User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
ADHD in preschool kids: Adrenergic agonists may be a better fit
A new study finds that alpha2-adrenergic agonists may be of benefit and have fewer side effects than stimulant medications for the treatment of attention-deficit/hyperactivity disorder in preschool-age children.
The study was published online May 4 in JAMA.
As part of a retrospective analysis, Elizabeth Harstad, MD, MPH, of Boston Children’s Hospital and colleagues evaluated health record data from 497 preschool-age children with ADHD across seven developmental-behavioral pediatric practices in the United States. Children included in the evaluation were younger than 6 years and were treated for ADHD between Jan. 1, 2013, and July 1, 2017, with either an alpha2-adrenergic agonist or a stimulant.
Overall, 175 children (35%) were prescribed an alpha2-adrenergic agonist (most often guanfacine) as first-line ADHD medication, and 322 children (65%) were prescribed a stimulant (most often a methylphenidate-based preparation). Before any medication regimens were initiated, 62% of children received behavioral therapy.
“These findings suggest that for some children there may be a concern about either how well a stimulant will work or how well a stimulant will be tolerated that is leading clinicians to instead prescribe an alpha2-adrenergic agonist as the first medication tried,” Dr. Harstad said in an interview.
Clinical improvement was noted in 66% of children treated with alpha2-adrenergic agonists (95% confidence interval, 57.5%-73.9%) and in 78% of children treated with stimulants (95% CI, 72.4%-83.4%).
Most adverse effects were more common among children who received stimulants than among those who received alpha2-adrenergic agonists. These adverse effects included difficulty falling asleep (21% vs. 11%), decreased appetite (38% vs. 7%), increased stomachaches (13% vs. 5%), and increased skin picking/repetitive behaviors (11% vs. 5%). Only daytime sleepiness was more frequent among children who received an alpha2-adrenergic agonist rather than a stimulant (38% vs. 3%).
“We also found that for the youngest children (<4 years old), those initiated on alpha2-adrenergic agonists stayed on these medications longer than those initiated on stimulants, which may indicate that they are better tolerated, although more research is needed to confirm this,” Dr. Harstad said.
“While our study focused on how well medications work and how well they are tolerated when used to treat preschool-age children with ADHD, it is important to remember that behavioral therapy is recommended as first-line treatment for ADHD in preschool-age children, not medication,” Dr. Harstad added.
Mark Wolraich, MD, of the University of Oklahoma, echoed that sentiment. “The article mentions that behavioral interventions, in the form of parent training in behavior management, is an effective first-line treatment” and, per the American Academy of Pediatrics guidelines, “is the first line of treatment recommended for preschool-age children before medication should be considered.”
Dr. Wolraich also noted that “neither drug has official FDA [U.S. Food and Drug Administration] approval in this age group” but that “methylphenidate comes the closest to having met the FDA requirements for approval in this age group, which is why the AAP guidelines recommended its use if parent training in behavior management is not sufficient.”
Although Dr. Harstad and colleagues note that the study included a large and diverse sample size from across the United States, they acknowledge that “further research, including from randomized clinical trials, is needed to assess comparative effectiveness of alpha2-adrenergic agonists versus stimulants.”
Funding for the study was provided through a cooperative agreement with the Maternal and Child Health Bureau, the Health Resources and Services Administration, and the U.S. Department of Health & Human Services. Dr. Harstad has reported receiving reported receiving compensation for serving as a medical reviewer for Understood.org and grant funding from the Palmer Family Fund for Autism Research to conduct research related to autism spectrum disorder at Boston Children’s Hospital. Disclosures for the other authors are listed in the original article. Dr. Wolraich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study finds that alpha2-adrenergic agonists may be of benefit and have fewer side effects than stimulant medications for the treatment of attention-deficit/hyperactivity disorder in preschool-age children.
The study was published online May 4 in JAMA.
As part of a retrospective analysis, Elizabeth Harstad, MD, MPH, of Boston Children’s Hospital and colleagues evaluated health record data from 497 preschool-age children with ADHD across seven developmental-behavioral pediatric practices in the United States. Children included in the evaluation were younger than 6 years and were treated for ADHD between Jan. 1, 2013, and July 1, 2017, with either an alpha2-adrenergic agonist or a stimulant.
Overall, 175 children (35%) were prescribed an alpha2-adrenergic agonist (most often guanfacine) as first-line ADHD medication, and 322 children (65%) were prescribed a stimulant (most often a methylphenidate-based preparation). Before any medication regimens were initiated, 62% of children received behavioral therapy.
“These findings suggest that for some children there may be a concern about either how well a stimulant will work or how well a stimulant will be tolerated that is leading clinicians to instead prescribe an alpha2-adrenergic agonist as the first medication tried,” Dr. Harstad said in an interview.
Clinical improvement was noted in 66% of children treated with alpha2-adrenergic agonists (95% confidence interval, 57.5%-73.9%) and in 78% of children treated with stimulants (95% CI, 72.4%-83.4%).
Most adverse effects were more common among children who received stimulants than among those who received alpha2-adrenergic agonists. These adverse effects included difficulty falling asleep (21% vs. 11%), decreased appetite (38% vs. 7%), increased stomachaches (13% vs. 5%), and increased skin picking/repetitive behaviors (11% vs. 5%). Only daytime sleepiness was more frequent among children who received an alpha2-adrenergic agonist rather than a stimulant (38% vs. 3%).
“We also found that for the youngest children (<4 years old), those initiated on alpha2-adrenergic agonists stayed on these medications longer than those initiated on stimulants, which may indicate that they are better tolerated, although more research is needed to confirm this,” Dr. Harstad said.
“While our study focused on how well medications work and how well they are tolerated when used to treat preschool-age children with ADHD, it is important to remember that behavioral therapy is recommended as first-line treatment for ADHD in preschool-age children, not medication,” Dr. Harstad added.
Mark Wolraich, MD, of the University of Oklahoma, echoed that sentiment. “The article mentions that behavioral interventions, in the form of parent training in behavior management, is an effective first-line treatment” and, per the American Academy of Pediatrics guidelines, “is the first line of treatment recommended for preschool-age children before medication should be considered.”
Dr. Wolraich also noted that “neither drug has official FDA [U.S. Food and Drug Administration] approval in this age group” but that “methylphenidate comes the closest to having met the FDA requirements for approval in this age group, which is why the AAP guidelines recommended its use if parent training in behavior management is not sufficient.”
Although Dr. Harstad and colleagues note that the study included a large and diverse sample size from across the United States, they acknowledge that “further research, including from randomized clinical trials, is needed to assess comparative effectiveness of alpha2-adrenergic agonists versus stimulants.”
Funding for the study was provided through a cooperative agreement with the Maternal and Child Health Bureau, the Health Resources and Services Administration, and the U.S. Department of Health & Human Services. Dr. Harstad has reported receiving reported receiving compensation for serving as a medical reviewer for Understood.org and grant funding from the Palmer Family Fund for Autism Research to conduct research related to autism spectrum disorder at Boston Children’s Hospital. Disclosures for the other authors are listed in the original article. Dr. Wolraich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study finds that alpha2-adrenergic agonists may be of benefit and have fewer side effects than stimulant medications for the treatment of attention-deficit/hyperactivity disorder in preschool-age children.
The study was published online May 4 in JAMA.
As part of a retrospective analysis, Elizabeth Harstad, MD, MPH, of Boston Children’s Hospital and colleagues evaluated health record data from 497 preschool-age children with ADHD across seven developmental-behavioral pediatric practices in the United States. Children included in the evaluation were younger than 6 years and were treated for ADHD between Jan. 1, 2013, and July 1, 2017, with either an alpha2-adrenergic agonist or a stimulant.
Overall, 175 children (35%) were prescribed an alpha2-adrenergic agonist (most often guanfacine) as first-line ADHD medication, and 322 children (65%) were prescribed a stimulant (most often a methylphenidate-based preparation). Before any medication regimens were initiated, 62% of children received behavioral therapy.
“These findings suggest that for some children there may be a concern about either how well a stimulant will work or how well a stimulant will be tolerated that is leading clinicians to instead prescribe an alpha2-adrenergic agonist as the first medication tried,” Dr. Harstad said in an interview.
Clinical improvement was noted in 66% of children treated with alpha2-adrenergic agonists (95% confidence interval, 57.5%-73.9%) and in 78% of children treated with stimulants (95% CI, 72.4%-83.4%).
Most adverse effects were more common among children who received stimulants than among those who received alpha2-adrenergic agonists. These adverse effects included difficulty falling asleep (21% vs. 11%), decreased appetite (38% vs. 7%), increased stomachaches (13% vs. 5%), and increased skin picking/repetitive behaviors (11% vs. 5%). Only daytime sleepiness was more frequent among children who received an alpha2-adrenergic agonist rather than a stimulant (38% vs. 3%).
“We also found that for the youngest children (<4 years old), those initiated on alpha2-adrenergic agonists stayed on these medications longer than those initiated on stimulants, which may indicate that they are better tolerated, although more research is needed to confirm this,” Dr. Harstad said.
“While our study focused on how well medications work and how well they are tolerated when used to treat preschool-age children with ADHD, it is important to remember that behavioral therapy is recommended as first-line treatment for ADHD in preschool-age children, not medication,” Dr. Harstad added.
Mark Wolraich, MD, of the University of Oklahoma, echoed that sentiment. “The article mentions that behavioral interventions, in the form of parent training in behavior management, is an effective first-line treatment” and, per the American Academy of Pediatrics guidelines, “is the first line of treatment recommended for preschool-age children before medication should be considered.”
Dr. Wolraich also noted that “neither drug has official FDA [U.S. Food and Drug Administration] approval in this age group” but that “methylphenidate comes the closest to having met the FDA requirements for approval in this age group, which is why the AAP guidelines recommended its use if parent training in behavior management is not sufficient.”
Although Dr. Harstad and colleagues note that the study included a large and diverse sample size from across the United States, they acknowledge that “further research, including from randomized clinical trials, is needed to assess comparative effectiveness of alpha2-adrenergic agonists versus stimulants.”
Funding for the study was provided through a cooperative agreement with the Maternal and Child Health Bureau, the Health Resources and Services Administration, and the U.S. Department of Health & Human Services. Dr. Harstad has reported receiving reported receiving compensation for serving as a medical reviewer for Understood.org and grant funding from the Palmer Family Fund for Autism Research to conduct research related to autism spectrum disorder at Boston Children’s Hospital. Disclosures for the other authors are listed in the original article. Dr. Wolraich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Attending a patient’s funeral: How psychiatrists decide
Psychiatrists often develop long-term relationships with their patients, but what happens when a patient dies? Should the psychiatrist attend the patient’s funeral?
It’s a question Ashley Pettaway, MD, faced as a medical resident at the University of Alabama School of Medicine.
For 2 months, Dr. Pettaway was involved in the day-to-day care of a woman in her 40s who ultimately died. As part of that care, Dr. Pettaway had regular meetings with the patient’s husband and family members.
“The patient was about my mother’s age, so I naturally was kind of attached to her,” Dr. Pettaway told this news organization. After she died, her family invited Dr. Pettaway to the funeral.
“While I couldn’t make it to the funeral, it got me thinking. Should I go? If I go, what do I say? Who do I sit with? How do I introduce myself?” wondered Dr. Pettaway, now a resident in the department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville.
She turned to the literature but found very little regarding psychiatrists attending their patients’ funerals. “This was surprising to me because in psychiatry, you can get so engrossed in patients’ lives,” Dr. Pettaway said.
Given the lack of rules or formal guidance on psychiatrists attending patients’ funerals, Dr. Pettaway and her mentor, Gabrielle Marzani, MD, conducted an informal survey of 12 supervising psychiatrists at the University of Virginia.
The survey results were presented at the virtual American Psychiatric Association 2021 Annual Meeting.
Ten of the 12 psychiatrists who were surveyed were caring for a patient who died while under their care. Five of those psychiatrists reported going to at least one patient’s funeral over the course of their career.
Among the psychiatrists who attended a patient’s funeral, their attendance was often based on their clinical intuition, their relationship with the family, or whether the patient was an established presence in the community. In the latter case, the psychiatrist attended as a community member.
The number of years in practice also mattered. Fewer senior faculty reported that they would be hesitant to attend and that they would not attend without a formal invitation from the family. Senior career psychiatrists were more likely to attend and felt that an invitation was not required.
None of the psychiatrists surveyed had received training or guidance on attending patients’ funerals at any point in their career.
Given the absence of formal recommendations, Dr. Pettaway believes increased conversation on this topic as part of residency training programs would help psychiatrists navigate these complex situations.
A complex issue
Commenting on the topic for an interview, Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, said this is an “interesting and important topic that is underdiscussed.”
“I don’t think there’s a right answer that applies to every situation,” said Dr. Appelbaum, a past president of the APA.
There will be times, he said, when psychiatrists or other mental health professionals have worked closely with a patient for many years and may have interacted with the family over that period.
“When that patient passes away, they may feel, and the family may feel, that it would be comforting and appropriate for them to be at the funeral,” said Dr. Appelbaum.
However, he added,
“There are obviously a number of complexities involved. One is how the family feels about the relationship with the psychiatrist – whether they were accepting of the reality that the patient had a mental disorder and was in treatment,” he said.
There is also the question of confidentiality, said Dr. Appelbaum.
“If it’s a large funeral and the psychiatrist is just one face in the crowd, that’s not likely to be an issue. But if it’s a relatively small group of mourners, all of whom know each other, and an unknown figure pops up, that could raise questions and perhaps inadvertently reveal to family members or friends that the deceased had a psychiatric condition and was in treatment. That needs to be taken into account as well,” he added.
In cases in which the family invites the psychiatrist, confidentiality is not a concern, and attendance by the psychiatrist is something the patient would have wanted, said Dr. Appelbaum.
How the patient died may also be factor. When a patient dies by suicide, it’s an “emotionally charged situation for both sides,” said Dr. Appelbaum.
In the case of a suicide, he noted, the deceased was often an active patient, and both the psychiatrist and the family are dealing with strong emotions – the psychiatrist with regret over loss of the patient and perhaps with questions as to what could have been done differently, and the family with sorrow but “also sometimes with suspicion or anger in that the psychiatrist somehow failed to keep the patient alive,” Dr. Appelbaum noted.
“In this situation, it’s even more crucial for the psychiatrist or other mental health professionals to take the lead from the family – perhaps to initiate contact to express condolences and inquire delicately about the funeral arrangements and whether their presence would be welcomed,” he said.
The research had no specific funding. Dr. Pettaway and Dr. Appelbaum have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychiatrists often develop long-term relationships with their patients, but what happens when a patient dies? Should the psychiatrist attend the patient’s funeral?
It’s a question Ashley Pettaway, MD, faced as a medical resident at the University of Alabama School of Medicine.
For 2 months, Dr. Pettaway was involved in the day-to-day care of a woman in her 40s who ultimately died. As part of that care, Dr. Pettaway had regular meetings with the patient’s husband and family members.
“The patient was about my mother’s age, so I naturally was kind of attached to her,” Dr. Pettaway told this news organization. After she died, her family invited Dr. Pettaway to the funeral.
“While I couldn’t make it to the funeral, it got me thinking. Should I go? If I go, what do I say? Who do I sit with? How do I introduce myself?” wondered Dr. Pettaway, now a resident in the department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville.
She turned to the literature but found very little regarding psychiatrists attending their patients’ funerals. “This was surprising to me because in psychiatry, you can get so engrossed in patients’ lives,” Dr. Pettaway said.
Given the lack of rules or formal guidance on psychiatrists attending patients’ funerals, Dr. Pettaway and her mentor, Gabrielle Marzani, MD, conducted an informal survey of 12 supervising psychiatrists at the University of Virginia.
The survey results were presented at the virtual American Psychiatric Association 2021 Annual Meeting.
Ten of the 12 psychiatrists who were surveyed were caring for a patient who died while under their care. Five of those psychiatrists reported going to at least one patient’s funeral over the course of their career.
Among the psychiatrists who attended a patient’s funeral, their attendance was often based on their clinical intuition, their relationship with the family, or whether the patient was an established presence in the community. In the latter case, the psychiatrist attended as a community member.
The number of years in practice also mattered. Fewer senior faculty reported that they would be hesitant to attend and that they would not attend without a formal invitation from the family. Senior career psychiatrists were more likely to attend and felt that an invitation was not required.
None of the psychiatrists surveyed had received training or guidance on attending patients’ funerals at any point in their career.
Given the absence of formal recommendations, Dr. Pettaway believes increased conversation on this topic as part of residency training programs would help psychiatrists navigate these complex situations.
A complex issue
Commenting on the topic for an interview, Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, said this is an “interesting and important topic that is underdiscussed.”
“I don’t think there’s a right answer that applies to every situation,” said Dr. Appelbaum, a past president of the APA.
There will be times, he said, when psychiatrists or other mental health professionals have worked closely with a patient for many years and may have interacted with the family over that period.
“When that patient passes away, they may feel, and the family may feel, that it would be comforting and appropriate for them to be at the funeral,” said Dr. Appelbaum.
However, he added,
“There are obviously a number of complexities involved. One is how the family feels about the relationship with the psychiatrist – whether they were accepting of the reality that the patient had a mental disorder and was in treatment,” he said.
There is also the question of confidentiality, said Dr. Appelbaum.
“If it’s a large funeral and the psychiatrist is just one face in the crowd, that’s not likely to be an issue. But if it’s a relatively small group of mourners, all of whom know each other, and an unknown figure pops up, that could raise questions and perhaps inadvertently reveal to family members or friends that the deceased had a psychiatric condition and was in treatment. That needs to be taken into account as well,” he added.
In cases in which the family invites the psychiatrist, confidentiality is not a concern, and attendance by the psychiatrist is something the patient would have wanted, said Dr. Appelbaum.
How the patient died may also be factor. When a patient dies by suicide, it’s an “emotionally charged situation for both sides,” said Dr. Appelbaum.
In the case of a suicide, he noted, the deceased was often an active patient, and both the psychiatrist and the family are dealing with strong emotions – the psychiatrist with regret over loss of the patient and perhaps with questions as to what could have been done differently, and the family with sorrow but “also sometimes with suspicion or anger in that the psychiatrist somehow failed to keep the patient alive,” Dr. Appelbaum noted.
“In this situation, it’s even more crucial for the psychiatrist or other mental health professionals to take the lead from the family – perhaps to initiate contact to express condolences and inquire delicately about the funeral arrangements and whether their presence would be welcomed,” he said.
The research had no specific funding. Dr. Pettaway and Dr. Appelbaum have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychiatrists often develop long-term relationships with their patients, but what happens when a patient dies? Should the psychiatrist attend the patient’s funeral?
It’s a question Ashley Pettaway, MD, faced as a medical resident at the University of Alabama School of Medicine.
For 2 months, Dr. Pettaway was involved in the day-to-day care of a woman in her 40s who ultimately died. As part of that care, Dr. Pettaway had regular meetings with the patient’s husband and family members.
“The patient was about my mother’s age, so I naturally was kind of attached to her,” Dr. Pettaway told this news organization. After she died, her family invited Dr. Pettaway to the funeral.
“While I couldn’t make it to the funeral, it got me thinking. Should I go? If I go, what do I say? Who do I sit with? How do I introduce myself?” wondered Dr. Pettaway, now a resident in the department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville.
She turned to the literature but found very little regarding psychiatrists attending their patients’ funerals. “This was surprising to me because in psychiatry, you can get so engrossed in patients’ lives,” Dr. Pettaway said.
Given the lack of rules or formal guidance on psychiatrists attending patients’ funerals, Dr. Pettaway and her mentor, Gabrielle Marzani, MD, conducted an informal survey of 12 supervising psychiatrists at the University of Virginia.
The survey results were presented at the virtual American Psychiatric Association 2021 Annual Meeting.
Ten of the 12 psychiatrists who were surveyed were caring for a patient who died while under their care. Five of those psychiatrists reported going to at least one patient’s funeral over the course of their career.
Among the psychiatrists who attended a patient’s funeral, their attendance was often based on their clinical intuition, their relationship with the family, or whether the patient was an established presence in the community. In the latter case, the psychiatrist attended as a community member.
The number of years in practice also mattered. Fewer senior faculty reported that they would be hesitant to attend and that they would not attend without a formal invitation from the family. Senior career psychiatrists were more likely to attend and felt that an invitation was not required.
None of the psychiatrists surveyed had received training or guidance on attending patients’ funerals at any point in their career.
Given the absence of formal recommendations, Dr. Pettaway believes increased conversation on this topic as part of residency training programs would help psychiatrists navigate these complex situations.
A complex issue
Commenting on the topic for an interview, Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, said this is an “interesting and important topic that is underdiscussed.”
“I don’t think there’s a right answer that applies to every situation,” said Dr. Appelbaum, a past president of the APA.
There will be times, he said, when psychiatrists or other mental health professionals have worked closely with a patient for many years and may have interacted with the family over that period.
“When that patient passes away, they may feel, and the family may feel, that it would be comforting and appropriate for them to be at the funeral,” said Dr. Appelbaum.
However, he added,
“There are obviously a number of complexities involved. One is how the family feels about the relationship with the psychiatrist – whether they were accepting of the reality that the patient had a mental disorder and was in treatment,” he said.
There is also the question of confidentiality, said Dr. Appelbaum.
“If it’s a large funeral and the psychiatrist is just one face in the crowd, that’s not likely to be an issue. But if it’s a relatively small group of mourners, all of whom know each other, and an unknown figure pops up, that could raise questions and perhaps inadvertently reveal to family members or friends that the deceased had a psychiatric condition and was in treatment. That needs to be taken into account as well,” he added.
In cases in which the family invites the psychiatrist, confidentiality is not a concern, and attendance by the psychiatrist is something the patient would have wanted, said Dr. Appelbaum.
How the patient died may also be factor. When a patient dies by suicide, it’s an “emotionally charged situation for both sides,” said Dr. Appelbaum.
In the case of a suicide, he noted, the deceased was often an active patient, and both the psychiatrist and the family are dealing with strong emotions – the psychiatrist with regret over loss of the patient and perhaps with questions as to what could have been done differently, and the family with sorrow but “also sometimes with suspicion or anger in that the psychiatrist somehow failed to keep the patient alive,” Dr. Appelbaum noted.
“In this situation, it’s even more crucial for the psychiatrist or other mental health professionals to take the lead from the family – perhaps to initiate contact to express condolences and inquire delicately about the funeral arrangements and whether their presence would be welcomed,” he said.
The research had no specific funding. Dr. Pettaway and Dr. Appelbaum have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Support group for Asian Americans uses theater to cope with COVID
An online, culturally based peer support group that uses theater and other creative outlets is helping Asian Americans cope with the COVID-19 pandemic, new research shows.
The findings of the qualitative study suggest that the program could be a model to support the mental health of other minority community groups during the COVID pandemic and beyond, say investigators from the Yale University Child Study Center, New Haven, Conn.
The Yale Compassionate Home, Action Together (CHATogether) group was created to promote emotional wellness among Asian American youth, young adults, and their families.
Early in the pandemic, it expanded its purpose to serve as a COVID-19 support group. Through social media outreach, CHATogether encourages members to cope with COVID-19 by using productive and creative outlets.
“We are a community education program serving Asian American families,” said Eunice Yuen, MD, PhD, the program’s founder and director, who is with the Yale University Child Study Center.
“ such as family conflict and xenophobic attacks,” said Dr. Yuen.
She discussed the program at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Skits, role playing
CHATogether groups consist of people with similar experiences and challenges who support each other through weekly online group meetings, she explained.
Group members work together to create family conflict scenarios and role-play dialogues on topics amplified during the COVID-19 pandemic, such as cross-cultural challenges among Asian Americans, academic expectations in home schooling, and Black Lives Matter and LGBTQ conflicts within Asian families.
Group members create skits that are based on their personal experiences and that allow them to work through their own internal conflicts and gain a sense of agency, said Dr. Yuen.
“CHATogether is really the interface of mental health, art, and theater, and we’re trying to create a vehicle that can be a lighthearted way for people to talk about mental health, especially for Asian American families,” said Dr. Yuen.
Preliminary results from a focus group with 10 CHATogether members who joined the program since the pandemic started identified four major ways in which the program has had a positive impact on the mental health and well-being of participants:
- It provides a safe and supportive environment, strengthens bonds between members, and increases the sense of belonging, thus encouraging engagement.
- It provides structural consistency/stability through regular meetings and consistent group functions. Weekly meetings provide a sense of control and hope in the midst of uncertainty during periods of sheltering in place.
- Through adapting the group to virtual platforms, group members experience the inherent strengths of a growth mindset and cognitive flexibility when facing challenges.
- It supports healthy coping skills through sublimation and altruism.
Looking ahead, Dr. Yuen said, the team plans to investigate the validity and effectiveness of this model and to expand the group to include other minorities, school educators, and medical education for trainees and medical students.
Commenting on the program, briefing moderator Jeffrey Borenstein, MD, president and CEO of the Brain and Behavior Research Foundation and editor-in-chief of Psychiatric News, described the initiative as a “great project that serves as a model that can be used not only for Asian Americans but for other groups.
“I think the key to it is that cultural sensitivity that we need to really take into account and cultural differences among people in order to best engage them and help support them. I think this program does that beautifully,” said Dr. Borenstein.
The work was supported by the APA’s Substance Abuse and Mental Health Services Administration Minority Fellowship, which provides a 1-year fellowship to psychiatry residents committed to addressing minority psychiatric mental health issues. Dr. Yuen and Dr. Borenstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An online, culturally based peer support group that uses theater and other creative outlets is helping Asian Americans cope with the COVID-19 pandemic, new research shows.
The findings of the qualitative study suggest that the program could be a model to support the mental health of other minority community groups during the COVID pandemic and beyond, say investigators from the Yale University Child Study Center, New Haven, Conn.
The Yale Compassionate Home, Action Together (CHATogether) group was created to promote emotional wellness among Asian American youth, young adults, and their families.
Early in the pandemic, it expanded its purpose to serve as a COVID-19 support group. Through social media outreach, CHATogether encourages members to cope with COVID-19 by using productive and creative outlets.
“We are a community education program serving Asian American families,” said Eunice Yuen, MD, PhD, the program’s founder and director, who is with the Yale University Child Study Center.
“ such as family conflict and xenophobic attacks,” said Dr. Yuen.
She discussed the program at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Skits, role playing
CHATogether groups consist of people with similar experiences and challenges who support each other through weekly online group meetings, she explained.
Group members work together to create family conflict scenarios and role-play dialogues on topics amplified during the COVID-19 pandemic, such as cross-cultural challenges among Asian Americans, academic expectations in home schooling, and Black Lives Matter and LGBTQ conflicts within Asian families.
Group members create skits that are based on their personal experiences and that allow them to work through their own internal conflicts and gain a sense of agency, said Dr. Yuen.
“CHATogether is really the interface of mental health, art, and theater, and we’re trying to create a vehicle that can be a lighthearted way for people to talk about mental health, especially for Asian American families,” said Dr. Yuen.
Preliminary results from a focus group with 10 CHATogether members who joined the program since the pandemic started identified four major ways in which the program has had a positive impact on the mental health and well-being of participants:
- It provides a safe and supportive environment, strengthens bonds between members, and increases the sense of belonging, thus encouraging engagement.
- It provides structural consistency/stability through regular meetings and consistent group functions. Weekly meetings provide a sense of control and hope in the midst of uncertainty during periods of sheltering in place.
- Through adapting the group to virtual platforms, group members experience the inherent strengths of a growth mindset and cognitive flexibility when facing challenges.
- It supports healthy coping skills through sublimation and altruism.
Looking ahead, Dr. Yuen said, the team plans to investigate the validity and effectiveness of this model and to expand the group to include other minorities, school educators, and medical education for trainees and medical students.
Commenting on the program, briefing moderator Jeffrey Borenstein, MD, president and CEO of the Brain and Behavior Research Foundation and editor-in-chief of Psychiatric News, described the initiative as a “great project that serves as a model that can be used not only for Asian Americans but for other groups.
“I think the key to it is that cultural sensitivity that we need to really take into account and cultural differences among people in order to best engage them and help support them. I think this program does that beautifully,” said Dr. Borenstein.
The work was supported by the APA’s Substance Abuse and Mental Health Services Administration Minority Fellowship, which provides a 1-year fellowship to psychiatry residents committed to addressing minority psychiatric mental health issues. Dr. Yuen and Dr. Borenstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An online, culturally based peer support group that uses theater and other creative outlets is helping Asian Americans cope with the COVID-19 pandemic, new research shows.
The findings of the qualitative study suggest that the program could be a model to support the mental health of other minority community groups during the COVID pandemic and beyond, say investigators from the Yale University Child Study Center, New Haven, Conn.
The Yale Compassionate Home, Action Together (CHATogether) group was created to promote emotional wellness among Asian American youth, young adults, and their families.
Early in the pandemic, it expanded its purpose to serve as a COVID-19 support group. Through social media outreach, CHATogether encourages members to cope with COVID-19 by using productive and creative outlets.
“We are a community education program serving Asian American families,” said Eunice Yuen, MD, PhD, the program’s founder and director, who is with the Yale University Child Study Center.
“ such as family conflict and xenophobic attacks,” said Dr. Yuen.
She discussed the program at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Skits, role playing
CHATogether groups consist of people with similar experiences and challenges who support each other through weekly online group meetings, she explained.
Group members work together to create family conflict scenarios and role-play dialogues on topics amplified during the COVID-19 pandemic, such as cross-cultural challenges among Asian Americans, academic expectations in home schooling, and Black Lives Matter and LGBTQ conflicts within Asian families.
Group members create skits that are based on their personal experiences and that allow them to work through their own internal conflicts and gain a sense of agency, said Dr. Yuen.
“CHATogether is really the interface of mental health, art, and theater, and we’re trying to create a vehicle that can be a lighthearted way for people to talk about mental health, especially for Asian American families,” said Dr. Yuen.
Preliminary results from a focus group with 10 CHATogether members who joined the program since the pandemic started identified four major ways in which the program has had a positive impact on the mental health and well-being of participants:
- It provides a safe and supportive environment, strengthens bonds between members, and increases the sense of belonging, thus encouraging engagement.
- It provides structural consistency/stability through regular meetings and consistent group functions. Weekly meetings provide a sense of control and hope in the midst of uncertainty during periods of sheltering in place.
- Through adapting the group to virtual platforms, group members experience the inherent strengths of a growth mindset and cognitive flexibility when facing challenges.
- It supports healthy coping skills through sublimation and altruism.
Looking ahead, Dr. Yuen said, the team plans to investigate the validity and effectiveness of this model and to expand the group to include other minorities, school educators, and medical education for trainees and medical students.
Commenting on the program, briefing moderator Jeffrey Borenstein, MD, president and CEO of the Brain and Behavior Research Foundation and editor-in-chief of Psychiatric News, described the initiative as a “great project that serves as a model that can be used not only for Asian Americans but for other groups.
“I think the key to it is that cultural sensitivity that we need to really take into account and cultural differences among people in order to best engage them and help support them. I think this program does that beautifully,” said Dr. Borenstein.
The work was supported by the APA’s Substance Abuse and Mental Health Services Administration Minority Fellowship, which provides a 1-year fellowship to psychiatry residents committed to addressing minority psychiatric mental health issues. Dr. Yuen and Dr. Borenstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dr. Fauci: Feds may ease indoor mask mandates soon
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Suicide risk prediction tools fail people of color
Current models used to predict suicide risk fall short for racialized populations including Black, Indigenous, and people of color (BIPOC), new research shows.
Investigators developed two suicide prediction models to examine whether these types of tools are accurate in their predictive abilities, or whether they are flawed.
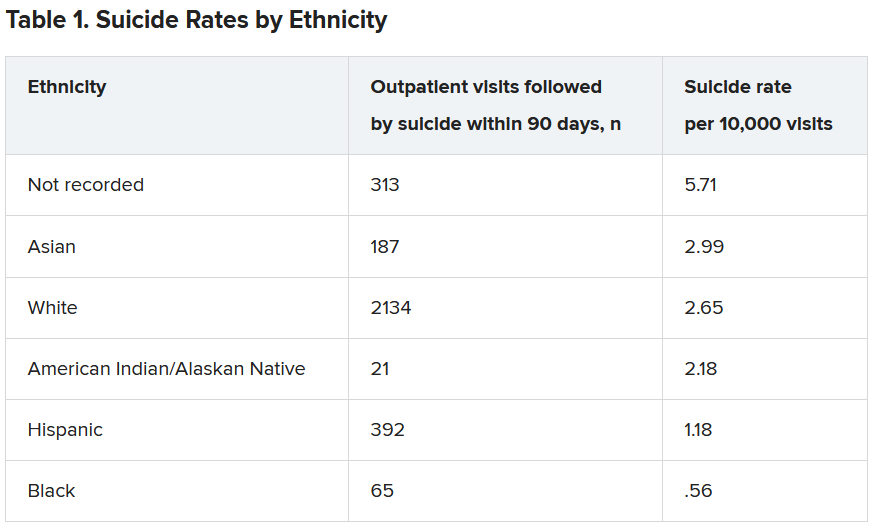
They found both prediction models failed to identify high-risk BIPOC individuals. In the first model, nearly half of outpatient visits followed by suicide were identified in White patients versus only 7% of visits followed by suicide in BIPOC patients. The second model had a sensitivity of 41% for White patients, but just 3% for Black patients and 7% for American Indian/Alaskan Native patients.
“You don’t know whether a prediction model will be useful or harmful until it’s evaluated. The take-home message of our study is this: You have to look,” lead author Yates Coley, PhD, assistant investigator, Kaiser Permanente Washington Health Research Institute, Seattle, said in an interview.
The study was published online April 28, 2021, in JAMA Psychiatry.
Racial inequities
Suicide risk prediction models have been “developed and validated in several settings” and are now in regular use at the Veterans Health Administration, HealthPartners, and Kaiser Permanente, the authors wrote.
But the performance of suicide risk prediction models, while accurate in the overall population, “remains unexamined” in particular subpopulations, they noted.
“Health records data reflect existing racial and ethnic inequities in health care access, quality, and outcomes; and prediction models using health records data may perpetuate these disparities by presuming that past healthcare patterns accurately reflect actual needs,” Dr. Coley said.
Dr. Coley and associates “wanted to make sure that any suicide prediction model we implemented in clinical care reduced health disparities rather than exacerbated them.”
To investigate, researchers examined all outpatient mental health visits to seven large integrated health care systems by patients 13 years and older (n = 13,980,570 visits by 1,422,534 patients; 64% female, mean age, 42 years). The study spanned from Jan. 1, 2009, to Sept. 30, 2017, with follow-up through Dec. 31, 2017.
In particular, researchers looked at suicides that took place within 90 days following the outpatient visit.
Researchers used two prediction models: logistic regression with LASSO (Least Absolute Shrinkage and Selection Operator) variable selection and random forest technique, a “tree-based method that explores interactions between predictors (including those with race and ethnicity) in estimating probability of an outcome.”
The models considered prespecified interactions between predictors, including prior diagnoses, suicide attempts, and PHQ-9 [Patient Health Questionnaire–9] responses, and race and ethnicity data.
Researchers evaluated performance of the prediction models in the overall validation set and within subgroups defined by race/ethnicity.
The area under the curve measured model discrimination, and sensitivity was estimated for global and race/ethnicity-specific thresholds.
‘Unacceptable’ scenario
Within the total population, there were 768 deaths by suicide within 90 days of 3,143 visits. Suicide rates were highest for visits by patients with no recorded race/ethnicity, followed by visits by Asian, White, American Indian/Alaskan Native, Hispanic, and Black patients.
Both models showed “high” AUC sensitivity for White, Hispanic, and Asian patients but “poor” AUC sensitivity for BIPOC and patients without recorded race/ethnicity, the authors reported.
“Implementation of prediction models has to be considered in the broader context of unmet health care needs,” said Dr. Coley.
“In our specific example of suicide prediction, BIPOC populations already face substantial barriers in accessing quality mental health care and, as a result, have poorer outcomes, and using either of the suicide prediction models examined in our study will provide less benefit to already-underserved populations and widen existing care gaps,” a scenario Dr. Coley said is “unacceptable.”
“ she added.
Biased algorithms
Commenting on the study, Jonathan Singer, PhD, LCSW, associate professor at Loyola University, Chicago, described it as an “important contribution because it points to a systemic problem and also to the fact that the algorithms we create are biased, created by humans, and humans are biased.”
Although the study focused on the health care system, Dr. Singer believes the findings have implications for individual clinicians.
“If clinicians may be biased against identifying suicide risk in Black and Native American patients, they may attribute suicidal risk to something else. For example, we know that in Black Americans, expressions of intense emotions are oftentimes interpreted as aggression or being threatening, as opposed to indicators of sadness or fear,” noted Dr. Singer, who is also president of the American Academy of Suicidology and was not involved with the study,
“Clinicians who misinterpret these intense emotions are less likely to identify a Black client or patient who is suicidal,” Dr. Singer said.
The research was supported by the Mental Health Research Network from the National Institute of Mental Health. Dr. Coley has reported receiving support through a grant from the Agency for Healthcare Research and Quality. Dr. Singer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Current models used to predict suicide risk fall short for racialized populations including Black, Indigenous, and people of color (BIPOC), new research shows.
Investigators developed two suicide prediction models to examine whether these types of tools are accurate in their predictive abilities, or whether they are flawed.
They found both prediction models failed to identify high-risk BIPOC individuals. In the first model, nearly half of outpatient visits followed by suicide were identified in White patients versus only 7% of visits followed by suicide in BIPOC patients. The second model had a sensitivity of 41% for White patients, but just 3% for Black patients and 7% for American Indian/Alaskan Native patients.
“You don’t know whether a prediction model will be useful or harmful until it’s evaluated. The take-home message of our study is this: You have to look,” lead author Yates Coley, PhD, assistant investigator, Kaiser Permanente Washington Health Research Institute, Seattle, said in an interview.
The study was published online April 28, 2021, in JAMA Psychiatry.
Racial inequities
Suicide risk prediction models have been “developed and validated in several settings” and are now in regular use at the Veterans Health Administration, HealthPartners, and Kaiser Permanente, the authors wrote.
But the performance of suicide risk prediction models, while accurate in the overall population, “remains unexamined” in particular subpopulations, they noted.
“Health records data reflect existing racial and ethnic inequities in health care access, quality, and outcomes; and prediction models using health records data may perpetuate these disparities by presuming that past healthcare patterns accurately reflect actual needs,” Dr. Coley said.
Dr. Coley and associates “wanted to make sure that any suicide prediction model we implemented in clinical care reduced health disparities rather than exacerbated them.”
To investigate, researchers examined all outpatient mental health visits to seven large integrated health care systems by patients 13 years and older (n = 13,980,570 visits by 1,422,534 patients; 64% female, mean age, 42 years). The study spanned from Jan. 1, 2009, to Sept. 30, 2017, with follow-up through Dec. 31, 2017.
In particular, researchers looked at suicides that took place within 90 days following the outpatient visit.
Researchers used two prediction models: logistic regression with LASSO (Least Absolute Shrinkage and Selection Operator) variable selection and random forest technique, a “tree-based method that explores interactions between predictors (including those with race and ethnicity) in estimating probability of an outcome.”
The models considered prespecified interactions between predictors, including prior diagnoses, suicide attempts, and PHQ-9 [Patient Health Questionnaire–9] responses, and race and ethnicity data.
Researchers evaluated performance of the prediction models in the overall validation set and within subgroups defined by race/ethnicity.
The area under the curve measured model discrimination, and sensitivity was estimated for global and race/ethnicity-specific thresholds.
‘Unacceptable’ scenario
Within the total population, there were 768 deaths by suicide within 90 days of 3,143 visits. Suicide rates were highest for visits by patients with no recorded race/ethnicity, followed by visits by Asian, White, American Indian/Alaskan Native, Hispanic, and Black patients.
Both models showed “high” AUC sensitivity for White, Hispanic, and Asian patients but “poor” AUC sensitivity for BIPOC and patients without recorded race/ethnicity, the authors reported.
“Implementation of prediction models has to be considered in the broader context of unmet health care needs,” said Dr. Coley.
“In our specific example of suicide prediction, BIPOC populations already face substantial barriers in accessing quality mental health care and, as a result, have poorer outcomes, and using either of the suicide prediction models examined in our study will provide less benefit to already-underserved populations and widen existing care gaps,” a scenario Dr. Coley said is “unacceptable.”
“ she added.
Biased algorithms
Commenting on the study, Jonathan Singer, PhD, LCSW, associate professor at Loyola University, Chicago, described it as an “important contribution because it points to a systemic problem and also to the fact that the algorithms we create are biased, created by humans, and humans are biased.”
Although the study focused on the health care system, Dr. Singer believes the findings have implications for individual clinicians.
“If clinicians may be biased against identifying suicide risk in Black and Native American patients, they may attribute suicidal risk to something else. For example, we know that in Black Americans, expressions of intense emotions are oftentimes interpreted as aggression or being threatening, as opposed to indicators of sadness or fear,” noted Dr. Singer, who is also president of the American Academy of Suicidology and was not involved with the study,
“Clinicians who misinterpret these intense emotions are less likely to identify a Black client or patient who is suicidal,” Dr. Singer said.
The research was supported by the Mental Health Research Network from the National Institute of Mental Health. Dr. Coley has reported receiving support through a grant from the Agency for Healthcare Research and Quality. Dr. Singer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Current models used to predict suicide risk fall short for racialized populations including Black, Indigenous, and people of color (BIPOC), new research shows.
Investigators developed two suicide prediction models to examine whether these types of tools are accurate in their predictive abilities, or whether they are flawed.
They found both prediction models failed to identify high-risk BIPOC individuals. In the first model, nearly half of outpatient visits followed by suicide were identified in White patients versus only 7% of visits followed by suicide in BIPOC patients. The second model had a sensitivity of 41% for White patients, but just 3% for Black patients and 7% for American Indian/Alaskan Native patients.
“You don’t know whether a prediction model will be useful or harmful until it’s evaluated. The take-home message of our study is this: You have to look,” lead author Yates Coley, PhD, assistant investigator, Kaiser Permanente Washington Health Research Institute, Seattle, said in an interview.
The study was published online April 28, 2021, in JAMA Psychiatry.
Racial inequities
Suicide risk prediction models have been “developed and validated in several settings” and are now in regular use at the Veterans Health Administration, HealthPartners, and Kaiser Permanente, the authors wrote.
But the performance of suicide risk prediction models, while accurate in the overall population, “remains unexamined” in particular subpopulations, they noted.
“Health records data reflect existing racial and ethnic inequities in health care access, quality, and outcomes; and prediction models using health records data may perpetuate these disparities by presuming that past healthcare patterns accurately reflect actual needs,” Dr. Coley said.
Dr. Coley and associates “wanted to make sure that any suicide prediction model we implemented in clinical care reduced health disparities rather than exacerbated them.”
To investigate, researchers examined all outpatient mental health visits to seven large integrated health care systems by patients 13 years and older (n = 13,980,570 visits by 1,422,534 patients; 64% female, mean age, 42 years). The study spanned from Jan. 1, 2009, to Sept. 30, 2017, with follow-up through Dec. 31, 2017.
In particular, researchers looked at suicides that took place within 90 days following the outpatient visit.
Researchers used two prediction models: logistic regression with LASSO (Least Absolute Shrinkage and Selection Operator) variable selection and random forest technique, a “tree-based method that explores interactions between predictors (including those with race and ethnicity) in estimating probability of an outcome.”
The models considered prespecified interactions between predictors, including prior diagnoses, suicide attempts, and PHQ-9 [Patient Health Questionnaire–9] responses, and race and ethnicity data.
Researchers evaluated performance of the prediction models in the overall validation set and within subgroups defined by race/ethnicity.
The area under the curve measured model discrimination, and sensitivity was estimated for global and race/ethnicity-specific thresholds.
‘Unacceptable’ scenario
Within the total population, there were 768 deaths by suicide within 90 days of 3,143 visits. Suicide rates were highest for visits by patients with no recorded race/ethnicity, followed by visits by Asian, White, American Indian/Alaskan Native, Hispanic, and Black patients.
Both models showed “high” AUC sensitivity for White, Hispanic, and Asian patients but “poor” AUC sensitivity for BIPOC and patients without recorded race/ethnicity, the authors reported.
“Implementation of prediction models has to be considered in the broader context of unmet health care needs,” said Dr. Coley.
“In our specific example of suicide prediction, BIPOC populations already face substantial barriers in accessing quality mental health care and, as a result, have poorer outcomes, and using either of the suicide prediction models examined in our study will provide less benefit to already-underserved populations and widen existing care gaps,” a scenario Dr. Coley said is “unacceptable.”
“ she added.
Biased algorithms
Commenting on the study, Jonathan Singer, PhD, LCSW, associate professor at Loyola University, Chicago, described it as an “important contribution because it points to a systemic problem and also to the fact that the algorithms we create are biased, created by humans, and humans are biased.”
Although the study focused on the health care system, Dr. Singer believes the findings have implications for individual clinicians.
“If clinicians may be biased against identifying suicide risk in Black and Native American patients, they may attribute suicidal risk to something else. For example, we know that in Black Americans, expressions of intense emotions are oftentimes interpreted as aggression or being threatening, as opposed to indicators of sadness or fear,” noted Dr. Singer, who is also president of the American Academy of Suicidology and was not involved with the study,
“Clinicians who misinterpret these intense emotions are less likely to identify a Black client or patient who is suicidal,” Dr. Singer said.
The research was supported by the Mental Health Research Network from the National Institute of Mental Health. Dr. Coley has reported receiving support through a grant from the Agency for Healthcare Research and Quality. Dr. Singer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA authorizes Pfizer COVID vaccine for teens 12-15
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
TIPP the scales in managing stress
The past year presented unprecedented challenges for many. In addition, mental health services have also been stretched to capacity. Anecdotally, some hospitals and emergency departments note that more youth have been presenting in mental health crises, and the severity of symptoms has also been higher. Safety planning is important, including working with patients to identify skills they can use in distress. Even those who do not experience suicidal thoughts may struggle with dysregulation or may use coping strategies that may not be the healthiest in the long term.
Within my practice, I see some families who are still waiting for an available therapist, or some may not wish to participate in therapy despite its being recommended. For these families, supporting them in using strategies that they may be willing and able to use in the moment to help them get through the moment of crisis can been helpful:
Case example (identifying details have been changed)
Emily is a 17-year-old girl who has a history of generalized anxiety disorder and obsessive-compulsive disorder. She has had multiple medication trials and a course of cognitive behavioral therapy when younger, with significant improvement in symptoms. She returns to clinic because of increased anxiety related to stressors of the pandemic. She wishes to not return to therapy because of feeling that she received maximal benefit and that further sessions would not be fruitful. However, she struggles with identifying what skills she can use, and her anxiety heightens significantly to near-panic and hyperventilating with even cursory exploration of triggers for her symptoms. Medications are also discussed during this appointment, and it is noted that it may take some time to see therapeutic effect. Of note, she reports no acute safety concerns. She has engaged in skin picking. No reported substance use. As she hyperventilates, she was asked to identify items in the room matching the colors of the rainbow in order. She was able to quickly do this, and then was asked to do it again. Afterward, she noted feeling much less anxious because it distracted her from her thoughts.
Distress tolerance skills can be very helpful to navigate getting through a crisis. When under stress, some may be more likely to engage in behaviors that are not helpful in the long term such as using avoidance; procrastinating; consuming tobacco, alcohol, or other substances; spending too much time on screens; or engaging in self-harm behaviors. While some of these activities may be okay in moderation, others are always harmful. At times, when encouraging patients to use skills with which they may be more familiar, e.g., deep breathing, progressive muscle relaxation, the response may be, “these don’t work!” It can be important to distinguish that the function of these skills is not to make someone feel good or to eliminate the stressor, but to “take some of the edge off” so they are less likely to slide into problematic behaviors. It can be beneficial to have multiple tools at one’s disposal because not all skills will always be effective or available.
TIPP skills (temperature, intense exercise, paced breathing, progressive muscle relaxation) are distress tolerance skills from dialectical behavioral therapy (DBT),1 which was initially developed to treat individuals with borderline personality disorder. More recently, the therapy modality has been applied to individuals who may struggle with emotion regulation for a variety of reasons. TIPP skills work quickly (within seconds to minutes) with the aim to decrease physiological arousal. They do not require a lot of thinking, and many are portable or easy to use. Given the speed of effect, these skills can also be used in lieu of p.r.n. medications or patients can be counseled about trying these instead of turning to substance use. The effect is brief (5-20 minutes), although this may lower the affective temperature sufficiently for someone to get through the intense moment or to be able to then utilize other skills that may require more cognitive reserves.
T – Temperature
Holding one’s breath and placing one’s face in cold water (above 50°) for 10-20 seconds to stimulate the diving response and decrease heart rate. Patients can repeat this up to 3 times. Alternatively, cold compresses or gel eye masks can be used.
I – Intense exercise
Aerobic exercise for 10-20 minutes. This can include running, jumping jacks, dancing to loud music in a way that feels intense. The parasympathetic nervous system (PNS) is activated for approximately 20 minutes after cessation of intense exercise.
P – Paced breathing
Decreasing rate of breathing, with each inhalation/exhalation cycle lasting 10-12 seconds and the exhale being longer than the inhale also activates the PNS.
P – Progressive muscle relaxation (PMR)
Sequentially tensing and relaxing muscles from head to toes. Having at least 5-10 minutes to perform this exercise is preferred.2 Children’s Hospital of Philadelphia offerssample PMR recordings.
Body scans can also be helpful. This practice differs from PMR in that it is a mindfulness practice noting body sensations without trying to change them. The University of Vermont offers some sample exercises.3
These skills were described to Emily. She noted that dunking her face in cold water was effective and it was reassuring knowing she had a tool to help her anxiety. She started to push herself to go outside to exercise. We additionally incorporated other distraction techniques such as identifying items from colors of the rainbow that were around her. She appreciated that she could even do this discreetly while at school. At times she had to do a couple of rounds, but this could help stop her repetitive thoughts so she could use other skills.
Helping patients identify skills that can help in the moment can help them feel supported and gain traction in other areas.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. She has no relevant financial disclosures
References
1. Rathus JH, Miller AL. DBT® Skills manual for adolescents. 2015. Guilford Press.
2. Guided Relaxation Exercises, Children’s Hospital of Philadelphia.
3. Vermont Center for Children, Youth, and Families: Staying Close While Keeping Your Distance.
The past year presented unprecedented challenges for many. In addition, mental health services have also been stretched to capacity. Anecdotally, some hospitals and emergency departments note that more youth have been presenting in mental health crises, and the severity of symptoms has also been higher. Safety planning is important, including working with patients to identify skills they can use in distress. Even those who do not experience suicidal thoughts may struggle with dysregulation or may use coping strategies that may not be the healthiest in the long term.
Within my practice, I see some families who are still waiting for an available therapist, or some may not wish to participate in therapy despite its being recommended. For these families, supporting them in using strategies that they may be willing and able to use in the moment to help them get through the moment of crisis can been helpful:
Case example (identifying details have been changed)
Emily is a 17-year-old girl who has a history of generalized anxiety disorder and obsessive-compulsive disorder. She has had multiple medication trials and a course of cognitive behavioral therapy when younger, with significant improvement in symptoms. She returns to clinic because of increased anxiety related to stressors of the pandemic. She wishes to not return to therapy because of feeling that she received maximal benefit and that further sessions would not be fruitful. However, she struggles with identifying what skills she can use, and her anxiety heightens significantly to near-panic and hyperventilating with even cursory exploration of triggers for her symptoms. Medications are also discussed during this appointment, and it is noted that it may take some time to see therapeutic effect. Of note, she reports no acute safety concerns. She has engaged in skin picking. No reported substance use. As she hyperventilates, she was asked to identify items in the room matching the colors of the rainbow in order. She was able to quickly do this, and then was asked to do it again. Afterward, she noted feeling much less anxious because it distracted her from her thoughts.
Distress tolerance skills can be very helpful to navigate getting through a crisis. When under stress, some may be more likely to engage in behaviors that are not helpful in the long term such as using avoidance; procrastinating; consuming tobacco, alcohol, or other substances; spending too much time on screens; or engaging in self-harm behaviors. While some of these activities may be okay in moderation, others are always harmful. At times, when encouraging patients to use skills with which they may be more familiar, e.g., deep breathing, progressive muscle relaxation, the response may be, “these don’t work!” It can be important to distinguish that the function of these skills is not to make someone feel good or to eliminate the stressor, but to “take some of the edge off” so they are less likely to slide into problematic behaviors. It can be beneficial to have multiple tools at one’s disposal because not all skills will always be effective or available.
TIPP skills (temperature, intense exercise, paced breathing, progressive muscle relaxation) are distress tolerance skills from dialectical behavioral therapy (DBT),1 which was initially developed to treat individuals with borderline personality disorder. More recently, the therapy modality has been applied to individuals who may struggle with emotion regulation for a variety of reasons. TIPP skills work quickly (within seconds to minutes) with the aim to decrease physiological arousal. They do not require a lot of thinking, and many are portable or easy to use. Given the speed of effect, these skills can also be used in lieu of p.r.n. medications or patients can be counseled about trying these instead of turning to substance use. The effect is brief (5-20 minutes), although this may lower the affective temperature sufficiently for someone to get through the intense moment or to be able to then utilize other skills that may require more cognitive reserves.
T – Temperature
Holding one’s breath and placing one’s face in cold water (above 50°) for 10-20 seconds to stimulate the diving response and decrease heart rate. Patients can repeat this up to 3 times. Alternatively, cold compresses or gel eye masks can be used.
I – Intense exercise
Aerobic exercise for 10-20 minutes. This can include running, jumping jacks, dancing to loud music in a way that feels intense. The parasympathetic nervous system (PNS) is activated for approximately 20 minutes after cessation of intense exercise.
P – Paced breathing
Decreasing rate of breathing, with each inhalation/exhalation cycle lasting 10-12 seconds and the exhale being longer than the inhale also activates the PNS.
P – Progressive muscle relaxation (PMR)
Sequentially tensing and relaxing muscles from head to toes. Having at least 5-10 minutes to perform this exercise is preferred.2 Children’s Hospital of Philadelphia offerssample PMR recordings.
Body scans can also be helpful. This practice differs from PMR in that it is a mindfulness practice noting body sensations without trying to change them. The University of Vermont offers some sample exercises.3
These skills were described to Emily. She noted that dunking her face in cold water was effective and it was reassuring knowing she had a tool to help her anxiety. She started to push herself to go outside to exercise. We additionally incorporated other distraction techniques such as identifying items from colors of the rainbow that were around her. She appreciated that she could even do this discreetly while at school. At times she had to do a couple of rounds, but this could help stop her repetitive thoughts so she could use other skills.
Helping patients identify skills that can help in the moment can help them feel supported and gain traction in other areas.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. She has no relevant financial disclosures
References
1. Rathus JH, Miller AL. DBT® Skills manual for adolescents. 2015. Guilford Press.
2. Guided Relaxation Exercises, Children’s Hospital of Philadelphia.
3. Vermont Center for Children, Youth, and Families: Staying Close While Keeping Your Distance.
The past year presented unprecedented challenges for many. In addition, mental health services have also been stretched to capacity. Anecdotally, some hospitals and emergency departments note that more youth have been presenting in mental health crises, and the severity of symptoms has also been higher. Safety planning is important, including working with patients to identify skills they can use in distress. Even those who do not experience suicidal thoughts may struggle with dysregulation or may use coping strategies that may not be the healthiest in the long term.
Within my practice, I see some families who are still waiting for an available therapist, or some may not wish to participate in therapy despite its being recommended. For these families, supporting them in using strategies that they may be willing and able to use in the moment to help them get through the moment of crisis can been helpful:
Case example (identifying details have been changed)
Emily is a 17-year-old girl who has a history of generalized anxiety disorder and obsessive-compulsive disorder. She has had multiple medication trials and a course of cognitive behavioral therapy when younger, with significant improvement in symptoms. She returns to clinic because of increased anxiety related to stressors of the pandemic. She wishes to not return to therapy because of feeling that she received maximal benefit and that further sessions would not be fruitful. However, she struggles with identifying what skills she can use, and her anxiety heightens significantly to near-panic and hyperventilating with even cursory exploration of triggers for her symptoms. Medications are also discussed during this appointment, and it is noted that it may take some time to see therapeutic effect. Of note, she reports no acute safety concerns. She has engaged in skin picking. No reported substance use. As she hyperventilates, she was asked to identify items in the room matching the colors of the rainbow in order. She was able to quickly do this, and then was asked to do it again. Afterward, she noted feeling much less anxious because it distracted her from her thoughts.
Distress tolerance skills can be very helpful to navigate getting through a crisis. When under stress, some may be more likely to engage in behaviors that are not helpful in the long term such as using avoidance; procrastinating; consuming tobacco, alcohol, or other substances; spending too much time on screens; or engaging in self-harm behaviors. While some of these activities may be okay in moderation, others are always harmful. At times, when encouraging patients to use skills with which they may be more familiar, e.g., deep breathing, progressive muscle relaxation, the response may be, “these don’t work!” It can be important to distinguish that the function of these skills is not to make someone feel good or to eliminate the stressor, but to “take some of the edge off” so they are less likely to slide into problematic behaviors. It can be beneficial to have multiple tools at one’s disposal because not all skills will always be effective or available.
TIPP skills (temperature, intense exercise, paced breathing, progressive muscle relaxation) are distress tolerance skills from dialectical behavioral therapy (DBT),1 which was initially developed to treat individuals with borderline personality disorder. More recently, the therapy modality has been applied to individuals who may struggle with emotion regulation for a variety of reasons. TIPP skills work quickly (within seconds to minutes) with the aim to decrease physiological arousal. They do not require a lot of thinking, and many are portable or easy to use. Given the speed of effect, these skills can also be used in lieu of p.r.n. medications or patients can be counseled about trying these instead of turning to substance use. The effect is brief (5-20 minutes), although this may lower the affective temperature sufficiently for someone to get through the intense moment or to be able to then utilize other skills that may require more cognitive reserves.
T – Temperature
Holding one’s breath and placing one’s face in cold water (above 50°) for 10-20 seconds to stimulate the diving response and decrease heart rate. Patients can repeat this up to 3 times. Alternatively, cold compresses or gel eye masks can be used.
I – Intense exercise
Aerobic exercise for 10-20 minutes. This can include running, jumping jacks, dancing to loud music in a way that feels intense. The parasympathetic nervous system (PNS) is activated for approximately 20 minutes after cessation of intense exercise.
P – Paced breathing
Decreasing rate of breathing, with each inhalation/exhalation cycle lasting 10-12 seconds and the exhale being longer than the inhale also activates the PNS.
P – Progressive muscle relaxation (PMR)
Sequentially tensing and relaxing muscles from head to toes. Having at least 5-10 minutes to perform this exercise is preferred.2 Children’s Hospital of Philadelphia offerssample PMR recordings.
Body scans can also be helpful. This practice differs from PMR in that it is a mindfulness practice noting body sensations without trying to change them. The University of Vermont offers some sample exercises.3
These skills were described to Emily. She noted that dunking her face in cold water was effective and it was reassuring knowing she had a tool to help her anxiety. She started to push herself to go outside to exercise. We additionally incorporated other distraction techniques such as identifying items from colors of the rainbow that were around her. She appreciated that she could even do this discreetly while at school. At times she had to do a couple of rounds, but this could help stop her repetitive thoughts so she could use other skills.
Helping patients identify skills that can help in the moment can help them feel supported and gain traction in other areas.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. She has no relevant financial disclosures
References
1. Rathus JH, Miller AL. DBT® Skills manual for adolescents. 2015. Guilford Press.
2. Guided Relaxation Exercises, Children’s Hospital of Philadelphia.
3. Vermont Center for Children, Youth, and Families: Staying Close While Keeping Your Distance.
Transcranial brain stimulation can modulate placebo and nocebo experiences
, according to results of a new study published in the Proceedings of the National Academy of Sciences (PNAS).
“Placebo and nocebo effects are a critical component of clinical care and efficacy studies,” said senior author Jian Kong, MD, associate professor in the department of psychiatry at Massachusetts General Hospital, Charlestown campus. “Harnessing these effects in clinical practice and research could facilitate the development of new pain management methods,” he said. “Healing may involve multiple components: the self-healing properties of the body; the nonspecific effects of treatment (i.e., placebo effect); and the specific effect of a physical or pharmacologic intervention. Therefore, enhancing the placebo effect may ultimately boost the overall therapeutic effect of existing treatment,” he explained, emphasizing that the results are preliminary and should be interpreted with caution.
The authors noted that reducing nocebo effects could also be a major benefit “since patients discontinue prescribed medications, make unnecessary medical visits, and take additional medications to counteract adverse effects that are actually nocebo effects.”
Testing the hypothesis
The randomized, double-blind, sham-controlled study used transcranial direct current stimulation (tDCS), which delivers an electrical current to the brain via scalp electrodes. The aim was to see if stimulating the dorsolateral prefrontal cortex with tDCS could alter the brain’s perception of placebo and nocebo experiences.
The study included 81 participants (37 females, mean age: 27.4 years), who were randomized into one of three tDCS groups (anodal, cathodal, or sham).
All participants were first conditioned to believe that an inert cream was either lidocaine or capsaicin and that this cream could either dull the impact of a painful heat stimulus (placebo analgesia) or exacerbate it (nocebo hyperalgesia). Participants were then placed into a functional MRI scanner where tDCS was initiated. Painful stimuli were then applied to spots on their forearms where they believed they had either lidocaine, capsaicin, or a neutral control cream and they rated the pain using the Gracely Sensory Scale.
Placebo analgesia was defined as the difference between perceived pain intensity where participants believed they had lidocaine cream compared with where they believed they had control cream. Nocebo hyperalgesia was defined as the difference between perceived pain intensity where they believed they had capsaicin cream compared with where they believed they had control cream.
The researchers found that compared with sham tDCS, cathodal tDCS showed significant effects in increasing placebo analgesia and brain responses in the ventromedial prefrontal cortex (vmPFC), while anodal tDCS showed significant effects in inhibiting nocebo hyperalgesia and brain responses in the insula.
“The potential to enhance salubrious placebo effects and/or diminish treatment-interfering nocebo effects may have clinical significance,” the authors noted. “For example, clinical studies have suggested that expectancy is positively associated with chronic pain improvement, and using conditioning-like expectancy manipulation, we have shown that significantly boosting expectancy can improve treatment outcome.”
Proof of concept
Asked to comment on the study, Brian E. McGeeney, MD, of the John R. Graham Headache Center at Brigham and Women’s Faulkner Hospital in Boston, said “the findings are a proof of concept that it is possible to use noninvasive brain stimulation to modulate placebo and nocebo pain effects.”
Although the findings do not have immediate clinical application, they are “exciting” and “break new ground in expectancy research,” he said.
“It is important to recognize that the researchers are trying to utilize a purported expectancy mechanism rather than attempting to alter placebo/nocebo by verbal and other cues. It remains to be seen whether the manipulation of brief experimental pain like this can translate into altered chronic pain over time, the main clinical goal. Current tDCS therapy for various reasons is necessarily brief and one can ask whether there are meaningful changes from brief stimulation. Such results can foster speculation as to whether direct strategic placement of intracranial stimulation leads could result in more longstanding similar benefits.”
Dr. Kong holds equity in a startup company (MNT) and a pending patent to develop new peripheral neuromodulation tools, but declares no conflict of interest. All other authors declare no conflict of interest.
, according to results of a new study published in the Proceedings of the National Academy of Sciences (PNAS).
“Placebo and nocebo effects are a critical component of clinical care and efficacy studies,” said senior author Jian Kong, MD, associate professor in the department of psychiatry at Massachusetts General Hospital, Charlestown campus. “Harnessing these effects in clinical practice and research could facilitate the development of new pain management methods,” he said. “Healing may involve multiple components: the self-healing properties of the body; the nonspecific effects of treatment (i.e., placebo effect); and the specific effect of a physical or pharmacologic intervention. Therefore, enhancing the placebo effect may ultimately boost the overall therapeutic effect of existing treatment,” he explained, emphasizing that the results are preliminary and should be interpreted with caution.
The authors noted that reducing nocebo effects could also be a major benefit “since patients discontinue prescribed medications, make unnecessary medical visits, and take additional medications to counteract adverse effects that are actually nocebo effects.”
Testing the hypothesis
The randomized, double-blind, sham-controlled study used transcranial direct current stimulation (tDCS), which delivers an electrical current to the brain via scalp electrodes. The aim was to see if stimulating the dorsolateral prefrontal cortex with tDCS could alter the brain’s perception of placebo and nocebo experiences.
The study included 81 participants (37 females, mean age: 27.4 years), who were randomized into one of three tDCS groups (anodal, cathodal, or sham).
All participants were first conditioned to believe that an inert cream was either lidocaine or capsaicin and that this cream could either dull the impact of a painful heat stimulus (placebo analgesia) or exacerbate it (nocebo hyperalgesia). Participants were then placed into a functional MRI scanner where tDCS was initiated. Painful stimuli were then applied to spots on their forearms where they believed they had either lidocaine, capsaicin, or a neutral control cream and they rated the pain using the Gracely Sensory Scale.
Placebo analgesia was defined as the difference between perceived pain intensity where participants believed they had lidocaine cream compared with where they believed they had control cream. Nocebo hyperalgesia was defined as the difference between perceived pain intensity where they believed they had capsaicin cream compared with where they believed they had control cream.
The researchers found that compared with sham tDCS, cathodal tDCS showed significant effects in increasing placebo analgesia and brain responses in the ventromedial prefrontal cortex (vmPFC), while anodal tDCS showed significant effects in inhibiting nocebo hyperalgesia and brain responses in the insula.
“The potential to enhance salubrious placebo effects and/or diminish treatment-interfering nocebo effects may have clinical significance,” the authors noted. “For example, clinical studies have suggested that expectancy is positively associated with chronic pain improvement, and using conditioning-like expectancy manipulation, we have shown that significantly boosting expectancy can improve treatment outcome.”
Proof of concept
Asked to comment on the study, Brian E. McGeeney, MD, of the John R. Graham Headache Center at Brigham and Women’s Faulkner Hospital in Boston, said “the findings are a proof of concept that it is possible to use noninvasive brain stimulation to modulate placebo and nocebo pain effects.”
Although the findings do not have immediate clinical application, they are “exciting” and “break new ground in expectancy research,” he said.
“It is important to recognize that the researchers are trying to utilize a purported expectancy mechanism rather than attempting to alter placebo/nocebo by verbal and other cues. It remains to be seen whether the manipulation of brief experimental pain like this can translate into altered chronic pain over time, the main clinical goal. Current tDCS therapy for various reasons is necessarily brief and one can ask whether there are meaningful changes from brief stimulation. Such results can foster speculation as to whether direct strategic placement of intracranial stimulation leads could result in more longstanding similar benefits.”
Dr. Kong holds equity in a startup company (MNT) and a pending patent to develop new peripheral neuromodulation tools, but declares no conflict of interest. All other authors declare no conflict of interest.
, according to results of a new study published in the Proceedings of the National Academy of Sciences (PNAS).
“Placebo and nocebo effects are a critical component of clinical care and efficacy studies,” said senior author Jian Kong, MD, associate professor in the department of psychiatry at Massachusetts General Hospital, Charlestown campus. “Harnessing these effects in clinical practice and research could facilitate the development of new pain management methods,” he said. “Healing may involve multiple components: the self-healing properties of the body; the nonspecific effects of treatment (i.e., placebo effect); and the specific effect of a physical or pharmacologic intervention. Therefore, enhancing the placebo effect may ultimately boost the overall therapeutic effect of existing treatment,” he explained, emphasizing that the results are preliminary and should be interpreted with caution.
The authors noted that reducing nocebo effects could also be a major benefit “since patients discontinue prescribed medications, make unnecessary medical visits, and take additional medications to counteract adverse effects that are actually nocebo effects.”
Testing the hypothesis
The randomized, double-blind, sham-controlled study used transcranial direct current stimulation (tDCS), which delivers an electrical current to the brain via scalp electrodes. The aim was to see if stimulating the dorsolateral prefrontal cortex with tDCS could alter the brain’s perception of placebo and nocebo experiences.
The study included 81 participants (37 females, mean age: 27.4 years), who were randomized into one of three tDCS groups (anodal, cathodal, or sham).
All participants were first conditioned to believe that an inert cream was either lidocaine or capsaicin and that this cream could either dull the impact of a painful heat stimulus (placebo analgesia) or exacerbate it (nocebo hyperalgesia). Participants were then placed into a functional MRI scanner where tDCS was initiated. Painful stimuli were then applied to spots on their forearms where they believed they had either lidocaine, capsaicin, or a neutral control cream and they rated the pain using the Gracely Sensory Scale.
Placebo analgesia was defined as the difference between perceived pain intensity where participants believed they had lidocaine cream compared with where they believed they had control cream. Nocebo hyperalgesia was defined as the difference between perceived pain intensity where they believed they had capsaicin cream compared with where they believed they had control cream.
The researchers found that compared with sham tDCS, cathodal tDCS showed significant effects in increasing placebo analgesia and brain responses in the ventromedial prefrontal cortex (vmPFC), while anodal tDCS showed significant effects in inhibiting nocebo hyperalgesia and brain responses in the insula.
“The potential to enhance salubrious placebo effects and/or diminish treatment-interfering nocebo effects may have clinical significance,” the authors noted. “For example, clinical studies have suggested that expectancy is positively associated with chronic pain improvement, and using conditioning-like expectancy manipulation, we have shown that significantly boosting expectancy can improve treatment outcome.”
Proof of concept
Asked to comment on the study, Brian E. McGeeney, MD, of the John R. Graham Headache Center at Brigham and Women’s Faulkner Hospital in Boston, said “the findings are a proof of concept that it is possible to use noninvasive brain stimulation to modulate placebo and nocebo pain effects.”
Although the findings do not have immediate clinical application, they are “exciting” and “break new ground in expectancy research,” he said.
“It is important to recognize that the researchers are trying to utilize a purported expectancy mechanism rather than attempting to alter placebo/nocebo by verbal and other cues. It remains to be seen whether the manipulation of brief experimental pain like this can translate into altered chronic pain over time, the main clinical goal. Current tDCS therapy for various reasons is necessarily brief and one can ask whether there are meaningful changes from brief stimulation. Such results can foster speculation as to whether direct strategic placement of intracranial stimulation leads could result in more longstanding similar benefits.”
Dr. Kong holds equity in a startup company (MNT) and a pending patent to develop new peripheral neuromodulation tools, but declares no conflict of interest. All other authors declare no conflict of interest.
FROM PNAS
‘Malicious peer review’ destroyed doc’s career, he says
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
New digital ADHD intervention tools are emerging
New digital tools are on the horizon to help patients with attention-deficit/hyperactivity disorder (ADHD) manage the condition.
Speakers at the World Congress on ADHD – Virtual Event described innovations aimed at improving medication compliance or reducing symptoms through the use of smartphone technology such as apps and text messaging, and video games. Some of these technologies have shown promising results in clinical trials, but the experts called for additional studies to further vet their efficacy.
Digital technologies have limitations and should be seen as adjunctive rather than standalone tools that can aid clinicians and educators, said Hannah Kirk, PhD, a psychology research fellow at Monash University’s Turner Institute for Brain and Mental Health in Clayton, Australia. Dr. Kirk joined three other speakers for the session: “ADHD in the digital age – From pitfalls to challenges.”
An explosion in technology
ADHD, the most common neurodevelopmental disorder, has global prevalence rates ranging from 5% to 7%, said Dr. Kirk. Digital technology and digital health “have been heralded as having enormous potential to improve early access and to improve the increasing demand in child support services,” she said.
The world has seen an explosion of digital technology innovation in the last decade, spurred on most recently by the COVID-19 pandemic. New demand exists for tools in educational and health care settings to provide information and support through websites, apps, SMS, video conferencing, and wearable devices, Dr. Kirk said.
Looking at the landscape of ADHD digital therapeutics, “there are probably tens of thousands of apps and other digital products to treat and manage conditions across the spectrum,” said Scott H. Kollins, PhD, MS, a clinical psychologist at Duke Health’s ADHD Clinic in Durham, N.C.
In general, few developers of these products have conducted rigorous, well-controlled trials, he noted.
Video game interventions
AKL-T01, a tool that pairs continuous fine motor tasks and perceptual reaction time tasks, went through several rounds of clinical trials to achieve federal approval as a digital therapeutic.
“This not just another video game,” said Dr. Kollins, who helped developed it. The tool’s adaptive algorithms adjust and monitor task difficulty based on performance, using a video game format and rewards to engage users.
Two phase 3 trials provided the basis for the Food and Drug Administration’s approval of AKL-T01, also known as EndeavorRx, in 2020. The first trial, published in The Lancet, randomized 348 children 1:1 to receive either the AKL-T01 treatment or a controlled intervention, which was a word game. Participating children aged 8-12 with a confirmed ADHD diagnosis were asked to play the game for about 25 minutes a day, 5 days a week over 4 weeks. The study excluded children who were taking medicine.
The researchers reported statistically significant improvements in attentional functioning in the AKL-T01 group as rated by test of variables of attention. The trial reported no serious adverse events, although one child in the AKL-T01 group withdrew from the study.
“As kids go through this treatment, it’s challenging and the difficulty levels increase, so it’s not surprising that kids get frustrated with that, or have emotional outbursts,” Dr. Kollins said. Those reactions suggest that the intervention was working, he added.
A follow-up study, published in npj Digital Medicine, broadened the scope. That study included children who had taken medication and extended the study period. Overall, 206 children aged 8-14 (130 on stimulants and 76 on no medication) played the game for 28 days, taking a pause for another 28 days, then reinitiating the treatment.
As in the first trial, AKL-T01 significantly improved ADHD-related impairment, a metric that continued to improve in the second round of treatment. Looking at secondary outcomes, the proportion of children deemed as clinical responders on the Impairment Rating Scale, 68.3% of all of the participants were responders by the end of the study on the ADHD ratings scale, meaning there was a greater than 30% improvement in symptoms. Upward of 50% of participants at the end of the second round of treatment showed substantial improvement in their ADHD ratings scale scores.
“This was really a substantial move ... the first-ever app-based video game approved by the FDA,” noted Dr. Kollins, who is affiliated with the Duke Clinical Research Institute. Some skeptics have called this a marketing ploy or have questioned the integrity of the FDA approval process.
“I would submit and argue that the rigor of the trial speaks for itself,” he said. “But it’s not surprising that there’s skepticism in the clinical community about something like this – a brand new treatment modality.”
In her own research, Dr. Kirk has studied game-based interventions aimed at assessing ADHD and improving cognitive training. In 2018, her team developed a touch screen game–based intervention for early evaluation of attention skills, using six activities. In a visual search task, children were asked to locate red lobsters on a screen that showed a variety of underwater creatures. In another selection attention task, children were asked to scan the screen for a particular target, such as a yellow star, and to indicate whether that target was absent or present on the screen. Other tasks assessed for sustained attention abilities and information processing speed.
She and her colleagues recruited 340 children aged 4-7 years to evaluate whether the tool produced consistent results over time, and compared favorably to existing measures of attention. None of the participants had been diagnosed with ADHD. To assess reliability, a subset of children completed another assessment 2 weeks after the first one. The study showed varying results according to activity. The visual search task had high test-retest reliability and the strongest validity, compared with the other tasks. The sustained attention tasks exhibited the weakest validity.
The next steps are to assess whether this tool is sensitive enough to detect differences between children with or without clinical attention difficulties such as ADHD, Dr. Kirk said.
Apps improve adherence
As some technologies focus on reducing symptoms through games, others seek to improve medication compliance through SMS and smartphone apps.
Studies have shown that medication can decrease incidence of smoking, mood disorders, traumatic brain injuries, car crashes, and educational outcomes. However, risk decreases only if compliance is good, said Joseph Biederman, MD. Right now, “there’s extremely poor adherence to stimulant medications in ADHD” across the world, said Dr. Biederman, chief of clinical and research programs in pediatric psychopharmacology and adult ADHD at Massachusetts General Hospital in Boston.
“This is a problem that’s driven by ADHD itself,” he continued. Prescribers don’t always have the time to educate the patient on medications, deal with misconceptions, or provide support for management of daily activities.
Text reminders may offer a solution. Partnering with a Canadian technology company, MEMOTEXT, Dr. Biederman and colleagues at Massachusetts General Hospital developed an SMS-based disease management intervention for ADHD.
The tool aims to manage work, home life, and social relationships by supporting the timely renewal of medications. It doesn’t just remind people to take their ADHD medication, it reminds them to take any other medication they need, and provides the reasons why it’s important to take these drugs. Through interactive questions, it also assesses the progress and knowledge of patients and families about ADHD.
Testing this app in pediatric settings, Dr. Biederman and colleagues published a study in the Journal of Psychopharmacology showing a dramatic increase in compliance – from 60% to 90%.
In another study, this one published in the Journal of Clinical Psychopharmacology, Dr. Biederman and colleagues found that compliance improved, from 35% to 70% in adults. The SMS program in these settings not only improved adherence, but it also reduced costs of ADHD-associated complications while adding beneficial support and value to patients, families, and prescribers, Dr. Biederman said.
Promising findings about the power of apps to increase ADHD medication adherence led Luis Augusto Rohde, MD, PhD, and colleagues to develop the FOCUS app in 2016, for use in his home country of Brazil. The app objectively monitors symptoms of ADHD and establishes cooperative relationships between the patient, their families, and caregivers, said Dr. Rohde, professor of child and adolescent psychiatry at the Federal University of Rio Grande do Sul’s department of psychiatry, Porto Alegre, Brazil.
FOCUS works through collaboration. Anyone involved in the patient’s care: teachers, family members, and health care professionals, can download the app. Through this shared connection with the patient, they can participate in weekly assessments of symptoms and adverse events. A task manager sends medication reminders to the patient, who can select activities to help monitor daily performance and customize rewards.
All of those features “make it much easier to plan and individualize treatments and discuss compliance and issues with the patient,” Dr. Rohde said.
FOCUS traffic ranges from 1,200 to 1,500 active users each week, offering a wealth of data to mine on compliance, behavior, and adverse events. An upcoming randomized clinical trial in three groups of patients will further explore FOCUS’s ability to increase adherence to treatment, Dr. Rohde said.
Digital tech pros and cons
The accessibility of digital technology to children living in remote areas is one of its biggest assets, Dr. Kirk said.
Digital technologies capture real time data, are easy to use, are suitable for young children with developmental disorders, have few adverse effects, and can be easily updated. However, there are some limitations, she added. Attitudes toward technology, time required to supervise their use, and funding to facilitate the use of such technology can hinder implementation. Given that digital technology is increasingly being used to collect sensitive medical data and assess clinical conditions, it’s crucial for these new technologies to be compliant with HIPAA requirements, Dr. Kirk said.
Developers need to be thoughtful and deliberate in how they design clinical evidence strategies for digital therapeutics for ADHD.
“There’s much work that needs to be done from a clinical, statistical, regulatory, and policy perspective, but this journey illustrates this can be done with ADHD and other mental health conditions.”
Dr. Kirk disclosed working previously for a small technology company in Melbourne that developed medical technologies for children. Dr. Kollins’ work has been supported by numerous U.S. agencies, including the National Institute of Mental Health. He has served as a consultant to numerous pharmaceutical companies tied to ADHD clinical psychopharmacology. Dr. Biederman has provided research support to Genentech, Headspace, Pfizer, Roche Translational & Clinical Research Center, and other pharmaceutical companies. Also, Dr. Biederman has a partnership with MEMOTEXT through Partners Healthcare Innovation. Dr. Rohde has received grant or research support from, and served as a consultant to, several companies, including Bial, Novartis, Pfizer, and Shire/Takeda. He has received authorship royalties from Oxford University Press and ArtMed, and travel grants from Shire.
New digital tools are on the horizon to help patients with attention-deficit/hyperactivity disorder (ADHD) manage the condition.
Speakers at the World Congress on ADHD – Virtual Event described innovations aimed at improving medication compliance or reducing symptoms through the use of smartphone technology such as apps and text messaging, and video games. Some of these technologies have shown promising results in clinical trials, but the experts called for additional studies to further vet their efficacy.
Digital technologies have limitations and should be seen as adjunctive rather than standalone tools that can aid clinicians and educators, said Hannah Kirk, PhD, a psychology research fellow at Monash University’s Turner Institute for Brain and Mental Health in Clayton, Australia. Dr. Kirk joined three other speakers for the session: “ADHD in the digital age – From pitfalls to challenges.”
An explosion in technology
ADHD, the most common neurodevelopmental disorder, has global prevalence rates ranging from 5% to 7%, said Dr. Kirk. Digital technology and digital health “have been heralded as having enormous potential to improve early access and to improve the increasing demand in child support services,” she said.
The world has seen an explosion of digital technology innovation in the last decade, spurred on most recently by the COVID-19 pandemic. New demand exists for tools in educational and health care settings to provide information and support through websites, apps, SMS, video conferencing, and wearable devices, Dr. Kirk said.
Looking at the landscape of ADHD digital therapeutics, “there are probably tens of thousands of apps and other digital products to treat and manage conditions across the spectrum,” said Scott H. Kollins, PhD, MS, a clinical psychologist at Duke Health’s ADHD Clinic in Durham, N.C.
In general, few developers of these products have conducted rigorous, well-controlled trials, he noted.
Video game interventions
AKL-T01, a tool that pairs continuous fine motor tasks and perceptual reaction time tasks, went through several rounds of clinical trials to achieve federal approval as a digital therapeutic.
“This not just another video game,” said Dr. Kollins, who helped developed it. The tool’s adaptive algorithms adjust and monitor task difficulty based on performance, using a video game format and rewards to engage users.
Two phase 3 trials provided the basis for the Food and Drug Administration’s approval of AKL-T01, also known as EndeavorRx, in 2020. The first trial, published in The Lancet, randomized 348 children 1:1 to receive either the AKL-T01 treatment or a controlled intervention, which was a word game. Participating children aged 8-12 with a confirmed ADHD diagnosis were asked to play the game for about 25 minutes a day, 5 days a week over 4 weeks. The study excluded children who were taking medicine.
The researchers reported statistically significant improvements in attentional functioning in the AKL-T01 group as rated by test of variables of attention. The trial reported no serious adverse events, although one child in the AKL-T01 group withdrew from the study.
“As kids go through this treatment, it’s challenging and the difficulty levels increase, so it’s not surprising that kids get frustrated with that, or have emotional outbursts,” Dr. Kollins said. Those reactions suggest that the intervention was working, he added.
A follow-up study, published in npj Digital Medicine, broadened the scope. That study included children who had taken medication and extended the study period. Overall, 206 children aged 8-14 (130 on stimulants and 76 on no medication) played the game for 28 days, taking a pause for another 28 days, then reinitiating the treatment.
As in the first trial, AKL-T01 significantly improved ADHD-related impairment, a metric that continued to improve in the second round of treatment. Looking at secondary outcomes, the proportion of children deemed as clinical responders on the Impairment Rating Scale, 68.3% of all of the participants were responders by the end of the study on the ADHD ratings scale, meaning there was a greater than 30% improvement in symptoms. Upward of 50% of participants at the end of the second round of treatment showed substantial improvement in their ADHD ratings scale scores.
“This was really a substantial move ... the first-ever app-based video game approved by the FDA,” noted Dr. Kollins, who is affiliated with the Duke Clinical Research Institute. Some skeptics have called this a marketing ploy or have questioned the integrity of the FDA approval process.
“I would submit and argue that the rigor of the trial speaks for itself,” he said. “But it’s not surprising that there’s skepticism in the clinical community about something like this – a brand new treatment modality.”
In her own research, Dr. Kirk has studied game-based interventions aimed at assessing ADHD and improving cognitive training. In 2018, her team developed a touch screen game–based intervention for early evaluation of attention skills, using six activities. In a visual search task, children were asked to locate red lobsters on a screen that showed a variety of underwater creatures. In another selection attention task, children were asked to scan the screen for a particular target, such as a yellow star, and to indicate whether that target was absent or present on the screen. Other tasks assessed for sustained attention abilities and information processing speed.
She and her colleagues recruited 340 children aged 4-7 years to evaluate whether the tool produced consistent results over time, and compared favorably to existing measures of attention. None of the participants had been diagnosed with ADHD. To assess reliability, a subset of children completed another assessment 2 weeks after the first one. The study showed varying results according to activity. The visual search task had high test-retest reliability and the strongest validity, compared with the other tasks. The sustained attention tasks exhibited the weakest validity.
The next steps are to assess whether this tool is sensitive enough to detect differences between children with or without clinical attention difficulties such as ADHD, Dr. Kirk said.
Apps improve adherence
As some technologies focus on reducing symptoms through games, others seek to improve medication compliance through SMS and smartphone apps.
Studies have shown that medication can decrease incidence of smoking, mood disorders, traumatic brain injuries, car crashes, and educational outcomes. However, risk decreases only if compliance is good, said Joseph Biederman, MD. Right now, “there’s extremely poor adherence to stimulant medications in ADHD” across the world, said Dr. Biederman, chief of clinical and research programs in pediatric psychopharmacology and adult ADHD at Massachusetts General Hospital in Boston.
“This is a problem that’s driven by ADHD itself,” he continued. Prescribers don’t always have the time to educate the patient on medications, deal with misconceptions, or provide support for management of daily activities.
Text reminders may offer a solution. Partnering with a Canadian technology company, MEMOTEXT, Dr. Biederman and colleagues at Massachusetts General Hospital developed an SMS-based disease management intervention for ADHD.
The tool aims to manage work, home life, and social relationships by supporting the timely renewal of medications. It doesn’t just remind people to take their ADHD medication, it reminds them to take any other medication they need, and provides the reasons why it’s important to take these drugs. Through interactive questions, it also assesses the progress and knowledge of patients and families about ADHD.
Testing this app in pediatric settings, Dr. Biederman and colleagues published a study in the Journal of Psychopharmacology showing a dramatic increase in compliance – from 60% to 90%.
In another study, this one published in the Journal of Clinical Psychopharmacology, Dr. Biederman and colleagues found that compliance improved, from 35% to 70% in adults. The SMS program in these settings not only improved adherence, but it also reduced costs of ADHD-associated complications while adding beneficial support and value to patients, families, and prescribers, Dr. Biederman said.
Promising findings about the power of apps to increase ADHD medication adherence led Luis Augusto Rohde, MD, PhD, and colleagues to develop the FOCUS app in 2016, for use in his home country of Brazil. The app objectively monitors symptoms of ADHD and establishes cooperative relationships between the patient, their families, and caregivers, said Dr. Rohde, professor of child and adolescent psychiatry at the Federal University of Rio Grande do Sul’s department of psychiatry, Porto Alegre, Brazil.
FOCUS works through collaboration. Anyone involved in the patient’s care: teachers, family members, and health care professionals, can download the app. Through this shared connection with the patient, they can participate in weekly assessments of symptoms and adverse events. A task manager sends medication reminders to the patient, who can select activities to help monitor daily performance and customize rewards.
All of those features “make it much easier to plan and individualize treatments and discuss compliance and issues with the patient,” Dr. Rohde said.
FOCUS traffic ranges from 1,200 to 1,500 active users each week, offering a wealth of data to mine on compliance, behavior, and adverse events. An upcoming randomized clinical trial in three groups of patients will further explore FOCUS’s ability to increase adherence to treatment, Dr. Rohde said.
Digital tech pros and cons
The accessibility of digital technology to children living in remote areas is one of its biggest assets, Dr. Kirk said.
Digital technologies capture real time data, are easy to use, are suitable for young children with developmental disorders, have few adverse effects, and can be easily updated. However, there are some limitations, she added. Attitudes toward technology, time required to supervise their use, and funding to facilitate the use of such technology can hinder implementation. Given that digital technology is increasingly being used to collect sensitive medical data and assess clinical conditions, it’s crucial for these new technologies to be compliant with HIPAA requirements, Dr. Kirk said.
Developers need to be thoughtful and deliberate in how they design clinical evidence strategies for digital therapeutics for ADHD.
“There’s much work that needs to be done from a clinical, statistical, regulatory, and policy perspective, but this journey illustrates this can be done with ADHD and other mental health conditions.”
Dr. Kirk disclosed working previously for a small technology company in Melbourne that developed medical technologies for children. Dr. Kollins’ work has been supported by numerous U.S. agencies, including the National Institute of Mental Health. He has served as a consultant to numerous pharmaceutical companies tied to ADHD clinical psychopharmacology. Dr. Biederman has provided research support to Genentech, Headspace, Pfizer, Roche Translational & Clinical Research Center, and other pharmaceutical companies. Also, Dr. Biederman has a partnership with MEMOTEXT through Partners Healthcare Innovation. Dr. Rohde has received grant or research support from, and served as a consultant to, several companies, including Bial, Novartis, Pfizer, and Shire/Takeda. He has received authorship royalties from Oxford University Press and ArtMed, and travel grants from Shire.
New digital tools are on the horizon to help patients with attention-deficit/hyperactivity disorder (ADHD) manage the condition.
Speakers at the World Congress on ADHD – Virtual Event described innovations aimed at improving medication compliance or reducing symptoms through the use of smartphone technology such as apps and text messaging, and video games. Some of these technologies have shown promising results in clinical trials, but the experts called for additional studies to further vet their efficacy.
Digital technologies have limitations and should be seen as adjunctive rather than standalone tools that can aid clinicians and educators, said Hannah Kirk, PhD, a psychology research fellow at Monash University’s Turner Institute for Brain and Mental Health in Clayton, Australia. Dr. Kirk joined three other speakers for the session: “ADHD in the digital age – From pitfalls to challenges.”
An explosion in technology
ADHD, the most common neurodevelopmental disorder, has global prevalence rates ranging from 5% to 7%, said Dr. Kirk. Digital technology and digital health “have been heralded as having enormous potential to improve early access and to improve the increasing demand in child support services,” she said.
The world has seen an explosion of digital technology innovation in the last decade, spurred on most recently by the COVID-19 pandemic. New demand exists for tools in educational and health care settings to provide information and support through websites, apps, SMS, video conferencing, and wearable devices, Dr. Kirk said.
Looking at the landscape of ADHD digital therapeutics, “there are probably tens of thousands of apps and other digital products to treat and manage conditions across the spectrum,” said Scott H. Kollins, PhD, MS, a clinical psychologist at Duke Health’s ADHD Clinic in Durham, N.C.
In general, few developers of these products have conducted rigorous, well-controlled trials, he noted.
Video game interventions
AKL-T01, a tool that pairs continuous fine motor tasks and perceptual reaction time tasks, went through several rounds of clinical trials to achieve federal approval as a digital therapeutic.
“This not just another video game,” said Dr. Kollins, who helped developed it. The tool’s adaptive algorithms adjust and monitor task difficulty based on performance, using a video game format and rewards to engage users.
Two phase 3 trials provided the basis for the Food and Drug Administration’s approval of AKL-T01, also known as EndeavorRx, in 2020. The first trial, published in The Lancet, randomized 348 children 1:1 to receive either the AKL-T01 treatment or a controlled intervention, which was a word game. Participating children aged 8-12 with a confirmed ADHD diagnosis were asked to play the game for about 25 minutes a day, 5 days a week over 4 weeks. The study excluded children who were taking medicine.
The researchers reported statistically significant improvements in attentional functioning in the AKL-T01 group as rated by test of variables of attention. The trial reported no serious adverse events, although one child in the AKL-T01 group withdrew from the study.
“As kids go through this treatment, it’s challenging and the difficulty levels increase, so it’s not surprising that kids get frustrated with that, or have emotional outbursts,” Dr. Kollins said. Those reactions suggest that the intervention was working, he added.
A follow-up study, published in npj Digital Medicine, broadened the scope. That study included children who had taken medication and extended the study period. Overall, 206 children aged 8-14 (130 on stimulants and 76 on no medication) played the game for 28 days, taking a pause for another 28 days, then reinitiating the treatment.
As in the first trial, AKL-T01 significantly improved ADHD-related impairment, a metric that continued to improve in the second round of treatment. Looking at secondary outcomes, the proportion of children deemed as clinical responders on the Impairment Rating Scale, 68.3% of all of the participants were responders by the end of the study on the ADHD ratings scale, meaning there was a greater than 30% improvement in symptoms. Upward of 50% of participants at the end of the second round of treatment showed substantial improvement in their ADHD ratings scale scores.
“This was really a substantial move ... the first-ever app-based video game approved by the FDA,” noted Dr. Kollins, who is affiliated with the Duke Clinical Research Institute. Some skeptics have called this a marketing ploy or have questioned the integrity of the FDA approval process.
“I would submit and argue that the rigor of the trial speaks for itself,” he said. “But it’s not surprising that there’s skepticism in the clinical community about something like this – a brand new treatment modality.”
In her own research, Dr. Kirk has studied game-based interventions aimed at assessing ADHD and improving cognitive training. In 2018, her team developed a touch screen game–based intervention for early evaluation of attention skills, using six activities. In a visual search task, children were asked to locate red lobsters on a screen that showed a variety of underwater creatures. In another selection attention task, children were asked to scan the screen for a particular target, such as a yellow star, and to indicate whether that target was absent or present on the screen. Other tasks assessed for sustained attention abilities and information processing speed.
She and her colleagues recruited 340 children aged 4-7 years to evaluate whether the tool produced consistent results over time, and compared favorably to existing measures of attention. None of the participants had been diagnosed with ADHD. To assess reliability, a subset of children completed another assessment 2 weeks after the first one. The study showed varying results according to activity. The visual search task had high test-retest reliability and the strongest validity, compared with the other tasks. The sustained attention tasks exhibited the weakest validity.
The next steps are to assess whether this tool is sensitive enough to detect differences between children with or without clinical attention difficulties such as ADHD, Dr. Kirk said.
Apps improve adherence
As some technologies focus on reducing symptoms through games, others seek to improve medication compliance through SMS and smartphone apps.
Studies have shown that medication can decrease incidence of smoking, mood disorders, traumatic brain injuries, car crashes, and educational outcomes. However, risk decreases only if compliance is good, said Joseph Biederman, MD. Right now, “there’s extremely poor adherence to stimulant medications in ADHD” across the world, said Dr. Biederman, chief of clinical and research programs in pediatric psychopharmacology and adult ADHD at Massachusetts General Hospital in Boston.
“This is a problem that’s driven by ADHD itself,” he continued. Prescribers don’t always have the time to educate the patient on medications, deal with misconceptions, or provide support for management of daily activities.
Text reminders may offer a solution. Partnering with a Canadian technology company, MEMOTEXT, Dr. Biederman and colleagues at Massachusetts General Hospital developed an SMS-based disease management intervention for ADHD.
The tool aims to manage work, home life, and social relationships by supporting the timely renewal of medications. It doesn’t just remind people to take their ADHD medication, it reminds them to take any other medication they need, and provides the reasons why it’s important to take these drugs. Through interactive questions, it also assesses the progress and knowledge of patients and families about ADHD.
Testing this app in pediatric settings, Dr. Biederman and colleagues published a study in the Journal of Psychopharmacology showing a dramatic increase in compliance – from 60% to 90%.
In another study, this one published in the Journal of Clinical Psychopharmacology, Dr. Biederman and colleagues found that compliance improved, from 35% to 70% in adults. The SMS program in these settings not only improved adherence, but it also reduced costs of ADHD-associated complications while adding beneficial support and value to patients, families, and prescribers, Dr. Biederman said.
Promising findings about the power of apps to increase ADHD medication adherence led Luis Augusto Rohde, MD, PhD, and colleagues to develop the FOCUS app in 2016, for use in his home country of Brazil. The app objectively monitors symptoms of ADHD and establishes cooperative relationships between the patient, their families, and caregivers, said Dr. Rohde, professor of child and adolescent psychiatry at the Federal University of Rio Grande do Sul’s department of psychiatry, Porto Alegre, Brazil.
FOCUS works through collaboration. Anyone involved in the patient’s care: teachers, family members, and health care professionals, can download the app. Through this shared connection with the patient, they can participate in weekly assessments of symptoms and adverse events. A task manager sends medication reminders to the patient, who can select activities to help monitor daily performance and customize rewards.
All of those features “make it much easier to plan and individualize treatments and discuss compliance and issues with the patient,” Dr. Rohde said.
FOCUS traffic ranges from 1,200 to 1,500 active users each week, offering a wealth of data to mine on compliance, behavior, and adverse events. An upcoming randomized clinical trial in three groups of patients will further explore FOCUS’s ability to increase adherence to treatment, Dr. Rohde said.
Digital tech pros and cons
The accessibility of digital technology to children living in remote areas is one of its biggest assets, Dr. Kirk said.
Digital technologies capture real time data, are easy to use, are suitable for young children with developmental disorders, have few adverse effects, and can be easily updated. However, there are some limitations, she added. Attitudes toward technology, time required to supervise their use, and funding to facilitate the use of such technology can hinder implementation. Given that digital technology is increasingly being used to collect sensitive medical data and assess clinical conditions, it’s crucial for these new technologies to be compliant with HIPAA requirements, Dr. Kirk said.
Developers need to be thoughtful and deliberate in how they design clinical evidence strategies for digital therapeutics for ADHD.
“There’s much work that needs to be done from a clinical, statistical, regulatory, and policy perspective, but this journey illustrates this can be done with ADHD and other mental health conditions.”
Dr. Kirk disclosed working previously for a small technology company in Melbourne that developed medical technologies for children. Dr. Kollins’ work has been supported by numerous U.S. agencies, including the National Institute of Mental Health. He has served as a consultant to numerous pharmaceutical companies tied to ADHD clinical psychopharmacology. Dr. Biederman has provided research support to Genentech, Headspace, Pfizer, Roche Translational & Clinical Research Center, and other pharmaceutical companies. Also, Dr. Biederman has a partnership with MEMOTEXT through Partners Healthcare Innovation. Dr. Rohde has received grant or research support from, and served as a consultant to, several companies, including Bial, Novartis, Pfizer, and Shire/Takeda. He has received authorship royalties from Oxford University Press and ArtMed, and travel grants from Shire.
FROM ADHD 2021