User login
Skeletal-Related Events in Patients With Multiple Myeloma and Prostate Cancer Who Receive Standard vs Extended-Interval Bisphosphonate Dosing (FULL)
In patients with multiple myeloma and prostate cancer, extending the bisphosphonatedosing interval may help decrease medication-related morbidity without compromising therapeutic benefit.
Bone pain is one of the most common causes of morbidity in multiple myeloma (MM) and metastatic prostate cancer (CaP). This pain originates with the underlying pathologic processes of the cancer and with downstream skeletal-related events (SREs). SREs—fractures, spinal cord compression, and irradiation or surgery performed in ≥ 1 bone sites—represent a significant health care burden, particularly given the incidence of the underlying malignancies. According to American Cancer Society statistics, CaP is the second most common cancer in American men, and MM the second most common hematologic malignancy, despite its relatively low overall lifetime risk.1,2 Regardless of the underlying malignancy, bisphosphonates are the cornerstone of SRE prevention, though the optimal dosing strategy is the subject of clinical debate.
Although similar in SRE incidence, MM and CaP have distinct pathophysiologic processes in the dysregulation of bone resorption. MM is a hematologic malignancy that increases the risk of SREs by osteoclast up-regulation, primarily through the RANK (receptor activator of nuclear factor α-B) signaling pathway.3 CaP is a solid tumor malignancy that metastasizes to bone. Dysregulation of the bone resorption or formation cycle and net bone loss are a result of endogenous osteoclast up-regulation in response to abnormal bone formation in osteoblastic bone metastases.4 Androgen-deprivation therapy, the cornerstone of CaP treatment, further predisposes CaP patients to osteoporosis and SREs.
Prevention of SREs is pharmacologically driven by bisphosphonates, which have antiresorptive effects on bone through promotion of osteoclast apoptosis.5 Two IV formulations, pamidronate and zoledronic acid (ZA), are US Food and Drug Administration approved for use in bone metastases from MM or solid tumors.6-10 Although generally well tolerated, bisphosphonates can cause osteonecrosis of the jaw (ONJ), an avascular death of bone tissue, particularly with prolonged use.11 With its documented incidence of 5% to 6.7% in bone metastasis, ONJ represents a significant morbidity risk in patients with MM and CaP who are treated with IV bisphosphonates.12
Investigators are exploring bisphosphonate dosing intervals to determine which is most appropriate in mitigating the risk of ONJ. Before 2006, bisphosphonates were consistently dosed once monthly in patients with MM or metastatic bone disease—a standard derived empirically rather than from comparative studies or compelling pharmacodynamic data.13-15 In a 2006 consensus statement, the Mayo Clinic issued an expert opinion recommendation for increasing the bisphosphonate dosing interval to every 3 months in patients with MM.16 The first objective evidence for the clinical applicability of extending the ZA dosing interval was reported by Himelstein and colleagues in 2017.17 The randomized clinical trial found no differences in SRE rates when ZA was dosed every 12 weeks,17 prompting a conditional recommendation for dosing interval extension in the American Society of Clinical Oncology MM treatment guidelines (2018).13 Because of the age and racial demographics of the patients in these studies, many questions remain unanswered.
For the US Department of Veterans Affairs (VA) population, the pharmacokinetic and dynamic differences imposed by age and race limit the applicability of the available data. However, in veterans with MM or CaP, extending the bisphosphonate dosing interval may help decrease medication-related morbidity (eg, ONJ, nephrotoxicity) without compromising therapeutic benefit. To this end at the Memphis VA Medical Center (VAMC), we assessed for differences in SRE rates by comparing outcomes of patients who received ZA in standard- vs extended-interval dosing.
Methods
We retrospectively reviewed the Computerized Patient Record System for veterans with MM or metastatic CaP treated with ZA at the Memphis VAMC. Study inclusion criteria were aged > 18 years and care provided by a Memphis VAMC oncologist between January 2003 and January 2018. The study was approved by the Memphis VAMC’s Institutional Review Board, and procedures were followed in accordance with the ethical standards of its committee on human experimentation.
Using Microsoft SQL 2016 (Redmond, WA), we performed a query to identify patients who were prescribed ZA during the study period. Exclusion criteria were ZA prescribed for an indication other than MM or CaP (ie, osteoporosis) and receipt of ≤ 1 dose of ZA. Once a list was compiled, patients were stratified by ZA dosing interval: standard (mean, every month) or extended (mean, every 3 months). Patients whose ZA dosing interval was changed during treatment were included as independent data points in each group.
Skeletal-related events included fractures, spinal compression, irradiation, and surgery. Fractures and spinal compression were pertinent in the presence of radiographic documentation (eg, X-ray, magnetic resonance imaging scan) during the period the patient received ZA or within 1 dosing interval of the last recorded ZA dose. Irradiation was defined as documented application of radiation therapy to ≥ 1 bone sites for palliation of pain or as an intervention in the setting of spinal compression. Surgery was defined as any procedure performed to correct a fracture or spinal compression. Each SRE was counted as a single occurrence.
Osteonecrosis of the jaw was defined as radiographically documented necrosis of the mandible or associated structures with assessment by a VA dentist. Records from non-VA dental practices were not available for assessment. Documentation of dental assessment before the first dose of ZA and any assessments during treatment were recorded.
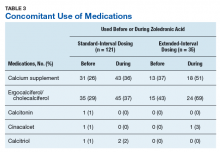
Medication use was assessed before and during ZA treatment. Number of ZA doses and reasons for any discontinuations were documented, as was concomitant use of calcium supplements, vitamin D supplements, calcitriol, paricalcitol, calcitonin, cinacalcet, and pamidronate.
The primary study outcome was observed difference in incidence of SREs between standard- and extended-interval dosing of ZA. Secondary outcomes included difference in incidence of ONJ as well as incidence of SREs and ONJ by disease subtype (MM, CaP).
Descriptive statistics were used to summarize demographic data and assess prespecified outcomes. Differences in rates of SREs and ONJ between dosing interval groups were analyzed with the Pearson χ2 test. The predetermined a priori level of significance was .05.
Results
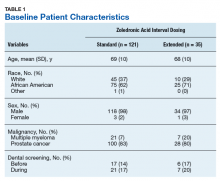
Of the 300 patients prescribed ZA at the Memphis VAMC, 177 were excluded (96 for indication,78 for receiving only 1 dose of ZA, 3 for not receiving any doses of ZA). The remaining 123 patients were stratified into a standard-interval dosing group (121) and an extended-interval dosing group (35). Of the 123 patients, 33 received both standard- and extended-interval dosing of ZA over the course of the study period and were included discretely in each group for the duration of each dosing strategy.
Pre-ZA dental screenings were documented in 14% of standard-interval patients and 17% of extended-interval patients, and during-ZA screenings were documented in 17% of standard-interval patients and 20% of extended-interval patients. Chi-square analysis revealed no significant difference in rates of dental screening before or during use of ZA.
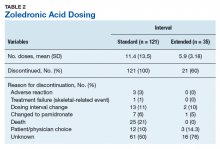
Standard-interval patients received a mean (SD) 11.4 (13.5) doses of ZA (range, 2-124). Extended-interval patients received a mean (SD) of 5.9 (3.18) doses (range, 2-14). All standard-interval patients had discontinued treatment at the time of the study, most commonly because of death or for an unknown reason. Sixty percent of extended-interval patients had discontinued treatment, most commonly because of patient/physician choice or for an unknown reason (Table 2).
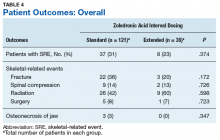
Skeletal-related events were observed in 31% of standard-interval patients and 23% of extended-interval patients. There were no statistically significant differences in SRE rates between groups (P = .374). The most common SRE in both groups was bone irradiation (42% and 60%, respectively), with no statistically significant difference in proportion between groups (Table 4).
Discussion
This retrospective review of patients with MM and CaP receiving ZA for bone metastasesfound no differences in the rates of SREs when ZA was dosed monthly vs every 3 months.
Earlier studies found that ZA can decrease SRE rates, but a major concern is that frequent, prolonged exposure to IV bisphosphonates may increase the risk of ONJ. No significant differences in ONJ rates existed between dosing groups, but all documented cases of ONJ occurred in the standard-interval group, suggesting a trend toward decreased incidence with an extension of the dosing interval.
Limitations
This study had several limitations. Geriatric African American men comprised the majority of the study population, and patients with MM accounted for only 22% of included regimens, limiting external validity. Patient overlap between groups may have confounded the results. The retrospective design precluded the ability to control for confounding variables, such as concomitant medication use and medication adherence, and significant heterogeneity was noted in rates of adherence with ZA infusion schedules regardless of dosing group. Use of medications associated with increased risk of osteoporosis—including corticosteroids and proton pump inhibitors—was not assessed.
Assessment of ONJ incidence was limited by the lack of access to dental records from providers outside the VA. Many patients in this review were not eligible for VA dental benefits because of requirements involving time and service connection, a reimbursement measurement that reflects health conditions “incurred or aggravated during active military service.”18
The results of this study provide further support for extended-interval dosing of ZA as a potential method of increasing patient adherence and decreasing the possibility of adverse drug reactions without compromising therapeutic benefit. Further randomized controlled trials are needed to define the potential decrease in ONJ incidence.
Conclusion
In comparisons of standard- and extended-interval dosing of ZA, there was no difference in the incidence of skeletal-related events in veteran patients with bone metastases from MM or CaP.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review (CSR), 1975-2014 [based on November 2016 SEER data submission posted to SEER website April 2017]. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed January 12, 2019.
3. Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23(3):435-441.
4. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657.
5. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032-1045.
6. Zometa [package insert]. East Hanover, NJ: Novartis; 2016.
7. Aredia [package insert]. East Hanover, NJ: Novartis; 2011.
8. Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases: a double-blind, randomized dose-response study [published correction appears in Cancer. 2001;91(10):1956]. Cancer. 2001;91(7):1191-1200.
9. Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488-493.
10. Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012;(5):CD003188.
11. Wu S, Dahut WL, Gulley JL. The use of bisphosphonates in cancer patients. Acta Oncol. 2007;46(5):581-591.
12. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580-8587.
13. Anderson K, Ismaila N, Flynn PJ, et al. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(8):812-818.
14. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Multiple Myeloma. Version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed January 29, 2019.
15. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Prostate Cancer. Version 4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 29, 2019.
16. Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo Clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81(8):1047-1053.
17. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58.
18. Office of Public and Intergovernmental Affairs, US Department of Veterans Affairs. Service connected disabilities. In: Federal Benefits for Veterans, Dependents, and Survivors. https://www.va.gov/opa/publications/benefits_book/benefits_chap02.asp. Published April 2015. Accessed May 22, 2018.
In patients with multiple myeloma and prostate cancer, extending the bisphosphonatedosing interval may help decrease medication-related morbidity without compromising therapeutic benefit.
In patients with multiple myeloma and prostate cancer, extending the bisphosphonatedosing interval may help decrease medication-related morbidity without compromising therapeutic benefit.
Bone pain is one of the most common causes of morbidity in multiple myeloma (MM) and metastatic prostate cancer (CaP). This pain originates with the underlying pathologic processes of the cancer and with downstream skeletal-related events (SREs). SREs—fractures, spinal cord compression, and irradiation or surgery performed in ≥ 1 bone sites—represent a significant health care burden, particularly given the incidence of the underlying malignancies. According to American Cancer Society statistics, CaP is the second most common cancer in American men, and MM the second most common hematologic malignancy, despite its relatively low overall lifetime risk.1,2 Regardless of the underlying malignancy, bisphosphonates are the cornerstone of SRE prevention, though the optimal dosing strategy is the subject of clinical debate.
Although similar in SRE incidence, MM and CaP have distinct pathophysiologic processes in the dysregulation of bone resorption. MM is a hematologic malignancy that increases the risk of SREs by osteoclast up-regulation, primarily through the RANK (receptor activator of nuclear factor α-B) signaling pathway.3 CaP is a solid tumor malignancy that metastasizes to bone. Dysregulation of the bone resorption or formation cycle and net bone loss are a result of endogenous osteoclast up-regulation in response to abnormal bone formation in osteoblastic bone metastases.4 Androgen-deprivation therapy, the cornerstone of CaP treatment, further predisposes CaP patients to osteoporosis and SREs.
Prevention of SREs is pharmacologically driven by bisphosphonates, which have antiresorptive effects on bone through promotion of osteoclast apoptosis.5 Two IV formulations, pamidronate and zoledronic acid (ZA), are US Food and Drug Administration approved for use in bone metastases from MM or solid tumors.6-10 Although generally well tolerated, bisphosphonates can cause osteonecrosis of the jaw (ONJ), an avascular death of bone tissue, particularly with prolonged use.11 With its documented incidence of 5% to 6.7% in bone metastasis, ONJ represents a significant morbidity risk in patients with MM and CaP who are treated with IV bisphosphonates.12
Investigators are exploring bisphosphonate dosing intervals to determine which is most appropriate in mitigating the risk of ONJ. Before 2006, bisphosphonates were consistently dosed once monthly in patients with MM or metastatic bone disease—a standard derived empirically rather than from comparative studies or compelling pharmacodynamic data.13-15 In a 2006 consensus statement, the Mayo Clinic issued an expert opinion recommendation for increasing the bisphosphonate dosing interval to every 3 months in patients with MM.16 The first objective evidence for the clinical applicability of extending the ZA dosing interval was reported by Himelstein and colleagues in 2017.17 The randomized clinical trial found no differences in SRE rates when ZA was dosed every 12 weeks,17 prompting a conditional recommendation for dosing interval extension in the American Society of Clinical Oncology MM treatment guidelines (2018).13 Because of the age and racial demographics of the patients in these studies, many questions remain unanswered.
For the US Department of Veterans Affairs (VA) population, the pharmacokinetic and dynamic differences imposed by age and race limit the applicability of the available data. However, in veterans with MM or CaP, extending the bisphosphonate dosing interval may help decrease medication-related morbidity (eg, ONJ, nephrotoxicity) without compromising therapeutic benefit. To this end at the Memphis VA Medical Center (VAMC), we assessed for differences in SRE rates by comparing outcomes of patients who received ZA in standard- vs extended-interval dosing.
Methods
We retrospectively reviewed the Computerized Patient Record System for veterans with MM or metastatic CaP treated with ZA at the Memphis VAMC. Study inclusion criteria were aged > 18 years and care provided by a Memphis VAMC oncologist between January 2003 and January 2018. The study was approved by the Memphis VAMC’s Institutional Review Board, and procedures were followed in accordance with the ethical standards of its committee on human experimentation.
Using Microsoft SQL 2016 (Redmond, WA), we performed a query to identify patients who were prescribed ZA during the study period. Exclusion criteria were ZA prescribed for an indication other than MM or CaP (ie, osteoporosis) and receipt of ≤ 1 dose of ZA. Once a list was compiled, patients were stratified by ZA dosing interval: standard (mean, every month) or extended (mean, every 3 months). Patients whose ZA dosing interval was changed during treatment were included as independent data points in each group.
Skeletal-related events included fractures, spinal compression, irradiation, and surgery. Fractures and spinal compression were pertinent in the presence of radiographic documentation (eg, X-ray, magnetic resonance imaging scan) during the period the patient received ZA or within 1 dosing interval of the last recorded ZA dose. Irradiation was defined as documented application of radiation therapy to ≥ 1 bone sites for palliation of pain or as an intervention in the setting of spinal compression. Surgery was defined as any procedure performed to correct a fracture or spinal compression. Each SRE was counted as a single occurrence.
Osteonecrosis of the jaw was defined as radiographically documented necrosis of the mandible or associated structures with assessment by a VA dentist. Records from non-VA dental practices were not available for assessment. Documentation of dental assessment before the first dose of ZA and any assessments during treatment were recorded.
Medication use was assessed before and during ZA treatment. Number of ZA doses and reasons for any discontinuations were documented, as was concomitant use of calcium supplements, vitamin D supplements, calcitriol, paricalcitol, calcitonin, cinacalcet, and pamidronate.
The primary study outcome was observed difference in incidence of SREs between standard- and extended-interval dosing of ZA. Secondary outcomes included difference in incidence of ONJ as well as incidence of SREs and ONJ by disease subtype (MM, CaP).
Descriptive statistics were used to summarize demographic data and assess prespecified outcomes. Differences in rates of SREs and ONJ between dosing interval groups were analyzed with the Pearson χ2 test. The predetermined a priori level of significance was .05.
Results
Of the 300 patients prescribed ZA at the Memphis VAMC, 177 were excluded (96 for indication,78 for receiving only 1 dose of ZA, 3 for not receiving any doses of ZA). The remaining 123 patients were stratified into a standard-interval dosing group (121) and an extended-interval dosing group (35). Of the 123 patients, 33 received both standard- and extended-interval dosing of ZA over the course of the study period and were included discretely in each group for the duration of each dosing strategy.
Pre-ZA dental screenings were documented in 14% of standard-interval patients and 17% of extended-interval patients, and during-ZA screenings were documented in 17% of standard-interval patients and 20% of extended-interval patients. Chi-square analysis revealed no significant difference in rates of dental screening before or during use of ZA.
Standard-interval patients received a mean (SD) 11.4 (13.5) doses of ZA (range, 2-124). Extended-interval patients received a mean (SD) of 5.9 (3.18) doses (range, 2-14). All standard-interval patients had discontinued treatment at the time of the study, most commonly because of death or for an unknown reason. Sixty percent of extended-interval patients had discontinued treatment, most commonly because of patient/physician choice or for an unknown reason (Table 2).
Skeletal-related events were observed in 31% of standard-interval patients and 23% of extended-interval patients. There were no statistically significant differences in SRE rates between groups (P = .374). The most common SRE in both groups was bone irradiation (42% and 60%, respectively), with no statistically significant difference in proportion between groups (Table 4).
Discussion
This retrospective review of patients with MM and CaP receiving ZA for bone metastasesfound no differences in the rates of SREs when ZA was dosed monthly vs every 3 months.
Earlier studies found that ZA can decrease SRE rates, but a major concern is that frequent, prolonged exposure to IV bisphosphonates may increase the risk of ONJ. No significant differences in ONJ rates existed between dosing groups, but all documented cases of ONJ occurred in the standard-interval group, suggesting a trend toward decreased incidence with an extension of the dosing interval.
Limitations
This study had several limitations. Geriatric African American men comprised the majority of the study population, and patients with MM accounted for only 22% of included regimens, limiting external validity. Patient overlap between groups may have confounded the results. The retrospective design precluded the ability to control for confounding variables, such as concomitant medication use and medication adherence, and significant heterogeneity was noted in rates of adherence with ZA infusion schedules regardless of dosing group. Use of medications associated with increased risk of osteoporosis—including corticosteroids and proton pump inhibitors—was not assessed.
Assessment of ONJ incidence was limited by the lack of access to dental records from providers outside the VA. Many patients in this review were not eligible for VA dental benefits because of requirements involving time and service connection, a reimbursement measurement that reflects health conditions “incurred or aggravated during active military service.”18
The results of this study provide further support for extended-interval dosing of ZA as a potential method of increasing patient adherence and decreasing the possibility of adverse drug reactions without compromising therapeutic benefit. Further randomized controlled trials are needed to define the potential decrease in ONJ incidence.
Conclusion
In comparisons of standard- and extended-interval dosing of ZA, there was no difference in the incidence of skeletal-related events in veteran patients with bone metastases from MM or CaP.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Bone pain is one of the most common causes of morbidity in multiple myeloma (MM) and metastatic prostate cancer (CaP). This pain originates with the underlying pathologic processes of the cancer and with downstream skeletal-related events (SREs). SREs—fractures, spinal cord compression, and irradiation or surgery performed in ≥ 1 bone sites—represent a significant health care burden, particularly given the incidence of the underlying malignancies. According to American Cancer Society statistics, CaP is the second most common cancer in American men, and MM the second most common hematologic malignancy, despite its relatively low overall lifetime risk.1,2 Regardless of the underlying malignancy, bisphosphonates are the cornerstone of SRE prevention, though the optimal dosing strategy is the subject of clinical debate.
Although similar in SRE incidence, MM and CaP have distinct pathophysiologic processes in the dysregulation of bone resorption. MM is a hematologic malignancy that increases the risk of SREs by osteoclast up-regulation, primarily through the RANK (receptor activator of nuclear factor α-B) signaling pathway.3 CaP is a solid tumor malignancy that metastasizes to bone. Dysregulation of the bone resorption or formation cycle and net bone loss are a result of endogenous osteoclast up-regulation in response to abnormal bone formation in osteoblastic bone metastases.4 Androgen-deprivation therapy, the cornerstone of CaP treatment, further predisposes CaP patients to osteoporosis and SREs.
Prevention of SREs is pharmacologically driven by bisphosphonates, which have antiresorptive effects on bone through promotion of osteoclast apoptosis.5 Two IV formulations, pamidronate and zoledronic acid (ZA), are US Food and Drug Administration approved for use in bone metastases from MM or solid tumors.6-10 Although generally well tolerated, bisphosphonates can cause osteonecrosis of the jaw (ONJ), an avascular death of bone tissue, particularly with prolonged use.11 With its documented incidence of 5% to 6.7% in bone metastasis, ONJ represents a significant morbidity risk in patients with MM and CaP who are treated with IV bisphosphonates.12
Investigators are exploring bisphosphonate dosing intervals to determine which is most appropriate in mitigating the risk of ONJ. Before 2006, bisphosphonates were consistently dosed once monthly in patients with MM or metastatic bone disease—a standard derived empirically rather than from comparative studies or compelling pharmacodynamic data.13-15 In a 2006 consensus statement, the Mayo Clinic issued an expert opinion recommendation for increasing the bisphosphonate dosing interval to every 3 months in patients with MM.16 The first objective evidence for the clinical applicability of extending the ZA dosing interval was reported by Himelstein and colleagues in 2017.17 The randomized clinical trial found no differences in SRE rates when ZA was dosed every 12 weeks,17 prompting a conditional recommendation for dosing interval extension in the American Society of Clinical Oncology MM treatment guidelines (2018).13 Because of the age and racial demographics of the patients in these studies, many questions remain unanswered.
For the US Department of Veterans Affairs (VA) population, the pharmacokinetic and dynamic differences imposed by age and race limit the applicability of the available data. However, in veterans with MM or CaP, extending the bisphosphonate dosing interval may help decrease medication-related morbidity (eg, ONJ, nephrotoxicity) without compromising therapeutic benefit. To this end at the Memphis VA Medical Center (VAMC), we assessed for differences in SRE rates by comparing outcomes of patients who received ZA in standard- vs extended-interval dosing.
Methods
We retrospectively reviewed the Computerized Patient Record System for veterans with MM or metastatic CaP treated with ZA at the Memphis VAMC. Study inclusion criteria were aged > 18 years and care provided by a Memphis VAMC oncologist between January 2003 and January 2018. The study was approved by the Memphis VAMC’s Institutional Review Board, and procedures were followed in accordance with the ethical standards of its committee on human experimentation.
Using Microsoft SQL 2016 (Redmond, WA), we performed a query to identify patients who were prescribed ZA during the study period. Exclusion criteria were ZA prescribed for an indication other than MM or CaP (ie, osteoporosis) and receipt of ≤ 1 dose of ZA. Once a list was compiled, patients were stratified by ZA dosing interval: standard (mean, every month) or extended (mean, every 3 months). Patients whose ZA dosing interval was changed during treatment were included as independent data points in each group.
Skeletal-related events included fractures, spinal compression, irradiation, and surgery. Fractures and spinal compression were pertinent in the presence of radiographic documentation (eg, X-ray, magnetic resonance imaging scan) during the period the patient received ZA or within 1 dosing interval of the last recorded ZA dose. Irradiation was defined as documented application of radiation therapy to ≥ 1 bone sites for palliation of pain or as an intervention in the setting of spinal compression. Surgery was defined as any procedure performed to correct a fracture or spinal compression. Each SRE was counted as a single occurrence.
Osteonecrosis of the jaw was defined as radiographically documented necrosis of the mandible or associated structures with assessment by a VA dentist. Records from non-VA dental practices were not available for assessment. Documentation of dental assessment before the first dose of ZA and any assessments during treatment were recorded.
Medication use was assessed before and during ZA treatment. Number of ZA doses and reasons for any discontinuations were documented, as was concomitant use of calcium supplements, vitamin D supplements, calcitriol, paricalcitol, calcitonin, cinacalcet, and pamidronate.
The primary study outcome was observed difference in incidence of SREs between standard- and extended-interval dosing of ZA. Secondary outcomes included difference in incidence of ONJ as well as incidence of SREs and ONJ by disease subtype (MM, CaP).
Descriptive statistics were used to summarize demographic data and assess prespecified outcomes. Differences in rates of SREs and ONJ between dosing interval groups were analyzed with the Pearson χ2 test. The predetermined a priori level of significance was .05.
Results
Of the 300 patients prescribed ZA at the Memphis VAMC, 177 were excluded (96 for indication,78 for receiving only 1 dose of ZA, 3 for not receiving any doses of ZA). The remaining 123 patients were stratified into a standard-interval dosing group (121) and an extended-interval dosing group (35). Of the 123 patients, 33 received both standard- and extended-interval dosing of ZA over the course of the study period and were included discretely in each group for the duration of each dosing strategy.
Pre-ZA dental screenings were documented in 14% of standard-interval patients and 17% of extended-interval patients, and during-ZA screenings were documented in 17% of standard-interval patients and 20% of extended-interval patients. Chi-square analysis revealed no significant difference in rates of dental screening before or during use of ZA.
Standard-interval patients received a mean (SD) 11.4 (13.5) doses of ZA (range, 2-124). Extended-interval patients received a mean (SD) of 5.9 (3.18) doses (range, 2-14). All standard-interval patients had discontinued treatment at the time of the study, most commonly because of death or for an unknown reason. Sixty percent of extended-interval patients had discontinued treatment, most commonly because of patient/physician choice or for an unknown reason (Table 2).
Skeletal-related events were observed in 31% of standard-interval patients and 23% of extended-interval patients. There were no statistically significant differences in SRE rates between groups (P = .374). The most common SRE in both groups was bone irradiation (42% and 60%, respectively), with no statistically significant difference in proportion between groups (Table 4).
Discussion
This retrospective review of patients with MM and CaP receiving ZA for bone metastasesfound no differences in the rates of SREs when ZA was dosed monthly vs every 3 months.
Earlier studies found that ZA can decrease SRE rates, but a major concern is that frequent, prolonged exposure to IV bisphosphonates may increase the risk of ONJ. No significant differences in ONJ rates existed between dosing groups, but all documented cases of ONJ occurred in the standard-interval group, suggesting a trend toward decreased incidence with an extension of the dosing interval.
Limitations
This study had several limitations. Geriatric African American men comprised the majority of the study population, and patients with MM accounted for only 22% of included regimens, limiting external validity. Patient overlap between groups may have confounded the results. The retrospective design precluded the ability to control for confounding variables, such as concomitant medication use and medication adherence, and significant heterogeneity was noted in rates of adherence with ZA infusion schedules regardless of dosing group. Use of medications associated with increased risk of osteoporosis—including corticosteroids and proton pump inhibitors—was not assessed.
Assessment of ONJ incidence was limited by the lack of access to dental records from providers outside the VA. Many patients in this review were not eligible for VA dental benefits because of requirements involving time and service connection, a reimbursement measurement that reflects health conditions “incurred or aggravated during active military service.”18
The results of this study provide further support for extended-interval dosing of ZA as a potential method of increasing patient adherence and decreasing the possibility of adverse drug reactions without compromising therapeutic benefit. Further randomized controlled trials are needed to define the potential decrease in ONJ incidence.
Conclusion
In comparisons of standard- and extended-interval dosing of ZA, there was no difference in the incidence of skeletal-related events in veteran patients with bone metastases from MM or CaP.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review (CSR), 1975-2014 [based on November 2016 SEER data submission posted to SEER website April 2017]. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed January 12, 2019.
3. Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23(3):435-441.
4. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657.
5. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032-1045.
6. Zometa [package insert]. East Hanover, NJ: Novartis; 2016.
7. Aredia [package insert]. East Hanover, NJ: Novartis; 2011.
8. Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases: a double-blind, randomized dose-response study [published correction appears in Cancer. 2001;91(10):1956]. Cancer. 2001;91(7):1191-1200.
9. Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488-493.
10. Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012;(5):CD003188.
11. Wu S, Dahut WL, Gulley JL. The use of bisphosphonates in cancer patients. Acta Oncol. 2007;46(5):581-591.
12. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580-8587.
13. Anderson K, Ismaila N, Flynn PJ, et al. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(8):812-818.
14. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Multiple Myeloma. Version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed January 29, 2019.
15. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Prostate Cancer. Version 4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 29, 2019.
16. Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo Clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81(8):1047-1053.
17. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58.
18. Office of Public and Intergovernmental Affairs, US Department of Veterans Affairs. Service connected disabilities. In: Federal Benefits for Veterans, Dependents, and Survivors. https://www.va.gov/opa/publications/benefits_book/benefits_chap02.asp. Published April 2015. Accessed May 22, 2018.
1. American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review (CSR), 1975-2014 [based on November 2016 SEER data submission posted to SEER website April 2017]. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed January 12, 2019.
3. Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23(3):435-441.
4. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657.
5. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032-1045.
6. Zometa [package insert]. East Hanover, NJ: Novartis; 2016.
7. Aredia [package insert]. East Hanover, NJ: Novartis; 2011.
8. Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases: a double-blind, randomized dose-response study [published correction appears in Cancer. 2001;91(10):1956]. Cancer. 2001;91(7):1191-1200.
9. Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488-493.
10. Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012;(5):CD003188.
11. Wu S, Dahut WL, Gulley JL. The use of bisphosphonates in cancer patients. Acta Oncol. 2007;46(5):581-591.
12. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580-8587.
13. Anderson K, Ismaila N, Flynn PJ, et al. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(8):812-818.
14. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Multiple Myeloma. Version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed January 29, 2019.
15. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Prostate Cancer. Version 4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 29, 2019.
16. Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo Clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81(8):1047-1053.
17. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58.
18. Office of Public and Intergovernmental Affairs, US Department of Veterans Affairs. Service connected disabilities. In: Federal Benefits for Veterans, Dependents, and Survivors. https://www.va.gov/opa/publications/benefits_book/benefits_chap02.asp. Published April 2015. Accessed May 22, 2018.
Prostate Cancer Surveillance After Radiation Therapy in a National Delivery System (FULL)
Guideline concordance with PSA surveillance among veterans treated with definitiveradiation therapy was generally high, but opportunities may exist to improve surveillance among select groups.
Guidelines recommend prostate-specific antigen (PSA) surveillance among men treated with definitive radiation therapy (RT) for prostate cancer. Specifically, the National Comprehensive Cancer Network recommends testing every 6 to 12 months for 5 years and annually thereafter (with no specific stopping period specified), while the American Urology Association recommends testing for at least 10 years, with the frequency to be determined by the risk of relapse and patient preferences for monitoring.1,2 Salvage treatments exist for men with localized recurrence identified early through PSA testing, so adherence to follow-up guidelines is important for quality prostate cancer survivorship care.1,2
However, few studies focus on adherence to PSA surveillance following radiation therapy. Posttreatment surveillance among surgical patients is generally high, but sociodemographic disparities exist. Racial and ethnic minorities and unmarried men are less likely to undergo guideline concordant surveillance than is the general population, potentially preventing effective salvage therapy.3,4 A recent Department of Veterans Affairs (VA) study on posttreatment surveillance included radiation therapy patients but did not examine the impact of younger age, concurrent androgen deprivation therapy (ADT), or treatment facility (ie, diagnosed and treated at the same vs different facilities, with the latter including a separate VA facility or the community) on surveillance patterns.5 The latter is particularly relevant given increasing efforts to coordinate care outside the VA delivery system supported by the 2018 VA Maintaining Systems and Strengthening Integrated Outside Networks (MISSION) Act. Furthermore, these patient, treatment, and delivery system factors may each uniquely contribute to whether patients receive guideline-recommended PSA surveillance after prostate cancer treatment.
For these reasons, we conducted a study to better understand determinants of adherence to guideline-recommended PSA surveillance among veterans undergoing definitive radiation therapy with or without concurrent ADT. Our study uniquely included both elderly and nonelderly patients as well as investigated relationships between treatment at or away from the diagnosing facility. Although we found high overall levels of adherence to PSA surveillance, our findings do offer insights into determinants associated with worse adherence and provide opportunities to improve prostate cancer survivorship care after RT.
Methods
This study population included men with biopsy-proven nonmetastatic incident prostate cancer diagnosed between January 2005 and December 2008, with follow-up through 2012, identified using the VA Central Cancer Registry. We included men who underwent definitive RT with or without concurrent ADT injections, determined using the VA pharmacy files. We excluded men with a prior diagnosis of prostate or other malignancy (given the presence of other malignancies might affect life expectancy and surveillance patterns), hospice enrollment within 30 days, diagnosis at autopsy, and those treated with radical prostatectomy. We extracted cancer registry data, including biopsy Gleason score, pretreatment PSA level, clinical tumor stage, and whether RT was delivered at the patient’s diagnosing facility. For the latter, we used data on radiation location coded by the tumor registrar. We also collected demographic information, including age at diagnosis, race, ethnicity, marital status, and ZIP code. We used diagnosis codes to determine Charlson comorbidity scores similar to prior studies.6-8
Primary Outcome
The primary outcome was receipt of guideline concordant annual PSA surveillance in the initial 5 years following RT. We used laboratory files within the VA Corporate Data Warehouse to identify the date and value for each PSA test after RT for the entire cohort. Specifically, we defined the surveillance period as 60 days after initiation of RT through December 31, 2012. We defined guideline concordance as receiving at least 1 PSA test for each 12-month period after RT.
Statistical Analysis
We used descriptive statistics to characterize our cohort of veterans with prostate cancer treated with RT with or without concurrent ADT. To handle missing data, we performed multiple imputation, generating 10 imputations using all baseline clinical and demographic variables, year of diagnosis, and the regional VA network (ie, the Veterans Integrated Services Network [VISN]) for each patient.
Next, we calculated the annual guideline concordance rate for each year of follow-up for each patient, for the overall cohort, as well as by age, race/ethnicity, and concurrent ADT use. We examined bivariable relationships between guideline concordance and baseline demographic, clinical, and delivery system factors, including year of diagnosis and whether patients were treated at the diagnosing facility, using multilevel logistic regression modeling to account for clustering at the patient level.
Analyses were performed using Stata Version 15 (College Station, TX). We considered a 2-sided P value of < .05 as statistically significant. This study was approved by the VA Ann Arbor Health Care System Institution Review Board.
Results
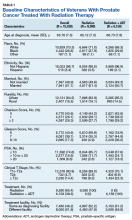
We evaluated annual PSA surveillance for 15,538 men treated with RT with or without concurrent ADT (Table 1).
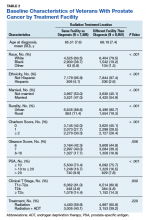
On unadjusted analysis, annual guideline concordance was less common among patients who were at the extremes of age, white, had Gleason 6 disease, PSA ≤ 10 ng/mL, did not receive concurrent ADT, and were treated away from their diagnosing facility (P < .05) (data not shown). We did find slight differences in patient characteristics based on whether patients were treated at their diagnosing facility (Table 2).
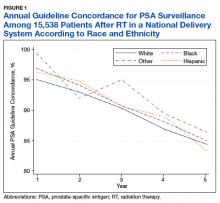
Overall, we found annual guideline concordance was initially very high, though declined slightly over the study period. For example, guideline concordance dropped from 96% in year 1 to 85% in year 5, with an average patient-level guideline concordance of 91% during the study period. We found minimal differences in annual surveillance after RT by race/ethnicity (Figure 1).
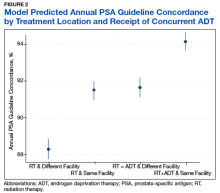
On multilevel multivariable analysis to adjust for clustering at the patient level, we found that race and PSA level were no longer significant predictors of annual surveillance (Table 3).
Discussion
We investigated adherence to guideline-recommended annual surveillance PSA testing in a national cohort of veterans treated with definitive RT for prostate cancer. We found guideline concordance was initially high and decreased slightly over time. We also found guideline concordance with PSA surveillance varied based on a number of clinical and delivery system factors, including marital status, rurality, receipt of concurrent ADT, as well as whether the veteran was treated at his diagnosing facility. Taken together, these overall results are promising, however, also point to unique considerations for some patient groups and potentially those treated in the community.
Our finding of lower guideline concordance among nonmarried patients is consistent with prior research, including our study of patients undergoing surgery for prostate cancer.4 Addressing surveillance in this population is important, as they may have less social support than do their married counterparts. We also found surveillance was lower at the extremes of age, which may be appropriate in elderly patients with limited life expectancy but is concerning for younger men with low competing mortality risks.7 Future work should explore whether younger patients experience barriers to care, including employment challenges, as these men are at greatest risk of cancer progression if recurrence goes undetected.
Although rural patients are less likely to undergo definitive prostate cancer treatment, possibly reflecting barriers to care, in our study, surveillance was actually higher among this population than that for urban patients.9 This could reflect the VA’s success in connecting rural patients to appropriate services despite travel distances to maintain quality of cancer care.10 Given annual PSA surveillance is relatively infrequent and not particularly resource intensive, these high surveillance rates might not apply to patients with cancers who need more frequent survivorship care, such as those with head and neck cancer. Future work should examine why surveillance rates among urban patients might be slightly lower, as living in a metropolitan area does not equate to the absence of barriers to survivorship care, especially for veterans who may not be able to take time off from work or have transportation barriers.
We found guideline concordance was higher among patients with higher Gleason scores, which is important given their higher likelihood of failure. However, low- and intermediate-risk patients also are at risk for treatment failure, so annual PSA surveillance should be optimized in this population unless future studies support the safety and feasibility of less frequent surveillance.10-13 Our finding of increased surveillance in patients who receive concurrent ADT may relate to the increased frequency of survivorship care given the need for injections, often every 3 to 6 months. Future studies might examine whether surveillance decreases in this population once they complete their short or long-term ADT, typically given for a maximum of 3 years.
A particularly relevant finding given recent VA policy changes includes lower guideline concordance for patients receiving RT at a different facility than where they were diagnosed. One possible explanation is that a proportion of patients treated outside of their home facilities use Medicare or private insurance and may have surveillance performed outside of the VA, which would not have been captured in our study.14 However, it remains plausible that there are challenges related to coordination and fragmentation of survivorship care for veterans who receive care at separate VA facilities or receive their initial treatment in the community.15 Future studies can help quantify how much this difference is driven by diagnosis and treatment at separate VA sites vs treatment outside of the VA, as different strategies might be necessary to improve surveillance in these 2 populations. Moreover, electronic health record-based tracking has been proposed as a strategy to identify patients who have not received guideline concordant PSA surveillance.14 This strategy may help increase guideline concordance regardless of initial treatment location if VA survivorship care is intended.
Although our study examined receipt of PSA testing, it did not examine whether patients are physically seen back in radiation oncology clinics, or whether their PSAs have been reviewed by radiation oncology providers. Although many surgical patients return to primary care providers for PSA surveillance, surveillance after RT is more complex and likely best managed in the initial years by radiation oncologists. Unlike the postoperative setting in which the definition of PSA failure is straightforward at > 0.2 ng/mL, the definition of treatment failure after RT is more complicated as described below.
For patients who did not receive concurrent ADT, failure is defined as a PSA nadir + 2 ng/mL, which first requires establishing the nadir using the first few postradiation PSA values.15 It becomes even more complex in the setting of ADT as it causes PSA suppression even in the absence of RT due to testosterone suppression.2 At the conclusion of ADT (short term 4-6 months or long term 18-36 months), the PSA may rise as testosterone recovers.15,16 This is not necessarily indicative of treatment failure, as some normal PSA-producing prostatic tissue may remain after treatment. Given these complexities, ongoing survivorship care with radiation oncology is recommended at least in the short term.
Physical visits are a challenge for some patients undergoing prostate cancer surveillance after treatment. Therefore, exploring the safety and feasibility of automated PSA tracking15 and strategies for increasing utilization of telemedicine, including clinical video telehealth appointments that are already used for survivorship and other urologic care in a number of VA clinics, represents opportunities to systematically provide highest quality survivorship care in VA.17,18
Conclusion
Most veterans receive guideline concordant PSA surveillance after RT for prostate cancer. Nonetheless, at the beginning of treatment, providers should screen veterans for risk factors for loss to follow-up (eg, care at a different or non-VA facility), discuss geographic, financial, and other barriers, and plan to leverage existing VA resources (eg, travel support) to continue to achieve high-quality PSA surveillance and survivorship care. Future research should investigate ways to take advantage of the VA’s robust electronic health record system and telemedicine infrastructure to further optimize prostate cancer survivorship care and PSA surveillance particularly among vulnerable patient groups and those treated outside of their diagnosing facility.
Acknowledgments
Funding Sources: VA HSR&D Career Development Award: 2 (CDA 12−171) and NCI R37 R37CA222885 (TAS).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
1. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prostate cancer v4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated August 15, 2018. Accessed January 23, 2019.
2. Sanda MG, Chen RC, Crispino T, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. https://www.auanet.org/guidelines/prostate-cancer-clinically-localized-(2017). Published 2017. Accessed January 22,2019.
3. Zeliadt SB, Penson DF, Albertsen PC, Concato J, Etzioni RD. Race independently predicts prostate specific antigen testing frequency following a prostate carcinoma diagnosis. Cancer. 2003;98(3):496-503.
4. Trantham LC, Nielsen ME, Mobley LR, Wheeler SB, Carpenter WR, Biddle AK. Use of prostate-specific antigen testing as a disease surveillance tool following radical prostatectomy. Cancer. 2013;119(19):3523-3530.
5. Shi Y, Fung KZ, John Boscardin W, et al. Individualizing PSA monitoring among older prostate cancer survivors. J Gen Intern Med. 2018;33(5):602-604.
6. Chapman C, Burns J, Caram M, Zaslavsky A, Tsodikov A, Skolarus TA. Multilevel predictors of surveillance PSA guideline concordance after radical prostatectomy: a national Veterans Affairs study. Paper presented at: Association of VA Hematology/Oncology Annual Meeting;
September 28-30, 2018; Chicago, IL. Abstract 34. https://www.mdedge.com/fedprac/avaho/article/175094/prostate-cancer/multilevel-predictors-surveillance-psa-guideline. Accessed January 22, 2019.
7. Kirk PS, Borza T, Caram MEV, et al. Characterising potential bone scan overuse amongst men treated with radical prostatectomy. BJU Int. 2018. [Epub ahead of print.]
8. Kirk PS, Borza T, Shahinian VB, et al. The implications of baseline bone-health assessment at initiation of androgen-deprivation therapy for prostate cancer. BJU Int. 2018;121(4):558-564.
9. Baldwin LM, Andrilla CH, Porter MP, Rosenblatt RA, Patel S, Doescher MP. Treatment of early-stage prostate cancer among rural and urban patients. Cancer. 2013;119(16):3067-3075.
10. Skolarus TA, Chan S, Shelton JB, et al. Quality of prostate cancer care among rural men in the Veterans Health Administration. Cancer. 2013;119(20):3629-3635.
11. Hamdy FC, Donovan JL, Lane JA, et al; ProtecT Study Group. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424.
12. Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG Oncology RTOG 0126 randomized clinical trial. JAMA Oncol.2018;4(6):e180039.
13. Chang MG, DeSotto K, Taibi P, Troeschel S. Development of a PSA tracking system for patients with prostate cancer following definitive radiotherapy to enhance rural health. J Clin Oncol. 2016;34(suppl 2):39-39.
14. Skolarus TA, Zhang Y, Hollenbeck BK. Understanding fragmentation of prostate cancer survivorship care: implications for cost and quality. Cancer. 2012;118(11):2837-2845.
15. Roach M, 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974.
16. Buyyounouski MK, Hanlon AL, Horwitz EM, Uzzo RG, Pollack A. Biochemical failure and the temporal kinetics of prostate-specific antigen after radiation therapy with androgen deprivation. Int J Radiat Oncol Biol Phys. 2005;61(5):1291-1298.
17. Chu S, Boxer R, Madison P, et al. Veterans Affairs telemedicine: bringing urologic care to remote clinics. Urology. 2015;86(2):255-260.
18. Safir IJ, Gabale S, David SA, et al. Implementation of a tele-urology program for outpatient hematuria referrals: initial results and patient satisfaction. Urology. 2016;97:33-39.
Guideline concordance with PSA surveillance among veterans treated with definitiveradiation therapy was generally high, but opportunities may exist to improve surveillance among select groups.
Guideline concordance with PSA surveillance among veterans treated with definitiveradiation therapy was generally high, but opportunities may exist to improve surveillance among select groups.
Guidelines recommend prostate-specific antigen (PSA) surveillance among men treated with definitive radiation therapy (RT) for prostate cancer. Specifically, the National Comprehensive Cancer Network recommends testing every 6 to 12 months for 5 years and annually thereafter (with no specific stopping period specified), while the American Urology Association recommends testing for at least 10 years, with the frequency to be determined by the risk of relapse and patient preferences for monitoring.1,2 Salvage treatments exist for men with localized recurrence identified early through PSA testing, so adherence to follow-up guidelines is important for quality prostate cancer survivorship care.1,2
However, few studies focus on adherence to PSA surveillance following radiation therapy. Posttreatment surveillance among surgical patients is generally high, but sociodemographic disparities exist. Racial and ethnic minorities and unmarried men are less likely to undergo guideline concordant surveillance than is the general population, potentially preventing effective salvage therapy.3,4 A recent Department of Veterans Affairs (VA) study on posttreatment surveillance included radiation therapy patients but did not examine the impact of younger age, concurrent androgen deprivation therapy (ADT), or treatment facility (ie, diagnosed and treated at the same vs different facilities, with the latter including a separate VA facility or the community) on surveillance patterns.5 The latter is particularly relevant given increasing efforts to coordinate care outside the VA delivery system supported by the 2018 VA Maintaining Systems and Strengthening Integrated Outside Networks (MISSION) Act. Furthermore, these patient, treatment, and delivery system factors may each uniquely contribute to whether patients receive guideline-recommended PSA surveillance after prostate cancer treatment.
For these reasons, we conducted a study to better understand determinants of adherence to guideline-recommended PSA surveillance among veterans undergoing definitive radiation therapy with or without concurrent ADT. Our study uniquely included both elderly and nonelderly patients as well as investigated relationships between treatment at or away from the diagnosing facility. Although we found high overall levels of adherence to PSA surveillance, our findings do offer insights into determinants associated with worse adherence and provide opportunities to improve prostate cancer survivorship care after RT.
Methods
This study population included men with biopsy-proven nonmetastatic incident prostate cancer diagnosed between January 2005 and December 2008, with follow-up through 2012, identified using the VA Central Cancer Registry. We included men who underwent definitive RT with or without concurrent ADT injections, determined using the VA pharmacy files. We excluded men with a prior diagnosis of prostate or other malignancy (given the presence of other malignancies might affect life expectancy and surveillance patterns), hospice enrollment within 30 days, diagnosis at autopsy, and those treated with radical prostatectomy. We extracted cancer registry data, including biopsy Gleason score, pretreatment PSA level, clinical tumor stage, and whether RT was delivered at the patient’s diagnosing facility. For the latter, we used data on radiation location coded by the tumor registrar. We also collected demographic information, including age at diagnosis, race, ethnicity, marital status, and ZIP code. We used diagnosis codes to determine Charlson comorbidity scores similar to prior studies.6-8
Primary Outcome
The primary outcome was receipt of guideline concordant annual PSA surveillance in the initial 5 years following RT. We used laboratory files within the VA Corporate Data Warehouse to identify the date and value for each PSA test after RT for the entire cohort. Specifically, we defined the surveillance period as 60 days after initiation of RT through December 31, 2012. We defined guideline concordance as receiving at least 1 PSA test for each 12-month period after RT.
Statistical Analysis
We used descriptive statistics to characterize our cohort of veterans with prostate cancer treated with RT with or without concurrent ADT. To handle missing data, we performed multiple imputation, generating 10 imputations using all baseline clinical and demographic variables, year of diagnosis, and the regional VA network (ie, the Veterans Integrated Services Network [VISN]) for each patient.
Next, we calculated the annual guideline concordance rate for each year of follow-up for each patient, for the overall cohort, as well as by age, race/ethnicity, and concurrent ADT use. We examined bivariable relationships between guideline concordance and baseline demographic, clinical, and delivery system factors, including year of diagnosis and whether patients were treated at the diagnosing facility, using multilevel logistic regression modeling to account for clustering at the patient level.
Analyses were performed using Stata Version 15 (College Station, TX). We considered a 2-sided P value of < .05 as statistically significant. This study was approved by the VA Ann Arbor Health Care System Institution Review Board.
Results
We evaluated annual PSA surveillance for 15,538 men treated with RT with or without concurrent ADT (Table 1).
On unadjusted analysis, annual guideline concordance was less common among patients who were at the extremes of age, white, had Gleason 6 disease, PSA ≤ 10 ng/mL, did not receive concurrent ADT, and were treated away from their diagnosing facility (P < .05) (data not shown). We did find slight differences in patient characteristics based on whether patients were treated at their diagnosing facility (Table 2).
Overall, we found annual guideline concordance was initially very high, though declined slightly over the study period. For example, guideline concordance dropped from 96% in year 1 to 85% in year 5, with an average patient-level guideline concordance of 91% during the study period. We found minimal differences in annual surveillance after RT by race/ethnicity (Figure 1).
On multilevel multivariable analysis to adjust for clustering at the patient level, we found that race and PSA level were no longer significant predictors of annual surveillance (Table 3).
Discussion
We investigated adherence to guideline-recommended annual surveillance PSA testing in a national cohort of veterans treated with definitive RT for prostate cancer. We found guideline concordance was initially high and decreased slightly over time. We also found guideline concordance with PSA surveillance varied based on a number of clinical and delivery system factors, including marital status, rurality, receipt of concurrent ADT, as well as whether the veteran was treated at his diagnosing facility. Taken together, these overall results are promising, however, also point to unique considerations for some patient groups and potentially those treated in the community.
Our finding of lower guideline concordance among nonmarried patients is consistent with prior research, including our study of patients undergoing surgery for prostate cancer.4 Addressing surveillance in this population is important, as they may have less social support than do their married counterparts. We also found surveillance was lower at the extremes of age, which may be appropriate in elderly patients with limited life expectancy but is concerning for younger men with low competing mortality risks.7 Future work should explore whether younger patients experience barriers to care, including employment challenges, as these men are at greatest risk of cancer progression if recurrence goes undetected.
Although rural patients are less likely to undergo definitive prostate cancer treatment, possibly reflecting barriers to care, in our study, surveillance was actually higher among this population than that for urban patients.9 This could reflect the VA’s success in connecting rural patients to appropriate services despite travel distances to maintain quality of cancer care.10 Given annual PSA surveillance is relatively infrequent and not particularly resource intensive, these high surveillance rates might not apply to patients with cancers who need more frequent survivorship care, such as those with head and neck cancer. Future work should examine why surveillance rates among urban patients might be slightly lower, as living in a metropolitan area does not equate to the absence of barriers to survivorship care, especially for veterans who may not be able to take time off from work or have transportation barriers.
We found guideline concordance was higher among patients with higher Gleason scores, which is important given their higher likelihood of failure. However, low- and intermediate-risk patients also are at risk for treatment failure, so annual PSA surveillance should be optimized in this population unless future studies support the safety and feasibility of less frequent surveillance.10-13 Our finding of increased surveillance in patients who receive concurrent ADT may relate to the increased frequency of survivorship care given the need for injections, often every 3 to 6 months. Future studies might examine whether surveillance decreases in this population once they complete their short or long-term ADT, typically given for a maximum of 3 years.
A particularly relevant finding given recent VA policy changes includes lower guideline concordance for patients receiving RT at a different facility than where they were diagnosed. One possible explanation is that a proportion of patients treated outside of their home facilities use Medicare or private insurance and may have surveillance performed outside of the VA, which would not have been captured in our study.14 However, it remains plausible that there are challenges related to coordination and fragmentation of survivorship care for veterans who receive care at separate VA facilities or receive their initial treatment in the community.15 Future studies can help quantify how much this difference is driven by diagnosis and treatment at separate VA sites vs treatment outside of the VA, as different strategies might be necessary to improve surveillance in these 2 populations. Moreover, electronic health record-based tracking has been proposed as a strategy to identify patients who have not received guideline concordant PSA surveillance.14 This strategy may help increase guideline concordance regardless of initial treatment location if VA survivorship care is intended.
Although our study examined receipt of PSA testing, it did not examine whether patients are physically seen back in radiation oncology clinics, or whether their PSAs have been reviewed by radiation oncology providers. Although many surgical patients return to primary care providers for PSA surveillance, surveillance after RT is more complex and likely best managed in the initial years by radiation oncologists. Unlike the postoperative setting in which the definition of PSA failure is straightforward at > 0.2 ng/mL, the definition of treatment failure after RT is more complicated as described below.
For patients who did not receive concurrent ADT, failure is defined as a PSA nadir + 2 ng/mL, which first requires establishing the nadir using the first few postradiation PSA values.15 It becomes even more complex in the setting of ADT as it causes PSA suppression even in the absence of RT due to testosterone suppression.2 At the conclusion of ADT (short term 4-6 months or long term 18-36 months), the PSA may rise as testosterone recovers.15,16 This is not necessarily indicative of treatment failure, as some normal PSA-producing prostatic tissue may remain after treatment. Given these complexities, ongoing survivorship care with radiation oncology is recommended at least in the short term.
Physical visits are a challenge for some patients undergoing prostate cancer surveillance after treatment. Therefore, exploring the safety and feasibility of automated PSA tracking15 and strategies for increasing utilization of telemedicine, including clinical video telehealth appointments that are already used for survivorship and other urologic care in a number of VA clinics, represents opportunities to systematically provide highest quality survivorship care in VA.17,18
Conclusion
Most veterans receive guideline concordant PSA surveillance after RT for prostate cancer. Nonetheless, at the beginning of treatment, providers should screen veterans for risk factors for loss to follow-up (eg, care at a different or non-VA facility), discuss geographic, financial, and other barriers, and plan to leverage existing VA resources (eg, travel support) to continue to achieve high-quality PSA surveillance and survivorship care. Future research should investigate ways to take advantage of the VA’s robust electronic health record system and telemedicine infrastructure to further optimize prostate cancer survivorship care and PSA surveillance particularly among vulnerable patient groups and those treated outside of their diagnosing facility.
Acknowledgments
Funding Sources: VA HSR&D Career Development Award: 2 (CDA 12−171) and NCI R37 R37CA222885 (TAS).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Guidelines recommend prostate-specific antigen (PSA) surveillance among men treated with definitive radiation therapy (RT) for prostate cancer. Specifically, the National Comprehensive Cancer Network recommends testing every 6 to 12 months for 5 years and annually thereafter (with no specific stopping period specified), while the American Urology Association recommends testing for at least 10 years, with the frequency to be determined by the risk of relapse and patient preferences for monitoring.1,2 Salvage treatments exist for men with localized recurrence identified early through PSA testing, so adherence to follow-up guidelines is important for quality prostate cancer survivorship care.1,2
However, few studies focus on adherence to PSA surveillance following radiation therapy. Posttreatment surveillance among surgical patients is generally high, but sociodemographic disparities exist. Racial and ethnic minorities and unmarried men are less likely to undergo guideline concordant surveillance than is the general population, potentially preventing effective salvage therapy.3,4 A recent Department of Veterans Affairs (VA) study on posttreatment surveillance included radiation therapy patients but did not examine the impact of younger age, concurrent androgen deprivation therapy (ADT), or treatment facility (ie, diagnosed and treated at the same vs different facilities, with the latter including a separate VA facility or the community) on surveillance patterns.5 The latter is particularly relevant given increasing efforts to coordinate care outside the VA delivery system supported by the 2018 VA Maintaining Systems and Strengthening Integrated Outside Networks (MISSION) Act. Furthermore, these patient, treatment, and delivery system factors may each uniquely contribute to whether patients receive guideline-recommended PSA surveillance after prostate cancer treatment.
For these reasons, we conducted a study to better understand determinants of adherence to guideline-recommended PSA surveillance among veterans undergoing definitive radiation therapy with or without concurrent ADT. Our study uniquely included both elderly and nonelderly patients as well as investigated relationships between treatment at or away from the diagnosing facility. Although we found high overall levels of adherence to PSA surveillance, our findings do offer insights into determinants associated with worse adherence and provide opportunities to improve prostate cancer survivorship care after RT.
Methods
This study population included men with biopsy-proven nonmetastatic incident prostate cancer diagnosed between January 2005 and December 2008, with follow-up through 2012, identified using the VA Central Cancer Registry. We included men who underwent definitive RT with or without concurrent ADT injections, determined using the VA pharmacy files. We excluded men with a prior diagnosis of prostate or other malignancy (given the presence of other malignancies might affect life expectancy and surveillance patterns), hospice enrollment within 30 days, diagnosis at autopsy, and those treated with radical prostatectomy. We extracted cancer registry data, including biopsy Gleason score, pretreatment PSA level, clinical tumor stage, and whether RT was delivered at the patient’s diagnosing facility. For the latter, we used data on radiation location coded by the tumor registrar. We also collected demographic information, including age at diagnosis, race, ethnicity, marital status, and ZIP code. We used diagnosis codes to determine Charlson comorbidity scores similar to prior studies.6-8
Primary Outcome
The primary outcome was receipt of guideline concordant annual PSA surveillance in the initial 5 years following RT. We used laboratory files within the VA Corporate Data Warehouse to identify the date and value for each PSA test after RT for the entire cohort. Specifically, we defined the surveillance period as 60 days after initiation of RT through December 31, 2012. We defined guideline concordance as receiving at least 1 PSA test for each 12-month period after RT.
Statistical Analysis
We used descriptive statistics to characterize our cohort of veterans with prostate cancer treated with RT with or without concurrent ADT. To handle missing data, we performed multiple imputation, generating 10 imputations using all baseline clinical and demographic variables, year of diagnosis, and the regional VA network (ie, the Veterans Integrated Services Network [VISN]) for each patient.
Next, we calculated the annual guideline concordance rate for each year of follow-up for each patient, for the overall cohort, as well as by age, race/ethnicity, and concurrent ADT use. We examined bivariable relationships between guideline concordance and baseline demographic, clinical, and delivery system factors, including year of diagnosis and whether patients were treated at the diagnosing facility, using multilevel logistic regression modeling to account for clustering at the patient level.
Analyses were performed using Stata Version 15 (College Station, TX). We considered a 2-sided P value of < .05 as statistically significant. This study was approved by the VA Ann Arbor Health Care System Institution Review Board.
Results
We evaluated annual PSA surveillance for 15,538 men treated with RT with or without concurrent ADT (Table 1).
On unadjusted analysis, annual guideline concordance was less common among patients who were at the extremes of age, white, had Gleason 6 disease, PSA ≤ 10 ng/mL, did not receive concurrent ADT, and were treated away from their diagnosing facility (P < .05) (data not shown). We did find slight differences in patient characteristics based on whether patients were treated at their diagnosing facility (Table 2).
Overall, we found annual guideline concordance was initially very high, though declined slightly over the study period. For example, guideline concordance dropped from 96% in year 1 to 85% in year 5, with an average patient-level guideline concordance of 91% during the study period. We found minimal differences in annual surveillance after RT by race/ethnicity (Figure 1).
On multilevel multivariable analysis to adjust for clustering at the patient level, we found that race and PSA level were no longer significant predictors of annual surveillance (Table 3).
Discussion
We investigated adherence to guideline-recommended annual surveillance PSA testing in a national cohort of veterans treated with definitive RT for prostate cancer. We found guideline concordance was initially high and decreased slightly over time. We also found guideline concordance with PSA surveillance varied based on a number of clinical and delivery system factors, including marital status, rurality, receipt of concurrent ADT, as well as whether the veteran was treated at his diagnosing facility. Taken together, these overall results are promising, however, also point to unique considerations for some patient groups and potentially those treated in the community.
Our finding of lower guideline concordance among nonmarried patients is consistent with prior research, including our study of patients undergoing surgery for prostate cancer.4 Addressing surveillance in this population is important, as they may have less social support than do their married counterparts. We also found surveillance was lower at the extremes of age, which may be appropriate in elderly patients with limited life expectancy but is concerning for younger men with low competing mortality risks.7 Future work should explore whether younger patients experience barriers to care, including employment challenges, as these men are at greatest risk of cancer progression if recurrence goes undetected.
Although rural patients are less likely to undergo definitive prostate cancer treatment, possibly reflecting barriers to care, in our study, surveillance was actually higher among this population than that for urban patients.9 This could reflect the VA’s success in connecting rural patients to appropriate services despite travel distances to maintain quality of cancer care.10 Given annual PSA surveillance is relatively infrequent and not particularly resource intensive, these high surveillance rates might not apply to patients with cancers who need more frequent survivorship care, such as those with head and neck cancer. Future work should examine why surveillance rates among urban patients might be slightly lower, as living in a metropolitan area does not equate to the absence of barriers to survivorship care, especially for veterans who may not be able to take time off from work or have transportation barriers.
We found guideline concordance was higher among patients with higher Gleason scores, which is important given their higher likelihood of failure. However, low- and intermediate-risk patients also are at risk for treatment failure, so annual PSA surveillance should be optimized in this population unless future studies support the safety and feasibility of less frequent surveillance.10-13 Our finding of increased surveillance in patients who receive concurrent ADT may relate to the increased frequency of survivorship care given the need for injections, often every 3 to 6 months. Future studies might examine whether surveillance decreases in this population once they complete their short or long-term ADT, typically given for a maximum of 3 years.
A particularly relevant finding given recent VA policy changes includes lower guideline concordance for patients receiving RT at a different facility than where they were diagnosed. One possible explanation is that a proportion of patients treated outside of their home facilities use Medicare or private insurance and may have surveillance performed outside of the VA, which would not have been captured in our study.14 However, it remains plausible that there are challenges related to coordination and fragmentation of survivorship care for veterans who receive care at separate VA facilities or receive their initial treatment in the community.15 Future studies can help quantify how much this difference is driven by diagnosis and treatment at separate VA sites vs treatment outside of the VA, as different strategies might be necessary to improve surveillance in these 2 populations. Moreover, electronic health record-based tracking has been proposed as a strategy to identify patients who have not received guideline concordant PSA surveillance.14 This strategy may help increase guideline concordance regardless of initial treatment location if VA survivorship care is intended.
Although our study examined receipt of PSA testing, it did not examine whether patients are physically seen back in radiation oncology clinics, or whether their PSAs have been reviewed by radiation oncology providers. Although many surgical patients return to primary care providers for PSA surveillance, surveillance after RT is more complex and likely best managed in the initial years by radiation oncologists. Unlike the postoperative setting in which the definition of PSA failure is straightforward at > 0.2 ng/mL, the definition of treatment failure after RT is more complicated as described below.
For patients who did not receive concurrent ADT, failure is defined as a PSA nadir + 2 ng/mL, which first requires establishing the nadir using the first few postradiation PSA values.15 It becomes even more complex in the setting of ADT as it causes PSA suppression even in the absence of RT due to testosterone suppression.2 At the conclusion of ADT (short term 4-6 months or long term 18-36 months), the PSA may rise as testosterone recovers.15,16 This is not necessarily indicative of treatment failure, as some normal PSA-producing prostatic tissue may remain after treatment. Given these complexities, ongoing survivorship care with radiation oncology is recommended at least in the short term.
Physical visits are a challenge for some patients undergoing prostate cancer surveillance after treatment. Therefore, exploring the safety and feasibility of automated PSA tracking15 and strategies for increasing utilization of telemedicine, including clinical video telehealth appointments that are already used for survivorship and other urologic care in a number of VA clinics, represents opportunities to systematically provide highest quality survivorship care in VA.17,18
Conclusion
Most veterans receive guideline concordant PSA surveillance after RT for prostate cancer. Nonetheless, at the beginning of treatment, providers should screen veterans for risk factors for loss to follow-up (eg, care at a different or non-VA facility), discuss geographic, financial, and other barriers, and plan to leverage existing VA resources (eg, travel support) to continue to achieve high-quality PSA surveillance and survivorship care. Future research should investigate ways to take advantage of the VA’s robust electronic health record system and telemedicine infrastructure to further optimize prostate cancer survivorship care and PSA surveillance particularly among vulnerable patient groups and those treated outside of their diagnosing facility.
Acknowledgments
Funding Sources: VA HSR&D Career Development Award: 2 (CDA 12−171) and NCI R37 R37CA222885 (TAS).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
1. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prostate cancer v4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated August 15, 2018. Accessed January 23, 2019.
2. Sanda MG, Chen RC, Crispino T, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. https://www.auanet.org/guidelines/prostate-cancer-clinically-localized-(2017). Published 2017. Accessed January 22,2019.
3. Zeliadt SB, Penson DF, Albertsen PC, Concato J, Etzioni RD. Race independently predicts prostate specific antigen testing frequency following a prostate carcinoma diagnosis. Cancer. 2003;98(3):496-503.
4. Trantham LC, Nielsen ME, Mobley LR, Wheeler SB, Carpenter WR, Biddle AK. Use of prostate-specific antigen testing as a disease surveillance tool following radical prostatectomy. Cancer. 2013;119(19):3523-3530.
5. Shi Y, Fung KZ, John Boscardin W, et al. Individualizing PSA monitoring among older prostate cancer survivors. J Gen Intern Med. 2018;33(5):602-604.
6. Chapman C, Burns J, Caram M, Zaslavsky A, Tsodikov A, Skolarus TA. Multilevel predictors of surveillance PSA guideline concordance after radical prostatectomy: a national Veterans Affairs study. Paper presented at: Association of VA Hematology/Oncology Annual Meeting;
September 28-30, 2018; Chicago, IL. Abstract 34. https://www.mdedge.com/fedprac/avaho/article/175094/prostate-cancer/multilevel-predictors-surveillance-psa-guideline. Accessed January 22, 2019.
7. Kirk PS, Borza T, Caram MEV, et al. Characterising potential bone scan overuse amongst men treated with radical prostatectomy. BJU Int. 2018. [Epub ahead of print.]
8. Kirk PS, Borza T, Shahinian VB, et al. The implications of baseline bone-health assessment at initiation of androgen-deprivation therapy for prostate cancer. BJU Int. 2018;121(4):558-564.
9. Baldwin LM, Andrilla CH, Porter MP, Rosenblatt RA, Patel S, Doescher MP. Treatment of early-stage prostate cancer among rural and urban patients. Cancer. 2013;119(16):3067-3075.
10. Skolarus TA, Chan S, Shelton JB, et al. Quality of prostate cancer care among rural men in the Veterans Health Administration. Cancer. 2013;119(20):3629-3635.
11. Hamdy FC, Donovan JL, Lane JA, et al; ProtecT Study Group. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424.
12. Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG Oncology RTOG 0126 randomized clinical trial. JAMA Oncol.2018;4(6):e180039.
13. Chang MG, DeSotto K, Taibi P, Troeschel S. Development of a PSA tracking system for patients with prostate cancer following definitive radiotherapy to enhance rural health. J Clin Oncol. 2016;34(suppl 2):39-39.
14. Skolarus TA, Zhang Y, Hollenbeck BK. Understanding fragmentation of prostate cancer survivorship care: implications for cost and quality. Cancer. 2012;118(11):2837-2845.
15. Roach M, 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974.
16. Buyyounouski MK, Hanlon AL, Horwitz EM, Uzzo RG, Pollack A. Biochemical failure and the temporal kinetics of prostate-specific antigen after radiation therapy with androgen deprivation. Int J Radiat Oncol Biol Phys. 2005;61(5):1291-1298.
17. Chu S, Boxer R, Madison P, et al. Veterans Affairs telemedicine: bringing urologic care to remote clinics. Urology. 2015;86(2):255-260.
18. Safir IJ, Gabale S, David SA, et al. Implementation of a tele-urology program for outpatient hematuria referrals: initial results and patient satisfaction. Urology. 2016;97:33-39.
1. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prostate cancer v4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated August 15, 2018. Accessed January 23, 2019.
2. Sanda MG, Chen RC, Crispino T, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. https://www.auanet.org/guidelines/prostate-cancer-clinically-localized-(2017). Published 2017. Accessed January 22,2019.
3. Zeliadt SB, Penson DF, Albertsen PC, Concato J, Etzioni RD. Race independently predicts prostate specific antigen testing frequency following a prostate carcinoma diagnosis. Cancer. 2003;98(3):496-503.
4. Trantham LC, Nielsen ME, Mobley LR, Wheeler SB, Carpenter WR, Biddle AK. Use of prostate-specific antigen testing as a disease surveillance tool following radical prostatectomy. Cancer. 2013;119(19):3523-3530.
5. Shi Y, Fung KZ, John Boscardin W, et al. Individualizing PSA monitoring among older prostate cancer survivors. J Gen Intern Med. 2018;33(5):602-604.
6. Chapman C, Burns J, Caram M, Zaslavsky A, Tsodikov A, Skolarus TA. Multilevel predictors of surveillance PSA guideline concordance after radical prostatectomy: a national Veterans Affairs study. Paper presented at: Association of VA Hematology/Oncology Annual Meeting;
September 28-30, 2018; Chicago, IL. Abstract 34. https://www.mdedge.com/fedprac/avaho/article/175094/prostate-cancer/multilevel-predictors-surveillance-psa-guideline. Accessed January 22, 2019.
7. Kirk PS, Borza T, Caram MEV, et al. Characterising potential bone scan overuse amongst men treated with radical prostatectomy. BJU Int. 2018. [Epub ahead of print.]
8. Kirk PS, Borza T, Shahinian VB, et al. The implications of baseline bone-health assessment at initiation of androgen-deprivation therapy for prostate cancer. BJU Int. 2018;121(4):558-564.
9. Baldwin LM, Andrilla CH, Porter MP, Rosenblatt RA, Patel S, Doescher MP. Treatment of early-stage prostate cancer among rural and urban patients. Cancer. 2013;119(16):3067-3075.
10. Skolarus TA, Chan S, Shelton JB, et al. Quality of prostate cancer care among rural men in the Veterans Health Administration. Cancer. 2013;119(20):3629-3635.
11. Hamdy FC, Donovan JL, Lane JA, et al; ProtecT Study Group. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424.
12. Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG Oncology RTOG 0126 randomized clinical trial. JAMA Oncol.2018;4(6):e180039.
13. Chang MG, DeSotto K, Taibi P, Troeschel S. Development of a PSA tracking system for patients with prostate cancer following definitive radiotherapy to enhance rural health. J Clin Oncol. 2016;34(suppl 2):39-39.
14. Skolarus TA, Zhang Y, Hollenbeck BK. Understanding fragmentation of prostate cancer survivorship care: implications for cost and quality. Cancer. 2012;118(11):2837-2845.
15. Roach M, 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974.
16. Buyyounouski MK, Hanlon AL, Horwitz EM, Uzzo RG, Pollack A. Biochemical failure and the temporal kinetics of prostate-specific antigen after radiation therapy with androgen deprivation. Int J Radiat Oncol Biol Phys. 2005;61(5):1291-1298.
17. Chu S, Boxer R, Madison P, et al. Veterans Affairs telemedicine: bringing urologic care to remote clinics. Urology. 2015;86(2):255-260.
18. Safir IJ, Gabale S, David SA, et al. Implementation of a tele-urology program for outpatient hematuria referrals: initial results and patient satisfaction. Urology. 2016;97:33-39.
Novel mutations contribute to progression of venetoclax-treated CLL
Newly discovered gene mutations in the progression of venetoclax-treated relapsed chronic lymphocytic leukemia (CLL) may improve understanding of clinical resistance mechanisms underlying the disease, according to recent research.
“We investigated patients with progressive CLL on venetoclax harboring subclonal BCL2 Gly101Val mutations for the presence of additional acquired BCL2 resistance mutations,” wrote Piers Blombery, MBBS, of the University of Melbourne in Victoria, Australia, and his colleagues in Blood.
Among 67 patients with relapsed disease treated with the BCL2 inhibitor venetoclax, the researchers identified a total of 11 patients with co-occurring BCL2 Gly101Val mutations. Each patient was enrolled in an early phase clinical trial at an institution in Australia.
With respect to testing methods, next-generation sequencing (NGS) and hybridization-based target enrichment technologies were used to detect novel acquired mutations in the BCL2 coding region.
Among those harboring the Gly101Val mutation, additional BCL2 mutations were identified in 10 patients (91%), with a median of three mutations detected per patient (range, 1-7). Previously undescribed mutations included an in-frame insertion mutation (Arg107_Arg110dup), and other substitutions (Asp103/Val156) in the BCL2 gene.
“As with the Gly101Val, these observations support the specificity of these mutations for the context of venetoclax resistance,” they wrote.
The investigators further explained that the BCL2 Asp103Glu mutation could have particular significance in the context of venetoclax sensitivity because of selective targeting of the BCL2 gene.
In comparison to wild-type aspartic acid, the BCL2 Asp103Glu substitution was linked to an approximate 20-fold reduction in affinity for venetoclax, they reported.
“[Our findings] consolidate the paradigm emerging across hematological malignancies of multiple independent molecular mechanisms underpinning an ‘oligoclonal’ pattern of clinical relapse on targeted therapies,” they concluded.
Further studies are needed to fully characterize the relationship between acquired BCL2 mutations and venetoclax resistance.
The study was funded by the Snowdome Foundation, Vision Super and the Wilson Centre for Lymphoma Genomics, the Leukemia and Lymphoma Society, the National Health and Medical Research Council of Australia, and other grant funding sources provided to the study authors. The authors reported financial affiliations with AbbVie, Genentech, and the Walter and Eliza Hall Institute.
Newly discovered gene mutations in the progression of venetoclax-treated relapsed chronic lymphocytic leukemia (CLL) may improve understanding of clinical resistance mechanisms underlying the disease, according to recent research.
“We investigated patients with progressive CLL on venetoclax harboring subclonal BCL2 Gly101Val mutations for the presence of additional acquired BCL2 resistance mutations,” wrote Piers Blombery, MBBS, of the University of Melbourne in Victoria, Australia, and his colleagues in Blood.
Among 67 patients with relapsed disease treated with the BCL2 inhibitor venetoclax, the researchers identified a total of 11 patients with co-occurring BCL2 Gly101Val mutations. Each patient was enrolled in an early phase clinical trial at an institution in Australia.
With respect to testing methods, next-generation sequencing (NGS) and hybridization-based target enrichment technologies were used to detect novel acquired mutations in the BCL2 coding region.
Among those harboring the Gly101Val mutation, additional BCL2 mutations were identified in 10 patients (91%), with a median of three mutations detected per patient (range, 1-7). Previously undescribed mutations included an in-frame insertion mutation (Arg107_Arg110dup), and other substitutions (Asp103/Val156) in the BCL2 gene.
“As with the Gly101Val, these observations support the specificity of these mutations for the context of venetoclax resistance,” they wrote.
The investigators further explained that the BCL2 Asp103Glu mutation could have particular significance in the context of venetoclax sensitivity because of selective targeting of the BCL2 gene.
In comparison to wild-type aspartic acid, the BCL2 Asp103Glu substitution was linked to an approximate 20-fold reduction in affinity for venetoclax, they reported.
“[Our findings] consolidate the paradigm emerging across hematological malignancies of multiple independent molecular mechanisms underpinning an ‘oligoclonal’ pattern of clinical relapse on targeted therapies,” they concluded.
Further studies are needed to fully characterize the relationship between acquired BCL2 mutations and venetoclax resistance.
The study was funded by the Snowdome Foundation, Vision Super and the Wilson Centre for Lymphoma Genomics, the Leukemia and Lymphoma Society, the National Health and Medical Research Council of Australia, and other grant funding sources provided to the study authors. The authors reported financial affiliations with AbbVie, Genentech, and the Walter and Eliza Hall Institute.
Newly discovered gene mutations in the progression of venetoclax-treated relapsed chronic lymphocytic leukemia (CLL) may improve understanding of clinical resistance mechanisms underlying the disease, according to recent research.
“We investigated patients with progressive CLL on venetoclax harboring subclonal BCL2 Gly101Val mutations for the presence of additional acquired BCL2 resistance mutations,” wrote Piers Blombery, MBBS, of the University of Melbourne in Victoria, Australia, and his colleagues in Blood.
Among 67 patients with relapsed disease treated with the BCL2 inhibitor venetoclax, the researchers identified a total of 11 patients with co-occurring BCL2 Gly101Val mutations. Each patient was enrolled in an early phase clinical trial at an institution in Australia.
With respect to testing methods, next-generation sequencing (NGS) and hybridization-based target enrichment technologies were used to detect novel acquired mutations in the BCL2 coding region.
Among those harboring the Gly101Val mutation, additional BCL2 mutations were identified in 10 patients (91%), with a median of three mutations detected per patient (range, 1-7). Previously undescribed mutations included an in-frame insertion mutation (Arg107_Arg110dup), and other substitutions (Asp103/Val156) in the BCL2 gene.
“As with the Gly101Val, these observations support the specificity of these mutations for the context of venetoclax resistance,” they wrote.
The investigators further explained that the BCL2 Asp103Glu mutation could have particular significance in the context of venetoclax sensitivity because of selective targeting of the BCL2 gene.
In comparison to wild-type aspartic acid, the BCL2 Asp103Glu substitution was linked to an approximate 20-fold reduction in affinity for venetoclax, they reported.
“[Our findings] consolidate the paradigm emerging across hematological malignancies of multiple independent molecular mechanisms underpinning an ‘oligoclonal’ pattern of clinical relapse on targeted therapies,” they concluded.
Further studies are needed to fully characterize the relationship between acquired BCL2 mutations and venetoclax resistance.
The study was funded by the Snowdome Foundation, Vision Super and the Wilson Centre for Lymphoma Genomics, the Leukemia and Lymphoma Society, the National Health and Medical Research Council of Australia, and other grant funding sources provided to the study authors. The authors reported financial affiliations with AbbVie, Genentech, and the Walter and Eliza Hall Institute.
FROM BLOOD
ECHELON-1 update: A+AVD bests ABVD in Hodgkin lymphoma
Brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) provides “robust, sustained efficacy” in patients with Hodgkin lymphoma, according to investigators.
In the ECHELON-1 trial, investigators compared A+AVD to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for stage III or IV Hodgkin lymphoma. The 3-year progression-free survival (PFS) was superior in patients who received A+AVD, and this benefit was seen across most subgroups.
David J. Straus, MD, of Memorial Sloan Kettering Cancer Center in New York and his colleagues detailed these findings in Blood.
The phase 3 trial (NCT01712490) enrolled 1,334 patients with stage III or IV classical Hodgkin lymphoma. They were randomized to receive A+AVD (n = 664) or ABVD (n = 670). Baseline characteristics were similar between the treatment arms.
Positron emission tomography status after cycle 2 (PET2) was similar between the treatment arms as well. Most patients – 89% of the A+AVD arm and 86% of the ABVD arm – were PET2 negative. Treating physicians used PET2 status as a guide to potentially switch patients to an alternative regimen (radiotherapy or chemotherapy with or without transplant).
In a prior analysis, the study’s primary endpoint was modified PFS (time to progression, death, or noncomplete response after frontline therapy) per an independent review committee (N Engl J Med. 2018;378:331-44). The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm (hazard ratio, 0.77; P = .04).
PFS update
In the current analysis, the main exploratory endpoint was PFS per investigator. The 3-year PFS rate was significantly higher in the A+AVD arm than in the ABVD arm – 83.1% and 76.0%, respectively (HR, 0.704; P = .005).
The investigators observed a “consistent improvement in PFS” in the A+AVD arm, regardless of disease stage, International Prognostic score, Eastern Cooperative Oncology Group status, sex, or age. There was a significant improvement in PFS with A+AVD in PET2-negative patients and a trend toward improvement in PET2-positive patients. In the PET2-negative patients, the 3-year PFS was 85.8% in the A+AVD arm and 79.5% in the ABVD arm (HR, 0.69; P = .009). In PET2-positive patients, the 3-year PFS was 67.7% and 51.5%, respectively (HR, 0.59; P = .077).
“These data highlight that A+AVD provides a durable efficacy benefit, compared with ABVD, for frontline stage III/IV cHL [classical Hodgkin lymphoma], which is consistent across key subgroups regardless of patient status at PET2,” Dr. Straus and his colleagues wrote.
Safety update
In both treatment arms, peripheral neuropathy continued to improve or resolve with longer follow-up. Among patients who developed peripheral neuropathy, 78% in the A+AVD arm and 83% in the ABVD arm had improvement or resolution of the condition at 3 years.
Most patients had complete resolution of peripheral neuropathy; 62% in the A+AVD arm and 73% in the ABVD arm. The median time to complete resolution was 28 weeks (range, 0-167 weeks) after stopping A+AVD and 14 weeks (range, 0-188 weeks) after stopping ABVD.
The incidence of secondary malignancies was similar between the treatment arms. There were 14 secondary malignancies in the A+AVD arm (6 solid tumors, 8 hematologic malignancies) and 20 in the ABVD arm (9 solid tumors, 11 hematologic malignancies).
“A+AVD provided a sustained PFS benefit with a predictable and manageable safety profile,” Dr. Straus and colleagues wrote. “These data further support the advantages of A+AVD versus ABVD as frontline treatment of patients with advanced stage III or IV cHL [classical Hodgkin lymphoma].”
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals (a subsidiary of Takeda) and Seattle Genetics. The investigators disclosed relationships with Millennium, Takeda, Seattle Genetics, and a range of other companies.
SOURCE: Straus DJ et al. Blood. 2020 Jan 16. pii: blood.2019003127. doi: 10.1182/blood.2019003127.
Brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) provides “robust, sustained efficacy” in patients with Hodgkin lymphoma, according to investigators.
In the ECHELON-1 trial, investigators compared A+AVD to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for stage III or IV Hodgkin lymphoma. The 3-year progression-free survival (PFS) was superior in patients who received A+AVD, and this benefit was seen across most subgroups.
David J. Straus, MD, of Memorial Sloan Kettering Cancer Center in New York and his colleagues detailed these findings in Blood.
The phase 3 trial (NCT01712490) enrolled 1,334 patients with stage III or IV classical Hodgkin lymphoma. They were randomized to receive A+AVD (n = 664) or ABVD (n = 670). Baseline characteristics were similar between the treatment arms.
Positron emission tomography status after cycle 2 (PET2) was similar between the treatment arms as well. Most patients – 89% of the A+AVD arm and 86% of the ABVD arm – were PET2 negative. Treating physicians used PET2 status as a guide to potentially switch patients to an alternative regimen (radiotherapy or chemotherapy with or without transplant).
In a prior analysis, the study’s primary endpoint was modified PFS (time to progression, death, or noncomplete response after frontline therapy) per an independent review committee (N Engl J Med. 2018;378:331-44). The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm (hazard ratio, 0.77; P = .04).
PFS update
In the current analysis, the main exploratory endpoint was PFS per investigator. The 3-year PFS rate was significantly higher in the A+AVD arm than in the ABVD arm – 83.1% and 76.0%, respectively (HR, 0.704; P = .005).
The investigators observed a “consistent improvement in PFS” in the A+AVD arm, regardless of disease stage, International Prognostic score, Eastern Cooperative Oncology Group status, sex, or age. There was a significant improvement in PFS with A+AVD in PET2-negative patients and a trend toward improvement in PET2-positive patients. In the PET2-negative patients, the 3-year PFS was 85.8% in the A+AVD arm and 79.5% in the ABVD arm (HR, 0.69; P = .009). In PET2-positive patients, the 3-year PFS was 67.7% and 51.5%, respectively (HR, 0.59; P = .077).
“These data highlight that A+AVD provides a durable efficacy benefit, compared with ABVD, for frontline stage III/IV cHL [classical Hodgkin lymphoma], which is consistent across key subgroups regardless of patient status at PET2,” Dr. Straus and his colleagues wrote.
Safety update
In both treatment arms, peripheral neuropathy continued to improve or resolve with longer follow-up. Among patients who developed peripheral neuropathy, 78% in the A+AVD arm and 83% in the ABVD arm had improvement or resolution of the condition at 3 years.
Most patients had complete resolution of peripheral neuropathy; 62% in the A+AVD arm and 73% in the ABVD arm. The median time to complete resolution was 28 weeks (range, 0-167 weeks) after stopping A+AVD and 14 weeks (range, 0-188 weeks) after stopping ABVD.
The incidence of secondary malignancies was similar between the treatment arms. There were 14 secondary malignancies in the A+AVD arm (6 solid tumors, 8 hematologic malignancies) and 20 in the ABVD arm (9 solid tumors, 11 hematologic malignancies).
“A+AVD provided a sustained PFS benefit with a predictable and manageable safety profile,” Dr. Straus and colleagues wrote. “These data further support the advantages of A+AVD versus ABVD as frontline treatment of patients with advanced stage III or IV cHL [classical Hodgkin lymphoma].”
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals (a subsidiary of Takeda) and Seattle Genetics. The investigators disclosed relationships with Millennium, Takeda, Seattle Genetics, and a range of other companies.
SOURCE: Straus DJ et al. Blood. 2020 Jan 16. pii: blood.2019003127. doi: 10.1182/blood.2019003127.
Brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) provides “robust, sustained efficacy” in patients with Hodgkin lymphoma, according to investigators.
In the ECHELON-1 trial, investigators compared A+AVD to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for stage III or IV Hodgkin lymphoma. The 3-year progression-free survival (PFS) was superior in patients who received A+AVD, and this benefit was seen across most subgroups.
David J. Straus, MD, of Memorial Sloan Kettering Cancer Center in New York and his colleagues detailed these findings in Blood.
The phase 3 trial (NCT01712490) enrolled 1,334 patients with stage III or IV classical Hodgkin lymphoma. They were randomized to receive A+AVD (n = 664) or ABVD (n = 670). Baseline characteristics were similar between the treatment arms.
Positron emission tomography status after cycle 2 (PET2) was similar between the treatment arms as well. Most patients – 89% of the A+AVD arm and 86% of the ABVD arm – were PET2 negative. Treating physicians used PET2 status as a guide to potentially switch patients to an alternative regimen (radiotherapy or chemotherapy with or without transplant).
In a prior analysis, the study’s primary endpoint was modified PFS (time to progression, death, or noncomplete response after frontline therapy) per an independent review committee (N Engl J Med. 2018;378:331-44). The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm (hazard ratio, 0.77; P = .04).
PFS update
In the current analysis, the main exploratory endpoint was PFS per investigator. The 3-year PFS rate was significantly higher in the A+AVD arm than in the ABVD arm – 83.1% and 76.0%, respectively (HR, 0.704; P = .005).
The investigators observed a “consistent improvement in PFS” in the A+AVD arm, regardless of disease stage, International Prognostic score, Eastern Cooperative Oncology Group status, sex, or age. There was a significant improvement in PFS with A+AVD in PET2-negative patients and a trend toward improvement in PET2-positive patients. In the PET2-negative patients, the 3-year PFS was 85.8% in the A+AVD arm and 79.5% in the ABVD arm (HR, 0.69; P = .009). In PET2-positive patients, the 3-year PFS was 67.7% and 51.5%, respectively (HR, 0.59; P = .077).
“These data highlight that A+AVD provides a durable efficacy benefit, compared with ABVD, for frontline stage III/IV cHL [classical Hodgkin lymphoma], which is consistent across key subgroups regardless of patient status at PET2,” Dr. Straus and his colleagues wrote.
Safety update
In both treatment arms, peripheral neuropathy continued to improve or resolve with longer follow-up. Among patients who developed peripheral neuropathy, 78% in the A+AVD arm and 83% in the ABVD arm had improvement or resolution of the condition at 3 years.
Most patients had complete resolution of peripheral neuropathy; 62% in the A+AVD arm and 73% in the ABVD arm. The median time to complete resolution was 28 weeks (range, 0-167 weeks) after stopping A+AVD and 14 weeks (range, 0-188 weeks) after stopping ABVD.
The incidence of secondary malignancies was similar between the treatment arms. There were 14 secondary malignancies in the A+AVD arm (6 solid tumors, 8 hematologic malignancies) and 20 in the ABVD arm (9 solid tumors, 11 hematologic malignancies).
“A+AVD provided a sustained PFS benefit with a predictable and manageable safety profile,” Dr. Straus and colleagues wrote. “These data further support the advantages of A+AVD versus ABVD as frontline treatment of patients with advanced stage III or IV cHL [classical Hodgkin lymphoma].”
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals (a subsidiary of Takeda) and Seattle Genetics. The investigators disclosed relationships with Millennium, Takeda, Seattle Genetics, and a range of other companies.
SOURCE: Straus DJ et al. Blood. 2020 Jan 16. pii: blood.2019003127. doi: 10.1182/blood.2019003127.
FROM BLOOD
Dependent trait in chronic migraine may predict nonresponse to onabotulinumtoxin A
according to research published in the January issue of Headache. The research may be the first to show that personality traits predict response to onabotulinumtoxin A in this population.
“These findings point out that conducting an evaluation of personality traits in patients with chronic migraine might be helpful in the prediction of the course and election of the treatment, as well as identifying patients who might benefit from a multidisciplinary approach,” wrote Alicia Gonzalez-Martinez, MD, of the Hospital Universitario de La Princesa and Instituto de Investigación Sanitaria de La Princesa in Madrid and colleagues. “Categorical questionnaires such as the Salamanca screening test seem to be useful for this purpose.”
Researchers used ICD-10 personality criteria
Personality patterns in patients with migraine and other primary headaches have been the subject of decades of research. Munoz et al. found that certain personality traits are associated with migraine and chronic migraine, and this association may influence clinical management and treatment. The effect of personality traits on response to treatment, however, had not been studied previously.
Dr. Gonzalez-Martinez and colleagues hypothesized that cluster C traits (e.g., obsessive-compulsive, dependent, and anxious), as defined by ICD-10, are associated with nonresponse to onabotulinumtoxin A. To test this hypothesis, they conducted a case-control observational study in a cohort of patients with chronic migraine. Eligible patients presented to one of two headache units of a tertiary hospital between January and May 2018. The investigators obtained a complete headache history and demographic information from each patient. Patients had at least two treatment cycles of onabotulinumtoxin A. Dr. Gonzalez-Martinez and colleagues defined treatment response as a reduction in the number of monthly migraine days of at least 50% after at least two treatment cycles.
The investigators assessed participants’ personality traits by administering the Salamanca test, a brief categorical inventory that examines 11 personality traits using 22 questions. Patients completed the test at the beginning of the study period and before they were classified as responders or nonresponders.
Medication overuse was a potential confounder
The study population included 112 patients with chronic migraine. One hundred patients (89%) were women. Participants’ mean age at initiation of onabotulinumtoxin A treatment was 43 years. The population’s mean duration of chronic migraine was 29 months. Eighty-three patients (74.1%) had medication overuse, and 96 (85.7%) responded to onabotulinumtoxin A.
Cluster A traits in the population included paranoid (prevalence, 10.7%), schizoid (38.4%), and schizotypal (7.1%). Cluster B traits included histrionic (50%), antisocial (1.8%), narcissistic (9.8%), emotional instability subtype impulsive (27.7%), and emotional instability subtype limit (EISL, 24.1%). Cluster C traits were anxious (58.9%) anancastic (i.e., obsessive-compulsive, 54.5%), and dependent (32.1%).
The investigators found no differences in demographics between responders and nonresponders. In a univariate analysis, dependent traits (e.g., passivity and emotional overdependence on others) and EISL traits (e.g., impulsivity and disturbed self-image) were significantly more common among nonresponders. In a multivariate analysis, dependent traits remained significantly associated with nonresponse to onabotulinumtoxin A.
Medication overuse was a potential confounder in the study, according to Dr. Gonzalez-Martinez and colleagues. One of the study’s limitations was its absence of a healthy control group. Another was the fact that the psychometrics of the Salamanca screening test have not been published in a peer-reviewed journal and may need further examination.
Dependent personality “may also be part of the proposed chronic pain sufferer personality,” wrote the investigators. “Early detection of personality traits could improve management and outcome of chronic migraine patients. Additionally, the possibility to predict the effectiveness of onabotulinumtoxin A therapy may reduce costs and latency time of effect in patients with improbable effectiveness.”
The study had no outside funding, and the authors reported no conflicts of interest.
SOURCE: Gonzalez-Martinez A et al. Headache. 2020;60(1):153-61.
according to research published in the January issue of Headache. The research may be the first to show that personality traits predict response to onabotulinumtoxin A in this population.
“These findings point out that conducting an evaluation of personality traits in patients with chronic migraine might be helpful in the prediction of the course and election of the treatment, as well as identifying patients who might benefit from a multidisciplinary approach,” wrote Alicia Gonzalez-Martinez, MD, of the Hospital Universitario de La Princesa and Instituto de Investigación Sanitaria de La Princesa in Madrid and colleagues. “Categorical questionnaires such as the Salamanca screening test seem to be useful for this purpose.”
Researchers used ICD-10 personality criteria
Personality patterns in patients with migraine and other primary headaches have been the subject of decades of research. Munoz et al. found that certain personality traits are associated with migraine and chronic migraine, and this association may influence clinical management and treatment. The effect of personality traits on response to treatment, however, had not been studied previously.
Dr. Gonzalez-Martinez and colleagues hypothesized that cluster C traits (e.g., obsessive-compulsive, dependent, and anxious), as defined by ICD-10, are associated with nonresponse to onabotulinumtoxin A. To test this hypothesis, they conducted a case-control observational study in a cohort of patients with chronic migraine. Eligible patients presented to one of two headache units of a tertiary hospital between January and May 2018. The investigators obtained a complete headache history and demographic information from each patient. Patients had at least two treatment cycles of onabotulinumtoxin A. Dr. Gonzalez-Martinez and colleagues defined treatment response as a reduction in the number of monthly migraine days of at least 50% after at least two treatment cycles.
The investigators assessed participants’ personality traits by administering the Salamanca test, a brief categorical inventory that examines 11 personality traits using 22 questions. Patients completed the test at the beginning of the study period and before they were classified as responders or nonresponders.
Medication overuse was a potential confounder
The study population included 112 patients with chronic migraine. One hundred patients (89%) were women. Participants’ mean age at initiation of onabotulinumtoxin A treatment was 43 years. The population’s mean duration of chronic migraine was 29 months. Eighty-three patients (74.1%) had medication overuse, and 96 (85.7%) responded to onabotulinumtoxin A.
Cluster A traits in the population included paranoid (prevalence, 10.7%), schizoid (38.4%), and schizotypal (7.1%). Cluster B traits included histrionic (50%), antisocial (1.8%), narcissistic (9.8%), emotional instability subtype impulsive (27.7%), and emotional instability subtype limit (EISL, 24.1%). Cluster C traits were anxious (58.9%) anancastic (i.e., obsessive-compulsive, 54.5%), and dependent (32.1%).
The investigators found no differences in demographics between responders and nonresponders. In a univariate analysis, dependent traits (e.g., passivity and emotional overdependence on others) and EISL traits (e.g., impulsivity and disturbed self-image) were significantly more common among nonresponders. In a multivariate analysis, dependent traits remained significantly associated with nonresponse to onabotulinumtoxin A.
Medication overuse was a potential confounder in the study, according to Dr. Gonzalez-Martinez and colleagues. One of the study’s limitations was its absence of a healthy control group. Another was the fact that the psychometrics of the Salamanca screening test have not been published in a peer-reviewed journal and may need further examination.
Dependent personality “may also be part of the proposed chronic pain sufferer personality,” wrote the investigators. “Early detection of personality traits could improve management and outcome of chronic migraine patients. Additionally, the possibility to predict the effectiveness of onabotulinumtoxin A therapy may reduce costs and latency time of effect in patients with improbable effectiveness.”
The study had no outside funding, and the authors reported no conflicts of interest.
SOURCE: Gonzalez-Martinez A et al. Headache. 2020;60(1):153-61.
according to research published in the January issue of Headache. The research may be the first to show that personality traits predict response to onabotulinumtoxin A in this population.
“These findings point out that conducting an evaluation of personality traits in patients with chronic migraine might be helpful in the prediction of the course and election of the treatment, as well as identifying patients who might benefit from a multidisciplinary approach,” wrote Alicia Gonzalez-Martinez, MD, of the Hospital Universitario de La Princesa and Instituto de Investigación Sanitaria de La Princesa in Madrid and colleagues. “Categorical questionnaires such as the Salamanca screening test seem to be useful for this purpose.”
Researchers used ICD-10 personality criteria
Personality patterns in patients with migraine and other primary headaches have been the subject of decades of research. Munoz et al. found that certain personality traits are associated with migraine and chronic migraine, and this association may influence clinical management and treatment. The effect of personality traits on response to treatment, however, had not been studied previously.
Dr. Gonzalez-Martinez and colleagues hypothesized that cluster C traits (e.g., obsessive-compulsive, dependent, and anxious), as defined by ICD-10, are associated with nonresponse to onabotulinumtoxin A. To test this hypothesis, they conducted a case-control observational study in a cohort of patients with chronic migraine. Eligible patients presented to one of two headache units of a tertiary hospital between January and May 2018. The investigators obtained a complete headache history and demographic information from each patient. Patients had at least two treatment cycles of onabotulinumtoxin A. Dr. Gonzalez-Martinez and colleagues defined treatment response as a reduction in the number of monthly migraine days of at least 50% after at least two treatment cycles.
The investigators assessed participants’ personality traits by administering the Salamanca test, a brief categorical inventory that examines 11 personality traits using 22 questions. Patients completed the test at the beginning of the study period and before they were classified as responders or nonresponders.
Medication overuse was a potential confounder
The study population included 112 patients with chronic migraine. One hundred patients (89%) were women. Participants’ mean age at initiation of onabotulinumtoxin A treatment was 43 years. The population’s mean duration of chronic migraine was 29 months. Eighty-three patients (74.1%) had medication overuse, and 96 (85.7%) responded to onabotulinumtoxin A.
Cluster A traits in the population included paranoid (prevalence, 10.7%), schizoid (38.4%), and schizotypal (7.1%). Cluster B traits included histrionic (50%), antisocial (1.8%), narcissistic (9.8%), emotional instability subtype impulsive (27.7%), and emotional instability subtype limit (EISL, 24.1%). Cluster C traits were anxious (58.9%) anancastic (i.e., obsessive-compulsive, 54.5%), and dependent (32.1%).
The investigators found no differences in demographics between responders and nonresponders. In a univariate analysis, dependent traits (e.g., passivity and emotional overdependence on others) and EISL traits (e.g., impulsivity and disturbed self-image) were significantly more common among nonresponders. In a multivariate analysis, dependent traits remained significantly associated with nonresponse to onabotulinumtoxin A.
Medication overuse was a potential confounder in the study, according to Dr. Gonzalez-Martinez and colleagues. One of the study’s limitations was its absence of a healthy control group. Another was the fact that the psychometrics of the Salamanca screening test have not been published in a peer-reviewed journal and may need further examination.
Dependent personality “may also be part of the proposed chronic pain sufferer personality,” wrote the investigators. “Early detection of personality traits could improve management and outcome of chronic migraine patients. Additionally, the possibility to predict the effectiveness of onabotulinumtoxin A therapy may reduce costs and latency time of effect in patients with improbable effectiveness.”
The study had no outside funding, and the authors reported no conflicts of interest.
SOURCE: Gonzalez-Martinez A et al. Headache. 2020;60(1):153-61.
FROM HEADACHE
Dietary flavonol intake linked to reduced risk of Alzheimer’s
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
FROM NEUROLOGY
Celecoxib oral solution treats migraine effectively in randomized trial
, according to trial results published in the January issue of Headache.
Two hours after treatment, a significantly greater proportion of patients who received the liquid solution, known as DFN-15, had freedom from pain and freedom from their most bothersome accompanying symptom – nausea, photophobia, or phonophobia – compared with patients who received placebo. The pain freedom rates were 35.6% with celecoxib oral solution and 21.7% with placebo. The rates of freedom from the most bothersome symptom were 57.8% with celecoxib oral solution and 44.8% with placebo.
About 9% of patients who received celecoxib oral solution had treatment-emergent adverse events related to the study drug, the most common of which were dysgeusia (4.2%) and nausea (3.2%). In comparison, about 6% of patients who received placebo had treatment-emergent adverse events. There were no serious treatment-emergent adverse events.
“DFN‐15 has the potential to become a reliable and convenient acute therapeutic option for patients with migraine,” said lead author Richard B. Lipton, MD, and colleagues. Dr. Lipton is affiliated with the Albert Einstein College of Medicine in New York.
Assessing celecoxib in migraineurs
Evidence-based guidelines recommend nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, diclofenac, ibuprofen, and naproxen, as effective acute migraine treatments, but these medications may increase the risk of adverse gastrointestinal events, the authors said. Celecoxib, a selective cyclooxygenase (COX)-2 inhibitor, is indicated for the treatment of acute pain in patients with ankylosing spondylitis, osteoarthritis, primary dysmenorrhea, and rheumatoid arthritis. Although it produces analgesia similar to other NSAIDs, among patients with osteoarthritis and rheumatoid arthritis, celecoxib is associated with significantly lower risk of gastrointestinal events, compared with naproxen and ibuprofen, and significantly lower risk of renal events, compared with ibuprofen.
Researchers have studied an oral capsule form of celecoxib (Celebrex, Pfizer) as an acute treatment for migraine in an open-label study that compared celecoxib with naproxen sodium. “While preliminary results suggest comparable efficacy but better tolerability than widely used and guideline-recommended NSAIDs, celecoxib is not currently approved for migraine,” the authors said.
Compared with the oral capsule formulation, the oral liquid solution DFN-15 has a faster median time to peak concentration under fasting conditions (within 1 hour vs. 2.5 hours), which “could translate into more rapid onset of pain relief,” the authors said. In addition, DFN-15 may have greater bioavailability, which could lower dose requirements and improve safety and tolerability. To compare the efficacy, tolerability, and safety of 120-mg DFN-15 with placebo for the acute treatment of migraine, researchers conducted a randomized, double-blind, placebo-controlled study.
Participants used single-dose bottles
Researchers randomized 622 patients 1:1 to DFN-15 or placebo, and 567 treated a migraine during the trial. Patients had a mean age of 40 years, and 87% were female. Patients had episodic migraine with or without aura, no signs of medication overuse, and two-eight migraine attacks per month. For the trial, patients treated a single migraine attack of moderate to severe intensity within 1 hour of onset. “Each subject was given a single‐dose bottle of DFN‐15 120 mg or matching placebo containing 4.8 mL liquid,” Dr. Lipton and colleagues said. “They were instructed to drink the entire contents of the bottle to ensure complete consumption of study medication.”
Freedom from pain and freedom from the most bothersome symptom at 2 hours were the coprimary endpoints. “DFN‐15 was also significantly superior to placebo on multiple secondary 2‐hour endpoints, including freedom from photophobia, pain relief, change in functional disability from baseline, overall and 24‐hour satisfaction with treatment, and use of rescue medication,” they reported.
“A new COX‐2 inhibitor that is effective and rapidly absorbed could provide an important new option for a wide range of patients,” the authors said. “Though cross‐study comparisons are problematic, the current results for DFN‐15 indicate that its efficacy is similar to that of NSAIDs and small‐molecule calcitonin gene‐related peptide receptor antagonists (gepants), based on placebo‐subtracted rates pain freedom in acute treatment trials (14%‐21%). DFN‐15 may also be useful among triptan users, who are at elevated risk of medication‐overuse headache and for whom TEAEs within 24 hours postdose are common. ... The form and delivery system of DFN‐15 – a ready‐to‐use solution in a 4.8‐mL single‐use bottle – may support patient adherence.”
The trial had robust placebo response rates, which may have been influenced by “the novelty of a ready‐made oral solution, which has not been previously tested for the acute treatment of migraine,” the authors noted. In addition, the trial does not address the treatment of mild pain or treatment across multiple attacks.
The trial was supported by Dr. Reddy’s Laboratories, manufacturer of DFN-15. Two authors are employed by and own stock in Dr. Reddy’s. Dr. Lipton and a coauthor disclosed research support from and consulting for Dr. Reddy’s.
SOURCE: Lipton RB et al. Headache. 2020;60(1):58-70. doi: 10.1111/head.13663.
, according to trial results published in the January issue of Headache.
Two hours after treatment, a significantly greater proportion of patients who received the liquid solution, known as DFN-15, had freedom from pain and freedom from their most bothersome accompanying symptom – nausea, photophobia, or phonophobia – compared with patients who received placebo. The pain freedom rates were 35.6% with celecoxib oral solution and 21.7% with placebo. The rates of freedom from the most bothersome symptom were 57.8% with celecoxib oral solution and 44.8% with placebo.
About 9% of patients who received celecoxib oral solution had treatment-emergent adverse events related to the study drug, the most common of which were dysgeusia (4.2%) and nausea (3.2%). In comparison, about 6% of patients who received placebo had treatment-emergent adverse events. There were no serious treatment-emergent adverse events.
“DFN‐15 has the potential to become a reliable and convenient acute therapeutic option for patients with migraine,” said lead author Richard B. Lipton, MD, and colleagues. Dr. Lipton is affiliated with the Albert Einstein College of Medicine in New York.
Assessing celecoxib in migraineurs
Evidence-based guidelines recommend nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, diclofenac, ibuprofen, and naproxen, as effective acute migraine treatments, but these medications may increase the risk of adverse gastrointestinal events, the authors said. Celecoxib, a selective cyclooxygenase (COX)-2 inhibitor, is indicated for the treatment of acute pain in patients with ankylosing spondylitis, osteoarthritis, primary dysmenorrhea, and rheumatoid arthritis. Although it produces analgesia similar to other NSAIDs, among patients with osteoarthritis and rheumatoid arthritis, celecoxib is associated with significantly lower risk of gastrointestinal events, compared with naproxen and ibuprofen, and significantly lower risk of renal events, compared with ibuprofen.
Researchers have studied an oral capsule form of celecoxib (Celebrex, Pfizer) as an acute treatment for migraine in an open-label study that compared celecoxib with naproxen sodium. “While preliminary results suggest comparable efficacy but better tolerability than widely used and guideline-recommended NSAIDs, celecoxib is not currently approved for migraine,” the authors said.
Compared with the oral capsule formulation, the oral liquid solution DFN-15 has a faster median time to peak concentration under fasting conditions (within 1 hour vs. 2.5 hours), which “could translate into more rapid onset of pain relief,” the authors said. In addition, DFN-15 may have greater bioavailability, which could lower dose requirements and improve safety and tolerability. To compare the efficacy, tolerability, and safety of 120-mg DFN-15 with placebo for the acute treatment of migraine, researchers conducted a randomized, double-blind, placebo-controlled study.
Participants used single-dose bottles
Researchers randomized 622 patients 1:1 to DFN-15 or placebo, and 567 treated a migraine during the trial. Patients had a mean age of 40 years, and 87% were female. Patients had episodic migraine with or without aura, no signs of medication overuse, and two-eight migraine attacks per month. For the trial, patients treated a single migraine attack of moderate to severe intensity within 1 hour of onset. “Each subject was given a single‐dose bottle of DFN‐15 120 mg or matching placebo containing 4.8 mL liquid,” Dr. Lipton and colleagues said. “They were instructed to drink the entire contents of the bottle to ensure complete consumption of study medication.”
Freedom from pain and freedom from the most bothersome symptom at 2 hours were the coprimary endpoints. “DFN‐15 was also significantly superior to placebo on multiple secondary 2‐hour endpoints, including freedom from photophobia, pain relief, change in functional disability from baseline, overall and 24‐hour satisfaction with treatment, and use of rescue medication,” they reported.
“A new COX‐2 inhibitor that is effective and rapidly absorbed could provide an important new option for a wide range of patients,” the authors said. “Though cross‐study comparisons are problematic, the current results for DFN‐15 indicate that its efficacy is similar to that of NSAIDs and small‐molecule calcitonin gene‐related peptide receptor antagonists (gepants), based on placebo‐subtracted rates pain freedom in acute treatment trials (14%‐21%). DFN‐15 may also be useful among triptan users, who are at elevated risk of medication‐overuse headache and for whom TEAEs within 24 hours postdose are common. ... The form and delivery system of DFN‐15 – a ready‐to‐use solution in a 4.8‐mL single‐use bottle – may support patient adherence.”
The trial had robust placebo response rates, which may have been influenced by “the novelty of a ready‐made oral solution, which has not been previously tested for the acute treatment of migraine,” the authors noted. In addition, the trial does not address the treatment of mild pain or treatment across multiple attacks.
The trial was supported by Dr. Reddy’s Laboratories, manufacturer of DFN-15. Two authors are employed by and own stock in Dr. Reddy’s. Dr. Lipton and a coauthor disclosed research support from and consulting for Dr. Reddy’s.
SOURCE: Lipton RB et al. Headache. 2020;60(1):58-70. doi: 10.1111/head.13663.
, according to trial results published in the January issue of Headache.
Two hours after treatment, a significantly greater proportion of patients who received the liquid solution, known as DFN-15, had freedom from pain and freedom from their most bothersome accompanying symptom – nausea, photophobia, or phonophobia – compared with patients who received placebo. The pain freedom rates were 35.6% with celecoxib oral solution and 21.7% with placebo. The rates of freedom from the most bothersome symptom were 57.8% with celecoxib oral solution and 44.8% with placebo.
About 9% of patients who received celecoxib oral solution had treatment-emergent adverse events related to the study drug, the most common of which were dysgeusia (4.2%) and nausea (3.2%). In comparison, about 6% of patients who received placebo had treatment-emergent adverse events. There were no serious treatment-emergent adverse events.
“DFN‐15 has the potential to become a reliable and convenient acute therapeutic option for patients with migraine,” said lead author Richard B. Lipton, MD, and colleagues. Dr. Lipton is affiliated with the Albert Einstein College of Medicine in New York.
Assessing celecoxib in migraineurs
Evidence-based guidelines recommend nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, diclofenac, ibuprofen, and naproxen, as effective acute migraine treatments, but these medications may increase the risk of adverse gastrointestinal events, the authors said. Celecoxib, a selective cyclooxygenase (COX)-2 inhibitor, is indicated for the treatment of acute pain in patients with ankylosing spondylitis, osteoarthritis, primary dysmenorrhea, and rheumatoid arthritis. Although it produces analgesia similar to other NSAIDs, among patients with osteoarthritis and rheumatoid arthritis, celecoxib is associated with significantly lower risk of gastrointestinal events, compared with naproxen and ibuprofen, and significantly lower risk of renal events, compared with ibuprofen.
Researchers have studied an oral capsule form of celecoxib (Celebrex, Pfizer) as an acute treatment for migraine in an open-label study that compared celecoxib with naproxen sodium. “While preliminary results suggest comparable efficacy but better tolerability than widely used and guideline-recommended NSAIDs, celecoxib is not currently approved for migraine,” the authors said.
Compared with the oral capsule formulation, the oral liquid solution DFN-15 has a faster median time to peak concentration under fasting conditions (within 1 hour vs. 2.5 hours), which “could translate into more rapid onset of pain relief,” the authors said. In addition, DFN-15 may have greater bioavailability, which could lower dose requirements and improve safety and tolerability. To compare the efficacy, tolerability, and safety of 120-mg DFN-15 with placebo for the acute treatment of migraine, researchers conducted a randomized, double-blind, placebo-controlled study.
Participants used single-dose bottles
Researchers randomized 622 patients 1:1 to DFN-15 or placebo, and 567 treated a migraine during the trial. Patients had a mean age of 40 years, and 87% were female. Patients had episodic migraine with or without aura, no signs of medication overuse, and two-eight migraine attacks per month. For the trial, patients treated a single migraine attack of moderate to severe intensity within 1 hour of onset. “Each subject was given a single‐dose bottle of DFN‐15 120 mg or matching placebo containing 4.8 mL liquid,” Dr. Lipton and colleagues said. “They were instructed to drink the entire contents of the bottle to ensure complete consumption of study medication.”
Freedom from pain and freedom from the most bothersome symptom at 2 hours were the coprimary endpoints. “DFN‐15 was also significantly superior to placebo on multiple secondary 2‐hour endpoints, including freedom from photophobia, pain relief, change in functional disability from baseline, overall and 24‐hour satisfaction with treatment, and use of rescue medication,” they reported.
“A new COX‐2 inhibitor that is effective and rapidly absorbed could provide an important new option for a wide range of patients,” the authors said. “Though cross‐study comparisons are problematic, the current results for DFN‐15 indicate that its efficacy is similar to that of NSAIDs and small‐molecule calcitonin gene‐related peptide receptor antagonists (gepants), based on placebo‐subtracted rates pain freedom in acute treatment trials (14%‐21%). DFN‐15 may also be useful among triptan users, who are at elevated risk of medication‐overuse headache and for whom TEAEs within 24 hours postdose are common. ... The form and delivery system of DFN‐15 – a ready‐to‐use solution in a 4.8‐mL single‐use bottle – may support patient adherence.”
The trial had robust placebo response rates, which may have been influenced by “the novelty of a ready‐made oral solution, which has not been previously tested for the acute treatment of migraine,” the authors noted. In addition, the trial does not address the treatment of mild pain or treatment across multiple attacks.
The trial was supported by Dr. Reddy’s Laboratories, manufacturer of DFN-15. Two authors are employed by and own stock in Dr. Reddy’s. Dr. Lipton and a coauthor disclosed research support from and consulting for Dr. Reddy’s.
SOURCE: Lipton RB et al. Headache. 2020;60(1):58-70. doi: 10.1111/head.13663.
FROM HEADACHE
Genetic factor linked to impaired memory after heading many soccer balls
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
FROM JAMA Neurology
Adding lymphopenia component ‘improves’ FLIPI
Incorporating lymphopenia into the Follicular Lymphoma International Prognostic Index (FLIPI) can improve prognostication, according to researchers.
The team added lymphopenia as a point in a revised FLIPI scoring system, called FLIPI-L, and found the new system could better predict overall survival (OS), progression-free survival, and histologic transformation in patients with follicular lymphoma.
George Yang, MD, of Moffitt Cancer Center in Tampa, Fla., and his colleagues described results with the FLIPI-L in a letter published in Blood Cancer Journal.
“Prior studies have demonstrated that lymphopenia was associated with worsened OS in [follicular lymphoma],” Dr. Yang and his colleagues wrote. “Therefore, we hypothesized that lymphopenia may be integrated with existing FLIPI to better stratify long-term survival outcomes and predict for transformation.”
The researchers tested this theory in 736 follicular lymphoma patients who were followed for a median of 72 months (range, 2-211 months). The 5-year OS in this cohort was 81.3%, the 10-year OS was 67.3%, and 18% of patients experienced transformation to high-grade lymphoma.
The researchers defined absolute lymphopenia as less than 1.0 × 109 lymphocytes per liter. In multivariate analyses, lymphopenia was an independent predictor of OS (hazard ratio, 1.74; P less than .01) and transformation (odds ratio, 2.1; P less than .01).
To incorporate lymphopenia into the FLIPI, the researchers created a model in which 1 point was given for each of the standard FLIPI components (age, Ann Arbor stage, number of nodal areas, lactate dehydrogenase, and hemoglobin level), and one point was given for the presence of lymphopenia. Patients in the low-risk FLIPI-L category had 0-1 points, those in the intermediate-risk category had 2-3 points, and patients in the high-risk FLIPI-L category had 4-6 points.
Using the original FLIPI, the 5-year OS was 91% in the low-risk group (0-1), 82.7% in the intermediate-risk group (2), and 66% in the high-risk group (3-5). The 10-year OS was 80.4%, 66%, and 45.8%, respectively.
Using the FLIPI-L, the 5-year OS was 94.5% in the low-risk group (0-1), 89% in the intermediate-risk group (2-3), and 61% in the high-risk group (4-6). The 10-year OS was 83.9%, 68.5%, and 34.5%, respectively.
In a univariate Cox regression analysis of OS, each point increase in FLIPI-L score was associated with a significant increase in hazard ratio. For example, the hazard ratio was 3.4 for patients with a FLIPI-L score of 1 and 30.9 for those with a FLIPI-L score of 6 (P less than .02 for all FLIPI-L scores). Conversely, increases in hazard ratio were not significant with the original FLIPI (P greater than .05 for all FLIPI scores).
The FLIPI-L was prognostic for OS in different treatment groups. In patients who received rituximab alone, radiation alone, or rituximab plus chemotherapy, the scoring system differentiated low-, intermediate-, and high-risk groups (P less than .04). In patients under observation, the FLIPI-L distinguished low/intermediate-risk and high-risk groups (P less than .01).
For patients who progressed within 24 months, the FLIPI-L was more predictive of progression-free survival (P = .05) than was the original FLIPI (P = .11).
Increasing FLIPI-L was an independent predictor of transformation, both when assessed as a continuous variable (P less than .01) and stepwise for FLIPI-L 3-5 (P = .004-.01). The original FLIPI, on the other hand, was not an independent predictor of transformation.
“Our analysis of a lymphopenia cutoff as an addition to the original FLIPI is simple yet improves risk stratification to differentiate between prognostic groups and, importantly, to predict transformation,” Dr. Yang and his colleagues wrote.
The authors reported having no conflicts of interest.
SOURCE: Yang G et al. Blood Cancer J. 2020 Jan 2;9(12):104. doi: 10.1038/s41408-019-0269-6.
Incorporating lymphopenia into the Follicular Lymphoma International Prognostic Index (FLIPI) can improve prognostication, according to researchers.
The team added lymphopenia as a point in a revised FLIPI scoring system, called FLIPI-L, and found the new system could better predict overall survival (OS), progression-free survival, and histologic transformation in patients with follicular lymphoma.
George Yang, MD, of Moffitt Cancer Center in Tampa, Fla., and his colleagues described results with the FLIPI-L in a letter published in Blood Cancer Journal.
“Prior studies have demonstrated that lymphopenia was associated with worsened OS in [follicular lymphoma],” Dr. Yang and his colleagues wrote. “Therefore, we hypothesized that lymphopenia may be integrated with existing FLIPI to better stratify long-term survival outcomes and predict for transformation.”
The researchers tested this theory in 736 follicular lymphoma patients who were followed for a median of 72 months (range, 2-211 months). The 5-year OS in this cohort was 81.3%, the 10-year OS was 67.3%, and 18% of patients experienced transformation to high-grade lymphoma.
The researchers defined absolute lymphopenia as less than 1.0 × 109 lymphocytes per liter. In multivariate analyses, lymphopenia was an independent predictor of OS (hazard ratio, 1.74; P less than .01) and transformation (odds ratio, 2.1; P less than .01).
To incorporate lymphopenia into the FLIPI, the researchers created a model in which 1 point was given for each of the standard FLIPI components (age, Ann Arbor stage, number of nodal areas, lactate dehydrogenase, and hemoglobin level), and one point was given for the presence of lymphopenia. Patients in the low-risk FLIPI-L category had 0-1 points, those in the intermediate-risk category had 2-3 points, and patients in the high-risk FLIPI-L category had 4-6 points.
Using the original FLIPI, the 5-year OS was 91% in the low-risk group (0-1), 82.7% in the intermediate-risk group (2), and 66% in the high-risk group (3-5). The 10-year OS was 80.4%, 66%, and 45.8%, respectively.
Using the FLIPI-L, the 5-year OS was 94.5% in the low-risk group (0-1), 89% in the intermediate-risk group (2-3), and 61% in the high-risk group (4-6). The 10-year OS was 83.9%, 68.5%, and 34.5%, respectively.
In a univariate Cox regression analysis of OS, each point increase in FLIPI-L score was associated with a significant increase in hazard ratio. For example, the hazard ratio was 3.4 for patients with a FLIPI-L score of 1 and 30.9 for those with a FLIPI-L score of 6 (P less than .02 for all FLIPI-L scores). Conversely, increases in hazard ratio were not significant with the original FLIPI (P greater than .05 for all FLIPI scores).
The FLIPI-L was prognostic for OS in different treatment groups. In patients who received rituximab alone, radiation alone, or rituximab plus chemotherapy, the scoring system differentiated low-, intermediate-, and high-risk groups (P less than .04). In patients under observation, the FLIPI-L distinguished low/intermediate-risk and high-risk groups (P less than .01).
For patients who progressed within 24 months, the FLIPI-L was more predictive of progression-free survival (P = .05) than was the original FLIPI (P = .11).
Increasing FLIPI-L was an independent predictor of transformation, both when assessed as a continuous variable (P less than .01) and stepwise for FLIPI-L 3-5 (P = .004-.01). The original FLIPI, on the other hand, was not an independent predictor of transformation.
“Our analysis of a lymphopenia cutoff as an addition to the original FLIPI is simple yet improves risk stratification to differentiate between prognostic groups and, importantly, to predict transformation,” Dr. Yang and his colleagues wrote.
The authors reported having no conflicts of interest.
SOURCE: Yang G et al. Blood Cancer J. 2020 Jan 2;9(12):104. doi: 10.1038/s41408-019-0269-6.
Incorporating lymphopenia into the Follicular Lymphoma International Prognostic Index (FLIPI) can improve prognostication, according to researchers.
The team added lymphopenia as a point in a revised FLIPI scoring system, called FLIPI-L, and found the new system could better predict overall survival (OS), progression-free survival, and histologic transformation in patients with follicular lymphoma.
George Yang, MD, of Moffitt Cancer Center in Tampa, Fla., and his colleagues described results with the FLIPI-L in a letter published in Blood Cancer Journal.
“Prior studies have demonstrated that lymphopenia was associated with worsened OS in [follicular lymphoma],” Dr. Yang and his colleagues wrote. “Therefore, we hypothesized that lymphopenia may be integrated with existing FLIPI to better stratify long-term survival outcomes and predict for transformation.”
The researchers tested this theory in 736 follicular lymphoma patients who were followed for a median of 72 months (range, 2-211 months). The 5-year OS in this cohort was 81.3%, the 10-year OS was 67.3%, and 18% of patients experienced transformation to high-grade lymphoma.
The researchers defined absolute lymphopenia as less than 1.0 × 109 lymphocytes per liter. In multivariate analyses, lymphopenia was an independent predictor of OS (hazard ratio, 1.74; P less than .01) and transformation (odds ratio, 2.1; P less than .01).
To incorporate lymphopenia into the FLIPI, the researchers created a model in which 1 point was given for each of the standard FLIPI components (age, Ann Arbor stage, number of nodal areas, lactate dehydrogenase, and hemoglobin level), and one point was given for the presence of lymphopenia. Patients in the low-risk FLIPI-L category had 0-1 points, those in the intermediate-risk category had 2-3 points, and patients in the high-risk FLIPI-L category had 4-6 points.
Using the original FLIPI, the 5-year OS was 91% in the low-risk group (0-1), 82.7% in the intermediate-risk group (2), and 66% in the high-risk group (3-5). The 10-year OS was 80.4%, 66%, and 45.8%, respectively.
Using the FLIPI-L, the 5-year OS was 94.5% in the low-risk group (0-1), 89% in the intermediate-risk group (2-3), and 61% in the high-risk group (4-6). The 10-year OS was 83.9%, 68.5%, and 34.5%, respectively.
In a univariate Cox regression analysis of OS, each point increase in FLIPI-L score was associated with a significant increase in hazard ratio. For example, the hazard ratio was 3.4 for patients with a FLIPI-L score of 1 and 30.9 for those with a FLIPI-L score of 6 (P less than .02 for all FLIPI-L scores). Conversely, increases in hazard ratio were not significant with the original FLIPI (P greater than .05 for all FLIPI scores).
The FLIPI-L was prognostic for OS in different treatment groups. In patients who received rituximab alone, radiation alone, or rituximab plus chemotherapy, the scoring system differentiated low-, intermediate-, and high-risk groups (P less than .04). In patients under observation, the FLIPI-L distinguished low/intermediate-risk and high-risk groups (P less than .01).
For patients who progressed within 24 months, the FLIPI-L was more predictive of progression-free survival (P = .05) than was the original FLIPI (P = .11).
Increasing FLIPI-L was an independent predictor of transformation, both when assessed as a continuous variable (P less than .01) and stepwise for FLIPI-L 3-5 (P = .004-.01). The original FLIPI, on the other hand, was not an independent predictor of transformation.
“Our analysis of a lymphopenia cutoff as an addition to the original FLIPI is simple yet improves risk stratification to differentiate between prognostic groups and, importantly, to predict transformation,” Dr. Yang and his colleagues wrote.
The authors reported having no conflicts of interest.
SOURCE: Yang G et al. Blood Cancer J. 2020 Jan 2;9(12):104. doi: 10.1038/s41408-019-0269-6.
FROM BLOOD CANCER JOURNAL
Race, ethnicity may influence outcomes after supratentorial intracerebral hemorrhage
researchers reported Jan. 22 in Neurology.
“There has been considerable research on stroke in older people, but there is still much to be learned about stroke in younger people and how it affects people of different races and ethnicities,” study author Daniel Woo, MD, professor of neurology at the University of Cincinnati, said in a news release. “Our study found that, even when you account for factors that affect outcomes, such as how big the stroke is, race and ethnicity were still independent predictors of how well people would recover.”
A subset of ERICH participants
To examine predictors of functional outcome in young patients with ICH, researchers analyzed data from a subset of patients in the Ethnic/Racial Variations in Intracerebral Hemorrhage (ERICH) study. ERICH enrolled patients with nontraumatic ICHs at 42 sites in the United States. It included 1,000 non-Hispanic black patients, 1,000 non-Hispanic white patients, and 1,000 Hispanic patients. Participants self-reported race and ethnicity.
Lead author Laura C. Miyares from Yale School of Medicine in New Haven, Conn., and colleagues analyzed data from 418 patients in ERICH who were aged 18-49 years and had supratentorial ICH. The cohort had an average age of 43 years, and 69% were male. In this subset, 41% were black, 12% were white, and 47% were Hispanic.
The primary outcome was modified Rankin Scale (mRS) score 3 months after the ICH, and the investigators defined a poor outcome as a score of 4 or greater. At 3 months, 35% had a poor functional outcome. Approximately 18% were unable to walk without assistance and attend to their bodily needs (mRS 4); 8% were bedridden, incontinent, and required nursing care (mRS 5); and 10% had died (mRS 6).
The percentage of patients with a poor functional outcome was 52% among white patients, 35% among black patients, and 31% among Hispanic patients. In a univariable analysis, black patients had a 51% reduction in odds of a poor outcome, compared with white patients, and Hispanic patients had a 59% reduction.
“The association between race/ethnicity and 3-month post-ICH functional outcome remained significant after adjusting for age, sex, premorbid disability, ICH location, ICH volume, [intraventricular hemorrhage] extension, systolic blood pressure, and [Glasgow Coma Scale] score on admission,” the researchers said. “In multivariable analysis, using white patients as the reference category, black patients had a 58% reduction in the odds of poor functional outcome at 3 months and Hispanic patients had a 66% reduction in the same odds.”
Their analysis identified the importance of other risk factors as well. “The volume of the hematoma, the most powerful predictor of outcome in older patients with ICH, was also found to be the most significant predictor of poor outcome in young patients,” they said.
Vascular risks and oral anticoagulants
About 80% of the young adults with ICH had a history of diagnosed hypertension. In nearly half, the condition was untreated. “After hypertension, the most common stroke risk factors in the young were diabetes, high cholesterol, smoking, and alcohol abuse,” the authors said. “In combination, these results indicate that vascular risk factors, especially untreated, could explain a large proportion of cases of ICH in the young.”
“Our results also point to treatment with oral anticoagulants before hospitalization as a potential mediator of the effect of race/ethnicity on short-term functional outcomes,” they said. About 8% of the white patients used oral anticoagulants, compared with 4% of the black patients and 1% of the Hispanic patients. Oral anticoagulant treatment “is a known risk factor for ICH and an established predictor of poor outcome in this condition. However, because only a small proportion of enrolled young patients with ICH were on [oral anticoagulants] prior to presentation, these results should be further validated by future studies.”
The study’s limitations include the broad categorization of racial and ethnic groups, the fact that younger patients with supratentorial ICH were more likely to be black or Hispanic and less likely to be white, and the exclusion of a significant proportion of cases of young white patients with smaller ICH volumes because of missing data, the researchers noted. Although the cohort was large, researchers may need to study more patients to capture differences among racial and ethnic groups, the investigators said.
The association between race/ethnicity and functional outcome could relate to “distinct pathophysiologies of the initial bleed or unique mechanisms of secondary injury,” the researchers suggested. “Future studies are necessary to probe the potential biological and social mediators of these findings to elucidate the role of race/ethnicity in ICH severity and functional recovery, and to develop improved prognostication for a racially varied population.”
ERICH is supported by the National Institute of Neurological Disorders and Stroke. Authors disclosed grants from the government, professional societies, and a university.
SOURCE: Miyares LC et al. Neurology. 2020 Jan 22. doi: 10.1212/WNL.0000000000008930.
researchers reported Jan. 22 in Neurology.
“There has been considerable research on stroke in older people, but there is still much to be learned about stroke in younger people and how it affects people of different races and ethnicities,” study author Daniel Woo, MD, professor of neurology at the University of Cincinnati, said in a news release. “Our study found that, even when you account for factors that affect outcomes, such as how big the stroke is, race and ethnicity were still independent predictors of how well people would recover.”
A subset of ERICH participants
To examine predictors of functional outcome in young patients with ICH, researchers analyzed data from a subset of patients in the Ethnic/Racial Variations in Intracerebral Hemorrhage (ERICH) study. ERICH enrolled patients with nontraumatic ICHs at 42 sites in the United States. It included 1,000 non-Hispanic black patients, 1,000 non-Hispanic white patients, and 1,000 Hispanic patients. Participants self-reported race and ethnicity.
Lead author Laura C. Miyares from Yale School of Medicine in New Haven, Conn., and colleagues analyzed data from 418 patients in ERICH who were aged 18-49 years and had supratentorial ICH. The cohort had an average age of 43 years, and 69% were male. In this subset, 41% were black, 12% were white, and 47% were Hispanic.
The primary outcome was modified Rankin Scale (mRS) score 3 months after the ICH, and the investigators defined a poor outcome as a score of 4 or greater. At 3 months, 35% had a poor functional outcome. Approximately 18% were unable to walk without assistance and attend to their bodily needs (mRS 4); 8% were bedridden, incontinent, and required nursing care (mRS 5); and 10% had died (mRS 6).
The percentage of patients with a poor functional outcome was 52% among white patients, 35% among black patients, and 31% among Hispanic patients. In a univariable analysis, black patients had a 51% reduction in odds of a poor outcome, compared with white patients, and Hispanic patients had a 59% reduction.
“The association between race/ethnicity and 3-month post-ICH functional outcome remained significant after adjusting for age, sex, premorbid disability, ICH location, ICH volume, [intraventricular hemorrhage] extension, systolic blood pressure, and [Glasgow Coma Scale] score on admission,” the researchers said. “In multivariable analysis, using white patients as the reference category, black patients had a 58% reduction in the odds of poor functional outcome at 3 months and Hispanic patients had a 66% reduction in the same odds.”
Their analysis identified the importance of other risk factors as well. “The volume of the hematoma, the most powerful predictor of outcome in older patients with ICH, was also found to be the most significant predictor of poor outcome in young patients,” they said.
Vascular risks and oral anticoagulants
About 80% of the young adults with ICH had a history of diagnosed hypertension. In nearly half, the condition was untreated. “After hypertension, the most common stroke risk factors in the young were diabetes, high cholesterol, smoking, and alcohol abuse,” the authors said. “In combination, these results indicate that vascular risk factors, especially untreated, could explain a large proportion of cases of ICH in the young.”
“Our results also point to treatment with oral anticoagulants before hospitalization as a potential mediator of the effect of race/ethnicity on short-term functional outcomes,” they said. About 8% of the white patients used oral anticoagulants, compared with 4% of the black patients and 1% of the Hispanic patients. Oral anticoagulant treatment “is a known risk factor for ICH and an established predictor of poor outcome in this condition. However, because only a small proportion of enrolled young patients with ICH were on [oral anticoagulants] prior to presentation, these results should be further validated by future studies.”
The study’s limitations include the broad categorization of racial and ethnic groups, the fact that younger patients with supratentorial ICH were more likely to be black or Hispanic and less likely to be white, and the exclusion of a significant proportion of cases of young white patients with smaller ICH volumes because of missing data, the researchers noted. Although the cohort was large, researchers may need to study more patients to capture differences among racial and ethnic groups, the investigators said.
The association between race/ethnicity and functional outcome could relate to “distinct pathophysiologies of the initial bleed or unique mechanisms of secondary injury,” the researchers suggested. “Future studies are necessary to probe the potential biological and social mediators of these findings to elucidate the role of race/ethnicity in ICH severity and functional recovery, and to develop improved prognostication for a racially varied population.”
ERICH is supported by the National Institute of Neurological Disorders and Stroke. Authors disclosed grants from the government, professional societies, and a university.
SOURCE: Miyares LC et al. Neurology. 2020 Jan 22. doi: 10.1212/WNL.0000000000008930.
researchers reported Jan. 22 in Neurology.
“There has been considerable research on stroke in older people, but there is still much to be learned about stroke in younger people and how it affects people of different races and ethnicities,” study author Daniel Woo, MD, professor of neurology at the University of Cincinnati, said in a news release. “Our study found that, even when you account for factors that affect outcomes, such as how big the stroke is, race and ethnicity were still independent predictors of how well people would recover.”
A subset of ERICH participants
To examine predictors of functional outcome in young patients with ICH, researchers analyzed data from a subset of patients in the Ethnic/Racial Variations in Intracerebral Hemorrhage (ERICH) study. ERICH enrolled patients with nontraumatic ICHs at 42 sites in the United States. It included 1,000 non-Hispanic black patients, 1,000 non-Hispanic white patients, and 1,000 Hispanic patients. Participants self-reported race and ethnicity.
Lead author Laura C. Miyares from Yale School of Medicine in New Haven, Conn., and colleagues analyzed data from 418 patients in ERICH who were aged 18-49 years and had supratentorial ICH. The cohort had an average age of 43 years, and 69% were male. In this subset, 41% were black, 12% were white, and 47% were Hispanic.
The primary outcome was modified Rankin Scale (mRS) score 3 months after the ICH, and the investigators defined a poor outcome as a score of 4 or greater. At 3 months, 35% had a poor functional outcome. Approximately 18% were unable to walk without assistance and attend to their bodily needs (mRS 4); 8% were bedridden, incontinent, and required nursing care (mRS 5); and 10% had died (mRS 6).
The percentage of patients with a poor functional outcome was 52% among white patients, 35% among black patients, and 31% among Hispanic patients. In a univariable analysis, black patients had a 51% reduction in odds of a poor outcome, compared with white patients, and Hispanic patients had a 59% reduction.
“The association between race/ethnicity and 3-month post-ICH functional outcome remained significant after adjusting for age, sex, premorbid disability, ICH location, ICH volume, [intraventricular hemorrhage] extension, systolic blood pressure, and [Glasgow Coma Scale] score on admission,” the researchers said. “In multivariable analysis, using white patients as the reference category, black patients had a 58% reduction in the odds of poor functional outcome at 3 months and Hispanic patients had a 66% reduction in the same odds.”
Their analysis identified the importance of other risk factors as well. “The volume of the hematoma, the most powerful predictor of outcome in older patients with ICH, was also found to be the most significant predictor of poor outcome in young patients,” they said.
Vascular risks and oral anticoagulants
About 80% of the young adults with ICH had a history of diagnosed hypertension. In nearly half, the condition was untreated. “After hypertension, the most common stroke risk factors in the young were diabetes, high cholesterol, smoking, and alcohol abuse,” the authors said. “In combination, these results indicate that vascular risk factors, especially untreated, could explain a large proportion of cases of ICH in the young.”
“Our results also point to treatment with oral anticoagulants before hospitalization as a potential mediator of the effect of race/ethnicity on short-term functional outcomes,” they said. About 8% of the white patients used oral anticoagulants, compared with 4% of the black patients and 1% of the Hispanic patients. Oral anticoagulant treatment “is a known risk factor for ICH and an established predictor of poor outcome in this condition. However, because only a small proportion of enrolled young patients with ICH were on [oral anticoagulants] prior to presentation, these results should be further validated by future studies.”
The study’s limitations include the broad categorization of racial and ethnic groups, the fact that younger patients with supratentorial ICH were more likely to be black or Hispanic and less likely to be white, and the exclusion of a significant proportion of cases of young white patients with smaller ICH volumes because of missing data, the researchers noted. Although the cohort was large, researchers may need to study more patients to capture differences among racial and ethnic groups, the investigators said.
The association between race/ethnicity and functional outcome could relate to “distinct pathophysiologies of the initial bleed or unique mechanisms of secondary injury,” the researchers suggested. “Future studies are necessary to probe the potential biological and social mediators of these findings to elucidate the role of race/ethnicity in ICH severity and functional recovery, and to develop improved prognostication for a racially varied population.”
ERICH is supported by the National Institute of Neurological Disorders and Stroke. Authors disclosed grants from the government, professional societies, and a university.
SOURCE: Miyares LC et al. Neurology. 2020 Jan 22. doi: 10.1212/WNL.0000000000008930.
FROM NEUROLOGY
Key clinical point: Among young adults with supratentorial intracerebral hemorrhage (ICH), black race and Hispanic ethnicity are associated with better functional outcomes, compared with white race.
Major finding: In multivariable analysis, black patients had a 58% reduction in the odds of poor functional outcome at 3 months, compared with white patients, and Hispanic patients had a 66% reduction.
Study details: An analysis of data from a subset of 418 patients in the Ethnic/Racial Variations in Intracerebral Hemorrhage (ERICH) study.
Disclosures: ERICH is supported by the National Institute of Neurological Disorders and Stroke. Authors disclosed grants from the government, professional societies, and a university.
Source: Miyares LC et al. Neurology. 2020 Jan 22. doi: 10.1212/WNL.0000000000008930.