotherapy resulted in bone marrow suppression and pancytopenia. Mr. A’s ANC steadily decreased during the next 10 days until it reached 0 µL. This was consistent with the predicted ANC nadir between Day 10 and Day 14, after which recovery was expected. However, Mr. A’s ANC remained at 0 µL on Day 15.
[polldaddy:11125947]
Continue to: The authors' observations
The authors’ observations
Temporary decreases in ANC are expected during chemotherapy, and the timing of onset and recovery is often well characterized. Prior to Day 15, the observed progressive marrow suppression was solely due to chemotherapy. However, because Mr. A’s ANC remained 0 µL longer than anticipated, reevaluation of clozapine’s effects was warranted.
Timing, clinical course, and comprehensive hematologic monitoring can provide important clues as to whether clozapine may be responsible for prolonged neutropenia. Though a prolonged ANC of 0 µL raised concern for clozapine-induced agranulocytosis (CIAG), comprehensive monitoring of hematologic cell lines was reassuring because CIAG selectively targets granulocytic cells (neutrophils).2 In contrast, chemotherapy can affect other cell lineages, including lymphocytes, red blood cells, and platelets, which causes pancytopenia.3 For Mr. A, though the clinical presentation of pancytopenia was significant and concerning, it was inconsistent with CIAG.
Additionally, the patient’s baseline risk of CIAG should be considered. After 18 weeks of clozapine treatment, the risk of CIAG decreases to a level similar to that associated with other antipsychotics.4,5 Therefore, CIAG would be unlikely in a patient treated with clozapine for more than 1 year and who did not have a history of neutropenia, as was the case with Mr. A.
While bone marrow biopsy can help differentiate between the causes of agranulocytosis,6 it is highly invasive and may not be necessary if laboratory evidence is sufficient. However, if a treatment team is strongly considering discontinuing clozapine and there are no suitable alternatives, a biopsy may provide additional clarification.
TREATMENT CAR T-cell therapy and cancer remission
Clozapine is continued with daily monitoring. On Day 19, Mr. A’s ANC increases, reaching 2,600 µL by discharge on Day 40. Mr. A remains psychiatrically stable throughout his hospitalization and does not experience any complications associated with neutropenia, despite its prolonged duration.
Continue to: Unfortunately, multiple cycles of...
Unfortunately, multiple cycles of chemotherapy fail to induce remission. Mr. A is referred for CD19/CD22 chimeric antigen receptor (CAR) T-cell therapy, which helps achieve remission. Allogeneic hematopoietic stem cell transplant (HSCT) is recommended to maximize the likelihood of sustained remission.7 As with chemotherapy, Mr. A and his family agree with the multidisciplinary treatment recommendation to continue clozapine during both CAR T-cell therapy and HSCT, because the risks associated with psychiatric decompensation were greater than a potential increased risk of agranulocytosis. Clozapine treatment is continued throughout both therapies without issue.
Four months after HSCT, Mr. A is admitted for neutropenic fever and left face cellulitis. Upon admission, his ANC is 30 µL and subsequently decreases to 0 µL. In addition to neutropenia, Mr. A is also anemic and thrombocytopenic. He undergoes a bone marrow biopsy.
[polldaddy:11125950]
The authors’ observations
While no published cases have examined the bone marrow of patients experiencing CIAG, 2 retrospective studies have characterized 2 classes of bone marrow findings associated with drug-induced agranulocytosis resulting from nonchemotherapeutic agents (Table).8,9 Type I marrow appears hypercellular with adequate neutrophil precursors but an arrested neutrophil maturation, with few or no mature forms of neutrophils beyond myelocytes.8,9 Type II demonstrates a severe reduction or complete absence of granulocytic precursors with normal or increased erythropoiesis and megakaryocytes.8,9 These findings have been used to accurately differentiate between chemotherapy and nonchemotherapy drug-induced agranulocytosis,6 resulting in successful identification and discontinuation of the responsible agent.

Mr. A’s bone marrow biopsy showed severe pancytopenia with profound neutropenia and normocytic anemia, without evidence of residual leukemia, inconsistent with Type I or Type II. Findings were suggestive of a myelodysplastic syndrome, consistent with secondary graft failure. Symptoms resolved after treatment with antibiotics, granulocyte colony-stimulating factor, epoetin alfa, and thrombopoietin. Mr. A’s ANC remained 0 µL for 22 days before returning to normal (>1,500 µL) by Day 29. He had no secondary complications resulting from neutropenia. As the clinical evidence suggested, Mr. A’s neutropenia was unlikely to be due to clozapine. Clozapine was continued throughout his cancer treatment, and he remained psychiatrically stable.
Clozapine, cancer treatments, and agranulocytosis
This case demonstrates that clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even with the development of agranulocytosis and prolonged neutropenia. Evidence to guide psychiatric clinicians to evaluate the likelihood that agranulocytosis is clozapine-induced is limited.
Continue to: We offer an algorithm...
We offer an algorithm to assist clinicians faced with this challenging clinical dilemma (Figure). Based on our experience and limited current evidence, we recommend continuing clozapine during cancer treatment unless there is clear evidence to suggest otherwise. Presently, no evidence in published literature suggests worsened outcomes in patients treated concurrently with clozapine and cancer therapies.

OUTCOME Cancer-free and psychiatrically stable
Mr. A continues clozapine therapy throughout all phases of treatment, without interruption. No adverse effects are determined to be secondary to clozapine. He remains psychiatrically stable throughout treatment, and able to participate and engage in his oncologic therapy. Mr. A is now more than 1 year in remission with no recurrence of graft failure, and his psychiatric symptoms continue to be well controlled with clozapine.
Bottom Line
Clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even in patients who develop agranulocytosis and prolonged neutropenia. Based on our experience and limited evidence, we offer an algorithm to assist clinicians faced with this challenging clinical dilemma.
Related Resources
- Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286. doi:10.1111/ejh.13285
- Daniel JS, Gross T. Managing clozapine-induced neutropenia and agranulocytosis. Current Psychiatry. 2016;15(12):51-53.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Divalproex sodium • Depakote
Epoetin alfa • Epogen
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
CASE Schizophrenia, leukemia, and chemotherapy
Mr. A, age 30, has schizophrenia but has been stable on clozapine 600 mg/d. He presents to the emergency department with generalized pain that started in his right scapula, arm, elbow, and back. Laboratory tests and a diagnostic examination reveal severe leukocytosis, thrombocytopenia, and anemia, and clinicians diagnose Mr. A with B-cell acute lymphocytic leukemia (B-ALL). Upon admission, Mr. A is neutropenic with an absolute neutrophil count (ANC) of 1,420 µL (reference range 2,500 to 6,000 µL). The hematology team recommends chemotherapy. The treating clinicians also consult the psychiatry team for recommendations on how to best manage Mr. A’s schizophrenia during chemotherapy, including whether clozapine should be discontinued.
HISTORY Stable on clozapine for >10 years
Mr. A was diagnosed with schizophrenia at age 15 after developing paranoia and auditory hallucinations of people talking to him and to each other. He had been hospitalized multiple times for worsened auditory hallucinations and paranoia that led to significant agitation and violence. Previous treatment with multiple antipsychotics, including haloperidol, quetiapine, aripiprazole, olanzapine, risperidone, and ziprasidone, was not successful. Mr. A began clozapine >10 years ago, and his symptoms have been stable since, without any further psychiatric hospitalizations. Mr. A takes clozapine 600 mg/d and divalproex sodium 1,500 mg/d, which he tolerates well and without significant adverse effects. Though he continues to have intermittent auditory hallucinations, they are mild and manageable. Mr. A lives with his mother, who reports he occasionally talks to himself but when he does not take clozapine, the auditory hallucinations worsen and cause him to become paranoid and aggressive. His ANC is monitored monthly and had been normal for several years until he was diagnosed with B-ALL.
[polldaddy:11125941]
The authors’ observations
The decision to continue clozapine during chemotherapy is challenging and should weigh the risk of agranulocytosis against that of psychiatric destabilization. Because clozapine and chemotherapy are both associated with agranulocytosis, there is concern that concurrent treatment could increase this risk in an additive or synergistic manner. To the best of our knowledge, there are currently no controlled studies investigating the interactions between clozapine and chemotherapeutic agents. Evidence on the hematopoietic consequences of concurrent clozapine and chemotherapy treatment has been limited to case reports because the topic does not lend itself well to randomized controlled trials.
A recent systematic review found no adverse outcomes among the 27 published cases in which clozapine was continued during myelosuppressive chemotherapy.1 The most notable finding was an association between clozapine discontinuation and psychiatric decompensation, which was reported in 12 of 13 cases in which clozapine was prophylactically discontinued to minimize the risk of agranulocytosis.
Patient-specific factors must also be considered, such as the likelihood that psychotic symptoms will recur or worsen if clozapine is discontinued, as well as the extent to which symptom recurrence would interfere with cancer treatment. Clinicians should evaluate the feasibility of switching to another antipsychotic by obtaining a thorough history of the patient’s previous antipsychotics, doses, treatment duration, and response. However, many patients are treated with clozapine because their psychotic symptoms did not improve with other treatments. The character and severity of the patient’s psychotic symptoms when untreated or prior to clozapine treatment can provide a clearer understanding of how a recurrence of symptoms may interfere with cancer treatment. To formulate an accurate assessment of risks and benefits, it is necessary to consider both available evidence and patient-specific factors. The significant agitation and paranoia that Mr. A experienced when not taking clozapine was likely to disrupt chemotherapy. Thus, the adverse consequences of discontinuing clozapine were both severe and likely.
TREATMENT Continuing clozapine
After an extensive discussion of risks, benefits, and alternative treatments with the hematology and psychiatry teams, Mr. A and his family decide to continue clozapine with increased ANC monitoring during chemotherapy. Concurrent treatment was pursued with close collaboration among the patient, the patient’s family, and the hematology and pharmacy teams, and in careful consideration of the clozapine risk evaluation and mitigation strategy. Mr. A’s ANC was monitored daily during chemotherapy treatments and weekly in the intervals between treatments.
As expected, chemotherapy resulted in bone marrow suppression and pancytopenia. Mr. A’s ANC steadily decreased during the next 10 days until it reached 0 µL. This was consistent with the predicted ANC nadir between Day 10 and Day 14, after which recovery was expected. However, Mr. A’s ANC remained at 0 µL on Day 15.
[polldaddy:11125947]
Continue to: The authors' observations
The authors’ observations
Temporary decreases in ANC are expected during chemotherapy, and the timing of onset and recovery is often well characterized. Prior to Day 15, the observed progressive marrow suppression was solely due to chemotherapy. However, because Mr. A’s ANC remained 0 µL longer than anticipated, reevaluation of clozapine’s effects was warranted.
Timing, clinical course, and comprehensive hematologic monitoring can provide important clues as to whether clozapine may be responsible for prolonged neutropenia. Though a prolonged ANC of 0 µL raised concern for clozapine-induced agranulocytosis (CIAG), comprehensive monitoring of hematologic cell lines was reassuring because CIAG selectively targets granulocytic cells (neutrophils).2 In contrast, chemotherapy can affect other cell lineages, including lymphocytes, red blood cells, and platelets, which causes pancytopenia.3 For Mr. A, though the clinical presentation of pancytopenia was significant and concerning, it was inconsistent with CIAG.
Additionally, the patient’s baseline risk of CIAG should be considered. After 18 weeks of clozapine treatment, the risk of CIAG decreases to a level similar to that associated with other antipsychotics.4,5 Therefore, CIAG would be unlikely in a patient treated with clozapine for more than 1 year and who did not have a history of neutropenia, as was the case with Mr. A.
While bone marrow biopsy can help differentiate between the causes of agranulocytosis,6 it is highly invasive and may not be necessary if laboratory evidence is sufficient. However, if a treatment team is strongly considering discontinuing clozapine and there are no suitable alternatives, a biopsy may provide additional clarification.
TREATMENT CAR T-cell therapy and cancer remission
Clozapine is continued with daily monitoring. On Day 19, Mr. A’s ANC increases, reaching 2,600 µL by discharge on Day 40. Mr. A remains psychiatrically stable throughout his hospitalization and does not experience any complications associated with neutropenia, despite its prolonged duration.
Continue to: Unfortunately, multiple cycles of...
Unfortunately, multiple cycles of chemotherapy fail to induce remission. Mr. A is referred for CD19/CD22 chimeric antigen receptor (CAR) T-cell therapy, which helps achieve remission. Allogeneic hematopoietic stem cell transplant (HSCT) is recommended to maximize the likelihood of sustained remission.7 As with chemotherapy, Mr. A and his family agree with the multidisciplinary treatment recommendation to continue clozapine during both CAR T-cell therapy and HSCT, because the risks associated with psychiatric decompensation were greater than a potential increased risk of agranulocytosis. Clozapine treatment is continued throughout both therapies without issue.
Four months after HSCT, Mr. A is admitted for neutropenic fever and left face cellulitis. Upon admission, his ANC is 30 µL and subsequently decreases to 0 µL. In addition to neutropenia, Mr. A is also anemic and thrombocytopenic. He undergoes a bone marrow biopsy.
[polldaddy:11125950]
The authors’ observations
While no published cases have examined the bone marrow of patients experiencing CIAG, 2 retrospective studies have characterized 2 classes of bone marrow findings associated with drug-induced agranulocytosis resulting from nonchemotherapeutic agents (Table).8,9 Type I marrow appears hypercellular with adequate neutrophil precursors but an arrested neutrophil maturation, with few or no mature forms of neutrophils beyond myelocytes.8,9 Type II demonstrates a severe reduction or complete absence of granulocytic precursors with normal or increased erythropoiesis and megakaryocytes.8,9 These findings have been used to accurately differentiate between chemotherapy and nonchemotherapy drug-induced agranulocytosis,6 resulting in successful identification and discontinuation of the responsible agent.

Mr. A’s bone marrow biopsy showed severe pancytopenia with profound neutropenia and normocytic anemia, without evidence of residual leukemia, inconsistent with Type I or Type II. Findings were suggestive of a myelodysplastic syndrome, consistent with secondary graft failure. Symptoms resolved after treatment with antibiotics, granulocyte colony-stimulating factor, epoetin alfa, and thrombopoietin. Mr. A’s ANC remained 0 µL for 22 days before returning to normal (>1,500 µL) by Day 29. He had no secondary complications resulting from neutropenia. As the clinical evidence suggested, Mr. A’s neutropenia was unlikely to be due to clozapine. Clozapine was continued throughout his cancer treatment, and he remained psychiatrically stable.
Clozapine, cancer treatments, and agranulocytosis
This case demonstrates that clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even with the development of agranulocytosis and prolonged neutropenia. Evidence to guide psychiatric clinicians to evaluate the likelihood that agranulocytosis is clozapine-induced is limited.
Continue to: We offer an algorithm...
We offer an algorithm to assist clinicians faced with this challenging clinical dilemma (Figure). Based on our experience and limited current evidence, we recommend continuing clozapine during cancer treatment unless there is clear evidence to suggest otherwise. Presently, no evidence in published literature suggests worsened outcomes in patients treated concurrently with clozapine and cancer therapies.

OUTCOME Cancer-free and psychiatrically stable
Mr. A continues clozapine therapy throughout all phases of treatment, without interruption. No adverse effects are determined to be secondary to clozapine. He remains psychiatrically stable throughout treatment, and able to participate and engage in his oncologic therapy. Mr. A is now more than 1 year in remission with no recurrence of graft failure, and his psychiatric symptoms continue to be well controlled with clozapine.
Bottom Line
Clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even in patients who develop agranulocytosis and prolonged neutropenia. Based on our experience and limited evidence, we offer an algorithm to assist clinicians faced with this challenging clinical dilemma.
Related Resources
- Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286. doi:10.1111/ejh.13285
- Daniel JS, Gross T. Managing clozapine-induced neutropenia and agranulocytosis. Current Psychiatry. 2016;15(12):51-53.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Divalproex sodium • Depakote
Epoetin alfa • Epogen
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
1. Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286.
2. Pick AM, Nystrom KK. Nonchemotherapy drug-induced neutropenia and agranulocytosis: could medications be the culprit? J Pharm Pract. 2014:27(5):447-452.
3. Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606-3618.
4. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
5. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483-488.
6. Azadeh N, Kelemen K, Fonseca R. Amitriptyline-induced agranulocytosis with bone marrow confirmation. Clin Lymphoma Myeloma Leuk. 2014;14(5):e183-e185.
7. Liu J, Zhang X, Zhong JF, et al. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9(13):1115-1125.
8. Apinantriyo B, Lekhakula A, Rujirojindakul P. Incidence, etiology and bone marrow characteristics of non-chemotherapy-induced agranulocytosis. Hematology. 2011;16(1):50-53.
9. Yang J, Zhong J, Xiao XH, et al. The relationship between bone marrow characteristics and the clinical prognosis of antithyroid drug-induced agranulocytosis. Endocr J. 2013;60(2):185-189.
1. Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286.
2. Pick AM, Nystrom KK. Nonchemotherapy drug-induced neutropenia and agranulocytosis: could medications be the culprit? J Pharm Pract. 2014:27(5):447-452.
3. Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606-3618.
4. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
5. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483-488.
6. Azadeh N, Kelemen K, Fonseca R. Amitriptyline-induced agranulocytosis with bone marrow confirmation. Clin Lymphoma Myeloma Leuk. 2014;14(5):e183-e185.
7. Liu J, Zhang X, Zhong JF, et al. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9(13):1115-1125.
8. Apinantriyo B, Lekhakula A, Rujirojindakul P. Incidence, etiology and bone marrow characteristics of non-chemotherapy-induced agranulocytosis. Hematology. 2011;16(1):50-53.
9. Yang J, Zhong J, Xiao XH, et al. The relationship between bone marrow characteristics and the clinical prognosis of antithyroid drug-induced agranulocytosis. Endocr J. 2013;60(2):185-189.
BOARDING psychiatric patients in the ED: Key strategies
Boarding of psychiatric patients in the emergency department (ED) has been well documented.1 Numerous researchers have discussed ways to address this public health crisis. In this Pearl, I use the acronym BOARDING to provide key strategies for psychiatric clinicians managing psychiatric patients who are boarding in an ED.
Be vigilant. As a patient’s time waiting in the ED increases, watch for clinical blind spots. New medical problems,2 psychiatric issues, or medication errors3 may unexpectedly arise since the patient was originally stabilized by emergency medicine clinicians.
Orders. Since the patient could be waiting in the ED for 24 hours or longer, consider starting orders (eg, precautions, medications, diet, vital sign checks, labs, etc) as you would for a patient in an inpatient psychiatric unit or a dedicated psychiatric ED.
AWOL. Unlike inpatient psychiatric units, EDs generally are not locked. Extra resources (eg, sitter, safety alarm bracelet) may be needed to help prevent patients from leaving this setting unnoticed, especially those on involuntary psychiatric holds.
Re-evaluate. Ideally, re-evaluate the patient every shift. Does the patient still need an inpatient psychiatric setting? Can the involuntary psychiatric hold be discontinued?
Disposition. Is there a family member or reliable caregiver to whom the patient can be discharged? Can the patient go to a shelter or be stabilized in a short-term residential program, instead of an inpatient psychiatric unit?
Inpatient. If the patient waits 24 hours or longer, begin thinking like an inpatient psychiatric clinician. Are there any interventions you can reasonably begin in the ED that you would otherwise begin on an inpatient psychiatric unit?
Nursing. Work with ED nursing staff to familiarize them with the patient’s specific needs.
Guidelines. With the input of clinical and administrative leadership, establish local hospital-based guidelines for managing psychiatric patients who are boarding in the ED.
1. Nordstrom K, Berlin JS, Nash SS, et al. Boarding of mentally ill patients in emergency departments: American Psychiatric Association Resource Document. West J Emerg Med. 2019;20(5):690-695.
2. Garfinkel E, Rose D, Strouse K, et al. Psychiatric emergency department boarding: from catatonia to cardiac arrest. Am J Emerg Med. 2019;37(3):543-544.
3. Bakhsh HT, Perona SJ, Shields WA, et al. Medication errors in psychiatric patients boarded in the emergency department. Int J Risk Saf Med. 2014;26(4):191-198.
Boarding of psychiatric patients in the emergency department (ED) has been well documented.1 Numerous researchers have discussed ways to address this public health crisis. In this Pearl, I use the acronym BOARDING to provide key strategies for psychiatric clinicians managing psychiatric patients who are boarding in an ED.
Be vigilant. As a patient’s time waiting in the ED increases, watch for clinical blind spots. New medical problems,2 psychiatric issues, or medication errors3 may unexpectedly arise since the patient was originally stabilized by emergency medicine clinicians.
Orders. Since the patient could be waiting in the ED for 24 hours or longer, consider starting orders (eg, precautions, medications, diet, vital sign checks, labs, etc) as you would for a patient in an inpatient psychiatric unit or a dedicated psychiatric ED.
AWOL. Unlike inpatient psychiatric units, EDs generally are not locked. Extra resources (eg, sitter, safety alarm bracelet) may be needed to help prevent patients from leaving this setting unnoticed, especially those on involuntary psychiatric holds.
Re-evaluate. Ideally, re-evaluate the patient every shift. Does the patient still need an inpatient psychiatric setting? Can the involuntary psychiatric hold be discontinued?
Disposition. Is there a family member or reliable caregiver to whom the patient can be discharged? Can the patient go to a shelter or be stabilized in a short-term residential program, instead of an inpatient psychiatric unit?
Inpatient. If the patient waits 24 hours or longer, begin thinking like an inpatient psychiatric clinician. Are there any interventions you can reasonably begin in the ED that you would otherwise begin on an inpatient psychiatric unit?
Nursing. Work with ED nursing staff to familiarize them with the patient’s specific needs.
Guidelines. With the input of clinical and administrative leadership, establish local hospital-based guidelines for managing psychiatric patients who are boarding in the ED.
Boarding of psychiatric patients in the emergency department (ED) has been well documented.1 Numerous researchers have discussed ways to address this public health crisis. In this Pearl, I use the acronym BOARDING to provide key strategies for psychiatric clinicians managing psychiatric patients who are boarding in an ED.
Be vigilant. As a patient’s time waiting in the ED increases, watch for clinical blind spots. New medical problems,2 psychiatric issues, or medication errors3 may unexpectedly arise since the patient was originally stabilized by emergency medicine clinicians.
Orders. Since the patient could be waiting in the ED for 24 hours or longer, consider starting orders (eg, precautions, medications, diet, vital sign checks, labs, etc) as you would for a patient in an inpatient psychiatric unit or a dedicated psychiatric ED.
AWOL. Unlike inpatient psychiatric units, EDs generally are not locked. Extra resources (eg, sitter, safety alarm bracelet) may be needed to help prevent patients from leaving this setting unnoticed, especially those on involuntary psychiatric holds.
Re-evaluate. Ideally, re-evaluate the patient every shift. Does the patient still need an inpatient psychiatric setting? Can the involuntary psychiatric hold be discontinued?
Disposition. Is there a family member or reliable caregiver to whom the patient can be discharged? Can the patient go to a shelter or be stabilized in a short-term residential program, instead of an inpatient psychiatric unit?
Inpatient. If the patient waits 24 hours or longer, begin thinking like an inpatient psychiatric clinician. Are there any interventions you can reasonably begin in the ED that you would otherwise begin on an inpatient psychiatric unit?
Nursing. Work with ED nursing staff to familiarize them with the patient’s specific needs.
Guidelines. With the input of clinical and administrative leadership, establish local hospital-based guidelines for managing psychiatric patients who are boarding in the ED.
1. Nordstrom K, Berlin JS, Nash SS, et al. Boarding of mentally ill patients in emergency departments: American Psychiatric Association Resource Document. West J Emerg Med. 2019;20(5):690-695.
2. Garfinkel E, Rose D, Strouse K, et al. Psychiatric emergency department boarding: from catatonia to cardiac arrest. Am J Emerg Med. 2019;37(3):543-544.
3. Bakhsh HT, Perona SJ, Shields WA, et al. Medication errors in psychiatric patients boarded in the emergency department. Int J Risk Saf Med. 2014;26(4):191-198.
1. Nordstrom K, Berlin JS, Nash SS, et al. Boarding of mentally ill patients in emergency departments: American Psychiatric Association Resource Document. West J Emerg Med. 2019;20(5):690-695.
2. Garfinkel E, Rose D, Strouse K, et al. Psychiatric emergency department boarding: from catatonia to cardiac arrest. Am J Emerg Med. 2019;37(3):543-544.
3. Bakhsh HT, Perona SJ, Shields WA, et al. Medication errors in psychiatric patients boarded in the emergency department. Int J Risk Saf Med. 2014;26(4):191-198.
Caring for Muslim patients who fast during Ramadan
Ramadan is one of the obligatory pillars in Islam during which healthy Muslims are required to fast from dawn until sunset every day for 1 month. There are an estimated 3.45 million Muslims in the United States, and this population will continue to grow by 100,000 per year.1 With the increased growth of the Muslim population, it is important for clinicians to be aware of how patients of Muslim faith are affected during Ramadan. In this article, we explore the potential risks, as well as the benefits, the month of Ramadan brings to patients. We will also explain how being religiously aware is necessary to provide optimal care for these individuals.
For some patients, fasting may pose risks
Similar to other communities in the United States, individuals who are Muslim experience mood disorders, anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, substance use disorders, and other psychiatric illnesses.2 During the month of Ramadan, Muslims are to abstain completely from eating and drinking from dawn until sunset. This includes medications as well as food and drink.
Due to these circumstances, patients will often change the timing, frequency, and dosing of their medications to allow them to fast. One study found 60% of Muslims made medication adjustments during Ramadan without seeking medical advice.3 It is possible that such alterations may be detrimental. During Ramadan, some Muslims wake up early in the morning to eat a pre-dawn meal, and often go back to sleep. This has been reported to cause a delay in sleep-wake times and to reduce rapid eye movement sleep.4 These circadian rhythm changes can be detrimental to patients with bipolar disorder. One study found higher rates of relapse to depression and mania in patients with bipolar disorder who were fasting during Ramadan.5 Circadian rhythm disturbances also may worsen depression.6 Another point of concern is patients with eating disorders. One small case series (N = 6) found that fasting during Ramadan exacerbated symptoms in patients with eating disorders.7
Another concern is that dehydration while fasting can lead to lithium toxicity. However, one study found lithium levels remained stable while fasting for 10 to 12 hours.5 Another showed that changing lithium dosing from twice a day to once a day allowed for easier administration without causing a subtherapeutic change in blood lithium levels.8
The practice also may have benefits for mental health
For many Muslims, Ramadan is the best time of the year, where they reconnect with their religion and experience the utmost spiritual growth. Studies have shown that the incidence of suicide is lowest during Ramadan compared to other months.9 A study of older men found that intermittent fasting and calorie restriction (not during Ramadan) resulted in decreases in tension, confusion, anger, and mood disturbance.10 Another study found that fasting during Ramadan had a positive impact on depression, anxiety, stress, and cognitive function.11
Clinical considerations
To provide the best care for Muslim patients during Ramadan, clinicians should take a holistic approach and take all factors into consideration. It is common for circadian rhythm disruptions to exacerbate mood disorders, so encourage patients to maintain healthy sleep hygiene to their best ability during this month. Another important consideration is medication timing and dosing.12 For patients prescribed a medication that typically is taken twice a day, determine if this dosing can be changed to once a day, or if both doses can be taken when it is permissible to eat (sunset to dawn). For medications that are absorbed with food, consider how these medications might be adjusted and maintained while a patient is fasting. Some medications may be sedating or activating, so the timing of administration may need to be adjusted to meet the patient’s needs. Lastly, keep in mind that certain medications can have withdrawal effects, and the likelihood of this occurring while a patient is fasting.
One vital point is that if a patient is at high risk of clinically decompensating due to fasting or medication adjustments or discontinuation, advise them to not fast. Muslims with physical or mental illnesses are excused from fasting. Bear in mind that because Ramadan is meant to be a month of heightened spirituality, many Muslims will prefer to fast.
1. Pew Research Center. Demographic portrait of Muslim Americans. Published July 26, 2017. Accessed January 15, 2019. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans
2. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
3. Aslam M, Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public Health. 1986;100(1):49-53.
4. Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017;21(3):577-586.
5. Eddahby S, Kadri N, Moussaoui D. Fasting during Ramadan is associated with a higher recurrence rate in patients with bipolar disorder. World Psychiatry. 2014;13(1):97.
6. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571-585.
7. Akgül S, Derman O, Kanbur NÖ. Fasting during Ramadan: a religious factor as a possible trigger or exacerbator for eating disorders in adolescents. Int J Eat Disord. 2014;47(8):905-910.
8. Kadri N, Mouchtaq N, Hakkou F, et al. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol. 2000;3(1):45-49.
9. Taktak S, Kumral B, Unsal A, et al. Evidence for an association between suicide and religion: a 33-year retrospective autopsy analysis of suicide by hanging during the month of Ramadan in Istanbul. Aust J Forensic Sci. 2016;48(2):121-131.
10. Hussin NM, Shahar S, Teng NI, et al. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Health Aging. 2013;17(8):674-680.
11. Amin A, Sai Sailesh K, Mishra S, et al. Effects of fasting during Ramadan month on depression, anxiety and stress and cognition. Int J Med Res Rev. 2016;4(5):771-774.
12. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
Ramadan is one of the obligatory pillars in Islam during which healthy Muslims are required to fast from dawn until sunset every day for 1 month. There are an estimated 3.45 million Muslims in the United States, and this population will continue to grow by 100,000 per year.1 With the increased growth of the Muslim population, it is important for clinicians to be aware of how patients of Muslim faith are affected during Ramadan. In this article, we explore the potential risks, as well as the benefits, the month of Ramadan brings to patients. We will also explain how being religiously aware is necessary to provide optimal care for these individuals.
For some patients, fasting may pose risks
Similar to other communities in the United States, individuals who are Muslim experience mood disorders, anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, substance use disorders, and other psychiatric illnesses.2 During the month of Ramadan, Muslims are to abstain completely from eating and drinking from dawn until sunset. This includes medications as well as food and drink.
Due to these circumstances, patients will often change the timing, frequency, and dosing of their medications to allow them to fast. One study found 60% of Muslims made medication adjustments during Ramadan without seeking medical advice.3 It is possible that such alterations may be detrimental. During Ramadan, some Muslims wake up early in the morning to eat a pre-dawn meal, and often go back to sleep. This has been reported to cause a delay in sleep-wake times and to reduce rapid eye movement sleep.4 These circadian rhythm changes can be detrimental to patients with bipolar disorder. One study found higher rates of relapse to depression and mania in patients with bipolar disorder who were fasting during Ramadan.5 Circadian rhythm disturbances also may worsen depression.6 Another point of concern is patients with eating disorders. One small case series (N = 6) found that fasting during Ramadan exacerbated symptoms in patients with eating disorders.7
Another concern is that dehydration while fasting can lead to lithium toxicity. However, one study found lithium levels remained stable while fasting for 10 to 12 hours.5 Another showed that changing lithium dosing from twice a day to once a day allowed for easier administration without causing a subtherapeutic change in blood lithium levels.8
The practice also may have benefits for mental health
For many Muslims, Ramadan is the best time of the year, where they reconnect with their religion and experience the utmost spiritual growth. Studies have shown that the incidence of suicide is lowest during Ramadan compared to other months.9 A study of older men found that intermittent fasting and calorie restriction (not during Ramadan) resulted in decreases in tension, confusion, anger, and mood disturbance.10 Another study found that fasting during Ramadan had a positive impact on depression, anxiety, stress, and cognitive function.11
Clinical considerations
To provide the best care for Muslim patients during Ramadan, clinicians should take a holistic approach and take all factors into consideration. It is common for circadian rhythm disruptions to exacerbate mood disorders, so encourage patients to maintain healthy sleep hygiene to their best ability during this month. Another important consideration is medication timing and dosing.12 For patients prescribed a medication that typically is taken twice a day, determine if this dosing can be changed to once a day, or if both doses can be taken when it is permissible to eat (sunset to dawn). For medications that are absorbed with food, consider how these medications might be adjusted and maintained while a patient is fasting. Some medications may be sedating or activating, so the timing of administration may need to be adjusted to meet the patient’s needs. Lastly, keep in mind that certain medications can have withdrawal effects, and the likelihood of this occurring while a patient is fasting.
One vital point is that if a patient is at high risk of clinically decompensating due to fasting or medication adjustments or discontinuation, advise them to not fast. Muslims with physical or mental illnesses are excused from fasting. Bear in mind that because Ramadan is meant to be a month of heightened spirituality, many Muslims will prefer to fast.
Ramadan is one of the obligatory pillars in Islam during which healthy Muslims are required to fast from dawn until sunset every day for 1 month. There are an estimated 3.45 million Muslims in the United States, and this population will continue to grow by 100,000 per year.1 With the increased growth of the Muslim population, it is important for clinicians to be aware of how patients of Muslim faith are affected during Ramadan. In this article, we explore the potential risks, as well as the benefits, the month of Ramadan brings to patients. We will also explain how being religiously aware is necessary to provide optimal care for these individuals.
For some patients, fasting may pose risks
Similar to other communities in the United States, individuals who are Muslim experience mood disorders, anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, substance use disorders, and other psychiatric illnesses.2 During the month of Ramadan, Muslims are to abstain completely from eating and drinking from dawn until sunset. This includes medications as well as food and drink.
Due to these circumstances, patients will often change the timing, frequency, and dosing of their medications to allow them to fast. One study found 60% of Muslims made medication adjustments during Ramadan without seeking medical advice.3 It is possible that such alterations may be detrimental. During Ramadan, some Muslims wake up early in the morning to eat a pre-dawn meal, and often go back to sleep. This has been reported to cause a delay in sleep-wake times and to reduce rapid eye movement sleep.4 These circadian rhythm changes can be detrimental to patients with bipolar disorder. One study found higher rates of relapse to depression and mania in patients with bipolar disorder who were fasting during Ramadan.5 Circadian rhythm disturbances also may worsen depression.6 Another point of concern is patients with eating disorders. One small case series (N = 6) found that fasting during Ramadan exacerbated symptoms in patients with eating disorders.7
Another concern is that dehydration while fasting can lead to lithium toxicity. However, one study found lithium levels remained stable while fasting for 10 to 12 hours.5 Another showed that changing lithium dosing from twice a day to once a day allowed for easier administration without causing a subtherapeutic change in blood lithium levels.8
The practice also may have benefits for mental health
For many Muslims, Ramadan is the best time of the year, where they reconnect with their religion and experience the utmost spiritual growth. Studies have shown that the incidence of suicide is lowest during Ramadan compared to other months.9 A study of older men found that intermittent fasting and calorie restriction (not during Ramadan) resulted in decreases in tension, confusion, anger, and mood disturbance.10 Another study found that fasting during Ramadan had a positive impact on depression, anxiety, stress, and cognitive function.11
Clinical considerations
To provide the best care for Muslim patients during Ramadan, clinicians should take a holistic approach and take all factors into consideration. It is common for circadian rhythm disruptions to exacerbate mood disorders, so encourage patients to maintain healthy sleep hygiene to their best ability during this month. Another important consideration is medication timing and dosing.12 For patients prescribed a medication that typically is taken twice a day, determine if this dosing can be changed to once a day, or if both doses can be taken when it is permissible to eat (sunset to dawn). For medications that are absorbed with food, consider how these medications might be adjusted and maintained while a patient is fasting. Some medications may be sedating or activating, so the timing of administration may need to be adjusted to meet the patient’s needs. Lastly, keep in mind that certain medications can have withdrawal effects, and the likelihood of this occurring while a patient is fasting.
One vital point is that if a patient is at high risk of clinically decompensating due to fasting or medication adjustments or discontinuation, advise them to not fast. Muslims with physical or mental illnesses are excused from fasting. Bear in mind that because Ramadan is meant to be a month of heightened spirituality, many Muslims will prefer to fast.
1. Pew Research Center. Demographic portrait of Muslim Americans. Published July 26, 2017. Accessed January 15, 2019. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans
2. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
3. Aslam M, Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public Health. 1986;100(1):49-53.
4. Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017;21(3):577-586.
5. Eddahby S, Kadri N, Moussaoui D. Fasting during Ramadan is associated with a higher recurrence rate in patients with bipolar disorder. World Psychiatry. 2014;13(1):97.
6. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571-585.
7. Akgül S, Derman O, Kanbur NÖ. Fasting during Ramadan: a religious factor as a possible trigger or exacerbator for eating disorders in adolescents. Int J Eat Disord. 2014;47(8):905-910.
8. Kadri N, Mouchtaq N, Hakkou F, et al. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol. 2000;3(1):45-49.
9. Taktak S, Kumral B, Unsal A, et al. Evidence for an association between suicide and religion: a 33-year retrospective autopsy analysis of suicide by hanging during the month of Ramadan in Istanbul. Aust J Forensic Sci. 2016;48(2):121-131.
10. Hussin NM, Shahar S, Teng NI, et al. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Health Aging. 2013;17(8):674-680.
11. Amin A, Sai Sailesh K, Mishra S, et al. Effects of fasting during Ramadan month on depression, anxiety and stress and cognition. Int J Med Res Rev. 2016;4(5):771-774.
12. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
1. Pew Research Center. Demographic portrait of Muslim Americans. Published July 26, 2017. Accessed January 15, 2019. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans
2. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
3. Aslam M, Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public Health. 1986;100(1):49-53.
4. Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017;21(3):577-586.
5. Eddahby S, Kadri N, Moussaoui D. Fasting during Ramadan is associated with a higher recurrence rate in patients with bipolar disorder. World Psychiatry. 2014;13(1):97.
6. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571-585.
7. Akgül S, Derman O, Kanbur NÖ. Fasting during Ramadan: a religious factor as a possible trigger or exacerbator for eating disorders in adolescents. Int J Eat Disord. 2014;47(8):905-910.
8. Kadri N, Mouchtaq N, Hakkou F, et al. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol. 2000;3(1):45-49.
9. Taktak S, Kumral B, Unsal A, et al. Evidence for an association between suicide and religion: a 33-year retrospective autopsy analysis of suicide by hanging during the month of Ramadan in Istanbul. Aust J Forensic Sci. 2016;48(2):121-131.
10. Hussin NM, Shahar S, Teng NI, et al. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Health Aging. 2013;17(8):674-680.
11. Amin A, Sai Sailesh K, Mishra S, et al. Effects of fasting during Ramadan month on depression, anxiety and stress and cognition. Int J Med Res Rev. 2016;4(5):771-774.
12. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
Cats, toxoplasmosis, and psychosis: Understanding the risks
It has been clearly established that most human infectious diseases are caused by infectious agents that have been transmitted from animals to humans.1 Based on published estimates from the 2000s, 60% to 76% of emerging infectious disease events are transmitted from animals to humans.2
When we consider animals that cause human diseases, we usually think of rats and bats. We rarely think of the 90 million cats owned as pets in the United States, or the approximately 30 to 80 million feral cats. Many consider cats as family members, and three-fourths of cats owned in the United States are allowed to sleep on the beds of their owners.1 These cats may be a substantial source of human disease. Researchers at the University of Liverpool have identified 273 infectious agents carried by cats, of which 151 are known to be shared with humans.1 The most widely known of these agents are Lyssavirus, the virus that causes rabies; Bartonella henselae, the bacteria that causes cat scratch disease; and Toxoplasma gondii (T. gondii), the parasite that causes toxoplasmosis.
In my new open-access book Parasites, Pussycats and Psychosis (available at https://link.springer.com/book/10.1007/978-3-030-86811-6), I describe the relationship between cats, T. gondii, and toxoplasmosis, and detail the evidence linking T. gondii to some cases of schizophrenia, bipolar disorder, and other diseases.1 Though human T. gondii infection is typically asymptomatic or produces minor, flu-like symptoms, there are a few important exceptions. This article outlines those exceptions, and investigates evidence that implicates a link between T. gondii and psychosis.
How T. gondii can be transmitted
T. gondii has been called “one of the most successful parasites on earth.”3 Globally, approximately one-third of the human population is infected with T. gondii, though this varies widely by country and is dependent on dietary habits and exposure to cats. A 2014 survey reported that 11% of Americans—approximately 40 million people—have been infected, as evidenced by the presence of antibodies in their blood.1
T. gondii begins its life cycle when a cat becomes infected, usually as a kitten. Most infected cats are asymptomatic, but for approximately 8 days they excrete up to 50 million infectious oocysts in their feces daily. Depending on the temperature, these oocysts can live for 2 years or longer.It is thought that a single oocyst can cause human infection.1 Since cats like loose soil for defecation, the infective oocysts commonly end up in gardens, uncovered sandboxes, or animal feed piles in barns. After 24 hours, the oocysts dry out and may become aerosolized. For this reason, cat owners are advised to change their cat’s litter daily.
The number of ways T. gondii can be transmitted to humans is extensive. Farm animals can become infected from contaminated feed; this causes T. gondii oocysts in animals’ muscles, which later may cause human infection if eaten as undercooked meat. Many such family outbreaks of toxoplasmosis have been described.1
If infective oocysts get into the water supply, they may also cause outbreaks of disease. More than 200 such outbreaks have been described, including an instance in Victoria, British Columbia, in which 100 people became clinically infected.4
Continue to: Family outbreaks...
Family outbreaks have also been described that involve multiple children who played in an infected sandbox or dirt pile.5 Similarly, an outbreak has been reported in a riding stable that was home to infected cats. Infective oocysts were thought to have become aerosolized and breathed in by the patrons.6 Multiple other possible modes of transmission are being investigated, including sexual transmission among humans.7
Human infections are not always benign
In most human T. gondii cases, the infected individual experiences mild, flu-like symptoms, often with enlarged lymph nodes, or has no symptoms.1 Thus, most people who have been infected with
There are 3 exceptions to this otherwise benign clinical picture. The first is cerebral toxoplasmosis, which occurs in individuals who are immunosuppressed because they have AIDS or are receiving treatment for cancer or organ transplantation. Cerebral toxoplasmosis can be severe and was a common cause of death in patients with AIDS before the development of effective AIDS treatments.
The second exception is congenital toxoplasmosis, when an infection occurs in a pregnant woman. Such infections can cause severe damage to the developing fetus, including abortion, stillbirth, and brain damage. Congenital toxoplasmosis infections occur in approximately 1 of every 10,000 births in the United States, or approximately 3,800 each year.8 As a result, pregnant women are advised not to change their cat’s litter and to be tested for evidence of T. gondii infection.
The third exception is eye disease. Toxoplasmosis is one of the most common causes of eye disease, especially of the retina. Each year in the United States, approximately 4,800 individuals develop systematic ocular toxoplasmosis.9
Continue to: Toxoplasmosis and psychosis
Toxoplasmosis and psychosis: What evidence supports a link?
Until recently, cerebral infections, congenital infections, and eye disease were thought to be the main clinical problems associated with toxoplasmosis. However, accumulating evidence suggests that psychosis should be added to this list. Five lines of evidence support this.
1. T. gondii can cause psychotic symptoms. It has been known for decades that T. gondii can cause delusions, auditory hallucinations, and other psychotic symptoms.1 In one of the earliest publications (1966), Ladee10 concluded “The literature not infrequently focuses attention on psychosis with schizophrenia or schizophreniform features that accompany chronic toxoplasmosis.” Among the cases Ladee10 described was a laboratory worker who became infected with T. gondii and developed delusions and hallucinations.10
2. Patients with schizophrenia who are infected with T. gondii have more severe psychotic symptoms. This finding has been reported in at least 7 studies.1 Holub et al11 evaluated 251 patients with schizophrenia who were treated in Prague Psychiatric Centre between 2000 and 2010. Overall, 57 participants were infected with T. gondii and 194 were not infected. Compared to those who were not infected, the infected group:
- had significantly more severe symptoms (P = .032) as measured on the Positive and Negative Symptom Scale
- were prescribed higher doses of antipsychotic medications
- had been hospitalized longer.11
3. Compared with controls, patients with psychosis are significantly more likely to have antibodies against T. gondii, indicating previous infection. To date there have been approximately 100 such studies, of which at least three-fourths reported a positive association. In a 2012 meta-analysis of 38 such studies, Torrey et al12 reported an odds ratio (OR) of 2.7—compared to persons who have not been infected, those who have been infected with T. gondii were 2.7 times more likely to have schizophrenia.12 This study replicated the findings of a previous meta-analysis of 23 antibody studies, which also found an OR of 2.7.13
4. Compared with controls, individuals with schizophrenia or bipolar disorder are significantly more likely as a child to have lived in a home with a cat. Since 1995, 10 such studies have been published; 7 were positive, 2 were negative, and 1 was inconclusive.1 Torrey et al14 reviewed 2,025 individuals with schizophrenia or bipolar disorder and 4,847 controls and found that 51% of the cases and 43% of the controls had owned a cat before age 13; this difference was highly significant (P < .001). In fact, it is surprising that any study can find a statistically significant association between cat ownership and childhood psychosis. This is because a child who did not own a cat could become infected in many locations where cats have been present, including sandboxes at school, a babysitter’s or friend’s house, or a public park. And even if a child became infected at home, they would not necessarily have owned a cat, since the neighbor’s cat could have been responsible for the oocyst contamination.
Continue to: Epidemiologically...
5. Epidemiologically, there is a close temporal correlation between the rise of cats as pets and the rise of psychosis. This can be illustrated most clearly in England, where the rise of cat ownership has been documented by writers and where there is data on the rise of psychosis, especially in the 18th and 19th centuries.1
How many cases of psychosis might be caused by T. gondii?
In 2014, using data from the antibody studies discussed above,12,13 Smith15 sought to discover how many cases of psychosis might be caused by T. gondii. He concluded that 21% of cases of schizophrenia might have been caused by T. gondii. Based on the annual incidence of schizophrenia in the United States, this would mean an estimated >10,000 new cases of schizophrenia each year are attributable to this parasite.
Some researchers have found links between T. gondii and several nonpsychiatric diseases and conditions, including epilepsy and brain cancer (Box1,16-19).
Box
As interest in Toxoplasma gondii (T. gondii) has increased, researchers have looked for associations between this parasite with other diseases and conditions. Based on the literature, the following are of most interest:
Epilepsy. Since 1995, 16 studies1 have explored the relationship between T. gondii and epilepsy. A recent meta-analysis reported a statistically significant association between T. gondii and epilepsy.16
Brain cancer. Authors in 2 of 3 studies of meningiomas and 4 of 5 studies of gliomas reported statistically significant associations between these brain tumors and infection with T. gondii.1,17
Rheumatoid arthritis. Eight studies reported an increased prevalence of T. gondii antibodies in individuals with rheumatoid arthritis.1,18
Motor vehicle accidents. Infection with T. gondii is known to decrease motor reaction times in humans. At least 11 studies1 have examined whether infected individuals are more likely to have been involved in motor vehicle accidents. The results are mixed; the largest study reported a weak but statistically significant association.19
Clinical implications: What to tell patients about cats
What do these studies of toxoplasmosis imply for psychiatric care? As mental health professionals, part of our job is to educate our patients. Anything that appears to be a risk factor for the development of psychosis is thus of interest. Consider discussing the following with your patients.
Are cats safe? Cats that are kept exclusively indoors are safe pets because they are unlikely to become infected with T. gondii. However, cats that are allowed to go outdoors may not be safe, especially for children and young adults. What is needed is an effective vaccine that could be given to newborn kittens to prevent infection, but development of this type of vaccine has never been prioritized. At the community level, programs to decrease the number of stray and feral cats would also decrease the risk of infection.
Continue to: How to decrease risk
How to decrease risk. On a personal level, we can decrease T. gondii infections by not eating undercooked meat. Pregnant women and individuals who are immunocompromised should not change cat litter. When gardening, we should wear gloves because cats favor loose soil for depositing their feces. We should also protect children by covering sandboxes when not in use and by not allowing children to play in uncovered public sandboxes.
Treatment. Toxoplasmosis typically is treated with pyrimethamine, usually in combination with a sulfa drug. However, pyrimethamine does not cross the blood brain barrier and thus is ineffective when T. gondii infects the brain. The development of a drug that will effectively treat T. gondii in the brain should be a high priority.
For additional details on the studies discussed in this article as well as more resources on the impact T. gondii can have if proper precautions are not taken, see my open-access book at https://link.springer.com/book/10.1007/978-3-030-86811-6.
Bottom Line
Some evidence suggests that infection with Toxoplasma gondii (T. gondii) may cause psychotic symptoms, may increase an individual’s risk of developing psychosis, and may result in more severe psychotic symptoms. Cats can transmit T. gondii to humans. Educate patients that they can reduce their risk by keeping their cats inside, avoiding exposure to cat feces, particularly while pregnant or if immunocompromised, and not eating undercooked meat.
Related Resources
- Torrey EF. Parasites, Pussycats, and Psychosis: The Unknown Dangers of Human Toxoplasmosis. Springer Nature; 2022. https://link.springer.com/book/10.1007/978-3-030-86811-6
Drug Brand Names
Pyrimethamine • Daraprim
1. Torrey EF. Parasites, Pussycats, and Psychosis: The Unknown Dangers of Human Toxoplasmosis. Springer Nature; 2022. https://link.springer.com/book/10.1007/978-3-030-86811-6
2. Rohr JR, Barrett CB, Civitello DJ, et al. Emerging human infectious diseases and the links to global food production. Nat Sustain. 2019;2(6):445-456.
3. Joynson DHM. Preface. In: Joynson DHM, Wreghitt TG, eds. Toxoplasmosis: A Comprehensive Clinical Guide. Cambridge University Press; 2001:xi.
4. Bowie WR, King AS, Werker DH, et al. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet. 1997;350(9072):173-177.
5. Stagno S, Dykes AC, Amos CS, et al. An outbreak of toxoplasmosis linked to cats. Pediatrics. 1980;65(4):706-712.
6. Teutsch SM, Juranek DD, Sulzer A, et al. Epidemic toxoplasmosis associated with infected cats. N Engl J Med. 1979;300(13):695-699.
7. Kaňková Š, Hlaváčová J, Flegr J. Oral sex: a new, and possibly the most dangerous, route of toxoplasmosis transmission. Med Hypotheses. 2020;141:109725.
8. Guerina NG, Hsu HW, Meissner HC, et al. Neonatal serologic screening and early treatment for congenital T. gondii infection. N Engl J Med. 1994;330(26):1858-1863.
9. Jones JL, Holland GN. Annual burden of ocular toxoplasmosis in the US. Am J Trop Med Hyg. 2010;82(3):464-465.
10. Ladee GA. Diagnostic problems in psychiatry with regard to acquired toxoplasmosis. Psychiatr Neurol Neurochir. 1966;69(1):65-82.
11. Holub D, Flegr J, Dragomirecká E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
12. Torrey EF, Bartko JJ, Yolken RH. T. gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38(3):642-647.
13. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:729-736.
14. Torrey EF, Simmons W, Yolken RH. Is childhood cat ownership a risk factor for schizophrenia later in life? Schizophr Res. 2015;165(1):1-2.
15. Smith G. Estimating the population attributable fraction for schizophrenia when T. gondii is assumed absent in human populations. Prev Vet Med. 2014;117(3-4):425-435.
16. Sadeghi M, Riahi SM, Mohammadi M, et al. An updated meta-analysis of the association between T. gondii infection and risk of epilepsy. Trans R Soc Trop Med Hyg. 2019;113(8):453-462.
17. Hodge JM, Coghill AE, Kim Y, et al. T. gondii infection and the risk of adult glioma in two prospective studies. Int J Cancer. 2021;148(10):2449-2456.
18. Hosseininejad Z, Sharif M, Sarvi S, et al. Toxoplasmosis seroprevalence in rheumatoid arthritis patients: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(6):e0006545.
19. Burgdorf KS, Trabjerg BB, Pedersen MG, et al. Large-scale study of Toxoplasma and Cytomegalovirus shows an association between infection and serious psychiatric disorders. Brain Behav Immun. 2019; 79:152-158.
It has been clearly established that most human infectious diseases are caused by infectious agents that have been transmitted from animals to humans.1 Based on published estimates from the 2000s, 60% to 76% of emerging infectious disease events are transmitted from animals to humans.2
When we consider animals that cause human diseases, we usually think of rats and bats. We rarely think of the 90 million cats owned as pets in the United States, or the approximately 30 to 80 million feral cats. Many consider cats as family members, and three-fourths of cats owned in the United States are allowed to sleep on the beds of their owners.1 These cats may be a substantial source of human disease. Researchers at the University of Liverpool have identified 273 infectious agents carried by cats, of which 151 are known to be shared with humans.1 The most widely known of these agents are Lyssavirus, the virus that causes rabies; Bartonella henselae, the bacteria that causes cat scratch disease; and Toxoplasma gondii (T. gondii), the parasite that causes toxoplasmosis.
In my new open-access book Parasites, Pussycats and Psychosis (available at https://link.springer.com/book/10.1007/978-3-030-86811-6), I describe the relationship between cats, T. gondii, and toxoplasmosis, and detail the evidence linking T. gondii to some cases of schizophrenia, bipolar disorder, and other diseases.1 Though human T. gondii infection is typically asymptomatic or produces minor, flu-like symptoms, there are a few important exceptions. This article outlines those exceptions, and investigates evidence that implicates a link between T. gondii and psychosis.
How T. gondii can be transmitted
T. gondii has been called “one of the most successful parasites on earth.”3 Globally, approximately one-third of the human population is infected with T. gondii, though this varies widely by country and is dependent on dietary habits and exposure to cats. A 2014 survey reported that 11% of Americans—approximately 40 million people—have been infected, as evidenced by the presence of antibodies in their blood.1
T. gondii begins its life cycle when a cat becomes infected, usually as a kitten. Most infected cats are asymptomatic, but for approximately 8 days they excrete up to 50 million infectious oocysts in their feces daily. Depending on the temperature, these oocysts can live for 2 years or longer.It is thought that a single oocyst can cause human infection.1 Since cats like loose soil for defecation, the infective oocysts commonly end up in gardens, uncovered sandboxes, or animal feed piles in barns. After 24 hours, the oocysts dry out and may become aerosolized. For this reason, cat owners are advised to change their cat’s litter daily.
The number of ways T. gondii can be transmitted to humans is extensive. Farm animals can become infected from contaminated feed; this causes T. gondii oocysts in animals’ muscles, which later may cause human infection if eaten as undercooked meat. Many such family outbreaks of toxoplasmosis have been described.1
If infective oocysts get into the water supply, they may also cause outbreaks of disease. More than 200 such outbreaks have been described, including an instance in Victoria, British Columbia, in which 100 people became clinically infected.4
Continue to: Family outbreaks...
Family outbreaks have also been described that involve multiple children who played in an infected sandbox or dirt pile.5 Similarly, an outbreak has been reported in a riding stable that was home to infected cats. Infective oocysts were thought to have become aerosolized and breathed in by the patrons.6 Multiple other possible modes of transmission are being investigated, including sexual transmission among humans.7
Human infections are not always benign
In most human T. gondii cases, the infected individual experiences mild, flu-like symptoms, often with enlarged lymph nodes, or has no symptoms.1 Thus, most people who have been infected with
There are 3 exceptions to this otherwise benign clinical picture. The first is cerebral toxoplasmosis, which occurs in individuals who are immunosuppressed because they have AIDS or are receiving treatment for cancer or organ transplantation. Cerebral toxoplasmosis can be severe and was a common cause of death in patients with AIDS before the development of effective AIDS treatments.
The second exception is congenital toxoplasmosis, when an infection occurs in a pregnant woman. Such infections can cause severe damage to the developing fetus, including abortion, stillbirth, and brain damage. Congenital toxoplasmosis infections occur in approximately 1 of every 10,000 births in the United States, or approximately 3,800 each year.8 As a result, pregnant women are advised not to change their cat’s litter and to be tested for evidence of T. gondii infection.
The third exception is eye disease. Toxoplasmosis is one of the most common causes of eye disease, especially of the retina. Each year in the United States, approximately 4,800 individuals develop systematic ocular toxoplasmosis.9
Continue to: Toxoplasmosis and psychosis
Toxoplasmosis and psychosis: What evidence supports a link?
Until recently, cerebral infections, congenital infections, and eye disease were thought to be the main clinical problems associated with toxoplasmosis. However, accumulating evidence suggests that psychosis should be added to this list. Five lines of evidence support this.
1. T. gondii can cause psychotic symptoms. It has been known for decades that T. gondii can cause delusions, auditory hallucinations, and other psychotic symptoms.1 In one of the earliest publications (1966), Ladee10 concluded “The literature not infrequently focuses attention on psychosis with schizophrenia or schizophreniform features that accompany chronic toxoplasmosis.” Among the cases Ladee10 described was a laboratory worker who became infected with T. gondii and developed delusions and hallucinations.10
2. Patients with schizophrenia who are infected with T. gondii have more severe psychotic symptoms. This finding has been reported in at least 7 studies.1 Holub et al11 evaluated 251 patients with schizophrenia who were treated in Prague Psychiatric Centre between 2000 and 2010. Overall, 57 participants were infected with T. gondii and 194 were not infected. Compared to those who were not infected, the infected group:
- had significantly more severe symptoms (P = .032) as measured on the Positive and Negative Symptom Scale
- were prescribed higher doses of antipsychotic medications
- had been hospitalized longer.11
3. Compared with controls, patients with psychosis are significantly more likely to have antibodies against T. gondii, indicating previous infection. To date there have been approximately 100 such studies, of which at least three-fourths reported a positive association. In a 2012 meta-analysis of 38 such studies, Torrey et al12 reported an odds ratio (OR) of 2.7—compared to persons who have not been infected, those who have been infected with T. gondii were 2.7 times more likely to have schizophrenia.12 This study replicated the findings of a previous meta-analysis of 23 antibody studies, which also found an OR of 2.7.13
4. Compared with controls, individuals with schizophrenia or bipolar disorder are significantly more likely as a child to have lived in a home with a cat. Since 1995, 10 such studies have been published; 7 were positive, 2 were negative, and 1 was inconclusive.1 Torrey et al14 reviewed 2,025 individuals with schizophrenia or bipolar disorder and 4,847 controls and found that 51% of the cases and 43% of the controls had owned a cat before age 13; this difference was highly significant (P < .001). In fact, it is surprising that any study can find a statistically significant association between cat ownership and childhood psychosis. This is because a child who did not own a cat could become infected in many locations where cats have been present, including sandboxes at school, a babysitter’s or friend’s house, or a public park. And even if a child became infected at home, they would not necessarily have owned a cat, since the neighbor’s cat could have been responsible for the oocyst contamination.
Continue to: Epidemiologically...
5. Epidemiologically, there is a close temporal correlation between the rise of cats as pets and the rise of psychosis. This can be illustrated most clearly in England, where the rise of cat ownership has been documented by writers and where there is data on the rise of psychosis, especially in the 18th and 19th centuries.1
How many cases of psychosis might be caused by T. gondii?
In 2014, using data from the antibody studies discussed above,12,13 Smith15 sought to discover how many cases of psychosis might be caused by T. gondii. He concluded that 21% of cases of schizophrenia might have been caused by T. gondii. Based on the annual incidence of schizophrenia in the United States, this would mean an estimated >10,000 new cases of schizophrenia each year are attributable to this parasite.
Some researchers have found links between T. gondii and several nonpsychiatric diseases and conditions, including epilepsy and brain cancer (Box1,16-19).
Box
As interest in Toxoplasma gondii (T. gondii) has increased, researchers have looked for associations between this parasite with other diseases and conditions. Based on the literature, the following are of most interest:
Epilepsy. Since 1995, 16 studies1 have explored the relationship between T. gondii and epilepsy. A recent meta-analysis reported a statistically significant association between T. gondii and epilepsy.16
Brain cancer. Authors in 2 of 3 studies of meningiomas and 4 of 5 studies of gliomas reported statistically significant associations between these brain tumors and infection with T. gondii.1,17
Rheumatoid arthritis. Eight studies reported an increased prevalence of T. gondii antibodies in individuals with rheumatoid arthritis.1,18
Motor vehicle accidents. Infection with T. gondii is known to decrease motor reaction times in humans. At least 11 studies1 have examined whether infected individuals are more likely to have been involved in motor vehicle accidents. The results are mixed; the largest study reported a weak but statistically significant association.19
Clinical implications: What to tell patients about cats
What do these studies of toxoplasmosis imply for psychiatric care? As mental health professionals, part of our job is to educate our patients. Anything that appears to be a risk factor for the development of psychosis is thus of interest. Consider discussing the following with your patients.
Are cats safe? Cats that are kept exclusively indoors are safe pets because they are unlikely to become infected with T. gondii. However, cats that are allowed to go outdoors may not be safe, especially for children and young adults. What is needed is an effective vaccine that could be given to newborn kittens to prevent infection, but development of this type of vaccine has never been prioritized. At the community level, programs to decrease the number of stray and feral cats would also decrease the risk of infection.
Continue to: How to decrease risk
How to decrease risk. On a personal level, we can decrease T. gondii infections by not eating undercooked meat. Pregnant women and individuals who are immunocompromised should not change cat litter. When gardening, we should wear gloves because cats favor loose soil for depositing their feces. We should also protect children by covering sandboxes when not in use and by not allowing children to play in uncovered public sandboxes.
Treatment. Toxoplasmosis typically is treated with pyrimethamine, usually in combination with a sulfa drug. However, pyrimethamine does not cross the blood brain barrier and thus is ineffective when T. gondii infects the brain. The development of a drug that will effectively treat T. gondii in the brain should be a high priority.
For additional details on the studies discussed in this article as well as more resources on the impact T. gondii can have if proper precautions are not taken, see my open-access book at https://link.springer.com/book/10.1007/978-3-030-86811-6.
Bottom Line
Some evidence suggests that infection with Toxoplasma gondii (T. gondii) may cause psychotic symptoms, may increase an individual’s risk of developing psychosis, and may result in more severe psychotic symptoms. Cats can transmit T. gondii to humans. Educate patients that they can reduce their risk by keeping their cats inside, avoiding exposure to cat feces, particularly while pregnant or if immunocompromised, and not eating undercooked meat.
Related Resources
- Torrey EF. Parasites, Pussycats, and Psychosis: The Unknown Dangers of Human Toxoplasmosis. Springer Nature; 2022. https://link.springer.com/book/10.1007/978-3-030-86811-6
Drug Brand Names
Pyrimethamine • Daraprim
It has been clearly established that most human infectious diseases are caused by infectious agents that have been transmitted from animals to humans.1 Based on published estimates from the 2000s, 60% to 76% of emerging infectious disease events are transmitted from animals to humans.2
When we consider animals that cause human diseases, we usually think of rats and bats. We rarely think of the 90 million cats owned as pets in the United States, or the approximately 30 to 80 million feral cats. Many consider cats as family members, and three-fourths of cats owned in the United States are allowed to sleep on the beds of their owners.1 These cats may be a substantial source of human disease. Researchers at the University of Liverpool have identified 273 infectious agents carried by cats, of which 151 are known to be shared with humans.1 The most widely known of these agents are Lyssavirus, the virus that causes rabies; Bartonella henselae, the bacteria that causes cat scratch disease; and Toxoplasma gondii (T. gondii), the parasite that causes toxoplasmosis.
In my new open-access book Parasites, Pussycats and Psychosis (available at https://link.springer.com/book/10.1007/978-3-030-86811-6), I describe the relationship between cats, T. gondii, and toxoplasmosis, and detail the evidence linking T. gondii to some cases of schizophrenia, bipolar disorder, and other diseases.1 Though human T. gondii infection is typically asymptomatic or produces minor, flu-like symptoms, there are a few important exceptions. This article outlines those exceptions, and investigates evidence that implicates a link between T. gondii and psychosis.
How T. gondii can be transmitted
T. gondii has been called “one of the most successful parasites on earth.”3 Globally, approximately one-third of the human population is infected with T. gondii, though this varies widely by country and is dependent on dietary habits and exposure to cats. A 2014 survey reported that 11% of Americans—approximately 40 million people—have been infected, as evidenced by the presence of antibodies in their blood.1
T. gondii begins its life cycle when a cat becomes infected, usually as a kitten. Most infected cats are asymptomatic, but for approximately 8 days they excrete up to 50 million infectious oocysts in their feces daily. Depending on the temperature, these oocysts can live for 2 years or longer.It is thought that a single oocyst can cause human infection.1 Since cats like loose soil for defecation, the infective oocysts commonly end up in gardens, uncovered sandboxes, or animal feed piles in barns. After 24 hours, the oocysts dry out and may become aerosolized. For this reason, cat owners are advised to change their cat’s litter daily.
The number of ways T. gondii can be transmitted to humans is extensive. Farm animals can become infected from contaminated feed; this causes T. gondii oocysts in animals’ muscles, which later may cause human infection if eaten as undercooked meat. Many such family outbreaks of toxoplasmosis have been described.1
If infective oocysts get into the water supply, they may also cause outbreaks of disease. More than 200 such outbreaks have been described, including an instance in Victoria, British Columbia, in which 100 people became clinically infected.4
Continue to: Family outbreaks...
Family outbreaks have also been described that involve multiple children who played in an infected sandbox or dirt pile.5 Similarly, an outbreak has been reported in a riding stable that was home to infected cats. Infective oocysts were thought to have become aerosolized and breathed in by the patrons.6 Multiple other possible modes of transmission are being investigated, including sexual transmission among humans.7
Human infections are not always benign
In most human T. gondii cases, the infected individual experiences mild, flu-like symptoms, often with enlarged lymph nodes, or has no symptoms.1 Thus, most people who have been infected with
There are 3 exceptions to this otherwise benign clinical picture. The first is cerebral toxoplasmosis, which occurs in individuals who are immunosuppressed because they have AIDS or are receiving treatment for cancer or organ transplantation. Cerebral toxoplasmosis can be severe and was a common cause of death in patients with AIDS before the development of effective AIDS treatments.
The second exception is congenital toxoplasmosis, when an infection occurs in a pregnant woman. Such infections can cause severe damage to the developing fetus, including abortion, stillbirth, and brain damage. Congenital toxoplasmosis infections occur in approximately 1 of every 10,000 births in the United States, or approximately 3,800 each year.8 As a result, pregnant women are advised not to change their cat’s litter and to be tested for evidence of T. gondii infection.
The third exception is eye disease. Toxoplasmosis is one of the most common causes of eye disease, especially of the retina. Each year in the United States, approximately 4,800 individuals develop systematic ocular toxoplasmosis.9
Continue to: Toxoplasmosis and psychosis
Toxoplasmosis and psychosis: What evidence supports a link?
Until recently, cerebral infections, congenital infections, and eye disease were thought to be the main clinical problems associated with toxoplasmosis. However, accumulating evidence suggests that psychosis should be added to this list. Five lines of evidence support this.
1. T. gondii can cause psychotic symptoms. It has been known for decades that T. gondii can cause delusions, auditory hallucinations, and other psychotic symptoms.1 In one of the earliest publications (1966), Ladee10 concluded “The literature not infrequently focuses attention on psychosis with schizophrenia or schizophreniform features that accompany chronic toxoplasmosis.” Among the cases Ladee10 described was a laboratory worker who became infected with T. gondii and developed delusions and hallucinations.10
2. Patients with schizophrenia who are infected with T. gondii have more severe psychotic symptoms. This finding has been reported in at least 7 studies.1 Holub et al11 evaluated 251 patients with schizophrenia who were treated in Prague Psychiatric Centre between 2000 and 2010. Overall, 57 participants were infected with T. gondii and 194 were not infected. Compared to those who were not infected, the infected group:
- had significantly more severe symptoms (P = .032) as measured on the Positive and Negative Symptom Scale
- were prescribed higher doses of antipsychotic medications
- had been hospitalized longer.11
3. Compared with controls, patients with psychosis are significantly more likely to have antibodies against T. gondii, indicating previous infection. To date there have been approximately 100 such studies, of which at least three-fourths reported a positive association. In a 2012 meta-analysis of 38 such studies, Torrey et al12 reported an odds ratio (OR) of 2.7—compared to persons who have not been infected, those who have been infected with T. gondii were 2.7 times more likely to have schizophrenia.12 This study replicated the findings of a previous meta-analysis of 23 antibody studies, which also found an OR of 2.7.13
4. Compared with controls, individuals with schizophrenia or bipolar disorder are significantly more likely as a child to have lived in a home with a cat. Since 1995, 10 such studies have been published; 7 were positive, 2 were negative, and 1 was inconclusive.1 Torrey et al14 reviewed 2,025 individuals with schizophrenia or bipolar disorder and 4,847 controls and found that 51% of the cases and 43% of the controls had owned a cat before age 13; this difference was highly significant (P < .001). In fact, it is surprising that any study can find a statistically significant association between cat ownership and childhood psychosis. This is because a child who did not own a cat could become infected in many locations where cats have been present, including sandboxes at school, a babysitter’s or friend’s house, or a public park. And even if a child became infected at home, they would not necessarily have owned a cat, since the neighbor’s cat could have been responsible for the oocyst contamination.
Continue to: Epidemiologically...
5. Epidemiologically, there is a close temporal correlation between the rise of cats as pets and the rise of psychosis. This can be illustrated most clearly in England, where the rise of cat ownership has been documented by writers and where there is data on the rise of psychosis, especially in the 18th and 19th centuries.1
How many cases of psychosis might be caused by T. gondii?
In 2014, using data from the antibody studies discussed above,12,13 Smith15 sought to discover how many cases of psychosis might be caused by T. gondii. He concluded that 21% of cases of schizophrenia might have been caused by T. gondii. Based on the annual incidence of schizophrenia in the United States, this would mean an estimated >10,000 new cases of schizophrenia each year are attributable to this parasite.
Some researchers have found links between T. gondii and several nonpsychiatric diseases and conditions, including epilepsy and brain cancer (Box1,16-19).
Box
As interest in Toxoplasma gondii (T. gondii) has increased, researchers have looked for associations between this parasite with other diseases and conditions. Based on the literature, the following are of most interest:
Epilepsy. Since 1995, 16 studies1 have explored the relationship between T. gondii and epilepsy. A recent meta-analysis reported a statistically significant association between T. gondii and epilepsy.16
Brain cancer. Authors in 2 of 3 studies of meningiomas and 4 of 5 studies of gliomas reported statistically significant associations between these brain tumors and infection with T. gondii.1,17
Rheumatoid arthritis. Eight studies reported an increased prevalence of T. gondii antibodies in individuals with rheumatoid arthritis.1,18
Motor vehicle accidents. Infection with T. gondii is known to decrease motor reaction times in humans. At least 11 studies1 have examined whether infected individuals are more likely to have been involved in motor vehicle accidents. The results are mixed; the largest study reported a weak but statistically significant association.19
Clinical implications: What to tell patients about cats
What do these studies of toxoplasmosis imply for psychiatric care? As mental health professionals, part of our job is to educate our patients. Anything that appears to be a risk factor for the development of psychosis is thus of interest. Consider discussing the following with your patients.
Are cats safe? Cats that are kept exclusively indoors are safe pets because they are unlikely to become infected with T. gondii. However, cats that are allowed to go outdoors may not be safe, especially for children and young adults. What is needed is an effective vaccine that could be given to newborn kittens to prevent infection, but development of this type of vaccine has never been prioritized. At the community level, programs to decrease the number of stray and feral cats would also decrease the risk of infection.
Continue to: How to decrease risk
How to decrease risk. On a personal level, we can decrease T. gondii infections by not eating undercooked meat. Pregnant women and individuals who are immunocompromised should not change cat litter. When gardening, we should wear gloves because cats favor loose soil for depositing their feces. We should also protect children by covering sandboxes when not in use and by not allowing children to play in uncovered public sandboxes.
Treatment. Toxoplasmosis typically is treated with pyrimethamine, usually in combination with a sulfa drug. However, pyrimethamine does not cross the blood brain barrier and thus is ineffective when T. gondii infects the brain. The development of a drug that will effectively treat T. gondii in the brain should be a high priority.
For additional details on the studies discussed in this article as well as more resources on the impact T. gondii can have if proper precautions are not taken, see my open-access book at https://link.springer.com/book/10.1007/978-3-030-86811-6.
Bottom Line
Some evidence suggests that infection with Toxoplasma gondii (T. gondii) may cause psychotic symptoms, may increase an individual’s risk of developing psychosis, and may result in more severe psychotic symptoms. Cats can transmit T. gondii to humans. Educate patients that they can reduce their risk by keeping their cats inside, avoiding exposure to cat feces, particularly while pregnant or if immunocompromised, and not eating undercooked meat.
Related Resources
- Torrey EF. Parasites, Pussycats, and Psychosis: The Unknown Dangers of Human Toxoplasmosis. Springer Nature; 2022. https://link.springer.com/book/10.1007/978-3-030-86811-6
Drug Brand Names
Pyrimethamine • Daraprim
1. Torrey EF. Parasites, Pussycats, and Psychosis: The Unknown Dangers of Human Toxoplasmosis. Springer Nature; 2022. https://link.springer.com/book/10.1007/978-3-030-86811-6
2. Rohr JR, Barrett CB, Civitello DJ, et al. Emerging human infectious diseases and the links to global food production. Nat Sustain. 2019;2(6):445-456.
3. Joynson DHM. Preface. In: Joynson DHM, Wreghitt TG, eds. Toxoplasmosis: A Comprehensive Clinical Guide. Cambridge University Press; 2001:xi.
4. Bowie WR, King AS, Werker DH, et al. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet. 1997;350(9072):173-177.
5. Stagno S, Dykes AC, Amos CS, et al. An outbreak of toxoplasmosis linked to cats. Pediatrics. 1980;65(4):706-712.
6. Teutsch SM, Juranek DD, Sulzer A, et al. Epidemic toxoplasmosis associated with infected cats. N Engl J Med. 1979;300(13):695-699.
7. Kaňková Š, Hlaváčová J, Flegr J. Oral sex: a new, and possibly the most dangerous, route of toxoplasmosis transmission. Med Hypotheses. 2020;141:109725.
8. Guerina NG, Hsu HW, Meissner HC, et al. Neonatal serologic screening and early treatment for congenital T. gondii infection. N Engl J Med. 1994;330(26):1858-1863.
9. Jones JL, Holland GN. Annual burden of ocular toxoplasmosis in the US. Am J Trop Med Hyg. 2010;82(3):464-465.
10. Ladee GA. Diagnostic problems in psychiatry with regard to acquired toxoplasmosis. Psychiatr Neurol Neurochir. 1966;69(1):65-82.
11. Holub D, Flegr J, Dragomirecká E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
12. Torrey EF, Bartko JJ, Yolken RH. T. gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38(3):642-647.
13. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:729-736.
14. Torrey EF, Simmons W, Yolken RH. Is childhood cat ownership a risk factor for schizophrenia later in life? Schizophr Res. 2015;165(1):1-2.
15. Smith G. Estimating the population attributable fraction for schizophrenia when T. gondii is assumed absent in human populations. Prev Vet Med. 2014;117(3-4):425-435.
16. Sadeghi M, Riahi SM, Mohammadi M, et al. An updated meta-analysis of the association between T. gondii infection and risk of epilepsy. Trans R Soc Trop Med Hyg. 2019;113(8):453-462.
17. Hodge JM, Coghill AE, Kim Y, et al. T. gondii infection and the risk of adult glioma in two prospective studies. Int J Cancer. 2021;148(10):2449-2456.
18. Hosseininejad Z, Sharif M, Sarvi S, et al. Toxoplasmosis seroprevalence in rheumatoid arthritis patients: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(6):e0006545.
19. Burgdorf KS, Trabjerg BB, Pedersen MG, et al. Large-scale study of Toxoplasma and Cytomegalovirus shows an association between infection and serious psychiatric disorders. Brain Behav Immun. 2019; 79:152-158.
1. Torrey EF. Parasites, Pussycats, and Psychosis: The Unknown Dangers of Human Toxoplasmosis. Springer Nature; 2022. https://link.springer.com/book/10.1007/978-3-030-86811-6
2. Rohr JR, Barrett CB, Civitello DJ, et al. Emerging human infectious diseases and the links to global food production. Nat Sustain. 2019;2(6):445-456.
3. Joynson DHM. Preface. In: Joynson DHM, Wreghitt TG, eds. Toxoplasmosis: A Comprehensive Clinical Guide. Cambridge University Press; 2001:xi.
4. Bowie WR, King AS, Werker DH, et al. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet. 1997;350(9072):173-177.
5. Stagno S, Dykes AC, Amos CS, et al. An outbreak of toxoplasmosis linked to cats. Pediatrics. 1980;65(4):706-712.
6. Teutsch SM, Juranek DD, Sulzer A, et al. Epidemic toxoplasmosis associated with infected cats. N Engl J Med. 1979;300(13):695-699.
7. Kaňková Š, Hlaváčová J, Flegr J. Oral sex: a new, and possibly the most dangerous, route of toxoplasmosis transmission. Med Hypotheses. 2020;141:109725.
8. Guerina NG, Hsu HW, Meissner HC, et al. Neonatal serologic screening and early treatment for congenital T. gondii infection. N Engl J Med. 1994;330(26):1858-1863.
9. Jones JL, Holland GN. Annual burden of ocular toxoplasmosis in the US. Am J Trop Med Hyg. 2010;82(3):464-465.
10. Ladee GA. Diagnostic problems in psychiatry with regard to acquired toxoplasmosis. Psychiatr Neurol Neurochir. 1966;69(1):65-82.
11. Holub D, Flegr J, Dragomirecká E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
12. Torrey EF, Bartko JJ, Yolken RH. T. gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38(3):642-647.
13. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:729-736.
14. Torrey EF, Simmons W, Yolken RH. Is childhood cat ownership a risk factor for schizophrenia later in life? Schizophr Res. 2015;165(1):1-2.
15. Smith G. Estimating the population attributable fraction for schizophrenia when T. gondii is assumed absent in human populations. Prev Vet Med. 2014;117(3-4):425-435.
16. Sadeghi M, Riahi SM, Mohammadi M, et al. An updated meta-analysis of the association between T. gondii infection and risk of epilepsy. Trans R Soc Trop Med Hyg. 2019;113(8):453-462.
17. Hodge JM, Coghill AE, Kim Y, et al. T. gondii infection and the risk of adult glioma in two prospective studies. Int J Cancer. 2021;148(10):2449-2456.
18. Hosseininejad Z, Sharif M, Sarvi S, et al. Toxoplasmosis seroprevalence in rheumatoid arthritis patients: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(6):e0006545.
19. Burgdorf KS, Trabjerg BB, Pedersen MG, et al. Large-scale study of Toxoplasma and Cytomegalovirus shows an association between infection and serious psychiatric disorders. Brain Behav Immun. 2019; 79:152-158.
Psychodynamic factors in psychotropic prescribing
Medical noncompliance and patient resistance to treatment are frequent problems in medical practice. According to an older report by the US Office of Inspector General, approximately 125,000 people die each year in the United States because they do not take their medication properly.1 The World Health Organization reported that 10% to 25% of hospital and nursing home admissions are a result of patient noncompliance.2 In addition, approximately 50% of prescriptions filled for chronic diseases in developed nations are not taken correctly, and up to 40% of patients do not adhere to their treatment regimens.2 Among psychiatric patients, noncompliance with medications and other treatments ranges from 25% to 75%.3
In recent years, combining pharmacotherapy with psychodynamic psychotherapy has become a fairly common form of psychiatric practice. A main reason for combining these treatments is that a patient with severe psychiatric symptoms may be unable to engage in self-reflective insightful therapy until those symptoms are substantially relieved with pharmacotherapy. The efficacy of combined pharmacotherapy/psychotherapy may also be more than additive and result in a therapeutic alliance that is greater than the sum of the 2 individual treatments.4 Establishing a therapeutic alliance is critical to successful treatment, but this alliance can be distorted by the needs and expectations of both the patient and the clinician.
A psychodynamic understanding of the patient and the therapeutic alliance can facilitate combined treatment in several ways. It can lead to better communication, which in turn can lead to a realistic discussion of a patient’s fears and worries about any medications they have been prescribed. A dynamically aware clinician may better understand what the symptoms mean to the patient. Such clinicians will not only be able to explain the value of a medication, its target symptoms, and the rationale for taking it, but will also be able to discuss the psychological significance of the medication, along with its medical and biological significance.5
This article briefly reviews the therapeutic alliance and the influence of transference (the emotional reactions of the patient towards the clinician),6 countertransference (the emotional reactions of the clinician towards the patient),6 and patient resistance/nonadherence to treatment on the failure or success of pharmacotherapy. We provide case examples to illustrate how these psychodynamic factors can be at play in prescribing.
The therapeutic alliance
The therapeutic alliance is a rational agreement or contract between a patient and the clinician; it is a cornerstone of treatment in medicine.6 Its basic premise is that the patient’s rational expectation that their physician is appropriately qualified, will perform a suitable evaluation, and will prescribe relevant treatment is matched by the physician’s expectation that the patient will do their best to comply with treatment recommendations. For this to succeed, the contract needs to be straightforward, and there needs to be no covert agenda. A covert agenda may be in the form of unrealistic expectations and wishes rooted in insecure experiences in childhood by either party. A patient under stress may react to the physician with mistrust, excessive demands, and noncompliance. A physician under stress may react to a patient by becoming authoritative or indecisive, or by overmedicating or underprescribing.
Transference
Transference is a phenomenon whereby a patient’s feelings and attitudes are unconsciously transferred from a person or situation in the past to the clinician or treatment in the present.6 For example, a patient who is scared of a serious illness may adopt a helpless, childlike role and project an omnipotent, parentlike quality on the clinician (positive transference) that may be unrealistic. Positive transference may underlie a placebo response to medication in which a patient’s response is too quick or too complete, and it may be a way of unconsciously pleasing an authoritative parent figure from childhood. On the other hand, a patient may unconsciously view their physician as a controlling parent (negative transference) and react angrily or rebelliously. A patient’s flirtatious behavior toward their physician may be a form of transference from unresolved sexual trauma during childhood. However, not all patient reactions should be considered transference; a patient may be appropriately thankful and deferential, or irritated and questioning, depending on the clinician’s demeanor and treatment approach.
Countertransference
Countertransference is the response elicited in the physician by a patient’s appearance and behaviors, or by a patient’s transference projections.6 This response can be positive or negative and includes both feelings and associated thoughts related to the physician’s past experiences. For example, a physician in the emergency department may get angry with a patient with an alcohol use disorder because of the physician’s negative experiences with an alcoholic parent during childhood. On the other hand, a physician raised by a compulsive mother may order unnecessary tests on a demanding older female patient. Or, a clinician raised by a sheltering parent may react to a hapless and dependent patient by spending excessive time with them or providing additional medication samples. However, not all clinician reactions are countertransference. For example, a physician’s empathic or stoic demeanor may be an appropriate emotional response to a patient’s diagnosis such as cancer.
Continue to: Patient resistance/nonadherence
Patient resistance/nonadherence
In 1920, Freud conceptualized the psychodynamic factors in patient resistance to treatment and theorized that many patients were unconsciously reluctant to give up their symptoms or were driven, for transference reasons, to resist the physician.7 This same concept may underlie patient resistance to pharmacotherapy. When symptoms constitute an important defense mechanism, patients are likely to resist medication effects until they have developed more mature defenses or more effective ways of coping.8 Even when patients do not resist symptom relief, they may still resist the physician’s choice of treatment due to negative transference. Such patients often negotiate the type of medication, dose, timing of the dose, and start date as a way of trying to “keep control” of a “doctor they don’t quite trust.”8 They may manage their own medication regimen by taking more or less than the prescribed dose. This resistance might lead to a “nocebo” effect in which a medication trial fails not because of its ineffectiveness but instead from the unconscious mind influencing the patient’s body to resist. Nonadherence to treatment may occur in patients who have attachment difficulties that make it difficult for them to trust anyone as a result of negative childhood experiences.9 Clinicians need to recognize the dynamics of power struggles, control, and trust. A warm, collaborative and cooperative stance is likely to be more beneficial than an authoritative and detached approach.10
The following 3 case examples illustrate how psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and the outcomes of pharmacotherapy.
CASE 1
Mr. A, age 63, has posttraumatic stress disorder originating from his father’s death by a self-inflicted gunshot wound when Mr. A was 19, and later from the symbolic loss of his mother when she remarried. He reported vivid memories of his father sexually assaulting his mother when he was 6. This fostered a protective nature in him for his mother, as well as for his 3 younger siblings. After his father’s suicide, Mr. A had to take on a paternal role for his 3 siblings. He often feels he grew up too quickly, and resents this. He feels his mother betrayed him when she got remarried. Mr. A attempts suicide, is admitted to a local hospital, and then follows up at a university hospital outpatient psychiatry clinic.
At the clinic, Mr. A begins psychodynamic psychotherapy with a female resident physician. They establish a good rapport. Mr. A begins working through his past traumas and looks forward to his therapy sessions. The physician views this as positive transference, perhaps because her personality style and appearance are similar to that of Mr. A’s mother. She also often notes a positive countertransference during sessions; Mr. A seemingly reminds her of her father in personality and appearance. Perhaps due to this positive transference/positive countertransference dynamic, Mr. A feels comfortable with having his medication regimen simplified after years of unsuccessful medication trials and a course of electroconvulsive therapy. His regimen soon consists of only a selective serotonin reuptake inhibitor and a glutamate modulator as an adjunct for anxiety. Psychotherapy sessions remain the mainstay of his treatment plan. Mr. A’s mood and anxiety improve significantly over a short time.
CASE 2
Ms. G, age 24, is admitted to a partial hospitalization program (PHP). Her diagnoses include seasonal affective disorder, anxiety, and attention-deficit/hyperactivity disorder (ADHD); she might have a genetic disposition to bipolar disorder. Ms. G recently had attempted suicide and was discharged from an inpatient unit. She is a middle child and was raised by emotionally and verbally abusive parents in a tumultuous household. Her father rarely kept a job for more than a few months, displayed rage, and lacked empathy. Ms. G feels unloved by her mother and says that her mother is emotionally unstable. Upon admission to the PHP, Ms. G is quick to question the credentials of every staff member she meets, and suggests the abuse and lack of trust she had experienced during her formative years have made her aggressive and paranoid.
Continue to: Since her teens...
Since her teens, Ms. G had received treatment for ADHD with various stimulant and nonstimulant medications that were prescribed by an outpatient psychiatrist. During her sophomore year of college, she was also prescribed medications for depression and anxiety. Ms. G speaks very highly of and praises the skill of her previous psychiatrist while voicing concerns about having to see new clinicians in the PHP. She had recently seen a therapist who moved out of state after a few sessions. Ms. G has abandonment fears and appears to react with anger toward new clinicians.
A negative transference towards Ms. G’s treatment team and the PHP as a whole are evident during the first week. She skips most group therapy sessions and criticizes the clinicians’ skills and training as ineffective. When her psychiatrist recommends changes in medication, she initially argues. She eventually agrees to take a new medication but soon reports intolerable adverse effects, which suggests negative transference toward the psychiatrist as an authority figure, and toward the medication as an extension of the psychiatrist. The treatment team also interprets this as nocebo effect. Ms. G engages in “splitting” by complaining about her psychiatrist to her therapist. The psychiatrist resents having been belittled. Ms. G demands to see a different psychiatrist, and when her demands are not met, she discharges herself from the PHP against medical advice. The treatment team interprets Ms. G’s resistance to treatment to have resulted from poor attachment during childhood and subsequent negative transference.
CASE 3
Ms. U, age 60, is seen at a local mental health center and diagnosed with major depressive disorder, likely resulting from grief and loss from her husband’s recent death. She was raised by her single mother and mostly absent father. Ms. U is a homemaker and had been married for more than 30 years. She participates in weekly psychotherapy with a young male psychiatrist, who prescribes an antidepressant. Ms. U is eager to please and makes every effort to be the perfect patient: she is always early for her appointments, takes her medications as prescribed, and frequently expresses her respect and appreciation for her psychiatrist. Within a few weeks, Ms. U’s depressive symptoms rapidly improve.
Ms. U is a talented and avid knit and crochet expert. At an appointment soon before Christmas, she gives her psychiatrist a pair of socks she knitted. While the gift is of little monetary value, the psychiatrist interprets this as part of transference, but the intimate nature of the gift makes him uncomfortable. He and Ms. U discuss this at length, which reveals definite transference as Ms. U says the psychiatrist perhaps reminds her of her husband, who also had brown skin. It is also apparent that Ms. U’s tendency to please perhaps comes from the lack of having a father figure, which her husband had fulfilled. The psychiatrist believes that Ms. U’s rapid response may be a placebo effect from positive transference. Upon further reflection, the psychiatrist realizes that Ms. U is a motherly figure to him, and that positive countertransference is at play in that he could not turn down the gift and had looked forward to the therapy sessions with her.
Bottom Line
Even clinicians who do not provide psychodynamic psychotherapy can use an awareness of psychodynamic factors to improve treatment. Psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and patient outcomes. Patients’ experiences and difficulties with attachment during childhood should be recognized and addressed as part of pharmacotherapy.
Related Resources
- Neumann M, Silva N, Opler DJ. ARISE to supportive psychotherapy. Current Psychiatry. 2021;20(5):48-49. doi:10.12788/cp.0123
- Mintz D. Psychodynamic psychopharmacology. Psychiatric Times. 2011;28(9). https://www.psychiatrictimes.com/view/psychodynamic-psychopharmacology
1. Office of Inspector General, Office of Evaluation and Inspections. Medication Regimens: Causes of Noncompliance. 1990. Accessed April 13, 2022. https://oig.hhs.gov/oei/reports/oei-04-89-89121.pdf
2. World Health Organization. Adherence to Long Term Therapies: Evidence for Action. World Health Organization; 2003.
3. Powell AD. The medication life. J Psychother Pract Res. 2001;10(4):217-222.
4. Wright JH, Hollifield M. Combining pharmacotherapy and psychotherapy. Psychiatric Annals. 2006;36(5):302-305.
5. Summers RF, Barber JP. Psychodynamic Therapy: A Guide to Evidence-Based Practice. Guilford Press; 2013:265-290.
6. Hughes P, Kerr I. Transference and countertransference in communication between doctor and patient. Advances in Psychiatric Treatment. 2000;6(1):57-64.
7. Freud S. Resistance and suppression. In: Freud S. A General Introduction to Psychoanalysis. Boni and Liveright Publishers; 1920:248-261.
8. Vlastelica M. Psychodynamic approach as a creative factor in psychopharmacotherapy. Psychiatr Danub. 2013;25(3):316-319.
9. Alfonso CA. Understanding the psychodynamics of nonadherence. Psychiatric Times. 2011;28(5). Accessed April 13, 2022. https://www.psychiatrictimes.com/view/understanding-psychodynamics-nonadherence
10. Wallin DJ. Attachment in Psychotherapy. Guilford Press; 2007.
Medical noncompliance and patient resistance to treatment are frequent problems in medical practice. According to an older report by the US Office of Inspector General, approximately 125,000 people die each year in the United States because they do not take their medication properly.1 The World Health Organization reported that 10% to 25% of hospital and nursing home admissions are a result of patient noncompliance.2 In addition, approximately 50% of prescriptions filled for chronic diseases in developed nations are not taken correctly, and up to 40% of patients do not adhere to their treatment regimens.2 Among psychiatric patients, noncompliance with medications and other treatments ranges from 25% to 75%.3
In recent years, combining pharmacotherapy with psychodynamic psychotherapy has become a fairly common form of psychiatric practice. A main reason for combining these treatments is that a patient with severe psychiatric symptoms may be unable to engage in self-reflective insightful therapy until those symptoms are substantially relieved with pharmacotherapy. The efficacy of combined pharmacotherapy/psychotherapy may also be more than additive and result in a therapeutic alliance that is greater than the sum of the 2 individual treatments.4 Establishing a therapeutic alliance is critical to successful treatment, but this alliance can be distorted by the needs and expectations of both the patient and the clinician.
A psychodynamic understanding of the patient and the therapeutic alliance can facilitate combined treatment in several ways. It can lead to better communication, which in turn can lead to a realistic discussion of a patient’s fears and worries about any medications they have been prescribed. A dynamically aware clinician may better understand what the symptoms mean to the patient. Such clinicians will not only be able to explain the value of a medication, its target symptoms, and the rationale for taking it, but will also be able to discuss the psychological significance of the medication, along with its medical and biological significance.5
This article briefly reviews the therapeutic alliance and the influence of transference (the emotional reactions of the patient towards the clinician),6 countertransference (the emotional reactions of the clinician towards the patient),6 and patient resistance/nonadherence to treatment on the failure or success of pharmacotherapy. We provide case examples to illustrate how these psychodynamic factors can be at play in prescribing.
The therapeutic alliance
The therapeutic alliance is a rational agreement or contract between a patient and the clinician; it is a cornerstone of treatment in medicine.6 Its basic premise is that the patient’s rational expectation that their physician is appropriately qualified, will perform a suitable evaluation, and will prescribe relevant treatment is matched by the physician’s expectation that the patient will do their best to comply with treatment recommendations. For this to succeed, the contract needs to be straightforward, and there needs to be no covert agenda. A covert agenda may be in the form of unrealistic expectations and wishes rooted in insecure experiences in childhood by either party. A patient under stress may react to the physician with mistrust, excessive demands, and noncompliance. A physician under stress may react to a patient by becoming authoritative or indecisive, or by overmedicating or underprescribing.
Transference
Transference is a phenomenon whereby a patient’s feelings and attitudes are unconsciously transferred from a person or situation in the past to the clinician or treatment in the present.6 For example, a patient who is scared of a serious illness may adopt a helpless, childlike role and project an omnipotent, parentlike quality on the clinician (positive transference) that may be unrealistic. Positive transference may underlie a placebo response to medication in which a patient’s response is too quick or too complete, and it may be a way of unconsciously pleasing an authoritative parent figure from childhood. On the other hand, a patient may unconsciously view their physician as a controlling parent (negative transference) and react angrily or rebelliously. A patient’s flirtatious behavior toward their physician may be a form of transference from unresolved sexual trauma during childhood. However, not all patient reactions should be considered transference; a patient may be appropriately thankful and deferential, or irritated and questioning, depending on the clinician’s demeanor and treatment approach.
Countertransference
Countertransference is the response elicited in the physician by a patient’s appearance and behaviors, or by a patient’s transference projections.6 This response can be positive or negative and includes both feelings and associated thoughts related to the physician’s past experiences. For example, a physician in the emergency department may get angry with a patient with an alcohol use disorder because of the physician’s negative experiences with an alcoholic parent during childhood. On the other hand, a physician raised by a compulsive mother may order unnecessary tests on a demanding older female patient. Or, a clinician raised by a sheltering parent may react to a hapless and dependent patient by spending excessive time with them or providing additional medication samples. However, not all clinician reactions are countertransference. For example, a physician’s empathic or stoic demeanor may be an appropriate emotional response to a patient’s diagnosis such as cancer.
Continue to: Patient resistance/nonadherence
Patient resistance/nonadherence
In 1920, Freud conceptualized the psychodynamic factors in patient resistance to treatment and theorized that many patients were unconsciously reluctant to give up their symptoms or were driven, for transference reasons, to resist the physician.7 This same concept may underlie patient resistance to pharmacotherapy. When symptoms constitute an important defense mechanism, patients are likely to resist medication effects until they have developed more mature defenses or more effective ways of coping.8 Even when patients do not resist symptom relief, they may still resist the physician’s choice of treatment due to negative transference. Such patients often negotiate the type of medication, dose, timing of the dose, and start date as a way of trying to “keep control” of a “doctor they don’t quite trust.”8 They may manage their own medication regimen by taking more or less than the prescribed dose. This resistance might lead to a “nocebo” effect in which a medication trial fails not because of its ineffectiveness but instead from the unconscious mind influencing the patient’s body to resist. Nonadherence to treatment may occur in patients who have attachment difficulties that make it difficult for them to trust anyone as a result of negative childhood experiences.9 Clinicians need to recognize the dynamics of power struggles, control, and trust. A warm, collaborative and cooperative stance is likely to be more beneficial than an authoritative and detached approach.10
The following 3 case examples illustrate how psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and the outcomes of pharmacotherapy.
CASE 1
Mr. A, age 63, has posttraumatic stress disorder originating from his father’s death by a self-inflicted gunshot wound when Mr. A was 19, and later from the symbolic loss of his mother when she remarried. He reported vivid memories of his father sexually assaulting his mother when he was 6. This fostered a protective nature in him for his mother, as well as for his 3 younger siblings. After his father’s suicide, Mr. A had to take on a paternal role for his 3 siblings. He often feels he grew up too quickly, and resents this. He feels his mother betrayed him when she got remarried. Mr. A attempts suicide, is admitted to a local hospital, and then follows up at a university hospital outpatient psychiatry clinic.
At the clinic, Mr. A begins psychodynamic psychotherapy with a female resident physician. They establish a good rapport. Mr. A begins working through his past traumas and looks forward to his therapy sessions. The physician views this as positive transference, perhaps because her personality style and appearance are similar to that of Mr. A’s mother. She also often notes a positive countertransference during sessions; Mr. A seemingly reminds her of her father in personality and appearance. Perhaps due to this positive transference/positive countertransference dynamic, Mr. A feels comfortable with having his medication regimen simplified after years of unsuccessful medication trials and a course of electroconvulsive therapy. His regimen soon consists of only a selective serotonin reuptake inhibitor and a glutamate modulator as an adjunct for anxiety. Psychotherapy sessions remain the mainstay of his treatment plan. Mr. A’s mood and anxiety improve significantly over a short time.
CASE 2
Ms. G, age 24, is admitted to a partial hospitalization program (PHP). Her diagnoses include seasonal affective disorder, anxiety, and attention-deficit/hyperactivity disorder (ADHD); she might have a genetic disposition to bipolar disorder. Ms. G recently had attempted suicide and was discharged from an inpatient unit. She is a middle child and was raised by emotionally and verbally abusive parents in a tumultuous household. Her father rarely kept a job for more than a few months, displayed rage, and lacked empathy. Ms. G feels unloved by her mother and says that her mother is emotionally unstable. Upon admission to the PHP, Ms. G is quick to question the credentials of every staff member she meets, and suggests the abuse and lack of trust she had experienced during her formative years have made her aggressive and paranoid.
Continue to: Since her teens...
Since her teens, Ms. G had received treatment for ADHD with various stimulant and nonstimulant medications that were prescribed by an outpatient psychiatrist. During her sophomore year of college, she was also prescribed medications for depression and anxiety. Ms. G speaks very highly of and praises the skill of her previous psychiatrist while voicing concerns about having to see new clinicians in the PHP. She had recently seen a therapist who moved out of state after a few sessions. Ms. G has abandonment fears and appears to react with anger toward new clinicians.
A negative transference towards Ms. G’s treatment team and the PHP as a whole are evident during the first week. She skips most group therapy sessions and criticizes the clinicians’ skills and training as ineffective. When her psychiatrist recommends changes in medication, she initially argues. She eventually agrees to take a new medication but soon reports intolerable adverse effects, which suggests negative transference toward the psychiatrist as an authority figure, and toward the medication as an extension of the psychiatrist. The treatment team also interprets this as nocebo effect. Ms. G engages in “splitting” by complaining about her psychiatrist to her therapist. The psychiatrist resents having been belittled. Ms. G demands to see a different psychiatrist, and when her demands are not met, she discharges herself from the PHP against medical advice. The treatment team interprets Ms. G’s resistance to treatment to have resulted from poor attachment during childhood and subsequent negative transference.
CASE 3
Ms. U, age 60, is seen at a local mental health center and diagnosed with major depressive disorder, likely resulting from grief and loss from her husband’s recent death. She was raised by her single mother and mostly absent father. Ms. U is a homemaker and had been married for more than 30 years. She participates in weekly psychotherapy with a young male psychiatrist, who prescribes an antidepressant. Ms. U is eager to please and makes every effort to be the perfect patient: she is always early for her appointments, takes her medications as prescribed, and frequently expresses her respect and appreciation for her psychiatrist. Within a few weeks, Ms. U’s depressive symptoms rapidly improve.
Ms. U is a talented and avid knit and crochet expert. At an appointment soon before Christmas, she gives her psychiatrist a pair of socks she knitted. While the gift is of little monetary value, the psychiatrist interprets this as part of transference, but the intimate nature of the gift makes him uncomfortable. He and Ms. U discuss this at length, which reveals definite transference as Ms. U says the psychiatrist perhaps reminds her of her husband, who also had brown skin. It is also apparent that Ms. U’s tendency to please perhaps comes from the lack of having a father figure, which her husband had fulfilled. The psychiatrist believes that Ms. U’s rapid response may be a placebo effect from positive transference. Upon further reflection, the psychiatrist realizes that Ms. U is a motherly figure to him, and that positive countertransference is at play in that he could not turn down the gift and had looked forward to the therapy sessions with her.
Bottom Line
Even clinicians who do not provide psychodynamic psychotherapy can use an awareness of psychodynamic factors to improve treatment. Psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and patient outcomes. Patients’ experiences and difficulties with attachment during childhood should be recognized and addressed as part of pharmacotherapy.
Related Resources
- Neumann M, Silva N, Opler DJ. ARISE to supportive psychotherapy. Current Psychiatry. 2021;20(5):48-49. doi:10.12788/cp.0123
- Mintz D. Psychodynamic psychopharmacology. Psychiatric Times. 2011;28(9). https://www.psychiatrictimes.com/view/psychodynamic-psychopharmacology
Medical noncompliance and patient resistance to treatment are frequent problems in medical practice. According to an older report by the US Office of Inspector General, approximately 125,000 people die each year in the United States because they do not take their medication properly.1 The World Health Organization reported that 10% to 25% of hospital and nursing home admissions are a result of patient noncompliance.2 In addition, approximately 50% of prescriptions filled for chronic diseases in developed nations are not taken correctly, and up to 40% of patients do not adhere to their treatment regimens.2 Among psychiatric patients, noncompliance with medications and other treatments ranges from 25% to 75%.3
In recent years, combining pharmacotherapy with psychodynamic psychotherapy has become a fairly common form of psychiatric practice. A main reason for combining these treatments is that a patient with severe psychiatric symptoms may be unable to engage in self-reflective insightful therapy until those symptoms are substantially relieved with pharmacotherapy. The efficacy of combined pharmacotherapy/psychotherapy may also be more than additive and result in a therapeutic alliance that is greater than the sum of the 2 individual treatments.4 Establishing a therapeutic alliance is critical to successful treatment, but this alliance can be distorted by the needs and expectations of both the patient and the clinician.
A psychodynamic understanding of the patient and the therapeutic alliance can facilitate combined treatment in several ways. It can lead to better communication, which in turn can lead to a realistic discussion of a patient’s fears and worries about any medications they have been prescribed. A dynamically aware clinician may better understand what the symptoms mean to the patient. Such clinicians will not only be able to explain the value of a medication, its target symptoms, and the rationale for taking it, but will also be able to discuss the psychological significance of the medication, along with its medical and biological significance.5
This article briefly reviews the therapeutic alliance and the influence of transference (the emotional reactions of the patient towards the clinician),6 countertransference (the emotional reactions of the clinician towards the patient),6 and patient resistance/nonadherence to treatment on the failure or success of pharmacotherapy. We provide case examples to illustrate how these psychodynamic factors can be at play in prescribing.
The therapeutic alliance
The therapeutic alliance is a rational agreement or contract between a patient and the clinician; it is a cornerstone of treatment in medicine.6 Its basic premise is that the patient’s rational expectation that their physician is appropriately qualified, will perform a suitable evaluation, and will prescribe relevant treatment is matched by the physician’s expectation that the patient will do their best to comply with treatment recommendations. For this to succeed, the contract needs to be straightforward, and there needs to be no covert agenda. A covert agenda may be in the form of unrealistic expectations and wishes rooted in insecure experiences in childhood by either party. A patient under stress may react to the physician with mistrust, excessive demands, and noncompliance. A physician under stress may react to a patient by becoming authoritative or indecisive, or by overmedicating or underprescribing.
Transference
Transference is a phenomenon whereby a patient’s feelings and attitudes are unconsciously transferred from a person or situation in the past to the clinician or treatment in the present.6 For example, a patient who is scared of a serious illness may adopt a helpless, childlike role and project an omnipotent, parentlike quality on the clinician (positive transference) that may be unrealistic. Positive transference may underlie a placebo response to medication in which a patient’s response is too quick or too complete, and it may be a way of unconsciously pleasing an authoritative parent figure from childhood. On the other hand, a patient may unconsciously view their physician as a controlling parent (negative transference) and react angrily or rebelliously. A patient’s flirtatious behavior toward their physician may be a form of transference from unresolved sexual trauma during childhood. However, not all patient reactions should be considered transference; a patient may be appropriately thankful and deferential, or irritated and questioning, depending on the clinician’s demeanor and treatment approach.
Countertransference
Countertransference is the response elicited in the physician by a patient’s appearance and behaviors, or by a patient’s transference projections.6 This response can be positive or negative and includes both feelings and associated thoughts related to the physician’s past experiences. For example, a physician in the emergency department may get angry with a patient with an alcohol use disorder because of the physician’s negative experiences with an alcoholic parent during childhood. On the other hand, a physician raised by a compulsive mother may order unnecessary tests on a demanding older female patient. Or, a clinician raised by a sheltering parent may react to a hapless and dependent patient by spending excessive time with them or providing additional medication samples. However, not all clinician reactions are countertransference. For example, a physician’s empathic or stoic demeanor may be an appropriate emotional response to a patient’s diagnosis such as cancer.
Continue to: Patient resistance/nonadherence
Patient resistance/nonadherence
In 1920, Freud conceptualized the psychodynamic factors in patient resistance to treatment and theorized that many patients were unconsciously reluctant to give up their symptoms or were driven, for transference reasons, to resist the physician.7 This same concept may underlie patient resistance to pharmacotherapy. When symptoms constitute an important defense mechanism, patients are likely to resist medication effects until they have developed more mature defenses or more effective ways of coping.8 Even when patients do not resist symptom relief, they may still resist the physician’s choice of treatment due to negative transference. Such patients often negotiate the type of medication, dose, timing of the dose, and start date as a way of trying to “keep control” of a “doctor they don’t quite trust.”8 They may manage their own medication regimen by taking more or less than the prescribed dose. This resistance might lead to a “nocebo” effect in which a medication trial fails not because of its ineffectiveness but instead from the unconscious mind influencing the patient’s body to resist. Nonadherence to treatment may occur in patients who have attachment difficulties that make it difficult for them to trust anyone as a result of negative childhood experiences.9 Clinicians need to recognize the dynamics of power struggles, control, and trust. A warm, collaborative and cooperative stance is likely to be more beneficial than an authoritative and detached approach.10
The following 3 case examples illustrate how psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and the outcomes of pharmacotherapy.
CASE 1
Mr. A, age 63, has posttraumatic stress disorder originating from his father’s death by a self-inflicted gunshot wound when Mr. A was 19, and later from the symbolic loss of his mother when she remarried. He reported vivid memories of his father sexually assaulting his mother when he was 6. This fostered a protective nature in him for his mother, as well as for his 3 younger siblings. After his father’s suicide, Mr. A had to take on a paternal role for his 3 siblings. He often feels he grew up too quickly, and resents this. He feels his mother betrayed him when she got remarried. Mr. A attempts suicide, is admitted to a local hospital, and then follows up at a university hospital outpatient psychiatry clinic.
At the clinic, Mr. A begins psychodynamic psychotherapy with a female resident physician. They establish a good rapport. Mr. A begins working through his past traumas and looks forward to his therapy sessions. The physician views this as positive transference, perhaps because her personality style and appearance are similar to that of Mr. A’s mother. She also often notes a positive countertransference during sessions; Mr. A seemingly reminds her of her father in personality and appearance. Perhaps due to this positive transference/positive countertransference dynamic, Mr. A feels comfortable with having his medication regimen simplified after years of unsuccessful medication trials and a course of electroconvulsive therapy. His regimen soon consists of only a selective serotonin reuptake inhibitor and a glutamate modulator as an adjunct for anxiety. Psychotherapy sessions remain the mainstay of his treatment plan. Mr. A’s mood and anxiety improve significantly over a short time.
CASE 2
Ms. G, age 24, is admitted to a partial hospitalization program (PHP). Her diagnoses include seasonal affective disorder, anxiety, and attention-deficit/hyperactivity disorder (ADHD); she might have a genetic disposition to bipolar disorder. Ms. G recently had attempted suicide and was discharged from an inpatient unit. She is a middle child and was raised by emotionally and verbally abusive parents in a tumultuous household. Her father rarely kept a job for more than a few months, displayed rage, and lacked empathy. Ms. G feels unloved by her mother and says that her mother is emotionally unstable. Upon admission to the PHP, Ms. G is quick to question the credentials of every staff member she meets, and suggests the abuse and lack of trust she had experienced during her formative years have made her aggressive and paranoid.
Continue to: Since her teens...
Since her teens, Ms. G had received treatment for ADHD with various stimulant and nonstimulant medications that were prescribed by an outpatient psychiatrist. During her sophomore year of college, she was also prescribed medications for depression and anxiety. Ms. G speaks very highly of and praises the skill of her previous psychiatrist while voicing concerns about having to see new clinicians in the PHP. She had recently seen a therapist who moved out of state after a few sessions. Ms. G has abandonment fears and appears to react with anger toward new clinicians.
A negative transference towards Ms. G’s treatment team and the PHP as a whole are evident during the first week. She skips most group therapy sessions and criticizes the clinicians’ skills and training as ineffective. When her psychiatrist recommends changes in medication, she initially argues. She eventually agrees to take a new medication but soon reports intolerable adverse effects, which suggests negative transference toward the psychiatrist as an authority figure, and toward the medication as an extension of the psychiatrist. The treatment team also interprets this as nocebo effect. Ms. G engages in “splitting” by complaining about her psychiatrist to her therapist. The psychiatrist resents having been belittled. Ms. G demands to see a different psychiatrist, and when her demands are not met, she discharges herself from the PHP against medical advice. The treatment team interprets Ms. G’s resistance to treatment to have resulted from poor attachment during childhood and subsequent negative transference.
CASE 3
Ms. U, age 60, is seen at a local mental health center and diagnosed with major depressive disorder, likely resulting from grief and loss from her husband’s recent death. She was raised by her single mother and mostly absent father. Ms. U is a homemaker and had been married for more than 30 years. She participates in weekly psychotherapy with a young male psychiatrist, who prescribes an antidepressant. Ms. U is eager to please and makes every effort to be the perfect patient: she is always early for her appointments, takes her medications as prescribed, and frequently expresses her respect and appreciation for her psychiatrist. Within a few weeks, Ms. U’s depressive symptoms rapidly improve.
Ms. U is a talented and avid knit and crochet expert. At an appointment soon before Christmas, she gives her psychiatrist a pair of socks she knitted. While the gift is of little monetary value, the psychiatrist interprets this as part of transference, but the intimate nature of the gift makes him uncomfortable. He and Ms. U discuss this at length, which reveals definite transference as Ms. U says the psychiatrist perhaps reminds her of her husband, who also had brown skin. It is also apparent that Ms. U’s tendency to please perhaps comes from the lack of having a father figure, which her husband had fulfilled. The psychiatrist believes that Ms. U’s rapid response may be a placebo effect from positive transference. Upon further reflection, the psychiatrist realizes that Ms. U is a motherly figure to him, and that positive countertransference is at play in that he could not turn down the gift and had looked forward to the therapy sessions with her.
Bottom Line
Even clinicians who do not provide psychodynamic psychotherapy can use an awareness of psychodynamic factors to improve treatment. Psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and patient outcomes. Patients’ experiences and difficulties with attachment during childhood should be recognized and addressed as part of pharmacotherapy.
Related Resources
- Neumann M, Silva N, Opler DJ. ARISE to supportive psychotherapy. Current Psychiatry. 2021;20(5):48-49. doi:10.12788/cp.0123
- Mintz D. Psychodynamic psychopharmacology. Psychiatric Times. 2011;28(9). https://www.psychiatrictimes.com/view/psychodynamic-psychopharmacology
1. Office of Inspector General, Office of Evaluation and Inspections. Medication Regimens: Causes of Noncompliance. 1990. Accessed April 13, 2022. https://oig.hhs.gov/oei/reports/oei-04-89-89121.pdf
2. World Health Organization. Adherence to Long Term Therapies: Evidence for Action. World Health Organization; 2003.
3. Powell AD. The medication life. J Psychother Pract Res. 2001;10(4):217-222.
4. Wright JH, Hollifield M. Combining pharmacotherapy and psychotherapy. Psychiatric Annals. 2006;36(5):302-305.
5. Summers RF, Barber JP. Psychodynamic Therapy: A Guide to Evidence-Based Practice. Guilford Press; 2013:265-290.
6. Hughes P, Kerr I. Transference and countertransference in communication between doctor and patient. Advances in Psychiatric Treatment. 2000;6(1):57-64.
7. Freud S. Resistance and suppression. In: Freud S. A General Introduction to Psychoanalysis. Boni and Liveright Publishers; 1920:248-261.
8. Vlastelica M. Psychodynamic approach as a creative factor in psychopharmacotherapy. Psychiatr Danub. 2013;25(3):316-319.
9. Alfonso CA. Understanding the psychodynamics of nonadherence. Psychiatric Times. 2011;28(5). Accessed April 13, 2022. https://www.psychiatrictimes.com/view/understanding-psychodynamics-nonadherence
10. Wallin DJ. Attachment in Psychotherapy. Guilford Press; 2007.
1. Office of Inspector General, Office of Evaluation and Inspections. Medication Regimens: Causes of Noncompliance. 1990. Accessed April 13, 2022. https://oig.hhs.gov/oei/reports/oei-04-89-89121.pdf
2. World Health Organization. Adherence to Long Term Therapies: Evidence for Action. World Health Organization; 2003.
3. Powell AD. The medication life. J Psychother Pract Res. 2001;10(4):217-222.
4. Wright JH, Hollifield M. Combining pharmacotherapy and psychotherapy. Psychiatric Annals. 2006;36(5):302-305.
5. Summers RF, Barber JP. Psychodynamic Therapy: A Guide to Evidence-Based Practice. Guilford Press; 2013:265-290.
6. Hughes P, Kerr I. Transference and countertransference in communication between doctor and patient. Advances in Psychiatric Treatment. 2000;6(1):57-64.
7. Freud S. Resistance and suppression. In: Freud S. A General Introduction to Psychoanalysis. Boni and Liveright Publishers; 1920:248-261.
8. Vlastelica M. Psychodynamic approach as a creative factor in psychopharmacotherapy. Psychiatr Danub. 2013;25(3):316-319.
9. Alfonso CA. Understanding the psychodynamics of nonadherence. Psychiatric Times. 2011;28(5). Accessed April 13, 2022. https://www.psychiatrictimes.com/view/understanding-psychodynamics-nonadherence
10. Wallin DJ. Attachment in Psychotherapy. Guilford Press; 2007.