User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Scientists find microplastics in human lung tissue
U.K. scientists said microplastics may pose even more of a threat than previously thought after confirming their presence in lung tissue taken from living people.
Microplastics were identified in all lung regions, but significantly higher levels were found in the lower lung.
The results supported inhalation as an exposure risk, according to the team from the University of Hull and Hull York Medical School (England), who said their findings could support further investigations into the effects of airborne microplastics on respiratory health.
The study, published in Science of the Total Environment, used lung tissue collected from surgical procedures on patients during routine medical care at Castle Hill Hospital in East Yorkshire.
Polypropylene and polyethylene
It found 39 microplastics in 11 of the 13 lung tissue samples tested using micro-Fourier-transform infrared (μFTIR) analysis, which the scientists said was considerably higher than results from previous laboratory tests.
Of microplastics detected, 12 polymer types were identified, of which the most common were polypropylene, (23%) polyethylene terephthalate (18%), and resin (15%). The fibers are commonly found in packaging, bottles, clothing, rope and twine manufacture, and other industries, the scientists said.
Microplastics with dimensions as small as 4 μm were found, but the scientists said they were surprised to discover samples as large as greater than 2 mm within all lung region samples, with the majority being fibrous and fragmented.
The study identified 11 microplastics in the upper part of the lung, seven in the mid part, and 21 in the lower part of the lung.
Laura Sadofsky, the study’s lead author, said: “Microplastics have previously been found in human cadaver autopsy samples. This is the first robust study to show microplastics in lungs from live people. It also shows that they are in the lower parts of the lung. Lung airways are very narrow, so no one thought they could possibly get there, but they clearly have.”
There were also considerably higher levels of microplastics found in male patients, compared with female patients.
Future investigations into health implications
“The characterization of types and levels of microplastics we have found can now inform realistic conditions for laboratory exposure experiments with the aim of determining health impacts,” said Laura Sadofsky, who is a senior lecturer in respiratory medicine in the Centre for Atherothrombotic and Metabolic Research at Hull York Medical School.
The latest investigation followed previous research by the medical school and the University of Hull, which found high levels of atmospheric microplastics within the Humber region.
That study, published in Atmosphere, identified resins, which could have originated from degraded roads, paint marking, or tire rubber, as well as polyethylene fibers.
A version of this article first appeared on Medscape UK.
U.K. scientists said microplastics may pose even more of a threat than previously thought after confirming their presence in lung tissue taken from living people.
Microplastics were identified in all lung regions, but significantly higher levels were found in the lower lung.
The results supported inhalation as an exposure risk, according to the team from the University of Hull and Hull York Medical School (England), who said their findings could support further investigations into the effects of airborne microplastics on respiratory health.
The study, published in Science of the Total Environment, used lung tissue collected from surgical procedures on patients during routine medical care at Castle Hill Hospital in East Yorkshire.
Polypropylene and polyethylene
It found 39 microplastics in 11 of the 13 lung tissue samples tested using micro-Fourier-transform infrared (μFTIR) analysis, which the scientists said was considerably higher than results from previous laboratory tests.
Of microplastics detected, 12 polymer types were identified, of which the most common were polypropylene, (23%) polyethylene terephthalate (18%), and resin (15%). The fibers are commonly found in packaging, bottles, clothing, rope and twine manufacture, and other industries, the scientists said.
Microplastics with dimensions as small as 4 μm were found, but the scientists said they were surprised to discover samples as large as greater than 2 mm within all lung region samples, with the majority being fibrous and fragmented.
The study identified 11 microplastics in the upper part of the lung, seven in the mid part, and 21 in the lower part of the lung.
Laura Sadofsky, the study’s lead author, said: “Microplastics have previously been found in human cadaver autopsy samples. This is the first robust study to show microplastics in lungs from live people. It also shows that they are in the lower parts of the lung. Lung airways are very narrow, so no one thought they could possibly get there, but they clearly have.”
There were also considerably higher levels of microplastics found in male patients, compared with female patients.
Future investigations into health implications
“The characterization of types and levels of microplastics we have found can now inform realistic conditions for laboratory exposure experiments with the aim of determining health impacts,” said Laura Sadofsky, who is a senior lecturer in respiratory medicine in the Centre for Atherothrombotic and Metabolic Research at Hull York Medical School.
The latest investigation followed previous research by the medical school and the University of Hull, which found high levels of atmospheric microplastics within the Humber region.
That study, published in Atmosphere, identified resins, which could have originated from degraded roads, paint marking, or tire rubber, as well as polyethylene fibers.
A version of this article first appeared on Medscape UK.
U.K. scientists said microplastics may pose even more of a threat than previously thought after confirming their presence in lung tissue taken from living people.
Microplastics were identified in all lung regions, but significantly higher levels were found in the lower lung.
The results supported inhalation as an exposure risk, according to the team from the University of Hull and Hull York Medical School (England), who said their findings could support further investigations into the effects of airborne microplastics on respiratory health.
The study, published in Science of the Total Environment, used lung tissue collected from surgical procedures on patients during routine medical care at Castle Hill Hospital in East Yorkshire.
Polypropylene and polyethylene
It found 39 microplastics in 11 of the 13 lung tissue samples tested using micro-Fourier-transform infrared (μFTIR) analysis, which the scientists said was considerably higher than results from previous laboratory tests.
Of microplastics detected, 12 polymer types were identified, of which the most common were polypropylene, (23%) polyethylene terephthalate (18%), and resin (15%). The fibers are commonly found in packaging, bottles, clothing, rope and twine manufacture, and other industries, the scientists said.
Microplastics with dimensions as small as 4 μm were found, but the scientists said they were surprised to discover samples as large as greater than 2 mm within all lung region samples, with the majority being fibrous and fragmented.
The study identified 11 microplastics in the upper part of the lung, seven in the mid part, and 21 in the lower part of the lung.
Laura Sadofsky, the study’s lead author, said: “Microplastics have previously been found in human cadaver autopsy samples. This is the first robust study to show microplastics in lungs from live people. It also shows that they are in the lower parts of the lung. Lung airways are very narrow, so no one thought they could possibly get there, but they clearly have.”
There were also considerably higher levels of microplastics found in male patients, compared with female patients.
Future investigations into health implications
“The characterization of types and levels of microplastics we have found can now inform realistic conditions for laboratory exposure experiments with the aim of determining health impacts,” said Laura Sadofsky, who is a senior lecturer in respiratory medicine in the Centre for Atherothrombotic and Metabolic Research at Hull York Medical School.
The latest investigation followed previous research by the medical school and the University of Hull, which found high levels of atmospheric microplastics within the Humber region.
That study, published in Atmosphere, identified resins, which could have originated from degraded roads, paint marking, or tire rubber, as well as polyethylene fibers.
A version of this article first appeared on Medscape UK.
Sex differences in COPD slow to be recognized, treated
When Sigmund Freud claimed that “anatomy is destiny” he was referring to anatomical sex as a determinant of personality traits. Expert consensus statements have previously offered some recommendations for managing these syndromes, but clinical data are scarce, so the present review “is intended to establish a starting point for future research,”
That notion has been widely discredited, but Freud appears to be inadvertently right in one respect: When it comes to chronic obstructive pulmonary disease (COPD), anatomy really is destiny, and sex may be as well, pulmonary researchers say.
There is a growing body of evidence to indicate that COPD affects men and women differently, and that men and women patients with COPD require different clinical management. Yet women are often underdiagnosed or misdiagnosed, partly because of poorly understood sex differences, but also because of cultural biases.
But plunging any farther into the weeds, it’s important to define terms. Although various investigators have used the terms “sex” and “gender” interchangeably, sex is the preferred term when referring to biological attributes of individual patients, while gender refers to personal identity.
These distinctions are important, contended Amik Sodhi, MBBS, MPH, from the division of allergy, pulmonology, and critical care medicine at the University of Wisconsin–Madison.
“Sex is essentially a biologic construct, so it’s got to do with the sex chromosomes, the genetics of that person, and it refers to the anatomic variations that can change susceptibility to different diseases,” she said in an interview.
An example of sex differences or “sexual dimorphism” can be found in a recent meta-analysis of sex-based genetic associations by Megan Hardin, MD, MPH from Brigham & Women’s Hospital in Boston and colleagues.
They reported that CELSR1, a gene involved in fetal lung development, was expressed more among women than among men and that a single nucleotide polymorphism in the gene was associated with COPD among women smokers, but not among men smokers.
The finding points to a potential risk locus for COPD in women, and could help shed light on sexual dimorphism in COPD, Dr. Hardin and colleagues said.
In contrast to sex, “gender is more of a psychosocial construct which can impact how diseases manifest themselves, how they are potentially managed, and what outcomes might occur for that particular disease,” Dr. Sodhi said.
She and her colleagues recently published a review of sex and gender in common lung disorders and sleep in the journal CHEST, where they wrote that the “influence of sex and gender is portrayed in epidemiological data, disease pathogenesis and pathophysiology, clinical manifestations, response to treatment, access to care, and health outcomes. Hence, sex and gender should be considered in all types of research, clinical practice and educational curricula.”
For example, as previously reported at the 2021 annual meeting of the American Thoracic Society, sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with COPD may point to different criteria for diagnosing cardiac comorbidities in women and men.
Those conclusions came from a retrospective analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease.
The investigators looked at the patients’ clinical history, comorbidities, lung function, COPD Assessment Test scores, and modified Medical Research Council (mMRC) dyspnea score, and found significant differences between men and women for most functional parameters and comorbidities, and for CAT items of cough, phlegm, and energy.
In logistic regression analysis, predictors for cardiac disease in men were energy, mMRC score, smoking status, body mass index, age, and spirometric lung function, but in women only age was significantly predictive for cardiac disease.
An example of gender effects on COPD differences in men and women is the increase in cigarette advertising aimed at women in the 1960s and the advent of women-targeted brands such as Virginia Slims, which in turn lead to increased smoking rates among women. In addition, in the developing world, where the sex/gender gap in COPD is narrowing, women tend to have greater exposure to wood smoke and cooking fuels in unventilated or poorly ventilated spaces, compared with men.
Increasing incidence among women
According to the Centers for Disease Control and Prevention, chronic lower respiratory diseases, primarily COPD, were the fourth-leading cause of death in women in the United States in 2018, following only heart disease, cancer, and accidents/injuries.
And as a CDC analysis of data from the 2013 Behavioral Risk Factor Surveillance System showed, women were more likely to report being told by a physician that they had COPD than did men (6.6%, compared with 5.4%).
Dr. Sodhi and colleagues noted that, at all time points examined from 2005 to 2014, women had a higher proportion than men of COPD hospitalizations and in-hospital deaths. They also noted that female sex is associated with a threefold risk for severe early-onset COPD, and that women with COPD have lower diffusion capacity of lungs for carbon monoxide, despite having higher predicted forced expiratory volume in 1 second, compared with men.
“Historically, COPD wasn’t a disease that was so prevalent in women. It’s been in the past 20 years that the trends have changed,” said Patricia Silveyra, MSc, PhD, ATSF, associate professor of environmental and occupational health at Indiana University, Bloomington.
The increasing prevalence of COPD among women cannot be explained by smoking alone, she said in an interview.
“It used to be thought that it was because more women smoked, but actually a lot of women who don’t smoke can develop COPD, so it appears to be probably something environmental, but because it used to be a disease of older men, in the clinic there was also a bias to diagnose men with COPD, and women with asthma, so a lot of women went underdiagnosed,” Dr. Silveyra said.
In their review, Dr. Sodhi and colleagues noted that women with COPD “may be underdiagnosed as a result of having different symptoms from those classically recognized. Reasons for underdiagnosis or a delay in diagnosis may also be due to lack of a formal evaluation with spirometry, women seeking care later in the course of disease, physician bias, or associated fatigue or depression misdirecting diagnostic strategies. Underdiagnosis may be associated with psychological distress and worse health-related quality of life.”
Although the evidence is mixed, women tend to present more frequently with the chronic bronchitis phenotype of COPD, compared with the emphysema phenotype, and women tend to have greater degrees of pulmonary function impairment when exposed to tobacco smoke, even after controlling for differences in height and weight.
“For the same amount of exposure to tobacco smoke, females are likely to develop more severe airflow limitation at an earlier age than males, and have more exacerbation,” Dr. Sodhi and colleagues wrote.
Both Dr. Silveyra and Dr. Sodhi said that reason why men and women differ in their physiological reactions to smoke are still unknown.
Sex differences in drug responses
There is only limited evidence to indicate that women and men respond differently to various therapeutic agents, but what is clear is that more research into this area is needed, Dr. Sodhi and Dr. Silveyra said.
For example, among the few studies that have documented sex differences, one showed no sex differences in the efficacy of salmeterol/fluticasone combination therapy for reducing exacerbations or improving quality of life, whereas another showed that women were more likely than men to experience COPD symptoms or exacerbations after stopping inhaled corticosteroids, Dr. Sodhi and colleagues noted.
Both Dr. Sodhi and Dr. Silveyra emphasized the need for clinical trials that study the effects of sex on treatment outcomes in COPD, which could lead to better, more personalized therapeutic regimens that take sex and gender into account.
Dr. Sodhi and colleagues offered the following advice to clinicians: “Interaction with female patients should take into account that their symptoms may not conform to traditionally accepted presentations. Challenges exist for female patients at all levels of health care interaction and as clinicians we need to acknowledge the bias and willfully work toward recognition and elimination of unconscious and conscious bias. Empowering our patients to have frank discussions with their health care team when they perceive bias is another step to help promote equity.”
The review by Dr. Sodhi and colleagues was supported by grants from the National Institutes of Health. Dr. Sodhi and Dr. Silveyra reported having no conflicts of interest to disclose.
When Sigmund Freud claimed that “anatomy is destiny” he was referring to anatomical sex as a determinant of personality traits. Expert consensus statements have previously offered some recommendations for managing these syndromes, but clinical data are scarce, so the present review “is intended to establish a starting point for future research,”
That notion has been widely discredited, but Freud appears to be inadvertently right in one respect: When it comes to chronic obstructive pulmonary disease (COPD), anatomy really is destiny, and sex may be as well, pulmonary researchers say.
There is a growing body of evidence to indicate that COPD affects men and women differently, and that men and women patients with COPD require different clinical management. Yet women are often underdiagnosed or misdiagnosed, partly because of poorly understood sex differences, but also because of cultural biases.
But plunging any farther into the weeds, it’s important to define terms. Although various investigators have used the terms “sex” and “gender” interchangeably, sex is the preferred term when referring to biological attributes of individual patients, while gender refers to personal identity.
These distinctions are important, contended Amik Sodhi, MBBS, MPH, from the division of allergy, pulmonology, and critical care medicine at the University of Wisconsin–Madison.
“Sex is essentially a biologic construct, so it’s got to do with the sex chromosomes, the genetics of that person, and it refers to the anatomic variations that can change susceptibility to different diseases,” she said in an interview.
An example of sex differences or “sexual dimorphism” can be found in a recent meta-analysis of sex-based genetic associations by Megan Hardin, MD, MPH from Brigham & Women’s Hospital in Boston and colleagues.
They reported that CELSR1, a gene involved in fetal lung development, was expressed more among women than among men and that a single nucleotide polymorphism in the gene was associated with COPD among women smokers, but not among men smokers.
The finding points to a potential risk locus for COPD in women, and could help shed light on sexual dimorphism in COPD, Dr. Hardin and colleagues said.
In contrast to sex, “gender is more of a psychosocial construct which can impact how diseases manifest themselves, how they are potentially managed, and what outcomes might occur for that particular disease,” Dr. Sodhi said.
She and her colleagues recently published a review of sex and gender in common lung disorders and sleep in the journal CHEST, where they wrote that the “influence of sex and gender is portrayed in epidemiological data, disease pathogenesis and pathophysiology, clinical manifestations, response to treatment, access to care, and health outcomes. Hence, sex and gender should be considered in all types of research, clinical practice and educational curricula.”
For example, as previously reported at the 2021 annual meeting of the American Thoracic Society, sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with COPD may point to different criteria for diagnosing cardiac comorbidities in women and men.
Those conclusions came from a retrospective analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease.
The investigators looked at the patients’ clinical history, comorbidities, lung function, COPD Assessment Test scores, and modified Medical Research Council (mMRC) dyspnea score, and found significant differences between men and women for most functional parameters and comorbidities, and for CAT items of cough, phlegm, and energy.
In logistic regression analysis, predictors for cardiac disease in men were energy, mMRC score, smoking status, body mass index, age, and spirometric lung function, but in women only age was significantly predictive for cardiac disease.
An example of gender effects on COPD differences in men and women is the increase in cigarette advertising aimed at women in the 1960s and the advent of women-targeted brands such as Virginia Slims, which in turn lead to increased smoking rates among women. In addition, in the developing world, where the sex/gender gap in COPD is narrowing, women tend to have greater exposure to wood smoke and cooking fuels in unventilated or poorly ventilated spaces, compared with men.
Increasing incidence among women
According to the Centers for Disease Control and Prevention, chronic lower respiratory diseases, primarily COPD, were the fourth-leading cause of death in women in the United States in 2018, following only heart disease, cancer, and accidents/injuries.
And as a CDC analysis of data from the 2013 Behavioral Risk Factor Surveillance System showed, women were more likely to report being told by a physician that they had COPD than did men (6.6%, compared with 5.4%).
Dr. Sodhi and colleagues noted that, at all time points examined from 2005 to 2014, women had a higher proportion than men of COPD hospitalizations and in-hospital deaths. They also noted that female sex is associated with a threefold risk for severe early-onset COPD, and that women with COPD have lower diffusion capacity of lungs for carbon monoxide, despite having higher predicted forced expiratory volume in 1 second, compared with men.
“Historically, COPD wasn’t a disease that was so prevalent in women. It’s been in the past 20 years that the trends have changed,” said Patricia Silveyra, MSc, PhD, ATSF, associate professor of environmental and occupational health at Indiana University, Bloomington.
The increasing prevalence of COPD among women cannot be explained by smoking alone, she said in an interview.
“It used to be thought that it was because more women smoked, but actually a lot of women who don’t smoke can develop COPD, so it appears to be probably something environmental, but because it used to be a disease of older men, in the clinic there was also a bias to diagnose men with COPD, and women with asthma, so a lot of women went underdiagnosed,” Dr. Silveyra said.
In their review, Dr. Sodhi and colleagues noted that women with COPD “may be underdiagnosed as a result of having different symptoms from those classically recognized. Reasons for underdiagnosis or a delay in diagnosis may also be due to lack of a formal evaluation with spirometry, women seeking care later in the course of disease, physician bias, or associated fatigue or depression misdirecting diagnostic strategies. Underdiagnosis may be associated with psychological distress and worse health-related quality of life.”
Although the evidence is mixed, women tend to present more frequently with the chronic bronchitis phenotype of COPD, compared with the emphysema phenotype, and women tend to have greater degrees of pulmonary function impairment when exposed to tobacco smoke, even after controlling for differences in height and weight.
“For the same amount of exposure to tobacco smoke, females are likely to develop more severe airflow limitation at an earlier age than males, and have more exacerbation,” Dr. Sodhi and colleagues wrote.
Both Dr. Silveyra and Dr. Sodhi said that reason why men and women differ in their physiological reactions to smoke are still unknown.
Sex differences in drug responses
There is only limited evidence to indicate that women and men respond differently to various therapeutic agents, but what is clear is that more research into this area is needed, Dr. Sodhi and Dr. Silveyra said.
For example, among the few studies that have documented sex differences, one showed no sex differences in the efficacy of salmeterol/fluticasone combination therapy for reducing exacerbations or improving quality of life, whereas another showed that women were more likely than men to experience COPD symptoms or exacerbations after stopping inhaled corticosteroids, Dr. Sodhi and colleagues noted.
Both Dr. Sodhi and Dr. Silveyra emphasized the need for clinical trials that study the effects of sex on treatment outcomes in COPD, which could lead to better, more personalized therapeutic regimens that take sex and gender into account.
Dr. Sodhi and colleagues offered the following advice to clinicians: “Interaction with female patients should take into account that their symptoms may not conform to traditionally accepted presentations. Challenges exist for female patients at all levels of health care interaction and as clinicians we need to acknowledge the bias and willfully work toward recognition and elimination of unconscious and conscious bias. Empowering our patients to have frank discussions with their health care team when they perceive bias is another step to help promote equity.”
The review by Dr. Sodhi and colleagues was supported by grants from the National Institutes of Health. Dr. Sodhi and Dr. Silveyra reported having no conflicts of interest to disclose.
When Sigmund Freud claimed that “anatomy is destiny” he was referring to anatomical sex as a determinant of personality traits. Expert consensus statements have previously offered some recommendations for managing these syndromes, but clinical data are scarce, so the present review “is intended to establish a starting point for future research,”
That notion has been widely discredited, but Freud appears to be inadvertently right in one respect: When it comes to chronic obstructive pulmonary disease (COPD), anatomy really is destiny, and sex may be as well, pulmonary researchers say.
There is a growing body of evidence to indicate that COPD affects men and women differently, and that men and women patients with COPD require different clinical management. Yet women are often underdiagnosed or misdiagnosed, partly because of poorly understood sex differences, but also because of cultural biases.
But plunging any farther into the weeds, it’s important to define terms. Although various investigators have used the terms “sex” and “gender” interchangeably, sex is the preferred term when referring to biological attributes of individual patients, while gender refers to personal identity.
These distinctions are important, contended Amik Sodhi, MBBS, MPH, from the division of allergy, pulmonology, and critical care medicine at the University of Wisconsin–Madison.
“Sex is essentially a biologic construct, so it’s got to do with the sex chromosomes, the genetics of that person, and it refers to the anatomic variations that can change susceptibility to different diseases,” she said in an interview.
An example of sex differences or “sexual dimorphism” can be found in a recent meta-analysis of sex-based genetic associations by Megan Hardin, MD, MPH from Brigham & Women’s Hospital in Boston and colleagues.
They reported that CELSR1, a gene involved in fetal lung development, was expressed more among women than among men and that a single nucleotide polymorphism in the gene was associated with COPD among women smokers, but not among men smokers.
The finding points to a potential risk locus for COPD in women, and could help shed light on sexual dimorphism in COPD, Dr. Hardin and colleagues said.
In contrast to sex, “gender is more of a psychosocial construct which can impact how diseases manifest themselves, how they are potentially managed, and what outcomes might occur for that particular disease,” Dr. Sodhi said.
She and her colleagues recently published a review of sex and gender in common lung disorders and sleep in the journal CHEST, where they wrote that the “influence of sex and gender is portrayed in epidemiological data, disease pathogenesis and pathophysiology, clinical manifestations, response to treatment, access to care, and health outcomes. Hence, sex and gender should be considered in all types of research, clinical practice and educational curricula.”
For example, as previously reported at the 2021 annual meeting of the American Thoracic Society, sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with COPD may point to different criteria for diagnosing cardiac comorbidities in women and men.
Those conclusions came from a retrospective analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease.
The investigators looked at the patients’ clinical history, comorbidities, lung function, COPD Assessment Test scores, and modified Medical Research Council (mMRC) dyspnea score, and found significant differences between men and women for most functional parameters and comorbidities, and for CAT items of cough, phlegm, and energy.
In logistic regression analysis, predictors for cardiac disease in men were energy, mMRC score, smoking status, body mass index, age, and spirometric lung function, but in women only age was significantly predictive for cardiac disease.
An example of gender effects on COPD differences in men and women is the increase in cigarette advertising aimed at women in the 1960s and the advent of women-targeted brands such as Virginia Slims, which in turn lead to increased smoking rates among women. In addition, in the developing world, where the sex/gender gap in COPD is narrowing, women tend to have greater exposure to wood smoke and cooking fuels in unventilated or poorly ventilated spaces, compared with men.
Increasing incidence among women
According to the Centers for Disease Control and Prevention, chronic lower respiratory diseases, primarily COPD, were the fourth-leading cause of death in women in the United States in 2018, following only heart disease, cancer, and accidents/injuries.
And as a CDC analysis of data from the 2013 Behavioral Risk Factor Surveillance System showed, women were more likely to report being told by a physician that they had COPD than did men (6.6%, compared with 5.4%).
Dr. Sodhi and colleagues noted that, at all time points examined from 2005 to 2014, women had a higher proportion than men of COPD hospitalizations and in-hospital deaths. They also noted that female sex is associated with a threefold risk for severe early-onset COPD, and that women with COPD have lower diffusion capacity of lungs for carbon monoxide, despite having higher predicted forced expiratory volume in 1 second, compared with men.
“Historically, COPD wasn’t a disease that was so prevalent in women. It’s been in the past 20 years that the trends have changed,” said Patricia Silveyra, MSc, PhD, ATSF, associate professor of environmental and occupational health at Indiana University, Bloomington.
The increasing prevalence of COPD among women cannot be explained by smoking alone, she said in an interview.
“It used to be thought that it was because more women smoked, but actually a lot of women who don’t smoke can develop COPD, so it appears to be probably something environmental, but because it used to be a disease of older men, in the clinic there was also a bias to diagnose men with COPD, and women with asthma, so a lot of women went underdiagnosed,” Dr. Silveyra said.
In their review, Dr. Sodhi and colleagues noted that women with COPD “may be underdiagnosed as a result of having different symptoms from those classically recognized. Reasons for underdiagnosis or a delay in diagnosis may also be due to lack of a formal evaluation with spirometry, women seeking care later in the course of disease, physician bias, or associated fatigue or depression misdirecting diagnostic strategies. Underdiagnosis may be associated with psychological distress and worse health-related quality of life.”
Although the evidence is mixed, women tend to present more frequently with the chronic bronchitis phenotype of COPD, compared with the emphysema phenotype, and women tend to have greater degrees of pulmonary function impairment when exposed to tobacco smoke, even after controlling for differences in height and weight.
“For the same amount of exposure to tobacco smoke, females are likely to develop more severe airflow limitation at an earlier age than males, and have more exacerbation,” Dr. Sodhi and colleagues wrote.
Both Dr. Silveyra and Dr. Sodhi said that reason why men and women differ in their physiological reactions to smoke are still unknown.
Sex differences in drug responses
There is only limited evidence to indicate that women and men respond differently to various therapeutic agents, but what is clear is that more research into this area is needed, Dr. Sodhi and Dr. Silveyra said.
For example, among the few studies that have documented sex differences, one showed no sex differences in the efficacy of salmeterol/fluticasone combination therapy for reducing exacerbations or improving quality of life, whereas another showed that women were more likely than men to experience COPD symptoms or exacerbations after stopping inhaled corticosteroids, Dr. Sodhi and colleagues noted.
Both Dr. Sodhi and Dr. Silveyra emphasized the need for clinical trials that study the effects of sex on treatment outcomes in COPD, which could lead to better, more personalized therapeutic regimens that take sex and gender into account.
Dr. Sodhi and colleagues offered the following advice to clinicians: “Interaction with female patients should take into account that their symptoms may not conform to traditionally accepted presentations. Challenges exist for female patients at all levels of health care interaction and as clinicians we need to acknowledge the bias and willfully work toward recognition and elimination of unconscious and conscious bias. Empowering our patients to have frank discussions with their health care team when they perceive bias is another step to help promote equity.”
The review by Dr. Sodhi and colleagues was supported by grants from the National Institutes of Health. Dr. Sodhi and Dr. Silveyra reported having no conflicts of interest to disclose.
Children and COVID: Cases drop again, admission rate up slightly
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.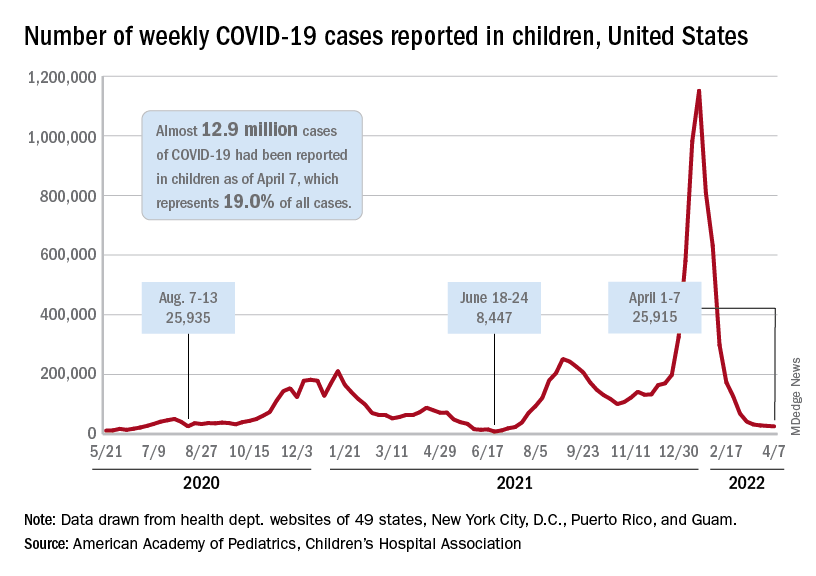
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
Can gram stains guide antibiotics for pneumonia in critical care?
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Belmont Stakes to support initiatives focused on improving the patient experience
There is a variety of ways to support the many impactful new programs the CHEST Foundation will launch in 2022, but one of the most anticipated options is the annual Belmont Stakes Dinner and Auction on June 11 in New York City. This fun-filled evening will include a viewing of the 154th running of “The Championship Track,” a cocktail reception and plated dinner, a silent auction, a rooftop party, and much more.
This year, the dinner and auction will support the CHEST Foundation’s work in patient education and CHEST initiatives to improve patient care. Two areas of focus are disparities in care delivery and improving patients’ quality of life through partnerships designed to encourage earlier diagnosis and treatment.
With these goals in mind, new initiatives include an extension of the 2020 Foundation Listening Tour designed to help clinicians increase trust, equity, and access to health care for patients in traditionally marginalized communities.
In addition, CHEST is partnering with the Three Lakes Foundation on a program dedicated to shortening the time to diagnosis for pulmonary fibrosis (PF). This initiative will bring together pulmonologists and primary care physicians to develop a strategy for identifying PF more quickly, ensuring treatment can begin earlier in the disease trajectory. Early detection of PF is associated with better quality of life for patients, so improving clinicians’ understanding of the signs and symptoms of this rare disease and formulating better guidance for diagnosing it could result in drastic improvements for those living with PF.
To highlight the importance of these efforts, the evening also will include speeches from two patient advocates who have turned their own experiences with living with chronic lung disease into incredible action on behalf of patients.
To learn more about the CHEST Foundation’s initiatives in 2022 and how you can attend the Belmont Stakes Dinner and Auction to support these efforts, visit foundation.chestnet.org.
There is a variety of ways to support the many impactful new programs the CHEST Foundation will launch in 2022, but one of the most anticipated options is the annual Belmont Stakes Dinner and Auction on June 11 in New York City. This fun-filled evening will include a viewing of the 154th running of “The Championship Track,” a cocktail reception and plated dinner, a silent auction, a rooftop party, and much more.
This year, the dinner and auction will support the CHEST Foundation’s work in patient education and CHEST initiatives to improve patient care. Two areas of focus are disparities in care delivery and improving patients’ quality of life through partnerships designed to encourage earlier diagnosis and treatment.
With these goals in mind, new initiatives include an extension of the 2020 Foundation Listening Tour designed to help clinicians increase trust, equity, and access to health care for patients in traditionally marginalized communities.
In addition, CHEST is partnering with the Three Lakes Foundation on a program dedicated to shortening the time to diagnosis for pulmonary fibrosis (PF). This initiative will bring together pulmonologists and primary care physicians to develop a strategy for identifying PF more quickly, ensuring treatment can begin earlier in the disease trajectory. Early detection of PF is associated with better quality of life for patients, so improving clinicians’ understanding of the signs and symptoms of this rare disease and formulating better guidance for diagnosing it could result in drastic improvements for those living with PF.
To highlight the importance of these efforts, the evening also will include speeches from two patient advocates who have turned their own experiences with living with chronic lung disease into incredible action on behalf of patients.
To learn more about the CHEST Foundation’s initiatives in 2022 and how you can attend the Belmont Stakes Dinner and Auction to support these efforts, visit foundation.chestnet.org.
There is a variety of ways to support the many impactful new programs the CHEST Foundation will launch in 2022, but one of the most anticipated options is the annual Belmont Stakes Dinner and Auction on June 11 in New York City. This fun-filled evening will include a viewing of the 154th running of “The Championship Track,” a cocktail reception and plated dinner, a silent auction, a rooftop party, and much more.
This year, the dinner and auction will support the CHEST Foundation’s work in patient education and CHEST initiatives to improve patient care. Two areas of focus are disparities in care delivery and improving patients’ quality of life through partnerships designed to encourage earlier diagnosis and treatment.
With these goals in mind, new initiatives include an extension of the 2020 Foundation Listening Tour designed to help clinicians increase trust, equity, and access to health care for patients in traditionally marginalized communities.
In addition, CHEST is partnering with the Three Lakes Foundation on a program dedicated to shortening the time to diagnosis for pulmonary fibrosis (PF). This initiative will bring together pulmonologists and primary care physicians to develop a strategy for identifying PF more quickly, ensuring treatment can begin earlier in the disease trajectory. Early detection of PF is associated with better quality of life for patients, so improving clinicians’ understanding of the signs and symptoms of this rare disease and formulating better guidance for diagnosing it could result in drastic improvements for those living with PF.
To highlight the importance of these efforts, the evening also will include speeches from two patient advocates who have turned their own experiences with living with chronic lung disease into incredible action on behalf of patients.
To learn more about the CHEST Foundation’s initiatives in 2022 and how you can attend the Belmont Stakes Dinner and Auction to support these efforts, visit foundation.chestnet.org.
What COVID-19 taught us: The challenge of maintaining contingency level care to proactively forestall crisis care
In 2014, the Task Force for Mass Critical Care (TFMCC) published a CHEST consensus statement on disaster preparedness principles in caring for the critically ill during disasters and pandemics (Christian et al. CHEST. 2014;146[4_suppl]:8s-34s). This publication attempted to guide preparedness for both single-event disasters and more prolonged events, including a feared influenza pandemic.
Despite the foundation of planning and support this guidance provided, the COVID-19 pandemic response revealed substantial gaps in our understanding and preparedness for these more prolonged and widespread events.
In New York City, as the first COVID-19 wave began in March and April of 2020, area hospitals responded with surge plans that prioritized what was felt to be most important (Griffin et al. Am J Respir Crit Care Med. 2020 Jun 1;201[11]:1337-44). Tiered, creative staffing structures were rapidly created with intensivists supervising non-ICU physicians and APPs. Procedure teams were created for intubation, proning, and central line placement. ICU space was created with adaptations to ORs and PACUs, and rooms on med-surg floors and step-down units underwent emergency renovations to allow creation of new “pop-up” ICUs. Triage protocols were altered: patients on high levels of supplemental oxygen, who would under normal circumstances have been admitted to an ICU, were triaged to floors and stepdown units. Equipment was reused, modified, and substituted creatively to optimize care for the maximum number of patients.
In the face of all of these struggles, many around the country and the world felt the efforts, though heroic, resulted in less than standard of care. Two subsequent publications validated this concern (Kadri et al. Ann Int Med. 2021,174;9:1240-51; Bravata DM et al. JAMA Open Network. 2021;4[1]:e2034266), demonstrating during severe surge, COVID-19 patients’ mortality increased significantly beyond that seen in non-surging or less-severe surging times, demonstrating a mortality effect of surge itself. Though these studies observed COVID-19 patients only, there is every reason to believe the findings applied to all critically ill patients cared for during these surges.
These experiences led the TFMCC to report updated strategies for remaining in contingency care levels and avoiding crisis care (Dichter JR et al. CHEST. 2022;161[2]:429-47). Contingency is equivalent to routine care though may require adaptations and employment of otherwise non-traditional resources. The ultimate goal of mass critical care in a public health emergency is to avoid crisis-operating conditions, crisis standards of care, and their associated challenging triage decisions regarding allocation of scarce resources.
The 10 suggestions included in the most recent TFMCC publication include staffing strategies and suggestions based on COVID-19 experiences for graded staff-to-patient ratios, and support processes to preserve the existing health care work force. Strategies also include reduction of redundant documentation, limiting overtime, and most importantly, approaches for improving teamwork and supporting psychological well-being and resilience. Examples include daily unit huddles to update care and share experiences, genuine intra-team recognition and appreciation, and embedding emotional health experts within teams to provide ongoing support.
Consistent communication between incident command and frontline clinicians was also a suggested priority, perhaps with a newly proposed position of physician clinical support supervisor. This would be a formal role within hospital incident command, a liaison between the two groups.
Surge strategies should include empowerment of bedside clinicians and leaders with both planning and real-time assessment of the clinical situation, as being at the front line of care enables the situational awareness to assess ICU strain most effectively. Further, ICU clinicians must recognize when progression deeper into contingency operations occurs and they become perilously close to crisis mode. At this point, decisions are made and scarce resources are modified beyond routine standards of care to preserve life. TFMCC designates this gray area between contingency and crisis as the Critical Clinical Prioritization level (Figure).
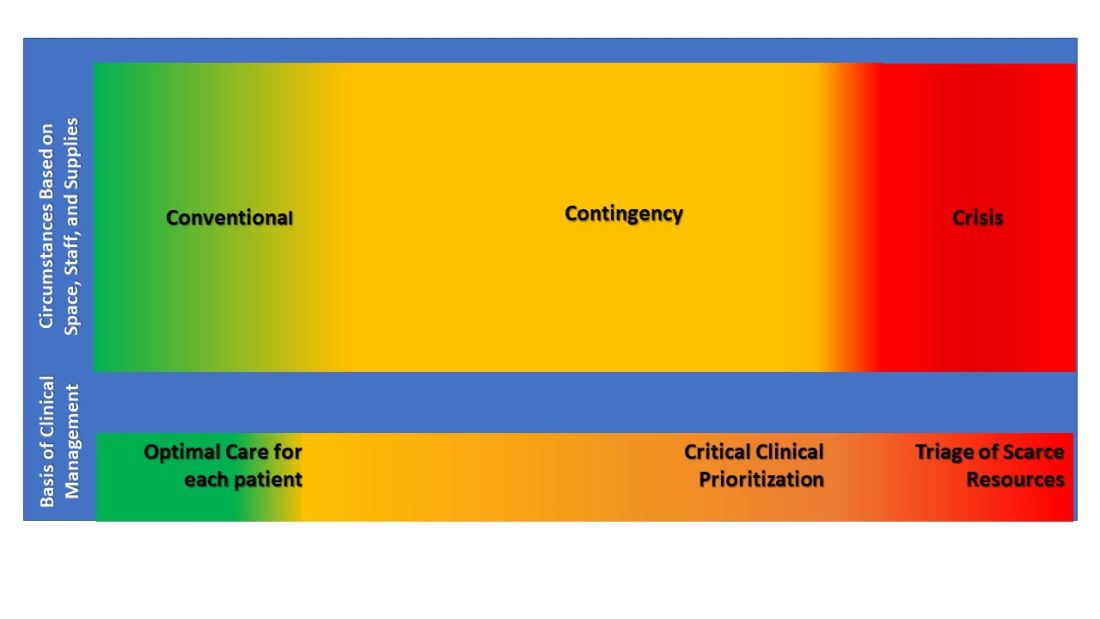
At this point, more resources must be provided, or patients must be transferred to other resourced hospitals.
Critical Clinical Prioritization is an illustration of necessity being the mother of invention, as these are adaptations clinicians devised under duress. Some particularly poignant examples are the spreading of 24 hours of continuous renal replacement therapy (CRRT) resource between two and sometimes three patients to provide life sustainment to all; and when ventilators were in short supply, determining which patients required full ICU ventilator support vs those who could manage with lower functioning ventilators, and trading them between patients when demands changed.
These adaptations can only be done by experienced clinicians proactively managing bedside critical care under duress, further underscoring the importance of our suggestion that Critical Clinical Prioritization and ICU strain be managed by bedside clinicians and leaders.
The response of early transfer of patients – load-balancing - should be considered as soon as any hospital enters contingency conditions. This strategy is commonly implemented within larger health systems, ideally before reaching Critical Clinical Prioritization. Formal, organized state or regional load-balancing coordination, now referred to as medical operations command centers (MOCCs), were highly effective and proved lifesaving for those states that implemented them (including Arizona, Washington, California, Minnesota, and others). Support for establishment of MOCC’s is crucial in prolonging contingency operations and further helps support and protect disadvantaged populations (White et al. N Engl J Med. 2021;385[24]:2211-4).
Establishment of MOCCs has met resistance due to challenges that include interhospital/intersystem competition, logistics of moving critically ill patients sometimes across significant physical distance, and the costs of assuming care of uninsured or underinsured patients. Nevertheless, the benefits to the population as a whole necessitate working through these obstacles as successful MOCCs have done, usually with government and hospital association support.
In their final suggestion of the 2022 updated strategies, TFMCC suggests that hospitals use telemedicine technology both to expand specialists’ ability to provide care and facilitate families virtually visiting their critically ill loved one when safety precludes in-person visits.
These suggestions are pivotal in planning for future public health emergencies that include mass critical care, even during events that are limited in scope and duration.
Lastly, intensivists struggled with legal and ethical concerns when mired in crisis care circumstances and decisions of allocation, and potential reallocation, of scarce resources. These issues were not well addressed during the COVID-19 pandemic, further emphasizing the importance of maintaining contingency level care and requiring further involvement from legal and medical ethics professionals for future planning.
The guiding principle of disaster preparedness is that we must do all the planning we can to ensure that we never need crisis standards of care (National Academies of Sciences, Engineering, and Medicine. 2020 Mar 28. Rapid Expert Consultation on Crisis Standards of Care for the COVID-19 Pandemic. Washington, DC: The National Academies Press.).
We must be prepared. Guidelines and suggestions laid out through decades of experience gained a real-world test in the COVID-19 pandemic. Now we must all reorganize and create new plans or augment old ones with the information we have gained. The time is now. The work must continue.
Dr. Griffin is Assistant Professor of Medicine, New York Presbyterian Hospital – Weill Cornell Medicine. Dr. Dichter is Associate Professor of Medicine, University of Minnesota.
In 2014, the Task Force for Mass Critical Care (TFMCC) published a CHEST consensus statement on disaster preparedness principles in caring for the critically ill during disasters and pandemics (Christian et al. CHEST. 2014;146[4_suppl]:8s-34s). This publication attempted to guide preparedness for both single-event disasters and more prolonged events, including a feared influenza pandemic.
Despite the foundation of planning and support this guidance provided, the COVID-19 pandemic response revealed substantial gaps in our understanding and preparedness for these more prolonged and widespread events.
In New York City, as the first COVID-19 wave began in March and April of 2020, area hospitals responded with surge plans that prioritized what was felt to be most important (Griffin et al. Am J Respir Crit Care Med. 2020 Jun 1;201[11]:1337-44). Tiered, creative staffing structures were rapidly created with intensivists supervising non-ICU physicians and APPs. Procedure teams were created for intubation, proning, and central line placement. ICU space was created with adaptations to ORs and PACUs, and rooms on med-surg floors and step-down units underwent emergency renovations to allow creation of new “pop-up” ICUs. Triage protocols were altered: patients on high levels of supplemental oxygen, who would under normal circumstances have been admitted to an ICU, were triaged to floors and stepdown units. Equipment was reused, modified, and substituted creatively to optimize care for the maximum number of patients.
In the face of all of these struggles, many around the country and the world felt the efforts, though heroic, resulted in less than standard of care. Two subsequent publications validated this concern (Kadri et al. Ann Int Med. 2021,174;9:1240-51; Bravata DM et al. JAMA Open Network. 2021;4[1]:e2034266), demonstrating during severe surge, COVID-19 patients’ mortality increased significantly beyond that seen in non-surging or less-severe surging times, demonstrating a mortality effect of surge itself. Though these studies observed COVID-19 patients only, there is every reason to believe the findings applied to all critically ill patients cared for during these surges.
These experiences led the TFMCC to report updated strategies for remaining in contingency care levels and avoiding crisis care (Dichter JR et al. CHEST. 2022;161[2]:429-47). Contingency is equivalent to routine care though may require adaptations and employment of otherwise non-traditional resources. The ultimate goal of mass critical care in a public health emergency is to avoid crisis-operating conditions, crisis standards of care, and their associated challenging triage decisions regarding allocation of scarce resources.
The 10 suggestions included in the most recent TFMCC publication include staffing strategies and suggestions based on COVID-19 experiences for graded staff-to-patient ratios, and support processes to preserve the existing health care work force. Strategies also include reduction of redundant documentation, limiting overtime, and most importantly, approaches for improving teamwork and supporting psychological well-being and resilience. Examples include daily unit huddles to update care and share experiences, genuine intra-team recognition and appreciation, and embedding emotional health experts within teams to provide ongoing support.
Consistent communication between incident command and frontline clinicians was also a suggested priority, perhaps with a newly proposed position of physician clinical support supervisor. This would be a formal role within hospital incident command, a liaison between the two groups.
Surge strategies should include empowerment of bedside clinicians and leaders with both planning and real-time assessment of the clinical situation, as being at the front line of care enables the situational awareness to assess ICU strain most effectively. Further, ICU clinicians must recognize when progression deeper into contingency operations occurs and they become perilously close to crisis mode. At this point, decisions are made and scarce resources are modified beyond routine standards of care to preserve life. TFMCC designates this gray area between contingency and crisis as the Critical Clinical Prioritization level (Figure).

At this point, more resources must be provided, or patients must be transferred to other resourced hospitals.
Critical Clinical Prioritization is an illustration of necessity being the mother of invention, as these are adaptations clinicians devised under duress. Some particularly poignant examples are the spreading of 24 hours of continuous renal replacement therapy (CRRT) resource between two and sometimes three patients to provide life sustainment to all; and when ventilators were in short supply, determining which patients required full ICU ventilator support vs those who could manage with lower functioning ventilators, and trading them between patients when demands changed.
These adaptations can only be done by experienced clinicians proactively managing bedside critical care under duress, further underscoring the importance of our suggestion that Critical Clinical Prioritization and ICU strain be managed by bedside clinicians and leaders.
The response of early transfer of patients – load-balancing - should be considered as soon as any hospital enters contingency conditions. This strategy is commonly implemented within larger health systems, ideally before reaching Critical Clinical Prioritization. Formal, organized state or regional load-balancing coordination, now referred to as medical operations command centers (MOCCs), were highly effective and proved lifesaving for those states that implemented them (including Arizona, Washington, California, Minnesota, and others). Support for establishment of MOCC’s is crucial in prolonging contingency operations and further helps support and protect disadvantaged populations (White et al. N Engl J Med. 2021;385[24]:2211-4).
Establishment of MOCCs has met resistance due to challenges that include interhospital/intersystem competition, logistics of moving critically ill patients sometimes across significant physical distance, and the costs of assuming care of uninsured or underinsured patients. Nevertheless, the benefits to the population as a whole necessitate working through these obstacles as successful MOCCs have done, usually with government and hospital association support.
In their final suggestion of the 2022 updated strategies, TFMCC suggests that hospitals use telemedicine technology both to expand specialists’ ability to provide care and facilitate families virtually visiting their critically ill loved one when safety precludes in-person visits.
These suggestions are pivotal in planning for future public health emergencies that include mass critical care, even during events that are limited in scope and duration.
Lastly, intensivists struggled with legal and ethical concerns when mired in crisis care circumstances and decisions of allocation, and potential reallocation, of scarce resources. These issues were not well addressed during the COVID-19 pandemic, further emphasizing the importance of maintaining contingency level care and requiring further involvement from legal and medical ethics professionals for future planning.
The guiding principle of disaster preparedness is that we must do all the planning we can to ensure that we never need crisis standards of care (National Academies of Sciences, Engineering, and Medicine. 2020 Mar 28. Rapid Expert Consultation on Crisis Standards of Care for the COVID-19 Pandemic. Washington, DC: The National Academies Press.).
We must be prepared. Guidelines and suggestions laid out through decades of experience gained a real-world test in the COVID-19 pandemic. Now we must all reorganize and create new plans or augment old ones with the information we have gained. The time is now. The work must continue.
Dr. Griffin is Assistant Professor of Medicine, New York Presbyterian Hospital – Weill Cornell Medicine. Dr. Dichter is Associate Professor of Medicine, University of Minnesota.
In 2014, the Task Force for Mass Critical Care (TFMCC) published a CHEST consensus statement on disaster preparedness principles in caring for the critically ill during disasters and pandemics (Christian et al. CHEST. 2014;146[4_suppl]:8s-34s). This publication attempted to guide preparedness for both single-event disasters and more prolonged events, including a feared influenza pandemic.
Despite the foundation of planning and support this guidance provided, the COVID-19 pandemic response revealed substantial gaps in our understanding and preparedness for these more prolonged and widespread events.
In New York City, as the first COVID-19 wave began in March and April of 2020, area hospitals responded with surge plans that prioritized what was felt to be most important (Griffin et al. Am J Respir Crit Care Med. 2020 Jun 1;201[11]:1337-44). Tiered, creative staffing structures were rapidly created with intensivists supervising non-ICU physicians and APPs. Procedure teams were created for intubation, proning, and central line placement. ICU space was created with adaptations to ORs and PACUs, and rooms on med-surg floors and step-down units underwent emergency renovations to allow creation of new “pop-up” ICUs. Triage protocols were altered: patients on high levels of supplemental oxygen, who would under normal circumstances have been admitted to an ICU, were triaged to floors and stepdown units. Equipment was reused, modified, and substituted creatively to optimize care for the maximum number of patients.
In the face of all of these struggles, many around the country and the world felt the efforts, though heroic, resulted in less than standard of care. Two subsequent publications validated this concern (Kadri et al. Ann Int Med. 2021,174;9:1240-51; Bravata DM et al. JAMA Open Network. 2021;4[1]:e2034266), demonstrating during severe surge, COVID-19 patients’ mortality increased significantly beyond that seen in non-surging or less-severe surging times, demonstrating a mortality effect of surge itself. Though these studies observed COVID-19 patients only, there is every reason to believe the findings applied to all critically ill patients cared for during these surges.
These experiences led the TFMCC to report updated strategies for remaining in contingency care levels and avoiding crisis care (Dichter JR et al. CHEST. 2022;161[2]:429-47). Contingency is equivalent to routine care though may require adaptations and employment of otherwise non-traditional resources. The ultimate goal of mass critical care in a public health emergency is to avoid crisis-operating conditions, crisis standards of care, and their associated challenging triage decisions regarding allocation of scarce resources.
The 10 suggestions included in the most recent TFMCC publication include staffing strategies and suggestions based on COVID-19 experiences for graded staff-to-patient ratios, and support processes to preserve the existing health care work force. Strategies also include reduction of redundant documentation, limiting overtime, and most importantly, approaches for improving teamwork and supporting psychological well-being and resilience. Examples include daily unit huddles to update care and share experiences, genuine intra-team recognition and appreciation, and embedding emotional health experts within teams to provide ongoing support.
Consistent communication between incident command and frontline clinicians was also a suggested priority, perhaps with a newly proposed position of physician clinical support supervisor. This would be a formal role within hospital incident command, a liaison between the two groups.
Surge strategies should include empowerment of bedside clinicians and leaders with both planning and real-time assessment of the clinical situation, as being at the front line of care enables the situational awareness to assess ICU strain most effectively. Further, ICU clinicians must recognize when progression deeper into contingency operations occurs and they become perilously close to crisis mode. At this point, decisions are made and scarce resources are modified beyond routine standards of care to preserve life. TFMCC designates this gray area between contingency and crisis as the Critical Clinical Prioritization level (Figure).

At this point, more resources must be provided, or patients must be transferred to other resourced hospitals.
Critical Clinical Prioritization is an illustration of necessity being the mother of invention, as these are adaptations clinicians devised under duress. Some particularly poignant examples are the spreading of 24 hours of continuous renal replacement therapy (CRRT) resource between two and sometimes three patients to provide life sustainment to all; and when ventilators were in short supply, determining which patients required full ICU ventilator support vs those who could manage with lower functioning ventilators, and trading them between patients when demands changed.
These adaptations can only be done by experienced clinicians proactively managing bedside critical care under duress, further underscoring the importance of our suggestion that Critical Clinical Prioritization and ICU strain be managed by bedside clinicians and leaders.
The response of early transfer of patients – load-balancing - should be considered as soon as any hospital enters contingency conditions. This strategy is commonly implemented within larger health systems, ideally before reaching Critical Clinical Prioritization. Formal, organized state or regional load-balancing coordination, now referred to as medical operations command centers (MOCCs), were highly effective and proved lifesaving for those states that implemented them (including Arizona, Washington, California, Minnesota, and others). Support for establishment of MOCC’s is crucial in prolonging contingency operations and further helps support and protect disadvantaged populations (White et al. N Engl J Med. 2021;385[24]:2211-4).
Establishment of MOCCs has met resistance due to challenges that include interhospital/intersystem competition, logistics of moving critically ill patients sometimes across significant physical distance, and the costs of assuming care of uninsured or underinsured patients. Nevertheless, the benefits to the population as a whole necessitate working through these obstacles as successful MOCCs have done, usually with government and hospital association support.
In their final suggestion of the 2022 updated strategies, TFMCC suggests that hospitals use telemedicine technology both to expand specialists’ ability to provide care and facilitate families virtually visiting their critically ill loved one when safety precludes in-person visits.
These suggestions are pivotal in planning for future public health emergencies that include mass critical care, even during events that are limited in scope and duration.
Lastly, intensivists struggled with legal and ethical concerns when mired in crisis care circumstances and decisions of allocation, and potential reallocation, of scarce resources. These issues were not well addressed during the COVID-19 pandemic, further emphasizing the importance of maintaining contingency level care and requiring further involvement from legal and medical ethics professionals for future planning.
The guiding principle of disaster preparedness is that we must do all the planning we can to ensure that we never need crisis standards of care (National Academies of Sciences, Engineering, and Medicine. 2020 Mar 28. Rapid Expert Consultation on Crisis Standards of Care for the COVID-19 Pandemic. Washington, DC: The National Academies Press.).
We must be prepared. Guidelines and suggestions laid out through decades of experience gained a real-world test in the COVID-19 pandemic. Now we must all reorganize and create new plans or augment old ones with the information we have gained. The time is now. The work must continue.
Dr. Griffin is Assistant Professor of Medicine, New York Presbyterian Hospital – Weill Cornell Medicine. Dr. Dichter is Associate Professor of Medicine, University of Minnesota.
Building CHEST 2022: A look into the Scientific Program Committee Meeting
A quality educational meeting starts with a great slate of programs tailored to its audience, and CHEST 2022 is on-track to offer the highest tier of education for those in pulmonary, critical care, and sleep medicine.
Although planning for the meeting started after CHEST 2021 wrapped up, the real magic started to happen a few months ago when the schedule began coming together. In mid-February, members of the Scientific Planning Committee gathered both virtually and in-person at the CHEST headquarters to solidify the schedule for the upcoming CHEST 2022 meeting taking place in Nashville, TN, October 16-19.
The excitement in the room was palpable as committee members gathered for the first time in over a year to plan what will be the first in-person meeting since CHEST 2019 in New Orleans.
Chair of CHEST 2022, Subani Chandra, MD, FCCP, has high expectations for the meeting and is excited for everyone to be together in Nashville. “There is something special about an in-person meeting and my goal for CHEST 2022 is to not only meet the academic needs of the attendees, but also to serve as a chance to recharge after a long haul in managing COVID-19,” says Dr. Chandra. “Many first-time CHEST attendees are fellows and, with the last two meetings being virtual, there are a lot of fellows who have yet to attend a meeting in-person, so that is a big responsibility for us and opportunity for them. We want to make sure they have a fun and productive meeting – learn from the best, understand how to apply the latest research, get to present their work, network, participate, and have fun doing it all!”
With something for everyone in chest medicine, the CHEST 2022 meeting will feature over 200 sessions covering eight curriculum groups:
- Obstructive lung disease
- Sleep
- Chest infections
- Cardiovascular/pulmonary vascular disease
- Pulmonary procedures/lung cancer/cardiothoracic surgery
- Interstitial lung disease/radiology
- Interdisciplinary/practice operations/education
- Critical care
Covering a large breadth of information, the sessions will include the latest trends in COVID-19 care – recommended protocols, surge-planning and best practices; deeper looks into the latest CHEST guidelines – thromboprophylaxis in patients with COVID-19, antithrombotic therapy for VTE disease, and the guidelines for lung cancer screening; and sessions speaking to diversity, inclusion, and equity within medicine, including how lung disease affects populations differently.
Dr. Chandra says diversity was top of mind throughout the planning process. When submitting session ideas, it was noted that “submissions with speakers representing one gender and/or one institution will not be considered,” and that “selection priority will be given to outstanding submissions with proposed speakers who represent diversity of race, ethnicity, and professional status.”
During February’s meeting, as the committee members confirmed each of the sessions, they took the time to ensure every single one had presenters from a variety of backgrounds, including diversity of gender, race, credentialing, and years of experience in medicine.
It was important to the committee that this not be a physician-only meeting, because both CHEST and Pulmonary/Critical Care Medicine feature an array of team members including physicians, advance practice providers, respiratory therapists, nurses and other members of the care team and the sessions will reflect that.
When asked what she hopes attendees will gain from CHEST 2022, Dr. Chandra says, “I want attendees to feel the joy that comes from not only being together, but learning together.”
She continued, “I want this meeting to remind clinicians why they fell in love with medicine and to remember why it is that we do what we do, especially after two grueling years. Attendees should leave feeling reinvigorated and charged with the latest literature and clinical expertise ready to be implemented into practice. Most of all, I want all of the attendees to have fun, because we are there to learn, but CHEST is also about enjoying medicine and those around you. I just cannot wait.”
A quality educational meeting starts with a great slate of programs tailored to its audience, and CHEST 2022 is on-track to offer the highest tier of education for those in pulmonary, critical care, and sleep medicine.
Although planning for the meeting started after CHEST 2021 wrapped up, the real magic started to happen a few months ago when the schedule began coming together. In mid-February, members of the Scientific Planning Committee gathered both virtually and in-person at the CHEST headquarters to solidify the schedule for the upcoming CHEST 2022 meeting taking place in Nashville, TN, October 16-19.
The excitement in the room was palpable as committee members gathered for the first time in over a year to plan what will be the first in-person meeting since CHEST 2019 in New Orleans.
Chair of CHEST 2022, Subani Chandra, MD, FCCP, has high expectations for the meeting and is excited for everyone to be together in Nashville. “There is something special about an in-person meeting and my goal for CHEST 2022 is to not only meet the academic needs of the attendees, but also to serve as a chance to recharge after a long haul in managing COVID-19,” says Dr. Chandra. “Many first-time CHEST attendees are fellows and, with the last two meetings being virtual, there are a lot of fellows who have yet to attend a meeting in-person, so that is a big responsibility for us and opportunity for them. We want to make sure they have a fun and productive meeting – learn from the best, understand how to apply the latest research, get to present their work, network, participate, and have fun doing it all!”
With something for everyone in chest medicine, the CHEST 2022 meeting will feature over 200 sessions covering eight curriculum groups:
- Obstructive lung disease
- Sleep
- Chest infections
- Cardiovascular/pulmonary vascular disease
- Pulmonary procedures/lung cancer/cardiothoracic surgery
- Interstitial lung disease/radiology
- Interdisciplinary/practice operations/education
- Critical care
Covering a large breadth of information, the sessions will include the latest trends in COVID-19 care – recommended protocols, surge-planning and best practices; deeper looks into the latest CHEST guidelines – thromboprophylaxis in patients with COVID-19, antithrombotic therapy for VTE disease, and the guidelines for lung cancer screening; and sessions speaking to diversity, inclusion, and equity within medicine, including how lung disease affects populations differently.
Dr. Chandra says diversity was top of mind throughout the planning process. When submitting session ideas, it was noted that “submissions with speakers representing one gender and/or one institution will not be considered,” and that “selection priority will be given to outstanding submissions with proposed speakers who represent diversity of race, ethnicity, and professional status.”
During February’s meeting, as the committee members confirmed each of the sessions, they took the time to ensure every single one had presenters from a variety of backgrounds, including diversity of gender, race, credentialing, and years of experience in medicine.
It was important to the committee that this not be a physician-only meeting, because both CHEST and Pulmonary/Critical Care Medicine feature an array of team members including physicians, advance practice providers, respiratory therapists, nurses and other members of the care team and the sessions will reflect that.
When asked what she hopes attendees will gain from CHEST 2022, Dr. Chandra says, “I want attendees to feel the joy that comes from not only being together, but learning together.”
She continued, “I want this meeting to remind clinicians why they fell in love with medicine and to remember why it is that we do what we do, especially after two grueling years. Attendees should leave feeling reinvigorated and charged with the latest literature and clinical expertise ready to be implemented into practice. Most of all, I want all of the attendees to have fun, because we are there to learn, but CHEST is also about enjoying medicine and those around you. I just cannot wait.”
A quality educational meeting starts with a great slate of programs tailored to its audience, and CHEST 2022 is on-track to offer the highest tier of education for those in pulmonary, critical care, and sleep medicine.
Although planning for the meeting started after CHEST 2021 wrapped up, the real magic started to happen a few months ago when the schedule began coming together. In mid-February, members of the Scientific Planning Committee gathered both virtually and in-person at the CHEST headquarters to solidify the schedule for the upcoming CHEST 2022 meeting taking place in Nashville, TN, October 16-19.
The excitement in the room was palpable as committee members gathered for the first time in over a year to plan what will be the first in-person meeting since CHEST 2019 in New Orleans.
Chair of CHEST 2022, Subani Chandra, MD, FCCP, has high expectations for the meeting and is excited for everyone to be together in Nashville. “There is something special about an in-person meeting and my goal for CHEST 2022 is to not only meet the academic needs of the attendees, but also to serve as a chance to recharge after a long haul in managing COVID-19,” says Dr. Chandra. “Many first-time CHEST attendees are fellows and, with the last two meetings being virtual, there are a lot of fellows who have yet to attend a meeting in-person, so that is a big responsibility for us and opportunity for them. We want to make sure they have a fun and productive meeting – learn from the best, understand how to apply the latest research, get to present their work, network, participate, and have fun doing it all!”
With something for everyone in chest medicine, the CHEST 2022 meeting will feature over 200 sessions covering eight curriculum groups:
- Obstructive lung disease
- Sleep
- Chest infections
- Cardiovascular/pulmonary vascular disease
- Pulmonary procedures/lung cancer/cardiothoracic surgery
- Interstitial lung disease/radiology
- Interdisciplinary/practice operations/education
- Critical care
Covering a large breadth of information, the sessions will include the latest trends in COVID-19 care – recommended protocols, surge-planning and best practices; deeper looks into the latest CHEST guidelines – thromboprophylaxis in patients with COVID-19, antithrombotic therapy for VTE disease, and the guidelines for lung cancer screening; and sessions speaking to diversity, inclusion, and equity within medicine, including how lung disease affects populations differently.
Dr. Chandra says diversity was top of mind throughout the planning process. When submitting session ideas, it was noted that “submissions with speakers representing one gender and/or one institution will not be considered,” and that “selection priority will be given to outstanding submissions with proposed speakers who represent diversity of race, ethnicity, and professional status.”
During February’s meeting, as the committee members confirmed each of the sessions, they took the time to ensure every single one had presenters from a variety of backgrounds, including diversity of gender, race, credentialing, and years of experience in medicine.
It was important to the committee that this not be a physician-only meeting, because both CHEST and Pulmonary/Critical Care Medicine feature an array of team members including physicians, advance practice providers, respiratory therapists, nurses and other members of the care team and the sessions will reflect that.
When asked what she hopes attendees will gain from CHEST 2022, Dr. Chandra says, “I want attendees to feel the joy that comes from not only being together, but learning together.”
She continued, “I want this meeting to remind clinicians why they fell in love with medicine and to remember why it is that we do what we do, especially after two grueling years. Attendees should leave feeling reinvigorated and charged with the latest literature and clinical expertise ready to be implemented into practice. Most of all, I want all of the attendees to have fun, because we are there to learn, but CHEST is also about enjoying medicine and those around you. I just cannot wait.”
Continuous remote patient monitoring
The SARS-CoV-2 pandemic required health care systems around the world to rapidly innovate and adapt to unprecedented operational and clinical strain. Many health care systems leveraged virtual care capabilities as an innovative approach to safely and efficiently manage patients while reducing staff exposure and medical resource constraints (Healthcare [Basel]. 2020 Nov;8[4]:517; JMIR Form Res. 2021 Jan; 5[1]:e23190). With Medicare insurance claims data demonstrating a 30% reduction of in-person health visits, telemedicine has become an essential means to fill the gaps in providing essential medical services (JAMA Intern Med. 2021 Mar;181[3]:388-91). A vast majority of virtual health care visits come via telephonic encounters, which have inherent limitations in the ability to monitor patients with complex or critical medical conditions (Front Public Health. 2020;8:410; N Engl J Med. 2020 Apr;382[18]:1679-81). Remote patient monitoring (RPM) has been established in multiple clinical models as an effective adjunct in telemedicine encounters in order to ensure treatment regimen adherence, make real-time treatment adjustments, and identify patients at risk for early decompensation.
Long-term RPM data has demonstrated cost reduction, reduced burden of in-office visits, expedited management of significant clinical events, and decreased all-cause mortality rates. Previously RPM was limited to the care of patients with chronic conditions, particularly cardiac patients with congestive heart failure and invasive devices, such as pacemakers or implantable cardioverter–defibrillators (JMIR Form Res. 2021 Jan;5[1]:e23190; Front Public Health. 2020; 8:410). In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) added RPM billing codes in 2019 and then included coverage of acute conditions in 2020 that permitted a more extensive role of RPM in telemedicine. This change in financial reimbursement led to a more aggressive expansion of RPM devices to assess physiologic parameters, such as weight, blood pressure, oxygen saturation, and blood glucose levels for clinicians to review.
Currently, RPM devices fall within a low-risk FDA category that do not require clinical trials for validation prior to being cleared for CMS billing in a fee-for-service reimbursement model (N Engl J Med. 2021 Apr;384[15]:1384-6). A shortage of evidence-based publications to guide clinicians in this new landscape creates challenges from underuse, misuse, or abuse of RPM tools. In order to maximize the clinical benefits of RPM, standardized processes and device specifications derived from up-to-date research need to be established in professional society guidelines.
Formalized RPM protocols should play a key role in overcoming the hesitancy of health institutions becoming early adopters of RPM technologies. Some significant challenges leading to reluctance of executing an RPM program were recently highlighted at the REPROGRAM international consortium of telemedicine. These concerns involved building a technological infrastructure, training clinical staff, ensuring remote connectivity with broadband Internet, and working with patients of various technologic literacy (Front Public Health. 2020;8:410). We attempted to address these challenges by using a COVID-19 remote patient monitoring (CRPM) strategy within our Military Health System (MHS). By using the well-established responsible, accountable, consulted, and informed (RACI) matrix process mapping tool, we created a standardized enrollment process of high-risk patients across eight military treatment facilities (MTFs). High risk patients included those with COVID-19 pneumonia and persistent hypoxemia, those recovering from acute exacerbations of congestive heart failure, those with cardiopulmonary instability associated with malignancy, and other conditions that required continuous monitoring outside of the hospital setting.
In our CRPM process, the hospital inpatient unit or ED refer high-risk patients to a primary designated provider at each MTF for enrollment prior to discharge. Enrolled patients are equipped with an FDA-approved home monitoring kit that contains an electronic tablet, a network hub that operates independently of and/or in conjunction with Wi-Fi, and an armband containing a coin-sized monitor. The system has the capability to pair with additional smart-enabled accessories, such as a blood pressure cuff, temperature patch, and digital spirometer. With continuous bio-physiologic and symptom-based monitoring, a team of teleworking critical-care nurses monitor patients continuously. In case of a decompensation necessitating a higher level of care, an emergency action plan (EAP) is activated to ensure patients urgently receive emergency medical services. Once released from the CRPM program, discharged patients use prepaid shipping boxes to facilitate contactless repackaging, sanitization, and pickup for redistribution of devices to the MTF.
Given the increased number of hospital admissions noted during the COVID-19 global pandemic, the CRPM program has allowed us to address overutilization of hospital beds. Furthermore, it has allowed us to address issues of screening and resource utilization as we consider patients for safe implementation of home monitoring. While data concerning the outcome of the CRPM program are pending, we are encouraged about the ability to provide high quality care in a remote setting. To that end, we have addressed technologic difficulties, communication between remote providers and patients in the home environment, and communication between health care providers in various settings, such as the ED, inpatient wards, and the outpatient clinic.
To be sure, there are many challenges in making sure that CRPM adequately addresses the needs of patients, who may have persistent perturbations in cardiopulmonary status, tremendous anxiety about the progress or deterioration in their health status, and lack of understanding about their medical condition. Furthermore, providers face the challenge of making clinical decisions sometimes without the advantage of in-person examinations. Sometimes decisions must be made with incomplete information or when the status of the patient does not follow presupposed algorithms. Nevertheless, like many issues during the COVID-19 pandemic, patients and providers have evolved, pivoted, and made necessary adjustments to address an unprecedented time in recent history.
Ultimately, we believe that a continuous remote patient monitoring program can be designed, implemented, and maintained across a multifacility health care system for safe, effective, and efficient health care delivery. Limitations in implementing such a program might include lack of adequate Internet services, lack of telephonic communication, inadequate home facilities, lack of adequate home support, and, perhaps, lack of available emergency services. However, if the conditions for home monitoring are optimized, CRPM holds the promise of reducing the burden on emergency and inpatient hospital services, particularly when those services are strained in circumstances such as the ongoing global pandemic due to COVID-19. With further study, standardization, and evolution, remote monitoring will likely become a more acceptable and necessary form of health care delivery in the future.
Dr. Salomon is an Internal Medicine Resident (PGY-2); Dr. Muller is an Internal Medicine Resident (PGY-2); Dr. Boster is a Pulmonary and Critical Care Fellow; Dr. Loudermilk is a Pulmonary and Critical Care Fellow; and Dr. Kemp is Pulmonary and Critical Care staff, San Antonio Military Medical Center, Fort Sam Houston, Texas.
The SARS-CoV-2 pandemic required health care systems around the world to rapidly innovate and adapt to unprecedented operational and clinical strain. Many health care systems leveraged virtual care capabilities as an innovative approach to safely and efficiently manage patients while reducing staff exposure and medical resource constraints (Healthcare [Basel]. 2020 Nov;8[4]:517; JMIR Form Res. 2021 Jan; 5[1]:e23190). With Medicare insurance claims data demonstrating a 30% reduction of in-person health visits, telemedicine has become an essential means to fill the gaps in providing essential medical services (JAMA Intern Med. 2021 Mar;181[3]:388-91). A vast majority of virtual health care visits come via telephonic encounters, which have inherent limitations in the ability to monitor patients with complex or critical medical conditions (Front Public Health. 2020;8:410; N Engl J Med. 2020 Apr;382[18]:1679-81). Remote patient monitoring (RPM) has been established in multiple clinical models as an effective adjunct in telemedicine encounters in order to ensure treatment regimen adherence, make real-time treatment adjustments, and identify patients at risk for early decompensation.
Long-term RPM data has demonstrated cost reduction, reduced burden of in-office visits, expedited management of significant clinical events, and decreased all-cause mortality rates. Previously RPM was limited to the care of patients with chronic conditions, particularly cardiac patients with congestive heart failure and invasive devices, such as pacemakers or implantable cardioverter–defibrillators (JMIR Form Res. 2021 Jan;5[1]:e23190; Front Public Health. 2020; 8:410). In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) added RPM billing codes in 2019 and then included coverage of acute conditions in 2020 that permitted a more extensive role of RPM in telemedicine. This change in financial reimbursement led to a more aggressive expansion of RPM devices to assess physiologic parameters, such as weight, blood pressure, oxygen saturation, and blood glucose levels for clinicians to review.
Currently, RPM devices fall within a low-risk FDA category that do not require clinical trials for validation prior to being cleared for CMS billing in a fee-for-service reimbursement model (N Engl J Med. 2021 Apr;384[15]:1384-6). A shortage of evidence-based publications to guide clinicians in this new landscape creates challenges from underuse, misuse, or abuse of RPM tools. In order to maximize the clinical benefits of RPM, standardized processes and device specifications derived from up-to-date research need to be established in professional society guidelines.
Formalized RPM protocols should play a key role in overcoming the hesitancy of health institutions becoming early adopters of RPM technologies. Some significant challenges leading to reluctance of executing an RPM program were recently highlighted at the REPROGRAM international consortium of telemedicine. These concerns involved building a technological infrastructure, training clinical staff, ensuring remote connectivity with broadband Internet, and working with patients of various technologic literacy (Front Public Health. 2020;8:410). We attempted to address these challenges by using a COVID-19 remote patient monitoring (CRPM) strategy within our Military Health System (MHS). By using the well-established responsible, accountable, consulted, and informed (RACI) matrix process mapping tool, we created a standardized enrollment process of high-risk patients across eight military treatment facilities (MTFs). High risk patients included those with COVID-19 pneumonia and persistent hypoxemia, those recovering from acute exacerbations of congestive heart failure, those with cardiopulmonary instability associated with malignancy, and other conditions that required continuous monitoring outside of the hospital setting.
In our CRPM process, the hospital inpatient unit or ED refer high-risk patients to a primary designated provider at each MTF for enrollment prior to discharge. Enrolled patients are equipped with an FDA-approved home monitoring kit that contains an electronic tablet, a network hub that operates independently of and/or in conjunction with Wi-Fi, and an armband containing a coin-sized monitor. The system has the capability to pair with additional smart-enabled accessories, such as a blood pressure cuff, temperature patch, and digital spirometer. With continuous bio-physiologic and symptom-based monitoring, a team of teleworking critical-care nurses monitor patients continuously. In case of a decompensation necessitating a higher level of care, an emergency action plan (EAP) is activated to ensure patients urgently receive emergency medical services. Once released from the CRPM program, discharged patients use prepaid shipping boxes to facilitate contactless repackaging, sanitization, and pickup for redistribution of devices to the MTF.
Given the increased number of hospital admissions noted during the COVID-19 global pandemic, the CRPM program has allowed us to address overutilization of hospital beds. Furthermore, it has allowed us to address issues of screening and resource utilization as we consider patients for safe implementation of home monitoring. While data concerning the outcome of the CRPM program are pending, we are encouraged about the ability to provide high quality care in a remote setting. To that end, we have addressed technologic difficulties, communication between remote providers and patients in the home environment, and communication between health care providers in various settings, such as the ED, inpatient wards, and the outpatient clinic.
To be sure, there are many challenges in making sure that CRPM adequately addresses the needs of patients, who may have persistent perturbations in cardiopulmonary status, tremendous anxiety about the progress or deterioration in their health status, and lack of understanding about their medical condition. Furthermore, providers face the challenge of making clinical decisions sometimes without the advantage of in-person examinations. Sometimes decisions must be made with incomplete information or when the status of the patient does not follow presupposed algorithms. Nevertheless, like many issues during the COVID-19 pandemic, patients and providers have evolved, pivoted, and made necessary adjustments to address an unprecedented time in recent history.
Ultimately, we believe that a continuous remote patient monitoring program can be designed, implemented, and maintained across a multifacility health care system for safe, effective, and efficient health care delivery. Limitations in implementing such a program might include lack of adequate Internet services, lack of telephonic communication, inadequate home facilities, lack of adequate home support, and, perhaps, lack of available emergency services. However, if the conditions for home monitoring are optimized, CRPM holds the promise of reducing the burden on emergency and inpatient hospital services, particularly when those services are strained in circumstances such as the ongoing global pandemic due to COVID-19. With further study, standardization, and evolution, remote monitoring will likely become a more acceptable and necessary form of health care delivery in the future.
Dr. Salomon is an Internal Medicine Resident (PGY-2); Dr. Muller is an Internal Medicine Resident (PGY-2); Dr. Boster is a Pulmonary and Critical Care Fellow; Dr. Loudermilk is a Pulmonary and Critical Care Fellow; and Dr. Kemp is Pulmonary and Critical Care staff, San Antonio Military Medical Center, Fort Sam Houston, Texas.
The SARS-CoV-2 pandemic required health care systems around the world to rapidly innovate and adapt to unprecedented operational and clinical strain. Many health care systems leveraged virtual care capabilities as an innovative approach to safely and efficiently manage patients while reducing staff exposure and medical resource constraints (Healthcare [Basel]. 2020 Nov;8[4]:517; JMIR Form Res. 2021 Jan; 5[1]:e23190). With Medicare insurance claims data demonstrating a 30% reduction of in-person health visits, telemedicine has become an essential means to fill the gaps in providing essential medical services (JAMA Intern Med. 2021 Mar;181[3]:388-91). A vast majority of virtual health care visits come via telephonic encounters, which have inherent limitations in the ability to monitor patients with complex or critical medical conditions (Front Public Health. 2020;8:410; N Engl J Med. 2020 Apr;382[18]:1679-81). Remote patient monitoring (RPM) has been established in multiple clinical models as an effective adjunct in telemedicine encounters in order to ensure treatment regimen adherence, make real-time treatment adjustments, and identify patients at risk for early decompensation.
Long-term RPM data has demonstrated cost reduction, reduced burden of in-office visits, expedited management of significant clinical events, and decreased all-cause mortality rates. Previously RPM was limited to the care of patients with chronic conditions, particularly cardiac patients with congestive heart failure and invasive devices, such as pacemakers or implantable cardioverter–defibrillators (JMIR Form Res. 2021 Jan;5[1]:e23190; Front Public Health. 2020; 8:410). In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) added RPM billing codes in 2019 and then included coverage of acute conditions in 2020 that permitted a more extensive role of RPM in telemedicine. This change in financial reimbursement led to a more aggressive expansion of RPM devices to assess physiologic parameters, such as weight, blood pressure, oxygen saturation, and blood glucose levels for clinicians to review.
Currently, RPM devices fall within a low-risk FDA category that do not require clinical trials for validation prior to being cleared for CMS billing in a fee-for-service reimbursement model (N Engl J Med. 2021 Apr;384[15]:1384-6). A shortage of evidence-based publications to guide clinicians in this new landscape creates challenges from underuse, misuse, or abuse of RPM tools. In order to maximize the clinical benefits of RPM, standardized processes and device specifications derived from up-to-date research need to be established in professional society guidelines.
Formalized RPM protocols should play a key role in overcoming the hesitancy of health institutions becoming early adopters of RPM technologies. Some significant challenges leading to reluctance of executing an RPM program were recently highlighted at the REPROGRAM international consortium of telemedicine. These concerns involved building a technological infrastructure, training clinical staff, ensuring remote connectivity with broadband Internet, and working with patients of various technologic literacy (Front Public Health. 2020;8:410). We attempted to address these challenges by using a COVID-19 remote patient monitoring (CRPM) strategy within our Military Health System (MHS). By using the well-established responsible, accountable, consulted, and informed (RACI) matrix process mapping tool, we created a standardized enrollment process of high-risk patients across eight military treatment facilities (MTFs). High risk patients included those with COVID-19 pneumonia and persistent hypoxemia, those recovering from acute exacerbations of congestive heart failure, those with cardiopulmonary instability associated with malignancy, and other conditions that required continuous monitoring outside of the hospital setting.
In our CRPM process, the hospital inpatient unit or ED refer high-risk patients to a primary designated provider at each MTF for enrollment prior to discharge. Enrolled patients are equipped with an FDA-approved home monitoring kit that contains an electronic tablet, a network hub that operates independently of and/or in conjunction with Wi-Fi, and an armband containing a coin-sized monitor. The system has the capability to pair with additional smart-enabled accessories, such as a blood pressure cuff, temperature patch, and digital spirometer. With continuous bio-physiologic and symptom-based monitoring, a team of teleworking critical-care nurses monitor patients continuously. In case of a decompensation necessitating a higher level of care, an emergency action plan (EAP) is activated to ensure patients urgently receive emergency medical services. Once released from the CRPM program, discharged patients use prepaid shipping boxes to facilitate contactless repackaging, sanitization, and pickup for redistribution of devices to the MTF.
Given the increased number of hospital admissions noted during the COVID-19 global pandemic, the CRPM program has allowed us to address overutilization of hospital beds. Furthermore, it has allowed us to address issues of screening and resource utilization as we consider patients for safe implementation of home monitoring. While data concerning the outcome of the CRPM program are pending, we are encouraged about the ability to provide high quality care in a remote setting. To that end, we have addressed technologic difficulties, communication between remote providers and patients in the home environment, and communication between health care providers in various settings, such as the ED, inpatient wards, and the outpatient clinic.
To be sure, there are many challenges in making sure that CRPM adequately addresses the needs of patients, who may have persistent perturbations in cardiopulmonary status, tremendous anxiety about the progress or deterioration in their health status, and lack of understanding about their medical condition. Furthermore, providers face the challenge of making clinical decisions sometimes without the advantage of in-person examinations. Sometimes decisions must be made with incomplete information or when the status of the patient does not follow presupposed algorithms. Nevertheless, like many issues during the COVID-19 pandemic, patients and providers have evolved, pivoted, and made necessary adjustments to address an unprecedented time in recent history.
Ultimately, we believe that a continuous remote patient monitoring program can be designed, implemented, and maintained across a multifacility health care system for safe, effective, and efficient health care delivery. Limitations in implementing such a program might include lack of adequate Internet services, lack of telephonic communication, inadequate home facilities, lack of adequate home support, and, perhaps, lack of available emergency services. However, if the conditions for home monitoring are optimized, CRPM holds the promise of reducing the burden on emergency and inpatient hospital services, particularly when those services are strained in circumstances such as the ongoing global pandemic due to COVID-19. With further study, standardization, and evolution, remote monitoring will likely become a more acceptable and necessary form of health care delivery in the future.
Dr. Salomon is an Internal Medicine Resident (PGY-2); Dr. Muller is an Internal Medicine Resident (PGY-2); Dr. Boster is a Pulmonary and Critical Care Fellow; Dr. Loudermilk is a Pulmonary and Critical Care Fellow; and Dr. Kemp is Pulmonary and Critical Care staff, San Antonio Military Medical Center, Fort Sam Houston, Texas.
About ABIM’s Longitudinal Knowledge Assessment
Physicians from every specialty have stepped up in extraordinary ways during the pandemic; however, ABIM recognizes that pulmonary disease and critical care physicians, along with hospitalists and infectious disease specialists, have been especially burdened. ABIM has heard from many pulmonary disease and critical care medicine physicians asking for greater flexibility and choice in how they can maintain their board certifications.
For that reason, ABIM has extended deadlines for all Maintenance of Certification (MOC) requirements to 12/31/22 and to 2023 for Critical Care Medicine, Hospital Medicine, Infectious Disease, and Pulmonary Disease.
What assessment options does ABIM offer?
If you haven’t needed to take an MOC exam for a while, you might not be aware of ABIM’s current options and how they might work for you:
- The traditional, 10-year MOC assessment (a point-in-time exam taken at a test center)
- The new Longitudinal Knowledge Assessment (LKATM) (available in 12 specialties including Internal Medicine and Sleep Medicine now, and in Critical Care Medicine and Pulmonary Disease in 2023)
The 2-year Knowledge Check-In was retired at the end of 2021 with the introduction of the LKA.
How the new LKA works
As a longitudinal assessment, the LKA is designed to help you measure your medical knowledge over time and better melds assessment and learning. It consists of a 5-year cycle, during which you’ll be offered 30 questions each quarter, and need to open at least 500 out of 600 questions to meet the LKA Participation Requirement. You can choose not to open up to 100 questions over 5 years, allowing you to take breaks when you need them.
Once enrolled, you can take questions on your laptop, desktop, or smartphone. You’ll also be able to answer questions where and when it’s convenient for you, such as at your home or office – with no need to schedule an appointment or go to a test center. You can use all the same resources you use in practice – journals, apps, and your own personal notes—anything except another person. For most questions, you’ll find out immediately if your answer was correct or not, and you’ll receive a rationale explaining why, along with one or more references.
You’ll have 4 minutes to answer each question and can add extra time if needed by drawing from an annual 30-minute time bank. For each correct answer, you’ll earn 0.2 MOC points, and if you choose to participate in LKA for more than one of your certificates, you’ll have even more opportunities to earn points. In addition, beginning in your second year of participation, interim score reports will give you helpful information to let you know how you’re doing, so you can re-adjust your approach and focus your studies as needed. A pass/fail decision is made at the end of the 5-year cycle.
About eligibility
If you are currently certified in Critical Care Medicine or Pulmonary Disease and had an assessment due in 2020, 2021 or 2022, you don’t need to take an assessment this year and will be eligible to enroll in the LKA in 2023, or you can choose to take the traditional 10-year MOC exam.
Upon enrolling, you will continue to be reported as “Certified” as long as you are meeting the LKA Participation Requirement. If your next assessment isn’t due for a while, you will be able to enroll in the LKA in your assessment due year—not before then.
More information about eligibility can be found in a special section of ABIM’s website.
How much does it cost?
ABIM revised its MOC fees in 2022 to provide an option to pay less over time than previously, and the LKA will be included in your annual MOC fee at no additional cost. Here’s how it works:
In closing
Thousands of physicians have already started taking the LKA in 2022 and are reporting positive experiences with it. The ABIM is excited that physicians in additional disciplines, including Critical Care Medicine and Pulmonary Disease, will get to experience it themselves in 2023.
Physicians from every specialty have stepped up in extraordinary ways during the pandemic; however, ABIM recognizes that pulmonary disease and critical care physicians, along with hospitalists and infectious disease specialists, have been especially burdened. ABIM has heard from many pulmonary disease and critical care medicine physicians asking for greater flexibility and choice in how they can maintain their board certifications.
For that reason, ABIM has extended deadlines for all Maintenance of Certification (MOC) requirements to 12/31/22 and to 2023 for Critical Care Medicine, Hospital Medicine, Infectious Disease, and Pulmonary Disease.
What assessment options does ABIM offer?
If you haven’t needed to take an MOC exam for a while, you might not be aware of ABIM’s current options and how they might work for you:
- The traditional, 10-year MOC assessment (a point-in-time exam taken at a test center)
- The new Longitudinal Knowledge Assessment (LKATM) (available in 12 specialties including Internal Medicine and Sleep Medicine now, and in Critical Care Medicine and Pulmonary Disease in 2023)
The 2-year Knowledge Check-In was retired at the end of 2021 with the introduction of the LKA.
How the new LKA works
As a longitudinal assessment, the LKA is designed to help you measure your medical knowledge over time and better melds assessment and learning. It consists of a 5-year cycle, during which you’ll be offered 30 questions each quarter, and need to open at least 500 out of 600 questions to meet the LKA Participation Requirement. You can choose not to open up to 100 questions over 5 years, allowing you to take breaks when you need them.
Once enrolled, you can take questions on your laptop, desktop, or smartphone. You’ll also be able to answer questions where and when it’s convenient for you, such as at your home or office – with no need to schedule an appointment or go to a test center. You can use all the same resources you use in practice – journals, apps, and your own personal notes—anything except another person. For most questions, you’ll find out immediately if your answer was correct or not, and you’ll receive a rationale explaining why, along with one or more references.
You’ll have 4 minutes to answer each question and can add extra time if needed by drawing from an annual 30-minute time bank. For each correct answer, you’ll earn 0.2 MOC points, and if you choose to participate in LKA for more than one of your certificates, you’ll have even more opportunities to earn points. In addition, beginning in your second year of participation, interim score reports will give you helpful information to let you know how you’re doing, so you can re-adjust your approach and focus your studies as needed. A pass/fail decision is made at the end of the 5-year cycle.
About eligibility
If you are currently certified in Critical Care Medicine or Pulmonary Disease and had an assessment due in 2020, 2021 or 2022, you don’t need to take an assessment this year and will be eligible to enroll in the LKA in 2023, or you can choose to take the traditional 10-year MOC exam.
Upon enrolling, you will continue to be reported as “Certified” as long as you are meeting the LKA Participation Requirement. If your next assessment isn’t due for a while, you will be able to enroll in the LKA in your assessment due year—not before then.
More information about eligibility can be found in a special section of ABIM’s website.
How much does it cost?
ABIM revised its MOC fees in 2022 to provide an option to pay less over time than previously, and the LKA will be included in your annual MOC fee at no additional cost. Here’s how it works:

In closing
Thousands of physicians have already started taking the LKA in 2022 and are reporting positive experiences with it. The ABIM is excited that physicians in additional disciplines, including Critical Care Medicine and Pulmonary Disease, will get to experience it themselves in 2023.
Physicians from every specialty have stepped up in extraordinary ways during the pandemic; however, ABIM recognizes that pulmonary disease and critical care physicians, along with hospitalists and infectious disease specialists, have been especially burdened. ABIM has heard from many pulmonary disease and critical care medicine physicians asking for greater flexibility and choice in how they can maintain their board certifications.
For that reason, ABIM has extended deadlines for all Maintenance of Certification (MOC) requirements to 12/31/22 and to 2023 for Critical Care Medicine, Hospital Medicine, Infectious Disease, and Pulmonary Disease.
What assessment options does ABIM offer?
If you haven’t needed to take an MOC exam for a while, you might not be aware of ABIM’s current options and how they might work for you:
- The traditional, 10-year MOC assessment (a point-in-time exam taken at a test center)
- The new Longitudinal Knowledge Assessment (LKATM) (available in 12 specialties including Internal Medicine and Sleep Medicine now, and in Critical Care Medicine and Pulmonary Disease in 2023)
The 2-year Knowledge Check-In was retired at the end of 2021 with the introduction of the LKA.
How the new LKA works
As a longitudinal assessment, the LKA is designed to help you measure your medical knowledge over time and better melds assessment and learning. It consists of a 5-year cycle, during which you’ll be offered 30 questions each quarter, and need to open at least 500 out of 600 questions to meet the LKA Participation Requirement. You can choose not to open up to 100 questions over 5 years, allowing you to take breaks when you need them.
Once enrolled, you can take questions on your laptop, desktop, or smartphone. You’ll also be able to answer questions where and when it’s convenient for you, such as at your home or office – with no need to schedule an appointment or go to a test center. You can use all the same resources you use in practice – journals, apps, and your own personal notes—anything except another person. For most questions, you’ll find out immediately if your answer was correct or not, and you’ll receive a rationale explaining why, along with one or more references.
You’ll have 4 minutes to answer each question and can add extra time if needed by drawing from an annual 30-minute time bank. For each correct answer, you’ll earn 0.2 MOC points, and if you choose to participate in LKA for more than one of your certificates, you’ll have even more opportunities to earn points. In addition, beginning in your second year of participation, interim score reports will give you helpful information to let you know how you’re doing, so you can re-adjust your approach and focus your studies as needed. A pass/fail decision is made at the end of the 5-year cycle.
About eligibility
If you are currently certified in Critical Care Medicine or Pulmonary Disease and had an assessment due in 2020, 2021 or 2022, you don’t need to take an assessment this year and will be eligible to enroll in the LKA in 2023, or you can choose to take the traditional 10-year MOC exam.
Upon enrolling, you will continue to be reported as “Certified” as long as you are meeting the LKA Participation Requirement. If your next assessment isn’t due for a while, you will be able to enroll in the LKA in your assessment due year—not before then.
More information about eligibility can be found in a special section of ABIM’s website.
How much does it cost?
ABIM revised its MOC fees in 2022 to provide an option to pay less over time than previously, and the LKA will be included in your annual MOC fee at no additional cost. Here’s how it works:

In closing
Thousands of physicians have already started taking the LKA in 2022 and are reporting positive experiences with it. The ABIM is excited that physicians in additional disciplines, including Critical Care Medicine and Pulmonary Disease, will get to experience it themselves in 2023.
Patients need Sep-1: Why don’t some doctors like it?
Since its inception, the CMS Sep-1 Core Quality Measure has been unpopular in some circles. It is now under official attack by the American College of Emergency Physicians (ACEP) and the Infectious Diseases Society of America (IDSA), along with a handful of smaller professional societies. These societies appealed the National Quality Forum’s (NQF) 2021 recommendation that the measure be renewed. The NQF is the multidisciplinary and broadly representative group of evaluators who evaluate proposals for CMS-sponsored quality improvement on behalf of the American people and of the Centers for Medicare & Medicaid Services (CMS). Readers of CHEST Physician are likely familiar with core measures, in general, and with Sep-1, in particular. CMS requires hospitals to publicly report their compliance with several Core Quality Measures, and the failure to do so results in across the board reductions in Medicare payments. As of now, no penalties are levied for the degree of compliance but only for failure to report the degree of compliance.
The measure asks, in the main, for hospitals to perform what most physicians can agree should be standard care for patients with sepsis. Depending on whether shock is present, the measure requires:
1. Blood cultures before antibiotics
2. Antibiotics within 3 hours of recognition of sepsis
3. Serum lactate measurement in the first 3 hours and, if increased, a repeat measurement by 6 hours
4. If the patient is hypotensive, 30 mL/kg IV crystalloid within 3 hours, or documentation of why that is not appropriate for the patient
5. If hypotension persists, vasopressors within 6 hours
6. Repeat cardiovascular assessment within 6 hours for patients with shock
If I evaluate these criteria as a patient who has been hospitalized for a serious infection, which I am, they do not seem particularly stringent. In fact, as a patient, I would want my doctors and nurses to act substantially faster than this if I had sepsis or septic shock. If my doctor did not come back in less than 6 hours to check on my shock status, I would be disappointed, to say the least. Nevertheless, some physicians and professional societies see no reason why these should be standards and state that the data underlying them are of low quality. Meanwhile, according to CMS’ own careful evaluation, national compliance with the measures is less than 50%, while being compliant with the measures reduces absolute overall mortality by approximately 4%, from 26.3% to 22.2% (Townsend SR et al. Chest. 2022;161[2]:392-406). This would translate to between 14,000 and 15,000 fewer patients dying from sepsis per year, if all patients received bundled, measure-compliant care. These are patients I don’t care to ignore.
ACEP and IDSA point specifically to the new Surviving Sepsis Campaign Guidelines (SSC) recommendations as evidence that the antibiotic measure is based on low quality evidence (Evans L et al. Crit Care Med. 2021;49[11]:1974-82). In this regard, they are technically correct; the system of evidence review that the SSC panel uses, Grading of Assessment, Recommendations, and Evaluation (GRADE), considers that retrospective analyses, which nearly all of these studies are, can be graded no higher than low quality. Clearly, retrospective studies will never achieve the level of certainty that we achieve with randomized controlled trials, but the NQF, itself, typically views that when a number of well-performed retrospective studies point in the same direction, the level of evidence is at least moderate. After all, just as it would be inappropriate to randomize participants to decades of smoking vs nonsmoking in order prove that smoking causes lung cancer, it is not appropriate to randomize patients with sepsis to receive delayed antibiotics before we accept that such delays are harmful to them.
ACEP and IDSA also assert that the association of early antibiotics with survival is “stronger” for septic shock than for sepsis. In fact, the association is quite strong for both severities of illness. Until it progresses to septic shock, the expected mortality of sepsis is lower, and the percent reduction in mortality is less than for septic shock. However, the opportunity for lives preserved is quite large, because the number of patients with sepsis at presentation is approximately 10 times higher than the number with septic shock at presentation. Antibiotic delays are also associated with progression from infection or sepsis to septic shock (Whiles BB et al. Crit Care Med. 2017;45[4]:623-29; Bisarya R et al. Chest. 2022;161[1]:112-20). Importantly, SSC gave a strong recommendation for all patients with suspected sepsis to receive antibiotics within 3 hours of suspecting sepsis and within 1 hour of suspecting septic shock, a recommendation even stronger than that of Sep-1.
Critics opine that CMS should stop looking at the process measures and focus only on the outcomes of sepsis care. There is a certain attractiveness to this proposition. One could say that it does not matter so much how a hospital achieves lower mortality as long as they do achieve it. However, the question would then become – how low should the mortality rate be? I have a notion that whatever the number, the Sep-1 critics would find it unbearable.
There is a core principle embedded in the Sep-1 process measures, in SSC guidelines, and in the concept of early goal-directed therapy that preceded them: success is not dependent only on what we do but on when we do it. All of you have experienced this. Each of you has attended a professional school, whether medical, nursing, respiratory therapy, etc. None of you showed up unannounced on opening day of the semester and was admitted to that school. All of you garnered the grades, solicited the letters of recommendation, took the entrance exams, and submitted an application. Some of you went to an interview. All of these things were done in a timely fashion; professional schools do not accept incomplete applications or late applications. Doing the right things at the wrong time would have left us all pursuing different careers.
Very early in my career as an attending physician in the ICU, I found myself exasperated by the circumstances of many patients who we received in the ICU with sepsis. I would peruse their medical records and find that they had been septic, ie, had met criteria for severe sepsis, 1 to 2 days before their deterioration to septic shock, yet they had not been diagnosed with sepsis until shock developed. In the ICU, we began resuscitative fluids, ensured appropriate antibiotics, and started vasopressors, but it was often to no avail. The treatments we gave made no difference for many patients, because they were given too late. For me, this was career altering; much of my career since that time has focused on teaching medical personnel how to recognize sepsis, how to give timely and appropriate treatments, and how to keep the data to show when they have done that and when they have not.
Before Sep-1 many, if not most, of the hospitals in the United States had no particular strategy in place to recognize and treat patients with sepsis, even though it was and is the most common cause of death and the costliest condition in American hospitals. Now, most hospitals do have such strategies. Assertions by professional societies that it is difficult to collect the data for Sep-1 reporting are likely true. However, keeping patients safe and alive is a hospital’s primary reason for existing. As long as hospitals are tracking each antibiotic and every liter of fluid so that they can bill for them, my own ears are deaf to hearing that it is too difficult to make sure that we are doing our job. Modifying or eliminating Sep-1 for any reason except data that show we can clearly further improve the outcome for all patients with sepsis is the wrong move to make. So far, other professional societies want to remove elements of Sep-1 without evidence that it would improve our care for patients with sepsis or their outcomes. Thankfully, from the time we proposed the first criteria for diagnosing sepsis, CHEST has promoted what is best for patients, whether it is difficult or not.
Dr. Simpson is a pulmonologist and intensivist with an extensive background in sepsis and in critical care quality improvement, including by serving as a senior adviser to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the U.S. Department of Health and Human Services and an author of the 2016 and 2020 updates of the Surviving Sepsis Campaign Guidelines. Dr. Simpson is the senior medical adviser for Sepsis Alliance, a nationwide patient information and advocacy organization. He is the immediate past president of CHEST.
Since its inception, the CMS Sep-1 Core Quality Measure has been unpopular in some circles. It is now under official attack by the American College of Emergency Physicians (ACEP) and the Infectious Diseases Society of America (IDSA), along with a handful of smaller professional societies. These societies appealed the National Quality Forum’s (NQF) 2021 recommendation that the measure be renewed. The NQF is the multidisciplinary and broadly representative group of evaluators who evaluate proposals for CMS-sponsored quality improvement on behalf of the American people and of the Centers for Medicare & Medicaid Services (CMS). Readers of CHEST Physician are likely familiar with core measures, in general, and with Sep-1, in particular. CMS requires hospitals to publicly report their compliance with several Core Quality Measures, and the failure to do so results in across the board reductions in Medicare payments. As of now, no penalties are levied for the degree of compliance but only for failure to report the degree of compliance.
The measure asks, in the main, for hospitals to perform what most physicians can agree should be standard care for patients with sepsis. Depending on whether shock is present, the measure requires:
1. Blood cultures before antibiotics
2. Antibiotics within 3 hours of recognition of sepsis
3. Serum lactate measurement in the first 3 hours and, if increased, a repeat measurement by 6 hours
4. If the patient is hypotensive, 30 mL/kg IV crystalloid within 3 hours, or documentation of why that is not appropriate for the patient
5. If hypotension persists, vasopressors within 6 hours
6. Repeat cardiovascular assessment within 6 hours for patients with shock
If I evaluate these criteria as a patient who has been hospitalized for a serious infection, which I am, they do not seem particularly stringent. In fact, as a patient, I would want my doctors and nurses to act substantially faster than this if I had sepsis or septic shock. If my doctor did not come back in less than 6 hours to check on my shock status, I would be disappointed, to say the least. Nevertheless, some physicians and professional societies see no reason why these should be standards and state that the data underlying them are of low quality. Meanwhile, according to CMS’ own careful evaluation, national compliance with the measures is less than 50%, while being compliant with the measures reduces absolute overall mortality by approximately 4%, from 26.3% to 22.2% (Townsend SR et al. Chest. 2022;161[2]:392-406). This would translate to between 14,000 and 15,000 fewer patients dying from sepsis per year, if all patients received bundled, measure-compliant care. These are patients I don’t care to ignore.
ACEP and IDSA point specifically to the new Surviving Sepsis Campaign Guidelines (SSC) recommendations as evidence that the antibiotic measure is based on low quality evidence (Evans L et al. Crit Care Med. 2021;49[11]:1974-82). In this regard, they are technically correct; the system of evidence review that the SSC panel uses, Grading of Assessment, Recommendations, and Evaluation (GRADE), considers that retrospective analyses, which nearly all of these studies are, can be graded no higher than low quality. Clearly, retrospective studies will never achieve the level of certainty that we achieve with randomized controlled trials, but the NQF, itself, typically views that when a number of well-performed retrospective studies point in the same direction, the level of evidence is at least moderate. After all, just as it would be inappropriate to randomize participants to decades of smoking vs nonsmoking in order prove that smoking causes lung cancer, it is not appropriate to randomize patients with sepsis to receive delayed antibiotics before we accept that such delays are harmful to them.
ACEP and IDSA also assert that the association of early antibiotics with survival is “stronger” for septic shock than for sepsis. In fact, the association is quite strong for both severities of illness. Until it progresses to septic shock, the expected mortality of sepsis is lower, and the percent reduction in mortality is less than for septic shock. However, the opportunity for lives preserved is quite large, because the number of patients with sepsis at presentation is approximately 10 times higher than the number with septic shock at presentation. Antibiotic delays are also associated with progression from infection or sepsis to septic shock (Whiles BB et al. Crit Care Med. 2017;45[4]:623-29; Bisarya R et al. Chest. 2022;161[1]:112-20). Importantly, SSC gave a strong recommendation for all patients with suspected sepsis to receive antibiotics within 3 hours of suspecting sepsis and within 1 hour of suspecting septic shock, a recommendation even stronger than that of Sep-1.
Critics opine that CMS should stop looking at the process measures and focus only on the outcomes of sepsis care. There is a certain attractiveness to this proposition. One could say that it does not matter so much how a hospital achieves lower mortality as long as they do achieve it. However, the question would then become – how low should the mortality rate be? I have a notion that whatever the number, the Sep-1 critics would find it unbearable.
There is a core principle embedded in the Sep-1 process measures, in SSC guidelines, and in the concept of early goal-directed therapy that preceded them: success is not dependent only on what we do but on when we do it. All of you have experienced this. Each of you has attended a professional school, whether medical, nursing, respiratory therapy, etc. None of you showed up unannounced on opening day of the semester and was admitted to that school. All of you garnered the grades, solicited the letters of recommendation, took the entrance exams, and submitted an application. Some of you went to an interview. All of these things were done in a timely fashion; professional schools do not accept incomplete applications or late applications. Doing the right things at the wrong time would have left us all pursuing different careers.
Very early in my career as an attending physician in the ICU, I found myself exasperated by the circumstances of many patients who we received in the ICU with sepsis. I would peruse their medical records and find that they had been septic, ie, had met criteria for severe sepsis, 1 to 2 days before their deterioration to septic shock, yet they had not been diagnosed with sepsis until shock developed. In the ICU, we began resuscitative fluids, ensured appropriate antibiotics, and started vasopressors, but it was often to no avail. The treatments we gave made no difference for many patients, because they were given too late. For me, this was career altering; much of my career since that time has focused on teaching medical personnel how to recognize sepsis, how to give timely and appropriate treatments, and how to keep the data to show when they have done that and when they have not.
Before Sep-1 many, if not most, of the hospitals in the United States had no particular strategy in place to recognize and treat patients with sepsis, even though it was and is the most common cause of death and the costliest condition in American hospitals. Now, most hospitals do have such strategies. Assertions by professional societies that it is difficult to collect the data for Sep-1 reporting are likely true. However, keeping patients safe and alive is a hospital’s primary reason for existing. As long as hospitals are tracking each antibiotic and every liter of fluid so that they can bill for them, my own ears are deaf to hearing that it is too difficult to make sure that we are doing our job. Modifying or eliminating Sep-1 for any reason except data that show we can clearly further improve the outcome for all patients with sepsis is the wrong move to make. So far, other professional societies want to remove elements of Sep-1 without evidence that it would improve our care for patients with sepsis or their outcomes. Thankfully, from the time we proposed the first criteria for diagnosing sepsis, CHEST has promoted what is best for patients, whether it is difficult or not.
Dr. Simpson is a pulmonologist and intensivist with an extensive background in sepsis and in critical care quality improvement, including by serving as a senior adviser to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the U.S. Department of Health and Human Services and an author of the 2016 and 2020 updates of the Surviving Sepsis Campaign Guidelines. Dr. Simpson is the senior medical adviser for Sepsis Alliance, a nationwide patient information and advocacy organization. He is the immediate past president of CHEST.
Since its inception, the CMS Sep-1 Core Quality Measure has been unpopular in some circles. It is now under official attack by the American College of Emergency Physicians (ACEP) and the Infectious Diseases Society of America (IDSA), along with a handful of smaller professional societies. These societies appealed the National Quality Forum’s (NQF) 2021 recommendation that the measure be renewed. The NQF is the multidisciplinary and broadly representative group of evaluators who evaluate proposals for CMS-sponsored quality improvement on behalf of the American people and of the Centers for Medicare & Medicaid Services (CMS). Readers of CHEST Physician are likely familiar with core measures, in general, and with Sep-1, in particular. CMS requires hospitals to publicly report their compliance with several Core Quality Measures, and the failure to do so results in across the board reductions in Medicare payments. As of now, no penalties are levied for the degree of compliance but only for failure to report the degree of compliance.
The measure asks, in the main, for hospitals to perform what most physicians can agree should be standard care for patients with sepsis. Depending on whether shock is present, the measure requires:
1. Blood cultures before antibiotics
2. Antibiotics within 3 hours of recognition of sepsis
3. Serum lactate measurement in the first 3 hours and, if increased, a repeat measurement by 6 hours
4. If the patient is hypotensive, 30 mL/kg IV crystalloid within 3 hours, or documentation of why that is not appropriate for the patient
5. If hypotension persists, vasopressors within 6 hours
6. Repeat cardiovascular assessment within 6 hours for patients with shock
If I evaluate these criteria as a patient who has been hospitalized for a serious infection, which I am, they do not seem particularly stringent. In fact, as a patient, I would want my doctors and nurses to act substantially faster than this if I had sepsis or septic shock. If my doctor did not come back in less than 6 hours to check on my shock status, I would be disappointed, to say the least. Nevertheless, some physicians and professional societies see no reason why these should be standards and state that the data underlying them are of low quality. Meanwhile, according to CMS’ own careful evaluation, national compliance with the measures is less than 50%, while being compliant with the measures reduces absolute overall mortality by approximately 4%, from 26.3% to 22.2% (Townsend SR et al. Chest. 2022;161[2]:392-406). This would translate to between 14,000 and 15,000 fewer patients dying from sepsis per year, if all patients received bundled, measure-compliant care. These are patients I don’t care to ignore.
ACEP and IDSA point specifically to the new Surviving Sepsis Campaign Guidelines (SSC) recommendations as evidence that the antibiotic measure is based on low quality evidence (Evans L et al. Crit Care Med. 2021;49[11]:1974-82). In this regard, they are technically correct; the system of evidence review that the SSC panel uses, Grading of Assessment, Recommendations, and Evaluation (GRADE), considers that retrospective analyses, which nearly all of these studies are, can be graded no higher than low quality. Clearly, retrospective studies will never achieve the level of certainty that we achieve with randomized controlled trials, but the NQF, itself, typically views that when a number of well-performed retrospective studies point in the same direction, the level of evidence is at least moderate. After all, just as it would be inappropriate to randomize participants to decades of smoking vs nonsmoking in order prove that smoking causes lung cancer, it is not appropriate to randomize patients with sepsis to receive delayed antibiotics before we accept that such delays are harmful to them.
ACEP and IDSA also assert that the association of early antibiotics with survival is “stronger” for septic shock than for sepsis. In fact, the association is quite strong for both severities of illness. Until it progresses to septic shock, the expected mortality of sepsis is lower, and the percent reduction in mortality is less than for septic shock. However, the opportunity for lives preserved is quite large, because the number of patients with sepsis at presentation is approximately 10 times higher than the number with septic shock at presentation. Antibiotic delays are also associated with progression from infection or sepsis to septic shock (Whiles BB et al. Crit Care Med. 2017;45[4]:623-29; Bisarya R et al. Chest. 2022;161[1]:112-20). Importantly, SSC gave a strong recommendation for all patients with suspected sepsis to receive antibiotics within 3 hours of suspecting sepsis and within 1 hour of suspecting septic shock, a recommendation even stronger than that of Sep-1.
Critics opine that CMS should stop looking at the process measures and focus only on the outcomes of sepsis care. There is a certain attractiveness to this proposition. One could say that it does not matter so much how a hospital achieves lower mortality as long as they do achieve it. However, the question would then become – how low should the mortality rate be? I have a notion that whatever the number, the Sep-1 critics would find it unbearable.
There is a core principle embedded in the Sep-1 process measures, in SSC guidelines, and in the concept of early goal-directed therapy that preceded them: success is not dependent only on what we do but on when we do it. All of you have experienced this. Each of you has attended a professional school, whether medical, nursing, respiratory therapy, etc. None of you showed up unannounced on opening day of the semester and was admitted to that school. All of you garnered the grades, solicited the letters of recommendation, took the entrance exams, and submitted an application. Some of you went to an interview. All of these things were done in a timely fashion; professional schools do not accept incomplete applications or late applications. Doing the right things at the wrong time would have left us all pursuing different careers.
Very early in my career as an attending physician in the ICU, I found myself exasperated by the circumstances of many patients who we received in the ICU with sepsis. I would peruse their medical records and find that they had been septic, ie, had met criteria for severe sepsis, 1 to 2 days before their deterioration to septic shock, yet they had not been diagnosed with sepsis until shock developed. In the ICU, we began resuscitative fluids, ensured appropriate antibiotics, and started vasopressors, but it was often to no avail. The treatments we gave made no difference for many patients, because they were given too late. For me, this was career altering; much of my career since that time has focused on teaching medical personnel how to recognize sepsis, how to give timely and appropriate treatments, and how to keep the data to show when they have done that and when they have not.
Before Sep-1 many, if not most, of the hospitals in the United States had no particular strategy in place to recognize and treat patients with sepsis, even though it was and is the most common cause of death and the costliest condition in American hospitals. Now, most hospitals do have such strategies. Assertions by professional societies that it is difficult to collect the data for Sep-1 reporting are likely true. However, keeping patients safe and alive is a hospital’s primary reason for existing. As long as hospitals are tracking each antibiotic and every liter of fluid so that they can bill for them, my own ears are deaf to hearing that it is too difficult to make sure that we are doing our job. Modifying or eliminating Sep-1 for any reason except data that show we can clearly further improve the outcome for all patients with sepsis is the wrong move to make. So far, other professional societies want to remove elements of Sep-1 without evidence that it would improve our care for patients with sepsis or their outcomes. Thankfully, from the time we proposed the first criteria for diagnosing sepsis, CHEST has promoted what is best for patients, whether it is difficult or not.
Dr. Simpson is a pulmonologist and intensivist with an extensive background in sepsis and in critical care quality improvement, including by serving as a senior adviser to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the U.S. Department of Health and Human Services and an author of the 2016 and 2020 updates of the Surviving Sepsis Campaign Guidelines. Dr. Simpson is the senior medical adviser for Sepsis Alliance, a nationwide patient information and advocacy organization. He is the immediate past president of CHEST.












