User login
Cancer risk-reducing strategies: Focus on chemoprevention
In her presentation at The North American Menopause Society (NAMS) 2021 annual meeting (September 22–25, 2021, in Washington, DC), Dr. Holly J. Pederson offered her expert perspectives on breast cancer prevention in at-risk women in “Chemoprevention for risk reduction: Women’s health clinicians have a role.”
Which patients would benefit from chemoprevention?
Holly J. Pederson, MD: Obviously, women with significant family history are at risk. And approximately 10% of biopsies that are done for other reasons incidentally show atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS)—which are not precancers or cancers but are markers for the development of the disease—and they markedly increase risk. Atypical hyperplasia confers a 30% risk for developing breast cancer over the next 25 years, and LCIS is associated with up to a 2% per year risk. In this setting, preventive medication has been shown to cut risk by 56% to 86%; this is a targeted population that is often overlooked.
Mathematical risk models can be used to assess risk by assessing women’s risk factors. The United States Preventive Services Task Force (USPSTF) has set forth a threshold at which they believe the benefits outweigh the risks of preventive medications. That threshold is 3% or greater over the next 5 years using the Gail breast cancer risk assessment tool.1 The American Society of Clinical Oncology (ASCO) uses the Tyrer-Cuzick breast cancer risk evaluation model with a threshold of 5% over the next 10 years.2 In general, those are the situations in which chemoprevention is a no-brainer.
Certain genetic mutations also predispose to estrogen-sensitive breast cancer. While preventive medications specifically have not been studied in large groups of gene carriers, chemoprevention makes sense because these medications prevent estrogen-sensitive breast cancers that those patients are prone to. Examples would be patients with ATM and CHEK2 gene mutations, which are very common, and patients with BRCA2 and even BRCA1 variants in the postmenopausal years. Those are the big targets.
Risk assessment models
Dr. Pederson: Yes, I almost exclusively use the Tyrer-Cuzick risk model, version 8, which incorporates breast density. This model is intimidating to some practitioners initially, but once you get used to it, you can complete it very quickly.
The Gail model is very limited. It assesses only first-degree relatives, so you don’t get the paternal information at all, and you don’t use age at diagnosis, family structure, genetic testing, results of breast density, or body mass index (BMI). There are many limitations of the Gail model, but most people use it because it is so easy and they are familiar with it.
Possibly the best model is the CanRisk tool, which incorporates the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), but it takes too much time to use in clinic; it’s too complicated. The Tyrer-Cuzick model is easy to use once you get used to it.
Dr. Pederson: Risk doesn’t always need to be formally calculated, which can be time-consuming. It’s one of those situations where most practitioners know it when they see it. Benign atypical biopsies, a strong family history, or, obviously, the presence of a genetic mutation are huge red flags.
If a practitioner has a nearby high-risk center where they can refer patients, that can be so useful, even for a one-time consultation to guide management. For example, with the virtual world now, I do a lot of consultations for patients and outline a plan, and then the referring practitioner can carry out the plan with confidence and then send the patient back periodically. There are so many more options now that previously did not exist for the busy ObGyn or primary care provider to rely on.
Continue to: Chemoprevention uptake in at-risk women...
Chemoprevention uptake in at-risk women
Dr. Pederson: We really never practice medicine using numbers. We use clinical judgment, and we use relationships with patients in terms of developing confidence and trust. I think that the uptake that we exhibit in our center probably is more based on the patients’ perception that we are confident in our recommendations. I think that many practitioners simply are not comfortable with explaining medications, explaining and managing adverse effects, and using alternative medications. While the modeling helps, I think the personal expertise really makes the difference.
Going forward, the addition of the polygenic risk score to the mathematical risk models is going to make a big difference. Right now, the mathematical risk model is simply that: it takes the traditional risk factors that a patient has and spits out a number. But adding the patient’s genomic data—that is, a weighted summation of SNPs, or single nucleotide polymorphisms, now numbering over 300 for breast cancer—can explain more about their personalized risk, which is going to be more powerful in influencing a woman to take medication or not to take medication, in my opinion. Knowing their actual genomic risk will be a big step forward in individualized risk stratification and increased medication uptake as well as vigilance with high risk screening and attention to diet, exercise, and drinking alcohol in moderation.
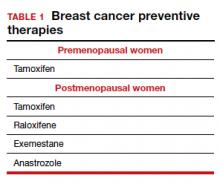
Dr. Pederson: The only drug that can be used in the premenopausal setting is tamoxifen (TABLE 1). Women can’t take it if they are pregnant, planning to become pregnant, or if they don’t use a reliable form of birth control because it is teratogenic. Women also cannot take tamoxifen if they have had a history of blood clots, stroke, or transient ischemic attack; if they are on warfarin or estrogen preparations; or if they have had atypical endometrial biopsies or endometrial cancer. Those are the absolute contraindications for tamoxifen use.
Tamoxifen is generally very well tolerated in most women; some women experience hot flashes and night sweats that often will subside (or become tolerable) over the first 90 days. In addition, some women experience vaginal discharge rather than dryness, but it is not as bothersome to patients as dryness can be.
Tamoxifen can be used in the pre- or postmenopausal setting. In healthy premenopausal women, there’s no increased risk of the serious adverse effects that are seen with tamoxifen use in postmenopausal women, such as the 1% risk of blood clots and the 1% risk of endometrial cancer.
In postmenopausal women who still have their uterus, I’ll preferentially use raloxifene over tamoxifen. If they don’t have their uterus, tamoxifen is slightly more effective than the raloxifene, and I’ll use that.
Tamoxifen and raloxifene are both selective estrogen receptor modulators, or SERMs, which means that they stimulate receptors in some tissues, like bone, keeping bones strong, and block the receptors in other tissues, like the breast, reducing risk. And so you get kind of a two-for-one in terms of breast cancer risk reduction and osteoporosis prevention.
Another class of preventive drugs is the aromatase inhibitors (AIs). They block the enzyme aromatase, which converts androgens to estrogens peripherally; that is, the androgens that are produced primarily in the adrenal gland, but in part in postmenopausal ovaries.
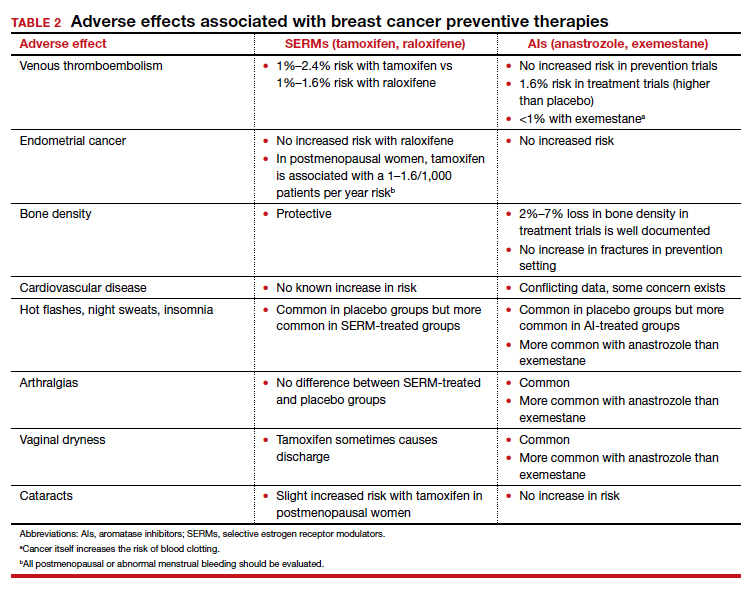
In general, AIs are less well tolerated. There are generally more hot flashes and night sweats, and more vaginal dryness than with the SERMs. Anastrozole use is associated with arthralgias; and with exemestane use, there can be some hair loss (TABLE 2). Relative contraindications to SERMs become more important in the postmenopausal setting because of the increased frequency of both blood clots and uterine cancer in the postmenopausal years. I won’t give it to smokers. I won’t give tamoxifen to smokers in the premenopausal period either. With obese women, care must be taken because of the risk of blood clots with the SERMS, so then I’ll resort to the AIs. In the postmenopausal setting, you have to think a lot harder about the choices you use for preventive medication. Preferentially, I’ll use the SERMS if possible as they have fewer adverse effects.
Dr. Pederson: All of them are recommended to be given for 5 years, but the MAP.3 trial, which studied exemestane compared with placebo, showed a 65% risk reduction with 3 years of therapy.3 So occasionally, we’ll use 3 years of therapy. Why the treatment recommendation is universally 5 years is unclear, given that the trial with that particular drug was done in 3 years. And with low-dose tamoxifen, the recommended duration is 3 years. That study was done in Italy with 5 mg daily for 3 years.4 In the United States we use 10 mg every other day for 3 years because the 5-mg tablet is not available here.
Continue to: Counseling points...
Counseling points
Dr. Pederson: Patients’ fears about adverse effects are often worse than the adverse effects themselves. Women will fester over, Should I take it? Should I take it possibly for years? And then they take the medication and they tell me, “I don’t even notice that I’m taking it, and I know I’m being proactive.” The majority of patients who take these medications don’t have a lot of significant adverse effects.
Severe hot flashes can be managed in a number of ways, primarily and most effectively with certain antidepressants. Oxybutynin use is another good way to manage vasomotor symptoms. Sometimes we use local vaginal estrogen if a patient has vaginal dryness. In general, however, I would say at least 80% of my patients who take preventive medications do not require management of adverse side effects, that they are tolerable.
I counsel women this way, “Don’t think of this as a 5-year course of medication. Think of it as a 90-day trial, and let’s see how you do. If you hate it, then we don’t do it.” They often are pleasantly surprised that the medication is much easier to tolerate than they thought it would be.
Dr. Pederson: It would be neat if a trial would directly compare lifestyle interventions with medications, because probably lifestyle change is as effective as medication is—but we don’t know that and probably will never have that data. We do know that alcohol consumption, every drink per day, increases risk by 10%. We know that obesity is responsible for 30% of breast cancers in this country, and that hormone replacement probably is overrated as a significant risk factor. Updated data from the Women’s Health Initiative study suggest that hormone replacement may actually reduce both breast cancer and cardiovascular risk in women in their 50s, but that’s in average-risk women and not in high-risk women, so we can’t generalize. We do recommend lifestyle measures including weight loss, exercise, and limiting alcohol consumption for all of our patients and certainly for our high-risk patients.
The only 2 things a woman can do to reduce the risk of triple negative breast cancer are to achieve and maintain ideal body weight and to breastfeed. The medications that I have mentioned don’t reduce the risk of triple negative breast cancer. Staying thin and breastfeeding do. It’s a problem in this country because at least 35% of all women and 58% of Black women are obese in America, and Black women tend to be prone to triple-negative breast cancer. That’s a real public health issue that we need to address. If we were going to focus on one thing, it would be focusing on obesity in terms of risk reduction.
Final thoughts
Dr. Pederson: I would like to direct attention to the American Heart Association scientific statement published at the end of 2020 that reported that hormone replacement in average-risk women reduced both cardiovascular events and overall mortality in women in their 50s by 30%.5 While that’s not directly related to what we are talking about, we need to weigh the pros and cons of estrogen versus estrogen blockade in women in terms of breast cancer risk management discussions. Part of shared decision making now needs to include cardiovascular risk factors and how estrogen is going to play into that.
In women with atypical hyperplasia or LCIS, they may benefit from the preventive medications we discussed. But in women with family history or in women with genetic mutations who have not had benign atypical biopsies, they may choose to consider estrogen during their 50s and perhaps take tamoxifen either beforehand or raloxifene afterward.
We need to look at patients holistically and consider all their risk factors together. We can’t look at one dimension alone.
- US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867.
- Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152-3165.
- Goss PE, Ingle JN, Alex-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37:1629-1637.
- El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532.
In her presentation at The North American Menopause Society (NAMS) 2021 annual meeting (September 22–25, 2021, in Washington, DC), Dr. Holly J. Pederson offered her expert perspectives on breast cancer prevention in at-risk women in “Chemoprevention for risk reduction: Women’s health clinicians have a role.”
Which patients would benefit from chemoprevention?
Holly J. Pederson, MD: Obviously, women with significant family history are at risk. And approximately 10% of biopsies that are done for other reasons incidentally show atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS)—which are not precancers or cancers but are markers for the development of the disease—and they markedly increase risk. Atypical hyperplasia confers a 30% risk for developing breast cancer over the next 25 years, and LCIS is associated with up to a 2% per year risk. In this setting, preventive medication has been shown to cut risk by 56% to 86%; this is a targeted population that is often overlooked.
Mathematical risk models can be used to assess risk by assessing women’s risk factors. The United States Preventive Services Task Force (USPSTF) has set forth a threshold at which they believe the benefits outweigh the risks of preventive medications. That threshold is 3% or greater over the next 5 years using the Gail breast cancer risk assessment tool.1 The American Society of Clinical Oncology (ASCO) uses the Tyrer-Cuzick breast cancer risk evaluation model with a threshold of 5% over the next 10 years.2 In general, those are the situations in which chemoprevention is a no-brainer.
Certain genetic mutations also predispose to estrogen-sensitive breast cancer. While preventive medications specifically have not been studied in large groups of gene carriers, chemoprevention makes sense because these medications prevent estrogen-sensitive breast cancers that those patients are prone to. Examples would be patients with ATM and CHEK2 gene mutations, which are very common, and patients with BRCA2 and even BRCA1 variants in the postmenopausal years. Those are the big targets.
Risk assessment models
Dr. Pederson: Yes, I almost exclusively use the Tyrer-Cuzick risk model, version 8, which incorporates breast density. This model is intimidating to some practitioners initially, but once you get used to it, you can complete it very quickly.
The Gail model is very limited. It assesses only first-degree relatives, so you don’t get the paternal information at all, and you don’t use age at diagnosis, family structure, genetic testing, results of breast density, or body mass index (BMI). There are many limitations of the Gail model, but most people use it because it is so easy and they are familiar with it.
Possibly the best model is the CanRisk tool, which incorporates the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), but it takes too much time to use in clinic; it’s too complicated. The Tyrer-Cuzick model is easy to use once you get used to it.
Dr. Pederson: Risk doesn’t always need to be formally calculated, which can be time-consuming. It’s one of those situations where most practitioners know it when they see it. Benign atypical biopsies, a strong family history, or, obviously, the presence of a genetic mutation are huge red flags.
If a practitioner has a nearby high-risk center where they can refer patients, that can be so useful, even for a one-time consultation to guide management. For example, with the virtual world now, I do a lot of consultations for patients and outline a plan, and then the referring practitioner can carry out the plan with confidence and then send the patient back periodically. There are so many more options now that previously did not exist for the busy ObGyn or primary care provider to rely on.
Continue to: Chemoprevention uptake in at-risk women...
Chemoprevention uptake in at-risk women
Dr. Pederson: We really never practice medicine using numbers. We use clinical judgment, and we use relationships with patients in terms of developing confidence and trust. I think that the uptake that we exhibit in our center probably is more based on the patients’ perception that we are confident in our recommendations. I think that many practitioners simply are not comfortable with explaining medications, explaining and managing adverse effects, and using alternative medications. While the modeling helps, I think the personal expertise really makes the difference.
Going forward, the addition of the polygenic risk score to the mathematical risk models is going to make a big difference. Right now, the mathematical risk model is simply that: it takes the traditional risk factors that a patient has and spits out a number. But adding the patient’s genomic data—that is, a weighted summation of SNPs, or single nucleotide polymorphisms, now numbering over 300 for breast cancer—can explain more about their personalized risk, which is going to be more powerful in influencing a woman to take medication or not to take medication, in my opinion. Knowing their actual genomic risk will be a big step forward in individualized risk stratification and increased medication uptake as well as vigilance with high risk screening and attention to diet, exercise, and drinking alcohol in moderation.
Dr. Pederson: The only drug that can be used in the premenopausal setting is tamoxifen (TABLE 1). Women can’t take it if they are pregnant, planning to become pregnant, or if they don’t use a reliable form of birth control because it is teratogenic. Women also cannot take tamoxifen if they have had a history of blood clots, stroke, or transient ischemic attack; if they are on warfarin or estrogen preparations; or if they have had atypical endometrial biopsies or endometrial cancer. Those are the absolute contraindications for tamoxifen use.
Tamoxifen is generally very well tolerated in most women; some women experience hot flashes and night sweats that often will subside (or become tolerable) over the first 90 days. In addition, some women experience vaginal discharge rather than dryness, but it is not as bothersome to patients as dryness can be.
Tamoxifen can be used in the pre- or postmenopausal setting. In healthy premenopausal women, there’s no increased risk of the serious adverse effects that are seen with tamoxifen use in postmenopausal women, such as the 1% risk of blood clots and the 1% risk of endometrial cancer.
In postmenopausal women who still have their uterus, I’ll preferentially use raloxifene over tamoxifen. If they don’t have their uterus, tamoxifen is slightly more effective than the raloxifene, and I’ll use that.
Tamoxifen and raloxifene are both selective estrogen receptor modulators, or SERMs, which means that they stimulate receptors in some tissues, like bone, keeping bones strong, and block the receptors in other tissues, like the breast, reducing risk. And so you get kind of a two-for-one in terms of breast cancer risk reduction and osteoporosis prevention.
Another class of preventive drugs is the aromatase inhibitors (AIs). They block the enzyme aromatase, which converts androgens to estrogens peripherally; that is, the androgens that are produced primarily in the adrenal gland, but in part in postmenopausal ovaries.
In general, AIs are less well tolerated. There are generally more hot flashes and night sweats, and more vaginal dryness than with the SERMs. Anastrozole use is associated with arthralgias; and with exemestane use, there can be some hair loss (TABLE 2). Relative contraindications to SERMs become more important in the postmenopausal setting because of the increased frequency of both blood clots and uterine cancer in the postmenopausal years. I won’t give it to smokers. I won’t give tamoxifen to smokers in the premenopausal period either. With obese women, care must be taken because of the risk of blood clots with the SERMS, so then I’ll resort to the AIs. In the postmenopausal setting, you have to think a lot harder about the choices you use for preventive medication. Preferentially, I’ll use the SERMS if possible as they have fewer adverse effects.

Dr. Pederson: All of them are recommended to be given for 5 years, but the MAP.3 trial, which studied exemestane compared with placebo, showed a 65% risk reduction with 3 years of therapy.3 So occasionally, we’ll use 3 years of therapy. Why the treatment recommendation is universally 5 years is unclear, given that the trial with that particular drug was done in 3 years. And with low-dose tamoxifen, the recommended duration is 3 years. That study was done in Italy with 5 mg daily for 3 years.4 In the United States we use 10 mg every other day for 3 years because the 5-mg tablet is not available here.
Continue to: Counseling points...
Counseling points
Dr. Pederson: Patients’ fears about adverse effects are often worse than the adverse effects themselves. Women will fester over, Should I take it? Should I take it possibly for years? And then they take the medication and they tell me, “I don’t even notice that I’m taking it, and I know I’m being proactive.” The majority of patients who take these medications don’t have a lot of significant adverse effects.
Severe hot flashes can be managed in a number of ways, primarily and most effectively with certain antidepressants. Oxybutynin use is another good way to manage vasomotor symptoms. Sometimes we use local vaginal estrogen if a patient has vaginal dryness. In general, however, I would say at least 80% of my patients who take preventive medications do not require management of adverse side effects, that they are tolerable.
I counsel women this way, “Don’t think of this as a 5-year course of medication. Think of it as a 90-day trial, and let’s see how you do. If you hate it, then we don’t do it.” They often are pleasantly surprised that the medication is much easier to tolerate than they thought it would be.
Dr. Pederson: It would be neat if a trial would directly compare lifestyle interventions with medications, because probably lifestyle change is as effective as medication is—but we don’t know that and probably will never have that data. We do know that alcohol consumption, every drink per day, increases risk by 10%. We know that obesity is responsible for 30% of breast cancers in this country, and that hormone replacement probably is overrated as a significant risk factor. Updated data from the Women’s Health Initiative study suggest that hormone replacement may actually reduce both breast cancer and cardiovascular risk in women in their 50s, but that’s in average-risk women and not in high-risk women, so we can’t generalize. We do recommend lifestyle measures including weight loss, exercise, and limiting alcohol consumption for all of our patients and certainly for our high-risk patients.
The only 2 things a woman can do to reduce the risk of triple negative breast cancer are to achieve and maintain ideal body weight and to breastfeed. The medications that I have mentioned don’t reduce the risk of triple negative breast cancer. Staying thin and breastfeeding do. It’s a problem in this country because at least 35% of all women and 58% of Black women are obese in America, and Black women tend to be prone to triple-negative breast cancer. That’s a real public health issue that we need to address. If we were going to focus on one thing, it would be focusing on obesity in terms of risk reduction.
Final thoughts
Dr. Pederson: I would like to direct attention to the American Heart Association scientific statement published at the end of 2020 that reported that hormone replacement in average-risk women reduced both cardiovascular events and overall mortality in women in their 50s by 30%.5 While that’s not directly related to what we are talking about, we need to weigh the pros and cons of estrogen versus estrogen blockade in women in terms of breast cancer risk management discussions. Part of shared decision making now needs to include cardiovascular risk factors and how estrogen is going to play into that.
In women with atypical hyperplasia or LCIS, they may benefit from the preventive medications we discussed. But in women with family history or in women with genetic mutations who have not had benign atypical biopsies, they may choose to consider estrogen during their 50s and perhaps take tamoxifen either beforehand or raloxifene afterward.
We need to look at patients holistically and consider all their risk factors together. We can’t look at one dimension alone.
In her presentation at The North American Menopause Society (NAMS) 2021 annual meeting (September 22–25, 2021, in Washington, DC), Dr. Holly J. Pederson offered her expert perspectives on breast cancer prevention in at-risk women in “Chemoprevention for risk reduction: Women’s health clinicians have a role.”
Which patients would benefit from chemoprevention?
Holly J. Pederson, MD: Obviously, women with significant family history are at risk. And approximately 10% of biopsies that are done for other reasons incidentally show atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS)—which are not precancers or cancers but are markers for the development of the disease—and they markedly increase risk. Atypical hyperplasia confers a 30% risk for developing breast cancer over the next 25 years, and LCIS is associated with up to a 2% per year risk. In this setting, preventive medication has been shown to cut risk by 56% to 86%; this is a targeted population that is often overlooked.
Mathematical risk models can be used to assess risk by assessing women’s risk factors. The United States Preventive Services Task Force (USPSTF) has set forth a threshold at which they believe the benefits outweigh the risks of preventive medications. That threshold is 3% or greater over the next 5 years using the Gail breast cancer risk assessment tool.1 The American Society of Clinical Oncology (ASCO) uses the Tyrer-Cuzick breast cancer risk evaluation model with a threshold of 5% over the next 10 years.2 In general, those are the situations in which chemoprevention is a no-brainer.
Certain genetic mutations also predispose to estrogen-sensitive breast cancer. While preventive medications specifically have not been studied in large groups of gene carriers, chemoprevention makes sense because these medications prevent estrogen-sensitive breast cancers that those patients are prone to. Examples would be patients with ATM and CHEK2 gene mutations, which are very common, and patients with BRCA2 and even BRCA1 variants in the postmenopausal years. Those are the big targets.
Risk assessment models
Dr. Pederson: Yes, I almost exclusively use the Tyrer-Cuzick risk model, version 8, which incorporates breast density. This model is intimidating to some practitioners initially, but once you get used to it, you can complete it very quickly.
The Gail model is very limited. It assesses only first-degree relatives, so you don’t get the paternal information at all, and you don’t use age at diagnosis, family structure, genetic testing, results of breast density, or body mass index (BMI). There are many limitations of the Gail model, but most people use it because it is so easy and they are familiar with it.
Possibly the best model is the CanRisk tool, which incorporates the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), but it takes too much time to use in clinic; it’s too complicated. The Tyrer-Cuzick model is easy to use once you get used to it.
Dr. Pederson: Risk doesn’t always need to be formally calculated, which can be time-consuming. It’s one of those situations where most practitioners know it when they see it. Benign atypical biopsies, a strong family history, or, obviously, the presence of a genetic mutation are huge red flags.
If a practitioner has a nearby high-risk center where they can refer patients, that can be so useful, even for a one-time consultation to guide management. For example, with the virtual world now, I do a lot of consultations for patients and outline a plan, and then the referring practitioner can carry out the plan with confidence and then send the patient back periodically. There are so many more options now that previously did not exist for the busy ObGyn or primary care provider to rely on.
Continue to: Chemoprevention uptake in at-risk women...
Chemoprevention uptake in at-risk women
Dr. Pederson: We really never practice medicine using numbers. We use clinical judgment, and we use relationships with patients in terms of developing confidence and trust. I think that the uptake that we exhibit in our center probably is more based on the patients’ perception that we are confident in our recommendations. I think that many practitioners simply are not comfortable with explaining medications, explaining and managing adverse effects, and using alternative medications. While the modeling helps, I think the personal expertise really makes the difference.
Going forward, the addition of the polygenic risk score to the mathematical risk models is going to make a big difference. Right now, the mathematical risk model is simply that: it takes the traditional risk factors that a patient has and spits out a number. But adding the patient’s genomic data—that is, a weighted summation of SNPs, or single nucleotide polymorphisms, now numbering over 300 for breast cancer—can explain more about their personalized risk, which is going to be more powerful in influencing a woman to take medication or not to take medication, in my opinion. Knowing their actual genomic risk will be a big step forward in individualized risk stratification and increased medication uptake as well as vigilance with high risk screening and attention to diet, exercise, and drinking alcohol in moderation.
Dr. Pederson: The only drug that can be used in the premenopausal setting is tamoxifen (TABLE 1). Women can’t take it if they are pregnant, planning to become pregnant, or if they don’t use a reliable form of birth control because it is teratogenic. Women also cannot take tamoxifen if they have had a history of blood clots, stroke, or transient ischemic attack; if they are on warfarin or estrogen preparations; or if they have had atypical endometrial biopsies or endometrial cancer. Those are the absolute contraindications for tamoxifen use.
Tamoxifen is generally very well tolerated in most women; some women experience hot flashes and night sweats that often will subside (or become tolerable) over the first 90 days. In addition, some women experience vaginal discharge rather than dryness, but it is not as bothersome to patients as dryness can be.
Tamoxifen can be used in the pre- or postmenopausal setting. In healthy premenopausal women, there’s no increased risk of the serious adverse effects that are seen with tamoxifen use in postmenopausal women, such as the 1% risk of blood clots and the 1% risk of endometrial cancer.
In postmenopausal women who still have their uterus, I’ll preferentially use raloxifene over tamoxifen. If they don’t have their uterus, tamoxifen is slightly more effective than the raloxifene, and I’ll use that.
Tamoxifen and raloxifene are both selective estrogen receptor modulators, or SERMs, which means that they stimulate receptors in some tissues, like bone, keeping bones strong, and block the receptors in other tissues, like the breast, reducing risk. And so you get kind of a two-for-one in terms of breast cancer risk reduction and osteoporosis prevention.
Another class of preventive drugs is the aromatase inhibitors (AIs). They block the enzyme aromatase, which converts androgens to estrogens peripherally; that is, the androgens that are produced primarily in the adrenal gland, but in part in postmenopausal ovaries.
In general, AIs are less well tolerated. There are generally more hot flashes and night sweats, and more vaginal dryness than with the SERMs. Anastrozole use is associated with arthralgias; and with exemestane use, there can be some hair loss (TABLE 2). Relative contraindications to SERMs become more important in the postmenopausal setting because of the increased frequency of both blood clots and uterine cancer in the postmenopausal years. I won’t give it to smokers. I won’t give tamoxifen to smokers in the premenopausal period either. With obese women, care must be taken because of the risk of blood clots with the SERMS, so then I’ll resort to the AIs. In the postmenopausal setting, you have to think a lot harder about the choices you use for preventive medication. Preferentially, I’ll use the SERMS if possible as they have fewer adverse effects.

Dr. Pederson: All of them are recommended to be given for 5 years, but the MAP.3 trial, which studied exemestane compared with placebo, showed a 65% risk reduction with 3 years of therapy.3 So occasionally, we’ll use 3 years of therapy. Why the treatment recommendation is universally 5 years is unclear, given that the trial with that particular drug was done in 3 years. And with low-dose tamoxifen, the recommended duration is 3 years. That study was done in Italy with 5 mg daily for 3 years.4 In the United States we use 10 mg every other day for 3 years because the 5-mg tablet is not available here.
Continue to: Counseling points...
Counseling points
Dr. Pederson: Patients’ fears about adverse effects are often worse than the adverse effects themselves. Women will fester over, Should I take it? Should I take it possibly for years? And then they take the medication and they tell me, “I don’t even notice that I’m taking it, and I know I’m being proactive.” The majority of patients who take these medications don’t have a lot of significant adverse effects.
Severe hot flashes can be managed in a number of ways, primarily and most effectively with certain antidepressants. Oxybutynin use is another good way to manage vasomotor symptoms. Sometimes we use local vaginal estrogen if a patient has vaginal dryness. In general, however, I would say at least 80% of my patients who take preventive medications do not require management of adverse side effects, that they are tolerable.
I counsel women this way, “Don’t think of this as a 5-year course of medication. Think of it as a 90-day trial, and let’s see how you do. If you hate it, then we don’t do it.” They often are pleasantly surprised that the medication is much easier to tolerate than they thought it would be.
Dr. Pederson: It would be neat if a trial would directly compare lifestyle interventions with medications, because probably lifestyle change is as effective as medication is—but we don’t know that and probably will never have that data. We do know that alcohol consumption, every drink per day, increases risk by 10%. We know that obesity is responsible for 30% of breast cancers in this country, and that hormone replacement probably is overrated as a significant risk factor. Updated data from the Women’s Health Initiative study suggest that hormone replacement may actually reduce both breast cancer and cardiovascular risk in women in their 50s, but that’s in average-risk women and not in high-risk women, so we can’t generalize. We do recommend lifestyle measures including weight loss, exercise, and limiting alcohol consumption for all of our patients and certainly for our high-risk patients.
The only 2 things a woman can do to reduce the risk of triple negative breast cancer are to achieve and maintain ideal body weight and to breastfeed. The medications that I have mentioned don’t reduce the risk of triple negative breast cancer. Staying thin and breastfeeding do. It’s a problem in this country because at least 35% of all women and 58% of Black women are obese in America, and Black women tend to be prone to triple-negative breast cancer. That’s a real public health issue that we need to address. If we were going to focus on one thing, it would be focusing on obesity in terms of risk reduction.
Final thoughts
Dr. Pederson: I would like to direct attention to the American Heart Association scientific statement published at the end of 2020 that reported that hormone replacement in average-risk women reduced both cardiovascular events and overall mortality in women in their 50s by 30%.5 While that’s not directly related to what we are talking about, we need to weigh the pros and cons of estrogen versus estrogen blockade in women in terms of breast cancer risk management discussions. Part of shared decision making now needs to include cardiovascular risk factors and how estrogen is going to play into that.
In women with atypical hyperplasia or LCIS, they may benefit from the preventive medications we discussed. But in women with family history or in women with genetic mutations who have not had benign atypical biopsies, they may choose to consider estrogen during their 50s and perhaps take tamoxifen either beforehand or raloxifene afterward.
We need to look at patients holistically and consider all their risk factors together. We can’t look at one dimension alone.
- US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867.
- Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152-3165.
- Goss PE, Ingle JN, Alex-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37:1629-1637.
- El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532.
- US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867.
- Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152-3165.
- Goss PE, Ingle JN, Alex-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37:1629-1637.
- El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532.
Cancer prevention through cascade genetic testing: A review of the current practice guidelines, barriers to testing and proposed solutions

CASE Woman with BRCA2 mutation
An 80-year-old woman presents for evaluation of newly diagnosed metastatic pancreatic adenocarcinoma. Her medical history is notable for breast cancer. Genetic testing of pancreatic tumor tissue detected a pathogenic variant in BRCA2. Family history revealed a history of melanoma as well as bladder, prostate, breast, and colon cancer. The patient subsequently underwent germline genetic testing with an 86-gene panel and a pathogenic mutation in BRCA2 was identified.
Watch a video of this patient and her clinician, Dr. Andrea Hagemann: https://www.youtube.com/watch?
Methods of genetic testing
It is estimated that 1 in 300 to 1 in 500 women in the United States carry a deleterious mutation in BRCA1 or BRCA2. This equates to between 250,000 and 415,000 women who are at high risk for breast and ovarian cancer.1 Looking at all women with cancer, 20% with ovarian,2 10% with breast,3 2% to 3% with endometrial,4 and 5% with colon cancer5 will have a germline mutation predisposing them to cancer. Identification of germline or somatic (tumor) mutations now inform treatment for patients with cancer. An equally important goal of germline genetic testing is cancer prevention. Cancer prevention strategies include risk-based screening for breast, colon, melanoma, and pancreatic cancer and prophylactic surgeries to reduce the risk of breast and ovarian cancer based on mutation type. Evidence-based screening guidelines by mutation type and absolute risk of associated cancers can be found on the National Comprehensive Cancer Network (NCCN).6,7
Multiple strategies have been proposed to identify patients for germline genetic testing. Patients can be identified based on a detailed multigenerational family history. This strategy requires clinicians or genetic counselors to take and update family histories, to recognize when a patient requires referral for testing, and for such testing to be completed. Even then the generation of a detailed pedigree is not very sensitive or specific. Population-based screening for high-penetrance breast and ovarian cancer susceptibility genes, regardless of family history, also has been proposed.8 Such a strategy has become increasingly realistic with decreasing cost and increasing availability of genetic testing. However, it would require increased genetic counseling resources to feasibly and equitably reach the target population and to explain the results to those patients and their relatives.
An alternative is to test the enriched population of family members of a patient with cancer who has been found to carry a pathogenic variant in a clinically relevant cancer susceptibility gene. This type of testing is termed cascade genetic testing. Cascade testing in first-degree family members carries a 50% probability of detecting the same pathogenic mutation. A related testing model is traceback testing where genetic testing is performed on pathology or tumor registry specimens from deceased patients with cancer.9 This genetic testing information is then provided to the family. Traceback models of genetic testing are an active area of research but can introduce ethical dilemmas. The more widely accepted cascade testing starts with the testing of a living patient affected with cancer. A recent article demonstrated the feasibility of a cascade testing model. Using a multiple linear regression model, the authors determined that all carriers of pathogenic mutations in 18 clinically relevant cancer susceptibility genes in the United States could be identified in 9.9 years if there was a 70% cascade testing rate of first-, second- and third-degree relatives, compared to 59.5 years with no cascade testing.10
Gaps in practice
Identification of mutation carriers, either through screening triggered by family history or through testing of patients affected with cancer, represents a gap between guidelines and clinical practice. Current NCCN guidelines outline genetic testing criteria for hereditary breast and ovarian cancer syndrome and for hereditary colorectal cancer. Despite well-established criteria, a survey in the United States revealed that only 19% of primary care providers were able to accurately assess family history for BRCA1 and 2 testing.11 Looking at patients who meet criteria for testing for Lynch syndrome, only 1 in 4 individuals have undergone genetic testing.12 Among patients diagnosed with breast and ovarian cancer, current NCCN guidelines recommend germline genetic testing for all patients with epithelial ovarian cancer; emerging evidence suggests all patients with breast cancer should be offered germline genetic testing.7,13 Large population-based studies have repeatedly demonstrated that testing rates fall short of this goal, with only 10% to 30% of patients undergoing genetic testing.9,14
Among families with a known hereditary mutation, rates of cascade genetic testing are also low, ranging from 17% to 50%.15-18 Evidence-based management guidelines, for both hereditary breast and ovarian cancer as well as Lynch syndrome, have been shown to reduce mortality.19,20 Failure to identify patients who carry these genetic mutations equates to increased mortality for our patients.
Barriers to cascade genetic testing
Cascade genetic testing ideally would be performed on entire families. Actual practice is far from ideal, and barriers to cascade testing exist. Barriers encompass resistance on the part of the family and provider as well as environmental or system factors.
Family factors
Because of privacy laws, the responsibility of disclosure of genetic testing results to family members falls primarily to the patient. Proband education is critical to ensure disclosure amongst family members. Family dynamics and geographic distribution of family members can further complicate disclosure. Following disclosure, family member gender, education, and demographics as well as personal views, attitudes, and emotions affect whether a family member decides to undergo testing.21 Furthermore, insurance status and awareness of and access to specialty-specific care for the proband’s family members may influence cascade genetic testing rates.
Provider factors
Provider factors that affect cascade genetic testing include awareness of testing guidelines, interpretation of genetic testing results, and education and knowledge of specific mutations. For instance, providers must recognize that cascade testing is not appropriate for variants of uncertain significance. This can lead to unnecessary surveillance testing and prophylactic surgeries. Providers, however, must continue to follow patients and periodically update testing results as variants may be reclassified over time. Additionally, providers must be knowledgeable about the complex and nuanced nature of the screening guidelines for each mutation. The NCCN provides detailed recommendations by mutation.7 Patients may benefit from care with cancer specialists who are aware of the guidelines, particularly for moderate-penetrance genes like BRIP1 and PALB2, as discussions about the timing of risk-reducing surgery are more nuanced in this population. Finally, which providers are responsible for facilitating cascade testing may be unclear; oncologists and genetic counselors not primarily treating probands’ relatives may assume the proper information has been passed along to family members without a practical means to follow up, and primary care providers may assume it is being taken care of by the oncology provider.
Continue to: Environmental or system factors...
Environmental or system factors
Accessibility of genetic counseling and testing is a common barrier to cascade testing. Family members may be geographically remote and connecting them to counseling and testing can be challenging. Working with local genetic counselors can facilitate this process. Insurance coverage of testing is a common perceived barrier; however, many testing companies now provide cascade testing free of charge if within a certain window from the initial test. Despite this, patients often site cost as a barrier to undergoing testing. Concerns about insurance coverage are common after a positive result. The Genetic Information Nondiscrimination Act of 2008 prohibits discrimination against employees or insurance applicants because of genetic information. Life insurance or long-term care policies, however, can incorporate genetic testing information into policy rates, so patients should be recommended to consider purchasing life insurance prior to undergoing genetic testing. This is especially important if the person considering testing has not yet been diagnosed with cancer.
Implications of a positive result
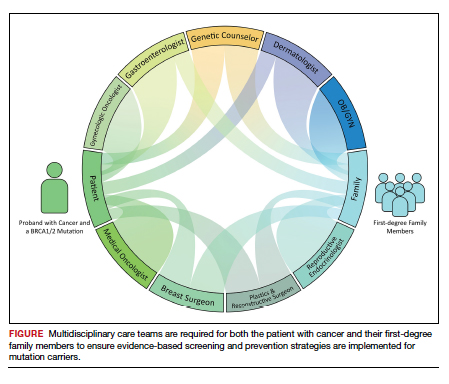
Family members who receive a positive test result should be referred for genetic counseling and to the appropriate specialists for evidence-based screening and discussion for risk-reducing surgery (FIGURE).7 For mutations associated with hereditary breast and ovarian cancer, referral to breast and gynecologic surgeons with expertise in risk reducing surgery is critical as the risk of diagnosing an occult malignancy is approximately 1%.22 Surgical technique with a 2-cm margin on the
Patient resources: decision aids, websites
As genetic testing becomes more accessible and people are tested at younger ages, studies examining the balance of risk reduction and quality of life (QOL) are increasingly important. Fertility concerns, effects of early menopause, and the interrelatedness between decisions for breast and gynecologic risk reduction should all be considered in the counseling for surgical risk reduction. Patient decision aids can help mutation carriers navigate the complex information and decisions.25 Websites specifically designed by advocacy groups can be useful adjuncts to in-office counseling (Facing Our Risk Empowered, FORCE; Facingourrisk.org).
Family letters
The American College of Obstetricians and Gynecologists recommends an ObGyn have a letter or documentation stating that the patient’s relative has a specific mutation before initiating cascade testing for an at-risk family member. The indicated test (such as BRCA1) should be ordered only after the patient has been counseled about potential outcomes and has expressly decided to be tested.26 Letters, such as the example given in the American College of Obstetricians and Gynecologists practice bulletin,26 are a key component of communication between oncology providers, probands, family members, and their primary care providers. ObGyn providers should work together with genetic counselors and gynecologic oncologists to determine the most efficient strategies in their communities.
Technology
Access to genetic testing and genetic counseling has been improved with the rise in telemedicine. Geographically remote patients can now access genetic counseling through medical center–based counselors as well as company-provided genetic counseling over the phone. Patients also can submit samples remotely without needing to be tested in a doctor’s office.
Databases from cancer centers that detail cascade genetic testing rates. As the preventive impact of cascade genetic testing becomes clearer, strategies to have recurrent discussions with cancer patients regarding their family members’ risk should be implemented. It is still unclear which providers—genetic counselors, gynecologic oncologists, medical oncologists, breast surgeons, ObGyns, to name a few—are primarily responsible for remembering to have these follow-up discussions, and despite advances, the burden still rests on the cancer patient themselves. Databases with automated follow-up surveys done every 6 to 12 months could provide some aid to busy providers in this regard.
Emerging research
If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged. The Women Choosing Surgical Prevention (NCT02760849) in the United States and the TUBA study (NCT02321228) in the Netherlands were designed to compare menopause-related QOL between standard risk-reducing salpingo-oophorectomy (RRSO) and the innovative risk-reducing salpingectomy with delayed oophorectomy for mutation carriers. Results from the nonrandomized controlled TUBA trial suggest that patients have better menopause-related QOL after risk-reducing salpingectomy than after RRSO, regardless of hormone replacement therapy.27 International collaboration is continuing to better understand oncologic safety. In the United States, the SOROCk trial (NCT04251052) is a noninferiority surgical choice study underway for BRCA1 mutation carriers aged 35 to 50, powered to determine oncologic outcome differences in addition to QOL outcomes between RRSO and delayed oophorectomy arms.
Returning to the case
The patient and her family underwent genetic counseling. The patient’s 2 daughters, each in their 50s, underwent cascade genetic testing and were found to carry the same pathogenic mutation in BRCA2. After counseling from both breast and gynecologic surgeons, they both elected to undergo risk reducing bilateral salpingo-oophorectomy with hysterectomy. Both now complete regular screening for breast cancer and melanoma with plans to start screening for pancreatic cancer. Both are currently cancer free.
Summary
Cascade genetic testing is an efficient strategy to identify mutation carriers for hereditary breast and ovarian cancer syndrome. Implementation of the best patient-centric care will require continued collaboration and communication across and within disciplines. ●
Cascade, or targeted, genetic testing within families known to carry a hereditary mutation in a cancer susceptibility gene should be performed on all living first-degree family members over the age of 18. All mutation carriers should be connected to a multidisciplinary care team (FIGURE) to ensure implementation of evidence-based screening and risk-reducing surgery for cancer prevention. If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged.
- Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111:14205-14210.
- Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482-490.
- Yamauchi H, Takei J. Management of hereditary breast and ovarian cancer. Int J Clin Oncol. 2018;23:45-51.
- Kahn RM, Gordhandas S, Maddy BP, et al. Universal endometrial cancer tumor typing: how much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer. 2019;125:3172-3183.
- Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
- Gupta S, Provenzale D, Llor X, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 2.2019. J Natl Compr Canc Netw. 2019;17:1032-1041.
- Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:77-102.
- King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312:1091-1092.
- Samimi G, et al. Traceback: a proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation carriers through family-based outreach. J Clin Oncol. 2017;35:2329-2337.
- Offit K, Tkachuk KA, Stadler ZK, et al. Cascading after peridiagnostic cancer genetic testing: an alternative to population-based screening. J Clin Oncol. 2020;38:1398-1408.
- Bellcross CA, Kolor K, Goddard KAB, et al. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40:61-66.
- Cross DS, Rahm AK, Kauffman TL, et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15:933-940.
- Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37:453-460.
- Childers CP, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7:230-237.
- Sharaf RN, Myer P, Stave CD, et al. Uptake of genetic testing by relatives of Lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. 2013;11:1093-1100.
- Menko FH, Ter Stege JA, van der Kolk LE, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer. 2019;18:127-135.
- Griffin NE, Buchanan TR, Smith SH, et al. Low rates of cascade genetic testing among families with hereditary gynecologic cancer: an opportunity to improve cancer prevention. Gynecol Oncol. 2020;156:140-146.
- Roberts MC, Dotson WD, DeVore CS, et al. Delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff (Millwood). 2018;37:801-808.
- Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Srinivasan S, Won NY, Dotson WD, et al. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28:1631-1644.
- Piedimonte S, Frank C, Laprise C, et al. Occult tubal carcinoma after risk-reducing salpingo-oophorectomy: a systematic review. Obstet Gynecol. 2020;135:498-508.
- Shu CA, Pike MC, Jotwani AR, et al. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. 2016;2:1434-1440.
- Gordhandas S, Norquist BM, Pennington KP, et al. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol. 2019;153:192-200.
- Steenbeek MP, van Bommel MHD, Harmsen MG, et al. Evaluation of a patient decision aid for BRCA1/2 pathogenic variant carriers choosing an ovarian cancer prevention strategy. Gynecol Oncol. 2021;163:371-377.
- Committee on Gynecologic Practice. ACOG committee opinion No. 727: Cascade testing: testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol. 2018;131:E31-E34.
- Steenbeek MP, Harmsen MG, Hoogerbrugge N, et al. Association of salpingectomy with delayed oophorectomy versus salpingo-oophorectomy with quality of life in BRCA1/2 pathogenic variant carriers: a nonrandomized controlled trial. JAMA Oncol. 2021;7:1203-1212.

CASE Woman with BRCA2 mutation
An 80-year-old woman presents for evaluation of newly diagnosed metastatic pancreatic adenocarcinoma. Her medical history is notable for breast cancer. Genetic testing of pancreatic tumor tissue detected a pathogenic variant in BRCA2. Family history revealed a history of melanoma as well as bladder, prostate, breast, and colon cancer. The patient subsequently underwent germline genetic testing with an 86-gene panel and a pathogenic mutation in BRCA2 was identified.
Watch a video of this patient and her clinician, Dr. Andrea Hagemann: https://www.youtube.com/watch?
Methods of genetic testing
It is estimated that 1 in 300 to 1 in 500 women in the United States carry a deleterious mutation in BRCA1 or BRCA2. This equates to between 250,000 and 415,000 women who are at high risk for breast and ovarian cancer.1 Looking at all women with cancer, 20% with ovarian,2 10% with breast,3 2% to 3% with endometrial,4 and 5% with colon cancer5 will have a germline mutation predisposing them to cancer. Identification of germline or somatic (tumor) mutations now inform treatment for patients with cancer. An equally important goal of germline genetic testing is cancer prevention. Cancer prevention strategies include risk-based screening for breast, colon, melanoma, and pancreatic cancer and prophylactic surgeries to reduce the risk of breast and ovarian cancer based on mutation type. Evidence-based screening guidelines by mutation type and absolute risk of associated cancers can be found on the National Comprehensive Cancer Network (NCCN).6,7
Multiple strategies have been proposed to identify patients for germline genetic testing. Patients can be identified based on a detailed multigenerational family history. This strategy requires clinicians or genetic counselors to take and update family histories, to recognize when a patient requires referral for testing, and for such testing to be completed. Even then the generation of a detailed pedigree is not very sensitive or specific. Population-based screening for high-penetrance breast and ovarian cancer susceptibility genes, regardless of family history, also has been proposed.8 Such a strategy has become increasingly realistic with decreasing cost and increasing availability of genetic testing. However, it would require increased genetic counseling resources to feasibly and equitably reach the target population and to explain the results to those patients and their relatives.
An alternative is to test the enriched population of family members of a patient with cancer who has been found to carry a pathogenic variant in a clinically relevant cancer susceptibility gene. This type of testing is termed cascade genetic testing. Cascade testing in first-degree family members carries a 50% probability of detecting the same pathogenic mutation. A related testing model is traceback testing where genetic testing is performed on pathology or tumor registry specimens from deceased patients with cancer.9 This genetic testing information is then provided to the family. Traceback models of genetic testing are an active area of research but can introduce ethical dilemmas. The more widely accepted cascade testing starts with the testing of a living patient affected with cancer. A recent article demonstrated the feasibility of a cascade testing model. Using a multiple linear regression model, the authors determined that all carriers of pathogenic mutations in 18 clinically relevant cancer susceptibility genes in the United States could be identified in 9.9 years if there was a 70% cascade testing rate of first-, second- and third-degree relatives, compared to 59.5 years with no cascade testing.10
Gaps in practice
Identification of mutation carriers, either through screening triggered by family history or through testing of patients affected with cancer, represents a gap between guidelines and clinical practice. Current NCCN guidelines outline genetic testing criteria for hereditary breast and ovarian cancer syndrome and for hereditary colorectal cancer. Despite well-established criteria, a survey in the United States revealed that only 19% of primary care providers were able to accurately assess family history for BRCA1 and 2 testing.11 Looking at patients who meet criteria for testing for Lynch syndrome, only 1 in 4 individuals have undergone genetic testing.12 Among patients diagnosed with breast and ovarian cancer, current NCCN guidelines recommend germline genetic testing for all patients with epithelial ovarian cancer; emerging evidence suggests all patients with breast cancer should be offered germline genetic testing.7,13 Large population-based studies have repeatedly demonstrated that testing rates fall short of this goal, with only 10% to 30% of patients undergoing genetic testing.9,14
Among families with a known hereditary mutation, rates of cascade genetic testing are also low, ranging from 17% to 50%.15-18 Evidence-based management guidelines, for both hereditary breast and ovarian cancer as well as Lynch syndrome, have been shown to reduce mortality.19,20 Failure to identify patients who carry these genetic mutations equates to increased mortality for our patients.
Barriers to cascade genetic testing
Cascade genetic testing ideally would be performed on entire families. Actual practice is far from ideal, and barriers to cascade testing exist. Barriers encompass resistance on the part of the family and provider as well as environmental or system factors.
Family factors
Because of privacy laws, the responsibility of disclosure of genetic testing results to family members falls primarily to the patient. Proband education is critical to ensure disclosure amongst family members. Family dynamics and geographic distribution of family members can further complicate disclosure. Following disclosure, family member gender, education, and demographics as well as personal views, attitudes, and emotions affect whether a family member decides to undergo testing.21 Furthermore, insurance status and awareness of and access to specialty-specific care for the proband’s family members may influence cascade genetic testing rates.
Provider factors
Provider factors that affect cascade genetic testing include awareness of testing guidelines, interpretation of genetic testing results, and education and knowledge of specific mutations. For instance, providers must recognize that cascade testing is not appropriate for variants of uncertain significance. This can lead to unnecessary surveillance testing and prophylactic surgeries. Providers, however, must continue to follow patients and periodically update testing results as variants may be reclassified over time. Additionally, providers must be knowledgeable about the complex and nuanced nature of the screening guidelines for each mutation. The NCCN provides detailed recommendations by mutation.7 Patients may benefit from care with cancer specialists who are aware of the guidelines, particularly for moderate-penetrance genes like BRIP1 and PALB2, as discussions about the timing of risk-reducing surgery are more nuanced in this population. Finally, which providers are responsible for facilitating cascade testing may be unclear; oncologists and genetic counselors not primarily treating probands’ relatives may assume the proper information has been passed along to family members without a practical means to follow up, and primary care providers may assume it is being taken care of by the oncology provider.
Continue to: Environmental or system factors...
Environmental or system factors
Accessibility of genetic counseling and testing is a common barrier to cascade testing. Family members may be geographically remote and connecting them to counseling and testing can be challenging. Working with local genetic counselors can facilitate this process. Insurance coverage of testing is a common perceived barrier; however, many testing companies now provide cascade testing free of charge if within a certain window from the initial test. Despite this, patients often site cost as a barrier to undergoing testing. Concerns about insurance coverage are common after a positive result. The Genetic Information Nondiscrimination Act of 2008 prohibits discrimination against employees or insurance applicants because of genetic information. Life insurance or long-term care policies, however, can incorporate genetic testing information into policy rates, so patients should be recommended to consider purchasing life insurance prior to undergoing genetic testing. This is especially important if the person considering testing has not yet been diagnosed with cancer.
Implications of a positive result
Family members who receive a positive test result should be referred for genetic counseling and to the appropriate specialists for evidence-based screening and discussion for risk-reducing surgery (FIGURE).7 For mutations associated with hereditary breast and ovarian cancer, referral to breast and gynecologic surgeons with expertise in risk reducing surgery is critical as the risk of diagnosing an occult malignancy is approximately 1%.22 Surgical technique with a 2-cm margin on the

Patient resources: decision aids, websites
As genetic testing becomes more accessible and people are tested at younger ages, studies examining the balance of risk reduction and quality of life (QOL) are increasingly important. Fertility concerns, effects of early menopause, and the interrelatedness between decisions for breast and gynecologic risk reduction should all be considered in the counseling for surgical risk reduction. Patient decision aids can help mutation carriers navigate the complex information and decisions.25 Websites specifically designed by advocacy groups can be useful adjuncts to in-office counseling (Facing Our Risk Empowered, FORCE; Facingourrisk.org).
Family letters
The American College of Obstetricians and Gynecologists recommends an ObGyn have a letter or documentation stating that the patient’s relative has a specific mutation before initiating cascade testing for an at-risk family member. The indicated test (such as BRCA1) should be ordered only after the patient has been counseled about potential outcomes and has expressly decided to be tested.26 Letters, such as the example given in the American College of Obstetricians and Gynecologists practice bulletin,26 are a key component of communication between oncology providers, probands, family members, and their primary care providers. ObGyn providers should work together with genetic counselors and gynecologic oncologists to determine the most efficient strategies in their communities.
Technology
Access to genetic testing and genetic counseling has been improved with the rise in telemedicine. Geographically remote patients can now access genetic counseling through medical center–based counselors as well as company-provided genetic counseling over the phone. Patients also can submit samples remotely without needing to be tested in a doctor’s office.
Databases from cancer centers that detail cascade genetic testing rates. As the preventive impact of cascade genetic testing becomes clearer, strategies to have recurrent discussions with cancer patients regarding their family members’ risk should be implemented. It is still unclear which providers—genetic counselors, gynecologic oncologists, medical oncologists, breast surgeons, ObGyns, to name a few—are primarily responsible for remembering to have these follow-up discussions, and despite advances, the burden still rests on the cancer patient themselves. Databases with automated follow-up surveys done every 6 to 12 months could provide some aid to busy providers in this regard.
Emerging research
If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged. The Women Choosing Surgical Prevention (NCT02760849) in the United States and the TUBA study (NCT02321228) in the Netherlands were designed to compare menopause-related QOL between standard risk-reducing salpingo-oophorectomy (RRSO) and the innovative risk-reducing salpingectomy with delayed oophorectomy for mutation carriers. Results from the nonrandomized controlled TUBA trial suggest that patients have better menopause-related QOL after risk-reducing salpingectomy than after RRSO, regardless of hormone replacement therapy.27 International collaboration is continuing to better understand oncologic safety. In the United States, the SOROCk trial (NCT04251052) is a noninferiority surgical choice study underway for BRCA1 mutation carriers aged 35 to 50, powered to determine oncologic outcome differences in addition to QOL outcomes between RRSO and delayed oophorectomy arms.
Returning to the case
The patient and her family underwent genetic counseling. The patient’s 2 daughters, each in their 50s, underwent cascade genetic testing and were found to carry the same pathogenic mutation in BRCA2. After counseling from both breast and gynecologic surgeons, they both elected to undergo risk reducing bilateral salpingo-oophorectomy with hysterectomy. Both now complete regular screening for breast cancer and melanoma with plans to start screening for pancreatic cancer. Both are currently cancer free.
Summary
Cascade genetic testing is an efficient strategy to identify mutation carriers for hereditary breast and ovarian cancer syndrome. Implementation of the best patient-centric care will require continued collaboration and communication across and within disciplines. ●
Cascade, or targeted, genetic testing within families known to carry a hereditary mutation in a cancer susceptibility gene should be performed on all living first-degree family members over the age of 18. All mutation carriers should be connected to a multidisciplinary care team (FIGURE) to ensure implementation of evidence-based screening and risk-reducing surgery for cancer prevention. If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged.

CASE Woman with BRCA2 mutation
An 80-year-old woman presents for evaluation of newly diagnosed metastatic pancreatic adenocarcinoma. Her medical history is notable for breast cancer. Genetic testing of pancreatic tumor tissue detected a pathogenic variant in BRCA2. Family history revealed a history of melanoma as well as bladder, prostate, breast, and colon cancer. The patient subsequently underwent germline genetic testing with an 86-gene panel and a pathogenic mutation in BRCA2 was identified.
Watch a video of this patient and her clinician, Dr. Andrea Hagemann: https://www.youtube.com/watch?
Methods of genetic testing
It is estimated that 1 in 300 to 1 in 500 women in the United States carry a deleterious mutation in BRCA1 or BRCA2. This equates to between 250,000 and 415,000 women who are at high risk for breast and ovarian cancer.1 Looking at all women with cancer, 20% with ovarian,2 10% with breast,3 2% to 3% with endometrial,4 and 5% with colon cancer5 will have a germline mutation predisposing them to cancer. Identification of germline or somatic (tumor) mutations now inform treatment for patients with cancer. An equally important goal of germline genetic testing is cancer prevention. Cancer prevention strategies include risk-based screening for breast, colon, melanoma, and pancreatic cancer and prophylactic surgeries to reduce the risk of breast and ovarian cancer based on mutation type. Evidence-based screening guidelines by mutation type and absolute risk of associated cancers can be found on the National Comprehensive Cancer Network (NCCN).6,7
Multiple strategies have been proposed to identify patients for germline genetic testing. Patients can be identified based on a detailed multigenerational family history. This strategy requires clinicians or genetic counselors to take and update family histories, to recognize when a patient requires referral for testing, and for such testing to be completed. Even then the generation of a detailed pedigree is not very sensitive or specific. Population-based screening for high-penetrance breast and ovarian cancer susceptibility genes, regardless of family history, also has been proposed.8 Such a strategy has become increasingly realistic with decreasing cost and increasing availability of genetic testing. However, it would require increased genetic counseling resources to feasibly and equitably reach the target population and to explain the results to those patients and their relatives.
An alternative is to test the enriched population of family members of a patient with cancer who has been found to carry a pathogenic variant in a clinically relevant cancer susceptibility gene. This type of testing is termed cascade genetic testing. Cascade testing in first-degree family members carries a 50% probability of detecting the same pathogenic mutation. A related testing model is traceback testing where genetic testing is performed on pathology or tumor registry specimens from deceased patients with cancer.9 This genetic testing information is then provided to the family. Traceback models of genetic testing are an active area of research but can introduce ethical dilemmas. The more widely accepted cascade testing starts with the testing of a living patient affected with cancer. A recent article demonstrated the feasibility of a cascade testing model. Using a multiple linear regression model, the authors determined that all carriers of pathogenic mutations in 18 clinically relevant cancer susceptibility genes in the United States could be identified in 9.9 years if there was a 70% cascade testing rate of first-, second- and third-degree relatives, compared to 59.5 years with no cascade testing.10
Gaps in practice
Identification of mutation carriers, either through screening triggered by family history or through testing of patients affected with cancer, represents a gap between guidelines and clinical practice. Current NCCN guidelines outline genetic testing criteria for hereditary breast and ovarian cancer syndrome and for hereditary colorectal cancer. Despite well-established criteria, a survey in the United States revealed that only 19% of primary care providers were able to accurately assess family history for BRCA1 and 2 testing.11 Looking at patients who meet criteria for testing for Lynch syndrome, only 1 in 4 individuals have undergone genetic testing.12 Among patients diagnosed with breast and ovarian cancer, current NCCN guidelines recommend germline genetic testing for all patients with epithelial ovarian cancer; emerging evidence suggests all patients with breast cancer should be offered germline genetic testing.7,13 Large population-based studies have repeatedly demonstrated that testing rates fall short of this goal, with only 10% to 30% of patients undergoing genetic testing.9,14
Among families with a known hereditary mutation, rates of cascade genetic testing are also low, ranging from 17% to 50%.15-18 Evidence-based management guidelines, for both hereditary breast and ovarian cancer as well as Lynch syndrome, have been shown to reduce mortality.19,20 Failure to identify patients who carry these genetic mutations equates to increased mortality for our patients.
Barriers to cascade genetic testing
Cascade genetic testing ideally would be performed on entire families. Actual practice is far from ideal, and barriers to cascade testing exist. Barriers encompass resistance on the part of the family and provider as well as environmental or system factors.
Family factors
Because of privacy laws, the responsibility of disclosure of genetic testing results to family members falls primarily to the patient. Proband education is critical to ensure disclosure amongst family members. Family dynamics and geographic distribution of family members can further complicate disclosure. Following disclosure, family member gender, education, and demographics as well as personal views, attitudes, and emotions affect whether a family member decides to undergo testing.21 Furthermore, insurance status and awareness of and access to specialty-specific care for the proband’s family members may influence cascade genetic testing rates.
Provider factors
Provider factors that affect cascade genetic testing include awareness of testing guidelines, interpretation of genetic testing results, and education and knowledge of specific mutations. For instance, providers must recognize that cascade testing is not appropriate for variants of uncertain significance. This can lead to unnecessary surveillance testing and prophylactic surgeries. Providers, however, must continue to follow patients and periodically update testing results as variants may be reclassified over time. Additionally, providers must be knowledgeable about the complex and nuanced nature of the screening guidelines for each mutation. The NCCN provides detailed recommendations by mutation.7 Patients may benefit from care with cancer specialists who are aware of the guidelines, particularly for moderate-penetrance genes like BRIP1 and PALB2, as discussions about the timing of risk-reducing surgery are more nuanced in this population. Finally, which providers are responsible for facilitating cascade testing may be unclear; oncologists and genetic counselors not primarily treating probands’ relatives may assume the proper information has been passed along to family members without a practical means to follow up, and primary care providers may assume it is being taken care of by the oncology provider.
Continue to: Environmental or system factors...
Environmental or system factors
Accessibility of genetic counseling and testing is a common barrier to cascade testing. Family members may be geographically remote and connecting them to counseling and testing can be challenging. Working with local genetic counselors can facilitate this process. Insurance coverage of testing is a common perceived barrier; however, many testing companies now provide cascade testing free of charge if within a certain window from the initial test. Despite this, patients often site cost as a barrier to undergoing testing. Concerns about insurance coverage are common after a positive result. The Genetic Information Nondiscrimination Act of 2008 prohibits discrimination against employees or insurance applicants because of genetic information. Life insurance or long-term care policies, however, can incorporate genetic testing information into policy rates, so patients should be recommended to consider purchasing life insurance prior to undergoing genetic testing. This is especially important if the person considering testing has not yet been diagnosed with cancer.
Implications of a positive result
Family members who receive a positive test result should be referred for genetic counseling and to the appropriate specialists for evidence-based screening and discussion for risk-reducing surgery (FIGURE).7 For mutations associated with hereditary breast and ovarian cancer, referral to breast and gynecologic surgeons with expertise in risk reducing surgery is critical as the risk of diagnosing an occult malignancy is approximately 1%.22 Surgical technique with a 2-cm margin on the

Patient resources: decision aids, websites
As genetic testing becomes more accessible and people are tested at younger ages, studies examining the balance of risk reduction and quality of life (QOL) are increasingly important. Fertility concerns, effects of early menopause, and the interrelatedness between decisions for breast and gynecologic risk reduction should all be considered in the counseling for surgical risk reduction. Patient decision aids can help mutation carriers navigate the complex information and decisions.25 Websites specifically designed by advocacy groups can be useful adjuncts to in-office counseling (Facing Our Risk Empowered, FORCE; Facingourrisk.org).
Family letters
The American College of Obstetricians and Gynecologists recommends an ObGyn have a letter or documentation stating that the patient’s relative has a specific mutation before initiating cascade testing for an at-risk family member. The indicated test (such as BRCA1) should be ordered only after the patient has been counseled about potential outcomes and has expressly decided to be tested.26 Letters, such as the example given in the American College of Obstetricians and Gynecologists practice bulletin,26 are a key component of communication between oncology providers, probands, family members, and their primary care providers. ObGyn providers should work together with genetic counselors and gynecologic oncologists to determine the most efficient strategies in their communities.
Technology
Access to genetic testing and genetic counseling has been improved with the rise in telemedicine. Geographically remote patients can now access genetic counseling through medical center–based counselors as well as company-provided genetic counseling over the phone. Patients also can submit samples remotely without needing to be tested in a doctor’s office.
Databases from cancer centers that detail cascade genetic testing rates. As the preventive impact of cascade genetic testing becomes clearer, strategies to have recurrent discussions with cancer patients regarding their family members’ risk should be implemented. It is still unclear which providers—genetic counselors, gynecologic oncologists, medical oncologists, breast surgeons, ObGyns, to name a few—are primarily responsible for remembering to have these follow-up discussions, and despite advances, the burden still rests on the cancer patient themselves. Databases with automated follow-up surveys done every 6 to 12 months could provide some aid to busy providers in this regard.
Emerging research
If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged. The Women Choosing Surgical Prevention (NCT02760849) in the United States and the TUBA study (NCT02321228) in the Netherlands were designed to compare menopause-related QOL between standard risk-reducing salpingo-oophorectomy (RRSO) and the innovative risk-reducing salpingectomy with delayed oophorectomy for mutation carriers. Results from the nonrandomized controlled TUBA trial suggest that patients have better menopause-related QOL after risk-reducing salpingectomy than after RRSO, regardless of hormone replacement therapy.27 International collaboration is continuing to better understand oncologic safety. In the United States, the SOROCk trial (NCT04251052) is a noninferiority surgical choice study underway for BRCA1 mutation carriers aged 35 to 50, powered to determine oncologic outcome differences in addition to QOL outcomes between RRSO and delayed oophorectomy arms.
Returning to the case
The patient and her family underwent genetic counseling. The patient’s 2 daughters, each in their 50s, underwent cascade genetic testing and were found to carry the same pathogenic mutation in BRCA2. After counseling from both breast and gynecologic surgeons, they both elected to undergo risk reducing bilateral salpingo-oophorectomy with hysterectomy. Both now complete regular screening for breast cancer and melanoma with plans to start screening for pancreatic cancer. Both are currently cancer free.
Summary
Cascade genetic testing is an efficient strategy to identify mutation carriers for hereditary breast and ovarian cancer syndrome. Implementation of the best patient-centric care will require continued collaboration and communication across and within disciplines. ●
Cascade, or targeted, genetic testing within families known to carry a hereditary mutation in a cancer susceptibility gene should be performed on all living first-degree family members over the age of 18. All mutation carriers should be connected to a multidisciplinary care team (FIGURE) to ensure implementation of evidence-based screening and risk-reducing surgery for cancer prevention. If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged.
- Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111:14205-14210.
- Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482-490.
- Yamauchi H, Takei J. Management of hereditary breast and ovarian cancer. Int J Clin Oncol. 2018;23:45-51.
- Kahn RM, Gordhandas S, Maddy BP, et al. Universal endometrial cancer tumor typing: how much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer. 2019;125:3172-3183.
- Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
- Gupta S, Provenzale D, Llor X, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 2.2019. J Natl Compr Canc Netw. 2019;17:1032-1041.
- Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:77-102.
- King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312:1091-1092.
- Samimi G, et al. Traceback: a proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation carriers through family-based outreach. J Clin Oncol. 2017;35:2329-2337.
- Offit K, Tkachuk KA, Stadler ZK, et al. Cascading after peridiagnostic cancer genetic testing: an alternative to population-based screening. J Clin Oncol. 2020;38:1398-1408.
- Bellcross CA, Kolor K, Goddard KAB, et al. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40:61-66.
- Cross DS, Rahm AK, Kauffman TL, et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15:933-940.
- Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37:453-460.
- Childers CP, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7:230-237.
- Sharaf RN, Myer P, Stave CD, et al. Uptake of genetic testing by relatives of Lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. 2013;11:1093-1100.
- Menko FH, Ter Stege JA, van der Kolk LE, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer. 2019;18:127-135.
- Griffin NE, Buchanan TR, Smith SH, et al. Low rates of cascade genetic testing among families with hereditary gynecologic cancer: an opportunity to improve cancer prevention. Gynecol Oncol. 2020;156:140-146.
- Roberts MC, Dotson WD, DeVore CS, et al. Delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff (Millwood). 2018;37:801-808.
- Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Srinivasan S, Won NY, Dotson WD, et al. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28:1631-1644.
- Piedimonte S, Frank C, Laprise C, et al. Occult tubal carcinoma after risk-reducing salpingo-oophorectomy: a systematic review. Obstet Gynecol. 2020;135:498-508.
- Shu CA, Pike MC, Jotwani AR, et al. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. 2016;2:1434-1440.
- Gordhandas S, Norquist BM, Pennington KP, et al. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol. 2019;153:192-200.
- Steenbeek MP, van Bommel MHD, Harmsen MG, et al. Evaluation of a patient decision aid for BRCA1/2 pathogenic variant carriers choosing an ovarian cancer prevention strategy. Gynecol Oncol. 2021;163:371-377.
- Committee on Gynecologic Practice. ACOG committee opinion No. 727: Cascade testing: testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol. 2018;131:E31-E34.
- Steenbeek MP, Harmsen MG, Hoogerbrugge N, et al. Association of salpingectomy with delayed oophorectomy versus salpingo-oophorectomy with quality of life in BRCA1/2 pathogenic variant carriers: a nonrandomized controlled trial. JAMA Oncol. 2021;7:1203-1212.
- Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111:14205-14210.
- Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482-490.
- Yamauchi H, Takei J. Management of hereditary breast and ovarian cancer. Int J Clin Oncol. 2018;23:45-51.
- Kahn RM, Gordhandas S, Maddy BP, et al. Universal endometrial cancer tumor typing: how much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer. 2019;125:3172-3183.
- Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
- Gupta S, Provenzale D, Llor X, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 2.2019. J Natl Compr Canc Netw. 2019;17:1032-1041.
- Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:77-102.
- King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312:1091-1092.
- Samimi G, et al. Traceback: a proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation carriers through family-based outreach. J Clin Oncol. 2017;35:2329-2337.
- Offit K, Tkachuk KA, Stadler ZK, et al. Cascading after peridiagnostic cancer genetic testing: an alternative to population-based screening. J Clin Oncol. 2020;38:1398-1408.
- Bellcross CA, Kolor K, Goddard KAB, et al. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40:61-66.
- Cross DS, Rahm AK, Kauffman TL, et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15:933-940.
- Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37:453-460.
- Childers CP, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7:230-237.
- Sharaf RN, Myer P, Stave CD, et al. Uptake of genetic testing by relatives of Lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. 2013;11:1093-1100.
- Menko FH, Ter Stege JA, van der Kolk LE, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer. 2019;18:127-135.
- Griffin NE, Buchanan TR, Smith SH, et al. Low rates of cascade genetic testing among families with hereditary gynecologic cancer: an opportunity to improve cancer prevention. Gynecol Oncol. 2020;156:140-146.
- Roberts MC, Dotson WD, DeVore CS, et al. Delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff (Millwood). 2018;37:801-808.
- Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Srinivasan S, Won NY, Dotson WD, et al. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28:1631-1644.
- Piedimonte S, Frank C, Laprise C, et al. Occult tubal carcinoma after risk-reducing salpingo-oophorectomy: a systematic review. Obstet Gynecol. 2020;135:498-508.
- Shu CA, Pike MC, Jotwani AR, et al. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. 2016;2:1434-1440.
- Gordhandas S, Norquist BM, Pennington KP, et al. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol. 2019;153:192-200.
- Steenbeek MP, van Bommel MHD, Harmsen MG, et al. Evaluation of a patient decision aid for BRCA1/2 pathogenic variant carriers choosing an ovarian cancer prevention strategy. Gynecol Oncol. 2021;163:371-377.
- Committee on Gynecologic Practice. ACOG committee opinion No. 727: Cascade testing: testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol. 2018;131:E31-E34.
- Steenbeek MP, Harmsen MG, Hoogerbrugge N, et al. Association of salpingectomy with delayed oophorectomy versus salpingo-oophorectomy with quality of life in BRCA1/2 pathogenic variant carriers: a nonrandomized controlled trial. JAMA Oncol. 2021;7:1203-1212.
‘Highest survival’ with combo immunotherapy in advanced melanoma
An researchers say.
Nearly half the patients treated with nivolumab (Opdivo) and ipilimumab (Yervoy) were alive at 6½ years. Within this group, 77% had not received further systemic treatment after coming off the study drugs.
After a minimum follow-up of 77 months, median overall survival was 72.1 months in patients on the combination, which was more than three times longer than the 19.9 months with ipilimumab alone (hazard ratio, 0.52; 95% confidence interval, 0.43-0.64) and twice as long as the 36.9 months with nivolumab alone (HR, 0.84; 95% CI, 0.67-1.04).
The results represent the longest median overall survival seen in a phase 3 trial of advanced melanoma and are evidence of “a substantial development in the melanoma treatment landscape versus the standard median survival of 8 months a decade ago,” researchers wrote in a study published online in the Journal of Clinical Oncology.
However, lead author Jedd D. Wolchok, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, noted that the study was not designed to compare nivolumab alone with the combination. “It wasn’t powered for that. [But] what we can say is that the highest survival was in the combination group,” Dr. Wolchok told this news organization.
Dr. Wolchok cautioned that the combination therapy is not currently standard of care. “PD-1 blockade – either nivolumab or the combination – are both excellent options for care,” he added. “I can’t tell you that one of them is the standard of care because that’s too complex of a decision.”
For example, he explained, “for a patient who only has lung metastases, a single-agent PD-1 blockade might be sufficient. But if it has spread to other organs, such as the liver or bones, which are more difficult to treat, that’s when we often reach for the combination.”
Other factors that weigh into the therapeutic decision are the patient’s performance status and their so-called clinical reserve for tolerating side effects. “The likelihood of having a high-grade side effect with the combination is more than twice that of the single agent,” Dr. Wolchok said.
Until 2011, only two therapies were approved for metastatic melanoma: Chemotherapy with dacarbazine and immunotherapy with high-dose interleukin-2, neither of which was very effective at prolonging life. But patient survival changed with the advent of targeted therapies and immunotherapy. Some patients are now living for years, and as the current study shows, many have surpassed the 5-year mark and are treatment free.
The updated CheckMate 067 analysis included patients with previously untreated, unresectable stage III/IV melanoma who were randomly assigned to receive nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (four doses) followed by nivolumab 3 mg/kg every 2 weeks (n = 314), nivolumab 3 mg/kg every 2 weeks (n = 316), or ipilimumab 3 mg/ kg every 3 weeks (four doses; n = 315).
The authors reported the 5-year overall survival rates from the trial, published in the New England Journal of Medicine in 2019 – 52% with the combination, 44% with nivolumab alone, and 26% with ipilimumab alone.
In the updated study, overall survival at 6½ years had dropped slightly to 49%, 42%, and 23%, respectively. Patients with BRAF-mutant tumors had overall survival rates of 57%, 43%, and 25% versus 46%, 42%, and 22% in those with BRAF wild-type tumors.
Overall, median investigator-assessed progression-free survival was 11.5 months with the combination, 6.9 months with nivolumab alone, and 2.9 months with ipilimumab.
The new analysis also evaluated melanoma-specific survival (MSS), which removes competing causes of deaths from the long-term follow-up. The MSS was not reached in the combination group, and was 58.7 months in the nivolumab group and 21.9 months for ipilimumab, with MSS rates at 6.5 years of 56%, 48%, and 27%, respectively.
No new safety signals were detected, but there was more immune-mediated toxicity in the combination group, the researchers reported.
“The patients will continue to be followed,” said Dr. Wolchok, “And data are still being collected.”
The trial was supported by Bristol-Myers Squibb, the National Cancer Institute, and the National Institute for Health Research Royal Marsden–Institute of Cancer Research Biomedical Research Centre. Dr. Wolchok and coauthors reported relationships with Bristol-Myers Squibb and other drugmakers.
A version of this article first appeared on Medscape.com.
An researchers say.
Nearly half the patients treated with nivolumab (Opdivo) and ipilimumab (Yervoy) were alive at 6½ years. Within this group, 77% had not received further systemic treatment after coming off the study drugs.
After a minimum follow-up of 77 months, median overall survival was 72.1 months in patients on the combination, which was more than three times longer than the 19.9 months with ipilimumab alone (hazard ratio, 0.52; 95% confidence interval, 0.43-0.64) and twice as long as the 36.9 months with nivolumab alone (HR, 0.84; 95% CI, 0.67-1.04).
The results represent the longest median overall survival seen in a phase 3 trial of advanced melanoma and are evidence of “a substantial development in the melanoma treatment landscape versus the standard median survival of 8 months a decade ago,” researchers wrote in a study published online in the Journal of Clinical Oncology.
However, lead author Jedd D. Wolchok, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, noted that the study was not designed to compare nivolumab alone with the combination. “It wasn’t powered for that. [But] what we can say is that the highest survival was in the combination group,” Dr. Wolchok told this news organization.
Dr. Wolchok cautioned that the combination therapy is not currently standard of care. “PD-1 blockade – either nivolumab or the combination – are both excellent options for care,” he added. “I can’t tell you that one of them is the standard of care because that’s too complex of a decision.”
For example, he explained, “for a patient who only has lung metastases, a single-agent PD-1 blockade might be sufficient. But if it has spread to other organs, such as the liver or bones, which are more difficult to treat, that’s when we often reach for the combination.”
Other factors that weigh into the therapeutic decision are the patient’s performance status and their so-called clinical reserve for tolerating side effects. “The likelihood of having a high-grade side effect with the combination is more than twice that of the single agent,” Dr. Wolchok said.
Until 2011, only two therapies were approved for metastatic melanoma: Chemotherapy with dacarbazine and immunotherapy with high-dose interleukin-2, neither of which was very effective at prolonging life. But patient survival changed with the advent of targeted therapies and immunotherapy. Some patients are now living for years, and as the current study shows, many have surpassed the 5-year mark and are treatment free.
The updated CheckMate 067 analysis included patients with previously untreated, unresectable stage III/IV melanoma who were randomly assigned to receive nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (four doses) followed by nivolumab 3 mg/kg every 2 weeks (n = 314), nivolumab 3 mg/kg every 2 weeks (n = 316), or ipilimumab 3 mg/ kg every 3 weeks (four doses; n = 315).
The authors reported the 5-year overall survival rates from the trial, published in the New England Journal of Medicine in 2019 – 52% with the combination, 44% with nivolumab alone, and 26% with ipilimumab alone.
In the updated study, overall survival at 6½ years had dropped slightly to 49%, 42%, and 23%, respectively. Patients with BRAF-mutant tumors had overall survival rates of 57%, 43%, and 25% versus 46%, 42%, and 22% in those with BRAF wild-type tumors.
Overall, median investigator-assessed progression-free survival was 11.5 months with the combination, 6.9 months with nivolumab alone, and 2.9 months with ipilimumab.
The new analysis also evaluated melanoma-specific survival (MSS), which removes competing causes of deaths from the long-term follow-up. The MSS was not reached in the combination group, and was 58.7 months in the nivolumab group and 21.9 months for ipilimumab, with MSS rates at 6.5 years of 56%, 48%, and 27%, respectively.
No new safety signals were detected, but there was more immune-mediated toxicity in the combination group, the researchers reported.
“The patients will continue to be followed,” said Dr. Wolchok, “And data are still being collected.”
The trial was supported by Bristol-Myers Squibb, the National Cancer Institute, and the National Institute for Health Research Royal Marsden–Institute of Cancer Research Biomedical Research Centre. Dr. Wolchok and coauthors reported relationships with Bristol-Myers Squibb and other drugmakers.
A version of this article first appeared on Medscape.com.
An researchers say.
Nearly half the patients treated with nivolumab (Opdivo) and ipilimumab (Yervoy) were alive at 6½ years. Within this group, 77% had not received further systemic treatment after coming off the study drugs.
After a minimum follow-up of 77 months, median overall survival was 72.1 months in patients on the combination, which was more than three times longer than the 19.9 months with ipilimumab alone (hazard ratio, 0.52; 95% confidence interval, 0.43-0.64) and twice as long as the 36.9 months with nivolumab alone (HR, 0.84; 95% CI, 0.67-1.04).
The results represent the longest median overall survival seen in a phase 3 trial of advanced melanoma and are evidence of “a substantial development in the melanoma treatment landscape versus the standard median survival of 8 months a decade ago,” researchers wrote in a study published online in the Journal of Clinical Oncology.
However, lead author Jedd D. Wolchok, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, noted that the study was not designed to compare nivolumab alone with the combination. “It wasn’t powered for that. [But] what we can say is that the highest survival was in the combination group,” Dr. Wolchok told this news organization.
Dr. Wolchok cautioned that the combination therapy is not currently standard of care. “PD-1 blockade – either nivolumab or the combination – are both excellent options for care,” he added. “I can’t tell you that one of them is the standard of care because that’s too complex of a decision.”
For example, he explained, “for a patient who only has lung metastases, a single-agent PD-1 blockade might be sufficient. But if it has spread to other organs, such as the liver or bones, which are more difficult to treat, that’s when we often reach for the combination.”
Other factors that weigh into the therapeutic decision are the patient’s performance status and their so-called clinical reserve for tolerating side effects. “The likelihood of having a high-grade side effect with the combination is more than twice that of the single agent,” Dr. Wolchok said.
Until 2011, only two therapies were approved for metastatic melanoma: Chemotherapy with dacarbazine and immunotherapy with high-dose interleukin-2, neither of which was very effective at prolonging life. But patient survival changed with the advent of targeted therapies and immunotherapy. Some patients are now living for years, and as the current study shows, many have surpassed the 5-year mark and are treatment free.
The updated CheckMate 067 analysis included patients with previously untreated, unresectable stage III/IV melanoma who were randomly assigned to receive nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (four doses) followed by nivolumab 3 mg/kg every 2 weeks (n = 314), nivolumab 3 mg/kg every 2 weeks (n = 316), or ipilimumab 3 mg/ kg every 3 weeks (four doses; n = 315).
The authors reported the 5-year overall survival rates from the trial, published in the New England Journal of Medicine in 2019 – 52% with the combination, 44% with nivolumab alone, and 26% with ipilimumab alone.
In the updated study, overall survival at 6½ years had dropped slightly to 49%, 42%, and 23%, respectively. Patients with BRAF-mutant tumors had overall survival rates of 57%, 43%, and 25% versus 46%, 42%, and 22% in those with BRAF wild-type tumors.
Overall, median investigator-assessed progression-free survival was 11.5 months with the combination, 6.9 months with nivolumab alone, and 2.9 months with ipilimumab.
The new analysis also evaluated melanoma-specific survival (MSS), which removes competing causes of deaths from the long-term follow-up. The MSS was not reached in the combination group, and was 58.7 months in the nivolumab group and 21.9 months for ipilimumab, with MSS rates at 6.5 years of 56%, 48%, and 27%, respectively.
No new safety signals were detected, but there was more immune-mediated toxicity in the combination group, the researchers reported.
“The patients will continue to be followed,” said Dr. Wolchok, “And data are still being collected.”
The trial was supported by Bristol-Myers Squibb, the National Cancer Institute, and the National Institute for Health Research Royal Marsden–Institute of Cancer Research Biomedical Research Centre. Dr. Wolchok and coauthors reported relationships with Bristol-Myers Squibb and other drugmakers.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Sleep disorders and cancer: It’s complicated
Sleep apnea and other types of sleep disorders appear to elevate the risk for some types of cancer, specifically prostate cancer, more so than others. But the overall risk can be highly variable, and some sleep problems were found to be associated with a lower risk for cancer and cancer-related death, an analysis of a large observational cohort study of cardiovascular patients found.
Results of the analysis were published online in the journal Cancer Epidemiology. Investigators analyzed the presence of sleep apnea and insomnia and cancer risk in more than 8,500 patients in the Cardiovascular Health Study (CHS). “The fact that we observed certain sleep problems, like apneas, to be associated with elevated risk of some cancers but not others reflects the fact that cancer is a heterogeneous disease,” senior author Amanda Phipps, PhD, said in an interview. Dr. Phipps is an associate professor of epidemiology at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
Variable cancer links
The researchers assessed sleep problems in two groups in the CHS: an incident cancer group of 3,930 patients and a cancer mortality group of 4,580 patients. Within those respective groups, the investigators identified 885 first-incident cancers and 804 cancer deaths with a median follow-up of 12 and 14 years. The average age of the study population was 73 years, and 57% were women.
Sleep apnea symptoms (SAS) were associated with a lower risk for incident cancers – a 16% lower baseline risk and a 24% lower time-dependent risk. The study showed no association between cancer incidence and daytime sleepiness and apneas.
However, there was a significantly elevated risk relationship between sleep problems and prostate cancer. A time-dependent analysis of apnea showed more than double the risk (hazard ratio, 2.34), and baseline snoring carried a 69% greater risk. There was also a dose-response relationship for baseline cumulative SAS, compared with not having symptoms: an HR of 1.30 for one symptom, and 2.22 for two or more symptoms.
Risks for lymphatic or hematopoietic cancers were also associated with baseline daytime sleepiness (HR, 1.81), but not with insomnia (HR, 0.54).
With regard to cancer mortality, the study found no relationship between sleep problems and cancer death. In fact, it found an overall inverse relationship with snoring (time-dependent HR, 0.73; cumulative average HR, 0.67) and baseline apnea (HR, 0.69). Likewise, patients reporting SAS had lower risks than those having no SAS: an HR of 0.90 for one symptom and 0.75 for multiple symptoms. No relationships were found between any insomnia symptom and cancer death.
“We know the pathways that lead to prostate cancer can be very different than the pathways that lead to colorectal cancer,” Dr. Phipps said. “What we don’t yet understand is why these associations differ or what mechanisms are responsible for these cancer site-specific associations.”
Need for sleep assessment
The findings don’t change much for how clinicians should evaluate cancer risks in patients with sleep problems, Dr. Phipps said. “Other studies have clearly demonstrated the implications that sleep apnea has for a variety of other important health conditions – such as cardiovascular disease – so there are already plenty of good reasons for clinicians to ask their patients about their sleep and to connect patients with resources for the diagnosis and treatment of sleep apnea,” she added. “This study provides another possible reason.”
These findings provide context for future studies of the relationship between sleep problems and cancer. “But, given that sleep is something we all do and given that sleep problems are so pervasive, it’s important that we keep trying to better understand this relationship,” Dr. Phipps said.
“My hope is that future cancer studies will build in more detailed, longitudinal information on sleep patterns to help us fill current gaps in knowledge.”
Dr. Phipps has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sleep apnea and other types of sleep disorders appear to elevate the risk for some types of cancer, specifically prostate cancer, more so than others. But the overall risk can be highly variable, and some sleep problems were found to be associated with a lower risk for cancer and cancer-related death, an analysis of a large observational cohort study of cardiovascular patients found.
Results of the analysis were published online in the journal Cancer Epidemiology. Investigators analyzed the presence of sleep apnea and insomnia and cancer risk in more than 8,500 patients in the Cardiovascular Health Study (CHS). “The fact that we observed certain sleep problems, like apneas, to be associated with elevated risk of some cancers but not others reflects the fact that cancer is a heterogeneous disease,” senior author Amanda Phipps, PhD, said in an interview. Dr. Phipps is an associate professor of epidemiology at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
Variable cancer links
The researchers assessed sleep problems in two groups in the CHS: an incident cancer group of 3,930 patients and a cancer mortality group of 4,580 patients. Within those respective groups, the investigators identified 885 first-incident cancers and 804 cancer deaths with a median follow-up of 12 and 14 years. The average age of the study population was 73 years, and 57% were women.
Sleep apnea symptoms (SAS) were associated with a lower risk for incident cancers – a 16% lower baseline risk and a 24% lower time-dependent risk. The study showed no association between cancer incidence and daytime sleepiness and apneas.
However, there was a significantly elevated risk relationship between sleep problems and prostate cancer. A time-dependent analysis of apnea showed more than double the risk (hazard ratio, 2.34), and baseline snoring carried a 69% greater risk. There was also a dose-response relationship for baseline cumulative SAS, compared with not having symptoms: an HR of 1.30 for one symptom, and 2.22 for two or more symptoms.
Risks for lymphatic or hematopoietic cancers were also associated with baseline daytime sleepiness (HR, 1.81), but not with insomnia (HR, 0.54).
With regard to cancer mortality, the study found no relationship between sleep problems and cancer death. In fact, it found an overall inverse relationship with snoring (time-dependent HR, 0.73; cumulative average HR, 0.67) and baseline apnea (HR, 0.69). Likewise, patients reporting SAS had lower risks than those having no SAS: an HR of 0.90 for one symptom and 0.75 for multiple symptoms. No relationships were found between any insomnia symptom and cancer death.
“We know the pathways that lead to prostate cancer can be very different than the pathways that lead to colorectal cancer,” Dr. Phipps said. “What we don’t yet understand is why these associations differ or what mechanisms are responsible for these cancer site-specific associations.”
Need for sleep assessment
The findings don’t change much for how clinicians should evaluate cancer risks in patients with sleep problems, Dr. Phipps said. “Other studies have clearly demonstrated the implications that sleep apnea has for a variety of other important health conditions – such as cardiovascular disease – so there are already plenty of good reasons for clinicians to ask their patients about their sleep and to connect patients with resources for the diagnosis and treatment of sleep apnea,” she added. “This study provides another possible reason.”
These findings provide context for future studies of the relationship between sleep problems and cancer. “But, given that sleep is something we all do and given that sleep problems are so pervasive, it’s important that we keep trying to better understand this relationship,” Dr. Phipps said.
“My hope is that future cancer studies will build in more detailed, longitudinal information on sleep patterns to help us fill current gaps in knowledge.”
Dr. Phipps has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sleep apnea and other types of sleep disorders appear to elevate the risk for some types of cancer, specifically prostate cancer, more so than others. But the overall risk can be highly variable, and some sleep problems were found to be associated with a lower risk for cancer and cancer-related death, an analysis of a large observational cohort study of cardiovascular patients found.
Results of the analysis were published online in the journal Cancer Epidemiology. Investigators analyzed the presence of sleep apnea and insomnia and cancer risk in more than 8,500 patients in the Cardiovascular Health Study (CHS). “The fact that we observed certain sleep problems, like apneas, to be associated with elevated risk of some cancers but not others reflects the fact that cancer is a heterogeneous disease,” senior author Amanda Phipps, PhD, said in an interview. Dr. Phipps is an associate professor of epidemiology at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
Variable cancer links
The researchers assessed sleep problems in two groups in the CHS: an incident cancer group of 3,930 patients and a cancer mortality group of 4,580 patients. Within those respective groups, the investigators identified 885 first-incident cancers and 804 cancer deaths with a median follow-up of 12 and 14 years. The average age of the study population was 73 years, and 57% were women.
Sleep apnea symptoms (SAS) were associated with a lower risk for incident cancers – a 16% lower baseline risk and a 24% lower time-dependent risk. The study showed no association between cancer incidence and daytime sleepiness and apneas.
However, there was a significantly elevated risk relationship between sleep problems and prostate cancer. A time-dependent analysis of apnea showed more than double the risk (hazard ratio, 2.34), and baseline snoring carried a 69% greater risk. There was also a dose-response relationship for baseline cumulative SAS, compared with not having symptoms: an HR of 1.30 for one symptom, and 2.22 for two or more symptoms.
Risks for lymphatic or hematopoietic cancers were also associated with baseline daytime sleepiness (HR, 1.81), but not with insomnia (HR, 0.54).
With regard to cancer mortality, the study found no relationship between sleep problems and cancer death. In fact, it found an overall inverse relationship with snoring (time-dependent HR, 0.73; cumulative average HR, 0.67) and baseline apnea (HR, 0.69). Likewise, patients reporting SAS had lower risks than those having no SAS: an HR of 0.90 for one symptom and 0.75 for multiple symptoms. No relationships were found between any insomnia symptom and cancer death.
“We know the pathways that lead to prostate cancer can be very different than the pathways that lead to colorectal cancer,” Dr. Phipps said. “What we don’t yet understand is why these associations differ or what mechanisms are responsible for these cancer site-specific associations.”
Need for sleep assessment
The findings don’t change much for how clinicians should evaluate cancer risks in patients with sleep problems, Dr. Phipps said. “Other studies have clearly demonstrated the implications that sleep apnea has for a variety of other important health conditions – such as cardiovascular disease – so there are already plenty of good reasons for clinicians to ask their patients about their sleep and to connect patients with resources for the diagnosis and treatment of sleep apnea,” she added. “This study provides another possible reason.”
These findings provide context for future studies of the relationship between sleep problems and cancer. “But, given that sleep is something we all do and given that sleep problems are so pervasive, it’s important that we keep trying to better understand this relationship,” Dr. Phipps said.
“My hope is that future cancer studies will build in more detailed, longitudinal information on sleep patterns to help us fill current gaps in knowledge.”
Dr. Phipps has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANCER EPIDEMIOLOGY
Large analysis confirms safety of nipple-sparing mastectomy
A new analysis of over 22,000 mastectomy patients confirms what smaller studies have indicated: Patients who undergo nipple-sparing mastectomy have overall and disease-free survival similar to that of those who receive a total mastectomy.
When nipple-sparing mastectomy was introduced, many experts felt uneasy about opting for the less invasive procedure, recalled Rosa Hwang, MD, associate medical director for breast surgery at MD Anderson Cancer Center in Houston. “The concern was leaving all this skin,” said Dr. Hwang. “Are you going to leave cancer behind” and increase the risk of local recurrence?
Over the past 2 decades, the number of patients undergoing nipple-sparing mastectomy increased and, in turn, studies began to demonstrate the safety of the procedure.
However, large analyses evaluating long-term outcomes – namely, overall survival and breast cancer-specific survival – of nipple-sparing mastectomy were still lacking.
The latest study, published online Nov. 20 in Annals of Surgical Oncology, compared the long-term prognosis and survival benefits of nipple-sparing to total mastectomy in thousands of women. The analysis, which pulled data from the SEER cancer database, included 5,765 patients who underwent the nipple-sparing procedure and 17,289 patients who had a total mastectomy.
The authors found that overall survival and breast cancer–specific survival were similar for women undergoing nipple-sparing mastectomy and those receiving a total mastectomy. In fact, over the long-term, the nipple-sparing group slightly edged out the total mastectomy group in overall survival (94.61% vs. 93% at 5 years and 86.34% vs. 83.48% at 10 years, respectively) and in breast cancer-specific survival rates (96.16% vs. 95.74% at 5 years, and 92.2% vs. 91.37% at 10 years). The differences, however, were not significant.
The study also found that certain subgroups – including White women, women over age 46, those with a median household income of $70,000 or more, hormone receptor-positive, and HER2 negative – had significantly better overall survival rate with the nipple-sparing procedure (P < .05). However, the authors noted, the survival advantage in the nipple-sparing group did not extend to breast cancer–specific survival.
Dr. Hwang, who was not involved in the current analysis, said the significant overall survival result in the subgroup analysis was surprising because “there’s no biological reason why one would expect that to be true.”
Given that the subgroups did not demonstrate better breast cancer–specific survival, Dr. Hwang believes the overall survival finding may have more to do with comorbidities, which the study did not account for, than type of mastectomy.
When choosing who is eligible for a nipple-sparing mastectomy, “We’re more selective,” Dr. Hwang said. For instance, patients with uncontrolled diabetes or who smoke are unlikely to be candidates. “So, I think it’s possible that medical comorbidities and medical conditions between these groups [were] different.”
According to the authors, coding inconsistencies represent another possible weakness of the study. From 1998 to 2010, “the term ‘nipple-sparing mastectomy’ was coded as a [total mastectomy] with the ‘subcutaneous mastectomy’ code.” It’s possible that some patients receiving the nipple-sparing procedure before 2011 were not appropriately coded in the current study.
Moving forward, a large prospective study that includes comorbidities would be helpful, but overall the study helps validate that “nipple-sparing mastectomy is a safe operation for selected patients,” Dr. Hwang said.
A version of this article first appeared on Medscape.com.
A new analysis of over 22,000 mastectomy patients confirms what smaller studies have indicated: Patients who undergo nipple-sparing mastectomy have overall and disease-free survival similar to that of those who receive a total mastectomy.
When nipple-sparing mastectomy was introduced, many experts felt uneasy about opting for the less invasive procedure, recalled Rosa Hwang, MD, associate medical director for breast surgery at MD Anderson Cancer Center in Houston. “The concern was leaving all this skin,” said Dr. Hwang. “Are you going to leave cancer behind” and increase the risk of local recurrence?
Over the past 2 decades, the number of patients undergoing nipple-sparing mastectomy increased and, in turn, studies began to demonstrate the safety of the procedure.
However, large analyses evaluating long-term outcomes – namely, overall survival and breast cancer-specific survival – of nipple-sparing mastectomy were still lacking.
The latest study, published online Nov. 20 in Annals of Surgical Oncology, compared the long-term prognosis and survival benefits of nipple-sparing to total mastectomy in thousands of women. The analysis, which pulled data from the SEER cancer database, included 5,765 patients who underwent the nipple-sparing procedure and 17,289 patients who had a total mastectomy.
The authors found that overall survival and breast cancer–specific survival were similar for women undergoing nipple-sparing mastectomy and those receiving a total mastectomy. In fact, over the long-term, the nipple-sparing group slightly edged out the total mastectomy group in overall survival (94.61% vs. 93% at 5 years and 86.34% vs. 83.48% at 10 years, respectively) and in breast cancer-specific survival rates (96.16% vs. 95.74% at 5 years, and 92.2% vs. 91.37% at 10 years). The differences, however, were not significant.
The study also found that certain subgroups – including White women, women over age 46, those with a median household income of $70,000 or more, hormone receptor-positive, and HER2 negative – had significantly better overall survival rate with the nipple-sparing procedure (P < .05). However, the authors noted, the survival advantage in the nipple-sparing group did not extend to breast cancer–specific survival.
Dr. Hwang, who was not involved in the current analysis, said the significant overall survival result in the subgroup analysis was surprising because “there’s no biological reason why one would expect that to be true.”
Given that the subgroups did not demonstrate better breast cancer–specific survival, Dr. Hwang believes the overall survival finding may have more to do with comorbidities, which the study did not account for, than type of mastectomy.
When choosing who is eligible for a nipple-sparing mastectomy, “We’re more selective,” Dr. Hwang said. For instance, patients with uncontrolled diabetes or who smoke are unlikely to be candidates. “So, I think it’s possible that medical comorbidities and medical conditions between these groups [were] different.”
According to the authors, coding inconsistencies represent another possible weakness of the study. From 1998 to 2010, “the term ‘nipple-sparing mastectomy’ was coded as a [total mastectomy] with the ‘subcutaneous mastectomy’ code.” It’s possible that some patients receiving the nipple-sparing procedure before 2011 were not appropriately coded in the current study.
Moving forward, a large prospective study that includes comorbidities would be helpful, but overall the study helps validate that “nipple-sparing mastectomy is a safe operation for selected patients,” Dr. Hwang said.
A version of this article first appeared on Medscape.com.
A new analysis of over 22,000 mastectomy patients confirms what smaller studies have indicated: Patients who undergo nipple-sparing mastectomy have overall and disease-free survival similar to that of those who receive a total mastectomy.
When nipple-sparing mastectomy was introduced, many experts felt uneasy about opting for the less invasive procedure, recalled Rosa Hwang, MD, associate medical director for breast surgery at MD Anderson Cancer Center in Houston. “The concern was leaving all this skin,” said Dr. Hwang. “Are you going to leave cancer behind” and increase the risk of local recurrence?
Over the past 2 decades, the number of patients undergoing nipple-sparing mastectomy increased and, in turn, studies began to demonstrate the safety of the procedure.
However, large analyses evaluating long-term outcomes – namely, overall survival and breast cancer-specific survival – of nipple-sparing mastectomy were still lacking.
The latest study, published online Nov. 20 in Annals of Surgical Oncology, compared the long-term prognosis and survival benefits of nipple-sparing to total mastectomy in thousands of women. The analysis, which pulled data from the SEER cancer database, included 5,765 patients who underwent the nipple-sparing procedure and 17,289 patients who had a total mastectomy.
The authors found that overall survival and breast cancer–specific survival were similar for women undergoing nipple-sparing mastectomy and those receiving a total mastectomy. In fact, over the long-term, the nipple-sparing group slightly edged out the total mastectomy group in overall survival (94.61% vs. 93% at 5 years and 86.34% vs. 83.48% at 10 years, respectively) and in breast cancer-specific survival rates (96.16% vs. 95.74% at 5 years, and 92.2% vs. 91.37% at 10 years). The differences, however, were not significant.
The study also found that certain subgroups – including White women, women over age 46, those with a median household income of $70,000 or more, hormone receptor-positive, and HER2 negative – had significantly better overall survival rate with the nipple-sparing procedure (P < .05). However, the authors noted, the survival advantage in the nipple-sparing group did not extend to breast cancer–specific survival.
Dr. Hwang, who was not involved in the current analysis, said the significant overall survival result in the subgroup analysis was surprising because “there’s no biological reason why one would expect that to be true.”
Given that the subgroups did not demonstrate better breast cancer–specific survival, Dr. Hwang believes the overall survival finding may have more to do with comorbidities, which the study did not account for, than type of mastectomy.
When choosing who is eligible for a nipple-sparing mastectomy, “We’re more selective,” Dr. Hwang said. For instance, patients with uncontrolled diabetes or who smoke are unlikely to be candidates. “So, I think it’s possible that medical comorbidities and medical conditions between these groups [were] different.”
According to the authors, coding inconsistencies represent another possible weakness of the study. From 1998 to 2010, “the term ‘nipple-sparing mastectomy’ was coded as a [total mastectomy] with the ‘subcutaneous mastectomy’ code.” It’s possible that some patients receiving the nipple-sparing procedure before 2011 were not appropriately coded in the current study.
Moving forward, a large prospective study that includes comorbidities would be helpful, but overall the study helps validate that “nipple-sparing mastectomy is a safe operation for selected patients,” Dr. Hwang said.
A version of this article first appeared on Medscape.com.
IUDs may increase background enhancement on breast MRI
Intrauterine contraceptive devices (IUDs) have been linked to increased background enhancement on breast MRI, according to research presented at the Radiological Society of North America 2021 annual meeting.
About 10.4% of women 15-49 years of age who use contraception have an IUD or contraceptive implant, according to the Centers for Disease Control and Prevention. Unlike oral or transdermal hormonal contraceptives and hormone replacement therapy, levonorgestrel-releasing IUDs release a small amount of the hormone directly into the uterus and are thought to have a much more localized effect, Luisa Huck, MD, the lead author of the study, said in an interview.
But women with IUDs have long reported adverse effects associated with other hormonal medication. “In the past, some women reported depression, headaches, sleep disorders, and panic attacks,” noted Dr. Huck, a radiology resident at RWTH Aachen University in Germany.
Christiane Kuhl, MD, chief of the department of radiology at RWTH Aachen University and senior author of the research, had also observed that women with hormonal IUDs often have increased background parenchymal enhancement (BPE) on contrast-enhanced MRI. BPE “has been established as a sensitive marker of hormonal stimulation of breast,” the study authors wrote, and previous studies have shown that women using hormonal medications have higher BPE on breast MRIs.
To better understand whether IUDs can increase BPE, Dr. Huck and colleagues used the hospital database to search for premenopausal women who had undergone breast MRIs for screening between January 2014 and July 2020. To be included, women had to have had at least two scans: one with and one without an IUD in place, with the scan conducted at least 4 weeks after IUD placement or removal. All women in the study had no history of breast cancer or hormone or antihormone intake.
The study involved 48 women with an average age of 45 years and a median of 27 months between the two scans. Forty-six of the women had the Mirena levonorgestrel-releasing IUD and two had the Jaydess IUD. To account for hormone variations between patients, the researchers used each patient as their own reference point. To control for age-related effects, 25 women had their first MRI without an IUD and their second scan with an IUD in place. The second group of 23 women underwent their first MRI with an IUD and had it removed before the second scan.
Hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging.
For 23 women in the study, background enhancement was higher on scans with the IUD than without (P < .001). For 24 women, there was no change in BPE with or without an IUD, and one woman had lower BPE with an IUD than without.
“It is very interesting and relevant to practice to consider that the presence of an intrauterine device would have potential impact on the enhancement we see in the breast on MRI imaging,” Samantha Heller, MD, PhD, associate professor of radiology at New York University, said in an interview.
However, the study used BPE as a measure for hormonal shifts, and “hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging,” she noted. BPE on MRI can fluctuate, so testing actual hormone levels in patients with elevated BPE could be helpful to identify hormonal shifts, she added. It is also important to understand why half of the women in the study showed no variation in BPE, she said.
The study findings are not very surprising, considering that it is known that low levels of progesterone from IUDs circulate in the blood stream, Frances Casey, MD, MPH, associate professor in the department of obstetrics and gynecology at Virginia Commonwealth University in Richmond, said in an interview. They do not suggest that there should be any changes to IUD guidelines, she added.
However, “the study findings raise the question as to whether IUD status should be documented as a matter of course prior to performing breast MRI,” said Dr. Heller. “It is standard to document the timing of a woman’s menstrual cycle, as well as to note any hormone suppression or replacement therapy. This is in part so that the radiologist may understand the etiology of any observed variation in background enhancement,” she explained.
Although increased enhancement on MRI has sometimes been linked to higher chances of recommendations for additional imaging or biopsies, she noted, “more work would be needed to understand the impact – if any – of an IUD on breast MRI recommendations due to enhancement changes.”
Dr. Huck, Dr. Heller, and Dr. Casey disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Intrauterine contraceptive devices (IUDs) have been linked to increased background enhancement on breast MRI, according to research presented at the Radiological Society of North America 2021 annual meeting.
About 10.4% of women 15-49 years of age who use contraception have an IUD or contraceptive implant, according to the Centers for Disease Control and Prevention. Unlike oral or transdermal hormonal contraceptives and hormone replacement therapy, levonorgestrel-releasing IUDs release a small amount of the hormone directly into the uterus and are thought to have a much more localized effect, Luisa Huck, MD, the lead author of the study, said in an interview.
But women with IUDs have long reported adverse effects associated with other hormonal medication. “In the past, some women reported depression, headaches, sleep disorders, and panic attacks,” noted Dr. Huck, a radiology resident at RWTH Aachen University in Germany.
Christiane Kuhl, MD, chief of the department of radiology at RWTH Aachen University and senior author of the research, had also observed that women with hormonal IUDs often have increased background parenchymal enhancement (BPE) on contrast-enhanced MRI. BPE “has been established as a sensitive marker of hormonal stimulation of breast,” the study authors wrote, and previous studies have shown that women using hormonal medications have higher BPE on breast MRIs.
To better understand whether IUDs can increase BPE, Dr. Huck and colleagues used the hospital database to search for premenopausal women who had undergone breast MRIs for screening between January 2014 and July 2020. To be included, women had to have had at least two scans: one with and one without an IUD in place, with the scan conducted at least 4 weeks after IUD placement or removal. All women in the study had no history of breast cancer or hormone or antihormone intake.
The study involved 48 women with an average age of 45 years and a median of 27 months between the two scans. Forty-six of the women had the Mirena levonorgestrel-releasing IUD and two had the Jaydess IUD. To account for hormone variations between patients, the researchers used each patient as their own reference point. To control for age-related effects, 25 women had their first MRI without an IUD and their second scan with an IUD in place. The second group of 23 women underwent their first MRI with an IUD and had it removed before the second scan.
Hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging.
For 23 women in the study, background enhancement was higher on scans with the IUD than without (P < .001). For 24 women, there was no change in BPE with or without an IUD, and one woman had lower BPE with an IUD than without.
“It is very interesting and relevant to practice to consider that the presence of an intrauterine device would have potential impact on the enhancement we see in the breast on MRI imaging,” Samantha Heller, MD, PhD, associate professor of radiology at New York University, said in an interview.
However, the study used BPE as a measure for hormonal shifts, and “hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging,” she noted. BPE on MRI can fluctuate, so testing actual hormone levels in patients with elevated BPE could be helpful to identify hormonal shifts, she added. It is also important to understand why half of the women in the study showed no variation in BPE, she said.
The study findings are not very surprising, considering that it is known that low levels of progesterone from IUDs circulate in the blood stream, Frances Casey, MD, MPH, associate professor in the department of obstetrics and gynecology at Virginia Commonwealth University in Richmond, said in an interview. They do not suggest that there should be any changes to IUD guidelines, she added.
However, “the study findings raise the question as to whether IUD status should be documented as a matter of course prior to performing breast MRI,” said Dr. Heller. “It is standard to document the timing of a woman’s menstrual cycle, as well as to note any hormone suppression or replacement therapy. This is in part so that the radiologist may understand the etiology of any observed variation in background enhancement,” she explained.
Although increased enhancement on MRI has sometimes been linked to higher chances of recommendations for additional imaging or biopsies, she noted, “more work would be needed to understand the impact – if any – of an IUD on breast MRI recommendations due to enhancement changes.”
Dr. Huck, Dr. Heller, and Dr. Casey disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Intrauterine contraceptive devices (IUDs) have been linked to increased background enhancement on breast MRI, according to research presented at the Radiological Society of North America 2021 annual meeting.
About 10.4% of women 15-49 years of age who use contraception have an IUD or contraceptive implant, according to the Centers for Disease Control and Prevention. Unlike oral or transdermal hormonal contraceptives and hormone replacement therapy, levonorgestrel-releasing IUDs release a small amount of the hormone directly into the uterus and are thought to have a much more localized effect, Luisa Huck, MD, the lead author of the study, said in an interview.
But women with IUDs have long reported adverse effects associated with other hormonal medication. “In the past, some women reported depression, headaches, sleep disorders, and panic attacks,” noted Dr. Huck, a radiology resident at RWTH Aachen University in Germany.
Christiane Kuhl, MD, chief of the department of radiology at RWTH Aachen University and senior author of the research, had also observed that women with hormonal IUDs often have increased background parenchymal enhancement (BPE) on contrast-enhanced MRI. BPE “has been established as a sensitive marker of hormonal stimulation of breast,” the study authors wrote, and previous studies have shown that women using hormonal medications have higher BPE on breast MRIs.
To better understand whether IUDs can increase BPE, Dr. Huck and colleagues used the hospital database to search for premenopausal women who had undergone breast MRIs for screening between January 2014 and July 2020. To be included, women had to have had at least two scans: one with and one without an IUD in place, with the scan conducted at least 4 weeks after IUD placement or removal. All women in the study had no history of breast cancer or hormone or antihormone intake.
The study involved 48 women with an average age of 45 years and a median of 27 months between the two scans. Forty-six of the women had the Mirena levonorgestrel-releasing IUD and two had the Jaydess IUD. To account for hormone variations between patients, the researchers used each patient as their own reference point. To control for age-related effects, 25 women had their first MRI without an IUD and their second scan with an IUD in place. The second group of 23 women underwent their first MRI with an IUD and had it removed before the second scan.
Hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging.
For 23 women in the study, background enhancement was higher on scans with the IUD than without (P < .001). For 24 women, there was no change in BPE with or without an IUD, and one woman had lower BPE with an IUD than without.
“It is very interesting and relevant to practice to consider that the presence of an intrauterine device would have potential impact on the enhancement we see in the breast on MRI imaging,” Samantha Heller, MD, PhD, associate professor of radiology at New York University, said in an interview.
However, the study used BPE as a measure for hormonal shifts, and “hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging,” she noted. BPE on MRI can fluctuate, so testing actual hormone levels in patients with elevated BPE could be helpful to identify hormonal shifts, she added. It is also important to understand why half of the women in the study showed no variation in BPE, she said.
The study findings are not very surprising, considering that it is known that low levels of progesterone from IUDs circulate in the blood stream, Frances Casey, MD, MPH, associate professor in the department of obstetrics and gynecology at Virginia Commonwealth University in Richmond, said in an interview. They do not suggest that there should be any changes to IUD guidelines, she added.
However, “the study findings raise the question as to whether IUD status should be documented as a matter of course prior to performing breast MRI,” said Dr. Heller. “It is standard to document the timing of a woman’s menstrual cycle, as well as to note any hormone suppression or replacement therapy. This is in part so that the radiologist may understand the etiology of any observed variation in background enhancement,” she explained.
Although increased enhancement on MRI has sometimes been linked to higher chances of recommendations for additional imaging or biopsies, she noted, “more work would be needed to understand the impact – if any – of an IUD on breast MRI recommendations due to enhancement changes.”
Dr. Huck, Dr. Heller, and Dr. Casey disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Can aspirin prolong survival in patients with NSCLC?
(NSCLC), according to a new study from Taiwan.
The analysis, published online Nov. 22 in BMC Cancer , adds another data point to a small and inconsistent evidence base.
“Despite the need for future prospective randomized clinical trials, aspirin may be considered as an additional treatment for inoperable NSCLC patients,” Ming-Szu Hung, MD, of Chang-Gung University, Taoyuan City, and colleagues write.
The current literature suggests that the over-the-counter medication may help ward off various types of cancer, including lung cancer, but the various study findings do not always align. For lung-cancer survival, in particular, a few observational studies have found increased survival among aspirin users while others have not.
To help bring clarity to the literature, Dr. Hung’s team examined data from Taiwan’s National Health Insurance Research Database on more than 38,000 patients diagnosed with NSCLC between 2000 and 2012, almost 5,000 of whom were taking aspirin at the time of diagnosis.
The researchers found that aspirin users survived for a median of 1.73 years, compared with 1.30 years for nonusers. Taking the drug was associated with longer overall survival in time-varying covariate analysis (hazard ratio, 0.83; 95% CI, 0.80-0.86). This finding was confirmed in a propensity-score analysis of 4,932 matched pairs (HR, 0.79; 95% CI, 0.75-0.83).
“These results warrant further randomized clinical trials to evaluate the actual role of aspirin in the treatment of NSCLC patients,” the researchers conclude.
But Úna McMenamin, PhD, a cancer epidemiologist at Queen’s University Belfast, Ireland, was not convinced by the study’s methods.
While she praised its large size and use of population-based health registers, she expressed concern about the potential for reverse causation, “as it is unclear whether authors lagged the aspirin exposure in the cohort of lung cancer patients.”
There is evidence that common medications such as aspirin may be withdrawn from patients who are thought to be near the end of their life, Dr. McMenamin told this news organization. When not factored into the statistical analysis, aspirin may appear “to be spuriously associated with a reduced risk of death when, in fact, no association may be present.”
Previous studies of aspirin use in lung cancer patients that have included a lag, such as one Dr. McMenamin and colleagues conducted in 2015, have found no evidence of a protective effect.
That is why, according to Dr. McMenamin, “additional population-based studies, in diverse populations, are required to investigate the association between aspirin use and survival outcomes in lung-cancer patients to determine whether randomized controlled trials are warranted in this patient group.”
In addition, she noted, “any potential benefit of aspirin in lung cancer patients needs to be balanced against known adverse events associated with prolonged aspirin use, such as gastrointestinal bleeding.”
Dr. Hung did not reply to requests for comment.
The study had no funding, and the researchers report no conflicts of interest.
A version of this article first appeared on Medscape.com.
(NSCLC), according to a new study from Taiwan.
The analysis, published online Nov. 22 in BMC Cancer , adds another data point to a small and inconsistent evidence base.
“Despite the need for future prospective randomized clinical trials, aspirin may be considered as an additional treatment for inoperable NSCLC patients,” Ming-Szu Hung, MD, of Chang-Gung University, Taoyuan City, and colleagues write.
The current literature suggests that the over-the-counter medication may help ward off various types of cancer, including lung cancer, but the various study findings do not always align. For lung-cancer survival, in particular, a few observational studies have found increased survival among aspirin users while others have not.
To help bring clarity to the literature, Dr. Hung’s team examined data from Taiwan’s National Health Insurance Research Database on more than 38,000 patients diagnosed with NSCLC between 2000 and 2012, almost 5,000 of whom were taking aspirin at the time of diagnosis.
The researchers found that aspirin users survived for a median of 1.73 years, compared with 1.30 years for nonusers. Taking the drug was associated with longer overall survival in time-varying covariate analysis (hazard ratio, 0.83; 95% CI, 0.80-0.86). This finding was confirmed in a propensity-score analysis of 4,932 matched pairs (HR, 0.79; 95% CI, 0.75-0.83).
“These results warrant further randomized clinical trials to evaluate the actual role of aspirin in the treatment of NSCLC patients,” the researchers conclude.
But Úna McMenamin, PhD, a cancer epidemiologist at Queen’s University Belfast, Ireland, was not convinced by the study’s methods.
While she praised its large size and use of population-based health registers, she expressed concern about the potential for reverse causation, “as it is unclear whether authors lagged the aspirin exposure in the cohort of lung cancer patients.”
There is evidence that common medications such as aspirin may be withdrawn from patients who are thought to be near the end of their life, Dr. McMenamin told this news organization. When not factored into the statistical analysis, aspirin may appear “to be spuriously associated with a reduced risk of death when, in fact, no association may be present.”
Previous studies of aspirin use in lung cancer patients that have included a lag, such as one Dr. McMenamin and colleagues conducted in 2015, have found no evidence of a protective effect.
That is why, according to Dr. McMenamin, “additional population-based studies, in diverse populations, are required to investigate the association between aspirin use and survival outcomes in lung-cancer patients to determine whether randomized controlled trials are warranted in this patient group.”
In addition, she noted, “any potential benefit of aspirin in lung cancer patients needs to be balanced against known adverse events associated with prolonged aspirin use, such as gastrointestinal bleeding.”
Dr. Hung did not reply to requests for comment.
The study had no funding, and the researchers report no conflicts of interest.
A version of this article first appeared on Medscape.com.
(NSCLC), according to a new study from Taiwan.
The analysis, published online Nov. 22 in BMC Cancer , adds another data point to a small and inconsistent evidence base.
“Despite the need for future prospective randomized clinical trials, aspirin may be considered as an additional treatment for inoperable NSCLC patients,” Ming-Szu Hung, MD, of Chang-Gung University, Taoyuan City, and colleagues write.
The current literature suggests that the over-the-counter medication may help ward off various types of cancer, including lung cancer, but the various study findings do not always align. For lung-cancer survival, in particular, a few observational studies have found increased survival among aspirin users while others have not.
To help bring clarity to the literature, Dr. Hung’s team examined data from Taiwan’s National Health Insurance Research Database on more than 38,000 patients diagnosed with NSCLC between 2000 and 2012, almost 5,000 of whom were taking aspirin at the time of diagnosis.
The researchers found that aspirin users survived for a median of 1.73 years, compared with 1.30 years for nonusers. Taking the drug was associated with longer overall survival in time-varying covariate analysis (hazard ratio, 0.83; 95% CI, 0.80-0.86). This finding was confirmed in a propensity-score analysis of 4,932 matched pairs (HR, 0.79; 95% CI, 0.75-0.83).
“These results warrant further randomized clinical trials to evaluate the actual role of aspirin in the treatment of NSCLC patients,” the researchers conclude.
But Úna McMenamin, PhD, a cancer epidemiologist at Queen’s University Belfast, Ireland, was not convinced by the study’s methods.
While she praised its large size and use of population-based health registers, she expressed concern about the potential for reverse causation, “as it is unclear whether authors lagged the aspirin exposure in the cohort of lung cancer patients.”
There is evidence that common medications such as aspirin may be withdrawn from patients who are thought to be near the end of their life, Dr. McMenamin told this news organization. When not factored into the statistical analysis, aspirin may appear “to be spuriously associated with a reduced risk of death when, in fact, no association may be present.”
Previous studies of aspirin use in lung cancer patients that have included a lag, such as one Dr. McMenamin and colleagues conducted in 2015, have found no evidence of a protective effect.
That is why, according to Dr. McMenamin, “additional population-based studies, in diverse populations, are required to investigate the association between aspirin use and survival outcomes in lung-cancer patients to determine whether randomized controlled trials are warranted in this patient group.”
In addition, she noted, “any potential benefit of aspirin in lung cancer patients needs to be balanced against known adverse events associated with prolonged aspirin use, such as gastrointestinal bleeding.”
Dr. Hung did not reply to requests for comment.
The study had no funding, and the researchers report no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM BMC CANCER
What’s hot at the world’s premiere breast cancer meeting
Dr. Arteaga, the meeting’s codirector, said the first-ever hybrid symposium will take place virtually from Dec. 7 to 10 as well as in person. Online availability appears to be a boon to attendance, with a record 9,325 registrants for the 2020 symposium, held only virtually because of the COVID-19 pandemic.
The meeting will have an app available, which can be accessed by searching “San Antonio Breast Cancer Symposium” (Google Play for Android, Apple for iOS) and downloading, or by going to www.core-apps.com/dl/sabcs from a desktop computer.
Dr. Arteaga provided a sneak peek of the most exciting research being presented at the upcoming meeting.
On the horizon for advanced breast cancer
A “very important” study of an investigational oral agent employed in heavily pretreated postmenopausal women with estrogen receptor–positive (ER+) advanced breast cancer headlines the meeting.
This international, multicenter trial could have “practice-changing implications,” Dr. Arteaga said in an interview.
The phase 3 EMERALD trial (abstract GS2-02) pits elacestrant, a selective estrogen receptor degrader (SERD), against standard endocrine therapy (fulvestrant or an aromatase inhibitor) in patients with metastatic breast cancer whose disease has progressed after treatment with at least one endocrine therapy and a CDK4/6 inhibitor.
The trial is important because many patients with breast cancer have estrogen receptor mutations, which are a “major mechanism of [drug] resistance” and thus progression on earlier therapy, Dr. Arteaga said.
Elacestrant is in good company among a plethora of oral SERDs under investigation in advanced breast cancer; however, currently, fulvestrant – which requires an intramuscular injection in the buttocks every month – is the only approved SERD.
“There’s plenty of preclinical data that suggest that these drugs [SERDs] may have activity against these mutant forms of the receptor, which occur in up to 40% of patients with advanced ER+ breast cancer,” he explained.
Researchers will present data on two primary outcome measures from the phase 3 trial: progression-free survival (PFS) based on mutations of the estrogen receptor 1 gene (ESR1-mut) and PFS in all subjects regardless of ESR1 status.
In addition to the EMERALD trial, PADA-1 (abstract GS3-05) is another important randomized, phase 3 trial focused on treating estrogen receptor mutations in patients with metastatic disease, said Dr. Arteaga.
The trial has enrolled patients with ER+ metastatic breast cancer who received an aromatase inhibitor (letrozole, anastrozole, or exemestane) and the CDK4/6 inhibitor palbociclib as first-line therapy.
In step 1 of the trial, approximately 1,000 patients were screened for circulating blood ESR1 mutation detection at regular intervals while being treated with palbociclib and an aromatase inhibitor in a continuous scheme until tumor progression or ESR1 mutation detection.
In step 2, up to 200 patients with a rising circulating ESR1 mutation and no tumor progression were randomized 1:1 to no change in therapy until tumor progression or to receive palbociclib plus fulvestrant until tumor progression.
The trial examines the safety and efficacy of “a clinical conundrum that we face” in this setting: whether or not to switch treatment from an aromatase inhibitor to fulvestrant while continuing a CDK4/6 inhibitor at the sign of mutation detection, Dr. Arteaga explained.
Refining who gets the ‘kitchen sink’
Dr. Arteaga highlighted two trials focused on the immune checkpoint inhibitor pembrolizumab.
The phase 3 KEYNOTE-522 study led to the approval of neoadjuvant pembrolizumab plus chemotherapy for early-stage triple-negative breast cancer (TNBC) in July 2021. At this year’s SABCS, researchers will present new data from KEYNOTE-522 (abstract GS1-01), representing final results from the trial’s event-free survival (EFS) outcome.
Previously, investigators reported a statistically significant and clinically meaningful improvement in EFS. These data suggest “that deploying immunotherapy early before surgery ... may be curative in some patients,” Dr. Arteaga said. The new data will allow the “robustness and consistency” of the earlier findings to be assessed.
But, he added, this is a “tough” treatment, which includes five drugs. “It’s the kitchen sink, and not everybody needs the kitchen sink. It’s important to refine these findings. Some patients may not need pembrolizumab, but some do.”
The second trial exploring pembrolizumab – KEYNOTE-355 (abstract GS1-02) – mirrors KEYNOTE-522 but in patients with previously untreated locally recurrent inoperable or metastatic TNBC whose tumors expressed PD-L1.
Previously, investigators reported that pembrolizumab combined with chemotherapy showed statistically significant improvements in overall survival and PFS compared to placebo plus chemotherapy. At the 2021 SABCS, researchers will provide final study results, including outcomes in subgroups of patients by additional combined positive score cutoffs.
Metformin trial: ‘This is it’
Dr. Arteaga highlighted CCTGMA.32 (abstract GS1-08), a phase 3 randomized, placebo-controlled adjuvant trial of the diabetes drug metformin versus placebo in early breast cancer. Results of the primary efficacy analysis of the trial will be presented at the meeting.
The Canadian-led study seeks to determine if metformin can decrease breast cancer cell growth and work with cancer therapies to prevent disease recurrence. The study design calls for patients to take twice-daily oral metformin or placebo pills for up to 5 years in the absence of disease progression.
The primary outcome of the 3,500-plus patient trial is invasive disease-free survival in hormone receptor (ER and PgR) negative and positive (ER and/or PgR) subgroups.
“Metformin has actually been associated with improved survival [in breast cancer] in patients on chemotherapy. But we don’t know exactly how,” he said. “There’s never been a head-to-head comparison in the adjuvant setting [before]. This is it.”
TKI for breast cancer with brain mets
The SABCS codirector spotlighted an updated overall survival analysis of the randomized phase 3 PHOEBE trial (abstract GS3-02).
Previous research confirmed the superiority of pyrotinib, a novel TKI targeting HER1, HER2, and HER4, over lapatinib when given in combination with capecitabine in HER2-positive metastatic breast cancer.
In the United States, the lapatinib-capecitabine combination is “mostly used” in patients with HER2 metastatic disease and brain metastases who also undergo stereotactic radiation, Dr. Arteaga said.
This use has continued despite groundbreaking results from the HER2CLIMB trial, featuring the TKI tucatinib, he said.
As reported last year, adding tucatinib to trastuzumab and capecitabine in patients with HER2-positive breast cancer and brain metastases increased median overall survival from 12 months to 18.1 months. The results were called the first of their kind at that time.
The pyrotinib study may matter to American clinicians because pyrotinib is used mostly in China, not the United States, and this analysis suggests that pyrotinib could be part of the armamentarium in the United States, alongside tucatinib.
TKIs are like Coke and Pepsi, Dr. Arteaga said: “Similar but not identical.” Therefore, it is worth taking a look at the new study, he said. “There may be some benefit in having more than one [TKI] in the therapeutic armamentarium.”
Dr. Arteaga receives or has received grant support from Pfizer and Lilly and serves or has served in a scientific advisory role with Novartis, Lilly, TAIHO Oncology, Daiichi Sankyo, Merck, AstraZeneca, OrigiMed, Immunomedics, ARVINAS, Sanofi, Athenex, and the Susan G. Komen Foundation. He also holds minor stock options from Provista.
A version of this article first appeared on Medscape.com.
Dr. Arteaga, the meeting’s codirector, said the first-ever hybrid symposium will take place virtually from Dec. 7 to 10 as well as in person. Online availability appears to be a boon to attendance, with a record 9,325 registrants for the 2020 symposium, held only virtually because of the COVID-19 pandemic.
The meeting will have an app available, which can be accessed by searching “San Antonio Breast Cancer Symposium” (Google Play for Android, Apple for iOS) and downloading, or by going to www.core-apps.com/dl/sabcs from a desktop computer.
Dr. Arteaga provided a sneak peek of the most exciting research being presented at the upcoming meeting.
On the horizon for advanced breast cancer
A “very important” study of an investigational oral agent employed in heavily pretreated postmenopausal women with estrogen receptor–positive (ER+) advanced breast cancer headlines the meeting.
This international, multicenter trial could have “practice-changing implications,” Dr. Arteaga said in an interview.
The phase 3 EMERALD trial (abstract GS2-02) pits elacestrant, a selective estrogen receptor degrader (SERD), against standard endocrine therapy (fulvestrant or an aromatase inhibitor) in patients with metastatic breast cancer whose disease has progressed after treatment with at least one endocrine therapy and a CDK4/6 inhibitor.
The trial is important because many patients with breast cancer have estrogen receptor mutations, which are a “major mechanism of [drug] resistance” and thus progression on earlier therapy, Dr. Arteaga said.
Elacestrant is in good company among a plethora of oral SERDs under investigation in advanced breast cancer; however, currently, fulvestrant – which requires an intramuscular injection in the buttocks every month – is the only approved SERD.
“There’s plenty of preclinical data that suggest that these drugs [SERDs] may have activity against these mutant forms of the receptor, which occur in up to 40% of patients with advanced ER+ breast cancer,” he explained.
Researchers will present data on two primary outcome measures from the phase 3 trial: progression-free survival (PFS) based on mutations of the estrogen receptor 1 gene (ESR1-mut) and PFS in all subjects regardless of ESR1 status.
In addition to the EMERALD trial, PADA-1 (abstract GS3-05) is another important randomized, phase 3 trial focused on treating estrogen receptor mutations in patients with metastatic disease, said Dr. Arteaga.
The trial has enrolled patients with ER+ metastatic breast cancer who received an aromatase inhibitor (letrozole, anastrozole, or exemestane) and the CDK4/6 inhibitor palbociclib as first-line therapy.
In step 1 of the trial, approximately 1,000 patients were screened for circulating blood ESR1 mutation detection at regular intervals while being treated with palbociclib and an aromatase inhibitor in a continuous scheme until tumor progression or ESR1 mutation detection.
In step 2, up to 200 patients with a rising circulating ESR1 mutation and no tumor progression were randomized 1:1 to no change in therapy until tumor progression or to receive palbociclib plus fulvestrant until tumor progression.
The trial examines the safety and efficacy of “a clinical conundrum that we face” in this setting: whether or not to switch treatment from an aromatase inhibitor to fulvestrant while continuing a CDK4/6 inhibitor at the sign of mutation detection, Dr. Arteaga explained.
Refining who gets the ‘kitchen sink’
Dr. Arteaga highlighted two trials focused on the immune checkpoint inhibitor pembrolizumab.
The phase 3 KEYNOTE-522 study led to the approval of neoadjuvant pembrolizumab plus chemotherapy for early-stage triple-negative breast cancer (TNBC) in July 2021. At this year’s SABCS, researchers will present new data from KEYNOTE-522 (abstract GS1-01), representing final results from the trial’s event-free survival (EFS) outcome.
Previously, investigators reported a statistically significant and clinically meaningful improvement in EFS. These data suggest “that deploying immunotherapy early before surgery ... may be curative in some patients,” Dr. Arteaga said. The new data will allow the “robustness and consistency” of the earlier findings to be assessed.
But, he added, this is a “tough” treatment, which includes five drugs. “It’s the kitchen sink, and not everybody needs the kitchen sink. It’s important to refine these findings. Some patients may not need pembrolizumab, but some do.”
The second trial exploring pembrolizumab – KEYNOTE-355 (abstract GS1-02) – mirrors KEYNOTE-522 but in patients with previously untreated locally recurrent inoperable or metastatic TNBC whose tumors expressed PD-L1.
Previously, investigators reported that pembrolizumab combined with chemotherapy showed statistically significant improvements in overall survival and PFS compared to placebo plus chemotherapy. At the 2021 SABCS, researchers will provide final study results, including outcomes in subgroups of patients by additional combined positive score cutoffs.
Metformin trial: ‘This is it’
Dr. Arteaga highlighted CCTGMA.32 (abstract GS1-08), a phase 3 randomized, placebo-controlled adjuvant trial of the diabetes drug metformin versus placebo in early breast cancer. Results of the primary efficacy analysis of the trial will be presented at the meeting.
The Canadian-led study seeks to determine if metformin can decrease breast cancer cell growth and work with cancer therapies to prevent disease recurrence. The study design calls for patients to take twice-daily oral metformin or placebo pills for up to 5 years in the absence of disease progression.
The primary outcome of the 3,500-plus patient trial is invasive disease-free survival in hormone receptor (ER and PgR) negative and positive (ER and/or PgR) subgroups.
“Metformin has actually been associated with improved survival [in breast cancer] in patients on chemotherapy. But we don’t know exactly how,” he said. “There’s never been a head-to-head comparison in the adjuvant setting [before]. This is it.”
TKI for breast cancer with brain mets
The SABCS codirector spotlighted an updated overall survival analysis of the randomized phase 3 PHOEBE trial (abstract GS3-02).
Previous research confirmed the superiority of pyrotinib, a novel TKI targeting HER1, HER2, and HER4, over lapatinib when given in combination with capecitabine in HER2-positive metastatic breast cancer.
In the United States, the lapatinib-capecitabine combination is “mostly used” in patients with HER2 metastatic disease and brain metastases who also undergo stereotactic radiation, Dr. Arteaga said.
This use has continued despite groundbreaking results from the HER2CLIMB trial, featuring the TKI tucatinib, he said.
As reported last year, adding tucatinib to trastuzumab and capecitabine in patients with HER2-positive breast cancer and brain metastases increased median overall survival from 12 months to 18.1 months. The results were called the first of their kind at that time.
The pyrotinib study may matter to American clinicians because pyrotinib is used mostly in China, not the United States, and this analysis suggests that pyrotinib could be part of the armamentarium in the United States, alongside tucatinib.
TKIs are like Coke and Pepsi, Dr. Arteaga said: “Similar but not identical.” Therefore, it is worth taking a look at the new study, he said. “There may be some benefit in having more than one [TKI] in the therapeutic armamentarium.”
Dr. Arteaga receives or has received grant support from Pfizer and Lilly and serves or has served in a scientific advisory role with Novartis, Lilly, TAIHO Oncology, Daiichi Sankyo, Merck, AstraZeneca, OrigiMed, Immunomedics, ARVINAS, Sanofi, Athenex, and the Susan G. Komen Foundation. He also holds minor stock options from Provista.
A version of this article first appeared on Medscape.com.
Dr. Arteaga, the meeting’s codirector, said the first-ever hybrid symposium will take place virtually from Dec. 7 to 10 as well as in person. Online availability appears to be a boon to attendance, with a record 9,325 registrants for the 2020 symposium, held only virtually because of the COVID-19 pandemic.
The meeting will have an app available, which can be accessed by searching “San Antonio Breast Cancer Symposium” (Google Play for Android, Apple for iOS) and downloading, or by going to www.core-apps.com/dl/sabcs from a desktop computer.
Dr. Arteaga provided a sneak peek of the most exciting research being presented at the upcoming meeting.
On the horizon for advanced breast cancer
A “very important” study of an investigational oral agent employed in heavily pretreated postmenopausal women with estrogen receptor–positive (ER+) advanced breast cancer headlines the meeting.
This international, multicenter trial could have “practice-changing implications,” Dr. Arteaga said in an interview.
The phase 3 EMERALD trial (abstract GS2-02) pits elacestrant, a selective estrogen receptor degrader (SERD), against standard endocrine therapy (fulvestrant or an aromatase inhibitor) in patients with metastatic breast cancer whose disease has progressed after treatment with at least one endocrine therapy and a CDK4/6 inhibitor.
The trial is important because many patients with breast cancer have estrogen receptor mutations, which are a “major mechanism of [drug] resistance” and thus progression on earlier therapy, Dr. Arteaga said.
Elacestrant is in good company among a plethora of oral SERDs under investigation in advanced breast cancer; however, currently, fulvestrant – which requires an intramuscular injection in the buttocks every month – is the only approved SERD.
“There’s plenty of preclinical data that suggest that these drugs [SERDs] may have activity against these mutant forms of the receptor, which occur in up to 40% of patients with advanced ER+ breast cancer,” he explained.
Researchers will present data on two primary outcome measures from the phase 3 trial: progression-free survival (PFS) based on mutations of the estrogen receptor 1 gene (ESR1-mut) and PFS in all subjects regardless of ESR1 status.
In addition to the EMERALD trial, PADA-1 (abstract GS3-05) is another important randomized, phase 3 trial focused on treating estrogen receptor mutations in patients with metastatic disease, said Dr. Arteaga.
The trial has enrolled patients with ER+ metastatic breast cancer who received an aromatase inhibitor (letrozole, anastrozole, or exemestane) and the CDK4/6 inhibitor palbociclib as first-line therapy.
In step 1 of the trial, approximately 1,000 patients were screened for circulating blood ESR1 mutation detection at regular intervals while being treated with palbociclib and an aromatase inhibitor in a continuous scheme until tumor progression or ESR1 mutation detection.
In step 2, up to 200 patients with a rising circulating ESR1 mutation and no tumor progression were randomized 1:1 to no change in therapy until tumor progression or to receive palbociclib plus fulvestrant until tumor progression.
The trial examines the safety and efficacy of “a clinical conundrum that we face” in this setting: whether or not to switch treatment from an aromatase inhibitor to fulvestrant while continuing a CDK4/6 inhibitor at the sign of mutation detection, Dr. Arteaga explained.
Refining who gets the ‘kitchen sink’
Dr. Arteaga highlighted two trials focused on the immune checkpoint inhibitor pembrolizumab.
The phase 3 KEYNOTE-522 study led to the approval of neoadjuvant pembrolizumab plus chemotherapy for early-stage triple-negative breast cancer (TNBC) in July 2021. At this year’s SABCS, researchers will present new data from KEYNOTE-522 (abstract GS1-01), representing final results from the trial’s event-free survival (EFS) outcome.
Previously, investigators reported a statistically significant and clinically meaningful improvement in EFS. These data suggest “that deploying immunotherapy early before surgery ... may be curative in some patients,” Dr. Arteaga said. The new data will allow the “robustness and consistency” of the earlier findings to be assessed.
But, he added, this is a “tough” treatment, which includes five drugs. “It’s the kitchen sink, and not everybody needs the kitchen sink. It’s important to refine these findings. Some patients may not need pembrolizumab, but some do.”
The second trial exploring pembrolizumab – KEYNOTE-355 (abstract GS1-02) – mirrors KEYNOTE-522 but in patients with previously untreated locally recurrent inoperable or metastatic TNBC whose tumors expressed PD-L1.
Previously, investigators reported that pembrolizumab combined with chemotherapy showed statistically significant improvements in overall survival and PFS compared to placebo plus chemotherapy. At the 2021 SABCS, researchers will provide final study results, including outcomes in subgroups of patients by additional combined positive score cutoffs.
Metformin trial: ‘This is it’
Dr. Arteaga highlighted CCTGMA.32 (abstract GS1-08), a phase 3 randomized, placebo-controlled adjuvant trial of the diabetes drug metformin versus placebo in early breast cancer. Results of the primary efficacy analysis of the trial will be presented at the meeting.
The Canadian-led study seeks to determine if metformin can decrease breast cancer cell growth and work with cancer therapies to prevent disease recurrence. The study design calls for patients to take twice-daily oral metformin or placebo pills for up to 5 years in the absence of disease progression.
The primary outcome of the 3,500-plus patient trial is invasive disease-free survival in hormone receptor (ER and PgR) negative and positive (ER and/or PgR) subgroups.
“Metformin has actually been associated with improved survival [in breast cancer] in patients on chemotherapy. But we don’t know exactly how,” he said. “There’s never been a head-to-head comparison in the adjuvant setting [before]. This is it.”
TKI for breast cancer with brain mets
The SABCS codirector spotlighted an updated overall survival analysis of the randomized phase 3 PHOEBE trial (abstract GS3-02).
Previous research confirmed the superiority of pyrotinib, a novel TKI targeting HER1, HER2, and HER4, over lapatinib when given in combination with capecitabine in HER2-positive metastatic breast cancer.
In the United States, the lapatinib-capecitabine combination is “mostly used” in patients with HER2 metastatic disease and brain metastases who also undergo stereotactic radiation, Dr. Arteaga said.
This use has continued despite groundbreaking results from the HER2CLIMB trial, featuring the TKI tucatinib, he said.
As reported last year, adding tucatinib to trastuzumab and capecitabine in patients with HER2-positive breast cancer and brain metastases increased median overall survival from 12 months to 18.1 months. The results were called the first of their kind at that time.
The pyrotinib study may matter to American clinicians because pyrotinib is used mostly in China, not the United States, and this analysis suggests that pyrotinib could be part of the armamentarium in the United States, alongside tucatinib.
TKIs are like Coke and Pepsi, Dr. Arteaga said: “Similar but not identical.” Therefore, it is worth taking a look at the new study, he said. “There may be some benefit in having more than one [TKI] in the therapeutic armamentarium.”
Dr. Arteaga receives or has received grant support from Pfizer and Lilly and serves or has served in a scientific advisory role with Novartis, Lilly, TAIHO Oncology, Daiichi Sankyo, Merck, AstraZeneca, OrigiMed, Immunomedics, ARVINAS, Sanofi, Athenex, and the Susan G. Komen Foundation. He also holds minor stock options from Provista.
A version of this article first appeared on Medscape.com.
FROM SABCS 2021
Microbleeds, age contribute to ARIA risk with aducanumab
Though primary efficacy results have yet to be published,
Amyloid-related imaging abnormalities, or ARIA, have been seen linked to a variety of experimental amyloid-lowering treatments for Alzheimer’s disease. The abnormalities include brain bleeding (ARIA-H) and brain edema (ARIA-E), detected on magnetic resonance imaging.
Safety findings
In a study published Nov. 22 in JAMA Neurology, Stephen Salloway, MD, director of neurology and the memory and aging program at Butler Hospital and the Martin M. Zucker Professor of Psychiatry and Human Behavior and Professor of Neurology at the Warren Alpert Medical School of Brown University in Providence, R.I., and his colleagues, reported that 41% of 1,029 patients in the high-dose (10 mg/kg) treatment groups of aducanumab (Aduhelm, Biogen) developed ARIA.
Thirty-five percent of the high-dose patients (n = 362) developed ARIA-E, and 94 had symptoms, with headache the most commonly reported, followed by confusion. ARIA-E occurred only sporadically in the placebo groups, while ARIA-H was more common. Microbleeds were seen in 19% of the high-dose patients compared with 6.6% in the placebo group, while superficial siderosis occurred in about 15%, versus 2.2% on placebo. Most of the ARIA-E events occurred during the first eight doses of the infusion treatment. People with one or more copies of the APOE4 genetic variant saw higher risk of ARIA-E associated with treatment compared with noncarriers (hazard ratio [HR] 2.5; 95% confidence interval [CI], 1.90-3.20). Evidence of brain micro-hemorrhages at baseline was associated with higher risk of ARIA-E (HR 1.7; 95% CI, 1.31-2.27) compared with patients without MRI evidence of brain bleeds in the year before treatment began.
Older age independently increased risk of ARIA-H, with a risk that was seen increasing 6% with each additional year of age.
The identically designed EMERGE and ENGAGE trials of aducanumab enrolled nearly 3,300 patients worldwide (mean age 70.4, 52% female). Participants were screened to include only those with amyloid-positive mild cognitive impairment (81% of the cohort) or mild Alzheimer’s dementia. Both trials were halted early after a futility analysis concluded that treatment was unlikely to result in benefit.
A post hoc analysis later determined that patients in one trial, EMERGE, showed slight clinical benefit on follow-up in the high-dose group only. The Food and Drug Administration approved the drug in July 2021 on the basis of that finding, overriding the consensus of its independent advisory committee, which was not persuaded. Since then the drug has become synonymous with controversy, not aided by its high list price of more than $50,000 per year, with many insurers and large health care systems refusing to deliver it. The recent reported death of a woman participating in an open-label extension trial of aducanumab, who was admitted to the hospital with brain swelling, has added to safety concerns.
Brain bleeds and age affect risk
In an interview with MDedge Neurology, neurologist Madhav Thambisetty, MD, PhD, a senior investigator with the National Institute on Aging in Baltimore, and a member of the FDA advisory committee that recommended against approval for aducanumab, said that while physicians are aware that APOE4 carriers face higher risks of treatment-related complications, the new safety findings offer additional guidance on patient selection.
“The older you are the greater your risk of ARIA, and the more micro-hemorrhages you have at baseline the greater your risk. Those are important findings that were not previously well publicized before,” Dr. Thambisetty said.
In the EMERGE and ENGAGE trials, Dr. Thambisetty pointed out, patients with four or more micro-hemorrhages at baseline were excluded. The new findings reveal that even a small number of bleeds at baseline can contribute to ARIA risk.
“Patients in real-world clinical practice are going to be very different from the tightly controlled, well-screened participants who were enrolled in these trials. Microbleeds are very common in Alzheimer’s patients, occurring in 18-32%. Now that these findings are available, it’s important for a practicing physician to obtain a baseline MRI scan and really pay attention to microbleeds, because that will affect treatment decisions.”
Additional concerns
Dr. Thambisetty cautioned that the new results made no mention of another important safety outcome: loss of brain volume associated with treatment.
Changes in brain volume have been seen associated with other amyloid-lowering treatments, though the reasons for this are poorly understood. Participants in EMERGE and ENGAGE “received numerous MRI scans,” Dr. Thambisetty said. “This was one of the strengths of the trials. Thanks to an open-label extension we now have more than 2 years of MRI data from meticulously monitored patients, and there has been no mention of brain volume changes despite this being a prespecified outcome. This, for me, is one of the glaring omissions of this paper, and the fact that it’s not even mentioned is really worrisome.”
The sponsor of the aducanumab trials, Biogen, has yet to publish efficacy findings in a peer-reviewed journal, instead presenting them piecemeal at conferences.
“The current paper was a secondary analysis,” Dr. Thambisetty said. “The authors say the primary analysis will be published elsewhere. I think it’s important to reflect upon the fact that these clinical trials enrolled more than 3,000 participants at more than 300 trial centers in 20 countries. We now have an approved drug that’s commercially available. And yet we don’t have a single peer-reviewed publication discussing the efficacy data. None of this is in the interest of our patients, or in advancing the science.”
The EMERGE and ENGAGE trials were funded by Biogen. Eight of the current paper’s 14 authors are Biogen employees. Dr. Salloway, the lead author, disclosed financial support from Biogen and other manufacturers, as did two of his coauthors. Dr. Thambisetty disclosed no financial conflicts of interest.
Though primary efficacy results have yet to be published,
Amyloid-related imaging abnormalities, or ARIA, have been seen linked to a variety of experimental amyloid-lowering treatments for Alzheimer’s disease. The abnormalities include brain bleeding (ARIA-H) and brain edema (ARIA-E), detected on magnetic resonance imaging.
Safety findings
In a study published Nov. 22 in JAMA Neurology, Stephen Salloway, MD, director of neurology and the memory and aging program at Butler Hospital and the Martin M. Zucker Professor of Psychiatry and Human Behavior and Professor of Neurology at the Warren Alpert Medical School of Brown University in Providence, R.I., and his colleagues, reported that 41% of 1,029 patients in the high-dose (10 mg/kg) treatment groups of aducanumab (Aduhelm, Biogen) developed ARIA.
Thirty-five percent of the high-dose patients (n = 362) developed ARIA-E, and 94 had symptoms, with headache the most commonly reported, followed by confusion. ARIA-E occurred only sporadically in the placebo groups, while ARIA-H was more common. Microbleeds were seen in 19% of the high-dose patients compared with 6.6% in the placebo group, while superficial siderosis occurred in about 15%, versus 2.2% on placebo. Most of the ARIA-E events occurred during the first eight doses of the infusion treatment. People with one or more copies of the APOE4 genetic variant saw higher risk of ARIA-E associated with treatment compared with noncarriers (hazard ratio [HR] 2.5; 95% confidence interval [CI], 1.90-3.20). Evidence of brain micro-hemorrhages at baseline was associated with higher risk of ARIA-E (HR 1.7; 95% CI, 1.31-2.27) compared with patients without MRI evidence of brain bleeds in the year before treatment began.
Older age independently increased risk of ARIA-H, with a risk that was seen increasing 6% with each additional year of age.
The identically designed EMERGE and ENGAGE trials of aducanumab enrolled nearly 3,300 patients worldwide (mean age 70.4, 52% female). Participants were screened to include only those with amyloid-positive mild cognitive impairment (81% of the cohort) or mild Alzheimer’s dementia. Both trials were halted early after a futility analysis concluded that treatment was unlikely to result in benefit.
A post hoc analysis later determined that patients in one trial, EMERGE, showed slight clinical benefit on follow-up in the high-dose group only. The Food and Drug Administration approved the drug in July 2021 on the basis of that finding, overriding the consensus of its independent advisory committee, which was not persuaded. Since then the drug has become synonymous with controversy, not aided by its high list price of more than $50,000 per year, with many insurers and large health care systems refusing to deliver it. The recent reported death of a woman participating in an open-label extension trial of aducanumab, who was admitted to the hospital with brain swelling, has added to safety concerns.
Brain bleeds and age affect risk
In an interview with MDedge Neurology, neurologist Madhav Thambisetty, MD, PhD, a senior investigator with the National Institute on Aging in Baltimore, and a member of the FDA advisory committee that recommended against approval for aducanumab, said that while physicians are aware that APOE4 carriers face higher risks of treatment-related complications, the new safety findings offer additional guidance on patient selection.
“The older you are the greater your risk of ARIA, and the more micro-hemorrhages you have at baseline the greater your risk. Those are important findings that were not previously well publicized before,” Dr. Thambisetty said.
In the EMERGE and ENGAGE trials, Dr. Thambisetty pointed out, patients with four or more micro-hemorrhages at baseline were excluded. The new findings reveal that even a small number of bleeds at baseline can contribute to ARIA risk.
“Patients in real-world clinical practice are going to be very different from the tightly controlled, well-screened participants who were enrolled in these trials. Microbleeds are very common in Alzheimer’s patients, occurring in 18-32%. Now that these findings are available, it’s important for a practicing physician to obtain a baseline MRI scan and really pay attention to microbleeds, because that will affect treatment decisions.”
Additional concerns
Dr. Thambisetty cautioned that the new results made no mention of another important safety outcome: loss of brain volume associated with treatment.
Changes in brain volume have been seen associated with other amyloid-lowering treatments, though the reasons for this are poorly understood. Participants in EMERGE and ENGAGE “received numerous MRI scans,” Dr. Thambisetty said. “This was one of the strengths of the trials. Thanks to an open-label extension we now have more than 2 years of MRI data from meticulously monitored patients, and there has been no mention of brain volume changes despite this being a prespecified outcome. This, for me, is one of the glaring omissions of this paper, and the fact that it’s not even mentioned is really worrisome.”
The sponsor of the aducanumab trials, Biogen, has yet to publish efficacy findings in a peer-reviewed journal, instead presenting them piecemeal at conferences.
“The current paper was a secondary analysis,” Dr. Thambisetty said. “The authors say the primary analysis will be published elsewhere. I think it’s important to reflect upon the fact that these clinical trials enrolled more than 3,000 participants at more than 300 trial centers in 20 countries. We now have an approved drug that’s commercially available. And yet we don’t have a single peer-reviewed publication discussing the efficacy data. None of this is in the interest of our patients, or in advancing the science.”
The EMERGE and ENGAGE trials were funded by Biogen. Eight of the current paper’s 14 authors are Biogen employees. Dr. Salloway, the lead author, disclosed financial support from Biogen and other manufacturers, as did two of his coauthors. Dr. Thambisetty disclosed no financial conflicts of interest.
Though primary efficacy results have yet to be published,
Amyloid-related imaging abnormalities, or ARIA, have been seen linked to a variety of experimental amyloid-lowering treatments for Alzheimer’s disease. The abnormalities include brain bleeding (ARIA-H) and brain edema (ARIA-E), detected on magnetic resonance imaging.
Safety findings
In a study published Nov. 22 in JAMA Neurology, Stephen Salloway, MD, director of neurology and the memory and aging program at Butler Hospital and the Martin M. Zucker Professor of Psychiatry and Human Behavior and Professor of Neurology at the Warren Alpert Medical School of Brown University in Providence, R.I., and his colleagues, reported that 41% of 1,029 patients in the high-dose (10 mg/kg) treatment groups of aducanumab (Aduhelm, Biogen) developed ARIA.
Thirty-five percent of the high-dose patients (n = 362) developed ARIA-E, and 94 had symptoms, with headache the most commonly reported, followed by confusion. ARIA-E occurred only sporadically in the placebo groups, while ARIA-H was more common. Microbleeds were seen in 19% of the high-dose patients compared with 6.6% in the placebo group, while superficial siderosis occurred in about 15%, versus 2.2% on placebo. Most of the ARIA-E events occurred during the first eight doses of the infusion treatment. People with one or more copies of the APOE4 genetic variant saw higher risk of ARIA-E associated with treatment compared with noncarriers (hazard ratio [HR] 2.5; 95% confidence interval [CI], 1.90-3.20). Evidence of brain micro-hemorrhages at baseline was associated with higher risk of ARIA-E (HR 1.7; 95% CI, 1.31-2.27) compared with patients without MRI evidence of brain bleeds in the year before treatment began.
Older age independently increased risk of ARIA-H, with a risk that was seen increasing 6% with each additional year of age.
The identically designed EMERGE and ENGAGE trials of aducanumab enrolled nearly 3,300 patients worldwide (mean age 70.4, 52% female). Participants were screened to include only those with amyloid-positive mild cognitive impairment (81% of the cohort) or mild Alzheimer’s dementia. Both trials were halted early after a futility analysis concluded that treatment was unlikely to result in benefit.
A post hoc analysis later determined that patients in one trial, EMERGE, showed slight clinical benefit on follow-up in the high-dose group only. The Food and Drug Administration approved the drug in July 2021 on the basis of that finding, overriding the consensus of its independent advisory committee, which was not persuaded. Since then the drug has become synonymous with controversy, not aided by its high list price of more than $50,000 per year, with many insurers and large health care systems refusing to deliver it. The recent reported death of a woman participating in an open-label extension trial of aducanumab, who was admitted to the hospital with brain swelling, has added to safety concerns.
Brain bleeds and age affect risk
In an interview with MDedge Neurology, neurologist Madhav Thambisetty, MD, PhD, a senior investigator with the National Institute on Aging in Baltimore, and a member of the FDA advisory committee that recommended against approval for aducanumab, said that while physicians are aware that APOE4 carriers face higher risks of treatment-related complications, the new safety findings offer additional guidance on patient selection.
“The older you are the greater your risk of ARIA, and the more micro-hemorrhages you have at baseline the greater your risk. Those are important findings that were not previously well publicized before,” Dr. Thambisetty said.
In the EMERGE and ENGAGE trials, Dr. Thambisetty pointed out, patients with four or more micro-hemorrhages at baseline were excluded. The new findings reveal that even a small number of bleeds at baseline can contribute to ARIA risk.
“Patients in real-world clinical practice are going to be very different from the tightly controlled, well-screened participants who were enrolled in these trials. Microbleeds are very common in Alzheimer’s patients, occurring in 18-32%. Now that these findings are available, it’s important for a practicing physician to obtain a baseline MRI scan and really pay attention to microbleeds, because that will affect treatment decisions.”
Additional concerns
Dr. Thambisetty cautioned that the new results made no mention of another important safety outcome: loss of brain volume associated with treatment.
Changes in brain volume have been seen associated with other amyloid-lowering treatments, though the reasons for this are poorly understood. Participants in EMERGE and ENGAGE “received numerous MRI scans,” Dr. Thambisetty said. “This was one of the strengths of the trials. Thanks to an open-label extension we now have more than 2 years of MRI data from meticulously monitored patients, and there has been no mention of brain volume changes despite this being a prespecified outcome. This, for me, is one of the glaring omissions of this paper, and the fact that it’s not even mentioned is really worrisome.”
The sponsor of the aducanumab trials, Biogen, has yet to publish efficacy findings in a peer-reviewed journal, instead presenting them piecemeal at conferences.
“The current paper was a secondary analysis,” Dr. Thambisetty said. “The authors say the primary analysis will be published elsewhere. I think it’s important to reflect upon the fact that these clinical trials enrolled more than 3,000 participants at more than 300 trial centers in 20 countries. We now have an approved drug that’s commercially available. And yet we don’t have a single peer-reviewed publication discussing the efficacy data. None of this is in the interest of our patients, or in advancing the science.”
The EMERGE and ENGAGE trials were funded by Biogen. Eight of the current paper’s 14 authors are Biogen employees. Dr. Salloway, the lead author, disclosed financial support from Biogen and other manufacturers, as did two of his coauthors. Dr. Thambisetty disclosed no financial conflicts of interest.
FROM JAMA NEUROLOGY
Pfizer COVID vaccine is 100% effective in adolescents: Study
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.