User login
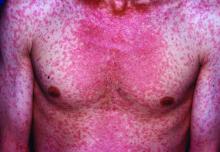
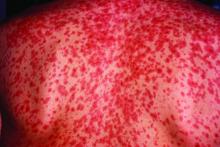
Measles complications in the U.S. unchanged in posteradication era
CHICAGO – An evaluation of the measles threat in the modern era gives no indication that the risk of complications or death is any different than it was before a vaccine became available, according to an analysis of inpatient complications between 2002 and 2013.
In 2000, measles was declared eliminated in the United States, but for those who have been infected since that time, the risk of serious complications and death has not diminished, noted Raj Chovatiya, MD, PhD, in a session at the annual meeting of the Society for Investigative Dermatology.
By eliminated, the Centers of Disease Control and Prevention – which reported 86 confirmed cases of measles in 2000 – was referring to a technical definition of no new endemic or continuous transmissions in the previous 12 months. It was expected that a modest number of cases of this reportable disease would continue to accrue for an infection that remains common elsewhere in the world.
“Worldwide there are about 20 million cases of measles annually with an estimated 100,000 deaths attributed to this cause,” said Dr. Chovatiya, who is a dermatology resident at Northwestern University, Chicago.
In the United States, posteradication infection rates remained at low levels for several years but were already rising from 2002 to 2013, when Dr. Chovatiya and his coinvestigators sought to describe the incidence, associations, comorbidities, and outcomes of hospitalizations for measles. Toward the end of the period the researchers were examining the incidence rates climbed more steeply.
“So far this year, 764 CDC cases of measles [were] reported. That is the most we have seen in the U.S. since 1994,” Dr. Chovatiya said.
Based on his analysis of hospitalizations from 2002 to 2013, the threat of these outbreaks is no different then that before the disease was declared eliminated or before a vaccine became available.
The cross-sectional study was conducted with data from the Nationwide Inpatient Sample, an all-payer database that is considered to be a representative of national trends.
Characteristic of measles, the majority of the 582 hospitalizations evaluated over this period occurred in children aged between 1 and 9 years. The proportion of patients with preexisting chronic comorbid conditions was low. Rather, “most were pretty healthy” prior to admission, according to Dr. Chovatiya, who said that the majority of admissions were from an emergency department.
Measles, which targets epithelial cells and depresses the immune system, is a potentially serious disease because of its ability to produce complications in essentially every organ of the body, including the lungs, kidneys, blood, and central nervous system. Consistent with past studies, the most common complication in this series was pneumonia, observed in 20% of patients. The list of other serious complications identified in this study period, including encephalitis and acute renal failure, was long.
“We observed death in 4.3% of our 582 cases, or about 25 cases,” reported Dr. Chovatiya. He indicated that this is a high percentage among a population composed largely of children who were well before hospitalization.
The mortality rate from measles was numerically but not statistically higher than that of overall hospital admissions during this period, but an admission for measles was associated with significantly longer average length of stay (3.7 vs. 3.5 days) and slightly but significantly higher direct costs ($18,907 vs. $18,474).
“I want to point out that these are just direct inpatient costs,” Dr. Chovatiya said. Extrapolating from published data about indirect expenses, he said that the total health cost burden “is absolutely staggering.”
Previous studies have suggested that about 25% of patients with measles require hospitalization and 1 in every 1,000 patients will die. The data collected by Dr. Chovatiya support these often-cited figures, indicating that they remain unchanged in the modern era.
particularly insufficient penetration of vaccination in many communities.
The vaccine “is inexpensive, extremely effective, and lifesaving,” said Dr. Chovatiya, making the point that all of the morbidity, mortality, and costs he described are largely avoidable.
Attempting to provide perspective of the measles threat and the impact of the vaccine, Dr. Chovatiya cited a hypothetical calculation that 732,000 deaths from measles would have been expected in the United States among the pool of children born between 1994 and 2013 had no vaccine been offered. Again, most of these deaths would have occurred in otherwise healthy children.
Dr. Chovatiya reported no potential conflicts of interest.
CHICAGO – An evaluation of the measles threat in the modern era gives no indication that the risk of complications or death is any different than it was before a vaccine became available, according to an analysis of inpatient complications between 2002 and 2013.
In 2000, measles was declared eliminated in the United States, but for those who have been infected since that time, the risk of serious complications and death has not diminished, noted Raj Chovatiya, MD, PhD, in a session at the annual meeting of the Society for Investigative Dermatology.
By eliminated, the Centers of Disease Control and Prevention – which reported 86 confirmed cases of measles in 2000 – was referring to a technical definition of no new endemic or continuous transmissions in the previous 12 months. It was expected that a modest number of cases of this reportable disease would continue to accrue for an infection that remains common elsewhere in the world.
“Worldwide there are about 20 million cases of measles annually with an estimated 100,000 deaths attributed to this cause,” said Dr. Chovatiya, who is a dermatology resident at Northwestern University, Chicago.
In the United States, posteradication infection rates remained at low levels for several years but were already rising from 2002 to 2013, when Dr. Chovatiya and his coinvestigators sought to describe the incidence, associations, comorbidities, and outcomes of hospitalizations for measles. Toward the end of the period the researchers were examining the incidence rates climbed more steeply.
“So far this year, 764 CDC cases of measles [were] reported. That is the most we have seen in the U.S. since 1994,” Dr. Chovatiya said.
Based on his analysis of hospitalizations from 2002 to 2013, the threat of these outbreaks is no different then that before the disease was declared eliminated or before a vaccine became available.
The cross-sectional study was conducted with data from the Nationwide Inpatient Sample, an all-payer database that is considered to be a representative of national trends.
Characteristic of measles, the majority of the 582 hospitalizations evaluated over this period occurred in children aged between 1 and 9 years. The proportion of patients with preexisting chronic comorbid conditions was low. Rather, “most were pretty healthy” prior to admission, according to Dr. Chovatiya, who said that the majority of admissions were from an emergency department.
Measles, which targets epithelial cells and depresses the immune system, is a potentially serious disease because of its ability to produce complications in essentially every organ of the body, including the lungs, kidneys, blood, and central nervous system. Consistent with past studies, the most common complication in this series was pneumonia, observed in 20% of patients. The list of other serious complications identified in this study period, including encephalitis and acute renal failure, was long.
“We observed death in 4.3% of our 582 cases, or about 25 cases,” reported Dr. Chovatiya. He indicated that this is a high percentage among a population composed largely of children who were well before hospitalization.
The mortality rate from measles was numerically but not statistically higher than that of overall hospital admissions during this period, but an admission for measles was associated with significantly longer average length of stay (3.7 vs. 3.5 days) and slightly but significantly higher direct costs ($18,907 vs. $18,474).
“I want to point out that these are just direct inpatient costs,” Dr. Chovatiya said. Extrapolating from published data about indirect expenses, he said that the total health cost burden “is absolutely staggering.”
Previous studies have suggested that about 25% of patients with measles require hospitalization and 1 in every 1,000 patients will die. The data collected by Dr. Chovatiya support these often-cited figures, indicating that they remain unchanged in the modern era.
particularly insufficient penetration of vaccination in many communities.
The vaccine “is inexpensive, extremely effective, and lifesaving,” said Dr. Chovatiya, making the point that all of the morbidity, mortality, and costs he described are largely avoidable.
Attempting to provide perspective of the measles threat and the impact of the vaccine, Dr. Chovatiya cited a hypothetical calculation that 732,000 deaths from measles would have been expected in the United States among the pool of children born between 1994 and 2013 had no vaccine been offered. Again, most of these deaths would have occurred in otherwise healthy children.
Dr. Chovatiya reported no potential conflicts of interest.
CHICAGO – An evaluation of the measles threat in the modern era gives no indication that the risk of complications or death is any different than it was before a vaccine became available, according to an analysis of inpatient complications between 2002 and 2013.
In 2000, measles was declared eliminated in the United States, but for those who have been infected since that time, the risk of serious complications and death has not diminished, noted Raj Chovatiya, MD, PhD, in a session at the annual meeting of the Society for Investigative Dermatology.
By eliminated, the Centers of Disease Control and Prevention – which reported 86 confirmed cases of measles in 2000 – was referring to a technical definition of no new endemic or continuous transmissions in the previous 12 months. It was expected that a modest number of cases of this reportable disease would continue to accrue for an infection that remains common elsewhere in the world.
“Worldwide there are about 20 million cases of measles annually with an estimated 100,000 deaths attributed to this cause,” said Dr. Chovatiya, who is a dermatology resident at Northwestern University, Chicago.
In the United States, posteradication infection rates remained at low levels for several years but were already rising from 2002 to 2013, when Dr. Chovatiya and his coinvestigators sought to describe the incidence, associations, comorbidities, and outcomes of hospitalizations for measles. Toward the end of the period the researchers were examining the incidence rates climbed more steeply.
“So far this year, 764 CDC cases of measles [were] reported. That is the most we have seen in the U.S. since 1994,” Dr. Chovatiya said.
Based on his analysis of hospitalizations from 2002 to 2013, the threat of these outbreaks is no different then that before the disease was declared eliminated or before a vaccine became available.
The cross-sectional study was conducted with data from the Nationwide Inpatient Sample, an all-payer database that is considered to be a representative of national trends.
Characteristic of measles, the majority of the 582 hospitalizations evaluated over this period occurred in children aged between 1 and 9 years. The proportion of patients with preexisting chronic comorbid conditions was low. Rather, “most were pretty healthy” prior to admission, according to Dr. Chovatiya, who said that the majority of admissions were from an emergency department.
Measles, which targets epithelial cells and depresses the immune system, is a potentially serious disease because of its ability to produce complications in essentially every organ of the body, including the lungs, kidneys, blood, and central nervous system. Consistent with past studies, the most common complication in this series was pneumonia, observed in 20% of patients. The list of other serious complications identified in this study period, including encephalitis and acute renal failure, was long.
“We observed death in 4.3% of our 582 cases, or about 25 cases,” reported Dr. Chovatiya. He indicated that this is a high percentage among a population composed largely of children who were well before hospitalization.
The mortality rate from measles was numerically but not statistically higher than that of overall hospital admissions during this period, but an admission for measles was associated with significantly longer average length of stay (3.7 vs. 3.5 days) and slightly but significantly higher direct costs ($18,907 vs. $18,474).
“I want to point out that these are just direct inpatient costs,” Dr. Chovatiya said. Extrapolating from published data about indirect expenses, he said that the total health cost burden “is absolutely staggering.”
Previous studies have suggested that about 25% of patients with measles require hospitalization and 1 in every 1,000 patients will die. The data collected by Dr. Chovatiya support these often-cited figures, indicating that they remain unchanged in the modern era.
particularly insufficient penetration of vaccination in many communities.
The vaccine “is inexpensive, extremely effective, and lifesaving,” said Dr. Chovatiya, making the point that all of the morbidity, mortality, and costs he described are largely avoidable.
Attempting to provide perspective of the measles threat and the impact of the vaccine, Dr. Chovatiya cited a hypothetical calculation that 732,000 deaths from measles would have been expected in the United States among the pool of children born between 1994 and 2013 had no vaccine been offered. Again, most of these deaths would have occurred in otherwise healthy children.
Dr. Chovatiya reported no potential conflicts of interest.
REPORTING FROM SID 2019
GI disease screening with artificial intelligence is close
SAN FRANCISCO – As a tool for the screening and diagnosis of diseases in the gastrointestinal (GI) tract, artificial intelligence (AI) is advancing rapidly, according to a review of this technology presented at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
Much of the focus of the update was on screening colonoscopy, but the same principles are relevant and being pursued for other GI conditions, such as dysplasia screening in patients with Barrett’s esophagus and the assessment of mucosal healing in inflammatory bowel disease, according to Michael F. Byrne, MD, a clinical professor in the division of gastroenterology at Vancouver General Hospital.
“There are many technologies [to improve screening and diagnosis of GI diseases], but I believe these will struggle if they do not also have some kind of built-in machine intelligence,” Dr. Byrne said. In addition to his practice in gastroenterology, Dr. Byrne is CEO of Satis Operations and founder of AI4GI, a commercial joint venture focused on clinical applications of AI in colon polyp disease.
In this context, AI is being built on the principle of deep learning, which employs neural networks or a set of algorithms that permits a computer to recognize patterns when “trained” with data. In the machine learning process, the computer can use a large number of features in the task of discrimination.
This might suggest that AI could, in turn, train physicians to recognize the same features, but this underestimates the complexity and sophistication of machine learning, according to Dr. Byrne. The current status of machine learning for screening colonoscopy underscores this point.
“A computer can consider a thousand features when evaluating a polyp, which is way beyond what we can do,” Dr. Byrne said. Even with advances to improve visualization in screening colonoscopy, such as improved resolution and better lighting, the reason that AI is expected to prevail is that “the human eye is just not accurate enough.”
Many groups have developed advanced machine learning systems for screening colonoscopy. Dr. Byrne reviewed some of the early work done in Japan and that performed with a system in development by his group. In a study with the AI4GI model, published recently in Gut (2019;68:94-100), greater than 94% accuracy was achieved in distinguishing adenomas from hyperplastic polyps using histopathology as a gold standard.
Because of the ability of machine learning to see what the human eye cannot, Dr. Byrne predicts that AI-centric classification will replace current polyp classification systems, which could offer categories that are more clinically useful and reliable.
However, the work in screening colonoscopy is just the beginning, according to Dr. Byrne. “The opportunity of machine learning goes way beyond polyps.”
Recognizing dysplasia associated with Barrett’s esophagus has parallels with identifying adenomatous polyps in screening colonoscopy, but Dr. Byrne also discussed machine learning as an “optical biopsy” for evaluating the mucosa of patients with IBD. No longer a screening approach, the characterization of IBD tissue could help with therapeutic decisions.
With an AI approach to optical biopsy, “there is a great opportunity to assign an inflammatory burden in IBD,” he suggested, explaining how evidence of disease activity could guide escalation or de-escalation of treatment within the context of the treat-to-target approach to prolonging remission.
Overall, there is abundant evidence that “optical biopsy is feasible,” Dr. Byrne said. He indicated that clinical applications are approaching quickly. While he acknowledged that the technology “will need a human in the loop” as it enters clinical practice initially, he believes that this technology will play a significant role in GI practice because of the clear limitations of the human eye in assessing endoscopic images of GI tissue.
SAN FRANCISCO – As a tool for the screening and diagnosis of diseases in the gastrointestinal (GI) tract, artificial intelligence (AI) is advancing rapidly, according to a review of this technology presented at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
Much of the focus of the update was on screening colonoscopy, but the same principles are relevant and being pursued for other GI conditions, such as dysplasia screening in patients with Barrett’s esophagus and the assessment of mucosal healing in inflammatory bowel disease, according to Michael F. Byrne, MD, a clinical professor in the division of gastroenterology at Vancouver General Hospital.
“There are many technologies [to improve screening and diagnosis of GI diseases], but I believe these will struggle if they do not also have some kind of built-in machine intelligence,” Dr. Byrne said. In addition to his practice in gastroenterology, Dr. Byrne is CEO of Satis Operations and founder of AI4GI, a commercial joint venture focused on clinical applications of AI in colon polyp disease.
In this context, AI is being built on the principle of deep learning, which employs neural networks or a set of algorithms that permits a computer to recognize patterns when “trained” with data. In the machine learning process, the computer can use a large number of features in the task of discrimination.
This might suggest that AI could, in turn, train physicians to recognize the same features, but this underestimates the complexity and sophistication of machine learning, according to Dr. Byrne. The current status of machine learning for screening colonoscopy underscores this point.
“A computer can consider a thousand features when evaluating a polyp, which is way beyond what we can do,” Dr. Byrne said. Even with advances to improve visualization in screening colonoscopy, such as improved resolution and better lighting, the reason that AI is expected to prevail is that “the human eye is just not accurate enough.”
Many groups have developed advanced machine learning systems for screening colonoscopy. Dr. Byrne reviewed some of the early work done in Japan and that performed with a system in development by his group. In a study with the AI4GI model, published recently in Gut (2019;68:94-100), greater than 94% accuracy was achieved in distinguishing adenomas from hyperplastic polyps using histopathology as a gold standard.
Because of the ability of machine learning to see what the human eye cannot, Dr. Byrne predicts that AI-centric classification will replace current polyp classification systems, which could offer categories that are more clinically useful and reliable.
However, the work in screening colonoscopy is just the beginning, according to Dr. Byrne. “The opportunity of machine learning goes way beyond polyps.”
Recognizing dysplasia associated with Barrett’s esophagus has parallels with identifying adenomatous polyps in screening colonoscopy, but Dr. Byrne also discussed machine learning as an “optical biopsy” for evaluating the mucosa of patients with IBD. No longer a screening approach, the characterization of IBD tissue could help with therapeutic decisions.
With an AI approach to optical biopsy, “there is a great opportunity to assign an inflammatory burden in IBD,” he suggested, explaining how evidence of disease activity could guide escalation or de-escalation of treatment within the context of the treat-to-target approach to prolonging remission.
Overall, there is abundant evidence that “optical biopsy is feasible,” Dr. Byrne said. He indicated that clinical applications are approaching quickly. While he acknowledged that the technology “will need a human in the loop” as it enters clinical practice initially, he believes that this technology will play a significant role in GI practice because of the clear limitations of the human eye in assessing endoscopic images of GI tissue.
SAN FRANCISCO – As a tool for the screening and diagnosis of diseases in the gastrointestinal (GI) tract, artificial intelligence (AI) is advancing rapidly, according to a review of this technology presented at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
Much of the focus of the update was on screening colonoscopy, but the same principles are relevant and being pursued for other GI conditions, such as dysplasia screening in patients with Barrett’s esophagus and the assessment of mucosal healing in inflammatory bowel disease, according to Michael F. Byrne, MD, a clinical professor in the division of gastroenterology at Vancouver General Hospital.
“There are many technologies [to improve screening and diagnosis of GI diseases], but I believe these will struggle if they do not also have some kind of built-in machine intelligence,” Dr. Byrne said. In addition to his practice in gastroenterology, Dr. Byrne is CEO of Satis Operations and founder of AI4GI, a commercial joint venture focused on clinical applications of AI in colon polyp disease.
In this context, AI is being built on the principle of deep learning, which employs neural networks or a set of algorithms that permits a computer to recognize patterns when “trained” with data. In the machine learning process, the computer can use a large number of features in the task of discrimination.
This might suggest that AI could, in turn, train physicians to recognize the same features, but this underestimates the complexity and sophistication of machine learning, according to Dr. Byrne. The current status of machine learning for screening colonoscopy underscores this point.
“A computer can consider a thousand features when evaluating a polyp, which is way beyond what we can do,” Dr. Byrne said. Even with advances to improve visualization in screening colonoscopy, such as improved resolution and better lighting, the reason that AI is expected to prevail is that “the human eye is just not accurate enough.”
Many groups have developed advanced machine learning systems for screening colonoscopy. Dr. Byrne reviewed some of the early work done in Japan and that performed with a system in development by his group. In a study with the AI4GI model, published recently in Gut (2019;68:94-100), greater than 94% accuracy was achieved in distinguishing adenomas from hyperplastic polyps using histopathology as a gold standard.
Because of the ability of machine learning to see what the human eye cannot, Dr. Byrne predicts that AI-centric classification will replace current polyp classification systems, which could offer categories that are more clinically useful and reliable.
However, the work in screening colonoscopy is just the beginning, according to Dr. Byrne. “The opportunity of machine learning goes way beyond polyps.”
Recognizing dysplasia associated with Barrett’s esophagus has parallels with identifying adenomatous polyps in screening colonoscopy, but Dr. Byrne also discussed machine learning as an “optical biopsy” for evaluating the mucosa of patients with IBD. No longer a screening approach, the characterization of IBD tissue could help with therapeutic decisions.
With an AI approach to optical biopsy, “there is a great opportunity to assign an inflammatory burden in IBD,” he suggested, explaining how evidence of disease activity could guide escalation or de-escalation of treatment within the context of the treat-to-target approach to prolonging remission.
Overall, there is abundant evidence that “optical biopsy is feasible,” Dr. Byrne said. He indicated that clinical applications are approaching quickly. While he acknowledged that the technology “will need a human in the loop” as it enters clinical practice initially, he believes that this technology will play a significant role in GI practice because of the clear limitations of the human eye in assessing endoscopic images of GI tissue.
EXPERT ANALYSIS FROM 2019 AGA TECH SUMMIT
Relatively high starting infliximab doses recommended for hidradenitis suppurativa
CHICAGO – In patients with hidradenitis suppurativa (HS), infliximab started at 7.5 mg/kg was highly effective and well tolerated and may be a more appropriate starting dose than the 5 mg/kg that is recommended in the treatment of plaque psoriasis, according to a chart review presented as a late breaker at the annual meeting of the Society for Investigative Dermatology.
“Based on these data, we believe that a high starting dose of infliximab should be the new paradigm for this disease,” reported Justine Potemkin, a medical student at Albert Einstein College of Medicine, New York. The analysis was performed under the mentorship of Steven R. Cohen, MD, professor of dermatology and chief of the division of dermatology at Albert Einstein.
Infliximab, a tumor necrosis factor (TNF) blocker, is not approved for the treatment of HS, but it has been widely used following numerous reports of benefit in the literature, including a phase 2 randomized study published several years ago (J Am Acad Dermatol. 2010 Feb;62[2]:205-17), according to Ms. Potemkin.
“Based on dosing recommended for psoriasis, many hidradenitis suppurativa patients are started on 5 mg/kg, but doses of up to 10 mg/kg are within labeling for psoriasis, and our data support more aggressive initial treatment in patients with HS,” said Mondana Ghias, a research assistant in the department of dermatology at Albert Einstein, who was also involved in this study and joined Ms. Potemkin in presenting the data at the meeting.
In the chart review, 58 patients with moderate to severe HS initiated on 7.5 mg/kg of intravenous infliximab were identified. Due to a suboptimal response, 16 of these patients received 10 mg/kg infliximab in subsequent infusions, but the average response scores at 4 weeks compared favorably to previous reports with lower doses of infliximab, without increased toxicity.
“Starting with the 7.5 mg/kg dose with dose escalation if needed appears to provide a maximal response with minimal safety issues, based on our experience,” Ms. Potemkin reported.
At 4 weeks, a 10-point pain scale fell from a baseline mean of 5.65 to 1.34 (P less than .01). It reached 0.52 at week 12. The HS Physician Global Assessment (HS-PGA) fell from a baseline mean of 4.2 to 2.1 at week 4 (P less than .01) and then to 1.9 at week 12. The control of disease activity correlated with significant reductions in C-reactive protein and erythrocyte sedimentation rate.
The mean age in this patient population was 34.6 years. Slightly more than half (53.3%) were female. The average body mass index was 34.
“Obese patients also achieved a good response, which is consistent with weight-based dosing,” Ms. Potemkin reported.
The TNF blocker adalimumab is the only biologic and the only systemic therapy given regulatory approval for the treatment of HS, but Ms. Potemkin indicated that infliximab remains a widely used treatment option. She suggested that the responses observed in this patient series suggest infliximab is effective when used aggressively.
“No previous reports have examined the efficacy of high-dose infliximab,” Ms. Potemkin noted. “We think these findings are relevant to HS management.”
CHICAGO – In patients with hidradenitis suppurativa (HS), infliximab started at 7.5 mg/kg was highly effective and well tolerated and may be a more appropriate starting dose than the 5 mg/kg that is recommended in the treatment of plaque psoriasis, according to a chart review presented as a late breaker at the annual meeting of the Society for Investigative Dermatology.
“Based on these data, we believe that a high starting dose of infliximab should be the new paradigm for this disease,” reported Justine Potemkin, a medical student at Albert Einstein College of Medicine, New York. The analysis was performed under the mentorship of Steven R. Cohen, MD, professor of dermatology and chief of the division of dermatology at Albert Einstein.
Infliximab, a tumor necrosis factor (TNF) blocker, is not approved for the treatment of HS, but it has been widely used following numerous reports of benefit in the literature, including a phase 2 randomized study published several years ago (J Am Acad Dermatol. 2010 Feb;62[2]:205-17), according to Ms. Potemkin.
“Based on dosing recommended for psoriasis, many hidradenitis suppurativa patients are started on 5 mg/kg, but doses of up to 10 mg/kg are within labeling for psoriasis, and our data support more aggressive initial treatment in patients with HS,” said Mondana Ghias, a research assistant in the department of dermatology at Albert Einstein, who was also involved in this study and joined Ms. Potemkin in presenting the data at the meeting.
In the chart review, 58 patients with moderate to severe HS initiated on 7.5 mg/kg of intravenous infliximab were identified. Due to a suboptimal response, 16 of these patients received 10 mg/kg infliximab in subsequent infusions, but the average response scores at 4 weeks compared favorably to previous reports with lower doses of infliximab, without increased toxicity.
“Starting with the 7.5 mg/kg dose with dose escalation if needed appears to provide a maximal response with minimal safety issues, based on our experience,” Ms. Potemkin reported.
At 4 weeks, a 10-point pain scale fell from a baseline mean of 5.65 to 1.34 (P less than .01). It reached 0.52 at week 12. The HS Physician Global Assessment (HS-PGA) fell from a baseline mean of 4.2 to 2.1 at week 4 (P less than .01) and then to 1.9 at week 12. The control of disease activity correlated with significant reductions in C-reactive protein and erythrocyte sedimentation rate.
The mean age in this patient population was 34.6 years. Slightly more than half (53.3%) were female. The average body mass index was 34.
“Obese patients also achieved a good response, which is consistent with weight-based dosing,” Ms. Potemkin reported.
The TNF blocker adalimumab is the only biologic and the only systemic therapy given regulatory approval for the treatment of HS, but Ms. Potemkin indicated that infliximab remains a widely used treatment option. She suggested that the responses observed in this patient series suggest infliximab is effective when used aggressively.
“No previous reports have examined the efficacy of high-dose infliximab,” Ms. Potemkin noted. “We think these findings are relevant to HS management.”
CHICAGO – In patients with hidradenitis suppurativa (HS), infliximab started at 7.5 mg/kg was highly effective and well tolerated and may be a more appropriate starting dose than the 5 mg/kg that is recommended in the treatment of plaque psoriasis, according to a chart review presented as a late breaker at the annual meeting of the Society for Investigative Dermatology.
“Based on these data, we believe that a high starting dose of infliximab should be the new paradigm for this disease,” reported Justine Potemkin, a medical student at Albert Einstein College of Medicine, New York. The analysis was performed under the mentorship of Steven R. Cohen, MD, professor of dermatology and chief of the division of dermatology at Albert Einstein.
Infliximab, a tumor necrosis factor (TNF) blocker, is not approved for the treatment of HS, but it has been widely used following numerous reports of benefit in the literature, including a phase 2 randomized study published several years ago (J Am Acad Dermatol. 2010 Feb;62[2]:205-17), according to Ms. Potemkin.
“Based on dosing recommended for psoriasis, many hidradenitis suppurativa patients are started on 5 mg/kg, but doses of up to 10 mg/kg are within labeling for psoriasis, and our data support more aggressive initial treatment in patients with HS,” said Mondana Ghias, a research assistant in the department of dermatology at Albert Einstein, who was also involved in this study and joined Ms. Potemkin in presenting the data at the meeting.
In the chart review, 58 patients with moderate to severe HS initiated on 7.5 mg/kg of intravenous infliximab were identified. Due to a suboptimal response, 16 of these patients received 10 mg/kg infliximab in subsequent infusions, but the average response scores at 4 weeks compared favorably to previous reports with lower doses of infliximab, without increased toxicity.
“Starting with the 7.5 mg/kg dose with dose escalation if needed appears to provide a maximal response with minimal safety issues, based on our experience,” Ms. Potemkin reported.
At 4 weeks, a 10-point pain scale fell from a baseline mean of 5.65 to 1.34 (P less than .01). It reached 0.52 at week 12. The HS Physician Global Assessment (HS-PGA) fell from a baseline mean of 4.2 to 2.1 at week 4 (P less than .01) and then to 1.9 at week 12. The control of disease activity correlated with significant reductions in C-reactive protein and erythrocyte sedimentation rate.
The mean age in this patient population was 34.6 years. Slightly more than half (53.3%) were female. The average body mass index was 34.
“Obese patients also achieved a good response, which is consistent with weight-based dosing,” Ms. Potemkin reported.
The TNF blocker adalimumab is the only biologic and the only systemic therapy given regulatory approval for the treatment of HS, but Ms. Potemkin indicated that infliximab remains a widely used treatment option. She suggested that the responses observed in this patient series suggest infliximab is effective when used aggressively.
“No previous reports have examined the efficacy of high-dose infliximab,” Ms. Potemkin noted. “We think these findings are relevant to HS management.”
REPORTING FROM SID 2019
Baseline imaging recommended in all Merkel cell carcinoma patients
CHICAGO – , including those without palpable lymph nodes, according to results of an analysis of a large MCC registry presented at the annual meeting of the Society for Investigative Dermatology.
The results were a “surprise,” according to Neha Singh, a researcher in the division of dermatology at the University of Washington, Seattle. She contended that many treatment guidelines for MCC, including imaging at the time of diagnosis, are borrowed from those developed for melanoma but should not be.
“MCC is much more frequently metastatic to regional and distant sites than melanoma, so current melanoma guidelines may not be appropriate for use with MCC,” she said. According to the data she cited, 41% of MCC patients, versus 14% of melanoma patients, already have metastatic disease at the time of diagnosis.
The presence of more aggressive disease and the need for scanning was confirmed in the analysis of the MCC Registry in Seattle, which contains 1,439 patients. Of 586 patients who met inclusion criteria for this analysis, 493 MCC patients had no palpable lymph nodes at the time of diagnosis. Yet, 60 (12%) proved to already have regional or distant metastases on the basis of scans.
This contrasts starkly with melanoma data, according to Ms. Singh. Guidelines from the National Comprehensive Cancer Network (NCCN) do not recommend scans in melanoma patients without palpable lymph nodes based on evidence that only 1% of these will be upstaged by imaging. This figure was judged too small to justify routine scans, she said.
In melanoma patients with palpable lymph nodes, NCCN guidelines do recommend imaging at diagnosis because upstaging is common, and the same is true in MCC, Ms. Singh noted. In the Seattle registry, 10 (11%) of the 93 patients with palpable lymph nodes were upstaged for distant metastases found on imaging.
In those without palpable lymph nodes, “even a small tumor does not guarantee the absence of distant metastases,” Ms. Singh cautioned. Although the median tumor size in this group was 2.3 cm, tumors of less than 1 cm were still associated with distant disease.
The likelihood of distant disease in MCC patients without palpable lymph nodes might be even greater than that identified in this analysis. At least some of the patients in this series were evaluated with CT rather than PET imaging, which is more sensitive. Ms. Singh reported that no stratification to determine rates of distant disease by imaging type have yet been undertaken in this dataset.
Based on these findings, guidelines for MCC should include consideration of baseline imaging in all patients, Ms. Singh said. In making this point, she also emphasized the guidelines for melanoma should not be considered transferable to MCC.
“Why is this important?” Ms. Singh asked. Understaging MCC “may lead to inadequate surgery, overaggressive local therapy, and a potential delay to effective systemic therapy.”
CHICAGO – , including those without palpable lymph nodes, according to results of an analysis of a large MCC registry presented at the annual meeting of the Society for Investigative Dermatology.
The results were a “surprise,” according to Neha Singh, a researcher in the division of dermatology at the University of Washington, Seattle. She contended that many treatment guidelines for MCC, including imaging at the time of diagnosis, are borrowed from those developed for melanoma but should not be.
“MCC is much more frequently metastatic to regional and distant sites than melanoma, so current melanoma guidelines may not be appropriate for use with MCC,” she said. According to the data she cited, 41% of MCC patients, versus 14% of melanoma patients, already have metastatic disease at the time of diagnosis.
The presence of more aggressive disease and the need for scanning was confirmed in the analysis of the MCC Registry in Seattle, which contains 1,439 patients. Of 586 patients who met inclusion criteria for this analysis, 493 MCC patients had no palpable lymph nodes at the time of diagnosis. Yet, 60 (12%) proved to already have regional or distant metastases on the basis of scans.
This contrasts starkly with melanoma data, according to Ms. Singh. Guidelines from the National Comprehensive Cancer Network (NCCN) do not recommend scans in melanoma patients without palpable lymph nodes based on evidence that only 1% of these will be upstaged by imaging. This figure was judged too small to justify routine scans, she said.
In melanoma patients with palpable lymph nodes, NCCN guidelines do recommend imaging at diagnosis because upstaging is common, and the same is true in MCC, Ms. Singh noted. In the Seattle registry, 10 (11%) of the 93 patients with palpable lymph nodes were upstaged for distant metastases found on imaging.
In those without palpable lymph nodes, “even a small tumor does not guarantee the absence of distant metastases,” Ms. Singh cautioned. Although the median tumor size in this group was 2.3 cm, tumors of less than 1 cm were still associated with distant disease.
The likelihood of distant disease in MCC patients without palpable lymph nodes might be even greater than that identified in this analysis. At least some of the patients in this series were evaluated with CT rather than PET imaging, which is more sensitive. Ms. Singh reported that no stratification to determine rates of distant disease by imaging type have yet been undertaken in this dataset.
Based on these findings, guidelines for MCC should include consideration of baseline imaging in all patients, Ms. Singh said. In making this point, she also emphasized the guidelines for melanoma should not be considered transferable to MCC.
“Why is this important?” Ms. Singh asked. Understaging MCC “may lead to inadequate surgery, overaggressive local therapy, and a potential delay to effective systemic therapy.”
CHICAGO – , including those without palpable lymph nodes, according to results of an analysis of a large MCC registry presented at the annual meeting of the Society for Investigative Dermatology.
The results were a “surprise,” according to Neha Singh, a researcher in the division of dermatology at the University of Washington, Seattle. She contended that many treatment guidelines for MCC, including imaging at the time of diagnosis, are borrowed from those developed for melanoma but should not be.
“MCC is much more frequently metastatic to regional and distant sites than melanoma, so current melanoma guidelines may not be appropriate for use with MCC,” she said. According to the data she cited, 41% of MCC patients, versus 14% of melanoma patients, already have metastatic disease at the time of diagnosis.
The presence of more aggressive disease and the need for scanning was confirmed in the analysis of the MCC Registry in Seattle, which contains 1,439 patients. Of 586 patients who met inclusion criteria for this analysis, 493 MCC patients had no palpable lymph nodes at the time of diagnosis. Yet, 60 (12%) proved to already have regional or distant metastases on the basis of scans.
This contrasts starkly with melanoma data, according to Ms. Singh. Guidelines from the National Comprehensive Cancer Network (NCCN) do not recommend scans in melanoma patients without palpable lymph nodes based on evidence that only 1% of these will be upstaged by imaging. This figure was judged too small to justify routine scans, she said.
In melanoma patients with palpable lymph nodes, NCCN guidelines do recommend imaging at diagnosis because upstaging is common, and the same is true in MCC, Ms. Singh noted. In the Seattle registry, 10 (11%) of the 93 patients with palpable lymph nodes were upstaged for distant metastases found on imaging.
In those without palpable lymph nodes, “even a small tumor does not guarantee the absence of distant metastases,” Ms. Singh cautioned. Although the median tumor size in this group was 2.3 cm, tumors of less than 1 cm were still associated with distant disease.
The likelihood of distant disease in MCC patients without palpable lymph nodes might be even greater than that identified in this analysis. At least some of the patients in this series were evaluated with CT rather than PET imaging, which is more sensitive. Ms. Singh reported that no stratification to determine rates of distant disease by imaging type have yet been undertaken in this dataset.
Based on these findings, guidelines for MCC should include consideration of baseline imaging in all patients, Ms. Singh said. In making this point, she also emphasized the guidelines for melanoma should not be considered transferable to MCC.
“Why is this important?” Ms. Singh asked. Understaging MCC “may lead to inadequate surgery, overaggressive local therapy, and a potential delay to effective systemic therapy.”
REPORTING FROM SID 2019
Gentamicin restores wound healing in hereditary epidermolysis bullosa
Rare progress seen in challenging disease
CHICAGO – Topical gentamicin counters the nonsense mutations that inhibit production of laminin 332 in infants with Herlitz junctional epidermolysis bullosa (H-JEB) to allow lesion healing, according to results of a small clinical study presented at the annual meeting of the Society for Investigative Dermatology.
“All of the children treated so far have responded,” reported Andrew Kwong, who will soon graduate from the Keck School of Medicine at the University of Southern California, Los Angeles.
H-JEB is an inherited blistering skin disease associated with nonsense mutations in the LAMA3, LAMB3, or LAMC2 genes that result in impaired production of functional laminin 332, an essential protein for epidermal-dermal adherence. At this time there are no effective therapies, and the disease is fatal.
The small clinical study was initiated after in vitro studies demonstrated that gentamicin restored functional laminin 332 in cultured keratinocytes from infants with H-JEB. The dose-dependent effect was credited to the ability of gentamicin to induce readthrough of premature stop codons that block production of laminin 332.
Data were presented on the first three infants with H-JEB treated with oral gentamicin. In each child, lesions were treated with topical 0.5% gentamicin twice daily for two weeks. Biopsies were taken prior to the initiation of treatment and at one and three months after treatment. The primary outcome was change in laminin 332, but clinical improvement was also monitored.
Although none of the infants had measurable laminin 332 prior to treatment, all lesions treated with topical gentamicin developed localized laminin 332 at the dermal-epidermal junction of the skin, Mr. Kwong reported. This expression, which was about 40% to 60% of that seen in normal skin, still persisted when evaluated three months after treatment.
The expression was associated with resolution of existing lesions and a reduced risk of developing new lesions, according to Mr. Kwong. In lesions that went untreated, there was no change.
Other molecular changes in the skin, such as increased expression and polarization of beta-4 integrin, were consistent with the ability of gentamicin to address the underlying pathophysiology of H-JEB. There were no adverse events observed.
By restoring functional laminin 332 in the skin, topical gentamicin appears to address the underlying cause of the bullae associated with H-JEB, but Mr. Kwong said that the next step is to determine whether intravenous gentamicin can address the systemic effects. If so, this treatment has the potential to improve survival. He reported that an infant with H-JEB was recently started on intravenous treatment, and initial results were encouraging.
Asked whether he would recommend topical gentamicin on the basis of these findings, Mr. Kwong cautioned that the case series remains very small, but he noted that the uniformity of the positive response is encouraging. He expects that off-label use of this novel and low-cost approach might be warranted in a population that has very limited therapeutic options.
SOURCE: Kwong A. SID 2019;S102, Abstract 594. Annual meeting of the Society for Investigative Dermatology.
Rare progress seen in challenging disease
Rare progress seen in challenging disease
CHICAGO – Topical gentamicin counters the nonsense mutations that inhibit production of laminin 332 in infants with Herlitz junctional epidermolysis bullosa (H-JEB) to allow lesion healing, according to results of a small clinical study presented at the annual meeting of the Society for Investigative Dermatology.
“All of the children treated so far have responded,” reported Andrew Kwong, who will soon graduate from the Keck School of Medicine at the University of Southern California, Los Angeles.
H-JEB is an inherited blistering skin disease associated with nonsense mutations in the LAMA3, LAMB3, or LAMC2 genes that result in impaired production of functional laminin 332, an essential protein for epidermal-dermal adherence. At this time there are no effective therapies, and the disease is fatal.
The small clinical study was initiated after in vitro studies demonstrated that gentamicin restored functional laminin 332 in cultured keratinocytes from infants with H-JEB. The dose-dependent effect was credited to the ability of gentamicin to induce readthrough of premature stop codons that block production of laminin 332.
Data were presented on the first three infants with H-JEB treated with oral gentamicin. In each child, lesions were treated with topical 0.5% gentamicin twice daily for two weeks. Biopsies were taken prior to the initiation of treatment and at one and three months after treatment. The primary outcome was change in laminin 332, but clinical improvement was also monitored.
Although none of the infants had measurable laminin 332 prior to treatment, all lesions treated with topical gentamicin developed localized laminin 332 at the dermal-epidermal junction of the skin, Mr. Kwong reported. This expression, which was about 40% to 60% of that seen in normal skin, still persisted when evaluated three months after treatment.
The expression was associated with resolution of existing lesions and a reduced risk of developing new lesions, according to Mr. Kwong. In lesions that went untreated, there was no change.
Other molecular changes in the skin, such as increased expression and polarization of beta-4 integrin, were consistent with the ability of gentamicin to address the underlying pathophysiology of H-JEB. There were no adverse events observed.
By restoring functional laminin 332 in the skin, topical gentamicin appears to address the underlying cause of the bullae associated with H-JEB, but Mr. Kwong said that the next step is to determine whether intravenous gentamicin can address the systemic effects. If so, this treatment has the potential to improve survival. He reported that an infant with H-JEB was recently started on intravenous treatment, and initial results were encouraging.
Asked whether he would recommend topical gentamicin on the basis of these findings, Mr. Kwong cautioned that the case series remains very small, but he noted that the uniformity of the positive response is encouraging. He expects that off-label use of this novel and low-cost approach might be warranted in a population that has very limited therapeutic options.
SOURCE: Kwong A. SID 2019;S102, Abstract 594. Annual meeting of the Society for Investigative Dermatology.
CHICAGO – Topical gentamicin counters the nonsense mutations that inhibit production of laminin 332 in infants with Herlitz junctional epidermolysis bullosa (H-JEB) to allow lesion healing, according to results of a small clinical study presented at the annual meeting of the Society for Investigative Dermatology.
“All of the children treated so far have responded,” reported Andrew Kwong, who will soon graduate from the Keck School of Medicine at the University of Southern California, Los Angeles.
H-JEB is an inherited blistering skin disease associated with nonsense mutations in the LAMA3, LAMB3, or LAMC2 genes that result in impaired production of functional laminin 332, an essential protein for epidermal-dermal adherence. At this time there are no effective therapies, and the disease is fatal.
The small clinical study was initiated after in vitro studies demonstrated that gentamicin restored functional laminin 332 in cultured keratinocytes from infants with H-JEB. The dose-dependent effect was credited to the ability of gentamicin to induce readthrough of premature stop codons that block production of laminin 332.
Data were presented on the first three infants with H-JEB treated with oral gentamicin. In each child, lesions were treated with topical 0.5% gentamicin twice daily for two weeks. Biopsies were taken prior to the initiation of treatment and at one and three months after treatment. The primary outcome was change in laminin 332, but clinical improvement was also monitored.
Although none of the infants had measurable laminin 332 prior to treatment, all lesions treated with topical gentamicin developed localized laminin 332 at the dermal-epidermal junction of the skin, Mr. Kwong reported. This expression, which was about 40% to 60% of that seen in normal skin, still persisted when evaluated three months after treatment.
The expression was associated with resolution of existing lesions and a reduced risk of developing new lesions, according to Mr. Kwong. In lesions that went untreated, there was no change.
Other molecular changes in the skin, such as increased expression and polarization of beta-4 integrin, were consistent with the ability of gentamicin to address the underlying pathophysiology of H-JEB. There were no adverse events observed.
By restoring functional laminin 332 in the skin, topical gentamicin appears to address the underlying cause of the bullae associated with H-JEB, but Mr. Kwong said that the next step is to determine whether intravenous gentamicin can address the systemic effects. If so, this treatment has the potential to improve survival. He reported that an infant with H-JEB was recently started on intravenous treatment, and initial results were encouraging.
Asked whether he would recommend topical gentamicin on the basis of these findings, Mr. Kwong cautioned that the case series remains very small, but he noted that the uniformity of the positive response is encouraging. He expects that off-label use of this novel and low-cost approach might be warranted in a population that has very limited therapeutic options.
SOURCE: Kwong A. SID 2019;S102, Abstract 594. Annual meeting of the Society for Investigative Dermatology.
REPORTING FROM SID 2019
Predictive analytics with large data sets are being pursued to individualize IBD therapy
SAN FRANCISCO – Predictive analytics of large quantities of data using machine learning present a powerful tool for improving therapeutic choices, according to a summary of work performed in inflammatory bowel disease (IBD) and presented at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
This type of work is relevant to many fields of medicine, but studies conducted in IBD have provided particularly compelling evidence that predictive analytics will improve outcomes and lead to more cost effective delivery of care, according to Akbar K. Waljee, MD, MSc, an associate professor in the division of gastroenterology at University of Michigan, Ann Arbor, and a staff physician and researcher at the VA Ann Arbor Healthcare system.
“We collect large amounts of clinical data every day in the delivery of health care, but we are now only just beginning to leverage [these] data to guide treatment,” Dr. Waljee said. He has now published several papers on the role of precision analytics of big data to improve treatment choices in IBD, as well as other diseases. These analyses are relevant for determining both who to treat with a certain drug and who to not treat with it.
In one example, data from 1,080 IBD patients taking thiopurines were used to develop a machine learning algorithm that analyzed multiple readily available variables, such as a complete blood count with differential and a chemistry panel, to predict whether someone was or was not in remission. This was then used to compare the mean yearly clinical event rates (new steroids prescriptions, hospitalizations, and abdominal surgeries) between the two groups (1.08 vs. 3.95 events) to show the associated clinical benefit of using this algorithm.
“The heterogeneity of response to therapies for IBD is well established. If machine learning predicts effective choices, there will be an opportunity to accelerate the time to disease control, as well as save costs by avoiding therapies not likely to be effective,” Dr. Waljee explained.
In another example, an algorithm was developed to predict the likelihood of achieving a corticosteroid-free biologic remission at 1 year in Crohn’s disease patients when patients were evaluated 6 weeks after initiating the gut-selective biologic vedolizumab. Again, it was based on an analysis of numerous variables, including laboratory data, sex, and race. Based on the model drawn from the analysis of 472 patients, 35.8% of the patients predicted to be in corticosteroid-free biologic remission at 1 year achieved this endpoint, whereas only 6.7% of the patients predicted to fail achieved the endpoint.
“This suggests that we can use an algorithm relatively early in the course of this biologic to predict who is going to respond,” reported Dr. Waljee. Again, patients with a low likelihood of response at 6 weeks can be started on an alternative treatment, which could potentially accelerate the time to disease control and avoid the costs of an ineffective and expensive treatment.
IBD is a particularly attractive focus of precision analytics with big data. IBD has a relatively unpredictable relapsing/remitting course and a heterogeneous response to available therapies. Algorithms predictive of response circumvent the inherent delays from evaluating disease control over an extended period.
“With ever increasing concern about costs of care and access to care, these treatment algorithms promise to use resources more efficiently,” Dr. Waljee said.
SAN FRANCISCO – Predictive analytics of large quantities of data using machine learning present a powerful tool for improving therapeutic choices, according to a summary of work performed in inflammatory bowel disease (IBD) and presented at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
This type of work is relevant to many fields of medicine, but studies conducted in IBD have provided particularly compelling evidence that predictive analytics will improve outcomes and lead to more cost effective delivery of care, according to Akbar K. Waljee, MD, MSc, an associate professor in the division of gastroenterology at University of Michigan, Ann Arbor, and a staff physician and researcher at the VA Ann Arbor Healthcare system.
“We collect large amounts of clinical data every day in the delivery of health care, but we are now only just beginning to leverage [these] data to guide treatment,” Dr. Waljee said. He has now published several papers on the role of precision analytics of big data to improve treatment choices in IBD, as well as other diseases. These analyses are relevant for determining both who to treat with a certain drug and who to not treat with it.
In one example, data from 1,080 IBD patients taking thiopurines were used to develop a machine learning algorithm that analyzed multiple readily available variables, such as a complete blood count with differential and a chemistry panel, to predict whether someone was or was not in remission. This was then used to compare the mean yearly clinical event rates (new steroids prescriptions, hospitalizations, and abdominal surgeries) between the two groups (1.08 vs. 3.95 events) to show the associated clinical benefit of using this algorithm.
“The heterogeneity of response to therapies for IBD is well established. If machine learning predicts effective choices, there will be an opportunity to accelerate the time to disease control, as well as save costs by avoiding therapies not likely to be effective,” Dr. Waljee explained.
In another example, an algorithm was developed to predict the likelihood of achieving a corticosteroid-free biologic remission at 1 year in Crohn’s disease patients when patients were evaluated 6 weeks after initiating the gut-selective biologic vedolizumab. Again, it was based on an analysis of numerous variables, including laboratory data, sex, and race. Based on the model drawn from the analysis of 472 patients, 35.8% of the patients predicted to be in corticosteroid-free biologic remission at 1 year achieved this endpoint, whereas only 6.7% of the patients predicted to fail achieved the endpoint.
“This suggests that we can use an algorithm relatively early in the course of this biologic to predict who is going to respond,” reported Dr. Waljee. Again, patients with a low likelihood of response at 6 weeks can be started on an alternative treatment, which could potentially accelerate the time to disease control and avoid the costs of an ineffective and expensive treatment.
IBD is a particularly attractive focus of precision analytics with big data. IBD has a relatively unpredictable relapsing/remitting course and a heterogeneous response to available therapies. Algorithms predictive of response circumvent the inherent delays from evaluating disease control over an extended period.
“With ever increasing concern about costs of care and access to care, these treatment algorithms promise to use resources more efficiently,” Dr. Waljee said.
SAN FRANCISCO – Predictive analytics of large quantities of data using machine learning present a powerful tool for improving therapeutic choices, according to a summary of work performed in inflammatory bowel disease (IBD) and presented at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
This type of work is relevant to many fields of medicine, but studies conducted in IBD have provided particularly compelling evidence that predictive analytics will improve outcomes and lead to more cost effective delivery of care, according to Akbar K. Waljee, MD, MSc, an associate professor in the division of gastroenterology at University of Michigan, Ann Arbor, and a staff physician and researcher at the VA Ann Arbor Healthcare system.
“We collect large amounts of clinical data every day in the delivery of health care, but we are now only just beginning to leverage [these] data to guide treatment,” Dr. Waljee said. He has now published several papers on the role of precision analytics of big data to improve treatment choices in IBD, as well as other diseases. These analyses are relevant for determining both who to treat with a certain drug and who to not treat with it.
In one example, data from 1,080 IBD patients taking thiopurines were used to develop a machine learning algorithm that analyzed multiple readily available variables, such as a complete blood count with differential and a chemistry panel, to predict whether someone was or was not in remission. This was then used to compare the mean yearly clinical event rates (new steroids prescriptions, hospitalizations, and abdominal surgeries) between the two groups (1.08 vs. 3.95 events) to show the associated clinical benefit of using this algorithm.
“The heterogeneity of response to therapies for IBD is well established. If machine learning predicts effective choices, there will be an opportunity to accelerate the time to disease control, as well as save costs by avoiding therapies not likely to be effective,” Dr. Waljee explained.
In another example, an algorithm was developed to predict the likelihood of achieving a corticosteroid-free biologic remission at 1 year in Crohn’s disease patients when patients were evaluated 6 weeks after initiating the gut-selective biologic vedolizumab. Again, it was based on an analysis of numerous variables, including laboratory data, sex, and race. Based on the model drawn from the analysis of 472 patients, 35.8% of the patients predicted to be in corticosteroid-free biologic remission at 1 year achieved this endpoint, whereas only 6.7% of the patients predicted to fail achieved the endpoint.
“This suggests that we can use an algorithm relatively early in the course of this biologic to predict who is going to respond,” reported Dr. Waljee. Again, patients with a low likelihood of response at 6 weeks can be started on an alternative treatment, which could potentially accelerate the time to disease control and avoid the costs of an ineffective and expensive treatment.
IBD is a particularly attractive focus of precision analytics with big data. IBD has a relatively unpredictable relapsing/remitting course and a heterogeneous response to available therapies. Algorithms predictive of response circumvent the inherent delays from evaluating disease control over an extended period.
“With ever increasing concern about costs of care and access to care, these treatment algorithms promise to use resources more efficiently,” Dr. Waljee said.
EXPERT ANALYSIS FROM 2019 AGA TECH SUMMIT
Five enter the Shark Tank, one emerges
SAN FRANCISCO – All five innovative startups pitched at the Shark Tank at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology, are in advanced stages of development, but only one is given the opportunity to be declared the winner of the competition. The ideas ranged from a smart toilet for early disease detection to a unique strategy for obesity phenotyping, but the winner by both official decision and popular vote was a smartphone app to help patients with inflammatory bowel disease (IBD) manage the condition.
“As always, this year’s Shark Tank was a highlight of the AGA Tech Summit and represents the progress our field is making when it comes to innovation. Our panel of sharks was focused on understanding the problem each innovation solved – that’s the key when determining if an idea is novel or innovation for innovation’s sake. We were impressed with all of the technologies presented, but ultimately chose the Oshi Health IBD app as our winner because of the impact it is already having on improving the health and care of IBD patients,” said V. Raman Muthusamy, MD, AGAF, chair of the AGA Center for GI Innovation and Technology.
The winner: Oshi pitches “all-in-one” IBD app
By both popular vote from those attending the AGA Tech Summit as well as the six-member Shark Tank panel, Oshi Health was selected as the 2019 Shark Tank winner for its IBD app. The app was designed to help patients track symptoms, a first step in understanding flare patterns, which differ substantially between patients and emphasize the need for a personalized plan for controlling disease.

“Since we launched last June at DDW® we have had 40,000 downloads. We are the number one IBD management app,” reported Dan Weinstein, MBA, CEO of Oshi Health.
The available app represents the first of three phases as the functionality is expanded. Currently, in addition to using the app as a tracking tool, patients can find resources to learn about their disease and to communicate with other patients about their experiences. In a second phase, information gathered by the app will be made available to physicians to provide accurate current information about disease status to better individualize therapy.
Ultimately, the app is expected to guide treatment based on information it has collected on symptom patterns and other data collected over time, although this application is further down the road and will require regulatory approval if it is designed to provide clinical advice as expected, according to Mr. Weinstein.
However, benefits have already been seen. Mr. Weinstein cited data that associated the app with a 40% improvement in medication adherence and a nearly 60-day reduction in flare duration. Calling the app “the next chapter in treat-to-target” IBD management, he believes that this is an important step forward in digital health that will improve IBD outcomes. The Shark Tank panel agreed.
Runners-up: Other potential innovations to improve GI health
With or without Shark Tank endorsement, the other four startups described in the competition are moving forward. Each is designed to address an important unmet need with the potential to improve patient outcomes, which is a criterion for their inclusion in the competition.
The smart toilet seat
One involves a technologically advanced toilet seat. The new seat is based on the fact that fecal matter provides insight into a broad array of disease states, but specimen collection is a hurdle for a variety of reasons, including patient resistance. A toilet seat developed by Toi Labs, called TrueLoo, is equipped with lighting and cameras that captures images of bowel movements and urination for subsequent analysis.
“The toilet seat sees what the eye cannot,” according to Vikram Kashyap, CEO of Toi Labs. He believes it has major potential for early detection of conditions ranging from dehydration to gastrointestinal cancer.

Others agree. According to Mr. Kashyap, executives of a chain of senior living facilities have already expressed interest in installing this seat to better monitor health among residents. The seat is bolted into position in place of any standard toilet seat. It collects images and data that are transmitted directly to a cellular network.
“Using our technology, the goal is to catch disease states early before they progress,” said Mr. Kashyap, who called the surveillance system a low-cost disease-screening tool. He believes the smart toilet seat could be of the most important disease detection devices developed in recent years.
AI to aid screening endoscopy
A third entrant in this year’s Shark Tank described a strategy to employ artificial intelligence (AI) to aid endoscopists in screening for dysplasia. The tool is called Ultivision and is being developed by a startup called Docbot. The CEO, Andrew Ninh, and a senior executive, Jason B. Samarasena, MD, outlined an idea that could be used in either screening colonoscopy or in surveillance of Barrett’s esophagus).
“Dysplasia is difficult to find. It is subtle and it is often missed. With better detection of dysplasia, artificial intelligence offers an opportunity to reduce risk of cancer,” Dr. Samarasena said.
The tool integrates seamlessly with existing endoscopic tools, according to Mr. Ninh. As tissue is visualized, the AI is programmed to highlight suspected dysplasia with a colored box to alert the endoscopist. The colonoscopy application is a more advanced stage of development and might be submitted for regulatory approval this year, he said. The same technology will be adapted for Barrett’s esophagus.
“It is like facial recognition for dysplasia,” said Dr. Samarasena.
Obesity phenotyping tool
A fourth Shark Tank entrant employs technology to phenotype obese patients to better tailor therapy. The Pheno Test, developed by Phenomix Sciences, applies “multi-omics” to a blood-based test to separate patients with obesity into four phenotypes. When therapy is tailored to the phenotype, weight loss is greater, according to Andres J. Acosta, MD, PhD, assistant professor of medicine and consultant in gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
In an initial study that compared weight loss in 55 patients treated based on phenotype with 175 patients managed with standard of care, the total body weight loss “more than doubled,” Dr. Acosta reported.
According to Dr. Acosta, obesity is driven by very different mechanisms. He described the four major phenotypes identified with his test as hungry brain (satiation signal is impaired), hungry gut (signals to eat are upregulated), emotional hunger (psychological reasons drive eating behavior), and slow metabolism (failure to burn fat at normal rates).
With the blood test, which utilizes hormones, metabolites, DNA, and other biomarkers to separate these phenotypes, treatment can be tailored appropriately, according to Dr. Acosta. His company is now seeking Food and Drug Administration clearance of the test, which he believes will have a major impact on obesity control.
Capsule diagnostic tool
The final entrant selected to participate in this year’s Shark Tank described an ingestible capsule that diagnoses diseases by detecting gases as it descends the gastrointestinal tract. The Atmo Gas Capsule from Atmo Biosciences measures gases at the source, accelerating the diagnosis of such diseases as irritable bowel syndrome (IBS) and IBD.
“By measuring gases at their source, the accuracy is far better than a breath test,” said Malcolm Hebblewhite, MBA, CEO of Atmo Biosciences. The capsule is an alternative to more invasive and expensive diagnostic tools and it is highly accurate.
Providing examples, Mr. Hebblewhite said that elevated levels of oxygen suggest a disorder of motility while an elevated level of carbon dioxide and hydrogen suggest IBS. The capsule transmits data to a small receiver and then on to a smartphone.
“The real-time data is displayed for the user with more complex information accessible by the practitioner remotely via the cloud,” Mr. Hebblewhite said. He cited several papers that have already been published documenting the potential of this technology.
“The capsule is a single-use disposable device that is not retrieved,” according to Mr. Hebblewhite. He reported that his company plans to pursue the diagnosis of motility as an initial clinical application. The diagnosis of IBS and other GI conditions will follow. Clinical studies are already planned.
SAN FRANCISCO – All five innovative startups pitched at the Shark Tank at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology, are in advanced stages of development, but only one is given the opportunity to be declared the winner of the competition. The ideas ranged from a smart toilet for early disease detection to a unique strategy for obesity phenotyping, but the winner by both official decision and popular vote was a smartphone app to help patients with inflammatory bowel disease (IBD) manage the condition.
“As always, this year’s Shark Tank was a highlight of the AGA Tech Summit and represents the progress our field is making when it comes to innovation. Our panel of sharks was focused on understanding the problem each innovation solved – that’s the key when determining if an idea is novel or innovation for innovation’s sake. We were impressed with all of the technologies presented, but ultimately chose the Oshi Health IBD app as our winner because of the impact it is already having on improving the health and care of IBD patients,” said V. Raman Muthusamy, MD, AGAF, chair of the AGA Center for GI Innovation and Technology.
The winner: Oshi pitches “all-in-one” IBD app
By both popular vote from those attending the AGA Tech Summit as well as the six-member Shark Tank panel, Oshi Health was selected as the 2019 Shark Tank winner for its IBD app. The app was designed to help patients track symptoms, a first step in understanding flare patterns, which differ substantially between patients and emphasize the need for a personalized plan for controlling disease.

“Since we launched last June at DDW® we have had 40,000 downloads. We are the number one IBD management app,” reported Dan Weinstein, MBA, CEO of Oshi Health.
The available app represents the first of three phases as the functionality is expanded. Currently, in addition to using the app as a tracking tool, patients can find resources to learn about their disease and to communicate with other patients about their experiences. In a second phase, information gathered by the app will be made available to physicians to provide accurate current information about disease status to better individualize therapy.
Ultimately, the app is expected to guide treatment based on information it has collected on symptom patterns and other data collected over time, although this application is further down the road and will require regulatory approval if it is designed to provide clinical advice as expected, according to Mr. Weinstein.
However, benefits have already been seen. Mr. Weinstein cited data that associated the app with a 40% improvement in medication adherence and a nearly 60-day reduction in flare duration. Calling the app “the next chapter in treat-to-target” IBD management, he believes that this is an important step forward in digital health that will improve IBD outcomes. The Shark Tank panel agreed.
Runners-up: Other potential innovations to improve GI health
With or without Shark Tank endorsement, the other four startups described in the competition are moving forward. Each is designed to address an important unmet need with the potential to improve patient outcomes, which is a criterion for their inclusion in the competition.
The smart toilet seat
One involves a technologically advanced toilet seat. The new seat is based on the fact that fecal matter provides insight into a broad array of disease states, but specimen collection is a hurdle for a variety of reasons, including patient resistance. A toilet seat developed by Toi Labs, called TrueLoo, is equipped with lighting and cameras that captures images of bowel movements and urination for subsequent analysis.
“The toilet seat sees what the eye cannot,” according to Vikram Kashyap, CEO of Toi Labs. He believes it has major potential for early detection of conditions ranging from dehydration to gastrointestinal cancer.

Others agree. According to Mr. Kashyap, executives of a chain of senior living facilities have already expressed interest in installing this seat to better monitor health among residents. The seat is bolted into position in place of any standard toilet seat. It collects images and data that are transmitted directly to a cellular network.
“Using our technology, the goal is to catch disease states early before they progress,” said Mr. Kashyap, who called the surveillance system a low-cost disease-screening tool. He believes the smart toilet seat could be of the most important disease detection devices developed in recent years.
AI to aid screening endoscopy
A third entrant in this year’s Shark Tank described a strategy to employ artificial intelligence (AI) to aid endoscopists in screening for dysplasia. The tool is called Ultivision and is being developed by a startup called Docbot. The CEO, Andrew Ninh, and a senior executive, Jason B. Samarasena, MD, outlined an idea that could be used in either screening colonoscopy or in surveillance of Barrett’s esophagus).
“Dysplasia is difficult to find. It is subtle and it is often missed. With better detection of dysplasia, artificial intelligence offers an opportunity to reduce risk of cancer,” Dr. Samarasena said.
The tool integrates seamlessly with existing endoscopic tools, according to Mr. Ninh. As tissue is visualized, the AI is programmed to highlight suspected dysplasia with a colored box to alert the endoscopist. The colonoscopy application is a more advanced stage of development and might be submitted for regulatory approval this year, he said. The same technology will be adapted for Barrett’s esophagus.
“It is like facial recognition for dysplasia,” said Dr. Samarasena.
Obesity phenotyping tool
A fourth Shark Tank entrant employs technology to phenotype obese patients to better tailor therapy. The Pheno Test, developed by Phenomix Sciences, applies “multi-omics” to a blood-based test to separate patients with obesity into four phenotypes. When therapy is tailored to the phenotype, weight loss is greater, according to Andres J. Acosta, MD, PhD, assistant professor of medicine and consultant in gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
In an initial study that compared weight loss in 55 patients treated based on phenotype with 175 patients managed with standard of care, the total body weight loss “more than doubled,” Dr. Acosta reported.
According to Dr. Acosta, obesity is driven by very different mechanisms. He described the four major phenotypes identified with his test as hungry brain (satiation signal is impaired), hungry gut (signals to eat are upregulated), emotional hunger (psychological reasons drive eating behavior), and slow metabolism (failure to burn fat at normal rates).
With the blood test, which utilizes hormones, metabolites, DNA, and other biomarkers to separate these phenotypes, treatment can be tailored appropriately, according to Dr. Acosta. His company is now seeking Food and Drug Administration clearance of the test, which he believes will have a major impact on obesity control.
Capsule diagnostic tool
The final entrant selected to participate in this year’s Shark Tank described an ingestible capsule that diagnoses diseases by detecting gases as it descends the gastrointestinal tract. The Atmo Gas Capsule from Atmo Biosciences measures gases at the source, accelerating the diagnosis of such diseases as irritable bowel syndrome (IBS) and IBD.
“By measuring gases at their source, the accuracy is far better than a breath test,” said Malcolm Hebblewhite, MBA, CEO of Atmo Biosciences. The capsule is an alternative to more invasive and expensive diagnostic tools and it is highly accurate.
Providing examples, Mr. Hebblewhite said that elevated levels of oxygen suggest a disorder of motility while an elevated level of carbon dioxide and hydrogen suggest IBS. The capsule transmits data to a small receiver and then on to a smartphone.
“The real-time data is displayed for the user with more complex information accessible by the practitioner remotely via the cloud,” Mr. Hebblewhite said. He cited several papers that have already been published documenting the potential of this technology.
“The capsule is a single-use disposable device that is not retrieved,” according to Mr. Hebblewhite. He reported that his company plans to pursue the diagnosis of motility as an initial clinical application. The diagnosis of IBS and other GI conditions will follow. Clinical studies are already planned.
SAN FRANCISCO – All five innovative startups pitched at the Shark Tank at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology, are in advanced stages of development, but only one is given the opportunity to be declared the winner of the competition. The ideas ranged from a smart toilet for early disease detection to a unique strategy for obesity phenotyping, but the winner by both official decision and popular vote was a smartphone app to help patients with inflammatory bowel disease (IBD) manage the condition.
“As always, this year’s Shark Tank was a highlight of the AGA Tech Summit and represents the progress our field is making when it comes to innovation. Our panel of sharks was focused on understanding the problem each innovation solved – that’s the key when determining if an idea is novel or innovation for innovation’s sake. We were impressed with all of the technologies presented, but ultimately chose the Oshi Health IBD app as our winner because of the impact it is already having on improving the health and care of IBD patients,” said V. Raman Muthusamy, MD, AGAF, chair of the AGA Center for GI Innovation and Technology.
The winner: Oshi pitches “all-in-one” IBD app
By both popular vote from those attending the AGA Tech Summit as well as the six-member Shark Tank panel, Oshi Health was selected as the 2019 Shark Tank winner for its IBD app. The app was designed to help patients track symptoms, a first step in understanding flare patterns, which differ substantially between patients and emphasize the need for a personalized plan for controlling disease.

“Since we launched last June at DDW® we have had 40,000 downloads. We are the number one IBD management app,” reported Dan Weinstein, MBA, CEO of Oshi Health.
The available app represents the first of three phases as the functionality is expanded. Currently, in addition to using the app as a tracking tool, patients can find resources to learn about their disease and to communicate with other patients about their experiences. In a second phase, information gathered by the app will be made available to physicians to provide accurate current information about disease status to better individualize therapy.
Ultimately, the app is expected to guide treatment based on information it has collected on symptom patterns and other data collected over time, although this application is further down the road and will require regulatory approval if it is designed to provide clinical advice as expected, according to Mr. Weinstein.
However, benefits have already been seen. Mr. Weinstein cited data that associated the app with a 40% improvement in medication adherence and a nearly 60-day reduction in flare duration. Calling the app “the next chapter in treat-to-target” IBD management, he believes that this is an important step forward in digital health that will improve IBD outcomes. The Shark Tank panel agreed.
Runners-up: Other potential innovations to improve GI health
With or without Shark Tank endorsement, the other four startups described in the competition are moving forward. Each is designed to address an important unmet need with the potential to improve patient outcomes, which is a criterion for their inclusion in the competition.
The smart toilet seat
One involves a technologically advanced toilet seat. The new seat is based on the fact that fecal matter provides insight into a broad array of disease states, but specimen collection is a hurdle for a variety of reasons, including patient resistance. A toilet seat developed by Toi Labs, called TrueLoo, is equipped with lighting and cameras that captures images of bowel movements and urination for subsequent analysis.
“The toilet seat sees what the eye cannot,” according to Vikram Kashyap, CEO of Toi Labs. He believes it has major potential for early detection of conditions ranging from dehydration to gastrointestinal cancer.

Others agree. According to Mr. Kashyap, executives of a chain of senior living facilities have already expressed interest in installing this seat to better monitor health among residents. The seat is bolted into position in place of any standard toilet seat. It collects images and data that are transmitted directly to a cellular network.
“Using our technology, the goal is to catch disease states early before they progress,” said Mr. Kashyap, who called the surveillance system a low-cost disease-screening tool. He believes the smart toilet seat could be of the most important disease detection devices developed in recent years.
AI to aid screening endoscopy
A third entrant in this year’s Shark Tank described a strategy to employ artificial intelligence (AI) to aid endoscopists in screening for dysplasia. The tool is called Ultivision and is being developed by a startup called Docbot. The CEO, Andrew Ninh, and a senior executive, Jason B. Samarasena, MD, outlined an idea that could be used in either screening colonoscopy or in surveillance of Barrett’s esophagus).
“Dysplasia is difficult to find. It is subtle and it is often missed. With better detection of dysplasia, artificial intelligence offers an opportunity to reduce risk of cancer,” Dr. Samarasena said.
The tool integrates seamlessly with existing endoscopic tools, according to Mr. Ninh. As tissue is visualized, the AI is programmed to highlight suspected dysplasia with a colored box to alert the endoscopist. The colonoscopy application is a more advanced stage of development and might be submitted for regulatory approval this year, he said. The same technology will be adapted for Barrett’s esophagus.
“It is like facial recognition for dysplasia,” said Dr. Samarasena.
Obesity phenotyping tool
A fourth Shark Tank entrant employs technology to phenotype obese patients to better tailor therapy. The Pheno Test, developed by Phenomix Sciences, applies “multi-omics” to a blood-based test to separate patients with obesity into four phenotypes. When therapy is tailored to the phenotype, weight loss is greater, according to Andres J. Acosta, MD, PhD, assistant professor of medicine and consultant in gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
In an initial study that compared weight loss in 55 patients treated based on phenotype with 175 patients managed with standard of care, the total body weight loss “more than doubled,” Dr. Acosta reported.
According to Dr. Acosta, obesity is driven by very different mechanisms. He described the four major phenotypes identified with his test as hungry brain (satiation signal is impaired), hungry gut (signals to eat are upregulated), emotional hunger (psychological reasons drive eating behavior), and slow metabolism (failure to burn fat at normal rates).
With the blood test, which utilizes hormones, metabolites, DNA, and other biomarkers to separate these phenotypes, treatment can be tailored appropriately, according to Dr. Acosta. His company is now seeking Food and Drug Administration clearance of the test, which he believes will have a major impact on obesity control.
Capsule diagnostic tool
The final entrant selected to participate in this year’s Shark Tank described an ingestible capsule that diagnoses diseases by detecting gases as it descends the gastrointestinal tract. The Atmo Gas Capsule from Atmo Biosciences measures gases at the source, accelerating the diagnosis of such diseases as irritable bowel syndrome (IBS) and IBD.
“By measuring gases at their source, the accuracy is far better than a breath test,” said Malcolm Hebblewhite, MBA, CEO of Atmo Biosciences. The capsule is an alternative to more invasive and expensive diagnostic tools and it is highly accurate.
Providing examples, Mr. Hebblewhite said that elevated levels of oxygen suggest a disorder of motility while an elevated level of carbon dioxide and hydrogen suggest IBS. The capsule transmits data to a small receiver and then on to a smartphone.
“The real-time data is displayed for the user with more complex information accessible by the practitioner remotely via the cloud,” Mr. Hebblewhite said. He cited several papers that have already been published documenting the potential of this technology.
“The capsule is a single-use disposable device that is not retrieved,” according to Mr. Hebblewhite. He reported that his company plans to pursue the diagnosis of motility as an initial clinical application. The diagnosis of IBS and other GI conditions will follow. Clinical studies are already planned.
REPORTING FROM 2019 AGA TECH SUMMIT
Telemedicine proving to be an efficient platform for delivering nutritional services
SAN FRANCISCO – A telemedicine platform that connects patients to registered dietitians is providing a solution for a number of interrelated unmet needs, according to a description at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
Although not limited to patients with gastrointestinal diseases, the applications of this platform are illustrative of the value to both patients and the physicians who prescribe diet as part of the management of chronic conditions, according to Jonah Cohen, MD, who is a founder of the digital therapeutics company Nutrimedy, which created the platform for virtual nutritional counseling.
“As gastroenterologists, we are experts in the function of the gastrointestinal tract but not necessarily in nutrition. Most physicians get very little training in this area,” said Dr. Cohen, who is a gastroenterologist affiliated with Harvard Medical School and Beth Israel Deaconess Medical Center in Boston.
Even for those physicians who have expertise and an interest in nutrition, dietary counseling requires a substantial investment of time and continuity to affect behavior change. Helping patients develop an effective diet and implementation strategy to which they are willing to adhere is not a simple task. It requires recognizing and managing nuanced preferences and tastes. For most patients, frequent engagement with an expert is essential to remain on track.
“Nutrimedy’s proprietary matching system connects patients to their ideal dietitian who will help establish a specific nutrition plan for their medical conditions through video visits, unlimited messaging, photo food logs, recipes, and biometric trackers,” Dr. Cohen explained. “The key to the success of these relationships is based on the ease of clinical touch points we’re able to achieve through telenutrition when patients can ask questions, make modifications, and get positive feedback on their own time.”
Launched in 2016, Nutrimedy now has over 1,000 dietitians on its roster and a HIPAA-compliant device-agnostic platform to deliver best-in-class nutritional care remotely.
“The breadth of expertise of our providers enables us to provide medical nutrition therapy across a range of conditions, including irritable bowel syndrome (IBS), celiac disease, acid reflux, fatty liver disease, and gastroparesis to name just several, for patients anytime, anywhere,” according to Dr. Cohen.
In many places within the United States, there are few expert nutritionists with the specific expertise needed to manage a disease condition like IBS through low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet, and thus appointments are often hard to get for these providers, according to Dr. Cohen. Moreover, he explained that office visits are not just inconvenient but can be an obstacle to a successfully implemented dietary plan.
Other challenges may include cultural or language barriers. Dr. Cohen gave the example of Sophia, a busy mother of two with IBS, who is vegetarian, eats predominantly Spanish cuisine, lives in rural Massachusetts, and speaks Spanish. She prefers not to take medications and hopes to better manage her condition with a low FODMAP diet.
Nutrimedy draws on its roster of registered dietitians to find a good match for patients like Sophia. “Our vision is that everyone deserves to have an expert literally in their back pocket to help them on their journey to better health through food as medicine,” Dr. Cohen explained. He called Nutrimedy “a turn-key solution for GI practices who want to improve medical nutrition therapy for their patients.”
According to Dr. Cohen, Nutrimedy has already proven effective for its core mission of making effective nutritional counseling easier to obtain, and is now working to extend its reach. For example, Dr. Cohen said that the company has actively engaged with employers to provide corporate wellness solutions, and it is partnering with pharmaceutical and other life sciences companies who offer therapies relevant to nutritional health where Nutrimedy has potential to serve as a digital therapeutic companion.
“In almost every chronic condition, diet plays an important role in disease prevention or management,” said Dr. Cohen who believes his company is participating in the effort to reduce the burden of chronic disease related to poor diet. “I feel that in 2019 we’re at a tipping point where health care entities are finally recognizing that we can transform wellness in America through healthier eating.” He believes that Nutrimedy is poised “to play a part in this revolution.
SAN FRANCISCO – A telemedicine platform that connects patients to registered dietitians is providing a solution for a number of interrelated unmet needs, according to a description at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
Although not limited to patients with gastrointestinal diseases, the applications of this platform are illustrative of the value to both patients and the physicians who prescribe diet as part of the management of chronic conditions, according to Jonah Cohen, MD, who is a founder of the digital therapeutics company Nutrimedy, which created the platform for virtual nutritional counseling.
“As gastroenterologists, we are experts in the function of the gastrointestinal tract but not necessarily in nutrition. Most physicians get very little training in this area,” said Dr. Cohen, who is a gastroenterologist affiliated with Harvard Medical School and Beth Israel Deaconess Medical Center in Boston.
Even for those physicians who have expertise and an interest in nutrition, dietary counseling requires a substantial investment of time and continuity to affect behavior change. Helping patients develop an effective diet and implementation strategy to which they are willing to adhere is not a simple task. It requires recognizing and managing nuanced preferences and tastes. For most patients, frequent engagement with an expert is essential to remain on track.
“Nutrimedy’s proprietary matching system connects patients to their ideal dietitian who will help establish a specific nutrition plan for their medical conditions through video visits, unlimited messaging, photo food logs, recipes, and biometric trackers,” Dr. Cohen explained. “The key to the success of these relationships is based on the ease of clinical touch points we’re able to achieve through telenutrition when patients can ask questions, make modifications, and get positive feedback on their own time.”
Launched in 2016, Nutrimedy now has over 1,000 dietitians on its roster and a HIPAA-compliant device-agnostic platform to deliver best-in-class nutritional care remotely.
“The breadth of expertise of our providers enables us to provide medical nutrition therapy across a range of conditions, including irritable bowel syndrome (IBS), celiac disease, acid reflux, fatty liver disease, and gastroparesis to name just several, for patients anytime, anywhere,” according to Dr. Cohen.
In many places within the United States, there are few expert nutritionists with the specific expertise needed to manage a disease condition like IBS through low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet, and thus appointments are often hard to get for these providers, according to Dr. Cohen. Moreover, he explained that office visits are not just inconvenient but can be an obstacle to a successfully implemented dietary plan.
Other challenges may include cultural or language barriers. Dr. Cohen gave the example of Sophia, a busy mother of two with IBS, who is vegetarian, eats predominantly Spanish cuisine, lives in rural Massachusetts, and speaks Spanish. She prefers not to take medications and hopes to better manage her condition with a low FODMAP diet.
Nutrimedy draws on its roster of registered dietitians to find a good match for patients like Sophia. “Our vision is that everyone deserves to have an expert literally in their back pocket to help them on their journey to better health through food as medicine,” Dr. Cohen explained. He called Nutrimedy “a turn-key solution for GI practices who want to improve medical nutrition therapy for their patients.”
According to Dr. Cohen, Nutrimedy has already proven effective for its core mission of making effective nutritional counseling easier to obtain, and is now working to extend its reach. For example, Dr. Cohen said that the company has actively engaged with employers to provide corporate wellness solutions, and it is partnering with pharmaceutical and other life sciences companies who offer therapies relevant to nutritional health where Nutrimedy has potential to serve as a digital therapeutic companion.
“In almost every chronic condition, diet plays an important role in disease prevention or management,” said Dr. Cohen who believes his company is participating in the effort to reduce the burden of chronic disease related to poor diet. “I feel that in 2019 we’re at a tipping point where health care entities are finally recognizing that we can transform wellness in America through healthier eating.” He believes that Nutrimedy is poised “to play a part in this revolution.
SAN FRANCISCO – A telemedicine platform that connects patients to registered dietitians is providing a solution for a number of interrelated unmet needs, according to a description at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
Although not limited to patients with gastrointestinal diseases, the applications of this platform are illustrative of the value to both patients and the physicians who prescribe diet as part of the management of chronic conditions, according to Jonah Cohen, MD, who is a founder of the digital therapeutics company Nutrimedy, which created the platform for virtual nutritional counseling.
“As gastroenterologists, we are experts in the function of the gastrointestinal tract but not necessarily in nutrition. Most physicians get very little training in this area,” said Dr. Cohen, who is a gastroenterologist affiliated with Harvard Medical School and Beth Israel Deaconess Medical Center in Boston.
Even for those physicians who have expertise and an interest in nutrition, dietary counseling requires a substantial investment of time and continuity to affect behavior change. Helping patients develop an effective diet and implementation strategy to which they are willing to adhere is not a simple task. It requires recognizing and managing nuanced preferences and tastes. For most patients, frequent engagement with an expert is essential to remain on track.
“Nutrimedy’s proprietary matching system connects patients to their ideal dietitian who will help establish a specific nutrition plan for their medical conditions through video visits, unlimited messaging, photo food logs, recipes, and biometric trackers,” Dr. Cohen explained. “The key to the success of these relationships is based on the ease of clinical touch points we’re able to achieve through telenutrition when patients can ask questions, make modifications, and get positive feedback on their own time.”
Launched in 2016, Nutrimedy now has over 1,000 dietitians on its roster and a HIPAA-compliant device-agnostic platform to deliver best-in-class nutritional care remotely.
“The breadth of expertise of our providers enables us to provide medical nutrition therapy across a range of conditions, including irritable bowel syndrome (IBS), celiac disease, acid reflux, fatty liver disease, and gastroparesis to name just several, for patients anytime, anywhere,” according to Dr. Cohen.
In many places within the United States, there are few expert nutritionists with the specific expertise needed to manage a disease condition like IBS through low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet, and thus appointments are often hard to get for these providers, according to Dr. Cohen. Moreover, he explained that office visits are not just inconvenient but can be an obstacle to a successfully implemented dietary plan.
Other challenges may include cultural or language barriers. Dr. Cohen gave the example of Sophia, a busy mother of two with IBS, who is vegetarian, eats predominantly Spanish cuisine, lives in rural Massachusetts, and speaks Spanish. She prefers not to take medications and hopes to better manage her condition with a low FODMAP diet.
Nutrimedy draws on its roster of registered dietitians to find a good match for patients like Sophia. “Our vision is that everyone deserves to have an expert literally in their back pocket to help them on their journey to better health through food as medicine,” Dr. Cohen explained. He called Nutrimedy “a turn-key solution for GI practices who want to improve medical nutrition therapy for their patients.”
According to Dr. Cohen, Nutrimedy has already proven effective for its core mission of making effective nutritional counseling easier to obtain, and is now working to extend its reach. For example, Dr. Cohen said that the company has actively engaged with employers to provide corporate wellness solutions, and it is partnering with pharmaceutical and other life sciences companies who offer therapies relevant to nutritional health where Nutrimedy has potential to serve as a digital therapeutic companion.
“In almost every chronic condition, diet plays an important role in disease prevention or management,” said Dr. Cohen who believes his company is participating in the effort to reduce the burden of chronic disease related to poor diet. “I feel that in 2019 we’re at a tipping point where health care entities are finally recognizing that we can transform wellness in America through healthier eating.” He believes that Nutrimedy is poised “to play a part in this revolution.
EXPERT ANALYSIS FROM 2019 AGA TECH SUMMIT
Adoption rates high for smartphone tool that prepares patients for procedures
SAN FRANCISCO – Patients are being better prepared for medical procedures, such as screening colonoscopy, through a new service based on smartphone texts that remind patients of steps to take prior to their procedure, according to a description at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“The reminders mean fewer no shows and fewer cancellations, but in the case of colonoscopy we are also seeing better rates of adequate bowel prep and lower rates of aborted procedures,” reported Andy Pfau, chief operating officer, RxHealth, New York.
The texts are based on a patient-care pathway integrated with the electronic medical record (EMR) system. In the case of colonoscopy, once a physician creates an appointment in the EMR for a colonoscopy and selects the bowel prep, the system takes over, automatically obtaining access to the patient’s cell phone number in order to send reminder texts at intervals relevant to their appointment.
“The texts provide links to additional information so that patients are not only reminded to begin their bowel prep but can access instructions and supportive educational material,” Mr. Pfau explained.
“The messaging is not just limited to reminders. We can provide driving and parking instructions. We have also partnered with ride sharing companies to make it easier for patients to get to the facility. We can adjust the platform in a variety of ways to help patients show up prepared for the procedure,” he added.
The commercial tool, known as RxUniverse, was a spin off of a program developed at the Icahn School of Medicine at Mount Sinai. The problem of no shows and the importance of bowel prep makes this service particularly attractive in colonoscopy, according to Mr. Pfau, who cited data associating this tool with a 34% improvement in bowel preps and a more than 90% rate of patient satisfaction.
The same approach can and already is being employed in other procedures in GI, such as guiding patients scheduled for an upper endoscopy.
“We see a large role for this tool in comprehensive care plans because it is versatile and could be applied to a variety of care pathways, including forms of telemedicine, where timely communication through smartphone messaging could help patients adhere to goals of treatment,” Mr. Pfau said.
The tool can already be integrated with four EMR systems, including EPIC, but Mr. Pfau said the tool will ultimately be EMR agonistic according to current plans. While the company spun out of Mount Sinai in late 2016, RxHealth began marketing the RxUniverse prescription platform in earnest in 2018.
“In the last eight or so months, growth has been exponential,” Mr. Pfau said. In addition to growth in the U.S., the program is now being marketed overseas. He named several large medical systems that have already adopted the technology, including the Arizona Centers for Digestive Health and Yale New Haven Health. The American Gastroenterological Association, recognizing the value of this innovation for GI, has also partnered with RxHealth to help bring this product to its members.
As the automated pathway is integrated into existing EMR systems, the per-patient cost of the pathway is relatively low, according to Mr. Pfau. In situations in which the service increases the proportion of procedures completed successfully, it is reasonable to expect a highly favorable return on investment.
“There is a lot of interest in the potential of mobile devices to be employed in various ways to communicate and inform patients. This is part of that, and we think it is just the beginning. We are looking at a number of ways in which we can expand the platform and make it even more valuable,” Mr. Pfau said.
SAN FRANCISCO – Patients are being better prepared for medical procedures, such as screening colonoscopy, through a new service based on smartphone texts that remind patients of steps to take prior to their procedure, according to a description at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“The reminders mean fewer no shows and fewer cancellations, but in the case of colonoscopy we are also seeing better rates of adequate bowel prep and lower rates of aborted procedures,” reported Andy Pfau, chief operating officer, RxHealth, New York.
The texts are based on a patient-care pathway integrated with the electronic medical record (EMR) system. In the case of colonoscopy, once a physician creates an appointment in the EMR for a colonoscopy and selects the bowel prep, the system takes over, automatically obtaining access to the patient’s cell phone number in order to send reminder texts at intervals relevant to their appointment.
“The texts provide links to additional information so that patients are not only reminded to begin their bowel prep but can access instructions and supportive educational material,” Mr. Pfau explained.
“The messaging is not just limited to reminders. We can provide driving and parking instructions. We have also partnered with ride sharing companies to make it easier for patients to get to the facility. We can adjust the platform in a variety of ways to help patients show up prepared for the procedure,” he added.
The commercial tool, known as RxUniverse, was a spin off of a program developed at the Icahn School of Medicine at Mount Sinai. The problem of no shows and the importance of bowel prep makes this service particularly attractive in colonoscopy, according to Mr. Pfau, who cited data associating this tool with a 34% improvement in bowel preps and a more than 90% rate of patient satisfaction.
The same approach can and already is being employed in other procedures in GI, such as guiding patients scheduled for an upper endoscopy.
“We see a large role for this tool in comprehensive care plans because it is versatile and could be applied to a variety of care pathways, including forms of telemedicine, where timely communication through smartphone messaging could help patients adhere to goals of treatment,” Mr. Pfau said.
The tool can already be integrated with four EMR systems, including EPIC, but Mr. Pfau said the tool will ultimately be EMR agonistic according to current plans. While the company spun out of Mount Sinai in late 2016, RxHealth began marketing the RxUniverse prescription platform in earnest in 2018.
“In the last eight or so months, growth has been exponential,” Mr. Pfau said. In addition to growth in the U.S., the program is now being marketed overseas. He named several large medical systems that have already adopted the technology, including the Arizona Centers for Digestive Health and Yale New Haven Health. The American Gastroenterological Association, recognizing the value of this innovation for GI, has also partnered with RxHealth to help bring this product to its members.
As the automated pathway is integrated into existing EMR systems, the per-patient cost of the pathway is relatively low, according to Mr. Pfau. In situations in which the service increases the proportion of procedures completed successfully, it is reasonable to expect a highly favorable return on investment.
“There is a lot of interest in the potential of mobile devices to be employed in various ways to communicate and inform patients. This is part of that, and we think it is just the beginning. We are looking at a number of ways in which we can expand the platform and make it even more valuable,” Mr. Pfau said.
SAN FRANCISCO – Patients are being better prepared for medical procedures, such as screening colonoscopy, through a new service based on smartphone texts that remind patients of steps to take prior to their procedure, according to a description at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“The reminders mean fewer no shows and fewer cancellations, but in the case of colonoscopy we are also seeing better rates of adequate bowel prep and lower rates of aborted procedures,” reported Andy Pfau, chief operating officer, RxHealth, New York.
The texts are based on a patient-care pathway integrated with the electronic medical record (EMR) system. In the case of colonoscopy, once a physician creates an appointment in the EMR for a colonoscopy and selects the bowel prep, the system takes over, automatically obtaining access to the patient’s cell phone number in order to send reminder texts at intervals relevant to their appointment.
“The texts provide links to additional information so that patients are not only reminded to begin their bowel prep but can access instructions and supportive educational material,” Mr. Pfau explained.
“The messaging is not just limited to reminders. We can provide driving and parking instructions. We have also partnered with ride sharing companies to make it easier for patients to get to the facility. We can adjust the platform in a variety of ways to help patients show up prepared for the procedure,” he added.
The commercial tool, known as RxUniverse, was a spin off of a program developed at the Icahn School of Medicine at Mount Sinai. The problem of no shows and the importance of bowel prep makes this service particularly attractive in colonoscopy, according to Mr. Pfau, who cited data associating this tool with a 34% improvement in bowel preps and a more than 90% rate of patient satisfaction.
The same approach can and already is being employed in other procedures in GI, such as guiding patients scheduled for an upper endoscopy.
“We see a large role for this tool in comprehensive care plans because it is versatile and could be applied to a variety of care pathways, including forms of telemedicine, where timely communication through smartphone messaging could help patients adhere to goals of treatment,” Mr. Pfau said.
The tool can already be integrated with four EMR systems, including EPIC, but Mr. Pfau said the tool will ultimately be EMR agonistic according to current plans. While the company spun out of Mount Sinai in late 2016, RxHealth began marketing the RxUniverse prescription platform in earnest in 2018.
“In the last eight or so months, growth has been exponential,” Mr. Pfau said. In addition to growth in the U.S., the program is now being marketed overseas. He named several large medical systems that have already adopted the technology, including the Arizona Centers for Digestive Health and Yale New Haven Health. The American Gastroenterological Association, recognizing the value of this innovation for GI, has also partnered with RxHealth to help bring this product to its members.
As the automated pathway is integrated into existing EMR systems, the per-patient cost of the pathway is relatively low, according to Mr. Pfau. In situations in which the service increases the proportion of procedures completed successfully, it is reasonable to expect a highly favorable return on investment.
“There is a lot of interest in the potential of mobile devices to be employed in various ways to communicate and inform patients. This is part of that, and we think it is just the beginning. We are looking at a number of ways in which we can expand the platform and make it even more valuable,” Mr. Pfau said.
EXPERT ANALYSIS FROM 2019 AGA TECH SUMMIT
Evidence for endoscopic GERD treatments approaching critical mass
SAN FRANCISCO – It is now reasonable to conclude that many of the endoscopic devices and procedures developed for the treatment of gastroesophageal reflux disease (GERD) offer good short-term efficacy, leaving only the task to understand how these fit with competing options to improve quality of life long term, according to a state-of-the-art summary at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“The quality of the data for many of these devices has improved substantially, putting us in a much better place than we were in 4 or 5 years ago in considering their role,” reported Michael F. Vaezi, MD, PhD, professor of medicine, Vanderbilt University, Nashville, Tenn.
Over the past 15 years, an array of endoscopic approaches to treatment of GERD has received FDA approval. Examples of the very different techniques include plication devices that can suture, staple, or otherwise prevent reflux at the gastroesophageal junction (GEJ), and interventions aimed at the lower esophageal sphincter (LES), where placement of magnets or radiofrequency ablation has been employed to achieve a tighter defense against transient reflux episodes.
Despite FDA approval, the supportive evidence for many of these endoscopic interventions was criticized. In some cases, the number of patients evaluated in pivotal studies was considered too small. In others, there were objections to methodology, particularly to the choice of control arm. In all cases, there has been concern that follow-up was insufficient to confirm persistent benefit. Many of these criticisms are dissipating under the weight of more data.
“For most of the currently available, FDA-approved devices, there is now a substantial body of at least short-term data showing efficacy and safety,” reported Dr. Vaezi, who is a coauthor of an expert review now being prepared for publication. “This includes evidence that they improve quality of life, reduce the need for acid-suppressing therapy, and reduce esophageal acid exposure.”
Additional follow-up represents the final hurdle for understanding how these endoscopic interventions fit for extended symptom control. The long-term efficacy of the current standards of chronic proton pump inhibitor (PPI) therapy and surgical fundoplication has been established. Among these options, the choice is indefinite pharmacologic therapy or a surgical procedure. Endoscopic devices add additional options, but not with clear conclusions to be drawn on persistence of benefit.
Patient selection is an important consideration. Dr. Vaezi outlined three groups of patients: Patients who have responded to once-daily PPIs and are doing well, but would prefer not to take them indefinitely; PPI non-responders; and patients with improved heartburn but no improvement in regurgitation. Responders are reasonable candidates for endoscopic interventions, but non-responders are not, according to Dr. Vaezi. “You’re exposing the patient to the risk without the benefit, because they don’t have reflux. It’s something else,” he said.
Patients with improved heartburn but no change in regurgitation may be a candidate for endoscopic devices, as long as the clinician rules out non-reflux causes such as achalasia or gastroparesis.
“In patients being considered for alternative nonmedical therapy, it is essential to show that their symptoms are acid related. Those who do not respond to a PPI have traditionally not been good surgical candidates because the lack of a response suggests that acid reflux is not the source of their complaints. For patients being considered for an endoscopic treatment, we must apply the same time proven strategy. At this point, what is uncertain about the device therapies is the long-term durability for reflux control,” Dr. Vaezi said.
PPIs are effective for acid control, so the reason to consider an invasive treatment strategy is to avoid chronic PPI treatment. This is an increasingly attractive goal for many patients as a result of well-publicized case-control studies associating PPI use with a variety of increased risks, such as osteoporosis, chronic kidney disease, and gastrointestinal infections, but many gastroenterologists have been slow to recommend endoscopic interventions due to enduring concerns about safety and efficacy.
From his survey of the evidence, Dr. Vaezi characterized himself as “cautiously optimistic” that many of the endoscopic interventions will be included among standard options for durable GERD treatment.
SAN FRANCISCO – It is now reasonable to conclude that many of the endoscopic devices and procedures developed for the treatment of gastroesophageal reflux disease (GERD) offer good short-term efficacy, leaving only the task to understand how these fit with competing options to improve quality of life long term, according to a state-of-the-art summary at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“The quality of the data for many of these devices has improved substantially, putting us in a much better place than we were in 4 or 5 years ago in considering their role,” reported Michael F. Vaezi, MD, PhD, professor of medicine, Vanderbilt University, Nashville, Tenn.
Over the past 15 years, an array of endoscopic approaches to treatment of GERD has received FDA approval. Examples of the very different techniques include plication devices that can suture, staple, or otherwise prevent reflux at the gastroesophageal junction (GEJ), and interventions aimed at the lower esophageal sphincter (LES), where placement of magnets or radiofrequency ablation has been employed to achieve a tighter defense against transient reflux episodes.
Despite FDA approval, the supportive evidence for many of these endoscopic interventions was criticized. In some cases, the number of patients evaluated in pivotal studies was considered too small. In others, there were objections to methodology, particularly to the choice of control arm. In all cases, there has been concern that follow-up was insufficient to confirm persistent benefit. Many of these criticisms are dissipating under the weight of more data.
“For most of the currently available, FDA-approved devices, there is now a substantial body of at least short-term data showing efficacy and safety,” reported Dr. Vaezi, who is a coauthor of an expert review now being prepared for publication. “This includes evidence that they improve quality of life, reduce the need for acid-suppressing therapy, and reduce esophageal acid exposure.”
Additional follow-up represents the final hurdle for understanding how these endoscopic interventions fit for extended symptom control. The long-term efficacy of the current standards of chronic proton pump inhibitor (PPI) therapy and surgical fundoplication has been established. Among these options, the choice is indefinite pharmacologic therapy or a surgical procedure. Endoscopic devices add additional options, but not with clear conclusions to be drawn on persistence of benefit.
Patient selection is an important consideration. Dr. Vaezi outlined three groups of patients: Patients who have responded to once-daily PPIs and are doing well, but would prefer not to take them indefinitely; PPI non-responders; and patients with improved heartburn but no improvement in regurgitation. Responders are reasonable candidates for endoscopic interventions, but non-responders are not, according to Dr. Vaezi. “You’re exposing the patient to the risk without the benefit, because they don’t have reflux. It’s something else,” he said.
Patients with improved heartburn but no change in regurgitation may be a candidate for endoscopic devices, as long as the clinician rules out non-reflux causes such as achalasia or gastroparesis.
“In patients being considered for alternative nonmedical therapy, it is essential to show that their symptoms are acid related. Those who do not respond to a PPI have traditionally not been good surgical candidates because the lack of a response suggests that acid reflux is not the source of their complaints. For patients being considered for an endoscopic treatment, we must apply the same time proven strategy. At this point, what is uncertain about the device therapies is the long-term durability for reflux control,” Dr. Vaezi said.
PPIs are effective for acid control, so the reason to consider an invasive treatment strategy is to avoid chronic PPI treatment. This is an increasingly attractive goal for many patients as a result of well-publicized case-control studies associating PPI use with a variety of increased risks, such as osteoporosis, chronic kidney disease, and gastrointestinal infections, but many gastroenterologists have been slow to recommend endoscopic interventions due to enduring concerns about safety and efficacy.
From his survey of the evidence, Dr. Vaezi characterized himself as “cautiously optimistic” that many of the endoscopic interventions will be included among standard options for durable GERD treatment.
SAN FRANCISCO – It is now reasonable to conclude that many of the endoscopic devices and procedures developed for the treatment of gastroesophageal reflux disease (GERD) offer good short-term efficacy, leaving only the task to understand how these fit with competing options to improve quality of life long term, according to a state-of-the-art summary at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“The quality of the data for many of these devices has improved substantially, putting us in a much better place than we were in 4 or 5 years ago in considering their role,” reported Michael F. Vaezi, MD, PhD, professor of medicine, Vanderbilt University, Nashville, Tenn.
Over the past 15 years, an array of endoscopic approaches to treatment of GERD has received FDA approval. Examples of the very different techniques include plication devices that can suture, staple, or otherwise prevent reflux at the gastroesophageal junction (GEJ), and interventions aimed at the lower esophageal sphincter (LES), where placement of magnets or radiofrequency ablation has been employed to achieve a tighter defense against transient reflux episodes.
Despite FDA approval, the supportive evidence for many of these endoscopic interventions was criticized. In some cases, the number of patients evaluated in pivotal studies was considered too small. In others, there were objections to methodology, particularly to the choice of control arm. In all cases, there has been concern that follow-up was insufficient to confirm persistent benefit. Many of these criticisms are dissipating under the weight of more data.
“For most of the currently available, FDA-approved devices, there is now a substantial body of at least short-term data showing efficacy and safety,” reported Dr. Vaezi, who is a coauthor of an expert review now being prepared for publication. “This includes evidence that they improve quality of life, reduce the need for acid-suppressing therapy, and reduce esophageal acid exposure.”
Additional follow-up represents the final hurdle for understanding how these endoscopic interventions fit for extended symptom control. The long-term efficacy of the current standards of chronic proton pump inhibitor (PPI) therapy and surgical fundoplication has been established. Among these options, the choice is indefinite pharmacologic therapy or a surgical procedure. Endoscopic devices add additional options, but not with clear conclusions to be drawn on persistence of benefit.
Patient selection is an important consideration. Dr. Vaezi outlined three groups of patients: Patients who have responded to once-daily PPIs and are doing well, but would prefer not to take them indefinitely; PPI non-responders; and patients with improved heartburn but no improvement in regurgitation. Responders are reasonable candidates for endoscopic interventions, but non-responders are not, according to Dr. Vaezi. “You’re exposing the patient to the risk without the benefit, because they don’t have reflux. It’s something else,” he said.
Patients with improved heartburn but no change in regurgitation may be a candidate for endoscopic devices, as long as the clinician rules out non-reflux causes such as achalasia or gastroparesis.
“In patients being considered for alternative nonmedical therapy, it is essential to show that their symptoms are acid related. Those who do not respond to a PPI have traditionally not been good surgical candidates because the lack of a response suggests that acid reflux is not the source of their complaints. For patients being considered for an endoscopic treatment, we must apply the same time proven strategy. At this point, what is uncertain about the device therapies is the long-term durability for reflux control,” Dr. Vaezi said.
PPIs are effective for acid control, so the reason to consider an invasive treatment strategy is to avoid chronic PPI treatment. This is an increasingly attractive goal for many patients as a result of well-publicized case-control studies associating PPI use with a variety of increased risks, such as osteoporosis, chronic kidney disease, and gastrointestinal infections, but many gastroenterologists have been slow to recommend endoscopic interventions due to enduring concerns about safety and efficacy.
From his survey of the evidence, Dr. Vaezi characterized himself as “cautiously optimistic” that many of the endoscopic interventions will be included among standard options for durable GERD treatment.
EXPERT ANALYSIS FROM 2019 AGA TECH SUMMIT