User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Delta variant key to breakthrough infections in vaccinated Israelis
Israeli officials are reporting a 30% decrease in the effectiveness of the Pfizer/BioNTech vaccine to prevent SARS-CoV-2 infection and mild to moderate cases of COVID-19. At the same time, protection against hospitalization and severe illness remains robust.
The country’s Ministry of Health data cited high levels of circulating Delta variant and a relaxation of public health measures in early June for the drop in the vaccine’s prevention of “breakthrough” cases from 94% to 64% in recent weeks.
However, it is important to consider the findings in context, experts cautioned.
“My overall take on this that the vaccine is highly protective against the endpoints that matter – hospitalization and severe disease,” Anna Durbin, MD, told this news organization.
“I was very pleasantly surprised with the very high efficacy against hospitalization and severe disease – even against the Delta variant,” added Dr. Durbin, professor of medicine at Johns Hopkins University, Baltimore.
Ali Mokdad, PhD, of the Institute for Health Metrics at the University of Washington, Seattle, agreed that the high degree of protection against severe outcomes should be the focus.
“That’s the whole idea. You want to defend against COVID-19. So even if someone is infected, they don’t end up in the hospital or in the morgue,” he said in an interview.
Compared with an earlier report, the efficacy of the Pfizer vaccine against hospitalization fell slightly from 98% to 93%.
“For me, the fact that there is increased infection from the Delta variant after the vaccines such as Pfizer is of course a concern. But the positive news is that there is 93% prevention against severe disease or mortality,” added Dr. Mokdad, who is also professor of global health at University of Washington.
In addition, the absolute numbers remain relatively small. The Ministry of Health data show that, of the 63 Israelis hospitalized with COVID-19 nationwide on July 3, 34 were in critical condition.
Unrealistic expectations?
People may have unrealistic expectations regarding breakthrough infections, Dr. Durbin said. “It seems that people are almost expecting ‘sterilizing immunity’ from these vaccines,” she said, explaining that would mean complete protection from infection.
Expectations may be high “because these vaccines have been so effective,” added Dr. Durbin, who is also affiliated with the Johns Hopkins Center for Global Health.
The higher the number of vaccinated residents, the more breakthrough cases will be reported, epidemiologist Katelyn Jetelina, PhD, MPH, assistant professor of epidemiology, human genetics, and environmental sciences at the University of Texas Science Center at Houston, wrote in her “Your Local Epidemiologist” blog.
This could apply to Israel, with an estimated 60% of adults in Israel fully vaccinated and 65% receiving at least one dose as of July 5, Our World in Data figures show.
How the updated figures were reported could be confusing, Dr. Jetelina said. Israel’s Health Minister Chezy Levy noted that “55% of the newly infected had been vaccinated” in a radio interview announcing the results.
“This language is important because it’s very different than ‘half of vaccinated people were infected,’ ” Dr. Jetelina noted.
Israel had a 7-day rolling average of 324 new confirmed COVID-19 cases as of July 5. Assuming 55% of these cases were among vaccinated people, that would mean 178 people experienced breakthrough infections.
In contrast, almost 6 million people in Israel are fully vaccinated. If 55% of them experienced breakthrough infections, the number would be much higher – more than 3 million.
Dr. Jetelina added that more details about the new Israel figures would be helpful, including the severity of COVID-19 among the vaccinated cases and breakdown of infections between adults and children.
Next steps
Israeli health officials are weighing the necessity of a third or booster dose of the vaccine. Whether they will reinstate public health measures to prevent spread of COVID-19 also remains unknown.
Going forward, Israel intends to study whether factors such as age, comorbidities, or time since immunization affect risk for breakthrough infections among people vaccinated against COVID-19.
“We want to prevent people from getting hospitalized, seriously ill, and of course, dying. It’s encouraging these vaccines will be able to have a high impact on those outcomes,” Dr. Durbin said. “We just need to get people vaccinated.”
A call for better global surveillance
A global surveillance system is a potential solution to track and respond to the growing threat of the Delta variant and other variants of concern, Scott P. Layne, MD, and Jeffery K. Taubenberger, MD, PhD, wrote in a July 7, 2021, editorial in Science Translational Medicine.
One goal, Dr. Layne said in an interview, is to highlight “the compelling need for a new global COVID-19 program of surveillance and offer a blueprint for building it.” A second aim is to promote global cooperation among key advisers and leaders in the G7, G20, and Asia-Pacific Economic Cooperation nations.
“It’s an uphill struggle with superpower discords, global warming, cybersecurity, and pandemics all competing for finite attention,” Dr. Layne said. “However, what other options do we have for taming the so-called forever virus?”
Dr. Mokdad and Dr. Jetelina had no relevant disclosures. Dr. Durban disclosed she was the site primary investigator for the phase 3 AstraZeneca vaccine trial and an investigator on the Pfizer COVID-19 vaccine trial.
A version of this article first appeared on Medscape.com.
Israeli officials are reporting a 30% decrease in the effectiveness of the Pfizer/BioNTech vaccine to prevent SARS-CoV-2 infection and mild to moderate cases of COVID-19. At the same time, protection against hospitalization and severe illness remains robust.
The country’s Ministry of Health data cited high levels of circulating Delta variant and a relaxation of public health measures in early June for the drop in the vaccine’s prevention of “breakthrough” cases from 94% to 64% in recent weeks.
However, it is important to consider the findings in context, experts cautioned.
“My overall take on this that the vaccine is highly protective against the endpoints that matter – hospitalization and severe disease,” Anna Durbin, MD, told this news organization.
“I was very pleasantly surprised with the very high efficacy against hospitalization and severe disease – even against the Delta variant,” added Dr. Durbin, professor of medicine at Johns Hopkins University, Baltimore.
Ali Mokdad, PhD, of the Institute for Health Metrics at the University of Washington, Seattle, agreed that the high degree of protection against severe outcomes should be the focus.
“That’s the whole idea. You want to defend against COVID-19. So even if someone is infected, they don’t end up in the hospital or in the morgue,” he said in an interview.
Compared with an earlier report, the efficacy of the Pfizer vaccine against hospitalization fell slightly from 98% to 93%.
“For me, the fact that there is increased infection from the Delta variant after the vaccines such as Pfizer is of course a concern. But the positive news is that there is 93% prevention against severe disease or mortality,” added Dr. Mokdad, who is also professor of global health at University of Washington.
In addition, the absolute numbers remain relatively small. The Ministry of Health data show that, of the 63 Israelis hospitalized with COVID-19 nationwide on July 3, 34 were in critical condition.
Unrealistic expectations?
People may have unrealistic expectations regarding breakthrough infections, Dr. Durbin said. “It seems that people are almost expecting ‘sterilizing immunity’ from these vaccines,” she said, explaining that would mean complete protection from infection.
Expectations may be high “because these vaccines have been so effective,” added Dr. Durbin, who is also affiliated with the Johns Hopkins Center for Global Health.
The higher the number of vaccinated residents, the more breakthrough cases will be reported, epidemiologist Katelyn Jetelina, PhD, MPH, assistant professor of epidemiology, human genetics, and environmental sciences at the University of Texas Science Center at Houston, wrote in her “Your Local Epidemiologist” blog.
This could apply to Israel, with an estimated 60% of adults in Israel fully vaccinated and 65% receiving at least one dose as of July 5, Our World in Data figures show.
How the updated figures were reported could be confusing, Dr. Jetelina said. Israel’s Health Minister Chezy Levy noted that “55% of the newly infected had been vaccinated” in a radio interview announcing the results.
“This language is important because it’s very different than ‘half of vaccinated people were infected,’ ” Dr. Jetelina noted.
Israel had a 7-day rolling average of 324 new confirmed COVID-19 cases as of July 5. Assuming 55% of these cases were among vaccinated people, that would mean 178 people experienced breakthrough infections.
In contrast, almost 6 million people in Israel are fully vaccinated. If 55% of them experienced breakthrough infections, the number would be much higher – more than 3 million.
Dr. Jetelina added that more details about the new Israel figures would be helpful, including the severity of COVID-19 among the vaccinated cases and breakdown of infections between adults and children.
Next steps
Israeli health officials are weighing the necessity of a third or booster dose of the vaccine. Whether they will reinstate public health measures to prevent spread of COVID-19 also remains unknown.
Going forward, Israel intends to study whether factors such as age, comorbidities, or time since immunization affect risk for breakthrough infections among people vaccinated against COVID-19.
“We want to prevent people from getting hospitalized, seriously ill, and of course, dying. It’s encouraging these vaccines will be able to have a high impact on those outcomes,” Dr. Durbin said. “We just need to get people vaccinated.”
A call for better global surveillance
A global surveillance system is a potential solution to track and respond to the growing threat of the Delta variant and other variants of concern, Scott P. Layne, MD, and Jeffery K. Taubenberger, MD, PhD, wrote in a July 7, 2021, editorial in Science Translational Medicine.
One goal, Dr. Layne said in an interview, is to highlight “the compelling need for a new global COVID-19 program of surveillance and offer a blueprint for building it.” A second aim is to promote global cooperation among key advisers and leaders in the G7, G20, and Asia-Pacific Economic Cooperation nations.
“It’s an uphill struggle with superpower discords, global warming, cybersecurity, and pandemics all competing for finite attention,” Dr. Layne said. “However, what other options do we have for taming the so-called forever virus?”
Dr. Mokdad and Dr. Jetelina had no relevant disclosures. Dr. Durban disclosed she was the site primary investigator for the phase 3 AstraZeneca vaccine trial and an investigator on the Pfizer COVID-19 vaccine trial.
A version of this article first appeared on Medscape.com.
Israeli officials are reporting a 30% decrease in the effectiveness of the Pfizer/BioNTech vaccine to prevent SARS-CoV-2 infection and mild to moderate cases of COVID-19. At the same time, protection against hospitalization and severe illness remains robust.
The country’s Ministry of Health data cited high levels of circulating Delta variant and a relaxation of public health measures in early June for the drop in the vaccine’s prevention of “breakthrough” cases from 94% to 64% in recent weeks.
However, it is important to consider the findings in context, experts cautioned.
“My overall take on this that the vaccine is highly protective against the endpoints that matter – hospitalization and severe disease,” Anna Durbin, MD, told this news organization.
“I was very pleasantly surprised with the very high efficacy against hospitalization and severe disease – even against the Delta variant,” added Dr. Durbin, professor of medicine at Johns Hopkins University, Baltimore.
Ali Mokdad, PhD, of the Institute for Health Metrics at the University of Washington, Seattle, agreed that the high degree of protection against severe outcomes should be the focus.
“That’s the whole idea. You want to defend against COVID-19. So even if someone is infected, they don’t end up in the hospital or in the morgue,” he said in an interview.
Compared with an earlier report, the efficacy of the Pfizer vaccine against hospitalization fell slightly from 98% to 93%.
“For me, the fact that there is increased infection from the Delta variant after the vaccines such as Pfizer is of course a concern. But the positive news is that there is 93% prevention against severe disease or mortality,” added Dr. Mokdad, who is also professor of global health at University of Washington.
In addition, the absolute numbers remain relatively small. The Ministry of Health data show that, of the 63 Israelis hospitalized with COVID-19 nationwide on July 3, 34 were in critical condition.
Unrealistic expectations?
People may have unrealistic expectations regarding breakthrough infections, Dr. Durbin said. “It seems that people are almost expecting ‘sterilizing immunity’ from these vaccines,” she said, explaining that would mean complete protection from infection.
Expectations may be high “because these vaccines have been so effective,” added Dr. Durbin, who is also affiliated with the Johns Hopkins Center for Global Health.
The higher the number of vaccinated residents, the more breakthrough cases will be reported, epidemiologist Katelyn Jetelina, PhD, MPH, assistant professor of epidemiology, human genetics, and environmental sciences at the University of Texas Science Center at Houston, wrote in her “Your Local Epidemiologist” blog.
This could apply to Israel, with an estimated 60% of adults in Israel fully vaccinated and 65% receiving at least one dose as of July 5, Our World in Data figures show.
How the updated figures were reported could be confusing, Dr. Jetelina said. Israel’s Health Minister Chezy Levy noted that “55% of the newly infected had been vaccinated” in a radio interview announcing the results.
“This language is important because it’s very different than ‘half of vaccinated people were infected,’ ” Dr. Jetelina noted.
Israel had a 7-day rolling average of 324 new confirmed COVID-19 cases as of July 5. Assuming 55% of these cases were among vaccinated people, that would mean 178 people experienced breakthrough infections.
In contrast, almost 6 million people in Israel are fully vaccinated. If 55% of them experienced breakthrough infections, the number would be much higher – more than 3 million.
Dr. Jetelina added that more details about the new Israel figures would be helpful, including the severity of COVID-19 among the vaccinated cases and breakdown of infections between adults and children.
Next steps
Israeli health officials are weighing the necessity of a third or booster dose of the vaccine. Whether they will reinstate public health measures to prevent spread of COVID-19 also remains unknown.
Going forward, Israel intends to study whether factors such as age, comorbidities, or time since immunization affect risk for breakthrough infections among people vaccinated against COVID-19.
“We want to prevent people from getting hospitalized, seriously ill, and of course, dying. It’s encouraging these vaccines will be able to have a high impact on those outcomes,” Dr. Durbin said. “We just need to get people vaccinated.”
A call for better global surveillance
A global surveillance system is a potential solution to track and respond to the growing threat of the Delta variant and other variants of concern, Scott P. Layne, MD, and Jeffery K. Taubenberger, MD, PhD, wrote in a July 7, 2021, editorial in Science Translational Medicine.
One goal, Dr. Layne said in an interview, is to highlight “the compelling need for a new global COVID-19 program of surveillance and offer a blueprint for building it.” A second aim is to promote global cooperation among key advisers and leaders in the G7, G20, and Asia-Pacific Economic Cooperation nations.
“It’s an uphill struggle with superpower discords, global warming, cybersecurity, and pandemics all competing for finite attention,” Dr. Layne said. “However, what other options do we have for taming the so-called forever virus?”
Dr. Mokdad and Dr. Jetelina had no relevant disclosures. Dr. Durban disclosed she was the site primary investigator for the phase 3 AstraZeneca vaccine trial and an investigator on the Pfizer COVID-19 vaccine trial.
A version of this article first appeared on Medscape.com.
Sublingual immunotherapy: Where does it stand?
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Direct-care allergy clinic specializes in sublingual immunotherapy
With degrees in electrical engineering and computer science from the Massachusetts Institute of Technology, Nikhila Schroeder, MD, MEng, brings a problem-solving mindset to medicine.
Being a doctor means having to “figure out all aspects of [a patient’s] situation and do my best to come up with an answer,” said Dr. Schroeder, who founded Allergenuity Health, a solo allergy practice in Huntersville, N.C., with her husband James, who serves as practice executive. It’s “being a medical detective for your patient.”
Yet, during her training, Dr. Schroeder found that market-driven health care makes it hard to practice medicine with a patient’s best interest foremost. Procedures for diagnosing and treating disease cater to insurance companies’ reimbursement policies. “You wind up having to tailor your care to whatever insurance will cover,” she said.
Insurers, in turn, look for evidence from large, peer-reviewed studies to prove that a treatment works. Many physicians hesitate to offer therapies that aren’t covered by insurance, for both liability and financial reasons. So treatment tends to be limited to those options that were rigorously vetted in long, costly, multisite trials that are difficult to conduct without a corporate sponsor.
This is why there is still only one licensed treatment for people with food allergies – a set of standardized peanut powder capsules (Palforzia) that was approved by the Food and Drug Administration in early 2020 for peanut-allergic children aged 4-17 years. A small but growing number of allergists offer unapproved oral immunotherapy (OIT) using commercial food products to treat allergies to peanuts and other foods.
Even fewer allergists treat food allergy patients with another immune-modifying treatment, sublingual immunotherapy (SLIT), which delivers allergens through liquid drops held for several minutes under the tongue. Since 2018, Allergenuity Health, which offers SLIT to treat food and environmental allergies, has provided care to more than 400 patients. More than a third have come from out of state.
The clinic uses a direct-care approach. Rather than taking insurance, the clinic offers a monthly billing program that includes tests, SLIT bottles, and access to Dr. Schroeder via phone, email, or text. “I’m only contracted with the patient, and my only focus is the patient,” Dr. Schroeder said in an interview.
Unforgettable day
Allergy was not on Dr. Schroeder’s radar in medical school. She wanted to be a surgeon. But she loved working with children, so she did a pediatrics residency at the University of Virginia Children’s Hospital in Charlottesville. There Dr. Schroeder started seeing kids with eczema and allergies. While covering a friend’s clinic shift in 2010, she was thrust into an emergency. A family who didn’t speak English had just brought in their screaming 6-month-old baby, red and puffy with hives. “We didn’t know what was going on with this child,” Dr. Schroeder said. “Somehow I was elected to go in there.”
All of a sudden, things got quiet. Yet the baby was still screaming, mouth wide open. Dr. Schroeder had learned about anaphylaxis but had never witnessed it – until that day. The baby›s airways swelled so much that the crying became hoarse and soft. After working with a nurse to administer epinephrine, Dr. Schroeder saw something equally unforgettable: The baby’s heart rate soared, but within minutes the hives and swelling subsided and smiles returned. “It was incredible how quickly things changed,” Dr. Schroeder said. The baby had a reaction to rice, an uncommon allergen.
Dr. Schroeder stayed at UVA 2 more years to complete an allergy and immunology fellowship. She learned to diagnose food allergies but became frustrated having to tell patients they had little recourse but to avoid the food and to check in every year or 2. “I was, like, aren’t we specialists? Shouldn’t we have a little more expertise and maybe see if there are ways we could change this?” Dr. Schroeder said.
During those years, allergy shots were the only form of immunotherapy being taught to fellows. At clinic, Dr. Schroeder served as backup to the nurses when someone reacted to shots. She was troubled that some patients needed epinephrine to stop asthma attacks caused by injections they had received as treatment. The idea of injecting substances under the skin seemed akin to vaccination – where “you want to aggravate the immune system, you want it to get revved up, you want to build it up to fight,” she said. “But that’s not what you want for allergy. You want to tone it down. It didn’t really, to be honest, make a lot of sense to me.”
Dr. Schroeder started digging and asking questions. How does the immune system decide what is safe? Which cells and molecules communicate these decisions? She thought about babies and how they “learn” by putting stuff into their mouths. “If we don’t tolerate most of what we take in there, we wouldn’t survive,” Dr. Schroeder said. “It makes a lot of sense that a lot of tolerance begins with cells of the mouth.”
Dr. Schroeder discussed these concepts with her attendings. “They were all, like, no, there’s really no good evidence for that,” she said. But at some point, someone mentioned sublingual immunotherapy, and Schroeder came across Allergy Associates of La Crosse (Wis.).
The clinic’s late founder, David Morris, MD, learned about SLIT in the 1960s as an alternative option for farmers who suffered terrible side effects from injection immunotherapy they received to treat their mold allergies. Dr. Morris attended conferences, learned more about sublingual techniques – at times seeking advice from European allergists who offered SLIT – and became board certified in allergy before opening the La Crosse clinic in 1970. According to the clinic, more than 200,000 patients with environmental and food allergies have been treated with its SLIT protocol.
Dr. Schroeder was shocked to discover that this clinic had existed for 40 years, yet “I, as an allergist, had heard nothing about them,” she said.
Toward the end of her fellowship, OIT was becoming more well known. But she felt its risks were often downplayed. After years of talking with food allergy patients, Schroeder realized that most didn’t actually care about eating peanut butter sandwiches or sesame or walnuts. “Often I would hear, through tears: ‘I just want my child to be able to sit with their friends at lunch, to not be put at this other table, to not feel so isolated,’ ” she said. What mattered most to many families was gaining enough protection to not feel anxious about participating in social activities involving food.
Dr. Schroeder had a growing sense that SLIT – given its ease, safety, and sensible route of allergen delivery – seemed more useful. She wanted to learn more.
Her mentors urged her to stay in academia instead. “They were, like, you have a good academic reputation. You’re a solid thinker. You’re great at what you do. Do the traditional stuff,” Dr. Schroeder said.
Despite these admonitions, Dr. Schroeder left academia and took a job at La Crosse after completing her allergy fellowship. Determined to see whether SLIT could be effective, “I decided in the end, you know what, I have to go do this,” she said. “I need to know, and the only way I’m going to know is to do it, because no one was giving me good information.”
Before treating anyone with SLIT, Dr. Schroeder tried it herself – as a La Crosse patient. Growing up with severe eczema, eye swelling, and chronic nasal congestion leading to sinus infections, “I myself was a severely allergic person,” she said. Within several months, Dr. Schroeder saw dramatic improvement in her symptoms – “a night and day difference.” She experienced some mouth tingling, one of SLIT’s most common side effects, but found it “very tolerable, very mild.”
Allergenuity Health doesn’t aim to promote SLIT as the best treatment, said Dr. Schroeder, who has helped some families use avoidance or OIT as a better option. “An initial evaluation is always about proper diagnosis and education about all the treatment options available. Really, the point is education – be a detective for them and figure out what’s going on, be honest about what we know and what we don’t know, and give them the tools to figure out how to proceed.”
A version of this article first appeared on Medscape.com. This is part two of a three-part series. Part one is here. Part three is here.
With degrees in electrical engineering and computer science from the Massachusetts Institute of Technology, Nikhila Schroeder, MD, MEng, brings a problem-solving mindset to medicine.
Being a doctor means having to “figure out all aspects of [a patient’s] situation and do my best to come up with an answer,” said Dr. Schroeder, who founded Allergenuity Health, a solo allergy practice in Huntersville, N.C., with her husband James, who serves as practice executive. It’s “being a medical detective for your patient.”
Yet, during her training, Dr. Schroeder found that market-driven health care makes it hard to practice medicine with a patient’s best interest foremost. Procedures for diagnosing and treating disease cater to insurance companies’ reimbursement policies. “You wind up having to tailor your care to whatever insurance will cover,” she said.
Insurers, in turn, look for evidence from large, peer-reviewed studies to prove that a treatment works. Many physicians hesitate to offer therapies that aren’t covered by insurance, for both liability and financial reasons. So treatment tends to be limited to those options that were rigorously vetted in long, costly, multisite trials that are difficult to conduct without a corporate sponsor.
This is why there is still only one licensed treatment for people with food allergies – a set of standardized peanut powder capsules (Palforzia) that was approved by the Food and Drug Administration in early 2020 for peanut-allergic children aged 4-17 years. A small but growing number of allergists offer unapproved oral immunotherapy (OIT) using commercial food products to treat allergies to peanuts and other foods.
Even fewer allergists treat food allergy patients with another immune-modifying treatment, sublingual immunotherapy (SLIT), which delivers allergens through liquid drops held for several minutes under the tongue. Since 2018, Allergenuity Health, which offers SLIT to treat food and environmental allergies, has provided care to more than 400 patients. More than a third have come from out of state.
The clinic uses a direct-care approach. Rather than taking insurance, the clinic offers a monthly billing program that includes tests, SLIT bottles, and access to Dr. Schroeder via phone, email, or text. “I’m only contracted with the patient, and my only focus is the patient,” Dr. Schroeder said in an interview.
Unforgettable day
Allergy was not on Dr. Schroeder’s radar in medical school. She wanted to be a surgeon. But she loved working with children, so she did a pediatrics residency at the University of Virginia Children’s Hospital in Charlottesville. There Dr. Schroeder started seeing kids with eczema and allergies. While covering a friend’s clinic shift in 2010, she was thrust into an emergency. A family who didn’t speak English had just brought in their screaming 6-month-old baby, red and puffy with hives. “We didn’t know what was going on with this child,” Dr. Schroeder said. “Somehow I was elected to go in there.”
All of a sudden, things got quiet. Yet the baby was still screaming, mouth wide open. Dr. Schroeder had learned about anaphylaxis but had never witnessed it – until that day. The baby›s airways swelled so much that the crying became hoarse and soft. After working with a nurse to administer epinephrine, Dr. Schroeder saw something equally unforgettable: The baby’s heart rate soared, but within minutes the hives and swelling subsided and smiles returned. “It was incredible how quickly things changed,” Dr. Schroeder said. The baby had a reaction to rice, an uncommon allergen.
Dr. Schroeder stayed at UVA 2 more years to complete an allergy and immunology fellowship. She learned to diagnose food allergies but became frustrated having to tell patients they had little recourse but to avoid the food and to check in every year or 2. “I was, like, aren’t we specialists? Shouldn’t we have a little more expertise and maybe see if there are ways we could change this?” Dr. Schroeder said.
During those years, allergy shots were the only form of immunotherapy being taught to fellows. At clinic, Dr. Schroeder served as backup to the nurses when someone reacted to shots. She was troubled that some patients needed epinephrine to stop asthma attacks caused by injections they had received as treatment. The idea of injecting substances under the skin seemed akin to vaccination – where “you want to aggravate the immune system, you want it to get revved up, you want to build it up to fight,” she said. “But that’s not what you want for allergy. You want to tone it down. It didn’t really, to be honest, make a lot of sense to me.”
Dr. Schroeder started digging and asking questions. How does the immune system decide what is safe? Which cells and molecules communicate these decisions? She thought about babies and how they “learn” by putting stuff into their mouths. “If we don’t tolerate most of what we take in there, we wouldn’t survive,” Dr. Schroeder said. “It makes a lot of sense that a lot of tolerance begins with cells of the mouth.”
Dr. Schroeder discussed these concepts with her attendings. “They were all, like, no, there’s really no good evidence for that,” she said. But at some point, someone mentioned sublingual immunotherapy, and Schroeder came across Allergy Associates of La Crosse (Wis.).
The clinic’s late founder, David Morris, MD, learned about SLIT in the 1960s as an alternative option for farmers who suffered terrible side effects from injection immunotherapy they received to treat their mold allergies. Dr. Morris attended conferences, learned more about sublingual techniques – at times seeking advice from European allergists who offered SLIT – and became board certified in allergy before opening the La Crosse clinic in 1970. According to the clinic, more than 200,000 patients with environmental and food allergies have been treated with its SLIT protocol.
Dr. Schroeder was shocked to discover that this clinic had existed for 40 years, yet “I, as an allergist, had heard nothing about them,” she said.
Toward the end of her fellowship, OIT was becoming more well known. But she felt its risks were often downplayed. After years of talking with food allergy patients, Schroeder realized that most didn’t actually care about eating peanut butter sandwiches or sesame or walnuts. “Often I would hear, through tears: ‘I just want my child to be able to sit with their friends at lunch, to not be put at this other table, to not feel so isolated,’ ” she said. What mattered most to many families was gaining enough protection to not feel anxious about participating in social activities involving food.
Dr. Schroeder had a growing sense that SLIT – given its ease, safety, and sensible route of allergen delivery – seemed more useful. She wanted to learn more.
Her mentors urged her to stay in academia instead. “They were, like, you have a good academic reputation. You’re a solid thinker. You’re great at what you do. Do the traditional stuff,” Dr. Schroeder said.
Despite these admonitions, Dr. Schroeder left academia and took a job at La Crosse after completing her allergy fellowship. Determined to see whether SLIT could be effective, “I decided in the end, you know what, I have to go do this,” she said. “I need to know, and the only way I’m going to know is to do it, because no one was giving me good information.”
Before treating anyone with SLIT, Dr. Schroeder tried it herself – as a La Crosse patient. Growing up with severe eczema, eye swelling, and chronic nasal congestion leading to sinus infections, “I myself was a severely allergic person,” she said. Within several months, Dr. Schroeder saw dramatic improvement in her symptoms – “a night and day difference.” She experienced some mouth tingling, one of SLIT’s most common side effects, but found it “very tolerable, very mild.”
Allergenuity Health doesn’t aim to promote SLIT as the best treatment, said Dr. Schroeder, who has helped some families use avoidance or OIT as a better option. “An initial evaluation is always about proper diagnosis and education about all the treatment options available. Really, the point is education – be a detective for them and figure out what’s going on, be honest about what we know and what we don’t know, and give them the tools to figure out how to proceed.”
A version of this article first appeared on Medscape.com. This is part two of a three-part series. Part one is here. Part three is here.
With degrees in electrical engineering and computer science from the Massachusetts Institute of Technology, Nikhila Schroeder, MD, MEng, brings a problem-solving mindset to medicine.
Being a doctor means having to “figure out all aspects of [a patient’s] situation and do my best to come up with an answer,” said Dr. Schroeder, who founded Allergenuity Health, a solo allergy practice in Huntersville, N.C., with her husband James, who serves as practice executive. It’s “being a medical detective for your patient.”
Yet, during her training, Dr. Schroeder found that market-driven health care makes it hard to practice medicine with a patient’s best interest foremost. Procedures for diagnosing and treating disease cater to insurance companies’ reimbursement policies. “You wind up having to tailor your care to whatever insurance will cover,” she said.
Insurers, in turn, look for evidence from large, peer-reviewed studies to prove that a treatment works. Many physicians hesitate to offer therapies that aren’t covered by insurance, for both liability and financial reasons. So treatment tends to be limited to those options that were rigorously vetted in long, costly, multisite trials that are difficult to conduct without a corporate sponsor.
This is why there is still only one licensed treatment for people with food allergies – a set of standardized peanut powder capsules (Palforzia) that was approved by the Food and Drug Administration in early 2020 for peanut-allergic children aged 4-17 years. A small but growing number of allergists offer unapproved oral immunotherapy (OIT) using commercial food products to treat allergies to peanuts and other foods.
Even fewer allergists treat food allergy patients with another immune-modifying treatment, sublingual immunotherapy (SLIT), which delivers allergens through liquid drops held for several minutes under the tongue. Since 2018, Allergenuity Health, which offers SLIT to treat food and environmental allergies, has provided care to more than 400 patients. More than a third have come from out of state.
The clinic uses a direct-care approach. Rather than taking insurance, the clinic offers a monthly billing program that includes tests, SLIT bottles, and access to Dr. Schroeder via phone, email, or text. “I’m only contracted with the patient, and my only focus is the patient,” Dr. Schroeder said in an interview.
Unforgettable day
Allergy was not on Dr. Schroeder’s radar in medical school. She wanted to be a surgeon. But she loved working with children, so she did a pediatrics residency at the University of Virginia Children’s Hospital in Charlottesville. There Dr. Schroeder started seeing kids with eczema and allergies. While covering a friend’s clinic shift in 2010, she was thrust into an emergency. A family who didn’t speak English had just brought in their screaming 6-month-old baby, red and puffy with hives. “We didn’t know what was going on with this child,” Dr. Schroeder said. “Somehow I was elected to go in there.”
All of a sudden, things got quiet. Yet the baby was still screaming, mouth wide open. Dr. Schroeder had learned about anaphylaxis but had never witnessed it – until that day. The baby›s airways swelled so much that the crying became hoarse and soft. After working with a nurse to administer epinephrine, Dr. Schroeder saw something equally unforgettable: The baby’s heart rate soared, but within minutes the hives and swelling subsided and smiles returned. “It was incredible how quickly things changed,” Dr. Schroeder said. The baby had a reaction to rice, an uncommon allergen.
Dr. Schroeder stayed at UVA 2 more years to complete an allergy and immunology fellowship. She learned to diagnose food allergies but became frustrated having to tell patients they had little recourse but to avoid the food and to check in every year or 2. “I was, like, aren’t we specialists? Shouldn’t we have a little more expertise and maybe see if there are ways we could change this?” Dr. Schroeder said.
During those years, allergy shots were the only form of immunotherapy being taught to fellows. At clinic, Dr. Schroeder served as backup to the nurses when someone reacted to shots. She was troubled that some patients needed epinephrine to stop asthma attacks caused by injections they had received as treatment. The idea of injecting substances under the skin seemed akin to vaccination – where “you want to aggravate the immune system, you want it to get revved up, you want to build it up to fight,” she said. “But that’s not what you want for allergy. You want to tone it down. It didn’t really, to be honest, make a lot of sense to me.”
Dr. Schroeder started digging and asking questions. How does the immune system decide what is safe? Which cells and molecules communicate these decisions? She thought about babies and how they “learn” by putting stuff into their mouths. “If we don’t tolerate most of what we take in there, we wouldn’t survive,” Dr. Schroeder said. “It makes a lot of sense that a lot of tolerance begins with cells of the mouth.”
Dr. Schroeder discussed these concepts with her attendings. “They were all, like, no, there’s really no good evidence for that,” she said. But at some point, someone mentioned sublingual immunotherapy, and Schroeder came across Allergy Associates of La Crosse (Wis.).
The clinic’s late founder, David Morris, MD, learned about SLIT in the 1960s as an alternative option for farmers who suffered terrible side effects from injection immunotherapy they received to treat their mold allergies. Dr. Morris attended conferences, learned more about sublingual techniques – at times seeking advice from European allergists who offered SLIT – and became board certified in allergy before opening the La Crosse clinic in 1970. According to the clinic, more than 200,000 patients with environmental and food allergies have been treated with its SLIT protocol.
Dr. Schroeder was shocked to discover that this clinic had existed for 40 years, yet “I, as an allergist, had heard nothing about them,” she said.
Toward the end of her fellowship, OIT was becoming more well known. But she felt its risks were often downplayed. After years of talking with food allergy patients, Schroeder realized that most didn’t actually care about eating peanut butter sandwiches or sesame or walnuts. “Often I would hear, through tears: ‘I just want my child to be able to sit with their friends at lunch, to not be put at this other table, to not feel so isolated,’ ” she said. What mattered most to many families was gaining enough protection to not feel anxious about participating in social activities involving food.
Dr. Schroeder had a growing sense that SLIT – given its ease, safety, and sensible route of allergen delivery – seemed more useful. She wanted to learn more.
Her mentors urged her to stay in academia instead. “They were, like, you have a good academic reputation. You’re a solid thinker. You’re great at what you do. Do the traditional stuff,” Dr. Schroeder said.
Despite these admonitions, Dr. Schroeder left academia and took a job at La Crosse after completing her allergy fellowship. Determined to see whether SLIT could be effective, “I decided in the end, you know what, I have to go do this,” she said. “I need to know, and the only way I’m going to know is to do it, because no one was giving me good information.”
Before treating anyone with SLIT, Dr. Schroeder tried it herself – as a La Crosse patient. Growing up with severe eczema, eye swelling, and chronic nasal congestion leading to sinus infections, “I myself was a severely allergic person,” she said. Within several months, Dr. Schroeder saw dramatic improvement in her symptoms – “a night and day difference.” She experienced some mouth tingling, one of SLIT’s most common side effects, but found it “very tolerable, very mild.”
Allergenuity Health doesn’t aim to promote SLIT as the best treatment, said Dr. Schroeder, who has helped some families use avoidance or OIT as a better option. “An initial evaluation is always about proper diagnosis and education about all the treatment options available. Really, the point is education – be a detective for them and figure out what’s going on, be honest about what we know and what we don’t know, and give them the tools to figure out how to proceed.”
A version of this article first appeared on Medscape.com. This is part two of a three-part series. Part one is here. Part three is here.
There’s a much safer food allergy immunotherapy – why don’t more doctors offer it?
For the 32 million people in the United States with food allergies, those who seek relief beyond constant vigilance and EpiPens face a confusing treatment landscape. In January 2020, the Food and Drug Administration approved an oral immunotherapy product (Palforzia) for peanut-allergic children. Yet the product’s ill-timed release during a pandemic and its black-box warning about the risk for anaphylaxis has slowed uptake.
A small number of allergists offer home-grown oral immunotherapy (OIT), which builds protection by exposing patients to increasing daily doses of commercial food products over months. However, as with Palforzia, allergic reactions are common during treatment, and the hard-earned protection can fade if not maintained with regular dosing.
An alternate approach, sublingual immunotherapy (SLIT), delivers food proteins through liquid drops held in the mouth – a site rich in tolerance-inducing immune cells. In a 2019 study of peanut-allergic children aged 1-11 years, SLIT offered a level of protection on par with Palforzia while causing considerably fewer adverse events. And at the 2021 annual meeting of the American Academy of Allergy, Asthma and Immunology, researchers reported that SLIT produced stronger, more durable benefits in toddlers aged 1-4.
Sublingual immunotherapy is “a bunch of drops you put under your tongue, you hold it for a couple minutes, and then you’re done for the day,” said Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, who led the two recent studies. For protecting against accidental ingestions, SLIT “is pushing pretty close to what OIT is able to provide but seemingly with a superior ease of administration and safety profile.”
Many parents don’t necessarily want their allergic kids to be able to eat a peanut butter sandwich – but do want them to be able to safely sit at the same lunch table and attend birthday parties with other kids. SLIT achieves this level of protection about as well as OIT, with fewer side effects.
Still, because of concerns about the treatment’s cost, unclear dosing regimens, and lack of FDA approval, very few U.S. allergists – likely less than 5% – offer sublingual immunotherapy to treat food allergies, making SLIT even less available than OIT.
Concerns about SLIT
One possible reason: Success is slower and less visible for SLIT. When patients undergo OIT, they build up to dosing with the actual food. “To a family who has a concern about their kid reacting, they can see them eating chunks of peanut in our office. That is really encouraging,” said Douglas Mack, MD, FRCPC, an allergist with Halton Pediatric Allergy and assistant clinical professor of pediatrics at McMaster University, Hamilton, Ont.
On the other hand, ingestion isn’t the focus for SLIT, so progress is harder to measure using metrics in published trials. After holding SLIT drops under the tongue, some patients spit them out. If they swallow the dose, it’s a vanishingly small amount. Immune changes that reflect increasing tolerance, such as a decrease in IgE antibodies, tend to be more gradual with SLIT than with OIT. And because SLIT is only offered in private clinics, such tests are not conducted as regularly as they would be for published trials.
But there may be a bigger factor: Some think earlier trials comparing the two immunotherapy regimens gave SLIT a bad rap. For example, in studies of milk- and peanut-allergic children conducted in 2011 and 2014, investigators concluded that SLIT was safer and that OIT appeared to be more effective. However, those trials compared SLIT with OIT using a much higher dose (2,000 mg) than is used in the licensed product (300 mg).
Over the years, endpoints for food allergy treatment trials have shifted from enabling patients to eat a full serving of their allergen to merely raising their threshold to guard against accidental exposures. So in those earlier articles, “we would probably write the discussion section differently now,” said Corinne Keet, MD, PhD, first author on the 2011 milk study and an associate professor of pediatrics at Johns Hopkins University, Baltimore.
Indeed, “when you compare [SLIT] to Palforzia or other studies of low-dose OIT (300 mg/d), they look equal in terms of their efficacy,” said senior author Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins. Yet, “I’m afraid we had a major [negative] impact on pharma’s interest in pursuing SLIT.”
Without corporate funding, it’s nearly impossible to conduct the large, multisite trials required for FDA approval of a treatment. And without approved products, many allergists are reluctant to offer the therapy, Dr. Wood said. It “makes your life a lot more complicated to be dabbling in things that are not approved,” he noted.
But at least one company is giving it a go. Applying the SLIT principle of delivering food allergens to tolerance-promoting immune cells in the mouth, New York–based Intrommune Therapeutics recently started enrolling peanut-allergic adults for a phase 1 trial of its experimental toothpaste.
Interest in food-allergy SLIT seems to be growing. “I definitely think that it could be an option for the future,” said Jaclyn Bjelac, MD, associate director of the Food Allergy Center of Excellence at the Cleveland Clinic. “Up until a few months ago, it really wasn’t on our radar.”
On conversations with Dr. Kim, philanthropists and drug developers said they found the recent data on SLIT promising, yet pointed out that food SLIT protocols and products are already in the public domain – they are described in published research using allergen extracts that are on the market. They “can’t see a commercial path forward,” Dr. Kim said in an interview. “And that’s kind of where many of my conversations end.”
Although there are no licensed SLIT products for food allergies, between 2014 and 2017, the FDA approved four sublingual immunotherapy tablets to treat environmental allergies – Stallergenes-Greer’s Oralair and ALK’s Grastek for grass pollens, ALK’s Odactra for dust mites, and ALK’s Ragwitek for short ragweed.
SLIT tablets work as well as allergy shots (subcutaneous immunotherapy) for controlling environmental allergy symptoms, they have a better safety profile, according to AAAAI guidelines, and they can be self-administered at home, which has made them a popular option globally. “Our European colleagues have used sublingual immunotherapy much more frequently than, for example, in the U.S.,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
Use of SLIT is also increasing in the United States, especially as FDA-approved products become available. In a 2019 survey, the percentage of U.S. allergists who said they were offering sublingual treatment for environmental allergies increased from 5.9% in 2007 to 73.5% in 2019. However, only 11.2% reported extensive SLIT use; the remainder reported some (50.5%) or little (38.3%) use.
As noted above, considerably fewer U.S. allergists use SLIT to treat food allergies. Similarly, a 2021 survey of allergists in Canada found that only 7% offered food sublingual immunotherapy; more than half reported offering OIT.
One practice, Allergy Associates of La Crosse (Wis.), has offered SLIT drops for food and environmental allergies for decades. Since the clinic opened in 1970, more than 200,000 people have been treated with its protocol. Every patient receives customized sublingual drops – “exactly what they’re allergic to, exactly how allergic they are, and then we build from there,” said Jeff Kessler, MBA, FACHE, practice executive at Allergy Associates of La Crosse. “Quite frankly, it’s the way immunotherapy should be done.
A version of this article first appeared on Medscape.com. This is part one of a three-part series. Part two is here. Part three is here.
For the 32 million people in the United States with food allergies, those who seek relief beyond constant vigilance and EpiPens face a confusing treatment landscape. In January 2020, the Food and Drug Administration approved an oral immunotherapy product (Palforzia) for peanut-allergic children. Yet the product’s ill-timed release during a pandemic and its black-box warning about the risk for anaphylaxis has slowed uptake.
A small number of allergists offer home-grown oral immunotherapy (OIT), which builds protection by exposing patients to increasing daily doses of commercial food products over months. However, as with Palforzia, allergic reactions are common during treatment, and the hard-earned protection can fade if not maintained with regular dosing.
An alternate approach, sublingual immunotherapy (SLIT), delivers food proteins through liquid drops held in the mouth – a site rich in tolerance-inducing immune cells. In a 2019 study of peanut-allergic children aged 1-11 years, SLIT offered a level of protection on par with Palforzia while causing considerably fewer adverse events. And at the 2021 annual meeting of the American Academy of Allergy, Asthma and Immunology, researchers reported that SLIT produced stronger, more durable benefits in toddlers aged 1-4.
Sublingual immunotherapy is “a bunch of drops you put under your tongue, you hold it for a couple minutes, and then you’re done for the day,” said Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, who led the two recent studies. For protecting against accidental ingestions, SLIT “is pushing pretty close to what OIT is able to provide but seemingly with a superior ease of administration and safety profile.”
Many parents don’t necessarily want their allergic kids to be able to eat a peanut butter sandwich – but do want them to be able to safely sit at the same lunch table and attend birthday parties with other kids. SLIT achieves this level of protection about as well as OIT, with fewer side effects.
Still, because of concerns about the treatment’s cost, unclear dosing regimens, and lack of FDA approval, very few U.S. allergists – likely less than 5% – offer sublingual immunotherapy to treat food allergies, making SLIT even less available than OIT.
Concerns about SLIT
One possible reason: Success is slower and less visible for SLIT. When patients undergo OIT, they build up to dosing with the actual food. “To a family who has a concern about their kid reacting, they can see them eating chunks of peanut in our office. That is really encouraging,” said Douglas Mack, MD, FRCPC, an allergist with Halton Pediatric Allergy and assistant clinical professor of pediatrics at McMaster University, Hamilton, Ont.
On the other hand, ingestion isn’t the focus for SLIT, so progress is harder to measure using metrics in published trials. After holding SLIT drops under the tongue, some patients spit them out. If they swallow the dose, it’s a vanishingly small amount. Immune changes that reflect increasing tolerance, such as a decrease in IgE antibodies, tend to be more gradual with SLIT than with OIT. And because SLIT is only offered in private clinics, such tests are not conducted as regularly as they would be for published trials.
But there may be a bigger factor: Some think earlier trials comparing the two immunotherapy regimens gave SLIT a bad rap. For example, in studies of milk- and peanut-allergic children conducted in 2011 and 2014, investigators concluded that SLIT was safer and that OIT appeared to be more effective. However, those trials compared SLIT with OIT using a much higher dose (2,000 mg) than is used in the licensed product (300 mg).
Over the years, endpoints for food allergy treatment trials have shifted from enabling patients to eat a full serving of their allergen to merely raising their threshold to guard against accidental exposures. So in those earlier articles, “we would probably write the discussion section differently now,” said Corinne Keet, MD, PhD, first author on the 2011 milk study and an associate professor of pediatrics at Johns Hopkins University, Baltimore.
Indeed, “when you compare [SLIT] to Palforzia or other studies of low-dose OIT (300 mg/d), they look equal in terms of their efficacy,” said senior author Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins. Yet, “I’m afraid we had a major [negative] impact on pharma’s interest in pursuing SLIT.”
Without corporate funding, it’s nearly impossible to conduct the large, multisite trials required for FDA approval of a treatment. And without approved products, many allergists are reluctant to offer the therapy, Dr. Wood said. It “makes your life a lot more complicated to be dabbling in things that are not approved,” he noted.
But at least one company is giving it a go. Applying the SLIT principle of delivering food allergens to tolerance-promoting immune cells in the mouth, New York–based Intrommune Therapeutics recently started enrolling peanut-allergic adults for a phase 1 trial of its experimental toothpaste.
Interest in food-allergy SLIT seems to be growing. “I definitely think that it could be an option for the future,” said Jaclyn Bjelac, MD, associate director of the Food Allergy Center of Excellence at the Cleveland Clinic. “Up until a few months ago, it really wasn’t on our radar.”
On conversations with Dr. Kim, philanthropists and drug developers said they found the recent data on SLIT promising, yet pointed out that food SLIT protocols and products are already in the public domain – they are described in published research using allergen extracts that are on the market. They “can’t see a commercial path forward,” Dr. Kim said in an interview. “And that’s kind of where many of my conversations end.”
Although there are no licensed SLIT products for food allergies, between 2014 and 2017, the FDA approved four sublingual immunotherapy tablets to treat environmental allergies – Stallergenes-Greer’s Oralair and ALK’s Grastek for grass pollens, ALK’s Odactra for dust mites, and ALK’s Ragwitek for short ragweed.
SLIT tablets work as well as allergy shots (subcutaneous immunotherapy) for controlling environmental allergy symptoms, they have a better safety profile, according to AAAAI guidelines, and they can be self-administered at home, which has made them a popular option globally. “Our European colleagues have used sublingual immunotherapy much more frequently than, for example, in the U.S.,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
Use of SLIT is also increasing in the United States, especially as FDA-approved products become available. In a 2019 survey, the percentage of U.S. allergists who said they were offering sublingual treatment for environmental allergies increased from 5.9% in 2007 to 73.5% in 2019. However, only 11.2% reported extensive SLIT use; the remainder reported some (50.5%) or little (38.3%) use.
As noted above, considerably fewer U.S. allergists use SLIT to treat food allergies. Similarly, a 2021 survey of allergists in Canada found that only 7% offered food sublingual immunotherapy; more than half reported offering OIT.
One practice, Allergy Associates of La Crosse (Wis.), has offered SLIT drops for food and environmental allergies for decades. Since the clinic opened in 1970, more than 200,000 people have been treated with its protocol. Every patient receives customized sublingual drops – “exactly what they’re allergic to, exactly how allergic they are, and then we build from there,” said Jeff Kessler, MBA, FACHE, practice executive at Allergy Associates of La Crosse. “Quite frankly, it’s the way immunotherapy should be done.
A version of this article first appeared on Medscape.com. This is part one of a three-part series. Part two is here. Part three is here.
For the 32 million people in the United States with food allergies, those who seek relief beyond constant vigilance and EpiPens face a confusing treatment landscape. In January 2020, the Food and Drug Administration approved an oral immunotherapy product (Palforzia) for peanut-allergic children. Yet the product’s ill-timed release during a pandemic and its black-box warning about the risk for anaphylaxis has slowed uptake.
A small number of allergists offer home-grown oral immunotherapy (OIT), which builds protection by exposing patients to increasing daily doses of commercial food products over months. However, as with Palforzia, allergic reactions are common during treatment, and the hard-earned protection can fade if not maintained with regular dosing.
An alternate approach, sublingual immunotherapy (SLIT), delivers food proteins through liquid drops held in the mouth – a site rich in tolerance-inducing immune cells. In a 2019 study of peanut-allergic children aged 1-11 years, SLIT offered a level of protection on par with Palforzia while causing considerably fewer adverse events. And at the 2021 annual meeting of the American Academy of Allergy, Asthma and Immunology, researchers reported that SLIT produced stronger, more durable benefits in toddlers aged 1-4.
Sublingual immunotherapy is “a bunch of drops you put under your tongue, you hold it for a couple minutes, and then you’re done for the day,” said Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, who led the two recent studies. For protecting against accidental ingestions, SLIT “is pushing pretty close to what OIT is able to provide but seemingly with a superior ease of administration and safety profile.”
Many parents don’t necessarily want their allergic kids to be able to eat a peanut butter sandwich – but do want them to be able to safely sit at the same lunch table and attend birthday parties with other kids. SLIT achieves this level of protection about as well as OIT, with fewer side effects.
Still, because of concerns about the treatment’s cost, unclear dosing regimens, and lack of FDA approval, very few U.S. allergists – likely less than 5% – offer sublingual immunotherapy to treat food allergies, making SLIT even less available than OIT.
Concerns about SLIT
One possible reason: Success is slower and less visible for SLIT. When patients undergo OIT, they build up to dosing with the actual food. “To a family who has a concern about their kid reacting, they can see them eating chunks of peanut in our office. That is really encouraging,” said Douglas Mack, MD, FRCPC, an allergist with Halton Pediatric Allergy and assistant clinical professor of pediatrics at McMaster University, Hamilton, Ont.
On the other hand, ingestion isn’t the focus for SLIT, so progress is harder to measure using metrics in published trials. After holding SLIT drops under the tongue, some patients spit them out. If they swallow the dose, it’s a vanishingly small amount. Immune changes that reflect increasing tolerance, such as a decrease in IgE antibodies, tend to be more gradual with SLIT than with OIT. And because SLIT is only offered in private clinics, such tests are not conducted as regularly as they would be for published trials.
But there may be a bigger factor: Some think earlier trials comparing the two immunotherapy regimens gave SLIT a bad rap. For example, in studies of milk- and peanut-allergic children conducted in 2011 and 2014, investigators concluded that SLIT was safer and that OIT appeared to be more effective. However, those trials compared SLIT with OIT using a much higher dose (2,000 mg) than is used in the licensed product (300 mg).
Over the years, endpoints for food allergy treatment trials have shifted from enabling patients to eat a full serving of their allergen to merely raising their threshold to guard against accidental exposures. So in those earlier articles, “we would probably write the discussion section differently now,” said Corinne Keet, MD, PhD, first author on the 2011 milk study and an associate professor of pediatrics at Johns Hopkins University, Baltimore.
Indeed, “when you compare [SLIT] to Palforzia or other studies of low-dose OIT (300 mg/d), they look equal in terms of their efficacy,” said senior author Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins. Yet, “I’m afraid we had a major [negative] impact on pharma’s interest in pursuing SLIT.”
Without corporate funding, it’s nearly impossible to conduct the large, multisite trials required for FDA approval of a treatment. And without approved products, many allergists are reluctant to offer the therapy, Dr. Wood said. It “makes your life a lot more complicated to be dabbling in things that are not approved,” he noted.
But at least one company is giving it a go. Applying the SLIT principle of delivering food allergens to tolerance-promoting immune cells in the mouth, New York–based Intrommune Therapeutics recently started enrolling peanut-allergic adults for a phase 1 trial of its experimental toothpaste.
Interest in food-allergy SLIT seems to be growing. “I definitely think that it could be an option for the future,” said Jaclyn Bjelac, MD, associate director of the Food Allergy Center of Excellence at the Cleveland Clinic. “Up until a few months ago, it really wasn’t on our radar.”
On conversations with Dr. Kim, philanthropists and drug developers said they found the recent data on SLIT promising, yet pointed out that food SLIT protocols and products are already in the public domain – they are described in published research using allergen extracts that are on the market. They “can’t see a commercial path forward,” Dr. Kim said in an interview. “And that’s kind of where many of my conversations end.”
Although there are no licensed SLIT products for food allergies, between 2014 and 2017, the FDA approved four sublingual immunotherapy tablets to treat environmental allergies – Stallergenes-Greer’s Oralair and ALK’s Grastek for grass pollens, ALK’s Odactra for dust mites, and ALK’s Ragwitek for short ragweed.
SLIT tablets work as well as allergy shots (subcutaneous immunotherapy) for controlling environmental allergy symptoms, they have a better safety profile, according to AAAAI guidelines, and they can be self-administered at home, which has made them a popular option globally. “Our European colleagues have used sublingual immunotherapy much more frequently than, for example, in the U.S.,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
Use of SLIT is also increasing in the United States, especially as FDA-approved products become available. In a 2019 survey, the percentage of U.S. allergists who said they were offering sublingual treatment for environmental allergies increased from 5.9% in 2007 to 73.5% in 2019. However, only 11.2% reported extensive SLIT use; the remainder reported some (50.5%) or little (38.3%) use.
As noted above, considerably fewer U.S. allergists use SLIT to treat food allergies. Similarly, a 2021 survey of allergists in Canada found that only 7% offered food sublingual immunotherapy; more than half reported offering OIT.
One practice, Allergy Associates of La Crosse (Wis.), has offered SLIT drops for food and environmental allergies for decades. Since the clinic opened in 1970, more than 200,000 people have been treated with its protocol. Every patient receives customized sublingual drops – “exactly what they’re allergic to, exactly how allergic they are, and then we build from there,” said Jeff Kessler, MBA, FACHE, practice executive at Allergy Associates of La Crosse. “Quite frankly, it’s the way immunotherapy should be done.
A version of this article first appeared on Medscape.com. This is part one of a three-part series. Part two is here. Part three is here.
California’s highest COVID infection rates shift to rural counties
Most of us are familiar with the good news: In recent weeks, rates of COVID-19 infection and death have plummeted in California, falling to levels not seen since the early days of the pandemic. The average number of new COVID infections reported each day dropped by an astounding 98% from December to June, according to figures from the California Department of Public Health.
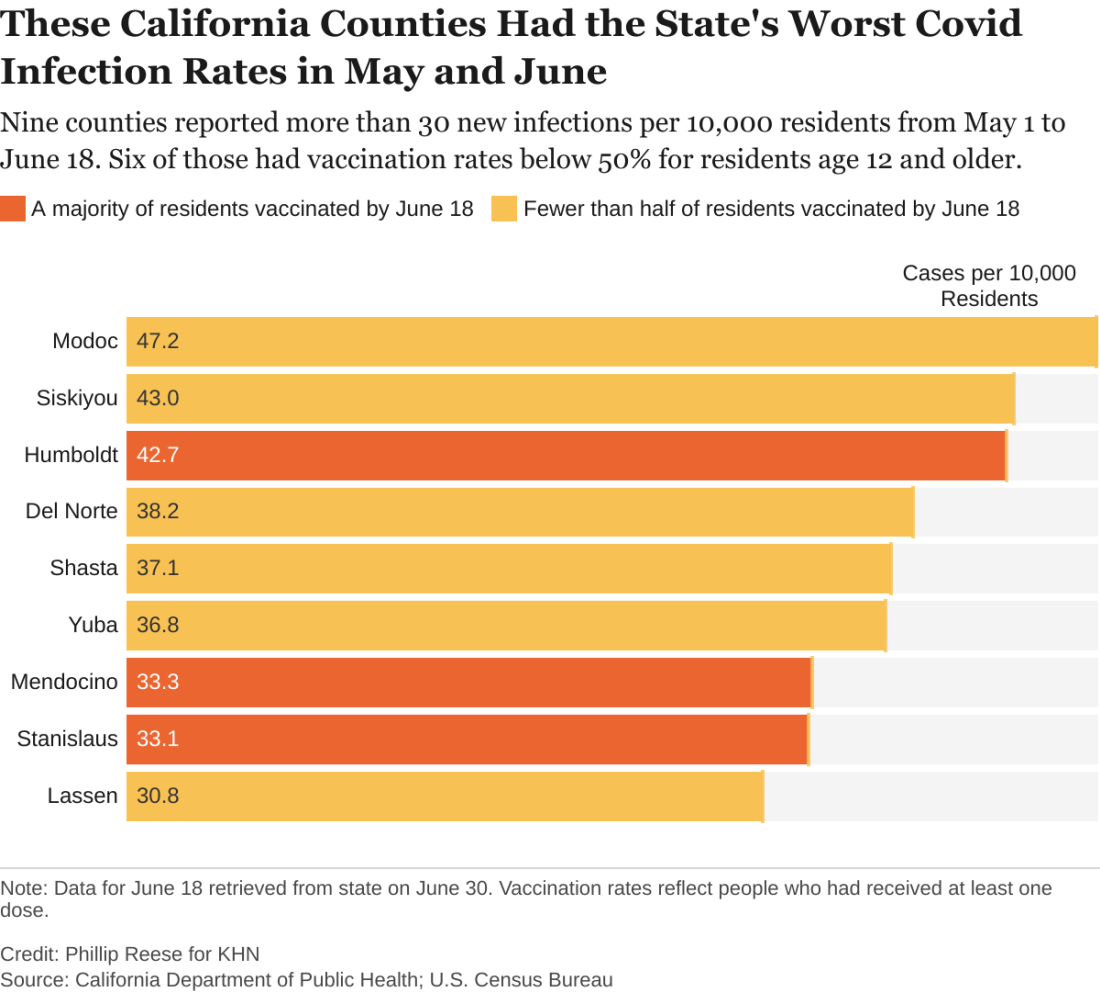
And bolstering that trend, nearly 70% of Californians 12 and older are partially or fully vaccinated.
But state health officials are still reporting nearly 1,000 new COVID cases and more than 2 dozen COVID-related deaths per day. So,
An analysis of state data shows some clear patterns at this stage of the pandemic: As vaccination rates rose across the state, the overall numbers of cases and deaths plunged. But within that broader trend are pronounced regional discrepancies. Counties with relatively low rates of vaccination reported much higher rates of COVID infections and deaths in May and June than counties with high vaccination rates.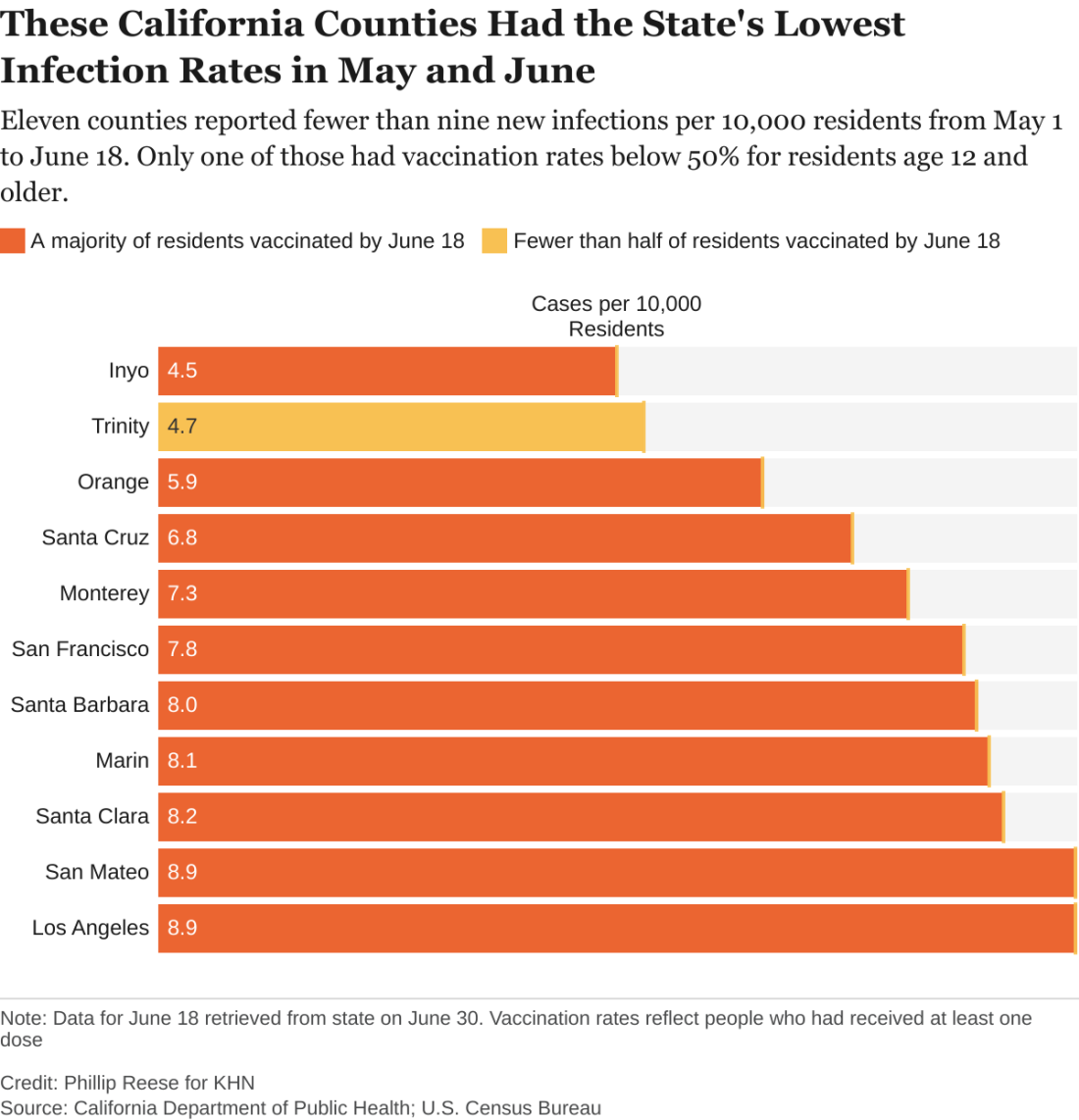
There were about 182 new COVID infections per 100,000 residents from May 1 to June 18 in California counties where fewer than half of residents age 12 and older had received at least one vaccine dose, CDPH data show. By comparison, there were about 102 COVID infections per 100,000 residents in counties where more than two-thirds of residents 12 and up had gotten at least one dose.
“If you live in an area that has low vaccination rates and you have a few people who start to develop a disease, it’s going to spread quickly among those who aren’t vaccinated,” said Rita Burke, PhD, assistant professor of clinical preventive medicine at the University of Southern California, Los Angeles. Dr. Burke noted that the highly contagious Delta variant of the coronavirus now circulating in California amplifies the threat of serious outbreaks in areas with low vaccination rates.
The regional discrepancies in COVID-related deaths are also striking. There were about 3.2 COVID-related deaths per 100,000 residents from May 1 to June 18 in counties where first-dose vaccination rates were below 50%. That is almost twice as high as the death rate in counties where more than two-thirds of residents had at least one dose.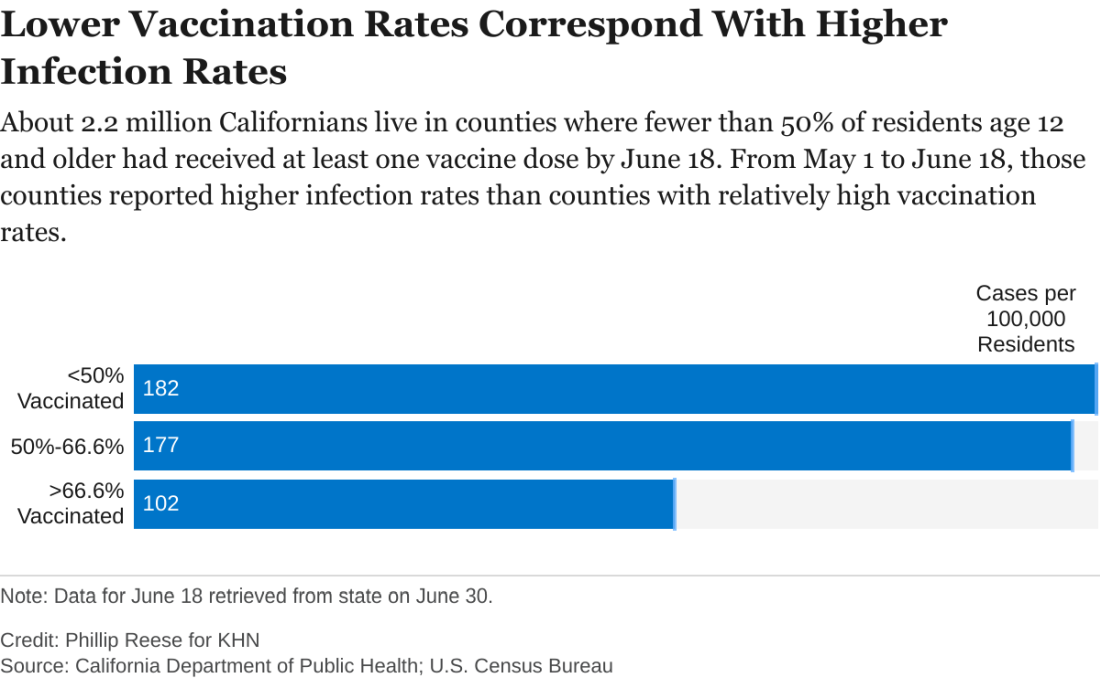
While the pattern is clear, there are exceptions. A couple of sparsely populated mountain counties with low vaccination rates – Trinity and Mariposa – also had relatively low rates of new infections in May and June. Likewise, a few suburban counties with high vaccination rates – among them Sonoma and Contra Costa – had relatively high rates of new infections.
“There are three things that are going on,” said George Rutherford, MD, a professor of epidemiology and biostatistics at the University of California, San Francisco. “One is the vaccine – very important, but not the whole story. One is naturally acquired immunity, which is huge in some places.” A third, he said, is people still managing to evade infection, whether by taking precautions or simply by living in areas with few infections.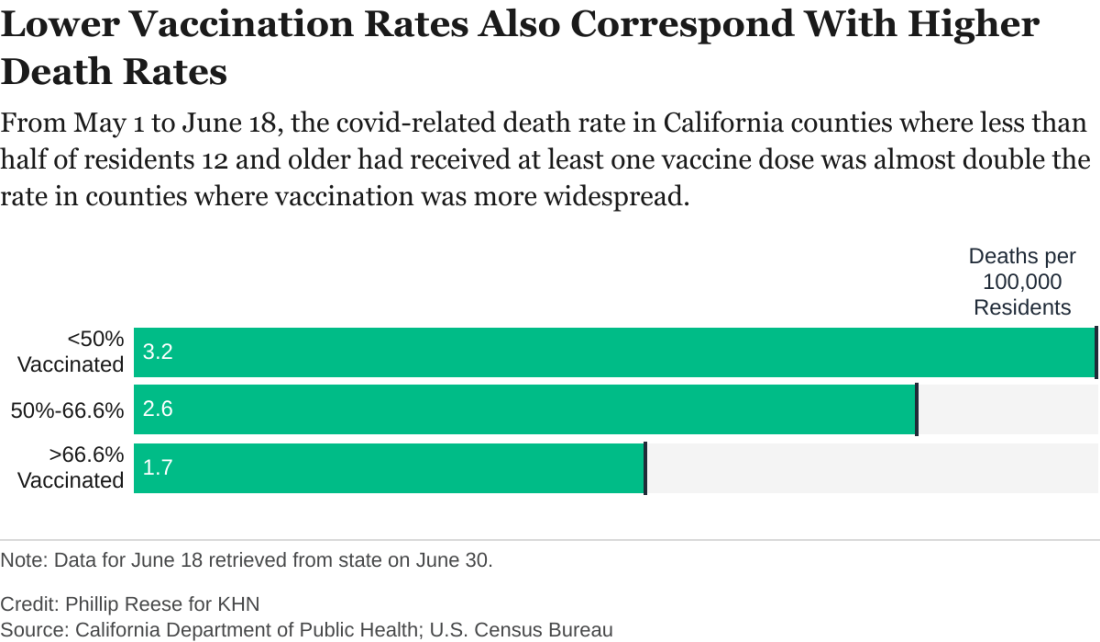
As of June 18, about 67% of Californians age 12 and older had received at least one dose of COVID vaccine, according to the state health department. But that masks a wide variance among the state’s 58 counties. In 14 counties, for example, fewer than half of residents 12 and older had received a shot. In 19 counties, more than two-thirds had.
The counties with low vaccination rates are largely rugged and rural. Nearly all are politically conservative. In January, about 6% of the state’s COVID infections were in the 23 counties where a majority of voters cast ballots for President Donald Trump in November. By May and June, that figure had risen to 11%.
While surveys indicate politics plays a role in vaccine hesitancy in many communities, access also remains an issue in many of California’s rural outposts. It can be hard, or at least inconvenient, for people who live far from the nearest medical facility to get two shots a month apart.
“If you have to drive 30 minutes out to the nearest vaccination site, you may not be as inclined to do that versus if it’s 5 minutes from your house,” Dr. Burke said. “And so we, the public health community, recognize that and have really made a concerted effort in order to eliminate or alleviate that access issue.”
Many of the counties with low vaccination rates had relatively low infection rates in the early months of the pandemic, largely thanks to their remoteness. But, as COVID reaches those communities, that lack of prior exposure and acquired immunity magnifies their vulnerability, Dr. Rutherford said. “We’re going to see cases where people are unvaccinated or where there’s not been a big background level of immunity already.”
As it becomes clearer that new infections will be disproportionately concentrated in areas with low vaccination rates, state officials are working to persuade hesitant Californians to get a vaccine, even introducing a vaccine lottery.
But most persuasive are friends and family members who can help counter the disinformation rampant in some communities, said Lorena Garcia, DrPH, an associate professor of epidemiology at the University of California, Davis. Belittling people for their hesitancy or getting into a political argument likely won’t work.
When talking to her own skeptical relatives, Dr. Garcia avoided politics: “I just explained any questions that they had.”
“Vaccines are a good part of our life,” she said. “It’s something that we’ve done since we were babies. So, it’s just something we’re going to do again.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Most of us are familiar with the good news: In recent weeks, rates of COVID-19 infection and death have plummeted in California, falling to levels not seen since the early days of the pandemic. The average number of new COVID infections reported each day dropped by an astounding 98% from December to June, according to figures from the California Department of Public Health.

And bolstering that trend, nearly 70% of Californians 12 and older are partially or fully vaccinated.
But state health officials are still reporting nearly 1,000 new COVID cases and more than 2 dozen COVID-related deaths per day. So,
An analysis of state data shows some clear patterns at this stage of the pandemic: As vaccination rates rose across the state, the overall numbers of cases and deaths plunged. But within that broader trend are pronounced regional discrepancies. Counties with relatively low rates of vaccination reported much higher rates of COVID infections and deaths in May and June than counties with high vaccination rates.
There were about 182 new COVID infections per 100,000 residents from May 1 to June 18 in California counties where fewer than half of residents age 12 and older had received at least one vaccine dose, CDPH data show. By comparison, there were about 102 COVID infections per 100,000 residents in counties where more than two-thirds of residents 12 and up had gotten at least one dose.
“If you live in an area that has low vaccination rates and you have a few people who start to develop a disease, it’s going to spread quickly among those who aren’t vaccinated,” said Rita Burke, PhD, assistant professor of clinical preventive medicine at the University of Southern California, Los Angeles. Dr. Burke noted that the highly contagious Delta variant of the coronavirus now circulating in California amplifies the threat of serious outbreaks in areas with low vaccination rates.
The regional discrepancies in COVID-related deaths are also striking. There were about 3.2 COVID-related deaths per 100,000 residents from May 1 to June 18 in counties where first-dose vaccination rates were below 50%. That is almost twice as high as the death rate in counties where more than two-thirds of residents had at least one dose.
While the pattern is clear, there are exceptions. A couple of sparsely populated mountain counties with low vaccination rates – Trinity and Mariposa – also had relatively low rates of new infections in May and June. Likewise, a few suburban counties with high vaccination rates – among them Sonoma and Contra Costa – had relatively high rates of new infections.
“There are three things that are going on,” said George Rutherford, MD, a professor of epidemiology and biostatistics at the University of California, San Francisco. “One is the vaccine – very important, but not the whole story. One is naturally acquired immunity, which is huge in some places.” A third, he said, is people still managing to evade infection, whether by taking precautions or simply by living in areas with few infections.
As of June 18, about 67% of Californians age 12 and older had received at least one dose of COVID vaccine, according to the state health department. But that masks a wide variance among the state’s 58 counties. In 14 counties, for example, fewer than half of residents 12 and older had received a shot. In 19 counties, more than two-thirds had.
The counties with low vaccination rates are largely rugged and rural. Nearly all are politically conservative. In January, about 6% of the state’s COVID infections were in the 23 counties where a majority of voters cast ballots for President Donald Trump in November. By May and June, that figure had risen to 11%.
While surveys indicate politics plays a role in vaccine hesitancy in many communities, access also remains an issue in many of California’s rural outposts. It can be hard, or at least inconvenient, for people who live far from the nearest medical facility to get two shots a month apart.
“If you have to drive 30 minutes out to the nearest vaccination site, you may not be as inclined to do that versus if it’s 5 minutes from your house,” Dr. Burke said. “And so we, the public health community, recognize that and have really made a concerted effort in order to eliminate or alleviate that access issue.”
Many of the counties with low vaccination rates had relatively low infection rates in the early months of the pandemic, largely thanks to their remoteness. But, as COVID reaches those communities, that lack of prior exposure and acquired immunity magnifies their vulnerability, Dr. Rutherford said. “We’re going to see cases where people are unvaccinated or where there’s not been a big background level of immunity already.”
As it becomes clearer that new infections will be disproportionately concentrated in areas with low vaccination rates, state officials are working to persuade hesitant Californians to get a vaccine, even introducing a vaccine lottery.
But most persuasive are friends and family members who can help counter the disinformation rampant in some communities, said Lorena Garcia, DrPH, an associate professor of epidemiology at the University of California, Davis. Belittling people for their hesitancy or getting into a political argument likely won’t work.
When talking to her own skeptical relatives, Dr. Garcia avoided politics: “I just explained any questions that they had.”
“Vaccines are a good part of our life,” she said. “It’s something that we’ve done since we were babies. So, it’s just something we’re going to do again.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Most of us are familiar with the good news: In recent weeks, rates of COVID-19 infection and death have plummeted in California, falling to levels not seen since the early days of the pandemic. The average number of new COVID infections reported each day dropped by an astounding 98% from December to June, according to figures from the California Department of Public Health.

And bolstering that trend, nearly 70% of Californians 12 and older are partially or fully vaccinated.
But state health officials are still reporting nearly 1,000 new COVID cases and more than 2 dozen COVID-related deaths per day. So,
An analysis of state data shows some clear patterns at this stage of the pandemic: As vaccination rates rose across the state, the overall numbers of cases and deaths plunged. But within that broader trend are pronounced regional discrepancies. Counties with relatively low rates of vaccination reported much higher rates of COVID infections and deaths in May and June than counties with high vaccination rates.
There were about 182 new COVID infections per 100,000 residents from May 1 to June 18 in California counties where fewer than half of residents age 12 and older had received at least one vaccine dose, CDPH data show. By comparison, there were about 102 COVID infections per 100,000 residents in counties where more than two-thirds of residents 12 and up had gotten at least one dose.
“If you live in an area that has low vaccination rates and you have a few people who start to develop a disease, it’s going to spread quickly among those who aren’t vaccinated,” said Rita Burke, PhD, assistant professor of clinical preventive medicine at the University of Southern California, Los Angeles. Dr. Burke noted that the highly contagious Delta variant of the coronavirus now circulating in California amplifies the threat of serious outbreaks in areas with low vaccination rates.
The regional discrepancies in COVID-related deaths are also striking. There were about 3.2 COVID-related deaths per 100,000 residents from May 1 to June 18 in counties where first-dose vaccination rates were below 50%. That is almost twice as high as the death rate in counties where more than two-thirds of residents had at least one dose.
While the pattern is clear, there are exceptions. A couple of sparsely populated mountain counties with low vaccination rates – Trinity and Mariposa – also had relatively low rates of new infections in May and June. Likewise, a few suburban counties with high vaccination rates – among them Sonoma and Contra Costa – had relatively high rates of new infections.
“There are three things that are going on,” said George Rutherford, MD, a professor of epidemiology and biostatistics at the University of California, San Francisco. “One is the vaccine – very important, but not the whole story. One is naturally acquired immunity, which is huge in some places.” A third, he said, is people still managing to evade infection, whether by taking precautions or simply by living in areas with few infections.
As of June 18, about 67% of Californians age 12 and older had received at least one dose of COVID vaccine, according to the state health department. But that masks a wide variance among the state’s 58 counties. In 14 counties, for example, fewer than half of residents 12 and older had received a shot. In 19 counties, more than two-thirds had.
The counties with low vaccination rates are largely rugged and rural. Nearly all are politically conservative. In January, about 6% of the state’s COVID infections were in the 23 counties where a majority of voters cast ballots for President Donald Trump in November. By May and June, that figure had risen to 11%.
While surveys indicate politics plays a role in vaccine hesitancy in many communities, access also remains an issue in many of California’s rural outposts. It can be hard, or at least inconvenient, for people who live far from the nearest medical facility to get two shots a month apart.
“If you have to drive 30 minutes out to the nearest vaccination site, you may not be as inclined to do that versus if it’s 5 minutes from your house,” Dr. Burke said. “And so we, the public health community, recognize that and have really made a concerted effort in order to eliminate or alleviate that access issue.”
Many of the counties with low vaccination rates had relatively low infection rates in the early months of the pandemic, largely thanks to their remoteness. But, as COVID reaches those communities, that lack of prior exposure and acquired immunity magnifies their vulnerability, Dr. Rutherford said. “We’re going to see cases where people are unvaccinated or where there’s not been a big background level of immunity already.”
As it becomes clearer that new infections will be disproportionately concentrated in areas with low vaccination rates, state officials are working to persuade hesitant Californians to get a vaccine, even introducing a vaccine lottery.
But most persuasive are friends and family members who can help counter the disinformation rampant in some communities, said Lorena Garcia, DrPH, an associate professor of epidemiology at the University of California, Davis. Belittling people for their hesitancy or getting into a political argument likely won’t work.
When talking to her own skeptical relatives, Dr. Garcia avoided politics: “I just explained any questions that they had.”
“Vaccines are a good part of our life,” she said. “It’s something that we’ve done since we were babies. So, it’s just something we’re going to do again.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Sleep-disordered breathing in neuromuscular disease: Early noninvasive ventilation needed
Sleep-disordered breathing is common in patients with neuromuscular disease and is increasingly addressed with noninvasive ventilation, but its patterns go beyond obstructive sleep apnea (OSA) and include hypoventilation, hypoxemia, central sleep apnea, pseudocentrals, periodic breathing, and Cheyne-Stokes respiration, Gaurav Singh, MD, MPH said at the virtual annual meeting of the Associated Professional Sleep Societies.
The prevalence of sleep-related disordered breathing surpasses 40% in patients diagnosed with neuromuscular disease, but “sleep disordered breathing [in these patients] does not equal obstructive sleep apnea,” said Dr. Singh, staff physician at the Veteran Affairs Palo Alto (Calif.) Health Care System in the section of pulmonary, critical care and sleep medicine, and an affiliated clinical assistant professor at Stanford (Calif.) University.
“The most common sleep-related breathing disorder in neuromuscular disease is probably hypopnea and hypoventilation with the sawtooth pattern of dips in oxygen saturation that occur during REM sleep,” he said. As neuromuscular diseases progress, hypoventilation may occur during non-REM sleep as well.
Evaluation is usually performed with polysomnography and pulmonary function testing, he said, but supplementary testing including serum bicarbonate levels, arterial blood gases, and echocardiography to assess for left ventricular ejection fraction and cardiomyopathy may be useful as well.
While a sleep study is not required per Centers for Medicare & Medicaid coverage criteria for the use of respiratory assist devices in patients with neuromuscular disease, polysomnography is valuable for identifying early nocturnal respiratory impairment before the appearance of symptoms and daytime abnormalities in gas exchange, and is better than home testing for distinguishing different types of events (including pseudocentrals). It also is helpful for determining the appropriate pressures needed for ventilatory support and for assessing the need for a backup rate, Dr. Singh said.
Commonly used types of noninvasive ventilation include bilevel positive airway pressure on the spontaneous/timed or pressure control modes, with or without volume-assured pressure support, he said.
Expiratory positive airway pressure (EPAP) is usually set low initially to help decrease the work of breathing and improve triggering, then titrated up to ensure that upper airway obstructive events are treated. Pressure support (the difference between the inspiratory positive airway pressure and EPAP) is set to achieve target tidal volume and to rest the respiratory muscles. And inspiratory time is set “on the longer end” to achieve maximal target volume and ensure appropriate gas exchange, Dr. Singh said.
Data from randomized controlled trials evaluating the effectiveness of NIV are limited, he said. A study published 15 years ago showed a survival benefit and improvement in quality of life measures in patients with amyotrophic lateral sclerosis (ALS) with normal or moderately impaired bulbar function but not in those with severe bulbar weakness.
Regarding the timing of initiating NIV, a retrospective study published several years ago looked at almost 200 ALS patients and evaluated differences in survival amongst those started earlier with NIV (forced vital capacity ≥80%) and those started later (FVC <80%). At 36 months from diagnosis, mortality was 35% for the early group and 53% for the later group. “Improved survival was driven by benefit in patients with non–bulbar-onset ALS, compared with bulbar-onset disease,” Dr. Singh said.
“This study and several other similar studies seem to indicate that the earlier NIV [noninvasive ventilation] is started in patients with neuromuscular disease, the better in terms of improving survival and other relevant measures such as quality of life,” he said.
Asked about Dr. Singh’s presentation, Michelle Cao, DO, clinical associate professor at Stanford University, said that NIV is an “invaluable tool in the treatment of conditions leading to chronic respiratory failure,” such as neuromuscular disease, and that it’s important to incorporate NIV training for future pulmonary, critical care and sleep physicians. Dr. Cao directs the adult NIV program for the neuromuscular medical program at Stanford Health Care.
Saiprakash B. Venkateshiah, MD, of Emory University, Atlanta, also said in introducing Dr. Singh at the meeting that earlier diagnosis and appropriate NIV therapy “may improve quality of life and possibly even lower survival in certain disorders.”
In addition, he noted that sleep disturbances “may be the earliest sign of muscle weakness in [patients with neuromuscular disease], sometimes being detected before their underlying neuromuscular disease is diagnosed.”
Dr. Singh, Dr. Cao, and Dr. Venkateshiah each reported that they had no potential conflicts of interest.
Sleep-disordered breathing is common in patients with neuromuscular disease and is increasingly addressed with noninvasive ventilation, but its patterns go beyond obstructive sleep apnea (OSA) and include hypoventilation, hypoxemia, central sleep apnea, pseudocentrals, periodic breathing, and Cheyne-Stokes respiration, Gaurav Singh, MD, MPH said at the virtual annual meeting of the Associated Professional Sleep Societies.
The prevalence of sleep-related disordered breathing surpasses 40% in patients diagnosed with neuromuscular disease, but “sleep disordered breathing [in these patients] does not equal obstructive sleep apnea,” said Dr. Singh, staff physician at the Veteran Affairs Palo Alto (Calif.) Health Care System in the section of pulmonary, critical care and sleep medicine, and an affiliated clinical assistant professor at Stanford (Calif.) University.
“The most common sleep-related breathing disorder in neuromuscular disease is probably hypopnea and hypoventilation with the sawtooth pattern of dips in oxygen saturation that occur during REM sleep,” he said. As neuromuscular diseases progress, hypoventilation may occur during non-REM sleep as well.
Evaluation is usually performed with polysomnography and pulmonary function testing, he said, but supplementary testing including serum bicarbonate levels, arterial blood gases, and echocardiography to assess for left ventricular ejection fraction and cardiomyopathy may be useful as well.
While a sleep study is not required per Centers for Medicare & Medicaid coverage criteria for the use of respiratory assist devices in patients with neuromuscular disease, polysomnography is valuable for identifying early nocturnal respiratory impairment before the appearance of symptoms and daytime abnormalities in gas exchange, and is better than home testing for distinguishing different types of events (including pseudocentrals). It also is helpful for determining the appropriate pressures needed for ventilatory support and for assessing the need for a backup rate, Dr. Singh said.
Commonly used types of noninvasive ventilation include bilevel positive airway pressure on the spontaneous/timed or pressure control modes, with or without volume-assured pressure support, he said.
Expiratory positive airway pressure (EPAP) is usually set low initially to help decrease the work of breathing and improve triggering, then titrated up to ensure that upper airway obstructive events are treated. Pressure support (the difference between the inspiratory positive airway pressure and EPAP) is set to achieve target tidal volume and to rest the respiratory muscles. And inspiratory time is set “on the longer end” to achieve maximal target volume and ensure appropriate gas exchange, Dr. Singh said.
Data from randomized controlled trials evaluating the effectiveness of NIV are limited, he said. A study published 15 years ago showed a survival benefit and improvement in quality of life measures in patients with amyotrophic lateral sclerosis (ALS) with normal or moderately impaired bulbar function but not in those with severe bulbar weakness.
Regarding the timing of initiating NIV, a retrospective study published several years ago looked at almost 200 ALS patients and evaluated differences in survival amongst those started earlier with NIV (forced vital capacity ≥80%) and those started later (FVC <80%). At 36 months from diagnosis, mortality was 35% for the early group and 53% for the later group. “Improved survival was driven by benefit in patients with non–bulbar-onset ALS, compared with bulbar-onset disease,” Dr. Singh said.
“This study and several other similar studies seem to indicate that the earlier NIV [noninvasive ventilation] is started in patients with neuromuscular disease, the better in terms of improving survival and other relevant measures such as quality of life,” he said.
Asked about Dr. Singh’s presentation, Michelle Cao, DO, clinical associate professor at Stanford University, said that NIV is an “invaluable tool in the treatment of conditions leading to chronic respiratory failure,” such as neuromuscular disease, and that it’s important to incorporate NIV training for future pulmonary, critical care and sleep physicians. Dr. Cao directs the adult NIV program for the neuromuscular medical program at Stanford Health Care.
Saiprakash B. Venkateshiah, MD, of Emory University, Atlanta, also said in introducing Dr. Singh at the meeting that earlier diagnosis and appropriate NIV therapy “may improve quality of life and possibly even lower survival in certain disorders.”
In addition, he noted that sleep disturbances “may be the earliest sign of muscle weakness in [patients with neuromuscular disease], sometimes being detected before their underlying neuromuscular disease is diagnosed.”
Dr. Singh, Dr. Cao, and Dr. Venkateshiah each reported that they had no potential conflicts of interest.
Sleep-disordered breathing is common in patients with neuromuscular disease and is increasingly addressed with noninvasive ventilation, but its patterns go beyond obstructive sleep apnea (OSA) and include hypoventilation, hypoxemia, central sleep apnea, pseudocentrals, periodic breathing, and Cheyne-Stokes respiration, Gaurav Singh, MD, MPH said at the virtual annual meeting of the Associated Professional Sleep Societies.
The prevalence of sleep-related disordered breathing surpasses 40% in patients diagnosed with neuromuscular disease, but “sleep disordered breathing [in these patients] does not equal obstructive sleep apnea,” said Dr. Singh, staff physician at the Veteran Affairs Palo Alto (Calif.) Health Care System in the section of pulmonary, critical care and sleep medicine, and an affiliated clinical assistant professor at Stanford (Calif.) University.
“The most common sleep-related breathing disorder in neuromuscular disease is probably hypopnea and hypoventilation with the sawtooth pattern of dips in oxygen saturation that occur during REM sleep,” he said. As neuromuscular diseases progress, hypoventilation may occur during non-REM sleep as well.
Evaluation is usually performed with polysomnography and pulmonary function testing, he said, but supplementary testing including serum bicarbonate levels, arterial blood gases, and echocardiography to assess for left ventricular ejection fraction and cardiomyopathy may be useful as well.
While a sleep study is not required per Centers for Medicare & Medicaid coverage criteria for the use of respiratory assist devices in patients with neuromuscular disease, polysomnography is valuable for identifying early nocturnal respiratory impairment before the appearance of symptoms and daytime abnormalities in gas exchange, and is better than home testing for distinguishing different types of events (including pseudocentrals). It also is helpful for determining the appropriate pressures needed for ventilatory support and for assessing the need for a backup rate, Dr. Singh said.
Commonly used types of noninvasive ventilation include bilevel positive airway pressure on the spontaneous/timed or pressure control modes, with or without volume-assured pressure support, he said.
Expiratory positive airway pressure (EPAP) is usually set low initially to help decrease the work of breathing and improve triggering, then titrated up to ensure that upper airway obstructive events are treated. Pressure support (the difference between the inspiratory positive airway pressure and EPAP) is set to achieve target tidal volume and to rest the respiratory muscles. And inspiratory time is set “on the longer end” to achieve maximal target volume and ensure appropriate gas exchange, Dr. Singh said.
Data from randomized controlled trials evaluating the effectiveness of NIV are limited, he said. A study published 15 years ago showed a survival benefit and improvement in quality of life measures in patients with amyotrophic lateral sclerosis (ALS) with normal or moderately impaired bulbar function but not in those with severe bulbar weakness.
Regarding the timing of initiating NIV, a retrospective study published several years ago looked at almost 200 ALS patients and evaluated differences in survival amongst those started earlier with NIV (forced vital capacity ≥80%) and those started later (FVC <80%). At 36 months from diagnosis, mortality was 35% for the early group and 53% for the later group. “Improved survival was driven by benefit in patients with non–bulbar-onset ALS, compared with bulbar-onset disease,” Dr. Singh said.
“This study and several other similar studies seem to indicate that the earlier NIV [noninvasive ventilation] is started in patients with neuromuscular disease, the better in terms of improving survival and other relevant measures such as quality of life,” he said.
Asked about Dr. Singh’s presentation, Michelle Cao, DO, clinical associate professor at Stanford University, said that NIV is an “invaluable tool in the treatment of conditions leading to chronic respiratory failure,” such as neuromuscular disease, and that it’s important to incorporate NIV training for future pulmonary, critical care and sleep physicians. Dr. Cao directs the adult NIV program for the neuromuscular medical program at Stanford Health Care.
Saiprakash B. Venkateshiah, MD, of Emory University, Atlanta, also said in introducing Dr. Singh at the meeting that earlier diagnosis and appropriate NIV therapy “may improve quality of life and possibly even lower survival in certain disorders.”
In addition, he noted that sleep disturbances “may be the earliest sign of muscle weakness in [patients with neuromuscular disease], sometimes being detected before their underlying neuromuscular disease is diagnosed.”
Dr. Singh, Dr. Cao, and Dr. Venkateshiah each reported that they had no potential conflicts of interest.
FROM SLEEP 2021
Delta becomes dominant coronavirus variant in U.S.
The contagious Delta variant has become the dominant form of the coronavirus in the United States, now accounting for more than 51% of COVID-19 cases in the country, according to new CDC data to updated on July 6.
The variant, also known as B.1.617.2 and first detected in India, makes up more than 80% of new cases in some Midwestern states, including Iowa, Kansas, and Missouri. Delta also accounts for 74% of cases in Western states such as Colorado and Utah and 59% of cases in Southern states such as Louisiana and Texas.
Communities with low vaccination rates are bearing the brunt of new Delta cases. Public health experts are urging those who are unvaccinated to get a shot to protect themselves and their communities against future surges.
“Right now we have two Americas: the vaccinated and the unvaccinated,” Paul Offit, MD, an infectious disease specialist at Children’s Hospital of Philadelphia, told NPR.
“We’re feeling pretty good right now because it’s the summer,” he said. “But come winter, if we still have a significant percentage of the population that is unvaccinated, we’re going to see this virus surge again.”
So far, COVID-19 vaccines appear to protect people against the Delta variant. But health officials are watching other variants that could evade vaccine protection and lead to major outbreaks this year.
For instance, certain mutations in the Epsilon variant may allow it to evade the immunity from past infections and current COVID-19 vaccines, according to a new study published July 1 in the Science. The variant, also known as B.1.427/B.1.429 and first identified in California, has now been reported in 34 countries and could become widespread in the United States.
Researchers from the University of Washington and clinics in Switzerland tested the variant in blood samples from vaccinated people, as well as those who were previously infected with COVID-19. They found that the neutralizing power was reduced by about 2 to 3½ times.
The research team also visualized the variant and found that three mutations on Epsilon’s spike protein allow the virus to escape certain antibodies and lower the strength of vaccines.
Epsilon “relies on an indirect and unusual neutralization-escape strategy,” they wrote, saying that understanding these escape routes could help scientists track new variants, curb the pandemic, and create booster shots.
In Australia, for instance, public health officials have detected the Lambda variant, which could be more infectious than the Delta variant and resistant to vaccines, according to Sky News.
A hotel quarantine program in New South Wales identified the variant in someone who had returned from travel, the news outlet reported. Also known as C.37, Lambda was named a “variant of interest” by the World Health Organization in June.
Lambda was first identified in Peru in December and now accounts for more than 80% of the country’s cases, according to the Financial Times. It has since been found in 27 countries, including the U.S., U.K., and Germany.
The variant has seven mutations on the spike protein that allow the virus to infect human cells, the news outlet reported. One mutation is like another mutation on the Delta variant, which could make it more contagious.
In a preprint study published July 1, researchers at the University of Chile at Santiago found that Lambda is better able to escape antibodies created by the CoronaVac vaccine made by Sinovac in China. In the paper, which hasn’t yet been peer-reviewed, researchers tested blood samples from local health care workers in Santiago who had received two doses of the vaccine.
“Our data revealed that the spike protein ... carries mutations conferring increased infectivity and the ability to escape from neutralizing antibodies,” they wrote.
The research team urged countries to continue testing for contagious variants, even in areas with high vaccination rates, so scientists can identify mutations quickly and analyze whether new variants can escape vaccines.
“The world has to get its act together,” Saad Omer, PhD, director of the Yale Institute for Global Health, told NPR. “Otherwise yet another, potentially more dangerous, variant could emerge.”
A version of this article first appeared on WebMD.com.
The contagious Delta variant has become the dominant form of the coronavirus in the United States, now accounting for more than 51% of COVID-19 cases in the country, according to new CDC data to updated on July 6.
The variant, also known as B.1.617.2 and first detected in India, makes up more than 80% of new cases in some Midwestern states, including Iowa, Kansas, and Missouri. Delta also accounts for 74% of cases in Western states such as Colorado and Utah and 59% of cases in Southern states such as Louisiana and Texas.
Communities with low vaccination rates are bearing the brunt of new Delta cases. Public health experts are urging those who are unvaccinated to get a shot to protect themselves and their communities against future surges.
“Right now we have two Americas: the vaccinated and the unvaccinated,” Paul Offit, MD, an infectious disease specialist at Children’s Hospital of Philadelphia, told NPR.
“We’re feeling pretty good right now because it’s the summer,” he said. “But come winter, if we still have a significant percentage of the population that is unvaccinated, we’re going to see this virus surge again.”
So far, COVID-19 vaccines appear to protect people against the Delta variant. But health officials are watching other variants that could evade vaccine protection and lead to major outbreaks this year.
For instance, certain mutations in the Epsilon variant may allow it to evade the immunity from past infections and current COVID-19 vaccines, according to a new study published July 1 in the Science. The variant, also known as B.1.427/B.1.429 and first identified in California, has now been reported in 34 countries and could become widespread in the United States.
Researchers from the University of Washington and clinics in Switzerland tested the variant in blood samples from vaccinated people, as well as those who were previously infected with COVID-19. They found that the neutralizing power was reduced by about 2 to 3½ times.
The research team also visualized the variant and found that three mutations on Epsilon’s spike protein allow the virus to escape certain antibodies and lower the strength of vaccines.
Epsilon “relies on an indirect and unusual neutralization-escape strategy,” they wrote, saying that understanding these escape routes could help scientists track new variants, curb the pandemic, and create booster shots.
In Australia, for instance, public health officials have detected the Lambda variant, which could be more infectious than the Delta variant and resistant to vaccines, according to Sky News.
A hotel quarantine program in New South Wales identified the variant in someone who had returned from travel, the news outlet reported. Also known as C.37, Lambda was named a “variant of interest” by the World Health Organization in June.
Lambda was first identified in Peru in December and now accounts for more than 80% of the country’s cases, according to the Financial Times. It has since been found in 27 countries, including the U.S., U.K., and Germany.
The variant has seven mutations on the spike protein that allow the virus to infect human cells, the news outlet reported. One mutation is like another mutation on the Delta variant, which could make it more contagious.
In a preprint study published July 1, researchers at the University of Chile at Santiago found that Lambda is better able to escape antibodies created by the CoronaVac vaccine made by Sinovac in China. In the paper, which hasn’t yet been peer-reviewed, researchers tested blood samples from local health care workers in Santiago who had received two doses of the vaccine.
“Our data revealed that the spike protein ... carries mutations conferring increased infectivity and the ability to escape from neutralizing antibodies,” they wrote.
The research team urged countries to continue testing for contagious variants, even in areas with high vaccination rates, so scientists can identify mutations quickly and analyze whether new variants can escape vaccines.
“The world has to get its act together,” Saad Omer, PhD, director of the Yale Institute for Global Health, told NPR. “Otherwise yet another, potentially more dangerous, variant could emerge.”
A version of this article first appeared on WebMD.com.
The contagious Delta variant has become the dominant form of the coronavirus in the United States, now accounting for more than 51% of COVID-19 cases in the country, according to new CDC data to updated on July 6.
The variant, also known as B.1.617.2 and first detected in India, makes up more than 80% of new cases in some Midwestern states, including Iowa, Kansas, and Missouri. Delta also accounts for 74% of cases in Western states such as Colorado and Utah and 59% of cases in Southern states such as Louisiana and Texas.
Communities with low vaccination rates are bearing the brunt of new Delta cases. Public health experts are urging those who are unvaccinated to get a shot to protect themselves and their communities against future surges.
“Right now we have two Americas: the vaccinated and the unvaccinated,” Paul Offit, MD, an infectious disease specialist at Children’s Hospital of Philadelphia, told NPR.
“We’re feeling pretty good right now because it’s the summer,” he said. “But come winter, if we still have a significant percentage of the population that is unvaccinated, we’re going to see this virus surge again.”
So far, COVID-19 vaccines appear to protect people against the Delta variant. But health officials are watching other variants that could evade vaccine protection and lead to major outbreaks this year.
For instance, certain mutations in the Epsilon variant may allow it to evade the immunity from past infections and current COVID-19 vaccines, according to a new study published July 1 in the Science. The variant, also known as B.1.427/B.1.429 and first identified in California, has now been reported in 34 countries and could become widespread in the United States.
Researchers from the University of Washington and clinics in Switzerland tested the variant in blood samples from vaccinated people, as well as those who were previously infected with COVID-19. They found that the neutralizing power was reduced by about 2 to 3½ times.
The research team also visualized the variant and found that three mutations on Epsilon’s spike protein allow the virus to escape certain antibodies and lower the strength of vaccines.
Epsilon “relies on an indirect and unusual neutralization-escape strategy,” they wrote, saying that understanding these escape routes could help scientists track new variants, curb the pandemic, and create booster shots.
In Australia, for instance, public health officials have detected the Lambda variant, which could be more infectious than the Delta variant and resistant to vaccines, according to Sky News.
A hotel quarantine program in New South Wales identified the variant in someone who had returned from travel, the news outlet reported. Also known as C.37, Lambda was named a “variant of interest” by the World Health Organization in June.
Lambda was first identified in Peru in December and now accounts for more than 80% of the country’s cases, according to the Financial Times. It has since been found in 27 countries, including the U.S., U.K., and Germany.
The variant has seven mutations on the spike protein that allow the virus to infect human cells, the news outlet reported. One mutation is like another mutation on the Delta variant, which could make it more contagious.
In a preprint study published July 1, researchers at the University of Chile at Santiago found that Lambda is better able to escape antibodies created by the CoronaVac vaccine made by Sinovac in China. In the paper, which hasn’t yet been peer-reviewed, researchers tested blood samples from local health care workers in Santiago who had received two doses of the vaccine.
“Our data revealed that the spike protein ... carries mutations conferring increased infectivity and the ability to escape from neutralizing antibodies,” they wrote.
The research team urged countries to continue testing for contagious variants, even in areas with high vaccination rates, so scientists can identify mutations quickly and analyze whether new variants can escape vaccines.
“The world has to get its act together,” Saad Omer, PhD, director of the Yale Institute for Global Health, told NPR. “Otherwise yet another, potentially more dangerous, variant could emerge.”
A version of this article first appeared on WebMD.com.
What’s my number? Do I really need $10 million to retire from my medical practice?
“What’s my number?” When I hear this from my financial planning clients, I know they mean: In my 20-year career, this “magic number” is by far the most common thing physicians want to know.
If you look online, articles may recommend having a portfolio valued at $2 million, $5 million, and not uncommonly $10 million or more to retire. Really? $10 million? You might be thinking that surely not everyone needs that amount. Luckily, that’s true.
There’s no magic number your portfolio should be – just your number.
It’s human nature to want a simple, clear target to shoot for. But unfortunately, there’s no generic answer when it comes to saving for retirement. Even after a comprehensive hour-long review of a client’s financial plan – including insurance, investments, estate planning, and other items – the most honest answer I can give is: “It depends.” Not satisfying, I know. But there are still too many holes to fill.
By far the most important factor in getting beyond “it depends” is having an accurate estimate of annual retirement expenses. I have clients who live comfortably on $50,000 a year in retirement and others who need $250,000 or more. Knowing how much you need – your personal number – depends on the individual’s unique dream for retirement and calculating what that dream will cost.
Form a guesstimate based on savings and anticipated expenses
The total portfolio value needed to sustain an annual expense of $50,000 a year in retirement spending versus the portfolio size needed for $250,000 or more, blows apart the fiction of a universal “magic number.” It’s just not that simple. While it’s hard to gauge exactly what you will need, the right information can lead to a logical guesstimate about what size portfolio will provide you with financial independence.
In the end, it’s up to you to determine your desired retirement lifestyle. Then, the only way to get there is to calculate how much it will cost and save up for it by following a well-informed financial plan. This plan will be based on strategy that shifts from the middle to the later stages of your medical career and into retirement.
Let’s see how it works.
Early to mid-career: Focus on building up retirement savings
We ultimately want to save enough to meet our retirement expenses. But figuring out how much to save when you’re in your 40s and 50s is difficult. A mid-career physician likely has significant family- and child-related expenses. When we become empty-nesters, those expenses will decline. In retirement they may disappear entirely, but new expenses may arise.
With large variations in expenses at different life stages, it’s hard to calculate exactly how much you will need to save. Early on, the most sensible thing is putting aside a “reasonable” percentage of gross income for retirement savings.
What is a ‘reasonable’ savings goal for retirement?
As is often the case with high-income earners, many of our clients don’t have a budget or a clear picture of their current expenses and spending habits. That’s alright as long as they are building up a reasonable nest egg for the future – which begs the question of what is reasonable.
For mid-career docs, a reasonable goal to aim for is putting aside 20% of gross income for retirement. What you spend the rest of your money on is less important than how much you’re saving.
This is quite different from how you’ll handle expenses during retirement, when you no longer have a steady stream of income; rather, you have a pot of money that needs to last you another 20, 30, or even 40 years. At that point, thinking about specific expenses becomes more important (more on this topic later). That said, if you’re a mid-career doctor who is not meeting this 20% savings goal, it’s time to make a plan that will free up cash for retirement savings and investments.
Later-career docs: Calculate your spending level in retirement
Financial success means having a portfolio that can support your retirement dreams – with the confidence that your money will last and you won’t need to watch every dollar you spend. As you near retirement, your focus will shift away from accumulating savings to calculating the annual expenses you will have to meet in retirement.
A good place to start is figuring out which expenses will be necessary and which will be more flexible. To do this, separate your anticipated spending into these two categories:
- Fixed expenses: You can confidently forecast your “must-have” fixed expenses – such as property taxes, property/casualty insurance, health care costs, utilities, and groceries – because they remain steady from month to month.
- Discretionary expenses: These “like-to-have” expenses vary from month to month. This makes them harder to predict but easier to control. They might include dining out, travel, and charitable contributions.
As a retiree, understanding your fixed and discretionary expenses can help you prepare for a bear market, when the stock market can decline by 20% or more. Your portfolio won’t consist entirely of stocks, so it shouldn’t drop to that degree. Still, it will decline significantly. You may need to cut back on spending for a year or 2 to allow your portfolio to recover, particularly if the portfolio declines early in retirement.
Are you ready for retirement?
During the long bull market preceding the great recession of 2007 and 2009, many physicians retired –only to return to their practices when their portfolio values plummeted. In the exuberance of the moment, many failed to heed the warnings of many economists and got caught flat-footed.
Right now it’s a bull market, but we’re seeing concerning signs, such as an out-of-control housing market and rumblings about inflation and rising consumer costs. Sound familiar? If you hope to retire soon, take the time to objectively look around the corner so you can plan appropriately – whether your goal is to retire completely, stay in practice part-time, or even take on a new opportunity.
In an “it-depends” world, don’t be lured by a fictitious magic number, no matter what comes up when you Google: “When can I retire?” Instead, save early, imagine your dream retirement, and calculate expenses later to see what’s possible.
Dr. Greenwald is a graduate of the Albert Einstein College of Medicine, New York. Dr. Greenwald completed his internal medicine residency at the University of Minnesota, Minneapolis. He practiced internal medicine in the Twin Cities for 11 years before making the transition to financial planning for physicians, beginning in 1998.
A version of this article first appeared on Medscape.com.
“What’s my number?” When I hear this from my financial planning clients, I know they mean: In my 20-year career, this “magic number” is by far the most common thing physicians want to know.
If you look online, articles may recommend having a portfolio valued at $2 million, $5 million, and not uncommonly $10 million or more to retire. Really? $10 million? You might be thinking that surely not everyone needs that amount. Luckily, that’s true.
There’s no magic number your portfolio should be – just your number.
It’s human nature to want a simple, clear target to shoot for. But unfortunately, there’s no generic answer when it comes to saving for retirement. Even after a comprehensive hour-long review of a client’s financial plan – including insurance, investments, estate planning, and other items – the most honest answer I can give is: “It depends.” Not satisfying, I know. But there are still too many holes to fill.
By far the most important factor in getting beyond “it depends” is having an accurate estimate of annual retirement expenses. I have clients who live comfortably on $50,000 a year in retirement and others who need $250,000 or more. Knowing how much you need – your personal number – depends on the individual’s unique dream for retirement and calculating what that dream will cost.
Form a guesstimate based on savings and anticipated expenses
The total portfolio value needed to sustain an annual expense of $50,000 a year in retirement spending versus the portfolio size needed for $250,000 or more, blows apart the fiction of a universal “magic number.” It’s just not that simple. While it’s hard to gauge exactly what you will need, the right information can lead to a logical guesstimate about what size portfolio will provide you with financial independence.
In the end, it’s up to you to determine your desired retirement lifestyle. Then, the only way to get there is to calculate how much it will cost and save up for it by following a well-informed financial plan. This plan will be based on strategy that shifts from the middle to the later stages of your medical career and into retirement.
Let’s see how it works.
Early to mid-career: Focus on building up retirement savings
We ultimately want to save enough to meet our retirement expenses. But figuring out how much to save when you’re in your 40s and 50s is difficult. A mid-career physician likely has significant family- and child-related expenses. When we become empty-nesters, those expenses will decline. In retirement they may disappear entirely, but new expenses may arise.
With large variations in expenses at different life stages, it’s hard to calculate exactly how much you will need to save. Early on, the most sensible thing is putting aside a “reasonable” percentage of gross income for retirement savings.
What is a ‘reasonable’ savings goal for retirement?
As is often the case with high-income earners, many of our clients don’t have a budget or a clear picture of their current expenses and spending habits. That’s alright as long as they are building up a reasonable nest egg for the future – which begs the question of what is reasonable.
For mid-career docs, a reasonable goal to aim for is putting aside 20% of gross income for retirement. What you spend the rest of your money on is less important than how much you’re saving.
This is quite different from how you’ll handle expenses during retirement, when you no longer have a steady stream of income; rather, you have a pot of money that needs to last you another 20, 30, or even 40 years. At that point, thinking about specific expenses becomes more important (more on this topic later). That said, if you’re a mid-career doctor who is not meeting this 20% savings goal, it’s time to make a plan that will free up cash for retirement savings and investments.
Later-career docs: Calculate your spending level in retirement
Financial success means having a portfolio that can support your retirement dreams – with the confidence that your money will last and you won’t need to watch every dollar you spend. As you near retirement, your focus will shift away from accumulating savings to calculating the annual expenses you will have to meet in retirement.
A good place to start is figuring out which expenses will be necessary and which will be more flexible. To do this, separate your anticipated spending into these two categories:
- Fixed expenses: You can confidently forecast your “must-have” fixed expenses – such as property taxes, property/casualty insurance, health care costs, utilities, and groceries – because they remain steady from month to month.
- Discretionary expenses: These “like-to-have” expenses vary from month to month. This makes them harder to predict but easier to control. They might include dining out, travel, and charitable contributions.
As a retiree, understanding your fixed and discretionary expenses can help you prepare for a bear market, when the stock market can decline by 20% or more. Your portfolio won’t consist entirely of stocks, so it shouldn’t drop to that degree. Still, it will decline significantly. You may need to cut back on spending for a year or 2 to allow your portfolio to recover, particularly if the portfolio declines early in retirement.
Are you ready for retirement?
During the long bull market preceding the great recession of 2007 and 2009, many physicians retired –only to return to their practices when their portfolio values plummeted. In the exuberance of the moment, many failed to heed the warnings of many economists and got caught flat-footed.
Right now it’s a bull market, but we’re seeing concerning signs, such as an out-of-control housing market and rumblings about inflation and rising consumer costs. Sound familiar? If you hope to retire soon, take the time to objectively look around the corner so you can plan appropriately – whether your goal is to retire completely, stay in practice part-time, or even take on a new opportunity.
In an “it-depends” world, don’t be lured by a fictitious magic number, no matter what comes up when you Google: “When can I retire?” Instead, save early, imagine your dream retirement, and calculate expenses later to see what’s possible.
Dr. Greenwald is a graduate of the Albert Einstein College of Medicine, New York. Dr. Greenwald completed his internal medicine residency at the University of Minnesota, Minneapolis. He practiced internal medicine in the Twin Cities for 11 years before making the transition to financial planning for physicians, beginning in 1998.
A version of this article first appeared on Medscape.com.
“What’s my number?” When I hear this from my financial planning clients, I know they mean: In my 20-year career, this “magic number” is by far the most common thing physicians want to know.
If you look online, articles may recommend having a portfolio valued at $2 million, $5 million, and not uncommonly $10 million or more to retire. Really? $10 million? You might be thinking that surely not everyone needs that amount. Luckily, that’s true.
There’s no magic number your portfolio should be – just your number.
It’s human nature to want a simple, clear target to shoot for. But unfortunately, there’s no generic answer when it comes to saving for retirement. Even after a comprehensive hour-long review of a client’s financial plan – including insurance, investments, estate planning, and other items – the most honest answer I can give is: “It depends.” Not satisfying, I know. But there are still too many holes to fill.
By far the most important factor in getting beyond “it depends” is having an accurate estimate of annual retirement expenses. I have clients who live comfortably on $50,000 a year in retirement and others who need $250,000 or more. Knowing how much you need – your personal number – depends on the individual’s unique dream for retirement and calculating what that dream will cost.
Form a guesstimate based on savings and anticipated expenses
The total portfolio value needed to sustain an annual expense of $50,000 a year in retirement spending versus the portfolio size needed for $250,000 or more, blows apart the fiction of a universal “magic number.” It’s just not that simple. While it’s hard to gauge exactly what you will need, the right information can lead to a logical guesstimate about what size portfolio will provide you with financial independence.
In the end, it’s up to you to determine your desired retirement lifestyle. Then, the only way to get there is to calculate how much it will cost and save up for it by following a well-informed financial plan. This plan will be based on strategy that shifts from the middle to the later stages of your medical career and into retirement.
Let’s see how it works.
Early to mid-career: Focus on building up retirement savings
We ultimately want to save enough to meet our retirement expenses. But figuring out how much to save when you’re in your 40s and 50s is difficult. A mid-career physician likely has significant family- and child-related expenses. When we become empty-nesters, those expenses will decline. In retirement they may disappear entirely, but new expenses may arise.
With large variations in expenses at different life stages, it’s hard to calculate exactly how much you will need to save. Early on, the most sensible thing is putting aside a “reasonable” percentage of gross income for retirement savings.
What is a ‘reasonable’ savings goal for retirement?
As is often the case with high-income earners, many of our clients don’t have a budget or a clear picture of their current expenses and spending habits. That’s alright as long as they are building up a reasonable nest egg for the future – which begs the question of what is reasonable.
For mid-career docs, a reasonable goal to aim for is putting aside 20% of gross income for retirement. What you spend the rest of your money on is less important than how much you’re saving.
This is quite different from how you’ll handle expenses during retirement, when you no longer have a steady stream of income; rather, you have a pot of money that needs to last you another 20, 30, or even 40 years. At that point, thinking about specific expenses becomes more important (more on this topic later). That said, if you’re a mid-career doctor who is not meeting this 20% savings goal, it’s time to make a plan that will free up cash for retirement savings and investments.
Later-career docs: Calculate your spending level in retirement
Financial success means having a portfolio that can support your retirement dreams – with the confidence that your money will last and you won’t need to watch every dollar you spend. As you near retirement, your focus will shift away from accumulating savings to calculating the annual expenses you will have to meet in retirement.
A good place to start is figuring out which expenses will be necessary and which will be more flexible. To do this, separate your anticipated spending into these two categories:
- Fixed expenses: You can confidently forecast your “must-have” fixed expenses – such as property taxes, property/casualty insurance, health care costs, utilities, and groceries – because they remain steady from month to month.
- Discretionary expenses: These “like-to-have” expenses vary from month to month. This makes them harder to predict but easier to control. They might include dining out, travel, and charitable contributions.
As a retiree, understanding your fixed and discretionary expenses can help you prepare for a bear market, when the stock market can decline by 20% or more. Your portfolio won’t consist entirely of stocks, so it shouldn’t drop to that degree. Still, it will decline significantly. You may need to cut back on spending for a year or 2 to allow your portfolio to recover, particularly if the portfolio declines early in retirement.
Are you ready for retirement?
During the long bull market preceding the great recession of 2007 and 2009, many physicians retired –only to return to their practices when their portfolio values plummeted. In the exuberance of the moment, many failed to heed the warnings of many economists and got caught flat-footed.
Right now it’s a bull market, but we’re seeing concerning signs, such as an out-of-control housing market and rumblings about inflation and rising consumer costs. Sound familiar? If you hope to retire soon, take the time to objectively look around the corner so you can plan appropriately – whether your goal is to retire completely, stay in practice part-time, or even take on a new opportunity.
In an “it-depends” world, don’t be lured by a fictitious magic number, no matter what comes up when you Google: “When can I retire?” Instead, save early, imagine your dream retirement, and calculate expenses later to see what’s possible.
Dr. Greenwald is a graduate of the Albert Einstein College of Medicine, New York. Dr. Greenwald completed his internal medicine residency at the University of Minnesota, Minneapolis. He practiced internal medicine in the Twin Cities for 11 years before making the transition to financial planning for physicians, beginning in 1998.
A version of this article first appeared on Medscape.com.
Small uptick in children’s COVID vaccinations can’t change overall decline
The weekly number of 12- to 15-year-olds receiving a first dose of COVID-19 vaccine rose slightly, but the age group’s share of all first vaccinations continues to drop, according to data from the Centers for Disease Control and Prevention.

the CDC reported on its COVID Data Tracker site.
As of July 5, not quite one-third (32.2%) of 12- to 15-year-olds had received at least one dose of the vaccine and 23.4% were fully vaccinated. For those aged 16-17 years, 44.5% have gotten at least one dose and 35.9% are fully vaccinated. Total numbers of fully vaccinated individuals in each age group are 4.9 million (12-15) and 3.4 million (16-17), the CDC said.
Looking at another measure, percentage of all vaccines initiated by each age group over the previous 14 days, shows that the decline has not stopped for those aged 12-15. They represented 12.1% of all first vaccines administered during the 2 weeks ending July 4, compared with 14.3% on June 28 and 23.4% (the highest proportion reached) on May 30. The 16- and 17-year olds were at 4.6% on July 4, but that figure has only ranged from 4.2% to 4.9% since late May, based on CDC data.
The numbers for full vaccination follow a similar trajectory. Children aged 12-15 represented 12.1% of all those completing the vaccine regimen over the 2 weeks ending July 4, down from 16.7% a week earlier (June 28) and from a high of 21.5% for the 2 weeks ending June 21. Full vaccination for 16- and 17-year-olds matched their pattern for first doses: nothing lower than 4.2% or higher than 4.6%, the COVID Data Tracker shows.
The weekly number of 12- to 15-year-olds receiving a first dose of COVID-19 vaccine rose slightly, but the age group’s share of all first vaccinations continues to drop, according to data from the Centers for Disease Control and Prevention.

the CDC reported on its COVID Data Tracker site.
As of July 5, not quite one-third (32.2%) of 12- to 15-year-olds had received at least one dose of the vaccine and 23.4% were fully vaccinated. For those aged 16-17 years, 44.5% have gotten at least one dose and 35.9% are fully vaccinated. Total numbers of fully vaccinated individuals in each age group are 4.9 million (12-15) and 3.4 million (16-17), the CDC said.
Looking at another measure, percentage of all vaccines initiated by each age group over the previous 14 days, shows that the decline has not stopped for those aged 12-15. They represented 12.1% of all first vaccines administered during the 2 weeks ending July 4, compared with 14.3% on June 28 and 23.4% (the highest proportion reached) on May 30. The 16- and 17-year olds were at 4.6% on July 4, but that figure has only ranged from 4.2% to 4.9% since late May, based on CDC data.
The numbers for full vaccination follow a similar trajectory. Children aged 12-15 represented 12.1% of all those completing the vaccine regimen over the 2 weeks ending July 4, down from 16.7% a week earlier (June 28) and from a high of 21.5% for the 2 weeks ending June 21. Full vaccination for 16- and 17-year-olds matched their pattern for first doses: nothing lower than 4.2% or higher than 4.6%, the COVID Data Tracker shows.
The weekly number of 12- to 15-year-olds receiving a first dose of COVID-19 vaccine rose slightly, but the age group’s share of all first vaccinations continues to drop, according to data from the Centers for Disease Control and Prevention.

the CDC reported on its COVID Data Tracker site.
As of July 5, not quite one-third (32.2%) of 12- to 15-year-olds had received at least one dose of the vaccine and 23.4% were fully vaccinated. For those aged 16-17 years, 44.5% have gotten at least one dose and 35.9% are fully vaccinated. Total numbers of fully vaccinated individuals in each age group are 4.9 million (12-15) and 3.4 million (16-17), the CDC said.
Looking at another measure, percentage of all vaccines initiated by each age group over the previous 14 days, shows that the decline has not stopped for those aged 12-15. They represented 12.1% of all first vaccines administered during the 2 weeks ending July 4, compared with 14.3% on June 28 and 23.4% (the highest proportion reached) on May 30. The 16- and 17-year olds were at 4.6% on July 4, but that figure has only ranged from 4.2% to 4.9% since late May, based on CDC data.
The numbers for full vaccination follow a similar trajectory. Children aged 12-15 represented 12.1% of all those completing the vaccine regimen over the 2 weeks ending July 4, down from 16.7% a week earlier (June 28) and from a high of 21.5% for the 2 weeks ending June 21. Full vaccination for 16- and 17-year-olds matched their pattern for first doses: nothing lower than 4.2% or higher than 4.6%, the COVID Data Tracker shows.
Antimicrobial resistance threat continues during COVID-19
The stark realities of antimicrobial resistance – including rising rates of difficult-to-treat infections, lack of a robust pipeline of future antimicrobials, and COVID-19 treatments that leave people more vulnerable to infections – remain urgent priorities, experts say.
For some patients, the pandemic and antimicrobial resistance (AMR) are intertwined.
“One patient I’m seeing now in service really underscores how the two interact,” Vance Fowler, MD, said during a June 30 media briefing sponsored by the Infectious Diseases Society of America (IDSA). A man in his mid-40s, married with a small child, developed COVID-19 in early January 2021. He was intubated, spent about 1 month in the ICU, and managed to survive.
“But since then he has been struck with a series of progressively more drug resistant bacteria,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C., and chair of the IDSA Antimicrobial Resistance Committee.
The patient acquired Pseudomonas ventilator-associated pneumonia. Although the infection initially responded to standard antibiotics, he has experienced relapses over the past few months. Through these multiple infections the Pseudomonas grew increasingly pan-resistant to treatment.
The only remaining antimicrobial agent for this patient, Dr. Fowler said, is “a case study in what we are describing ... a drug that is used relatively infrequently, that is fairly expensive, but for that particular patient is absolutely vital.”
A ‘terrifying’ personal experience
Tori Kinamon, a Duke University medical student and Food and Drug Administration antibacterial drug resistance fellow, joined Dr. Fowler at the IDSA briefing. She shared her personal journey of surviving a methicillin-resistant Staphylococcus aureus (MRSA) infection, one that sparked her interest in becoming a physician.
“I had a very frightening and unexpected confrontation with antimicrobial resistance when I was a freshman in college,” Ms. Kinamon said.
A few days after competing in a Division One gymnastics championship, she felt a gradual onset of pain in her left hamstring. The pain grew acutely worse and, within days, her leg become red, swollen, and painful to the touch.
Ms. Kinamon was admitted to the hospital for suspected cellulitis and put on intravenous antibiotics.
“However, my clinical condition continued to decline,” she recalled. “Imaging studies revealed a 15-cm abscess deep in my hamstring.”
The limb- and life-threatening infection left her wondering if she would come out of surgery with both legs.
“Ultimately, I had eight surgeries in 2 weeks,” she said.
“As a 19-year-old collegiate athlete, that’s terrifying. And I never imagined that something like that would happen to me – until it did,” said Ms. Kinamon, who is an NCAA infection prevention advocate.
When Ms. Kinamon’s kidneys could no longer tolerate vancomycin, she was switched to daptomycin.
“I reflect quite frequently on how having that one extra drug in the stockpile had a significant impact on my outcome,” she said.
Incentivizing new antimicrobial agents
A lack of new antimicrobials in development is not a new story.
“There’s been a chill that’s been sustained on the antibiotic development field. Most large pharmaceutical companies have left the area of anti-infectants and the bulk of research and development is now in small pharmaceutical companies,” Dr. Fowler said. “And they’re struggling.”
One potential solution is the Pasteur Act, a bipartisan bill reintroduced in Congress and supported by IDSA. The bill encourages pharmaceutical companies to develop new antimicrobial agents with funding not linked to sales or use of the drugs.
Furthermore, the bill emphasizes appropriate use of these agents through effective stewardship programs.
Although some institutions shifted resources away from AMR out of necessity when COVID-19 struck, “I can say certainly from our experience at Duke that at least stewardship was alive and well. It was not relegated to the side,” Dr. Fowler said.
“In fact,” he added, “if anything, COVID really emphasized the importance of stewardship” by helping clinicians with guidance on the use of remdesivir and other antivirals during the pandemic.
Also, in some instances, treatments used to keep people with COVID-19 alive can paradoxically place them at higher risk for other infections, Dr. Fowler said, citing corticosteroids as an example.
Everyone’s concern
AMR isn’t just an issue in hospital settings, either. Ms. Kinamon reiterated that she picked up the infection in an athletic environment.
“Antimicrobial resistance is not just a problem for ICU patients in the hospital. I was the healthiest I had ever been and just very nearly escaped death due to one of these infections,” she said. ”As rates of resistance rise as these pathogens become more virulent, AMR is becoming more and more of a community threat,” she added.
Furthermore, consumers are partially to blame as well, Dr. Fowler noted.
“It’s interesting when you look at the surveys of the numbers of patients that have used someone else’s antibiotics” or leftover antimicrobial agents from a prior infection.
“It’s really startling ... that’s the sort of antibiotic overuse that directly contributes to antibacterial resistance,” he said.
Reasons for optimism
Promising advances in diagnostics, treatment, and prevention of AMRs are underway, Dr. Fowler said.
“It always gets me really excited to talk about it. It’s amazing what technology and scientific discovery can bring to this discussion and to this threat,” he said.
For example, there is a “silent revolution” in diagnostics with the aim to rapidly provide life-saving actionable data on a real patient in nearly real time.
Traditionally, “you start off by treating what should be there” while awaiting results of tests to narrow down therapy, Dr. Fowler said. However, a whole host of new platforms are in development to reduce the time to susceptibility results. This kind of technology has “the potential to transform our ability to take care of patients, giving them the right drug at the right time and no more,” he said.
Another promising avenue of research involves bacteriophages. Dr. Fowler is principal investigator on a clinical trial underway to evaluate bacteriophages as adjunct therapy for MRSA bacteremia.
When it comes to prevention on AMR infections in the future, “I continue to be optimistic about the possibility of vaccines to prevent many of these infections,” Dr. Fowler said, adding that companies are working on vaccines against these kinds of infections caused by MRSA or Escherichia coli, for example.
Patient outcomes
The man in his 40s with the multidrug resistant Pseudomonas infections “is now to the point where he’s walking in the halls and I think he’ll get out of the hospital eventually,” Dr. Fowler said.
“But his life is forever changed,” he added.
Ms. Kinamon’s recovery from MRSA included time in the ICU, 1 month in a regular hospital setting, and 5 months at home.
“It sparked my interest in antibiotic research and development because I see myself as a direct beneficiary of the stockpile of antibiotics that were available to treat my infection,” Ms. Kinamon said. “Now as a medical student working with patients who have similar infections, I feel a deep empathy and connectedness to them because they ask the same questions that I did.”
A version of this article first appeared on WebMD.com.
The stark realities of antimicrobial resistance – including rising rates of difficult-to-treat infections, lack of a robust pipeline of future antimicrobials, and COVID-19 treatments that leave people more vulnerable to infections – remain urgent priorities, experts say.
For some patients, the pandemic and antimicrobial resistance (AMR) are intertwined.
“One patient I’m seeing now in service really underscores how the two interact,” Vance Fowler, MD, said during a June 30 media briefing sponsored by the Infectious Diseases Society of America (IDSA). A man in his mid-40s, married with a small child, developed COVID-19 in early January 2021. He was intubated, spent about 1 month in the ICU, and managed to survive.
“But since then he has been struck with a series of progressively more drug resistant bacteria,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C., and chair of the IDSA Antimicrobial Resistance Committee.
The patient acquired Pseudomonas ventilator-associated pneumonia. Although the infection initially responded to standard antibiotics, he has experienced relapses over the past few months. Through these multiple infections the Pseudomonas grew increasingly pan-resistant to treatment.
The only remaining antimicrobial agent for this patient, Dr. Fowler said, is “a case study in what we are describing ... a drug that is used relatively infrequently, that is fairly expensive, but for that particular patient is absolutely vital.”
A ‘terrifying’ personal experience
Tori Kinamon, a Duke University medical student and Food and Drug Administration antibacterial drug resistance fellow, joined Dr. Fowler at the IDSA briefing. She shared her personal journey of surviving a methicillin-resistant Staphylococcus aureus (MRSA) infection, one that sparked her interest in becoming a physician.
“I had a very frightening and unexpected confrontation with antimicrobial resistance when I was a freshman in college,” Ms. Kinamon said.
A few days after competing in a Division One gymnastics championship, she felt a gradual onset of pain in her left hamstring. The pain grew acutely worse and, within days, her leg become red, swollen, and painful to the touch.
Ms. Kinamon was admitted to the hospital for suspected cellulitis and put on intravenous antibiotics.
“However, my clinical condition continued to decline,” she recalled. “Imaging studies revealed a 15-cm abscess deep in my hamstring.”
The limb- and life-threatening infection left her wondering if she would come out of surgery with both legs.
“Ultimately, I had eight surgeries in 2 weeks,” she said.
“As a 19-year-old collegiate athlete, that’s terrifying. And I never imagined that something like that would happen to me – until it did,” said Ms. Kinamon, who is an NCAA infection prevention advocate.
When Ms. Kinamon’s kidneys could no longer tolerate vancomycin, she was switched to daptomycin.
“I reflect quite frequently on how having that one extra drug in the stockpile had a significant impact on my outcome,” she said.
Incentivizing new antimicrobial agents
A lack of new antimicrobials in development is not a new story.
“There’s been a chill that’s been sustained on the antibiotic development field. Most large pharmaceutical companies have left the area of anti-infectants and the bulk of research and development is now in small pharmaceutical companies,” Dr. Fowler said. “And they’re struggling.”
One potential solution is the Pasteur Act, a bipartisan bill reintroduced in Congress and supported by IDSA. The bill encourages pharmaceutical companies to develop new antimicrobial agents with funding not linked to sales or use of the drugs.
Furthermore, the bill emphasizes appropriate use of these agents through effective stewardship programs.
Although some institutions shifted resources away from AMR out of necessity when COVID-19 struck, “I can say certainly from our experience at Duke that at least stewardship was alive and well. It was not relegated to the side,” Dr. Fowler said.
“In fact,” he added, “if anything, COVID really emphasized the importance of stewardship” by helping clinicians with guidance on the use of remdesivir and other antivirals during the pandemic.
Also, in some instances, treatments used to keep people with COVID-19 alive can paradoxically place them at higher risk for other infections, Dr. Fowler said, citing corticosteroids as an example.
Everyone’s concern
AMR isn’t just an issue in hospital settings, either. Ms. Kinamon reiterated that she picked up the infection in an athletic environment.
“Antimicrobial resistance is not just a problem for ICU patients in the hospital. I was the healthiest I had ever been and just very nearly escaped death due to one of these infections,” she said. ”As rates of resistance rise as these pathogens become more virulent, AMR is becoming more and more of a community threat,” she added.
Furthermore, consumers are partially to blame as well, Dr. Fowler noted.
“It’s interesting when you look at the surveys of the numbers of patients that have used someone else’s antibiotics” or leftover antimicrobial agents from a prior infection.
“It’s really startling ... that’s the sort of antibiotic overuse that directly contributes to antibacterial resistance,” he said.
Reasons for optimism
Promising advances in diagnostics, treatment, and prevention of AMRs are underway, Dr. Fowler said.
“It always gets me really excited to talk about it. It’s amazing what technology and scientific discovery can bring to this discussion and to this threat,” he said.
For example, there is a “silent revolution” in diagnostics with the aim to rapidly provide life-saving actionable data on a real patient in nearly real time.
Traditionally, “you start off by treating what should be there” while awaiting results of tests to narrow down therapy, Dr. Fowler said. However, a whole host of new platforms are in development to reduce the time to susceptibility results. This kind of technology has “the potential to transform our ability to take care of patients, giving them the right drug at the right time and no more,” he said.
Another promising avenue of research involves bacteriophages. Dr. Fowler is principal investigator on a clinical trial underway to evaluate bacteriophages as adjunct therapy for MRSA bacteremia.
When it comes to prevention on AMR infections in the future, “I continue to be optimistic about the possibility of vaccines to prevent many of these infections,” Dr. Fowler said, adding that companies are working on vaccines against these kinds of infections caused by MRSA or Escherichia coli, for example.
Patient outcomes
The man in his 40s with the multidrug resistant Pseudomonas infections “is now to the point where he’s walking in the halls and I think he’ll get out of the hospital eventually,” Dr. Fowler said.
“But his life is forever changed,” he added.
Ms. Kinamon’s recovery from MRSA included time in the ICU, 1 month in a regular hospital setting, and 5 months at home.
“It sparked my interest in antibiotic research and development because I see myself as a direct beneficiary of the stockpile of antibiotics that were available to treat my infection,” Ms. Kinamon said. “Now as a medical student working with patients who have similar infections, I feel a deep empathy and connectedness to them because they ask the same questions that I did.”
A version of this article first appeared on WebMD.com.
The stark realities of antimicrobial resistance – including rising rates of difficult-to-treat infections, lack of a robust pipeline of future antimicrobials, and COVID-19 treatments that leave people more vulnerable to infections – remain urgent priorities, experts say.
For some patients, the pandemic and antimicrobial resistance (AMR) are intertwined.
“One patient I’m seeing now in service really underscores how the two interact,” Vance Fowler, MD, said during a June 30 media briefing sponsored by the Infectious Diseases Society of America (IDSA). A man in his mid-40s, married with a small child, developed COVID-19 in early January 2021. He was intubated, spent about 1 month in the ICU, and managed to survive.
“But since then he has been struck with a series of progressively more drug resistant bacteria,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C., and chair of the IDSA Antimicrobial Resistance Committee.
The patient acquired Pseudomonas ventilator-associated pneumonia. Although the infection initially responded to standard antibiotics, he has experienced relapses over the past few months. Through these multiple infections the Pseudomonas grew increasingly pan-resistant to treatment.
The only remaining antimicrobial agent for this patient, Dr. Fowler said, is “a case study in what we are describing ... a drug that is used relatively infrequently, that is fairly expensive, but for that particular patient is absolutely vital.”
A ‘terrifying’ personal experience
Tori Kinamon, a Duke University medical student and Food and Drug Administration antibacterial drug resistance fellow, joined Dr. Fowler at the IDSA briefing. She shared her personal journey of surviving a methicillin-resistant Staphylococcus aureus (MRSA) infection, one that sparked her interest in becoming a physician.
“I had a very frightening and unexpected confrontation with antimicrobial resistance when I was a freshman in college,” Ms. Kinamon said.
A few days after competing in a Division One gymnastics championship, she felt a gradual onset of pain in her left hamstring. The pain grew acutely worse and, within days, her leg become red, swollen, and painful to the touch.
Ms. Kinamon was admitted to the hospital for suspected cellulitis and put on intravenous antibiotics.
“However, my clinical condition continued to decline,” she recalled. “Imaging studies revealed a 15-cm abscess deep in my hamstring.”
The limb- and life-threatening infection left her wondering if she would come out of surgery with both legs.
“Ultimately, I had eight surgeries in 2 weeks,” she said.
“As a 19-year-old collegiate athlete, that’s terrifying. And I never imagined that something like that would happen to me – until it did,” said Ms. Kinamon, who is an NCAA infection prevention advocate.
When Ms. Kinamon’s kidneys could no longer tolerate vancomycin, she was switched to daptomycin.
“I reflect quite frequently on how having that one extra drug in the stockpile had a significant impact on my outcome,” she said.
Incentivizing new antimicrobial agents
A lack of new antimicrobials in development is not a new story.
“There’s been a chill that’s been sustained on the antibiotic development field. Most large pharmaceutical companies have left the area of anti-infectants and the bulk of research and development is now in small pharmaceutical companies,” Dr. Fowler said. “And they’re struggling.”
One potential solution is the Pasteur Act, a bipartisan bill reintroduced in Congress and supported by IDSA. The bill encourages pharmaceutical companies to develop new antimicrobial agents with funding not linked to sales or use of the drugs.
Furthermore, the bill emphasizes appropriate use of these agents through effective stewardship programs.
Although some institutions shifted resources away from AMR out of necessity when COVID-19 struck, “I can say certainly from our experience at Duke that at least stewardship was alive and well. It was not relegated to the side,” Dr. Fowler said.
“In fact,” he added, “if anything, COVID really emphasized the importance of stewardship” by helping clinicians with guidance on the use of remdesivir and other antivirals during the pandemic.
Also, in some instances, treatments used to keep people with COVID-19 alive can paradoxically place them at higher risk for other infections, Dr. Fowler said, citing corticosteroids as an example.
Everyone’s concern
AMR isn’t just an issue in hospital settings, either. Ms. Kinamon reiterated that she picked up the infection in an athletic environment.
“Antimicrobial resistance is not just a problem for ICU patients in the hospital. I was the healthiest I had ever been and just very nearly escaped death due to one of these infections,” she said. ”As rates of resistance rise as these pathogens become more virulent, AMR is becoming more and more of a community threat,” she added.
Furthermore, consumers are partially to blame as well, Dr. Fowler noted.
“It’s interesting when you look at the surveys of the numbers of patients that have used someone else’s antibiotics” or leftover antimicrobial agents from a prior infection.
“It’s really startling ... that’s the sort of antibiotic overuse that directly contributes to antibacterial resistance,” he said.
Reasons for optimism
Promising advances in diagnostics, treatment, and prevention of AMRs are underway, Dr. Fowler said.
“It always gets me really excited to talk about it. It’s amazing what technology and scientific discovery can bring to this discussion and to this threat,” he said.
For example, there is a “silent revolution” in diagnostics with the aim to rapidly provide life-saving actionable data on a real patient in nearly real time.
Traditionally, “you start off by treating what should be there” while awaiting results of tests to narrow down therapy, Dr. Fowler said. However, a whole host of new platforms are in development to reduce the time to susceptibility results. This kind of technology has “the potential to transform our ability to take care of patients, giving them the right drug at the right time and no more,” he said.
Another promising avenue of research involves bacteriophages. Dr. Fowler is principal investigator on a clinical trial underway to evaluate bacteriophages as adjunct therapy for MRSA bacteremia.
When it comes to prevention on AMR infections in the future, “I continue to be optimistic about the possibility of vaccines to prevent many of these infections,” Dr. Fowler said, adding that companies are working on vaccines against these kinds of infections caused by MRSA or Escherichia coli, for example.
Patient outcomes
The man in his 40s with the multidrug resistant Pseudomonas infections “is now to the point where he’s walking in the halls and I think he’ll get out of the hospital eventually,” Dr. Fowler said.
“But his life is forever changed,” he added.
Ms. Kinamon’s recovery from MRSA included time in the ICU, 1 month in a regular hospital setting, and 5 months at home.
“It sparked my interest in antibiotic research and development because I see myself as a direct beneficiary of the stockpile of antibiotics that were available to treat my infection,” Ms. Kinamon said. “Now as a medical student working with patients who have similar infections, I feel a deep empathy and connectedness to them because they ask the same questions that I did.”
A version of this article first appeared on WebMD.com.

