User login
Artificial intelligence matches cancer genotypes to patient phenotypes
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR 2020
Hypertension often goes undertreated in patients with a history of stroke
A new study of hypertension treatment trends found that “To our knowledge, the present study is the first to analyze and report national antihypertensive medication trends exclusively among individuals with a history of stroke in the United States,” wrote Daniel Santos, MD, and Mandip S. Dhamoon, MD, DrPH, of the Icahn School of Medicine at Mount Sinai, New York. Their study was published in JAMA Neurology.
To examine blood pressure control and treatment trends among stroke survivors, the researchers examined more than a decade of data from the National Health and Nutrition Examination Survey (NHANES). The cross-sectional survey is conducted in 2-year cycles; the authors analyzed the results from 2005 to 2016 and uncovered a total of 4,971,136 eligible individuals with a history of both stroke and hypertension.
The mean age of the study population was 67.1 (95% confidence interval, 66.1-68.1), and 2,790,518 (56.1%) were women. Their mean blood pressure was 134/68 mm Hg (95% CI, 133/67–136/69), and the average number of antihypertensive medications they were taking was 1.8 (95% CI, 1.7-1.9). Of the 4,971,136 analyzed individuals, 4,721,409 (95%) were aware of their hypertension diagnosis yet more than 10% of that group had not previously been prescribed an antihypertensive medication.
More than 37% (n = 1,846,470) of the participants had uncontrolled high blood pressure upon examination (95% CI, 33.5%-40.8%), and 15.3% (95% CI, 12.5%-18.0%) were not taking any medication for it at all. The most commonly used antihypertensive medications included ACE inhibitors or angiotensin receptor blockers (59.2%; 95% CI, 54.9%-63.4%), beta-blockers (43.8%; 95% CI, 40.3%-47.3%), diuretics (41.6%; 95% CI, 37.3%-45.9%) and calcium-channel blockers (31.5%; 95% CI, 28.2%-34.8%).* Roughly 57% of the sample was taking more than one antihypertensive medication (95% CI, 52.8%-60.6%) while 28% (95% CI, 24.6%-31.5%) were taking only one.
Continued surveillance is key
“All the studies that have ever been done show that hypertension is inadequately treated,” Louis Caplan, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston, said in an interview. “One of the reasons is that it can be hard to get some of the patients to seek treatment, particularly Black Americans. Also, a lot of the medicines to treat high blood pressure have side effects, so many patients don’t want to take the pills.
“Treating hypertension really requires continued surveillance,” he added. “It’s not one visit where the doctor gives you a pill. It’s taking the pill, following your blood pressure, and seeing if it works. If it doesn’t, then maybe you change the dose, get another pill, and are followed once again. That doesn’t happen as often as it should.”
In regard to next steps, Dr. Caplan urged that hypertension “be evaluated more seriously. Even as home blood pressure kits and monitoring become increasingly available, many doctors are still going by a casual blood pressure test in the office, which doesn’t tell you how serious the problem is. There needs to be more use of technology and more conditioning of patients to monitor their own blood pressure as a guide, and then we go from there.”
The authors acknowledged their study’s limitations, including the NHANES’s reliance on self-reporting a history of stroke and the inability to distinguish between subtypes of stroke. In addition, they noted that many antihypertensive medications have uses beyond treating hypertension, which introduces “another confounding factor to medication trends.”
The authors and Dr. Caplan reported no conflicts of interest.
SOURCE: Santos D et al. JAMA Neurol. 2020 Jul 27. doi: 10.1001/jamaneurol.2020.2499.
Correction, 8/20/20: An earlier version of this article misstated the confidence interval for diuretics.
A new study of hypertension treatment trends found that “To our knowledge, the present study is the first to analyze and report national antihypertensive medication trends exclusively among individuals with a history of stroke in the United States,” wrote Daniel Santos, MD, and Mandip S. Dhamoon, MD, DrPH, of the Icahn School of Medicine at Mount Sinai, New York. Their study was published in JAMA Neurology.
To examine blood pressure control and treatment trends among stroke survivors, the researchers examined more than a decade of data from the National Health and Nutrition Examination Survey (NHANES). The cross-sectional survey is conducted in 2-year cycles; the authors analyzed the results from 2005 to 2016 and uncovered a total of 4,971,136 eligible individuals with a history of both stroke and hypertension.
The mean age of the study population was 67.1 (95% confidence interval, 66.1-68.1), and 2,790,518 (56.1%) were women. Their mean blood pressure was 134/68 mm Hg (95% CI, 133/67–136/69), and the average number of antihypertensive medications they were taking was 1.8 (95% CI, 1.7-1.9). Of the 4,971,136 analyzed individuals, 4,721,409 (95%) were aware of their hypertension diagnosis yet more than 10% of that group had not previously been prescribed an antihypertensive medication.
More than 37% (n = 1,846,470) of the participants had uncontrolled high blood pressure upon examination (95% CI, 33.5%-40.8%), and 15.3% (95% CI, 12.5%-18.0%) were not taking any medication for it at all. The most commonly used antihypertensive medications included ACE inhibitors or angiotensin receptor blockers (59.2%; 95% CI, 54.9%-63.4%), beta-blockers (43.8%; 95% CI, 40.3%-47.3%), diuretics (41.6%; 95% CI, 37.3%-45.9%) and calcium-channel blockers (31.5%; 95% CI, 28.2%-34.8%).* Roughly 57% of the sample was taking more than one antihypertensive medication (95% CI, 52.8%-60.6%) while 28% (95% CI, 24.6%-31.5%) were taking only one.
Continued surveillance is key
“All the studies that have ever been done show that hypertension is inadequately treated,” Louis Caplan, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston, said in an interview. “One of the reasons is that it can be hard to get some of the patients to seek treatment, particularly Black Americans. Also, a lot of the medicines to treat high blood pressure have side effects, so many patients don’t want to take the pills.
“Treating hypertension really requires continued surveillance,” he added. “It’s not one visit where the doctor gives you a pill. It’s taking the pill, following your blood pressure, and seeing if it works. If it doesn’t, then maybe you change the dose, get another pill, and are followed once again. That doesn’t happen as often as it should.”
In regard to next steps, Dr. Caplan urged that hypertension “be evaluated more seriously. Even as home blood pressure kits and monitoring become increasingly available, many doctors are still going by a casual blood pressure test in the office, which doesn’t tell you how serious the problem is. There needs to be more use of technology and more conditioning of patients to monitor their own blood pressure as a guide, and then we go from there.”
The authors acknowledged their study’s limitations, including the NHANES’s reliance on self-reporting a history of stroke and the inability to distinguish between subtypes of stroke. In addition, they noted that many antihypertensive medications have uses beyond treating hypertension, which introduces “another confounding factor to medication trends.”
The authors and Dr. Caplan reported no conflicts of interest.
SOURCE: Santos D et al. JAMA Neurol. 2020 Jul 27. doi: 10.1001/jamaneurol.2020.2499.
Correction, 8/20/20: An earlier version of this article misstated the confidence interval for diuretics.
A new study of hypertension treatment trends found that “To our knowledge, the present study is the first to analyze and report national antihypertensive medication trends exclusively among individuals with a history of stroke in the United States,” wrote Daniel Santos, MD, and Mandip S. Dhamoon, MD, DrPH, of the Icahn School of Medicine at Mount Sinai, New York. Their study was published in JAMA Neurology.
To examine blood pressure control and treatment trends among stroke survivors, the researchers examined more than a decade of data from the National Health and Nutrition Examination Survey (NHANES). The cross-sectional survey is conducted in 2-year cycles; the authors analyzed the results from 2005 to 2016 and uncovered a total of 4,971,136 eligible individuals with a history of both stroke and hypertension.
The mean age of the study population was 67.1 (95% confidence interval, 66.1-68.1), and 2,790,518 (56.1%) were women. Their mean blood pressure was 134/68 mm Hg (95% CI, 133/67–136/69), and the average number of antihypertensive medications they were taking was 1.8 (95% CI, 1.7-1.9). Of the 4,971,136 analyzed individuals, 4,721,409 (95%) were aware of their hypertension diagnosis yet more than 10% of that group had not previously been prescribed an antihypertensive medication.
More than 37% (n = 1,846,470) of the participants had uncontrolled high blood pressure upon examination (95% CI, 33.5%-40.8%), and 15.3% (95% CI, 12.5%-18.0%) were not taking any medication for it at all. The most commonly used antihypertensive medications included ACE inhibitors or angiotensin receptor blockers (59.2%; 95% CI, 54.9%-63.4%), beta-blockers (43.8%; 95% CI, 40.3%-47.3%), diuretics (41.6%; 95% CI, 37.3%-45.9%) and calcium-channel blockers (31.5%; 95% CI, 28.2%-34.8%).* Roughly 57% of the sample was taking more than one antihypertensive medication (95% CI, 52.8%-60.6%) while 28% (95% CI, 24.6%-31.5%) were taking only one.
Continued surveillance is key
“All the studies that have ever been done show that hypertension is inadequately treated,” Louis Caplan, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston, said in an interview. “One of the reasons is that it can be hard to get some of the patients to seek treatment, particularly Black Americans. Also, a lot of the medicines to treat high blood pressure have side effects, so many patients don’t want to take the pills.
“Treating hypertension really requires continued surveillance,” he added. “It’s not one visit where the doctor gives you a pill. It’s taking the pill, following your blood pressure, and seeing if it works. If it doesn’t, then maybe you change the dose, get another pill, and are followed once again. That doesn’t happen as often as it should.”
In regard to next steps, Dr. Caplan urged that hypertension “be evaluated more seriously. Even as home blood pressure kits and monitoring become increasingly available, many doctors are still going by a casual blood pressure test in the office, which doesn’t tell you how serious the problem is. There needs to be more use of technology and more conditioning of patients to monitor their own blood pressure as a guide, and then we go from there.”
The authors acknowledged their study’s limitations, including the NHANES’s reliance on self-reporting a history of stroke and the inability to distinguish between subtypes of stroke. In addition, they noted that many antihypertensive medications have uses beyond treating hypertension, which introduces “another confounding factor to medication trends.”
The authors and Dr. Caplan reported no conflicts of interest.
SOURCE: Santos D et al. JAMA Neurol. 2020 Jul 27. doi: 10.1001/jamaneurol.2020.2499.
Correction, 8/20/20: An earlier version of this article misstated the confidence interval for diuretics.
FROM JAMA NEUROLOGY
Chronicles of cancer: A history of mammography, part 1
Technological imperatives
The history of mammography provides a powerful example of the connection between social factors and the rise of a medical technology. It is also an object lesson in the profound difficulties that the medical community faces when trying to evaluate and embrace new discoveries in such a complex area as cancer diagnosis and treatment, especially when tied to issues of sex-based bias and gender identity. Given its profound ties to women’s lives and women’s bodies, mammography holds a unique place in the history of cancer. Part 1 will examine the technological imperatives driving mammography forward, and part 2 will address the social factors that promoted and inhibited the developing technology.
All that glitters
Innovations in technology have contributed so greatly to the progress of medical science in saving and improving patients’ lives that the lure of new technology and the desire to see it succeed and to embrace it has become profound.
In a debate on the adoption of new technologies, Michael Rosen, MD, a surgeon at the Cleveland Clinic, Ohio, pointed out the inherent risks in the life cycle of medical technology: “The stages of surgical innovation have been well described as moving from the generation of a hypothesis with an early promising report to being accepted conclusively as a new standard without formal testing. As the life cycle continues and comparative effectiveness data begin to emerge slowly through appropriately designed trials, the procedure or device is often ultimately abandoned.”1
The history of mammography bears out this grim warning in example after example as an object lesson, revealing not only the difficulties involved in the development of new medical technologies, but also the profound problems involved in validating the effectiveness and appropriateness of a new technology from its inception to the present.
A modern failure?
In fact, one of the more modern developments in mammography technology – digital imaging – has recently been called into question with regard to its effectiveness in saving lives, even as the technology continues to spread throughout the medical community.
A recent meta-analysis has shown that there is little or no improvement in outcomes of breast cancer screening when using digital analysis and screening mammograms vs. traditional film recording.
The meta-analysis assessed 24 studies with a combined total of 16,583,743 screening examinations (10,968,843 film and 5,614,900 digital). The study found that the difference in cancer detection rate using digital rather than film screening showed an increase of only 0.51 detections per 1,000 screens.
The researchers concluded “that while digital mammography is beneficial for medical facilities due to easier storage and handling of images, these results suggest the transition from film to digital mammography has not resulted in health benefits for screened women.”2
In fact, the researchers added that “This analysis reinforces the need to carefully evaluate effects of future changes in technology, such as tomosynthesis, to ensure new technology leads to improved health outcomes and beyond technical gains.”2
None of the nine main randomized clinical trials that were used to determine the effectiveness of mammography screening from the 1960s to the 1990s used digital or 3-D digital mammography (digital breast tomosynthesis or DBT). The earliest trial used direct-exposure film mammography and the others relied upon screen-film mammography.3 And yet the assumptions of the validity of the new digital technologies were predicated on the generalized acceptance of the validity of screening derived from these studies, and a corollary assumption that any technological improvement in the quality of the image must inherently be an improvement of the overall results of screening.
The failure of new technologies to meet expectations is a sobering corrective to the high hopes of researchers, practitioners, and patient groups alike, and is perhaps destined to contribute more to the parallel history of controversy and distrust concerning the risk/benefits of mammography that has been a media and scientific mainstay.
Too often the history of medical technology has found disappointment at the end of the road for new discoveries. But although the disappointing results of digital screening might be considered a failure in the progress of mammography, it is likely just another pause on the road of this technology, the history of which has been rocky from the start.
The need for a new way of looking
The rationale behind the original and continuing development of mammography is a simple one, common to all cancer screening methods – the belief that the earlier the detection of a cancer, the more likely it is to be treated effectively with the therapeutic regimens at hand. While there is some controversy regarding the cost-benefit ratio of screening, especially when therapies for breast cancer are not perfect and vary widely in expense and availability globally, the driving belief has been that mammography provides an outcomes benefit in allowing early surgical and chemoradiation therapy with a curative intent.
There were two main driving forces behind the early development of mammography. The first was the highly lethal nature of breast cancer, especially when it was caught too late and had spread too far to benefit from the only available option at the time – surgery. The second was the severity of the surgical treatment, the only therapeutic option at the time, and the distressing number of women who faced the radical mastectomy procedure pioneered by physicians William Stewart Halsted (1852-1922) at Johns Hopkins University, Baltimore, and Willy Meyer (1858-1932) in New York.
In 1894, in an era when the development of anesthetics and antisepsis made ever more difficult surgical procedures possible without inevitably killing the patient, both men separately published their results of a highly extensive operation that consisted of removal of the breast, chest muscles, and axillary lymph nodes.
As long as there was no presurgical method of determining the extent of a breast cancer’s spread, much less an ability to visually distinguish malignant from benign growths, this “better safe than sorry” approach became the default approach of an increasing number of surgeons, and the drastic solution of radical mastectomy was increasingly applied universally.
But in 1895, with the discovery of x-rays, medical science recognized a nearly miraculous technology for visualizing the inside of the body, and radioactive materials were also routinely used in medical therapies, by both legitimate practitioners and hucksters.
However, in the very early days, the users of x-rays were unaware that large radiation doses could have serious biological effects and had no way of determining radiation field strength and accumulating dosage.
In fact, early calibration of x-ray tubes was based on the amount of skin reddening (erythema) produced when the operator placed a hand directly in the x-ray beam.
It was in this environment that, within only a few decades, the new x-rays, especially with the development of improvements in mammography imaging, were able in many cases to identify smaller, more curable breast cancers. This eventually allowed surgeons to develop and use less extensive operations than the highly disfiguring radical mastectomy that was simultaneously dreaded for its invasiveness and embraced for its life-saving potential.4
Pioneering era
The technological history of mammography was thus driven by the quest for better imaging and reproducibility in order to further the hopes of curative surgical approaches.
In 1913, the German surgeon Albert Salomon (1883-1976) was the first to detect breast cancer using x-rays, but its clinical use was not established, as the images published in his “Beiträge zur pathologie und klinik der mammakarzinome (Contributions to the pathology and clinic of breast cancers)” were photographs of postsurgical breast specimens that illustrated the anatomy and spread of breast cancer tumors but were not adapted to presurgical screening.
After Salomon’s work was published in 1913, there was no new mammography literature published until 1927, when German surgeon Otto Kleinschmidt (1880-1948) published a report describing the world’s first authentic mammography, which he attributed to his mentor, the plastic surgeon Erwin Payr (1871-1946).5
This was followed soon after in 1930 by the work of radiologist Stafford L. Warren (1896-1981), of the University of Rochester (N.Y.), who published a paper on the use of standard roentgenograms for the in vivo preoperative assessment of breast malignancies. His technique involved the use of a stereoscopic system with a grid mechanism and intensifying screens to amplify the image. Breast compression was not involved in his mammogram technique. “Dr. Warren claimed to be correct 92% of the time when using this technique to predict malignancy.”5
His study of 119 women with a histopathologic diagnosis (61 benign and 58 malignant) demonstrated the feasibility of the technique for routine use and “created a surge of interest.”6
But the technology of the time proved difficult to use, and the results difficult to reproduce from laboratory to laboratory, and ultimately did not gain wide acceptance. Among Warren’s other claims to fame, he was a participant in the Manhattan Project and was a member of the teams sent to assess radiation damage in Hiroshima and Nagasaki after the dropping of the atomic bombs.
And in fact, future developments in mammography and all other x-ray screening techniques included attempts to minimize radiation exposure; such attempts were driven, in part, by the tragic impact of atomic bomb radiation and the medical studies carried out on the survivors.
An image more deadly than the disease
Further improvements in mammography technique occurred through the 1930s and 1940s, including better visualization of the mammary ducts based upon the pioneering studies of Emil Ries, MD, in Chicago, who, along with Nymphus Frederick Hicken, MD (1900-1998), reported on the use of contrast mammography (also known as ductography or galactography). On a side note, Dr. Hicken was responsible for introducing the terms mammogram and mammography in 1937.
Problems with ductography, which involved the injection of a radiographically opaque contrast agent into the nipple, occurred when the early contrast agents, such as oil-based lipiodol, proved to be toxic and capable of causing abscesses.7This advance led to the development of other agents, and among the most popular at the time was one that would prove deadly to many.
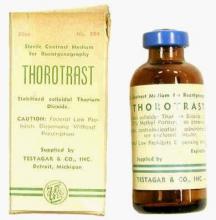
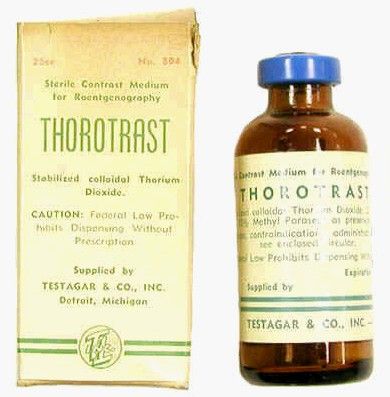
Thorotrast, first used in 1928, was widely embraced because of its lack of immediately noticeable side effects and the high-quality contrast it provided. Thorotrast was a suspension of radioactive thorium dioxide particles, which gained popularity for use as a radiological imaging agent from the 1930s to 1950s throughout the world, being used in an estimated 2-10 million radiographic exams, primarily for neurosurgery.
In the 1920s and 1930s, world governments had begun to recognize the dangers of radiation exposure, especially among workers, but thorotrast was a unique case because, unbeknownst to most practitioners at the time, thorium dioxide was retained in the body for the lifetime of the patient, with 70% deposited in the liver, 20% in the spleen, and the remaining in the bony medulla and in the peripheral lymph nodes.
Nineteen years after the first use of thorotrast, the first case of a human malignant tumor attributed to its exposure was reported. “Besides the liver neoplasm cases, aplastic anemia, leukemia and an impressive incidence of chromosome aberrations were registered in exposed individuals.”8
Despite its widespread adoption elsewhere, especially in Japan, the use of thorotrast never became popular in the United States, in part because in 1932 and 1937, warnings were issued by the American Medical Association to restrict its use.9
There was a shift to the use of iodinated hydrophilic molecules as contrast agents for conventional x-ray, computed tomography, and fluoroscopy procedures.9 However, it was discovered that these agents, too, have their own risks and dangerous side effects. They can cause severe adverse effects, including allergies, cardiovascular diseases, and nephrotoxicity in some patients.
Slow adoption and limited results
Between 1930 and 1950, Dr. Warren, Jacob Gershon-Cohen, MD (1899-1971) of Philadelphia, and radiologist Raul Leborgne of Uruguay “spread the gospel of mammography as an adjunct to physical examination for the diagnosis of breast cancer.”4 The latter also developed the breast compression technique to produce better quality images and lower the radiation exposure needed, and described the differences that could be visualized between benign and malign microcalcifications.
But despite the introduction of improvements such as double-emulsion film and breast compression to produce higher-quality images, “mammographic films often remained dark and hazy. Moreover, the new techniques, while improving the images, were not easily reproduced by other investigators and clinicians,” and therefore were still not widely adopted.4
Little noticeable effect of mammography
Although the technology existed and had its popularizers, mammography had little impact on an epidemiological level.
There was no major change in the mean maximum breast cancer tumor diameter and node positivity rate detected over the 20 years from 1929 to 1948.10 However, starting in the late 1940s, the American Cancer Society began public education campaigns and early detection education, and thereafter, there was a 3% decline in mean maximum diameter of tumor size seen every 10 years until 1968.
“We have interpreted this as the effect of public education and professional education about early detection through television, print media, and professional publications that began in 1947 because no other event was known to occur that would affect cancer detection beginning in the late 1940s.”10
However, the early detection methods at the time were self-examination and clinical examination for lumps, with mammography remaining a relatively limited tool until its general acceptance broadened a few decades later.
Robert Egan, “Father of Mammography,” et al.
The broad acceptance of mammography as a screening tool and its impacts on a broad population level resulted in large part from the work of Robert L. Egan, MD (1921-2001) in the late 1950s and 1960s.
Dr. Egan’s work was inspired in 1956 by a presentation by a visiting fellow, Jean Pierre Batiani, who brought a mammogram clearly showing a breast cancer from his institution, the Curie Foundation in Paris. The image had been made using very low kilowattage, high tube currents, and fine-grain film.
Dr. Egan, then a resident in radiology, was given the task by the head of his department of reproducing the results.
In 1959, Dr. Egan, then at the University of Texas MD Anderson Cancer Center, Houston, published a combined technique that used a high-milliamperage–low-voltage technique, a fine-grain intensifying screen, and single-emulsion films for mammography, thereby decreasing the radiation exposure significantly from previous x-ray techniques and improving the visualization and reproducibility of screening.
By 1960, Dr. Egan reported on 1,000 mammography cases at MD Anderson, demonstrating the ability of proper screening to detect unsuspected cancers and to limit mastectomies on benign masses. Of 245 breast cancers ultimately confirmed by biopsy, 238 were discovered by mammography, 19 of which were in women whose physical examinations had revealed no breast pathology. One of the cancers was only 8 mm in diameter when sectioned at biopsy.
Dr. Egan’s findings prompted an investigation by the Cancer Control Program (CCP) of the U.S. Public Health Service and led to a study jointly conducted by the National Cancer Institute and MD Anderson Hospital and the CCP, which involved 24 institutions and 1,500 patients.
“The results showed a 21% false-negative rate and a 79% true-positive rate for screening studies using Egan’s technique. This was a milestone for women’s imaging in the United States. Screening mammography was off to a tentative start.”5
“Egan was the man who developed a smooth-riding automobile compared to a Model T. He put mammography on the map and made it an intelligible, reproducible study. In short, he was the father of modern mammography,” according to his professor, mentor, and fellow mammography pioneer Gerald Dodd, MD (Emory School of Medicine website biography).
In 1964 Dr. Egan published his definitive book, “Mammography,” and in 1965 he hosted a 30-minute audiovisual presentation describing in detail his technique.11
The use of mammography was further powered by improved methods of preoperative needle localization, pioneered by Richard H. Gold, MD, in 1963 at Jefferson Medical College, Philadelphia, which eased obtaining a tissue diagnosis for any suspicious lesions detected in the mammogram. Dr. Gold performed needle localization of nonpalpable, mammographically visible lesions before biopsy, which allowed surgical resection of a smaller volume of breast tissue than was possible before.
Throughout the era, there were also incremental improvements in mammography machines and an increase in the number of commercial manufacturers.
Xeroradiography, an imaging technique adapted from xerographic photocopying, was seen as a major improvement over direct film imaging, and the technology became popular throughout the 1970s based on the research of John N. Wolfe, MD (1923-1993), who worked closely with the Xerox Corporation to improve the breast imaging process.6 However, this technology had all the same problems associated with running an office copying machine, including paper jams and toner issues, and the worst aspect was the high dose of radiation required. For this reason, it would quickly be superseded by the use of screen-film mammography, which eventually completely replaced the use of both xeromammography and direct-exposure film mammography.
The march of mammography
A series of nine randomized clinical trials (RCTs) between the 1960s and 1990s formed the foundation of the clinical use of mammography. These studies enrolled more than 600,000 women in the United States, Canada, the United Kingdom, and Sweden. The nine main RCTs of breast cancer screening were the Health Insurance Plan of Greater New York (HIP) trial, the Edinburgh trial, the Canadian National Breast Screening Study, the Canadian National Breast Screening Study 2, the United Kingdom Age trial, the Stockholm trial, the Malmö Mammographic Screening Trial, the Gothenburg trial, and the Swedish Two-County Study.3
These trials incorporated improvements in the technology as it developed, as seen in the fact that the earliest, the HIP trial, used direct-exposure film mammography and the other trials used screen-film mammography.3
Meta-analyses of the major nine screening trials indicated that reduced breast cancer mortality with screening was dependent on age. In particular, the results for women aged 40-49 years and 50-59 years showed only borderline statistical significance, and they varied depending on how cases were accrued in individual trials. “Assuming that differences actually exist, the absolute breast cancer mortality reduction per 10,000 women screened for 10 years ranged from 3 for age 39-49 years; 5-8 for age 50-59 years; and 12-21 for age 60-69 years.”3 In addition the estimates for women aged 70-74 years were limited by low numbers of events in trials that had smaller numbers of women in this age group.
However, at the time, the studies had a profound influence on increasing the popularity and spread of mammography.
As mammographies became more common, standardization became an important issue and a Mammography Accreditation Program began in 1987. Originally a voluntary program, it became mandatory with the Mammography Quality Standards Act of 1992, which required all U.S. mammography facilities to become accredited and certified.
In 1986, the American College of Radiology proposed its Breast Imaging Reporting and Data System (BI-RADS) initiative to enable standardized reporting of mammography; the first report was released in 1993.
BI-RADS is now on its fifth edition and has addressed the use of mammography, breast ultrasonography, and breast magnetic resonance imaging, developing standardized auditing approaches for all three techniques of breast cancer imaging.6
The digital era and beyond
With the dawn of the 21st century, the era of digital breast cancer screening began.
The screen-film mammography (SFM) technique employed throughout the 1980s and 1990s had significant advantages over earlier x-ray films for producing more vivid images of dense breast tissues. The next technology, digital mammography, was introduced in the late 20th century, and the first system was approved by the U.S. FDA in 2000.
One of the key benefits touted for digital mammograms is the fact that the radiologist can manipulate the contrast of the images, which allows for masses to be identified that might otherwise not be visible on standard film.
However, the recent meta-analysis discussed in the introduction calls such benefits into question, and a new controversy is likely to ensue on the question of the effectiveness of digital mammography on overall clinical outcomes.
But the technology continues to evolve.
“There has been a continuous and substantial technical development from SFM to full-field digital mammography and very recently also the introduction of digital breast tomosynthesis (DBT). This technical evolution calls for new evidence regarding the performance of screening using new mammography technologies, and the evidence needed to translate new technologies into screening practice,” according to an updated assessment by the U.S. Preventive Services Task Force.12
DBT was approved by the Food and Drug Administration in 2011. The technology involves the creation of a series of images, which are assembled into a 3-D–like image of breast slices. Traditional digital mammography creates a 2-D image of a flattened breast, and the radiologist must peer through the layers to find abnormalities. DBT uses a computer algorithm to reconstruct multiple low-dose digital images of the breast that can be displayed individually or in cinematic mode.13
Early trials showed a significant benefit of DBT in detecting new and smaller breast cancers, compared with standard digital mammography.
In women in their 40s, DBT found 1.7 more cancers than digital mammography for every 1,000 exams of women with normal breast tissue. In addition, 16.3% of women in this age group who were screened using digital mammography received callbacks, versus 11.7% of those screened using DBT. For younger women with dense breasts, the advantage of DBT was even greater, with 2.27 more cancers found for every 1,000 women screened. Whether such results will lead to clinically improved outcomes remains a question. “It can still miss cancers. Also, like traditional mammography, DBT might not reduce deaths from tumors that are very aggressive and fast-growing. And some women will still be called back unnecessarily for false-positive results.”14
But such technological advances further the hopes of researchers and patients alike.
Conclusion
Medical technology is driven both by advances in science and by the demands of patients and physicians for improved outcomes. The history of mammography, for example, is tied to the scientific advancements in x-ray technology, which allowed physicians for the first time to peer inside a living body without a scalpel at hand. But mammography was also an outgrowth of the profound need of the surgeon to identify cancerous masses in the breast at an early-enough stage to attempt a cure, while simultaneously minimizing the radical nature of the surgery required.
And while seeing is believing, the need to see and verify what was seen in order to make life-and-death decisions drove the demand for improvements in the technology of mammography throughout most of the 20th century and beyond.
The tortuous path from the early and continuing snafus with contrast agents to the apparent failure of the promise of digital technology serves as a continuing reminder of the hopes and perils that developing medical technologies present. It will be interesting to see if further refinements to mammography, such as DBT, will enhance the technology enough to have a major impact on countless women’s lives, or if new developments in magnetic resonance imaging and ultrasound make traditional mammography a relic of the past.
Part 2 of this history will present the social dynamics intimately involved with the rise and promulgation of mammography and how social need and public fears and controversies affected its development and spread as much, if not more, than technological innovation.
This article could only touch upon the myriad of details and technologies involved in the history of mammography, and I urge interested readers to check out the relevant references for far more in-depth and fascinating stories from its complex and controversial past.
References
1. Felix EL, Rosen M, Earle D. “Curbing Our Enthusiasm for Surgical Innovation: Is It a Good Thing or Bad Thing?” The Great Debates, General Surgery News, 2018 Oct 17
2. J Natl Cancer Inst. 2020 Jun 23. doi: 10.1093/jnci/djaa080.
3. Nelson H et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Evidence Synthesis No. 124. (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan, pp. 29-49)4. Lerner, BH. “To See Today With the Eyes of Tomorrow: A History of Screening Mammography,” background paper for Patlak M et al., Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer (Washington: National Academies Press, 2001).
5. Grady I, Hansen P. Chapter 28: Mammography in “Kuerer’s Breast Surgical Oncology”(New York: McGaw-Hill Medical, 2010)
6. Radiology. 2014 Nov;273(2 Suppl):S23-44.
7. Bassett LW, Kim CH. (2003) Chapter 1: Ductography in Dershaw DD (eds) “Imaging-Guided Interventional Breast Techniques” (New York: Springer, 2003, pp. 1-30).
8. Cuperschmid EM, Ribeiro de Campos TP. 2009 International Nuclear Atlantic Conference, Rio de Janeiro, Sept 27–Oct 2, 2009
9. Bioscience Microflora. 2000;19(2):107-16.
10. Cady B. New era in breast cancer. Impact of screening on disease presentation. Surg Oncol Clin N Am. 1997 Apr;6(2):195-202.
11. Egan R. “Mammography Technique.” Audiovisual presentation. (Washington: U.S. Public Health Service, 1965).
12. Zackrisson S, Houssami N. Chapter 13: Evolution of Mammography Screening: From Film Screen to Digital Breast Tomosynthesis in “Breast Cancer Screening: An Examination of Scientific Evidence” (Cambridge, Mass.: Academic Press, 2016, pp. 323-46).13. Melnikow J et al. Screening for breast cancer with digital breast tomosynthesis. Evidence Synthesis No. 125 (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan).
14. Newer breast screening technology may spot more cancers. Harvard Women’s Health Watch online, June 2019.
Mark Lesney is the editor of Hematology News and the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has worked as a writer/editor for the American Chemical Society, and has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
Technological imperatives
Technological imperatives
The history of mammography provides a powerful example of the connection between social factors and the rise of a medical technology. It is also an object lesson in the profound difficulties that the medical community faces when trying to evaluate and embrace new discoveries in such a complex area as cancer diagnosis and treatment, especially when tied to issues of sex-based bias and gender identity. Given its profound ties to women’s lives and women’s bodies, mammography holds a unique place in the history of cancer. Part 1 will examine the technological imperatives driving mammography forward, and part 2 will address the social factors that promoted and inhibited the developing technology.
All that glitters
Innovations in technology have contributed so greatly to the progress of medical science in saving and improving patients’ lives that the lure of new technology and the desire to see it succeed and to embrace it has become profound.
In a debate on the adoption of new technologies, Michael Rosen, MD, a surgeon at the Cleveland Clinic, Ohio, pointed out the inherent risks in the life cycle of medical technology: “The stages of surgical innovation have been well described as moving from the generation of a hypothesis with an early promising report to being accepted conclusively as a new standard without formal testing. As the life cycle continues and comparative effectiveness data begin to emerge slowly through appropriately designed trials, the procedure or device is often ultimately abandoned.”1
The history of mammography bears out this grim warning in example after example as an object lesson, revealing not only the difficulties involved in the development of new medical technologies, but also the profound problems involved in validating the effectiveness and appropriateness of a new technology from its inception to the present.
A modern failure?
In fact, one of the more modern developments in mammography technology – digital imaging – has recently been called into question with regard to its effectiveness in saving lives, even as the technology continues to spread throughout the medical community.
A recent meta-analysis has shown that there is little or no improvement in outcomes of breast cancer screening when using digital analysis and screening mammograms vs. traditional film recording.
The meta-analysis assessed 24 studies with a combined total of 16,583,743 screening examinations (10,968,843 film and 5,614,900 digital). The study found that the difference in cancer detection rate using digital rather than film screening showed an increase of only 0.51 detections per 1,000 screens.
The researchers concluded “that while digital mammography is beneficial for medical facilities due to easier storage and handling of images, these results suggest the transition from film to digital mammography has not resulted in health benefits for screened women.”2
In fact, the researchers added that “This analysis reinforces the need to carefully evaluate effects of future changes in technology, such as tomosynthesis, to ensure new technology leads to improved health outcomes and beyond technical gains.”2
None of the nine main randomized clinical trials that were used to determine the effectiveness of mammography screening from the 1960s to the 1990s used digital or 3-D digital mammography (digital breast tomosynthesis or DBT). The earliest trial used direct-exposure film mammography and the others relied upon screen-film mammography.3 And yet the assumptions of the validity of the new digital technologies were predicated on the generalized acceptance of the validity of screening derived from these studies, and a corollary assumption that any technological improvement in the quality of the image must inherently be an improvement of the overall results of screening.
The failure of new technologies to meet expectations is a sobering corrective to the high hopes of researchers, practitioners, and patient groups alike, and is perhaps destined to contribute more to the parallel history of controversy and distrust concerning the risk/benefits of mammography that has been a media and scientific mainstay.
Too often the history of medical technology has found disappointment at the end of the road for new discoveries. But although the disappointing results of digital screening might be considered a failure in the progress of mammography, it is likely just another pause on the road of this technology, the history of which has been rocky from the start.
The need for a new way of looking
The rationale behind the original and continuing development of mammography is a simple one, common to all cancer screening methods – the belief that the earlier the detection of a cancer, the more likely it is to be treated effectively with the therapeutic regimens at hand. While there is some controversy regarding the cost-benefit ratio of screening, especially when therapies for breast cancer are not perfect and vary widely in expense and availability globally, the driving belief has been that mammography provides an outcomes benefit in allowing early surgical and chemoradiation therapy with a curative intent.
There were two main driving forces behind the early development of mammography. The first was the highly lethal nature of breast cancer, especially when it was caught too late and had spread too far to benefit from the only available option at the time – surgery. The second was the severity of the surgical treatment, the only therapeutic option at the time, and the distressing number of women who faced the radical mastectomy procedure pioneered by physicians William Stewart Halsted (1852-1922) at Johns Hopkins University, Baltimore, and Willy Meyer (1858-1932) in New York.
In 1894, in an era when the development of anesthetics and antisepsis made ever more difficult surgical procedures possible without inevitably killing the patient, both men separately published their results of a highly extensive operation that consisted of removal of the breast, chest muscles, and axillary lymph nodes.
As long as there was no presurgical method of determining the extent of a breast cancer’s spread, much less an ability to visually distinguish malignant from benign growths, this “better safe than sorry” approach became the default approach of an increasing number of surgeons, and the drastic solution of radical mastectomy was increasingly applied universally.
But in 1895, with the discovery of x-rays, medical science recognized a nearly miraculous technology for visualizing the inside of the body, and radioactive materials were also routinely used in medical therapies, by both legitimate practitioners and hucksters.
However, in the very early days, the users of x-rays were unaware that large radiation doses could have serious biological effects and had no way of determining radiation field strength and accumulating dosage.
In fact, early calibration of x-ray tubes was based on the amount of skin reddening (erythema) produced when the operator placed a hand directly in the x-ray beam.
It was in this environment that, within only a few decades, the new x-rays, especially with the development of improvements in mammography imaging, were able in many cases to identify smaller, more curable breast cancers. This eventually allowed surgeons to develop and use less extensive operations than the highly disfiguring radical mastectomy that was simultaneously dreaded for its invasiveness and embraced for its life-saving potential.4
Pioneering era
The technological history of mammography was thus driven by the quest for better imaging and reproducibility in order to further the hopes of curative surgical approaches.
In 1913, the German surgeon Albert Salomon (1883-1976) was the first to detect breast cancer using x-rays, but its clinical use was not established, as the images published in his “Beiträge zur pathologie und klinik der mammakarzinome (Contributions to the pathology and clinic of breast cancers)” were photographs of postsurgical breast specimens that illustrated the anatomy and spread of breast cancer tumors but were not adapted to presurgical screening.
After Salomon’s work was published in 1913, there was no new mammography literature published until 1927, when German surgeon Otto Kleinschmidt (1880-1948) published a report describing the world’s first authentic mammography, which he attributed to his mentor, the plastic surgeon Erwin Payr (1871-1946).5
This was followed soon after in 1930 by the work of radiologist Stafford L. Warren (1896-1981), of the University of Rochester (N.Y.), who published a paper on the use of standard roentgenograms for the in vivo preoperative assessment of breast malignancies. His technique involved the use of a stereoscopic system with a grid mechanism and intensifying screens to amplify the image. Breast compression was not involved in his mammogram technique. “Dr. Warren claimed to be correct 92% of the time when using this technique to predict malignancy.”5
His study of 119 women with a histopathologic diagnosis (61 benign and 58 malignant) demonstrated the feasibility of the technique for routine use and “created a surge of interest.”6
But the technology of the time proved difficult to use, and the results difficult to reproduce from laboratory to laboratory, and ultimately did not gain wide acceptance. Among Warren’s other claims to fame, he was a participant in the Manhattan Project and was a member of the teams sent to assess radiation damage in Hiroshima and Nagasaki after the dropping of the atomic bombs.
And in fact, future developments in mammography and all other x-ray screening techniques included attempts to minimize radiation exposure; such attempts were driven, in part, by the tragic impact of atomic bomb radiation and the medical studies carried out on the survivors.
An image more deadly than the disease
Further improvements in mammography technique occurred through the 1930s and 1940s, including better visualization of the mammary ducts based upon the pioneering studies of Emil Ries, MD, in Chicago, who, along with Nymphus Frederick Hicken, MD (1900-1998), reported on the use of contrast mammography (also known as ductography or galactography). On a side note, Dr. Hicken was responsible for introducing the terms mammogram and mammography in 1937.
Problems with ductography, which involved the injection of a radiographically opaque contrast agent into the nipple, occurred when the early contrast agents, such as oil-based lipiodol, proved to be toxic and capable of causing abscesses.7This advance led to the development of other agents, and among the most popular at the time was one that would prove deadly to many.
Thorotrast, first used in 1928, was widely embraced because of its lack of immediately noticeable side effects and the high-quality contrast it provided. Thorotrast was a suspension of radioactive thorium dioxide particles, which gained popularity for use as a radiological imaging agent from the 1930s to 1950s throughout the world, being used in an estimated 2-10 million radiographic exams, primarily for neurosurgery.
In the 1920s and 1930s, world governments had begun to recognize the dangers of radiation exposure, especially among workers, but thorotrast was a unique case because, unbeknownst to most practitioners at the time, thorium dioxide was retained in the body for the lifetime of the patient, with 70% deposited in the liver, 20% in the spleen, and the remaining in the bony medulla and in the peripheral lymph nodes.
Nineteen years after the first use of thorotrast, the first case of a human malignant tumor attributed to its exposure was reported. “Besides the liver neoplasm cases, aplastic anemia, leukemia and an impressive incidence of chromosome aberrations were registered in exposed individuals.”8
Despite its widespread adoption elsewhere, especially in Japan, the use of thorotrast never became popular in the United States, in part because in 1932 and 1937, warnings were issued by the American Medical Association to restrict its use.9
There was a shift to the use of iodinated hydrophilic molecules as contrast agents for conventional x-ray, computed tomography, and fluoroscopy procedures.9 However, it was discovered that these agents, too, have their own risks and dangerous side effects. They can cause severe adverse effects, including allergies, cardiovascular diseases, and nephrotoxicity in some patients.
Slow adoption and limited results
Between 1930 and 1950, Dr. Warren, Jacob Gershon-Cohen, MD (1899-1971) of Philadelphia, and radiologist Raul Leborgne of Uruguay “spread the gospel of mammography as an adjunct to physical examination for the diagnosis of breast cancer.”4 The latter also developed the breast compression technique to produce better quality images and lower the radiation exposure needed, and described the differences that could be visualized between benign and malign microcalcifications.
But despite the introduction of improvements such as double-emulsion film and breast compression to produce higher-quality images, “mammographic films often remained dark and hazy. Moreover, the new techniques, while improving the images, were not easily reproduced by other investigators and clinicians,” and therefore were still not widely adopted.4
Little noticeable effect of mammography
Although the technology existed and had its popularizers, mammography had little impact on an epidemiological level.
There was no major change in the mean maximum breast cancer tumor diameter and node positivity rate detected over the 20 years from 1929 to 1948.10 However, starting in the late 1940s, the American Cancer Society began public education campaigns and early detection education, and thereafter, there was a 3% decline in mean maximum diameter of tumor size seen every 10 years until 1968.
“We have interpreted this as the effect of public education and professional education about early detection through television, print media, and professional publications that began in 1947 because no other event was known to occur that would affect cancer detection beginning in the late 1940s.”10
However, the early detection methods at the time were self-examination and clinical examination for lumps, with mammography remaining a relatively limited tool until its general acceptance broadened a few decades later.
Robert Egan, “Father of Mammography,” et al.
The broad acceptance of mammography as a screening tool and its impacts on a broad population level resulted in large part from the work of Robert L. Egan, MD (1921-2001) in the late 1950s and 1960s.
Dr. Egan’s work was inspired in 1956 by a presentation by a visiting fellow, Jean Pierre Batiani, who brought a mammogram clearly showing a breast cancer from his institution, the Curie Foundation in Paris. The image had been made using very low kilowattage, high tube currents, and fine-grain film.
Dr. Egan, then a resident in radiology, was given the task by the head of his department of reproducing the results.
In 1959, Dr. Egan, then at the University of Texas MD Anderson Cancer Center, Houston, published a combined technique that used a high-milliamperage–low-voltage technique, a fine-grain intensifying screen, and single-emulsion films for mammography, thereby decreasing the radiation exposure significantly from previous x-ray techniques and improving the visualization and reproducibility of screening.
By 1960, Dr. Egan reported on 1,000 mammography cases at MD Anderson, demonstrating the ability of proper screening to detect unsuspected cancers and to limit mastectomies on benign masses. Of 245 breast cancers ultimately confirmed by biopsy, 238 were discovered by mammography, 19 of which were in women whose physical examinations had revealed no breast pathology. One of the cancers was only 8 mm in diameter when sectioned at biopsy.
Dr. Egan’s findings prompted an investigation by the Cancer Control Program (CCP) of the U.S. Public Health Service and led to a study jointly conducted by the National Cancer Institute and MD Anderson Hospital and the CCP, which involved 24 institutions and 1,500 patients.
“The results showed a 21% false-negative rate and a 79% true-positive rate for screening studies using Egan’s technique. This was a milestone for women’s imaging in the United States. Screening mammography was off to a tentative start.”5
“Egan was the man who developed a smooth-riding automobile compared to a Model T. He put mammography on the map and made it an intelligible, reproducible study. In short, he was the father of modern mammography,” according to his professor, mentor, and fellow mammography pioneer Gerald Dodd, MD (Emory School of Medicine website biography).
In 1964 Dr. Egan published his definitive book, “Mammography,” and in 1965 he hosted a 30-minute audiovisual presentation describing in detail his technique.11
The use of mammography was further powered by improved methods of preoperative needle localization, pioneered by Richard H. Gold, MD, in 1963 at Jefferson Medical College, Philadelphia, which eased obtaining a tissue diagnosis for any suspicious lesions detected in the mammogram. Dr. Gold performed needle localization of nonpalpable, mammographically visible lesions before biopsy, which allowed surgical resection of a smaller volume of breast tissue than was possible before.
Throughout the era, there were also incremental improvements in mammography machines and an increase in the number of commercial manufacturers.
Xeroradiography, an imaging technique adapted from xerographic photocopying, was seen as a major improvement over direct film imaging, and the technology became popular throughout the 1970s based on the research of John N. Wolfe, MD (1923-1993), who worked closely with the Xerox Corporation to improve the breast imaging process.6 However, this technology had all the same problems associated with running an office copying machine, including paper jams and toner issues, and the worst aspect was the high dose of radiation required. For this reason, it would quickly be superseded by the use of screen-film mammography, which eventually completely replaced the use of both xeromammography and direct-exposure film mammography.
The march of mammography
A series of nine randomized clinical trials (RCTs) between the 1960s and 1990s formed the foundation of the clinical use of mammography. These studies enrolled more than 600,000 women in the United States, Canada, the United Kingdom, and Sweden. The nine main RCTs of breast cancer screening were the Health Insurance Plan of Greater New York (HIP) trial, the Edinburgh trial, the Canadian National Breast Screening Study, the Canadian National Breast Screening Study 2, the United Kingdom Age trial, the Stockholm trial, the Malmö Mammographic Screening Trial, the Gothenburg trial, and the Swedish Two-County Study.3
These trials incorporated improvements in the technology as it developed, as seen in the fact that the earliest, the HIP trial, used direct-exposure film mammography and the other trials used screen-film mammography.3
Meta-analyses of the major nine screening trials indicated that reduced breast cancer mortality with screening was dependent on age. In particular, the results for women aged 40-49 years and 50-59 years showed only borderline statistical significance, and they varied depending on how cases were accrued in individual trials. “Assuming that differences actually exist, the absolute breast cancer mortality reduction per 10,000 women screened for 10 years ranged from 3 for age 39-49 years; 5-8 for age 50-59 years; and 12-21 for age 60-69 years.”3 In addition the estimates for women aged 70-74 years were limited by low numbers of events in trials that had smaller numbers of women in this age group.
However, at the time, the studies had a profound influence on increasing the popularity and spread of mammography.
As mammographies became more common, standardization became an important issue and a Mammography Accreditation Program began in 1987. Originally a voluntary program, it became mandatory with the Mammography Quality Standards Act of 1992, which required all U.S. mammography facilities to become accredited and certified.
In 1986, the American College of Radiology proposed its Breast Imaging Reporting and Data System (BI-RADS) initiative to enable standardized reporting of mammography; the first report was released in 1993.
BI-RADS is now on its fifth edition and has addressed the use of mammography, breast ultrasonography, and breast magnetic resonance imaging, developing standardized auditing approaches for all three techniques of breast cancer imaging.6
The digital era and beyond
With the dawn of the 21st century, the era of digital breast cancer screening began.
The screen-film mammography (SFM) technique employed throughout the 1980s and 1990s had significant advantages over earlier x-ray films for producing more vivid images of dense breast tissues. The next technology, digital mammography, was introduced in the late 20th century, and the first system was approved by the U.S. FDA in 2000.
One of the key benefits touted for digital mammograms is the fact that the radiologist can manipulate the contrast of the images, which allows for masses to be identified that might otherwise not be visible on standard film.
However, the recent meta-analysis discussed in the introduction calls such benefits into question, and a new controversy is likely to ensue on the question of the effectiveness of digital mammography on overall clinical outcomes.
But the technology continues to evolve.
“There has been a continuous and substantial technical development from SFM to full-field digital mammography and very recently also the introduction of digital breast tomosynthesis (DBT). This technical evolution calls for new evidence regarding the performance of screening using new mammography technologies, and the evidence needed to translate new technologies into screening practice,” according to an updated assessment by the U.S. Preventive Services Task Force.12
DBT was approved by the Food and Drug Administration in 2011. The technology involves the creation of a series of images, which are assembled into a 3-D–like image of breast slices. Traditional digital mammography creates a 2-D image of a flattened breast, and the radiologist must peer through the layers to find abnormalities. DBT uses a computer algorithm to reconstruct multiple low-dose digital images of the breast that can be displayed individually or in cinematic mode.13
Early trials showed a significant benefit of DBT in detecting new and smaller breast cancers, compared with standard digital mammography.
In women in their 40s, DBT found 1.7 more cancers than digital mammography for every 1,000 exams of women with normal breast tissue. In addition, 16.3% of women in this age group who were screened using digital mammography received callbacks, versus 11.7% of those screened using DBT. For younger women with dense breasts, the advantage of DBT was even greater, with 2.27 more cancers found for every 1,000 women screened. Whether such results will lead to clinically improved outcomes remains a question. “It can still miss cancers. Also, like traditional mammography, DBT might not reduce deaths from tumors that are very aggressive and fast-growing. And some women will still be called back unnecessarily for false-positive results.”14
But such technological advances further the hopes of researchers and patients alike.
Conclusion
Medical technology is driven both by advances in science and by the demands of patients and physicians for improved outcomes. The history of mammography, for example, is tied to the scientific advancements in x-ray technology, which allowed physicians for the first time to peer inside a living body without a scalpel at hand. But mammography was also an outgrowth of the profound need of the surgeon to identify cancerous masses in the breast at an early-enough stage to attempt a cure, while simultaneously minimizing the radical nature of the surgery required.
And while seeing is believing, the need to see and verify what was seen in order to make life-and-death decisions drove the demand for improvements in the technology of mammography throughout most of the 20th century and beyond.
The tortuous path from the early and continuing snafus with contrast agents to the apparent failure of the promise of digital technology serves as a continuing reminder of the hopes and perils that developing medical technologies present. It will be interesting to see if further refinements to mammography, such as DBT, will enhance the technology enough to have a major impact on countless women’s lives, or if new developments in magnetic resonance imaging and ultrasound make traditional mammography a relic of the past.
Part 2 of this history will present the social dynamics intimately involved with the rise and promulgation of mammography and how social need and public fears and controversies affected its development and spread as much, if not more, than technological innovation.
This article could only touch upon the myriad of details and technologies involved in the history of mammography, and I urge interested readers to check out the relevant references for far more in-depth and fascinating stories from its complex and controversial past.
References
1. Felix EL, Rosen M, Earle D. “Curbing Our Enthusiasm for Surgical Innovation: Is It a Good Thing or Bad Thing?” The Great Debates, General Surgery News, 2018 Oct 17
2. J Natl Cancer Inst. 2020 Jun 23. doi: 10.1093/jnci/djaa080.
3. Nelson H et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Evidence Synthesis No. 124. (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan, pp. 29-49)4. Lerner, BH. “To See Today With the Eyes of Tomorrow: A History of Screening Mammography,” background paper for Patlak M et al., Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer (Washington: National Academies Press, 2001).
5. Grady I, Hansen P. Chapter 28: Mammography in “Kuerer’s Breast Surgical Oncology”(New York: McGaw-Hill Medical, 2010)
6. Radiology. 2014 Nov;273(2 Suppl):S23-44.
7. Bassett LW, Kim CH. (2003) Chapter 1: Ductography in Dershaw DD (eds) “Imaging-Guided Interventional Breast Techniques” (New York: Springer, 2003, pp. 1-30).
8. Cuperschmid EM, Ribeiro de Campos TP. 2009 International Nuclear Atlantic Conference, Rio de Janeiro, Sept 27–Oct 2, 2009
9. Bioscience Microflora. 2000;19(2):107-16.
10. Cady B. New era in breast cancer. Impact of screening on disease presentation. Surg Oncol Clin N Am. 1997 Apr;6(2):195-202.
11. Egan R. “Mammography Technique.” Audiovisual presentation. (Washington: U.S. Public Health Service, 1965).
12. Zackrisson S, Houssami N. Chapter 13: Evolution of Mammography Screening: From Film Screen to Digital Breast Tomosynthesis in “Breast Cancer Screening: An Examination of Scientific Evidence” (Cambridge, Mass.: Academic Press, 2016, pp. 323-46).13. Melnikow J et al. Screening for breast cancer with digital breast tomosynthesis. Evidence Synthesis No. 125 (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan).
14. Newer breast screening technology may spot more cancers. Harvard Women’s Health Watch online, June 2019.
Mark Lesney is the editor of Hematology News and the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has worked as a writer/editor for the American Chemical Society, and has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
The history of mammography provides a powerful example of the connection between social factors and the rise of a medical technology. It is also an object lesson in the profound difficulties that the medical community faces when trying to evaluate and embrace new discoveries in such a complex area as cancer diagnosis and treatment, especially when tied to issues of sex-based bias and gender identity. Given its profound ties to women’s lives and women’s bodies, mammography holds a unique place in the history of cancer. Part 1 will examine the technological imperatives driving mammography forward, and part 2 will address the social factors that promoted and inhibited the developing technology.
All that glitters
Innovations in technology have contributed so greatly to the progress of medical science in saving and improving patients’ lives that the lure of new technology and the desire to see it succeed and to embrace it has become profound.
In a debate on the adoption of new technologies, Michael Rosen, MD, a surgeon at the Cleveland Clinic, Ohio, pointed out the inherent risks in the life cycle of medical technology: “The stages of surgical innovation have been well described as moving from the generation of a hypothesis with an early promising report to being accepted conclusively as a new standard without formal testing. As the life cycle continues and comparative effectiveness data begin to emerge slowly through appropriately designed trials, the procedure or device is often ultimately abandoned.”1
The history of mammography bears out this grim warning in example after example as an object lesson, revealing not only the difficulties involved in the development of new medical technologies, but also the profound problems involved in validating the effectiveness and appropriateness of a new technology from its inception to the present.
A modern failure?
In fact, one of the more modern developments in mammography technology – digital imaging – has recently been called into question with regard to its effectiveness in saving lives, even as the technology continues to spread throughout the medical community.
A recent meta-analysis has shown that there is little or no improvement in outcomes of breast cancer screening when using digital analysis and screening mammograms vs. traditional film recording.
The meta-analysis assessed 24 studies with a combined total of 16,583,743 screening examinations (10,968,843 film and 5,614,900 digital). The study found that the difference in cancer detection rate using digital rather than film screening showed an increase of only 0.51 detections per 1,000 screens.
The researchers concluded “that while digital mammography is beneficial for medical facilities due to easier storage and handling of images, these results suggest the transition from film to digital mammography has not resulted in health benefits for screened women.”2
In fact, the researchers added that “This analysis reinforces the need to carefully evaluate effects of future changes in technology, such as tomosynthesis, to ensure new technology leads to improved health outcomes and beyond technical gains.”2
None of the nine main randomized clinical trials that were used to determine the effectiveness of mammography screening from the 1960s to the 1990s used digital or 3-D digital mammography (digital breast tomosynthesis or DBT). The earliest trial used direct-exposure film mammography and the others relied upon screen-film mammography.3 And yet the assumptions of the validity of the new digital technologies were predicated on the generalized acceptance of the validity of screening derived from these studies, and a corollary assumption that any technological improvement in the quality of the image must inherently be an improvement of the overall results of screening.
The failure of new technologies to meet expectations is a sobering corrective to the high hopes of researchers, practitioners, and patient groups alike, and is perhaps destined to contribute more to the parallel history of controversy and distrust concerning the risk/benefits of mammography that has been a media and scientific mainstay.
Too often the history of medical technology has found disappointment at the end of the road for new discoveries. But although the disappointing results of digital screening might be considered a failure in the progress of mammography, it is likely just another pause on the road of this technology, the history of which has been rocky from the start.
The need for a new way of looking
The rationale behind the original and continuing development of mammography is a simple one, common to all cancer screening methods – the belief that the earlier the detection of a cancer, the more likely it is to be treated effectively with the therapeutic regimens at hand. While there is some controversy regarding the cost-benefit ratio of screening, especially when therapies for breast cancer are not perfect and vary widely in expense and availability globally, the driving belief has been that mammography provides an outcomes benefit in allowing early surgical and chemoradiation therapy with a curative intent.
There were two main driving forces behind the early development of mammography. The first was the highly lethal nature of breast cancer, especially when it was caught too late and had spread too far to benefit from the only available option at the time – surgery. The second was the severity of the surgical treatment, the only therapeutic option at the time, and the distressing number of women who faced the radical mastectomy procedure pioneered by physicians William Stewart Halsted (1852-1922) at Johns Hopkins University, Baltimore, and Willy Meyer (1858-1932) in New York.
In 1894, in an era when the development of anesthetics and antisepsis made ever more difficult surgical procedures possible without inevitably killing the patient, both men separately published their results of a highly extensive operation that consisted of removal of the breast, chest muscles, and axillary lymph nodes.
As long as there was no presurgical method of determining the extent of a breast cancer’s spread, much less an ability to visually distinguish malignant from benign growths, this “better safe than sorry” approach became the default approach of an increasing number of surgeons, and the drastic solution of radical mastectomy was increasingly applied universally.
But in 1895, with the discovery of x-rays, medical science recognized a nearly miraculous technology for visualizing the inside of the body, and radioactive materials were also routinely used in medical therapies, by both legitimate practitioners and hucksters.
However, in the very early days, the users of x-rays were unaware that large radiation doses could have serious biological effects and had no way of determining radiation field strength and accumulating dosage.
In fact, early calibration of x-ray tubes was based on the amount of skin reddening (erythema) produced when the operator placed a hand directly in the x-ray beam.
It was in this environment that, within only a few decades, the new x-rays, especially with the development of improvements in mammography imaging, were able in many cases to identify smaller, more curable breast cancers. This eventually allowed surgeons to develop and use less extensive operations than the highly disfiguring radical mastectomy that was simultaneously dreaded for its invasiveness and embraced for its life-saving potential.4
Pioneering era
The technological history of mammography was thus driven by the quest for better imaging and reproducibility in order to further the hopes of curative surgical approaches.
In 1913, the German surgeon Albert Salomon (1883-1976) was the first to detect breast cancer using x-rays, but its clinical use was not established, as the images published in his “Beiträge zur pathologie und klinik der mammakarzinome (Contributions to the pathology and clinic of breast cancers)” were photographs of postsurgical breast specimens that illustrated the anatomy and spread of breast cancer tumors but were not adapted to presurgical screening.
After Salomon’s work was published in 1913, there was no new mammography literature published until 1927, when German surgeon Otto Kleinschmidt (1880-1948) published a report describing the world’s first authentic mammography, which he attributed to his mentor, the plastic surgeon Erwin Payr (1871-1946).5
This was followed soon after in 1930 by the work of radiologist Stafford L. Warren (1896-1981), of the University of Rochester (N.Y.), who published a paper on the use of standard roentgenograms for the in vivo preoperative assessment of breast malignancies. His technique involved the use of a stereoscopic system with a grid mechanism and intensifying screens to amplify the image. Breast compression was not involved in his mammogram technique. “Dr. Warren claimed to be correct 92% of the time when using this technique to predict malignancy.”5
His study of 119 women with a histopathologic diagnosis (61 benign and 58 malignant) demonstrated the feasibility of the technique for routine use and “created a surge of interest.”6
But the technology of the time proved difficult to use, and the results difficult to reproduce from laboratory to laboratory, and ultimately did not gain wide acceptance. Among Warren’s other claims to fame, he was a participant in the Manhattan Project and was a member of the teams sent to assess radiation damage in Hiroshima and Nagasaki after the dropping of the atomic bombs.
And in fact, future developments in mammography and all other x-ray screening techniques included attempts to minimize radiation exposure; such attempts were driven, in part, by the tragic impact of atomic bomb radiation and the medical studies carried out on the survivors.
An image more deadly than the disease
Further improvements in mammography technique occurred through the 1930s and 1940s, including better visualization of the mammary ducts based upon the pioneering studies of Emil Ries, MD, in Chicago, who, along with Nymphus Frederick Hicken, MD (1900-1998), reported on the use of contrast mammography (also known as ductography or galactography). On a side note, Dr. Hicken was responsible for introducing the terms mammogram and mammography in 1937.
Problems with ductography, which involved the injection of a radiographically opaque contrast agent into the nipple, occurred when the early contrast agents, such as oil-based lipiodol, proved to be toxic and capable of causing abscesses.7This advance led to the development of other agents, and among the most popular at the time was one that would prove deadly to many.
Thorotrast, first used in 1928, was widely embraced because of its lack of immediately noticeable side effects and the high-quality contrast it provided. Thorotrast was a suspension of radioactive thorium dioxide particles, which gained popularity for use as a radiological imaging agent from the 1930s to 1950s throughout the world, being used in an estimated 2-10 million radiographic exams, primarily for neurosurgery.
In the 1920s and 1930s, world governments had begun to recognize the dangers of radiation exposure, especially among workers, but thorotrast was a unique case because, unbeknownst to most practitioners at the time, thorium dioxide was retained in the body for the lifetime of the patient, with 70% deposited in the liver, 20% in the spleen, and the remaining in the bony medulla and in the peripheral lymph nodes.
Nineteen years after the first use of thorotrast, the first case of a human malignant tumor attributed to its exposure was reported. “Besides the liver neoplasm cases, aplastic anemia, leukemia and an impressive incidence of chromosome aberrations were registered in exposed individuals.”8
Despite its widespread adoption elsewhere, especially in Japan, the use of thorotrast never became popular in the United States, in part because in 1932 and 1937, warnings were issued by the American Medical Association to restrict its use.9
There was a shift to the use of iodinated hydrophilic molecules as contrast agents for conventional x-ray, computed tomography, and fluoroscopy procedures.9 However, it was discovered that these agents, too, have their own risks and dangerous side effects. They can cause severe adverse effects, including allergies, cardiovascular diseases, and nephrotoxicity in some patients.
Slow adoption and limited results
Between 1930 and 1950, Dr. Warren, Jacob Gershon-Cohen, MD (1899-1971) of Philadelphia, and radiologist Raul Leborgne of Uruguay “spread the gospel of mammography as an adjunct to physical examination for the diagnosis of breast cancer.”4 The latter also developed the breast compression technique to produce better quality images and lower the radiation exposure needed, and described the differences that could be visualized between benign and malign microcalcifications.
But despite the introduction of improvements such as double-emulsion film and breast compression to produce higher-quality images, “mammographic films often remained dark and hazy. Moreover, the new techniques, while improving the images, were not easily reproduced by other investigators and clinicians,” and therefore were still not widely adopted.4
Little noticeable effect of mammography
Although the technology existed and had its popularizers, mammography had little impact on an epidemiological level.
There was no major change in the mean maximum breast cancer tumor diameter and node positivity rate detected over the 20 years from 1929 to 1948.10 However, starting in the late 1940s, the American Cancer Society began public education campaigns and early detection education, and thereafter, there was a 3% decline in mean maximum diameter of tumor size seen every 10 years until 1968.
“We have interpreted this as the effect of public education and professional education about early detection through television, print media, and professional publications that began in 1947 because no other event was known to occur that would affect cancer detection beginning in the late 1940s.”10
However, the early detection methods at the time were self-examination and clinical examination for lumps, with mammography remaining a relatively limited tool until its general acceptance broadened a few decades later.
Robert Egan, “Father of Mammography,” et al.
The broad acceptance of mammography as a screening tool and its impacts on a broad population level resulted in large part from the work of Robert L. Egan, MD (1921-2001) in the late 1950s and 1960s.
Dr. Egan’s work was inspired in 1956 by a presentation by a visiting fellow, Jean Pierre Batiani, who brought a mammogram clearly showing a breast cancer from his institution, the Curie Foundation in Paris. The image had been made using very low kilowattage, high tube currents, and fine-grain film.
Dr. Egan, then a resident in radiology, was given the task by the head of his department of reproducing the results.
In 1959, Dr. Egan, then at the University of Texas MD Anderson Cancer Center, Houston, published a combined technique that used a high-milliamperage–low-voltage technique, a fine-grain intensifying screen, and single-emulsion films for mammography, thereby decreasing the radiation exposure significantly from previous x-ray techniques and improving the visualization and reproducibility of screening.
By 1960, Dr. Egan reported on 1,000 mammography cases at MD Anderson, demonstrating the ability of proper screening to detect unsuspected cancers and to limit mastectomies on benign masses. Of 245 breast cancers ultimately confirmed by biopsy, 238 were discovered by mammography, 19 of which were in women whose physical examinations had revealed no breast pathology. One of the cancers was only 8 mm in diameter when sectioned at biopsy.
Dr. Egan’s findings prompted an investigation by the Cancer Control Program (CCP) of the U.S. Public Health Service and led to a study jointly conducted by the National Cancer Institute and MD Anderson Hospital and the CCP, which involved 24 institutions and 1,500 patients.
“The results showed a 21% false-negative rate and a 79% true-positive rate for screening studies using Egan’s technique. This was a milestone for women’s imaging in the United States. Screening mammography was off to a tentative start.”5
“Egan was the man who developed a smooth-riding automobile compared to a Model T. He put mammography on the map and made it an intelligible, reproducible study. In short, he was the father of modern mammography,” according to his professor, mentor, and fellow mammography pioneer Gerald Dodd, MD (Emory School of Medicine website biography).
In 1964 Dr. Egan published his definitive book, “Mammography,” and in 1965 he hosted a 30-minute audiovisual presentation describing in detail his technique.11
The use of mammography was further powered by improved methods of preoperative needle localization, pioneered by Richard H. Gold, MD, in 1963 at Jefferson Medical College, Philadelphia, which eased obtaining a tissue diagnosis for any suspicious lesions detected in the mammogram. Dr. Gold performed needle localization of nonpalpable, mammographically visible lesions before biopsy, which allowed surgical resection of a smaller volume of breast tissue than was possible before.
Throughout the era, there were also incremental improvements in mammography machines and an increase in the number of commercial manufacturers.
Xeroradiography, an imaging technique adapted from xerographic photocopying, was seen as a major improvement over direct film imaging, and the technology became popular throughout the 1970s based on the research of John N. Wolfe, MD (1923-1993), who worked closely with the Xerox Corporation to improve the breast imaging process.6 However, this technology had all the same problems associated with running an office copying machine, including paper jams and toner issues, and the worst aspect was the high dose of radiation required. For this reason, it would quickly be superseded by the use of screen-film mammography, which eventually completely replaced the use of both xeromammography and direct-exposure film mammography.
The march of mammography
A series of nine randomized clinical trials (RCTs) between the 1960s and 1990s formed the foundation of the clinical use of mammography. These studies enrolled more than 600,000 women in the United States, Canada, the United Kingdom, and Sweden. The nine main RCTs of breast cancer screening were the Health Insurance Plan of Greater New York (HIP) trial, the Edinburgh trial, the Canadian National Breast Screening Study, the Canadian National Breast Screening Study 2, the United Kingdom Age trial, the Stockholm trial, the Malmö Mammographic Screening Trial, the Gothenburg trial, and the Swedish Two-County Study.3
These trials incorporated improvements in the technology as it developed, as seen in the fact that the earliest, the HIP trial, used direct-exposure film mammography and the other trials used screen-film mammography.3
Meta-analyses of the major nine screening trials indicated that reduced breast cancer mortality with screening was dependent on age. In particular, the results for women aged 40-49 years and 50-59 years showed only borderline statistical significance, and they varied depending on how cases were accrued in individual trials. “Assuming that differences actually exist, the absolute breast cancer mortality reduction per 10,000 women screened for 10 years ranged from 3 for age 39-49 years; 5-8 for age 50-59 years; and 12-21 for age 60-69 years.”3 In addition the estimates for women aged 70-74 years were limited by low numbers of events in trials that had smaller numbers of women in this age group.
However, at the time, the studies had a profound influence on increasing the popularity and spread of mammography.
As mammographies became more common, standardization became an important issue and a Mammography Accreditation Program began in 1987. Originally a voluntary program, it became mandatory with the Mammography Quality Standards Act of 1992, which required all U.S. mammography facilities to become accredited and certified.
In 1986, the American College of Radiology proposed its Breast Imaging Reporting and Data System (BI-RADS) initiative to enable standardized reporting of mammography; the first report was released in 1993.
BI-RADS is now on its fifth edition and has addressed the use of mammography, breast ultrasonography, and breast magnetic resonance imaging, developing standardized auditing approaches for all three techniques of breast cancer imaging.6
The digital era and beyond
With the dawn of the 21st century, the era of digital breast cancer screening began.
The screen-film mammography (SFM) technique employed throughout the 1980s and 1990s had significant advantages over earlier x-ray films for producing more vivid images of dense breast tissues. The next technology, digital mammography, was introduced in the late 20th century, and the first system was approved by the U.S. FDA in 2000.
One of the key benefits touted for digital mammograms is the fact that the radiologist can manipulate the contrast of the images, which allows for masses to be identified that might otherwise not be visible on standard film.
However, the recent meta-analysis discussed in the introduction calls such benefits into question, and a new controversy is likely to ensue on the question of the effectiveness of digital mammography on overall clinical outcomes.
But the technology continues to evolve.
“There has been a continuous and substantial technical development from SFM to full-field digital mammography and very recently also the introduction of digital breast tomosynthesis (DBT). This technical evolution calls for new evidence regarding the performance of screening using new mammography technologies, and the evidence needed to translate new technologies into screening practice,” according to an updated assessment by the U.S. Preventive Services Task Force.12
DBT was approved by the Food and Drug Administration in 2011. The technology involves the creation of a series of images, which are assembled into a 3-D–like image of breast slices. Traditional digital mammography creates a 2-D image of a flattened breast, and the radiologist must peer through the layers to find abnormalities. DBT uses a computer algorithm to reconstruct multiple low-dose digital images of the breast that can be displayed individually or in cinematic mode.13
Early trials showed a significant benefit of DBT in detecting new and smaller breast cancers, compared with standard digital mammography.
In women in their 40s, DBT found 1.7 more cancers than digital mammography for every 1,000 exams of women with normal breast tissue. In addition, 16.3% of women in this age group who were screened using digital mammography received callbacks, versus 11.7% of those screened using DBT. For younger women with dense breasts, the advantage of DBT was even greater, with 2.27 more cancers found for every 1,000 women screened. Whether such results will lead to clinically improved outcomes remains a question. “It can still miss cancers. Also, like traditional mammography, DBT might not reduce deaths from tumors that are very aggressive and fast-growing. And some women will still be called back unnecessarily for false-positive results.”14
But such technological advances further the hopes of researchers and patients alike.
Conclusion
Medical technology is driven both by advances in science and by the demands of patients and physicians for improved outcomes. The history of mammography, for example, is tied to the scientific advancements in x-ray technology, which allowed physicians for the first time to peer inside a living body without a scalpel at hand. But mammography was also an outgrowth of the profound need of the surgeon to identify cancerous masses in the breast at an early-enough stage to attempt a cure, while simultaneously minimizing the radical nature of the surgery required.
And while seeing is believing, the need to see and verify what was seen in order to make life-and-death decisions drove the demand for improvements in the technology of mammography throughout most of the 20th century and beyond.
The tortuous path from the early and continuing snafus with contrast agents to the apparent failure of the promise of digital technology serves as a continuing reminder of the hopes and perils that developing medical technologies present. It will be interesting to see if further refinements to mammography, such as DBT, will enhance the technology enough to have a major impact on countless women’s lives, or if new developments in magnetic resonance imaging and ultrasound make traditional mammography a relic of the past.
Part 2 of this history will present the social dynamics intimately involved with the rise and promulgation of mammography and how social need and public fears and controversies affected its development and spread as much, if not more, than technological innovation.
This article could only touch upon the myriad of details and technologies involved in the history of mammography, and I urge interested readers to check out the relevant references for far more in-depth and fascinating stories from its complex and controversial past.
References
1. Felix EL, Rosen M, Earle D. “Curbing Our Enthusiasm for Surgical Innovation: Is It a Good Thing or Bad Thing?” The Great Debates, General Surgery News, 2018 Oct 17
2. J Natl Cancer Inst. 2020 Jun 23. doi: 10.1093/jnci/djaa080.
3. Nelson H et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Evidence Synthesis No. 124. (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan, pp. 29-49)4. Lerner, BH. “To See Today With the Eyes of Tomorrow: A History of Screening Mammography,” background paper for Patlak M et al., Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer (Washington: National Academies Press, 2001).
5. Grady I, Hansen P. Chapter 28: Mammography in “Kuerer’s Breast Surgical Oncology”(New York: McGaw-Hill Medical, 2010)
6. Radiology. 2014 Nov;273(2 Suppl):S23-44.
7. Bassett LW, Kim CH. (2003) Chapter 1: Ductography in Dershaw DD (eds) “Imaging-Guided Interventional Breast Techniques” (New York: Springer, 2003, pp. 1-30).
8. Cuperschmid EM, Ribeiro de Campos TP. 2009 International Nuclear Atlantic Conference, Rio de Janeiro, Sept 27–Oct 2, 2009
9. Bioscience Microflora. 2000;19(2):107-16.
10. Cady B. New era in breast cancer. Impact of screening on disease presentation. Surg Oncol Clin N Am. 1997 Apr;6(2):195-202.
11. Egan R. “Mammography Technique.” Audiovisual presentation. (Washington: U.S. Public Health Service, 1965).
12. Zackrisson S, Houssami N. Chapter 13: Evolution of Mammography Screening: From Film Screen to Digital Breast Tomosynthesis in “Breast Cancer Screening: An Examination of Scientific Evidence” (Cambridge, Mass.: Academic Press, 2016, pp. 323-46).13. Melnikow J et al. Screening for breast cancer with digital breast tomosynthesis. Evidence Synthesis No. 125 (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan).
14. Newer breast screening technology may spot more cancers. Harvard Women’s Health Watch online, June 2019.
Mark Lesney is the editor of Hematology News and the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has worked as a writer/editor for the American Chemical Society, and has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
System provides ‘faster, less invasive’ method for breast cancer detection
A system designed for resource-limited settings provides rapid cancer profiling and requires “scant” cellular specimens, according to researchers.
The automated image cytometry system is called CytoPAN. In preclinical experiments, CytoPAN provided accurate cancer detection in 1 hour using as few as 50 cells.
In a prospective study of 68 breast cancer patients, CytoPAN detected cancer with 100% accuracy. The receptor subtyping accuracy was 96% for HER2 and 93% for estrogen and progesterone receptors.
Jouha Min, PhD, of Massachusetts General Hospital, Boston, and colleagues reported these findings in Science Translational Medicine.
The authors explained that the CytoPAN system is designed to address one of the biggest cancer challenges in low- and middle-income countries (LMICs), where more than two-thirds of cancer deaths occur: providing rapid, affordable diagnostics that enable patients to obtain locally available treatments.
Unfortunately, because of bottlenecks in specimen acquisition, complex handling logistics, a lack of pathologists, and limited laboratory infrastructure, diagnosis in many LMICs frequently takes months. Cancers typically are not diagnosed until advanced symptoms such as palpable mass lesions, weight loss, and malaise have become manifest.
Lesion assessment guides management
For women with suspicion of breast cancer, the authors noted, preoperative assessment of focal lesions for receptor status, presence of invasion, histologic type, and tumor grade are crucial for planning therapeutic management. For physicians to provide, for example, tamoxifen, which is commonly available at low cost in LMICs, they must know a patients’ hormone receptor status.
While core and open surgical biopsies yield abundant tissue for embedding, sectioning, and staining for subsequent histopathological analysis, they entail lengthy work flow and call for expensive instrumentation and a trained workforce, the authors noted.
Fine needle aspirations (FNAs) can be performed by nonphysicians after minimal training with very low complication rates. The much smaller–gauge needles (20-25 gauge) used in FNAs are generally well tolerated, the authors added.
This is why CytoPAN was designed for use with specimens obtained via FNA of palpable mass lesions.
Self-contained design
The CytoPAN system was engineered as a self-contained, integrated cytometry platform enabling same-day diagnosis and treatment of breast lesions.
The system was designed to comply with the World Health Organization’s “ASSURED” criteria (affordable, sensitive, specific, user friendly, rapid and robust, equipment free, and deliverable to end users), and to be potentially operable by nonphysicians after brief training.
CytoPAN operators collect cells through minimally invasive FNAs and use lyophilized immunostaining kits with relevant antibodies (not requiring refrigeration). Operators perform imaging using the CytoPAN device, which is then subjected to an automated analysis algorithm with results displayed on a user interface.
CytoPAN classifies detected malignant cells according to four subtypes reflecting estrogen receptor (ER), progesterone receptor (PR), and HER2 status – luminal HER2-negative, luminal HER2-positive, HER2-positive, and triple-negative breast cancer. This is intended to facilitate informed treatment choices (e.g., a selective ER modulator, antiestrogen or aromatase inhibitor for ER/PR-positive patients; an anti-HER2 agent for HER2-positive patients).
The final diagnostic report from a given patient sample includes cancer cell population and molecular subtype distribution. The entire diagnostic procedure takes less than an hour. A repeat biopsy, should the sample be nondiagnostic, can be taken within an hour.
Murine, then human testing
A test of CytoPAN on FNA samples from mouse xenografts representing the spectrum of human breast cancer subtypes showed correct and reproducible molecular subtyping that matched well with flow cytometry reports derived from the same tumors.
To determine the clinical utility of CytoPAN, investigators enrolled 68 treatment-naive breast cancer patients who were referred for primary surgery at the Kyungpook National University Chilgok Hospital in Daegu, South Korea. FNA samples were obtained after visualization of breast masses by ultrasound or CT.
Surgical specimens and/or core biopsies were processed by routine pathology for “gold-standard” comparison. FNA samples had sufficient numbers of cells in 63 (93%) patients, with a mean number of cells among them of 1,308 (range, 93-11,985).
CytoPAN analysis correctly identified malignant breast cancer in 55 patients and benign lesions in 5 patients. Three cases were inconclusive because of low numbers of Quad-positive cells for further analysis. The authors pointed out that roughly 20% of biopsy samples in developed countries are deemed “nondiagnostic” because of insufficient cellular contents.
The authors’ summary underscored CytoPAN’s affordable system using cellular rather than tissue specimens, actionable results in an hour, lack of moving parts, multiplexed analysis, and user-friendly interface.
Cancer detection accuracy was 100% (no false negatives or false positives), and receptor subtyping accuracy was 97% for HER2 and 93% for ER/PR.
“Based on these results, we envision prospective clinical trials in remote, decentralized locations,” the authors wrote. The system is being tested further in Botswana.
“I find the data in this paper compelling – particularly for those patients presenting with a palpable mass on clinical exam. ... Certainly in resource-limited settings, this would have significant appeal,” William J. Gradishar, MD, of Northwestern University, Chicago, said in an interview.
Dr. Gradishar explained that, while palpable masses that lead to a diagnosis of noninvasive disease alone are uncommon in the United States because of routine screening mammography, they may still be an issue in other parts of the world.
The authors received funding from the National Institutes of Health, the MGH Scholar Fund, and Robert Wood Johnson Foundation. Some authors disclosed relationships with Akili, Accure Health, ModeRNA, Tarveda, Lumicell, and Noul. Dr. Gradishar reported having no disclosures.
SOURCE: Min J et al. Sci Transl Med. 2020 Aug 5. doi: 10.1126/scitranslmed.aaz9746.
A system designed for resource-limited settings provides rapid cancer profiling and requires “scant” cellular specimens, according to researchers.
The automated image cytometry system is called CytoPAN. In preclinical experiments, CytoPAN provided accurate cancer detection in 1 hour using as few as 50 cells.
In a prospective study of 68 breast cancer patients, CytoPAN detected cancer with 100% accuracy. The receptor subtyping accuracy was 96% for HER2 and 93% for estrogen and progesterone receptors.
Jouha Min, PhD, of Massachusetts General Hospital, Boston, and colleagues reported these findings in Science Translational Medicine.
The authors explained that the CytoPAN system is designed to address one of the biggest cancer challenges in low- and middle-income countries (LMICs), where more than two-thirds of cancer deaths occur: providing rapid, affordable diagnostics that enable patients to obtain locally available treatments.
Unfortunately, because of bottlenecks in specimen acquisition, complex handling logistics, a lack of pathologists, and limited laboratory infrastructure, diagnosis in many LMICs frequently takes months. Cancers typically are not diagnosed until advanced symptoms such as palpable mass lesions, weight loss, and malaise have become manifest.
Lesion assessment guides management
For women with suspicion of breast cancer, the authors noted, preoperative assessment of focal lesions for receptor status, presence of invasion, histologic type, and tumor grade are crucial for planning therapeutic management. For physicians to provide, for example, tamoxifen, which is commonly available at low cost in LMICs, they must know a patients’ hormone receptor status.
While core and open surgical biopsies yield abundant tissue for embedding, sectioning, and staining for subsequent histopathological analysis, they entail lengthy work flow and call for expensive instrumentation and a trained workforce, the authors noted.
Fine needle aspirations (FNAs) can be performed by nonphysicians after minimal training with very low complication rates. The much smaller–gauge needles (20-25 gauge) used in FNAs are generally well tolerated, the authors added.
This is why CytoPAN was designed for use with specimens obtained via FNA of palpable mass lesions.
Self-contained design
The CytoPAN system was engineered as a self-contained, integrated cytometry platform enabling same-day diagnosis and treatment of breast lesions.
The system was designed to comply with the World Health Organization’s “ASSURED” criteria (affordable, sensitive, specific, user friendly, rapid and robust, equipment free, and deliverable to end users), and to be potentially operable by nonphysicians after brief training.
CytoPAN operators collect cells through minimally invasive FNAs and use lyophilized immunostaining kits with relevant antibodies (not requiring refrigeration). Operators perform imaging using the CytoPAN device, which is then subjected to an automated analysis algorithm with results displayed on a user interface.
CytoPAN classifies detected malignant cells according to four subtypes reflecting estrogen receptor (ER), progesterone receptor (PR), and HER2 status – luminal HER2-negative, luminal HER2-positive, HER2-positive, and triple-negative breast cancer. This is intended to facilitate informed treatment choices (e.g., a selective ER modulator, antiestrogen or aromatase inhibitor for ER/PR-positive patients; an anti-HER2 agent for HER2-positive patients).
The final diagnostic report from a given patient sample includes cancer cell population and molecular subtype distribution. The entire diagnostic procedure takes less than an hour. A repeat biopsy, should the sample be nondiagnostic, can be taken within an hour.
Murine, then human testing
A test of CytoPAN on FNA samples from mouse xenografts representing the spectrum of human breast cancer subtypes showed correct and reproducible molecular subtyping that matched well with flow cytometry reports derived from the same tumors.
To determine the clinical utility of CytoPAN, investigators enrolled 68 treatment-naive breast cancer patients who were referred for primary surgery at the Kyungpook National University Chilgok Hospital in Daegu, South Korea. FNA samples were obtained after visualization of breast masses by ultrasound or CT.
Surgical specimens and/or core biopsies were processed by routine pathology for “gold-standard” comparison. FNA samples had sufficient numbers of cells in 63 (93%) patients, with a mean number of cells among them of 1,308 (range, 93-11,985).
CytoPAN analysis correctly identified malignant breast cancer in 55 patients and benign lesions in 5 patients. Three cases were inconclusive because of low numbers of Quad-positive cells for further analysis. The authors pointed out that roughly 20% of biopsy samples in developed countries are deemed “nondiagnostic” because of insufficient cellular contents.
The authors’ summary underscored CytoPAN’s affordable system using cellular rather than tissue specimens, actionable results in an hour, lack of moving parts, multiplexed analysis, and user-friendly interface.
Cancer detection accuracy was 100% (no false negatives or false positives), and receptor subtyping accuracy was 97% for HER2 and 93% for ER/PR.
“Based on these results, we envision prospective clinical trials in remote, decentralized locations,” the authors wrote. The system is being tested further in Botswana.
“I find the data in this paper compelling – particularly for those patients presenting with a palpable mass on clinical exam. ... Certainly in resource-limited settings, this would have significant appeal,” William J. Gradishar, MD, of Northwestern University, Chicago, said in an interview.
Dr. Gradishar explained that, while palpable masses that lead to a diagnosis of noninvasive disease alone are uncommon in the United States because of routine screening mammography, they may still be an issue in other parts of the world.
The authors received funding from the National Institutes of Health, the MGH Scholar Fund, and Robert Wood Johnson Foundation. Some authors disclosed relationships with Akili, Accure Health, ModeRNA, Tarveda, Lumicell, and Noul. Dr. Gradishar reported having no disclosures.
SOURCE: Min J et al. Sci Transl Med. 2020 Aug 5. doi: 10.1126/scitranslmed.aaz9746.
A system designed for resource-limited settings provides rapid cancer profiling and requires “scant” cellular specimens, according to researchers.
The automated image cytometry system is called CytoPAN. In preclinical experiments, CytoPAN provided accurate cancer detection in 1 hour using as few as 50 cells.
In a prospective study of 68 breast cancer patients, CytoPAN detected cancer with 100% accuracy. The receptor subtyping accuracy was 96% for HER2 and 93% for estrogen and progesterone receptors.
Jouha Min, PhD, of Massachusetts General Hospital, Boston, and colleagues reported these findings in Science Translational Medicine.
The authors explained that the CytoPAN system is designed to address one of the biggest cancer challenges in low- and middle-income countries (LMICs), where more than two-thirds of cancer deaths occur: providing rapid, affordable diagnostics that enable patients to obtain locally available treatments.
Unfortunately, because of bottlenecks in specimen acquisition, complex handling logistics, a lack of pathologists, and limited laboratory infrastructure, diagnosis in many LMICs frequently takes months. Cancers typically are not diagnosed until advanced symptoms such as palpable mass lesions, weight loss, and malaise have become manifest.
Lesion assessment guides management
For women with suspicion of breast cancer, the authors noted, preoperative assessment of focal lesions for receptor status, presence of invasion, histologic type, and tumor grade are crucial for planning therapeutic management. For physicians to provide, for example, tamoxifen, which is commonly available at low cost in LMICs, they must know a patients’ hormone receptor status.
While core and open surgical biopsies yield abundant tissue for embedding, sectioning, and staining for subsequent histopathological analysis, they entail lengthy work flow and call for expensive instrumentation and a trained workforce, the authors noted.
Fine needle aspirations (FNAs) can be performed by nonphysicians after minimal training with very low complication rates. The much smaller–gauge needles (20-25 gauge) used in FNAs are generally well tolerated, the authors added.
This is why CytoPAN was designed for use with specimens obtained via FNA of palpable mass lesions.
Self-contained design
The CytoPAN system was engineered as a self-contained, integrated cytometry platform enabling same-day diagnosis and treatment of breast lesions.
The system was designed to comply with the World Health Organization’s “ASSURED” criteria (affordable, sensitive, specific, user friendly, rapid and robust, equipment free, and deliverable to end users), and to be potentially operable by nonphysicians after brief training.
CytoPAN operators collect cells through minimally invasive FNAs and use lyophilized immunostaining kits with relevant antibodies (not requiring refrigeration). Operators perform imaging using the CytoPAN device, which is then subjected to an automated analysis algorithm with results displayed on a user interface.
CytoPAN classifies detected malignant cells according to four subtypes reflecting estrogen receptor (ER), progesterone receptor (PR), and HER2 status – luminal HER2-negative, luminal HER2-positive, HER2-positive, and triple-negative breast cancer. This is intended to facilitate informed treatment choices (e.g., a selective ER modulator, antiestrogen or aromatase inhibitor for ER/PR-positive patients; an anti-HER2 agent for HER2-positive patients).
The final diagnostic report from a given patient sample includes cancer cell population and molecular subtype distribution. The entire diagnostic procedure takes less than an hour. A repeat biopsy, should the sample be nondiagnostic, can be taken within an hour.
Murine, then human testing
A test of CytoPAN on FNA samples from mouse xenografts representing the spectrum of human breast cancer subtypes showed correct and reproducible molecular subtyping that matched well with flow cytometry reports derived from the same tumors.
To determine the clinical utility of CytoPAN, investigators enrolled 68 treatment-naive breast cancer patients who were referred for primary surgery at the Kyungpook National University Chilgok Hospital in Daegu, South Korea. FNA samples were obtained after visualization of breast masses by ultrasound or CT.
Surgical specimens and/or core biopsies were processed by routine pathology for “gold-standard” comparison. FNA samples had sufficient numbers of cells in 63 (93%) patients, with a mean number of cells among them of 1,308 (range, 93-11,985).
CytoPAN analysis correctly identified malignant breast cancer in 55 patients and benign lesions in 5 patients. Three cases were inconclusive because of low numbers of Quad-positive cells for further analysis. The authors pointed out that roughly 20% of biopsy samples in developed countries are deemed “nondiagnostic” because of insufficient cellular contents.
The authors’ summary underscored CytoPAN’s affordable system using cellular rather than tissue specimens, actionable results in an hour, lack of moving parts, multiplexed analysis, and user-friendly interface.
Cancer detection accuracy was 100% (no false negatives or false positives), and receptor subtyping accuracy was 97% for HER2 and 93% for ER/PR.
“Based on these results, we envision prospective clinical trials in remote, decentralized locations,” the authors wrote. The system is being tested further in Botswana.
“I find the data in this paper compelling – particularly for those patients presenting with a palpable mass on clinical exam. ... Certainly in resource-limited settings, this would have significant appeal,” William J. Gradishar, MD, of Northwestern University, Chicago, said in an interview.
Dr. Gradishar explained that, while palpable masses that lead to a diagnosis of noninvasive disease alone are uncommon in the United States because of routine screening mammography, they may still be an issue in other parts of the world.
The authors received funding from the National Institutes of Health, the MGH Scholar Fund, and Robert Wood Johnson Foundation. Some authors disclosed relationships with Akili, Accure Health, ModeRNA, Tarveda, Lumicell, and Noul. Dr. Gradishar reported having no disclosures.
SOURCE: Min J et al. Sci Transl Med. 2020 Aug 5. doi: 10.1126/scitranslmed.aaz9746.
FROM SCIENCE TRANSLATIONAL MEDICINE
Hepatitis screening now for all patients with cancer on therapy
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
MSBase study validates therapy for relapse in secondary progressive MS
An observational cohort study of a prospective international database of patients with multiple sclerosis has reported that medical therapy can reduce disability progression in patients with active secondary progressive MS (SPMS) who are prone to inflammatory relapses.
The international researchers, led by Nathanial Lizak, MMBS, of the University of Melbourne, conducted the cohort study of 1,621 patients with SPMS from the MSBase international registry, which prospectively collected the information from 1995 to 2018. Their findings were published in JAMA Neurology.
“ wrote Dr. Lizak and colleagues of the MSBase Study Group.
Therapy’s impact on disease progression
To ensure that they had timely data on the early disease course of all study patients, they researchers only included patients whose diagnosis and first documented Expanded Disability Status Scale (EDSS) score were no more than 24 months apart. At SPMS conversion, 1,494 patients had an EDSS score of less than 7 (on a scale of 0-10); during the follow-up period, 267 of them (17.9%) crossed over the threshold of 7.
Dr. Lizak and colleagues noted that early treatment during relapsing remitting MS didn’t impact outcomes after SPMS conversion.
For evaluating the MS Severity Score (MSSS), the study split the cohort into three groups depending on the efficacy of therapy: low-efficacy treatments, mostly consisting of interferon-beta and glatiramer acetate; medium-efficacy treatments, mostly fingolimod and dimethyl fumarate; and high-efficacy treatments, predominately natalizumab and mitoxantrone.
The MSSS progression slope in the cohort had an average reduction of 0.02 points per year. “For patients who experienced superimposed relapses during SPMS, a reduced MSSS progression slope during SPMS was observed among those who received disease-modifying therapies for a greater proportion of time during SPMS,” Dr. Lizak and colleagues wrote.
MSSS progression slope reduction was more pronounced in the medium- and high-efficacy groups, with a reduction of beta 0.22 (P = .06) and 0.034 (P = .002), respectively.
“Based on our models, the level of disability in patients with active SPMS who are continuously treated with high-efficacy immunotherapies would progress more slowly in comparison with the general population with MS by a mean (standard deviation) of 1.56 (4.60) deciles over 10 years,” Dr. Lizak and colleagues wrote.
While the researchers cited a number of studies that didn’t support immunotherapy for SPMS, they also did cite the EXPAND trial to support treatment with siponimod in SPMS patients (Lancet. 2018;391:1263-73). The ASCEND trial of natalizumab enrolled a largely relapse-free cohort and found no link between treatment and disability progression in SPMS (Lancet Neurol. 2018;17:405-15), and a previous report by the MSBase Study Group found no benefit of therapy when adjusted for SPMS relapse rates (Neurology. 2017;89:1050-9).
“Together with the present study, the existing data converge on the suggestion that relapses during SPMS provide a therapeutic target and a marker of future response to immunotherapy during SPMS,” Dr. Lizak and colleagues wrote.
Challenging dogma
Commenting on the research, Mark Freedman, HBSc, MSc, MD, said that the MSBase study makes an important contribution to the literature on management of SPMS. “Up until this point we’ve been basing our assumptions on secondary progressive MS on natural history studies, which are actually quite old, dating back 20-30 years.” Dr. Freedman is senior scientist in the Neuroscience Program at the Ottawa Hospital Research Institute and professor of medicine in neurology at the University of Ottawa.
He said “the most damaging” of those studies was by the late Christian Confavreaux, MD, and colleagues in Lyon, France (N Engl J Med. 2000;343:1430-8), that reported relapses didn’t alter the progression of disability. “In other words, once you’re in EDSS of 4, it’s a runaway train; it doesn’t matter what you do,” Dr. Freedman said.
“That was kind of dogma for years,” he said. “The reason this publication is important is because it’s suggesting that’s not the case.” In other words, the MSBase cohort study is validating what neurologists have been doing in the real world for years: treating patients with SPMS who have relapses.
Dr Lizak reported receiving travel compensation from Merck outside the scope of the study. His coauthors reported numerous financial relationships.
SOURCE: Lizak N et al. JAMA Neurol. 2020 Jul 27. doi: 10.1001/jamaneurol.2020.2453
An observational cohort study of a prospective international database of patients with multiple sclerosis has reported that medical therapy can reduce disability progression in patients with active secondary progressive MS (SPMS) who are prone to inflammatory relapses.
The international researchers, led by Nathanial Lizak, MMBS, of the University of Melbourne, conducted the cohort study of 1,621 patients with SPMS from the MSBase international registry, which prospectively collected the information from 1995 to 2018. Their findings were published in JAMA Neurology.
“ wrote Dr. Lizak and colleagues of the MSBase Study Group.
Therapy’s impact on disease progression
To ensure that they had timely data on the early disease course of all study patients, they researchers only included patients whose diagnosis and first documented Expanded Disability Status Scale (EDSS) score were no more than 24 months apart. At SPMS conversion, 1,494 patients had an EDSS score of less than 7 (on a scale of 0-10); during the follow-up period, 267 of them (17.9%) crossed over the threshold of 7.
Dr. Lizak and colleagues noted that early treatment during relapsing remitting MS didn’t impact outcomes after SPMS conversion.
For evaluating the MS Severity Score (MSSS), the study split the cohort into three groups depending on the efficacy of therapy: low-efficacy treatments, mostly consisting of interferon-beta and glatiramer acetate; medium-efficacy treatments, mostly fingolimod and dimethyl fumarate; and high-efficacy treatments, predominately natalizumab and mitoxantrone.
The MSSS progression slope in the cohort had an average reduction of 0.02 points per year. “For patients who experienced superimposed relapses during SPMS, a reduced MSSS progression slope during SPMS was observed among those who received disease-modifying therapies for a greater proportion of time during SPMS,” Dr. Lizak and colleagues wrote.
MSSS progression slope reduction was more pronounced in the medium- and high-efficacy groups, with a reduction of beta 0.22 (P = .06) and 0.034 (P = .002), respectively.
“Based on our models, the level of disability in patients with active SPMS who are continuously treated with high-efficacy immunotherapies would progress more slowly in comparison with the general population with MS by a mean (standard deviation) of 1.56 (4.60) deciles over 10 years,” Dr. Lizak and colleagues wrote.
While the researchers cited a number of studies that didn’t support immunotherapy for SPMS, they also did cite the EXPAND trial to support treatment with siponimod in SPMS patients (Lancet. 2018;391:1263-73). The ASCEND trial of natalizumab enrolled a largely relapse-free cohort and found no link between treatment and disability progression in SPMS (Lancet Neurol. 2018;17:405-15), and a previous report by the MSBase Study Group found no benefit of therapy when adjusted for SPMS relapse rates (Neurology. 2017;89:1050-9).
“Together with the present study, the existing data converge on the suggestion that relapses during SPMS provide a therapeutic target and a marker of future response to immunotherapy during SPMS,” Dr. Lizak and colleagues wrote.
Challenging dogma
Commenting on the research, Mark Freedman, HBSc, MSc, MD, said that the MSBase study makes an important contribution to the literature on management of SPMS. “Up until this point we’ve been basing our assumptions on secondary progressive MS on natural history studies, which are actually quite old, dating back 20-30 years.” Dr. Freedman is senior scientist in the Neuroscience Program at the Ottawa Hospital Research Institute and professor of medicine in neurology at the University of Ottawa.
He said “the most damaging” of those studies was by the late Christian Confavreaux, MD, and colleagues in Lyon, France (N Engl J Med. 2000;343:1430-8), that reported relapses didn’t alter the progression of disability. “In other words, once you’re in EDSS of 4, it’s a runaway train; it doesn’t matter what you do,” Dr. Freedman said.
“That was kind of dogma for years,” he said. “The reason this publication is important is because it’s suggesting that’s not the case.” In other words, the MSBase cohort study is validating what neurologists have been doing in the real world for years: treating patients with SPMS who have relapses.
Dr Lizak reported receiving travel compensation from Merck outside the scope of the study. His coauthors reported numerous financial relationships.
SOURCE: Lizak N et al. JAMA Neurol. 2020 Jul 27. doi: 10.1001/jamaneurol.2020.2453
An observational cohort study of a prospective international database of patients with multiple sclerosis has reported that medical therapy can reduce disability progression in patients with active secondary progressive MS (SPMS) who are prone to inflammatory relapses.
The international researchers, led by Nathanial Lizak, MMBS, of the University of Melbourne, conducted the cohort study of 1,621 patients with SPMS from the MSBase international registry, which prospectively collected the information from 1995 to 2018. Their findings were published in JAMA Neurology.
“ wrote Dr. Lizak and colleagues of the MSBase Study Group.
Therapy’s impact on disease progression
To ensure that they had timely data on the early disease course of all study patients, they researchers only included patients whose diagnosis and first documented Expanded Disability Status Scale (EDSS) score were no more than 24 months apart. At SPMS conversion, 1,494 patients had an EDSS score of less than 7 (on a scale of 0-10); during the follow-up period, 267 of them (17.9%) crossed over the threshold of 7.
Dr. Lizak and colleagues noted that early treatment during relapsing remitting MS didn’t impact outcomes after SPMS conversion.
For evaluating the MS Severity Score (MSSS), the study split the cohort into three groups depending on the efficacy of therapy: low-efficacy treatments, mostly consisting of interferon-beta and glatiramer acetate; medium-efficacy treatments, mostly fingolimod and dimethyl fumarate; and high-efficacy treatments, predominately natalizumab and mitoxantrone.
The MSSS progression slope in the cohort had an average reduction of 0.02 points per year. “For patients who experienced superimposed relapses during SPMS, a reduced MSSS progression slope during SPMS was observed among those who received disease-modifying therapies for a greater proportion of time during SPMS,” Dr. Lizak and colleagues wrote.
MSSS progression slope reduction was more pronounced in the medium- and high-efficacy groups, with a reduction of beta 0.22 (P = .06) and 0.034 (P = .002), respectively.
“Based on our models, the level of disability in patients with active SPMS who are continuously treated with high-efficacy immunotherapies would progress more slowly in comparison with the general population with MS by a mean (standard deviation) of 1.56 (4.60) deciles over 10 years,” Dr. Lizak and colleagues wrote.
While the researchers cited a number of studies that didn’t support immunotherapy for SPMS, they also did cite the EXPAND trial to support treatment with siponimod in SPMS patients (Lancet. 2018;391:1263-73). The ASCEND trial of natalizumab enrolled a largely relapse-free cohort and found no link between treatment and disability progression in SPMS (Lancet Neurol. 2018;17:405-15), and a previous report by the MSBase Study Group found no benefit of therapy when adjusted for SPMS relapse rates (Neurology. 2017;89:1050-9).
“Together with the present study, the existing data converge on the suggestion that relapses during SPMS provide a therapeutic target and a marker of future response to immunotherapy during SPMS,” Dr. Lizak and colleagues wrote.
Challenging dogma
Commenting on the research, Mark Freedman, HBSc, MSc, MD, said that the MSBase study makes an important contribution to the literature on management of SPMS. “Up until this point we’ve been basing our assumptions on secondary progressive MS on natural history studies, which are actually quite old, dating back 20-30 years.” Dr. Freedman is senior scientist in the Neuroscience Program at the Ottawa Hospital Research Institute and professor of medicine in neurology at the University of Ottawa.
He said “the most damaging” of those studies was by the late Christian Confavreaux, MD, and colleagues in Lyon, France (N Engl J Med. 2000;343:1430-8), that reported relapses didn’t alter the progression of disability. “In other words, once you’re in EDSS of 4, it’s a runaway train; it doesn’t matter what you do,” Dr. Freedman said.
“That was kind of dogma for years,” he said. “The reason this publication is important is because it’s suggesting that’s not the case.” In other words, the MSBase cohort study is validating what neurologists have been doing in the real world for years: treating patients with SPMS who have relapses.
Dr Lizak reported receiving travel compensation from Merck outside the scope of the study. His coauthors reported numerous financial relationships.
SOURCE: Lizak N et al. JAMA Neurol. 2020 Jul 27. doi: 10.1001/jamaneurol.2020.2453
FROM JAMA NEUROLOGY
ASCO says ‘no’ to home infusions of cancer treatment, with exceptions
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
FDA approves cannabidiol for tuberous sclerosis complex
The cannabidiol (CBD) oral solution Epidiolex has been approved by the Food and Drug Administration for the new indication of treatment of seizures associated with tuberous sclerosis complex in patients 1 year of age and older.
The drug was approved by the FDA in 2018 for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, as reported by Medscape Medical News.
This is the only FDA-approved drug that contains a purified drug substance derived from cannabis. It is also the second FDA approval of a drug for the treatment of seizures associated with tuberous sclerosis complex.
CBD is a chemical component of the cannabis sativa plant, but it does not cause intoxication or euphoria (the “high”) that comes from tetrahydrocannabinol (THC), which is the primary psychoactive component of cannabis.
“The FDA continues to believe the drug approval process represents the best way to make new medicines, including any drugs derived from cannabis, available to patients in need of appropriate medical therapy such as the treatment of seizures associated with these rare conditions,” Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said in an agency press release.
“This paradigm ensures new therapies are safe, effective, and manufactured to a high quality that provides uniform and reliable dosing for patients,” Dr. Throckmorton said.
He added that the FDA is committed to supporting research on the potential medical uses of cannabis-derived products.
Rare genetic disease
Tuberous sclerosis complex is a rare genetic disease that causes benign tumors to grow in the brain and other parts of the body, such as the eyes, heart, kidneys, lungs, and skin.
It usually affects the central nervous system and can result in a combination of symptoms, including seizures, developmental delay, and behavioral problems. The signs and symptoms of the condition, as well as the severity of symptoms, vary widely. The disease affects about 1 in 6,000 individuals.
The effectiveness of Epidiolex in the treatment of seizures associated with tuberous sclerosis complex was established in a randomized, double-blind, placebo-controlled trial in which 148 patients of a total of 224 in the study received the active drug, the FDA noted.
Results showed that for patients treated with CBD, there was a significantly greater reduction in seizure frequency during the treatment period than for patients who received placebo.
This effect was seen within 8 weeks and remained consistent throughout the 16-week treatment period.
The most common side effects that occurred in CBD-treated participants were diarrhea, elevated liver enzyme levels, decreased appetite, sleepiness, fever, and vomiting. Additional side effects that have been reported with the product include liver injury, decreased weight, anemia, and increased creatinine level.
As is true for all drugs that currently treat epilepsy, including Epidiolex, the most serious risks may include an increase in suicidal thoughts and behavior or thoughts of self-harm, the FDA reports.
Patients, their caregivers, and their families should be advised to monitor for any unusual changes in mood or behavior, such as worsening depression or suicidal thoughts or behavior. They should report behaviors of concern immediately to health care providers, the agency notes.
It also points out that Epidiolex can cause liver injury, of which most cases are generally mild. However, there is a risk for rare but more severe liver injury. More severe liver injury can cause nausea, vomiting, abdominal pain, fatigue, anorexia, jaundice, and/or dark urine.
A version of this story originally appeared on Medscape.com.
The cannabidiol (CBD) oral solution Epidiolex has been approved by the Food and Drug Administration for the new indication of treatment of seizures associated with tuberous sclerosis complex in patients 1 year of age and older.
The drug was approved by the FDA in 2018 for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, as reported by Medscape Medical News.
This is the only FDA-approved drug that contains a purified drug substance derived from cannabis. It is also the second FDA approval of a drug for the treatment of seizures associated with tuberous sclerosis complex.
CBD is a chemical component of the cannabis sativa plant, but it does not cause intoxication or euphoria (the “high”) that comes from tetrahydrocannabinol (THC), which is the primary psychoactive component of cannabis.
“The FDA continues to believe the drug approval process represents the best way to make new medicines, including any drugs derived from cannabis, available to patients in need of appropriate medical therapy such as the treatment of seizures associated with these rare conditions,” Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said in an agency press release.
“This paradigm ensures new therapies are safe, effective, and manufactured to a high quality that provides uniform and reliable dosing for patients,” Dr. Throckmorton said.
He added that the FDA is committed to supporting research on the potential medical uses of cannabis-derived products.
Rare genetic disease
Tuberous sclerosis complex is a rare genetic disease that causes benign tumors to grow in the brain and other parts of the body, such as the eyes, heart, kidneys, lungs, and skin.
It usually affects the central nervous system and can result in a combination of symptoms, including seizures, developmental delay, and behavioral problems. The signs and symptoms of the condition, as well as the severity of symptoms, vary widely. The disease affects about 1 in 6,000 individuals.
The effectiveness of Epidiolex in the treatment of seizures associated with tuberous sclerosis complex was established in a randomized, double-blind, placebo-controlled trial in which 148 patients of a total of 224 in the study received the active drug, the FDA noted.
Results showed that for patients treated with CBD, there was a significantly greater reduction in seizure frequency during the treatment period than for patients who received placebo.
This effect was seen within 8 weeks and remained consistent throughout the 16-week treatment period.
The most common side effects that occurred in CBD-treated participants were diarrhea, elevated liver enzyme levels, decreased appetite, sleepiness, fever, and vomiting. Additional side effects that have been reported with the product include liver injury, decreased weight, anemia, and increased creatinine level.
As is true for all drugs that currently treat epilepsy, including Epidiolex, the most serious risks may include an increase in suicidal thoughts and behavior or thoughts of self-harm, the FDA reports.
Patients, their caregivers, and their families should be advised to monitor for any unusual changes in mood or behavior, such as worsening depression or suicidal thoughts or behavior. They should report behaviors of concern immediately to health care providers, the agency notes.
It also points out that Epidiolex can cause liver injury, of which most cases are generally mild. However, there is a risk for rare but more severe liver injury. More severe liver injury can cause nausea, vomiting, abdominal pain, fatigue, anorexia, jaundice, and/or dark urine.
A version of this story originally appeared on Medscape.com.
The cannabidiol (CBD) oral solution Epidiolex has been approved by the Food and Drug Administration for the new indication of treatment of seizures associated with tuberous sclerosis complex in patients 1 year of age and older.
The drug was approved by the FDA in 2018 for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, as reported by Medscape Medical News.
This is the only FDA-approved drug that contains a purified drug substance derived from cannabis. It is also the second FDA approval of a drug for the treatment of seizures associated with tuberous sclerosis complex.
CBD is a chemical component of the cannabis sativa plant, but it does not cause intoxication or euphoria (the “high”) that comes from tetrahydrocannabinol (THC), which is the primary psychoactive component of cannabis.
“The FDA continues to believe the drug approval process represents the best way to make new medicines, including any drugs derived from cannabis, available to patients in need of appropriate medical therapy such as the treatment of seizures associated with these rare conditions,” Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said in an agency press release.
“This paradigm ensures new therapies are safe, effective, and manufactured to a high quality that provides uniform and reliable dosing for patients,” Dr. Throckmorton said.
He added that the FDA is committed to supporting research on the potential medical uses of cannabis-derived products.
Rare genetic disease
Tuberous sclerosis complex is a rare genetic disease that causes benign tumors to grow in the brain and other parts of the body, such as the eyes, heart, kidneys, lungs, and skin.
It usually affects the central nervous system and can result in a combination of symptoms, including seizures, developmental delay, and behavioral problems. The signs and symptoms of the condition, as well as the severity of symptoms, vary widely. The disease affects about 1 in 6,000 individuals.
The effectiveness of Epidiolex in the treatment of seizures associated with tuberous sclerosis complex was established in a randomized, double-blind, placebo-controlled trial in which 148 patients of a total of 224 in the study received the active drug, the FDA noted.
Results showed that for patients treated with CBD, there was a significantly greater reduction in seizure frequency during the treatment period than for patients who received placebo.
This effect was seen within 8 weeks and remained consistent throughout the 16-week treatment period.
The most common side effects that occurred in CBD-treated participants were diarrhea, elevated liver enzyme levels, decreased appetite, sleepiness, fever, and vomiting. Additional side effects that have been reported with the product include liver injury, decreased weight, anemia, and increased creatinine level.
As is true for all drugs that currently treat epilepsy, including Epidiolex, the most serious risks may include an increase in suicidal thoughts and behavior or thoughts of self-harm, the FDA reports.
Patients, their caregivers, and their families should be advised to monitor for any unusual changes in mood or behavior, such as worsening depression or suicidal thoughts or behavior. They should report behaviors of concern immediately to health care providers, the agency notes.
It also points out that Epidiolex can cause liver injury, of which most cases are generally mild. However, there is a risk for rare but more severe liver injury. More severe liver injury can cause nausea, vomiting, abdominal pain, fatigue, anorexia, jaundice, and/or dark urine.
A version of this story originally appeared on Medscape.com.
Many older adults ‘overscreened’ for cancer
Older adults are being “overscreened” for cancer, say researchers who discovered that many patients reported undergoing screening for cancer even though they were older than the upper age limit recommended.
The U.S. Preventive Services Task Force recommends an upper age limit on cancer screening that varies by cancer type – 75 years old for colorectal cancer, 74 for breast cancer, and 65 for cervical cancer.
The study found that 59.3% of men and 56.2% of women being screening for colorectal cancer were above that cut-off age, as were 45.8% of women being screened for cervical cancer and 74.1% of women being screened for breast cancer.
Overscreening was particularly high for women living in metropolitan areas.
The finding is of concern, say the researchers, because “continuing to screen patients who are older and/or who have limited life expectancy may cause more harms than benefits.”
“The development of successful interventions to address this problem are thus essential,” they write.
The study was published online July 27 in JAMA Network Open.
Clinicians, patients, and health care systems can be changed – and should be changed – to minimize overscreening,” said lead author Jennifer L. Moss, PhD, assistant professor of family and community medicine and public health sciences at Penn State University, Hershey.
“It will probably take many changes to meaningfully decrease overscreening,” she told Medscape Medical News.
One change that would help is if health insurance companies stopped reimbursing providers for screening after the recommended upper age limit, she continued. “Another change is if providers had evidence-based tools to guide conversations about stopping screening, given an individual patient’s demographics, health status, and risks and benefits of the screening test.”
Approached for comment on the study, Nancy Schoenborn, MD, MHS, an associate professor of medicine in the Division of Geriatric Medicine and Gerontology at Johns Hopkins University, Baltimore, noted that the finding of high overscreening is not surprising and is consistent with prior works that found similar results.
“One value of this paper is that the timing of the study is more recent and confirms that the issue of overscreening is one that is still ongoing,” she told Medscape Medical News. Schoenborn was not associated with the study.
As for what physicians should do about the findings in this study, Schoenborn suggested the first step is to simply recognize that overscreening is likely a problem and “to reflect if there are instances in one’s own practice where overscreening may occur.”
In her own work, Schoenborn continued, “I was recently surprised that a substantial minority of clinicians actually do not believe overscreening to be a problem in older adults, and they have a number of concerns about how overscreening is defined and about unintended consequences that can occur from efforts to reduce overscreening.”
She added that there are a number of reasons why overscreening occurs. These include guideline inconsistencies, inertia, patient request, clinician knowledge gaps, and discomfort with discussing stopping. “A lot of work is ongoing to address each of these issues, but I think the first step would be the clinician recognizing and agreeing that this is a problem that needs to be addressed,” she said.
Unnecessary screening
The authors note that the prevalence estimates for overscreening have not been reported on a national level, and it is also unclear how overscreening may vary among subgroups.
“The reason I focused on colorectal, cervical, and breast cancers is because USPSTF has very clear, age-based recommendations for these cancers in terms of who should and should not get screened routinely,” explained Moss. “This was important because it allowed me and my coauthors to clearly say, based on age alone, this person probably was screened unnecessarily, and this person was not.”
She noted that the age-based recommendations for routine screening are based on very large clinical trials to examine the effectiveness of the screening tool. “The recommendations for lung and prostate cancer screening are not so clear cut, and we would not be able to tell, based only on the available survey data, if someone was overscreened,” she said.
For their study, the team used data from the 2018 Behavioral Risk Factor Surveillance System, administered by the Centers for Disease Control and Prevention.
Overscreening was assessed in a cohort of 20,937 men and 34,244 women for colorectal cancer, 82,811 women for cervical cancer, and 38,356 women for breast cancer. Most the participants lived in a metropolitan area (about 80%) and were white (about 80%).
Being overscreened was also more common in metropolitan vs. nonmetropolitan areas for colorectal cancer in women (adjusted odds ratio, 1.23), cervical cancer (aOR, 1.20), and breast cancer (aOR, 1.36).
Overscreening for cervical and breast cancers was also associated with having a usual source of care, good/very good/excellent self-reported health, education beyond a high school diploma, and being married or living as married.
The study was carried out in 2018, and the situation is likely to have changed over recent months during the COVID-19 pandemic.
“We have already seen dramatic reductions in routine cancer screening among age-eligible adults, so part of this problem of overscreening among older adults will likely diminish,” said Moss. “State and national cancer surveillance systems will continue to monitor trends in cancer screening, including overscreening, cancer incidence, and cancer mortality.”
Johns Hopkins’ Schoenborn said one finding of particular interest was that the colorectal cancer overscreening rate was higher in those older than 80 and in those with higher mortality risk.
“It makes me wonder if this is due to the increasing use of noninvasive colorectal cancer screening modalities, such as the fecal immunochemical test FIT or Cologuard,” Schoenborn commented. “It would be important for clinicians to consider downstream effects even when the initial test is low risk, such as if the stool test screens positive, would the patient still need a colonoscopy, and is that something the patient can undergo and wants to undergo?”
The study was funded by the National Cancer Institute and American Cancer Society. Moss, study coauthors, and Schoenborn have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Older adults are being “overscreened” for cancer, say researchers who discovered that many patients reported undergoing screening for cancer even though they were older than the upper age limit recommended.
The U.S. Preventive Services Task Force recommends an upper age limit on cancer screening that varies by cancer type – 75 years old for colorectal cancer, 74 for breast cancer, and 65 for cervical cancer.
The study found that 59.3% of men and 56.2% of women being screening for colorectal cancer were above that cut-off age, as were 45.8% of women being screened for cervical cancer and 74.1% of women being screened for breast cancer.
Overscreening was particularly high for women living in metropolitan areas.
The finding is of concern, say the researchers, because “continuing to screen patients who are older and/or who have limited life expectancy may cause more harms than benefits.”
“The development of successful interventions to address this problem are thus essential,” they write.
The study was published online July 27 in JAMA Network Open.
Clinicians, patients, and health care systems can be changed – and should be changed – to minimize overscreening,” said lead author Jennifer L. Moss, PhD, assistant professor of family and community medicine and public health sciences at Penn State University, Hershey.
“It will probably take many changes to meaningfully decrease overscreening,” she told Medscape Medical News.
One change that would help is if health insurance companies stopped reimbursing providers for screening after the recommended upper age limit, she continued. “Another change is if providers had evidence-based tools to guide conversations about stopping screening, given an individual patient’s demographics, health status, and risks and benefits of the screening test.”
Approached for comment on the study, Nancy Schoenborn, MD, MHS, an associate professor of medicine in the Division of Geriatric Medicine and Gerontology at Johns Hopkins University, Baltimore, noted that the finding of high overscreening is not surprising and is consistent with prior works that found similar results.
“One value of this paper is that the timing of the study is more recent and confirms that the issue of overscreening is one that is still ongoing,” she told Medscape Medical News. Schoenborn was not associated with the study.
As for what physicians should do about the findings in this study, Schoenborn suggested the first step is to simply recognize that overscreening is likely a problem and “to reflect if there are instances in one’s own practice where overscreening may occur.”
In her own work, Schoenborn continued, “I was recently surprised that a substantial minority of clinicians actually do not believe overscreening to be a problem in older adults, and they have a number of concerns about how overscreening is defined and about unintended consequences that can occur from efforts to reduce overscreening.”
She added that there are a number of reasons why overscreening occurs. These include guideline inconsistencies, inertia, patient request, clinician knowledge gaps, and discomfort with discussing stopping. “A lot of work is ongoing to address each of these issues, but I think the first step would be the clinician recognizing and agreeing that this is a problem that needs to be addressed,” she said.
Unnecessary screening
The authors note that the prevalence estimates for overscreening have not been reported on a national level, and it is also unclear how overscreening may vary among subgroups.
“The reason I focused on colorectal, cervical, and breast cancers is because USPSTF has very clear, age-based recommendations for these cancers in terms of who should and should not get screened routinely,” explained Moss. “This was important because it allowed me and my coauthors to clearly say, based on age alone, this person probably was screened unnecessarily, and this person was not.”
She noted that the age-based recommendations for routine screening are based on very large clinical trials to examine the effectiveness of the screening tool. “The recommendations for lung and prostate cancer screening are not so clear cut, and we would not be able to tell, based only on the available survey data, if someone was overscreened,” she said.
For their study, the team used data from the 2018 Behavioral Risk Factor Surveillance System, administered by the Centers for Disease Control and Prevention.
Overscreening was assessed in a cohort of 20,937 men and 34,244 women for colorectal cancer, 82,811 women for cervical cancer, and 38,356 women for breast cancer. Most the participants lived in a metropolitan area (about 80%) and were white (about 80%).
Being overscreened was also more common in metropolitan vs. nonmetropolitan areas for colorectal cancer in women (adjusted odds ratio, 1.23), cervical cancer (aOR, 1.20), and breast cancer (aOR, 1.36).
Overscreening for cervical and breast cancers was also associated with having a usual source of care, good/very good/excellent self-reported health, education beyond a high school diploma, and being married or living as married.
The study was carried out in 2018, and the situation is likely to have changed over recent months during the COVID-19 pandemic.
“We have already seen dramatic reductions in routine cancer screening among age-eligible adults, so part of this problem of overscreening among older adults will likely diminish,” said Moss. “State and national cancer surveillance systems will continue to monitor trends in cancer screening, including overscreening, cancer incidence, and cancer mortality.”
Johns Hopkins’ Schoenborn said one finding of particular interest was that the colorectal cancer overscreening rate was higher in those older than 80 and in those with higher mortality risk.
“It makes me wonder if this is due to the increasing use of noninvasive colorectal cancer screening modalities, such as the fecal immunochemical test FIT or Cologuard,” Schoenborn commented. “It would be important for clinicians to consider downstream effects even when the initial test is low risk, such as if the stool test screens positive, would the patient still need a colonoscopy, and is that something the patient can undergo and wants to undergo?”
The study was funded by the National Cancer Institute and American Cancer Society. Moss, study coauthors, and Schoenborn have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Older adults are being “overscreened” for cancer, say researchers who discovered that many patients reported undergoing screening for cancer even though they were older than the upper age limit recommended.
The U.S. Preventive Services Task Force recommends an upper age limit on cancer screening that varies by cancer type – 75 years old for colorectal cancer, 74 for breast cancer, and 65 for cervical cancer.
The study found that 59.3% of men and 56.2% of women being screening for colorectal cancer were above that cut-off age, as were 45.8% of women being screened for cervical cancer and 74.1% of women being screened for breast cancer.
Overscreening was particularly high for women living in metropolitan areas.
The finding is of concern, say the researchers, because “continuing to screen patients who are older and/or who have limited life expectancy may cause more harms than benefits.”
“The development of successful interventions to address this problem are thus essential,” they write.
The study was published online July 27 in JAMA Network Open.
Clinicians, patients, and health care systems can be changed – and should be changed – to minimize overscreening,” said lead author Jennifer L. Moss, PhD, assistant professor of family and community medicine and public health sciences at Penn State University, Hershey.
“It will probably take many changes to meaningfully decrease overscreening,” she told Medscape Medical News.
One change that would help is if health insurance companies stopped reimbursing providers for screening after the recommended upper age limit, she continued. “Another change is if providers had evidence-based tools to guide conversations about stopping screening, given an individual patient’s demographics, health status, and risks and benefits of the screening test.”
Approached for comment on the study, Nancy Schoenborn, MD, MHS, an associate professor of medicine in the Division of Geriatric Medicine and Gerontology at Johns Hopkins University, Baltimore, noted that the finding of high overscreening is not surprising and is consistent with prior works that found similar results.
“One value of this paper is that the timing of the study is more recent and confirms that the issue of overscreening is one that is still ongoing,” she told Medscape Medical News. Schoenborn was not associated with the study.
As for what physicians should do about the findings in this study, Schoenborn suggested the first step is to simply recognize that overscreening is likely a problem and “to reflect if there are instances in one’s own practice where overscreening may occur.”
In her own work, Schoenborn continued, “I was recently surprised that a substantial minority of clinicians actually do not believe overscreening to be a problem in older adults, and they have a number of concerns about how overscreening is defined and about unintended consequences that can occur from efforts to reduce overscreening.”
She added that there are a number of reasons why overscreening occurs. These include guideline inconsistencies, inertia, patient request, clinician knowledge gaps, and discomfort with discussing stopping. “A lot of work is ongoing to address each of these issues, but I think the first step would be the clinician recognizing and agreeing that this is a problem that needs to be addressed,” she said.
Unnecessary screening
The authors note that the prevalence estimates for overscreening have not been reported on a national level, and it is also unclear how overscreening may vary among subgroups.
“The reason I focused on colorectal, cervical, and breast cancers is because USPSTF has very clear, age-based recommendations for these cancers in terms of who should and should not get screened routinely,” explained Moss. “This was important because it allowed me and my coauthors to clearly say, based on age alone, this person probably was screened unnecessarily, and this person was not.”
She noted that the age-based recommendations for routine screening are based on very large clinical trials to examine the effectiveness of the screening tool. “The recommendations for lung and prostate cancer screening are not so clear cut, and we would not be able to tell, based only on the available survey data, if someone was overscreened,” she said.
For their study, the team used data from the 2018 Behavioral Risk Factor Surveillance System, administered by the Centers for Disease Control and Prevention.
Overscreening was assessed in a cohort of 20,937 men and 34,244 women for colorectal cancer, 82,811 women for cervical cancer, and 38,356 women for breast cancer. Most the participants lived in a metropolitan area (about 80%) and were white (about 80%).
Being overscreened was also more common in metropolitan vs. nonmetropolitan areas for colorectal cancer in women (adjusted odds ratio, 1.23), cervical cancer (aOR, 1.20), and breast cancer (aOR, 1.36).
Overscreening for cervical and breast cancers was also associated with having a usual source of care, good/very good/excellent self-reported health, education beyond a high school diploma, and being married or living as married.
The study was carried out in 2018, and the situation is likely to have changed over recent months during the COVID-19 pandemic.
“We have already seen dramatic reductions in routine cancer screening among age-eligible adults, so part of this problem of overscreening among older adults will likely diminish,” said Moss. “State and national cancer surveillance systems will continue to monitor trends in cancer screening, including overscreening, cancer incidence, and cancer mortality.”
Johns Hopkins’ Schoenborn said one finding of particular interest was that the colorectal cancer overscreening rate was higher in those older than 80 and in those with higher mortality risk.
“It makes me wonder if this is due to the increasing use of noninvasive colorectal cancer screening modalities, such as the fecal immunochemical test FIT or Cologuard,” Schoenborn commented. “It would be important for clinicians to consider downstream effects even when the initial test is low risk, such as if the stool test screens positive, would the patient still need a colonoscopy, and is that something the patient can undergo and wants to undergo?”
The study was funded by the National Cancer Institute and American Cancer Society. Moss, study coauthors, and Schoenborn have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Positive phase 3 top-line results for migraine prevention drug
AbbVie, the company developing the drug, has announced.
Top-line results from the ADVANCE trial, which evaluated atogepant 10, 30, and 60 mg, showed all three doses were associated with a significant reduction from baseline in mean monthly migraine days, compared with placebo.
There were also significant improvements in all six secondary endpoints with the two higher doses.
Data from the ADVANCE trial and a previous phase 2/3 trial will be the basis for regulatory submissions in the United States and other countries, AbbVie reported.
Decreased migraine days
The phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group trial was designed to evaluate the efficacy, safety, and tolerability of oral atogepant for the prevention of migraine in those who experienced 4-14 migraine days per month.
A total of 910 patients were randomized to one of four treatment groups: 10 mg, 30 mg, or 60 mg of atogepant once daily or placebo. Efficacy analyses were based on the modified intent-to-treat population of 873 patients.
The primary endpoint was change from baseline in mean monthly migraine days during the 12-week treatment period. All atogepant dose groups met the primary endpoint and demonstrated significantly greater decreases in mean monthly migraine days, compared with placebo.
Mean monthly migraine days were reduced by 3.69 days with the 10-mg dose, 3.86 days with the 30-mg dose, and 4.2 days with the 60-mg dose of atogepant, compared with a reduction of 2.48 migraine days in the placebo group (P < .0001, all dose groups vs. placebo).
A key secondary endpoint measured the proportion of patients who achieved at least a 50% reduction in mean monthly migraine days over 12 weeks. This outcome occurred in 55.6% of the 10-mg atogepant group, 58.7% of the 30-mg group, and 60.8% of the 60-mg group, compared with 29% of the placebo group (P < .0001, all dose groups vs. placebo).
Significant improvements
Additional secondary endpoints measured during the 12-week treatment period included change from baseline in mean monthly headache days, mean monthly acute–medication use days, mean monthly performance of daily activities and physical impairment domain scores on the Activity Impairment in Migraine-Diary, and change from baseline in the Migraine-Specific Quality of Life Questionnaire Role Function-Restrictive domain score at week 12. Treatment with the 30-mg and 60-mg doses resulted in significant improvements in all secondary endpoints, and treatment with the 10-mg dose resulted in significant improvements in four of the six secondary endpoints.
No new safety risks were observed when compared with the safety profile of atogepant observed in previous trials, AbbVie said. Serious adverse events occurred in 0.9% of patients in the atogepant 10-mg group versus 0.9% of patients in the placebo group. No patients in the atogepant 30-mg or 60-mg groups experienced a serious adverse event. The most common adverse events (reported in at least 5% of patients and at least one atogepant group and at a rate greater than placebo), across all doses versus placebo, were constipation (6.9%-7.7% vs. 0.5%), nausea (4.4%-6.1% vs. 1.8%), and upper respiratory tract infection (3.9%-5.7% vs. 4.5%).
Most cases of constipation, nausea, and upper respiratory tract infection were mild or moderate in severity and did not lead to discontinuation. There were no hepatic safety issues identified in the trial.
Full data results will be presented at an upcoming medical congress and/or published in a peer-reviewed journal, the company said.
A version of this article originally appeared on Medscape.com.
AbbVie, the company developing the drug, has announced.
Top-line results from the ADVANCE trial, which evaluated atogepant 10, 30, and 60 mg, showed all three doses were associated with a significant reduction from baseline in mean monthly migraine days, compared with placebo.
There were also significant improvements in all six secondary endpoints with the two higher doses.
Data from the ADVANCE trial and a previous phase 2/3 trial will be the basis for regulatory submissions in the United States and other countries, AbbVie reported.
Decreased migraine days
The phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group trial was designed to evaluate the efficacy, safety, and tolerability of oral atogepant for the prevention of migraine in those who experienced 4-14 migraine days per month.
A total of 910 patients were randomized to one of four treatment groups: 10 mg, 30 mg, or 60 mg of atogepant once daily or placebo. Efficacy analyses were based on the modified intent-to-treat population of 873 patients.
The primary endpoint was change from baseline in mean monthly migraine days during the 12-week treatment period. All atogepant dose groups met the primary endpoint and demonstrated significantly greater decreases in mean monthly migraine days, compared with placebo.
Mean monthly migraine days were reduced by 3.69 days with the 10-mg dose, 3.86 days with the 30-mg dose, and 4.2 days with the 60-mg dose of atogepant, compared with a reduction of 2.48 migraine days in the placebo group (P < .0001, all dose groups vs. placebo).
A key secondary endpoint measured the proportion of patients who achieved at least a 50% reduction in mean monthly migraine days over 12 weeks. This outcome occurred in 55.6% of the 10-mg atogepant group, 58.7% of the 30-mg group, and 60.8% of the 60-mg group, compared with 29% of the placebo group (P < .0001, all dose groups vs. placebo).
Significant improvements
Additional secondary endpoints measured during the 12-week treatment period included change from baseline in mean monthly headache days, mean monthly acute–medication use days, mean monthly performance of daily activities and physical impairment domain scores on the Activity Impairment in Migraine-Diary, and change from baseline in the Migraine-Specific Quality of Life Questionnaire Role Function-Restrictive domain score at week 12. Treatment with the 30-mg and 60-mg doses resulted in significant improvements in all secondary endpoints, and treatment with the 10-mg dose resulted in significant improvements in four of the six secondary endpoints.
No new safety risks were observed when compared with the safety profile of atogepant observed in previous trials, AbbVie said. Serious adverse events occurred in 0.9% of patients in the atogepant 10-mg group versus 0.9% of patients in the placebo group. No patients in the atogepant 30-mg or 60-mg groups experienced a serious adverse event. The most common adverse events (reported in at least 5% of patients and at least one atogepant group and at a rate greater than placebo), across all doses versus placebo, were constipation (6.9%-7.7% vs. 0.5%), nausea (4.4%-6.1% vs. 1.8%), and upper respiratory tract infection (3.9%-5.7% vs. 4.5%).
Most cases of constipation, nausea, and upper respiratory tract infection were mild or moderate in severity and did not lead to discontinuation. There were no hepatic safety issues identified in the trial.
Full data results will be presented at an upcoming medical congress and/or published in a peer-reviewed journal, the company said.
A version of this article originally appeared on Medscape.com.
AbbVie, the company developing the drug, has announced.
Top-line results from the ADVANCE trial, which evaluated atogepant 10, 30, and 60 mg, showed all three doses were associated with a significant reduction from baseline in mean monthly migraine days, compared with placebo.
There were also significant improvements in all six secondary endpoints with the two higher doses.
Data from the ADVANCE trial and a previous phase 2/3 trial will be the basis for regulatory submissions in the United States and other countries, AbbVie reported.
Decreased migraine days
The phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group trial was designed to evaluate the efficacy, safety, and tolerability of oral atogepant for the prevention of migraine in those who experienced 4-14 migraine days per month.
A total of 910 patients were randomized to one of four treatment groups: 10 mg, 30 mg, or 60 mg of atogepant once daily or placebo. Efficacy analyses were based on the modified intent-to-treat population of 873 patients.
The primary endpoint was change from baseline in mean monthly migraine days during the 12-week treatment period. All atogepant dose groups met the primary endpoint and demonstrated significantly greater decreases in mean monthly migraine days, compared with placebo.
Mean monthly migraine days were reduced by 3.69 days with the 10-mg dose, 3.86 days with the 30-mg dose, and 4.2 days with the 60-mg dose of atogepant, compared with a reduction of 2.48 migraine days in the placebo group (P < .0001, all dose groups vs. placebo).
A key secondary endpoint measured the proportion of patients who achieved at least a 50% reduction in mean monthly migraine days over 12 weeks. This outcome occurred in 55.6% of the 10-mg atogepant group, 58.7% of the 30-mg group, and 60.8% of the 60-mg group, compared with 29% of the placebo group (P < .0001, all dose groups vs. placebo).
Significant improvements
Additional secondary endpoints measured during the 12-week treatment period included change from baseline in mean monthly headache days, mean monthly acute–medication use days, mean monthly performance of daily activities and physical impairment domain scores on the Activity Impairment in Migraine-Diary, and change from baseline in the Migraine-Specific Quality of Life Questionnaire Role Function-Restrictive domain score at week 12. Treatment with the 30-mg and 60-mg doses resulted in significant improvements in all secondary endpoints, and treatment with the 10-mg dose resulted in significant improvements in four of the six secondary endpoints.
No new safety risks were observed when compared with the safety profile of atogepant observed in previous trials, AbbVie said. Serious adverse events occurred in 0.9% of patients in the atogepant 10-mg group versus 0.9% of patients in the placebo group. No patients in the atogepant 30-mg or 60-mg groups experienced a serious adverse event. The most common adverse events (reported in at least 5% of patients and at least one atogepant group and at a rate greater than placebo), across all doses versus placebo, were constipation (6.9%-7.7% vs. 0.5%), nausea (4.4%-6.1% vs. 1.8%), and upper respiratory tract infection (3.9%-5.7% vs. 4.5%).
Most cases of constipation, nausea, and upper respiratory tract infection were mild or moderate in severity and did not lead to discontinuation. There were no hepatic safety issues identified in the trial.
Full data results will be presented at an upcoming medical congress and/or published in a peer-reviewed journal, the company said.
A version of this article originally appeared on Medscape.com.