User login
Clinical Endocrinology News is an independent news source that provides endocrinologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the endocrinologist's practice. Specialty topics include Diabetes, Lipid & Metabolic Disorders Menopause, Obesity, Osteoporosis, Pediatric Endocrinology, Pituitary, Thyroid & Adrenal Disorders, and Reproductive Endocrinology. Featured content includes Commentaries, Implementin Health Reform, Law & Medicine, and In the Loop, the blog of Clinical Endocrinology News. Clinical Endocrinology News is owned by Frontline Medical Communications.
addict
addicted
addicting
addiction
adult sites
alcohol
antibody
ass
attorney
audit
auditor
babies
babpa
baby
ban
banned
banning
best
bisexual
bitch
bleach
blog
blow job
bondage
boobs
booty
buy
cannabis
certificate
certification
certified
cheap
cheapest
class action
cocaine
cock
counterfeit drug
crack
crap
crime
criminal
cunt
curable
cure
dangerous
dangers
dead
deadly
death
defend
defended
depedent
dependence
dependent
detergent
dick
die
dildo
drug abuse
drug recall
dying
fag
fake
fatal
fatalities
fatality
free
fuck
gangs
gingivitis
guns
hardcore
herbal
herbs
heroin
herpes
home remedies
homo
horny
hypersensitivity
hypoglycemia treatment
illegal drug use
illegal use of prescription
incest
infant
infants
job
ketoacidosis
kill
killer
killing
kinky
law suit
lawsuit
lawyer
lesbian
marijuana
medicine for hypoglycemia
murder
naked
natural
newborn
nigger
noise
nude
nudity
orgy
over the counter
overdosage
overdose
overdosed
overdosing
penis
pimp
pistol
porn
porno
pornographic
pornography
prison
profanity
purchase
purchasing
pussy
queer
rape
rapist
recall
recreational drug
rob
robberies
sale
sales
sex
sexual
shit
shoot
slut
slutty
stole
stolen
store
sue
suicidal
suicide
supplements
supply company
theft
thief
thieves
tit
toddler
toddlers
toxic
toxin
tragedy
treating dka
treating hypoglycemia
treatment for hypoglycemia
vagina
violence
whore
withdrawal
without prescription
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-imn')]
div[contains(@class, 'pane-pub-home-imn')]
div[contains(@class, 'pane-pub-topic-imn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Early or delayed menopause and irregular periods tied to new-onset atrial fibrillation
Takeaway
- Early or delayed menopause and a history of irregular menstrual cycles were significantly associated with a greater risk of new-onset atrial fibrillation (AF) in women.
- Women with nulliparity and multiparity had a greater risk of new-onset AF compared with those with one to two live births.
Why this matters
- Findings highlight the significance of considering the reproductive history of women while developing tailored screening and prevention strategies for AF.
Study design
- A population-based cohort study of 235,191 women (age, 40-69 years) without AF and a history of hysterectomy and/or bilateral oophorectomy, identified from the UK Biobank (2006-2010).
- Funding: Gender and Prevention Grant from ZonMw and other.
Key results
- During a median follow-up of 11.6 years, 4,629 (2.0%) women were diagnosed with new-onset AF.
- A history of irregular menstrual cycle was associated with higher risk of new-onset AF (adjusted HR, 1.34; 95% confidence interval, 1.01-1.79; P = .04).
- Compared with women who experienced menarche at the age of 12 years, the risk of new-onset AF was significantly higher in those who experienced menarche:
- –Earlier between the ages of 7 and 11 years (aHR, 1.10; 95% CI, 1.00-1.21; P = .04) and
- –Later between the ages of 13 and 18 years (aHR, 1.08; 95% CI, 1.00-1.17; P = .05).
- The risk of new-onset AF was significantly higher in women who experienced menopause:
- –At the age of < 35 years (aHR, 2.25; 95% CI, 1.48-3.43; P < .001);
- –Between the ages of 35 and 44 years (aHR, 1.24; 95% CI, 1.10-1.39; P < .001); and
- –At the age of ≥ 60 years (aHR, 1.34; 95% CI, 1.10-1.78; P = .04).
- Women with no live births (aHR, 1.13; 95% CI, 1.04-1.24; P < .01), four to six live births (aHR, 1.12; 95% CI, 1.01-1.24; P = .04), and ≥ seven live births (aHR, 1.67; 95% CI, 1.03-2.70; P = .03) vs. those with one to two live births had a significantly higher risk of new-onset AF.
Limitations
- Observational design.
A version of this article first appeared on Medscape UK.
Reference
Lu Z, Aribas E, Geurts S, Roeters van Lennep JE, Ikram MA, Bos MM, de Groot NMS, Kavousi M. Association Between Sex-Specific Risk Factors and Risk of New-Onset Atrial Fibrillation Among Women. JAMA Netw Open. 2022;5(9):e2229716. doi: 10.1001/jamanetworkopen.2022.29716. PMID: 36048441.
Takeaway
- Early or delayed menopause and a history of irregular menstrual cycles were significantly associated with a greater risk of new-onset atrial fibrillation (AF) in women.
- Women with nulliparity and multiparity had a greater risk of new-onset AF compared with those with one to two live births.
Why this matters
- Findings highlight the significance of considering the reproductive history of women while developing tailored screening and prevention strategies for AF.
Study design
- A population-based cohort study of 235,191 women (age, 40-69 years) without AF and a history of hysterectomy and/or bilateral oophorectomy, identified from the UK Biobank (2006-2010).
- Funding: Gender and Prevention Grant from ZonMw and other.
Key results
- During a median follow-up of 11.6 years, 4,629 (2.0%) women were diagnosed with new-onset AF.
- A history of irregular menstrual cycle was associated with higher risk of new-onset AF (adjusted HR, 1.34; 95% confidence interval, 1.01-1.79; P = .04).
- Compared with women who experienced menarche at the age of 12 years, the risk of new-onset AF was significantly higher in those who experienced menarche:
- –Earlier between the ages of 7 and 11 years (aHR, 1.10; 95% CI, 1.00-1.21; P = .04) and
- –Later between the ages of 13 and 18 years (aHR, 1.08; 95% CI, 1.00-1.17; P = .05).
- The risk of new-onset AF was significantly higher in women who experienced menopause:
- –At the age of < 35 years (aHR, 2.25; 95% CI, 1.48-3.43; P < .001);
- –Between the ages of 35 and 44 years (aHR, 1.24; 95% CI, 1.10-1.39; P < .001); and
- –At the age of ≥ 60 years (aHR, 1.34; 95% CI, 1.10-1.78; P = .04).
- Women with no live births (aHR, 1.13; 95% CI, 1.04-1.24; P < .01), four to six live births (aHR, 1.12; 95% CI, 1.01-1.24; P = .04), and ≥ seven live births (aHR, 1.67; 95% CI, 1.03-2.70; P = .03) vs. those with one to two live births had a significantly higher risk of new-onset AF.
Limitations
- Observational design.
A version of this article first appeared on Medscape UK.
Reference
Lu Z, Aribas E, Geurts S, Roeters van Lennep JE, Ikram MA, Bos MM, de Groot NMS, Kavousi M. Association Between Sex-Specific Risk Factors and Risk of New-Onset Atrial Fibrillation Among Women. JAMA Netw Open. 2022;5(9):e2229716. doi: 10.1001/jamanetworkopen.2022.29716. PMID: 36048441.
Takeaway
- Early or delayed menopause and a history of irregular menstrual cycles were significantly associated with a greater risk of new-onset atrial fibrillation (AF) in women.
- Women with nulliparity and multiparity had a greater risk of new-onset AF compared with those with one to two live births.
Why this matters
- Findings highlight the significance of considering the reproductive history of women while developing tailored screening and prevention strategies for AF.
Study design
- A population-based cohort study of 235,191 women (age, 40-69 years) without AF and a history of hysterectomy and/or bilateral oophorectomy, identified from the UK Biobank (2006-2010).
- Funding: Gender and Prevention Grant from ZonMw and other.
Key results
- During a median follow-up of 11.6 years, 4,629 (2.0%) women were diagnosed with new-onset AF.
- A history of irregular menstrual cycle was associated with higher risk of new-onset AF (adjusted HR, 1.34; 95% confidence interval, 1.01-1.79; P = .04).
- Compared with women who experienced menarche at the age of 12 years, the risk of new-onset AF was significantly higher in those who experienced menarche:
- –Earlier between the ages of 7 and 11 years (aHR, 1.10; 95% CI, 1.00-1.21; P = .04) and
- –Later between the ages of 13 and 18 years (aHR, 1.08; 95% CI, 1.00-1.17; P = .05).
- The risk of new-onset AF was significantly higher in women who experienced menopause:
- –At the age of < 35 years (aHR, 2.25; 95% CI, 1.48-3.43; P < .001);
- –Between the ages of 35 and 44 years (aHR, 1.24; 95% CI, 1.10-1.39; P < .001); and
- –At the age of ≥ 60 years (aHR, 1.34; 95% CI, 1.10-1.78; P = .04).
- Women with no live births (aHR, 1.13; 95% CI, 1.04-1.24; P < .01), four to six live births (aHR, 1.12; 95% CI, 1.01-1.24; P = .04), and ≥ seven live births (aHR, 1.67; 95% CI, 1.03-2.70; P = .03) vs. those with one to two live births had a significantly higher risk of new-onset AF.
Limitations
- Observational design.
A version of this article first appeared on Medscape UK.
Reference
Lu Z, Aribas E, Geurts S, Roeters van Lennep JE, Ikram MA, Bos MM, de Groot NMS, Kavousi M. Association Between Sex-Specific Risk Factors and Risk of New-Onset Atrial Fibrillation Among Women. JAMA Netw Open. 2022;5(9):e2229716. doi: 10.1001/jamanetworkopen.2022.29716. PMID: 36048441.
FROM JAMA NETWORK OPEN
Waist-hip ratio beats BMI for predicting obesity’s mortality risk
STOCKHOLM – New evidence continues to show that alternative measures of adiposity than body mass index, such as waist-to-hip ratio, work better for predicting the risk a person with overweight or obesity faces from their excess weight.
A direct comparison of waist-to-hip ratio (WHR), body mass index (BMI), and fat mass index (FMI) in a total of more than 380,000 United Kingdom residents included in the UK Biobank showed that WHR had the strongest and most consistent relationship to all-cause death, compared with the other two measures, indicating that clinicians should pay more attention to adiposity distribution than they do to BMI when prioritizing obesity interventions, Irfan Khan said at the annual meeting of the European Association for the Study of Diabetes.
Although it’s likely “way too early” to fully replace BMI as a measure of adiposity, because it is so established in guidelines and in practice, it is now time to “use WHR as an adjunct to BMI” suggested Mr. Khan in an interview.
“A lot of work still needs to be done to translate WHR into practice, but I think it’s getting closer,” said Mr. Khan, a medical student at McMaster University, Hamilton, Ont., who performed his analyses in collaboration with a research team based primarily at McMaster.
Moving away from BMI-centric obesity
“This is a timely topic, because guidelines for treating people with obesity have depended so much on BMI. We want to go from a BMI-centric view to a view of obesity that depends more on disease burden,” commented Matthias Blüher, MD, professor of molecular endocrinology and head of the Obesity Outpatient Clinic for Adults at the University of Leipzig (Germany).
For example, the 2016 obesity management guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology called for a “complications-centric” approach to assessing and intervening in people with obesity rather than a “BMI-centric” approach.
But Dr. Blüher went a step further in an interview, adding that “waist-to-hip ratio is now outdated,” with adjusted measures of WHR such as waist-to-height ratio “considered a better proxy for all-cause death.” He also gave high marks to the Edmonton Obesity Staging System, which independently added to BMI as well as to a diagnosis of metabolic syndrome for predicting mortality in a sample from the U.S. National Health and Nutrition Examination Survey (NHANES). The Edmonton System also surpassed BMI for disease-severity staging using data from more than 23,000 Canadians with a BMI that denoted obesity.
1 standard deviation increase in WHR linked with a 41% increased mortality
The study reported by Mr. Khan used both epidemiologic and Mendelian randomization analyses on data collected from more than 380,000 U.K. residents included in the UK Biobank database to examine the statistical associations between BMI, FMI, and WHR and all-cause death. This showed that while BMI and FMI both had significant, independent associations with all-cause mortality, with hazard ratios of 1.14 for each 1 standard deviation increase in BMI and of 1.17 for each standard deviation increase in FMI, the link was a stronger 1.41 per standard deviation increase in WHR, he said.
Another analysis that divided the entire UK Biobank study cohort into 20 roughly similar subgroups by their BMI showed that WHR had the most consistent association across the BMI spectrum.
Further analyses showed that WHR also strongly and significantly linked with cardiovascular disease death and with other causes of death that were not cardiovascular, cancer-related, or associated with respiratory diseases. And the WHR link to all-cause mortality was strongest in men, and much less robust in women, likely because visceral adiposity is much more common among men, even compared with the postmenopausal women who predominate in the UK Biobank cohort.
One more feature of WHR that makes it an attractive metric is its relative ease of measurement, about as easy as BMI, Mr. Khan said.
The study received no commercial funding, and Mr. Khan had no disclosures. Dr. Blüher has been a consultant to or speaker on behalf of Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, and Sanofi.
STOCKHOLM – New evidence continues to show that alternative measures of adiposity than body mass index, such as waist-to-hip ratio, work better for predicting the risk a person with overweight or obesity faces from their excess weight.
A direct comparison of waist-to-hip ratio (WHR), body mass index (BMI), and fat mass index (FMI) in a total of more than 380,000 United Kingdom residents included in the UK Biobank showed that WHR had the strongest and most consistent relationship to all-cause death, compared with the other two measures, indicating that clinicians should pay more attention to adiposity distribution than they do to BMI when prioritizing obesity interventions, Irfan Khan said at the annual meeting of the European Association for the Study of Diabetes.
Although it’s likely “way too early” to fully replace BMI as a measure of adiposity, because it is so established in guidelines and in practice, it is now time to “use WHR as an adjunct to BMI” suggested Mr. Khan in an interview.
“A lot of work still needs to be done to translate WHR into practice, but I think it’s getting closer,” said Mr. Khan, a medical student at McMaster University, Hamilton, Ont., who performed his analyses in collaboration with a research team based primarily at McMaster.
Moving away from BMI-centric obesity
“This is a timely topic, because guidelines for treating people with obesity have depended so much on BMI. We want to go from a BMI-centric view to a view of obesity that depends more on disease burden,” commented Matthias Blüher, MD, professor of molecular endocrinology and head of the Obesity Outpatient Clinic for Adults at the University of Leipzig (Germany).
For example, the 2016 obesity management guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology called for a “complications-centric” approach to assessing and intervening in people with obesity rather than a “BMI-centric” approach.
But Dr. Blüher went a step further in an interview, adding that “waist-to-hip ratio is now outdated,” with adjusted measures of WHR such as waist-to-height ratio “considered a better proxy for all-cause death.” He also gave high marks to the Edmonton Obesity Staging System, which independently added to BMI as well as to a diagnosis of metabolic syndrome for predicting mortality in a sample from the U.S. National Health and Nutrition Examination Survey (NHANES). The Edmonton System also surpassed BMI for disease-severity staging using data from more than 23,000 Canadians with a BMI that denoted obesity.
1 standard deviation increase in WHR linked with a 41% increased mortality
The study reported by Mr. Khan used both epidemiologic and Mendelian randomization analyses on data collected from more than 380,000 U.K. residents included in the UK Biobank database to examine the statistical associations between BMI, FMI, and WHR and all-cause death. This showed that while BMI and FMI both had significant, independent associations with all-cause mortality, with hazard ratios of 1.14 for each 1 standard deviation increase in BMI and of 1.17 for each standard deviation increase in FMI, the link was a stronger 1.41 per standard deviation increase in WHR, he said.
Another analysis that divided the entire UK Biobank study cohort into 20 roughly similar subgroups by their BMI showed that WHR had the most consistent association across the BMI spectrum.
Further analyses showed that WHR also strongly and significantly linked with cardiovascular disease death and with other causes of death that were not cardiovascular, cancer-related, or associated with respiratory diseases. And the WHR link to all-cause mortality was strongest in men, and much less robust in women, likely because visceral adiposity is much more common among men, even compared with the postmenopausal women who predominate in the UK Biobank cohort.
One more feature of WHR that makes it an attractive metric is its relative ease of measurement, about as easy as BMI, Mr. Khan said.
The study received no commercial funding, and Mr. Khan had no disclosures. Dr. Blüher has been a consultant to or speaker on behalf of Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, and Sanofi.
STOCKHOLM – New evidence continues to show that alternative measures of adiposity than body mass index, such as waist-to-hip ratio, work better for predicting the risk a person with overweight or obesity faces from their excess weight.
A direct comparison of waist-to-hip ratio (WHR), body mass index (BMI), and fat mass index (FMI) in a total of more than 380,000 United Kingdom residents included in the UK Biobank showed that WHR had the strongest and most consistent relationship to all-cause death, compared with the other two measures, indicating that clinicians should pay more attention to adiposity distribution than they do to BMI when prioritizing obesity interventions, Irfan Khan said at the annual meeting of the European Association for the Study of Diabetes.
Although it’s likely “way too early” to fully replace BMI as a measure of adiposity, because it is so established in guidelines and in practice, it is now time to “use WHR as an adjunct to BMI” suggested Mr. Khan in an interview.
“A lot of work still needs to be done to translate WHR into practice, but I think it’s getting closer,” said Mr. Khan, a medical student at McMaster University, Hamilton, Ont., who performed his analyses in collaboration with a research team based primarily at McMaster.
Moving away from BMI-centric obesity
“This is a timely topic, because guidelines for treating people with obesity have depended so much on BMI. We want to go from a BMI-centric view to a view of obesity that depends more on disease burden,” commented Matthias Blüher, MD, professor of molecular endocrinology and head of the Obesity Outpatient Clinic for Adults at the University of Leipzig (Germany).
For example, the 2016 obesity management guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology called for a “complications-centric” approach to assessing and intervening in people with obesity rather than a “BMI-centric” approach.
But Dr. Blüher went a step further in an interview, adding that “waist-to-hip ratio is now outdated,” with adjusted measures of WHR such as waist-to-height ratio “considered a better proxy for all-cause death.” He also gave high marks to the Edmonton Obesity Staging System, which independently added to BMI as well as to a diagnosis of metabolic syndrome for predicting mortality in a sample from the U.S. National Health and Nutrition Examination Survey (NHANES). The Edmonton System also surpassed BMI for disease-severity staging using data from more than 23,000 Canadians with a BMI that denoted obesity.
1 standard deviation increase in WHR linked with a 41% increased mortality
The study reported by Mr. Khan used both epidemiologic and Mendelian randomization analyses on data collected from more than 380,000 U.K. residents included in the UK Biobank database to examine the statistical associations between BMI, FMI, and WHR and all-cause death. This showed that while BMI and FMI both had significant, independent associations with all-cause mortality, with hazard ratios of 1.14 for each 1 standard deviation increase in BMI and of 1.17 for each standard deviation increase in FMI, the link was a stronger 1.41 per standard deviation increase in WHR, he said.
Another analysis that divided the entire UK Biobank study cohort into 20 roughly similar subgroups by their BMI showed that WHR had the most consistent association across the BMI spectrum.
Further analyses showed that WHR also strongly and significantly linked with cardiovascular disease death and with other causes of death that were not cardiovascular, cancer-related, or associated with respiratory diseases. And the WHR link to all-cause mortality was strongest in men, and much less robust in women, likely because visceral adiposity is much more common among men, even compared with the postmenopausal women who predominate in the UK Biobank cohort.
One more feature of WHR that makes it an attractive metric is its relative ease of measurement, about as easy as BMI, Mr. Khan said.
The study received no commercial funding, and Mr. Khan had no disclosures. Dr. Blüher has been a consultant to or speaker on behalf of Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, and Sanofi.
AT EASD 2022
Dignity
Queen Elizabeth is everywhere. She was even on the last slide of a presentation on COVID, monkeypox, and influenza vaccines given by our physician in charge of quality. This was odd. The presenter wasn’t English. The Queen had nothing to do with vaccines. Nor apparently would she have said even if she did have an opinion about them. But there we were, an audience of physicians and staff pausing for a moment of remembrance of her.
I’m not a Monarchist – except perhaps for the Kennedys. I grew up in New England. I don’t have an opinion on whether or not the British Crown should endure. But I do marvel at the astounding effect Queen Elizabeth’s passing had on so many around the world. Her personal qualities, particularly her steadiness and humane sympathy, might explain why so many are sad hearing the news. But also I think there was something in her role that we all wished for: Not the owning of palaces and sceptres, but rather, the respect that was given to her.
She was a stateswoman of “unmatched dignity,” the White House wrote. That was true, but it seems being the Queen might have been the last job on earth where such dignity is still possible. Certainly in politics, education, and even health care, there doesn’t seem to be much left lately.
The same day of that presentation I walked into the room of a patient 22 minutes late, she held her arm forth tapping her watch to indicate the time and my tardiness. Unnecessary, if not impertinent. Covering for one of my female physician colleagues, I read an email from a patient which began, “Dear Julie, With all due respect …” Another patient submitted a photo for us to review that was clearly taken from her car while waiting at a stop light. Hardly the consideration a clinical encounter should be given.
Much has been lost for patients. too. There are patients trying to make appointments lately who are told: “There are none. Call back later.” . There is no dignified way to remove exam paper stuck to your backside before introducing yourself to the doctor. Maybe that last slide of Her Majesty was in fact for us to have a moment of silence for what we’ve all lost.
Walter Bagehot (pronounce it “Baj-et” if you tell this story to your Harlan wine friends) was a political writer and editor of The Economist in the 1860s. He famously said that the secret to the English government was having two kinds of institutions, the dignified and the efficient. The efficient, Parliament, was responsible for all the work. The dignified, the Crown, gives significance and holds everyone’s respect. If medicine ever once was both dignified and efficient, we aren’t lately. We push to reduce backlogs, offer same-time virtual care, work to reduce costs. We’ve driven medicine to the efficient and left little of the dignity it seems.
The Queen will be remembered for her lifelong dedication to the laborious service of others. Even though each of us in medicine pledges the same, we also mourn this week the loss of dignity that once came with it.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Queen Elizabeth is everywhere. She was even on the last slide of a presentation on COVID, monkeypox, and influenza vaccines given by our physician in charge of quality. This was odd. The presenter wasn’t English. The Queen had nothing to do with vaccines. Nor apparently would she have said even if she did have an opinion about them. But there we were, an audience of physicians and staff pausing for a moment of remembrance of her.
I’m not a Monarchist – except perhaps for the Kennedys. I grew up in New England. I don’t have an opinion on whether or not the British Crown should endure. But I do marvel at the astounding effect Queen Elizabeth’s passing had on so many around the world. Her personal qualities, particularly her steadiness and humane sympathy, might explain why so many are sad hearing the news. But also I think there was something in her role that we all wished for: Not the owning of palaces and sceptres, but rather, the respect that was given to her.
She was a stateswoman of “unmatched dignity,” the White House wrote. That was true, but it seems being the Queen might have been the last job on earth where such dignity is still possible. Certainly in politics, education, and even health care, there doesn’t seem to be much left lately.
The same day of that presentation I walked into the room of a patient 22 minutes late, she held her arm forth tapping her watch to indicate the time and my tardiness. Unnecessary, if not impertinent. Covering for one of my female physician colleagues, I read an email from a patient which began, “Dear Julie, With all due respect …” Another patient submitted a photo for us to review that was clearly taken from her car while waiting at a stop light. Hardly the consideration a clinical encounter should be given.
Much has been lost for patients. too. There are patients trying to make appointments lately who are told: “There are none. Call back later.” . There is no dignified way to remove exam paper stuck to your backside before introducing yourself to the doctor. Maybe that last slide of Her Majesty was in fact for us to have a moment of silence for what we’ve all lost.
Walter Bagehot (pronounce it “Baj-et” if you tell this story to your Harlan wine friends) was a political writer and editor of The Economist in the 1860s. He famously said that the secret to the English government was having two kinds of institutions, the dignified and the efficient. The efficient, Parliament, was responsible for all the work. The dignified, the Crown, gives significance and holds everyone’s respect. If medicine ever once was both dignified and efficient, we aren’t lately. We push to reduce backlogs, offer same-time virtual care, work to reduce costs. We’ve driven medicine to the efficient and left little of the dignity it seems.
The Queen will be remembered for her lifelong dedication to the laborious service of others. Even though each of us in medicine pledges the same, we also mourn this week the loss of dignity that once came with it.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Queen Elizabeth is everywhere. She was even on the last slide of a presentation on COVID, monkeypox, and influenza vaccines given by our physician in charge of quality. This was odd. The presenter wasn’t English. The Queen had nothing to do with vaccines. Nor apparently would she have said even if she did have an opinion about them. But there we were, an audience of physicians and staff pausing for a moment of remembrance of her.
I’m not a Monarchist – except perhaps for the Kennedys. I grew up in New England. I don’t have an opinion on whether or not the British Crown should endure. But I do marvel at the astounding effect Queen Elizabeth’s passing had on so many around the world. Her personal qualities, particularly her steadiness and humane sympathy, might explain why so many are sad hearing the news. But also I think there was something in her role that we all wished for: Not the owning of palaces and sceptres, but rather, the respect that was given to her.
She was a stateswoman of “unmatched dignity,” the White House wrote. That was true, but it seems being the Queen might have been the last job on earth where such dignity is still possible. Certainly in politics, education, and even health care, there doesn’t seem to be much left lately.
The same day of that presentation I walked into the room of a patient 22 minutes late, she held her arm forth tapping her watch to indicate the time and my tardiness. Unnecessary, if not impertinent. Covering for one of my female physician colleagues, I read an email from a patient which began, “Dear Julie, With all due respect …” Another patient submitted a photo for us to review that was clearly taken from her car while waiting at a stop light. Hardly the consideration a clinical encounter should be given.
Much has been lost for patients. too. There are patients trying to make appointments lately who are told: “There are none. Call back later.” . There is no dignified way to remove exam paper stuck to your backside before introducing yourself to the doctor. Maybe that last slide of Her Majesty was in fact for us to have a moment of silence for what we’ve all lost.
Walter Bagehot (pronounce it “Baj-et” if you tell this story to your Harlan wine friends) was a political writer and editor of The Economist in the 1860s. He famously said that the secret to the English government was having two kinds of institutions, the dignified and the efficient. The efficient, Parliament, was responsible for all the work. The dignified, the Crown, gives significance and holds everyone’s respect. If medicine ever once was both dignified and efficient, we aren’t lately. We push to reduce backlogs, offer same-time virtual care, work to reduce costs. We’ve driven medicine to the efficient and left little of the dignity it seems.
The Queen will be remembered for her lifelong dedication to the laborious service of others. Even though each of us in medicine pledges the same, we also mourn this week the loss of dignity that once came with it.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
‘Game changer’ semaglutide halves diabetes risk from obesity
Treatment of people with obesity but without diabetes with the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy) – hailed at its approval in 2021 as a “game changer” for the treatment of obesity – led to beneficial changes in body mass index (BMI), glycemic control, and other clinical measures.
This collectively cut the calculated risk for possible future development of type 2 diabetes in study participants by more than half, based on post-hoc analysis of data from two pivotal trials that compared semaglutide with placebo.
The findings “suggest that semaglutide could help prevent type 2 diabetes in people with overweight or obesity,” said W. Timothy Garvey, MD, in a presentation at the annual meeting of the European Association for the Study of Diabetes.
Asked to comment, Rodolfo J. Galindo, MD, said: “We devote a significant amount of effort to treating people with diabetes but very little effort for diabetes prevention. We hope that further scientific findings showing the benefits of weight loss, as illustrated by [Dr.] Garvey [and colleagues], for diabetes prevention will change the pandemic of adiposity-based chronic disease.”
GLP-1 agonists as complication-reducing agents
Finding a link between treatment with semaglutide and a reduced future risk of developing type 2 diabetes is important because it shows that this regimen is not just a BMI-centric approach to treating people with obesity but is also a way to potentially reduce complications of obesity such as diabetes onset, explained Dr. Garvey, a professor and director of the Diabetes Research Center at the University of Alabama at Birmingham.
Recent obesity-management recommendations have focused on interventions aimed at avoiding complications, as in 2016 guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology, he noted.
Having evidence that treatment with a GLP-1 agonist such as semaglutide can reduce the incidence of diabetes in people with obesity might also help convince payers to more uniformly reimburse for this type of obesity intervention, which up to now has commonly faced coverage limitations, especially in the United States, he said in an interview.
Dr. Garvey added that evidence for a reduction in the incidence of cardiovascular disease complications such as myocardial infarction and stroke may need to join diabetes prevention as proven effects from obesity intervention before coverage decisions change.
He cited the SELECT trial, which is testing the hypothesis that semaglutide treatment of people with overweight or obesity can reduce the incidence of cardiovascular events in about 17,500 participants and with expected completion toward the end of 2023.
“A complication-centric approach to management of people with obesity needs prediction tools that allow a focus on prevention strategies for people with obesity who are at increased risk of developing diabetes,” commented Dr. Galindo, an endocrinologist at Emory University, Atlanta, in an interview.
Combined analysis of STEP 1 and STEP 4 data
The analysis conducted by Dr. Garvey and colleagues used data from the STEP 1 trial, which compared semaglutide 2.4 mg subcutaneous once weekly with placebo for weight loss in more than 1,500 people predominantly with obesity (about 6% were overweight) and showed that after 68 weeks semaglutide cut the calculated risk of developing type 2 diabetes over the subsequent 10 years from 18% at baseline to 7%, compared with a drop from 18% at baseline to 16% among those who received placebo.
A second, similar analysis of data from people predominantly with obesity in the STEP 4 trial – which treated around 800 people with semaglutide 2.4 mg for 20 weeks and then randomized them to placebo or continued semaglutide treatment – showed that semaglutide treatment cut their calculated 10-year risk for incident type 2 diabetes from 20% at baseline to about 11% after 20 weeks. The risk rebounded in the study participants who then switched from semaglutide to placebo. Among those randomized to remain on semaglutide for a total of 68 weeks, the 10-year risk fell further to 8%.
Dr. Garvey and associates used a validated prognostic formula, the cardiometabolic disease staging (CMDS) tool, they had previously developed and reported to calculate 10-year risk for development of type 2 diabetes based on three unmodifiable factors (age, sex, and race) and five modifiable factors (BMI, blood pressure, glucose level, HDL cholesterol, and triglycerides). They applied the analysis to data from 1,561 of the STEP 1 participants and 766 participants in the STEP 4 study.
“There is no better tool I know of to predict diabetes incidence,” commented Michael A. Nauck, MD, professor and chief of clinical research, diabetes division, St. Josef Hospital, Bochum, Germany.
In his opinion, the CMDS tool is appropriate for estimating the risk of developing incident type 2 diabetes in populations but not in specific individuals.
The new analyses also showed that, in STEP 1, the impact of semaglutide on reducing future risk of developing type 2 diabetes was roughly the same regardless of whether participants entered the study with prediabetes or were normoglycemic at entry.
Blood glucose changes confer the biggest effect
The biggest contributor among the five modifiable components of the CMDS tool for altering the predicted risk for incident diabetes was the reduction in blood glucose produced by semaglutide treatment, which influenced just under half of the change in predicted risk, Dr. Garvey said. The four other modifiable components had roughly similar individual effects on predicted risk, with change in BMI influencing about 15% of the observed effect.
“Our analysis shows that semaglutide treatment is preventing diabetes via several mechanisms. It’s not just a reduction in glucose,” Dr. Garvey said.
Dr. Nauck cautioned, however, that it is hard to judge the efficacy of an intervention like semaglutide for preventing incident diabetes when one of its effects is to dampen down hyperglycemia, the signal indicator of diabetes onset.
Indeed, semaglutide was first approved as a treatment for type 2 diabetes (known as Ozempic, Novo Nordisk) at slightly lower doses than it is approved for obesity. It is also available as an oral agent to treat diabetes (Rybelsus).
Dr. Nauck also noted that the results from at least one previously reported study had already shown the same relationship between treatment with the GLP-1 agonist liraglutide as an anti-obesity agent (3.0 mg dose daily, known as Saxenda) and a reduced subsequent incidence of type 2 diabetes but using actual clinical outcomes during 3 years of follow-up rather than a calculated projection of diabetes likelihood.
The SCALE Obesity and Prediabetes trial randomized 2,254 people with prediabetes and overweight or obesity to weekly treatment with 3.0 mg of liraglutide or placebo. After 160 weeks on treatment, the cumulative incidence of type 2 diabetes was 2% in those who received liraglutide and 6% among those on placebo, with a significant hazard ratio reduction of 79% in the incidence of diabetes on liraglutide treatment.
The STEP 1 and STEP 4 trials were sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Garvey has reported serving as an advisor without compensation to Novo Nordisk as well as Boehringer Ingelheim, Eli Lilly, Jazz, and Pfizer. He is also a site principal investigator for multicentered clinical trials sponsored by the University of Alabama at Birmingham and funded by Novo Nordisk, Eli Lilly, Epitomee, and Pfizer. Dr .Galindo has reported being a consultant or advisor for Boehringer Ingelheim, Eli Lilly, Pfizer, Sanofi, and Weight Watchers and receiving research funding from Dexcom, Eli Lilly, and Novo Nordisk. Dr. Nauck has reported being an advisor or consultant to Novo Nordisk as well as to Boehringer Ingelheim, Eli Lilly, Menarini/Berlin Chemie, MSD, Regor, and ShouTi/Gasherbrum, receiving research funding from MSD, being a member of a data monitoring and safety board for Inventiva, and being a speaker on behalf of Novo Nordisk as well as for Eli Lilly, Menarini/Berlin Chemie, MSD, and Sun Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Treatment of people with obesity but without diabetes with the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy) – hailed at its approval in 2021 as a “game changer” for the treatment of obesity – led to beneficial changes in body mass index (BMI), glycemic control, and other clinical measures.
This collectively cut the calculated risk for possible future development of type 2 diabetes in study participants by more than half, based on post-hoc analysis of data from two pivotal trials that compared semaglutide with placebo.
The findings “suggest that semaglutide could help prevent type 2 diabetes in people with overweight or obesity,” said W. Timothy Garvey, MD, in a presentation at the annual meeting of the European Association for the Study of Diabetes.
Asked to comment, Rodolfo J. Galindo, MD, said: “We devote a significant amount of effort to treating people with diabetes but very little effort for diabetes prevention. We hope that further scientific findings showing the benefits of weight loss, as illustrated by [Dr.] Garvey [and colleagues], for diabetes prevention will change the pandemic of adiposity-based chronic disease.”
GLP-1 agonists as complication-reducing agents
Finding a link between treatment with semaglutide and a reduced future risk of developing type 2 diabetes is important because it shows that this regimen is not just a BMI-centric approach to treating people with obesity but is also a way to potentially reduce complications of obesity such as diabetes onset, explained Dr. Garvey, a professor and director of the Diabetes Research Center at the University of Alabama at Birmingham.
Recent obesity-management recommendations have focused on interventions aimed at avoiding complications, as in 2016 guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology, he noted.
Having evidence that treatment with a GLP-1 agonist such as semaglutide can reduce the incidence of diabetes in people with obesity might also help convince payers to more uniformly reimburse for this type of obesity intervention, which up to now has commonly faced coverage limitations, especially in the United States, he said in an interview.
Dr. Garvey added that evidence for a reduction in the incidence of cardiovascular disease complications such as myocardial infarction and stroke may need to join diabetes prevention as proven effects from obesity intervention before coverage decisions change.
He cited the SELECT trial, which is testing the hypothesis that semaglutide treatment of people with overweight or obesity can reduce the incidence of cardiovascular events in about 17,500 participants and with expected completion toward the end of 2023.
“A complication-centric approach to management of people with obesity needs prediction tools that allow a focus on prevention strategies for people with obesity who are at increased risk of developing diabetes,” commented Dr. Galindo, an endocrinologist at Emory University, Atlanta, in an interview.
Combined analysis of STEP 1 and STEP 4 data
The analysis conducted by Dr. Garvey and colleagues used data from the STEP 1 trial, which compared semaglutide 2.4 mg subcutaneous once weekly with placebo for weight loss in more than 1,500 people predominantly with obesity (about 6% were overweight) and showed that after 68 weeks semaglutide cut the calculated risk of developing type 2 diabetes over the subsequent 10 years from 18% at baseline to 7%, compared with a drop from 18% at baseline to 16% among those who received placebo.
A second, similar analysis of data from people predominantly with obesity in the STEP 4 trial – which treated around 800 people with semaglutide 2.4 mg for 20 weeks and then randomized them to placebo or continued semaglutide treatment – showed that semaglutide treatment cut their calculated 10-year risk for incident type 2 diabetes from 20% at baseline to about 11% after 20 weeks. The risk rebounded in the study participants who then switched from semaglutide to placebo. Among those randomized to remain on semaglutide for a total of 68 weeks, the 10-year risk fell further to 8%.
Dr. Garvey and associates used a validated prognostic formula, the cardiometabolic disease staging (CMDS) tool, they had previously developed and reported to calculate 10-year risk for development of type 2 diabetes based on three unmodifiable factors (age, sex, and race) and five modifiable factors (BMI, blood pressure, glucose level, HDL cholesterol, and triglycerides). They applied the analysis to data from 1,561 of the STEP 1 participants and 766 participants in the STEP 4 study.
“There is no better tool I know of to predict diabetes incidence,” commented Michael A. Nauck, MD, professor and chief of clinical research, diabetes division, St. Josef Hospital, Bochum, Germany.
In his opinion, the CMDS tool is appropriate for estimating the risk of developing incident type 2 diabetes in populations but not in specific individuals.
The new analyses also showed that, in STEP 1, the impact of semaglutide on reducing future risk of developing type 2 diabetes was roughly the same regardless of whether participants entered the study with prediabetes or were normoglycemic at entry.
Blood glucose changes confer the biggest effect
The biggest contributor among the five modifiable components of the CMDS tool for altering the predicted risk for incident diabetes was the reduction in blood glucose produced by semaglutide treatment, which influenced just under half of the change in predicted risk, Dr. Garvey said. The four other modifiable components had roughly similar individual effects on predicted risk, with change in BMI influencing about 15% of the observed effect.
“Our analysis shows that semaglutide treatment is preventing diabetes via several mechanisms. It’s not just a reduction in glucose,” Dr. Garvey said.
Dr. Nauck cautioned, however, that it is hard to judge the efficacy of an intervention like semaglutide for preventing incident diabetes when one of its effects is to dampen down hyperglycemia, the signal indicator of diabetes onset.
Indeed, semaglutide was first approved as a treatment for type 2 diabetes (known as Ozempic, Novo Nordisk) at slightly lower doses than it is approved for obesity. It is also available as an oral agent to treat diabetes (Rybelsus).
Dr. Nauck also noted that the results from at least one previously reported study had already shown the same relationship between treatment with the GLP-1 agonist liraglutide as an anti-obesity agent (3.0 mg dose daily, known as Saxenda) and a reduced subsequent incidence of type 2 diabetes but using actual clinical outcomes during 3 years of follow-up rather than a calculated projection of diabetes likelihood.
The SCALE Obesity and Prediabetes trial randomized 2,254 people with prediabetes and overweight or obesity to weekly treatment with 3.0 mg of liraglutide or placebo. After 160 weeks on treatment, the cumulative incidence of type 2 diabetes was 2% in those who received liraglutide and 6% among those on placebo, with a significant hazard ratio reduction of 79% in the incidence of diabetes on liraglutide treatment.
The STEP 1 and STEP 4 trials were sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Garvey has reported serving as an advisor without compensation to Novo Nordisk as well as Boehringer Ingelheim, Eli Lilly, Jazz, and Pfizer. He is also a site principal investigator for multicentered clinical trials sponsored by the University of Alabama at Birmingham and funded by Novo Nordisk, Eli Lilly, Epitomee, and Pfizer. Dr .Galindo has reported being a consultant or advisor for Boehringer Ingelheim, Eli Lilly, Pfizer, Sanofi, and Weight Watchers and receiving research funding from Dexcom, Eli Lilly, and Novo Nordisk. Dr. Nauck has reported being an advisor or consultant to Novo Nordisk as well as to Boehringer Ingelheim, Eli Lilly, Menarini/Berlin Chemie, MSD, Regor, and ShouTi/Gasherbrum, receiving research funding from MSD, being a member of a data monitoring and safety board for Inventiva, and being a speaker on behalf of Novo Nordisk as well as for Eli Lilly, Menarini/Berlin Chemie, MSD, and Sun Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Treatment of people with obesity but without diabetes with the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy) – hailed at its approval in 2021 as a “game changer” for the treatment of obesity – led to beneficial changes in body mass index (BMI), glycemic control, and other clinical measures.
This collectively cut the calculated risk for possible future development of type 2 diabetes in study participants by more than half, based on post-hoc analysis of data from two pivotal trials that compared semaglutide with placebo.
The findings “suggest that semaglutide could help prevent type 2 diabetes in people with overweight or obesity,” said W. Timothy Garvey, MD, in a presentation at the annual meeting of the European Association for the Study of Diabetes.
Asked to comment, Rodolfo J. Galindo, MD, said: “We devote a significant amount of effort to treating people with diabetes but very little effort for diabetes prevention. We hope that further scientific findings showing the benefits of weight loss, as illustrated by [Dr.] Garvey [and colleagues], for diabetes prevention will change the pandemic of adiposity-based chronic disease.”
GLP-1 agonists as complication-reducing agents
Finding a link between treatment with semaglutide and a reduced future risk of developing type 2 diabetes is important because it shows that this regimen is not just a BMI-centric approach to treating people with obesity but is also a way to potentially reduce complications of obesity such as diabetes onset, explained Dr. Garvey, a professor and director of the Diabetes Research Center at the University of Alabama at Birmingham.
Recent obesity-management recommendations have focused on interventions aimed at avoiding complications, as in 2016 guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology, he noted.
Having evidence that treatment with a GLP-1 agonist such as semaglutide can reduce the incidence of diabetes in people with obesity might also help convince payers to more uniformly reimburse for this type of obesity intervention, which up to now has commonly faced coverage limitations, especially in the United States, he said in an interview.
Dr. Garvey added that evidence for a reduction in the incidence of cardiovascular disease complications such as myocardial infarction and stroke may need to join diabetes prevention as proven effects from obesity intervention before coverage decisions change.
He cited the SELECT trial, which is testing the hypothesis that semaglutide treatment of people with overweight or obesity can reduce the incidence of cardiovascular events in about 17,500 participants and with expected completion toward the end of 2023.
“A complication-centric approach to management of people with obesity needs prediction tools that allow a focus on prevention strategies for people with obesity who are at increased risk of developing diabetes,” commented Dr. Galindo, an endocrinologist at Emory University, Atlanta, in an interview.
Combined analysis of STEP 1 and STEP 4 data
The analysis conducted by Dr. Garvey and colleagues used data from the STEP 1 trial, which compared semaglutide 2.4 mg subcutaneous once weekly with placebo for weight loss in more than 1,500 people predominantly with obesity (about 6% were overweight) and showed that after 68 weeks semaglutide cut the calculated risk of developing type 2 diabetes over the subsequent 10 years from 18% at baseline to 7%, compared with a drop from 18% at baseline to 16% among those who received placebo.
A second, similar analysis of data from people predominantly with obesity in the STEP 4 trial – which treated around 800 people with semaglutide 2.4 mg for 20 weeks and then randomized them to placebo or continued semaglutide treatment – showed that semaglutide treatment cut their calculated 10-year risk for incident type 2 diabetes from 20% at baseline to about 11% after 20 weeks. The risk rebounded in the study participants who then switched from semaglutide to placebo. Among those randomized to remain on semaglutide for a total of 68 weeks, the 10-year risk fell further to 8%.
Dr. Garvey and associates used a validated prognostic formula, the cardiometabolic disease staging (CMDS) tool, they had previously developed and reported to calculate 10-year risk for development of type 2 diabetes based on three unmodifiable factors (age, sex, and race) and five modifiable factors (BMI, blood pressure, glucose level, HDL cholesterol, and triglycerides). They applied the analysis to data from 1,561 of the STEP 1 participants and 766 participants in the STEP 4 study.
“There is no better tool I know of to predict diabetes incidence,” commented Michael A. Nauck, MD, professor and chief of clinical research, diabetes division, St. Josef Hospital, Bochum, Germany.
In his opinion, the CMDS tool is appropriate for estimating the risk of developing incident type 2 diabetes in populations but not in specific individuals.
The new analyses also showed that, in STEP 1, the impact of semaglutide on reducing future risk of developing type 2 diabetes was roughly the same regardless of whether participants entered the study with prediabetes or were normoglycemic at entry.
Blood glucose changes confer the biggest effect
The biggest contributor among the five modifiable components of the CMDS tool for altering the predicted risk for incident diabetes was the reduction in blood glucose produced by semaglutide treatment, which influenced just under half of the change in predicted risk, Dr. Garvey said. The four other modifiable components had roughly similar individual effects on predicted risk, with change in BMI influencing about 15% of the observed effect.
“Our analysis shows that semaglutide treatment is preventing diabetes via several mechanisms. It’s not just a reduction in glucose,” Dr. Garvey said.
Dr. Nauck cautioned, however, that it is hard to judge the efficacy of an intervention like semaglutide for preventing incident diabetes when one of its effects is to dampen down hyperglycemia, the signal indicator of diabetes onset.
Indeed, semaglutide was first approved as a treatment for type 2 diabetes (known as Ozempic, Novo Nordisk) at slightly lower doses than it is approved for obesity. It is also available as an oral agent to treat diabetes (Rybelsus).
Dr. Nauck also noted that the results from at least one previously reported study had already shown the same relationship between treatment with the GLP-1 agonist liraglutide as an anti-obesity agent (3.0 mg dose daily, known as Saxenda) and a reduced subsequent incidence of type 2 diabetes but using actual clinical outcomes during 3 years of follow-up rather than a calculated projection of diabetes likelihood.
The SCALE Obesity and Prediabetes trial randomized 2,254 people with prediabetes and overweight or obesity to weekly treatment with 3.0 mg of liraglutide or placebo. After 160 weeks on treatment, the cumulative incidence of type 2 diabetes was 2% in those who received liraglutide and 6% among those on placebo, with a significant hazard ratio reduction of 79% in the incidence of diabetes on liraglutide treatment.
The STEP 1 and STEP 4 trials were sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Garvey has reported serving as an advisor without compensation to Novo Nordisk as well as Boehringer Ingelheim, Eli Lilly, Jazz, and Pfizer. He is also a site principal investigator for multicentered clinical trials sponsored by the University of Alabama at Birmingham and funded by Novo Nordisk, Eli Lilly, Epitomee, and Pfizer. Dr .Galindo has reported being a consultant or advisor for Boehringer Ingelheim, Eli Lilly, Pfizer, Sanofi, and Weight Watchers and receiving research funding from Dexcom, Eli Lilly, and Novo Nordisk. Dr. Nauck has reported being an advisor or consultant to Novo Nordisk as well as to Boehringer Ingelheim, Eli Lilly, Menarini/Berlin Chemie, MSD, Regor, and ShouTi/Gasherbrum, receiving research funding from MSD, being a member of a data monitoring and safety board for Inventiva, and being a speaker on behalf of Novo Nordisk as well as for Eli Lilly, Menarini/Berlin Chemie, MSD, and Sun Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Home BP monitoring in older adults falls short of recommendations
Just over 51% of older hypertensive adults regularly check their own blood pressure, compared with 48% of those with blood pressure–related health conditions (BPHCs), based on a 2021 survey of individuals aged 50-80 years.
“Guidelines recommend that patients use self-measured blood pressure monitoring (SBPM) outside the clinic to diagnose and manage hypertension,” but just 61% of respondents with a BPHC and 68% of those with hypertension said that they had received such a recommendation from a physician, nurse, or other health care professional, Melanie V. Springer, MD, and associates said in JAMA Network Open.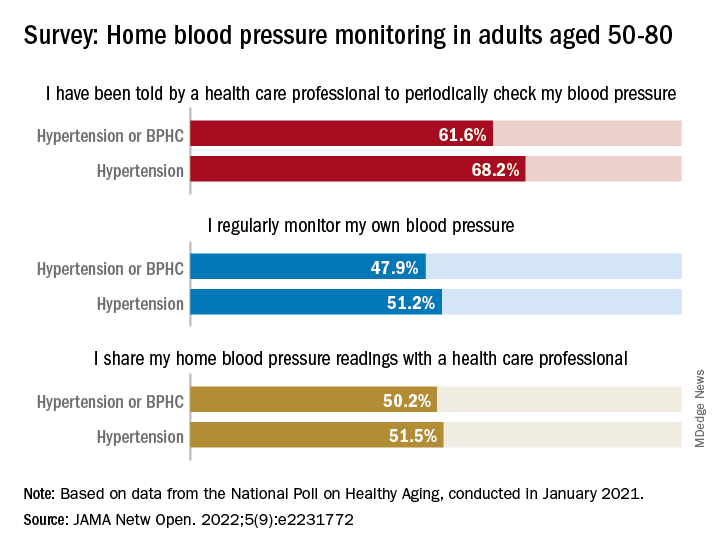
The prevalence of regular monitoring among those with hypertension, 51.2%, does, however, compare favorably with an earlier study showing that 43% of adults aged 18 and older regularly monitored their BP in 2005 and 2008, “which is perhaps associated with our sample’s older age,” said Dr. Springer and associates of the University of Michigan, Ann Arbor.
The current study, they noted, is the first to report “SBPM prevalence in adults ages 50 to 80 years with hypertension or BPHCs, who have a higher risk of adverse outcomes from uncontrolled BP than younger adults.” The analysis is based on data from the National Poll on Healthy Aging, conducted by the University of Michigan in January 2021 and completed by 2,023 individuals.
The frequency of home monitoring varied among adults with BPHCs, as just under 15% reported daily checks and the largest proportion, about 28%, used their device one to three times per month. The results of home monitoring were shared with health care professionals by 50.2% of respondents with a BPHC and by 51.5% of those with hypertension, they said in the research letter.
Home monitoring’s less-than-universal recommendation by providers and use by patients “suggest that protocols should be developed to educate patients about the importance of SBPM and sharing readings with clinicians and the frequency that SBPM should be performed,” Dr. Springer and associates wrote.
The study was funded by AARP, Michigan Medicine, the National Institute of Neurological Disorders and Stroke, and the Department of Veterans Affairs. One investigator has received consulting fees or honoraria from SeeChange Health, HealthMine, the Kaiser Permanente Washington Health Research Institute, the Robert Wood Johnson Foundation, AbilTo, Kansas City Area Life Sciences Institute, American Diabetes Association, Donaghue Foundation, and Luxembourg National Research Fund.
Just over 51% of older hypertensive adults regularly check their own blood pressure, compared with 48% of those with blood pressure–related health conditions (BPHCs), based on a 2021 survey of individuals aged 50-80 years.
“Guidelines recommend that patients use self-measured blood pressure monitoring (SBPM) outside the clinic to diagnose and manage hypertension,” but just 61% of respondents with a BPHC and 68% of those with hypertension said that they had received such a recommendation from a physician, nurse, or other health care professional, Melanie V. Springer, MD, and associates said in JAMA Network Open.
The prevalence of regular monitoring among those with hypertension, 51.2%, does, however, compare favorably with an earlier study showing that 43% of adults aged 18 and older regularly monitored their BP in 2005 and 2008, “which is perhaps associated with our sample’s older age,” said Dr. Springer and associates of the University of Michigan, Ann Arbor.
The current study, they noted, is the first to report “SBPM prevalence in adults ages 50 to 80 years with hypertension or BPHCs, who have a higher risk of adverse outcomes from uncontrolled BP than younger adults.” The analysis is based on data from the National Poll on Healthy Aging, conducted by the University of Michigan in January 2021 and completed by 2,023 individuals.
The frequency of home monitoring varied among adults with BPHCs, as just under 15% reported daily checks and the largest proportion, about 28%, used their device one to three times per month. The results of home monitoring were shared with health care professionals by 50.2% of respondents with a BPHC and by 51.5% of those with hypertension, they said in the research letter.
Home monitoring’s less-than-universal recommendation by providers and use by patients “suggest that protocols should be developed to educate patients about the importance of SBPM and sharing readings with clinicians and the frequency that SBPM should be performed,” Dr. Springer and associates wrote.
The study was funded by AARP, Michigan Medicine, the National Institute of Neurological Disorders and Stroke, and the Department of Veterans Affairs. One investigator has received consulting fees or honoraria from SeeChange Health, HealthMine, the Kaiser Permanente Washington Health Research Institute, the Robert Wood Johnson Foundation, AbilTo, Kansas City Area Life Sciences Institute, American Diabetes Association, Donaghue Foundation, and Luxembourg National Research Fund.
Just over 51% of older hypertensive adults regularly check their own blood pressure, compared with 48% of those with blood pressure–related health conditions (BPHCs), based on a 2021 survey of individuals aged 50-80 years.
“Guidelines recommend that patients use self-measured blood pressure monitoring (SBPM) outside the clinic to diagnose and manage hypertension,” but just 61% of respondents with a BPHC and 68% of those with hypertension said that they had received such a recommendation from a physician, nurse, or other health care professional, Melanie V. Springer, MD, and associates said in JAMA Network Open.
The prevalence of regular monitoring among those with hypertension, 51.2%, does, however, compare favorably with an earlier study showing that 43% of adults aged 18 and older regularly monitored their BP in 2005 and 2008, “which is perhaps associated with our sample’s older age,” said Dr. Springer and associates of the University of Michigan, Ann Arbor.
The current study, they noted, is the first to report “SBPM prevalence in adults ages 50 to 80 years with hypertension or BPHCs, who have a higher risk of adverse outcomes from uncontrolled BP than younger adults.” The analysis is based on data from the National Poll on Healthy Aging, conducted by the University of Michigan in January 2021 and completed by 2,023 individuals.
The frequency of home monitoring varied among adults with BPHCs, as just under 15% reported daily checks and the largest proportion, about 28%, used their device one to three times per month. The results of home monitoring were shared with health care professionals by 50.2% of respondents with a BPHC and by 51.5% of those with hypertension, they said in the research letter.
Home monitoring’s less-than-universal recommendation by providers and use by patients “suggest that protocols should be developed to educate patients about the importance of SBPM and sharing readings with clinicians and the frequency that SBPM should be performed,” Dr. Springer and associates wrote.
The study was funded by AARP, Michigan Medicine, the National Institute of Neurological Disorders and Stroke, and the Department of Veterans Affairs. One investigator has received consulting fees or honoraria from SeeChange Health, HealthMine, the Kaiser Permanente Washington Health Research Institute, the Robert Wood Johnson Foundation, AbilTo, Kansas City Area Life Sciences Institute, American Diabetes Association, Donaghue Foundation, and Luxembourg National Research Fund.
FROM JAMA NETWORK OPEN
Continuous cuffless monitoring may fuel lifestyle change to lower BP
Wearing a cuffless device on the wrist to continuously monitor blood pressure was associated with a significantly lower systolic BP at 6 months among hypertensive adults, real-world results from Europe show.
“We don’t know what they did to reduce their blood pressure,” Jay Shah, MD, Division of Cardiology, Mayo Clinic Arizona, Phoenix, told this news organization.
“The idea is that because they were exposed to their data on a continual basis, that may have prompted them to do something that led to an improvement in their blood pressure, whether it be exercise more, go to their doctor, or change their medication,” said Dr. Shah, who is also chief medical officer for Aktiia.
Dr. Shah presented the study at the Hypertension Scientific Sessions, San Diego.
Empowering data
The study used the Aktiia 24/7 BP monitor; Atkiia funded the trial. The monitor passively and continually monitors BP values from photoplethysmography signals collected via optical sensors at the wrist.
After initial individualized calibration using a cuff-based reference, BP measurements are displayed on a smartphone app, allowing users to consistently monitor their own BP for long periods of time.
Aktiia received CE mark in Europe in January 2021 and is currently under review by the U.S. Food and Drug Administration.
Dr. Shah and colleagues analyzed systolic BP (SBP) trends among 838 real-world Aktiia users in Europe (age 57 ± 11 years; 14% women) who consistently used the monitor for 6 months.
Altogether, they had data on 375 (± 287) app interactions, 3,646 (± 1,417) cuffless readings per user, and 9 (± 7) cuff readings per user.
Traditional cuff SBP averages were calculated monthly and compared with the SBP average of the first month. A t-test analysis was used to detect the difference in SBP between the first and successive months.
On the basis of the mean SBP calculated over 6 months, 136 participants were hypertensive (SBP > 140 mm Hg) and the rest had SBP less than 140 mm Hg.
Hypertensive users saw a statistically significant reduction in SBP of –3.2 mm Hg (95% CI, –0.70 to –5.59; P < .02), beginning at 3 months of continual cuffless BP monitoring, which was sustained through 6 months.
Among users with SBP less than 140 mm Hg, the mean SBP remained unchanged.
“The magnitude of improvement might look modest, but even a 5 mm Hg reduction in systolic BP correlates to a 10% decrease in cardiovascular risk,” Dr. Shah told this news organization.
He noted that “one of the major hurdles is that people may not be aware they have high blood pressure because they don’t feel it. And with a regular cuff, they’ll only see that number when they actually check their blood pressure, which is extremely rare, even for people who have hypertension.”
“Having the ability to show someone their continual blood pressure picture really empowers them to do something to make changes and to be aware, [as well as] to be a more active participant in their health,” Dr. Shah said.
He said that a good analogy is diabetes management, which has transitioned from single finger-stick glucose monitoring to continuous glucose monitoring that provides a complete 24/7 picture of glucose levels.
Transforming technology
Offering perspective on the study, Harlan Krumholz, MD, SM, with Yale New Haven Hospital and Yale School of Medicine, New Haven, Conn., said that having an accurate, affordable, unobtrusive cuffless continuous BP monitor would “transform” BP management.
“This could unlock an era of precision BP management with empowerment of patients to view and act on their numbers,” Dr. Krumholz said in an interview.
“We need data to be confident in the devices – and then research to best leverage the streams of information – and strategies to optimize its use in practice,” Dr. Krumholz added.
“Like any new innovation, we need to mitigate risks and monitor for unintended adverse consequences, but I am bullish on the future of cuffless continuous BP monitors,” Dr. Krumholz said.
Dr. Krumholz said that he “applauds Aktiia for doing studies that assess the effect of the information they are producing on BP over time. We need to know that new approaches not only generate valid information but that they can improve health.”
Ready for prime time?
In June, the European Society of Hypertension issued a statement noting that cuffless BP measurement is a fast-growing and promising field with considerable potential for improving hypertension awareness, management, and control, but because the accuracy of these new devices has not yet been validated, they are not yet suitable for clinical use.
Also providing perspective, Stephen Juraschek, MD, PhD, research director, Hypertension Center of Excellence at Healthcare Associates, Beth Israel Deaconess Medical Center, Boston, said that “there is a lot of interest in cuffless BP monitors due to their ease of measurement, comfort, and ability to obtain BP measurements in multiple settings and environments, and this study showed that the monitoring improved BP over time.”
“It is believed that the increased awareness and feedback may promote healthier behaviors aimed at lowering BP. However, this result should not be conflated with the accuracy of these monitors,” Dr. Juraschek cautioned.
He also noted that there is still no formally approved validation protocol by the Association for the Advancement of Medical Instrumentation.
“While a number of cuffless devices are cleared by the FDA through its 510k mechanism (that is, demonstration of device equivalence), there is no formal stamp of approval or attestation that the measurements are accurate,” Dr. Juraschek said in an interview.
In his view, “more work is needed to understand the validity of these devices. For now, validated, cuff-based home devices are recommended for BP measurement at home, while further work is done to determine the accuracy of these cuffless technologies.”
The study was funded by Aktiia. Dr. Shah is an employee of the company. Dr. Krumholz has no relevant disclosures. Dr. Juraschek is a member of the Validate BP review committee and the AAMI sphygmomanometer committee.
A version of this article first appeared on Medscape.com.
Wearing a cuffless device on the wrist to continuously monitor blood pressure was associated with a significantly lower systolic BP at 6 months among hypertensive adults, real-world results from Europe show.
“We don’t know what they did to reduce their blood pressure,” Jay Shah, MD, Division of Cardiology, Mayo Clinic Arizona, Phoenix, told this news organization.
“The idea is that because they were exposed to their data on a continual basis, that may have prompted them to do something that led to an improvement in their blood pressure, whether it be exercise more, go to their doctor, or change their medication,” said Dr. Shah, who is also chief medical officer for Aktiia.
Dr. Shah presented the study at the Hypertension Scientific Sessions, San Diego.
Empowering data
The study used the Aktiia 24/7 BP monitor; Atkiia funded the trial. The monitor passively and continually monitors BP values from photoplethysmography signals collected via optical sensors at the wrist.
After initial individualized calibration using a cuff-based reference, BP measurements are displayed on a smartphone app, allowing users to consistently monitor their own BP for long periods of time.
Aktiia received CE mark in Europe in January 2021 and is currently under review by the U.S. Food and Drug Administration.
Dr. Shah and colleagues analyzed systolic BP (SBP) trends among 838 real-world Aktiia users in Europe (age 57 ± 11 years; 14% women) who consistently used the monitor for 6 months.
Altogether, they had data on 375 (± 287) app interactions, 3,646 (± 1,417) cuffless readings per user, and 9 (± 7) cuff readings per user.
Traditional cuff SBP averages were calculated monthly and compared with the SBP average of the first month. A t-test analysis was used to detect the difference in SBP between the first and successive months.
On the basis of the mean SBP calculated over 6 months, 136 participants were hypertensive (SBP > 140 mm Hg) and the rest had SBP less than 140 mm Hg.
Hypertensive users saw a statistically significant reduction in SBP of –3.2 mm Hg (95% CI, –0.70 to –5.59; P < .02), beginning at 3 months of continual cuffless BP monitoring, which was sustained through 6 months.
Among users with SBP less than 140 mm Hg, the mean SBP remained unchanged.
“The magnitude of improvement might look modest, but even a 5 mm Hg reduction in systolic BP correlates to a 10% decrease in cardiovascular risk,” Dr. Shah told this news organization.
He noted that “one of the major hurdles is that people may not be aware they have high blood pressure because they don’t feel it. And with a regular cuff, they’ll only see that number when they actually check their blood pressure, which is extremely rare, even for people who have hypertension.”
“Having the ability to show someone their continual blood pressure picture really empowers them to do something to make changes and to be aware, [as well as] to be a more active participant in their health,” Dr. Shah said.
He said that a good analogy is diabetes management, which has transitioned from single finger-stick glucose monitoring to continuous glucose monitoring that provides a complete 24/7 picture of glucose levels.
Transforming technology
Offering perspective on the study, Harlan Krumholz, MD, SM, with Yale New Haven Hospital and Yale School of Medicine, New Haven, Conn., said that having an accurate, affordable, unobtrusive cuffless continuous BP monitor would “transform” BP management.
“This could unlock an era of precision BP management with empowerment of patients to view and act on their numbers,” Dr. Krumholz said in an interview.
“We need data to be confident in the devices – and then research to best leverage the streams of information – and strategies to optimize its use in practice,” Dr. Krumholz added.
“Like any new innovation, we need to mitigate risks and monitor for unintended adverse consequences, but I am bullish on the future of cuffless continuous BP monitors,” Dr. Krumholz said.
Dr. Krumholz said that he “applauds Aktiia for doing studies that assess the effect of the information they are producing on BP over time. We need to know that new approaches not only generate valid information but that they can improve health.”
Ready for prime time?
In June, the European Society of Hypertension issued a statement noting that cuffless BP measurement is a fast-growing and promising field with considerable potential for improving hypertension awareness, management, and control, but because the accuracy of these new devices has not yet been validated, they are not yet suitable for clinical use.
Also providing perspective, Stephen Juraschek, MD, PhD, research director, Hypertension Center of Excellence at Healthcare Associates, Beth Israel Deaconess Medical Center, Boston, said that “there is a lot of interest in cuffless BP monitors due to their ease of measurement, comfort, and ability to obtain BP measurements in multiple settings and environments, and this study showed that the monitoring improved BP over time.”
“It is believed that the increased awareness and feedback may promote healthier behaviors aimed at lowering BP. However, this result should not be conflated with the accuracy of these monitors,” Dr. Juraschek cautioned.
He also noted that there is still no formally approved validation protocol by the Association for the Advancement of Medical Instrumentation.
“While a number of cuffless devices are cleared by the FDA through its 510k mechanism (that is, demonstration of device equivalence), there is no formal stamp of approval or attestation that the measurements are accurate,” Dr. Juraschek said in an interview.
In his view, “more work is needed to understand the validity of these devices. For now, validated, cuff-based home devices are recommended for BP measurement at home, while further work is done to determine the accuracy of these cuffless technologies.”
The study was funded by Aktiia. Dr. Shah is an employee of the company. Dr. Krumholz has no relevant disclosures. Dr. Juraschek is a member of the Validate BP review committee and the AAMI sphygmomanometer committee.
A version of this article first appeared on Medscape.com.
Wearing a cuffless device on the wrist to continuously monitor blood pressure was associated with a significantly lower systolic BP at 6 months among hypertensive adults, real-world results from Europe show.
“We don’t know what they did to reduce their blood pressure,” Jay Shah, MD, Division of Cardiology, Mayo Clinic Arizona, Phoenix, told this news organization.
“The idea is that because they were exposed to their data on a continual basis, that may have prompted them to do something that led to an improvement in their blood pressure, whether it be exercise more, go to their doctor, or change their medication,” said Dr. Shah, who is also chief medical officer for Aktiia.
Dr. Shah presented the study at the Hypertension Scientific Sessions, San Diego.
Empowering data
The study used the Aktiia 24/7 BP monitor; Atkiia funded the trial. The monitor passively and continually monitors BP values from photoplethysmography signals collected via optical sensors at the wrist.
After initial individualized calibration using a cuff-based reference, BP measurements are displayed on a smartphone app, allowing users to consistently monitor their own BP for long periods of time.
Aktiia received CE mark in Europe in January 2021 and is currently under review by the U.S. Food and Drug Administration.
Dr. Shah and colleagues analyzed systolic BP (SBP) trends among 838 real-world Aktiia users in Europe (age 57 ± 11 years; 14% women) who consistently used the monitor for 6 months.
Altogether, they had data on 375 (± 287) app interactions, 3,646 (± 1,417) cuffless readings per user, and 9 (± 7) cuff readings per user.
Traditional cuff SBP averages were calculated monthly and compared with the SBP average of the first month. A t-test analysis was used to detect the difference in SBP between the first and successive months.
On the basis of the mean SBP calculated over 6 months, 136 participants were hypertensive (SBP > 140 mm Hg) and the rest had SBP less than 140 mm Hg.
Hypertensive users saw a statistically significant reduction in SBP of –3.2 mm Hg (95% CI, –0.70 to –5.59; P < .02), beginning at 3 months of continual cuffless BP monitoring, which was sustained through 6 months.
Among users with SBP less than 140 mm Hg, the mean SBP remained unchanged.
“The magnitude of improvement might look modest, but even a 5 mm Hg reduction in systolic BP correlates to a 10% decrease in cardiovascular risk,” Dr. Shah told this news organization.
He noted that “one of the major hurdles is that people may not be aware they have high blood pressure because they don’t feel it. And with a regular cuff, they’ll only see that number when they actually check their blood pressure, which is extremely rare, even for people who have hypertension.”
“Having the ability to show someone their continual blood pressure picture really empowers them to do something to make changes and to be aware, [as well as] to be a more active participant in their health,” Dr. Shah said.
He said that a good analogy is diabetes management, which has transitioned from single finger-stick glucose monitoring to continuous glucose monitoring that provides a complete 24/7 picture of glucose levels.
Transforming technology
Offering perspective on the study, Harlan Krumholz, MD, SM, with Yale New Haven Hospital and Yale School of Medicine, New Haven, Conn., said that having an accurate, affordable, unobtrusive cuffless continuous BP monitor would “transform” BP management.
“This could unlock an era of precision BP management with empowerment of patients to view and act on their numbers,” Dr. Krumholz said in an interview.
“We need data to be confident in the devices – and then research to best leverage the streams of information – and strategies to optimize its use in practice,” Dr. Krumholz added.
“Like any new innovation, we need to mitigate risks and monitor for unintended adverse consequences, but I am bullish on the future of cuffless continuous BP monitors,” Dr. Krumholz said.
Dr. Krumholz said that he “applauds Aktiia for doing studies that assess the effect of the information they are producing on BP over time. We need to know that new approaches not only generate valid information but that they can improve health.”
Ready for prime time?
In June, the European Society of Hypertension issued a statement noting that cuffless BP measurement is a fast-growing and promising field with considerable potential for improving hypertension awareness, management, and control, but because the accuracy of these new devices has not yet been validated, they are not yet suitable for clinical use.
Also providing perspective, Stephen Juraschek, MD, PhD, research director, Hypertension Center of Excellence at Healthcare Associates, Beth Israel Deaconess Medical Center, Boston, said that “there is a lot of interest in cuffless BP monitors due to their ease of measurement, comfort, and ability to obtain BP measurements in multiple settings and environments, and this study showed that the monitoring improved BP over time.”
“It is believed that the increased awareness and feedback may promote healthier behaviors aimed at lowering BP. However, this result should not be conflated with the accuracy of these monitors,” Dr. Juraschek cautioned.
He also noted that there is still no formally approved validation protocol by the Association for the Advancement of Medical Instrumentation.
“While a number of cuffless devices are cleared by the FDA through its 510k mechanism (that is, demonstration of device equivalence), there is no formal stamp of approval or attestation that the measurements are accurate,” Dr. Juraschek said in an interview.
In his view, “more work is needed to understand the validity of these devices. For now, validated, cuff-based home devices are recommended for BP measurement at home, while further work is done to determine the accuracy of these cuffless technologies.”
The study was funded by Aktiia. Dr. Shah is an employee of the company. Dr. Krumholz has no relevant disclosures. Dr. Juraschek is a member of the Validate BP review committee and the AAMI sphygmomanometer committee.
A version of this article first appeared on Medscape.com.
FROM AHA HYPERTENSION 2022
House passes prior authorization bill, Senate path unclear
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
WPATH removes age limits from transgender treatment guidelines
Long-awaited global transgender care guidelines have dropped, with no recommendations regarding age limits for treatment and surgery in teenagers but acknowledging the complexity of dealing with such adolescents amid lack of longitudinal research on the impact of transitioning gender.
The World Professional Association of Transgender Health published its latest standards of care (SOC8) as it opens its annual meeting on Sept. 16 in Montreal.
These are “the most comprehensive set of guidelines ever produced to assist health care professionals around the world in support of transgender and gender diverse adults, adolescents, and children who are taking steps to live their lives authentically,” wrote WPATH President Walter Bouman, MD, PhD, and WPATH President-Elect Marci Bowers, MD, in a news release.
The SOC8 is the first update to guidance on the treatment of transgender individuals in 10 years and appears online in the International Journal of Transgender Health.
For the first time, the association wrote a chapter dedicated to transgender and gender-diverse adolescents – distinct from the child chapter.
The complexity of treating adolescents
WPATH officials said that this was owed to exponential growth in adolescent referral rates, more research on adolescent gender diversity–related care, and the unique developmental and care issues of this age group.
Until recently, there was limited information regarding the prevalence of gender diversity among adolescents. Studies from high-school samples indicate much higher rates than was earlier thought, with reports of up to 1.2% of participants identifying as transgender and up to 2.7% or more (for example, 7%-9%) experiencing some level of self-reported gender diversity, WPATH said.
The new chapter “applies to adolescents from the start of puberty until the legal age of majority (in most cases 18 years),” it stated.
However, WPATH did not go as far as to recommend lowering the age at which youth can receive cross-sex hormone therapy or gender-affirming surgeries, as earlier decreed in a draft of the guidelines. That draft suggested that young people could receive hormone therapy at age 14 years and surgeries for double mastectomies at age 15 years and for genital reassignment at age 17 years.
The exception was phalloplasty – surgery to construct a penis in female-to-male individuals – which WPATH stressed should not be performed under the age of 18 years owing to its complexity.
Now, the final SOC8 emphasizes that each transgender adolescent is unique, and decisions must be made on an individual basis, with no recommendations on specific ages for any treatment. This could be interpreted in many ways.
The SOC8 also acknowledges the “very rare” regret of individuals who have transitioned to the opposite gender and then changed their minds.
“[Health care] providers may consider the possibility an adolescent may regret gender-affirming decisions made during adolescence, and a young person will want to stop treatment and return to living in the birth-assigned gender role in the future. Providers may discuss this topic in a collaborative and trusting manner with the adolescent and their parents/caregivers before gender-affirming medical treatments are started,” it states.
WPATH, in addition, stressed the importance of counseling and supporting regretting patients, many who “expressed difficulties finding help during their detransition process and reported their detransition was an isolating experience during which they did not receive either sufficient or appropriate support.”
Although it doesn’t put a firm figure on the rate of regret overall, in its chapter on surgery, WPATH estimates that 0.3%-3.8% of transgender individuals regret gender-affirming surgery.
SOC8 also acknowledges “A pattern of uneven ratios by assigned sex has been reported in gender clinics, with assigned female-at-birth patients initiating care 2.5-7.1 times more frequently” than patients who were assigned male at birth.
And WPATH states in SOC8 that another phenomenon is the growing number of adolescents seeking care who had not previously experienced or expressed gender diversity during their childhood years.
It goes on to cite the 2018 paper of Lisa Littman, MD, MPH, now president of the Institute for Comprehensive Gender Dysphoria Research. Dr. Littman coined the term, “rapid-onset gender dysphoria” to describe this phenomenon; SOC8 refrains from using this phrase, but does acknowledge: “For a select subgroup of young people, susceptibility to social influence impacting gender may be an important differential to consider.”
SOC8 recommends that before any medical or surgical treatment is considered, health care professionals “undertake a comprehensive biopsychosocial assessment of adolescents who present with gender identity-related concerns and seek medical/surgical transition-related care.”
And it specifically mentions that transgender adolescents “show high rates of autism spectrum disorder/characteristics,” and notes that “other neurodevelopmental presentations and/or mental health challenges may also be present, (e.g., ADHD, intellectual disability, and psychotic disorders).”
Who uses WPATH to guide care? This is ‘a big unknown’
WPATH is an umbrella organization with offshoots in most Western nations, such as USPATH in the United States, EPATH in Europe, and AUSPATH and NZPATH in Australia and New Zealand.
However, it is not the only organization to issue guidance on the care of transgender individuals; several specialties take care of this patient population, including, but not limited to: pediatricians, endocrinologists, psychiatrists, psychologists and plastic surgeons.
The extent to which any health care professional, or professional body, follows WPATH guidance is extremely varied.
“There is nothing binding clinicians to the SOC, and the SOC is so broad and vague that anyone can say they’re following it but according to their own biases and interpretation,” Aaron Kimberly, a trans man and mental health clinician from the Gender Dysphoria Alliance, said in an interview.
In North America, some clinics practice full “informed consent” with no assessment and prescriptions at the first visit, Mr. Kimberly said, whereas others do comprehensive assessments.
“I think SOC should be observed. It shouldn’t just be people going rogue,” Erica Anderson, a clinical psychologist in Berkeley, Calif., former president of USPATH, and former member of WPATH, who is herself transgender, said in an interview. “The reason there are standards of care is because hundreds of scientists have weighed in – is it perfect? No. We have a long way to go. But you can’t just ignore whatever it is that we know and let people make their own decisions.”
A version of this article first appeared on Medscape.com.
Long-awaited global transgender care guidelines have dropped, with no recommendations regarding age limits for treatment and surgery in teenagers but acknowledging the complexity of dealing with such adolescents amid lack of longitudinal research on the impact of transitioning gender.
The World Professional Association of Transgender Health published its latest standards of care (SOC8) as it opens its annual meeting on Sept. 16 in Montreal.
These are “the most comprehensive set of guidelines ever produced to assist health care professionals around the world in support of transgender and gender diverse adults, adolescents, and children who are taking steps to live their lives authentically,” wrote WPATH President Walter Bouman, MD, PhD, and WPATH President-Elect Marci Bowers, MD, in a news release.
The SOC8 is the first update to guidance on the treatment of transgender individuals in 10 years and appears online in the International Journal of Transgender Health.
For the first time, the association wrote a chapter dedicated to transgender and gender-diverse adolescents – distinct from the child chapter.
The complexity of treating adolescents
WPATH officials said that this was owed to exponential growth in adolescent referral rates, more research on adolescent gender diversity–related care, and the unique developmental and care issues of this age group.
Until recently, there was limited information regarding the prevalence of gender diversity among adolescents. Studies from high-school samples indicate much higher rates than was earlier thought, with reports of up to 1.2% of participants identifying as transgender and up to 2.7% or more (for example, 7%-9%) experiencing some level of self-reported gender diversity, WPATH said.
The new chapter “applies to adolescents from the start of puberty until the legal age of majority (in most cases 18 years),” it stated.
However, WPATH did not go as far as to recommend lowering the age at which youth can receive cross-sex hormone therapy or gender-affirming surgeries, as earlier decreed in a draft of the guidelines. That draft suggested that young people could receive hormone therapy at age 14 years and surgeries for double mastectomies at age 15 years and for genital reassignment at age 17 years.
The exception was phalloplasty – surgery to construct a penis in female-to-male individuals – which WPATH stressed should not be performed under the age of 18 years owing to its complexity.
Now, the final SOC8 emphasizes that each transgender adolescent is unique, and decisions must be made on an individual basis, with no recommendations on specific ages for any treatment. This could be interpreted in many ways.
The SOC8 also acknowledges the “very rare” regret of individuals who have transitioned to the opposite gender and then changed their minds.
“[Health care] providers may consider the possibility an adolescent may regret gender-affirming decisions made during adolescence, and a young person will want to stop treatment and return to living in the birth-assigned gender role in the future. Providers may discuss this topic in a collaborative and trusting manner with the adolescent and their parents/caregivers before gender-affirming medical treatments are started,” it states.
WPATH, in addition, stressed the importance of counseling and supporting regretting patients, many who “expressed difficulties finding help during their detransition process and reported their detransition was an isolating experience during which they did not receive either sufficient or appropriate support.”
Although it doesn’t put a firm figure on the rate of regret overall, in its chapter on surgery, WPATH estimates that 0.3%-3.8% of transgender individuals regret gender-affirming surgery.
SOC8 also acknowledges “A pattern of uneven ratios by assigned sex has been reported in gender clinics, with assigned female-at-birth patients initiating care 2.5-7.1 times more frequently” than patients who were assigned male at birth.
And WPATH states in SOC8 that another phenomenon is the growing number of adolescents seeking care who had not previously experienced or expressed gender diversity during their childhood years.
It goes on to cite the 2018 paper of Lisa Littman, MD, MPH, now president of the Institute for Comprehensive Gender Dysphoria Research. Dr. Littman coined the term, “rapid-onset gender dysphoria” to describe this phenomenon; SOC8 refrains from using this phrase, but does acknowledge: “For a select subgroup of young people, susceptibility to social influence impacting gender may be an important differential to consider.”
SOC8 recommends that before any medical or surgical treatment is considered, health care professionals “undertake a comprehensive biopsychosocial assessment of adolescents who present with gender identity-related concerns and seek medical/surgical transition-related care.”
And it specifically mentions that transgender adolescents “show high rates of autism spectrum disorder/characteristics,” and notes that “other neurodevelopmental presentations and/or mental health challenges may also be present, (e.g., ADHD, intellectual disability, and psychotic disorders).”
Who uses WPATH to guide care? This is ‘a big unknown’
WPATH is an umbrella organization with offshoots in most Western nations, such as USPATH in the United States, EPATH in Europe, and AUSPATH and NZPATH in Australia and New Zealand.
However, it is not the only organization to issue guidance on the care of transgender individuals; several specialties take care of this patient population, including, but not limited to: pediatricians, endocrinologists, psychiatrists, psychologists and plastic surgeons.
The extent to which any health care professional, or professional body, follows WPATH guidance is extremely varied.
“There is nothing binding clinicians to the SOC, and the SOC is so broad and vague that anyone can say they’re following it but according to their own biases and interpretation,” Aaron Kimberly, a trans man and mental health clinician from the Gender Dysphoria Alliance, said in an interview.
In North America, some clinics practice full “informed consent” with no assessment and prescriptions at the first visit, Mr. Kimberly said, whereas others do comprehensive assessments.
“I think SOC should be observed. It shouldn’t just be people going rogue,” Erica Anderson, a clinical psychologist in Berkeley, Calif., former president of USPATH, and former member of WPATH, who is herself transgender, said in an interview. “The reason there are standards of care is because hundreds of scientists have weighed in – is it perfect? No. We have a long way to go. But you can’t just ignore whatever it is that we know and let people make their own decisions.”
A version of this article first appeared on Medscape.com.
Long-awaited global transgender care guidelines have dropped, with no recommendations regarding age limits for treatment and surgery in teenagers but acknowledging the complexity of dealing with such adolescents amid lack of longitudinal research on the impact of transitioning gender.
The World Professional Association of Transgender Health published its latest standards of care (SOC8) as it opens its annual meeting on Sept. 16 in Montreal.
These are “the most comprehensive set of guidelines ever produced to assist health care professionals around the world in support of transgender and gender diverse adults, adolescents, and children who are taking steps to live their lives authentically,” wrote WPATH President Walter Bouman, MD, PhD, and WPATH President-Elect Marci Bowers, MD, in a news release.
The SOC8 is the first update to guidance on the treatment of transgender individuals in 10 years and appears online in the International Journal of Transgender Health.
For the first time, the association wrote a chapter dedicated to transgender and gender-diverse adolescents – distinct from the child chapter.
The complexity of treating adolescents
WPATH officials said that this was owed to exponential growth in adolescent referral rates, more research on adolescent gender diversity–related care, and the unique developmental and care issues of this age group.
Until recently, there was limited information regarding the prevalence of gender diversity among adolescents. Studies from high-school samples indicate much higher rates than was earlier thought, with reports of up to 1.2% of participants identifying as transgender and up to 2.7% or more (for example, 7%-9%) experiencing some level of self-reported gender diversity, WPATH said.
The new chapter “applies to adolescents from the start of puberty until the legal age of majority (in most cases 18 years),” it stated.
However, WPATH did not go as far as to recommend lowering the age at which youth can receive cross-sex hormone therapy or gender-affirming surgeries, as earlier decreed in a draft of the guidelines. That draft suggested that young people could receive hormone therapy at age 14 years and surgeries for double mastectomies at age 15 years and for genital reassignment at age 17 years.
The exception was phalloplasty – surgery to construct a penis in female-to-male individuals – which WPATH stressed should not be performed under the age of 18 years owing to its complexity.
Now, the final SOC8 emphasizes that each transgender adolescent is unique, and decisions must be made on an individual basis, with no recommendations on specific ages for any treatment. This could be interpreted in many ways.
The SOC8 also acknowledges the “very rare” regret of individuals who have transitioned to the opposite gender and then changed their minds.
“[Health care] providers may consider the possibility an adolescent may regret gender-affirming decisions made during adolescence, and a young person will want to stop treatment and return to living in the birth-assigned gender role in the future. Providers may discuss this topic in a collaborative and trusting manner with the adolescent and their parents/caregivers before gender-affirming medical treatments are started,” it states.
WPATH, in addition, stressed the importance of counseling and supporting regretting patients, many who “expressed difficulties finding help during their detransition process and reported their detransition was an isolating experience during which they did not receive either sufficient or appropriate support.”
Although it doesn’t put a firm figure on the rate of regret overall, in its chapter on surgery, WPATH estimates that 0.3%-3.8% of transgender individuals regret gender-affirming surgery.
SOC8 also acknowledges “A pattern of uneven ratios by assigned sex has been reported in gender clinics, with assigned female-at-birth patients initiating care 2.5-7.1 times more frequently” than patients who were assigned male at birth.
And WPATH states in SOC8 that another phenomenon is the growing number of adolescents seeking care who had not previously experienced or expressed gender diversity during their childhood years.
It goes on to cite the 2018 paper of Lisa Littman, MD, MPH, now president of the Institute for Comprehensive Gender Dysphoria Research. Dr. Littman coined the term, “rapid-onset gender dysphoria” to describe this phenomenon; SOC8 refrains from using this phrase, but does acknowledge: “For a select subgroup of young people, susceptibility to social influence impacting gender may be an important differential to consider.”
SOC8 recommends that before any medical or surgical treatment is considered, health care professionals “undertake a comprehensive biopsychosocial assessment of adolescents who present with gender identity-related concerns and seek medical/surgical transition-related care.”
And it specifically mentions that transgender adolescents “show high rates of autism spectrum disorder/characteristics,” and notes that “other neurodevelopmental presentations and/or mental health challenges may also be present, (e.g., ADHD, intellectual disability, and psychotic disorders).”
Who uses WPATH to guide care? This is ‘a big unknown’
WPATH is an umbrella organization with offshoots in most Western nations, such as USPATH in the United States, EPATH in Europe, and AUSPATH and NZPATH in Australia and New Zealand.
However, it is not the only organization to issue guidance on the care of transgender individuals; several specialties take care of this patient population, including, but not limited to: pediatricians, endocrinologists, psychiatrists, psychologists and plastic surgeons.
The extent to which any health care professional, or professional body, follows WPATH guidance is extremely varied.
“There is nothing binding clinicians to the SOC, and the SOC is so broad and vague that anyone can say they’re following it but according to their own biases and interpretation,” Aaron Kimberly, a trans man and mental health clinician from the Gender Dysphoria Alliance, said in an interview.
In North America, some clinics practice full “informed consent” with no assessment and prescriptions at the first visit, Mr. Kimberly said, whereas others do comprehensive assessments.
“I think SOC should be observed. It shouldn’t just be people going rogue,” Erica Anderson, a clinical psychologist in Berkeley, Calif., former president of USPATH, and former member of WPATH, who is herself transgender, said in an interview. “The reason there are standards of care is because hundreds of scientists have weighed in – is it perfect? No. We have a long way to go. But you can’t just ignore whatever it is that we know and let people make their own decisions.”
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF TRANSGENDER HEALTH
Physicians can’t be bystanders in ‘silent scourge’ of medical bullying
Maya Iyer, MD, MEd, experienced bullying as a faculty member, and she sensed that she wasn’t alone. “The best ideas for research often come from individual experiences, in both personal and the professional academic medicine setting,” she said in an interview.
“And I was correct. I was not the only one who experienced bullying. In fact, the most severe bullying experiences among ... women physician leaders occurred when they were in leadership positions,” said Dr. Iyer, a pediatric emergency medicine physician at Nationwide Children’s Hospital in Columbus, Ohio.
She is a coauthor of a study that was published in JAMA Network Open in which investigators surveyed the existence of antibullying policies for faculty at almost 100 U.S. medical schools.
The researchers defined bullying as “a severe form of mistreatment [that] occurs in the medical setting when a power differential allows offenders to consciously target individuals through persistent negative actions to impede the education or career of the target.”
The study included 91 medical schools, of which 4 schools had antibullying policies that included the reporting of procedures. Of the 87 medical schools without antibullying policies, 60 had antiharrassment policies; of those schools, 10 of the schools’ websites cited bullying and antiharassment policies. Five schools required a login to access policies, and one school’s website had a broken webpage link, per the study.
“We need to bring the silent scourge of bullying to the forefront because bullying is causing a brain drain on the medical profession,” said Dr. Iyer. “Bullying has numerous downstream negative effects, including depression, anxiety, burnout stress, decreased patient care satisfaction, increased medical errors, and job attrition.”
She added: “Through bullying, we are losing voices in medicine just at that point in time where we are trying to diversify the workforce to improve representation of all physicians.”
Dr. Iyer’s team sampled the top 25 schools for research and the top 25 schools for primary care. They also took a random sampling from 25 schools for research and a random sampling from top 25 schools for primary care. They assessed antibullying policies, antiharassment policies that mentioned bullying, antiharrassment policies that did not mention bullying, and the absence of policies addressing these issues.
Policy comprehensiveness was another focus for the researchers. They evaluated whether the relevant policies included faculty members and articulated the institution’s commitment to providing a safe and healthy workplace. Other factors included defining bullying and the roles and responsibilities of employees and procedures for reporting bullying.
Physicians can’t be bystanders to bullying
This means transitioning from being a bystander to an upstander.”
She doesn’t let medical schools off the hook, however. Instead, she advocated having institutions “provide safe spaces and opportunities for near-peer mentoring so that targets of bullying can share stories.”
Regarding who is responsible for addressing bullying, Dr. Iyer is emphatic. “I do want to be clear that the onus of disrupting does not fall on the targets. Rather, we need to fix the systems in which such behavior is tolerated.”
Her advice to leaders in academic medicine is to create comprehensive, zero-retaliation bullying policies that include detailed reporting procedures. Dr. Iyer advised leaders to partner with colleagues in human resources, offices of equity, and ombudspersons to develop, implement, and enforce these policies.
The study authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Maya Iyer, MD, MEd, experienced bullying as a faculty member, and she sensed that she wasn’t alone. “The best ideas for research often come from individual experiences, in both personal and the professional academic medicine setting,” she said in an interview.
“And I was correct. I was not the only one who experienced bullying. In fact, the most severe bullying experiences among ... women physician leaders occurred when they were in leadership positions,” said Dr. Iyer, a pediatric emergency medicine physician at Nationwide Children’s Hospital in Columbus, Ohio.
She is a coauthor of a study that was published in JAMA Network Open in which investigators surveyed the existence of antibullying policies for faculty at almost 100 U.S. medical schools.
The researchers defined bullying as “a severe form of mistreatment [that] occurs in the medical setting when a power differential allows offenders to consciously target individuals through persistent negative actions to impede the education or career of the target.”
The study included 91 medical schools, of which 4 schools had antibullying policies that included the reporting of procedures. Of the 87 medical schools without antibullying policies, 60 had antiharrassment policies; of those schools, 10 of the schools’ websites cited bullying and antiharassment policies. Five schools required a login to access policies, and one school’s website had a broken webpage link, per the study.
“We need to bring the silent scourge of bullying to the forefront because bullying is causing a brain drain on the medical profession,” said Dr. Iyer. “Bullying has numerous downstream negative effects, including depression, anxiety, burnout stress, decreased patient care satisfaction, increased medical errors, and job attrition.”
She added: “Through bullying, we are losing voices in medicine just at that point in time where we are trying to diversify the workforce to improve representation of all physicians.”
Dr. Iyer’s team sampled the top 25 schools for research and the top 25 schools for primary care. They also took a random sampling from 25 schools for research and a random sampling from top 25 schools for primary care. They assessed antibullying policies, antiharassment policies that mentioned bullying, antiharrassment policies that did not mention bullying, and the absence of policies addressing these issues.
Policy comprehensiveness was another focus for the researchers. They evaluated whether the relevant policies included faculty members and articulated the institution’s commitment to providing a safe and healthy workplace. Other factors included defining bullying and the roles and responsibilities of employees and procedures for reporting bullying.
Physicians can’t be bystanders to bullying
This means transitioning from being a bystander to an upstander.”
She doesn’t let medical schools off the hook, however. Instead, she advocated having institutions “provide safe spaces and opportunities for near-peer mentoring so that targets of bullying can share stories.”
Regarding who is responsible for addressing bullying, Dr. Iyer is emphatic. “I do want to be clear that the onus of disrupting does not fall on the targets. Rather, we need to fix the systems in which such behavior is tolerated.”
Her advice to leaders in academic medicine is to create comprehensive, zero-retaliation bullying policies that include detailed reporting procedures. Dr. Iyer advised leaders to partner with colleagues in human resources, offices of equity, and ombudspersons to develop, implement, and enforce these policies.
The study authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Maya Iyer, MD, MEd, experienced bullying as a faculty member, and she sensed that she wasn’t alone. “The best ideas for research often come from individual experiences, in both personal and the professional academic medicine setting,” she said in an interview.
“And I was correct. I was not the only one who experienced bullying. In fact, the most severe bullying experiences among ... women physician leaders occurred when they were in leadership positions,” said Dr. Iyer, a pediatric emergency medicine physician at Nationwide Children’s Hospital in Columbus, Ohio.
She is a coauthor of a study that was published in JAMA Network Open in which investigators surveyed the existence of antibullying policies for faculty at almost 100 U.S. medical schools.
The researchers defined bullying as “a severe form of mistreatment [that] occurs in the medical setting when a power differential allows offenders to consciously target individuals through persistent negative actions to impede the education or career of the target.”
The study included 91 medical schools, of which 4 schools had antibullying policies that included the reporting of procedures. Of the 87 medical schools without antibullying policies, 60 had antiharrassment policies; of those schools, 10 of the schools’ websites cited bullying and antiharassment policies. Five schools required a login to access policies, and one school’s website had a broken webpage link, per the study.
“We need to bring the silent scourge of bullying to the forefront because bullying is causing a brain drain on the medical profession,” said Dr. Iyer. “Bullying has numerous downstream negative effects, including depression, anxiety, burnout stress, decreased patient care satisfaction, increased medical errors, and job attrition.”
She added: “Through bullying, we are losing voices in medicine just at that point in time where we are trying to diversify the workforce to improve representation of all physicians.”
Dr. Iyer’s team sampled the top 25 schools for research and the top 25 schools for primary care. They also took a random sampling from 25 schools for research and a random sampling from top 25 schools for primary care. They assessed antibullying policies, antiharassment policies that mentioned bullying, antiharrassment policies that did not mention bullying, and the absence of policies addressing these issues.
Policy comprehensiveness was another focus for the researchers. They evaluated whether the relevant policies included faculty members and articulated the institution’s commitment to providing a safe and healthy workplace. Other factors included defining bullying and the roles and responsibilities of employees and procedures for reporting bullying.
Physicians can’t be bystanders to bullying
This means transitioning from being a bystander to an upstander.”
She doesn’t let medical schools off the hook, however. Instead, she advocated having institutions “provide safe spaces and opportunities for near-peer mentoring so that targets of bullying can share stories.”
Regarding who is responsible for addressing bullying, Dr. Iyer is emphatic. “I do want to be clear that the onus of disrupting does not fall on the targets. Rather, we need to fix the systems in which such behavior is tolerated.”
Her advice to leaders in academic medicine is to create comprehensive, zero-retaliation bullying policies that include detailed reporting procedures. Dr. Iyer advised leaders to partner with colleagues in human resources, offices of equity, and ombudspersons to develop, implement, and enforce these policies.
The study authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Experts express caution over type 2 diabetes/tea-drinking claim
type 2 diabetes/tea-drinking claim
A claim that drinking tea might protect people against developing type 2 diabetes has been met with caution from multiple experts ahead of the annual meeting of the European Association for the Study of Diabetes.
The claim is that people who drink four or more cups of tea every day – specifically green, Oolong, or black tea – are 17% less likely to develop type 2 diabetes than those who do not drink tea. Drinking fewer cups of tea per day was not found to confer any benefit.
“Our results are exciting because they suggest that people can do something as simple as drinking four cups of tea a day to potentially lessen their risk of developing type 2 diabetes,” Xiaying Li of Wuhan (China) University of Science and Technology is quoted as saying in an official EASD press release.
“It is possible that particular components in tea, such as polyphenols, may reduce blood glucose levels, but a sufficient amount of these bioactive compounds may be needed to be effective,” Dr. Li added.
“The words ‘suggest’ and ‘potentially’ are crucial here,” said Kevin McConway, PhD, MSc, MBA, emeritus professor of applied statistics at The Open University, said in a separate statement to the press that reeled in Dr. Li’s enthusiasm.
“Tea drinking would only be useful for reducing diabetes risk if the tea drinking causes reductions in risk, that is, if the risk is reduced if you drink the tea and not if you don’t – and this study simply can’t show whether it does this or not,” Dr. Conway stressed.
Naveed Sattar, FMedSci FRCPath FRCPGlas FRSE, professor of metabolic medicine at the University of Glasgow, was also cautiously critical. “There is no good trial evidence whatsoever that the chemicals in tea prevent diabetes,” he observed separately.
“So, I suspect its more about tea being healthier (less calorific) than many alternative drinks or tea drinkers leading healthier lives more generally.”
Dr. Sattar added that it could be that people who drink tea might also be avoiding drinking more harmful sugary drinks and have other health behaviors that might lead them to have a lower risk for type 2 diabetes.
Time for tea?
Dr. Li will present the findings of two analyses on Sept. 21 at the EASD meeting: the first a large observational cohort study and the second an updated systematic review and meta-analysis.
For the cohort study, Dr. Li and her coauthors took data on more than 5,100 adults who had participated in the long-running and ongoing China Health and Nutrition Survey (CHNS). Information on tea drinking behavior was extracted from questionnaires that had been filled out at two time points – 1997 and 2009 – and they determined whether people had developed type 2 diabetes according to American Diabetes Association criteria.
Nearly half, 45.8%, were found to be tea drinkers, and 10% of the population they sampled had developed type 2 diabetes. No association between tea drinking and type 2 diabetes development was found, however, with the hazard ratio comparing tea drinkers and non–tea drinkers sitting firmly at 1.02. Moreover, a sensitivity analysis that excluded participants who had developed type 2 diabetes in the first 3 years of follow-up did not change the result.
Things were slightly different when Dr. Li and associates performed their meta-analysis that involved analyzing data on more than 1 million participants in 19 studies conducted in eight countries that had been published up to September 2021.
Here, they found there was a significant (P < .003) linear association between tea consumption and having type 2 diabetes, with the relative risk of developing type 2 diabetes decreasing by 0.986 for every additional cup of tea that was drunk.
HRs for the development of type 2 diabetes in tea drinkers versus non–tea drinkers were 1.00 for those who drank less than one cup per day, 0.96 for those who had one to two cups, and 0.84 for those who drank four or more cups.
“While more research needs to be done to determine the exact dosage and mechanisms behind these observations, our findings suggest that drinking tea is beneficial in reducing the risk of type 2 diabetes, but only at high doses (at least 4 cups a day)”, said Dr. Li.
Perhaps, “we did not find an association between tea drinking and type 2 diabetes in our cohort study because we did not look at higher tea consumption,” she added.
Tempest in a teacup
“This is large, observational data. It’s not a randomized controlled trial so there’s plenty of room for data to be misunderstood,” warned Matt Sydes, MSc, professor of clinical trials & methodology at the MRC Clinical Trials Unit, University College London.
“Everyone drinks fluids. If there is an effect here (and that’s a big if), it might be not about the tea they drink, but about what they don’t drink. One can’t tell at the moment. It seems unlikely that a large randomized controlled trial could be done to disambiguate” added Dr. Sydes
“Being only a conference abstract, it is difficult to assess the quality of this research,” Baptiste Leurent, PhD, a medical statistician also working at University College London, said. Not only was the cohort study observational, so were all the other studies included in the meta-analysis, he pointed out.
“Therefore, no cause-effect conclusions can be drawn. The association could simply be due to other factors, such as those drinking more tea having a healthier lifestyle. It does not seem that the authors tried to control for confounders, which is usually difficult in meta-analysis,” Dr. Leurent said.
“There is reason to be a bit skeptical at this point; we really need to have the full details to assess it properly,” said Jonathan Cook of the Centre for Statistics in Medicine at the University of Oxford (England). “It’s a fair attempt to look at this, but not cutting edge, [using] fairly standard approaches.”
Similar studies have shown a reduced risk associated with coffee drinking, noted Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University in Birmingham.
“The important take-home message is that lifestyle is important in managing risk of developing type 2 diabetes,” Dr. Mellor said.
“That includes choosing low-calorie drinks including mainly water as well as unsweetened tea and coffee as your drinks of choice as part of a healthy lifestyle.”
The study was funded by the Young Talents Project of Hubei Provincial Health Commission, the Science and Technology Research Key Project of Education Department of Hubei Province, the Sanuo Diabetes Charity Foundation, and the Xiangyang Science and Technology Plan Project, all based in China. Dr. Li had no conflicts of interest to disclose. Dr. McConway is a Trustee and on the advisory committee of The Science Media Centre. Dr. Sattar has consulted for many companies that make diabetes and cardiovascular drugs and has been involved in multiple trials of lifestyle approaches for the prevention and remission of diabetes. Dr. Sydes, Dr. Leurent, Dr. Cook, and Dr. Mellor had no conflicts of interest to report.
A claim that drinking tea might protect people against developing type 2 diabetes has been met with caution from multiple experts ahead of the annual meeting of the European Association for the Study of Diabetes.
The claim is that people who drink four or more cups of tea every day – specifically green, Oolong, or black tea – are 17% less likely to develop type 2 diabetes than those who do not drink tea. Drinking fewer cups of tea per day was not found to confer any benefit.
“Our results are exciting because they suggest that people can do something as simple as drinking four cups of tea a day to potentially lessen their risk of developing type 2 diabetes,” Xiaying Li of Wuhan (China) University of Science and Technology is quoted as saying in an official EASD press release.
“It is possible that particular components in tea, such as polyphenols, may reduce blood glucose levels, but a sufficient amount of these bioactive compounds may be needed to be effective,” Dr. Li added.
“The words ‘suggest’ and ‘potentially’ are crucial here,” said Kevin McConway, PhD, MSc, MBA, emeritus professor of applied statistics at The Open University, said in a separate statement to the press that reeled in Dr. Li’s enthusiasm.
“Tea drinking would only be useful for reducing diabetes risk if the tea drinking causes reductions in risk, that is, if the risk is reduced if you drink the tea and not if you don’t – and this study simply can’t show whether it does this or not,” Dr. Conway stressed.
Naveed Sattar, FMedSci FRCPath FRCPGlas FRSE, professor of metabolic medicine at the University of Glasgow, was also cautiously critical. “There is no good trial evidence whatsoever that the chemicals in tea prevent diabetes,” he observed separately.
“So, I suspect its more about tea being healthier (less calorific) than many alternative drinks or tea drinkers leading healthier lives more generally.”
Dr. Sattar added that it could be that people who drink tea might also be avoiding drinking more harmful sugary drinks and have other health behaviors that might lead them to have a lower risk for type 2 diabetes.
Time for tea?
Dr. Li will present the findings of two analyses on Sept. 21 at the EASD meeting: the first a large observational cohort study and the second an updated systematic review and meta-analysis.
For the cohort study, Dr. Li and her coauthors took data on more than 5,100 adults who had participated in the long-running and ongoing China Health and Nutrition Survey (CHNS). Information on tea drinking behavior was extracted from questionnaires that had been filled out at two time points – 1997 and 2009 – and they determined whether people had developed type 2 diabetes according to American Diabetes Association criteria.
Nearly half, 45.8%, were found to be tea drinkers, and 10% of the population they sampled had developed type 2 diabetes. No association between tea drinking and type 2 diabetes development was found, however, with the hazard ratio comparing tea drinkers and non–tea drinkers sitting firmly at 1.02. Moreover, a sensitivity analysis that excluded participants who had developed type 2 diabetes in the first 3 years of follow-up did not change the result.
Things were slightly different when Dr. Li and associates performed their meta-analysis that involved analyzing data on more than 1 million participants in 19 studies conducted in eight countries that had been published up to September 2021.
Here, they found there was a significant (P < .003) linear association between tea consumption and having type 2 diabetes, with the relative risk of developing type 2 diabetes decreasing by 0.986 for every additional cup of tea that was drunk.
HRs for the development of type 2 diabetes in tea drinkers versus non–tea drinkers were 1.00 for those who drank less than one cup per day, 0.96 for those who had one to two cups, and 0.84 for those who drank four or more cups.
“While more research needs to be done to determine the exact dosage and mechanisms behind these observations, our findings suggest that drinking tea is beneficial in reducing the risk of type 2 diabetes, but only at high doses (at least 4 cups a day)”, said Dr. Li.
Perhaps, “we did not find an association between tea drinking and type 2 diabetes in our cohort study because we did not look at higher tea consumption,” she added.
Tempest in a teacup
“This is large, observational data. It’s not a randomized controlled trial so there’s plenty of room for data to be misunderstood,” warned Matt Sydes, MSc, professor of clinical trials & methodology at the MRC Clinical Trials Unit, University College London.
“Everyone drinks fluids. If there is an effect here (and that’s a big if), it might be not about the tea they drink, but about what they don’t drink. One can’t tell at the moment. It seems unlikely that a large randomized controlled trial could be done to disambiguate” added Dr. Sydes
“Being only a conference abstract, it is difficult to assess the quality of this research,” Baptiste Leurent, PhD, a medical statistician also working at University College London, said. Not only was the cohort study observational, so were all the other studies included in the meta-analysis, he pointed out.
“Therefore, no cause-effect conclusions can be drawn. The association could simply be due to other factors, such as those drinking more tea having a healthier lifestyle. It does not seem that the authors tried to control for confounders, which is usually difficult in meta-analysis,” Dr. Leurent said.
“There is reason to be a bit skeptical at this point; we really need to have the full details to assess it properly,” said Jonathan Cook of the Centre for Statistics in Medicine at the University of Oxford (England). “It’s a fair attempt to look at this, but not cutting edge, [using] fairly standard approaches.”
Similar studies have shown a reduced risk associated with coffee drinking, noted Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University in Birmingham.
“The important take-home message is that lifestyle is important in managing risk of developing type 2 diabetes,” Dr. Mellor said.
“That includes choosing low-calorie drinks including mainly water as well as unsweetened tea and coffee as your drinks of choice as part of a healthy lifestyle.”
The study was funded by the Young Talents Project of Hubei Provincial Health Commission, the Science and Technology Research Key Project of Education Department of Hubei Province, the Sanuo Diabetes Charity Foundation, and the Xiangyang Science and Technology Plan Project, all based in China. Dr. Li had no conflicts of interest to disclose. Dr. McConway is a Trustee and on the advisory committee of The Science Media Centre. Dr. Sattar has consulted for many companies that make diabetes and cardiovascular drugs and has been involved in multiple trials of lifestyle approaches for the prevention and remission of diabetes. Dr. Sydes, Dr. Leurent, Dr. Cook, and Dr. Mellor had no conflicts of interest to report.
A claim that drinking tea might protect people against developing type 2 diabetes has been met with caution from multiple experts ahead of the annual meeting of the European Association for the Study of Diabetes.
The claim is that people who drink four or more cups of tea every day – specifically green, Oolong, or black tea – are 17% less likely to develop type 2 diabetes than those who do not drink tea. Drinking fewer cups of tea per day was not found to confer any benefit.
“Our results are exciting because they suggest that people can do something as simple as drinking four cups of tea a day to potentially lessen their risk of developing type 2 diabetes,” Xiaying Li of Wuhan (China) University of Science and Technology is quoted as saying in an official EASD press release.
“It is possible that particular components in tea, such as polyphenols, may reduce blood glucose levels, but a sufficient amount of these bioactive compounds may be needed to be effective,” Dr. Li added.
“The words ‘suggest’ and ‘potentially’ are crucial here,” said Kevin McConway, PhD, MSc, MBA, emeritus professor of applied statistics at The Open University, said in a separate statement to the press that reeled in Dr. Li’s enthusiasm.
“Tea drinking would only be useful for reducing diabetes risk if the tea drinking causes reductions in risk, that is, if the risk is reduced if you drink the tea and not if you don’t – and this study simply can’t show whether it does this or not,” Dr. Conway stressed.
Naveed Sattar, FMedSci FRCPath FRCPGlas FRSE, professor of metabolic medicine at the University of Glasgow, was also cautiously critical. “There is no good trial evidence whatsoever that the chemicals in tea prevent diabetes,” he observed separately.
“So, I suspect its more about tea being healthier (less calorific) than many alternative drinks or tea drinkers leading healthier lives more generally.”
Dr. Sattar added that it could be that people who drink tea might also be avoiding drinking more harmful sugary drinks and have other health behaviors that might lead them to have a lower risk for type 2 diabetes.
Time for tea?
Dr. Li will present the findings of two analyses on Sept. 21 at the EASD meeting: the first a large observational cohort study and the second an updated systematic review and meta-analysis.
For the cohort study, Dr. Li and her coauthors took data on more than 5,100 adults who had participated in the long-running and ongoing China Health and Nutrition Survey (CHNS). Information on tea drinking behavior was extracted from questionnaires that had been filled out at two time points – 1997 and 2009 – and they determined whether people had developed type 2 diabetes according to American Diabetes Association criteria.
Nearly half, 45.8%, were found to be tea drinkers, and 10% of the population they sampled had developed type 2 diabetes. No association between tea drinking and type 2 diabetes development was found, however, with the hazard ratio comparing tea drinkers and non–tea drinkers sitting firmly at 1.02. Moreover, a sensitivity analysis that excluded participants who had developed type 2 diabetes in the first 3 years of follow-up did not change the result.
Things were slightly different when Dr. Li and associates performed their meta-analysis that involved analyzing data on more than 1 million participants in 19 studies conducted in eight countries that had been published up to September 2021.
Here, they found there was a significant (P < .003) linear association between tea consumption and having type 2 diabetes, with the relative risk of developing type 2 diabetes decreasing by 0.986 for every additional cup of tea that was drunk.
HRs for the development of type 2 diabetes in tea drinkers versus non–tea drinkers were 1.00 for those who drank less than one cup per day, 0.96 for those who had one to two cups, and 0.84 for those who drank four or more cups.
“While more research needs to be done to determine the exact dosage and mechanisms behind these observations, our findings suggest that drinking tea is beneficial in reducing the risk of type 2 diabetes, but only at high doses (at least 4 cups a day)”, said Dr. Li.
Perhaps, “we did not find an association between tea drinking and type 2 diabetes in our cohort study because we did not look at higher tea consumption,” she added.
Tempest in a teacup
“This is large, observational data. It’s not a randomized controlled trial so there’s plenty of room for data to be misunderstood,” warned Matt Sydes, MSc, professor of clinical trials & methodology at the MRC Clinical Trials Unit, University College London.
“Everyone drinks fluids. If there is an effect here (and that’s a big if), it might be not about the tea they drink, but about what they don’t drink. One can’t tell at the moment. It seems unlikely that a large randomized controlled trial could be done to disambiguate” added Dr. Sydes
“Being only a conference abstract, it is difficult to assess the quality of this research,” Baptiste Leurent, PhD, a medical statistician also working at University College London, said. Not only was the cohort study observational, so were all the other studies included in the meta-analysis, he pointed out.
“Therefore, no cause-effect conclusions can be drawn. The association could simply be due to other factors, such as those drinking more tea having a healthier lifestyle. It does not seem that the authors tried to control for confounders, which is usually difficult in meta-analysis,” Dr. Leurent said.
“There is reason to be a bit skeptical at this point; we really need to have the full details to assess it properly,” said Jonathan Cook of the Centre for Statistics in Medicine at the University of Oxford (England). “It’s a fair attempt to look at this, but not cutting edge, [using] fairly standard approaches.”
Similar studies have shown a reduced risk associated with coffee drinking, noted Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University in Birmingham.
“The important take-home message is that lifestyle is important in managing risk of developing type 2 diabetes,” Dr. Mellor said.
“That includes choosing low-calorie drinks including mainly water as well as unsweetened tea and coffee as your drinks of choice as part of a healthy lifestyle.”
The study was funded by the Young Talents Project of Hubei Provincial Health Commission, the Science and Technology Research Key Project of Education Department of Hubei Province, the Sanuo Diabetes Charity Foundation, and the Xiangyang Science and Technology Plan Project, all based in China. Dr. Li had no conflicts of interest to disclose. Dr. McConway is a Trustee and on the advisory committee of The Science Media Centre. Dr. Sattar has consulted for many companies that make diabetes and cardiovascular drugs and has been involved in multiple trials of lifestyle approaches for the prevention and remission of diabetes. Dr. Sydes, Dr. Leurent, Dr. Cook, and Dr. Mellor had no conflicts of interest to report.
type 2 diabetes/tea-drinking claim
type 2 diabetes/tea-drinking claim
FROM EASD 2022