User login
A total of 34% of children who underwent solid organ transplantation subsequently developed eczema, food allergy, rhinitis, eosinophilic gastrointestinal disease, or asthma, according to the results of a single-center retrospective cohort study.
Another 6.6% of patients developed autoimmunity, usually autoimmune cytopenia, inflammatory bowel disease, or vasculitis, wrote Nufar Marcus, MD, of the University of Toronto, and her associates.
Posttransplant allergy, autoimmunity, and immune-mediated disorders (PTAA) likely share a common pathogenesis “and may represent a unique state of post-transplant immune-dysregulation,” they wrote. The report was published in the Journal of Pediatrics.
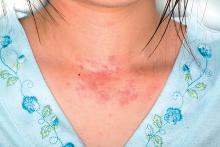
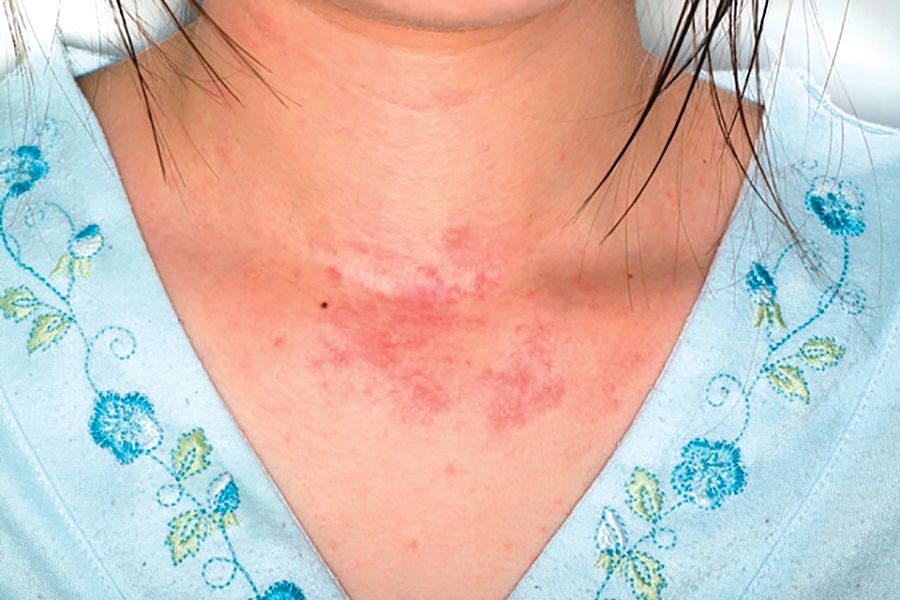
The study included 273 children who underwent solid organ transplantation and were followed for a median 3.6 years (range, 1.7-6.3 years). None had immune-mediated conditions or allergies diagnosed at baseline. Posttransplantation allergies most commonly included eczema (51%), asthma (32%), food allergy (25%, including 5% with associated anaphylaxis), rhinitis (17%), and eosinophilic esophagitis, gastritis, or enteritis (13%).
Although only 31% of patients had information available on family history of allergy, those with a positive family history of allergy had a fivefold greater odds of posttransplantation PTAA, compared with other patients. Other risk factors for PTAA included female sex, young age at transplantation, eosinophilia, and a positive test for Epstein-Barr virus after transplantation, Dr. Marcus and associates said.
“The association of blood eosinophilia and PTAA reached statistical significance only when the transplant recipient was at least 6 months of age, demonstrating the nonspecific nature of abnormally high eosinophil counts during the first months of life,” they noted. The longer patients had eosinophilia after transplantation, the more likely they were to develop PTAA, “suggest[ing] a potential detrimental effect of prolonged activation of the eosinophilic-associated immune arms.”
Factors that appeared unlinked with PTAA included acute organ rejection, duration of posttransplantation steroidal treatment, organ type (living versus cadaveric), donor/recipient blood type and compatibility, infections besides Epstein-Barr virus, and posttransplant lymphoproliferative disease. “The specific type of post-transplantation immunosuppression regimen was neither associated nor protective of PTAA,” the investigators wrote. “However, a significant limitation was our inability to assess the effect of tacrolimus, as nearly all the cohort (97.8%) was treated with this medication.”
Ashley’s Angels fund provided support. The researchers reported having no conflicts of interest.
SOURCE: Marcus N et al. J Pediatr. 2018;196:154-60.
The study is one of several to highlight the occurrence of atopy and allergy following solid organ transplantation in children, Helen M. Evans, MBChB, wrote in an editorial accompanying the report by Marcus et al.
This report differed because it studied the differences in rates of atopy and allergy between transplanted solid organ groups. These occurred in 41% and 40% of liver and heart recipients, respectively, but in only 4% of kidney recipients. Atopy or allergy developed in 57% of multivisceral transplant patients, but the number of patients was very small (n = 7). The majority of the conditions developed within 1 year of transplantation.
The recent spike in these reports could signify better recognition of the problem or “the widespread switch of primary immunosuppression from cyclosporine to tacrolimus over the last few decades,” wrote Dr. Evans.
Most of these reports have been single-center retrospective studies, which are subject to inconsistent case definitions and recall bias, she noted. “The time is right for well-conducted multicenter prospective studies to better inform the true extent of these conditions after solid organ transplantation.”
In the meantime, transplantation centers should routinely track de novo eczema, allergy, and eosinophilic gastrointestinal disease in children being assessed for solid organ transplantation, and should take “rigorous” personal and family histories, said Dr. Evans. Ultimately, this work will help “minimize the risk of children developing these conditions” and “effectively treat them in the setting of immunosuppression after transplantation.”
Dr. Evans is a pediatric gastroenterologist at Starship Child Health in Aukland, New Zealand. She reported having no conflicts of interest. These comments summarize her editorial ( J Pediatr. 2018;196:10-11 ).
The study is one of several to highlight the occurrence of atopy and allergy following solid organ transplantation in children, Helen M. Evans, MBChB, wrote in an editorial accompanying the report by Marcus et al.
This report differed because it studied the differences in rates of atopy and allergy between transplanted solid organ groups. These occurred in 41% and 40% of liver and heart recipients, respectively, but in only 4% of kidney recipients. Atopy or allergy developed in 57% of multivisceral transplant patients, but the number of patients was very small (n = 7). The majority of the conditions developed within 1 year of transplantation.
The recent spike in these reports could signify better recognition of the problem or “the widespread switch of primary immunosuppression from cyclosporine to tacrolimus over the last few decades,” wrote Dr. Evans.
Most of these reports have been single-center retrospective studies, which are subject to inconsistent case definitions and recall bias, she noted. “The time is right for well-conducted multicenter prospective studies to better inform the true extent of these conditions after solid organ transplantation.”
In the meantime, transplantation centers should routinely track de novo eczema, allergy, and eosinophilic gastrointestinal disease in children being assessed for solid organ transplantation, and should take “rigorous” personal and family histories, said Dr. Evans. Ultimately, this work will help “minimize the risk of children developing these conditions” and “effectively treat them in the setting of immunosuppression after transplantation.”
Dr. Evans is a pediatric gastroenterologist at Starship Child Health in Aukland, New Zealand. She reported having no conflicts of interest. These comments summarize her editorial ( J Pediatr. 2018;196:10-11 ).
The study is one of several to highlight the occurrence of atopy and allergy following solid organ transplantation in children, Helen M. Evans, MBChB, wrote in an editorial accompanying the report by Marcus et al.
This report differed because it studied the differences in rates of atopy and allergy between transplanted solid organ groups. These occurred in 41% and 40% of liver and heart recipients, respectively, but in only 4% of kidney recipients. Atopy or allergy developed in 57% of multivisceral transplant patients, but the number of patients was very small (n = 7). The majority of the conditions developed within 1 year of transplantation.
The recent spike in these reports could signify better recognition of the problem or “the widespread switch of primary immunosuppression from cyclosporine to tacrolimus over the last few decades,” wrote Dr. Evans.
Most of these reports have been single-center retrospective studies, which are subject to inconsistent case definitions and recall bias, she noted. “The time is right for well-conducted multicenter prospective studies to better inform the true extent of these conditions after solid organ transplantation.”
In the meantime, transplantation centers should routinely track de novo eczema, allergy, and eosinophilic gastrointestinal disease in children being assessed for solid organ transplantation, and should take “rigorous” personal and family histories, said Dr. Evans. Ultimately, this work will help “minimize the risk of children developing these conditions” and “effectively treat them in the setting of immunosuppression after transplantation.”
Dr. Evans is a pediatric gastroenterologist at Starship Child Health in Aukland, New Zealand. She reported having no conflicts of interest. These comments summarize her editorial ( J Pediatr. 2018;196:10-11 ).
A total of 34% of children who underwent solid organ transplantation subsequently developed eczema, food allergy, rhinitis, eosinophilic gastrointestinal disease, or asthma, according to the results of a single-center retrospective cohort study.
Another 6.6% of patients developed autoimmunity, usually autoimmune cytopenia, inflammatory bowel disease, or vasculitis, wrote Nufar Marcus, MD, of the University of Toronto, and her associates.
Posttransplant allergy, autoimmunity, and immune-mediated disorders (PTAA) likely share a common pathogenesis “and may represent a unique state of post-transplant immune-dysregulation,” they wrote. The report was published in the Journal of Pediatrics.
The study included 273 children who underwent solid organ transplantation and were followed for a median 3.6 years (range, 1.7-6.3 years). None had immune-mediated conditions or allergies diagnosed at baseline. Posttransplantation allergies most commonly included eczema (51%), asthma (32%), food allergy (25%, including 5% with associated anaphylaxis), rhinitis (17%), and eosinophilic esophagitis, gastritis, or enteritis (13%).
Although only 31% of patients had information available on family history of allergy, those with a positive family history of allergy had a fivefold greater odds of posttransplantation PTAA, compared with other patients. Other risk factors for PTAA included female sex, young age at transplantation, eosinophilia, and a positive test for Epstein-Barr virus after transplantation, Dr. Marcus and associates said.
“The association of blood eosinophilia and PTAA reached statistical significance only when the transplant recipient was at least 6 months of age, demonstrating the nonspecific nature of abnormally high eosinophil counts during the first months of life,” they noted. The longer patients had eosinophilia after transplantation, the more likely they were to develop PTAA, “suggest[ing] a potential detrimental effect of prolonged activation of the eosinophilic-associated immune arms.”
Factors that appeared unlinked with PTAA included acute organ rejection, duration of posttransplantation steroidal treatment, organ type (living versus cadaveric), donor/recipient blood type and compatibility, infections besides Epstein-Barr virus, and posttransplant lymphoproliferative disease. “The specific type of post-transplantation immunosuppression regimen was neither associated nor protective of PTAA,” the investigators wrote. “However, a significant limitation was our inability to assess the effect of tacrolimus, as nearly all the cohort (97.8%) was treated with this medication.”
Ashley’s Angels fund provided support. The researchers reported having no conflicts of interest.
SOURCE: Marcus N et al. J Pediatr. 2018;196:154-60.
A total of 34% of children who underwent solid organ transplantation subsequently developed eczema, food allergy, rhinitis, eosinophilic gastrointestinal disease, or asthma, according to the results of a single-center retrospective cohort study.
Another 6.6% of patients developed autoimmunity, usually autoimmune cytopenia, inflammatory bowel disease, or vasculitis, wrote Nufar Marcus, MD, of the University of Toronto, and her associates.
Posttransplant allergy, autoimmunity, and immune-mediated disorders (PTAA) likely share a common pathogenesis “and may represent a unique state of post-transplant immune-dysregulation,” they wrote. The report was published in the Journal of Pediatrics.
The study included 273 children who underwent solid organ transplantation and were followed for a median 3.6 years (range, 1.7-6.3 years). None had immune-mediated conditions or allergies diagnosed at baseline. Posttransplantation allergies most commonly included eczema (51%), asthma (32%), food allergy (25%, including 5% with associated anaphylaxis), rhinitis (17%), and eosinophilic esophagitis, gastritis, or enteritis (13%).
Although only 31% of patients had information available on family history of allergy, those with a positive family history of allergy had a fivefold greater odds of posttransplantation PTAA, compared with other patients. Other risk factors for PTAA included female sex, young age at transplantation, eosinophilia, and a positive test for Epstein-Barr virus after transplantation, Dr. Marcus and associates said.
“The association of blood eosinophilia and PTAA reached statistical significance only when the transplant recipient was at least 6 months of age, demonstrating the nonspecific nature of abnormally high eosinophil counts during the first months of life,” they noted. The longer patients had eosinophilia after transplantation, the more likely they were to develop PTAA, “suggest[ing] a potential detrimental effect of prolonged activation of the eosinophilic-associated immune arms.”
Factors that appeared unlinked with PTAA included acute organ rejection, duration of posttransplantation steroidal treatment, organ type (living versus cadaveric), donor/recipient blood type and compatibility, infections besides Epstein-Barr virus, and posttransplant lymphoproliferative disease. “The specific type of post-transplantation immunosuppression regimen was neither associated nor protective of PTAA,” the investigators wrote. “However, a significant limitation was our inability to assess the effect of tacrolimus, as nearly all the cohort (97.8%) was treated with this medication.”
Ashley’s Angels fund provided support. The researchers reported having no conflicts of interest.
SOURCE: Marcus N et al. J Pediatr. 2018;196:154-60.
FROM JOURNAL OF PEDIATRICS
Key clinical point: Children undergoing solid organ transplantation often developed allergy or autoimmunity.
Major finding: Study details: Single-center retrospective cross-sectional study of 273 patients aged 18 and under who underwent solid organ transplantation followed for a median 3.6 years.
Disclosures: Ashley’s Angels fund provided support. The researchers reported having no conflicts of interest.
Source: Marcus N et al. J Pediatr. 2018;196:154-60.