User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
The neurobiology of Jeopardy! champions
As a regular viewer of Jeopardy! I find it both interesting and educational. But the psychiatric neuroscientist in me marvels at the splendid cerebral attributes embedded in the brains of Jeopardy! champions.
Back in my college days, I participated in what were then called “general knowledge contests” and won a couple of trophies, the most gratifying of which was when our team of medical students beat the faculty team! Later, when my wife and I had children, Trivial Pursuit was a game frequently played in our household. So it is no wonder I have often thought of the remarkable, sometimes stunning intellectual performances of Jeopardy! champions.
What does it take to excel at Jeopardy!?
Watching contestants successfully answer a bewildering array of questions across an extensive spectrum of topics is simply dazzling and prompts me to ask: Which neurologic structures play a central role in the brains of Jeopardy! champions? So I channeled my inner neurobiologist and came up with the following prerequisites to excel at Jeopardy!:
- A hippocampus on steroids! Memory is obviously a core ingredient for responding to Jeopardy! questions. Unlike ordinary mortals, Jeopardy! champions appear to retain and instantaneously, accurately recall everything they have read, saw, or heard.
- A sublime network of dendritic spines, where learning is immediately transduced to biological memories, thanks to the wonders of experiential neuroplasticity in homo sapiens.
- A superlative frontal lobe, which provides the champion with an ultra-rapid abstraction ability in the dorsolateral prefrontal cortex, along with razor-sharp concentration and attention.
- An extremely well-myelinated network of the 137,000 miles of white matter fibers in the human brain. This is what leads to fabulous processing speed. Rapid neurotransmission is impossible without very well-myelinated axons and dendrites. It is not enough for a Jeopardy! champion to know the answer and retrieve it from the hippocampus—they also must transmit the answer at lightning speed to the speech area, and then activate the motor area to enunciate the answer. Processing speed is the foundation of overall cognitive functioning.
- A first-rate Broca’s area, referred to as “the brain’s scriptwriter,” which shapes human speech. It receives the flow of sensory information from the temporal cortex, devises a plan for speaking, and passes that plan seamlessly to the motor cortex, which controls the movements of the mouth.
- Blistering speed reflexes to click the handheld response buzzer within a fraction of a millisecond after the host finishes reading the clue (not before, or a penalty is incurred). Jeopardy! champions always click the buzzer faster than their competitors, who may know the answer but have ordinary motor reflexes (also related to the degree of myelination and a motoric component of processing speed).
- A thick corpus callosum, the largest interhemispheric commissure, a bundle of 200 million white matter fibers connecting analogous regions in the right and left hemispheres, is vital for the rapid bidirectional transfer of bits of information from the intuitive/nonverbal right hemisphere to the mathematical/verbal left hemisphere, when the answer requires right hemispheric input.
- A bright occipital cortex and exceptional optic nerve and retina, so that champions can recognize faces or locations and read the questions before the host finishes reading them, which gives them an awesome edge on other contestants.
Obviously, the brains of Jeopardy! champions are a breed of their own, with exceptional performances by multiple regions converging to produce a winning performance. But during their childhood and youthful years, such brains also generate motivation, curiosity, and interest in a wide range of topics, from cultures, regions, music genres, and word games to history, geography, sports, science, medicine, astronomy, and Greek mythology.
Jeopardy! champions may appear to have regular jobs and ordinary lives, but they have resplendent “renaissance” brains. I wonder how they spent their childhood, who mentored them, what type of family lives they had, and what they dream of accomplishing other than winning on Jeopardy!. Will their awe-inspiring performance in Jeopardy! translate to overall success in life? That’s a story that remains to be told.
As a regular viewer of Jeopardy! I find it both interesting and educational. But the psychiatric neuroscientist in me marvels at the splendid cerebral attributes embedded in the brains of Jeopardy! champions.
Back in my college days, I participated in what were then called “general knowledge contests” and won a couple of trophies, the most gratifying of which was when our team of medical students beat the faculty team! Later, when my wife and I had children, Trivial Pursuit was a game frequently played in our household. So it is no wonder I have often thought of the remarkable, sometimes stunning intellectual performances of Jeopardy! champions.
What does it take to excel at Jeopardy!?
Watching contestants successfully answer a bewildering array of questions across an extensive spectrum of topics is simply dazzling and prompts me to ask: Which neurologic structures play a central role in the brains of Jeopardy! champions? So I channeled my inner neurobiologist and came up with the following prerequisites to excel at Jeopardy!:
- A hippocampus on steroids! Memory is obviously a core ingredient for responding to Jeopardy! questions. Unlike ordinary mortals, Jeopardy! champions appear to retain and instantaneously, accurately recall everything they have read, saw, or heard.
- A sublime network of dendritic spines, where learning is immediately transduced to biological memories, thanks to the wonders of experiential neuroplasticity in homo sapiens.
- A superlative frontal lobe, which provides the champion with an ultra-rapid abstraction ability in the dorsolateral prefrontal cortex, along with razor-sharp concentration and attention.
- An extremely well-myelinated network of the 137,000 miles of white matter fibers in the human brain. This is what leads to fabulous processing speed. Rapid neurotransmission is impossible without very well-myelinated axons and dendrites. It is not enough for a Jeopardy! champion to know the answer and retrieve it from the hippocampus—they also must transmit the answer at lightning speed to the speech area, and then activate the motor area to enunciate the answer. Processing speed is the foundation of overall cognitive functioning.
- A first-rate Broca’s area, referred to as “the brain’s scriptwriter,” which shapes human speech. It receives the flow of sensory information from the temporal cortex, devises a plan for speaking, and passes that plan seamlessly to the motor cortex, which controls the movements of the mouth.
- Blistering speed reflexes to click the handheld response buzzer within a fraction of a millisecond after the host finishes reading the clue (not before, or a penalty is incurred). Jeopardy! champions always click the buzzer faster than their competitors, who may know the answer but have ordinary motor reflexes (also related to the degree of myelination and a motoric component of processing speed).
- A thick corpus callosum, the largest interhemispheric commissure, a bundle of 200 million white matter fibers connecting analogous regions in the right and left hemispheres, is vital for the rapid bidirectional transfer of bits of information from the intuitive/nonverbal right hemisphere to the mathematical/verbal left hemisphere, when the answer requires right hemispheric input.
- A bright occipital cortex and exceptional optic nerve and retina, so that champions can recognize faces or locations and read the questions before the host finishes reading them, which gives them an awesome edge on other contestants.
Obviously, the brains of Jeopardy! champions are a breed of their own, with exceptional performances by multiple regions converging to produce a winning performance. But during their childhood and youthful years, such brains also generate motivation, curiosity, and interest in a wide range of topics, from cultures, regions, music genres, and word games to history, geography, sports, science, medicine, astronomy, and Greek mythology.
Jeopardy! champions may appear to have regular jobs and ordinary lives, but they have resplendent “renaissance” brains. I wonder how they spent their childhood, who mentored them, what type of family lives they had, and what they dream of accomplishing other than winning on Jeopardy!. Will their awe-inspiring performance in Jeopardy! translate to overall success in life? That’s a story that remains to be told.
As a regular viewer of Jeopardy! I find it both interesting and educational. But the psychiatric neuroscientist in me marvels at the splendid cerebral attributes embedded in the brains of Jeopardy! champions.
Back in my college days, I participated in what were then called “general knowledge contests” and won a couple of trophies, the most gratifying of which was when our team of medical students beat the faculty team! Later, when my wife and I had children, Trivial Pursuit was a game frequently played in our household. So it is no wonder I have often thought of the remarkable, sometimes stunning intellectual performances of Jeopardy! champions.
What does it take to excel at Jeopardy!?
Watching contestants successfully answer a bewildering array of questions across an extensive spectrum of topics is simply dazzling and prompts me to ask: Which neurologic structures play a central role in the brains of Jeopardy! champions? So I channeled my inner neurobiologist and came up with the following prerequisites to excel at Jeopardy!:
- A hippocampus on steroids! Memory is obviously a core ingredient for responding to Jeopardy! questions. Unlike ordinary mortals, Jeopardy! champions appear to retain and instantaneously, accurately recall everything they have read, saw, or heard.
- A sublime network of dendritic spines, where learning is immediately transduced to biological memories, thanks to the wonders of experiential neuroplasticity in homo sapiens.
- A superlative frontal lobe, which provides the champion with an ultra-rapid abstraction ability in the dorsolateral prefrontal cortex, along with razor-sharp concentration and attention.
- An extremely well-myelinated network of the 137,000 miles of white matter fibers in the human brain. This is what leads to fabulous processing speed. Rapid neurotransmission is impossible without very well-myelinated axons and dendrites. It is not enough for a Jeopardy! champion to know the answer and retrieve it from the hippocampus—they also must transmit the answer at lightning speed to the speech area, and then activate the motor area to enunciate the answer. Processing speed is the foundation of overall cognitive functioning.
- A first-rate Broca’s area, referred to as “the brain’s scriptwriter,” which shapes human speech. It receives the flow of sensory information from the temporal cortex, devises a plan for speaking, and passes that plan seamlessly to the motor cortex, which controls the movements of the mouth.
- Blistering speed reflexes to click the handheld response buzzer within a fraction of a millisecond after the host finishes reading the clue (not before, or a penalty is incurred). Jeopardy! champions always click the buzzer faster than their competitors, who may know the answer but have ordinary motor reflexes (also related to the degree of myelination and a motoric component of processing speed).
- A thick corpus callosum, the largest interhemispheric commissure, a bundle of 200 million white matter fibers connecting analogous regions in the right and left hemispheres, is vital for the rapid bidirectional transfer of bits of information from the intuitive/nonverbal right hemisphere to the mathematical/verbal left hemisphere, when the answer requires right hemispheric input.
- A bright occipital cortex and exceptional optic nerve and retina, so that champions can recognize faces or locations and read the questions before the host finishes reading them, which gives them an awesome edge on other contestants.
Obviously, the brains of Jeopardy! champions are a breed of their own, with exceptional performances by multiple regions converging to produce a winning performance. But during their childhood and youthful years, such brains also generate motivation, curiosity, and interest in a wide range of topics, from cultures, regions, music genres, and word games to history, geography, sports, science, medicine, astronomy, and Greek mythology.
Jeopardy! champions may appear to have regular jobs and ordinary lives, but they have resplendent “renaissance” brains. I wonder how they spent their childhood, who mentored them, what type of family lives they had, and what they dream of accomplishing other than winning on Jeopardy!. Will their awe-inspiring performance in Jeopardy! translate to overall success in life? That’s a story that remains to be told.
The case for pursuing a consultation-liaison psychiatry fellowship
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Four years ago, pursuing a consultation-liaison psychiatry (CL) fellowship was the last thing on my mind. I had recently started my third year as a CL attending at the University of Cincinnati Medical Center and was becoming more of an integral part of its academic department. I felt that I had found my calling. I wanted to be an educator, with the hope of becoming a psychiatry residency program director. This idea was validated when I was awarded the Golden Apple for Excellence in Clinical Teaching, voted by the psychiatry residents, as well as a medical student teaching award. Both awards related to my CL duties.
And then, life happened. My wife and I decided to move east to be closer to family. I planned to continue my path at an academic institution while teaching CL psychiatry. Yet, each institution I interviewed with explained that while my recent experience was “great,” I would need to be formally CL fellowship–trained if I wanted to work in the CL division. On the one hand, this was frustrating to hear; however, the Accreditation Council for Graduate Medical Education has established rules regarding how many faculty at institutions that offer CL fellowships need to be CL fellowship–trained. After much consideration, specifically about my personal career aspirations and family situation, I decided to go “backwards” and pursue a CL fellowship.
Parts of the fellowship year were easier than the previous 3 years. For example, my caseload was much lighter, as were my supervision duties. However, almost immediately, there was an ego-check—for instance, recognizing that I would not always agree with my attendings, and other services would no longer view me as “the attending.” Despite that, as I now discuss with my trainees, I am never above further learning and gaining more clinical experience. Early in that first year of fellowship, I was involved in a complicated case of a patient with autoimmune encephalitis with severe catatonia who warranted electroconvulsive therapy. I gained experience using phenobarbital for the treatment of alcohol withdrawal, something I did not use during my residency or first 3 years as an attending.
Furthermore, my academic project that year was to revamp the fellowship. This included resetting the fellowship’s mission statement, as well as updating our rotations and curriculum, to better align with fellowship best practices around the country. It afforded me time to develop my own “educational” pathway and think of ways in which CL is expanding its footprint. Consistent with this, Park et al1 demonstrated the most common “major reason” for pursuing a CL fellowship was to obtain clinical training; the “moderate reason” of teaching opportunities cannot be overlooked.
The value of a CL fellowship
CL is about the intersection of behavioral health with medicine. As such, I believe CL fellowships will be part of the solution for addressing the current health care cost crisis2 as well as improving access to mental health treatment.3 We already see this solution in collaborative care programs. According to the National Resident Matching Program, in 2021 there were 60 CL fellowship programs and 124 CL fellowship positions offered nationwide, with a total of 89 fellowship applicants and 84 spots filled.4 Looking back 5 years, there were only 52 CL fellowship programs nationwide.4 While there are currently fewer applicants than spots, the fact that the number of available programs is increasing demonstrates the value that each institution puts into CL as well as the importance of our presence in the health care system. The CL fellowship year can create a special opportunity for the fellow that dovetails with their passions. If an applicant wants a program that has expert subspecialty services and focuses on teaching and social determinates of health, they can assuredly find that program.
The decision to pursue fellowship is a personal choice. I believe that CL as a subspecialty will demonstrate its importance to both the psychiatric and medical fields. CL fellowships can continue to innovate and move forward by recognizing the changing landscape of CL psychiatry and matching the fellowship experience to those needs. This will only make the draw for fellowship more powerful. Four years ago, I did not want to pursue fellowship—today I am truly grateful I did.
1. Park EM, Sockalingam S, Ravindranath D, et al; Academy of Psychosomatic Medicine’s Early Career Psychiatrist Special Interest Group. Psychosomatic medicine training as a bridge to practice: training and professional practice patterns of early career psychosomatic medicine specialists. Psychosomatics. 2015;56(1):52-58. doi:10.1016/j.psym.2014.05.003
2. Organisation for Economic Cooperation and Development. Health at a Glance 2021: OECD Indicators. OECD Publishing; 2021. https://doi.org/10.1787/ae3016b9-en
3. Center for Behavioral Health Statistics and Quality. Results from the 2015 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration; 2016.
4. National Resident Matching Program. Results and data: specialties matching service 2021 appointment year. National Resident Matching Program; 2021.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Four years ago, pursuing a consultation-liaison psychiatry (CL) fellowship was the last thing on my mind. I had recently started my third year as a CL attending at the University of Cincinnati Medical Center and was becoming more of an integral part of its academic department. I felt that I had found my calling. I wanted to be an educator, with the hope of becoming a psychiatry residency program director. This idea was validated when I was awarded the Golden Apple for Excellence in Clinical Teaching, voted by the psychiatry residents, as well as a medical student teaching award. Both awards related to my CL duties.
And then, life happened. My wife and I decided to move east to be closer to family. I planned to continue my path at an academic institution while teaching CL psychiatry. Yet, each institution I interviewed with explained that while my recent experience was “great,” I would need to be formally CL fellowship–trained if I wanted to work in the CL division. On the one hand, this was frustrating to hear; however, the Accreditation Council for Graduate Medical Education has established rules regarding how many faculty at institutions that offer CL fellowships need to be CL fellowship–trained. After much consideration, specifically about my personal career aspirations and family situation, I decided to go “backwards” and pursue a CL fellowship.
Parts of the fellowship year were easier than the previous 3 years. For example, my caseload was much lighter, as were my supervision duties. However, almost immediately, there was an ego-check—for instance, recognizing that I would not always agree with my attendings, and other services would no longer view me as “the attending.” Despite that, as I now discuss with my trainees, I am never above further learning and gaining more clinical experience. Early in that first year of fellowship, I was involved in a complicated case of a patient with autoimmune encephalitis with severe catatonia who warranted electroconvulsive therapy. I gained experience using phenobarbital for the treatment of alcohol withdrawal, something I did not use during my residency or first 3 years as an attending.
Furthermore, my academic project that year was to revamp the fellowship. This included resetting the fellowship’s mission statement, as well as updating our rotations and curriculum, to better align with fellowship best practices around the country. It afforded me time to develop my own “educational” pathway and think of ways in which CL is expanding its footprint. Consistent with this, Park et al1 demonstrated the most common “major reason” for pursuing a CL fellowship was to obtain clinical training; the “moderate reason” of teaching opportunities cannot be overlooked.
The value of a CL fellowship
CL is about the intersection of behavioral health with medicine. As such, I believe CL fellowships will be part of the solution for addressing the current health care cost crisis2 as well as improving access to mental health treatment.3 We already see this solution in collaborative care programs. According to the National Resident Matching Program, in 2021 there were 60 CL fellowship programs and 124 CL fellowship positions offered nationwide, with a total of 89 fellowship applicants and 84 spots filled.4 Looking back 5 years, there were only 52 CL fellowship programs nationwide.4 While there are currently fewer applicants than spots, the fact that the number of available programs is increasing demonstrates the value that each institution puts into CL as well as the importance of our presence in the health care system. The CL fellowship year can create a special opportunity for the fellow that dovetails with their passions. If an applicant wants a program that has expert subspecialty services and focuses on teaching and social determinates of health, they can assuredly find that program.
The decision to pursue fellowship is a personal choice. I believe that CL as a subspecialty will demonstrate its importance to both the psychiatric and medical fields. CL fellowships can continue to innovate and move forward by recognizing the changing landscape of CL psychiatry and matching the fellowship experience to those needs. This will only make the draw for fellowship more powerful. Four years ago, I did not want to pursue fellowship—today I am truly grateful I did.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Four years ago, pursuing a consultation-liaison psychiatry (CL) fellowship was the last thing on my mind. I had recently started my third year as a CL attending at the University of Cincinnati Medical Center and was becoming more of an integral part of its academic department. I felt that I had found my calling. I wanted to be an educator, with the hope of becoming a psychiatry residency program director. This idea was validated when I was awarded the Golden Apple for Excellence in Clinical Teaching, voted by the psychiatry residents, as well as a medical student teaching award. Both awards related to my CL duties.
And then, life happened. My wife and I decided to move east to be closer to family. I planned to continue my path at an academic institution while teaching CL psychiatry. Yet, each institution I interviewed with explained that while my recent experience was “great,” I would need to be formally CL fellowship–trained if I wanted to work in the CL division. On the one hand, this was frustrating to hear; however, the Accreditation Council for Graduate Medical Education has established rules regarding how many faculty at institutions that offer CL fellowships need to be CL fellowship–trained. After much consideration, specifically about my personal career aspirations and family situation, I decided to go “backwards” and pursue a CL fellowship.
Parts of the fellowship year were easier than the previous 3 years. For example, my caseload was much lighter, as were my supervision duties. However, almost immediately, there was an ego-check—for instance, recognizing that I would not always agree with my attendings, and other services would no longer view me as “the attending.” Despite that, as I now discuss with my trainees, I am never above further learning and gaining more clinical experience. Early in that first year of fellowship, I was involved in a complicated case of a patient with autoimmune encephalitis with severe catatonia who warranted electroconvulsive therapy. I gained experience using phenobarbital for the treatment of alcohol withdrawal, something I did not use during my residency or first 3 years as an attending.
Furthermore, my academic project that year was to revamp the fellowship. This included resetting the fellowship’s mission statement, as well as updating our rotations and curriculum, to better align with fellowship best practices around the country. It afforded me time to develop my own “educational” pathway and think of ways in which CL is expanding its footprint. Consistent with this, Park et al1 demonstrated the most common “major reason” for pursuing a CL fellowship was to obtain clinical training; the “moderate reason” of teaching opportunities cannot be overlooked.
The value of a CL fellowship
CL is about the intersection of behavioral health with medicine. As such, I believe CL fellowships will be part of the solution for addressing the current health care cost crisis2 as well as improving access to mental health treatment.3 We already see this solution in collaborative care programs. According to the National Resident Matching Program, in 2021 there were 60 CL fellowship programs and 124 CL fellowship positions offered nationwide, with a total of 89 fellowship applicants and 84 spots filled.4 Looking back 5 years, there were only 52 CL fellowship programs nationwide.4 While there are currently fewer applicants than spots, the fact that the number of available programs is increasing demonstrates the value that each institution puts into CL as well as the importance of our presence in the health care system. The CL fellowship year can create a special opportunity for the fellow that dovetails with their passions. If an applicant wants a program that has expert subspecialty services and focuses on teaching and social determinates of health, they can assuredly find that program.
The decision to pursue fellowship is a personal choice. I believe that CL as a subspecialty will demonstrate its importance to both the psychiatric and medical fields. CL fellowships can continue to innovate and move forward by recognizing the changing landscape of CL psychiatry and matching the fellowship experience to those needs. This will only make the draw for fellowship more powerful. Four years ago, I did not want to pursue fellowship—today I am truly grateful I did.
1. Park EM, Sockalingam S, Ravindranath D, et al; Academy of Psychosomatic Medicine’s Early Career Psychiatrist Special Interest Group. Psychosomatic medicine training as a bridge to practice: training and professional practice patterns of early career psychosomatic medicine specialists. Psychosomatics. 2015;56(1):52-58. doi:10.1016/j.psym.2014.05.003
2. Organisation for Economic Cooperation and Development. Health at a Glance 2021: OECD Indicators. OECD Publishing; 2021. https://doi.org/10.1787/ae3016b9-en
3. Center for Behavioral Health Statistics and Quality. Results from the 2015 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration; 2016.
4. National Resident Matching Program. Results and data: specialties matching service 2021 appointment year. National Resident Matching Program; 2021.
1. Park EM, Sockalingam S, Ravindranath D, et al; Academy of Psychosomatic Medicine’s Early Career Psychiatrist Special Interest Group. Psychosomatic medicine training as a bridge to practice: training and professional practice patterns of early career psychosomatic medicine specialists. Psychosomatics. 2015;56(1):52-58. doi:10.1016/j.psym.2014.05.003
2. Organisation for Economic Cooperation and Development. Health at a Glance 2021: OECD Indicators. OECD Publishing; 2021. https://doi.org/10.1787/ae3016b9-en
3. Center for Behavioral Health Statistics and Quality. Results from the 2015 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration; 2016.
4. National Resident Matching Program. Results and data: specialties matching service 2021 appointment year. National Resident Matching Program; 2021.
Deprescribing in older adults: An overview
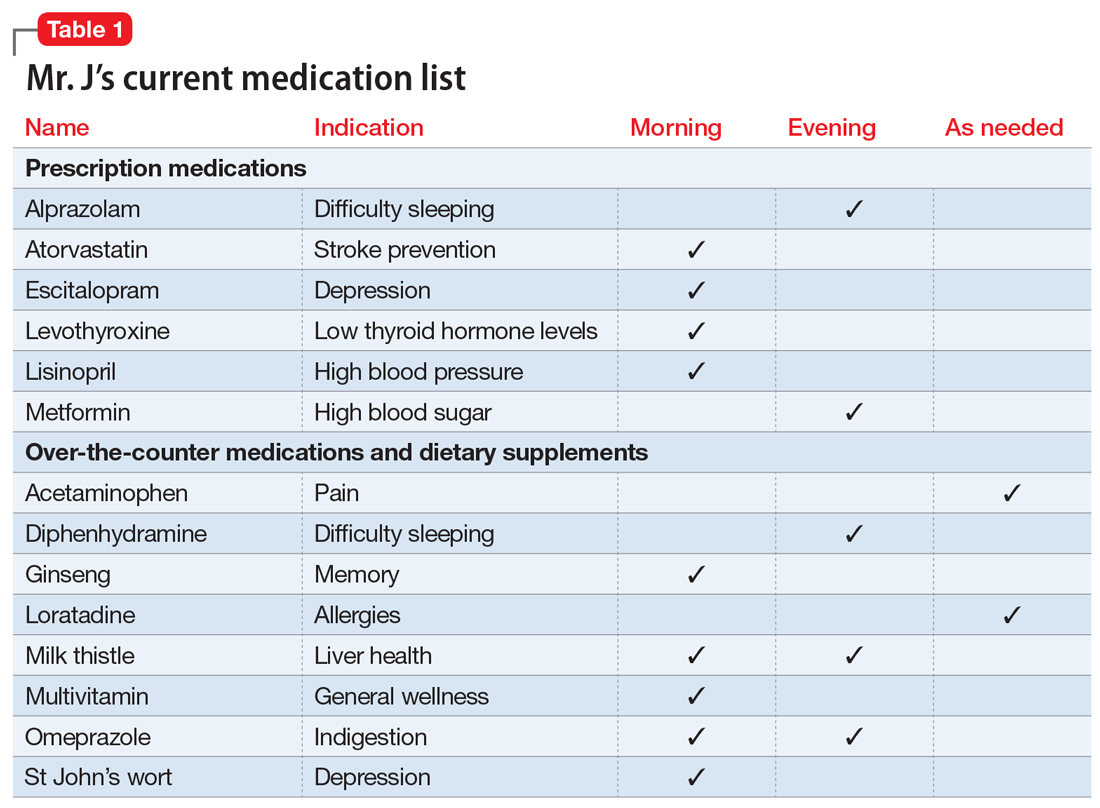
Mr. J, age 73, has a 25-year history of generalized anxiety disorder and major depressive disorder. His medical history includes hypertension, hyperlipidemia, type 2 diabetes mellitus, hypothyroidism, osteoarthritis, insomnia, and allergic rhinitis. His last laboratory test results indicate his hemoglobin A1c, thyroid-stimulating hormone, low-density lipoprotein, and blood pressure measurements are at goal. He believes his conditions are well controlled but cites concerns about taking multiple medications each day and being able to afford his medications.
You review the list of Mr. J’s current prescription medications, which include alprazolam 0.5 mg/d, atorvastatin 40 mg/d, escitalopram 10 mg/d, levothyroxine 0.125 mg/d, lisinopril 20 mg/d, and metformin XR 1,000 mg/d. Mr. J reports taking over-the-counter (OTC) acetaminophen as needed for pain, diphenhydramine for insomnia, loratadine as needed for allergic rhinitis, and omeprazole for 2 years for indigestion. After further questioning, he also reports taking ginseng, milk thistle, a multivitamin, and, based on a friend’s recommendation, St John’s Wort (Table 1).
Similar to Mr. J, many older adults take multiple medications to manage chronic health conditions and promote their overall health. On average, 30% of older adults take ≥5 medications.1 Among commonly prescribed medications for these patients, an estimated 1 in 5 of may be inappropriate.1 Older adults have high rates of polypharmacy (often defined as taking ≥5 medications1), age-related physiological changes, increased number of comorbidities, and frailty, all of which can increase the risk of medication-related adverse events.2 As a result, older patients’ medications should be regularly evaluated to determine if each medication is appropriate to continue or should be tapered or stopped.
Deprescribing, in which medications are tapered or discontinued using a patient-centered approach, should be considered when a patient is no longer receiving benefit from a medication, or when the harm may exceed the benefit.1,3
Several researchers1,3 and organizations have published detailed descriptions of and guidelines for the process of deprescribing (see Related Resources). Here we provide a brief overview of this process (Figure1,3). The first step is to assemble a list of all prescription and OTC medications, herbal products, vitamins, or nutritional supplements the patient is taking. It is important to specifically ask patients about their use of nonprescription products, because these products are infrequently documented in medical records.
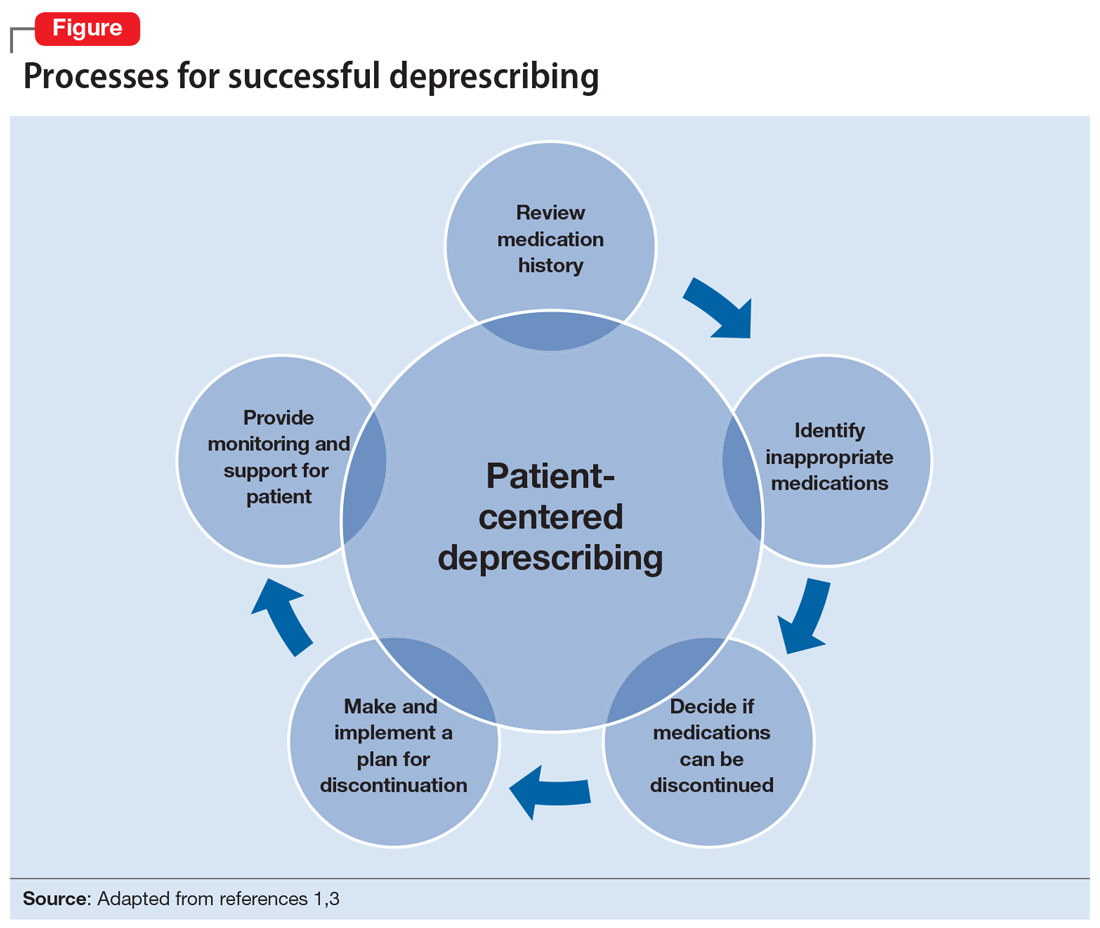
The second step is to evaluate the indication, effectiveness, safety, and patient’s adherence to each medication while beginning to consider opportunities to limit treatment burden and the risk of harm from medications. Ideally, this assessment should involve a patient-centered conversation that considers the patient’s goals, preferences, and treatment values. Many resources can be used to evaluate which medications might be inappropriate for an older adult. Two examples are the American Geriatrics Society Beers Criteria5 and STOPP/START criteria.6 By looking at these resources, you could identify that (for example) anticholinergic medications should be avoided in older patients due to an increased risk of adverse effects, change in cognitive status, and falls.5,6 These resources can aid in identifying, prioritizing, and deprescribing potentially harmful and/or inappropriate medications.
The next step is to decide whether any medications should be discontinued. Whenever possible, include the patient in this conversation, as they may have strong feelings about their current medication regimen. When there are multiple medications that can be discontinued, consider which medication to stop first based on potential harm, patient resistance, and other factors.
Continue to: Subsequently, work with...
Subsequently, work with the patient to create a plan for stopping or lowering the dose or frequency of the medication. These changes should be individualized based on the patient’s preferences as well as the properties of the medication. For example, some medications can be immediately discontinued, while others (eg, benzodiazepines) may need to be slowly tapered. It is important to consider if the patient will need to switch to a safer medication, change their behaviors (eg, lifestyle changes), or engage in alternative treatments (such as cognitive-behavioral therapy for insomnia) when they stop their current medication. Take an active role in monitoring your patient during this process, and encourage them to reach out to you or to their primary clinician if they have concerns.
CASE CONTINUED
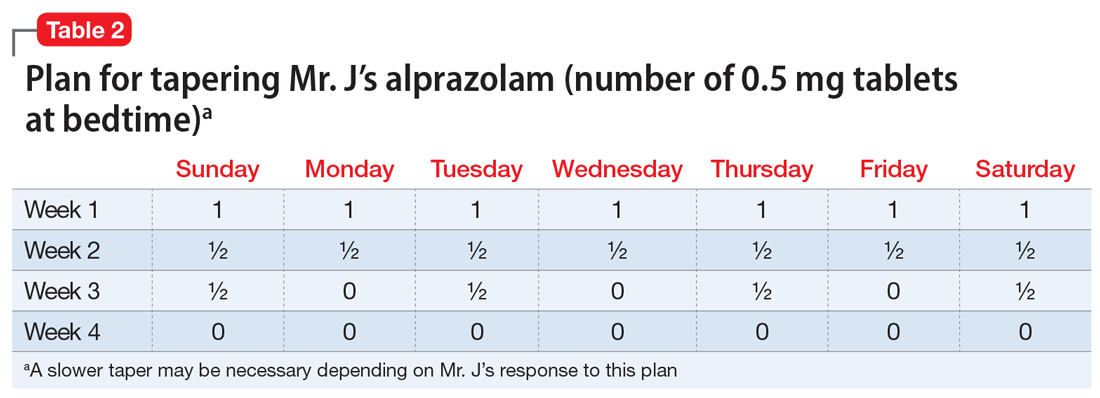
Mr. J is a candidate for deprescribing because he has expressed concerns about his current regimen, and because he is taking potentially unsafe medications. The 2 medications he’s taking that may cause the most harm are diphenhydramine and alprazolam, due to the risk of cognitive impairment and falls. Through a patient-centered conversation, Mr. J says he is willing to stop diphenhydramine immediately and taper off the alprazolam over the next month, with the support of a tapering chart (Table 2). You explain to him that a long tapering of alprazolam may be necessary. He is willing to try good sleep hygiene practices and will put off starting trazodone as an alternative to diphenhydramine until he sees if it will be necessary. You make a note to follow up with him in 1 week to assess his insomnia and adherence to the new treatment plan. You also teach Mr. J that some of his supplements may interact with his prescription medications, such as St John’s Wort with escitalopram (ie, risk of serotonin syndrome) and ginseng with metformin (ie, risk for hypoglycemia). He says he doesn’t take ginseng, milk thistle, or St John’s Wort regularly, and because he feels they do not offer any benefit, he will stop taking them. He says that at his next visit with his primary care physician, he will bring up the idea of stopping omeprazole.
Related Resources
- Deprescribing.org. Deprescribing guidelines and algorithms. https://deprescribing.org/resources/deprescribing-guidelines-algorithms/
- US Deprescribing Research Network. Resources for Clinicians. https://deprescribingresearch.org/resources-2/resources-for-clinicians/
Drug Brand Names
Alprazolam • Xanax
Atorvastatin • Lipitor
Escitalopram • Lexapro
Levothyroxine • Synthroid
Lisinopril • Zestril
Metformin XR • Glucophage XR
Trazodone • Desyrel
1. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
2. Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
3. Reeve E, Shakib S, Hendrix I, et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmcol. 2014;78(4):738-747.
4. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25(12):1021-1031.
5. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.

Mr. J, age 73, has a 25-year history of generalized anxiety disorder and major depressive disorder. His medical history includes hypertension, hyperlipidemia, type 2 diabetes mellitus, hypothyroidism, osteoarthritis, insomnia, and allergic rhinitis. His last laboratory test results indicate his hemoglobin A1c, thyroid-stimulating hormone, low-density lipoprotein, and blood pressure measurements are at goal. He believes his conditions are well controlled but cites concerns about taking multiple medications each day and being able to afford his medications.
You review the list of Mr. J’s current prescription medications, which include alprazolam 0.5 mg/d, atorvastatin 40 mg/d, escitalopram 10 mg/d, levothyroxine 0.125 mg/d, lisinopril 20 mg/d, and metformin XR 1,000 mg/d. Mr. J reports taking over-the-counter (OTC) acetaminophen as needed for pain, diphenhydramine for insomnia, loratadine as needed for allergic rhinitis, and omeprazole for 2 years for indigestion. After further questioning, he also reports taking ginseng, milk thistle, a multivitamin, and, based on a friend’s recommendation, St John’s Wort (Table 1).

Similar to Mr. J, many older adults take multiple medications to manage chronic health conditions and promote their overall health. On average, 30% of older adults take ≥5 medications.1 Among commonly prescribed medications for these patients, an estimated 1 in 5 of may be inappropriate.1 Older adults have high rates of polypharmacy (often defined as taking ≥5 medications1), age-related physiological changes, increased number of comorbidities, and frailty, all of which can increase the risk of medication-related adverse events.2 As a result, older patients’ medications should be regularly evaluated to determine if each medication is appropriate to continue or should be tapered or stopped.
Deprescribing, in which medications are tapered or discontinued using a patient-centered approach, should be considered when a patient is no longer receiving benefit from a medication, or when the harm may exceed the benefit.1,3
Several researchers1,3 and organizations have published detailed descriptions of and guidelines for the process of deprescribing (see Related Resources). Here we provide a brief overview of this process (Figure1,3). The first step is to assemble a list of all prescription and OTC medications, herbal products, vitamins, or nutritional supplements the patient is taking. It is important to specifically ask patients about their use of nonprescription products, because these products are infrequently documented in medical records.

The second step is to evaluate the indication, effectiveness, safety, and patient’s adherence to each medication while beginning to consider opportunities to limit treatment burden and the risk of harm from medications. Ideally, this assessment should involve a patient-centered conversation that considers the patient’s goals, preferences, and treatment values. Many resources can be used to evaluate which medications might be inappropriate for an older adult. Two examples are the American Geriatrics Society Beers Criteria5 and STOPP/START criteria.6 By looking at these resources, you could identify that (for example) anticholinergic medications should be avoided in older patients due to an increased risk of adverse effects, change in cognitive status, and falls.5,6 These resources can aid in identifying, prioritizing, and deprescribing potentially harmful and/or inappropriate medications.
The next step is to decide whether any medications should be discontinued. Whenever possible, include the patient in this conversation, as they may have strong feelings about their current medication regimen. When there are multiple medications that can be discontinued, consider which medication to stop first based on potential harm, patient resistance, and other factors.
Continue to: Subsequently, work with...
Subsequently, work with the patient to create a plan for stopping or lowering the dose or frequency of the medication. These changes should be individualized based on the patient’s preferences as well as the properties of the medication. For example, some medications can be immediately discontinued, while others (eg, benzodiazepines) may need to be slowly tapered. It is important to consider if the patient will need to switch to a safer medication, change their behaviors (eg, lifestyle changes), or engage in alternative treatments (such as cognitive-behavioral therapy for insomnia) when they stop their current medication. Take an active role in monitoring your patient during this process, and encourage them to reach out to you or to their primary clinician if they have concerns.
CASE CONTINUED
Mr. J is a candidate for deprescribing because he has expressed concerns about his current regimen, and because he is taking potentially unsafe medications. The 2 medications he’s taking that may cause the most harm are diphenhydramine and alprazolam, due to the risk of cognitive impairment and falls. Through a patient-centered conversation, Mr. J says he is willing to stop diphenhydramine immediately and taper off the alprazolam over the next month, with the support of a tapering chart (Table 2). You explain to him that a long tapering of alprazolam may be necessary. He is willing to try good sleep hygiene practices and will put off starting trazodone as an alternative to diphenhydramine until he sees if it will be necessary. You make a note to follow up with him in 1 week to assess his insomnia and adherence to the new treatment plan. You also teach Mr. J that some of his supplements may interact with his prescription medications, such as St John’s Wort with escitalopram (ie, risk of serotonin syndrome) and ginseng with metformin (ie, risk for hypoglycemia). He says he doesn’t take ginseng, milk thistle, or St John’s Wort regularly, and because he feels they do not offer any benefit, he will stop taking them. He says that at his next visit with his primary care physician, he will bring up the idea of stopping omeprazole.

Related Resources
- Deprescribing.org. Deprescribing guidelines and algorithms. https://deprescribing.org/resources/deprescribing-guidelines-algorithms/
- US Deprescribing Research Network. Resources for Clinicians. https://deprescribingresearch.org/resources-2/resources-for-clinicians/
Drug Brand Names
Alprazolam • Xanax
Atorvastatin • Lipitor
Escitalopram • Lexapro
Levothyroxine • Synthroid
Lisinopril • Zestril
Metformin XR • Glucophage XR
Trazodone • Desyrel

Mr. J, age 73, has a 25-year history of generalized anxiety disorder and major depressive disorder. His medical history includes hypertension, hyperlipidemia, type 2 diabetes mellitus, hypothyroidism, osteoarthritis, insomnia, and allergic rhinitis. His last laboratory test results indicate his hemoglobin A1c, thyroid-stimulating hormone, low-density lipoprotein, and blood pressure measurements are at goal. He believes his conditions are well controlled but cites concerns about taking multiple medications each day and being able to afford his medications.
You review the list of Mr. J’s current prescription medications, which include alprazolam 0.5 mg/d, atorvastatin 40 mg/d, escitalopram 10 mg/d, levothyroxine 0.125 mg/d, lisinopril 20 mg/d, and metformin XR 1,000 mg/d. Mr. J reports taking over-the-counter (OTC) acetaminophen as needed for pain, diphenhydramine for insomnia, loratadine as needed for allergic rhinitis, and omeprazole for 2 years for indigestion. After further questioning, he also reports taking ginseng, milk thistle, a multivitamin, and, based on a friend’s recommendation, St John’s Wort (Table 1).

Similar to Mr. J, many older adults take multiple medications to manage chronic health conditions and promote their overall health. On average, 30% of older adults take ≥5 medications.1 Among commonly prescribed medications for these patients, an estimated 1 in 5 of may be inappropriate.1 Older adults have high rates of polypharmacy (often defined as taking ≥5 medications1), age-related physiological changes, increased number of comorbidities, and frailty, all of which can increase the risk of medication-related adverse events.2 As a result, older patients’ medications should be regularly evaluated to determine if each medication is appropriate to continue or should be tapered or stopped.
Deprescribing, in which medications are tapered or discontinued using a patient-centered approach, should be considered when a patient is no longer receiving benefit from a medication, or when the harm may exceed the benefit.1,3
Several researchers1,3 and organizations have published detailed descriptions of and guidelines for the process of deprescribing (see Related Resources). Here we provide a brief overview of this process (Figure1,3). The first step is to assemble a list of all prescription and OTC medications, herbal products, vitamins, or nutritional supplements the patient is taking. It is important to specifically ask patients about their use of nonprescription products, because these products are infrequently documented in medical records.

The second step is to evaluate the indication, effectiveness, safety, and patient’s adherence to each medication while beginning to consider opportunities to limit treatment burden and the risk of harm from medications. Ideally, this assessment should involve a patient-centered conversation that considers the patient’s goals, preferences, and treatment values. Many resources can be used to evaluate which medications might be inappropriate for an older adult. Two examples are the American Geriatrics Society Beers Criteria5 and STOPP/START criteria.6 By looking at these resources, you could identify that (for example) anticholinergic medications should be avoided in older patients due to an increased risk of adverse effects, change in cognitive status, and falls.5,6 These resources can aid in identifying, prioritizing, and deprescribing potentially harmful and/or inappropriate medications.
The next step is to decide whether any medications should be discontinued. Whenever possible, include the patient in this conversation, as they may have strong feelings about their current medication regimen. When there are multiple medications that can be discontinued, consider which medication to stop first based on potential harm, patient resistance, and other factors.
Continue to: Subsequently, work with...
Subsequently, work with the patient to create a plan for stopping or lowering the dose or frequency of the medication. These changes should be individualized based on the patient’s preferences as well as the properties of the medication. For example, some medications can be immediately discontinued, while others (eg, benzodiazepines) may need to be slowly tapered. It is important to consider if the patient will need to switch to a safer medication, change their behaviors (eg, lifestyle changes), or engage in alternative treatments (such as cognitive-behavioral therapy for insomnia) when they stop their current medication. Take an active role in monitoring your patient during this process, and encourage them to reach out to you or to their primary clinician if they have concerns.
CASE CONTINUED
Mr. J is a candidate for deprescribing because he has expressed concerns about his current regimen, and because he is taking potentially unsafe medications. The 2 medications he’s taking that may cause the most harm are diphenhydramine and alprazolam, due to the risk of cognitive impairment and falls. Through a patient-centered conversation, Mr. J says he is willing to stop diphenhydramine immediately and taper off the alprazolam over the next month, with the support of a tapering chart (Table 2). You explain to him that a long tapering of alprazolam may be necessary. He is willing to try good sleep hygiene practices and will put off starting trazodone as an alternative to diphenhydramine until he sees if it will be necessary. You make a note to follow up with him in 1 week to assess his insomnia and adherence to the new treatment plan. You also teach Mr. J that some of his supplements may interact with his prescription medications, such as St John’s Wort with escitalopram (ie, risk of serotonin syndrome) and ginseng with metformin (ie, risk for hypoglycemia). He says he doesn’t take ginseng, milk thistle, or St John’s Wort regularly, and because he feels they do not offer any benefit, he will stop taking them. He says that at his next visit with his primary care physician, he will bring up the idea of stopping omeprazole.

Related Resources
- Deprescribing.org. Deprescribing guidelines and algorithms. https://deprescribing.org/resources/deprescribing-guidelines-algorithms/
- US Deprescribing Research Network. Resources for Clinicians. https://deprescribingresearch.org/resources-2/resources-for-clinicians/
Drug Brand Names
Alprazolam • Xanax
Atorvastatin • Lipitor
Escitalopram • Lexapro
Levothyroxine • Synthroid
Lisinopril • Zestril
Metformin XR • Glucophage XR
Trazodone • Desyrel
1. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
2. Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
3. Reeve E, Shakib S, Hendrix I, et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmcol. 2014;78(4):738-747.
4. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25(12):1021-1031.
5. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
1. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
2. Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
3. Reeve E, Shakib S, Hendrix I, et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmcol. 2014;78(4):738-747.
4. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25(12):1021-1031.
5. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
The woman who kept passing out
CASE An apparent code blue
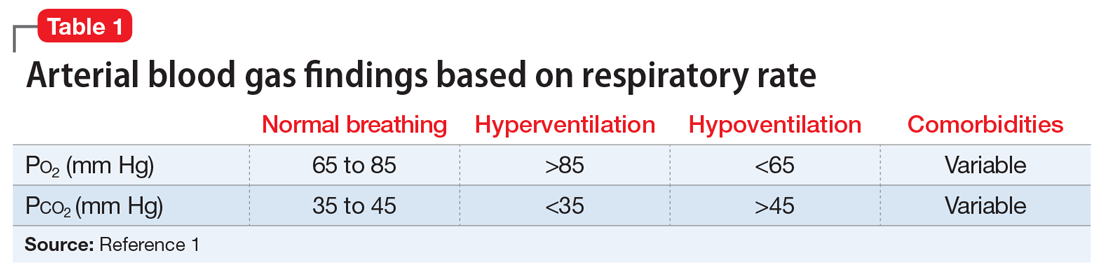
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
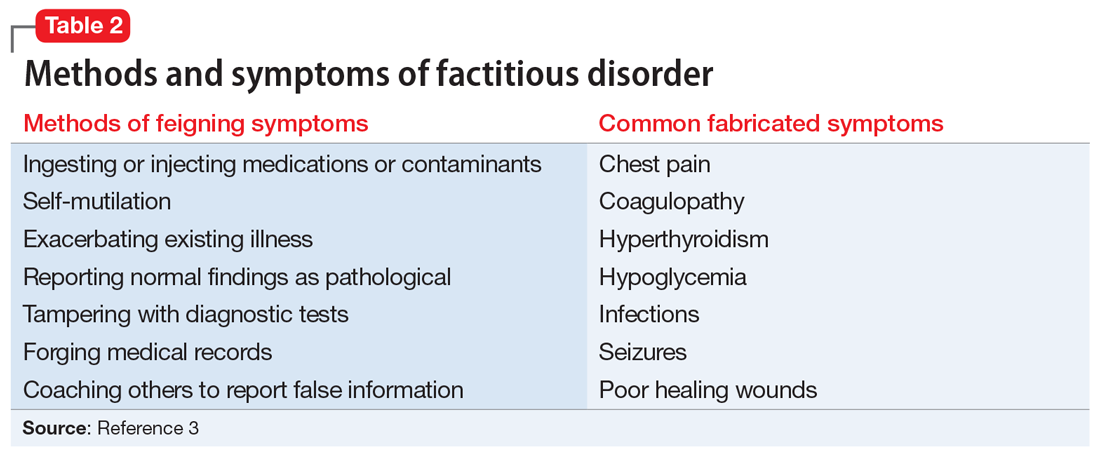
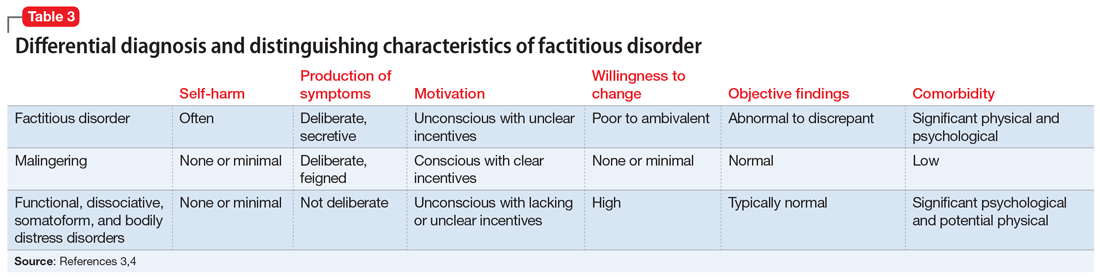
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
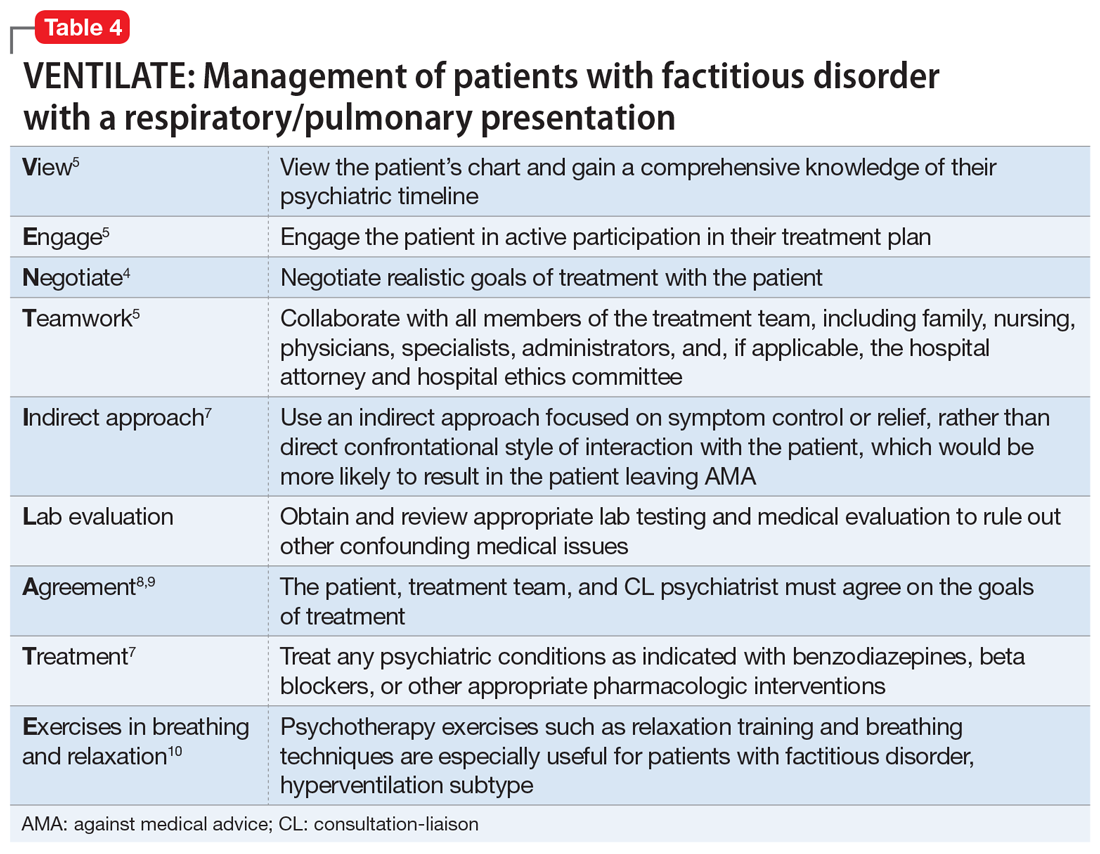
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.

HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).

Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3

Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).

Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.

HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).

Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3

Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).

Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
How to ‘cybersecure’ your practice
The health care sector is not immune from cybersecurity attacks (malicious attempts to access or damage a computer or network system). Between October 2019 and October 2021, 857 data breaches were reported to the United States Department of Health and Human Services.1 The 3 main types of breaches reported were theft, hacking/IT incident, or unauthorized access/disclosure.1 Health care has become a common target due to the availability of valuable patient information (health, personal, and financial), the industry’s financial stability and resource capacity, and network susceptibility.2 The top 2 cybersecurity threats facing physician practices are:
- ransomware attacks, in which an external party uses a type of malicious software (malware) that prevents you from accessing your computer files, systems, or networks, and demands you pay a ransom for their return.
- employee-related threats, such as the theft or destruction of sensitive information by a disgruntled employee.3
The financial implications of health care–related cybersecurity threats coupled with exposure to potential litigation associated with breaches of confidentiality result in a need to “cybersecure” your practice.2 In this article, I outline steps to take to protect your practice against such threats. Although the recommendations I provide will increase your practice’s cybersecurity fortification, they are not exhaustive, and you may need to consult with an IT specialist to help protect your data and network.
Improve your network protection. A broadband internet connection is always operating, which makes it continuously susceptible to cybersecurity attacks. Install a firewall (a network security system that monitors and controls network traffic and permits or blocks traffic based on a defined set of rules) between your practice’s internal computer network and the internet.4 For maximum protection, enable all available firewall settings in your operating software.2 Prevent unauthorized access by ensuring that all network passwords are strong (ie, they include a combination of uppercase and lowercase letters, numbers, and symbols). Consider using different networks for online communication and for storing sensitive information.2 Create separate Wi-Fi networks for your practice and for your patients, and use unique passwords for each that are not easily guessed.4 If you or your employees use a virtual private network (VPN) to remotely access your practice’s network, ensure that all devices used to do so (cell phones, tablets, etc) are encrypted and secured with strong passwords.
Reduce employee-related threats. Not every employee in your practice will need to access to your patients’ clinical or financial data. Limiting employee access to sensitive clinical or financial data can reduce the risks of employee-related cybersecurity threats.3 In addition, restrict an employee’s ability to install software on computers and other devices that belong to your practice.2
Frequently incorporate cybersecurity training, such as teaching your employees about the risks of clicking on links and attachments in emails and how to identify phishing attacks (in which an individual sends a fraudulent communication that appears to come from a reputable source in order to trick the recipient into revealing financial information, system credentials, or other sensitive data).2,3 Use multifactor authentication to verify an employee’s login identity, and change passwords often. Reinforce these policies at staff meetings and educate new employees about this process.3 If you need to fire an employee, consider deploying cybersurveillance software to monitor the behavior of all employees before the employee is terminated.3 Once the employee has been terminated, change all logins and passwords.
1. U.S. Department of Health and Human Services. Office for Civil Rights. Breach portal: Notice to the Secretary of HHS breach of unsecured protected health information. Accessed December 26, 2021. https://ocrportal.hhs.gov/ocr/breach/breach_report.jsf
2. Umali G. How to safeguard your practice from cybersecurity threats. Psychiatric News. 2021;56(12):23.
3. Cryts A. Top two cybersecurity threats facing physician practices. Physicians Practice. March 13, 2020. Accessed December 26, 2021. https://www.physicianspractice.com/view/top-two-cybersecurity-threats-facing-physician-practices
4. American Medical Association. Protect your practice and patients from cybersecurity threats. 2017. Accessed December 26, 2021. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/network-security.pdf
The health care sector is not immune from cybersecurity attacks (malicious attempts to access or damage a computer or network system). Between October 2019 and October 2021, 857 data breaches were reported to the United States Department of Health and Human Services.1 The 3 main types of breaches reported were theft, hacking/IT incident, or unauthorized access/disclosure.1 Health care has become a common target due to the availability of valuable patient information (health, personal, and financial), the industry’s financial stability and resource capacity, and network susceptibility.2 The top 2 cybersecurity threats facing physician practices are:
- ransomware attacks, in which an external party uses a type of malicious software (malware) that prevents you from accessing your computer files, systems, or networks, and demands you pay a ransom for their return.
- employee-related threats, such as the theft or destruction of sensitive information by a disgruntled employee.3
The financial implications of health care–related cybersecurity threats coupled with exposure to potential litigation associated with breaches of confidentiality result in a need to “cybersecure” your practice.2 In this article, I outline steps to take to protect your practice against such threats. Although the recommendations I provide will increase your practice’s cybersecurity fortification, they are not exhaustive, and you may need to consult with an IT specialist to help protect your data and network.
Improve your network protection. A broadband internet connection is always operating, which makes it continuously susceptible to cybersecurity attacks. Install a firewall (a network security system that monitors and controls network traffic and permits or blocks traffic based on a defined set of rules) between your practice’s internal computer network and the internet.4 For maximum protection, enable all available firewall settings in your operating software.2 Prevent unauthorized access by ensuring that all network passwords are strong (ie, they include a combination of uppercase and lowercase letters, numbers, and symbols). Consider using different networks for online communication and for storing sensitive information.2 Create separate Wi-Fi networks for your practice and for your patients, and use unique passwords for each that are not easily guessed.4 If you or your employees use a virtual private network (VPN) to remotely access your practice’s network, ensure that all devices used to do so (cell phones, tablets, etc) are encrypted and secured with strong passwords.
Reduce employee-related threats. Not every employee in your practice will need to access to your patients’ clinical or financial data. Limiting employee access to sensitive clinical or financial data can reduce the risks of employee-related cybersecurity threats.3 In addition, restrict an employee’s ability to install software on computers and other devices that belong to your practice.2
Frequently incorporate cybersecurity training, such as teaching your employees about the risks of clicking on links and attachments in emails and how to identify phishing attacks (in which an individual sends a fraudulent communication that appears to come from a reputable source in order to trick the recipient into revealing financial information, system credentials, or other sensitive data).2,3 Use multifactor authentication to verify an employee’s login identity, and change passwords often. Reinforce these policies at staff meetings and educate new employees about this process.3 If you need to fire an employee, consider deploying cybersurveillance software to monitor the behavior of all employees before the employee is terminated.3 Once the employee has been terminated, change all logins and passwords.
The health care sector is not immune from cybersecurity attacks (malicious attempts to access or damage a computer or network system). Between October 2019 and October 2021, 857 data breaches were reported to the United States Department of Health and Human Services.1 The 3 main types of breaches reported were theft, hacking/IT incident, or unauthorized access/disclosure.1 Health care has become a common target due to the availability of valuable patient information (health, personal, and financial), the industry’s financial stability and resource capacity, and network susceptibility.2 The top 2 cybersecurity threats facing physician practices are:
- ransomware attacks, in which an external party uses a type of malicious software (malware) that prevents you from accessing your computer files, systems, or networks, and demands you pay a ransom for their return.
- employee-related threats, such as the theft or destruction of sensitive information by a disgruntled employee.3
The financial implications of health care–related cybersecurity threats coupled with exposure to potential litigation associated with breaches of confidentiality result in a need to “cybersecure” your practice.2 In this article, I outline steps to take to protect your practice against such threats. Although the recommendations I provide will increase your practice’s cybersecurity fortification, they are not exhaustive, and you may need to consult with an IT specialist to help protect your data and network.
Improve your network protection. A broadband internet connection is always operating, which makes it continuously susceptible to cybersecurity attacks. Install a firewall (a network security system that monitors and controls network traffic and permits or blocks traffic based on a defined set of rules) between your practice’s internal computer network and the internet.4 For maximum protection, enable all available firewall settings in your operating software.2 Prevent unauthorized access by ensuring that all network passwords are strong (ie, they include a combination of uppercase and lowercase letters, numbers, and symbols). Consider using different networks for online communication and for storing sensitive information.2 Create separate Wi-Fi networks for your practice and for your patients, and use unique passwords for each that are not easily guessed.4 If you or your employees use a virtual private network (VPN) to remotely access your practice’s network, ensure that all devices used to do so (cell phones, tablets, etc) are encrypted and secured with strong passwords.
Reduce employee-related threats. Not every employee in your practice will need to access to your patients’ clinical or financial data. Limiting employee access to sensitive clinical or financial data can reduce the risks of employee-related cybersecurity threats.3 In addition, restrict an employee’s ability to install software on computers and other devices that belong to your practice.2
Frequently incorporate cybersecurity training, such as teaching your employees about the risks of clicking on links and attachments in emails and how to identify phishing attacks (in which an individual sends a fraudulent communication that appears to come from a reputable source in order to trick the recipient into revealing financial information, system credentials, or other sensitive data).2,3 Use multifactor authentication to verify an employee’s login identity, and change passwords often. Reinforce these policies at staff meetings and educate new employees about this process.3 If you need to fire an employee, consider deploying cybersurveillance software to monitor the behavior of all employees before the employee is terminated.3 Once the employee has been terminated, change all logins and passwords.
1. U.S. Department of Health and Human Services. Office for Civil Rights. Breach portal: Notice to the Secretary of HHS breach of unsecured protected health information. Accessed December 26, 2021. https://ocrportal.hhs.gov/ocr/breach/breach_report.jsf
2. Umali G. How to safeguard your practice from cybersecurity threats. Psychiatric News. 2021;56(12):23.
3. Cryts A. Top two cybersecurity threats facing physician practices. Physicians Practice. March 13, 2020. Accessed December 26, 2021. https://www.physicianspractice.com/view/top-two-cybersecurity-threats-facing-physician-practices
4. American Medical Association. Protect your practice and patients from cybersecurity threats. 2017. Accessed December 26, 2021. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/network-security.pdf
1. U.S. Department of Health and Human Services. Office for Civil Rights. Breach portal: Notice to the Secretary of HHS breach of unsecured protected health information. Accessed December 26, 2021. https://ocrportal.hhs.gov/ocr/breach/breach_report.jsf
2. Umali G. How to safeguard your practice from cybersecurity threats. Psychiatric News. 2021;56(12):23.
3. Cryts A. Top two cybersecurity threats facing physician practices. Physicians Practice. March 13, 2020. Accessed December 26, 2021. https://www.physicianspractice.com/view/top-two-cybersecurity-threats-facing-physician-practices
4. American Medical Association. Protect your practice and patients from cybersecurity threats. 2017. Accessed December 26, 2021. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/network-security.pdf
Managing bipolar disorder in women who are pregnant
Psychiatrists who treat women of childbearing age should consider that those women may become pregnant, and that women with psychiatric illness are more likely to have unplanned pregnancies.1 Thus, thoughtful perinatal medication choices should begin before pregnancy. Pregnancy is a time of vulnerability to psychiatric illness for many reasons, including physiologic changes that can affect mental status; changes in medication efficacy; and numerous stressors, such as new responsibilities and limited sleep.1,2 For the treatment of pregnant—or potentially pregnant—patients, we recommend the following.
Do not panic! Knee-jerk medication changes in response to learning a patient is pregnant can lead to an exacerbation of psychiatric symptoms, as well as decrease trust in clinicians.2 Switching to a medication with a purportedly “safer” reproductive profile may worsen psychiatric illness, while also exposing the fetus to a medication of unknown benefit. 2
Recognize the risk of untreated or undertreated psychiatric illness, either of which has the potential to harm both the woman and her fetus. For example, a pregnant woman in a manic state may be more likely to engage in risky behaviors, such as drug use or risky sexual activity, which can lead to adverse fetal outcomes. They may also present with a higher risk of suicide. Compared to nonpregnant women, pregnant women for whom lithium was discontinued were equally likely to experience illness recurrence and significantly more likely to experience postpartum illness recurrence.3 In addition, the risk of recurrence was greater after rapid discontinuation compared with gradual discontinuation.3
Accurately communicate research findings. Pregnancy risk categories are no longer used. A nuanced interpretation of the potential adverse effects of a medication, such as malformations, impaired fetal growth, birth outcomes (such as preterm birth), and neurodevelopmental sequelae is necessary. Physicians must accurately convey information about risks to their patients, including both the absolute risk of an adverse event and the possible range of severity. For example, lithium use during pregnancy confers a higher relative risk of Ebstein’s anomaly (a cardiac defect).4 However, the absolute incidence of this risk remains low: 0.6% of lithium-exposed infants vs 0.18% among unexposed infants.4 Ebstein’s anomaly also varies significantly in severity—serious cases may require surgery, but less serious cases need only monitoring. A reliable database that compiles the latest evidence may help in staying abreast of the latest data.
Treat the psychiatric illness. Consider the optimal treatment for the psychiatric illness. Lithium remains the gold standard treatment for bipolar I disorder, regardless of reproductive status. Olanzapine and quetiapine are also commonly used and effective during pregnancy. This is an opportunity to conduct a detailed review of the patient’s previous medication regimens, including a review of medication trials and efficacy. Keep in mind that untreated bipolar disorder also carries an increased risk of adverse pregnancy outcomes.5
Consider pregnancy timing. Most organs form between weeks 3 to 8 of pregnancy. For example, if a medication potentially affects heart formation, but the patient is in the third trimester, explain to her that the heart has already been formed. Consider that medication may be required long-term and affect future pregnancies. Pregnant women require more frequent monitoring, because blood volume changes in pregnancy and postpartum can affect medication levels and efficacy. In addition, note whether a woman plans to breastfeed and be mindful of a medication’s profile in breastfeeding.
Ensure the patient can provide informed consent. Communicate your diagnostic formulation and treatment options. Consider involving the patient’s partner and/or support system in the discussion, if the patient consents. If a patient cannot provide informed consent, a surrogate decision-maker should be identified.6
Continue to: Collaborate with other clinicians
Collaborate with other clinicians, such as the patient’s OB/GYN and family medicine physician when possible. This will ensure that all clinicians are on the same page.
Plan for future pregnancies. Psychiatric medications can be long-term. Even patients who say they do not wish to become pregnant may someday become pregnant. Having discussions about medication choices, and their reproductive implications, prior to pregnancy allows patients to take an active role in their health.1,2
Consult a reproductive psychiatrist when indicated, and as early in the pregnancy as possible.
1. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
2. Friedman SH, Reed E. Treating psychosis in pregnant women: a measured approach. Current Psychiatry. 2021; 20(7):34-35.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157(2):179-184.
4. Patorno E, Huybrechts KF, Bateman BT, et al. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
5. Bodén R, Lundgren M, Brandt L, et al. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi:10.1136/bmj.e7085
6. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022:63(1);61-70.
Psychiatrists who treat women of childbearing age should consider that those women may become pregnant, and that women with psychiatric illness are more likely to have unplanned pregnancies.1 Thus, thoughtful perinatal medication choices should begin before pregnancy. Pregnancy is a time of vulnerability to psychiatric illness for many reasons, including physiologic changes that can affect mental status; changes in medication efficacy; and numerous stressors, such as new responsibilities and limited sleep.1,2 For the treatment of pregnant—or potentially pregnant—patients, we recommend the following.
Do not panic! Knee-jerk medication changes in response to learning a patient is pregnant can lead to an exacerbation of psychiatric symptoms, as well as decrease trust in clinicians.2 Switching to a medication with a purportedly “safer” reproductive profile may worsen psychiatric illness, while also exposing the fetus to a medication of unknown benefit. 2
Recognize the risk of untreated or undertreated psychiatric illness, either of which has the potential to harm both the woman and her fetus. For example, a pregnant woman in a manic state may be more likely to engage in risky behaviors, such as drug use or risky sexual activity, which can lead to adverse fetal outcomes. They may also present with a higher risk of suicide. Compared to nonpregnant women, pregnant women for whom lithium was discontinued were equally likely to experience illness recurrence and significantly more likely to experience postpartum illness recurrence.3 In addition, the risk of recurrence was greater after rapid discontinuation compared with gradual discontinuation.3
Accurately communicate research findings. Pregnancy risk categories are no longer used. A nuanced interpretation of the potential adverse effects of a medication, such as malformations, impaired fetal growth, birth outcomes (such as preterm birth), and neurodevelopmental sequelae is necessary. Physicians must accurately convey information about risks to their patients, including both the absolute risk of an adverse event and the possible range of severity. For example, lithium use during pregnancy confers a higher relative risk of Ebstein’s anomaly (a cardiac defect).4 However, the absolute incidence of this risk remains low: 0.6% of lithium-exposed infants vs 0.18% among unexposed infants.4 Ebstein’s anomaly also varies significantly in severity—serious cases may require surgery, but less serious cases need only monitoring. A reliable database that compiles the latest evidence may help in staying abreast of the latest data.
Treat the psychiatric illness. Consider the optimal treatment for the psychiatric illness. Lithium remains the gold standard treatment for bipolar I disorder, regardless of reproductive status. Olanzapine and quetiapine are also commonly used and effective during pregnancy. This is an opportunity to conduct a detailed review of the patient’s previous medication regimens, including a review of medication trials and efficacy. Keep in mind that untreated bipolar disorder also carries an increased risk of adverse pregnancy outcomes.5
Consider pregnancy timing. Most organs form between weeks 3 to 8 of pregnancy. For example, if a medication potentially affects heart formation, but the patient is in the third trimester, explain to her that the heart has already been formed. Consider that medication may be required long-term and affect future pregnancies. Pregnant women require more frequent monitoring, because blood volume changes in pregnancy and postpartum can affect medication levels and efficacy. In addition, note whether a woman plans to breastfeed and be mindful of a medication’s profile in breastfeeding.
Ensure the patient can provide informed consent. Communicate your diagnostic formulation and treatment options. Consider involving the patient’s partner and/or support system in the discussion, if the patient consents. If a patient cannot provide informed consent, a surrogate decision-maker should be identified.6
Continue to: Collaborate with other clinicians
Collaborate with other clinicians, such as the patient’s OB/GYN and family medicine physician when possible. This will ensure that all clinicians are on the same page.
Plan for future pregnancies. Psychiatric medications can be long-term. Even patients who say they do not wish to become pregnant may someday become pregnant. Having discussions about medication choices, and their reproductive implications, prior to pregnancy allows patients to take an active role in their health.1,2
Consult a reproductive psychiatrist when indicated, and as early in the pregnancy as possible.
Psychiatrists who treat women of childbearing age should consider that those women may become pregnant, and that women with psychiatric illness are more likely to have unplanned pregnancies.1 Thus, thoughtful perinatal medication choices should begin before pregnancy. Pregnancy is a time of vulnerability to psychiatric illness for many reasons, including physiologic changes that can affect mental status; changes in medication efficacy; and numerous stressors, such as new responsibilities and limited sleep.1,2 For the treatment of pregnant—or potentially pregnant—patients, we recommend the following.
Do not panic! Knee-jerk medication changes in response to learning a patient is pregnant can lead to an exacerbation of psychiatric symptoms, as well as decrease trust in clinicians.2 Switching to a medication with a purportedly “safer” reproductive profile may worsen psychiatric illness, while also exposing the fetus to a medication of unknown benefit. 2
Recognize the risk of untreated or undertreated psychiatric illness, either of which has the potential to harm both the woman and her fetus. For example, a pregnant woman in a manic state may be more likely to engage in risky behaviors, such as drug use or risky sexual activity, which can lead to adverse fetal outcomes. They may also present with a higher risk of suicide. Compared to nonpregnant women, pregnant women for whom lithium was discontinued were equally likely to experience illness recurrence and significantly more likely to experience postpartum illness recurrence.3 In addition, the risk of recurrence was greater after rapid discontinuation compared with gradual discontinuation.3
Accurately communicate research findings. Pregnancy risk categories are no longer used. A nuanced interpretation of the potential adverse effects of a medication, such as malformations, impaired fetal growth, birth outcomes (such as preterm birth), and neurodevelopmental sequelae is necessary. Physicians must accurately convey information about risks to their patients, including both the absolute risk of an adverse event and the possible range of severity. For example, lithium use during pregnancy confers a higher relative risk of Ebstein’s anomaly (a cardiac defect).4 However, the absolute incidence of this risk remains low: 0.6% of lithium-exposed infants vs 0.18% among unexposed infants.4 Ebstein’s anomaly also varies significantly in severity—serious cases may require surgery, but less serious cases need only monitoring. A reliable database that compiles the latest evidence may help in staying abreast of the latest data.
Treat the psychiatric illness. Consider the optimal treatment for the psychiatric illness. Lithium remains the gold standard treatment for bipolar I disorder, regardless of reproductive status. Olanzapine and quetiapine are also commonly used and effective during pregnancy. This is an opportunity to conduct a detailed review of the patient’s previous medication regimens, including a review of medication trials and efficacy. Keep in mind that untreated bipolar disorder also carries an increased risk of adverse pregnancy outcomes.5
Consider pregnancy timing. Most organs form between weeks 3 to 8 of pregnancy. For example, if a medication potentially affects heart formation, but the patient is in the third trimester, explain to her that the heart has already been formed. Consider that medication may be required long-term and affect future pregnancies. Pregnant women require more frequent monitoring, because blood volume changes in pregnancy and postpartum can affect medication levels and efficacy. In addition, note whether a woman plans to breastfeed and be mindful of a medication’s profile in breastfeeding.
Ensure the patient can provide informed consent. Communicate your diagnostic formulation and treatment options. Consider involving the patient’s partner and/or support system in the discussion, if the patient consents. If a patient cannot provide informed consent, a surrogate decision-maker should be identified.6
Continue to: Collaborate with other clinicians
Collaborate with other clinicians, such as the patient’s OB/GYN and family medicine physician when possible. This will ensure that all clinicians are on the same page.
Plan for future pregnancies. Psychiatric medications can be long-term. Even patients who say they do not wish to become pregnant may someday become pregnant. Having discussions about medication choices, and their reproductive implications, prior to pregnancy allows patients to take an active role in their health.1,2
Consult a reproductive psychiatrist when indicated, and as early in the pregnancy as possible.
1. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
2. Friedman SH, Reed E. Treating psychosis in pregnant women: a measured approach. Current Psychiatry. 2021; 20(7):34-35.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157(2):179-184.
4. Patorno E, Huybrechts KF, Bateman BT, et al. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
5. Bodén R, Lundgren M, Brandt L, et al. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi:10.1136/bmj.e7085
6. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022:63(1);61-70.
1. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
2. Friedman SH, Reed E. Treating psychosis in pregnant women: a measured approach. Current Psychiatry. 2021; 20(7):34-35.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157(2):179-184.
4. Patorno E, Huybrechts KF, Bateman BT, et al. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
5. Bodén R, Lundgren M, Brandt L, et al. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi:10.1136/bmj.e7085
6. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022:63(1);61-70.
Loneliness: How psychiatry can help
Loneliness is distress that occurs when the quality or quantity of social relationships are less than desired.1 It is a symptom of many psychiatric disorders, and can lead to multiple negative health consequences, including depression, sleep deprivation, executive dysfunction, accelerated cognitive decline, and hypertension. Loneliness can increase the likelihood of immunocompromising conditions, including (but not limited to) stroke, anxiety, and depression, resulting in frequent emergency department visits and costly health expenses.2 Up to 80% of individuals younger than age 18 and 40% of adults older than age 65 report being lonely at least sometimes, with levels of loneliness gradually diminishing during middle age and then increasing in older adults.1 Loneliness is such a common and pervasive problem that in 2017, the government of the United Kingdom created a commission on loneliness and developed a Minister of Loneliness to find solutions to reduce it.3 In this article, I discuss the detrimental impact loneliness can have on our patients, and steps we can take to address it.
What contributes to loneliness?
Most people prefer the company of others, but some psychiatric disorders can cause individuals to become antisocial. For example, patients with schizoid personality disorder avoid social activities and interaction with others. Other patients may want to form bonds with others but their psychiatric disorder hinders this. For example, those with paranoia and social anxiety may avoid interacting with people due to their mistrust of others or their actions. Patients with substance use disorders can drive away those closest to them and lose familial bonds as a result of their behaviors. Patients with depression might not have the energy to pursue relationships and often have faulty cognitive patterns that lead them to believe they are unloved and unwanted.
Situational factors play a significant role in feelings of loneliness. Loss of a job or friends, ending a relationship, death of a loved one, or social isolation as experienced by COVID-19 or other illnesses can lead to loneliness. Social factors such as lack of income or transportation can make it difficult to attend or take part in social activities and events.
Some patients with dementia express feeling lonely, even after a visit from loved ones, because they forget the visit occurred. Nursing home residents often experience loneliness. Children may feel lonely after being subjected to bullying. College students, especially freshmen who are away from home for the first time, report significant levels of loneliness. Members of the LGBTQ+ community are often lonely due to familial rejection, prejudice, and religious beliefs. Anyone can experience loneliness, even married individuals if the marriage is unsatisfying.
What can psychiatry do to help?
Fortunately, psychiatric clinicians can play a large role in helping patients with loneliness.
Assessment. Ask the patient about the status of their present relationships and if they are feeling lonely. If yes, ask additional questions to identify possible causes. Are there conflicts that can be resolved? Is there abuse? What do they believe is the cause of their loneliness, and what might be the solution? How would their life be different if they weren’t lonely?
Treatment. When indicated, pharmacologic interventions might relieve symptoms that interfere with relationships and social interactions. For example, several types of antidepressants can improve mood and reduce anxiety, and selective serotonin reuptake inhibitors may relieve panic symptoms. Benzodiazepines and beta-blockers can reduce symptoms of social anxiety. Antipsychotics can reduce paranoia. Stimulants can aid patients with attention-deficit/hyperactivity disorder by improving their ability to interact with others.
Continue to: Psychotherapy and counseling...
Psychotherapy and counseling can specifically target loneliness. Solution-focused therapy, for example, involves solving the problem by deciding which actions the patient needs to take to relieve symptoms of loneliness. Dialectal behavior therapy can help patients with borderline and other personality disorders regulate their emotions and accept their feelings. Cognitive therapy and rational emotive therapy use various techniques to assist patients in changing their negative thought patterns. For example, a therapist might assign a patient to introduce themselves to a stranger or attend an event with others. The assignment is then discussed at the next session. Client-centered, psychodynamic, and behavior therapies also may be appropriate for a patient experiencing loneliness. Positive psychology can aid patients by helping them appreciate and not discount others in their lives. Meditation and mindfulness can motivate individuals to live in the present and enjoy those around them.
Referral and psychosocial support can be offered to direct patients to social services for help in improving their living circumstances. For example, a patient with an alcohol use disorder may benefit from a referral to a self-help organization such as Alcoholics Anonymous, where they can receive additional support and develop friendships. Other resources might offer patients the ability to discuss solutions, such as the benefits of owning a pet, attending a class, or volunteering opportunities, to combat loneliness.
Living a purposeful life is essential to engaging with others and avoiding isolation. Many people have turned to online support rooms, chat rooms, gaming, and social media to maintain relationships and meet others. Excessive computer use can be detrimental, however, if used in a manner that doesn’t involve interaction with others.
Regardless of the specific intervention, psychiatrists and other psychiatric clinicians can play a major role in reducing a patient’s loneliness. Simply by being present, you are showing the patient that at least one person in their life listens and cares.
1. Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40(2):218-227.
2. Pimlott N. The ministry of loneliness. Can Fam Physician. 2018;64(3):166.
3. Leach N. The health consequences of loneliness. Causes and health consequences of being lonely. 2020. Accessed March 24, 2022. https://www.awpnow.com/main/2020/02/04/the-health-consequences-of-loneliness/
Loneliness is distress that occurs when the quality or quantity of social relationships are less than desired.1 It is a symptom of many psychiatric disorders, and can lead to multiple negative health consequences, including depression, sleep deprivation, executive dysfunction, accelerated cognitive decline, and hypertension. Loneliness can increase the likelihood of immunocompromising conditions, including (but not limited to) stroke, anxiety, and depression, resulting in frequent emergency department visits and costly health expenses.2 Up to 80% of individuals younger than age 18 and 40% of adults older than age 65 report being lonely at least sometimes, with levels of loneliness gradually diminishing during middle age and then increasing in older adults.1 Loneliness is such a common and pervasive problem that in 2017, the government of the United Kingdom created a commission on loneliness and developed a Minister of Loneliness to find solutions to reduce it.3 In this article, I discuss the detrimental impact loneliness can have on our patients, and steps we can take to address it.
What contributes to loneliness?
Most people prefer the company of others, but some psychiatric disorders can cause individuals to become antisocial. For example, patients with schizoid personality disorder avoid social activities and interaction with others. Other patients may want to form bonds with others but their psychiatric disorder hinders this. For example, those with paranoia and social anxiety may avoid interacting with people due to their mistrust of others or their actions. Patients with substance use disorders can drive away those closest to them and lose familial bonds as a result of their behaviors. Patients with depression might not have the energy to pursue relationships and often have faulty cognitive patterns that lead them to believe they are unloved and unwanted.
Situational factors play a significant role in feelings of loneliness. Loss of a job or friends, ending a relationship, death of a loved one, or social isolation as experienced by COVID-19 or other illnesses can lead to loneliness. Social factors such as lack of income or transportation can make it difficult to attend or take part in social activities and events.
Some patients with dementia express feeling lonely, even after a visit from loved ones, because they forget the visit occurred. Nursing home residents often experience loneliness. Children may feel lonely after being subjected to bullying. College students, especially freshmen who are away from home for the first time, report significant levels of loneliness. Members of the LGBTQ+ community are often lonely due to familial rejection, prejudice, and religious beliefs. Anyone can experience loneliness, even married individuals if the marriage is unsatisfying.
What can psychiatry do to help?
Fortunately, psychiatric clinicians can play a large role in helping patients with loneliness.
Assessment. Ask the patient about the status of their present relationships and if they are feeling lonely. If yes, ask additional questions to identify possible causes. Are there conflicts that can be resolved? Is there abuse? What do they believe is the cause of their loneliness, and what might be the solution? How would their life be different if they weren’t lonely?
Treatment. When indicated, pharmacologic interventions might relieve symptoms that interfere with relationships and social interactions. For example, several types of antidepressants can improve mood and reduce anxiety, and selective serotonin reuptake inhibitors may relieve panic symptoms. Benzodiazepines and beta-blockers can reduce symptoms of social anxiety. Antipsychotics can reduce paranoia. Stimulants can aid patients with attention-deficit/hyperactivity disorder by improving their ability to interact with others.
Continue to: Psychotherapy and counseling...
Psychotherapy and counseling can specifically target loneliness. Solution-focused therapy, for example, involves solving the problem by deciding which actions the patient needs to take to relieve symptoms of loneliness. Dialectal behavior therapy can help patients with borderline and other personality disorders regulate their emotions and accept their feelings. Cognitive therapy and rational emotive therapy use various techniques to assist patients in changing their negative thought patterns. For example, a therapist might assign a patient to introduce themselves to a stranger or attend an event with others. The assignment is then discussed at the next session. Client-centered, psychodynamic, and behavior therapies also may be appropriate for a patient experiencing loneliness. Positive psychology can aid patients by helping them appreciate and not discount others in their lives. Meditation and mindfulness can motivate individuals to live in the present and enjoy those around them.
Referral and psychosocial support can be offered to direct patients to social services for help in improving their living circumstances. For example, a patient with an alcohol use disorder may benefit from a referral to a self-help organization such as Alcoholics Anonymous, where they can receive additional support and develop friendships. Other resources might offer patients the ability to discuss solutions, such as the benefits of owning a pet, attending a class, or volunteering opportunities, to combat loneliness.
Living a purposeful life is essential to engaging with others and avoiding isolation. Many people have turned to online support rooms, chat rooms, gaming, and social media to maintain relationships and meet others. Excessive computer use can be detrimental, however, if used in a manner that doesn’t involve interaction with others.
Regardless of the specific intervention, psychiatrists and other psychiatric clinicians can play a major role in reducing a patient’s loneliness. Simply by being present, you are showing the patient that at least one person in their life listens and cares.
Loneliness is distress that occurs when the quality or quantity of social relationships are less than desired.1 It is a symptom of many psychiatric disorders, and can lead to multiple negative health consequences, including depression, sleep deprivation, executive dysfunction, accelerated cognitive decline, and hypertension. Loneliness can increase the likelihood of immunocompromising conditions, including (but not limited to) stroke, anxiety, and depression, resulting in frequent emergency department visits and costly health expenses.2 Up to 80% of individuals younger than age 18 and 40% of adults older than age 65 report being lonely at least sometimes, with levels of loneliness gradually diminishing during middle age and then increasing in older adults.1 Loneliness is such a common and pervasive problem that in 2017, the government of the United Kingdom created a commission on loneliness and developed a Minister of Loneliness to find solutions to reduce it.3 In this article, I discuss the detrimental impact loneliness can have on our patients, and steps we can take to address it.
What contributes to loneliness?
Most people prefer the company of others, but some psychiatric disorders can cause individuals to become antisocial. For example, patients with schizoid personality disorder avoid social activities and interaction with others. Other patients may want to form bonds with others but their psychiatric disorder hinders this. For example, those with paranoia and social anxiety may avoid interacting with people due to their mistrust of others or their actions. Patients with substance use disorders can drive away those closest to them and lose familial bonds as a result of their behaviors. Patients with depression might not have the energy to pursue relationships and often have faulty cognitive patterns that lead them to believe they are unloved and unwanted.
Situational factors play a significant role in feelings of loneliness. Loss of a job or friends, ending a relationship, death of a loved one, or social isolation as experienced by COVID-19 or other illnesses can lead to loneliness. Social factors such as lack of income or transportation can make it difficult to attend or take part in social activities and events.
Some patients with dementia express feeling lonely, even after a visit from loved ones, because they forget the visit occurred. Nursing home residents often experience loneliness. Children may feel lonely after being subjected to bullying. College students, especially freshmen who are away from home for the first time, report significant levels of loneliness. Members of the LGBTQ+ community are often lonely due to familial rejection, prejudice, and religious beliefs. Anyone can experience loneliness, even married individuals if the marriage is unsatisfying.
What can psychiatry do to help?
Fortunately, psychiatric clinicians can play a large role in helping patients with loneliness.
Assessment. Ask the patient about the status of their present relationships and if they are feeling lonely. If yes, ask additional questions to identify possible causes. Are there conflicts that can be resolved? Is there abuse? What do they believe is the cause of their loneliness, and what might be the solution? How would their life be different if they weren’t lonely?
Treatment. When indicated, pharmacologic interventions might relieve symptoms that interfere with relationships and social interactions. For example, several types of antidepressants can improve mood and reduce anxiety, and selective serotonin reuptake inhibitors may relieve panic symptoms. Benzodiazepines and beta-blockers can reduce symptoms of social anxiety. Antipsychotics can reduce paranoia. Stimulants can aid patients with attention-deficit/hyperactivity disorder by improving their ability to interact with others.
Continue to: Psychotherapy and counseling...
Psychotherapy and counseling can specifically target loneliness. Solution-focused therapy, for example, involves solving the problem by deciding which actions the patient needs to take to relieve symptoms of loneliness. Dialectal behavior therapy can help patients with borderline and other personality disorders regulate their emotions and accept their feelings. Cognitive therapy and rational emotive therapy use various techniques to assist patients in changing their negative thought patterns. For example, a therapist might assign a patient to introduce themselves to a stranger or attend an event with others. The assignment is then discussed at the next session. Client-centered, psychodynamic, and behavior therapies also may be appropriate for a patient experiencing loneliness. Positive psychology can aid patients by helping them appreciate and not discount others in their lives. Meditation and mindfulness can motivate individuals to live in the present and enjoy those around them.
Referral and psychosocial support can be offered to direct patients to social services for help in improving their living circumstances. For example, a patient with an alcohol use disorder may benefit from a referral to a self-help organization such as Alcoholics Anonymous, where they can receive additional support and develop friendships. Other resources might offer patients the ability to discuss solutions, such as the benefits of owning a pet, attending a class, or volunteering opportunities, to combat loneliness.
Living a purposeful life is essential to engaging with others and avoiding isolation. Many people have turned to online support rooms, chat rooms, gaming, and social media to maintain relationships and meet others. Excessive computer use can be detrimental, however, if used in a manner that doesn’t involve interaction with others.
Regardless of the specific intervention, psychiatrists and other psychiatric clinicians can play a major role in reducing a patient’s loneliness. Simply by being present, you are showing the patient that at least one person in their life listens and cares.
1. Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40(2):218-227.
2. Pimlott N. The ministry of loneliness. Can Fam Physician. 2018;64(3):166.
3. Leach N. The health consequences of loneliness. Causes and health consequences of being lonely. 2020. Accessed March 24, 2022. https://www.awpnow.com/main/2020/02/04/the-health-consequences-of-loneliness/
1. Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40(2):218-227.
2. Pimlott N. The ministry of loneliness. Can Fam Physician. 2018;64(3):166.
3. Leach N. The health consequences of loneliness. Causes and health consequences of being lonely. 2020. Accessed March 24, 2022. https://www.awpnow.com/main/2020/02/04/the-health-consequences-of-loneliness/
A new era and a new paradox for mental health
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in Current Psychiatry. All submissions to Readers’ Forum undergo peer review and are subject to editing for length and style. For more information, contact [email protected].
As we see the end of the COVID-19 era through a collective windshield, there is hope and optimism at the exit ramps ahead of us. This unforeseen era has brought not only unprecedented change to the practice of medicine, but also a resurgent focus on the impact of medical care. The rapid adoption of telemedicine, the medical heroism lauded in the press during the early days of the pandemic, and the subsequent psychosocial impact of quarantines and lockdowns have brought increased attention to our citizens’ mental health, and not just during a crisis, but in a more holistic sense.
In fact, with the most recent annual Presidential State of the Union Address, mental health has finally received an invitation to the national agenda. This is an admirable achievement, a nod from Uncle Sam that says, “Here’s your seat at the table.” Now that we have earned this seat, have we improved our understanding of mental illness, treatment options, and our access to them? Or have we lost sight of our real challenges? Shouldn’t achieving national prominence have resulted in newfound treatments and strategies to increase access and understanding?
Instead, we are still touting the same (although perhaps nuanced) monoamine hypothesis underlying most of our conditions, as we have for decades. Vast areas of the country are out of reach of a local psychiatrist. Our treatments, largely centered on medications, though hopeful and promising at times, would fall short of the hurdle to become mainstay treatments in other medical specialties. Of course, the counterpoints are obvious: there are novel treatments (eg, ketamine, transcranial magnetic stimulation) and new understandings of glutamate and gamma aminobutyric acid systems in mood regulation and addiction. We also can use telemedicine to improve access to psychiatric care in underserved areas. But the overarching truth remains: an understanding of psychiatric illnesses, specifically the pathophysiology underlying those conditions, remains elusive or partially understood. Until we have a pathophysiology to treat, we can only continue to describe phenomenology and treat symptomatology.
Since we are treating symptoms, we must rely on verbal descriptions of psychiatric conditions. Descriptions and discussions of mental illness have pervaded the airwaves and media. It is not uncommon or unusual to hear people talk about depression, anxiety, insomnia, addiction, or even psychosis in a very normal, unjarring way. These words, which represent severe medical conditions, have now become part of the national nosology and colloquial description of individuals’ day-to-day lives. Have we stripped the severity and seriousness of our conditions from their descriptors in order to increase awareness and make mental health care a more “normal” part of health care?
We see it in clinics, the media, our schools, and our workplaces. Children and teens are talking about social anxiety because they feel a bit nervous on stage as their part in a school play begins. Teens are asking for extra time on a difficult test in a challenging class that is supposed to be strenuous. Employees are asking for mental health leave when a demanding new boss arrives on the scene.
Has our own campaign to increase awareness and destigmatize mental illness caused it to become diluted? Have we raised awareness by diluting its severity and seriousness, by making our nosology equivalent to everyday stressors? Was this a marketing strategy, a failure of our own nosology, or an inadvertent fallout of a decades-long campaign to raise mental health awareness?
Continue to: Until we have clear...
Until we have clear, delineated pathophysiology to treat, we will remain wed to our descriptive nosology. This nosology is flawed, at times ambiguous and overlapping, and now has become diluted to be more palatable to a national and consumer audience.
So yes, let’s grab a chair at the national table, but let’s make sure we’re not just chairwarmers. It’s time we redouble our focus on unraveling the pathophysiology of psychiatric illnesses, and to focus on a new scientific nosology, as opposed to our current, almost colloquial and now diluted descriptors that may raise awareness but do little to advance a real understanding of mental illness. A more holistic understanding of the pathophysiology of psychiatric disorders may provide us with a more scientific nosology. Ultimately, we can hope for more effective, and perhaps even curative treatments. That, my colleagues, is what will give us not just a seat at the table, but maybe even a table of our own.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in Current Psychiatry. All submissions to Readers’ Forum undergo peer review and are subject to editing for length and style. For more information, contact [email protected].
As we see the end of the COVID-19 era through a collective windshield, there is hope and optimism at the exit ramps ahead of us. This unforeseen era has brought not only unprecedented change to the practice of medicine, but also a resurgent focus on the impact of medical care. The rapid adoption of telemedicine, the medical heroism lauded in the press during the early days of the pandemic, and the subsequent psychosocial impact of quarantines and lockdowns have brought increased attention to our citizens’ mental health, and not just during a crisis, but in a more holistic sense.
In fact, with the most recent annual Presidential State of the Union Address, mental health has finally received an invitation to the national agenda. This is an admirable achievement, a nod from Uncle Sam that says, “Here’s your seat at the table.” Now that we have earned this seat, have we improved our understanding of mental illness, treatment options, and our access to them? Or have we lost sight of our real challenges? Shouldn’t achieving national prominence have resulted in newfound treatments and strategies to increase access and understanding?
Instead, we are still touting the same (although perhaps nuanced) monoamine hypothesis underlying most of our conditions, as we have for decades. Vast areas of the country are out of reach of a local psychiatrist. Our treatments, largely centered on medications, though hopeful and promising at times, would fall short of the hurdle to become mainstay treatments in other medical specialties. Of course, the counterpoints are obvious: there are novel treatments (eg, ketamine, transcranial magnetic stimulation) and new understandings of glutamate and gamma aminobutyric acid systems in mood regulation and addiction. We also can use telemedicine to improve access to psychiatric care in underserved areas. But the overarching truth remains: an understanding of psychiatric illnesses, specifically the pathophysiology underlying those conditions, remains elusive or partially understood. Until we have a pathophysiology to treat, we can only continue to describe phenomenology and treat symptomatology.
Since we are treating symptoms, we must rely on verbal descriptions of psychiatric conditions. Descriptions and discussions of mental illness have pervaded the airwaves and media. It is not uncommon or unusual to hear people talk about depression, anxiety, insomnia, addiction, or even psychosis in a very normal, unjarring way. These words, which represent severe medical conditions, have now become part of the national nosology and colloquial description of individuals’ day-to-day lives. Have we stripped the severity and seriousness of our conditions from their descriptors in order to increase awareness and make mental health care a more “normal” part of health care?
We see it in clinics, the media, our schools, and our workplaces. Children and teens are talking about social anxiety because they feel a bit nervous on stage as their part in a school play begins. Teens are asking for extra time on a difficult test in a challenging class that is supposed to be strenuous. Employees are asking for mental health leave when a demanding new boss arrives on the scene.
Has our own campaign to increase awareness and destigmatize mental illness caused it to become diluted? Have we raised awareness by diluting its severity and seriousness, by making our nosology equivalent to everyday stressors? Was this a marketing strategy, a failure of our own nosology, or an inadvertent fallout of a decades-long campaign to raise mental health awareness?
Continue to: Until we have clear...
Until we have clear, delineated pathophysiology to treat, we will remain wed to our descriptive nosology. This nosology is flawed, at times ambiguous and overlapping, and now has become diluted to be more palatable to a national and consumer audience.
So yes, let’s grab a chair at the national table, but let’s make sure we’re not just chairwarmers. It’s time we redouble our focus on unraveling the pathophysiology of psychiatric illnesses, and to focus on a new scientific nosology, as opposed to our current, almost colloquial and now diluted descriptors that may raise awareness but do little to advance a real understanding of mental illness. A more holistic understanding of the pathophysiology of psychiatric disorders may provide us with a more scientific nosology. Ultimately, we can hope for more effective, and perhaps even curative treatments. That, my colleagues, is what will give us not just a seat at the table, but maybe even a table of our own.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in Current Psychiatry. All submissions to Readers’ Forum undergo peer review and are subject to editing for length and style. For more information, contact [email protected].
As we see the end of the COVID-19 era through a collective windshield, there is hope and optimism at the exit ramps ahead of us. This unforeseen era has brought not only unprecedented change to the practice of medicine, but also a resurgent focus on the impact of medical care. The rapid adoption of telemedicine, the medical heroism lauded in the press during the early days of the pandemic, and the subsequent psychosocial impact of quarantines and lockdowns have brought increased attention to our citizens’ mental health, and not just during a crisis, but in a more holistic sense.
In fact, with the most recent annual Presidential State of the Union Address, mental health has finally received an invitation to the national agenda. This is an admirable achievement, a nod from Uncle Sam that says, “Here’s your seat at the table.” Now that we have earned this seat, have we improved our understanding of mental illness, treatment options, and our access to them? Or have we lost sight of our real challenges? Shouldn’t achieving national prominence have resulted in newfound treatments and strategies to increase access and understanding?
Instead, we are still touting the same (although perhaps nuanced) monoamine hypothesis underlying most of our conditions, as we have for decades. Vast areas of the country are out of reach of a local psychiatrist. Our treatments, largely centered on medications, though hopeful and promising at times, would fall short of the hurdle to become mainstay treatments in other medical specialties. Of course, the counterpoints are obvious: there are novel treatments (eg, ketamine, transcranial magnetic stimulation) and new understandings of glutamate and gamma aminobutyric acid systems in mood regulation and addiction. We also can use telemedicine to improve access to psychiatric care in underserved areas. But the overarching truth remains: an understanding of psychiatric illnesses, specifically the pathophysiology underlying those conditions, remains elusive or partially understood. Until we have a pathophysiology to treat, we can only continue to describe phenomenology and treat symptomatology.
Since we are treating symptoms, we must rely on verbal descriptions of psychiatric conditions. Descriptions and discussions of mental illness have pervaded the airwaves and media. It is not uncommon or unusual to hear people talk about depression, anxiety, insomnia, addiction, or even psychosis in a very normal, unjarring way. These words, which represent severe medical conditions, have now become part of the national nosology and colloquial description of individuals’ day-to-day lives. Have we stripped the severity and seriousness of our conditions from their descriptors in order to increase awareness and make mental health care a more “normal” part of health care?
We see it in clinics, the media, our schools, and our workplaces. Children and teens are talking about social anxiety because they feel a bit nervous on stage as their part in a school play begins. Teens are asking for extra time on a difficult test in a challenging class that is supposed to be strenuous. Employees are asking for mental health leave when a demanding new boss arrives on the scene.
Has our own campaign to increase awareness and destigmatize mental illness caused it to become diluted? Have we raised awareness by diluting its severity and seriousness, by making our nosology equivalent to everyday stressors? Was this a marketing strategy, a failure of our own nosology, or an inadvertent fallout of a decades-long campaign to raise mental health awareness?
Continue to: Until we have clear...
Until we have clear, delineated pathophysiology to treat, we will remain wed to our descriptive nosology. This nosology is flawed, at times ambiguous and overlapping, and now has become diluted to be more palatable to a national and consumer audience.
So yes, let’s grab a chair at the national table, but let’s make sure we’re not just chairwarmers. It’s time we redouble our focus on unraveling the pathophysiology of psychiatric illnesses, and to focus on a new scientific nosology, as opposed to our current, almost colloquial and now diluted descriptors that may raise awareness but do little to advance a real understanding of mental illness. A more holistic understanding of the pathophysiology of psychiatric disorders may provide us with a more scientific nosology. Ultimately, we can hope for more effective, and perhaps even curative treatments. That, my colleagues, is what will give us not just a seat at the table, but maybe even a table of our own.
Borderline personality disorder: Remember empathy and compassion
Oh, great!” a senior resident sardonically remarked with a smirk as they read up on the next patient in the clinic. “A borderline patient. Get ready for a rough one ... Ugh.”
Before ever stepping foot into the patient’s room, this resident had prematurely established and demonstrated an unfortunate dynamic for any student or trainee within earshot. This is an all-too-familiar occurrence when caring for individuals with borderline personality disorder (BPD), or any other patients deemed to be “difficult.” The patient, however, likely walked into the room with a traumatic past that they continue to suffer from, in addition to any other issues for which they were seeking care.
Consider what these patients have experienced
A typical profile of these resilient patients with BPD: They were born emotionally sensitive. They grew up in homes with caretakers who knowingly or unknowingly invalidated their complaints about having their feelings hurt, about being abused emotionally, sexually, or otherwise, or about their worries concerning their interactions with peers at school. These caretakers may have been frightening and unpredictable, randomly showing affection or arbitrarily punishing for any perceived misstep, which led these patients to develop (for their own safety’s sake) a hypersensitivity to the affect of others. Their wariness and distrust of their social surroundings may have led to a skeptical view of kindness from others. Over time, without any guidance from prior demonstrations of healthy coping skills or interpersonal outlets from their caregivers, the emotional pressure builds. This pressure finally erupts in the form of impulsivity, self-harm, desperation, and defensiveness—in other words, survival. This is often followed by these patients’ first experience with receiving some degree of appropriate response to their complaints—their first experience with feeling seen and heard by their caretakers. They learn that their needs are met only when they cry out in desperation.1-3
These patients typically bring these maladaptive coping skills with them into adulthood, which often leads to a series of intense, unhealthy, and short-lived interpersonal and professional connections. They desire healthy, lasting connections with others, but through no fault of their own are unable to appropriately manage the normal stressors therein.1 Often, these patients do not know of their eventual BPD diagnosis, or even reject it due to its ever-negative valence. For other patients, receiving a personality disorder diagnosis is incredibly validating because they are no longer alone regarding this type of suffering, and a doctor—a caretaker—is finally making sense of this tumultuous world.
The countertransference of frustration, anxiety, doubt, and annoyance we may feel when caring for patients with BPD pales in comparison to living in their shoes and carrying the weight of what they have had to endure before presenting to our care. As these resilient patients wait in the exam room for the chance to be heard, let this be a reminder to greet them with the patience, understanding, empathy, and compassion that physicians are known to embody.
Suggestions for working with ‘difficult’ patients
The following tips may be helpful for building rapport with patients with BPD or other “difficult” patients:
- validate their complaints, and the difficulties they cause
- be genuine and honest when discussing their complaints
- acknowledge your own mistakes and misunderstandings in their care
- don’t be defensive—accept criticism with an open mind
- practice listening with intent, and reflective listening
- set ground rules and stick to them (eg, time limits, prescribing expectations, patient-physician relationship boundaries)
- educate and support the patient and their loved ones.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:947.
2. Porter C, Palmier-Claus J, Branitsky A, et al. Childhood adversity and borderline personality disorder: a meta-analysis. Acta Psychiatr Scand. 2020;141(1):6-20.
3. Sansone RA, Sansone LA. Emotional hyper-reactivity in borderline personality disorder. Psychiatry (Edgmont). 2010;7(9):16-20.
Oh, great!” a senior resident sardonically remarked with a smirk as they read up on the next patient in the clinic. “A borderline patient. Get ready for a rough one ... Ugh.”
Before ever stepping foot into the patient’s room, this resident had prematurely established and demonstrated an unfortunate dynamic for any student or trainee within earshot. This is an all-too-familiar occurrence when caring for individuals with borderline personality disorder (BPD), or any other patients deemed to be “difficult.” The patient, however, likely walked into the room with a traumatic past that they continue to suffer from, in addition to any other issues for which they were seeking care.
Consider what these patients have experienced
A typical profile of these resilient patients with BPD: They were born emotionally sensitive. They grew up in homes with caretakers who knowingly or unknowingly invalidated their complaints about having their feelings hurt, about being abused emotionally, sexually, or otherwise, or about their worries concerning their interactions with peers at school. These caretakers may have been frightening and unpredictable, randomly showing affection or arbitrarily punishing for any perceived misstep, which led these patients to develop (for their own safety’s sake) a hypersensitivity to the affect of others. Their wariness and distrust of their social surroundings may have led to a skeptical view of kindness from others. Over time, without any guidance from prior demonstrations of healthy coping skills or interpersonal outlets from their caregivers, the emotional pressure builds. This pressure finally erupts in the form of impulsivity, self-harm, desperation, and defensiveness—in other words, survival. This is often followed by these patients’ first experience with receiving some degree of appropriate response to their complaints—their first experience with feeling seen and heard by their caretakers. They learn that their needs are met only when they cry out in desperation.1-3
These patients typically bring these maladaptive coping skills with them into adulthood, which often leads to a series of intense, unhealthy, and short-lived interpersonal and professional connections. They desire healthy, lasting connections with others, but through no fault of their own are unable to appropriately manage the normal stressors therein.1 Often, these patients do not know of their eventual BPD diagnosis, or even reject it due to its ever-negative valence. For other patients, receiving a personality disorder diagnosis is incredibly validating because they are no longer alone regarding this type of suffering, and a doctor—a caretaker—is finally making sense of this tumultuous world.
The countertransference of frustration, anxiety, doubt, and annoyance we may feel when caring for patients with BPD pales in comparison to living in their shoes and carrying the weight of what they have had to endure before presenting to our care. As these resilient patients wait in the exam room for the chance to be heard, let this be a reminder to greet them with the patience, understanding, empathy, and compassion that physicians are known to embody.
Suggestions for working with ‘difficult’ patients
The following tips may be helpful for building rapport with patients with BPD or other “difficult” patients:
- validate their complaints, and the difficulties they cause
- be genuine and honest when discussing their complaints
- acknowledge your own mistakes and misunderstandings in their care
- don’t be defensive—accept criticism with an open mind
- practice listening with intent, and reflective listening
- set ground rules and stick to them (eg, time limits, prescribing expectations, patient-physician relationship boundaries)
- educate and support the patient and their loved ones.
Oh, great!” a senior resident sardonically remarked with a smirk as they read up on the next patient in the clinic. “A borderline patient. Get ready for a rough one ... Ugh.”
Before ever stepping foot into the patient’s room, this resident had prematurely established and demonstrated an unfortunate dynamic for any student or trainee within earshot. This is an all-too-familiar occurrence when caring for individuals with borderline personality disorder (BPD), or any other patients deemed to be “difficult.” The patient, however, likely walked into the room with a traumatic past that they continue to suffer from, in addition to any other issues for which they were seeking care.
Consider what these patients have experienced
A typical profile of these resilient patients with BPD: They were born emotionally sensitive. They grew up in homes with caretakers who knowingly or unknowingly invalidated their complaints about having their feelings hurt, about being abused emotionally, sexually, or otherwise, or about their worries concerning their interactions with peers at school. These caretakers may have been frightening and unpredictable, randomly showing affection or arbitrarily punishing for any perceived misstep, which led these patients to develop (for their own safety’s sake) a hypersensitivity to the affect of others. Their wariness and distrust of their social surroundings may have led to a skeptical view of kindness from others. Over time, without any guidance from prior demonstrations of healthy coping skills or interpersonal outlets from their caregivers, the emotional pressure builds. This pressure finally erupts in the form of impulsivity, self-harm, desperation, and defensiveness—in other words, survival. This is often followed by these patients’ first experience with receiving some degree of appropriate response to their complaints—their first experience with feeling seen and heard by their caretakers. They learn that their needs are met only when they cry out in desperation.1-3
These patients typically bring these maladaptive coping skills with them into adulthood, which often leads to a series of intense, unhealthy, and short-lived interpersonal and professional connections. They desire healthy, lasting connections with others, but through no fault of their own are unable to appropriately manage the normal stressors therein.1 Often, these patients do not know of their eventual BPD diagnosis, or even reject it due to its ever-negative valence. For other patients, receiving a personality disorder diagnosis is incredibly validating because they are no longer alone regarding this type of suffering, and a doctor—a caretaker—is finally making sense of this tumultuous world.
The countertransference of frustration, anxiety, doubt, and annoyance we may feel when caring for patients with BPD pales in comparison to living in their shoes and carrying the weight of what they have had to endure before presenting to our care. As these resilient patients wait in the exam room for the chance to be heard, let this be a reminder to greet them with the patience, understanding, empathy, and compassion that physicians are known to embody.
Suggestions for working with ‘difficult’ patients
The following tips may be helpful for building rapport with patients with BPD or other “difficult” patients:
- validate their complaints, and the difficulties they cause
- be genuine and honest when discussing their complaints
- acknowledge your own mistakes and misunderstandings in their care
- don’t be defensive—accept criticism with an open mind
- practice listening with intent, and reflective listening
- set ground rules and stick to them (eg, time limits, prescribing expectations, patient-physician relationship boundaries)
- educate and support the patient and their loved ones.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:947.
2. Porter C, Palmier-Claus J, Branitsky A, et al. Childhood adversity and borderline personality disorder: a meta-analysis. Acta Psychiatr Scand. 2020;141(1):6-20.
3. Sansone RA, Sansone LA. Emotional hyper-reactivity in borderline personality disorder. Psychiatry (Edgmont). 2010;7(9):16-20.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:947.
2. Porter C, Palmier-Claus J, Branitsky A, et al. Childhood adversity and borderline personality disorder: a meta-analysis. Acta Psychiatr Scand. 2020;141(1):6-20.
3. Sansone RA, Sansone LA. Emotional hyper-reactivity in borderline personality disorder. Psychiatry (Edgmont). 2010;7(9):16-20.
Neurotransmitter-based diagnosis and treatment: A hypothesis (Part 1)
It is unfortunate that, in some clinical areas, medical conditions are still treated by name and not based on the underlying pathological process. It would be odd in 2022 to treat “dropsy” instead of heart or kidney disease (2 very different causes of edema). Similarly, if the FDA had been approving drugs 150 years ago, we would have medications on label for “dementia praecox,” not schizophrenia or Alzheimer disease. With the help of DSM-5, psychiatry still resides in the descriptive symptomatic world of disorders.
In the United States, thanks to Freud, psychiatric symptoms became separated from medical symptoms, which made it more difficult to associate psychiatric manifestations with the underlying pathophysiology. Though the physical manifestations that parallel emotional symptoms—such as the dry mouth of anxiety, the tremor and leg weakness of fear, the constipation and blurry vision of depression, the breathing difficulty of anger, the abdominal pain of stress, the blushing of shyness, the palpitations of flashbacks, and endless others—are well known, the present classification of psychiatric disorders is blind to it. Neurochemical causes of gastrointestinal spasm or muscle tension are better researched than underlying central neurochemistry, though the same neurotransmitters drive them.
Can the biochemistry of psychiatric symptoms be judged on the basis of peripheral symptoms? Can the mental manifestations be connected to biological causation, and vice versa? Would psychiatrists be better off selecting treatments by recognizing involved neurotransmitters instead of addressing descriptive “depression, anxiety, and psychosis”? Each of these clinical syndromes may be caused by entirely different underlying neuronal mechanisms. Such mechanisms could be suggested if medical symptoms (which are measurable and objective) would become part of the psychiatric diagnosis. Is treating the “cough” sufficient, or would recognition that tuberculosis caused the cough guide better treatment? Is it time to abandon descriptive conditions and replace them with a specific “mechanism-based” viewpoint?
Ample research has shown that serotonin, dopamine, norepinephrine, endorphins, glutamate, and gamma aminobutyric acid (GABA) are the neurotransmitters most responsible in the process of both psychiatric disorders and chronic pain. These neurotransmitters are involved in much more than emotions (including the feeling of pain). An abundance of medical symptom clusters point toward which neurotransmitter dysfunction may be leading in specific cases of distinct types of depression, psychosis, anxiety, or “chronic pain.” Even presently, there are medications available (both for FDA-approved indications and off-label) that can be used to regulate these neurotransmitters, allowing practitioners to target the possible biological underlining of psychiatric or pain pathology. Hopefully, in the not-so-distant future, there will be specific medications for serotonin, dopamine, and noradrenergic depression as well as for GABAergic anxiety, endorphin psychosis, noradrenergic insomnia, and similar conditions.
Numerous neurotransmitters may be connected to both depression and pain in all their forms. These include (but are not limited to) prostaglandins, bradykinins, substance P, potassium, magnesium, calcium, histamine, adenosine triphosphate, calcitonin gene-related peptide (CGRP), nitric oxide (NO), cholecystokinin 7 (CCK7), neurotrophic growth factor (NGF), neurotensin, acetylcholine (Ach), oxytocin, cannabinoids, and others. These have not been researched sufficiently to identify their clinical presentation of excessive or insufficient availability at the sites of neurotransmission. It is difficult to draw conclusions about what kind of clinical symptoms they may cause (outside of pain), and therefore, they are not addressed in this article.
Both high and low levels of certain neurotransmitters may be associated with psychiatric conditions and chronic pain. Too much is as bad as too little.1 This applies to both quantity of neurotransmitters as well as quality of the corresponding receptor activity. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation. Reading indirect signs of bodily functions is a basic clinical skill that should not be forgotten, even in the time of advanced technology.
A different way of viewing psychiatric disorders
In this article, we present 4 hypothetical clinical cases to emphasize a possible way of analyzing symptoms to identify underlying pathology and guide more effective treatment. In no way do these descriptions reflect the entire set of symptoms caused by neurotransmitters; we created them based on what is presently known or suspected, and extensive research is required to confirm or disprove what we describe here.
Continue to: There are no well-recognized...
There are no well-recognized, well-established, reliable, or validated syndromes described in our work. Our goal is to suggest an alternative way of looking at psychiatric disorders by viewing syndromal presentation through the lens of specific neurotransmitters. The collection of symptoms associated with various neurotransmitters as presented in this hypothesis is not complete. We have assembled what is described in the literature as a suggestion for specific future research. We simplified these clinical presentations by omitting scenarios in which a specific neurotransmitter increases in one area but not another. For example, all the symptoms of dopamine excess we describe would not have to occur concurrently in the same patient, but they may develop in certain patients depending on which dopaminergic pathway is exhibiting excess activity. Such distinctions may be established only by exhaustive research not yet conducted.
Our proposal may seem radical, but it truly is not. For example, if we know that dopamine excess may cause seizures, psychosis, and blood pressure elevation, why not consider dopamine excess as an underlying cause in a patient with depression who exhibits these symptoms simultaneously? And why not call it “dopamine excess syndrome”? We already have “serotonin syndrome” for a patient experiencing a serotonin storm. However, using the same logic, it should be called “serotonin excess syndrome.” And if we know of “serotonin excess syndrome,” why not consider “serotonin deficiency syndrome”?
In Part 1 of this article, we discuss serotonin and dopamine. Table 1 outlines medical and psychiatric symptoms that likely reflect serotonin excess2-18 and deficiency,14,19-29 and Table 2 lists symptoms that likely reflect dopamine excess14,30-41 and deficiency.4,14,20,38,40-43 In Part 2 we will touch on endorphins and norepinephrine, and in Part 3 we will conclude by looking at GABA and glutamate.
Serotonin excess (Table 12-18)
On a recent office visit, Ms. H reports that most of the time she does not feel much of anything, but she still experiences panic attacks8,9,13,15 and is easily agitated.6,8 Her mother died recently, and Ms. H is concerned that she did not grieve.15-18 She failed her last semester in college and was indifferent to her failure.18 She sleeps poorly,8 is failing her creative classes, and wonders why she has lost her artistic inclination.16-18 Ms. H has difficulty with amotivation, planning, social interactions, and speech.16,17 All of those symptoms worsened after she was prescribed fluoxetine approximately 1 year ago for her “blues.” Ms. H is obese and continues to gain weight,2 though she frequently has diarrhea,3,4,7,8 loud peristalsis, and abdominal cramps.4,7,8 She sweats easily6-8 and her heart frequently races.8,9 Additionally, Ms. H’s primary care physician told her that she has “borderline diabetes.”2 She is prone to frequent bruising11 and is easy to shake, even when she is experiencing minimal anxiety.6-9 Ms. H had consulted with a neurologist because of unusual electrical “zapping” in her brain and muscle twitches.5,8,9,13 She had experienced a seizure as a child, but this was possibly related to hypernatremia,2 and she has not taken any anticonvulsant medication for several years.8 She exhibits hyperactive deep tendon reflexes and tremors5,7,9 and blinks frequently.6,9 She experiences hot flashes,3,6-8,14 does not tolerate heat, and prefers cooler weather.8,9 Her pains and aches,12,14 to which she has been prone all of her life, have recently become much worse, and she was diagnosed with fibromyalgia in part because she frequently feels stiff all over.10 She complains of strange tingling and prickling sensations in her hands and feet, especially when anxious.7,9,10 Her headaches also worsened and may be precipitated by bright light, as her pupils are usually dilated.5,7,9 Her hypertension is fairly controlled with medication.6,8-10 Ms. H says she experienced a psychotic episode when she was in her mid-teens,6,8 but reassures you that “she is not that bad now,” although she remains hypervigilant.13 Also while in her teens, Ms. H was treated with paroxetine and experienced restlessness, agitation, delirium, tachycardia, fluctuating blood pressure, diaphoresis, diarrhea, and neuromuscular excitation, which prompted discontinuation of the antidepressant.5-7,9,10
Impression. Ms. H exhibits symptoms associated with serotonin hyperactivity. Discontinuing and avoiding selective serotonin reuptake inhibitors (SSRIs) would be prudent; prescribing an anticonvulsant would be reasonable. Using a GABAergic medication to suppress serotonin (eg, baclofen) is likely to help. Avoiding dopaminergic medications is a must. Antidepressive antipsychotics would be logical to use. The use of serotonin-suppressing medications may be considered. One may argue for the use of beta-blockers in such a patient.
Continue to: Serotonin deficiency
Serotonin deficiency (Table 114,19-29)
Mr. A is chronically depressed, hopeless,19 and easily angered.21 He does not believe anyone can help him.19 You are concerned for his safety because he had attempted to end his life by shooting himself in the chest.19,21,22,25 Even when he’s not particularly depressed, Mr. A does not enjoy much of anything.21,26,27 He becomes particularly agitated when he drinks alcohol,25 which unfortunately is common for him.29 He engages in binge eating to feel better; he knows this is not healthy but he cannot control his behavior.20,29 Mr. A is poorly compliant with his medications, even with a blood thinner, which he was prescribed due to an episode of deep vein thrombosis. He complains of chronic daily headaches and episodic migraines.23,24 He rarely blinks,23,28 his skin is dry and cool, his hair is brittle,23 his mouth is dry,14,23,27 and he constantly licks his chapped lips.14,26,27 Mr. A frequently has general body pain26,31 but is dismissive of his body aches and completely stops reporting pain when his depression gets particularly severe. When depressed, he is slow in movement and thinking.14,21,26,27 He is more concerned with anxiety than depression.21 Mr. A is plagued by constipation, abdominal pain, muscle tension, and episodes of shaking.14,26,27 He also frequently complains about chronic tinnitus.28
Impression. Mr. A shows symptoms associated with serotonin hypoactivity. SSRIs and any other antidepressants with serotonin activity would be an obvious choice for treatment. A mood-stabilizing antipsychotic with serotonin activity would be welcome in treatment. Thyroid hormone supplementation may be of value, especially if thyroid stimulating hormone level is high. Light therapy, a diet with food that contains tryptophan, psychotherapy, and exercise are desirable. Avoiding benzodiazepines would be a good idea.
Dopamine excess (Table 214,30-41)
Ms. L presents with complaints of “fibromyalgia” and “daily headaches,”14 and also dissociation (finding herself in places when she does not know how she got there) and “out-of-body experiences.”32 She is odd, and states that people do not understand her and that she is “different.”38 Her friend, who is present at the appointment, elaborates on Ms. L’s bizarreness and oddness in behavior, out-of-context emotions, suspiciousness, paranoia, and possible hallucinations.35,36,38 Ms. L discloses frequent diffuse body pains, headaches, nausea, excessive salivation, and tongue burning, as well as muscle twitching.14 Sex worsens her headaches and body pain. She reports seizures that are not registered on EEG. In the office, she is suspicious, exhibits odd posturing, tends to misinterpret your words, and makes you feel uncomfortable. Anxiety38 and multiple obsessive-compulsive symptoms, especially excessive cleaning and grooming, complicate Ms. L’s life.31,32,34 On examination, she is hypertensive, and she has scars caused by self-cutting and skin picking on her arms.30-32 An electrocardiogram shows an elevated heart rate, widened QRS complex, and ectopic heartbeats.14 Ms. L has experienced trichotillomania since adolescence32-34 and her fingernails are bitten to the skin.34 She has difficulty with impulse control, and thrill-seeking is a prominent part of her life, mainly via gambling, compulsive sex, and compulsive buying.35,36 She also says she experiences indigestion and delayed gastric emptying.37
Impression. Ms. L exhibits multiple symptoms associated with dopamine excess. Dopamine antagonists should be considered and may help not only with her psychiatric symptoms but also with her pain symptoms. Bupropion (as a dopamine agonist), caffeine, and stimulants should be avoided.
Excessive dopamine is, in extreme cases, associated with somatic psychosis, somatic symptom disorder, factitious disorder, pain disorder, and hypochondria.39 It may come with odd and bizarre/peculiar symptoms out of proportion with objectively identified pathology. These symptoms are common in chronic pain and headache patients, and need to be addressed by appropriate use of dopamine antagonizing medications.39
Continue to: Dopamine deficiency
Dopamine deficiency (Table 24,14,20,38,40-43)
Mr. W experiences widespread pain, including chronic back pain, headaches, and abdominal pain. He also has substantial anhedonia, lack of interest, procrastination, and hypersomnia.41,42 He is apathetic and has difficulty getting up in the morning.41,42 Unusual tiredness and weakness drive him to overuse caffeine; he states that 5 Mountain Dews and 4 cups of regular coffee a day make his headaches bearable.38,41-43 Sex also improves his headaches. Since childhood, he has taken stimulants for attention-deficit/hyperactivity disorder. He reports that occasional use of cocaine helps ease his pain and depression. Mr. W’s wife is concerned with her husband’s low sexual drive and alcohol consumption, and discloses that he has periodic trouble with gambling. Mr. W was forced into psychotherapy but never was able to work productively with his therapist.38,41-43 He loves eating and cannot control his weight.40 This contrasts with episodic anorexia he experienced when he was younger.20 His face is usually emotionless.43 Mr. W is prone to constipation.14 His restless leg syndrome and periodic limb movement disorder are so bad that his wife refuses to share a bed with him.14 He is clumsy and has a problem with repetitive motor tasks.43 A paucity of speech, limited eye contact, poor grooming, and difficulty forming therapeutic alliances have long been part of Mr. W’s history.38,42,43 On physical examination, he has a dry mouth; he is stiff, tremulous, and hypotensive.14
Impression. Mr. W shows multiple symptoms associated with dopamine deficiency. Bupropion may be reasonable to consider. Dopamine augmentation via the use of stimulants is warranted in such patients, especially if stimulants had not been tried before (lisdexamfetamine would be a good choice to minimize addictive potential). For a patient with dopamine deficiency, levodopa may improve more than just restless legs. Amantadine may improve dopaminergic signaling through the accelerated dopamine release and decrease in presynaptic uptake, so this medication may be carefully tried.44 Pain treatment would not be successful for Mr. W without simultaneous treatment for his substance use.
Bottom Line
Both high and low levels of serotonin and dopamine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: How to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Amantadine • Gocovri
Baclofen • Ozobax
Bupropion • Wellbutrin
Fluoxetine • Prozac
Lisdexamfetamine • Vyvanse
Paroxetine • Paxil
1. Stahl SM. Dazzled by the dominions of dopamine: clinical roles of D3, D2, and D1 receptors. CNS Spectr. 2017;22(4):305-311.
2. Young RL, Lumsden AL, Martin AM, et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018;42(11):1880-1889.
3. Ahlman H. Serotonin and carcinoid tumors. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S79-S85.
4. Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319-342.
5. Prakash S, Belani P, Trivedi A. Headache as a presenting feature in patients with serotonin syndrome: a case series. Cephalalgia. 2014;34(2):148-153.
6. van Ewijk CE, Jacobs GE, Girbes ARJ. Unsuspected serotonin toxicity in the ICU. Ann Intensive Care. 2016;6(1):85.
7. Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care. 2014;21(1):108-113.
8. Nguyen H, Pan A, Smollin C, et al. An 11-year retrospective review of cyproheptadine use in serotonin syndrome cases reported to the California Poison Control System. J Clin Pharm Ther. 2019;44(2):327-334.
9. Ansari H, Kouti L. Drug interaction and serotonin toxicity with opioid use: another reason to avoid opioids in headache and migraine treatment. Curr Pain Headache Rep. 2016;20(8):50.
10. Ott M, Mannchen JK, Jamshidi F, et al. Management of severe arterial hypertension associated with serotonin syndrome: a case report analysis based on systematic review techniques. Ther Adv Psychopharmacol. 2019;9:2045125318818814. doi:10.1177/2045125318818814
11. Cerrito F, Lazzaro MP, Gaudio E, et al. 5HT2-receptors and serotonin release: their role in human platelet aggregation. Life Sci. 1993;53(3):209-215.
12. Ohayon MM. Pain sensitivity, depression, and sleep deprivation: links with serotoninergic dysfunction. J Psychiatr Res. 2009;43(16):1243-1245.
13. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31(1):1-11.
14. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:120,199,201-204,730-740.
15. Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child Adolesc Psychopharmacol. 2001;11(2):181-186.
16. George MS, Trimble MR. A fluvoxamine-induced frontal lobe syndrome in a patient with comorbid Gilles de la Tourette’s syndrome and obsessive compulsive disorder. J Clin Psychiatry. 1992;53(10):379-380.
17. Hoehn-Saric R, Harris GJ, Pearlson GD, et al. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry. 1991;52(3):131-133.
18. Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol. 1990;10(5):343-345.
19. Samuelsson M, Jokinen J, Nordström AL, et al. CSF 5-HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatr Scand. 2006;113(1):44-47.
20. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
21. Mann JJ, Oquendo M, Underwood MD, et al. The neurobiology of suicide risk: a review for the clinician. J Clin Psychiatry. 1999;60 Suppl 2:7-116.
22. Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41(2):162-171.
23. Joseph R, Welch KM, D’Andrea G. Serotonergic hypofunction in migraine: a synthesis of evidence based on platelet dense body dysfunction. Cephalalgia. 1989;9(4):293-299.
24. Pakalnis A, Splaingard M, Splaingard D, et al. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49(10):1486-1492.
25. Virkkunen M, Goldman D, Nielsen DA, et al. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20(4):271-275.
26. Liu Y, Zhao J, Fan X, et al. Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Front Psychiatry. 2019;10:286.
27. Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179-1187.
28. O’Malley PG, Jackson JL, Santoro J, et al. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract. 1999;48(12):980-990.
29. Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42(2):147-151.
30. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
31. Graybiel AM, Saka E. A genetic basis for obsessive grooming. Neuron. 2002;33(1):1-2.
32. Tse W, Hälbig TD. Skin picking in Parkinson’s disease: a behavioral side-effect of dopaminergic treatment? Psychiatry Clin Neurosci. 2010;64(2):214.
33. Ayaydın H. Probable emergence of symptoms of trichotillomania by atomoxetine: a case report. Psychiatry and Clinical Psychopharmacology. 2019;29(2)220-222.
34. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702. doi:10.1155/2016/9782702
35. Clark CA, Dagher A. The role of dopamine in risk taking: a specific look at Parkinson’s disease and gambling. Front Behav Neurosci. 2014;8:196.
36. Norbury A, Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79-93.
37. Chen TS, Chang FY. Elevated serum dopamine increases while coffee consumption decreases the occurrence of reddish streaks in the intact stomach. J Gastroenterol Hepatol. 2013;28(12):1810-1814.
38. Wong-Riley MT. Neuroscience Secrets. 1st edition. Spanish version. Hanley & Belfus; 1999:420-429.
39. Arbuck DM. Antipsychotics, dopamine, and pain. Current Psychiatry. 2020;19(1):25-29,31.
40. Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97(1):25-33.
41. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1-46.
42. Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506.
43. Gepshtein S, Li X, Snider J, et al. Dopamine function and the efficiency of human movement. J Cogn Neurosci. 2014;26(3):645-657.
44. Scarff JR. The ABCDs of treating tardive dyskinesia. Current Psychiatry. 2020;19(4):21,55.
It is unfortunate that, in some clinical areas, medical conditions are still treated by name and not based on the underlying pathological process. It would be odd in 2022 to treat “dropsy” instead of heart or kidney disease (2 very different causes of edema). Similarly, if the FDA had been approving drugs 150 years ago, we would have medications on label for “dementia praecox,” not schizophrenia or Alzheimer disease. With the help of DSM-5, psychiatry still resides in the descriptive symptomatic world of disorders.
In the United States, thanks to Freud, psychiatric symptoms became separated from medical symptoms, which made it more difficult to associate psychiatric manifestations with the underlying pathophysiology. Though the physical manifestations that parallel emotional symptoms—such as the dry mouth of anxiety, the tremor and leg weakness of fear, the constipation and blurry vision of depression, the breathing difficulty of anger, the abdominal pain of stress, the blushing of shyness, the palpitations of flashbacks, and endless others—are well known, the present classification of psychiatric disorders is blind to it. Neurochemical causes of gastrointestinal spasm or muscle tension are better researched than underlying central neurochemistry, though the same neurotransmitters drive them.
Can the biochemistry of psychiatric symptoms be judged on the basis of peripheral symptoms? Can the mental manifestations be connected to biological causation, and vice versa? Would psychiatrists be better off selecting treatments by recognizing involved neurotransmitters instead of addressing descriptive “depression, anxiety, and psychosis”? Each of these clinical syndromes may be caused by entirely different underlying neuronal mechanisms. Such mechanisms could be suggested if medical symptoms (which are measurable and objective) would become part of the psychiatric diagnosis. Is treating the “cough” sufficient, or would recognition that tuberculosis caused the cough guide better treatment? Is it time to abandon descriptive conditions and replace them with a specific “mechanism-based” viewpoint?
Ample research has shown that serotonin, dopamine, norepinephrine, endorphins, glutamate, and gamma aminobutyric acid (GABA) are the neurotransmitters most responsible in the process of both psychiatric disorders and chronic pain. These neurotransmitters are involved in much more than emotions (including the feeling of pain). An abundance of medical symptom clusters point toward which neurotransmitter dysfunction may be leading in specific cases of distinct types of depression, psychosis, anxiety, or “chronic pain.” Even presently, there are medications available (both for FDA-approved indications and off-label) that can be used to regulate these neurotransmitters, allowing practitioners to target the possible biological underlining of psychiatric or pain pathology. Hopefully, in the not-so-distant future, there will be specific medications for serotonin, dopamine, and noradrenergic depression as well as for GABAergic anxiety, endorphin psychosis, noradrenergic insomnia, and similar conditions.
Numerous neurotransmitters may be connected to both depression and pain in all their forms. These include (but are not limited to) prostaglandins, bradykinins, substance P, potassium, magnesium, calcium, histamine, adenosine triphosphate, calcitonin gene-related peptide (CGRP), nitric oxide (NO), cholecystokinin 7 (CCK7), neurotrophic growth factor (NGF), neurotensin, acetylcholine (Ach), oxytocin, cannabinoids, and others. These have not been researched sufficiently to identify their clinical presentation of excessive or insufficient availability at the sites of neurotransmission. It is difficult to draw conclusions about what kind of clinical symptoms they may cause (outside of pain), and therefore, they are not addressed in this article.
Both high and low levels of certain neurotransmitters may be associated with psychiatric conditions and chronic pain. Too much is as bad as too little.1 This applies to both quantity of neurotransmitters as well as quality of the corresponding receptor activity. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation. Reading indirect signs of bodily functions is a basic clinical skill that should not be forgotten, even in the time of advanced technology.
A different way of viewing psychiatric disorders
In this article, we present 4 hypothetical clinical cases to emphasize a possible way of analyzing symptoms to identify underlying pathology and guide more effective treatment. In no way do these descriptions reflect the entire set of symptoms caused by neurotransmitters; we created them based on what is presently known or suspected, and extensive research is required to confirm or disprove what we describe here.
Continue to: There are no well-recognized...
There are no well-recognized, well-established, reliable, or validated syndromes described in our work. Our goal is to suggest an alternative way of looking at psychiatric disorders by viewing syndromal presentation through the lens of specific neurotransmitters. The collection of symptoms associated with various neurotransmitters as presented in this hypothesis is not complete. We have assembled what is described in the literature as a suggestion for specific future research. We simplified these clinical presentations by omitting scenarios in which a specific neurotransmitter increases in one area but not another. For example, all the symptoms of dopamine excess we describe would not have to occur concurrently in the same patient, but they may develop in certain patients depending on which dopaminergic pathway is exhibiting excess activity. Such distinctions may be established only by exhaustive research not yet conducted.
Our proposal may seem radical, but it truly is not. For example, if we know that dopamine excess may cause seizures, psychosis, and blood pressure elevation, why not consider dopamine excess as an underlying cause in a patient with depression who exhibits these symptoms simultaneously? And why not call it “dopamine excess syndrome”? We already have “serotonin syndrome” for a patient experiencing a serotonin storm. However, using the same logic, it should be called “serotonin excess syndrome.” And if we know of “serotonin excess syndrome,” why not consider “serotonin deficiency syndrome”?

In Part 1 of this article, we discuss serotonin and dopamine. Table 1 outlines medical and psychiatric symptoms that likely reflect serotonin excess2-18 and deficiency,14,19-29 and Table 2 lists symptoms that likely reflect dopamine excess14,30-41 and deficiency.4,14,20,38,40-43 In Part 2 we will touch on endorphins and norepinephrine, and in Part 3 we will conclude by looking at GABA and glutamate.

Serotonin excess (Table 12-18)
On a recent office visit, Ms. H reports that most of the time she does not feel much of anything, but she still experiences panic attacks8,9,13,15 and is easily agitated.6,8 Her mother died recently, and Ms. H is concerned that she did not grieve.15-18 She failed her last semester in college and was indifferent to her failure.18 She sleeps poorly,8 is failing her creative classes, and wonders why she has lost her artistic inclination.16-18 Ms. H has difficulty with amotivation, planning, social interactions, and speech.16,17 All of those symptoms worsened after she was prescribed fluoxetine approximately 1 year ago for her “blues.” Ms. H is obese and continues to gain weight,2 though she frequently has diarrhea,3,4,7,8 loud peristalsis, and abdominal cramps.4,7,8 She sweats easily6-8 and her heart frequently races.8,9 Additionally, Ms. H’s primary care physician told her that she has “borderline diabetes.”2 She is prone to frequent bruising11 and is easy to shake, even when she is experiencing minimal anxiety.6-9 Ms. H had consulted with a neurologist because of unusual electrical “zapping” in her brain and muscle twitches.5,8,9,13 She had experienced a seizure as a child, but this was possibly related to hypernatremia,2 and she has not taken any anticonvulsant medication for several years.8 She exhibits hyperactive deep tendon reflexes and tremors5,7,9 and blinks frequently.6,9 She experiences hot flashes,3,6-8,14 does not tolerate heat, and prefers cooler weather.8,9 Her pains and aches,12,14 to which she has been prone all of her life, have recently become much worse, and she was diagnosed with fibromyalgia in part because she frequently feels stiff all over.10 She complains of strange tingling and prickling sensations in her hands and feet, especially when anxious.7,9,10 Her headaches also worsened and may be precipitated by bright light, as her pupils are usually dilated.5,7,9 Her hypertension is fairly controlled with medication.6,8-10 Ms. H says she experienced a psychotic episode when she was in her mid-teens,6,8 but reassures you that “she is not that bad now,” although she remains hypervigilant.13 Also while in her teens, Ms. H was treated with paroxetine and experienced restlessness, agitation, delirium, tachycardia, fluctuating blood pressure, diaphoresis, diarrhea, and neuromuscular excitation, which prompted discontinuation of the antidepressant.5-7,9,10
Impression. Ms. H exhibits symptoms associated with serotonin hyperactivity. Discontinuing and avoiding selective serotonin reuptake inhibitors (SSRIs) would be prudent; prescribing an anticonvulsant would be reasonable. Using a GABAergic medication to suppress serotonin (eg, baclofen) is likely to help. Avoiding dopaminergic medications is a must. Antidepressive antipsychotics would be logical to use. The use of serotonin-suppressing medications may be considered. One may argue for the use of beta-blockers in such a patient.
Continue to: Serotonin deficiency
Serotonin deficiency (Table 114,19-29)
Mr. A is chronically depressed, hopeless,19 and easily angered.21 He does not believe anyone can help him.19 You are concerned for his safety because he had attempted to end his life by shooting himself in the chest.19,21,22,25 Even when he’s not particularly depressed, Mr. A does not enjoy much of anything.21,26,27 He becomes particularly agitated when he drinks alcohol,25 which unfortunately is common for him.29 He engages in binge eating to feel better; he knows this is not healthy but he cannot control his behavior.20,29 Mr. A is poorly compliant with his medications, even with a blood thinner, which he was prescribed due to an episode of deep vein thrombosis. He complains of chronic daily headaches and episodic migraines.23,24 He rarely blinks,23,28 his skin is dry and cool, his hair is brittle,23 his mouth is dry,14,23,27 and he constantly licks his chapped lips.14,26,27 Mr. A frequently has general body pain26,31 but is dismissive of his body aches and completely stops reporting pain when his depression gets particularly severe. When depressed, he is slow in movement and thinking.14,21,26,27 He is more concerned with anxiety than depression.21 Mr. A is plagued by constipation, abdominal pain, muscle tension, and episodes of shaking.14,26,27 He also frequently complains about chronic tinnitus.28
Impression. Mr. A shows symptoms associated with serotonin hypoactivity. SSRIs and any other antidepressants with serotonin activity would be an obvious choice for treatment. A mood-stabilizing antipsychotic with serotonin activity would be welcome in treatment. Thyroid hormone supplementation may be of value, especially if thyroid stimulating hormone level is high. Light therapy, a diet with food that contains tryptophan, psychotherapy, and exercise are desirable. Avoiding benzodiazepines would be a good idea.
Dopamine excess (Table 214,30-41)
Ms. L presents with complaints of “fibromyalgia” and “daily headaches,”14 and also dissociation (finding herself in places when she does not know how she got there) and “out-of-body experiences.”32 She is odd, and states that people do not understand her and that she is “different.”38 Her friend, who is present at the appointment, elaborates on Ms. L’s bizarreness and oddness in behavior, out-of-context emotions, suspiciousness, paranoia, and possible hallucinations.35,36,38 Ms. L discloses frequent diffuse body pains, headaches, nausea, excessive salivation, and tongue burning, as well as muscle twitching.14 Sex worsens her headaches and body pain. She reports seizures that are not registered on EEG. In the office, she is suspicious, exhibits odd posturing, tends to misinterpret your words, and makes you feel uncomfortable. Anxiety38 and multiple obsessive-compulsive symptoms, especially excessive cleaning and grooming, complicate Ms. L’s life.31,32,34 On examination, she is hypertensive, and she has scars caused by self-cutting and skin picking on her arms.30-32 An electrocardiogram shows an elevated heart rate, widened QRS complex, and ectopic heartbeats.14 Ms. L has experienced trichotillomania since adolescence32-34 and her fingernails are bitten to the skin.34 She has difficulty with impulse control, and thrill-seeking is a prominent part of her life, mainly via gambling, compulsive sex, and compulsive buying.35,36 She also says she experiences indigestion and delayed gastric emptying.37
Impression. Ms. L exhibits multiple symptoms associated with dopamine excess. Dopamine antagonists should be considered and may help not only with her psychiatric symptoms but also with her pain symptoms. Bupropion (as a dopamine agonist), caffeine, and stimulants should be avoided.
Excessive dopamine is, in extreme cases, associated with somatic psychosis, somatic symptom disorder, factitious disorder, pain disorder, and hypochondria.39 It may come with odd and bizarre/peculiar symptoms out of proportion with objectively identified pathology. These symptoms are common in chronic pain and headache patients, and need to be addressed by appropriate use of dopamine antagonizing medications.39
Continue to: Dopamine deficiency
Dopamine deficiency (Table 24,14,20,38,40-43)
Mr. W experiences widespread pain, including chronic back pain, headaches, and abdominal pain. He also has substantial anhedonia, lack of interest, procrastination, and hypersomnia.41,42 He is apathetic and has difficulty getting up in the morning.41,42 Unusual tiredness and weakness drive him to overuse caffeine; he states that 5 Mountain Dews and 4 cups of regular coffee a day make his headaches bearable.38,41-43 Sex also improves his headaches. Since childhood, he has taken stimulants for attention-deficit/hyperactivity disorder. He reports that occasional use of cocaine helps ease his pain and depression. Mr. W’s wife is concerned with her husband’s low sexual drive and alcohol consumption, and discloses that he has periodic trouble with gambling. Mr. W was forced into psychotherapy but never was able to work productively with his therapist.38,41-43 He loves eating and cannot control his weight.40 This contrasts with episodic anorexia he experienced when he was younger.20 His face is usually emotionless.43 Mr. W is prone to constipation.14 His restless leg syndrome and periodic limb movement disorder are so bad that his wife refuses to share a bed with him.14 He is clumsy and has a problem with repetitive motor tasks.43 A paucity of speech, limited eye contact, poor grooming, and difficulty forming therapeutic alliances have long been part of Mr. W’s history.38,42,43 On physical examination, he has a dry mouth; he is stiff, tremulous, and hypotensive.14
Impression. Mr. W shows multiple symptoms associated with dopamine deficiency. Bupropion may be reasonable to consider. Dopamine augmentation via the use of stimulants is warranted in such patients, especially if stimulants had not been tried before (lisdexamfetamine would be a good choice to minimize addictive potential). For a patient with dopamine deficiency, levodopa may improve more than just restless legs. Amantadine may improve dopaminergic signaling through the accelerated dopamine release and decrease in presynaptic uptake, so this medication may be carefully tried.44 Pain treatment would not be successful for Mr. W without simultaneous treatment for his substance use.
Bottom Line
Both high and low levels of serotonin and dopamine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: How to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Amantadine • Gocovri
Baclofen • Ozobax
Bupropion • Wellbutrin
Fluoxetine • Prozac
Lisdexamfetamine • Vyvanse
Paroxetine • Paxil
It is unfortunate that, in some clinical areas, medical conditions are still treated by name and not based on the underlying pathological process. It would be odd in 2022 to treat “dropsy” instead of heart or kidney disease (2 very different causes of edema). Similarly, if the FDA had been approving drugs 150 years ago, we would have medications on label for “dementia praecox,” not schizophrenia or Alzheimer disease. With the help of DSM-5, psychiatry still resides in the descriptive symptomatic world of disorders.
In the United States, thanks to Freud, psychiatric symptoms became separated from medical symptoms, which made it more difficult to associate psychiatric manifestations with the underlying pathophysiology. Though the physical manifestations that parallel emotional symptoms—such as the dry mouth of anxiety, the tremor and leg weakness of fear, the constipation and blurry vision of depression, the breathing difficulty of anger, the abdominal pain of stress, the blushing of shyness, the palpitations of flashbacks, and endless others—are well known, the present classification of psychiatric disorders is blind to it. Neurochemical causes of gastrointestinal spasm or muscle tension are better researched than underlying central neurochemistry, though the same neurotransmitters drive them.
Can the biochemistry of psychiatric symptoms be judged on the basis of peripheral symptoms? Can the mental manifestations be connected to biological causation, and vice versa? Would psychiatrists be better off selecting treatments by recognizing involved neurotransmitters instead of addressing descriptive “depression, anxiety, and psychosis”? Each of these clinical syndromes may be caused by entirely different underlying neuronal mechanisms. Such mechanisms could be suggested if medical symptoms (which are measurable and objective) would become part of the psychiatric diagnosis. Is treating the “cough” sufficient, or would recognition that tuberculosis caused the cough guide better treatment? Is it time to abandon descriptive conditions and replace them with a specific “mechanism-based” viewpoint?
Ample research has shown that serotonin, dopamine, norepinephrine, endorphins, glutamate, and gamma aminobutyric acid (GABA) are the neurotransmitters most responsible in the process of both psychiatric disorders and chronic pain. These neurotransmitters are involved in much more than emotions (including the feeling of pain). An abundance of medical symptom clusters point toward which neurotransmitter dysfunction may be leading in specific cases of distinct types of depression, psychosis, anxiety, or “chronic pain.” Even presently, there are medications available (both for FDA-approved indications and off-label) that can be used to regulate these neurotransmitters, allowing practitioners to target the possible biological underlining of psychiatric or pain pathology. Hopefully, in the not-so-distant future, there will be specific medications for serotonin, dopamine, and noradrenergic depression as well as for GABAergic anxiety, endorphin psychosis, noradrenergic insomnia, and similar conditions.
Numerous neurotransmitters may be connected to both depression and pain in all their forms. These include (but are not limited to) prostaglandins, bradykinins, substance P, potassium, magnesium, calcium, histamine, adenosine triphosphate, calcitonin gene-related peptide (CGRP), nitric oxide (NO), cholecystokinin 7 (CCK7), neurotrophic growth factor (NGF), neurotensin, acetylcholine (Ach), oxytocin, cannabinoids, and others. These have not been researched sufficiently to identify their clinical presentation of excessive or insufficient availability at the sites of neurotransmission. It is difficult to draw conclusions about what kind of clinical symptoms they may cause (outside of pain), and therefore, they are not addressed in this article.
Both high and low levels of certain neurotransmitters may be associated with psychiatric conditions and chronic pain. Too much is as bad as too little.1 This applies to both quantity of neurotransmitters as well as quality of the corresponding receptor activity. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation. Reading indirect signs of bodily functions is a basic clinical skill that should not be forgotten, even in the time of advanced technology.
A different way of viewing psychiatric disorders
In this article, we present 4 hypothetical clinical cases to emphasize a possible way of analyzing symptoms to identify underlying pathology and guide more effective treatment. In no way do these descriptions reflect the entire set of symptoms caused by neurotransmitters; we created them based on what is presently known or suspected, and extensive research is required to confirm or disprove what we describe here.
Continue to: There are no well-recognized...
There are no well-recognized, well-established, reliable, or validated syndromes described in our work. Our goal is to suggest an alternative way of looking at psychiatric disorders by viewing syndromal presentation through the lens of specific neurotransmitters. The collection of symptoms associated with various neurotransmitters as presented in this hypothesis is not complete. We have assembled what is described in the literature as a suggestion for specific future research. We simplified these clinical presentations by omitting scenarios in which a specific neurotransmitter increases in one area but not another. For example, all the symptoms of dopamine excess we describe would not have to occur concurrently in the same patient, but they may develop in certain patients depending on which dopaminergic pathway is exhibiting excess activity. Such distinctions may be established only by exhaustive research not yet conducted.
Our proposal may seem radical, but it truly is not. For example, if we know that dopamine excess may cause seizures, psychosis, and blood pressure elevation, why not consider dopamine excess as an underlying cause in a patient with depression who exhibits these symptoms simultaneously? And why not call it “dopamine excess syndrome”? We already have “serotonin syndrome” for a patient experiencing a serotonin storm. However, using the same logic, it should be called “serotonin excess syndrome.” And if we know of “serotonin excess syndrome,” why not consider “serotonin deficiency syndrome”?

In Part 1 of this article, we discuss serotonin and dopamine. Table 1 outlines medical and psychiatric symptoms that likely reflect serotonin excess2-18 and deficiency,14,19-29 and Table 2 lists symptoms that likely reflect dopamine excess14,30-41 and deficiency.4,14,20,38,40-43 In Part 2 we will touch on endorphins and norepinephrine, and in Part 3 we will conclude by looking at GABA and glutamate.

Serotonin excess (Table 12-18)
On a recent office visit, Ms. H reports that most of the time she does not feel much of anything, but she still experiences panic attacks8,9,13,15 and is easily agitated.6,8 Her mother died recently, and Ms. H is concerned that she did not grieve.15-18 She failed her last semester in college and was indifferent to her failure.18 She sleeps poorly,8 is failing her creative classes, and wonders why she has lost her artistic inclination.16-18 Ms. H has difficulty with amotivation, planning, social interactions, and speech.16,17 All of those symptoms worsened after she was prescribed fluoxetine approximately 1 year ago for her “blues.” Ms. H is obese and continues to gain weight,2 though she frequently has diarrhea,3,4,7,8 loud peristalsis, and abdominal cramps.4,7,8 She sweats easily6-8 and her heart frequently races.8,9 Additionally, Ms. H’s primary care physician told her that she has “borderline diabetes.”2 She is prone to frequent bruising11 and is easy to shake, even when she is experiencing minimal anxiety.6-9 Ms. H had consulted with a neurologist because of unusual electrical “zapping” in her brain and muscle twitches.5,8,9,13 She had experienced a seizure as a child, but this was possibly related to hypernatremia,2 and she has not taken any anticonvulsant medication for several years.8 She exhibits hyperactive deep tendon reflexes and tremors5,7,9 and blinks frequently.6,9 She experiences hot flashes,3,6-8,14 does not tolerate heat, and prefers cooler weather.8,9 Her pains and aches,12,14 to which she has been prone all of her life, have recently become much worse, and she was diagnosed with fibromyalgia in part because she frequently feels stiff all over.10 She complains of strange tingling and prickling sensations in her hands and feet, especially when anxious.7,9,10 Her headaches also worsened and may be precipitated by bright light, as her pupils are usually dilated.5,7,9 Her hypertension is fairly controlled with medication.6,8-10 Ms. H says she experienced a psychotic episode when she was in her mid-teens,6,8 but reassures you that “she is not that bad now,” although she remains hypervigilant.13 Also while in her teens, Ms. H was treated with paroxetine and experienced restlessness, agitation, delirium, tachycardia, fluctuating blood pressure, diaphoresis, diarrhea, and neuromuscular excitation, which prompted discontinuation of the antidepressant.5-7,9,10
Impression. Ms. H exhibits symptoms associated with serotonin hyperactivity. Discontinuing and avoiding selective serotonin reuptake inhibitors (SSRIs) would be prudent; prescribing an anticonvulsant would be reasonable. Using a GABAergic medication to suppress serotonin (eg, baclofen) is likely to help. Avoiding dopaminergic medications is a must. Antidepressive antipsychotics would be logical to use. The use of serotonin-suppressing medications may be considered. One may argue for the use of beta-blockers in such a patient.
Continue to: Serotonin deficiency
Serotonin deficiency (Table 114,19-29)
Mr. A is chronically depressed, hopeless,19 and easily angered.21 He does not believe anyone can help him.19 You are concerned for his safety because he had attempted to end his life by shooting himself in the chest.19,21,22,25 Even when he’s not particularly depressed, Mr. A does not enjoy much of anything.21,26,27 He becomes particularly agitated when he drinks alcohol,25 which unfortunately is common for him.29 He engages in binge eating to feel better; he knows this is not healthy but he cannot control his behavior.20,29 Mr. A is poorly compliant with his medications, even with a blood thinner, which he was prescribed due to an episode of deep vein thrombosis. He complains of chronic daily headaches and episodic migraines.23,24 He rarely blinks,23,28 his skin is dry and cool, his hair is brittle,23 his mouth is dry,14,23,27 and he constantly licks his chapped lips.14,26,27 Mr. A frequently has general body pain26,31 but is dismissive of his body aches and completely stops reporting pain when his depression gets particularly severe. When depressed, he is slow in movement and thinking.14,21,26,27 He is more concerned with anxiety than depression.21 Mr. A is plagued by constipation, abdominal pain, muscle tension, and episodes of shaking.14,26,27 He also frequently complains about chronic tinnitus.28
Impression. Mr. A shows symptoms associated with serotonin hypoactivity. SSRIs and any other antidepressants with serotonin activity would be an obvious choice for treatment. A mood-stabilizing antipsychotic with serotonin activity would be welcome in treatment. Thyroid hormone supplementation may be of value, especially if thyroid stimulating hormone level is high. Light therapy, a diet with food that contains tryptophan, psychotherapy, and exercise are desirable. Avoiding benzodiazepines would be a good idea.
Dopamine excess (Table 214,30-41)
Ms. L presents with complaints of “fibromyalgia” and “daily headaches,”14 and also dissociation (finding herself in places when she does not know how she got there) and “out-of-body experiences.”32 She is odd, and states that people do not understand her and that she is “different.”38 Her friend, who is present at the appointment, elaborates on Ms. L’s bizarreness and oddness in behavior, out-of-context emotions, suspiciousness, paranoia, and possible hallucinations.35,36,38 Ms. L discloses frequent diffuse body pains, headaches, nausea, excessive salivation, and tongue burning, as well as muscle twitching.14 Sex worsens her headaches and body pain. She reports seizures that are not registered on EEG. In the office, she is suspicious, exhibits odd posturing, tends to misinterpret your words, and makes you feel uncomfortable. Anxiety38 and multiple obsessive-compulsive symptoms, especially excessive cleaning and grooming, complicate Ms. L’s life.31,32,34 On examination, she is hypertensive, and she has scars caused by self-cutting and skin picking on her arms.30-32 An electrocardiogram shows an elevated heart rate, widened QRS complex, and ectopic heartbeats.14 Ms. L has experienced trichotillomania since adolescence32-34 and her fingernails are bitten to the skin.34 She has difficulty with impulse control, and thrill-seeking is a prominent part of her life, mainly via gambling, compulsive sex, and compulsive buying.35,36 She also says she experiences indigestion and delayed gastric emptying.37
Impression. Ms. L exhibits multiple symptoms associated with dopamine excess. Dopamine antagonists should be considered and may help not only with her psychiatric symptoms but also with her pain symptoms. Bupropion (as a dopamine agonist), caffeine, and stimulants should be avoided.
Excessive dopamine is, in extreme cases, associated with somatic psychosis, somatic symptom disorder, factitious disorder, pain disorder, and hypochondria.39 It may come with odd and bizarre/peculiar symptoms out of proportion with objectively identified pathology. These symptoms are common in chronic pain and headache patients, and need to be addressed by appropriate use of dopamine antagonizing medications.39
Continue to: Dopamine deficiency
Dopamine deficiency (Table 24,14,20,38,40-43)
Mr. W experiences widespread pain, including chronic back pain, headaches, and abdominal pain. He also has substantial anhedonia, lack of interest, procrastination, and hypersomnia.41,42 He is apathetic and has difficulty getting up in the morning.41,42 Unusual tiredness and weakness drive him to overuse caffeine; he states that 5 Mountain Dews and 4 cups of regular coffee a day make his headaches bearable.38,41-43 Sex also improves his headaches. Since childhood, he has taken stimulants for attention-deficit/hyperactivity disorder. He reports that occasional use of cocaine helps ease his pain and depression. Mr. W’s wife is concerned with her husband’s low sexual drive and alcohol consumption, and discloses that he has periodic trouble with gambling. Mr. W was forced into psychotherapy but never was able to work productively with his therapist.38,41-43 He loves eating and cannot control his weight.40 This contrasts with episodic anorexia he experienced when he was younger.20 His face is usually emotionless.43 Mr. W is prone to constipation.14 His restless leg syndrome and periodic limb movement disorder are so bad that his wife refuses to share a bed with him.14 He is clumsy and has a problem with repetitive motor tasks.43 A paucity of speech, limited eye contact, poor grooming, and difficulty forming therapeutic alliances have long been part of Mr. W’s history.38,42,43 On physical examination, he has a dry mouth; he is stiff, tremulous, and hypotensive.14
Impression. Mr. W shows multiple symptoms associated with dopamine deficiency. Bupropion may be reasonable to consider. Dopamine augmentation via the use of stimulants is warranted in such patients, especially if stimulants had not been tried before (lisdexamfetamine would be a good choice to minimize addictive potential). For a patient with dopamine deficiency, levodopa may improve more than just restless legs. Amantadine may improve dopaminergic signaling through the accelerated dopamine release and decrease in presynaptic uptake, so this medication may be carefully tried.44 Pain treatment would not be successful for Mr. W without simultaneous treatment for his substance use.
Bottom Line
Both high and low levels of serotonin and dopamine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: How to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Amantadine • Gocovri
Baclofen • Ozobax
Bupropion • Wellbutrin
Fluoxetine • Prozac
Lisdexamfetamine • Vyvanse
Paroxetine • Paxil
1. Stahl SM. Dazzled by the dominions of dopamine: clinical roles of D3, D2, and D1 receptors. CNS Spectr. 2017;22(4):305-311.
2. Young RL, Lumsden AL, Martin AM, et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018;42(11):1880-1889.
3. Ahlman H. Serotonin and carcinoid tumors. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S79-S85.
4. Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319-342.
5. Prakash S, Belani P, Trivedi A. Headache as a presenting feature in patients with serotonin syndrome: a case series. Cephalalgia. 2014;34(2):148-153.
6. van Ewijk CE, Jacobs GE, Girbes ARJ. Unsuspected serotonin toxicity in the ICU. Ann Intensive Care. 2016;6(1):85.
7. Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care. 2014;21(1):108-113.
8. Nguyen H, Pan A, Smollin C, et al. An 11-year retrospective review of cyproheptadine use in serotonin syndrome cases reported to the California Poison Control System. J Clin Pharm Ther. 2019;44(2):327-334.
9. Ansari H, Kouti L. Drug interaction and serotonin toxicity with opioid use: another reason to avoid opioids in headache and migraine treatment. Curr Pain Headache Rep. 2016;20(8):50.
10. Ott M, Mannchen JK, Jamshidi F, et al. Management of severe arterial hypertension associated with serotonin syndrome: a case report analysis based on systematic review techniques. Ther Adv Psychopharmacol. 2019;9:2045125318818814. doi:10.1177/2045125318818814
11. Cerrito F, Lazzaro MP, Gaudio E, et al. 5HT2-receptors and serotonin release: their role in human platelet aggregation. Life Sci. 1993;53(3):209-215.
12. Ohayon MM. Pain sensitivity, depression, and sleep deprivation: links with serotoninergic dysfunction. J Psychiatr Res. 2009;43(16):1243-1245.
13. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31(1):1-11.
14. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:120,199,201-204,730-740.
15. Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child Adolesc Psychopharmacol. 2001;11(2):181-186.
16. George MS, Trimble MR. A fluvoxamine-induced frontal lobe syndrome in a patient with comorbid Gilles de la Tourette’s syndrome and obsessive compulsive disorder. J Clin Psychiatry. 1992;53(10):379-380.
17. Hoehn-Saric R, Harris GJ, Pearlson GD, et al. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry. 1991;52(3):131-133.
18. Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol. 1990;10(5):343-345.
19. Samuelsson M, Jokinen J, Nordström AL, et al. CSF 5-HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatr Scand. 2006;113(1):44-47.
20. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
21. Mann JJ, Oquendo M, Underwood MD, et al. The neurobiology of suicide risk: a review for the clinician. J Clin Psychiatry. 1999;60 Suppl 2:7-116.
22. Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41(2):162-171.
23. Joseph R, Welch KM, D’Andrea G. Serotonergic hypofunction in migraine: a synthesis of evidence based on platelet dense body dysfunction. Cephalalgia. 1989;9(4):293-299.
24. Pakalnis A, Splaingard M, Splaingard D, et al. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49(10):1486-1492.
25. Virkkunen M, Goldman D, Nielsen DA, et al. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20(4):271-275.
26. Liu Y, Zhao J, Fan X, et al. Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Front Psychiatry. 2019;10:286.
27. Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179-1187.
28. O’Malley PG, Jackson JL, Santoro J, et al. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract. 1999;48(12):980-990.
29. Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42(2):147-151.
30. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
31. Graybiel AM, Saka E. A genetic basis for obsessive grooming. Neuron. 2002;33(1):1-2.
32. Tse W, Hälbig TD. Skin picking in Parkinson’s disease: a behavioral side-effect of dopaminergic treatment? Psychiatry Clin Neurosci. 2010;64(2):214.
33. Ayaydın H. Probable emergence of symptoms of trichotillomania by atomoxetine: a case report. Psychiatry and Clinical Psychopharmacology. 2019;29(2)220-222.
34. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702. doi:10.1155/2016/9782702
35. Clark CA, Dagher A. The role of dopamine in risk taking: a specific look at Parkinson’s disease and gambling. Front Behav Neurosci. 2014;8:196.
36. Norbury A, Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79-93.
37. Chen TS, Chang FY. Elevated serum dopamine increases while coffee consumption decreases the occurrence of reddish streaks in the intact stomach. J Gastroenterol Hepatol. 2013;28(12):1810-1814.
38. Wong-Riley MT. Neuroscience Secrets. 1st edition. Spanish version. Hanley & Belfus; 1999:420-429.
39. Arbuck DM. Antipsychotics, dopamine, and pain. Current Psychiatry. 2020;19(1):25-29,31.
40. Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97(1):25-33.
41. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1-46.
42. Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506.
43. Gepshtein S, Li X, Snider J, et al. Dopamine function and the efficiency of human movement. J Cogn Neurosci. 2014;26(3):645-657.
44. Scarff JR. The ABCDs of treating tardive dyskinesia. Current Psychiatry. 2020;19(4):21,55.
1. Stahl SM. Dazzled by the dominions of dopamine: clinical roles of D3, D2, and D1 receptors. CNS Spectr. 2017;22(4):305-311.
2. Young RL, Lumsden AL, Martin AM, et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018;42(11):1880-1889.
3. Ahlman H. Serotonin and carcinoid tumors. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S79-S85.
4. Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319-342.
5. Prakash S, Belani P, Trivedi A. Headache as a presenting feature in patients with serotonin syndrome: a case series. Cephalalgia. 2014;34(2):148-153.
6. van Ewijk CE, Jacobs GE, Girbes ARJ. Unsuspected serotonin toxicity in the ICU. Ann Intensive Care. 2016;6(1):85.
7. Pedavally S, Fugate JE, Rabinstein AA. Serotonin syndrome in the intensive care unit: clinical presentations and precipitating medications. Neurocrit Care. 2014;21(1):108-113.
8. Nguyen H, Pan A, Smollin C, et al. An 11-year retrospective review of cyproheptadine use in serotonin syndrome cases reported to the California Poison Control System. J Clin Pharm Ther. 2019;44(2):327-334.
9. Ansari H, Kouti L. Drug interaction and serotonin toxicity with opioid use: another reason to avoid opioids in headache and migraine treatment. Curr Pain Headache Rep. 2016;20(8):50.
10. Ott M, Mannchen JK, Jamshidi F, et al. Management of severe arterial hypertension associated with serotonin syndrome: a case report analysis based on systematic review techniques. Ther Adv Psychopharmacol. 2019;9:2045125318818814. doi:10.1177/2045125318818814
11. Cerrito F, Lazzaro MP, Gaudio E, et al. 5HT2-receptors and serotonin release: their role in human platelet aggregation. Life Sci. 1993;53(3):209-215.
12. Ohayon MM. Pain sensitivity, depression, and sleep deprivation: links with serotoninergic dysfunction. J Psychiatr Res. 2009;43(16):1243-1245.
13. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31(1):1-11.
14. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:120,199,201-204,730-740.
15. Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child Adolesc Psychopharmacol. 2001;11(2):181-186.
16. George MS, Trimble MR. A fluvoxamine-induced frontal lobe syndrome in a patient with comorbid Gilles de la Tourette’s syndrome and obsessive compulsive disorder. J Clin Psychiatry. 1992;53(10):379-380.
17. Hoehn-Saric R, Harris GJ, Pearlson GD, et al. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry. 1991;52(3):131-133.
18. Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol. 1990;10(5):343-345.
19. Samuelsson M, Jokinen J, Nordström AL, et al. CSF 5-HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatr Scand. 2006;113(1):44-47.
20. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
21. Mann JJ, Oquendo M, Underwood MD, et al. The neurobiology of suicide risk: a review for the clinician. J Clin Psychiatry. 1999;60 Suppl 2:7-116.
22. Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41(2):162-171.
23. Joseph R, Welch KM, D’Andrea G. Serotonergic hypofunction in migraine: a synthesis of evidence based on platelet dense body dysfunction. Cephalalgia. 1989;9(4):293-299.
24. Pakalnis A, Splaingard M, Splaingard D, et al. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49(10):1486-1492.
25. Virkkunen M, Goldman D, Nielsen DA, et al. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20(4):271-275.
26. Liu Y, Zhao J, Fan X, et al. Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Front Psychiatry. 2019;10:286.
27. Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179-1187.
28. O’Malley PG, Jackson JL, Santoro J, et al. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract. 1999;48(12):980-990.
29. Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42(2):147-151.
30. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
31. Graybiel AM, Saka E. A genetic basis for obsessive grooming. Neuron. 2002;33(1):1-2.
32. Tse W, Hälbig TD. Skin picking in Parkinson’s disease: a behavioral side-effect of dopaminergic treatment? Psychiatry Clin Neurosci. 2010;64(2):214.
33. Ayaydın H. Probable emergence of symptoms of trichotillomania by atomoxetine: a case report. Psychiatry and Clinical Psychopharmacology. 2019;29(2)220-222.
34. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702. doi:10.1155/2016/9782702
35. Clark CA, Dagher A. The role of dopamine in risk taking: a specific look at Parkinson’s disease and gambling. Front Behav Neurosci. 2014;8:196.
36. Norbury A, Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79-93.
37. Chen TS, Chang FY. Elevated serum dopamine increases while coffee consumption decreases the occurrence of reddish streaks in the intact stomach. J Gastroenterol Hepatol. 2013;28(12):1810-1814.
38. Wong-Riley MT. Neuroscience Secrets. 1st edition. Spanish version. Hanley & Belfus; 1999:420-429.
39. Arbuck DM. Antipsychotics, dopamine, and pain. Current Psychiatry. 2020;19(1):25-29,31.
40. Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97(1):25-33.
41. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1-46.
42. Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506.
43. Gepshtein S, Li X, Snider J, et al. Dopamine function and the efficiency of human movement. J Cogn Neurosci. 2014;26(3):645-657.
44. Scarff JR. The ABCDs of treating tardive dyskinesia. Current Psychiatry. 2020;19(4):21,55.