User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Use the SCAI stages to identify and treat cardiogenic shock
Cardiogenic shock (CS) is being recognized more often in critically ill patients. This increased prevalence is likely due to a better understanding of CS and the benefit of improving cardiac output (CO) to ensure adequate oxygen delivery (DO2).
CS is often, but not always, caused by a cardiac dysfunction. The heart is not able to provide adequate DO2 to the tissues. Hypoperfusion ensues. The body attempts to compensate for the poor perfusion by increasing heart rate, vasoconstriction, and shunting blood flow to vital organs. These compensatory mechanisms worsen perfusion by increasing myocardial ischemia which further worsens cardiac dysfunction. This is known as the downward spiral of CS (Ann Intern Med. 1999 Jul 6;131[1]).
There is a number of different etiologies for CS. Historically, acute myocardial infarctions (AMI) was the most common cause. In the last 20 years, AMI-induced CS has become less prevalent due to more aggressive reperfusion strategies. CS due to etiologies such as cardiomyopathy, myocarditis, right ventricle failure, and valvular pathologies have become more common. While the overarching goal is to restore DO2 to the tissue, the optimal treatment may differ based on the etiology of the CS. The Society for Cardiovascular Angiography and Intervention (SCAI) published CS classification stages in 2019 and then updated the stages 2022 (J Am Coll Cardiol. 2022 Mar 8;79[9]:933-46). In addition to the stages, there is now a three-axis model to address risk stratification. These classifications are a practically means of identifying and treating patients presenting with or concern for acute CS.
Stage A (At Risk) patients are not experiencing CS, but they are the at risk population. The patient’s hemodynamics, physical exam, and markers of hypoperfusion are normal. Stage A includes patients who have had a recent AMI or have heart failure.
Stage B (Beginning) patients have evidence of hemodynamic instability but are able to maintain tissue perfusion. These patients will have true or relative hypotension or tachycardia (in an attempt to maintain CO). Distal perfusion is adequate, but signs of ensuing decompensation (eg, elevated jugular venous pressure [JVP]) are present. Lactate is <2.0 mmol/L. Clinicians must be vigilant and treat these patients aggressively, so they do not decompensate further. It can be difficult to identify these patients because their blood pressure may be “normal,” but upon investigation, the blood pressure is actually a drop from the patient’s baseline.
Chronic heart failure patients with a history of depressed cardiac function will often have periods of cardiac decompensation between stages A and B. These patients are able to maintain perfusion for longer periods of time before further decompensation with hypoperfusion. If and when they do decompensate, they will often have a steep downward trajectory, so it is advantageous to the patient to be aggressive early.
Stage C (Classic) patients have evidence of tissue hypoperfusion. While these patients will often have true or relative hypotension, it is not a definition of stage C. These patients have evidence of volume overload with elevated JVP and rales throughout their lung fields. They will have poor distal perfusion and cool extremities that may become mottled. Lactate is ≥ 2 mmol/L. B-type natriuretic peptide (BNP) and liver function test (LFTs) results are elevated, and urine output is diminished. If a pulmonary arterial catheter is placed (highly recommended), the cardiac index (CI) is < 2.2 L/min/m2 and the pulmonary capillary wedge pressure (PCWP) is > 15 mm Hg. These patients look like what many clinicians think of when they think of CS.
These patients need better tissue perfusion. Inotropic support is needed to augment CO and DO2. Pharmacologic support is often the initial step. These patients also benefit from volume removal. This is usually accomplished with aggressive diuresis with a loop diuretic.
Stage D (Deteriorating) patients have failed initial treatment with single inotropic support. Hypoperfusion is not getting better and is often worsening. Lactate is staying > 2 mmol/L or rising. BNP and LFTs are also rising. These patients require additional inotropes and usually need vasopressors. Mechanical cardiac support (MCS) is often needed in addition to pharmacologic inotropic support.
Stage E (Extremis) patients have actual or impending circulatory collapse. These patients are peri-arrest with profound hypotension, lactic acidosis (often > 8 mmol/L), and unconsciousness. These patients are worsening despite multiple strategies to augment CO and DO2. These patients will likely die without emergent veno-arterial (VA) extracorporeal membrane oxygenation (ECMO). The goal of treatment is to stabilize the patient as quickly as possible to prevent cardiac arrest.
In addition to the stage of CS, SCAI developed the three-axis model of risk stratification as a conceptual model to be used for evaluation and prognostication. Etiology and phenotype, shock severity, and risk modifiers are factors related to patient outcomes from CS. This model is a way to individualize treatment to a specific patient.
Shock severity: What is the patient’s shock stage? What are the hemodynamics and metabolic abnormalities? What are the doses of the inotropes or vasopressors? Risk goes up with higher shock stages and vasoactive agent doses and worsening metabolic disturbances or hemodynamics.
Phenotype and etiology: what is the clinical etiology of the patient’s CS? Is this acute or acute on chronic? Which ventricle is involved? Is this cardiac driven or are other organs the driving factor? Single ventricle involvement is better than bi-ventricular failure. Cardiogenic collapse due to an overdose may have a better outcome than a massive AMI.
Risk modifiers: how old is the patient? What are the comorbidities? Did the patient have a cardiac arrest? What is the patient’s mental status? Some factors are modifiable, but others are not. The concept of chronologic vs. physiologic age may come into play. A frail 40 year old with stage 4 cancer and end stage renal failure may be assessed differently than a 70 year old with mild hypertension and an AMI.
The SCAI stages of CS are a pragmatic way to assess patients with an acute presentation of CS. These stages have defined criteria and treatment recommendations for all patients. The three-axis model allows the clinician to individualize patient care based on shock severity, etiology/phenotype, and risk modification. The goal of these stages is to identify and aggressively treat patients with CS, as well as identify when treatment is failing and additional therapies may be needed.
Dr. Gaillard is Associate Professor in the Departments of Anesthesiology, Section on Critical Care; Internal Medicine, Section on Pulmonology, Critical Care, Allergy, and Immunologic Diseases; and Emergency Medicine; Wake Forest School of Medicine, Winston-Salem, N.C.
Cardiogenic shock (CS) is being recognized more often in critically ill patients. This increased prevalence is likely due to a better understanding of CS and the benefit of improving cardiac output (CO) to ensure adequate oxygen delivery (DO2).
CS is often, but not always, caused by a cardiac dysfunction. The heart is not able to provide adequate DO2 to the tissues. Hypoperfusion ensues. The body attempts to compensate for the poor perfusion by increasing heart rate, vasoconstriction, and shunting blood flow to vital organs. These compensatory mechanisms worsen perfusion by increasing myocardial ischemia which further worsens cardiac dysfunction. This is known as the downward spiral of CS (Ann Intern Med. 1999 Jul 6;131[1]).
There is a number of different etiologies for CS. Historically, acute myocardial infarctions (AMI) was the most common cause. In the last 20 years, AMI-induced CS has become less prevalent due to more aggressive reperfusion strategies. CS due to etiologies such as cardiomyopathy, myocarditis, right ventricle failure, and valvular pathologies have become more common. While the overarching goal is to restore DO2 to the tissue, the optimal treatment may differ based on the etiology of the CS. The Society for Cardiovascular Angiography and Intervention (SCAI) published CS classification stages in 2019 and then updated the stages 2022 (J Am Coll Cardiol. 2022 Mar 8;79[9]:933-46). In addition to the stages, there is now a three-axis model to address risk stratification. These classifications are a practically means of identifying and treating patients presenting with or concern for acute CS.
Stage A (At Risk) patients are not experiencing CS, but they are the at risk population. The patient’s hemodynamics, physical exam, and markers of hypoperfusion are normal. Stage A includes patients who have had a recent AMI or have heart failure.
Stage B (Beginning) patients have evidence of hemodynamic instability but are able to maintain tissue perfusion. These patients will have true or relative hypotension or tachycardia (in an attempt to maintain CO). Distal perfusion is adequate, but signs of ensuing decompensation (eg, elevated jugular venous pressure [JVP]) are present. Lactate is <2.0 mmol/L. Clinicians must be vigilant and treat these patients aggressively, so they do not decompensate further. It can be difficult to identify these patients because their blood pressure may be “normal,” but upon investigation, the blood pressure is actually a drop from the patient’s baseline.
Chronic heart failure patients with a history of depressed cardiac function will often have periods of cardiac decompensation between stages A and B. These patients are able to maintain perfusion for longer periods of time before further decompensation with hypoperfusion. If and when they do decompensate, they will often have a steep downward trajectory, so it is advantageous to the patient to be aggressive early.
Stage C (Classic) patients have evidence of tissue hypoperfusion. While these patients will often have true or relative hypotension, it is not a definition of stage C. These patients have evidence of volume overload with elevated JVP and rales throughout their lung fields. They will have poor distal perfusion and cool extremities that may become mottled. Lactate is ≥ 2 mmol/L. B-type natriuretic peptide (BNP) and liver function test (LFTs) results are elevated, and urine output is diminished. If a pulmonary arterial catheter is placed (highly recommended), the cardiac index (CI) is < 2.2 L/min/m2 and the pulmonary capillary wedge pressure (PCWP) is > 15 mm Hg. These patients look like what many clinicians think of when they think of CS.
These patients need better tissue perfusion. Inotropic support is needed to augment CO and DO2. Pharmacologic support is often the initial step. These patients also benefit from volume removal. This is usually accomplished with aggressive diuresis with a loop diuretic.
Stage D (Deteriorating) patients have failed initial treatment with single inotropic support. Hypoperfusion is not getting better and is often worsening. Lactate is staying > 2 mmol/L or rising. BNP and LFTs are also rising. These patients require additional inotropes and usually need vasopressors. Mechanical cardiac support (MCS) is often needed in addition to pharmacologic inotropic support.
Stage E (Extremis) patients have actual or impending circulatory collapse. These patients are peri-arrest with profound hypotension, lactic acidosis (often > 8 mmol/L), and unconsciousness. These patients are worsening despite multiple strategies to augment CO and DO2. These patients will likely die without emergent veno-arterial (VA) extracorporeal membrane oxygenation (ECMO). The goal of treatment is to stabilize the patient as quickly as possible to prevent cardiac arrest.
In addition to the stage of CS, SCAI developed the three-axis model of risk stratification as a conceptual model to be used for evaluation and prognostication. Etiology and phenotype, shock severity, and risk modifiers are factors related to patient outcomes from CS. This model is a way to individualize treatment to a specific patient.
Shock severity: What is the patient’s shock stage? What are the hemodynamics and metabolic abnormalities? What are the doses of the inotropes or vasopressors? Risk goes up with higher shock stages and vasoactive agent doses and worsening metabolic disturbances or hemodynamics.
Phenotype and etiology: what is the clinical etiology of the patient’s CS? Is this acute or acute on chronic? Which ventricle is involved? Is this cardiac driven or are other organs the driving factor? Single ventricle involvement is better than bi-ventricular failure. Cardiogenic collapse due to an overdose may have a better outcome than a massive AMI.
Risk modifiers: how old is the patient? What are the comorbidities? Did the patient have a cardiac arrest? What is the patient’s mental status? Some factors are modifiable, but others are not. The concept of chronologic vs. physiologic age may come into play. A frail 40 year old with stage 4 cancer and end stage renal failure may be assessed differently than a 70 year old with mild hypertension and an AMI.
The SCAI stages of CS are a pragmatic way to assess patients with an acute presentation of CS. These stages have defined criteria and treatment recommendations for all patients. The three-axis model allows the clinician to individualize patient care based on shock severity, etiology/phenotype, and risk modification. The goal of these stages is to identify and aggressively treat patients with CS, as well as identify when treatment is failing and additional therapies may be needed.
Dr. Gaillard is Associate Professor in the Departments of Anesthesiology, Section on Critical Care; Internal Medicine, Section on Pulmonology, Critical Care, Allergy, and Immunologic Diseases; and Emergency Medicine; Wake Forest School of Medicine, Winston-Salem, N.C.
Cardiogenic shock (CS) is being recognized more often in critically ill patients. This increased prevalence is likely due to a better understanding of CS and the benefit of improving cardiac output (CO) to ensure adequate oxygen delivery (DO2).
CS is often, but not always, caused by a cardiac dysfunction. The heart is not able to provide adequate DO2 to the tissues. Hypoperfusion ensues. The body attempts to compensate for the poor perfusion by increasing heart rate, vasoconstriction, and shunting blood flow to vital organs. These compensatory mechanisms worsen perfusion by increasing myocardial ischemia which further worsens cardiac dysfunction. This is known as the downward spiral of CS (Ann Intern Med. 1999 Jul 6;131[1]).
There is a number of different etiologies for CS. Historically, acute myocardial infarctions (AMI) was the most common cause. In the last 20 years, AMI-induced CS has become less prevalent due to more aggressive reperfusion strategies. CS due to etiologies such as cardiomyopathy, myocarditis, right ventricle failure, and valvular pathologies have become more common. While the overarching goal is to restore DO2 to the tissue, the optimal treatment may differ based on the etiology of the CS. The Society for Cardiovascular Angiography and Intervention (SCAI) published CS classification stages in 2019 and then updated the stages 2022 (J Am Coll Cardiol. 2022 Mar 8;79[9]:933-46). In addition to the stages, there is now a three-axis model to address risk stratification. These classifications are a practically means of identifying and treating patients presenting with or concern for acute CS.
Stage A (At Risk) patients are not experiencing CS, but they are the at risk population. The patient’s hemodynamics, physical exam, and markers of hypoperfusion are normal. Stage A includes patients who have had a recent AMI or have heart failure.
Stage B (Beginning) patients have evidence of hemodynamic instability but are able to maintain tissue perfusion. These patients will have true or relative hypotension or tachycardia (in an attempt to maintain CO). Distal perfusion is adequate, but signs of ensuing decompensation (eg, elevated jugular venous pressure [JVP]) are present. Lactate is <2.0 mmol/L. Clinicians must be vigilant and treat these patients aggressively, so they do not decompensate further. It can be difficult to identify these patients because their blood pressure may be “normal,” but upon investigation, the blood pressure is actually a drop from the patient’s baseline.
Chronic heart failure patients with a history of depressed cardiac function will often have periods of cardiac decompensation between stages A and B. These patients are able to maintain perfusion for longer periods of time before further decompensation with hypoperfusion. If and when they do decompensate, they will often have a steep downward trajectory, so it is advantageous to the patient to be aggressive early.
Stage C (Classic) patients have evidence of tissue hypoperfusion. While these patients will often have true or relative hypotension, it is not a definition of stage C. These patients have evidence of volume overload with elevated JVP and rales throughout their lung fields. They will have poor distal perfusion and cool extremities that may become mottled. Lactate is ≥ 2 mmol/L. B-type natriuretic peptide (BNP) and liver function test (LFTs) results are elevated, and urine output is diminished. If a pulmonary arterial catheter is placed (highly recommended), the cardiac index (CI) is < 2.2 L/min/m2 and the pulmonary capillary wedge pressure (PCWP) is > 15 mm Hg. These patients look like what many clinicians think of when they think of CS.
These patients need better tissue perfusion. Inotropic support is needed to augment CO and DO2. Pharmacologic support is often the initial step. These patients also benefit from volume removal. This is usually accomplished with aggressive diuresis with a loop diuretic.
Stage D (Deteriorating) patients have failed initial treatment with single inotropic support. Hypoperfusion is not getting better and is often worsening. Lactate is staying > 2 mmol/L or rising. BNP and LFTs are also rising. These patients require additional inotropes and usually need vasopressors. Mechanical cardiac support (MCS) is often needed in addition to pharmacologic inotropic support.
Stage E (Extremis) patients have actual or impending circulatory collapse. These patients are peri-arrest with profound hypotension, lactic acidosis (often > 8 mmol/L), and unconsciousness. These patients are worsening despite multiple strategies to augment CO and DO2. These patients will likely die without emergent veno-arterial (VA) extracorporeal membrane oxygenation (ECMO). The goal of treatment is to stabilize the patient as quickly as possible to prevent cardiac arrest.
In addition to the stage of CS, SCAI developed the three-axis model of risk stratification as a conceptual model to be used for evaluation and prognostication. Etiology and phenotype, shock severity, and risk modifiers are factors related to patient outcomes from CS. This model is a way to individualize treatment to a specific patient.
Shock severity: What is the patient’s shock stage? What are the hemodynamics and metabolic abnormalities? What are the doses of the inotropes or vasopressors? Risk goes up with higher shock stages and vasoactive agent doses and worsening metabolic disturbances or hemodynamics.
Phenotype and etiology: what is the clinical etiology of the patient’s CS? Is this acute or acute on chronic? Which ventricle is involved? Is this cardiac driven or are other organs the driving factor? Single ventricle involvement is better than bi-ventricular failure. Cardiogenic collapse due to an overdose may have a better outcome than a massive AMI.
Risk modifiers: how old is the patient? What are the comorbidities? Did the patient have a cardiac arrest? What is the patient’s mental status? Some factors are modifiable, but others are not. The concept of chronologic vs. physiologic age may come into play. A frail 40 year old with stage 4 cancer and end stage renal failure may be assessed differently than a 70 year old with mild hypertension and an AMI.
The SCAI stages of CS are a pragmatic way to assess patients with an acute presentation of CS. These stages have defined criteria and treatment recommendations for all patients. The three-axis model allows the clinician to individualize patient care based on shock severity, etiology/phenotype, and risk modification. The goal of these stages is to identify and aggressively treat patients with CS, as well as identify when treatment is failing and additional therapies may be needed.
Dr. Gaillard is Associate Professor in the Departments of Anesthesiology, Section on Critical Care; Internal Medicine, Section on Pulmonology, Critical Care, Allergy, and Immunologic Diseases; and Emergency Medicine; Wake Forest School of Medicine, Winston-Salem, N.C.
Add hands-on and interactive learning opportunities to your CHEST 2023 schedule
Explore the many ticketed sessions, and sign up early in case they sell out.
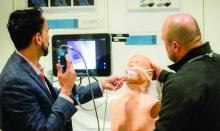
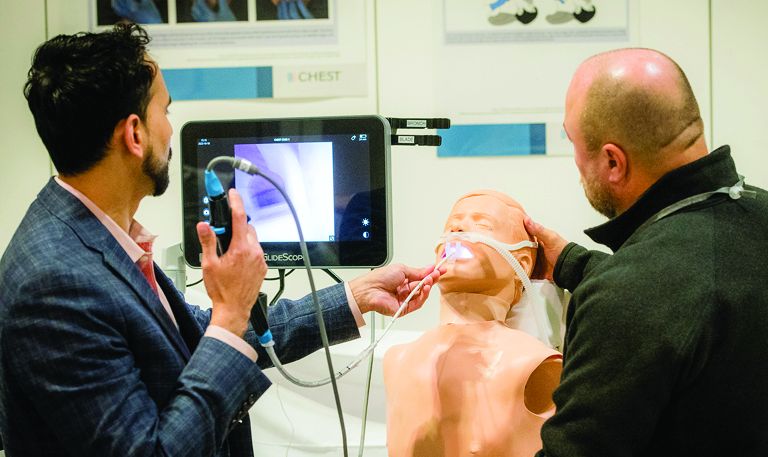
Simulation sessions
If you’re looking to gain hands-on exposure to equipment and tools that may not be available at your home institution, look no further than these simulation sessions. Choose from 25 different sessions offering firsthand experience with procedures relevant to your clinical practice.
“It’s a great opportunity to teach higher stakes procedures in a very low stakes environment where everybody’s comfortable and everybody’s learning from each other,” said Live Learning Subcommittee Chair, Nicholas Pastis, MD, FCCP.
CHEST 2023 simulation sessions will address clinical topics, including endobronchial ultrasound, cardiopulmonary exercise testing (CPET), intubation and cricothyrotomy, bronchoscopy management, and more. These sessions are taught by experts who use these real-world strategies in their daily practice.
CHEST 2022 attendee, Weston Bowker, MD, found value in the simulation courses he was able to attend in Nashville.
“It’s fantastic just to work with some of the leading experts in the field, especially from an interventional pulmonology standpoint. And, you truly get a different experience than maybe what your home institution offers,” he said.
Problem-based learning sessions
Exercise your critical thinking skills by working to resolve real-world clinical problems during these small group sessions. Refine your expertise on topics like lung cancer screening and staging, biologics in asthma, pneumonia, and more.
“Problem-based learning courses take a clinical problem or case study that is somewhat controversial to create a learning environment where the problem itself drives the learning with participants,” said CHEST 2023 Scientific Program Committee Chair, Aneesa Das, MD, FCCP. “These are small group sessions where learners can actively participate and collaborate to discuss various perspectives on the issue and work toward potential solutions.”
This year’s problem-based learning courses were chosen based on common controversies in chest medicine and current hot topics in medicine.
Dr. Das is excited for the Using CPET to Solve Your Difficult Cases course. “Cardiopulmonary exercise tests can sometimes be difficult even for seasoned physicians. This is always an amazing problem-based learning topic,” she added.
Meet the Professor sessions
Connect with leading chest medicine experts during these limited-capacity discussions capped at 24 registrants per session. Meet the Professor attendees will have the opportunity to engage in stimulating conversations on bronchiectasis, central airway obstructions, obesity hypoventilation, and sublobar resection.
“Meet the Professor sessions are a unique opportunity to interact and learn from a leader in the field in a very small group setting on a high-yield topic,” said Dr. Das. “These sessions allow for a learning environment that is personalized and intimate.”
Explore the many ticketed sessions, and sign up early in case they sell out.
Simulation sessions
If you’re looking to gain hands-on exposure to equipment and tools that may not be available at your home institution, look no further than these simulation sessions. Choose from 25 different sessions offering firsthand experience with procedures relevant to your clinical practice.
“It’s a great opportunity to teach higher stakes procedures in a very low stakes environment where everybody’s comfortable and everybody’s learning from each other,” said Live Learning Subcommittee Chair, Nicholas Pastis, MD, FCCP.
CHEST 2023 simulation sessions will address clinical topics, including endobronchial ultrasound, cardiopulmonary exercise testing (CPET), intubation and cricothyrotomy, bronchoscopy management, and more. These sessions are taught by experts who use these real-world strategies in their daily practice.
CHEST 2022 attendee, Weston Bowker, MD, found value in the simulation courses he was able to attend in Nashville.
“It’s fantastic just to work with some of the leading experts in the field, especially from an interventional pulmonology standpoint. And, you truly get a different experience than maybe what your home institution offers,” he said.
Problem-based learning sessions
Exercise your critical thinking skills by working to resolve real-world clinical problems during these small group sessions. Refine your expertise on topics like lung cancer screening and staging, biologics in asthma, pneumonia, and more.
“Problem-based learning courses take a clinical problem or case study that is somewhat controversial to create a learning environment where the problem itself drives the learning with participants,” said CHEST 2023 Scientific Program Committee Chair, Aneesa Das, MD, FCCP. “These are small group sessions where learners can actively participate and collaborate to discuss various perspectives on the issue and work toward potential solutions.”
This year’s problem-based learning courses were chosen based on common controversies in chest medicine and current hot topics in medicine.
Dr. Das is excited for the Using CPET to Solve Your Difficult Cases course. “Cardiopulmonary exercise tests can sometimes be difficult even for seasoned physicians. This is always an amazing problem-based learning topic,” she added.
Meet the Professor sessions
Connect with leading chest medicine experts during these limited-capacity discussions capped at 24 registrants per session. Meet the Professor attendees will have the opportunity to engage in stimulating conversations on bronchiectasis, central airway obstructions, obesity hypoventilation, and sublobar resection.
“Meet the Professor sessions are a unique opportunity to interact and learn from a leader in the field in a very small group setting on a high-yield topic,” said Dr. Das. “These sessions allow for a learning environment that is personalized and intimate.”
Explore the many ticketed sessions, and sign up early in case they sell out.
Simulation sessions
If you’re looking to gain hands-on exposure to equipment and tools that may not be available at your home institution, look no further than these simulation sessions. Choose from 25 different sessions offering firsthand experience with procedures relevant to your clinical practice.
“It’s a great opportunity to teach higher stakes procedures in a very low stakes environment where everybody’s comfortable and everybody’s learning from each other,” said Live Learning Subcommittee Chair, Nicholas Pastis, MD, FCCP.
CHEST 2023 simulation sessions will address clinical topics, including endobronchial ultrasound, cardiopulmonary exercise testing (CPET), intubation and cricothyrotomy, bronchoscopy management, and more. These sessions are taught by experts who use these real-world strategies in their daily practice.
CHEST 2022 attendee, Weston Bowker, MD, found value in the simulation courses he was able to attend in Nashville.
“It’s fantastic just to work with some of the leading experts in the field, especially from an interventional pulmonology standpoint. And, you truly get a different experience than maybe what your home institution offers,” he said.
Problem-based learning sessions
Exercise your critical thinking skills by working to resolve real-world clinical problems during these small group sessions. Refine your expertise on topics like lung cancer screening and staging, biologics in asthma, pneumonia, and more.
“Problem-based learning courses take a clinical problem or case study that is somewhat controversial to create a learning environment where the problem itself drives the learning with participants,” said CHEST 2023 Scientific Program Committee Chair, Aneesa Das, MD, FCCP. “These are small group sessions where learners can actively participate and collaborate to discuss various perspectives on the issue and work toward potential solutions.”
This year’s problem-based learning courses were chosen based on common controversies in chest medicine and current hot topics in medicine.
Dr. Das is excited for the Using CPET to Solve Your Difficult Cases course. “Cardiopulmonary exercise tests can sometimes be difficult even for seasoned physicians. This is always an amazing problem-based learning topic,” she added.
Meet the Professor sessions
Connect with leading chest medicine experts during these limited-capacity discussions capped at 24 registrants per session. Meet the Professor attendees will have the opportunity to engage in stimulating conversations on bronchiectasis, central airway obstructions, obesity hypoventilation, and sublobar resection.
“Meet the Professor sessions are a unique opportunity to interact and learn from a leader in the field in a very small group setting on a high-yield topic,” said Dr. Das. “These sessions allow for a learning environment that is personalized and intimate.”
We asked doctors using AI scribes: Just how good are they?
Andrea Partida, DO, an obstetrician and gynecologist in Enid, Okla., loves her new assistant.
The 15 or 20 minutes she used to spend on documentation for each patient visit is now 3. The 2-3 hours she’d spend charting outside clinic hours is maybe 1.
All that time saved allows her to see two to five more patients a day, provide better care to each patient, and get more involved in hospital leadership at Integris Health, where she works.
“I have a better work-life balance with my family,” Dr. Partida said. “I leave work at work and get home earlier.”
You’ve probably figured out the plot twist: Dr. Partida’s assistant is not a person – it’s artificial intelligence (AI).
Dr. Partida uses IRIS, a tool from OnPoint Healthcare Partners, part of a fast-growing niche of AI medical scribes designed to automate onerous data entry. The evolution of generative AI – specifically, large language models, such as ChatGPT – has led to a rapid explosion of these tools. Other companies in the space include Abridge, Ambience Healthcare, Augmedix, DeepScribe, Nuance (part of Microsoft), and Suki. The newest kid on the block, Amazon Web Services, announced the launch of HealthScribe in July.
These tools – some of which are already on the market, with more on the way – record patient visits and generate notes for treatment and billing. Earlier iterations combine AI with offsite human scribes who provide quality control. But more and more are fully automated, no human required. Some also offer video recording and foreign language translation.
The promise is alluring: Ease your workload and reclaim hours in your day so you can spend more time with patients or try that “work-life balance” thing you’ve heard so much about.
But do these tools fulfill that promise?
According to Dr. Partida and other doctors who spoke with this news organization, the answer is a resounding yes.
A tech solution for a tech problem
“I believe a lot of doctors see patients for free. They get paid to do paperwork,” said Anthony J. Mazzarelli, MD, JD, MBE, co-president and CEO of Cooper University Health Care, in Camden, N.J.
Indeed, for every hour U.S. clinicians spend with their patients, they may spend 2 more hours documenting in electronic health records (EHRs), estimates show. About half of doctors, especially those in primary care, report feeling burned out, and some 42% say they want to quit clinical practice.
Enter AI scribes.
“The holy grail in medicine right now is improving burnout while also maintaining or improving productivity and quality,” said Patricia Garcia, MD, associate clinical information officer for ambulatory care at Stanford (Calif.) Health Care. “These ambient digital scribes have the potential to do just that.”
While anyone can buy these products, their use has been mostly limited to pilot programs and early adopters so far, said Dr. Garcia, who has been helping to pilot Nuance’s digital scribe, DAX, at Stanford.
But that’s expected to change quickly. “I don’t think the time horizon is a decade,” Dr. Garcia said. “I think within a matter of 2 or 3 years, these tools will be pervasive throughout health care.”
Since introducing these tools at Cooper, “our doctors’ paperwork burden is significantly lighter,” said Dr. Mazzarelli, who decides which technologies Cooper should invest in and who monitors their results. In Cooper studies, physicians who used DAX more than half the time spent 43% less time working on notes.
“They spend more time connecting with their patients, talking with them, and looking them in the eye,” Dr. Mazzarelli said. That, in turn, seems to improve patient outcomes, reduce doctor burnout and turnover, and lower costs.
The AI scribes, by virtue of eliminating the distraction of note taking, also allow doctors to give their full attention to the patient. “The patient relationship is the most important aspect of medicine,” said Raul Ayala, MD, MHCM, a family medicine physician at Adventist Health, in Hanford, Calif., who uses Augmedix. The digital scribe “helps us strengthen that relationship.”
What’s it like to use an AI medical scribe?
The scribes feature hardware (typically a smartphone or tablet) and software built on automatic speech recognition, natural language processing, and machine learning. Download an app to your device, and you’re ready to go. Use it to record in-person or telehealth visits.
In the first week, a company may help train you to use the hardware and software. You’ll likely start by using it for a few patient visits per day, ramping up gradually. Dr. Partida said she was comfortable using the system for all her patients in 6 weeks.
Each day, Dr. Partida logs in to a dedicated smartphone or tablet, opens the app, and reviews her schedule, including details she needs to prepare for each patient.
At the start of each patient visit, Dr. Partida taps the app icon to begin recording and lays the device nearby. She can pause as needed. At the end of the visit, she taps the icon again to stop recording.
The AI listens, creates the note, and updates relevant data in the EHR. The note includes patient problems, assessment, treatment plan, patient history, orders, and tasks for staff, along with medications, referrals, and preauthorizations. A human scribe, who is also a physician, reviews the information for accuracy and edits it as needed. By the next morning, the data are ready for Dr. Partida to review.
Fully automated versions can generate notes much faster. Jack Shilling, MD, MBA, an orthopedic surgeon at Cooper University Health Care, in Voorhees, N.J., uses DAX. A new feature called DAX Express – which uses OpenAI’s GPT-4 but no humans – provides him with a draft of his clinical notes in just seconds.
How accurate are AI notes?
The accuracy of those notes remains an open question, Dr. Garcia said – mostly because accuracy can be hard to define.
“If you asked five docs to write a note based on the same patient encounter, you’d get five different notes,” Dr. Garcia said. “That makes it hard to assess these technologies in a scientifically rigorous way.”
Still, the onus is on the physician to review the notes and edit them as needed, Dr. Garcia said. How light or heavy those edits are can depend on your unique preferences.
Dr. Shilling said he may need to lightly edit transcripts of his conversations with patients. “When someone tells me how long their knee hurts, slight variability in their transcribed words is tolerable,” he said. But for some things – such as physical exam notes and x-ray readings – he dictates directly into the device, speaking at a closer range and being less conversational, more exact in his speech.
Should you let patients know they’re being recorded?
The federal Health Insurance Portability and Accountability Act (HIPAA) does not require providers to inform patients that their face-to-face conversations are being recorded, said Daniel Lebovic, JD, corporate legal counsel at Compliancy Group, in Greenlawn, N.Y., a company that helps providers adhere to HIPAA rules.
But make sure you know the laws in your state and the policies at your health care practice. State laws may require providers to inform patients and to get patients’ consent in advance of being recorded.
All the doctors who spoke to this news organization said their patients are informed that they’ll be recorded and that they can opt out if they wish.
How much do AI scribes cost?
As the marketplace for these tools expands, companies are offering more products and services at different price points that target a range of organizations, from large health care systems to small private practices.
Price models vary, said Dr. Garcia. Some are based on the number of users, others on the number of notes, and still others on minutes.
Amazon’s HealthScribe is priced at 10 cents per minute. For 1,000 consultation transcripts per month, with each call averaging 15 minutes, it would take 15,000 minutes at a total cost of $1,500 for the month.
In general, the rapidly growing competition in this space could mean prices become more affordable, Dr. Garcia said. “It’s good that so many are getting into this game, because that means the price will come down and it will be a lot more accessible to everybody.”
A version of this article appeared on Medscape.com.
Andrea Partida, DO, an obstetrician and gynecologist in Enid, Okla., loves her new assistant.
The 15 or 20 minutes she used to spend on documentation for each patient visit is now 3. The 2-3 hours she’d spend charting outside clinic hours is maybe 1.
All that time saved allows her to see two to five more patients a day, provide better care to each patient, and get more involved in hospital leadership at Integris Health, where she works.
“I have a better work-life balance with my family,” Dr. Partida said. “I leave work at work and get home earlier.”
You’ve probably figured out the plot twist: Dr. Partida’s assistant is not a person – it’s artificial intelligence (AI).
Dr. Partida uses IRIS, a tool from OnPoint Healthcare Partners, part of a fast-growing niche of AI medical scribes designed to automate onerous data entry. The evolution of generative AI – specifically, large language models, such as ChatGPT – has led to a rapid explosion of these tools. Other companies in the space include Abridge, Ambience Healthcare, Augmedix, DeepScribe, Nuance (part of Microsoft), and Suki. The newest kid on the block, Amazon Web Services, announced the launch of HealthScribe in July.
These tools – some of which are already on the market, with more on the way – record patient visits and generate notes for treatment and billing. Earlier iterations combine AI with offsite human scribes who provide quality control. But more and more are fully automated, no human required. Some also offer video recording and foreign language translation.
The promise is alluring: Ease your workload and reclaim hours in your day so you can spend more time with patients or try that “work-life balance” thing you’ve heard so much about.
But do these tools fulfill that promise?
According to Dr. Partida and other doctors who spoke with this news organization, the answer is a resounding yes.
A tech solution for a tech problem
“I believe a lot of doctors see patients for free. They get paid to do paperwork,” said Anthony J. Mazzarelli, MD, JD, MBE, co-president and CEO of Cooper University Health Care, in Camden, N.J.
Indeed, for every hour U.S. clinicians spend with their patients, they may spend 2 more hours documenting in electronic health records (EHRs), estimates show. About half of doctors, especially those in primary care, report feeling burned out, and some 42% say they want to quit clinical practice.
Enter AI scribes.
“The holy grail in medicine right now is improving burnout while also maintaining or improving productivity and quality,” said Patricia Garcia, MD, associate clinical information officer for ambulatory care at Stanford (Calif.) Health Care. “These ambient digital scribes have the potential to do just that.”
While anyone can buy these products, their use has been mostly limited to pilot programs and early adopters so far, said Dr. Garcia, who has been helping to pilot Nuance’s digital scribe, DAX, at Stanford.
But that’s expected to change quickly. “I don’t think the time horizon is a decade,” Dr. Garcia said. “I think within a matter of 2 or 3 years, these tools will be pervasive throughout health care.”
Since introducing these tools at Cooper, “our doctors’ paperwork burden is significantly lighter,” said Dr. Mazzarelli, who decides which technologies Cooper should invest in and who monitors their results. In Cooper studies, physicians who used DAX more than half the time spent 43% less time working on notes.
“They spend more time connecting with their patients, talking with them, and looking them in the eye,” Dr. Mazzarelli said. That, in turn, seems to improve patient outcomes, reduce doctor burnout and turnover, and lower costs.
The AI scribes, by virtue of eliminating the distraction of note taking, also allow doctors to give their full attention to the patient. “The patient relationship is the most important aspect of medicine,” said Raul Ayala, MD, MHCM, a family medicine physician at Adventist Health, in Hanford, Calif., who uses Augmedix. The digital scribe “helps us strengthen that relationship.”
What’s it like to use an AI medical scribe?
The scribes feature hardware (typically a smartphone or tablet) and software built on automatic speech recognition, natural language processing, and machine learning. Download an app to your device, and you’re ready to go. Use it to record in-person or telehealth visits.
In the first week, a company may help train you to use the hardware and software. You’ll likely start by using it for a few patient visits per day, ramping up gradually. Dr. Partida said she was comfortable using the system for all her patients in 6 weeks.
Each day, Dr. Partida logs in to a dedicated smartphone or tablet, opens the app, and reviews her schedule, including details she needs to prepare for each patient.
At the start of each patient visit, Dr. Partida taps the app icon to begin recording and lays the device nearby. She can pause as needed. At the end of the visit, she taps the icon again to stop recording.
The AI listens, creates the note, and updates relevant data in the EHR. The note includes patient problems, assessment, treatment plan, patient history, orders, and tasks for staff, along with medications, referrals, and preauthorizations. A human scribe, who is also a physician, reviews the information for accuracy and edits it as needed. By the next morning, the data are ready for Dr. Partida to review.
Fully automated versions can generate notes much faster. Jack Shilling, MD, MBA, an orthopedic surgeon at Cooper University Health Care, in Voorhees, N.J., uses DAX. A new feature called DAX Express – which uses OpenAI’s GPT-4 but no humans – provides him with a draft of his clinical notes in just seconds.
How accurate are AI notes?
The accuracy of those notes remains an open question, Dr. Garcia said – mostly because accuracy can be hard to define.
“If you asked five docs to write a note based on the same patient encounter, you’d get five different notes,” Dr. Garcia said. “That makes it hard to assess these technologies in a scientifically rigorous way.”
Still, the onus is on the physician to review the notes and edit them as needed, Dr. Garcia said. How light or heavy those edits are can depend on your unique preferences.
Dr. Shilling said he may need to lightly edit transcripts of his conversations with patients. “When someone tells me how long their knee hurts, slight variability in their transcribed words is tolerable,” he said. But for some things – such as physical exam notes and x-ray readings – he dictates directly into the device, speaking at a closer range and being less conversational, more exact in his speech.
Should you let patients know they’re being recorded?
The federal Health Insurance Portability and Accountability Act (HIPAA) does not require providers to inform patients that their face-to-face conversations are being recorded, said Daniel Lebovic, JD, corporate legal counsel at Compliancy Group, in Greenlawn, N.Y., a company that helps providers adhere to HIPAA rules.
But make sure you know the laws in your state and the policies at your health care practice. State laws may require providers to inform patients and to get patients’ consent in advance of being recorded.
All the doctors who spoke to this news organization said their patients are informed that they’ll be recorded and that they can opt out if they wish.
How much do AI scribes cost?
As the marketplace for these tools expands, companies are offering more products and services at different price points that target a range of organizations, from large health care systems to small private practices.
Price models vary, said Dr. Garcia. Some are based on the number of users, others on the number of notes, and still others on minutes.
Amazon’s HealthScribe is priced at 10 cents per minute. For 1,000 consultation transcripts per month, with each call averaging 15 minutes, it would take 15,000 minutes at a total cost of $1,500 for the month.
In general, the rapidly growing competition in this space could mean prices become more affordable, Dr. Garcia said. “It’s good that so many are getting into this game, because that means the price will come down and it will be a lot more accessible to everybody.”
A version of this article appeared on Medscape.com.
Andrea Partida, DO, an obstetrician and gynecologist in Enid, Okla., loves her new assistant.
The 15 or 20 minutes she used to spend on documentation for each patient visit is now 3. The 2-3 hours she’d spend charting outside clinic hours is maybe 1.
All that time saved allows her to see two to five more patients a day, provide better care to each patient, and get more involved in hospital leadership at Integris Health, where she works.
“I have a better work-life balance with my family,” Dr. Partida said. “I leave work at work and get home earlier.”
You’ve probably figured out the plot twist: Dr. Partida’s assistant is not a person – it’s artificial intelligence (AI).
Dr. Partida uses IRIS, a tool from OnPoint Healthcare Partners, part of a fast-growing niche of AI medical scribes designed to automate onerous data entry. The evolution of generative AI – specifically, large language models, such as ChatGPT – has led to a rapid explosion of these tools. Other companies in the space include Abridge, Ambience Healthcare, Augmedix, DeepScribe, Nuance (part of Microsoft), and Suki. The newest kid on the block, Amazon Web Services, announced the launch of HealthScribe in July.
These tools – some of which are already on the market, with more on the way – record patient visits and generate notes for treatment and billing. Earlier iterations combine AI with offsite human scribes who provide quality control. But more and more are fully automated, no human required. Some also offer video recording and foreign language translation.
The promise is alluring: Ease your workload and reclaim hours in your day so you can spend more time with patients or try that “work-life balance” thing you’ve heard so much about.
But do these tools fulfill that promise?
According to Dr. Partida and other doctors who spoke with this news organization, the answer is a resounding yes.
A tech solution for a tech problem
“I believe a lot of doctors see patients for free. They get paid to do paperwork,” said Anthony J. Mazzarelli, MD, JD, MBE, co-president and CEO of Cooper University Health Care, in Camden, N.J.
Indeed, for every hour U.S. clinicians spend with their patients, they may spend 2 more hours documenting in electronic health records (EHRs), estimates show. About half of doctors, especially those in primary care, report feeling burned out, and some 42% say they want to quit clinical practice.
Enter AI scribes.
“The holy grail in medicine right now is improving burnout while also maintaining or improving productivity and quality,” said Patricia Garcia, MD, associate clinical information officer for ambulatory care at Stanford (Calif.) Health Care. “These ambient digital scribes have the potential to do just that.”
While anyone can buy these products, their use has been mostly limited to pilot programs and early adopters so far, said Dr. Garcia, who has been helping to pilot Nuance’s digital scribe, DAX, at Stanford.
But that’s expected to change quickly. “I don’t think the time horizon is a decade,” Dr. Garcia said. “I think within a matter of 2 or 3 years, these tools will be pervasive throughout health care.”
Since introducing these tools at Cooper, “our doctors’ paperwork burden is significantly lighter,” said Dr. Mazzarelli, who decides which technologies Cooper should invest in and who monitors their results. In Cooper studies, physicians who used DAX more than half the time spent 43% less time working on notes.
“They spend more time connecting with their patients, talking with them, and looking them in the eye,” Dr. Mazzarelli said. That, in turn, seems to improve patient outcomes, reduce doctor burnout and turnover, and lower costs.
The AI scribes, by virtue of eliminating the distraction of note taking, also allow doctors to give their full attention to the patient. “The patient relationship is the most important aspect of medicine,” said Raul Ayala, MD, MHCM, a family medicine physician at Adventist Health, in Hanford, Calif., who uses Augmedix. The digital scribe “helps us strengthen that relationship.”
What’s it like to use an AI medical scribe?
The scribes feature hardware (typically a smartphone or tablet) and software built on automatic speech recognition, natural language processing, and machine learning. Download an app to your device, and you’re ready to go. Use it to record in-person or telehealth visits.
In the first week, a company may help train you to use the hardware and software. You’ll likely start by using it for a few patient visits per day, ramping up gradually. Dr. Partida said she was comfortable using the system for all her patients in 6 weeks.
Each day, Dr. Partida logs in to a dedicated smartphone or tablet, opens the app, and reviews her schedule, including details she needs to prepare for each patient.
At the start of each patient visit, Dr. Partida taps the app icon to begin recording and lays the device nearby. She can pause as needed. At the end of the visit, she taps the icon again to stop recording.
The AI listens, creates the note, and updates relevant data in the EHR. The note includes patient problems, assessment, treatment plan, patient history, orders, and tasks for staff, along with medications, referrals, and preauthorizations. A human scribe, who is also a physician, reviews the information for accuracy and edits it as needed. By the next morning, the data are ready for Dr. Partida to review.
Fully automated versions can generate notes much faster. Jack Shilling, MD, MBA, an orthopedic surgeon at Cooper University Health Care, in Voorhees, N.J., uses DAX. A new feature called DAX Express – which uses OpenAI’s GPT-4 but no humans – provides him with a draft of his clinical notes in just seconds.
How accurate are AI notes?
The accuracy of those notes remains an open question, Dr. Garcia said – mostly because accuracy can be hard to define.
“If you asked five docs to write a note based on the same patient encounter, you’d get five different notes,” Dr. Garcia said. “That makes it hard to assess these technologies in a scientifically rigorous way.”
Still, the onus is on the physician to review the notes and edit them as needed, Dr. Garcia said. How light or heavy those edits are can depend on your unique preferences.
Dr. Shilling said he may need to lightly edit transcripts of his conversations with patients. “When someone tells me how long their knee hurts, slight variability in their transcribed words is tolerable,” he said. But for some things – such as physical exam notes and x-ray readings – he dictates directly into the device, speaking at a closer range and being less conversational, more exact in his speech.
Should you let patients know they’re being recorded?
The federal Health Insurance Portability and Accountability Act (HIPAA) does not require providers to inform patients that their face-to-face conversations are being recorded, said Daniel Lebovic, JD, corporate legal counsel at Compliancy Group, in Greenlawn, N.Y., a company that helps providers adhere to HIPAA rules.
But make sure you know the laws in your state and the policies at your health care practice. State laws may require providers to inform patients and to get patients’ consent in advance of being recorded.
All the doctors who spoke to this news organization said their patients are informed that they’ll be recorded and that they can opt out if they wish.
How much do AI scribes cost?
As the marketplace for these tools expands, companies are offering more products and services at different price points that target a range of organizations, from large health care systems to small private practices.
Price models vary, said Dr. Garcia. Some are based on the number of users, others on the number of notes, and still others on minutes.
Amazon’s HealthScribe is priced at 10 cents per minute. For 1,000 consultation transcripts per month, with each call averaging 15 minutes, it would take 15,000 minutes at a total cost of $1,500 for the month.
In general, the rapidly growing competition in this space could mean prices become more affordable, Dr. Garcia said. “It’s good that so many are getting into this game, because that means the price will come down and it will be a lot more accessible to everybody.”
A version of this article appeared on Medscape.com.
Generic inhalers for COPD support hold their own
Sometimes we get what we pay for. Other times we pay too much.
That’s the message of a study published in Annals of Internal Medicine, which finds that a generic maintenance inhaler is as effective at managing symptoms of chronic obstructive pulmonary disorder (COPD) as a pricier branded alternative.
In 2019, the Food and Drug Administration approved Wixela Inhub (the combination corticosteroid/long-acting beta2 adrenergic agonist fluticasone-salmeterol; Viatris) as a generic dry powder inhaler for managing symptoms of COPD. This approval was based on evidence of the generic’s effectiveness against asthma, although COPD also was on the product label. The study authors compared Wixela’s effectiveness in controlling symptoms of COPD with that of the brand name inhaler Advair Diskus (fluticasone-salmeterol; GlaxoSmithKline), which uses the same active ingredients.
The result: “The generic looks to be as safe and effective as the brand name. I don’t see a clinical reason why one would ever need to get the brand name over the generic version,” said study author William Feldman, MD, DPhil, MPH, a health services researcher and pulmonologist at Harvard Medical School and Brigham and Women’s Hospital, both in Boston.
Same types of patients, different inhalers, same outcomes
Dr. Feldman and colleagues compared the medical records of 10,000 patients with COPD who began using the branded inhaler to the records of another 10,000 patients with COPD who opted for the generic alternative. Participants in the two groups were evenly matched by age, sex, race, and ethnicity, region, severity of COPD, and presence of other comorbidities, according to the researchers. Participants were all older than age 40, and the average age in both groups was 72 years.
The researchers looked for a difference in a first episode of a moderate exacerbation of COPD, defined as requiring a course of prednisone for 5-14 days. They also looked for cases of severe COPD exacerbation requiring hospitalization in the year after people began using either the generic or brand name inhaler. And they looked for differences across 1 year in rates of hospitalization for pneumonia.
For none of those outcomes, however, did the type of inhaler appear to matter. Compared with the brand-name drug, using the generic was associated with nearly identical rates of moderate or severe COPD exacerbation (hazard ratio, 0.97; 95% confidence interval, 0.90-1.04. The same was true for the proportion of people who went to the hospital for pneumonia at least once (HR, 0.99; 95% CI, 0.86-1.15).
“To get through the FDA as an interchangeable generic, the generic firms have to show that their product can be used in just the same way as the brand-name version,” Dr. Feldman said, which may explain why the generic and brand-name versions of the inhaler performed so similarly.
Dr. Feldman cautioned that the price savings for patients who opt for the generic over the branded product are hard to determine, given the vagaries of different insurance plans and potential rebates when using the branded project. As a general matter, having a single generic competitor will not lower costs much, Dr. Feldman noted, pointing to 2017 research from Harvard that found a profusion of generic competitors is needed to significantly lower health care costs.
“I don’t want to in any way underestimate the importance of getting that first generic onto the market, because it sets the stage for future generics,” Dr. Feldman said.
“There are very few generic options for patients with COPD,” said Surya Bhatt, MD, director of the Pulmonary Function and Exercise Physiology Lab at the University of Alabama at Birmingham. Even the rescue inhalers that people with COPD use to manage acute episodes of the condition are usually branded at this time, Dr. Bhatt noted, with few generic options.*
“The results are quite compelling,” said Dr. Bhatt, who was not involved in the research. Although the trial was not randomized, he commended the researchers for stratifying participants in the two groups to be as comparable as possible.
Dr. Bhatt noted that the FDA’s 2019 approval – given that the agency requires bioequivalence studies between branded and generic products – was enough to cause him to begin prescribing the generic inhaler. The fact that this approval was based on asthma but not also COPD is not a concern.
“There are so many similarities between asthma, COPD, and some obstructive lung diseases,” Dr. Bhatt noted.
In his experience, the only time someone with COPD continues using the branded inhaler – now that a potentially cheaper generic is available – is when their insurance plan makes their out-of-pocket cost minimal. Otherwise, brand loyalty does not exist.
“Patients are generally okay with being on a generic for inhalers, just because of the high cost,” Dr. Bhatt said.
The study was primarily supported by the National Heart, Lung, and Blood Institute. Dr. Feldman reported funding from Arnold Ventures, the Commonwealth Fund, and the FDA, and consulting relationships with Alosa Health and Aetion. Dr. Bhatt reported no relevant financial relationships.
*Correction, 8/16/23: An earlier version of this article mischaracterized Dr. Bhatt's comments on the availability of generic options.
A version of this article first appeared on Medscape.com.
Sometimes we get what we pay for. Other times we pay too much.
That’s the message of a study published in Annals of Internal Medicine, which finds that a generic maintenance inhaler is as effective at managing symptoms of chronic obstructive pulmonary disorder (COPD) as a pricier branded alternative.
In 2019, the Food and Drug Administration approved Wixela Inhub (the combination corticosteroid/long-acting beta2 adrenergic agonist fluticasone-salmeterol; Viatris) as a generic dry powder inhaler for managing symptoms of COPD. This approval was based on evidence of the generic’s effectiveness against asthma, although COPD also was on the product label. The study authors compared Wixela’s effectiveness in controlling symptoms of COPD with that of the brand name inhaler Advair Diskus (fluticasone-salmeterol; GlaxoSmithKline), which uses the same active ingredients.
The result: “The generic looks to be as safe and effective as the brand name. I don’t see a clinical reason why one would ever need to get the brand name over the generic version,” said study author William Feldman, MD, DPhil, MPH, a health services researcher and pulmonologist at Harvard Medical School and Brigham and Women’s Hospital, both in Boston.
Same types of patients, different inhalers, same outcomes
Dr. Feldman and colleagues compared the medical records of 10,000 patients with COPD who began using the branded inhaler to the records of another 10,000 patients with COPD who opted for the generic alternative. Participants in the two groups were evenly matched by age, sex, race, and ethnicity, region, severity of COPD, and presence of other comorbidities, according to the researchers. Participants were all older than age 40, and the average age in both groups was 72 years.
The researchers looked for a difference in a first episode of a moderate exacerbation of COPD, defined as requiring a course of prednisone for 5-14 days. They also looked for cases of severe COPD exacerbation requiring hospitalization in the year after people began using either the generic or brand name inhaler. And they looked for differences across 1 year in rates of hospitalization for pneumonia.
For none of those outcomes, however, did the type of inhaler appear to matter. Compared with the brand-name drug, using the generic was associated with nearly identical rates of moderate or severe COPD exacerbation (hazard ratio, 0.97; 95% confidence interval, 0.90-1.04. The same was true for the proportion of people who went to the hospital for pneumonia at least once (HR, 0.99; 95% CI, 0.86-1.15).
“To get through the FDA as an interchangeable generic, the generic firms have to show that their product can be used in just the same way as the brand-name version,” Dr. Feldman said, which may explain why the generic and brand-name versions of the inhaler performed so similarly.
Dr. Feldman cautioned that the price savings for patients who opt for the generic over the branded product are hard to determine, given the vagaries of different insurance plans and potential rebates when using the branded project. As a general matter, having a single generic competitor will not lower costs much, Dr. Feldman noted, pointing to 2017 research from Harvard that found a profusion of generic competitors is needed to significantly lower health care costs.
“I don’t want to in any way underestimate the importance of getting that first generic onto the market, because it sets the stage for future generics,” Dr. Feldman said.
“There are very few generic options for patients with COPD,” said Surya Bhatt, MD, director of the Pulmonary Function and Exercise Physiology Lab at the University of Alabama at Birmingham. Even the rescue inhalers that people with COPD use to manage acute episodes of the condition are usually branded at this time, Dr. Bhatt noted, with few generic options.*
“The results are quite compelling,” said Dr. Bhatt, who was not involved in the research. Although the trial was not randomized, he commended the researchers for stratifying participants in the two groups to be as comparable as possible.
Dr. Bhatt noted that the FDA’s 2019 approval – given that the agency requires bioequivalence studies between branded and generic products – was enough to cause him to begin prescribing the generic inhaler. The fact that this approval was based on asthma but not also COPD is not a concern.
“There are so many similarities between asthma, COPD, and some obstructive lung diseases,” Dr. Bhatt noted.
In his experience, the only time someone with COPD continues using the branded inhaler – now that a potentially cheaper generic is available – is when their insurance plan makes their out-of-pocket cost minimal. Otherwise, brand loyalty does not exist.
“Patients are generally okay with being on a generic for inhalers, just because of the high cost,” Dr. Bhatt said.
The study was primarily supported by the National Heart, Lung, and Blood Institute. Dr. Feldman reported funding from Arnold Ventures, the Commonwealth Fund, and the FDA, and consulting relationships with Alosa Health and Aetion. Dr. Bhatt reported no relevant financial relationships.
*Correction, 8/16/23: An earlier version of this article mischaracterized Dr. Bhatt's comments on the availability of generic options.
A version of this article first appeared on Medscape.com.
Sometimes we get what we pay for. Other times we pay too much.
That’s the message of a study published in Annals of Internal Medicine, which finds that a generic maintenance inhaler is as effective at managing symptoms of chronic obstructive pulmonary disorder (COPD) as a pricier branded alternative.
In 2019, the Food and Drug Administration approved Wixela Inhub (the combination corticosteroid/long-acting beta2 adrenergic agonist fluticasone-salmeterol; Viatris) as a generic dry powder inhaler for managing symptoms of COPD. This approval was based on evidence of the generic’s effectiveness against asthma, although COPD also was on the product label. The study authors compared Wixela’s effectiveness in controlling symptoms of COPD with that of the brand name inhaler Advair Diskus (fluticasone-salmeterol; GlaxoSmithKline), which uses the same active ingredients.
The result: “The generic looks to be as safe and effective as the brand name. I don’t see a clinical reason why one would ever need to get the brand name over the generic version,” said study author William Feldman, MD, DPhil, MPH, a health services researcher and pulmonologist at Harvard Medical School and Brigham and Women’s Hospital, both in Boston.
Same types of patients, different inhalers, same outcomes
Dr. Feldman and colleagues compared the medical records of 10,000 patients with COPD who began using the branded inhaler to the records of another 10,000 patients with COPD who opted for the generic alternative. Participants in the two groups were evenly matched by age, sex, race, and ethnicity, region, severity of COPD, and presence of other comorbidities, according to the researchers. Participants were all older than age 40, and the average age in both groups was 72 years.
The researchers looked for a difference in a first episode of a moderate exacerbation of COPD, defined as requiring a course of prednisone for 5-14 days. They also looked for cases of severe COPD exacerbation requiring hospitalization in the year after people began using either the generic or brand name inhaler. And they looked for differences across 1 year in rates of hospitalization for pneumonia.
For none of those outcomes, however, did the type of inhaler appear to matter. Compared with the brand-name drug, using the generic was associated with nearly identical rates of moderate or severe COPD exacerbation (hazard ratio, 0.97; 95% confidence interval, 0.90-1.04. The same was true for the proportion of people who went to the hospital for pneumonia at least once (HR, 0.99; 95% CI, 0.86-1.15).
“To get through the FDA as an interchangeable generic, the generic firms have to show that their product can be used in just the same way as the brand-name version,” Dr. Feldman said, which may explain why the generic and brand-name versions of the inhaler performed so similarly.
Dr. Feldman cautioned that the price savings for patients who opt for the generic over the branded product are hard to determine, given the vagaries of different insurance plans and potential rebates when using the branded project. As a general matter, having a single generic competitor will not lower costs much, Dr. Feldman noted, pointing to 2017 research from Harvard that found a profusion of generic competitors is needed to significantly lower health care costs.
“I don’t want to in any way underestimate the importance of getting that first generic onto the market, because it sets the stage for future generics,” Dr. Feldman said.
“There are very few generic options for patients with COPD,” said Surya Bhatt, MD, director of the Pulmonary Function and Exercise Physiology Lab at the University of Alabama at Birmingham. Even the rescue inhalers that people with COPD use to manage acute episodes of the condition are usually branded at this time, Dr. Bhatt noted, with few generic options.*
“The results are quite compelling,” said Dr. Bhatt, who was not involved in the research. Although the trial was not randomized, he commended the researchers for stratifying participants in the two groups to be as comparable as possible.
Dr. Bhatt noted that the FDA’s 2019 approval – given that the agency requires bioequivalence studies between branded and generic products – was enough to cause him to begin prescribing the generic inhaler. The fact that this approval was based on asthma but not also COPD is not a concern.
“There are so many similarities between asthma, COPD, and some obstructive lung diseases,” Dr. Bhatt noted.
In his experience, the only time someone with COPD continues using the branded inhaler – now that a potentially cheaper generic is available – is when their insurance plan makes their out-of-pocket cost minimal. Otherwise, brand loyalty does not exist.
“Patients are generally okay with being on a generic for inhalers, just because of the high cost,” Dr. Bhatt said.
The study was primarily supported by the National Heart, Lung, and Blood Institute. Dr. Feldman reported funding from Arnold Ventures, the Commonwealth Fund, and the FDA, and consulting relationships with Alosa Health and Aetion. Dr. Bhatt reported no relevant financial relationships.
*Correction, 8/16/23: An earlier version of this article mischaracterized Dr. Bhatt's comments on the availability of generic options.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
COPD plus PRISm may promote frailty progression
Chronic obstructive pulmonary disease and a new phenotype of lung function impairment predicted progression of frailty in older adults, based on data from more than 5,000 individuals.
COPD has been associated with frailty, but longitudinal data on the association of COPD with progression of frailty are limited, as are data on the potential association of preserved ratio impaired spirometry (PRISm) with frailty progression, wrote Di He, BS, of Zhejiang University, China, and colleagues.
PRISm has been defined in recent studies as “proportional impairments in FEV1 and FVC, resulting in the normal ratio of FEV1 and FVC.” Individuals with PRISm may transition to normal spirometry or COPD over time, the researchers wrote.
In a study published in the journal Chest, the researchers reviewed data from 5,901 adults aged 50 years and older who were participating on the English Longitudinal Study of Ageing (ELSA), a prospective cohort study. Of these, 3,765 were included in an additional analysis of the association between transitions from normal spirometry to PRISm and the progression of frailty. The mean age of the participants was 65.5 years; 54.9% were women.
The median follow-up period for analysis with frailty progression was 9.5 years for PRISm and COPD and 5.8 years for PRISm transitions. Lung function data were collected at baseline. Based on spirometry data, participants were divided into three lung function groups – normal spirometry, PRISm, and COPD – and each of these was classified based on severity. Frailty was assessed using the frailty index (FI) during the follow-up period.
with additional annual increases of 0.301 and 0.172, respectively (P < .001 for both).
When stratified by severity, individuals with more severe PRISm and with more COPD had higher baseline FI and faster FI progression, compared with those with mild PRISm and COPD.
PRISm transitions were assessed over a 4-year interval at the start of the ELSA. Individuals with normal spirometry who transitioned to PRISm during the study had accelerated progression of frailty, as did those with COPD who transitioned to PRISm. However, no significant frailty progression occurred in those who changed from PRISm to normal spirometry.
The mechanisms behind the associations of PRISm and COPD with frailty remain unclear, but the results were consistent after controlling for multiple confounders, “suggesting PRISm and COPD had independent pathophysiological mechanisms for frailty,” the researchers write in their discussion. Other recent studies have identified sarcopenia as a complication for individuals with lung function impairment, they noted. “Therefore, another plausible explanation could be that PRISm and COPD caused sarcopenia, which accelerated frailty progression,” they say.
The findings were limited by several factors, including the observational design and the potential underestimation of lung function in participants with reversible airflow obstruction because of the use of prebronchodilator spirometry in the cohort study, the researchers noted.
However, the results were strengthened by the large sample size and high-quality data from the ELSA, as well as by the repeat measures of FI and lung function. The results were consistent after controlling for multiple confounders, and support the need for more research to explore the causality behind the association of PRISm and COPD with frailty, the researchers concluded.
The study was supported by the Zhejiang Provincial Basic Public Welfare Research Project, the Zhoushan Science and Technology Project, and the Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province. The researchers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Chronic obstructive pulmonary disease and a new phenotype of lung function impairment predicted progression of frailty in older adults, based on data from more than 5,000 individuals.
COPD has been associated with frailty, but longitudinal data on the association of COPD with progression of frailty are limited, as are data on the potential association of preserved ratio impaired spirometry (PRISm) with frailty progression, wrote Di He, BS, of Zhejiang University, China, and colleagues.
PRISm has been defined in recent studies as “proportional impairments in FEV1 and FVC, resulting in the normal ratio of FEV1 and FVC.” Individuals with PRISm may transition to normal spirometry or COPD over time, the researchers wrote.
In a study published in the journal Chest, the researchers reviewed data from 5,901 adults aged 50 years and older who were participating on the English Longitudinal Study of Ageing (ELSA), a prospective cohort study. Of these, 3,765 were included in an additional analysis of the association between transitions from normal spirometry to PRISm and the progression of frailty. The mean age of the participants was 65.5 years; 54.9% were women.
The median follow-up period for analysis with frailty progression was 9.5 years for PRISm and COPD and 5.8 years for PRISm transitions. Lung function data were collected at baseline. Based on spirometry data, participants were divided into three lung function groups – normal spirometry, PRISm, and COPD – and each of these was classified based on severity. Frailty was assessed using the frailty index (FI) during the follow-up period.
with additional annual increases of 0.301 and 0.172, respectively (P < .001 for both).
When stratified by severity, individuals with more severe PRISm and with more COPD had higher baseline FI and faster FI progression, compared with those with mild PRISm and COPD.
PRISm transitions were assessed over a 4-year interval at the start of the ELSA. Individuals with normal spirometry who transitioned to PRISm during the study had accelerated progression of frailty, as did those with COPD who transitioned to PRISm. However, no significant frailty progression occurred in those who changed from PRISm to normal spirometry.
The mechanisms behind the associations of PRISm and COPD with frailty remain unclear, but the results were consistent after controlling for multiple confounders, “suggesting PRISm and COPD had independent pathophysiological mechanisms for frailty,” the researchers write in their discussion. Other recent studies have identified sarcopenia as a complication for individuals with lung function impairment, they noted. “Therefore, another plausible explanation could be that PRISm and COPD caused sarcopenia, which accelerated frailty progression,” they say.
The findings were limited by several factors, including the observational design and the potential underestimation of lung function in participants with reversible airflow obstruction because of the use of prebronchodilator spirometry in the cohort study, the researchers noted.
However, the results were strengthened by the large sample size and high-quality data from the ELSA, as well as by the repeat measures of FI and lung function. The results were consistent after controlling for multiple confounders, and support the need for more research to explore the causality behind the association of PRISm and COPD with frailty, the researchers concluded.
The study was supported by the Zhejiang Provincial Basic Public Welfare Research Project, the Zhoushan Science and Technology Project, and the Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province. The researchers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Chronic obstructive pulmonary disease and a new phenotype of lung function impairment predicted progression of frailty in older adults, based on data from more than 5,000 individuals.
COPD has been associated with frailty, but longitudinal data on the association of COPD with progression of frailty are limited, as are data on the potential association of preserved ratio impaired spirometry (PRISm) with frailty progression, wrote Di He, BS, of Zhejiang University, China, and colleagues.
PRISm has been defined in recent studies as “proportional impairments in FEV1 and FVC, resulting in the normal ratio of FEV1 and FVC.” Individuals with PRISm may transition to normal spirometry or COPD over time, the researchers wrote.
In a study published in the journal Chest, the researchers reviewed data from 5,901 adults aged 50 years and older who were participating on the English Longitudinal Study of Ageing (ELSA), a prospective cohort study. Of these, 3,765 were included in an additional analysis of the association between transitions from normal spirometry to PRISm and the progression of frailty. The mean age of the participants was 65.5 years; 54.9% were women.
The median follow-up period for analysis with frailty progression was 9.5 years for PRISm and COPD and 5.8 years for PRISm transitions. Lung function data were collected at baseline. Based on spirometry data, participants were divided into three lung function groups – normal spirometry, PRISm, and COPD – and each of these was classified based on severity. Frailty was assessed using the frailty index (FI) during the follow-up period.
with additional annual increases of 0.301 and 0.172, respectively (P < .001 for both).
When stratified by severity, individuals with more severe PRISm and with more COPD had higher baseline FI and faster FI progression, compared with those with mild PRISm and COPD.
PRISm transitions were assessed over a 4-year interval at the start of the ELSA. Individuals with normal spirometry who transitioned to PRISm during the study had accelerated progression of frailty, as did those with COPD who transitioned to PRISm. However, no significant frailty progression occurred in those who changed from PRISm to normal spirometry.
The mechanisms behind the associations of PRISm and COPD with frailty remain unclear, but the results were consistent after controlling for multiple confounders, “suggesting PRISm and COPD had independent pathophysiological mechanisms for frailty,” the researchers write in their discussion. Other recent studies have identified sarcopenia as a complication for individuals with lung function impairment, they noted. “Therefore, another plausible explanation could be that PRISm and COPD caused sarcopenia, which accelerated frailty progression,” they say.
The findings were limited by several factors, including the observational design and the potential underestimation of lung function in participants with reversible airflow obstruction because of the use of prebronchodilator spirometry in the cohort study, the researchers noted.
However, the results were strengthened by the large sample size and high-quality data from the ELSA, as well as by the repeat measures of FI and lung function. The results were consistent after controlling for multiple confounders, and support the need for more research to explore the causality behind the association of PRISm and COPD with frailty, the researchers concluded.
The study was supported by the Zhejiang Provincial Basic Public Welfare Research Project, the Zhoushan Science and Technology Project, and the Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province. The researchers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL CHEST
Classification of COPD exacerbation predicts prognosis
Adults with exacerbations of chronic obstructive pulmonary disease (ECOPD) whose condition was classified as severe using the Rome criteria had a higher risk of death at 1 year than those who were classified as having moderate or mild disease, as determined from data from more than 300 individuals.
Patients hospitalized with severe exacerbations of ECOPD are at increased risk for worse clinical outcomes and death, so early identification is important, Ernesto Crisafulli, MD, of the University of Verona (Italy) and Azienda Ospedaliera Universitaria Integrata of Verona, and colleagues wrote.
which grades ECOPD as mild, moderate, or severe on the basis of more objective and disease-related aspects. However, data on the clinical usefulness of the Rome criteria are limited.
In a study published in the journal Chest, the researchers retrospectively categorized 347 adults hospitalized with ECOPD using the Rome severity classifications of mild, moderate, and severe.
Classifications were made using baseline, clinical and microbiological factors, as well as gas analysis and laboratory variables. The researchers also reviewed data on the length of hospital stay and mortality (in-hospital and over a follow-up of 6 months to 3 years).
Approximately one-third of the patients (39%) were classified as having mild disease, 31% as having moderate disease, and 30% as having severe illness. Overall, hospital stay was significantly longer for the patients with severe disease, although in-hospital mortality was similar across all three groups.
Patients classified as having severe disease also had a worse prognosis at all follow-up time points, and severe classification was significantly associated with worse cumulative survival at 1 year and 3 years (Gehan-Breslow-Wilson test, P = .032 and P = .004, respectively).
In a multivariate analysis, the risk of death at 1 year was significantly higher among patients classified as severe or moderate (hazard ratio, 1.99 and 1.47, respectively), compared with those classified as mild.
Mortality risk also was higher among patients aged 80 years and older and among those requiring long-term oxygen therapy or with a history of ECOPD episodes, the researchers noted. Body mass index in the range of 25-29 kg/m2 was associated with lower risk.
The study was limited by several factors, including the replacement of dyspnea perception in the Rome classification with other objective measures, the researchers wrote. Other limitations include the retrospective design, small sample size, use of data from a single center, and lack of data on causes of mortality. Women were underrepresented in the study, and so additional research involving women is needed.
The results suggest that the Rome classification allows for the effective identification of patients with ECOPD who have a worse prognosis. The Rome classification may help guide disease management through targeted interventions and personalized care programs for this population, the researchers concluded.
The study received no outside funding. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adults with exacerbations of chronic obstructive pulmonary disease (ECOPD) whose condition was classified as severe using the Rome criteria had a higher risk of death at 1 year than those who were classified as having moderate or mild disease, as determined from data from more than 300 individuals.
Patients hospitalized with severe exacerbations of ECOPD are at increased risk for worse clinical outcomes and death, so early identification is important, Ernesto Crisafulli, MD, of the University of Verona (Italy) and Azienda Ospedaliera Universitaria Integrata of Verona, and colleagues wrote.
which grades ECOPD as mild, moderate, or severe on the basis of more objective and disease-related aspects. However, data on the clinical usefulness of the Rome criteria are limited.
In a study published in the journal Chest, the researchers retrospectively categorized 347 adults hospitalized with ECOPD using the Rome severity classifications of mild, moderate, and severe.
Classifications were made using baseline, clinical and microbiological factors, as well as gas analysis and laboratory variables. The researchers also reviewed data on the length of hospital stay and mortality (in-hospital and over a follow-up of 6 months to 3 years).
Approximately one-third of the patients (39%) were classified as having mild disease, 31% as having moderate disease, and 30% as having severe illness. Overall, hospital stay was significantly longer for the patients with severe disease, although in-hospital mortality was similar across all three groups.
Patients classified as having severe disease also had a worse prognosis at all follow-up time points, and severe classification was significantly associated with worse cumulative survival at 1 year and 3 years (Gehan-Breslow-Wilson test, P = .032 and P = .004, respectively).
In a multivariate analysis, the risk of death at 1 year was significantly higher among patients classified as severe or moderate (hazard ratio, 1.99 and 1.47, respectively), compared with those classified as mild.
Mortality risk also was higher among patients aged 80 years and older and among those requiring long-term oxygen therapy or with a history of ECOPD episodes, the researchers noted. Body mass index in the range of 25-29 kg/m2 was associated with lower risk.
The study was limited by several factors, including the replacement of dyspnea perception in the Rome classification with other objective measures, the researchers wrote. Other limitations include the retrospective design, small sample size, use of data from a single center, and lack of data on causes of mortality. Women were underrepresented in the study, and so additional research involving women is needed.
The results suggest that the Rome classification allows for the effective identification of patients with ECOPD who have a worse prognosis. The Rome classification may help guide disease management through targeted interventions and personalized care programs for this population, the researchers concluded.
The study received no outside funding. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adults with exacerbations of chronic obstructive pulmonary disease (ECOPD) whose condition was classified as severe using the Rome criteria had a higher risk of death at 1 year than those who were classified as having moderate or mild disease, as determined from data from more than 300 individuals.
Patients hospitalized with severe exacerbations of ECOPD are at increased risk for worse clinical outcomes and death, so early identification is important, Ernesto Crisafulli, MD, of the University of Verona (Italy) and Azienda Ospedaliera Universitaria Integrata of Verona, and colleagues wrote.
which grades ECOPD as mild, moderate, or severe on the basis of more objective and disease-related aspects. However, data on the clinical usefulness of the Rome criteria are limited.
In a study published in the journal Chest, the researchers retrospectively categorized 347 adults hospitalized with ECOPD using the Rome severity classifications of mild, moderate, and severe.
Classifications were made using baseline, clinical and microbiological factors, as well as gas analysis and laboratory variables. The researchers also reviewed data on the length of hospital stay and mortality (in-hospital and over a follow-up of 6 months to 3 years).
Approximately one-third of the patients (39%) were classified as having mild disease, 31% as having moderate disease, and 30% as having severe illness. Overall, hospital stay was significantly longer for the patients with severe disease, although in-hospital mortality was similar across all three groups.
Patients classified as having severe disease also had a worse prognosis at all follow-up time points, and severe classification was significantly associated with worse cumulative survival at 1 year and 3 years (Gehan-Breslow-Wilson test, P = .032 and P = .004, respectively).
In a multivariate analysis, the risk of death at 1 year was significantly higher among patients classified as severe or moderate (hazard ratio, 1.99 and 1.47, respectively), compared with those classified as mild.
Mortality risk also was higher among patients aged 80 years and older and among those requiring long-term oxygen therapy or with a history of ECOPD episodes, the researchers noted. Body mass index in the range of 25-29 kg/m2 was associated with lower risk.
The study was limited by several factors, including the replacement of dyspnea perception in the Rome classification with other objective measures, the researchers wrote. Other limitations include the retrospective design, small sample size, use of data from a single center, and lack of data on causes of mortality. Women were underrepresented in the study, and so additional research involving women is needed.
The results suggest that the Rome classification allows for the effective identification of patients with ECOPD who have a worse prognosis. The Rome classification may help guide disease management through targeted interventions and personalized care programs for this population, the researchers concluded.
The study received no outside funding. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL CHEST
Drug name confusion: More than 80 new drug pairs added to the list
Zolpidem (Ambien) is a well-known sedative for sleep. Letairis (Ambrisentan) is a vasodilator for the treatment of pulmonary arterial hypertension. Citalopram (Celexa) is an antidepressant; escitalopram (Lexapro) is prescribed for anxiety and depression.
The aim is to increase awareness about the potential for a serious medication mistake when the wrong drug is given because of drug names that look and sound similar.
Awareness of these drug names, however, is just the first step in preventing medication mistakes. Health care providers should take a number of other steps as well, experts said.
ISMP launched its confusing drug names list, previously called look-alike, sound-alike (LASA) drugs, in 2008. The new list is an update of the 2019 version, said Michael J. Gaunt, PharmD, senior manager of error reporting programs for the ISMP, which focuses on the prevention of medication mistakes. The new entries were chosen on the basis of a number of factors, including ISMP’s analysis of recent medication mishap reports that were submitted to it.
The ISMP list now includes about 528 drug pairs, Dr. Gaunt said. The list is long, he said, partly because each pair is listed twice, so readers can cross reference. For instance, hydralazine and hydroxyzine are listed in one entry in the list, and hydroxyzine and hydralazine are listed in another.
Brand Institute in Miami has named, among other drugs, Entresto, Rybelsus, and Lunesta. The regulatory arm of the company, the Drug Safety Institute, “considers drug names that have been confused as an important part of our comprehensive drug name assessments,” Todd Bridges, global president of the institute, said in an emailed statement. Information on the confusing drug names are incorporated into the company’s proprietary algorithm and is used when developing brand names for drugs. “We continually update this algorithm as new drug names that are often confused are identified,” Mr. Bridges said.
Confusing drug names: Ongoing issue
The length of the list, as well as the latest additions, are not surprising, said Mary Ann Kliethermes, PharmD, director of medication safety and quality for the American Society of Health-System Pharmacists, a membership organization of about 60,000 pharmacists who practice in inpatient and outpatient settings.
“I’ve been in practice over 45 years,” she said, “and this has been a problem ever since I have been in practice.” The sheer volume of new drugs is one reason, she said. From 2013 through 2022, the U.S. Food and Drug Administration approved an average of 43 novel drugs per year, according to a report from its Center for Drug Evaluation and Research. “Since the 90s, this [confusion about similar drug names] has happened,” Dr. Kliethermes said.
According to a 2023 report, about 7,000-9,000 people die each year in the United States as the result of a medication error. However, it’s impossible to say for sure what percentage of those errors involve name confusion, Dr. Gaunt said.
Not all the mistakes are reported. Some that are reported are dramatic and deadly. In 2022, a Tennessee nurse was convicted of gross neglect and negligent homicide. She was sentenced to 3 years’ probation after she mistakenly gave vercuronium, an anesthetic agent, instead of the sedative Versed to a patient, and the woman died.
Updated list: A closer look
Many of the new drug pairs that are listed in the update are cephalosporins, said Dr. Kliethermes, who reviewed the new list for this news organization. In all, 20 of the latest 82 additions are cephalosporins. These include drugs such as cefazolin, which can be confused with cefotetan, and vice versa. These drugs have been around since the 1980s, she said, but “they needed to be on there.” Even in the 1980s, it was becoming difficult to differentiate them, and there were fewer drugs in that class then, she said.
Influenza vaccines made the new list, too. Fluzone High-Dose Quadrivalent can be confused with fluzone quadrivalent. Other new additions: hydrochlorothiazide and hydroxychloroquine, Lasik and Wakix, Pitressin and Pitocin, Remeron and Rozerem.
Beyond the list
While it’s not possible to pinpoint how big a problem name confusion is in causing medication mistakes, “it is certainly still an issue,” Dr. Gaunt said. A variety of practices can reduce that risk substantially, Dr. Gaunt and Dr. Kliethermes agreed.
Tall-man lettering. Both the FDA and the ISMP recommend the use of so-called tall-man lettering (TML), which involves the use of uppercase letters, sometimes in boldface, to distinguish similar names on product labels and elsewhere. Examples include vinBLAStine and vinCRIStine.
Electronic prescribing. “It eliminates the risk of handwriting confusion,” Dr. Gaunt said. However, electronic prescribing can have a downside, Dr. Kliethermes said. When ordering medication, a person may type in a few letters and may then be presented with a prompt that lists several drug names, and it can be easy to click the wrong one. For that reason, ISMP and other experts recommend typing at least five letters when searching for a medication in an electronic system.
Use both brand and generic names on labels and prescriptions.
Write the indication. That can serve as a double check. If a prescription for Ambien says “For sleep,” there’s probably less risk of filling a prescription for ambrisentan, the vasodilator.
Smart formulary additions. When hospitals add medications to their formularies, “part of that formulary assessment should include looking at the potential risk for errors,” Dr. Gaunt said. This involves keeping an eye out for confusing names and similar packaging. “Do that analysis up front and put in strategies to minimize that. Maybe you look for a different drug [for the same use] that has a different name.” Or choose a different manufacturer, so the medication would at least have a different container.
Use bar code scanning. Suppose a pharmacist goes to the shelf and pulls the wrong drug. “Bar code scanning provides the opportunity to catch the error,” Dr. Gaunt said. Many community pharmacies now have bar code scanning. ISMP just issued best practices for community pharmacies, Dr. Gaunt said, and these include the use of bar code scanning and other measures.
Educate consumers. Health care providers can educate consumers on how to minimize the risk of getting the wrong drug, Dr. Gaunt said. When patients are picking up a prescription, suggest they look at the container label; if it looks different from previous prescriptions of the same medicine, ask the pharmacist for an explanation. Some patients just pass it off, Dr. Gaunt said, figuring the pharmacist or health plan switched manufacturers of their medication.
Access the list. The entire list is on the ISMP site and is accessible after free registration.
Goal: Preventing confusion
The FDA has provided guidance for industry on naming drugs not yet approved so that the proposed names are not too similar in sound or appearance to those already on the market. Included in the lengthy document are checklists, such as, “Across a range of dialects, are the names consistently pronounced differently?” and “Are the lengths of the names dissimilar when scripted?” (Lengths are considered different if they differ by two or more letters.)
The FDA also offers the phonetic and orthographic computer analysis (POCA) program, a software tool that employs an advanced algorithm to evaluate similarities between two drug names. The data sources are updated regularly as new drugs are approved.
Liability update
The problem may be decreasing. In a 2020 report, researchers used pharmacists’ professional liability claim data from the Healthcare Providers Service Organization. They compared 2018 data on claims with 2013 data. The percentage of claims associated with wrong drug dispensing errors declined from 43.8% in 2013 to 36.8% in 2018. Wrong dose claims also declined, from 31.5% to 15.3%.
These researchers concluded that technology and automation have contributed to the prevention of medication errors caused by the use of the wrong drug and the wrong dose, but mistakes continue, owing to system and human errors.
A version of this article first appeared on Medscape.com.
Zolpidem (Ambien) is a well-known sedative for sleep. Letairis (Ambrisentan) is a vasodilator for the treatment of pulmonary arterial hypertension. Citalopram (Celexa) is an antidepressant; escitalopram (Lexapro) is prescribed for anxiety and depression.
The aim is to increase awareness about the potential for a serious medication mistake when the wrong drug is given because of drug names that look and sound similar.
Awareness of these drug names, however, is just the first step in preventing medication mistakes. Health care providers should take a number of other steps as well, experts said.
ISMP launched its confusing drug names list, previously called look-alike, sound-alike (LASA) drugs, in 2008. The new list is an update of the 2019 version, said Michael J. Gaunt, PharmD, senior manager of error reporting programs for the ISMP, which focuses on the prevention of medication mistakes. The new entries were chosen on the basis of a number of factors, including ISMP’s analysis of recent medication mishap reports that were submitted to it.
The ISMP list now includes about 528 drug pairs, Dr. Gaunt said. The list is long, he said, partly because each pair is listed twice, so readers can cross reference. For instance, hydralazine and hydroxyzine are listed in one entry in the list, and hydroxyzine and hydralazine are listed in another.
Brand Institute in Miami has named, among other drugs, Entresto, Rybelsus, and Lunesta. The regulatory arm of the company, the Drug Safety Institute, “considers drug names that have been confused as an important part of our comprehensive drug name assessments,” Todd Bridges, global president of the institute, said in an emailed statement. Information on the confusing drug names are incorporated into the company’s proprietary algorithm and is used when developing brand names for drugs. “We continually update this algorithm as new drug names that are often confused are identified,” Mr. Bridges said.
Confusing drug names: Ongoing issue
The length of the list, as well as the latest additions, are not surprising, said Mary Ann Kliethermes, PharmD, director of medication safety and quality for the American Society of Health-System Pharmacists, a membership organization of about 60,000 pharmacists who practice in inpatient and outpatient settings.
“I’ve been in practice over 45 years,” she said, “and this has been a problem ever since I have been in practice.” The sheer volume of new drugs is one reason, she said. From 2013 through 2022, the U.S. Food and Drug Administration approved an average of 43 novel drugs per year, according to a report from its Center for Drug Evaluation and Research. “Since the 90s, this [confusion about similar drug names] has happened,” Dr. Kliethermes said.
According to a 2023 report, about 7,000-9,000 people die each year in the United States as the result of a medication error. However, it’s impossible to say for sure what percentage of those errors involve name confusion, Dr. Gaunt said.
Not all the mistakes are reported. Some that are reported are dramatic and deadly. In 2022, a Tennessee nurse was convicted of gross neglect and negligent homicide. She was sentenced to 3 years’ probation after she mistakenly gave vercuronium, an anesthetic agent, instead of the sedative Versed to a patient, and the woman died.
Updated list: A closer look
Many of the new drug pairs that are listed in the update are cephalosporins, said Dr. Kliethermes, who reviewed the new list for this news organization. In all, 20 of the latest 82 additions are cephalosporins. These include drugs such as cefazolin, which can be confused with cefotetan, and vice versa. These drugs have been around since the 1980s, she said, but “they needed to be on there.” Even in the 1980s, it was becoming difficult to differentiate them, and there were fewer drugs in that class then, she said.
Influenza vaccines made the new list, too. Fluzone High-Dose Quadrivalent can be confused with fluzone quadrivalent. Other new additions: hydrochlorothiazide and hydroxychloroquine, Lasik and Wakix, Pitressin and Pitocin, Remeron and Rozerem.
Beyond the list
While it’s not possible to pinpoint how big a problem name confusion is in causing medication mistakes, “it is certainly still an issue,” Dr. Gaunt said. A variety of practices can reduce that risk substantially, Dr. Gaunt and Dr. Kliethermes agreed.
Tall-man lettering. Both the FDA and the ISMP recommend the use of so-called tall-man lettering (TML), which involves the use of uppercase letters, sometimes in boldface, to distinguish similar names on product labels and elsewhere. Examples include vinBLAStine and vinCRIStine.
Electronic prescribing. “It eliminates the risk of handwriting confusion,” Dr. Gaunt said. However, electronic prescribing can have a downside, Dr. Kliethermes said. When ordering medication, a person may type in a few letters and may then be presented with a prompt that lists several drug names, and it can be easy to click the wrong one. For that reason, ISMP and other experts recommend typing at least five letters when searching for a medication in an electronic system.
Use both brand and generic names on labels and prescriptions.
Write the indication. That can serve as a double check. If a prescription for Ambien says “For sleep,” there’s probably less risk of filling a prescription for ambrisentan, the vasodilator.
Smart formulary additions. When hospitals add medications to their formularies, “part of that formulary assessment should include looking at the potential risk for errors,” Dr. Gaunt said. This involves keeping an eye out for confusing names and similar packaging. “Do that analysis up front and put in strategies to minimize that. Maybe you look for a different drug [for the same use] that has a different name.” Or choose a different manufacturer, so the medication would at least have a different container.
Use bar code scanning. Suppose a pharmacist goes to the shelf and pulls the wrong drug. “Bar code scanning provides the opportunity to catch the error,” Dr. Gaunt said. Many community pharmacies now have bar code scanning. ISMP just issued best practices for community pharmacies, Dr. Gaunt said, and these include the use of bar code scanning and other measures.
Educate consumers. Health care providers can educate consumers on how to minimize the risk of getting the wrong drug, Dr. Gaunt said. When patients are picking up a prescription, suggest they look at the container label; if it looks different from previous prescriptions of the same medicine, ask the pharmacist for an explanation. Some patients just pass it off, Dr. Gaunt said, figuring the pharmacist or health plan switched manufacturers of their medication.
Access the list. The entire list is on the ISMP site and is accessible after free registration.
Goal: Preventing confusion
The FDA has provided guidance for industry on naming drugs not yet approved so that the proposed names are not too similar in sound or appearance to those already on the market. Included in the lengthy document are checklists, such as, “Across a range of dialects, are the names consistently pronounced differently?” and “Are the lengths of the names dissimilar when scripted?” (Lengths are considered different if they differ by two or more letters.)
The FDA also offers the phonetic and orthographic computer analysis (POCA) program, a software tool that employs an advanced algorithm to evaluate similarities between two drug names. The data sources are updated regularly as new drugs are approved.
Liability update
The problem may be decreasing. In a 2020 report, researchers used pharmacists’ professional liability claim data from the Healthcare Providers Service Organization. They compared 2018 data on claims with 2013 data. The percentage of claims associated with wrong drug dispensing errors declined from 43.8% in 2013 to 36.8% in 2018. Wrong dose claims also declined, from 31.5% to 15.3%.
These researchers concluded that technology and automation have contributed to the prevention of medication errors caused by the use of the wrong drug and the wrong dose, but mistakes continue, owing to system and human errors.
A version of this article first appeared on Medscape.com.
Zolpidem (Ambien) is a well-known sedative for sleep. Letairis (Ambrisentan) is a vasodilator for the treatment of pulmonary arterial hypertension. Citalopram (Celexa) is an antidepressant; escitalopram (Lexapro) is prescribed for anxiety and depression.
The aim is to increase awareness about the potential for a serious medication mistake when the wrong drug is given because of drug names that look and sound similar.
Awareness of these drug names, however, is just the first step in preventing medication mistakes. Health care providers should take a number of other steps as well, experts said.
ISMP launched its confusing drug names list, previously called look-alike, sound-alike (LASA) drugs, in 2008. The new list is an update of the 2019 version, said Michael J. Gaunt, PharmD, senior manager of error reporting programs for the ISMP, which focuses on the prevention of medication mistakes. The new entries were chosen on the basis of a number of factors, including ISMP’s analysis of recent medication mishap reports that were submitted to it.
The ISMP list now includes about 528 drug pairs, Dr. Gaunt said. The list is long, he said, partly because each pair is listed twice, so readers can cross reference. For instance, hydralazine and hydroxyzine are listed in one entry in the list, and hydroxyzine and hydralazine are listed in another.
Brand Institute in Miami has named, among other drugs, Entresto, Rybelsus, and Lunesta. The regulatory arm of the company, the Drug Safety Institute, “considers drug names that have been confused as an important part of our comprehensive drug name assessments,” Todd Bridges, global president of the institute, said in an emailed statement. Information on the confusing drug names are incorporated into the company’s proprietary algorithm and is used when developing brand names for drugs. “We continually update this algorithm as new drug names that are often confused are identified,” Mr. Bridges said.
Confusing drug names: Ongoing issue
The length of the list, as well as the latest additions, are not surprising, said Mary Ann Kliethermes, PharmD, director of medication safety and quality for the American Society of Health-System Pharmacists, a membership organization of about 60,000 pharmacists who practice in inpatient and outpatient settings.
“I’ve been in practice over 45 years,” she said, “and this has been a problem ever since I have been in practice.” The sheer volume of new drugs is one reason, she said. From 2013 through 2022, the U.S. Food and Drug Administration approved an average of 43 novel drugs per year, according to a report from its Center for Drug Evaluation and Research. “Since the 90s, this [confusion about similar drug names] has happened,” Dr. Kliethermes said.
According to a 2023 report, about 7,000-9,000 people die each year in the United States as the result of a medication error. However, it’s impossible to say for sure what percentage of those errors involve name confusion, Dr. Gaunt said.
Not all the mistakes are reported. Some that are reported are dramatic and deadly. In 2022, a Tennessee nurse was convicted of gross neglect and negligent homicide. She was sentenced to 3 years’ probation after she mistakenly gave vercuronium, an anesthetic agent, instead of the sedative Versed to a patient, and the woman died.
Updated list: A closer look
Many of the new drug pairs that are listed in the update are cephalosporins, said Dr. Kliethermes, who reviewed the new list for this news organization. In all, 20 of the latest 82 additions are cephalosporins. These include drugs such as cefazolin, which can be confused with cefotetan, and vice versa. These drugs have been around since the 1980s, she said, but “they needed to be on there.” Even in the 1980s, it was becoming difficult to differentiate them, and there were fewer drugs in that class then, she said.
Influenza vaccines made the new list, too. Fluzone High-Dose Quadrivalent can be confused with fluzone quadrivalent. Other new additions: hydrochlorothiazide and hydroxychloroquine, Lasik and Wakix, Pitressin and Pitocin, Remeron and Rozerem.
Beyond the list
While it’s not possible to pinpoint how big a problem name confusion is in causing medication mistakes, “it is certainly still an issue,” Dr. Gaunt said. A variety of practices can reduce that risk substantially, Dr. Gaunt and Dr. Kliethermes agreed.
Tall-man lettering. Both the FDA and the ISMP recommend the use of so-called tall-man lettering (TML), which involves the use of uppercase letters, sometimes in boldface, to distinguish similar names on product labels and elsewhere. Examples include vinBLAStine and vinCRIStine.
Electronic prescribing. “It eliminates the risk of handwriting confusion,” Dr. Gaunt said. However, electronic prescribing can have a downside, Dr. Kliethermes said. When ordering medication, a person may type in a few letters and may then be presented with a prompt that lists several drug names, and it can be easy to click the wrong one. For that reason, ISMP and other experts recommend typing at least five letters when searching for a medication in an electronic system.
Use both brand and generic names on labels and prescriptions.
Write the indication. That can serve as a double check. If a prescription for Ambien says “For sleep,” there’s probably less risk of filling a prescription for ambrisentan, the vasodilator.
Smart formulary additions. When hospitals add medications to their formularies, “part of that formulary assessment should include looking at the potential risk for errors,” Dr. Gaunt said. This involves keeping an eye out for confusing names and similar packaging. “Do that analysis up front and put in strategies to minimize that. Maybe you look for a different drug [for the same use] that has a different name.” Or choose a different manufacturer, so the medication would at least have a different container.
Use bar code scanning. Suppose a pharmacist goes to the shelf and pulls the wrong drug. “Bar code scanning provides the opportunity to catch the error,” Dr. Gaunt said. Many community pharmacies now have bar code scanning. ISMP just issued best practices for community pharmacies, Dr. Gaunt said, and these include the use of bar code scanning and other measures.
Educate consumers. Health care providers can educate consumers on how to minimize the risk of getting the wrong drug, Dr. Gaunt said. When patients are picking up a prescription, suggest they look at the container label; if it looks different from previous prescriptions of the same medicine, ask the pharmacist for an explanation. Some patients just pass it off, Dr. Gaunt said, figuring the pharmacist or health plan switched manufacturers of their medication.
Access the list. The entire list is on the ISMP site and is accessible after free registration.
Goal: Preventing confusion
The FDA has provided guidance for industry on naming drugs not yet approved so that the proposed names are not too similar in sound or appearance to those already on the market. Included in the lengthy document are checklists, such as, “Across a range of dialects, are the names consistently pronounced differently?” and “Are the lengths of the names dissimilar when scripted?” (Lengths are considered different if they differ by two or more letters.)
The FDA also offers the phonetic and orthographic computer analysis (POCA) program, a software tool that employs an advanced algorithm to evaluate similarities between two drug names. The data sources are updated regularly as new drugs are approved.
Liability update
The problem may be decreasing. In a 2020 report, researchers used pharmacists’ professional liability claim data from the Healthcare Providers Service Organization. They compared 2018 data on claims with 2013 data. The percentage of claims associated with wrong drug dispensing errors declined from 43.8% in 2013 to 36.8% in 2018. Wrong dose claims also declined, from 31.5% to 15.3%.
These researchers concluded that technology and automation have contributed to the prevention of medication errors caused by the use of the wrong drug and the wrong dose, but mistakes continue, owing to system and human errors.
A version of this article first appeared on Medscape.com.
U.S. has new dominant COVID variant called EG.5
Called “Eris” among avid COVID trackers, the strain EG.5 now accounts for 17% of all U.S. COVID infections, according to the latest Centers for Disease Control and Prevention estimates. That’s up from 12% the week prior.
EG.5 has been rising worldwide, just weeks after the World Health Organization added the strain to its official monitoring list. In the United Kingdom, it now accounts for 1 in 10 COVID cases, The Independent reported.
EG.5 is a descendant of the XBB strains that have dominated tracking lists in recent months. It has the same makeup as XBB.1.9.2 but carries an extra spike mutation, according to a summary published by the Center for Infectious Disease Research and Policy at the University of Minnesota. The spike protein is the part of the virus that allows it to enter human cells. But there’s no indication so far that EG.5 is more contagious or severe than other recent variants, according to the CIDRAP summary and a recent podcast from the American Medical Association. The CDC said that current vaccines protect against the variant.
U.S. hospitals saw a 12% increase in COVID admissions during the week ending on July 22, with 8,047 people being admitted because of the virus, up from an all-time low of 6,306 the week of June 24. In 17 states, the past-week increase in hospitalizations was 20% or greater. In Minnesota, the rate jumped by 50%, and in West Virginia, it jumped by 63%. Meanwhile, deaths reached their lowest weekly rate ever for the week of data ending July 29, with just 176 deaths reported by the CDC.
A version of this article first appeared on WebMD.com.
Called “Eris” among avid COVID trackers, the strain EG.5 now accounts for 17% of all U.S. COVID infections, according to the latest Centers for Disease Control and Prevention estimates. That’s up from 12% the week prior.
EG.5 has been rising worldwide, just weeks after the World Health Organization added the strain to its official monitoring list. In the United Kingdom, it now accounts for 1 in 10 COVID cases, The Independent reported.
EG.5 is a descendant of the XBB strains that have dominated tracking lists in recent months. It has the same makeup as XBB.1.9.2 but carries an extra spike mutation, according to a summary published by the Center for Infectious Disease Research and Policy at the University of Minnesota. The spike protein is the part of the virus that allows it to enter human cells. But there’s no indication so far that EG.5 is more contagious or severe than other recent variants, according to the CIDRAP summary and a recent podcast from the American Medical Association. The CDC said that current vaccines protect against the variant.
U.S. hospitals saw a 12% increase in COVID admissions during the week ending on July 22, with 8,047 people being admitted because of the virus, up from an all-time low of 6,306 the week of June 24. In 17 states, the past-week increase in hospitalizations was 20% or greater. In Minnesota, the rate jumped by 50%, and in West Virginia, it jumped by 63%. Meanwhile, deaths reached their lowest weekly rate ever for the week of data ending July 29, with just 176 deaths reported by the CDC.
A version of this article first appeared on WebMD.com.
Called “Eris” among avid COVID trackers, the strain EG.5 now accounts for 17% of all U.S. COVID infections, according to the latest Centers for Disease Control and Prevention estimates. That’s up from 12% the week prior.
EG.5 has been rising worldwide, just weeks after the World Health Organization added the strain to its official monitoring list. In the United Kingdom, it now accounts for 1 in 10 COVID cases, The Independent reported.
EG.5 is a descendant of the XBB strains that have dominated tracking lists in recent months. It has the same makeup as XBB.1.9.2 but carries an extra spike mutation, according to a summary published by the Center for Infectious Disease Research and Policy at the University of Minnesota. The spike protein is the part of the virus that allows it to enter human cells. But there’s no indication so far that EG.5 is more contagious or severe than other recent variants, according to the CIDRAP summary and a recent podcast from the American Medical Association. The CDC said that current vaccines protect against the variant.
U.S. hospitals saw a 12% increase in COVID admissions during the week ending on July 22, with 8,047 people being admitted because of the virus, up from an all-time low of 6,306 the week of June 24. In 17 states, the past-week increase in hospitalizations was 20% or greater. In Minnesota, the rate jumped by 50%, and in West Virginia, it jumped by 63%. Meanwhile, deaths reached their lowest weekly rate ever for the week of data ending July 29, with just 176 deaths reported by the CDC.
A version of this article first appeared on WebMD.com.
Cigna accused of using AI, not doctors, to deny claims: Lawsuit
and forcing providers to bill patients in full.
In a complaint filed recently in California’s eastern district court, plaintiffs and Cigna health plan members Suzanne Kisting-Leung and Ayesha Smiley and their attorneys say that Cigna violates state insurance regulations by failing to conduct a “thorough, fair, and objective” review of their and other members’ claims.
The lawsuit says that, instead, Cigna relies on an algorithm, PxDx, to review and frequently deny medically necessary claims. According to court records, the system allows Cigna’s doctors to “instantly reject claims on medical grounds without ever opening patient files.” With use of the system, the average claims processing time is 1.2 seconds.
Cigna says it uses technology to verify coding on standard, low-cost procedures and to expedite physician reimbursement. In a statement to CBS News, the company called the lawsuit “highly questionable.”
The case highlights growing concerns about AI and its ability to replace humans for tasks and interactions in health care, business, and beyond. Public advocacy law firm Clarkson, which is representing the plaintiffs, has previously sued tech giants Google and ChatGPT creator OpenAI for harvesting Internet users’ personal and professional data to train their AI systems.
According to the complaint, Cigna denied the plaintiffs medically necessary tests, including blood work to screen for vitamin D deficiency and ultrasounds for patients suspected of having ovarian cancer. The plaintiffs’ attempts to appeal were unfruitful, and they were forced to pay out of pocket.
The plaintiff’s attorneys argue that the claims do not undergo more detailed reviews by physicians and employees, as mandated by California insurance laws, and that Cigna benefits by saving on labor costs.
Clarkson is demanding a jury trial and has asked the court to certify the Cigna case as a federal class action, potentially allowing the insurer’s other 2 million health plan members in California to join the lawsuit.
I. Glenn Cohen, JD, deputy dean and professor at Harvard Law School, Cambridge, Mass., said in an interview that this is the first lawsuit he’s aware of in which AI was involved in denying health insurance claims and that it is probably an uphill battle for the plaintiffs.
“In the last 25 years, the U.S. Supreme Court’s decisions have made getting a class action approved more difficult. If allowed to go forward as a class action, which Cigna is likely to vigorously oppose, then the pressure on Cigna to settle the case becomes enormous,” he said.
The allegations come after a recent deep dive by the nonprofit ProPublica uncovered similar claim denial issues. One physician who worked for Cigna told the nonprofit that he and other company doctors essentially rubber-stamped the denials in batches, which took “all of 10 seconds to do 50 at a time.”
In 2022, the American Medical Association and two state physician groups joined another class action against Cigna stemming from allegations that the insurer’s intermediary, Multiplan, intentionally underpaid medical claims. And in March, Cigna’s pharmacy benefit manager, Express Scripts, was accused of conspiring with other PBMs to drive up prescription drug prices for Ohio consumers, violating state antitrust laws.
Mr. Cohen said he expects Cigna to push back in court about the California class size, which the plaintiff’s attorneys hope will encompass all Cigna health plan members in the state.
“The injury is primarily to those whose claims were denied by AI, presumably a much smaller set of individuals and harder to identify,” said Mr. Cohen.
A version of this article first appeared on Medscape.com.
and forcing providers to bill patients in full.
In a complaint filed recently in California’s eastern district court, plaintiffs and Cigna health plan members Suzanne Kisting-Leung and Ayesha Smiley and their attorneys say that Cigna violates state insurance regulations by failing to conduct a “thorough, fair, and objective” review of their and other members’ claims.
The lawsuit says that, instead, Cigna relies on an algorithm, PxDx, to review and frequently deny medically necessary claims. According to court records, the system allows Cigna’s doctors to “instantly reject claims on medical grounds without ever opening patient files.” With use of the system, the average claims processing time is 1.2 seconds.
Cigna says it uses technology to verify coding on standard, low-cost procedures and to expedite physician reimbursement. In a statement to CBS News, the company called the lawsuit “highly questionable.”
The case highlights growing concerns about AI and its ability to replace humans for tasks and interactions in health care, business, and beyond. Public advocacy law firm Clarkson, which is representing the plaintiffs, has previously sued tech giants Google and ChatGPT creator OpenAI for harvesting Internet users’ personal and professional data to train their AI systems.
According to the complaint, Cigna denied the plaintiffs medically necessary tests, including blood work to screen for vitamin D deficiency and ultrasounds for patients suspected of having ovarian cancer. The plaintiffs’ attempts to appeal were unfruitful, and they were forced to pay out of pocket.
The plaintiff’s attorneys argue that the claims do not undergo more detailed reviews by physicians and employees, as mandated by California insurance laws, and that Cigna benefits by saving on labor costs.
Clarkson is demanding a jury trial and has asked the court to certify the Cigna case as a federal class action, potentially allowing the insurer’s other 2 million health plan members in California to join the lawsuit.
I. Glenn Cohen, JD, deputy dean and professor at Harvard Law School, Cambridge, Mass., said in an interview that this is the first lawsuit he’s aware of in which AI was involved in denying health insurance claims and that it is probably an uphill battle for the plaintiffs.
“In the last 25 years, the U.S. Supreme Court’s decisions have made getting a class action approved more difficult. If allowed to go forward as a class action, which Cigna is likely to vigorously oppose, then the pressure on Cigna to settle the case becomes enormous,” he said.
The allegations come after a recent deep dive by the nonprofit ProPublica uncovered similar claim denial issues. One physician who worked for Cigna told the nonprofit that he and other company doctors essentially rubber-stamped the denials in batches, which took “all of 10 seconds to do 50 at a time.”
In 2022, the American Medical Association and two state physician groups joined another class action against Cigna stemming from allegations that the insurer’s intermediary, Multiplan, intentionally underpaid medical claims. And in March, Cigna’s pharmacy benefit manager, Express Scripts, was accused of conspiring with other PBMs to drive up prescription drug prices for Ohio consumers, violating state antitrust laws.
Mr. Cohen said he expects Cigna to push back in court about the California class size, which the plaintiff’s attorneys hope will encompass all Cigna health plan members in the state.
“The injury is primarily to those whose claims were denied by AI, presumably a much smaller set of individuals and harder to identify,” said Mr. Cohen.
A version of this article first appeared on Medscape.com.
and forcing providers to bill patients in full.
In a complaint filed recently in California’s eastern district court, plaintiffs and Cigna health plan members Suzanne Kisting-Leung and Ayesha Smiley and their attorneys say that Cigna violates state insurance regulations by failing to conduct a “thorough, fair, and objective” review of their and other members’ claims.
The lawsuit says that, instead, Cigna relies on an algorithm, PxDx, to review and frequently deny medically necessary claims. According to court records, the system allows Cigna’s doctors to “instantly reject claims on medical grounds without ever opening patient files.” With use of the system, the average claims processing time is 1.2 seconds.
Cigna says it uses technology to verify coding on standard, low-cost procedures and to expedite physician reimbursement. In a statement to CBS News, the company called the lawsuit “highly questionable.”
The case highlights growing concerns about AI and its ability to replace humans for tasks and interactions in health care, business, and beyond. Public advocacy law firm Clarkson, which is representing the plaintiffs, has previously sued tech giants Google and ChatGPT creator OpenAI for harvesting Internet users’ personal and professional data to train their AI systems.
According to the complaint, Cigna denied the plaintiffs medically necessary tests, including blood work to screen for vitamin D deficiency and ultrasounds for patients suspected of having ovarian cancer. The plaintiffs’ attempts to appeal were unfruitful, and they were forced to pay out of pocket.
The plaintiff’s attorneys argue that the claims do not undergo more detailed reviews by physicians and employees, as mandated by California insurance laws, and that Cigna benefits by saving on labor costs.
Clarkson is demanding a jury trial and has asked the court to certify the Cigna case as a federal class action, potentially allowing the insurer’s other 2 million health plan members in California to join the lawsuit.
I. Glenn Cohen, JD, deputy dean and professor at Harvard Law School, Cambridge, Mass., said in an interview that this is the first lawsuit he’s aware of in which AI was involved in denying health insurance claims and that it is probably an uphill battle for the plaintiffs.
“In the last 25 years, the U.S. Supreme Court’s decisions have made getting a class action approved more difficult. If allowed to go forward as a class action, which Cigna is likely to vigorously oppose, then the pressure on Cigna to settle the case becomes enormous,” he said.
The allegations come after a recent deep dive by the nonprofit ProPublica uncovered similar claim denial issues. One physician who worked for Cigna told the nonprofit that he and other company doctors essentially rubber-stamped the denials in batches, which took “all of 10 seconds to do 50 at a time.”
In 2022, the American Medical Association and two state physician groups joined another class action against Cigna stemming from allegations that the insurer’s intermediary, Multiplan, intentionally underpaid medical claims. And in March, Cigna’s pharmacy benefit manager, Express Scripts, was accused of conspiring with other PBMs to drive up prescription drug prices for Ohio consumers, violating state antitrust laws.
Mr. Cohen said he expects Cigna to push back in court about the California class size, which the plaintiff’s attorneys hope will encompass all Cigna health plan members in the state.
“The injury is primarily to those whose claims were denied by AI, presumably a much smaller set of individuals and harder to identify,” said Mr. Cohen.
A version of this article first appeared on Medscape.com.
PRISm and nonspecific pattern: New insights in lung testing interpretation
The recent statement on interpretive strategies for lung testing uses the acronym PRISm for preserved ratio impaired spirometry. PRISm identifies patients with a normal forced expiratory volume in 1 second/forced vital capacity ratio but abnormal FEV1 and/or FVC (usually both). Most medical students are taught to call this a “restrictive pattern,” and every first-year pulmonary fellow orders full lung volumes when they see it. If total lung capacity (TLC) is normal, PRISm becomes the nonspecific pattern. If TLC is low, then the patient has “true” restriction, and if it’s elevated, then hyperinflation may be present.
The traditional classification scheme for basic spirometry interpretation (normal, restricted, obstructed, or mixed) is simple and conceptually clear. It turns out that many with this pattern won’t have an abnormal TLC, so the name is, in some ways, a misnomer and can be misleading. Enter PRISm, a more descriptive and inclusive term. The phrase also lends itself to a phonetic acronym that is fun to say, easy to remember, and likely to catch on with learners.
Information on occurrence and clinical behavior comes from large cohorts with basic spirometry, but without full lung volumes because PRISm no longer applies once TLC is determined. As may be expected, prevalence varies by the population studied. Estimates for general populations have been in the 7%-12% range; however, one study examining a database of patients with clinical spirometry referrals found a prevalence of 22.3%. Rates may be far higher in low- and middle-income countries. Identified risk factors include sex, tobacco use, and body mass index; the presence of PRISm is associated with respiratory symptoms and mortality. Thus, PRISm is common and it matters.
Along with PRISm, the nonspecific pattern is a new addition, if not a new concept, to the 2022 interpretative strategies statement. As with PRISm, the title is necessarily broad, though far less imaginative. Defined by reductions in FEV1 and FVC and a normal TLC, the nonspecific pattern has classically been considered a marker of early airway disease. The idea is that early, heterogeneous closure of distal segments of the bronchial tree can reduce total volume during a forced expiration before affecting the FEV1/FVC. The fact that the TLC is not a forced maneuver means there is proportionately less effect from more collapsible/susceptible smaller units. More recent data suggest that there are additional causes.
Because the nonspecific pattern requires full lung volumes, we have less population-level data than for PRISm. Estimated prevalence is approximately 9.5% in patients with complete test results. The two most common causes are obesity and airway obstruction, and the pattern is relatively stable over time. Notably, an increase in specific airway resistance or TLC minus alveolar volume difference predicts progression to frank obstruction on spirometry.
The physiologic changes that obesity inflicts on the lung have been well described. Patients with obesity breathe at lower lung volumes and are therefore susceptible to small airway closure at rest and during forced expiration. There is no doubt that the increased recognition of PRISm and the nonspecific pattern is in part related to the worldwide rise in obesity rates.
Key takeaways
In summary, PRISm and the nonspecific pattern are now part of the classification scheme we use for spirometry and full lung volumes, respectively. They should be included in interpretations given their diagnostic and predictive value. Airway disease and obesity are common causes and often coexist with either pattern. Many will not have a true, restrictive lung deficit, and a reductionist approach to interpretation is likely to lead to erroneous diagnoses. There were many important updates included in the 2022 iteration on lung testing interpretation that should not fly under the radar.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He disclosed ties with CHEST College, Metapharm, and WebMD.
A version of this article appeared on Medscape.com.
The recent statement on interpretive strategies for lung testing uses the acronym PRISm for preserved ratio impaired spirometry. PRISm identifies patients with a normal forced expiratory volume in 1 second/forced vital capacity ratio but abnormal FEV1 and/or FVC (usually both). Most medical students are taught to call this a “restrictive pattern,” and every first-year pulmonary fellow orders full lung volumes when they see it. If total lung capacity (TLC) is normal, PRISm becomes the nonspecific pattern. If TLC is low, then the patient has “true” restriction, and if it’s elevated, then hyperinflation may be present.
The traditional classification scheme for basic spirometry interpretation (normal, restricted, obstructed, or mixed) is simple and conceptually clear. It turns out that many with this pattern won’t have an abnormal TLC, so the name is, in some ways, a misnomer and can be misleading. Enter PRISm, a more descriptive and inclusive term. The phrase also lends itself to a phonetic acronym that is fun to say, easy to remember, and likely to catch on with learners.
Information on occurrence and clinical behavior comes from large cohorts with basic spirometry, but without full lung volumes because PRISm no longer applies once TLC is determined. As may be expected, prevalence varies by the population studied. Estimates for general populations have been in the 7%-12% range; however, one study examining a database of patients with clinical spirometry referrals found a prevalence of 22.3%. Rates may be far higher in low- and middle-income countries. Identified risk factors include sex, tobacco use, and body mass index; the presence of PRISm is associated with respiratory symptoms and mortality. Thus, PRISm is common and it matters.
Along with PRISm, the nonspecific pattern is a new addition, if not a new concept, to the 2022 interpretative strategies statement. As with PRISm, the title is necessarily broad, though far less imaginative. Defined by reductions in FEV1 and FVC and a normal TLC, the nonspecific pattern has classically been considered a marker of early airway disease. The idea is that early, heterogeneous closure of distal segments of the bronchial tree can reduce total volume during a forced expiration before affecting the FEV1/FVC. The fact that the TLC is not a forced maneuver means there is proportionately less effect from more collapsible/susceptible smaller units. More recent data suggest that there are additional causes.
Because the nonspecific pattern requires full lung volumes, we have less population-level data than for PRISm. Estimated prevalence is approximately 9.5% in patients with complete test results. The two most common causes are obesity and airway obstruction, and the pattern is relatively stable over time. Notably, an increase in specific airway resistance or TLC minus alveolar volume difference predicts progression to frank obstruction on spirometry.
The physiologic changes that obesity inflicts on the lung have been well described. Patients with obesity breathe at lower lung volumes and are therefore susceptible to small airway closure at rest and during forced expiration. There is no doubt that the increased recognition of PRISm and the nonspecific pattern is in part related to the worldwide rise in obesity rates.
Key takeaways
In summary, PRISm and the nonspecific pattern are now part of the classification scheme we use for spirometry and full lung volumes, respectively. They should be included in interpretations given their diagnostic and predictive value. Airway disease and obesity are common causes and often coexist with either pattern. Many will not have a true, restrictive lung deficit, and a reductionist approach to interpretation is likely to lead to erroneous diagnoses. There were many important updates included in the 2022 iteration on lung testing interpretation that should not fly under the radar.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He disclosed ties with CHEST College, Metapharm, and WebMD.
A version of this article appeared on Medscape.com.
The recent statement on interpretive strategies for lung testing uses the acronym PRISm for preserved ratio impaired spirometry. PRISm identifies patients with a normal forced expiratory volume in 1 second/forced vital capacity ratio but abnormal FEV1 and/or FVC (usually both). Most medical students are taught to call this a “restrictive pattern,” and every first-year pulmonary fellow orders full lung volumes when they see it. If total lung capacity (TLC) is normal, PRISm becomes the nonspecific pattern. If TLC is low, then the patient has “true” restriction, and if it’s elevated, then hyperinflation may be present.
The traditional classification scheme for basic spirometry interpretation (normal, restricted, obstructed, or mixed) is simple and conceptually clear. It turns out that many with this pattern won’t have an abnormal TLC, so the name is, in some ways, a misnomer and can be misleading. Enter PRISm, a more descriptive and inclusive term. The phrase also lends itself to a phonetic acronym that is fun to say, easy to remember, and likely to catch on with learners.
Information on occurrence and clinical behavior comes from large cohorts with basic spirometry, but without full lung volumes because PRISm no longer applies once TLC is determined. As may be expected, prevalence varies by the population studied. Estimates for general populations have been in the 7%-12% range; however, one study examining a database of patients with clinical spirometry referrals found a prevalence of 22.3%. Rates may be far higher in low- and middle-income countries. Identified risk factors include sex, tobacco use, and body mass index; the presence of PRISm is associated with respiratory symptoms and mortality. Thus, PRISm is common and it matters.
Along with PRISm, the nonspecific pattern is a new addition, if not a new concept, to the 2022 interpretative strategies statement. As with PRISm, the title is necessarily broad, though far less imaginative. Defined by reductions in FEV1 and FVC and a normal TLC, the nonspecific pattern has classically been considered a marker of early airway disease. The idea is that early, heterogeneous closure of distal segments of the bronchial tree can reduce total volume during a forced expiration before affecting the FEV1/FVC. The fact that the TLC is not a forced maneuver means there is proportionately less effect from more collapsible/susceptible smaller units. More recent data suggest that there are additional causes.
Because the nonspecific pattern requires full lung volumes, we have less population-level data than for PRISm. Estimated prevalence is approximately 9.5% in patients with complete test results. The two most common causes are obesity and airway obstruction, and the pattern is relatively stable over time. Notably, an increase in specific airway resistance or TLC minus alveolar volume difference predicts progression to frank obstruction on spirometry.
The physiologic changes that obesity inflicts on the lung have been well described. Patients with obesity breathe at lower lung volumes and are therefore susceptible to small airway closure at rest and during forced expiration. There is no doubt that the increased recognition of PRISm and the nonspecific pattern is in part related to the worldwide rise in obesity rates.
Key takeaways
In summary, PRISm and the nonspecific pattern are now part of the classification scheme we use for spirometry and full lung volumes, respectively. They should be included in interpretations given their diagnostic and predictive value. Airway disease and obesity are common causes and often coexist with either pattern. Many will not have a true, restrictive lung deficit, and a reductionist approach to interpretation is likely to lead to erroneous diagnoses. There were many important updates included in the 2022 iteration on lung testing interpretation that should not fly under the radar.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He disclosed ties with CHEST College, Metapharm, and WebMD.
A version of this article appeared on Medscape.com.