User login
Clinical Endocrinology News is an independent news source that provides endocrinologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the endocrinologist's practice. Specialty topics include Diabetes, Lipid & Metabolic Disorders Menopause, Obesity, Osteoporosis, Pediatric Endocrinology, Pituitary, Thyroid & Adrenal Disorders, and Reproductive Endocrinology. Featured content includes Commentaries, Implementin Health Reform, Law & Medicine, and In the Loop, the blog of Clinical Endocrinology News. Clinical Endocrinology News is owned by Frontline Medical Communications.
addict
addicted
addicting
addiction
adult sites
alcohol
antibody
ass
attorney
audit
auditor
babies
babpa
baby
ban
banned
banning
best
bisexual
bitch
bleach
blog
blow job
bondage
boobs
booty
buy
cannabis
certificate
certification
certified
cheap
cheapest
class action
cocaine
cock
counterfeit drug
crack
crap
crime
criminal
cunt
curable
cure
dangerous
dangers
dead
deadly
death
defend
defended
depedent
dependence
dependent
detergent
dick
die
dildo
drug abuse
drug recall
dying
fag
fake
fatal
fatalities
fatality
free
fuck
gangs
gingivitis
guns
hardcore
herbal
herbs
heroin
herpes
home remedies
homo
horny
hypersensitivity
hypoglycemia treatment
illegal drug use
illegal use of prescription
incest
infant
infants
job
ketoacidosis
kill
killer
killing
kinky
law suit
lawsuit
lawyer
lesbian
marijuana
medicine for hypoglycemia
murder
naked
natural
newborn
nigger
noise
nude
nudity
orgy
over the counter
overdosage
overdose
overdosed
overdosing
penis
pimp
pistol
porn
porno
pornographic
pornography
prison
profanity
purchase
purchasing
pussy
queer
rape
rapist
recall
recreational drug
rob
robberies
sale
sales
sex
sexual
shit
shoot
slut
slutty
stole
stolen
store
sue
suicidal
suicide
supplements
supply company
theft
thief
thieves
tit
toddler
toddlers
toxic
toxin
tragedy
treating dka
treating hypoglycemia
treatment for hypoglycemia
vagina
violence
whore
withdrawal
without prescription
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-imn')]
div[contains(@class, 'pane-pub-home-imn')]
div[contains(@class, 'pane-pub-topic-imn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
AI takes root in primary care. First stop: Diabetic retinopathy
At a routine doctor’s visit, a member of the clinic staff takes digital pictures of a patient’s retinas.
Within seconds, an artificial intelligence (AI) algorithm determines if the patient has diabetic retinopathy, a complication of diabetes that can lead to blindness.
If they do, the physician refers the patient to an eye care specialist for further evaluation and treatment.
This scene already is playing out in primary care clinics around the United States and in other countries, and it may become more common.
In May, OSF HealthCare, a network of medical facilities headquartered in Peoria, Ill., piloted an AI system to diagnose diabetic retinopathy, a condition that affects an estimated 4 million Americans. In 2023, the health care system plans to expand the technology to 34 locations.
Meanwhile, the Food and Drug Administration in November approved a new AI system to diagnose diabetic retinopathy, making AEYE-DS from AEYE Health the third such product on the market.
Roomasa Channa, MD, a clinician-scientist with the McPherson Eye Research Institute at the University of Wisconsin–Madison, has studied the use of AI in teenage patients with diabetes. She said she soon plans to use AI screening in federally qualified health centers to screen adults with diabetes.
Dr. Channa welcomed the latest regulatory clearance and said she hopes another Food and Drug Administration–cleared algorithm product will improve accessibility to the technology.
“It is good to see more players in the field: We need this technology to be readily available and affordable,” she said in an interview.
A mixed reception
Responses from physicians to this type of AI have been mixed. Some worry, for instance, that the algorithms might be programmed with unrecognized biases that could lead them to less accurately interpret images from certain patient groups. Researchers should be on the lookout out for this possibility, Dr. Channa said.
“We need more real-world studies in different settings,” she said. “We also need to keep collecting data on AI performance post approval,” like investigators do for newly approved drugs.
The first AI system to diagnose diabetic retinopathy, IDx-DR, was approved by the FDA in 2018 and rolled out in retail clinics soon after. A second system, EyeArt, gained clearance by the agency in 2020.
Adding AI algorithms into primary care practice has changed how patients with diabetes can receive a screening. It also has introduced a new way for certain medical conditions to be diagnosed in primary care.
The American Medical Association in 2021 released a new CPT code to allow clinicians to bill government and private insurers for use of these services. CPT code 92229 refers to imaging of the retina to detect disease with an automated analysis and report at the point of care.
Meeting a need
Health care clinics in underserved areas often do not have eye care providers onsite to conduct recommended screening exams, so AI could help patients receive screening who otherwise would not get it, Dr. Channa said.
Dr. Channa and colleagues successfully used one AI system, IDx-DR, to screen children at a pediatric diabetes clinic. Over a year, screening rates jumped from 49% to 95%.
This technology “can potentially help us in decreasing disparities in care and focusing our efforts on patients with the most severe diseases,” she said.
OSF HealthCare recently obtained an approximately $1 million grant from drug company Regeneron to expand the use of AI-based screening for diabetic retinopathy, following a successful pilot. Regeneron markets a treatment for diabetic retinopathy.
Without an AI option, recommended eye screening for patients with diabetes often falls through the cracks, according to Mark Meeker, DO, vice president of community medicine at OSF. Primary care physicians may refer patients elsewhere for their annual retinopathy screening exam.
“That often doesn’t get completed because it’s another trip, another appointment, another time away from work,” Dr. Meeker said.
All patients with diabetes should have their eyes screened each year, but between one- to two-thirds of patients nationwide do not, he said.
A member of the clinic staff takes digital pictures of the retina, almost always through undilated pupils.
If the result is normal, the patient is scheduled for another follow-up screening in a year. If early signs of diabetic retinopathy are spotted, patients are referred to an eye care specialist.
After 7 months of the pilot program, OSF had screened about 350 patients. Approximately 20% had diabetic retinopathy, according to OSF.
‘A huge impact’
OSF has about 66,000 patients with diabetes. About two-thirds do not receive annual screening, Dr. Meeker estimated. “This can have a huge impact on the quality of life in the coming years for our diabetic patients. It’s pretty profound.”
Eye care specialists typically treat diabetic retinopathy with lasers, surgery, or medication. For primary care clinicians, however, AI screening for retinopathy is an opportunity to emphasize how important it is to manage the disease and what its consequences can be.
AI screening is “another tool for us to use to get patients more engaged in their own care,” Dr. Meeker said. “This is probably the biggest advance in AI affecting our day-to-day interaction with patients that we’ve seen in primary care.”
A business opportunity, too?
The IDx-DR platform OSF is using in its clinics is owned by the company Digital Diagnostics. OSF Ventures, an investment arm of OSF HealthCare, has invested in the company, the health care system announced in August.
Other companies have had their products used in practice. In 2019, for example, Eyenuk described how its EyeArt system had been used to screen thousands of patients in Germany and in Italy.
And in 2021, Eyenuk reported that its customer base in the United States had expanded to more than 25 locations. The company credited a Centers for Medicare & Medicaid Services plan to cover CPT code 92229 with supporting this growth.
Zack Dvey-Aharon, PhD, the CEO of AEYE Health, said the company was motivated to enter this space when regulators decided that AI could be used to diagnose a condition — not just as a tool to help doctors arrive at a diagnosis.
With proper training, a person can diagnose diabetic retinopathy relatively easily if the image of the retina is of excellent quality. If image is dark or blurry, however, it’s a different story.
AI has its advantages in this scenario, according to Dr. Dvey-Aharon. “For AI, those darker, more blurry images are actually highly readable with fantastic accuracy.”
More to come?
The possibilities of AI in analyzing retinal images are vast.
New research shows that AI may be able to detect Alzheimer’s disease or predict a person’s risk for heart attack and stroke based on snapshots of the retina.
The retina may also shed light on kidney disease, control of blood glucose and blood pressure, hepatobiliary disease, and coronary artery calcium, according to Eric J. Topol, MD, director of Scripps Research Translational Institute in La Jolla, Calif.
Beyond retinas, interpretation of electrocardiograms (ECGs) may be another frontier for AI in primary care. In one trial, an AI-enhanced ECG reading facilitated early diagnosis of low ejection fraction, and some doctors now receive these reports routinely, Dr. Topol wrote.
The potential value of AI in medicine “extends to virtually all forms of medical images that have been assessed to date,” Dr. Topol wrote on his “Ground Truths” Substack.
Although much of the focus has been on what AI can see, researchers also are exploring what AI can do with what it hears. Early research suggests that algorithms may be able to diagnose disease by analyzing patients’ voices.
A version of this article first appeared on Medscape.com.
At a routine doctor’s visit, a member of the clinic staff takes digital pictures of a patient’s retinas.
Within seconds, an artificial intelligence (AI) algorithm determines if the patient has diabetic retinopathy, a complication of diabetes that can lead to blindness.
If they do, the physician refers the patient to an eye care specialist for further evaluation and treatment.
This scene already is playing out in primary care clinics around the United States and in other countries, and it may become more common.
In May, OSF HealthCare, a network of medical facilities headquartered in Peoria, Ill., piloted an AI system to diagnose diabetic retinopathy, a condition that affects an estimated 4 million Americans. In 2023, the health care system plans to expand the technology to 34 locations.
Meanwhile, the Food and Drug Administration in November approved a new AI system to diagnose diabetic retinopathy, making AEYE-DS from AEYE Health the third such product on the market.
Roomasa Channa, MD, a clinician-scientist with the McPherson Eye Research Institute at the University of Wisconsin–Madison, has studied the use of AI in teenage patients with diabetes. She said she soon plans to use AI screening in federally qualified health centers to screen adults with diabetes.
Dr. Channa welcomed the latest regulatory clearance and said she hopes another Food and Drug Administration–cleared algorithm product will improve accessibility to the technology.
“It is good to see more players in the field: We need this technology to be readily available and affordable,” she said in an interview.
A mixed reception
Responses from physicians to this type of AI have been mixed. Some worry, for instance, that the algorithms might be programmed with unrecognized biases that could lead them to less accurately interpret images from certain patient groups. Researchers should be on the lookout out for this possibility, Dr. Channa said.
“We need more real-world studies in different settings,” she said. “We also need to keep collecting data on AI performance post approval,” like investigators do for newly approved drugs.
The first AI system to diagnose diabetic retinopathy, IDx-DR, was approved by the FDA in 2018 and rolled out in retail clinics soon after. A second system, EyeArt, gained clearance by the agency in 2020.
Adding AI algorithms into primary care practice has changed how patients with diabetes can receive a screening. It also has introduced a new way for certain medical conditions to be diagnosed in primary care.
The American Medical Association in 2021 released a new CPT code to allow clinicians to bill government and private insurers for use of these services. CPT code 92229 refers to imaging of the retina to detect disease with an automated analysis and report at the point of care.
Meeting a need
Health care clinics in underserved areas often do not have eye care providers onsite to conduct recommended screening exams, so AI could help patients receive screening who otherwise would not get it, Dr. Channa said.
Dr. Channa and colleagues successfully used one AI system, IDx-DR, to screen children at a pediatric diabetes clinic. Over a year, screening rates jumped from 49% to 95%.
This technology “can potentially help us in decreasing disparities in care and focusing our efforts on patients with the most severe diseases,” she said.
OSF HealthCare recently obtained an approximately $1 million grant from drug company Regeneron to expand the use of AI-based screening for diabetic retinopathy, following a successful pilot. Regeneron markets a treatment for diabetic retinopathy.
Without an AI option, recommended eye screening for patients with diabetes often falls through the cracks, according to Mark Meeker, DO, vice president of community medicine at OSF. Primary care physicians may refer patients elsewhere for their annual retinopathy screening exam.
“That often doesn’t get completed because it’s another trip, another appointment, another time away from work,” Dr. Meeker said.
All patients with diabetes should have their eyes screened each year, but between one- to two-thirds of patients nationwide do not, he said.
A member of the clinic staff takes digital pictures of the retina, almost always through undilated pupils.
If the result is normal, the patient is scheduled for another follow-up screening in a year. If early signs of diabetic retinopathy are spotted, patients are referred to an eye care specialist.
After 7 months of the pilot program, OSF had screened about 350 patients. Approximately 20% had diabetic retinopathy, according to OSF.
‘A huge impact’
OSF has about 66,000 patients with diabetes. About two-thirds do not receive annual screening, Dr. Meeker estimated. “This can have a huge impact on the quality of life in the coming years for our diabetic patients. It’s pretty profound.”
Eye care specialists typically treat diabetic retinopathy with lasers, surgery, or medication. For primary care clinicians, however, AI screening for retinopathy is an opportunity to emphasize how important it is to manage the disease and what its consequences can be.
AI screening is “another tool for us to use to get patients more engaged in their own care,” Dr. Meeker said. “This is probably the biggest advance in AI affecting our day-to-day interaction with patients that we’ve seen in primary care.”
A business opportunity, too?
The IDx-DR platform OSF is using in its clinics is owned by the company Digital Diagnostics. OSF Ventures, an investment arm of OSF HealthCare, has invested in the company, the health care system announced in August.
Other companies have had their products used in practice. In 2019, for example, Eyenuk described how its EyeArt system had been used to screen thousands of patients in Germany and in Italy.
And in 2021, Eyenuk reported that its customer base in the United States had expanded to more than 25 locations. The company credited a Centers for Medicare & Medicaid Services plan to cover CPT code 92229 with supporting this growth.
Zack Dvey-Aharon, PhD, the CEO of AEYE Health, said the company was motivated to enter this space when regulators decided that AI could be used to diagnose a condition — not just as a tool to help doctors arrive at a diagnosis.
With proper training, a person can diagnose diabetic retinopathy relatively easily if the image of the retina is of excellent quality. If image is dark or blurry, however, it’s a different story.
AI has its advantages in this scenario, according to Dr. Dvey-Aharon. “For AI, those darker, more blurry images are actually highly readable with fantastic accuracy.”
More to come?
The possibilities of AI in analyzing retinal images are vast.
New research shows that AI may be able to detect Alzheimer’s disease or predict a person’s risk for heart attack and stroke based on snapshots of the retina.
The retina may also shed light on kidney disease, control of blood glucose and blood pressure, hepatobiliary disease, and coronary artery calcium, according to Eric J. Topol, MD, director of Scripps Research Translational Institute in La Jolla, Calif.
Beyond retinas, interpretation of electrocardiograms (ECGs) may be another frontier for AI in primary care. In one trial, an AI-enhanced ECG reading facilitated early diagnosis of low ejection fraction, and some doctors now receive these reports routinely, Dr. Topol wrote.
The potential value of AI in medicine “extends to virtually all forms of medical images that have been assessed to date,” Dr. Topol wrote on his “Ground Truths” Substack.
Although much of the focus has been on what AI can see, researchers also are exploring what AI can do with what it hears. Early research suggests that algorithms may be able to diagnose disease by analyzing patients’ voices.
A version of this article first appeared on Medscape.com.
At a routine doctor’s visit, a member of the clinic staff takes digital pictures of a patient’s retinas.
Within seconds, an artificial intelligence (AI) algorithm determines if the patient has diabetic retinopathy, a complication of diabetes that can lead to blindness.
If they do, the physician refers the patient to an eye care specialist for further evaluation and treatment.
This scene already is playing out in primary care clinics around the United States and in other countries, and it may become more common.
In May, OSF HealthCare, a network of medical facilities headquartered in Peoria, Ill., piloted an AI system to diagnose diabetic retinopathy, a condition that affects an estimated 4 million Americans. In 2023, the health care system plans to expand the technology to 34 locations.
Meanwhile, the Food and Drug Administration in November approved a new AI system to diagnose diabetic retinopathy, making AEYE-DS from AEYE Health the third such product on the market.
Roomasa Channa, MD, a clinician-scientist with the McPherson Eye Research Institute at the University of Wisconsin–Madison, has studied the use of AI in teenage patients with diabetes. She said she soon plans to use AI screening in federally qualified health centers to screen adults with diabetes.
Dr. Channa welcomed the latest regulatory clearance and said she hopes another Food and Drug Administration–cleared algorithm product will improve accessibility to the technology.
“It is good to see more players in the field: We need this technology to be readily available and affordable,” she said in an interview.
A mixed reception
Responses from physicians to this type of AI have been mixed. Some worry, for instance, that the algorithms might be programmed with unrecognized biases that could lead them to less accurately interpret images from certain patient groups. Researchers should be on the lookout out for this possibility, Dr. Channa said.
“We need more real-world studies in different settings,” she said. “We also need to keep collecting data on AI performance post approval,” like investigators do for newly approved drugs.
The first AI system to diagnose diabetic retinopathy, IDx-DR, was approved by the FDA in 2018 and rolled out in retail clinics soon after. A second system, EyeArt, gained clearance by the agency in 2020.
Adding AI algorithms into primary care practice has changed how patients with diabetes can receive a screening. It also has introduced a new way for certain medical conditions to be diagnosed in primary care.
The American Medical Association in 2021 released a new CPT code to allow clinicians to bill government and private insurers for use of these services. CPT code 92229 refers to imaging of the retina to detect disease with an automated analysis and report at the point of care.
Meeting a need
Health care clinics in underserved areas often do not have eye care providers onsite to conduct recommended screening exams, so AI could help patients receive screening who otherwise would not get it, Dr. Channa said.
Dr. Channa and colleagues successfully used one AI system, IDx-DR, to screen children at a pediatric diabetes clinic. Over a year, screening rates jumped from 49% to 95%.
This technology “can potentially help us in decreasing disparities in care and focusing our efforts on patients with the most severe diseases,” she said.
OSF HealthCare recently obtained an approximately $1 million grant from drug company Regeneron to expand the use of AI-based screening for diabetic retinopathy, following a successful pilot. Regeneron markets a treatment for diabetic retinopathy.
Without an AI option, recommended eye screening for patients with diabetes often falls through the cracks, according to Mark Meeker, DO, vice president of community medicine at OSF. Primary care physicians may refer patients elsewhere for their annual retinopathy screening exam.
“That often doesn’t get completed because it’s another trip, another appointment, another time away from work,” Dr. Meeker said.
All patients with diabetes should have their eyes screened each year, but between one- to two-thirds of patients nationwide do not, he said.
A member of the clinic staff takes digital pictures of the retina, almost always through undilated pupils.
If the result is normal, the patient is scheduled for another follow-up screening in a year. If early signs of diabetic retinopathy are spotted, patients are referred to an eye care specialist.
After 7 months of the pilot program, OSF had screened about 350 patients. Approximately 20% had diabetic retinopathy, according to OSF.
‘A huge impact’
OSF has about 66,000 patients with diabetes. About two-thirds do not receive annual screening, Dr. Meeker estimated. “This can have a huge impact on the quality of life in the coming years for our diabetic patients. It’s pretty profound.”
Eye care specialists typically treat diabetic retinopathy with lasers, surgery, or medication. For primary care clinicians, however, AI screening for retinopathy is an opportunity to emphasize how important it is to manage the disease and what its consequences can be.
AI screening is “another tool for us to use to get patients more engaged in their own care,” Dr. Meeker said. “This is probably the biggest advance in AI affecting our day-to-day interaction with patients that we’ve seen in primary care.”
A business opportunity, too?
The IDx-DR platform OSF is using in its clinics is owned by the company Digital Diagnostics. OSF Ventures, an investment arm of OSF HealthCare, has invested in the company, the health care system announced in August.
Other companies have had their products used in practice. In 2019, for example, Eyenuk described how its EyeArt system had been used to screen thousands of patients in Germany and in Italy.
And in 2021, Eyenuk reported that its customer base in the United States had expanded to more than 25 locations. The company credited a Centers for Medicare & Medicaid Services plan to cover CPT code 92229 with supporting this growth.
Zack Dvey-Aharon, PhD, the CEO of AEYE Health, said the company was motivated to enter this space when regulators decided that AI could be used to diagnose a condition — not just as a tool to help doctors arrive at a diagnosis.
With proper training, a person can diagnose diabetic retinopathy relatively easily if the image of the retina is of excellent quality. If image is dark or blurry, however, it’s a different story.
AI has its advantages in this scenario, according to Dr. Dvey-Aharon. “For AI, those darker, more blurry images are actually highly readable with fantastic accuracy.”
More to come?
The possibilities of AI in analyzing retinal images are vast.
New research shows that AI may be able to detect Alzheimer’s disease or predict a person’s risk for heart attack and stroke based on snapshots of the retina.
The retina may also shed light on kidney disease, control of blood glucose and blood pressure, hepatobiliary disease, and coronary artery calcium, according to Eric J. Topol, MD, director of Scripps Research Translational Institute in La Jolla, Calif.
Beyond retinas, interpretation of electrocardiograms (ECGs) may be another frontier for AI in primary care. In one trial, an AI-enhanced ECG reading facilitated early diagnosis of low ejection fraction, and some doctors now receive these reports routinely, Dr. Topol wrote.
The potential value of AI in medicine “extends to virtually all forms of medical images that have been assessed to date,” Dr. Topol wrote on his “Ground Truths” Substack.
Although much of the focus has been on what AI can see, researchers also are exploring what AI can do with what it hears. Early research suggests that algorithms may be able to diagnose disease by analyzing patients’ voices.
A version of this article first appeared on Medscape.com.
Not all children with type 2 diabetes have obesity
Obesity is not a universal phenotype in children with type 2 diabetes (T2D), a global systematic review and meta-analysis reported. In fact, the study found, as many as one in four children with T2D do not have obesity and some have normal reference-range body mass measurements. Further studies should consider other mechanisms beyond obesity in the genesis of pediatric diabetes, the authors of the international analysis concluded, writing for JAMA Network Open.
“We were aware that some children and adolescents with T2D did not have obesity, but we didn’t know the scale of obesity in T2D, or what variables may impact the occurrence of diabetes in this group,” endocrinologist M. Constantine Samaan, MD, MSc, associate professor of pediatrics at McMaster University in Hamilton, Ont., told this news organization. “So, the analysis did help us understand the body mass distribution of this group in more detail.”
The international investigators included in their meta-analysis 53 articles with 8,942 participants from multiple world regions and races/ethnicities. The overall prevalence of obesity in pediatric patients with T2D was 75.27% (95% confidence interval [CI], 70.47%-79.78%). The prevalence of obesity at time of diagnosis in 4,688 participants was 77.24% (95% CI, 70.55%-83.34%). Male participants had higher odds of obesity than females: odds ratio, 2.10 (95% CI, 1.33-3.31) – although girls are generally more likely to develop T2D. The highest prevalence of obesity occurred in Whites at 89.86% (95% CI, 71.50%-99.74%), while prevalence was lowest in Asian participants at 64.50% (95% CI, 53.28%-74.99%).
The authors noted that childhood obesity affects approximately 340 million children worldwide and is a major driver of pediatric T2D, an aggressive disease with a high treatment failure rate. Understanding the contribution of body mass to the evolution of insulin resistance, glucose intolerance, and T2D with its attendant comorbidities and complications, such as nonalcoholic fatty liver disease, remains crucial for developing personalized interventions.
Known risk factors for T2D include interactions between genetics and the environment, including lifestyle factors such as diet and low physical activity levels, Dr. Samaan noted. Certain ethnic groups have higher T2D risks, as do babies exposed in the womb to maternal obesity or diabetes, he said. “And there are likely many other factors that contribute to the risk of T2D, though these remain to be defined.”
Is “lean” T2D in children without obesity likely then to be hereditary, more severe, and harder to control with lifestyle modification? “That’s a great question, but the answer is we don’t know,” Dr. Samaan said.
Commenting on the study but not involved in it, Timothy J. Joos, MD, a pediatrician in Seattle affiliated with the Swedish Medical Center, said the findings raise the question of how many pediatric T2D patients are being missed because they don’t meet current screening criteria. “In nonobese T2D pediatric patients, genetics (and by proxy family history) obviously play a heavier role. In my practice, I often get parents asking me to screen their skinny teenager for diabetes because of diabetes in a family member. In the past I would begrudgingly comply with a smirk on my face. Now the smirk will be gone.”
Dr. Joos said it would be interesting to see what percentage of these T2D patients without obesity (body mass index < 95th percentile) would still meet the criteria for being overweight (BMI > 85th percentile) as this is the primary criterion for screening according to the American Diabetes Association guidelines.
Current guidelines generally look for elevated body mass measures as a main screening indication, Dr. Samaan’s group noted. But in their view, while factors such as ethnicity and in utero exposure to diabetes are already used in combination with BMI-based measures to justify screening, more sophisticated prediabetes and diabetes prediction models are needed to support a more comprehensive screening approach.
“Because being overweight is the initial criterion, children with multiple other criteria are not being screened,” Dr. Joos said. He agreed that more research is needed to sort out the other risk factors for pediatric T2D without obesity so these patients may be detected earlier.
New models may need to incorporate lifestyle factors, hormones, puberty, growth, and sex as well, the authors wrote. Markers of insulin resistance, insulin production capacity, and other markers are needed to refine the identification of those who should be screened.
Dr. Samaan’s group is planning to study the findings in more detail to clarify the effect of body mass on the comorbidities and complications of pediatric T2D.
In addition to the study limitation of significant interstudy heterogeneity, the authors acknowledged varying degrees of glycemic control and dyslipidemia among participants.
No specific funding was provided for this review and meta-analysis. The authors disclosed no conflicts of interest. Dr. Joos disclosed no competing interests with regard to his comments.
Obesity is not a universal phenotype in children with type 2 diabetes (T2D), a global systematic review and meta-analysis reported. In fact, the study found, as many as one in four children with T2D do not have obesity and some have normal reference-range body mass measurements. Further studies should consider other mechanisms beyond obesity in the genesis of pediatric diabetes, the authors of the international analysis concluded, writing for JAMA Network Open.
“We were aware that some children and adolescents with T2D did not have obesity, but we didn’t know the scale of obesity in T2D, or what variables may impact the occurrence of diabetes in this group,” endocrinologist M. Constantine Samaan, MD, MSc, associate professor of pediatrics at McMaster University in Hamilton, Ont., told this news organization. “So, the analysis did help us understand the body mass distribution of this group in more detail.”
The international investigators included in their meta-analysis 53 articles with 8,942 participants from multiple world regions and races/ethnicities. The overall prevalence of obesity in pediatric patients with T2D was 75.27% (95% confidence interval [CI], 70.47%-79.78%). The prevalence of obesity at time of diagnosis in 4,688 participants was 77.24% (95% CI, 70.55%-83.34%). Male participants had higher odds of obesity than females: odds ratio, 2.10 (95% CI, 1.33-3.31) – although girls are generally more likely to develop T2D. The highest prevalence of obesity occurred in Whites at 89.86% (95% CI, 71.50%-99.74%), while prevalence was lowest in Asian participants at 64.50% (95% CI, 53.28%-74.99%).
The authors noted that childhood obesity affects approximately 340 million children worldwide and is a major driver of pediatric T2D, an aggressive disease with a high treatment failure rate. Understanding the contribution of body mass to the evolution of insulin resistance, glucose intolerance, and T2D with its attendant comorbidities and complications, such as nonalcoholic fatty liver disease, remains crucial for developing personalized interventions.
Known risk factors for T2D include interactions between genetics and the environment, including lifestyle factors such as diet and low physical activity levels, Dr. Samaan noted. Certain ethnic groups have higher T2D risks, as do babies exposed in the womb to maternal obesity or diabetes, he said. “And there are likely many other factors that contribute to the risk of T2D, though these remain to be defined.”
Is “lean” T2D in children without obesity likely then to be hereditary, more severe, and harder to control with lifestyle modification? “That’s a great question, but the answer is we don’t know,” Dr. Samaan said.
Commenting on the study but not involved in it, Timothy J. Joos, MD, a pediatrician in Seattle affiliated with the Swedish Medical Center, said the findings raise the question of how many pediatric T2D patients are being missed because they don’t meet current screening criteria. “In nonobese T2D pediatric patients, genetics (and by proxy family history) obviously play a heavier role. In my practice, I often get parents asking me to screen their skinny teenager for diabetes because of diabetes in a family member. In the past I would begrudgingly comply with a smirk on my face. Now the smirk will be gone.”
Dr. Joos said it would be interesting to see what percentage of these T2D patients without obesity (body mass index < 95th percentile) would still meet the criteria for being overweight (BMI > 85th percentile) as this is the primary criterion for screening according to the American Diabetes Association guidelines.
Current guidelines generally look for elevated body mass measures as a main screening indication, Dr. Samaan’s group noted. But in their view, while factors such as ethnicity and in utero exposure to diabetes are already used in combination with BMI-based measures to justify screening, more sophisticated prediabetes and diabetes prediction models are needed to support a more comprehensive screening approach.
“Because being overweight is the initial criterion, children with multiple other criteria are not being screened,” Dr. Joos said. He agreed that more research is needed to sort out the other risk factors for pediatric T2D without obesity so these patients may be detected earlier.
New models may need to incorporate lifestyle factors, hormones, puberty, growth, and sex as well, the authors wrote. Markers of insulin resistance, insulin production capacity, and other markers are needed to refine the identification of those who should be screened.
Dr. Samaan’s group is planning to study the findings in more detail to clarify the effect of body mass on the comorbidities and complications of pediatric T2D.
In addition to the study limitation of significant interstudy heterogeneity, the authors acknowledged varying degrees of glycemic control and dyslipidemia among participants.
No specific funding was provided for this review and meta-analysis. The authors disclosed no conflicts of interest. Dr. Joos disclosed no competing interests with regard to his comments.
Obesity is not a universal phenotype in children with type 2 diabetes (T2D), a global systematic review and meta-analysis reported. In fact, the study found, as many as one in four children with T2D do not have obesity and some have normal reference-range body mass measurements. Further studies should consider other mechanisms beyond obesity in the genesis of pediatric diabetes, the authors of the international analysis concluded, writing for JAMA Network Open.
“We were aware that some children and adolescents with T2D did not have obesity, but we didn’t know the scale of obesity in T2D, or what variables may impact the occurrence of diabetes in this group,” endocrinologist M. Constantine Samaan, MD, MSc, associate professor of pediatrics at McMaster University in Hamilton, Ont., told this news organization. “So, the analysis did help us understand the body mass distribution of this group in more detail.”
The international investigators included in their meta-analysis 53 articles with 8,942 participants from multiple world regions and races/ethnicities. The overall prevalence of obesity in pediatric patients with T2D was 75.27% (95% confidence interval [CI], 70.47%-79.78%). The prevalence of obesity at time of diagnosis in 4,688 participants was 77.24% (95% CI, 70.55%-83.34%). Male participants had higher odds of obesity than females: odds ratio, 2.10 (95% CI, 1.33-3.31) – although girls are generally more likely to develop T2D. The highest prevalence of obesity occurred in Whites at 89.86% (95% CI, 71.50%-99.74%), while prevalence was lowest in Asian participants at 64.50% (95% CI, 53.28%-74.99%).
The authors noted that childhood obesity affects approximately 340 million children worldwide and is a major driver of pediatric T2D, an aggressive disease with a high treatment failure rate. Understanding the contribution of body mass to the evolution of insulin resistance, glucose intolerance, and T2D with its attendant comorbidities and complications, such as nonalcoholic fatty liver disease, remains crucial for developing personalized interventions.
Known risk factors for T2D include interactions between genetics and the environment, including lifestyle factors such as diet and low physical activity levels, Dr. Samaan noted. Certain ethnic groups have higher T2D risks, as do babies exposed in the womb to maternal obesity or diabetes, he said. “And there are likely many other factors that contribute to the risk of T2D, though these remain to be defined.”
Is “lean” T2D in children without obesity likely then to be hereditary, more severe, and harder to control with lifestyle modification? “That’s a great question, but the answer is we don’t know,” Dr. Samaan said.
Commenting on the study but not involved in it, Timothy J. Joos, MD, a pediatrician in Seattle affiliated with the Swedish Medical Center, said the findings raise the question of how many pediatric T2D patients are being missed because they don’t meet current screening criteria. “In nonobese T2D pediatric patients, genetics (and by proxy family history) obviously play a heavier role. In my practice, I often get parents asking me to screen their skinny teenager for diabetes because of diabetes in a family member. In the past I would begrudgingly comply with a smirk on my face. Now the smirk will be gone.”
Dr. Joos said it would be interesting to see what percentage of these T2D patients without obesity (body mass index < 95th percentile) would still meet the criteria for being overweight (BMI > 85th percentile) as this is the primary criterion for screening according to the American Diabetes Association guidelines.
Current guidelines generally look for elevated body mass measures as a main screening indication, Dr. Samaan’s group noted. But in their view, while factors such as ethnicity and in utero exposure to diabetes are already used in combination with BMI-based measures to justify screening, more sophisticated prediabetes and diabetes prediction models are needed to support a more comprehensive screening approach.
“Because being overweight is the initial criterion, children with multiple other criteria are not being screened,” Dr. Joos said. He agreed that more research is needed to sort out the other risk factors for pediatric T2D without obesity so these patients may be detected earlier.
New models may need to incorporate lifestyle factors, hormones, puberty, growth, and sex as well, the authors wrote. Markers of insulin resistance, insulin production capacity, and other markers are needed to refine the identification of those who should be screened.
Dr. Samaan’s group is planning to study the findings in more detail to clarify the effect of body mass on the comorbidities and complications of pediatric T2D.
In addition to the study limitation of significant interstudy heterogeneity, the authors acknowledged varying degrees of glycemic control and dyslipidemia among participants.
No specific funding was provided for this review and meta-analysis. The authors disclosed no conflicts of interest. Dr. Joos disclosed no competing interests with regard to his comments.
FROM JAMA NETWORK OPEN
Chlorthalidone, HCTZ equally effective in hypertension: DCP published
The Diuretic Comparison Project (DCP) trial, showing no difference in reduction of clinical events between the thiazide diuretics chlorthalidone and hydrochlorothiazide when used for the treatment of hypertension, has now been published.
The trial was first presented at the 2022 annual scientific sessions of the American Heart Association.
In the current paper, published online in the New England Journal of Medicine, the authors, led by Areef Ishani, MD, Minneapolis Veterans Affairs Health Care System, explained that early studies suggested that chlorthalidone was superior to hydrochlorothiazide in patients with hypertension, but more recent observational studies have shown that the two drugs reduced cardiovascular events at a similar rate. Chlorthalidone may be associated with an increased risk of adverse events, including hypokalemia.
They noted that, in 2020, Part D Medicare expenditures showed that approximately 1.5 million persons received prescriptions for chlorthalidone, compared with 11.5 million who received prescriptions for hydrochlorothiazide, despite guidelines that recommended chlorthalidone as the preferred agent. The discrepancy between guideline recommendation and real-world use is possibly related to the belief that chlorthalidone has a greater risk for adverse effects without clear evidence for differences in cardiovascular outcomes, the authors suggested.
They conducted the current study to directly compare the effect of the two agents on cardiovascular outcomes in patients with hypertension.
The pragmatic DCP trial was carried out within the VA Healthcare System, and randomly assigned 13,523 patients (mean age, 72.5 years) with hypertension who were receiving hydrochlorothiazide at baseline (25 or 50 mg per day) to continue hydrochlorothiazide at their baseline dose or to switch to chlorthalidone (12.5 or 25 mg per day).
The mean baseline systolic blood pressure was 139 mm Hg in both trial groups and did not change substantially during the trial.
Over a median follow-up of 2.4 years, there was no difference in the primary outcome – a composite of MI, stroke, hospitalization for heart failure, urgent coronary revascularization for unstable angina, and non–cancer-related death – between the chlorthalidone group (10.4%) and the hydrochlorothiazide group (10.0%), giving a hazard ratio of 1.04 (95% CI, 0.94-1.16; P = .45).
In addition, there were no treatment differences between the two groups in any primary outcome component. Hypokalemia and potassium supplement use were more common in the chlorthalidone group than in the hydrochlorothiazide group.
‘Importance lies in the design’
In an accompanying editorial, Julie R. Ingelfinger, MD, deputy editor of the New England Journal of Medicine, said the results are not surprising and may not change clinical practice. But she suggested that the importance of the trial lies in its design, which shows that a high-quality pragmatic comparative effectiveness trial can be accomplished in a cost-effective manner within a health care system with little disruption in patient care.
Dr. Ingelfinger pointed out several limitations of the trial. These include a lower-than-expected occurrence of primary outcome events, and the stipulation that patients were eligible to participate only if they continued to have hypertension while receiving hydrochlorothiazide.
In addition, 95% of the participants were receiving 25 mg of hydrochlorothiazide and only 5% were receiving 50 mg, which limited comparisons of the dose generally used in practice. Also, only approximately 13% of the patients were receiving hydrochlorothiazide alone for the treatment of hypertension at baseline.
She noted that the DCP is the first head-to-head comparison of hydrochlorothiazide and chlorthalidone in a randomized, prospective outcome trial.
“Without an apparent difference in the hazard ratios for the primary outcome in the two groups over the median follow-up of 2.4 years, results suggest that chlorthalidone therapy remains a good choice for hypertension despite the secondary observation that hypokalemia was more common with chlorthalidone than with hydrochlorothiazide,” Dr. Ingelfinger said.
“Although a subgroup analysis suggested that chlorthalidone was better than hydrochlorothiazide for participants with a history of myocardial infarction or stroke, that result may have been by chance,” she added.
As clinicians generally prefer using hydrochlorothiazide, she suggested that these DCP results will not provide any impetus for change.
“Furthermore, combined therapy and polypills may alter therapy beyond the results of this well-done, highly anticipated trial. Thus, its major effect may be as a model for other pragmatic study programs, which are greatly needed,” she concluded.
This study was supported by the Veterans Affairs Cooperative Studies Program through a grant to the Diuretic Comparison Project. Dr. Ishani reported no relevant financial relationships. Dr. Ingelfinger reported book royalties from Springer and from St. Martin’s Press, outside the submitted work, and that she is employed by the New England Journal of Medicine as deputy editor.
A version of this article first appeared on Medscape.com.
The Diuretic Comparison Project (DCP) trial, showing no difference in reduction of clinical events between the thiazide diuretics chlorthalidone and hydrochlorothiazide when used for the treatment of hypertension, has now been published.
The trial was first presented at the 2022 annual scientific sessions of the American Heart Association.
In the current paper, published online in the New England Journal of Medicine, the authors, led by Areef Ishani, MD, Minneapolis Veterans Affairs Health Care System, explained that early studies suggested that chlorthalidone was superior to hydrochlorothiazide in patients with hypertension, but more recent observational studies have shown that the two drugs reduced cardiovascular events at a similar rate. Chlorthalidone may be associated with an increased risk of adverse events, including hypokalemia.
They noted that, in 2020, Part D Medicare expenditures showed that approximately 1.5 million persons received prescriptions for chlorthalidone, compared with 11.5 million who received prescriptions for hydrochlorothiazide, despite guidelines that recommended chlorthalidone as the preferred agent. The discrepancy between guideline recommendation and real-world use is possibly related to the belief that chlorthalidone has a greater risk for adverse effects without clear evidence for differences in cardiovascular outcomes, the authors suggested.
They conducted the current study to directly compare the effect of the two agents on cardiovascular outcomes in patients with hypertension.
The pragmatic DCP trial was carried out within the VA Healthcare System, and randomly assigned 13,523 patients (mean age, 72.5 years) with hypertension who were receiving hydrochlorothiazide at baseline (25 or 50 mg per day) to continue hydrochlorothiazide at their baseline dose or to switch to chlorthalidone (12.5 or 25 mg per day).
The mean baseline systolic blood pressure was 139 mm Hg in both trial groups and did not change substantially during the trial.
Over a median follow-up of 2.4 years, there was no difference in the primary outcome – a composite of MI, stroke, hospitalization for heart failure, urgent coronary revascularization for unstable angina, and non–cancer-related death – between the chlorthalidone group (10.4%) and the hydrochlorothiazide group (10.0%), giving a hazard ratio of 1.04 (95% CI, 0.94-1.16; P = .45).
In addition, there were no treatment differences between the two groups in any primary outcome component. Hypokalemia and potassium supplement use were more common in the chlorthalidone group than in the hydrochlorothiazide group.
‘Importance lies in the design’
In an accompanying editorial, Julie R. Ingelfinger, MD, deputy editor of the New England Journal of Medicine, said the results are not surprising and may not change clinical practice. But she suggested that the importance of the trial lies in its design, which shows that a high-quality pragmatic comparative effectiveness trial can be accomplished in a cost-effective manner within a health care system with little disruption in patient care.
Dr. Ingelfinger pointed out several limitations of the trial. These include a lower-than-expected occurrence of primary outcome events, and the stipulation that patients were eligible to participate only if they continued to have hypertension while receiving hydrochlorothiazide.
In addition, 95% of the participants were receiving 25 mg of hydrochlorothiazide and only 5% were receiving 50 mg, which limited comparisons of the dose generally used in practice. Also, only approximately 13% of the patients were receiving hydrochlorothiazide alone for the treatment of hypertension at baseline.
She noted that the DCP is the first head-to-head comparison of hydrochlorothiazide and chlorthalidone in a randomized, prospective outcome trial.
“Without an apparent difference in the hazard ratios for the primary outcome in the two groups over the median follow-up of 2.4 years, results suggest that chlorthalidone therapy remains a good choice for hypertension despite the secondary observation that hypokalemia was more common with chlorthalidone than with hydrochlorothiazide,” Dr. Ingelfinger said.
“Although a subgroup analysis suggested that chlorthalidone was better than hydrochlorothiazide for participants with a history of myocardial infarction or stroke, that result may have been by chance,” she added.
As clinicians generally prefer using hydrochlorothiazide, she suggested that these DCP results will not provide any impetus for change.
“Furthermore, combined therapy and polypills may alter therapy beyond the results of this well-done, highly anticipated trial. Thus, its major effect may be as a model for other pragmatic study programs, which are greatly needed,” she concluded.
This study was supported by the Veterans Affairs Cooperative Studies Program through a grant to the Diuretic Comparison Project. Dr. Ishani reported no relevant financial relationships. Dr. Ingelfinger reported book royalties from Springer and from St. Martin’s Press, outside the submitted work, and that she is employed by the New England Journal of Medicine as deputy editor.
A version of this article first appeared on Medscape.com.
The Diuretic Comparison Project (DCP) trial, showing no difference in reduction of clinical events between the thiazide diuretics chlorthalidone and hydrochlorothiazide when used for the treatment of hypertension, has now been published.
The trial was first presented at the 2022 annual scientific sessions of the American Heart Association.
In the current paper, published online in the New England Journal of Medicine, the authors, led by Areef Ishani, MD, Minneapolis Veterans Affairs Health Care System, explained that early studies suggested that chlorthalidone was superior to hydrochlorothiazide in patients with hypertension, but more recent observational studies have shown that the two drugs reduced cardiovascular events at a similar rate. Chlorthalidone may be associated with an increased risk of adverse events, including hypokalemia.
They noted that, in 2020, Part D Medicare expenditures showed that approximately 1.5 million persons received prescriptions for chlorthalidone, compared with 11.5 million who received prescriptions for hydrochlorothiazide, despite guidelines that recommended chlorthalidone as the preferred agent. The discrepancy between guideline recommendation and real-world use is possibly related to the belief that chlorthalidone has a greater risk for adverse effects without clear evidence for differences in cardiovascular outcomes, the authors suggested.
They conducted the current study to directly compare the effect of the two agents on cardiovascular outcomes in patients with hypertension.
The pragmatic DCP trial was carried out within the VA Healthcare System, and randomly assigned 13,523 patients (mean age, 72.5 years) with hypertension who were receiving hydrochlorothiazide at baseline (25 or 50 mg per day) to continue hydrochlorothiazide at their baseline dose or to switch to chlorthalidone (12.5 or 25 mg per day).
The mean baseline systolic blood pressure was 139 mm Hg in both trial groups and did not change substantially during the trial.
Over a median follow-up of 2.4 years, there was no difference in the primary outcome – a composite of MI, stroke, hospitalization for heart failure, urgent coronary revascularization for unstable angina, and non–cancer-related death – between the chlorthalidone group (10.4%) and the hydrochlorothiazide group (10.0%), giving a hazard ratio of 1.04 (95% CI, 0.94-1.16; P = .45).
In addition, there were no treatment differences between the two groups in any primary outcome component. Hypokalemia and potassium supplement use were more common in the chlorthalidone group than in the hydrochlorothiazide group.
‘Importance lies in the design’
In an accompanying editorial, Julie R. Ingelfinger, MD, deputy editor of the New England Journal of Medicine, said the results are not surprising and may not change clinical practice. But she suggested that the importance of the trial lies in its design, which shows that a high-quality pragmatic comparative effectiveness trial can be accomplished in a cost-effective manner within a health care system with little disruption in patient care.
Dr. Ingelfinger pointed out several limitations of the trial. These include a lower-than-expected occurrence of primary outcome events, and the stipulation that patients were eligible to participate only if they continued to have hypertension while receiving hydrochlorothiazide.
In addition, 95% of the participants were receiving 25 mg of hydrochlorothiazide and only 5% were receiving 50 mg, which limited comparisons of the dose generally used in practice. Also, only approximately 13% of the patients were receiving hydrochlorothiazide alone for the treatment of hypertension at baseline.
She noted that the DCP is the first head-to-head comparison of hydrochlorothiazide and chlorthalidone in a randomized, prospective outcome trial.
“Without an apparent difference in the hazard ratios for the primary outcome in the two groups over the median follow-up of 2.4 years, results suggest that chlorthalidone therapy remains a good choice for hypertension despite the secondary observation that hypokalemia was more common with chlorthalidone than with hydrochlorothiazide,” Dr. Ingelfinger said.
“Although a subgroup analysis suggested that chlorthalidone was better than hydrochlorothiazide for participants with a history of myocardial infarction or stroke, that result may have been by chance,” she added.
As clinicians generally prefer using hydrochlorothiazide, she suggested that these DCP results will not provide any impetus for change.
“Furthermore, combined therapy and polypills may alter therapy beyond the results of this well-done, highly anticipated trial. Thus, its major effect may be as a model for other pragmatic study programs, which are greatly needed,” she concluded.
This study was supported by the Veterans Affairs Cooperative Studies Program through a grant to the Diuretic Comparison Project. Dr. Ishani reported no relevant financial relationships. Dr. Ingelfinger reported book royalties from Springer and from St. Martin’s Press, outside the submitted work, and that she is employed by the New England Journal of Medicine as deputy editor.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Cancer researcher banned from federal funding for faking data in nearly 400 images in 16 grant applications
according to a U.S. government research watchdog.
Alice C. Chang, PhD, whose publications and grants listed her name as Chun-Ju Chang, received nearly $700,000 in funding from the National Institutes of Health through grant applications that the U.S. Office of Research Integrity said contained fake data. She will be banned from receiving federal grants for a decade – a more severe sanction than ORI has typically imposed in recent years.
In its findings, ORI said Dr. Chang, who was an associate professor of basic medical sciences at Purdue’s College of Veterinary Medicine, West Lafayette, Ind., “knowingly, intentionally, or recklessly falsified and/or fabricated data from the same mouse models or cell lines by reusing the data, with or without manipulation, to represent unrelated experiments from different mouse models or cell lines with different treatments in three hundred eighty-four (384) figure panels in sixteen (16) grant applications.”
Two of the grant applications were funded. Dr. Chang received $688,196 from the National Cancer Institute, a division of NIH, from 2018 to 2019 for “Targeting metformin-directed stem cell fate in triple negative breast cancer.” The other grant ORI says was submitted in 2014 and funded, “Targeting cell polarity machinery to exhaust breast cancer stem cell pool,” does not show up in NIH RePorter. The rest of the grants were not approved.
We found a Chun-Ju Chang who is dean of the College of Life Sciences at China Medical University in Taiwan and has published papers with a group that Chun-Ju Chang at Purdue also published with. She did not immediately respond to our request for comment.
ORI’s finding also stated Dr. Chang faked data in two papers supported by government funding by reusing figures reporting gene expression in mice and cells after drug treatments, relabeling them to say they showed the results of different experiments. According to the agency, she has agreed to request corrections for the papers:
“Leptin–STAT3–G9a Signaling Promotes Obesity-Mediated Breast Cancer Progression,” published in May 2015 in Cancer Research and cited 83 times, according to Clarivate’s Web of Science.
“Retinoic acid directs breast cancer cell state changes through regulation of TET2-PKC-zeta pathway,” published in February 2017 in Oncogene and cited 26 times.
Between the two papers and 15 of the grant applications, ORI said that Dr. Chang reused gene expression data, sometimes with manipulation, in 119 figure panels. She reused other types of data and images in hundreds of figures across multiple grant applications, ORI found.
As well as correcting the two papers, Dr. Chang agreed to a 10-year ban from all federal contracting, including grant funding. She also agreed not to serve in any advisory or consulting role with the U.S. Public Health Service, which includes the NIH, for that time period.
A version of this article first appeared on Retraction Watch.
according to a U.S. government research watchdog.
Alice C. Chang, PhD, whose publications and grants listed her name as Chun-Ju Chang, received nearly $700,000 in funding from the National Institutes of Health through grant applications that the U.S. Office of Research Integrity said contained fake data. She will be banned from receiving federal grants for a decade – a more severe sanction than ORI has typically imposed in recent years.
In its findings, ORI said Dr. Chang, who was an associate professor of basic medical sciences at Purdue’s College of Veterinary Medicine, West Lafayette, Ind., “knowingly, intentionally, or recklessly falsified and/or fabricated data from the same mouse models or cell lines by reusing the data, with or without manipulation, to represent unrelated experiments from different mouse models or cell lines with different treatments in three hundred eighty-four (384) figure panels in sixteen (16) grant applications.”
Two of the grant applications were funded. Dr. Chang received $688,196 from the National Cancer Institute, a division of NIH, from 2018 to 2019 for “Targeting metformin-directed stem cell fate in triple negative breast cancer.” The other grant ORI says was submitted in 2014 and funded, “Targeting cell polarity machinery to exhaust breast cancer stem cell pool,” does not show up in NIH RePorter. The rest of the grants were not approved.
We found a Chun-Ju Chang who is dean of the College of Life Sciences at China Medical University in Taiwan and has published papers with a group that Chun-Ju Chang at Purdue also published with. She did not immediately respond to our request for comment.
ORI’s finding also stated Dr. Chang faked data in two papers supported by government funding by reusing figures reporting gene expression in mice and cells after drug treatments, relabeling them to say they showed the results of different experiments. According to the agency, she has agreed to request corrections for the papers:
“Leptin–STAT3–G9a Signaling Promotes Obesity-Mediated Breast Cancer Progression,” published in May 2015 in Cancer Research and cited 83 times, according to Clarivate’s Web of Science.
“Retinoic acid directs breast cancer cell state changes through regulation of TET2-PKC-zeta pathway,” published in February 2017 in Oncogene and cited 26 times.
Between the two papers and 15 of the grant applications, ORI said that Dr. Chang reused gene expression data, sometimes with manipulation, in 119 figure panels. She reused other types of data and images in hundreds of figures across multiple grant applications, ORI found.
As well as correcting the two papers, Dr. Chang agreed to a 10-year ban from all federal contracting, including grant funding. She also agreed not to serve in any advisory or consulting role with the U.S. Public Health Service, which includes the NIH, for that time period.
A version of this article first appeared on Retraction Watch.
according to a U.S. government research watchdog.
Alice C. Chang, PhD, whose publications and grants listed her name as Chun-Ju Chang, received nearly $700,000 in funding from the National Institutes of Health through grant applications that the U.S. Office of Research Integrity said contained fake data. She will be banned from receiving federal grants for a decade – a more severe sanction than ORI has typically imposed in recent years.
In its findings, ORI said Dr. Chang, who was an associate professor of basic medical sciences at Purdue’s College of Veterinary Medicine, West Lafayette, Ind., “knowingly, intentionally, or recklessly falsified and/or fabricated data from the same mouse models or cell lines by reusing the data, with or without manipulation, to represent unrelated experiments from different mouse models or cell lines with different treatments in three hundred eighty-four (384) figure panels in sixteen (16) grant applications.”
Two of the grant applications were funded. Dr. Chang received $688,196 from the National Cancer Institute, a division of NIH, from 2018 to 2019 for “Targeting metformin-directed stem cell fate in triple negative breast cancer.” The other grant ORI says was submitted in 2014 and funded, “Targeting cell polarity machinery to exhaust breast cancer stem cell pool,” does not show up in NIH RePorter. The rest of the grants were not approved.
We found a Chun-Ju Chang who is dean of the College of Life Sciences at China Medical University in Taiwan and has published papers with a group that Chun-Ju Chang at Purdue also published with. She did not immediately respond to our request for comment.
ORI’s finding also stated Dr. Chang faked data in two papers supported by government funding by reusing figures reporting gene expression in mice and cells after drug treatments, relabeling them to say they showed the results of different experiments. According to the agency, she has agreed to request corrections for the papers:
“Leptin–STAT3–G9a Signaling Promotes Obesity-Mediated Breast Cancer Progression,” published in May 2015 in Cancer Research and cited 83 times, according to Clarivate’s Web of Science.
“Retinoic acid directs breast cancer cell state changes through regulation of TET2-PKC-zeta pathway,” published in February 2017 in Oncogene and cited 26 times.
Between the two papers and 15 of the grant applications, ORI said that Dr. Chang reused gene expression data, sometimes with manipulation, in 119 figure panels. She reused other types of data and images in hundreds of figures across multiple grant applications, ORI found.
As well as correcting the two papers, Dr. Chang agreed to a 10-year ban from all federal contracting, including grant funding. She also agreed not to serve in any advisory or consulting role with the U.S. Public Health Service, which includes the NIH, for that time period.
A version of this article first appeared on Retraction Watch.
Intermittent fasting can lead to type 2 diabetes remission
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
DELIVER subanalysis ‘seals deal’ for dapagliflozin in HF
A prespecified analysis of a large global trial of patients with symptomatic heart failure with mildly reduced and preserved ejection fraction “seals the deal” on the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors to manage and improve their symptoms.
The prespecified analysis of the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial included 5,795 patients with mildly reduced and preserved ejection fraction who completed the Kansas City Cardiomyopathy Questionnaire (KCCQ) after taking the SGLT2 inhibitor dapagliflozin or placebo. The results were published online in the Journal of the American College of Cardiology.
“We’ve known from studies prior to DELIVER that SGLT2 inhibitors have been shown to improve health status, patient symptoms and quality of life among those that are living with heart failure and mildly reduced [HFmrEF] and preserved [HFpEF] ejection fraction,” lead author Mikhail N. Kosiborod, MD, vice president for research at Saint Luke’s Health System, and codirector of the St. Luke’s Michael and Marly Haverty Cardiometabolic Center of Excellence at St. Luke’s Mid America Heart Institute, Kansas City, Mo., said in an interview. “But the picture was incomplete for a number of different reasons, partly because the previous studies were either relatively modest in size, geographically limited, or suggested potential attenuation of these benefits in patients with completely normal ejection fraction.”
Specifically, the study authors noted the EMPEROR-Preserved trial of the SGLT2 inhibitor empagliflozin showed improvement in health status vs. placebo across the range of EF except in those with normal EF of 65% or greater. The PRESERVED-HF trial of dapagliflozin demonstrated a more robust response than EMPEROR-Preserved or DELIVER, but PRESERVED-HF patients were recruited only in the United States and had more debilitating HF symptoms at baseline.
“Because of the results of the DELIVER trial and because of how large, extensive, and inclusive the trial was, it really seals the deal on the value of SGLT2 inhibitors in patients with heart failure,” said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City.
The DELIVER analysis found that the effects of dapagliflozin on reducing cardiovascular death and worsening HF were greatest in patients who had the most debilitating symptoms at baseline, measured as KCCQ total symptom score (TSS) as 63 or less, the lowest of three tertiles used in the analysis. At baseline, these patients had the highest rates of CV death or worsening HF than those in the other two tertiles: KCCQ-TSS of 63-84, and greater than 84.
Compared with placebo, treated patients in the lowest KCCQ-TSS quartile had a 30% reduction in risk for the primary composite outcome, which consisted of time to first CV death or HF event (hazard ratio, 0.70; 95% confidence interval, 0.58-0.84; P < .001). In the second tertile, the relative risk reduction was 19% (HR, 0.81; 95% CI, 0.65-1.01; P < .006), and the highest quartile showed no significant difference between treatment and placebo (HR, 1.07; 95% CI, 0.83-1.37; P < .62).
“The most important take home message is that the SGLT2 inhibitor dapagliflozin significantly improved patient symptoms as measured by the Kansas City Cardiomyopathy Questionnaire symptom score,” Dr. Kosiborod said. “It improved those symptoms within 1 month and those benefits were sustained out to 8 months.”
DELIVER patients also showed improvement in all other key KCCQ domains across the board, he added. “In addition, dapagliflozin also improved the proportion of patients who had small, moderate, and large improvements in a responder analysis. So really, by every measure that we had, dapagliflozin had a significant beneficial effect.”
The DELIVER results taken collectively with the EMPEROR-Preserved and PRESERVED-HF trials cinch the deal for SGLT2 inhibitors, Dr. Kosiborod said. “They deliver on the triple goal of care in patients with heart failure. They reduce the risk of cardiovascular death and worsening heart failure and they improve patient symptoms, function and quality of life – and they accomplish that across the entire continuum of heart failure regardless of ejection fraction, regardless of whether patients are hospitalized or in an ambulatory setting, regardless of age or background therapies or other comorbidities.”
He added: “It’s a pretty historic development because we haven’t had that before.”
AstraZeneca funded the DELIVER trial. Dr. Kosiborod disclosed financial relationships with Alnylam, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma.
A prespecified analysis of a large global trial of patients with symptomatic heart failure with mildly reduced and preserved ejection fraction “seals the deal” on the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors to manage and improve their symptoms.
The prespecified analysis of the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial included 5,795 patients with mildly reduced and preserved ejection fraction who completed the Kansas City Cardiomyopathy Questionnaire (KCCQ) after taking the SGLT2 inhibitor dapagliflozin or placebo. The results were published online in the Journal of the American College of Cardiology.
“We’ve known from studies prior to DELIVER that SGLT2 inhibitors have been shown to improve health status, patient symptoms and quality of life among those that are living with heart failure and mildly reduced [HFmrEF] and preserved [HFpEF] ejection fraction,” lead author Mikhail N. Kosiborod, MD, vice president for research at Saint Luke’s Health System, and codirector of the St. Luke’s Michael and Marly Haverty Cardiometabolic Center of Excellence at St. Luke’s Mid America Heart Institute, Kansas City, Mo., said in an interview. “But the picture was incomplete for a number of different reasons, partly because the previous studies were either relatively modest in size, geographically limited, or suggested potential attenuation of these benefits in patients with completely normal ejection fraction.”
Specifically, the study authors noted the EMPEROR-Preserved trial of the SGLT2 inhibitor empagliflozin showed improvement in health status vs. placebo across the range of EF except in those with normal EF of 65% or greater. The PRESERVED-HF trial of dapagliflozin demonstrated a more robust response than EMPEROR-Preserved or DELIVER, but PRESERVED-HF patients were recruited only in the United States and had more debilitating HF symptoms at baseline.
“Because of the results of the DELIVER trial and because of how large, extensive, and inclusive the trial was, it really seals the deal on the value of SGLT2 inhibitors in patients with heart failure,” said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City.
The DELIVER analysis found that the effects of dapagliflozin on reducing cardiovascular death and worsening HF were greatest in patients who had the most debilitating symptoms at baseline, measured as KCCQ total symptom score (TSS) as 63 or less, the lowest of three tertiles used in the analysis. At baseline, these patients had the highest rates of CV death or worsening HF than those in the other two tertiles: KCCQ-TSS of 63-84, and greater than 84.
Compared with placebo, treated patients in the lowest KCCQ-TSS quartile had a 30% reduction in risk for the primary composite outcome, which consisted of time to first CV death or HF event (hazard ratio, 0.70; 95% confidence interval, 0.58-0.84; P < .001). In the second tertile, the relative risk reduction was 19% (HR, 0.81; 95% CI, 0.65-1.01; P < .006), and the highest quartile showed no significant difference between treatment and placebo (HR, 1.07; 95% CI, 0.83-1.37; P < .62).
“The most important take home message is that the SGLT2 inhibitor dapagliflozin significantly improved patient symptoms as measured by the Kansas City Cardiomyopathy Questionnaire symptom score,” Dr. Kosiborod said. “It improved those symptoms within 1 month and those benefits were sustained out to 8 months.”
DELIVER patients also showed improvement in all other key KCCQ domains across the board, he added. “In addition, dapagliflozin also improved the proportion of patients who had small, moderate, and large improvements in a responder analysis. So really, by every measure that we had, dapagliflozin had a significant beneficial effect.”
The DELIVER results taken collectively with the EMPEROR-Preserved and PRESERVED-HF trials cinch the deal for SGLT2 inhibitors, Dr. Kosiborod said. “They deliver on the triple goal of care in patients with heart failure. They reduce the risk of cardiovascular death and worsening heart failure and they improve patient symptoms, function and quality of life – and they accomplish that across the entire continuum of heart failure regardless of ejection fraction, regardless of whether patients are hospitalized or in an ambulatory setting, regardless of age or background therapies or other comorbidities.”
He added: “It’s a pretty historic development because we haven’t had that before.”
AstraZeneca funded the DELIVER trial. Dr. Kosiborod disclosed financial relationships with Alnylam, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma.
A prespecified analysis of a large global trial of patients with symptomatic heart failure with mildly reduced and preserved ejection fraction “seals the deal” on the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors to manage and improve their symptoms.
The prespecified analysis of the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial included 5,795 patients with mildly reduced and preserved ejection fraction who completed the Kansas City Cardiomyopathy Questionnaire (KCCQ) after taking the SGLT2 inhibitor dapagliflozin or placebo. The results were published online in the Journal of the American College of Cardiology.
“We’ve known from studies prior to DELIVER that SGLT2 inhibitors have been shown to improve health status, patient symptoms and quality of life among those that are living with heart failure and mildly reduced [HFmrEF] and preserved [HFpEF] ejection fraction,” lead author Mikhail N. Kosiborod, MD, vice president for research at Saint Luke’s Health System, and codirector of the St. Luke’s Michael and Marly Haverty Cardiometabolic Center of Excellence at St. Luke’s Mid America Heart Institute, Kansas City, Mo., said in an interview. “But the picture was incomplete for a number of different reasons, partly because the previous studies were either relatively modest in size, geographically limited, or suggested potential attenuation of these benefits in patients with completely normal ejection fraction.”
Specifically, the study authors noted the EMPEROR-Preserved trial of the SGLT2 inhibitor empagliflozin showed improvement in health status vs. placebo across the range of EF except in those with normal EF of 65% or greater. The PRESERVED-HF trial of dapagliflozin demonstrated a more robust response than EMPEROR-Preserved or DELIVER, but PRESERVED-HF patients were recruited only in the United States and had more debilitating HF symptoms at baseline.
“Because of the results of the DELIVER trial and because of how large, extensive, and inclusive the trial was, it really seals the deal on the value of SGLT2 inhibitors in patients with heart failure,” said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City.
The DELIVER analysis found that the effects of dapagliflozin on reducing cardiovascular death and worsening HF were greatest in patients who had the most debilitating symptoms at baseline, measured as KCCQ total symptom score (TSS) as 63 or less, the lowest of three tertiles used in the analysis. At baseline, these patients had the highest rates of CV death or worsening HF than those in the other two tertiles: KCCQ-TSS of 63-84, and greater than 84.
Compared with placebo, treated patients in the lowest KCCQ-TSS quartile had a 30% reduction in risk for the primary composite outcome, which consisted of time to first CV death or HF event (hazard ratio, 0.70; 95% confidence interval, 0.58-0.84; P < .001). In the second tertile, the relative risk reduction was 19% (HR, 0.81; 95% CI, 0.65-1.01; P < .006), and the highest quartile showed no significant difference between treatment and placebo (HR, 1.07; 95% CI, 0.83-1.37; P < .62).
“The most important take home message is that the SGLT2 inhibitor dapagliflozin significantly improved patient symptoms as measured by the Kansas City Cardiomyopathy Questionnaire symptom score,” Dr. Kosiborod said. “It improved those symptoms within 1 month and those benefits were sustained out to 8 months.”
DELIVER patients also showed improvement in all other key KCCQ domains across the board, he added. “In addition, dapagliflozin also improved the proportion of patients who had small, moderate, and large improvements in a responder analysis. So really, by every measure that we had, dapagliflozin had a significant beneficial effect.”
The DELIVER results taken collectively with the EMPEROR-Preserved and PRESERVED-HF trials cinch the deal for SGLT2 inhibitors, Dr. Kosiborod said. “They deliver on the triple goal of care in patients with heart failure. They reduce the risk of cardiovascular death and worsening heart failure and they improve patient symptoms, function and quality of life – and they accomplish that across the entire continuum of heart failure regardless of ejection fraction, regardless of whether patients are hospitalized or in an ambulatory setting, regardless of age or background therapies or other comorbidities.”
He added: “It’s a pretty historic development because we haven’t had that before.”
AstraZeneca funded the DELIVER trial. Dr. Kosiborod disclosed financial relationships with Alnylam, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Have you heard the one about the cow in the doctor’s office?
Maybe the cow was late for its appointment
It’s been a long day running the front desk at your doctor’s office. People calling in prescriptions, a million appointments, you’ve been running yourself ragged keeping things together. Finally, it’s almost closing time. The last patient of the day has just checked out and you turn back to the waiting room, expecting to see it blessedly empty.
Instead, a 650-pound cow is staring at you.
“I’m sorry, sir or madam, we’re about to close.”
Moo.
“I understand it’s important, but seriously, the doctor’s about to …”
Moo.
“Fine, I’ll see what we can do for you. What’s your insurance?”
Moo Cross Moo Shield.
“Sorry, we don’t take that. You’ll have to go someplace else.”
This is probably not how things went down recently at Orange (Va.) Family Physicians, when they had a cow break into the office. Cows don’t have health insurance.
The intrepid bovine was being transferred to a new home when it jumped off the trailer and wandered an eighth of a mile to Orange Family Physicians, where the cow wranglers found it hanging around outside. Unfortunately, this was a smart cow, and it bolted as it saw the wranglers, crashing through the glass doors into the doctor’s office. Though neither man had ever wrangled a cow from inside a building, they ultimately secured a rope around the cow’s neck and escorted it back outside, tying it to a nearby pole to keep it from further adventures.
One of the wranglers summed up the situation quite nicely on his Facebook page: “You ain’t no cowboy if you don’t rope a calf out of a [doctor’s] office.”
We can see that decision in your eyes
The cliché that eyes are the windows to the soul doesn’t tell the whole story about how telling eyes really are. It’s all about how they move. In a recent study, researchers determined that a type of eye movement known as a saccade reveals your choice before you even decide.
Saccades involve the eyes jumping from one fixation point to another, senior author Alaa Ahmed of the University of Colorado, Boulder, explained in a statement from the university. Saccade vigor was the key in how aligned the type of decisions were made by the 22 study participants.
In the study, subjects walked on a treadmill at varied inclines for a period of time. Then they sat in front of a monitor and a high-speed camera that tracked their eye movements as the monitor presented them with a series of exercise options. The participants had only 4 seconds to choose between them.
After they made their choices, participants went back on the treadmill to perform the exercises they had chosen. The researchers found that participants’ eyes jumped between the options slowly then faster to the option they eventually picked. The more impulsive decision-makers also tended to move their eyes even more rapidly before slowing down after a decision was made, making it pretty conclusive that the eyes were revealing their choices.
The way your eyes shift gives you away without saying a thing. Might be wise, then, to wear sunglasses to your next poker tournament.
Let them eat soap
Okay, we admit it: LOTME spends a lot of time in the bathroom. Today, though, we’re interested in the sinks. Specifically, the P-traps under the sinks. You know, the curvy bit that keeps sewer gas from wafting back into the room?
Well, researchers from the University of Reading (England) recently found some fungi while examining a bunch of sinks on the university’s Whiteknights campus. “It isn’t a big surprise to find fungi in a warm, wet environment. But sinks and P-traps have thus far been overlooked as potential reservoirs of these microorganisms,” they said in a written statement.
Samples collected from 289 P-traps contained “a very similar community of yeasts and molds, showing that sinks in use in public environments share a role as reservoirs of fungal organisms,” they noted.
The fungi living in the traps survived conditions with high temperatures, low pH, and little in the way of nutrients. So what were they eating? Some varieties, they said, “use detergents, found in soap, as a source of carbon-rich food.” We’ll repeat that last part: They used the soap as food.
WARNING: Rant Ahead.
There are a lot of cleaning products for sale that say they will make your home safe by killing 99.9% of germs and bacteria. Not fungi, exactly, but we’re still talking microorganisms. Molds, bacteria, and viruses are all stuff that can infect humans and make them sick.
So you kill 99.9% of them. Great, but that leaves 0.1% that you just made angry. And what do they do next? They learn to eat soap. Then University of Reading investigators find out that all the extra hand washing going on during the COVID-19 pandemic was “clogging up sinks with nasty disease-causing bacteria.”
These are microorganisms we’re talking about people. They’ve been at this for a billion years! Rats can’t beat them, cockroaches won’t stop them – Earth’s ultimate survivors are powerless against the invisible horde.
We’re doomed.
Maybe the cow was late for its appointment
It’s been a long day running the front desk at your doctor’s office. People calling in prescriptions, a million appointments, you’ve been running yourself ragged keeping things together. Finally, it’s almost closing time. The last patient of the day has just checked out and you turn back to the waiting room, expecting to see it blessedly empty.
Instead, a 650-pound cow is staring at you.
“I’m sorry, sir or madam, we’re about to close.”
Moo.
“I understand it’s important, but seriously, the doctor’s about to …”
Moo.
“Fine, I’ll see what we can do for you. What’s your insurance?”
Moo Cross Moo Shield.
“Sorry, we don’t take that. You’ll have to go someplace else.”
This is probably not how things went down recently at Orange (Va.) Family Physicians, when they had a cow break into the office. Cows don’t have health insurance.
The intrepid bovine was being transferred to a new home when it jumped off the trailer and wandered an eighth of a mile to Orange Family Physicians, where the cow wranglers found it hanging around outside. Unfortunately, this was a smart cow, and it bolted as it saw the wranglers, crashing through the glass doors into the doctor’s office. Though neither man had ever wrangled a cow from inside a building, they ultimately secured a rope around the cow’s neck and escorted it back outside, tying it to a nearby pole to keep it from further adventures.
One of the wranglers summed up the situation quite nicely on his Facebook page: “You ain’t no cowboy if you don’t rope a calf out of a [doctor’s] office.”
We can see that decision in your eyes
The cliché that eyes are the windows to the soul doesn’t tell the whole story about how telling eyes really are. It’s all about how they move. In a recent study, researchers determined that a type of eye movement known as a saccade reveals your choice before you even decide.
Saccades involve the eyes jumping from one fixation point to another, senior author Alaa Ahmed of the University of Colorado, Boulder, explained in a statement from the university. Saccade vigor was the key in how aligned the type of decisions were made by the 22 study participants.
In the study, subjects walked on a treadmill at varied inclines for a period of time. Then they sat in front of a monitor and a high-speed camera that tracked their eye movements as the monitor presented them with a series of exercise options. The participants had only 4 seconds to choose between them.
After they made their choices, participants went back on the treadmill to perform the exercises they had chosen. The researchers found that participants’ eyes jumped between the options slowly then faster to the option they eventually picked. The more impulsive decision-makers also tended to move their eyes even more rapidly before slowing down after a decision was made, making it pretty conclusive that the eyes were revealing their choices.
The way your eyes shift gives you away without saying a thing. Might be wise, then, to wear sunglasses to your next poker tournament.
Let them eat soap
Okay, we admit it: LOTME spends a lot of time in the bathroom. Today, though, we’re interested in the sinks. Specifically, the P-traps under the sinks. You know, the curvy bit that keeps sewer gas from wafting back into the room?
Well, researchers from the University of Reading (England) recently found some fungi while examining a bunch of sinks on the university’s Whiteknights campus. “It isn’t a big surprise to find fungi in a warm, wet environment. But sinks and P-traps have thus far been overlooked as potential reservoirs of these microorganisms,” they said in a written statement.
Samples collected from 289 P-traps contained “a very similar community of yeasts and molds, showing that sinks in use in public environments share a role as reservoirs of fungal organisms,” they noted.
The fungi living in the traps survived conditions with high temperatures, low pH, and little in the way of nutrients. So what were they eating? Some varieties, they said, “use detergents, found in soap, as a source of carbon-rich food.” We’ll repeat that last part: They used the soap as food.
WARNING: Rant Ahead.
There are a lot of cleaning products for sale that say they will make your home safe by killing 99.9% of germs and bacteria. Not fungi, exactly, but we’re still talking microorganisms. Molds, bacteria, and viruses are all stuff that can infect humans and make them sick.
So you kill 99.9% of them. Great, but that leaves 0.1% that you just made angry. And what do they do next? They learn to eat soap. Then University of Reading investigators find out that all the extra hand washing going on during the COVID-19 pandemic was “clogging up sinks with nasty disease-causing bacteria.”
These are microorganisms we’re talking about people. They’ve been at this for a billion years! Rats can’t beat them, cockroaches won’t stop them – Earth’s ultimate survivors are powerless against the invisible horde.
We’re doomed.
Maybe the cow was late for its appointment
It’s been a long day running the front desk at your doctor’s office. People calling in prescriptions, a million appointments, you’ve been running yourself ragged keeping things together. Finally, it’s almost closing time. The last patient of the day has just checked out and you turn back to the waiting room, expecting to see it blessedly empty.
Instead, a 650-pound cow is staring at you.
“I’m sorry, sir or madam, we’re about to close.”
Moo.
“I understand it’s important, but seriously, the doctor’s about to …”
Moo.
“Fine, I’ll see what we can do for you. What’s your insurance?”
Moo Cross Moo Shield.
“Sorry, we don’t take that. You’ll have to go someplace else.”
This is probably not how things went down recently at Orange (Va.) Family Physicians, when they had a cow break into the office. Cows don’t have health insurance.
The intrepid bovine was being transferred to a new home when it jumped off the trailer and wandered an eighth of a mile to Orange Family Physicians, where the cow wranglers found it hanging around outside. Unfortunately, this was a smart cow, and it bolted as it saw the wranglers, crashing through the glass doors into the doctor’s office. Though neither man had ever wrangled a cow from inside a building, they ultimately secured a rope around the cow’s neck and escorted it back outside, tying it to a nearby pole to keep it from further adventures.
One of the wranglers summed up the situation quite nicely on his Facebook page: “You ain’t no cowboy if you don’t rope a calf out of a [doctor’s] office.”
We can see that decision in your eyes
The cliché that eyes are the windows to the soul doesn’t tell the whole story about how telling eyes really are. It’s all about how they move. In a recent study, researchers determined that a type of eye movement known as a saccade reveals your choice before you even decide.
Saccades involve the eyes jumping from one fixation point to another, senior author Alaa Ahmed of the University of Colorado, Boulder, explained in a statement from the university. Saccade vigor was the key in how aligned the type of decisions were made by the 22 study participants.
In the study, subjects walked on a treadmill at varied inclines for a period of time. Then they sat in front of a monitor and a high-speed camera that tracked their eye movements as the monitor presented them with a series of exercise options. The participants had only 4 seconds to choose between them.
After they made their choices, participants went back on the treadmill to perform the exercises they had chosen. The researchers found that participants’ eyes jumped between the options slowly then faster to the option they eventually picked. The more impulsive decision-makers also tended to move their eyes even more rapidly before slowing down after a decision was made, making it pretty conclusive that the eyes were revealing their choices.
The way your eyes shift gives you away without saying a thing. Might be wise, then, to wear sunglasses to your next poker tournament.
Let them eat soap
Okay, we admit it: LOTME spends a lot of time in the bathroom. Today, though, we’re interested in the sinks. Specifically, the P-traps under the sinks. You know, the curvy bit that keeps sewer gas from wafting back into the room?
Well, researchers from the University of Reading (England) recently found some fungi while examining a bunch of sinks on the university’s Whiteknights campus. “It isn’t a big surprise to find fungi in a warm, wet environment. But sinks and P-traps have thus far been overlooked as potential reservoirs of these microorganisms,” they said in a written statement.
Samples collected from 289 P-traps contained “a very similar community of yeasts and molds, showing that sinks in use in public environments share a role as reservoirs of fungal organisms,” they noted.
The fungi living in the traps survived conditions with high temperatures, low pH, and little in the way of nutrients. So what were they eating? Some varieties, they said, “use detergents, found in soap, as a source of carbon-rich food.” We’ll repeat that last part: They used the soap as food.
WARNING: Rant Ahead.
There are a lot of cleaning products for sale that say they will make your home safe by killing 99.9% of germs and bacteria. Not fungi, exactly, but we’re still talking microorganisms. Molds, bacteria, and viruses are all stuff that can infect humans and make them sick.
So you kill 99.9% of them. Great, but that leaves 0.1% that you just made angry. And what do they do next? They learn to eat soap. Then University of Reading investigators find out that all the extra hand washing going on during the COVID-19 pandemic was “clogging up sinks with nasty disease-causing bacteria.”
These are microorganisms we’re talking about people. They’ve been at this for a billion years! Rats can’t beat them, cockroaches won’t stop them – Earth’s ultimate survivors are powerless against the invisible horde.
We’re doomed.
The new obesity breakthrough drugs
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.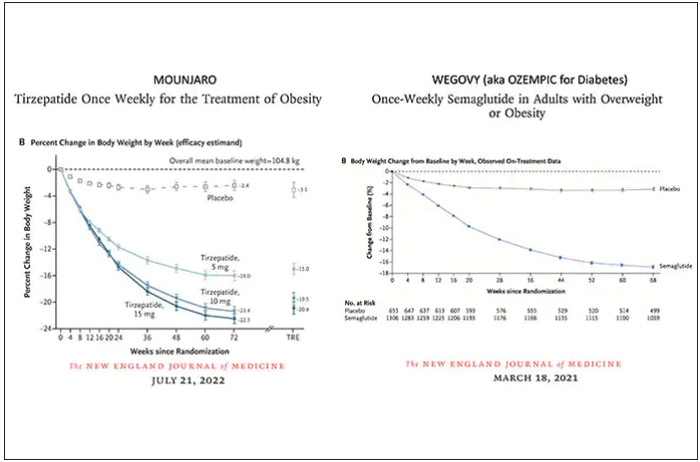
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction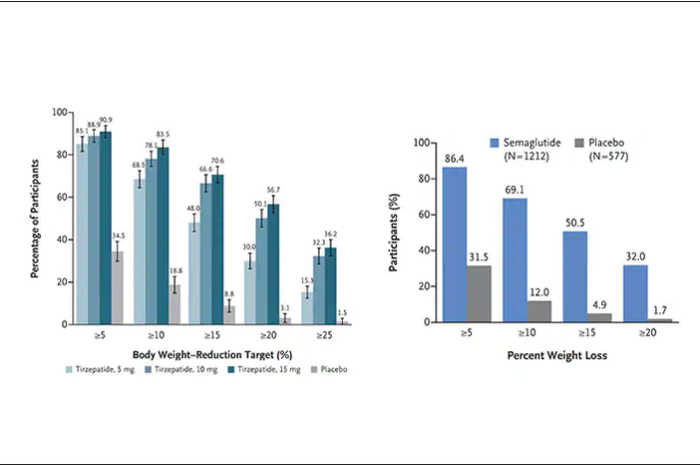
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.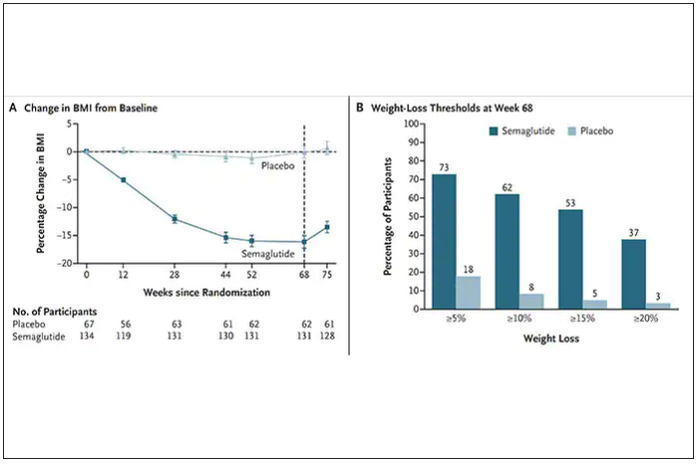
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.
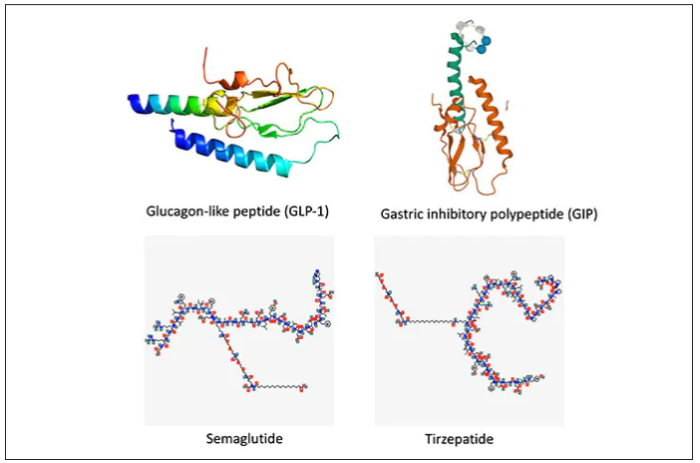
These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.
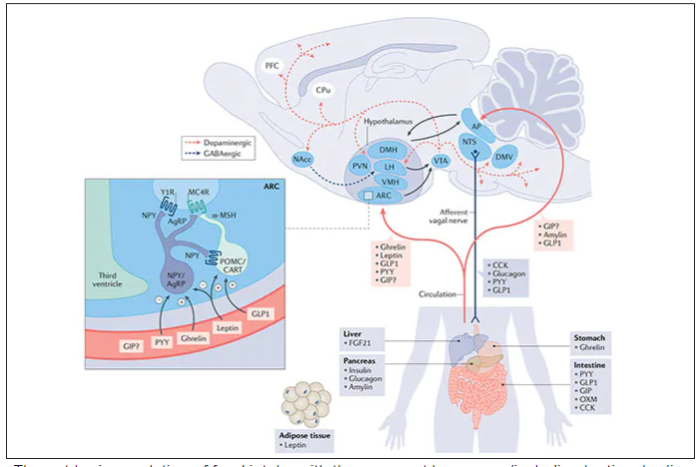
Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.
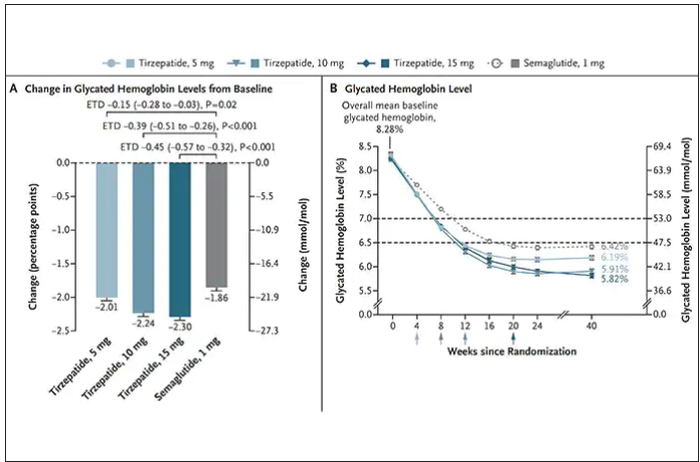
While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.
Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.

These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.

Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.

While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.

Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.

These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.

Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.

While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.

Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
Should you quit employment to open a practice? These docs share how they did it
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
Lifestyle choices could curb genetic risk for thyroid cancer
A healthier lifestyle mitigated the impact of genetic factors on the risk of thyroid cancer, in a study based on data from more than 260,000 individuals.
Thyroid cancer has increased globally in recent years and ranks 9th among 36 cancers worldwide, at a considerable cost to health care systems, wrote Xiuming Feng of Guangxi Medical University, Nanning, Guangxi, China, and colleagues.
Both genetic and lifestyle factors are related to thyroid cancer; previous research suggests a heritability of about 50%, but data on the impact of modifiable lifestyle factors on thyroid cancer are limited, the researchers said.
In a prospective cohort study published in JAMA Network Open, the researchers used data from the UK Biobank and recruited adults aged 40-69 years during March 2006–October 2010. The final study population included 264,956 individuals of European descent. The median age of the participants was 57 years, and 52% were women.
Data on lifestyle behaviors were collected using interviews and questionnaires. The researchers constructed a total lifestyle score based on five variables: diet, physical activity, weight, smoking, and alcohol consumption. Each variable was assigned a score of 0 or 1, with 1 being favorable lifestyle behavior. Lifestyle was divided into three categories: unfavorable (scores 0-1), intermediate (score 2), and favorable (scores 3-5).
Each individual’s polygenic risk score (PRS) was categorized as low, intermediate, or high based on a meta–genome-wide association study of three cohorts.
The main outcome was the development of thyroid cancer.
The researchers identified 423 incident thyroid cancer cases over a median follow-up of 11.1 years.
Overall, higher PRSs were significantly associated with thyroid cancer (hazard ratio, 2.25; 95% confidence interval [CI], 1.91-2.64; P < .00001) as was an unfavorable lifestyle score (HR, 1.93; 95% CI, 1.50-2.49; P < .001 for trend).
An unfavorable lifestyle was significantly associated with thyroid cancer in the highest PRS group, and individuals with high PRS and unfavorable lifestyle had a nearly fivefold increased risk of thyroid cancer (HR, 4.89; 95% CI, 3.03-7.91; P < .001). By extension, “Adherence to a healthier lifestyle could decrease the incidence of thyroid cancer in individuals with a higher PRS,” the researchers wrote in their discussion.
The findings were limited by several factors, including the availability of only baseline lifestyle data, and lack of data on iodine intake, radiation exposure, experience, and family history, the researchers noted. Other limitations include the potential lack of generalizability to populations other than the individuals of European descent in the current study, they said.
However, the study is the first known to address the association among lifestyle, genetic factors, and risk of thyroid cancer, and was strengthened by the large study population, and the results suggest that lifestyle interventions may help reduce the risk of thyroid cancer in those with a genetic predisposition, they concluded.
Healthy living can make a difference
The incidence of thyroid cancer has increased annually, and exploring the possible risk factors could prevent the occurrence of thyroid cancer, corresponding author Xiaobo Yang, PhD, said in an interview.
Previous studies have reported that thyroid cancer is related to genetics and lifestyle, said Dr. Yang. “However, whether healthy lifestyle was associated with thyroid cancer risk and could attenuate the impact of genetic variants on thyroid cancer remains equivocal; therefore, it is crucial to determine the associations between genetic and lifestyle with thyroid cancer,” he said.
“To our surprise, we found that adherence to healthier lifestyle also could reduce the risk of thyroid cancer in those with high genetic predispositions,” said Dr. Yang. “The findings highlight the potential role of lifestyle interventions on thyroid cancer, especially in those with high genetic risk, because the heritability of thyroid cancer was very high, approximately 50%,” he said. “More attention should be paid to the role of healthier lifestyle in the prevention of cancer,” he added.
“Adherence to a healthier lifestyle could decrease the risk of thyroid cancer, which is the important message for clinicians,” said Dr. Yang. “It is not too soon to comment on implications for clinical practice, because many studies have maintained the consistent comment that healthier lifestyle could prevent the occurrence of cancer,” he said.
The relationship between sex-specific lifestyle factors such as smoking and alcohol use and thyroid cancer remains uncertain, and more research is needed to validate these associations, Dr. Yang said. More research also is needed to confirm the complex mechanism between lifestyle and genetics in thyroid cancer, he added.
The study was supported by the National Key R&D Program of China and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
A healthier lifestyle mitigated the impact of genetic factors on the risk of thyroid cancer, in a study based on data from more than 260,000 individuals.
Thyroid cancer has increased globally in recent years and ranks 9th among 36 cancers worldwide, at a considerable cost to health care systems, wrote Xiuming Feng of Guangxi Medical University, Nanning, Guangxi, China, and colleagues.
Both genetic and lifestyle factors are related to thyroid cancer; previous research suggests a heritability of about 50%, but data on the impact of modifiable lifestyle factors on thyroid cancer are limited, the researchers said.
In a prospective cohort study published in JAMA Network Open, the researchers used data from the UK Biobank and recruited adults aged 40-69 years during March 2006–October 2010. The final study population included 264,956 individuals of European descent. The median age of the participants was 57 years, and 52% were women.
Data on lifestyle behaviors were collected using interviews and questionnaires. The researchers constructed a total lifestyle score based on five variables: diet, physical activity, weight, smoking, and alcohol consumption. Each variable was assigned a score of 0 or 1, with 1 being favorable lifestyle behavior. Lifestyle was divided into three categories: unfavorable (scores 0-1), intermediate (score 2), and favorable (scores 3-5).
Each individual’s polygenic risk score (PRS) was categorized as low, intermediate, or high based on a meta–genome-wide association study of three cohorts.
The main outcome was the development of thyroid cancer.
The researchers identified 423 incident thyroid cancer cases over a median follow-up of 11.1 years.
Overall, higher PRSs were significantly associated with thyroid cancer (hazard ratio, 2.25; 95% confidence interval [CI], 1.91-2.64; P < .00001) as was an unfavorable lifestyle score (HR, 1.93; 95% CI, 1.50-2.49; P < .001 for trend).
An unfavorable lifestyle was significantly associated with thyroid cancer in the highest PRS group, and individuals with high PRS and unfavorable lifestyle had a nearly fivefold increased risk of thyroid cancer (HR, 4.89; 95% CI, 3.03-7.91; P < .001). By extension, “Adherence to a healthier lifestyle could decrease the incidence of thyroid cancer in individuals with a higher PRS,” the researchers wrote in their discussion.
The findings were limited by several factors, including the availability of only baseline lifestyle data, and lack of data on iodine intake, radiation exposure, experience, and family history, the researchers noted. Other limitations include the potential lack of generalizability to populations other than the individuals of European descent in the current study, they said.
However, the study is the first known to address the association among lifestyle, genetic factors, and risk of thyroid cancer, and was strengthened by the large study population, and the results suggest that lifestyle interventions may help reduce the risk of thyroid cancer in those with a genetic predisposition, they concluded.
Healthy living can make a difference
The incidence of thyroid cancer has increased annually, and exploring the possible risk factors could prevent the occurrence of thyroid cancer, corresponding author Xiaobo Yang, PhD, said in an interview.
Previous studies have reported that thyroid cancer is related to genetics and lifestyle, said Dr. Yang. “However, whether healthy lifestyle was associated with thyroid cancer risk and could attenuate the impact of genetic variants on thyroid cancer remains equivocal; therefore, it is crucial to determine the associations between genetic and lifestyle with thyroid cancer,” he said.
“To our surprise, we found that adherence to healthier lifestyle also could reduce the risk of thyroid cancer in those with high genetic predispositions,” said Dr. Yang. “The findings highlight the potential role of lifestyle interventions on thyroid cancer, especially in those with high genetic risk, because the heritability of thyroid cancer was very high, approximately 50%,” he said. “More attention should be paid to the role of healthier lifestyle in the prevention of cancer,” he added.
“Adherence to a healthier lifestyle could decrease the risk of thyroid cancer, which is the important message for clinicians,” said Dr. Yang. “It is not too soon to comment on implications for clinical practice, because many studies have maintained the consistent comment that healthier lifestyle could prevent the occurrence of cancer,” he said.
The relationship between sex-specific lifestyle factors such as smoking and alcohol use and thyroid cancer remains uncertain, and more research is needed to validate these associations, Dr. Yang said. More research also is needed to confirm the complex mechanism between lifestyle and genetics in thyroid cancer, he added.
The study was supported by the National Key R&D Program of China and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
A healthier lifestyle mitigated the impact of genetic factors on the risk of thyroid cancer, in a study based on data from more than 260,000 individuals.
Thyroid cancer has increased globally in recent years and ranks 9th among 36 cancers worldwide, at a considerable cost to health care systems, wrote Xiuming Feng of Guangxi Medical University, Nanning, Guangxi, China, and colleagues.
Both genetic and lifestyle factors are related to thyroid cancer; previous research suggests a heritability of about 50%, but data on the impact of modifiable lifestyle factors on thyroid cancer are limited, the researchers said.
In a prospective cohort study published in JAMA Network Open, the researchers used data from the UK Biobank and recruited adults aged 40-69 years during March 2006–October 2010. The final study population included 264,956 individuals of European descent. The median age of the participants was 57 years, and 52% were women.
Data on lifestyle behaviors were collected using interviews and questionnaires. The researchers constructed a total lifestyle score based on five variables: diet, physical activity, weight, smoking, and alcohol consumption. Each variable was assigned a score of 0 or 1, with 1 being favorable lifestyle behavior. Lifestyle was divided into three categories: unfavorable (scores 0-1), intermediate (score 2), and favorable (scores 3-5).
Each individual’s polygenic risk score (PRS) was categorized as low, intermediate, or high based on a meta–genome-wide association study of three cohorts.
The main outcome was the development of thyroid cancer.
The researchers identified 423 incident thyroid cancer cases over a median follow-up of 11.1 years.
Overall, higher PRSs were significantly associated with thyroid cancer (hazard ratio, 2.25; 95% confidence interval [CI], 1.91-2.64; P < .00001) as was an unfavorable lifestyle score (HR, 1.93; 95% CI, 1.50-2.49; P < .001 for trend).
An unfavorable lifestyle was significantly associated with thyroid cancer in the highest PRS group, and individuals with high PRS and unfavorable lifestyle had a nearly fivefold increased risk of thyroid cancer (HR, 4.89; 95% CI, 3.03-7.91; P < .001). By extension, “Adherence to a healthier lifestyle could decrease the incidence of thyroid cancer in individuals with a higher PRS,” the researchers wrote in their discussion.
The findings were limited by several factors, including the availability of only baseline lifestyle data, and lack of data on iodine intake, radiation exposure, experience, and family history, the researchers noted. Other limitations include the potential lack of generalizability to populations other than the individuals of European descent in the current study, they said.
However, the study is the first known to address the association among lifestyle, genetic factors, and risk of thyroid cancer, and was strengthened by the large study population, and the results suggest that lifestyle interventions may help reduce the risk of thyroid cancer in those with a genetic predisposition, they concluded.
Healthy living can make a difference
The incidence of thyroid cancer has increased annually, and exploring the possible risk factors could prevent the occurrence of thyroid cancer, corresponding author Xiaobo Yang, PhD, said in an interview.
Previous studies have reported that thyroid cancer is related to genetics and lifestyle, said Dr. Yang. “However, whether healthy lifestyle was associated with thyroid cancer risk and could attenuate the impact of genetic variants on thyroid cancer remains equivocal; therefore, it is crucial to determine the associations between genetic and lifestyle with thyroid cancer,” he said.
“To our surprise, we found that adherence to healthier lifestyle also could reduce the risk of thyroid cancer in those with high genetic predispositions,” said Dr. Yang. “The findings highlight the potential role of lifestyle interventions on thyroid cancer, especially in those with high genetic risk, because the heritability of thyroid cancer was very high, approximately 50%,” he said. “More attention should be paid to the role of healthier lifestyle in the prevention of cancer,” he added.
“Adherence to a healthier lifestyle could decrease the risk of thyroid cancer, which is the important message for clinicians,” said Dr. Yang. “It is not too soon to comment on implications for clinical practice, because many studies have maintained the consistent comment that healthier lifestyle could prevent the occurrence of cancer,” he said.
The relationship between sex-specific lifestyle factors such as smoking and alcohol use and thyroid cancer remains uncertain, and more research is needed to validate these associations, Dr. Yang said. More research also is needed to confirm the complex mechanism between lifestyle and genetics in thyroid cancer, he added.
The study was supported by the National Key R&D Program of China and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN