User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
ACC/AHA issues updated guidance on aortic disease
focusing on surgical intervention considerations, consistent imaging practices, genetic and familial screenings, and the importance of multidisciplinary care.
“There has been a host of new evidence-based research available for clinicians in the past decade when it comes to aortic disease. It was time to reevaluate and update the previous, existing guidelines,” Eric M. Isselbacher, MD, MSc, chair of the writing committee, said in a statement.
“We hope this new guideline can inform clinical practices with up-to-date and synthesized recommendations, targeted toward a full multidisciplinary aortic team working to provide the best possible care for this vulnerable patient population,” added Dr. Isselbacher, codirector of the Thoracic Aortic Center at Massachusetts General Hospital, Boston.
The 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease was simultaneously published online in the Journal of the American College of Cardiology and Circulation.
The new guideline replaces the 2010 ACCF/AHA Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease and the 2015 Surgery for Aortic Dilation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the ACC/AHA Task Force on Clinical Practice Guidelines.
The new guideline is intended to be used with the 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease.
It brings together guidelines for both the thoracic and abdominal aorta and is targeted to cardiovascular clinicians involved in the care of people with aortic disease, including general cardiovascular care clinicians and emergency medicine clinicians, the writing group says.
Among the key recommendations in the new guideline are the following:
- Screen first-degree relatives of individuals diagnosed with aneurysms of the aortic root or ascending thoracic aorta, or those with aortic dissection to identify individuals most at risk for aortic disease. Screening would include genetic testing and imaging.
- Be consistent in the way CT or MRI are obtained and reported; in the measurement of aortic size and features; and in how often images are used for monitoring before and after repair surgery or other intervention. Ideally, all surveillance imaging for an individual should be done using the same modality and in the same lab, the guideline notes.
- For individuals who require aortic intervention, know that outcomes are optimized when surgery is performed by an experienced surgeon working in a multidisciplinary aortic team. The new guideline recommends “a specialized hospital team with expertise in the evaluation and management of aortic disease, in which care is delivered in a comprehensive, multidisciplinary manner.”
- At centers with multidisciplinary aortic teams and experienced surgeons, the threshold for surgical intervention for sporadic aortic root and ascending aortic aneurysms has been lowered from 5.5 cm to 5.0 cm in select individuals, and even lower in specific scenarios among patients with heritable thoracic aortic aneurysms.
- In patients who are significantly smaller or taller than average, surgical thresholds may incorporate indexing of the aortic root or ascending aortic diameter to either patient body surface area or height, or aortic cross-sectional area to patient height.
- Rapid aortic growth is a risk factor for rupture and the definition for rapid aneurysm growth rate has been updated. Surgery is now recommended for patients with aneurysms of aortic root and ascending thoracic aorta with a confirmed growth rate of ≥ 0.3 cm per year across 2 consecutive years or ≥ 0.5 cm in 1 year.
- In patients undergoing aortic root replacement surgery, valve-sparing aortic root replacement is reasonable if the valve is suitable for repair and when performed by experienced surgeons in a multidisciplinary aortic team.
- Patients with acute type A aortic dissection, if clinically stable, should be considered for transfer to a high-volume aortic center to improve survival. The operative repair of type A aortic dissection should entail at least an open distal anastomosis rather than just a simple supracoronary interposition graft.
- For management of uncomplicated type B aortic dissection, there is an increasing role for . Clinical trials of repair of thoracoabdominal aortic aneurysms with endografts are reporting results that suggest endovascular repair is an option for patients with suitable anatomy.
- Shared decision-making between the patient and multidisciplinary aortic team is highly encouraged, especially when the patient is on the borderline of thresholds for repair or eligible for different types of surgical repair.
- Shared decision-making should also be used with individuals who are pregnant or may become pregnant to consider the risks of pregnancy in individuals with aortic disease.
The guideline was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American College of Radiology, the Society of Cardiovascular Anesthesiologists, the Society for Cardiovascular Angiography and Interventions, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
It has been endorsed by the Society of Interventional Radiology and the Society for Vascular Surgery.
A version of this article first appeared on Medscape.com.
focusing on surgical intervention considerations, consistent imaging practices, genetic and familial screenings, and the importance of multidisciplinary care.
“There has been a host of new evidence-based research available for clinicians in the past decade when it comes to aortic disease. It was time to reevaluate and update the previous, existing guidelines,” Eric M. Isselbacher, MD, MSc, chair of the writing committee, said in a statement.
“We hope this new guideline can inform clinical practices with up-to-date and synthesized recommendations, targeted toward a full multidisciplinary aortic team working to provide the best possible care for this vulnerable patient population,” added Dr. Isselbacher, codirector of the Thoracic Aortic Center at Massachusetts General Hospital, Boston.
The 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease was simultaneously published online in the Journal of the American College of Cardiology and Circulation.
The new guideline replaces the 2010 ACCF/AHA Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease and the 2015 Surgery for Aortic Dilation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the ACC/AHA Task Force on Clinical Practice Guidelines.
The new guideline is intended to be used with the 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease.
It brings together guidelines for both the thoracic and abdominal aorta and is targeted to cardiovascular clinicians involved in the care of people with aortic disease, including general cardiovascular care clinicians and emergency medicine clinicians, the writing group says.
Among the key recommendations in the new guideline are the following:
- Screen first-degree relatives of individuals diagnosed with aneurysms of the aortic root or ascending thoracic aorta, or those with aortic dissection to identify individuals most at risk for aortic disease. Screening would include genetic testing and imaging.
- Be consistent in the way CT or MRI are obtained and reported; in the measurement of aortic size and features; and in how often images are used for monitoring before and after repair surgery or other intervention. Ideally, all surveillance imaging for an individual should be done using the same modality and in the same lab, the guideline notes.
- For individuals who require aortic intervention, know that outcomes are optimized when surgery is performed by an experienced surgeon working in a multidisciplinary aortic team. The new guideline recommends “a specialized hospital team with expertise in the evaluation and management of aortic disease, in which care is delivered in a comprehensive, multidisciplinary manner.”
- At centers with multidisciplinary aortic teams and experienced surgeons, the threshold for surgical intervention for sporadic aortic root and ascending aortic aneurysms has been lowered from 5.5 cm to 5.0 cm in select individuals, and even lower in specific scenarios among patients with heritable thoracic aortic aneurysms.
- In patients who are significantly smaller or taller than average, surgical thresholds may incorporate indexing of the aortic root or ascending aortic diameter to either patient body surface area or height, or aortic cross-sectional area to patient height.
- Rapid aortic growth is a risk factor for rupture and the definition for rapid aneurysm growth rate has been updated. Surgery is now recommended for patients with aneurysms of aortic root and ascending thoracic aorta with a confirmed growth rate of ≥ 0.3 cm per year across 2 consecutive years or ≥ 0.5 cm in 1 year.
- In patients undergoing aortic root replacement surgery, valve-sparing aortic root replacement is reasonable if the valve is suitable for repair and when performed by experienced surgeons in a multidisciplinary aortic team.
- Patients with acute type A aortic dissection, if clinically stable, should be considered for transfer to a high-volume aortic center to improve survival. The operative repair of type A aortic dissection should entail at least an open distal anastomosis rather than just a simple supracoronary interposition graft.
- For management of uncomplicated type B aortic dissection, there is an increasing role for . Clinical trials of repair of thoracoabdominal aortic aneurysms with endografts are reporting results that suggest endovascular repair is an option for patients with suitable anatomy.
- Shared decision-making between the patient and multidisciplinary aortic team is highly encouraged, especially when the patient is on the borderline of thresholds for repair or eligible for different types of surgical repair.
- Shared decision-making should also be used with individuals who are pregnant or may become pregnant to consider the risks of pregnancy in individuals with aortic disease.
The guideline was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American College of Radiology, the Society of Cardiovascular Anesthesiologists, the Society for Cardiovascular Angiography and Interventions, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
It has been endorsed by the Society of Interventional Radiology and the Society for Vascular Surgery.
A version of this article first appeared on Medscape.com.
focusing on surgical intervention considerations, consistent imaging practices, genetic and familial screenings, and the importance of multidisciplinary care.
“There has been a host of new evidence-based research available for clinicians in the past decade when it comes to aortic disease. It was time to reevaluate and update the previous, existing guidelines,” Eric M. Isselbacher, MD, MSc, chair of the writing committee, said in a statement.
“We hope this new guideline can inform clinical practices with up-to-date and synthesized recommendations, targeted toward a full multidisciplinary aortic team working to provide the best possible care for this vulnerable patient population,” added Dr. Isselbacher, codirector of the Thoracic Aortic Center at Massachusetts General Hospital, Boston.
The 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease was simultaneously published online in the Journal of the American College of Cardiology and Circulation.
The new guideline replaces the 2010 ACCF/AHA Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease and the 2015 Surgery for Aortic Dilation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the ACC/AHA Task Force on Clinical Practice Guidelines.
The new guideline is intended to be used with the 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease.
It brings together guidelines for both the thoracic and abdominal aorta and is targeted to cardiovascular clinicians involved in the care of people with aortic disease, including general cardiovascular care clinicians and emergency medicine clinicians, the writing group says.
Among the key recommendations in the new guideline are the following:
- Screen first-degree relatives of individuals diagnosed with aneurysms of the aortic root or ascending thoracic aorta, or those with aortic dissection to identify individuals most at risk for aortic disease. Screening would include genetic testing and imaging.
- Be consistent in the way CT or MRI are obtained and reported; in the measurement of aortic size and features; and in how often images are used for monitoring before and after repair surgery or other intervention. Ideally, all surveillance imaging for an individual should be done using the same modality and in the same lab, the guideline notes.
- For individuals who require aortic intervention, know that outcomes are optimized when surgery is performed by an experienced surgeon working in a multidisciplinary aortic team. The new guideline recommends “a specialized hospital team with expertise in the evaluation and management of aortic disease, in which care is delivered in a comprehensive, multidisciplinary manner.”
- At centers with multidisciplinary aortic teams and experienced surgeons, the threshold for surgical intervention for sporadic aortic root and ascending aortic aneurysms has been lowered from 5.5 cm to 5.0 cm in select individuals, and even lower in specific scenarios among patients with heritable thoracic aortic aneurysms.
- In patients who are significantly smaller or taller than average, surgical thresholds may incorporate indexing of the aortic root or ascending aortic diameter to either patient body surface area or height, or aortic cross-sectional area to patient height.
- Rapid aortic growth is a risk factor for rupture and the definition for rapid aneurysm growth rate has been updated. Surgery is now recommended for patients with aneurysms of aortic root and ascending thoracic aorta with a confirmed growth rate of ≥ 0.3 cm per year across 2 consecutive years or ≥ 0.5 cm in 1 year.
- In patients undergoing aortic root replacement surgery, valve-sparing aortic root replacement is reasonable if the valve is suitable for repair and when performed by experienced surgeons in a multidisciplinary aortic team.
- Patients with acute type A aortic dissection, if clinically stable, should be considered for transfer to a high-volume aortic center to improve survival. The operative repair of type A aortic dissection should entail at least an open distal anastomosis rather than just a simple supracoronary interposition graft.
- For management of uncomplicated type B aortic dissection, there is an increasing role for . Clinical trials of repair of thoracoabdominal aortic aneurysms with endografts are reporting results that suggest endovascular repair is an option for patients with suitable anatomy.
- Shared decision-making between the patient and multidisciplinary aortic team is highly encouraged, especially when the patient is on the borderline of thresholds for repair or eligible for different types of surgical repair.
- Shared decision-making should also be used with individuals who are pregnant or may become pregnant to consider the risks of pregnancy in individuals with aortic disease.
The guideline was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American College of Radiology, the Society of Cardiovascular Anesthesiologists, the Society for Cardiovascular Angiography and Interventions, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
It has been endorsed by the Society of Interventional Radiology and the Society for Vascular Surgery.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Working while sick: Why doctors don’t stay home when ill
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
Stroke risk rises with years of drinking in young adults
“The rate of stroke among young adults has been increasing over the last few decades, and stroke in young adults causes death and serious disability,” study coauthor Eue-Keun Choi, MD, PhD, of Seoul National University, Republic of Korea, said in a statement.
“If we could prevent stroke in young adults by reducing alcohol consumption, that could potentially have a substantial impact on the health of individuals and the overall burden of stroke on society,” Dr. Choi added.
The study was published online in Neurology.
Compounding effects
Using data from a Korean national health database, the researchers identified roughly 1.5 million adults aged 20-39 years (mean age 29.5 years, 72% male) who had four consecutive annual health examinations during which they were asked about their alcohol use.
During a median follow-up of roughly 6 years, 3,153 individuals suffered a stroke.
After multivariate adjustment accounting for other factors that could affect the risk for stroke, such as hypertension, smoking, and body mass index, the risk of stroke increased steadily with the number of years of moderate to heavy drinking, defined as 105 grams or more of alcohol per week.
Compared with light drinkers or teetotalers, stroke risk increased 19% with 2 years of moderate to heavy drinking and 22% and 23%, respectively, for 3 and 4 years of moderate or heaving drinking.
The positive dose-response relationship was chiefly driven by increased risk of hemorrhagic stroke; with 2, 3 and 4 years of moderate to heavy drinking, hemorrhagic stroke risk increased 30%, 42% and 36%, respectively, relative to light/no drinking.
“Since more than 90% of the burden of stroke overall can be attributed to potentially modifiable risk factors, including alcohol consumption, and since stroke in young adults severely impacts both the individual and society by limiting their activities during their most productive years, reducing alcohol consumption should be emphasized in young adults with heavy drinking habits as part of any strategy to prevent stroke,” Dr. Choi said.
A limitation of the study is that only Korean people were included, so the results may not apply to people of other races and ethnicities, they noted. In addition, people filled out questionnaires about their alcohol consumption, which may introduce recall bias.
Consistent evidence
“For decades, the evidence was suggestive that a moderate amount of alcohol daily is actually beneficial – one to two drinks in men and one drink in women – at reducing major vascular outcomes,” Pierre Fayad, MD, professor, department of neurological sciences, and director of the Nebraska Stroke Center, University of Nebraska Medical Center, Omaha, said in commenting on the research.
Yet, over the past few years, some research has found no evidence of benefit with moderate alcohol intake. There is, however, “consistent evidence” that shows a detrimental effect of excessive alcohol, particularly binge drinking, especially in young adults, Dr. Fayad said.
This study, he said, “adds to the known detrimental effects of excessive alcohol intake, in increasing the risk of stroke, particularly in young adults.
“The bottom line: Young adults who usually have a low risk of stroke increase their risk significantly by heavy alcohol drinking, and Koreans are equally at risk as other populations,” Dr. Fayad said.
The study was supported by the Korea Medical Device Development Fund and the Korea National Research Foundation. Dr. Choi and Dr. Fayad report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“The rate of stroke among young adults has been increasing over the last few decades, and stroke in young adults causes death and serious disability,” study coauthor Eue-Keun Choi, MD, PhD, of Seoul National University, Republic of Korea, said in a statement.
“If we could prevent stroke in young adults by reducing alcohol consumption, that could potentially have a substantial impact on the health of individuals and the overall burden of stroke on society,” Dr. Choi added.
The study was published online in Neurology.
Compounding effects
Using data from a Korean national health database, the researchers identified roughly 1.5 million adults aged 20-39 years (mean age 29.5 years, 72% male) who had four consecutive annual health examinations during which they were asked about their alcohol use.
During a median follow-up of roughly 6 years, 3,153 individuals suffered a stroke.
After multivariate adjustment accounting for other factors that could affect the risk for stroke, such as hypertension, smoking, and body mass index, the risk of stroke increased steadily with the number of years of moderate to heavy drinking, defined as 105 grams or more of alcohol per week.
Compared with light drinkers or teetotalers, stroke risk increased 19% with 2 years of moderate to heavy drinking and 22% and 23%, respectively, for 3 and 4 years of moderate or heaving drinking.
The positive dose-response relationship was chiefly driven by increased risk of hemorrhagic stroke; with 2, 3 and 4 years of moderate to heavy drinking, hemorrhagic stroke risk increased 30%, 42% and 36%, respectively, relative to light/no drinking.
“Since more than 90% of the burden of stroke overall can be attributed to potentially modifiable risk factors, including alcohol consumption, and since stroke in young adults severely impacts both the individual and society by limiting their activities during their most productive years, reducing alcohol consumption should be emphasized in young adults with heavy drinking habits as part of any strategy to prevent stroke,” Dr. Choi said.
A limitation of the study is that only Korean people were included, so the results may not apply to people of other races and ethnicities, they noted. In addition, people filled out questionnaires about their alcohol consumption, which may introduce recall bias.
Consistent evidence
“For decades, the evidence was suggestive that a moderate amount of alcohol daily is actually beneficial – one to two drinks in men and one drink in women – at reducing major vascular outcomes,” Pierre Fayad, MD, professor, department of neurological sciences, and director of the Nebraska Stroke Center, University of Nebraska Medical Center, Omaha, said in commenting on the research.
Yet, over the past few years, some research has found no evidence of benefit with moderate alcohol intake. There is, however, “consistent evidence” that shows a detrimental effect of excessive alcohol, particularly binge drinking, especially in young adults, Dr. Fayad said.
This study, he said, “adds to the known detrimental effects of excessive alcohol intake, in increasing the risk of stroke, particularly in young adults.
“The bottom line: Young adults who usually have a low risk of stroke increase their risk significantly by heavy alcohol drinking, and Koreans are equally at risk as other populations,” Dr. Fayad said.
The study was supported by the Korea Medical Device Development Fund and the Korea National Research Foundation. Dr. Choi and Dr. Fayad report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“The rate of stroke among young adults has been increasing over the last few decades, and stroke in young adults causes death and serious disability,” study coauthor Eue-Keun Choi, MD, PhD, of Seoul National University, Republic of Korea, said in a statement.
“If we could prevent stroke in young adults by reducing alcohol consumption, that could potentially have a substantial impact on the health of individuals and the overall burden of stroke on society,” Dr. Choi added.
The study was published online in Neurology.
Compounding effects
Using data from a Korean national health database, the researchers identified roughly 1.5 million adults aged 20-39 years (mean age 29.5 years, 72% male) who had four consecutive annual health examinations during which they were asked about their alcohol use.
During a median follow-up of roughly 6 years, 3,153 individuals suffered a stroke.
After multivariate adjustment accounting for other factors that could affect the risk for stroke, such as hypertension, smoking, and body mass index, the risk of stroke increased steadily with the number of years of moderate to heavy drinking, defined as 105 grams or more of alcohol per week.
Compared with light drinkers or teetotalers, stroke risk increased 19% with 2 years of moderate to heavy drinking and 22% and 23%, respectively, for 3 and 4 years of moderate or heaving drinking.
The positive dose-response relationship was chiefly driven by increased risk of hemorrhagic stroke; with 2, 3 and 4 years of moderate to heavy drinking, hemorrhagic stroke risk increased 30%, 42% and 36%, respectively, relative to light/no drinking.
“Since more than 90% of the burden of stroke overall can be attributed to potentially modifiable risk factors, including alcohol consumption, and since stroke in young adults severely impacts both the individual and society by limiting their activities during their most productive years, reducing alcohol consumption should be emphasized in young adults with heavy drinking habits as part of any strategy to prevent stroke,” Dr. Choi said.
A limitation of the study is that only Korean people were included, so the results may not apply to people of other races and ethnicities, they noted. In addition, people filled out questionnaires about their alcohol consumption, which may introduce recall bias.
Consistent evidence
“For decades, the evidence was suggestive that a moderate amount of alcohol daily is actually beneficial – one to two drinks in men and one drink in women – at reducing major vascular outcomes,” Pierre Fayad, MD, professor, department of neurological sciences, and director of the Nebraska Stroke Center, University of Nebraska Medical Center, Omaha, said in commenting on the research.
Yet, over the past few years, some research has found no evidence of benefit with moderate alcohol intake. There is, however, “consistent evidence” that shows a detrimental effect of excessive alcohol, particularly binge drinking, especially in young adults, Dr. Fayad said.
This study, he said, “adds to the known detrimental effects of excessive alcohol intake, in increasing the risk of stroke, particularly in young adults.
“The bottom line: Young adults who usually have a low risk of stroke increase their risk significantly by heavy alcohol drinking, and Koreans are equally at risk as other populations,” Dr. Fayad said.
The study was supported by the Korea Medical Device Development Fund and the Korea National Research Foundation. Dr. Choi and Dr. Fayad report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
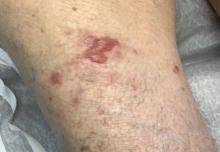
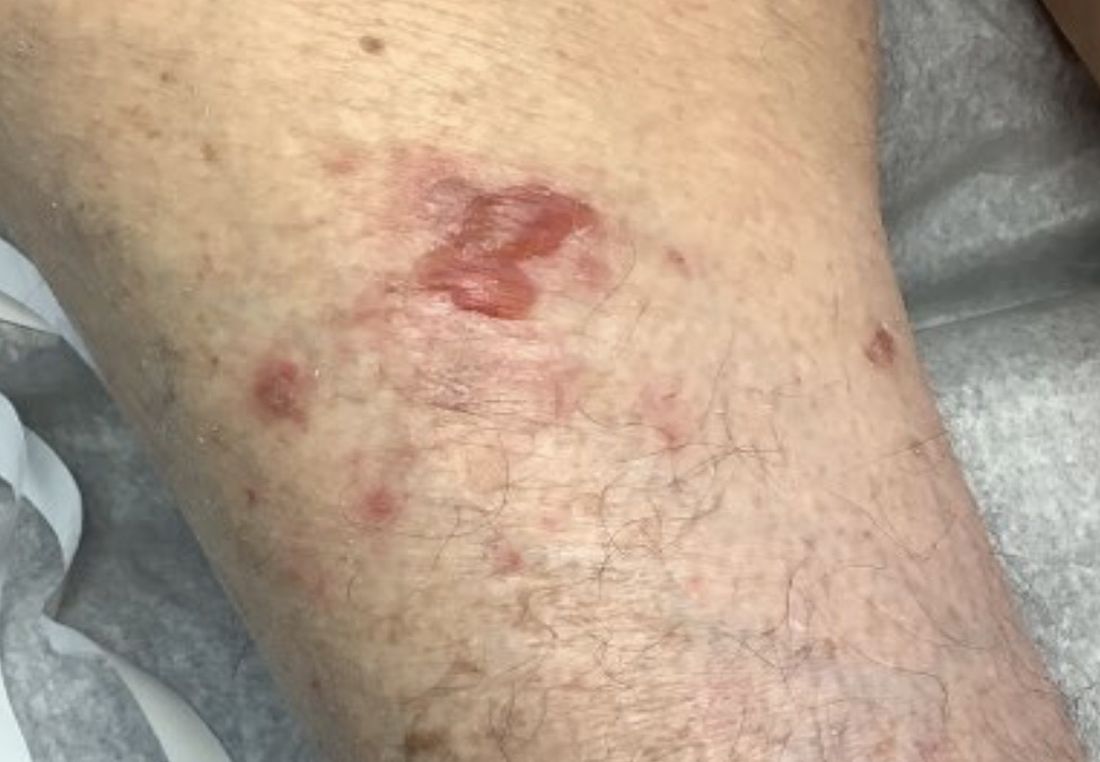
A 95-year-old White male with hypertension presented with itchy patches and bullae on the trunk and extremities
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
Combo thrombolytic approach fails to reduce ICH in stroke
A study evaluating a new approach using a combination of two thrombolytics designed to reduce bleeding risk in patients with acute ischemic stroke has not shown any benefit on the primary outcome of all intracranial hemorrhage (ICH).
However, there were some encouraging findings including a trend towards a reduction in symptomatic ICH, researchers report, and the combination approach did not show any depletion of fibrinogen levels, which suggests a potential lower bleeding risk.
“Although the main results of this study are neutral, we are encouraged that the combination approach with a low dose of alteplase followed by the new mutant pro-urokinase product looked as effective as full-dose alteplase alone, and there were some promising signs signaling a potential lower bleeding risk,” senior investigator, Diederik Dippel, MD, Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
The DUMAS study (Dual Thrombolytic Therapy With Mutant Pro-Urokinase and Low Dose Alteplase for Ischemic Stroke) was presented at the World Stroke Congress in Singapore by study coauthor Nadinda van der Ende, MD, also from Erasmus University Medical Center.
She pointed out that thrombolysis with intravenous alteplase increases the likelihood of a good outcome in acute ischemic stroke but can cause symptomatic intracranial hemorrhage, which can be associated with death and major disability.
Mutant pro-urokinase is a new thrombolytic agent, in development by Thrombolytic Science, Cambridge, Mass., formed by changing one amino acid in pro-urokinase to make it more stable. It is more fibrin specific than alteplase and therefore believed to have a lower risk of intracranial hemorrhage.
Fibrin is formed as the last step in the clotting process, and the precursor of fibrin in the blood is fibrinogen, Dr. van der Ende noted. Alteplase depletes fibrinogen, contributing to its increased bleeding risk, but mutant pro-urokinase is not believed to affect fibrinogen.
“Mutant pro-urokinase does not bind to intact fibrin. It only binds to fibrin that has already been primed by alteplase,” she explained.
The hypothesis behind the current study is that giving a small dose of alteplase will break down fibrin in the clot enough to expose the binding sites for mutant pro-urokinase, which can then be given to continue to lyse the clot.
As alteplase has a short half-life, it disappears quickly, and new fibrin is not affected. As mutant pro-urokinase can only lyse fibrin that is primed with alteplase, new hemostatic clots should stay intact. Animal studies have shown less bleeding from distant sites with this approach, Dr. van der Ende said.
The primary analysis of the phase 2 DUMAS study included 238 patients with mild ischemic stroke (median National Institutes of Health Stroke Scale [NIHSS] score 3) who met the standard criteria for IV alteplase.
They were randomized to alteplase alone at the regular dose of 0.9 mg/kg (max 90 mg) with a 10% bolus and the remaining given over 60 minutes; or to a combination of a 5-mg bolus of IV alteplase followed by mutant pro-urokinase at a dose of 40 mg given over 60 minutes.
The primary outcome was the rate of all intracranial hemorrhage (symptomatic and asymptomatic) detected by neuroimaging.
This occurred in 14% of patients in the full-dose alteplase group vs. 13% of patients in the combined alteplase/mutant pro-urokinase group, a nonsignificant difference: adjusted odds ratio, 0.99 (95% confidence interval, 0.46-2.14).
Secondary outcomes showed no significant differences in NIHSS scores at 24 hours or 5-7 days; functional outcome as measured by a shift analysis of the Modified Rankin Scale (mRS); final infarct volume; or perfusion deficit.
However, blood fibrinogen levels were not depleted and significantly higher in the alteplase/mutant pro-urokinase group than in the full-dose alteplase alone group.
In terms of safety, symptomatic ICH occurred in three patients in the alteplase group (3%) and in none (0%) in the combined alteplase/mutant pro-urokinase group; death occurred in 4% vs. 2% patients respectively; and major extracranial hemorrhage occurred in 1% in both groups.
Dr. Van der Ende concluded that the study showed an overall low rate of ICH; a combination of alteplase and mutant pro-urokinase was not superior to alteplase alone in reducing ICH rates in this population of patients with minor stroke; and mutant pro-urokinase appeared to be safe and, unlike alteplase, did not show any reduction in fibrinogen levels.
“We think the lack of an effect on fibrinogen with this new combination of a small alteplase bolus followed by mutant pro-urokinase infusion is promising,” Dr. Dippel commented. “The fact that there was no symptomatic ICH with the combination treatment is also encouraging. Although the primary endpoint of this trial was neutral, we still believe this is a very interesting approach, with the potential for reduced bleeding, compared with alteplase alone, but we need larger numbers to see an effect on outcomes.”
Dr. Dippel also pointed out that the study included only patients with minor stroke who were not eligible for endovascular therapy, and these patients have a low risk of a poor outcome and a low bleeding risk.
They are hoping to do another study in patients with more severe stroke, who have a higher bleeding risk and would have more to gain from this combination approach.
Because many patients with severe stroke now have immediate thrombectomy if they present to a comprehensive stroke center, a trial in severe stroke patients would have to be done in primary stroke centers, so if the patents are referred to thrombectomy, the thrombolytic would have a chance to work, Dr. Dippel added.
Commenting on the study for this news organization, Stefan Kiechl, MD, Medical University of Innsbruck (Austria), who is cochair of the World Stroke Congress scientific committee, said, “Alteplase is not fibrin specific, and also causes a degeneration of fibrinogen, which results in ‘fibrinogen depletion coagulopathy.’ It is assumed that 20%-40% of intracerebral bleeding after thrombolysis with alteplase is caused by this problem. DUMAS tests the combination of a substantially reduced alteplase [5 mg] dose plus mutant pro-urokinase to avoid this problem.”
The new thrombolysis protocol, however, did not result in a lower bleeding risk, compared to the comparator alteplase,” he added. “The main limitation of this study is that mainly patients with minor strokes were included. Patients with moderate and severe strokes, who have a substantial risk of bleeding, were not adequately addressed.”
The DUMAS trial was funded by an unrestricted grant from Thrombolytic Science, paid to the institution. Dr. Van der Ende and Dr. Dippel report no relevant disclosures.
A version of this article first appeared on Medscape.com.
A study evaluating a new approach using a combination of two thrombolytics designed to reduce bleeding risk in patients with acute ischemic stroke has not shown any benefit on the primary outcome of all intracranial hemorrhage (ICH).
However, there were some encouraging findings including a trend towards a reduction in symptomatic ICH, researchers report, and the combination approach did not show any depletion of fibrinogen levels, which suggests a potential lower bleeding risk.
“Although the main results of this study are neutral, we are encouraged that the combination approach with a low dose of alteplase followed by the new mutant pro-urokinase product looked as effective as full-dose alteplase alone, and there were some promising signs signaling a potential lower bleeding risk,” senior investigator, Diederik Dippel, MD, Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
The DUMAS study (Dual Thrombolytic Therapy With Mutant Pro-Urokinase and Low Dose Alteplase for Ischemic Stroke) was presented at the World Stroke Congress in Singapore by study coauthor Nadinda van der Ende, MD, also from Erasmus University Medical Center.
She pointed out that thrombolysis with intravenous alteplase increases the likelihood of a good outcome in acute ischemic stroke but can cause symptomatic intracranial hemorrhage, which can be associated with death and major disability.
Mutant pro-urokinase is a new thrombolytic agent, in development by Thrombolytic Science, Cambridge, Mass., formed by changing one amino acid in pro-urokinase to make it more stable. It is more fibrin specific than alteplase and therefore believed to have a lower risk of intracranial hemorrhage.
Fibrin is formed as the last step in the clotting process, and the precursor of fibrin in the blood is fibrinogen, Dr. van der Ende noted. Alteplase depletes fibrinogen, contributing to its increased bleeding risk, but mutant pro-urokinase is not believed to affect fibrinogen.
“Mutant pro-urokinase does not bind to intact fibrin. It only binds to fibrin that has already been primed by alteplase,” she explained.
The hypothesis behind the current study is that giving a small dose of alteplase will break down fibrin in the clot enough to expose the binding sites for mutant pro-urokinase, which can then be given to continue to lyse the clot.
As alteplase has a short half-life, it disappears quickly, and new fibrin is not affected. As mutant pro-urokinase can only lyse fibrin that is primed with alteplase, new hemostatic clots should stay intact. Animal studies have shown less bleeding from distant sites with this approach, Dr. van der Ende said.
The primary analysis of the phase 2 DUMAS study included 238 patients with mild ischemic stroke (median National Institutes of Health Stroke Scale [NIHSS] score 3) who met the standard criteria for IV alteplase.
They were randomized to alteplase alone at the regular dose of 0.9 mg/kg (max 90 mg) with a 10% bolus and the remaining given over 60 minutes; or to a combination of a 5-mg bolus of IV alteplase followed by mutant pro-urokinase at a dose of 40 mg given over 60 minutes.
The primary outcome was the rate of all intracranial hemorrhage (symptomatic and asymptomatic) detected by neuroimaging.
This occurred in 14% of patients in the full-dose alteplase group vs. 13% of patients in the combined alteplase/mutant pro-urokinase group, a nonsignificant difference: adjusted odds ratio, 0.99 (95% confidence interval, 0.46-2.14).
Secondary outcomes showed no significant differences in NIHSS scores at 24 hours or 5-7 days; functional outcome as measured by a shift analysis of the Modified Rankin Scale (mRS); final infarct volume; or perfusion deficit.
However, blood fibrinogen levels were not depleted and significantly higher in the alteplase/mutant pro-urokinase group than in the full-dose alteplase alone group.
In terms of safety, symptomatic ICH occurred in three patients in the alteplase group (3%) and in none (0%) in the combined alteplase/mutant pro-urokinase group; death occurred in 4% vs. 2% patients respectively; and major extracranial hemorrhage occurred in 1% in both groups.
Dr. Van der Ende concluded that the study showed an overall low rate of ICH; a combination of alteplase and mutant pro-urokinase was not superior to alteplase alone in reducing ICH rates in this population of patients with minor stroke; and mutant pro-urokinase appeared to be safe and, unlike alteplase, did not show any reduction in fibrinogen levels.
“We think the lack of an effect on fibrinogen with this new combination of a small alteplase bolus followed by mutant pro-urokinase infusion is promising,” Dr. Dippel commented. “The fact that there was no symptomatic ICH with the combination treatment is also encouraging. Although the primary endpoint of this trial was neutral, we still believe this is a very interesting approach, with the potential for reduced bleeding, compared with alteplase alone, but we need larger numbers to see an effect on outcomes.”
Dr. Dippel also pointed out that the study included only patients with minor stroke who were not eligible for endovascular therapy, and these patients have a low risk of a poor outcome and a low bleeding risk.
They are hoping to do another study in patients with more severe stroke, who have a higher bleeding risk and would have more to gain from this combination approach.
Because many patients with severe stroke now have immediate thrombectomy if they present to a comprehensive stroke center, a trial in severe stroke patients would have to be done in primary stroke centers, so if the patents are referred to thrombectomy, the thrombolytic would have a chance to work, Dr. Dippel added.
Commenting on the study for this news organization, Stefan Kiechl, MD, Medical University of Innsbruck (Austria), who is cochair of the World Stroke Congress scientific committee, said, “Alteplase is not fibrin specific, and also causes a degeneration of fibrinogen, which results in ‘fibrinogen depletion coagulopathy.’ It is assumed that 20%-40% of intracerebral bleeding after thrombolysis with alteplase is caused by this problem. DUMAS tests the combination of a substantially reduced alteplase [5 mg] dose plus mutant pro-urokinase to avoid this problem.”
The new thrombolysis protocol, however, did not result in a lower bleeding risk, compared to the comparator alteplase,” he added. “The main limitation of this study is that mainly patients with minor strokes were included. Patients with moderate and severe strokes, who have a substantial risk of bleeding, were not adequately addressed.”
The DUMAS trial was funded by an unrestricted grant from Thrombolytic Science, paid to the institution. Dr. Van der Ende and Dr. Dippel report no relevant disclosures.
A version of this article first appeared on Medscape.com.
A study evaluating a new approach using a combination of two thrombolytics designed to reduce bleeding risk in patients with acute ischemic stroke has not shown any benefit on the primary outcome of all intracranial hemorrhage (ICH).
However, there were some encouraging findings including a trend towards a reduction in symptomatic ICH, researchers report, and the combination approach did not show any depletion of fibrinogen levels, which suggests a potential lower bleeding risk.
“Although the main results of this study are neutral, we are encouraged that the combination approach with a low dose of alteplase followed by the new mutant pro-urokinase product looked as effective as full-dose alteplase alone, and there were some promising signs signaling a potential lower bleeding risk,” senior investigator, Diederik Dippel, MD, Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
The DUMAS study (Dual Thrombolytic Therapy With Mutant Pro-Urokinase and Low Dose Alteplase for Ischemic Stroke) was presented at the World Stroke Congress in Singapore by study coauthor Nadinda van der Ende, MD, also from Erasmus University Medical Center.
She pointed out that thrombolysis with intravenous alteplase increases the likelihood of a good outcome in acute ischemic stroke but can cause symptomatic intracranial hemorrhage, which can be associated with death and major disability.
Mutant pro-urokinase is a new thrombolytic agent, in development by Thrombolytic Science, Cambridge, Mass., formed by changing one amino acid in pro-urokinase to make it more stable. It is more fibrin specific than alteplase and therefore believed to have a lower risk of intracranial hemorrhage.
Fibrin is formed as the last step in the clotting process, and the precursor of fibrin in the blood is fibrinogen, Dr. van der Ende noted. Alteplase depletes fibrinogen, contributing to its increased bleeding risk, but mutant pro-urokinase is not believed to affect fibrinogen.
“Mutant pro-urokinase does not bind to intact fibrin. It only binds to fibrin that has already been primed by alteplase,” she explained.
The hypothesis behind the current study is that giving a small dose of alteplase will break down fibrin in the clot enough to expose the binding sites for mutant pro-urokinase, which can then be given to continue to lyse the clot.
As alteplase has a short half-life, it disappears quickly, and new fibrin is not affected. As mutant pro-urokinase can only lyse fibrin that is primed with alteplase, new hemostatic clots should stay intact. Animal studies have shown less bleeding from distant sites with this approach, Dr. van der Ende said.
The primary analysis of the phase 2 DUMAS study included 238 patients with mild ischemic stroke (median National Institutes of Health Stroke Scale [NIHSS] score 3) who met the standard criteria for IV alteplase.
They were randomized to alteplase alone at the regular dose of 0.9 mg/kg (max 90 mg) with a 10% bolus and the remaining given over 60 minutes; or to a combination of a 5-mg bolus of IV alteplase followed by mutant pro-urokinase at a dose of 40 mg given over 60 minutes.
The primary outcome was the rate of all intracranial hemorrhage (symptomatic and asymptomatic) detected by neuroimaging.
This occurred in 14% of patients in the full-dose alteplase group vs. 13% of patients in the combined alteplase/mutant pro-urokinase group, a nonsignificant difference: adjusted odds ratio, 0.99 (95% confidence interval, 0.46-2.14).
Secondary outcomes showed no significant differences in NIHSS scores at 24 hours or 5-7 days; functional outcome as measured by a shift analysis of the Modified Rankin Scale (mRS); final infarct volume; or perfusion deficit.
However, blood fibrinogen levels were not depleted and significantly higher in the alteplase/mutant pro-urokinase group than in the full-dose alteplase alone group.
In terms of safety, symptomatic ICH occurred in three patients in the alteplase group (3%) and in none (0%) in the combined alteplase/mutant pro-urokinase group; death occurred in 4% vs. 2% patients respectively; and major extracranial hemorrhage occurred in 1% in both groups.
Dr. Van der Ende concluded that the study showed an overall low rate of ICH; a combination of alteplase and mutant pro-urokinase was not superior to alteplase alone in reducing ICH rates in this population of patients with minor stroke; and mutant pro-urokinase appeared to be safe and, unlike alteplase, did not show any reduction in fibrinogen levels.
“We think the lack of an effect on fibrinogen with this new combination of a small alteplase bolus followed by mutant pro-urokinase infusion is promising,” Dr. Dippel commented. “The fact that there was no symptomatic ICH with the combination treatment is also encouraging. Although the primary endpoint of this trial was neutral, we still believe this is a very interesting approach, with the potential for reduced bleeding, compared with alteplase alone, but we need larger numbers to see an effect on outcomes.”
Dr. Dippel also pointed out that the study included only patients with minor stroke who were not eligible for endovascular therapy, and these patients have a low risk of a poor outcome and a low bleeding risk.
They are hoping to do another study in patients with more severe stroke, who have a higher bleeding risk and would have more to gain from this combination approach.
Because many patients with severe stroke now have immediate thrombectomy if they present to a comprehensive stroke center, a trial in severe stroke patients would have to be done in primary stroke centers, so if the patents are referred to thrombectomy, the thrombolytic would have a chance to work, Dr. Dippel added.
Commenting on the study for this news organization, Stefan Kiechl, MD, Medical University of Innsbruck (Austria), who is cochair of the World Stroke Congress scientific committee, said, “Alteplase is not fibrin specific, and also causes a degeneration of fibrinogen, which results in ‘fibrinogen depletion coagulopathy.’ It is assumed that 20%-40% of intracerebral bleeding after thrombolysis with alteplase is caused by this problem. DUMAS tests the combination of a substantially reduced alteplase [5 mg] dose plus mutant pro-urokinase to avoid this problem.”
The new thrombolysis protocol, however, did not result in a lower bleeding risk, compared to the comparator alteplase,” he added. “The main limitation of this study is that mainly patients with minor strokes were included. Patients with moderate and severe strokes, who have a substantial risk of bleeding, were not adequately addressed.”
The DUMAS trial was funded by an unrestricted grant from Thrombolytic Science, paid to the institution. Dr. Van der Ende and Dr. Dippel report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM WSC 2022
Uptake of high-sensitivity troponin assays lags in U.S. hospitals
Most hospitals in the United States have yet to transition from conventional to high-sensitivity cardiac troponin (hs-cTn) assays, despite their greater sensitivity for myocardial injury, a new National Cardiovascular Data Registry (NCDR) registry study indicates.
hs-cTn assays have been used in routine clinical practice in Europe, Canada, and Australia since 2010, but the first such assay did not gain approval in the United States until 2017. Although single-center studies have examined their efficacy and potential downstream consequences, few data exist on hs-cTn implementation nationally, explained study author Cian McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston.
The results were published online in the Journal of the American College of Cardiology and will be presented Nov. 5 at the American Heart Association scientific sessions.
For the study, Dr. McCarthy and colleagues examined 550 hospitals participating in the NCDR Chest Pain-MI registry from January 2019 through September 2021.
Of the 251,000 patients included in the analysis (mean age, 64 years; 41.5% female), 155,049 had a non–ST-segment myocardial infarction (NSTEMI), 15,989 had unstable angina, and 79,962 had low-risk chest pain.
The hs-cTn assays included Roche Diagnostic’s Elecsys Gen5 STAT troponin T assay (23%); Abbott’s ARCHITECT STAT (17%); Beckman Coulter’s ACCESS (21%); and Siemens’ Atellica IM (18%), Dimension VISTA (14%), Dimension EXL (4%), and ADVIA Centaur (2%) troponin I assays.
During the study period, 11.5% of patients were evaluated with hs-cTn assays and the remainder were evaluated with conventional troponin assays. These patients were slightly older (65.0 vs. 64.0 years), more commonly White (83.1% vs. 79.9%), less likely to be of Hispanic or Latino ethnicity (8.9% vs. 10.0%), and less likely to be uninsured (6.8% vs. 8.3%; P for all < .001).
A slightly higher proportion of patients evaluated with hs-cTn assays were diagnosed with unstable angina (7.1% vs. 6.3%), a lower proportion with NSTEMI (61.1% vs. 61.9%), and a similar proportion with low-risk chest pain (31.8% vs. 31.9%) compared with those evaluated by conventional troponin assays.
Implementation, defined as at least 25% of patients evaluated by hs-cTn in each quarter, increased from 3.3% in the first quarter of 2019 to 32.6% in the third quarter of 2021 (P trend < .001).
Using higher implementation thresholds of at least 50% and 75% of patients evaluated by hs-cTn, the prevalence in 2021 was 28.9% and 24.7%, respectively.
“So still, the majority of the hospitals by the end of the third quarter 2021 were not using these assays,” Dr. McCarthy said.
Potential explanations for the slow uptake are that prospective comparative effectiveness trials of These assays have predominantly been in international populations and real-world data on U.S. implementation have been limited to integrated health networks at academic institutions.
Approval of several assays was also delayed and the study data cut off just before the October publication of the 2021 AHA/ACC Chest Pain guideline. “So, whether the chest pain guideline with the new class 1 recommendation for hs-cTn will lead to further uptake is something that will need to be looked at in the future,” he said.
Downstream testing
In adjusted analyses, hs-cTn use was associated with more echocardiography among patients with non-ST elevation–acute coronary syndrome (NSTE-ACS) (82.4% vs. 75.0%; odds ratio [OR], 1.43; 95% confidence interval [CI], 1.19-1.73), but not among those with low-risk chest pain (19.7% vs. 19.4%; OR, 0.93; 95% CI, 0.71-1.22) compared with conventional cTn assays.
Importantly, hs-cTn was not associated with a difference in stress testing or CT coronary angiography utilization.
Use of hs-cTn was associated with lower use of invasive coronary angiography among patients with low-risk chest pain (3.7% vs. 4.5%; OR, 0.73, 95% CI, 0.58-0.92) but similar use for NSTE-ACS (96.3% vs. 95.8%; OR, 0.99, 95% CI, 0.82-1.19).
Among patients with NSTE-ACS, there also was no difference in revascularization with percutaneous coronary intervention (PCI) (52.7% vs. 52.3%; OR, 0.99; 95% CI, 0.94-1.04) or coronary bypass graft surgery (9.4% vs. 9.1%; OR, 1.06; 95% CI, 0.94-1.18).
PCI (0.1% vs. 0.2%; P = .05) and bypass graft surgery (both 0.1%) were uncommon among patients with low-risk chest pain.
In-hospital mortality was similar among patients with low-risk chest pain evaluated using hs-cTn assays vs. conventional troponin assays (0% vs. 0.02%; P = .16) and among patients with NSTE-ACS (2.8% vs. 3.2%; OR, 0.98, 95% CI, 0.87-1.11).
Length of stay was slightly shorter with hs-cTn use for patients with low-risk chest pain (median, 5.8 vs. 6.2 hours; P < .001) and patients with NSTE-ACS (66.9 vs. 67.8 hours; P = .01).
“There was always a concern that maybe high-sensitivity cardiac troponin would dramatically increase testing and could even increase length of stay, but I think these data are reassuring, in that this study suggests high-sensitivity cardiac troponin is associated with a small reduction in length of stay and possibly more appropriate use of testing with echocardiography in STEMI and a reduction in invasive angiography in low-risk patients,” Dr. McCarthy said. “But the majority of hospitals haven’t implemented the assay.”
The authors pointed out that because registry entry of patients with low-risk chest pain and unstable angina is optional for participating sites, the percentage of patients with NSTEMI is higher than in typical chest pain analyses. This higher pretest probability for MI may thus affect post-test accuracy for a true positive result. “That stated, this is the exact scenario where higher sensitivity might be associated with favorable impact on utilization.”
Among other limitations: There was the potential for unmeasured confounders, the accuracy of diagnoses could not be confirmed, patients with type 2 MI were excluded from the registry, and post-discharge safety was not assessed.
“These data indicate further opportunities to more widely and effectively implement hs-cTn in the U.S. hospitals persist that could optimize care for patients with possible or definitive ACS,” Dr. McCarthy and colleagues concluded.
The study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. McCarthy is supported by the National Heart, Lung, and Blood Institute and has received consulting income from Abbott Laboratories.
A version of this article first appeared on Medscape.com.
Most hospitals in the United States have yet to transition from conventional to high-sensitivity cardiac troponin (hs-cTn) assays, despite their greater sensitivity for myocardial injury, a new National Cardiovascular Data Registry (NCDR) registry study indicates.
hs-cTn assays have been used in routine clinical practice in Europe, Canada, and Australia since 2010, but the first such assay did not gain approval in the United States until 2017. Although single-center studies have examined their efficacy and potential downstream consequences, few data exist on hs-cTn implementation nationally, explained study author Cian McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston.
The results were published online in the Journal of the American College of Cardiology and will be presented Nov. 5 at the American Heart Association scientific sessions.
For the study, Dr. McCarthy and colleagues examined 550 hospitals participating in the NCDR Chest Pain-MI registry from January 2019 through September 2021.
Of the 251,000 patients included in the analysis (mean age, 64 years; 41.5% female), 155,049 had a non–ST-segment myocardial infarction (NSTEMI), 15,989 had unstable angina, and 79,962 had low-risk chest pain.
The hs-cTn assays included Roche Diagnostic’s Elecsys Gen5 STAT troponin T assay (23%); Abbott’s ARCHITECT STAT (17%); Beckman Coulter’s ACCESS (21%); and Siemens’ Atellica IM (18%), Dimension VISTA (14%), Dimension EXL (4%), and ADVIA Centaur (2%) troponin I assays.
During the study period, 11.5% of patients were evaluated with hs-cTn assays and the remainder were evaluated with conventional troponin assays. These patients were slightly older (65.0 vs. 64.0 years), more commonly White (83.1% vs. 79.9%), less likely to be of Hispanic or Latino ethnicity (8.9% vs. 10.0%), and less likely to be uninsured (6.8% vs. 8.3%; P for all < .001).
A slightly higher proportion of patients evaluated with hs-cTn assays were diagnosed with unstable angina (7.1% vs. 6.3%), a lower proportion with NSTEMI (61.1% vs. 61.9%), and a similar proportion with low-risk chest pain (31.8% vs. 31.9%) compared with those evaluated by conventional troponin assays.
Implementation, defined as at least 25% of patients evaluated by hs-cTn in each quarter, increased from 3.3% in the first quarter of 2019 to 32.6% in the third quarter of 2021 (P trend < .001).
Using higher implementation thresholds of at least 50% and 75% of patients evaluated by hs-cTn, the prevalence in 2021 was 28.9% and 24.7%, respectively.
“So still, the majority of the hospitals by the end of the third quarter 2021 were not using these assays,” Dr. McCarthy said.
Potential explanations for the slow uptake are that prospective comparative effectiveness trials of These assays have predominantly been in international populations and real-world data on U.S. implementation have been limited to integrated health networks at academic institutions.
Approval of several assays was also delayed and the study data cut off just before the October publication of the 2021 AHA/ACC Chest Pain guideline. “So, whether the chest pain guideline with the new class 1 recommendation for hs-cTn will lead to further uptake is something that will need to be looked at in the future,” he said.
Downstream testing
In adjusted analyses, hs-cTn use was associated with more echocardiography among patients with non-ST elevation–acute coronary syndrome (NSTE-ACS) (82.4% vs. 75.0%; odds ratio [OR], 1.43; 95% confidence interval [CI], 1.19-1.73), but not among those with low-risk chest pain (19.7% vs. 19.4%; OR, 0.93; 95% CI, 0.71-1.22) compared with conventional cTn assays.
Importantly, hs-cTn was not associated with a difference in stress testing or CT coronary angiography utilization.
Use of hs-cTn was associated with lower use of invasive coronary angiography among patients with low-risk chest pain (3.7% vs. 4.5%; OR, 0.73, 95% CI, 0.58-0.92) but similar use for NSTE-ACS (96.3% vs. 95.8%; OR, 0.99, 95% CI, 0.82-1.19).
Among patients with NSTE-ACS, there also was no difference in revascularization with percutaneous coronary intervention (PCI) (52.7% vs. 52.3%; OR, 0.99; 95% CI, 0.94-1.04) or coronary bypass graft surgery (9.4% vs. 9.1%; OR, 1.06; 95% CI, 0.94-1.18).
PCI (0.1% vs. 0.2%; P = .05) and bypass graft surgery (both 0.1%) were uncommon among patients with low-risk chest pain.
In-hospital mortality was similar among patients with low-risk chest pain evaluated using hs-cTn assays vs. conventional troponin assays (0% vs. 0.02%; P = .16) and among patients with NSTE-ACS (2.8% vs. 3.2%; OR, 0.98, 95% CI, 0.87-1.11).
Length of stay was slightly shorter with hs-cTn use for patients with low-risk chest pain (median, 5.8 vs. 6.2 hours; P < .001) and patients with NSTE-ACS (66.9 vs. 67.8 hours; P = .01).
“There was always a concern that maybe high-sensitivity cardiac troponin would dramatically increase testing and could even increase length of stay, but I think these data are reassuring, in that this study suggests high-sensitivity cardiac troponin is associated with a small reduction in length of stay and possibly more appropriate use of testing with echocardiography in STEMI and a reduction in invasive angiography in low-risk patients,” Dr. McCarthy said. “But the majority of hospitals haven’t implemented the assay.”
The authors pointed out that because registry entry of patients with low-risk chest pain and unstable angina is optional for participating sites, the percentage of patients with NSTEMI is higher than in typical chest pain analyses. This higher pretest probability for MI may thus affect post-test accuracy for a true positive result. “That stated, this is the exact scenario where higher sensitivity might be associated with favorable impact on utilization.”
Among other limitations: There was the potential for unmeasured confounders, the accuracy of diagnoses could not be confirmed, patients with type 2 MI were excluded from the registry, and post-discharge safety was not assessed.
“These data indicate further opportunities to more widely and effectively implement hs-cTn in the U.S. hospitals persist that could optimize care for patients with possible or definitive ACS,” Dr. McCarthy and colleagues concluded.
The study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. McCarthy is supported by the National Heart, Lung, and Blood Institute and has received consulting income from Abbott Laboratories.
A version of this article first appeared on Medscape.com.
Most hospitals in the United States have yet to transition from conventional to high-sensitivity cardiac troponin (hs-cTn) assays, despite their greater sensitivity for myocardial injury, a new National Cardiovascular Data Registry (NCDR) registry study indicates.
hs-cTn assays have been used in routine clinical practice in Europe, Canada, and Australia since 2010, but the first such assay did not gain approval in the United States until 2017. Although single-center studies have examined their efficacy and potential downstream consequences, few data exist on hs-cTn implementation nationally, explained study author Cian McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston.
The results were published online in the Journal of the American College of Cardiology and will be presented Nov. 5 at the American Heart Association scientific sessions.
For the study, Dr. McCarthy and colleagues examined 550 hospitals participating in the NCDR Chest Pain-MI registry from January 2019 through September 2021.
Of the 251,000 patients included in the analysis (mean age, 64 years; 41.5% female), 155,049 had a non–ST-segment myocardial infarction (NSTEMI), 15,989 had unstable angina, and 79,962 had low-risk chest pain.
The hs-cTn assays included Roche Diagnostic’s Elecsys Gen5 STAT troponin T assay (23%); Abbott’s ARCHITECT STAT (17%); Beckman Coulter’s ACCESS (21%); and Siemens’ Atellica IM (18%), Dimension VISTA (14%), Dimension EXL (4%), and ADVIA Centaur (2%) troponin I assays.
During the study period, 11.5% of patients were evaluated with hs-cTn assays and the remainder were evaluated with conventional troponin assays. These patients were slightly older (65.0 vs. 64.0 years), more commonly White (83.1% vs. 79.9%), less likely to be of Hispanic or Latino ethnicity (8.9% vs. 10.0%), and less likely to be uninsured (6.8% vs. 8.3%; P for all < .001).
A slightly higher proportion of patients evaluated with hs-cTn assays were diagnosed with unstable angina (7.1% vs. 6.3%), a lower proportion with NSTEMI (61.1% vs. 61.9%), and a similar proportion with low-risk chest pain (31.8% vs. 31.9%) compared with those evaluated by conventional troponin assays.
Implementation, defined as at least 25% of patients evaluated by hs-cTn in each quarter, increased from 3.3% in the first quarter of 2019 to 32.6% in the third quarter of 2021 (P trend < .001).
Using higher implementation thresholds of at least 50% and 75% of patients evaluated by hs-cTn, the prevalence in 2021 was 28.9% and 24.7%, respectively.
“So still, the majority of the hospitals by the end of the third quarter 2021 were not using these assays,” Dr. McCarthy said.
Potential explanations for the slow uptake are that prospective comparative effectiveness trials of These assays have predominantly been in international populations and real-world data on U.S. implementation have been limited to integrated health networks at academic institutions.
Approval of several assays was also delayed and the study data cut off just before the October publication of the 2021 AHA/ACC Chest Pain guideline. “So, whether the chest pain guideline with the new class 1 recommendation for hs-cTn will lead to further uptake is something that will need to be looked at in the future,” he said.
Downstream testing
In adjusted analyses, hs-cTn use was associated with more echocardiography among patients with non-ST elevation–acute coronary syndrome (NSTE-ACS) (82.4% vs. 75.0%; odds ratio [OR], 1.43; 95% confidence interval [CI], 1.19-1.73), but not among those with low-risk chest pain (19.7% vs. 19.4%; OR, 0.93; 95% CI, 0.71-1.22) compared with conventional cTn assays.
Importantly, hs-cTn was not associated with a difference in stress testing or CT coronary angiography utilization.
Use of hs-cTn was associated with lower use of invasive coronary angiography among patients with low-risk chest pain (3.7% vs. 4.5%; OR, 0.73, 95% CI, 0.58-0.92) but similar use for NSTE-ACS (96.3% vs. 95.8%; OR, 0.99, 95% CI, 0.82-1.19).
Among patients with NSTE-ACS, there also was no difference in revascularization with percutaneous coronary intervention (PCI) (52.7% vs. 52.3%; OR, 0.99; 95% CI, 0.94-1.04) or coronary bypass graft surgery (9.4% vs. 9.1%; OR, 1.06; 95% CI, 0.94-1.18).
PCI (0.1% vs. 0.2%; P = .05) and bypass graft surgery (both 0.1%) were uncommon among patients with low-risk chest pain.
In-hospital mortality was similar among patients with low-risk chest pain evaluated using hs-cTn assays vs. conventional troponin assays (0% vs. 0.02%; P = .16) and among patients with NSTE-ACS (2.8% vs. 3.2%; OR, 0.98, 95% CI, 0.87-1.11).
Length of stay was slightly shorter with hs-cTn use for patients with low-risk chest pain (median, 5.8 vs. 6.2 hours; P < .001) and patients with NSTE-ACS (66.9 vs. 67.8 hours; P = .01).
“There was always a concern that maybe high-sensitivity cardiac troponin would dramatically increase testing and could even increase length of stay, but I think these data are reassuring, in that this study suggests high-sensitivity cardiac troponin is associated with a small reduction in length of stay and possibly more appropriate use of testing with echocardiography in STEMI and a reduction in invasive angiography in low-risk patients,” Dr. McCarthy said. “But the majority of hospitals haven’t implemented the assay.”
The authors pointed out that because registry entry of patients with low-risk chest pain and unstable angina is optional for participating sites, the percentage of patients with NSTEMI is higher than in typical chest pain analyses. This higher pretest probability for MI may thus affect post-test accuracy for a true positive result. “That stated, this is the exact scenario where higher sensitivity might be associated with favorable impact on utilization.”
Among other limitations: There was the potential for unmeasured confounders, the accuracy of diagnoses could not be confirmed, patients with type 2 MI were excluded from the registry, and post-discharge safety was not assessed.
“These data indicate further opportunities to more widely and effectively implement hs-cTn in the U.S. hospitals persist that could optimize care for patients with possible or definitive ACS,” Dr. McCarthy and colleagues concluded.
The study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. McCarthy is supported by the National Heart, Lung, and Blood Institute and has received consulting income from Abbott Laboratories.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
Marital stress tied to worse outcome in young MI patients
Severe marital stress was associated with worse recovery after myocardial infarction in a large U.S. cohort of married/partnered patients aged 55 years or younger.
Compared with patients who reported no or mild marital stress a month after their MI, patients who reported severe marital stress had worse physical and mental health, worse generic and cardiovascular quality of life, more frequent angina symptoms, and a greater likelihood of having a hospital readmission a year later.
These findings held true after adjusting for gender, age, race/ethnicity, and baseline health status (model 1) and after further adjusting for education and income levels and employment and insurance status (model 2).
A greater percentage of women than men reported having severe marital stress (39% vs. 30%; P = .001).
Cenjing Zhu, MPhil, a PhD candidate at Yale University, New Haven, Conn., and colleagues will present this study at the American Heart Association scientific sessions.
The results show that “both patients and care providers should be aware that stress experienced in one’s everyday life, such as marital stress, can affect AMI [acute MI] recovery,” Ms. Zhu said in an email.
Health care providers should consider incorporating screening for everyday stress during follow-up patient visits to better spot people at high risk of a poor recovery and further hospitalizations, she added. When possible, they could guide patients to resources to help them manage and reduce their stress levels.
According to Ms. Zhu, the findings suggest that “managing personal stress may be as important as managing other clinical risk factors during the recovery process.”
This study in younger patients with MI “shows that high levels of marital stress impair heart attack recovery, and women have greater impairment in their heart attack recovery compared to men,” AHA spokesperson Nieca Goldberg, MD, who was not involved with this research, told this news organization.
The study shows that “clinicians have to incorporate mental health as part of their assessment of all patients,” said Dr. Goldberg, a clinical associate professor of medicine at New York University and medical director of Atria New York City.
“Our mental health impacts our physical health,” she noted. “Questions about marital stress should be included as part of an overall assessment of mental health. This means assessing all patients for stress, anxiety, and depression.”
Patients who are experiencing marital stress should share the information with their doctor and discuss ways to be referred to therapists and cardiac rehabilitation providers, she said. “My final thought is, women have often been told that their cardiac symptoms are due to stress by doctors. Now we know stress impacts physical health and [is] no longer an excuse but a contributing factor to our physical health.”
Does marital stress affect young MI recovery?
Previous literature has linked psychological stress with worse cardiovascular outcomes, Ms. Zhu noted.
However, little is known about the prognostic impact of marital stress on 1-year health outcomes for younger people who survive an MI.
To investigate this, the researchers analyzed data from participants in the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study.
The current study comprised 1,593 adults, including 1,020 female participants (64%), who were treated for MI at 103 hospitals in 30 U.S. states.
VIRGO enrolled participants in a 2:1 female-to-male ratio so as to enrich the inclusion of women, Ms. Zhu explained.
In the study, “partnered” participants were individuals who self-reported as “living as married/living with a partner.” There were 126 such patients (8%) in the current study.
The mean age of the patients was 47, and about 90% were 40-55 years old. Three quarters were White, 13% were Black, and 7% were Hispanic.
Marital stress was assessed on the basis of patients’ replies to 17 questions in the Stockholm Marital Stress Scale regarding the quality of their emotional and sexual relationships with their spouses/partners.
The researchers divided patients into three groups on the basis of their marital stress: mild or absent (lowest quartile), moderate (second quartile), and severe (upper two quartiles).
At 1 year after their MI, patients replied to questionnaires that assessed their health, quality of life, and depressive and angina symptoms. Hospital readmissions were determined on the basis of self-reports and medical records.
Compared to participants who reported no or mild marital stress, those who reported severe mental stress had significantly worse scores for physical and mental health and generic and cardiovascular quality of life, after adjusting for baseline health and demographics. They had worse scores for mental health and quality of life, after further adjusting for socioeconomic status.
In the fully adjusted model, patients who reported severe marital stress were significantly more likely to report more frequent chest pain/angina (odds ratio, 1.49; 95% confidence interval, 1.06-2.10; P = .023) and to have been readmitted to hospital for any cause (OR, 1.45; 95% CI, 1.04-2.00; P = .006), compared with the patients who reported no or mild marital stress.
Study limitations include the fact that the findings are based on self-reported questionnaire replies; they may not be generalizable to patients in other countries; and they do not extend beyond a period of 1 year.
The researchers call for further research “to understand this complex relationship and potential causal pathway associated with these findings.”
“Additional stressors beyond marital stress, such as financial strain or work stress, may also play a role in young adults’ recovery, and the interaction between these factors require further research,” Ms. Zhu noted in a press release from the AHA.
The study was funded by Canadian Institutes of Health Research. The VIRGO study was funded by the National Heart, Lung, and Blood Institute. Ms. Zhu and Dr. Goldberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Severe marital stress was associated with worse recovery after myocardial infarction in a large U.S. cohort of married/partnered patients aged 55 years or younger.
Compared with patients who reported no or mild marital stress a month after their MI, patients who reported severe marital stress had worse physical and mental health, worse generic and cardiovascular quality of life, more frequent angina symptoms, and a greater likelihood of having a hospital readmission a year later.
These findings held true after adjusting for gender, age, race/ethnicity, and baseline health status (model 1) and after further adjusting for education and income levels and employment and insurance status (model 2).
A greater percentage of women than men reported having severe marital stress (39% vs. 30%; P = .001).
Cenjing Zhu, MPhil, a PhD candidate at Yale University, New Haven, Conn., and colleagues will present this study at the American Heart Association scientific sessions.
The results show that “both patients and care providers should be aware that stress experienced in one’s everyday life, such as marital stress, can affect AMI [acute MI] recovery,” Ms. Zhu said in an email.
Health care providers should consider incorporating screening for everyday stress during follow-up patient visits to better spot people at high risk of a poor recovery and further hospitalizations, she added. When possible, they could guide patients to resources to help them manage and reduce their stress levels.
According to Ms. Zhu, the findings suggest that “managing personal stress may be as important as managing other clinical risk factors during the recovery process.”
This study in younger patients with MI “shows that high levels of marital stress impair heart attack recovery, and women have greater impairment in their heart attack recovery compared to men,” AHA spokesperson Nieca Goldberg, MD, who was not involved with this research, told this news organization.
The study shows that “clinicians have to incorporate mental health as part of their assessment of all patients,” said Dr. Goldberg, a clinical associate professor of medicine at New York University and medical director of Atria New York City.
“Our mental health impacts our physical health,” she noted. “Questions about marital stress should be included as part of an overall assessment of mental health. This means assessing all patients for stress, anxiety, and depression.”
Patients who are experiencing marital stress should share the information with their doctor and discuss ways to be referred to therapists and cardiac rehabilitation providers, she said. “My final thought is, women have often been told that their cardiac symptoms are due to stress by doctors. Now we know stress impacts physical health and [is] no longer an excuse but a contributing factor to our physical health.”
Does marital stress affect young MI recovery?
Previous literature has linked psychological stress with worse cardiovascular outcomes, Ms. Zhu noted.
However, little is known about the prognostic impact of marital stress on 1-year health outcomes for younger people who survive an MI.
To investigate this, the researchers analyzed data from participants in the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study.
The current study comprised 1,593 adults, including 1,020 female participants (64%), who were treated for MI at 103 hospitals in 30 U.S. states.
VIRGO enrolled participants in a 2:1 female-to-male ratio so as to enrich the inclusion of women, Ms. Zhu explained.
In the study, “partnered” participants were individuals who self-reported as “living as married/living with a partner.” There were 126 such patients (8%) in the current study.
The mean age of the patients was 47, and about 90% were 40-55 years old. Three quarters were White, 13% were Black, and 7% were Hispanic.
Marital stress was assessed on the basis of patients’ replies to 17 questions in the Stockholm Marital Stress Scale regarding the quality of their emotional and sexual relationships with their spouses/partners.
The researchers divided patients into three groups on the basis of their marital stress: mild or absent (lowest quartile), moderate (second quartile), and severe (upper two quartiles).
At 1 year after their MI, patients replied to questionnaires that assessed their health, quality of life, and depressive and angina symptoms. Hospital readmissions were determined on the basis of self-reports and medical records.
Compared to participants who reported no or mild marital stress, those who reported severe mental stress had significantly worse scores for physical and mental health and generic and cardiovascular quality of life, after adjusting for baseline health and demographics. They had worse scores for mental health and quality of life, after further adjusting for socioeconomic status.
In the fully adjusted model, patients who reported severe marital stress were significantly more likely to report more frequent chest pain/angina (odds ratio, 1.49; 95% confidence interval, 1.06-2.10; P = .023) and to have been readmitted to hospital for any cause (OR, 1.45; 95% CI, 1.04-2.00; P = .006), compared with the patients who reported no or mild marital stress.
Study limitations include the fact that the findings are based on self-reported questionnaire replies; they may not be generalizable to patients in other countries; and they do not extend beyond a period of 1 year.
The researchers call for further research “to understand this complex relationship and potential causal pathway associated with these findings.”
“Additional stressors beyond marital stress, such as financial strain or work stress, may also play a role in young adults’ recovery, and the interaction between these factors require further research,” Ms. Zhu noted in a press release from the AHA.
The study was funded by Canadian Institutes of Health Research. The VIRGO study was funded by the National Heart, Lung, and Blood Institute. Ms. Zhu and Dr. Goldberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Severe marital stress was associated with worse recovery after myocardial infarction in a large U.S. cohort of married/partnered patients aged 55 years or younger.
Compared with patients who reported no or mild marital stress a month after their MI, patients who reported severe marital stress had worse physical and mental health, worse generic and cardiovascular quality of life, more frequent angina symptoms, and a greater likelihood of having a hospital readmission a year later.
These findings held true after adjusting for gender, age, race/ethnicity, and baseline health status (model 1) and after further adjusting for education and income levels and employment and insurance status (model 2).
A greater percentage of women than men reported having severe marital stress (39% vs. 30%; P = .001).
Cenjing Zhu, MPhil, a PhD candidate at Yale University, New Haven, Conn., and colleagues will present this study at the American Heart Association scientific sessions.
The results show that “both patients and care providers should be aware that stress experienced in one’s everyday life, such as marital stress, can affect AMI [acute MI] recovery,” Ms. Zhu said in an email.
Health care providers should consider incorporating screening for everyday stress during follow-up patient visits to better spot people at high risk of a poor recovery and further hospitalizations, she added. When possible, they could guide patients to resources to help them manage and reduce their stress levels.
According to Ms. Zhu, the findings suggest that “managing personal stress may be as important as managing other clinical risk factors during the recovery process.”
This study in younger patients with MI “shows that high levels of marital stress impair heart attack recovery, and women have greater impairment in their heart attack recovery compared to men,” AHA spokesperson Nieca Goldberg, MD, who was not involved with this research, told this news organization.
The study shows that “clinicians have to incorporate mental health as part of their assessment of all patients,” said Dr. Goldberg, a clinical associate professor of medicine at New York University and medical director of Atria New York City.
“Our mental health impacts our physical health,” she noted. “Questions about marital stress should be included as part of an overall assessment of mental health. This means assessing all patients for stress, anxiety, and depression.”
Patients who are experiencing marital stress should share the information with their doctor and discuss ways to be referred to therapists and cardiac rehabilitation providers, she said. “My final thought is, women have often been told that their cardiac symptoms are due to stress by doctors. Now we know stress impacts physical health and [is] no longer an excuse but a contributing factor to our physical health.”
Does marital stress affect young MI recovery?
Previous literature has linked psychological stress with worse cardiovascular outcomes, Ms. Zhu noted.
However, little is known about the prognostic impact of marital stress on 1-year health outcomes for younger people who survive an MI.
To investigate this, the researchers analyzed data from participants in the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study.
The current study comprised 1,593 adults, including 1,020 female participants (64%), who were treated for MI at 103 hospitals in 30 U.S. states.
VIRGO enrolled participants in a 2:1 female-to-male ratio so as to enrich the inclusion of women, Ms. Zhu explained.
In the study, “partnered” participants were individuals who self-reported as “living as married/living with a partner.” There were 126 such patients (8%) in the current study.
The mean age of the patients was 47, and about 90% were 40-55 years old. Three quarters were White, 13% were Black, and 7% were Hispanic.
Marital stress was assessed on the basis of patients’ replies to 17 questions in the Stockholm Marital Stress Scale regarding the quality of their emotional and sexual relationships with their spouses/partners.
The researchers divided patients into three groups on the basis of their marital stress: mild or absent (lowest quartile), moderate (second quartile), and severe (upper two quartiles).
At 1 year after their MI, patients replied to questionnaires that assessed their health, quality of life, and depressive and angina symptoms. Hospital readmissions were determined on the basis of self-reports and medical records.
Compared to participants who reported no or mild marital stress, those who reported severe mental stress had significantly worse scores for physical and mental health and generic and cardiovascular quality of life, after adjusting for baseline health and demographics. They had worse scores for mental health and quality of life, after further adjusting for socioeconomic status.
In the fully adjusted model, patients who reported severe marital stress were significantly more likely to report more frequent chest pain/angina (odds ratio, 1.49; 95% confidence interval, 1.06-2.10; P = .023) and to have been readmitted to hospital for any cause (OR, 1.45; 95% CI, 1.04-2.00; P = .006), compared with the patients who reported no or mild marital stress.
Study limitations include the fact that the findings are based on self-reported questionnaire replies; they may not be generalizable to patients in other countries; and they do not extend beyond a period of 1 year.
The researchers call for further research “to understand this complex relationship and potential causal pathway associated with these findings.”
“Additional stressors beyond marital stress, such as financial strain or work stress, may also play a role in young adults’ recovery, and the interaction between these factors require further research,” Ms. Zhu noted in a press release from the AHA.
The study was funded by Canadian Institutes of Health Research. The VIRGO study was funded by the National Heart, Lung, and Blood Institute. Ms. Zhu and Dr. Goldberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
The truth of alcohol consequences
Bad drinking consequence No. 87: Joining the LOTME team
Alcohol and college students go together like peanut butter and jelly. Or peanut butter and chocolate. Or peanut butter and toothpaste. Peanut butter goes with a lot of things.
Naturally, when you combine alcohol and college students, bad decisions are sure to follow. But have you ever wondered just how many bad decisions alcohol causes? A team of researchers from Penn State University, the undisputed champion of poor drinking decisions (trust us, we know), sure has. They’ve even conducted a 4-year study of 1,700 students as they carved a drunken swath through the many fine local drinking establishments, such as East Halls or that one frat house that hosts medieval battle–style ping pong tournaments.
The students were surveyed twice a year throughout the study, and the researchers compiled a list of all the various consequences their subjects experienced. Ultimately, college students will experience an average of 102 consequences from drinking during their 4-year college careers, which is an impressive number. Try thinking up a hundred consequences for anything.
Some consequences are less common than others – we imagine “missing the Renaissance Faire because you felt drunker the morning after than while you were drinking” is pretty low on the list – but more than 96% of students reported that they’d experienced a hangover and that drinking had caused them to say or do embarrassing things. Also, more than 70% said they needed additional alcohol to feel any effect, a potential sign of alcohol use disorder.
Once they had their list, the researchers focused on 12 of the more common and severe consequences, such as blacking out, hangovers, and missing work/class, and asked the study participants how their parents would react to their drinking and those specific consequences. Students who believed their parents would disapprove of alcohol-related consequences actually experienced fewer consequences overall.
College students, it seems, really do care what their parents think, even if they don’t express it, the researchers said. That gives space for parents to offer advice about the consequences of hard drinking, making decisions while drunk, or bringing godawful Fireball whiskey to parties. Seriously, don’t do that. Stuff’s bad, and you should feel bad for bringing it. Your parents raised you better than that.
COVID ‘expert’ discusses data sharing
We interrupt our regularly scheduled programming to bring you this special news event. Elon Musk, the world’s second-most annoying human, is holding a press conference to discuss, of all things, COVID-19.
Reporter: Hey, Mr. Musketeer, what qualifies you to talk about a global pandemic?
EM: As the official king of the Twitterverse, I’m pretty much an expert on any topic.
Reporter: Okay then, Mr. Muskmelon, what can you tell us about the new study in Agricultural Economics, which looked at consumers’ knowledge of local COVID infection rates and their willingness to eat at restaurants?
EM: Well, I know that one of the investigators, Rigoberto Lopez, PhD, of the University of Connecticut, said “no news is bad news.” Restaurants located in cities where local regulations required COVID tracking recovered faster than those in areas that did not, according to data from 87 restaurants in 10 Chinese cities that were gathered between Dec. 1, 2019, and March 27, 2020. Having access to local infection rate data made customers more comfortable going out to eat, the investigators explained.
Second reporter: Interesting, Mr. Muskox, but how about this headline from CNN: “Workers flee China’s biggest iPhone factory over Covid outbreak”? Do you agree with analysts, who said that “the chaos at Zhengzhou could jeopardize Apple and Foxconn’s output in the coming weeks,” as CNN put it?
EM: I did see that a manager at Foxconn, which owns the factory and is known to its friends as Hon Hai Precision Industry, told a Chinese media outlet that “workers are panicking over the spread of the virus at the factory and lack of access to official information.” As we’ve already discussed, no news is bad news.
That’s all the time I have to chat with you today. I’m off to fire some more Twitter employees.
In case you hadn’t already guessed, Vlad Putin is officially more annoying than Elon Musk. We now return to this week’s typical LOTME shenanigans, already in progress.
The deadliest month
With climate change making the world hotter, leading to more heat stroke and organ failure, you would think the summer months would be the most deadly. In reality, though, it’s quite the opposite.
There are multiple factors that make January the most deadly month out of the year, as LiveScience discovered in a recent analysis.
Let’s go through them, shall we?
Respiratory viruses: Robert Glatter, MD, of Lenox Hill Hospital in New York, told LiveScence that winter is the time for illnesses like the flu, bacterial pneumonia, and RSV. Millions of people worldwide die from the flu, according to the CDC. And the World Health Organization reported lower respiratory infections as the fourth-leading cause of death worldwide before COVID came along.
Heart disease: Heart conditions are actually more fatal in the winter months, according to a study published in Circulation. The cold puts more stress on the heart to keep the body warm, which can be a challenge for people who already have preexisting heart conditions.
Space heaters: Dr. Glatter also told Live Science that the use of space heaters could be a factor in the cold winter months since they can lead to carbon monoxide poisoning and even fires. Silent killers.
Holiday season: A time for joy and merriment, certainly, but Christmas et al. have their downsides. By January we’re coming off a 3-month food and alcohol binge, which leads to cardiac stress. There’s also the psychological stress that comes with the season. Sometimes the most wonderful time of the year just isn’t.
So even though summer is hot, fall has hurricanes, and spring tends to have the highest suicide rate, winter still ends up being the deadliest season.
Bad drinking consequence No. 87: Joining the LOTME team
Alcohol and college students go together like peanut butter and jelly. Or peanut butter and chocolate. Or peanut butter and toothpaste. Peanut butter goes with a lot of things.
Naturally, when you combine alcohol and college students, bad decisions are sure to follow. But have you ever wondered just how many bad decisions alcohol causes? A team of researchers from Penn State University, the undisputed champion of poor drinking decisions (trust us, we know), sure has. They’ve even conducted a 4-year study of 1,700 students as they carved a drunken swath through the many fine local drinking establishments, such as East Halls or that one frat house that hosts medieval battle–style ping pong tournaments.
The students were surveyed twice a year throughout the study, and the researchers compiled a list of all the various consequences their subjects experienced. Ultimately, college students will experience an average of 102 consequences from drinking during their 4-year college careers, which is an impressive number. Try thinking up a hundred consequences for anything.
Some consequences are less common than others – we imagine “missing the Renaissance Faire because you felt drunker the morning after than while you were drinking” is pretty low on the list – but more than 96% of students reported that they’d experienced a hangover and that drinking had caused them to say or do embarrassing things. Also, more than 70% said they needed additional alcohol to feel any effect, a potential sign of alcohol use disorder.
Once they had their list, the researchers focused on 12 of the more common and severe consequences, such as blacking out, hangovers, and missing work/class, and asked the study participants how their parents would react to their drinking and those specific consequences. Students who believed their parents would disapprove of alcohol-related consequences actually experienced fewer consequences overall.
College students, it seems, really do care what their parents think, even if they don’t express it, the researchers said. That gives space for parents to offer advice about the consequences of hard drinking, making decisions while drunk, or bringing godawful Fireball whiskey to parties. Seriously, don’t do that. Stuff’s bad, and you should feel bad for bringing it. Your parents raised you better than that.
COVID ‘expert’ discusses data sharing
We interrupt our regularly scheduled programming to bring you this special news event. Elon Musk, the world’s second-most annoying human, is holding a press conference to discuss, of all things, COVID-19.
Reporter: Hey, Mr. Musketeer, what qualifies you to talk about a global pandemic?
EM: As the official king of the Twitterverse, I’m pretty much an expert on any topic.
Reporter: Okay then, Mr. Muskmelon, what can you tell us about the new study in Agricultural Economics, which looked at consumers’ knowledge of local COVID infection rates and their willingness to eat at restaurants?
EM: Well, I know that one of the investigators, Rigoberto Lopez, PhD, of the University of Connecticut, said “no news is bad news.” Restaurants located in cities where local regulations required COVID tracking recovered faster than those in areas that did not, according to data from 87 restaurants in 10 Chinese cities that were gathered between Dec. 1, 2019, and March 27, 2020. Having access to local infection rate data made customers more comfortable going out to eat, the investigators explained.
Second reporter: Interesting, Mr. Muskox, but how about this headline from CNN: “Workers flee China’s biggest iPhone factory over Covid outbreak”? Do you agree with analysts, who said that “the chaos at Zhengzhou could jeopardize Apple and Foxconn’s output in the coming weeks,” as CNN put it?
EM: I did see that a manager at Foxconn, which owns the factory and is known to its friends as Hon Hai Precision Industry, told a Chinese media outlet that “workers are panicking over the spread of the virus at the factory and lack of access to official information.” As we’ve already discussed, no news is bad news.
That’s all the time I have to chat with you today. I’m off to fire some more Twitter employees.
In case you hadn’t already guessed, Vlad Putin is officially more annoying than Elon Musk. We now return to this week’s typical LOTME shenanigans, already in progress.
The deadliest month
With climate change making the world hotter, leading to more heat stroke and organ failure, you would think the summer months would be the most deadly. In reality, though, it’s quite the opposite.
There are multiple factors that make January the most deadly month out of the year, as LiveScience discovered in a recent analysis.
Let’s go through them, shall we?
Respiratory viruses: Robert Glatter, MD, of Lenox Hill Hospital in New York, told LiveScence that winter is the time for illnesses like the flu, bacterial pneumonia, and RSV. Millions of people worldwide die from the flu, according to the CDC. And the World Health Organization reported lower respiratory infections as the fourth-leading cause of death worldwide before COVID came along.
Heart disease: Heart conditions are actually more fatal in the winter months, according to a study published in Circulation. The cold puts more stress on the heart to keep the body warm, which can be a challenge for people who already have preexisting heart conditions.
Space heaters: Dr. Glatter also told Live Science that the use of space heaters could be a factor in the cold winter months since they can lead to carbon monoxide poisoning and even fires. Silent killers.
Holiday season: A time for joy and merriment, certainly, but Christmas et al. have their downsides. By January we’re coming off a 3-month food and alcohol binge, which leads to cardiac stress. There’s also the psychological stress that comes with the season. Sometimes the most wonderful time of the year just isn’t.
So even though summer is hot, fall has hurricanes, and spring tends to have the highest suicide rate, winter still ends up being the deadliest season.
Bad drinking consequence No. 87: Joining the LOTME team
Alcohol and college students go together like peanut butter and jelly. Or peanut butter and chocolate. Or peanut butter and toothpaste. Peanut butter goes with a lot of things.
Naturally, when you combine alcohol and college students, bad decisions are sure to follow. But have you ever wondered just how many bad decisions alcohol causes? A team of researchers from Penn State University, the undisputed champion of poor drinking decisions (trust us, we know), sure has. They’ve even conducted a 4-year study of 1,700 students as they carved a drunken swath through the many fine local drinking establishments, such as East Halls or that one frat house that hosts medieval battle–style ping pong tournaments.
The students were surveyed twice a year throughout the study, and the researchers compiled a list of all the various consequences their subjects experienced. Ultimately, college students will experience an average of 102 consequences from drinking during their 4-year college careers, which is an impressive number. Try thinking up a hundred consequences for anything.
Some consequences are less common than others – we imagine “missing the Renaissance Faire because you felt drunker the morning after than while you were drinking” is pretty low on the list – but more than 96% of students reported that they’d experienced a hangover and that drinking had caused them to say or do embarrassing things. Also, more than 70% said they needed additional alcohol to feel any effect, a potential sign of alcohol use disorder.
Once they had their list, the researchers focused on 12 of the more common and severe consequences, such as blacking out, hangovers, and missing work/class, and asked the study participants how their parents would react to their drinking and those specific consequences. Students who believed their parents would disapprove of alcohol-related consequences actually experienced fewer consequences overall.
College students, it seems, really do care what their parents think, even if they don’t express it, the researchers said. That gives space for parents to offer advice about the consequences of hard drinking, making decisions while drunk, or bringing godawful Fireball whiskey to parties. Seriously, don’t do that. Stuff’s bad, and you should feel bad for bringing it. Your parents raised you better than that.
COVID ‘expert’ discusses data sharing
We interrupt our regularly scheduled programming to bring you this special news event. Elon Musk, the world’s second-most annoying human, is holding a press conference to discuss, of all things, COVID-19.
Reporter: Hey, Mr. Musketeer, what qualifies you to talk about a global pandemic?
EM: As the official king of the Twitterverse, I’m pretty much an expert on any topic.
Reporter: Okay then, Mr. Muskmelon, what can you tell us about the new study in Agricultural Economics, which looked at consumers’ knowledge of local COVID infection rates and their willingness to eat at restaurants?
EM: Well, I know that one of the investigators, Rigoberto Lopez, PhD, of the University of Connecticut, said “no news is bad news.” Restaurants located in cities where local regulations required COVID tracking recovered faster than those in areas that did not, according to data from 87 restaurants in 10 Chinese cities that were gathered between Dec. 1, 2019, and March 27, 2020. Having access to local infection rate data made customers more comfortable going out to eat, the investigators explained.
Second reporter: Interesting, Mr. Muskox, but how about this headline from CNN: “Workers flee China’s biggest iPhone factory over Covid outbreak”? Do you agree with analysts, who said that “the chaos at Zhengzhou could jeopardize Apple and Foxconn’s output in the coming weeks,” as CNN put it?
EM: I did see that a manager at Foxconn, which owns the factory and is known to its friends as Hon Hai Precision Industry, told a Chinese media outlet that “workers are panicking over the spread of the virus at the factory and lack of access to official information.” As we’ve already discussed, no news is bad news.
That’s all the time I have to chat with you today. I’m off to fire some more Twitter employees.
In case you hadn’t already guessed, Vlad Putin is officially more annoying than Elon Musk. We now return to this week’s typical LOTME shenanigans, already in progress.
The deadliest month
With climate change making the world hotter, leading to more heat stroke and organ failure, you would think the summer months would be the most deadly. In reality, though, it’s quite the opposite.
There are multiple factors that make January the most deadly month out of the year, as LiveScience discovered in a recent analysis.
Let’s go through them, shall we?
Respiratory viruses: Robert Glatter, MD, of Lenox Hill Hospital in New York, told LiveScence that winter is the time for illnesses like the flu, bacterial pneumonia, and RSV. Millions of people worldwide die from the flu, according to the CDC. And the World Health Organization reported lower respiratory infections as the fourth-leading cause of death worldwide before COVID came along.
Heart disease: Heart conditions are actually more fatal in the winter months, according to a study published in Circulation. The cold puts more stress on the heart to keep the body warm, which can be a challenge for people who already have preexisting heart conditions.
Space heaters: Dr. Glatter also told Live Science that the use of space heaters could be a factor in the cold winter months since they can lead to carbon monoxide poisoning and even fires. Silent killers.
Holiday season: A time for joy and merriment, certainly, but Christmas et al. have their downsides. By January we’re coming off a 3-month food and alcohol binge, which leads to cardiac stress. There’s also the psychological stress that comes with the season. Sometimes the most wonderful time of the year just isn’t.
So even though summer is hot, fall has hurricanes, and spring tends to have the highest suicide rate, winter still ends up being the deadliest season.
Exercise later in the day for better blood glucose control?
The data come from 775 participants with a mean body mass index (BMI) of 26.2 kg/m2 in the observational Netherlands Epidemiology of Obesity (NEO) study. Use of activity monitors for four consecutive days showed that performance of MVPA (defined as activity with intensity of > 3 metabolic equivalents of task) in the afternoon or evening was associated with up to 25% reduced insulin resistance compared with an even distribution of activity during the day.
“This is one of the first studies where in humans the relation between timing of physical activity and insulin resistance was examined,” lead author Jeroen van der Velde of the department of clinical epidemiology, Leiden (the Netherlands) University Medical Center, said in an interview.
Moreover, he noted that, while previous intervention studies have shown greater blood glucose reduction with high-intensity exercise performed in the afternoon, compared with the morning, in people with impaired glucose metabolism or type 2 diabetes, “as far as I am aware, we were the first to use a population-based study in a general population to study this.”
Katarina Kos, MD, PhD, senior lecturer in diabetes and obesity, University of Exeter (England), said: “This study is novel in that it relates the timing of physical activity if performed in the morning, afternoon, or evening to insulin resistance and fat content. This is from a cohort of middle-aged Dutch people between ages 45-65 studied 10 years ago and based on self-reports of weight and eating behavior and who were found to be generally overweight.”
Is it down to circadian rhythm?
“The results are of interest in that if the chosen timing was in the afternoon [63% of studied population] or evening (8% of the studied population), it seemed to relate with improved metabolism when compared to the morning exercising [16% of population]. ... Whether this was due to the (timing) of activity is yet to be shown,” Dr. Kos told the UK Science Media Centre.
Mr. van der Velde agrees that the effect may be explained at least in part by the circadian rhythm of the body. “Physical activity may act as ... a cue for the activation of clock genes. Previous research has suggested that our body’s muscular system and oxidative system are also affected by our circadian rhythm and their peak activity seems to be in the late afternoon. So, being mostly active in this time period ... may elicit greater metabolic responses compared to being active in the morning.”
But, he cautioned, “I think it is important to realize that we are just beginning to understand the potential impact of physical activity timing. At this stage, I believe it is most important to be physically active in general. So ... if the morning is the only time of the day to go for a walk or a run, certainly do this.”
Dr. Kos concurred: “As this is not an intervention study, further research is needed to explain the cause of the observed association.”
Mr. van der Velde also added that it’s not yet clear which individuals or subgroups might experience additional benefits from timed activities. That’s the current research focus of a large consortium of several research institutes in the Netherlands and Canada.
Timed exercise reduces insulin resistance but not liver fat
The findings were published online in Diabetologia.
The study population included men and women living in the greater Leiden area in the western Netherlands who were aged 45-65 years and self-reported a BMI of 27 or higher. A second cohort included inhabitants of one municipality who were invited to participate regardless of their BMI. All wore the activity monitors for 4 consecutive days and nights during their usual activities.
Neither sedentary time nor breaks in sedentary time (defined as a period of activity with an acceleration greater than 0.75 m/s2 following a sedentary period) were associated with lower insulin resistance, as calculated by blood sampling.
However, the number of breaks in sedentary time was associated with a significant 22% higher liver fat content, assessed with proton magnetic resonance spectroscopy.
One reason for the lack of effect of breaks on insulin resistance, the authors theorized, is that this was a real-world observational study where regular breaks aren’t common. Alternatively, people might not have been intensively active enough during breaks to make a difference.
After adjustment for total body fat, an additional hour of MVPA was associated with a 5% drop in insulin resistance. An additional hour of MVPA in 5-minute bouts was associated with 9% lower insulin resistance.
Also after adjustments, insulin resistance was reduced significantly in participants who were most active in the afternoon, by 18%, or evening, by 25%, whereas insulin resistance was not affected among those who were most active in the morning (–3%), all compared with people who distributed their MVPA throughout the day.
Timing of MVPA was not associated with liver fat content, and there were no significant differences in liver fat content and insulin resistance between groups based on timing of light physical activity.
“This is just speculation, but perhaps for fat accumulation in the liver the circadian system is less involved. Or perhaps timing of other lifestyle variables are more important here, such as dietary intake,” Mr. van der Velde said.
Finally, he observed, “timing of physical activity is most likely just a piece of the puzzle. Timing of other lifestyle behavior, such as sleep, and food intake are important cues for our circadian system as well, and it is likely that all these behaviors interact with each other.”
The NEO study is supported by Leiden University Medical Center, the Netherlands Cardiovascular Research Initiative, an initiative supported by the Dutch Heart Foundation, and the Netherlands Organisation for Health Research and Development/Partnership Diabetes/Dutch Diabetes foundation Breakthrough. Mr. van der Velde has reported no further disclosures.
A version of this article first appeared on Medscape.com.
The data come from 775 participants with a mean body mass index (BMI) of 26.2 kg/m2 in the observational Netherlands Epidemiology of Obesity (NEO) study. Use of activity monitors for four consecutive days showed that performance of MVPA (defined as activity with intensity of > 3 metabolic equivalents of task) in the afternoon or evening was associated with up to 25% reduced insulin resistance compared with an even distribution of activity during the day.
“This is one of the first studies where in humans the relation between timing of physical activity and insulin resistance was examined,” lead author Jeroen van der Velde of the department of clinical epidemiology, Leiden (the Netherlands) University Medical Center, said in an interview.
Moreover, he noted that, while previous intervention studies have shown greater blood glucose reduction with high-intensity exercise performed in the afternoon, compared with the morning, in people with impaired glucose metabolism or type 2 diabetes, “as far as I am aware, we were the first to use a population-based study in a general population to study this.”
Katarina Kos, MD, PhD, senior lecturer in diabetes and obesity, University of Exeter (England), said: “This study is novel in that it relates the timing of physical activity if performed in the morning, afternoon, or evening to insulin resistance and fat content. This is from a cohort of middle-aged Dutch people between ages 45-65 studied 10 years ago and based on self-reports of weight and eating behavior and who were found to be generally overweight.”
Is it down to circadian rhythm?
“The results are of interest in that if the chosen timing was in the afternoon [63% of studied population] or evening (8% of the studied population), it seemed to relate with improved metabolism when compared to the morning exercising [16% of population]. ... Whether this was due to the (timing) of activity is yet to be shown,” Dr. Kos told the UK Science Media Centre.
Mr. van der Velde agrees that the effect may be explained at least in part by the circadian rhythm of the body. “Physical activity may act as ... a cue for the activation of clock genes. Previous research has suggested that our body’s muscular system and oxidative system are also affected by our circadian rhythm and their peak activity seems to be in the late afternoon. So, being mostly active in this time period ... may elicit greater metabolic responses compared to being active in the morning.”
But, he cautioned, “I think it is important to realize that we are just beginning to understand the potential impact of physical activity timing. At this stage, I believe it is most important to be physically active in general. So ... if the morning is the only time of the day to go for a walk or a run, certainly do this.”
Dr. Kos concurred: “As this is not an intervention study, further research is needed to explain the cause of the observed association.”
Mr. van der Velde also added that it’s not yet clear which individuals or subgroups might experience additional benefits from timed activities. That’s the current research focus of a large consortium of several research institutes in the Netherlands and Canada.
Timed exercise reduces insulin resistance but not liver fat
The findings were published online in Diabetologia.
The study population included men and women living in the greater Leiden area in the western Netherlands who were aged 45-65 years and self-reported a BMI of 27 or higher. A second cohort included inhabitants of one municipality who were invited to participate regardless of their BMI. All wore the activity monitors for 4 consecutive days and nights during their usual activities.
Neither sedentary time nor breaks in sedentary time (defined as a period of activity with an acceleration greater than 0.75 m/s2 following a sedentary period) were associated with lower insulin resistance, as calculated by blood sampling.
However, the number of breaks in sedentary time was associated with a significant 22% higher liver fat content, assessed with proton magnetic resonance spectroscopy.
One reason for the lack of effect of breaks on insulin resistance, the authors theorized, is that this was a real-world observational study where regular breaks aren’t common. Alternatively, people might not have been intensively active enough during breaks to make a difference.
After adjustment for total body fat, an additional hour of MVPA was associated with a 5% drop in insulin resistance. An additional hour of MVPA in 5-minute bouts was associated with 9% lower insulin resistance.
Also after adjustments, insulin resistance was reduced significantly in participants who were most active in the afternoon, by 18%, or evening, by 25%, whereas insulin resistance was not affected among those who were most active in the morning (–3%), all compared with people who distributed their MVPA throughout the day.
Timing of MVPA was not associated with liver fat content, and there were no significant differences in liver fat content and insulin resistance between groups based on timing of light physical activity.
“This is just speculation, but perhaps for fat accumulation in the liver the circadian system is less involved. Or perhaps timing of other lifestyle variables are more important here, such as dietary intake,” Mr. van der Velde said.
Finally, he observed, “timing of physical activity is most likely just a piece of the puzzle. Timing of other lifestyle behavior, such as sleep, and food intake are important cues for our circadian system as well, and it is likely that all these behaviors interact with each other.”
The NEO study is supported by Leiden University Medical Center, the Netherlands Cardiovascular Research Initiative, an initiative supported by the Dutch Heart Foundation, and the Netherlands Organisation for Health Research and Development/Partnership Diabetes/Dutch Diabetes foundation Breakthrough. Mr. van der Velde has reported no further disclosures.
A version of this article first appeared on Medscape.com.
The data come from 775 participants with a mean body mass index (BMI) of 26.2 kg/m2 in the observational Netherlands Epidemiology of Obesity (NEO) study. Use of activity monitors for four consecutive days showed that performance of MVPA (defined as activity with intensity of > 3 metabolic equivalents of task) in the afternoon or evening was associated with up to 25% reduced insulin resistance compared with an even distribution of activity during the day.
“This is one of the first studies where in humans the relation between timing of physical activity and insulin resistance was examined,” lead author Jeroen van der Velde of the department of clinical epidemiology, Leiden (the Netherlands) University Medical Center, said in an interview.
Moreover, he noted that, while previous intervention studies have shown greater blood glucose reduction with high-intensity exercise performed in the afternoon, compared with the morning, in people with impaired glucose metabolism or type 2 diabetes, “as far as I am aware, we were the first to use a population-based study in a general population to study this.”
Katarina Kos, MD, PhD, senior lecturer in diabetes and obesity, University of Exeter (England), said: “This study is novel in that it relates the timing of physical activity if performed in the morning, afternoon, or evening to insulin resistance and fat content. This is from a cohort of middle-aged Dutch people between ages 45-65 studied 10 years ago and based on self-reports of weight and eating behavior and who were found to be generally overweight.”
Is it down to circadian rhythm?
“The results are of interest in that if the chosen timing was in the afternoon [63% of studied population] or evening (8% of the studied population), it seemed to relate with improved metabolism when compared to the morning exercising [16% of population]. ... Whether this was due to the (timing) of activity is yet to be shown,” Dr. Kos told the UK Science Media Centre.
Mr. van der Velde agrees that the effect may be explained at least in part by the circadian rhythm of the body. “Physical activity may act as ... a cue for the activation of clock genes. Previous research has suggested that our body’s muscular system and oxidative system are also affected by our circadian rhythm and their peak activity seems to be in the late afternoon. So, being mostly active in this time period ... may elicit greater metabolic responses compared to being active in the morning.”
But, he cautioned, “I think it is important to realize that we are just beginning to understand the potential impact of physical activity timing. At this stage, I believe it is most important to be physically active in general. So ... if the morning is the only time of the day to go for a walk or a run, certainly do this.”
Dr. Kos concurred: “As this is not an intervention study, further research is needed to explain the cause of the observed association.”
Mr. van der Velde also added that it’s not yet clear which individuals or subgroups might experience additional benefits from timed activities. That’s the current research focus of a large consortium of several research institutes in the Netherlands and Canada.
Timed exercise reduces insulin resistance but not liver fat
The findings were published online in Diabetologia.
The study population included men and women living in the greater Leiden area in the western Netherlands who were aged 45-65 years and self-reported a BMI of 27 or higher. A second cohort included inhabitants of one municipality who were invited to participate regardless of their BMI. All wore the activity monitors for 4 consecutive days and nights during their usual activities.
Neither sedentary time nor breaks in sedentary time (defined as a period of activity with an acceleration greater than 0.75 m/s2 following a sedentary period) were associated with lower insulin resistance, as calculated by blood sampling.
However, the number of breaks in sedentary time was associated with a significant 22% higher liver fat content, assessed with proton magnetic resonance spectroscopy.
One reason for the lack of effect of breaks on insulin resistance, the authors theorized, is that this was a real-world observational study where regular breaks aren’t common. Alternatively, people might not have been intensively active enough during breaks to make a difference.
After adjustment for total body fat, an additional hour of MVPA was associated with a 5% drop in insulin resistance. An additional hour of MVPA in 5-minute bouts was associated with 9% lower insulin resistance.
Also after adjustments, insulin resistance was reduced significantly in participants who were most active in the afternoon, by 18%, or evening, by 25%, whereas insulin resistance was not affected among those who were most active in the morning (–3%), all compared with people who distributed their MVPA throughout the day.
Timing of MVPA was not associated with liver fat content, and there were no significant differences in liver fat content and insulin resistance between groups based on timing of light physical activity.
“This is just speculation, but perhaps for fat accumulation in the liver the circadian system is less involved. Or perhaps timing of other lifestyle variables are more important here, such as dietary intake,” Mr. van der Velde said.
Finally, he observed, “timing of physical activity is most likely just a piece of the puzzle. Timing of other lifestyle behavior, such as sleep, and food intake are important cues for our circadian system as well, and it is likely that all these behaviors interact with each other.”
The NEO study is supported by Leiden University Medical Center, the Netherlands Cardiovascular Research Initiative, an initiative supported by the Dutch Heart Foundation, and the Netherlands Organisation for Health Research and Development/Partnership Diabetes/Dutch Diabetes foundation Breakthrough. Mr. van der Velde has reported no further disclosures.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA
USPSTF holds firm on postmenopausal hormone recommendations
The U.S. Preventive Services Task Force moved forward their recommendations for using hormone therapy to prevent chronic conditions in postmenopausal women by keeping them the same.
The central message of the new recommendations, released on Nov. 1 as a statement published in JAMA, remains unchanged from the last update in 2017.
The message also remains simple: Don’t use hormone therapy for preventing chronic conditions, such as cardiovascular disease, cancer, and osteoporosis, or bone fracture.
The USPSTF summarized its recommendations in two brief statements: the group “recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal persons” and “recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal persons who have had a hysterectomy.”
This wording is identical to that used in the 2017 guidance (except it now refers to postmenopausal persons instead of specifically women). The recommendation against use of estrogen and progestin for prevention of chronic conditions in postmenopausal women was first made by the USPSTF in 2002.
An editorial accompanying the 2022 revision notes that the evidence cited by the USPSTF includes “only two additional, modest-sized trials” (that focused on the effects of hormone therapy on cognition and brain structure) compared with 2017, “as well as ancillary analyses of previous trials.”
A standard 5-year update
The 2022 revision and revisiting of the evidence base by the Task Force regarding the benefits and risks of postmenopausal hormone therapy occurred “as part of the Task Force’s standard approach, which includes updating each recommendation approximately every 5 years,” explained Carol M. Mangione, MD, who is USPSTF chair and chief of the division of general internal medicine and health services research at the University of California, Los Angeles.
“In our review we again found that while hormone therapy may reduce the risk of some conditions, it can also lead to serious harms such as an increase in the risk of blood clots and stroke,” Dr. Mangione said in an interview. “The harms cancel out any potential benefits overall.”
This new statement only applies to using menopausal hormone treatment for preventing chronic conditions in asymptomatic people but does not speak to using this treatment in managing people with perimenopausal symptoms such as hot flashes or vaginal dryness or treating people with premature or surgical menopause, Dr. Mangione highlighted.
No review for treating menopausal symptoms
“The Task Force encourages people who are experiencing symptoms of menopause to talk with their health care professional about the best treatment for them,” explained Dr. Mangione. “The Task Force did not review the evidence on the use of hormone therapy to treat symptoms of menopause.”
Osteoporosis and increased risk for bone fracture were among the conditions that accompany menopause reviewed by the USPSTF. The Task Force concluded that while “hormone therapy was associated with decreased risk of fractures,” after weighing the benefits and harms for preventing this condition, “there is no net benefit at the population level.”
This conclusion seems to contrast with the 2022 hormone therapy position statement of the North American Menopause Society (NAMS), released in July, which states: “For women aged younger than 60 years or who are within 10 years of menopause onset and have no contraindications, the benefit-risk ratio is favorable for treatment of bothersome vasomotor symptoms and prevention of bone loss.”
USPSTF, NAMS are ‘completely consistent’
However, Stephanie S. Faubion, MD, medical director of NAMS and director of the women’s health clinic at Mayo Clinic, Rochester, Minn., said the new USPSTF recommendations “are completely consistent” with the recent NAMS statement.
“We are entirely aligned with the recommendation to use hormone therapy for management of menopausal symptoms and not for chronic disease prevention or as an anti-aging strategy,” Dr. Faubion commented in an interview.
Dr. Faubion also stressed that “menopausal hormone therapy remains the most effective treatment for menopausal symptoms,” and that “women should not be reflexively directed to other pharmacologic therapies for management of menopausal symptoms.”
The distinction the USPSTF makes between its recommendations against using hormone therapy to prevent chronic conditions and its deferral of comment on use of the same treatment to manage perimenopausal symptoms is often forgotten, note Alison J. Huang, MD, and Deborah Grady, MD, in their editorial.
A problem of conflation
“Many patients and clinicians conflate these two different indications,” they write.
The notion that the net harms of menopausal hormone therapy outweigh the benefits “is now widely adopted as a rationale for foregoing menopausal hormone therapy for symptomatic treatment,” even though “nonhormonal treatments that are as effective as menopausal hormone therapy have not yet been identified,” say Dr. Huang and Dr. Grady, both physicians at the University of California, San Francisco.
In addition, alternative, nonhormonal options for treating perimenopausal symptoms have not received the same level of scrutiny as hormonal treatment, they say.
“It is arguably problematic to avoid menopausal hormone therapy and favor potentially less effective treatments, when the longer-term implications of those treatments for health have not been evaluated,” Dr. Huang and Dr. Grady write in their editorial.
In short, during menopause, people are at risk of being “frightened away from considering using menopausal hormone therapy for distressing symptoms,” they say.
“We can’t speak to whether or how often clinicians might be conflating the role of hormone therapy in treating symptoms and preventing chronic conditions,” answered Dr. Mangione.
“We hope to ensure that health professionals know that hormone therapy is not a beneficial way to reduce the risk of chronic conditions such as heart disease, cancer, and strokes,” she added. The new recommendations are an effort to “raise awareness about the value of considering other safe and effective ways for people to reduce their risk of chronic health problems as they age.”
The issue of timing
Another critique offered by Dr. Huang and Dr. Grady in their editorial is that “the scientific and medical community should let go of the past,” and should no longer invest additional resources in “trying to parse out subsets of menopausal patients who may derive some preventive benefit from menopausal hormone therapy for a limited amount of time.”
But Dr. Mangione disagreed.
The USPSTF “calls for more research that can help us understand whether health outcomes – both benefits and harms – differ depending on a person’s age or when they started hormone therapy related to when they went through menopause,” she said.
Dr. Mangione also highlighted the need for additional research on whether the benefits and risks of menopausal hormone therapy vary across racial and ethnic groups.
USPSTF receives no commercial funding. Dr. Mangione, Dr. Huang, and Dr. Grady have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force moved forward their recommendations for using hormone therapy to prevent chronic conditions in postmenopausal women by keeping them the same.
The central message of the new recommendations, released on Nov. 1 as a statement published in JAMA, remains unchanged from the last update in 2017.
The message also remains simple: Don’t use hormone therapy for preventing chronic conditions, such as cardiovascular disease, cancer, and osteoporosis, or bone fracture.
The USPSTF summarized its recommendations in two brief statements: the group “recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal persons” and “recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal persons who have had a hysterectomy.”
This wording is identical to that used in the 2017 guidance (except it now refers to postmenopausal persons instead of specifically women). The recommendation against use of estrogen and progestin for prevention of chronic conditions in postmenopausal women was first made by the USPSTF in 2002.
An editorial accompanying the 2022 revision notes that the evidence cited by the USPSTF includes “only two additional, modest-sized trials” (that focused on the effects of hormone therapy on cognition and brain structure) compared with 2017, “as well as ancillary analyses of previous trials.”
A standard 5-year update
The 2022 revision and revisiting of the evidence base by the Task Force regarding the benefits and risks of postmenopausal hormone therapy occurred “as part of the Task Force’s standard approach, which includes updating each recommendation approximately every 5 years,” explained Carol M. Mangione, MD, who is USPSTF chair and chief of the division of general internal medicine and health services research at the University of California, Los Angeles.
“In our review we again found that while hormone therapy may reduce the risk of some conditions, it can also lead to serious harms such as an increase in the risk of blood clots and stroke,” Dr. Mangione said in an interview. “The harms cancel out any potential benefits overall.”
This new statement only applies to using menopausal hormone treatment for preventing chronic conditions in asymptomatic people but does not speak to using this treatment in managing people with perimenopausal symptoms such as hot flashes or vaginal dryness or treating people with premature or surgical menopause, Dr. Mangione highlighted.
No review for treating menopausal symptoms
“The Task Force encourages people who are experiencing symptoms of menopause to talk with their health care professional about the best treatment for them,” explained Dr. Mangione. “The Task Force did not review the evidence on the use of hormone therapy to treat symptoms of menopause.”
Osteoporosis and increased risk for bone fracture were among the conditions that accompany menopause reviewed by the USPSTF. The Task Force concluded that while “hormone therapy was associated with decreased risk of fractures,” after weighing the benefits and harms for preventing this condition, “there is no net benefit at the population level.”
This conclusion seems to contrast with the 2022 hormone therapy position statement of the North American Menopause Society (NAMS), released in July, which states: “For women aged younger than 60 years or who are within 10 years of menopause onset and have no contraindications, the benefit-risk ratio is favorable for treatment of bothersome vasomotor symptoms and prevention of bone loss.”
USPSTF, NAMS are ‘completely consistent’
However, Stephanie S. Faubion, MD, medical director of NAMS and director of the women’s health clinic at Mayo Clinic, Rochester, Minn., said the new USPSTF recommendations “are completely consistent” with the recent NAMS statement.
“We are entirely aligned with the recommendation to use hormone therapy for management of menopausal symptoms and not for chronic disease prevention or as an anti-aging strategy,” Dr. Faubion commented in an interview.
Dr. Faubion also stressed that “menopausal hormone therapy remains the most effective treatment for menopausal symptoms,” and that “women should not be reflexively directed to other pharmacologic therapies for management of menopausal symptoms.”
The distinction the USPSTF makes between its recommendations against using hormone therapy to prevent chronic conditions and its deferral of comment on use of the same treatment to manage perimenopausal symptoms is often forgotten, note Alison J. Huang, MD, and Deborah Grady, MD, in their editorial.
A problem of conflation
“Many patients and clinicians conflate these two different indications,” they write.
The notion that the net harms of menopausal hormone therapy outweigh the benefits “is now widely adopted as a rationale for foregoing menopausal hormone therapy for symptomatic treatment,” even though “nonhormonal treatments that are as effective as menopausal hormone therapy have not yet been identified,” say Dr. Huang and Dr. Grady, both physicians at the University of California, San Francisco.
In addition, alternative, nonhormonal options for treating perimenopausal symptoms have not received the same level of scrutiny as hormonal treatment, they say.
“It is arguably problematic to avoid menopausal hormone therapy and favor potentially less effective treatments, when the longer-term implications of those treatments for health have not been evaluated,” Dr. Huang and Dr. Grady write in their editorial.
In short, during menopause, people are at risk of being “frightened away from considering using menopausal hormone therapy for distressing symptoms,” they say.
“We can’t speak to whether or how often clinicians might be conflating the role of hormone therapy in treating symptoms and preventing chronic conditions,” answered Dr. Mangione.
“We hope to ensure that health professionals know that hormone therapy is not a beneficial way to reduce the risk of chronic conditions such as heart disease, cancer, and strokes,” she added. The new recommendations are an effort to “raise awareness about the value of considering other safe and effective ways for people to reduce their risk of chronic health problems as they age.”
The issue of timing
Another critique offered by Dr. Huang and Dr. Grady in their editorial is that “the scientific and medical community should let go of the past,” and should no longer invest additional resources in “trying to parse out subsets of menopausal patients who may derive some preventive benefit from menopausal hormone therapy for a limited amount of time.”
But Dr. Mangione disagreed.
The USPSTF “calls for more research that can help us understand whether health outcomes – both benefits and harms – differ depending on a person’s age or when they started hormone therapy related to when they went through menopause,” she said.
Dr. Mangione also highlighted the need for additional research on whether the benefits and risks of menopausal hormone therapy vary across racial and ethnic groups.
USPSTF receives no commercial funding. Dr. Mangione, Dr. Huang, and Dr. Grady have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force moved forward their recommendations for using hormone therapy to prevent chronic conditions in postmenopausal women by keeping them the same.
The central message of the new recommendations, released on Nov. 1 as a statement published in JAMA, remains unchanged from the last update in 2017.
The message also remains simple: Don’t use hormone therapy for preventing chronic conditions, such as cardiovascular disease, cancer, and osteoporosis, or bone fracture.
The USPSTF summarized its recommendations in two brief statements: the group “recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal persons” and “recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal persons who have had a hysterectomy.”
This wording is identical to that used in the 2017 guidance (except it now refers to postmenopausal persons instead of specifically women). The recommendation against use of estrogen and progestin for prevention of chronic conditions in postmenopausal women was first made by the USPSTF in 2002.
An editorial accompanying the 2022 revision notes that the evidence cited by the USPSTF includes “only two additional, modest-sized trials” (that focused on the effects of hormone therapy on cognition and brain structure) compared with 2017, “as well as ancillary analyses of previous trials.”
A standard 5-year update
The 2022 revision and revisiting of the evidence base by the Task Force regarding the benefits and risks of postmenopausal hormone therapy occurred “as part of the Task Force’s standard approach, which includes updating each recommendation approximately every 5 years,” explained Carol M. Mangione, MD, who is USPSTF chair and chief of the division of general internal medicine and health services research at the University of California, Los Angeles.
“In our review we again found that while hormone therapy may reduce the risk of some conditions, it can also lead to serious harms such as an increase in the risk of blood clots and stroke,” Dr. Mangione said in an interview. “The harms cancel out any potential benefits overall.”
This new statement only applies to using menopausal hormone treatment for preventing chronic conditions in asymptomatic people but does not speak to using this treatment in managing people with perimenopausal symptoms such as hot flashes or vaginal dryness or treating people with premature or surgical menopause, Dr. Mangione highlighted.
No review for treating menopausal symptoms
“The Task Force encourages people who are experiencing symptoms of menopause to talk with their health care professional about the best treatment for them,” explained Dr. Mangione. “The Task Force did not review the evidence on the use of hormone therapy to treat symptoms of menopause.”
Osteoporosis and increased risk for bone fracture were among the conditions that accompany menopause reviewed by the USPSTF. The Task Force concluded that while “hormone therapy was associated with decreased risk of fractures,” after weighing the benefits and harms for preventing this condition, “there is no net benefit at the population level.”
This conclusion seems to contrast with the 2022 hormone therapy position statement of the North American Menopause Society (NAMS), released in July, which states: “For women aged younger than 60 years or who are within 10 years of menopause onset and have no contraindications, the benefit-risk ratio is favorable for treatment of bothersome vasomotor symptoms and prevention of bone loss.”
USPSTF, NAMS are ‘completely consistent’
However, Stephanie S. Faubion, MD, medical director of NAMS and director of the women’s health clinic at Mayo Clinic, Rochester, Minn., said the new USPSTF recommendations “are completely consistent” with the recent NAMS statement.
“We are entirely aligned with the recommendation to use hormone therapy for management of menopausal symptoms and not for chronic disease prevention or as an anti-aging strategy,” Dr. Faubion commented in an interview.
Dr. Faubion also stressed that “menopausal hormone therapy remains the most effective treatment for menopausal symptoms,” and that “women should not be reflexively directed to other pharmacologic therapies for management of menopausal symptoms.”
The distinction the USPSTF makes between its recommendations against using hormone therapy to prevent chronic conditions and its deferral of comment on use of the same treatment to manage perimenopausal symptoms is often forgotten, note Alison J. Huang, MD, and Deborah Grady, MD, in their editorial.
A problem of conflation
“Many patients and clinicians conflate these two different indications,” they write.
The notion that the net harms of menopausal hormone therapy outweigh the benefits “is now widely adopted as a rationale for foregoing menopausal hormone therapy for symptomatic treatment,” even though “nonhormonal treatments that are as effective as menopausal hormone therapy have not yet been identified,” say Dr. Huang and Dr. Grady, both physicians at the University of California, San Francisco.
In addition, alternative, nonhormonal options for treating perimenopausal symptoms have not received the same level of scrutiny as hormonal treatment, they say.
“It is arguably problematic to avoid menopausal hormone therapy and favor potentially less effective treatments, when the longer-term implications of those treatments for health have not been evaluated,” Dr. Huang and Dr. Grady write in their editorial.
In short, during menopause, people are at risk of being “frightened away from considering using menopausal hormone therapy for distressing symptoms,” they say.
“We can’t speak to whether or how often clinicians might be conflating the role of hormone therapy in treating symptoms and preventing chronic conditions,” answered Dr. Mangione.
“We hope to ensure that health professionals know that hormone therapy is not a beneficial way to reduce the risk of chronic conditions such as heart disease, cancer, and strokes,” she added. The new recommendations are an effort to “raise awareness about the value of considering other safe and effective ways for people to reduce their risk of chronic health problems as they age.”
The issue of timing
Another critique offered by Dr. Huang and Dr. Grady in their editorial is that “the scientific and medical community should let go of the past,” and should no longer invest additional resources in “trying to parse out subsets of menopausal patients who may derive some preventive benefit from menopausal hormone therapy for a limited amount of time.”
But Dr. Mangione disagreed.
The USPSTF “calls for more research that can help us understand whether health outcomes – both benefits and harms – differ depending on a person’s age or when they started hormone therapy related to when they went through menopause,” she said.
Dr. Mangione also highlighted the need for additional research on whether the benefits and risks of menopausal hormone therapy vary across racial and ethnic groups.
USPSTF receives no commercial funding. Dr. Mangione, Dr. Huang, and Dr. Grady have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA