User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Do Patients Benefit from Cancer Trial Participation?
TOPLINE:
METHODOLOGY:
- The view that patients with cancer benefit from access to investigational drugs in the clinical trial setting is widely held but does necessarily align with trial findings, which often show limited evidence of a clinical benefit. First, most investigational treatments assessed in clinical trials fail to gain regulatory approval, and the minority that are approved tend to offer minimal clinical benefit, experts explained.
- To estimate the survival benefit and toxicities associated with receiving experimental treatments, researchers conducted a meta-analysis of 128 trials comprising 141 comparisons of an investigational drug and a control treatment, which included immunotherapies and targeted therapies.
- The analysis included 42 trials in non–small cell lung cancer (NSCLC), 37 in breast cancer, 15 in hepatobiliary cancer, 13 in pancreatic cancer, 12 in colorectal cancer, and 10 in prostate cancer, involving a total of 47,050 patients.
- The primary outcome was PFS and secondary outcomes were overall survival and grades 3-5 serious adverse events.
TAKEAWAY:
- Overall, the experimental treatment was associated with a 20% improvement in PFS (pooled hazard ratio [HR], 0.80), corresponding to a median 1.25-month PFS advantage. The PFS benefit was seen across all cancer types, except pancreatic cancer.
- Overall survival improved by 8% with experimental agents (HR, 0.92), corresponding to 1.18 additional months. A significant overall survival benefit was seen across NSCLC, breast cancer, and hepatobiliary cancer trials but not pancreatic, prostate, colorectal cancer trials.
- Patients in the experimental intervention group, however, experienced much higher risk for grade 3-5 serious adverse events (risk ratio [RR], 1.27), corresponding to 7.40% increase in absolute risk. The greater risk for serious adverse events was significant for all indications except prostate cancer (RR, 1.13; 95% CI, 0.91-1.40).
IN PRACTICE:
“We believe our findings are best interpreted as suggesting that access to experimental interventions that have not yet received full FDA approval is associated with a marginal but nonzero clinical benefit,” the authors wrote.
“Although our findings seem to reflect poorly on trials as a vehicle for extending survival for participants, they have reassuring implications for clinical investigators, policymakers, and institutional review boards,” the researchers said, explaining that this “scenario allows clinical trials to continue to pursue promising new treatments — supporting incremental advances that sum to large gains over extended periods of research — without disadvantaging patients in comparator groups.”
SOURCE:
Renata Iskander, MSc, of McGill University, Montreal, Quebec, Canada, led this work, which was published online on April 29, 2024, in Annals of Internal Medicine.
LIMITATIONS:
There was high heterogeneity across studies due to variations in drugs tested, comparators used, and populations involved. The use of comparators below standard care could have inflated survival benefits. Additionally, data collected from ClinicalTrials.gov might be biased due to some trials not being reported.
DISCLOSURES:
Canadian Institutes of Health Research supported this work. The authors received grants for this work from McGill University, Rossy Cancer Network, and National Science Foundation. One author received consulting fees outside this work. The other authors declared no competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The view that patients with cancer benefit from access to investigational drugs in the clinical trial setting is widely held but does necessarily align with trial findings, which often show limited evidence of a clinical benefit. First, most investigational treatments assessed in clinical trials fail to gain regulatory approval, and the minority that are approved tend to offer minimal clinical benefit, experts explained.
- To estimate the survival benefit and toxicities associated with receiving experimental treatments, researchers conducted a meta-analysis of 128 trials comprising 141 comparisons of an investigational drug and a control treatment, which included immunotherapies and targeted therapies.
- The analysis included 42 trials in non–small cell lung cancer (NSCLC), 37 in breast cancer, 15 in hepatobiliary cancer, 13 in pancreatic cancer, 12 in colorectal cancer, and 10 in prostate cancer, involving a total of 47,050 patients.
- The primary outcome was PFS and secondary outcomes were overall survival and grades 3-5 serious adverse events.
TAKEAWAY:
- Overall, the experimental treatment was associated with a 20% improvement in PFS (pooled hazard ratio [HR], 0.80), corresponding to a median 1.25-month PFS advantage. The PFS benefit was seen across all cancer types, except pancreatic cancer.
- Overall survival improved by 8% with experimental agents (HR, 0.92), corresponding to 1.18 additional months. A significant overall survival benefit was seen across NSCLC, breast cancer, and hepatobiliary cancer trials but not pancreatic, prostate, colorectal cancer trials.
- Patients in the experimental intervention group, however, experienced much higher risk for grade 3-5 serious adverse events (risk ratio [RR], 1.27), corresponding to 7.40% increase in absolute risk. The greater risk for serious adverse events was significant for all indications except prostate cancer (RR, 1.13; 95% CI, 0.91-1.40).
IN PRACTICE:
“We believe our findings are best interpreted as suggesting that access to experimental interventions that have not yet received full FDA approval is associated with a marginal but nonzero clinical benefit,” the authors wrote.
“Although our findings seem to reflect poorly on trials as a vehicle for extending survival for participants, they have reassuring implications for clinical investigators, policymakers, and institutional review boards,” the researchers said, explaining that this “scenario allows clinical trials to continue to pursue promising new treatments — supporting incremental advances that sum to large gains over extended periods of research — without disadvantaging patients in comparator groups.”
SOURCE:
Renata Iskander, MSc, of McGill University, Montreal, Quebec, Canada, led this work, which was published online on April 29, 2024, in Annals of Internal Medicine.
LIMITATIONS:
There was high heterogeneity across studies due to variations in drugs tested, comparators used, and populations involved. The use of comparators below standard care could have inflated survival benefits. Additionally, data collected from ClinicalTrials.gov might be biased due to some trials not being reported.
DISCLOSURES:
Canadian Institutes of Health Research supported this work. The authors received grants for this work from McGill University, Rossy Cancer Network, and National Science Foundation. One author received consulting fees outside this work. The other authors declared no competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The view that patients with cancer benefit from access to investigational drugs in the clinical trial setting is widely held but does necessarily align with trial findings, which often show limited evidence of a clinical benefit. First, most investigational treatments assessed in clinical trials fail to gain regulatory approval, and the minority that are approved tend to offer minimal clinical benefit, experts explained.
- To estimate the survival benefit and toxicities associated with receiving experimental treatments, researchers conducted a meta-analysis of 128 trials comprising 141 comparisons of an investigational drug and a control treatment, which included immunotherapies and targeted therapies.
- The analysis included 42 trials in non–small cell lung cancer (NSCLC), 37 in breast cancer, 15 in hepatobiliary cancer, 13 in pancreatic cancer, 12 in colorectal cancer, and 10 in prostate cancer, involving a total of 47,050 patients.
- The primary outcome was PFS and secondary outcomes were overall survival and grades 3-5 serious adverse events.
TAKEAWAY:
- Overall, the experimental treatment was associated with a 20% improvement in PFS (pooled hazard ratio [HR], 0.80), corresponding to a median 1.25-month PFS advantage. The PFS benefit was seen across all cancer types, except pancreatic cancer.
- Overall survival improved by 8% with experimental agents (HR, 0.92), corresponding to 1.18 additional months. A significant overall survival benefit was seen across NSCLC, breast cancer, and hepatobiliary cancer trials but not pancreatic, prostate, colorectal cancer trials.
- Patients in the experimental intervention group, however, experienced much higher risk for grade 3-5 serious adverse events (risk ratio [RR], 1.27), corresponding to 7.40% increase in absolute risk. The greater risk for serious adverse events was significant for all indications except prostate cancer (RR, 1.13; 95% CI, 0.91-1.40).
IN PRACTICE:
“We believe our findings are best interpreted as suggesting that access to experimental interventions that have not yet received full FDA approval is associated with a marginal but nonzero clinical benefit,” the authors wrote.
“Although our findings seem to reflect poorly on trials as a vehicle for extending survival for participants, they have reassuring implications for clinical investigators, policymakers, and institutional review boards,” the researchers said, explaining that this “scenario allows clinical trials to continue to pursue promising new treatments — supporting incremental advances that sum to large gains over extended periods of research — without disadvantaging patients in comparator groups.”
SOURCE:
Renata Iskander, MSc, of McGill University, Montreal, Quebec, Canada, led this work, which was published online on April 29, 2024, in Annals of Internal Medicine.
LIMITATIONS:
There was high heterogeneity across studies due to variations in drugs tested, comparators used, and populations involved. The use of comparators below standard care could have inflated survival benefits. Additionally, data collected from ClinicalTrials.gov might be biased due to some trials not being reported.
DISCLOSURES:
Canadian Institutes of Health Research supported this work. The authors received grants for this work from McGill University, Rossy Cancer Network, and National Science Foundation. One author received consulting fees outside this work. The other authors declared no competing interests.
A version of this article appeared on Medscape.com.
From Pharma’s Factories Direct to You
Pharmaceutical giant Eli Lilly recently announced that its newly approved weight loss medication Zepbound — a glucagon-like peptide 1 receptor agonist (GLP-1 RA) akin to Mounjaro, Ozempic, and Wegovy — will be prescribed by independent telehealth providers on a platform managed by the company itself. The drug can be subsequently shipped direct to consumer (DTC), allowing delivery straight to patients’ homes.
This arrangement raises serious concerns about an inherent conflict of interest, as we previously discussed. What happens when a pharmaceutical company influences access to remote providers who prescribe the very medications it manufactures?
Without new guardrails, the potential for misleading messaging to result in dangerous prescribing patterns looms large. The United States is one of only two countries to allow DTC advertising of prescription drugs, and the explosion in demand for GLP-1 RAs is partly attributable to this model (Oh, oh, Ozempic, anyone?). Americans spent over $78 billion on weight loss goods and services in 2019; time-intensive approaches such as diet and exercise are understandably difficult, and the public has always looked for a magic cure. Although GLP-1 RAs are promising, they may present a path to disaster without proper supervision.
LillyDirect, which in addition to Zepbound offers migraine medications and other products in the company’s catalogue, primarily aims to increase access to medication and reduce costs of the drugs for consumers. The stated mission is noble: By cutting out the middlemen of traditional pharmacies and benefit managers, administrative costs drop. LillyDirect goes a step further by reducing the need for patients to visit their regular family doctor to receive these medications.
On the surface, this design appears promising. Wait times for doctor’s appointments will fall. Patients can order drugs from the comfort of their home. Everyone benefits. Or do they?
Although easier access and reduced cost may be an apparent win for patients, DTC arrangements complicate the ethics of prescriptions and patient follow-up. This model reminds us of the roots of the opioid crisis, where powerful advertising and relationships between prescribers and drugmakers led to great harm. Providers often faced a conflict of interest when prescribing dangerous drugs to patients who requested them. We must learn from these mistakes to ensure there is critical oversight into the independence of prescribers used by LillyDirect and other DTC platforms.
Adding to these parallels, once a patient begins a GLP-1 medication such as Zepbound, stopping treatment will probably lead to regaining lost weight, serving as negative reinforcement. Hence, patients may decide never to discontinue these medications.
Obtaining what amounts to a lifelong prescription from a telehealth provider who may never follow a patient sets a dangerous precedent that will be difficult to unravel once begun. Recent challenges in access to medications such as Zepbound have been complicated by supply chain and manufacturing issues, leading to potential interruptions in patient access, ultimately affecting compliance. The rapid increase in online providers indicates competition for distribution channels has sharply increased and poses a threat to Lilly’s DTC site.
Furthermore, the lack of a regular physician to monitor patients introduces uncertainty in safety and continuity of care. These are important tenets in protecting patients, especially patients who are not diabetic and desire a quick fix. We have already seen a huge, arguably unrestrained, rise in prescriptions of GLP-1 RAs for weight loss — up to a 352% increase in 2023.
These drugs have shown great promise and are generally safe when used in the right patient, but important contraindications exist — namely, serious gastrointestinal side effects and low blood glucose in nondiabetic persons — that an astute physician must consider. Patients desiring these medications often must undergo comprehensive laboratory testing and cardiac evaluation, both before initiation and during regular follow-up, to check for comorbidities.
The American College of Physicians cautioned against such prescribing practices in a recent position statement, emphasizing that the lack of an established care provider could adversely affect patients. We note that the potential harms of DTC sales would concentrate in economically and racially underserved communities, where obesity, lack of insurance, and low health literacy are more common.
But the DTC genie is out of the pill bottle, and as such platforms become more common, patients will inherently take more ownership over their medical care. Remote providers will of course not be following these patients and evaluating for side effects. As a result, we in medical practice must be abreast of new downsides of these medications if and when they arise.
Every clinician must be aware of the medications a patient is taking, even those that they did not prescribe. They should educate their patients about drug-drug interactions and side effects and order lab tests to monitor for side effects.
Independent physicians abide by an underlying oath: First, do no harm. They serve as a trusted check on industry and a valuable long-term partner for patients. Where are the guardrails to protect patients and ensure that pharmaceutical companies are not essentially pushing prescriptions for their own products? Will traditional healthcare providers be effectively relegated to a bystander role in Lilly’s transactional approach to medication distribution? Unlike other commercial goods, pharmacologics have great nuance; not every approved medication is meant for every patient.
A version of this article appeared on Medscape.com.
Pharmaceutical giant Eli Lilly recently announced that its newly approved weight loss medication Zepbound — a glucagon-like peptide 1 receptor agonist (GLP-1 RA) akin to Mounjaro, Ozempic, and Wegovy — will be prescribed by independent telehealth providers on a platform managed by the company itself. The drug can be subsequently shipped direct to consumer (DTC), allowing delivery straight to patients’ homes.
This arrangement raises serious concerns about an inherent conflict of interest, as we previously discussed. What happens when a pharmaceutical company influences access to remote providers who prescribe the very medications it manufactures?
Without new guardrails, the potential for misleading messaging to result in dangerous prescribing patterns looms large. The United States is one of only two countries to allow DTC advertising of prescription drugs, and the explosion in demand for GLP-1 RAs is partly attributable to this model (Oh, oh, Ozempic, anyone?). Americans spent over $78 billion on weight loss goods and services in 2019; time-intensive approaches such as diet and exercise are understandably difficult, and the public has always looked for a magic cure. Although GLP-1 RAs are promising, they may present a path to disaster without proper supervision.
LillyDirect, which in addition to Zepbound offers migraine medications and other products in the company’s catalogue, primarily aims to increase access to medication and reduce costs of the drugs for consumers. The stated mission is noble: By cutting out the middlemen of traditional pharmacies and benefit managers, administrative costs drop. LillyDirect goes a step further by reducing the need for patients to visit their regular family doctor to receive these medications.
On the surface, this design appears promising. Wait times for doctor’s appointments will fall. Patients can order drugs from the comfort of their home. Everyone benefits. Or do they?
Although easier access and reduced cost may be an apparent win for patients, DTC arrangements complicate the ethics of prescriptions and patient follow-up. This model reminds us of the roots of the opioid crisis, where powerful advertising and relationships between prescribers and drugmakers led to great harm. Providers often faced a conflict of interest when prescribing dangerous drugs to patients who requested them. We must learn from these mistakes to ensure there is critical oversight into the independence of prescribers used by LillyDirect and other DTC platforms.
Adding to these parallels, once a patient begins a GLP-1 medication such as Zepbound, stopping treatment will probably lead to regaining lost weight, serving as negative reinforcement. Hence, patients may decide never to discontinue these medications.
Obtaining what amounts to a lifelong prescription from a telehealth provider who may never follow a patient sets a dangerous precedent that will be difficult to unravel once begun. Recent challenges in access to medications such as Zepbound have been complicated by supply chain and manufacturing issues, leading to potential interruptions in patient access, ultimately affecting compliance. The rapid increase in online providers indicates competition for distribution channels has sharply increased and poses a threat to Lilly’s DTC site.
Furthermore, the lack of a regular physician to monitor patients introduces uncertainty in safety and continuity of care. These are important tenets in protecting patients, especially patients who are not diabetic and desire a quick fix. We have already seen a huge, arguably unrestrained, rise in prescriptions of GLP-1 RAs for weight loss — up to a 352% increase in 2023.
These drugs have shown great promise and are generally safe when used in the right patient, but important contraindications exist — namely, serious gastrointestinal side effects and low blood glucose in nondiabetic persons — that an astute physician must consider. Patients desiring these medications often must undergo comprehensive laboratory testing and cardiac evaluation, both before initiation and during regular follow-up, to check for comorbidities.
The American College of Physicians cautioned against such prescribing practices in a recent position statement, emphasizing that the lack of an established care provider could adversely affect patients. We note that the potential harms of DTC sales would concentrate in economically and racially underserved communities, where obesity, lack of insurance, and low health literacy are more common.
But the DTC genie is out of the pill bottle, and as such platforms become more common, patients will inherently take more ownership over their medical care. Remote providers will of course not be following these patients and evaluating for side effects. As a result, we in medical practice must be abreast of new downsides of these medications if and when they arise.
Every clinician must be aware of the medications a patient is taking, even those that they did not prescribe. They should educate their patients about drug-drug interactions and side effects and order lab tests to monitor for side effects.
Independent physicians abide by an underlying oath: First, do no harm. They serve as a trusted check on industry and a valuable long-term partner for patients. Where are the guardrails to protect patients and ensure that pharmaceutical companies are not essentially pushing prescriptions for their own products? Will traditional healthcare providers be effectively relegated to a bystander role in Lilly’s transactional approach to medication distribution? Unlike other commercial goods, pharmacologics have great nuance; not every approved medication is meant for every patient.
A version of this article appeared on Medscape.com.
Pharmaceutical giant Eli Lilly recently announced that its newly approved weight loss medication Zepbound — a glucagon-like peptide 1 receptor agonist (GLP-1 RA) akin to Mounjaro, Ozempic, and Wegovy — will be prescribed by independent telehealth providers on a platform managed by the company itself. The drug can be subsequently shipped direct to consumer (DTC), allowing delivery straight to patients’ homes.
This arrangement raises serious concerns about an inherent conflict of interest, as we previously discussed. What happens when a pharmaceutical company influences access to remote providers who prescribe the very medications it manufactures?
Without new guardrails, the potential for misleading messaging to result in dangerous prescribing patterns looms large. The United States is one of only two countries to allow DTC advertising of prescription drugs, and the explosion in demand for GLP-1 RAs is partly attributable to this model (Oh, oh, Ozempic, anyone?). Americans spent over $78 billion on weight loss goods and services in 2019; time-intensive approaches such as diet and exercise are understandably difficult, and the public has always looked for a magic cure. Although GLP-1 RAs are promising, they may present a path to disaster without proper supervision.
LillyDirect, which in addition to Zepbound offers migraine medications and other products in the company’s catalogue, primarily aims to increase access to medication and reduce costs of the drugs for consumers. The stated mission is noble: By cutting out the middlemen of traditional pharmacies and benefit managers, administrative costs drop. LillyDirect goes a step further by reducing the need for patients to visit their regular family doctor to receive these medications.
On the surface, this design appears promising. Wait times for doctor’s appointments will fall. Patients can order drugs from the comfort of their home. Everyone benefits. Or do they?
Although easier access and reduced cost may be an apparent win for patients, DTC arrangements complicate the ethics of prescriptions and patient follow-up. This model reminds us of the roots of the opioid crisis, where powerful advertising and relationships between prescribers and drugmakers led to great harm. Providers often faced a conflict of interest when prescribing dangerous drugs to patients who requested them. We must learn from these mistakes to ensure there is critical oversight into the independence of prescribers used by LillyDirect and other DTC platforms.
Adding to these parallels, once a patient begins a GLP-1 medication such as Zepbound, stopping treatment will probably lead to regaining lost weight, serving as negative reinforcement. Hence, patients may decide never to discontinue these medications.
Obtaining what amounts to a lifelong prescription from a telehealth provider who may never follow a patient sets a dangerous precedent that will be difficult to unravel once begun. Recent challenges in access to medications such as Zepbound have been complicated by supply chain and manufacturing issues, leading to potential interruptions in patient access, ultimately affecting compliance. The rapid increase in online providers indicates competition for distribution channels has sharply increased and poses a threat to Lilly’s DTC site.
Furthermore, the lack of a regular physician to monitor patients introduces uncertainty in safety and continuity of care. These are important tenets in protecting patients, especially patients who are not diabetic and desire a quick fix. We have already seen a huge, arguably unrestrained, rise in prescriptions of GLP-1 RAs for weight loss — up to a 352% increase in 2023.
These drugs have shown great promise and are generally safe when used in the right patient, but important contraindications exist — namely, serious gastrointestinal side effects and low blood glucose in nondiabetic persons — that an astute physician must consider. Patients desiring these medications often must undergo comprehensive laboratory testing and cardiac evaluation, both before initiation and during regular follow-up, to check for comorbidities.
The American College of Physicians cautioned against such prescribing practices in a recent position statement, emphasizing that the lack of an established care provider could adversely affect patients. We note that the potential harms of DTC sales would concentrate in economically and racially underserved communities, where obesity, lack of insurance, and low health literacy are more common.
But the DTC genie is out of the pill bottle, and as such platforms become more common, patients will inherently take more ownership over their medical care. Remote providers will of course not be following these patients and evaluating for side effects. As a result, we in medical practice must be abreast of new downsides of these medications if and when they arise.
Every clinician must be aware of the medications a patient is taking, even those that they did not prescribe. They should educate their patients about drug-drug interactions and side effects and order lab tests to monitor for side effects.
Independent physicians abide by an underlying oath: First, do no harm. They serve as a trusted check on industry and a valuable long-term partner for patients. Where are the guardrails to protect patients and ensure that pharmaceutical companies are not essentially pushing prescriptions for their own products? Will traditional healthcare providers be effectively relegated to a bystander role in Lilly’s transactional approach to medication distribution? Unlike other commercial goods, pharmacologics have great nuance; not every approved medication is meant for every patient.
A version of this article appeared on Medscape.com.
Do Health-Related Social Needs Raise Mortality Risk in Cancer Survivors?
Little is known about the specific association between health-related social needs (HRSNs) and mortality risk even though HRSNs, defined as challenges in affording food, housing, and other necessities of daily living, are potential challenges for cancer survivors, wrote Zhiyuan Zheng, PhD, of the American Cancer Society, Atlanta, and colleagues.
A 2020 study by Dr. Zheng and colleagues published in the Journal of the National Comprehensive Cancer Network (NCCN) showed that food insecurity and financial worries had a negative impact on cancer survivorship. In the new study, published in Cancer, the researchers identified cancer survivors using the 2013-2018 National Health Interview Survey (NHIS) and the NHIS Mortality File through December 31, 2019. The researchers examined mortality using the data from the Centers for Disease Control and Prevention’s National Death Index (NDI) through December 31, 2019, which links to the National Health Interview Survey Data used in the study.
Individuals’ HRSNs were categorized into three groups: severe, moderate, and minor/none. HRSNs included food insecurity and nonmedical financial concerns, such as housing costs (rent, mortgage). Medical financial hardship included material, psychological, and behavioral domains and was divided into three groups: 2-3 domains, 1 domain, or 0 domains.
What Are the Potential Financial Implications of this Research?
The high costs of cancer care often cause medical financial hardships for cancer survivors, and expenses also may cause psychological distress and nonmedical financial hardship as survivors try to make ends meet while facing medical bills, wrote Dr. Zheng and colleagues.
Policy makers are increasingly interested in adding HRSNs to insurance coverage; recent guidance from the Centers for Medicare & Medicaid Services (CMS) allows individual states to apply to provide nutrition and housing supports through state Medicaid programs, according to authors of a 2023 article published in JAMA Health Forum.
The new study adds to the understanding of how HRSNs impact people with cancer by examining the association with mortality risk, Yelak Biru, MSc, president and chief executive officer of the International Myeloma Foundation, said in an interview.
“This is a key area of study for addressing the disparities in treatments and outcomes that result in inequities,” said Mr. Biru, a patient advocate and multiple myeloma survivor who was not involved in the study.
What Does the New Study Show?
The new study characterized HRSNs in 5,855 adult cancer survivors aged 18-64 years and 5,918 aged 65-79 years. In the 18- to 64-year-old group, 25.5% reported moderate levels of HRSNs, and 18.3% reported severe HRSNs. In patients aged 65-79 years, 15.6% and 6.6% reported moderate HRSNs and severe HRSNs, respectively.
Severe HRSN was significantly associated with higher mortality risk in an adjusted analysis in patients aged 18-64 years (hazard ratio 2.00, P < .001).
Among adults aged 65-79 years, severe HRSN was not associated with higher mortality risk; however, in this older age group, those with 2-3 domains of medical financial hardship had significantly increased mortality risk compared with adults aged 65-79 years with zero domains of medical financial hardship (HR 1.58, P = .007).
Although the findings that HRSNs were associated with increased mortality risk, especially in the younger group, were not surprising, they serve as a call to action to address how HRSNs are contributing to cancer mortality, Mr. Biru said in an interview. “HRSNs, like food or housing insecurity, can lead to patients being unable to undergo the best treatment approach for their cancer,” he said.
What Are the Limitations and Research Gaps?
The study findings were limited by several factors including the use of self-reports to measure medical financial hardship, food insecurity, and nonmedical financial concerns in the NHIS, the researchers wrote in their discussion. More research with longer follow-up time beyond 1-5 years is needed, wrote Dr. Zheng and colleagues.
Studies also are needed to illustrate how patient navigation can help prevent patients from falling through the cracks with regard to social needs and financial hardships, Mr. Biru told this news organization.
Other areas for research include how addressing social needs affects health outcomes and whether programs designed to address social needs are effective, he said.
“Finally, qualitative research is needed to capture the lived experiences of cancer survivors facing these challenges. This knowledge can inform the development of more patient-centered interventions and policies that effectively address the social determinants of health and improve overall outcomes for all cancer survivors,” Mr. Biru said.
What Is the Takeaway Message for Clinicians?
HRSNs and financial hardship are significantly associated with increased risk of mortality in adult cancer survivors, Dr. Zheng and colleagues concluded. Looking ahead, comprehensive assessment of HRSNs and financial hardship may help clinicians connect patients with relevant services to mitigate the social and financial impacts of cancer, they wrote.
“The takeaway message for oncologists in practice is that addressing [HRSNs] and financial hardship is crucial for providing comprehensive and equitable cancer care,” Mr. Biru said during his interview.
“The impact of social determinants of health on cancer outcomes cannot be ignored, and oncologists play a vital role in identifying and addressing these needs,” he said. Sensitive, discussion-based screenings are needed to identify core needs such as food and transportation, but clinicians also can consider broader social factors and work with a team to connect patients to appropriate resources, he added.
“By recognizing the importance of HRSN screening and taking proactive steps to address these needs, oncologists can contribute to improving health outcomes, reducing healthcare disparities, and providing more equitable cancer care for their patients,” he said.
What Other Guidance Is Available?
“High-quality cancer care requires treating the whole person; measuring and addressing anything in their life that could result in poorer health outcomes is a key component of comprehensive care,” Mr. Biru emphasized.
In September 2023, the National Comprehensive Cancer Network (NCCN) convened a working group cochaired by Mr. Biru that developed recommendations for how oncology practices should routinely measure HRSNs (NCCN.org/social-needs).
“The working group proposed that every cancer patient be assessed for food, transportation access, and financial and housing security at least once a year, and be reassessed at every care transition point as well,” Mr. Biru said. Such screenings should include follow-up to connect patients with services to address any HRSNs they are experiencing, he added.
Lead author Dr. Zheng is employed by the American Cancer Society, which as a nonprofit receives funds from the public through fundraising and contributions, as well as some support from corporations and industry to support its mission programs and services. Mr. Biru had no financial conflicts to disclose.
Little is known about the specific association between health-related social needs (HRSNs) and mortality risk even though HRSNs, defined as challenges in affording food, housing, and other necessities of daily living, are potential challenges for cancer survivors, wrote Zhiyuan Zheng, PhD, of the American Cancer Society, Atlanta, and colleagues.
A 2020 study by Dr. Zheng and colleagues published in the Journal of the National Comprehensive Cancer Network (NCCN) showed that food insecurity and financial worries had a negative impact on cancer survivorship. In the new study, published in Cancer, the researchers identified cancer survivors using the 2013-2018 National Health Interview Survey (NHIS) and the NHIS Mortality File through December 31, 2019. The researchers examined mortality using the data from the Centers for Disease Control and Prevention’s National Death Index (NDI) through December 31, 2019, which links to the National Health Interview Survey Data used in the study.
Individuals’ HRSNs were categorized into three groups: severe, moderate, and minor/none. HRSNs included food insecurity and nonmedical financial concerns, such as housing costs (rent, mortgage). Medical financial hardship included material, psychological, and behavioral domains and was divided into three groups: 2-3 domains, 1 domain, or 0 domains.
What Are the Potential Financial Implications of this Research?
The high costs of cancer care often cause medical financial hardships for cancer survivors, and expenses also may cause psychological distress and nonmedical financial hardship as survivors try to make ends meet while facing medical bills, wrote Dr. Zheng and colleagues.
Policy makers are increasingly interested in adding HRSNs to insurance coverage; recent guidance from the Centers for Medicare & Medicaid Services (CMS) allows individual states to apply to provide nutrition and housing supports through state Medicaid programs, according to authors of a 2023 article published in JAMA Health Forum.
The new study adds to the understanding of how HRSNs impact people with cancer by examining the association with mortality risk, Yelak Biru, MSc, president and chief executive officer of the International Myeloma Foundation, said in an interview.
“This is a key area of study for addressing the disparities in treatments and outcomes that result in inequities,” said Mr. Biru, a patient advocate and multiple myeloma survivor who was not involved in the study.
What Does the New Study Show?
The new study characterized HRSNs in 5,855 adult cancer survivors aged 18-64 years and 5,918 aged 65-79 years. In the 18- to 64-year-old group, 25.5% reported moderate levels of HRSNs, and 18.3% reported severe HRSNs. In patients aged 65-79 years, 15.6% and 6.6% reported moderate HRSNs and severe HRSNs, respectively.
Severe HRSN was significantly associated with higher mortality risk in an adjusted analysis in patients aged 18-64 years (hazard ratio 2.00, P < .001).
Among adults aged 65-79 years, severe HRSN was not associated with higher mortality risk; however, in this older age group, those with 2-3 domains of medical financial hardship had significantly increased mortality risk compared with adults aged 65-79 years with zero domains of medical financial hardship (HR 1.58, P = .007).
Although the findings that HRSNs were associated with increased mortality risk, especially in the younger group, were not surprising, they serve as a call to action to address how HRSNs are contributing to cancer mortality, Mr. Biru said in an interview. “HRSNs, like food or housing insecurity, can lead to patients being unable to undergo the best treatment approach for their cancer,” he said.
What Are the Limitations and Research Gaps?
The study findings were limited by several factors including the use of self-reports to measure medical financial hardship, food insecurity, and nonmedical financial concerns in the NHIS, the researchers wrote in their discussion. More research with longer follow-up time beyond 1-5 years is needed, wrote Dr. Zheng and colleagues.
Studies also are needed to illustrate how patient navigation can help prevent patients from falling through the cracks with regard to social needs and financial hardships, Mr. Biru told this news organization.
Other areas for research include how addressing social needs affects health outcomes and whether programs designed to address social needs are effective, he said.
“Finally, qualitative research is needed to capture the lived experiences of cancer survivors facing these challenges. This knowledge can inform the development of more patient-centered interventions and policies that effectively address the social determinants of health and improve overall outcomes for all cancer survivors,” Mr. Biru said.
What Is the Takeaway Message for Clinicians?
HRSNs and financial hardship are significantly associated with increased risk of mortality in adult cancer survivors, Dr. Zheng and colleagues concluded. Looking ahead, comprehensive assessment of HRSNs and financial hardship may help clinicians connect patients with relevant services to mitigate the social and financial impacts of cancer, they wrote.
“The takeaway message for oncologists in practice is that addressing [HRSNs] and financial hardship is crucial for providing comprehensive and equitable cancer care,” Mr. Biru said during his interview.
“The impact of social determinants of health on cancer outcomes cannot be ignored, and oncologists play a vital role in identifying and addressing these needs,” he said. Sensitive, discussion-based screenings are needed to identify core needs such as food and transportation, but clinicians also can consider broader social factors and work with a team to connect patients to appropriate resources, he added.
“By recognizing the importance of HRSN screening and taking proactive steps to address these needs, oncologists can contribute to improving health outcomes, reducing healthcare disparities, and providing more equitable cancer care for their patients,” he said.
What Other Guidance Is Available?
“High-quality cancer care requires treating the whole person; measuring and addressing anything in their life that could result in poorer health outcomes is a key component of comprehensive care,” Mr. Biru emphasized.
In September 2023, the National Comprehensive Cancer Network (NCCN) convened a working group cochaired by Mr. Biru that developed recommendations for how oncology practices should routinely measure HRSNs (NCCN.org/social-needs).
“The working group proposed that every cancer patient be assessed for food, transportation access, and financial and housing security at least once a year, and be reassessed at every care transition point as well,” Mr. Biru said. Such screenings should include follow-up to connect patients with services to address any HRSNs they are experiencing, he added.
Lead author Dr. Zheng is employed by the American Cancer Society, which as a nonprofit receives funds from the public through fundraising and contributions, as well as some support from corporations and industry to support its mission programs and services. Mr. Biru had no financial conflicts to disclose.
Little is known about the specific association between health-related social needs (HRSNs) and mortality risk even though HRSNs, defined as challenges in affording food, housing, and other necessities of daily living, are potential challenges for cancer survivors, wrote Zhiyuan Zheng, PhD, of the American Cancer Society, Atlanta, and colleagues.
A 2020 study by Dr. Zheng and colleagues published in the Journal of the National Comprehensive Cancer Network (NCCN) showed that food insecurity and financial worries had a negative impact on cancer survivorship. In the new study, published in Cancer, the researchers identified cancer survivors using the 2013-2018 National Health Interview Survey (NHIS) and the NHIS Mortality File through December 31, 2019. The researchers examined mortality using the data from the Centers for Disease Control and Prevention’s National Death Index (NDI) through December 31, 2019, which links to the National Health Interview Survey Data used in the study.
Individuals’ HRSNs were categorized into three groups: severe, moderate, and minor/none. HRSNs included food insecurity and nonmedical financial concerns, such as housing costs (rent, mortgage). Medical financial hardship included material, psychological, and behavioral domains and was divided into three groups: 2-3 domains, 1 domain, or 0 domains.
What Are the Potential Financial Implications of this Research?
The high costs of cancer care often cause medical financial hardships for cancer survivors, and expenses also may cause psychological distress and nonmedical financial hardship as survivors try to make ends meet while facing medical bills, wrote Dr. Zheng and colleagues.
Policy makers are increasingly interested in adding HRSNs to insurance coverage; recent guidance from the Centers for Medicare & Medicaid Services (CMS) allows individual states to apply to provide nutrition and housing supports through state Medicaid programs, according to authors of a 2023 article published in JAMA Health Forum.
The new study adds to the understanding of how HRSNs impact people with cancer by examining the association with mortality risk, Yelak Biru, MSc, president and chief executive officer of the International Myeloma Foundation, said in an interview.
“This is a key area of study for addressing the disparities in treatments and outcomes that result in inequities,” said Mr. Biru, a patient advocate and multiple myeloma survivor who was not involved in the study.
What Does the New Study Show?
The new study characterized HRSNs in 5,855 adult cancer survivors aged 18-64 years and 5,918 aged 65-79 years. In the 18- to 64-year-old group, 25.5% reported moderate levels of HRSNs, and 18.3% reported severe HRSNs. In patients aged 65-79 years, 15.6% and 6.6% reported moderate HRSNs and severe HRSNs, respectively.
Severe HRSN was significantly associated with higher mortality risk in an adjusted analysis in patients aged 18-64 years (hazard ratio 2.00, P < .001).
Among adults aged 65-79 years, severe HRSN was not associated with higher mortality risk; however, in this older age group, those with 2-3 domains of medical financial hardship had significantly increased mortality risk compared with adults aged 65-79 years with zero domains of medical financial hardship (HR 1.58, P = .007).
Although the findings that HRSNs were associated with increased mortality risk, especially in the younger group, were not surprising, they serve as a call to action to address how HRSNs are contributing to cancer mortality, Mr. Biru said in an interview. “HRSNs, like food or housing insecurity, can lead to patients being unable to undergo the best treatment approach for their cancer,” he said.
What Are the Limitations and Research Gaps?
The study findings were limited by several factors including the use of self-reports to measure medical financial hardship, food insecurity, and nonmedical financial concerns in the NHIS, the researchers wrote in their discussion. More research with longer follow-up time beyond 1-5 years is needed, wrote Dr. Zheng and colleagues.
Studies also are needed to illustrate how patient navigation can help prevent patients from falling through the cracks with regard to social needs and financial hardships, Mr. Biru told this news organization.
Other areas for research include how addressing social needs affects health outcomes and whether programs designed to address social needs are effective, he said.
“Finally, qualitative research is needed to capture the lived experiences of cancer survivors facing these challenges. This knowledge can inform the development of more patient-centered interventions and policies that effectively address the social determinants of health and improve overall outcomes for all cancer survivors,” Mr. Biru said.
What Is the Takeaway Message for Clinicians?
HRSNs and financial hardship are significantly associated with increased risk of mortality in adult cancer survivors, Dr. Zheng and colleagues concluded. Looking ahead, comprehensive assessment of HRSNs and financial hardship may help clinicians connect patients with relevant services to mitigate the social and financial impacts of cancer, they wrote.
“The takeaway message for oncologists in practice is that addressing [HRSNs] and financial hardship is crucial for providing comprehensive and equitable cancer care,” Mr. Biru said during his interview.
“The impact of social determinants of health on cancer outcomes cannot be ignored, and oncologists play a vital role in identifying and addressing these needs,” he said. Sensitive, discussion-based screenings are needed to identify core needs such as food and transportation, but clinicians also can consider broader social factors and work with a team to connect patients to appropriate resources, he added.
“By recognizing the importance of HRSN screening and taking proactive steps to address these needs, oncologists can contribute to improving health outcomes, reducing healthcare disparities, and providing more equitable cancer care for their patients,” he said.
What Other Guidance Is Available?
“High-quality cancer care requires treating the whole person; measuring and addressing anything in their life that could result in poorer health outcomes is a key component of comprehensive care,” Mr. Biru emphasized.
In September 2023, the National Comprehensive Cancer Network (NCCN) convened a working group cochaired by Mr. Biru that developed recommendations for how oncology practices should routinely measure HRSNs (NCCN.org/social-needs).
“The working group proposed that every cancer patient be assessed for food, transportation access, and financial and housing security at least once a year, and be reassessed at every care transition point as well,” Mr. Biru said. Such screenings should include follow-up to connect patients with services to address any HRSNs they are experiencing, he added.
Lead author Dr. Zheng is employed by the American Cancer Society, which as a nonprofit receives funds from the public through fundraising and contributions, as well as some support from corporations and industry to support its mission programs and services. Mr. Biru had no financial conflicts to disclose.
FROM CANCER
Terminal Cancer: What Matters to Patients and Caregivers
New research found that patients and caregivers both tend to prioritize symptom control over life extension but often preferring a balance. Patients and caregivers, however, are less aligned on decisions about cost containment, with patients more likely to prioritize cost containment.
“Our research has revealed that patients and caregivers generally share similar end-of-life goals,” with a “notable exception” when it comes to costs, first author Semra Ozdemir, PhD, with the Lien Centre for Palliative Care, Duke-NUS Medical School, Singapore, told this news organization.
However, when patients and caregivers have a better understanding of the patient’s prognosis, both may be more inclined to avoid costly life-extending treatments and prioritize symptom management.
In other words, the survey suggests that “knowing the prognosis helps patients and their families set realistic expectations for care and adequately prepare for end-of-life decisions,” said Dr. Ozdemir.
This study was published online in JAMA Network Open.
Patients with advanced cancer often face difficult decisions: Do they opt for treatments that may — or may not — extend life or do they focus more on symptom control?
Family caregivers, who also play an important role in this decision-making process, may have different care goals. Some research suggests that caregivers tend to prioritize treatments that could extend life, whereas patients prioritize symptom management, but it’s less clear how these priorities may change over time and how patients and caregivers may influence each other.
In the current study, the researchers examined goals of care among patients with stage IV solid tumors and caregivers during the last 2 years of life, focusing on life extension vs symptom management and cost containment, as well as how these goals changed over time.
The survey included 210 patient-caregiver pairs, recruited from outpatient clinics at two major cancer centers in Singapore. Patients had a mean age of 63 years, and about half were men. The caregivers had a mean age of 49 years, and almost two third (63%) were women.
Overall, 34% patients and 29% caregivers prioritized symptom management over life extension, whereas 24% patients and 19% caregivers prioritized life extension. Most patients and caregivers preferred balancing the two, with 34%-47% patients and 37%-69% caregivers supporting this approach.
When balancing cost and treatment decisions, however, patients were more likely to prioritize containing costs — 28% vs 17% for caregivers — over extending life — 26% of patients vs 35% of caregivers.
Cost containment tended to be more of a priority for older patients, those with a higher symptom burden, and those with less family caregiver support. For caregivers, cost containment was more of a priority for those who reported that caregiving had a big impact on their finances, those with worse self-esteem related to their caregiving abilities, as well as those caring for older patients.
To better align cost containment priorities between patients and caregivers, it’s essential for families to engage in open and thorough discussions about the allocation of resources, Dr. Ozdemir said.
Although “patients, families, and physicians often avoid discussions about prognosis,” such conversations are essential for setting realistic expectations for care and adequately preparing for end-of-life decisions, Dr. Ozdemir told this news organization.
“These conversations should aim to balance competing interests and create care plans that are mutually acceptable to both patients and caregivers,” she said, adding that “this approach will help in minimizing any potential conflicts and ensure that both parties feel respected and understood in their decision-making process.”
Managing Unrealistic Expectations
As patients approached the end of life, neither patients nor caregivers shifted their priorities from life extension to symptom management.
This finding raises concerns because it suggests that many patients hold unrealistic expectations regarding their care and “underscores the need for continuous dialogue and reassessment of care goals throughout the progression of illness,” Dr. Ozdemir said.
“This stability in preferences over time suggests that initial care decisions are deeply ingrained or that there may be a lack of ongoing communication about evolving care needs and possibilities as conditions change,” Ozdemir said.
Yet, it can be hard to define what unrealistic expectations mean, said Olivia Seecof, MD, who wasn’t involved in the study.
“I think people are hopeful that a devastating diagnosis won’t lead to the end of their life and that there will be a treatment or something that will change [their prognosis], and they’ll get better,” said Dr. Seecof, palliative care expert with the Supportive Oncology Program at NYU Langone Health’s Perlmutter Cancer Center in New York City.
Giving patients and caregivers a realistic understanding of the prognosis is important, but “there’s more to it than just telling the patient their diagnosis,” she said.
“We have to plan for end of life, what it can look like,” said Dr. Seecof, adding that “often we don’t do a very good job of talking about that early on in an illness course.”
Overall, though, Dr. Seecof stressed that no two patients or situations are the same, and it’s important to understand what’s important in each scenario. End-of-life care requires “an individual approach because every patient is different, even if they have the same diagnosis as someone else,” she said.
This work was supported by funding from the Singapore Millennium Foundation and the Lien Centre for Palliative Care. Dr. Ozdemir and Dr. Seecof had no relevant disclosures.
A version of this article appeared on Medscape.com.
New research found that patients and caregivers both tend to prioritize symptom control over life extension but often preferring a balance. Patients and caregivers, however, are less aligned on decisions about cost containment, with patients more likely to prioritize cost containment.
“Our research has revealed that patients and caregivers generally share similar end-of-life goals,” with a “notable exception” when it comes to costs, first author Semra Ozdemir, PhD, with the Lien Centre for Palliative Care, Duke-NUS Medical School, Singapore, told this news organization.
However, when patients and caregivers have a better understanding of the patient’s prognosis, both may be more inclined to avoid costly life-extending treatments and prioritize symptom management.
In other words, the survey suggests that “knowing the prognosis helps patients and their families set realistic expectations for care and adequately prepare for end-of-life decisions,” said Dr. Ozdemir.
This study was published online in JAMA Network Open.
Patients with advanced cancer often face difficult decisions: Do they opt for treatments that may — or may not — extend life or do they focus more on symptom control?
Family caregivers, who also play an important role in this decision-making process, may have different care goals. Some research suggests that caregivers tend to prioritize treatments that could extend life, whereas patients prioritize symptom management, but it’s less clear how these priorities may change over time and how patients and caregivers may influence each other.
In the current study, the researchers examined goals of care among patients with stage IV solid tumors and caregivers during the last 2 years of life, focusing on life extension vs symptom management and cost containment, as well as how these goals changed over time.
The survey included 210 patient-caregiver pairs, recruited from outpatient clinics at two major cancer centers in Singapore. Patients had a mean age of 63 years, and about half were men. The caregivers had a mean age of 49 years, and almost two third (63%) were women.
Overall, 34% patients and 29% caregivers prioritized symptom management over life extension, whereas 24% patients and 19% caregivers prioritized life extension. Most patients and caregivers preferred balancing the two, with 34%-47% patients and 37%-69% caregivers supporting this approach.
When balancing cost and treatment decisions, however, patients were more likely to prioritize containing costs — 28% vs 17% for caregivers — over extending life — 26% of patients vs 35% of caregivers.
Cost containment tended to be more of a priority for older patients, those with a higher symptom burden, and those with less family caregiver support. For caregivers, cost containment was more of a priority for those who reported that caregiving had a big impact on their finances, those with worse self-esteem related to their caregiving abilities, as well as those caring for older patients.
To better align cost containment priorities between patients and caregivers, it’s essential for families to engage in open and thorough discussions about the allocation of resources, Dr. Ozdemir said.
Although “patients, families, and physicians often avoid discussions about prognosis,” such conversations are essential for setting realistic expectations for care and adequately preparing for end-of-life decisions, Dr. Ozdemir told this news organization.
“These conversations should aim to balance competing interests and create care plans that are mutually acceptable to both patients and caregivers,” she said, adding that “this approach will help in minimizing any potential conflicts and ensure that both parties feel respected and understood in their decision-making process.”
Managing Unrealistic Expectations
As patients approached the end of life, neither patients nor caregivers shifted their priorities from life extension to symptom management.
This finding raises concerns because it suggests that many patients hold unrealistic expectations regarding their care and “underscores the need for continuous dialogue and reassessment of care goals throughout the progression of illness,” Dr. Ozdemir said.
“This stability in preferences over time suggests that initial care decisions are deeply ingrained or that there may be a lack of ongoing communication about evolving care needs and possibilities as conditions change,” Ozdemir said.
Yet, it can be hard to define what unrealistic expectations mean, said Olivia Seecof, MD, who wasn’t involved in the study.
“I think people are hopeful that a devastating diagnosis won’t lead to the end of their life and that there will be a treatment or something that will change [their prognosis], and they’ll get better,” said Dr. Seecof, palliative care expert with the Supportive Oncology Program at NYU Langone Health’s Perlmutter Cancer Center in New York City.
Giving patients and caregivers a realistic understanding of the prognosis is important, but “there’s more to it than just telling the patient their diagnosis,” she said.
“We have to plan for end of life, what it can look like,” said Dr. Seecof, adding that “often we don’t do a very good job of talking about that early on in an illness course.”
Overall, though, Dr. Seecof stressed that no two patients or situations are the same, and it’s important to understand what’s important in each scenario. End-of-life care requires “an individual approach because every patient is different, even if they have the same diagnosis as someone else,” she said.
This work was supported by funding from the Singapore Millennium Foundation and the Lien Centre for Palliative Care. Dr. Ozdemir and Dr. Seecof had no relevant disclosures.
A version of this article appeared on Medscape.com.
New research found that patients and caregivers both tend to prioritize symptom control over life extension but often preferring a balance. Patients and caregivers, however, are less aligned on decisions about cost containment, with patients more likely to prioritize cost containment.
“Our research has revealed that patients and caregivers generally share similar end-of-life goals,” with a “notable exception” when it comes to costs, first author Semra Ozdemir, PhD, with the Lien Centre for Palliative Care, Duke-NUS Medical School, Singapore, told this news organization.
However, when patients and caregivers have a better understanding of the patient’s prognosis, both may be more inclined to avoid costly life-extending treatments and prioritize symptom management.
In other words, the survey suggests that “knowing the prognosis helps patients and their families set realistic expectations for care and adequately prepare for end-of-life decisions,” said Dr. Ozdemir.
This study was published online in JAMA Network Open.
Patients with advanced cancer often face difficult decisions: Do they opt for treatments that may — or may not — extend life or do they focus more on symptom control?
Family caregivers, who also play an important role in this decision-making process, may have different care goals. Some research suggests that caregivers tend to prioritize treatments that could extend life, whereas patients prioritize symptom management, but it’s less clear how these priorities may change over time and how patients and caregivers may influence each other.
In the current study, the researchers examined goals of care among patients with stage IV solid tumors and caregivers during the last 2 years of life, focusing on life extension vs symptom management and cost containment, as well as how these goals changed over time.
The survey included 210 patient-caregiver pairs, recruited from outpatient clinics at two major cancer centers in Singapore. Patients had a mean age of 63 years, and about half were men. The caregivers had a mean age of 49 years, and almost two third (63%) were women.
Overall, 34% patients and 29% caregivers prioritized symptom management over life extension, whereas 24% patients and 19% caregivers prioritized life extension. Most patients and caregivers preferred balancing the two, with 34%-47% patients and 37%-69% caregivers supporting this approach.
When balancing cost and treatment decisions, however, patients were more likely to prioritize containing costs — 28% vs 17% for caregivers — over extending life — 26% of patients vs 35% of caregivers.
Cost containment tended to be more of a priority for older patients, those with a higher symptom burden, and those with less family caregiver support. For caregivers, cost containment was more of a priority for those who reported that caregiving had a big impact on their finances, those with worse self-esteem related to their caregiving abilities, as well as those caring for older patients.
To better align cost containment priorities between patients and caregivers, it’s essential for families to engage in open and thorough discussions about the allocation of resources, Dr. Ozdemir said.
Although “patients, families, and physicians often avoid discussions about prognosis,” such conversations are essential for setting realistic expectations for care and adequately preparing for end-of-life decisions, Dr. Ozdemir told this news organization.
“These conversations should aim to balance competing interests and create care plans that are mutually acceptable to both patients and caregivers,” she said, adding that “this approach will help in minimizing any potential conflicts and ensure that both parties feel respected and understood in their decision-making process.”
Managing Unrealistic Expectations
As patients approached the end of life, neither patients nor caregivers shifted their priorities from life extension to symptom management.
This finding raises concerns because it suggests that many patients hold unrealistic expectations regarding their care and “underscores the need for continuous dialogue and reassessment of care goals throughout the progression of illness,” Dr. Ozdemir said.
“This stability in preferences over time suggests that initial care decisions are deeply ingrained or that there may be a lack of ongoing communication about evolving care needs and possibilities as conditions change,” Ozdemir said.
Yet, it can be hard to define what unrealistic expectations mean, said Olivia Seecof, MD, who wasn’t involved in the study.
“I think people are hopeful that a devastating diagnosis won’t lead to the end of their life and that there will be a treatment or something that will change [their prognosis], and they’ll get better,” said Dr. Seecof, palliative care expert with the Supportive Oncology Program at NYU Langone Health’s Perlmutter Cancer Center in New York City.
Giving patients and caregivers a realistic understanding of the prognosis is important, but “there’s more to it than just telling the patient their diagnosis,” she said.
“We have to plan for end of life, what it can look like,” said Dr. Seecof, adding that “often we don’t do a very good job of talking about that early on in an illness course.”
Overall, though, Dr. Seecof stressed that no two patients or situations are the same, and it’s important to understand what’s important in each scenario. End-of-life care requires “an individual approach because every patient is different, even if they have the same diagnosis as someone else,” she said.
This work was supported by funding from the Singapore Millennium Foundation and the Lien Centre for Palliative Care. Dr. Ozdemir and Dr. Seecof had no relevant disclosures.
A version of this article appeared on Medscape.com.
Home ventilation consult
Specialist input on a sudden shift in device availability
Philips Respironics released a public statement on January 25, 2024, that would dramatically change the landscape of home mechanical ventilation and sleep-disordered breathing management in the United States. The company announced that, effective immediately in the US and US territories, Philips Respironics would stop production and sale of all hospital and home mechanical ventilation products, home and hospital ventilation devices, and oxygen concentrators.
There are many unknowns and uncertainties about how to proceed with care for patients requiring these devices. So we gathered an expert panel of clinicians from CHEST’s Home-Based Mechanical Ventilation and Neuromuscular Section within the Sleep Medicine Network to explain the current situation and offer suggestions on moving forward in caring for these patients.
Why is this happening?
John M. Coleman III, MD, FCCP: To understand the current Philips Respironics announcement, we must go back to June 2021. At that time, Philips recalled certain home mechanical ventilators, CPAP machines, and BiPAP machines due to potential health risks related to breakdown of the polyester-based polyurethane (PE-PUR) foam placed in these devices for noise reduction. Small and microscopic particles of this foam were at risk for being inhaled or ingested by patients using these devices. It was suspected that inhalation of these particles could potentially result in temporary or permanent injury. Machines in hot temperatures or using ozone cleaning were at increased risk. The US Food and Drug Administration (FDA) issued a class 1 recall, defined as “a situation in which there is reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
In the months following the initial recall, there were additional recalls of both in-hospital and home ventilators related to the potential of these foam particles to move and block the air path, reducing airflow and causing the device to alarm.
Over the next few years, tens of thousands of medical device reports were filed about PE-PUR foam-related injuries, with some cases resulting in death. At this time, the Department of Justice began collaborating with the FDA on a consent decree. There were ongoing recalls of the CoughAssist T70 device, as well as the newest generation of Philips Respironics home ventilators, the Trilogy EVO.
Ultimately, after years of ongoing recalls and reports of numerous deaths and injuries, with multiple class action lawsuits, the consent decree was finalized. Philips Respironics agreed to stop production of all respiratory-related products in the US and US territories.
What devices does this apply to?
Jason Ackrivo, MD: This notice affects the devices shown in Table 1. All sales and device shipments have been discontinued as of January 25, 2024. Philips Respironics will continue to service the devices, subject to part availability, up to 5 years after sales discontinuation. However, Philips Respironics will continue to sell consumables and accessories, including masks.
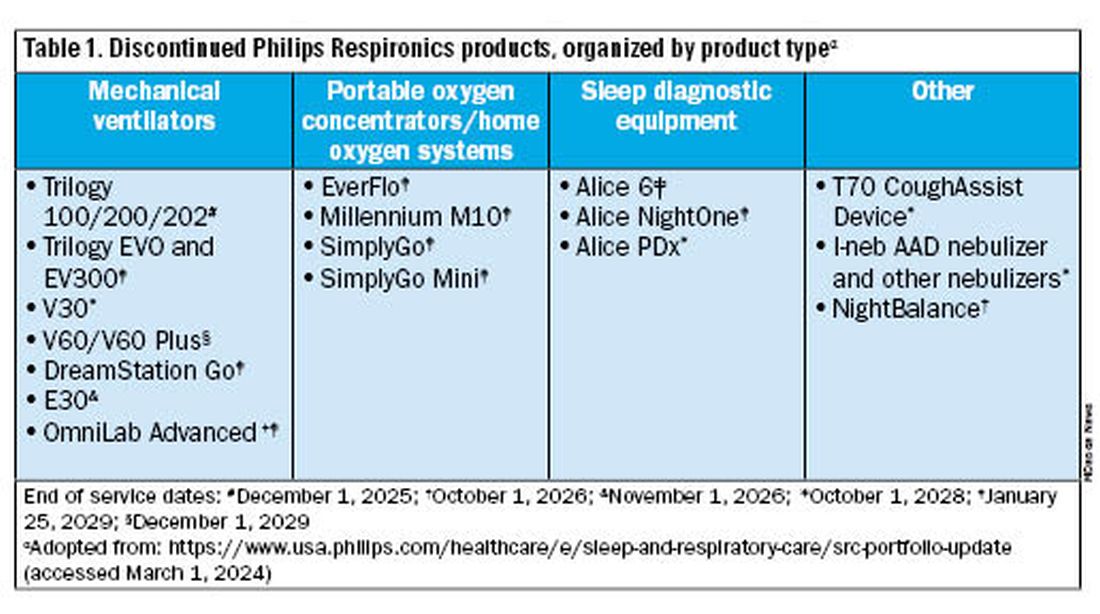
What are my options for home mechanical ventilators?
Bethany L. Lussier, MD, FCCP: In the US, alternative approved home mechanical ventilator (HMV) devices include Astral by ResMed, Vivo 45 and Vivo 65 by Breas, and VOCSN by Ventec. Additional options made available through emergency use authorization by the FDA between 2020 and 2022 included Luisa by Löwenstein Medical, the V+ by Ventec, and Life2000 by Baxter. Many of us expedite disposition from the hospital by prescribing HMVs rather than respiratory assist devices (RADs) because it is easier to meet qualifying criteria for insurance. In efforts to promote just allocation of resources, now might be the ideal time to reconsider higher utilization of RADs over HMVs. Reasonable RAD candidates are those who do not need autotitration of EPAP, dual mode therapy, or invasive ventilation. In these cases, the qualifying criteria and patient needs may be met with a RAD capable of VAPS or BPAP-ST mode.
How are these alternative devices similar to and different from the Trilogy EVO?
Dr. Ackrivo:All these devices are portable ventilators that can deliver noninvasive or invasive ventilation. They have internal batteries for enabling portability. They offer multiple programmable presets and mouthpiece ventilation, and some offer both oxygenation and CO2 monitoring (both TcCO2 and EtCO2).
All alternative portable ventilators include a proprietary ventilation mode analogous to the Trilogy AVAPS algorithm (Table 2). The ResMed Astral has a safety tidal volume feature that targets a minimum tidal volume in PS, S/T, or P(A)C modes. The ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. The Breas Vivo can target a tidal volume (TgV) in either PSV or PCV mode.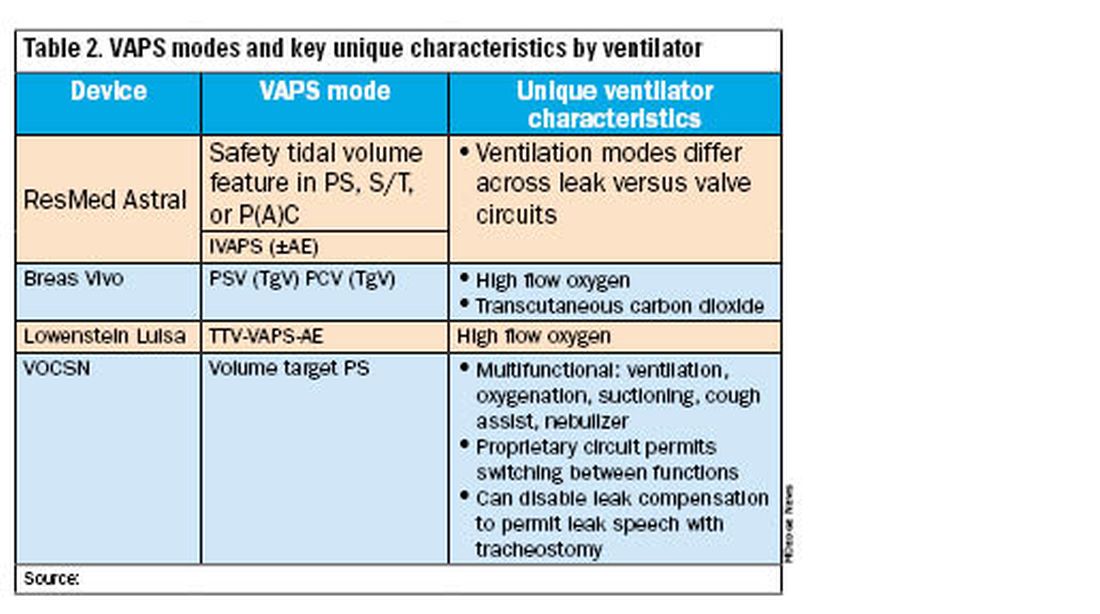
Unique ventilator characteristics are shown in Table 2. ResMed Astral mode options will differ between leak (passive) or valve (active) circuits. Both the Breas Vivo and Löwenstein Luisa enable high-flow oxygen delivery. Only the Breas Vivo enables connecting to a transcutaneous carbon dioxide monitor. The VOCSN name is an acronym for its multifunctional capabilities: ventilation, oxygenation, cough assist, suction, and nebulizer treatments. Lastly, the VOCSN can disable leak compensation, which may be advantageous for enabling leak speech with a tracheostomy.
I just provided my patient with a Trilogy EVO. Do I need to change this immediately?
Dr. Coleman: No, but you should start conversations with your patient/caregiving support and with your durable medical equipment (DME) provider about alternative options. The ripple effects of the Philips Respironics recall will be ongoing for years. The silver lining of this situation is that there are numerous HMV options on the market currently. It is important to review the differences between these new devices and consider what will work best for your patient and your practice. In addition, it is critical that your DME provider is familiar with these new devices, both for support and education, and is taking steps to make alternate devices available. We anticipate a push in coming months to switch patients off Trilogy EVO, so it important to get this process started.
For patients not interested in switching just yet, Philips Respironics will continue to service and offer supplies for these devices for up to 5 years, depending on part availability (Table 1). Refer to the Philips Respironics Sleep & Respiratory Product Portfolio Changes website for the most up-to-date information.
I have a patient on AVAPS, and I must change to iVAPS. What now?
Dr. Lussier: As mentioned previously by Dr. Ackrivo, the ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. A download from a current VAPS setting can be helpful in defining target ventilation and pressure ranges for a tailored prescription. ResMed has an online iVAPS calculator (resmed.com) to assist in making this switch. Close clinical monitoring with data downloads is recommended to assure desired targets are still achieved.
What will happen to Philips Respironics’ cloud patient data?
Dr. Lussier: Representatives have reported that both providers and DME companies will have continued access to Care Orchestrator going forward. Currently, the logistics of data maintenance and ownership remain unclear, which poses additional questions about global access to patients’ data downloads.
----------
The recent discontinuation of Philips Respironics ventilation devices will induce a dramatic shift in home ventilation options in the US. Clinicians and DME companies should begin familiarizing themselves with alternative ventilators and their unique features. While significant uncertainty exists, we encourage a proactive approach to education and communication to ensure a smooth transition for patients on home ventilation.
John M. Coleman III, MD, FCCP, is Associate Professor, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Northwestern University Feinberg School of Medicine. Bethany L. Lussier, MD, FCCP, is in the Department of Internal Medicine, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Division of Neurocritical Care, UT Southwestern Medical Center. Jason Ackrivo, MD, is Assistant Professor of Medicine and Neurology, and Associate Director, Jay and Randy Fishman Program for Home Assisted Ventilation, Pulmonary, Allergy, and Critical Care Division, Perelman School of Medicine, University of Pennsylvania.
Specialist input on a sudden shift in device availability
Specialist input on a sudden shift in device availability
Philips Respironics released a public statement on January 25, 2024, that would dramatically change the landscape of home mechanical ventilation and sleep-disordered breathing management in the United States. The company announced that, effective immediately in the US and US territories, Philips Respironics would stop production and sale of all hospital and home mechanical ventilation products, home and hospital ventilation devices, and oxygen concentrators.
There are many unknowns and uncertainties about how to proceed with care for patients requiring these devices. So we gathered an expert panel of clinicians from CHEST’s Home-Based Mechanical Ventilation and Neuromuscular Section within the Sleep Medicine Network to explain the current situation and offer suggestions on moving forward in caring for these patients.
Why is this happening?
John M. Coleman III, MD, FCCP: To understand the current Philips Respironics announcement, we must go back to June 2021. At that time, Philips recalled certain home mechanical ventilators, CPAP machines, and BiPAP machines due to potential health risks related to breakdown of the polyester-based polyurethane (PE-PUR) foam placed in these devices for noise reduction. Small and microscopic particles of this foam were at risk for being inhaled or ingested by patients using these devices. It was suspected that inhalation of these particles could potentially result in temporary or permanent injury. Machines in hot temperatures or using ozone cleaning were at increased risk. The US Food and Drug Administration (FDA) issued a class 1 recall, defined as “a situation in which there is reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
In the months following the initial recall, there were additional recalls of both in-hospital and home ventilators related to the potential of these foam particles to move and block the air path, reducing airflow and causing the device to alarm.
Over the next few years, tens of thousands of medical device reports were filed about PE-PUR foam-related injuries, with some cases resulting in death. At this time, the Department of Justice began collaborating with the FDA on a consent decree. There were ongoing recalls of the CoughAssist T70 device, as well as the newest generation of Philips Respironics home ventilators, the Trilogy EVO.
Ultimately, after years of ongoing recalls and reports of numerous deaths and injuries, with multiple class action lawsuits, the consent decree was finalized. Philips Respironics agreed to stop production of all respiratory-related products in the US and US territories.
What devices does this apply to?
Jason Ackrivo, MD: This notice affects the devices shown in Table 1. All sales and device shipments have been discontinued as of January 25, 2024. Philips Respironics will continue to service the devices, subject to part availability, up to 5 years after sales discontinuation. However, Philips Respironics will continue to sell consumables and accessories, including masks.

What are my options for home mechanical ventilators?
Bethany L. Lussier, MD, FCCP: In the US, alternative approved home mechanical ventilator (HMV) devices include Astral by ResMed, Vivo 45 and Vivo 65 by Breas, and VOCSN by Ventec. Additional options made available through emergency use authorization by the FDA between 2020 and 2022 included Luisa by Löwenstein Medical, the V+ by Ventec, and Life2000 by Baxter. Many of us expedite disposition from the hospital by prescribing HMVs rather than respiratory assist devices (RADs) because it is easier to meet qualifying criteria for insurance. In efforts to promote just allocation of resources, now might be the ideal time to reconsider higher utilization of RADs over HMVs. Reasonable RAD candidates are those who do not need autotitration of EPAP, dual mode therapy, or invasive ventilation. In these cases, the qualifying criteria and patient needs may be met with a RAD capable of VAPS or BPAP-ST mode.
How are these alternative devices similar to and different from the Trilogy EVO?
Dr. Ackrivo:All these devices are portable ventilators that can deliver noninvasive or invasive ventilation. They have internal batteries for enabling portability. They offer multiple programmable presets and mouthpiece ventilation, and some offer both oxygenation and CO2 monitoring (both TcCO2 and EtCO2).
All alternative portable ventilators include a proprietary ventilation mode analogous to the Trilogy AVAPS algorithm (Table 2). The ResMed Astral has a safety tidal volume feature that targets a minimum tidal volume in PS, S/T, or P(A)C modes. The ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. The Breas Vivo can target a tidal volume (TgV) in either PSV or PCV mode.
Unique ventilator characteristics are shown in Table 2. ResMed Astral mode options will differ between leak (passive) or valve (active) circuits. Both the Breas Vivo and Löwenstein Luisa enable high-flow oxygen delivery. Only the Breas Vivo enables connecting to a transcutaneous carbon dioxide monitor. The VOCSN name is an acronym for its multifunctional capabilities: ventilation, oxygenation, cough assist, suction, and nebulizer treatments. Lastly, the VOCSN can disable leak compensation, which may be advantageous for enabling leak speech with a tracheostomy.
I just provided my patient with a Trilogy EVO. Do I need to change this immediately?
Dr. Coleman: No, but you should start conversations with your patient/caregiving support and with your durable medical equipment (DME) provider about alternative options. The ripple effects of the Philips Respironics recall will be ongoing for years. The silver lining of this situation is that there are numerous HMV options on the market currently. It is important to review the differences between these new devices and consider what will work best for your patient and your practice. In addition, it is critical that your DME provider is familiar with these new devices, both for support and education, and is taking steps to make alternate devices available. We anticipate a push in coming months to switch patients off Trilogy EVO, so it important to get this process started.
For patients not interested in switching just yet, Philips Respironics will continue to service and offer supplies for these devices for up to 5 years, depending on part availability (Table 1). Refer to the Philips Respironics Sleep & Respiratory Product Portfolio Changes website for the most up-to-date information.
I have a patient on AVAPS, and I must change to iVAPS. What now?
Dr. Lussier: As mentioned previously by Dr. Ackrivo, the ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. A download from a current VAPS setting can be helpful in defining target ventilation and pressure ranges for a tailored prescription. ResMed has an online iVAPS calculator (resmed.com) to assist in making this switch. Close clinical monitoring with data downloads is recommended to assure desired targets are still achieved.
What will happen to Philips Respironics’ cloud patient data?
Dr. Lussier: Representatives have reported that both providers and DME companies will have continued access to Care Orchestrator going forward. Currently, the logistics of data maintenance and ownership remain unclear, which poses additional questions about global access to patients’ data downloads.
----------
The recent discontinuation of Philips Respironics ventilation devices will induce a dramatic shift in home ventilation options in the US. Clinicians and DME companies should begin familiarizing themselves with alternative ventilators and their unique features. While significant uncertainty exists, we encourage a proactive approach to education and communication to ensure a smooth transition for patients on home ventilation.
John M. Coleman III, MD, FCCP, is Associate Professor, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Northwestern University Feinberg School of Medicine. Bethany L. Lussier, MD, FCCP, is in the Department of Internal Medicine, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Division of Neurocritical Care, UT Southwestern Medical Center. Jason Ackrivo, MD, is Assistant Professor of Medicine and Neurology, and Associate Director, Jay and Randy Fishman Program for Home Assisted Ventilation, Pulmonary, Allergy, and Critical Care Division, Perelman School of Medicine, University of Pennsylvania.
Philips Respironics released a public statement on January 25, 2024, that would dramatically change the landscape of home mechanical ventilation and sleep-disordered breathing management in the United States. The company announced that, effective immediately in the US and US territories, Philips Respironics would stop production and sale of all hospital and home mechanical ventilation products, home and hospital ventilation devices, and oxygen concentrators.
There are many unknowns and uncertainties about how to proceed with care for patients requiring these devices. So we gathered an expert panel of clinicians from CHEST’s Home-Based Mechanical Ventilation and Neuromuscular Section within the Sleep Medicine Network to explain the current situation and offer suggestions on moving forward in caring for these patients.
Why is this happening?
John M. Coleman III, MD, FCCP: To understand the current Philips Respironics announcement, we must go back to June 2021. At that time, Philips recalled certain home mechanical ventilators, CPAP machines, and BiPAP machines due to potential health risks related to breakdown of the polyester-based polyurethane (PE-PUR) foam placed in these devices for noise reduction. Small and microscopic particles of this foam were at risk for being inhaled or ingested by patients using these devices. It was suspected that inhalation of these particles could potentially result in temporary or permanent injury. Machines in hot temperatures or using ozone cleaning were at increased risk. The US Food and Drug Administration (FDA) issued a class 1 recall, defined as “a situation in which there is reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
In the months following the initial recall, there were additional recalls of both in-hospital and home ventilators related to the potential of these foam particles to move and block the air path, reducing airflow and causing the device to alarm.
Over the next few years, tens of thousands of medical device reports were filed about PE-PUR foam-related injuries, with some cases resulting in death. At this time, the Department of Justice began collaborating with the FDA on a consent decree. There were ongoing recalls of the CoughAssist T70 device, as well as the newest generation of Philips Respironics home ventilators, the Trilogy EVO.
Ultimately, after years of ongoing recalls and reports of numerous deaths and injuries, with multiple class action lawsuits, the consent decree was finalized. Philips Respironics agreed to stop production of all respiratory-related products in the US and US territories.
What devices does this apply to?
Jason Ackrivo, MD: This notice affects the devices shown in Table 1. All sales and device shipments have been discontinued as of January 25, 2024. Philips Respironics will continue to service the devices, subject to part availability, up to 5 years after sales discontinuation. However, Philips Respironics will continue to sell consumables and accessories, including masks.

What are my options for home mechanical ventilators?
Bethany L. Lussier, MD, FCCP: In the US, alternative approved home mechanical ventilator (HMV) devices include Astral by ResMed, Vivo 45 and Vivo 65 by Breas, and VOCSN by Ventec. Additional options made available through emergency use authorization by the FDA between 2020 and 2022 included Luisa by Löwenstein Medical, the V+ by Ventec, and Life2000 by Baxter. Many of us expedite disposition from the hospital by prescribing HMVs rather than respiratory assist devices (RADs) because it is easier to meet qualifying criteria for insurance. In efforts to promote just allocation of resources, now might be the ideal time to reconsider higher utilization of RADs over HMVs. Reasonable RAD candidates are those who do not need autotitration of EPAP, dual mode therapy, or invasive ventilation. In these cases, the qualifying criteria and patient needs may be met with a RAD capable of VAPS or BPAP-ST mode.
How are these alternative devices similar to and different from the Trilogy EVO?
Dr. Ackrivo:All these devices are portable ventilators that can deliver noninvasive or invasive ventilation. They have internal batteries for enabling portability. They offer multiple programmable presets and mouthpiece ventilation, and some offer both oxygenation and CO2 monitoring (both TcCO2 and EtCO2).
All alternative portable ventilators include a proprietary ventilation mode analogous to the Trilogy AVAPS algorithm (Table 2). The ResMed Astral has a safety tidal volume feature that targets a minimum tidal volume in PS, S/T, or P(A)C modes. The ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. The Breas Vivo can target a tidal volume (TgV) in either PSV or PCV mode.
Unique ventilator characteristics are shown in Table 2. ResMed Astral mode options will differ between leak (passive) or valve (active) circuits. Both the Breas Vivo and Löwenstein Luisa enable high-flow oxygen delivery. Only the Breas Vivo enables connecting to a transcutaneous carbon dioxide monitor. The VOCSN name is an acronym for its multifunctional capabilities: ventilation, oxygenation, cough assist, suction, and nebulizer treatments. Lastly, the VOCSN can disable leak compensation, which may be advantageous for enabling leak speech with a tracheostomy.
I just provided my patient with a Trilogy EVO. Do I need to change this immediately?
Dr. Coleman: No, but you should start conversations with your patient/caregiving support and with your durable medical equipment (DME) provider about alternative options. The ripple effects of the Philips Respironics recall will be ongoing for years. The silver lining of this situation is that there are numerous HMV options on the market currently. It is important to review the differences between these new devices and consider what will work best for your patient and your practice. In addition, it is critical that your DME provider is familiar with these new devices, both for support and education, and is taking steps to make alternate devices available. We anticipate a push in coming months to switch patients off Trilogy EVO, so it important to get this process started.
For patients not interested in switching just yet, Philips Respironics will continue to service and offer supplies for these devices for up to 5 years, depending on part availability (Table 1). Refer to the Philips Respironics Sleep & Respiratory Product Portfolio Changes website for the most up-to-date information.
I have a patient on AVAPS, and I must change to iVAPS. What now?
Dr. Lussier: As mentioned previously by Dr. Ackrivo, the ResMed iVAPS algorithm adjusts inspiratory pressure and respiratory rate to target an alveolar ventilation based on patient-entered height. A download from a current VAPS setting can be helpful in defining target ventilation and pressure ranges for a tailored prescription. ResMed has an online iVAPS calculator (resmed.com) to assist in making this switch. Close clinical monitoring with data downloads is recommended to assure desired targets are still achieved.
What will happen to Philips Respironics’ cloud patient data?
Dr. Lussier: Representatives have reported that both providers and DME companies will have continued access to Care Orchestrator going forward. Currently, the logistics of data maintenance and ownership remain unclear, which poses additional questions about global access to patients’ data downloads.
----------
The recent discontinuation of Philips Respironics ventilation devices will induce a dramatic shift in home ventilation options in the US. Clinicians and DME companies should begin familiarizing themselves with alternative ventilators and their unique features. While significant uncertainty exists, we encourage a proactive approach to education and communication to ensure a smooth transition for patients on home ventilation.
John M. Coleman III, MD, FCCP, is Associate Professor, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Northwestern University Feinberg School of Medicine. Bethany L. Lussier, MD, FCCP, is in the Department of Internal Medicine, Division of Pulmonary & Critical Care Medicine, Department of Neurology, Division of Neurocritical Care, UT Southwestern Medical Center. Jason Ackrivo, MD, is Assistant Professor of Medicine and Neurology, and Associate Director, Jay and Randy Fishman Program for Home Assisted Ventilation, Pulmonary, Allergy, and Critical Care Division, Perelman School of Medicine, University of Pennsylvania.
Making invisible problems visible
How Erika Mosesón, MD, educates on the effects of air pollution and encourages community-level advocacy
For Erika Mosesón, MD, a pulmonologist and ICU doctor, advocacy for clean air and climate action started small: signing petitions and writing letters.
Even as she attended conferences and learned about the health impacts of air pollution, her impression was that experts were handling it. “I didn’t really think my voice was worth highlighting,” Dr. Mosesón said.
But her concerns grew with the repeal of the Clean Power Plan in 2019 and rolled-back federal protections around particulate matter and other environmental guidelines.
In response, Dr. Mosesón moved from writing letters to educating people in her home state of Oregon on the lung-related effects of pollution. She spoke at organization meetings and town halls and met with legislators. One way or another, she knew she needed to get the word out.
After all, problem-causing particulates are teeny-tiny; too small to be seen. “It’s literally invisible,” Dr. Mosesón said. But the impact on patients is not.
That’s how the Air Health Our Health podcast was born.
The podcast has a straightforward tagline — ”Clean air saves lives” — and a blunt recommendation: “If you do nothing else, don’t light things on fire and breathe them into your lungs.”
Giving a voice to the voiceless
In early 2017, the Oregon legislature was considering bills aimed at transitioning from diesel-fueled engines to cleaner alternatives. At the time, Dr. Mosesón was on the executive committee for the Oregon Thoracic Society, and, in partnership with the American Lung Association, she was tapped to speak to legislators about clean air and the health impacts of air pollution.
This role made it clear to her that lawmakers don’t hear diverse perspectives. A trucking company may budget for full-time lobbyists, whereas parents of kids with asthma aren’t in the room.
So there’s an asymmetry to who is and is not heard from, Dr. Mosesón said. That’s why in her conversations and presentations, she advocates for those who might not otherwise be represented in the rooms where big decisions are made.
Automating advocacy
Over time, Dr. Mosesón found her schedule was filling up with meetings and presentations.
“I’m a full-time clinician,” Dr. Mosesón noted. She’s also a parent to three kids. When she was asked to attend a hearing, sometimes her schedule required her to decline. And so, early in the pandemic, the Air Health Our Health podcast and the accompanying website were born.
“The podcast and website were honestly a way to automate advocacy,” Dr. Mosesón said.
In many ways, the pandemic was an ideal time to launch the podcast. For one thing, the idea of podcasting from your closet or living room (as opposed to a professional audio studio) became commonplace. Plus, for a pulmonologist, these years were full of relevant topics like how climate change and particulate matter interacted with COVID-19 , Dr. Mosesón noted.
Then, in 2020, the Labor Day fires led to Oregon’s having the worst air quality in the world. That same year, there were George Floyd protests around the country, including in Portland, which led to rampant use of tear gas and prompted Dr. Mosesón to dig into studies about these chemicals.
Given just how much air pollution affects health — and the continued extreme weather events (such as Oregon’s heat dome in summer 2021) — there was no shortage of topics for the podcast.
Next steps to empower physicians
Confronting climate change is daunting, and it is made more challenging by a partisan environment, distrust of experts, and disinformation. On her podcast, Dr. Mosesón aims to make it easier.
In each episode, she shares information and interviews experts. She shares how a patient might be affected by particular issues — radon, wildfires, and so on. The goal is to provide clinicians with a foundation on everyday issues.
“Every single doctor feels like they can talk to a patient about smoking, even if they don’t know all the deep nitty-gritty studies about it,” Dr. Mosesón said. The exact effects of smoking — cancer, heart disease, and lung disease — occur due to air pollution. “When I give talks, I tell people, if you can talk about smoking, you can talk about air pollution.”
Each podcast also features an array of action items.
Some steps are practical, such as creating a plan for heat events or encouraging radon testing. The solution could also be as simple as asking the right questions.
For example, at a doctor’s visit for asthma, common recommendations are to use a HEPA filter or place a sheet protector on the bed, Dr. Mosesón said. It won’t typically come up that a patient’s asthma may be caused or exacerbated by living beside a highway.
Dr. Mosesón also encourages advocacy. “There are all these different levels [of response],” she said. Next steps might involve writing a letter, contacting a councilperson, or advocating for a program (like retiring gas-powered leaf blowers).
For many patients, their doctor is the only person they routinely interact with who has advanced scientific training. Rather than presenting dry data, Dr. Mosesón recommends framing changes and recommendations in ways that are meaningful to neighbors.
“Each physician or clinician is going to know the values of their community,” Dr. Mosesón said. If you’re in a military town, advocating for electric cars may be easier if framed around decreasing dependence on foreign oil. If the region recently experienced back-to-back heat events, advocating for a cooling center might be galvanizing.
What is Dr. Mosesón’s ultimate goal? Inform others so well that she can retire her podcasting equipment.
“I would love,” Dr. Mosesón said, “for every physician in their local community to be a clean air and climate advocate.”
------
Be sure to check out a special episode of the Air Health Our Health podcast, where Dr. Mosesón and CHEST Advocates Editor in Chief, Drew Harris, MD, FCCP, discuss the serious health issues impacting coal miners. They take a deep dive into black lung disease and silica dust, highlighting the science and research, prevention efforts and challenges to implementation, and the importance of advocacy work.
LISTEN NOW »
This article was adapted from the Winter 2024 online issue of CHEST Advocates. For the full article — and to engage with the other content from this issue — visit chestnet.org/chest-advocates.
How Erika Mosesón, MD, educates on the effects of air pollution and encourages community-level advocacy
How Erika Mosesón, MD, educates on the effects of air pollution and encourages community-level advocacy
For Erika Mosesón, MD, a pulmonologist and ICU doctor, advocacy for clean air and climate action started small: signing petitions and writing letters.
Even as she attended conferences and learned about the health impacts of air pollution, her impression was that experts were handling it. “I didn’t really think my voice was worth highlighting,” Dr. Mosesón said.
But her concerns grew with the repeal of the Clean Power Plan in 2019 and rolled-back federal protections around particulate matter and other environmental guidelines.
In response, Dr. Mosesón moved from writing letters to educating people in her home state of Oregon on the lung-related effects of pollution. She spoke at organization meetings and town halls and met with legislators. One way or another, she knew she needed to get the word out.
After all, problem-causing particulates are teeny-tiny; too small to be seen. “It’s literally invisible,” Dr. Mosesón said. But the impact on patients is not.
That’s how the Air Health Our Health podcast was born.
The podcast has a straightforward tagline — ”Clean air saves lives” — and a blunt recommendation: “If you do nothing else, don’t light things on fire and breathe them into your lungs.”
Giving a voice to the voiceless
In early 2017, the Oregon legislature was considering bills aimed at transitioning from diesel-fueled engines to cleaner alternatives. At the time, Dr. Mosesón was on the executive committee for the Oregon Thoracic Society, and, in partnership with the American Lung Association, she was tapped to speak to legislators about clean air and the health impacts of air pollution.
This role made it clear to her that lawmakers don’t hear diverse perspectives. A trucking company may budget for full-time lobbyists, whereas parents of kids with asthma aren’t in the room.
So there’s an asymmetry to who is and is not heard from, Dr. Mosesón said. That’s why in her conversations and presentations, she advocates for those who might not otherwise be represented in the rooms where big decisions are made.
Automating advocacy
Over time, Dr. Mosesón found her schedule was filling up with meetings and presentations.
“I’m a full-time clinician,” Dr. Mosesón noted. She’s also a parent to three kids. When she was asked to attend a hearing, sometimes her schedule required her to decline. And so, early in the pandemic, the Air Health Our Health podcast and the accompanying website were born.
“The podcast and website were honestly a way to automate advocacy,” Dr. Mosesón said.
In many ways, the pandemic was an ideal time to launch the podcast. For one thing, the idea of podcasting from your closet or living room (as opposed to a professional audio studio) became commonplace. Plus, for a pulmonologist, these years were full of relevant topics like how climate change and particulate matter interacted with COVID-19 , Dr. Mosesón noted.
Then, in 2020, the Labor Day fires led to Oregon’s having the worst air quality in the world. That same year, there were George Floyd protests around the country, including in Portland, which led to rampant use of tear gas and prompted Dr. Mosesón to dig into studies about these chemicals.
Given just how much air pollution affects health — and the continued extreme weather events (such as Oregon’s heat dome in summer 2021) — there was no shortage of topics for the podcast.
Next steps to empower physicians
Confronting climate change is daunting, and it is made more challenging by a partisan environment, distrust of experts, and disinformation. On her podcast, Dr. Mosesón aims to make it easier.
In each episode, she shares information and interviews experts. She shares how a patient might be affected by particular issues — radon, wildfires, and so on. The goal is to provide clinicians with a foundation on everyday issues.
“Every single doctor feels like they can talk to a patient about smoking, even if they don’t know all the deep nitty-gritty studies about it,” Dr. Mosesón said. The exact effects of smoking — cancer, heart disease, and lung disease — occur due to air pollution. “When I give talks, I tell people, if you can talk about smoking, you can talk about air pollution.”
Each podcast also features an array of action items.
Some steps are practical, such as creating a plan for heat events or encouraging radon testing. The solution could also be as simple as asking the right questions.
For example, at a doctor’s visit for asthma, common recommendations are to use a HEPA filter or place a sheet protector on the bed, Dr. Mosesón said. It won’t typically come up that a patient’s asthma may be caused or exacerbated by living beside a highway.
Dr. Mosesón also encourages advocacy. “There are all these different levels [of response],” she said. Next steps might involve writing a letter, contacting a councilperson, or advocating for a program (like retiring gas-powered leaf blowers).
For many patients, their doctor is the only person they routinely interact with who has advanced scientific training. Rather than presenting dry data, Dr. Mosesón recommends framing changes and recommendations in ways that are meaningful to neighbors.
“Each physician or clinician is going to know the values of their community,” Dr. Mosesón said. If you’re in a military town, advocating for electric cars may be easier if framed around decreasing dependence on foreign oil. If the region recently experienced back-to-back heat events, advocating for a cooling center might be galvanizing.
What is Dr. Mosesón’s ultimate goal? Inform others so well that she can retire her podcasting equipment.
“I would love,” Dr. Mosesón said, “for every physician in their local community to be a clean air and climate advocate.”
------
Be sure to check out a special episode of the Air Health Our Health podcast, where Dr. Mosesón and CHEST Advocates Editor in Chief, Drew Harris, MD, FCCP, discuss the serious health issues impacting coal miners. They take a deep dive into black lung disease and silica dust, highlighting the science and research, prevention efforts and challenges to implementation, and the importance of advocacy work.
LISTEN NOW »
This article was adapted from the Winter 2024 online issue of CHEST Advocates. For the full article — and to engage with the other content from this issue — visit chestnet.org/chest-advocates.
For Erika Mosesón, MD, a pulmonologist and ICU doctor, advocacy for clean air and climate action started small: signing petitions and writing letters.
Even as she attended conferences and learned about the health impacts of air pollution, her impression was that experts were handling it. “I didn’t really think my voice was worth highlighting,” Dr. Mosesón said.
But her concerns grew with the repeal of the Clean Power Plan in 2019 and rolled-back federal protections around particulate matter and other environmental guidelines.
In response, Dr. Mosesón moved from writing letters to educating people in her home state of Oregon on the lung-related effects of pollution. She spoke at organization meetings and town halls and met with legislators. One way or another, she knew she needed to get the word out.
After all, problem-causing particulates are teeny-tiny; too small to be seen. “It’s literally invisible,” Dr. Mosesón said. But the impact on patients is not.
That’s how the Air Health Our Health podcast was born.
The podcast has a straightforward tagline — ”Clean air saves lives” — and a blunt recommendation: “If you do nothing else, don’t light things on fire and breathe them into your lungs.”
Giving a voice to the voiceless
In early 2017, the Oregon legislature was considering bills aimed at transitioning from diesel-fueled engines to cleaner alternatives. At the time, Dr. Mosesón was on the executive committee for the Oregon Thoracic Society, and, in partnership with the American Lung Association, she was tapped to speak to legislators about clean air and the health impacts of air pollution.
This role made it clear to her that lawmakers don’t hear diverse perspectives. A trucking company may budget for full-time lobbyists, whereas parents of kids with asthma aren’t in the room.
So there’s an asymmetry to who is and is not heard from, Dr. Mosesón said. That’s why in her conversations and presentations, she advocates for those who might not otherwise be represented in the rooms where big decisions are made.
Automating advocacy
Over time, Dr. Mosesón found her schedule was filling up with meetings and presentations.
“I’m a full-time clinician,” Dr. Mosesón noted. She’s also a parent to three kids. When she was asked to attend a hearing, sometimes her schedule required her to decline. And so, early in the pandemic, the Air Health Our Health podcast and the accompanying website were born.
“The podcast and website were honestly a way to automate advocacy,” Dr. Mosesón said.
In many ways, the pandemic was an ideal time to launch the podcast. For one thing, the idea of podcasting from your closet or living room (as opposed to a professional audio studio) became commonplace. Plus, for a pulmonologist, these years were full of relevant topics like how climate change and particulate matter interacted with COVID-19 , Dr. Mosesón noted.
Then, in 2020, the Labor Day fires led to Oregon’s having the worst air quality in the world. That same year, there were George Floyd protests around the country, including in Portland, which led to rampant use of tear gas and prompted Dr. Mosesón to dig into studies about these chemicals.
Given just how much air pollution affects health — and the continued extreme weather events (such as Oregon’s heat dome in summer 2021) — there was no shortage of topics for the podcast.
Next steps to empower physicians
Confronting climate change is daunting, and it is made more challenging by a partisan environment, distrust of experts, and disinformation. On her podcast, Dr. Mosesón aims to make it easier.
In each episode, she shares information and interviews experts. She shares how a patient might be affected by particular issues — radon, wildfires, and so on. The goal is to provide clinicians with a foundation on everyday issues.
“Every single doctor feels like they can talk to a patient about smoking, even if they don’t know all the deep nitty-gritty studies about it,” Dr. Mosesón said. The exact effects of smoking — cancer, heart disease, and lung disease — occur due to air pollution. “When I give talks, I tell people, if you can talk about smoking, you can talk about air pollution.”
Each podcast also features an array of action items.
Some steps are practical, such as creating a plan for heat events or encouraging radon testing. The solution could also be as simple as asking the right questions.
For example, at a doctor’s visit for asthma, common recommendations are to use a HEPA filter or place a sheet protector on the bed, Dr. Mosesón said. It won’t typically come up that a patient’s asthma may be caused or exacerbated by living beside a highway.
Dr. Mosesón also encourages advocacy. “There are all these different levels [of response],” she said. Next steps might involve writing a letter, contacting a councilperson, or advocating for a program (like retiring gas-powered leaf blowers).
For many patients, their doctor is the only person they routinely interact with who has advanced scientific training. Rather than presenting dry data, Dr. Mosesón recommends framing changes and recommendations in ways that are meaningful to neighbors.
“Each physician or clinician is going to know the values of their community,” Dr. Mosesón said. If you’re in a military town, advocating for electric cars may be easier if framed around decreasing dependence on foreign oil. If the region recently experienced back-to-back heat events, advocating for a cooling center might be galvanizing.
What is Dr. Mosesón’s ultimate goal? Inform others so well that she can retire her podcasting equipment.
“I would love,” Dr. Mosesón said, “for every physician in their local community to be a clean air and climate advocate.”
------
Be sure to check out a special episode of the Air Health Our Health podcast, where Dr. Mosesón and CHEST Advocates Editor in Chief, Drew Harris, MD, FCCP, discuss the serious health issues impacting coal miners. They take a deep dive into black lung disease and silica dust, highlighting the science and research, prevention efforts and challenges to implementation, and the importance of advocacy work.
LISTEN NOW »
This article was adapted from the Winter 2024 online issue of CHEST Advocates. For the full article — and to engage with the other content from this issue — visit chestnet.org/chest-advocates.
Fighting for fresh air: RSV’s connection to environmental pollution
Diffuse Lung Disease and Lung Transplant Network
Occupational and Environmental Health Section
Poor air quality has numerous health hazards for patients with chronic lung disease. Now mounting evidence from pediatric studies suggests a concerning link between air pollution and viral infections, specifically respiratory syncytial virus (RSV).
Multiple studies have shown increased incidence and severity of disease in children with exposure to air pollutants such as particulate matter and nitrogen dioxide.1,2,3 Researchers speculate that these pollutants potentiate viral entry to airway epithelium, increase viral load, and dysregulate the immune response.4 Air pollution, increasingly worsened by climate change, is also associated with acute respiratory infections in adults, though adult research remains sparse.5
The adoption of viral testing during the pandemic has revealed a previously under-recognized prevalence of RSV in adults.
RSV accounts for an estimated 60,000 to 160,000 hospitalizations and 6,000 to 10,000 deaths annually among elderly adults. This newfound awareness coincides with the exciting development of a new RSV vaccine that has shown around 85% efficacy at preventing symptomatic RSV infection in the first year, and new data suggest benefits persisting even into the second year after vaccination.6 With an estimated 60 million adults at high risk for RSV in the US, RSV prevention has become an increasingly important aspect of respiratory care.
While more research is needed to definitively quantify the link between air pollution and RSV in adults, the existing data offer valuable insights for all pulmonologists. These findings suggest a benefit in counseling patients with chronic lung conditions on taking steps to mitigate exposure to air pollutants, either through avoidance of outdoor activities or mask-wearing when air quality levels exceed healthy ranges, as well as promoting RSV vaccination for patients who are at risk.7
References
1. Milani GP, Cafora M, Favero C, et al. PM2.5, PM10 and bronchiolitis severity: a cohort study. Pediatr Allergy Immunol. 2022;33(10). https://doi.org/10.1111/pai.13853
2. Wrotek A, Badyda A, Czechowski PO, Owczarek T, Dąbrowiecki P, Jackowska T. Air pollutants’ concentrations are associated with increased number of RSV hospitalizations in Polish children. J Clin Med. 2021;10(15):3224. https://doi.org/10.3390/jcm10153224
3. Horne BD, Joy EA, Hofmann MG, et al. Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am J Respir Crit Care Med. 2018;198(6):759-766. https://doi.org/10.1164/rccm.201709-1883oc
4. Wrotek A, Jackowska T. Molecular mechanisms of RSV and air pollution interaction: a scoping review. Int J Mol Sci. 2022;23(20):12704. https://doi.org/10.3390/ijms232012704
5. Kirwa K, Eckert CM, Vedal S, Hajat A, Kaufman JD. Ambient air pollution and risk of respiratory infection among adults: evidence from the multiethnic study of atherosclerosis (MESA). BMJ Open Respir Res. 2021;8(1). https://doi.org/10.1136/bmjresp-2020-000866
6. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793-801. http://dx.doi.org/10.15585/mmwr.mm7229a4
7. Kodros JK, O’Dell K, Samet JM, L’Orange C, Pierce JR, Volckens J. Quantifying the health benefits of face masks and respirators to mitigate exposure to severe air pollution. GeoHealth. 2021;5(9). https://doi.org/10.1029/2021gh000482
Diffuse Lung Disease and Lung Transplant Network
Occupational and Environmental Health Section
Poor air quality has numerous health hazards for patients with chronic lung disease. Now mounting evidence from pediatric studies suggests a concerning link between air pollution and viral infections, specifically respiratory syncytial virus (RSV).
Multiple studies have shown increased incidence and severity of disease in children with exposure to air pollutants such as particulate matter and nitrogen dioxide.1,2,3 Researchers speculate that these pollutants potentiate viral entry to airway epithelium, increase viral load, and dysregulate the immune response.4 Air pollution, increasingly worsened by climate change, is also associated with acute respiratory infections in adults, though adult research remains sparse.5
The adoption of viral testing during the pandemic has revealed a previously under-recognized prevalence of RSV in adults.
RSV accounts for an estimated 60,000 to 160,000 hospitalizations and 6,000 to 10,000 deaths annually among elderly adults. This newfound awareness coincides with the exciting development of a new RSV vaccine that has shown around 85% efficacy at preventing symptomatic RSV infection in the first year, and new data suggest benefits persisting even into the second year after vaccination.6 With an estimated 60 million adults at high risk for RSV in the US, RSV prevention has become an increasingly important aspect of respiratory care.
While more research is needed to definitively quantify the link between air pollution and RSV in adults, the existing data offer valuable insights for all pulmonologists. These findings suggest a benefit in counseling patients with chronic lung conditions on taking steps to mitigate exposure to air pollutants, either through avoidance of outdoor activities or mask-wearing when air quality levels exceed healthy ranges, as well as promoting RSV vaccination for patients who are at risk.7
References
1. Milani GP, Cafora M, Favero C, et al. PM2.5, PM10 and bronchiolitis severity: a cohort study. Pediatr Allergy Immunol. 2022;33(10). https://doi.org/10.1111/pai.13853
2. Wrotek A, Badyda A, Czechowski PO, Owczarek T, Dąbrowiecki P, Jackowska T. Air pollutants’ concentrations are associated with increased number of RSV hospitalizations in Polish children. J Clin Med. 2021;10(15):3224. https://doi.org/10.3390/jcm10153224
3. Horne BD, Joy EA, Hofmann MG, et al. Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am J Respir Crit Care Med. 2018;198(6):759-766. https://doi.org/10.1164/rccm.201709-1883oc
4. Wrotek A, Jackowska T. Molecular mechanisms of RSV and air pollution interaction: a scoping review. Int J Mol Sci. 2022;23(20):12704. https://doi.org/10.3390/ijms232012704
5. Kirwa K, Eckert CM, Vedal S, Hajat A, Kaufman JD. Ambient air pollution and risk of respiratory infection among adults: evidence from the multiethnic study of atherosclerosis (MESA). BMJ Open Respir Res. 2021;8(1). https://doi.org/10.1136/bmjresp-2020-000866
6. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793-801. http://dx.doi.org/10.15585/mmwr.mm7229a4
7. Kodros JK, O’Dell K, Samet JM, L’Orange C, Pierce JR, Volckens J. Quantifying the health benefits of face masks and respirators to mitigate exposure to severe air pollution. GeoHealth. 2021;5(9). https://doi.org/10.1029/2021gh000482
Diffuse Lung Disease and Lung Transplant Network
Occupational and Environmental Health Section
Poor air quality has numerous health hazards for patients with chronic lung disease. Now mounting evidence from pediatric studies suggests a concerning link between air pollution and viral infections, specifically respiratory syncytial virus (RSV).
Multiple studies have shown increased incidence and severity of disease in children with exposure to air pollutants such as particulate matter and nitrogen dioxide.1,2,3 Researchers speculate that these pollutants potentiate viral entry to airway epithelium, increase viral load, and dysregulate the immune response.4 Air pollution, increasingly worsened by climate change, is also associated with acute respiratory infections in adults, though adult research remains sparse.5
The adoption of viral testing during the pandemic has revealed a previously under-recognized prevalence of RSV in adults.
RSV accounts for an estimated 60,000 to 160,000 hospitalizations and 6,000 to 10,000 deaths annually among elderly adults. This newfound awareness coincides with the exciting development of a new RSV vaccine that has shown around 85% efficacy at preventing symptomatic RSV infection in the first year, and new data suggest benefits persisting even into the second year after vaccination.6 With an estimated 60 million adults at high risk for RSV in the US, RSV prevention has become an increasingly important aspect of respiratory care.
While more research is needed to definitively quantify the link between air pollution and RSV in adults, the existing data offer valuable insights for all pulmonologists. These findings suggest a benefit in counseling patients with chronic lung conditions on taking steps to mitigate exposure to air pollutants, either through avoidance of outdoor activities or mask-wearing when air quality levels exceed healthy ranges, as well as promoting RSV vaccination for patients who are at risk.7
References
1. Milani GP, Cafora M, Favero C, et al. PM2.5, PM10 and bronchiolitis severity: a cohort study. Pediatr Allergy Immunol. 2022;33(10). https://doi.org/10.1111/pai.13853
2. Wrotek A, Badyda A, Czechowski PO, Owczarek T, Dąbrowiecki P, Jackowska T. Air pollutants’ concentrations are associated with increased number of RSV hospitalizations in Polish children. J Clin Med. 2021;10(15):3224. https://doi.org/10.3390/jcm10153224
3. Horne BD, Joy EA, Hofmann MG, et al. Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am J Respir Crit Care Med. 2018;198(6):759-766. https://doi.org/10.1164/rccm.201709-1883oc
4. Wrotek A, Jackowska T. Molecular mechanisms of RSV and air pollution interaction: a scoping review. Int J Mol Sci. 2022;23(20):12704. https://doi.org/10.3390/ijms232012704
5. Kirwa K, Eckert CM, Vedal S, Hajat A, Kaufman JD. Ambient air pollution and risk of respiratory infection among adults: evidence from the multiethnic study of atherosclerosis (MESA). BMJ Open Respir Res. 2021;8(1). https://doi.org/10.1136/bmjresp-2020-000866
6. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793-801. http://dx.doi.org/10.15585/mmwr.mm7229a4
7. Kodros JK, O’Dell K, Samet JM, L’Orange C, Pierce JR, Volckens J. Quantifying the health benefits of face masks and respirators to mitigate exposure to severe air pollution. GeoHealth. 2021;5(9). https://doi.org/10.1029/2021gh000482
A word of caution on e-cigarettes: Retracted paper
Editor’s note: On March 29, 2024, the authors of the study, “Efficacy of Electronic Cigarettes vs Varenicline and Nicotine Chewing Gum as an Aid to Stop Smoking: A Randomized Clinical Trial,” published in JAMA Internal Medicine, issued a formal retraction of their article. The CHEST Physician® Editorial Board apologizes for any confusion this may have caused.
An article in the April issue of the CHEST Physician publication headlined, “E-cigarettes beat nicotine gum for smoking cessation,” was based on an article in JAMA Internal Medicine by Liu Z and colleagues which was subsequently retracted by the author due to coding errors and discrepancies in calculations that cast doubt on the accuracy and reliability of the reported findings.
One should be cautious in evaluating claims of the benefits of electronic cigarettes (e-cigarettes). e-Cigarettes are a highly addictive and largely unregulated product. The fine print in previous clinical trials of e-cigarettes shows greater rates of stopping nicotine products—including e-cigarettes—in the groups assigned to recommendation for nicotine replacement therapy. e-Cigarettes have substantial acute and chronic harms.
Although much of the research to date is from animal models, there is a growing body of evidence in humans that validates the findings from the animal models. In laboratory animal models, e-cigarettes impair airway defenses, contribute to epithelial dysfunction, lead to apoptosis of airway cells, cause emphysematous changes, and lead to increased cancer rates.
Adverse effects on cardiovascular health have also been demonstrated. There is evidence of genotoxicity from e-cigarette exposure, with increased rates of DNA damage and decreased rates of DNA repair. Carcinogenic substances are present in e-cigarettes, and we may not see the carcinogenic effects in humans for several years or even decades. Commonly used flavoring chemicals have substantial pulmonary toxicity. There is evidence that the dual use of e-cigarettes and combustible tobacco can be more harmful than the use of combustible tobacco alone, as the person who smokes is now exposed to additional toxins unique to the e-cigarette.
E-cigarettes can cause severe acute lung disease; 14% of the severe e-cigarette or vaping product use-associated lung injury (EVALI) cases reported use of only nicotine-containing e-cigarette products. There are reports of people who used e-cigarettes who required lung transplant due to complications of their e-cigarette use.
The tobacco industry has a long history of “harm reduction” products that were anything but—from filter cigarettes (the “advanced” Kent Micronite filter contained asbestos) to the so-called low tar and nicotine cigarettes (which were no less harmful). There is a long history of physicians endorsing these products as “must be better.” The growing evidence that e-cigarettes carry distinct health risks of their own should prompt us to consider a broader picture beyond just comparing them with traditional cigarettes to assess their impact on health.
Physicians treating tobacco dependence should recommend US Food and Drug Administration-approved medications for pharmacotherapy. These have a robust evidence base documenting that they help people who smoke to break free of nicotine addiction. The goal of tobacco dependence treatment should be stopping ALL harmful tobacco/nicotine products—including e-cigarettes—not simply changing from one harmful product to another.
References
Liu Z. Notice of retraction: Lin HX et al. Efficacy of electronic cigarettes vs varenicline and nicotine chewing gum as an aid to stop smoking: a randomized clinical trial. JAMA Intern Med. 2024;184(3):291-299. JAMA Intern Med. Preprint. Posted online March 29, 2024. PMID: 38551593. doi: 10.1001/jamainternmed.2024.1125
Farber HJ, Conrado Pacheco Gallego M, Galiatsatos P, Folan P, Lamphere T, Pakhale S. Harms of electronic cigarettes: what the healthcare provider needs to know. Ann Am Thorac Soc. 2021;18(4):567-572. PMID: 33284731. doi: 10.1513/AnnalsATS.202009-1113CME
Proctor RN. Golden Holocaust: Origins of the Cigarette Catastrophe and the Case for Abolition. University of California Press; 2011.
Auer R, Schoeni A, Humair JP, et al. Electronic nicotine-delivery systems for smoking cessation. N Engl J Med. 2024;390(7):601-610. PMID: 38354139. doi: 10.1056/NEJMoa2308815
Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629-637. Preprint. Posted online January 30, 2019. PMID: 30699054. doi: 10.1056/NEJMoa1808779
Editor’s note: On March 29, 2024, the authors of the study, “Efficacy of Electronic Cigarettes vs Varenicline and Nicotine Chewing Gum as an Aid to Stop Smoking: A Randomized Clinical Trial,” published in JAMA Internal Medicine, issued a formal retraction of their article. The CHEST Physician® Editorial Board apologizes for any confusion this may have caused.
An article in the April issue of the CHEST Physician publication headlined, “E-cigarettes beat nicotine gum for smoking cessation,” was based on an article in JAMA Internal Medicine by Liu Z and colleagues which was subsequently retracted by the author due to coding errors and discrepancies in calculations that cast doubt on the accuracy and reliability of the reported findings.
One should be cautious in evaluating claims of the benefits of electronic cigarettes (e-cigarettes). e-Cigarettes are a highly addictive and largely unregulated product. The fine print in previous clinical trials of e-cigarettes shows greater rates of stopping nicotine products—including e-cigarettes—in the groups assigned to recommendation for nicotine replacement therapy. e-Cigarettes have substantial acute and chronic harms.
Although much of the research to date is from animal models, there is a growing body of evidence in humans that validates the findings from the animal models. In laboratory animal models, e-cigarettes impair airway defenses, contribute to epithelial dysfunction, lead to apoptosis of airway cells, cause emphysematous changes, and lead to increased cancer rates.
Adverse effects on cardiovascular health have also been demonstrated. There is evidence of genotoxicity from e-cigarette exposure, with increased rates of DNA damage and decreased rates of DNA repair. Carcinogenic substances are present in e-cigarettes, and we may not see the carcinogenic effects in humans for several years or even decades. Commonly used flavoring chemicals have substantial pulmonary toxicity. There is evidence that the dual use of e-cigarettes and combustible tobacco can be more harmful than the use of combustible tobacco alone, as the person who smokes is now exposed to additional toxins unique to the e-cigarette.
E-cigarettes can cause severe acute lung disease; 14% of the severe e-cigarette or vaping product use-associated lung injury (EVALI) cases reported use of only nicotine-containing e-cigarette products. There are reports of people who used e-cigarettes who required lung transplant due to complications of their e-cigarette use.
The tobacco industry has a long history of “harm reduction” products that were anything but—from filter cigarettes (the “advanced” Kent Micronite filter contained asbestos) to the so-called low tar and nicotine cigarettes (which were no less harmful). There is a long history of physicians endorsing these products as “must be better.” The growing evidence that e-cigarettes carry distinct health risks of their own should prompt us to consider a broader picture beyond just comparing them with traditional cigarettes to assess their impact on health.
Physicians treating tobacco dependence should recommend US Food and Drug Administration-approved medications for pharmacotherapy. These have a robust evidence base documenting that they help people who smoke to break free of nicotine addiction. The goal of tobacco dependence treatment should be stopping ALL harmful tobacco/nicotine products—including e-cigarettes—not simply changing from one harmful product to another.
References
Liu Z. Notice of retraction: Lin HX et al. Efficacy of electronic cigarettes vs varenicline and nicotine chewing gum as an aid to stop smoking: a randomized clinical trial. JAMA Intern Med. 2024;184(3):291-299. JAMA Intern Med. Preprint. Posted online March 29, 2024. PMID: 38551593. doi: 10.1001/jamainternmed.2024.1125
Farber HJ, Conrado Pacheco Gallego M, Galiatsatos P, Folan P, Lamphere T, Pakhale S. Harms of electronic cigarettes: what the healthcare provider needs to know. Ann Am Thorac Soc. 2021;18(4):567-572. PMID: 33284731. doi: 10.1513/AnnalsATS.202009-1113CME
Proctor RN. Golden Holocaust: Origins of the Cigarette Catastrophe and the Case for Abolition. University of California Press; 2011.
Auer R, Schoeni A, Humair JP, et al. Electronic nicotine-delivery systems for smoking cessation. N Engl J Med. 2024;390(7):601-610. PMID: 38354139. doi: 10.1056/NEJMoa2308815
Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629-637. Preprint. Posted online January 30, 2019. PMID: 30699054. doi: 10.1056/NEJMoa1808779
Editor’s note: On March 29, 2024, the authors of the study, “Efficacy of Electronic Cigarettes vs Varenicline and Nicotine Chewing Gum as an Aid to Stop Smoking: A Randomized Clinical Trial,” published in JAMA Internal Medicine, issued a formal retraction of their article. The CHEST Physician® Editorial Board apologizes for any confusion this may have caused.
An article in the April issue of the CHEST Physician publication headlined, “E-cigarettes beat nicotine gum for smoking cessation,” was based on an article in JAMA Internal Medicine by Liu Z and colleagues which was subsequently retracted by the author due to coding errors and discrepancies in calculations that cast doubt on the accuracy and reliability of the reported findings.
One should be cautious in evaluating claims of the benefits of electronic cigarettes (e-cigarettes). e-Cigarettes are a highly addictive and largely unregulated product. The fine print in previous clinical trials of e-cigarettes shows greater rates of stopping nicotine products—including e-cigarettes—in the groups assigned to recommendation for nicotine replacement therapy. e-Cigarettes have substantial acute and chronic harms.
Although much of the research to date is from animal models, there is a growing body of evidence in humans that validates the findings from the animal models. In laboratory animal models, e-cigarettes impair airway defenses, contribute to epithelial dysfunction, lead to apoptosis of airway cells, cause emphysematous changes, and lead to increased cancer rates.
Adverse effects on cardiovascular health have also been demonstrated. There is evidence of genotoxicity from e-cigarette exposure, with increased rates of DNA damage and decreased rates of DNA repair. Carcinogenic substances are present in e-cigarettes, and we may not see the carcinogenic effects in humans for several years or even decades. Commonly used flavoring chemicals have substantial pulmonary toxicity. There is evidence that the dual use of e-cigarettes and combustible tobacco can be more harmful than the use of combustible tobacco alone, as the person who smokes is now exposed to additional toxins unique to the e-cigarette.
E-cigarettes can cause severe acute lung disease; 14% of the severe e-cigarette or vaping product use-associated lung injury (EVALI) cases reported use of only nicotine-containing e-cigarette products. There are reports of people who used e-cigarettes who required lung transplant due to complications of their e-cigarette use.
The tobacco industry has a long history of “harm reduction” products that were anything but—from filter cigarettes (the “advanced” Kent Micronite filter contained asbestos) to the so-called low tar and nicotine cigarettes (which were no less harmful). There is a long history of physicians endorsing these products as “must be better.” The growing evidence that e-cigarettes carry distinct health risks of their own should prompt us to consider a broader picture beyond just comparing them with traditional cigarettes to assess their impact on health.
Physicians treating tobacco dependence should recommend US Food and Drug Administration-approved medications for pharmacotherapy. These have a robust evidence base documenting that they help people who smoke to break free of nicotine addiction. The goal of tobacco dependence treatment should be stopping ALL harmful tobacco/nicotine products—including e-cigarettes—not simply changing from one harmful product to another.
References
Liu Z. Notice of retraction: Lin HX et al. Efficacy of electronic cigarettes vs varenicline and nicotine chewing gum as an aid to stop smoking: a randomized clinical trial. JAMA Intern Med. 2024;184(3):291-299. JAMA Intern Med. Preprint. Posted online March 29, 2024. PMID: 38551593. doi: 10.1001/jamainternmed.2024.1125
Farber HJ, Conrado Pacheco Gallego M, Galiatsatos P, Folan P, Lamphere T, Pakhale S. Harms of electronic cigarettes: what the healthcare provider needs to know. Ann Am Thorac Soc. 2021;18(4):567-572. PMID: 33284731. doi: 10.1513/AnnalsATS.202009-1113CME
Proctor RN. Golden Holocaust: Origins of the Cigarette Catastrophe and the Case for Abolition. University of California Press; 2011.
Auer R, Schoeni A, Humair JP, et al. Electronic nicotine-delivery systems for smoking cessation. N Engl J Med. 2024;390(7):601-610. PMID: 38354139. doi: 10.1056/NEJMoa2308815
Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629-637. Preprint. Posted online January 30, 2019. PMID: 30699054. doi: 10.1056/NEJMoa1808779
Fellow to use diversity scholar mentorship to strengthen care in pediatric-to-adult transitions
During residency training at the Rush University Medical Center in Internal Medicine and Pediatrics, Esha Kapania, MD, quickly became interested in the pulmonary pathologies that span the life of a patient, beginning in childhood and lasting into adulthood.
Now in her first year of fellowship at the University of Louisville and as the recipient of the 2024 Medical Educator Scholar Diversity Fellowship from CHEST and the Association of Pulmonary and Critical Care Medicine Program Directors (APCCMPD), Dr. Kapania will utilize the support of the program to explore this space.
“Recent advancements in pediatric pulmonary medicine have prolonged the expected lifespan of many previously fatal diagnoses, and I have realized that, despite these innovations, there remains very little communication between the adult and pediatric subspecialists,” Dr. Kapania said. “There is minimal education on congenital pulmonary pathology in adult medicine and, perhaps equally as important, negligible instruction on the cultural and social changes that patients experience when they transition from pediatric to adult providers.”
In residency, Dr. Kapania witnessed the success of cystic fibrosis (CF) clinics and hopes to leverage that experience to advance transitional care across disease states. Using the guidelines set to transition patients with CF from pediatric to adult care as a model, Dr. Kapania will focus her time on creating a streamlined process for patients living with severe asthma and patients with neuromuscular diseases who are chronically vented.
“Patients who are chronically vented tend not to have a lot of resources dedicated to them and are a resource- and time-heavy population,” Dr. Kapania said. “Because there is no defined process to transition these patients, we tend to see pediatric providers hold on to these patients for a lot longer than they do with [patients with CF]. A set of evidence-based practices would go a long way in this space.”
Through the APCCMPD and CHEST Medical Educator Scholar Diversity Fellowship, Dr. Kapania will work closely with the program’s selected mentor, Başak Çoruh, MD, FCCP, who is an Associate Professor of Pulmonary, Critical Care, and Sleep Medicine and Director of the Pulmonary and Critical Care Medicine fellowship program at the University of Washington.
“I’m looking forward to working with Dr. Çoruh for career guidance and for support of my area of interest within [pulmonary and critical care medicine],” Dr. Kapania said. “She is an established physician who has a lot of insight to share, and this is a great opportunity to make the best of my fellowship.”
This is the first year for the APCCMPD and CHEST Medical Educator Scholar Diversity Fellowship. To learn more about the scholarship, visit the CHEST website.
During residency training at the Rush University Medical Center in Internal Medicine and Pediatrics, Esha Kapania, MD, quickly became interested in the pulmonary pathologies that span the life of a patient, beginning in childhood and lasting into adulthood.
Now in her first year of fellowship at the University of Louisville and as the recipient of the 2024 Medical Educator Scholar Diversity Fellowship from CHEST and the Association of Pulmonary and Critical Care Medicine Program Directors (APCCMPD), Dr. Kapania will utilize the support of the program to explore this space.
“Recent advancements in pediatric pulmonary medicine have prolonged the expected lifespan of many previously fatal diagnoses, and I have realized that, despite these innovations, there remains very little communication between the adult and pediatric subspecialists,” Dr. Kapania said. “There is minimal education on congenital pulmonary pathology in adult medicine and, perhaps equally as important, negligible instruction on the cultural and social changes that patients experience when they transition from pediatric to adult providers.”
In residency, Dr. Kapania witnessed the success of cystic fibrosis (CF) clinics and hopes to leverage that experience to advance transitional care across disease states. Using the guidelines set to transition patients with CF from pediatric to adult care as a model, Dr. Kapania will focus her time on creating a streamlined process for patients living with severe asthma and patients with neuromuscular diseases who are chronically vented.
“Patients who are chronically vented tend not to have a lot of resources dedicated to them and are a resource- and time-heavy population,” Dr. Kapania said. “Because there is no defined process to transition these patients, we tend to see pediatric providers hold on to these patients for a lot longer than they do with [patients with CF]. A set of evidence-based practices would go a long way in this space.”
Through the APCCMPD and CHEST Medical Educator Scholar Diversity Fellowship, Dr. Kapania will work closely with the program’s selected mentor, Başak Çoruh, MD, FCCP, who is an Associate Professor of Pulmonary, Critical Care, and Sleep Medicine and Director of the Pulmonary and Critical Care Medicine fellowship program at the University of Washington.
“I’m looking forward to working with Dr. Çoruh for career guidance and for support of my area of interest within [pulmonary and critical care medicine],” Dr. Kapania said. “She is an established physician who has a lot of insight to share, and this is a great opportunity to make the best of my fellowship.”
This is the first year for the APCCMPD and CHEST Medical Educator Scholar Diversity Fellowship. To learn more about the scholarship, visit the CHEST website.
During residency training at the Rush University Medical Center in Internal Medicine and Pediatrics, Esha Kapania, MD, quickly became interested in the pulmonary pathologies that span the life of a patient, beginning in childhood and lasting into adulthood.
Now in her first year of fellowship at the University of Louisville and as the recipient of the 2024 Medical Educator Scholar Diversity Fellowship from CHEST and the Association of Pulmonary and Critical Care Medicine Program Directors (APCCMPD), Dr. Kapania will utilize the support of the program to explore this space.
“Recent advancements in pediatric pulmonary medicine have prolonged the expected lifespan of many previously fatal diagnoses, and I have realized that, despite these innovations, there remains very little communication between the adult and pediatric subspecialists,” Dr. Kapania said. “There is minimal education on congenital pulmonary pathology in adult medicine and, perhaps equally as important, negligible instruction on the cultural and social changes that patients experience when they transition from pediatric to adult providers.”
In residency, Dr. Kapania witnessed the success of cystic fibrosis (CF) clinics and hopes to leverage that experience to advance transitional care across disease states. Using the guidelines set to transition patients with CF from pediatric to adult care as a model, Dr. Kapania will focus her time on creating a streamlined process for patients living with severe asthma and patients with neuromuscular diseases who are chronically vented.
“Patients who are chronically vented tend not to have a lot of resources dedicated to them and are a resource- and time-heavy population,” Dr. Kapania said. “Because there is no defined process to transition these patients, we tend to see pediatric providers hold on to these patients for a lot longer than they do with [patients with CF]. A set of evidence-based practices would go a long way in this space.”
Through the APCCMPD and CHEST Medical Educator Scholar Diversity Fellowship, Dr. Kapania will work closely with the program’s selected mentor, Başak Çoruh, MD, FCCP, who is an Associate Professor of Pulmonary, Critical Care, and Sleep Medicine and Director of the Pulmonary and Critical Care Medicine fellowship program at the University of Washington.
“I’m looking forward to working with Dr. Çoruh for career guidance and for support of my area of interest within [pulmonary and critical care medicine],” Dr. Kapania said. “She is an established physician who has a lot of insight to share, and this is a great opportunity to make the best of my fellowship.”
This is the first year for the APCCMPD and CHEST Medical Educator Scholar Diversity Fellowship. To learn more about the scholarship, visit the CHEST website.
Transesophageal ultrasound: The future of ultrasound in the ICU
Thoracic Oncology and Chest Procedures Network
Ultrasound and Chest Imaging Section
Historically, transesophageal ultrasound (TEE) has been regarded as a diagnostic and management tool for structural heart disease in relatively stable patients. However, TEE is more commonly being utilized by intensivists as a first-line tool in the diagnostics and management of patients in the ICU.
TEE, with its unobstructed superior cardiac views, facilitates rapid diagnosis in undifferentiated shock and guides appropriate resuscitation efforts. Studies have shown that TEE alters management strategies in 40% of cases, following transthoracic echocardiography with an extremely low complication rate of 2% to 3% (primarily in the form of self-limited gastrointestinal bleeding).1,2,3,4
TEE also provides ultrasonographic evaluation of the lungs through transesophageal lung ultrasound (TELUS). TELUS allows for visualization of all six traditional lung zones utilized in traditional lung ultrasound.5 Patients with severe acute respiratory distress syndrome may greatly benefit from TEE utilization. TEE enables early detection of right ventricular dysfunction, aids in fluid management, and assesses the severity of lung consolidation, thereby facilitating prompt utilization of prone positioning or adjustments in positive end-expiratory pressure.
Cardiac arrest is another unique opportunity for TEE utilization by providing real-time cardiac visualization during active cardiopulmonary resuscitation. This facilitates optimal chest compression positioning, early recognition of arrhythmia, timely identification of reversible cause, and procedural guidance for ECMO-assisted CPR.6 TEE is an invaluable tool for the modern-day intensivist, providing rapid and accurate assessments, and therefore holds the potential to become standard of care in the ICU.
References
1. Prager R, Bowdridge J, Pratte M, Cheng J, McInnes MD, Arntfield R. Indications, clinical impact, and complications of critical care transesophageal echocardiography: a scoping review. J Intensive Care Med. 2023;38(3):245-272. Preprint. Posted online July 19, 2022. PMID: 35854414; PMCID: PMC9806486. doi: 10.1177/08850666221115348
2. Hüttemann E, Schelenz C, Kara F, Chatzinikolaou K, Reinhart K. The use and safety of transoesophageal echocardiography in the general ICU – a minireview. Acta Anaesthesiol Scand. 2004;48(7):827-36. PMID: 15242426. doi: 10.1111/j.0001-5172.2004.00423.x
3. Mayo PH, Narasimhan M, Koenig S. Critical care transesophageal echocardiography. Chest. 2015;148(5):1323-1332. PMID: 26204465. doi: 10.1378/chest.15-0260
4. Prager R, Ainsworth C, Arntfield R. Critical care transesophageal echocardiography for the resuscitation of shock: an important diagnostic skill for the modern intensivist. Chest. 2023;163(2):268-269. PMID: 36759112. doi: 10.1016/j.chest.2022.09.001
5. Cavayas YA, Girard M, Desjardins G, Denault AY. Transesophageal lung ultrasonography: a novel technique for investigating hypoxemia. Can J Anaesth. 2016;63(11):1266-76. Preprint. Posted online July 29, 2016. PMID: 27473720. doi: 10.1007/s12630-016-0702-2
6. Teran F, Prats MI, Nelson BP, et al. Focused transesophageal echocardiography during cardiac arrest resuscitation: JACC review wopic of the Week. J Am Coll Cardiol. 2020;76(6):745-754. PMID: 32762909. doi: 10.1016/j.jacc.2020.05.074
Thoracic Oncology and Chest Procedures Network
Ultrasound and Chest Imaging Section
Historically, transesophageal ultrasound (TEE) has been regarded as a diagnostic and management tool for structural heart disease in relatively stable patients. However, TEE is more commonly being utilized by intensivists as a first-line tool in the diagnostics and management of patients in the ICU.
TEE, with its unobstructed superior cardiac views, facilitates rapid diagnosis in undifferentiated shock and guides appropriate resuscitation efforts. Studies have shown that TEE alters management strategies in 40% of cases, following transthoracic echocardiography with an extremely low complication rate of 2% to 3% (primarily in the form of self-limited gastrointestinal bleeding).1,2,3,4
TEE also provides ultrasonographic evaluation of the lungs through transesophageal lung ultrasound (TELUS). TELUS allows for visualization of all six traditional lung zones utilized in traditional lung ultrasound.5 Patients with severe acute respiratory distress syndrome may greatly benefit from TEE utilization. TEE enables early detection of right ventricular dysfunction, aids in fluid management, and assesses the severity of lung consolidation, thereby facilitating prompt utilization of prone positioning or adjustments in positive end-expiratory pressure.
Cardiac arrest is another unique opportunity for TEE utilization by providing real-time cardiac visualization during active cardiopulmonary resuscitation. This facilitates optimal chest compression positioning, early recognition of arrhythmia, timely identification of reversible cause, and procedural guidance for ECMO-assisted CPR.6 TEE is an invaluable tool for the modern-day intensivist, providing rapid and accurate assessments, and therefore holds the potential to become standard of care in the ICU.
References
1. Prager R, Bowdridge J, Pratte M, Cheng J, McInnes MD, Arntfield R. Indications, clinical impact, and complications of critical care transesophageal echocardiography: a scoping review. J Intensive Care Med. 2023;38(3):245-272. Preprint. Posted online July 19, 2022. PMID: 35854414; PMCID: PMC9806486. doi: 10.1177/08850666221115348
2. Hüttemann E, Schelenz C, Kara F, Chatzinikolaou K, Reinhart K. The use and safety of transoesophageal echocardiography in the general ICU – a minireview. Acta Anaesthesiol Scand. 2004;48(7):827-36. PMID: 15242426. doi: 10.1111/j.0001-5172.2004.00423.x
3. Mayo PH, Narasimhan M, Koenig S. Critical care transesophageal echocardiography. Chest. 2015;148(5):1323-1332. PMID: 26204465. doi: 10.1378/chest.15-0260
4. Prager R, Ainsworth C, Arntfield R. Critical care transesophageal echocardiography for the resuscitation of shock: an important diagnostic skill for the modern intensivist. Chest. 2023;163(2):268-269. PMID: 36759112. doi: 10.1016/j.chest.2022.09.001
5. Cavayas YA, Girard M, Desjardins G, Denault AY. Transesophageal lung ultrasonography: a novel technique for investigating hypoxemia. Can J Anaesth. 2016;63(11):1266-76. Preprint. Posted online July 29, 2016. PMID: 27473720. doi: 10.1007/s12630-016-0702-2
6. Teran F, Prats MI, Nelson BP, et al. Focused transesophageal echocardiography during cardiac arrest resuscitation: JACC review wopic of the Week. J Am Coll Cardiol. 2020;76(6):745-754. PMID: 32762909. doi: 10.1016/j.jacc.2020.05.074
Thoracic Oncology and Chest Procedures Network
Ultrasound and Chest Imaging Section
Historically, transesophageal ultrasound (TEE) has been regarded as a diagnostic and management tool for structural heart disease in relatively stable patients. However, TEE is more commonly being utilized by intensivists as a first-line tool in the diagnostics and management of patients in the ICU.
TEE, with its unobstructed superior cardiac views, facilitates rapid diagnosis in undifferentiated shock and guides appropriate resuscitation efforts. Studies have shown that TEE alters management strategies in 40% of cases, following transthoracic echocardiography with an extremely low complication rate of 2% to 3% (primarily in the form of self-limited gastrointestinal bleeding).1,2,3,4
TEE also provides ultrasonographic evaluation of the lungs through transesophageal lung ultrasound (TELUS). TELUS allows for visualization of all six traditional lung zones utilized in traditional lung ultrasound.5 Patients with severe acute respiratory distress syndrome may greatly benefit from TEE utilization. TEE enables early detection of right ventricular dysfunction, aids in fluid management, and assesses the severity of lung consolidation, thereby facilitating prompt utilization of prone positioning or adjustments in positive end-expiratory pressure.
Cardiac arrest is another unique opportunity for TEE utilization by providing real-time cardiac visualization during active cardiopulmonary resuscitation. This facilitates optimal chest compression positioning, early recognition of arrhythmia, timely identification of reversible cause, and procedural guidance for ECMO-assisted CPR.6 TEE is an invaluable tool for the modern-day intensivist, providing rapid and accurate assessments, and therefore holds the potential to become standard of care in the ICU.
References
1. Prager R, Bowdridge J, Pratte M, Cheng J, McInnes MD, Arntfield R. Indications, clinical impact, and complications of critical care transesophageal echocardiography: a scoping review. J Intensive Care Med. 2023;38(3):245-272. Preprint. Posted online July 19, 2022. PMID: 35854414; PMCID: PMC9806486. doi: 10.1177/08850666221115348
2. Hüttemann E, Schelenz C, Kara F, Chatzinikolaou K, Reinhart K. The use and safety of transoesophageal echocardiography in the general ICU – a minireview. Acta Anaesthesiol Scand. 2004;48(7):827-36. PMID: 15242426. doi: 10.1111/j.0001-5172.2004.00423.x
3. Mayo PH, Narasimhan M, Koenig S. Critical care transesophageal echocardiography. Chest. 2015;148(5):1323-1332. PMID: 26204465. doi: 10.1378/chest.15-0260
4. Prager R, Ainsworth C, Arntfield R. Critical care transesophageal echocardiography for the resuscitation of shock: an important diagnostic skill for the modern intensivist. Chest. 2023;163(2):268-269. PMID: 36759112. doi: 10.1016/j.chest.2022.09.001
5. Cavayas YA, Girard M, Desjardins G, Denault AY. Transesophageal lung ultrasonography: a novel technique for investigating hypoxemia. Can J Anaesth. 2016;63(11):1266-76. Preprint. Posted online July 29, 2016. PMID: 27473720. doi: 10.1007/s12630-016-0702-2
6. Teran F, Prats MI, Nelson BP, et al. Focused transesophageal echocardiography during cardiac arrest resuscitation: JACC review wopic of the Week. J Am Coll Cardiol. 2020;76(6):745-754. PMID: 32762909. doi: 10.1016/j.jacc.2020.05.074
















