User login
Clinical Endocrinology News is an independent news source that provides endocrinologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the endocrinologist's practice. Specialty topics include Diabetes, Lipid & Metabolic Disorders Menopause, Obesity, Osteoporosis, Pediatric Endocrinology, Pituitary, Thyroid & Adrenal Disorders, and Reproductive Endocrinology. Featured content includes Commentaries, Implementin Health Reform, Law & Medicine, and In the Loop, the blog of Clinical Endocrinology News. Clinical Endocrinology News is owned by Frontline Medical Communications.
addict
addicted
addicting
addiction
adult sites
alcohol
antibody
ass
attorney
audit
auditor
babies
babpa
baby
ban
banned
banning
best
bisexual
bitch
bleach
blog
blow job
bondage
boobs
booty
buy
cannabis
certificate
certification
certified
cheap
cheapest
class action
cocaine
cock
counterfeit drug
crack
crap
crime
criminal
cunt
curable
cure
dangerous
dangers
dead
deadly
death
defend
defended
depedent
dependence
dependent
detergent
dick
die
dildo
drug abuse
drug recall
dying
fag
fake
fatal
fatalities
fatality
free
fuck
gangs
gingivitis
guns
hardcore
herbal
herbs
heroin
herpes
home remedies
homo
horny
hypersensitivity
hypoglycemia treatment
illegal drug use
illegal use of prescription
incest
infant
infants
job
ketoacidosis
kill
killer
killing
kinky
law suit
lawsuit
lawyer
lesbian
marijuana
medicine for hypoglycemia
murder
naked
natural
newborn
nigger
noise
nude
nudity
orgy
over the counter
overdosage
overdose
overdosed
overdosing
penis
pimp
pistol
porn
porno
pornographic
pornography
prison
profanity
purchase
purchasing
pussy
queer
rape
rapist
recall
recreational drug
rob
robberies
sale
sales
sex
sexual
shit
shoot
slut
slutty
stole
stolen
store
sue
suicidal
suicide
supplements
supply company
theft
thief
thieves
tit
toddler
toddlers
toxic
toxin
tragedy
treating dka
treating hypoglycemia
treatment for hypoglycemia
vagina
violence
whore
withdrawal
without prescription
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-imn')]
div[contains(@class, 'pane-pub-home-imn')]
div[contains(@class, 'pane-pub-topic-imn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Triglyceride puzzle: Do TG metabolites better predict risk?
Triglyceride levels are a measure of cardiovascular risk and a target for therapy, but a focus on TG levels as a bad guy in CV risk assessments may be missing the mark, a population-based cohort study suggests.
The analysis, based on 30,000 participants in the Copenhagen General Population Study, saw sharply increased risks for all-cause mortality, CV mortality, and cancer mortality over 10 years among those with robust TG metabolism.
Those significant risks, gauged by concentrations of two molecules considered markers of TG metabolic rate, were independent of body mass index (BMI) and a range of other TG-linked risk factors, including plasma TG levels themselves.
All-cause mortality jumped 31% for plasma levels of glycerol in the highest versus lowest quartiles and rose 18% for highest-quartile levels of beta-hydroxybutyrate. In parallel, CV mortality climbed 37% for glycerol and 18% for beta-hydroxybutyrate in the study, published in the European Heart Journal.
The findings “implicate triglyceride metabolic rate as a risk factor for mortality not explained by high plasma triglycerides or high BMI,” the report states. The study, it continues, may be the first to link increased mortality to more active TG metabolism – according to levels of the two biomarkers – in the general population.
The results were “really, really surprising,” senior author Børge G. Nordestgaard, MD, DMSc, said in an interview. They are “completely novel” and “may make people think differently” about TG and mortality risk.
Given their unexpected findings, the group conducted further analyses for evidence that the metabolite-mortality associations weren’t independent. “We tried to stratify them away, but they stayed,” said Dr. Nordestgaard, of the University of Copenhagen.
In a weight-stratified analysis, for example, findings were similar in people with normal weight and with overweight and who were obese, Dr. Nordestgaard observed. “Even in the ones with normal weight by World Health Organization criteria, we saw the same and maybe even stronger relationships” between TG metabolism and mortality.
The study authors were is careful to note the retrospective cohort study’s limitations, but its findings “at most support an association, not causation,” Michael Miller, MD, Hospital of the University of Pennsylvania, Philadelphia, observed in an interview. Therefore, it can’t answer “whether and to what extent glycerol and/or beta-hydroxybutyrate independently contribute to mortality beyond triglyceride levels per se.”
Assessing levels of the two biomarkers “was an interesting way to indirectly assess whole-body TG metabolism,” but they were not fasting levels, said Dr. Miller, who wasn’t part of the study.
Also, the analysis doesn’t account for heparinization and other factors “that artificially raise glycerol levels” and suffers in other ways “from the inherent limitations of residual confounding,” said Dr. Miller, who is also chief of medicine at Corporal Michael J Crescenz VA Medical Center, Philadelphia.
The analysis tracked 30,000 men and women, participants in the much larger Copenhagen General Population Study cohort, for a median of 10.7 years. During that time, 9,897 of them died.
Plasma levels of glycerol and beta-hydroxybutyrate, the study authors noted, were measured using high-throughput nuclear magnetic resonance spectroscopy.
Glycerol levels greater than 80 mcmol/L represented the highest quartile and those less than 52 mcmol/L the lowest quartile. The corresponding beta-hydroxybutyrate quartiles were greater than 154 mcmol/L and less than 91 mcmol/L, respectively.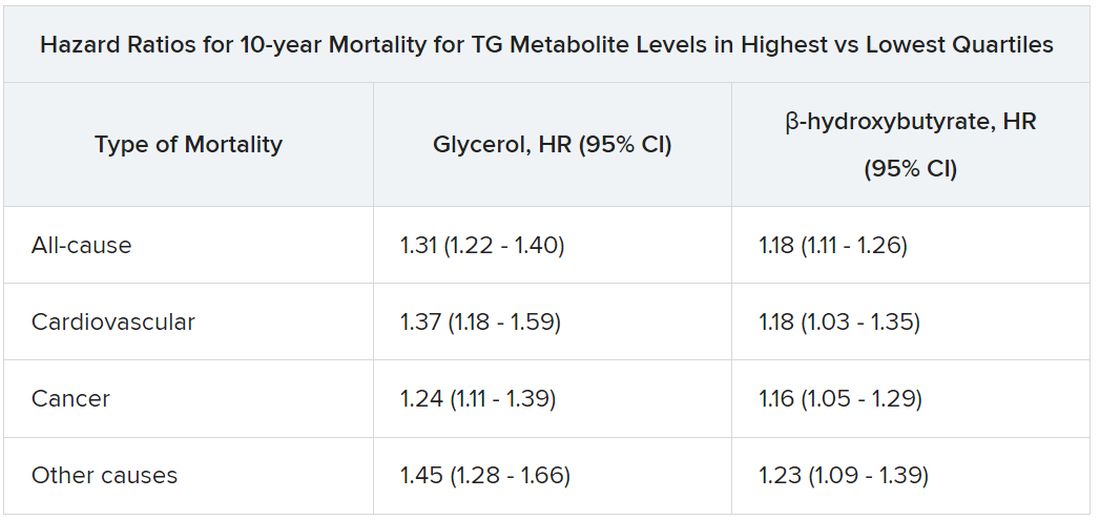
Mortality risks were independent not only of BMI and TG levels but also of age, greater waist circumference, many other standard CV risk factors, chronic obstructive pulmonary disease, diabetes, insulin use, and CV comorbidities and medications.
Dr. Nordestgaard, who also stressed that the findings are only hypothesis generating, speculated that glycerol and beta-hydroxybutyrate could potentially serve as biomarkers for predicting risk or guiding therapy and, indeed, might be amenable to risk-factor modification. “But I have absolutely no data to support that.”
The study was funded by the Independent Research Fund, and by Johan Boserup and Lise Boserups Grant. Dr. Nordestgaard reported consulting for or giving talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. The other authors reported no conflicts. Dr. Miller disclosed serving as a scientific adviser for Amarin and 89bio.
A version of this article first appeared on Medscape.com.
Triglyceride levels are a measure of cardiovascular risk and a target for therapy, but a focus on TG levels as a bad guy in CV risk assessments may be missing the mark, a population-based cohort study suggests.
The analysis, based on 30,000 participants in the Copenhagen General Population Study, saw sharply increased risks for all-cause mortality, CV mortality, and cancer mortality over 10 years among those with robust TG metabolism.
Those significant risks, gauged by concentrations of two molecules considered markers of TG metabolic rate, were independent of body mass index (BMI) and a range of other TG-linked risk factors, including plasma TG levels themselves.
All-cause mortality jumped 31% for plasma levels of glycerol in the highest versus lowest quartiles and rose 18% for highest-quartile levels of beta-hydroxybutyrate. In parallel, CV mortality climbed 37% for glycerol and 18% for beta-hydroxybutyrate in the study, published in the European Heart Journal.
The findings “implicate triglyceride metabolic rate as a risk factor for mortality not explained by high plasma triglycerides or high BMI,” the report states. The study, it continues, may be the first to link increased mortality to more active TG metabolism – according to levels of the two biomarkers – in the general population.
The results were “really, really surprising,” senior author Børge G. Nordestgaard, MD, DMSc, said in an interview. They are “completely novel” and “may make people think differently” about TG and mortality risk.
Given their unexpected findings, the group conducted further analyses for evidence that the metabolite-mortality associations weren’t independent. “We tried to stratify them away, but they stayed,” said Dr. Nordestgaard, of the University of Copenhagen.
In a weight-stratified analysis, for example, findings were similar in people with normal weight and with overweight and who were obese, Dr. Nordestgaard observed. “Even in the ones with normal weight by World Health Organization criteria, we saw the same and maybe even stronger relationships” between TG metabolism and mortality.
The study authors were is careful to note the retrospective cohort study’s limitations, but its findings “at most support an association, not causation,” Michael Miller, MD, Hospital of the University of Pennsylvania, Philadelphia, observed in an interview. Therefore, it can’t answer “whether and to what extent glycerol and/or beta-hydroxybutyrate independently contribute to mortality beyond triglyceride levels per se.”
Assessing levels of the two biomarkers “was an interesting way to indirectly assess whole-body TG metabolism,” but they were not fasting levels, said Dr. Miller, who wasn’t part of the study.
Also, the analysis doesn’t account for heparinization and other factors “that artificially raise glycerol levels” and suffers in other ways “from the inherent limitations of residual confounding,” said Dr. Miller, who is also chief of medicine at Corporal Michael J Crescenz VA Medical Center, Philadelphia.
The analysis tracked 30,000 men and women, participants in the much larger Copenhagen General Population Study cohort, for a median of 10.7 years. During that time, 9,897 of them died.
Plasma levels of glycerol and beta-hydroxybutyrate, the study authors noted, were measured using high-throughput nuclear magnetic resonance spectroscopy.
Glycerol levels greater than 80 mcmol/L represented the highest quartile and those less than 52 mcmol/L the lowest quartile. The corresponding beta-hydroxybutyrate quartiles were greater than 154 mcmol/L and less than 91 mcmol/L, respectively.
Mortality risks were independent not only of BMI and TG levels but also of age, greater waist circumference, many other standard CV risk factors, chronic obstructive pulmonary disease, diabetes, insulin use, and CV comorbidities and medications.
Dr. Nordestgaard, who also stressed that the findings are only hypothesis generating, speculated that glycerol and beta-hydroxybutyrate could potentially serve as biomarkers for predicting risk or guiding therapy and, indeed, might be amenable to risk-factor modification. “But I have absolutely no data to support that.”
The study was funded by the Independent Research Fund, and by Johan Boserup and Lise Boserups Grant. Dr. Nordestgaard reported consulting for or giving talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. The other authors reported no conflicts. Dr. Miller disclosed serving as a scientific adviser for Amarin and 89bio.
A version of this article first appeared on Medscape.com.
Triglyceride levels are a measure of cardiovascular risk and a target for therapy, but a focus on TG levels as a bad guy in CV risk assessments may be missing the mark, a population-based cohort study suggests.
The analysis, based on 30,000 participants in the Copenhagen General Population Study, saw sharply increased risks for all-cause mortality, CV mortality, and cancer mortality over 10 years among those with robust TG metabolism.
Those significant risks, gauged by concentrations of two molecules considered markers of TG metabolic rate, were independent of body mass index (BMI) and a range of other TG-linked risk factors, including plasma TG levels themselves.
All-cause mortality jumped 31% for plasma levels of glycerol in the highest versus lowest quartiles and rose 18% for highest-quartile levels of beta-hydroxybutyrate. In parallel, CV mortality climbed 37% for glycerol and 18% for beta-hydroxybutyrate in the study, published in the European Heart Journal.
The findings “implicate triglyceride metabolic rate as a risk factor for mortality not explained by high plasma triglycerides or high BMI,” the report states. The study, it continues, may be the first to link increased mortality to more active TG metabolism – according to levels of the two biomarkers – in the general population.
The results were “really, really surprising,” senior author Børge G. Nordestgaard, MD, DMSc, said in an interview. They are “completely novel” and “may make people think differently” about TG and mortality risk.
Given their unexpected findings, the group conducted further analyses for evidence that the metabolite-mortality associations weren’t independent. “We tried to stratify them away, but they stayed,” said Dr. Nordestgaard, of the University of Copenhagen.
In a weight-stratified analysis, for example, findings were similar in people with normal weight and with overweight and who were obese, Dr. Nordestgaard observed. “Even in the ones with normal weight by World Health Organization criteria, we saw the same and maybe even stronger relationships” between TG metabolism and mortality.
The study authors were is careful to note the retrospective cohort study’s limitations, but its findings “at most support an association, not causation,” Michael Miller, MD, Hospital of the University of Pennsylvania, Philadelphia, observed in an interview. Therefore, it can’t answer “whether and to what extent glycerol and/or beta-hydroxybutyrate independently contribute to mortality beyond triglyceride levels per se.”
Assessing levels of the two biomarkers “was an interesting way to indirectly assess whole-body TG metabolism,” but they were not fasting levels, said Dr. Miller, who wasn’t part of the study.
Also, the analysis doesn’t account for heparinization and other factors “that artificially raise glycerol levels” and suffers in other ways “from the inherent limitations of residual confounding,” said Dr. Miller, who is also chief of medicine at Corporal Michael J Crescenz VA Medical Center, Philadelphia.
The analysis tracked 30,000 men and women, participants in the much larger Copenhagen General Population Study cohort, for a median of 10.7 years. During that time, 9,897 of them died.
Plasma levels of glycerol and beta-hydroxybutyrate, the study authors noted, were measured using high-throughput nuclear magnetic resonance spectroscopy.
Glycerol levels greater than 80 mcmol/L represented the highest quartile and those less than 52 mcmol/L the lowest quartile. The corresponding beta-hydroxybutyrate quartiles were greater than 154 mcmol/L and less than 91 mcmol/L, respectively.
Mortality risks were independent not only of BMI and TG levels but also of age, greater waist circumference, many other standard CV risk factors, chronic obstructive pulmonary disease, diabetes, insulin use, and CV comorbidities and medications.
Dr. Nordestgaard, who also stressed that the findings are only hypothesis generating, speculated that glycerol and beta-hydroxybutyrate could potentially serve as biomarkers for predicting risk or guiding therapy and, indeed, might be amenable to risk-factor modification. “But I have absolutely no data to support that.”
The study was funded by the Independent Research Fund, and by Johan Boserup and Lise Boserups Grant. Dr. Nordestgaard reported consulting for or giving talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. The other authors reported no conflicts. Dr. Miller disclosed serving as a scientific adviser for Amarin and 89bio.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN HEART JOURNAL
FDA approves new tubeless insulin pump
The product received CE Mark in Europe in 2018 and is now available in 19 markets worldwide. It offers users a choice of bolusing directly from the pump or from a handheld remote-control device. The pump can be detached and reattached without wasting insulin.
The remote-control device also incorporates blood glucose monitoring and bolus advice, although it currently does not integrate with continuous glucose monitoring (CGM) devices.
A Roche spokesperson said in an interview, “For future product generations, we are exploring possibilities to integrate CGM data into the system. Already today, the diabetes manager allows users to manually enter a glucose value that can be used to calculate a bolus. To do so, people with diabetes could use their CGM device of choice in conjunction with the Accu-Chek Solo micropump system.”
Roche will provide an update on next steps for further developments and time lines for launch “in due course,” according to a company statement.
A version of this article appeared on Medscape.com.
The product received CE Mark in Europe in 2018 and is now available in 19 markets worldwide. It offers users a choice of bolusing directly from the pump or from a handheld remote-control device. The pump can be detached and reattached without wasting insulin.
The remote-control device also incorporates blood glucose monitoring and bolus advice, although it currently does not integrate with continuous glucose monitoring (CGM) devices.
A Roche spokesperson said in an interview, “For future product generations, we are exploring possibilities to integrate CGM data into the system. Already today, the diabetes manager allows users to manually enter a glucose value that can be used to calculate a bolus. To do so, people with diabetes could use their CGM device of choice in conjunction with the Accu-Chek Solo micropump system.”
Roche will provide an update on next steps for further developments and time lines for launch “in due course,” according to a company statement.
A version of this article appeared on Medscape.com.
The product received CE Mark in Europe in 2018 and is now available in 19 markets worldwide. It offers users a choice of bolusing directly from the pump or from a handheld remote-control device. The pump can be detached and reattached without wasting insulin.
The remote-control device also incorporates blood glucose monitoring and bolus advice, although it currently does not integrate with continuous glucose monitoring (CGM) devices.
A Roche spokesperson said in an interview, “For future product generations, we are exploring possibilities to integrate CGM data into the system. Already today, the diabetes manager allows users to manually enter a glucose value that can be used to calculate a bolus. To do so, people with diabetes could use their CGM device of choice in conjunction with the Accu-Chek Solo micropump system.”
Roche will provide an update on next steps for further developments and time lines for launch “in due course,” according to a company statement.
A version of this article appeared on Medscape.com.
Can caffeine improve thyroid function?
“Although the causal relationship between caffeine intake and thyroid function requires further verification, as an easily obtainable and widely consumed dietary ingredient, caffeine is a potential candidate for improving thyroid health in people with metabolic disorders,” reported the authors in the study, published in Nutritional Journal.
Caffeine intake, within established healthy ranges, showed a nonlinear association with thyroid levels.
Moderate caffeine intake has been associated with reducing the risk of metabolic disorders in addition to showing some mental health benefits. However, research on its effects on thyroid hormone, which importantly plays a key role in systemic metabolism and neurologic development, is lacking.
To investigate the effects, Yu Zhou, of the Department of Rehabilitation Medicine, School of Health, Fujian Medical University, Fuzhou, China, and colleagues evaluated data from the National Health and Nutrition Examination Survey (NHANES) III 2007-2012 study involving 2,582 participants for whom data were available regarding medical conditions, dietary intake, thyroid function, and demographic background.
The participants were divided into three subgroups based on sex, age, body mass index, hyperglycemia, hypertension, and cardio-cerebral vascular disease (CVD).
Group 1 (n = 208) was the most metabolically unhealthy. Patients in that group had the highest BMI and were of oldest age. In addition, that group had higher rates of hypertension, hyperglycemia, and CVD, but, notably, it had the lowest level of caffeine consumption.
In group 2 (n = 543), all participants were current smokers, and 90.4% had a habit of drinking alcohol. That group also had the highest percentage of men.
Group 3 (n = 1,183) was the most metabolically healthy, with more women, younger age, and lowest BMI. No participants in that group had hyperglycemia, hypertension, or CVD.
Group 1, the most metabolically unhealthy, had the highest serum thyroid-stimulating hormone (TSH) levels. Of note, while participants with thyroid diseases were initially excluded from the analysis, higher TSH levels are predictive of subclinical hypothyroidism or progression to overt hypothyroidism.
Overall, there was no association between caffeine and TSH levels.
However, a subgroup analysis of the groups showed that in group 1, caffeine intake correlated with TSH nonlinearly (P = .0019), with minimal average consumption of caffeine (< 9.97 mg/d). There was an association with slightly higher TSH levels (P = .035) after adjustment for age, sex, race, drink, disease state, micronutrients, and macronutrients.
However, in higher, moderate amounts of caffeine consumption (9.97 – 264.97 mg/d), there was an inverse association, with lower TSH (P = .001).
There was no association between daily caffeine consumption of more than 264.97 mg and TSH levels.
For context, a typical 8-ounce cup of coffee generally contains 80-100 mg of caffeine, and the Food and Drug Administration indicates that 400 mg/d of caffeine is safe for healthy adults.
Group 2 consumed the highest amount of caffeine. Notably, that group had the lowest serum TSH levels of the three groups. There were no significant associations between caffeine consumption and TSH levels in group 2 or group 3.
There were no significant associations between caffeine consumption and levels of serum FT4 or FT3, also linked to thyroid dysfunction, in any of the groups.
The findings show that “caffeine consumption was correlated with serum TSH nonlinearly, and when taken in moderate amounts (9.97-264.97 mg/d), caffeine demonstrated a positive correlation with serum TSH levels in patients with metabolic disorders,” the authors concluded.
Mechanisms?
Caffeine is believed to modulate pituitary hormone secretion, which has been shown to influence the hypothalamic-pituitary-adrenal axis. The authors speculated that caffeine could potentially affect thyroid activity by affecting pituitary function.
“However, the effects of transient and chronic caffeine administration on human thyroid function need to be verified further, and the related mechanisms remain unclear,” they noted.
Commenting on the study, Maik Pietzner, PhD, of the Berlin Institute of Health, noted that an important limitation of the study is that various patient groups were excluded, including those with abnormal TSH levels.
“What makes me wonder is the high number of exclusions and the focus on very specific groups of people. This almost certainly introduces bias, e.g., what is specific to people not reporting coffee consumption,” Dr. Pietzner said.
Furthermore, “we already know that patients with poor metabolic health do also have slight variations in thyroid hormone levels and also have different dietary patterns,” he explained.
“So reverse confounding might occur in which the poor metabolic health is associated with both poor thyroid hormone levels and coffee consumption,” Dr. Pietzner said.
He also noted the “somewhat odd” finding that the group with the highest metabolic disorders had the lowest coffee consumption, yet the highest TSH levels.
“My guess would be that this might also be a chance finding, given that the distribution of TSH values is very skewed, which can have a strong effect in linear regression models,” Dr. Pietzner said.
In general, “the evidence generated by the study is rather weak, but there is good evidence that higher coffee consumption is linked to better metabolic health, although the exact mechanisms is not known, if indeed causal,” Dr. Pietzner added. “Prospective studies are needed to evaluate whether higher coffee consumption indeed lowers the risk for thyroid disease.”
A version of this article first appeared on Medscape.com.
“Although the causal relationship between caffeine intake and thyroid function requires further verification, as an easily obtainable and widely consumed dietary ingredient, caffeine is a potential candidate for improving thyroid health in people with metabolic disorders,” reported the authors in the study, published in Nutritional Journal.
Caffeine intake, within established healthy ranges, showed a nonlinear association with thyroid levels.
Moderate caffeine intake has been associated with reducing the risk of metabolic disorders in addition to showing some mental health benefits. However, research on its effects on thyroid hormone, which importantly plays a key role in systemic metabolism and neurologic development, is lacking.
To investigate the effects, Yu Zhou, of the Department of Rehabilitation Medicine, School of Health, Fujian Medical University, Fuzhou, China, and colleagues evaluated data from the National Health and Nutrition Examination Survey (NHANES) III 2007-2012 study involving 2,582 participants for whom data were available regarding medical conditions, dietary intake, thyroid function, and demographic background.
The participants were divided into three subgroups based on sex, age, body mass index, hyperglycemia, hypertension, and cardio-cerebral vascular disease (CVD).
Group 1 (n = 208) was the most metabolically unhealthy. Patients in that group had the highest BMI and were of oldest age. In addition, that group had higher rates of hypertension, hyperglycemia, and CVD, but, notably, it had the lowest level of caffeine consumption.
In group 2 (n = 543), all participants were current smokers, and 90.4% had a habit of drinking alcohol. That group also had the highest percentage of men.
Group 3 (n = 1,183) was the most metabolically healthy, with more women, younger age, and lowest BMI. No participants in that group had hyperglycemia, hypertension, or CVD.
Group 1, the most metabolically unhealthy, had the highest serum thyroid-stimulating hormone (TSH) levels. Of note, while participants with thyroid diseases were initially excluded from the analysis, higher TSH levels are predictive of subclinical hypothyroidism or progression to overt hypothyroidism.
Overall, there was no association between caffeine and TSH levels.
However, a subgroup analysis of the groups showed that in group 1, caffeine intake correlated with TSH nonlinearly (P = .0019), with minimal average consumption of caffeine (< 9.97 mg/d). There was an association with slightly higher TSH levels (P = .035) after adjustment for age, sex, race, drink, disease state, micronutrients, and macronutrients.
However, in higher, moderate amounts of caffeine consumption (9.97 – 264.97 mg/d), there was an inverse association, with lower TSH (P = .001).
There was no association between daily caffeine consumption of more than 264.97 mg and TSH levels.
For context, a typical 8-ounce cup of coffee generally contains 80-100 mg of caffeine, and the Food and Drug Administration indicates that 400 mg/d of caffeine is safe for healthy adults.
Group 2 consumed the highest amount of caffeine. Notably, that group had the lowest serum TSH levels of the three groups. There were no significant associations between caffeine consumption and TSH levels in group 2 or group 3.
There were no significant associations between caffeine consumption and levels of serum FT4 or FT3, also linked to thyroid dysfunction, in any of the groups.
The findings show that “caffeine consumption was correlated with serum TSH nonlinearly, and when taken in moderate amounts (9.97-264.97 mg/d), caffeine demonstrated a positive correlation with serum TSH levels in patients with metabolic disorders,” the authors concluded.
Mechanisms?
Caffeine is believed to modulate pituitary hormone secretion, which has been shown to influence the hypothalamic-pituitary-adrenal axis. The authors speculated that caffeine could potentially affect thyroid activity by affecting pituitary function.
“However, the effects of transient and chronic caffeine administration on human thyroid function need to be verified further, and the related mechanisms remain unclear,” they noted.
Commenting on the study, Maik Pietzner, PhD, of the Berlin Institute of Health, noted that an important limitation of the study is that various patient groups were excluded, including those with abnormal TSH levels.
“What makes me wonder is the high number of exclusions and the focus on very specific groups of people. This almost certainly introduces bias, e.g., what is specific to people not reporting coffee consumption,” Dr. Pietzner said.
Furthermore, “we already know that patients with poor metabolic health do also have slight variations in thyroid hormone levels and also have different dietary patterns,” he explained.
“So reverse confounding might occur in which the poor metabolic health is associated with both poor thyroid hormone levels and coffee consumption,” Dr. Pietzner said.
He also noted the “somewhat odd” finding that the group with the highest metabolic disorders had the lowest coffee consumption, yet the highest TSH levels.
“My guess would be that this might also be a chance finding, given that the distribution of TSH values is very skewed, which can have a strong effect in linear regression models,” Dr. Pietzner said.
In general, “the evidence generated by the study is rather weak, but there is good evidence that higher coffee consumption is linked to better metabolic health, although the exact mechanisms is not known, if indeed causal,” Dr. Pietzner added. “Prospective studies are needed to evaluate whether higher coffee consumption indeed lowers the risk for thyroid disease.”
A version of this article first appeared on Medscape.com.
“Although the causal relationship between caffeine intake and thyroid function requires further verification, as an easily obtainable and widely consumed dietary ingredient, caffeine is a potential candidate for improving thyroid health in people with metabolic disorders,” reported the authors in the study, published in Nutritional Journal.
Caffeine intake, within established healthy ranges, showed a nonlinear association with thyroid levels.
Moderate caffeine intake has been associated with reducing the risk of metabolic disorders in addition to showing some mental health benefits. However, research on its effects on thyroid hormone, which importantly plays a key role in systemic metabolism and neurologic development, is lacking.
To investigate the effects, Yu Zhou, of the Department of Rehabilitation Medicine, School of Health, Fujian Medical University, Fuzhou, China, and colleagues evaluated data from the National Health and Nutrition Examination Survey (NHANES) III 2007-2012 study involving 2,582 participants for whom data were available regarding medical conditions, dietary intake, thyroid function, and demographic background.
The participants were divided into three subgroups based on sex, age, body mass index, hyperglycemia, hypertension, and cardio-cerebral vascular disease (CVD).
Group 1 (n = 208) was the most metabolically unhealthy. Patients in that group had the highest BMI and were of oldest age. In addition, that group had higher rates of hypertension, hyperglycemia, and CVD, but, notably, it had the lowest level of caffeine consumption.
In group 2 (n = 543), all participants were current smokers, and 90.4% had a habit of drinking alcohol. That group also had the highest percentage of men.
Group 3 (n = 1,183) was the most metabolically healthy, with more women, younger age, and lowest BMI. No participants in that group had hyperglycemia, hypertension, or CVD.
Group 1, the most metabolically unhealthy, had the highest serum thyroid-stimulating hormone (TSH) levels. Of note, while participants with thyroid diseases were initially excluded from the analysis, higher TSH levels are predictive of subclinical hypothyroidism or progression to overt hypothyroidism.
Overall, there was no association between caffeine and TSH levels.
However, a subgroup analysis of the groups showed that in group 1, caffeine intake correlated with TSH nonlinearly (P = .0019), with minimal average consumption of caffeine (< 9.97 mg/d). There was an association with slightly higher TSH levels (P = .035) after adjustment for age, sex, race, drink, disease state, micronutrients, and macronutrients.
However, in higher, moderate amounts of caffeine consumption (9.97 – 264.97 mg/d), there was an inverse association, with lower TSH (P = .001).
There was no association between daily caffeine consumption of more than 264.97 mg and TSH levels.
For context, a typical 8-ounce cup of coffee generally contains 80-100 mg of caffeine, and the Food and Drug Administration indicates that 400 mg/d of caffeine is safe for healthy adults.
Group 2 consumed the highest amount of caffeine. Notably, that group had the lowest serum TSH levels of the three groups. There were no significant associations between caffeine consumption and TSH levels in group 2 or group 3.
There were no significant associations between caffeine consumption and levels of serum FT4 or FT3, also linked to thyroid dysfunction, in any of the groups.
The findings show that “caffeine consumption was correlated with serum TSH nonlinearly, and when taken in moderate amounts (9.97-264.97 mg/d), caffeine demonstrated a positive correlation with serum TSH levels in patients with metabolic disorders,” the authors concluded.
Mechanisms?
Caffeine is believed to modulate pituitary hormone secretion, which has been shown to influence the hypothalamic-pituitary-adrenal axis. The authors speculated that caffeine could potentially affect thyroid activity by affecting pituitary function.
“However, the effects of transient and chronic caffeine administration on human thyroid function need to be verified further, and the related mechanisms remain unclear,” they noted.
Commenting on the study, Maik Pietzner, PhD, of the Berlin Institute of Health, noted that an important limitation of the study is that various patient groups were excluded, including those with abnormal TSH levels.
“What makes me wonder is the high number of exclusions and the focus on very specific groups of people. This almost certainly introduces bias, e.g., what is specific to people not reporting coffee consumption,” Dr. Pietzner said.
Furthermore, “we already know that patients with poor metabolic health do also have slight variations in thyroid hormone levels and also have different dietary patterns,” he explained.
“So reverse confounding might occur in which the poor metabolic health is associated with both poor thyroid hormone levels and coffee consumption,” Dr. Pietzner said.
He also noted the “somewhat odd” finding that the group with the highest metabolic disorders had the lowest coffee consumption, yet the highest TSH levels.
“My guess would be that this might also be a chance finding, given that the distribution of TSH values is very skewed, which can have a strong effect in linear regression models,” Dr. Pietzner said.
In general, “the evidence generated by the study is rather weak, but there is good evidence that higher coffee consumption is linked to better metabolic health, although the exact mechanisms is not known, if indeed causal,” Dr. Pietzner added. “Prospective studies are needed to evaluate whether higher coffee consumption indeed lowers the risk for thyroid disease.”
A version of this article first appeared on Medscape.com.
FROM NUTRITIONAL JOURNAL
Type 1 diabetes management improves as technology advances
Significant reductions in hemoglobin A1c have occurred over time among adults with type 1 diabetes as their use of diabetes technology has increased, yet there is still room for improvement, new data suggest.
The new findings are from a study involving patients at the Barbara Davis Center for Diabetes Adult Clinic between Jan. 1, 2014, and Dec. 31, 2021. They show that as technology use has increased, A1c levels have dropped in parallel. Moreover, progression from use of stand-alone continuous glucose monitors (CGMs) to automated insulin delivery systems (AIDs), which comprise insulin pumps and connected CGMs, furthered that progress.
The findings “are in agreement with American Diabetes Association standards of care, and recent international consensus recommending CGM and AID for most people with type 1 diabetes, and early initiation of diabetes technology from the onset of type 1 diabetes,” write Kagan E. Karakus, MD, of the University of Colorado’s Barbara Davis Center, Aurora, and colleagues in the article, which was published online in Diabetes Care.
“It’s very rewarding to us. We can see clearly that the uptake is going up and the A1c is dropping,” lead author Viral N. Shah, MD, of the Barbara Davis Center, told this news organization.
On the flip side, A1c levels rose significantly over the study period among nonusers of technology. “We cannot rule out provider bias for not prescribing diabetes technology among those with higher A1c or from disadvantaged socioeconomic backgrounds,” Dr. Karakus and colleagues write.
Also of note, even with use of the most advanced AID systems available during the study period, just under half of patients were still not achieving A1c levels below 7%. “The technology helps, but it’s not perfect,” Dr. Shah observed.
This study is the first to examine the relationship of A1c with technology use over time, in contrast to prior cross-sectional studies. “The intention here was to look at the landscape over a decade,” Dr. Shah said.
As overall use of technology use rose, A1c levels fell
The analysis included data for 4,174 unique patients (mean number of patients, 1,988/yr); 15,903 clinic visits were included over the 8-year study period. Technology use was defined as CGM use without an AID system or with an AID system.
Over the study period, diabetes technology use increased from 26.9% to 82.7% of the clinic population (P < .001). At the same time, the overall proportion patients who achieved the A1c goal of less than 7% increased from 32.3% to 41.7%, while the mean A1c level dropped from 7.7% to 7.5% (P < .001).
But among the technology nonusers, A1c rose from 7.85% in 2014 to 8.4% in 2021 (P < .001).
Regardless of diabetes technology use, White patients (about 80% of the total study population) had significantly lower A1c than non-White patients (7.5% vs. 7.7% for technology users [P = .02]; 8.0% vs. 8.3% for nontechnology users [P < .001]).
The non-White group was too small to enable the researchers to break down the data by technology type. Nonetheless, Dr. Shah said, “As a clinician, I can say that the penetration of diabetes technology in non-White populations remains low. These are also the people more vulnerable for socioeconomic and psychosocial reasons.”
The A1c increase among technology nonusers may be a result of a statistical artifact, as the number of those individuals was much lower in 2021 than in 2014. It’s possible that those remaining individuals have exceedingly high A1c levels, bringing the average up. “It’s still not good, though,” Dr. Shah said.
The more technology, the lower the A1c
Over the study period, the proportion of stand-alone CGM users rose from 26.9% to 44.1%, while use of AIDs rose from 0% in 2014 and 2015 to 38.6% in 2021. The latter group included patients who used first-generation Medtronic 670G and 770G devices and second-generation Tandem t:slim X2 with Control-IQ devices.
Between 2017 and 2021, AIDs users had significantly lower A1c levels than nontechnology users: 7.4% vs. 8.1% in 2017, and 7.3% vs. 8.4% in 2021 (P < .001 for every year). CGM users also had significantly lower A1c levels than nonusers at all time points (P < .001 per year).
The proportions achieving an A1c less than 7% differed significantly across users of CGMs, AIDs, and no technology (P < .01 for all years). In 2021, the percentage of people who achieved an A1c less than 7% were 50.9% with AIDs and 44.1% for CGMs vs, just 15.2% with no technology.
Work to be done: Why aren’t more achieving < 7% with AIDs?
Asked why only slightly more than half of patients who used AIDs achieved A1c levels below 7%, Dr. Shah listed three possibilities:
First, the 7% goal doesn’t apply to everyone with type 1 diabetes, including those with multiple comorbidities or with short life expectancy, for whom the recommended goal is 7.5%-8.0% to prevent hypoglycemia. “We didn’t separate out patients by A1c goals. If we add that, the number might go up,” Dr. Shah said.
Second, AID technology is continually improving, but it’s not perfect. Users still must enter carbohydrate counts and signal the devices for exercise, which can lead to errors. “It’s a wonderful technology for overnight control, but still, during the daytime, there are so many factors with the user interface and how much a person is engaged with the technology,” Dr. Shah explained.
Third, he said, “Unfortunately, obesity is increasing in type 1 diabetes, and insulin doses are increasing. Higher BMI [body mass index] and more insulin resistance can mean higher A1c. I really think for many patients, we probably will need an adjunct therapy, such as an SGLT2 [sodium-glucose cotransporter-2] inhibitor or a GLP-1 [glucagonlike peptide-1] agonist, even though they’re not approved in type 1 diabetes, for both glycemic and metabolic control including weight. I think that’s another missing piece.”
He also pointed out, “If someone has an A1c of 7.5%, I don’t expect a huge change. But if they’re at 10%, a drop to 8% is a huge change.”
Overall, Dr. Shah said, the news from the study is good. “In the past, only 30% were achieving an A1c less than 7%. Now we’re 20% above that. ... It’s a glass half full.”
Dr. Karakus has disclosed no relevant financial relationships. Dr. Shah has received, through the University of Colorado, research support from Novo Nordisk, Insulet, Tandem Diabetes, and Dexcom, and honoraria from Medscape, Lifescan, Novo Nordisk, and DKSH Singapore for advisory board attendance and from Insulet and Dexcom for speaking engagements.
A version of this article first appeared on Medscape.com.
Significant reductions in hemoglobin A1c have occurred over time among adults with type 1 diabetes as their use of diabetes technology has increased, yet there is still room for improvement, new data suggest.
The new findings are from a study involving patients at the Barbara Davis Center for Diabetes Adult Clinic between Jan. 1, 2014, and Dec. 31, 2021. They show that as technology use has increased, A1c levels have dropped in parallel. Moreover, progression from use of stand-alone continuous glucose monitors (CGMs) to automated insulin delivery systems (AIDs), which comprise insulin pumps and connected CGMs, furthered that progress.
The findings “are in agreement with American Diabetes Association standards of care, and recent international consensus recommending CGM and AID for most people with type 1 diabetes, and early initiation of diabetes technology from the onset of type 1 diabetes,” write Kagan E. Karakus, MD, of the University of Colorado’s Barbara Davis Center, Aurora, and colleagues in the article, which was published online in Diabetes Care.
“It’s very rewarding to us. We can see clearly that the uptake is going up and the A1c is dropping,” lead author Viral N. Shah, MD, of the Barbara Davis Center, told this news organization.
On the flip side, A1c levels rose significantly over the study period among nonusers of technology. “We cannot rule out provider bias for not prescribing diabetes technology among those with higher A1c or from disadvantaged socioeconomic backgrounds,” Dr. Karakus and colleagues write.
Also of note, even with use of the most advanced AID systems available during the study period, just under half of patients were still not achieving A1c levels below 7%. “The technology helps, but it’s not perfect,” Dr. Shah observed.
This study is the first to examine the relationship of A1c with technology use over time, in contrast to prior cross-sectional studies. “The intention here was to look at the landscape over a decade,” Dr. Shah said.
As overall use of technology use rose, A1c levels fell
The analysis included data for 4,174 unique patients (mean number of patients, 1,988/yr); 15,903 clinic visits were included over the 8-year study period. Technology use was defined as CGM use without an AID system or with an AID system.
Over the study period, diabetes technology use increased from 26.9% to 82.7% of the clinic population (P < .001). At the same time, the overall proportion patients who achieved the A1c goal of less than 7% increased from 32.3% to 41.7%, while the mean A1c level dropped from 7.7% to 7.5% (P < .001).
But among the technology nonusers, A1c rose from 7.85% in 2014 to 8.4% in 2021 (P < .001).
Regardless of diabetes technology use, White patients (about 80% of the total study population) had significantly lower A1c than non-White patients (7.5% vs. 7.7% for technology users [P = .02]; 8.0% vs. 8.3% for nontechnology users [P < .001]).
The non-White group was too small to enable the researchers to break down the data by technology type. Nonetheless, Dr. Shah said, “As a clinician, I can say that the penetration of diabetes technology in non-White populations remains low. These are also the people more vulnerable for socioeconomic and psychosocial reasons.”
The A1c increase among technology nonusers may be a result of a statistical artifact, as the number of those individuals was much lower in 2021 than in 2014. It’s possible that those remaining individuals have exceedingly high A1c levels, bringing the average up. “It’s still not good, though,” Dr. Shah said.
The more technology, the lower the A1c
Over the study period, the proportion of stand-alone CGM users rose from 26.9% to 44.1%, while use of AIDs rose from 0% in 2014 and 2015 to 38.6% in 2021. The latter group included patients who used first-generation Medtronic 670G and 770G devices and second-generation Tandem t:slim X2 with Control-IQ devices.
Between 2017 and 2021, AIDs users had significantly lower A1c levels than nontechnology users: 7.4% vs. 8.1% in 2017, and 7.3% vs. 8.4% in 2021 (P < .001 for every year). CGM users also had significantly lower A1c levels than nonusers at all time points (P < .001 per year).
The proportions achieving an A1c less than 7% differed significantly across users of CGMs, AIDs, and no technology (P < .01 for all years). In 2021, the percentage of people who achieved an A1c less than 7% were 50.9% with AIDs and 44.1% for CGMs vs, just 15.2% with no technology.
Work to be done: Why aren’t more achieving < 7% with AIDs?
Asked why only slightly more than half of patients who used AIDs achieved A1c levels below 7%, Dr. Shah listed three possibilities:
First, the 7% goal doesn’t apply to everyone with type 1 diabetes, including those with multiple comorbidities or with short life expectancy, for whom the recommended goal is 7.5%-8.0% to prevent hypoglycemia. “We didn’t separate out patients by A1c goals. If we add that, the number might go up,” Dr. Shah said.
Second, AID technology is continually improving, but it’s not perfect. Users still must enter carbohydrate counts and signal the devices for exercise, which can lead to errors. “It’s a wonderful technology for overnight control, but still, during the daytime, there are so many factors with the user interface and how much a person is engaged with the technology,” Dr. Shah explained.
Third, he said, “Unfortunately, obesity is increasing in type 1 diabetes, and insulin doses are increasing. Higher BMI [body mass index] and more insulin resistance can mean higher A1c. I really think for many patients, we probably will need an adjunct therapy, such as an SGLT2 [sodium-glucose cotransporter-2] inhibitor or a GLP-1 [glucagonlike peptide-1] agonist, even though they’re not approved in type 1 diabetes, for both glycemic and metabolic control including weight. I think that’s another missing piece.”
He also pointed out, “If someone has an A1c of 7.5%, I don’t expect a huge change. But if they’re at 10%, a drop to 8% is a huge change.”
Overall, Dr. Shah said, the news from the study is good. “In the past, only 30% were achieving an A1c less than 7%. Now we’re 20% above that. ... It’s a glass half full.”
Dr. Karakus has disclosed no relevant financial relationships. Dr. Shah has received, through the University of Colorado, research support from Novo Nordisk, Insulet, Tandem Diabetes, and Dexcom, and honoraria from Medscape, Lifescan, Novo Nordisk, and DKSH Singapore for advisory board attendance and from Insulet and Dexcom for speaking engagements.
A version of this article first appeared on Medscape.com.
Significant reductions in hemoglobin A1c have occurred over time among adults with type 1 diabetes as their use of diabetes technology has increased, yet there is still room for improvement, new data suggest.
The new findings are from a study involving patients at the Barbara Davis Center for Diabetes Adult Clinic between Jan. 1, 2014, and Dec. 31, 2021. They show that as technology use has increased, A1c levels have dropped in parallel. Moreover, progression from use of stand-alone continuous glucose monitors (CGMs) to automated insulin delivery systems (AIDs), which comprise insulin pumps and connected CGMs, furthered that progress.
The findings “are in agreement with American Diabetes Association standards of care, and recent international consensus recommending CGM and AID for most people with type 1 diabetes, and early initiation of diabetes technology from the onset of type 1 diabetes,” write Kagan E. Karakus, MD, of the University of Colorado’s Barbara Davis Center, Aurora, and colleagues in the article, which was published online in Diabetes Care.
“It’s very rewarding to us. We can see clearly that the uptake is going up and the A1c is dropping,” lead author Viral N. Shah, MD, of the Barbara Davis Center, told this news organization.
On the flip side, A1c levels rose significantly over the study period among nonusers of technology. “We cannot rule out provider bias for not prescribing diabetes technology among those with higher A1c or from disadvantaged socioeconomic backgrounds,” Dr. Karakus and colleagues write.
Also of note, even with use of the most advanced AID systems available during the study period, just under half of patients were still not achieving A1c levels below 7%. “The technology helps, but it’s not perfect,” Dr. Shah observed.
This study is the first to examine the relationship of A1c with technology use over time, in contrast to prior cross-sectional studies. “The intention here was to look at the landscape over a decade,” Dr. Shah said.
As overall use of technology use rose, A1c levels fell
The analysis included data for 4,174 unique patients (mean number of patients, 1,988/yr); 15,903 clinic visits were included over the 8-year study period. Technology use was defined as CGM use without an AID system or with an AID system.
Over the study period, diabetes technology use increased from 26.9% to 82.7% of the clinic population (P < .001). At the same time, the overall proportion patients who achieved the A1c goal of less than 7% increased from 32.3% to 41.7%, while the mean A1c level dropped from 7.7% to 7.5% (P < .001).
But among the technology nonusers, A1c rose from 7.85% in 2014 to 8.4% in 2021 (P < .001).
Regardless of diabetes technology use, White patients (about 80% of the total study population) had significantly lower A1c than non-White patients (7.5% vs. 7.7% for technology users [P = .02]; 8.0% vs. 8.3% for nontechnology users [P < .001]).
The non-White group was too small to enable the researchers to break down the data by technology type. Nonetheless, Dr. Shah said, “As a clinician, I can say that the penetration of diabetes technology in non-White populations remains low. These are also the people more vulnerable for socioeconomic and psychosocial reasons.”
The A1c increase among technology nonusers may be a result of a statistical artifact, as the number of those individuals was much lower in 2021 than in 2014. It’s possible that those remaining individuals have exceedingly high A1c levels, bringing the average up. “It’s still not good, though,” Dr. Shah said.
The more technology, the lower the A1c
Over the study period, the proportion of stand-alone CGM users rose from 26.9% to 44.1%, while use of AIDs rose from 0% in 2014 and 2015 to 38.6% in 2021. The latter group included patients who used first-generation Medtronic 670G and 770G devices and second-generation Tandem t:slim X2 with Control-IQ devices.
Between 2017 and 2021, AIDs users had significantly lower A1c levels than nontechnology users: 7.4% vs. 8.1% in 2017, and 7.3% vs. 8.4% in 2021 (P < .001 for every year). CGM users also had significantly lower A1c levels than nonusers at all time points (P < .001 per year).
The proportions achieving an A1c less than 7% differed significantly across users of CGMs, AIDs, and no technology (P < .01 for all years). In 2021, the percentage of people who achieved an A1c less than 7% were 50.9% with AIDs and 44.1% for CGMs vs, just 15.2% with no technology.
Work to be done: Why aren’t more achieving < 7% with AIDs?
Asked why only slightly more than half of patients who used AIDs achieved A1c levels below 7%, Dr. Shah listed three possibilities:
First, the 7% goal doesn’t apply to everyone with type 1 diabetes, including those with multiple comorbidities or with short life expectancy, for whom the recommended goal is 7.5%-8.0% to prevent hypoglycemia. “We didn’t separate out patients by A1c goals. If we add that, the number might go up,” Dr. Shah said.
Second, AID technology is continually improving, but it’s not perfect. Users still must enter carbohydrate counts and signal the devices for exercise, which can lead to errors. “It’s a wonderful technology for overnight control, but still, during the daytime, there are so many factors with the user interface and how much a person is engaged with the technology,” Dr. Shah explained.
Third, he said, “Unfortunately, obesity is increasing in type 1 diabetes, and insulin doses are increasing. Higher BMI [body mass index] and more insulin resistance can mean higher A1c. I really think for many patients, we probably will need an adjunct therapy, such as an SGLT2 [sodium-glucose cotransporter-2] inhibitor or a GLP-1 [glucagonlike peptide-1] agonist, even though they’re not approved in type 1 diabetes, for both glycemic and metabolic control including weight. I think that’s another missing piece.”
He also pointed out, “If someone has an A1c of 7.5%, I don’t expect a huge change. But if they’re at 10%, a drop to 8% is a huge change.”
Overall, Dr. Shah said, the news from the study is good. “In the past, only 30% were achieving an A1c less than 7%. Now we’re 20% above that. ... It’s a glass half full.”
Dr. Karakus has disclosed no relevant financial relationships. Dr. Shah has received, through the University of Colorado, research support from Novo Nordisk, Insulet, Tandem Diabetes, and Dexcom, and honoraria from Medscape, Lifescan, Novo Nordisk, and DKSH Singapore for advisory board attendance and from Insulet and Dexcom for speaking engagements.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Artificial sweeteners no help for weight loss: Review
It also shows evidence that these products are not beneficial for controlling excess weight.
Francisco Gómez-Delgado, MD, PhD, and Pablo Pérez-Martínez, MD, PhD, are members of the Spanish Society of Arteriosclerosis and of the Spanish Society of Internal Medicine. They have coordinated an updated review of the leading scientific evidence surrounding artificial sweeteners: evidence showing that far from positively affecting our health, they have “negative effects for the cardiometabolic system.”
The paper, published in Current Opinion in Cardiology, delves into the consumption of these sweeteners and their negative influence on the development of obesity and of several of the most important cardiometabolic risk factors (hypertension, dyslipidemia, and diabetes).
Globalization and the increase in consumption of ultraprocessed foods have led to a need for greater knowledge on the health impacts of certain nutrients such as artificial sweeteners (nutritive and nonnutritive). This review aims to analyze their role and their effect on cardiometabolic and cardiovascular disease risk.
Cardiovascular risk
The detrimental effects of a high-calorie, high-sugar diet have been well established. For this reason, health authorities recommend limiting sugar consumption. The recommendation has led the food industry to develop different artificial sweeteners with specific properties, such as flavor and stability (nutritive artificial sweeteners), and others aimed at limiting sugar in the diet (nonnutritive artificial sweeteners). Recent evidence explores the influence of these two types of artificial sweeteners on cardiovascular disease risk through risk factors such as obesity and type 2 diabetes, among others.
Initially, the consumption of artificial sweeteners was presented as an alternative for reducing calorie intake in the diet as an option for people with excess weight and obesity. However, as this paper explains, the consumption of these artificial sweeteners favors weight gain because of neuroendocrine mechanisms related to satiety that are abnormally activated when artificial sweeteners are consumed.
Weight gain
On the other hand, evidence shows that consuming artificial sweeteners does not encourage weight loss. “Quite the contrary,” Dr. Pérez-Martínez, scientific director at the Maimonides Biomedical Research Institute and internist at the University Hospital Reina Sofia, both in Córdoba, told this news organization. “There is evidence showing weight gain resulting from the effect that artificial sweetener consumption has at the neurohormonal level by altering the mechanisms involved in regulating the feeling of satiety.”
However, on the basis of current evidence, sugar cannot be claimed to be less harmful. “What we do know is that in both cases, we should reduce or remove them from our diets and replace them with other healthier alternatives for weight management, such as eating plant-based products or being physically active.”
Confronting ignorance
Nonetheless, these recommendations are conditional, “because the weight of the evidence is not extremely high, since there have not been a whole lot of studies. All nutritional studies must be viewed with caution,” Manuel Anguita, MD, PhD, said in an interview. Dr. Anguita is department head of clinical cardiology at the University Hospital Reina Sofia in Córdoba and past president of the Spanish Society of Cardiology.
“It’s something that should be included within the medical record when you’re assessing cardiovascular risk. In addition to identifying patients who use artificial sweeteners, it’s especially important to emphasize that it’s not an appropriate recommendation for weight management.” Healthier measures include moderate exercise and the Mediterranean diet.
Explaining why this research is valuable, he said, “It’s generally useful because there’s ignorance not only in the population but among physicians as well [about] these negative effects of sweeteners.”
Diabetes and metabolic syndrome
Artificial sweeteners cause significant disruptions in the endocrine system, leading our metabolism to function abnormally. The review revealed that consuming artificial sweeteners raises the risk for type 2 diabetes by between 18% and 24% and raises the risk for metabolic syndrome by up to 44%.
Dr. Gómez-Delgado, an internal medicine specialist at the University Hospital of Jaen in Spain and first author of the study, discussed the deleterious effects of sweeteners on metabolism. “On one hand, neurohormonal disorders impact appetite, and the feeling of satiety is abnormally delayed.” On the other hand, “they induce excessive insulin secretion in the pancreas,” which in the long run, encourages metabolic disorders that lead to diabetes. Ultimately, this process produces what we know as “dysbiosis, since our microbiota is unable to process these artificial sweeteners.” Dysbiosis triggers specific pathophysiologic processes that negatively affect cardiometabolic and cardiovascular systems.
No differences
Regarding the type of sweetener, Dr. Gómez-Delgado noted that currently available studies assess the consumption of special dietary products that, in most cases, include various types of artificial sweeteners. “So, it’s not possible to define specific differences between them as to how they impact our health.” Additional studies are needed to confirm this effect at the cardiometabolic level and to analyze the different types of artificial sweeteners individually.
“There’s enough evidence to confirm that consuming artificial sweeteners negatively interferes with our metabolism – especially glucose metabolism – and increases the risk of developing diabetes,” said Dr. Gómez-Delgado.
High-sodium drinks
When it comes to the influence of artificial sweeteners on hypertension, “there is no single explanation. The World Health Organization already discussed this issue 4-5 years ago, not only due to their carcinogenic risk, but also due to this cardiovascular risk in terms of a lack of control of obesity, diabetes, and hypertension,” said Dr. Anguita.
Another important point “is that this is not in reference to the sweeteners themselves, but to soft drinks containing those components, which is where we have more studies,” he added. There are two factors explaining this increase in hypertension, which poses a problem at the population level, with medium- to long-term follow-up. “The sugary beverages that we mentioned have a higher sodium content. That is, the sweeteners add this element, which is a factor that’s directly linked to the increase in blood pressure levels.” Another factor that can influence blood pressure is “the increase in insulin secretion that has been described as resulting from sweeteners. In the medium and long term, this is associated with increased blood pressure levels.”
Cardiovascular risk factor?
Are artificial sweeteners considered to be a new cardiovascular risk factor? “What they really do is increase the incidence of the other classic risk factors,” including obesity, said Dr. Anguita. It has been shown that artificial sweeteners don’t reduce obesity when used continuously. Nonetheless, “there is still not enough evidence to view it in the same light as the classic risk factors,” added Dr. Anguita. However, it is a factor that can clearly worsen the control of the other factors. Therefore, “it’s appropriate to sound an alarm and explain that it’s not the best way to lose weight; there are many other healthier choices.”
“We need more robust evidence to take a clear position on the use of this type of sweetener and its detrimental effect on health. Meanwhile, it would be ideal to limit their consumption or even avoid adding artificial sweeteners to coffee or teas,” added Dr. Pérez-Martínez.
Regulate consumption
Dr. Pérez-Martínez mentioned that the measures proposed to regulate the consumption of artificial sweeteners and to modify the current legislation must involve “minimizing the consumption of these special dietary products as much as possible and even avoiding adding these artificial sweeteners to the foods that we consume; for example, to coffee and tea.” On the other hand, “we must provide consumers with information that is as clear and simple as possible regarding the composition of the food they consume and how it impacts their health.”
However, “we need more evidence to be able to take a clear position on what type of sweeteners we can consume in our diet and also to what extent we should limit their presence in the foods we consume,” said Dr. Pérez-Martínez.
Last, “most of the evidence is from short-term observational studies that assess frequencies and patterns of consumption of foods containing these artificial sweeteners.” Of course, “we need studies that specifically analyze their effects at the metabolic level as well as longer-term studies where the nutritional follow-up of participants is more accurate and rigorous, especially when it comes to the consumption of this type of food,” concluded Dr. Gómez-Delgado.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
It also shows evidence that these products are not beneficial for controlling excess weight.
Francisco Gómez-Delgado, MD, PhD, and Pablo Pérez-Martínez, MD, PhD, are members of the Spanish Society of Arteriosclerosis and of the Spanish Society of Internal Medicine. They have coordinated an updated review of the leading scientific evidence surrounding artificial sweeteners: evidence showing that far from positively affecting our health, they have “negative effects for the cardiometabolic system.”
The paper, published in Current Opinion in Cardiology, delves into the consumption of these sweeteners and their negative influence on the development of obesity and of several of the most important cardiometabolic risk factors (hypertension, dyslipidemia, and diabetes).
Globalization and the increase in consumption of ultraprocessed foods have led to a need for greater knowledge on the health impacts of certain nutrients such as artificial sweeteners (nutritive and nonnutritive). This review aims to analyze their role and their effect on cardiometabolic and cardiovascular disease risk.
Cardiovascular risk
The detrimental effects of a high-calorie, high-sugar diet have been well established. For this reason, health authorities recommend limiting sugar consumption. The recommendation has led the food industry to develop different artificial sweeteners with specific properties, such as flavor and stability (nutritive artificial sweeteners), and others aimed at limiting sugar in the diet (nonnutritive artificial sweeteners). Recent evidence explores the influence of these two types of artificial sweeteners on cardiovascular disease risk through risk factors such as obesity and type 2 diabetes, among others.
Initially, the consumption of artificial sweeteners was presented as an alternative for reducing calorie intake in the diet as an option for people with excess weight and obesity. However, as this paper explains, the consumption of these artificial sweeteners favors weight gain because of neuroendocrine mechanisms related to satiety that are abnormally activated when artificial sweeteners are consumed.
Weight gain
On the other hand, evidence shows that consuming artificial sweeteners does not encourage weight loss. “Quite the contrary,” Dr. Pérez-Martínez, scientific director at the Maimonides Biomedical Research Institute and internist at the University Hospital Reina Sofia, both in Córdoba, told this news organization. “There is evidence showing weight gain resulting from the effect that artificial sweetener consumption has at the neurohormonal level by altering the mechanisms involved in regulating the feeling of satiety.”
However, on the basis of current evidence, sugar cannot be claimed to be less harmful. “What we do know is that in both cases, we should reduce or remove them from our diets and replace them with other healthier alternatives for weight management, such as eating plant-based products or being physically active.”
Confronting ignorance
Nonetheless, these recommendations are conditional, “because the weight of the evidence is not extremely high, since there have not been a whole lot of studies. All nutritional studies must be viewed with caution,” Manuel Anguita, MD, PhD, said in an interview. Dr. Anguita is department head of clinical cardiology at the University Hospital Reina Sofia in Córdoba and past president of the Spanish Society of Cardiology.
“It’s something that should be included within the medical record when you’re assessing cardiovascular risk. In addition to identifying patients who use artificial sweeteners, it’s especially important to emphasize that it’s not an appropriate recommendation for weight management.” Healthier measures include moderate exercise and the Mediterranean diet.
Explaining why this research is valuable, he said, “It’s generally useful because there’s ignorance not only in the population but among physicians as well [about] these negative effects of sweeteners.”
Diabetes and metabolic syndrome
Artificial sweeteners cause significant disruptions in the endocrine system, leading our metabolism to function abnormally. The review revealed that consuming artificial sweeteners raises the risk for type 2 diabetes by between 18% and 24% and raises the risk for metabolic syndrome by up to 44%.
Dr. Gómez-Delgado, an internal medicine specialist at the University Hospital of Jaen in Spain and first author of the study, discussed the deleterious effects of sweeteners on metabolism. “On one hand, neurohormonal disorders impact appetite, and the feeling of satiety is abnormally delayed.” On the other hand, “they induce excessive insulin secretion in the pancreas,” which in the long run, encourages metabolic disorders that lead to diabetes. Ultimately, this process produces what we know as “dysbiosis, since our microbiota is unable to process these artificial sweeteners.” Dysbiosis triggers specific pathophysiologic processes that negatively affect cardiometabolic and cardiovascular systems.
No differences
Regarding the type of sweetener, Dr. Gómez-Delgado noted that currently available studies assess the consumption of special dietary products that, in most cases, include various types of artificial sweeteners. “So, it’s not possible to define specific differences between them as to how they impact our health.” Additional studies are needed to confirm this effect at the cardiometabolic level and to analyze the different types of artificial sweeteners individually.
“There’s enough evidence to confirm that consuming artificial sweeteners negatively interferes with our metabolism – especially glucose metabolism – and increases the risk of developing diabetes,” said Dr. Gómez-Delgado.
High-sodium drinks
When it comes to the influence of artificial sweeteners on hypertension, “there is no single explanation. The World Health Organization already discussed this issue 4-5 years ago, not only due to their carcinogenic risk, but also due to this cardiovascular risk in terms of a lack of control of obesity, diabetes, and hypertension,” said Dr. Anguita.
Another important point “is that this is not in reference to the sweeteners themselves, but to soft drinks containing those components, which is where we have more studies,” he added. There are two factors explaining this increase in hypertension, which poses a problem at the population level, with medium- to long-term follow-up. “The sugary beverages that we mentioned have a higher sodium content. That is, the sweeteners add this element, which is a factor that’s directly linked to the increase in blood pressure levels.” Another factor that can influence blood pressure is “the increase in insulin secretion that has been described as resulting from sweeteners. In the medium and long term, this is associated with increased blood pressure levels.”
Cardiovascular risk factor?
Are artificial sweeteners considered to be a new cardiovascular risk factor? “What they really do is increase the incidence of the other classic risk factors,” including obesity, said Dr. Anguita. It has been shown that artificial sweeteners don’t reduce obesity when used continuously. Nonetheless, “there is still not enough evidence to view it in the same light as the classic risk factors,” added Dr. Anguita. However, it is a factor that can clearly worsen the control of the other factors. Therefore, “it’s appropriate to sound an alarm and explain that it’s not the best way to lose weight; there are many other healthier choices.”
“We need more robust evidence to take a clear position on the use of this type of sweetener and its detrimental effect on health. Meanwhile, it would be ideal to limit their consumption or even avoid adding artificial sweeteners to coffee or teas,” added Dr. Pérez-Martínez.
Regulate consumption
Dr. Pérez-Martínez mentioned that the measures proposed to regulate the consumption of artificial sweeteners and to modify the current legislation must involve “minimizing the consumption of these special dietary products as much as possible and even avoiding adding these artificial sweeteners to the foods that we consume; for example, to coffee and tea.” On the other hand, “we must provide consumers with information that is as clear and simple as possible regarding the composition of the food they consume and how it impacts their health.”
However, “we need more evidence to be able to take a clear position on what type of sweeteners we can consume in our diet and also to what extent we should limit their presence in the foods we consume,” said Dr. Pérez-Martínez.
Last, “most of the evidence is from short-term observational studies that assess frequencies and patterns of consumption of foods containing these artificial sweeteners.” Of course, “we need studies that specifically analyze their effects at the metabolic level as well as longer-term studies where the nutritional follow-up of participants is more accurate and rigorous, especially when it comes to the consumption of this type of food,” concluded Dr. Gómez-Delgado.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
It also shows evidence that these products are not beneficial for controlling excess weight.
Francisco Gómez-Delgado, MD, PhD, and Pablo Pérez-Martínez, MD, PhD, are members of the Spanish Society of Arteriosclerosis and of the Spanish Society of Internal Medicine. They have coordinated an updated review of the leading scientific evidence surrounding artificial sweeteners: evidence showing that far from positively affecting our health, they have “negative effects for the cardiometabolic system.”
The paper, published in Current Opinion in Cardiology, delves into the consumption of these sweeteners and their negative influence on the development of obesity and of several of the most important cardiometabolic risk factors (hypertension, dyslipidemia, and diabetes).
Globalization and the increase in consumption of ultraprocessed foods have led to a need for greater knowledge on the health impacts of certain nutrients such as artificial sweeteners (nutritive and nonnutritive). This review aims to analyze their role and their effect on cardiometabolic and cardiovascular disease risk.
Cardiovascular risk
The detrimental effects of a high-calorie, high-sugar diet have been well established. For this reason, health authorities recommend limiting sugar consumption. The recommendation has led the food industry to develop different artificial sweeteners with specific properties, such as flavor and stability (nutritive artificial sweeteners), and others aimed at limiting sugar in the diet (nonnutritive artificial sweeteners). Recent evidence explores the influence of these two types of artificial sweeteners on cardiovascular disease risk through risk factors such as obesity and type 2 diabetes, among others.
Initially, the consumption of artificial sweeteners was presented as an alternative for reducing calorie intake in the diet as an option for people with excess weight and obesity. However, as this paper explains, the consumption of these artificial sweeteners favors weight gain because of neuroendocrine mechanisms related to satiety that are abnormally activated when artificial sweeteners are consumed.
Weight gain
On the other hand, evidence shows that consuming artificial sweeteners does not encourage weight loss. “Quite the contrary,” Dr. Pérez-Martínez, scientific director at the Maimonides Biomedical Research Institute and internist at the University Hospital Reina Sofia, both in Córdoba, told this news organization. “There is evidence showing weight gain resulting from the effect that artificial sweetener consumption has at the neurohormonal level by altering the mechanisms involved in regulating the feeling of satiety.”
However, on the basis of current evidence, sugar cannot be claimed to be less harmful. “What we do know is that in both cases, we should reduce or remove them from our diets and replace them with other healthier alternatives for weight management, such as eating plant-based products or being physically active.”
Confronting ignorance
Nonetheless, these recommendations are conditional, “because the weight of the evidence is not extremely high, since there have not been a whole lot of studies. All nutritional studies must be viewed with caution,” Manuel Anguita, MD, PhD, said in an interview. Dr. Anguita is department head of clinical cardiology at the University Hospital Reina Sofia in Córdoba and past president of the Spanish Society of Cardiology.
“It’s something that should be included within the medical record when you’re assessing cardiovascular risk. In addition to identifying patients who use artificial sweeteners, it’s especially important to emphasize that it’s not an appropriate recommendation for weight management.” Healthier measures include moderate exercise and the Mediterranean diet.
Explaining why this research is valuable, he said, “It’s generally useful because there’s ignorance not only in the population but among physicians as well [about] these negative effects of sweeteners.”
Diabetes and metabolic syndrome
Artificial sweeteners cause significant disruptions in the endocrine system, leading our metabolism to function abnormally. The review revealed that consuming artificial sweeteners raises the risk for type 2 diabetes by between 18% and 24% and raises the risk for metabolic syndrome by up to 44%.
Dr. Gómez-Delgado, an internal medicine specialist at the University Hospital of Jaen in Spain and first author of the study, discussed the deleterious effects of sweeteners on metabolism. “On one hand, neurohormonal disorders impact appetite, and the feeling of satiety is abnormally delayed.” On the other hand, “they induce excessive insulin secretion in the pancreas,” which in the long run, encourages metabolic disorders that lead to diabetes. Ultimately, this process produces what we know as “dysbiosis, since our microbiota is unable to process these artificial sweeteners.” Dysbiosis triggers specific pathophysiologic processes that negatively affect cardiometabolic and cardiovascular systems.
No differences
Regarding the type of sweetener, Dr. Gómez-Delgado noted that currently available studies assess the consumption of special dietary products that, in most cases, include various types of artificial sweeteners. “So, it’s not possible to define specific differences between them as to how they impact our health.” Additional studies are needed to confirm this effect at the cardiometabolic level and to analyze the different types of artificial sweeteners individually.
“There’s enough evidence to confirm that consuming artificial sweeteners negatively interferes with our metabolism – especially glucose metabolism – and increases the risk of developing diabetes,” said Dr. Gómez-Delgado.
High-sodium drinks
When it comes to the influence of artificial sweeteners on hypertension, “there is no single explanation. The World Health Organization already discussed this issue 4-5 years ago, not only due to their carcinogenic risk, but also due to this cardiovascular risk in terms of a lack of control of obesity, diabetes, and hypertension,” said Dr. Anguita.
Another important point “is that this is not in reference to the sweeteners themselves, but to soft drinks containing those components, which is where we have more studies,” he added. There are two factors explaining this increase in hypertension, which poses a problem at the population level, with medium- to long-term follow-up. “The sugary beverages that we mentioned have a higher sodium content. That is, the sweeteners add this element, which is a factor that’s directly linked to the increase in blood pressure levels.” Another factor that can influence blood pressure is “the increase in insulin secretion that has been described as resulting from sweeteners. In the medium and long term, this is associated with increased blood pressure levels.”
Cardiovascular risk factor?
Are artificial sweeteners considered to be a new cardiovascular risk factor? “What they really do is increase the incidence of the other classic risk factors,” including obesity, said Dr. Anguita. It has been shown that artificial sweeteners don’t reduce obesity when used continuously. Nonetheless, “there is still not enough evidence to view it in the same light as the classic risk factors,” added Dr. Anguita. However, it is a factor that can clearly worsen the control of the other factors. Therefore, “it’s appropriate to sound an alarm and explain that it’s not the best way to lose weight; there are many other healthier choices.”
“We need more robust evidence to take a clear position on the use of this type of sweetener and its detrimental effect on health. Meanwhile, it would be ideal to limit their consumption or even avoid adding artificial sweeteners to coffee or teas,” added Dr. Pérez-Martínez.
Regulate consumption
Dr. Pérez-Martínez mentioned that the measures proposed to regulate the consumption of artificial sweeteners and to modify the current legislation must involve “minimizing the consumption of these special dietary products as much as possible and even avoiding adding these artificial sweeteners to the foods that we consume; for example, to coffee and tea.” On the other hand, “we must provide consumers with information that is as clear and simple as possible regarding the composition of the food they consume and how it impacts their health.”
However, “we need more evidence to be able to take a clear position on what type of sweeteners we can consume in our diet and also to what extent we should limit their presence in the foods we consume,” said Dr. Pérez-Martínez.
Last, “most of the evidence is from short-term observational studies that assess frequencies and patterns of consumption of foods containing these artificial sweeteners.” Of course, “we need studies that specifically analyze their effects at the metabolic level as well as longer-term studies where the nutritional follow-up of participants is more accurate and rigorous, especially when it comes to the consumption of this type of food,” concluded Dr. Gómez-Delgado.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
FROM CURRENT OPINION IN CARDIOLOGY
Dementia diagnosis a good time to reduce polypharmacy
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
FROM JAMA INTERNAL MEDICINE
Lobbying allowed insurers to charge physicians fees to receive payments online: Report
, according to a new investigation by the nonprofit news organization ProPublica.
The Affordable Care Act requires that health plans give providers the option of being paid electronically to improve efficiency and save money. In 2017, the Centers for Medicare & Medicaid Services issued guidance that prohibited insurers and their payment processing vendors from “engaging in unfair business practices that do not support an efficient healthcare system,” according to a recent Medical Group Management Association position paper.
But that guidance, which appeared to forbid requiring fees to receive payments online, disappeared from the CMS site 6 months later.
According to ProPublica’s reporting, the change was the result of a quiet insurance industry lobbying campaign led by Matthew Albright, a former CMS employee who left government service to work for Zelis, a payment processing company co-owned by private equity giant Bain Capital.
The details of the lobbying effort were discovered by Alex Shteynshlyuger, a New York urologist, who through public records requests received the email correspondence between Mr. Albright and CMS and shared that material with ProPublica.
Mr. Albright had been able to influence CMS policy to protect what ProPublica called a “crucial revenue stream” for payment processors. The fee notice was removed just 3 days after Mr. Albright requested the change, ProPublica found.
When CMS resisted further changes, including eliminating guidance forbidding insurers and payment processors from charging excess fees for online payments, Mr. Albright brought in a law firm. The threat of a lawsuit by deep-pocketed Zelis was enough to bring CMS in line, ProPublica reported. Today, these fees can cost larger medical practices more than $1 million a year, according to the MGMA report.
“It took less than a decade for a new industry of middlemen, owned by private equity funds and giant conglomerates like UnitedHealth Group, to cash in,” writes Cezary Podkul, the author of the ProPublica report.
Predatory practices
It might seem that avoiding the fees would be as simple as requesting to be paid by check. However, a 2021 poll by the MGMA found that 57% of doctors were being charged these fees when they hadn’t agreed to them. According to the ProPublica report, physicians who have requested to be paid by check often find themselves being bounced back to electronic fund transfer (EFT) payments, where they are again charged fees.
In October 2021, more than 90 physician organizations, including the American Medical Association and the MGMA, signed a letter calling on the Biden administration to reinstate guidance to protect physicians’ right to receive EFT payments without paying fees. The letter describes the practice as “outrageous” and analogous to “an employee being required to enroll in a program that would deduct a percentage of each paycheck to receive direct deposit payments from an employer.”
So far, however, the situation remains unchanged. The language on the CMS site has changed, though. In 2022, the guidelines were adjusted to clarify that EFT fees are allowed.
A version of this article first appeared on Medscape.com.
, according to a new investigation by the nonprofit news organization ProPublica.
The Affordable Care Act requires that health plans give providers the option of being paid electronically to improve efficiency and save money. In 2017, the Centers for Medicare & Medicaid Services issued guidance that prohibited insurers and their payment processing vendors from “engaging in unfair business practices that do not support an efficient healthcare system,” according to a recent Medical Group Management Association position paper.
But that guidance, which appeared to forbid requiring fees to receive payments online, disappeared from the CMS site 6 months later.
According to ProPublica’s reporting, the change was the result of a quiet insurance industry lobbying campaign led by Matthew Albright, a former CMS employee who left government service to work for Zelis, a payment processing company co-owned by private equity giant Bain Capital.
The details of the lobbying effort were discovered by Alex Shteynshlyuger, a New York urologist, who through public records requests received the email correspondence between Mr. Albright and CMS and shared that material with ProPublica.
Mr. Albright had been able to influence CMS policy to protect what ProPublica called a “crucial revenue stream” for payment processors. The fee notice was removed just 3 days after Mr. Albright requested the change, ProPublica found.
When CMS resisted further changes, including eliminating guidance forbidding insurers and payment processors from charging excess fees for online payments, Mr. Albright brought in a law firm. The threat of a lawsuit by deep-pocketed Zelis was enough to bring CMS in line, ProPublica reported. Today, these fees can cost larger medical practices more than $1 million a year, according to the MGMA report.
“It took less than a decade for a new industry of middlemen, owned by private equity funds and giant conglomerates like UnitedHealth Group, to cash in,” writes Cezary Podkul, the author of the ProPublica report.
Predatory practices
It might seem that avoiding the fees would be as simple as requesting to be paid by check. However, a 2021 poll by the MGMA found that 57% of doctors were being charged these fees when they hadn’t agreed to them. According to the ProPublica report, physicians who have requested to be paid by check often find themselves being bounced back to electronic fund transfer (EFT) payments, where they are again charged fees.
In October 2021, more than 90 physician organizations, including the American Medical Association and the MGMA, signed a letter calling on the Biden administration to reinstate guidance to protect physicians’ right to receive EFT payments without paying fees. The letter describes the practice as “outrageous” and analogous to “an employee being required to enroll in a program that would deduct a percentage of each paycheck to receive direct deposit payments from an employer.”
So far, however, the situation remains unchanged. The language on the CMS site has changed, though. In 2022, the guidelines were adjusted to clarify that EFT fees are allowed.
A version of this article first appeared on Medscape.com.
, according to a new investigation by the nonprofit news organization ProPublica.
The Affordable Care Act requires that health plans give providers the option of being paid electronically to improve efficiency and save money. In 2017, the Centers for Medicare & Medicaid Services issued guidance that prohibited insurers and their payment processing vendors from “engaging in unfair business practices that do not support an efficient healthcare system,” according to a recent Medical Group Management Association position paper.
But that guidance, which appeared to forbid requiring fees to receive payments online, disappeared from the CMS site 6 months later.
According to ProPublica’s reporting, the change was the result of a quiet insurance industry lobbying campaign led by Matthew Albright, a former CMS employee who left government service to work for Zelis, a payment processing company co-owned by private equity giant Bain Capital.
The details of the lobbying effort were discovered by Alex Shteynshlyuger, a New York urologist, who through public records requests received the email correspondence between Mr. Albright and CMS and shared that material with ProPublica.
Mr. Albright had been able to influence CMS policy to protect what ProPublica called a “crucial revenue stream” for payment processors. The fee notice was removed just 3 days after Mr. Albright requested the change, ProPublica found.
When CMS resisted further changes, including eliminating guidance forbidding insurers and payment processors from charging excess fees for online payments, Mr. Albright brought in a law firm. The threat of a lawsuit by deep-pocketed Zelis was enough to bring CMS in line, ProPublica reported. Today, these fees can cost larger medical practices more than $1 million a year, according to the MGMA report.
“It took less than a decade for a new industry of middlemen, owned by private equity funds and giant conglomerates like UnitedHealth Group, to cash in,” writes Cezary Podkul, the author of the ProPublica report.
Predatory practices
It might seem that avoiding the fees would be as simple as requesting to be paid by check. However, a 2021 poll by the MGMA found that 57% of doctors were being charged these fees when they hadn’t agreed to them. According to the ProPublica report, physicians who have requested to be paid by check often find themselves being bounced back to electronic fund transfer (EFT) payments, where they are again charged fees.
In October 2021, more than 90 physician organizations, including the American Medical Association and the MGMA, signed a letter calling on the Biden administration to reinstate guidance to protect physicians’ right to receive EFT payments without paying fees. The letter describes the practice as “outrageous” and analogous to “an employee being required to enroll in a program that would deduct a percentage of each paycheck to receive direct deposit payments from an employer.”
So far, however, the situation remains unchanged. The language on the CMS site has changed, though. In 2022, the guidelines were adjusted to clarify that EFT fees are allowed.
A version of this article first appeared on Medscape.com.
Simple blood test may predict heart and kidney risk in T2D
, suggests an analysis of the CREDENCE trial.
The research, published online in the journal Circulation, also revealed that patients treated with the sodium-glucose cotransporter-2 inhibitor canagliflozin (Invokana, Invokamet) had lower levels of the biomarkers after 1 year compared with those given placebo.
Examination of biomarker levels in more than 2,600 patients from CREDENCE showed that high baseline concentrations of the individual biomarkers were able to predict the future risk for a composite endpoint of renal and heart outcomes.
The combination of all four biomarkers into a single panel revealed that patients with the highest levels were more than four times as likely to experience the composite endpoint than were those with the lowest levels.
As two of the biomarkers used in the study have yet to have established prognostic thresholds, the results remain exploratory.
Lead author James L. Januzzi, MD, director of the Heart Failure and Biomarker Trials at the Baim Institute for Clinical Research, Boston, said that further study will help refine the predictive value of the panel.
“Given that the American Heart Association/American College of Cardiology and the American Diabetes Association now all recommend measurement of biomarkers to enhance the ability to predict risk in persons with type 2 diabetes, these results may considerably extend the reach of biomarker-based testing, refining accuracy even further,” he said in a press release.
In an interview, Dr. Januzzi said that “three out of the four biomarkers are already clinically and commercially available,” while the fourth, for insulin-like growth factor binding protein 7 (IGFBP7), is “on the near horizon.”
He stressed that the “future for multiple biomarker testing, however, will be less about ordering each individual test, and ultimately will revolve around panels of blood work that are ordered as a single test.”
Dr. Januzzi added that “rather than using the rather primitive approach that we took” of looking at the individual biomarkers in adjusted models, the next stage “will be to utilize algorithms to combine the results into a single value.
“A clinician will not have to struggle with looking at individual results but will just receive one aggregated test result that informs them whether a patient is at low, medium or higher risk,” he explained.
However, this will require determining the relative importance of each biomarker and weighting them in the final model.
Consequently, the current results “set the foundation for identifying some very powerful individual tests that may ultimately, in aggregate, help us to help our patients with diabetes avoid a major complication,” Dr. Januzzi said.
By revealing that some individuals with both type 2 diabetes and kidney disease are at higher risk than others, he also hopes the findings can be leveraged to treat patients with “varying degrees of intensity with proven therapies, including weight loss, dietary adjustment, and pharmacologic intervention.”
Dr. Januzzi added: “Diabetes affects a dramatic, and growing, percentage of our population, and this type of personalized strategy to reduce the major complications of this rather common disease is an important step forward.”
The authors noted that there is a “bidirectional relationship” between cardiovascular disease and chronic kidney disease (CKD), such that either diagnosis may increase the risk of, or exacerbate, the other.
Individuals with type 2 diabetes and CKD albuminuria, they added, are at particularly high risk for major cardiovascular events, and studies have shown that several circulating cardiorenal stress biomarkers may predict the onset and progression of CKD in type 2 diabetes, as well as predict cardiovascular events.
Several biomarkers associated with myocardial stress and necrosis
The recent CANVAS trial revealed that, among individuals with type 2 diabetes with and without CKD, several biomarkers were associated with myocardial stress and necrosis, and renal tubular injury, predicting the progression of CKD with albuminuria, and the risk for heart failure events.
Taking inspiration from those findings, the current researchers studied a panel of similar cardiac and renal biomarkers among participants from the CREDENCE trial, for which 4,401 patients with type 2 diabetes and CKD at high risk of progression were randomly assigned to canagliflozin or placebo.
The current analysis involved 2,627 participants who had baseline plasma samples available for analysis of four circulating biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), growth differentiation factor-15 (GDF-15), and IGFBP7.
Among those, 2,385 participants also had year 1 plasma samples available for analysis, while year 3 plasma samples were available for 895 individuals.
The results showed that, in general, median baseline concentrations of each biomarker in both treatment groups were elevated compared with healthy reference populations.
Baseline log-transformed concentrations of each biomarker were also strongly predictive of cardiac and renal outcomes, including heart failure and progression of CKD.
For example, each unit increase in baseline NT-proBNP concentrations was associated with a hazard ratio of 1.35 for the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine levels, renal death, or cardiovascular disease (P < .001).
For each unit increase in hs-cTnT levels, the hazard ratio for the primary composite was 1.73 (P < .001), for GDF-15 it was 1.84 (P < .0001), and for IGFBP7 the hazard ratio was 3.14 (P < .001).
Combining the four biomarkers into a single multimarker panel revealed that, compared with individuals with a low-risk score, those with a high-risk score had a hazard ratio for the primary outcome of 4.01, whereas those with a moderate risk score had a hazard ratio of 2.39 (P < .001 for both).
For the individual outcome of heart failure hospitalization, the effect was even greater. A high-risk score was associated with a hazard ratio vs. a low-risk score of 6.04 (P < .001), whereas patients with a moderate risk score had a hazard ratio of 2.45 (P = .04).
The researchers also reported that, between baseline and year 1, concentrations of all four biomarkers rose from 6% to 29% in the placebo group, but from 3% to just 10% in those treated with canagliflozin.
“It was reassuring to discover that canagliflozin helped reduce risks the most in people with the highest chances for complications,” said Dr. Januzzi.
The CREDENCE trial and the current analysis were funded by Janssen Research & Development LLC. NT-proBNP, hs-cTnT, GDF-15, and IGFBP7 reagents were provided by Roche Diagnostics. Dr. Januzzi is funded in part by the Hutter Family Professorship. Dr. Januzzi declared relationships with Imbria Pharmaceuticals, Jana Care, Abbott, Applied Therapeutics, HeartFlow, Innolife, Roche Diagnostics, Beckman, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, Pfizer, Siemens, Abbott, AbbVie, CVRx, Intercept, and Takeda.
A version of this article first appeared on Medscape.com.
, suggests an analysis of the CREDENCE trial.
The research, published online in the journal Circulation, also revealed that patients treated with the sodium-glucose cotransporter-2 inhibitor canagliflozin (Invokana, Invokamet) had lower levels of the biomarkers after 1 year compared with those given placebo.
Examination of biomarker levels in more than 2,600 patients from CREDENCE showed that high baseline concentrations of the individual biomarkers were able to predict the future risk for a composite endpoint of renal and heart outcomes.
The combination of all four biomarkers into a single panel revealed that patients with the highest levels were more than four times as likely to experience the composite endpoint than were those with the lowest levels.
As two of the biomarkers used in the study have yet to have established prognostic thresholds, the results remain exploratory.
Lead author James L. Januzzi, MD, director of the Heart Failure and Biomarker Trials at the Baim Institute for Clinical Research, Boston, said that further study will help refine the predictive value of the panel.
“Given that the American Heart Association/American College of Cardiology and the American Diabetes Association now all recommend measurement of biomarkers to enhance the ability to predict risk in persons with type 2 diabetes, these results may considerably extend the reach of biomarker-based testing, refining accuracy even further,” he said in a press release.
In an interview, Dr. Januzzi said that “three out of the four biomarkers are already clinically and commercially available,” while the fourth, for insulin-like growth factor binding protein 7 (IGFBP7), is “on the near horizon.”
He stressed that the “future for multiple biomarker testing, however, will be less about ordering each individual test, and ultimately will revolve around panels of blood work that are ordered as a single test.”
Dr. Januzzi added that “rather than using the rather primitive approach that we took” of looking at the individual biomarkers in adjusted models, the next stage “will be to utilize algorithms to combine the results into a single value.
“A clinician will not have to struggle with looking at individual results but will just receive one aggregated test result that informs them whether a patient is at low, medium or higher risk,” he explained.
However, this will require determining the relative importance of each biomarker and weighting them in the final model.
Consequently, the current results “set the foundation for identifying some very powerful individual tests that may ultimately, in aggregate, help us to help our patients with diabetes avoid a major complication,” Dr. Januzzi said.
By revealing that some individuals with both type 2 diabetes and kidney disease are at higher risk than others, he also hopes the findings can be leveraged to treat patients with “varying degrees of intensity with proven therapies, including weight loss, dietary adjustment, and pharmacologic intervention.”
Dr. Januzzi added: “Diabetes affects a dramatic, and growing, percentage of our population, and this type of personalized strategy to reduce the major complications of this rather common disease is an important step forward.”
The authors noted that there is a “bidirectional relationship” between cardiovascular disease and chronic kidney disease (CKD), such that either diagnosis may increase the risk of, or exacerbate, the other.
Individuals with type 2 diabetes and CKD albuminuria, they added, are at particularly high risk for major cardiovascular events, and studies have shown that several circulating cardiorenal stress biomarkers may predict the onset and progression of CKD in type 2 diabetes, as well as predict cardiovascular events.
Several biomarkers associated with myocardial stress and necrosis
The recent CANVAS trial revealed that, among individuals with type 2 diabetes with and without CKD, several biomarkers were associated with myocardial stress and necrosis, and renal tubular injury, predicting the progression of CKD with albuminuria, and the risk for heart failure events.
Taking inspiration from those findings, the current researchers studied a panel of similar cardiac and renal biomarkers among participants from the CREDENCE trial, for which 4,401 patients with type 2 diabetes and CKD at high risk of progression were randomly assigned to canagliflozin or placebo.
The current analysis involved 2,627 participants who had baseline plasma samples available for analysis of four circulating biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), growth differentiation factor-15 (GDF-15), and IGFBP7.
Among those, 2,385 participants also had year 1 plasma samples available for analysis, while year 3 plasma samples were available for 895 individuals.
The results showed that, in general, median baseline concentrations of each biomarker in both treatment groups were elevated compared with healthy reference populations.
Baseline log-transformed concentrations of each biomarker were also strongly predictive of cardiac and renal outcomes, including heart failure and progression of CKD.
For example, each unit increase in baseline NT-proBNP concentrations was associated with a hazard ratio of 1.35 for the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine levels, renal death, or cardiovascular disease (P < .001).
For each unit increase in hs-cTnT levels, the hazard ratio for the primary composite was 1.73 (P < .001), for GDF-15 it was 1.84 (P < .0001), and for IGFBP7 the hazard ratio was 3.14 (P < .001).
Combining the four biomarkers into a single multimarker panel revealed that, compared with individuals with a low-risk score, those with a high-risk score had a hazard ratio for the primary outcome of 4.01, whereas those with a moderate risk score had a hazard ratio of 2.39 (P < .001 for both).
For the individual outcome of heart failure hospitalization, the effect was even greater. A high-risk score was associated with a hazard ratio vs. a low-risk score of 6.04 (P < .001), whereas patients with a moderate risk score had a hazard ratio of 2.45 (P = .04).
The researchers also reported that, between baseline and year 1, concentrations of all four biomarkers rose from 6% to 29% in the placebo group, but from 3% to just 10% in those treated with canagliflozin.
“It was reassuring to discover that canagliflozin helped reduce risks the most in people with the highest chances for complications,” said Dr. Januzzi.
The CREDENCE trial and the current analysis were funded by Janssen Research & Development LLC. NT-proBNP, hs-cTnT, GDF-15, and IGFBP7 reagents were provided by Roche Diagnostics. Dr. Januzzi is funded in part by the Hutter Family Professorship. Dr. Januzzi declared relationships with Imbria Pharmaceuticals, Jana Care, Abbott, Applied Therapeutics, HeartFlow, Innolife, Roche Diagnostics, Beckman, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, Pfizer, Siemens, Abbott, AbbVie, CVRx, Intercept, and Takeda.
A version of this article first appeared on Medscape.com.
, suggests an analysis of the CREDENCE trial.
The research, published online in the journal Circulation, also revealed that patients treated with the sodium-glucose cotransporter-2 inhibitor canagliflozin (Invokana, Invokamet) had lower levels of the biomarkers after 1 year compared with those given placebo.
Examination of biomarker levels in more than 2,600 patients from CREDENCE showed that high baseline concentrations of the individual biomarkers were able to predict the future risk for a composite endpoint of renal and heart outcomes.
The combination of all four biomarkers into a single panel revealed that patients with the highest levels were more than four times as likely to experience the composite endpoint than were those with the lowest levels.
As two of the biomarkers used in the study have yet to have established prognostic thresholds, the results remain exploratory.
Lead author James L. Januzzi, MD, director of the Heart Failure and Biomarker Trials at the Baim Institute for Clinical Research, Boston, said that further study will help refine the predictive value of the panel.
“Given that the American Heart Association/American College of Cardiology and the American Diabetes Association now all recommend measurement of biomarkers to enhance the ability to predict risk in persons with type 2 diabetes, these results may considerably extend the reach of biomarker-based testing, refining accuracy even further,” he said in a press release.
In an interview, Dr. Januzzi said that “three out of the four biomarkers are already clinically and commercially available,” while the fourth, for insulin-like growth factor binding protein 7 (IGFBP7), is “on the near horizon.”
He stressed that the “future for multiple biomarker testing, however, will be less about ordering each individual test, and ultimately will revolve around panels of blood work that are ordered as a single test.”
Dr. Januzzi added that “rather than using the rather primitive approach that we took” of looking at the individual biomarkers in adjusted models, the next stage “will be to utilize algorithms to combine the results into a single value.
“A clinician will not have to struggle with looking at individual results but will just receive one aggregated test result that informs them whether a patient is at low, medium or higher risk,” he explained.
However, this will require determining the relative importance of each biomarker and weighting them in the final model.
Consequently, the current results “set the foundation for identifying some very powerful individual tests that may ultimately, in aggregate, help us to help our patients with diabetes avoid a major complication,” Dr. Januzzi said.
By revealing that some individuals with both type 2 diabetes and kidney disease are at higher risk than others, he also hopes the findings can be leveraged to treat patients with “varying degrees of intensity with proven therapies, including weight loss, dietary adjustment, and pharmacologic intervention.”
Dr. Januzzi added: “Diabetes affects a dramatic, and growing, percentage of our population, and this type of personalized strategy to reduce the major complications of this rather common disease is an important step forward.”
The authors noted that there is a “bidirectional relationship” between cardiovascular disease and chronic kidney disease (CKD), such that either diagnosis may increase the risk of, or exacerbate, the other.
Individuals with type 2 diabetes and CKD albuminuria, they added, are at particularly high risk for major cardiovascular events, and studies have shown that several circulating cardiorenal stress biomarkers may predict the onset and progression of CKD in type 2 diabetes, as well as predict cardiovascular events.
Several biomarkers associated with myocardial stress and necrosis
The recent CANVAS trial revealed that, among individuals with type 2 diabetes with and without CKD, several biomarkers were associated with myocardial stress and necrosis, and renal tubular injury, predicting the progression of CKD with albuminuria, and the risk for heart failure events.
Taking inspiration from those findings, the current researchers studied a panel of similar cardiac and renal biomarkers among participants from the CREDENCE trial, for which 4,401 patients with type 2 diabetes and CKD at high risk of progression were randomly assigned to canagliflozin or placebo.
The current analysis involved 2,627 participants who had baseline plasma samples available for analysis of four circulating biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), growth differentiation factor-15 (GDF-15), and IGFBP7.
Among those, 2,385 participants also had year 1 plasma samples available for analysis, while year 3 plasma samples were available for 895 individuals.
The results showed that, in general, median baseline concentrations of each biomarker in both treatment groups were elevated compared with healthy reference populations.
Baseline log-transformed concentrations of each biomarker were also strongly predictive of cardiac and renal outcomes, including heart failure and progression of CKD.
For example, each unit increase in baseline NT-proBNP concentrations was associated with a hazard ratio of 1.35 for the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine levels, renal death, or cardiovascular disease (P < .001).
For each unit increase in hs-cTnT levels, the hazard ratio for the primary composite was 1.73 (P < .001), for GDF-15 it was 1.84 (P < .0001), and for IGFBP7 the hazard ratio was 3.14 (P < .001).
Combining the four biomarkers into a single multimarker panel revealed that, compared with individuals with a low-risk score, those with a high-risk score had a hazard ratio for the primary outcome of 4.01, whereas those with a moderate risk score had a hazard ratio of 2.39 (P < .001 for both).
For the individual outcome of heart failure hospitalization, the effect was even greater. A high-risk score was associated with a hazard ratio vs. a low-risk score of 6.04 (P < .001), whereas patients with a moderate risk score had a hazard ratio of 2.45 (P = .04).
The researchers also reported that, between baseline and year 1, concentrations of all four biomarkers rose from 6% to 29% in the placebo group, but from 3% to just 10% in those treated with canagliflozin.
“It was reassuring to discover that canagliflozin helped reduce risks the most in people with the highest chances for complications,” said Dr. Januzzi.
The CREDENCE trial and the current analysis were funded by Janssen Research & Development LLC. NT-proBNP, hs-cTnT, GDF-15, and IGFBP7 reagents were provided by Roche Diagnostics. Dr. Januzzi is funded in part by the Hutter Family Professorship. Dr. Januzzi declared relationships with Imbria Pharmaceuticals, Jana Care, Abbott, Applied Therapeutics, HeartFlow, Innolife, Roche Diagnostics, Beckman, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, Pfizer, Siemens, Abbott, AbbVie, CVRx, Intercept, and Takeda.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Could a malpractice insurer drop you when you need it most?
You’ve practiced medicine for years without issues, but now you are facing a medical malpractice case. No worries – you’ve had professional liability insurance all this time, so surely there’s nothing to be concerned about. Undoubtedly, your medical malpractice insurer will cover the costs of defending you. Or will they? One case casts questions on just this issue.
Professional liability insurance
According to the American Medical Association, almost one in three physicians (31%) have had a medical malpractice lawsuit filed against them at some point in their careers. These numbers only increase the longer a physician practices; almost half of doctors 55 and over have been sued, compared with less than 10% of physicians under 40.
And while the majority of cases are dropped or dismissed, and the small minority of cases that do go to trial are mostly won by the defense, the cost of defending these cases can be extremely high. Physicians have medical malpractice insurance to defray these costs.
Malpractice insurance generally covers the costs of attorney fees, court costs, arbitration, compensatory damages, and settlements related to patient injury or death. Insurance sometimes, but not always, pays for the costs of malpractice lawsuits arising out of Health Insurance Portability and Accountability Act (HIPAA) violations.
But it is what the policies don’t pay for that should be of most interest to practitioners.
Exclusions to medical malpractice insurance
All professional liability insurance policies contain exclusions, and it is essential that you know what they are. While the exclusions may vary by policy, most malpractice insurance policies exclude claims stemming from:
- Reckless or intentional acts.
- Illegal/criminal activities, including theft.
- Misrepresentation, including dishonesty, fraudulent activity, falsification, and misrepresentation on forms.
- Practicing under the influence of alcohol or drugs.
- Altering patient or hospital records.
- Sexual misconduct.
- Cyber security issues, which typically require a separate cyber liability policy to protect against cyber attacks and data breaches affecting patient medical records.
It’s essential to know what your specific policy’s exclusions are, or you may be surprised to find that your malpractice liability insurance doesn’t cover you when you expected that it would. Such was the situation in a recently decided case.
Also essential is knowing what type of coverage your policy provides – claims-made or occurrence-based. Occurrence policies offer lifetime coverage for incidents that occurred during the policy period, no matter when the claim is made. Claims-made policies cover only incidents that occur and are reported within the policy’s time period (unless a “tail” policy is purchased to extend the reporting period).
The case
Dr. P was a neurologist specializing in pain management. He had a professional liability insurance policy with an insurance company. In 2012, Dr. P’s insurance agent saw a television news story about the physician being accused by the state medical board for overprescribing opioids, resulting in the deaths of 17 patients. The next day, the agent obtained copies of documents from the state medical board, including a summary suspension order and a notice of contemplated action.
The notice of contemplated action specified that Dr. P had deviated from the standard of care through injudicious prescribing, leading to approximately 17 patient deaths due to drug toxicity. Because the agent realized that lawsuits could be filed against Dr. P for the deaths, she sent the insurance company the paperwork from the medical board so the insurer would be aware of the potential claims.
However, when the insurer received the information, it did not investigate or seek more information as it was required to do. The insurer failed to get medical records, or specific patient names, and none of the 17 deaths were recorded in the insurance company’s claims system (a failure to follow company procedure). Instead, the insurance company decided to cancel Dr. P’s policy effective the following month.
The company sent Dr. P a cancellation letter advising him that his policy was being terminated due to “license suspension, nature of allegations, and practice profile,” and offered him a tail policy to purchase.
The insurance company did not advise Dr. P that he should ensure all potential claims were reported, including the 17 deaths, before his policy expired. The company also did not advise him that he had a claims-made policy and what that meant regarding future lawsuits that might be filed after his policy period expired.
A year later, Dr. P was sued in two wrongful death lawsuits by the families of two of the 17 opioid-related deaths. When he was served with the papers, he promptly notified the insurance company. The insurance company issued a denial letter, incorrectly asserting that the 17 drug-toxicity deaths that they were aware of did not qualify as claims under Dr. P’s policy.
After his insurance company failed to represent him, Dr. P divorced his wife of 35 years and filed for bankruptcy. The only creditors with claims were the two families who had sued him. The bankruptcy trustee filed a lawsuit against the insurance company on behalf of Dr. P for the insurer’s failure to defend and indemnify Dr. P against the wrongful death lawsuits. In 2017, the bankruptcy trustee settled the two wrongful death cases by paying the families a certain amount of cash and assigning the insurance bad faith lawsuit to them.
Court and jury decide
In 2020, the case against the insurance company ended up in court. By 2022, the court had decided some of the issues and left some for the jury to determine.
The court found that the insurance company had breached its obligation to defend and indemnify Dr. P, committed unfair insurance claims practices, and committed bad faith in failing to defend the physician. The court limited the compensation to the amount of cash that had been paid to settle the two cases, and any fees and costs that Dr. P had incurred defending himself.
However, this still left the jury to decide whether the insurance company had committed bad faith in failing to indemnify (secure a person against legal liability for his/her actions) Dr. P, whether it had violated the state’s Unfair Insurance Practices Act, and whether punitive damages should be levied against the insurer.
The jury trial ended in a stunning $52 million verdict against the insurance company after less than 2 hours of deliberation. The jury found that the insurance company had acted in bad faith and willfully violated the Unfair Insurances Practices Act.
While the jury ultimately decided against the insurance company and sent it a strong message with a large verdict, Dr. P’s career was still over. He had stopped practicing medicine, was bankrupt, and his personal life was in shambles. The litigation had taken about a decade. Sometimes a win isn’t a victory.
Protecting yourself
The best way to protect yourself from a situation in which your insurer will not defend you is to really know and understand your insurance policy. Is it occurrence-based or claims-made insurance? What exactly does it cover? How are claims supposed to be made? Your professional liability insurance can be extremely important if you get sued, so it is equally important to choose it carefully and to really understand what is being covered.
Other ways to protect yourself:
- Know your agent. Your agent is key to explaining your policy as well as helping in the event that you need to make a claim. Dr. P’s agent saw a news story about him on television, which is why she submitted the information to the insurance company. Dr. P would have been far better off calling the agent directly when he was being investigated by the state medical board to explain the situation and seek advice.
- Be aware of exclusions to your policy. Many – such as criminal acts, reckless or intentional acts, or practicing under the influence – were mentioned earlier in this article. Some may be unexpected, so it is extremely important that you understand the specific exclusions to your particular policy.
- Be aware of your state law, and how changes might affect you. For example, in states that have outlawed or criminalized abortion, an insurance company would probably not have to represent a policy holder who was sued for malpractice involving an abortion. On the other hand, be aware that not treating a patient who needs life-saving care because you are afraid of running afoul of the law can also get you in trouble if the patient is harmed by not being treated. (For example, the Centers for Medicare & Medicaid Services is currently investigating two hospitals that failed to provide necessary stabilizing abortion care to a patient with an emergency medication condition resulting from a miscarriage.)
- Know how your policy defines ‘intentional’ acts (which are typically excluded from coverage). This is important. In some jurisdictions, the insured clinician has to merely intend to commit the acts in order for the claim to be excluded. In other jurisdictions, the insured doctor has to intend to cause the resulting damage. This can result in a very different outcome.
- The best thing doctors can do is to really understand what the policy covers and be prepared to make some noise if the company is not covering something that it should. Don’t be afraid to ask questions if you think your insurer is doing something wrong, and if the answers don’t satisfy you, consult an attorney.
The future
In the fall of 2022, at least partially in response to the Dobbs v. Jackson Women’s Health Organization decision regarding abortion, one professional liability company (Physician’s Insurance) launched criminal defense reimbursement coverage for physicians and hospitals to pay for defense costs incurred in responding to criminal allegations arising directly from patient care.
The add-on Criminal Defense Reimbursement Endorsement was made available in Washington State in January 2023, and will be offered in other states pending regulatory approval. It reimburses defense costs up to $250,000 when criminal actions have arisen from direct patient care.
In a press release announcing the new coverage, Physician’s Insurance CEO Bill Cotter explained the company’s reasoning in providing it: “The already challenging environment for physicians and hospitals has been made even more difficult as they now navigate the legal ramifications of increased criminal medical negligence claims as seen in the case of the Nashville nurse at the Vanderbilt University Medical Center, the potential for criminal state claims arising out of the U.S. Supreme Court decision in Dobbs v. Jackson Women’s Health Organization, and the subsequent state criminalization of healthcare practices that have long been the professionally accepted standard of care.”
Expect to see more insurance companies offering new coverage options for physicians in the future as they recognize that physicians may be facing more than just medical malpractice lawsuits arising out of patient care.
A version of this article first appeared on Medscape.com.
You’ve practiced medicine for years without issues, but now you are facing a medical malpractice case. No worries – you’ve had professional liability insurance all this time, so surely there’s nothing to be concerned about. Undoubtedly, your medical malpractice insurer will cover the costs of defending you. Or will they? One case casts questions on just this issue.
Professional liability insurance
According to the American Medical Association, almost one in three physicians (31%) have had a medical malpractice lawsuit filed against them at some point in their careers. These numbers only increase the longer a physician practices; almost half of doctors 55 and over have been sued, compared with less than 10% of physicians under 40.
And while the majority of cases are dropped or dismissed, and the small minority of cases that do go to trial are mostly won by the defense, the cost of defending these cases can be extremely high. Physicians have medical malpractice insurance to defray these costs.
Malpractice insurance generally covers the costs of attorney fees, court costs, arbitration, compensatory damages, and settlements related to patient injury or death. Insurance sometimes, but not always, pays for the costs of malpractice lawsuits arising out of Health Insurance Portability and Accountability Act (HIPAA) violations.
But it is what the policies don’t pay for that should be of most interest to practitioners.
Exclusions to medical malpractice insurance
All professional liability insurance policies contain exclusions, and it is essential that you know what they are. While the exclusions may vary by policy, most malpractice insurance policies exclude claims stemming from:
- Reckless or intentional acts.
- Illegal/criminal activities, including theft.
- Misrepresentation, including dishonesty, fraudulent activity, falsification, and misrepresentation on forms.
- Practicing under the influence of alcohol or drugs.
- Altering patient or hospital records.
- Sexual misconduct.
- Cyber security issues, which typically require a separate cyber liability policy to protect against cyber attacks and data breaches affecting patient medical records.
It’s essential to know what your specific policy’s exclusions are, or you may be surprised to find that your malpractice liability insurance doesn’t cover you when you expected that it would. Such was the situation in a recently decided case.
Also essential is knowing what type of coverage your policy provides – claims-made or occurrence-based. Occurrence policies offer lifetime coverage for incidents that occurred during the policy period, no matter when the claim is made. Claims-made policies cover only incidents that occur and are reported within the policy’s time period (unless a “tail” policy is purchased to extend the reporting period).
The case
Dr. P was a neurologist specializing in pain management. He had a professional liability insurance policy with an insurance company. In 2012, Dr. P’s insurance agent saw a television news story about the physician being accused by the state medical board for overprescribing opioids, resulting in the deaths of 17 patients. The next day, the agent obtained copies of documents from the state medical board, including a summary suspension order and a notice of contemplated action.
The notice of contemplated action specified that Dr. P had deviated from the standard of care through injudicious prescribing, leading to approximately 17 patient deaths due to drug toxicity. Because the agent realized that lawsuits could be filed against Dr. P for the deaths, she sent the insurance company the paperwork from the medical board so the insurer would be aware of the potential claims.
However, when the insurer received the information, it did not investigate or seek more information as it was required to do. The insurer failed to get medical records, or specific patient names, and none of the 17 deaths were recorded in the insurance company’s claims system (a failure to follow company procedure). Instead, the insurance company decided to cancel Dr. P’s policy effective the following month.
The company sent Dr. P a cancellation letter advising him that his policy was being terminated due to “license suspension, nature of allegations, and practice profile,” and offered him a tail policy to purchase.
The insurance company did not advise Dr. P that he should ensure all potential claims were reported, including the 17 deaths, before his policy expired. The company also did not advise him that he had a claims-made policy and what that meant regarding future lawsuits that might be filed after his policy period expired.
A year later, Dr. P was sued in two wrongful death lawsuits by the families of two of the 17 opioid-related deaths. When he was served with the papers, he promptly notified the insurance company. The insurance company issued a denial letter, incorrectly asserting that the 17 drug-toxicity deaths that they were aware of did not qualify as claims under Dr. P’s policy.
After his insurance company failed to represent him, Dr. P divorced his wife of 35 years and filed for bankruptcy. The only creditors with claims were the two families who had sued him. The bankruptcy trustee filed a lawsuit against the insurance company on behalf of Dr. P for the insurer’s failure to defend and indemnify Dr. P against the wrongful death lawsuits. In 2017, the bankruptcy trustee settled the two wrongful death cases by paying the families a certain amount of cash and assigning the insurance bad faith lawsuit to them.
Court and jury decide
In 2020, the case against the insurance company ended up in court. By 2022, the court had decided some of the issues and left some for the jury to determine.
The court found that the insurance company had breached its obligation to defend and indemnify Dr. P, committed unfair insurance claims practices, and committed bad faith in failing to defend the physician. The court limited the compensation to the amount of cash that had been paid to settle the two cases, and any fees and costs that Dr. P had incurred defending himself.
However, this still left the jury to decide whether the insurance company had committed bad faith in failing to indemnify (secure a person against legal liability for his/her actions) Dr. P, whether it had violated the state’s Unfair Insurance Practices Act, and whether punitive damages should be levied against the insurer.
The jury trial ended in a stunning $52 million verdict against the insurance company after less than 2 hours of deliberation. The jury found that the insurance company had acted in bad faith and willfully violated the Unfair Insurances Practices Act.
While the jury ultimately decided against the insurance company and sent it a strong message with a large verdict, Dr. P’s career was still over. He had stopped practicing medicine, was bankrupt, and his personal life was in shambles. The litigation had taken about a decade. Sometimes a win isn’t a victory.
Protecting yourself
The best way to protect yourself from a situation in which your insurer will not defend you is to really know and understand your insurance policy. Is it occurrence-based or claims-made insurance? What exactly does it cover? How are claims supposed to be made? Your professional liability insurance can be extremely important if you get sued, so it is equally important to choose it carefully and to really understand what is being covered.
Other ways to protect yourself:
- Know your agent. Your agent is key to explaining your policy as well as helping in the event that you need to make a claim. Dr. P’s agent saw a news story about him on television, which is why she submitted the information to the insurance company. Dr. P would have been far better off calling the agent directly when he was being investigated by the state medical board to explain the situation and seek advice.
- Be aware of exclusions to your policy. Many – such as criminal acts, reckless or intentional acts, or practicing under the influence – were mentioned earlier in this article. Some may be unexpected, so it is extremely important that you understand the specific exclusions to your particular policy.
- Be aware of your state law, and how changes might affect you. For example, in states that have outlawed or criminalized abortion, an insurance company would probably not have to represent a policy holder who was sued for malpractice involving an abortion. On the other hand, be aware that not treating a patient who needs life-saving care because you are afraid of running afoul of the law can also get you in trouble if the patient is harmed by not being treated. (For example, the Centers for Medicare & Medicaid Services is currently investigating two hospitals that failed to provide necessary stabilizing abortion care to a patient with an emergency medication condition resulting from a miscarriage.)
- Know how your policy defines ‘intentional’ acts (which are typically excluded from coverage). This is important. In some jurisdictions, the insured clinician has to merely intend to commit the acts in order for the claim to be excluded. In other jurisdictions, the insured doctor has to intend to cause the resulting damage. This can result in a very different outcome.
- The best thing doctors can do is to really understand what the policy covers and be prepared to make some noise if the company is not covering something that it should. Don’t be afraid to ask questions if you think your insurer is doing something wrong, and if the answers don’t satisfy you, consult an attorney.
The future
In the fall of 2022, at least partially in response to the Dobbs v. Jackson Women’s Health Organization decision regarding abortion, one professional liability company (Physician’s Insurance) launched criminal defense reimbursement coverage for physicians and hospitals to pay for defense costs incurred in responding to criminal allegations arising directly from patient care.
The add-on Criminal Defense Reimbursement Endorsement was made available in Washington State in January 2023, and will be offered in other states pending regulatory approval. It reimburses defense costs up to $250,000 when criminal actions have arisen from direct patient care.
In a press release announcing the new coverage, Physician’s Insurance CEO Bill Cotter explained the company’s reasoning in providing it: “The already challenging environment for physicians and hospitals has been made even more difficult as they now navigate the legal ramifications of increased criminal medical negligence claims as seen in the case of the Nashville nurse at the Vanderbilt University Medical Center, the potential for criminal state claims arising out of the U.S. Supreme Court decision in Dobbs v. Jackson Women’s Health Organization, and the subsequent state criminalization of healthcare practices that have long been the professionally accepted standard of care.”
Expect to see more insurance companies offering new coverage options for physicians in the future as they recognize that physicians may be facing more than just medical malpractice lawsuits arising out of patient care.
A version of this article first appeared on Medscape.com.
You’ve practiced medicine for years without issues, but now you are facing a medical malpractice case. No worries – you’ve had professional liability insurance all this time, so surely there’s nothing to be concerned about. Undoubtedly, your medical malpractice insurer will cover the costs of defending you. Or will they? One case casts questions on just this issue.
Professional liability insurance
According to the American Medical Association, almost one in three physicians (31%) have had a medical malpractice lawsuit filed against them at some point in their careers. These numbers only increase the longer a physician practices; almost half of doctors 55 and over have been sued, compared with less than 10% of physicians under 40.
And while the majority of cases are dropped or dismissed, and the small minority of cases that do go to trial are mostly won by the defense, the cost of defending these cases can be extremely high. Physicians have medical malpractice insurance to defray these costs.
Malpractice insurance generally covers the costs of attorney fees, court costs, arbitration, compensatory damages, and settlements related to patient injury or death. Insurance sometimes, but not always, pays for the costs of malpractice lawsuits arising out of Health Insurance Portability and Accountability Act (HIPAA) violations.
But it is what the policies don’t pay for that should be of most interest to practitioners.
Exclusions to medical malpractice insurance
All professional liability insurance policies contain exclusions, and it is essential that you know what they are. While the exclusions may vary by policy, most malpractice insurance policies exclude claims stemming from:
- Reckless or intentional acts.
- Illegal/criminal activities, including theft.
- Misrepresentation, including dishonesty, fraudulent activity, falsification, and misrepresentation on forms.
- Practicing under the influence of alcohol or drugs.
- Altering patient or hospital records.
- Sexual misconduct.
- Cyber security issues, which typically require a separate cyber liability policy to protect against cyber attacks and data breaches affecting patient medical records.
It’s essential to know what your specific policy’s exclusions are, or you may be surprised to find that your malpractice liability insurance doesn’t cover you when you expected that it would. Such was the situation in a recently decided case.
Also essential is knowing what type of coverage your policy provides – claims-made or occurrence-based. Occurrence policies offer lifetime coverage for incidents that occurred during the policy period, no matter when the claim is made. Claims-made policies cover only incidents that occur and are reported within the policy’s time period (unless a “tail” policy is purchased to extend the reporting period).
The case
Dr. P was a neurologist specializing in pain management. He had a professional liability insurance policy with an insurance company. In 2012, Dr. P’s insurance agent saw a television news story about the physician being accused by the state medical board for overprescribing opioids, resulting in the deaths of 17 patients. The next day, the agent obtained copies of documents from the state medical board, including a summary suspension order and a notice of contemplated action.
The notice of contemplated action specified that Dr. P had deviated from the standard of care through injudicious prescribing, leading to approximately 17 patient deaths due to drug toxicity. Because the agent realized that lawsuits could be filed against Dr. P for the deaths, she sent the insurance company the paperwork from the medical board so the insurer would be aware of the potential claims.
However, when the insurer received the information, it did not investigate or seek more information as it was required to do. The insurer failed to get medical records, or specific patient names, and none of the 17 deaths were recorded in the insurance company’s claims system (a failure to follow company procedure). Instead, the insurance company decided to cancel Dr. P’s policy effective the following month.
The company sent Dr. P a cancellation letter advising him that his policy was being terminated due to “license suspension, nature of allegations, and practice profile,” and offered him a tail policy to purchase.
The insurance company did not advise Dr. P that he should ensure all potential claims were reported, including the 17 deaths, before his policy expired. The company also did not advise him that he had a claims-made policy and what that meant regarding future lawsuits that might be filed after his policy period expired.
A year later, Dr. P was sued in two wrongful death lawsuits by the families of two of the 17 opioid-related deaths. When he was served with the papers, he promptly notified the insurance company. The insurance company issued a denial letter, incorrectly asserting that the 17 drug-toxicity deaths that they were aware of did not qualify as claims under Dr. P’s policy.
After his insurance company failed to represent him, Dr. P divorced his wife of 35 years and filed for bankruptcy. The only creditors with claims were the two families who had sued him. The bankruptcy trustee filed a lawsuit against the insurance company on behalf of Dr. P for the insurer’s failure to defend and indemnify Dr. P against the wrongful death lawsuits. In 2017, the bankruptcy trustee settled the two wrongful death cases by paying the families a certain amount of cash and assigning the insurance bad faith lawsuit to them.
Court and jury decide
In 2020, the case against the insurance company ended up in court. By 2022, the court had decided some of the issues and left some for the jury to determine.
The court found that the insurance company had breached its obligation to defend and indemnify Dr. P, committed unfair insurance claims practices, and committed bad faith in failing to defend the physician. The court limited the compensation to the amount of cash that had been paid to settle the two cases, and any fees and costs that Dr. P had incurred defending himself.
However, this still left the jury to decide whether the insurance company had committed bad faith in failing to indemnify (secure a person against legal liability for his/her actions) Dr. P, whether it had violated the state’s Unfair Insurance Practices Act, and whether punitive damages should be levied against the insurer.
The jury trial ended in a stunning $52 million verdict against the insurance company after less than 2 hours of deliberation. The jury found that the insurance company had acted in bad faith and willfully violated the Unfair Insurances Practices Act.
While the jury ultimately decided against the insurance company and sent it a strong message with a large verdict, Dr. P’s career was still over. He had stopped practicing medicine, was bankrupt, and his personal life was in shambles. The litigation had taken about a decade. Sometimes a win isn’t a victory.
Protecting yourself
The best way to protect yourself from a situation in which your insurer will not defend you is to really know and understand your insurance policy. Is it occurrence-based or claims-made insurance? What exactly does it cover? How are claims supposed to be made? Your professional liability insurance can be extremely important if you get sued, so it is equally important to choose it carefully and to really understand what is being covered.
Other ways to protect yourself:
- Know your agent. Your agent is key to explaining your policy as well as helping in the event that you need to make a claim. Dr. P’s agent saw a news story about him on television, which is why she submitted the information to the insurance company. Dr. P would have been far better off calling the agent directly when he was being investigated by the state medical board to explain the situation and seek advice.
- Be aware of exclusions to your policy. Many – such as criminal acts, reckless or intentional acts, or practicing under the influence – were mentioned earlier in this article. Some may be unexpected, so it is extremely important that you understand the specific exclusions to your particular policy.
- Be aware of your state law, and how changes might affect you. For example, in states that have outlawed or criminalized abortion, an insurance company would probably not have to represent a policy holder who was sued for malpractice involving an abortion. On the other hand, be aware that not treating a patient who needs life-saving care because you are afraid of running afoul of the law can also get you in trouble if the patient is harmed by not being treated. (For example, the Centers for Medicare & Medicaid Services is currently investigating two hospitals that failed to provide necessary stabilizing abortion care to a patient with an emergency medication condition resulting from a miscarriage.)
- Know how your policy defines ‘intentional’ acts (which are typically excluded from coverage). This is important. In some jurisdictions, the insured clinician has to merely intend to commit the acts in order for the claim to be excluded. In other jurisdictions, the insured doctor has to intend to cause the resulting damage. This can result in a very different outcome.
- The best thing doctors can do is to really understand what the policy covers and be prepared to make some noise if the company is not covering something that it should. Don’t be afraid to ask questions if you think your insurer is doing something wrong, and if the answers don’t satisfy you, consult an attorney.
The future
In the fall of 2022, at least partially in response to the Dobbs v. Jackson Women’s Health Organization decision regarding abortion, one professional liability company (Physician’s Insurance) launched criminal defense reimbursement coverage for physicians and hospitals to pay for defense costs incurred in responding to criminal allegations arising directly from patient care.
The add-on Criminal Defense Reimbursement Endorsement was made available in Washington State in January 2023, and will be offered in other states pending regulatory approval. It reimburses defense costs up to $250,000 when criminal actions have arisen from direct patient care.
In a press release announcing the new coverage, Physician’s Insurance CEO Bill Cotter explained the company’s reasoning in providing it: “The already challenging environment for physicians and hospitals has been made even more difficult as they now navigate the legal ramifications of increased criminal medical negligence claims as seen in the case of the Nashville nurse at the Vanderbilt University Medical Center, the potential for criminal state claims arising out of the U.S. Supreme Court decision in Dobbs v. Jackson Women’s Health Organization, and the subsequent state criminalization of healthcare practices that have long been the professionally accepted standard of care.”
Expect to see more insurance companies offering new coverage options for physicians in the future as they recognize that physicians may be facing more than just medical malpractice lawsuits arising out of patient care.
A version of this article first appeared on Medscape.com.
Docs using AI? Some love it, most remain wary
When OpenAI released ChatGPT-3 publicly last November, some doctors decided to try out the free AI tool that learns language and writes human-like text. Some physicians found the chatbot made mistakes and stopped using it, while others were happy with the results and plan to use it more often.
“We’ve played around with it. It was very early on in AI and we noticed it gave us incorrect information with regards to clinical guidance,” said Monalisa Tailor, MD, an internal medicine physician at Norton Health Care in Louisville, Ky. “We decided not to pursue it further,” she said.
Orthopedic spine surgeon Daniel Choi, MD, who owns a small medical/surgical practice in Long Island, New York, tested the chatbot’s performance with a few administrative tasks, including writing a job listing for an administrator and prior authorization letters.
He was enthusiastic. “A well-polished job posting that would usually take me 2-3 hours to write was done in 5 minutes,” Dr. Choi said. “I was blown away by the writing – it was much better than anything I could write.”
The chatbot can also automate administrative tasks in doctors’ practices from appointment scheduling and billing to clinical documentation, saving doctors time and money, experts say.
Most physicians are proceeding cautiously. About 10% of more than 500 medical group leaders, responding to a March poll by the Medical Group Management Association, said their practices regularly use AI tools.
More than half of the respondents not using AI said they first want more evidence that the technology works as intended.
“None of them work as advertised,” said one respondent.
MGMA practice management consultant Dawn Plested acknowledges that many of the physician practices she’s worked with are still wary. “I have yet to encounter a practice that is using any AI tool, even something as low-risk as appointment scheduling,” she said.
Physician groups may be concerned about the costs and logistics of integrating ChatGPT with their electronic health record systems (EHRs) and how that would work, said Ms. Plested.
Doctors may also be skeptical of AI based on their experience with EHRs, she said.
“They were promoted as a panacea to many problems; they were supposed to automate business practice, reduce staff and clinician’s work, and improve billing/coding/documentation. Unfortunately, they have become a major source of frustration for doctors,” said Ms. Plested.
Drawing the line at patient care
Patients are worried about their doctors relying on AI for their care, according to a Pew Research Center poll released in February. About 60% of U.S. adults say they would feel uncomfortable if their own health care professional relied on artificial intelligence to do things like diagnose disease and recommend treatments; about 40% say they would feel comfortable with this.
“We have not yet gone into using ChatGPT for clinical purposes and will be very cautious with these types of applications due to concerns about inaccuracies,” Dr. Choi said.
Practice leaders reported in the MGMA poll that the most common uses of AI were nonclinical, such as:
- Patient communications, including call center answering service to help triage calls, to sort/distribute incoming fax messages, and outreach such as appointment reminders and marketing materials.
- Capturing clinical documentation, often with natural language processing or speech recognition platforms to help virtually scribe.
- Improving billing operations and predictive analytics.
Some doctors told The New York Times that ChatGPT helped them communicate with patients in a more compassionate way.
They used chatbots “to find words to break bad news and express concerns about a patient’s suffering, or to just more clearly explain medical recommendations,” the story noted.
Is regulation needed?
Some legal scholars and medical groups say that AI should be regulated to protect patients and doctors from risks, including medical errors, that could harm patients.
“It’s very important to evaluate the accuracy, safety, and privacy of language learning models (LLMs) before integrating them into the medical system. The same should be true of any new medical tool,” said Mason Marks, MD, JD, a health law professor at the Florida State University College of Law in Tallahassee.
In mid-June, the American Medical Association approved two resolutions calling for greater government oversight of AI. The AMA will develop proposed state and federal regulations and work with the federal government and other organizations to protect patients from false or misleading AI-generated medical advice.
Dr. Marks pointed to existing federal rules that apply to AI. “The Federal Trade Commission already has regulation that can potentially be used to combat unfair or deceptive trade practices associated with chatbots,” he said.
In addition, “the U.S. Food and Drug Administration can also regulate these tools, but it needs to update how it approaches risk when it comes to AI. The FDA has an outdated view of risk as physical harm, for instance, from traditional medical devices. That view of risk needs to be updated and expanded to encompass the unique harms of AI,” Dr. Marks said.
There should also be more transparency about how LLM software is used in medicine, he said. “That could be a norm implemented by the LLM developers and it could also be enforced by federal agencies. For instance, the FDA could require developers to be more transparent regarding training data and methods, and the FTC could require greater transparency regarding how consumer data might be used and opportunities to opt out of certain uses,” said Dr. Marks.
What should doctors do?
Dr. Marks advised doctors to be cautious when using ChatGPT and other LLMs, especially for medical advice. “The same would apply to any new medical tool, but we know that the current generation of LLMs [is] particularly prone to making things up, which could lead to medical errors if relied on in clinical settings,” he said.
There is also potential for breaches of patient confidentiality if doctors input clinical information. ChatGPT and OpenAI-enabled tools may not be compliant with the Health Insurance Portability and Accountability Act, which set national standards to protect individuals’ medical records and individually identifiable health information.
“The best approach is to use chatbots cautiously and with skepticism. Don’t input patient information, confirm the accuracy of information produced, and don’t use them as replacements for professional judgment,” Dr. Marks recommended.
Ms. Plested suggested that doctors who want to experiment with AI start with a low-risk tool such as appointment reminders that could save staff time and money. “I never recommend they start with something as high-stakes as coding/billing,” she said.
A version of this article appeared on Medscape.com.
When OpenAI released ChatGPT-3 publicly last November, some doctors decided to try out the free AI tool that learns language and writes human-like text. Some physicians found the chatbot made mistakes and stopped using it, while others were happy with the results and plan to use it more often.
“We’ve played around with it. It was very early on in AI and we noticed it gave us incorrect information with regards to clinical guidance,” said Monalisa Tailor, MD, an internal medicine physician at Norton Health Care in Louisville, Ky. “We decided not to pursue it further,” she said.
Orthopedic spine surgeon Daniel Choi, MD, who owns a small medical/surgical practice in Long Island, New York, tested the chatbot’s performance with a few administrative tasks, including writing a job listing for an administrator and prior authorization letters.
He was enthusiastic. “A well-polished job posting that would usually take me 2-3 hours to write was done in 5 minutes,” Dr. Choi said. “I was blown away by the writing – it was much better than anything I could write.”
The chatbot can also automate administrative tasks in doctors’ practices from appointment scheduling and billing to clinical documentation, saving doctors time and money, experts say.
Most physicians are proceeding cautiously. About 10% of more than 500 medical group leaders, responding to a March poll by the Medical Group Management Association, said their practices regularly use AI tools.
More than half of the respondents not using AI said they first want more evidence that the technology works as intended.
“None of them work as advertised,” said one respondent.
MGMA practice management consultant Dawn Plested acknowledges that many of the physician practices she’s worked with are still wary. “I have yet to encounter a practice that is using any AI tool, even something as low-risk as appointment scheduling,” she said.
Physician groups may be concerned about the costs and logistics of integrating ChatGPT with their electronic health record systems (EHRs) and how that would work, said Ms. Plested.
Doctors may also be skeptical of AI based on their experience with EHRs, she said.
“They were promoted as a panacea to many problems; they were supposed to automate business practice, reduce staff and clinician’s work, and improve billing/coding/documentation. Unfortunately, they have become a major source of frustration for doctors,” said Ms. Plested.
Drawing the line at patient care
Patients are worried about their doctors relying on AI for their care, according to a Pew Research Center poll released in February. About 60% of U.S. adults say they would feel uncomfortable if their own health care professional relied on artificial intelligence to do things like diagnose disease and recommend treatments; about 40% say they would feel comfortable with this.
“We have not yet gone into using ChatGPT for clinical purposes and will be very cautious with these types of applications due to concerns about inaccuracies,” Dr. Choi said.
Practice leaders reported in the MGMA poll that the most common uses of AI were nonclinical, such as:
- Patient communications, including call center answering service to help triage calls, to sort/distribute incoming fax messages, and outreach such as appointment reminders and marketing materials.
- Capturing clinical documentation, often with natural language processing or speech recognition platforms to help virtually scribe.
- Improving billing operations and predictive analytics.
Some doctors told The New York Times that ChatGPT helped them communicate with patients in a more compassionate way.
They used chatbots “to find words to break bad news and express concerns about a patient’s suffering, or to just more clearly explain medical recommendations,” the story noted.
Is regulation needed?
Some legal scholars and medical groups say that AI should be regulated to protect patients and doctors from risks, including medical errors, that could harm patients.
“It’s very important to evaluate the accuracy, safety, and privacy of language learning models (LLMs) before integrating them into the medical system. The same should be true of any new medical tool,” said Mason Marks, MD, JD, a health law professor at the Florida State University College of Law in Tallahassee.
In mid-June, the American Medical Association approved two resolutions calling for greater government oversight of AI. The AMA will develop proposed state and federal regulations and work with the federal government and other organizations to protect patients from false or misleading AI-generated medical advice.
Dr. Marks pointed to existing federal rules that apply to AI. “The Federal Trade Commission already has regulation that can potentially be used to combat unfair or deceptive trade practices associated with chatbots,” he said.
In addition, “the U.S. Food and Drug Administration can also regulate these tools, but it needs to update how it approaches risk when it comes to AI. The FDA has an outdated view of risk as physical harm, for instance, from traditional medical devices. That view of risk needs to be updated and expanded to encompass the unique harms of AI,” Dr. Marks said.
There should also be more transparency about how LLM software is used in medicine, he said. “That could be a norm implemented by the LLM developers and it could also be enforced by federal agencies. For instance, the FDA could require developers to be more transparent regarding training data and methods, and the FTC could require greater transparency regarding how consumer data might be used and opportunities to opt out of certain uses,” said Dr. Marks.
What should doctors do?
Dr. Marks advised doctors to be cautious when using ChatGPT and other LLMs, especially for medical advice. “The same would apply to any new medical tool, but we know that the current generation of LLMs [is] particularly prone to making things up, which could lead to medical errors if relied on in clinical settings,” he said.
There is also potential for breaches of patient confidentiality if doctors input clinical information. ChatGPT and OpenAI-enabled tools may not be compliant with the Health Insurance Portability and Accountability Act, which set national standards to protect individuals’ medical records and individually identifiable health information.
“The best approach is to use chatbots cautiously and with skepticism. Don’t input patient information, confirm the accuracy of information produced, and don’t use them as replacements for professional judgment,” Dr. Marks recommended.
Ms. Plested suggested that doctors who want to experiment with AI start with a low-risk tool such as appointment reminders that could save staff time and money. “I never recommend they start with something as high-stakes as coding/billing,” she said.
A version of this article appeared on Medscape.com.
When OpenAI released ChatGPT-3 publicly last November, some doctors decided to try out the free AI tool that learns language and writes human-like text. Some physicians found the chatbot made mistakes and stopped using it, while others were happy with the results and plan to use it more often.
“We’ve played around with it. It was very early on in AI and we noticed it gave us incorrect information with regards to clinical guidance,” said Monalisa Tailor, MD, an internal medicine physician at Norton Health Care in Louisville, Ky. “We decided not to pursue it further,” she said.
Orthopedic spine surgeon Daniel Choi, MD, who owns a small medical/surgical practice in Long Island, New York, tested the chatbot’s performance with a few administrative tasks, including writing a job listing for an administrator and prior authorization letters.
He was enthusiastic. “A well-polished job posting that would usually take me 2-3 hours to write was done in 5 minutes,” Dr. Choi said. “I was blown away by the writing – it was much better than anything I could write.”
The chatbot can also automate administrative tasks in doctors’ practices from appointment scheduling and billing to clinical documentation, saving doctors time and money, experts say.
Most physicians are proceeding cautiously. About 10% of more than 500 medical group leaders, responding to a March poll by the Medical Group Management Association, said their practices regularly use AI tools.
More than half of the respondents not using AI said they first want more evidence that the technology works as intended.
“None of them work as advertised,” said one respondent.
MGMA practice management consultant Dawn Plested acknowledges that many of the physician practices she’s worked with are still wary. “I have yet to encounter a practice that is using any AI tool, even something as low-risk as appointment scheduling,” she said.
Physician groups may be concerned about the costs and logistics of integrating ChatGPT with their electronic health record systems (EHRs) and how that would work, said Ms. Plested.
Doctors may also be skeptical of AI based on their experience with EHRs, she said.
“They were promoted as a panacea to many problems; they were supposed to automate business practice, reduce staff and clinician’s work, and improve billing/coding/documentation. Unfortunately, they have become a major source of frustration for doctors,” said Ms. Plested.
Drawing the line at patient care
Patients are worried about their doctors relying on AI for their care, according to a Pew Research Center poll released in February. About 60% of U.S. adults say they would feel uncomfortable if their own health care professional relied on artificial intelligence to do things like diagnose disease and recommend treatments; about 40% say they would feel comfortable with this.
“We have not yet gone into using ChatGPT for clinical purposes and will be very cautious with these types of applications due to concerns about inaccuracies,” Dr. Choi said.
Practice leaders reported in the MGMA poll that the most common uses of AI were nonclinical, such as:
- Patient communications, including call center answering service to help triage calls, to sort/distribute incoming fax messages, and outreach such as appointment reminders and marketing materials.
- Capturing clinical documentation, often with natural language processing or speech recognition platforms to help virtually scribe.
- Improving billing operations and predictive analytics.
Some doctors told The New York Times that ChatGPT helped them communicate with patients in a more compassionate way.
They used chatbots “to find words to break bad news and express concerns about a patient’s suffering, or to just more clearly explain medical recommendations,” the story noted.
Is regulation needed?
Some legal scholars and medical groups say that AI should be regulated to protect patients and doctors from risks, including medical errors, that could harm patients.
“It’s very important to evaluate the accuracy, safety, and privacy of language learning models (LLMs) before integrating them into the medical system. The same should be true of any new medical tool,” said Mason Marks, MD, JD, a health law professor at the Florida State University College of Law in Tallahassee.
In mid-June, the American Medical Association approved two resolutions calling for greater government oversight of AI. The AMA will develop proposed state and federal regulations and work with the federal government and other organizations to protect patients from false or misleading AI-generated medical advice.
Dr. Marks pointed to existing federal rules that apply to AI. “The Federal Trade Commission already has regulation that can potentially be used to combat unfair or deceptive trade practices associated with chatbots,” he said.
In addition, “the U.S. Food and Drug Administration can also regulate these tools, but it needs to update how it approaches risk when it comes to AI. The FDA has an outdated view of risk as physical harm, for instance, from traditional medical devices. That view of risk needs to be updated and expanded to encompass the unique harms of AI,” Dr. Marks said.
There should also be more transparency about how LLM software is used in medicine, he said. “That could be a norm implemented by the LLM developers and it could also be enforced by federal agencies. For instance, the FDA could require developers to be more transparent regarding training data and methods, and the FTC could require greater transparency regarding how consumer data might be used and opportunities to opt out of certain uses,” said Dr. Marks.
What should doctors do?
Dr. Marks advised doctors to be cautious when using ChatGPT and other LLMs, especially for medical advice. “The same would apply to any new medical tool, but we know that the current generation of LLMs [is] particularly prone to making things up, which could lead to medical errors if relied on in clinical settings,” he said.
There is also potential for breaches of patient confidentiality if doctors input clinical information. ChatGPT and OpenAI-enabled tools may not be compliant with the Health Insurance Portability and Accountability Act, which set national standards to protect individuals’ medical records and individually identifiable health information.
“The best approach is to use chatbots cautiously and with skepticism. Don’t input patient information, confirm the accuracy of information produced, and don’t use them as replacements for professional judgment,” Dr. Marks recommended.
Ms. Plested suggested that doctors who want to experiment with AI start with a low-risk tool such as appointment reminders that could save staff time and money. “I never recommend they start with something as high-stakes as coding/billing,” she said.
A version of this article appeared on Medscape.com.



