User login
Liquid biopsy may help guide treatment decisions in prostate cancer
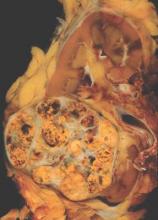
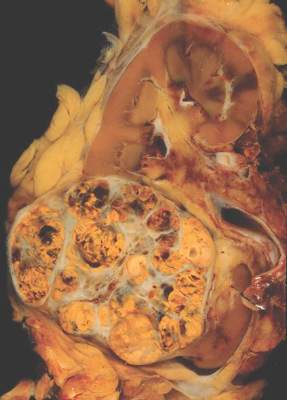
Analysis of the phenotypic and genotypic profile of circulating tumor cells (CTCs) – a so-called liquid biopsy – may help guide treatment decisions in men with castration-resistant metastatic prostate cancer, according to a cohort study being reported at the Genitourinary Cancers Symposium.
Investigators analyzed CTCs in 221 blood samples from 179 patients with metastatic prostate cancer about to begin either hormonal therapy (enzalutamide or abiraterone) or chemotherapy based on a taxane (docetaxel or cabazitaxel).
Among patients given hormonal therapy, those whose CTCs exhibited high versus low scores for phenotypic heterogeneity had poorer radiographic progression-free survival (5 months vs. 17 months) and overall survival (9 months vs. not reached), first author Dr. Howard I. Scher reported in a press briefing held before the 2016 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
In contrast, among those given chemotherapy, heterogeneity score did not affect these outcomes.
In other key findings, the heterogeneity score increased with each additional line of therapy patients received in the metastatic setting.
“We were able to show that single cell morphology, protein, and genomic characterization is feasible and can be used to assess tumor heterogeneity,” commented Dr. Scher of the Genitourinary Oncology Service at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York. “For characterizing disease at the point of decision making, a noninvasive liquid biopsy that enables the characterization of individual cells from a patient with metastatic prostate cancer can be used to guide treatment selection. Clinical trials to validate these findings are currently in development.”
He speculated that CTC heterogeneity was important only for hormonal therapy because it is a targeted therapy; as the disease becomes more diverse, essentially evolving into multiple diseases, this therapy is less likely to be effective. “The mechanism of taxanes is more general and not quite as targeted as these specific agents, which is why we believe they are more effective in a more diverse population,” he explained.
ASCO spokesperson and moderator of the press briefing Dr. Sumanta K. Pal, commented, “This is really fascinating work. We’ve always relied on a patient’s tumor to get genetic information, but that can be incredibly complicated for a number of reasons.” Among them, metastases may be located in hard-to-biopsy locations, and it is not practical to repeatedly perform needle biopsies over time.
The new findings show that “prostate cancer appears to get more complex over time as the disease evolves. The genetic diversity, quote unquote, increases, potentially suggesting that the cancer cell is crafting machinery to resist treatment,” he said.
From the clinical perspective, the ability to link CTC characteristics to response or lack thereof to a given therapy “is of incredible value,” according to Dr. Pal of the City of Hope, Duarte, Calif. “We have a number of new treatments for advanced prostate cancer and right now, we have little means of personalizing them and offering the right treatment to the right patient. With the studies that Dr. Scher has proposed to potentially validate this modality, we would have this personalized selection tool in our hands.”
Giving some background to the research, Dr. Scher noted, “Tissues are composed of a mixture of cells that differ morphologically and biologically. The result is diversity at single sites and multiple sites, broadly termed heterogeneity, so that we are not treating a single disease but a collection of diseases, and that is one reason we have difficulty achieving cures.”
“One could think of the blood as sampling, at least in theory, from all of the metastatic sites that are present. And we think you’ll have a greater chance of getting a more relevant characterization of the disease as a whole, in particular, by looking at the individual cells,” he further noted.
In the study, the investigators collected CTCs from blood samples, deposited them onto slides, stained them with DAPI and for various proteins (cytokeratin, androgen receptor, and CD45), and then scanned them, using a commercial platform (Epic Sciences).
They analyzed morphologic and phenotypic features of 9,225 single CTCs, splitting them into 15 distinct subtypes. They also performed whole-genome sequencing in a subset of 741 CTCs to assess copy number variation, clonality, and gene amplifications and deletions.
“The samples can be run quickly, and you can get results within 48 hours, which is important if you are trying to make a treatment decision,” Dr. Scher noted.
Findings showed that among patients given hormonal therapy, a high versus low CTC heterogeneity score was associated with much greater risks of radiographic progression-free survival events (hazard ratio, 2.2; P = .00182) and death (HR, 5.5; P less than .0001). In contrast, among patients who received taxane-based chemotherapy, CTC heterogeneity did not significantly affect either outcome.
Analysis of the phenotypic and genotypic profile of circulating tumor cells (CTCs) – a so-called liquid biopsy – may help guide treatment decisions in men with castration-resistant metastatic prostate cancer, according to a cohort study being reported at the Genitourinary Cancers Symposium.
Investigators analyzed CTCs in 221 blood samples from 179 patients with metastatic prostate cancer about to begin either hormonal therapy (enzalutamide or abiraterone) or chemotherapy based on a taxane (docetaxel or cabazitaxel).
Among patients given hormonal therapy, those whose CTCs exhibited high versus low scores for phenotypic heterogeneity had poorer radiographic progression-free survival (5 months vs. 17 months) and overall survival (9 months vs. not reached), first author Dr. Howard I. Scher reported in a press briefing held before the 2016 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
In contrast, among those given chemotherapy, heterogeneity score did not affect these outcomes.
In other key findings, the heterogeneity score increased with each additional line of therapy patients received in the metastatic setting.
“We were able to show that single cell morphology, protein, and genomic characterization is feasible and can be used to assess tumor heterogeneity,” commented Dr. Scher of the Genitourinary Oncology Service at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York. “For characterizing disease at the point of decision making, a noninvasive liquid biopsy that enables the characterization of individual cells from a patient with metastatic prostate cancer can be used to guide treatment selection. Clinical trials to validate these findings are currently in development.”
He speculated that CTC heterogeneity was important only for hormonal therapy because it is a targeted therapy; as the disease becomes more diverse, essentially evolving into multiple diseases, this therapy is less likely to be effective. “The mechanism of taxanes is more general and not quite as targeted as these specific agents, which is why we believe they are more effective in a more diverse population,” he explained.
ASCO spokesperson and moderator of the press briefing Dr. Sumanta K. Pal, commented, “This is really fascinating work. We’ve always relied on a patient’s tumor to get genetic information, but that can be incredibly complicated for a number of reasons.” Among them, metastases may be located in hard-to-biopsy locations, and it is not practical to repeatedly perform needle biopsies over time.
The new findings show that “prostate cancer appears to get more complex over time as the disease evolves. The genetic diversity, quote unquote, increases, potentially suggesting that the cancer cell is crafting machinery to resist treatment,” he said.
From the clinical perspective, the ability to link CTC characteristics to response or lack thereof to a given therapy “is of incredible value,” according to Dr. Pal of the City of Hope, Duarte, Calif. “We have a number of new treatments for advanced prostate cancer and right now, we have little means of personalizing them and offering the right treatment to the right patient. With the studies that Dr. Scher has proposed to potentially validate this modality, we would have this personalized selection tool in our hands.”
Giving some background to the research, Dr. Scher noted, “Tissues are composed of a mixture of cells that differ morphologically and biologically. The result is diversity at single sites and multiple sites, broadly termed heterogeneity, so that we are not treating a single disease but a collection of diseases, and that is one reason we have difficulty achieving cures.”
“One could think of the blood as sampling, at least in theory, from all of the metastatic sites that are present. And we think you’ll have a greater chance of getting a more relevant characterization of the disease as a whole, in particular, by looking at the individual cells,” he further noted.
In the study, the investigators collected CTCs from blood samples, deposited them onto slides, stained them with DAPI and for various proteins (cytokeratin, androgen receptor, and CD45), and then scanned them, using a commercial platform (Epic Sciences).
They analyzed morphologic and phenotypic features of 9,225 single CTCs, splitting them into 15 distinct subtypes. They also performed whole-genome sequencing in a subset of 741 CTCs to assess copy number variation, clonality, and gene amplifications and deletions.
“The samples can be run quickly, and you can get results within 48 hours, which is important if you are trying to make a treatment decision,” Dr. Scher noted.
Findings showed that among patients given hormonal therapy, a high versus low CTC heterogeneity score was associated with much greater risks of radiographic progression-free survival events (hazard ratio, 2.2; P = .00182) and death (HR, 5.5; P less than .0001). In contrast, among patients who received taxane-based chemotherapy, CTC heterogeneity did not significantly affect either outcome.
Analysis of the phenotypic and genotypic profile of circulating tumor cells (CTCs) – a so-called liquid biopsy – may help guide treatment decisions in men with castration-resistant metastatic prostate cancer, according to a cohort study being reported at the Genitourinary Cancers Symposium.
Investigators analyzed CTCs in 221 blood samples from 179 patients with metastatic prostate cancer about to begin either hormonal therapy (enzalutamide or abiraterone) or chemotherapy based on a taxane (docetaxel or cabazitaxel).
Among patients given hormonal therapy, those whose CTCs exhibited high versus low scores for phenotypic heterogeneity had poorer radiographic progression-free survival (5 months vs. 17 months) and overall survival (9 months vs. not reached), first author Dr. Howard I. Scher reported in a press briefing held before the 2016 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
In contrast, among those given chemotherapy, heterogeneity score did not affect these outcomes.
In other key findings, the heterogeneity score increased with each additional line of therapy patients received in the metastatic setting.
“We were able to show that single cell morphology, protein, and genomic characterization is feasible and can be used to assess tumor heterogeneity,” commented Dr. Scher of the Genitourinary Oncology Service at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York. “For characterizing disease at the point of decision making, a noninvasive liquid biopsy that enables the characterization of individual cells from a patient with metastatic prostate cancer can be used to guide treatment selection. Clinical trials to validate these findings are currently in development.”
He speculated that CTC heterogeneity was important only for hormonal therapy because it is a targeted therapy; as the disease becomes more diverse, essentially evolving into multiple diseases, this therapy is less likely to be effective. “The mechanism of taxanes is more general and not quite as targeted as these specific agents, which is why we believe they are more effective in a more diverse population,” he explained.
ASCO spokesperson and moderator of the press briefing Dr. Sumanta K. Pal, commented, “This is really fascinating work. We’ve always relied on a patient’s tumor to get genetic information, but that can be incredibly complicated for a number of reasons.” Among them, metastases may be located in hard-to-biopsy locations, and it is not practical to repeatedly perform needle biopsies over time.
The new findings show that “prostate cancer appears to get more complex over time as the disease evolves. The genetic diversity, quote unquote, increases, potentially suggesting that the cancer cell is crafting machinery to resist treatment,” he said.
From the clinical perspective, the ability to link CTC characteristics to response or lack thereof to a given therapy “is of incredible value,” according to Dr. Pal of the City of Hope, Duarte, Calif. “We have a number of new treatments for advanced prostate cancer and right now, we have little means of personalizing them and offering the right treatment to the right patient. With the studies that Dr. Scher has proposed to potentially validate this modality, we would have this personalized selection tool in our hands.”
Giving some background to the research, Dr. Scher noted, “Tissues are composed of a mixture of cells that differ morphologically and biologically. The result is diversity at single sites and multiple sites, broadly termed heterogeneity, so that we are not treating a single disease but a collection of diseases, and that is one reason we have difficulty achieving cures.”
“One could think of the blood as sampling, at least in theory, from all of the metastatic sites that are present. And we think you’ll have a greater chance of getting a more relevant characterization of the disease as a whole, in particular, by looking at the individual cells,” he further noted.
In the study, the investigators collected CTCs from blood samples, deposited them onto slides, stained them with DAPI and for various proteins (cytokeratin, androgen receptor, and CD45), and then scanned them, using a commercial platform (Epic Sciences).
They analyzed morphologic and phenotypic features of 9,225 single CTCs, splitting them into 15 distinct subtypes. They also performed whole-genome sequencing in a subset of 741 CTCs to assess copy number variation, clonality, and gene amplifications and deletions.
“The samples can be run quickly, and you can get results within 48 hours, which is important if you are trying to make a treatment decision,” Dr. Scher noted.
Findings showed that among patients given hormonal therapy, a high versus low CTC heterogeneity score was associated with much greater risks of radiographic progression-free survival events (hazard ratio, 2.2; P = .00182) and death (HR, 5.5; P less than .0001). In contrast, among patients who received taxane-based chemotherapy, CTC heterogeneity did not significantly affect either outcome.
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point: In patients with castration-resistant metastatic prostate cancer, CTC heterogeneity affects the response to hormonal therapy but not to chemotherapy.
Major finding: A high vs. low CTC heterogeneity score was associated with shorter median progression-free survival (5 vs. 17 months) and overall survival (9 months vs. not reached) with hormonal therapy but did not affect these outcomes with chemotherapy.
Data source: A cohort study of 179 patients with castration-resistant metastatic prostate cancer about to begin either hormonal therapy or taxane-based chemotherapy.
Disclosures: Dr. Scher disclosed that he has a consulting or advisory role, and receives travel, accommodations, and/or expenses from numerous pharmaceutical companies. He receives research funding (institutional) from BIND Biosciences, Exelixis, Janssen Pharmaceuticals, Medivation, Janssen Diagnostics, and Innocrin Pharmaceuticals. The study received funding from the Prostate Cancer Foundation, MSKCC SPORE, and MSKCC Core Grant. Dr. Pal disclosed that he receives honoraria from Astellas Pharma, Medivation, and Novartis; that he has a consulting or advisory role with Aveo, Genentech, Myriad Pharmaceuticals, Novartis, and Pfizer; and that he receives research funding from Medivation.
METEOR: Cabozantinib bests everolimus across renal cancer subgroups
The oral multitargeted tyrosine kinase inhibitor cabozantinib is more efficacious than everolimus as therapy for advanced renal cell carcinoma across a wide range of patients, suggests a subgroup analysis of the phase III METEOR trial being reported at the genitourinary cancers symposium.
Trial participants were 658 patients with advanced renal cell carcinoma and clear cell histology who had experienced progression on a tyrosine kinase inhibitor targeting the vascular endothelial growth factor receptor (VEGFR). There was no limit on the number of prior therapies.
The patients were randomized evenly to cabozantinib (Cometriq), which inhibits the VEGFR, MET, and AXL tyrosine kinases – all of which are up-regulated in this cancer – or to everolimus (Afinitor), an inhibitor of the mammalian target of rapamycin that is considered a standard of care. (At present, cabozantinib is approved by the Food and Drug Administration for the treatment of medullary thyroid cancer.)
Results for the entire trial population showed that patients in the cabozantinib group were about half as likely as were their counterparts in the everolimus group to experience progression-free survival events, lead author Dr. Bernard Escudier reported in a press briefing held before the 2016 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology. All patients appeared to derive benefit, with the reduction in risk ranging from 16% to 78% depending on the specific subgroup.
“Cabozantinib improved progression-free survival, compared to one of our standard therapies, everolimus, in advanced renal cell carcinoma,” concluded Dr. Escudier, who is chair of the Genitourinary Oncology Committee at the Institut Gustave Roussy in Villejuif, France. “Benefit was observed across prespecified subgroups,” including a small subgroup who had previously received immunotherapies targeting the programmed death 1 (PD-1) signaling pathway.
Toxicity was somewhat problematic with cabozantinib, despite starting the drug at a lower dose than has typically been used in the past, he acknowledged. The most common side effects were diarrhea, fatigue, nausea, decreased appetite, and hand-foot syndrome, and they often necessitated further dose reductions.
“Benefit with cabozantinib treatment is supported by a trend in overall survival, and hopefully, we will give this final overall survival analysis at ASCO this year,” Dr. Escudier added. Findings of an interim analysis reported last year were very promising with respect to this outcome (hazard ratio, 0.67; P = .005) (N Engl J Med. 2015 Nov 5;373:1814-23).
“This study is unique compared to others in that it allowed a broad range of patients: Patients could have had spread of cancer to the brain, they could have received any number of prior therapies, and they could have been exposed to immune-based treatments,” commented ASCO spokesperson and moderator of the press briefing Dr. Sumanta K. Pal. “The magnitude of benefit that patients got from cabozantinib far exceeds, in my opinion, what we have seen to date in this setting in terms of both delay in tumor growth and improving survival.”
In fact, for some oncologists, the findings may be strong enough to prompt use of cabozantinib as second-line therapy, according to Dr. Pal, who is a medical oncologist at the City of Hope in Duarte, Calif.
“Given the fact that cabozantinib has a very compelling benefit in terms of both delay in tumor growth and a hint toward a benefit in terms of overall survival, I would perhaps tend to favor that as a second-line option as compared to other comparators, such as nivolumab (Opdivo), in that setting,” he said, referring to an antibody that targets the cell surface receptor PD-1. “Now that’s a personal opinion. I certainly think there are some merits with nivolumab, such as the toxicity profile. But, in broad terms, patients are very focused on clinical efficacy, and with that in mind, the data for cabozantinib truly speaks for itself.”
But Dr. Escudier offered a more-reserved perspective. “I think what people are going to do will be to use nivolumab as second-line [therapy] in most patients and keep cabozantinib for nivolumab failure,” he predicted. “Based on that, this subgroup, although small, is of importance. I don’t think it’s good enough to say we should use cabozantinib or nivolumab in second line based on the subgroup analyses we have.”
The eagerly awaited overall survival results will also help determine cabozantinib’s position in treatment sequence, he added. “If we get a survival advantage [that] is the same magnitude that we have with nivolumab, with such an impressive improvement in progression-free survival, maybe despite the toxicity with cabozantinib, people will be willing to use cabozantinib early on.”
In the new analysis, median progression-free survival in the entire trial population was 7.4 months with cabozantinib and 3.9 months with everolimus, translating to a near halving of the risk of events (hazard ratio, 0.52; P less than .001).
Subgroup analyses showed that patients in the cabozantinib group consistently had a lower risk of events, with hazard ratios ranging from 0.22 to 0.84, regardless of their Memorial Sloan Kettering Cancer Center risk group, number of organs with metastases, presence of both visceral and bone metastases, number of prior VEGFR tyrosine kinase inhibitors, the specific VEGFR tyrosine kinase inhibitor in patients who had received only one, and prior immunotherapy targeting the programmed death pathway.
The 42 patients who had previously received immunotherapy targeting that pathway were among those seeming to derive most benefit, Dr. Escudier reported. “Of course, this is a small number, but certainly an observation [of interest] when many patients are going to receive nivolumab as second-line in kidney cancer. This drug is still very active after PD-1 or PD-L1 antibodies.”
The oral multitargeted tyrosine kinase inhibitor cabozantinib is more efficacious than everolimus as therapy for advanced renal cell carcinoma across a wide range of patients, suggests a subgroup analysis of the phase III METEOR trial being reported at the genitourinary cancers symposium.
Trial participants were 658 patients with advanced renal cell carcinoma and clear cell histology who had experienced progression on a tyrosine kinase inhibitor targeting the vascular endothelial growth factor receptor (VEGFR). There was no limit on the number of prior therapies.
The patients were randomized evenly to cabozantinib (Cometriq), which inhibits the VEGFR, MET, and AXL tyrosine kinases – all of which are up-regulated in this cancer – or to everolimus (Afinitor), an inhibitor of the mammalian target of rapamycin that is considered a standard of care. (At present, cabozantinib is approved by the Food and Drug Administration for the treatment of medullary thyroid cancer.)
Results for the entire trial population showed that patients in the cabozantinib group were about half as likely as were their counterparts in the everolimus group to experience progression-free survival events, lead author Dr. Bernard Escudier reported in a press briefing held before the 2016 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology. All patients appeared to derive benefit, with the reduction in risk ranging from 16% to 78% depending on the specific subgroup.
“Cabozantinib improved progression-free survival, compared to one of our standard therapies, everolimus, in advanced renal cell carcinoma,” concluded Dr. Escudier, who is chair of the Genitourinary Oncology Committee at the Institut Gustave Roussy in Villejuif, France. “Benefit was observed across prespecified subgroups,” including a small subgroup who had previously received immunotherapies targeting the programmed death 1 (PD-1) signaling pathway.
Toxicity was somewhat problematic with cabozantinib, despite starting the drug at a lower dose than has typically been used in the past, he acknowledged. The most common side effects were diarrhea, fatigue, nausea, decreased appetite, and hand-foot syndrome, and they often necessitated further dose reductions.
“Benefit with cabozantinib treatment is supported by a trend in overall survival, and hopefully, we will give this final overall survival analysis at ASCO this year,” Dr. Escudier added. Findings of an interim analysis reported last year were very promising with respect to this outcome (hazard ratio, 0.67; P = .005) (N Engl J Med. 2015 Nov 5;373:1814-23).
“This study is unique compared to others in that it allowed a broad range of patients: Patients could have had spread of cancer to the brain, they could have received any number of prior therapies, and they could have been exposed to immune-based treatments,” commented ASCO spokesperson and moderator of the press briefing Dr. Sumanta K. Pal. “The magnitude of benefit that patients got from cabozantinib far exceeds, in my opinion, what we have seen to date in this setting in terms of both delay in tumor growth and improving survival.”
In fact, for some oncologists, the findings may be strong enough to prompt use of cabozantinib as second-line therapy, according to Dr. Pal, who is a medical oncologist at the City of Hope in Duarte, Calif.
“Given the fact that cabozantinib has a very compelling benefit in terms of both delay in tumor growth and a hint toward a benefit in terms of overall survival, I would perhaps tend to favor that as a second-line option as compared to other comparators, such as nivolumab (Opdivo), in that setting,” he said, referring to an antibody that targets the cell surface receptor PD-1. “Now that’s a personal opinion. I certainly think there are some merits with nivolumab, such as the toxicity profile. But, in broad terms, patients are very focused on clinical efficacy, and with that in mind, the data for cabozantinib truly speaks for itself.”
But Dr. Escudier offered a more-reserved perspective. “I think what people are going to do will be to use nivolumab as second-line [therapy] in most patients and keep cabozantinib for nivolumab failure,” he predicted. “Based on that, this subgroup, although small, is of importance. I don’t think it’s good enough to say we should use cabozantinib or nivolumab in second line based on the subgroup analyses we have.”
The eagerly awaited overall survival results will also help determine cabozantinib’s position in treatment sequence, he added. “If we get a survival advantage [that] is the same magnitude that we have with nivolumab, with such an impressive improvement in progression-free survival, maybe despite the toxicity with cabozantinib, people will be willing to use cabozantinib early on.”
In the new analysis, median progression-free survival in the entire trial population was 7.4 months with cabozantinib and 3.9 months with everolimus, translating to a near halving of the risk of events (hazard ratio, 0.52; P less than .001).
Subgroup analyses showed that patients in the cabozantinib group consistently had a lower risk of events, with hazard ratios ranging from 0.22 to 0.84, regardless of their Memorial Sloan Kettering Cancer Center risk group, number of organs with metastases, presence of both visceral and bone metastases, number of prior VEGFR tyrosine kinase inhibitors, the specific VEGFR tyrosine kinase inhibitor in patients who had received only one, and prior immunotherapy targeting the programmed death pathway.
The 42 patients who had previously received immunotherapy targeting that pathway were among those seeming to derive most benefit, Dr. Escudier reported. “Of course, this is a small number, but certainly an observation [of interest] when many patients are going to receive nivolumab as second-line in kidney cancer. This drug is still very active after PD-1 or PD-L1 antibodies.”
The oral multitargeted tyrosine kinase inhibitor cabozantinib is more efficacious than everolimus as therapy for advanced renal cell carcinoma across a wide range of patients, suggests a subgroup analysis of the phase III METEOR trial being reported at the genitourinary cancers symposium.
Trial participants were 658 patients with advanced renal cell carcinoma and clear cell histology who had experienced progression on a tyrosine kinase inhibitor targeting the vascular endothelial growth factor receptor (VEGFR). There was no limit on the number of prior therapies.
The patients were randomized evenly to cabozantinib (Cometriq), which inhibits the VEGFR, MET, and AXL tyrosine kinases – all of which are up-regulated in this cancer – or to everolimus (Afinitor), an inhibitor of the mammalian target of rapamycin that is considered a standard of care. (At present, cabozantinib is approved by the Food and Drug Administration for the treatment of medullary thyroid cancer.)
Results for the entire trial population showed that patients in the cabozantinib group were about half as likely as were their counterparts in the everolimus group to experience progression-free survival events, lead author Dr. Bernard Escudier reported in a press briefing held before the 2016 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology. All patients appeared to derive benefit, with the reduction in risk ranging from 16% to 78% depending on the specific subgroup.
“Cabozantinib improved progression-free survival, compared to one of our standard therapies, everolimus, in advanced renal cell carcinoma,” concluded Dr. Escudier, who is chair of the Genitourinary Oncology Committee at the Institut Gustave Roussy in Villejuif, France. “Benefit was observed across prespecified subgroups,” including a small subgroup who had previously received immunotherapies targeting the programmed death 1 (PD-1) signaling pathway.
Toxicity was somewhat problematic with cabozantinib, despite starting the drug at a lower dose than has typically been used in the past, he acknowledged. The most common side effects were diarrhea, fatigue, nausea, decreased appetite, and hand-foot syndrome, and they often necessitated further dose reductions.
“Benefit with cabozantinib treatment is supported by a trend in overall survival, and hopefully, we will give this final overall survival analysis at ASCO this year,” Dr. Escudier added. Findings of an interim analysis reported last year were very promising with respect to this outcome (hazard ratio, 0.67; P = .005) (N Engl J Med. 2015 Nov 5;373:1814-23).
“This study is unique compared to others in that it allowed a broad range of patients: Patients could have had spread of cancer to the brain, they could have received any number of prior therapies, and they could have been exposed to immune-based treatments,” commented ASCO spokesperson and moderator of the press briefing Dr. Sumanta K. Pal. “The magnitude of benefit that patients got from cabozantinib far exceeds, in my opinion, what we have seen to date in this setting in terms of both delay in tumor growth and improving survival.”
In fact, for some oncologists, the findings may be strong enough to prompt use of cabozantinib as second-line therapy, according to Dr. Pal, who is a medical oncologist at the City of Hope in Duarte, Calif.
“Given the fact that cabozantinib has a very compelling benefit in terms of both delay in tumor growth and a hint toward a benefit in terms of overall survival, I would perhaps tend to favor that as a second-line option as compared to other comparators, such as nivolumab (Opdivo), in that setting,” he said, referring to an antibody that targets the cell surface receptor PD-1. “Now that’s a personal opinion. I certainly think there are some merits with nivolumab, such as the toxicity profile. But, in broad terms, patients are very focused on clinical efficacy, and with that in mind, the data for cabozantinib truly speaks for itself.”
But Dr. Escudier offered a more-reserved perspective. “I think what people are going to do will be to use nivolumab as second-line [therapy] in most patients and keep cabozantinib for nivolumab failure,” he predicted. “Based on that, this subgroup, although small, is of importance. I don’t think it’s good enough to say we should use cabozantinib or nivolumab in second line based on the subgroup analyses we have.”
The eagerly awaited overall survival results will also help determine cabozantinib’s position in treatment sequence, he added. “If we get a survival advantage [that] is the same magnitude that we have with nivolumab, with such an impressive improvement in progression-free survival, maybe despite the toxicity with cabozantinib, people will be willing to use cabozantinib early on.”
In the new analysis, median progression-free survival in the entire trial population was 7.4 months with cabozantinib and 3.9 months with everolimus, translating to a near halving of the risk of events (hazard ratio, 0.52; P less than .001).
Subgroup analyses showed that patients in the cabozantinib group consistently had a lower risk of events, with hazard ratios ranging from 0.22 to 0.84, regardless of their Memorial Sloan Kettering Cancer Center risk group, number of organs with metastases, presence of both visceral and bone metastases, number of prior VEGFR tyrosine kinase inhibitors, the specific VEGFR tyrosine kinase inhibitor in patients who had received only one, and prior immunotherapy targeting the programmed death pathway.
The 42 patients who had previously received immunotherapy targeting that pathway were among those seeming to derive most benefit, Dr. Escudier reported. “Of course, this is a small number, but certainly an observation [of interest] when many patients are going to receive nivolumab as second-line in kidney cancer. This drug is still very active after PD-1 or PD-L1 antibodies.”
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point: Cabozantinib appears more efficacious than does everolimus for a wide range of patients.
Major finding: Progression-free survival was better with cabozantinib than with everolimus across subgroups (hazard ratios, 0.22-0.84).
Data source: A subgroup analysis of 658 patients with previously treated advanced renal cell carcinoma in a randomized phase III trial (METEOR).
Disclosures: Dr. Escudier disclosed that he has a consulting or advisory role with Bayer, GlaxoSmithKline, Novartis, Pfizer, Exelixis, Bristol-Myers Squibb, and that he receives honoraria from Pfizer, Novartis, GlaxoSmithKline, and Bayer. The study was funded in part by Exelixis. Dr. Pal disclosed that he receives honoraria from Astellas Pharma, Medivation, and Novartis; that he has a consulting or advisory role with Aveo, Genentech, Myriad Pharmaceuticals, Novartis, and Pfizer; and that he receives research funding from Medivation.
Risk of lethal prostate cancer is lower for regular aspirin users
Regular aspirin use appears to protect against the development of metastatic and fatal prostate cancer, according to an analysis of the Physicians’ Health Study.
Investigators led by Dr. Christopher Brian Allard analyzed data from 22,071 male physicians who were initially free of prostate cancer and were prospectively followed from 1982 through 2009.
Results showed that after adjustment for age, race, body mass index, and smoking status, men who took aspirin regularly (more than three tablets a week) were 24% less likely to develop lethal prostate cancer, which was defined in the study as metastatic disease or death from prostate cancer, Dr. Allard reported in a press briefing held before the 2016 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
However, regular aspirin use did not reduce the risk of prostate cancer overall, of high-grade prostate cancer, or of locally advanced prostate cancer.
Further analyses restricted to the men who developed prostate cancer showed that regular aspirin use after diagnosis was associated with a 39% lower risk of dying from the disease. In contrast, use before diagnosis did not have a protective effect.
“Our study demonstrates that regular aspirin intake may inhibit lethal prostate cancer, probably by preventing cancer progression,” said Dr. Allard, a urologic oncology fellow at Brigham and Women’s Hospital and Massachusetts General Hospital, both in Boston. Also, “men with prostate cancer who took aspirin regularly after diagnosis had a significantly reduced risk of death.”
Although aspirin’s exact mechanism in preventing lethal disease is unknown, preclinical data have implicated its antiplatelet action, which is consistent with evidence suggesting that circulating cancer cells may use platelets to escape immune detection, he said. “That would explain why there is no effect on the local cancer, but it is preventing deposition of metastases into metastatic environments.”
The main shortcoming of the research was the lack of information on aspirin dose, Dr. Allard acknowledged. Although the Physicians’ Health Study began as a randomized trial in 1982 testing 325 mg of the drug every other day, it was formally stopped 5 years later after cardiovascular benefit was established, and participants were free to take any dose thereafter. “We think most men started at 325 [mg] but then 81 mg did become a popular dose, and we really don’t know what they were taking,” he said.
“More work is needed to identify particular subsets of men most likely to benefit from aspirin and to determine the optimal aspirin dose,” Dr. Allard said.
In terms of applying the findings to clinical care, he recommended an individualized approach. “The main thing to keep in mind is that although aspirin is over the counter, there are side effects and potential harms. That being said, we don’t have the results of a randomized clinical trial looking at aspirin for prostate cancer survival yet,” he said. So men who are interested in aspirin for prevention of lethal prostate cancer should talk to their physicians, he added, “and look at their personal risks of side effects and harms from aspirin, as well as their benefits in terms of both prostate cancer and also potential cardiovascular benefits. It needs to be a personalized decision for every individual patient.”
Dr. Sumanta Pal, ASCO spokesperson and moderator of the press briefing, agreed that the trial left unanswered some critical questions and that clinicians must weigh the potential harms of aspirin therapy against the observed benefits.
“While this work is provocative, it’s important to keep in mind that the findings were from an observational study in which surveys and a review of hospital records were used to obtain information,” added Dr. Pal, a medical oncologist at City of Hope in Duarte, Calif. “These studies are certainly thought provoking but are perhaps best followed by formal clinical trials where we compare use of aspirin either to no treatment or perhaps to placebo.”
Dr. Allard disclosed that he had no conflicts of interests. The Prostate Cancer Foundation and the National Institutes of Health/National Cancer Institute funded the trial. Dr. Pal disclosed that he receives honoraria from Astellas Pharma, Medivation, and Novartis; that he has a consulting or advisory role with Aveo, Genentech, Myriad Pharmaceuticals, Novartis, and Pfizer; and that he receives research funding from Medivation.
*This article was updated 1/6/2015.
Regular aspirin use appears to protect against the development of metastatic and fatal prostate cancer, according to an analysis of the Physicians’ Health Study.
Investigators led by Dr. Christopher Brian Allard analyzed data from 22,071 male physicians who were initially free of prostate cancer and were prospectively followed from 1982 through 2009.
Results showed that after adjustment for age, race, body mass index, and smoking status, men who took aspirin regularly (more than three tablets a week) were 24% less likely to develop lethal prostate cancer, which was defined in the study as metastatic disease or death from prostate cancer, Dr. Allard reported in a press briefing held before the 2016 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
However, regular aspirin use did not reduce the risk of prostate cancer overall, of high-grade prostate cancer, or of locally advanced prostate cancer.
Further analyses restricted to the men who developed prostate cancer showed that regular aspirin use after diagnosis was associated with a 39% lower risk of dying from the disease. In contrast, use before diagnosis did not have a protective effect.
“Our study demonstrates that regular aspirin intake may inhibit lethal prostate cancer, probably by preventing cancer progression,” said Dr. Allard, a urologic oncology fellow at Brigham and Women’s Hospital and Massachusetts General Hospital, both in Boston. Also, “men with prostate cancer who took aspirin regularly after diagnosis had a significantly reduced risk of death.”
Although aspirin’s exact mechanism in preventing lethal disease is unknown, preclinical data have implicated its antiplatelet action, which is consistent with evidence suggesting that circulating cancer cells may use platelets to escape immune detection, he said. “That would explain why there is no effect on the local cancer, but it is preventing deposition of metastases into metastatic environments.”
The main shortcoming of the research was the lack of information on aspirin dose, Dr. Allard acknowledged. Although the Physicians’ Health Study began as a randomized trial in 1982 testing 325 mg of the drug every other day, it was formally stopped 5 years later after cardiovascular benefit was established, and participants were free to take any dose thereafter. “We think most men started at 325 [mg] but then 81 mg did become a popular dose, and we really don’t know what they were taking,” he said.
“More work is needed to identify particular subsets of men most likely to benefit from aspirin and to determine the optimal aspirin dose,” Dr. Allard said.
In terms of applying the findings to clinical care, he recommended an individualized approach. “The main thing to keep in mind is that although aspirin is over the counter, there are side effects and potential harms. That being said, we don’t have the results of a randomized clinical trial looking at aspirin for prostate cancer survival yet,” he said. So men who are interested in aspirin for prevention of lethal prostate cancer should talk to their physicians, he added, “and look at their personal risks of side effects and harms from aspirin, as well as their benefits in terms of both prostate cancer and also potential cardiovascular benefits. It needs to be a personalized decision for every individual patient.”
Dr. Sumanta Pal, ASCO spokesperson and moderator of the press briefing, agreed that the trial left unanswered some critical questions and that clinicians must weigh the potential harms of aspirin therapy against the observed benefits.
“While this work is provocative, it’s important to keep in mind that the findings were from an observational study in which surveys and a review of hospital records were used to obtain information,” added Dr. Pal, a medical oncologist at City of Hope in Duarte, Calif. “These studies are certainly thought provoking but are perhaps best followed by formal clinical trials where we compare use of aspirin either to no treatment or perhaps to placebo.”
Dr. Allard disclosed that he had no conflicts of interests. The Prostate Cancer Foundation and the National Institutes of Health/National Cancer Institute funded the trial. Dr. Pal disclosed that he receives honoraria from Astellas Pharma, Medivation, and Novartis; that he has a consulting or advisory role with Aveo, Genentech, Myriad Pharmaceuticals, Novartis, and Pfizer; and that he receives research funding from Medivation.
*This article was updated 1/6/2015.
Regular aspirin use appears to protect against the development of metastatic and fatal prostate cancer, according to an analysis of the Physicians’ Health Study.
Investigators led by Dr. Christopher Brian Allard analyzed data from 22,071 male physicians who were initially free of prostate cancer and were prospectively followed from 1982 through 2009.
Results showed that after adjustment for age, race, body mass index, and smoking status, men who took aspirin regularly (more than three tablets a week) were 24% less likely to develop lethal prostate cancer, which was defined in the study as metastatic disease or death from prostate cancer, Dr. Allard reported in a press briefing held before the 2016 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
However, regular aspirin use did not reduce the risk of prostate cancer overall, of high-grade prostate cancer, or of locally advanced prostate cancer.
Further analyses restricted to the men who developed prostate cancer showed that regular aspirin use after diagnosis was associated with a 39% lower risk of dying from the disease. In contrast, use before diagnosis did not have a protective effect.
“Our study demonstrates that regular aspirin intake may inhibit lethal prostate cancer, probably by preventing cancer progression,” said Dr. Allard, a urologic oncology fellow at Brigham and Women’s Hospital and Massachusetts General Hospital, both in Boston. Also, “men with prostate cancer who took aspirin regularly after diagnosis had a significantly reduced risk of death.”
Although aspirin’s exact mechanism in preventing lethal disease is unknown, preclinical data have implicated its antiplatelet action, which is consistent with evidence suggesting that circulating cancer cells may use platelets to escape immune detection, he said. “That would explain why there is no effect on the local cancer, but it is preventing deposition of metastases into metastatic environments.”
The main shortcoming of the research was the lack of information on aspirin dose, Dr. Allard acknowledged. Although the Physicians’ Health Study began as a randomized trial in 1982 testing 325 mg of the drug every other day, it was formally stopped 5 years later after cardiovascular benefit was established, and participants were free to take any dose thereafter. “We think most men started at 325 [mg] but then 81 mg did become a popular dose, and we really don’t know what they were taking,” he said.
“More work is needed to identify particular subsets of men most likely to benefit from aspirin and to determine the optimal aspirin dose,” Dr. Allard said.
In terms of applying the findings to clinical care, he recommended an individualized approach. “The main thing to keep in mind is that although aspirin is over the counter, there are side effects and potential harms. That being said, we don’t have the results of a randomized clinical trial looking at aspirin for prostate cancer survival yet,” he said. So men who are interested in aspirin for prevention of lethal prostate cancer should talk to their physicians, he added, “and look at their personal risks of side effects and harms from aspirin, as well as their benefits in terms of both prostate cancer and also potential cardiovascular benefits. It needs to be a personalized decision for every individual patient.”
Dr. Sumanta Pal, ASCO spokesperson and moderator of the press briefing, agreed that the trial left unanswered some critical questions and that clinicians must weigh the potential harms of aspirin therapy against the observed benefits.
“While this work is provocative, it’s important to keep in mind that the findings were from an observational study in which surveys and a review of hospital records were used to obtain information,” added Dr. Pal, a medical oncologist at City of Hope in Duarte, Calif. “These studies are certainly thought provoking but are perhaps best followed by formal clinical trials where we compare use of aspirin either to no treatment or perhaps to placebo.”
Dr. Allard disclosed that he had no conflicts of interests. The Prostate Cancer Foundation and the National Institutes of Health/National Cancer Institute funded the trial. Dr. Pal disclosed that he receives honoraria from Astellas Pharma, Medivation, and Novartis; that he has a consulting or advisory role with Aveo, Genentech, Myriad Pharmaceuticals, Novartis, and Pfizer; and that he receives research funding from Medivation.
*This article was updated 1/6/2015.
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point: Regular aspirin use may reduce the risk of developing advanced prostate cancer and dying from the disease.
Major finding: Men who regularly took aspirin had a 24% lower risk of developing lethal prostate cancer and, among all who developed the disease, a 39% lower risk of dying from it.
Data source: A prospective cohort study of 22,071 men from the Physicians’ Health Study followed up for 27 years.
Disclosures: Dr. Allard disclosed that he had no conflicts of interests. The Prostate Cancer Foundation and the National Institutes of Health/National Cancer Institute funded the trial. Dr. Pal disclosed that he receives honoraria from Astellas Pharma, Medivation, and Novartis; that he has a consulting or advisory role with Aveo, Genentech, Myriad Pharmaceuticals, Novartis, and Pfizer; and that he receives research funding from Medivation.
Late risks of breast cancer RT are higher for smokers
SAN ANTONIO – The late side effects of modern radiation therapy for breast cancer depend in part on a woman’s smoking status, suggests a meta-analysis of data from more than 40,000 women presented at the San Antonio Breast Cancer Symposium.
For nonsmokers, radiation therapy had little impact on the absolute risks of lung cancer or cardiac death, the main risks identified, which combined totaled less than 1%, Dr. Carolyn Taylor reported on behalf of the Early Breast Cancer Trialists’ Collaborative Group. But for women who had smoked throughout their adult life and continued to do so during and after treatment, it increased that absolute risk to roughly 2%.
“Smoking status can determine the net long-term effects of breast cancer radiotherapy on mortality. Stopping smoking at the time of radiotherapy may avoid much of the risk, and that’s because most of the risk of lung cancer starts more than 10 years after radiotherapy,” said Dr. Taylor, a radiation oncologist at the University of Oxford (England).
Radiation therapy remains an important tool in treating breast cancer, ultimately reducing the likelihood of death from the disease, she reminded symposium attendees. “The absolute benefit in women treated according to current guidelines is a few percent. Let’s remember the magnitude of that benefit as we think about the risks of radiotherapy.”
Attendee Dr. Steven Vogl of Montefiore Medical Center, New York, asked whether information was available on the location of the lung cancers that occurred in the trials.
“We didn’t have location of lung cancers. We didn’t even know if it was ipsilateral or contralateral to the previous breast cancer in this study,” Dr. Taylor replied. “But we’ve done other studies where we have known the location of the lung cancer, and there were similar findings in those studies.”
“In the last 4 years, we’ve had very good information that annual CT screening substantially and very quickly reduces the mortality from lung cancer,” Dr. Vogl added as a comment. “Any of us who care for patients who have been radiated where, really, any lung has been treated, who continue to smoke, should be screened – and screened and screened and screened again,” he recommended.
The investigators analyzed data from 40,781 women with breast cancer from 75 randomized trials conducted worldwide that compared outcomes with versus without radiation therapy. The median year of trial entry was 1983. On average, women in the trials received 10 Gy to both lungs combined and 6 Gy to the heart.
Comparing women who did and did not receive radiation therapy, the rate ratio for lung cancer was 2.10 at 10 or more years out, and the rate ratio for cardiac mortality was 1.30 overall. Given the mean radiation doses in the trials, the excess risk translated to 12% per Gray for lung cancer and 4% per Gray for cardiac mortality. “These rate ratios are likely to apply today,” Dr. Taylor maintained.
However, she noted, contemporary breast cancer radiation therapy techniques are much better at sparing normal tissues. To derive absolute risk estimates that are relevant today, she and her colleagues reviewed the literature for 2010-2015 and determined that women now receive an average of 5 Gy to both lungs combined and 4 Gy to the heart, with some centers achieving even lower values.
Among nonsmokers, the estimated cumulative 30-year risk of lung cancer was 0.5% for women who did not receive radiation therapy and 0.8% for those who received radiation therapy with a mean dose of 5 Gy to both lungs combined, Dr. Taylor reported. However, among long-term smokers, it was 9.4% without radiation and a substantially higher 13.8% with it.
Similarly, among nonsmokers, the estimated cumulative 30-year risk of ischemic heart disease death was 1.8% for women who did not receive radiation therapy and 2.0% for women who received radiation therapy with a mean dose of 2 Gy to the heart. Among long-term smokers, it was 8.0% without radiation and a slightly higher 8.6% with it.
Additional analyses looking at other late side effects showed no radiation therapy–related excess risk of sarcomas, according to Dr. Taylor. The risk of leukemia was increased with radiation, but actual numbers of cases were very small, she cautioned.
Attendee Dr. Pamela Goodwin, University of Toronto, said, “I’m just wondering whether you considered if it was valid to assume that there was a linear relationship between radiation dose and the risk of lung cancer in the range of radiation doses that you looked at, so, from the higher range in the earlier studies to the much lower dose now.”
Numbers of heart disease events were sufficient to establish a linear relationship, according to Dr. Taylor. Numbers of lung cancers were not, but case-control studies in the literature with adequate numbers have identified a linear relationship there, too. “We use what we can, and we have got now several hundred events, if you combine all of the literature together. And they do suggest the dose-response relationship is linear, but we can’t know that for certain,” she said.
SAN ANTONIO – The late side effects of modern radiation therapy for breast cancer depend in part on a woman’s smoking status, suggests a meta-analysis of data from more than 40,000 women presented at the San Antonio Breast Cancer Symposium.
For nonsmokers, radiation therapy had little impact on the absolute risks of lung cancer or cardiac death, the main risks identified, which combined totaled less than 1%, Dr. Carolyn Taylor reported on behalf of the Early Breast Cancer Trialists’ Collaborative Group. But for women who had smoked throughout their adult life and continued to do so during and after treatment, it increased that absolute risk to roughly 2%.
“Smoking status can determine the net long-term effects of breast cancer radiotherapy on mortality. Stopping smoking at the time of radiotherapy may avoid much of the risk, and that’s because most of the risk of lung cancer starts more than 10 years after radiotherapy,” said Dr. Taylor, a radiation oncologist at the University of Oxford (England).
Radiation therapy remains an important tool in treating breast cancer, ultimately reducing the likelihood of death from the disease, she reminded symposium attendees. “The absolute benefit in women treated according to current guidelines is a few percent. Let’s remember the magnitude of that benefit as we think about the risks of radiotherapy.”
Attendee Dr. Steven Vogl of Montefiore Medical Center, New York, asked whether information was available on the location of the lung cancers that occurred in the trials.
“We didn’t have location of lung cancers. We didn’t even know if it was ipsilateral or contralateral to the previous breast cancer in this study,” Dr. Taylor replied. “But we’ve done other studies where we have known the location of the lung cancer, and there were similar findings in those studies.”
“In the last 4 years, we’ve had very good information that annual CT screening substantially and very quickly reduces the mortality from lung cancer,” Dr. Vogl added as a comment. “Any of us who care for patients who have been radiated where, really, any lung has been treated, who continue to smoke, should be screened – and screened and screened and screened again,” he recommended.
The investigators analyzed data from 40,781 women with breast cancer from 75 randomized trials conducted worldwide that compared outcomes with versus without radiation therapy. The median year of trial entry was 1983. On average, women in the trials received 10 Gy to both lungs combined and 6 Gy to the heart.
Comparing women who did and did not receive radiation therapy, the rate ratio for lung cancer was 2.10 at 10 or more years out, and the rate ratio for cardiac mortality was 1.30 overall. Given the mean radiation doses in the trials, the excess risk translated to 12% per Gray for lung cancer and 4% per Gray for cardiac mortality. “These rate ratios are likely to apply today,” Dr. Taylor maintained.
However, she noted, contemporary breast cancer radiation therapy techniques are much better at sparing normal tissues. To derive absolute risk estimates that are relevant today, she and her colleagues reviewed the literature for 2010-2015 and determined that women now receive an average of 5 Gy to both lungs combined and 4 Gy to the heart, with some centers achieving even lower values.
Among nonsmokers, the estimated cumulative 30-year risk of lung cancer was 0.5% for women who did not receive radiation therapy and 0.8% for those who received radiation therapy with a mean dose of 5 Gy to both lungs combined, Dr. Taylor reported. However, among long-term smokers, it was 9.4% without radiation and a substantially higher 13.8% with it.
Similarly, among nonsmokers, the estimated cumulative 30-year risk of ischemic heart disease death was 1.8% for women who did not receive radiation therapy and 2.0% for women who received radiation therapy with a mean dose of 2 Gy to the heart. Among long-term smokers, it was 8.0% without radiation and a slightly higher 8.6% with it.
Additional analyses looking at other late side effects showed no radiation therapy–related excess risk of sarcomas, according to Dr. Taylor. The risk of leukemia was increased with radiation, but actual numbers of cases were very small, she cautioned.
Attendee Dr. Pamela Goodwin, University of Toronto, said, “I’m just wondering whether you considered if it was valid to assume that there was a linear relationship between radiation dose and the risk of lung cancer in the range of radiation doses that you looked at, so, from the higher range in the earlier studies to the much lower dose now.”
Numbers of heart disease events were sufficient to establish a linear relationship, according to Dr. Taylor. Numbers of lung cancers were not, but case-control studies in the literature with adequate numbers have identified a linear relationship there, too. “We use what we can, and we have got now several hundred events, if you combine all of the literature together. And they do suggest the dose-response relationship is linear, but we can’t know that for certain,” she said.
SAN ANTONIO – The late side effects of modern radiation therapy for breast cancer depend in part on a woman’s smoking status, suggests a meta-analysis of data from more than 40,000 women presented at the San Antonio Breast Cancer Symposium.
For nonsmokers, radiation therapy had little impact on the absolute risks of lung cancer or cardiac death, the main risks identified, which combined totaled less than 1%, Dr. Carolyn Taylor reported on behalf of the Early Breast Cancer Trialists’ Collaborative Group. But for women who had smoked throughout their adult life and continued to do so during and after treatment, it increased that absolute risk to roughly 2%.
“Smoking status can determine the net long-term effects of breast cancer radiotherapy on mortality. Stopping smoking at the time of radiotherapy may avoid much of the risk, and that’s because most of the risk of lung cancer starts more than 10 years after radiotherapy,” said Dr. Taylor, a radiation oncologist at the University of Oxford (England).
Radiation therapy remains an important tool in treating breast cancer, ultimately reducing the likelihood of death from the disease, she reminded symposium attendees. “The absolute benefit in women treated according to current guidelines is a few percent. Let’s remember the magnitude of that benefit as we think about the risks of radiotherapy.”
Attendee Dr. Steven Vogl of Montefiore Medical Center, New York, asked whether information was available on the location of the lung cancers that occurred in the trials.
“We didn’t have location of lung cancers. We didn’t even know if it was ipsilateral or contralateral to the previous breast cancer in this study,” Dr. Taylor replied. “But we’ve done other studies where we have known the location of the lung cancer, and there were similar findings in those studies.”
“In the last 4 years, we’ve had very good information that annual CT screening substantially and very quickly reduces the mortality from lung cancer,” Dr. Vogl added as a comment. “Any of us who care for patients who have been radiated where, really, any lung has been treated, who continue to smoke, should be screened – and screened and screened and screened again,” he recommended.
The investigators analyzed data from 40,781 women with breast cancer from 75 randomized trials conducted worldwide that compared outcomes with versus without radiation therapy. The median year of trial entry was 1983. On average, women in the trials received 10 Gy to both lungs combined and 6 Gy to the heart.
Comparing women who did and did not receive radiation therapy, the rate ratio for lung cancer was 2.10 at 10 or more years out, and the rate ratio for cardiac mortality was 1.30 overall. Given the mean radiation doses in the trials, the excess risk translated to 12% per Gray for lung cancer and 4% per Gray for cardiac mortality. “These rate ratios are likely to apply today,” Dr. Taylor maintained.
However, she noted, contemporary breast cancer radiation therapy techniques are much better at sparing normal tissues. To derive absolute risk estimates that are relevant today, she and her colleagues reviewed the literature for 2010-2015 and determined that women now receive an average of 5 Gy to both lungs combined and 4 Gy to the heart, with some centers achieving even lower values.
Among nonsmokers, the estimated cumulative 30-year risk of lung cancer was 0.5% for women who did not receive radiation therapy and 0.8% for those who received radiation therapy with a mean dose of 5 Gy to both lungs combined, Dr. Taylor reported. However, among long-term smokers, it was 9.4% without radiation and a substantially higher 13.8% with it.
Similarly, among nonsmokers, the estimated cumulative 30-year risk of ischemic heart disease death was 1.8% for women who did not receive radiation therapy and 2.0% for women who received radiation therapy with a mean dose of 2 Gy to the heart. Among long-term smokers, it was 8.0% without radiation and a slightly higher 8.6% with it.
Additional analyses looking at other late side effects showed no radiation therapy–related excess risk of sarcomas, according to Dr. Taylor. The risk of leukemia was increased with radiation, but actual numbers of cases were very small, she cautioned.
Attendee Dr. Pamela Goodwin, University of Toronto, said, “I’m just wondering whether you considered if it was valid to assume that there was a linear relationship between radiation dose and the risk of lung cancer in the range of radiation doses that you looked at, so, from the higher range in the earlier studies to the much lower dose now.”
Numbers of heart disease events were sufficient to establish a linear relationship, according to Dr. Taylor. Numbers of lung cancers were not, but case-control studies in the literature with adequate numbers have identified a linear relationship there, too. “We use what we can, and we have got now several hundred events, if you combine all of the literature together. And they do suggest the dose-response relationship is linear, but we can’t know that for certain,” she said.
AT SABCS 2015
Key clinical point: Receipt of radiation therapy had little effect on risks among nonsmokers, but increased the risk of lung cancer and cardiac mortality among smokers.
Major finding: The cumulative 30-year risk of lung cancer differed little with radiation versus without radiation in nonsmokers (0.8% vs. 0.5%) but was much higher with radiation in smokers (13.8% vs. 9.4%).
Data source: A meta-analysis of 40,781 women with breast cancer in 75 randomized trials.
Disclosures: Dr. Taylor disclosed that she had no relevant conflicts of interest.
BRCA mutation predicts neoadjuvant therapy benefit but is not strongly prognostic
SAN ANTONIO – In patients with triple-negative breast cancer, the presence of a mutation in the breast cancer susceptibility genes BRCA1 and BRCA2 appears helpful for identifying those who will benefit from neoadjuvant chemotherapy but not very helpful for estimating prognosis, finds a subgroup analysis from the GeparQuinto trial.
Dr. Peter A. Fasching and colleagues studied 471 patients with triple-negative disease who were treated with neoadjuvant therapy in the trial, underwent surgery, and did not receive any postoperative therapy.
Prospectively specified genome-wide association studies showed that 17.4% of the patients had a BRCA1/2 mutation, he reported at the San Antonio Breast Cancer Symposium.
The rate of pathologic complete response (pCR), defined as ypT0/ypN0 stage, was 50% in the patients with a mutation, compared with 30.8% in those with wild-type genes (P = .001).
The patients with a mutation also had better disease-free survival than their wild-type peers (hazard ratio, 0.64), but this difference missed statistical significance (P = .06).
“BRCA mutation carriers had a significantly higher pathologic complete response rate after neoadjuvant chemotherapy,” commented Dr. Fasching, an oncologist the University Hospital Erlangen (Germany), Comprehensive Cancer Center Erlangen-EMN. “And BRCA mutation carriers had a better prognosis.”
Attendee Dr. George Somlo of City of Hope, Duarte, California, wondered if surgical management played a role in the findings. “Can you clarify…whether mastectomies on the ipsilateral side or contralateral side had any effect on the disease-free survival, since I assume once the mutations were known, other interventions might have taken place?”
“Unfortunately, I cannot,” Dr. Fasching replied. “We did not extend the exploratory analysis to that subpoint.”
Giving some background to the analysis, he commented, “Neoadjuvant studies may serve as a very good research ground to look for answers to both questions: whether BRCA mutation status predicts response to a chemotherapy and, furthermore, whether this translates into a prognostic effect.”
All patients with triple-negative disease in the GeparQuinto randomized phase III trial received neoadjuvant chemotherapy (epirubicin, cyclophosphamide, and docetaxel), with or without the antiangiogenic antibody bevacizumab (Avastin).
Additional findings showed that among BRCA mutation carriers, a pCR was not significantly associated with better disease-free survival. In contrast, among patients with wild-type genes, it was associated with a sharply reduced risk of events (HR, 0.21; P less than .0001). However, the interaction was not statistically significant.
“The prognostic information of pCR with regard to prognosis appeared to be somewhat weaker in patients with a BRCA mutation, compared to wild-type patients, but you have to keep in mind the test for interaction in this exploratory analysis was not significant,” Dr. Fasching commented.
The investigators also performed exploratory analyses looking at the impact of achieving a pCR in each treatment arm.
As far as a rationale, hypoxia is known to cause DNA damage, and synthetic lethality has been described in BRCA mutation carriers, Dr. Fasching explained. “There are some data that show that angiogenic factors are as a matter of fact increased in tumors with a BRCA mutation, compared to wild-type patients,” he added.
Results here showed that addition of bevacizumab improved pCR rate among both BRCA1/2 mutation carriers (65.7% vs. 38.3%, P = .025) and patients with wild-type (35.8% vs. 26.2%, P = .048). But addition of bevacizumab did not significantly improve disease-free survival in either group.
SAN ANTONIO – In patients with triple-negative breast cancer, the presence of a mutation in the breast cancer susceptibility genes BRCA1 and BRCA2 appears helpful for identifying those who will benefit from neoadjuvant chemotherapy but not very helpful for estimating prognosis, finds a subgroup analysis from the GeparQuinto trial.
Dr. Peter A. Fasching and colleagues studied 471 patients with triple-negative disease who were treated with neoadjuvant therapy in the trial, underwent surgery, and did not receive any postoperative therapy.
Prospectively specified genome-wide association studies showed that 17.4% of the patients had a BRCA1/2 mutation, he reported at the San Antonio Breast Cancer Symposium.
The rate of pathologic complete response (pCR), defined as ypT0/ypN0 stage, was 50% in the patients with a mutation, compared with 30.8% in those with wild-type genes (P = .001).
The patients with a mutation also had better disease-free survival than their wild-type peers (hazard ratio, 0.64), but this difference missed statistical significance (P = .06).
“BRCA mutation carriers had a significantly higher pathologic complete response rate after neoadjuvant chemotherapy,” commented Dr. Fasching, an oncologist the University Hospital Erlangen (Germany), Comprehensive Cancer Center Erlangen-EMN. “And BRCA mutation carriers had a better prognosis.”
Attendee Dr. George Somlo of City of Hope, Duarte, California, wondered if surgical management played a role in the findings. “Can you clarify…whether mastectomies on the ipsilateral side or contralateral side had any effect on the disease-free survival, since I assume once the mutations were known, other interventions might have taken place?”
“Unfortunately, I cannot,” Dr. Fasching replied. “We did not extend the exploratory analysis to that subpoint.”
Giving some background to the analysis, he commented, “Neoadjuvant studies may serve as a very good research ground to look for answers to both questions: whether BRCA mutation status predicts response to a chemotherapy and, furthermore, whether this translates into a prognostic effect.”
All patients with triple-negative disease in the GeparQuinto randomized phase III trial received neoadjuvant chemotherapy (epirubicin, cyclophosphamide, and docetaxel), with or without the antiangiogenic antibody bevacizumab (Avastin).
Additional findings showed that among BRCA mutation carriers, a pCR was not significantly associated with better disease-free survival. In contrast, among patients with wild-type genes, it was associated with a sharply reduced risk of events (HR, 0.21; P less than .0001). However, the interaction was not statistically significant.
“The prognostic information of pCR with regard to prognosis appeared to be somewhat weaker in patients with a BRCA mutation, compared to wild-type patients, but you have to keep in mind the test for interaction in this exploratory analysis was not significant,” Dr. Fasching commented.
The investigators also performed exploratory analyses looking at the impact of achieving a pCR in each treatment arm.
As far as a rationale, hypoxia is known to cause DNA damage, and synthetic lethality has been described in BRCA mutation carriers, Dr. Fasching explained. “There are some data that show that angiogenic factors are as a matter of fact increased in tumors with a BRCA mutation, compared to wild-type patients,” he added.
Results here showed that addition of bevacizumab improved pCR rate among both BRCA1/2 mutation carriers (65.7% vs. 38.3%, P = .025) and patients with wild-type (35.8% vs. 26.2%, P = .048). But addition of bevacizumab did not significantly improve disease-free survival in either group.
SAN ANTONIO – In patients with triple-negative breast cancer, the presence of a mutation in the breast cancer susceptibility genes BRCA1 and BRCA2 appears helpful for identifying those who will benefit from neoadjuvant chemotherapy but not very helpful for estimating prognosis, finds a subgroup analysis from the GeparQuinto trial.
Dr. Peter A. Fasching and colleagues studied 471 patients with triple-negative disease who were treated with neoadjuvant therapy in the trial, underwent surgery, and did not receive any postoperative therapy.
Prospectively specified genome-wide association studies showed that 17.4% of the patients had a BRCA1/2 mutation, he reported at the San Antonio Breast Cancer Symposium.
The rate of pathologic complete response (pCR), defined as ypT0/ypN0 stage, was 50% in the patients with a mutation, compared with 30.8% in those with wild-type genes (P = .001).
The patients with a mutation also had better disease-free survival than their wild-type peers (hazard ratio, 0.64), but this difference missed statistical significance (P = .06).
“BRCA mutation carriers had a significantly higher pathologic complete response rate after neoadjuvant chemotherapy,” commented Dr. Fasching, an oncologist the University Hospital Erlangen (Germany), Comprehensive Cancer Center Erlangen-EMN. “And BRCA mutation carriers had a better prognosis.”
Attendee Dr. George Somlo of City of Hope, Duarte, California, wondered if surgical management played a role in the findings. “Can you clarify…whether mastectomies on the ipsilateral side or contralateral side had any effect on the disease-free survival, since I assume once the mutations were known, other interventions might have taken place?”
“Unfortunately, I cannot,” Dr. Fasching replied. “We did not extend the exploratory analysis to that subpoint.”
Giving some background to the analysis, he commented, “Neoadjuvant studies may serve as a very good research ground to look for answers to both questions: whether BRCA mutation status predicts response to a chemotherapy and, furthermore, whether this translates into a prognostic effect.”
All patients with triple-negative disease in the GeparQuinto randomized phase III trial received neoadjuvant chemotherapy (epirubicin, cyclophosphamide, and docetaxel), with or without the antiangiogenic antibody bevacizumab (Avastin).
Additional findings showed that among BRCA mutation carriers, a pCR was not significantly associated with better disease-free survival. In contrast, among patients with wild-type genes, it was associated with a sharply reduced risk of events (HR, 0.21; P less than .0001). However, the interaction was not statistically significant.
“The prognostic information of pCR with regard to prognosis appeared to be somewhat weaker in patients with a BRCA mutation, compared to wild-type patients, but you have to keep in mind the test for interaction in this exploratory analysis was not significant,” Dr. Fasching commented.
The investigators also performed exploratory analyses looking at the impact of achieving a pCR in each treatment arm.
As far as a rationale, hypoxia is known to cause DNA damage, and synthetic lethality has been described in BRCA mutation carriers, Dr. Fasching explained. “There are some data that show that angiogenic factors are as a matter of fact increased in tumors with a BRCA mutation, compared to wild-type patients,” he added.
Results here showed that addition of bevacizumab improved pCR rate among both BRCA1/2 mutation carriers (65.7% vs. 38.3%, P = .025) and patients with wild-type (35.8% vs. 26.2%, P = .048). But addition of bevacizumab did not significantly improve disease-free survival in either group.
AT SABCS 2015
Key clinical point: BRCA1/2 mutation in triple-negative breast cancer is predictive but only weakly prognostic.
Major finding: Patients with a BRCA1/2 mutation were more likely than peers with wild-type genes to have a pCR to neoadjuvant therapy (50% vs. 30.8%), but they did not have significantly better disease-free survival.
Data source: A subgroup analysis of 471 patients with triple-negative breast cancer given neoadjuvant therapy in the GeparQuinto trial.
Disclosures: Dr. Fasching disclosed that he consults for Novartis, Genomic Health, Nanostring, Pfizer, Roche, and Teva; that he is a speaker for Novartis, Pfizer, Roche, Amgen, GSK, Genomic Health, and Teva; and that he receives contracts from Amgen and Novartis. The trial was sponsored by the German Breast Group in collaboration with the AGO Study Group.
Pembrolizumab shows promise in PD-L1–positive breast cancer
SAN ANTONIO – The immune checkpoint inhibitor pembrolizumab appears safe and modestly active in women with estrogen receptor (ER)–positive, HER2-negative advanced breast cancer that expresses programmed death–ligand 1 (PD-L1), according to preliminary results of a phase Ib trial presented at the San Antonio Breast Cancer Symposium.
Nineteen percent of women screened for the trial, KEYNOTE-028, had archival or fresh tumors from nonirradiated sites that tested positive for this ligand by immunohistochemistry, reported lead investigator Dr. Hope S. Rugo, clinical professor in the department of medicine (hematology/oncology) and director of the breast oncology clinical trials program at the University of California, San Francisco.
A total of 25 of these women received at least one dose of pembrolizumab (Keytruda), an antibody to the PD-1 (programmed death–1) cell surface receptor that blocks interaction of the receptor with its ligands, thereby preventing the inactivation of T cells. (The antibody is currently approved by the Food and Drug Administration for the treatment of melanoma and non–small cell lung cancer.)
With a median duration of follow-up of 7.3 months, the overall response rate as assessed by investigators using RECIST criteria was 12%, and the clinical benefit rate was 20%, Dr. Rugo reported. Responses were durable, lasting anywhere from about 9 weeks to more than 44 weeks.
The rate of grade 3 or 4 adverse events was 16%. Only a single patient had to have interruption of pembrolizumab because of the development of an immune adverse event.
“Based on these data, we believe that further investigation of immune therapies in ER-positive, HER2-negative breast cancer, particularly using combination therapies that can expand the T-cell compartment, are warranted,” Dr. Rugo maintained.
She noted that the trial’s findings differ somewhat from those of a similar trial testing avelumab, an investigational antibody against the PD-L1 ligand itself, that was presented at the symposium (abstract S1-04). That trial found a much higher PD-L1 positivity rate in screened women, 55%, and a much lower overall response rate, 3%.
These differences may have been due to use of different immunohistochemical assays for PD-L1, lack of data for some patients in the other trial, and/or differences in the target of the agent used (PD-1 vs PD-L1), she speculated. “It’s clear that as we move forward in this field of immunotherapy, which we are all very excited about, that standardization of assays assessing PD-L1 positivity are going to be critical,” she concluded.
“After how many studies will we be convinced [given] the discordance between different antibodies [and] scoring mechanisms, and the very, very weak enrichment in responders, that PD-L1 staining is not an adequate biomarker for these agents?” asked session attendee Justin Balko, Pharm.D., Ph.D., of Vanderbilt University, Nashville, Tenn.
“I think that we are going to need a consortium as well as development of guidelines for the best methods to test PD-L1 expression, whether that’s an immunohistochemical test, looking at mRNA, or looking at pathway activation,” Dr. Rugo replied. “Whether we need to include for example TILs [tumor-infiltrating lymphocytes] in evaluation or quantification of lymphocytes, which may or may not be practical, remains to be seen,” she said, adding that this research will require a collaborative effort.
Another attendee speculated that the disparate findings for the two trials may have been due to reliance on RECIST criteria in the KEYNOTE-028 trial. “I wonder if you had the opportunity to explore any of the immune-related response criteria that have been evaluated in other settings like melanoma,” she said.
“As a breast cancer field in immunotherapy, we are kind of behind everybody else, and we are just now starting to use immune RECIST as part of our assessments for the studies going on now prospectively in breast cancer,” Dr. Rugo replied. “ So no, we didn’t use immune RECIST. But it’s an interesting area to go back and look at, to see whether or not we can reclassify any of the responses.”
Giving some background to the research, she noted that PD-L1 expression has been inversely correlated with ER expression. A previous trial found an overall response rate of about 19% in triple-negative breast cancer (SABCS 2014, abstract S1-09).
For KEYNOTE-028, patients were screened for PD-L1 positivity, even though pembrolizumab targets PD-1, because there is much greater variability in ligand levels than in receptor levels when it comes to determining T cell inactivation, Dr. Rugo explained.
Fully 80% of the 25 women treated had received three or more prior lines of therapy for their advanced disease. They were give pembrolizumab every 2 weeks with assessments every 8 weeks initially.
The grade 3 or 4 adverse events observed were cases of autoimmune hepatitis, increased gamma-glutamyl transferase levels, muscle weakness, nausea, and septic shock. However, there were no treatment-related deaths.
In patients who developed any-grade immune-related adverse events of interest, just one – a patient with autoimmune hepatitis – had to have treatment interruption.
All of the responses observed were partial responses. The median time to response was 8 weeks, and the median duration of response was not reached, with a range from about 9 to 44 weeks. The three patients with a response had been in the study for at least 26 weeks as of the data cutoff for analysis.
“Cost is an issue with these drugs,” said attendee Dr. Mark Graham II, an oncologist with Waverly Hematology Oncology, Cary, N.C. “I’m particularly interested in your nonresponders and the fact that we can see pseudoprogression with these drugs. So for the eventual nonresponders, …what is the likely number of cycles that will be necessary to conclude that a patient is not an initial responder?”
That number is difficult to accurately pin down, as patients in the trial were taken off the agent as early as 8 weeks along if they had any evidence of progression, Dr. Rugo said. “I really don’t think we know the answer yet, but if you went out to 16 weeks, all the patients that we saw who were going to respond had responded by 16 weeks.”
An accurate answer will likely require future studies, she added. “I think it’s most complicated in triple-negative disease, where a small phase Ib trial showed a very long median time to response. So this is a good question and it remains to be answered.”
If sufficient tissue is available, the investigators plan to look for other biomarkers of pembrolizumab benefit, such as proliferation markers and intrinsic subtype, according to Dr. Rugo. “Probably those more in-depth investigations are going to occur with future studies, just because of the need to acquire enough tissue to do them. This trial was exploratory,” she said.
Dr. Rugo disclosed that her institution receives research funding from Merck & Co. – the trial sponsor – and Genentech.
SAN ANTONIO – The immune checkpoint inhibitor pembrolizumab appears safe and modestly active in women with estrogen receptor (ER)–positive, HER2-negative advanced breast cancer that expresses programmed death–ligand 1 (PD-L1), according to preliminary results of a phase Ib trial presented at the San Antonio Breast Cancer Symposium.
Nineteen percent of women screened for the trial, KEYNOTE-028, had archival or fresh tumors from nonirradiated sites that tested positive for this ligand by immunohistochemistry, reported lead investigator Dr. Hope S. Rugo, clinical professor in the department of medicine (hematology/oncology) and director of the breast oncology clinical trials program at the University of California, San Francisco.
A total of 25 of these women received at least one dose of pembrolizumab (Keytruda), an antibody to the PD-1 (programmed death–1) cell surface receptor that blocks interaction of the receptor with its ligands, thereby preventing the inactivation of T cells. (The antibody is currently approved by the Food and Drug Administration for the treatment of melanoma and non–small cell lung cancer.)
With a median duration of follow-up of 7.3 months, the overall response rate as assessed by investigators using RECIST criteria was 12%, and the clinical benefit rate was 20%, Dr. Rugo reported. Responses were durable, lasting anywhere from about 9 weeks to more than 44 weeks.
The rate of grade 3 or 4 adverse events was 16%. Only a single patient had to have interruption of pembrolizumab because of the development of an immune adverse event.
“Based on these data, we believe that further investigation of immune therapies in ER-positive, HER2-negative breast cancer, particularly using combination therapies that can expand the T-cell compartment, are warranted,” Dr. Rugo maintained.
She noted that the trial’s findings differ somewhat from those of a similar trial testing avelumab, an investigational antibody against the PD-L1 ligand itself, that was presented at the symposium (abstract S1-04). That trial found a much higher PD-L1 positivity rate in screened women, 55%, and a much lower overall response rate, 3%.
These differences may have been due to use of different immunohistochemical assays for PD-L1, lack of data for some patients in the other trial, and/or differences in the target of the agent used (PD-1 vs PD-L1), she speculated. “It’s clear that as we move forward in this field of immunotherapy, which we are all very excited about, that standardization of assays assessing PD-L1 positivity are going to be critical,” she concluded.
“After how many studies will we be convinced [given] the discordance between different antibodies [and] scoring mechanisms, and the very, very weak enrichment in responders, that PD-L1 staining is not an adequate biomarker for these agents?” asked session attendee Justin Balko, Pharm.D., Ph.D., of Vanderbilt University, Nashville, Tenn.
“I think that we are going to need a consortium as well as development of guidelines for the best methods to test PD-L1 expression, whether that’s an immunohistochemical test, looking at mRNA, or looking at pathway activation,” Dr. Rugo replied. “Whether we need to include for example TILs [tumor-infiltrating lymphocytes] in evaluation or quantification of lymphocytes, which may or may not be practical, remains to be seen,” she said, adding that this research will require a collaborative effort.
Another attendee speculated that the disparate findings for the two trials may have been due to reliance on RECIST criteria in the KEYNOTE-028 trial. “I wonder if you had the opportunity to explore any of the immune-related response criteria that have been evaluated in other settings like melanoma,” she said.
“As a breast cancer field in immunotherapy, we are kind of behind everybody else, and we are just now starting to use immune RECIST as part of our assessments for the studies going on now prospectively in breast cancer,” Dr. Rugo replied. “ So no, we didn’t use immune RECIST. But it’s an interesting area to go back and look at, to see whether or not we can reclassify any of the responses.”
Giving some background to the research, she noted that PD-L1 expression has been inversely correlated with ER expression. A previous trial found an overall response rate of about 19% in triple-negative breast cancer (SABCS 2014, abstract S1-09).
For KEYNOTE-028, patients were screened for PD-L1 positivity, even though pembrolizumab targets PD-1, because there is much greater variability in ligand levels than in receptor levels when it comes to determining T cell inactivation, Dr. Rugo explained.
Fully 80% of the 25 women treated had received three or more prior lines of therapy for their advanced disease. They were give pembrolizumab every 2 weeks with assessments every 8 weeks initially.
The grade 3 or 4 adverse events observed were cases of autoimmune hepatitis, increased gamma-glutamyl transferase levels, muscle weakness, nausea, and septic shock. However, there were no treatment-related deaths.
In patients who developed any-grade immune-related adverse events of interest, just one – a patient with autoimmune hepatitis – had to have treatment interruption.
All of the responses observed were partial responses. The median time to response was 8 weeks, and the median duration of response was not reached, with a range from about 9 to 44 weeks. The three patients with a response had been in the study for at least 26 weeks as of the data cutoff for analysis.
“Cost is an issue with these drugs,” said attendee Dr. Mark Graham II, an oncologist with Waverly Hematology Oncology, Cary, N.C. “I’m particularly interested in your nonresponders and the fact that we can see pseudoprogression with these drugs. So for the eventual nonresponders, …what is the likely number of cycles that will be necessary to conclude that a patient is not an initial responder?”
That number is difficult to accurately pin down, as patients in the trial were taken off the agent as early as 8 weeks along if they had any evidence of progression, Dr. Rugo said. “I really don’t think we know the answer yet, but if you went out to 16 weeks, all the patients that we saw who were going to respond had responded by 16 weeks.”
An accurate answer will likely require future studies, she added. “I think it’s most complicated in triple-negative disease, where a small phase Ib trial showed a very long median time to response. So this is a good question and it remains to be answered.”
If sufficient tissue is available, the investigators plan to look for other biomarkers of pembrolizumab benefit, such as proliferation markers and intrinsic subtype, according to Dr. Rugo. “Probably those more in-depth investigations are going to occur with future studies, just because of the need to acquire enough tissue to do them. This trial was exploratory,” she said.
Dr. Rugo disclosed that her institution receives research funding from Merck & Co. – the trial sponsor – and Genentech.
SAN ANTONIO – The immune checkpoint inhibitor pembrolizumab appears safe and modestly active in women with estrogen receptor (ER)–positive, HER2-negative advanced breast cancer that expresses programmed death–ligand 1 (PD-L1), according to preliminary results of a phase Ib trial presented at the San Antonio Breast Cancer Symposium.
Nineteen percent of women screened for the trial, KEYNOTE-028, had archival or fresh tumors from nonirradiated sites that tested positive for this ligand by immunohistochemistry, reported lead investigator Dr. Hope S. Rugo, clinical professor in the department of medicine (hematology/oncology) and director of the breast oncology clinical trials program at the University of California, San Francisco.
A total of 25 of these women received at least one dose of pembrolizumab (Keytruda), an antibody to the PD-1 (programmed death–1) cell surface receptor that blocks interaction of the receptor with its ligands, thereby preventing the inactivation of T cells. (The antibody is currently approved by the Food and Drug Administration for the treatment of melanoma and non–small cell lung cancer.)
With a median duration of follow-up of 7.3 months, the overall response rate as assessed by investigators using RECIST criteria was 12%, and the clinical benefit rate was 20%, Dr. Rugo reported. Responses were durable, lasting anywhere from about 9 weeks to more than 44 weeks.
The rate of grade 3 or 4 adverse events was 16%. Only a single patient had to have interruption of pembrolizumab because of the development of an immune adverse event.
“Based on these data, we believe that further investigation of immune therapies in ER-positive, HER2-negative breast cancer, particularly using combination therapies that can expand the T-cell compartment, are warranted,” Dr. Rugo maintained.
She noted that the trial’s findings differ somewhat from those of a similar trial testing avelumab, an investigational antibody against the PD-L1 ligand itself, that was presented at the symposium (abstract S1-04). That trial found a much higher PD-L1 positivity rate in screened women, 55%, and a much lower overall response rate, 3%.
These differences may have been due to use of different immunohistochemical assays for PD-L1, lack of data for some patients in the other trial, and/or differences in the target of the agent used (PD-1 vs PD-L1), she speculated. “It’s clear that as we move forward in this field of immunotherapy, which we are all very excited about, that standardization of assays assessing PD-L1 positivity are going to be critical,” she concluded.
“After how many studies will we be convinced [given] the discordance between different antibodies [and] scoring mechanisms, and the very, very weak enrichment in responders, that PD-L1 staining is not an adequate biomarker for these agents?” asked session attendee Justin Balko, Pharm.D., Ph.D., of Vanderbilt University, Nashville, Tenn.
“I think that we are going to need a consortium as well as development of guidelines for the best methods to test PD-L1 expression, whether that’s an immunohistochemical test, looking at mRNA, or looking at pathway activation,” Dr. Rugo replied. “Whether we need to include for example TILs [tumor-infiltrating lymphocytes] in evaluation or quantification of lymphocytes, which may or may not be practical, remains to be seen,” she said, adding that this research will require a collaborative effort.
Another attendee speculated that the disparate findings for the two trials may have been due to reliance on RECIST criteria in the KEYNOTE-028 trial. “I wonder if you had the opportunity to explore any of the immune-related response criteria that have been evaluated in other settings like melanoma,” she said.
“As a breast cancer field in immunotherapy, we are kind of behind everybody else, and we are just now starting to use immune RECIST as part of our assessments for the studies going on now prospectively in breast cancer,” Dr. Rugo replied. “ So no, we didn’t use immune RECIST. But it’s an interesting area to go back and look at, to see whether or not we can reclassify any of the responses.”
Giving some background to the research, she noted that PD-L1 expression has been inversely correlated with ER expression. A previous trial found an overall response rate of about 19% in triple-negative breast cancer (SABCS 2014, abstract S1-09).
For KEYNOTE-028, patients were screened for PD-L1 positivity, even though pembrolizumab targets PD-1, because there is much greater variability in ligand levels than in receptor levels when it comes to determining T cell inactivation, Dr. Rugo explained.
Fully 80% of the 25 women treated had received three or more prior lines of therapy for their advanced disease. They were give pembrolizumab every 2 weeks with assessments every 8 weeks initially.
The grade 3 or 4 adverse events observed were cases of autoimmune hepatitis, increased gamma-glutamyl transferase levels, muscle weakness, nausea, and septic shock. However, there were no treatment-related deaths.
In patients who developed any-grade immune-related adverse events of interest, just one – a patient with autoimmune hepatitis – had to have treatment interruption.
All of the responses observed were partial responses. The median time to response was 8 weeks, and the median duration of response was not reached, with a range from about 9 to 44 weeks. The three patients with a response had been in the study for at least 26 weeks as of the data cutoff for analysis.
“Cost is an issue with these drugs,” said attendee Dr. Mark Graham II, an oncologist with Waverly Hematology Oncology, Cary, N.C. “I’m particularly interested in your nonresponders and the fact that we can see pseudoprogression with these drugs. So for the eventual nonresponders, …what is the likely number of cycles that will be necessary to conclude that a patient is not an initial responder?”
That number is difficult to accurately pin down, as patients in the trial were taken off the agent as early as 8 weeks along if they had any evidence of progression, Dr. Rugo said. “I really don’t think we know the answer yet, but if you went out to 16 weeks, all the patients that we saw who were going to respond had responded by 16 weeks.”
An accurate answer will likely require future studies, she added. “I think it’s most complicated in triple-negative disease, where a small phase Ib trial showed a very long median time to response. So this is a good question and it remains to be answered.”
If sufficient tissue is available, the investigators plan to look for other biomarkers of pembrolizumab benefit, such as proliferation markers and intrinsic subtype, according to Dr. Rugo. “Probably those more in-depth investigations are going to occur with future studies, just because of the need to acquire enough tissue to do them. This trial was exploratory,” she said.
Dr. Rugo disclosed that her institution receives research funding from Merck & Co. – the trial sponsor – and Genentech.
AT SABCS 2015
Key clinical point: Pembrolizumab appears safe and modestly active for treatment of ER-positive, HER2-negative advanced breast cancer that expresses PD-L1.
Major finding: The overall response rate was 12%, and the rate of grade 3 or 4 adverse events was 16%.
Data source: An analysis of 25 women with PD-L1–positive, ER-positive, HER2-negative advanced breast cancer enrolled in a phase Ib trial of pembrolizumab monotherapy in solid tumors (KEYNOTE-028).
Disclosures: Dr. Rugo disclosed that her institution receives research funding from Merck & Co. – the trial sponsor – and Genentech.
Cotransplant boosted insulin secretion in type 1 diabetes
VANCOUVER – A new stem cell transplant approach addressed both underlying autoimmunity and pancreatic beta-cell repair and regeneration in a pilot study of patients with established type 1 diabetes mellitus.
In an open-label, randomized, controlled trial of 42 patients, cotransplantation of umbilical cord mesenchymal stromal cells and autologous bone marrow mononuclear cells, without any immunotherapy, was tested against usual care.
At 1 year, the area under the curve for C-peptide during an oral glucose tolerance test had more than doubled among the transplant recipients, whereas it had fallen among those managed with usual care, presenting author Xiumin Xu reported at the World Diabetes Congress. The transplant group also saw greater improvements in other metabolic measures, in immune indicators, and in scores for mental well-being and quality of life.
Overall, the transplant procedure was safe and well tolerated, she reported. Severe hypoglycemic events were less common in the transplant group. One patient had transient abdominal pain during the procedure, and another had bleeding at the site where the infusion catheter was placed.
“Although the absolute change in C-peptide is marginal, it is relatively significant in view of the long disease duration,” commented Ms. Xu, a researcher with the Diabetes Research Institute, Cell Transplant Center, University of Miami; The Cure Alliance, Miami; and the Diabetes Research Institute Federation in Hollywood, Fla.
The trial was unusual in enrolling patients with established diabetes, Ms. Xu pointed out. Most type 1 diabetes studies have enrolled patients with recent disease onset, but increasing evidence suggests some preservation of beta-cell mass and C-peptide production in those with established disease.
The researchers tapped mesenchymal cells for transplant because of their known ability to modulate immune response and tissue repair through paracrine mechanisms. In a preclinical model of diabetes, combining these cells with bone marrow cells synergistically improved glycemia and insulin levels (Stem Cells. 2008;26:244-53).
The trial, conducted in China, enrolled patients 18-40 years old who had type 1 diabetes for at least 2 years and were otherwise generally healthy. They were required to have a hemoglobin A1c level of 7.5%-10.5%, a fasting serum C-peptide level of less than 0.1 pmol/mL, and a daily insulin requirement of less than 100 IU.
The transplant group underwent sequential infusion of autologous bone marrow cells and umbilical cord mesenchymal stromal cells over 30 minutes through supraselective pancreatic artery cannulation. The control group continued to receive usual clinical care.
Trial results, reported at the congress and recently published (Diabetes Care January 2016. 39[1]:149-157), showed that transplant recipients, compared with their usual care counterparts, had improvements in the area under the curve for C-peptide (+105.7% vs. –7.7%, P = .013), the trial’s primary endpoint.
They also had improvements in the area under the curve for insulin (+49.3% vs. –5.7%, P = .027), hemoglobin A1c (–12.6% vs. +1.2%, P less than .01), fasting blood glucose (–24.4% vs. –4.3%, P less than .05), and daily insulin requirements (–29.2% vs. 0%, P less than .01).
“Although metabolic control improved in patients receiving stem cell transplant, insulin independence was not achieved,” noted Ms. Xu, who disclosed that she had no relevant conflicts of interest.
The transplant group also had more favorable changes in markers of immune function, such as an increase in interleukin-10 levels and a decrease in serum interferon-gamma levels.
Additionally, they had significant improvements in scores for anxiety, depression, and quality of life relative to their usual care peers.
VANCOUVER – A new stem cell transplant approach addressed both underlying autoimmunity and pancreatic beta-cell repair and regeneration in a pilot study of patients with established type 1 diabetes mellitus.
In an open-label, randomized, controlled trial of 42 patients, cotransplantation of umbilical cord mesenchymal stromal cells and autologous bone marrow mononuclear cells, without any immunotherapy, was tested against usual care.
At 1 year, the area under the curve for C-peptide during an oral glucose tolerance test had more than doubled among the transplant recipients, whereas it had fallen among those managed with usual care, presenting author Xiumin Xu reported at the World Diabetes Congress. The transplant group also saw greater improvements in other metabolic measures, in immune indicators, and in scores for mental well-being and quality of life.
Overall, the transplant procedure was safe and well tolerated, she reported. Severe hypoglycemic events were less common in the transplant group. One patient had transient abdominal pain during the procedure, and another had bleeding at the site where the infusion catheter was placed.
“Although the absolute change in C-peptide is marginal, it is relatively significant in view of the long disease duration,” commented Ms. Xu, a researcher with the Diabetes Research Institute, Cell Transplant Center, University of Miami; The Cure Alliance, Miami; and the Diabetes Research Institute Federation in Hollywood, Fla.
The trial was unusual in enrolling patients with established diabetes, Ms. Xu pointed out. Most type 1 diabetes studies have enrolled patients with recent disease onset, but increasing evidence suggests some preservation of beta-cell mass and C-peptide production in those with established disease.
The researchers tapped mesenchymal cells for transplant because of their known ability to modulate immune response and tissue repair through paracrine mechanisms. In a preclinical model of diabetes, combining these cells with bone marrow cells synergistically improved glycemia and insulin levels (Stem Cells. 2008;26:244-53).
The trial, conducted in China, enrolled patients 18-40 years old who had type 1 diabetes for at least 2 years and were otherwise generally healthy. They were required to have a hemoglobin A1c level of 7.5%-10.5%, a fasting serum C-peptide level of less than 0.1 pmol/mL, and a daily insulin requirement of less than 100 IU.
The transplant group underwent sequential infusion of autologous bone marrow cells and umbilical cord mesenchymal stromal cells over 30 minutes through supraselective pancreatic artery cannulation. The control group continued to receive usual clinical care.
Trial results, reported at the congress and recently published (Diabetes Care January 2016. 39[1]:149-157), showed that transplant recipients, compared with their usual care counterparts, had improvements in the area under the curve for C-peptide (+105.7% vs. –7.7%, P = .013), the trial’s primary endpoint.
They also had improvements in the area under the curve for insulin (+49.3% vs. –5.7%, P = .027), hemoglobin A1c (–12.6% vs. +1.2%, P less than .01), fasting blood glucose (–24.4% vs. –4.3%, P less than .05), and daily insulin requirements (–29.2% vs. 0%, P less than .01).
“Although metabolic control improved in patients receiving stem cell transplant, insulin independence was not achieved,” noted Ms. Xu, who disclosed that she had no relevant conflicts of interest.
The transplant group also had more favorable changes in markers of immune function, such as an increase in interleukin-10 levels and a decrease in serum interferon-gamma levels.
Additionally, they had significant improvements in scores for anxiety, depression, and quality of life relative to their usual care peers.
VANCOUVER – A new stem cell transplant approach addressed both underlying autoimmunity and pancreatic beta-cell repair and regeneration in a pilot study of patients with established type 1 diabetes mellitus.
In an open-label, randomized, controlled trial of 42 patients, cotransplantation of umbilical cord mesenchymal stromal cells and autologous bone marrow mononuclear cells, without any immunotherapy, was tested against usual care.
At 1 year, the area under the curve for C-peptide during an oral glucose tolerance test had more than doubled among the transplant recipients, whereas it had fallen among those managed with usual care, presenting author Xiumin Xu reported at the World Diabetes Congress. The transplant group also saw greater improvements in other metabolic measures, in immune indicators, and in scores for mental well-being and quality of life.
Overall, the transplant procedure was safe and well tolerated, she reported. Severe hypoglycemic events were less common in the transplant group. One patient had transient abdominal pain during the procedure, and another had bleeding at the site where the infusion catheter was placed.
“Although the absolute change in C-peptide is marginal, it is relatively significant in view of the long disease duration,” commented Ms. Xu, a researcher with the Diabetes Research Institute, Cell Transplant Center, University of Miami; The Cure Alliance, Miami; and the Diabetes Research Institute Federation in Hollywood, Fla.
The trial was unusual in enrolling patients with established diabetes, Ms. Xu pointed out. Most type 1 diabetes studies have enrolled patients with recent disease onset, but increasing evidence suggests some preservation of beta-cell mass and C-peptide production in those with established disease.
The researchers tapped mesenchymal cells for transplant because of their known ability to modulate immune response and tissue repair through paracrine mechanisms. In a preclinical model of diabetes, combining these cells with bone marrow cells synergistically improved glycemia and insulin levels (Stem Cells. 2008;26:244-53).
The trial, conducted in China, enrolled patients 18-40 years old who had type 1 diabetes for at least 2 years and were otherwise generally healthy. They were required to have a hemoglobin A1c level of 7.5%-10.5%, a fasting serum C-peptide level of less than 0.1 pmol/mL, and a daily insulin requirement of less than 100 IU.
The transplant group underwent sequential infusion of autologous bone marrow cells and umbilical cord mesenchymal stromal cells over 30 minutes through supraselective pancreatic artery cannulation. The control group continued to receive usual clinical care.
Trial results, reported at the congress and recently published (Diabetes Care January 2016. 39[1]:149-157), showed that transplant recipients, compared with their usual care counterparts, had improvements in the area under the curve for C-peptide (+105.7% vs. –7.7%, P = .013), the trial’s primary endpoint.
They also had improvements in the area under the curve for insulin (+49.3% vs. –5.7%, P = .027), hemoglobin A1c (–12.6% vs. +1.2%, P less than .01), fasting blood glucose (–24.4% vs. –4.3%, P less than .05), and daily insulin requirements (–29.2% vs. 0%, P less than .01).
“Although metabolic control improved in patients receiving stem cell transplant, insulin independence was not achieved,” noted Ms. Xu, who disclosed that she had no relevant conflicts of interest.
The transplant group also had more favorable changes in markers of immune function, such as an increase in interleukin-10 levels and a decrease in serum interferon-gamma levels.
Additionally, they had significant improvements in scores for anxiety, depression, and quality of life relative to their usual care peers.
AT THE WORLD DIABETES CONGRESS
Key clinical point: Cotransplantation with umbilical cord mesenchymal stromal cells and autologous bone marrow cells improved metabolic, immune, and quality of life indicators in patients with type 1 diabetes.
Major finding: The area under the curve for C-peptide increased by 105.7% with transplant, whereas it fell by 7.7% with usual care.
Data source: A pilot trial in 42 patients with established type 1 diabetes.
Disclosures: Ms. Xu disclosed that she had no relevant conflicts of interest.
Buparlisib overcomes endocrine resistance in hormone receptor–positive breast cancer
SAN ANTONIO – Buparlisib, an oral investigational pan-phosphoinositide 3-kinase (pan-PI3) kinase inhibitor, appears to be efficacious for overcoming endocrine resistance in advanced breast cancer, according to first results of the BELLE-2 trialreported at the San Antonio Breast Cancer Symposium. But benefit is likely restricted to patients whose tumors have a mutation of the PI3 kinase alpha gene (PIK3CA) detectable in circulating tumor DNA.
Trial participants were 1,147 postmenopausal women with hormone receptor–positive, HER2-negative locally advanced or metastatic breast cancer that had progressed on or after prior aromatase inhibitor therapy. They were randomized to treatment with buparlisib (formerly BKM120) or placebo, each added to the estrogen-receptor antagonist fulvestrant (Faslodex).
Results showed that buparlisib prolonged progression-free survival by about 2 months relative to placebo, meeting the trial’s primary endpoint, according to data reported in a session and related press briefing.
The presence of a PIK3CA mutation and/or loss of PTEN in archival tumor tissue was not helpful in identifying women who would benefit. But the presence of a PIK3CA mutation detected in circulating cell-free tumor DNA (a so-called liquid biopsy) was, with a progression-free survival advantage from buparlisib approaching 4 months in this subset.
“This is the first time that we showed that eliminating the PI3 kinase pathway may be a viable option for patients with hormone therapy–resistant breast cancer,” coinvestigator Dr. Mario Campone, Institut de Cancérologie de l’Ouest – René Gauducheau Centre de Recherche en Cancérologie, in Nantes, France, commented in the press briefing. “Frequent discontinuations due to adverse events reduced treatment duration in the buparlisib arm, potentially limiting the efficacy of combination therapy.”
“The BELLE-2 study suggests that assessment of PIK3CA mutations in a liquid biopsy may help select patients who benefit from adding a PI3 kinase inhibitor to endocrine therapy,” he added. “Further studies are warranted to confirm the utility and predictive value of PIK3CA mutations detected by liquid biopsy and tumor tissue, which we see in the SOLAR-1 clinical trial” testing the drug alpelisib (BYL719).
He speculated that the lack of predictive value of mutational status in the tumor vs. mutational status in the circulating tumor DNA was due in part to the timing of collection of these samples. In about 80% of cases, the tumor was the primary tumor, collected before any treatment, whereas the circulating tumor DNA was obtained at trial enrollment, after patients had been exposed to aromatase inhibitors.
Press briefing moderator Dr. C. Kent Osborne, a professor and director of the Dan L. Duncan Comprehensive Cancer Center, Baylor College of Medicine, Houston, commented that buparlisib “is not a home run, if you will, in terms of patients benefiting, probably because there are many other pathways that can contribute [to resistance] … And we just have to identify those and then determine which treatments we can combine together to have a better, more optimal effect.”
The 2-month benefit from buparlisib seen in the entire trial population was modest but based on median values, he cautioned. Looking at the progression-free survival curves at 2-3 years, the proportion of patients still free of events was about 20% with the drug, compared with 10% with placebo.
“It will take time to know whether we see an overall survival benefit. I wouldn’t be surprised if we didn’t,” given that patients in the control group will be given effective therapies at the time of progression, Dr. Osborne further commented. “A lot of studies in metastatic breast cancer show these sort of modest benefits, but then when you take the treatment earlier in the course of disease, such as in the adjuvant setting, for instance, you see a much more dramatic benefit. So this is the first time that a PI3 kinase inhibitor has shown this, and I would say that this is still an advance and tells us that this gives us more information about the importance of this pathway in endocrine-resistant disease.”
BELLE-2 was designed on the basis of data suggesting that activation of the PI3 kinase/mTOR pathway is a hallmark of hormone receptor–positive breast cancers that have become resistant to endocrine therapy, according to the investigators. Thus, a dual blockade of signaling through both the estrogen receptor pathway and the PI3 kinase pathway may circumvent resistance.
Trial results showed that the median duration of treatment was 4.2 months with buparlisib plus fulvestrant (1.9 months, specifically with buparlisib) and 5.0 months with placebo plus fulvestrant (4.0 months, specifically with placebo), reported Dr. Campone.
The drug was associated with a higher rate of grade 3 or 4 adverse events (77% vs. 32%), mainly driven by more cases of transaminitis, hyperglycemia, rash, and mood disorders.
In the entire trial population, median progression-free survival was 6.9 months with buparlisib and 5.0 months with placebo (hazard ratio, 0.78; P less than .001), meeting the trial’s primary endpoint.
In the subset of 372 patients with PI3K activation that was defined by presence of a PIK3CA mutation of any type (activating or not) and/or PTEN loss in archival tumor tissue, median progression-free survival was 6.8 months with buparlisib and 4.0 months with placebo (HR, 0.76), but the P value missed the threshold for significance in this analysis.
In a preplanned exploratory analysis, the investigators analyzed circulating tumor DNA in 587 patients for two PIK3CA mutations known to be activating mutations. Results here suggested that the progression-free survival benefit was limited to those who had a mutation – 7.0 months with buparlisib vs. 3.2 months with placebo (HR, 0.56; P less than .001) – with no significant benefit in patients having the wild-type (nonmutated) gene.
SAN ANTONIO – Buparlisib, an oral investigational pan-phosphoinositide 3-kinase (pan-PI3) kinase inhibitor, appears to be efficacious for overcoming endocrine resistance in advanced breast cancer, according to first results of the BELLE-2 trialreported at the San Antonio Breast Cancer Symposium. But benefit is likely restricted to patients whose tumors have a mutation of the PI3 kinase alpha gene (PIK3CA) detectable in circulating tumor DNA.
Trial participants were 1,147 postmenopausal women with hormone receptor–positive, HER2-negative locally advanced or metastatic breast cancer that had progressed on or after prior aromatase inhibitor therapy. They were randomized to treatment with buparlisib (formerly BKM120) or placebo, each added to the estrogen-receptor antagonist fulvestrant (Faslodex).
Results showed that buparlisib prolonged progression-free survival by about 2 months relative to placebo, meeting the trial’s primary endpoint, according to data reported in a session and related press briefing.
The presence of a PIK3CA mutation and/or loss of PTEN in archival tumor tissue was not helpful in identifying women who would benefit. But the presence of a PIK3CA mutation detected in circulating cell-free tumor DNA (a so-called liquid biopsy) was, with a progression-free survival advantage from buparlisib approaching 4 months in this subset.
“This is the first time that we showed that eliminating the PI3 kinase pathway may be a viable option for patients with hormone therapy–resistant breast cancer,” coinvestigator Dr. Mario Campone, Institut de Cancérologie de l’Ouest – René Gauducheau Centre de Recherche en Cancérologie, in Nantes, France, commented in the press briefing. “Frequent discontinuations due to adverse events reduced treatment duration in the buparlisib arm, potentially limiting the efficacy of combination therapy.”
“The BELLE-2 study suggests that assessment of PIK3CA mutations in a liquid biopsy may help select patients who benefit from adding a PI3 kinase inhibitor to endocrine therapy,” he added. “Further studies are warranted to confirm the utility and predictive value of PIK3CA mutations detected by liquid biopsy and tumor tissue, which we see in the SOLAR-1 clinical trial” testing the drug alpelisib (BYL719).
He speculated that the lack of predictive value of mutational status in the tumor vs. mutational status in the circulating tumor DNA was due in part to the timing of collection of these samples. In about 80% of cases, the tumor was the primary tumor, collected before any treatment, whereas the circulating tumor DNA was obtained at trial enrollment, after patients had been exposed to aromatase inhibitors.
Press briefing moderator Dr. C. Kent Osborne, a professor and director of the Dan L. Duncan Comprehensive Cancer Center, Baylor College of Medicine, Houston, commented that buparlisib “is not a home run, if you will, in terms of patients benefiting, probably because there are many other pathways that can contribute [to resistance] … And we just have to identify those and then determine which treatments we can combine together to have a better, more optimal effect.”
The 2-month benefit from buparlisib seen in the entire trial population was modest but based on median values, he cautioned. Looking at the progression-free survival curves at 2-3 years, the proportion of patients still free of events was about 20% with the drug, compared with 10% with placebo.
“It will take time to know whether we see an overall survival benefit. I wouldn’t be surprised if we didn’t,” given that patients in the control group will be given effective therapies at the time of progression, Dr. Osborne further commented. “A lot of studies in metastatic breast cancer show these sort of modest benefits, but then when you take the treatment earlier in the course of disease, such as in the adjuvant setting, for instance, you see a much more dramatic benefit. So this is the first time that a PI3 kinase inhibitor has shown this, and I would say that this is still an advance and tells us that this gives us more information about the importance of this pathway in endocrine-resistant disease.”
BELLE-2 was designed on the basis of data suggesting that activation of the PI3 kinase/mTOR pathway is a hallmark of hormone receptor–positive breast cancers that have become resistant to endocrine therapy, according to the investigators. Thus, a dual blockade of signaling through both the estrogen receptor pathway and the PI3 kinase pathway may circumvent resistance.
Trial results showed that the median duration of treatment was 4.2 months with buparlisib plus fulvestrant (1.9 months, specifically with buparlisib) and 5.0 months with placebo plus fulvestrant (4.0 months, specifically with placebo), reported Dr. Campone.
The drug was associated with a higher rate of grade 3 or 4 adverse events (77% vs. 32%), mainly driven by more cases of transaminitis, hyperglycemia, rash, and mood disorders.
In the entire trial population, median progression-free survival was 6.9 months with buparlisib and 5.0 months with placebo (hazard ratio, 0.78; P less than .001), meeting the trial’s primary endpoint.
In the subset of 372 patients with PI3K activation that was defined by presence of a PIK3CA mutation of any type (activating or not) and/or PTEN loss in archival tumor tissue, median progression-free survival was 6.8 months with buparlisib and 4.0 months with placebo (HR, 0.76), but the P value missed the threshold for significance in this analysis.
In a preplanned exploratory analysis, the investigators analyzed circulating tumor DNA in 587 patients for two PIK3CA mutations known to be activating mutations. Results here suggested that the progression-free survival benefit was limited to those who had a mutation – 7.0 months with buparlisib vs. 3.2 months with placebo (HR, 0.56; P less than .001) – with no significant benefit in patients having the wild-type (nonmutated) gene.
SAN ANTONIO – Buparlisib, an oral investigational pan-phosphoinositide 3-kinase (pan-PI3) kinase inhibitor, appears to be efficacious for overcoming endocrine resistance in advanced breast cancer, according to first results of the BELLE-2 trialreported at the San Antonio Breast Cancer Symposium. But benefit is likely restricted to patients whose tumors have a mutation of the PI3 kinase alpha gene (PIK3CA) detectable in circulating tumor DNA.
Trial participants were 1,147 postmenopausal women with hormone receptor–positive, HER2-negative locally advanced or metastatic breast cancer that had progressed on or after prior aromatase inhibitor therapy. They were randomized to treatment with buparlisib (formerly BKM120) or placebo, each added to the estrogen-receptor antagonist fulvestrant (Faslodex).
Results showed that buparlisib prolonged progression-free survival by about 2 months relative to placebo, meeting the trial’s primary endpoint, according to data reported in a session and related press briefing.
The presence of a PIK3CA mutation and/or loss of PTEN in archival tumor tissue was not helpful in identifying women who would benefit. But the presence of a PIK3CA mutation detected in circulating cell-free tumor DNA (a so-called liquid biopsy) was, with a progression-free survival advantage from buparlisib approaching 4 months in this subset.
“This is the first time that we showed that eliminating the PI3 kinase pathway may be a viable option for patients with hormone therapy–resistant breast cancer,” coinvestigator Dr. Mario Campone, Institut de Cancérologie de l’Ouest – René Gauducheau Centre de Recherche en Cancérologie, in Nantes, France, commented in the press briefing. “Frequent discontinuations due to adverse events reduced treatment duration in the buparlisib arm, potentially limiting the efficacy of combination therapy.”
“The BELLE-2 study suggests that assessment of PIK3CA mutations in a liquid biopsy may help select patients who benefit from adding a PI3 kinase inhibitor to endocrine therapy,” he added. “Further studies are warranted to confirm the utility and predictive value of PIK3CA mutations detected by liquid biopsy and tumor tissue, which we see in the SOLAR-1 clinical trial” testing the drug alpelisib (BYL719).
He speculated that the lack of predictive value of mutational status in the tumor vs. mutational status in the circulating tumor DNA was due in part to the timing of collection of these samples. In about 80% of cases, the tumor was the primary tumor, collected before any treatment, whereas the circulating tumor DNA was obtained at trial enrollment, after patients had been exposed to aromatase inhibitors.
Press briefing moderator Dr. C. Kent Osborne, a professor and director of the Dan L. Duncan Comprehensive Cancer Center, Baylor College of Medicine, Houston, commented that buparlisib “is not a home run, if you will, in terms of patients benefiting, probably because there are many other pathways that can contribute [to resistance] … And we just have to identify those and then determine which treatments we can combine together to have a better, more optimal effect.”
The 2-month benefit from buparlisib seen in the entire trial population was modest but based on median values, he cautioned. Looking at the progression-free survival curves at 2-3 years, the proportion of patients still free of events was about 20% with the drug, compared with 10% with placebo.
“It will take time to know whether we see an overall survival benefit. I wouldn’t be surprised if we didn’t,” given that patients in the control group will be given effective therapies at the time of progression, Dr. Osborne further commented. “A lot of studies in metastatic breast cancer show these sort of modest benefits, but then when you take the treatment earlier in the course of disease, such as in the adjuvant setting, for instance, you see a much more dramatic benefit. So this is the first time that a PI3 kinase inhibitor has shown this, and I would say that this is still an advance and tells us that this gives us more information about the importance of this pathway in endocrine-resistant disease.”
BELLE-2 was designed on the basis of data suggesting that activation of the PI3 kinase/mTOR pathway is a hallmark of hormone receptor–positive breast cancers that have become resistant to endocrine therapy, according to the investigators. Thus, a dual blockade of signaling through both the estrogen receptor pathway and the PI3 kinase pathway may circumvent resistance.
Trial results showed that the median duration of treatment was 4.2 months with buparlisib plus fulvestrant (1.9 months, specifically with buparlisib) and 5.0 months with placebo plus fulvestrant (4.0 months, specifically with placebo), reported Dr. Campone.
The drug was associated with a higher rate of grade 3 or 4 adverse events (77% vs. 32%), mainly driven by more cases of transaminitis, hyperglycemia, rash, and mood disorders.
In the entire trial population, median progression-free survival was 6.9 months with buparlisib and 5.0 months with placebo (hazard ratio, 0.78; P less than .001), meeting the trial’s primary endpoint.
In the subset of 372 patients with PI3K activation that was defined by presence of a PIK3CA mutation of any type (activating or not) and/or PTEN loss in archival tumor tissue, median progression-free survival was 6.8 months with buparlisib and 4.0 months with placebo (HR, 0.76), but the P value missed the threshold for significance in this analysis.
In a preplanned exploratory analysis, the investigators analyzed circulating tumor DNA in 587 patients for two PIK3CA mutations known to be activating mutations. Results here suggested that the progression-free survival benefit was limited to those who had a mutation – 7.0 months with buparlisib vs. 3.2 months with placebo (HR, 0.56; P less than .001) – with no significant benefit in patients having the wild-type (nonmutated) gene.
AT SABCS 2015
Key clinical point: Buparlisib modestly improves progression-free survival among women with progressive hormone receptor–positive, HER2-negative advanced breast cancer.
Major finding: Median progression-free survival was 6.9 months with buparlisib vs. 5.0 months with placebo.
Data source: A phase III randomized trial of buparlisib vs. placebo, each with fulvestrant, in 1,147 postmenopausal women with progressive hormone receptor–positive, HER2-negative advanced breast cancer (BELLE-2 trial).
Disclosures: Dr. Campone disclosed that he receives consulting fees from Novartis, Servier, and Menarini, and non-CME service fees from Novartis and Sanofi. The trial was sponsored by Novartis.
DNA-mutating enzyme is implicated in tamoxifen resistance
SAN ANTONIO – Women whose estrogen receptor–positive breast cancers have high expression of a DNA-mutating enzyme called APOBEC3B (A3B for short) are more likely to experience tamoxifen resistance, suggest data reported in a session and press briefing at the San Antonio Breast Cancer Symposium.
In a study of 285 women, the risk of progression or death on first-line tamoxifen was 67% higher for those whose primaries had high versus low expression of the enzyme, a DNA cytosine deaminase that is overexpressed in about half of all breast cancers.
Additionally, companion experiments in a xenograft breast cancer model showed that suppressing the enzyme delayed the development of tamoxifen resistance in tumors, whereas overexpressing it accelerated the development of resistance.
“High APOBECB3 expression levels correlate with poor outcomes for estrogen receptor–positive breast cancer, including recurrence during tamoxifen treatment,” said lead author Reuben Harris, Ph.D., an investigator at the Howard Hughes Medical Institute, professor at the University of Minnesota, Minneapolis, and member of the Masonic Cancer Center.
“One point to emphasize is that this is a gain-of-function mutating enzyme. A lot of things we hear about are loss of functions that are happening in cancer,” and at present, not much can be done about those, he said. But “because [APOBECB3] is an enzyme with an active site, I think that’s targetable.”
Dr. Harris drew an analogy between APOBEC mutagenesis and UV mutagenesis, noting that once the molecular mechanism was defined for the latter, interventions such as UV-blocking sunscreens and protective clothing were harnessed for prevention. “Now we have identified a significant source of mutation in breast cancer (as well as other cancers) and we can begin to think about innovations to control the mutating activity of this enzyme,” he elaborated.
Press briefing moderator and codirector of the San Antonio Breast Cancer Symposium, Dr. Carlos Arteaga, professor of medicine at Vanderbilt University, Nashville, Tenn,, and director of the Breast Cancer Program, asked, “Do you think this is ready to be addressed in the clinic? If you think so, can you speculate how we can do that?”
“I think it’s nearing readiness,” Dr. Harris replied. “We need more clinical studies. So far we’ve been focused on retrospective studies. … Some prospective studies need to be done. Obviously this needs to expand to include other treatments, and we also have to get a better handle on which subtypes are most affected by this mutagenic process.”
Previous research his team did in collaboration with their coinvestigators in the Netherlands showed that women with estrogen receptor–positive primary tumors treated only with resection had poorer progression-free and overall survival if their primaries had up-regulation of the A3B enzyme (Horm Cancer. 2014;5:405-13).
“This suggests that this is a prognostic marker for outcome, but we wanted to go further and ask if this process is contributing to or correlating with poor outcomes such as recurrence and resistance to tamoxifen therapy,” Dr. Harris commented.
In the new retrospective study, this time among women who experienced a recurrence of estrogen receptor–positive breast cancer and were then given first-line tamoxifen, the median progression-free survival was just 7.5 months for those with high A3B expression in the primary tumor, compared with 13.3 months for those with low expression (hazard ratio, 1.67; P = .0001).
“These are correlative studies that really suggest what many of the cellular experiments and genetic experiments in my lab and others have indicated, that this would be an adverse effector in estrogen receptor–positive breast cancer, but we wanted to test this hypothesis with a cause-and-effect experiment,” he said.
In xenograft experiments, they engineered estrogen receptor–positive MCF7L breast cancer cells to have either high or low expression of A3B and grew the cells into small tumors. They then grafted the tumors into female nude mice.
In a first set of experiments, knocking down expression of A3B slowed the development of tamoxifen resistance, and in fact, few tumors had become resistant to the drug even after a year, Dr. Harris reported. In a second set, overexpressing the enzyme in tumor cells by about 100-fold led to almost immediate tamoxifen resistance.
“So we can slow it down, and we can speed it up, and all of this is consistent with the clinical data indicating that those primary breast tumors that have high levels of this enzyme result in poorer outcomes than those tumors that have low levels,” he concluded.
As mutations induced by A3B might also affect the response to other treatments, ongoing research is now looking at its impact on the development of chemotherapy resistance, noted Dr. Harris.
SAN ANTONIO – Women whose estrogen receptor–positive breast cancers have high expression of a DNA-mutating enzyme called APOBEC3B (A3B for short) are more likely to experience tamoxifen resistance, suggest data reported in a session and press briefing at the San Antonio Breast Cancer Symposium.
In a study of 285 women, the risk of progression or death on first-line tamoxifen was 67% higher for those whose primaries had high versus low expression of the enzyme, a DNA cytosine deaminase that is overexpressed in about half of all breast cancers.
Additionally, companion experiments in a xenograft breast cancer model showed that suppressing the enzyme delayed the development of tamoxifen resistance in tumors, whereas overexpressing it accelerated the development of resistance.
“High APOBECB3 expression levels correlate with poor outcomes for estrogen receptor–positive breast cancer, including recurrence during tamoxifen treatment,” said lead author Reuben Harris, Ph.D., an investigator at the Howard Hughes Medical Institute, professor at the University of Minnesota, Minneapolis, and member of the Masonic Cancer Center.
“One point to emphasize is that this is a gain-of-function mutating enzyme. A lot of things we hear about are loss of functions that are happening in cancer,” and at present, not much can be done about those, he said. But “because [APOBECB3] is an enzyme with an active site, I think that’s targetable.”
Dr. Harris drew an analogy between APOBEC mutagenesis and UV mutagenesis, noting that once the molecular mechanism was defined for the latter, interventions such as UV-blocking sunscreens and protective clothing were harnessed for prevention. “Now we have identified a significant source of mutation in breast cancer (as well as other cancers) and we can begin to think about innovations to control the mutating activity of this enzyme,” he elaborated.
Press briefing moderator and codirector of the San Antonio Breast Cancer Symposium, Dr. Carlos Arteaga, professor of medicine at Vanderbilt University, Nashville, Tenn,, and director of the Breast Cancer Program, asked, “Do you think this is ready to be addressed in the clinic? If you think so, can you speculate how we can do that?”
“I think it’s nearing readiness,” Dr. Harris replied. “We need more clinical studies. So far we’ve been focused on retrospective studies. … Some prospective studies need to be done. Obviously this needs to expand to include other treatments, and we also have to get a better handle on which subtypes are most affected by this mutagenic process.”
Previous research his team did in collaboration with their coinvestigators in the Netherlands showed that women with estrogen receptor–positive primary tumors treated only with resection had poorer progression-free and overall survival if their primaries had up-regulation of the A3B enzyme (Horm Cancer. 2014;5:405-13).
“This suggests that this is a prognostic marker for outcome, but we wanted to go further and ask if this process is contributing to or correlating with poor outcomes such as recurrence and resistance to tamoxifen therapy,” Dr. Harris commented.
In the new retrospective study, this time among women who experienced a recurrence of estrogen receptor–positive breast cancer and were then given first-line tamoxifen, the median progression-free survival was just 7.5 months for those with high A3B expression in the primary tumor, compared with 13.3 months for those with low expression (hazard ratio, 1.67; P = .0001).
“These are correlative studies that really suggest what many of the cellular experiments and genetic experiments in my lab and others have indicated, that this would be an adverse effector in estrogen receptor–positive breast cancer, but we wanted to test this hypothesis with a cause-and-effect experiment,” he said.
In xenograft experiments, they engineered estrogen receptor–positive MCF7L breast cancer cells to have either high or low expression of A3B and grew the cells into small tumors. They then grafted the tumors into female nude mice.
In a first set of experiments, knocking down expression of A3B slowed the development of tamoxifen resistance, and in fact, few tumors had become resistant to the drug even after a year, Dr. Harris reported. In a second set, overexpressing the enzyme in tumor cells by about 100-fold led to almost immediate tamoxifen resistance.
“So we can slow it down, and we can speed it up, and all of this is consistent with the clinical data indicating that those primary breast tumors that have high levels of this enzyme result in poorer outcomes than those tumors that have low levels,” he concluded.
As mutations induced by A3B might also affect the response to other treatments, ongoing research is now looking at its impact on the development of chemotherapy resistance, noted Dr. Harris.
SAN ANTONIO – Women whose estrogen receptor–positive breast cancers have high expression of a DNA-mutating enzyme called APOBEC3B (A3B for short) are more likely to experience tamoxifen resistance, suggest data reported in a session and press briefing at the San Antonio Breast Cancer Symposium.
In a study of 285 women, the risk of progression or death on first-line tamoxifen was 67% higher for those whose primaries had high versus low expression of the enzyme, a DNA cytosine deaminase that is overexpressed in about half of all breast cancers.
Additionally, companion experiments in a xenograft breast cancer model showed that suppressing the enzyme delayed the development of tamoxifen resistance in tumors, whereas overexpressing it accelerated the development of resistance.
“High APOBECB3 expression levels correlate with poor outcomes for estrogen receptor–positive breast cancer, including recurrence during tamoxifen treatment,” said lead author Reuben Harris, Ph.D., an investigator at the Howard Hughes Medical Institute, professor at the University of Minnesota, Minneapolis, and member of the Masonic Cancer Center.
“One point to emphasize is that this is a gain-of-function mutating enzyme. A lot of things we hear about are loss of functions that are happening in cancer,” and at present, not much can be done about those, he said. But “because [APOBECB3] is an enzyme with an active site, I think that’s targetable.”
Dr. Harris drew an analogy between APOBEC mutagenesis and UV mutagenesis, noting that once the molecular mechanism was defined for the latter, interventions such as UV-blocking sunscreens and protective clothing were harnessed for prevention. “Now we have identified a significant source of mutation in breast cancer (as well as other cancers) and we can begin to think about innovations to control the mutating activity of this enzyme,” he elaborated.
Press briefing moderator and codirector of the San Antonio Breast Cancer Symposium, Dr. Carlos Arteaga, professor of medicine at Vanderbilt University, Nashville, Tenn,, and director of the Breast Cancer Program, asked, “Do you think this is ready to be addressed in the clinic? If you think so, can you speculate how we can do that?”
“I think it’s nearing readiness,” Dr. Harris replied. “We need more clinical studies. So far we’ve been focused on retrospective studies. … Some prospective studies need to be done. Obviously this needs to expand to include other treatments, and we also have to get a better handle on which subtypes are most affected by this mutagenic process.”
Previous research his team did in collaboration with their coinvestigators in the Netherlands showed that women with estrogen receptor–positive primary tumors treated only with resection had poorer progression-free and overall survival if their primaries had up-regulation of the A3B enzyme (Horm Cancer. 2014;5:405-13).
“This suggests that this is a prognostic marker for outcome, but we wanted to go further and ask if this process is contributing to or correlating with poor outcomes such as recurrence and resistance to tamoxifen therapy,” Dr. Harris commented.
In the new retrospective study, this time among women who experienced a recurrence of estrogen receptor–positive breast cancer and were then given first-line tamoxifen, the median progression-free survival was just 7.5 months for those with high A3B expression in the primary tumor, compared with 13.3 months for those with low expression (hazard ratio, 1.67; P = .0001).
“These are correlative studies that really suggest what many of the cellular experiments and genetic experiments in my lab and others have indicated, that this would be an adverse effector in estrogen receptor–positive breast cancer, but we wanted to test this hypothesis with a cause-and-effect experiment,” he said.
In xenograft experiments, they engineered estrogen receptor–positive MCF7L breast cancer cells to have either high or low expression of A3B and grew the cells into small tumors. They then grafted the tumors into female nude mice.
In a first set of experiments, knocking down expression of A3B slowed the development of tamoxifen resistance, and in fact, few tumors had become resistant to the drug even after a year, Dr. Harris reported. In a second set, overexpressing the enzyme in tumor cells by about 100-fold led to almost immediate tamoxifen resistance.
“So we can slow it down, and we can speed it up, and all of this is consistent with the clinical data indicating that those primary breast tumors that have high levels of this enzyme result in poorer outcomes than those tumors that have low levels,” he concluded.
As mutations induced by A3B might also affect the response to other treatments, ongoing research is now looking at its impact on the development of chemotherapy resistance, noted Dr. Harris.
AT SABCS 2015
Key clinical point: A high level of APOBEC3B expression in estrogen receptor–positive breast cancer is a marker for tamoxifen resistance.
Major finding: Median progression-free survival on first-line tamoxifen was 7.5 vs. 13.3 months for those with high vs. low APOBEC3B expression in their primary.
Data source: A retrospective cohort study of 285 women with recurrent estrogen receptor–positive breast cancer, and companion preclinical experiments in a xenograft breast cancer model.
Disclosures: Dr. Harris disclosed that he is a cofounder of ApoGen Biotechnologies and that he receives research support from Biogen.
WDC: Data help clarify which diabetic patients benefit from bariatric surgery
VANCOUVER – It may be time to reconsider the criteria used to select obese patients with type 2 diabetes mellitus for bariatric surgery, according to data from a pooled cohort study.
Typically, diabetic patients are considered eligible only if they have a body mass index of at least 35 kg/m2 and poorly controlled glycemia, according to lead author Dr. Geltrude Mingrone, department of internal medicine, Catholic University, Rome, and department of diabetes and nutritional sciences, King’s College, London.
But in her team’s new analysis of 727 patients, the best predictors of remission after bariatric surgery were a lower fasting glucose level, shorter diabetes duration at baseline, and having a gastric procedure with diversion, she reported at the World Diabetes Congress. And the best baseline predictors of improved glycemic control after bariatric surgery were lower waist circumference, better diabetes control, and lower triglyceride levels.
“We can say that there is a clear advantage of an early operation on diabetes remission that is independent of baseline body mass index. Baseline waist circumference and HOMA-IR [homeostasis model assessment of insulin resistance] are better predictors of glycemic control after bariatric surgery than body mass index,” Dr. Mingrone maintained.
“So I would like to advise the scientific community … to try to define new criteria for the selection of diabetic patients for metabolic surgery. Also, [it is important] because all over the world, the number of bariatric operations is only 250,000, a quarter of a million, while the potential eligible patients are many, many millions,” she concluded.
“I completely concur with Professor Mingrone – we need to come up with criteria for utilizing this procedure,” session comoderator Dr. Robert E. Ratner said in an interview.
“There are so many millions of individuals with diabetes, we cannot be doing surgery on all of them. We need to be selective, and we need to be very specific about why we are doing it and what we are looking for,” elaborated Dr. Rattner, who is a professor of medicine at Georgetown University and senior research scientist at the MedStar Health Research Institute, Washington, as well as chief scientific and medical officer of the American Diabetes Association, Alexandria, Va.
In the new research, the investigators merged databases of the Swedish Obese Subjects (SOS) prospective controlled study (N Engl J Med. 2004;351:2683-93) and two randomized, controlled trials conducted in Australia (JAMA. 2008;299:316-23) and Italy (N Engl J Med. 2012;366:1577-85).
Overall, 415 patients had bariatric surgery (about three-fourths had a gastric-only procedure, while the rest had a gastric procedure plus diversion) and 312 patients had medical management. The mean duration of diabetes at baseline was roughly 3.5 years.
After 2 years the proportion of patients achieving diabetes remission, defined as a fasting plasma glucose of less than 5.6 mmol/L in the absence of any antidiabetes medication, was higher in the surgical group than in the medical group (64% vs. 15%, P less than .001). And within the surgical group, it was higher for those whose operation included a diversion (76% vs. 60%, P = .016).
Among the patients who had surgery, the probability of remission fell with increasing baseline diabetes duration (–0.210), and fasting blood glucose level (–0.145), whereas it rose with increasing body mass index (+0.059). But when the type of surgery was added to the model, body mass index was no longer a significant predictor, and two additional predictors emerged: use of only oral antidiabetic medications versus none (–1.22) and receipt of a procedure with diversion (2.180).
In the subgroup who had a gastric-only procedure, the probability of remission fell with increasing diabetes duration (–0.197), and increasing fasting blood glucose level (–0.186), and it was lower for patients who used only oral antidiabetic medications (–1.364) or insulin with or without oral medications (–1.783). In the subgroup who had a gastric procedure with diversion, the only predictor was diabetes duration (–0.273).
Of note, there was no significant difference in the odds of remission between patients with a body mass index of 35 kg/m2 or lower and patients with a body index between 35 and 40 kg/m2.
Dr. Mingrone disclosed that she receives lecture fees and travel expenses from Novo Nordisk, and research grants from AstraZeneca, and is a consultant to Fractyl.
VANCOUVER – It may be time to reconsider the criteria used to select obese patients with type 2 diabetes mellitus for bariatric surgery, according to data from a pooled cohort study.
Typically, diabetic patients are considered eligible only if they have a body mass index of at least 35 kg/m2 and poorly controlled glycemia, according to lead author Dr. Geltrude Mingrone, department of internal medicine, Catholic University, Rome, and department of diabetes and nutritional sciences, King’s College, London.
But in her team’s new analysis of 727 patients, the best predictors of remission after bariatric surgery were a lower fasting glucose level, shorter diabetes duration at baseline, and having a gastric procedure with diversion, she reported at the World Diabetes Congress. And the best baseline predictors of improved glycemic control after bariatric surgery were lower waist circumference, better diabetes control, and lower triglyceride levels.
“We can say that there is a clear advantage of an early operation on diabetes remission that is independent of baseline body mass index. Baseline waist circumference and HOMA-IR [homeostasis model assessment of insulin resistance] are better predictors of glycemic control after bariatric surgery than body mass index,” Dr. Mingrone maintained.
“So I would like to advise the scientific community … to try to define new criteria for the selection of diabetic patients for metabolic surgery. Also, [it is important] because all over the world, the number of bariatric operations is only 250,000, a quarter of a million, while the potential eligible patients are many, many millions,” she concluded.
“I completely concur with Professor Mingrone – we need to come up with criteria for utilizing this procedure,” session comoderator Dr. Robert E. Ratner said in an interview.
“There are so many millions of individuals with diabetes, we cannot be doing surgery on all of them. We need to be selective, and we need to be very specific about why we are doing it and what we are looking for,” elaborated Dr. Rattner, who is a professor of medicine at Georgetown University and senior research scientist at the MedStar Health Research Institute, Washington, as well as chief scientific and medical officer of the American Diabetes Association, Alexandria, Va.
In the new research, the investigators merged databases of the Swedish Obese Subjects (SOS) prospective controlled study (N Engl J Med. 2004;351:2683-93) and two randomized, controlled trials conducted in Australia (JAMA. 2008;299:316-23) and Italy (N Engl J Med. 2012;366:1577-85).
Overall, 415 patients had bariatric surgery (about three-fourths had a gastric-only procedure, while the rest had a gastric procedure plus diversion) and 312 patients had medical management. The mean duration of diabetes at baseline was roughly 3.5 years.
After 2 years the proportion of patients achieving diabetes remission, defined as a fasting plasma glucose of less than 5.6 mmol/L in the absence of any antidiabetes medication, was higher in the surgical group than in the medical group (64% vs. 15%, P less than .001). And within the surgical group, it was higher for those whose operation included a diversion (76% vs. 60%, P = .016).
Among the patients who had surgery, the probability of remission fell with increasing baseline diabetes duration (–0.210), and fasting blood glucose level (–0.145), whereas it rose with increasing body mass index (+0.059). But when the type of surgery was added to the model, body mass index was no longer a significant predictor, and two additional predictors emerged: use of only oral antidiabetic medications versus none (–1.22) and receipt of a procedure with diversion (2.180).
In the subgroup who had a gastric-only procedure, the probability of remission fell with increasing diabetes duration (–0.197), and increasing fasting blood glucose level (–0.186), and it was lower for patients who used only oral antidiabetic medications (–1.364) or insulin with or without oral medications (–1.783). In the subgroup who had a gastric procedure with diversion, the only predictor was diabetes duration (–0.273).
Of note, there was no significant difference in the odds of remission between patients with a body mass index of 35 kg/m2 or lower and patients with a body index between 35 and 40 kg/m2.
Dr. Mingrone disclosed that she receives lecture fees and travel expenses from Novo Nordisk, and research grants from AstraZeneca, and is a consultant to Fractyl.
VANCOUVER – It may be time to reconsider the criteria used to select obese patients with type 2 diabetes mellitus for bariatric surgery, according to data from a pooled cohort study.
Typically, diabetic patients are considered eligible only if they have a body mass index of at least 35 kg/m2 and poorly controlled glycemia, according to lead author Dr. Geltrude Mingrone, department of internal medicine, Catholic University, Rome, and department of diabetes and nutritional sciences, King’s College, London.
But in her team’s new analysis of 727 patients, the best predictors of remission after bariatric surgery were a lower fasting glucose level, shorter diabetes duration at baseline, and having a gastric procedure with diversion, she reported at the World Diabetes Congress. And the best baseline predictors of improved glycemic control after bariatric surgery were lower waist circumference, better diabetes control, and lower triglyceride levels.
“We can say that there is a clear advantage of an early operation on diabetes remission that is independent of baseline body mass index. Baseline waist circumference and HOMA-IR [homeostasis model assessment of insulin resistance] are better predictors of glycemic control after bariatric surgery than body mass index,” Dr. Mingrone maintained.
“So I would like to advise the scientific community … to try to define new criteria for the selection of diabetic patients for metabolic surgery. Also, [it is important] because all over the world, the number of bariatric operations is only 250,000, a quarter of a million, while the potential eligible patients are many, many millions,” she concluded.
“I completely concur with Professor Mingrone – we need to come up with criteria for utilizing this procedure,” session comoderator Dr. Robert E. Ratner said in an interview.
“There are so many millions of individuals with diabetes, we cannot be doing surgery on all of them. We need to be selective, and we need to be very specific about why we are doing it and what we are looking for,” elaborated Dr. Rattner, who is a professor of medicine at Georgetown University and senior research scientist at the MedStar Health Research Institute, Washington, as well as chief scientific and medical officer of the American Diabetes Association, Alexandria, Va.
In the new research, the investigators merged databases of the Swedish Obese Subjects (SOS) prospective controlled study (N Engl J Med. 2004;351:2683-93) and two randomized, controlled trials conducted in Australia (JAMA. 2008;299:316-23) and Italy (N Engl J Med. 2012;366:1577-85).
Overall, 415 patients had bariatric surgery (about three-fourths had a gastric-only procedure, while the rest had a gastric procedure plus diversion) and 312 patients had medical management. The mean duration of diabetes at baseline was roughly 3.5 years.
After 2 years the proportion of patients achieving diabetes remission, defined as a fasting plasma glucose of less than 5.6 mmol/L in the absence of any antidiabetes medication, was higher in the surgical group than in the medical group (64% vs. 15%, P less than .001). And within the surgical group, it was higher for those whose operation included a diversion (76% vs. 60%, P = .016).
Among the patients who had surgery, the probability of remission fell with increasing baseline diabetes duration (–0.210), and fasting blood glucose level (–0.145), whereas it rose with increasing body mass index (+0.059). But when the type of surgery was added to the model, body mass index was no longer a significant predictor, and two additional predictors emerged: use of only oral antidiabetic medications versus none (–1.22) and receipt of a procedure with diversion (2.180).
In the subgroup who had a gastric-only procedure, the probability of remission fell with increasing diabetes duration (–0.197), and increasing fasting blood glucose level (–0.186), and it was lower for patients who used only oral antidiabetic medications (–1.364) or insulin with or without oral medications (–1.783). In the subgroup who had a gastric procedure with diversion, the only predictor was diabetes duration (–0.273).
Of note, there was no significant difference in the odds of remission between patients with a body mass index of 35 kg/m2 or lower and patients with a body index between 35 and 40 kg/m2.
Dr. Mingrone disclosed that she receives lecture fees and travel expenses from Novo Nordisk, and research grants from AstraZeneca, and is a consultant to Fractyl.
AT THE WORLD DIABETES CONGRESS
Key clinical point: Compared with BMI, disease duration and control and type of procedure are better predictors of diabetes remission and control after bariatric surgery.
Major finding: Patients had a higher probability of remission at 2 years if they had lower baseline glycemia and shorter diabetes duration, and if they underwent a gastric procedure with diversion.
Data source: A pooled cohort study of 727 obese patients with type 2 diabetes who were managed with bariatric surgery or with medical therapy.
Disclosures: Dr. Mingrone disclosed that she receives lecture fees and travel expenses from Novo Nordisk and research grants from AstraZeneca, and that she is a consultant to Fractyl.