User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Children and COVID: Decline of summer surge continues
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
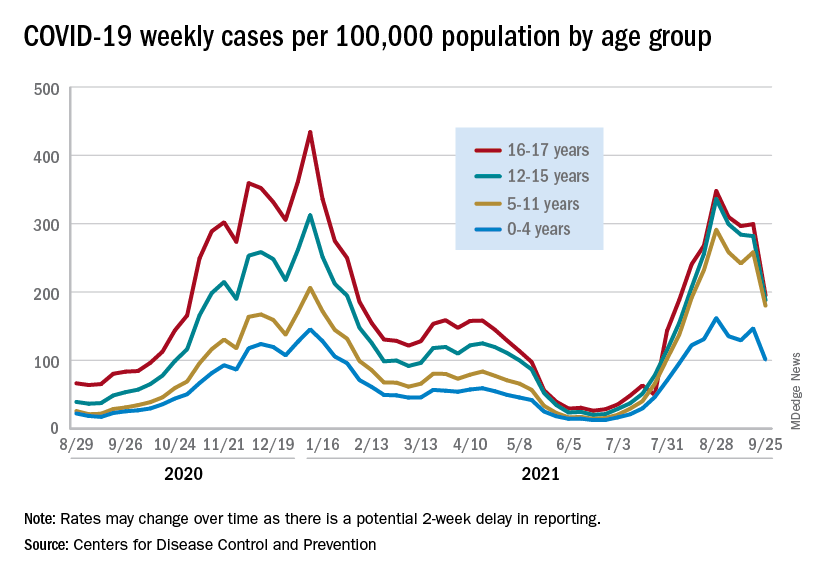
Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
Johnson & Johnson requests FDA approval for vaccine booster doses
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
The Importance of Guideline-Recommended Biomarker Testing and Multidisciplinary Treatment in Resectable Stage IB-IIIA Non-Small Cell Lung Cancer
Pfizer COVID vaccine antibodies may disappear in 7 months, study says
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
Antibody cocktail reduces chance of developing COVID
A one-time dose of two long-acting monoclonal antibodies reduced the risk of developing symptomatic COVID by 77% in comparison with placebo (P < .001) in a randomized, double-blind, placebo-controlled, phase 3 trial in adults, according to researchers who presented results at IDWeek 2021, an annual scientific meeting on infectious diseases.
The mix of tixagevimab and cilgavimab (AZD7442, Astra Zeneca) in a 300-mg dose is delivered in two intramuscular injections.
“This is the first long-acting combination of monoclonal antibodies that represents a potential new option to augment COVID-19 prevention,” said lead author Myron J. Levin, MD, a professor and pediatric infectious disease specialist at the University of Colorado at Denver, Aurora, who presented the findings of the PROVENT trial.
Both antibodies were taken from B cells donated by patients who had been infected with SARS-CoV-2, and they work synergistically, Dr. Levin said.
“The combination of them is better than adding results of each individually,” he said. “In vitro experiments have already shown that variants of interest and concern, including the Delta variant, are successfully neutralized by this cocktail.”
The trial was conducted in 87 sites in the United States, the United Kingdom, Spain, France, and Belgium. Participants included 5,197 unvaccinated adults who had never been infected with SARS-CoV-2 and either were at higher risk for inadequate response to COVID-19 vaccines because they were immunocompromised or were at high risk for exposure.
“Efficacy was observed through at least 3 months,” Dr. Levin said. “Preliminary pharmacokinetic modeling predicts potential protection for up to 12 months.”
Raymund Razonable, MD, an infectious disease expert with the Mayo Clinic in Rochester, Minn., who was not involved with the trial, told this news organization he was particularly interested in this combination because the developers made use of novel technology that extends the half-life of the antibodies and because of the large number of participants in the study.
Modeling that shows protection could last up to a year is novel and important, he said.
“People won’t need frequent injections,” Dr. Razonable said. With postexposure prophylaxis monoclonal cocktails, people may be given a dose a month, he noted.
Dr. Razonable said, “This is something intended to prevent COVID in people who are unvaccinated. The downside to that is we want people to get vaccinated. The best strategy so far is really vaccination.”
He said AZD7442 could potentially help fill the void for patients who are not able to respond to the COVID vaccines, including some who are immunocompromised or are undergoing chemotherapy.
Dr. Razonable said that, although the 77% reduction for developing symptomatic COVID-19 (95% confidence interval vs. placebo, 46.0-90.0; P < .001) is impressive, it is a reduction in relative risk. Still unknown is how much an individual’s absolute risk is reduced.
He also said it would be helpful to know how many people in the study population were immunocompromised, “because I think that’s where this product will be useful for prevention.”
The primary study endpoints were the first case of SARS-CoV-2 RT-PCR-positive symptomatic illness post dose and prior to day 183 (efficacy) as well as the safety of the product.
The cocktail appeared to be well tolerated. Adverse events occurred in 35% of participants administered AZD7442 and in 34% of the placebo group. Injection-site reactions occurred in 2.4% of the AZD7442 group and in 2.1% of the placebo group. There was one case of severe or critical COVID-19; two COVID-19–related deaths occurred in the placebo group.
AZD7442 is being developed with the help of funding from the U.S. government. Dr. Levin has received support from GlaxoSmithKline companies. Many of the coauthors are employed by AstraZeneca and hold stock in the company. Dr. Razonable has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A one-time dose of two long-acting monoclonal antibodies reduced the risk of developing symptomatic COVID by 77% in comparison with placebo (P < .001) in a randomized, double-blind, placebo-controlled, phase 3 trial in adults, according to researchers who presented results at IDWeek 2021, an annual scientific meeting on infectious diseases.
The mix of tixagevimab and cilgavimab (AZD7442, Astra Zeneca) in a 300-mg dose is delivered in two intramuscular injections.
“This is the first long-acting combination of monoclonal antibodies that represents a potential new option to augment COVID-19 prevention,” said lead author Myron J. Levin, MD, a professor and pediatric infectious disease specialist at the University of Colorado at Denver, Aurora, who presented the findings of the PROVENT trial.
Both antibodies were taken from B cells donated by patients who had been infected with SARS-CoV-2, and they work synergistically, Dr. Levin said.
“The combination of them is better than adding results of each individually,” he said. “In vitro experiments have already shown that variants of interest and concern, including the Delta variant, are successfully neutralized by this cocktail.”
The trial was conducted in 87 sites in the United States, the United Kingdom, Spain, France, and Belgium. Participants included 5,197 unvaccinated adults who had never been infected with SARS-CoV-2 and either were at higher risk for inadequate response to COVID-19 vaccines because they were immunocompromised or were at high risk for exposure.
“Efficacy was observed through at least 3 months,” Dr. Levin said. “Preliminary pharmacokinetic modeling predicts potential protection for up to 12 months.”
Raymund Razonable, MD, an infectious disease expert with the Mayo Clinic in Rochester, Minn., who was not involved with the trial, told this news organization he was particularly interested in this combination because the developers made use of novel technology that extends the half-life of the antibodies and because of the large number of participants in the study.
Modeling that shows protection could last up to a year is novel and important, he said.
“People won’t need frequent injections,” Dr. Razonable said. With postexposure prophylaxis monoclonal cocktails, people may be given a dose a month, he noted.
Dr. Razonable said, “This is something intended to prevent COVID in people who are unvaccinated. The downside to that is we want people to get vaccinated. The best strategy so far is really vaccination.”
He said AZD7442 could potentially help fill the void for patients who are not able to respond to the COVID vaccines, including some who are immunocompromised or are undergoing chemotherapy.
Dr. Razonable said that, although the 77% reduction for developing symptomatic COVID-19 (95% confidence interval vs. placebo, 46.0-90.0; P < .001) is impressive, it is a reduction in relative risk. Still unknown is how much an individual’s absolute risk is reduced.
He also said it would be helpful to know how many people in the study population were immunocompromised, “because I think that’s where this product will be useful for prevention.”
The primary study endpoints were the first case of SARS-CoV-2 RT-PCR-positive symptomatic illness post dose and prior to day 183 (efficacy) as well as the safety of the product.
The cocktail appeared to be well tolerated. Adverse events occurred in 35% of participants administered AZD7442 and in 34% of the placebo group. Injection-site reactions occurred in 2.4% of the AZD7442 group and in 2.1% of the placebo group. There was one case of severe or critical COVID-19; two COVID-19–related deaths occurred in the placebo group.
AZD7442 is being developed with the help of funding from the U.S. government. Dr. Levin has received support from GlaxoSmithKline companies. Many of the coauthors are employed by AstraZeneca and hold stock in the company. Dr. Razonable has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A one-time dose of two long-acting monoclonal antibodies reduced the risk of developing symptomatic COVID by 77% in comparison with placebo (P < .001) in a randomized, double-blind, placebo-controlled, phase 3 trial in adults, according to researchers who presented results at IDWeek 2021, an annual scientific meeting on infectious diseases.
The mix of tixagevimab and cilgavimab (AZD7442, Astra Zeneca) in a 300-mg dose is delivered in two intramuscular injections.
“This is the first long-acting combination of monoclonal antibodies that represents a potential new option to augment COVID-19 prevention,” said lead author Myron J. Levin, MD, a professor and pediatric infectious disease specialist at the University of Colorado at Denver, Aurora, who presented the findings of the PROVENT trial.
Both antibodies were taken from B cells donated by patients who had been infected with SARS-CoV-2, and they work synergistically, Dr. Levin said.
“The combination of them is better than adding results of each individually,” he said. “In vitro experiments have already shown that variants of interest and concern, including the Delta variant, are successfully neutralized by this cocktail.”
The trial was conducted in 87 sites in the United States, the United Kingdom, Spain, France, and Belgium. Participants included 5,197 unvaccinated adults who had never been infected with SARS-CoV-2 and either were at higher risk for inadequate response to COVID-19 vaccines because they were immunocompromised or were at high risk for exposure.
“Efficacy was observed through at least 3 months,” Dr. Levin said. “Preliminary pharmacokinetic modeling predicts potential protection for up to 12 months.”
Raymund Razonable, MD, an infectious disease expert with the Mayo Clinic in Rochester, Minn., who was not involved with the trial, told this news organization he was particularly interested in this combination because the developers made use of novel technology that extends the half-life of the antibodies and because of the large number of participants in the study.
Modeling that shows protection could last up to a year is novel and important, he said.
“People won’t need frequent injections,” Dr. Razonable said. With postexposure prophylaxis monoclonal cocktails, people may be given a dose a month, he noted.
Dr. Razonable said, “This is something intended to prevent COVID in people who are unvaccinated. The downside to that is we want people to get vaccinated. The best strategy so far is really vaccination.”
He said AZD7442 could potentially help fill the void for patients who are not able to respond to the COVID vaccines, including some who are immunocompromised or are undergoing chemotherapy.
Dr. Razonable said that, although the 77% reduction for developing symptomatic COVID-19 (95% confidence interval vs. placebo, 46.0-90.0; P < .001) is impressive, it is a reduction in relative risk. Still unknown is how much an individual’s absolute risk is reduced.
He also said it would be helpful to know how many people in the study population were immunocompromised, “because I think that’s where this product will be useful for prevention.”
The primary study endpoints were the first case of SARS-CoV-2 RT-PCR-positive symptomatic illness post dose and prior to day 183 (efficacy) as well as the safety of the product.
The cocktail appeared to be well tolerated. Adverse events occurred in 35% of participants administered AZD7442 and in 34% of the placebo group. Injection-site reactions occurred in 2.4% of the AZD7442 group and in 2.1% of the placebo group. There was one case of severe or critical COVID-19; two COVID-19–related deaths occurred in the placebo group.
AZD7442 is being developed with the help of funding from the U.S. government. Dr. Levin has received support from GlaxoSmithKline companies. Many of the coauthors are employed by AstraZeneca and hold stock in the company. Dr. Razonable has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Unprecedented’ 3-year sustained survival with lung cancer combo treatment
The durable overall survival (OS) benefit and the well-tolerated safety profile of the durvalumab with EP therapy further establishes the combination as the standard of care for the first-line treatment of ES-SCLC, Luis Paz-Ares, MD, reported at the 2021 European Society for Medical Oncology Congress on Sept. 18 (abstract LBA61).
At 3 years, there is more than three times the survival in patients with durvalumab and EP versus EP, and at the same time, the adverse-event profile continues to be favorable,” said Dr. Paz-Ares of Universidad Complutense & Ciberonc, Madrid.
This is the longest follow-up reported to date for a phase 3 trial of a programmed death–ligand 1 inhibitor and EP in this setting, he said.
The CASPIAN trial included 805 treatment-naive patients with ES-SCLC who were randomized 1:1:1 to receive 1,500 mg of durvalumab with EP every 3 weeks, 1,500 mg of durvalumab at 75 mg of tremelimumab and EP every 3 weeks, or EP alone. Patients in the durvalumab arms received four cycles of treatment followed by maintenance durvalumab, and those in the EP-only arm received up to six cycles of EP.
Primary outcomes data from the trial showed a significant overall survival benefit with durvalumab and EP versus EP alone (hazard ratio, 0.73), as did a subsequent analysis after a median follow-up of 25.1 mo (HR, 0.75).
Durvalumab with tremelimumab and EP numerically improved overall survival versus EP (HR, 0.82), but did not reach statistical significance.
At median follow up of 39.4 months, the durvalumab and EP combination showed sustained improvement in overall survival versus EP alone (HR, 0.71).
Median overall survival was 12.9 versus 10.5 months. OS was 22.9% versus 13.9% at 24 months, and 17.6% versus 5.8% at 36 months with durvalumab with EP versus EP, respectively, Dr. Paz-Ares said.
Durvalumab plus tremelimumab plus EP continued to numerically improve overall survival, compared with EP alone (HR, 0.81); median OS in that arm was 10.4 months, and 15.3% of patients were alive at 36 months, he noted.
Serious adverse events occurred in 32.5%, 47.4%, and 36.5% of patients in the durvalumab with EP, durvalumab plus tremelimumab plus EP, and EP arms respectively, and adverse events leading to death occurred in 5.3%, 10.9%, and 6.0%, respectively.
The findings are “really encouraging and unprecedented, frankly,” said session chair Alfredo Addeo, MD, of University Hospital, Geneva.
“They are setting the bar for competitors,” he said, referencing the IMpower 133 trial looking at atezolizumab with chemotherapy in ES-SCLC.
The CASPIAN study was funded by AstraZeneca. Dr. Paz-Ares reported relationships with multiple pharmaceutical companies.
The durable overall survival (OS) benefit and the well-tolerated safety profile of the durvalumab with EP therapy further establishes the combination as the standard of care for the first-line treatment of ES-SCLC, Luis Paz-Ares, MD, reported at the 2021 European Society for Medical Oncology Congress on Sept. 18 (abstract LBA61).
At 3 years, there is more than three times the survival in patients with durvalumab and EP versus EP, and at the same time, the adverse-event profile continues to be favorable,” said Dr. Paz-Ares of Universidad Complutense & Ciberonc, Madrid.
This is the longest follow-up reported to date for a phase 3 trial of a programmed death–ligand 1 inhibitor and EP in this setting, he said.
The CASPIAN trial included 805 treatment-naive patients with ES-SCLC who were randomized 1:1:1 to receive 1,500 mg of durvalumab with EP every 3 weeks, 1,500 mg of durvalumab at 75 mg of tremelimumab and EP every 3 weeks, or EP alone. Patients in the durvalumab arms received four cycles of treatment followed by maintenance durvalumab, and those in the EP-only arm received up to six cycles of EP.
Primary outcomes data from the trial showed a significant overall survival benefit with durvalumab and EP versus EP alone (hazard ratio, 0.73), as did a subsequent analysis after a median follow-up of 25.1 mo (HR, 0.75).
Durvalumab with tremelimumab and EP numerically improved overall survival versus EP (HR, 0.82), but did not reach statistical significance.
At median follow up of 39.4 months, the durvalumab and EP combination showed sustained improvement in overall survival versus EP alone (HR, 0.71).
Median overall survival was 12.9 versus 10.5 months. OS was 22.9% versus 13.9% at 24 months, and 17.6% versus 5.8% at 36 months with durvalumab with EP versus EP, respectively, Dr. Paz-Ares said.
Durvalumab plus tremelimumab plus EP continued to numerically improve overall survival, compared with EP alone (HR, 0.81); median OS in that arm was 10.4 months, and 15.3% of patients were alive at 36 months, he noted.
Serious adverse events occurred in 32.5%, 47.4%, and 36.5% of patients in the durvalumab with EP, durvalumab plus tremelimumab plus EP, and EP arms respectively, and adverse events leading to death occurred in 5.3%, 10.9%, and 6.0%, respectively.
The findings are “really encouraging and unprecedented, frankly,” said session chair Alfredo Addeo, MD, of University Hospital, Geneva.
“They are setting the bar for competitors,” he said, referencing the IMpower 133 trial looking at atezolizumab with chemotherapy in ES-SCLC.
The CASPIAN study was funded by AstraZeneca. Dr. Paz-Ares reported relationships with multiple pharmaceutical companies.
The durable overall survival (OS) benefit and the well-tolerated safety profile of the durvalumab with EP therapy further establishes the combination as the standard of care for the first-line treatment of ES-SCLC, Luis Paz-Ares, MD, reported at the 2021 European Society for Medical Oncology Congress on Sept. 18 (abstract LBA61).
At 3 years, there is more than three times the survival in patients with durvalumab and EP versus EP, and at the same time, the adverse-event profile continues to be favorable,” said Dr. Paz-Ares of Universidad Complutense & Ciberonc, Madrid.
This is the longest follow-up reported to date for a phase 3 trial of a programmed death–ligand 1 inhibitor and EP in this setting, he said.
The CASPIAN trial included 805 treatment-naive patients with ES-SCLC who were randomized 1:1:1 to receive 1,500 mg of durvalumab with EP every 3 weeks, 1,500 mg of durvalumab at 75 mg of tremelimumab and EP every 3 weeks, or EP alone. Patients in the durvalumab arms received four cycles of treatment followed by maintenance durvalumab, and those in the EP-only arm received up to six cycles of EP.
Primary outcomes data from the trial showed a significant overall survival benefit with durvalumab and EP versus EP alone (hazard ratio, 0.73), as did a subsequent analysis after a median follow-up of 25.1 mo (HR, 0.75).
Durvalumab with tremelimumab and EP numerically improved overall survival versus EP (HR, 0.82), but did not reach statistical significance.
At median follow up of 39.4 months, the durvalumab and EP combination showed sustained improvement in overall survival versus EP alone (HR, 0.71).
Median overall survival was 12.9 versus 10.5 months. OS was 22.9% versus 13.9% at 24 months, and 17.6% versus 5.8% at 36 months with durvalumab with EP versus EP, respectively, Dr. Paz-Ares said.
Durvalumab plus tremelimumab plus EP continued to numerically improve overall survival, compared with EP alone (HR, 0.81); median OS in that arm was 10.4 months, and 15.3% of patients were alive at 36 months, he noted.
Serious adverse events occurred in 32.5%, 47.4%, and 36.5% of patients in the durvalumab with EP, durvalumab plus tremelimumab plus EP, and EP arms respectively, and adverse events leading to death occurred in 5.3%, 10.9%, and 6.0%, respectively.
The findings are “really encouraging and unprecedented, frankly,” said session chair Alfredo Addeo, MD, of University Hospital, Geneva.
“They are setting the bar for competitors,” he said, referencing the IMpower 133 trial looking at atezolizumab with chemotherapy in ES-SCLC.
The CASPIAN study was funded by AstraZeneca. Dr. Paz-Ares reported relationships with multiple pharmaceutical companies.
FROM ESMO 2021
More U.S. adults vaccinated, but partisan divide remains: Survey
The number of U.S. adults who are at least partially vaccinated rose five percentage points to 72% in August, a slightly faster increase than in previous months, according to the latest monthly COVID-19 Vaccine Monitor report of the Kaiser Family Foundation (KFF).
The largest increases in vaccine uptake between July and September were among Hispanic adults and people aged 18-29 years. Roughly equal shares of adults now report being vaccinated across racial and ethnic groups: 71% of White adults, 70% of Black adults, and 73% of Hispanic adults.
Overall, the big takeaway of the latest Kaiser COVID-19 survey is that the partisan divide on all aspects of the pandemic, from vaccination status to attitudes toward vaccination and mask mandates, remains as wide as ever.
The only thing that Republicans, Democrats, and Independents mostly agree on is that COVID-19 will probably become an endemic disease like influenza. Seventy-nine percent of respondents agreed with that statement; 14% predicted that COVID-19 would probably be largely eliminated in future years, like polio.
Delta motivated many
The most important factor that recently led people toward being vaccinated against COVID-19 was the surge in cases, hospitalizations, and deaths due to the Delta variant, KFF reports.
Full approval of the Pfizer vaccine by the Food and Drug Administration and the increasing prevalence of vaccine mandates played secondary roles in the vaccination uptick.
Specifically, 10% of the recently vaccinated said the main reason they got shots was the increase in COVID-19 cases due to the Delta variant. Concern about reports of local hospitals and intensive care units filling up with COVID-19 patients was the main motivator for 12% of those who just got shots. Fourteen percent of the recently vaccinated got inoculated mainly because someone they knew had become seriously ill or had died from COVID-19.
The role of the Delta threat is also evident with regard to where those who were recently vaccinated live. Twenty-four percent of those who received their first dose of vaccine after June 1, 2021, reside in counties with a high COVID-19 case rate; 15% of them live in counties with a relatively low case rate.
Despite the recent surge in vaccinations, however, 7% of adults are still taking a wait-and-see approach; 4% said they’d get a shot only if required; and 12% said they definitely wouldn’t get vaccinated. The latter figure has barely budged since January of this year.
Ninety percent of Democrats said they had received at least one dose of the COVID-19 vaccine, vs. 68% of Independents, and 58% of Republicans.
Wealthier, better educated, urban, and older people were more likely to be vaccinated, with one exception: Sixty-eight percent of those aged 18-29 were vaccinated, vs. 66% of those aged 30-49. The group least likely to be vaccinated were uninsured people younger than 65, suggesting that some of them were unaware that the shot is free.
Partisan affiliation
Attitudes toward vaccine booster shots – which are now recommended for people older than 65, the immunocompromised, and certain frontline workers – largely fell along party lines and/or reflected whether respondents had been vaccinated.
Discussion of the boosters, KFF said, “appears to be a net positive for people who are already vaccinated, but a net negative for the unvaccinated. While a larger share of vaccinated adults say the information they have seen about boosters has been helpful (54%) than find it confusing (35%), among the unvaccinated almost twice as many find the information confusing as find it helpful (45% vs. 24%).”
Among fully vaccinated adults, 68% of Democrats say they’d definitely get a booster, and 20% say they probably would. Among Republicans, those percentages are 36% and 33%, respectively. Independents fall in between the other groups.
Although 82% of Democrats say the boosters show that scientists are continuing to make vaccines more effective, 52% of Republicans say that it shows that the vaccines are not working as well as promised.
Similarly, partisan attitudes emerged in questions about breakthrough infections. The fact that COVID-19 cases are fairly mild when they occur among persons who have been vaccinated indicates that the vaccines are working, said 87% of Democrats, but only 55% of Republicans agreed with that assessment.
In contrast, just 10% of Democrats but 39% of Republicans said that breakthrough infections mean the vaccines are not working.
Vaccine requirements
The public is more evenly divided on vaccine requirements. About 6 in 10 respondents said that vaccines should be required for health care workers (62%) and schoolteachers (58%). Slim majorities supported mandates for federal government employees (55%), college students (55%), and state and local government employees (54%).
On the question of whether employers in general should require their workers to be vaccinated, 48% of respondents said they should, and 50% said they should not.
Similarly, 52% said that all schools should mandate vaccines for eligible students; 46% didn’t approve of such requirements.
Three in four Democrats supported employer vaccine mandates, compared to 45% of Independents and just 20% of Republicans. Large partisan gaps were also seen for government, school, and health care vaccine mandates.
In contrast, 78% of the public favored requiring large employers to give their workers paid time off to get vaccinated and to recover from any side effects.
Twenty percent of workers under mandates
One in five workers said their employers require COVID-19 vaccination. Twenty-eight percent of employed people want their employers to require vaccination, and 50% don’t.
Again, the responses broke down along party lines, with 52% of Democrats, 21% of Independents, and 10% of Republicans favoring a vaccine mandate by their employers.
Most unvaccinated people didn’t support an employer mandate. A third of unvaccinated workers said they’d be likely to get vaccinated if their companies required it, but most of them said they would choose weekly testing if offered the option.
Being unable to use gyms, restaurants, or indoor entertainment venues that require vaccination was cited by 13% of the recently vaccinated as the main reason why they got shots.
The public was evenly divided on whether states or local governments should require such businesses to mandate that staff and customers show proof of vaccination. Although the views of the public were tied on this issue overall, 79% of Democrats, 43% of Independents, and 21% of Republicans supported having these kinds of businesses require proof of vaccination.
On school mask mandates, 56% of the respondents supported requiring all students and staff to wear masks. Favoring this kind of mandate were 83% of Democrats, 53% of Independents, and 29% of Republicans.
Partisanship also defined how Democrats and Republicans viewed the current high number of COVID-19 cases. Most Democrats blamed people who don’t wear masks and those who don’t get vaccinated, whereas Republicans were more likely to blame immigrants and tourists bringing COVID-19 into the United States.
A version of this article first appeared on Medscape.com.
The number of U.S. adults who are at least partially vaccinated rose five percentage points to 72% in August, a slightly faster increase than in previous months, according to the latest monthly COVID-19 Vaccine Monitor report of the Kaiser Family Foundation (KFF).
The largest increases in vaccine uptake between July and September were among Hispanic adults and people aged 18-29 years. Roughly equal shares of adults now report being vaccinated across racial and ethnic groups: 71% of White adults, 70% of Black adults, and 73% of Hispanic adults.
Overall, the big takeaway of the latest Kaiser COVID-19 survey is that the partisan divide on all aspects of the pandemic, from vaccination status to attitudes toward vaccination and mask mandates, remains as wide as ever.
The only thing that Republicans, Democrats, and Independents mostly agree on is that COVID-19 will probably become an endemic disease like influenza. Seventy-nine percent of respondents agreed with that statement; 14% predicted that COVID-19 would probably be largely eliminated in future years, like polio.
Delta motivated many
The most important factor that recently led people toward being vaccinated against COVID-19 was the surge in cases, hospitalizations, and deaths due to the Delta variant, KFF reports.
Full approval of the Pfizer vaccine by the Food and Drug Administration and the increasing prevalence of vaccine mandates played secondary roles in the vaccination uptick.
Specifically, 10% of the recently vaccinated said the main reason they got shots was the increase in COVID-19 cases due to the Delta variant. Concern about reports of local hospitals and intensive care units filling up with COVID-19 patients was the main motivator for 12% of those who just got shots. Fourteen percent of the recently vaccinated got inoculated mainly because someone they knew had become seriously ill or had died from COVID-19.
The role of the Delta threat is also evident with regard to where those who were recently vaccinated live. Twenty-four percent of those who received their first dose of vaccine after June 1, 2021, reside in counties with a high COVID-19 case rate; 15% of them live in counties with a relatively low case rate.
Despite the recent surge in vaccinations, however, 7% of adults are still taking a wait-and-see approach; 4% said they’d get a shot only if required; and 12% said they definitely wouldn’t get vaccinated. The latter figure has barely budged since January of this year.
Ninety percent of Democrats said they had received at least one dose of the COVID-19 vaccine, vs. 68% of Independents, and 58% of Republicans.
Wealthier, better educated, urban, and older people were more likely to be vaccinated, with one exception: Sixty-eight percent of those aged 18-29 were vaccinated, vs. 66% of those aged 30-49. The group least likely to be vaccinated were uninsured people younger than 65, suggesting that some of them were unaware that the shot is free.
Partisan affiliation
Attitudes toward vaccine booster shots – which are now recommended for people older than 65, the immunocompromised, and certain frontline workers – largely fell along party lines and/or reflected whether respondents had been vaccinated.
Discussion of the boosters, KFF said, “appears to be a net positive for people who are already vaccinated, but a net negative for the unvaccinated. While a larger share of vaccinated adults say the information they have seen about boosters has been helpful (54%) than find it confusing (35%), among the unvaccinated almost twice as many find the information confusing as find it helpful (45% vs. 24%).”
Among fully vaccinated adults, 68% of Democrats say they’d definitely get a booster, and 20% say they probably would. Among Republicans, those percentages are 36% and 33%, respectively. Independents fall in between the other groups.
Although 82% of Democrats say the boosters show that scientists are continuing to make vaccines more effective, 52% of Republicans say that it shows that the vaccines are not working as well as promised.
Similarly, partisan attitudes emerged in questions about breakthrough infections. The fact that COVID-19 cases are fairly mild when they occur among persons who have been vaccinated indicates that the vaccines are working, said 87% of Democrats, but only 55% of Republicans agreed with that assessment.
In contrast, just 10% of Democrats but 39% of Republicans said that breakthrough infections mean the vaccines are not working.
Vaccine requirements
The public is more evenly divided on vaccine requirements. About 6 in 10 respondents said that vaccines should be required for health care workers (62%) and schoolteachers (58%). Slim majorities supported mandates for federal government employees (55%), college students (55%), and state and local government employees (54%).
On the question of whether employers in general should require their workers to be vaccinated, 48% of respondents said they should, and 50% said they should not.
Similarly, 52% said that all schools should mandate vaccines for eligible students; 46% didn’t approve of such requirements.
Three in four Democrats supported employer vaccine mandates, compared to 45% of Independents and just 20% of Republicans. Large partisan gaps were also seen for government, school, and health care vaccine mandates.
In contrast, 78% of the public favored requiring large employers to give their workers paid time off to get vaccinated and to recover from any side effects.
Twenty percent of workers under mandates
One in five workers said their employers require COVID-19 vaccination. Twenty-eight percent of employed people want their employers to require vaccination, and 50% don’t.
Again, the responses broke down along party lines, with 52% of Democrats, 21% of Independents, and 10% of Republicans favoring a vaccine mandate by their employers.
Most unvaccinated people didn’t support an employer mandate. A third of unvaccinated workers said they’d be likely to get vaccinated if their companies required it, but most of them said they would choose weekly testing if offered the option.
Being unable to use gyms, restaurants, or indoor entertainment venues that require vaccination was cited by 13% of the recently vaccinated as the main reason why they got shots.
The public was evenly divided on whether states or local governments should require such businesses to mandate that staff and customers show proof of vaccination. Although the views of the public were tied on this issue overall, 79% of Democrats, 43% of Independents, and 21% of Republicans supported having these kinds of businesses require proof of vaccination.
On school mask mandates, 56% of the respondents supported requiring all students and staff to wear masks. Favoring this kind of mandate were 83% of Democrats, 53% of Independents, and 29% of Republicans.
Partisanship also defined how Democrats and Republicans viewed the current high number of COVID-19 cases. Most Democrats blamed people who don’t wear masks and those who don’t get vaccinated, whereas Republicans were more likely to blame immigrants and tourists bringing COVID-19 into the United States.
A version of this article first appeared on Medscape.com.
The number of U.S. adults who are at least partially vaccinated rose five percentage points to 72% in August, a slightly faster increase than in previous months, according to the latest monthly COVID-19 Vaccine Monitor report of the Kaiser Family Foundation (KFF).
The largest increases in vaccine uptake between July and September were among Hispanic adults and people aged 18-29 years. Roughly equal shares of adults now report being vaccinated across racial and ethnic groups: 71% of White adults, 70% of Black adults, and 73% of Hispanic adults.
Overall, the big takeaway of the latest Kaiser COVID-19 survey is that the partisan divide on all aspects of the pandemic, from vaccination status to attitudes toward vaccination and mask mandates, remains as wide as ever.
The only thing that Republicans, Democrats, and Independents mostly agree on is that COVID-19 will probably become an endemic disease like influenza. Seventy-nine percent of respondents agreed with that statement; 14% predicted that COVID-19 would probably be largely eliminated in future years, like polio.
Delta motivated many
The most important factor that recently led people toward being vaccinated against COVID-19 was the surge in cases, hospitalizations, and deaths due to the Delta variant, KFF reports.
Full approval of the Pfizer vaccine by the Food and Drug Administration and the increasing prevalence of vaccine mandates played secondary roles in the vaccination uptick.
Specifically, 10% of the recently vaccinated said the main reason they got shots was the increase in COVID-19 cases due to the Delta variant. Concern about reports of local hospitals and intensive care units filling up with COVID-19 patients was the main motivator for 12% of those who just got shots. Fourteen percent of the recently vaccinated got inoculated mainly because someone they knew had become seriously ill or had died from COVID-19.
The role of the Delta threat is also evident with regard to where those who were recently vaccinated live. Twenty-four percent of those who received their first dose of vaccine after June 1, 2021, reside in counties with a high COVID-19 case rate; 15% of them live in counties with a relatively low case rate.
Despite the recent surge in vaccinations, however, 7% of adults are still taking a wait-and-see approach; 4% said they’d get a shot only if required; and 12% said they definitely wouldn’t get vaccinated. The latter figure has barely budged since January of this year.
Ninety percent of Democrats said they had received at least one dose of the COVID-19 vaccine, vs. 68% of Independents, and 58% of Republicans.
Wealthier, better educated, urban, and older people were more likely to be vaccinated, with one exception: Sixty-eight percent of those aged 18-29 were vaccinated, vs. 66% of those aged 30-49. The group least likely to be vaccinated were uninsured people younger than 65, suggesting that some of them were unaware that the shot is free.
Partisan affiliation
Attitudes toward vaccine booster shots – which are now recommended for people older than 65, the immunocompromised, and certain frontline workers – largely fell along party lines and/or reflected whether respondents had been vaccinated.
Discussion of the boosters, KFF said, “appears to be a net positive for people who are already vaccinated, but a net negative for the unvaccinated. While a larger share of vaccinated adults say the information they have seen about boosters has been helpful (54%) than find it confusing (35%), among the unvaccinated almost twice as many find the information confusing as find it helpful (45% vs. 24%).”
Among fully vaccinated adults, 68% of Democrats say they’d definitely get a booster, and 20% say they probably would. Among Republicans, those percentages are 36% and 33%, respectively. Independents fall in between the other groups.
Although 82% of Democrats say the boosters show that scientists are continuing to make vaccines more effective, 52% of Republicans say that it shows that the vaccines are not working as well as promised.
Similarly, partisan attitudes emerged in questions about breakthrough infections. The fact that COVID-19 cases are fairly mild when they occur among persons who have been vaccinated indicates that the vaccines are working, said 87% of Democrats, but only 55% of Republicans agreed with that assessment.
In contrast, just 10% of Democrats but 39% of Republicans said that breakthrough infections mean the vaccines are not working.
Vaccine requirements
The public is more evenly divided on vaccine requirements. About 6 in 10 respondents said that vaccines should be required for health care workers (62%) and schoolteachers (58%). Slim majorities supported mandates for federal government employees (55%), college students (55%), and state and local government employees (54%).
On the question of whether employers in general should require their workers to be vaccinated, 48% of respondents said they should, and 50% said they should not.
Similarly, 52% said that all schools should mandate vaccines for eligible students; 46% didn’t approve of such requirements.
Three in four Democrats supported employer vaccine mandates, compared to 45% of Independents and just 20% of Republicans. Large partisan gaps were also seen for government, school, and health care vaccine mandates.
In contrast, 78% of the public favored requiring large employers to give their workers paid time off to get vaccinated and to recover from any side effects.
Twenty percent of workers under mandates
One in five workers said their employers require COVID-19 vaccination. Twenty-eight percent of employed people want their employers to require vaccination, and 50% don’t.
Again, the responses broke down along party lines, with 52% of Democrats, 21% of Independents, and 10% of Republicans favoring a vaccine mandate by their employers.
Most unvaccinated people didn’t support an employer mandate. A third of unvaccinated workers said they’d be likely to get vaccinated if their companies required it, but most of them said they would choose weekly testing if offered the option.
Being unable to use gyms, restaurants, or indoor entertainment venues that require vaccination was cited by 13% of the recently vaccinated as the main reason why they got shots.
The public was evenly divided on whether states or local governments should require such businesses to mandate that staff and customers show proof of vaccination. Although the views of the public were tied on this issue overall, 79% of Democrats, 43% of Independents, and 21% of Republicans supported having these kinds of businesses require proof of vaccination.
On school mask mandates, 56% of the respondents supported requiring all students and staff to wear masks. Favoring this kind of mandate were 83% of Democrats, 53% of Independents, and 29% of Republicans.
Partisanship also defined how Democrats and Republicans viewed the current high number of COVID-19 cases. Most Democrats blamed people who don’t wear masks and those who don’t get vaccinated, whereas Republicans were more likely to blame immigrants and tourists bringing COVID-19 into the United States.
A version of this article first appeared on Medscape.com.
Drug cocktail significantly reduced severe COVID, death in outpatients
A monoclonal antibody combination of casirivimab and imdevimab (REGEN-COV) significantly reduced the risk of COVID-19–related hospitalizations and death from any cause in the phase 3 portion of an adaptive trial of outpatients.
Researchers, led by David Weinreich, MD, MBA, executive vice president of the drug cocktail’s manufacturer Regeneron, found in the randomized trial that the combination also resolved symptoms and reduced the SARS-CoV-2 viral load more quickly, compared with placebo.
Findings were published in the New England Journal of Medicine.
COVID-related hospitalization or death from any cause occurred in 18 of 1,355 patients (1.3%) in the group getting 2,400 mg infusions of the study drug, compared with 62 (4.6%) of 1,341 in the matching placebo group, indicating a relative risk reduction of 71.3%; P < .001.
Sunil Joshi, MD, president of the Duval County Medical Society Foundation and an immunologist in Jacksonville, Fla., said in an interview that these findings confirm benefits of REGEN-COV and are very good news for a patient group that includes those age 65 and older with high blood pressure, diabetes, or obesity; and for people not vaccinated, who are all at high risk of hospitalization or death if they get COVID-19.
“Vaccines are critically important,” he said, “but if you were to be infected and know that there’s a way to keep yourself out of the hospital, this is very good news.”
Researchers seek lowest doses
This trial found that the effect was similar when researchers cut the doses in half. These outcomes occurred in 7 of 736 (1%) of patients given 1,200 mg of REGEN-COV and in 24 (3.2%) of 748 in the matching placebo group (relative risk reduction, 70.4%; P = .002).
Symptoms were resolved on average 4 days earlier with each REGEN-COV dose than with placebo (10 days vs. 14 days; P < .001 for both comparisons).
Dr. Weinreich said in an interview that trials will continue to find the lowest effective doses that can stand up to all evolving variants.
“This is one of those settings where you don’t want to underdose. You’ve got one shot at this,” he said. “We’d love to do lower doses. It would be more convenient and we could treat more patients, but if it generates more clinical failures or doesn’t work with certain variants, then you’ve done a huge disservice to the world.”
Also new in this study is that researchers tested not only seronegative patients, but patients at high risk regardless of blood antibody status, he said.
“It’s the first suggestion of data that if you’re breaking through a vaccine and you’re at high risk, the use of the cocktail is something to strongly consider because treatment early is better than treatment later,” Dr. Weinreich said.
In addition to efficacy, the phase 3 trial demonstrated the cocktail had a good safety profile. Serious adverse events occurred more often in the placebo group (4%) than in the 1,200-mg group (1.1%) and the 2,400-mg group (1.3%). Infusion reactions (grade 2 or higher) occurred in less than 0.3% of patients in all groups.
William Fales, MD, state medical director for the Michigan Department of Health and Human Services, said the results confirm the promise of REGEN-COV for reducing hospitalizations and death in a peer-reviewed publication.
COVID-19 a moving target
However, Dr. Fales noted that COVID-19 is a moving target with emerging variants. The criteria for populations at high risk have also broadened since the start of the study, he said.
“A great example is pregnancy is now included as high risk, and that would have likely been a specific contraindication of patients in this clinical trial,” he said.
Dr. Fales said Michigan has been using both REGEN-COV and the Eli Lilly combination of bamlanivimab and etesevimab, which also has an emergency use authorization (EUA) from the Food and Drug Administration, with positive results.
REGEN-COV has an EUA to treat people who are at high risk of serious consequences from COVID-19, including those who are already infected (nonhospitalized) or those in certain postexposure prophylaxis settings.
“We’re seeing very low hospitalization rates and few deaths in a state that is predominately Delta,” Dr. Fales said. “So, this makes us feel that we’re doing the right thing and supports the current efforts around the country to make monoclonal antibody therapy available to high-risk patients.”
Dr. Joshi noted that trial results have been emerging from other monoclonal antibody cocktails with different COVID-19 patient groups.
However, he said in an interview, “how much more effective they would be than this is something we’d have to look at, as 71% effectiveness in keeping people out of the hospital is pretty good for any treatment.”
“These are great numbers, but vaccination itself keeps you from getting the disease in the first place and not just for a short time period. This treatment is just that – a treatment. It gets you through that episode but it doesn’t mean you won’t get sick again. You don’t develop an immune response as you do with the vaccine,” he said.
Dr. Weinreich agreed: “This is not a substitute for a vaccine except for the small group who get the vaccine and their bodies can’t respond to it because they’re significantly immunocompromised.”
The results from this paper “are one piece of a large, multistudy, phase 3 program that basically spans from prophylaxis all the way to hospitalization and pretty much the gamut – all of them – have worked. All of these studies have shown dramatic improvement in whatever the definitive regulatory endpoint is,” Dr. Weinreich said.
He said discussions are ongoing for full regulatory approval in the United States and for expanding the EUA for other populations, including pre-exposure prophylaxis, “which the [United Kingdom’s] authority has already granted us but the FDA has not.”
The study is funded by Regeneron and the Department of Health & Human Services. Dr. Weinreich is a vice president of Regeneron. Dr. Joshi reported no relevant financial relationships. Dr. Fales holds stock in Eli Lilly.
A version of this article first appeared on Medscape.com.
A monoclonal antibody combination of casirivimab and imdevimab (REGEN-COV) significantly reduced the risk of COVID-19–related hospitalizations and death from any cause in the phase 3 portion of an adaptive trial of outpatients.
Researchers, led by David Weinreich, MD, MBA, executive vice president of the drug cocktail’s manufacturer Regeneron, found in the randomized trial that the combination also resolved symptoms and reduced the SARS-CoV-2 viral load more quickly, compared with placebo.
Findings were published in the New England Journal of Medicine.
COVID-related hospitalization or death from any cause occurred in 18 of 1,355 patients (1.3%) in the group getting 2,400 mg infusions of the study drug, compared with 62 (4.6%) of 1,341 in the matching placebo group, indicating a relative risk reduction of 71.3%; P < .001.
Sunil Joshi, MD, president of the Duval County Medical Society Foundation and an immunologist in Jacksonville, Fla., said in an interview that these findings confirm benefits of REGEN-COV and are very good news for a patient group that includes those age 65 and older with high blood pressure, diabetes, or obesity; and for people not vaccinated, who are all at high risk of hospitalization or death if they get COVID-19.
“Vaccines are critically important,” he said, “but if you were to be infected and know that there’s a way to keep yourself out of the hospital, this is very good news.”
Researchers seek lowest doses
This trial found that the effect was similar when researchers cut the doses in half. These outcomes occurred in 7 of 736 (1%) of patients given 1,200 mg of REGEN-COV and in 24 (3.2%) of 748 in the matching placebo group (relative risk reduction, 70.4%; P = .002).
Symptoms were resolved on average 4 days earlier with each REGEN-COV dose than with placebo (10 days vs. 14 days; P < .001 for both comparisons).
Dr. Weinreich said in an interview that trials will continue to find the lowest effective doses that can stand up to all evolving variants.
“This is one of those settings where you don’t want to underdose. You’ve got one shot at this,” he said. “We’d love to do lower doses. It would be more convenient and we could treat more patients, but if it generates more clinical failures or doesn’t work with certain variants, then you’ve done a huge disservice to the world.”
Also new in this study is that researchers tested not only seronegative patients, but patients at high risk regardless of blood antibody status, he said.
“It’s the first suggestion of data that if you’re breaking through a vaccine and you’re at high risk, the use of the cocktail is something to strongly consider because treatment early is better than treatment later,” Dr. Weinreich said.
In addition to efficacy, the phase 3 trial demonstrated the cocktail had a good safety profile. Serious adverse events occurred more often in the placebo group (4%) than in the 1,200-mg group (1.1%) and the 2,400-mg group (1.3%). Infusion reactions (grade 2 or higher) occurred in less than 0.3% of patients in all groups.
William Fales, MD, state medical director for the Michigan Department of Health and Human Services, said the results confirm the promise of REGEN-COV for reducing hospitalizations and death in a peer-reviewed publication.
COVID-19 a moving target
However, Dr. Fales noted that COVID-19 is a moving target with emerging variants. The criteria for populations at high risk have also broadened since the start of the study, he said.
“A great example is pregnancy is now included as high risk, and that would have likely been a specific contraindication of patients in this clinical trial,” he said.
Dr. Fales said Michigan has been using both REGEN-COV and the Eli Lilly combination of bamlanivimab and etesevimab, which also has an emergency use authorization (EUA) from the Food and Drug Administration, with positive results.
REGEN-COV has an EUA to treat people who are at high risk of serious consequences from COVID-19, including those who are already infected (nonhospitalized) or those in certain postexposure prophylaxis settings.
“We’re seeing very low hospitalization rates and few deaths in a state that is predominately Delta,” Dr. Fales said. “So, this makes us feel that we’re doing the right thing and supports the current efforts around the country to make monoclonal antibody therapy available to high-risk patients.”
Dr. Joshi noted that trial results have been emerging from other monoclonal antibody cocktails with different COVID-19 patient groups.
However, he said in an interview, “how much more effective they would be than this is something we’d have to look at, as 71% effectiveness in keeping people out of the hospital is pretty good for any treatment.”
“These are great numbers, but vaccination itself keeps you from getting the disease in the first place and not just for a short time period. This treatment is just that – a treatment. It gets you through that episode but it doesn’t mean you won’t get sick again. You don’t develop an immune response as you do with the vaccine,” he said.
Dr. Weinreich agreed: “This is not a substitute for a vaccine except for the small group who get the vaccine and their bodies can’t respond to it because they’re significantly immunocompromised.”
The results from this paper “are one piece of a large, multistudy, phase 3 program that basically spans from prophylaxis all the way to hospitalization and pretty much the gamut – all of them – have worked. All of these studies have shown dramatic improvement in whatever the definitive regulatory endpoint is,” Dr. Weinreich said.
He said discussions are ongoing for full regulatory approval in the United States and for expanding the EUA for other populations, including pre-exposure prophylaxis, “which the [United Kingdom’s] authority has already granted us but the FDA has not.”
The study is funded by Regeneron and the Department of Health & Human Services. Dr. Weinreich is a vice president of Regeneron. Dr. Joshi reported no relevant financial relationships. Dr. Fales holds stock in Eli Lilly.
A version of this article first appeared on Medscape.com.
A monoclonal antibody combination of casirivimab and imdevimab (REGEN-COV) significantly reduced the risk of COVID-19–related hospitalizations and death from any cause in the phase 3 portion of an adaptive trial of outpatients.
Researchers, led by David Weinreich, MD, MBA, executive vice president of the drug cocktail’s manufacturer Regeneron, found in the randomized trial that the combination also resolved symptoms and reduced the SARS-CoV-2 viral load more quickly, compared with placebo.
Findings were published in the New England Journal of Medicine.
COVID-related hospitalization or death from any cause occurred in 18 of 1,355 patients (1.3%) in the group getting 2,400 mg infusions of the study drug, compared with 62 (4.6%) of 1,341 in the matching placebo group, indicating a relative risk reduction of 71.3%; P < .001.
Sunil Joshi, MD, president of the Duval County Medical Society Foundation and an immunologist in Jacksonville, Fla., said in an interview that these findings confirm benefits of REGEN-COV and are very good news for a patient group that includes those age 65 and older with high blood pressure, diabetes, or obesity; and for people not vaccinated, who are all at high risk of hospitalization or death if they get COVID-19.
“Vaccines are critically important,” he said, “but if you were to be infected and know that there’s a way to keep yourself out of the hospital, this is very good news.”
Researchers seek lowest doses
This trial found that the effect was similar when researchers cut the doses in half. These outcomes occurred in 7 of 736 (1%) of patients given 1,200 mg of REGEN-COV and in 24 (3.2%) of 748 in the matching placebo group (relative risk reduction, 70.4%; P = .002).
Symptoms were resolved on average 4 days earlier with each REGEN-COV dose than with placebo (10 days vs. 14 days; P < .001 for both comparisons).
Dr. Weinreich said in an interview that trials will continue to find the lowest effective doses that can stand up to all evolving variants.
“This is one of those settings where you don’t want to underdose. You’ve got one shot at this,” he said. “We’d love to do lower doses. It would be more convenient and we could treat more patients, but if it generates more clinical failures or doesn’t work with certain variants, then you’ve done a huge disservice to the world.”
Also new in this study is that researchers tested not only seronegative patients, but patients at high risk regardless of blood antibody status, he said.
“It’s the first suggestion of data that if you’re breaking through a vaccine and you’re at high risk, the use of the cocktail is something to strongly consider because treatment early is better than treatment later,” Dr. Weinreich said.
In addition to efficacy, the phase 3 trial demonstrated the cocktail had a good safety profile. Serious adverse events occurred more often in the placebo group (4%) than in the 1,200-mg group (1.1%) and the 2,400-mg group (1.3%). Infusion reactions (grade 2 or higher) occurred in less than 0.3% of patients in all groups.
William Fales, MD, state medical director for the Michigan Department of Health and Human Services, said the results confirm the promise of REGEN-COV for reducing hospitalizations and death in a peer-reviewed publication.
COVID-19 a moving target
However, Dr. Fales noted that COVID-19 is a moving target with emerging variants. The criteria for populations at high risk have also broadened since the start of the study, he said.
“A great example is pregnancy is now included as high risk, and that would have likely been a specific contraindication of patients in this clinical trial,” he said.
Dr. Fales said Michigan has been using both REGEN-COV and the Eli Lilly combination of bamlanivimab and etesevimab, which also has an emergency use authorization (EUA) from the Food and Drug Administration, with positive results.
REGEN-COV has an EUA to treat people who are at high risk of serious consequences from COVID-19, including those who are already infected (nonhospitalized) or those in certain postexposure prophylaxis settings.
“We’re seeing very low hospitalization rates and few deaths in a state that is predominately Delta,” Dr. Fales said. “So, this makes us feel that we’re doing the right thing and supports the current efforts around the country to make monoclonal antibody therapy available to high-risk patients.”
Dr. Joshi noted that trial results have been emerging from other monoclonal antibody cocktails with different COVID-19 patient groups.
However, he said in an interview, “how much more effective they would be than this is something we’d have to look at, as 71% effectiveness in keeping people out of the hospital is pretty good for any treatment.”
“These are great numbers, but vaccination itself keeps you from getting the disease in the first place and not just for a short time period. This treatment is just that – a treatment. It gets you through that episode but it doesn’t mean you won’t get sick again. You don’t develop an immune response as you do with the vaccine,” he said.
Dr. Weinreich agreed: “This is not a substitute for a vaccine except for the small group who get the vaccine and their bodies can’t respond to it because they’re significantly immunocompromised.”
The results from this paper “are one piece of a large, multistudy, phase 3 program that basically spans from prophylaxis all the way to hospitalization and pretty much the gamut – all of them – have worked. All of these studies have shown dramatic improvement in whatever the definitive regulatory endpoint is,” Dr. Weinreich said.
He said discussions are ongoing for full regulatory approval in the United States and for expanding the EUA for other populations, including pre-exposure prophylaxis, “which the [United Kingdom’s] authority has already granted us but the FDA has not.”
The study is funded by Regeneron and the Department of Health & Human Services. Dr. Weinreich is a vice president of Regeneron. Dr. Joshi reported no relevant financial relationships. Dr. Fales holds stock in Eli Lilly.
A version of this article first appeared on Medscape.com.
Flu shot highly recommended this year
With the Delta variant of COVID-19 still raging in the United States and ICUs in parts of the country filled with patients with the coronavirus, experts are voicing concern about the added risk of a difficult flu season.
Two mathematical models are predicting a big rebound in the number and severity of flu cases in the 2021-22 season after 2020-2021’s flu season failed to show up when public health measures brought in to control COVID-19 seemed to have the added benefit of stopping the flu.
But both analyses, posted to the medRxiv preprint server and not yet peer reviewed by other experts, have come to the same conclusion: The flu could make a comeback this year.
In the worst-case scenario, the United States could see an extra 300,000-400,000 hospitalizations from the flu – almost double the usual number – according to senior study author Mark Roberts, MD, director of the Public Health Dynamics Laboratory at the University of Pittsburgh. These numbers could be a disaster in areas where hospitals are already filled with COVID-19 patients.
Waning natural immunity in the public because of 2020-2021’s missing flu season could make people, especially young children, more likely to get the virus.
“Usually, a combination of natural immunity and vaccination helps tamp down seasonal influenza,” said Dr. Roberts. “If we don’t have the first part, we’ll have to rely more on the vaccine.”
In a typical year, about half of Americans get the flu shot. The new mathematical models predict that the vaccination rate would need to rise to about 75% to avoid the extra hospitalizations. But even a 10% increase in vaccination rates could reduce hospitalizations by 6%-46%, depending on what strains are dominant.
Usually, the Southern Hemisphere flu season, from February to August, helps show what the Northern Hemisphere can expect over the coming winter. But with strict COVID-19 measures and limits on international travel still in place in countries like Australia and New Zealand and much of South America, it has been another record-low year for flu infections, said Ian Barr, PhD, deputy director of the World Health Organization’s Collaborating Center for Reference and Research on Influenza in Melbourne.
Australia detected only around 500 cases in 2021, compared with about 300,000 in a normal year, and recorded no hospitalizations or deaths from the flu. New Zealand recorded just two cases.
“I’ve never seen anything like this,” Dr. Barr said.
In Australia, the mild flu season led to fewer people getting their flu shot than usual. The rate fell from around 50% to just 33%, said Dr. Barr. “If that happens in the U.S., the population will be even more vulnerable because there has been almost no flu for more than 12 months,” he said.
Both Dr. Roberts and Dr. Barr say it is vital that as many people as possible get vaccinated during the upcoming flu season, especially children who will have almost no natural immunity to the virus.
“The vaccine is our best weapon against the flu, especially for the most at-risk groups,” said Dr. Barr.
Other parts of the world had mixed results. India saw a high number of flu cases, while neighboring Sri Lanka had very few. West Africa also saw quite a high level of circulating virus. Overall, the flu was detected in 45 countries during the Southern Hemisphere season, less than half of what might be expected in a normal year, said Dr. Barr.
Despite the overall low numbers, the WHO saw enough in the data to make two changes to 2022’s Southern Hemisphere vaccine formulation at its meeting on Sept. 24, after changing just one of the strains for the Northern Hemisphere vaccine at its meeting in February.
The CDC recommends that everyone 6 months or older get the flu shot, with few exceptions.
A version of this article first appeared on WebMD.com.
With the Delta variant of COVID-19 still raging in the United States and ICUs in parts of the country filled with patients with the coronavirus, experts are voicing concern about the added risk of a difficult flu season.
Two mathematical models are predicting a big rebound in the number and severity of flu cases in the 2021-22 season after 2020-2021’s flu season failed to show up when public health measures brought in to control COVID-19 seemed to have the added benefit of stopping the flu.
But both analyses, posted to the medRxiv preprint server and not yet peer reviewed by other experts, have come to the same conclusion: The flu could make a comeback this year.
In the worst-case scenario, the United States could see an extra 300,000-400,000 hospitalizations from the flu – almost double the usual number – according to senior study author Mark Roberts, MD, director of the Public Health Dynamics Laboratory at the University of Pittsburgh. These numbers could be a disaster in areas where hospitals are already filled with COVID-19 patients.
Waning natural immunity in the public because of 2020-2021’s missing flu season could make people, especially young children, more likely to get the virus.
“Usually, a combination of natural immunity and vaccination helps tamp down seasonal influenza,” said Dr. Roberts. “If we don’t have the first part, we’ll have to rely more on the vaccine.”
In a typical year, about half of Americans get the flu shot. The new mathematical models predict that the vaccination rate would need to rise to about 75% to avoid the extra hospitalizations. But even a 10% increase in vaccination rates could reduce hospitalizations by 6%-46%, depending on what strains are dominant.
Usually, the Southern Hemisphere flu season, from February to August, helps show what the Northern Hemisphere can expect over the coming winter. But with strict COVID-19 measures and limits on international travel still in place in countries like Australia and New Zealand and much of South America, it has been another record-low year for flu infections, said Ian Barr, PhD, deputy director of the World Health Organization’s Collaborating Center for Reference and Research on Influenza in Melbourne.
Australia detected only around 500 cases in 2021, compared with about 300,000 in a normal year, and recorded no hospitalizations or deaths from the flu. New Zealand recorded just two cases.
“I’ve never seen anything like this,” Dr. Barr said.
In Australia, the mild flu season led to fewer people getting their flu shot than usual. The rate fell from around 50% to just 33%, said Dr. Barr. “If that happens in the U.S., the population will be even more vulnerable because there has been almost no flu for more than 12 months,” he said.
Both Dr. Roberts and Dr. Barr say it is vital that as many people as possible get vaccinated during the upcoming flu season, especially children who will have almost no natural immunity to the virus.
“The vaccine is our best weapon against the flu, especially for the most at-risk groups,” said Dr. Barr.
Other parts of the world had mixed results. India saw a high number of flu cases, while neighboring Sri Lanka had very few. West Africa also saw quite a high level of circulating virus. Overall, the flu was detected in 45 countries during the Southern Hemisphere season, less than half of what might be expected in a normal year, said Dr. Barr.
Despite the overall low numbers, the WHO saw enough in the data to make two changes to 2022’s Southern Hemisphere vaccine formulation at its meeting on Sept. 24, after changing just one of the strains for the Northern Hemisphere vaccine at its meeting in February.
The CDC recommends that everyone 6 months or older get the flu shot, with few exceptions.
A version of this article first appeared on WebMD.com.
With the Delta variant of COVID-19 still raging in the United States and ICUs in parts of the country filled with patients with the coronavirus, experts are voicing concern about the added risk of a difficult flu season.
Two mathematical models are predicting a big rebound in the number and severity of flu cases in the 2021-22 season after 2020-2021’s flu season failed to show up when public health measures brought in to control COVID-19 seemed to have the added benefit of stopping the flu.
But both analyses, posted to the medRxiv preprint server and not yet peer reviewed by other experts, have come to the same conclusion: The flu could make a comeback this year.
In the worst-case scenario, the United States could see an extra 300,000-400,000 hospitalizations from the flu – almost double the usual number – according to senior study author Mark Roberts, MD, director of the Public Health Dynamics Laboratory at the University of Pittsburgh. These numbers could be a disaster in areas where hospitals are already filled with COVID-19 patients.
Waning natural immunity in the public because of 2020-2021’s missing flu season could make people, especially young children, more likely to get the virus.
“Usually, a combination of natural immunity and vaccination helps tamp down seasonal influenza,” said Dr. Roberts. “If we don’t have the first part, we’ll have to rely more on the vaccine.”
In a typical year, about half of Americans get the flu shot. The new mathematical models predict that the vaccination rate would need to rise to about 75% to avoid the extra hospitalizations. But even a 10% increase in vaccination rates could reduce hospitalizations by 6%-46%, depending on what strains are dominant.
Usually, the Southern Hemisphere flu season, from February to August, helps show what the Northern Hemisphere can expect over the coming winter. But with strict COVID-19 measures and limits on international travel still in place in countries like Australia and New Zealand and much of South America, it has been another record-low year for flu infections, said Ian Barr, PhD, deputy director of the World Health Organization’s Collaborating Center for Reference and Research on Influenza in Melbourne.
Australia detected only around 500 cases in 2021, compared with about 300,000 in a normal year, and recorded no hospitalizations or deaths from the flu. New Zealand recorded just two cases.
“I’ve never seen anything like this,” Dr. Barr said.
In Australia, the mild flu season led to fewer people getting their flu shot than usual. The rate fell from around 50% to just 33%, said Dr. Barr. “If that happens in the U.S., the population will be even more vulnerable because there has been almost no flu for more than 12 months,” he said.
Both Dr. Roberts and Dr. Barr say it is vital that as many people as possible get vaccinated during the upcoming flu season, especially children who will have almost no natural immunity to the virus.
“The vaccine is our best weapon against the flu, especially for the most at-risk groups,” said Dr. Barr.
Other parts of the world had mixed results. India saw a high number of flu cases, while neighboring Sri Lanka had very few. West Africa also saw quite a high level of circulating virus. Overall, the flu was detected in 45 countries during the Southern Hemisphere season, less than half of what might be expected in a normal year, said Dr. Barr.
Despite the overall low numbers, the WHO saw enough in the data to make two changes to 2022’s Southern Hemisphere vaccine formulation at its meeting on Sept. 24, after changing just one of the strains for the Northern Hemisphere vaccine at its meeting in February.
The CDC recommends that everyone 6 months or older get the flu shot, with few exceptions.
A version of this article first appeared on WebMD.com.
New data illustrate pandemic pivot to telehealth by patients, physicians
Telehealth use, although much higher than before the COVID-19 pandemic, accounted for less than 20% of weekly outpatient visits 6 months into the pandemic, according to a new report from the American Medical Association. Ten percent of weekly visits were conducted via videoconferencing, and 8.1% of visits were conducted using the telephone.
Those figures may overstate the true level of telehealth use in fall 2020. A study by the Commonwealth Fund, Harvard University, Boston, and Phreesia found that in December of that year, only 8% of outpatient visits involved the use of telemedicine – and that was up from 6% in October. In contrast to the AMA results, which came from its 2020 benchmark survey of physicians, the Commonwealth Fund study used data from practice management systems and an online patient registration platform, as well as electronic health record data.
A more recent survey of hospital executives found that as of September 2021, hospital telehealth visits had leveled off at 10% to 20% of appointments. Similarly, a McKinsey survey in July showed that telehealth encounters made up 13% to 17% of evaluation and management visits across all specialties.
Big jump during pandemic
The AMA report offers a wealth of data on how physicians use telehealth and the differences between specialties in this area.
The report found that 70.3% of physicians worked in practices that used videoconferencing to provide patient visits in September 2020, compared to 14.3% of physicians in September 2018. Sixty-seven percent of physicians worked in practices that used telephone visits (the comparable figure for 2018 was unavailable).
Overall, 79% of physicians worked in a practice that used telehealth, compared to 25% in 2018.
Not every doctor in practices that utilized telehealth conducted virtual visits. In contrast to the 70.3% of doctors who were in practices that had video visits, only 59.1% of the respondents had personally conducted a videoconferencing visit in the previous week. The average numbers of weekly video and telephone visits per physician were 9.9 and 7.6, respectively, including those who did none.
There were big differences in virtual visit use among specialties as well. Eighty-five percent of psychiatrists were in practices that provided online appointments, according to the AMA survey, and three-quarters of primary care physicians said their practices offered telehealth appointments. Pediatricians were much less likely than family practice/general practice physicians (FPs/GPs) or general internists to do so.
The practices of many medical specialists were also highly likely to provide telehealth. Over 75% of practices in cardiology, endocrinology/diabetes, gastroenterology, nephrology, and neurology offered telehealth visits. About 88% of hematologists/oncologists offered video visits. Far fewer surgeons reported that their practice used virtual visits; the exceptions were urologists and dermatologists, 87% of whose practices used telehealth.
How telehealth was used
Across all specialties, 58% of physicians said clinicians in their practices used it to diagnose or treat patients; 59.2%, to manage patients with chronic disease; 50.4%, to provide acute care; and 34.3%, to provide preventive care.
Seventy-two percent of FP/GP and pediatric practices used telehealth to diagnose or treat patients. Just 64.9% of internists said their practices did so, and only 61.9% of them said their practices provided acute care via telehealth, versus 70% of FPs/GPs and pediatricians.
Among medical specialties, endocrinologists/diabetes physicians were those most likely to report the practice-level use of telehealth to diagnose or treat patients (71.9%), manage patients with chronic disease (92.1%), and provide preventive care (52.6%).
Significantly, 33% of medical specialists said their practices used remote patient monitoring. This finding was driven by high rates of use among cardiology practices (63.3%) and endocrinology practices (41.6%). Overall, the practice-level use of remote patient monitoring rose from 10.4% of practices in 2018 to 19.9% in 2020.
Virtual consults with peers
Some practices used telehealth to enable physicians to consult with colleagues. Twelve percent of respondents said their practices used telehealth to seek a second opinion from a health care professional in 2020, compared to 6.9% in 2018. Formal consultations via telehealth were also increasingly common: 17.2% of doctors said their practices did this in 2020, compared to 11.3% in 2018.
Also of note, 22.4% of physicians said their practices used telehealth for after-hours care or night calls in 2020, versus 9.9% in 2018.
The AMA report credited telehealth and expanded coverage and payment rules for enabling physician practices to keep their revenue streams positive and their practices open. However, the Commonwealth Fund study found “a substantial cumulative reduction in visits across all specialties over the course of the pandemic in 2020.” These ranged from a drop of 27% in pediatric visits to a decline of 8% in rheumatology visits during the period from March to December 2020.
A version of this article first appeared on Medscape.com.
Telehealth use, although much higher than before the COVID-19 pandemic, accounted for less than 20% of weekly outpatient visits 6 months into the pandemic, according to a new report from the American Medical Association. Ten percent of weekly visits were conducted via videoconferencing, and 8.1% of visits were conducted using the telephone.
Those figures may overstate the true level of telehealth use in fall 2020. A study by the Commonwealth Fund, Harvard University, Boston, and Phreesia found that in December of that year, only 8% of outpatient visits involved the use of telemedicine – and that was up from 6% in October. In contrast to the AMA results, which came from its 2020 benchmark survey of physicians, the Commonwealth Fund study used data from practice management systems and an online patient registration platform, as well as electronic health record data.
A more recent survey of hospital executives found that as of September 2021, hospital telehealth visits had leveled off at 10% to 20% of appointments. Similarly, a McKinsey survey in July showed that telehealth encounters made up 13% to 17% of evaluation and management visits across all specialties.
Big jump during pandemic
The AMA report offers a wealth of data on how physicians use telehealth and the differences between specialties in this area.
The report found that 70.3% of physicians worked in practices that used videoconferencing to provide patient visits in September 2020, compared to 14.3% of physicians in September 2018. Sixty-seven percent of physicians worked in practices that used telephone visits (the comparable figure for 2018 was unavailable).
Overall, 79% of physicians worked in a practice that used telehealth, compared to 25% in 2018.
Not every doctor in practices that utilized telehealth conducted virtual visits. In contrast to the 70.3% of doctors who were in practices that had video visits, only 59.1% of the respondents had personally conducted a videoconferencing visit in the previous week. The average numbers of weekly video and telephone visits per physician were 9.9 and 7.6, respectively, including those who did none.
There were big differences in virtual visit use among specialties as well. Eighty-five percent of psychiatrists were in practices that provided online appointments, according to the AMA survey, and three-quarters of primary care physicians said their practices offered telehealth appointments. Pediatricians were much less likely than family practice/general practice physicians (FPs/GPs) or general internists to do so.
The practices of many medical specialists were also highly likely to provide telehealth. Over 75% of practices in cardiology, endocrinology/diabetes, gastroenterology, nephrology, and neurology offered telehealth visits. About 88% of hematologists/oncologists offered video visits. Far fewer surgeons reported that their practice used virtual visits; the exceptions were urologists and dermatologists, 87% of whose practices used telehealth.
How telehealth was used
Across all specialties, 58% of physicians said clinicians in their practices used it to diagnose or treat patients; 59.2%, to manage patients with chronic disease; 50.4%, to provide acute care; and 34.3%, to provide preventive care.
Seventy-two percent of FP/GP and pediatric practices used telehealth to diagnose or treat patients. Just 64.9% of internists said their practices did so, and only 61.9% of them said their practices provided acute care via telehealth, versus 70% of FPs/GPs and pediatricians.
Among medical specialties, endocrinologists/diabetes physicians were those most likely to report the practice-level use of telehealth to diagnose or treat patients (71.9%), manage patients with chronic disease (92.1%), and provide preventive care (52.6%).
Significantly, 33% of medical specialists said their practices used remote patient monitoring. This finding was driven by high rates of use among cardiology practices (63.3%) and endocrinology practices (41.6%). Overall, the practice-level use of remote patient monitoring rose from 10.4% of practices in 2018 to 19.9% in 2020.
Virtual consults with peers
Some practices used telehealth to enable physicians to consult with colleagues. Twelve percent of respondents said their practices used telehealth to seek a second opinion from a health care professional in 2020, compared to 6.9% in 2018. Formal consultations via telehealth were also increasingly common: 17.2% of doctors said their practices did this in 2020, compared to 11.3% in 2018.
Also of note, 22.4% of physicians said their practices used telehealth for after-hours care or night calls in 2020, versus 9.9% in 2018.
The AMA report credited telehealth and expanded coverage and payment rules for enabling physician practices to keep their revenue streams positive and their practices open. However, the Commonwealth Fund study found “a substantial cumulative reduction in visits across all specialties over the course of the pandemic in 2020.” These ranged from a drop of 27% in pediatric visits to a decline of 8% in rheumatology visits during the period from March to December 2020.
A version of this article first appeared on Medscape.com.
Telehealth use, although much higher than before the COVID-19 pandemic, accounted for less than 20% of weekly outpatient visits 6 months into the pandemic, according to a new report from the American Medical Association. Ten percent of weekly visits were conducted via videoconferencing, and 8.1% of visits were conducted using the telephone.
Those figures may overstate the true level of telehealth use in fall 2020. A study by the Commonwealth Fund, Harvard University, Boston, and Phreesia found that in December of that year, only 8% of outpatient visits involved the use of telemedicine – and that was up from 6% in October. In contrast to the AMA results, which came from its 2020 benchmark survey of physicians, the Commonwealth Fund study used data from practice management systems and an online patient registration platform, as well as electronic health record data.
A more recent survey of hospital executives found that as of September 2021, hospital telehealth visits had leveled off at 10% to 20% of appointments. Similarly, a McKinsey survey in July showed that telehealth encounters made up 13% to 17% of evaluation and management visits across all specialties.
Big jump during pandemic
The AMA report offers a wealth of data on how physicians use telehealth and the differences between specialties in this area.
The report found that 70.3% of physicians worked in practices that used videoconferencing to provide patient visits in September 2020, compared to 14.3% of physicians in September 2018. Sixty-seven percent of physicians worked in practices that used telephone visits (the comparable figure for 2018 was unavailable).
Overall, 79% of physicians worked in a practice that used telehealth, compared to 25% in 2018.
Not every doctor in practices that utilized telehealth conducted virtual visits. In contrast to the 70.3% of doctors who were in practices that had video visits, only 59.1% of the respondents had personally conducted a videoconferencing visit in the previous week. The average numbers of weekly video and telephone visits per physician were 9.9 and 7.6, respectively, including those who did none.
There were big differences in virtual visit use among specialties as well. Eighty-five percent of psychiatrists were in practices that provided online appointments, according to the AMA survey, and three-quarters of primary care physicians said their practices offered telehealth appointments. Pediatricians were much less likely than family practice/general practice physicians (FPs/GPs) or general internists to do so.
The practices of many medical specialists were also highly likely to provide telehealth. Over 75% of practices in cardiology, endocrinology/diabetes, gastroenterology, nephrology, and neurology offered telehealth visits. About 88% of hematologists/oncologists offered video visits. Far fewer surgeons reported that their practice used virtual visits; the exceptions were urologists and dermatologists, 87% of whose practices used telehealth.
How telehealth was used
Across all specialties, 58% of physicians said clinicians in their practices used it to diagnose or treat patients; 59.2%, to manage patients with chronic disease; 50.4%, to provide acute care; and 34.3%, to provide preventive care.
Seventy-two percent of FP/GP and pediatric practices used telehealth to diagnose or treat patients. Just 64.9% of internists said their practices did so, and only 61.9% of them said their practices provided acute care via telehealth, versus 70% of FPs/GPs and pediatricians.
Among medical specialties, endocrinologists/diabetes physicians were those most likely to report the practice-level use of telehealth to diagnose or treat patients (71.9%), manage patients with chronic disease (92.1%), and provide preventive care (52.6%).
Significantly, 33% of medical specialists said their practices used remote patient monitoring. This finding was driven by high rates of use among cardiology practices (63.3%) and endocrinology practices (41.6%). Overall, the practice-level use of remote patient monitoring rose from 10.4% of practices in 2018 to 19.9% in 2020.
Virtual consults with peers
Some practices used telehealth to enable physicians to consult with colleagues. Twelve percent of respondents said their practices used telehealth to seek a second opinion from a health care professional in 2020, compared to 6.9% in 2018. Formal consultations via telehealth were also increasingly common: 17.2% of doctors said their practices did this in 2020, compared to 11.3% in 2018.
Also of note, 22.4% of physicians said their practices used telehealth for after-hours care or night calls in 2020, versus 9.9% in 2018.
The AMA report credited telehealth and expanded coverage and payment rules for enabling physician practices to keep their revenue streams positive and their practices open. However, the Commonwealth Fund study found “a substantial cumulative reduction in visits across all specialties over the course of the pandemic in 2020.” These ranged from a drop of 27% in pediatric visits to a decline of 8% in rheumatology visits during the period from March to December 2020.
A version of this article first appeared on Medscape.com.