User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Summer flu, RSV in July, ‘super colds?’
Richard Martinello, MD, a professor of medicine and pediatric infectious diseases at Yale University, New haven, Conn., doesn’t expect to see a child hospitalized with respiratory syncytial virus (RSV) in the middle of summer. The illness, which can strike infants and older adults especially hard, is known as a “winter virus.”
But not this year. Over the last several weeks, he says, admissions for children with RSV have increased at the Yale New Haven Children’s Hospital. While the numbers aren’t large, they are out of the ordinary, he says, “because usually, at this time of year, we see zero. For lack of a better term, it’s weird.”
Likewise, William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville, says RSV is on the rise there. Tennessee is one of 10 states taking part in a Centers for Disease Control and Prevention surveillance system that tracks influenza, RSV, and COVID-19.
He says RSV cases have risen by at least a third during the past week, including all age ranges. At this time of year, he says, “We aren’t supposed to have any RSV.”
RSV isn’t the only virus thriving out of season or otherwise acting strangely. Since the pandemic began, flu seasons have been out of whack – sometimes nearly nonexistent and other times extending well beyond “normal” seasons. Some experts say one influenza “B” strain may now be extinct, while others say it will be back.
Severe colds – what some call “super colds” – also seem to be on the rise in recent warm-weather months, although that evidence is mostly based on personal experience, not science.
Trying to explain these out-of-season variations has sparked much discussion among epidemiologists and virologists, Dr. Schaffner says, with debates ongoing about whether human behavior and habits or the seasons play a bigger role in the transmission of viral illness.
On top of that, scientists are also looking at the interactions between the SARS-CoV-2 virus that causes COVID-19 and other viruses. When people get hit with COVID-19 and other viruses at the same time, does that make COVID-19 more severe, or less?
Research is conflicting.
Summer of 2022: A repeat of 2021?
RSV. Most children contract the virus by age 2, and while it’s generally mild, about 58,000 children under age 5 years are hospitalized each year. During the pandemic, RSV cases decreased from January to April 2020, the CDC reported, and then remained at “historically low levels”: less than 1% positive RSV results a week, for the next year.
But cases began rising in April 2021.
“Last year, we did have an unusual summer,” Dr. Schaffner says. After lockdown ended, to everyone’s surprise, RSV infections rose.
That increase triggered a CDC health advisory in June 2021, telling doctors and caregivers about the increase in “interseasonal” RSV cases across parts of the Southern United States, recommending broader testing for RSV in patients who had a respiratory illness but tested negative for COVID.
Because of the reduced circulation of RSV during the winter of 2020 to 2021, the CDC warned, older infants and toddlers might have a higher risk of RSV since they weren’t exposed to typical levels of RSV for the previous 15 months.
What about 2022? “At the moment,” Dr. Schaffner says, “it looks like we are having a repeat [of 2021].”
On Twitter, other pediatricians, including those from Maine and Texas, have reported an increase in RSV cases this summer.
Influenza. From October 2020 until May 2021, flu activity was lower than during any previous flu season since at least 1997, according to the CDC.
In late 2021, researchers suggested that one line of influenza known as B/Yamagata may have become extinct.
The 2021-2022 flu season has been mild, the CDC says, but it has come in two waves, with the second wave lingering longer than previous ones. While flu activity is decreasing, last week the CDC said doctors should be alert to flu infections throughout the summer.
Colds. In reports on colds that aren’t based on science, several doctors say they are seeing more colds than usual in the summer, and they’re more severe than usual. According to the CDC, common coronaviruses and respiratory adenoviruses have been increasing since early 2021, and rhinoviruses since June 2020.
Behavior vs. seasons
In explaining the spread of viral respiratory diseases, infectious disease doctors consider two things. “One is that temperature and humidity in the winter favors longer survival of some viruses, leading to longer periods of possible transmission,” says Dean Blumberg, MD, a professor of pediatrics and chief of pediatric infectious disease at University of California Davis Health.
“The other is differences in human behavior, with people spending more time outside in the summer, which results in more distancing and [less] virus concentration due to very large air volume,” he says, and vice versa in winter.
What about the “super colds?” COVID-19 lockdowns and social distancing greatly reduced people’s exposure to common viruses like those that cause colds, says Neil A. Mabbott, PhD, a professor of immunopathology at the University of Edinburgh (Scotland).
“Immunity to these common cold viruses gained through natural infection is considered to last around 8 or 9 months or so,” he says. “Each winter, when we are exposed to the new circulating variants of these viruses, our immunity receives a natural boost.”
That explains why most people get a cold that’s relatively mild. But with all the pandemic lockdowns and the use of hand sanitizers, most people had limited exposure to other viruses, including the common cold. When people emerged from lockdown, the common cold viruses were beginning to circulate again.
“Our immune systems were less able to clear the infection than previously,” Dr. Mabbott says. “As a consequence, some may have experienced increased symptoms, giving the impression of being infected with a ‘super cold.’ ”
“The colds themselves are probably not different to those we got prepandemic,” says Ian Mackay, PhD, a virologist at the University of Queensland, Brisbane, Australia. “But there might be more of them. So I doubt they are ‘super colds’ as much as they are ‘super-perfect circumstances.’ ”
The colds themselves are probably not different to those we got prepandemic. But there might be more of them.
Those super-perfect circumstances, he says, include people gathering after lockdown; a lack of immunity in new babies; viruses that have remained, even if at low levels, but continue to mutate; and our waning immunity to the range of viruses we’d normally encounter.
While lack of exposure may partly explain why some viruses become rampant out of season, it’s likely not the only reason. For example, the reduced circulation of RSV in the population as a whole also may have reduced the transfer of immunity from mothers to infants, some researchers say, making those infants more vulnerable than usual.
Interactions of viruses
Another thing that may be driving the different behavior of viruses is that the SARS-CoV-2 virus could somehow be interacting with other respiratory viruses, Dr. Schaffner says. “And if so, what sort of interactions?”
Many researchers are looking into that, and how coinfections with other respiratory diseases, including the common cold and flu, may affect the course of COVID-19. Some studies have found that the T cells – a source of deeper, cellular immunity in people – generated after a common cold “may also provide cross-protection in some people against COVID-19.”
But another study found immunity against common cold–causing coronaviruses might make COVID-19 more severe.
When researchers in the United Kingdom studied nearly 7,000 patients infected with COVID-19, including 583 also infected with RSV, flu, or adenoviruses (causing flulike or coldlike illness), those with flu or adenovirus, compared with the others, were at higher risk of death.
To be continued …
Exactly how COVID-19 will be changing what we know of other viruses is yet to be determined, too.
Even before the pandemic, Dr. Martinello says, there were already some shifts in RSV. Florida, for instance, has an RSV season longer than the rest of the country, mimicking the pattern in the tropics.
Will the atypical patterns continue? “My guess is that this will settle out,” he says, with some sort of pattern developing. At this point, there are many unknowns. “We still can’t answer whether there will be some seasonality to COVID.”
A version of this article first appeared on WebMD.com.
Richard Martinello, MD, a professor of medicine and pediatric infectious diseases at Yale University, New haven, Conn., doesn’t expect to see a child hospitalized with respiratory syncytial virus (RSV) in the middle of summer. The illness, which can strike infants and older adults especially hard, is known as a “winter virus.”
But not this year. Over the last several weeks, he says, admissions for children with RSV have increased at the Yale New Haven Children’s Hospital. While the numbers aren’t large, they are out of the ordinary, he says, “because usually, at this time of year, we see zero. For lack of a better term, it’s weird.”
Likewise, William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville, says RSV is on the rise there. Tennessee is one of 10 states taking part in a Centers for Disease Control and Prevention surveillance system that tracks influenza, RSV, and COVID-19.
He says RSV cases have risen by at least a third during the past week, including all age ranges. At this time of year, he says, “We aren’t supposed to have any RSV.”
RSV isn’t the only virus thriving out of season or otherwise acting strangely. Since the pandemic began, flu seasons have been out of whack – sometimes nearly nonexistent and other times extending well beyond “normal” seasons. Some experts say one influenza “B” strain may now be extinct, while others say it will be back.
Severe colds – what some call “super colds” – also seem to be on the rise in recent warm-weather months, although that evidence is mostly based on personal experience, not science.
Trying to explain these out-of-season variations has sparked much discussion among epidemiologists and virologists, Dr. Schaffner says, with debates ongoing about whether human behavior and habits or the seasons play a bigger role in the transmission of viral illness.
On top of that, scientists are also looking at the interactions between the SARS-CoV-2 virus that causes COVID-19 and other viruses. When people get hit with COVID-19 and other viruses at the same time, does that make COVID-19 more severe, or less?
Research is conflicting.
Summer of 2022: A repeat of 2021?
RSV. Most children contract the virus by age 2, and while it’s generally mild, about 58,000 children under age 5 years are hospitalized each year. During the pandemic, RSV cases decreased from January to April 2020, the CDC reported, and then remained at “historically low levels”: less than 1% positive RSV results a week, for the next year.
But cases began rising in April 2021.
“Last year, we did have an unusual summer,” Dr. Schaffner says. After lockdown ended, to everyone’s surprise, RSV infections rose.
That increase triggered a CDC health advisory in June 2021, telling doctors and caregivers about the increase in “interseasonal” RSV cases across parts of the Southern United States, recommending broader testing for RSV in patients who had a respiratory illness but tested negative for COVID.
Because of the reduced circulation of RSV during the winter of 2020 to 2021, the CDC warned, older infants and toddlers might have a higher risk of RSV since they weren’t exposed to typical levels of RSV for the previous 15 months.
What about 2022? “At the moment,” Dr. Schaffner says, “it looks like we are having a repeat [of 2021].”
On Twitter, other pediatricians, including those from Maine and Texas, have reported an increase in RSV cases this summer.
Influenza. From October 2020 until May 2021, flu activity was lower than during any previous flu season since at least 1997, according to the CDC.
In late 2021, researchers suggested that one line of influenza known as B/Yamagata may have become extinct.
The 2021-2022 flu season has been mild, the CDC says, but it has come in two waves, with the second wave lingering longer than previous ones. While flu activity is decreasing, last week the CDC said doctors should be alert to flu infections throughout the summer.
Colds. In reports on colds that aren’t based on science, several doctors say they are seeing more colds than usual in the summer, and they’re more severe than usual. According to the CDC, common coronaviruses and respiratory adenoviruses have been increasing since early 2021, and rhinoviruses since June 2020.
Behavior vs. seasons
In explaining the spread of viral respiratory diseases, infectious disease doctors consider two things. “One is that temperature and humidity in the winter favors longer survival of some viruses, leading to longer periods of possible transmission,” says Dean Blumberg, MD, a professor of pediatrics and chief of pediatric infectious disease at University of California Davis Health.
“The other is differences in human behavior, with people spending more time outside in the summer, which results in more distancing and [less] virus concentration due to very large air volume,” he says, and vice versa in winter.
What about the “super colds?” COVID-19 lockdowns and social distancing greatly reduced people’s exposure to common viruses like those that cause colds, says Neil A. Mabbott, PhD, a professor of immunopathology at the University of Edinburgh (Scotland).
“Immunity to these common cold viruses gained through natural infection is considered to last around 8 or 9 months or so,” he says. “Each winter, when we are exposed to the new circulating variants of these viruses, our immunity receives a natural boost.”
That explains why most people get a cold that’s relatively mild. But with all the pandemic lockdowns and the use of hand sanitizers, most people had limited exposure to other viruses, including the common cold. When people emerged from lockdown, the common cold viruses were beginning to circulate again.
“Our immune systems were less able to clear the infection than previously,” Dr. Mabbott says. “As a consequence, some may have experienced increased symptoms, giving the impression of being infected with a ‘super cold.’ ”
“The colds themselves are probably not different to those we got prepandemic,” says Ian Mackay, PhD, a virologist at the University of Queensland, Brisbane, Australia. “But there might be more of them. So I doubt they are ‘super colds’ as much as they are ‘super-perfect circumstances.’ ”
The colds themselves are probably not different to those we got prepandemic. But there might be more of them.
Those super-perfect circumstances, he says, include people gathering after lockdown; a lack of immunity in new babies; viruses that have remained, even if at low levels, but continue to mutate; and our waning immunity to the range of viruses we’d normally encounter.
While lack of exposure may partly explain why some viruses become rampant out of season, it’s likely not the only reason. For example, the reduced circulation of RSV in the population as a whole also may have reduced the transfer of immunity from mothers to infants, some researchers say, making those infants more vulnerable than usual.
Interactions of viruses
Another thing that may be driving the different behavior of viruses is that the SARS-CoV-2 virus could somehow be interacting with other respiratory viruses, Dr. Schaffner says. “And if so, what sort of interactions?”
Many researchers are looking into that, and how coinfections with other respiratory diseases, including the common cold and flu, may affect the course of COVID-19. Some studies have found that the T cells – a source of deeper, cellular immunity in people – generated after a common cold “may also provide cross-protection in some people against COVID-19.”
But another study found immunity against common cold–causing coronaviruses might make COVID-19 more severe.
When researchers in the United Kingdom studied nearly 7,000 patients infected with COVID-19, including 583 also infected with RSV, flu, or adenoviruses (causing flulike or coldlike illness), those with flu or adenovirus, compared with the others, were at higher risk of death.
To be continued …
Exactly how COVID-19 will be changing what we know of other viruses is yet to be determined, too.
Even before the pandemic, Dr. Martinello says, there were already some shifts in RSV. Florida, for instance, has an RSV season longer than the rest of the country, mimicking the pattern in the tropics.
Will the atypical patterns continue? “My guess is that this will settle out,” he says, with some sort of pattern developing. At this point, there are many unknowns. “We still can’t answer whether there will be some seasonality to COVID.”
A version of this article first appeared on WebMD.com.
Richard Martinello, MD, a professor of medicine and pediatric infectious diseases at Yale University, New haven, Conn., doesn’t expect to see a child hospitalized with respiratory syncytial virus (RSV) in the middle of summer. The illness, which can strike infants and older adults especially hard, is known as a “winter virus.”
But not this year. Over the last several weeks, he says, admissions for children with RSV have increased at the Yale New Haven Children’s Hospital. While the numbers aren’t large, they are out of the ordinary, he says, “because usually, at this time of year, we see zero. For lack of a better term, it’s weird.”
Likewise, William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville, says RSV is on the rise there. Tennessee is one of 10 states taking part in a Centers for Disease Control and Prevention surveillance system that tracks influenza, RSV, and COVID-19.
He says RSV cases have risen by at least a third during the past week, including all age ranges. At this time of year, he says, “We aren’t supposed to have any RSV.”
RSV isn’t the only virus thriving out of season or otherwise acting strangely. Since the pandemic began, flu seasons have been out of whack – sometimes nearly nonexistent and other times extending well beyond “normal” seasons. Some experts say one influenza “B” strain may now be extinct, while others say it will be back.
Severe colds – what some call “super colds” – also seem to be on the rise in recent warm-weather months, although that evidence is mostly based on personal experience, not science.
Trying to explain these out-of-season variations has sparked much discussion among epidemiologists and virologists, Dr. Schaffner says, with debates ongoing about whether human behavior and habits or the seasons play a bigger role in the transmission of viral illness.
On top of that, scientists are also looking at the interactions between the SARS-CoV-2 virus that causes COVID-19 and other viruses. When people get hit with COVID-19 and other viruses at the same time, does that make COVID-19 more severe, or less?
Research is conflicting.
Summer of 2022: A repeat of 2021?
RSV. Most children contract the virus by age 2, and while it’s generally mild, about 58,000 children under age 5 years are hospitalized each year. During the pandemic, RSV cases decreased from January to April 2020, the CDC reported, and then remained at “historically low levels”: less than 1% positive RSV results a week, for the next year.
But cases began rising in April 2021.
“Last year, we did have an unusual summer,” Dr. Schaffner says. After lockdown ended, to everyone’s surprise, RSV infections rose.
That increase triggered a CDC health advisory in June 2021, telling doctors and caregivers about the increase in “interseasonal” RSV cases across parts of the Southern United States, recommending broader testing for RSV in patients who had a respiratory illness but tested negative for COVID.
Because of the reduced circulation of RSV during the winter of 2020 to 2021, the CDC warned, older infants and toddlers might have a higher risk of RSV since they weren’t exposed to typical levels of RSV for the previous 15 months.
What about 2022? “At the moment,” Dr. Schaffner says, “it looks like we are having a repeat [of 2021].”
On Twitter, other pediatricians, including those from Maine and Texas, have reported an increase in RSV cases this summer.
Influenza. From October 2020 until May 2021, flu activity was lower than during any previous flu season since at least 1997, according to the CDC.
In late 2021, researchers suggested that one line of influenza known as B/Yamagata may have become extinct.
The 2021-2022 flu season has been mild, the CDC says, but it has come in two waves, with the second wave lingering longer than previous ones. While flu activity is decreasing, last week the CDC said doctors should be alert to flu infections throughout the summer.
Colds. In reports on colds that aren’t based on science, several doctors say they are seeing more colds than usual in the summer, and they’re more severe than usual. According to the CDC, common coronaviruses and respiratory adenoviruses have been increasing since early 2021, and rhinoviruses since June 2020.
Behavior vs. seasons
In explaining the spread of viral respiratory diseases, infectious disease doctors consider two things. “One is that temperature and humidity in the winter favors longer survival of some viruses, leading to longer periods of possible transmission,” says Dean Blumberg, MD, a professor of pediatrics and chief of pediatric infectious disease at University of California Davis Health.
“The other is differences in human behavior, with people spending more time outside in the summer, which results in more distancing and [less] virus concentration due to very large air volume,” he says, and vice versa in winter.
What about the “super colds?” COVID-19 lockdowns and social distancing greatly reduced people’s exposure to common viruses like those that cause colds, says Neil A. Mabbott, PhD, a professor of immunopathology at the University of Edinburgh (Scotland).
“Immunity to these common cold viruses gained through natural infection is considered to last around 8 or 9 months or so,” he says. “Each winter, when we are exposed to the new circulating variants of these viruses, our immunity receives a natural boost.”
That explains why most people get a cold that’s relatively mild. But with all the pandemic lockdowns and the use of hand sanitizers, most people had limited exposure to other viruses, including the common cold. When people emerged from lockdown, the common cold viruses were beginning to circulate again.
“Our immune systems were less able to clear the infection than previously,” Dr. Mabbott says. “As a consequence, some may have experienced increased symptoms, giving the impression of being infected with a ‘super cold.’ ”
“The colds themselves are probably not different to those we got prepandemic,” says Ian Mackay, PhD, a virologist at the University of Queensland, Brisbane, Australia. “But there might be more of them. So I doubt they are ‘super colds’ as much as they are ‘super-perfect circumstances.’ ”
The colds themselves are probably not different to those we got prepandemic. But there might be more of them.
Those super-perfect circumstances, he says, include people gathering after lockdown; a lack of immunity in new babies; viruses that have remained, even if at low levels, but continue to mutate; and our waning immunity to the range of viruses we’d normally encounter.
While lack of exposure may partly explain why some viruses become rampant out of season, it’s likely not the only reason. For example, the reduced circulation of RSV in the population as a whole also may have reduced the transfer of immunity from mothers to infants, some researchers say, making those infants more vulnerable than usual.
Interactions of viruses
Another thing that may be driving the different behavior of viruses is that the SARS-CoV-2 virus could somehow be interacting with other respiratory viruses, Dr. Schaffner says. “And if so, what sort of interactions?”
Many researchers are looking into that, and how coinfections with other respiratory diseases, including the common cold and flu, may affect the course of COVID-19. Some studies have found that the T cells – a source of deeper, cellular immunity in people – generated after a common cold “may also provide cross-protection in some people against COVID-19.”
But another study found immunity against common cold–causing coronaviruses might make COVID-19 more severe.
When researchers in the United Kingdom studied nearly 7,000 patients infected with COVID-19, including 583 also infected with RSV, flu, or adenoviruses (causing flulike or coldlike illness), those with flu or adenovirus, compared with the others, were at higher risk of death.
To be continued …
Exactly how COVID-19 will be changing what we know of other viruses is yet to be determined, too.
Even before the pandemic, Dr. Martinello says, there were already some shifts in RSV. Florida, for instance, has an RSV season longer than the rest of the country, mimicking the pattern in the tropics.
Will the atypical patterns continue? “My guess is that this will settle out,” he says, with some sort of pattern developing. At this point, there are many unknowns. “We still can’t answer whether there will be some seasonality to COVID.”
A version of this article first appeared on WebMD.com.
Children and COVID: Weekly cases top 95,000, admissions continue to rise
New pediatric COVID-19 cases increased for the third straight week as a substantial number of children under age 5 years started to receive their second doses of the vaccine.
Despite the 3-week trend, however, there are some positive signs. The new-case count for the latest reporting week (July 22-28) was over 95,000, but the 3.9% increase over the previous week’s 92,000 cases is much smaller than that week’s (July 15-21) corresponding jump of almost 22% over the July 8-14 total (75,000), according to the American Academy of Pediatrics and the Children’s Hospital Association.
On the not-so-positive side is the trend in admissions among children aged 0-17 years, which continue to climb steadily and have nearly equaled the highest rate seen during the Delta surge in 2021. The rate on July 29 was 0.46 admissions per 100,000 population, and the highest rate over the course of the Delta surge was 0.47 per 100,000, but the all-time high from the Omicron surge – 1.25 per 100,000 in mid-January – is still a long way off, based on data from the Centers for Disease Control and Prevention.
A similar situation is occurring with emergency department visits, but there is differentiation by age group. Among those aged 0-11 years, visits with diagnosed COVID made up 6.5% of all their ED visits on July 25, which was well above the high (4.0%) during the Delta surge, the CDC said.
That is not the case, however, for the older children, for whom rates are rising more slowly. Those aged 12-15 have reached 3.4% so far this summer, as have the 16- to 17-years-olds, versus Delta highs last year of around 7%, the CDC said on its COVID Data Tracker. As with admissions, though, current rates are well below the all-time Omicron high points, the CDC data show.
Joining the ranks of the fully vaccinated
Over the last 2 weeks, the first children to receive the COVID vaccine after its approval for those under age 5 years have been coming back for their second doses. Almost 50,000, about 0.3% of all those in that age group, had done so by July 27. Just over 662,000, about 3.4% of the total under-5 population, have received at least one dose, the CDC said.
Meanwhile, analysis of “data from the first several weeks following availability of the vaccine in this age group indicate high variability across states,” the AAP said in its weekly vaccination report. In the District of Columbia, 20.7% of all children under age 5 have received an initial dose as of July 27, as have 15.5% of those in Vermont and 12.5% in Massachusetts. No other state was above 10%, but Mississippi, at 0.7%, was the only one below 1%.
The older children, obviously, have a head start, so their numbers are much higher. At the state level, Vermont has the highest initial dose rate, 69%, for those aged 5-11 years, while Alabama, Mississippi, and Wyoming, at 17%, are looking up at everyone else in the country. Among children aged 12-17 years, D.C. is the highest with 100% vaccination – Massachusetts and Rhode Island are at 98% – and Wyoming is the lowest with 40%, the AAP said.
New pediatric COVID-19 cases increased for the third straight week as a substantial number of children under age 5 years started to receive their second doses of the vaccine.
Despite the 3-week trend, however, there are some positive signs. The new-case count for the latest reporting week (July 22-28) was over 95,000, but the 3.9% increase over the previous week’s 92,000 cases is much smaller than that week’s (July 15-21) corresponding jump of almost 22% over the July 8-14 total (75,000), according to the American Academy of Pediatrics and the Children’s Hospital Association.
On the not-so-positive side is the trend in admissions among children aged 0-17 years, which continue to climb steadily and have nearly equaled the highest rate seen during the Delta surge in 2021. The rate on July 29 was 0.46 admissions per 100,000 population, and the highest rate over the course of the Delta surge was 0.47 per 100,000, but the all-time high from the Omicron surge – 1.25 per 100,000 in mid-January – is still a long way off, based on data from the Centers for Disease Control and Prevention.
A similar situation is occurring with emergency department visits, but there is differentiation by age group. Among those aged 0-11 years, visits with diagnosed COVID made up 6.5% of all their ED visits on July 25, which was well above the high (4.0%) during the Delta surge, the CDC said.
That is not the case, however, for the older children, for whom rates are rising more slowly. Those aged 12-15 have reached 3.4% so far this summer, as have the 16- to 17-years-olds, versus Delta highs last year of around 7%, the CDC said on its COVID Data Tracker. As with admissions, though, current rates are well below the all-time Omicron high points, the CDC data show.
Joining the ranks of the fully vaccinated
Over the last 2 weeks, the first children to receive the COVID vaccine after its approval for those under age 5 years have been coming back for their second doses. Almost 50,000, about 0.3% of all those in that age group, had done so by July 27. Just over 662,000, about 3.4% of the total under-5 population, have received at least one dose, the CDC said.
Meanwhile, analysis of “data from the first several weeks following availability of the vaccine in this age group indicate high variability across states,” the AAP said in its weekly vaccination report. In the District of Columbia, 20.7% of all children under age 5 have received an initial dose as of July 27, as have 15.5% of those in Vermont and 12.5% in Massachusetts. No other state was above 10%, but Mississippi, at 0.7%, was the only one below 1%.
The older children, obviously, have a head start, so their numbers are much higher. At the state level, Vermont has the highest initial dose rate, 69%, for those aged 5-11 years, while Alabama, Mississippi, and Wyoming, at 17%, are looking up at everyone else in the country. Among children aged 12-17 years, D.C. is the highest with 100% vaccination – Massachusetts and Rhode Island are at 98% – and Wyoming is the lowest with 40%, the AAP said.
New pediatric COVID-19 cases increased for the third straight week as a substantial number of children under age 5 years started to receive their second doses of the vaccine.
Despite the 3-week trend, however, there are some positive signs. The new-case count for the latest reporting week (July 22-28) was over 95,000, but the 3.9% increase over the previous week’s 92,000 cases is much smaller than that week’s (July 15-21) corresponding jump of almost 22% over the July 8-14 total (75,000), according to the American Academy of Pediatrics and the Children’s Hospital Association.
On the not-so-positive side is the trend in admissions among children aged 0-17 years, which continue to climb steadily and have nearly equaled the highest rate seen during the Delta surge in 2021. The rate on July 29 was 0.46 admissions per 100,000 population, and the highest rate over the course of the Delta surge was 0.47 per 100,000, but the all-time high from the Omicron surge – 1.25 per 100,000 in mid-January – is still a long way off, based on data from the Centers for Disease Control and Prevention.
A similar situation is occurring with emergency department visits, but there is differentiation by age group. Among those aged 0-11 years, visits with diagnosed COVID made up 6.5% of all their ED visits on July 25, which was well above the high (4.0%) during the Delta surge, the CDC said.
That is not the case, however, for the older children, for whom rates are rising more slowly. Those aged 12-15 have reached 3.4% so far this summer, as have the 16- to 17-years-olds, versus Delta highs last year of around 7%, the CDC said on its COVID Data Tracker. As with admissions, though, current rates are well below the all-time Omicron high points, the CDC data show.
Joining the ranks of the fully vaccinated
Over the last 2 weeks, the first children to receive the COVID vaccine after its approval for those under age 5 years have been coming back for their second doses. Almost 50,000, about 0.3% of all those in that age group, had done so by July 27. Just over 662,000, about 3.4% of the total under-5 population, have received at least one dose, the CDC said.
Meanwhile, analysis of “data from the first several weeks following availability of the vaccine in this age group indicate high variability across states,” the AAP said in its weekly vaccination report. In the District of Columbia, 20.7% of all children under age 5 have received an initial dose as of July 27, as have 15.5% of those in Vermont and 12.5% in Massachusetts. No other state was above 10%, but Mississippi, at 0.7%, was the only one below 1%.
The older children, obviously, have a head start, so their numbers are much higher. At the state level, Vermont has the highest initial dose rate, 69%, for those aged 5-11 years, while Alabama, Mississippi, and Wyoming, at 17%, are looking up at everyone else in the country. Among children aged 12-17 years, D.C. is the highest with 100% vaccination – Massachusetts and Rhode Island are at 98% – and Wyoming is the lowest with 40%, the AAP said.
Evusheld for COVID-19: Lifesaving and free, but still few takers
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
‘Striking’ disparities in CVD deaths persist across COVID waves
Cardiovascular disease (CVD) mortality rose significantly during the COVID-19 pandemic and persists more than 2 years on and, once again, Blacks and African Americans have been disproportionately affected, an analysis of death certificates shows.
The findings “suggest that the pandemic may reverse years or decades of work aimed at reducing gaps in cardiovascular outcomes,” Sadeer G. Al-Kindi, MD, Case Western Reserve University, Cleveland, said in an interview.
Although the disparities are in line with previous research, he said, “what was surprising is the persistence of excess cardiovascular mortality approximately 2 years after the pandemic started, even during a period of low COVID-19 mortality.”
“This suggests that the pandemic resulted in a disruption of health care access and, along with disparities in COVID-19 infection and its complications, he said, “may have a long-lasting effect on health care disparities, especially among vulnerable populations.”
The study was published online in Mayo Clinic Proceedings with lead author Scott E. Janus, MD, also of Case Western Reserve University.
Impact consistently greater for Blacks
Dr. Al-Kindi and colleagues used 3,598,352 U.S. death files to investigate trends in deaths caused specifically by CVD as well as its subtypes myocardial infarction, stroke, and heart failure (HF) in 2018 and 2019 (prepandemic) and the pandemic years 2020 and 2021. Baseline demographics showed a higher percentage of older, female, and Black individuals among the CVD subtypes of interest.
Overall, there was an excess CVD mortality of 6.7% during the pandemic, compared with prepandemic years, including a 2.5% rise in MI deaths and an 8.5% rise in stroke deaths. HF mortality remained relatively steady, rising only 0.1%.
Subgroup analyses revealed “striking differences” in excess mortality between Blacks and Whites, the authors noted. Blacks had an overall excess mortality of 13.8% versus 5.1% for Whites, compared with the prepandemic years. The differences were consistent across subtypes: MI (9.6% vs. 1.0%); stroke (14.5% vs. 6.9%); and HF (5.1% vs. –1.2%; P value for all < .001).
When the investigators looked at deaths on a yearly basis with 2018 as the baseline, they found CVD deaths increased by 1.5% in 2019, 15.8% in 2020, and 13.5% in 2021 among Black Americans, compared with 0.5%, 5.1%, and 5.7%, respectively, among White Americans.
Excess deaths from MI rose by 9.5% in 2020 and by 6.7% in 2021 among Blacks but fell by 1.2% in 2020 and by 1.0% in 2021 among Whites.
Disparities in excess HF mortality were similar, rising 9.1% and 4.1% in 2020 and 2021 among Blacks, while dipping 0.1% and 0.8% in 2020 and 2021 among Whites.
The “most striking difference” was in excess stroke mortality, which doubled among Blacks compared with whites in 2020 (14.9% vs. 6.7%) and in 2021 (17.5% vs. 8.1%), according to the authors.
Awareness urged
Although the disparities were expected, “there is clear value in documenting and quantifying the magnitude of these disparities,” Amil M. Shah, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, both in Boston, said in an interview.
In addition to being observational, the main limitation of the study, he noted, is the quality and resolution of the death certificate data, which may limit the accuracy of the cause of death ascertainment and classification of race or ethnicity. “However, I think these potential inaccuracies are unlikely to materially impact the overall study findings.”
Dr. Shah, who was not involved in the study, said he would like to see additional research into the diversity and heterogeneity in risk among Black communities. “Understanding the environmental, social, and health care factors – both harmful and protective – that influence risk for CVD morbidity and mortality among Black individuals and communities offers the promise to provide actionable insights to mitigate these disparities.”
“Intervention studies testing approaches to mitigate disparities based on race/ethnicity” are also needed, he added. These may be at the policy, community, health system, or individual level, and community involvement in phases will be essential.”
Meanwhile, both Dr. Al-Kindi and Dr. Shah urged clinicians to be aware of the disparities and the need to improve access to care and address social determinants of health in vulnerable populations.
These disparities “are driven by structural factors, and are reinforced by individual behaviors. In this context, implicit bias training is important to help clinicians recognize and mitigate bias in their own practice,” Dr. Shah said. “Supporting diversity, equity, and inclusion efforts, and advocating for anti-racist policies and practices in their health systems” can also help.
Dr. Al-Kindi and Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular disease (CVD) mortality rose significantly during the COVID-19 pandemic and persists more than 2 years on and, once again, Blacks and African Americans have been disproportionately affected, an analysis of death certificates shows.
The findings “suggest that the pandemic may reverse years or decades of work aimed at reducing gaps in cardiovascular outcomes,” Sadeer G. Al-Kindi, MD, Case Western Reserve University, Cleveland, said in an interview.
Although the disparities are in line with previous research, he said, “what was surprising is the persistence of excess cardiovascular mortality approximately 2 years after the pandemic started, even during a period of low COVID-19 mortality.”
“This suggests that the pandemic resulted in a disruption of health care access and, along with disparities in COVID-19 infection and its complications, he said, “may have a long-lasting effect on health care disparities, especially among vulnerable populations.”
The study was published online in Mayo Clinic Proceedings with lead author Scott E. Janus, MD, also of Case Western Reserve University.
Impact consistently greater for Blacks
Dr. Al-Kindi and colleagues used 3,598,352 U.S. death files to investigate trends in deaths caused specifically by CVD as well as its subtypes myocardial infarction, stroke, and heart failure (HF) in 2018 and 2019 (prepandemic) and the pandemic years 2020 and 2021. Baseline demographics showed a higher percentage of older, female, and Black individuals among the CVD subtypes of interest.
Overall, there was an excess CVD mortality of 6.7% during the pandemic, compared with prepandemic years, including a 2.5% rise in MI deaths and an 8.5% rise in stroke deaths. HF mortality remained relatively steady, rising only 0.1%.
Subgroup analyses revealed “striking differences” in excess mortality between Blacks and Whites, the authors noted. Blacks had an overall excess mortality of 13.8% versus 5.1% for Whites, compared with the prepandemic years. The differences were consistent across subtypes: MI (9.6% vs. 1.0%); stroke (14.5% vs. 6.9%); and HF (5.1% vs. –1.2%; P value for all < .001).
When the investigators looked at deaths on a yearly basis with 2018 as the baseline, they found CVD deaths increased by 1.5% in 2019, 15.8% in 2020, and 13.5% in 2021 among Black Americans, compared with 0.5%, 5.1%, and 5.7%, respectively, among White Americans.
Excess deaths from MI rose by 9.5% in 2020 and by 6.7% in 2021 among Blacks but fell by 1.2% in 2020 and by 1.0% in 2021 among Whites.
Disparities in excess HF mortality were similar, rising 9.1% and 4.1% in 2020 and 2021 among Blacks, while dipping 0.1% and 0.8% in 2020 and 2021 among Whites.
The “most striking difference” was in excess stroke mortality, which doubled among Blacks compared with whites in 2020 (14.9% vs. 6.7%) and in 2021 (17.5% vs. 8.1%), according to the authors.
Awareness urged
Although the disparities were expected, “there is clear value in documenting and quantifying the magnitude of these disparities,” Amil M. Shah, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, both in Boston, said in an interview.
In addition to being observational, the main limitation of the study, he noted, is the quality and resolution of the death certificate data, which may limit the accuracy of the cause of death ascertainment and classification of race or ethnicity. “However, I think these potential inaccuracies are unlikely to materially impact the overall study findings.”
Dr. Shah, who was not involved in the study, said he would like to see additional research into the diversity and heterogeneity in risk among Black communities. “Understanding the environmental, social, and health care factors – both harmful and protective – that influence risk for CVD morbidity and mortality among Black individuals and communities offers the promise to provide actionable insights to mitigate these disparities.”
“Intervention studies testing approaches to mitigate disparities based on race/ethnicity” are also needed, he added. These may be at the policy, community, health system, or individual level, and community involvement in phases will be essential.”
Meanwhile, both Dr. Al-Kindi and Dr. Shah urged clinicians to be aware of the disparities and the need to improve access to care and address social determinants of health in vulnerable populations.
These disparities “are driven by structural factors, and are reinforced by individual behaviors. In this context, implicit bias training is important to help clinicians recognize and mitigate bias in their own practice,” Dr. Shah said. “Supporting diversity, equity, and inclusion efforts, and advocating for anti-racist policies and practices in their health systems” can also help.
Dr. Al-Kindi and Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular disease (CVD) mortality rose significantly during the COVID-19 pandemic and persists more than 2 years on and, once again, Blacks and African Americans have been disproportionately affected, an analysis of death certificates shows.
The findings “suggest that the pandemic may reverse years or decades of work aimed at reducing gaps in cardiovascular outcomes,” Sadeer G. Al-Kindi, MD, Case Western Reserve University, Cleveland, said in an interview.
Although the disparities are in line with previous research, he said, “what was surprising is the persistence of excess cardiovascular mortality approximately 2 years after the pandemic started, even during a period of low COVID-19 mortality.”
“This suggests that the pandemic resulted in a disruption of health care access and, along with disparities in COVID-19 infection and its complications, he said, “may have a long-lasting effect on health care disparities, especially among vulnerable populations.”
The study was published online in Mayo Clinic Proceedings with lead author Scott E. Janus, MD, also of Case Western Reserve University.
Impact consistently greater for Blacks
Dr. Al-Kindi and colleagues used 3,598,352 U.S. death files to investigate trends in deaths caused specifically by CVD as well as its subtypes myocardial infarction, stroke, and heart failure (HF) in 2018 and 2019 (prepandemic) and the pandemic years 2020 and 2021. Baseline demographics showed a higher percentage of older, female, and Black individuals among the CVD subtypes of interest.
Overall, there was an excess CVD mortality of 6.7% during the pandemic, compared with prepandemic years, including a 2.5% rise in MI deaths and an 8.5% rise in stroke deaths. HF mortality remained relatively steady, rising only 0.1%.
Subgroup analyses revealed “striking differences” in excess mortality between Blacks and Whites, the authors noted. Blacks had an overall excess mortality of 13.8% versus 5.1% for Whites, compared with the prepandemic years. The differences were consistent across subtypes: MI (9.6% vs. 1.0%); stroke (14.5% vs. 6.9%); and HF (5.1% vs. –1.2%; P value for all < .001).
When the investigators looked at deaths on a yearly basis with 2018 as the baseline, they found CVD deaths increased by 1.5% in 2019, 15.8% in 2020, and 13.5% in 2021 among Black Americans, compared with 0.5%, 5.1%, and 5.7%, respectively, among White Americans.
Excess deaths from MI rose by 9.5% in 2020 and by 6.7% in 2021 among Blacks but fell by 1.2% in 2020 and by 1.0% in 2021 among Whites.
Disparities in excess HF mortality were similar, rising 9.1% and 4.1% in 2020 and 2021 among Blacks, while dipping 0.1% and 0.8% in 2020 and 2021 among Whites.
The “most striking difference” was in excess stroke mortality, which doubled among Blacks compared with whites in 2020 (14.9% vs. 6.7%) and in 2021 (17.5% vs. 8.1%), according to the authors.
Awareness urged
Although the disparities were expected, “there is clear value in documenting and quantifying the magnitude of these disparities,” Amil M. Shah, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, both in Boston, said in an interview.
In addition to being observational, the main limitation of the study, he noted, is the quality and resolution of the death certificate data, which may limit the accuracy of the cause of death ascertainment and classification of race or ethnicity. “However, I think these potential inaccuracies are unlikely to materially impact the overall study findings.”
Dr. Shah, who was not involved in the study, said he would like to see additional research into the diversity and heterogeneity in risk among Black communities. “Understanding the environmental, social, and health care factors – both harmful and protective – that influence risk for CVD morbidity and mortality among Black individuals and communities offers the promise to provide actionable insights to mitigate these disparities.”
“Intervention studies testing approaches to mitigate disparities based on race/ethnicity” are also needed, he added. These may be at the policy, community, health system, or individual level, and community involvement in phases will be essential.”
Meanwhile, both Dr. Al-Kindi and Dr. Shah urged clinicians to be aware of the disparities and the need to improve access to care and address social determinants of health in vulnerable populations.
These disparities “are driven by structural factors, and are reinforced by individual behaviors. In this context, implicit bias training is important to help clinicians recognize and mitigate bias in their own practice,” Dr. Shah said. “Supporting diversity, equity, and inclusion efforts, and advocating for anti-racist policies and practices in their health systems” can also help.
Dr. Al-Kindi and Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MAYO CLINIC PROCEEDINGS
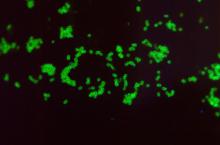
Potentially deadly bacteria detected in U.S. soil
, according to a new alert from the Centers for Disease Control and Prevention.
The bacteria, Burkholderia pseudomallei, was found along the Gulf Coast region in southern Mississippi. Typically, the bacteria are in tropical and subtropical climates, especially in parts of Southeast Asia, northern Australia, Central America, South America, Puerto Rico, and the U.S. Virgin Islands.
The bacteria can cause melioidosis, a rare and serious infectious disease that spreads to animals and humans through contact with contaminated soil and water via cuts, wounds, mucous membranes, breathing the bacteria in, or eating or drinking it. Worldwide, the disease is fatal in 10%-50% of those who become infected.
CDC and state officials are investigating the samples to find out how widespread the bacteria are within the United States. So far, modeling suggests that the environmental conditions on the Gulf Coast support the growth of B. pseudomallei.
“It is unclear how long the bacteria has been in the environment and where else it might be found in the U.S.,” according to the CDC statement. “CDC is alerting clinicians throughout the country of this discovery through a national health advisory, reminding them to be aware of the signs and symptoms of melioidosis and to consider melioidosis in patients that present with symptoms of the disease.”
Two unrelated people who live near the Gulf Coast region of Mississippi became sick with melioidosis recently – one in July 2020 and one in May 2022. Neither had traveled outside of the United States. The cases led the CDC and the Mississippi State Department of Health to collect environmental samples and test household products at the patients’ homes in June 2022. Three of the samples taken from soil and puddle water in the 2020 case tested positive for the bacteria.
Genomic sequencing revealed that both patients were infected with the same strain of the bacteria from the Western Hemisphere. They were hospitalized with sepsis due to pneumonia and had known risk factors for melioidosis. Both patients recovered after they were treated with antibiotics.
An average of 12 melioidosis cases are diagnosed in the United States each year, with most in people with recent travel to a country where the bacteria is endemic, or regularly found. Cases have also been linked to contaminated products imported from endemic countries. In late 2021, four cases in four states – Georgia, Kansas, Minnesota, and Texas – were linked to a contaminated aromatherapy spray that was imported, and Walmart issued a recall in November of that year, according to a CDC announcement. Two of the four people died.
Given the small number of cases found in the United States, the CDC believes the risk of melioidosis for the general population continues to be “very low,” and the risk of person-to-person spread is considered “extremely low.” But people who live on the Gulf Coast of Mississippi and who have health conditions that may put them at a higher risk, such as diabetes, chronic kidney disease, chronic lung disease, excessive alcohol use, and immunosuppressive conditions, should protect themselves.
The CDC recommends avoiding contact with soil or muddy water, particularly after heavy rains, and protecting open wounds with waterproof bandages. People should also wear waterproof boots when gardening, working in the yard, or doing agricultural work, which can prevent infection through the feet and lower legs, especially after flooding or storms. People should also wear gloves to protect their hands when working directly with soil.
Melioidosis has a wide range of symptoms, including fever, joint pain, headaches, coughing, chest pain, and belly pain. It can also cause conditions such as pneumonia, abscesses, and blood infections. The disease can infect any organ, including the brain. In most cases, symptoms appear within 1-21 days after exposure, with an average of 7 days after exposure.
The CDC’s health advisory for health professionals and public health officials shows that melioidosis is now considered to be locally endemic in areas of the Gulf Coast region in Mississippi.
“Once well-established in the soil, B. pseudomallei cannot feasibly be removed from the soil,” according to the advisory. “Public health efforts should focus primarily on improving identification of cases so that appropriate treatment can be administered.”
A version of this article first appeared on WebMD.com.
, according to a new alert from the Centers for Disease Control and Prevention.
The bacteria, Burkholderia pseudomallei, was found along the Gulf Coast region in southern Mississippi. Typically, the bacteria are in tropical and subtropical climates, especially in parts of Southeast Asia, northern Australia, Central America, South America, Puerto Rico, and the U.S. Virgin Islands.
The bacteria can cause melioidosis, a rare and serious infectious disease that spreads to animals and humans through contact with contaminated soil and water via cuts, wounds, mucous membranes, breathing the bacteria in, or eating or drinking it. Worldwide, the disease is fatal in 10%-50% of those who become infected.
CDC and state officials are investigating the samples to find out how widespread the bacteria are within the United States. So far, modeling suggests that the environmental conditions on the Gulf Coast support the growth of B. pseudomallei.
“It is unclear how long the bacteria has been in the environment and where else it might be found in the U.S.,” according to the CDC statement. “CDC is alerting clinicians throughout the country of this discovery through a national health advisory, reminding them to be aware of the signs and symptoms of melioidosis and to consider melioidosis in patients that present with symptoms of the disease.”
Two unrelated people who live near the Gulf Coast region of Mississippi became sick with melioidosis recently – one in July 2020 and one in May 2022. Neither had traveled outside of the United States. The cases led the CDC and the Mississippi State Department of Health to collect environmental samples and test household products at the patients’ homes in June 2022. Three of the samples taken from soil and puddle water in the 2020 case tested positive for the bacteria.
Genomic sequencing revealed that both patients were infected with the same strain of the bacteria from the Western Hemisphere. They were hospitalized with sepsis due to pneumonia and had known risk factors for melioidosis. Both patients recovered after they were treated with antibiotics.
An average of 12 melioidosis cases are diagnosed in the United States each year, with most in people with recent travel to a country where the bacteria is endemic, or regularly found. Cases have also been linked to contaminated products imported from endemic countries. In late 2021, four cases in four states – Georgia, Kansas, Minnesota, and Texas – were linked to a contaminated aromatherapy spray that was imported, and Walmart issued a recall in November of that year, according to a CDC announcement. Two of the four people died.
Given the small number of cases found in the United States, the CDC believes the risk of melioidosis for the general population continues to be “very low,” and the risk of person-to-person spread is considered “extremely low.” But people who live on the Gulf Coast of Mississippi and who have health conditions that may put them at a higher risk, such as diabetes, chronic kidney disease, chronic lung disease, excessive alcohol use, and immunosuppressive conditions, should protect themselves.
The CDC recommends avoiding contact with soil or muddy water, particularly after heavy rains, and protecting open wounds with waterproof bandages. People should also wear waterproof boots when gardening, working in the yard, or doing agricultural work, which can prevent infection through the feet and lower legs, especially after flooding or storms. People should also wear gloves to protect their hands when working directly with soil.
Melioidosis has a wide range of symptoms, including fever, joint pain, headaches, coughing, chest pain, and belly pain. It can also cause conditions such as pneumonia, abscesses, and blood infections. The disease can infect any organ, including the brain. In most cases, symptoms appear within 1-21 days after exposure, with an average of 7 days after exposure.
The CDC’s health advisory for health professionals and public health officials shows that melioidosis is now considered to be locally endemic in areas of the Gulf Coast region in Mississippi.
“Once well-established in the soil, B. pseudomallei cannot feasibly be removed from the soil,” according to the advisory. “Public health efforts should focus primarily on improving identification of cases so that appropriate treatment can be administered.”
A version of this article first appeared on WebMD.com.
, according to a new alert from the Centers for Disease Control and Prevention.
The bacteria, Burkholderia pseudomallei, was found along the Gulf Coast region in southern Mississippi. Typically, the bacteria are in tropical and subtropical climates, especially in parts of Southeast Asia, northern Australia, Central America, South America, Puerto Rico, and the U.S. Virgin Islands.
The bacteria can cause melioidosis, a rare and serious infectious disease that spreads to animals and humans through contact with contaminated soil and water via cuts, wounds, mucous membranes, breathing the bacteria in, or eating or drinking it. Worldwide, the disease is fatal in 10%-50% of those who become infected.
CDC and state officials are investigating the samples to find out how widespread the bacteria are within the United States. So far, modeling suggests that the environmental conditions on the Gulf Coast support the growth of B. pseudomallei.
“It is unclear how long the bacteria has been in the environment and where else it might be found in the U.S.,” according to the CDC statement. “CDC is alerting clinicians throughout the country of this discovery through a national health advisory, reminding them to be aware of the signs and symptoms of melioidosis and to consider melioidosis in patients that present with symptoms of the disease.”
Two unrelated people who live near the Gulf Coast region of Mississippi became sick with melioidosis recently – one in July 2020 and one in May 2022. Neither had traveled outside of the United States. The cases led the CDC and the Mississippi State Department of Health to collect environmental samples and test household products at the patients’ homes in June 2022. Three of the samples taken from soil and puddle water in the 2020 case tested positive for the bacteria.
Genomic sequencing revealed that both patients were infected with the same strain of the bacteria from the Western Hemisphere. They were hospitalized with sepsis due to pneumonia and had known risk factors for melioidosis. Both patients recovered after they were treated with antibiotics.
An average of 12 melioidosis cases are diagnosed in the United States each year, with most in people with recent travel to a country where the bacteria is endemic, or regularly found. Cases have also been linked to contaminated products imported from endemic countries. In late 2021, four cases in four states – Georgia, Kansas, Minnesota, and Texas – were linked to a contaminated aromatherapy spray that was imported, and Walmart issued a recall in November of that year, according to a CDC announcement. Two of the four people died.
Given the small number of cases found in the United States, the CDC believes the risk of melioidosis for the general population continues to be “very low,” and the risk of person-to-person spread is considered “extremely low.” But people who live on the Gulf Coast of Mississippi and who have health conditions that may put them at a higher risk, such as diabetes, chronic kidney disease, chronic lung disease, excessive alcohol use, and immunosuppressive conditions, should protect themselves.
The CDC recommends avoiding contact with soil or muddy water, particularly after heavy rains, and protecting open wounds with waterproof bandages. People should also wear waterproof boots when gardening, working in the yard, or doing agricultural work, which can prevent infection through the feet and lower legs, especially after flooding or storms. People should also wear gloves to protect their hands when working directly with soil.
Melioidosis has a wide range of symptoms, including fever, joint pain, headaches, coughing, chest pain, and belly pain. It can also cause conditions such as pneumonia, abscesses, and blood infections. The disease can infect any organ, including the brain. In most cases, symptoms appear within 1-21 days after exposure, with an average of 7 days after exposure.
The CDC’s health advisory for health professionals and public health officials shows that melioidosis is now considered to be locally endemic in areas of the Gulf Coast region in Mississippi.
“Once well-established in the soil, B. pseudomallei cannot feasibly be removed from the soil,” according to the advisory. “Public health efforts should focus primarily on improving identification of cases so that appropriate treatment can be administered.”
A version of this article first appeared on WebMD.com.
More evidence that COVID-19 started in Wuhan marketplace
The original spread of the virus was a one-two punch, the studies found. Twice, the virus jumped from animals to humans. Virus genetics and outbreak modeling in one study revealed two strains released a few weeks apart in November and December 2019.
“Now I realize it sounds like I just said that a once-in-a-generation event happened twice in short succession, and pandemics are indeed rare,” Joel O. Wertheim, PhD, said at a briefing sponsored by the American Association for the Advancement of Science.
A unique storm of factors had to be present for the outbreak to blow up into a pandemic: Animals carrying a virus that could spread to humans, close human contact with these animals, and a city large enough for the infection to take off before it could be contained are examples.
Unluckily for us humans, this coronavirus – SARS-CoV-2 – is a “generalist virus” capable of infecting many animals, including humans.
“Once all the conditions are in place ... the barriers to spillover have been lowered,” said Dr. Wertheim, a researcher in genetic and molecular networks at the University of California, San Diego. In fact, beyond the two strains of the virus that took hold, there were likely up to two dozen more times where people got the virus but did not spread it far and wide, and it died out.
Overall, the odds were against the virus – 78% of the time, the “introduction” to humans was likely to go extinct, the study showed.
The research revealed the COVID-19 pandemic started small.
“Our model shows that there were likely only a few dozen infections, and only several hospitalizations due to COVID-19, by early December,” said Jonathan Pekar, a graduate student working with Dr. Wertheim.
In Wuhan in late 2019, Mr. Pekar said, there was not a single positive coronavirus sample from thousands of samples from healthy blood donors tested between September and December. Likewise, not one blood sample from patients hospitalized with flu-like illness from October to December 2019 tested positive for SARS-CoV-2.
Mapping the outbreak
A second study published in the journal Science mapped out the earliest COVID-19 cases. This effort showed a tight cluster around the wholesale seafood market inside Wuhan, a city of 11 million residents.
When researchers tried other scenarios – modeling outbreaks in other parts of the city – the pattern did not hold. Again, the Wuhan market appeared to be ground zero for the start of the pandemic.
Michael Worobey, PhD, and colleagues used data from Chinese scientists and the World Health Organization for the study.
“There was this extraordinary pattern where the highest density of cases was both extremely near to and very centered on this market,” said Dr. Worobey, head of ecology and evolutionary biology at the University of Arizona, Tucson.
The highest density of cases, in a city of 8,000 square kilometers, was a “very, very small area of about a third of a kilometer square,” he said.
The outbreak pattern showed the Wuhan market “smack dab in the middle.”
So if it started with infected workers at the market, how did it spread from there? It’s likely the virus got into the community as the vendors at the market went to local shops, infecting people in those stores. Then local community members not linked to the market started getting the virus, Dr. Worobey said.
The investigators also identified which stalls in the market were most likely involved, a sort of internal clustering. “That clustering is very, very specifically in the parts of the market where ... they were selling wildlife, including, for example, raccoon dogs and other animals that we know are susceptible to infection with SARS-CoV-2,” said Kristian Andersen, PhD, director of infectious disease genomics at the Scripps Research Institute in La Jolla, Calif.
What remains unknown is which animal or animals carried the virus, although the raccoon dog – an animal similar to a fox that is native to parts of Asia – remains central to most theories. In addition, many of the farms supplying animals to the market have since been closed, making it challenging for researchers to figure out exactly where infected animals came from.
“We don’t know necessarily, but raccoon dogs were sold at this market all the way up to the beginning of the pandemic,” Dr. Andersen said.
Not ruling out other theories
People who believe SARS-CoV-2 was released from a laboratory in China at first included Dr. Worobey himself. “I’ve in the past been much more open to the lab leak idea,” he said. “And published that in a letter in Science” in November 2021.
The letter was “much more influential than I thought it would be in ways that I think it turned out to be quite damaging,” he said. As more evidence emerged since then, Dr. Worobey said he came around to the Wuhan market source theory.
Dr. Andersen agreed he was more open to the lab-leak theory at first. “I was quite convinced of the lab leak myself until we dove into this very carefully and looked at it much closer,” he said. Newer evidence convinced him “that actually, the data points to this particular market.”
“Have we disproved the lab-leak theory? No,” Dr. Anderson said. “Will we ever be able to? No.” But the Wuhan market origin scenario is more plausible. “I would say these two papers combined present the strongest evidence of that to date.”
Identifying the source of the outbreak that led to the COVID-19 pandemic is based in science, Dr. Andersen said. “What we’re trying to understand is the origin of the pandemic. We’re not trying to place blame.”
Future directions
“With pandemics being pandemics, they affect all of us,” Dr. Andersen said. “We can’t prevent these kinds of events that led to the COVID-19 pandemic. But what we can hope to do is to prevent outbreaks from becoming pandemics.”
Rapid reporting of data and cooperation are needed going forward, Dr. Andersen said. Very strong surveillance systems, including wastewater surveillance, could help monitor for SARS-CoV-2, and other pathogens of potential concern in the future as well.
It should be standard practice for medical professionals to be on alert for unusual respiratory infections too, the researchers said.
“It’s a bloody lucky thing that the doctors at the Shinwa hospital were so on the ball, that they noticed that these cases were something unusual at the end of December,” Dr. Worobey said. “It didn’t have to work out that way.”
A version of this article first appeared on WebMD.com.
The original spread of the virus was a one-two punch, the studies found. Twice, the virus jumped from animals to humans. Virus genetics and outbreak modeling in one study revealed two strains released a few weeks apart in November and December 2019.
“Now I realize it sounds like I just said that a once-in-a-generation event happened twice in short succession, and pandemics are indeed rare,” Joel O. Wertheim, PhD, said at a briefing sponsored by the American Association for the Advancement of Science.
A unique storm of factors had to be present for the outbreak to blow up into a pandemic: Animals carrying a virus that could spread to humans, close human contact with these animals, and a city large enough for the infection to take off before it could be contained are examples.
Unluckily for us humans, this coronavirus – SARS-CoV-2 – is a “generalist virus” capable of infecting many animals, including humans.
“Once all the conditions are in place ... the barriers to spillover have been lowered,” said Dr. Wertheim, a researcher in genetic and molecular networks at the University of California, San Diego. In fact, beyond the two strains of the virus that took hold, there were likely up to two dozen more times where people got the virus but did not spread it far and wide, and it died out.
Overall, the odds were against the virus – 78% of the time, the “introduction” to humans was likely to go extinct, the study showed.
The research revealed the COVID-19 pandemic started small.
“Our model shows that there were likely only a few dozen infections, and only several hospitalizations due to COVID-19, by early December,” said Jonathan Pekar, a graduate student working with Dr. Wertheim.
In Wuhan in late 2019, Mr. Pekar said, there was not a single positive coronavirus sample from thousands of samples from healthy blood donors tested between September and December. Likewise, not one blood sample from patients hospitalized with flu-like illness from October to December 2019 tested positive for SARS-CoV-2.
Mapping the outbreak
A second study published in the journal Science mapped out the earliest COVID-19 cases. This effort showed a tight cluster around the wholesale seafood market inside Wuhan, a city of 11 million residents.
When researchers tried other scenarios – modeling outbreaks in other parts of the city – the pattern did not hold. Again, the Wuhan market appeared to be ground zero for the start of the pandemic.
Michael Worobey, PhD, and colleagues used data from Chinese scientists and the World Health Organization for the study.
“There was this extraordinary pattern where the highest density of cases was both extremely near to and very centered on this market,” said Dr. Worobey, head of ecology and evolutionary biology at the University of Arizona, Tucson.
The highest density of cases, in a city of 8,000 square kilometers, was a “very, very small area of about a third of a kilometer square,” he said.
The outbreak pattern showed the Wuhan market “smack dab in the middle.”
So if it started with infected workers at the market, how did it spread from there? It’s likely the virus got into the community as the vendors at the market went to local shops, infecting people in those stores. Then local community members not linked to the market started getting the virus, Dr. Worobey said.
The investigators also identified which stalls in the market were most likely involved, a sort of internal clustering. “That clustering is very, very specifically in the parts of the market where ... they were selling wildlife, including, for example, raccoon dogs and other animals that we know are susceptible to infection with SARS-CoV-2,” said Kristian Andersen, PhD, director of infectious disease genomics at the Scripps Research Institute in La Jolla, Calif.
What remains unknown is which animal or animals carried the virus, although the raccoon dog – an animal similar to a fox that is native to parts of Asia – remains central to most theories. In addition, many of the farms supplying animals to the market have since been closed, making it challenging for researchers to figure out exactly where infected animals came from.
“We don’t know necessarily, but raccoon dogs were sold at this market all the way up to the beginning of the pandemic,” Dr. Andersen said.
Not ruling out other theories
People who believe SARS-CoV-2 was released from a laboratory in China at first included Dr. Worobey himself. “I’ve in the past been much more open to the lab leak idea,” he said. “And published that in a letter in Science” in November 2021.
The letter was “much more influential than I thought it would be in ways that I think it turned out to be quite damaging,” he said. As more evidence emerged since then, Dr. Worobey said he came around to the Wuhan market source theory.
Dr. Andersen agreed he was more open to the lab-leak theory at first. “I was quite convinced of the lab leak myself until we dove into this very carefully and looked at it much closer,” he said. Newer evidence convinced him “that actually, the data points to this particular market.”
“Have we disproved the lab-leak theory? No,” Dr. Anderson said. “Will we ever be able to? No.” But the Wuhan market origin scenario is more plausible. “I would say these two papers combined present the strongest evidence of that to date.”
Identifying the source of the outbreak that led to the COVID-19 pandemic is based in science, Dr. Andersen said. “What we’re trying to understand is the origin of the pandemic. We’re not trying to place blame.”
Future directions
“With pandemics being pandemics, they affect all of us,” Dr. Andersen said. “We can’t prevent these kinds of events that led to the COVID-19 pandemic. But what we can hope to do is to prevent outbreaks from becoming pandemics.”
Rapid reporting of data and cooperation are needed going forward, Dr. Andersen said. Very strong surveillance systems, including wastewater surveillance, could help monitor for SARS-CoV-2, and other pathogens of potential concern in the future as well.
It should be standard practice for medical professionals to be on alert for unusual respiratory infections too, the researchers said.
“It’s a bloody lucky thing that the doctors at the Shinwa hospital were so on the ball, that they noticed that these cases were something unusual at the end of December,” Dr. Worobey said. “It didn’t have to work out that way.”
A version of this article first appeared on WebMD.com.
The original spread of the virus was a one-two punch, the studies found. Twice, the virus jumped from animals to humans. Virus genetics and outbreak modeling in one study revealed two strains released a few weeks apart in November and December 2019.
“Now I realize it sounds like I just said that a once-in-a-generation event happened twice in short succession, and pandemics are indeed rare,” Joel O. Wertheim, PhD, said at a briefing sponsored by the American Association for the Advancement of Science.
A unique storm of factors had to be present for the outbreak to blow up into a pandemic: Animals carrying a virus that could spread to humans, close human contact with these animals, and a city large enough for the infection to take off before it could be contained are examples.
Unluckily for us humans, this coronavirus – SARS-CoV-2 – is a “generalist virus” capable of infecting many animals, including humans.
“Once all the conditions are in place ... the barriers to spillover have been lowered,” said Dr. Wertheim, a researcher in genetic and molecular networks at the University of California, San Diego. In fact, beyond the two strains of the virus that took hold, there were likely up to two dozen more times where people got the virus but did not spread it far and wide, and it died out.
Overall, the odds were against the virus – 78% of the time, the “introduction” to humans was likely to go extinct, the study showed.
The research revealed the COVID-19 pandemic started small.
“Our model shows that there were likely only a few dozen infections, and only several hospitalizations due to COVID-19, by early December,” said Jonathan Pekar, a graduate student working with Dr. Wertheim.
In Wuhan in late 2019, Mr. Pekar said, there was not a single positive coronavirus sample from thousands of samples from healthy blood donors tested between September and December. Likewise, not one blood sample from patients hospitalized with flu-like illness from October to December 2019 tested positive for SARS-CoV-2.
Mapping the outbreak
A second study published in the journal Science mapped out the earliest COVID-19 cases. This effort showed a tight cluster around the wholesale seafood market inside Wuhan, a city of 11 million residents.
When researchers tried other scenarios – modeling outbreaks in other parts of the city – the pattern did not hold. Again, the Wuhan market appeared to be ground zero for the start of the pandemic.
Michael Worobey, PhD, and colleagues used data from Chinese scientists and the World Health Organization for the study.
“There was this extraordinary pattern where the highest density of cases was both extremely near to and very centered on this market,” said Dr. Worobey, head of ecology and evolutionary biology at the University of Arizona, Tucson.
The highest density of cases, in a city of 8,000 square kilometers, was a “very, very small area of about a third of a kilometer square,” he said.
The outbreak pattern showed the Wuhan market “smack dab in the middle.”
So if it started with infected workers at the market, how did it spread from there? It’s likely the virus got into the community as the vendors at the market went to local shops, infecting people in those stores. Then local community members not linked to the market started getting the virus, Dr. Worobey said.
The investigators also identified which stalls in the market were most likely involved, a sort of internal clustering. “That clustering is very, very specifically in the parts of the market where ... they were selling wildlife, including, for example, raccoon dogs and other animals that we know are susceptible to infection with SARS-CoV-2,” said Kristian Andersen, PhD, director of infectious disease genomics at the Scripps Research Institute in La Jolla, Calif.
What remains unknown is which animal or animals carried the virus, although the raccoon dog – an animal similar to a fox that is native to parts of Asia – remains central to most theories. In addition, many of the farms supplying animals to the market have since been closed, making it challenging for researchers to figure out exactly where infected animals came from.
“We don’t know necessarily, but raccoon dogs were sold at this market all the way up to the beginning of the pandemic,” Dr. Andersen said.
Not ruling out other theories
People who believe SARS-CoV-2 was released from a laboratory in China at first included Dr. Worobey himself. “I’ve in the past been much more open to the lab leak idea,” he said. “And published that in a letter in Science” in November 2021.
The letter was “much more influential than I thought it would be in ways that I think it turned out to be quite damaging,” he said. As more evidence emerged since then, Dr. Worobey said he came around to the Wuhan market source theory.
Dr. Andersen agreed he was more open to the lab-leak theory at first. “I was quite convinced of the lab leak myself until we dove into this very carefully and looked at it much closer,” he said. Newer evidence convinced him “that actually, the data points to this particular market.”
“Have we disproved the lab-leak theory? No,” Dr. Anderson said. “Will we ever be able to? No.” But the Wuhan market origin scenario is more plausible. “I would say these two papers combined present the strongest evidence of that to date.”
Identifying the source of the outbreak that led to the COVID-19 pandemic is based in science, Dr. Andersen said. “What we’re trying to understand is the origin of the pandemic. We’re not trying to place blame.”
Future directions
“With pandemics being pandemics, they affect all of us,” Dr. Andersen said. “We can’t prevent these kinds of events that led to the COVID-19 pandemic. But what we can hope to do is to prevent outbreaks from becoming pandemics.”
Rapid reporting of data and cooperation are needed going forward, Dr. Andersen said. Very strong surveillance systems, including wastewater surveillance, could help monitor for SARS-CoV-2, and other pathogens of potential concern in the future as well.
It should be standard practice for medical professionals to be on alert for unusual respiratory infections too, the researchers said.
“It’s a bloody lucky thing that the doctors at the Shinwa hospital were so on the ball, that they noticed that these cases were something unusual at the end of December,” Dr. Worobey said. “It didn’t have to work out that way.”
A version of this article first appeared on WebMD.com.
Pandemic tied to misdiagnosis of rare pneumonia
Psittacosis, a rare disease, has been underdiagnosed or misdiagnosed during the COVID-19 pandemic, likely because the symptoms of the disease are similar to COVID-19 symptoms, researchers suggest on the basis of data from 32 individuals.
Diagnosis of and screening for COVID-19 continues to increase; however, cases of atypical pneumonia caused by uncommon pathogens, which presents with similar symptoms, may be missed, wrote Qiaoqiao Yin, MS, of Zhejiang Provincial People’s Hospital, China, and colleagues.
“The clinical manifestations of human psittacosis can present as rapidly progressing severe pneumonia, acute respiratory distress syndrome, sepsis, and multiple organ failure,” but human cases have not been well studied, they say.
In a study published in the International Journal of Infectious Diseases, the researchers reviewed data from 32 adults diagnosed with Chlamydia psittaci pneumonia during the COVID-19 pandemic between April 2020 and June 2021 in China. The median age of the patients was 63 years, 20 were men, and 20 had underlying diseases.
A total of 17 patients presented with fever, cough, and expectoration of yellow-white sputum. At the time of hospital admission, three patients had myalgia, two had headache, and two had hypertension. The patients were originally suspected of having COVID-19.
all of which could be observed in COVID-19 patients as well, the researchers wrote.
Reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) testing were used to rule out COVID-19. The researchers then used metagenomic next-generation sequencing (mNGS) to identify the disease-causing pathogens. They collected 18 bronchoalveolar lavage fluid (BALF) samples, 9 peripheral blood samples, and 5 sputum samples. The mNGS identified C. psittaci as the suspected pathogen within 48 hours. Suspected C. psittaci infections were confirmed by endpoint PCR for the BALF and sputum samples and six of nine blood samples, “indicating a lower sensitivity of PCR compared to mNGS for blood samples,” the researchers say. No other potential pathogens were identified.
Psittacosis is common in birds but is rare in humans. C. psittaci is responsible for 1%-8% of cases involving community-acquired pneumonia in China, the researchers note. Although poultry is a source of infection, 25 of the patients in the study did not report a history of exposure to poultry or pigeons at the time of their initial hospital admission. Many patients may be unaware of exposures to poultry, which further complicates the C. psittaci diagnosis, they note.
All patients were treated with doxycycline-based regimens and showed improvement.
The findings were limited by several factors, including the lack of a definitive diagnostic tool for C. psittaci and the lack of convalescent serum samples to confirm cases, the researchers note. In addition, molecular detections for PCR are unavailable in most hospitals in China, they say. The results represent the largest known collection of suspected C. psittaci pneumonia cases and highlight the need for clinician vigilance and awareness of this rare condition, especially in light of the potential for misdiagnosis during the ongoing COVID-19 pandemic, they conclude.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psittacosis, a rare disease, has been underdiagnosed or misdiagnosed during the COVID-19 pandemic, likely because the symptoms of the disease are similar to COVID-19 symptoms, researchers suggest on the basis of data from 32 individuals.
Diagnosis of and screening for COVID-19 continues to increase; however, cases of atypical pneumonia caused by uncommon pathogens, which presents with similar symptoms, may be missed, wrote Qiaoqiao Yin, MS, of Zhejiang Provincial People’s Hospital, China, and colleagues.
“The clinical manifestations of human psittacosis can present as rapidly progressing severe pneumonia, acute respiratory distress syndrome, sepsis, and multiple organ failure,” but human cases have not been well studied, they say.
In a study published in the International Journal of Infectious Diseases, the researchers reviewed data from 32 adults diagnosed with Chlamydia psittaci pneumonia during the COVID-19 pandemic between April 2020 and June 2021 in China. The median age of the patients was 63 years, 20 were men, and 20 had underlying diseases.
A total of 17 patients presented with fever, cough, and expectoration of yellow-white sputum. At the time of hospital admission, three patients had myalgia, two had headache, and two had hypertension. The patients were originally suspected of having COVID-19.
all of which could be observed in COVID-19 patients as well, the researchers wrote.
Reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) testing were used to rule out COVID-19. The researchers then used metagenomic next-generation sequencing (mNGS) to identify the disease-causing pathogens. They collected 18 bronchoalveolar lavage fluid (BALF) samples, 9 peripheral blood samples, and 5 sputum samples. The mNGS identified C. psittaci as the suspected pathogen within 48 hours. Suspected C. psittaci infections were confirmed by endpoint PCR for the BALF and sputum samples and six of nine blood samples, “indicating a lower sensitivity of PCR compared to mNGS for blood samples,” the researchers say. No other potential pathogens were identified.
Psittacosis is common in birds but is rare in humans. C. psittaci is responsible for 1%-8% of cases involving community-acquired pneumonia in China, the researchers note. Although poultry is a source of infection, 25 of the patients in the study did not report a history of exposure to poultry or pigeons at the time of their initial hospital admission. Many patients may be unaware of exposures to poultry, which further complicates the C. psittaci diagnosis, they note.
All patients were treated with doxycycline-based regimens and showed improvement.
The findings were limited by several factors, including the lack of a definitive diagnostic tool for C. psittaci and the lack of convalescent serum samples to confirm cases, the researchers note. In addition, molecular detections for PCR are unavailable in most hospitals in China, they say. The results represent the largest known collection of suspected C. psittaci pneumonia cases and highlight the need for clinician vigilance and awareness of this rare condition, especially in light of the potential for misdiagnosis during the ongoing COVID-19 pandemic, they conclude.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psittacosis, a rare disease, has been underdiagnosed or misdiagnosed during the COVID-19 pandemic, likely because the symptoms of the disease are similar to COVID-19 symptoms, researchers suggest on the basis of data from 32 individuals.
Diagnosis of and screening for COVID-19 continues to increase; however, cases of atypical pneumonia caused by uncommon pathogens, which presents with similar symptoms, may be missed, wrote Qiaoqiao Yin, MS, of Zhejiang Provincial People’s Hospital, China, and colleagues.
“The clinical manifestations of human psittacosis can present as rapidly progressing severe pneumonia, acute respiratory distress syndrome, sepsis, and multiple organ failure,” but human cases have not been well studied, they say.
In a study published in the International Journal of Infectious Diseases, the researchers reviewed data from 32 adults diagnosed with Chlamydia psittaci pneumonia during the COVID-19 pandemic between April 2020 and June 2021 in China. The median age of the patients was 63 years, 20 were men, and 20 had underlying diseases.
A total of 17 patients presented with fever, cough, and expectoration of yellow-white sputum. At the time of hospital admission, three patients had myalgia, two had headache, and two had hypertension. The patients were originally suspected of having COVID-19.
all of which could be observed in COVID-19 patients as well, the researchers wrote.
Reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) testing were used to rule out COVID-19. The researchers then used metagenomic next-generation sequencing (mNGS) to identify the disease-causing pathogens. They collected 18 bronchoalveolar lavage fluid (BALF) samples, 9 peripheral blood samples, and 5 sputum samples. The mNGS identified C. psittaci as the suspected pathogen within 48 hours. Suspected C. psittaci infections were confirmed by endpoint PCR for the BALF and sputum samples and six of nine blood samples, “indicating a lower sensitivity of PCR compared to mNGS for blood samples,” the researchers say. No other potential pathogens were identified.
Psittacosis is common in birds but is rare in humans. C. psittaci is responsible for 1%-8% of cases involving community-acquired pneumonia in China, the researchers note. Although poultry is a source of infection, 25 of the patients in the study did not report a history of exposure to poultry or pigeons at the time of their initial hospital admission. Many patients may be unaware of exposures to poultry, which further complicates the C. psittaci diagnosis, they note.
All patients were treated with doxycycline-based regimens and showed improvement.
The findings were limited by several factors, including the lack of a definitive diagnostic tool for C. psittaci and the lack of convalescent serum samples to confirm cases, the researchers note. In addition, molecular detections for PCR are unavailable in most hospitals in China, they say. The results represent the largest known collection of suspected C. psittaci pneumonia cases and highlight the need for clinician vigilance and awareness of this rare condition, especially in light of the potential for misdiagnosis during the ongoing COVID-19 pandemic, they conclude.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Linezolid succeeds against gram-positive bacterial infections in ICU
Approximately 80% of patients in intensive care showed clinical improvement in gram-positive bacterial infections after treatment with linezolid, based on data from more than 300 individuals.
Bacterial infections remain a challenge in the management of critically ill patients, as many gram-positive pathogens have become resistant to multiple drug options, Aijia Ma, MD, of West China Hospital of Sichuan University, and colleagues wrote.
Linezolid has demonstrated effectiveness against MRSA and skin and soft-tissue infections (SSTIs), but its use in critically ill patients with gram-positive infections in the ICU has not been characterized, they said.
In a multicenter, real-world study published in the Journal of Intensive Medicine, the researchers reviewed data from 52 hospitals between June 2018 and December 2019. The study population included 366 patients admitted to the ICU with a clinical or laboratory diagnosis of a gram-positive bacterial infection. Patients were treated with linezolid injections (200 mg/100 mL) and followed up once a day until 48 hours after discontinuing therapy, transferring out of the ICU, or death. Most of the patients (243) were older than 65 years; 90 were aged 18-65 years, and 30 were younger than 18 years. Approximately two-thirds (67%) were men. The primary outcome of clinical efficacy was success (cured or improved).
Linezolid was used as second-line and first-line treatment in 232 (63.4%) and 134 (36.6%) patients, respectively. The most common isolated strain was Staphylococcus aureus (31% MRSA; 12.6% methicillin-susceptible S. aureus [MSSA]) followed by Enterococci (6.7% vancomycin resistant, 9.2% vancomycin susceptible) and Streptococcus pneumoniae (3.4% multidrug resistant, 1.7% non–multidrug resistant).
Overall, 82.2% of patients met the criteria for clinical success; 34 (9.3%) were cured and 267 (73%) improved. Clinical success rates for first-line and second-line linezolid therapy were 79.9% and 83.6%, respectively. Failure rates for linezolid were higher for second-line versus first-line treatment (9.5% vs. 5.2%).
The clinical success rate was highest against MSSA (93.3%), followed by MRSA (83.8%). The average daily linezolid dose was 1,109 mg, and the mean treatment time was 5.1 days.
A total of eight patients (2.2%) reported linezolid-related adverse events, and four patients discontinued the medication because of them; none reported treatment-related serious adverse events. The low incidence of thrombocytopenia in the current study (two patients), compared with previous studies may have been related to avoidance of linezolid for at-risk patients as determined by clinicians, and the relatively short duration of linezolid use, the researchers wrote.
The study findings were limited by several factors, including the observational design and inability to compare the efficacy of different drugs; the small sample size; and the lack of data on drugs used prior to ICU admission, the researchers noted. Other limitations included the low detection rate of gram-positive bacteria and potential underreporting of adverse events.
However, the although clinicians will need to pay close attention to possible side effects and evaluate patient conditions on an individual basis before using linezolid in the clinic, they concluded.
The study was supported by grants from West China Hospital of Sichuan University. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately 80% of patients in intensive care showed clinical improvement in gram-positive bacterial infections after treatment with linezolid, based on data from more than 300 individuals.
Bacterial infections remain a challenge in the management of critically ill patients, as many gram-positive pathogens have become resistant to multiple drug options, Aijia Ma, MD, of West China Hospital of Sichuan University, and colleagues wrote.
Linezolid has demonstrated effectiveness against MRSA and skin and soft-tissue infections (SSTIs), but its use in critically ill patients with gram-positive infections in the ICU has not been characterized, they said.
In a multicenter, real-world study published in the Journal of Intensive Medicine, the researchers reviewed data from 52 hospitals between June 2018 and December 2019. The study population included 366 patients admitted to the ICU with a clinical or laboratory diagnosis of a gram-positive bacterial infection. Patients were treated with linezolid injections (200 mg/100 mL) and followed up once a day until 48 hours after discontinuing therapy, transferring out of the ICU, or death. Most of the patients (243) were older than 65 years; 90 were aged 18-65 years, and 30 were younger than 18 years. Approximately two-thirds (67%) were men. The primary outcome of clinical efficacy was success (cured or improved).
Linezolid was used as second-line and first-line treatment in 232 (63.4%) and 134 (36.6%) patients, respectively. The most common isolated strain was Staphylococcus aureus (31% MRSA; 12.6% methicillin-susceptible S. aureus [MSSA]) followed by Enterococci (6.7% vancomycin resistant, 9.2% vancomycin susceptible) and Streptococcus pneumoniae (3.4% multidrug resistant, 1.7% non–multidrug resistant).
Overall, 82.2% of patients met the criteria for clinical success; 34 (9.3%) were cured and 267 (73%) improved. Clinical success rates for first-line and second-line linezolid therapy were 79.9% and 83.6%, respectively. Failure rates for linezolid were higher for second-line versus first-line treatment (9.5% vs. 5.2%).
The clinical success rate was highest against MSSA (93.3%), followed by MRSA (83.8%). The average daily linezolid dose was 1,109 mg, and the mean treatment time was 5.1 days.
A total of eight patients (2.2%) reported linezolid-related adverse events, and four patients discontinued the medication because of them; none reported treatment-related serious adverse events. The low incidence of thrombocytopenia in the current study (two patients), compared with previous studies may have been related to avoidance of linezolid for at-risk patients as determined by clinicians, and the relatively short duration of linezolid use, the researchers wrote.
The study findings were limited by several factors, including the observational design and inability to compare the efficacy of different drugs; the small sample size; and the lack of data on drugs used prior to ICU admission, the researchers noted. Other limitations included the low detection rate of gram-positive bacteria and potential underreporting of adverse events.
However, the although clinicians will need to pay close attention to possible side effects and evaluate patient conditions on an individual basis before using linezolid in the clinic, they concluded.
The study was supported by grants from West China Hospital of Sichuan University. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately 80% of patients in intensive care showed clinical improvement in gram-positive bacterial infections after treatment with linezolid, based on data from more than 300 individuals.
Bacterial infections remain a challenge in the management of critically ill patients, as many gram-positive pathogens have become resistant to multiple drug options, Aijia Ma, MD, of West China Hospital of Sichuan University, and colleagues wrote.
Linezolid has demonstrated effectiveness against MRSA and skin and soft-tissue infections (SSTIs), but its use in critically ill patients with gram-positive infections in the ICU has not been characterized, they said.
In a multicenter, real-world study published in the Journal of Intensive Medicine, the researchers reviewed data from 52 hospitals between June 2018 and December 2019. The study population included 366 patients admitted to the ICU with a clinical or laboratory diagnosis of a gram-positive bacterial infection. Patients were treated with linezolid injections (200 mg/100 mL) and followed up once a day until 48 hours after discontinuing therapy, transferring out of the ICU, or death. Most of the patients (243) were older than 65 years; 90 were aged 18-65 years, and 30 were younger than 18 years. Approximately two-thirds (67%) were men. The primary outcome of clinical efficacy was success (cured or improved).
Linezolid was used as second-line and first-line treatment in 232 (63.4%) and 134 (36.6%) patients, respectively. The most common isolated strain was Staphylococcus aureus (31% MRSA; 12.6% methicillin-susceptible S. aureus [MSSA]) followed by Enterococci (6.7% vancomycin resistant, 9.2% vancomycin susceptible) and Streptococcus pneumoniae (3.4% multidrug resistant, 1.7% non–multidrug resistant).
Overall, 82.2% of patients met the criteria for clinical success; 34 (9.3%) were cured and 267 (73%) improved. Clinical success rates for first-line and second-line linezolid therapy were 79.9% and 83.6%, respectively. Failure rates for linezolid were higher for second-line versus first-line treatment (9.5% vs. 5.2%).
The clinical success rate was highest against MSSA (93.3%), followed by MRSA (83.8%). The average daily linezolid dose was 1,109 mg, and the mean treatment time was 5.1 days.
A total of eight patients (2.2%) reported linezolid-related adverse events, and four patients discontinued the medication because of them; none reported treatment-related serious adverse events. The low incidence of thrombocytopenia in the current study (two patients), compared with previous studies may have been related to avoidance of linezolid for at-risk patients as determined by clinicians, and the relatively short duration of linezolid use, the researchers wrote.
The study findings were limited by several factors, including the observational design and inability to compare the efficacy of different drugs; the small sample size; and the lack of data on drugs used prior to ICU admission, the researchers noted. Other limitations included the low detection rate of gram-positive bacteria and potential underreporting of adverse events.
However, the although clinicians will need to pay close attention to possible side effects and evaluate patient conditions on an individual basis before using linezolid in the clinic, they concluded.
The study was supported by grants from West China Hospital of Sichuan University. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF INTENSIVE MEDICINE
Coming to a pill near you: The exercise molecule
Exercise in a pill? Sign us up
You just got home from a long shift and you know you should go to the gym, but the bed is calling and you just answered. We know sometimes we have to make sacrifices in the name of fitness, but there just aren’t enough hours in the day. Unless our prayers have been answered. There could be a pill that has the benefits of working out without having to work out.
In a study published in Nature, investigators reported that they have identified a molecule made during exercise and used it on mice, which took in less food after being given the pill, which may open doors to understanding how exercise affects hunger.
In the first part of the study, the researchers found the molecule, known as Lac-Phe – which is synthesized from lactate and phenylalanine – in the blood plasma of mice after they had run on a treadmill.
The investigators then gave a Lac-Phe supplement to mice on high-fat diets and found that their food intake was about 50% of what other mice were eating. The supplement also improved their glucose tolerance.
Because the research also found Lac-Phe in humans who exercised, they hope that this pill will be in our future. “Our next steps include finding more details about how Lac-Phe mediates its effects in the body, including the brain,” Yong Xu, MD, of Baylor College of Medicine, Houston, said in a written statement. “Our goal is to learn to modulate this exercise pathway for therapeutic interventions.”
As always, we are rooting for you, science!
Gonorrhea and grandparents: A match made in prehistoric heaven
*Editorial note: LOTME takes no responsibility for any unfortunate imagery the reader may have experienced from the above headline.
Old people are the greatest. Back pains, cognitive decline, aches in all the diodes down your left side, there’s nothing quite like your golden years. Notably, however, humans are one of the few animals who experience true old age, as most creatures are adapted to maximize reproductive potential. As such, living past menopause is rare in the animal kingdom.
This is where the “grandmother hypothesis” comes in: Back in Ye Olde Stone Age, women who lived into old age could provide child care for younger women, because human babies require a lot more time and attention than other animal offspring. But how did humans end up living so long? Enter a group of Californian researchers, who believe they have an answer. It was gonorrhea.
When compared with the chimpanzee genome (as well as with Neanderthals and Denisovans, our closest ancestors), humans have a unique mutated version of the CD33 gene that lacks a sugar-binding site; the standard version uses the sugar-binding site to protect against autoimmune response in the body, but that same site actually suppresses the brain’s ability to clear away damaged brain cells and amyloid, which eventually leads to diseases like dementia. The mutated version allows microglia (brain immune cells) to attack and clear out this unwanted material. People with higher levels of this mutated CD33 variant actually have higher protection against Alzheimer’s.
Interestingly, gonorrhea bacteria are coated in the same sugar that standard CD33 receptors bind to, thus allowing them to bypass the body’s immune system. According to the researchers, the mutated CD33 version likely emerged as a protection against gonorrhea, depriving the bacteria of their “molecular mimicry” abilities. In one of life’s happy accidents, it turned out this mutation also protects against age-related diseases, thus allowing humans with the mutation to live longer. Obviously, this was a good thing, and we ran with it until the modern day. Now we have senior citizens climbing Everest, and all our politicians keep on politicking into their 70s and 80s ... well, everything has its drawbacks.
Parents raise a glass to children’s food addiction
There can be something pretty addicting about processed foods. Have you ever eaten just one french fry? Or taken just one cookie? If so, your willpower is incredible. For many of us, it can be a struggle to stop.
A recent study from the University of Michigan, which considered the existence of an eating phenotype, suggests our parents’ habits could be to blame.
By administering a series of questionnaires that inquired about food addiction, alcohol use disorders, cannabis use disorder, nicotine/e-cigarette dependence, and their family tree, investigators found that participants with a “paternal history of problematic alcohol use” had higher risk of food addiction but not obesity.
Apparently about one in five people display a clinically significant addiction to highly processed foods. It was noted that foods like ice cream, pizza, and french fries have high amounts of refined carbs and fats, which could trigger an addictive response.
Lindzey Hoover, a graduate student at the university who was the study’s lead author, noted that living in an environment where these foods are cheap and accessible can be really challenging for those with a family history of addiction. The investigators suggested that public health approaches, like restriction of other substances and marketing to kids, should be put in place for highly processed foods.
Maybe french fries should come with a warning label.
A prescription for America’s traffic problems
Nostalgia is a funny thing. Do you ever feel nostalgic about things that really weren’t very pleasant in the first place? Take, for instance, the morning commute. Here in the Washington area, more than 2 years into the COVID era, the traffic is still not what it used to be … and we kind of miss it.
Nah, not really. That was just a way to get everyone thinking about driving, because AAA has something of an explanation for the situation out there on the highways and byways of America. It’s drugs. No, not those kinds of drugs. This time it’s prescription drugs that are the problem. Well, part of the problem, anyway.
AAA did a survey last summer and found that nearly 50% of drivers “used one or more potentially impairing medications in the past 30 days. … The proportion of those choosing to drive is higher among those taking multiple medications.” How much higher? More than 63% of those with two or more prescriptions were driving within 2 hours of taking at least one of those meds, as were 71% of those taking three or more.
The 2,657 respondents also were asked about the types of potentially impairing drugs they were taking: 61% of those using antidepressants had been on the road within 2 hours of use at least once in the past 30 days, as had 73% of those taking an amphetamine, AAA said.
So there you have it. That guy in the BMW who’s been tailgating you for the last 3 miles? He may be a jerk, but there’s a good chance he’s a jerk with a prescription … or two … or three.
Exercise in a pill? Sign us up
You just got home from a long shift and you know you should go to the gym, but the bed is calling and you just answered. We know sometimes we have to make sacrifices in the name of fitness, but there just aren’t enough hours in the day. Unless our prayers have been answered. There could be a pill that has the benefits of working out without having to work out.
In a study published in Nature, investigators reported that they have identified a molecule made during exercise and used it on mice, which took in less food after being given the pill, which may open doors to understanding how exercise affects hunger.
In the first part of the study, the researchers found the molecule, known as Lac-Phe – which is synthesized from lactate and phenylalanine – in the blood plasma of mice after they had run on a treadmill.
The investigators then gave a Lac-Phe supplement to mice on high-fat diets and found that their food intake was about 50% of what other mice were eating. The supplement also improved their glucose tolerance.
Because the research also found Lac-Phe in humans who exercised, they hope that this pill will be in our future. “Our next steps include finding more details about how Lac-Phe mediates its effects in the body, including the brain,” Yong Xu, MD, of Baylor College of Medicine, Houston, said in a written statement. “Our goal is to learn to modulate this exercise pathway for therapeutic interventions.”
As always, we are rooting for you, science!
Gonorrhea and grandparents: A match made in prehistoric heaven
*Editorial note: LOTME takes no responsibility for any unfortunate imagery the reader may have experienced from the above headline.
Old people are the greatest. Back pains, cognitive decline, aches in all the diodes down your left side, there’s nothing quite like your golden years. Notably, however, humans are one of the few animals who experience true old age, as most creatures are adapted to maximize reproductive potential. As such, living past menopause is rare in the animal kingdom.
This is where the “grandmother hypothesis” comes in: Back in Ye Olde Stone Age, women who lived into old age could provide child care for younger women, because human babies require a lot more time and attention than other animal offspring. But how did humans end up living so long? Enter a group of Californian researchers, who believe they have an answer. It was gonorrhea.
When compared with the chimpanzee genome (as well as with Neanderthals and Denisovans, our closest ancestors), humans have a unique mutated version of the CD33 gene that lacks a sugar-binding site; the standard version uses the sugar-binding site to protect against autoimmune response in the body, but that same site actually suppresses the brain’s ability to clear away damaged brain cells and amyloid, which eventually leads to diseases like dementia. The mutated version allows microglia (brain immune cells) to attack and clear out this unwanted material. People with higher levels of this mutated CD33 variant actually have higher protection against Alzheimer’s.
Interestingly, gonorrhea bacteria are coated in the same sugar that standard CD33 receptors bind to, thus allowing them to bypass the body’s immune system. According to the researchers, the mutated CD33 version likely emerged as a protection against gonorrhea, depriving the bacteria of their “molecular mimicry” abilities. In one of life’s happy accidents, it turned out this mutation also protects against age-related diseases, thus allowing humans with the mutation to live longer. Obviously, this was a good thing, and we ran with it until the modern day. Now we have senior citizens climbing Everest, and all our politicians keep on politicking into their 70s and 80s ... well, everything has its drawbacks.
Parents raise a glass to children’s food addiction
There can be something pretty addicting about processed foods. Have you ever eaten just one french fry? Or taken just one cookie? If so, your willpower is incredible. For many of us, it can be a struggle to stop.
A recent study from the University of Michigan, which considered the existence of an eating phenotype, suggests our parents’ habits could be to blame.
By administering a series of questionnaires that inquired about food addiction, alcohol use disorders, cannabis use disorder, nicotine/e-cigarette dependence, and their family tree, investigators found that participants with a “paternal history of problematic alcohol use” had higher risk of food addiction but not obesity.
Apparently about one in five people display a clinically significant addiction to highly processed foods. It was noted that foods like ice cream, pizza, and french fries have high amounts of refined carbs and fats, which could trigger an addictive response.
Lindzey Hoover, a graduate student at the university who was the study’s lead author, noted that living in an environment where these foods are cheap and accessible can be really challenging for those with a family history of addiction. The investigators suggested that public health approaches, like restriction of other substances and marketing to kids, should be put in place for highly processed foods.
Maybe french fries should come with a warning label.
A prescription for America’s traffic problems
Nostalgia is a funny thing. Do you ever feel nostalgic about things that really weren’t very pleasant in the first place? Take, for instance, the morning commute. Here in the Washington area, more than 2 years into the COVID era, the traffic is still not what it used to be … and we kind of miss it.
Nah, not really. That was just a way to get everyone thinking about driving, because AAA has something of an explanation for the situation out there on the highways and byways of America. It’s drugs. No, not those kinds of drugs. This time it’s prescription drugs that are the problem. Well, part of the problem, anyway.
AAA did a survey last summer and found that nearly 50% of drivers “used one or more potentially impairing medications in the past 30 days. … The proportion of those choosing to drive is higher among those taking multiple medications.” How much higher? More than 63% of those with two or more prescriptions were driving within 2 hours of taking at least one of those meds, as were 71% of those taking three or more.
The 2,657 respondents also were asked about the types of potentially impairing drugs they were taking: 61% of those using antidepressants had been on the road within 2 hours of use at least once in the past 30 days, as had 73% of those taking an amphetamine, AAA said.
So there you have it. That guy in the BMW who’s been tailgating you for the last 3 miles? He may be a jerk, but there’s a good chance he’s a jerk with a prescription … or two … or three.
Exercise in a pill? Sign us up
You just got home from a long shift and you know you should go to the gym, but the bed is calling and you just answered. We know sometimes we have to make sacrifices in the name of fitness, but there just aren’t enough hours in the day. Unless our prayers have been answered. There could be a pill that has the benefits of working out without having to work out.
In a study published in Nature, investigators reported that they have identified a molecule made during exercise and used it on mice, which took in less food after being given the pill, which may open doors to understanding how exercise affects hunger.
In the first part of the study, the researchers found the molecule, known as Lac-Phe – which is synthesized from lactate and phenylalanine – in the blood plasma of mice after they had run on a treadmill.
The investigators then gave a Lac-Phe supplement to mice on high-fat diets and found that their food intake was about 50% of what other mice were eating. The supplement also improved their glucose tolerance.
Because the research also found Lac-Phe in humans who exercised, they hope that this pill will be in our future. “Our next steps include finding more details about how Lac-Phe mediates its effects in the body, including the brain,” Yong Xu, MD, of Baylor College of Medicine, Houston, said in a written statement. “Our goal is to learn to modulate this exercise pathway for therapeutic interventions.”
As always, we are rooting for you, science!
Gonorrhea and grandparents: A match made in prehistoric heaven
*Editorial note: LOTME takes no responsibility for any unfortunate imagery the reader may have experienced from the above headline.
Old people are the greatest. Back pains, cognitive decline, aches in all the diodes down your left side, there’s nothing quite like your golden years. Notably, however, humans are one of the few animals who experience true old age, as most creatures are adapted to maximize reproductive potential. As such, living past menopause is rare in the animal kingdom.
This is where the “grandmother hypothesis” comes in: Back in Ye Olde Stone Age, women who lived into old age could provide child care for younger women, because human babies require a lot more time and attention than other animal offspring. But how did humans end up living so long? Enter a group of Californian researchers, who believe they have an answer. It was gonorrhea.
When compared with the chimpanzee genome (as well as with Neanderthals and Denisovans, our closest ancestors), humans have a unique mutated version of the CD33 gene that lacks a sugar-binding site; the standard version uses the sugar-binding site to protect against autoimmune response in the body, but that same site actually suppresses the brain’s ability to clear away damaged brain cells and amyloid, which eventually leads to diseases like dementia. The mutated version allows microglia (brain immune cells) to attack and clear out this unwanted material. People with higher levels of this mutated CD33 variant actually have higher protection against Alzheimer’s.
Interestingly, gonorrhea bacteria are coated in the same sugar that standard CD33 receptors bind to, thus allowing them to bypass the body’s immune system. According to the researchers, the mutated CD33 version likely emerged as a protection against gonorrhea, depriving the bacteria of their “molecular mimicry” abilities. In one of life’s happy accidents, it turned out this mutation also protects against age-related diseases, thus allowing humans with the mutation to live longer. Obviously, this was a good thing, and we ran with it until the modern day. Now we have senior citizens climbing Everest, and all our politicians keep on politicking into their 70s and 80s ... well, everything has its drawbacks.
Parents raise a glass to children’s food addiction
There can be something pretty addicting about processed foods. Have you ever eaten just one french fry? Or taken just one cookie? If so, your willpower is incredible. For many of us, it can be a struggle to stop.
A recent study from the University of Michigan, which considered the existence of an eating phenotype, suggests our parents’ habits could be to blame.
By administering a series of questionnaires that inquired about food addiction, alcohol use disorders, cannabis use disorder, nicotine/e-cigarette dependence, and their family tree, investigators found that participants with a “paternal history of problematic alcohol use” had higher risk of food addiction but not obesity.
Apparently about one in five people display a clinically significant addiction to highly processed foods. It was noted that foods like ice cream, pizza, and french fries have high amounts of refined carbs and fats, which could trigger an addictive response.
Lindzey Hoover, a graduate student at the university who was the study’s lead author, noted that living in an environment where these foods are cheap and accessible can be really challenging for those with a family history of addiction. The investigators suggested that public health approaches, like restriction of other substances and marketing to kids, should be put in place for highly processed foods.
Maybe french fries should come with a warning label.
A prescription for America’s traffic problems
Nostalgia is a funny thing. Do you ever feel nostalgic about things that really weren’t very pleasant in the first place? Take, for instance, the morning commute. Here in the Washington area, more than 2 years into the COVID era, the traffic is still not what it used to be … and we kind of miss it.
Nah, not really. That was just a way to get everyone thinking about driving, because AAA has something of an explanation for the situation out there on the highways and byways of America. It’s drugs. No, not those kinds of drugs. This time it’s prescription drugs that are the problem. Well, part of the problem, anyway.
AAA did a survey last summer and found that nearly 50% of drivers “used one or more potentially impairing medications in the past 30 days. … The proportion of those choosing to drive is higher among those taking multiple medications.” How much higher? More than 63% of those with two or more prescriptions were driving within 2 hours of taking at least one of those meds, as were 71% of those taking three or more.
The 2,657 respondents also were asked about the types of potentially impairing drugs they were taking: 61% of those using antidepressants had been on the road within 2 hours of use at least once in the past 30 days, as had 73% of those taking an amphetamine, AAA said.
So there you have it. That guy in the BMW who’s been tailgating you for the last 3 miles? He may be a jerk, but there’s a good chance he’s a jerk with a prescription … or two … or three.
Scientists aim to combat COVID with a shot in the nose
Scientists seeking to stay ahead of an evolving SARS-Cov-2 virus are looking at new strategies, including developing intranasal vaccines, according to speakers at a conference on July 26.
inviting researchers to provide a public update on efforts to try to keep ahead of SARS-CoV-2.
Scientists and federal officials are looking to build on the successes seen in developing the original crop of COVID vaccines, which were authorized for use in the United States less than a year after the pandemic took hold.
But emerging variants are eroding these gains. For months now, officials at the Centers for Disease Control and Prevention and Food and Drug Administration have been keeping an eye on how the level of effectiveness of COVID vaccines has waned during the rise of the Omicron strain. And there’s continual concern about how SARS-CoV-2 might evolve over time.
“Our vaccines are terrific,” Ashish K. Jha, MD, the White House’s COVID-19 response coordinator, said at the summit. “[But] we have to do better.”
Among the approaches being considered are vaccines that would be applied intranasally, with the idea that this might be able to boost the immune response to SARS-CoV-2.
At the summit, Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., said the intranasal approach might be helpful in preventing transmission as well as reducing the burden of illness for those who are infected with SARS-CoV-2.
“We’re stopping the virus from spreading right at the border,” Dr. Iwasaki said at the summit. “This is akin to putting a guard outside of the house in order to patrol for invaders compared to putting the guards in the hallway of the building in the hope that they capture the invader.”
Dr. Iwasaki is one of the founders of Xanadu Bio, a private company created last year to focus on ways to kill SARS-CoV-2 in the nasosinus before it spreads deeper into the respiratory tract. In an editorial in Science Immunology, Dr. Iwasaki and Eric J. Topol, MD, director of the Scripps Research Translational Institute, urged greater federal investment in this approach to fighting SARS-CoV-2. (Dr. Topol is editor-in-chief of Medscape.)
Titled “Operation Nasal Vaccine – Lightning speed to counter COVID-19,” their editorial noted the “unprecedented success” seen in the rapid development of the first two mRNA shots. Dr. Iwasaki and Dr. Topol noted that these victories had been “fueled by the $10 billion governmental investment in Operation Warp Speed.
“During the first year of the pandemic, meaningful evolution of the virus was slow-paced, without any functional consequences, but since that time we have seen a succession of important variants of concern, with increasing transmissibility and immune evasion, culminating in the Omicron lineages,” wrote Dr. Iwasaki and Dr. Topol.
Recent developments have “spotlighted the possibility of nasal vaccines, with their allure for achieving mucosal immunity, complementing, and likely bolstering the circulating immunity achieved via intramuscular shots,” they added.
An early setback
Scientists at the National Institutes of Health and the Biomedical Advanced Research and Development Authority (BARDA) have for some time been looking to vet an array of next-generation vaccine concepts, including ones that trigger mucosal immunity, the Washington Post reported in April.
At the summit on July 26, several participants, including Dr. Jha, stressed the role that public-private partnerships were key to the rapid development of the initial COVID vaccines. They said continued U.S. government support will be needed to make advances in this field.
One of the presenters, Biao He, PhD, founder and president of CyanVac and Blue Lake Biotechnology, spoke of the federal support that his efforts have received over the years to develop intranasal vaccines. His Georgia-based firm already has an experimental intranasal vaccine candidate, CVXGA1-001, in phase 1 testing (NCT04954287).
The CVXGA-001 builds on technology already used in a veterinary product, an intranasal vaccine long used to prevent kennel cough in dogs, he said at the summit.
The emerging field of experimental intranasal COVID vaccines already has had at least one setback.
The biotech firm Altimmune in June 2021 announced that it would discontinue development of its experimental intranasal AdCOVID vaccine following disappointing phase 1 results. The vaccine appeared to be well tolerated in the test, but the immunogenicity data demonstrated lower than expected results in healthy volunteers, especially in light of the responses seen to already cleared vaccines, Altimmune said in a release.
In the statement, Scot Roberts, PhD, chief scientific officer at Altimmune, noted that the study participants lacked immunity from prior infection or vaccination. “We believe that prior immunity in humans may be important for a robust immune response to intranasal dosing with AdCOVID,” he said.
At the summit, Marty Moore, PhD, cofounder and chief scientific officer for Redwood City, Calif.–based Meissa Vaccines, noted the challenges that remain ahead for intranasal COVID vaccines, while also highlighting what he sees as the potential of this approach.
Meissa also has advanced an experimental intranasal COVID vaccine as far as phase 1 testing (NCT04798001).
“No one here today can tell you that mucosal COVID vaccines work. We’re not there yet. We need clinical efficacy data to answer that question,” Dr. Moore said.
But there’s a potential for a “knockout blow to COVID, a transmission-blocking vaccine” from the intranasal approach, he said.
“The virus is mutating faster than our ability to manage vaccines and not enough people are getting boosters. These injectable vaccines do a great job of preventing severe disease, but they do little to prevent infection” from spreading, Dr. Moore said.
A version of this article first appeared on Medscape.com.
Scientists seeking to stay ahead of an evolving SARS-Cov-2 virus are looking at new strategies, including developing intranasal vaccines, according to speakers at a conference on July 26.
inviting researchers to provide a public update on efforts to try to keep ahead of SARS-CoV-2.
Scientists and federal officials are looking to build on the successes seen in developing the original crop of COVID vaccines, which were authorized for use in the United States less than a year after the pandemic took hold.
But emerging variants are eroding these gains. For months now, officials at the Centers for Disease Control and Prevention and Food and Drug Administration have been keeping an eye on how the level of effectiveness of COVID vaccines has waned during the rise of the Omicron strain. And there’s continual concern about how SARS-CoV-2 might evolve over time.
“Our vaccines are terrific,” Ashish K. Jha, MD, the White House’s COVID-19 response coordinator, said at the summit. “[But] we have to do better.”
Among the approaches being considered are vaccines that would be applied intranasally, with the idea that this might be able to boost the immune response to SARS-CoV-2.
At the summit, Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., said the intranasal approach might be helpful in preventing transmission as well as reducing the burden of illness for those who are infected with SARS-CoV-2.
“We’re stopping the virus from spreading right at the border,” Dr. Iwasaki said at the summit. “This is akin to putting a guard outside of the house in order to patrol for invaders compared to putting the guards in the hallway of the building in the hope that they capture the invader.”
Dr. Iwasaki is one of the founders of Xanadu Bio, a private company created last year to focus on ways to kill SARS-CoV-2 in the nasosinus before it spreads deeper into the respiratory tract. In an editorial in Science Immunology, Dr. Iwasaki and Eric J. Topol, MD, director of the Scripps Research Translational Institute, urged greater federal investment in this approach to fighting SARS-CoV-2. (Dr. Topol is editor-in-chief of Medscape.)
Titled “Operation Nasal Vaccine – Lightning speed to counter COVID-19,” their editorial noted the “unprecedented success” seen in the rapid development of the first two mRNA shots. Dr. Iwasaki and Dr. Topol noted that these victories had been “fueled by the $10 billion governmental investment in Operation Warp Speed.
“During the first year of the pandemic, meaningful evolution of the virus was slow-paced, without any functional consequences, but since that time we have seen a succession of important variants of concern, with increasing transmissibility and immune evasion, culminating in the Omicron lineages,” wrote Dr. Iwasaki and Dr. Topol.
Recent developments have “spotlighted the possibility of nasal vaccines, with their allure for achieving mucosal immunity, complementing, and likely bolstering the circulating immunity achieved via intramuscular shots,” they added.
An early setback
Scientists at the National Institutes of Health and the Biomedical Advanced Research and Development Authority (BARDA) have for some time been looking to vet an array of next-generation vaccine concepts, including ones that trigger mucosal immunity, the Washington Post reported in April.
At the summit on July 26, several participants, including Dr. Jha, stressed the role that public-private partnerships were key to the rapid development of the initial COVID vaccines. They said continued U.S. government support will be needed to make advances in this field.
One of the presenters, Biao He, PhD, founder and president of CyanVac and Blue Lake Biotechnology, spoke of the federal support that his efforts have received over the years to develop intranasal vaccines. His Georgia-based firm already has an experimental intranasal vaccine candidate, CVXGA1-001, in phase 1 testing (NCT04954287).
The CVXGA-001 builds on technology already used in a veterinary product, an intranasal vaccine long used to prevent kennel cough in dogs, he said at the summit.
The emerging field of experimental intranasal COVID vaccines already has had at least one setback.
The biotech firm Altimmune in June 2021 announced that it would discontinue development of its experimental intranasal AdCOVID vaccine following disappointing phase 1 results. The vaccine appeared to be well tolerated in the test, but the immunogenicity data demonstrated lower than expected results in healthy volunteers, especially in light of the responses seen to already cleared vaccines, Altimmune said in a release.
In the statement, Scot Roberts, PhD, chief scientific officer at Altimmune, noted that the study participants lacked immunity from prior infection or vaccination. “We believe that prior immunity in humans may be important for a robust immune response to intranasal dosing with AdCOVID,” he said.
At the summit, Marty Moore, PhD, cofounder and chief scientific officer for Redwood City, Calif.–based Meissa Vaccines, noted the challenges that remain ahead for intranasal COVID vaccines, while also highlighting what he sees as the potential of this approach.
Meissa also has advanced an experimental intranasal COVID vaccine as far as phase 1 testing (NCT04798001).
“No one here today can tell you that mucosal COVID vaccines work. We’re not there yet. We need clinical efficacy data to answer that question,” Dr. Moore said.
But there’s a potential for a “knockout blow to COVID, a transmission-blocking vaccine” from the intranasal approach, he said.
“The virus is mutating faster than our ability to manage vaccines and not enough people are getting boosters. These injectable vaccines do a great job of preventing severe disease, but they do little to prevent infection” from spreading, Dr. Moore said.
A version of this article first appeared on Medscape.com.
Scientists seeking to stay ahead of an evolving SARS-Cov-2 virus are looking at new strategies, including developing intranasal vaccines, according to speakers at a conference on July 26.
inviting researchers to provide a public update on efforts to try to keep ahead of SARS-CoV-2.
Scientists and federal officials are looking to build on the successes seen in developing the original crop of COVID vaccines, which were authorized for use in the United States less than a year after the pandemic took hold.
But emerging variants are eroding these gains. For months now, officials at the Centers for Disease Control and Prevention and Food and Drug Administration have been keeping an eye on how the level of effectiveness of COVID vaccines has waned during the rise of the Omicron strain. And there’s continual concern about how SARS-CoV-2 might evolve over time.
“Our vaccines are terrific,” Ashish K. Jha, MD, the White House’s COVID-19 response coordinator, said at the summit. “[But] we have to do better.”
Among the approaches being considered are vaccines that would be applied intranasally, with the idea that this might be able to boost the immune response to SARS-CoV-2.
At the summit, Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., said the intranasal approach might be helpful in preventing transmission as well as reducing the burden of illness for those who are infected with SARS-CoV-2.
“We’re stopping the virus from spreading right at the border,” Dr. Iwasaki said at the summit. “This is akin to putting a guard outside of the house in order to patrol for invaders compared to putting the guards in the hallway of the building in the hope that they capture the invader.”
Dr. Iwasaki is one of the founders of Xanadu Bio, a private company created last year to focus on ways to kill SARS-CoV-2 in the nasosinus before it spreads deeper into the respiratory tract. In an editorial in Science Immunology, Dr. Iwasaki and Eric J. Topol, MD, director of the Scripps Research Translational Institute, urged greater federal investment in this approach to fighting SARS-CoV-2. (Dr. Topol is editor-in-chief of Medscape.)
Titled “Operation Nasal Vaccine – Lightning speed to counter COVID-19,” their editorial noted the “unprecedented success” seen in the rapid development of the first two mRNA shots. Dr. Iwasaki and Dr. Topol noted that these victories had been “fueled by the $10 billion governmental investment in Operation Warp Speed.
“During the first year of the pandemic, meaningful evolution of the virus was slow-paced, without any functional consequences, but since that time we have seen a succession of important variants of concern, with increasing transmissibility and immune evasion, culminating in the Omicron lineages,” wrote Dr. Iwasaki and Dr. Topol.
Recent developments have “spotlighted the possibility of nasal vaccines, with their allure for achieving mucosal immunity, complementing, and likely bolstering the circulating immunity achieved via intramuscular shots,” they added.
An early setback
Scientists at the National Institutes of Health and the Biomedical Advanced Research and Development Authority (BARDA) have for some time been looking to vet an array of next-generation vaccine concepts, including ones that trigger mucosal immunity, the Washington Post reported in April.
At the summit on July 26, several participants, including Dr. Jha, stressed the role that public-private partnerships were key to the rapid development of the initial COVID vaccines. They said continued U.S. government support will be needed to make advances in this field.
One of the presenters, Biao He, PhD, founder and president of CyanVac and Blue Lake Biotechnology, spoke of the federal support that his efforts have received over the years to develop intranasal vaccines. His Georgia-based firm already has an experimental intranasal vaccine candidate, CVXGA1-001, in phase 1 testing (NCT04954287).
The CVXGA-001 builds on technology already used in a veterinary product, an intranasal vaccine long used to prevent kennel cough in dogs, he said at the summit.
The emerging field of experimental intranasal COVID vaccines already has had at least one setback.
The biotech firm Altimmune in June 2021 announced that it would discontinue development of its experimental intranasal AdCOVID vaccine following disappointing phase 1 results. The vaccine appeared to be well tolerated in the test, but the immunogenicity data demonstrated lower than expected results in healthy volunteers, especially in light of the responses seen to already cleared vaccines, Altimmune said in a release.
In the statement, Scot Roberts, PhD, chief scientific officer at Altimmune, noted that the study participants lacked immunity from prior infection or vaccination. “We believe that prior immunity in humans may be important for a robust immune response to intranasal dosing with AdCOVID,” he said.
At the summit, Marty Moore, PhD, cofounder and chief scientific officer for Redwood City, Calif.–based Meissa Vaccines, noted the challenges that remain ahead for intranasal COVID vaccines, while also highlighting what he sees as the potential of this approach.
Meissa also has advanced an experimental intranasal COVID vaccine as far as phase 1 testing (NCT04798001).
“No one here today can tell you that mucosal COVID vaccines work. We’re not there yet. We need clinical efficacy data to answer that question,” Dr. Moore said.
But there’s a potential for a “knockout blow to COVID, a transmission-blocking vaccine” from the intranasal approach, he said.
“The virus is mutating faster than our ability to manage vaccines and not enough people are getting boosters. These injectable vaccines do a great job of preventing severe disease, but they do little to prevent infection” from spreading, Dr. Moore said.
A version of this article first appeared on Medscape.com.