User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Sleep problems in young children linked to lower QOL in later years
Sleep problems in children from birth to middle childhood may lead to decreased emotional well-being and quality of life by the time a child is 10-11 years old, a recent longitudinal study has found.
The effects of these impairments increased over time and included internalizing and externalizing concerns, self-control, and quality of life, but did not appear to significantly affect cognitive or academic skills, according to Ariel A. Williamson, PhD, DBSM, of Children’s Hospital of Philadelphia, and colleagues. While children with consistent sleep problems experienced the worse outcomes, mild sleep problems also were associated with impairment, the researchers said.
“The range of impairments across academic and psychosocial domains in middle childhood indicate that it is important to screen for sleep problems consistently over the course of a child’s development, especially to target children who experience persistent sleep problems over time,” said Dr. Williamson in a press release.
The researchers examined data from 5,107 children in the Longitudinal Study of Australian Children – Birth Cohort, where sleep problems and well-being outcomes were measured at multiple time points. Behaviors such as difficulty getting off to sleep at night, not happy to sleep alone, and waking during the night were defined as sleep problems. The investigators found five main domains of sleep issues: children who had persistent sleep problems through middle childhood (7.7%), limited sleep problems as an infant or during preschool (9.0%), mild sleep problems over time (14.4%), increased sleep problems during middle childhood (17.0%), and a group that did not experience sleep problems (51.9%).
Caregivers reported sleep issues in the cohort, while well-being outcomes were reported by caregivers and teachers, and tasks were completed by the children at 10-11 years of age. Dr. Williamson and colleagues examined well-being in terms of emotional and behavioral functioning, health-related quality of life, cognitive skills, and academic achievement.
Different reports from teacher and caregivers
Teacher and caregivers reported different effects in children with persistent sleep problems. Teachers reported moderate internalizing (effect size, –0.65; 95% confidence interval [CI],–0.87 to –0.43; P < .001) and externalizing concerns (ES, –0.40; 95% CI, –0.58 to –0.21; P less than .001), compared with children who did not have sleep problems, whereas caregivers reported large internalizing (ES, –0.75; 95% CI, –0.92 to –0.57; P less than .001) and externalizing concerns (ES, –0.70; 95% CI, –0.86 to –0.53; P < .001). Children with persistent sleep problems had moderate impairment of self-control as reported by caregivers, compared with children with no sleep problems (ES, –0.37; 95% CI, –0.52 to –0.21; P < .001). Psychosocial and health-related quality of life reported by caregivers were worse in children with persistent sleep problems, compared with children who did not have sleep problems (ES range, –0.78 to –0.90; 95% CI, –1.06 to –0.56; P < .001).
For children who exhibited increased sleep problems in middle childhood, caregivers (ES for both, –0.61; 95% CI, –0.76 to –0.46; P < .001) and teachers (ES range, –0.29 to –0.39; 95% CI, –0.53 to –0.15; P < .001) reported greater rates of internalizing and externalizing symptoms, compared with children who had no sleep issues.
Small impairments in internalizing internal or externalizing symptoms were seen in children who had limited sleep problems as an infant or in preschool (ES, –0.12; 95% CI, –0.23 to –0.01; P < .05) as reported by teachers, and in children with mild sleep problems over time (ES, –0.19; 95% CI, –0.30 to –0.08; P < .001) as reported by caregivers. There were no significant impairments in self-control for children in either the infant or preschool impairment group or in the group of children with mild sleep problems.
Across all groups, sleep problems did not significantly impair nonverbal reasoning, and most areas of academic competencies were not significantly impaired among groups except in language and literacy, and mathematical thinking for children with persistent sleep problems (ES, –0.41 for both; 95% CI, –0.60 to –0.23; P < .001). Children with increased sleep problems during middle childhood “had few academic and cognitive impairments,” and academic impairments among children with mild sleep problems were not significant.
Expert opinion
Brandon M. Seay MD, FAAP, pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta, said in an interview that the study is one of the first to offer longitudinal data for impairment in children with sleep problems. He said the paper emphasizes the need for recognizing when children are demonstrating sleep problems. “It just shows that problems that aren’t dealt with earlier on definitely have bigger impacts on sleep as you go through life,” he said.
Although primary care physicians and pediatricians should be already asking questions about sleep through anticipatory guidance, he said, intervening earlier for sleep problems is important. He noted children who exhibit sleep problems over time are more likely to have issues in handling their emotions and eventually may develop cognitive issues. “[W]e know that if these problems continue to go through, this paper’s showing us that they have worse effects down the road,” he said.
Impact of the COVID-19 crisis
These problems may also be worsened by the COVID-19 pandemic. Dr. Seay noted that with many parents working from home, sleep schedules can be affected and parents may also be co-sleeping with their children, which can cause chronic insomnia and early waking. To help address sleep issues, especially ones that may have arisen during COVID-19, parents should make sure their children show up for primary care visits to report problems, and clinicians should make a sleep routine a focus of conversations around sleep problems.
Prior to the pandemic, “we already were hitting upon that in sleep clinic, making sure [they] get the same schedule every day,” said Dr. Seay. For parents with children who have “issues with insomnia or waking up during the night, having that routine in place does help to mitigate that a little bit, so if that routine is not there, it can actually exacerbate the issues.”
This study was funded by the Australian federal government. The authors report no relevant conflicts of interest. Dr. Seay reports no relevant conflicts of interest.
SOURCE: Williamson AA et al. J Child Psychol Psychiatry. 2020 Jul 26. doi:10.1111/jcpp.13303.
Sleep problems in children from birth to middle childhood may lead to decreased emotional well-being and quality of life by the time a child is 10-11 years old, a recent longitudinal study has found.
The effects of these impairments increased over time and included internalizing and externalizing concerns, self-control, and quality of life, but did not appear to significantly affect cognitive or academic skills, according to Ariel A. Williamson, PhD, DBSM, of Children’s Hospital of Philadelphia, and colleagues. While children with consistent sleep problems experienced the worse outcomes, mild sleep problems also were associated with impairment, the researchers said.
“The range of impairments across academic and psychosocial domains in middle childhood indicate that it is important to screen for sleep problems consistently over the course of a child’s development, especially to target children who experience persistent sleep problems over time,” said Dr. Williamson in a press release.
The researchers examined data from 5,107 children in the Longitudinal Study of Australian Children – Birth Cohort, where sleep problems and well-being outcomes were measured at multiple time points. Behaviors such as difficulty getting off to sleep at night, not happy to sleep alone, and waking during the night were defined as sleep problems. The investigators found five main domains of sleep issues: children who had persistent sleep problems through middle childhood (7.7%), limited sleep problems as an infant or during preschool (9.0%), mild sleep problems over time (14.4%), increased sleep problems during middle childhood (17.0%), and a group that did not experience sleep problems (51.9%).
Caregivers reported sleep issues in the cohort, while well-being outcomes were reported by caregivers and teachers, and tasks were completed by the children at 10-11 years of age. Dr. Williamson and colleagues examined well-being in terms of emotional and behavioral functioning, health-related quality of life, cognitive skills, and academic achievement.
Different reports from teacher and caregivers
Teacher and caregivers reported different effects in children with persistent sleep problems. Teachers reported moderate internalizing (effect size, –0.65; 95% confidence interval [CI],–0.87 to –0.43; P < .001) and externalizing concerns (ES, –0.40; 95% CI, –0.58 to –0.21; P less than .001), compared with children who did not have sleep problems, whereas caregivers reported large internalizing (ES, –0.75; 95% CI, –0.92 to –0.57; P less than .001) and externalizing concerns (ES, –0.70; 95% CI, –0.86 to –0.53; P < .001). Children with persistent sleep problems had moderate impairment of self-control as reported by caregivers, compared with children with no sleep problems (ES, –0.37; 95% CI, –0.52 to –0.21; P < .001). Psychosocial and health-related quality of life reported by caregivers were worse in children with persistent sleep problems, compared with children who did not have sleep problems (ES range, –0.78 to –0.90; 95% CI, –1.06 to –0.56; P < .001).
For children who exhibited increased sleep problems in middle childhood, caregivers (ES for both, –0.61; 95% CI, –0.76 to –0.46; P < .001) and teachers (ES range, –0.29 to –0.39; 95% CI, –0.53 to –0.15; P < .001) reported greater rates of internalizing and externalizing symptoms, compared with children who had no sleep issues.
Small impairments in internalizing internal or externalizing symptoms were seen in children who had limited sleep problems as an infant or in preschool (ES, –0.12; 95% CI, –0.23 to –0.01; P < .05) as reported by teachers, and in children with mild sleep problems over time (ES, –0.19; 95% CI, –0.30 to –0.08; P < .001) as reported by caregivers. There were no significant impairments in self-control for children in either the infant or preschool impairment group or in the group of children with mild sleep problems.
Across all groups, sleep problems did not significantly impair nonverbal reasoning, and most areas of academic competencies were not significantly impaired among groups except in language and literacy, and mathematical thinking for children with persistent sleep problems (ES, –0.41 for both; 95% CI, –0.60 to –0.23; P < .001). Children with increased sleep problems during middle childhood “had few academic and cognitive impairments,” and academic impairments among children with mild sleep problems were not significant.
Expert opinion
Brandon M. Seay MD, FAAP, pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta, said in an interview that the study is one of the first to offer longitudinal data for impairment in children with sleep problems. He said the paper emphasizes the need for recognizing when children are demonstrating sleep problems. “It just shows that problems that aren’t dealt with earlier on definitely have bigger impacts on sleep as you go through life,” he said.
Although primary care physicians and pediatricians should be already asking questions about sleep through anticipatory guidance, he said, intervening earlier for sleep problems is important. He noted children who exhibit sleep problems over time are more likely to have issues in handling their emotions and eventually may develop cognitive issues. “[W]e know that if these problems continue to go through, this paper’s showing us that they have worse effects down the road,” he said.
Impact of the COVID-19 crisis
These problems may also be worsened by the COVID-19 pandemic. Dr. Seay noted that with many parents working from home, sleep schedules can be affected and parents may also be co-sleeping with their children, which can cause chronic insomnia and early waking. To help address sleep issues, especially ones that may have arisen during COVID-19, parents should make sure their children show up for primary care visits to report problems, and clinicians should make a sleep routine a focus of conversations around sleep problems.
Prior to the pandemic, “we already were hitting upon that in sleep clinic, making sure [they] get the same schedule every day,” said Dr. Seay. For parents with children who have “issues with insomnia or waking up during the night, having that routine in place does help to mitigate that a little bit, so if that routine is not there, it can actually exacerbate the issues.”
This study was funded by the Australian federal government. The authors report no relevant conflicts of interest. Dr. Seay reports no relevant conflicts of interest.
SOURCE: Williamson AA et al. J Child Psychol Psychiatry. 2020 Jul 26. doi:10.1111/jcpp.13303.
Sleep problems in children from birth to middle childhood may lead to decreased emotional well-being and quality of life by the time a child is 10-11 years old, a recent longitudinal study has found.
The effects of these impairments increased over time and included internalizing and externalizing concerns, self-control, and quality of life, but did not appear to significantly affect cognitive or academic skills, according to Ariel A. Williamson, PhD, DBSM, of Children’s Hospital of Philadelphia, and colleagues. While children with consistent sleep problems experienced the worse outcomes, mild sleep problems also were associated with impairment, the researchers said.
“The range of impairments across academic and psychosocial domains in middle childhood indicate that it is important to screen for sleep problems consistently over the course of a child’s development, especially to target children who experience persistent sleep problems over time,” said Dr. Williamson in a press release.
The researchers examined data from 5,107 children in the Longitudinal Study of Australian Children – Birth Cohort, where sleep problems and well-being outcomes were measured at multiple time points. Behaviors such as difficulty getting off to sleep at night, not happy to sleep alone, and waking during the night were defined as sleep problems. The investigators found five main domains of sleep issues: children who had persistent sleep problems through middle childhood (7.7%), limited sleep problems as an infant or during preschool (9.0%), mild sleep problems over time (14.4%), increased sleep problems during middle childhood (17.0%), and a group that did not experience sleep problems (51.9%).
Caregivers reported sleep issues in the cohort, while well-being outcomes were reported by caregivers and teachers, and tasks were completed by the children at 10-11 years of age. Dr. Williamson and colleagues examined well-being in terms of emotional and behavioral functioning, health-related quality of life, cognitive skills, and academic achievement.
Different reports from teacher and caregivers
Teacher and caregivers reported different effects in children with persistent sleep problems. Teachers reported moderate internalizing (effect size, –0.65; 95% confidence interval [CI],–0.87 to –0.43; P < .001) and externalizing concerns (ES, –0.40; 95% CI, –0.58 to –0.21; P less than .001), compared with children who did not have sleep problems, whereas caregivers reported large internalizing (ES, –0.75; 95% CI, –0.92 to –0.57; P less than .001) and externalizing concerns (ES, –0.70; 95% CI, –0.86 to –0.53; P < .001). Children with persistent sleep problems had moderate impairment of self-control as reported by caregivers, compared with children with no sleep problems (ES, –0.37; 95% CI, –0.52 to –0.21; P < .001). Psychosocial and health-related quality of life reported by caregivers were worse in children with persistent sleep problems, compared with children who did not have sleep problems (ES range, –0.78 to –0.90; 95% CI, –1.06 to –0.56; P < .001).
For children who exhibited increased sleep problems in middle childhood, caregivers (ES for both, –0.61; 95% CI, –0.76 to –0.46; P < .001) and teachers (ES range, –0.29 to –0.39; 95% CI, –0.53 to –0.15; P < .001) reported greater rates of internalizing and externalizing symptoms, compared with children who had no sleep issues.
Small impairments in internalizing internal or externalizing symptoms were seen in children who had limited sleep problems as an infant or in preschool (ES, –0.12; 95% CI, –0.23 to –0.01; P < .05) as reported by teachers, and in children with mild sleep problems over time (ES, –0.19; 95% CI, –0.30 to –0.08; P < .001) as reported by caregivers. There were no significant impairments in self-control for children in either the infant or preschool impairment group or in the group of children with mild sleep problems.
Across all groups, sleep problems did not significantly impair nonverbal reasoning, and most areas of academic competencies were not significantly impaired among groups except in language and literacy, and mathematical thinking for children with persistent sleep problems (ES, –0.41 for both; 95% CI, –0.60 to –0.23; P < .001). Children with increased sleep problems during middle childhood “had few academic and cognitive impairments,” and academic impairments among children with mild sleep problems were not significant.
Expert opinion
Brandon M. Seay MD, FAAP, pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta, said in an interview that the study is one of the first to offer longitudinal data for impairment in children with sleep problems. He said the paper emphasizes the need for recognizing when children are demonstrating sleep problems. “It just shows that problems that aren’t dealt with earlier on definitely have bigger impacts on sleep as you go through life,” he said.
Although primary care physicians and pediatricians should be already asking questions about sleep through anticipatory guidance, he said, intervening earlier for sleep problems is important. He noted children who exhibit sleep problems over time are more likely to have issues in handling their emotions and eventually may develop cognitive issues. “[W]e know that if these problems continue to go through, this paper’s showing us that they have worse effects down the road,” he said.
Impact of the COVID-19 crisis
These problems may also be worsened by the COVID-19 pandemic. Dr. Seay noted that with many parents working from home, sleep schedules can be affected and parents may also be co-sleeping with their children, which can cause chronic insomnia and early waking. To help address sleep issues, especially ones that may have arisen during COVID-19, parents should make sure their children show up for primary care visits to report problems, and clinicians should make a sleep routine a focus of conversations around sleep problems.
Prior to the pandemic, “we already were hitting upon that in sleep clinic, making sure [they] get the same schedule every day,” said Dr. Seay. For parents with children who have “issues with insomnia or waking up during the night, having that routine in place does help to mitigate that a little bit, so if that routine is not there, it can actually exacerbate the issues.”
This study was funded by the Australian federal government. The authors report no relevant conflicts of interest. Dr. Seay reports no relevant conflicts of interest.
SOURCE: Williamson AA et al. J Child Psychol Psychiatry. 2020 Jul 26. doi:10.1111/jcpp.13303.
FROM JOURNAL OF CHILD PSYCHOLOGY AND PSYCHIATRY
COVID-19 cases in children nearly doubled in just 4 weeks
The cumulative number of new COVID-19 cases among children in the United States jumped by 90% during a recent 4-week period, according to a report that confirms children are not immune to the coronavirus.
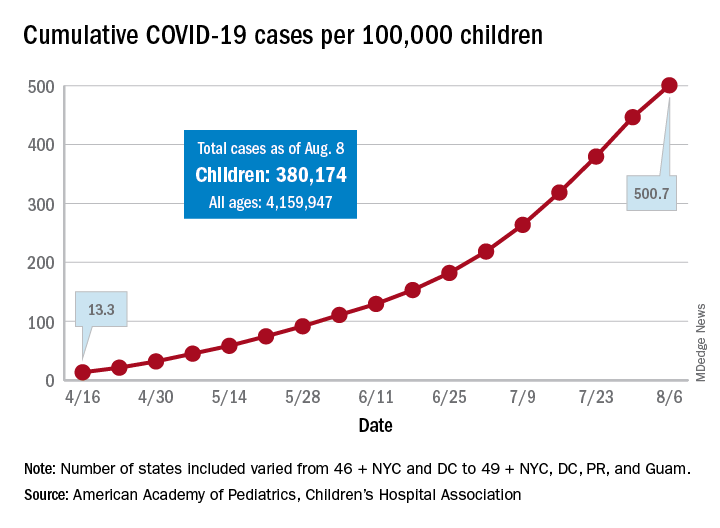
“In areas with rapid community spread, it’s likely that more children will also be infected, and these data show that,” Sally Goza, MD, president of the American Academy of Pediatrics, said in a written statement. “I urge people to wear cloth face coverings and be diligent in social distancing and hand-washing. It is up to us to make the difference, community by community.”
The joint report from the AAP and the Children’s Hospital Association draws on data from state and local health departments in 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
The cumulative number of COVID-19 cases in children as of Aug. 6, 2020, was 380,174, and that number is 90% higher – an increase of 179,990 cases – than the total on July 9, just 4 weeks earlier, the two organizations said in the report.
and 27 states out of 47 with available data now report that over 10% of their cases were children, with Wyoming the highest at 16.5% and New Jersey the lowest at 2.9%, the report data show.
Alabama has a higher percentage of 22.5%, but the state has been reporting cases in individuals aged 0-24 years as child cases since May 7. The report’s findings are somewhat limited by differences in reporting among the states and by “gaps in the data they are reporting [that affect] how the data can be interpreted,” the AAP said in its statement.
The cumulative number of cases per 100,000 children has risen from 13.3 in mid-April, when the total number was 9,259 cases, to 500.7 per 100,000 as of Aug. 6, and there are now 21 states, along with the District of Columbia, reporting a rate of over 500 cases per 100,000 children. Arizona has the highest rate at 1,206.4, followed by South Carolina (1,074.4) and Tennessee (1,050.8), the AAP and the CHA said.
In New York City, the early epicenter of the pandemic, the 390.5 cases per 100,000 children have been reported, and in New Jersey, which joined New York in the initial surge of cases, the number is 269.5. As of Aug. 6, Hawaii had the fewest cases of any state at 91.2 per 100,000, according to the report.
Children continue to represent a very low proportion of COVID-19 deaths, “but as case counts rise across the board, that is likely to impact more children with severe illness as well,” Sean O’Leary, MD, MPH, vice chair of the AAP’s committee on infectious diseases, said in the AAP statement.
It is possible that “some of the increase in numbers of cases in children could be due to more testing. Early in the pandemic, testing only occurred for the sickest individuals. Now that there is more testing capacity … the numbers reflect a broader slice of the population, including children who may have mild or few symptoms,” the AAP suggested.
This article was updated on 8/17/2020.
The cumulative number of new COVID-19 cases among children in the United States jumped by 90% during a recent 4-week period, according to a report that confirms children are not immune to the coronavirus.

“In areas with rapid community spread, it’s likely that more children will also be infected, and these data show that,” Sally Goza, MD, president of the American Academy of Pediatrics, said in a written statement. “I urge people to wear cloth face coverings and be diligent in social distancing and hand-washing. It is up to us to make the difference, community by community.”
The joint report from the AAP and the Children’s Hospital Association draws on data from state and local health departments in 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
The cumulative number of COVID-19 cases in children as of Aug. 6, 2020, was 380,174, and that number is 90% higher – an increase of 179,990 cases – than the total on July 9, just 4 weeks earlier, the two organizations said in the report.
and 27 states out of 47 with available data now report that over 10% of their cases were children, with Wyoming the highest at 16.5% and New Jersey the lowest at 2.9%, the report data show.
Alabama has a higher percentage of 22.5%, but the state has been reporting cases in individuals aged 0-24 years as child cases since May 7. The report’s findings are somewhat limited by differences in reporting among the states and by “gaps in the data they are reporting [that affect] how the data can be interpreted,” the AAP said in its statement.
The cumulative number of cases per 100,000 children has risen from 13.3 in mid-April, when the total number was 9,259 cases, to 500.7 per 100,000 as of Aug. 6, and there are now 21 states, along with the District of Columbia, reporting a rate of over 500 cases per 100,000 children. Arizona has the highest rate at 1,206.4, followed by South Carolina (1,074.4) and Tennessee (1,050.8), the AAP and the CHA said.
In New York City, the early epicenter of the pandemic, the 390.5 cases per 100,000 children have been reported, and in New Jersey, which joined New York in the initial surge of cases, the number is 269.5. As of Aug. 6, Hawaii had the fewest cases of any state at 91.2 per 100,000, according to the report.
Children continue to represent a very low proportion of COVID-19 deaths, “but as case counts rise across the board, that is likely to impact more children with severe illness as well,” Sean O’Leary, MD, MPH, vice chair of the AAP’s committee on infectious diseases, said in the AAP statement.
It is possible that “some of the increase in numbers of cases in children could be due to more testing. Early in the pandemic, testing only occurred for the sickest individuals. Now that there is more testing capacity … the numbers reflect a broader slice of the population, including children who may have mild or few symptoms,” the AAP suggested.
This article was updated on 8/17/2020.
The cumulative number of new COVID-19 cases among children in the United States jumped by 90% during a recent 4-week period, according to a report that confirms children are not immune to the coronavirus.

“In areas with rapid community spread, it’s likely that more children will also be infected, and these data show that,” Sally Goza, MD, president of the American Academy of Pediatrics, said in a written statement. “I urge people to wear cloth face coverings and be diligent in social distancing and hand-washing. It is up to us to make the difference, community by community.”
The joint report from the AAP and the Children’s Hospital Association draws on data from state and local health departments in 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
The cumulative number of COVID-19 cases in children as of Aug. 6, 2020, was 380,174, and that number is 90% higher – an increase of 179,990 cases – than the total on July 9, just 4 weeks earlier, the two organizations said in the report.
and 27 states out of 47 with available data now report that over 10% of their cases were children, with Wyoming the highest at 16.5% and New Jersey the lowest at 2.9%, the report data show.
Alabama has a higher percentage of 22.5%, but the state has been reporting cases in individuals aged 0-24 years as child cases since May 7. The report’s findings are somewhat limited by differences in reporting among the states and by “gaps in the data they are reporting [that affect] how the data can be interpreted,” the AAP said in its statement.
The cumulative number of cases per 100,000 children has risen from 13.3 in mid-April, when the total number was 9,259 cases, to 500.7 per 100,000 as of Aug. 6, and there are now 21 states, along with the District of Columbia, reporting a rate of over 500 cases per 100,000 children. Arizona has the highest rate at 1,206.4, followed by South Carolina (1,074.4) and Tennessee (1,050.8), the AAP and the CHA said.
In New York City, the early epicenter of the pandemic, the 390.5 cases per 100,000 children have been reported, and in New Jersey, which joined New York in the initial surge of cases, the number is 269.5. As of Aug. 6, Hawaii had the fewest cases of any state at 91.2 per 100,000, according to the report.
Children continue to represent a very low proportion of COVID-19 deaths, “but as case counts rise across the board, that is likely to impact more children with severe illness as well,” Sean O’Leary, MD, MPH, vice chair of the AAP’s committee on infectious diseases, said in the AAP statement.
It is possible that “some of the increase in numbers of cases in children could be due to more testing. Early in the pandemic, testing only occurred for the sickest individuals. Now that there is more testing capacity … the numbers reflect a broader slice of the population, including children who may have mild or few symptoms,” the AAP suggested.
This article was updated on 8/17/2020.
Sintilimab scintillates in first-line nonsquamous NSCLC
The investigational anti-PD-1 antibody sintilimab (Tyvyt, Innovent Biologics and Eli Lilly) has shown that it improves the efficacy of platinum-based chemotherapy in the first-line treatment of patients with advanced nonsquamous non–small cell lung cancer (NSCLC) in a phase 3 trial dubbed ORIENT-11.
The study was presented at the World Congress on Lung Cancer 2020 Virtual Presidential Symposium, held virtually due to the COVID-19 pandemic, on August 8. It was also published simultaneously in the Journal of Thoracic Oncology.
Sintilimab is a fully human IgG4 monoclonal antibody that blocks the binding of programmed death (PD)-1 to PD-ligand 1 (PD-L1) or PD-L2 with high affinity, and has received market authorization in China for the treatment of Hodgkin lymphoma.
For ORIENT-11, almost 400 patients with advanced nonsquamous NSCLC were randomly assigned to sintilimab or placebo plus pemetrexed and platinum-based chemotherapy in a 2:1 ratio.
“The addition of sintilimab to pemetrexed and platinum significantly improved PFS [progression-free survival], compared to placebo,” reducing progression rates by 52%, noted lead investigator Li Zhang, MD, professor of medical oncology, Sun Yat-Sen University Cancer Center, Guangzhou, China.
Crucially, this benefit “was seen across key clinical subgroups,” he added.
He noted that the overall response rate “was also improved, with a durable response,” while the results, which are not yet mature, suggest the experimental arm was associated with an overall survival (OS) benefit.
Study discussant Misako Nagasaka, MD, a thoracic oncologist and clinical investigator at Karmanos Cancer Institute, Detroit, said that the PFS benefit seen in the study is “certainly encouraging.”
Adding a note of caution, she continued: “But we have seen studies with PFS improvement which did not translate into OS improvement.
“Longer follow-up would allow events to mature and we will ultimately see if there would be a significant OS benefit,” she said.
Dr. Nagasaka also pointed out that the greater benefit with sintilimab seen in patients with high PD-L1 begs the question as to what would be the preferred regimen in those with higher or lower expression.
And, she said, this is not just about what regimen to choose but “more importantly, why?”
“Perhaps you’d like to use something with the best response rate, perhaps you’re sticking to a single agent immunotherapy because the toxicity profile is more favorable, or perhaps you [are] convinced with a certain regimen because of the robust PFS and OS data,” she said.
“Whatever you chose, there was a reason for your choice,” she said, adding that the sintilimab combination would have to “fulfill those reasons for you to consider choosing this regimen.”
Study details
Dr. Zhang began his presentation by noting that previous phase 1b studies have shown that sintilimab plus pemetrexed and platinum-based chemotherapy has a “tolerable safety profile and promising efficacy” in previously untreated non-squamous NSCLC.
They therefore conducted ORIENT-11, a randomized, double-blind, phase 3 study involving 397 patients with untreated stage IIIB/C or IV nonsquamous NSCLC who had neither EGFR nor ALK gene alterations.
The patients were randomly assigned in a 2:1 fashion to sintilimab plus pemetrexed and platinum-based chemotherapy (n = 266) or placebo plus pemetrexed and chemo (n = 131) for four cycles, followed by sintilimab or placebo plus pemetrexed for up to 24 months.
Thirty-five patients in the placebo arm crossed over to sintilimab monotherapy, representing 31.3% of the intention-to-treat population.
At the data cutoff of Nov. 15, 2019, 198 events had occurred, at a median follow-up of 8.9 months.
The team found that median PFS was significantly higher with sintilimab than placebo combination therapy, at 8.9 months vs. 5.0 months, or a hazard ratio of 0.482 (P < .00001).
Dr. Zhang noted that the benefit with sintilimab plus pemetrexed and platinum-based chemotherapy was seen across all subgroups.
However, it was notable that the impact of adding sintilimab on PFS was greater in patients with a tumor proportion score (TPS) ≥50%.
The HR for progression vs. the placebo treatment arm was 0.310, with median PFS not reached, which decreased to 0.503 in patients with a TPS of 1%-49% and 0.664 among those with a TPS <1%.
The results also showed that there was a “nominally significant improvement” in overall survival with sintilimab versus placebo, at a HR of 0.609 (P = 0.01921).
The ORR was markedly different between the sintilimab and placebo groups, at 51.9% vs. 29.8%, with the duration of response not reached in the sintilimab arm compared with 5.5 months in the placebo arm.
The sintilimab arm included three (1.1%) complete responses, which was not observed with pemetrexed and platinum-based chemotherapy alone.
Finally, Dr. Zhang observed that the safety profiles of the sintilimab and placebo arms were similar, with comparable rates of any, grade 3-5, and serious adverse events largely driven by high rates of chemotherapy-related events.
While there were fewer adverse events that led to death with sintilimab, at 2.3% vs. 6.9% with placebo, there were, as expected, more immune-related adverse events, at 43.2% vs. 36.6%, respectively.
Comparison with pembrolizumab
In her discussion, Dr. Nagasaka said that the first question that came to mind when she saw the results was: “How does the ORIENT-11 data compare with KEYNOTE-189?”
For that study, pembrolizumab (Keytruda, Merck) was added to pemetrexed plus carboplatin chemotherapy and compared with standard of care alone in patients with untreated metastatic nonsquamous NSCLC.
As reported by Medscape Medical News, pembrolizumab was associated with a 48% reduced risk of disease progression, as well as improved overall survival.
Dr. Nagasaka said that ORIENT-11 “had patients that tended to be younger, there were more males, more with performance status 1, and those who had never smoked” than those in KEYNOTE-189.
“But most importantly, KEYNOTE-189 had a very small number of patients from East Asia, only 1% in the pembro arm and 2.9% in the placebo arm.”
In contrast, all the patients included in ORIENT-11 were from East Asia, making the study of “high importance.”
She added that, “while across-trial comparisons must be taken with caution, the medium PFS of ORIENT-11 ... appears comparable to those of KEYNOTE-189,” while the HR “appears identical.”
This is despite median follow-up time in ORIENT-11 of “only” 8.9 months vs. a median of 23.1 months in the updated KEYNOTE-189 data.
There are plans to register the sintilimab combination therapy in China for the treatment of nonsquamous NSCLC, where it will go up against pembrolizumab as well as, potentially, tislelizumab (BeiGene).
The study was sponsored by Innovent Biologics and Eli Lilly. Dr. Zhang disclosed research grants from Eli Lilly and Pfizer. Dr. Nagasaka disclosed serving on the advisory boards of AstraZeneca, Daiichi Sankyo, Takeda, Novartis, and EMD Serono; as a consultant for Caris Life Sciences; and receiving travel support from An Hearts Therapeutics.
This article first appeared on Medscape.com.
The investigational anti-PD-1 antibody sintilimab (Tyvyt, Innovent Biologics and Eli Lilly) has shown that it improves the efficacy of platinum-based chemotherapy in the first-line treatment of patients with advanced nonsquamous non–small cell lung cancer (NSCLC) in a phase 3 trial dubbed ORIENT-11.
The study was presented at the World Congress on Lung Cancer 2020 Virtual Presidential Symposium, held virtually due to the COVID-19 pandemic, on August 8. It was also published simultaneously in the Journal of Thoracic Oncology.
Sintilimab is a fully human IgG4 monoclonal antibody that blocks the binding of programmed death (PD)-1 to PD-ligand 1 (PD-L1) or PD-L2 with high affinity, and has received market authorization in China for the treatment of Hodgkin lymphoma.
For ORIENT-11, almost 400 patients with advanced nonsquamous NSCLC were randomly assigned to sintilimab or placebo plus pemetrexed and platinum-based chemotherapy in a 2:1 ratio.
“The addition of sintilimab to pemetrexed and platinum significantly improved PFS [progression-free survival], compared to placebo,” reducing progression rates by 52%, noted lead investigator Li Zhang, MD, professor of medical oncology, Sun Yat-Sen University Cancer Center, Guangzhou, China.
Crucially, this benefit “was seen across key clinical subgroups,” he added.
He noted that the overall response rate “was also improved, with a durable response,” while the results, which are not yet mature, suggest the experimental arm was associated with an overall survival (OS) benefit.
Study discussant Misako Nagasaka, MD, a thoracic oncologist and clinical investigator at Karmanos Cancer Institute, Detroit, said that the PFS benefit seen in the study is “certainly encouraging.”
Adding a note of caution, she continued: “But we have seen studies with PFS improvement which did not translate into OS improvement.
“Longer follow-up would allow events to mature and we will ultimately see if there would be a significant OS benefit,” she said.
Dr. Nagasaka also pointed out that the greater benefit with sintilimab seen in patients with high PD-L1 begs the question as to what would be the preferred regimen in those with higher or lower expression.
And, she said, this is not just about what regimen to choose but “more importantly, why?”
“Perhaps you’d like to use something with the best response rate, perhaps you’re sticking to a single agent immunotherapy because the toxicity profile is more favorable, or perhaps you [are] convinced with a certain regimen because of the robust PFS and OS data,” she said.
“Whatever you chose, there was a reason for your choice,” she said, adding that the sintilimab combination would have to “fulfill those reasons for you to consider choosing this regimen.”
Study details
Dr. Zhang began his presentation by noting that previous phase 1b studies have shown that sintilimab plus pemetrexed and platinum-based chemotherapy has a “tolerable safety profile and promising efficacy” in previously untreated non-squamous NSCLC.
They therefore conducted ORIENT-11, a randomized, double-blind, phase 3 study involving 397 patients with untreated stage IIIB/C or IV nonsquamous NSCLC who had neither EGFR nor ALK gene alterations.
The patients were randomly assigned in a 2:1 fashion to sintilimab plus pemetrexed and platinum-based chemotherapy (n = 266) or placebo plus pemetrexed and chemo (n = 131) for four cycles, followed by sintilimab or placebo plus pemetrexed for up to 24 months.
Thirty-five patients in the placebo arm crossed over to sintilimab monotherapy, representing 31.3% of the intention-to-treat population.
At the data cutoff of Nov. 15, 2019, 198 events had occurred, at a median follow-up of 8.9 months.
The team found that median PFS was significantly higher with sintilimab than placebo combination therapy, at 8.9 months vs. 5.0 months, or a hazard ratio of 0.482 (P < .00001).
Dr. Zhang noted that the benefit with sintilimab plus pemetrexed and platinum-based chemotherapy was seen across all subgroups.
However, it was notable that the impact of adding sintilimab on PFS was greater in patients with a tumor proportion score (TPS) ≥50%.
The HR for progression vs. the placebo treatment arm was 0.310, with median PFS not reached, which decreased to 0.503 in patients with a TPS of 1%-49% and 0.664 among those with a TPS <1%.
The results also showed that there was a “nominally significant improvement” in overall survival with sintilimab versus placebo, at a HR of 0.609 (P = 0.01921).
The ORR was markedly different between the sintilimab and placebo groups, at 51.9% vs. 29.8%, with the duration of response not reached in the sintilimab arm compared with 5.5 months in the placebo arm.
The sintilimab arm included three (1.1%) complete responses, which was not observed with pemetrexed and platinum-based chemotherapy alone.
Finally, Dr. Zhang observed that the safety profiles of the sintilimab and placebo arms were similar, with comparable rates of any, grade 3-5, and serious adverse events largely driven by high rates of chemotherapy-related events.
While there were fewer adverse events that led to death with sintilimab, at 2.3% vs. 6.9% with placebo, there were, as expected, more immune-related adverse events, at 43.2% vs. 36.6%, respectively.
Comparison with pembrolizumab
In her discussion, Dr. Nagasaka said that the first question that came to mind when she saw the results was: “How does the ORIENT-11 data compare with KEYNOTE-189?”
For that study, pembrolizumab (Keytruda, Merck) was added to pemetrexed plus carboplatin chemotherapy and compared with standard of care alone in patients with untreated metastatic nonsquamous NSCLC.
As reported by Medscape Medical News, pembrolizumab was associated with a 48% reduced risk of disease progression, as well as improved overall survival.
Dr. Nagasaka said that ORIENT-11 “had patients that tended to be younger, there were more males, more with performance status 1, and those who had never smoked” than those in KEYNOTE-189.
“But most importantly, KEYNOTE-189 had a very small number of patients from East Asia, only 1% in the pembro arm and 2.9% in the placebo arm.”
In contrast, all the patients included in ORIENT-11 were from East Asia, making the study of “high importance.”
She added that, “while across-trial comparisons must be taken with caution, the medium PFS of ORIENT-11 ... appears comparable to those of KEYNOTE-189,” while the HR “appears identical.”
This is despite median follow-up time in ORIENT-11 of “only” 8.9 months vs. a median of 23.1 months in the updated KEYNOTE-189 data.
There are plans to register the sintilimab combination therapy in China for the treatment of nonsquamous NSCLC, where it will go up against pembrolizumab as well as, potentially, tislelizumab (BeiGene).
The study was sponsored by Innovent Biologics and Eli Lilly. Dr. Zhang disclosed research grants from Eli Lilly and Pfizer. Dr. Nagasaka disclosed serving on the advisory boards of AstraZeneca, Daiichi Sankyo, Takeda, Novartis, and EMD Serono; as a consultant for Caris Life Sciences; and receiving travel support from An Hearts Therapeutics.
This article first appeared on Medscape.com.
The investigational anti-PD-1 antibody sintilimab (Tyvyt, Innovent Biologics and Eli Lilly) has shown that it improves the efficacy of platinum-based chemotherapy in the first-line treatment of patients with advanced nonsquamous non–small cell lung cancer (NSCLC) in a phase 3 trial dubbed ORIENT-11.
The study was presented at the World Congress on Lung Cancer 2020 Virtual Presidential Symposium, held virtually due to the COVID-19 pandemic, on August 8. It was also published simultaneously in the Journal of Thoracic Oncology.
Sintilimab is a fully human IgG4 monoclonal antibody that blocks the binding of programmed death (PD)-1 to PD-ligand 1 (PD-L1) or PD-L2 with high affinity, and has received market authorization in China for the treatment of Hodgkin lymphoma.
For ORIENT-11, almost 400 patients with advanced nonsquamous NSCLC were randomly assigned to sintilimab or placebo plus pemetrexed and platinum-based chemotherapy in a 2:1 ratio.
“The addition of sintilimab to pemetrexed and platinum significantly improved PFS [progression-free survival], compared to placebo,” reducing progression rates by 52%, noted lead investigator Li Zhang, MD, professor of medical oncology, Sun Yat-Sen University Cancer Center, Guangzhou, China.
Crucially, this benefit “was seen across key clinical subgroups,” he added.
He noted that the overall response rate “was also improved, with a durable response,” while the results, which are not yet mature, suggest the experimental arm was associated with an overall survival (OS) benefit.
Study discussant Misako Nagasaka, MD, a thoracic oncologist and clinical investigator at Karmanos Cancer Institute, Detroit, said that the PFS benefit seen in the study is “certainly encouraging.”
Adding a note of caution, she continued: “But we have seen studies with PFS improvement which did not translate into OS improvement.
“Longer follow-up would allow events to mature and we will ultimately see if there would be a significant OS benefit,” she said.
Dr. Nagasaka also pointed out that the greater benefit with sintilimab seen in patients with high PD-L1 begs the question as to what would be the preferred regimen in those with higher or lower expression.
And, she said, this is not just about what regimen to choose but “more importantly, why?”
“Perhaps you’d like to use something with the best response rate, perhaps you’re sticking to a single agent immunotherapy because the toxicity profile is more favorable, or perhaps you [are] convinced with a certain regimen because of the robust PFS and OS data,” she said.
“Whatever you chose, there was a reason for your choice,” she said, adding that the sintilimab combination would have to “fulfill those reasons for you to consider choosing this regimen.”
Study details
Dr. Zhang began his presentation by noting that previous phase 1b studies have shown that sintilimab plus pemetrexed and platinum-based chemotherapy has a “tolerable safety profile and promising efficacy” in previously untreated non-squamous NSCLC.
They therefore conducted ORIENT-11, a randomized, double-blind, phase 3 study involving 397 patients with untreated stage IIIB/C or IV nonsquamous NSCLC who had neither EGFR nor ALK gene alterations.
The patients were randomly assigned in a 2:1 fashion to sintilimab plus pemetrexed and platinum-based chemotherapy (n = 266) or placebo plus pemetrexed and chemo (n = 131) for four cycles, followed by sintilimab or placebo plus pemetrexed for up to 24 months.
Thirty-five patients in the placebo arm crossed over to sintilimab monotherapy, representing 31.3% of the intention-to-treat population.
At the data cutoff of Nov. 15, 2019, 198 events had occurred, at a median follow-up of 8.9 months.
The team found that median PFS was significantly higher with sintilimab than placebo combination therapy, at 8.9 months vs. 5.0 months, or a hazard ratio of 0.482 (P < .00001).
Dr. Zhang noted that the benefit with sintilimab plus pemetrexed and platinum-based chemotherapy was seen across all subgroups.
However, it was notable that the impact of adding sintilimab on PFS was greater in patients with a tumor proportion score (TPS) ≥50%.
The HR for progression vs. the placebo treatment arm was 0.310, with median PFS not reached, which decreased to 0.503 in patients with a TPS of 1%-49% and 0.664 among those with a TPS <1%.
The results also showed that there was a “nominally significant improvement” in overall survival with sintilimab versus placebo, at a HR of 0.609 (P = 0.01921).
The ORR was markedly different between the sintilimab and placebo groups, at 51.9% vs. 29.8%, with the duration of response not reached in the sintilimab arm compared with 5.5 months in the placebo arm.
The sintilimab arm included three (1.1%) complete responses, which was not observed with pemetrexed and platinum-based chemotherapy alone.
Finally, Dr. Zhang observed that the safety profiles of the sintilimab and placebo arms were similar, with comparable rates of any, grade 3-5, and serious adverse events largely driven by high rates of chemotherapy-related events.
While there were fewer adverse events that led to death with sintilimab, at 2.3% vs. 6.9% with placebo, there were, as expected, more immune-related adverse events, at 43.2% vs. 36.6%, respectively.
Comparison with pembrolizumab
In her discussion, Dr. Nagasaka said that the first question that came to mind when she saw the results was: “How does the ORIENT-11 data compare with KEYNOTE-189?”
For that study, pembrolizumab (Keytruda, Merck) was added to pemetrexed plus carboplatin chemotherapy and compared with standard of care alone in patients with untreated metastatic nonsquamous NSCLC.
As reported by Medscape Medical News, pembrolizumab was associated with a 48% reduced risk of disease progression, as well as improved overall survival.
Dr. Nagasaka said that ORIENT-11 “had patients that tended to be younger, there were more males, more with performance status 1, and those who had never smoked” than those in KEYNOTE-189.
“But most importantly, KEYNOTE-189 had a very small number of patients from East Asia, only 1% in the pembro arm and 2.9% in the placebo arm.”
In contrast, all the patients included in ORIENT-11 were from East Asia, making the study of “high importance.”
She added that, “while across-trial comparisons must be taken with caution, the medium PFS of ORIENT-11 ... appears comparable to those of KEYNOTE-189,” while the HR “appears identical.”
This is despite median follow-up time in ORIENT-11 of “only” 8.9 months vs. a median of 23.1 months in the updated KEYNOTE-189 data.
There are plans to register the sintilimab combination therapy in China for the treatment of nonsquamous NSCLC, where it will go up against pembrolizumab as well as, potentially, tislelizumab (BeiGene).
The study was sponsored by Innovent Biologics and Eli Lilly. Dr. Zhang disclosed research grants from Eli Lilly and Pfizer. Dr. Nagasaka disclosed serving on the advisory boards of AstraZeneca, Daiichi Sankyo, Takeda, Novartis, and EMD Serono; as a consultant for Caris Life Sciences; and receiving travel support from An Hearts Therapeutics.
This article first appeared on Medscape.com.
Chemo-free management of mesothelioma on horizon
Patients with untreated mesothelioma may be able to avoid chemotherapy, say researchers reporting new survival data with the immunotherapy combination of nivolumab (Opdivo) and ipilimumab (Yervoy).
The two approaches were compared in more than 600 patients with treatment-naive mesothelioma in the phase 3 CheckMate 743 trial, which was supported by the manufacturer of both immunotherapies, Bristol-Myers Squibb.
The trial “met its primary endpoint of statistically improving overall survival for the experimental arm vs chemotherapy in a prespecified interim analysis,” reported Paul Baas, MD, PhD, Netherlands Cancer Institute, Amsterdam, The Netherlands,
The combined nivo+ipi immunotherapy regimen was associated with a 26% improvement in overall survival. At 2 years, 41% of patients in the immunotherapy arm were still alive, vs 27% in the chemotherapy group.
“This is the first positive randomized trial of dual immunotherapy in the first-line treatment of patients with mesothelioma,” he said. He suggested that it should therefore “be considered as a new standard of care.”
The data were presented on August 8 in the presidential symposium of the World Congress on Lung Cancer 2020, which was held online because of the COVID-19 pandemic.
A key analysis for the study was by histologic subgroup. It is known that standard-of-care chemotherapy performs better in patients with epithelioid as opposed to nonepithelioid tumor subtypes.
Bass highlighted that the performance of nivo+ipi was “almost the same” in patients with epithelioid and nonepithelioid tumors, at a median overall survival of 18.7 months and 18.1 months, respectively.
In contrast, overall survival in the chemotherapy arm was markedly lower in patients with nonepithelioid tumors, at 8.8 months vs 16.5 months among those with epithelioid tumors.
This was reflected in the hazard ratios for overall survival vs nivo+ipi, at 0.46 and 0.86, respectively, the latter nonsignificantly different from combination immunotherapy.
For study discussant Dean A. Fennell, MD, PhD, professor and consultant in thoracic medical oncology, University of Leicester, United Kingdom, the epithet of a “new standard of care” for nivo+ipi should be reserved for nonepithelioid disease.
In this setting, he described the overall survival improvement as “transformative,” considering the “marked chemo resistance” of nonepithelioid tumors, which is “almost certainly” associated with epithelial-to-mesenchymal transition (EMT).
In the future, he suggested, combinations of chemotherapy and immunotherapy involving all histologies or selective targeting of nonepithelioid mesothelioma “could further extend the benefit for patients.”
Improving survival in mesothelioma
“We have been trying to improve the overall survival of patients with mesothelioma now for many decades,” Bass commented. Platinum-based chemotherapy plus pemetrexed is a standard of care, although the 5-year survival rate «is still below 10%,” he noted.
Randomized trials of single-agent immune checkpoint inhibitor therapy in the second-line treatment of patients with mesothelioma have not shown any significant benefits.
However, nivolumab and ipilimumab have a “complementary mechanism of action,” and two previous reports have indicated that together, they have clinical activity in the second-line setting.
The team conducted CheckMate 743 to determine the efficacy of the combination in the first-line setting.
The study involved 605 patients with pleural mesothelioma who had received no prior systemic therapy and had good performance status.
They were randomly assigned in a 1:1 ratio to receive nivo+ipi for up to 2 years or six cycles of pemetrexed plus cisplatin or carboplatin until disease progression or unacceptable toxicity occurred.
“Patients could have a subsequent therapy,” Bass noted; 44.0% of patients in the experimental arm received subsequent therapy, vs 44.1% of those in the chemotherapy arm.
Of the latter, 20% received an immune checkpoint inhibitor as subsequent therapy.
The minimum follow-up for overall survival was 22.1 months; the median follow-up was 29.7 months.
Nivo+ipi was associated with a significant improvement in overall survival vs standard-of-care chemotherapy, at a median overall survival of 18.1 months vs 14.1 months, with a hazard ratio of 0.74 (P = .0020).
The results indicated that overall survival was similar across key subgroups, which suggests that “no subgroup was harmed” by nivo+ipi, Bass said.
Stratification by PD-LI expression
Stratifying the patients by the absence or presence of programmed cell death–ligand-1 (PD-L1) expression, the team found that the performance of nivo+ipi was “the same” as that of chemotherapy, Bass said.
“But in cases where there is any expression of PD-L1, the experimental arm performs better,” at an overall survival 18.0 months vs 13.3 months for chemotherapy and a hazard ratio of 0.69, he said.
There was no difference between the two treatment arms in progression-free survival. Chemotherapy performed better in the first 6 months of treatment, after which the nivo+ipi arm had lower event rates.
Nivo+ipi was also associated with a greater duration of response, at a median of 11.0 months vs 6.7 months for standard-of-care chemotherapy.
Moreover, at 24 months, 32% of nivo+ipi patients were still experiencing a response, whereas 8% of those in the chemotherapy arm were.
Treatment-related adverse events rates were almost identical between the two treatment groups, although treatment with nivo+ipi was associated with more grade 3/4 serious treatment-related adverse events, at 15 vs six for chemotherapy.
Choosing immunotherapy vs. chemotherapy
In his discussion of the new study, Fennell compared the current results with those from two studies, INITIATE and MAPS2. “What’s very clear is the response rate is slightly higher,” as is the disease control rate, he said.
This, he explained, “is perhaps not surprising, given that these two previous trials were in the relapse setting.”
He pointed out, however, that the progression-free survival data from those previous trials were “not a million miles away” from results seen in CheckMate 743, “suggesting that this immunotherapy does have significant activity in the relapse setting.”
For Fennell, the “pivotal data” are in patients with nonepithelioid tumors, particularly inasmuch as chemotherapy performed “poorly” in this setting, whereas it performs “as expected” in epithelioid mesothelioma.
He believes that the driver for this is the poor prognosis associated with sarcomatoid biphasic disease, a subtype characterized by increased expression of vimentin and ZEB1, proteins both associated with EMT.
“What does this mean?” Fennell asked.
“If you have have enrichment of EMT, what you see is increased drug resistance, increased invasiveness, something we know well with sarcomatoid mesotheliomas in particular, and this drug-resistance phenotype may account for the drug resistance that we see in CheckMate 743 with chemotherapy.
“This does not appear, however, to impact in any way the efficacy of the immunotherapy,” he noted.
Fennell believes that, with regard to both efficacy and safety, the balance is “very much in favor” of nivo+ipi in epithelioid mesothelioma, although there is less to choose between immunotherapy and chemotherapy in the nonepithelioid setting.
Indeed, the choice is “possible tilting slightly towards chemotherapy” in patients with the nonepithelioid tumors, owing to the lower rates of grade 3/4 serious treatment-related adverse events in comparison with combination immunotherapy.
The study was supported by Bristol-Myers Squibb. Bass has served on the advisory boards of MSD, AstraZeneca, and Takeda. Fennell has received research support from AstraZeneca, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly, MSD, and Roche; research funding from Astex Therapeutics, Bayer, and Boehringer Ingelheim; has served on the speaker bureau of AstraZeneca, Boehringer Ingelheim, and Roche; has acted as a consultant for Bayer and Lab 21; and has served on the advisory board of Atara Biotherapeutics, Boehringer Ingelheim, and Inventiva.
This article first appeared on Medscape.com.
Patients with untreated mesothelioma may be able to avoid chemotherapy, say researchers reporting new survival data with the immunotherapy combination of nivolumab (Opdivo) and ipilimumab (Yervoy).
The two approaches were compared in more than 600 patients with treatment-naive mesothelioma in the phase 3 CheckMate 743 trial, which was supported by the manufacturer of both immunotherapies, Bristol-Myers Squibb.
The trial “met its primary endpoint of statistically improving overall survival for the experimental arm vs chemotherapy in a prespecified interim analysis,” reported Paul Baas, MD, PhD, Netherlands Cancer Institute, Amsterdam, The Netherlands,
The combined nivo+ipi immunotherapy regimen was associated with a 26% improvement in overall survival. At 2 years, 41% of patients in the immunotherapy arm were still alive, vs 27% in the chemotherapy group.
“This is the first positive randomized trial of dual immunotherapy in the first-line treatment of patients with mesothelioma,” he said. He suggested that it should therefore “be considered as a new standard of care.”
The data were presented on August 8 in the presidential symposium of the World Congress on Lung Cancer 2020, which was held online because of the COVID-19 pandemic.
A key analysis for the study was by histologic subgroup. It is known that standard-of-care chemotherapy performs better in patients with epithelioid as opposed to nonepithelioid tumor subtypes.
Bass highlighted that the performance of nivo+ipi was “almost the same” in patients with epithelioid and nonepithelioid tumors, at a median overall survival of 18.7 months and 18.1 months, respectively.
In contrast, overall survival in the chemotherapy arm was markedly lower in patients with nonepithelioid tumors, at 8.8 months vs 16.5 months among those with epithelioid tumors.
This was reflected in the hazard ratios for overall survival vs nivo+ipi, at 0.46 and 0.86, respectively, the latter nonsignificantly different from combination immunotherapy.
For study discussant Dean A. Fennell, MD, PhD, professor and consultant in thoracic medical oncology, University of Leicester, United Kingdom, the epithet of a “new standard of care” for nivo+ipi should be reserved for nonepithelioid disease.
In this setting, he described the overall survival improvement as “transformative,” considering the “marked chemo resistance” of nonepithelioid tumors, which is “almost certainly” associated with epithelial-to-mesenchymal transition (EMT).
In the future, he suggested, combinations of chemotherapy and immunotherapy involving all histologies or selective targeting of nonepithelioid mesothelioma “could further extend the benefit for patients.”
Improving survival in mesothelioma
“We have been trying to improve the overall survival of patients with mesothelioma now for many decades,” Bass commented. Platinum-based chemotherapy plus pemetrexed is a standard of care, although the 5-year survival rate «is still below 10%,” he noted.
Randomized trials of single-agent immune checkpoint inhibitor therapy in the second-line treatment of patients with mesothelioma have not shown any significant benefits.
However, nivolumab and ipilimumab have a “complementary mechanism of action,” and two previous reports have indicated that together, they have clinical activity in the second-line setting.
The team conducted CheckMate 743 to determine the efficacy of the combination in the first-line setting.
The study involved 605 patients with pleural mesothelioma who had received no prior systemic therapy and had good performance status.
They were randomly assigned in a 1:1 ratio to receive nivo+ipi for up to 2 years or six cycles of pemetrexed plus cisplatin or carboplatin until disease progression or unacceptable toxicity occurred.
“Patients could have a subsequent therapy,” Bass noted; 44.0% of patients in the experimental arm received subsequent therapy, vs 44.1% of those in the chemotherapy arm.
Of the latter, 20% received an immune checkpoint inhibitor as subsequent therapy.
The minimum follow-up for overall survival was 22.1 months; the median follow-up was 29.7 months.
Nivo+ipi was associated with a significant improvement in overall survival vs standard-of-care chemotherapy, at a median overall survival of 18.1 months vs 14.1 months, with a hazard ratio of 0.74 (P = .0020).
The results indicated that overall survival was similar across key subgroups, which suggests that “no subgroup was harmed” by nivo+ipi, Bass said.
Stratification by PD-LI expression
Stratifying the patients by the absence or presence of programmed cell death–ligand-1 (PD-L1) expression, the team found that the performance of nivo+ipi was “the same” as that of chemotherapy, Bass said.
“But in cases where there is any expression of PD-L1, the experimental arm performs better,” at an overall survival 18.0 months vs 13.3 months for chemotherapy and a hazard ratio of 0.69, he said.
There was no difference between the two treatment arms in progression-free survival. Chemotherapy performed better in the first 6 months of treatment, after which the nivo+ipi arm had lower event rates.
Nivo+ipi was also associated with a greater duration of response, at a median of 11.0 months vs 6.7 months for standard-of-care chemotherapy.
Moreover, at 24 months, 32% of nivo+ipi patients were still experiencing a response, whereas 8% of those in the chemotherapy arm were.
Treatment-related adverse events rates were almost identical between the two treatment groups, although treatment with nivo+ipi was associated with more grade 3/4 serious treatment-related adverse events, at 15 vs six for chemotherapy.
Choosing immunotherapy vs. chemotherapy
In his discussion of the new study, Fennell compared the current results with those from two studies, INITIATE and MAPS2. “What’s very clear is the response rate is slightly higher,” as is the disease control rate, he said.
This, he explained, “is perhaps not surprising, given that these two previous trials were in the relapse setting.”
He pointed out, however, that the progression-free survival data from those previous trials were “not a million miles away” from results seen in CheckMate 743, “suggesting that this immunotherapy does have significant activity in the relapse setting.”
For Fennell, the “pivotal data” are in patients with nonepithelioid tumors, particularly inasmuch as chemotherapy performed “poorly” in this setting, whereas it performs “as expected” in epithelioid mesothelioma.
He believes that the driver for this is the poor prognosis associated with sarcomatoid biphasic disease, a subtype characterized by increased expression of vimentin and ZEB1, proteins both associated with EMT.
“What does this mean?” Fennell asked.
“If you have have enrichment of EMT, what you see is increased drug resistance, increased invasiveness, something we know well with sarcomatoid mesotheliomas in particular, and this drug-resistance phenotype may account for the drug resistance that we see in CheckMate 743 with chemotherapy.
“This does not appear, however, to impact in any way the efficacy of the immunotherapy,” he noted.
Fennell believes that, with regard to both efficacy and safety, the balance is “very much in favor” of nivo+ipi in epithelioid mesothelioma, although there is less to choose between immunotherapy and chemotherapy in the nonepithelioid setting.
Indeed, the choice is “possible tilting slightly towards chemotherapy” in patients with the nonepithelioid tumors, owing to the lower rates of grade 3/4 serious treatment-related adverse events in comparison with combination immunotherapy.
The study was supported by Bristol-Myers Squibb. Bass has served on the advisory boards of MSD, AstraZeneca, and Takeda. Fennell has received research support from AstraZeneca, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly, MSD, and Roche; research funding from Astex Therapeutics, Bayer, and Boehringer Ingelheim; has served on the speaker bureau of AstraZeneca, Boehringer Ingelheim, and Roche; has acted as a consultant for Bayer and Lab 21; and has served on the advisory board of Atara Biotherapeutics, Boehringer Ingelheim, and Inventiva.
This article first appeared on Medscape.com.
Patients with untreated mesothelioma may be able to avoid chemotherapy, say researchers reporting new survival data with the immunotherapy combination of nivolumab (Opdivo) and ipilimumab (Yervoy).
The two approaches were compared in more than 600 patients with treatment-naive mesothelioma in the phase 3 CheckMate 743 trial, which was supported by the manufacturer of both immunotherapies, Bristol-Myers Squibb.
The trial “met its primary endpoint of statistically improving overall survival for the experimental arm vs chemotherapy in a prespecified interim analysis,” reported Paul Baas, MD, PhD, Netherlands Cancer Institute, Amsterdam, The Netherlands,
The combined nivo+ipi immunotherapy regimen was associated with a 26% improvement in overall survival. At 2 years, 41% of patients in the immunotherapy arm were still alive, vs 27% in the chemotherapy group.
“This is the first positive randomized trial of dual immunotherapy in the first-line treatment of patients with mesothelioma,” he said. He suggested that it should therefore “be considered as a new standard of care.”
The data were presented on August 8 in the presidential symposium of the World Congress on Lung Cancer 2020, which was held online because of the COVID-19 pandemic.
A key analysis for the study was by histologic subgroup. It is known that standard-of-care chemotherapy performs better in patients with epithelioid as opposed to nonepithelioid tumor subtypes.
Bass highlighted that the performance of nivo+ipi was “almost the same” in patients with epithelioid and nonepithelioid tumors, at a median overall survival of 18.7 months and 18.1 months, respectively.
In contrast, overall survival in the chemotherapy arm was markedly lower in patients with nonepithelioid tumors, at 8.8 months vs 16.5 months among those with epithelioid tumors.
This was reflected in the hazard ratios for overall survival vs nivo+ipi, at 0.46 and 0.86, respectively, the latter nonsignificantly different from combination immunotherapy.
For study discussant Dean A. Fennell, MD, PhD, professor and consultant in thoracic medical oncology, University of Leicester, United Kingdom, the epithet of a “new standard of care” for nivo+ipi should be reserved for nonepithelioid disease.
In this setting, he described the overall survival improvement as “transformative,” considering the “marked chemo resistance” of nonepithelioid tumors, which is “almost certainly” associated with epithelial-to-mesenchymal transition (EMT).
In the future, he suggested, combinations of chemotherapy and immunotherapy involving all histologies or selective targeting of nonepithelioid mesothelioma “could further extend the benefit for patients.”
Improving survival in mesothelioma
“We have been trying to improve the overall survival of patients with mesothelioma now for many decades,” Bass commented. Platinum-based chemotherapy plus pemetrexed is a standard of care, although the 5-year survival rate «is still below 10%,” he noted.
Randomized trials of single-agent immune checkpoint inhibitor therapy in the second-line treatment of patients with mesothelioma have not shown any significant benefits.
However, nivolumab and ipilimumab have a “complementary mechanism of action,” and two previous reports have indicated that together, they have clinical activity in the second-line setting.
The team conducted CheckMate 743 to determine the efficacy of the combination in the first-line setting.
The study involved 605 patients with pleural mesothelioma who had received no prior systemic therapy and had good performance status.
They were randomly assigned in a 1:1 ratio to receive nivo+ipi for up to 2 years or six cycles of pemetrexed plus cisplatin or carboplatin until disease progression or unacceptable toxicity occurred.
“Patients could have a subsequent therapy,” Bass noted; 44.0% of patients in the experimental arm received subsequent therapy, vs 44.1% of those in the chemotherapy arm.
Of the latter, 20% received an immune checkpoint inhibitor as subsequent therapy.
The minimum follow-up for overall survival was 22.1 months; the median follow-up was 29.7 months.
Nivo+ipi was associated with a significant improvement in overall survival vs standard-of-care chemotherapy, at a median overall survival of 18.1 months vs 14.1 months, with a hazard ratio of 0.74 (P = .0020).
The results indicated that overall survival was similar across key subgroups, which suggests that “no subgroup was harmed” by nivo+ipi, Bass said.
Stratification by PD-LI expression
Stratifying the patients by the absence or presence of programmed cell death–ligand-1 (PD-L1) expression, the team found that the performance of nivo+ipi was “the same” as that of chemotherapy, Bass said.
“But in cases where there is any expression of PD-L1, the experimental arm performs better,” at an overall survival 18.0 months vs 13.3 months for chemotherapy and a hazard ratio of 0.69, he said.
There was no difference between the two treatment arms in progression-free survival. Chemotherapy performed better in the first 6 months of treatment, after which the nivo+ipi arm had lower event rates.
Nivo+ipi was also associated with a greater duration of response, at a median of 11.0 months vs 6.7 months for standard-of-care chemotherapy.
Moreover, at 24 months, 32% of nivo+ipi patients were still experiencing a response, whereas 8% of those in the chemotherapy arm were.
Treatment-related adverse events rates were almost identical between the two treatment groups, although treatment with nivo+ipi was associated with more grade 3/4 serious treatment-related adverse events, at 15 vs six for chemotherapy.
Choosing immunotherapy vs. chemotherapy
In his discussion of the new study, Fennell compared the current results with those from two studies, INITIATE and MAPS2. “What’s very clear is the response rate is slightly higher,” as is the disease control rate, he said.
This, he explained, “is perhaps not surprising, given that these two previous trials were in the relapse setting.”
He pointed out, however, that the progression-free survival data from those previous trials were “not a million miles away” from results seen in CheckMate 743, “suggesting that this immunotherapy does have significant activity in the relapse setting.”
For Fennell, the “pivotal data” are in patients with nonepithelioid tumors, particularly inasmuch as chemotherapy performed “poorly” in this setting, whereas it performs “as expected” in epithelioid mesothelioma.
He believes that the driver for this is the poor prognosis associated with sarcomatoid biphasic disease, a subtype characterized by increased expression of vimentin and ZEB1, proteins both associated with EMT.
“What does this mean?” Fennell asked.
“If you have have enrichment of EMT, what you see is increased drug resistance, increased invasiveness, something we know well with sarcomatoid mesotheliomas in particular, and this drug-resistance phenotype may account for the drug resistance that we see in CheckMate 743 with chemotherapy.
“This does not appear, however, to impact in any way the efficacy of the immunotherapy,” he noted.
Fennell believes that, with regard to both efficacy and safety, the balance is “very much in favor” of nivo+ipi in epithelioid mesothelioma, although there is less to choose between immunotherapy and chemotherapy in the nonepithelioid setting.
Indeed, the choice is “possible tilting slightly towards chemotherapy” in patients with the nonepithelioid tumors, owing to the lower rates of grade 3/4 serious treatment-related adverse events in comparison with combination immunotherapy.
The study was supported by Bristol-Myers Squibb. Bass has served on the advisory boards of MSD, AstraZeneca, and Takeda. Fennell has received research support from AstraZeneca, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly, MSD, and Roche; research funding from Astex Therapeutics, Bayer, and Boehringer Ingelheim; has served on the speaker bureau of AstraZeneca, Boehringer Ingelheim, and Roche; has acted as a consultant for Bayer and Lab 21; and has served on the advisory board of Atara Biotherapeutics, Boehringer Ingelheim, and Inventiva.
This article first appeared on Medscape.com.
Critical care readiness. Coding for telemedicine. Physical therapy teleconsultations. Physical therapy teleconsultations.
Preparation is key for disaster management. It includes identifying heath-care worker capability, surge capacity, disposable medical resources, and expert consultation availability.
Staff
In disaster, the hospital transitions to a mass casualty strategy, repurposing noncritical care staff to a tiered critical care model focusing on disaster triage and mass critical care. The goal is to provide care to minimize mortality.
Stuff
Critical care supplies improve survival and are implemented quickly and easily. Essential supplies include personal protective equipment, basic modes of mechanical ventilation, hemodynamic support, antimicrobial therapy or other disease-specific countermeasures, oxygen, and prophylactic treatments.
Structure
Disaster critical care can be delivered in noncritical care areas. Hospital policies should establish surge capacity strategies.
System
Providing quality lifesaving care to appropriately triaged patients by utilizing minimal qualifications for survival, predetermined ICU admission criteria, and dynamic protocols using the highest level of evidence available scalable to local resources.
Inappropriate triage results in suboptimal care and can lead to increased mortality.
Virtual critical care can augment critical care capacity and capability.
The implementation of mass critical care requires hospitals to rapidly increase its patient volume above its normal capacity. The essential four components are staff, stuff, space, and structure. Effective mass critical care requires a different mindset than critical care in day-to-day operations.
Patrick Moon, MD; and Alexis MacDonald, MD
(Drs. Reed and Tripp's Fellows)
Mary Jane Reed, MD, FCCP, and Michael Tripp, MD, FCCP
Steering Committee Members
Practice Operations
Over the years since telemedicine (TM) was developed in the 1960s, it has transformed into more mobile, compact, and interconnected forms. However, its widespread adoption has been limited by the regulatory, compensatory, and licensing status quo. The emergence of the COVID-19 pandemic and its necessity for physical distancing has brought TM into the limelight. With restrictions on TM use lifted by CMS, the scope of TM could extend from outpatient to inpatient care to emergency triaging and management of chronic medical conditions.
In February 2020, the comprehensive 2020 COVID-19 ICD 10 coding guidelines were released. To date, CMS has approved approximately 80 codes, which can be used with telehealth and non face-face-to-face (NFTF) encounters. They include telephone calls, online digital E/M services, interprofessional telephone/internet/electric health record consultations, digitally stored data services/remote physiologic monitoring, remote reporting of self-measure blood pressure, and remote physiologic monitoring treatment management services. Some of the key "rules of the game" are highlighted below.
- For telephone visits in the outpatient setting use the codes 99441 (5-10 minutes), 99442 (11-20 minutes), and 99443 (21-30 minutes).
- For interactive real-time audio and video telecommunication (RAVT) in the outpatient setting, use the codes normally used for outpatient E/M: 99201-99215.
- For using RAVT to perform an initial visit for an inpatient, use the codes that are normally used for inpatient E/M: 99221-99223.
- For using RAVT to perform a subsequent visit for an inpatient, use the codes that are normally used for subsequent hospital care service E/M: 99231-99233.
- Seeing a critically ill patient without being in the patient's room is allowed, as a physical exam is not required for either 99291 or 99292. Be sure to use 99292 for each 30 minutes beyond the initial 74 minutes and document the time spent on the patient.
The details of the coding/billing guidelines are intricate and full of nuances and for a better understanding on how to utilize TM both in an inpatient and outpatient setting, consider the following resources:
1. CHEST Experience presentation entitled "TELE MEDICINE/TELE HEALTH IN THE ERA OF PANDEMIC" at the CHEST Annual Meeting 2020.
1. Coding and Billing Guidelines by ATS:
https://www.thoracic.org/about/newsroom/newsletters/coding-and-billing/resources/2020/mostrecentcbqapril.pdf
2. Coding specific for management of COVID patients by the AMA:
https://www.ama-assn.org/system/files/2020-05/covid-19-coding-advice.pdf
Humayun Anjum, MD, FCCP
Vice-Chair, Practice Operations
Haala Rokadia, MD, FCCP
Practice Operations NetWork Steering Committee Member
Transplant
Physical therapy teleconsultations
The COVID 19 pandemic led the health-care community to rapidly adopt telecommunication tools allowing provision of care equivalent to in-person visits. Implementation of telemedicine visits demonstrated that providers can simultaneously distance and connect with patients to provide expert care.
The University of Pennsylvania lung transplant team adapted video communications to provide individualized physical therapy (PT) recommendations for lung transplantation candidates. The evaluation includes a systems review, musculoskeletal screen, submaximal aerobic capacity testing, and performance of the short physical performance battery test (SPPBT), a frequently used frailty evaluation tool focused on lower extremity function and balance. In the era of social distancing, telemedicine capabilities have made this crucial aspect of pretransplant evaluation possible.
In advance, patients are emailed a document outlining the telemedicine PT assessment, including the SPPBT. Patients receive videos of the SPPBT to ensure they understand the test and can prepare their home to safely perform the tasks. We are able to highlight the patient's functional capabilities and detail accurate assessments of their deficits. Our teleconsultations utilize BlueJeans for connectivity and typically last about 30 minutes. At this time, we are billing for these pretransplant visits but not for posttransplant PT follow-up.
Patient experiences with the PT teleconsultations have been overwhelmingly positive. Patients and their families appreciate the uninterrupted evaluation time and the individualized recommendations for improving their deficits. The providers can devote their full attention to the patient directly in front of them. Importantly, patients and providers report they have never felt a stronger connection than through these telemedicine encounters. Longitudinal telemedicine PT assessments will enable us to better monitor our patients throughout the lung transplantation process.
Joshua Diamond, MD
Steering Committee Member
Derek Zaleski, PT, DPT
Women's Lung Health
SARS-COV-2 and pregnancy
The SARS-COV-2 pandemic has brought on many fears and uncertainties with new information emerging daily, including the effect during pregnancy. At the time of this article,however, data pertaining to COVID-19 and pregnancy remain limited. Pregnant women do not seem to have a higher infection rate than the general population. In a correspondence where pregnant women admitted for delivery underwent universal screening in NY, 1.9% of women were symptomatic and tested positive, and 13.7% of the asymptomatic patients were found to be SARS-COV-2 positive.1 Furthermore, unlike H1NI, data suggest that pregnant women infected with SARS-COV-2 currently do not seem to have worse outcomes than the average person.2,3 As of now, there have not been any reports of maternal fetal vertical transmission from COVID-19 or any other coronavirus variants.4 Postpartum testing of infants has yielded a very small number of babies who have tested positive for virus, but this more likely represents transmission after birth. There are currently no specific FDA-approved medications for the treatment of moderate-severe infections with COVID-19 in pregnant women, although there are several clinical trials underway. Patients with moderate to severe symptoms should seek medical attention, while those with mild symptoms should continue with conservative therapies, as well as maintaining proper hygiene.5 Delivery methods and timing remain unchanged with cesarean delivery as currently indicated per established guidelines.5
Mariam Louis, MD
Steering Committee Member
Jorge Trabanco, MD
1. N Engl J Med. 2020 Apr 13;382:2163-4. April 13, 2020, DOI: 10.1056/NEJMc2009316
2. N Engl J Med. 2020 Jun 18; 382:e100. April 17, 2020 DOI: 10.1056/NEJMc2009226
3. Acta Obstet Gynecol Scand. 2020 Jul;99(7):823-829. 2020 Apr 7. doi: 10.1111/aogs.13867. [Epub ahead of print]
4. Arch Pathol Lab Med. 2020 Apr 27. doi: 10.5858/arpa.2020-0211-SA. [Epub ahead of print]
5. ACOG practice advisory, Novel Coronavirus 2019 (COVID-19) April 23, 2020.
Preparation is key for disaster management. It includes identifying heath-care worker capability, surge capacity, disposable medical resources, and expert consultation availability.
Staff
In disaster, the hospital transitions to a mass casualty strategy, repurposing noncritical care staff to a tiered critical care model focusing on disaster triage and mass critical care. The goal is to provide care to minimize mortality.
Stuff
Critical care supplies improve survival and are implemented quickly and easily. Essential supplies include personal protective equipment, basic modes of mechanical ventilation, hemodynamic support, antimicrobial therapy or other disease-specific countermeasures, oxygen, and prophylactic treatments.
Structure
Disaster critical care can be delivered in noncritical care areas. Hospital policies should establish surge capacity strategies.
System
Providing quality lifesaving care to appropriately triaged patients by utilizing minimal qualifications for survival, predetermined ICU admission criteria, and dynamic protocols using the highest level of evidence available scalable to local resources.
Inappropriate triage results in suboptimal care and can lead to increased mortality.
Virtual critical care can augment critical care capacity and capability.
The implementation of mass critical care requires hospitals to rapidly increase its patient volume above its normal capacity. The essential four components are staff, stuff, space, and structure. Effective mass critical care requires a different mindset than critical care in day-to-day operations.
Patrick Moon, MD; and Alexis MacDonald, MD
(Drs. Reed and Tripp's Fellows)
Mary Jane Reed, MD, FCCP, and Michael Tripp, MD, FCCP
Steering Committee Members
Practice Operations
Over the years since telemedicine (TM) was developed in the 1960s, it has transformed into more mobile, compact, and interconnected forms. However, its widespread adoption has been limited by the regulatory, compensatory, and licensing status quo. The emergence of the COVID-19 pandemic and its necessity for physical distancing has brought TM into the limelight. With restrictions on TM use lifted by CMS, the scope of TM could extend from outpatient to inpatient care to emergency triaging and management of chronic medical conditions.
In February 2020, the comprehensive 2020 COVID-19 ICD 10 coding guidelines were released. To date, CMS has approved approximately 80 codes, which can be used with telehealth and non face-face-to-face (NFTF) encounters. They include telephone calls, online digital E/M services, interprofessional telephone/internet/electric health record consultations, digitally stored data services/remote physiologic monitoring, remote reporting of self-measure blood pressure, and remote physiologic monitoring treatment management services. Some of the key "rules of the game" are highlighted below.
- For telephone visits in the outpatient setting use the codes 99441 (5-10 minutes), 99442 (11-20 minutes), and 99443 (21-30 minutes).
- For interactive real-time audio and video telecommunication (RAVT) in the outpatient setting, use the codes normally used for outpatient E/M: 99201-99215.
- For using RAVT to perform an initial visit for an inpatient, use the codes that are normally used for inpatient E/M: 99221-99223.
- For using RAVT to perform a subsequent visit for an inpatient, use the codes that are normally used for subsequent hospital care service E/M: 99231-99233.
- Seeing a critically ill patient without being in the patient's room is allowed, as a physical exam is not required for either 99291 or 99292. Be sure to use 99292 for each 30 minutes beyond the initial 74 minutes and document the time spent on the patient.
The details of the coding/billing guidelines are intricate and full of nuances and for a better understanding on how to utilize TM both in an inpatient and outpatient setting, consider the following resources:
1. CHEST Experience presentation entitled "TELE MEDICINE/TELE HEALTH IN THE ERA OF PANDEMIC" at the CHEST Annual Meeting 2020.
1. Coding and Billing Guidelines by ATS:
https://www.thoracic.org/about/newsroom/newsletters/coding-and-billing/resources/2020/mostrecentcbqapril.pdf
2. Coding specific for management of COVID patients by the AMA:
https://www.ama-assn.org/system/files/2020-05/covid-19-coding-advice.pdf
Humayun Anjum, MD, FCCP
Vice-Chair, Practice Operations
Haala Rokadia, MD, FCCP
Practice Operations NetWork Steering Committee Member
Transplant
Physical therapy teleconsultations
The COVID 19 pandemic led the health-care community to rapidly adopt telecommunication tools allowing provision of care equivalent to in-person visits. Implementation of telemedicine visits demonstrated that providers can simultaneously distance and connect with patients to provide expert care.
The University of Pennsylvania lung transplant team adapted video communications to provide individualized physical therapy (PT) recommendations for lung transplantation candidates. The evaluation includes a systems review, musculoskeletal screen, submaximal aerobic capacity testing, and performance of the short physical performance battery test (SPPBT), a frequently used frailty evaluation tool focused on lower extremity function and balance. In the era of social distancing, telemedicine capabilities have made this crucial aspect of pretransplant evaluation possible.
In advance, patients are emailed a document outlining the telemedicine PT assessment, including the SPPBT. Patients receive videos of the SPPBT to ensure they understand the test and can prepare their home to safely perform the tasks. We are able to highlight the patient's functional capabilities and detail accurate assessments of their deficits. Our teleconsultations utilize BlueJeans for connectivity and typically last about 30 minutes. At this time, we are billing for these pretransplant visits but not for posttransplant PT follow-up.
Patient experiences with the PT teleconsultations have been overwhelmingly positive. Patients and their families appreciate the uninterrupted evaluation time and the individualized recommendations for improving their deficits. The providers can devote their full attention to the patient directly in front of them. Importantly, patients and providers report they have never felt a stronger connection than through these telemedicine encounters. Longitudinal telemedicine PT assessments will enable us to better monitor our patients throughout the lung transplantation process.
Joshua Diamond, MD
Steering Committee Member
Derek Zaleski, PT, DPT
Women's Lung Health
SARS-COV-2 and pregnancy
The SARS-COV-2 pandemic has brought on many fears and uncertainties with new information emerging daily, including the effect during pregnancy. At the time of this article,however, data pertaining to COVID-19 and pregnancy remain limited. Pregnant women do not seem to have a higher infection rate than the general population. In a correspondence where pregnant women admitted for delivery underwent universal screening in NY, 1.9% of women were symptomatic and tested positive, and 13.7% of the asymptomatic patients were found to be SARS-COV-2 positive.1 Furthermore, unlike H1NI, data suggest that pregnant women infected with SARS-COV-2 currently do not seem to have worse outcomes than the average person.2,3 As of now, there have not been any reports of maternal fetal vertical transmission from COVID-19 or any other coronavirus variants.4 Postpartum testing of infants has yielded a very small number of babies who have tested positive for virus, but this more likely represents transmission after birth. There are currently no specific FDA-approved medications for the treatment of moderate-severe infections with COVID-19 in pregnant women, although there are several clinical trials underway. Patients with moderate to severe symptoms should seek medical attention, while those with mild symptoms should continue with conservative therapies, as well as maintaining proper hygiene.5 Delivery methods and timing remain unchanged with cesarean delivery as currently indicated per established guidelines.5
Mariam Louis, MD
Steering Committee Member
Jorge Trabanco, MD
1. N Engl J Med. 2020 Apr 13;382:2163-4. April 13, 2020, DOI: 10.1056/NEJMc2009316
2. N Engl J Med. 2020 Jun 18; 382:e100. April 17, 2020 DOI: 10.1056/NEJMc2009226
3. Acta Obstet Gynecol Scand. 2020 Jul;99(7):823-829. 2020 Apr 7. doi: 10.1111/aogs.13867. [Epub ahead of print]
4. Arch Pathol Lab Med. 2020 Apr 27. doi: 10.5858/arpa.2020-0211-SA. [Epub ahead of print]
5. ACOG practice advisory, Novel Coronavirus 2019 (COVID-19) April 23, 2020.
Preparation is key for disaster management. It includes identifying heath-care worker capability, surge capacity, disposable medical resources, and expert consultation availability.
Staff
In disaster, the hospital transitions to a mass casualty strategy, repurposing noncritical care staff to a tiered critical care model focusing on disaster triage and mass critical care. The goal is to provide care to minimize mortality.
Stuff
Critical care supplies improve survival and are implemented quickly and easily. Essential supplies include personal protective equipment, basic modes of mechanical ventilation, hemodynamic support, antimicrobial therapy or other disease-specific countermeasures, oxygen, and prophylactic treatments.
Structure
Disaster critical care can be delivered in noncritical care areas. Hospital policies should establish surge capacity strategies.
System
Providing quality lifesaving care to appropriately triaged patients by utilizing minimal qualifications for survival, predetermined ICU admission criteria, and dynamic protocols using the highest level of evidence available scalable to local resources.
Inappropriate triage results in suboptimal care and can lead to increased mortality.
Virtual critical care can augment critical care capacity and capability.
The implementation of mass critical care requires hospitals to rapidly increase its patient volume above its normal capacity. The essential four components are staff, stuff, space, and structure. Effective mass critical care requires a different mindset than critical care in day-to-day operations.
Patrick Moon, MD; and Alexis MacDonald, MD
(Drs. Reed and Tripp's Fellows)
Mary Jane Reed, MD, FCCP, and Michael Tripp, MD, FCCP
Steering Committee Members
Practice Operations
Over the years since telemedicine (TM) was developed in the 1960s, it has transformed into more mobile, compact, and interconnected forms. However, its widespread adoption has been limited by the regulatory, compensatory, and licensing status quo. The emergence of the COVID-19 pandemic and its necessity for physical distancing has brought TM into the limelight. With restrictions on TM use lifted by CMS, the scope of TM could extend from outpatient to inpatient care to emergency triaging and management of chronic medical conditions.
In February 2020, the comprehensive 2020 COVID-19 ICD 10 coding guidelines were released. To date, CMS has approved approximately 80 codes, which can be used with telehealth and non face-face-to-face (NFTF) encounters. They include telephone calls, online digital E/M services, interprofessional telephone/internet/electric health record consultations, digitally stored data services/remote physiologic monitoring, remote reporting of self-measure blood pressure, and remote physiologic monitoring treatment management services. Some of the key "rules of the game" are highlighted below.
- For telephone visits in the outpatient setting use the codes 99441 (5-10 minutes), 99442 (11-20 minutes), and 99443 (21-30 minutes).
- For interactive real-time audio and video telecommunication (RAVT) in the outpatient setting, use the codes normally used for outpatient E/M: 99201-99215.
- For using RAVT to perform an initial visit for an inpatient, use the codes that are normally used for inpatient E/M: 99221-99223.
- For using RAVT to perform a subsequent visit for an inpatient, use the codes that are normally used for subsequent hospital care service E/M: 99231-99233.
- Seeing a critically ill patient without being in the patient's room is allowed, as a physical exam is not required for either 99291 or 99292. Be sure to use 99292 for each 30 minutes beyond the initial 74 minutes and document the time spent on the patient.
The details of the coding/billing guidelines are intricate and full of nuances and for a better understanding on how to utilize TM both in an inpatient and outpatient setting, consider the following resources:
1. CHEST Experience presentation entitled "TELE MEDICINE/TELE HEALTH IN THE ERA OF PANDEMIC" at the CHEST Annual Meeting 2020.
1. Coding and Billing Guidelines by ATS:
https://www.thoracic.org/about/newsroom/newsletters/coding-and-billing/resources/2020/mostrecentcbqapril.pdf
2. Coding specific for management of COVID patients by the AMA:
https://www.ama-assn.org/system/files/2020-05/covid-19-coding-advice.pdf
Humayun Anjum, MD, FCCP
Vice-Chair, Practice Operations
Haala Rokadia, MD, FCCP
Practice Operations NetWork Steering Committee Member
Transplant
Physical therapy teleconsultations
The COVID 19 pandemic led the health-care community to rapidly adopt telecommunication tools allowing provision of care equivalent to in-person visits. Implementation of telemedicine visits demonstrated that providers can simultaneously distance and connect with patients to provide expert care.
The University of Pennsylvania lung transplant team adapted video communications to provide individualized physical therapy (PT) recommendations for lung transplantation candidates. The evaluation includes a systems review, musculoskeletal screen, submaximal aerobic capacity testing, and performance of the short physical performance battery test (SPPBT), a frequently used frailty evaluation tool focused on lower extremity function and balance. In the era of social distancing, telemedicine capabilities have made this crucial aspect of pretransplant evaluation possible.
In advance, patients are emailed a document outlining the telemedicine PT assessment, including the SPPBT. Patients receive videos of the SPPBT to ensure they understand the test and can prepare their home to safely perform the tasks. We are able to highlight the patient's functional capabilities and detail accurate assessments of their deficits. Our teleconsultations utilize BlueJeans for connectivity and typically last about 30 minutes. At this time, we are billing for these pretransplant visits but not for posttransplant PT follow-up.
Patient experiences with the PT teleconsultations have been overwhelmingly positive. Patients and their families appreciate the uninterrupted evaluation time and the individualized recommendations for improving their deficits. The providers can devote their full attention to the patient directly in front of them. Importantly, patients and providers report they have never felt a stronger connection than through these telemedicine encounters. Longitudinal telemedicine PT assessments will enable us to better monitor our patients throughout the lung transplantation process.
Joshua Diamond, MD
Steering Committee Member
Derek Zaleski, PT, DPT
Women's Lung Health
SARS-COV-2 and pregnancy
The SARS-COV-2 pandemic has brought on many fears and uncertainties with new information emerging daily, including the effect during pregnancy. At the time of this article,however, data pertaining to COVID-19 and pregnancy remain limited. Pregnant women do not seem to have a higher infection rate than the general population. In a correspondence where pregnant women admitted for delivery underwent universal screening in NY, 1.9% of women were symptomatic and tested positive, and 13.7% of the asymptomatic patients were found to be SARS-COV-2 positive.1 Furthermore, unlike H1NI, data suggest that pregnant women infected with SARS-COV-2 currently do not seem to have worse outcomes than the average person.2,3 As of now, there have not been any reports of maternal fetal vertical transmission from COVID-19 or any other coronavirus variants.4 Postpartum testing of infants has yielded a very small number of babies who have tested positive for virus, but this more likely represents transmission after birth. There are currently no specific FDA-approved medications for the treatment of moderate-severe infections with COVID-19 in pregnant women, although there are several clinical trials underway. Patients with moderate to severe symptoms should seek medical attention, while those with mild symptoms should continue with conservative therapies, as well as maintaining proper hygiene.5 Delivery methods and timing remain unchanged with cesarean delivery as currently indicated per established guidelines.5
Mariam Louis, MD
Steering Committee Member
Jorge Trabanco, MD
1. N Engl J Med. 2020 Apr 13;382:2163-4. April 13, 2020, DOI: 10.1056/NEJMc2009316
2. N Engl J Med. 2020 Jun 18; 382:e100. April 17, 2020 DOI: 10.1056/NEJMc2009226
3. Acta Obstet Gynecol Scand. 2020 Jul;99(7):823-829. 2020 Apr 7. doi: 10.1111/aogs.13867. [Epub ahead of print]
4. Arch Pathol Lab Med. 2020 Apr 27. doi: 10.5858/arpa.2020-0211-SA. [Epub ahead of print]
5. ACOG practice advisory, Novel Coronavirus 2019 (COVID-19) April 23, 2020.
Telehealth in the COVID-19 era: The New York experience
Big data scientists and health-care experts have tried preparing physicians and patients for the arrival of telemedicine for years. Health tracking applications are on our smartphones. Compact ambulatory devices diagnose hypertension and atrial fibrillation. Advanced imaging modalities make the stethoscope more of a neck accessory than a practical tool. Despite these efficient technologic advancements, the idea of making the sacred in-person office visit remote and through a screen appealed to few. In fact, prior to the COVID-19 pandemic, only 15% of medical practices offered telehealth services and 8% of Americans joined in remote visits annually (Mann DM et al. J Am Med Inform Assoc. 2019 Feb 1;26[2]:106-114).
When the COVID-19 pandemic hit New York City and admissions for hypoxemic respiratory failure skyrocketed, ED and in-person clinic visits for other acute and chronic conditions plummeted. Prior to clinics officially closing their doors, doctors in New York City asked their patients to reserve office visits for emergency issues only ,with most patients willingly staying home to avoid exposure to the virus. Suddenly, after years of disinterest in adopting telehealth, hospitals and clinics were catapulted into a full-on need for this technology. Overnight, our division’s secretaries and medical assistants became IT support staff. We all learned together what worked, what didn’t work, and how to adapt our workflow to meet everyone’s needs.
Previously, longstanding issues with accessibility and reimbursement presented barriers to widespread adoption of telemedicine. Once the pandemic hit, though, many regulatory changes were quickly made to accommodate telehealth.
Three such changes are worth highlighting (Centers for Medicare and Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. March 30, 2020).
First, patient privacy rules became more lenient. Prior to the pandemic, HIPAA mandated that both doctor and patient use embedded video interfaces with high levels of security. Now, health-care providers can use commonplace video chat applications such as FaceTime, Google Hangouts, Zoom, or Skype to provide telehealth without risk of penalty for HIPAA noncompliance. When connectivity concerns arose with our EMR’s embedded telehealth application, a quick transition to one of these platforms mitigated patient and provider frustration.
Second, prior to the pandemic, some private insurance providers reimbursed for televisits, but there were stipulations on how the visit could be conducted. Now, many of the commercial insurers plus Medicare and Medicaid in New York State reimburse the same amount for televisits as in-person visits (fee-for-service rate). Reimbursement rates of audio-only encounters were increased. If these changes are continued postpandemic, it will have an expansive impact on the future of an outpatient practice.
Third, restrictive government regulations relaxed with regard to telehealth deployment. Gone are the demands on providers and patients to be physically face-to-face. Many colleagues worked from home, safely social distancing.
Even though remote medical visits were a crucial part of flattening the curve during the peak of the pandemic in New York City, the telehealth experience is not without flaws.
An informal survey of providers in our own division garnered diverse and spirited viewpoints about seeing patients remotely. Instead of using a stethoscope to pick up a subtle finding, telehealth visits require the use of our eyes to scan a patient’s home environment for insights explaining their chronic cough (Where is the mold? Where is the water damage? Where is the bird?). We use our ears to hear the intonation of our patient’s voice to know when he or she is concerned, anxious, or are at their baselines. We would implore patients to put on their pulse oximeter and perform activities of daily living and/or exertion. On multiple occasions, patients would perform their own, unsolicited walks about their home to show us what they could and couldn’t do, where they place their concentrators, and where they are likely to trip over oxygen tubing. We learned to depend on them to reach the conclusion that they were at their normal state of health.
For straight-forward encounters with existing patients, most of our colleagues appreciated the simplicity and efficiency of telemedicine. But when it came to new patients, some colleagues struggled with whether they should see them for the first time over video. Universally, providers felt feelings of inadequacy without an in-person examination and review of diagnostic information.
Along those lines, many of our colleagues worried about their ability to perform the most fundamental role of a physician over the phone/internet for all patients: building trust with a patient. Eye contact, the physical exam, and verbal and nonv
Providers also noted that telehealth implementation is not the same for all individuals. Just as COVID-19 disproportionately affects the most vulnerable populations (NYC Health. COVID-19: data. Accessed July 1, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page), practicing telehealth has uncovered more ways in which racial/ethnic minorities, low income communities, and older patients are at a disadvantage (Garg S, et al. MMWR Morb Mortal Wkly Rep. 2020;69[15]:458). The relatively quick transition to telemedicine revealed that many of our patients don’t have emails or home computers to connect with online platforms. Similarly, some do not have smart phones with internet capabilities. Many do not speak English and cannot partake in video visits since translators are not yet embedded into the EMR’s video system. Elderly patients were frequently very anxious with telemedicine because of unfamiliarity with the technology, and many preferred a phone conversation. Thus, while more fortunate patients get to use a video interface and its association with higher patient understanding and satisfaction, our most vulnerable populations are often denied the same access to such care (Voils CI et al. J Genet Couns. 2018;27[2]:339).
Telemedicine will continue to have a significant impact on the future of health care long after the COVID-19 pandemic abates. There will be growing pains, refinement of technology, improvements in policy, and an ongoing general evolution of the system. Patients and providers will grow together as its utilization continues. We suspect patient surveys about their attitudes and preferences for telemedicine will be as varied as the providers surveyed here. A recent survey of 1000 patients about their telehealth experiences during the pandemic reported that over 75% were very or completely satisfied with their virtual care experiences and over 50% indicated they would be willing to switch providers to have virtual visits on a regular basis (Patient Perspectives on Virtual Care Report, Accessed July 7, 2020, https://www.kyruus.com/2020-virtual-care-report).
One hopes that with time and on-going feedback, the fundamental purpose of the physician-patient relationship can be maintained and both sides can still appreciate the conveniences and power of telehealth technology.
Dr. Fedyna and Dr. McGroder are affiliated with the Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University Medical Center, New York, NY.
Big data scientists and health-care experts have tried preparing physicians and patients for the arrival of telemedicine for years. Health tracking applications are on our smartphones. Compact ambulatory devices diagnose hypertension and atrial fibrillation. Advanced imaging modalities make the stethoscope more of a neck accessory than a practical tool. Despite these efficient technologic advancements, the idea of making the sacred in-person office visit remote and through a screen appealed to few. In fact, prior to the COVID-19 pandemic, only 15% of medical practices offered telehealth services and 8% of Americans joined in remote visits annually (Mann DM et al. J Am Med Inform Assoc. 2019 Feb 1;26[2]:106-114).
When the COVID-19 pandemic hit New York City and admissions for hypoxemic respiratory failure skyrocketed, ED and in-person clinic visits for other acute and chronic conditions plummeted. Prior to clinics officially closing their doors, doctors in New York City asked their patients to reserve office visits for emergency issues only ,with most patients willingly staying home to avoid exposure to the virus. Suddenly, after years of disinterest in adopting telehealth, hospitals and clinics were catapulted into a full-on need for this technology. Overnight, our division’s secretaries and medical assistants became IT support staff. We all learned together what worked, what didn’t work, and how to adapt our workflow to meet everyone’s needs.
Previously, longstanding issues with accessibility and reimbursement presented barriers to widespread adoption of telemedicine. Once the pandemic hit, though, many regulatory changes were quickly made to accommodate telehealth.
Three such changes are worth highlighting (Centers for Medicare and Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. March 30, 2020).
First, patient privacy rules became more lenient. Prior to the pandemic, HIPAA mandated that both doctor and patient use embedded video interfaces with high levels of security. Now, health-care providers can use commonplace video chat applications such as FaceTime, Google Hangouts, Zoom, or Skype to provide telehealth without risk of penalty for HIPAA noncompliance. When connectivity concerns arose with our EMR’s embedded telehealth application, a quick transition to one of these platforms mitigated patient and provider frustration.
Second, prior to the pandemic, some private insurance providers reimbursed for televisits, but there were stipulations on how the visit could be conducted. Now, many of the commercial insurers plus Medicare and Medicaid in New York State reimburse the same amount for televisits as in-person visits (fee-for-service rate). Reimbursement rates of audio-only encounters were increased. If these changes are continued postpandemic, it will have an expansive impact on the future of an outpatient practice.
Third, restrictive government regulations relaxed with regard to telehealth deployment. Gone are the demands on providers and patients to be physically face-to-face. Many colleagues worked from home, safely social distancing.
Even though remote medical visits were a crucial part of flattening the curve during the peak of the pandemic in New York City, the telehealth experience is not without flaws.
An informal survey of providers in our own division garnered diverse and spirited viewpoints about seeing patients remotely. Instead of using a stethoscope to pick up a subtle finding, telehealth visits require the use of our eyes to scan a patient’s home environment for insights explaining their chronic cough (Where is the mold? Where is the water damage? Where is the bird?). We use our ears to hear the intonation of our patient’s voice to know when he or she is concerned, anxious, or are at their baselines. We would implore patients to put on their pulse oximeter and perform activities of daily living and/or exertion. On multiple occasions, patients would perform their own, unsolicited walks about their home to show us what they could and couldn’t do, where they place their concentrators, and where they are likely to trip over oxygen tubing. We learned to depend on them to reach the conclusion that they were at their normal state of health.
For straight-forward encounters with existing patients, most of our colleagues appreciated the simplicity and efficiency of telemedicine. But when it came to new patients, some colleagues struggled with whether they should see them for the first time over video. Universally, providers felt feelings of inadequacy without an in-person examination and review of diagnostic information.
Along those lines, many of our colleagues worried about their ability to perform the most fundamental role of a physician over the phone/internet for all patients: building trust with a patient. Eye contact, the physical exam, and verbal and nonv
Providers also noted that telehealth implementation is not the same for all individuals. Just as COVID-19 disproportionately affects the most vulnerable populations (NYC Health. COVID-19: data. Accessed July 1, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page), practicing telehealth has uncovered more ways in which racial/ethnic minorities, low income communities, and older patients are at a disadvantage (Garg S, et al. MMWR Morb Mortal Wkly Rep. 2020;69[15]:458). The relatively quick transition to telemedicine revealed that many of our patients don’t have emails or home computers to connect with online platforms. Similarly, some do not have smart phones with internet capabilities. Many do not speak English and cannot partake in video visits since translators are not yet embedded into the EMR’s video system. Elderly patients were frequently very anxious with telemedicine because of unfamiliarity with the technology, and many preferred a phone conversation. Thus, while more fortunate patients get to use a video interface and its association with higher patient understanding and satisfaction, our most vulnerable populations are often denied the same access to such care (Voils CI et al. J Genet Couns. 2018;27[2]:339).
Telemedicine will continue to have a significant impact on the future of health care long after the COVID-19 pandemic abates. There will be growing pains, refinement of technology, improvements in policy, and an ongoing general evolution of the system. Patients and providers will grow together as its utilization continues. We suspect patient surveys about their attitudes and preferences for telemedicine will be as varied as the providers surveyed here. A recent survey of 1000 patients about their telehealth experiences during the pandemic reported that over 75% were very or completely satisfied with their virtual care experiences and over 50% indicated they would be willing to switch providers to have virtual visits on a regular basis (Patient Perspectives on Virtual Care Report, Accessed July 7, 2020, https://www.kyruus.com/2020-virtual-care-report).
One hopes that with time and on-going feedback, the fundamental purpose of the physician-patient relationship can be maintained and both sides can still appreciate the conveniences and power of telehealth technology.
Dr. Fedyna and Dr. McGroder are affiliated with the Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University Medical Center, New York, NY.
Big data scientists and health-care experts have tried preparing physicians and patients for the arrival of telemedicine for years. Health tracking applications are on our smartphones. Compact ambulatory devices diagnose hypertension and atrial fibrillation. Advanced imaging modalities make the stethoscope more of a neck accessory than a practical tool. Despite these efficient technologic advancements, the idea of making the sacred in-person office visit remote and through a screen appealed to few. In fact, prior to the COVID-19 pandemic, only 15% of medical practices offered telehealth services and 8% of Americans joined in remote visits annually (Mann DM et al. J Am Med Inform Assoc. 2019 Feb 1;26[2]:106-114).
When the COVID-19 pandemic hit New York City and admissions for hypoxemic respiratory failure skyrocketed, ED and in-person clinic visits for other acute and chronic conditions plummeted. Prior to clinics officially closing their doors, doctors in New York City asked their patients to reserve office visits for emergency issues only ,with most patients willingly staying home to avoid exposure to the virus. Suddenly, after years of disinterest in adopting telehealth, hospitals and clinics were catapulted into a full-on need for this technology. Overnight, our division’s secretaries and medical assistants became IT support staff. We all learned together what worked, what didn’t work, and how to adapt our workflow to meet everyone’s needs.
Previously, longstanding issues with accessibility and reimbursement presented barriers to widespread adoption of telemedicine. Once the pandemic hit, though, many regulatory changes were quickly made to accommodate telehealth.
Three such changes are worth highlighting (Centers for Medicare and Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. March 30, 2020).
First, patient privacy rules became more lenient. Prior to the pandemic, HIPAA mandated that both doctor and patient use embedded video interfaces with high levels of security. Now, health-care providers can use commonplace video chat applications such as FaceTime, Google Hangouts, Zoom, or Skype to provide telehealth without risk of penalty for HIPAA noncompliance. When connectivity concerns arose with our EMR’s embedded telehealth application, a quick transition to one of these platforms mitigated patient and provider frustration.
Second, prior to the pandemic, some private insurance providers reimbursed for televisits, but there were stipulations on how the visit could be conducted. Now, many of the commercial insurers plus Medicare and Medicaid in New York State reimburse the same amount for televisits as in-person visits (fee-for-service rate). Reimbursement rates of audio-only encounters were increased. If these changes are continued postpandemic, it will have an expansive impact on the future of an outpatient practice.
Third, restrictive government regulations relaxed with regard to telehealth deployment. Gone are the demands on providers and patients to be physically face-to-face. Many colleagues worked from home, safely social distancing.
Even though remote medical visits were a crucial part of flattening the curve during the peak of the pandemic in New York City, the telehealth experience is not without flaws.
An informal survey of providers in our own division garnered diverse and spirited viewpoints about seeing patients remotely. Instead of using a stethoscope to pick up a subtle finding, telehealth visits require the use of our eyes to scan a patient’s home environment for insights explaining their chronic cough (Where is the mold? Where is the water damage? Where is the bird?). We use our ears to hear the intonation of our patient’s voice to know when he or she is concerned, anxious, or are at their baselines. We would implore patients to put on their pulse oximeter and perform activities of daily living and/or exertion. On multiple occasions, patients would perform their own, unsolicited walks about their home to show us what they could and couldn’t do, where they place their concentrators, and where they are likely to trip over oxygen tubing. We learned to depend on them to reach the conclusion that they were at their normal state of health.
For straight-forward encounters with existing patients, most of our colleagues appreciated the simplicity and efficiency of telemedicine. But when it came to new patients, some colleagues struggled with whether they should see them for the first time over video. Universally, providers felt feelings of inadequacy without an in-person examination and review of diagnostic information.
Along those lines, many of our colleagues worried about their ability to perform the most fundamental role of a physician over the phone/internet for all patients: building trust with a patient. Eye contact, the physical exam, and verbal and nonv
Providers also noted that telehealth implementation is not the same for all individuals. Just as COVID-19 disproportionately affects the most vulnerable populations (NYC Health. COVID-19: data. Accessed July 1, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page), practicing telehealth has uncovered more ways in which racial/ethnic minorities, low income communities, and older patients are at a disadvantage (Garg S, et al. MMWR Morb Mortal Wkly Rep. 2020;69[15]:458). The relatively quick transition to telemedicine revealed that many of our patients don’t have emails or home computers to connect with online platforms. Similarly, some do not have smart phones with internet capabilities. Many do not speak English and cannot partake in video visits since translators are not yet embedded into the EMR’s video system. Elderly patients were frequently very anxious with telemedicine because of unfamiliarity with the technology, and many preferred a phone conversation. Thus, while more fortunate patients get to use a video interface and its association with higher patient understanding and satisfaction, our most vulnerable populations are often denied the same access to such care (Voils CI et al. J Genet Couns. 2018;27[2]:339).
Telemedicine will continue to have a significant impact on the future of health care long after the COVID-19 pandemic abates. There will be growing pains, refinement of technology, improvements in policy, and an ongoing general evolution of the system. Patients and providers will grow together as its utilization continues. We suspect patient surveys about their attitudes and preferences for telemedicine will be as varied as the providers surveyed here. A recent survey of 1000 patients about their telehealth experiences during the pandemic reported that over 75% were very or completely satisfied with their virtual care experiences and over 50% indicated they would be willing to switch providers to have virtual visits on a regular basis (Patient Perspectives on Virtual Care Report, Accessed July 7, 2020, https://www.kyruus.com/2020-virtual-care-report).
One hopes that with time and on-going feedback, the fundamental purpose of the physician-patient relationship can be maintained and both sides can still appreciate the conveniences and power of telehealth technology.
Dr. Fedyna and Dr. McGroder are affiliated with the Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University Medical Center, New York, NY.
Management of EVALI in the ICU
Since 2019, more than 2,700 individuals have been hospitalized with electronic cigarette- (e-cigarette), or vaping-associated lung injury (EVALI). This entity first reached clinical attention after a series of otherwise healthy young adults presented with dyspnea, severe hypoxia, and diffuse pulmonary infiltrates in the Midwest (Layden J, et al. N Engl J Med. 2020;382[10]:903). Investigation of these cases revealed an association with the use of e-cigarettes, or vaping. As cases continued to mount, the link between vaping and acute lung injury became increasingly apparent.
How it presents
EVALI can present in variable ways, ranging from mild cough or dyspnea without hypoxia to severe acute respiratory distress syndrome (ARDS), requiring advanced life support. Although challenging in the ICU setting, obtaining a detailed history of vaping is crucial to make the diagnosis. Collateral history can be helpful, but if unrevealing, it should not be considered sufficient to exclude vaping as potential etiology, particularly in adolescent e-cigarette users, where parental awareness of substance use history may be limited. If a vaping history is obtained, it is important to assess the substance(s) vaped, how these substances were obtained, and methods of inhalation. While e-cigarettes are the most commonly recognized method of vaping, alternate methods such as “dabbing” and “dripping,” are increasingly popular among vape users, often utilizing modified e-liquid components that may not be reported by patients unless specifically queried.
About 82% of patients hospitalized with EVALI reported vaping tetrahydrocannabinol- (THC) containing fluid. This is important because, unlike nicotine based e-liquids that are primarily purchased over the counter, more than 70% of THC-containing e-liquids are reportedly obtained through informal sources, including illegal distributors. In contrast, only 14% of patients hospitalized with EVALI reported vaping of commercial nicotine products alone. Nicotine-based e-liquids can also be modified, and informal purchasing sources remain a concern, particularly among younger users.
The onset of respiratory symptoms in EVALI is often preceded by several days of a systemic prodrome, including low-grade fevers, myalgia, gastrointestinal complaints, and fatigue (MacMurdo M, et al. Chest. 2020;157[6]:e181). The diagnosis of EVALI is made clinically, and alternative etiologies of lung injury (eg, infections) should be excluded. As there is significant overlap between the presenting symptoms of EVALI and COVID-19 infection, patients should be tested for COVID-19 before a diagnosis of EVALI can be made.
Imaging patterns of EVALI include diffuse alveolar damage (the most common), comprising of diffuse ground-glass opacities, septal thickening, and heterogeneous consolidation (MacMurdo M, et al. Chest. 2020;157[6]:e181). Bilateral ground glass opacities suggestive of organizing pneumonia have also been described. Atypical patterns of nodularity suggestive of hypersensitivity pneumonitis are significantly less common. Given the variety of imaging patterns, EVALI should be considered as a differential diagnosis in all patients presenting with new bilateral pulmonary infiltrates and severe hypoxia.
Early evaluation of these patients revealed lipid-laden macrophages in the bronchoalveolar lavage (BAL) fluid of these patients, raising concern for exogenous lipid inhalation resulting in the development of lipoid pneumonia (Maddock SD, et al. N Engl J Med. 2019;381[15]:1488). Analysis of BAL fluid revealed the presence of vitamin E acetate, a diluent utilized to cut, or dilute, e-liquid (Blount BC, et al. MMWR. 2019;68[45]:1040). This supported the hypothesis that the outbreak of EVALI was being driven, at least in part, by contaminated or self-modified e-liquid. Evaluation of lung biopsies revealed different pathologic patterns of acute lung injury, including diffuse alveolar damage and organizing pneumonia. Importantly, while lipid-laden macrophages were detected, other characteristics of lipoid pneumonia were absent (Mukhopadhyay S, et al. Am J Clin Path. 2019;153[1]30).
How to manage EVALI
Approximately half of patients hospitalized with EVALI required ICU admission. However, there is likely a substantial portion of patients with mild disease who may not be represented in the current registry since they did not require hospitalization. The management is primarily supportive and, in patients who require mechanical ventilation, following lung-protective ventilator strategies is of paramount importance. Steroids have been used in some case series, particularly for patients presenting with more severe disease, but data on benefit, optimal dose, and duration are limited.
Vaping cessation is crucial and should be aggressively encouraged. Newer generations of e-cigarettes contain comparatively higher nicotine concentrations, and likely have high potential for nicotine addiction. Treatment for nicotine dependence, including pharmacologic therapy, needs to be considered in all patients following recovery from EVALI.
With supportive care and removal of ongoing exposure, recovery is anticipated in most patients. Long-term outcomes in patients who develop EVALI remain unclear. Although early fibrosis was present in some patients who had transbronchial biopsies, the long-term effects on pulmonary function that may be seen in patients with a history of EVALI are yet to be determined.
What about policy?
New regulations related to e-cigarette use have been proposed in response to the increasing prevalence of vaping and the EVALI outbreak. These regulations center primarily on limiting adolescent e-cigarette usage. Tobacco 21, federal legislation passed in 2019, makes it illegal to sell tobacco products to those under the age of 21. The FDA also issued an enforcement policy on unauthorized flavored e-cigarette products. However, this has been criticized for not being comprehensive enough. For example, tobacco and menthol flavors were not included in the ban. Furthermore, THC-containing e-liquid remains largely unregulated at the federal level, and state-level regulation varies significantly by marijuana legalization status.
Policy initiatives that restrict sales without also addressing drivers of e-cigarette use, such as nicotine dependence and aggressive marketing campaigns, are of particular concern and are likely to disproportionately impact younger users. Another unintended effect of e-cigarette sales restrictions may result in a new wave of illegal product distribution and e-liquid modification. Supporting this hypothesis was the finding that the risk of EVALI was higher in states without legalized recreational marijuana, suggesting that users who obtained e-liquid through these informal sources were at greater risk of exposure to contaminated product (Wing C, et al. JAMA Netw Open. 2020;3[4]:e202187). While the CDC is no longer actively tracking EVALI cases, they continue to be reported, and vape use remains common (Armatas C, et al. MMWR. 69[25]:801). As long as e-cigarettes remain in use, another EVALI outbreak remains possible.
It remains important for the intensivist to be familiar with the full spectrum of vaping methods, and to report suspected cases when they arise. While treatable, much remains unknown about the long-term effects on this patient population. Further research is needed to better understand the long-term outcomes in patients with EVALI, in addition to the treatment of nicotine dependence and substance use associated with vaping. Finally, comprehensive regulation to curb e-cigarette usage is needed, particularly among adolescents. However, legislation that is too narrow in scope runs the risk of channeling adolescent e-cigarette users to obtain product through informal sources, further increasing their risk for EVALI. As clinicians, we cannot afford to drop our guard!
Dr. Macmurdo and Dr. Choi are with Cleveland Clinic, Respiratory Institute, Cleveland, Ohio.
Since 2019, more than 2,700 individuals have been hospitalized with electronic cigarette- (e-cigarette), or vaping-associated lung injury (EVALI). This entity first reached clinical attention after a series of otherwise healthy young adults presented with dyspnea, severe hypoxia, and diffuse pulmonary infiltrates in the Midwest (Layden J, et al. N Engl J Med. 2020;382[10]:903). Investigation of these cases revealed an association with the use of e-cigarettes, or vaping. As cases continued to mount, the link between vaping and acute lung injury became increasingly apparent.
How it presents
EVALI can present in variable ways, ranging from mild cough or dyspnea without hypoxia to severe acute respiratory distress syndrome (ARDS), requiring advanced life support. Although challenging in the ICU setting, obtaining a detailed history of vaping is crucial to make the diagnosis. Collateral history can be helpful, but if unrevealing, it should not be considered sufficient to exclude vaping as potential etiology, particularly in adolescent e-cigarette users, where parental awareness of substance use history may be limited. If a vaping history is obtained, it is important to assess the substance(s) vaped, how these substances were obtained, and methods of inhalation. While e-cigarettes are the most commonly recognized method of vaping, alternate methods such as “dabbing” and “dripping,” are increasingly popular among vape users, often utilizing modified e-liquid components that may not be reported by patients unless specifically queried.
About 82% of patients hospitalized with EVALI reported vaping tetrahydrocannabinol- (THC) containing fluid. This is important because, unlike nicotine based e-liquids that are primarily purchased over the counter, more than 70% of THC-containing e-liquids are reportedly obtained through informal sources, including illegal distributors. In contrast, only 14% of patients hospitalized with EVALI reported vaping of commercial nicotine products alone. Nicotine-based e-liquids can also be modified, and informal purchasing sources remain a concern, particularly among younger users.
The onset of respiratory symptoms in EVALI is often preceded by several days of a systemic prodrome, including low-grade fevers, myalgia, gastrointestinal complaints, and fatigue (MacMurdo M, et al. Chest. 2020;157[6]:e181). The diagnosis of EVALI is made clinically, and alternative etiologies of lung injury (eg, infections) should be excluded. As there is significant overlap between the presenting symptoms of EVALI and COVID-19 infection, patients should be tested for COVID-19 before a diagnosis of EVALI can be made.
Imaging patterns of EVALI include diffuse alveolar damage (the most common), comprising of diffuse ground-glass opacities, septal thickening, and heterogeneous consolidation (MacMurdo M, et al. Chest. 2020;157[6]:e181). Bilateral ground glass opacities suggestive of organizing pneumonia have also been described. Atypical patterns of nodularity suggestive of hypersensitivity pneumonitis are significantly less common. Given the variety of imaging patterns, EVALI should be considered as a differential diagnosis in all patients presenting with new bilateral pulmonary infiltrates and severe hypoxia.
Early evaluation of these patients revealed lipid-laden macrophages in the bronchoalveolar lavage (BAL) fluid of these patients, raising concern for exogenous lipid inhalation resulting in the development of lipoid pneumonia (Maddock SD, et al. N Engl J Med. 2019;381[15]:1488). Analysis of BAL fluid revealed the presence of vitamin E acetate, a diluent utilized to cut, or dilute, e-liquid (Blount BC, et al. MMWR. 2019;68[45]:1040). This supported the hypothesis that the outbreak of EVALI was being driven, at least in part, by contaminated or self-modified e-liquid. Evaluation of lung biopsies revealed different pathologic patterns of acute lung injury, including diffuse alveolar damage and organizing pneumonia. Importantly, while lipid-laden macrophages were detected, other characteristics of lipoid pneumonia were absent (Mukhopadhyay S, et al. Am J Clin Path. 2019;153[1]30).
How to manage EVALI
Approximately half of patients hospitalized with EVALI required ICU admission. However, there is likely a substantial portion of patients with mild disease who may not be represented in the current registry since they did not require hospitalization. The management is primarily supportive and, in patients who require mechanical ventilation, following lung-protective ventilator strategies is of paramount importance. Steroids have been used in some case series, particularly for patients presenting with more severe disease, but data on benefit, optimal dose, and duration are limited.
Vaping cessation is crucial and should be aggressively encouraged. Newer generations of e-cigarettes contain comparatively higher nicotine concentrations, and likely have high potential for nicotine addiction. Treatment for nicotine dependence, including pharmacologic therapy, needs to be considered in all patients following recovery from EVALI.
With supportive care and removal of ongoing exposure, recovery is anticipated in most patients. Long-term outcomes in patients who develop EVALI remain unclear. Although early fibrosis was present in some patients who had transbronchial biopsies, the long-term effects on pulmonary function that may be seen in patients with a history of EVALI are yet to be determined.
What about policy?
New regulations related to e-cigarette use have been proposed in response to the increasing prevalence of vaping and the EVALI outbreak. These regulations center primarily on limiting adolescent e-cigarette usage. Tobacco 21, federal legislation passed in 2019, makes it illegal to sell tobacco products to those under the age of 21. The FDA also issued an enforcement policy on unauthorized flavored e-cigarette products. However, this has been criticized for not being comprehensive enough. For example, tobacco and menthol flavors were not included in the ban. Furthermore, THC-containing e-liquid remains largely unregulated at the federal level, and state-level regulation varies significantly by marijuana legalization status.
Policy initiatives that restrict sales without also addressing drivers of e-cigarette use, such as nicotine dependence and aggressive marketing campaigns, are of particular concern and are likely to disproportionately impact younger users. Another unintended effect of e-cigarette sales restrictions may result in a new wave of illegal product distribution and e-liquid modification. Supporting this hypothesis was the finding that the risk of EVALI was higher in states without legalized recreational marijuana, suggesting that users who obtained e-liquid through these informal sources were at greater risk of exposure to contaminated product (Wing C, et al. JAMA Netw Open. 2020;3[4]:e202187). While the CDC is no longer actively tracking EVALI cases, they continue to be reported, and vape use remains common (Armatas C, et al. MMWR. 69[25]:801). As long as e-cigarettes remain in use, another EVALI outbreak remains possible.
It remains important for the intensivist to be familiar with the full spectrum of vaping methods, and to report suspected cases when they arise. While treatable, much remains unknown about the long-term effects on this patient population. Further research is needed to better understand the long-term outcomes in patients with EVALI, in addition to the treatment of nicotine dependence and substance use associated with vaping. Finally, comprehensive regulation to curb e-cigarette usage is needed, particularly among adolescents. However, legislation that is too narrow in scope runs the risk of channeling adolescent e-cigarette users to obtain product through informal sources, further increasing their risk for EVALI. As clinicians, we cannot afford to drop our guard!
Dr. Macmurdo and Dr. Choi are with Cleveland Clinic, Respiratory Institute, Cleveland, Ohio.
Since 2019, more than 2,700 individuals have been hospitalized with electronic cigarette- (e-cigarette), or vaping-associated lung injury (EVALI). This entity first reached clinical attention after a series of otherwise healthy young adults presented with dyspnea, severe hypoxia, and diffuse pulmonary infiltrates in the Midwest (Layden J, et al. N Engl J Med. 2020;382[10]:903). Investigation of these cases revealed an association with the use of e-cigarettes, or vaping. As cases continued to mount, the link between vaping and acute lung injury became increasingly apparent.
How it presents
EVALI can present in variable ways, ranging from mild cough or dyspnea without hypoxia to severe acute respiratory distress syndrome (ARDS), requiring advanced life support. Although challenging in the ICU setting, obtaining a detailed history of vaping is crucial to make the diagnosis. Collateral history can be helpful, but if unrevealing, it should not be considered sufficient to exclude vaping as potential etiology, particularly in adolescent e-cigarette users, where parental awareness of substance use history may be limited. If a vaping history is obtained, it is important to assess the substance(s) vaped, how these substances were obtained, and methods of inhalation. While e-cigarettes are the most commonly recognized method of vaping, alternate methods such as “dabbing” and “dripping,” are increasingly popular among vape users, often utilizing modified e-liquid components that may not be reported by patients unless specifically queried.
About 82% of patients hospitalized with EVALI reported vaping tetrahydrocannabinol- (THC) containing fluid. This is important because, unlike nicotine based e-liquids that are primarily purchased over the counter, more than 70% of THC-containing e-liquids are reportedly obtained through informal sources, including illegal distributors. In contrast, only 14% of patients hospitalized with EVALI reported vaping of commercial nicotine products alone. Nicotine-based e-liquids can also be modified, and informal purchasing sources remain a concern, particularly among younger users.
The onset of respiratory symptoms in EVALI is often preceded by several days of a systemic prodrome, including low-grade fevers, myalgia, gastrointestinal complaints, and fatigue (MacMurdo M, et al. Chest. 2020;157[6]:e181). The diagnosis of EVALI is made clinically, and alternative etiologies of lung injury (eg, infections) should be excluded. As there is significant overlap between the presenting symptoms of EVALI and COVID-19 infection, patients should be tested for COVID-19 before a diagnosis of EVALI can be made.
Imaging patterns of EVALI include diffuse alveolar damage (the most common), comprising of diffuse ground-glass opacities, septal thickening, and heterogeneous consolidation (MacMurdo M, et al. Chest. 2020;157[6]:e181). Bilateral ground glass opacities suggestive of organizing pneumonia have also been described. Atypical patterns of nodularity suggestive of hypersensitivity pneumonitis are significantly less common. Given the variety of imaging patterns, EVALI should be considered as a differential diagnosis in all patients presenting with new bilateral pulmonary infiltrates and severe hypoxia.
Early evaluation of these patients revealed lipid-laden macrophages in the bronchoalveolar lavage (BAL) fluid of these patients, raising concern for exogenous lipid inhalation resulting in the development of lipoid pneumonia (Maddock SD, et al. N Engl J Med. 2019;381[15]:1488). Analysis of BAL fluid revealed the presence of vitamin E acetate, a diluent utilized to cut, or dilute, e-liquid (Blount BC, et al. MMWR. 2019;68[45]:1040). This supported the hypothesis that the outbreak of EVALI was being driven, at least in part, by contaminated or self-modified e-liquid. Evaluation of lung biopsies revealed different pathologic patterns of acute lung injury, including diffuse alveolar damage and organizing pneumonia. Importantly, while lipid-laden macrophages were detected, other characteristics of lipoid pneumonia were absent (Mukhopadhyay S, et al. Am J Clin Path. 2019;153[1]30).
How to manage EVALI
Approximately half of patients hospitalized with EVALI required ICU admission. However, there is likely a substantial portion of patients with mild disease who may not be represented in the current registry since they did not require hospitalization. The management is primarily supportive and, in patients who require mechanical ventilation, following lung-protective ventilator strategies is of paramount importance. Steroids have been used in some case series, particularly for patients presenting with more severe disease, but data on benefit, optimal dose, and duration are limited.
Vaping cessation is crucial and should be aggressively encouraged. Newer generations of e-cigarettes contain comparatively higher nicotine concentrations, and likely have high potential for nicotine addiction. Treatment for nicotine dependence, including pharmacologic therapy, needs to be considered in all patients following recovery from EVALI.
With supportive care and removal of ongoing exposure, recovery is anticipated in most patients. Long-term outcomes in patients who develop EVALI remain unclear. Although early fibrosis was present in some patients who had transbronchial biopsies, the long-term effects on pulmonary function that may be seen in patients with a history of EVALI are yet to be determined.
What about policy?
New regulations related to e-cigarette use have been proposed in response to the increasing prevalence of vaping and the EVALI outbreak. These regulations center primarily on limiting adolescent e-cigarette usage. Tobacco 21, federal legislation passed in 2019, makes it illegal to sell tobacco products to those under the age of 21. The FDA also issued an enforcement policy on unauthorized flavored e-cigarette products. However, this has been criticized for not being comprehensive enough. For example, tobacco and menthol flavors were not included in the ban. Furthermore, THC-containing e-liquid remains largely unregulated at the federal level, and state-level regulation varies significantly by marijuana legalization status.
Policy initiatives that restrict sales without also addressing drivers of e-cigarette use, such as nicotine dependence and aggressive marketing campaigns, are of particular concern and are likely to disproportionately impact younger users. Another unintended effect of e-cigarette sales restrictions may result in a new wave of illegal product distribution and e-liquid modification. Supporting this hypothesis was the finding that the risk of EVALI was higher in states without legalized recreational marijuana, suggesting that users who obtained e-liquid through these informal sources were at greater risk of exposure to contaminated product (Wing C, et al. JAMA Netw Open. 2020;3[4]:e202187). While the CDC is no longer actively tracking EVALI cases, they continue to be reported, and vape use remains common (Armatas C, et al. MMWR. 69[25]:801). As long as e-cigarettes remain in use, another EVALI outbreak remains possible.
It remains important for the intensivist to be familiar with the full spectrum of vaping methods, and to report suspected cases when they arise. While treatable, much remains unknown about the long-term effects on this patient population. Further research is needed to better understand the long-term outcomes in patients with EVALI, in addition to the treatment of nicotine dependence and substance use associated with vaping. Finally, comprehensive regulation to curb e-cigarette usage is needed, particularly among adolescents. However, legislation that is too narrow in scope runs the risk of channeling adolescent e-cigarette users to obtain product through informal sources, further increasing their risk for EVALI. As clinicians, we cannot afford to drop our guard!
Dr. Macmurdo and Dr. Choi are with Cleveland Clinic, Respiratory Institute, Cleveland, Ohio.
NetWorks Challenge 2020
The CHEST Foundation is excited to announce that the NetWorks Challenge will be reinvented for 2020! Instead of raising funds to support travel grants to CHEST’s Annual Meeting as in previous years, the NetWorks Challenge will focus on raising funds to support COVID-19 community service grants. With so many people suffering due to the pandemic, we believe this change will make a tangible impact on the lives of people who need it most.
To date, the CHEST Foundation has dispersed over $60,000 in payments for patient support groups that provide services to those living with chronic lung disease, and we hope this year’s efforts will enable us to continue this work. For every $2,500 raised by a NetWork, the CHEST Foundation will provide a grant to a community support group in need.
While providing vulnerable populations with funds to purchase essential items (PPE, cleaning supplies, emergency food purchases, etc), each grant will be named in honor of the NetWork raising the funds, and all stories of impact will be shared with NetWorks’ members, once they are available.
The NetWorks Challenge spans from Monday, July 20, to the end of Board Review on August 22, and members can easily designate their donation to their NetWork on the CHEST Foundation’s donor page.
In addition to receiving named recognition of your NetWork, the NetWork that raises the most funds, along with the NetWork with the highest percentage of participation, will receive additional prizes, including two complimentary registrations to CHEST 2020. These registrations are specifically for early-career clinicians and fellows-in-training, which will be selected by each NetWorks’s steering committee.
For every $5,000 raised by a NetWork, that NetWork will receive one complimentary registration to CHEST 2020, which will be awarded to their early-career and fellows-in-training as selected by that NetWorks’s steering committee.
In addition to directly impacting patients across the United States, NetWorks members will have a chance to test their knowledge against their peers by participating in a NetWork Challenge Game Series, where they will be asked a series of hand-selected board review questions each week through the end of Board Review.
For additional Information about the NetWorks Challenge, visit the CHEST Foundation’s website.
The CHEST Foundation is excited to announce that the NetWorks Challenge will be reinvented for 2020! Instead of raising funds to support travel grants to CHEST’s Annual Meeting as in previous years, the NetWorks Challenge will focus on raising funds to support COVID-19 community service grants. With so many people suffering due to the pandemic, we believe this change will make a tangible impact on the lives of people who need it most.
To date, the CHEST Foundation has dispersed over $60,000 in payments for patient support groups that provide services to those living with chronic lung disease, and we hope this year’s efforts will enable us to continue this work. For every $2,500 raised by a NetWork, the CHEST Foundation will provide a grant to a community support group in need.
While providing vulnerable populations with funds to purchase essential items (PPE, cleaning supplies, emergency food purchases, etc), each grant will be named in honor of the NetWork raising the funds, and all stories of impact will be shared with NetWorks’ members, once they are available.
The NetWorks Challenge spans from Monday, July 20, to the end of Board Review on August 22, and members can easily designate their donation to their NetWork on the CHEST Foundation’s donor page.
In addition to receiving named recognition of your NetWork, the NetWork that raises the most funds, along with the NetWork with the highest percentage of participation, will receive additional prizes, including two complimentary registrations to CHEST 2020. These registrations are specifically for early-career clinicians and fellows-in-training, which will be selected by each NetWorks’s steering committee.
For every $5,000 raised by a NetWork, that NetWork will receive one complimentary registration to CHEST 2020, which will be awarded to their early-career and fellows-in-training as selected by that NetWorks’s steering committee.
In addition to directly impacting patients across the United States, NetWorks members will have a chance to test their knowledge against their peers by participating in a NetWork Challenge Game Series, where they will be asked a series of hand-selected board review questions each week through the end of Board Review.
For additional Information about the NetWorks Challenge, visit the CHEST Foundation’s website.
The CHEST Foundation is excited to announce that the NetWorks Challenge will be reinvented for 2020! Instead of raising funds to support travel grants to CHEST’s Annual Meeting as in previous years, the NetWorks Challenge will focus on raising funds to support COVID-19 community service grants. With so many people suffering due to the pandemic, we believe this change will make a tangible impact on the lives of people who need it most.
To date, the CHEST Foundation has dispersed over $60,000 in payments for patient support groups that provide services to those living with chronic lung disease, and we hope this year’s efforts will enable us to continue this work. For every $2,500 raised by a NetWork, the CHEST Foundation will provide a grant to a community support group in need.
While providing vulnerable populations with funds to purchase essential items (PPE, cleaning supplies, emergency food purchases, etc), each grant will be named in honor of the NetWork raising the funds, and all stories of impact will be shared with NetWorks’ members, once they are available.
The NetWorks Challenge spans from Monday, July 20, to the end of Board Review on August 22, and members can easily designate their donation to their NetWork on the CHEST Foundation’s donor page.
In addition to receiving named recognition of your NetWork, the NetWork that raises the most funds, along with the NetWork with the highest percentage of participation, will receive additional prizes, including two complimentary registrations to CHEST 2020. These registrations are specifically for early-career clinicians and fellows-in-training, which will be selected by each NetWorks’s steering committee.
For every $5,000 raised by a NetWork, that NetWork will receive one complimentary registration to CHEST 2020, which will be awarded to their early-career and fellows-in-training as selected by that NetWorks’s steering committee.
In addition to directly impacting patients across the United States, NetWorks members will have a chance to test their knowledge against their peers by participating in a NetWork Challenge Game Series, where they will be asked a series of hand-selected board review questions each week through the end of Board Review.
For additional Information about the NetWorks Challenge, visit the CHEST Foundation’s website.
News from the Board of Regents: Progress during a pandemic – June 2020
The Board of Regents met remotely in June because of ongoing travel restrictions and safety concerns for staff and board members.
• The meeting was opened with Stephanie Levine, President; Steve Simpson, President-Elect; and Robert Musacchio, CEO/EVP discussing the impacts of the COVID-19 pandemic and Business Continuity Planning. The COVID-19 Task Force, chaired by Steve Simpson, continues to meet weekly to identify emerging content needs toward supporting membership and their patients through the pandemic, connecting with the Education Committee and Foundation to ensure robust coverage, drawing on the expertise of the NetWorks for content development, and leveraging the Social Media Workgroup for dissemination. Key activities include: a regular Thursday webinar series at 3:00 pm CDT titled: “Advice From the Front Lines”; clinical resources in the form of infographics and guides are posted in the resource center and circulated through social media; Alex Niven, MD FCCP, led a team to develop a wellness curriculum and series; the CHEST Foundation developed patient education videos and guides, a public service announcement in partnership with the American Thoracic Society, and a pilot partnership with AMITA Health enabling access to telehealth.
• The Finance Committee, chaired by John Howington, reported that CHEST is on track to meet its budget and exceed its debt covenants and operating reserve policy for the current fiscal year. The record attendance at the October 2019 annual meeting, along with strong performance from our digital offerings offset the financial impacts of the global pandemic. Bob Musacchio, CEO/EVP, reminded the Board why CHEST is switching from a fiscal year to calendar year budget. A calendar year budget process creates better alignment with budgets of pharma, other clients, and vendors; facilitates various accruals that are based on the calendar year, such as benefits, vacation, sick, and PTO days; provides for greater continuity for doing business throughout the year, and permits more planning time for staff in setting individual goals related to the annual meeting.
• CHEST’S Digital Transformation strategy that kicked off in 2019 was timely considering the pandemic. With education as one of our main foci, CHEST has hired and onboarded a Chief Learning Officer, Jim Young, to actively examine how we develop and deploy our educational products and services. Our first movement toward remote meetings occurred on June 26 with the Virtual Congress originally slated for Bologna, Italy. Here, we piloted a new platform and brought to life the tenets established in the new learning strategy—providing choice, demonstrating responsiveness, and fostering connection.
• CHEST’s Governance Committee reviewed the College bylaws for revisions, as per the group’s practice every 2-3 years, and the Board approved the revisions to the bylaws as proposed by the committee.
• CHEST’s newly formed Health Policy and Advocacy Committee (HPAC), chaired by Neil Freedman, MD, FCCP, is holding monthly meetings with a goal of making a recommendation to the Board of Regents on CHEST’s regulatory and policy priorities during the August meeting. The HPAC assists CHEST leadership and the BOR in developing and implementing health policy positions, setting chest advocacy agendas in the legislative and regulatory arenas, engaging with policymakers as directed by the BOR, and educating CHEST members of government affairs relevant to CHEST’s mission. The HPAC is currently setting its priorities to bring to the BOR for approval later this summer. Areas of focus include home mechanical ventilation and competitive bidding access to in education four home auction therapy, only rehabilitation and tobacco vaping education,
• Peter Mazzone, MD, FCCP; Editor in Chief, CHEST journal, reviewed his editorial team, which now consists of three Deputy Editors, nine Associate Editors, an Assistant Editor, a Statistical Editor, and three Case Series Editors and the publishing staff and partners.
The Board’s next meetings will be a scheduled teleconference in August, followed by their meeting that will occur concomitantly with the CHEST meeting in October.
The Board of Regents met remotely in June because of ongoing travel restrictions and safety concerns for staff and board members.
• The meeting was opened with Stephanie Levine, President; Steve Simpson, President-Elect; and Robert Musacchio, CEO/EVP discussing the impacts of the COVID-19 pandemic and Business Continuity Planning. The COVID-19 Task Force, chaired by Steve Simpson, continues to meet weekly to identify emerging content needs toward supporting membership and their patients through the pandemic, connecting with the Education Committee and Foundation to ensure robust coverage, drawing on the expertise of the NetWorks for content development, and leveraging the Social Media Workgroup for dissemination. Key activities include: a regular Thursday webinar series at 3:00 pm CDT titled: “Advice From the Front Lines”; clinical resources in the form of infographics and guides are posted in the resource center and circulated through social media; Alex Niven, MD FCCP, led a team to develop a wellness curriculum and series; the CHEST Foundation developed patient education videos and guides, a public service announcement in partnership with the American Thoracic Society, and a pilot partnership with AMITA Health enabling access to telehealth.
• The Finance Committee, chaired by John Howington, reported that CHEST is on track to meet its budget and exceed its debt covenants and operating reserve policy for the current fiscal year. The record attendance at the October 2019 annual meeting, along with strong performance from our digital offerings offset the financial impacts of the global pandemic. Bob Musacchio, CEO/EVP, reminded the Board why CHEST is switching from a fiscal year to calendar year budget. A calendar year budget process creates better alignment with budgets of pharma, other clients, and vendors; facilitates various accruals that are based on the calendar year, such as benefits, vacation, sick, and PTO days; provides for greater continuity for doing business throughout the year, and permits more planning time for staff in setting individual goals related to the annual meeting.
• CHEST’S Digital Transformation strategy that kicked off in 2019 was timely considering the pandemic. With education as one of our main foci, CHEST has hired and onboarded a Chief Learning Officer, Jim Young, to actively examine how we develop and deploy our educational products and services. Our first movement toward remote meetings occurred on June 26 with the Virtual Congress originally slated for Bologna, Italy. Here, we piloted a new platform and brought to life the tenets established in the new learning strategy—providing choice, demonstrating responsiveness, and fostering connection.
• CHEST’s Governance Committee reviewed the College bylaws for revisions, as per the group’s practice every 2-3 years, and the Board approved the revisions to the bylaws as proposed by the committee.
• CHEST’s newly formed Health Policy and Advocacy Committee (HPAC), chaired by Neil Freedman, MD, FCCP, is holding monthly meetings with a goal of making a recommendation to the Board of Regents on CHEST’s regulatory and policy priorities during the August meeting. The HPAC assists CHEST leadership and the BOR in developing and implementing health policy positions, setting chest advocacy agendas in the legislative and regulatory arenas, engaging with policymakers as directed by the BOR, and educating CHEST members of government affairs relevant to CHEST’s mission. The HPAC is currently setting its priorities to bring to the BOR for approval later this summer. Areas of focus include home mechanical ventilation and competitive bidding access to in education four home auction therapy, only rehabilitation and tobacco vaping education,
• Peter Mazzone, MD, FCCP; Editor in Chief, CHEST journal, reviewed his editorial team, which now consists of three Deputy Editors, nine Associate Editors, an Assistant Editor, a Statistical Editor, and three Case Series Editors and the publishing staff and partners.
The Board’s next meetings will be a scheduled teleconference in August, followed by their meeting that will occur concomitantly with the CHEST meeting in October.
The Board of Regents met remotely in June because of ongoing travel restrictions and safety concerns for staff and board members.
• The meeting was opened with Stephanie Levine, President; Steve Simpson, President-Elect; and Robert Musacchio, CEO/EVP discussing the impacts of the COVID-19 pandemic and Business Continuity Planning. The COVID-19 Task Force, chaired by Steve Simpson, continues to meet weekly to identify emerging content needs toward supporting membership and their patients through the pandemic, connecting with the Education Committee and Foundation to ensure robust coverage, drawing on the expertise of the NetWorks for content development, and leveraging the Social Media Workgroup for dissemination. Key activities include: a regular Thursday webinar series at 3:00 pm CDT titled: “Advice From the Front Lines”; clinical resources in the form of infographics and guides are posted in the resource center and circulated through social media; Alex Niven, MD FCCP, led a team to develop a wellness curriculum and series; the CHEST Foundation developed patient education videos and guides, a public service announcement in partnership with the American Thoracic Society, and a pilot partnership with AMITA Health enabling access to telehealth.
• The Finance Committee, chaired by John Howington, reported that CHEST is on track to meet its budget and exceed its debt covenants and operating reserve policy for the current fiscal year. The record attendance at the October 2019 annual meeting, along with strong performance from our digital offerings offset the financial impacts of the global pandemic. Bob Musacchio, CEO/EVP, reminded the Board why CHEST is switching from a fiscal year to calendar year budget. A calendar year budget process creates better alignment with budgets of pharma, other clients, and vendors; facilitates various accruals that are based on the calendar year, such as benefits, vacation, sick, and PTO days; provides for greater continuity for doing business throughout the year, and permits more planning time for staff in setting individual goals related to the annual meeting.
• CHEST’S Digital Transformation strategy that kicked off in 2019 was timely considering the pandemic. With education as one of our main foci, CHEST has hired and onboarded a Chief Learning Officer, Jim Young, to actively examine how we develop and deploy our educational products and services. Our first movement toward remote meetings occurred on June 26 with the Virtual Congress originally slated for Bologna, Italy. Here, we piloted a new platform and brought to life the tenets established in the new learning strategy—providing choice, demonstrating responsiveness, and fostering connection.
• CHEST’s Governance Committee reviewed the College bylaws for revisions, as per the group’s practice every 2-3 years, and the Board approved the revisions to the bylaws as proposed by the committee.
• CHEST’s newly formed Health Policy and Advocacy Committee (HPAC), chaired by Neil Freedman, MD, FCCP, is holding monthly meetings with a goal of making a recommendation to the Board of Regents on CHEST’s regulatory and policy priorities during the August meeting. The HPAC assists CHEST leadership and the BOR in developing and implementing health policy positions, setting chest advocacy agendas in the legislative and regulatory arenas, engaging with policymakers as directed by the BOR, and educating CHEST members of government affairs relevant to CHEST’s mission. The HPAC is currently setting its priorities to bring to the BOR for approval later this summer. Areas of focus include home mechanical ventilation and competitive bidding access to in education four home auction therapy, only rehabilitation and tobacco vaping education,
• Peter Mazzone, MD, FCCP; Editor in Chief, CHEST journal, reviewed his editorial team, which now consists of three Deputy Editors, nine Associate Editors, an Assistant Editor, a Statistical Editor, and three Case Series Editors and the publishing staff and partners.
The Board’s next meetings will be a scheduled teleconference in August, followed by their meeting that will occur concomitantly with the CHEST meeting in October.
Antibiotic resistance: Personal responsibility in somewhat short supply
Most primary care physicians agree that antibiotic resistance and inappropriate prescribing are problems in the United States, but they are much less inclined to recognize these issues in their own practices, according to the results of a nationwide survey.

Rachel M. Zetts, MPH, of the Pew Charitable Trusts, Washington, D.C., and associates wrote in Open Forum Infectious Diseases.
Almost all (94%) of the 1,550 internists, family physicians, and pediatricians who responded to the survey said that antibiotic resistance is a national problem, and nearly that many (91%) agreed that “inappropriate antibiotic prescribing is a problem in outpatient health care settings,” the investigators acknowledged.
Narrowing the focus to their own practices, however, changed some opinions. At that level, only 55% of the respondents said that resistance was a problem for their practices, and just 37% said that there any sort of inappropriate prescribing going on, based on data from the survey, which was conducted from August to October 2018 by Pew and the American Medical Association.
Antibiotic stewardship, defined as activities meant to ensure appropriate prescribing of antibiotics, should include “staff and patient education, clinician-level antibiotic prescribing feedback, and communications training on how to discuss antibiotic prescribing with patients,” Ms. Zetts and associates explained.
The need for such stewardship in health care settings was acknowledged by 72% of respondents, but 53% of those surveyed also said that all they need to do to support such efforts “is to talk with their patients about the value of an antibiotic for their symptoms,” they noted.
The bacteria, it seems, are not the only ones with some resistance. Half of the primary care physicians believe that it would be difficult to fairly and accurately track the appropriate use of antibiotics, and 52% agreed with the statement that “practice-based reporting requirements for antibiotic use would be too onerous,” the researchers pointed out.
“Antibiotic resistance is an impending public health crisis. We are seeing today, as we respond to the COVID-19 pandemic, what our health system looks like with no or limited treatments available to tackle an outbreak. … We must all remain vigilant in combating the spread of antibiotic resistant bacteria and be prudent when prescribing antibiotics,” AMA President Susan R. Bailey, MD, said in a written statement.
SOURCE: Zetts RM et al. Open Forum Infect Dis. 2020 July;7(7). doi: 10.1093/ofid/ofaa244.
Most primary care physicians agree that antibiotic resistance and inappropriate prescribing are problems in the United States, but they are much less inclined to recognize these issues in their own practices, according to the results of a nationwide survey.

Rachel M. Zetts, MPH, of the Pew Charitable Trusts, Washington, D.C., and associates wrote in Open Forum Infectious Diseases.
Almost all (94%) of the 1,550 internists, family physicians, and pediatricians who responded to the survey said that antibiotic resistance is a national problem, and nearly that many (91%) agreed that “inappropriate antibiotic prescribing is a problem in outpatient health care settings,” the investigators acknowledged.
Narrowing the focus to their own practices, however, changed some opinions. At that level, only 55% of the respondents said that resistance was a problem for their practices, and just 37% said that there any sort of inappropriate prescribing going on, based on data from the survey, which was conducted from August to October 2018 by Pew and the American Medical Association.
Antibiotic stewardship, defined as activities meant to ensure appropriate prescribing of antibiotics, should include “staff and patient education, clinician-level antibiotic prescribing feedback, and communications training on how to discuss antibiotic prescribing with patients,” Ms. Zetts and associates explained.
The need for such stewardship in health care settings was acknowledged by 72% of respondents, but 53% of those surveyed also said that all they need to do to support such efforts “is to talk with their patients about the value of an antibiotic for their symptoms,” they noted.
The bacteria, it seems, are not the only ones with some resistance. Half of the primary care physicians believe that it would be difficult to fairly and accurately track the appropriate use of antibiotics, and 52% agreed with the statement that “practice-based reporting requirements for antibiotic use would be too onerous,” the researchers pointed out.
“Antibiotic resistance is an impending public health crisis. We are seeing today, as we respond to the COVID-19 pandemic, what our health system looks like with no or limited treatments available to tackle an outbreak. … We must all remain vigilant in combating the spread of antibiotic resistant bacteria and be prudent when prescribing antibiotics,” AMA President Susan R. Bailey, MD, said in a written statement.
SOURCE: Zetts RM et al. Open Forum Infect Dis. 2020 July;7(7). doi: 10.1093/ofid/ofaa244.
Most primary care physicians agree that antibiotic resistance and inappropriate prescribing are problems in the United States, but they are much less inclined to recognize these issues in their own practices, according to the results of a nationwide survey.

Rachel M. Zetts, MPH, of the Pew Charitable Trusts, Washington, D.C., and associates wrote in Open Forum Infectious Diseases.
Almost all (94%) of the 1,550 internists, family physicians, and pediatricians who responded to the survey said that antibiotic resistance is a national problem, and nearly that many (91%) agreed that “inappropriate antibiotic prescribing is a problem in outpatient health care settings,” the investigators acknowledged.
Narrowing the focus to their own practices, however, changed some opinions. At that level, only 55% of the respondents said that resistance was a problem for their practices, and just 37% said that there any sort of inappropriate prescribing going on, based on data from the survey, which was conducted from August to October 2018 by Pew and the American Medical Association.
Antibiotic stewardship, defined as activities meant to ensure appropriate prescribing of antibiotics, should include “staff and patient education, clinician-level antibiotic prescribing feedback, and communications training on how to discuss antibiotic prescribing with patients,” Ms. Zetts and associates explained.
The need for such stewardship in health care settings was acknowledged by 72% of respondents, but 53% of those surveyed also said that all they need to do to support such efforts “is to talk with their patients about the value of an antibiotic for their symptoms,” they noted.
The bacteria, it seems, are not the only ones with some resistance. Half of the primary care physicians believe that it would be difficult to fairly and accurately track the appropriate use of antibiotics, and 52% agreed with the statement that “practice-based reporting requirements for antibiotic use would be too onerous,” the researchers pointed out.
“Antibiotic resistance is an impending public health crisis. We are seeing today, as we respond to the COVID-19 pandemic, what our health system looks like with no or limited treatments available to tackle an outbreak. … We must all remain vigilant in combating the spread of antibiotic resistant bacteria and be prudent when prescribing antibiotics,” AMA President Susan R. Bailey, MD, said in a written statement.
SOURCE: Zetts RM et al. Open Forum Infect Dis. 2020 July;7(7). doi: 10.1093/ofid/ofaa244.
FROM OPEN FORUM INFECTIOUS DISEASES













