User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Medical practice gave 8,000 patients cancer for Christmas
We wish you a merry Christmas and a happy heart failure
Does anyone really like it when places of business send out cards or messages for the holidays? A card from a truly small family business is one thing, but when you start getting emails from multibillion dollar corporations, it feels a bit dishonest. And that’s not even mentioning the potential blowback when things go wrong.
Now, you may wonder how a company could possibly mess up something so simple. “We wish you a merry Christmas and a happy New Year.” Not that difficult. Unless you’re Askern Medical Practice in Doncaster, England. Instead of expressing a simple expression of joy for the holiday season, Askern informed all 8,000 of its patients that they had aggressive lung cancer with metastases and they needed to fill out a DS1500 form, which entitles terminal patients to certain benefits.
It only took an hour for Askern to recognize its mistake and send a second text apologizing and adding in the appropriate season’s greetings, but obviously the damage was done. Presumably patients who were last at the doctor to have their cold treated were able to shrug off the text, or simply didn’t see it before the correction came through, but obviously many patients had concerns directly related to cancer and panicked. They called in but were by and large unable to reach anyone at the practice. Some patients close by even went to center itself to clear things up.
One patient, Mr. Carl Chegwin, raised an excellent point about the debacle: “What if that message was meant for someone, and then they are told it’s a Christmas message, then again told, ‘Oh no, that was actually meant for you?’ ” The old double backtrack into yes, you actually do have cancer has got to be a candidate for worst Christmas gift of all. Yes, even worse than socks.
Genes know it: You are when you eat
There’s been a lot of recent research on intermittent fasting and what it can and can’t do for one’s health. Much of it has focused on participants’ metabolic rates, but a study just published in Cell Metabolism shows how time-restricted feeding (TRF) has an impact on gene expression, the process through which genes are activated and respond to their environment by creating proteins.
The research conducted by Satchidananda Panda, PhD, of the Salk Institute and his team involved two groups of mice, one with free access to food and the other with a daily 9-hour feeding window. Analysis of tissue samples collected from 22 organ groups revealed that nearly 80% of mouse genes responded to TRF. Interestingly, 40% of the genes in the hypothalamus, adrenal gland, and pancreas, which handle hormone regulation, were affected, suggesting that TRF could potentially aid in diabetes and stress disorder management, the investigators said in a written statement.
The researchers also found that TRF aligned the circadian rhythms of multiple organs of the body, which brings sleep into the picture. “Time-restricted eating synchronized the circadian rhythms to have two major waves: one during fasting, and another just after eating. We suspect this allows the body to coordinate different processes,” said Dr. Panda, whose previous research looked at TRF in firefighters, who typically work on shift schedules.
Time-restricted eating, it appears, affects gene expression throughout the body and allows interconnected organ systems to work smoothly. It’s not just about eating. Go figure.
This group practice reduced stress for everyone
It’s been awhile since we checked in on the good folks at Maharishi International University in Fairfield, Iowa – fictional home of the Fighting Transcendentalists [MAHARISHI RULES!] – but we just have to mention their long-term effort to reduce the national stress.
Way back in the year 2000, a group from MIU began practicing transcendental meditation. The size of the group increased over the next few years and eventually reached 1,725 in 2006. That number is important because it represents the square root of 1% of the U.S. population. When that “transition threshold was achieved,” the university explained in a written statement, “all stress indicators immediately started decreasing.”
By stress indicators they mean the U.S. stress index, the mean of eight variables – murder, rape, assault, robbery, infant mortality, drug deaths, vehicle fatalities, and child deaths by injuries – that the study investigators used to track the effectiveness of the meditation program, they said in the World Journal of Social Science.
After 2011, “when the size of the group size began to decline the rate of decrease in stress slowed and then it reversed and began to increase,” MIU reported.
Coauthor Dr. Kenneth Cavanaugh of MIU explained the process: “This study used state-of-the-art methods of time series regression analysis for eliminating potential alternative explanations due to intrinsic preexisting trends and fluctuations in the data. We carefully studied potential alternative explanations in terms of changes in economic conditions, political leadership, population demographics, and policing strategies. None of these factors could account for the results.”
Since we here at LOTME are serious professional journalists, the use of quotes means we are not making this up. Here’s one more thing in quotes: “A grant for 75 million dollars from the Howard and Alice Settle Foundation provided stipends for participants to be in the group and provided funding to bring several hundred visiting [meditation] experts from India to further augment the MIU group.”
Who needs to make up stuff? Not us.
We wish you a merry Christmas and a happy heart failure
Does anyone really like it when places of business send out cards or messages for the holidays? A card from a truly small family business is one thing, but when you start getting emails from multibillion dollar corporations, it feels a bit dishonest. And that’s not even mentioning the potential blowback when things go wrong.
Now, you may wonder how a company could possibly mess up something so simple. “We wish you a merry Christmas and a happy New Year.” Not that difficult. Unless you’re Askern Medical Practice in Doncaster, England. Instead of expressing a simple expression of joy for the holiday season, Askern informed all 8,000 of its patients that they had aggressive lung cancer with metastases and they needed to fill out a DS1500 form, which entitles terminal patients to certain benefits.
It only took an hour for Askern to recognize its mistake and send a second text apologizing and adding in the appropriate season’s greetings, but obviously the damage was done. Presumably patients who were last at the doctor to have their cold treated were able to shrug off the text, or simply didn’t see it before the correction came through, but obviously many patients had concerns directly related to cancer and panicked. They called in but were by and large unable to reach anyone at the practice. Some patients close by even went to center itself to clear things up.
One patient, Mr. Carl Chegwin, raised an excellent point about the debacle: “What if that message was meant for someone, and then they are told it’s a Christmas message, then again told, ‘Oh no, that was actually meant for you?’ ” The old double backtrack into yes, you actually do have cancer has got to be a candidate for worst Christmas gift of all. Yes, even worse than socks.
Genes know it: You are when you eat
There’s been a lot of recent research on intermittent fasting and what it can and can’t do for one’s health. Much of it has focused on participants’ metabolic rates, but a study just published in Cell Metabolism shows how time-restricted feeding (TRF) has an impact on gene expression, the process through which genes are activated and respond to their environment by creating proteins.
The research conducted by Satchidananda Panda, PhD, of the Salk Institute and his team involved two groups of mice, one with free access to food and the other with a daily 9-hour feeding window. Analysis of tissue samples collected from 22 organ groups revealed that nearly 80% of mouse genes responded to TRF. Interestingly, 40% of the genes in the hypothalamus, adrenal gland, and pancreas, which handle hormone regulation, were affected, suggesting that TRF could potentially aid in diabetes and stress disorder management, the investigators said in a written statement.
The researchers also found that TRF aligned the circadian rhythms of multiple organs of the body, which brings sleep into the picture. “Time-restricted eating synchronized the circadian rhythms to have two major waves: one during fasting, and another just after eating. We suspect this allows the body to coordinate different processes,” said Dr. Panda, whose previous research looked at TRF in firefighters, who typically work on shift schedules.
Time-restricted eating, it appears, affects gene expression throughout the body and allows interconnected organ systems to work smoothly. It’s not just about eating. Go figure.
This group practice reduced stress for everyone
It’s been awhile since we checked in on the good folks at Maharishi International University in Fairfield, Iowa – fictional home of the Fighting Transcendentalists [MAHARISHI RULES!] – but we just have to mention their long-term effort to reduce the national stress.
Way back in the year 2000, a group from MIU began practicing transcendental meditation. The size of the group increased over the next few years and eventually reached 1,725 in 2006. That number is important because it represents the square root of 1% of the U.S. population. When that “transition threshold was achieved,” the university explained in a written statement, “all stress indicators immediately started decreasing.”
By stress indicators they mean the U.S. stress index, the mean of eight variables – murder, rape, assault, robbery, infant mortality, drug deaths, vehicle fatalities, and child deaths by injuries – that the study investigators used to track the effectiveness of the meditation program, they said in the World Journal of Social Science.
After 2011, “when the size of the group size began to decline the rate of decrease in stress slowed and then it reversed and began to increase,” MIU reported.
Coauthor Dr. Kenneth Cavanaugh of MIU explained the process: “This study used state-of-the-art methods of time series regression analysis for eliminating potential alternative explanations due to intrinsic preexisting trends and fluctuations in the data. We carefully studied potential alternative explanations in terms of changes in economic conditions, political leadership, population demographics, and policing strategies. None of these factors could account for the results.”
Since we here at LOTME are serious professional journalists, the use of quotes means we are not making this up. Here’s one more thing in quotes: “A grant for 75 million dollars from the Howard and Alice Settle Foundation provided stipends for participants to be in the group and provided funding to bring several hundred visiting [meditation] experts from India to further augment the MIU group.”
Who needs to make up stuff? Not us.
We wish you a merry Christmas and a happy heart failure
Does anyone really like it when places of business send out cards or messages for the holidays? A card from a truly small family business is one thing, but when you start getting emails from multibillion dollar corporations, it feels a bit dishonest. And that’s not even mentioning the potential blowback when things go wrong.
Now, you may wonder how a company could possibly mess up something so simple. “We wish you a merry Christmas and a happy New Year.” Not that difficult. Unless you’re Askern Medical Practice in Doncaster, England. Instead of expressing a simple expression of joy for the holiday season, Askern informed all 8,000 of its patients that they had aggressive lung cancer with metastases and they needed to fill out a DS1500 form, which entitles terminal patients to certain benefits.
It only took an hour for Askern to recognize its mistake and send a second text apologizing and adding in the appropriate season’s greetings, but obviously the damage was done. Presumably patients who were last at the doctor to have their cold treated were able to shrug off the text, or simply didn’t see it before the correction came through, but obviously many patients had concerns directly related to cancer and panicked. They called in but were by and large unable to reach anyone at the practice. Some patients close by even went to center itself to clear things up.
One patient, Mr. Carl Chegwin, raised an excellent point about the debacle: “What if that message was meant for someone, and then they are told it’s a Christmas message, then again told, ‘Oh no, that was actually meant for you?’ ” The old double backtrack into yes, you actually do have cancer has got to be a candidate for worst Christmas gift of all. Yes, even worse than socks.
Genes know it: You are when you eat
There’s been a lot of recent research on intermittent fasting and what it can and can’t do for one’s health. Much of it has focused on participants’ metabolic rates, but a study just published in Cell Metabolism shows how time-restricted feeding (TRF) has an impact on gene expression, the process through which genes are activated and respond to their environment by creating proteins.
The research conducted by Satchidananda Panda, PhD, of the Salk Institute and his team involved two groups of mice, one with free access to food and the other with a daily 9-hour feeding window. Analysis of tissue samples collected from 22 organ groups revealed that nearly 80% of mouse genes responded to TRF. Interestingly, 40% of the genes in the hypothalamus, adrenal gland, and pancreas, which handle hormone regulation, were affected, suggesting that TRF could potentially aid in diabetes and stress disorder management, the investigators said in a written statement.
The researchers also found that TRF aligned the circadian rhythms of multiple organs of the body, which brings sleep into the picture. “Time-restricted eating synchronized the circadian rhythms to have two major waves: one during fasting, and another just after eating. We suspect this allows the body to coordinate different processes,” said Dr. Panda, whose previous research looked at TRF in firefighters, who typically work on shift schedules.
Time-restricted eating, it appears, affects gene expression throughout the body and allows interconnected organ systems to work smoothly. It’s not just about eating. Go figure.
This group practice reduced stress for everyone
It’s been awhile since we checked in on the good folks at Maharishi International University in Fairfield, Iowa – fictional home of the Fighting Transcendentalists [MAHARISHI RULES!] – but we just have to mention their long-term effort to reduce the national stress.
Way back in the year 2000, a group from MIU began practicing transcendental meditation. The size of the group increased over the next few years and eventually reached 1,725 in 2006. That number is important because it represents the square root of 1% of the U.S. population. When that “transition threshold was achieved,” the university explained in a written statement, “all stress indicators immediately started decreasing.”
By stress indicators they mean the U.S. stress index, the mean of eight variables – murder, rape, assault, robbery, infant mortality, drug deaths, vehicle fatalities, and child deaths by injuries – that the study investigators used to track the effectiveness of the meditation program, they said in the World Journal of Social Science.
After 2011, “when the size of the group size began to decline the rate of decrease in stress slowed and then it reversed and began to increase,” MIU reported.
Coauthor Dr. Kenneth Cavanaugh of MIU explained the process: “This study used state-of-the-art methods of time series regression analysis for eliminating potential alternative explanations due to intrinsic preexisting trends and fluctuations in the data. We carefully studied potential alternative explanations in terms of changes in economic conditions, political leadership, population demographics, and policing strategies. None of these factors could account for the results.”
Since we here at LOTME are serious professional journalists, the use of quotes means we are not making this up. Here’s one more thing in quotes: “A grant for 75 million dollars from the Howard and Alice Settle Foundation provided stipends for participants to be in the group and provided funding to bring several hundred visiting [meditation] experts from India to further augment the MIU group.”
Who needs to make up stuff? Not us.
CHEST simulation courses support learning for every career stage
One mark of an excellent clinician is their commitment to lifelong learning, and CHEST’s hands-on simulation courses offer the chance for practitioners of all experience levels to enhance their knowledge.
A variety of interactive courses are offered at CHEST’s state-of-the-art Innovation, Simulation, and Training Center in Glenview, Illinois, covering topics like ultrasonography, bronchoscopy, and mechanical ventilation. And this year, our simulation schedule will offer several new sessions on advances in invasive and noninvasive ventilation, critical care transesophageal echocardiography, master-level EBUS practice, and mechanical circulatory support.
Each course is led by expert instructors and includes attendees from a full range of career stages, from trainees and mid-career clinicians to long-time CHEST faculty members.
At a fall 2022 session of the Ultrasonography: Essentials in Critical Care course, Adil Ahmed, MD, an intern at the University of Texas Health Science Center at San Antonio, shared his perspective attending as a resident.
“CHEST has lots of specialized resources and renowned faculty members, and they’re doing an exceptional job,” he said. “A lot of the things I’ve learned in the first workshop alone are completely brand new to me. I think more programs should start sending residents to these courses.”
Trainees don’t just attend simulation courses – they teach them, too. Carmen Mei, MD, a pulmonary and critical care fellow at Rutgers University, was a faculty member at the recent ultrasound course. She taught attendees representing a wide array of ages.
“It’s a learning environment. Everybody’s very engaged, no matter where they are in their career,” she said.
As a mid-career clinician, Yonatan Y. Greenstein, MD, FCCP – who serves as a co-chair of the ultrasonography course – appreciates the diversity of experiences among attendees.
“Over the years, we’ve found that the wide breadth enhances the course because learners appreciate the questions that are brought up from different angles,” he said.
For experienced clinicians like CHEST Immediate Past President David Schulman, MD, MPH, FCCP, the interactive courses provide an opportunity to continue expanding his expertise. At the ultrasound course, Dr. Schulman said he enjoyed the chance to extend and refine his skillset alongside clinicians with a broad range of experience levels.
“Ultrasound is one of those skills that many clinicians, even in their forties and older, have never trained in. It’s great to see how the more junior learners approach this with a very excited mindset, and they’re learning right beside mid-career faculty who didn’t have the exposure to ultrasound when they were young,” he said.
To find the simulation course that’s the best fit for your practice, visit chestnet.org/simulation.
One mark of an excellent clinician is their commitment to lifelong learning, and CHEST’s hands-on simulation courses offer the chance for practitioners of all experience levels to enhance their knowledge.
A variety of interactive courses are offered at CHEST’s state-of-the-art Innovation, Simulation, and Training Center in Glenview, Illinois, covering topics like ultrasonography, bronchoscopy, and mechanical ventilation. And this year, our simulation schedule will offer several new sessions on advances in invasive and noninvasive ventilation, critical care transesophageal echocardiography, master-level EBUS practice, and mechanical circulatory support.
Each course is led by expert instructors and includes attendees from a full range of career stages, from trainees and mid-career clinicians to long-time CHEST faculty members.
At a fall 2022 session of the Ultrasonography: Essentials in Critical Care course, Adil Ahmed, MD, an intern at the University of Texas Health Science Center at San Antonio, shared his perspective attending as a resident.
“CHEST has lots of specialized resources and renowned faculty members, and they’re doing an exceptional job,” he said. “A lot of the things I’ve learned in the first workshop alone are completely brand new to me. I think more programs should start sending residents to these courses.”
Trainees don’t just attend simulation courses – they teach them, too. Carmen Mei, MD, a pulmonary and critical care fellow at Rutgers University, was a faculty member at the recent ultrasound course. She taught attendees representing a wide array of ages.
“It’s a learning environment. Everybody’s very engaged, no matter where they are in their career,” she said.
As a mid-career clinician, Yonatan Y. Greenstein, MD, FCCP – who serves as a co-chair of the ultrasonography course – appreciates the diversity of experiences among attendees.
“Over the years, we’ve found that the wide breadth enhances the course because learners appreciate the questions that are brought up from different angles,” he said.
For experienced clinicians like CHEST Immediate Past President David Schulman, MD, MPH, FCCP, the interactive courses provide an opportunity to continue expanding his expertise. At the ultrasound course, Dr. Schulman said he enjoyed the chance to extend and refine his skillset alongside clinicians with a broad range of experience levels.
“Ultrasound is one of those skills that many clinicians, even in their forties and older, have never trained in. It’s great to see how the more junior learners approach this with a very excited mindset, and they’re learning right beside mid-career faculty who didn’t have the exposure to ultrasound when they were young,” he said.
To find the simulation course that’s the best fit for your practice, visit chestnet.org/simulation.
One mark of an excellent clinician is their commitment to lifelong learning, and CHEST’s hands-on simulation courses offer the chance for practitioners of all experience levels to enhance their knowledge.
A variety of interactive courses are offered at CHEST’s state-of-the-art Innovation, Simulation, and Training Center in Glenview, Illinois, covering topics like ultrasonography, bronchoscopy, and mechanical ventilation. And this year, our simulation schedule will offer several new sessions on advances in invasive and noninvasive ventilation, critical care transesophageal echocardiography, master-level EBUS practice, and mechanical circulatory support.
Each course is led by expert instructors and includes attendees from a full range of career stages, from trainees and mid-career clinicians to long-time CHEST faculty members.
At a fall 2022 session of the Ultrasonography: Essentials in Critical Care course, Adil Ahmed, MD, an intern at the University of Texas Health Science Center at San Antonio, shared his perspective attending as a resident.
“CHEST has lots of specialized resources and renowned faculty members, and they’re doing an exceptional job,” he said. “A lot of the things I’ve learned in the first workshop alone are completely brand new to me. I think more programs should start sending residents to these courses.”
Trainees don’t just attend simulation courses – they teach them, too. Carmen Mei, MD, a pulmonary and critical care fellow at Rutgers University, was a faculty member at the recent ultrasound course. She taught attendees representing a wide array of ages.
“It’s a learning environment. Everybody’s very engaged, no matter where they are in their career,” she said.
As a mid-career clinician, Yonatan Y. Greenstein, MD, FCCP – who serves as a co-chair of the ultrasonography course – appreciates the diversity of experiences among attendees.
“Over the years, we’ve found that the wide breadth enhances the course because learners appreciate the questions that are brought up from different angles,” he said.
For experienced clinicians like CHEST Immediate Past President David Schulman, MD, MPH, FCCP, the interactive courses provide an opportunity to continue expanding his expertise. At the ultrasound course, Dr. Schulman said he enjoyed the chance to extend and refine his skillset alongside clinicians with a broad range of experience levels.
“Ultrasound is one of those skills that many clinicians, even in their forties and older, have never trained in. It’s great to see how the more junior learners approach this with a very excited mindset, and they’re learning right beside mid-career faculty who didn’t have the exposure to ultrasound when they were young,” he said.
To find the simulation course that’s the best fit for your practice, visit chestnet.org/simulation.
Defining six asthma subtypes may promote personalized treatment
Six subtypes of asthma that may facilitate personalized treatment were identified and confirmed in a large database review of approximately 50,000 patients, according to a recent study.
Previous studies of asthma subtypes have involved age of disease onset, the presence of allergies, and level of eosinophilic inflammation, and have been limited by factors including small sample size and lack of formal validation, Elsie M.F. Horne, MD, of the Asthma UK Centre for Applied Research, Edinburgh, and colleagues wrote.
In a study published in the International Journal of Medical Informatics, the researchers used data from two databases in the United Kingdom: the Optimum Patient Care Research Database (OPCRD) and the Secure Anonymised Information Linkage Database (SAIL). Each dataset included 50,000 randomly selected nonoverlapping adult asthma patients.
The researchers identified 45 categorical features from primary care electronic health records. The features included those directly linked to asthma, such as medications; and features indirectly linked to asthma, such as comorbidities.
The subtypes were defined by the clinically applicable features of level of inhaled corticosteroid use, level of health care use, and the presence of comorbidities, using multiple correspondence analysis and k-means cluster analysis.
The six asthma subtypes were identified in the OPCRD study population as follows: low inhaled corticosteroid use and low health care utilization (30%); low to medium ICS use (36%); low to medium ICS use and comorbidities (12%); varied ICS use and comorbid chronic obstructive pulmonary disease (4%); high ICS use (10%); and very high ICS use (7%).
The researchers replicated the subtypes with 91%-92% accuracy in an internal dataset and 84%-86% accuracy in an external dataset. “These subtypes generalized well at two future time points, and in an additional EHR database from a different U.K. nation (the SAIL Databank),” they wrote in their discussion.
The findings were limited by several factors including the retrospective design, the possible inclusion of people without asthma because of the cohort selection criteria, and the possible biases associated with the use of EHRs; however, the results were strengthened by the large dataset and the additional validations, the researchers noted.
“Using these subtypes to summarize asthma populations could help with management and resource planning at the practice level, and could be useful for understanding regional differences in the asthma population,” they noted. For example, key clinical implications for individuals in a low health care utilization subtype could include being flagged for barriers to care and misdiagnoses, while those in a high health care utilization subtype could be considered for reassessment of medication and other options.
The study received no outside funding. Lead author Dr. Horne had no financial conflicts to disclose.
Six subtypes of asthma that may facilitate personalized treatment were identified and confirmed in a large database review of approximately 50,000 patients, according to a recent study.
Previous studies of asthma subtypes have involved age of disease onset, the presence of allergies, and level of eosinophilic inflammation, and have been limited by factors including small sample size and lack of formal validation, Elsie M.F. Horne, MD, of the Asthma UK Centre for Applied Research, Edinburgh, and colleagues wrote.
In a study published in the International Journal of Medical Informatics, the researchers used data from two databases in the United Kingdom: the Optimum Patient Care Research Database (OPCRD) and the Secure Anonymised Information Linkage Database (SAIL). Each dataset included 50,000 randomly selected nonoverlapping adult asthma patients.
The researchers identified 45 categorical features from primary care electronic health records. The features included those directly linked to asthma, such as medications; and features indirectly linked to asthma, such as comorbidities.
The subtypes were defined by the clinically applicable features of level of inhaled corticosteroid use, level of health care use, and the presence of comorbidities, using multiple correspondence analysis and k-means cluster analysis.
The six asthma subtypes were identified in the OPCRD study population as follows: low inhaled corticosteroid use and low health care utilization (30%); low to medium ICS use (36%); low to medium ICS use and comorbidities (12%); varied ICS use and comorbid chronic obstructive pulmonary disease (4%); high ICS use (10%); and very high ICS use (7%).
The researchers replicated the subtypes with 91%-92% accuracy in an internal dataset and 84%-86% accuracy in an external dataset. “These subtypes generalized well at two future time points, and in an additional EHR database from a different U.K. nation (the SAIL Databank),” they wrote in their discussion.
The findings were limited by several factors including the retrospective design, the possible inclusion of people without asthma because of the cohort selection criteria, and the possible biases associated with the use of EHRs; however, the results were strengthened by the large dataset and the additional validations, the researchers noted.
“Using these subtypes to summarize asthma populations could help with management and resource planning at the practice level, and could be useful for understanding regional differences in the asthma population,” they noted. For example, key clinical implications for individuals in a low health care utilization subtype could include being flagged for barriers to care and misdiagnoses, while those in a high health care utilization subtype could be considered for reassessment of medication and other options.
The study received no outside funding. Lead author Dr. Horne had no financial conflicts to disclose.
Six subtypes of asthma that may facilitate personalized treatment were identified and confirmed in a large database review of approximately 50,000 patients, according to a recent study.
Previous studies of asthma subtypes have involved age of disease onset, the presence of allergies, and level of eosinophilic inflammation, and have been limited by factors including small sample size and lack of formal validation, Elsie M.F. Horne, MD, of the Asthma UK Centre for Applied Research, Edinburgh, and colleagues wrote.
In a study published in the International Journal of Medical Informatics, the researchers used data from two databases in the United Kingdom: the Optimum Patient Care Research Database (OPCRD) and the Secure Anonymised Information Linkage Database (SAIL). Each dataset included 50,000 randomly selected nonoverlapping adult asthma patients.
The researchers identified 45 categorical features from primary care electronic health records. The features included those directly linked to asthma, such as medications; and features indirectly linked to asthma, such as comorbidities.
The subtypes were defined by the clinically applicable features of level of inhaled corticosteroid use, level of health care use, and the presence of comorbidities, using multiple correspondence analysis and k-means cluster analysis.
The six asthma subtypes were identified in the OPCRD study population as follows: low inhaled corticosteroid use and low health care utilization (30%); low to medium ICS use (36%); low to medium ICS use and comorbidities (12%); varied ICS use and comorbid chronic obstructive pulmonary disease (4%); high ICS use (10%); and very high ICS use (7%).
The researchers replicated the subtypes with 91%-92% accuracy in an internal dataset and 84%-86% accuracy in an external dataset. “These subtypes generalized well at two future time points, and in an additional EHR database from a different U.K. nation (the SAIL Databank),” they wrote in their discussion.
The findings were limited by several factors including the retrospective design, the possible inclusion of people without asthma because of the cohort selection criteria, and the possible biases associated with the use of EHRs; however, the results were strengthened by the large dataset and the additional validations, the researchers noted.
“Using these subtypes to summarize asthma populations could help with management and resource planning at the practice level, and could be useful for understanding regional differences in the asthma population,” they noted. For example, key clinical implications for individuals in a low health care utilization subtype could include being flagged for barriers to care and misdiagnoses, while those in a high health care utilization subtype could be considered for reassessment of medication and other options.
The study received no outside funding. Lead author Dr. Horne had no financial conflicts to disclose.
FROM THE INTERNATIONAL JOURNAL OF MEDICAL INFORMATICS
CHEST President shares inside look at priorities, plans for 2023
Attendees at the CHEST 2022 Opening Session on October 16 got a sneak peek into plans and priorities for CHEST President Doreen J. Addrizzo-Harris, MD, FCCP, in 2023 – and some insights into her own path to the role.
A longtime leader at CHEST, she shared how members’ pandemic response reminded her of the great impact the organization can have. In March 2020, Dr. Addrizzo-Harris was overseeing ICU staffing at NYU Langone Health’s Bellevue Hospital Center and organizing dozens of volunteer physicians to help meet the pandemic care burden.
“I knew all too quickly that we wouldn’t have enough intensivists,” said Dr. Addrizzo-Harris. “It was a quick call very late one night, probably around 1 am, that I made to CHEST CEO, Bob Musacchio, that helped materialize a monumental effort ... many of these physicians were CHEST members themselves. They were fearless and unselfish, and they came to help us in our time of need.”
She saw this same spirit of dedication and drive in CHEST’s leadership and staff, she said – one she will continue and expand upon during her presidency.
“I’ve watched our last three presidents lead by great example ... with innovation and nimbleness, in a time when we were so isolated from each other and so tired from the long hours that we worked each day,” she said. “They, along with the Board of Regents, the CEO, and our phenomenal staff, were able to keep CHEST amazingly alive and vibrant and more connected than ever. They are truly inspiring. For 2023, I hope to take this incredible energy to the next level.”
As CHEST president, Dr. in the United States and advancing international outreach initiatives launched by CHEST Past President David Schulman, MD, MPH, FCCP. This also includes supporting and building upon CHEST’s ongoing commitment to diversity, equity, and inclusion initiatives to encourage greater representation in the field and improve patient care.
“Whether it’s through supporting our clinical research grants, expanding patient education and advocacy, or programs like the First 5 Minutes™ and the Harold Amos scholarship program, we want to train our leaders for the future,” she said.
Revisit the September issue of CHEST Physician, and watch future issues to learn more about Dr. Addrizzo-Harris and her plans for the presidency.
Attendees at the CHEST 2022 Opening Session on October 16 got a sneak peek into plans and priorities for CHEST President Doreen J. Addrizzo-Harris, MD, FCCP, in 2023 – and some insights into her own path to the role.
A longtime leader at CHEST, she shared how members’ pandemic response reminded her of the great impact the organization can have. In March 2020, Dr. Addrizzo-Harris was overseeing ICU staffing at NYU Langone Health’s Bellevue Hospital Center and organizing dozens of volunteer physicians to help meet the pandemic care burden.
“I knew all too quickly that we wouldn’t have enough intensivists,” said Dr. Addrizzo-Harris. “It was a quick call very late one night, probably around 1 am, that I made to CHEST CEO, Bob Musacchio, that helped materialize a monumental effort ... many of these physicians were CHEST members themselves. They were fearless and unselfish, and they came to help us in our time of need.”
She saw this same spirit of dedication and drive in CHEST’s leadership and staff, she said – one she will continue and expand upon during her presidency.
“I’ve watched our last three presidents lead by great example ... with innovation and nimbleness, in a time when we were so isolated from each other and so tired from the long hours that we worked each day,” she said. “They, along with the Board of Regents, the CEO, and our phenomenal staff, were able to keep CHEST amazingly alive and vibrant and more connected than ever. They are truly inspiring. For 2023, I hope to take this incredible energy to the next level.”
As CHEST president, Dr. in the United States and advancing international outreach initiatives launched by CHEST Past President David Schulman, MD, MPH, FCCP. This also includes supporting and building upon CHEST’s ongoing commitment to diversity, equity, and inclusion initiatives to encourage greater representation in the field and improve patient care.
“Whether it’s through supporting our clinical research grants, expanding patient education and advocacy, or programs like the First 5 Minutes™ and the Harold Amos scholarship program, we want to train our leaders for the future,” she said.
Revisit the September issue of CHEST Physician, and watch future issues to learn more about Dr. Addrizzo-Harris and her plans for the presidency.
Attendees at the CHEST 2022 Opening Session on October 16 got a sneak peek into plans and priorities for CHEST President Doreen J. Addrizzo-Harris, MD, FCCP, in 2023 – and some insights into her own path to the role.
A longtime leader at CHEST, she shared how members’ pandemic response reminded her of the great impact the organization can have. In March 2020, Dr. Addrizzo-Harris was overseeing ICU staffing at NYU Langone Health’s Bellevue Hospital Center and organizing dozens of volunteer physicians to help meet the pandemic care burden.
“I knew all too quickly that we wouldn’t have enough intensivists,” said Dr. Addrizzo-Harris. “It was a quick call very late one night, probably around 1 am, that I made to CHEST CEO, Bob Musacchio, that helped materialize a monumental effort ... many of these physicians were CHEST members themselves. They were fearless and unselfish, and they came to help us in our time of need.”
She saw this same spirit of dedication and drive in CHEST’s leadership and staff, she said – one she will continue and expand upon during her presidency.
“I’ve watched our last three presidents lead by great example ... with innovation and nimbleness, in a time when we were so isolated from each other and so tired from the long hours that we worked each day,” she said. “They, along with the Board of Regents, the CEO, and our phenomenal staff, were able to keep CHEST amazingly alive and vibrant and more connected than ever. They are truly inspiring. For 2023, I hope to take this incredible energy to the next level.”
As CHEST president, Dr. in the United States and advancing international outreach initiatives launched by CHEST Past President David Schulman, MD, MPH, FCCP. This also includes supporting and building upon CHEST’s ongoing commitment to diversity, equity, and inclusion initiatives to encourage greater representation in the field and improve patient care.
“Whether it’s through supporting our clinical research grants, expanding patient education and advocacy, or programs like the First 5 Minutes™ and the Harold Amos scholarship program, we want to train our leaders for the future,” she said.
Revisit the September issue of CHEST Physician, and watch future issues to learn more about Dr. Addrizzo-Harris and her plans for the presidency.
CHEST 2022 award winners More award winners
Each year,
MASTER FELLOW AWARD
Gerard A. Silvestri, MD, MS, Master FCCP
DISTINGUISHED SERVICE AWARD
Aneesa M. Das, MD, FCCP
COLLEGE MEDALIST AWARD
William R. Auger, MD, FCCP
ALFRED SOFFER AWARD FOR EDITORIAL EXCELLENCE
Todd W. Rice, MD, FCCP
EARLY CAREER CLINICIAN EDUCATOR AWARD
Mauricio Danckers, MD, FCCP
MASTER CLINICIAN EDUCATOR AWARD
Neil R. MacIntyre, MD, FCCP
PRESIDENTIAL CITATION
CHEST Staff
EDWARD C. ROSENOW III, MD, MASTER FCCP/MASTER TEACHER ENDOWED HONOR LECTURE
Alexander S. Niven, MD, FCCP
THOMAS L. PETTY, MD, MASTER FCCP MEMORIAL LECTURE
Sandra G. Adams, MD, FCCP
2021 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Kenneth I. Berger, MD, FCCP
PRESIDENTIAL HONOR LECTURE
Jack D. Buckley, MD, MPH, FCCP
PASQUALE CIAGLIA MEMORIAL LECTURE IN INTERVENTIONAL MEDICINE
Nicholas J. Pastis, MD, FCCP
ROGER C. BONE MEMORIAL LECTURE IN CRITICAL CARE
E. Wesley Ely, MD, MPH, FCCP
MURRAY KORNFELD MEMORIAL FOUNDERS AWARD
Marin H. Kollef, MD, FCCP
OM P. SHARMA, MD, MASTER FCCP MEMORIAL LECTURE
Daniel A. Culver, DO, FCCP
RICHARD S. IRWIN, MD, MASTER FCCP HONOR LECTURE
Nneka O. Sederstrom, PhD, MS, MA, FCCP
2022 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Martin J. Tobin, MBBCh, FCCP
MARK J. ROSEN, MD, MASTER FCCP ENDOWED MEMORIAL LECTURE
Stephanie M. Levine, MD, FCCP
MARGARET PFROMMER ENDOWED MEMORIAL LECTURE IN HOME-BASED MECHANICAL VENTILATION
Lisa Wolfe, MD, FCCP
CHEST CHALLENGE FINALISTS
1st Place – Mayo Clinic
Amjad Kanj, MD
Paige Marty, MD
Zhenmei Zhang, MD
Program Director: Darlene Nelson, MD, FCCP
2nd Place – Brooke Army Medical Center
Joshua Boster, MD
Tyler Campbell, DO
Daniel Foster, MD
Program Director: Robert Walter, MD, PhD
3rd Place – NewYork-Presbyterian Brooklyn Methodist
Albina Guri, DO
Jahrul Islam, MD
Sylvana Salama, MD
Program Director: Anthony Saleh, MD, FCCP
Please Note: Award winners from the following categories will be listed in the February issue of CHEST Physician.
CHEST Foundation Grant Awards
Scientific Abstract Awards
Alfred Soffer Research Award Winners
Young Investigator Award Winners
Abstract Rapid Fire Winners
Case Report Session Winners
Case Report Rapid Fire Winners
Each year,
MASTER FELLOW AWARD
Gerard A. Silvestri, MD, MS, Master FCCP
DISTINGUISHED SERVICE AWARD
Aneesa M. Das, MD, FCCP
COLLEGE MEDALIST AWARD
William R. Auger, MD, FCCP
ALFRED SOFFER AWARD FOR EDITORIAL EXCELLENCE
Todd W. Rice, MD, FCCP
EARLY CAREER CLINICIAN EDUCATOR AWARD
Mauricio Danckers, MD, FCCP
MASTER CLINICIAN EDUCATOR AWARD
Neil R. MacIntyre, MD, FCCP
PRESIDENTIAL CITATION
CHEST Staff
EDWARD C. ROSENOW III, MD, MASTER FCCP/MASTER TEACHER ENDOWED HONOR LECTURE
Alexander S. Niven, MD, FCCP
THOMAS L. PETTY, MD, MASTER FCCP MEMORIAL LECTURE
Sandra G. Adams, MD, FCCP
2021 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Kenneth I. Berger, MD, FCCP
PRESIDENTIAL HONOR LECTURE
Jack D. Buckley, MD, MPH, FCCP
PASQUALE CIAGLIA MEMORIAL LECTURE IN INTERVENTIONAL MEDICINE
Nicholas J. Pastis, MD, FCCP
ROGER C. BONE MEMORIAL LECTURE IN CRITICAL CARE
E. Wesley Ely, MD, MPH, FCCP
MURRAY KORNFELD MEMORIAL FOUNDERS AWARD
Marin H. Kollef, MD, FCCP
OM P. SHARMA, MD, MASTER FCCP MEMORIAL LECTURE
Daniel A. Culver, DO, FCCP
RICHARD S. IRWIN, MD, MASTER FCCP HONOR LECTURE
Nneka O. Sederstrom, PhD, MS, MA, FCCP
2022 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Martin J. Tobin, MBBCh, FCCP
MARK J. ROSEN, MD, MASTER FCCP ENDOWED MEMORIAL LECTURE
Stephanie M. Levine, MD, FCCP
MARGARET PFROMMER ENDOWED MEMORIAL LECTURE IN HOME-BASED MECHANICAL VENTILATION
Lisa Wolfe, MD, FCCP
CHEST CHALLENGE FINALISTS
1st Place – Mayo Clinic
Amjad Kanj, MD
Paige Marty, MD
Zhenmei Zhang, MD
Program Director: Darlene Nelson, MD, FCCP
2nd Place – Brooke Army Medical Center
Joshua Boster, MD
Tyler Campbell, DO
Daniel Foster, MD
Program Director: Robert Walter, MD, PhD
3rd Place – NewYork-Presbyterian Brooklyn Methodist
Albina Guri, DO
Jahrul Islam, MD
Sylvana Salama, MD
Program Director: Anthony Saleh, MD, FCCP
Please Note: Award winners from the following categories will be listed in the February issue of CHEST Physician.
CHEST Foundation Grant Awards
Scientific Abstract Awards
Alfred Soffer Research Award Winners
Young Investigator Award Winners
Abstract Rapid Fire Winners
Case Report Session Winners
Case Report Rapid Fire Winners
Each year,
MASTER FELLOW AWARD
Gerard A. Silvestri, MD, MS, Master FCCP
DISTINGUISHED SERVICE AWARD
Aneesa M. Das, MD, FCCP
COLLEGE MEDALIST AWARD
William R. Auger, MD, FCCP
ALFRED SOFFER AWARD FOR EDITORIAL EXCELLENCE
Todd W. Rice, MD, FCCP
EARLY CAREER CLINICIAN EDUCATOR AWARD
Mauricio Danckers, MD, FCCP
MASTER CLINICIAN EDUCATOR AWARD
Neil R. MacIntyre, MD, FCCP
PRESIDENTIAL CITATION
CHEST Staff
EDWARD C. ROSENOW III, MD, MASTER FCCP/MASTER TEACHER ENDOWED HONOR LECTURE
Alexander S. Niven, MD, FCCP
THOMAS L. PETTY, MD, MASTER FCCP MEMORIAL LECTURE
Sandra G. Adams, MD, FCCP
2021 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Kenneth I. Berger, MD, FCCP
PRESIDENTIAL HONOR LECTURE
Jack D. Buckley, MD, MPH, FCCP
PASQUALE CIAGLIA MEMORIAL LECTURE IN INTERVENTIONAL MEDICINE
Nicholas J. Pastis, MD, FCCP
ROGER C. BONE MEMORIAL LECTURE IN CRITICAL CARE
E. Wesley Ely, MD, MPH, FCCP
MURRAY KORNFELD MEMORIAL FOUNDERS AWARD
Marin H. Kollef, MD, FCCP
OM P. SHARMA, MD, MASTER FCCP MEMORIAL LECTURE
Daniel A. Culver, DO, FCCP
RICHARD S. IRWIN, MD, MASTER FCCP HONOR LECTURE
Nneka O. Sederstrom, PhD, MS, MA, FCCP
2022 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Martin J. Tobin, MBBCh, FCCP
MARK J. ROSEN, MD, MASTER FCCP ENDOWED MEMORIAL LECTURE
Stephanie M. Levine, MD, FCCP
MARGARET PFROMMER ENDOWED MEMORIAL LECTURE IN HOME-BASED MECHANICAL VENTILATION
Lisa Wolfe, MD, FCCP
CHEST CHALLENGE FINALISTS
1st Place – Mayo Clinic
Amjad Kanj, MD
Paige Marty, MD
Zhenmei Zhang, MD
Program Director: Darlene Nelson, MD, FCCP
2nd Place – Brooke Army Medical Center
Joshua Boster, MD
Tyler Campbell, DO
Daniel Foster, MD
Program Director: Robert Walter, MD, PhD
3rd Place – NewYork-Presbyterian Brooklyn Methodist
Albina Guri, DO
Jahrul Islam, MD
Sylvana Salama, MD
Program Director: Anthony Saleh, MD, FCCP
Please Note: Award winners from the following categories will be listed in the February issue of CHEST Physician.
CHEST Foundation Grant Awards
Scientific Abstract Awards
Alfred Soffer Research Award Winners
Young Investigator Award Winners
Abstract Rapid Fire Winners
Case Report Session Winners
Case Report Rapid Fire Winners
CHEST Challenge returned to the stage in the Music City
After several years of virtual competitions, the CHEST Challenge Championship returned to the stage at CHEST 2022 in Nashville, where outstanding fellows from Brooke Army Medical Center, Mayo Clinic, and NewYork-Presbyterian Brooklyn Methodist battled to compete in unconventional skills challenges and clinical trivia.
After an excellent showing from all three institutions, (not to mention, the ultimate bragging rights and the chance to raise the coveted Rosen Cup). Runner-up Brooke Army Medical Center received $3,000, and NewYork-Presbyterian Brooklyn Methodist received $1,000.
This year’s Jeopardy-style championship included a variety of category types, including everything from straightforward clinical answers in “Asthmalogic” about asthma-related issues and “Under a Microscope” for topics related to histopathology, to brain-boggling alternate options, such as “Rhyme Time,” which twisted answers in rhyming phrases.
The competition also included timed skills challenges that tested the competitors physically – and presented some very special guests.
In “Bugs and Drugs,” Team Methodist sprinted to grab and then matched unlabeled pathogen photographs with their appropriate therapeutic agents in less than 35 seconds. In another, Team Brooke aced the challenge of performing timed procedures on three different body parts in Dr. Frankenstein’s laboratory, while the monster himself (played by Board of Regents member Victor J. Test, MD, FCCP) worked to distract them.
Mayo Clinic was already in the lead by the time the Final Challenge wager was presented by William Kelly, MD, FCCP, so the team responded to the answer “This disease is inherited as an autosomal recessive trait and is a variant in the SCL34A2 gene” with their own unique reply: “Thank you, CHEST,” and a symbolic wager of $22.
Drs. Kanj, Marty, and Zhang credited their success to their training program back home, as well as the support of friends and colleagues on-site, including Program Director, Darlene Nelson, MD, FCCP. The team also prepared with mock sessions days before the championship and had a strong fan base cheering them on in the audience.
Want to join rising stars in pulmonary, critical care, and sleep medicine for next year’s championship in Hawai’i? Watch CHEST’s social media in the spring for the first phase of CHEST Challenge.
After several years of virtual competitions, the CHEST Challenge Championship returned to the stage at CHEST 2022 in Nashville, where outstanding fellows from Brooke Army Medical Center, Mayo Clinic, and NewYork-Presbyterian Brooklyn Methodist battled to compete in unconventional skills challenges and clinical trivia.
After an excellent showing from all three institutions, (not to mention, the ultimate bragging rights and the chance to raise the coveted Rosen Cup). Runner-up Brooke Army Medical Center received $3,000, and NewYork-Presbyterian Brooklyn Methodist received $1,000.
This year’s Jeopardy-style championship included a variety of category types, including everything from straightforward clinical answers in “Asthmalogic” about asthma-related issues and “Under a Microscope” for topics related to histopathology, to brain-boggling alternate options, such as “Rhyme Time,” which twisted answers in rhyming phrases.
The competition also included timed skills challenges that tested the competitors physically – and presented some very special guests.
In “Bugs and Drugs,” Team Methodist sprinted to grab and then matched unlabeled pathogen photographs with their appropriate therapeutic agents in less than 35 seconds. In another, Team Brooke aced the challenge of performing timed procedures on three different body parts in Dr. Frankenstein’s laboratory, while the monster himself (played by Board of Regents member Victor J. Test, MD, FCCP) worked to distract them.
Mayo Clinic was already in the lead by the time the Final Challenge wager was presented by William Kelly, MD, FCCP, so the team responded to the answer “This disease is inherited as an autosomal recessive trait and is a variant in the SCL34A2 gene” with their own unique reply: “Thank you, CHEST,” and a symbolic wager of $22.
Drs. Kanj, Marty, and Zhang credited their success to their training program back home, as well as the support of friends and colleagues on-site, including Program Director, Darlene Nelson, MD, FCCP. The team also prepared with mock sessions days before the championship and had a strong fan base cheering them on in the audience.
Want to join rising stars in pulmonary, critical care, and sleep medicine for next year’s championship in Hawai’i? Watch CHEST’s social media in the spring for the first phase of CHEST Challenge.
After several years of virtual competitions, the CHEST Challenge Championship returned to the stage at CHEST 2022 in Nashville, where outstanding fellows from Brooke Army Medical Center, Mayo Clinic, and NewYork-Presbyterian Brooklyn Methodist battled to compete in unconventional skills challenges and clinical trivia.
After an excellent showing from all three institutions, (not to mention, the ultimate bragging rights and the chance to raise the coveted Rosen Cup). Runner-up Brooke Army Medical Center received $3,000, and NewYork-Presbyterian Brooklyn Methodist received $1,000.
This year’s Jeopardy-style championship included a variety of category types, including everything from straightforward clinical answers in “Asthmalogic” about asthma-related issues and “Under a Microscope” for topics related to histopathology, to brain-boggling alternate options, such as “Rhyme Time,” which twisted answers in rhyming phrases.
The competition also included timed skills challenges that tested the competitors physically – and presented some very special guests.
In “Bugs and Drugs,” Team Methodist sprinted to grab and then matched unlabeled pathogen photographs with their appropriate therapeutic agents in less than 35 seconds. In another, Team Brooke aced the challenge of performing timed procedures on three different body parts in Dr. Frankenstein’s laboratory, while the monster himself (played by Board of Regents member Victor J. Test, MD, FCCP) worked to distract them.
Mayo Clinic was already in the lead by the time the Final Challenge wager was presented by William Kelly, MD, FCCP, so the team responded to the answer “This disease is inherited as an autosomal recessive trait and is a variant in the SCL34A2 gene” with their own unique reply: “Thank you, CHEST,” and a symbolic wager of $22.
Drs. Kanj, Marty, and Zhang credited their success to their training program back home, as well as the support of friends and colleagues on-site, including Program Director, Darlene Nelson, MD, FCCP. The team also prepared with mock sessions days before the championship and had a strong fan base cheering them on in the audience.
Want to join rising stars in pulmonary, critical care, and sleep medicine for next year’s championship in Hawai’i? Watch CHEST’s social media in the spring for the first phase of CHEST Challenge.
The latest on ERS/ATS lung function interpretation guidelines and bronchodilator testing
The European Respiratory Society (ERS) and the American Thoracic Society (ATS) just published an updated technical standard on lung function interpretation. It’s impossible to review in its entirety without more space, so I’ll settle for covering what the authors say about bronchodilator testing. But before I do that, it’s worth reviewing what we think we know about having a patient perform spirometry, inhale a bronchodilator, and then repeat it. This is colloquially referred to as pre- and postbronchodilator testing.
Administering a bronchodilator and measuring changes in lung function seems simple and intuitive. It is biologically plausible that improvement would predict treatment response. It should allow for phenotyping airway diseases and quantifying exacerbation risk. It is easy to perform and can be done in the clinic. But in practice it falls short of its purpose, in part because of technical factors but also because it doesn’t really have a purpose.
The last interpretative strategies document from the ERS/ATS was published in 2005. Reading it many years ago, I was struck by the contrast between our reliance on bronchodilator response and its lack of standardization. It seemed that there was none. After making statements like, “There is no consensus on what constitutes reversibility in subjects with airflow obstruction” and “There is no consensus on how a bronchodilator response should be expressed, the variables to be used, and finally, the kind, dose, and inhalation mode of bronchodilator agent,” the 2005 ERS/ATS authors suggest using the criteria most clinicians are familiar with: A change of 12% and 200 cc in FEV1 or FVC marks a “significant” bronchodilator response. Four puffs of albuterol (100 mcg each for a total of 400 mcg) with a 15- to 20-minute wait before repeat spirometry is also suggested.
The 2005 iteration acknowledges that a significant bronchodilator response isn’t a very accurate predictor of, well, anything. It doesn’t reliably differentiate COPD from asthma and it’s never been as sensitive as bronchoprovocation testing for diagnosing airway reactivity. The absence of a significant bronchodilator response does not preclude a 2-month trial of the same medicine used to test for response. Given these problems with standardization and accuracy, I was left wondering why anyone bothers ordering the test at all.
In my own practice, I continued to order, conduct, and interpret bronchodilator response according to the suggestions made by the ERS/ATS in 2005 when trying to diagnose asthma. I recognized that a nonsignificant response meant nothing, but bronchodilator response testing was easier to obtain than bronchoprovocation at my hospital. It was a matter of convenience for me and the patient. According to the Global Initiative for Asthma (GINA) Guidelines, a significant bronchodilator response conducted and interpreted as recommended by the ERS/ATS 2005 standard provides objective confirmation of asthma in the presence of characteristic clinical symptoms.
The headline from the ERS/ATS 2022 Technical Standard is that the 12% and 200-cc criteria suggested in 2005 are being retired. Why? Well, much of the variability in the 2005 criteria is explained by height, age, sex, and baseline lung function. These factors obscure change related to intrinsic airway abnormalities. Instead, the authors suggest using a threshold change in the predicted values of FEV1 and FVC to determine a significant response. Because predicted values incorporate age, height, and sex, the impact from these variables is minimized. Using a percent predicted (PPD) threshold will also minimize the effect from the inverse relationship between measured values and bronchodilator response.
A 10% change in the PPD value for either FEV1 or FVC constitutes a significant bronchodilator response. Ten percent was chosen because it represents the statistically defined upper limit of normal response; and a greater than 8% change in bronchodilator response is associated with mortality, implying that values above this threshold connote disease. The technical standard seems to be on solid ground here; the rationale is mathematically appropriate and evidence based. The new definition will certainly improve precision.
There’s really no progress on accuracy, though. There are no comments on the protocol to be followed or clinical indications. The reader is referred to the ERS/ATS 2019 technical statement on standardization of spirometry. The statement on standardization is short on details, too, and refers the reader to an online supplement for a suggested protocol. The suggested protocol is identical to that suggested in 2005.
In summary, not a lot is different in the world of bronchodilator response testing. The definition is different now, and though it’s likely to be more precise, we still don’t know enough about accuracy. It’s nice to know that the new criteria will predict mortality, but in clinical practice we don’t use the test for that purpose. The 2022 technical standard acknowledges this and other limitations in a “future directions” paragraph. Perhaps we’ll know more when the next iteration is published.
Dr. Holley is a professor of medicine at Uniformed Services University of the Health Sciences. Bethesda, Md., and a pulmonary medicine/critical care physician at MedStar Washington Hospital Center, Washington. He reported conflicts of interest with Metapharm and the American College of Chest Physicians. A version of this article first appeared on Medscape.com.
The European Respiratory Society (ERS) and the American Thoracic Society (ATS) just published an updated technical standard on lung function interpretation. It’s impossible to review in its entirety without more space, so I’ll settle for covering what the authors say about bronchodilator testing. But before I do that, it’s worth reviewing what we think we know about having a patient perform spirometry, inhale a bronchodilator, and then repeat it. This is colloquially referred to as pre- and postbronchodilator testing.
Administering a bronchodilator and measuring changes in lung function seems simple and intuitive. It is biologically plausible that improvement would predict treatment response. It should allow for phenotyping airway diseases and quantifying exacerbation risk. It is easy to perform and can be done in the clinic. But in practice it falls short of its purpose, in part because of technical factors but also because it doesn’t really have a purpose.
The last interpretative strategies document from the ERS/ATS was published in 2005. Reading it many years ago, I was struck by the contrast between our reliance on bronchodilator response and its lack of standardization. It seemed that there was none. After making statements like, “There is no consensus on what constitutes reversibility in subjects with airflow obstruction” and “There is no consensus on how a bronchodilator response should be expressed, the variables to be used, and finally, the kind, dose, and inhalation mode of bronchodilator agent,” the 2005 ERS/ATS authors suggest using the criteria most clinicians are familiar with: A change of 12% and 200 cc in FEV1 or FVC marks a “significant” bronchodilator response. Four puffs of albuterol (100 mcg each for a total of 400 mcg) with a 15- to 20-minute wait before repeat spirometry is also suggested.
The 2005 iteration acknowledges that a significant bronchodilator response isn’t a very accurate predictor of, well, anything. It doesn’t reliably differentiate COPD from asthma and it’s never been as sensitive as bronchoprovocation testing for diagnosing airway reactivity. The absence of a significant bronchodilator response does not preclude a 2-month trial of the same medicine used to test for response. Given these problems with standardization and accuracy, I was left wondering why anyone bothers ordering the test at all.
In my own practice, I continued to order, conduct, and interpret bronchodilator response according to the suggestions made by the ERS/ATS in 2005 when trying to diagnose asthma. I recognized that a nonsignificant response meant nothing, but bronchodilator response testing was easier to obtain than bronchoprovocation at my hospital. It was a matter of convenience for me and the patient. According to the Global Initiative for Asthma (GINA) Guidelines, a significant bronchodilator response conducted and interpreted as recommended by the ERS/ATS 2005 standard provides objective confirmation of asthma in the presence of characteristic clinical symptoms.
The headline from the ERS/ATS 2022 Technical Standard is that the 12% and 200-cc criteria suggested in 2005 are being retired. Why? Well, much of the variability in the 2005 criteria is explained by height, age, sex, and baseline lung function. These factors obscure change related to intrinsic airway abnormalities. Instead, the authors suggest using a threshold change in the predicted values of FEV1 and FVC to determine a significant response. Because predicted values incorporate age, height, and sex, the impact from these variables is minimized. Using a percent predicted (PPD) threshold will also minimize the effect from the inverse relationship between measured values and bronchodilator response.
A 10% change in the PPD value for either FEV1 or FVC constitutes a significant bronchodilator response. Ten percent was chosen because it represents the statistically defined upper limit of normal response; and a greater than 8% change in bronchodilator response is associated with mortality, implying that values above this threshold connote disease. The technical standard seems to be on solid ground here; the rationale is mathematically appropriate and evidence based. The new definition will certainly improve precision.
There’s really no progress on accuracy, though. There are no comments on the protocol to be followed or clinical indications. The reader is referred to the ERS/ATS 2019 technical statement on standardization of spirometry. The statement on standardization is short on details, too, and refers the reader to an online supplement for a suggested protocol. The suggested protocol is identical to that suggested in 2005.
In summary, not a lot is different in the world of bronchodilator response testing. The definition is different now, and though it’s likely to be more precise, we still don’t know enough about accuracy. It’s nice to know that the new criteria will predict mortality, but in clinical practice we don’t use the test for that purpose. The 2022 technical standard acknowledges this and other limitations in a “future directions” paragraph. Perhaps we’ll know more when the next iteration is published.
Dr. Holley is a professor of medicine at Uniformed Services University of the Health Sciences. Bethesda, Md., and a pulmonary medicine/critical care physician at MedStar Washington Hospital Center, Washington. He reported conflicts of interest with Metapharm and the American College of Chest Physicians. A version of this article first appeared on Medscape.com.
The European Respiratory Society (ERS) and the American Thoracic Society (ATS) just published an updated technical standard on lung function interpretation. It’s impossible to review in its entirety without more space, so I’ll settle for covering what the authors say about bronchodilator testing. But before I do that, it’s worth reviewing what we think we know about having a patient perform spirometry, inhale a bronchodilator, and then repeat it. This is colloquially referred to as pre- and postbronchodilator testing.
Administering a bronchodilator and measuring changes in lung function seems simple and intuitive. It is biologically plausible that improvement would predict treatment response. It should allow for phenotyping airway diseases and quantifying exacerbation risk. It is easy to perform and can be done in the clinic. But in practice it falls short of its purpose, in part because of technical factors but also because it doesn’t really have a purpose.
The last interpretative strategies document from the ERS/ATS was published in 2005. Reading it many years ago, I was struck by the contrast between our reliance on bronchodilator response and its lack of standardization. It seemed that there was none. After making statements like, “There is no consensus on what constitutes reversibility in subjects with airflow obstruction” and “There is no consensus on how a bronchodilator response should be expressed, the variables to be used, and finally, the kind, dose, and inhalation mode of bronchodilator agent,” the 2005 ERS/ATS authors suggest using the criteria most clinicians are familiar with: A change of 12% and 200 cc in FEV1 or FVC marks a “significant” bronchodilator response. Four puffs of albuterol (100 mcg each for a total of 400 mcg) with a 15- to 20-minute wait before repeat spirometry is also suggested.
The 2005 iteration acknowledges that a significant bronchodilator response isn’t a very accurate predictor of, well, anything. It doesn’t reliably differentiate COPD from asthma and it’s never been as sensitive as bronchoprovocation testing for diagnosing airway reactivity. The absence of a significant bronchodilator response does not preclude a 2-month trial of the same medicine used to test for response. Given these problems with standardization and accuracy, I was left wondering why anyone bothers ordering the test at all.
In my own practice, I continued to order, conduct, and interpret bronchodilator response according to the suggestions made by the ERS/ATS in 2005 when trying to diagnose asthma. I recognized that a nonsignificant response meant nothing, but bronchodilator response testing was easier to obtain than bronchoprovocation at my hospital. It was a matter of convenience for me and the patient. According to the Global Initiative for Asthma (GINA) Guidelines, a significant bronchodilator response conducted and interpreted as recommended by the ERS/ATS 2005 standard provides objective confirmation of asthma in the presence of characteristic clinical symptoms.
The headline from the ERS/ATS 2022 Technical Standard is that the 12% and 200-cc criteria suggested in 2005 are being retired. Why? Well, much of the variability in the 2005 criteria is explained by height, age, sex, and baseline lung function. These factors obscure change related to intrinsic airway abnormalities. Instead, the authors suggest using a threshold change in the predicted values of FEV1 and FVC to determine a significant response. Because predicted values incorporate age, height, and sex, the impact from these variables is minimized. Using a percent predicted (PPD) threshold will also minimize the effect from the inverse relationship between measured values and bronchodilator response.
A 10% change in the PPD value for either FEV1 or FVC constitutes a significant bronchodilator response. Ten percent was chosen because it represents the statistically defined upper limit of normal response; and a greater than 8% change in bronchodilator response is associated with mortality, implying that values above this threshold connote disease. The technical standard seems to be on solid ground here; the rationale is mathematically appropriate and evidence based. The new definition will certainly improve precision.
There’s really no progress on accuracy, though. There are no comments on the protocol to be followed or clinical indications. The reader is referred to the ERS/ATS 2019 technical statement on standardization of spirometry. The statement on standardization is short on details, too, and refers the reader to an online supplement for a suggested protocol. The suggested protocol is identical to that suggested in 2005.
In summary, not a lot is different in the world of bronchodilator response testing. The definition is different now, and though it’s likely to be more precise, we still don’t know enough about accuracy. It’s nice to know that the new criteria will predict mortality, but in clinical practice we don’t use the test for that purpose. The 2022 technical standard acknowledges this and other limitations in a “future directions” paragraph. Perhaps we’ll know more when the next iteration is published.
Dr. Holley is a professor of medicine at Uniformed Services University of the Health Sciences. Bethesda, Md., and a pulmonary medicine/critical care physician at MedStar Washington Hospital Center, Washington. He reported conflicts of interest with Metapharm and the American College of Chest Physicians. A version of this article first appeared on Medscape.com.
Children and COVID: New cases fell as the old year ended
The end of 2022 saw a drop in new COVID-19 cases in children, even as rates of emergency department visits continued upward trends that began in late October.
New cases for the week of Dec. 23-29 fell for the first time since late November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP/CHA analysis of publicly available state data differs somewhat from figures reported by the Centers for Disease Control and Prevention, which has new cases for the latest available week, Dec.18-24, at just over 27,000 after 3 straight weeks of declines from a count of almost 63,000 for the week ending Nov. 26. The CDC, however, updates previously reported data on a regular basis, so that 27,000 is likely to increase in the coming weeks.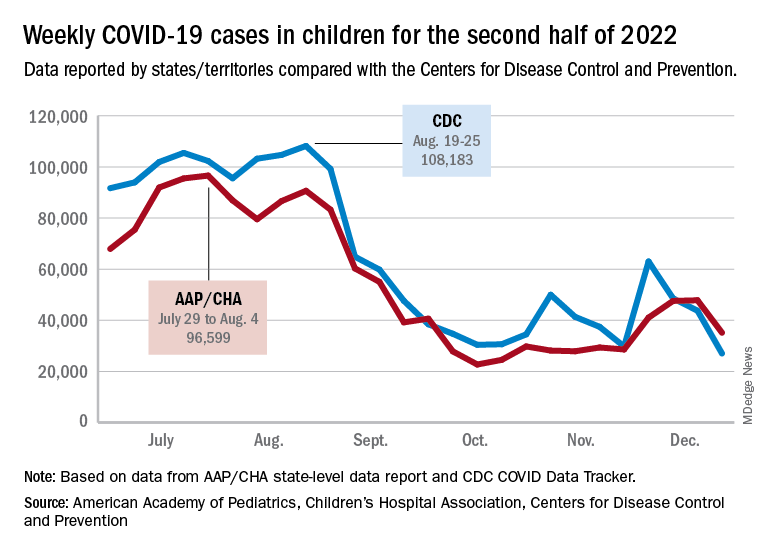
The CDC line on the graph also shows a peak for the week of Oct. 30 to Nov. 5 when new cases reached almost 50,000, compared with almost 30,000 reported for the week of Oct. 28 to Nov. 3 by the AAP and CHA in their report of state-level data. The AAP and CHA put the total number of child COVID cases since the start of the pandemic at 15.2 million as of Dec. 29, while the CDC reports 16.2 million cases as of Dec. 28.
There have been 1,975 deaths from COVID-19 in children aged 0-17 years, according to the CDC, which amounts to just over 0.2% of all COVID deaths for which age group data were available.
CDC data on emergency department visits involving diagnosed COVID-19 have been rising since late October. In children aged 0-11 years, for example, COVID was involved in 1.0% of ED visits (7-day average) as late as Nov. 4, but by Dec. 27 that rate was 2.6%. Children aged 12-15 years went from 0.6% on Oct. 28 to 1.5% on Dec. 27, while 16- to 17-year-olds had ED visit rates of 0.6% on Oct. 19 and 1.7% on Dec. 27, the CDC said on its COVID Data Tracker.
New hospital admissions with diagnosed COVID, which had been following the same upward trend as ED visits since late October, halted that rise in children aged 0-17 years and have gone no higher than 0.29 per 100,000 population since Dec. 9, the CDC data show.
The end of 2022 saw a drop in new COVID-19 cases in children, even as rates of emergency department visits continued upward trends that began in late October.
New cases for the week of Dec. 23-29 fell for the first time since late November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP/CHA analysis of publicly available state data differs somewhat from figures reported by the Centers for Disease Control and Prevention, which has new cases for the latest available week, Dec.18-24, at just over 27,000 after 3 straight weeks of declines from a count of almost 63,000 for the week ending Nov. 26. The CDC, however, updates previously reported data on a regular basis, so that 27,000 is likely to increase in the coming weeks.
The CDC line on the graph also shows a peak for the week of Oct. 30 to Nov. 5 when new cases reached almost 50,000, compared with almost 30,000 reported for the week of Oct. 28 to Nov. 3 by the AAP and CHA in their report of state-level data. The AAP and CHA put the total number of child COVID cases since the start of the pandemic at 15.2 million as of Dec. 29, while the CDC reports 16.2 million cases as of Dec. 28.
There have been 1,975 deaths from COVID-19 in children aged 0-17 years, according to the CDC, which amounts to just over 0.2% of all COVID deaths for which age group data were available.
CDC data on emergency department visits involving diagnosed COVID-19 have been rising since late October. In children aged 0-11 years, for example, COVID was involved in 1.0% of ED visits (7-day average) as late as Nov. 4, but by Dec. 27 that rate was 2.6%. Children aged 12-15 years went from 0.6% on Oct. 28 to 1.5% on Dec. 27, while 16- to 17-year-olds had ED visit rates of 0.6% on Oct. 19 and 1.7% on Dec. 27, the CDC said on its COVID Data Tracker.
New hospital admissions with diagnosed COVID, which had been following the same upward trend as ED visits since late October, halted that rise in children aged 0-17 years and have gone no higher than 0.29 per 100,000 population since Dec. 9, the CDC data show.
The end of 2022 saw a drop in new COVID-19 cases in children, even as rates of emergency department visits continued upward trends that began in late October.
New cases for the week of Dec. 23-29 fell for the first time since late November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP/CHA analysis of publicly available state data differs somewhat from figures reported by the Centers for Disease Control and Prevention, which has new cases for the latest available week, Dec.18-24, at just over 27,000 after 3 straight weeks of declines from a count of almost 63,000 for the week ending Nov. 26. The CDC, however, updates previously reported data on a regular basis, so that 27,000 is likely to increase in the coming weeks.
The CDC line on the graph also shows a peak for the week of Oct. 30 to Nov. 5 when new cases reached almost 50,000, compared with almost 30,000 reported for the week of Oct. 28 to Nov. 3 by the AAP and CHA in their report of state-level data. The AAP and CHA put the total number of child COVID cases since the start of the pandemic at 15.2 million as of Dec. 29, while the CDC reports 16.2 million cases as of Dec. 28.
There have been 1,975 deaths from COVID-19 in children aged 0-17 years, according to the CDC, which amounts to just over 0.2% of all COVID deaths for which age group data were available.
CDC data on emergency department visits involving diagnosed COVID-19 have been rising since late October. In children aged 0-11 years, for example, COVID was involved in 1.0% of ED visits (7-day average) as late as Nov. 4, but by Dec. 27 that rate was 2.6%. Children aged 12-15 years went from 0.6% on Oct. 28 to 1.5% on Dec. 27, while 16- to 17-year-olds had ED visit rates of 0.6% on Oct. 19 and 1.7% on Dec. 27, the CDC said on its COVID Data Tracker.
New hospital admissions with diagnosed COVID, which had been following the same upward trend as ED visits since late October, halted that rise in children aged 0-17 years and have gone no higher than 0.29 per 100,000 population since Dec. 9, the CDC data show.
SARS-CoV-2 seroprevalence grew rapidly in pandemic’s early waves
By August 2022, two-and-a-half years into the COVID-19 pandemic, most children as well as most adults under age 60 years had evidence of SARS-CoV-2 vaccination and/or infection, a Canadian seroprevalence study of almost 14,000 people over the first seven waves of the pandemic reports.
However, fewer than 50% people older than age 60, the age group most vulnerable to severe outcomes, showed evidence of immunity from infection or vaccination. The authors noted that older adults – who have the lowest infection rates but highest risk of severe outcomes – continue to warrant prioritized vaccination, writing online in the Canadian Medical Association Journal.
Previous evidence suggests that a combination of both infection and vaccination exposure may induce more robust and durable hybrid immunity than either exposure alone, according to lead author Danuta M. Skowronski, MD, MHSc, an epidemiologist at the British Columbia Centre for Disease Control in Vancouver.
“Our main objective was to chronicle the changing proportion of the population considered immunologically naive and therefore susceptible to SARS-CoV-2,” Dr. Skowronski said in an interview. “It’s relevant for risk assessment to know what proportion have acquired some priming for more efficient immune memory response to the virus because that reduces the likelihood of severe outcomes.” Standardized seroprevalence studies are essential to inform COVID-19 response, particularly in resource-limited regions.
The study
Conducted in British Columbia’s Greater Vancouver and Fraser Valley, the analysis found that in the first year of the pandemic, when extraordinary measures were in place to curtail transmission, virtually everyone remained immunologically naive. Thereafter, however, age-based vaccine rollouts dramatically changed the immuno-epidemiological landscape so that by September 2021, more than 80% of the study population had antibody evidence of immunological priming, while more than 85% remained uninfected.
By August 2022, after the Omicron-variant waves, overall vaccine- and infection-induced seroprevalence exceeded 95%, with 60% having been actually infected, including at least three-quarters of children. Fewer than 50% of older adults, however, showed immunological evidence of exposure.
The study results were based on anonymized residual sera from children and adults in an outpatient laboratory network. At least three immunoassays per serosurvey were used to detect antibodies to SARS-CoV-2 spike (from vaccine) and to nucleocapsid antibodies (from infection).
The researchers assessed any seroprevalence – vaccine-, infection-induced, or both – as defined by positivity on any two assays. Infection-induced seroprevalence was also defined by dual-assay positivity but required both antinucleocapsid and antispike detection. Their estimates of infection-induced seroprevalence indicated considerable under-ascertainment of infections by standard surveillance case reports.
Results
During the first year of the pandemic, fewer than 1% manifested seroprevalence during the first three snapshots and fewer than 5% by January 2021. With vaccine rollout, however, seroprevalence increased dramatically during the first half of 2021 to 56% by May/June 2021 and to 83% by September/October 2021.
In addition, infection-induced seroprevalence was low at less than 15% in September/October 2021 until the arrival of the Omicron waves, after which it rose to 42% by March 2022 and 61% by July/August 2022. Combined seroprevalence for vaccination or infection was more than 95% by the summer, with most children but less than half of adults older than 60 years showing evidence of having been infected.
“We found the highest infection rates among children, closely followed by young adults, which may reflect their greater interconnectedness, including between siblings and parents in the household, as well as with peers in schools and the community,” the authors wrote, adding that the low cumulative infection rates among older adults may reflect their higher vaccination rates and greater social isolation.
U.S. data show similar age-related infection rates, but data among children from other Canadian provinces are limited, the authors said.
Commenting on the survey but not involved in it, infectious diseases expert Marc Germain, MD, PhD, a vice president at Héma-Québec in Quebec City, believes the pattern observed in British Columbia is fairly representative of what happened across Canada and the United States, including the sweeping effect of the Omicron variant and the differential impact according to age.
“But regional differences might very well exist – for example, due to differential vaccine uptake – and are also probably related in part to the different testing platforms being used,” Dr. Germain told this news organization.
Caroline Quach-Tanh, MD, PhD, a pediatrician and epidemiologist/infectologist at the University of Montreal, pointed out that Quebec seroprevalence surveys using residual blood samples from children and adults visiting emergency departments for any reason showed higher rates of prior infection than did the BC surveys. “But Dr. Skowronski’s findings are likely applicable to settings where some nonpharmacological interventions were put in place, but without strict confinement – and thus are likely applicable to most settings in the U.S. and Canada.”
Dr. Quach-Tanh added that there is always a risk of bias with the use of residual blood samples, “but the fact that the study method was stable should have captured a similar population from time to time. It would be unlikely to result in a major overestimation in the proportion of individuals positive for SARS-CoV-2 antibodies.”
A recent global meta-analysis found that while global seroprevalence has risen considerably, albeit variably by region, more than a third of the world’s population is still seronegative to the SARS-CoV-2 virus.
Dr. Skowronski reported institutional grants from the Canadian Institutes of Health Research and the British Columbia Centre for Disease Control Foundation for Public Health for other SARSCoV-2 work. Coauthor Romina C. Reyes, MD, chairs the BC Diagnostic Accreditation Program committee. Coauthor Mel Krajden, MD, reported institutional grants from Roche, Hologic, and Siemens. Dr. Germain and Dr. Quach-Tanh disclosed no competing interests relevant to their comments.
By August 2022, two-and-a-half years into the COVID-19 pandemic, most children as well as most adults under age 60 years had evidence of SARS-CoV-2 vaccination and/or infection, a Canadian seroprevalence study of almost 14,000 people over the first seven waves of the pandemic reports.
However, fewer than 50% people older than age 60, the age group most vulnerable to severe outcomes, showed evidence of immunity from infection or vaccination. The authors noted that older adults – who have the lowest infection rates but highest risk of severe outcomes – continue to warrant prioritized vaccination, writing online in the Canadian Medical Association Journal.
Previous evidence suggests that a combination of both infection and vaccination exposure may induce more robust and durable hybrid immunity than either exposure alone, according to lead author Danuta M. Skowronski, MD, MHSc, an epidemiologist at the British Columbia Centre for Disease Control in Vancouver.
“Our main objective was to chronicle the changing proportion of the population considered immunologically naive and therefore susceptible to SARS-CoV-2,” Dr. Skowronski said in an interview. “It’s relevant for risk assessment to know what proportion have acquired some priming for more efficient immune memory response to the virus because that reduces the likelihood of severe outcomes.” Standardized seroprevalence studies are essential to inform COVID-19 response, particularly in resource-limited regions.
The study
Conducted in British Columbia’s Greater Vancouver and Fraser Valley, the analysis found that in the first year of the pandemic, when extraordinary measures were in place to curtail transmission, virtually everyone remained immunologically naive. Thereafter, however, age-based vaccine rollouts dramatically changed the immuno-epidemiological landscape so that by September 2021, more than 80% of the study population had antibody evidence of immunological priming, while more than 85% remained uninfected.
By August 2022, after the Omicron-variant waves, overall vaccine- and infection-induced seroprevalence exceeded 95%, with 60% having been actually infected, including at least three-quarters of children. Fewer than 50% of older adults, however, showed immunological evidence of exposure.
The study results were based on anonymized residual sera from children and adults in an outpatient laboratory network. At least three immunoassays per serosurvey were used to detect antibodies to SARS-CoV-2 spike (from vaccine) and to nucleocapsid antibodies (from infection).
The researchers assessed any seroprevalence – vaccine-, infection-induced, or both – as defined by positivity on any two assays. Infection-induced seroprevalence was also defined by dual-assay positivity but required both antinucleocapsid and antispike detection. Their estimates of infection-induced seroprevalence indicated considerable under-ascertainment of infections by standard surveillance case reports.
Results
During the first year of the pandemic, fewer than 1% manifested seroprevalence during the first three snapshots and fewer than 5% by January 2021. With vaccine rollout, however, seroprevalence increased dramatically during the first half of 2021 to 56% by May/June 2021 and to 83% by September/October 2021.
In addition, infection-induced seroprevalence was low at less than 15% in September/October 2021 until the arrival of the Omicron waves, after which it rose to 42% by March 2022 and 61% by July/August 2022. Combined seroprevalence for vaccination or infection was more than 95% by the summer, with most children but less than half of adults older than 60 years showing evidence of having been infected.
“We found the highest infection rates among children, closely followed by young adults, which may reflect their greater interconnectedness, including between siblings and parents in the household, as well as with peers in schools and the community,” the authors wrote, adding that the low cumulative infection rates among older adults may reflect their higher vaccination rates and greater social isolation.
U.S. data show similar age-related infection rates, but data among children from other Canadian provinces are limited, the authors said.
Commenting on the survey but not involved in it, infectious diseases expert Marc Germain, MD, PhD, a vice president at Héma-Québec in Quebec City, believes the pattern observed in British Columbia is fairly representative of what happened across Canada and the United States, including the sweeping effect of the Omicron variant and the differential impact according to age.
“But regional differences might very well exist – for example, due to differential vaccine uptake – and are also probably related in part to the different testing platforms being used,” Dr. Germain told this news organization.
Caroline Quach-Tanh, MD, PhD, a pediatrician and epidemiologist/infectologist at the University of Montreal, pointed out that Quebec seroprevalence surveys using residual blood samples from children and adults visiting emergency departments for any reason showed higher rates of prior infection than did the BC surveys. “But Dr. Skowronski’s findings are likely applicable to settings where some nonpharmacological interventions were put in place, but without strict confinement – and thus are likely applicable to most settings in the U.S. and Canada.”
Dr. Quach-Tanh added that there is always a risk of bias with the use of residual blood samples, “but the fact that the study method was stable should have captured a similar population from time to time. It would be unlikely to result in a major overestimation in the proportion of individuals positive for SARS-CoV-2 antibodies.”
A recent global meta-analysis found that while global seroprevalence has risen considerably, albeit variably by region, more than a third of the world’s population is still seronegative to the SARS-CoV-2 virus.
Dr. Skowronski reported institutional grants from the Canadian Institutes of Health Research and the British Columbia Centre for Disease Control Foundation for Public Health for other SARSCoV-2 work. Coauthor Romina C. Reyes, MD, chairs the BC Diagnostic Accreditation Program committee. Coauthor Mel Krajden, MD, reported institutional grants from Roche, Hologic, and Siemens. Dr. Germain and Dr. Quach-Tanh disclosed no competing interests relevant to their comments.
By August 2022, two-and-a-half years into the COVID-19 pandemic, most children as well as most adults under age 60 years had evidence of SARS-CoV-2 vaccination and/or infection, a Canadian seroprevalence study of almost 14,000 people over the first seven waves of the pandemic reports.
However, fewer than 50% people older than age 60, the age group most vulnerable to severe outcomes, showed evidence of immunity from infection or vaccination. The authors noted that older adults – who have the lowest infection rates but highest risk of severe outcomes – continue to warrant prioritized vaccination, writing online in the Canadian Medical Association Journal.
Previous evidence suggests that a combination of both infection and vaccination exposure may induce more robust and durable hybrid immunity than either exposure alone, according to lead author Danuta M. Skowronski, MD, MHSc, an epidemiologist at the British Columbia Centre for Disease Control in Vancouver.
“Our main objective was to chronicle the changing proportion of the population considered immunologically naive and therefore susceptible to SARS-CoV-2,” Dr. Skowronski said in an interview. “It’s relevant for risk assessment to know what proportion have acquired some priming for more efficient immune memory response to the virus because that reduces the likelihood of severe outcomes.” Standardized seroprevalence studies are essential to inform COVID-19 response, particularly in resource-limited regions.
The study
Conducted in British Columbia’s Greater Vancouver and Fraser Valley, the analysis found that in the first year of the pandemic, when extraordinary measures were in place to curtail transmission, virtually everyone remained immunologically naive. Thereafter, however, age-based vaccine rollouts dramatically changed the immuno-epidemiological landscape so that by September 2021, more than 80% of the study population had antibody evidence of immunological priming, while more than 85% remained uninfected.
By August 2022, after the Omicron-variant waves, overall vaccine- and infection-induced seroprevalence exceeded 95%, with 60% having been actually infected, including at least three-quarters of children. Fewer than 50% of older adults, however, showed immunological evidence of exposure.
The study results were based on anonymized residual sera from children and adults in an outpatient laboratory network. At least three immunoassays per serosurvey were used to detect antibodies to SARS-CoV-2 spike (from vaccine) and to nucleocapsid antibodies (from infection).
The researchers assessed any seroprevalence – vaccine-, infection-induced, or both – as defined by positivity on any two assays. Infection-induced seroprevalence was also defined by dual-assay positivity but required both antinucleocapsid and antispike detection. Their estimates of infection-induced seroprevalence indicated considerable under-ascertainment of infections by standard surveillance case reports.
Results
During the first year of the pandemic, fewer than 1% manifested seroprevalence during the first three snapshots and fewer than 5% by January 2021. With vaccine rollout, however, seroprevalence increased dramatically during the first half of 2021 to 56% by May/June 2021 and to 83% by September/October 2021.
In addition, infection-induced seroprevalence was low at less than 15% in September/October 2021 until the arrival of the Omicron waves, after which it rose to 42% by March 2022 and 61% by July/August 2022. Combined seroprevalence for vaccination or infection was more than 95% by the summer, with most children but less than half of adults older than 60 years showing evidence of having been infected.
“We found the highest infection rates among children, closely followed by young adults, which may reflect their greater interconnectedness, including between siblings and parents in the household, as well as with peers in schools and the community,” the authors wrote, adding that the low cumulative infection rates among older adults may reflect their higher vaccination rates and greater social isolation.
U.S. data show similar age-related infection rates, but data among children from other Canadian provinces are limited, the authors said.
Commenting on the survey but not involved in it, infectious diseases expert Marc Germain, MD, PhD, a vice president at Héma-Québec in Quebec City, believes the pattern observed in British Columbia is fairly representative of what happened across Canada and the United States, including the sweeping effect of the Omicron variant and the differential impact according to age.
“But regional differences might very well exist – for example, due to differential vaccine uptake – and are also probably related in part to the different testing platforms being used,” Dr. Germain told this news organization.
Caroline Quach-Tanh, MD, PhD, a pediatrician and epidemiologist/infectologist at the University of Montreal, pointed out that Quebec seroprevalence surveys using residual blood samples from children and adults visiting emergency departments for any reason showed higher rates of prior infection than did the BC surveys. “But Dr. Skowronski’s findings are likely applicable to settings where some nonpharmacological interventions were put in place, but without strict confinement – and thus are likely applicable to most settings in the U.S. and Canada.”
Dr. Quach-Tanh added that there is always a risk of bias with the use of residual blood samples, “but the fact that the study method was stable should have captured a similar population from time to time. It would be unlikely to result in a major overestimation in the proportion of individuals positive for SARS-CoV-2 antibodies.”
A recent global meta-analysis found that while global seroprevalence has risen considerably, albeit variably by region, more than a third of the world’s population is still seronegative to the SARS-CoV-2 virus.
Dr. Skowronski reported institutional grants from the Canadian Institutes of Health Research and the British Columbia Centre for Disease Control Foundation for Public Health for other SARSCoV-2 work. Coauthor Romina C. Reyes, MD, chairs the BC Diagnostic Accreditation Program committee. Coauthor Mel Krajden, MD, reported institutional grants from Roche, Hologic, and Siemens. Dr. Germain and Dr. Quach-Tanh disclosed no competing interests relevant to their comments.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Study of beliefs about what causes cancer sparks debate
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.








