User login
Systems Automation for Cancer Surveillance: A Lean Six Sigma Project for Tracking Care of Patients With Head and Neck Cancer (FULL)
The American Cancer Society estimates that there were 1.68 million newly diagnosed cases of cancer in the U.S. in 2016, with an associated 595,690 deaths.1 Of this number, about 3% was attributable to head and neck cancer (HNC), with 48,330 new cases and 9,570 deaths in 2016. Cancer is among the leading causes of death worldwide, and veterans have a prevalence of HNC nearly twice that of the general population.2 The number of people living with and beyond a cancer diagnosis in the U.S. has risen to an estimated 15.5 million survivors.
Head and neck cancer comprises several subsites, including the oral cavity (lips, buccal mucosa, anterior tongue, floor of mouth, hard palate, and gingiva), the pharynx (nasopharynx, oropharynx, and hypopharynx), the larynx (supraglottis, glottis, and subglottis), the nasal cavity, paranasal sinuses, and the saliva glands.3 The economic burden for HNC treatment was estimated at $3.64 billion in 2010.4
Treatment is based on primary site and staging, and staging is according to the tumor node metastasis system of the American Joint Committee on Cancer.5 In general, lower stages (in situ, stages I and II) are treated with single modalities of organ-sparing surgery or radiation, whereas higher stages (stages III and IV) are treated with multiple modalities, which may include radiation combined with chemotherapy or surgery before or after radiation/chemotherapy.
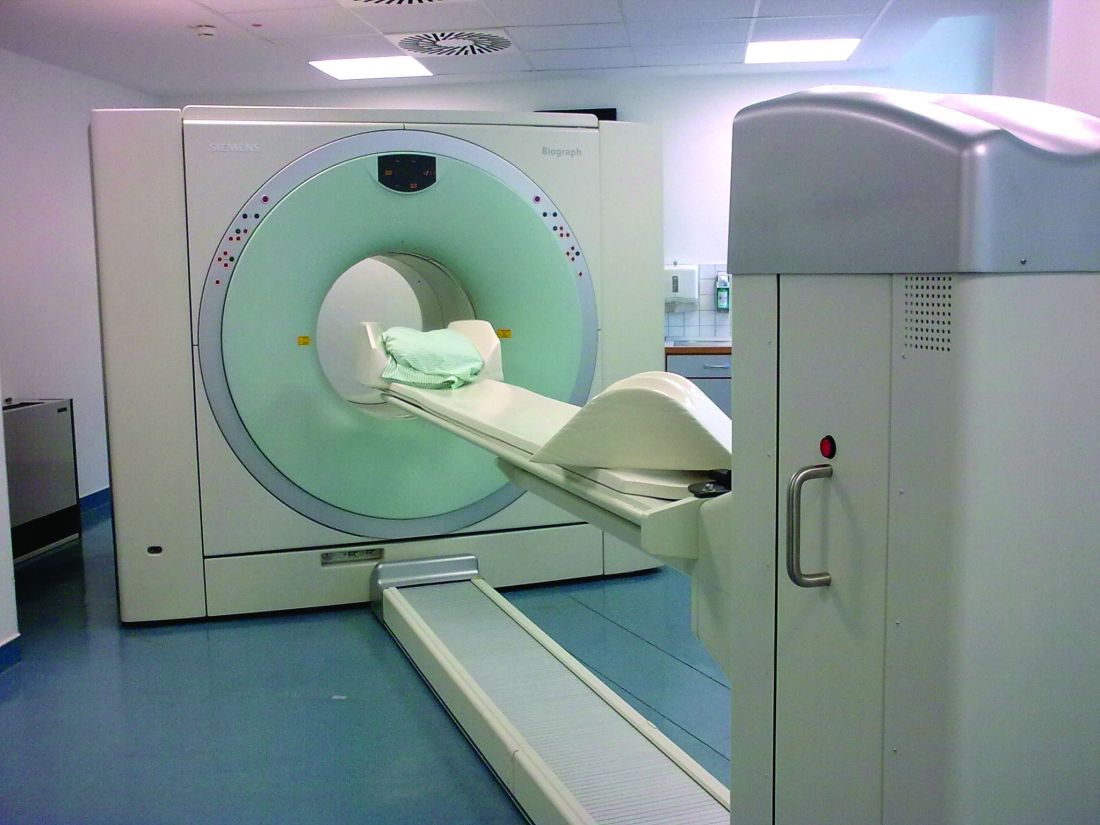
Survival rate after treatment varies by primary site, cancer stage at diagnosis, histopathologic cell type, viral association, tobacco use, chemical exposure, and treatment modality; survival ranges from 24% to 90% at 5 years based on these variables.6 There is not yet a reliable blood test or other biochemical marker for recurrence, and serial radiologic examinations are expensive and expose the survivor to large amounts of additional ionizing radiation.7,8 Surveillance for recurrence after treatment consists primarily of physical examination and reported symptoms, which may be difficult for the primary care provider (PCP) to perform and distinguish from treatment sequelae.9,10 Thus, HNC survivors are followed in the ear, nose, and throat (ENT) otolaryngology clinic on a decreasing frequency schedule based on risk of relapse, second primaries, treatment sequelae, and toxicities (every 1-3 months in year 1, 2-6 months in year 2, 4-8 months in years 3-5, and every 12 months after 5 years) according to the National Comprehensive Cancer Network (NCCN) guidelines.11
Adherence with posttreatment surveillance in HNC recently was associated with length of survival; however, this observation at a single tertiary academic center was discordant with earlier published reports.12-15 About 80% to 90% of all postcurative intent treatment recurrences and second primary cancers occur within the first 4 years, with a better functional outcome if the recurrence is surgically salvageable or amenable to adjuvant radiation or combined radiation and chemotherapy.16,17 Nonadherence is generally associated with worse clinical and acute care utilization outcomes.18
Problem
At the Raymond G. Murphy VAMC, a tertiary care center in Albuquerque, New Mexico, there was a propensity of veteran HNC patients who missed scheduled surveillance appointments or were lost to follow-up. An informal review of several VA ENT departments revealed similar issues without any consistent method to solve the problem. In an effort to recapture these patients, in 2011 an ENT registered nurse (RN) was added to the team as cancer care coordinator (CCC). After several weeks of chart review of clinic records, it was determined that 31% of HNC patients had missed 1 or more ongoing surveillance appointments, either by patient no-show, clinic cancellations that failed to reschedule patients, or patient cancellation without rescheduling. The CCC was tasked with recapturing these lost patients, returning them to regular follow-up per NCCN guidelines, and tracking new cancer patients as they were diagnosed and progressed through treatment and surveillance. As there had been no one previously in this role in the ENT clinic, there was no guidance about how to proceed.
The mechanism in place for rescheduling no-show patients at that time consisted of a mailed postcard reminder sent by a medical support assistant who requested that the veteran contact the clinic to reschedule. Veterans reported that these reminders often appeared in their mail mingled with so-called junk mail and were discarded without reading. The CCC spent several more weeks examining clinic records in the computerized patient record system (CPRS), looking for patients with cancer in the 5-year surveillance period, and compiling a database of survivors and newly diagnosed patients. This database was compiled initially on paper and then converted to a spreadsheet. Patients who had missed appointments were contacted by the CCC and rescheduled, which resulted in a 100% recovery rate.
Unfortunately, although the manual tracking process was successful, it was laborious and time consuming. Weekly and sometimes daily examination of CPRS clinic records for new patients and survivor adherence was followed by tedious data entry into the spreadsheet. The manual tracking system was deemed suboptimal and a Lean Six Sigma process improvement project was initiated. The project goal was to produce a dashboard database tool that was patient centered to improve the quality of cancer care to veterans.
Methods
Lean Six Sigma is a combination of 2 improvement processes and is embraced by large business and government entities with the goal of improving efficiencies, reducing waste, decreasing errors, and generating cost savings.19 The first improvement process, Six Sigma, is a statistical concept with the goal of producing no more than 3.4 defects per million opportunities.20Using specific tools, Six Sigma identifies the cause of the problem to help develop effective solutions. Six Sigma also helps uncover defects and problems by using a standardized and systematic method for each process improvement project in a sequence of steps known as DMAIC (Define, Measure, Analyze, Improve, and Control) to ensure a defect-free product at a rate of 99.99966%. Define, the first step, contains a written statement defining the problem and the goals; Measure scrutinizes the current baseline of the project in measureable data to identify possible contributing factors; Analyze uses data and tools to understand the cause-and-effect relationships in the process; Improve uses creative developments and changes that lead to process improvements; and Control takes measures to ensure the improvements are implemented, reliable, and constant.
Although slightly different but complementary, Lean focuses on streamlining improvement processes by identifying and eliminating waste that has little or no value to the customer. The 8 most common forms of waste are identified through the mnemonic DOWNTIME (Defects, Overproduction, Waiting, Not utilizing human talent, Transportation, Inventory excess, Motion excess, and Excess processing).21 When both Lean and Six Sigma are used together, the synergistic effects have a powerful impact on the complete quality improvement process and yield consistent reliability. The combined process then includes several methodologic tools for systems redesign, including root-cause analysis, defining waste barriers, measuring current and expected performance, analyzing the data collected, improving the target process, and controlling the improvements. Though already existing and used within the VA system, Lean Six Sigma training was included as a mandatory component of new employee orientation in a memo issued in August 2015 from the assistant secretary for human resources and administration (VA access-only memo VAIQ 7595924).
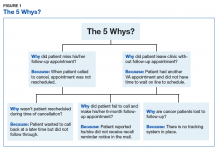
Root-cause analysis was accomplished using the “5 Why” technique adapted into Lean and Six Sigma from the Toyota Motor Corporation. For example, the question “Why do patients miss appointments?” was asked 5 different ways, and it was determined that many patients lacked transportation, some were not able to reschedule at the time they called to cancel their appointment, those with multiple same-day appointments at the tertiary medical center were not able to wait to schedule a follow-up appointment for fear of missing or being late to their next appointment, and others were placed on recall lists with appointment reminders that failed to accomplish the purpose of self-scheduling by veterans. Thus, the common denominator and answer to the question “why” was that there was no tracking system in place to identify and reschedule missed follow-ups, and before employing a dedicated coordinator, no one accountable for the process (Figure 1).
Wasteful barriers to efficiency were examined with particular attention to the rescheduling process. Rescheduling produced immediate duplication of work for scheduling staff and increased wait time for future appointments. There was potential for additional health care expenses related to costs of late and progressive salvage treatment or for less-than-timely correction of HNC treatment sequelae, such as scarring, lymphedema, or dysphagia. Ear, nose, and throat providers were concerned about missing occult recurrence or residual cancer.
In 2013, the Lean Six Sigma process was used again to critique efforts by the CCC to identify and track HNC patients. One suggestion was to automate the process, and the Information Resource Management (IRM) office was contacted via work order to explore options for mining CPRS data. Working with a committed health information analyst, further discussion was aimed at pulling in additional data that would simultaneously track required posttreatment laboratory results and imaging. It was decided that a secure dashboard format would provide greater utility than would an online report that the CCC had to request and generate daily.
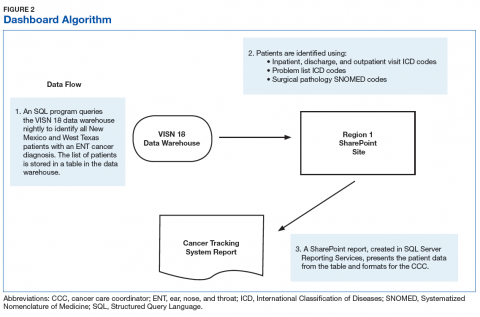
Integrated technologist Stephen Few defines a data dashboard as “… a visual display of the most important information needed to achieve one or more objectives; consolidated and arranged on a single screen so the information can be monitored at a glance.”22 The Head & Neck Cancer Tracking Dashboard (HNC Dashboard), designed by the IRM analyst, queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with HNC by examining outpatient visit and inpatient discharge International Classification of Disease (ICD) codes entered by providers when coding encounter notes in CPRS. It also adds those with a HNC diagnosis in the VistA problem list and the HNC pathology department Systematized Nomenclature of Medicine (SNOMED) codes (Figure 2).
The automated ENT cancer tracking dashboard prototype debuted in 2014, but several months of trial and error took place to reanalyze ICD codes and narrow the list. The dashboard underwent multiple tests to ensure accuracy. Identified patients are presented using an interactive report hosted on a secure SharePoint (Redmond,WA) site, which reduced the risk of a data breach as access requires multi-authenticated user identification from a VA computer.
Another characteristic of the dashboard’s format is the ability to add custom features as needed. Several features now included in the dashboard are location of residence, diagnosis date, ICD code, date captured in the tracking system, most recent ENT clinic visit, future scheduled ENT clinic appointment, date of last thyroid stimulating hormone (TSH) laboratory test, and date of last position emission tomography scan. In addition, cancellations, no-shows, and patients overdue for TSH testing are highlighted in bold. Highlighted fields alert the CCC to reschedule patients in a timely manner and can alert providers to order needed follow-up tests and procedures.
Among the merits of the ENT cancer tracking dashboard is ease of use. The CCC uses a simple ABC acronym to describe utilization:
- A—added: The CCC daily edits new patients added to the dashboard with a HNC diagnosis. Several times recently the CCC saw a new diagnosis before the provider had been notified by pathology of biopsy results (Figure 3).
- B—browse: The dashboard format allows for rapid perusal of critical information at a glance (Figure 4). Recent labs and imaging can be discussed with providers immediately or at weekly ENT team cancer update meetings. Notification to clinicians can be rapid if the results show suspicion for residual/recurrent disease, a second primary site, metastasis, or there is need to notify the patient’s primary care provider to treat elevated TSH levels (hypothyroidism incidence after head and neck radiation is reportedly as high as 44%, with most patients being asymptomatic or simply fatigued).10,23
- C—check: Appointments are checked for those in the future, cancelled without rescheduling, or no-show dates. Empty fields under the “Next ENT Appointment” header alert the CCC to reschedule a follow-up appointment within NCCN guidelines. Alerting providers to upcoming surveillance appointments allows timely coordination with other care providers and departments, including speech pathology, nutrition, audiology, and social work. The “ENT Recall Date” has a unique time-sensitive feature and will visually display a bold type font when ready to be scheduled for a physical appointment (Figure 5).
Results
The cancer dashboard has demonstrated its success by supporting consistent and reliable monthly data. Results recorded over a 24-month period (from January 1, 2015 through December 31, 2016) showed that the electronic tracker identified 101 new HNC patients. During this period, 1,067 HNC patients were scheduled for follow-up appointments for cancer surveillance. Of these, the authors found that 112 HNC patients had missed their appointments due to calling and cancelling or not showing up as scheduled; resulting in a no-show status. This yielded an appointment nonadherence rate of 10%. The authors also found that 73 (7%) HNC patients did not have an elected scheduled appointment to return to the clinic for continued cancer surveillance. This number comprises all HNC patients whose appointments were cancelled by clinic cancellation, self-cancellation, no-show appointments, or those who left the clinic without scheduling a subsequent follow-up appointment. The electronic tracker identified 100% of these patients as missing and needing a future appointment. These patients may have otherwise been lost through manual tracking.
Implementation and utilization of a robust automated dashboard format HNC patient tracking system has been rewarding for the ENT department. The CCC has saved an estimated 600 to 800 hours per year of chart review and data entry. Although a time study was never conducted to measure the work process of this task, it is reasonable to conclude based on the following multiple manual step-by-step processes that the CCC had to perform frequently were now performed within the dashboard: reviewing consults for HNC diagnosis, recording new patient profile data on the spreadsheet; reviewing VA hospital pathology reports for new HNC diagnoses, reviewing the clinic schedule to track patient appointment adherence, updating and recording recent appointment activity, and reviewing the electronic medical records daily for recommended treatment plan and follow-up.
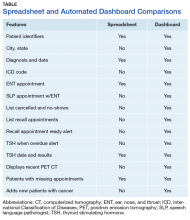
A side-by-side comparison of the functional features of tracking both manually and with automation showed that automation outnumbers the function of manual tracking by 36% and offers improved efficiency (Table). This has allowed time for the CCC to participate in simultaneous HNC care initiatives, including facilitating interfacility telehealth referrals for complex cancer surgery, scheduling and monitoring rural cancer surveillance telehealth appointments, and development of an ENT Survivorship Care Plan. These programs optimize time and workflow, reduce waste, reduce expenditures related to costly treatment modalities associated with advanced stages of malignancy, and improve the veteran experience. Further benefits to the veteran HNC patient population include increased self-efficacy and awareness for disease management through continuity of care, reduced cost associated with travel expense, and reduced potential copays due to additional medical care related to advanced stages of recurrent or residual disease.
In-house development of the HNC tracking dashboard has contributed to further cost savings for the VA. Specialized third-party acquired software can cost thousands of dollars for purchase and implementation and often includes ongoing fees for use. The Sustain and Spread concept of Lean Six Sigma is proven by a 100% recapture rate of HNC patients in the ENT clinic that potentially would have been lost to follow-up. The success in Spreading this innovation forward has resulted in adoption by other VAMCs for current use and implementation. After sharing information regarding the dashboard at 2 national conferences via presentations and poster, other VAMCs in neighboring states have requested the software and initiated custom versions. Because of this success and further demand, dashboard use is currently under consideration by the VA for nationwide availability.
Conclusion
Deficiencies in tracking cancer patients in the VA system exist in part due to little or no sophisticated electronic tracking systems that could perform multiple task functions to identify new cancer patients, the type of cancer, when appointments are missed, and notification when the required labs and procedures are completed. Often, the CCC is dependent on the arduous task of inputting of data to keep him/her up-to-date with patient care and coordination in a timely manner. As new VA policies attempts to perfect and streamline the scheduling process by way of providers placing “return to clinic” orders for patient follow-up care, there remains a potential risk of those patients not getting scheduled without a vigilant tracking process in place to monitor and ensure that all patients are scheduled.
The dashboard has proved to be an easy to use and vital tool in tracking HNC patients by the CCC. It will continue to assist in the identification of new HNC patients, provide ready access to patient information and follow-up care, and help facilitate CCC and provider communication on a daily basis, thereby meeting the goal of a patient-centered product that proves to improve the quality of cancer care of veterans.
Acknowledgment
The authors thank Mr. Dominic B. Ruiz, Visual Information Specialist, at the Raymond Murphy VAMC, who created images in high resolution for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner , Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Patil RD, Meinzen-Derr JK, Hendricks BL, Patil YJ. Improving access and timelines of care for veterans with head and neck squamous cell carcinoma: a multidisciplinary team’s approach. Laryngoscope. 2016;126(3):627-631.
3. Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865-882.
4. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
5. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
6. Cancer.net. Head and neck cancer: statistics. http://www.cancer.net/cancer-types/head-and-neck-cancer/Statistics. Updated September 2016. Accessed April 12, 2017.
7. Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1068-E1074.
8. Cheung PK, Chin RY, Eslick GD. Detecting residual/recurrent head neck squamous cell carcinomas using PET or PET/CT: systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2016;154(3):421-432.
9. Haddad RI, Limaye S. Overview of approach to long-term survivors of head and neck cancer. http://www .uptodate .com/contents/overview-of-approach-to-long-term-survivors-of-head-and-neck-cancer. Updated October 26, 2016. Accessed April 12, 2017.
10. Manikantan K, Khode S, Dwivedi RC, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35(8):744-753.
11. National Comprehensive Cancer Network. NCCN Clinical practice guidelines in onclology:head and neck cancers(2.2017).2017. Updated May 8, 2017. https://www.nccn.org/professionals/physician_gls/f_/pdf/head-and-neck.pdf. Accessed July 18, 2017.
12. Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Schnayder Y. The impact of compliance in post treatment surveillance in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(6):519-525.
13. Merkx MA, van Gulick JJ, Marres HA, et al. Effectiveness of routine follow-up of patients treated for T1-2N0 oral squamous cell carcinomas of the floor of mouth and tongue. Head Neck. 2006:28(1):1-7.
14. Ritoe SC, de Vegt F, Scheike IM, et al. Effect of routine follow-up after treatment for laryngeal cancer on life expectancy and mortality: results of a Markov model analysis. Cancer. 2007;109(2):239-247.
15. Agrawal A, Hammond TH, Young GS, Avon AL, Ozer E, Schuller DE. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope. 2009;119(11):2135-2140.
16. Roland NJ, Bradley PJ. The role of surgery in the palliation of head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):101-108.
17. Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111(3):382-387.
18. Hwang AS, Atlas SJ, Cronin P, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
19. Healthcare Daily Online. VA healthcare system adopts lean six sigma. http://www.healthcaredailyonline.com/news/va-lean-six-sigma-in-healthcare. Updated December 7, 2015. Accessed April 12, 2017.
20. Gygi C, Williams B. Six Sigma for Dummies. 2nd edition. Hoboken, NJ: John Wiley & Sons; 2012.
21. Kavanagh S, Krings D. The 8 sources of waste and how to eliminate them: improving performance with LEAN management techniques. http://www.gfoa.org/sites/default /files/GFR_DEC_11_18.pdf. Updated December, 2011. Accessed April 14, 2017.
22. Few S. What is a dashboard? In: Wheeler C, ed. Information Dashboard Design: The Effective Visual Communication of Data. 1st ed. Sebastopol, CA: O’Reilly Media; 2006:34.
23. Murthy V, Narang K, Ghosh-Laskar S, Gupta T, Budrukkar A, Agrawal JP. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck. 2014;36(11):1573-1780.
The American Cancer Society estimates that there were 1.68 million newly diagnosed cases of cancer in the U.S. in 2016, with an associated 595,690 deaths.1 Of this number, about 3% was attributable to head and neck cancer (HNC), with 48,330 new cases and 9,570 deaths in 2016. Cancer is among the leading causes of death worldwide, and veterans have a prevalence of HNC nearly twice that of the general population.2 The number of people living with and beyond a cancer diagnosis in the U.S. has risen to an estimated 15.5 million survivors.
Head and neck cancer comprises several subsites, including the oral cavity (lips, buccal mucosa, anterior tongue, floor of mouth, hard palate, and gingiva), the pharynx (nasopharynx, oropharynx, and hypopharynx), the larynx (supraglottis, glottis, and subglottis), the nasal cavity, paranasal sinuses, and the saliva glands.3 The economic burden for HNC treatment was estimated at $3.64 billion in 2010.4
Treatment is based on primary site and staging, and staging is according to the tumor node metastasis system of the American Joint Committee on Cancer.5 In general, lower stages (in situ, stages I and II) are treated with single modalities of organ-sparing surgery or radiation, whereas higher stages (stages III and IV) are treated with multiple modalities, which may include radiation combined with chemotherapy or surgery before or after radiation/chemotherapy.
Survival rate after treatment varies by primary site, cancer stage at diagnosis, histopathologic cell type, viral association, tobacco use, chemical exposure, and treatment modality; survival ranges from 24% to 90% at 5 years based on these variables.6 There is not yet a reliable blood test or other biochemical marker for recurrence, and serial radiologic examinations are expensive and expose the survivor to large amounts of additional ionizing radiation.7,8 Surveillance for recurrence after treatment consists primarily of physical examination and reported symptoms, which may be difficult for the primary care provider (PCP) to perform and distinguish from treatment sequelae.9,10 Thus, HNC survivors are followed in the ear, nose, and throat (ENT) otolaryngology clinic on a decreasing frequency schedule based on risk of relapse, second primaries, treatment sequelae, and toxicities (every 1-3 months in year 1, 2-6 months in year 2, 4-8 months in years 3-5, and every 12 months after 5 years) according to the National Comprehensive Cancer Network (NCCN) guidelines.11
Adherence with posttreatment surveillance in HNC recently was associated with length of survival; however, this observation at a single tertiary academic center was discordant with earlier published reports.12-15 About 80% to 90% of all postcurative intent treatment recurrences and second primary cancers occur within the first 4 years, with a better functional outcome if the recurrence is surgically salvageable or amenable to adjuvant radiation or combined radiation and chemotherapy.16,17 Nonadherence is generally associated with worse clinical and acute care utilization outcomes.18
Problem
At the Raymond G. Murphy VAMC, a tertiary care center in Albuquerque, New Mexico, there was a propensity of veteran HNC patients who missed scheduled surveillance appointments or were lost to follow-up. An informal review of several VA ENT departments revealed similar issues without any consistent method to solve the problem. In an effort to recapture these patients, in 2011 an ENT registered nurse (RN) was added to the team as cancer care coordinator (CCC). After several weeks of chart review of clinic records, it was determined that 31% of HNC patients had missed 1 or more ongoing surveillance appointments, either by patient no-show, clinic cancellations that failed to reschedule patients, or patient cancellation without rescheduling. The CCC was tasked with recapturing these lost patients, returning them to regular follow-up per NCCN guidelines, and tracking new cancer patients as they were diagnosed and progressed through treatment and surveillance. As there had been no one previously in this role in the ENT clinic, there was no guidance about how to proceed.
The mechanism in place for rescheduling no-show patients at that time consisted of a mailed postcard reminder sent by a medical support assistant who requested that the veteran contact the clinic to reschedule. Veterans reported that these reminders often appeared in their mail mingled with so-called junk mail and were discarded without reading. The CCC spent several more weeks examining clinic records in the computerized patient record system (CPRS), looking for patients with cancer in the 5-year surveillance period, and compiling a database of survivors and newly diagnosed patients. This database was compiled initially on paper and then converted to a spreadsheet. Patients who had missed appointments were contacted by the CCC and rescheduled, which resulted in a 100% recovery rate.
Unfortunately, although the manual tracking process was successful, it was laborious and time consuming. Weekly and sometimes daily examination of CPRS clinic records for new patients and survivor adherence was followed by tedious data entry into the spreadsheet. The manual tracking system was deemed suboptimal and a Lean Six Sigma process improvement project was initiated. The project goal was to produce a dashboard database tool that was patient centered to improve the quality of cancer care to veterans.
Methods
Lean Six Sigma is a combination of 2 improvement processes and is embraced by large business and government entities with the goal of improving efficiencies, reducing waste, decreasing errors, and generating cost savings.19 The first improvement process, Six Sigma, is a statistical concept with the goal of producing no more than 3.4 defects per million opportunities.20Using specific tools, Six Sigma identifies the cause of the problem to help develop effective solutions. Six Sigma also helps uncover defects and problems by using a standardized and systematic method for each process improvement project in a sequence of steps known as DMAIC (Define, Measure, Analyze, Improve, and Control) to ensure a defect-free product at a rate of 99.99966%. Define, the first step, contains a written statement defining the problem and the goals; Measure scrutinizes the current baseline of the project in measureable data to identify possible contributing factors; Analyze uses data and tools to understand the cause-and-effect relationships in the process; Improve uses creative developments and changes that lead to process improvements; and Control takes measures to ensure the improvements are implemented, reliable, and constant.
Although slightly different but complementary, Lean focuses on streamlining improvement processes by identifying and eliminating waste that has little or no value to the customer. The 8 most common forms of waste are identified through the mnemonic DOWNTIME (Defects, Overproduction, Waiting, Not utilizing human talent, Transportation, Inventory excess, Motion excess, and Excess processing).21 When both Lean and Six Sigma are used together, the synergistic effects have a powerful impact on the complete quality improvement process and yield consistent reliability. The combined process then includes several methodologic tools for systems redesign, including root-cause analysis, defining waste barriers, measuring current and expected performance, analyzing the data collected, improving the target process, and controlling the improvements. Though already existing and used within the VA system, Lean Six Sigma training was included as a mandatory component of new employee orientation in a memo issued in August 2015 from the assistant secretary for human resources and administration (VA access-only memo VAIQ 7595924).
Root-cause analysis was accomplished using the “5 Why” technique adapted into Lean and Six Sigma from the Toyota Motor Corporation. For example, the question “Why do patients miss appointments?” was asked 5 different ways, and it was determined that many patients lacked transportation, some were not able to reschedule at the time they called to cancel their appointment, those with multiple same-day appointments at the tertiary medical center were not able to wait to schedule a follow-up appointment for fear of missing or being late to their next appointment, and others were placed on recall lists with appointment reminders that failed to accomplish the purpose of self-scheduling by veterans. Thus, the common denominator and answer to the question “why” was that there was no tracking system in place to identify and reschedule missed follow-ups, and before employing a dedicated coordinator, no one accountable for the process (Figure 1).
Wasteful barriers to efficiency were examined with particular attention to the rescheduling process. Rescheduling produced immediate duplication of work for scheduling staff and increased wait time for future appointments. There was potential for additional health care expenses related to costs of late and progressive salvage treatment or for less-than-timely correction of HNC treatment sequelae, such as scarring, lymphedema, or dysphagia. Ear, nose, and throat providers were concerned about missing occult recurrence or residual cancer.
In 2013, the Lean Six Sigma process was used again to critique efforts by the CCC to identify and track HNC patients. One suggestion was to automate the process, and the Information Resource Management (IRM) office was contacted via work order to explore options for mining CPRS data. Working with a committed health information analyst, further discussion was aimed at pulling in additional data that would simultaneously track required posttreatment laboratory results and imaging. It was decided that a secure dashboard format would provide greater utility than would an online report that the CCC had to request and generate daily.
Integrated technologist Stephen Few defines a data dashboard as “… a visual display of the most important information needed to achieve one or more objectives; consolidated and arranged on a single screen so the information can be monitored at a glance.”22 The Head & Neck Cancer Tracking Dashboard (HNC Dashboard), designed by the IRM analyst, queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with HNC by examining outpatient visit and inpatient discharge International Classification of Disease (ICD) codes entered by providers when coding encounter notes in CPRS. It also adds those with a HNC diagnosis in the VistA problem list and the HNC pathology department Systematized Nomenclature of Medicine (SNOMED) codes (Figure 2).
The automated ENT cancer tracking dashboard prototype debuted in 2014, but several months of trial and error took place to reanalyze ICD codes and narrow the list. The dashboard underwent multiple tests to ensure accuracy. Identified patients are presented using an interactive report hosted on a secure SharePoint (Redmond,WA) site, which reduced the risk of a data breach as access requires multi-authenticated user identification from a VA computer.
Another characteristic of the dashboard’s format is the ability to add custom features as needed. Several features now included in the dashboard are location of residence, diagnosis date, ICD code, date captured in the tracking system, most recent ENT clinic visit, future scheduled ENT clinic appointment, date of last thyroid stimulating hormone (TSH) laboratory test, and date of last position emission tomography scan. In addition, cancellations, no-shows, and patients overdue for TSH testing are highlighted in bold. Highlighted fields alert the CCC to reschedule patients in a timely manner and can alert providers to order needed follow-up tests and procedures.
Among the merits of the ENT cancer tracking dashboard is ease of use. The CCC uses a simple ABC acronym to describe utilization:
- A—added: The CCC daily edits new patients added to the dashboard with a HNC diagnosis. Several times recently the CCC saw a new diagnosis before the provider had been notified by pathology of biopsy results (Figure 3).
- B—browse: The dashboard format allows for rapid perusal of critical information at a glance (Figure 4). Recent labs and imaging can be discussed with providers immediately or at weekly ENT team cancer update meetings. Notification to clinicians can be rapid if the results show suspicion for residual/recurrent disease, a second primary site, metastasis, or there is need to notify the patient’s primary care provider to treat elevated TSH levels (hypothyroidism incidence after head and neck radiation is reportedly as high as 44%, with most patients being asymptomatic or simply fatigued).10,23
- C—check: Appointments are checked for those in the future, cancelled without rescheduling, or no-show dates. Empty fields under the “Next ENT Appointment” header alert the CCC to reschedule a follow-up appointment within NCCN guidelines. Alerting providers to upcoming surveillance appointments allows timely coordination with other care providers and departments, including speech pathology, nutrition, audiology, and social work. The “ENT Recall Date” has a unique time-sensitive feature and will visually display a bold type font when ready to be scheduled for a physical appointment (Figure 5).
Results
The cancer dashboard has demonstrated its success by supporting consistent and reliable monthly data. Results recorded over a 24-month period (from January 1, 2015 through December 31, 2016) showed that the electronic tracker identified 101 new HNC patients. During this period, 1,067 HNC patients were scheduled for follow-up appointments for cancer surveillance. Of these, the authors found that 112 HNC patients had missed their appointments due to calling and cancelling or not showing up as scheduled; resulting in a no-show status. This yielded an appointment nonadherence rate of 10%. The authors also found that 73 (7%) HNC patients did not have an elected scheduled appointment to return to the clinic for continued cancer surveillance. This number comprises all HNC patients whose appointments were cancelled by clinic cancellation, self-cancellation, no-show appointments, or those who left the clinic without scheduling a subsequent follow-up appointment. The electronic tracker identified 100% of these patients as missing and needing a future appointment. These patients may have otherwise been lost through manual tracking.
Implementation and utilization of a robust automated dashboard format HNC patient tracking system has been rewarding for the ENT department. The CCC has saved an estimated 600 to 800 hours per year of chart review and data entry. Although a time study was never conducted to measure the work process of this task, it is reasonable to conclude based on the following multiple manual step-by-step processes that the CCC had to perform frequently were now performed within the dashboard: reviewing consults for HNC diagnosis, recording new patient profile data on the spreadsheet; reviewing VA hospital pathology reports for new HNC diagnoses, reviewing the clinic schedule to track patient appointment adherence, updating and recording recent appointment activity, and reviewing the electronic medical records daily for recommended treatment plan and follow-up.
A side-by-side comparison of the functional features of tracking both manually and with automation showed that automation outnumbers the function of manual tracking by 36% and offers improved efficiency (Table). This has allowed time for the CCC to participate in simultaneous HNC care initiatives, including facilitating interfacility telehealth referrals for complex cancer surgery, scheduling and monitoring rural cancer surveillance telehealth appointments, and development of an ENT Survivorship Care Plan. These programs optimize time and workflow, reduce waste, reduce expenditures related to costly treatment modalities associated with advanced stages of malignancy, and improve the veteran experience. Further benefits to the veteran HNC patient population include increased self-efficacy and awareness for disease management through continuity of care, reduced cost associated with travel expense, and reduced potential copays due to additional medical care related to advanced stages of recurrent or residual disease.
In-house development of the HNC tracking dashboard has contributed to further cost savings for the VA. Specialized third-party acquired software can cost thousands of dollars for purchase and implementation and often includes ongoing fees for use. The Sustain and Spread concept of Lean Six Sigma is proven by a 100% recapture rate of HNC patients in the ENT clinic that potentially would have been lost to follow-up. The success in Spreading this innovation forward has resulted in adoption by other VAMCs for current use and implementation. After sharing information regarding the dashboard at 2 national conferences via presentations and poster, other VAMCs in neighboring states have requested the software and initiated custom versions. Because of this success and further demand, dashboard use is currently under consideration by the VA for nationwide availability.
Conclusion
Deficiencies in tracking cancer patients in the VA system exist in part due to little or no sophisticated electronic tracking systems that could perform multiple task functions to identify new cancer patients, the type of cancer, when appointments are missed, and notification when the required labs and procedures are completed. Often, the CCC is dependent on the arduous task of inputting of data to keep him/her up-to-date with patient care and coordination in a timely manner. As new VA policies attempts to perfect and streamline the scheduling process by way of providers placing “return to clinic” orders for patient follow-up care, there remains a potential risk of those patients not getting scheduled without a vigilant tracking process in place to monitor and ensure that all patients are scheduled.
The dashboard has proved to be an easy to use and vital tool in tracking HNC patients by the CCC. It will continue to assist in the identification of new HNC patients, provide ready access to patient information and follow-up care, and help facilitate CCC and provider communication on a daily basis, thereby meeting the goal of a patient-centered product that proves to improve the quality of cancer care of veterans.
Acknowledgment
The authors thank Mr. Dominic B. Ruiz, Visual Information Specialist, at the Raymond Murphy VAMC, who created images in high resolution for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner , Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
The American Cancer Society estimates that there were 1.68 million newly diagnosed cases of cancer in the U.S. in 2016, with an associated 595,690 deaths.1 Of this number, about 3% was attributable to head and neck cancer (HNC), with 48,330 new cases and 9,570 deaths in 2016. Cancer is among the leading causes of death worldwide, and veterans have a prevalence of HNC nearly twice that of the general population.2 The number of people living with and beyond a cancer diagnosis in the U.S. has risen to an estimated 15.5 million survivors.
Head and neck cancer comprises several subsites, including the oral cavity (lips, buccal mucosa, anterior tongue, floor of mouth, hard palate, and gingiva), the pharynx (nasopharynx, oropharynx, and hypopharynx), the larynx (supraglottis, glottis, and subglottis), the nasal cavity, paranasal sinuses, and the saliva glands.3 The economic burden for HNC treatment was estimated at $3.64 billion in 2010.4
Treatment is based on primary site and staging, and staging is according to the tumor node metastasis system of the American Joint Committee on Cancer.5 In general, lower stages (in situ, stages I and II) are treated with single modalities of organ-sparing surgery or radiation, whereas higher stages (stages III and IV) are treated with multiple modalities, which may include radiation combined with chemotherapy or surgery before or after radiation/chemotherapy.
Survival rate after treatment varies by primary site, cancer stage at diagnosis, histopathologic cell type, viral association, tobacco use, chemical exposure, and treatment modality; survival ranges from 24% to 90% at 5 years based on these variables.6 There is not yet a reliable blood test or other biochemical marker for recurrence, and serial radiologic examinations are expensive and expose the survivor to large amounts of additional ionizing radiation.7,8 Surveillance for recurrence after treatment consists primarily of physical examination and reported symptoms, which may be difficult for the primary care provider (PCP) to perform and distinguish from treatment sequelae.9,10 Thus, HNC survivors are followed in the ear, nose, and throat (ENT) otolaryngology clinic on a decreasing frequency schedule based on risk of relapse, second primaries, treatment sequelae, and toxicities (every 1-3 months in year 1, 2-6 months in year 2, 4-8 months in years 3-5, and every 12 months after 5 years) according to the National Comprehensive Cancer Network (NCCN) guidelines.11
Adherence with posttreatment surveillance in HNC recently was associated with length of survival; however, this observation at a single tertiary academic center was discordant with earlier published reports.12-15 About 80% to 90% of all postcurative intent treatment recurrences and second primary cancers occur within the first 4 years, with a better functional outcome if the recurrence is surgically salvageable or amenable to adjuvant radiation or combined radiation and chemotherapy.16,17 Nonadherence is generally associated with worse clinical and acute care utilization outcomes.18
Problem
At the Raymond G. Murphy VAMC, a tertiary care center in Albuquerque, New Mexico, there was a propensity of veteran HNC patients who missed scheduled surveillance appointments or were lost to follow-up. An informal review of several VA ENT departments revealed similar issues without any consistent method to solve the problem. In an effort to recapture these patients, in 2011 an ENT registered nurse (RN) was added to the team as cancer care coordinator (CCC). After several weeks of chart review of clinic records, it was determined that 31% of HNC patients had missed 1 or more ongoing surveillance appointments, either by patient no-show, clinic cancellations that failed to reschedule patients, or patient cancellation without rescheduling. The CCC was tasked with recapturing these lost patients, returning them to regular follow-up per NCCN guidelines, and tracking new cancer patients as they were diagnosed and progressed through treatment and surveillance. As there had been no one previously in this role in the ENT clinic, there was no guidance about how to proceed.
The mechanism in place for rescheduling no-show patients at that time consisted of a mailed postcard reminder sent by a medical support assistant who requested that the veteran contact the clinic to reschedule. Veterans reported that these reminders often appeared in their mail mingled with so-called junk mail and were discarded without reading. The CCC spent several more weeks examining clinic records in the computerized patient record system (CPRS), looking for patients with cancer in the 5-year surveillance period, and compiling a database of survivors and newly diagnosed patients. This database was compiled initially on paper and then converted to a spreadsheet. Patients who had missed appointments were contacted by the CCC and rescheduled, which resulted in a 100% recovery rate.
Unfortunately, although the manual tracking process was successful, it was laborious and time consuming. Weekly and sometimes daily examination of CPRS clinic records for new patients and survivor adherence was followed by tedious data entry into the spreadsheet. The manual tracking system was deemed suboptimal and a Lean Six Sigma process improvement project was initiated. The project goal was to produce a dashboard database tool that was patient centered to improve the quality of cancer care to veterans.
Methods
Lean Six Sigma is a combination of 2 improvement processes and is embraced by large business and government entities with the goal of improving efficiencies, reducing waste, decreasing errors, and generating cost savings.19 The first improvement process, Six Sigma, is a statistical concept with the goal of producing no more than 3.4 defects per million opportunities.20Using specific tools, Six Sigma identifies the cause of the problem to help develop effective solutions. Six Sigma also helps uncover defects and problems by using a standardized and systematic method for each process improvement project in a sequence of steps known as DMAIC (Define, Measure, Analyze, Improve, and Control) to ensure a defect-free product at a rate of 99.99966%. Define, the first step, contains a written statement defining the problem and the goals; Measure scrutinizes the current baseline of the project in measureable data to identify possible contributing factors; Analyze uses data and tools to understand the cause-and-effect relationships in the process; Improve uses creative developments and changes that lead to process improvements; and Control takes measures to ensure the improvements are implemented, reliable, and constant.
Although slightly different but complementary, Lean focuses on streamlining improvement processes by identifying and eliminating waste that has little or no value to the customer. The 8 most common forms of waste are identified through the mnemonic DOWNTIME (Defects, Overproduction, Waiting, Not utilizing human talent, Transportation, Inventory excess, Motion excess, and Excess processing).21 When both Lean and Six Sigma are used together, the synergistic effects have a powerful impact on the complete quality improvement process and yield consistent reliability. The combined process then includes several methodologic tools for systems redesign, including root-cause analysis, defining waste barriers, measuring current and expected performance, analyzing the data collected, improving the target process, and controlling the improvements. Though already existing and used within the VA system, Lean Six Sigma training was included as a mandatory component of new employee orientation in a memo issued in August 2015 from the assistant secretary for human resources and administration (VA access-only memo VAIQ 7595924).
Root-cause analysis was accomplished using the “5 Why” technique adapted into Lean and Six Sigma from the Toyota Motor Corporation. For example, the question “Why do patients miss appointments?” was asked 5 different ways, and it was determined that many patients lacked transportation, some were not able to reschedule at the time they called to cancel their appointment, those with multiple same-day appointments at the tertiary medical center were not able to wait to schedule a follow-up appointment for fear of missing or being late to their next appointment, and others were placed on recall lists with appointment reminders that failed to accomplish the purpose of self-scheduling by veterans. Thus, the common denominator and answer to the question “why” was that there was no tracking system in place to identify and reschedule missed follow-ups, and before employing a dedicated coordinator, no one accountable for the process (Figure 1).
Wasteful barriers to efficiency were examined with particular attention to the rescheduling process. Rescheduling produced immediate duplication of work for scheduling staff and increased wait time for future appointments. There was potential for additional health care expenses related to costs of late and progressive salvage treatment or for less-than-timely correction of HNC treatment sequelae, such as scarring, lymphedema, or dysphagia. Ear, nose, and throat providers were concerned about missing occult recurrence or residual cancer.
In 2013, the Lean Six Sigma process was used again to critique efforts by the CCC to identify and track HNC patients. One suggestion was to automate the process, and the Information Resource Management (IRM) office was contacted via work order to explore options for mining CPRS data. Working with a committed health information analyst, further discussion was aimed at pulling in additional data that would simultaneously track required posttreatment laboratory results and imaging. It was decided that a secure dashboard format would provide greater utility than would an online report that the CCC had to request and generate daily.
Integrated technologist Stephen Few defines a data dashboard as “… a visual display of the most important information needed to achieve one or more objectives; consolidated and arranged on a single screen so the information can be monitored at a glance.”22 The Head & Neck Cancer Tracking Dashboard (HNC Dashboard), designed by the IRM analyst, queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with HNC by examining outpatient visit and inpatient discharge International Classification of Disease (ICD) codes entered by providers when coding encounter notes in CPRS. It also adds those with a HNC diagnosis in the VistA problem list and the HNC pathology department Systematized Nomenclature of Medicine (SNOMED) codes (Figure 2).
The automated ENT cancer tracking dashboard prototype debuted in 2014, but several months of trial and error took place to reanalyze ICD codes and narrow the list. The dashboard underwent multiple tests to ensure accuracy. Identified patients are presented using an interactive report hosted on a secure SharePoint (Redmond,WA) site, which reduced the risk of a data breach as access requires multi-authenticated user identification from a VA computer.
Another characteristic of the dashboard’s format is the ability to add custom features as needed. Several features now included in the dashboard are location of residence, diagnosis date, ICD code, date captured in the tracking system, most recent ENT clinic visit, future scheduled ENT clinic appointment, date of last thyroid stimulating hormone (TSH) laboratory test, and date of last position emission tomography scan. In addition, cancellations, no-shows, and patients overdue for TSH testing are highlighted in bold. Highlighted fields alert the CCC to reschedule patients in a timely manner and can alert providers to order needed follow-up tests and procedures.
Among the merits of the ENT cancer tracking dashboard is ease of use. The CCC uses a simple ABC acronym to describe utilization:
- A—added: The CCC daily edits new patients added to the dashboard with a HNC diagnosis. Several times recently the CCC saw a new diagnosis before the provider had been notified by pathology of biopsy results (Figure 3).
- B—browse: The dashboard format allows for rapid perusal of critical information at a glance (Figure 4). Recent labs and imaging can be discussed with providers immediately or at weekly ENT team cancer update meetings. Notification to clinicians can be rapid if the results show suspicion for residual/recurrent disease, a second primary site, metastasis, or there is need to notify the patient’s primary care provider to treat elevated TSH levels (hypothyroidism incidence after head and neck radiation is reportedly as high as 44%, with most patients being asymptomatic or simply fatigued).10,23
- C—check: Appointments are checked for those in the future, cancelled without rescheduling, or no-show dates. Empty fields under the “Next ENT Appointment” header alert the CCC to reschedule a follow-up appointment within NCCN guidelines. Alerting providers to upcoming surveillance appointments allows timely coordination with other care providers and departments, including speech pathology, nutrition, audiology, and social work. The “ENT Recall Date” has a unique time-sensitive feature and will visually display a bold type font when ready to be scheduled for a physical appointment (Figure 5).
Results
The cancer dashboard has demonstrated its success by supporting consistent and reliable monthly data. Results recorded over a 24-month period (from January 1, 2015 through December 31, 2016) showed that the electronic tracker identified 101 new HNC patients. During this period, 1,067 HNC patients were scheduled for follow-up appointments for cancer surveillance. Of these, the authors found that 112 HNC patients had missed their appointments due to calling and cancelling or not showing up as scheduled; resulting in a no-show status. This yielded an appointment nonadherence rate of 10%. The authors also found that 73 (7%) HNC patients did not have an elected scheduled appointment to return to the clinic for continued cancer surveillance. This number comprises all HNC patients whose appointments were cancelled by clinic cancellation, self-cancellation, no-show appointments, or those who left the clinic without scheduling a subsequent follow-up appointment. The electronic tracker identified 100% of these patients as missing and needing a future appointment. These patients may have otherwise been lost through manual tracking.
Implementation and utilization of a robust automated dashboard format HNC patient tracking system has been rewarding for the ENT department. The CCC has saved an estimated 600 to 800 hours per year of chart review and data entry. Although a time study was never conducted to measure the work process of this task, it is reasonable to conclude based on the following multiple manual step-by-step processes that the CCC had to perform frequently were now performed within the dashboard: reviewing consults for HNC diagnosis, recording new patient profile data on the spreadsheet; reviewing VA hospital pathology reports for new HNC diagnoses, reviewing the clinic schedule to track patient appointment adherence, updating and recording recent appointment activity, and reviewing the electronic medical records daily for recommended treatment plan and follow-up.
A side-by-side comparison of the functional features of tracking both manually and with automation showed that automation outnumbers the function of manual tracking by 36% and offers improved efficiency (Table). This has allowed time for the CCC to participate in simultaneous HNC care initiatives, including facilitating interfacility telehealth referrals for complex cancer surgery, scheduling and monitoring rural cancer surveillance telehealth appointments, and development of an ENT Survivorship Care Plan. These programs optimize time and workflow, reduce waste, reduce expenditures related to costly treatment modalities associated with advanced stages of malignancy, and improve the veteran experience. Further benefits to the veteran HNC patient population include increased self-efficacy and awareness for disease management through continuity of care, reduced cost associated with travel expense, and reduced potential copays due to additional medical care related to advanced stages of recurrent or residual disease.
In-house development of the HNC tracking dashboard has contributed to further cost savings for the VA. Specialized third-party acquired software can cost thousands of dollars for purchase and implementation and often includes ongoing fees for use. The Sustain and Spread concept of Lean Six Sigma is proven by a 100% recapture rate of HNC patients in the ENT clinic that potentially would have been lost to follow-up. The success in Spreading this innovation forward has resulted in adoption by other VAMCs for current use and implementation. After sharing information regarding the dashboard at 2 national conferences via presentations and poster, other VAMCs in neighboring states have requested the software and initiated custom versions. Because of this success and further demand, dashboard use is currently under consideration by the VA for nationwide availability.
Conclusion
Deficiencies in tracking cancer patients in the VA system exist in part due to little or no sophisticated electronic tracking systems that could perform multiple task functions to identify new cancer patients, the type of cancer, when appointments are missed, and notification when the required labs and procedures are completed. Often, the CCC is dependent on the arduous task of inputting of data to keep him/her up-to-date with patient care and coordination in a timely manner. As new VA policies attempts to perfect and streamline the scheduling process by way of providers placing “return to clinic” orders for patient follow-up care, there remains a potential risk of those patients not getting scheduled without a vigilant tracking process in place to monitor and ensure that all patients are scheduled.
The dashboard has proved to be an easy to use and vital tool in tracking HNC patients by the CCC. It will continue to assist in the identification of new HNC patients, provide ready access to patient information and follow-up care, and help facilitate CCC and provider communication on a daily basis, thereby meeting the goal of a patient-centered product that proves to improve the quality of cancer care of veterans.
Acknowledgment
The authors thank Mr. Dominic B. Ruiz, Visual Information Specialist, at the Raymond Murphy VAMC, who created images in high resolution for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner , Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Patil RD, Meinzen-Derr JK, Hendricks BL, Patil YJ. Improving access and timelines of care for veterans with head and neck squamous cell carcinoma: a multidisciplinary team’s approach. Laryngoscope. 2016;126(3):627-631.
3. Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865-882.
4. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
5. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
6. Cancer.net. Head and neck cancer: statistics. http://www.cancer.net/cancer-types/head-and-neck-cancer/Statistics. Updated September 2016. Accessed April 12, 2017.
7. Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1068-E1074.
8. Cheung PK, Chin RY, Eslick GD. Detecting residual/recurrent head neck squamous cell carcinomas using PET or PET/CT: systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2016;154(3):421-432.
9. Haddad RI, Limaye S. Overview of approach to long-term survivors of head and neck cancer. http://www .uptodate .com/contents/overview-of-approach-to-long-term-survivors-of-head-and-neck-cancer. Updated October 26, 2016. Accessed April 12, 2017.
10. Manikantan K, Khode S, Dwivedi RC, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35(8):744-753.
11. National Comprehensive Cancer Network. NCCN Clinical practice guidelines in onclology:head and neck cancers(2.2017).2017. Updated May 8, 2017. https://www.nccn.org/professionals/physician_gls/f_/pdf/head-and-neck.pdf. Accessed July 18, 2017.
12. Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Schnayder Y. The impact of compliance in post treatment surveillance in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(6):519-525.
13. Merkx MA, van Gulick JJ, Marres HA, et al. Effectiveness of routine follow-up of patients treated for T1-2N0 oral squamous cell carcinomas of the floor of mouth and tongue. Head Neck. 2006:28(1):1-7.
14. Ritoe SC, de Vegt F, Scheike IM, et al. Effect of routine follow-up after treatment for laryngeal cancer on life expectancy and mortality: results of a Markov model analysis. Cancer. 2007;109(2):239-247.
15. Agrawal A, Hammond TH, Young GS, Avon AL, Ozer E, Schuller DE. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope. 2009;119(11):2135-2140.
16. Roland NJ, Bradley PJ. The role of surgery in the palliation of head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):101-108.
17. Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111(3):382-387.
18. Hwang AS, Atlas SJ, Cronin P, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
19. Healthcare Daily Online. VA healthcare system adopts lean six sigma. http://www.healthcaredailyonline.com/news/va-lean-six-sigma-in-healthcare. Updated December 7, 2015. Accessed April 12, 2017.
20. Gygi C, Williams B. Six Sigma for Dummies. 2nd edition. Hoboken, NJ: John Wiley & Sons; 2012.
21. Kavanagh S, Krings D. The 8 sources of waste and how to eliminate them: improving performance with LEAN management techniques. http://www.gfoa.org/sites/default /files/GFR_DEC_11_18.pdf. Updated December, 2011. Accessed April 14, 2017.
22. Few S. What is a dashboard? In: Wheeler C, ed. Information Dashboard Design: The Effective Visual Communication of Data. 1st ed. Sebastopol, CA: O’Reilly Media; 2006:34.
23. Murthy V, Narang K, Ghosh-Laskar S, Gupta T, Budrukkar A, Agrawal JP. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck. 2014;36(11):1573-1780.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Patil RD, Meinzen-Derr JK, Hendricks BL, Patil YJ. Improving access and timelines of care for veterans with head and neck squamous cell carcinoma: a multidisciplinary team’s approach. Laryngoscope. 2016;126(3):627-631.
3. Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865-882.
4. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
5. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
6. Cancer.net. Head and neck cancer: statistics. http://www.cancer.net/cancer-types/head-and-neck-cancer/Statistics. Updated September 2016. Accessed April 12, 2017.
7. Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1068-E1074.
8. Cheung PK, Chin RY, Eslick GD. Detecting residual/recurrent head neck squamous cell carcinomas using PET or PET/CT: systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2016;154(3):421-432.
9. Haddad RI, Limaye S. Overview of approach to long-term survivors of head and neck cancer. http://www .uptodate .com/contents/overview-of-approach-to-long-term-survivors-of-head-and-neck-cancer. Updated October 26, 2016. Accessed April 12, 2017.
10. Manikantan K, Khode S, Dwivedi RC, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35(8):744-753.
11. National Comprehensive Cancer Network. NCCN Clinical practice guidelines in onclology:head and neck cancers(2.2017).2017. Updated May 8, 2017. https://www.nccn.org/professionals/physician_gls/f_/pdf/head-and-neck.pdf. Accessed July 18, 2017.
12. Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Schnayder Y. The impact of compliance in post treatment surveillance in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(6):519-525.
13. Merkx MA, van Gulick JJ, Marres HA, et al. Effectiveness of routine follow-up of patients treated for T1-2N0 oral squamous cell carcinomas of the floor of mouth and tongue. Head Neck. 2006:28(1):1-7.
14. Ritoe SC, de Vegt F, Scheike IM, et al. Effect of routine follow-up after treatment for laryngeal cancer on life expectancy and mortality: results of a Markov model analysis. Cancer. 2007;109(2):239-247.
15. Agrawal A, Hammond TH, Young GS, Avon AL, Ozer E, Schuller DE. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope. 2009;119(11):2135-2140.
16. Roland NJ, Bradley PJ. The role of surgery in the palliation of head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):101-108.
17. Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111(3):382-387.
18. Hwang AS, Atlas SJ, Cronin P, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
19. Healthcare Daily Online. VA healthcare system adopts lean six sigma. http://www.healthcaredailyonline.com/news/va-lean-six-sigma-in-healthcare. Updated December 7, 2015. Accessed April 12, 2017.
20. Gygi C, Williams B. Six Sigma for Dummies. 2nd edition. Hoboken, NJ: John Wiley & Sons; 2012.
21. Kavanagh S, Krings D. The 8 sources of waste and how to eliminate them: improving performance with LEAN management techniques. http://www.gfoa.org/sites/default /files/GFR_DEC_11_18.pdf. Updated December, 2011. Accessed April 14, 2017.
22. Few S. What is a dashboard? In: Wheeler C, ed. Information Dashboard Design: The Effective Visual Communication of Data. 1st ed. Sebastopol, CA: O’Reilly Media; 2006:34.
23. Murthy V, Narang K, Ghosh-Laskar S, Gupta T, Budrukkar A, Agrawal JP. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck. 2014;36(11):1573-1780.
Company narrows focus of development for tazemetostat
Epizyme, Inc., has announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with diffuse large B-cell lymphoma (DLBCL).
However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Tazemetostat is an EZH2 inhibitor being developed to treat multiple hematologic and solid tumor malignancies.
Epizyme has been conducting a phase 1/2 trial of tazemetostat in patients with relapsed and/or refractory DLBCL as well as other B-cell lymphomas and solid tumors (NCT01897571).
The trial includes DLBCL patients with and without EZH2 activating mutations. Some patients were assigned to receive tazemetostat monotherapy, and some were assigned to tazemetostat in combination with prednisolone.
Epizyme has conducted an interim assessment of data from this trial and concluded that the clinical activity observed “is not sufficient to warrant further development of tazemetostat in DLBCL as a monotherapy or in combination with prednisolone.”
Epizyme said it plans to present data from this trial at a medical meeting in the second half of 2018.
The company is still conducting other studies of tazemetostat in patients with DLBCL.
In one study (NCT02889523), Epizyme and the Lymphoma Academic Research Organisation are evaluating tazemetostat in combination with R-CHOP (rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisolone) in patients with newly diagnosed DLBCL.
In another study (NCT03028103), Epizyme is evaluating tazemetostat in combination with fluconazole or omeprazole and repaglinide in patients with relapsed/refractory DLBCL, other B-cell lymphomas, or solid tumor malignancies.
Epizyme, Inc., has announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with diffuse large B-cell lymphoma (DLBCL).
However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Tazemetostat is an EZH2 inhibitor being developed to treat multiple hematologic and solid tumor malignancies.
Epizyme has been conducting a phase 1/2 trial of tazemetostat in patients with relapsed and/or refractory DLBCL as well as other B-cell lymphomas and solid tumors (NCT01897571).
The trial includes DLBCL patients with and without EZH2 activating mutations. Some patients were assigned to receive tazemetostat monotherapy, and some were assigned to tazemetostat in combination with prednisolone.
Epizyme has conducted an interim assessment of data from this trial and concluded that the clinical activity observed “is not sufficient to warrant further development of tazemetostat in DLBCL as a monotherapy or in combination with prednisolone.”
Epizyme said it plans to present data from this trial at a medical meeting in the second half of 2018.
The company is still conducting other studies of tazemetostat in patients with DLBCL.
In one study (NCT02889523), Epizyme and the Lymphoma Academic Research Organisation are evaluating tazemetostat in combination with R-CHOP (rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisolone) in patients with newly diagnosed DLBCL.
In another study (NCT03028103), Epizyme is evaluating tazemetostat in combination with fluconazole or omeprazole and repaglinide in patients with relapsed/refractory DLBCL, other B-cell lymphomas, or solid tumor malignancies.
Epizyme, Inc., has announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with diffuse large B-cell lymphoma (DLBCL).
However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Tazemetostat is an EZH2 inhibitor being developed to treat multiple hematologic and solid tumor malignancies.
Epizyme has been conducting a phase 1/2 trial of tazemetostat in patients with relapsed and/or refractory DLBCL as well as other B-cell lymphomas and solid tumors (NCT01897571).
The trial includes DLBCL patients with and without EZH2 activating mutations. Some patients were assigned to receive tazemetostat monotherapy, and some were assigned to tazemetostat in combination with prednisolone.
Epizyme has conducted an interim assessment of data from this trial and concluded that the clinical activity observed “is not sufficient to warrant further development of tazemetostat in DLBCL as a monotherapy or in combination with prednisolone.”
Epizyme said it plans to present data from this trial at a medical meeting in the second half of 2018.
The company is still conducting other studies of tazemetostat in patients with DLBCL.
In one study (NCT02889523), Epizyme and the Lymphoma Academic Research Organisation are evaluating tazemetostat in combination with R-CHOP (rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisolone) in patients with newly diagnosed DLBCL.
In another study (NCT03028103), Epizyme is evaluating tazemetostat in combination with fluconazole or omeprazole and repaglinide in patients with relapsed/refractory DLBCL, other B-cell lymphomas, or solid tumor malignancies.
PET/CT accurately predicts MCL stage
Bone marrow involvement in mantle cell lymphoma could be assessed using just 18fluorodeoxyglucose (FDG)–PET/CT, according to findings from a small, retrospective study published in Clinical Lymphoma, Myeloma & Leukemia.
Rustain Morgan, MD, of the University of Colorado, Aurora, and his colleagues found that, at a certain threshold of bone marrow voxels in standard uptake value (SUV), there was 100% sensitivity and 80% specificity in determining bone marrow involvement in mantle cell lymphoma (MCL).
Currently, National Comprehensive Cancer Network guidelines call for bone marrow biopsy and whole body FDG PET/CT scan to complete an initial diagnosis of MCL.
“One of the most important factors for correct staging is the identification of bone marrow involvement, occurring in approximately 55% of patients with MCL, which classifies patients as advanced stage. However, accurate analysis of bone marrow involvement can be challenging due to sampling error,” the researchers wrote. “While bone marrow biopsy remains the gold standard, it is not a perfect standard given unilateral variability.”
In previous studies, FDG PET/CT was not considered sensitive enough to detect gastrointestinal or bone marrow involvement. However, these earlier studies used SUV maximum or mean or a visual assessment of the bone marrow activity, compared with hepatic uptake. To address this issue, the researchers developed a new method of examining SUV distribution throughout the pelvic bones by analyzing thousands of bone marrow voxels within the bilateral iliacs.
During the developmental phase, an institutional dataset of 11 patients with MCL was used to define the voxel-based analysis. These patients had undergone both unilateral iliac bone marrow biopsy and FDG PET/CT at the initial diagnosis. Then, FDG PET/CT scans from another 12 patients with MCL from a different institution were used to validate the developmental phase findings. Finally, a control group of 5 people with no known malignancy were referred for FDG PET/CT pulmonary nodule evaluation.
“The hypothesis of the study was that, if the bone marrow was involved by lymphoma, then there would be a small increase in the SUV of each voxel, reflecting involvement by the lymphoma. In order to capture such changes, we analyzed the percent of total voxels in SUV ranging from 0.75 to 1.20, in increments of 0.05, as this is where the greatest divergence was visually identified,” the researchers wrote. “The goal was to identify if a percentage of voxels at a set SUV could detect lymphomatous involvement.”
The researchers identified 10 candidate thresholds in the developmental phase; 4 of these performed better than the others in the validation phase. Using those thresholds, 10 of the 12 patients in the validation cohort could be correctly staged using FDG PET/CT.
Further analysis identified a single threshold that performed best: If greater than 38% of the voxels (averaging 1,734 voxels) demonstrated an SUV of less than 0.95, the sensitivity was 100% and the specificity was 80%.
The researchers acknowledged that the findings are limited because of the study’s small sample size and said the results should be validated in a larger trial.
There was no external funding for the study and the researchers reported having no financial disclosures.
SOURCE: Morgan R et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 4. doi: 10.1016/j.clml.2018.06.024.
Bone marrow involvement in mantle cell lymphoma could be assessed using just 18fluorodeoxyglucose (FDG)–PET/CT, according to findings from a small, retrospective study published in Clinical Lymphoma, Myeloma & Leukemia.
Rustain Morgan, MD, of the University of Colorado, Aurora, and his colleagues found that, at a certain threshold of bone marrow voxels in standard uptake value (SUV), there was 100% sensitivity and 80% specificity in determining bone marrow involvement in mantle cell lymphoma (MCL).
Currently, National Comprehensive Cancer Network guidelines call for bone marrow biopsy and whole body FDG PET/CT scan to complete an initial diagnosis of MCL.
“One of the most important factors for correct staging is the identification of bone marrow involvement, occurring in approximately 55% of patients with MCL, which classifies patients as advanced stage. However, accurate analysis of bone marrow involvement can be challenging due to sampling error,” the researchers wrote. “While bone marrow biopsy remains the gold standard, it is not a perfect standard given unilateral variability.”
In previous studies, FDG PET/CT was not considered sensitive enough to detect gastrointestinal or bone marrow involvement. However, these earlier studies used SUV maximum or mean or a visual assessment of the bone marrow activity, compared with hepatic uptake. To address this issue, the researchers developed a new method of examining SUV distribution throughout the pelvic bones by analyzing thousands of bone marrow voxels within the bilateral iliacs.
During the developmental phase, an institutional dataset of 11 patients with MCL was used to define the voxel-based analysis. These patients had undergone both unilateral iliac bone marrow biopsy and FDG PET/CT at the initial diagnosis. Then, FDG PET/CT scans from another 12 patients with MCL from a different institution were used to validate the developmental phase findings. Finally, a control group of 5 people with no known malignancy were referred for FDG PET/CT pulmonary nodule evaluation.
“The hypothesis of the study was that, if the bone marrow was involved by lymphoma, then there would be a small increase in the SUV of each voxel, reflecting involvement by the lymphoma. In order to capture such changes, we analyzed the percent of total voxels in SUV ranging from 0.75 to 1.20, in increments of 0.05, as this is where the greatest divergence was visually identified,” the researchers wrote. “The goal was to identify if a percentage of voxels at a set SUV could detect lymphomatous involvement.”
The researchers identified 10 candidate thresholds in the developmental phase; 4 of these performed better than the others in the validation phase. Using those thresholds, 10 of the 12 patients in the validation cohort could be correctly staged using FDG PET/CT.
Further analysis identified a single threshold that performed best: If greater than 38% of the voxels (averaging 1,734 voxels) demonstrated an SUV of less than 0.95, the sensitivity was 100% and the specificity was 80%.
The researchers acknowledged that the findings are limited because of the study’s small sample size and said the results should be validated in a larger trial.
There was no external funding for the study and the researchers reported having no financial disclosures.
SOURCE: Morgan R et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 4. doi: 10.1016/j.clml.2018.06.024.
Bone marrow involvement in mantle cell lymphoma could be assessed using just 18fluorodeoxyglucose (FDG)–PET/CT, according to findings from a small, retrospective study published in Clinical Lymphoma, Myeloma & Leukemia.
Rustain Morgan, MD, of the University of Colorado, Aurora, and his colleagues found that, at a certain threshold of bone marrow voxels in standard uptake value (SUV), there was 100% sensitivity and 80% specificity in determining bone marrow involvement in mantle cell lymphoma (MCL).
Currently, National Comprehensive Cancer Network guidelines call for bone marrow biopsy and whole body FDG PET/CT scan to complete an initial diagnosis of MCL.
“One of the most important factors for correct staging is the identification of bone marrow involvement, occurring in approximately 55% of patients with MCL, which classifies patients as advanced stage. However, accurate analysis of bone marrow involvement can be challenging due to sampling error,” the researchers wrote. “While bone marrow biopsy remains the gold standard, it is not a perfect standard given unilateral variability.”
In previous studies, FDG PET/CT was not considered sensitive enough to detect gastrointestinal or bone marrow involvement. However, these earlier studies used SUV maximum or mean or a visual assessment of the bone marrow activity, compared with hepatic uptake. To address this issue, the researchers developed a new method of examining SUV distribution throughout the pelvic bones by analyzing thousands of bone marrow voxels within the bilateral iliacs.
During the developmental phase, an institutional dataset of 11 patients with MCL was used to define the voxel-based analysis. These patients had undergone both unilateral iliac bone marrow biopsy and FDG PET/CT at the initial diagnosis. Then, FDG PET/CT scans from another 12 patients with MCL from a different institution were used to validate the developmental phase findings. Finally, a control group of 5 people with no known malignancy were referred for FDG PET/CT pulmonary nodule evaluation.
“The hypothesis of the study was that, if the bone marrow was involved by lymphoma, then there would be a small increase in the SUV of each voxel, reflecting involvement by the lymphoma. In order to capture such changes, we analyzed the percent of total voxels in SUV ranging from 0.75 to 1.20, in increments of 0.05, as this is where the greatest divergence was visually identified,” the researchers wrote. “The goal was to identify if a percentage of voxels at a set SUV could detect lymphomatous involvement.”
The researchers identified 10 candidate thresholds in the developmental phase; 4 of these performed better than the others in the validation phase. Using those thresholds, 10 of the 12 patients in the validation cohort could be correctly staged using FDG PET/CT.
Further analysis identified a single threshold that performed best: If greater than 38% of the voxels (averaging 1,734 voxels) demonstrated an SUV of less than 0.95, the sensitivity was 100% and the specificity was 80%.
The researchers acknowledged that the findings are limited because of the study’s small sample size and said the results should be validated in a larger trial.
There was no external funding for the study and the researchers reported having no financial disclosures.
SOURCE: Morgan R et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 4. doi: 10.1016/j.clml.2018.06.024.
REPORTING FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: If greater than 38% of the voxels demonstrated an standard uptake value of less than 0.95, there was a sensitivity of 100% and a specificity of 80%.
Study details: A retrospective cohort study of 23 patients with mantle cell leukemia and 5 controls.
Disclosures: There was no external funding for the study and the researchers reported having no financial disclosures.
Source: Morgan R et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 4. doi: 10.1016/j.clml.2018.06.024.
Status Epilepticus in Pregnancy
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Clinical History
A 37-year-old pregnant African American woman with a history of epilepsy and polysubstance abuse was found unresponsive in a hotel room. She had four convulsions en route to the hospital. In transit, she received levetiracetam and phenytoin, resulting in the cessation of the clinical seizures.
According to her mother, seizures began at age 16 during her first pregnancy, which was complicated by hypertension. She was prescribed medications for hypertension and phenytoin for seizures. The patient provided a different history, claiming that her seizures began 2 years ago. She denied taking medication for seizures or other health problems.
The patient has two children, ages 22 and 11 years. Past medical history is otherwise unremarkable. She has no allergies. Social history includes cigarette smoking, and alcohol and substance abuse. She lives with her boyfriend and does not work. She is 25 weeks pregnant. Family history was notable only for migraine in her mother and grandmother.
Physical Examination
In the emergency department, blood pressure was 135/65, pulse 121 beats per minute, and oxygen saturation was 97%. She was oriented only to self and did not follow commands. Pupils were equal and reactive. There was no facial asymmetry. She moved all 4 extremities spontaneously. Reflexes were brisk. Oral mucosa was dry. She had no edema in the lower extremities.
Laboratories
Chest x-ray was normal. EKG revealed tachycardia and nonspecific ST changes. Hemoglobin was 11.1 g/dl, hematocrit 32%, white blood cell count 10,900, and platelets 181,000. Electrolytes were normal except for a low sodium of 132 mmol/l (135-145) and bicarbonate of 17 mmol/l (21-31). Glucose was initially 67 mg/dl and dropped to 46 mg/dl. Total protein was 6 g/dl (6.7-8.2) and albumin was 2.7 g/dl (3.2-5.5). Metabolic panel was otherwise normal. Urinalysis was positive for glucose, ketones, and a small amount of blood and protein. There were no bacteria. Blood and urine cultures were negative. Phenytoin level was undetectable. Urine drug screen was positive for cannabinoids and cocaine.
Hospital Course
Hypoglycemia was treated with an ampule of D50 and intravenous fluids. On the obstetrics ward, nurses observed several episodes of head and eye deviation to the right accompanied by decreased responsiveness that lasted approximately 30 seconds. The patient was sent to the electrophysiology lab where an EEG revealed a diffusely slow background (Figure 1).
Figure 1. Generalized Slowing
During the 20-minute EEG recording, the patient had six clinical seizures similar to those described by the nurses. These events correlated with an ictal pattern consisting of 11 HZ_sharp activity in the right occipital temporal region that spread to the right parietal and left occipital temporal regions (Figure 2). Head CT revealed mild generalized atrophy and an enlarged right occipital horn, but no acute lesions (Figure 3).
Figure 2. Partial seizure originating in right occipital temporal region
Figure 3. Mild generalized arophy, greater in right hemisphere
The patient was transferred to intensive care and received fosphenytoin. No further clinical and /or electrographic seizures were identified. The following day, an EEG revealed diffuse slowing without focal seizures (Figure 4). The patient gradually became more alert and cooperative over the next 24 hours. However, the next day no fetal heartbeat was detected. Labor was induced and a stillborn baby delivered. The pathology report indicated that the placenta was between the 5th and 10th percentile for gestational age.
Figure 4. Improved generalized slowing
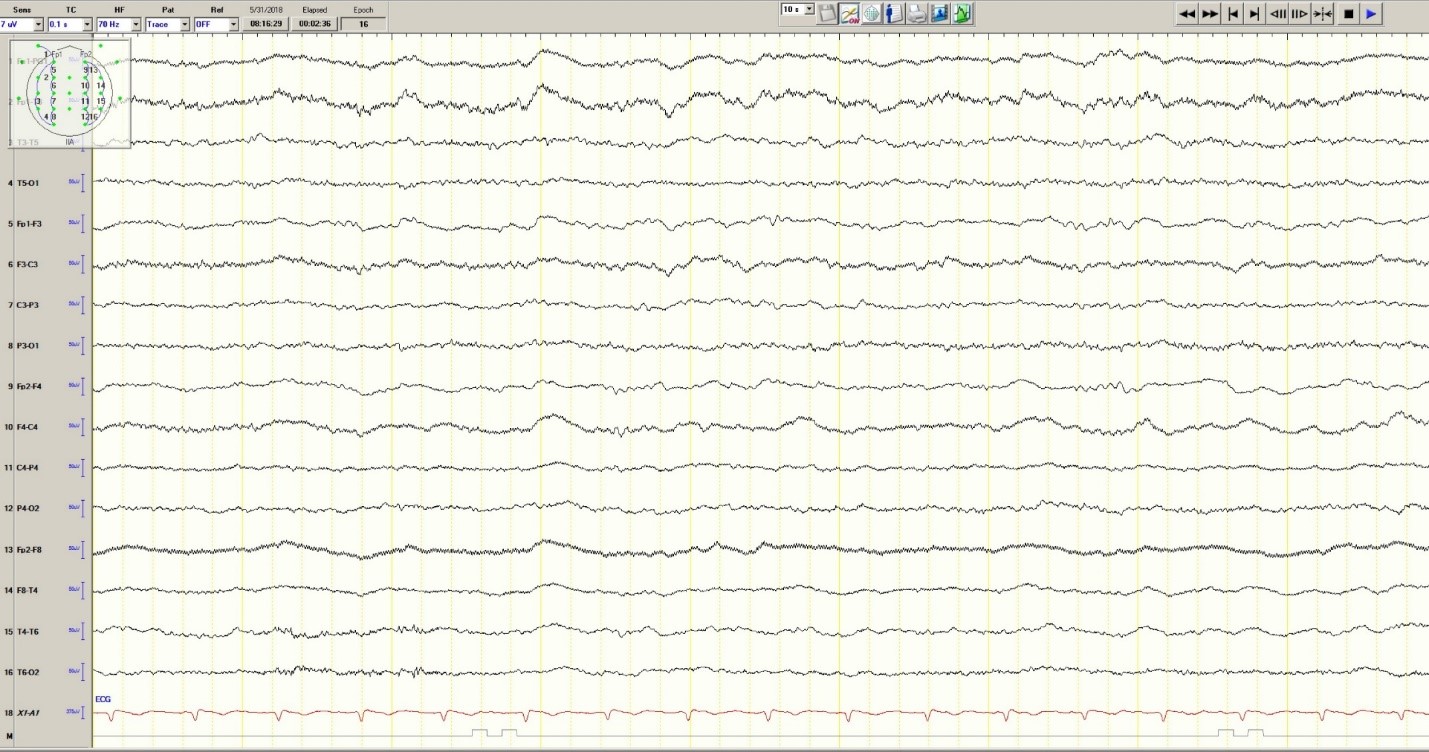
Discussion
Status epilepticus is associated with significant morbidity and mortality (Claassen et al. 2002). This 37-year-old pregnant woman had an episode of focal status epilepticus with impaired awareness likely provoked by nonadherence to antiepileptic drugs (AEDs). Cocaine may have contributed to the episode of status epilepticus (Majlesi et al. 2010). The obstetric service did not diagnose preeclampsia.
The patient’s seizures started in the right occipital region, which was abnormal on neuroimaging. An MRI might have revealed more subtle structural abnormalities such as cortical dysplasia as the etiology of her epilepsy, but she refused the scan.
Women with epilepsy are at increased risk for adverse pregnancy outcomes such as preeclampsia, preterm labor, and stillbirth and should be considered high risk (MacDonald et al. 2015). Serum levels of AEDs such as lamotrigine, levetiracetam and phenytoin may decrease during pregnancy and contribute to breakthrough seizures. Accordingly, monthly measurements of serum levels of AEDs during the entire course of the pregnancy are strongly recommended. These measurements allow for a timely adjustment of AED doses to prevent significant drop in their serum concentrations and minimize the occurrence of breakthrough seizures. In the case of phenytoin, measurement of free and total serum concentrations are recommended. Supplementation with at least 0.4 mg/day to 1 mg /day of folic acid (and up to 4 mg /day) has been recommended (Harden et al. 2009a). Of note, there is no increase in the incidence of status epilepticus due to pregnancy per se (Harden et al. 2009b).
Although the patient survived this episode of status epilepticus, her fetus did not. Antiseizure drug nonadherence and polysubstance abuse probably contributed to fetal demise.
References
Claassen J, Lokin JK, Fitzsimons BFM et al. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002;58:139-142.
Harden CL, Pennell PB, Koppel BS et al. Practice Parameter update: Management issues for women with epilepsy Focus on pregnancy (an evidence-based review): Vitamin K, folic acid, blood levels, and breastfeeding: Neurology 2009a;73:142-149.
Harden CL, Hopp J, Ting TY et al. Practice Parameter update: Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency. Neurology 2009b;50(5):1229-36.
MacDonald SC, Bateman BT, McElrath TF, Hernandez-Diaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Majlesi N, Shih R, Fiesseler FW et al. Cocaine-associated seizures and incidence of status epilepticus. Western Journal of Emergency Medicine. 2010;XI(2):157-160.
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Clinical History
A 37-year-old pregnant African American woman with a history of epilepsy and polysubstance abuse was found unresponsive in a hotel room. She had four convulsions en route to the hospital. In transit, she received levetiracetam and phenytoin, resulting in the cessation of the clinical seizures.
According to her mother, seizures began at age 16 during her first pregnancy, which was complicated by hypertension. She was prescribed medications for hypertension and phenytoin for seizures. The patient provided a different history, claiming that her seizures began 2 years ago. She denied taking medication for seizures or other health problems.
The patient has two children, ages 22 and 11 years. Past medical history is otherwise unremarkable. She has no allergies. Social history includes cigarette smoking, and alcohol and substance abuse. She lives with her boyfriend and does not work. She is 25 weeks pregnant. Family history was notable only for migraine in her mother and grandmother.
Physical Examination
In the emergency department, blood pressure was 135/65, pulse 121 beats per minute, and oxygen saturation was 97%. She was oriented only to self and did not follow commands. Pupils were equal and reactive. There was no facial asymmetry. She moved all 4 extremities spontaneously. Reflexes were brisk. Oral mucosa was dry. She had no edema in the lower extremities.
Laboratories
Chest x-ray was normal. EKG revealed tachycardia and nonspecific ST changes. Hemoglobin was 11.1 g/dl, hematocrit 32%, white blood cell count 10,900, and platelets 181,000. Electrolytes were normal except for a low sodium of 132 mmol/l (135-145) and bicarbonate of 17 mmol/l (21-31). Glucose was initially 67 mg/dl and dropped to 46 mg/dl. Total protein was 6 g/dl (6.7-8.2) and albumin was 2.7 g/dl (3.2-5.5). Metabolic panel was otherwise normal. Urinalysis was positive for glucose, ketones, and a small amount of blood and protein. There were no bacteria. Blood and urine cultures were negative. Phenytoin level was undetectable. Urine drug screen was positive for cannabinoids and cocaine.
Hospital Course
Hypoglycemia was treated with an ampule of D50 and intravenous fluids. On the obstetrics ward, nurses observed several episodes of head and eye deviation to the right accompanied by decreased responsiveness that lasted approximately 30 seconds. The patient was sent to the electrophysiology lab where an EEG revealed a diffusely slow background (Figure 1).
Figure 1. Generalized Slowing

During the 20-minute EEG recording, the patient had six clinical seizures similar to those described by the nurses. These events correlated with an ictal pattern consisting of 11 HZ_sharp activity in the right occipital temporal region that spread to the right parietal and left occipital temporal regions (Figure 2). Head CT revealed mild generalized atrophy and an enlarged right occipital horn, but no acute lesions (Figure 3).
Figure 2. Partial seizure originating in right occipital temporal region

Figure 3. Mild generalized arophy, greater in right hemisphere

The patient was transferred to intensive care and received fosphenytoin. No further clinical and /or electrographic seizures were identified. The following day, an EEG revealed diffuse slowing without focal seizures (Figure 4). The patient gradually became more alert and cooperative over the next 24 hours. However, the next day no fetal heartbeat was detected. Labor was induced and a stillborn baby delivered. The pathology report indicated that the placenta was between the 5th and 10th percentile for gestational age.
Figure 4. Improved generalized slowing

Discussion
Status epilepticus is associated with significant morbidity and mortality (Claassen et al. 2002). This 37-year-old pregnant woman had an episode of focal status epilepticus with impaired awareness likely provoked by nonadherence to antiepileptic drugs (AEDs). Cocaine may have contributed to the episode of status epilepticus (Majlesi et al. 2010). The obstetric service did not diagnose preeclampsia.
The patient’s seizures started in the right occipital region, which was abnormal on neuroimaging. An MRI might have revealed more subtle structural abnormalities such as cortical dysplasia as the etiology of her epilepsy, but she refused the scan.
Women with epilepsy are at increased risk for adverse pregnancy outcomes such as preeclampsia, preterm labor, and stillbirth and should be considered high risk (MacDonald et al. 2015). Serum levels of AEDs such as lamotrigine, levetiracetam and phenytoin may decrease during pregnancy and contribute to breakthrough seizures. Accordingly, monthly measurements of serum levels of AEDs during the entire course of the pregnancy are strongly recommended. These measurements allow for a timely adjustment of AED doses to prevent significant drop in their serum concentrations and minimize the occurrence of breakthrough seizures. In the case of phenytoin, measurement of free and total serum concentrations are recommended. Supplementation with at least 0.4 mg/day to 1 mg /day of folic acid (and up to 4 mg /day) has been recommended (Harden et al. 2009a). Of note, there is no increase in the incidence of status epilepticus due to pregnancy per se (Harden et al. 2009b).
Although the patient survived this episode of status epilepticus, her fetus did not. Antiseizure drug nonadherence and polysubstance abuse probably contributed to fetal demise.
References
Claassen J, Lokin JK, Fitzsimons BFM et al. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002;58:139-142.
Harden CL, Pennell PB, Koppel BS et al. Practice Parameter update: Management issues for women with epilepsy Focus on pregnancy (an evidence-based review): Vitamin K, folic acid, blood levels, and breastfeeding: Neurology 2009a;73:142-149.
Harden CL, Hopp J, Ting TY et al. Practice Parameter update: Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency. Neurology 2009b;50(5):1229-36.
MacDonald SC, Bateman BT, McElrath TF, Hernandez-Diaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Majlesi N, Shih R, Fiesseler FW et al. Cocaine-associated seizures and incidence of status epilepticus. Western Journal of Emergency Medicine. 2010;XI(2):157-160.
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Clinical History
A 37-year-old pregnant African American woman with a history of epilepsy and polysubstance abuse was found unresponsive in a hotel room. She had four convulsions en route to the hospital. In transit, she received levetiracetam and phenytoin, resulting in the cessation of the clinical seizures.
According to her mother, seizures began at age 16 during her first pregnancy, which was complicated by hypertension. She was prescribed medications for hypertension and phenytoin for seizures. The patient provided a different history, claiming that her seizures began 2 years ago. She denied taking medication for seizures or other health problems.
The patient has two children, ages 22 and 11 years. Past medical history is otherwise unremarkable. She has no allergies. Social history includes cigarette smoking, and alcohol and substance abuse. She lives with her boyfriend and does not work. She is 25 weeks pregnant. Family history was notable only for migraine in her mother and grandmother.
Physical Examination
In the emergency department, blood pressure was 135/65, pulse 121 beats per minute, and oxygen saturation was 97%. She was oriented only to self and did not follow commands. Pupils were equal and reactive. There was no facial asymmetry. She moved all 4 extremities spontaneously. Reflexes were brisk. Oral mucosa was dry. She had no edema in the lower extremities.
Laboratories
Chest x-ray was normal. EKG revealed tachycardia and nonspecific ST changes. Hemoglobin was 11.1 g/dl, hematocrit 32%, white blood cell count 10,900, and platelets 181,000. Electrolytes were normal except for a low sodium of 132 mmol/l (135-145) and bicarbonate of 17 mmol/l (21-31). Glucose was initially 67 mg/dl and dropped to 46 mg/dl. Total protein was 6 g/dl (6.7-8.2) and albumin was 2.7 g/dl (3.2-5.5). Metabolic panel was otherwise normal. Urinalysis was positive for glucose, ketones, and a small amount of blood and protein. There were no bacteria. Blood and urine cultures were negative. Phenytoin level was undetectable. Urine drug screen was positive for cannabinoids and cocaine.
Hospital Course
Hypoglycemia was treated with an ampule of D50 and intravenous fluids. On the obstetrics ward, nurses observed several episodes of head and eye deviation to the right accompanied by decreased responsiveness that lasted approximately 30 seconds. The patient was sent to the electrophysiology lab where an EEG revealed a diffusely slow background (Figure 1).
Figure 1. Generalized Slowing

During the 20-minute EEG recording, the patient had six clinical seizures similar to those described by the nurses. These events correlated with an ictal pattern consisting of 11 HZ_sharp activity in the right occipital temporal region that spread to the right parietal and left occipital temporal regions (Figure 2). Head CT revealed mild generalized atrophy and an enlarged right occipital horn, but no acute lesions (Figure 3).
Figure 2. Partial seizure originating in right occipital temporal region

Figure 3. Mild generalized arophy, greater in right hemisphere

The patient was transferred to intensive care and received fosphenytoin. No further clinical and /or electrographic seizures were identified. The following day, an EEG revealed diffuse slowing without focal seizures (Figure 4). The patient gradually became more alert and cooperative over the next 24 hours. However, the next day no fetal heartbeat was detected. Labor was induced and a stillborn baby delivered. The pathology report indicated that the placenta was between the 5th and 10th percentile for gestational age.
Figure 4. Improved generalized slowing

Discussion
Status epilepticus is associated with significant morbidity and mortality (Claassen et al. 2002). This 37-year-old pregnant woman had an episode of focal status epilepticus with impaired awareness likely provoked by nonadherence to antiepileptic drugs (AEDs). Cocaine may have contributed to the episode of status epilepticus (Majlesi et al. 2010). The obstetric service did not diagnose preeclampsia.
The patient’s seizures started in the right occipital region, which was abnormal on neuroimaging. An MRI might have revealed more subtle structural abnormalities such as cortical dysplasia as the etiology of her epilepsy, but she refused the scan.
Women with epilepsy are at increased risk for adverse pregnancy outcomes such as preeclampsia, preterm labor, and stillbirth and should be considered high risk (MacDonald et al. 2015). Serum levels of AEDs such as lamotrigine, levetiracetam and phenytoin may decrease during pregnancy and contribute to breakthrough seizures. Accordingly, monthly measurements of serum levels of AEDs during the entire course of the pregnancy are strongly recommended. These measurements allow for a timely adjustment of AED doses to prevent significant drop in their serum concentrations and minimize the occurrence of breakthrough seizures. In the case of phenytoin, measurement of free and total serum concentrations are recommended. Supplementation with at least 0.4 mg/day to 1 mg /day of folic acid (and up to 4 mg /day) has been recommended (Harden et al. 2009a). Of note, there is no increase in the incidence of status epilepticus due to pregnancy per se (Harden et al. 2009b).
Although the patient survived this episode of status epilepticus, her fetus did not. Antiseizure drug nonadherence and polysubstance abuse probably contributed to fetal demise.
References
Claassen J, Lokin JK, Fitzsimons BFM et al. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002;58:139-142.
Harden CL, Pennell PB, Koppel BS et al. Practice Parameter update: Management issues for women with epilepsy Focus on pregnancy (an evidence-based review): Vitamin K, folic acid, blood levels, and breastfeeding: Neurology 2009a;73:142-149.
Harden CL, Hopp J, Ting TY et al. Practice Parameter update: Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency. Neurology 2009b;50(5):1229-36.
MacDonald SC, Bateman BT, McElrath TF, Hernandez-Diaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Majlesi N, Shih R, Fiesseler FW et al. Cocaine-associated seizures and incidence of status epilepticus. Western Journal of Emergency Medicine. 2010;XI(2):157-160.
Latex Hypersensitivity to Injection Devices for Biologic Therapies in Psoriasis Patients
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4
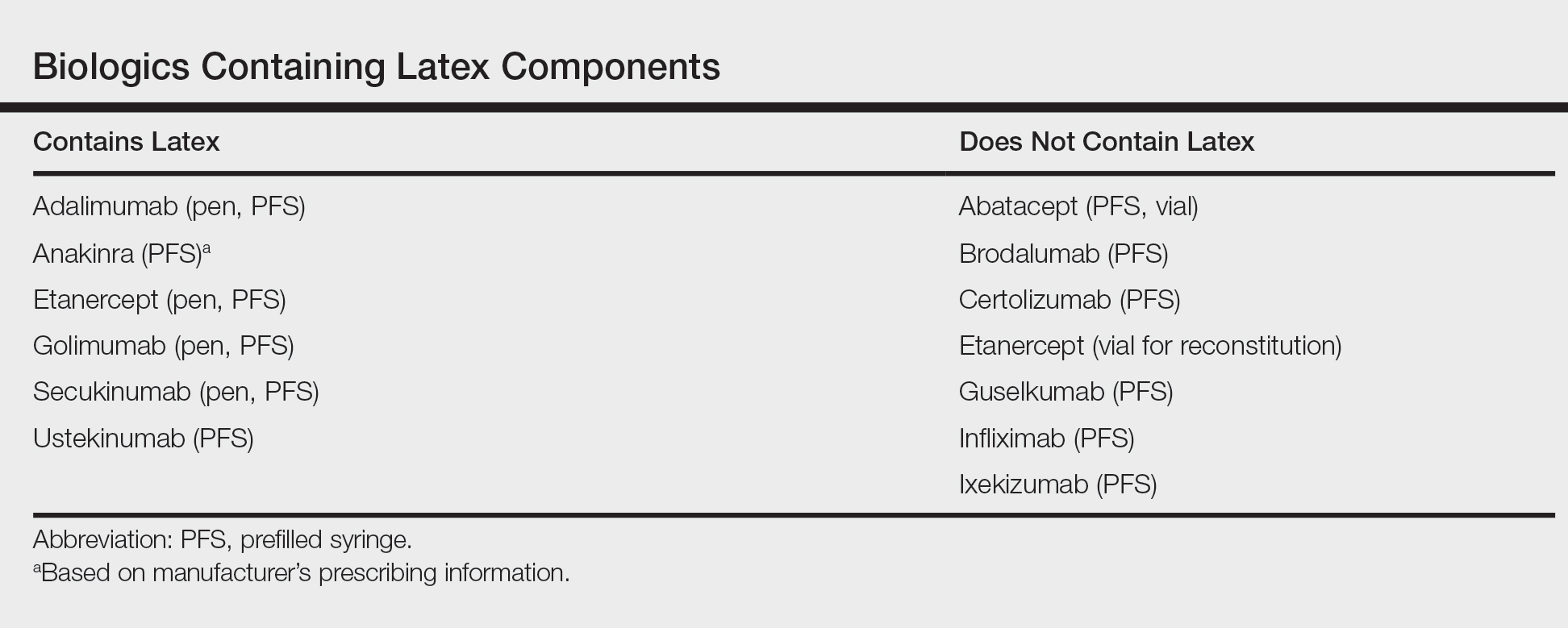
Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4

Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4

Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
Inflammatory Linear Verrucous Epidermal Nevus Responsive to 308-nm Excimer Laser Treatment
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).
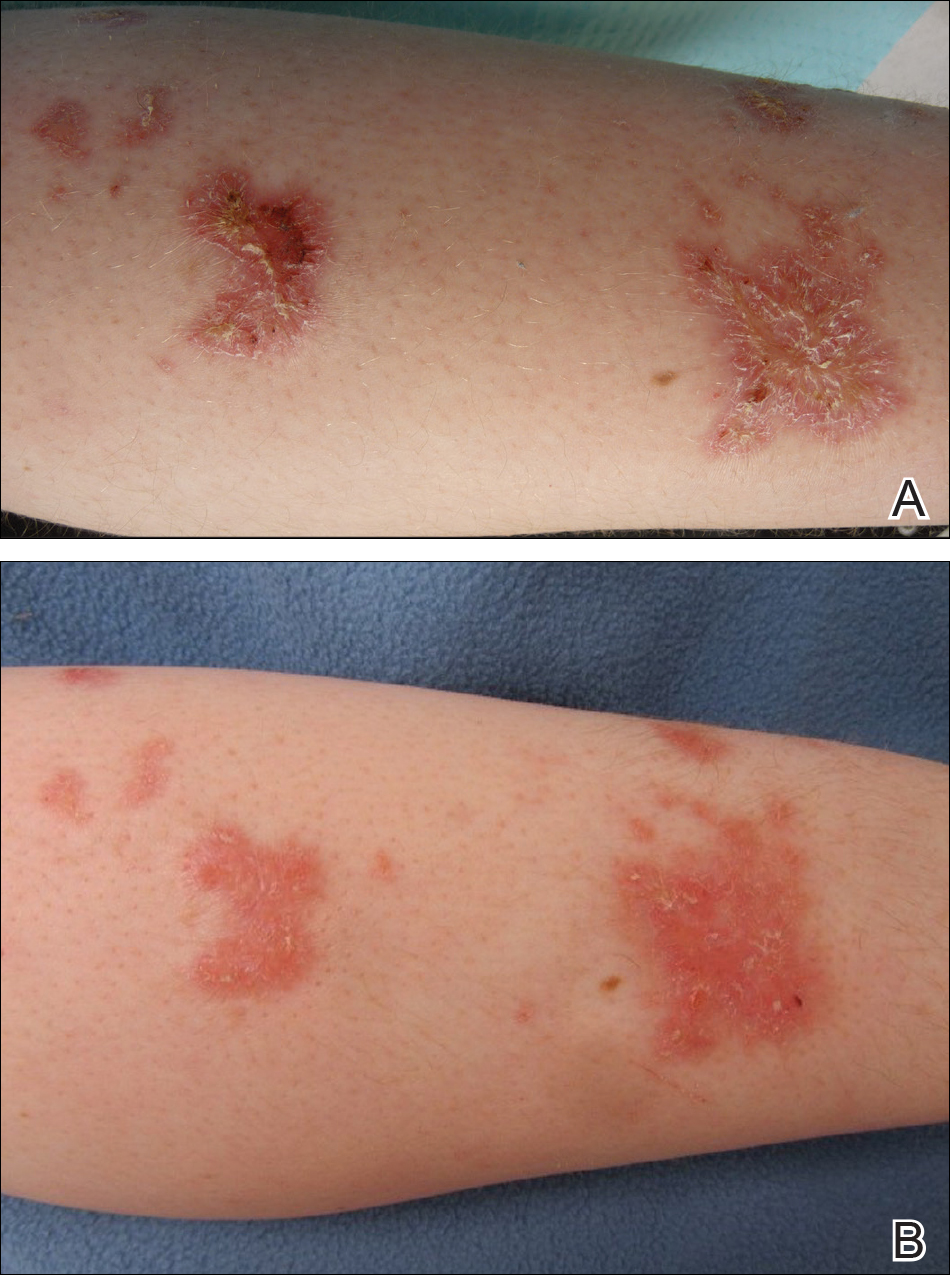
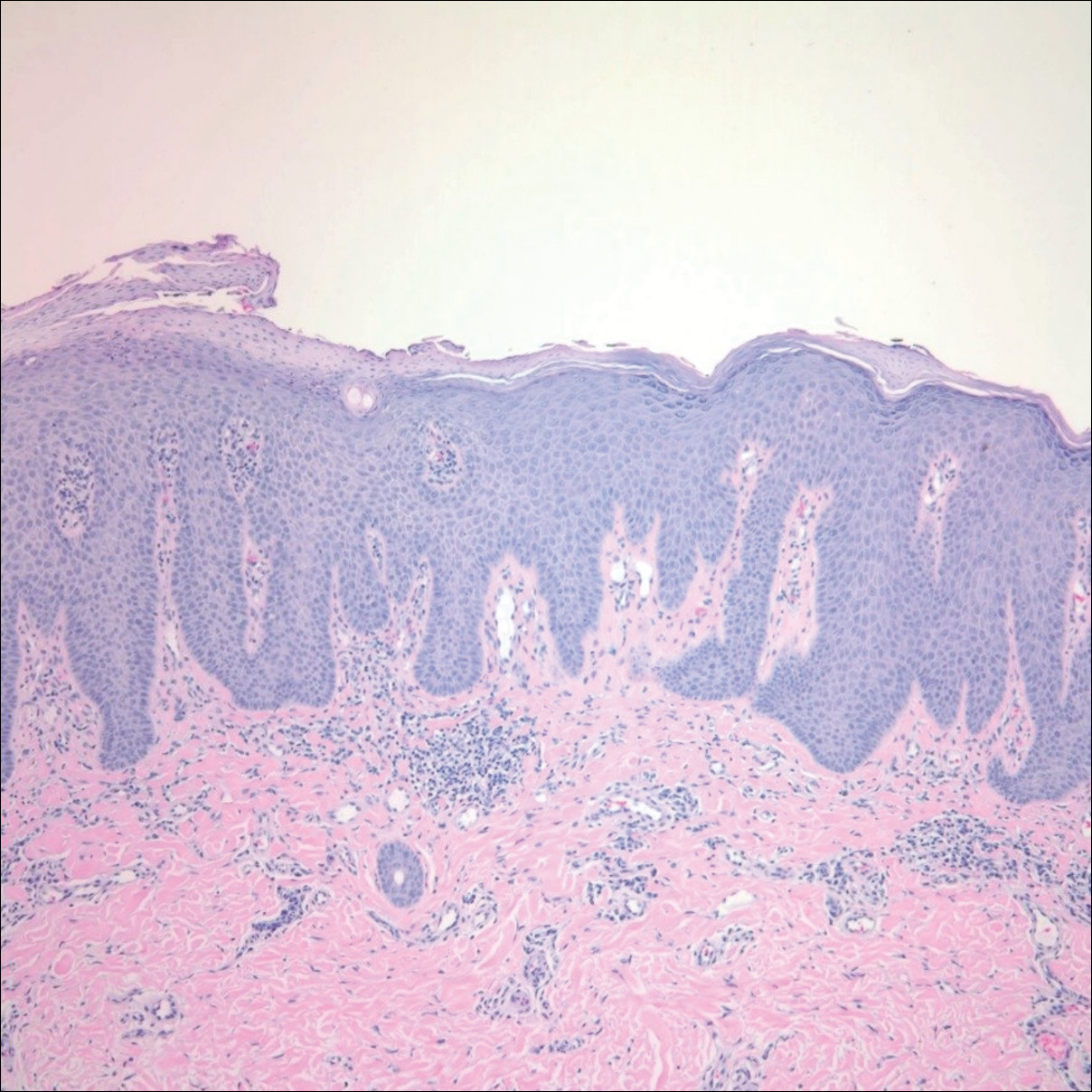
Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).


Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).


Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
Do Erythropoiesis-Stimulating Agents Have a Risk Evaluation and Mitigation Strategy? (FULL)
Epoetin alfa and darbepoetin alfa are erythropoiesis-stimulating agents (ESAs), approved for the treatment of anemia (low red blood cells [RBCs]) resulting from chronic kidney disease, chemotherapy, and certain treatments for HIV. These ESAs also are used to reduce the number of blood transfusions during and after certain major surgeries. Erythropoiesis-stimulating agents work like the human protein erythropoietin, which stimulates bone marrow to make RBCs. Epoetin alfa (marketed as Procrit and Epogen) and darbepoetin alfa (marketed as Aranesp) are manufactured by Amgen, Inc. (Thousand Oaks, CA).
In 1989 epoetin alfa was approved for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 1993 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy. Epoetin alfa also is indicated for anemia due to zidovudine in patients with HIV and reduction of RBC transfusions during certain surgeries.
Darbepoetin alfa was approved in 2001 for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 2006 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy.
Risk Evaluation and Mitigation Strategies
Both epoetin alfa and darbepoetin alfa increase the risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access and tumor progression or recurrence. Epoetin alfa also can lead to an increase in adverse cardiovascular events, hypertension, seizures, and severe anemia.
In 2008, the FDA determined that Risk Evaluation and Mitigation Strategies (REMS) were necessary for ESAs (darbopoetin alfa and epoetin alfa), to ensure that the benefits for use as treatment for anemia associated with myelosuppressive chemotherapy outweigh the risk of shortened overall survival (OS) and/or the increased risk of tumor progression or recurrence in patients with cancer. The REMS was approved in 2010.
Under the ESA REMS program, referred to as the ESA APPRISE Oncology Program, health care providers (HCPs) that prescribed and/or dispensed darbopoetin alfa to patients with cancer and hospitals that dispensed darbopoetin alfa to patients with cancer were required to enroll and become certified in the ESA REMS. The ESA REMS also required the completion of a Patient and Healthcare Provider Acknowledgement Form for each patient with cancer before the new ESA treatment course to ensure patients were counseled about the benefits and risks of these products.
In April 2017, the FDA determined that the ESA REMS that was limited to the use of epoetin alfa and darbopoetin alfa to treat patients with anemia due to associated myelosuppressive chemotherapy was no longer necessary; the benefits of ESAs outweighed the risks of shortened OS and/or increased risk of tumor progression or recurrence in patients with cancer. 1 The FDA recognized the burden that some REMS can place on HCPs and patients. The agency has authority to modify or remove the REMS to minimize the burden on the health care delivery system of complying with the strategy.
Data
The FDA discontinued the REMS based on an evaluation of the results of the REMS Assessments submitted by Amgen and additional FDA analyses to understand the impact of the various regulatory and other actions on the use of ESAs. The REMS Assessment showed the following:
- The results from surveyed prescribers demonstrated acceptable knowledge of the product risks of decreased survival and/or the increased risk of tumor progression or recurrence and the need to counsel patients about these risks; and
- The drug utilization data indicated appropriate prescribing of ESAs consistent with the intended use as a treatment alternative to RBC transfusion for anemia associated with myelosuppressive chemotherapy.
The FDA also conducted an evaluation of the impact of multiple actions, including the ESA REMS, on the use of the ESAs using sponsor-submitted data from outpatient oncology practices between 2006 and 2014. During 2004 to 2009, the FDA took multiple regulatory actions, including labeling changes. In 2007, the Center for Medicare and Medicaid Services (CMS) made a National Coverage Determination (NCD) to limit coverage of ESAs for nonrenal disease indications. These actions coincided with the following:
- A decrease in the proportion of patients receiving chemotherapy using ESAs;
- An increase in the proportion of patients receiving chemotherapy who initiate ESAs at a hemoglobin level < 10 g/dL; and
- An increase in the proportion of patients who initiate ESAs at a dosage consistent with product prescribing information.
Full implementation of the ESA REMS in 2011 had minimal impact on trends in these 3 ESA utilization metrics beyond the changes observed after the CMS coverage determination and multiple other FDA regulatory actions.
This information led the FDA to conclude that it was no longer necessary to require the certification of prescribers and hospitals that prescribe and/or dispense ESAs to patients with cancer in order to ensure that the benefits outweigh the risks.
The FDA has released the REMS requirements for the epoetin alfa and darbopoetin alfa ESA products, and the risks can be communicated by the current product prescribing information. The appropriate use of ESAs is supported by the CMS NCD, the American Society of Clinical Oncology, and American Society of Hematology clinical guidelines, which are evidence-based guidelines intended to provide a basis for the standard of care in clinical oncology.
Education
While the REMS is no longer necessary to ensure the benefits outweigh the risks, the serious risks of shortened OS and/or increased risk of tumor progression or recurrence associated with these drugs remain. The boxed warning language remains as follows: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE. Health care providers are encouraged to discuss the risks and benefits of using ESAs with each patient before initiating use.
Click here to read the digital edition.
1. U.S. Food & Drug Administration. Information on erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). https://www.fda.gov/Drugs/DrugSafety/ucm109375.htm. Updated April 13, 2017. Accessed July 13, 2017.
Epoetin alfa and darbepoetin alfa are erythropoiesis-stimulating agents (ESAs), approved for the treatment of anemia (low red blood cells [RBCs]) resulting from chronic kidney disease, chemotherapy, and certain treatments for HIV. These ESAs also are used to reduce the number of blood transfusions during and after certain major surgeries. Erythropoiesis-stimulating agents work like the human protein erythropoietin, which stimulates bone marrow to make RBCs. Epoetin alfa (marketed as Procrit and Epogen) and darbepoetin alfa (marketed as Aranesp) are manufactured by Amgen, Inc. (Thousand Oaks, CA).
In 1989 epoetin alfa was approved for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 1993 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy. Epoetin alfa also is indicated for anemia due to zidovudine in patients with HIV and reduction of RBC transfusions during certain surgeries.
Darbepoetin alfa was approved in 2001 for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 2006 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy.
Risk Evaluation and Mitigation Strategies
Both epoetin alfa and darbepoetin alfa increase the risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access and tumor progression or recurrence. Epoetin alfa also can lead to an increase in adverse cardiovascular events, hypertension, seizures, and severe anemia.
In 2008, the FDA determined that Risk Evaluation and Mitigation Strategies (REMS) were necessary for ESAs (darbopoetin alfa and epoetin alfa), to ensure that the benefits for use as treatment for anemia associated with myelosuppressive chemotherapy outweigh the risk of shortened overall survival (OS) and/or the increased risk of tumor progression or recurrence in patients with cancer. The REMS was approved in 2010.
Under the ESA REMS program, referred to as the ESA APPRISE Oncology Program, health care providers (HCPs) that prescribed and/or dispensed darbopoetin alfa to patients with cancer and hospitals that dispensed darbopoetin alfa to patients with cancer were required to enroll and become certified in the ESA REMS. The ESA REMS also required the completion of a Patient and Healthcare Provider Acknowledgement Form for each patient with cancer before the new ESA treatment course to ensure patients were counseled about the benefits and risks of these products.
In April 2017, the FDA determined that the ESA REMS that was limited to the use of epoetin alfa and darbopoetin alfa to treat patients with anemia due to associated myelosuppressive chemotherapy was no longer necessary; the benefits of ESAs outweighed the risks of shortened OS and/or increased risk of tumor progression or recurrence in patients with cancer. 1 The FDA recognized the burden that some REMS can place on HCPs and patients. The agency has authority to modify or remove the REMS to minimize the burden on the health care delivery system of complying with the strategy.
Data
The FDA discontinued the REMS based on an evaluation of the results of the REMS Assessments submitted by Amgen and additional FDA analyses to understand the impact of the various regulatory and other actions on the use of ESAs. The REMS Assessment showed the following:
- The results from surveyed prescribers demonstrated acceptable knowledge of the product risks of decreased survival and/or the increased risk of tumor progression or recurrence and the need to counsel patients about these risks; and
- The drug utilization data indicated appropriate prescribing of ESAs consistent with the intended use as a treatment alternative to RBC transfusion for anemia associated with myelosuppressive chemotherapy.
The FDA also conducted an evaluation of the impact of multiple actions, including the ESA REMS, on the use of the ESAs using sponsor-submitted data from outpatient oncology practices between 2006 and 2014. During 2004 to 2009, the FDA took multiple regulatory actions, including labeling changes. In 2007, the Center for Medicare and Medicaid Services (CMS) made a National Coverage Determination (NCD) to limit coverage of ESAs for nonrenal disease indications. These actions coincided with the following:
- A decrease in the proportion of patients receiving chemotherapy using ESAs;
- An increase in the proportion of patients receiving chemotherapy who initiate ESAs at a hemoglobin level < 10 g/dL; and
- An increase in the proportion of patients who initiate ESAs at a dosage consistent with product prescribing information.
Full implementation of the ESA REMS in 2011 had minimal impact on trends in these 3 ESA utilization metrics beyond the changes observed after the CMS coverage determination and multiple other FDA regulatory actions.
This information led the FDA to conclude that it was no longer necessary to require the certification of prescribers and hospitals that prescribe and/or dispense ESAs to patients with cancer in order to ensure that the benefits outweigh the risks.
The FDA has released the REMS requirements for the epoetin alfa and darbopoetin alfa ESA products, and the risks can be communicated by the current product prescribing information. The appropriate use of ESAs is supported by the CMS NCD, the American Society of Clinical Oncology, and American Society of Hematology clinical guidelines, which are evidence-based guidelines intended to provide a basis for the standard of care in clinical oncology.
Education
While the REMS is no longer necessary to ensure the benefits outweigh the risks, the serious risks of shortened OS and/or increased risk of tumor progression or recurrence associated with these drugs remain. The boxed warning language remains as follows: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE. Health care providers are encouraged to discuss the risks and benefits of using ESAs with each patient before initiating use.
Click here to read the digital edition.
Epoetin alfa and darbepoetin alfa are erythropoiesis-stimulating agents (ESAs), approved for the treatment of anemia (low red blood cells [RBCs]) resulting from chronic kidney disease, chemotherapy, and certain treatments for HIV. These ESAs also are used to reduce the number of blood transfusions during and after certain major surgeries. Erythropoiesis-stimulating agents work like the human protein erythropoietin, which stimulates bone marrow to make RBCs. Epoetin alfa (marketed as Procrit and Epogen) and darbepoetin alfa (marketed as Aranesp) are manufactured by Amgen, Inc. (Thousand Oaks, CA).
In 1989 epoetin alfa was approved for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 1993 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy. Epoetin alfa also is indicated for anemia due to zidovudine in patients with HIV and reduction of RBC transfusions during certain surgeries.
Darbepoetin alfa was approved in 2001 for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 2006 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy.
Risk Evaluation and Mitigation Strategies
Both epoetin alfa and darbepoetin alfa increase the risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access and tumor progression or recurrence. Epoetin alfa also can lead to an increase in adverse cardiovascular events, hypertension, seizures, and severe anemia.
In 2008, the FDA determined that Risk Evaluation and Mitigation Strategies (REMS) were necessary for ESAs (darbopoetin alfa and epoetin alfa), to ensure that the benefits for use as treatment for anemia associated with myelosuppressive chemotherapy outweigh the risk of shortened overall survival (OS) and/or the increased risk of tumor progression or recurrence in patients with cancer. The REMS was approved in 2010.
Under the ESA REMS program, referred to as the ESA APPRISE Oncology Program, health care providers (HCPs) that prescribed and/or dispensed darbopoetin alfa to patients with cancer and hospitals that dispensed darbopoetin alfa to patients with cancer were required to enroll and become certified in the ESA REMS. The ESA REMS also required the completion of a Patient and Healthcare Provider Acknowledgement Form for each patient with cancer before the new ESA treatment course to ensure patients were counseled about the benefits and risks of these products.
In April 2017, the FDA determined that the ESA REMS that was limited to the use of epoetin alfa and darbopoetin alfa to treat patients with anemia due to associated myelosuppressive chemotherapy was no longer necessary; the benefits of ESAs outweighed the risks of shortened OS and/or increased risk of tumor progression or recurrence in patients with cancer. 1 The FDA recognized the burden that some REMS can place on HCPs and patients. The agency has authority to modify or remove the REMS to minimize the burden on the health care delivery system of complying with the strategy.
Data
The FDA discontinued the REMS based on an evaluation of the results of the REMS Assessments submitted by Amgen and additional FDA analyses to understand the impact of the various regulatory and other actions on the use of ESAs. The REMS Assessment showed the following:
- The results from surveyed prescribers demonstrated acceptable knowledge of the product risks of decreased survival and/or the increased risk of tumor progression or recurrence and the need to counsel patients about these risks; and
- The drug utilization data indicated appropriate prescribing of ESAs consistent with the intended use as a treatment alternative to RBC transfusion for anemia associated with myelosuppressive chemotherapy.
The FDA also conducted an evaluation of the impact of multiple actions, including the ESA REMS, on the use of the ESAs using sponsor-submitted data from outpatient oncology practices between 2006 and 2014. During 2004 to 2009, the FDA took multiple regulatory actions, including labeling changes. In 2007, the Center for Medicare and Medicaid Services (CMS) made a National Coverage Determination (NCD) to limit coverage of ESAs for nonrenal disease indications. These actions coincided with the following:
- A decrease in the proportion of patients receiving chemotherapy using ESAs;
- An increase in the proportion of patients receiving chemotherapy who initiate ESAs at a hemoglobin level < 10 g/dL; and
- An increase in the proportion of patients who initiate ESAs at a dosage consistent with product prescribing information.
Full implementation of the ESA REMS in 2011 had minimal impact on trends in these 3 ESA utilization metrics beyond the changes observed after the CMS coverage determination and multiple other FDA regulatory actions.
This information led the FDA to conclude that it was no longer necessary to require the certification of prescribers and hospitals that prescribe and/or dispense ESAs to patients with cancer in order to ensure that the benefits outweigh the risks.
The FDA has released the REMS requirements for the epoetin alfa and darbopoetin alfa ESA products, and the risks can be communicated by the current product prescribing information. The appropriate use of ESAs is supported by the CMS NCD, the American Society of Clinical Oncology, and American Society of Hematology clinical guidelines, which are evidence-based guidelines intended to provide a basis for the standard of care in clinical oncology.
Education
While the REMS is no longer necessary to ensure the benefits outweigh the risks, the serious risks of shortened OS and/or increased risk of tumor progression or recurrence associated with these drugs remain. The boxed warning language remains as follows: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE. Health care providers are encouraged to discuss the risks and benefits of using ESAs with each patient before initiating use.
Click here to read the digital edition.
1. U.S. Food & Drug Administration. Information on erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). https://www.fda.gov/Drugs/DrugSafety/ucm109375.htm. Updated April 13, 2017. Accessed July 13, 2017.
1. U.S. Food & Drug Administration. Information on erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). https://www.fda.gov/Drugs/DrugSafety/ucm109375.htm. Updated April 13, 2017. Accessed July 13, 2017.
Treatment improves PFS in early stage FL
A multidrug regimen can improve upon involved-field radiotherapy (IFRT) in patients with early stage follicular lymphoma (FL), according to research published in the Journal of Clinical Oncology.
FL patients who received IFRT plus cyclophosphamide, vincristine, and prednisolone (CVP)—with or without rituximab—had a significant improvement in progression-free survival (PFS) compared to patients who received standard treatment with IFRT alone.
However, there was no significant difference in overall survival (OS) between the treatment arms.
“This is the first successful randomized study ever to be conducted in early stage follicular lymphoma comparing standard therapy to standard therapy plus effective chemotherapy or immunochemotherapy,” said Michael MacManus, MBBCh, of Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
“It shows that the initial treatment received by patients can significantly affect their long-term chance of staying free from disease. Moving forward, we are interested in determining whether there is a benefit in overall long-term survival for patients treated with the combination with further follow-up, and if there is any way to predict if a person will benefit from combined treatment based on analyses of blood or biopsy specimens.”
Dr MacManus and his colleagues studied 150 patients with stage I to II, low-grade FL who were enrolled in this trial between 2000 and 2012.
At randomization, the patients’ median age was 57, 52% were male, 75% had stage I disease, and 48% had PET staging.
Half of patients (n=75) were randomized to receive IFRT (30-36 Gy) alone, and half were randomized to IFRT (30-36 Gy) plus 6 cycles of CVP. From 2006 on, patients in the CVP arm received rituximab (R) as well (n=31).
Baseline characteristics were well-balanced between the treatment arms.
Efficacy
The median follow-up was 9.6 years (range, 3.1 to 15.8 years).
PFS was significantly better among patients randomized to receive CVP±R (hazard ratio [HR]=0.57; P=0.033). The estimated 10-year PFS rate was 41% in the IFRT arm and 59% in the CVP±R arm.
Patients randomized to receive CVP plus R (n=31) had significantly better PFS than patients randomized to receive IFRT alone (n=31) over the same time period (HR=0.26; P=0.045).
There were 10 deaths in the IRFT arm and 5 in the CVP±R arm, but there was no significant difference in OS between the arms (HR=0.62; P=0.40). The 10-year OS rate was 86% in the IFRT arm and 95% in the CVP±R arm.
There was no significant between-arm difference in transformation to aggressive lymphoma (P=0.1). Transformation occurred in 10 patients in the IFRT arm and 4 in the CVP±R arm.
Safety
There were 148 patients from both arms who ultimately received IFRT, and 69 patients who received CVP±R.
Grade 2 toxicities occurring in more than 10% of IFRT recipients included upper gastrointestinal (n=27; 18%), skin (n=21; 14%), and mucous membrane (n=19; 12%) toxicity. One IFRT recipient had grade 3 mucositis, and 1 had grade 4 esophageal/pharyngeal mucosal toxicity.
Grade 3 toxicities occurring in at least 2 patients in the CVP±R arm included neutropenia (n=10; 14%), infection (n=8; 12%), diarrhea (n=3; 4%), elevated gamma-glutamyl transferase (n=3; 4%), fatigue (n=3; 4%), and febrile neutropenia (n=3; 4%).
Three patients (4%) in the CVP±R arm had acute grade 3 neuropathy related to vincristine. Ten patients (14%) had grade 4 neutropenia.
The most common late toxicities for the entire patient cohort were salivary gland (n=8; 5%) and skin (n=4; 3%) toxicities.
Grade 3 lung and menopausal toxicities occurred in 1 patient each. Two patients had late grade 3 vincristine neuropathy. One patient who had grade 3 neuropathy during chemotherapy progressed to grade 4.
A multidrug regimen can improve upon involved-field radiotherapy (IFRT) in patients with early stage follicular lymphoma (FL), according to research published in the Journal of Clinical Oncology.
FL patients who received IFRT plus cyclophosphamide, vincristine, and prednisolone (CVP)—with or without rituximab—had a significant improvement in progression-free survival (PFS) compared to patients who received standard treatment with IFRT alone.
However, there was no significant difference in overall survival (OS) between the treatment arms.
“This is the first successful randomized study ever to be conducted in early stage follicular lymphoma comparing standard therapy to standard therapy plus effective chemotherapy or immunochemotherapy,” said Michael MacManus, MBBCh, of Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
“It shows that the initial treatment received by patients can significantly affect their long-term chance of staying free from disease. Moving forward, we are interested in determining whether there is a benefit in overall long-term survival for patients treated with the combination with further follow-up, and if there is any way to predict if a person will benefit from combined treatment based on analyses of blood or biopsy specimens.”
Dr MacManus and his colleagues studied 150 patients with stage I to II, low-grade FL who were enrolled in this trial between 2000 and 2012.
At randomization, the patients’ median age was 57, 52% were male, 75% had stage I disease, and 48% had PET staging.
Half of patients (n=75) were randomized to receive IFRT (30-36 Gy) alone, and half were randomized to IFRT (30-36 Gy) plus 6 cycles of CVP. From 2006 on, patients in the CVP arm received rituximab (R) as well (n=31).
Baseline characteristics were well-balanced between the treatment arms.
Efficacy
The median follow-up was 9.6 years (range, 3.1 to 15.8 years).
PFS was significantly better among patients randomized to receive CVP±R (hazard ratio [HR]=0.57; P=0.033). The estimated 10-year PFS rate was 41% in the IFRT arm and 59% in the CVP±R arm.
Patients randomized to receive CVP plus R (n=31) had significantly better PFS than patients randomized to receive IFRT alone (n=31) over the same time period (HR=0.26; P=0.045).
There were 10 deaths in the IRFT arm and 5 in the CVP±R arm, but there was no significant difference in OS between the arms (HR=0.62; P=0.40). The 10-year OS rate was 86% in the IFRT arm and 95% in the CVP±R arm.
There was no significant between-arm difference in transformation to aggressive lymphoma (P=0.1). Transformation occurred in 10 patients in the IFRT arm and 4 in the CVP±R arm.
Safety
There were 148 patients from both arms who ultimately received IFRT, and 69 patients who received CVP±R.
Grade 2 toxicities occurring in more than 10% of IFRT recipients included upper gastrointestinal (n=27; 18%), skin (n=21; 14%), and mucous membrane (n=19; 12%) toxicity. One IFRT recipient had grade 3 mucositis, and 1 had grade 4 esophageal/pharyngeal mucosal toxicity.
Grade 3 toxicities occurring in at least 2 patients in the CVP±R arm included neutropenia (n=10; 14%), infection (n=8; 12%), diarrhea (n=3; 4%), elevated gamma-glutamyl transferase (n=3; 4%), fatigue (n=3; 4%), and febrile neutropenia (n=3; 4%).
Three patients (4%) in the CVP±R arm had acute grade 3 neuropathy related to vincristine. Ten patients (14%) had grade 4 neutropenia.
The most common late toxicities for the entire patient cohort were salivary gland (n=8; 5%) and skin (n=4; 3%) toxicities.
Grade 3 lung and menopausal toxicities occurred in 1 patient each. Two patients had late grade 3 vincristine neuropathy. One patient who had grade 3 neuropathy during chemotherapy progressed to grade 4.
A multidrug regimen can improve upon involved-field radiotherapy (IFRT) in patients with early stage follicular lymphoma (FL), according to research published in the Journal of Clinical Oncology.
FL patients who received IFRT plus cyclophosphamide, vincristine, and prednisolone (CVP)—with or without rituximab—had a significant improvement in progression-free survival (PFS) compared to patients who received standard treatment with IFRT alone.
However, there was no significant difference in overall survival (OS) between the treatment arms.
“This is the first successful randomized study ever to be conducted in early stage follicular lymphoma comparing standard therapy to standard therapy plus effective chemotherapy or immunochemotherapy,” said Michael MacManus, MBBCh, of Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
“It shows that the initial treatment received by patients can significantly affect their long-term chance of staying free from disease. Moving forward, we are interested in determining whether there is a benefit in overall long-term survival for patients treated with the combination with further follow-up, and if there is any way to predict if a person will benefit from combined treatment based on analyses of blood or biopsy specimens.”
Dr MacManus and his colleagues studied 150 patients with stage I to II, low-grade FL who were enrolled in this trial between 2000 and 2012.
At randomization, the patients’ median age was 57, 52% were male, 75% had stage I disease, and 48% had PET staging.
Half of patients (n=75) were randomized to receive IFRT (30-36 Gy) alone, and half were randomized to IFRT (30-36 Gy) plus 6 cycles of CVP. From 2006 on, patients in the CVP arm received rituximab (R) as well (n=31).
Baseline characteristics were well-balanced between the treatment arms.
Efficacy
The median follow-up was 9.6 years (range, 3.1 to 15.8 years).
PFS was significantly better among patients randomized to receive CVP±R (hazard ratio [HR]=0.57; P=0.033). The estimated 10-year PFS rate was 41% in the IFRT arm and 59% in the CVP±R arm.
Patients randomized to receive CVP plus R (n=31) had significantly better PFS than patients randomized to receive IFRT alone (n=31) over the same time period (HR=0.26; P=0.045).
There were 10 deaths in the IRFT arm and 5 in the CVP±R arm, but there was no significant difference in OS between the arms (HR=0.62; P=0.40). The 10-year OS rate was 86% in the IFRT arm and 95% in the CVP±R arm.
There was no significant between-arm difference in transformation to aggressive lymphoma (P=0.1). Transformation occurred in 10 patients in the IFRT arm and 4 in the CVP±R arm.
Safety
There were 148 patients from both arms who ultimately received IFRT, and 69 patients who received CVP±R.
Grade 2 toxicities occurring in more than 10% of IFRT recipients included upper gastrointestinal (n=27; 18%), skin (n=21; 14%), and mucous membrane (n=19; 12%) toxicity. One IFRT recipient had grade 3 mucositis, and 1 had grade 4 esophageal/pharyngeal mucosal toxicity.
Grade 3 toxicities occurring in at least 2 patients in the CVP±R arm included neutropenia (n=10; 14%), infection (n=8; 12%), diarrhea (n=3; 4%), elevated gamma-glutamyl transferase (n=3; 4%), fatigue (n=3; 4%), and febrile neutropenia (n=3; 4%).
Three patients (4%) in the CVP±R arm had acute grade 3 neuropathy related to vincristine. Ten patients (14%) had grade 4 neutropenia.
The most common late toxicities for the entire patient cohort were salivary gland (n=8; 5%) and skin (n=4; 3%) toxicities.
Grade 3 lung and menopausal toxicities occurred in 1 patient each. Two patients had late grade 3 vincristine neuropathy. One patient who had grade 3 neuropathy during chemotherapy progressed to grade 4.
Investigators Describe the MS Prodrome
Patients who later develop MS are more likely than others to consult physicians for nervous system and genitourinary symptoms.
The prodrome of multiple sclerosis (MS) may include an increased risk of nervous system, sensory, and musculoskeletal disorders, according to research published online ahead of print July 1 in Multiple Sclerosis Journal. Patients who later develop MS also may be more likely to have genitourinary and psychiatric symptoms in the five years before diagnosis.
“The existence of such warning signs is well-accepted for Alzheimer’s disease and Parkinson’s disease, but there has been little investigation into a similar pattern for MS,” said Helen Tremlett, PhD, Professor in the Division of Neurology at the University of British Columbia in Canada. “We now need to delve deeper into this phenomenon, perhaps using data-mining techniques. We want to see if there are discernible patterns related to sex, age, or the type of MS they eventually develop.”
Clinical and Administrative Matched Cohorts
Dr. Tremlett and colleagues analyzed data from a matched-cohort record-linkage study to examine the MS prodrome. The investigators used population-based health administrative data and clinical data from the Canadian provinces of British Columbia, Saskatchewan, Manitoba, and Nova Scotia. The information included demographics, hospital visits, physician encounters, and prescriptions filled. Clinical data were for patients diagnosed by a neurologist at an MS clinic and included first clinical visit (or date of diagnosis) and date of symptom onset. Data were collected from April 1984 to April 2014.
Using the data, Dr. Tremlett and colleagues created a health-administrative cohort and a clinical cohort. The clinical cohort did not include data from Saskatchewan. To create the cohorts, the investigators identified patients with MS and matched them by sex, year of birth, and postal code with as many as five controls. The index date was the earliest recorded claim for a demyelinating disease for the health-administrative cohort and the date of MS symptom onset for the clinical cohort. Study outcomes were the number of physician and hospital encounters per ICD-10 chapter, the number of physician encounters per physician specialty, and the percentage of people with one or more prescriptions per drug class in the five years before the index date.
Clinical Cohort Results May Be More Accurate
The administrative cohort included 13,951 cases and 66,940 controls. The clinical cohort included 3,202 cases and 16,006 controls. Compared with controls, people with MS had more physician and hospital encounters for the nervous (rate ratio [RR], 2.31 to 4.75), sensory (RR, 1.40 to 2.28), musculoskeletal (RR, 1.19 to 1.70), and genitourinary systems (RR, 1.17 to 1.59) in the five years before the first demyelinating claim or symptom onset. Cases had more visits with psychiatrists and urologists (RR, 1.48 to 1.80) and higher proportions of musculoskeletal, genitourinary, or hormonal-related prescriptions (1.1–1.5 times higher), compared with controls. People with MS had fewer pregnancy-related encounters than controls, however (RR, 0.78 to 0.88).
The “more conservative” results for the clinical cohort are more likely to reflect the MS prodrome accurately because they are “unlikely to be influenced by a physician’s suspicion or consideration of MS,” said Dr. Tremlett and colleagues. “Although not all individuals with MS attend an MS specialty clinic, the clinical cohort represents a subgroup of the population that may differ with respect to demographic and clinical characteristics from nonclinic attendees (eg, have fewer comorbidities),” they added. NR
—Erik Greb
Suggested Reading
Wijnands JM, Zhu F, Kingwell E, et al. Five years before multiple sclerosis onset: Phenotyping the prodrome. Mult Scler. 2018 Jul 1 [Epub ahead of print].
Patients who later develop MS are more likely than others to consult physicians for nervous system and genitourinary symptoms.
Patients who later develop MS are more likely than others to consult physicians for nervous system and genitourinary symptoms.
The prodrome of multiple sclerosis (MS) may include an increased risk of nervous system, sensory, and musculoskeletal disorders, according to research published online ahead of print July 1 in Multiple Sclerosis Journal. Patients who later develop MS also may be more likely to have genitourinary and psychiatric symptoms in the five years before diagnosis.
“The existence of such warning signs is well-accepted for Alzheimer’s disease and Parkinson’s disease, but there has been little investigation into a similar pattern for MS,” said Helen Tremlett, PhD, Professor in the Division of Neurology at the University of British Columbia in Canada. “We now need to delve deeper into this phenomenon, perhaps using data-mining techniques. We want to see if there are discernible patterns related to sex, age, or the type of MS they eventually develop.”
Clinical and Administrative Matched Cohorts
Dr. Tremlett and colleagues analyzed data from a matched-cohort record-linkage study to examine the MS prodrome. The investigators used population-based health administrative data and clinical data from the Canadian provinces of British Columbia, Saskatchewan, Manitoba, and Nova Scotia. The information included demographics, hospital visits, physician encounters, and prescriptions filled. Clinical data were for patients diagnosed by a neurologist at an MS clinic and included first clinical visit (or date of diagnosis) and date of symptom onset. Data were collected from April 1984 to April 2014.
Using the data, Dr. Tremlett and colleagues created a health-administrative cohort and a clinical cohort. The clinical cohort did not include data from Saskatchewan. To create the cohorts, the investigators identified patients with MS and matched them by sex, year of birth, and postal code with as many as five controls. The index date was the earliest recorded claim for a demyelinating disease for the health-administrative cohort and the date of MS symptom onset for the clinical cohort. Study outcomes were the number of physician and hospital encounters per ICD-10 chapter, the number of physician encounters per physician specialty, and the percentage of people with one or more prescriptions per drug class in the five years before the index date.
Clinical Cohort Results May Be More Accurate
The administrative cohort included 13,951 cases and 66,940 controls. The clinical cohort included 3,202 cases and 16,006 controls. Compared with controls, people with MS had more physician and hospital encounters for the nervous (rate ratio [RR], 2.31 to 4.75), sensory (RR, 1.40 to 2.28), musculoskeletal (RR, 1.19 to 1.70), and genitourinary systems (RR, 1.17 to 1.59) in the five years before the first demyelinating claim or symptom onset. Cases had more visits with psychiatrists and urologists (RR, 1.48 to 1.80) and higher proportions of musculoskeletal, genitourinary, or hormonal-related prescriptions (1.1–1.5 times higher), compared with controls. People with MS had fewer pregnancy-related encounters than controls, however (RR, 0.78 to 0.88).
The “more conservative” results for the clinical cohort are more likely to reflect the MS prodrome accurately because they are “unlikely to be influenced by a physician’s suspicion or consideration of MS,” said Dr. Tremlett and colleagues. “Although not all individuals with MS attend an MS specialty clinic, the clinical cohort represents a subgroup of the population that may differ with respect to demographic and clinical characteristics from nonclinic attendees (eg, have fewer comorbidities),” they added. NR
—Erik Greb
Suggested Reading
Wijnands JM, Zhu F, Kingwell E, et al. Five years before multiple sclerosis onset: Phenotyping the prodrome. Mult Scler. 2018 Jul 1 [Epub ahead of print].
The prodrome of multiple sclerosis (MS) may include an increased risk of nervous system, sensory, and musculoskeletal disorders, according to research published online ahead of print July 1 in Multiple Sclerosis Journal. Patients who later develop MS also may be more likely to have genitourinary and psychiatric symptoms in the five years before diagnosis.
“The existence of such warning signs is well-accepted for Alzheimer’s disease and Parkinson’s disease, but there has been little investigation into a similar pattern for MS,” said Helen Tremlett, PhD, Professor in the Division of Neurology at the University of British Columbia in Canada. “We now need to delve deeper into this phenomenon, perhaps using data-mining techniques. We want to see if there are discernible patterns related to sex, age, or the type of MS they eventually develop.”
Clinical and Administrative Matched Cohorts
Dr. Tremlett and colleagues analyzed data from a matched-cohort record-linkage study to examine the MS prodrome. The investigators used population-based health administrative data and clinical data from the Canadian provinces of British Columbia, Saskatchewan, Manitoba, and Nova Scotia. The information included demographics, hospital visits, physician encounters, and prescriptions filled. Clinical data were for patients diagnosed by a neurologist at an MS clinic and included first clinical visit (or date of diagnosis) and date of symptom onset. Data were collected from April 1984 to April 2014.
Using the data, Dr. Tremlett and colleagues created a health-administrative cohort and a clinical cohort. The clinical cohort did not include data from Saskatchewan. To create the cohorts, the investigators identified patients with MS and matched them by sex, year of birth, and postal code with as many as five controls. The index date was the earliest recorded claim for a demyelinating disease for the health-administrative cohort and the date of MS symptom onset for the clinical cohort. Study outcomes were the number of physician and hospital encounters per ICD-10 chapter, the number of physician encounters per physician specialty, and the percentage of people with one or more prescriptions per drug class in the five years before the index date.
Clinical Cohort Results May Be More Accurate
The administrative cohort included 13,951 cases and 66,940 controls. The clinical cohort included 3,202 cases and 16,006 controls. Compared with controls, people with MS had more physician and hospital encounters for the nervous (rate ratio [RR], 2.31 to 4.75), sensory (RR, 1.40 to 2.28), musculoskeletal (RR, 1.19 to 1.70), and genitourinary systems (RR, 1.17 to 1.59) in the five years before the first demyelinating claim or symptom onset. Cases had more visits with psychiatrists and urologists (RR, 1.48 to 1.80) and higher proportions of musculoskeletal, genitourinary, or hormonal-related prescriptions (1.1–1.5 times higher), compared with controls. People with MS had fewer pregnancy-related encounters than controls, however (RR, 0.78 to 0.88).
The “more conservative” results for the clinical cohort are more likely to reflect the MS prodrome accurately because they are “unlikely to be influenced by a physician’s suspicion or consideration of MS,” said Dr. Tremlett and colleagues. “Although not all individuals with MS attend an MS specialty clinic, the clinical cohort represents a subgroup of the population that may differ with respect to demographic and clinical characteristics from nonclinic attendees (eg, have fewer comorbidities),” they added. NR
—Erik Greb
Suggested Reading
Wijnands JM, Zhu F, Kingwell E, et al. Five years before multiple sclerosis onset: Phenotyping the prodrome. Mult Scler. 2018 Jul 1 [Epub ahead of print].
Drug receives orphan designation for MM
The US Food and Drug Administration (FDA) has granted orphan drug designation to SRF231 for the treatment of multiple myeloma (MM).
SRF231 is a fully human antibody that inhibits the activity of CD47, a protein that is overexpressed on many cancer cells and prevents them from being engulfed and eliminated by macrophages.
Surface Oncology, the company developing SRF231, is currently conducting a phase 1 trial (NCT03512340) of SRF231 in patients with solid tumors and hematologic malignancies.
Preclinical research on SRF231 was presented at the 2016 ASH Annual Meeting.
SRF231 demonstrated “potent” activity against hematologic malignancies, according to researchers.
The team said SRF231 promoted macrophage-mediated phagocytic clearance of several hematologic primary tumor samples and cell lines in vitro.
SRF231 also demonstrated activity in murine xenograft models of hematologic malignancies. Specifically, the researchers observed “profound tumor growth inhibition” in models of MM, diffuse large B-cell lymphoma, and Burkitt lymphoma.
The team said SRF231 demonstrated activity when given alone or in combination with opsonizing antibodies.
Results also showed that SRF231 did not induce hemagglutination or phagocytosis of red blood cells in vitro.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to SRF231 for the treatment of multiple myeloma (MM).
SRF231 is a fully human antibody that inhibits the activity of CD47, a protein that is overexpressed on many cancer cells and prevents them from being engulfed and eliminated by macrophages.
Surface Oncology, the company developing SRF231, is currently conducting a phase 1 trial (NCT03512340) of SRF231 in patients with solid tumors and hematologic malignancies.
Preclinical research on SRF231 was presented at the 2016 ASH Annual Meeting.
SRF231 demonstrated “potent” activity against hematologic malignancies, according to researchers.
The team said SRF231 promoted macrophage-mediated phagocytic clearance of several hematologic primary tumor samples and cell lines in vitro.
SRF231 also demonstrated activity in murine xenograft models of hematologic malignancies. Specifically, the researchers observed “profound tumor growth inhibition” in models of MM, diffuse large B-cell lymphoma, and Burkitt lymphoma.
The team said SRF231 demonstrated activity when given alone or in combination with opsonizing antibodies.
Results also showed that SRF231 did not induce hemagglutination or phagocytosis of red blood cells in vitro.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to SRF231 for the treatment of multiple myeloma (MM).
SRF231 is a fully human antibody that inhibits the activity of CD47, a protein that is overexpressed on many cancer cells and prevents them from being engulfed and eliminated by macrophages.
Surface Oncology, the company developing SRF231, is currently conducting a phase 1 trial (NCT03512340) of SRF231 in patients with solid tumors and hematologic malignancies.
Preclinical research on SRF231 was presented at the 2016 ASH Annual Meeting.
SRF231 demonstrated “potent” activity against hematologic malignancies, according to researchers.
The team said SRF231 promoted macrophage-mediated phagocytic clearance of several hematologic primary tumor samples and cell lines in vitro.
SRF231 also demonstrated activity in murine xenograft models of hematologic malignancies. Specifically, the researchers observed “profound tumor growth inhibition” in models of MM, diffuse large B-cell lymphoma, and Burkitt lymphoma.
The team said SRF231 demonstrated activity when given alone or in combination with opsonizing antibodies.
Results also showed that SRF231 did not induce hemagglutination or phagocytosis of red blood cells in vitro.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.