User login
CCJM delivers practical clinical articles relevant to internists, cardiologists, endocrinologists, and other specialists, all written by known experts.
Copyright © 2019 Cleveland Clinic. All rights reserved. The information provided is for educational purposes only. Use of this website is subject to the disclaimer and privacy policy.
gambling
compulsive behaviors
ammunition
assault rifle
black jack
Boko Haram
bondage
child abuse
cocaine
Daech
drug paraphernalia
explosion
gun
human trafficking
ISIL
ISIS
Islamic caliphate
Islamic state
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
pedophile
pedophilia
poker
porn
pornography
psychedelic drug
recreational drug
sex slave rings
slot machine
terrorism
terrorist
Texas hold 'em
UFC
substance abuse
abuseed
abuseer
abusees
abuseing
abusely
abuses
aeolus
aeolused
aeoluser
aeoluses
aeolusing
aeolusly
aeoluss
ahole
aholeed
aholeer
aholees
aholeing
aholely
aholes
alcohol
alcoholed
alcoholer
alcoholes
alcoholing
alcoholly
alcohols
allman
allmaned
allmaner
allmanes
allmaning
allmanly
allmans
alted
altes
alting
altly
alts
analed
analer
anales
analing
anally
analprobe
analprobeed
analprobeer
analprobees
analprobeing
analprobely
analprobes
anals
anilingus
anilingused
anilinguser
anilinguses
anilingusing
anilingusly
anilinguss
anus
anused
anuser
anuses
anusing
anusly
anuss
areola
areolaed
areolaer
areolaes
areolaing
areolaly
areolas
areole
areoleed
areoleer
areolees
areoleing
areolely
areoles
arian
arianed
arianer
arianes
arianing
arianly
arians
aryan
aryaned
aryaner
aryanes
aryaning
aryanly
aryans
asiaed
asiaer
asiaes
asiaing
asialy
asias
ass
ass hole
ass lick
ass licked
ass licker
ass lickes
ass licking
ass lickly
ass licks
assbang
assbanged
assbangeded
assbangeder
assbangedes
assbangeding
assbangedly
assbangeds
assbanger
assbanges
assbanging
assbangly
assbangs
assbangsed
assbangser
assbangses
assbangsing
assbangsly
assbangss
assed
asser
asses
assesed
asseser
asseses
assesing
assesly
assess
assfuck
assfucked
assfucker
assfuckered
assfuckerer
assfuckeres
assfuckering
assfuckerly
assfuckers
assfuckes
assfucking
assfuckly
assfucks
asshat
asshated
asshater
asshates
asshating
asshatly
asshats
assholeed
assholeer
assholees
assholeing
assholely
assholes
assholesed
assholeser
assholeses
assholesing
assholesly
assholess
assing
assly
assmaster
assmastered
assmasterer
assmasteres
assmastering
assmasterly
assmasters
assmunch
assmunched
assmuncher
assmunches
assmunching
assmunchly
assmunchs
asss
asswipe
asswipeed
asswipeer
asswipees
asswipeing
asswipely
asswipes
asswipesed
asswipeser
asswipeses
asswipesing
asswipesly
asswipess
azz
azzed
azzer
azzes
azzing
azzly
azzs
babeed
babeer
babees
babeing
babely
babes
babesed
babeser
babeses
babesing
babesly
babess
ballsac
ballsaced
ballsacer
ballsaces
ballsacing
ballsack
ballsacked
ballsacker
ballsackes
ballsacking
ballsackly
ballsacks
ballsacly
ballsacs
ballsed
ballser
ballses
ballsing
ballsly
ballss
barf
barfed
barfer
barfes
barfing
barfly
barfs
bastard
bastarded
bastarder
bastardes
bastarding
bastardly
bastards
bastardsed
bastardser
bastardses
bastardsing
bastardsly
bastardss
bawdy
bawdyed
bawdyer
bawdyes
bawdying
bawdyly
bawdys
beaner
beanered
beanerer
beaneres
beanering
beanerly
beaners
beardedclam
beardedclamed
beardedclamer
beardedclames
beardedclaming
beardedclamly
beardedclams
beastiality
beastialityed
beastialityer
beastialityes
beastialitying
beastialityly
beastialitys
beatch
beatched
beatcher
beatches
beatching
beatchly
beatchs
beater
beatered
beaterer
beateres
beatering
beaterly
beaters
beered
beerer
beeres
beering
beerly
beeyotch
beeyotched
beeyotcher
beeyotches
beeyotching
beeyotchly
beeyotchs
beotch
beotched
beotcher
beotches
beotching
beotchly
beotchs
biatch
biatched
biatcher
biatches
biatching
biatchly
biatchs
big tits
big titsed
big titser
big titses
big titsing
big titsly
big titss
bigtits
bigtitsed
bigtitser
bigtitses
bigtitsing
bigtitsly
bigtitss
bimbo
bimboed
bimboer
bimboes
bimboing
bimboly
bimbos
bisexualed
bisexualer
bisexuales
bisexualing
bisexually
bisexuals
bitch
bitched
bitcheded
bitcheder
bitchedes
bitcheding
bitchedly
bitcheds
bitcher
bitches
bitchesed
bitcheser
bitcheses
bitchesing
bitchesly
bitchess
bitching
bitchly
bitchs
bitchy
bitchyed
bitchyer
bitchyes
bitchying
bitchyly
bitchys
bleached
bleacher
bleaches
bleaching
bleachly
bleachs
blow job
blow jobed
blow jober
blow jobes
blow jobing
blow jobly
blow jobs
blowed
blower
blowes
blowing
blowjob
blowjobed
blowjober
blowjobes
blowjobing
blowjobly
blowjobs
blowjobsed
blowjobser
blowjobses
blowjobsing
blowjobsly
blowjobss
blowly
blows
boink
boinked
boinker
boinkes
boinking
boinkly
boinks
bollock
bollocked
bollocker
bollockes
bollocking
bollockly
bollocks
bollocksed
bollockser
bollockses
bollocksing
bollocksly
bollockss
bollok
bolloked
bolloker
bollokes
bolloking
bollokly
bolloks
boner
bonered
bonerer
boneres
bonering
bonerly
boners
bonersed
bonerser
bonerses
bonersing
bonersly
bonerss
bong
bonged
bonger
bonges
bonging
bongly
bongs
boob
boobed
boober
boobes
boobies
boobiesed
boobieser
boobieses
boobiesing
boobiesly
boobiess
boobing
boobly
boobs
boobsed
boobser
boobses
boobsing
boobsly
boobss
booby
boobyed
boobyer
boobyes
boobying
boobyly
boobys
booger
boogered
boogerer
boogeres
boogering
boogerly
boogers
bookie
bookieed
bookieer
bookiees
bookieing
bookiely
bookies
bootee
booteeed
booteeer
booteees
booteeing
booteely
bootees
bootie
bootieed
bootieer
bootiees
bootieing
bootiely
booties
booty
bootyed
bootyer
bootyes
bootying
bootyly
bootys
boozeed
boozeer
boozees
boozeing
boozely
boozer
boozered
boozerer
boozeres
boozering
boozerly
boozers
boozes
boozy
boozyed
boozyer
boozyes
boozying
boozyly
boozys
bosomed
bosomer
bosomes
bosoming
bosomly
bosoms
bosomy
bosomyed
bosomyer
bosomyes
bosomying
bosomyly
bosomys
bugger
buggered
buggerer
buggeres
buggering
buggerly
buggers
bukkake
bukkakeed
bukkakeer
bukkakees
bukkakeing
bukkakely
bukkakes
bull shit
bull shited
bull shiter
bull shites
bull shiting
bull shitly
bull shits
bullshit
bullshited
bullshiter
bullshites
bullshiting
bullshitly
bullshits
bullshitsed
bullshitser
bullshitses
bullshitsing
bullshitsly
bullshitss
bullshitted
bullshitteded
bullshitteder
bullshittedes
bullshitteding
bullshittedly
bullshitteds
bullturds
bullturdsed
bullturdser
bullturdses
bullturdsing
bullturdsly
bullturdss
bung
bunged
bunger
bunges
bunging
bungly
bungs
busty
bustyed
bustyer
bustyes
bustying
bustyly
bustys
butt
butt fuck
butt fucked
butt fucker
butt fuckes
butt fucking
butt fuckly
butt fucks
butted
buttes
buttfuck
buttfucked
buttfucker
buttfuckered
buttfuckerer
buttfuckeres
buttfuckering
buttfuckerly
buttfuckers
buttfuckes
buttfucking
buttfuckly
buttfucks
butting
buttly
buttplug
buttpluged
buttpluger
buttpluges
buttpluging
buttplugly
buttplugs
butts
caca
cacaed
cacaer
cacaes
cacaing
cacaly
cacas
cahone
cahoneed
cahoneer
cahonees
cahoneing
cahonely
cahones
cameltoe
cameltoeed
cameltoeer
cameltoees
cameltoeing
cameltoely
cameltoes
carpetmuncher
carpetmunchered
carpetmuncherer
carpetmuncheres
carpetmunchering
carpetmuncherly
carpetmunchers
cawk
cawked
cawker
cawkes
cawking
cawkly
cawks
chinc
chinced
chincer
chinces
chincing
chincly
chincs
chincsed
chincser
chincses
chincsing
chincsly
chincss
chink
chinked
chinker
chinkes
chinking
chinkly
chinks
chode
chodeed
chodeer
chodees
chodeing
chodely
chodes
chodesed
chodeser
chodeses
chodesing
chodesly
chodess
clit
clited
cliter
clites
cliting
clitly
clitoris
clitorised
clitoriser
clitorises
clitorising
clitorisly
clitoriss
clitorus
clitorused
clitoruser
clitoruses
clitorusing
clitorusly
clitoruss
clits
clitsed
clitser
clitses
clitsing
clitsly
clitss
clitty
clittyed
clittyer
clittyes
clittying
clittyly
clittys
cocain
cocaine
cocained
cocaineed
cocaineer
cocainees
cocaineing
cocainely
cocainer
cocaines
cocaining
cocainly
cocains
cock
cock sucker
cock suckered
cock suckerer
cock suckeres
cock suckering
cock suckerly
cock suckers
cockblock
cockblocked
cockblocker
cockblockes
cockblocking
cockblockly
cockblocks
cocked
cocker
cockes
cockholster
cockholstered
cockholsterer
cockholsteres
cockholstering
cockholsterly
cockholsters
cocking
cockknocker
cockknockered
cockknockerer
cockknockeres
cockknockering
cockknockerly
cockknockers
cockly
cocks
cocksed
cockser
cockses
cocksing
cocksly
cocksmoker
cocksmokered
cocksmokerer
cocksmokeres
cocksmokering
cocksmokerly
cocksmokers
cockss
cocksucker
cocksuckered
cocksuckerer
cocksuckeres
cocksuckering
cocksuckerly
cocksuckers
coital
coitaled
coitaler
coitales
coitaling
coitally
coitals
commie
commieed
commieer
commiees
commieing
commiely
commies
condomed
condomer
condomes
condoming
condomly
condoms
coon
cooned
cooner
coones
cooning
coonly
coons
coonsed
coonser
coonses
coonsing
coonsly
coonss
corksucker
corksuckered
corksuckerer
corksuckeres
corksuckering
corksuckerly
corksuckers
cracked
crackwhore
crackwhoreed
crackwhoreer
crackwhorees
crackwhoreing
crackwhorely
crackwhores
crap
craped
craper
crapes
craping
craply
crappy
crappyed
crappyer
crappyes
crappying
crappyly
crappys
cum
cumed
cumer
cumes
cuming
cumly
cummin
cummined
cumminer
cummines
cumming
cumminged
cumminger
cumminges
cumminging
cummingly
cummings
cummining
cumminly
cummins
cums
cumshot
cumshoted
cumshoter
cumshotes
cumshoting
cumshotly
cumshots
cumshotsed
cumshotser
cumshotses
cumshotsing
cumshotsly
cumshotss
cumslut
cumsluted
cumsluter
cumslutes
cumsluting
cumslutly
cumsluts
cumstain
cumstained
cumstainer
cumstaines
cumstaining
cumstainly
cumstains
cunilingus
cunilingused
cunilinguser
cunilinguses
cunilingusing
cunilingusly
cunilinguss
cunnilingus
cunnilingused
cunnilinguser
cunnilinguses
cunnilingusing
cunnilingusly
cunnilinguss
cunny
cunnyed
cunnyer
cunnyes
cunnying
cunnyly
cunnys
cunt
cunted
cunter
cuntes
cuntface
cuntfaceed
cuntfaceer
cuntfacees
cuntfaceing
cuntfacely
cuntfaces
cunthunter
cunthuntered
cunthunterer
cunthunteres
cunthuntering
cunthunterly
cunthunters
cunting
cuntlick
cuntlicked
cuntlicker
cuntlickered
cuntlickerer
cuntlickeres
cuntlickering
cuntlickerly
cuntlickers
cuntlickes
cuntlicking
cuntlickly
cuntlicks
cuntly
cunts
cuntsed
cuntser
cuntses
cuntsing
cuntsly
cuntss
dago
dagoed
dagoer
dagoes
dagoing
dagoly
dagos
dagosed
dagoser
dagoses
dagosing
dagosly
dagoss
dammit
dammited
dammiter
dammites
dammiting
dammitly
dammits
damn
damned
damneded
damneder
damnedes
damneding
damnedly
damneds
damner
damnes
damning
damnit
damnited
damniter
damnites
damniting
damnitly
damnits
damnly
damns
dick
dickbag
dickbaged
dickbager
dickbages
dickbaging
dickbagly
dickbags
dickdipper
dickdippered
dickdipperer
dickdipperes
dickdippering
dickdipperly
dickdippers
dicked
dicker
dickes
dickface
dickfaceed
dickfaceer
dickfacees
dickfaceing
dickfacely
dickfaces
dickflipper
dickflippered
dickflipperer
dickflipperes
dickflippering
dickflipperly
dickflippers
dickhead
dickheaded
dickheader
dickheades
dickheading
dickheadly
dickheads
dickheadsed
dickheadser
dickheadses
dickheadsing
dickheadsly
dickheadss
dicking
dickish
dickished
dickisher
dickishes
dickishing
dickishly
dickishs
dickly
dickripper
dickrippered
dickripperer
dickripperes
dickrippering
dickripperly
dickrippers
dicks
dicksipper
dicksippered
dicksipperer
dicksipperes
dicksippering
dicksipperly
dicksippers
dickweed
dickweeded
dickweeder
dickweedes
dickweeding
dickweedly
dickweeds
dickwhipper
dickwhippered
dickwhipperer
dickwhipperes
dickwhippering
dickwhipperly
dickwhippers
dickzipper
dickzippered
dickzipperer
dickzipperes
dickzippering
dickzipperly
dickzippers
diddle
diddleed
diddleer
diddlees
diddleing
diddlely
diddles
dike
dikeed
dikeer
dikees
dikeing
dikely
dikes
dildo
dildoed
dildoer
dildoes
dildoing
dildoly
dildos
dildosed
dildoser
dildoses
dildosing
dildosly
dildoss
diligaf
diligafed
diligafer
diligafes
diligafing
diligafly
diligafs
dillweed
dillweeded
dillweeder
dillweedes
dillweeding
dillweedly
dillweeds
dimwit
dimwited
dimwiter
dimwites
dimwiting
dimwitly
dimwits
dingle
dingleed
dingleer
dinglees
dingleing
dinglely
dingles
dipship
dipshiped
dipshiper
dipshipes
dipshiping
dipshiply
dipships
dizzyed
dizzyer
dizzyes
dizzying
dizzyly
dizzys
doggiestyleed
doggiestyleer
doggiestylees
doggiestyleing
doggiestylely
doggiestyles
doggystyleed
doggystyleer
doggystylees
doggystyleing
doggystylely
doggystyles
dong
donged
donger
donges
donging
dongly
dongs
doofus
doofused
doofuser
doofuses
doofusing
doofusly
doofuss
doosh
dooshed
doosher
dooshes
dooshing
dooshly
dooshs
dopeyed
dopeyer
dopeyes
dopeying
dopeyly
dopeys
douchebag
douchebaged
douchebager
douchebages
douchebaging
douchebagly
douchebags
douchebagsed
douchebagser
douchebagses
douchebagsing
douchebagsly
douchebagss
doucheed
doucheer
douchees
doucheing
douchely
douches
douchey
doucheyed
doucheyer
doucheyes
doucheying
doucheyly
doucheys
drunk
drunked
drunker
drunkes
drunking
drunkly
drunks
dumass
dumassed
dumasser
dumasses
dumassing
dumassly
dumasss
dumbass
dumbassed
dumbasser
dumbasses
dumbassesed
dumbasseser
dumbasseses
dumbassesing
dumbassesly
dumbassess
dumbassing
dumbassly
dumbasss
dummy
dummyed
dummyer
dummyes
dummying
dummyly
dummys
dyke
dykeed
dykeer
dykees
dykeing
dykely
dykes
dykesed
dykeser
dykeses
dykesing
dykesly
dykess
erotic
eroticed
eroticer
erotices
eroticing
eroticly
erotics
extacy
extacyed
extacyer
extacyes
extacying
extacyly
extacys
extasy
extasyed
extasyer
extasyes
extasying
extasyly
extasys
fack
facked
facker
fackes
facking
fackly
facks
fag
faged
fager
fages
fagg
fagged
faggeded
faggeder
faggedes
faggeding
faggedly
faggeds
fagger
fagges
fagging
faggit
faggited
faggiter
faggites
faggiting
faggitly
faggits
faggly
faggot
faggoted
faggoter
faggotes
faggoting
faggotly
faggots
faggs
faging
fagly
fagot
fagoted
fagoter
fagotes
fagoting
fagotly
fagots
fags
fagsed
fagser
fagses
fagsing
fagsly
fagss
faig
faiged
faiger
faiges
faiging
faigly
faigs
faigt
faigted
faigter
faigtes
faigting
faigtly
faigts
fannybandit
fannybandited
fannybanditer
fannybandites
fannybanditing
fannybanditly
fannybandits
farted
farter
fartes
farting
fartknocker
fartknockered
fartknockerer
fartknockeres
fartknockering
fartknockerly
fartknockers
fartly
farts
felch
felched
felcher
felchered
felcherer
felcheres
felchering
felcherly
felchers
felches
felching
felchinged
felchinger
felchinges
felchinging
felchingly
felchings
felchly
felchs
fellate
fellateed
fellateer
fellatees
fellateing
fellately
fellates
fellatio
fellatioed
fellatioer
fellatioes
fellatioing
fellatioly
fellatios
feltch
feltched
feltcher
feltchered
feltcherer
feltcheres
feltchering
feltcherly
feltchers
feltches
feltching
feltchly
feltchs
feom
feomed
feomer
feomes
feoming
feomly
feoms
fisted
fisteded
fisteder
fistedes
fisteding
fistedly
fisteds
fisting
fistinged
fistinger
fistinges
fistinging
fistingly
fistings
fisty
fistyed
fistyer
fistyes
fistying
fistyly
fistys
floozy
floozyed
floozyer
floozyes
floozying
floozyly
floozys
foad
foaded
foader
foades
foading
foadly
foads
fondleed
fondleer
fondlees
fondleing
fondlely
fondles
foobar
foobared
foobarer
foobares
foobaring
foobarly
foobars
freex
freexed
freexer
freexes
freexing
freexly
freexs
frigg
frigga
friggaed
friggaer
friggaes
friggaing
friggaly
friggas
frigged
frigger
frigges
frigging
friggly
friggs
fubar
fubared
fubarer
fubares
fubaring
fubarly
fubars
fuck
fuckass
fuckassed
fuckasser
fuckasses
fuckassing
fuckassly
fuckasss
fucked
fuckeded
fuckeder
fuckedes
fuckeding
fuckedly
fuckeds
fucker
fuckered
fuckerer
fuckeres
fuckering
fuckerly
fuckers
fuckes
fuckface
fuckfaceed
fuckfaceer
fuckfacees
fuckfaceing
fuckfacely
fuckfaces
fuckin
fuckined
fuckiner
fuckines
fucking
fuckinged
fuckinger
fuckinges
fuckinging
fuckingly
fuckings
fuckining
fuckinly
fuckins
fuckly
fucknugget
fucknuggeted
fucknuggeter
fucknuggetes
fucknuggeting
fucknuggetly
fucknuggets
fucknut
fucknuted
fucknuter
fucknutes
fucknuting
fucknutly
fucknuts
fuckoff
fuckoffed
fuckoffer
fuckoffes
fuckoffing
fuckoffly
fuckoffs
fucks
fucksed
fuckser
fuckses
fucksing
fucksly
fuckss
fucktard
fucktarded
fucktarder
fucktardes
fucktarding
fucktardly
fucktards
fuckup
fuckuped
fuckuper
fuckupes
fuckuping
fuckuply
fuckups
fuckwad
fuckwaded
fuckwader
fuckwades
fuckwading
fuckwadly
fuckwads
fuckwit
fuckwited
fuckwiter
fuckwites
fuckwiting
fuckwitly
fuckwits
fudgepacker
fudgepackered
fudgepackerer
fudgepackeres
fudgepackering
fudgepackerly
fudgepackers
fuk
fuked
fuker
fukes
fuking
fukly
fuks
fvck
fvcked
fvcker
fvckes
fvcking
fvckly
fvcks
fxck
fxcked
fxcker
fxckes
fxcking
fxckly
fxcks
gae
gaeed
gaeer
gaees
gaeing
gaely
gaes
gai
gaied
gaier
gaies
gaiing
gaily
gais
ganja
ganjaed
ganjaer
ganjaes
ganjaing
ganjaly
ganjas
gayed
gayer
gayes
gaying
gayly
gays
gaysed
gayser
gayses
gaysing
gaysly
gayss
gey
geyed
geyer
geyes
geying
geyly
geys
gfc
gfced
gfcer
gfces
gfcing
gfcly
gfcs
gfy
gfyed
gfyer
gfyes
gfying
gfyly
gfys
ghay
ghayed
ghayer
ghayes
ghaying
ghayly
ghays
ghey
gheyed
gheyer
gheyes
gheying
gheyly
gheys
gigolo
gigoloed
gigoloer
gigoloes
gigoloing
gigololy
gigolos
goatse
goatseed
goatseer
goatsees
goatseing
goatsely
goatses
godamn
godamned
godamner
godamnes
godamning
godamnit
godamnited
godamniter
godamnites
godamniting
godamnitly
godamnits
godamnly
godamns
goddam
goddamed
goddamer
goddames
goddaming
goddamly
goddammit
goddammited
goddammiter
goddammites
goddammiting
goddammitly
goddammits
goddamn
goddamned
goddamner
goddamnes
goddamning
goddamnly
goddamns
goddams
goldenshower
goldenshowered
goldenshowerer
goldenshoweres
goldenshowering
goldenshowerly
goldenshowers
gonad
gonaded
gonader
gonades
gonading
gonadly
gonads
gonadsed
gonadser
gonadses
gonadsing
gonadsly
gonadss
gook
gooked
gooker
gookes
gooking
gookly
gooks
gooksed
gookser
gookses
gooksing
gooksly
gookss
gringo
gringoed
gringoer
gringoes
gringoing
gringoly
gringos
gspot
gspoted
gspoter
gspotes
gspoting
gspotly
gspots
gtfo
gtfoed
gtfoer
gtfoes
gtfoing
gtfoly
gtfos
guido
guidoed
guidoer
guidoes
guidoing
guidoly
guidos
handjob
handjobed
handjober
handjobes
handjobing
handjobly
handjobs
hard on
hard oned
hard oner
hard ones
hard oning
hard only
hard ons
hardknight
hardknighted
hardknighter
hardknightes
hardknighting
hardknightly
hardknights
hebe
hebeed
hebeer
hebees
hebeing
hebely
hebes
heeb
heebed
heeber
heebes
heebing
heebly
heebs
hell
helled
heller
helles
helling
hellly
hells
hemp
hemped
hemper
hempes
hemping
hemply
hemps
heroined
heroiner
heroines
heroining
heroinly
heroins
herp
herped
herper
herpes
herpesed
herpeser
herpeses
herpesing
herpesly
herpess
herping
herply
herps
herpy
herpyed
herpyer
herpyes
herpying
herpyly
herpys
hitler
hitlered
hitlerer
hitleres
hitlering
hitlerly
hitlers
hived
hiver
hives
hiving
hivly
hivs
hobag
hobaged
hobager
hobages
hobaging
hobagly
hobags
homey
homeyed
homeyer
homeyes
homeying
homeyly
homeys
homo
homoed
homoer
homoes
homoey
homoeyed
homoeyer
homoeyes
homoeying
homoeyly
homoeys
homoing
homoly
homos
honky
honkyed
honkyer
honkyes
honkying
honkyly
honkys
hooch
hooched
hoocher
hooches
hooching
hoochly
hoochs
hookah
hookahed
hookaher
hookahes
hookahing
hookahly
hookahs
hooker
hookered
hookerer
hookeres
hookering
hookerly
hookers
hoor
hoored
hoorer
hoores
hooring
hoorly
hoors
hootch
hootched
hootcher
hootches
hootching
hootchly
hootchs
hooter
hootered
hooterer
hooteres
hootering
hooterly
hooters
hootersed
hooterser
hooterses
hootersing
hootersly
hooterss
horny
hornyed
hornyer
hornyes
hornying
hornyly
hornys
houstoned
houstoner
houstones
houstoning
houstonly
houstons
hump
humped
humpeded
humpeder
humpedes
humpeding
humpedly
humpeds
humper
humpes
humping
humpinged
humpinger
humpinges
humpinging
humpingly
humpings
humply
humps
husbanded
husbander
husbandes
husbanding
husbandly
husbands
hussy
hussyed
hussyer
hussyes
hussying
hussyly
hussys
hymened
hymener
hymenes
hymening
hymenly
hymens
inbred
inbreded
inbreder
inbredes
inbreding
inbredly
inbreds
incest
incested
incester
incestes
incesting
incestly
incests
injun
injuned
injuner
injunes
injuning
injunly
injuns
jackass
jackassed
jackasser
jackasses
jackassing
jackassly
jackasss
jackhole
jackholeed
jackholeer
jackholees
jackholeing
jackholely
jackholes
jackoff
jackoffed
jackoffer
jackoffes
jackoffing
jackoffly
jackoffs
jap
japed
japer
japes
japing
japly
japs
japsed
japser
japses
japsing
japsly
japss
jerkoff
jerkoffed
jerkoffer
jerkoffes
jerkoffing
jerkoffly
jerkoffs
jerks
jism
jismed
jismer
jismes
jisming
jismly
jisms
jiz
jized
jizer
jizes
jizing
jizly
jizm
jizmed
jizmer
jizmes
jizming
jizmly
jizms
jizs
jizz
jizzed
jizzeded
jizzeder
jizzedes
jizzeding
jizzedly
jizzeds
jizzer
jizzes
jizzing
jizzly
jizzs
junkie
junkieed
junkieer
junkiees
junkieing
junkiely
junkies
junky
junkyed
junkyer
junkyes
junkying
junkyly
junkys
kike
kikeed
kikeer
kikees
kikeing
kikely
kikes
kikesed
kikeser
kikeses
kikesing
kikesly
kikess
killed
killer
killes
killing
killly
kills
kinky
kinkyed
kinkyer
kinkyes
kinkying
kinkyly
kinkys
kkk
kkked
kkker
kkkes
kkking
kkkly
kkks
klan
klaned
klaner
klanes
klaning
klanly
klans
knobend
knobended
knobender
knobendes
knobending
knobendly
knobends
kooch
kooched
koocher
kooches
koochesed
koocheser
koocheses
koochesing
koochesly
koochess
kooching
koochly
koochs
kootch
kootched
kootcher
kootches
kootching
kootchly
kootchs
kraut
krauted
krauter
krautes
krauting
krautly
krauts
kyke
kykeed
kykeer
kykees
kykeing
kykely
kykes
lech
leched
lecher
leches
leching
lechly
lechs
leper
lepered
leperer
leperes
lepering
leperly
lepers
lesbiansed
lesbianser
lesbianses
lesbiansing
lesbiansly
lesbianss
lesbo
lesboed
lesboer
lesboes
lesboing
lesboly
lesbos
lesbosed
lesboser
lesboses
lesbosing
lesbosly
lesboss
lez
lezbianed
lezbianer
lezbianes
lezbianing
lezbianly
lezbians
lezbiansed
lezbianser
lezbianses
lezbiansing
lezbiansly
lezbianss
lezbo
lezboed
lezboer
lezboes
lezboing
lezboly
lezbos
lezbosed
lezboser
lezboses
lezbosing
lezbosly
lezboss
lezed
lezer
lezes
lezing
lezly
lezs
lezzie
lezzieed
lezzieer
lezziees
lezzieing
lezziely
lezzies
lezziesed
lezzieser
lezzieses
lezziesing
lezziesly
lezziess
lezzy
lezzyed
lezzyer
lezzyes
lezzying
lezzyly
lezzys
lmaoed
lmaoer
lmaoes
lmaoing
lmaoly
lmaos
lmfao
lmfaoed
lmfaoer
lmfaoes
lmfaoing
lmfaoly
lmfaos
loined
loiner
loines
loining
loinly
loins
loinsed
loinser
loinses
loinsing
loinsly
loinss
lubeed
lubeer
lubees
lubeing
lubely
lubes
lusty
lustyed
lustyer
lustyes
lustying
lustyly
lustys
massa
massaed
massaer
massaes
massaing
massaly
massas
masterbate
masterbateed
masterbateer
masterbatees
masterbateing
masterbately
masterbates
masterbating
masterbatinged
masterbatinger
masterbatinges
masterbatinging
masterbatingly
masterbatings
masterbation
masterbationed
masterbationer
masterbationes
masterbationing
masterbationly
masterbations
masturbate
masturbateed
masturbateer
masturbatees
masturbateing
masturbately
masturbates
masturbating
masturbatinged
masturbatinger
masturbatinges
masturbatinging
masturbatingly
masturbatings
masturbation
masturbationed
masturbationer
masturbationes
masturbationing
masturbationly
masturbations
methed
mether
methes
mething
methly
meths
militaryed
militaryer
militaryes
militarying
militaryly
militarys
mofo
mofoed
mofoer
mofoes
mofoing
mofoly
mofos
molest
molested
molester
molestes
molesting
molestly
molests
moolie
moolieed
moolieer
mooliees
moolieing
mooliely
moolies
moron
moroned
moroner
morones
moroning
moronly
morons
motherfucka
motherfuckaed
motherfuckaer
motherfuckaes
motherfuckaing
motherfuckaly
motherfuckas
motherfucker
motherfuckered
motherfuckerer
motherfuckeres
motherfuckering
motherfuckerly
motherfuckers
motherfucking
motherfuckinged
motherfuckinger
motherfuckinges
motherfuckinging
motherfuckingly
motherfuckings
mtherfucker
mtherfuckered
mtherfuckerer
mtherfuckeres
mtherfuckering
mtherfuckerly
mtherfuckers
mthrfucker
mthrfuckered
mthrfuckerer
mthrfuckeres
mthrfuckering
mthrfuckerly
mthrfuckers
mthrfucking
mthrfuckinged
mthrfuckinger
mthrfuckinges
mthrfuckinging
mthrfuckingly
mthrfuckings
muff
muffdiver
muffdivered
muffdiverer
muffdiveres
muffdivering
muffdiverly
muffdivers
muffed
muffer
muffes
muffing
muffly
muffs
murdered
murderer
murderes
murdering
murderly
murders
muthafuckaz
muthafuckazed
muthafuckazer
muthafuckazes
muthafuckazing
muthafuckazly
muthafuckazs
muthafucker
muthafuckered
muthafuckerer
muthafuckeres
muthafuckering
muthafuckerly
muthafuckers
mutherfucker
mutherfuckered
mutherfuckerer
mutherfuckeres
mutherfuckering
mutherfuckerly
mutherfuckers
mutherfucking
mutherfuckinged
mutherfuckinger
mutherfuckinges
mutherfuckinging
mutherfuckingly
mutherfuckings
muthrfucking
muthrfuckinged
muthrfuckinger
muthrfuckinges
muthrfuckinging
muthrfuckingly
muthrfuckings
nad
naded
nader
nades
nading
nadly
nads
nadsed
nadser
nadses
nadsing
nadsly
nadss
nakeded
nakeder
nakedes
nakeding
nakedly
nakeds
napalm
napalmed
napalmer
napalmes
napalming
napalmly
napalms
nappy
nappyed
nappyer
nappyes
nappying
nappyly
nappys
nazi
nazied
nazier
nazies
naziing
nazily
nazis
nazism
nazismed
nazismer
nazismes
nazisming
nazismly
nazisms
negro
negroed
negroer
negroes
negroing
negroly
negros
nigga
niggaed
niggaer
niggaes
niggah
niggahed
niggaher
niggahes
niggahing
niggahly
niggahs
niggaing
niggaly
niggas
niggased
niggaser
niggases
niggasing
niggasly
niggass
niggaz
niggazed
niggazer
niggazes
niggazing
niggazly
niggazs
nigger
niggered
niggerer
niggeres
niggering
niggerly
niggers
niggersed
niggerser
niggerses
niggersing
niggersly
niggerss
niggle
niggleed
niggleer
nigglees
niggleing
nigglely
niggles
niglet
nigleted
nigleter
nigletes
nigleting
nigletly
niglets
nimrod
nimroded
nimroder
nimrodes
nimroding
nimrodly
nimrods
ninny
ninnyed
ninnyer
ninnyes
ninnying
ninnyly
ninnys
nooky
nookyed
nookyer
nookyes
nookying
nookyly
nookys
nuccitelli
nuccitellied
nuccitellier
nuccitellies
nuccitelliing
nuccitellily
nuccitellis
nympho
nymphoed
nymphoer
nymphoes
nymphoing
nympholy
nymphos
opium
opiumed
opiumer
opiumes
opiuming
opiumly
opiums
orgies
orgiesed
orgieser
orgieses
orgiesing
orgiesly
orgiess
orgy
orgyed
orgyer
orgyes
orgying
orgyly
orgys
paddy
paddyed
paddyer
paddyes
paddying
paddyly
paddys
paki
pakied
pakier
pakies
pakiing
pakily
pakis
pantie
pantieed
pantieer
pantiees
pantieing
pantiely
panties
pantiesed
pantieser
pantieses
pantiesing
pantiesly
pantiess
panty
pantyed
pantyer
pantyes
pantying
pantyly
pantys
pastie
pastieed
pastieer
pastiees
pastieing
pastiely
pasties
pasty
pastyed
pastyer
pastyes
pastying
pastyly
pastys
pecker
peckered
peckerer
peckeres
peckering
peckerly
peckers
pedo
pedoed
pedoer
pedoes
pedoing
pedoly
pedophile
pedophileed
pedophileer
pedophilees
pedophileing
pedophilely
pedophiles
pedophilia
pedophiliac
pedophiliaced
pedophiliacer
pedophiliaces
pedophiliacing
pedophiliacly
pedophiliacs
pedophiliaed
pedophiliaer
pedophiliaes
pedophiliaing
pedophilialy
pedophilias
pedos
penial
penialed
penialer
peniales
penialing
penially
penials
penile
penileed
penileer
penilees
penileing
penilely
peniles
penis
penised
peniser
penises
penising
penisly
peniss
perversion
perversioned
perversioner
perversiones
perversioning
perversionly
perversions
peyote
peyoteed
peyoteer
peyotees
peyoteing
peyotely
peyotes
phuck
phucked
phucker
phuckes
phucking
phuckly
phucks
pillowbiter
pillowbitered
pillowbiterer
pillowbiteres
pillowbitering
pillowbiterly
pillowbiters
pimp
pimped
pimper
pimpes
pimping
pimply
pimps
pinko
pinkoed
pinkoer
pinkoes
pinkoing
pinkoly
pinkos
pissed
pisseded
pisseder
pissedes
pisseding
pissedly
pisseds
pisser
pisses
pissing
pissly
pissoff
pissoffed
pissoffer
pissoffes
pissoffing
pissoffly
pissoffs
pisss
polack
polacked
polacker
polackes
polacking
polackly
polacks
pollock
pollocked
pollocker
pollockes
pollocking
pollockly
pollocks
poon
pooned
pooner
poones
pooning
poonly
poons
poontang
poontanged
poontanger
poontanges
poontanging
poontangly
poontangs
porn
porned
porner
pornes
porning
pornly
porno
pornoed
pornoer
pornoes
pornography
pornographyed
pornographyer
pornographyes
pornographying
pornographyly
pornographys
pornoing
pornoly
pornos
porns
prick
pricked
pricker
prickes
pricking
prickly
pricks
prig
priged
priger
priges
priging
prigly
prigs
prostitute
prostituteed
prostituteer
prostitutees
prostituteing
prostitutely
prostitutes
prude
prudeed
prudeer
prudees
prudeing
prudely
prudes
punkass
punkassed
punkasser
punkasses
punkassing
punkassly
punkasss
punky
punkyed
punkyer
punkyes
punkying
punkyly
punkys
puss
pussed
pusser
pusses
pussies
pussiesed
pussieser
pussieses
pussiesing
pussiesly
pussiess
pussing
pussly
pusss
pussy
pussyed
pussyer
pussyes
pussying
pussyly
pussypounder
pussypoundered
pussypounderer
pussypounderes
pussypoundering
pussypounderly
pussypounders
pussys
puto
putoed
putoer
putoes
putoing
putoly
putos
queaf
queafed
queafer
queafes
queafing
queafly
queafs
queef
queefed
queefer
queefes
queefing
queefly
queefs
queer
queered
queerer
queeres
queering
queerly
queero
queeroed
queeroer
queeroes
queeroing
queeroly
queeros
queers
queersed
queerser
queerses
queersing
queersly
queerss
quicky
quickyed
quickyer
quickyes
quickying
quickyly
quickys
quim
quimed
quimer
quimes
quiming
quimly
quims
racy
racyed
racyer
racyes
racying
racyly
racys
rape
raped
rapeded
rapeder
rapedes
rapeding
rapedly
rapeds
rapeed
rapeer
rapees
rapeing
rapely
raper
rapered
raperer
raperes
rapering
raperly
rapers
rapes
rapist
rapisted
rapister
rapistes
rapisting
rapistly
rapists
raunch
raunched
rauncher
raunches
raunching
raunchly
raunchs
rectus
rectused
rectuser
rectuses
rectusing
rectusly
rectuss
reefer
reefered
reeferer
reeferes
reefering
reeferly
reefers
reetard
reetarded
reetarder
reetardes
reetarding
reetardly
reetards
reich
reiched
reicher
reiches
reiching
reichly
reichs
retard
retarded
retardeded
retardeder
retardedes
retardeding
retardedly
retardeds
retarder
retardes
retarding
retardly
retards
rimjob
rimjobed
rimjober
rimjobes
rimjobing
rimjobly
rimjobs
ritard
ritarded
ritarder
ritardes
ritarding
ritardly
ritards
rtard
rtarded
rtarder
rtardes
rtarding
rtardly
rtards
rum
rumed
rumer
rumes
ruming
rumly
rump
rumped
rumper
rumpes
rumping
rumply
rumprammer
rumprammered
rumprammerer
rumprammeres
rumprammering
rumprammerly
rumprammers
rumps
rums
ruski
ruskied
ruskier
ruskies
ruskiing
ruskily
ruskis
sadism
sadismed
sadismer
sadismes
sadisming
sadismly
sadisms
sadist
sadisted
sadister
sadistes
sadisting
sadistly
sadists
scag
scaged
scager
scages
scaging
scagly
scags
scantily
scantilyed
scantilyer
scantilyes
scantilying
scantilyly
scantilys
schlong
schlonged
schlonger
schlonges
schlonging
schlongly
schlongs
scrog
scroged
scroger
scroges
scroging
scrogly
scrogs
scrot
scrote
scroted
scroteed
scroteer
scrotees
scroteing
scrotely
scroter
scrotes
scroting
scrotly
scrots
scrotum
scrotumed
scrotumer
scrotumes
scrotuming
scrotumly
scrotums
scrud
scruded
scruder
scrudes
scruding
scrudly
scruds
scum
scumed
scumer
scumes
scuming
scumly
scums
seaman
seamaned
seamaner
seamanes
seamaning
seamanly
seamans
seamen
seamened
seamener
seamenes
seamening
seamenly
seamens
seduceed
seduceer
seducees
seduceing
seducely
seduces
semen
semened
semener
semenes
semening
semenly
semens
shamedame
shamedameed
shamedameer
shamedamees
shamedameing
shamedamely
shamedames
shit
shite
shiteater
shiteatered
shiteaterer
shiteateres
shiteatering
shiteaterly
shiteaters
shited
shiteed
shiteer
shitees
shiteing
shitely
shiter
shites
shitface
shitfaceed
shitfaceer
shitfacees
shitfaceing
shitfacely
shitfaces
shithead
shitheaded
shitheader
shitheades
shitheading
shitheadly
shitheads
shithole
shitholeed
shitholeer
shitholees
shitholeing
shitholely
shitholes
shithouse
shithouseed
shithouseer
shithousees
shithouseing
shithousely
shithouses
shiting
shitly
shits
shitsed
shitser
shitses
shitsing
shitsly
shitss
shitt
shitted
shitteded
shitteder
shittedes
shitteding
shittedly
shitteds
shitter
shittered
shitterer
shitteres
shittering
shitterly
shitters
shittes
shitting
shittly
shitts
shitty
shittyed
shittyer
shittyes
shittying
shittyly
shittys
shiz
shized
shizer
shizes
shizing
shizly
shizs
shooted
shooter
shootes
shooting
shootly
shoots
sissy
sissyed
sissyer
sissyes
sissying
sissyly
sissys
skag
skaged
skager
skages
skaging
skagly
skags
skank
skanked
skanker
skankes
skanking
skankly
skanks
slave
slaveed
slaveer
slavees
slaveing
slavely
slaves
sleaze
sleazeed
sleazeer
sleazees
sleazeing
sleazely
sleazes
sleazy
sleazyed
sleazyer
sleazyes
sleazying
sleazyly
sleazys
slut
slutdumper
slutdumpered
slutdumperer
slutdumperes
slutdumpering
slutdumperly
slutdumpers
sluted
sluter
slutes
sluting
slutkiss
slutkissed
slutkisser
slutkisses
slutkissing
slutkissly
slutkisss
slutly
sluts
slutsed
slutser
slutses
slutsing
slutsly
slutss
smegma
smegmaed
smegmaer
smegmaes
smegmaing
smegmaly
smegmas
smut
smuted
smuter
smutes
smuting
smutly
smuts
smutty
smuttyed
smuttyer
smuttyes
smuttying
smuttyly
smuttys
snatch
snatched
snatcher
snatches
snatching
snatchly
snatchs
sniper
snipered
sniperer
sniperes
snipering
sniperly
snipers
snort
snorted
snorter
snortes
snorting
snortly
snorts
snuff
snuffed
snuffer
snuffes
snuffing
snuffly
snuffs
sodom
sodomed
sodomer
sodomes
sodoming
sodomly
sodoms
spic
spiced
spicer
spices
spicing
spick
spicked
spicker
spickes
spicking
spickly
spicks
spicly
spics
spik
spoof
spoofed
spoofer
spoofes
spoofing
spoofly
spoofs
spooge
spoogeed
spoogeer
spoogees
spoogeing
spoogely
spooges
spunk
spunked
spunker
spunkes
spunking
spunkly
spunks
steamyed
steamyer
steamyes
steamying
steamyly
steamys
stfu
stfued
stfuer
stfues
stfuing
stfuly
stfus
stiffy
stiffyed
stiffyer
stiffyes
stiffying
stiffyly
stiffys
stoneded
stoneder
stonedes
stoneding
stonedly
stoneds
stupided
stupider
stupides
stupiding
stupidly
stupids
suckeded
suckeder
suckedes
suckeding
suckedly
suckeds
sucker
suckes
sucking
suckinged
suckinger
suckinges
suckinging
suckingly
suckings
suckly
sucks
sumofabiatch
sumofabiatched
sumofabiatcher
sumofabiatches
sumofabiatching
sumofabiatchly
sumofabiatchs
tard
tarded
tarder
tardes
tarding
tardly
tards
tawdry
tawdryed
tawdryer
tawdryes
tawdrying
tawdryly
tawdrys
teabagging
teabagginged
teabagginger
teabagginges
teabagginging
teabaggingly
teabaggings
terd
terded
terder
terdes
terding
terdly
terds
teste
testee
testeed
testeeed
testeeer
testeees
testeeing
testeely
testeer
testees
testeing
testely
testes
testesed
testeser
testeses
testesing
testesly
testess
testicle
testicleed
testicleer
testiclees
testicleing
testiclely
testicles
testis
testised
testiser
testises
testising
testisly
testiss
thrusted
thruster
thrustes
thrusting
thrustly
thrusts
thug
thuged
thuger
thuges
thuging
thugly
thugs
tinkle
tinkleed
tinkleer
tinklees
tinkleing
tinklely
tinkles
tit
tited
titer
tites
titfuck
titfucked
titfucker
titfuckes
titfucking
titfuckly
titfucks
titi
titied
titier
tities
titiing
titily
titing
titis
titly
tits
titsed
titser
titses
titsing
titsly
titss
tittiefucker
tittiefuckered
tittiefuckerer
tittiefuckeres
tittiefuckering
tittiefuckerly
tittiefuckers
titties
tittiesed
tittieser
tittieses
tittiesing
tittiesly
tittiess
titty
tittyed
tittyer
tittyes
tittyfuck
tittyfucked
tittyfucker
tittyfuckered
tittyfuckerer
tittyfuckeres
tittyfuckering
tittyfuckerly
tittyfuckers
tittyfuckes
tittyfucking
tittyfuckly
tittyfucks
tittying
tittyly
tittys
toke
tokeed
tokeer
tokees
tokeing
tokely
tokes
toots
tootsed
tootser
tootses
tootsing
tootsly
tootss
tramp
tramped
tramper
trampes
tramping
tramply
tramps
transsexualed
transsexualer
transsexuales
transsexualing
transsexually
transsexuals
trashy
trashyed
trashyer
trashyes
trashying
trashyly
trashys
tubgirl
tubgirled
tubgirler
tubgirles
tubgirling
tubgirlly
tubgirls
turd
turded
turder
turdes
turding
turdly
turds
tush
tushed
tusher
tushes
tushing
tushly
tushs
twat
twated
twater
twates
twating
twatly
twats
twatsed
twatser
twatses
twatsing
twatsly
twatss
undies
undiesed
undieser
undieses
undiesing
undiesly
undiess
unweded
unweder
unwedes
unweding
unwedly
unweds
uzi
uzied
uzier
uzies
uziing
uzily
uzis
vag
vaged
vager
vages
vaging
vagly
vags
valium
valiumed
valiumer
valiumes
valiuming
valiumly
valiums
venous
virgined
virginer
virgines
virgining
virginly
virgins
vixen
vixened
vixener
vixenes
vixening
vixenly
vixens
vodkaed
vodkaer
vodkaes
vodkaing
vodkaly
vodkas
voyeur
voyeured
voyeurer
voyeures
voyeuring
voyeurly
voyeurs
vulgar
vulgared
vulgarer
vulgares
vulgaring
vulgarly
vulgars
wang
wanged
wanger
wanges
wanging
wangly
wangs
wank
wanked
wanker
wankered
wankerer
wankeres
wankering
wankerly
wankers
wankes
wanking
wankly
wanks
wazoo
wazooed
wazooer
wazooes
wazooing
wazooly
wazoos
wedgie
wedgieed
wedgieer
wedgiees
wedgieing
wedgiely
wedgies
weeded
weeder
weedes
weeding
weedly
weeds
weenie
weenieed
weenieer
weeniees
weenieing
weeniely
weenies
weewee
weeweeed
weeweeer
weeweees
weeweeing
weeweely
weewees
weiner
weinered
weinerer
weineres
weinering
weinerly
weiners
weirdo
weirdoed
weirdoer
weirdoes
weirdoing
weirdoly
weirdos
wench
wenched
wencher
wenches
wenching
wenchly
wenchs
wetback
wetbacked
wetbacker
wetbackes
wetbacking
wetbackly
wetbacks
whitey
whiteyed
whiteyer
whiteyes
whiteying
whiteyly
whiteys
whiz
whized
whizer
whizes
whizing
whizly
whizs
whoralicious
whoralicioused
whoraliciouser
whoraliciouses
whoraliciousing
whoraliciously
whoraliciouss
whore
whorealicious
whorealicioused
whorealiciouser
whorealiciouses
whorealiciousing
whorealiciously
whorealiciouss
whored
whoreded
whoreder
whoredes
whoreding
whoredly
whoreds
whoreed
whoreer
whorees
whoreface
whorefaceed
whorefaceer
whorefacees
whorefaceing
whorefacely
whorefaces
whorehopper
whorehoppered
whorehopperer
whorehopperes
whorehoppering
whorehopperly
whorehoppers
whorehouse
whorehouseed
whorehouseer
whorehousees
whorehouseing
whorehousely
whorehouses
whoreing
whorely
whores
whoresed
whoreser
whoreses
whoresing
whoresly
whoress
whoring
whoringed
whoringer
whoringes
whoringing
whoringly
whorings
wigger
wiggered
wiggerer
wiggeres
wiggering
wiggerly
wiggers
woody
woodyed
woodyer
woodyes
woodying
woodyly
woodys
wop
woped
woper
wopes
woping
woply
wops
wtf
wtfed
wtfer
wtfes
wtfing
wtfly
wtfs
xxx
xxxed
xxxer
xxxes
xxxing
xxxly
xxxs
yeasty
yeastyed
yeastyer
yeastyes
yeastying
yeastyly
yeastys
yobbo
yobboed
yobboer
yobboes
yobboing
yobboly
yobbos
zoophile
zoophileed
zoophileer
zoophilees
zoophileing
zoophilely
zoophiles
anal
ass
ass lick
balls
ballsac
bisexual
bleach
causas
cheap
cost of miracles
cunt
display network stats
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gfc
humira AND expensive
illegal
madvocate
masturbation
nuccitelli
overdose
porn
shit
snort
texarkana
direct\-acting antivirals
assistance
ombitasvir
support path
harvoni
abbvie
direct-acting antivirals
paritaprevir
advocacy
ledipasvir
vpak
ritonavir with dasabuvir
program
gilead
greedy
financial
needy
fake-ovir
viekira pak
v pak
sofosbuvir
support
oasis
discount
dasabuvir
protest
ritonavir
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cleveland-clinic')]
div[contains(@class, 'pane-pub-home-cleveland-clinic')]
div[contains(@class, 'pane-pub-topic-cleveland-clinic')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Palmoplantar exanthema and liver dysfunction
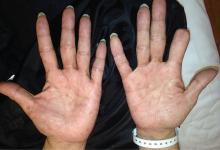
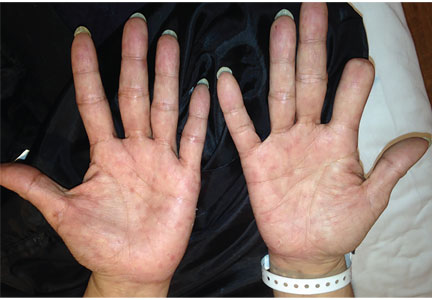
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
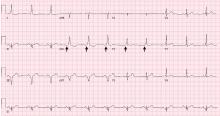
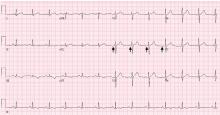
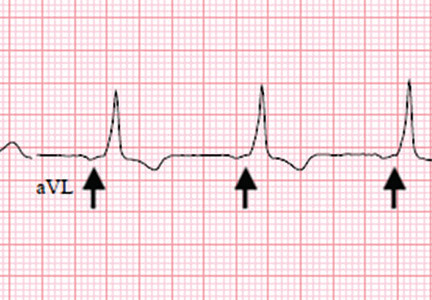
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
Angular cheilitis induced by iron deficiency anemia
A 20-year-old woman had a 4-month history of painful red erosions around the mouth. She had no dysphagia or fatigue and no history of diarrhea, gluten intolerance, or diabetes mellitus. An antifungal-antibacterial ointment prescribed by her dentist had provided no relief.
- Hemoglobin 8.0 g/dL (reference range for females 12.3–15.3)
- Mean corpuscular volume 62 fL (80–100)
- Serum ferritin 1.3 ng/mL (15–200)
- Reticulocyte count 0.86% (0.5–1.5)
- White blood cell count 9.8 × 109/L (4.5–11.0)
- Platelet count 450 × 109/L (150–400).
Vitamin B12 and folate levels were normal, and tests for antitissue transglutaminase and antinuclear antibodies were negative.
Based on these results, the diagnosis was angular cheilitis from iron deficiency anemia. Treatment with oral ferrous gluconate 300 mg twice daily cleared the cheilitis, and after 4 weeks of this treatment, the hemoglobin level increased to 9.8 g/dL, the serum ferritin increased to 7 ng/mL, and the reticulocyte count increased to 2.6%. She was advised to continue taking oral iron tablets for another 3 months until the hemoglobin level reached 12.0 g/dL.
During 2 years of follow-up, she had no recurrence of angular cheilitis, and her hemoglobin and serum ferritin levels remained normal. Ferrous gluconate was her only medication from the time of her diagnosis.
A BROAD DIFFERENTIAL DIAGNOSIS
Angular cheilitis (perlèche) is an inflammatory condition characterized by erosive inflammation at one or both angles of the mouth. It typically presents as erythema, scaling, fissuring, and ulceration. A wide variety of factors, including nutritional deficiencies, local and systemic factors, and drug side effects, may produce cheilitis.1,2
Nutritional deficiencies account for 25% of all cases of angular cheilitis3 and include iron deficiency and deficiencies of the B vitamins riboflavin (B2), niacin (B3), pyridoxine (B6), and cyanocobalamin (B12).1
Local causes include infection with Candida albicans or Staphylococcus aureus and allergic contact dermatitis. Common causes of allergic contact dermatitis include lipstick, toothpaste, mouthwash, cosmetics, sunscreen, fragrance, metals such as nickel, and dental appliances.1
Systemic diseases associated with angular cheilitis include xerostomia, inflammatory bowel disease, Sjögren syndrome, glucagonoma, and human immunodeficiency virus.1
Drugs that cause angular cheilitis include isotretinoin, sorafenib (antineoplastic kinase inhibitor), and ointments or creams such as neomycin sulfate–polymyxin B sulfate, bacitracin, idoxuridine, and steroids.1,4
Conditions that mimic angular cheilitis include herpes simplex type 1 (herpes labialis) and actinic cheilitis. Herpes labialis, characterized by burning sensation, itching, or paresthesia, usually precedes a recurrence of vesicles that eventually ulcerate or form a crust and heal without a crust. Herpes labialis often recurs, affecting the vermilion border and lasting approximately 1 week.
Actinic cheilitis, a premalignant condition that commonly involves the lower lip with sparing of the corners of the mouth, is caused by excessive sun exposure. Patients often have persistent dryness and cracking of the lips.
In our patient, angular cheilitis was the main clinical manifestation of iron deficiency anemia, highlighting the importance of looking for iron deficiency in affected patients without a more obvious cause.
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 2: nutritional, systemic, and drug-related causes and treatment. Cutis 2011; 88(1):27–32. pmid:21877503
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 1: local etiologies. Cutis 2011; 87(6):289–295. pmid:21838086
- Konstantinidis AB, Hatziotis JH. Angular cheilosis: an analysis of 156 cases. J Oral Med 1984; 39(4):199–206. pmid:6594458
- Yang CH, Lin WC, Chuang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 2008; 158(3):592–596. doi:10.1111/j.1365-2133.2007.08357.x
A 20-year-old woman had a 4-month history of painful red erosions around the mouth. She had no dysphagia or fatigue and no history of diarrhea, gluten intolerance, or diabetes mellitus. An antifungal-antibacterial ointment prescribed by her dentist had provided no relief.
- Hemoglobin 8.0 g/dL (reference range for females 12.3–15.3)
- Mean corpuscular volume 62 fL (80–100)
- Serum ferritin 1.3 ng/mL (15–200)
- Reticulocyte count 0.86% (0.5–1.5)
- White blood cell count 9.8 × 109/L (4.5–11.0)
- Platelet count 450 × 109/L (150–400).
Vitamin B12 and folate levels were normal, and tests for antitissue transglutaminase and antinuclear antibodies were negative.
Based on these results, the diagnosis was angular cheilitis from iron deficiency anemia. Treatment with oral ferrous gluconate 300 mg twice daily cleared the cheilitis, and after 4 weeks of this treatment, the hemoglobin level increased to 9.8 g/dL, the serum ferritin increased to 7 ng/mL, and the reticulocyte count increased to 2.6%. She was advised to continue taking oral iron tablets for another 3 months until the hemoglobin level reached 12.0 g/dL.
During 2 years of follow-up, she had no recurrence of angular cheilitis, and her hemoglobin and serum ferritin levels remained normal. Ferrous gluconate was her only medication from the time of her diagnosis.
A BROAD DIFFERENTIAL DIAGNOSIS
Angular cheilitis (perlèche) is an inflammatory condition characterized by erosive inflammation at one or both angles of the mouth. It typically presents as erythema, scaling, fissuring, and ulceration. A wide variety of factors, including nutritional deficiencies, local and systemic factors, and drug side effects, may produce cheilitis.1,2
Nutritional deficiencies account for 25% of all cases of angular cheilitis3 and include iron deficiency and deficiencies of the B vitamins riboflavin (B2), niacin (B3), pyridoxine (B6), and cyanocobalamin (B12).1
Local causes include infection with Candida albicans or Staphylococcus aureus and allergic contact dermatitis. Common causes of allergic contact dermatitis include lipstick, toothpaste, mouthwash, cosmetics, sunscreen, fragrance, metals such as nickel, and dental appliances.1
Systemic diseases associated with angular cheilitis include xerostomia, inflammatory bowel disease, Sjögren syndrome, glucagonoma, and human immunodeficiency virus.1
Drugs that cause angular cheilitis include isotretinoin, sorafenib (antineoplastic kinase inhibitor), and ointments or creams such as neomycin sulfate–polymyxin B sulfate, bacitracin, idoxuridine, and steroids.1,4
Conditions that mimic angular cheilitis include herpes simplex type 1 (herpes labialis) and actinic cheilitis. Herpes labialis, characterized by burning sensation, itching, or paresthesia, usually precedes a recurrence of vesicles that eventually ulcerate or form a crust and heal without a crust. Herpes labialis often recurs, affecting the vermilion border and lasting approximately 1 week.
Actinic cheilitis, a premalignant condition that commonly involves the lower lip with sparing of the corners of the mouth, is caused by excessive sun exposure. Patients often have persistent dryness and cracking of the lips.
In our patient, angular cheilitis was the main clinical manifestation of iron deficiency anemia, highlighting the importance of looking for iron deficiency in affected patients without a more obvious cause.
A 20-year-old woman had a 4-month history of painful red erosions around the mouth. She had no dysphagia or fatigue and no history of diarrhea, gluten intolerance, or diabetes mellitus. An antifungal-antibacterial ointment prescribed by her dentist had provided no relief.
- Hemoglobin 8.0 g/dL (reference range for females 12.3–15.3)
- Mean corpuscular volume 62 fL (80–100)
- Serum ferritin 1.3 ng/mL (15–200)
- Reticulocyte count 0.86% (0.5–1.5)
- White blood cell count 9.8 × 109/L (4.5–11.0)
- Platelet count 450 × 109/L (150–400).
Vitamin B12 and folate levels were normal, and tests for antitissue transglutaminase and antinuclear antibodies were negative.
Based on these results, the diagnosis was angular cheilitis from iron deficiency anemia. Treatment with oral ferrous gluconate 300 mg twice daily cleared the cheilitis, and after 4 weeks of this treatment, the hemoglobin level increased to 9.8 g/dL, the serum ferritin increased to 7 ng/mL, and the reticulocyte count increased to 2.6%. She was advised to continue taking oral iron tablets for another 3 months until the hemoglobin level reached 12.0 g/dL.
During 2 years of follow-up, she had no recurrence of angular cheilitis, and her hemoglobin and serum ferritin levels remained normal. Ferrous gluconate was her only medication from the time of her diagnosis.
A BROAD DIFFERENTIAL DIAGNOSIS
Angular cheilitis (perlèche) is an inflammatory condition characterized by erosive inflammation at one or both angles of the mouth. It typically presents as erythema, scaling, fissuring, and ulceration. A wide variety of factors, including nutritional deficiencies, local and systemic factors, and drug side effects, may produce cheilitis.1,2
Nutritional deficiencies account for 25% of all cases of angular cheilitis3 and include iron deficiency and deficiencies of the B vitamins riboflavin (B2), niacin (B3), pyridoxine (B6), and cyanocobalamin (B12).1
Local causes include infection with Candida albicans or Staphylococcus aureus and allergic contact dermatitis. Common causes of allergic contact dermatitis include lipstick, toothpaste, mouthwash, cosmetics, sunscreen, fragrance, metals such as nickel, and dental appliances.1
Systemic diseases associated with angular cheilitis include xerostomia, inflammatory bowel disease, Sjögren syndrome, glucagonoma, and human immunodeficiency virus.1
Drugs that cause angular cheilitis include isotretinoin, sorafenib (antineoplastic kinase inhibitor), and ointments or creams such as neomycin sulfate–polymyxin B sulfate, bacitracin, idoxuridine, and steroids.1,4
Conditions that mimic angular cheilitis include herpes simplex type 1 (herpes labialis) and actinic cheilitis. Herpes labialis, characterized by burning sensation, itching, or paresthesia, usually precedes a recurrence of vesicles that eventually ulcerate or form a crust and heal without a crust. Herpes labialis often recurs, affecting the vermilion border and lasting approximately 1 week.
Actinic cheilitis, a premalignant condition that commonly involves the lower lip with sparing of the corners of the mouth, is caused by excessive sun exposure. Patients often have persistent dryness and cracking of the lips.
In our patient, angular cheilitis was the main clinical manifestation of iron deficiency anemia, highlighting the importance of looking for iron deficiency in affected patients without a more obvious cause.
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 2: nutritional, systemic, and drug-related causes and treatment. Cutis 2011; 88(1):27–32. pmid:21877503
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 1: local etiologies. Cutis 2011; 87(6):289–295. pmid:21838086
- Konstantinidis AB, Hatziotis JH. Angular cheilosis: an analysis of 156 cases. J Oral Med 1984; 39(4):199–206. pmid:6594458
- Yang CH, Lin WC, Chuang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 2008; 158(3):592–596. doi:10.1111/j.1365-2133.2007.08357.x
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 2: nutritional, systemic, and drug-related causes and treatment. Cutis 2011; 88(1):27–32. pmid:21877503
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 1: local etiologies. Cutis 2011; 87(6):289–295. pmid:21838086
- Konstantinidis AB, Hatziotis JH. Angular cheilosis: an analysis of 156 cases. J Oral Med 1984; 39(4):199–206. pmid:6594458
- Yang CH, Lin WC, Chuang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 2008; 158(3):592–596. doi:10.1111/j.1365-2133.2007.08357.x
Diabetes and pregnancy: Risks and opportunities
A 29-year-old nulliparous woman presents for a routine checkup. She has hypertension and type 2 diabetes mellitus. Her current medications are chlorpropamide 500 mg daily, metformin 500 mg twice a day, lisinopril 40 mg daily, simvastatin 40 mg daily, and aspirin 81 mg daily. Her body mass index is 37 kg/m2 and her blood pressure is 130/80 mm Hg. Her hemoglobin A1c level is 7.8% and her low-density lipoprotein cholesterol 90 mg/dL.
She is considering pregnancy. How would you counsel her?
DEFINING DIABETES IN PREGNANCY
Diabetes in pregnant women, both gestational and pregestational, is the most common medical complication associated with pregnancy.1
- Gestational diabetes is defined as diabetes that is diagnosed during the second or third trimester of pregnancy and that is not clearly pregestational.2
- Pregestational diabetes exists before pregnancy and can be either type 1 or type 2.
Most cases of diabetes diagnosed during the first trimester reflect pregestational diabetes, as gestational diabetes occurs when insulin resistance increases in the later trimesters.
Type 1 diabetes involves autoimmune destruction of pancreatic islet cells, leading to insulin deficiency and the need for insulin therapy. Type 2 diabetes is characterized by insulin resistance rather than overall insulin deficiency. Type 2 diabetes tends to be associated with comorbidities such as obesity and hypertension, which are independent risk factors for adverse perinatal outcomes.3,4
Gestational diabetes accounts for most cases of diabetes during pregnancy. Although both pregestational and gestational diabetes increase the risk of maternal and fetal complications, pregestational diabetes is associated with significantly greater risks.1
IMPACT OF DIABETES ON THE MOTHER
Pregnancy increases the risk of maternal hypoglycemia, especially during the first trimester in patients with type 1 diabetes, as insulin sensitivity increases in early pregnancy.1 Pregnant women with diabetes may also have an altered counterregulatory response and less hypoglycemic awareness.1 Insulin resistance rises during the second and early third trimesters, increasing the risk of hyperglycemia in women with diabetes.1
Glycemic control during pregnancy is usually easier to achieve in patients with type 2 diabetes than with type 1, but it may require much higher insulin doses.
Because pregnancy is inherently a ketogenic state, women with type 1 diabetes are at higher risk of diabetic ketoacidosis, particularly during the second and third trimesters.1 There are reports of euglycemic diabetic ketoacidosis in pregnant women with either gestational or pregestational diabetes.5
Diabetes is associated with a risk of preeclampsia 4 times higher than in nondiabetic women.6 Other potential pregnancy-related complications include infections, polyhydramnios, spontaneous abortion, and cesarean delivery.1,7 The risk of pregnancy loss is similar in women with either type 1 or type 2 diabetes (2.6% and 3.7%, respectively), but the causes are different.8 Although preexisting diabetic complications such as retinopathy, nephropathy, and gastroparesis can be exacerbated during pregnancy,1 only severe gastroparesis and advanced renal disease are considered relative contraindications to pregnancy.
IMPACT OF DIABETES ON THE FETUS
Fetal complications of maternal diabetes include embryopathy (fetal malformations) and fetopathy (overgrowth, ie, fetus large for gestational age, and increased risk of fetal death or distress). Maternal hyperglycemia is associated with diabetic embryopathy, resulting in major birth defects in 5% to 25% of pregnancies and spontaneous abortions in 15% to 20%.9,10 There is a 2- to 6-fold increase in risk of congenital malformations.6
The most common diabetes-associated congenital malformations affect the cardiovascular system. Congenital heart disease includes tetralogy of Fallot, transposition of the great vessels, septal defects, and anomalous pulmonary venous return. Other relatively common defects involve the fetal central nervous system, spine, orofacial system, kidneys, urogenital system, gastrointestinal tract, and skeleton.11
The risk of fetopathy is proportional to the degree of maternal hyperglycemia. Excess maternal glucose and fatty acid levels can lead to fetal hyperglycemia and overgrowth, which increases fetal oxygen requirements. Erythropoietin levels rise, causing an increase in red cell mass, with subsequent hyperviscosity within the placenta and higher risk of fetal death.
Other complications include intrauterine growth restriction, prematurity, and preterm delivery. Fetal macrosomia (birth weight > 90th percentile or 4 kg, approximately 8 lb, 13 oz) occurs in 27% to 62% of children born to mothers with diabetes, a rate 10 times higher than in patients without diabetes. It contributes to shoulder dystocia (risk 2 to 4 times higher in diabetic pregnancies) and cesarean delivery.6 Infants born to mothers with diabetes also have higher risks of neonatal hypoglycemia, erythrocytosis, hyperbilirubinemia, hypocalcemia, respiratory distress, cardiomyopathy, and death, as well as for developing diabetes, obesity, and other adverse cardiometabolic outcomes later in life.11
GET GLUCOSE UNDER CONTROL BEFORE PREGNANCY
Nearly half of pregnancies in the general population are unplanned,15 so preconception diabetes assessment needs to be part of routine medical care for all reproductive-age women. Because most organogenesis occurs during the first 5 to 8 weeks after fertilization—potentially before a woman realizes she is pregnant—achieving optimal glycemic control before conception is necessary to improve pregnancy outcomes.1
EVERY VISIT IS AN OPPORTUNITY
Every medical visit with a reproductive-age woman with diabetes is an opportunity for counseling about pregnancy. Topics that need to be discussed include the risks of unplanned pregnancy and of poor metabolic control, and the benefits of improved maternal and fetal outcomes with appropriate pregnancy planning and diabetes management.
Referral to a registered dietitian for individualized counseling about proper nutrition, particularly during pregnancy, has been associated with positive outcomes.16 Patients with diabetes and at high risk of pregnancy complications should be referred to a clinic that specializes in high-risk pregnancies.
Practitioners also should emphasize the importance of regular exercise and encourage patients to maintain or achieve a medically optimal weight before conception. Ideally, this would be a normal body mass index; however, this is not always possible.
In women who are planning pregnancy or are not on effective contraception, medications should be reviewed for potential teratogenicity. If needed, discuss alternative medications or switch to safer ones. However, these changes should not interrupt diabetes treatment.
In addition, ensure that the patient is up to date on age- and disease-appropriate preventive care (eg, immunizations, screening for sexually transmitted disease and malignancy). Counseling and intervention for use of tobacco, alcohol, and recreational drugs are also important. As with any preconception counseling, the patient (and her partner, if possible) should be advised to avoid travel to areas where Zika virus is endemic, and informed about the availability of expanded carrier genetic screening through her obstetric provider.
Finally, pregnant women with diabetes benefit from screening for diabetic complications including hypertension, retinopathy, cardiovascular disease, neuropathy, and nephropathy.
ASSESSING RISKS
Blood pressure
Chronic (preexisting) hypertension is defined as a systolic pressure 140 mm Hg or higher or a diastolic pressure 90 mm Hg or higher, or both, that antedates pregnancy or is present before the 20th week of pregnancy.3 Chronic hypertension has been reported in up to 5% of pregnant women and is associated with increased risk of preterm delivery, superimposed preeclampsia, low birth weight, and perinatal death.3
Reproductive-age women with diabetes and high blood pressure benefit from lifestyle and behavioral modifications.17 If drug therapy is needed, antihypertensive drugs that are safe for the fetus should be used. Treatment of mild or moderate hypertension during pregnancy reduces the risk of progression to severe hypertension but may not improve obstetric outcomes.
Diabetic retinopathy
Diabetic retinopathy can significantly worsen during pregnancy: the risk of progression is double that in the nonpregnant state.18 Women with diabetes who are contemplating pregnancy should have a comprehensive eye examination before conception, and any active proliferative retinopathy needs to be treated. These patients may require ophthalmologic monitoring and treatment during pregnancy. (Note: laser photocoagulation is not contraindicated during pregnancy.)
Cardiovascular disease
Cardiovascular physiology changes dramatically during pregnancy. Cardiovascular disease, especially when superimposed on diabetes, can increase the risk of maternal death. Thus, evaluation for cardiovascular risk factors as well as cardiovascular system integrity before conception is important. Listen for arterial bruits and murmurs, and assess peripheral pulses. Consideration should be given to obtaining a preconception resting electrocardiogram in women with diabetes who are over age 35 or who are suspected of having cardiovascular disease.16
Neurologic disorders
Peripheral neuropathy, the most common neurologic complication of diabetes, is associated with injury and infection.19
Autonomic neuropathy is associated with decreased cardiac responsiveness and orthostatic hypotension.19 Diabetic gastroparesis alone can precipitate serious complications during pregnancy, including extreme hypoglycemia and hyperglycemia, increased risk of diabetic ketoacidosis, weight loss, malnutrition, frequent hospitalizations, and increased requirement for parenteral nutrition.20
Although diabetic neuropathy does not significantly worsen during pregnancy, women with preexisting gastroparesis should be counseled on the substantial risks associated with pregnancy. Screening for neuropathy should be part of all diabetic preconception examinations.
Renal complications
Pregnancy in women with diabetes and preexisting renal dysfunction increases their risk of accelerated progression of diabetic kidney disease.21 Preexisting renal dysfunction also increases the risk of pregnancy-related complications, such as stillbirth, intrauterine growth restriction, gestational hypertension, preeclampsia, and preterm delivery.19,21,22 Further, the risk of pregnancy complications correlates directly with the severity of renal dysfunction.22
Psychiatric disorders
Emotional wellness is essential for optimal diabetes management. It is important to recognize the emotional impact of diabetes in pregnant women and to conduct routine screening for depression, anxiety, stress, and eating disorders.16
LABORATORY TESTS TO CONSIDER
Hemoglobin A1c. The general consensus is to achieve the lowest hemoglobin A1c level possible that does not increase the risk of hypoglycemia. The American Diabetes Association (ADA) recommends that, before attempting to conceive, women should lower their hemoglobin A1c to below 6.5%.1
Thyroid measures. Autoimmune thyroid disease is the most common autoimmune disorder associated with diabetes and has been reported in 35% to 40% of women with type 1 diabetes.23 Recommendations are to check thyroid-stimulating hormone and thyroid peroxidase antibody levels before conception or early in pregnancy in all women with diabetes.1,24 Overt hypothyroidism should be treated before conception, given that early fetal brain development depends on maternal thyroxine.
Renal function testing. Preconception assessment of renal function is important for counseling and risk stratification. This assessment should include serum creatinine level, estimated glomerular filtration rate, and urinary albumin excretion.21
Celiac screening. Because women with type 1 diabetes are more susceptible to autoimmune diseases, they should be screened for celiac disease before conception, with testing for immunoglobulin A (IgA) and tissue transglutaminase antibodies, with or without IgA endomysial antibodies.16,25,26 An estimated 6% of patients with type 1 diabetes have celiac disease vs 1% of the general population.25 Celiac disease is 2 to 3 times more common in women, and asymptomatic people with type 1 diabetes are considered at increased risk for celiac disease.26
The association between type 1 diabetes and celiac disease most likely relates to the overlap in human leukocyte antigens of the diseases. There is no established link between type 2 diabetes and celiac disease.25
Undiagnosed celiac disease increases a woman’s risk of obstetric complications such as preterm birth, low birth weight, and stillbirth.26 The most likely explanation for these adverse effects is nutrient malabsorption, which is characteristic of celiac disease. Adherence to a gluten-free diet before and during gestation may reduce the risk of preterm delivery by as much as 20%.26
Vitamin B12 level. Celiac disease interferes with the absorption of vitamin B12-instrinsic factor in the ileum, which can lead to vitamin B12 deficiency. Therefore, baseline vitamin B12 levels should be checked before conception in women with celiac disease. Levels should also be checked in women taking metformin, which also decreases vitamin B12 absorption. Of note, increased folate levels due to taking supplements can potentially mask vitamin B12 deficiency.
MEDICATIONS TO REVIEW FOR PREGNANCY INTERACTIONS
Diabetic medications
Insulin is the first-line pharmacotherapy for pregnant patients with type 1, type 2, or gestational diabetes. Insulin does not cross the placenta to a measurable extent, and most insulin preparations have been classified as category B,1 meaning no risks to the fetus have been found in humans.
Insulin dosing during pregnancy is not static. Beginning around mid-gestation, insulin requirements increase,28,29 but after 32 weeks the need may decrease. These changes require practitioners to closely monitor blood glucose throughout pregnancy.
Both basal-bolus injections and continuous subcutaneous infusion are reasonable options during pregnancy.30 However, the need for multiple and potentially painful insulin injections daily can lead to poor compliance. This inconvenience has led to studies using oral hypoglycemic medications instead of insulin for patients with gestational and type 2 diabetes.
Metformin is an oral biguanide that decreases hepatic gluconeogenesis and intestinal glucose absorption while peripherally increasing glucose utilization and uptake. Metformin does not pose a risk of hypoglycemia because its mechanism of action does not involve increased insulin production.7
Metformin does cross the placenta, resulting in umbilical cord blood levels higher than maternal levels. Nevertheless, studies support the efficacy and short-term safety of metformin use during a pregnancy complicated by gestational or type 2 diabetes.7,31 Moreover, metformin has been associated with a lower risk of neonatal hypoglycemia and maternal weight gain than insulin.32 However, this agent should be used with caution, as long-term data are not yet available, and it may slightly increase the risk of premature delivery.
Glyburide is another oral hypoglycemic medication that has been used during pregnancy. This second-generation sulfonylurea enhances the release of insulin from the pancreas by binding beta islet cell ATP-calcium channel receptors. Compared with other sulfonylureas, glyburide has the lowest rate of maternal-to-fetal transfer, with umbilical cord plasma concentrations 70% of maternal levels.33 Although some trials support the efficacy and short-term safety of glyburide treatment for gestational diabetes,34 recent studies have associated glyburide use during pregnancy with a higher rate of neonatal hypoglycemia, neonatal respiratory distress, macrosomia, and neonatal intensive care unit admissions than insulin and metformin.1,35
Patients treated with oral agents should be informed that these drugs cross the placenta, and that although no adverse effects on the fetus have been demonstrated, long-term safety data are lacking. In addition, oral agents are ineffective in type 1 diabetes and may be insufficient to overcome the insulin resistance in type 2 diabetes.
Antihypertensive drugs
All antihypertensive drugs cross the placenta, but several have an acceptable safety profile in pregnancy, including methyldopa, labetalol, clonidine, prazosin, and nifedipine. Hydralazine and labetalol are short-acting, come in intravenous formulations, and can be used for urgent blood pressure control during pregnancy. Diltiazem may be used for heart rate control during pregnancy, and it has been shown to lower blood pressure and proteinuria in pregnant patients with underlying renal disease.36,37 The ADA recommends against chronic use of diuretics during pregnancy because of potential reductions in maternal plasma volume and uteroplacental perfusion.1
Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and direct renin inhibitors are contraindicated during pregnancy because of the risk of fetal defects, particularly in the renal system.21,38 Although there is evidence to question the association between first semester exposure and fetotoxicity,39 we avoid these drugs during pregnancy and switch to a different agent in women planning pregnancy.
Other drugs
Statins are contraindicated in pregnancy because they interfere with the development of the fetal nervous system.21 Although preliminary data from a small study did not identify safety risks associated with pravastatin use after 12 weeks of gestation,40 we recommend discontinuing statins in women attempting pregnancy.
Aspirin. The US Preventive Services Task Force41 recommends low-dose aspirin (81 mg/day) after 12 weeks of gestation for women with type 1 or type 2 diabetes, as well as those with renal disease or chronic hypertension, to prevent preeclampsia. Of note, higher doses need to be used with caution during pregnancy because fetal abnormalities have been reported, such as disruption of fetal vasculature (mesenteric vessels), gastroschisis, and small intestinal atresia.16
Folate supplementation (0.6–4 mg/day) is recommended in women with celiac disease to prevent neural tube defects in the offspring, and the US Preventive Services Task Force recommends 0.4 mg daily of folic acid supplementation for all women planning or capable of pregnancy.42–44 Higher doses, ranging from 0.6 to 5 mg/day, have been proposed for patients with diabetes,13 and we recommend at least 1 mg for this group, based on data suggesting that higher doses further reduce the risk of neural tube defects.43
IS BREASTFEEDING AFFECTED?
Maternal diabetes, insulin therapy, and oral hypoglycemic agents are not contraindications to breastfeeding. The US Preventive Services Task Force recommends interventions by primary care physicians to promote and support breastfeeding.45 Breastfeeding is encouraged based on various short- and long-term health benefits for both breastfed infants and breastfeeding mothers. Breastfeeding decreases a woman’s insulin requirements and increases the risk for hypoglycemia, especially in patients with insulin-dependent type 1 diabetes.1
Additionally, insulin sensitivity increases immediately following delivery of the placenta.1 Therefore, it is prudent to adjust insulin doses postpartum, especially while a patient is breastfeeding, or to suggest high-carbohydrate snacks before feeds.9,29
Antihypertensive drugs considered safe to use during lactation include captopril, enalapril, quinapril, labetalol, propranolol, nifedipine, and hydralazine.21,46 Methyldopa is not contraindicated, but it causes fatigue and worsened postpartum depression and should not be used as first-line therapy. Diuretics and ARBs are not recommended during lactation.21 Both metformin and glyburide enter breast milk in small enough amounts that they are not contraindicated during breastfeeding.16 The Lactmed database (www.toxnet.nlm.nih.gov) provides information about drugs and breastfeeding.
WHAT ABOUT CONTRACEPTIVES?
The ADA recommends contraception for women with diabetes because, just as in women without diabetes, the risks of unplanned pregnancy outweigh those of contraceptives.1
We recommend low-dose combination estrogen-progestin oral contraceptives to normotensive women under age 35 with diabetes but without underlying microvascular disease. For women over age 35 or for those with microvascular disease, additional options include intrauterine devices or progestin implants. We prefer not to use injectable depot medroxyprogesterone acetate because of its side effects of insulin resistance and weight gain.47
CASE DISCUSSION: NEXT STEPS
Our patient’s interest in family planning presents an opportunity for preconception counseling. We recommend a prenatal folic acid supplement, diet and regular exercise for weight loss, and screening tests including a comprehensive metabolic panel, hemoglobin A1c, thyroid-stimulating hormone, and dilated eye examination. We make sure she is up to date on her indicated health maintenance (eg, immunizations, disease screening), and we review her medications for potential teratogens. She denies any recreational drug use. Also, she has no plans for long-distance travel.
Our counseling includes discussions of pregnancy risks associated with pregestational diabetes and suboptimal glycemic control. We encourage her to use effective contraception until she is “medically optimized” for pregnancy—ie, until her hemoglobin A1c is lower than 6.5% and she has achieved a medically optimal weight. If feasible, a reduction of weight (7% or so) through lifestyle modification should be attempted, and if her hemoglobin A1c remains elevated, adding insulin would be recommended.
Pregnant patients or patients contemplating pregnancy are usually motivated to modify their behavior, making this a good time to reinforce lifestyle modifications. Many patients benefit from individualized counseling by a registered dietitian to help achieve the recommended weight and glycemic control.
Our physical examination in this patient includes screening for micro- and macrovascular complications of diabetes, and the test results are negative. Patients with active proliferative retinopathy should be referred to an ophthalmologist for assessment and treatment.
We review her medications for potential teratogenic effects and stop her ACE inhibitor (lisinopril) and statin (simvastatin). We switch her from a first-generation sulfonylurea (chlorpropamide) to glyburide, a second-generation sulfonylurea. Second-generation sulfonylureas are considered more “fetus-friendly” because first-generation sulfonylureas cross the placenta more easily and can cause fetal hyperinsulinemia, leading to macrosomia and neonatal hypoglycemia.7
The management of diabetes during pregnancy leans toward insulin use, given the lack of information regarding long-term outcomes with oral agents. If insulin is needed, it is best to initiate it before the patient conceives, and then to stop other diabetes medications. We would not make any changes to her aspirin or metformin use.
Educating the patient and her family about prevention, recognition, and treatment of hypoglycemia is important to prevent and manage the increased risk of hypoglycemia with insulin therapy and in early pregnancy.1 Consideration should be given to providing ketone strips as well as education on diabetic ketoacidosis prevention and detection.1 If the patient conceives, begin prenatal care early to allow adequate planning for care of her disease and evaluation of the fetus. Because of the complexity of insulin management in pregnancy, the ADA recommends referral, if possible, to a center offering team-based care, including an obstetrician specialized in high-risk pregnancies, an endocrinologist, and a dietitian.1
- American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S137–S143. doi:10.2337/dc18-S013
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S13–S27. doi:10.2337/dc18-S002
- Lawler J, Osman M, Shelton JA, Yeh J. Population-based analysis of hypertensive disorders in pregnancy. Hypertens Pregnancy 2007; 26(1):67–76. doi:10.1080/10641950601147945
- Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015; 16(8):621–638. doi:10.1111/obr.12288
- Garrison EA, Jagasia S. Inpatient management of women with gestational and pregestational diabetes in pregnancy. Curr Diab Rep 2014; 14(2):457. doi:10.1007/s11892-013-0457-x
- Ballas J, Moore TR, Ramos GA. Management of diabetes in pregnancy. Curr Diab Rep 2012; 12(1):33–42. doi:10.1007/s11892-011-0249-0
- Ryu RJ, Hays KE, Hebert MF. Gestational diabetes mellitus management with oral hypoglycemic agents. Semin Perinatol 2014; 38(8):508–515. doi:10.1053/j.semperi.2014.08.012
- Cundy T, Gamble G, Neale L, et al. Differing causes of pregnancy loss in type 1 and type 2 diabetes. Diabetes Care 2007; 30(10):2603–2607. doi:10.2337/dc07-0555
- Castorino K, Jovanovic L. Pregnancy and diabetes management: advances and controversies. Clin Chem 2011; 57(2):221–230. doi:10.1373/clinchem.2010.155382
- Hammouda SA, Hakeem R. Role of HbA1c in predicting risk for congenital malformations. Prim Care Diabetes 2015; 9(6):458–464. doi:10.1016/j.pcd.2015.01.004
- Chen CP. Congenital malformations associated with maternal diabetes. Taiwanese J Obstet Gynecol 2005; 44(1):1–7. doi:10.1016/S1028-4559(09)60099-1
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33(3):676–682. doi:10.2337/dc09-1848
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36(5):1384–1395. doi:10.2337/dc12-2480
- HAPO Study Cooperative Research Group; Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358(19):1991–2002. doi:10.1056/NEJMoa0707943
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health 2014; 104(suppl 1):S43–S48. doi:10.2105/AJPH.2013.301416
- Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care 2008; 31(5):1060–1079. doi:10.2337/dc08-9020
- Webster LM, Conti-Ramsden F, Seed PT, Webb AJ, Nelson-Piercy C, Chappell LC. Impact of antihypertensive treatment on maternal and perinatal outcomes in pregnancy complicated by chronic hypertension: a systematic review and meta-analysis. J Am Heart Assoc 2017; 6(5).pii:e005526. doi:10.1161/JAHA.117.005526
- Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy: the Diabetes in Early Pregnancy Study. Diabetes Care 1995; 18(5):631–637. pmid:8586000
- American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care 2016; 39 (suppl 1):S1–S109.
- Hawthorne, G. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):77–90. doi:10.1016/j.bpobgyn.2010.10.015
- Ringholm L, Damm JA, Vestgaard M, Damm P, Mathiesen ER. Diabetic nephropathy in women with preexisting diabetes: from pregnancy planning to breastfeeding. Curr Diab Rep 2016; 16(2):12. doi:10.1007/s11892-015-0705-3
- Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 2015; 10(11):1964–1978. doi:10.2215/CJN.09250914
- Umpierrez GE, Latif KA, Murphy MB, et al. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care 2003; 26(4):1181–1185. pmid:12663594
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Akirov A, Pinhas-Hamiel O. Co-occurrence of type 1 diabetes mellitus and celiac disease. World J Diabetes 2015; 6(5):707–714. doi:10.4239/wjd.v6.i5.707
- Saccone G, Berghella V, Sarno L, et al. Celiac disease and obstetric complications: a systematic review and metaanalysis. Am J Obstet Gynecol 2016; 214(2):225–234. doi:10.1016/j.ajog.2015.09.080
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
- de Valk HW, Visser GH. Insulin during pregnancy, labour and delivery. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):65–76. doi:10.1016/j.bpobgyn.2010.10.002
- Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int J Gen Med 2011; 4:827–835. doi:10.2147/IJGM.S26889
- Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2016; (6):CD005542. doi:10.1002/14651858.CD005542.pub2
- Charles B, Norris R, Xiao X, Hague W. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit 2006; 28(1):67–72. pmid:16418696
- Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 2015; 350:h102. doi:10.1136/bmj.h102
- Hebert MF, Ma X, Naraharisetti SB, et al; Obstetric-Fetal Pharmacology Research Unit Network. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009; 85(6):607–614. doi:10.1038/clpt.2009.5
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000; 343(16):1134–1138. doi:10.1056/NEJM200010193431601
- Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr, Jonsson Funk M. Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr 2015; 169:452–458. doi:10.1001/jamapediatrics.2015.74
- Gowda RM, Khan IA, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac arrhythmias in pregnancy: clinical and therapeutic considerations. Int J Cardiol 2003; 88(2):129–133. pmid:12714190
- Khandelwal M, Kumanova M, Gaughan JP, Reece EA. Role of diltiazem in pregnant women with chronic renal disease. J Matern Fetal Neonatal Med 2002; 12(6):408–412. doi:10.1080/jmf.12.6.408.412
- Magee LA, Abalos E, von Dadelszen P, Sibai B, Easterling T, Walkinshaw S; CHIPS Study Group. How to manage hypertension in pregnancy effectively. Br J Clin Pharmacol 2011; 72(3):394–401. doi:10.1111/j.1365-2125.2011.04002.x
- Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 2006; 354(23):2443–2451. doi:10.1056/NEJMoa055202
- Costantine MM, Cleary K, Hebert MF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 2016; 214(6):720.e1–720.e17. doi:10.1016/j.ajog.2015.12.038
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161(11):819–826. doi:10.7326/M14-1884
- Curry SJ, Grossman DC, Whitlock EP, Cantu A. Behavioral counseling research and evidence-based practice recommendations: US Preventive Services Task Force perspectives. Ann Intern Med 2014; 160(6):407–413. doi:10.7326/M13-2128
- Wald N, Law M, Morris J, Wald D. Quantifying the effect of folic acid. Lancet 2001; 358(9298):2069–2073. pmid:11755633
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. JAMA 2017; 317(2):183–189. doi:10.1001/jama.2016.19438
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Primary care interventions to support breastfeeding: US Preventive Services Task Force recommendation statement. JAMA 2016; 316(16):1688–1693. doi:10.1001/jama.2016.14697
- Newton ER, Hale TW. Drugs in breast milk. Clin Obstet Gynecol 2015; 58(4):868–884. doi:10.1097/GRF.0000000000000142
- Xiang AH, Kawakubo M, Kjos SL, Buchanan TA. Long-acting injectable progestin contraception and risk of type 2 diabetes in Latino women with prior gestational diabetes mellitus. Diabetes Care 2006; 29(3):613–617. pmid:16505515
A 29-year-old nulliparous woman presents for a routine checkup. She has hypertension and type 2 diabetes mellitus. Her current medications are chlorpropamide 500 mg daily, metformin 500 mg twice a day, lisinopril 40 mg daily, simvastatin 40 mg daily, and aspirin 81 mg daily. Her body mass index is 37 kg/m2 and her blood pressure is 130/80 mm Hg. Her hemoglobin A1c level is 7.8% and her low-density lipoprotein cholesterol 90 mg/dL.
She is considering pregnancy. How would you counsel her?
DEFINING DIABETES IN PREGNANCY
Diabetes in pregnant women, both gestational and pregestational, is the most common medical complication associated with pregnancy.1
- Gestational diabetes is defined as diabetes that is diagnosed during the second or third trimester of pregnancy and that is not clearly pregestational.2
- Pregestational diabetes exists before pregnancy and can be either type 1 or type 2.
Most cases of diabetes diagnosed during the first trimester reflect pregestational diabetes, as gestational diabetes occurs when insulin resistance increases in the later trimesters.
Type 1 diabetes involves autoimmune destruction of pancreatic islet cells, leading to insulin deficiency and the need for insulin therapy. Type 2 diabetes is characterized by insulin resistance rather than overall insulin deficiency. Type 2 diabetes tends to be associated with comorbidities such as obesity and hypertension, which are independent risk factors for adverse perinatal outcomes.3,4
Gestational diabetes accounts for most cases of diabetes during pregnancy. Although both pregestational and gestational diabetes increase the risk of maternal and fetal complications, pregestational diabetes is associated with significantly greater risks.1
IMPACT OF DIABETES ON THE MOTHER
Pregnancy increases the risk of maternal hypoglycemia, especially during the first trimester in patients with type 1 diabetes, as insulin sensitivity increases in early pregnancy.1 Pregnant women with diabetes may also have an altered counterregulatory response and less hypoglycemic awareness.1 Insulin resistance rises during the second and early third trimesters, increasing the risk of hyperglycemia in women with diabetes.1
Glycemic control during pregnancy is usually easier to achieve in patients with type 2 diabetes than with type 1, but it may require much higher insulin doses.
Because pregnancy is inherently a ketogenic state, women with type 1 diabetes are at higher risk of diabetic ketoacidosis, particularly during the second and third trimesters.1 There are reports of euglycemic diabetic ketoacidosis in pregnant women with either gestational or pregestational diabetes.5
Diabetes is associated with a risk of preeclampsia 4 times higher than in nondiabetic women.6 Other potential pregnancy-related complications include infections, polyhydramnios, spontaneous abortion, and cesarean delivery.1,7 The risk of pregnancy loss is similar in women with either type 1 or type 2 diabetes (2.6% and 3.7%, respectively), but the causes are different.8 Although preexisting diabetic complications such as retinopathy, nephropathy, and gastroparesis can be exacerbated during pregnancy,1 only severe gastroparesis and advanced renal disease are considered relative contraindications to pregnancy.
IMPACT OF DIABETES ON THE FETUS
Fetal complications of maternal diabetes include embryopathy (fetal malformations) and fetopathy (overgrowth, ie, fetus large for gestational age, and increased risk of fetal death or distress). Maternal hyperglycemia is associated with diabetic embryopathy, resulting in major birth defects in 5% to 25% of pregnancies and spontaneous abortions in 15% to 20%.9,10 There is a 2- to 6-fold increase in risk of congenital malformations.6
The most common diabetes-associated congenital malformations affect the cardiovascular system. Congenital heart disease includes tetralogy of Fallot, transposition of the great vessels, septal defects, and anomalous pulmonary venous return. Other relatively common defects involve the fetal central nervous system, spine, orofacial system, kidneys, urogenital system, gastrointestinal tract, and skeleton.11
The risk of fetopathy is proportional to the degree of maternal hyperglycemia. Excess maternal glucose and fatty acid levels can lead to fetal hyperglycemia and overgrowth, which increases fetal oxygen requirements. Erythropoietin levels rise, causing an increase in red cell mass, with subsequent hyperviscosity within the placenta and higher risk of fetal death.
Other complications include intrauterine growth restriction, prematurity, and preterm delivery. Fetal macrosomia (birth weight > 90th percentile or 4 kg, approximately 8 lb, 13 oz) occurs in 27% to 62% of children born to mothers with diabetes, a rate 10 times higher than in patients without diabetes. It contributes to shoulder dystocia (risk 2 to 4 times higher in diabetic pregnancies) and cesarean delivery.6 Infants born to mothers with diabetes also have higher risks of neonatal hypoglycemia, erythrocytosis, hyperbilirubinemia, hypocalcemia, respiratory distress, cardiomyopathy, and death, as well as for developing diabetes, obesity, and other adverse cardiometabolic outcomes later in life.11
GET GLUCOSE UNDER CONTROL BEFORE PREGNANCY
Nearly half of pregnancies in the general population are unplanned,15 so preconception diabetes assessment needs to be part of routine medical care for all reproductive-age women. Because most organogenesis occurs during the first 5 to 8 weeks after fertilization—potentially before a woman realizes she is pregnant—achieving optimal glycemic control before conception is necessary to improve pregnancy outcomes.1
EVERY VISIT IS AN OPPORTUNITY
Every medical visit with a reproductive-age woman with diabetes is an opportunity for counseling about pregnancy. Topics that need to be discussed include the risks of unplanned pregnancy and of poor metabolic control, and the benefits of improved maternal and fetal outcomes with appropriate pregnancy planning and diabetes management.
Referral to a registered dietitian for individualized counseling about proper nutrition, particularly during pregnancy, has been associated with positive outcomes.16 Patients with diabetes and at high risk of pregnancy complications should be referred to a clinic that specializes in high-risk pregnancies.
Practitioners also should emphasize the importance of regular exercise and encourage patients to maintain or achieve a medically optimal weight before conception. Ideally, this would be a normal body mass index; however, this is not always possible.
In women who are planning pregnancy or are not on effective contraception, medications should be reviewed for potential teratogenicity. If needed, discuss alternative medications or switch to safer ones. However, these changes should not interrupt diabetes treatment.
In addition, ensure that the patient is up to date on age- and disease-appropriate preventive care (eg, immunizations, screening for sexually transmitted disease and malignancy). Counseling and intervention for use of tobacco, alcohol, and recreational drugs are also important. As with any preconception counseling, the patient (and her partner, if possible) should be advised to avoid travel to areas where Zika virus is endemic, and informed about the availability of expanded carrier genetic screening through her obstetric provider.
Finally, pregnant women with diabetes benefit from screening for diabetic complications including hypertension, retinopathy, cardiovascular disease, neuropathy, and nephropathy.
ASSESSING RISKS
Blood pressure
Chronic (preexisting) hypertension is defined as a systolic pressure 140 mm Hg or higher or a diastolic pressure 90 mm Hg or higher, or both, that antedates pregnancy or is present before the 20th week of pregnancy.3 Chronic hypertension has been reported in up to 5% of pregnant women and is associated with increased risk of preterm delivery, superimposed preeclampsia, low birth weight, and perinatal death.3
Reproductive-age women with diabetes and high blood pressure benefit from lifestyle and behavioral modifications.17 If drug therapy is needed, antihypertensive drugs that are safe for the fetus should be used. Treatment of mild or moderate hypertension during pregnancy reduces the risk of progression to severe hypertension but may not improve obstetric outcomes.
Diabetic retinopathy
Diabetic retinopathy can significantly worsen during pregnancy: the risk of progression is double that in the nonpregnant state.18 Women with diabetes who are contemplating pregnancy should have a comprehensive eye examination before conception, and any active proliferative retinopathy needs to be treated. These patients may require ophthalmologic monitoring and treatment during pregnancy. (Note: laser photocoagulation is not contraindicated during pregnancy.)
Cardiovascular disease
Cardiovascular physiology changes dramatically during pregnancy. Cardiovascular disease, especially when superimposed on diabetes, can increase the risk of maternal death. Thus, evaluation for cardiovascular risk factors as well as cardiovascular system integrity before conception is important. Listen for arterial bruits and murmurs, and assess peripheral pulses. Consideration should be given to obtaining a preconception resting electrocardiogram in women with diabetes who are over age 35 or who are suspected of having cardiovascular disease.16
Neurologic disorders
Peripheral neuropathy, the most common neurologic complication of diabetes, is associated with injury and infection.19
Autonomic neuropathy is associated with decreased cardiac responsiveness and orthostatic hypotension.19 Diabetic gastroparesis alone can precipitate serious complications during pregnancy, including extreme hypoglycemia and hyperglycemia, increased risk of diabetic ketoacidosis, weight loss, malnutrition, frequent hospitalizations, and increased requirement for parenteral nutrition.20
Although diabetic neuropathy does not significantly worsen during pregnancy, women with preexisting gastroparesis should be counseled on the substantial risks associated with pregnancy. Screening for neuropathy should be part of all diabetic preconception examinations.
Renal complications
Pregnancy in women with diabetes and preexisting renal dysfunction increases their risk of accelerated progression of diabetic kidney disease.21 Preexisting renal dysfunction also increases the risk of pregnancy-related complications, such as stillbirth, intrauterine growth restriction, gestational hypertension, preeclampsia, and preterm delivery.19,21,22 Further, the risk of pregnancy complications correlates directly with the severity of renal dysfunction.22
Psychiatric disorders
Emotional wellness is essential for optimal diabetes management. It is important to recognize the emotional impact of diabetes in pregnant women and to conduct routine screening for depression, anxiety, stress, and eating disorders.16
LABORATORY TESTS TO CONSIDER
Hemoglobin A1c. The general consensus is to achieve the lowest hemoglobin A1c level possible that does not increase the risk of hypoglycemia. The American Diabetes Association (ADA) recommends that, before attempting to conceive, women should lower their hemoglobin A1c to below 6.5%.1
Thyroid measures. Autoimmune thyroid disease is the most common autoimmune disorder associated with diabetes and has been reported in 35% to 40% of women with type 1 diabetes.23 Recommendations are to check thyroid-stimulating hormone and thyroid peroxidase antibody levels before conception or early in pregnancy in all women with diabetes.1,24 Overt hypothyroidism should be treated before conception, given that early fetal brain development depends on maternal thyroxine.
Renal function testing. Preconception assessment of renal function is important for counseling and risk stratification. This assessment should include serum creatinine level, estimated glomerular filtration rate, and urinary albumin excretion.21
Celiac screening. Because women with type 1 diabetes are more susceptible to autoimmune diseases, they should be screened for celiac disease before conception, with testing for immunoglobulin A (IgA) and tissue transglutaminase antibodies, with or without IgA endomysial antibodies.16,25,26 An estimated 6% of patients with type 1 diabetes have celiac disease vs 1% of the general population.25 Celiac disease is 2 to 3 times more common in women, and asymptomatic people with type 1 diabetes are considered at increased risk for celiac disease.26
The association between type 1 diabetes and celiac disease most likely relates to the overlap in human leukocyte antigens of the diseases. There is no established link between type 2 diabetes and celiac disease.25
Undiagnosed celiac disease increases a woman’s risk of obstetric complications such as preterm birth, low birth weight, and stillbirth.26 The most likely explanation for these adverse effects is nutrient malabsorption, which is characteristic of celiac disease. Adherence to a gluten-free diet before and during gestation may reduce the risk of preterm delivery by as much as 20%.26
Vitamin B12 level. Celiac disease interferes with the absorption of vitamin B12-instrinsic factor in the ileum, which can lead to vitamin B12 deficiency. Therefore, baseline vitamin B12 levels should be checked before conception in women with celiac disease. Levels should also be checked in women taking metformin, which also decreases vitamin B12 absorption. Of note, increased folate levels due to taking supplements can potentially mask vitamin B12 deficiency.
MEDICATIONS TO REVIEW FOR PREGNANCY INTERACTIONS
Diabetic medications
Insulin is the first-line pharmacotherapy for pregnant patients with type 1, type 2, or gestational diabetes. Insulin does not cross the placenta to a measurable extent, and most insulin preparations have been classified as category B,1 meaning no risks to the fetus have been found in humans.
Insulin dosing during pregnancy is not static. Beginning around mid-gestation, insulin requirements increase,28,29 but after 32 weeks the need may decrease. These changes require practitioners to closely monitor blood glucose throughout pregnancy.
Both basal-bolus injections and continuous subcutaneous infusion are reasonable options during pregnancy.30 However, the need for multiple and potentially painful insulin injections daily can lead to poor compliance. This inconvenience has led to studies using oral hypoglycemic medications instead of insulin for patients with gestational and type 2 diabetes.
Metformin is an oral biguanide that decreases hepatic gluconeogenesis and intestinal glucose absorption while peripherally increasing glucose utilization and uptake. Metformin does not pose a risk of hypoglycemia because its mechanism of action does not involve increased insulin production.7
Metformin does cross the placenta, resulting in umbilical cord blood levels higher than maternal levels. Nevertheless, studies support the efficacy and short-term safety of metformin use during a pregnancy complicated by gestational or type 2 diabetes.7,31 Moreover, metformin has been associated with a lower risk of neonatal hypoglycemia and maternal weight gain than insulin.32 However, this agent should be used with caution, as long-term data are not yet available, and it may slightly increase the risk of premature delivery.
Glyburide is another oral hypoglycemic medication that has been used during pregnancy. This second-generation sulfonylurea enhances the release of insulin from the pancreas by binding beta islet cell ATP-calcium channel receptors. Compared with other sulfonylureas, glyburide has the lowest rate of maternal-to-fetal transfer, with umbilical cord plasma concentrations 70% of maternal levels.33 Although some trials support the efficacy and short-term safety of glyburide treatment for gestational diabetes,34 recent studies have associated glyburide use during pregnancy with a higher rate of neonatal hypoglycemia, neonatal respiratory distress, macrosomia, and neonatal intensive care unit admissions than insulin and metformin.1,35
Patients treated with oral agents should be informed that these drugs cross the placenta, and that although no adverse effects on the fetus have been demonstrated, long-term safety data are lacking. In addition, oral agents are ineffective in type 1 diabetes and may be insufficient to overcome the insulin resistance in type 2 diabetes.
Antihypertensive drugs
All antihypertensive drugs cross the placenta, but several have an acceptable safety profile in pregnancy, including methyldopa, labetalol, clonidine, prazosin, and nifedipine. Hydralazine and labetalol are short-acting, come in intravenous formulations, and can be used for urgent blood pressure control during pregnancy. Diltiazem may be used for heart rate control during pregnancy, and it has been shown to lower blood pressure and proteinuria in pregnant patients with underlying renal disease.36,37 The ADA recommends against chronic use of diuretics during pregnancy because of potential reductions in maternal plasma volume and uteroplacental perfusion.1
Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and direct renin inhibitors are contraindicated during pregnancy because of the risk of fetal defects, particularly in the renal system.21,38 Although there is evidence to question the association between first semester exposure and fetotoxicity,39 we avoid these drugs during pregnancy and switch to a different agent in women planning pregnancy.
Other drugs
Statins are contraindicated in pregnancy because they interfere with the development of the fetal nervous system.21 Although preliminary data from a small study did not identify safety risks associated with pravastatin use after 12 weeks of gestation,40 we recommend discontinuing statins in women attempting pregnancy.
Aspirin. The US Preventive Services Task Force41 recommends low-dose aspirin (81 mg/day) after 12 weeks of gestation for women with type 1 or type 2 diabetes, as well as those with renal disease or chronic hypertension, to prevent preeclampsia. Of note, higher doses need to be used with caution during pregnancy because fetal abnormalities have been reported, such as disruption of fetal vasculature (mesenteric vessels), gastroschisis, and small intestinal atresia.16
Folate supplementation (0.6–4 mg/day) is recommended in women with celiac disease to prevent neural tube defects in the offspring, and the US Preventive Services Task Force recommends 0.4 mg daily of folic acid supplementation for all women planning or capable of pregnancy.42–44 Higher doses, ranging from 0.6 to 5 mg/day, have been proposed for patients with diabetes,13 and we recommend at least 1 mg for this group, based on data suggesting that higher doses further reduce the risk of neural tube defects.43
IS BREASTFEEDING AFFECTED?
Maternal diabetes, insulin therapy, and oral hypoglycemic agents are not contraindications to breastfeeding. The US Preventive Services Task Force recommends interventions by primary care physicians to promote and support breastfeeding.45 Breastfeeding is encouraged based on various short- and long-term health benefits for both breastfed infants and breastfeeding mothers. Breastfeeding decreases a woman’s insulin requirements and increases the risk for hypoglycemia, especially in patients with insulin-dependent type 1 diabetes.1
Additionally, insulin sensitivity increases immediately following delivery of the placenta.1 Therefore, it is prudent to adjust insulin doses postpartum, especially while a patient is breastfeeding, or to suggest high-carbohydrate snacks before feeds.9,29
Antihypertensive drugs considered safe to use during lactation include captopril, enalapril, quinapril, labetalol, propranolol, nifedipine, and hydralazine.21,46 Methyldopa is not contraindicated, but it causes fatigue and worsened postpartum depression and should not be used as first-line therapy. Diuretics and ARBs are not recommended during lactation.21 Both metformin and glyburide enter breast milk in small enough amounts that they are not contraindicated during breastfeeding.16 The Lactmed database (www.toxnet.nlm.nih.gov) provides information about drugs and breastfeeding.
WHAT ABOUT CONTRACEPTIVES?
The ADA recommends contraception for women with diabetes because, just as in women without diabetes, the risks of unplanned pregnancy outweigh those of contraceptives.1
We recommend low-dose combination estrogen-progestin oral contraceptives to normotensive women under age 35 with diabetes but without underlying microvascular disease. For women over age 35 or for those with microvascular disease, additional options include intrauterine devices or progestin implants. We prefer not to use injectable depot medroxyprogesterone acetate because of its side effects of insulin resistance and weight gain.47
CASE DISCUSSION: NEXT STEPS
Our patient’s interest in family planning presents an opportunity for preconception counseling. We recommend a prenatal folic acid supplement, diet and regular exercise for weight loss, and screening tests including a comprehensive metabolic panel, hemoglobin A1c, thyroid-stimulating hormone, and dilated eye examination. We make sure she is up to date on her indicated health maintenance (eg, immunizations, disease screening), and we review her medications for potential teratogens. She denies any recreational drug use. Also, she has no plans for long-distance travel.
Our counseling includes discussions of pregnancy risks associated with pregestational diabetes and suboptimal glycemic control. We encourage her to use effective contraception until she is “medically optimized” for pregnancy—ie, until her hemoglobin A1c is lower than 6.5% and she has achieved a medically optimal weight. If feasible, a reduction of weight (7% or so) through lifestyle modification should be attempted, and if her hemoglobin A1c remains elevated, adding insulin would be recommended.
Pregnant patients or patients contemplating pregnancy are usually motivated to modify their behavior, making this a good time to reinforce lifestyle modifications. Many patients benefit from individualized counseling by a registered dietitian to help achieve the recommended weight and glycemic control.
Our physical examination in this patient includes screening for micro- and macrovascular complications of diabetes, and the test results are negative. Patients with active proliferative retinopathy should be referred to an ophthalmologist for assessment and treatment.
We review her medications for potential teratogenic effects and stop her ACE inhibitor (lisinopril) and statin (simvastatin). We switch her from a first-generation sulfonylurea (chlorpropamide) to glyburide, a second-generation sulfonylurea. Second-generation sulfonylureas are considered more “fetus-friendly” because first-generation sulfonylureas cross the placenta more easily and can cause fetal hyperinsulinemia, leading to macrosomia and neonatal hypoglycemia.7
The management of diabetes during pregnancy leans toward insulin use, given the lack of information regarding long-term outcomes with oral agents. If insulin is needed, it is best to initiate it before the patient conceives, and then to stop other diabetes medications. We would not make any changes to her aspirin or metformin use.
Educating the patient and her family about prevention, recognition, and treatment of hypoglycemia is important to prevent and manage the increased risk of hypoglycemia with insulin therapy and in early pregnancy.1 Consideration should be given to providing ketone strips as well as education on diabetic ketoacidosis prevention and detection.1 If the patient conceives, begin prenatal care early to allow adequate planning for care of her disease and evaluation of the fetus. Because of the complexity of insulin management in pregnancy, the ADA recommends referral, if possible, to a center offering team-based care, including an obstetrician specialized in high-risk pregnancies, an endocrinologist, and a dietitian.1
A 29-year-old nulliparous woman presents for a routine checkup. She has hypertension and type 2 diabetes mellitus. Her current medications are chlorpropamide 500 mg daily, metformin 500 mg twice a day, lisinopril 40 mg daily, simvastatin 40 mg daily, and aspirin 81 mg daily. Her body mass index is 37 kg/m2 and her blood pressure is 130/80 mm Hg. Her hemoglobin A1c level is 7.8% and her low-density lipoprotein cholesterol 90 mg/dL.
She is considering pregnancy. How would you counsel her?
DEFINING DIABETES IN PREGNANCY
Diabetes in pregnant women, both gestational and pregestational, is the most common medical complication associated with pregnancy.1
- Gestational diabetes is defined as diabetes that is diagnosed during the second or third trimester of pregnancy and that is not clearly pregestational.2
- Pregestational diabetes exists before pregnancy and can be either type 1 or type 2.
Most cases of diabetes diagnosed during the first trimester reflect pregestational diabetes, as gestational diabetes occurs when insulin resistance increases in the later trimesters.
Type 1 diabetes involves autoimmune destruction of pancreatic islet cells, leading to insulin deficiency and the need for insulin therapy. Type 2 diabetes is characterized by insulin resistance rather than overall insulin deficiency. Type 2 diabetes tends to be associated with comorbidities such as obesity and hypertension, which are independent risk factors for adverse perinatal outcomes.3,4
Gestational diabetes accounts for most cases of diabetes during pregnancy. Although both pregestational and gestational diabetes increase the risk of maternal and fetal complications, pregestational diabetes is associated with significantly greater risks.1
IMPACT OF DIABETES ON THE MOTHER
Pregnancy increases the risk of maternal hypoglycemia, especially during the first trimester in patients with type 1 diabetes, as insulin sensitivity increases in early pregnancy.1 Pregnant women with diabetes may also have an altered counterregulatory response and less hypoglycemic awareness.1 Insulin resistance rises during the second and early third trimesters, increasing the risk of hyperglycemia in women with diabetes.1
Glycemic control during pregnancy is usually easier to achieve in patients with type 2 diabetes than with type 1, but it may require much higher insulin doses.
Because pregnancy is inherently a ketogenic state, women with type 1 diabetes are at higher risk of diabetic ketoacidosis, particularly during the second and third trimesters.1 There are reports of euglycemic diabetic ketoacidosis in pregnant women with either gestational or pregestational diabetes.5
Diabetes is associated with a risk of preeclampsia 4 times higher than in nondiabetic women.6 Other potential pregnancy-related complications include infections, polyhydramnios, spontaneous abortion, and cesarean delivery.1,7 The risk of pregnancy loss is similar in women with either type 1 or type 2 diabetes (2.6% and 3.7%, respectively), but the causes are different.8 Although preexisting diabetic complications such as retinopathy, nephropathy, and gastroparesis can be exacerbated during pregnancy,1 only severe gastroparesis and advanced renal disease are considered relative contraindications to pregnancy.
IMPACT OF DIABETES ON THE FETUS
Fetal complications of maternal diabetes include embryopathy (fetal malformations) and fetopathy (overgrowth, ie, fetus large for gestational age, and increased risk of fetal death or distress). Maternal hyperglycemia is associated with diabetic embryopathy, resulting in major birth defects in 5% to 25% of pregnancies and spontaneous abortions in 15% to 20%.9,10 There is a 2- to 6-fold increase in risk of congenital malformations.6
The most common diabetes-associated congenital malformations affect the cardiovascular system. Congenital heart disease includes tetralogy of Fallot, transposition of the great vessels, septal defects, and anomalous pulmonary venous return. Other relatively common defects involve the fetal central nervous system, spine, orofacial system, kidneys, urogenital system, gastrointestinal tract, and skeleton.11
The risk of fetopathy is proportional to the degree of maternal hyperglycemia. Excess maternal glucose and fatty acid levels can lead to fetal hyperglycemia and overgrowth, which increases fetal oxygen requirements. Erythropoietin levels rise, causing an increase in red cell mass, with subsequent hyperviscosity within the placenta and higher risk of fetal death.
Other complications include intrauterine growth restriction, prematurity, and preterm delivery. Fetal macrosomia (birth weight > 90th percentile or 4 kg, approximately 8 lb, 13 oz) occurs in 27% to 62% of children born to mothers with diabetes, a rate 10 times higher than in patients without diabetes. It contributes to shoulder dystocia (risk 2 to 4 times higher in diabetic pregnancies) and cesarean delivery.6 Infants born to mothers with diabetes also have higher risks of neonatal hypoglycemia, erythrocytosis, hyperbilirubinemia, hypocalcemia, respiratory distress, cardiomyopathy, and death, as well as for developing diabetes, obesity, and other adverse cardiometabolic outcomes later in life.11
GET GLUCOSE UNDER CONTROL BEFORE PREGNANCY
Nearly half of pregnancies in the general population are unplanned,15 so preconception diabetes assessment needs to be part of routine medical care for all reproductive-age women. Because most organogenesis occurs during the first 5 to 8 weeks after fertilization—potentially before a woman realizes she is pregnant—achieving optimal glycemic control before conception is necessary to improve pregnancy outcomes.1
EVERY VISIT IS AN OPPORTUNITY
Every medical visit with a reproductive-age woman with diabetes is an opportunity for counseling about pregnancy. Topics that need to be discussed include the risks of unplanned pregnancy and of poor metabolic control, and the benefits of improved maternal and fetal outcomes with appropriate pregnancy planning and diabetes management.
Referral to a registered dietitian for individualized counseling about proper nutrition, particularly during pregnancy, has been associated with positive outcomes.16 Patients with diabetes and at high risk of pregnancy complications should be referred to a clinic that specializes in high-risk pregnancies.
Practitioners also should emphasize the importance of regular exercise and encourage patients to maintain or achieve a medically optimal weight before conception. Ideally, this would be a normal body mass index; however, this is not always possible.
In women who are planning pregnancy or are not on effective contraception, medications should be reviewed for potential teratogenicity. If needed, discuss alternative medications or switch to safer ones. However, these changes should not interrupt diabetes treatment.
In addition, ensure that the patient is up to date on age- and disease-appropriate preventive care (eg, immunizations, screening for sexually transmitted disease and malignancy). Counseling and intervention for use of tobacco, alcohol, and recreational drugs are also important. As with any preconception counseling, the patient (and her partner, if possible) should be advised to avoid travel to areas where Zika virus is endemic, and informed about the availability of expanded carrier genetic screening through her obstetric provider.
Finally, pregnant women with diabetes benefit from screening for diabetic complications including hypertension, retinopathy, cardiovascular disease, neuropathy, and nephropathy.
ASSESSING RISKS
Blood pressure
Chronic (preexisting) hypertension is defined as a systolic pressure 140 mm Hg or higher or a diastolic pressure 90 mm Hg or higher, or both, that antedates pregnancy or is present before the 20th week of pregnancy.3 Chronic hypertension has been reported in up to 5% of pregnant women and is associated with increased risk of preterm delivery, superimposed preeclampsia, low birth weight, and perinatal death.3
Reproductive-age women with diabetes and high blood pressure benefit from lifestyle and behavioral modifications.17 If drug therapy is needed, antihypertensive drugs that are safe for the fetus should be used. Treatment of mild or moderate hypertension during pregnancy reduces the risk of progression to severe hypertension but may not improve obstetric outcomes.
Diabetic retinopathy
Diabetic retinopathy can significantly worsen during pregnancy: the risk of progression is double that in the nonpregnant state.18 Women with diabetes who are contemplating pregnancy should have a comprehensive eye examination before conception, and any active proliferative retinopathy needs to be treated. These patients may require ophthalmologic monitoring and treatment during pregnancy. (Note: laser photocoagulation is not contraindicated during pregnancy.)
Cardiovascular disease
Cardiovascular physiology changes dramatically during pregnancy. Cardiovascular disease, especially when superimposed on diabetes, can increase the risk of maternal death. Thus, evaluation for cardiovascular risk factors as well as cardiovascular system integrity before conception is important. Listen for arterial bruits and murmurs, and assess peripheral pulses. Consideration should be given to obtaining a preconception resting electrocardiogram in women with diabetes who are over age 35 or who are suspected of having cardiovascular disease.16
Neurologic disorders
Peripheral neuropathy, the most common neurologic complication of diabetes, is associated with injury and infection.19
Autonomic neuropathy is associated with decreased cardiac responsiveness and orthostatic hypotension.19 Diabetic gastroparesis alone can precipitate serious complications during pregnancy, including extreme hypoglycemia and hyperglycemia, increased risk of diabetic ketoacidosis, weight loss, malnutrition, frequent hospitalizations, and increased requirement for parenteral nutrition.20
Although diabetic neuropathy does not significantly worsen during pregnancy, women with preexisting gastroparesis should be counseled on the substantial risks associated with pregnancy. Screening for neuropathy should be part of all diabetic preconception examinations.
Renal complications
Pregnancy in women with diabetes and preexisting renal dysfunction increases their risk of accelerated progression of diabetic kidney disease.21 Preexisting renal dysfunction also increases the risk of pregnancy-related complications, such as stillbirth, intrauterine growth restriction, gestational hypertension, preeclampsia, and preterm delivery.19,21,22 Further, the risk of pregnancy complications correlates directly with the severity of renal dysfunction.22
Psychiatric disorders
Emotional wellness is essential for optimal diabetes management. It is important to recognize the emotional impact of diabetes in pregnant women and to conduct routine screening for depression, anxiety, stress, and eating disorders.16
LABORATORY TESTS TO CONSIDER
Hemoglobin A1c. The general consensus is to achieve the lowest hemoglobin A1c level possible that does not increase the risk of hypoglycemia. The American Diabetes Association (ADA) recommends that, before attempting to conceive, women should lower their hemoglobin A1c to below 6.5%.1
Thyroid measures. Autoimmune thyroid disease is the most common autoimmune disorder associated with diabetes and has been reported in 35% to 40% of women with type 1 diabetes.23 Recommendations are to check thyroid-stimulating hormone and thyroid peroxidase antibody levels before conception or early in pregnancy in all women with diabetes.1,24 Overt hypothyroidism should be treated before conception, given that early fetal brain development depends on maternal thyroxine.
Renal function testing. Preconception assessment of renal function is important for counseling and risk stratification. This assessment should include serum creatinine level, estimated glomerular filtration rate, and urinary albumin excretion.21
Celiac screening. Because women with type 1 diabetes are more susceptible to autoimmune diseases, they should be screened for celiac disease before conception, with testing for immunoglobulin A (IgA) and tissue transglutaminase antibodies, with or without IgA endomysial antibodies.16,25,26 An estimated 6% of patients with type 1 diabetes have celiac disease vs 1% of the general population.25 Celiac disease is 2 to 3 times more common in women, and asymptomatic people with type 1 diabetes are considered at increased risk for celiac disease.26
The association between type 1 diabetes and celiac disease most likely relates to the overlap in human leukocyte antigens of the diseases. There is no established link between type 2 diabetes and celiac disease.25
Undiagnosed celiac disease increases a woman’s risk of obstetric complications such as preterm birth, low birth weight, and stillbirth.26 The most likely explanation for these adverse effects is nutrient malabsorption, which is characteristic of celiac disease. Adherence to a gluten-free diet before and during gestation may reduce the risk of preterm delivery by as much as 20%.26
Vitamin B12 level. Celiac disease interferes with the absorption of vitamin B12-instrinsic factor in the ileum, which can lead to vitamin B12 deficiency. Therefore, baseline vitamin B12 levels should be checked before conception in women with celiac disease. Levels should also be checked in women taking metformin, which also decreases vitamin B12 absorption. Of note, increased folate levels due to taking supplements can potentially mask vitamin B12 deficiency.
MEDICATIONS TO REVIEW FOR PREGNANCY INTERACTIONS
Diabetic medications
Insulin is the first-line pharmacotherapy for pregnant patients with type 1, type 2, or gestational diabetes. Insulin does not cross the placenta to a measurable extent, and most insulin preparations have been classified as category B,1 meaning no risks to the fetus have been found in humans.
Insulin dosing during pregnancy is not static. Beginning around mid-gestation, insulin requirements increase,28,29 but after 32 weeks the need may decrease. These changes require practitioners to closely monitor blood glucose throughout pregnancy.
Both basal-bolus injections and continuous subcutaneous infusion are reasonable options during pregnancy.30 However, the need for multiple and potentially painful insulin injections daily can lead to poor compliance. This inconvenience has led to studies using oral hypoglycemic medications instead of insulin for patients with gestational and type 2 diabetes.
Metformin is an oral biguanide that decreases hepatic gluconeogenesis and intestinal glucose absorption while peripherally increasing glucose utilization and uptake. Metformin does not pose a risk of hypoglycemia because its mechanism of action does not involve increased insulin production.7
Metformin does cross the placenta, resulting in umbilical cord blood levels higher than maternal levels. Nevertheless, studies support the efficacy and short-term safety of metformin use during a pregnancy complicated by gestational or type 2 diabetes.7,31 Moreover, metformin has been associated with a lower risk of neonatal hypoglycemia and maternal weight gain than insulin.32 However, this agent should be used with caution, as long-term data are not yet available, and it may slightly increase the risk of premature delivery.
Glyburide is another oral hypoglycemic medication that has been used during pregnancy. This second-generation sulfonylurea enhances the release of insulin from the pancreas by binding beta islet cell ATP-calcium channel receptors. Compared with other sulfonylureas, glyburide has the lowest rate of maternal-to-fetal transfer, with umbilical cord plasma concentrations 70% of maternal levels.33 Although some trials support the efficacy and short-term safety of glyburide treatment for gestational diabetes,34 recent studies have associated glyburide use during pregnancy with a higher rate of neonatal hypoglycemia, neonatal respiratory distress, macrosomia, and neonatal intensive care unit admissions than insulin and metformin.1,35
Patients treated with oral agents should be informed that these drugs cross the placenta, and that although no adverse effects on the fetus have been demonstrated, long-term safety data are lacking. In addition, oral agents are ineffective in type 1 diabetes and may be insufficient to overcome the insulin resistance in type 2 diabetes.
Antihypertensive drugs
All antihypertensive drugs cross the placenta, but several have an acceptable safety profile in pregnancy, including methyldopa, labetalol, clonidine, prazosin, and nifedipine. Hydralazine and labetalol are short-acting, come in intravenous formulations, and can be used for urgent blood pressure control during pregnancy. Diltiazem may be used for heart rate control during pregnancy, and it has been shown to lower blood pressure and proteinuria in pregnant patients with underlying renal disease.36,37 The ADA recommends against chronic use of diuretics during pregnancy because of potential reductions in maternal plasma volume and uteroplacental perfusion.1
Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and direct renin inhibitors are contraindicated during pregnancy because of the risk of fetal defects, particularly in the renal system.21,38 Although there is evidence to question the association between first semester exposure and fetotoxicity,39 we avoid these drugs during pregnancy and switch to a different agent in women planning pregnancy.
Other drugs
Statins are contraindicated in pregnancy because they interfere with the development of the fetal nervous system.21 Although preliminary data from a small study did not identify safety risks associated with pravastatin use after 12 weeks of gestation,40 we recommend discontinuing statins in women attempting pregnancy.
Aspirin. The US Preventive Services Task Force41 recommends low-dose aspirin (81 mg/day) after 12 weeks of gestation for women with type 1 or type 2 diabetes, as well as those with renal disease or chronic hypertension, to prevent preeclampsia. Of note, higher doses need to be used with caution during pregnancy because fetal abnormalities have been reported, such as disruption of fetal vasculature (mesenteric vessels), gastroschisis, and small intestinal atresia.16
Folate supplementation (0.6–4 mg/day) is recommended in women with celiac disease to prevent neural tube defects in the offspring, and the US Preventive Services Task Force recommends 0.4 mg daily of folic acid supplementation for all women planning or capable of pregnancy.42–44 Higher doses, ranging from 0.6 to 5 mg/day, have been proposed for patients with diabetes,13 and we recommend at least 1 mg for this group, based on data suggesting that higher doses further reduce the risk of neural tube defects.43
IS BREASTFEEDING AFFECTED?
Maternal diabetes, insulin therapy, and oral hypoglycemic agents are not contraindications to breastfeeding. The US Preventive Services Task Force recommends interventions by primary care physicians to promote and support breastfeeding.45 Breastfeeding is encouraged based on various short- and long-term health benefits for both breastfed infants and breastfeeding mothers. Breastfeeding decreases a woman’s insulin requirements and increases the risk for hypoglycemia, especially in patients with insulin-dependent type 1 diabetes.1
Additionally, insulin sensitivity increases immediately following delivery of the placenta.1 Therefore, it is prudent to adjust insulin doses postpartum, especially while a patient is breastfeeding, or to suggest high-carbohydrate snacks before feeds.9,29
Antihypertensive drugs considered safe to use during lactation include captopril, enalapril, quinapril, labetalol, propranolol, nifedipine, and hydralazine.21,46 Methyldopa is not contraindicated, but it causes fatigue and worsened postpartum depression and should not be used as first-line therapy. Diuretics and ARBs are not recommended during lactation.21 Both metformin and glyburide enter breast milk in small enough amounts that they are not contraindicated during breastfeeding.16 The Lactmed database (www.toxnet.nlm.nih.gov) provides information about drugs and breastfeeding.
WHAT ABOUT CONTRACEPTIVES?
The ADA recommends contraception for women with diabetes because, just as in women without diabetes, the risks of unplanned pregnancy outweigh those of contraceptives.1
We recommend low-dose combination estrogen-progestin oral contraceptives to normotensive women under age 35 with diabetes but without underlying microvascular disease. For women over age 35 or for those with microvascular disease, additional options include intrauterine devices or progestin implants. We prefer not to use injectable depot medroxyprogesterone acetate because of its side effects of insulin resistance and weight gain.47
CASE DISCUSSION: NEXT STEPS
Our patient’s interest in family planning presents an opportunity for preconception counseling. We recommend a prenatal folic acid supplement, diet and regular exercise for weight loss, and screening tests including a comprehensive metabolic panel, hemoglobin A1c, thyroid-stimulating hormone, and dilated eye examination. We make sure she is up to date on her indicated health maintenance (eg, immunizations, disease screening), and we review her medications for potential teratogens. She denies any recreational drug use. Also, she has no plans for long-distance travel.
Our counseling includes discussions of pregnancy risks associated with pregestational diabetes and suboptimal glycemic control. We encourage her to use effective contraception until she is “medically optimized” for pregnancy—ie, until her hemoglobin A1c is lower than 6.5% and she has achieved a medically optimal weight. If feasible, a reduction of weight (7% or so) through lifestyle modification should be attempted, and if her hemoglobin A1c remains elevated, adding insulin would be recommended.
Pregnant patients or patients contemplating pregnancy are usually motivated to modify their behavior, making this a good time to reinforce lifestyle modifications. Many patients benefit from individualized counseling by a registered dietitian to help achieve the recommended weight and glycemic control.
Our physical examination in this patient includes screening for micro- and macrovascular complications of diabetes, and the test results are negative. Patients with active proliferative retinopathy should be referred to an ophthalmologist for assessment and treatment.
We review her medications for potential teratogenic effects and stop her ACE inhibitor (lisinopril) and statin (simvastatin). We switch her from a first-generation sulfonylurea (chlorpropamide) to glyburide, a second-generation sulfonylurea. Second-generation sulfonylureas are considered more “fetus-friendly” because first-generation sulfonylureas cross the placenta more easily and can cause fetal hyperinsulinemia, leading to macrosomia and neonatal hypoglycemia.7
The management of diabetes during pregnancy leans toward insulin use, given the lack of information regarding long-term outcomes with oral agents. If insulin is needed, it is best to initiate it before the patient conceives, and then to stop other diabetes medications. We would not make any changes to her aspirin or metformin use.
Educating the patient and her family about prevention, recognition, and treatment of hypoglycemia is important to prevent and manage the increased risk of hypoglycemia with insulin therapy and in early pregnancy.1 Consideration should be given to providing ketone strips as well as education on diabetic ketoacidosis prevention and detection.1 If the patient conceives, begin prenatal care early to allow adequate planning for care of her disease and evaluation of the fetus. Because of the complexity of insulin management in pregnancy, the ADA recommends referral, if possible, to a center offering team-based care, including an obstetrician specialized in high-risk pregnancies, an endocrinologist, and a dietitian.1
- American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S137–S143. doi:10.2337/dc18-S013
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S13–S27. doi:10.2337/dc18-S002
- Lawler J, Osman M, Shelton JA, Yeh J. Population-based analysis of hypertensive disorders in pregnancy. Hypertens Pregnancy 2007; 26(1):67–76. doi:10.1080/10641950601147945
- Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015; 16(8):621–638. doi:10.1111/obr.12288
- Garrison EA, Jagasia S. Inpatient management of women with gestational and pregestational diabetes in pregnancy. Curr Diab Rep 2014; 14(2):457. doi:10.1007/s11892-013-0457-x
- Ballas J, Moore TR, Ramos GA. Management of diabetes in pregnancy. Curr Diab Rep 2012; 12(1):33–42. doi:10.1007/s11892-011-0249-0
- Ryu RJ, Hays KE, Hebert MF. Gestational diabetes mellitus management with oral hypoglycemic agents. Semin Perinatol 2014; 38(8):508–515. doi:10.1053/j.semperi.2014.08.012
- Cundy T, Gamble G, Neale L, et al. Differing causes of pregnancy loss in type 1 and type 2 diabetes. Diabetes Care 2007; 30(10):2603–2607. doi:10.2337/dc07-0555
- Castorino K, Jovanovic L. Pregnancy and diabetes management: advances and controversies. Clin Chem 2011; 57(2):221–230. doi:10.1373/clinchem.2010.155382
- Hammouda SA, Hakeem R. Role of HbA1c in predicting risk for congenital malformations. Prim Care Diabetes 2015; 9(6):458–464. doi:10.1016/j.pcd.2015.01.004
- Chen CP. Congenital malformations associated with maternal diabetes. Taiwanese J Obstet Gynecol 2005; 44(1):1–7. doi:10.1016/S1028-4559(09)60099-1
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33(3):676–682. doi:10.2337/dc09-1848
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36(5):1384–1395. doi:10.2337/dc12-2480
- HAPO Study Cooperative Research Group; Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358(19):1991–2002. doi:10.1056/NEJMoa0707943
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health 2014; 104(suppl 1):S43–S48. doi:10.2105/AJPH.2013.301416
- Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care 2008; 31(5):1060–1079. doi:10.2337/dc08-9020
- Webster LM, Conti-Ramsden F, Seed PT, Webb AJ, Nelson-Piercy C, Chappell LC. Impact of antihypertensive treatment on maternal and perinatal outcomes in pregnancy complicated by chronic hypertension: a systematic review and meta-analysis. J Am Heart Assoc 2017; 6(5).pii:e005526. doi:10.1161/JAHA.117.005526
- Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy: the Diabetes in Early Pregnancy Study. Diabetes Care 1995; 18(5):631–637. pmid:8586000
- American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care 2016; 39 (suppl 1):S1–S109.
- Hawthorne, G. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):77–90. doi:10.1016/j.bpobgyn.2010.10.015
- Ringholm L, Damm JA, Vestgaard M, Damm P, Mathiesen ER. Diabetic nephropathy in women with preexisting diabetes: from pregnancy planning to breastfeeding. Curr Diab Rep 2016; 16(2):12. doi:10.1007/s11892-015-0705-3
- Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 2015; 10(11):1964–1978. doi:10.2215/CJN.09250914
- Umpierrez GE, Latif KA, Murphy MB, et al. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care 2003; 26(4):1181–1185. pmid:12663594
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Akirov A, Pinhas-Hamiel O. Co-occurrence of type 1 diabetes mellitus and celiac disease. World J Diabetes 2015; 6(5):707–714. doi:10.4239/wjd.v6.i5.707
- Saccone G, Berghella V, Sarno L, et al. Celiac disease and obstetric complications: a systematic review and metaanalysis. Am J Obstet Gynecol 2016; 214(2):225–234. doi:10.1016/j.ajog.2015.09.080
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
- de Valk HW, Visser GH. Insulin during pregnancy, labour and delivery. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):65–76. doi:10.1016/j.bpobgyn.2010.10.002
- Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int J Gen Med 2011; 4:827–835. doi:10.2147/IJGM.S26889
- Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2016; (6):CD005542. doi:10.1002/14651858.CD005542.pub2
- Charles B, Norris R, Xiao X, Hague W. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit 2006; 28(1):67–72. pmid:16418696
- Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 2015; 350:h102. doi:10.1136/bmj.h102
- Hebert MF, Ma X, Naraharisetti SB, et al; Obstetric-Fetal Pharmacology Research Unit Network. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009; 85(6):607–614. doi:10.1038/clpt.2009.5
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000; 343(16):1134–1138. doi:10.1056/NEJM200010193431601
- Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr, Jonsson Funk M. Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr 2015; 169:452–458. doi:10.1001/jamapediatrics.2015.74
- Gowda RM, Khan IA, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac arrhythmias in pregnancy: clinical and therapeutic considerations. Int J Cardiol 2003; 88(2):129–133. pmid:12714190
- Khandelwal M, Kumanova M, Gaughan JP, Reece EA. Role of diltiazem in pregnant women with chronic renal disease. J Matern Fetal Neonatal Med 2002; 12(6):408–412. doi:10.1080/jmf.12.6.408.412
- Magee LA, Abalos E, von Dadelszen P, Sibai B, Easterling T, Walkinshaw S; CHIPS Study Group. How to manage hypertension in pregnancy effectively. Br J Clin Pharmacol 2011; 72(3):394–401. doi:10.1111/j.1365-2125.2011.04002.x
- Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 2006; 354(23):2443–2451. doi:10.1056/NEJMoa055202
- Costantine MM, Cleary K, Hebert MF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 2016; 214(6):720.e1–720.e17. doi:10.1016/j.ajog.2015.12.038
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161(11):819–826. doi:10.7326/M14-1884
- Curry SJ, Grossman DC, Whitlock EP, Cantu A. Behavioral counseling research and evidence-based practice recommendations: US Preventive Services Task Force perspectives. Ann Intern Med 2014; 160(6):407–413. doi:10.7326/M13-2128
- Wald N, Law M, Morris J, Wald D. Quantifying the effect of folic acid. Lancet 2001; 358(9298):2069–2073. pmid:11755633
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. JAMA 2017; 317(2):183–189. doi:10.1001/jama.2016.19438
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Primary care interventions to support breastfeeding: US Preventive Services Task Force recommendation statement. JAMA 2016; 316(16):1688–1693. doi:10.1001/jama.2016.14697
- Newton ER, Hale TW. Drugs in breast milk. Clin Obstet Gynecol 2015; 58(4):868–884. doi:10.1097/GRF.0000000000000142
- Xiang AH, Kawakubo M, Kjos SL, Buchanan TA. Long-acting injectable progestin contraception and risk of type 2 diabetes in Latino women with prior gestational diabetes mellitus. Diabetes Care 2006; 29(3):613–617. pmid:16505515
- American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S137–S143. doi:10.2337/dc18-S013
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S13–S27. doi:10.2337/dc18-S002
- Lawler J, Osman M, Shelton JA, Yeh J. Population-based analysis of hypertensive disorders in pregnancy. Hypertens Pregnancy 2007; 26(1):67–76. doi:10.1080/10641950601147945
- Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015; 16(8):621–638. doi:10.1111/obr.12288
- Garrison EA, Jagasia S. Inpatient management of women with gestational and pregestational diabetes in pregnancy. Curr Diab Rep 2014; 14(2):457. doi:10.1007/s11892-013-0457-x
- Ballas J, Moore TR, Ramos GA. Management of diabetes in pregnancy. Curr Diab Rep 2012; 12(1):33–42. doi:10.1007/s11892-011-0249-0
- Ryu RJ, Hays KE, Hebert MF. Gestational diabetes mellitus management with oral hypoglycemic agents. Semin Perinatol 2014; 38(8):508–515. doi:10.1053/j.semperi.2014.08.012
- Cundy T, Gamble G, Neale L, et al. Differing causes of pregnancy loss in type 1 and type 2 diabetes. Diabetes Care 2007; 30(10):2603–2607. doi:10.2337/dc07-0555
- Castorino K, Jovanovic L. Pregnancy and diabetes management: advances and controversies. Clin Chem 2011; 57(2):221–230. doi:10.1373/clinchem.2010.155382
- Hammouda SA, Hakeem R. Role of HbA1c in predicting risk for congenital malformations. Prim Care Diabetes 2015; 9(6):458–464. doi:10.1016/j.pcd.2015.01.004
- Chen CP. Congenital malformations associated with maternal diabetes. Taiwanese J Obstet Gynecol 2005; 44(1):1–7. doi:10.1016/S1028-4559(09)60099-1
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33(3):676–682. doi:10.2337/dc09-1848
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36(5):1384–1395. doi:10.2337/dc12-2480
- HAPO Study Cooperative Research Group; Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358(19):1991–2002. doi:10.1056/NEJMoa0707943
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health 2014; 104(suppl 1):S43–S48. doi:10.2105/AJPH.2013.301416
- Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care 2008; 31(5):1060–1079. doi:10.2337/dc08-9020
- Webster LM, Conti-Ramsden F, Seed PT, Webb AJ, Nelson-Piercy C, Chappell LC. Impact of antihypertensive treatment on maternal and perinatal outcomes in pregnancy complicated by chronic hypertension: a systematic review and meta-analysis. J Am Heart Assoc 2017; 6(5).pii:e005526. doi:10.1161/JAHA.117.005526
- Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy: the Diabetes in Early Pregnancy Study. Diabetes Care 1995; 18(5):631–637. pmid:8586000
- American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care 2016; 39 (suppl 1):S1–S109.
- Hawthorne, G. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):77–90. doi:10.1016/j.bpobgyn.2010.10.015
- Ringholm L, Damm JA, Vestgaard M, Damm P, Mathiesen ER. Diabetic nephropathy in women with preexisting diabetes: from pregnancy planning to breastfeeding. Curr Diab Rep 2016; 16(2):12. doi:10.1007/s11892-015-0705-3
- Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 2015; 10(11):1964–1978. doi:10.2215/CJN.09250914
- Umpierrez GE, Latif KA, Murphy MB, et al. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care 2003; 26(4):1181–1185. pmid:12663594
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Akirov A, Pinhas-Hamiel O. Co-occurrence of type 1 diabetes mellitus and celiac disease. World J Diabetes 2015; 6(5):707–714. doi:10.4239/wjd.v6.i5.707
- Saccone G, Berghella V, Sarno L, et al. Celiac disease and obstetric complications: a systematic review and metaanalysis. Am J Obstet Gynecol 2016; 214(2):225–234. doi:10.1016/j.ajog.2015.09.080
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
- de Valk HW, Visser GH. Insulin during pregnancy, labour and delivery. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):65–76. doi:10.1016/j.bpobgyn.2010.10.002
- Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int J Gen Med 2011; 4:827–835. doi:10.2147/IJGM.S26889
- Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2016; (6):CD005542. doi:10.1002/14651858.CD005542.pub2
- Charles B, Norris R, Xiao X, Hague W. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit 2006; 28(1):67–72. pmid:16418696
- Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 2015; 350:h102. doi:10.1136/bmj.h102
- Hebert MF, Ma X, Naraharisetti SB, et al; Obstetric-Fetal Pharmacology Research Unit Network. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009; 85(6):607–614. doi:10.1038/clpt.2009.5
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000; 343(16):1134–1138. doi:10.1056/NEJM200010193431601
- Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr, Jonsson Funk M. Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr 2015; 169:452–458. doi:10.1001/jamapediatrics.2015.74
- Gowda RM, Khan IA, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac arrhythmias in pregnancy: clinical and therapeutic considerations. Int J Cardiol 2003; 88(2):129–133. pmid:12714190
- Khandelwal M, Kumanova M, Gaughan JP, Reece EA. Role of diltiazem in pregnant women with chronic renal disease. J Matern Fetal Neonatal Med 2002; 12(6):408–412. doi:10.1080/jmf.12.6.408.412
- Magee LA, Abalos E, von Dadelszen P, Sibai B, Easterling T, Walkinshaw S; CHIPS Study Group. How to manage hypertension in pregnancy effectively. Br J Clin Pharmacol 2011; 72(3):394–401. doi:10.1111/j.1365-2125.2011.04002.x
- Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 2006; 354(23):2443–2451. doi:10.1056/NEJMoa055202
- Costantine MM, Cleary K, Hebert MF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 2016; 214(6):720.e1–720.e17. doi:10.1016/j.ajog.2015.12.038
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161(11):819–826. doi:10.7326/M14-1884
- Curry SJ, Grossman DC, Whitlock EP, Cantu A. Behavioral counseling research and evidence-based practice recommendations: US Preventive Services Task Force perspectives. Ann Intern Med 2014; 160(6):407–413. doi:10.7326/M13-2128
- Wald N, Law M, Morris J, Wald D. Quantifying the effect of folic acid. Lancet 2001; 358(9298):2069–2073. pmid:11755633
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. JAMA 2017; 317(2):183–189. doi:10.1001/jama.2016.19438
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Primary care interventions to support breastfeeding: US Preventive Services Task Force recommendation statement. JAMA 2016; 316(16):1688–1693. doi:10.1001/jama.2016.14697
- Newton ER, Hale TW. Drugs in breast milk. Clin Obstet Gynecol 2015; 58(4):868–884. doi:10.1097/GRF.0000000000000142
- Xiang AH, Kawakubo M, Kjos SL, Buchanan TA. Long-acting injectable progestin contraception and risk of type 2 diabetes in Latino women with prior gestational diabetes mellitus. Diabetes Care 2006; 29(3):613–617. pmid:16505515
KEY POINTS
- Aim for a hemoglobin A1c of 6.5% or lower, if it is attainable without increasing the risk of hypoglycemia.
- Avoid teratogenic drugs in sexually active women of childbearing age unless the patient uses effective contraception.
- Because about half of pregnancies are unplanned, it is important to routinely discuss family planning and provide preconception counseling that includes reducing risks associated with pregnancy.
- Screen for diabetic end-organ damage, especially retinopathy and nephropathy.
Navigating travel with diabetes
Travel, once reserved for wealthy vacationers and high-level executives, has become a regular experience for many people. The US Travel and Tourism Overview reported that US domestic travel climbed to more than 2.25 billion person-trips in 2017.1 The US Centers for Disease Control and Prevention (CDC) and the US Travel Association suggest that, based on this frequency and the known rate of diabetes, 17 million people with diabetes travel annually for leisure and 5.6 million for business, and these numbers are expected to increase.2
It stands to reason that as the number of people who travel continues to increase, so too will the number of patients with diabetes seeking medical travel advice. Despite resources available to travelers with diabetes, researchers at the 2016 meeting of the American Diabetes Association noted that only 30% of patients with diabetes who responded to a survey reported being satisfied with the resources available to help them manage their diabetes while traveling.2 This article discusses how clinicians can help patients manage their diabetes while traveling, address common travel questions, and prepare patients for emergencies that may arise while traveling.
PRE-TRIP PREPARATION
Provider visit before travel: Checking the bases
Advise patients to schedule an appointment 4 to 6 weeks before their trip.3 At this appointment, give the patient a healthcare provider travel letter (Figure 1) and prescriptions that the patient can hand-carry en route.3 The provider letter should state that the patient has diabetes and should list all supplies the patient needs. The letter should also include specific medications used by the patient and the devices that deliver these medications, eg, Humalog insulin and U-100 syringes4 to administer insulin, as well as any food and medication allergies.
Prescriptions should be written for patients to use in the event of an emergency during travel. Prescriptions for diabetes medications should be written with generic names to minimize confusion for those traveling internationally. Additionally, all prescriptions should provide enough medication to last throughout the trip.4
Advise patients that rules for filling prescriptions may vary between states and countries.3 Also, the strength of insulin may vary between the United States and other countries. Patients should understand that if they fill their insulin prescription in a foreign country, they may need to purchase new syringes to match the insulin dose. For example, if patients use U-100 syringes and purchase U-40 insulin, they will need to buy U-40 syringes or risk taking too little of a dose.
Remind patients that prescriptions are not necessary for all diabetes supplies but are essential for coverage by insurance companies. Blood glucose testing supplies, ketone strips, and glucose tablets may be purchased in a pharmacy without a prescription. Human insulin may also be purchased over the counter. However, oral medications, glucagon, and analog insulins require a prescription. We suggest that patients who travel have their prescriptions on file at a chain pharmacy rather than an independent one. If they are in the United States, they can go to any branch of the chain pharmacy and easily fill a prescription.
Work with the patient to compile a separate document that details the medication dosing, correction-scale instructions, carbohydrate-to-insulin ratios, and pump settings (basal rates, insulin sensitivity, active insulin time).4 Patients who use an insulin pump should record all pump settings in the event that they need to convert to insulin injections during travel.4 We suggest that all patients with an insulin pump have an alternate insulin method (eg, pens, vials) and that they carry this with them along with basal insulin in case the pump fails. This level of preparation empowers the patient to assume responsibility for his or her own care if a healthcare provider is not available during travel.
Like all travelers, patients with diabetes should confirm that their immunizations are up to date. Encourage patients to the CDC’s page (wwwnc.cdc.gov/travel) to check the list of vaccines necessary for their region of travel.4,5 Many special immunizations can be acquired only from a public health department and not from a clinician’s office.
Additionally, depending on the region of travel, prescribing antibiotics or antidiarrheal medications may be necessary to ensure patient safety and comfort. We also recommend that patients with type 1 diabetes obtain a supply of antibiotics and antidiarrheals because they can become sick quickly.
Packing with diabetes: Double is better
The American Diabetes Association recommends that patients pack at least twice the medication and blood-testing supplies they anticipate needing.3 Reinforce to patients the need to pack all medications and supplies in their carry-on bag and to keep this bag in their possession at all times to avoid damage, loss, and extreme changes in temperature and air pressure, which can adversely affect the activity and stability of insulin.
Ask patients about the activities they plan to participate in and how many days they will be traveling, and then recommend shoes that will encourage appropriate foot care.4 Patients with diabetes should choose comfort over style when selecting footwear. All new shoes should be purchased and “broken in” 2 to 3 weeks before the trip. Alternating shoes decreases the risk of blisters and calluses.4
Emergency abroad: Planning to be prepared
It is crucial to counsel patients on how to respond in an emergency.
Encourage patients with diabetes, especially those who use insulin, to obtain a medical identification bracelet, necklace, or in some cases, a tattoo, that states they use insulin and discloses any allergies.3 This ensures that emergency medical personnel will be aware of the patient’s condition when providing care. Also suggest that your patients have emergency contact information available on their person and their cell phone to expedite assistance in an emergency (Table 2).
Urge patients to determine prior to their departure if their health coverage will change once they leave the state or the country. Some insurance companies require patients to go to a specific healthcare system while others regulate the amount of time a patient can be in the hospital before being transferred home. It is important for patients to be aware of these terms in the event of hospitalization.4 Travel insurance should be considered for international travel.
AIRPORT SECURITY: WHAT TO EXPECT WITH DIABETES
The American Diabetes Association works with the US Transportation Security Administration (TSA) to ensure that passengers with diabetes have access to supplies. Travelers with diabetes are allowed to apply for an optional disability notification card, which discreetly informs officers that the passenger has a condition or device that may affect screening procedures.6
The TSA suggests that, before going through airport screening, patients with diabetes separate their diabetes supplies from their luggage and declare all items.6 Including prescription labels for medications and medical devices helps speed up the security process. Advise patients to carry glucose tablets and other solid foods for treating hypoglycemia when passing through airport security checkpoints.7
Since 2016, the TSA has allowed all diabetes-related supplies, medications, and equipment, including liquids and devices, through security after they have been screened by the x-ray scanner or by hand.7 People with diabetes are allowed to carry insulin and other liquid medications in amounts greater than 3.4 ounces (100 mLs) through airport security checkpoints.
Insulin can pass safely through x-ray scanners, but if patients are concerned, they may request that their insulin be inspected by hand.7 Patients must inform airport security of this decision before the screening process begins. A hand inspection may include swabbing for explosives.
Patients with an insulin pump and a continuous glucose monitoring device may feel uncomfortable during x-ray screening and special security screenings. Remind patients that it is TSA policy that patients do not need to disconnect their devices and can request screening by pat-down rather than x-ray scanner.6 It is the responsibility of the patient to research whether the pump can pass through x-ray scanners.
All patients have the right to request a pat-down and can opt out of passing through the x-ray scanner.6 However, patients need to inform officers about a pump before screening and must understand that the pump may be subject to further inspection. Usually, this additional inspection includes swabbing the patient’s hands to check for explosive material and a simple pat-down of the insulin pump.7
IN-FLIGHT TIPS
Time zones and insulin dosing
Diabetes management is often based on a 24-hour medication schedule. Travel can disrupt this schedule, making it challenging for patients to determine the appropriate medication adjustments. With some assistance, the patient can determine the best course of action based on the direction of travel and the number of time zones crossed.
According to Chandran and Edelman,7 medication adjustments are needed only when the patient is traveling east or west, not north or south. As time zones change, day length changes and, consequently, so does the 24-hour regimen many patients follow. As a general rule, traveling east results in a shortened day, requiring a potential reduction in insulin, while traveling west results in a longer day, possibly requiring an increase in insulin dose.7 However, this is a guideline and may not be applicable to all patients.7
Advise patients to follow local time to administer medications beginning the morning after arrival.7 It is not uncommon, due to changes in meal schedules and dosing, for patients to experience hyperglycemia during travel. They should be prepared to correct this if necessary.
Patients using insulin injections should plan to adjust to the new time zone as soon as possible. If the time change is only 1 or 2 hours, they should take their medications before departure according to their normal home time.7 Upon arrival, they should resume their insulin regimen based on the local time.
Westward travel. If the patient is traveling west with a time change of 3 or more hours, additional changes may be necessary. Advise patients to take their insulin according to their normal home time before departure. The change in dosing and schedule will depend largely on current glucose control, time of travel, and availability of food and glucose during travel. Encourage patients to discuss these matters with you in advance of any long travel.
Eastward travel. When the patient is traveling east with a time change greater than 3 hours, the day will be consequently shortened. On the day of travel, patients should take their morning dose according to home time. If they are concerned about hypoglycemia, suggest that they decrease the dose by 10%.6 On arrival, they should adhere to the new time zone and base insulin dosing on local time.
Advice for insulin pump users. Patients with an insulin pump need make only minimal changes to their dosing schedule. They should continue their routine of basal and bolus doses and change the time on their insulin pump to local time when they arrive. Insulin pump users should bring insulin and syringes as backup; in the event of pump malfunction, the patient should continue to use the same amount of bolus insulin to correct glucose readings and to cover meals.7 As for the basal dose, patients can administer a once-daily injection of long-acting insulin, which can be calculated from their pump or accessed from the list they created as part of their pre-travel preparation.7
Advice for patients on oral diabetes medications
If a patient is taking an oral medication, it is less crucial to adhere to a time schedule. In fact, in some cases it may be preferable to skip a dose and risk slight hyperglycemia for a few hours rather than take medication too close in time and risk hypoglycemia.7
Remind patients to anticipate a change in their oral medication regimen if they travel farther than 5 time zones.7 Encourage patients to research time changes and discuss the necessary changes in medication dosage on the day of travel as well as the specific aspects of their trip. A time-zone converter can be found at www.timeanddate.com.8
WHAT TO EXPECT WHILE ON LAND
Insulin 101
Storing insulin at the appropriate temperature may be a concern. Insulin should be kept between 40°F and 86°F (4°C–30°C).4 Remind patients to carry their insulin with them at all times and to not store it in a car glove compartment or backpack where it can be exposed to excessive sun. The Frio cold pack (ReadyCare, Walnut Creek, CA) is a helpful alternative to refrigeration and can be used to cool insulin when hiking or participating in activities where insulin can overheat. These cooling gel packs are activated when exposed to cold water for 5 to 7 minutes5 and are reusable.
Alert patients that insulin names and concentrations may vary among countries. Most insulins are U-100 concentration, which means that for every 1 mL of liquid there are 100 units of insulin. This is the standard insulin concentration used in the United States. There are U-200, U-300, and U-500 insulins as well. In Europe, the standard concentration is U-40 insulin. Syringe sizes are designed to accommodate either U-100 or U-40 insulin. Review these differences with patients and explain the consequences of mixing insulin concentration with syringes of different sizes. Figure 2 shows how to calculate equivalent doses.
Resort tips: Food, drinks, and excursions
A large component of travel is indulging in local cuisine. Patients with diabetes need to be aware of how different foods can affect their diabetes control. Encourage them to research the foods common to the local cuisine. Websites such as Calorie King, MyFitnessPal, Lose it!, and Nutrition Data can help identify the caloric and nutritional makeup of foods.9
Advise patients to actively monitor how their blood glucose is affected by new foods by checking blood glucose levels before and after each meal.9 Opting for vegetables and protein sources minimizes glucose fluctuations. Remind patients that drinks at resorts may contain more sugar than advertised. Patients should continue to manage their blood glucose by checking levels and by making appropriate insulin adjustments based on the readings. We often advise patients to pack a jar of peanut butter when traveling to ensure a ready source of protein.
Patients who plan to participate in physically challenging activities while travelling should inform all relevant members of the activity staff of their condition. In case of an emergency, hotel staff and guides will be better equipped to help with situations such as hypoglycemia. As noted above, patients should always carry snacks and supplies to treat hypoglycemia in case no alternative food options are available during an excursion. Also, warn patients to avoid walking barefoot. Water shoes are a good alternative to protect feet from cuts and sores.
Patients should inquire about the safety of high-elevation activities. With many glucose meters, every 1,000 feet of elevation results in a 1% to 2% underestimation of blood glucose,10 which could result in an inaccurate reading. If high-altitude activities are planned, advise patients to bring multiple meters to cross-check glucose readings in cases where inaccuracies (due to elevation) are possible.
- US Travel Association. US travel and tourism overview. www.ustravel.org/system/files/media_root/document/Research_Fact-Sheet_US-Travel-and-Tourism-Overview.pdf. Accessed June 14, 2018.
- Brunk D. Long haul travel turbulent for many with type 1 diabetes. Clinical Endocrinology News 2016. www.mdedge.com/clinicalendocrinologynews/article/109866/diabetes/long-haul-travel-turbulent-many-type-1-diabetes. Accessed June 14, 2018.
- American Diabetes Association. When you travel. www.diabetes.org/living-with-diabetes/treatment-and-care/when-you-travel.html?utm_source=DSH_BLOG&utm_medium=BlogPost&utm_content=051514-travel&utm_campaign=CON. Accessed June 14, 2018.
- Kruger DF. The Diabetes Travel Guide. How to travel with diabetes-anywhere in the world. Arlington, VA: American Diabetes Association; 2000.
- Centers for Disease Control and Prevention. Travelers’ health. wwwnc.cdc.gov/travel/. Accessed June 14, 2018.
- American Diabetes Association. What special concerns may arise? www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/what-special-concerns-may.html. Accessed June 14, 2018.
- Chandran M, Edelman SV. Have insulin, will fly: diabetes management during air travel and time zone adjustment strategies. Clinical Diabetes 2003; 21(2):82–85. doi:10.2337/diaclin.21.2.82
- Time and Date AS. Time zone converter. timeanddate.com. Accessed March 19, 2018.
- Joslin Diabetes Center. Diabetes and travel—10 tips for a safe trip. www.joslin.org/info/diabetes_and_travel_10_tips_for_a_safe_trip.html. Accessed June 14, 2018.
- Jendle J, Adolfsson P. Impact of high altitudes on glucose control. J Diabetes Sci Technol 2011; 5(6):1621–1622. doi:10.1177/193229681100500642
Travel, once reserved for wealthy vacationers and high-level executives, has become a regular experience for many people. The US Travel and Tourism Overview reported that US domestic travel climbed to more than 2.25 billion person-trips in 2017.1 The US Centers for Disease Control and Prevention (CDC) and the US Travel Association suggest that, based on this frequency and the known rate of diabetes, 17 million people with diabetes travel annually for leisure and 5.6 million for business, and these numbers are expected to increase.2
It stands to reason that as the number of people who travel continues to increase, so too will the number of patients with diabetes seeking medical travel advice. Despite resources available to travelers with diabetes, researchers at the 2016 meeting of the American Diabetes Association noted that only 30% of patients with diabetes who responded to a survey reported being satisfied with the resources available to help them manage their diabetes while traveling.2 This article discusses how clinicians can help patients manage their diabetes while traveling, address common travel questions, and prepare patients for emergencies that may arise while traveling.
PRE-TRIP PREPARATION
Provider visit before travel: Checking the bases
Advise patients to schedule an appointment 4 to 6 weeks before their trip.3 At this appointment, give the patient a healthcare provider travel letter (Figure 1) and prescriptions that the patient can hand-carry en route.3 The provider letter should state that the patient has diabetes and should list all supplies the patient needs. The letter should also include specific medications used by the patient and the devices that deliver these medications, eg, Humalog insulin and U-100 syringes4 to administer insulin, as well as any food and medication allergies.
Prescriptions should be written for patients to use in the event of an emergency during travel. Prescriptions for diabetes medications should be written with generic names to minimize confusion for those traveling internationally. Additionally, all prescriptions should provide enough medication to last throughout the trip.4
Advise patients that rules for filling prescriptions may vary between states and countries.3 Also, the strength of insulin may vary between the United States and other countries. Patients should understand that if they fill their insulin prescription in a foreign country, they may need to purchase new syringes to match the insulin dose. For example, if patients use U-100 syringes and purchase U-40 insulin, they will need to buy U-40 syringes or risk taking too little of a dose.
Remind patients that prescriptions are not necessary for all diabetes supplies but are essential for coverage by insurance companies. Blood glucose testing supplies, ketone strips, and glucose tablets may be purchased in a pharmacy without a prescription. Human insulin may also be purchased over the counter. However, oral medications, glucagon, and analog insulins require a prescription. We suggest that patients who travel have their prescriptions on file at a chain pharmacy rather than an independent one. If they are in the United States, they can go to any branch of the chain pharmacy and easily fill a prescription.
Work with the patient to compile a separate document that details the medication dosing, correction-scale instructions, carbohydrate-to-insulin ratios, and pump settings (basal rates, insulin sensitivity, active insulin time).4 Patients who use an insulin pump should record all pump settings in the event that they need to convert to insulin injections during travel.4 We suggest that all patients with an insulin pump have an alternate insulin method (eg, pens, vials) and that they carry this with them along with basal insulin in case the pump fails. This level of preparation empowers the patient to assume responsibility for his or her own care if a healthcare provider is not available during travel.
Like all travelers, patients with diabetes should confirm that their immunizations are up to date. Encourage patients to the CDC’s page (wwwnc.cdc.gov/travel) to check the list of vaccines necessary for their region of travel.4,5 Many special immunizations can be acquired only from a public health department and not from a clinician’s office.
Additionally, depending on the region of travel, prescribing antibiotics or antidiarrheal medications may be necessary to ensure patient safety and comfort. We also recommend that patients with type 1 diabetes obtain a supply of antibiotics and antidiarrheals because they can become sick quickly.
Packing with diabetes: Double is better
The American Diabetes Association recommends that patients pack at least twice the medication and blood-testing supplies they anticipate needing.3 Reinforce to patients the need to pack all medications and supplies in their carry-on bag and to keep this bag in their possession at all times to avoid damage, loss, and extreme changes in temperature and air pressure, which can adversely affect the activity and stability of insulin.
Ask patients about the activities they plan to participate in and how many days they will be traveling, and then recommend shoes that will encourage appropriate foot care.4 Patients with diabetes should choose comfort over style when selecting footwear. All new shoes should be purchased and “broken in” 2 to 3 weeks before the trip. Alternating shoes decreases the risk of blisters and calluses.4
Emergency abroad: Planning to be prepared
It is crucial to counsel patients on how to respond in an emergency.
Encourage patients with diabetes, especially those who use insulin, to obtain a medical identification bracelet, necklace, or in some cases, a tattoo, that states they use insulin and discloses any allergies.3 This ensures that emergency medical personnel will be aware of the patient’s condition when providing care. Also suggest that your patients have emergency contact information available on their person and their cell phone to expedite assistance in an emergency (Table 2).
Urge patients to determine prior to their departure if their health coverage will change once they leave the state or the country. Some insurance companies require patients to go to a specific healthcare system while others regulate the amount of time a patient can be in the hospital before being transferred home. It is important for patients to be aware of these terms in the event of hospitalization.4 Travel insurance should be considered for international travel.
AIRPORT SECURITY: WHAT TO EXPECT WITH DIABETES
The American Diabetes Association works with the US Transportation Security Administration (TSA) to ensure that passengers with diabetes have access to supplies. Travelers with diabetes are allowed to apply for an optional disability notification card, which discreetly informs officers that the passenger has a condition or device that may affect screening procedures.6
The TSA suggests that, before going through airport screening, patients with diabetes separate their diabetes supplies from their luggage and declare all items.6 Including prescription labels for medications and medical devices helps speed up the security process. Advise patients to carry glucose tablets and other solid foods for treating hypoglycemia when passing through airport security checkpoints.7
Since 2016, the TSA has allowed all diabetes-related supplies, medications, and equipment, including liquids and devices, through security after they have been screened by the x-ray scanner or by hand.7 People with diabetes are allowed to carry insulin and other liquid medications in amounts greater than 3.4 ounces (100 mLs) through airport security checkpoints.
Insulin can pass safely through x-ray scanners, but if patients are concerned, they may request that their insulin be inspected by hand.7 Patients must inform airport security of this decision before the screening process begins. A hand inspection may include swabbing for explosives.
Patients with an insulin pump and a continuous glucose monitoring device may feel uncomfortable during x-ray screening and special security screenings. Remind patients that it is TSA policy that patients do not need to disconnect their devices and can request screening by pat-down rather than x-ray scanner.6 It is the responsibility of the patient to research whether the pump can pass through x-ray scanners.
All patients have the right to request a pat-down and can opt out of passing through the x-ray scanner.6 However, patients need to inform officers about a pump before screening and must understand that the pump may be subject to further inspection. Usually, this additional inspection includes swabbing the patient’s hands to check for explosive material and a simple pat-down of the insulin pump.7
IN-FLIGHT TIPS
Time zones and insulin dosing
Diabetes management is often based on a 24-hour medication schedule. Travel can disrupt this schedule, making it challenging for patients to determine the appropriate medication adjustments. With some assistance, the patient can determine the best course of action based on the direction of travel and the number of time zones crossed.
According to Chandran and Edelman,7 medication adjustments are needed only when the patient is traveling east or west, not north or south. As time zones change, day length changes and, consequently, so does the 24-hour regimen many patients follow. As a general rule, traveling east results in a shortened day, requiring a potential reduction in insulin, while traveling west results in a longer day, possibly requiring an increase in insulin dose.7 However, this is a guideline and may not be applicable to all patients.7
Advise patients to follow local time to administer medications beginning the morning after arrival.7 It is not uncommon, due to changes in meal schedules and dosing, for patients to experience hyperglycemia during travel. They should be prepared to correct this if necessary.
Patients using insulin injections should plan to adjust to the new time zone as soon as possible. If the time change is only 1 or 2 hours, they should take their medications before departure according to their normal home time.7 Upon arrival, they should resume their insulin regimen based on the local time.
Westward travel. If the patient is traveling west with a time change of 3 or more hours, additional changes may be necessary. Advise patients to take their insulin according to their normal home time before departure. The change in dosing and schedule will depend largely on current glucose control, time of travel, and availability of food and glucose during travel. Encourage patients to discuss these matters with you in advance of any long travel.
Eastward travel. When the patient is traveling east with a time change greater than 3 hours, the day will be consequently shortened. On the day of travel, patients should take their morning dose according to home time. If they are concerned about hypoglycemia, suggest that they decrease the dose by 10%.6 On arrival, they should adhere to the new time zone and base insulin dosing on local time.
Advice for insulin pump users. Patients with an insulin pump need make only minimal changes to their dosing schedule. They should continue their routine of basal and bolus doses and change the time on their insulin pump to local time when they arrive. Insulin pump users should bring insulin and syringes as backup; in the event of pump malfunction, the patient should continue to use the same amount of bolus insulin to correct glucose readings and to cover meals.7 As for the basal dose, patients can administer a once-daily injection of long-acting insulin, which can be calculated from their pump or accessed from the list they created as part of their pre-travel preparation.7
Advice for patients on oral diabetes medications
If a patient is taking an oral medication, it is less crucial to adhere to a time schedule. In fact, in some cases it may be preferable to skip a dose and risk slight hyperglycemia for a few hours rather than take medication too close in time and risk hypoglycemia.7
Remind patients to anticipate a change in their oral medication regimen if they travel farther than 5 time zones.7 Encourage patients to research time changes and discuss the necessary changes in medication dosage on the day of travel as well as the specific aspects of their trip. A time-zone converter can be found at www.timeanddate.com.8
WHAT TO EXPECT WHILE ON LAND
Insulin 101
Storing insulin at the appropriate temperature may be a concern. Insulin should be kept between 40°F and 86°F (4°C–30°C).4 Remind patients to carry their insulin with them at all times and to not store it in a car glove compartment or backpack where it can be exposed to excessive sun. The Frio cold pack (ReadyCare, Walnut Creek, CA) is a helpful alternative to refrigeration and can be used to cool insulin when hiking or participating in activities where insulin can overheat. These cooling gel packs are activated when exposed to cold water for 5 to 7 minutes5 and are reusable.
Alert patients that insulin names and concentrations may vary among countries. Most insulins are U-100 concentration, which means that for every 1 mL of liquid there are 100 units of insulin. This is the standard insulin concentration used in the United States. There are U-200, U-300, and U-500 insulins as well. In Europe, the standard concentration is U-40 insulin. Syringe sizes are designed to accommodate either U-100 or U-40 insulin. Review these differences with patients and explain the consequences of mixing insulin concentration with syringes of different sizes. Figure 2 shows how to calculate equivalent doses.
Resort tips: Food, drinks, and excursions
A large component of travel is indulging in local cuisine. Patients with diabetes need to be aware of how different foods can affect their diabetes control. Encourage them to research the foods common to the local cuisine. Websites such as Calorie King, MyFitnessPal, Lose it!, and Nutrition Data can help identify the caloric and nutritional makeup of foods.9
Advise patients to actively monitor how their blood glucose is affected by new foods by checking blood glucose levels before and after each meal.9 Opting for vegetables and protein sources minimizes glucose fluctuations. Remind patients that drinks at resorts may contain more sugar than advertised. Patients should continue to manage their blood glucose by checking levels and by making appropriate insulin adjustments based on the readings. We often advise patients to pack a jar of peanut butter when traveling to ensure a ready source of protein.
Patients who plan to participate in physically challenging activities while travelling should inform all relevant members of the activity staff of their condition. In case of an emergency, hotel staff and guides will be better equipped to help with situations such as hypoglycemia. As noted above, patients should always carry snacks and supplies to treat hypoglycemia in case no alternative food options are available during an excursion. Also, warn patients to avoid walking barefoot. Water shoes are a good alternative to protect feet from cuts and sores.
Patients should inquire about the safety of high-elevation activities. With many glucose meters, every 1,000 feet of elevation results in a 1% to 2% underestimation of blood glucose,10 which could result in an inaccurate reading. If high-altitude activities are planned, advise patients to bring multiple meters to cross-check glucose readings in cases where inaccuracies (due to elevation) are possible.
Travel, once reserved for wealthy vacationers and high-level executives, has become a regular experience for many people. The US Travel and Tourism Overview reported that US domestic travel climbed to more than 2.25 billion person-trips in 2017.1 The US Centers for Disease Control and Prevention (CDC) and the US Travel Association suggest that, based on this frequency and the known rate of diabetes, 17 million people with diabetes travel annually for leisure and 5.6 million for business, and these numbers are expected to increase.2
It stands to reason that as the number of people who travel continues to increase, so too will the number of patients with diabetes seeking medical travel advice. Despite resources available to travelers with diabetes, researchers at the 2016 meeting of the American Diabetes Association noted that only 30% of patients with diabetes who responded to a survey reported being satisfied with the resources available to help them manage their diabetes while traveling.2 This article discusses how clinicians can help patients manage their diabetes while traveling, address common travel questions, and prepare patients for emergencies that may arise while traveling.
PRE-TRIP PREPARATION
Provider visit before travel: Checking the bases
Advise patients to schedule an appointment 4 to 6 weeks before their trip.3 At this appointment, give the patient a healthcare provider travel letter (Figure 1) and prescriptions that the patient can hand-carry en route.3 The provider letter should state that the patient has diabetes and should list all supplies the patient needs. The letter should also include specific medications used by the patient and the devices that deliver these medications, eg, Humalog insulin and U-100 syringes4 to administer insulin, as well as any food and medication allergies.
Prescriptions should be written for patients to use in the event of an emergency during travel. Prescriptions for diabetes medications should be written with generic names to minimize confusion for those traveling internationally. Additionally, all prescriptions should provide enough medication to last throughout the trip.4
Advise patients that rules for filling prescriptions may vary between states and countries.3 Also, the strength of insulin may vary between the United States and other countries. Patients should understand that if they fill their insulin prescription in a foreign country, they may need to purchase new syringes to match the insulin dose. For example, if patients use U-100 syringes and purchase U-40 insulin, they will need to buy U-40 syringes or risk taking too little of a dose.
Remind patients that prescriptions are not necessary for all diabetes supplies but are essential for coverage by insurance companies. Blood glucose testing supplies, ketone strips, and glucose tablets may be purchased in a pharmacy without a prescription. Human insulin may also be purchased over the counter. However, oral medications, glucagon, and analog insulins require a prescription. We suggest that patients who travel have their prescriptions on file at a chain pharmacy rather than an independent one. If they are in the United States, they can go to any branch of the chain pharmacy and easily fill a prescription.
Work with the patient to compile a separate document that details the medication dosing, correction-scale instructions, carbohydrate-to-insulin ratios, and pump settings (basal rates, insulin sensitivity, active insulin time).4 Patients who use an insulin pump should record all pump settings in the event that they need to convert to insulin injections during travel.4 We suggest that all patients with an insulin pump have an alternate insulin method (eg, pens, vials) and that they carry this with them along with basal insulin in case the pump fails. This level of preparation empowers the patient to assume responsibility for his or her own care if a healthcare provider is not available during travel.
Like all travelers, patients with diabetes should confirm that their immunizations are up to date. Encourage patients to the CDC’s page (wwwnc.cdc.gov/travel) to check the list of vaccines necessary for their region of travel.4,5 Many special immunizations can be acquired only from a public health department and not from a clinician’s office.
Additionally, depending on the region of travel, prescribing antibiotics or antidiarrheal medications may be necessary to ensure patient safety and comfort. We also recommend that patients with type 1 diabetes obtain a supply of antibiotics and antidiarrheals because they can become sick quickly.
Packing with diabetes: Double is better
The American Diabetes Association recommends that patients pack at least twice the medication and blood-testing supplies they anticipate needing.3 Reinforce to patients the need to pack all medications and supplies in their carry-on bag and to keep this bag in their possession at all times to avoid damage, loss, and extreme changes in temperature and air pressure, which can adversely affect the activity and stability of insulin.
Ask patients about the activities they plan to participate in and how many days they will be traveling, and then recommend shoes that will encourage appropriate foot care.4 Patients with diabetes should choose comfort over style when selecting footwear. All new shoes should be purchased and “broken in” 2 to 3 weeks before the trip. Alternating shoes decreases the risk of blisters and calluses.4
Emergency abroad: Planning to be prepared
It is crucial to counsel patients on how to respond in an emergency.
Encourage patients with diabetes, especially those who use insulin, to obtain a medical identification bracelet, necklace, or in some cases, a tattoo, that states they use insulin and discloses any allergies.3 This ensures that emergency medical personnel will be aware of the patient’s condition when providing care. Also suggest that your patients have emergency contact information available on their person and their cell phone to expedite assistance in an emergency (Table 2).
Urge patients to determine prior to their departure if their health coverage will change once they leave the state or the country. Some insurance companies require patients to go to a specific healthcare system while others regulate the amount of time a patient can be in the hospital before being transferred home. It is important for patients to be aware of these terms in the event of hospitalization.4 Travel insurance should be considered for international travel.
AIRPORT SECURITY: WHAT TO EXPECT WITH DIABETES
The American Diabetes Association works with the US Transportation Security Administration (TSA) to ensure that passengers with diabetes have access to supplies. Travelers with diabetes are allowed to apply for an optional disability notification card, which discreetly informs officers that the passenger has a condition or device that may affect screening procedures.6
The TSA suggests that, before going through airport screening, patients with diabetes separate their diabetes supplies from their luggage and declare all items.6 Including prescription labels for medications and medical devices helps speed up the security process. Advise patients to carry glucose tablets and other solid foods for treating hypoglycemia when passing through airport security checkpoints.7
Since 2016, the TSA has allowed all diabetes-related supplies, medications, and equipment, including liquids and devices, through security after they have been screened by the x-ray scanner or by hand.7 People with diabetes are allowed to carry insulin and other liquid medications in amounts greater than 3.4 ounces (100 mLs) through airport security checkpoints.
Insulin can pass safely through x-ray scanners, but if patients are concerned, they may request that their insulin be inspected by hand.7 Patients must inform airport security of this decision before the screening process begins. A hand inspection may include swabbing for explosives.
Patients with an insulin pump and a continuous glucose monitoring device may feel uncomfortable during x-ray screening and special security screenings. Remind patients that it is TSA policy that patients do not need to disconnect their devices and can request screening by pat-down rather than x-ray scanner.6 It is the responsibility of the patient to research whether the pump can pass through x-ray scanners.
All patients have the right to request a pat-down and can opt out of passing through the x-ray scanner.6 However, patients need to inform officers about a pump before screening and must understand that the pump may be subject to further inspection. Usually, this additional inspection includes swabbing the patient’s hands to check for explosive material and a simple pat-down of the insulin pump.7
IN-FLIGHT TIPS
Time zones and insulin dosing
Diabetes management is often based on a 24-hour medication schedule. Travel can disrupt this schedule, making it challenging for patients to determine the appropriate medication adjustments. With some assistance, the patient can determine the best course of action based on the direction of travel and the number of time zones crossed.
According to Chandran and Edelman,7 medication adjustments are needed only when the patient is traveling east or west, not north or south. As time zones change, day length changes and, consequently, so does the 24-hour regimen many patients follow. As a general rule, traveling east results in a shortened day, requiring a potential reduction in insulin, while traveling west results in a longer day, possibly requiring an increase in insulin dose.7 However, this is a guideline and may not be applicable to all patients.7
Advise patients to follow local time to administer medications beginning the morning after arrival.7 It is not uncommon, due to changes in meal schedules and dosing, for patients to experience hyperglycemia during travel. They should be prepared to correct this if necessary.
Patients using insulin injections should plan to adjust to the new time zone as soon as possible. If the time change is only 1 or 2 hours, they should take their medications before departure according to their normal home time.7 Upon arrival, they should resume their insulin regimen based on the local time.
Westward travel. If the patient is traveling west with a time change of 3 or more hours, additional changes may be necessary. Advise patients to take their insulin according to their normal home time before departure. The change in dosing and schedule will depend largely on current glucose control, time of travel, and availability of food and glucose during travel. Encourage patients to discuss these matters with you in advance of any long travel.
Eastward travel. When the patient is traveling east with a time change greater than 3 hours, the day will be consequently shortened. On the day of travel, patients should take their morning dose according to home time. If they are concerned about hypoglycemia, suggest that they decrease the dose by 10%.6 On arrival, they should adhere to the new time zone and base insulin dosing on local time.
Advice for insulin pump users. Patients with an insulin pump need make only minimal changes to their dosing schedule. They should continue their routine of basal and bolus doses and change the time on their insulin pump to local time when they arrive. Insulin pump users should bring insulin and syringes as backup; in the event of pump malfunction, the patient should continue to use the same amount of bolus insulin to correct glucose readings and to cover meals.7 As for the basal dose, patients can administer a once-daily injection of long-acting insulin, which can be calculated from their pump or accessed from the list they created as part of their pre-travel preparation.7
Advice for patients on oral diabetes medications
If a patient is taking an oral medication, it is less crucial to adhere to a time schedule. In fact, in some cases it may be preferable to skip a dose and risk slight hyperglycemia for a few hours rather than take medication too close in time and risk hypoglycemia.7
Remind patients to anticipate a change in their oral medication regimen if they travel farther than 5 time zones.7 Encourage patients to research time changes and discuss the necessary changes in medication dosage on the day of travel as well as the specific aspects of their trip. A time-zone converter can be found at www.timeanddate.com.8
WHAT TO EXPECT WHILE ON LAND
Insulin 101
Storing insulin at the appropriate temperature may be a concern. Insulin should be kept between 40°F and 86°F (4°C–30°C).4 Remind patients to carry their insulin with them at all times and to not store it in a car glove compartment or backpack where it can be exposed to excessive sun. The Frio cold pack (ReadyCare, Walnut Creek, CA) is a helpful alternative to refrigeration and can be used to cool insulin when hiking or participating in activities where insulin can overheat. These cooling gel packs are activated when exposed to cold water for 5 to 7 minutes5 and are reusable.
Alert patients that insulin names and concentrations may vary among countries. Most insulins are U-100 concentration, which means that for every 1 mL of liquid there are 100 units of insulin. This is the standard insulin concentration used in the United States. There are U-200, U-300, and U-500 insulins as well. In Europe, the standard concentration is U-40 insulin. Syringe sizes are designed to accommodate either U-100 or U-40 insulin. Review these differences with patients and explain the consequences of mixing insulin concentration with syringes of different sizes. Figure 2 shows how to calculate equivalent doses.
Resort tips: Food, drinks, and excursions
A large component of travel is indulging in local cuisine. Patients with diabetes need to be aware of how different foods can affect their diabetes control. Encourage them to research the foods common to the local cuisine. Websites such as Calorie King, MyFitnessPal, Lose it!, and Nutrition Data can help identify the caloric and nutritional makeup of foods.9
Advise patients to actively monitor how their blood glucose is affected by new foods by checking blood glucose levels before and after each meal.9 Opting for vegetables and protein sources minimizes glucose fluctuations. Remind patients that drinks at resorts may contain more sugar than advertised. Patients should continue to manage their blood glucose by checking levels and by making appropriate insulin adjustments based on the readings. We often advise patients to pack a jar of peanut butter when traveling to ensure a ready source of protein.
Patients who plan to participate in physically challenging activities while travelling should inform all relevant members of the activity staff of their condition. In case of an emergency, hotel staff and guides will be better equipped to help with situations such as hypoglycemia. As noted above, patients should always carry snacks and supplies to treat hypoglycemia in case no alternative food options are available during an excursion. Also, warn patients to avoid walking barefoot. Water shoes are a good alternative to protect feet from cuts and sores.
Patients should inquire about the safety of high-elevation activities. With many glucose meters, every 1,000 feet of elevation results in a 1% to 2% underestimation of blood glucose,10 which could result in an inaccurate reading. If high-altitude activities are planned, advise patients to bring multiple meters to cross-check glucose readings in cases where inaccuracies (due to elevation) are possible.
- US Travel Association. US travel and tourism overview. www.ustravel.org/system/files/media_root/document/Research_Fact-Sheet_US-Travel-and-Tourism-Overview.pdf. Accessed June 14, 2018.
- Brunk D. Long haul travel turbulent for many with type 1 diabetes. Clinical Endocrinology News 2016. www.mdedge.com/clinicalendocrinologynews/article/109866/diabetes/long-haul-travel-turbulent-many-type-1-diabetes. Accessed June 14, 2018.
- American Diabetes Association. When you travel. www.diabetes.org/living-with-diabetes/treatment-and-care/when-you-travel.html?utm_source=DSH_BLOG&utm_medium=BlogPost&utm_content=051514-travel&utm_campaign=CON. Accessed June 14, 2018.
- Kruger DF. The Diabetes Travel Guide. How to travel with diabetes-anywhere in the world. Arlington, VA: American Diabetes Association; 2000.
- Centers for Disease Control and Prevention. Travelers’ health. wwwnc.cdc.gov/travel/. Accessed June 14, 2018.
- American Diabetes Association. What special concerns may arise? www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/what-special-concerns-may.html. Accessed June 14, 2018.
- Chandran M, Edelman SV. Have insulin, will fly: diabetes management during air travel and time zone adjustment strategies. Clinical Diabetes 2003; 21(2):82–85. doi:10.2337/diaclin.21.2.82
- Time and Date AS. Time zone converter. timeanddate.com. Accessed March 19, 2018.
- Joslin Diabetes Center. Diabetes and travel—10 tips for a safe trip. www.joslin.org/info/diabetes_and_travel_10_tips_for_a_safe_trip.html. Accessed June 14, 2018.
- Jendle J, Adolfsson P. Impact of high altitudes on glucose control. J Diabetes Sci Technol 2011; 5(6):1621–1622. doi:10.1177/193229681100500642
- US Travel Association. US travel and tourism overview. www.ustravel.org/system/files/media_root/document/Research_Fact-Sheet_US-Travel-and-Tourism-Overview.pdf. Accessed June 14, 2018.
- Brunk D. Long haul travel turbulent for many with type 1 diabetes. Clinical Endocrinology News 2016. www.mdedge.com/clinicalendocrinologynews/article/109866/diabetes/long-haul-travel-turbulent-many-type-1-diabetes. Accessed June 14, 2018.
- American Diabetes Association. When you travel. www.diabetes.org/living-with-diabetes/treatment-and-care/when-you-travel.html?utm_source=DSH_BLOG&utm_medium=BlogPost&utm_content=051514-travel&utm_campaign=CON. Accessed June 14, 2018.
- Kruger DF. The Diabetes Travel Guide. How to travel with diabetes-anywhere in the world. Arlington, VA: American Diabetes Association; 2000.
- Centers for Disease Control and Prevention. Travelers’ health. wwwnc.cdc.gov/travel/. Accessed June 14, 2018.
- American Diabetes Association. What special concerns may arise? www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/what-special-concerns-may.html. Accessed June 14, 2018.
- Chandran M, Edelman SV. Have insulin, will fly: diabetes management during air travel and time zone adjustment strategies. Clinical Diabetes 2003; 21(2):82–85. doi:10.2337/diaclin.21.2.82
- Time and Date AS. Time zone converter. timeanddate.com. Accessed March 19, 2018.
- Joslin Diabetes Center. Diabetes and travel—10 tips for a safe trip. www.joslin.org/info/diabetes_and_travel_10_tips_for_a_safe_trip.html. Accessed June 14, 2018.
- Jendle J, Adolfsson P. Impact of high altitudes on glucose control. J Diabetes Sci Technol 2011; 5(6):1621–1622. doi:10.1177/193229681100500642
KEY POINTS
- Patients should pack all diabetes medications and supplies in a carry-on bag and keep it in their possession at all times.
- A travel letter will facilitate easy transfer through security and customs.
- Patients should always take more supplies than needed to accommodate changes in travel plans.
- If patients will cross multiple time zones during their travel, they will likely need to adjust their medication and food schedules.
‘Dry drowning’ and other myths
In June 2017, a 4-year-old boy died 1 week after being knocked over and briefly submerged while playing in knee-deep water. This story was widely reported as a case of a rare occurrence called “dry” or “secondary” drowning, depending on the source.1 The media accounts went viral, spreading fear in parents and others learning about these alleged conditions from the news and social media.
Many alleged cases of dry drowning are reported every year, but each has been found to have a recognized medical source that has a legitimate medically recognized diagnosis (which dry and secondary drowning are not).
Drowning is one of the most common causes of death in children, and so we ought to make sure that the information we share about it is accurate, as it is vital to effective prevention, rescue, and treatment.
Unfortunately, medical providers, medical journals, and the mass media continue to disseminate misinformation on drowning.2 These reports often prevail over updated information and hinder accurate understanding of the drowning problem and its solutions.
Every death is tragic, especially the death of a child, and our heartfelt sympathies go out to the family in this alleged drowning case, as well as to all families suffering the loss of a loved one to drowning. However, in the 2017 case, the cause of death was found on autopsy to be myocarditis not related in any way to drowning. As often happens in such situations, this clarification did not receive any media attention, despite the wide reporting and penetration of the original, erroneous story.
We hope our review will reduce misunderstanding among the public and healthcare providers, contribute to improved data collection, and help to promote interventions aimed at prevention, rescue, and mitigation of drowning incidents.
WHAT IS DROWNING?
A consensus committee of the World Health Organization defined drowning as “the process of experiencing respiratory impairment from submersion/immersion in liquid.”3 The process begins when the victim’s airway goes below the surface of the liquid (submersion) or when water splashes over the face (immersion). If the victim is rescued at any time, the process is interrupted, and this is termed a nonfatal drowning. If the victim dies at any time, this is a fatal drowning. Any water-distress incident without evidence of respiratory impairment (ie, without aspiration) should be considered a water rescue and not a drowning.
Rarely do minimally symptomatic cases progress to death, just as most cases of chest pain do not progress to cardiac arrest.4 Nonetheless, rescued drowning victims can deteriorate, which is why we encourage people to seek medical care immediately upon warning signs, as we do with chest pain. For drowning, such warning signs are any water distress followed by difficulty breathing, excessive coughing, foam in the mouth, or abnormal behavior.
A SERIOUS PUBLIC HEALTH ISSUE
Drowning is a serious and neglected public health issue, claiming the lives of 372,000 people a year worldwide.5 It is a leading cause of death in children ages 1 to 14. The toll continues largely unabated, and in low- and middle-income nations it does not attract the levels of funding that go to other forms of injury prevention, such as road safety.
Nonfatal drowning—with symptoms ranging from mild cough to severe pulmonary edema, and complications ranging from none to severe neurologic impairment—is far more common than fatal drowning.6 For every fatal drowning, there are at least 5 nonfatal drowning incidents in which medical care is needed, and 200 rescues are performed.7–10
In the United States, drowning accounts for almost 13,000 emergency department visits per year and about 3,500 deaths.7,8
In Brazil, with two-thirds the population of the United States, drowning accounts for far fewer hospital visits but about twice as many deaths. In Rio de Janeiro, where a highly effective and specialized prehospital service is provided at 3 drowning resuscitation centers staffed by medical doctors, an analysis of the 46,060 cases of rescue in 10 years from 1991 to 2000 showed that medical assistance was needed in only 930 cases (2%).10 The preventive and rescue actions of parents, bystanders, lifeguards, and prehospital rescue services significantly reduce the number of drowning deaths, but these groups do not consistently gather data on nonfatal drowning that can be included in a comprehensive database.
DROWNING IS A PROCESS
When a person in the water can no longer keep the airway clear, water that enters the mouth is voluntarily spit out or swallowed. Within a few seconds to minutes, the person can no longer clear the airways and water is aspirated, stimulating the cough reflex. Laryngospasm, another myth concerning drowning, is presumed to protect the airways but does not, as it is rare, occurring in less than 2% of cases.11,12
If the person is not rescued, aspiration of water continues, and hypoxemia leads to loss of consciousness and apnea within seconds to a few minutes, followed by cardiac arrest. As a consequence, hypoxemic cardiac arrest generally occurs after a period of tachycardia followed by bradycardia and pulseless electrical activity, usually leading to asystole.13,14
The entire drowning process, from water distress to cardiac arrest, usually takes a few minutes, but in rare situations, such as rapid hypothermia, it can go on for up to an hour.15 Most drowning patients have an otherwise healthy heart, and the apnea and hypoxemia precede the cardiac arrest by only a few seconds to minutes; thus, cardiac arrest is caused by the hypoxemic insult and not by ventricular dysrhythmias.6,16
Drowning can be interrupted at any point between distress and death. If the person is rescued early, the clinical picture is determined by the reactivity of the airway and the amount of water that has been aspirated, but not by the type of water (salt or fresh).
Another myth is that drowning in salt water is different from drowning in fresh water. Both salt water and fresh water cause similar surfactant destruction and washout and disrupt the alveolar-capillary membrane. Disruption of the alveolar-capillary membrane increases its permeability and exacerbates shifting of fluid, plasma, and electrolytes into the alveoli.13 The clinical picture of the damage is one of regional or generalized pulmonary edema, which interferes with gas exchange in the lungs.6,13,17
Animal studies by Modell et al showed that aspiration of just 2.2 mL of water per kilogram of body weight is sufficient to cause severe disturbances in oxygen exchange,17 reflected in a rise in arterial pH and a drop in partial pressure of oxygen. The situation must be similar in humans. In a 70-kg person, this is only about 154 mL of water—about two-thirds of a cup.
The combined effects of fluid in the lungs, the loss of surfactant, and the increase in capillary-alveolar permeability can result in decreased lung compliance, increased right-to-left shunting in the lungs, atelectasis, alveolitis, hypoxemia, and cerebral hypoxia.13
If the victim needs cardiopulmonary resuscitation, the possibility of neurologic damage is similar to that in other cardiac arrest situations, but exceptions exist. For example, in rare cases, hypothermia provides a protective mechanism that allows victims to survive prolonged submersion.4,15
The duration of submersion is the best predictor of death.18 Underwater, people are not taking in oxygen, and cerebral hypoxia causes both morbidity and death. For this reason, reversing cerebral hypoxia with effective ventilation, oxygen, and chest compression is the priority of treatment.
MYTHS AND SLOPPY TERMINOLOGY
“Near drowning,” “dry drowning,” “wet drowning,” “delayed drowning,” and “secondary drowning” are not medically accepted diagnoses,3,4,19 and many organizations and lifesaving institutions around the world discourage the use of these terms.19,20 Unfortunately, these terms still slip past the editors of medical journals and are thus perpetuated. The terms are most pervasive in the nonmedical media, where drowning seems to be synonymous with death.3,19,21 We urge all authors and stakeholders to abandon these terms in favor of understanding and communicating drowning as a process that can vary in severity and have a fatal or nonfatal outcome.
Near-drowning
Historically, drowning meant death, while near-drowning meant the victim survived, at least initially (usually for at least 24 hours).
Before 2002, there were 13 different published definitions of near-drowning.21,22 This variability has caused a great deal of confusion when trying to describe and monitor drowning.
A person can drown and survive, just as a person can have cardiac arrest and survive.4,21 Just as there is no recognized condition of “near-cardiac arrest,” there is also no condition of near-drowning. Using near-drowning as a medical diagnosis hides the true burden of drowning and consequently amplifies difficulties in developing effective prevention, rescue, and treatment programs.
Dry drowning
Dry drowning has never been an accepted medical term, although it has been used to describe different parts of the drowning process. While many authors use it as a synonym for secondary drowning (described below), in the past it was usually used in cases in which no water was found in the lungs at autopsy in persons who were found dead in the water.2–4,21 This occurred in about 10% to 15% of cases and was also called drowning “without water aspiration.”
Perhaps some victims suffer sudden cardiac death. It happens on land—why not in the water? Modell et al stated, “In the absence of the common finding of significant pulmonary edema in the victim’s respiratory system, to conclude his or her death was caused by ‘drowning without aspiration’ is unwise.”23
Laryngospasm is another proposed explanation. It could play a role in the fewer than 2% of cases in which no other cause of death is found on clinical examination or autopsy,11,12,19,23 but it does not occur in most cases of drowning, or it is brief and is terminated by the respiratory movements that allow the air in the lung to escape and water to be inhaled.
The problem with the term dry drowning is the harm caused by misdiagnosing cases of sudden death as drowning, when an alternative cause is present. Most importantly, the management is the same if small amounts of water are present or not; therefore, no clinical distinction is made between wet and dry drowning.
Secondary drowning
Secondary drowning, sometimes called delayed drowning, is another term that is not medically accepted. The historical use of this term reflects the reality that some patients may worsen due to pulmonary edema after aspirating small amounts of water.
Drowning starts with aspiration, and few or only mild symptoms may be present as soon as the person is removed from the water. Either the small amount of water in the lungs is absorbed and causes no complications or, rarely, the patient’s condition becomes progressively worse over the next few hours as the alveoli become inflamed and the alveolar-capillary membrane is disrupted. But people do not unexpectedly die of drowning days or weeks later with no preceding symptoms. The lungs and heart do not “fill up with water,” and water does not need to be pumped out of the lungs.
There has never been a case published in the medical literature of a patient who underwent clinical evaluation, was initially without symptoms, and later deteriorated and died more than 8 hours after the incident.6,10,21 People who have drowned and have minimal symptoms get better (usually) or worse (rarely) within 4 to 8 hours. In a study of more than 41,000 lifeguard rescues, only 0.5% of symptomatic patients died.6
Drowning secondary to injury or sudden illness
Any injury, trauma, or sudden illness that can cause loss of consciousness or mental or physical weakness can lead to drowning. Physicians need to recognize these situations to treat them appropriately. Drowning that is secondary to other primary insults can be classified as24:
- Drowning caused by injury or trauma (eg, a surfing, boating, or a hang-gliding accident)
- Drowning caused by a sudden illness such as cardiac disease (eg, myocardial ischemia, arrhythmias, prolonged QT syndrome, hypertrophic cardiomyopathy) or neurologic disease (eg, epilepsy, stroke)
- Diving disease (eg, decompression sickness, pulmonary overpressurization syndrome, compression barotrauma, narcosis [“rapture of the deep”], shallow water blackout, immersion pulmonary edema).
PREVENTION IS BEST
Drowning is a leading and preventable cause of death worldwide and for people of all ages. The danger is real, not esoteric or rare, and healthcare providers should use any opportunity to discuss with patients, parents, and the media the most important tool for treating drowning: primary prevention.
For example, small children should be continuously and uninterruptedly supervised within arm’s reach while in the water, even if a lifeguard is present. Other preventive measures are lifejackets, fences completely enclosing pools or ponds, and swimming and water safety lessons. Drowning often occurs in a deceptively pleasant environment that may not seem dangerous.
RECOGNIZE DISTRESS
When preventive measures fail, responders (usually a health professional is involved) need to be able to perform the necessary steps to interrupt the drowning process.
The first challenge is to recognize when someone in the water is at risk of drowning and needs to be rescued.25 Early self-rescue or rescue by others may stop the drowning process and prevent most cases of initial and subsequent water aspiration, respiratory distress, and medical complications.
DON’T BECOME A VICTIM
Rescuers must take care not to become victims themselves. Panicked swimmers can thrash about and injure the rescuer or clutch at anything they encounter, dragging the rescuer under. And the rescuer can succumb to the same hazards that got the victim into trouble, such as strong currents, deep water, or underwater hazards.
Certified lifeguards are trained to get victims out of the water safely. The American Red Cross slogan “Reach or throw, don’t go” means “Reach out with a pole or other object or throw something that floats; don’t get in the water yourself.”
WHAT TO TELL THE PUBLIC
While some journalists acknowledge that the terms dry drowning and secondary drowning are medically discredited, they still use them in their reports. The novelty of this story—and its appeal to media outlets—is precisely the unfamiliarity of these terms to the general public and the perceived mysterious, looming threat.
We often hear that these terms are more familiar to the public, which is likely true. More concerning, some physicians continue to use them (and older definitions of drowning that equate it with death) in media interviews, clinical care, and publications. The paradox is that we, the medical community, invented these terms, not patients or the media.
As clinicians and researchers, we should drive popular culture definitions, not the other way around. Rather than dismiss these terms as “semantics” or “technicalities,” we should take the opportunity to highlight the dangers of drowning and the importance of prevention, and to promote simpler language that is easier for us and our patients to understand.19,21
Healthcare providers should understand and share modern drowning science and best practices, which will reduce fear, improve resource utilization, and prevent potentially deadly consequences due to misunderstanding or misinterpretation of incorrect terminology.
WHEN PATIENTS SHOULD SEEK CARE
Anyone who experiences cough, breathlessness, or other worrisome symptoms such as abnormal mentation within 8 hours of a drowning incident (using the modern definition above) should seek medical advice immediately.
We tell people to seek care if symptoms seem any worse than the experience of a drink “going down the wrong pipe” at the dinner table.21 But symptoms can be minimal. Careful attention should be given to mild symptoms that get progressively worse during that time. These cases can rarely progress to acute respiratory distress syndrome.
Table 1 explores who needs further medical help after being rescued from the water.26
In most of these cases, it is most appropriate to call an ambulance, but care may involve seeing a doctor depending on the severity of the symptoms.6,21 Usually, drowning patients are observed for 4 to 8 hours in an emergency department and are discharged if normal. Symptoms that are more significant include persistent cough, foam at the mouth or nose, confusion, or abnormal behavior, and these require further medical evaluation.
Patients should also seek medical care even if they are 100% normal upon exiting the water but develop worrisome symptoms more than 8 hours later, and providers should consider diagnoses other than primary drowning. Spontaneous pneumothorax, chemical pneumonitis, bacterial or viral pneumonia, head injury, asthma, chest trauma, and acute respiratory distress syndrome have been mislabeled as delayed, dry, or secondary drowning.3,4,19,21
- Buffington B. Texas boy dies from ‘dry drowning’ days after swimming. USA Today, June 8, 2017. www.usatoday.com/story/news/nation-now/2017/06/08/texas-boy-dies-dry-drowning-days-after-swimming/379944001.
- Schmidt AC, Sempsrott JR, Szpilman D, et al. The use of non-uniform drowning terminology: a follow-up study. Scand J Trauma Resusc Emerg Med 2017; 25(1):72. doi:10.1186/s13049-017-0405-x
- van Beeck EF, Branche CM, Szpilman D, Modell JH, Bierens JJ. A new definition of drowning: towards documentation and prevention of a global public health problem. Bull World Health Organ 2005; 83(11):853–856. pmid:16302042
- Szpilman D, Bierens JJ, Handley AJ, Orlowski JP. Drowning. N Engl J Med 2012; 366(22):2102–2110. doi:10.1056/NEJMra1013317
- World Health Organization. Global report on drowning: preventing a leading killer. www.who.int/violence_injury_prevention/global_report_drowning/en. Accessed June 13, 2018.
- Szpilman D. Near-drowning and drowning classification: a proposal to stratify mortality based on the analysis of 1,831 cases. Chest 1997; 112(3):660–665. pmid:9315798
- Centers for Disease Control and Prevention. Welcome to WISQARS. www.cdc.gov/injury/wisqars. Accessed June 13, 2018.
- Centers for Disease Control and Prevention. WONDER. https://wonder.cdc.gov. Accessed June 13, 2018.
- Cummings P, Quan L. Trends in unintentional drowning: the role of alcohol and medical care. JAMA 1999; 281(23):2198–2202. pmid:10376572
- Szpilman D, Elmann J, Cruz-Filho FES. Drowning classification: a revalidation study based on the analysis of 930 cases over 10 years. World Congress on Drowning, Netherlands 2002. www.researchgate.net/publication/267981062_DROWNING_CLASSIFICATION_a_revalidation_study_based_on_the_analysis_of_930_cases_over_10_years. Accessed June 13, 2018.
- Szpilman D, Elmann J, Cruz-Filho FES. Dry-drowning—fact or myth? World Congress on Drowning. Netherlands, 2002. www.researchgate.net/publication/267981164_Dry-drowning_-Fact_or_Myth. Accessed June 13, 2018.
- Lunetta P, Modell JH, Sajantila A. What is the incidence and significance of "dry-lungs" in bodies found in water? Am J Forensic Med Pathol 2004; 25(4):291–301. pmid:15577518
- Orlowski JP, Abulleil MM, Phillips JM. The hemodynamic and cardiovascular effects of near-drowning in hypotonic, isotonic, or hypertonic solutions. Ann Emerg Med 1989; 18:1044–1049. pmid:2802278
- Grmec S, Strnad M, Podgorsek D. Comparison of the characteristics and outcome among patients suffering from out-of-hospital primary cardiac arrest and drowning victims in cardiac arrest. Int J Emerg Med 2009; 2(1):7–12. doi:10.1007/s12245-009-0084-0
- Tipton MJ, Golden FS. A proposed decision-making guide for the search, rescue and resuscitation of submersion (head under) victims based on expert opinion. Resuscitation 2011; 82(7):819–824. doi:10.1016/j.resuscitation.2011.02.021
- Orlowski JP, Szpilman D. Drowning. Rescue, resuscitation, and reanimation. Pediatr Clin North Am 2001; 48(3):627–646. pmid:11411297
- Modell JH, Moya F, Newby EJ, Ruiz BC, Showers AV. The effects of fluid volume in seawater drowning. Ann Intern Med 1967; 67(1):68–80. pmid:6028660
- Quan L, Wentz KR, Gore EJ, Copass MK. Outcome and predictors of outcome in pediatric submersion victims receiving prehospital care in King County, Washington. Pediatrics 1990; 86(4):586–593. pmid:2216625
- Szpilman D, Orlowski JP, Cruz-Filho FES. Hey “Near-drowning,” you’ve been messing up our minds! World Congress on Drowning. Amsterdam, 2002. www.researchgate.net/publication/267981173_HEY_Near-drowning_YOU%27VE_BEEN_MESSING_UP_OUR_MINDS. Accessed June 13, 2018.
- American College of Emergency Physicians. Death after swimming is extremely rare—and is not “dry drowning.” http://newsroom.acep.org/2017-07-11-Death-After-Swimming-Is-Extremely-Rare-And-Is-NOT-Dry-Drowning. Accessed June 13, 2018.
- Hawkins SC, Sempsrott J, Schmidt A. “Drowning” in a sea of misinformation. Emergency Medicine News 2017; 39*8):1. http://journals.lww.com/em-news/blog/BreakingNews/pages/post.aspx?PostID=377. Accessed June 5, 2018.
- Szpilman D, Tipton M, Sempsrott J, et al. Drowning timeline: a new systematic model of the drowning process. Am J Emerg Med 2016; 34(11):2224–2226. doi:10.1016/j.ajem.2016.07.063
- Modell JH, Bellefleur M, Davis JH. Drowning without aspiration: is this an appropriate diagnosis? J Forensic Sci 1999; 44(6):1119–1123. pmid:10582353
- Szpilman D, Orlowski JP. Sports related to drowning. Eur Respir Rev 2016; 25(141):348–359. doi:10.1183/16000617.0038-2016
- Szpilman D, Webber J, Quan L, et al. Creating a drowning chain of survival. Resuscitation 2014; 85(9):1149–1152. doi:10.1016/j.resuscitation.2014.05.034
- International Life Saving Federation. Who needs further medical help after rescue from the water. Medical Position Statement - MPS 06, 2016. www.ilsf.org/file/3916/download?token=pDnPDCrk. Accessed June 13, 2018.
In June 2017, a 4-year-old boy died 1 week after being knocked over and briefly submerged while playing in knee-deep water. This story was widely reported as a case of a rare occurrence called “dry” or “secondary” drowning, depending on the source.1 The media accounts went viral, spreading fear in parents and others learning about these alleged conditions from the news and social media.
Many alleged cases of dry drowning are reported every year, but each has been found to have a recognized medical source that has a legitimate medically recognized diagnosis (which dry and secondary drowning are not).
Drowning is one of the most common causes of death in children, and so we ought to make sure that the information we share about it is accurate, as it is vital to effective prevention, rescue, and treatment.
Unfortunately, medical providers, medical journals, and the mass media continue to disseminate misinformation on drowning.2 These reports often prevail over updated information and hinder accurate understanding of the drowning problem and its solutions.
Every death is tragic, especially the death of a child, and our heartfelt sympathies go out to the family in this alleged drowning case, as well as to all families suffering the loss of a loved one to drowning. However, in the 2017 case, the cause of death was found on autopsy to be myocarditis not related in any way to drowning. As often happens in such situations, this clarification did not receive any media attention, despite the wide reporting and penetration of the original, erroneous story.
We hope our review will reduce misunderstanding among the public and healthcare providers, contribute to improved data collection, and help to promote interventions aimed at prevention, rescue, and mitigation of drowning incidents.
WHAT IS DROWNING?
A consensus committee of the World Health Organization defined drowning as “the process of experiencing respiratory impairment from submersion/immersion in liquid.”3 The process begins when the victim’s airway goes below the surface of the liquid (submersion) or when water splashes over the face (immersion). If the victim is rescued at any time, the process is interrupted, and this is termed a nonfatal drowning. If the victim dies at any time, this is a fatal drowning. Any water-distress incident without evidence of respiratory impairment (ie, without aspiration) should be considered a water rescue and not a drowning.
Rarely do minimally symptomatic cases progress to death, just as most cases of chest pain do not progress to cardiac arrest.4 Nonetheless, rescued drowning victims can deteriorate, which is why we encourage people to seek medical care immediately upon warning signs, as we do with chest pain. For drowning, such warning signs are any water distress followed by difficulty breathing, excessive coughing, foam in the mouth, or abnormal behavior.
A SERIOUS PUBLIC HEALTH ISSUE
Drowning is a serious and neglected public health issue, claiming the lives of 372,000 people a year worldwide.5 It is a leading cause of death in children ages 1 to 14. The toll continues largely unabated, and in low- and middle-income nations it does not attract the levels of funding that go to other forms of injury prevention, such as road safety.
Nonfatal drowning—with symptoms ranging from mild cough to severe pulmonary edema, and complications ranging from none to severe neurologic impairment—is far more common than fatal drowning.6 For every fatal drowning, there are at least 5 nonfatal drowning incidents in which medical care is needed, and 200 rescues are performed.7–10
In the United States, drowning accounts for almost 13,000 emergency department visits per year and about 3,500 deaths.7,8
In Brazil, with two-thirds the population of the United States, drowning accounts for far fewer hospital visits but about twice as many deaths. In Rio de Janeiro, where a highly effective and specialized prehospital service is provided at 3 drowning resuscitation centers staffed by medical doctors, an analysis of the 46,060 cases of rescue in 10 years from 1991 to 2000 showed that medical assistance was needed in only 930 cases (2%).10 The preventive and rescue actions of parents, bystanders, lifeguards, and prehospital rescue services significantly reduce the number of drowning deaths, but these groups do not consistently gather data on nonfatal drowning that can be included in a comprehensive database.
DROWNING IS A PROCESS
When a person in the water can no longer keep the airway clear, water that enters the mouth is voluntarily spit out or swallowed. Within a few seconds to minutes, the person can no longer clear the airways and water is aspirated, stimulating the cough reflex. Laryngospasm, another myth concerning drowning, is presumed to protect the airways but does not, as it is rare, occurring in less than 2% of cases.11,12
If the person is not rescued, aspiration of water continues, and hypoxemia leads to loss of consciousness and apnea within seconds to a few minutes, followed by cardiac arrest. As a consequence, hypoxemic cardiac arrest generally occurs after a period of tachycardia followed by bradycardia and pulseless electrical activity, usually leading to asystole.13,14
The entire drowning process, from water distress to cardiac arrest, usually takes a few minutes, but in rare situations, such as rapid hypothermia, it can go on for up to an hour.15 Most drowning patients have an otherwise healthy heart, and the apnea and hypoxemia precede the cardiac arrest by only a few seconds to minutes; thus, cardiac arrest is caused by the hypoxemic insult and not by ventricular dysrhythmias.6,16
Drowning can be interrupted at any point between distress and death. If the person is rescued early, the clinical picture is determined by the reactivity of the airway and the amount of water that has been aspirated, but not by the type of water (salt or fresh).
Another myth is that drowning in salt water is different from drowning in fresh water. Both salt water and fresh water cause similar surfactant destruction and washout and disrupt the alveolar-capillary membrane. Disruption of the alveolar-capillary membrane increases its permeability and exacerbates shifting of fluid, plasma, and electrolytes into the alveoli.13 The clinical picture of the damage is one of regional or generalized pulmonary edema, which interferes with gas exchange in the lungs.6,13,17
Animal studies by Modell et al showed that aspiration of just 2.2 mL of water per kilogram of body weight is sufficient to cause severe disturbances in oxygen exchange,17 reflected in a rise in arterial pH and a drop in partial pressure of oxygen. The situation must be similar in humans. In a 70-kg person, this is only about 154 mL of water—about two-thirds of a cup.
The combined effects of fluid in the lungs, the loss of surfactant, and the increase in capillary-alveolar permeability can result in decreased lung compliance, increased right-to-left shunting in the lungs, atelectasis, alveolitis, hypoxemia, and cerebral hypoxia.13
If the victim needs cardiopulmonary resuscitation, the possibility of neurologic damage is similar to that in other cardiac arrest situations, but exceptions exist. For example, in rare cases, hypothermia provides a protective mechanism that allows victims to survive prolonged submersion.4,15
The duration of submersion is the best predictor of death.18 Underwater, people are not taking in oxygen, and cerebral hypoxia causes both morbidity and death. For this reason, reversing cerebral hypoxia with effective ventilation, oxygen, and chest compression is the priority of treatment.
MYTHS AND SLOPPY TERMINOLOGY
“Near drowning,” “dry drowning,” “wet drowning,” “delayed drowning,” and “secondary drowning” are not medically accepted diagnoses,3,4,19 and many organizations and lifesaving institutions around the world discourage the use of these terms.19,20 Unfortunately, these terms still slip past the editors of medical journals and are thus perpetuated. The terms are most pervasive in the nonmedical media, where drowning seems to be synonymous with death.3,19,21 We urge all authors and stakeholders to abandon these terms in favor of understanding and communicating drowning as a process that can vary in severity and have a fatal or nonfatal outcome.
Near-drowning
Historically, drowning meant death, while near-drowning meant the victim survived, at least initially (usually for at least 24 hours).
Before 2002, there were 13 different published definitions of near-drowning.21,22 This variability has caused a great deal of confusion when trying to describe and monitor drowning.
A person can drown and survive, just as a person can have cardiac arrest and survive.4,21 Just as there is no recognized condition of “near-cardiac arrest,” there is also no condition of near-drowning. Using near-drowning as a medical diagnosis hides the true burden of drowning and consequently amplifies difficulties in developing effective prevention, rescue, and treatment programs.
Dry drowning
Dry drowning has never been an accepted medical term, although it has been used to describe different parts of the drowning process. While many authors use it as a synonym for secondary drowning (described below), in the past it was usually used in cases in which no water was found in the lungs at autopsy in persons who were found dead in the water.2–4,21 This occurred in about 10% to 15% of cases and was also called drowning “without water aspiration.”
Perhaps some victims suffer sudden cardiac death. It happens on land—why not in the water? Modell et al stated, “In the absence of the common finding of significant pulmonary edema in the victim’s respiratory system, to conclude his or her death was caused by ‘drowning without aspiration’ is unwise.”23
Laryngospasm is another proposed explanation. It could play a role in the fewer than 2% of cases in which no other cause of death is found on clinical examination or autopsy,11,12,19,23 but it does not occur in most cases of drowning, or it is brief and is terminated by the respiratory movements that allow the air in the lung to escape and water to be inhaled.
The problem with the term dry drowning is the harm caused by misdiagnosing cases of sudden death as drowning, when an alternative cause is present. Most importantly, the management is the same if small amounts of water are present or not; therefore, no clinical distinction is made between wet and dry drowning.
Secondary drowning
Secondary drowning, sometimes called delayed drowning, is another term that is not medically accepted. The historical use of this term reflects the reality that some patients may worsen due to pulmonary edema after aspirating small amounts of water.
Drowning starts with aspiration, and few or only mild symptoms may be present as soon as the person is removed from the water. Either the small amount of water in the lungs is absorbed and causes no complications or, rarely, the patient’s condition becomes progressively worse over the next few hours as the alveoli become inflamed and the alveolar-capillary membrane is disrupted. But people do not unexpectedly die of drowning days or weeks later with no preceding symptoms. The lungs and heart do not “fill up with water,” and water does not need to be pumped out of the lungs.
There has never been a case published in the medical literature of a patient who underwent clinical evaluation, was initially without symptoms, and later deteriorated and died more than 8 hours after the incident.6,10,21 People who have drowned and have minimal symptoms get better (usually) or worse (rarely) within 4 to 8 hours. In a study of more than 41,000 lifeguard rescues, only 0.5% of symptomatic patients died.6
Drowning secondary to injury or sudden illness
Any injury, trauma, or sudden illness that can cause loss of consciousness or mental or physical weakness can lead to drowning. Physicians need to recognize these situations to treat them appropriately. Drowning that is secondary to other primary insults can be classified as24:
- Drowning caused by injury or trauma (eg, a surfing, boating, or a hang-gliding accident)
- Drowning caused by a sudden illness such as cardiac disease (eg, myocardial ischemia, arrhythmias, prolonged QT syndrome, hypertrophic cardiomyopathy) or neurologic disease (eg, epilepsy, stroke)
- Diving disease (eg, decompression sickness, pulmonary overpressurization syndrome, compression barotrauma, narcosis [“rapture of the deep”], shallow water blackout, immersion pulmonary edema).
PREVENTION IS BEST
Drowning is a leading and preventable cause of death worldwide and for people of all ages. The danger is real, not esoteric or rare, and healthcare providers should use any opportunity to discuss with patients, parents, and the media the most important tool for treating drowning: primary prevention.
For example, small children should be continuously and uninterruptedly supervised within arm’s reach while in the water, even if a lifeguard is present. Other preventive measures are lifejackets, fences completely enclosing pools or ponds, and swimming and water safety lessons. Drowning often occurs in a deceptively pleasant environment that may not seem dangerous.
RECOGNIZE DISTRESS
When preventive measures fail, responders (usually a health professional is involved) need to be able to perform the necessary steps to interrupt the drowning process.
The first challenge is to recognize when someone in the water is at risk of drowning and needs to be rescued.25 Early self-rescue or rescue by others may stop the drowning process and prevent most cases of initial and subsequent water aspiration, respiratory distress, and medical complications.
DON’T BECOME A VICTIM
Rescuers must take care not to become victims themselves. Panicked swimmers can thrash about and injure the rescuer or clutch at anything they encounter, dragging the rescuer under. And the rescuer can succumb to the same hazards that got the victim into trouble, such as strong currents, deep water, or underwater hazards.
Certified lifeguards are trained to get victims out of the water safely. The American Red Cross slogan “Reach or throw, don’t go” means “Reach out with a pole or other object or throw something that floats; don’t get in the water yourself.”
WHAT TO TELL THE PUBLIC
While some journalists acknowledge that the terms dry drowning and secondary drowning are medically discredited, they still use them in their reports. The novelty of this story—and its appeal to media outlets—is precisely the unfamiliarity of these terms to the general public and the perceived mysterious, looming threat.
We often hear that these terms are more familiar to the public, which is likely true. More concerning, some physicians continue to use them (and older definitions of drowning that equate it with death) in media interviews, clinical care, and publications. The paradox is that we, the medical community, invented these terms, not patients or the media.
As clinicians and researchers, we should drive popular culture definitions, not the other way around. Rather than dismiss these terms as “semantics” or “technicalities,” we should take the opportunity to highlight the dangers of drowning and the importance of prevention, and to promote simpler language that is easier for us and our patients to understand.19,21
Healthcare providers should understand and share modern drowning science and best practices, which will reduce fear, improve resource utilization, and prevent potentially deadly consequences due to misunderstanding or misinterpretation of incorrect terminology.
WHEN PATIENTS SHOULD SEEK CARE
Anyone who experiences cough, breathlessness, or other worrisome symptoms such as abnormal mentation within 8 hours of a drowning incident (using the modern definition above) should seek medical advice immediately.
We tell people to seek care if symptoms seem any worse than the experience of a drink “going down the wrong pipe” at the dinner table.21 But symptoms can be minimal. Careful attention should be given to mild symptoms that get progressively worse during that time. These cases can rarely progress to acute respiratory distress syndrome.
Table 1 explores who needs further medical help after being rescued from the water.26
In most of these cases, it is most appropriate to call an ambulance, but care may involve seeing a doctor depending on the severity of the symptoms.6,21 Usually, drowning patients are observed for 4 to 8 hours in an emergency department and are discharged if normal. Symptoms that are more significant include persistent cough, foam at the mouth or nose, confusion, or abnormal behavior, and these require further medical evaluation.
Patients should also seek medical care even if they are 100% normal upon exiting the water but develop worrisome symptoms more than 8 hours later, and providers should consider diagnoses other than primary drowning. Spontaneous pneumothorax, chemical pneumonitis, bacterial or viral pneumonia, head injury, asthma, chest trauma, and acute respiratory distress syndrome have been mislabeled as delayed, dry, or secondary drowning.3,4,19,21
In June 2017, a 4-year-old boy died 1 week after being knocked over and briefly submerged while playing in knee-deep water. This story was widely reported as a case of a rare occurrence called “dry” or “secondary” drowning, depending on the source.1 The media accounts went viral, spreading fear in parents and others learning about these alleged conditions from the news and social media.
Many alleged cases of dry drowning are reported every year, but each has been found to have a recognized medical source that has a legitimate medically recognized diagnosis (which dry and secondary drowning are not).
Drowning is one of the most common causes of death in children, and so we ought to make sure that the information we share about it is accurate, as it is vital to effective prevention, rescue, and treatment.
Unfortunately, medical providers, medical journals, and the mass media continue to disseminate misinformation on drowning.2 These reports often prevail over updated information and hinder accurate understanding of the drowning problem and its solutions.
Every death is tragic, especially the death of a child, and our heartfelt sympathies go out to the family in this alleged drowning case, as well as to all families suffering the loss of a loved one to drowning. However, in the 2017 case, the cause of death was found on autopsy to be myocarditis not related in any way to drowning. As often happens in such situations, this clarification did not receive any media attention, despite the wide reporting and penetration of the original, erroneous story.
We hope our review will reduce misunderstanding among the public and healthcare providers, contribute to improved data collection, and help to promote interventions aimed at prevention, rescue, and mitigation of drowning incidents.
WHAT IS DROWNING?
A consensus committee of the World Health Organization defined drowning as “the process of experiencing respiratory impairment from submersion/immersion in liquid.”3 The process begins when the victim’s airway goes below the surface of the liquid (submersion) or when water splashes over the face (immersion). If the victim is rescued at any time, the process is interrupted, and this is termed a nonfatal drowning. If the victim dies at any time, this is a fatal drowning. Any water-distress incident without evidence of respiratory impairment (ie, without aspiration) should be considered a water rescue and not a drowning.
Rarely do minimally symptomatic cases progress to death, just as most cases of chest pain do not progress to cardiac arrest.4 Nonetheless, rescued drowning victims can deteriorate, which is why we encourage people to seek medical care immediately upon warning signs, as we do with chest pain. For drowning, such warning signs are any water distress followed by difficulty breathing, excessive coughing, foam in the mouth, or abnormal behavior.
A SERIOUS PUBLIC HEALTH ISSUE
Drowning is a serious and neglected public health issue, claiming the lives of 372,000 people a year worldwide.5 It is a leading cause of death in children ages 1 to 14. The toll continues largely unabated, and in low- and middle-income nations it does not attract the levels of funding that go to other forms of injury prevention, such as road safety.
Nonfatal drowning—with symptoms ranging from mild cough to severe pulmonary edema, and complications ranging from none to severe neurologic impairment—is far more common than fatal drowning.6 For every fatal drowning, there are at least 5 nonfatal drowning incidents in which medical care is needed, and 200 rescues are performed.7–10
In the United States, drowning accounts for almost 13,000 emergency department visits per year and about 3,500 deaths.7,8
In Brazil, with two-thirds the population of the United States, drowning accounts for far fewer hospital visits but about twice as many deaths. In Rio de Janeiro, where a highly effective and specialized prehospital service is provided at 3 drowning resuscitation centers staffed by medical doctors, an analysis of the 46,060 cases of rescue in 10 years from 1991 to 2000 showed that medical assistance was needed in only 930 cases (2%).10 The preventive and rescue actions of parents, bystanders, lifeguards, and prehospital rescue services significantly reduce the number of drowning deaths, but these groups do not consistently gather data on nonfatal drowning that can be included in a comprehensive database.
DROWNING IS A PROCESS
When a person in the water can no longer keep the airway clear, water that enters the mouth is voluntarily spit out or swallowed. Within a few seconds to minutes, the person can no longer clear the airways and water is aspirated, stimulating the cough reflex. Laryngospasm, another myth concerning drowning, is presumed to protect the airways but does not, as it is rare, occurring in less than 2% of cases.11,12
If the person is not rescued, aspiration of water continues, and hypoxemia leads to loss of consciousness and apnea within seconds to a few minutes, followed by cardiac arrest. As a consequence, hypoxemic cardiac arrest generally occurs after a period of tachycardia followed by bradycardia and pulseless electrical activity, usually leading to asystole.13,14
The entire drowning process, from water distress to cardiac arrest, usually takes a few minutes, but in rare situations, such as rapid hypothermia, it can go on for up to an hour.15 Most drowning patients have an otherwise healthy heart, and the apnea and hypoxemia precede the cardiac arrest by only a few seconds to minutes; thus, cardiac arrest is caused by the hypoxemic insult and not by ventricular dysrhythmias.6,16
Drowning can be interrupted at any point between distress and death. If the person is rescued early, the clinical picture is determined by the reactivity of the airway and the amount of water that has been aspirated, but not by the type of water (salt or fresh).
Another myth is that drowning in salt water is different from drowning in fresh water. Both salt water and fresh water cause similar surfactant destruction and washout and disrupt the alveolar-capillary membrane. Disruption of the alveolar-capillary membrane increases its permeability and exacerbates shifting of fluid, plasma, and electrolytes into the alveoli.13 The clinical picture of the damage is one of regional or generalized pulmonary edema, which interferes with gas exchange in the lungs.6,13,17
Animal studies by Modell et al showed that aspiration of just 2.2 mL of water per kilogram of body weight is sufficient to cause severe disturbances in oxygen exchange,17 reflected in a rise in arterial pH and a drop in partial pressure of oxygen. The situation must be similar in humans. In a 70-kg person, this is only about 154 mL of water—about two-thirds of a cup.
The combined effects of fluid in the lungs, the loss of surfactant, and the increase in capillary-alveolar permeability can result in decreased lung compliance, increased right-to-left shunting in the lungs, atelectasis, alveolitis, hypoxemia, and cerebral hypoxia.13
If the victim needs cardiopulmonary resuscitation, the possibility of neurologic damage is similar to that in other cardiac arrest situations, but exceptions exist. For example, in rare cases, hypothermia provides a protective mechanism that allows victims to survive prolonged submersion.4,15
The duration of submersion is the best predictor of death.18 Underwater, people are not taking in oxygen, and cerebral hypoxia causes both morbidity and death. For this reason, reversing cerebral hypoxia with effective ventilation, oxygen, and chest compression is the priority of treatment.
MYTHS AND SLOPPY TERMINOLOGY
“Near drowning,” “dry drowning,” “wet drowning,” “delayed drowning,” and “secondary drowning” are not medically accepted diagnoses,3,4,19 and many organizations and lifesaving institutions around the world discourage the use of these terms.19,20 Unfortunately, these terms still slip past the editors of medical journals and are thus perpetuated. The terms are most pervasive in the nonmedical media, where drowning seems to be synonymous with death.3,19,21 We urge all authors and stakeholders to abandon these terms in favor of understanding and communicating drowning as a process that can vary in severity and have a fatal or nonfatal outcome.
Near-drowning
Historically, drowning meant death, while near-drowning meant the victim survived, at least initially (usually for at least 24 hours).
Before 2002, there were 13 different published definitions of near-drowning.21,22 This variability has caused a great deal of confusion when trying to describe and monitor drowning.
A person can drown and survive, just as a person can have cardiac arrest and survive.4,21 Just as there is no recognized condition of “near-cardiac arrest,” there is also no condition of near-drowning. Using near-drowning as a medical diagnosis hides the true burden of drowning and consequently amplifies difficulties in developing effective prevention, rescue, and treatment programs.
Dry drowning
Dry drowning has never been an accepted medical term, although it has been used to describe different parts of the drowning process. While many authors use it as a synonym for secondary drowning (described below), in the past it was usually used in cases in which no water was found in the lungs at autopsy in persons who were found dead in the water.2–4,21 This occurred in about 10% to 15% of cases and was also called drowning “without water aspiration.”
Perhaps some victims suffer sudden cardiac death. It happens on land—why not in the water? Modell et al stated, “In the absence of the common finding of significant pulmonary edema in the victim’s respiratory system, to conclude his or her death was caused by ‘drowning without aspiration’ is unwise.”23
Laryngospasm is another proposed explanation. It could play a role in the fewer than 2% of cases in which no other cause of death is found on clinical examination or autopsy,11,12,19,23 but it does not occur in most cases of drowning, or it is brief and is terminated by the respiratory movements that allow the air in the lung to escape and water to be inhaled.
The problem with the term dry drowning is the harm caused by misdiagnosing cases of sudden death as drowning, when an alternative cause is present. Most importantly, the management is the same if small amounts of water are present or not; therefore, no clinical distinction is made between wet and dry drowning.
Secondary drowning
Secondary drowning, sometimes called delayed drowning, is another term that is not medically accepted. The historical use of this term reflects the reality that some patients may worsen due to pulmonary edema after aspirating small amounts of water.
Drowning starts with aspiration, and few or only mild symptoms may be present as soon as the person is removed from the water. Either the small amount of water in the lungs is absorbed and causes no complications or, rarely, the patient’s condition becomes progressively worse over the next few hours as the alveoli become inflamed and the alveolar-capillary membrane is disrupted. But people do not unexpectedly die of drowning days or weeks later with no preceding symptoms. The lungs and heart do not “fill up with water,” and water does not need to be pumped out of the lungs.
There has never been a case published in the medical literature of a patient who underwent clinical evaluation, was initially without symptoms, and later deteriorated and died more than 8 hours after the incident.6,10,21 People who have drowned and have minimal symptoms get better (usually) or worse (rarely) within 4 to 8 hours. In a study of more than 41,000 lifeguard rescues, only 0.5% of symptomatic patients died.6
Drowning secondary to injury or sudden illness
Any injury, trauma, or sudden illness that can cause loss of consciousness or mental or physical weakness can lead to drowning. Physicians need to recognize these situations to treat them appropriately. Drowning that is secondary to other primary insults can be classified as24:
- Drowning caused by injury or trauma (eg, a surfing, boating, or a hang-gliding accident)
- Drowning caused by a sudden illness such as cardiac disease (eg, myocardial ischemia, arrhythmias, prolonged QT syndrome, hypertrophic cardiomyopathy) or neurologic disease (eg, epilepsy, stroke)
- Diving disease (eg, decompression sickness, pulmonary overpressurization syndrome, compression barotrauma, narcosis [“rapture of the deep”], shallow water blackout, immersion pulmonary edema).
PREVENTION IS BEST
Drowning is a leading and preventable cause of death worldwide and for people of all ages. The danger is real, not esoteric or rare, and healthcare providers should use any opportunity to discuss with patients, parents, and the media the most important tool for treating drowning: primary prevention.
For example, small children should be continuously and uninterruptedly supervised within arm’s reach while in the water, even if a lifeguard is present. Other preventive measures are lifejackets, fences completely enclosing pools or ponds, and swimming and water safety lessons. Drowning often occurs in a deceptively pleasant environment that may not seem dangerous.
RECOGNIZE DISTRESS
When preventive measures fail, responders (usually a health professional is involved) need to be able to perform the necessary steps to interrupt the drowning process.
The first challenge is to recognize when someone in the water is at risk of drowning and needs to be rescued.25 Early self-rescue or rescue by others may stop the drowning process and prevent most cases of initial and subsequent water aspiration, respiratory distress, and medical complications.
DON’T BECOME A VICTIM
Rescuers must take care not to become victims themselves. Panicked swimmers can thrash about and injure the rescuer or clutch at anything they encounter, dragging the rescuer under. And the rescuer can succumb to the same hazards that got the victim into trouble, such as strong currents, deep water, or underwater hazards.
Certified lifeguards are trained to get victims out of the water safely. The American Red Cross slogan “Reach or throw, don’t go” means “Reach out with a pole or other object or throw something that floats; don’t get in the water yourself.”
WHAT TO TELL THE PUBLIC
While some journalists acknowledge that the terms dry drowning and secondary drowning are medically discredited, they still use them in their reports. The novelty of this story—and its appeal to media outlets—is precisely the unfamiliarity of these terms to the general public and the perceived mysterious, looming threat.
We often hear that these terms are more familiar to the public, which is likely true. More concerning, some physicians continue to use them (and older definitions of drowning that equate it with death) in media interviews, clinical care, and publications. The paradox is that we, the medical community, invented these terms, not patients or the media.
As clinicians and researchers, we should drive popular culture definitions, not the other way around. Rather than dismiss these terms as “semantics” or “technicalities,” we should take the opportunity to highlight the dangers of drowning and the importance of prevention, and to promote simpler language that is easier for us and our patients to understand.19,21
Healthcare providers should understand and share modern drowning science and best practices, which will reduce fear, improve resource utilization, and prevent potentially deadly consequences due to misunderstanding or misinterpretation of incorrect terminology.
WHEN PATIENTS SHOULD SEEK CARE
Anyone who experiences cough, breathlessness, or other worrisome symptoms such as abnormal mentation within 8 hours of a drowning incident (using the modern definition above) should seek medical advice immediately.
We tell people to seek care if symptoms seem any worse than the experience of a drink “going down the wrong pipe” at the dinner table.21 But symptoms can be minimal. Careful attention should be given to mild symptoms that get progressively worse during that time. These cases can rarely progress to acute respiratory distress syndrome.
Table 1 explores who needs further medical help after being rescued from the water.26
In most of these cases, it is most appropriate to call an ambulance, but care may involve seeing a doctor depending on the severity of the symptoms.6,21 Usually, drowning patients are observed for 4 to 8 hours in an emergency department and are discharged if normal. Symptoms that are more significant include persistent cough, foam at the mouth or nose, confusion, or abnormal behavior, and these require further medical evaluation.
Patients should also seek medical care even if they are 100% normal upon exiting the water but develop worrisome symptoms more than 8 hours later, and providers should consider diagnoses other than primary drowning. Spontaneous pneumothorax, chemical pneumonitis, bacterial or viral pneumonia, head injury, asthma, chest trauma, and acute respiratory distress syndrome have been mislabeled as delayed, dry, or secondary drowning.3,4,19,21
- Buffington B. Texas boy dies from ‘dry drowning’ days after swimming. USA Today, June 8, 2017. www.usatoday.com/story/news/nation-now/2017/06/08/texas-boy-dies-dry-drowning-days-after-swimming/379944001.
- Schmidt AC, Sempsrott JR, Szpilman D, et al. The use of non-uniform drowning terminology: a follow-up study. Scand J Trauma Resusc Emerg Med 2017; 25(1):72. doi:10.1186/s13049-017-0405-x
- van Beeck EF, Branche CM, Szpilman D, Modell JH, Bierens JJ. A new definition of drowning: towards documentation and prevention of a global public health problem. Bull World Health Organ 2005; 83(11):853–856. pmid:16302042
- Szpilman D, Bierens JJ, Handley AJ, Orlowski JP. Drowning. N Engl J Med 2012; 366(22):2102–2110. doi:10.1056/NEJMra1013317
- World Health Organization. Global report on drowning: preventing a leading killer. www.who.int/violence_injury_prevention/global_report_drowning/en. Accessed June 13, 2018.
- Szpilman D. Near-drowning and drowning classification: a proposal to stratify mortality based on the analysis of 1,831 cases. Chest 1997; 112(3):660–665. pmid:9315798
- Centers for Disease Control and Prevention. Welcome to WISQARS. www.cdc.gov/injury/wisqars. Accessed June 13, 2018.
- Centers for Disease Control and Prevention. WONDER. https://wonder.cdc.gov. Accessed June 13, 2018.
- Cummings P, Quan L. Trends in unintentional drowning: the role of alcohol and medical care. JAMA 1999; 281(23):2198–2202. pmid:10376572
- Szpilman D, Elmann J, Cruz-Filho FES. Drowning classification: a revalidation study based on the analysis of 930 cases over 10 years. World Congress on Drowning, Netherlands 2002. www.researchgate.net/publication/267981062_DROWNING_CLASSIFICATION_a_revalidation_study_based_on_the_analysis_of_930_cases_over_10_years. Accessed June 13, 2018.
- Szpilman D, Elmann J, Cruz-Filho FES. Dry-drowning—fact or myth? World Congress on Drowning. Netherlands, 2002. www.researchgate.net/publication/267981164_Dry-drowning_-Fact_or_Myth. Accessed June 13, 2018.
- Lunetta P, Modell JH, Sajantila A. What is the incidence and significance of "dry-lungs" in bodies found in water? Am J Forensic Med Pathol 2004; 25(4):291–301. pmid:15577518
- Orlowski JP, Abulleil MM, Phillips JM. The hemodynamic and cardiovascular effects of near-drowning in hypotonic, isotonic, or hypertonic solutions. Ann Emerg Med 1989; 18:1044–1049. pmid:2802278
- Grmec S, Strnad M, Podgorsek D. Comparison of the characteristics and outcome among patients suffering from out-of-hospital primary cardiac arrest and drowning victims in cardiac arrest. Int J Emerg Med 2009; 2(1):7–12. doi:10.1007/s12245-009-0084-0
- Tipton MJ, Golden FS. A proposed decision-making guide for the search, rescue and resuscitation of submersion (head under) victims based on expert opinion. Resuscitation 2011; 82(7):819–824. doi:10.1016/j.resuscitation.2011.02.021
- Orlowski JP, Szpilman D. Drowning. Rescue, resuscitation, and reanimation. Pediatr Clin North Am 2001; 48(3):627–646. pmid:11411297
- Modell JH, Moya F, Newby EJ, Ruiz BC, Showers AV. The effects of fluid volume in seawater drowning. Ann Intern Med 1967; 67(1):68–80. pmid:6028660
- Quan L, Wentz KR, Gore EJ, Copass MK. Outcome and predictors of outcome in pediatric submersion victims receiving prehospital care in King County, Washington. Pediatrics 1990; 86(4):586–593. pmid:2216625
- Szpilman D, Orlowski JP, Cruz-Filho FES. Hey “Near-drowning,” you’ve been messing up our minds! World Congress on Drowning. Amsterdam, 2002. www.researchgate.net/publication/267981173_HEY_Near-drowning_YOU%27VE_BEEN_MESSING_UP_OUR_MINDS. Accessed June 13, 2018.
- American College of Emergency Physicians. Death after swimming is extremely rare—and is not “dry drowning.” http://newsroom.acep.org/2017-07-11-Death-After-Swimming-Is-Extremely-Rare-And-Is-NOT-Dry-Drowning. Accessed June 13, 2018.
- Hawkins SC, Sempsrott J, Schmidt A. “Drowning” in a sea of misinformation. Emergency Medicine News 2017; 39*8):1. http://journals.lww.com/em-news/blog/BreakingNews/pages/post.aspx?PostID=377. Accessed June 5, 2018.
- Szpilman D, Tipton M, Sempsrott J, et al. Drowning timeline: a new systematic model of the drowning process. Am J Emerg Med 2016; 34(11):2224–2226. doi:10.1016/j.ajem.2016.07.063
- Modell JH, Bellefleur M, Davis JH. Drowning without aspiration: is this an appropriate diagnosis? J Forensic Sci 1999; 44(6):1119–1123. pmid:10582353
- Szpilman D, Orlowski JP. Sports related to drowning. Eur Respir Rev 2016; 25(141):348–359. doi:10.1183/16000617.0038-2016
- Szpilman D, Webber J, Quan L, et al. Creating a drowning chain of survival. Resuscitation 2014; 85(9):1149–1152. doi:10.1016/j.resuscitation.2014.05.034
- International Life Saving Federation. Who needs further medical help after rescue from the water. Medical Position Statement - MPS 06, 2016. www.ilsf.org/file/3916/download?token=pDnPDCrk. Accessed June 13, 2018.
- Buffington B. Texas boy dies from ‘dry drowning’ days after swimming. USA Today, June 8, 2017. www.usatoday.com/story/news/nation-now/2017/06/08/texas-boy-dies-dry-drowning-days-after-swimming/379944001.
- Schmidt AC, Sempsrott JR, Szpilman D, et al. The use of non-uniform drowning terminology: a follow-up study. Scand J Trauma Resusc Emerg Med 2017; 25(1):72. doi:10.1186/s13049-017-0405-x
- van Beeck EF, Branche CM, Szpilman D, Modell JH, Bierens JJ. A new definition of drowning: towards documentation and prevention of a global public health problem. Bull World Health Organ 2005; 83(11):853–856. pmid:16302042
- Szpilman D, Bierens JJ, Handley AJ, Orlowski JP. Drowning. N Engl J Med 2012; 366(22):2102–2110. doi:10.1056/NEJMra1013317
- World Health Organization. Global report on drowning: preventing a leading killer. www.who.int/violence_injury_prevention/global_report_drowning/en. Accessed June 13, 2018.
- Szpilman D. Near-drowning and drowning classification: a proposal to stratify mortality based on the analysis of 1,831 cases. Chest 1997; 112(3):660–665. pmid:9315798
- Centers for Disease Control and Prevention. Welcome to WISQARS. www.cdc.gov/injury/wisqars. Accessed June 13, 2018.
- Centers for Disease Control and Prevention. WONDER. https://wonder.cdc.gov. Accessed June 13, 2018.
- Cummings P, Quan L. Trends in unintentional drowning: the role of alcohol and medical care. JAMA 1999; 281(23):2198–2202. pmid:10376572
- Szpilman D, Elmann J, Cruz-Filho FES. Drowning classification: a revalidation study based on the analysis of 930 cases over 10 years. World Congress on Drowning, Netherlands 2002. www.researchgate.net/publication/267981062_DROWNING_CLASSIFICATION_a_revalidation_study_based_on_the_analysis_of_930_cases_over_10_years. Accessed June 13, 2018.
- Szpilman D, Elmann J, Cruz-Filho FES. Dry-drowning—fact or myth? World Congress on Drowning. Netherlands, 2002. www.researchgate.net/publication/267981164_Dry-drowning_-Fact_or_Myth. Accessed June 13, 2018.
- Lunetta P, Modell JH, Sajantila A. What is the incidence and significance of "dry-lungs" in bodies found in water? Am J Forensic Med Pathol 2004; 25(4):291–301. pmid:15577518
- Orlowski JP, Abulleil MM, Phillips JM. The hemodynamic and cardiovascular effects of near-drowning in hypotonic, isotonic, or hypertonic solutions. Ann Emerg Med 1989; 18:1044–1049. pmid:2802278
- Grmec S, Strnad M, Podgorsek D. Comparison of the characteristics and outcome among patients suffering from out-of-hospital primary cardiac arrest and drowning victims in cardiac arrest. Int J Emerg Med 2009; 2(1):7–12. doi:10.1007/s12245-009-0084-0
- Tipton MJ, Golden FS. A proposed decision-making guide for the search, rescue and resuscitation of submersion (head under) victims based on expert opinion. Resuscitation 2011; 82(7):819–824. doi:10.1016/j.resuscitation.2011.02.021
- Orlowski JP, Szpilman D. Drowning. Rescue, resuscitation, and reanimation. Pediatr Clin North Am 2001; 48(3):627–646. pmid:11411297
- Modell JH, Moya F, Newby EJ, Ruiz BC, Showers AV. The effects of fluid volume in seawater drowning. Ann Intern Med 1967; 67(1):68–80. pmid:6028660
- Quan L, Wentz KR, Gore EJ, Copass MK. Outcome and predictors of outcome in pediatric submersion victims receiving prehospital care in King County, Washington. Pediatrics 1990; 86(4):586–593. pmid:2216625
- Szpilman D, Orlowski JP, Cruz-Filho FES. Hey “Near-drowning,” you’ve been messing up our minds! World Congress on Drowning. Amsterdam, 2002. www.researchgate.net/publication/267981173_HEY_Near-drowning_YOU%27VE_BEEN_MESSING_UP_OUR_MINDS. Accessed June 13, 2018.
- American College of Emergency Physicians. Death after swimming is extremely rare—and is not “dry drowning.” http://newsroom.acep.org/2017-07-11-Death-After-Swimming-Is-Extremely-Rare-And-Is-NOT-Dry-Drowning. Accessed June 13, 2018.
- Hawkins SC, Sempsrott J, Schmidt A. “Drowning” in a sea of misinformation. Emergency Medicine News 2017; 39*8):1. http://journals.lww.com/em-news/blog/BreakingNews/pages/post.aspx?PostID=377. Accessed June 5, 2018.
- Szpilman D, Tipton M, Sempsrott J, et al. Drowning timeline: a new systematic model of the drowning process. Am J Emerg Med 2016; 34(11):2224–2226. doi:10.1016/j.ajem.2016.07.063
- Modell JH, Bellefleur M, Davis JH. Drowning without aspiration: is this an appropriate diagnosis? J Forensic Sci 1999; 44(6):1119–1123. pmid:10582353
- Szpilman D, Orlowski JP. Sports related to drowning. Eur Respir Rev 2016; 25(141):348–359. doi:10.1183/16000617.0038-2016
- Szpilman D, Webber J, Quan L, et al. Creating a drowning chain of survival. Resuscitation 2014; 85(9):1149–1152. doi:10.1016/j.resuscitation.2014.05.034
- International Life Saving Federation. Who needs further medical help after rescue from the water. Medical Position Statement - MPS 06, 2016. www.ilsf.org/file/3916/download?token=pDnPDCrk. Accessed June 13, 2018.
KEY POINTS
- Drowning is a process of aspiration leading to hypoxia and eventually cardiac arrest. However, it is not synonymous with death: it can be interrupted.
- Patients who have been rescued from drowning and who have minimal symptoms generally get better within 4 to 8 hours of the event.
- Rescued victims should be warned that, although a rare condition, if they develop cough, breathlessness, or any other worrisome symptom within 8 hours of being in the water, they should seek medical attention immediately.
Wolff-Parkinson-White pattern unmasked by severe musculoskeletal pain
A 55-year-old man with no significant medical history presented to the emergency department with left-sided flank pain that had begun 3 days earlier. He described the pain as continuous, sharp, and aggravated by movement. He worked in construction, and before the pain started he had moved 8 sheets of drywall and lifted 5-gallon buckets of spackling compound. He denied any associated chest pain, palpitations, dyspnea, cough, or lightheadedness. His family history included sudden cardiac death in 2 second-degree relatives.
On arrival in the emergency department, his vital signs were normal, as were the rest of the findings on physical examination except for reproducible point tenderness below the left scapula.
Laboratory workup revealed normal blood cell counts, liver enzymes, and kidney function. His initial troponin test was negative.
The patient was referred to an electrophysiologist for further evaluation, but he returned to his home country (Haiti) after discharge and was lost to follow-up.
WOLFF-PARKINSON-WHITE PATTERN VS SYNDROME
WPW syndrome is a disorder of the conduction system leading to preexcitation of the ventricles by an accessory pathway between the atria and ventricles. It is characterized by preexcitation manifested on electrocardiography and by symptomatic arrhythmias.
In contrast, the WPW pattern is defined only by preexcitation findings on electrocardiography without symptomatic arrhythmias. Patients with WPW syndrome can present with palpitation, dizziness, and syncope resulting from underlying arrhythmia.1 This is not seen in patients with the WPW pattern.
A short PR interval with or without delta waves can also be seen in the absence of an accessory pathway, eg, in hypoplastic left heart syndrome, atrioventricular canal defect, and Ebstein anomaly. These conditions are termed pseudopreexcitation syndrome.2
Our patient presented with severe musculoskeletal pain that precipitated the electrocardiographic changes of the WPW pattern and resolved with adequate pain control. The WPW pattern can be unmasked under different scenarios, including anesthesia, sympathomimetic drugs, and postoperatively.3–5
Catecholamine challenge has been used to unmask high-risk features in WPW syndrome.3 Our patient may have had a transient spike in catecholamine levels because of severe musculoskeletal pain, leading to unmasking of accessory pathways and resulting in the WPW pattern on electrocardiography.
Most patients with the WPW pattern experience no symptoms, but a small percentage develop arrhythmias.
In rare cases, sudden cardiac death can be the presenting feature of WPW syndrome. The estimated risk of sudden cardiac death in patients with the WPW pattern is 1.25 per 1,000 person-years; ventricular fibrillation is the underlying mechanism.6 As our patient had a family history of sudden cardiac death, he was considered at high risk and was therefore referred to an electrophysiologist.
- Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation 1993; 87(3):866–873. pmid:8443907
- Carlson AM, Turek JW, Law IH, Von Bergen NH. Pseudo-preexcitation is prevalent among patients with repaired complex congenital heart disease. Pediatr Cardiol.2015; 36(1):8–13. doi:10.1007/s00246-014-0955-x
- Aleong RG, Singh SM, Levinson JR, Milan DJ. Catecholamine challenge unmasking high-risk features in the Wolff-Parkinson-White syndrome. Europace 2009; 11(10):1396–1398. doi:10.1093/europace/eup211
- Sahu S, Karna ST, Karna A, Lata I, Kapoor D. Anaesthetic management of Wolff-Parkinson-White syndrome for hysterectomy. Indian J Anaesth 2011; 55(4):378–380. doi:10.4103/0019-5049.84866
- Tseng ZH, Yadav AV, Scheinman MM. Catecholamine dependent accessory pathway automaticity. Pacing Clin Electrophysiol 2004; 27(7):1005–1007. doi:10.1111/j.1540-8159.2004.00574.x
- Obeyesekere MN, Leong-Sit P, Massel D, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation 2012; 125(19):2308–2315. doi:10.1161/CIRCULATIONAHA.111.055350
A 55-year-old man with no significant medical history presented to the emergency department with left-sided flank pain that had begun 3 days earlier. He described the pain as continuous, sharp, and aggravated by movement. He worked in construction, and before the pain started he had moved 8 sheets of drywall and lifted 5-gallon buckets of spackling compound. He denied any associated chest pain, palpitations, dyspnea, cough, or lightheadedness. His family history included sudden cardiac death in 2 second-degree relatives.
On arrival in the emergency department, his vital signs were normal, as were the rest of the findings on physical examination except for reproducible point tenderness below the left scapula.
Laboratory workup revealed normal blood cell counts, liver enzymes, and kidney function. His initial troponin test was negative.
The patient was referred to an electrophysiologist for further evaluation, but he returned to his home country (Haiti) after discharge and was lost to follow-up.
WOLFF-PARKINSON-WHITE PATTERN VS SYNDROME
WPW syndrome is a disorder of the conduction system leading to preexcitation of the ventricles by an accessory pathway between the atria and ventricles. It is characterized by preexcitation manifested on electrocardiography and by symptomatic arrhythmias.
In contrast, the WPW pattern is defined only by preexcitation findings on electrocardiography without symptomatic arrhythmias. Patients with WPW syndrome can present with palpitation, dizziness, and syncope resulting from underlying arrhythmia.1 This is not seen in patients with the WPW pattern.
A short PR interval with or without delta waves can also be seen in the absence of an accessory pathway, eg, in hypoplastic left heart syndrome, atrioventricular canal defect, and Ebstein anomaly. These conditions are termed pseudopreexcitation syndrome.2
Our patient presented with severe musculoskeletal pain that precipitated the electrocardiographic changes of the WPW pattern and resolved with adequate pain control. The WPW pattern can be unmasked under different scenarios, including anesthesia, sympathomimetic drugs, and postoperatively.3–5
Catecholamine challenge has been used to unmask high-risk features in WPW syndrome.3 Our patient may have had a transient spike in catecholamine levels because of severe musculoskeletal pain, leading to unmasking of accessory pathways and resulting in the WPW pattern on electrocardiography.
Most patients with the WPW pattern experience no symptoms, but a small percentage develop arrhythmias.
In rare cases, sudden cardiac death can be the presenting feature of WPW syndrome. The estimated risk of sudden cardiac death in patients with the WPW pattern is 1.25 per 1,000 person-years; ventricular fibrillation is the underlying mechanism.6 As our patient had a family history of sudden cardiac death, he was considered at high risk and was therefore referred to an electrophysiologist.
A 55-year-old man with no significant medical history presented to the emergency department with left-sided flank pain that had begun 3 days earlier. He described the pain as continuous, sharp, and aggravated by movement. He worked in construction, and before the pain started he had moved 8 sheets of drywall and lifted 5-gallon buckets of spackling compound. He denied any associated chest pain, palpitations, dyspnea, cough, or lightheadedness. His family history included sudden cardiac death in 2 second-degree relatives.
On arrival in the emergency department, his vital signs were normal, as were the rest of the findings on physical examination except for reproducible point tenderness below the left scapula.
Laboratory workup revealed normal blood cell counts, liver enzymes, and kidney function. His initial troponin test was negative.
The patient was referred to an electrophysiologist for further evaluation, but he returned to his home country (Haiti) after discharge and was lost to follow-up.
WOLFF-PARKINSON-WHITE PATTERN VS SYNDROME
WPW syndrome is a disorder of the conduction system leading to preexcitation of the ventricles by an accessory pathway between the atria and ventricles. It is characterized by preexcitation manifested on electrocardiography and by symptomatic arrhythmias.
In contrast, the WPW pattern is defined only by preexcitation findings on electrocardiography without symptomatic arrhythmias. Patients with WPW syndrome can present with palpitation, dizziness, and syncope resulting from underlying arrhythmia.1 This is not seen in patients with the WPW pattern.
A short PR interval with or without delta waves can also be seen in the absence of an accessory pathway, eg, in hypoplastic left heart syndrome, atrioventricular canal defect, and Ebstein anomaly. These conditions are termed pseudopreexcitation syndrome.2
Our patient presented with severe musculoskeletal pain that precipitated the electrocardiographic changes of the WPW pattern and resolved with adequate pain control. The WPW pattern can be unmasked under different scenarios, including anesthesia, sympathomimetic drugs, and postoperatively.3–5
Catecholamine challenge has been used to unmask high-risk features in WPW syndrome.3 Our patient may have had a transient spike in catecholamine levels because of severe musculoskeletal pain, leading to unmasking of accessory pathways and resulting in the WPW pattern on electrocardiography.
Most patients with the WPW pattern experience no symptoms, but a small percentage develop arrhythmias.
In rare cases, sudden cardiac death can be the presenting feature of WPW syndrome. The estimated risk of sudden cardiac death in patients with the WPW pattern is 1.25 per 1,000 person-years; ventricular fibrillation is the underlying mechanism.6 As our patient had a family history of sudden cardiac death, he was considered at high risk and was therefore referred to an electrophysiologist.
- Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation 1993; 87(3):866–873. pmid:8443907
- Carlson AM, Turek JW, Law IH, Von Bergen NH. Pseudo-preexcitation is prevalent among patients with repaired complex congenital heart disease. Pediatr Cardiol.2015; 36(1):8–13. doi:10.1007/s00246-014-0955-x
- Aleong RG, Singh SM, Levinson JR, Milan DJ. Catecholamine challenge unmasking high-risk features in the Wolff-Parkinson-White syndrome. Europace 2009; 11(10):1396–1398. doi:10.1093/europace/eup211
- Sahu S, Karna ST, Karna A, Lata I, Kapoor D. Anaesthetic management of Wolff-Parkinson-White syndrome for hysterectomy. Indian J Anaesth 2011; 55(4):378–380. doi:10.4103/0019-5049.84866
- Tseng ZH, Yadav AV, Scheinman MM. Catecholamine dependent accessory pathway automaticity. Pacing Clin Electrophysiol 2004; 27(7):1005–1007. doi:10.1111/j.1540-8159.2004.00574.x
- Obeyesekere MN, Leong-Sit P, Massel D, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation 2012; 125(19):2308–2315. doi:10.1161/CIRCULATIONAHA.111.055350
- Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation 1993; 87(3):866–873. pmid:8443907
- Carlson AM, Turek JW, Law IH, Von Bergen NH. Pseudo-preexcitation is prevalent among patients with repaired complex congenital heart disease. Pediatr Cardiol.2015; 36(1):8–13. doi:10.1007/s00246-014-0955-x
- Aleong RG, Singh SM, Levinson JR, Milan DJ. Catecholamine challenge unmasking high-risk features in the Wolff-Parkinson-White syndrome. Europace 2009; 11(10):1396–1398. doi:10.1093/europace/eup211
- Sahu S, Karna ST, Karna A, Lata I, Kapoor D. Anaesthetic management of Wolff-Parkinson-White syndrome for hysterectomy. Indian J Anaesth 2011; 55(4):378–380. doi:10.4103/0019-5049.84866
- Tseng ZH, Yadav AV, Scheinman MM. Catecholamine dependent accessory pathway automaticity. Pacing Clin Electrophysiol 2004; 27(7):1005–1007. doi:10.1111/j.1540-8159.2004.00574.x
- Obeyesekere MN, Leong-Sit P, Massel D, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation 2012; 125(19):2308–2315. doi:10.1161/CIRCULATIONAHA.111.055350
Osmotic demyelination syndrome due to hyperosmolar hyperglycemia
A 55-year-old man with a 20-year history of type 2 diabetes mellitus was admitted to the hospital after presenting to the emergency department with an acute change in mental status. Three days earlier, he had begun to feel abdominal discomfort and dizziness, which gradually worsened.
On presentation, his Glasgow Coma Scale score was 13 out of 15 (eye-opening response 3 of 4, verbal response 4 of 5, motor response 6 of 6), his blood pressure was 221/114 mm Hg, and other vital signs were normal. Physical examination including a neurologic examination was normal. No gait abnormality or ataxia was noted.
When asked about current medications, he said that 2 years earlier he had missed an appointment with his primary care physician and so had never obtained refills of his diabetes medications.
Results of laboratory testing were as follows:
- Blood glucose 1,011 mg/dL (reference range 65–110)
- Hemoglobin A1c 17.8% (4%–5.6%)
- Sodium 126 mmol/L (135–145)
- Sodium corrected for serum glucose 141 mmol/L
- Potassium 3.2 mmol/L (3.5–5.0)
- Blood urea nitrogen 43.8 mg/dL (8–21)
- Calculated serum osmolality 324 mosm/kg (275–295).
Blood gas analysis showed no acidosis. Tests for urinary and serum ketones were negative. Computed tomography (CT) of the head without contrast was normal.
Based on the results of the evaluation, the patient’s condition was diagnosed as a hyperosmolar hyperglycemic state, presumably from dehydration and noncompliance with diabetes medications. His altered mental status was also attributed to this diagnosis. He was started on aggressive hydration and insulin infusion to correct the blood glucose level. Repeat laboratory testing 7 hours after admission revealed a blood glucose of 49 mg/dL, sodium 148 mmol/L, blood urea nitrogen 43 mg/dL, and calculated serum osmolality 290 mosm/kg.
The insulin infusion was suspended, and glucose infusion was started. With this treatment, his blood glucose level stabilized, but his Glasgow Coma Scale score was unchanged from the time of presentation. A neurologic examination at this time showed bilateral dysmetria. Cranial nerves were normal. Motor examination showed normal tone with a Medical Research Council score of 5 of 5 in all extremities. Sensory examination revealed a glove-and-stocking pattern of loss of vibratory sensation. Tendon reflexes were normal except for diminished bilateral knee-jerk and ankle-jerk responses.
OSMOTIC DEMYELINATION SYNDROME
Central pontine myelinolysis is a pivotal manifestation of the syndrome and is characterized by progressive lethargy, quadriparesis, dysarthria, ophthalmoplegia, dysphasia, ataxia, and reflex changes. Clinical symptoms of extrapontine myelinolysis are variable.4
Although CT may underestimate osmotic demyelination syndrome, the typical radiologic findings on brain MRI are hyperintense lesions in the central pons or associated extrapontine structures on T2-weighted and fluid-attenuated inversion recovery sequences.4
A precise definition of hyperosmolar hyperglycemia does not exist. The Joint British Diabetes Societies for Inpatient Care suggested the following features: a measured osmolality of 320 mosm/kg or higher, a blood glucose level of 541 mg/dL or higher, severe dehydration, and feeling unwell.5
Our patient’s clinical course and high hemoglobin A1c suggested prolonged hyperglycemia and high serum osmolality before his admission. After his admission, aggressive hydration and insulin therapy corrected the hyperglycemia and serum osmolality too rapidly for his brain cells to adjust to the change. It was reasonable to suspect a hyperosmolar hyperglycemic state as one of the main causes of his mental status change and ataxia. This, along with lack of improvement in his impaired metal status and new-onset ataxia despite treatment, led to suspicion of osmotic demyelination syndrome. His diminished bilateral knee-jerk and ankle-jerk responses more likely represented diabetic neuropathy rather than osmotic demyelination syndrome.
Osmotic demyelination syndrome has seldom been reported as a complication of hyperosmolar hyperglycemia.6–13 And extrapontine myelinolysis with hyperosmolar hyperglycemia is extremely rare, with only 2 reports to date to the best of our knowledge.6,10
There is no specific treatment for osmotic demyelination syndrome except for supportive care and treatment of coexisting conditions. Once an osmotic derangement is identified, we recommend correcting chronically elevated serum glucose values gradually to avoid overtreatment, just as we would do with elevated serum sodium levels. Changes in neurologic findings, serum blood glucose level, and serum osmolality should be followed closely. A review showed that a favorable recovery from osmotic demyelination syndrome is possible even with severe neurologic deficits.4
TAKE-AWAY POINTS
- Osmotic demyelination syndrome is a rare but severe complication of a hyperosmolar hyperglycemic state.
- Physicians should be aware not only of changes in serum sodium, but also of changes in serum osmolality and serum glucose.
- When a new-onset neurologic deficit is found during treatment of a hyperosmolar hyperglycemic state, suspect osmotic demyelination syndrome, monitor changes in serum osmolality, and consider brain MRI.
- Brown WD. Osmotic demyelination disorders: central pontine and extrapontine myelinolysis. Curr Opin Neurol 2000; 13(6):691–697. pmid:11148672
- Laureno R, Karp BI. Myelinolysis after correction of hyponatraemia. Ann Intern Med 1997; 126(1):57–62. pmid:8992924
- Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry 1959; 81(2):154–172. pmid:13616772
- Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol 2014; 21(12):1443–1450. doi:10.1111/ene.12571
- Scott AR; Joint British Diabetes Societies (JBDS) for Inpatient Care; JBDS Hyperosmolar Hyperglycaemic Guidelines Group. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med 2015; 32(6):714–724. doi:10.1111/dme.12757
- McComb RD, Pfeiffer RF, Casey JH, Wolcott G, Till DJ. Lateral pontine and extrapontine myelinolysis associated with hypernatremia and hyperglycemia. Clin Neuropathol 1989; 8(6):284–288. pmid:2695277
- O’Malley G, Moran C, Draman MS, et al. Central pontine myelinolysis complicating treatment of the hyperglycaemic hyperosmolar state. Ann Clin Biochem 2008; 45(pt 4):440–443. doi:10.1258/acb.2008.007171
- Burns JD, Kosa SC, Wijdicks EF. Central pontine myelinolysis in a patient with hyperosmolar hyperglycemia and consistently normal serum sodium. Neurocrit Care 2009; 11(2):251–254. doi:10.1007/s12028-009-9241-9
- Mao S, Liu Z, Ding M. Central pontine myelinolysis in a patient with epilepsia partialis continua and hyperglycaemic hyperosmolar state. Ann Clin Biochem 2011; 48(pt 1):79–82. doi:10.1258/acb.2010.010152. Epub 2010 Nov 23.
- Guerrero WR, Dababneh H, Nadeau SE. Hemiparesis, encephalopathy, and extrapontine osmotic myelinolysis in the setting of hyperosmolar hyperglycemia. J Clin Neurosci 2013; 20(6):894–896. doi:10.1016/j.jocn.2012.05.045
- Hegazi MO, Mashankar A. Central pontine myelinolysis in the hyperosmolar hyperglycaemic state. Med Princ Pract 2013; 22(1):96–99. doi:10.1159/000341718
- Rodríguez-Velver KV, Soto-Garcia AJ, Zapata-Rivera MA, Montes-Villarreal J, Villarreal-Pérez JZ, Rodríguez-Gutiérrez R. Osmotic demyelination syndrome as the initial manifestation of a hyperosmolar hyperglycemic state. Case Rep Neurol Med 2014; 2014:652523. doi:10.1155/2014/652523
- Chang YM. Central pontine myelinolysis associated with diabetic hyperglycemia. JSM Clin Case Rep 2014; 2(6):1059.
A 55-year-old man with a 20-year history of type 2 diabetes mellitus was admitted to the hospital after presenting to the emergency department with an acute change in mental status. Three days earlier, he had begun to feel abdominal discomfort and dizziness, which gradually worsened.
On presentation, his Glasgow Coma Scale score was 13 out of 15 (eye-opening response 3 of 4, verbal response 4 of 5, motor response 6 of 6), his blood pressure was 221/114 mm Hg, and other vital signs were normal. Physical examination including a neurologic examination was normal. No gait abnormality or ataxia was noted.
When asked about current medications, he said that 2 years earlier he had missed an appointment with his primary care physician and so had never obtained refills of his diabetes medications.
Results of laboratory testing were as follows:
- Blood glucose 1,011 mg/dL (reference range 65–110)
- Hemoglobin A1c 17.8% (4%–5.6%)
- Sodium 126 mmol/L (135–145)
- Sodium corrected for serum glucose 141 mmol/L
- Potassium 3.2 mmol/L (3.5–5.0)
- Blood urea nitrogen 43.8 mg/dL (8–21)
- Calculated serum osmolality 324 mosm/kg (275–295).
Blood gas analysis showed no acidosis. Tests for urinary and serum ketones were negative. Computed tomography (CT) of the head without contrast was normal.
Based on the results of the evaluation, the patient’s condition was diagnosed as a hyperosmolar hyperglycemic state, presumably from dehydration and noncompliance with diabetes medications. His altered mental status was also attributed to this diagnosis. He was started on aggressive hydration and insulin infusion to correct the blood glucose level. Repeat laboratory testing 7 hours after admission revealed a blood glucose of 49 mg/dL, sodium 148 mmol/L, blood urea nitrogen 43 mg/dL, and calculated serum osmolality 290 mosm/kg.
The insulin infusion was suspended, and glucose infusion was started. With this treatment, his blood glucose level stabilized, but his Glasgow Coma Scale score was unchanged from the time of presentation. A neurologic examination at this time showed bilateral dysmetria. Cranial nerves were normal. Motor examination showed normal tone with a Medical Research Council score of 5 of 5 in all extremities. Sensory examination revealed a glove-and-stocking pattern of loss of vibratory sensation. Tendon reflexes were normal except for diminished bilateral knee-jerk and ankle-jerk responses.
OSMOTIC DEMYELINATION SYNDROME
Central pontine myelinolysis is a pivotal manifestation of the syndrome and is characterized by progressive lethargy, quadriparesis, dysarthria, ophthalmoplegia, dysphasia, ataxia, and reflex changes. Clinical symptoms of extrapontine myelinolysis are variable.4
Although CT may underestimate osmotic demyelination syndrome, the typical radiologic findings on brain MRI are hyperintense lesions in the central pons or associated extrapontine structures on T2-weighted and fluid-attenuated inversion recovery sequences.4
A precise definition of hyperosmolar hyperglycemia does not exist. The Joint British Diabetes Societies for Inpatient Care suggested the following features: a measured osmolality of 320 mosm/kg or higher, a blood glucose level of 541 mg/dL or higher, severe dehydration, and feeling unwell.5
Our patient’s clinical course and high hemoglobin A1c suggested prolonged hyperglycemia and high serum osmolality before his admission. After his admission, aggressive hydration and insulin therapy corrected the hyperglycemia and serum osmolality too rapidly for his brain cells to adjust to the change. It was reasonable to suspect a hyperosmolar hyperglycemic state as one of the main causes of his mental status change and ataxia. This, along with lack of improvement in his impaired metal status and new-onset ataxia despite treatment, led to suspicion of osmotic demyelination syndrome. His diminished bilateral knee-jerk and ankle-jerk responses more likely represented diabetic neuropathy rather than osmotic demyelination syndrome.
Osmotic demyelination syndrome has seldom been reported as a complication of hyperosmolar hyperglycemia.6–13 And extrapontine myelinolysis with hyperosmolar hyperglycemia is extremely rare, with only 2 reports to date to the best of our knowledge.6,10
There is no specific treatment for osmotic demyelination syndrome except for supportive care and treatment of coexisting conditions. Once an osmotic derangement is identified, we recommend correcting chronically elevated serum glucose values gradually to avoid overtreatment, just as we would do with elevated serum sodium levels. Changes in neurologic findings, serum blood glucose level, and serum osmolality should be followed closely. A review showed that a favorable recovery from osmotic demyelination syndrome is possible even with severe neurologic deficits.4
TAKE-AWAY POINTS
- Osmotic demyelination syndrome is a rare but severe complication of a hyperosmolar hyperglycemic state.
- Physicians should be aware not only of changes in serum sodium, but also of changes in serum osmolality and serum glucose.
- When a new-onset neurologic deficit is found during treatment of a hyperosmolar hyperglycemic state, suspect osmotic demyelination syndrome, monitor changes in serum osmolality, and consider brain MRI.
A 55-year-old man with a 20-year history of type 2 diabetes mellitus was admitted to the hospital after presenting to the emergency department with an acute change in mental status. Three days earlier, he had begun to feel abdominal discomfort and dizziness, which gradually worsened.
On presentation, his Glasgow Coma Scale score was 13 out of 15 (eye-opening response 3 of 4, verbal response 4 of 5, motor response 6 of 6), his blood pressure was 221/114 mm Hg, and other vital signs were normal. Physical examination including a neurologic examination was normal. No gait abnormality or ataxia was noted.
When asked about current medications, he said that 2 years earlier he had missed an appointment with his primary care physician and so had never obtained refills of his diabetes medications.
Results of laboratory testing were as follows:
- Blood glucose 1,011 mg/dL (reference range 65–110)
- Hemoglobin A1c 17.8% (4%–5.6%)
- Sodium 126 mmol/L (135–145)
- Sodium corrected for serum glucose 141 mmol/L
- Potassium 3.2 mmol/L (3.5–5.0)
- Blood urea nitrogen 43.8 mg/dL (8–21)
- Calculated serum osmolality 324 mosm/kg (275–295).
Blood gas analysis showed no acidosis. Tests for urinary and serum ketones were negative. Computed tomography (CT) of the head without contrast was normal.
Based on the results of the evaluation, the patient’s condition was diagnosed as a hyperosmolar hyperglycemic state, presumably from dehydration and noncompliance with diabetes medications. His altered mental status was also attributed to this diagnosis. He was started on aggressive hydration and insulin infusion to correct the blood glucose level. Repeat laboratory testing 7 hours after admission revealed a blood glucose of 49 mg/dL, sodium 148 mmol/L, blood urea nitrogen 43 mg/dL, and calculated serum osmolality 290 mosm/kg.
The insulin infusion was suspended, and glucose infusion was started. With this treatment, his blood glucose level stabilized, but his Glasgow Coma Scale score was unchanged from the time of presentation. A neurologic examination at this time showed bilateral dysmetria. Cranial nerves were normal. Motor examination showed normal tone with a Medical Research Council score of 5 of 5 in all extremities. Sensory examination revealed a glove-and-stocking pattern of loss of vibratory sensation. Tendon reflexes were normal except for diminished bilateral knee-jerk and ankle-jerk responses.
OSMOTIC DEMYELINATION SYNDROME
Central pontine myelinolysis is a pivotal manifestation of the syndrome and is characterized by progressive lethargy, quadriparesis, dysarthria, ophthalmoplegia, dysphasia, ataxia, and reflex changes. Clinical symptoms of extrapontine myelinolysis are variable.4
Although CT may underestimate osmotic demyelination syndrome, the typical radiologic findings on brain MRI are hyperintense lesions in the central pons or associated extrapontine structures on T2-weighted and fluid-attenuated inversion recovery sequences.4
A precise definition of hyperosmolar hyperglycemia does not exist. The Joint British Diabetes Societies for Inpatient Care suggested the following features: a measured osmolality of 320 mosm/kg or higher, a blood glucose level of 541 mg/dL or higher, severe dehydration, and feeling unwell.5
Our patient’s clinical course and high hemoglobin A1c suggested prolonged hyperglycemia and high serum osmolality before his admission. After his admission, aggressive hydration and insulin therapy corrected the hyperglycemia and serum osmolality too rapidly for his brain cells to adjust to the change. It was reasonable to suspect a hyperosmolar hyperglycemic state as one of the main causes of his mental status change and ataxia. This, along with lack of improvement in his impaired metal status and new-onset ataxia despite treatment, led to suspicion of osmotic demyelination syndrome. His diminished bilateral knee-jerk and ankle-jerk responses more likely represented diabetic neuropathy rather than osmotic demyelination syndrome.
Osmotic demyelination syndrome has seldom been reported as a complication of hyperosmolar hyperglycemia.6–13 And extrapontine myelinolysis with hyperosmolar hyperglycemia is extremely rare, with only 2 reports to date to the best of our knowledge.6,10
There is no specific treatment for osmotic demyelination syndrome except for supportive care and treatment of coexisting conditions. Once an osmotic derangement is identified, we recommend correcting chronically elevated serum glucose values gradually to avoid overtreatment, just as we would do with elevated serum sodium levels. Changes in neurologic findings, serum blood glucose level, and serum osmolality should be followed closely. A review showed that a favorable recovery from osmotic demyelination syndrome is possible even with severe neurologic deficits.4
TAKE-AWAY POINTS
- Osmotic demyelination syndrome is a rare but severe complication of a hyperosmolar hyperglycemic state.
- Physicians should be aware not only of changes in serum sodium, but also of changes in serum osmolality and serum glucose.
- When a new-onset neurologic deficit is found during treatment of a hyperosmolar hyperglycemic state, suspect osmotic demyelination syndrome, monitor changes in serum osmolality, and consider brain MRI.
- Brown WD. Osmotic demyelination disorders: central pontine and extrapontine myelinolysis. Curr Opin Neurol 2000; 13(6):691–697. pmid:11148672
- Laureno R, Karp BI. Myelinolysis after correction of hyponatraemia. Ann Intern Med 1997; 126(1):57–62. pmid:8992924
- Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry 1959; 81(2):154–172. pmid:13616772
- Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol 2014; 21(12):1443–1450. doi:10.1111/ene.12571
- Scott AR; Joint British Diabetes Societies (JBDS) for Inpatient Care; JBDS Hyperosmolar Hyperglycaemic Guidelines Group. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med 2015; 32(6):714–724. doi:10.1111/dme.12757
- McComb RD, Pfeiffer RF, Casey JH, Wolcott G, Till DJ. Lateral pontine and extrapontine myelinolysis associated with hypernatremia and hyperglycemia. Clin Neuropathol 1989; 8(6):284–288. pmid:2695277
- O’Malley G, Moran C, Draman MS, et al. Central pontine myelinolysis complicating treatment of the hyperglycaemic hyperosmolar state. Ann Clin Biochem 2008; 45(pt 4):440–443. doi:10.1258/acb.2008.007171
- Burns JD, Kosa SC, Wijdicks EF. Central pontine myelinolysis in a patient with hyperosmolar hyperglycemia and consistently normal serum sodium. Neurocrit Care 2009; 11(2):251–254. doi:10.1007/s12028-009-9241-9
- Mao S, Liu Z, Ding M. Central pontine myelinolysis in a patient with epilepsia partialis continua and hyperglycaemic hyperosmolar state. Ann Clin Biochem 2011; 48(pt 1):79–82. doi:10.1258/acb.2010.010152. Epub 2010 Nov 23.
- Guerrero WR, Dababneh H, Nadeau SE. Hemiparesis, encephalopathy, and extrapontine osmotic myelinolysis in the setting of hyperosmolar hyperglycemia. J Clin Neurosci 2013; 20(6):894–896. doi:10.1016/j.jocn.2012.05.045
- Hegazi MO, Mashankar A. Central pontine myelinolysis in the hyperosmolar hyperglycaemic state. Med Princ Pract 2013; 22(1):96–99. doi:10.1159/000341718
- Rodríguez-Velver KV, Soto-Garcia AJ, Zapata-Rivera MA, Montes-Villarreal J, Villarreal-Pérez JZ, Rodríguez-Gutiérrez R. Osmotic demyelination syndrome as the initial manifestation of a hyperosmolar hyperglycemic state. Case Rep Neurol Med 2014; 2014:652523. doi:10.1155/2014/652523
- Chang YM. Central pontine myelinolysis associated with diabetic hyperglycemia. JSM Clin Case Rep 2014; 2(6):1059.
- Brown WD. Osmotic demyelination disorders: central pontine and extrapontine myelinolysis. Curr Opin Neurol 2000; 13(6):691–697. pmid:11148672
- Laureno R, Karp BI. Myelinolysis after correction of hyponatraemia. Ann Intern Med 1997; 126(1):57–62. pmid:8992924
- Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry 1959; 81(2):154–172. pmid:13616772
- Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol 2014; 21(12):1443–1450. doi:10.1111/ene.12571
- Scott AR; Joint British Diabetes Societies (JBDS) for Inpatient Care; JBDS Hyperosmolar Hyperglycaemic Guidelines Group. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med 2015; 32(6):714–724. doi:10.1111/dme.12757
- McComb RD, Pfeiffer RF, Casey JH, Wolcott G, Till DJ. Lateral pontine and extrapontine myelinolysis associated with hypernatremia and hyperglycemia. Clin Neuropathol 1989; 8(6):284–288. pmid:2695277
- O’Malley G, Moran C, Draman MS, et al. Central pontine myelinolysis complicating treatment of the hyperglycaemic hyperosmolar state. Ann Clin Biochem 2008; 45(pt 4):440–443. doi:10.1258/acb.2008.007171
- Burns JD, Kosa SC, Wijdicks EF. Central pontine myelinolysis in a patient with hyperosmolar hyperglycemia and consistently normal serum sodium. Neurocrit Care 2009; 11(2):251–254. doi:10.1007/s12028-009-9241-9
- Mao S, Liu Z, Ding M. Central pontine myelinolysis in a patient with epilepsia partialis continua and hyperglycaemic hyperosmolar state. Ann Clin Biochem 2011; 48(pt 1):79–82. doi:10.1258/acb.2010.010152. Epub 2010 Nov 23.
- Guerrero WR, Dababneh H, Nadeau SE. Hemiparesis, encephalopathy, and extrapontine osmotic myelinolysis in the setting of hyperosmolar hyperglycemia. J Clin Neurosci 2013; 20(6):894–896. doi:10.1016/j.jocn.2012.05.045
- Hegazi MO, Mashankar A. Central pontine myelinolysis in the hyperosmolar hyperglycaemic state. Med Princ Pract 2013; 22(1):96–99. doi:10.1159/000341718
- Rodríguez-Velver KV, Soto-Garcia AJ, Zapata-Rivera MA, Montes-Villarreal J, Villarreal-Pérez JZ, Rodríguez-Gutiérrez R. Osmotic demyelination syndrome as the initial manifestation of a hyperosmolar hyperglycemic state. Case Rep Neurol Med 2014; 2014:652523. doi:10.1155/2014/652523
- Chang YM. Central pontine myelinolysis associated with diabetic hyperglycemia. JSM Clin Case Rep 2014; 2(6):1059.
What inpatient treatments do we have for acute intractable migraine?
We recommend the following combination treatment:
Normal saline (0.9% NaCl) 1 to 2 L by intravenous (IV) infusion over 2 to 4 hours. This can be repeated every 6 to 12 hours.
Ketorolac 30-mg IV bolus, which can be repeated every 6 hours. However, patients with coronary artery disease, uncontrolled hypertension, acute renal failure, or cerebrovascular disease should instead receive acetaminophen 1,000 mg, naproxen sodium 550 mg, or aspirin 325 mg by mouth.
Prochlorperazine or metoclopramide 10-mg IV infusion. This can be repeated every 6 hours. However, to reduce the extrapyramidal adverse effects of these drugs, patients should first receive diphenhydramine 25- to 50-mg IV bolus, which can be repeated every 6 to 8 hours.
Sodium valproate 500 to 1,000 mg by IV infusion over 20 minutes. This can be repeated after 8 hours.
Dexamethasone 4-mg IV bolus every 6 hours, or 10-mg IV bolus once in 24 hours.
Magnesium sulfate 500 to 1,000 mg by IV infusion over 1 hour. This can be repeated every 6 to 12 hours.
If the migraine has not improved after 3 cycles of this regimen, a neurologic consultation should be considered. Other options include dihydroergotamine and occipital nerve blocks1 performed at the bedside.
GENERAL PRINCIPLES
Managing acute intractable migraine can be frustrating for both the practitioner and the patient. Some general principles are helpful.
Use a combination of drugs. Aborting a severe migraine attack often requires a combination of medications that work synergistically.
Use IV and intramuscular formulations rather than oral formulations: they are more rapidly absorbed, provide faster pain relief, and can be given when the nausea that often accompanies migraine precludes oral treatments.
Rule out secondary causes. The mnemonic SNOOP—systemic signs, neurologic signs, onset, older age, progression of existing headache disorder—is useful for assessing underlying causes.2 Any patient presenting with intractable migraine should also have a thorough neurologic examination.
Screening electrocardiography may be helpful, as the pretreatment QTc interval may direct the choice of intravenous treatment. If the patient has a prolonged QTc or is taking another drug that could prolong the QTc, certain medications, specifically dopamine receptor antagonists and diphenhydramine, should be avoided.
Ask the patient what has worked previously. A particular agent may have been effective in aborting the migraine; thus, a single dose of it could abort the headache, expediting discharge.
Establish if a triptan or ergot derivative has been used during the 24 hours before presentation, as repeated dosing within this interval is not recommended.3
Establish the baseline headache severity. Complete headache relief is difficult to achieve in a patient with chronic daily headache, and establishing a more realistic goal (eg, 50% relief) from the outset is useful.
OPTIONS FOR DRUG THERAPY
Antiemetics
Dopamine receptor antagonists are assumed to merely treat nausea in patients with migraine; however, they act independently to abort migraine and thus should be considered, irrespective of the presence of nausea.
The two most commonly used agents are prochlorperazine and metoclopramide. The American Academy of Neurology guidelines recommend prochlorperazine as first-line therapy for acute migraine. Metoclopramide is rated slightly lower and is considered to have moderate benefit.4 The Canadian Headache Society cites a high level of evidence supporting prochlorperazine and a moderate level of evidence supporting metoclopramide.5 The American Headache Society assessment of parenteral pharmacotherapies gives prochlorperazine and metoclopramide a level B recommendation of “should offer” (a recommendation only additionally assigned to subcutaneous sumatriptan).3 Hence, either agent can be used.
To reduce the risk of posttreatment akathisia, diphenhydramine or benztropine may be given before starting a dopamine receptor antagonist. Diphenhydramine may be independently effective in migraine treatment,6,7 but data on this are limited.
Ketorolac, ibuprofen
Ketorolac and ibuprofen are the only available nonsteroidal antiinflammatory drugs (NSAIDs) for IV administration. The Canadian Headache Society guidelines strongly recommend ketorolac for the treatment of migraine in emergency settings.5 Doses range from 30 mg to 60 mg.1 Ibuprofen 400 to 800 mg by IV infusion is an acceptable alternative. These medications should be avoided in patients with renal failure or severe coronary artery disease.
Oral naproxen sodium is a possible alternative in patients with cardiovascular disease, as it has been shown to carry a lower cardiovascular risk than other NSAIDs.8
The same concerns in patients with renal dysfunction apply to any NSAID, as the enzyme cyclooxygenase plays a constitutive role in glomerular function.
Antiepileptic drugs
The antiepileptic drugs sodium valproate and levetiracetam are available in IV formulations that have demonstrated efficacy in the treatment of status migrainosus1 (ie, migraine lasting more than 72 hours). Valproate has the strongest track record, is well tolerated, and is effective in rapidly aborting migraine.9
Volume repletion
Although its use is anecdotal and to date no trial has measured its efficacy, IV volume repletion is often used in acute migraine, as most headache experts surmise it to be highly effective, especially in patients with prolonged nausea or vomiting.1
Magnesium
IV magnesium is effective, particularly for migraine with aura.10 Hypotension is a common side effect, and pretreatment or concurrent treatment with IV fluids is advised. Doses from 500 mg to 1,000 mg have demonstrated efficacy.10
Corticosteroids
Corticosteroids can be used in the treatment of status migrainosus. Most studies have shown benefit in preventing recurrences rather than merely aborting migraine.11 A systematic review suggested that recurrent headaches are milder with corticosteroid treatment; 19 of 25 studies indicated favorable benefit, and 6 of 19 studies indicated noninferior outcomes.12
Both IV methylprednisolone and IV dexamethasone may be considered.12 Dexamethasone appears to be particularly effective in preventing headache recurrence when combined with other IV therapies.13 It can be given as a single dose of 10 mg, or as repeated doses of 4 mg up to 16 mg/day.1 Patients with active psychosis or uncontrolled diabetes should be closely monitored for these conditions, which corticosteroids can worsen.
Serotoninergic agents
Serotonin agonists including subcutaneous sumatriptan and IV dihydroergotamine are highly effective, with proven statistical and clinical benefit.4 They should be considered in patients with no known history of coronary artery disease or other vaso-occlusive vascular disorder.1
Ideally, IV dihydroergotamine should be administered after consultation with a neurologist or headache specialist, given the pretreatment and cotreatment requirements often necessary to suppress its side effects. Careful titration is important to prevent transient headache exacerbations during infusion, as well as abdominal cramping, nausea, and diarrhea.
Avoid opioids
Opioids should be avoided. Evidence supporting their use in acute migraine is extremely limited,3 and the risks of migraine becoming chronic and of addiction are high.14 Safer, more effective alternatives have been detailed above.
A detailed algorithm for the management of patients with acute migraine has been published14 and is aimed at decreasing acute treatment with opioids and barbiturates.
- Rozen TD. Emergency department and inpatient management of status migrainosus and intractable headache. Continuum (Minneap Minn) 2015; 21(4):1004–1017. doi:10.1212/CON.0000000000000191
- Dodick D. Headache as a symptom of ominous disease. What are the warning signals? Postgrad Med 1997; 101(5):46–50, 55–56, 62–64. doi:10.3810/pgm.1997.05.217
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache 2016; 56(6):911–940. doi:10.1111/head.12835
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55(6):754–762. doi:10.1212/WNL.55.6.754
- Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia 2015; 35(3):271–284. doi:10.1177/0333102414535997
- Swidan SZ, Lake AE 3rd, Saper JR. Efficacy of intravenous diphenhydramine versus intravenous DHE-45 in the treatment of severe migraine headache. Curr Pain Headache Rep 2005; 9(1):65–70. doi:10.1007/s11916-005-0077-5
- Marmura MJ, Goldberg SW. Inpatient management of migraine. Curr Neurol Neurosci Rep 2015; 15(4):13. doi:10.1007/s11910-015-0539-z
- Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol 2009; 103(9):1227–1237. doi:10.1016/j.amjcard.2009.01.014
- Stillman MJ, Zajac D, Rybicki LA. Treatment of primary headache disorders with intravenous valproate: initial outpatient experience. Headache 2004; 44(1):65–69. doi:10.1111/j.1526-4610.2004.04010.x
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55(1):3–20. doi:10.1111/head.12499
- Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ 2008; 336(7657):1359–1361. doi:10.1136/bmj.39566.806725.BE
- Woldeamanuel YW, Rapoport AM, Cowan RP. The place of corticosteroids in migraine attack management: a 65-year systematic review with pooled analysis and critical appraisal. Cephalalgia 2015; 35(1):996–1024. doi:10.1177/0333102414566200
- Singh A, Alter HJ, Zaia B. Does the addition of dexamethasone to standard therapy for acute migraine headache decrease the incidence of recurrent headache for patients treated in the emergency department? A meta-analysis and systematic review of the literature. Acad Emerg Med 2008; 15(12):1223–1233. doi:10.1111/j.1553-2712.2008.00283.x
- Ahmed ZA, Nacopoulos DA, John S, Papesh N, Levine D, Bamford CC. An algorithm for opioid and barbiturate reduction in the acute management of headache in the emergency department. Headache 2017; 57(1):71–79. doi:10.1111/head.12961
We recommend the following combination treatment:
Normal saline (0.9% NaCl) 1 to 2 L by intravenous (IV) infusion over 2 to 4 hours. This can be repeated every 6 to 12 hours.
Ketorolac 30-mg IV bolus, which can be repeated every 6 hours. However, patients with coronary artery disease, uncontrolled hypertension, acute renal failure, or cerebrovascular disease should instead receive acetaminophen 1,000 mg, naproxen sodium 550 mg, or aspirin 325 mg by mouth.
Prochlorperazine or metoclopramide 10-mg IV infusion. This can be repeated every 6 hours. However, to reduce the extrapyramidal adverse effects of these drugs, patients should first receive diphenhydramine 25- to 50-mg IV bolus, which can be repeated every 6 to 8 hours.
Sodium valproate 500 to 1,000 mg by IV infusion over 20 minutes. This can be repeated after 8 hours.
Dexamethasone 4-mg IV bolus every 6 hours, or 10-mg IV bolus once in 24 hours.
Magnesium sulfate 500 to 1,000 mg by IV infusion over 1 hour. This can be repeated every 6 to 12 hours.
If the migraine has not improved after 3 cycles of this regimen, a neurologic consultation should be considered. Other options include dihydroergotamine and occipital nerve blocks1 performed at the bedside.
GENERAL PRINCIPLES
Managing acute intractable migraine can be frustrating for both the practitioner and the patient. Some general principles are helpful.
Use a combination of drugs. Aborting a severe migraine attack often requires a combination of medications that work synergistically.
Use IV and intramuscular formulations rather than oral formulations: they are more rapidly absorbed, provide faster pain relief, and can be given when the nausea that often accompanies migraine precludes oral treatments.
Rule out secondary causes. The mnemonic SNOOP—systemic signs, neurologic signs, onset, older age, progression of existing headache disorder—is useful for assessing underlying causes.2 Any patient presenting with intractable migraine should also have a thorough neurologic examination.
Screening electrocardiography may be helpful, as the pretreatment QTc interval may direct the choice of intravenous treatment. If the patient has a prolonged QTc or is taking another drug that could prolong the QTc, certain medications, specifically dopamine receptor antagonists and diphenhydramine, should be avoided.
Ask the patient what has worked previously. A particular agent may have been effective in aborting the migraine; thus, a single dose of it could abort the headache, expediting discharge.
Establish if a triptan or ergot derivative has been used during the 24 hours before presentation, as repeated dosing within this interval is not recommended.3
Establish the baseline headache severity. Complete headache relief is difficult to achieve in a patient with chronic daily headache, and establishing a more realistic goal (eg, 50% relief) from the outset is useful.
OPTIONS FOR DRUG THERAPY
Antiemetics
Dopamine receptor antagonists are assumed to merely treat nausea in patients with migraine; however, they act independently to abort migraine and thus should be considered, irrespective of the presence of nausea.
The two most commonly used agents are prochlorperazine and metoclopramide. The American Academy of Neurology guidelines recommend prochlorperazine as first-line therapy for acute migraine. Metoclopramide is rated slightly lower and is considered to have moderate benefit.4 The Canadian Headache Society cites a high level of evidence supporting prochlorperazine and a moderate level of evidence supporting metoclopramide.5 The American Headache Society assessment of parenteral pharmacotherapies gives prochlorperazine and metoclopramide a level B recommendation of “should offer” (a recommendation only additionally assigned to subcutaneous sumatriptan).3 Hence, either agent can be used.
To reduce the risk of posttreatment akathisia, diphenhydramine or benztropine may be given before starting a dopamine receptor antagonist. Diphenhydramine may be independently effective in migraine treatment,6,7 but data on this are limited.
Ketorolac, ibuprofen
Ketorolac and ibuprofen are the only available nonsteroidal antiinflammatory drugs (NSAIDs) for IV administration. The Canadian Headache Society guidelines strongly recommend ketorolac for the treatment of migraine in emergency settings.5 Doses range from 30 mg to 60 mg.1 Ibuprofen 400 to 800 mg by IV infusion is an acceptable alternative. These medications should be avoided in patients with renal failure or severe coronary artery disease.
Oral naproxen sodium is a possible alternative in patients with cardiovascular disease, as it has been shown to carry a lower cardiovascular risk than other NSAIDs.8
The same concerns in patients with renal dysfunction apply to any NSAID, as the enzyme cyclooxygenase plays a constitutive role in glomerular function.
Antiepileptic drugs
The antiepileptic drugs sodium valproate and levetiracetam are available in IV formulations that have demonstrated efficacy in the treatment of status migrainosus1 (ie, migraine lasting more than 72 hours). Valproate has the strongest track record, is well tolerated, and is effective in rapidly aborting migraine.9
Volume repletion
Although its use is anecdotal and to date no trial has measured its efficacy, IV volume repletion is often used in acute migraine, as most headache experts surmise it to be highly effective, especially in patients with prolonged nausea or vomiting.1
Magnesium
IV magnesium is effective, particularly for migraine with aura.10 Hypotension is a common side effect, and pretreatment or concurrent treatment with IV fluids is advised. Doses from 500 mg to 1,000 mg have demonstrated efficacy.10
Corticosteroids
Corticosteroids can be used in the treatment of status migrainosus. Most studies have shown benefit in preventing recurrences rather than merely aborting migraine.11 A systematic review suggested that recurrent headaches are milder with corticosteroid treatment; 19 of 25 studies indicated favorable benefit, and 6 of 19 studies indicated noninferior outcomes.12
Both IV methylprednisolone and IV dexamethasone may be considered.12 Dexamethasone appears to be particularly effective in preventing headache recurrence when combined with other IV therapies.13 It can be given as a single dose of 10 mg, or as repeated doses of 4 mg up to 16 mg/day.1 Patients with active psychosis or uncontrolled diabetes should be closely monitored for these conditions, which corticosteroids can worsen.
Serotoninergic agents
Serotonin agonists including subcutaneous sumatriptan and IV dihydroergotamine are highly effective, with proven statistical and clinical benefit.4 They should be considered in patients with no known history of coronary artery disease or other vaso-occlusive vascular disorder.1
Ideally, IV dihydroergotamine should be administered after consultation with a neurologist or headache specialist, given the pretreatment and cotreatment requirements often necessary to suppress its side effects. Careful titration is important to prevent transient headache exacerbations during infusion, as well as abdominal cramping, nausea, and diarrhea.
Avoid opioids
Opioids should be avoided. Evidence supporting their use in acute migraine is extremely limited,3 and the risks of migraine becoming chronic and of addiction are high.14 Safer, more effective alternatives have been detailed above.
A detailed algorithm for the management of patients with acute migraine has been published14 and is aimed at decreasing acute treatment with opioids and barbiturates.
We recommend the following combination treatment:
Normal saline (0.9% NaCl) 1 to 2 L by intravenous (IV) infusion over 2 to 4 hours. This can be repeated every 6 to 12 hours.
Ketorolac 30-mg IV bolus, which can be repeated every 6 hours. However, patients with coronary artery disease, uncontrolled hypertension, acute renal failure, or cerebrovascular disease should instead receive acetaminophen 1,000 mg, naproxen sodium 550 mg, or aspirin 325 mg by mouth.
Prochlorperazine or metoclopramide 10-mg IV infusion. This can be repeated every 6 hours. However, to reduce the extrapyramidal adverse effects of these drugs, patients should first receive diphenhydramine 25- to 50-mg IV bolus, which can be repeated every 6 to 8 hours.
Sodium valproate 500 to 1,000 mg by IV infusion over 20 minutes. This can be repeated after 8 hours.
Dexamethasone 4-mg IV bolus every 6 hours, or 10-mg IV bolus once in 24 hours.
Magnesium sulfate 500 to 1,000 mg by IV infusion over 1 hour. This can be repeated every 6 to 12 hours.
If the migraine has not improved after 3 cycles of this regimen, a neurologic consultation should be considered. Other options include dihydroergotamine and occipital nerve blocks1 performed at the bedside.
GENERAL PRINCIPLES
Managing acute intractable migraine can be frustrating for both the practitioner and the patient. Some general principles are helpful.
Use a combination of drugs. Aborting a severe migraine attack often requires a combination of medications that work synergistically.
Use IV and intramuscular formulations rather than oral formulations: they are more rapidly absorbed, provide faster pain relief, and can be given when the nausea that often accompanies migraine precludes oral treatments.
Rule out secondary causes. The mnemonic SNOOP—systemic signs, neurologic signs, onset, older age, progression of existing headache disorder—is useful for assessing underlying causes.2 Any patient presenting with intractable migraine should also have a thorough neurologic examination.
Screening electrocardiography may be helpful, as the pretreatment QTc interval may direct the choice of intravenous treatment. If the patient has a prolonged QTc or is taking another drug that could prolong the QTc, certain medications, specifically dopamine receptor antagonists and diphenhydramine, should be avoided.
Ask the patient what has worked previously. A particular agent may have been effective in aborting the migraine; thus, a single dose of it could abort the headache, expediting discharge.
Establish if a triptan or ergot derivative has been used during the 24 hours before presentation, as repeated dosing within this interval is not recommended.3
Establish the baseline headache severity. Complete headache relief is difficult to achieve in a patient with chronic daily headache, and establishing a more realistic goal (eg, 50% relief) from the outset is useful.
OPTIONS FOR DRUG THERAPY
Antiemetics
Dopamine receptor antagonists are assumed to merely treat nausea in patients with migraine; however, they act independently to abort migraine and thus should be considered, irrespective of the presence of nausea.
The two most commonly used agents are prochlorperazine and metoclopramide. The American Academy of Neurology guidelines recommend prochlorperazine as first-line therapy for acute migraine. Metoclopramide is rated slightly lower and is considered to have moderate benefit.4 The Canadian Headache Society cites a high level of evidence supporting prochlorperazine and a moderate level of evidence supporting metoclopramide.5 The American Headache Society assessment of parenteral pharmacotherapies gives prochlorperazine and metoclopramide a level B recommendation of “should offer” (a recommendation only additionally assigned to subcutaneous sumatriptan).3 Hence, either agent can be used.
To reduce the risk of posttreatment akathisia, diphenhydramine or benztropine may be given before starting a dopamine receptor antagonist. Diphenhydramine may be independently effective in migraine treatment,6,7 but data on this are limited.
Ketorolac, ibuprofen
Ketorolac and ibuprofen are the only available nonsteroidal antiinflammatory drugs (NSAIDs) for IV administration. The Canadian Headache Society guidelines strongly recommend ketorolac for the treatment of migraine in emergency settings.5 Doses range from 30 mg to 60 mg.1 Ibuprofen 400 to 800 mg by IV infusion is an acceptable alternative. These medications should be avoided in patients with renal failure or severe coronary artery disease.
Oral naproxen sodium is a possible alternative in patients with cardiovascular disease, as it has been shown to carry a lower cardiovascular risk than other NSAIDs.8
The same concerns in patients with renal dysfunction apply to any NSAID, as the enzyme cyclooxygenase plays a constitutive role in glomerular function.
Antiepileptic drugs
The antiepileptic drugs sodium valproate and levetiracetam are available in IV formulations that have demonstrated efficacy in the treatment of status migrainosus1 (ie, migraine lasting more than 72 hours). Valproate has the strongest track record, is well tolerated, and is effective in rapidly aborting migraine.9
Volume repletion
Although its use is anecdotal and to date no trial has measured its efficacy, IV volume repletion is often used in acute migraine, as most headache experts surmise it to be highly effective, especially in patients with prolonged nausea or vomiting.1
Magnesium
IV magnesium is effective, particularly for migraine with aura.10 Hypotension is a common side effect, and pretreatment or concurrent treatment with IV fluids is advised. Doses from 500 mg to 1,000 mg have demonstrated efficacy.10
Corticosteroids
Corticosteroids can be used in the treatment of status migrainosus. Most studies have shown benefit in preventing recurrences rather than merely aborting migraine.11 A systematic review suggested that recurrent headaches are milder with corticosteroid treatment; 19 of 25 studies indicated favorable benefit, and 6 of 19 studies indicated noninferior outcomes.12
Both IV methylprednisolone and IV dexamethasone may be considered.12 Dexamethasone appears to be particularly effective in preventing headache recurrence when combined with other IV therapies.13 It can be given as a single dose of 10 mg, or as repeated doses of 4 mg up to 16 mg/day.1 Patients with active psychosis or uncontrolled diabetes should be closely monitored for these conditions, which corticosteroids can worsen.
Serotoninergic agents
Serotonin agonists including subcutaneous sumatriptan and IV dihydroergotamine are highly effective, with proven statistical and clinical benefit.4 They should be considered in patients with no known history of coronary artery disease or other vaso-occlusive vascular disorder.1
Ideally, IV dihydroergotamine should be administered after consultation with a neurologist or headache specialist, given the pretreatment and cotreatment requirements often necessary to suppress its side effects. Careful titration is important to prevent transient headache exacerbations during infusion, as well as abdominal cramping, nausea, and diarrhea.
Avoid opioids
Opioids should be avoided. Evidence supporting their use in acute migraine is extremely limited,3 and the risks of migraine becoming chronic and of addiction are high.14 Safer, more effective alternatives have been detailed above.
A detailed algorithm for the management of patients with acute migraine has been published14 and is aimed at decreasing acute treatment with opioids and barbiturates.
- Rozen TD. Emergency department and inpatient management of status migrainosus and intractable headache. Continuum (Minneap Minn) 2015; 21(4):1004–1017. doi:10.1212/CON.0000000000000191
- Dodick D. Headache as a symptom of ominous disease. What are the warning signals? Postgrad Med 1997; 101(5):46–50, 55–56, 62–64. doi:10.3810/pgm.1997.05.217
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache 2016; 56(6):911–940. doi:10.1111/head.12835
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55(6):754–762. doi:10.1212/WNL.55.6.754
- Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia 2015; 35(3):271–284. doi:10.1177/0333102414535997
- Swidan SZ, Lake AE 3rd, Saper JR. Efficacy of intravenous diphenhydramine versus intravenous DHE-45 in the treatment of severe migraine headache. Curr Pain Headache Rep 2005; 9(1):65–70. doi:10.1007/s11916-005-0077-5
- Marmura MJ, Goldberg SW. Inpatient management of migraine. Curr Neurol Neurosci Rep 2015; 15(4):13. doi:10.1007/s11910-015-0539-z
- Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol 2009; 103(9):1227–1237. doi:10.1016/j.amjcard.2009.01.014
- Stillman MJ, Zajac D, Rybicki LA. Treatment of primary headache disorders with intravenous valproate: initial outpatient experience. Headache 2004; 44(1):65–69. doi:10.1111/j.1526-4610.2004.04010.x
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55(1):3–20. doi:10.1111/head.12499
- Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ 2008; 336(7657):1359–1361. doi:10.1136/bmj.39566.806725.BE
- Woldeamanuel YW, Rapoport AM, Cowan RP. The place of corticosteroids in migraine attack management: a 65-year systematic review with pooled analysis and critical appraisal. Cephalalgia 2015; 35(1):996–1024. doi:10.1177/0333102414566200
- Singh A, Alter HJ, Zaia B. Does the addition of dexamethasone to standard therapy for acute migraine headache decrease the incidence of recurrent headache for patients treated in the emergency department? A meta-analysis and systematic review of the literature. Acad Emerg Med 2008; 15(12):1223–1233. doi:10.1111/j.1553-2712.2008.00283.x
- Ahmed ZA, Nacopoulos DA, John S, Papesh N, Levine D, Bamford CC. An algorithm for opioid and barbiturate reduction in the acute management of headache in the emergency department. Headache 2017; 57(1):71–79. doi:10.1111/head.12961
- Rozen TD. Emergency department and inpatient management of status migrainosus and intractable headache. Continuum (Minneap Minn) 2015; 21(4):1004–1017. doi:10.1212/CON.0000000000000191
- Dodick D. Headache as a symptom of ominous disease. What are the warning signals? Postgrad Med 1997; 101(5):46–50, 55–56, 62–64. doi:10.3810/pgm.1997.05.217
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache 2016; 56(6):911–940. doi:10.1111/head.12835
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55(6):754–762. doi:10.1212/WNL.55.6.754
- Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia 2015; 35(3):271–284. doi:10.1177/0333102414535997
- Swidan SZ, Lake AE 3rd, Saper JR. Efficacy of intravenous diphenhydramine versus intravenous DHE-45 in the treatment of severe migraine headache. Curr Pain Headache Rep 2005; 9(1):65–70. doi:10.1007/s11916-005-0077-5
- Marmura MJ, Goldberg SW. Inpatient management of migraine. Curr Neurol Neurosci Rep 2015; 15(4):13. doi:10.1007/s11910-015-0539-z
- Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol 2009; 103(9):1227–1237. doi:10.1016/j.amjcard.2009.01.014
- Stillman MJ, Zajac D, Rybicki LA. Treatment of primary headache disorders with intravenous valproate: initial outpatient experience. Headache 2004; 44(1):65–69. doi:10.1111/j.1526-4610.2004.04010.x
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55(1):3–20. doi:10.1111/head.12499
- Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ 2008; 336(7657):1359–1361. doi:10.1136/bmj.39566.806725.BE
- Woldeamanuel YW, Rapoport AM, Cowan RP. The place of corticosteroids in migraine attack management: a 65-year systematic review with pooled analysis and critical appraisal. Cephalalgia 2015; 35(1):996–1024. doi:10.1177/0333102414566200
- Singh A, Alter HJ, Zaia B. Does the addition of dexamethasone to standard therapy for acute migraine headache decrease the incidence of recurrent headache for patients treated in the emergency department? A meta-analysis and systematic review of the literature. Acad Emerg Med 2008; 15(12):1223–1233. doi:10.1111/j.1553-2712.2008.00283.x
- Ahmed ZA, Nacopoulos DA, John S, Papesh N, Levine D, Bamford CC. An algorithm for opioid and barbiturate reduction in the acute management of headache in the emergency department. Headache 2017; 57(1):71–79. doi:10.1111/head.12961
When does S aureus bacteremia require transesophageal echocardiography?
Staphylococcus aureus is the most common infective agent in native and prosthetic valve endocarditis, and 13% to 22% of patients with S aureus bacteremia have infective endocarditis.1
Transthoracic echocardiography (TTE) is a good starting point in the workup of suspected infective endocarditis, but transesophageal echocardiography (TEE) plays a key role in diagnosis and is indicated in patients with a high pretest probability of infective endocarditis, as in the following scenarios:
- Clinical picture consistent with infective endocarditis
- Presence of previously placed port or other indwelling vascular device
- Presence of a prosthetic valve or other prosthetic material
- Presence of a pacemaker
- History of valve disease
- Injection drug use
- Positive blood cultures after 72 hours despite appropriate antibiotic treatment
- Abnormal TTE result requiring better visualization of valvular anatomy and function and confirmation of local complications
- Absence of another reasonable explanation for S aureus bacteremia.
Forgoing TEE is reasonable in patients with normal results on TTE, no predisposing risk factors, a reasonable alternative explanation for S aureus bacteremia, and a low pretest probability of infective endocarditis.1 TEE may also be unnecessary if there is another disease focus requiring extended treatment (eg, vertebral infection) and there are no findings suggesting complicated infective endocarditis, eg, persistent bacteremia, symptoms of heart failure, and conduction abnormality.1
TEE also may be unnecessary in patients at low risk who have identifiable foci of bacteremia due to soft-tissue infection or a newly placed vascular catheter and whose bacteremia clears within 72 hours of the start of antibiotic therapy. These patients may be followed clinically for the development of new findings such as metastatic foci of infection (eg, septic pulmonary emboli, renal infarction, splenic abscess or infarction), the new onset of heart failure or cardiac conduction abnormality, or recurrence of previously cleared S aureus bacteremia. If these should develop, then a more invasive study such as TEE may be warranted.
INFECTIVE ENDOCARDITIS: EPIDEMIOLOGY AND MICROBIOLOGY
The US incidence rate of infective endocarditis has steadily increased, with an estimated 457,052 hospitalizations from 2000 to 2011. During that period, from 2000 to 2007, there was a marked increase in valve replacement surgeries.2 This trend is likely explained by an increase in the at-risk population—eg, elderly patients, patients with opiate dependence or diabetes, and patients on hemodialysis.
Although S aureus is the predominant pathogen in infective endocarditis,2–5 S aureus bacteremia is often observed in patients with skin or soft-tissue infection, prosthetic device infection, vascular graft or catheter infection, and bone and joint infections. S aureus bacteremia necessitates a search for the source of infection.
S aureus is a major pathogen in bloodstream infections, and up to 14% of patients with S aureus bacteremia have infective endocarditis as the primary source of infection.3 The pathogenesis of S aureus infective endocarditis is thought to be mediated by cell-wall factors that promote adhesion to the extracellular matrix of intravascular structures.3
A new localizing symptom such as back pain, joint pain, or swelling in a patient with S aureus bacteremia should trigger an investigation for metastatic infection.
Infectious disease consultation in patients with S aureus bacteremia is associated with improved outcomes and, thus, should be pursued.3
A cardiac surgery consult is recommended early on in cases of infective endocarditis caused by vancomycin-resistant enterococci, Pseudomonas aeruginosa, and fungi, as well as in patients with complications such as valvular insufficiency, perivalvular abscess, conduction abnormalities, persistent bacteremia, and metastatic foci of infection.6
RISK FACTORS
Risk factors for infective endocarditis include injection drug abuse, valvular heart disease, congenital heart disease (unrepaired, repaired with residual defects, or fully repaired within the past 6 months), previous infective endocarditis, prosthetic heart valve, and cardiac transplant.2–4,6 Other risk factors are poor dentition, hemodialysis, ventriculoatrial shunts, intravascular devices including vascular grafts, and pacemakers.2,3 Many risk factors for infective endocarditis and S aureus bacteremia overlap.3
DIAGNOSTIC PRINCIPLES
The clinical presentation of infective endocarditis can vary from a nonspecific infectious syndrome, to overt organ failure (heart failure, kidney failure), to an acute vascular catastrophe (arterial ischemia, cerebrovascular accidents, myocardial infarction). Patients may present with indolent symptoms such as fever, fatigue, and weight loss,6 or they may present at an advanced stage, with fulminant acute heart failure due to valvular insufficiency or with arrhythmias due to a perivalvular abscess infiltrating the conduction system. Extracardiac clinical manifestations may be related to direct infective metastatic foci such as septic emboli or to immunologic phenomena such as glomerulonephritis or Osler nodes.
ECHOCARDIOGRAPHY’S ROLE IN DIAGNOSIS
TTE plays an important role in diagnosis and risk stratification of infective endocarditis.6 TTE is usually done first because of its low cost, wide availability, and safety; it has a sensitivity of 70% and a specificity over 95%.8 While a normal result on TTE does not completely rule out infective endocarditis, completely normal valvular morphology and function on TTE make the diagnosis less likely.8,9
If suspicion remains high despite a normal study, repeating TTE at a later time may result in a higher diagnostic yield because of growth of the suspected vegetation. Otherwise, TEE should be considered.
TEE provides a higher spatial resolution and diagnostic yield than TTE, especially for detecting complex pathology such as pseudoaneurysm, valve perforation, or valvular abscess. TEE has a sensitivity and specificity of approximately 95% for infective endocarditis.8 It should be performed early in patients with preexisting valve disease, prosthetic cardiac material (eg, valves), or a pacemaker or implantable cardioverter-defibrillator.6,7
Detecting valve vegetation provides answers about the cause of S aureus bacteremia with its complications (eg, septic emboli, mycotic aneurysm) and informs decisions about the duration of antibiotic therapy and the need for surgery.3,6
As with any diagnostic test, it is important to compare the results of any recent study with those of previous studies whenever possible to differentiate new from old findings.
WHEN TO FORGO TEE IN S AUREUS BACTEREMIA
Because TEE is invasive and requires the patient to swallow an endoscopic probe,10 it is important to screen patients for esophageal disease, cervical spine conditions, and baseline respiratory insufficiency. Complications are rare but include esophageal perforation, esophageal bleeding, pharyngeal hematoma, and reactions to anesthesia.10
As with any diagnostic test, the clinician first needs to consider the patient’s pretest probability of the disease, the diagnostic accuracy, the associated risks and costs, and the implications of the results.
While TEE provides better diagnostic images than TTE, a normal TEE study does not exclude the diagnosis of infective endocarditis: small lesions and complications such as paravalvular abscess of a prosthetic aortic valve may still be missed. In such patients, a repeat TEE examination or additional imaging study (eg, gated computed tomographic angiography) should be considered.6
Noninfective sterile echodensities, valvular tumors such as papillary fibroelastomas, Lambl excrescences, and suture lines of prosthetic valves are among the conditions and factors that can cause a false-positive result on TEE.
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65(19):2070–2076. doi:10.1016/j.jacc.2015.03.518
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28(3):603–661. doi:10.1128/CMR.00134-14
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Barton T, Moir S, Rehmani H, Woolley I, Korman TM, Stuart RL. Low rates of endocarditis in healthcare-associated Staphylococcus aureus bacteremia suggest that echocardiography might not always be required. Eur J Clin Microbiol Infect Dis 2016; 35(1):49–55. doi:10.1007/s10096-015-2505-8
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi10.1161/CIR.0000000000000296
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30(4):633–638. doi:10.1086/313753
- Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11(2):202–219. doi:10.1093/ejechocard/jeq004
- Irani WN, Grayburn PA, Afridi I. A negative transthoracic echocardiogram obviates the need for transesophageal echocardiography in patients with suspected native valve active infective endocarditis. Am J Cardiol 1996; 78(1):101–103. pmid:8712097
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013; 26(9):921–964. doi:10.1016/j.echo.2013.07.009
Staphylococcus aureus is the most common infective agent in native and prosthetic valve endocarditis, and 13% to 22% of patients with S aureus bacteremia have infective endocarditis.1
Transthoracic echocardiography (TTE) is a good starting point in the workup of suspected infective endocarditis, but transesophageal echocardiography (TEE) plays a key role in diagnosis and is indicated in patients with a high pretest probability of infective endocarditis, as in the following scenarios:
- Clinical picture consistent with infective endocarditis
- Presence of previously placed port or other indwelling vascular device
- Presence of a prosthetic valve or other prosthetic material
- Presence of a pacemaker
- History of valve disease
- Injection drug use
- Positive blood cultures after 72 hours despite appropriate antibiotic treatment
- Abnormal TTE result requiring better visualization of valvular anatomy and function and confirmation of local complications
- Absence of another reasonable explanation for S aureus bacteremia.
Forgoing TEE is reasonable in patients with normal results on TTE, no predisposing risk factors, a reasonable alternative explanation for S aureus bacteremia, and a low pretest probability of infective endocarditis.1 TEE may also be unnecessary if there is another disease focus requiring extended treatment (eg, vertebral infection) and there are no findings suggesting complicated infective endocarditis, eg, persistent bacteremia, symptoms of heart failure, and conduction abnormality.1
TEE also may be unnecessary in patients at low risk who have identifiable foci of bacteremia due to soft-tissue infection or a newly placed vascular catheter and whose bacteremia clears within 72 hours of the start of antibiotic therapy. These patients may be followed clinically for the development of new findings such as metastatic foci of infection (eg, septic pulmonary emboli, renal infarction, splenic abscess or infarction), the new onset of heart failure or cardiac conduction abnormality, or recurrence of previously cleared S aureus bacteremia. If these should develop, then a more invasive study such as TEE may be warranted.
INFECTIVE ENDOCARDITIS: EPIDEMIOLOGY AND MICROBIOLOGY
The US incidence rate of infective endocarditis has steadily increased, with an estimated 457,052 hospitalizations from 2000 to 2011. During that period, from 2000 to 2007, there was a marked increase in valve replacement surgeries.2 This trend is likely explained by an increase in the at-risk population—eg, elderly patients, patients with opiate dependence or diabetes, and patients on hemodialysis.
Although S aureus is the predominant pathogen in infective endocarditis,2–5 S aureus bacteremia is often observed in patients with skin or soft-tissue infection, prosthetic device infection, vascular graft or catheter infection, and bone and joint infections. S aureus bacteremia necessitates a search for the source of infection.
S aureus is a major pathogen in bloodstream infections, and up to 14% of patients with S aureus bacteremia have infective endocarditis as the primary source of infection.3 The pathogenesis of S aureus infective endocarditis is thought to be mediated by cell-wall factors that promote adhesion to the extracellular matrix of intravascular structures.3
A new localizing symptom such as back pain, joint pain, or swelling in a patient with S aureus bacteremia should trigger an investigation for metastatic infection.
Infectious disease consultation in patients with S aureus bacteremia is associated with improved outcomes and, thus, should be pursued.3
A cardiac surgery consult is recommended early on in cases of infective endocarditis caused by vancomycin-resistant enterococci, Pseudomonas aeruginosa, and fungi, as well as in patients with complications such as valvular insufficiency, perivalvular abscess, conduction abnormalities, persistent bacteremia, and metastatic foci of infection.6
RISK FACTORS
Risk factors for infective endocarditis include injection drug abuse, valvular heart disease, congenital heart disease (unrepaired, repaired with residual defects, or fully repaired within the past 6 months), previous infective endocarditis, prosthetic heart valve, and cardiac transplant.2–4,6 Other risk factors are poor dentition, hemodialysis, ventriculoatrial shunts, intravascular devices including vascular grafts, and pacemakers.2,3 Many risk factors for infective endocarditis and S aureus bacteremia overlap.3
DIAGNOSTIC PRINCIPLES
The clinical presentation of infective endocarditis can vary from a nonspecific infectious syndrome, to overt organ failure (heart failure, kidney failure), to an acute vascular catastrophe (arterial ischemia, cerebrovascular accidents, myocardial infarction). Patients may present with indolent symptoms such as fever, fatigue, and weight loss,6 or they may present at an advanced stage, with fulminant acute heart failure due to valvular insufficiency or with arrhythmias due to a perivalvular abscess infiltrating the conduction system. Extracardiac clinical manifestations may be related to direct infective metastatic foci such as septic emboli or to immunologic phenomena such as glomerulonephritis or Osler nodes.
ECHOCARDIOGRAPHY’S ROLE IN DIAGNOSIS
TTE plays an important role in diagnosis and risk stratification of infective endocarditis.6 TTE is usually done first because of its low cost, wide availability, and safety; it has a sensitivity of 70% and a specificity over 95%.8 While a normal result on TTE does not completely rule out infective endocarditis, completely normal valvular morphology and function on TTE make the diagnosis less likely.8,9
If suspicion remains high despite a normal study, repeating TTE at a later time may result in a higher diagnostic yield because of growth of the suspected vegetation. Otherwise, TEE should be considered.
TEE provides a higher spatial resolution and diagnostic yield than TTE, especially for detecting complex pathology such as pseudoaneurysm, valve perforation, or valvular abscess. TEE has a sensitivity and specificity of approximately 95% for infective endocarditis.8 It should be performed early in patients with preexisting valve disease, prosthetic cardiac material (eg, valves), or a pacemaker or implantable cardioverter-defibrillator.6,7
Detecting valve vegetation provides answers about the cause of S aureus bacteremia with its complications (eg, septic emboli, mycotic aneurysm) and informs decisions about the duration of antibiotic therapy and the need for surgery.3,6
As with any diagnostic test, it is important to compare the results of any recent study with those of previous studies whenever possible to differentiate new from old findings.
WHEN TO FORGO TEE IN S AUREUS BACTEREMIA
Because TEE is invasive and requires the patient to swallow an endoscopic probe,10 it is important to screen patients for esophageal disease, cervical spine conditions, and baseline respiratory insufficiency. Complications are rare but include esophageal perforation, esophageal bleeding, pharyngeal hematoma, and reactions to anesthesia.10
As with any diagnostic test, the clinician first needs to consider the patient’s pretest probability of the disease, the diagnostic accuracy, the associated risks and costs, and the implications of the results.
While TEE provides better diagnostic images than TTE, a normal TEE study does not exclude the diagnosis of infective endocarditis: small lesions and complications such as paravalvular abscess of a prosthetic aortic valve may still be missed. In such patients, a repeat TEE examination or additional imaging study (eg, gated computed tomographic angiography) should be considered.6
Noninfective sterile echodensities, valvular tumors such as papillary fibroelastomas, Lambl excrescences, and suture lines of prosthetic valves are among the conditions and factors that can cause a false-positive result on TEE.
Staphylococcus aureus is the most common infective agent in native and prosthetic valve endocarditis, and 13% to 22% of patients with S aureus bacteremia have infective endocarditis.1
Transthoracic echocardiography (TTE) is a good starting point in the workup of suspected infective endocarditis, but transesophageal echocardiography (TEE) plays a key role in diagnosis and is indicated in patients with a high pretest probability of infective endocarditis, as in the following scenarios:
- Clinical picture consistent with infective endocarditis
- Presence of previously placed port or other indwelling vascular device
- Presence of a prosthetic valve or other prosthetic material
- Presence of a pacemaker
- History of valve disease
- Injection drug use
- Positive blood cultures after 72 hours despite appropriate antibiotic treatment
- Abnormal TTE result requiring better visualization of valvular anatomy and function and confirmation of local complications
- Absence of another reasonable explanation for S aureus bacteremia.
Forgoing TEE is reasonable in patients with normal results on TTE, no predisposing risk factors, a reasonable alternative explanation for S aureus bacteremia, and a low pretest probability of infective endocarditis.1 TEE may also be unnecessary if there is another disease focus requiring extended treatment (eg, vertebral infection) and there are no findings suggesting complicated infective endocarditis, eg, persistent bacteremia, symptoms of heart failure, and conduction abnormality.1
TEE also may be unnecessary in patients at low risk who have identifiable foci of bacteremia due to soft-tissue infection or a newly placed vascular catheter and whose bacteremia clears within 72 hours of the start of antibiotic therapy. These patients may be followed clinically for the development of new findings such as metastatic foci of infection (eg, septic pulmonary emboli, renal infarction, splenic abscess or infarction), the new onset of heart failure or cardiac conduction abnormality, or recurrence of previously cleared S aureus bacteremia. If these should develop, then a more invasive study such as TEE may be warranted.
INFECTIVE ENDOCARDITIS: EPIDEMIOLOGY AND MICROBIOLOGY
The US incidence rate of infective endocarditis has steadily increased, with an estimated 457,052 hospitalizations from 2000 to 2011. During that period, from 2000 to 2007, there was a marked increase in valve replacement surgeries.2 This trend is likely explained by an increase in the at-risk population—eg, elderly patients, patients with opiate dependence or diabetes, and patients on hemodialysis.
Although S aureus is the predominant pathogen in infective endocarditis,2–5 S aureus bacteremia is often observed in patients with skin or soft-tissue infection, prosthetic device infection, vascular graft or catheter infection, and bone and joint infections. S aureus bacteremia necessitates a search for the source of infection.
S aureus is a major pathogen in bloodstream infections, and up to 14% of patients with S aureus bacteremia have infective endocarditis as the primary source of infection.3 The pathogenesis of S aureus infective endocarditis is thought to be mediated by cell-wall factors that promote adhesion to the extracellular matrix of intravascular structures.3
A new localizing symptom such as back pain, joint pain, or swelling in a patient with S aureus bacteremia should trigger an investigation for metastatic infection.
Infectious disease consultation in patients with S aureus bacteremia is associated with improved outcomes and, thus, should be pursued.3
A cardiac surgery consult is recommended early on in cases of infective endocarditis caused by vancomycin-resistant enterococci, Pseudomonas aeruginosa, and fungi, as well as in patients with complications such as valvular insufficiency, perivalvular abscess, conduction abnormalities, persistent bacteremia, and metastatic foci of infection.6
RISK FACTORS
Risk factors for infective endocarditis include injection drug abuse, valvular heart disease, congenital heart disease (unrepaired, repaired with residual defects, or fully repaired within the past 6 months), previous infective endocarditis, prosthetic heart valve, and cardiac transplant.2–4,6 Other risk factors are poor dentition, hemodialysis, ventriculoatrial shunts, intravascular devices including vascular grafts, and pacemakers.2,3 Many risk factors for infective endocarditis and S aureus bacteremia overlap.3
DIAGNOSTIC PRINCIPLES
The clinical presentation of infective endocarditis can vary from a nonspecific infectious syndrome, to overt organ failure (heart failure, kidney failure), to an acute vascular catastrophe (arterial ischemia, cerebrovascular accidents, myocardial infarction). Patients may present with indolent symptoms such as fever, fatigue, and weight loss,6 or they may present at an advanced stage, with fulminant acute heart failure due to valvular insufficiency or with arrhythmias due to a perivalvular abscess infiltrating the conduction system. Extracardiac clinical manifestations may be related to direct infective metastatic foci such as septic emboli or to immunologic phenomena such as glomerulonephritis or Osler nodes.
ECHOCARDIOGRAPHY’S ROLE IN DIAGNOSIS
TTE plays an important role in diagnosis and risk stratification of infective endocarditis.6 TTE is usually done first because of its low cost, wide availability, and safety; it has a sensitivity of 70% and a specificity over 95%.8 While a normal result on TTE does not completely rule out infective endocarditis, completely normal valvular morphology and function on TTE make the diagnosis less likely.8,9
If suspicion remains high despite a normal study, repeating TTE at a later time may result in a higher diagnostic yield because of growth of the suspected vegetation. Otherwise, TEE should be considered.
TEE provides a higher spatial resolution and diagnostic yield than TTE, especially for detecting complex pathology such as pseudoaneurysm, valve perforation, or valvular abscess. TEE has a sensitivity and specificity of approximately 95% for infective endocarditis.8 It should be performed early in patients with preexisting valve disease, prosthetic cardiac material (eg, valves), or a pacemaker or implantable cardioverter-defibrillator.6,7
Detecting valve vegetation provides answers about the cause of S aureus bacteremia with its complications (eg, septic emboli, mycotic aneurysm) and informs decisions about the duration of antibiotic therapy and the need for surgery.3,6
As with any diagnostic test, it is important to compare the results of any recent study with those of previous studies whenever possible to differentiate new from old findings.
WHEN TO FORGO TEE IN S AUREUS BACTEREMIA
Because TEE is invasive and requires the patient to swallow an endoscopic probe,10 it is important to screen patients for esophageal disease, cervical spine conditions, and baseline respiratory insufficiency. Complications are rare but include esophageal perforation, esophageal bleeding, pharyngeal hematoma, and reactions to anesthesia.10
As with any diagnostic test, the clinician first needs to consider the patient’s pretest probability of the disease, the diagnostic accuracy, the associated risks and costs, and the implications of the results.
While TEE provides better diagnostic images than TTE, a normal TEE study does not exclude the diagnosis of infective endocarditis: small lesions and complications such as paravalvular abscess of a prosthetic aortic valve may still be missed. In such patients, a repeat TEE examination or additional imaging study (eg, gated computed tomographic angiography) should be considered.6
Noninfective sterile echodensities, valvular tumors such as papillary fibroelastomas, Lambl excrescences, and suture lines of prosthetic valves are among the conditions and factors that can cause a false-positive result on TEE.
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65(19):2070–2076. doi:10.1016/j.jacc.2015.03.518
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28(3):603–661. doi:10.1128/CMR.00134-14
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Barton T, Moir S, Rehmani H, Woolley I, Korman TM, Stuart RL. Low rates of endocarditis in healthcare-associated Staphylococcus aureus bacteremia suggest that echocardiography might not always be required. Eur J Clin Microbiol Infect Dis 2016; 35(1):49–55. doi:10.1007/s10096-015-2505-8
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi10.1161/CIR.0000000000000296
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30(4):633–638. doi:10.1086/313753
- Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11(2):202–219. doi:10.1093/ejechocard/jeq004
- Irani WN, Grayburn PA, Afridi I. A negative transthoracic echocardiogram obviates the need for transesophageal echocardiography in patients with suspected native valve active infective endocarditis. Am J Cardiol 1996; 78(1):101–103. pmid:8712097
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013; 26(9):921–964. doi:10.1016/j.echo.2013.07.009
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65(19):2070–2076. doi:10.1016/j.jacc.2015.03.518
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28(3):603–661. doi:10.1128/CMR.00134-14
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Barton T, Moir S, Rehmani H, Woolley I, Korman TM, Stuart RL. Low rates of endocarditis in healthcare-associated Staphylococcus aureus bacteremia suggest that echocardiography might not always be required. Eur J Clin Microbiol Infect Dis 2016; 35(1):49–55. doi:10.1007/s10096-015-2505-8
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi10.1161/CIR.0000000000000296
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30(4):633–638. doi:10.1086/313753
- Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11(2):202–219. doi:10.1093/ejechocard/jeq004
- Irani WN, Grayburn PA, Afridi I. A negative transthoracic echocardiogram obviates the need for transesophageal echocardiography in patients with suspected native valve active infective endocarditis. Am J Cardiol 1996; 78(1):101–103. pmid:8712097
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013; 26(9):921–964. doi:10.1016/j.echo.2013.07.009