User login
Legacy of neutral renal denervation trial recast by long-term outcomes: SYMPLICITY HTN-3
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.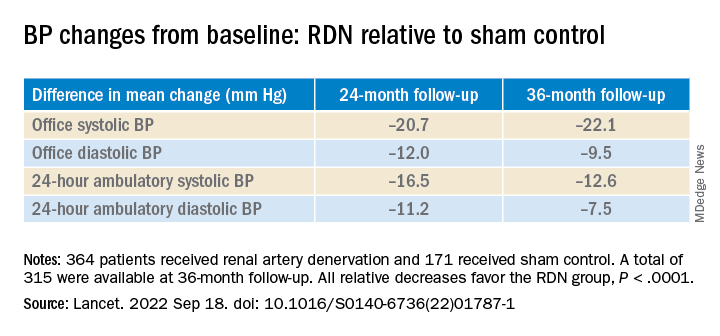
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
AT TCT 2022
PASCAL for MV repair noninferior to MitraClip in CLASP IID; FDA took notice
A newly available transcatheter device for edge-to-edge mitral valve (MV) repair, named for a famed scientist-inventor, is similar to the long-available MitraClip (Abbott) for short-term efficacy and safety, suggests an interim but prespecified analysis from a randomized trial.
In its comparison with MitraClip, the PASCAL transcatheter valve repair system (Edwards Lifesciences) was noninferior with respect to 30-day major adverse events and to success at achieving mitral regurgitation (MR) of no more than moderate severity within 6 months. The trial had entered patients with significant, symptomatic degenerative MR considered too high-risk for surgical repair or replacement.
The interim analysis covers 180 of the 300 patients followed in the study, of whom 117 received the PASCAL device and 63 were given MitraClip. Both groups showed significant gains in functional class, symptom status, and quality of life over 6 months, reported D. Scott Lim, MD, University of Virginia Health System Hospital, Charlottesville, and Konstantinos Koulogiannis, MD, Morristown Medical Center, N.J., jointly on Sept. 17 at the Transcatheter Cardiovascular Therapeutics (TCT) 2022 annual meeting in Boston.
Dr. Lim, one of the trial’s principal investigators, is also lead author on its same-day publication in JACC: Cardiovascular Interventions.
Based largely on those results from the CLASP IID pivotal trial, the U.S. Food and Drug Administration recently approved the PASCAL system for use in patients with degenerative MR, Edwards announced on Sept. 15. The device was approved in the European Union on Aug. 17.
MitraClip has been available in various iterations in the United States since 2013 and in Europe since 2008.
“It’s good for the field to be able to say we have two devices that are comparable,” giving clinicians more options, Vinod H. Thourani, MD, Piedmont Heart Institute, Atlanta, told this news organization.
The current analysis shows that “we’ve yet to figure out what patient pathologies will be beneficial” for each of the devices, Dr. Thourani said. “The goal will be to find out if there are certain anatomical considerations where one device is better than the other.”
It will be necessary to study “more patients, a larger cohort, with longer follow-up to allow us to see their true benefits,” he said, as well as to conduct more subgroup analyses. For now, the choice of device will probably be “operator-specific, which they feel comfortable with.”
Dr. Thourani, not an author on the current study, is the U.S. principal investigator for the CLASP IIF study looking at clinical outcomes with the two devices and says he consults for both Edwards and Abbott.
The findings are “preliminary for now,” said Michael Young, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., in part because, like most randomized trials, CLASP IID entered a select, not broadly representative population.
“They want to make, as best as they could, an apples-to-apples comparison, without confounding that might make it more difficult to interpret it afterwards,” Dr. Young, not associated with the trial, told this news organization.
But CLASP IID “did enroll patients that we do see and treat, so undoubtedly it’s a compelling study. We now have another device that is shown to be safe and effective. How we’re going to extrapolate it to all the patients that are being referred to our practices will, I think, be under debate and deliberation.”
The PASCAL and MitraClip devices each may be more suitable for different patients with varying mitral valve pathologies because of differences in their designs, Dr. Lim said. The PASCAL’s relative flexibility might make it preferable in patients with smaller mitral valves, and its ability to elongate during delivery could make it more suitable for patients with chordal-dense areas around the valve, he speculated.
MitraClip, Dr. Lim told this news organization, has a mechanical closure system for anchoring that may make it more appropriate for “more complicated, thicker leaflets with calcium.”
CLASP IID enrolled patients with grade 3+ or 4+ degenerative MR considered to be “at prohibitive surgical risk” at 43 sites in North America and Europe. It randomly assigned them 2-to-1 to receive the PASCAL device or MitraClip.
Either of two PASCAL versions were used, the original device or the “smaller, narrower” PASCAL Ace, Dr. Lim observed. Both versions are covered by the PASCAL Precision System FDA approval. About 40% of patients assigned to MitraClip received older versions of the device and about 60%, more recent versions, as they were entered into practice.
The mean procedure times were 88 minutes for PASCAL and 79 minutes for MitraClip (P = .023), with much of the difference attributable to the earliest PASCAL procedures. Procedure times for the device declined with greater operator experience, the published report states.
Rates of the primary safety endpoint of major adverse events at 30 days were 3.4% for PASCAL and 4.8% for MitraClip. The endpoint was a composite of cardiovascular mortality, stroke, myocardial infarction, new need for renal replacement therapy, severe bleeding, or nonelective MV reintervention.
The proportion of patients with MR grade 2+ or lower at 6 months, the primary effectiveness endpoint, assessed at a core laboratory, was 96.5% for the PASCAL group over a median follow-up of 179.5 days and 96.8% over a median of 184.5 days for those who received MitraClip.
Comparisons for both primary endpoints met the prespecified criteria for PASCAL noninferiority.
In a secondary analysis, the proportion of PASCAL patients with MR grade 1+ or less held about steady from postprocedure discharge out to 6 months, at 87.2% and 83.7%, respectively (P = .317).
But whereas 88.5% of MitraClip patients had MR grade 1+ or better at discharge, 71.2% were at that grade by 6 months (P = .003). That apparent hemodynamic deterioration raised some eyebrows at the TCT sessions as a potential sign that PASCAL functional results are more durable.
That sort of judgment is premature, offered Anita W. Asgar, MD, MSc, Montreal Heart Institute, Quebec City, as an invited discussant after the CLASP IID trial’s formal presentation at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The trial is notable in part for “showing how safe this procedure is and how successful it is for these patients – this is phenomenal,” she said, but “I would caution comparing one device being better than another with such a small number of patients.”
MitraClip, Dr. Young observed, “has been, up to this point, our only option for edge-to-edge repair of the mitral valve. And many of us have years of experience and a lot of patients that we treat with that device.” His center hasn’t yet used PASCAL, but that may change as the field gains more familiarity with the device. Operators may use either device in different cases, he said.
“Depending on the program, and depending on the volume of mitral patients that you see and edge-to-edge repair that you do, it could be that you stick with one, or switch to another, or you integrate both of them and try to decide which patients might be better suited for one or the other.”
CLASP IID was sponsored by Edwards Lifesciences. Dr. Lim discloses consulting for Philips, Venus, and Valgen and receiving research grants from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. Dr. Koulogiannis discloses consulting and serving on an advisory board for Edwards Lifesciences and as a speaker for Abbott and discloses holding equity, stocks, or stock options in 4C. Dr. Thourani discloses serving as a consultant to both Abbott and Edwards Lifesciences. Dr. Young discloses receiving consulting fees or honoraria or serving on a speaker’s bureau for Medtronic. Dr. Asgar discloses receiving research support from or holding a research contract with Abbott Vascular and receiving consulting fees or honoraria or serving on a speaker’s bureau for Medtronic, Edwards Lifesciences, and W. Gore & Associates.
A version of this article first appeared on Medscape.com.
A newly available transcatheter device for edge-to-edge mitral valve (MV) repair, named for a famed scientist-inventor, is similar to the long-available MitraClip (Abbott) for short-term efficacy and safety, suggests an interim but prespecified analysis from a randomized trial.
In its comparison with MitraClip, the PASCAL transcatheter valve repair system (Edwards Lifesciences) was noninferior with respect to 30-day major adverse events and to success at achieving mitral regurgitation (MR) of no more than moderate severity within 6 months. The trial had entered patients with significant, symptomatic degenerative MR considered too high-risk for surgical repair or replacement.
The interim analysis covers 180 of the 300 patients followed in the study, of whom 117 received the PASCAL device and 63 were given MitraClip. Both groups showed significant gains in functional class, symptom status, and quality of life over 6 months, reported D. Scott Lim, MD, University of Virginia Health System Hospital, Charlottesville, and Konstantinos Koulogiannis, MD, Morristown Medical Center, N.J., jointly on Sept. 17 at the Transcatheter Cardiovascular Therapeutics (TCT) 2022 annual meeting in Boston.
Dr. Lim, one of the trial’s principal investigators, is also lead author on its same-day publication in JACC: Cardiovascular Interventions.
Based largely on those results from the CLASP IID pivotal trial, the U.S. Food and Drug Administration recently approved the PASCAL system for use in patients with degenerative MR, Edwards announced on Sept. 15. The device was approved in the European Union on Aug. 17.
MitraClip has been available in various iterations in the United States since 2013 and in Europe since 2008.
“It’s good for the field to be able to say we have two devices that are comparable,” giving clinicians more options, Vinod H. Thourani, MD, Piedmont Heart Institute, Atlanta, told this news organization.
The current analysis shows that “we’ve yet to figure out what patient pathologies will be beneficial” for each of the devices, Dr. Thourani said. “The goal will be to find out if there are certain anatomical considerations where one device is better than the other.”
It will be necessary to study “more patients, a larger cohort, with longer follow-up to allow us to see their true benefits,” he said, as well as to conduct more subgroup analyses. For now, the choice of device will probably be “operator-specific, which they feel comfortable with.”
Dr. Thourani, not an author on the current study, is the U.S. principal investigator for the CLASP IIF study looking at clinical outcomes with the two devices and says he consults for both Edwards and Abbott.
The findings are “preliminary for now,” said Michael Young, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., in part because, like most randomized trials, CLASP IID entered a select, not broadly representative population.
“They want to make, as best as they could, an apples-to-apples comparison, without confounding that might make it more difficult to interpret it afterwards,” Dr. Young, not associated with the trial, told this news organization.
But CLASP IID “did enroll patients that we do see and treat, so undoubtedly it’s a compelling study. We now have another device that is shown to be safe and effective. How we’re going to extrapolate it to all the patients that are being referred to our practices will, I think, be under debate and deliberation.”
The PASCAL and MitraClip devices each may be more suitable for different patients with varying mitral valve pathologies because of differences in their designs, Dr. Lim said. The PASCAL’s relative flexibility might make it preferable in patients with smaller mitral valves, and its ability to elongate during delivery could make it more suitable for patients with chordal-dense areas around the valve, he speculated.
MitraClip, Dr. Lim told this news organization, has a mechanical closure system for anchoring that may make it more appropriate for “more complicated, thicker leaflets with calcium.”
CLASP IID enrolled patients with grade 3+ or 4+ degenerative MR considered to be “at prohibitive surgical risk” at 43 sites in North America and Europe. It randomly assigned them 2-to-1 to receive the PASCAL device or MitraClip.
Either of two PASCAL versions were used, the original device or the “smaller, narrower” PASCAL Ace, Dr. Lim observed. Both versions are covered by the PASCAL Precision System FDA approval. About 40% of patients assigned to MitraClip received older versions of the device and about 60%, more recent versions, as they were entered into practice.
The mean procedure times were 88 minutes for PASCAL and 79 minutes for MitraClip (P = .023), with much of the difference attributable to the earliest PASCAL procedures. Procedure times for the device declined with greater operator experience, the published report states.
Rates of the primary safety endpoint of major adverse events at 30 days were 3.4% for PASCAL and 4.8% for MitraClip. The endpoint was a composite of cardiovascular mortality, stroke, myocardial infarction, new need for renal replacement therapy, severe bleeding, or nonelective MV reintervention.
The proportion of patients with MR grade 2+ or lower at 6 months, the primary effectiveness endpoint, assessed at a core laboratory, was 96.5% for the PASCAL group over a median follow-up of 179.5 days and 96.8% over a median of 184.5 days for those who received MitraClip.
Comparisons for both primary endpoints met the prespecified criteria for PASCAL noninferiority.
In a secondary analysis, the proportion of PASCAL patients with MR grade 1+ or less held about steady from postprocedure discharge out to 6 months, at 87.2% and 83.7%, respectively (P = .317).
But whereas 88.5% of MitraClip patients had MR grade 1+ or better at discharge, 71.2% were at that grade by 6 months (P = .003). That apparent hemodynamic deterioration raised some eyebrows at the TCT sessions as a potential sign that PASCAL functional results are more durable.
That sort of judgment is premature, offered Anita W. Asgar, MD, MSc, Montreal Heart Institute, Quebec City, as an invited discussant after the CLASP IID trial’s formal presentation at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The trial is notable in part for “showing how safe this procedure is and how successful it is for these patients – this is phenomenal,” she said, but “I would caution comparing one device being better than another with such a small number of patients.”
MitraClip, Dr. Young observed, “has been, up to this point, our only option for edge-to-edge repair of the mitral valve. And many of us have years of experience and a lot of patients that we treat with that device.” His center hasn’t yet used PASCAL, but that may change as the field gains more familiarity with the device. Operators may use either device in different cases, he said.
“Depending on the program, and depending on the volume of mitral patients that you see and edge-to-edge repair that you do, it could be that you stick with one, or switch to another, or you integrate both of them and try to decide which patients might be better suited for one or the other.”
CLASP IID was sponsored by Edwards Lifesciences. Dr. Lim discloses consulting for Philips, Venus, and Valgen and receiving research grants from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. Dr. Koulogiannis discloses consulting and serving on an advisory board for Edwards Lifesciences and as a speaker for Abbott and discloses holding equity, stocks, or stock options in 4C. Dr. Thourani discloses serving as a consultant to both Abbott and Edwards Lifesciences. Dr. Young discloses receiving consulting fees or honoraria or serving on a speaker’s bureau for Medtronic. Dr. Asgar discloses receiving research support from or holding a research contract with Abbott Vascular and receiving consulting fees or honoraria or serving on a speaker’s bureau for Medtronic, Edwards Lifesciences, and W. Gore & Associates.
A version of this article first appeared on Medscape.com.
A newly available transcatheter device for edge-to-edge mitral valve (MV) repair, named for a famed scientist-inventor, is similar to the long-available MitraClip (Abbott) for short-term efficacy and safety, suggests an interim but prespecified analysis from a randomized trial.
In its comparison with MitraClip, the PASCAL transcatheter valve repair system (Edwards Lifesciences) was noninferior with respect to 30-day major adverse events and to success at achieving mitral regurgitation (MR) of no more than moderate severity within 6 months. The trial had entered patients with significant, symptomatic degenerative MR considered too high-risk for surgical repair or replacement.
The interim analysis covers 180 of the 300 patients followed in the study, of whom 117 received the PASCAL device and 63 were given MitraClip. Both groups showed significant gains in functional class, symptom status, and quality of life over 6 months, reported D. Scott Lim, MD, University of Virginia Health System Hospital, Charlottesville, and Konstantinos Koulogiannis, MD, Morristown Medical Center, N.J., jointly on Sept. 17 at the Transcatheter Cardiovascular Therapeutics (TCT) 2022 annual meeting in Boston.
Dr. Lim, one of the trial’s principal investigators, is also lead author on its same-day publication in JACC: Cardiovascular Interventions.
Based largely on those results from the CLASP IID pivotal trial, the U.S. Food and Drug Administration recently approved the PASCAL system for use in patients with degenerative MR, Edwards announced on Sept. 15. The device was approved in the European Union on Aug. 17.
MitraClip has been available in various iterations in the United States since 2013 and in Europe since 2008.
“It’s good for the field to be able to say we have two devices that are comparable,” giving clinicians more options, Vinod H. Thourani, MD, Piedmont Heart Institute, Atlanta, told this news organization.
The current analysis shows that “we’ve yet to figure out what patient pathologies will be beneficial” for each of the devices, Dr. Thourani said. “The goal will be to find out if there are certain anatomical considerations where one device is better than the other.”
It will be necessary to study “more patients, a larger cohort, with longer follow-up to allow us to see their true benefits,” he said, as well as to conduct more subgroup analyses. For now, the choice of device will probably be “operator-specific, which they feel comfortable with.”
Dr. Thourani, not an author on the current study, is the U.S. principal investigator for the CLASP IIF study looking at clinical outcomes with the two devices and says he consults for both Edwards and Abbott.
The findings are “preliminary for now,” said Michael Young, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., in part because, like most randomized trials, CLASP IID entered a select, not broadly representative population.
“They want to make, as best as they could, an apples-to-apples comparison, without confounding that might make it more difficult to interpret it afterwards,” Dr. Young, not associated with the trial, told this news organization.
But CLASP IID “did enroll patients that we do see and treat, so undoubtedly it’s a compelling study. We now have another device that is shown to be safe and effective. How we’re going to extrapolate it to all the patients that are being referred to our practices will, I think, be under debate and deliberation.”
The PASCAL and MitraClip devices each may be more suitable for different patients with varying mitral valve pathologies because of differences in their designs, Dr. Lim said. The PASCAL’s relative flexibility might make it preferable in patients with smaller mitral valves, and its ability to elongate during delivery could make it more suitable for patients with chordal-dense areas around the valve, he speculated.
MitraClip, Dr. Lim told this news organization, has a mechanical closure system for anchoring that may make it more appropriate for “more complicated, thicker leaflets with calcium.”
CLASP IID enrolled patients with grade 3+ or 4+ degenerative MR considered to be “at prohibitive surgical risk” at 43 sites in North America and Europe. It randomly assigned them 2-to-1 to receive the PASCAL device or MitraClip.
Either of two PASCAL versions were used, the original device or the “smaller, narrower” PASCAL Ace, Dr. Lim observed. Both versions are covered by the PASCAL Precision System FDA approval. About 40% of patients assigned to MitraClip received older versions of the device and about 60%, more recent versions, as they were entered into practice.
The mean procedure times were 88 minutes for PASCAL and 79 minutes for MitraClip (P = .023), with much of the difference attributable to the earliest PASCAL procedures. Procedure times for the device declined with greater operator experience, the published report states.
Rates of the primary safety endpoint of major adverse events at 30 days were 3.4% for PASCAL and 4.8% for MitraClip. The endpoint was a composite of cardiovascular mortality, stroke, myocardial infarction, new need for renal replacement therapy, severe bleeding, or nonelective MV reintervention.
The proportion of patients with MR grade 2+ or lower at 6 months, the primary effectiveness endpoint, assessed at a core laboratory, was 96.5% for the PASCAL group over a median follow-up of 179.5 days and 96.8% over a median of 184.5 days for those who received MitraClip.
Comparisons for both primary endpoints met the prespecified criteria for PASCAL noninferiority.
In a secondary analysis, the proportion of PASCAL patients with MR grade 1+ or less held about steady from postprocedure discharge out to 6 months, at 87.2% and 83.7%, respectively (P = .317).
But whereas 88.5% of MitraClip patients had MR grade 1+ or better at discharge, 71.2% were at that grade by 6 months (P = .003). That apparent hemodynamic deterioration raised some eyebrows at the TCT sessions as a potential sign that PASCAL functional results are more durable.
That sort of judgment is premature, offered Anita W. Asgar, MD, MSc, Montreal Heart Institute, Quebec City, as an invited discussant after the CLASP IID trial’s formal presentation at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The trial is notable in part for “showing how safe this procedure is and how successful it is for these patients – this is phenomenal,” she said, but “I would caution comparing one device being better than another with such a small number of patients.”
MitraClip, Dr. Young observed, “has been, up to this point, our only option for edge-to-edge repair of the mitral valve. And many of us have years of experience and a lot of patients that we treat with that device.” His center hasn’t yet used PASCAL, but that may change as the field gains more familiarity with the device. Operators may use either device in different cases, he said.
“Depending on the program, and depending on the volume of mitral patients that you see and edge-to-edge repair that you do, it could be that you stick with one, or switch to another, or you integrate both of them and try to decide which patients might be better suited for one or the other.”
CLASP IID was sponsored by Edwards Lifesciences. Dr. Lim discloses consulting for Philips, Venus, and Valgen and receiving research grants from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. Dr. Koulogiannis discloses consulting and serving on an advisory board for Edwards Lifesciences and as a speaker for Abbott and discloses holding equity, stocks, or stock options in 4C. Dr. Thourani discloses serving as a consultant to both Abbott and Edwards Lifesciences. Dr. Young discloses receiving consulting fees or honoraria or serving on a speaker’s bureau for Medtronic. Dr. Asgar discloses receiving research support from or holding a research contract with Abbott Vascular and receiving consulting fees or honoraria or serving on a speaker’s bureau for Medtronic, Edwards Lifesciences, and W. Gore & Associates.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
Ultrasonic renal denervation passes 2-month test in uncontrolled HTN: RADIANCE II
Systolic blood pressure went down safely and consistently 2 months after renal denervation achieved by ultrasound ablation in patients with uncontrolled, mild to moderate hypertension (HTN) in a key sham-controlled test of the balloon-equipped catheter.
The BP reductions were significant almost regardless of how they were measured – at home, in the office, during the day, at night, or over 24 hours – and weren’t dependent on baseline BP levels.
The 224-patient RADIANCE II Pivotal Study follows two earlier successful sham-controlled trials that used the same renal denervation catheter in other types of patients with HTN. They were RADIANCE-HTN SOLO, which entered patients with mild to moderate HTN not taking medication, and RADIANCE-HTN TRIO, which included patients with HTN despite fixed-dose, single-pill, triple-antihypertensive therapy.
The consistent results of all three trials suggest that the ultrasound renal denervation (uRDN) technique “lowers blood pressure across the spectrum of hypertension,” concluded co–principal investigator Ajay J. Kirtane, MD, SM, Columbia University Irving Medical Center, New York–Presbyterian Hospital, when presenting RADIANCE II at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
RADIANCE II, the largest of the three studies, met its prespecified primary efficacy endpoint of change in daytime ambulatory systolic BP at 2 months by showing a significant 6.3–mm Hg greater reduction in the uRDN group, compared with the sham-control group. There were no major adverse events at 30 days in either group.
The trial was similarly successful for the secondary endpoints of change in systolic BP measured in various other settings, including over 24 hours. Reductions after uRDN averaged 5-7 mm Hg greater than in the control group.
Sparse top-line results of the RADIANCE II pivotal trial were announced in July by the study’s sponsor, ReCor Medical.
Dr. Kirtane stressed in an interview that uRDN and likely any form of HTN renal denervation therapy is not a substitute for standard management. “This is really for patients in whom you’ve made best efforts to do the traditional things – lifestyle modification, medications, all of that – and yet they’re still uncontrolled.” At that point, assuming denervation therapy is available in practice, “it would be something to potentially consider.”
As a panelist after Dr. Kirtane’s formal presentation of RADIANCE II at the conference, Naomi D. Fisher, MD, who was a RADIANCE-HTN TRIO investigator, described how the treatment’s perceived intended patient population evolved over time.
“We all began with the idea that we were going to treat patients with resistant hypertension, that was going to be the first target. We have learned that those patients are far fewer than we thought,” said Dr. Fisher, who directs the hypertension service at Brigham and Women’s Hospital, Boston.
Initial estimates were that such patients with the resistant form, “meaning they require more than three drugs to control their blood pressure,” would represent 15%-20% of patients with HTN.
“We learned from our TRIO data that if you give these patients one single combined pill, lo and behold, many of them become controlled,” she said. “There is so much nonadherence out there in the world, about 50% of our patients aren’t taking their pills. It’s a hard and true fact.”
Exclude patients who aren’t adherent and “our true resistance population becomes minuscule. So, I don’t think that’s going to be the main population” for renal denervation therapy.
More likely, she said, it would be “patients who are uncontrolled and unable to take their medications. So that is going to include nonadherence, intolerance. It’s a very large category of patients. And the priorities can be stacked in favor of those who have higher cardiovascular risk.”
RADIANCE II can show the persistence of uRDN’s BP-lowering effect only out to 2 months so far, but the effect’s durability based on the RADIANCE program’s combined experience appears to be at least 2 years, Dr. Kirtane said in an interview.
“The RADIANCE II pivotal trial is a powerful, well-designed study attesting to the efficacy of renal denervation in BP lowering,” Franz H. Messerli, MD, Swiss Cardiovascular Center, University Hospital Bern, said in an interview.
The trial “shows the well-known unpredictability of antihypertensive response. We cannot predict who responds to renal denervation and who does not, and who even has a paradoxical increase in BP,” Dr. Messerli, an international hypertension expert not associated with the trial, said in an interview.
“As long as we cannot predict the antihypertensive response to renal denervation therapy, potential synergism/antagonism with drug therapy remains an educated guess,” he said.
“Hypertension is a disease that lasts years and decades. As impressive as RADIANCE II’s 2-month snapshot is, I look forward to similar or better BP data 12 and 24 months after renal denervation,” Dr. Messerli added.
RADIANCE II entered patients with mild to moderate uncontrolled HTN, that is, a systolic BP at least 140/90 mm Hg and less than 180/120 mm Hg, who were receiving no more than two antihypertensive medications. They could have no history of cardiovascular or cerebrovascular events or uncontrolled diabetes, and their estimated glomerular filtration rate (eGFR) had to be at least 40 mL/min per 1.73 m2.
After a 4-week drug washout period, patients who were clinically stable with an ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg underwent CT and renal angiography. Then, the 224 patients still anatomically eligible for the procedure were randomly assigned 2:1 to uRDN or a sham-control procedure: 150 and 74 patients, respectively.
At 2 months, daytime ambulatory systolic BP on average fell 7.9 mm Hg in the uRDN group and 1.8 mm Hg in the sham-control group, for a drop that was steeper by 6.3 mm Hg (P < .0001) after uRDN.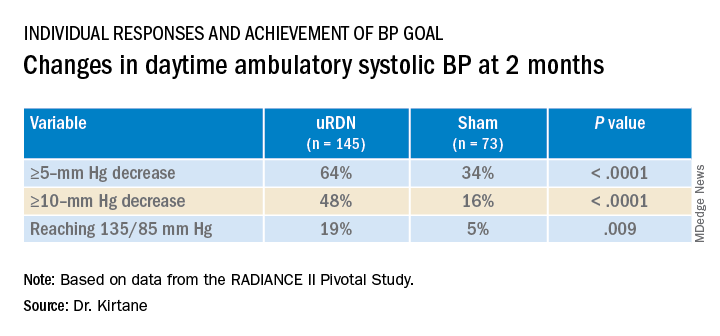
Also in the uRDN group, there was a 6.2–mm Hg larger decrease in 24-hour ambulatory systolic BP (P < .0001), a 5.8–mm Hg greater decline in nighttime ambulatory systolic BP (P < .0004), a 7.6–mm Hg steeper drop in mean home systolic BP (P < .0001), and 5.4 mm Hg more of a decrease in office-based systolic BP (P = .0035).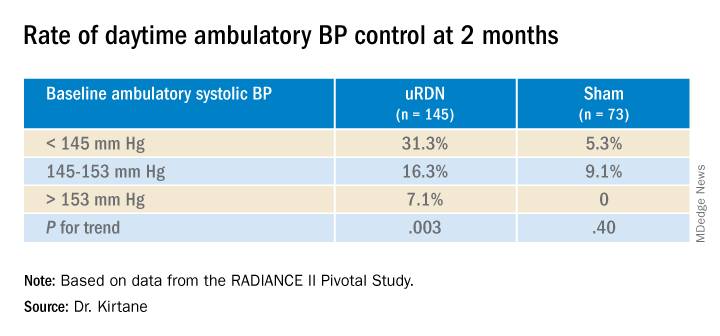
No significant differences were seen in subgroup analyses by sex, age, higher versus lower baseline systolic pressures, high versus low baseline eGFR, degree of abdominal obesity, U.S. versus European site, or whether patients entered before or during the COVID pandemic
Regulators have been accepting change in systolic BP as a surrogate for clinical endpoints in trials of antihypertensive therapy, whether pharmacologic or interventional, under consideration for approval. “That’s why safety endpoints are important to investigate” in these clinical trials, especially for invasive therapies like renal denervation, Dr. Kirtane observed.
That said, “in the longer-term follow-ups of the renal denervation therapies that are out there, including this one, there does not appear to be an appreciable decline in glomerular filtration rate, or any adverse safety signals that we see to date,” Dr. Kirtane said in an interview. “But we know that these are low-frequency events, so we have to be very vigilant, and we can’t get complacent about it.”
In RADIANCE II, there were zero adverse events within 30 days in both groups; the endpoint included death, new myocardial infarction, renal artery complications requiring invasive intervention, and hospitalization for major cardiovascular or hemodynamic-related events. Nor were there instances of new-onset renal artery stenosis greater than 70% documented by imaging at 6 months.
The ReCor uRDN catheter uses ultrasound energy to disrupt renal nerve signaling, a technology thought to deliver safer “burns,” compared with other renal denervation catheter technologies. It features an axially stabilizing balloon that transmits ultrasound energy – two to three sonications, each lasting 7 seconds, Dr. Kirtane said – outward through the arterial wall. The design is intended to ensure consistently circumferential ablation. Circulating saline within the balloon, Kirtane noted, directly cools the adjacent vessel wall to help it avoid thermal damage.
Dr. Kirtane reported receiving institutional funding from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, Shockwave Medical, and Merck; consulting for IMDS; and receiving travel and meal expenses from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr. Fisher disclosed receiving honoraria or fees for consulting or serving on a speaker’s bureau for Medtronic, ReCor Medical, and Aktiia and receiving grant support or holding research contracts for Recor Medical and Aktiia. Dr. Messerli disclosed receiving honoraria from Medtronic, Menarini, Krka, and Ipca.
A version of this article first appeared on Medscape.com.
Systolic blood pressure went down safely and consistently 2 months after renal denervation achieved by ultrasound ablation in patients with uncontrolled, mild to moderate hypertension (HTN) in a key sham-controlled test of the balloon-equipped catheter.
The BP reductions were significant almost regardless of how they were measured – at home, in the office, during the day, at night, or over 24 hours – and weren’t dependent on baseline BP levels.
The 224-patient RADIANCE II Pivotal Study follows two earlier successful sham-controlled trials that used the same renal denervation catheter in other types of patients with HTN. They were RADIANCE-HTN SOLO, which entered patients with mild to moderate HTN not taking medication, and RADIANCE-HTN TRIO, which included patients with HTN despite fixed-dose, single-pill, triple-antihypertensive therapy.
The consistent results of all three trials suggest that the ultrasound renal denervation (uRDN) technique “lowers blood pressure across the spectrum of hypertension,” concluded co–principal investigator Ajay J. Kirtane, MD, SM, Columbia University Irving Medical Center, New York–Presbyterian Hospital, when presenting RADIANCE II at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
RADIANCE II, the largest of the three studies, met its prespecified primary efficacy endpoint of change in daytime ambulatory systolic BP at 2 months by showing a significant 6.3–mm Hg greater reduction in the uRDN group, compared with the sham-control group. There were no major adverse events at 30 days in either group.
The trial was similarly successful for the secondary endpoints of change in systolic BP measured in various other settings, including over 24 hours. Reductions after uRDN averaged 5-7 mm Hg greater than in the control group.
Sparse top-line results of the RADIANCE II pivotal trial were announced in July by the study’s sponsor, ReCor Medical.
Dr. Kirtane stressed in an interview that uRDN and likely any form of HTN renal denervation therapy is not a substitute for standard management. “This is really for patients in whom you’ve made best efforts to do the traditional things – lifestyle modification, medications, all of that – and yet they’re still uncontrolled.” At that point, assuming denervation therapy is available in practice, “it would be something to potentially consider.”
As a panelist after Dr. Kirtane’s formal presentation of RADIANCE II at the conference, Naomi D. Fisher, MD, who was a RADIANCE-HTN TRIO investigator, described how the treatment’s perceived intended patient population evolved over time.
“We all began with the idea that we were going to treat patients with resistant hypertension, that was going to be the first target. We have learned that those patients are far fewer than we thought,” said Dr. Fisher, who directs the hypertension service at Brigham and Women’s Hospital, Boston.
Initial estimates were that such patients with the resistant form, “meaning they require more than three drugs to control their blood pressure,” would represent 15%-20% of patients with HTN.
“We learned from our TRIO data that if you give these patients one single combined pill, lo and behold, many of them become controlled,” she said. “There is so much nonadherence out there in the world, about 50% of our patients aren’t taking their pills. It’s a hard and true fact.”
Exclude patients who aren’t adherent and “our true resistance population becomes minuscule. So, I don’t think that’s going to be the main population” for renal denervation therapy.
More likely, she said, it would be “patients who are uncontrolled and unable to take their medications. So that is going to include nonadherence, intolerance. It’s a very large category of patients. And the priorities can be stacked in favor of those who have higher cardiovascular risk.”
RADIANCE II can show the persistence of uRDN’s BP-lowering effect only out to 2 months so far, but the effect’s durability based on the RADIANCE program’s combined experience appears to be at least 2 years, Dr. Kirtane said in an interview.
“The RADIANCE II pivotal trial is a powerful, well-designed study attesting to the efficacy of renal denervation in BP lowering,” Franz H. Messerli, MD, Swiss Cardiovascular Center, University Hospital Bern, said in an interview.
The trial “shows the well-known unpredictability of antihypertensive response. We cannot predict who responds to renal denervation and who does not, and who even has a paradoxical increase in BP,” Dr. Messerli, an international hypertension expert not associated with the trial, said in an interview.
“As long as we cannot predict the antihypertensive response to renal denervation therapy, potential synergism/antagonism with drug therapy remains an educated guess,” he said.
“Hypertension is a disease that lasts years and decades. As impressive as RADIANCE II’s 2-month snapshot is, I look forward to similar or better BP data 12 and 24 months after renal denervation,” Dr. Messerli added.
RADIANCE II entered patients with mild to moderate uncontrolled HTN, that is, a systolic BP at least 140/90 mm Hg and less than 180/120 mm Hg, who were receiving no more than two antihypertensive medications. They could have no history of cardiovascular or cerebrovascular events or uncontrolled diabetes, and their estimated glomerular filtration rate (eGFR) had to be at least 40 mL/min per 1.73 m2.
After a 4-week drug washout period, patients who were clinically stable with an ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg underwent CT and renal angiography. Then, the 224 patients still anatomically eligible for the procedure were randomly assigned 2:1 to uRDN or a sham-control procedure: 150 and 74 patients, respectively.
At 2 months, daytime ambulatory systolic BP on average fell 7.9 mm Hg in the uRDN group and 1.8 mm Hg in the sham-control group, for a drop that was steeper by 6.3 mm Hg (P < .0001) after uRDN.
Also in the uRDN group, there was a 6.2–mm Hg larger decrease in 24-hour ambulatory systolic BP (P < .0001), a 5.8–mm Hg greater decline in nighttime ambulatory systolic BP (P < .0004), a 7.6–mm Hg steeper drop in mean home systolic BP (P < .0001), and 5.4 mm Hg more of a decrease in office-based systolic BP (P = .0035).
No significant differences were seen in subgroup analyses by sex, age, higher versus lower baseline systolic pressures, high versus low baseline eGFR, degree of abdominal obesity, U.S. versus European site, or whether patients entered before or during the COVID pandemic
Regulators have been accepting change in systolic BP as a surrogate for clinical endpoints in trials of antihypertensive therapy, whether pharmacologic or interventional, under consideration for approval. “That’s why safety endpoints are important to investigate” in these clinical trials, especially for invasive therapies like renal denervation, Dr. Kirtane observed.
That said, “in the longer-term follow-ups of the renal denervation therapies that are out there, including this one, there does not appear to be an appreciable decline in glomerular filtration rate, or any adverse safety signals that we see to date,” Dr. Kirtane said in an interview. “But we know that these are low-frequency events, so we have to be very vigilant, and we can’t get complacent about it.”
In RADIANCE II, there were zero adverse events within 30 days in both groups; the endpoint included death, new myocardial infarction, renal artery complications requiring invasive intervention, and hospitalization for major cardiovascular or hemodynamic-related events. Nor were there instances of new-onset renal artery stenosis greater than 70% documented by imaging at 6 months.
The ReCor uRDN catheter uses ultrasound energy to disrupt renal nerve signaling, a technology thought to deliver safer “burns,” compared with other renal denervation catheter technologies. It features an axially stabilizing balloon that transmits ultrasound energy – two to three sonications, each lasting 7 seconds, Dr. Kirtane said – outward through the arterial wall. The design is intended to ensure consistently circumferential ablation. Circulating saline within the balloon, Kirtane noted, directly cools the adjacent vessel wall to help it avoid thermal damage.
Dr. Kirtane reported receiving institutional funding from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, Shockwave Medical, and Merck; consulting for IMDS; and receiving travel and meal expenses from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr. Fisher disclosed receiving honoraria or fees for consulting or serving on a speaker’s bureau for Medtronic, ReCor Medical, and Aktiia and receiving grant support or holding research contracts for Recor Medical and Aktiia. Dr. Messerli disclosed receiving honoraria from Medtronic, Menarini, Krka, and Ipca.
A version of this article first appeared on Medscape.com.
Systolic blood pressure went down safely and consistently 2 months after renal denervation achieved by ultrasound ablation in patients with uncontrolled, mild to moderate hypertension (HTN) in a key sham-controlled test of the balloon-equipped catheter.
The BP reductions were significant almost regardless of how they were measured – at home, in the office, during the day, at night, or over 24 hours – and weren’t dependent on baseline BP levels.
The 224-patient RADIANCE II Pivotal Study follows two earlier successful sham-controlled trials that used the same renal denervation catheter in other types of patients with HTN. They were RADIANCE-HTN SOLO, which entered patients with mild to moderate HTN not taking medication, and RADIANCE-HTN TRIO, which included patients with HTN despite fixed-dose, single-pill, triple-antihypertensive therapy.
The consistent results of all three trials suggest that the ultrasound renal denervation (uRDN) technique “lowers blood pressure across the spectrum of hypertension,” concluded co–principal investigator Ajay J. Kirtane, MD, SM, Columbia University Irving Medical Center, New York–Presbyterian Hospital, when presenting RADIANCE II at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
RADIANCE II, the largest of the three studies, met its prespecified primary efficacy endpoint of change in daytime ambulatory systolic BP at 2 months by showing a significant 6.3–mm Hg greater reduction in the uRDN group, compared with the sham-control group. There were no major adverse events at 30 days in either group.
The trial was similarly successful for the secondary endpoints of change in systolic BP measured in various other settings, including over 24 hours. Reductions after uRDN averaged 5-7 mm Hg greater than in the control group.
Sparse top-line results of the RADIANCE II pivotal trial were announced in July by the study’s sponsor, ReCor Medical.
Dr. Kirtane stressed in an interview that uRDN and likely any form of HTN renal denervation therapy is not a substitute for standard management. “This is really for patients in whom you’ve made best efforts to do the traditional things – lifestyle modification, medications, all of that – and yet they’re still uncontrolled.” At that point, assuming denervation therapy is available in practice, “it would be something to potentially consider.”
As a panelist after Dr. Kirtane’s formal presentation of RADIANCE II at the conference, Naomi D. Fisher, MD, who was a RADIANCE-HTN TRIO investigator, described how the treatment’s perceived intended patient population evolved over time.
“We all began with the idea that we were going to treat patients with resistant hypertension, that was going to be the first target. We have learned that those patients are far fewer than we thought,” said Dr. Fisher, who directs the hypertension service at Brigham and Women’s Hospital, Boston.
Initial estimates were that such patients with the resistant form, “meaning they require more than three drugs to control their blood pressure,” would represent 15%-20% of patients with HTN.
“We learned from our TRIO data that if you give these patients one single combined pill, lo and behold, many of them become controlled,” she said. “There is so much nonadherence out there in the world, about 50% of our patients aren’t taking their pills. It’s a hard and true fact.”
Exclude patients who aren’t adherent and “our true resistance population becomes minuscule. So, I don’t think that’s going to be the main population” for renal denervation therapy.
More likely, she said, it would be “patients who are uncontrolled and unable to take their medications. So that is going to include nonadherence, intolerance. It’s a very large category of patients. And the priorities can be stacked in favor of those who have higher cardiovascular risk.”
RADIANCE II can show the persistence of uRDN’s BP-lowering effect only out to 2 months so far, but the effect’s durability based on the RADIANCE program’s combined experience appears to be at least 2 years, Dr. Kirtane said in an interview.
“The RADIANCE II pivotal trial is a powerful, well-designed study attesting to the efficacy of renal denervation in BP lowering,” Franz H. Messerli, MD, Swiss Cardiovascular Center, University Hospital Bern, said in an interview.
The trial “shows the well-known unpredictability of antihypertensive response. We cannot predict who responds to renal denervation and who does not, and who even has a paradoxical increase in BP,” Dr. Messerli, an international hypertension expert not associated with the trial, said in an interview.
“As long as we cannot predict the antihypertensive response to renal denervation therapy, potential synergism/antagonism with drug therapy remains an educated guess,” he said.
“Hypertension is a disease that lasts years and decades. As impressive as RADIANCE II’s 2-month snapshot is, I look forward to similar or better BP data 12 and 24 months after renal denervation,” Dr. Messerli added.
RADIANCE II entered patients with mild to moderate uncontrolled HTN, that is, a systolic BP at least 140/90 mm Hg and less than 180/120 mm Hg, who were receiving no more than two antihypertensive medications. They could have no history of cardiovascular or cerebrovascular events or uncontrolled diabetes, and their estimated glomerular filtration rate (eGFR) had to be at least 40 mL/min per 1.73 m2.
After a 4-week drug washout period, patients who were clinically stable with an ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg underwent CT and renal angiography. Then, the 224 patients still anatomically eligible for the procedure were randomly assigned 2:1 to uRDN or a sham-control procedure: 150 and 74 patients, respectively.
At 2 months, daytime ambulatory systolic BP on average fell 7.9 mm Hg in the uRDN group and 1.8 mm Hg in the sham-control group, for a drop that was steeper by 6.3 mm Hg (P < .0001) after uRDN.
Also in the uRDN group, there was a 6.2–mm Hg larger decrease in 24-hour ambulatory systolic BP (P < .0001), a 5.8–mm Hg greater decline in nighttime ambulatory systolic BP (P < .0004), a 7.6–mm Hg steeper drop in mean home systolic BP (P < .0001), and 5.4 mm Hg more of a decrease in office-based systolic BP (P = .0035).
No significant differences were seen in subgroup analyses by sex, age, higher versus lower baseline systolic pressures, high versus low baseline eGFR, degree of abdominal obesity, U.S. versus European site, or whether patients entered before or during the COVID pandemic
Regulators have been accepting change in systolic BP as a surrogate for clinical endpoints in trials of antihypertensive therapy, whether pharmacologic or interventional, under consideration for approval. “That’s why safety endpoints are important to investigate” in these clinical trials, especially for invasive therapies like renal denervation, Dr. Kirtane observed.
That said, “in the longer-term follow-ups of the renal denervation therapies that are out there, including this one, there does not appear to be an appreciable decline in glomerular filtration rate, or any adverse safety signals that we see to date,” Dr. Kirtane said in an interview. “But we know that these are low-frequency events, so we have to be very vigilant, and we can’t get complacent about it.”
In RADIANCE II, there were zero adverse events within 30 days in both groups; the endpoint included death, new myocardial infarction, renal artery complications requiring invasive intervention, and hospitalization for major cardiovascular or hemodynamic-related events. Nor were there instances of new-onset renal artery stenosis greater than 70% documented by imaging at 6 months.
The ReCor uRDN catheter uses ultrasound energy to disrupt renal nerve signaling, a technology thought to deliver safer “burns,” compared with other renal denervation catheter technologies. It features an axially stabilizing balloon that transmits ultrasound energy – two to three sonications, each lasting 7 seconds, Dr. Kirtane said – outward through the arterial wall. The design is intended to ensure consistently circumferential ablation. Circulating saline within the balloon, Kirtane noted, directly cools the adjacent vessel wall to help it avoid thermal damage.
Dr. Kirtane reported receiving institutional funding from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, Shockwave Medical, and Merck; consulting for IMDS; and receiving travel and meal expenses from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr. Fisher disclosed receiving honoraria or fees for consulting or serving on a speaker’s bureau for Medtronic, ReCor Medical, and Aktiia and receiving grant support or holding research contracts for Recor Medical and Aktiia. Dr. Messerli disclosed receiving honoraria from Medtronic, Menarini, Krka, and Ipca.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
Heparin pretreatment may safely open arteries before STEMI cath
, suggests a large registry study.
An open infarct-related artery (IRA) at angiography on cath-lab arrival presents STEMI patients an opportunity for earlier reperfusion and a chance, in theory at least, for smaller infarcts and maybe improved clinical outcomes.
In the new analysis, which covers more than 40,000 patients with STEMI in Sweden, the 38% who received heparin before cath-lab arrival were 11% less likely to show IRA occlusion at angiography prior to direct percutaneous coronary intervention (PCI). They also showed a 13% lower 30-day mortality compared with patients who were started on heparin in the cath lab. Importantly, their risk of major bleeding in the hospital did not increase.
The “early reperfusion” associated with IRA patency at angiography “could have long-term benefit due to smaller infarct size,” potentially explaining the observed 30-day survival gain in the pretreatment group, Oskar Love Emilsson, Lund (Sweden) University, said in an interview.
Mr. Emilsson, a third-year medical student, reported the analysis at the annual congress of the European Society of Cardiology, and is lead author on its same-day publication in the journal EuroIntervention.
He mentioned a few cautions in interpreting the study, which is based primarily on data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). It included several sensitivity analyses that continued to back pretreatment heparin as a significant predictor of an unoccluded IRA but didn’t consistently support the 30-day mortality benefit seen in the primary analysis.
And, although the pretreatment group overall didn’t have more major bleeds, the risk did go up significantly for those older than 75 or those who weighed less than 60 kg (132 pounds) or underwent catheterization with an access route other than the radial artery. Extra caution should be exercised in such patients who receive heparin before cath-lab arrival for PCI, Mr. Emilsson observed.
“Our results suggest that heparin pretreatment might be a good option to improve patency of infarct related arteries in STEMI,” and potentially clinical outcomes, he said. “However, a definite answer would require a randomized controlled trial.”
Meanwhile, the current study may be the largest yet to look at clinical outcomes after pretreatment with unfractionated heparin before PCI for acute STEMI, the report states. There have been some observational studies, subanalyses of STEMI trials, and even a few limited randomized trials – including the HEAP trial published in 2000 – to weigh in on the subject. Some have supported the strategy, others have not.
“With rapid door-to-balloon times in STEMI, it can be challenging to show a significant difference between a prehospital heparin approach and heparin given in the lab,” observed Sunil V. Rao, MD, NYU Langone Health System, New York, who is not connected with the current study.
Many EDs in the United States have “a STEMI protocol that calls for an IV bolus of heparin. It would be tougher in the U.S. to give it in the ambulance but again, it’s not clear how much advantage that would really provide,” he told this news organization.
Support from randomized trials would be needed before the practice could be formally recommended. “The SCAAR registries have set the standard for how registries should be conducted,” Dr. Rao said. “This is a very well done observational study, but it is observational.”
The priority for STEMI patients, he added, “really should be to get them to the lab as fast as possible. If the ED protocol includes heparin before the cath lab, that’s great, but I don’t think we should delay getting these patients to the lab to accommodate pre–cath-lab heparin.”
The current analysis covered 41,631 patients with STEMI from 2008 through to 2016, of whom 38% were pretreated with heparin in an ambulance or the ED. The remaining 62% initiated heparin in the cath lab.
About one-third of the group had an open IRA at angiography. The adjusted risk ratio (RR) for IRA occlusion at angiography for patients pretreated vs. not pretreated with heparin was 0.89 (95% confidence interval [CI], 0.87-0.90).
The corresponding RR for death within 30 days was 0.87 (95% CI, 0.77-0.99), and for major in-hospital bleeding it was 1.01 (95% CI, 0.86-1.18).
The analysis was adjusted for other medications received before cath-lab arrival, especially a long list of antiplatelets and non-heparin antithrombins. That strengthens the case for heparin pretreatment as an independent predictor of an open IRA at initial angiography, Mr. Emilsson said.
Comparisons of propensity-score–matched subgroups of the total cohort, conducted separately for the IRA-occlusion endpoint and the endpoints of 30-day mortality and major bleeding, produced similar results.
Some observational data suggest that antiplatelet pretreatment with a P2Y12 inhibitor may promote IRA patency on angiography after cath lab arrival, Dr. Rao observed. “This indicates that there probably is a role of earlier antithrombotic therapy in STEMI patients, but the randomized trials have not shown a consistent benefit,” he said, referring in particular to the ATLANTIC trial.
Mr. Emilsson and Dr. Rao disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests a large registry study.
An open infarct-related artery (IRA) at angiography on cath-lab arrival presents STEMI patients an opportunity for earlier reperfusion and a chance, in theory at least, for smaller infarcts and maybe improved clinical outcomes.
In the new analysis, which covers more than 40,000 patients with STEMI in Sweden, the 38% who received heparin before cath-lab arrival were 11% less likely to show IRA occlusion at angiography prior to direct percutaneous coronary intervention (PCI). They also showed a 13% lower 30-day mortality compared with patients who were started on heparin in the cath lab. Importantly, their risk of major bleeding in the hospital did not increase.
The “early reperfusion” associated with IRA patency at angiography “could have long-term benefit due to smaller infarct size,” potentially explaining the observed 30-day survival gain in the pretreatment group, Oskar Love Emilsson, Lund (Sweden) University, said in an interview.
Mr. Emilsson, a third-year medical student, reported the analysis at the annual congress of the European Society of Cardiology, and is lead author on its same-day publication in the journal EuroIntervention.
He mentioned a few cautions in interpreting the study, which is based primarily on data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). It included several sensitivity analyses that continued to back pretreatment heparin as a significant predictor of an unoccluded IRA but didn’t consistently support the 30-day mortality benefit seen in the primary analysis.
And, although the pretreatment group overall didn’t have more major bleeds, the risk did go up significantly for those older than 75 or those who weighed less than 60 kg (132 pounds) or underwent catheterization with an access route other than the radial artery. Extra caution should be exercised in such patients who receive heparin before cath-lab arrival for PCI, Mr. Emilsson observed.
“Our results suggest that heparin pretreatment might be a good option to improve patency of infarct related arteries in STEMI,” and potentially clinical outcomes, he said. “However, a definite answer would require a randomized controlled trial.”
Meanwhile, the current study may be the largest yet to look at clinical outcomes after pretreatment with unfractionated heparin before PCI for acute STEMI, the report states. There have been some observational studies, subanalyses of STEMI trials, and even a few limited randomized trials – including the HEAP trial published in 2000 – to weigh in on the subject. Some have supported the strategy, others have not.
“With rapid door-to-balloon times in STEMI, it can be challenging to show a significant difference between a prehospital heparin approach and heparin given in the lab,” observed Sunil V. Rao, MD, NYU Langone Health System, New York, who is not connected with the current study.
Many EDs in the United States have “a STEMI protocol that calls for an IV bolus of heparin. It would be tougher in the U.S. to give it in the ambulance but again, it’s not clear how much advantage that would really provide,” he told this news organization.
Support from randomized trials would be needed before the practice could be formally recommended. “The SCAAR registries have set the standard for how registries should be conducted,” Dr. Rao said. “This is a very well done observational study, but it is observational.”
The priority for STEMI patients, he added, “really should be to get them to the lab as fast as possible. If the ED protocol includes heparin before the cath lab, that’s great, but I don’t think we should delay getting these patients to the lab to accommodate pre–cath-lab heparin.”
The current analysis covered 41,631 patients with STEMI from 2008 through to 2016, of whom 38% were pretreated with heparin in an ambulance or the ED. The remaining 62% initiated heparin in the cath lab.
About one-third of the group had an open IRA at angiography. The adjusted risk ratio (RR) for IRA occlusion at angiography for patients pretreated vs. not pretreated with heparin was 0.89 (95% confidence interval [CI], 0.87-0.90).
The corresponding RR for death within 30 days was 0.87 (95% CI, 0.77-0.99), and for major in-hospital bleeding it was 1.01 (95% CI, 0.86-1.18).
The analysis was adjusted for other medications received before cath-lab arrival, especially a long list of antiplatelets and non-heparin antithrombins. That strengthens the case for heparin pretreatment as an independent predictor of an open IRA at initial angiography, Mr. Emilsson said.
Comparisons of propensity-score–matched subgroups of the total cohort, conducted separately for the IRA-occlusion endpoint and the endpoints of 30-day mortality and major bleeding, produced similar results.
Some observational data suggest that antiplatelet pretreatment with a P2Y12 inhibitor may promote IRA patency on angiography after cath lab arrival, Dr. Rao observed. “This indicates that there probably is a role of earlier antithrombotic therapy in STEMI patients, but the randomized trials have not shown a consistent benefit,” he said, referring in particular to the ATLANTIC trial.
Mr. Emilsson and Dr. Rao disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests a large registry study.
An open infarct-related artery (IRA) at angiography on cath-lab arrival presents STEMI patients an opportunity for earlier reperfusion and a chance, in theory at least, for smaller infarcts and maybe improved clinical outcomes.
In the new analysis, which covers more than 40,000 patients with STEMI in Sweden, the 38% who received heparin before cath-lab arrival were 11% less likely to show IRA occlusion at angiography prior to direct percutaneous coronary intervention (PCI). They also showed a 13% lower 30-day mortality compared with patients who were started on heparin in the cath lab. Importantly, their risk of major bleeding in the hospital did not increase.
The “early reperfusion” associated with IRA patency at angiography “could have long-term benefit due to smaller infarct size,” potentially explaining the observed 30-day survival gain in the pretreatment group, Oskar Love Emilsson, Lund (Sweden) University, said in an interview.
Mr. Emilsson, a third-year medical student, reported the analysis at the annual congress of the European Society of Cardiology, and is lead author on its same-day publication in the journal EuroIntervention.
He mentioned a few cautions in interpreting the study, which is based primarily on data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). It included several sensitivity analyses that continued to back pretreatment heparin as a significant predictor of an unoccluded IRA but didn’t consistently support the 30-day mortality benefit seen in the primary analysis.
And, although the pretreatment group overall didn’t have more major bleeds, the risk did go up significantly for those older than 75 or those who weighed less than 60 kg (132 pounds) or underwent catheterization with an access route other than the radial artery. Extra caution should be exercised in such patients who receive heparin before cath-lab arrival for PCI, Mr. Emilsson observed.
“Our results suggest that heparin pretreatment might be a good option to improve patency of infarct related arteries in STEMI,” and potentially clinical outcomes, he said. “However, a definite answer would require a randomized controlled trial.”
Meanwhile, the current study may be the largest yet to look at clinical outcomes after pretreatment with unfractionated heparin before PCI for acute STEMI, the report states. There have been some observational studies, subanalyses of STEMI trials, and even a few limited randomized trials – including the HEAP trial published in 2000 – to weigh in on the subject. Some have supported the strategy, others have not.
“With rapid door-to-balloon times in STEMI, it can be challenging to show a significant difference between a prehospital heparin approach and heparin given in the lab,” observed Sunil V. Rao, MD, NYU Langone Health System, New York, who is not connected with the current study.
Many EDs in the United States have “a STEMI protocol that calls for an IV bolus of heparin. It would be tougher in the U.S. to give it in the ambulance but again, it’s not clear how much advantage that would really provide,” he told this news organization.
Support from randomized trials would be needed before the practice could be formally recommended. “The SCAAR registries have set the standard for how registries should be conducted,” Dr. Rao said. “This is a very well done observational study, but it is observational.”
The priority for STEMI patients, he added, “really should be to get them to the lab as fast as possible. If the ED protocol includes heparin before the cath lab, that’s great, but I don’t think we should delay getting these patients to the lab to accommodate pre–cath-lab heparin.”
The current analysis covered 41,631 patients with STEMI from 2008 through to 2016, of whom 38% were pretreated with heparin in an ambulance or the ED. The remaining 62% initiated heparin in the cath lab.
About one-third of the group had an open IRA at angiography. The adjusted risk ratio (RR) for IRA occlusion at angiography for patients pretreated vs. not pretreated with heparin was 0.89 (95% confidence interval [CI], 0.87-0.90).
The corresponding RR for death within 30 days was 0.87 (95% CI, 0.77-0.99), and for major in-hospital bleeding it was 1.01 (95% CI, 0.86-1.18).
The analysis was adjusted for other medications received before cath-lab arrival, especially a long list of antiplatelets and non-heparin antithrombins. That strengthens the case for heparin pretreatment as an independent predictor of an open IRA at initial angiography, Mr. Emilsson said.
Comparisons of propensity-score–matched subgroups of the total cohort, conducted separately for the IRA-occlusion endpoint and the endpoints of 30-day mortality and major bleeding, produced similar results.
Some observational data suggest that antiplatelet pretreatment with a P2Y12 inhibitor may promote IRA patency on angiography after cath lab arrival, Dr. Rao observed. “This indicates that there probably is a role of earlier antithrombotic therapy in STEMI patients, but the randomized trials have not shown a consistent benefit,” he said, referring in particular to the ATLANTIC trial.
Mr. Emilsson and Dr. Rao disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
No invasive strategy benefit at 5 years in ISCHEMIA-CKD extension study
A trip to the cath lab for possible revascularization after a positive stress test, compared with a wait-and-see approach backed by optimal medications, did not improve 5-year survival for patients with advanced chronic kidney disease (CKD) in the ISCHEMIA-CKD trial’s extension study, ISCHEMIA-CKD EXTEND.
The long-term results, from the same 777 patients followed for an average of 2.2 years in the main trial, are consistent with the overall findings of no survival advantage with an initially invasive strategy, compared with one that is initially conservative. The finding applies to patients like those in the trial who had moderate to severe ischemia at stress testing and whose CKD put them in an especially high-risk and little-studied coronary artery disease (CAD) category.
Indeed, in a reflection of that high-risk status, 5-year all-cause mortality reached about 40% and cardiovascular (CV) mortality approached 30%, with no significant differences between patients in the invasive- and conservative-strategy groups.
Those numbers arguably put CKD’s effect on CAD survival in about the same league as an ejection fraction (EF) of 35% or less. For context, all-cause mortality over 3-4 years was about 32% in the REVIVED-BCIS2 trial of such patients with ischemic reduced-EF cardiomyopathy, whether or not they were revascularized, observed Sripal Bangalore, MD, MHA.
Yet in ISCHEMIA-CKD EXTEND, “you’re seeing in a group of patients, with largely preserved EF but advanced CKD, a mortality rate close to 40% at 5 years,” said Dr. Bangalore of New York University.
Although the study doesn’t show benefit from the initially invasive approach in CKD patients with stable CAD, for those with acute coronary syndromes (ACS), it seems to suggest that “at least the invasive strategy is safe,” Dr. Bangalore said during a press conference preceding his presentation of the study Aug. 29 at the annual congress of the European Society of Cardiology, held in Barcelona.
REVIVED-BCIS2 was also presented at the ESC sessions on Aug. 27, as reported by this news organization.
ISCHEMIA-CKD EXTEND “is a large trial and a very well-done trial. The results are robust, and they should influence clinical practice,” Deepak L. Bhatt, MD, MPH, Brigham and Women’s Hospital Heart & Vascular Center, Boston, said as the invited discussant after Dr. Bangalore’s presentation.
“The main message here, really, is don’t just go looking for ischemia, at least with the modalities used in this trial, in your CKD patients as a routine practice, and then try to stomp out that ischemia with revascularization,” Dr. Bhatt said. “The right thing to do in these high-risk patients is to focus on lifestyle modification and intensive medical therapy.”
A caveat, he said, is that the trial’s results don’t apply to the types of patients excluded from it, including those with recent ACS and those who are highly symptomatic or have an EF of less than 35%.
“Those CKD patients likely benefit from an invasive strategy with anatomically appropriate revascularization,” whether percutaneous coronary intervention (PCI) or coronary bypass surgery, Dr. Bhatt said.
At a median follow-up of 5 years in the extension study, the rates of death from any cause were 40.6% for patients in the invasive-strategy group and 37.4% for those in the conservative-strategy group. That yielded a hazard ratio of 1.12 (95% confidence interval, 0.89-1.41; P = .32) after adjustment for age, sex, diabetes status, EF, dialysis status, and – for patients not on dialysis – baseline estimated glomerular filtration rate.
The rates of CV death were 29% for patients managed invasively and 27% for those initially managed conservatively, for a similarly adjusted HR of 1.04 (95% CI, 0.80-1.37; P = .75).
In subgroup analyses, Dr. Bangalore reported, there were no significant differences in all-cause or CV mortality by diabetes status, by severity of baseline ischemia, or by whether the patient had recently experienced new or more frequent angina at study entry, was on guideline-directed medical therapy at baseline, or was on dialysis.
Among the contributions of ISCHEMIA-CKD and its 5-year extension study, Dr. Bangalore told this news organization, is that the relative safety of revascularization they showed may help to counter “renalism,” that is, the aversion to invasive intervention in patients with advanced CKD in clinical practice.
For example, if a patient with advanced CKD presents with an acute myocardial infarction, “people are hesitant to take them to the cath lab,” Dr. Bangalore said. But “if you follow protocols, if you follow strategies to minimize the risk, you can safely go ahead and do it.”
But in patients with stable CAD, as the ISCHEMIA-CKD studies show, “routinely revascularizing them may not have significant benefits.”
ISCHEMIC-CKD and its extension study were funded by the National Heart, Lung, and Blood Institute. Dr. Bangalore discloses receiving research grants from NHLBI and serving as a consultant for Abbott Vascular, Biotronik, Boston Scientific, Amgen, Pfizer, Merck, and Reata. Dr. Bhatt has disclosed grants and/or personal fees from many companies; personal fees from WebMD and other publications or organizations; and having other relationships with Medscape Cardiology and other publications or organizations.
A version of this article first appeared on Medscape.com.
A trip to the cath lab for possible revascularization after a positive stress test, compared with a wait-and-see approach backed by optimal medications, did not improve 5-year survival for patients with advanced chronic kidney disease (CKD) in the ISCHEMIA-CKD trial’s extension study, ISCHEMIA-CKD EXTEND.
The long-term results, from the same 777 patients followed for an average of 2.2 years in the main trial, are consistent with the overall findings of no survival advantage with an initially invasive strategy, compared with one that is initially conservative. The finding applies to patients like those in the trial who had moderate to severe ischemia at stress testing and whose CKD put them in an especially high-risk and little-studied coronary artery disease (CAD) category.
Indeed, in a reflection of that high-risk status, 5-year all-cause mortality reached about 40% and cardiovascular (CV) mortality approached 30%, with no significant differences between patients in the invasive- and conservative-strategy groups.
Those numbers arguably put CKD’s effect on CAD survival in about the same league as an ejection fraction (EF) of 35% or less. For context, all-cause mortality over 3-4 years was about 32% in the REVIVED-BCIS2 trial of such patients with ischemic reduced-EF cardiomyopathy, whether or not they were revascularized, observed Sripal Bangalore, MD, MHA.
Yet in ISCHEMIA-CKD EXTEND, “you’re seeing in a group of patients, with largely preserved EF but advanced CKD, a mortality rate close to 40% at 5 years,” said Dr. Bangalore of New York University.
Although the study doesn’t show benefit from the initially invasive approach in CKD patients with stable CAD, for those with acute coronary syndromes (ACS), it seems to suggest that “at least the invasive strategy is safe,” Dr. Bangalore said during a press conference preceding his presentation of the study Aug. 29 at the annual congress of the European Society of Cardiology, held in Barcelona.
REVIVED-BCIS2 was also presented at the ESC sessions on Aug. 27, as reported by this news organization.
ISCHEMIA-CKD EXTEND “is a large trial and a very well-done trial. The results are robust, and they should influence clinical practice,” Deepak L. Bhatt, MD, MPH, Brigham and Women’s Hospital Heart & Vascular Center, Boston, said as the invited discussant after Dr. Bangalore’s presentation.
“The main message here, really, is don’t just go looking for ischemia, at least with the modalities used in this trial, in your CKD patients as a routine practice, and then try to stomp out that ischemia with revascularization,” Dr. Bhatt said. “The right thing to do in these high-risk patients is to focus on lifestyle modification and intensive medical therapy.”
A caveat, he said, is that the trial’s results don’t apply to the types of patients excluded from it, including those with recent ACS and those who are highly symptomatic or have an EF of less than 35%.
“Those CKD patients likely benefit from an invasive strategy with anatomically appropriate revascularization,” whether percutaneous coronary intervention (PCI) or coronary bypass surgery, Dr. Bhatt said.
At a median follow-up of 5 years in the extension study, the rates of death from any cause were 40.6% for patients in the invasive-strategy group and 37.4% for those in the conservative-strategy group. That yielded a hazard ratio of 1.12 (95% confidence interval, 0.89-1.41; P = .32) after adjustment for age, sex, diabetes status, EF, dialysis status, and – for patients not on dialysis – baseline estimated glomerular filtration rate.
The rates of CV death were 29% for patients managed invasively and 27% for those initially managed conservatively, for a similarly adjusted HR of 1.04 (95% CI, 0.80-1.37; P = .75).
In subgroup analyses, Dr. Bangalore reported, there were no significant differences in all-cause or CV mortality by diabetes status, by severity of baseline ischemia, or by whether the patient had recently experienced new or more frequent angina at study entry, was on guideline-directed medical therapy at baseline, or was on dialysis.
Among the contributions of ISCHEMIA-CKD and its 5-year extension study, Dr. Bangalore told this news organization, is that the relative safety of revascularization they showed may help to counter “renalism,” that is, the aversion to invasive intervention in patients with advanced CKD in clinical practice.
For example, if a patient with advanced CKD presents with an acute myocardial infarction, “people are hesitant to take them to the cath lab,” Dr. Bangalore said. But “if you follow protocols, if you follow strategies to minimize the risk, you can safely go ahead and do it.”
But in patients with stable CAD, as the ISCHEMIA-CKD studies show, “routinely revascularizing them may not have significant benefits.”
ISCHEMIC-CKD and its extension study were funded by the National Heart, Lung, and Blood Institute. Dr. Bangalore discloses receiving research grants from NHLBI and serving as a consultant for Abbott Vascular, Biotronik, Boston Scientific, Amgen, Pfizer, Merck, and Reata. Dr. Bhatt has disclosed grants and/or personal fees from many companies; personal fees from WebMD and other publications or organizations; and having other relationships with Medscape Cardiology and other publications or organizations.
A version of this article first appeared on Medscape.com.
A trip to the cath lab for possible revascularization after a positive stress test, compared with a wait-and-see approach backed by optimal medications, did not improve 5-year survival for patients with advanced chronic kidney disease (CKD) in the ISCHEMIA-CKD trial’s extension study, ISCHEMIA-CKD EXTEND.
The long-term results, from the same 777 patients followed for an average of 2.2 years in the main trial, are consistent with the overall findings of no survival advantage with an initially invasive strategy, compared with one that is initially conservative. The finding applies to patients like those in the trial who had moderate to severe ischemia at stress testing and whose CKD put them in an especially high-risk and little-studied coronary artery disease (CAD) category.
Indeed, in a reflection of that high-risk status, 5-year all-cause mortality reached about 40% and cardiovascular (CV) mortality approached 30%, with no significant differences between patients in the invasive- and conservative-strategy groups.
Those numbers arguably put CKD’s effect on CAD survival in about the same league as an ejection fraction (EF) of 35% or less. For context, all-cause mortality over 3-4 years was about 32% in the REVIVED-BCIS2 trial of such patients with ischemic reduced-EF cardiomyopathy, whether or not they were revascularized, observed Sripal Bangalore, MD, MHA.
Yet in ISCHEMIA-CKD EXTEND, “you’re seeing in a group of patients, with largely preserved EF but advanced CKD, a mortality rate close to 40% at 5 years,” said Dr. Bangalore of New York University.
Although the study doesn’t show benefit from the initially invasive approach in CKD patients with stable CAD, for those with acute coronary syndromes (ACS), it seems to suggest that “at least the invasive strategy is safe,” Dr. Bangalore said during a press conference preceding his presentation of the study Aug. 29 at the annual congress of the European Society of Cardiology, held in Barcelona.
REVIVED-BCIS2 was also presented at the ESC sessions on Aug. 27, as reported by this news organization.
ISCHEMIA-CKD EXTEND “is a large trial and a very well-done trial. The results are robust, and they should influence clinical practice,” Deepak L. Bhatt, MD, MPH, Brigham and Women’s Hospital Heart & Vascular Center, Boston, said as the invited discussant after Dr. Bangalore’s presentation.
“The main message here, really, is don’t just go looking for ischemia, at least with the modalities used in this trial, in your CKD patients as a routine practice, and then try to stomp out that ischemia with revascularization,” Dr. Bhatt said. “The right thing to do in these high-risk patients is to focus on lifestyle modification and intensive medical therapy.”
A caveat, he said, is that the trial’s results don’t apply to the types of patients excluded from it, including those with recent ACS and those who are highly symptomatic or have an EF of less than 35%.
“Those CKD patients likely benefit from an invasive strategy with anatomically appropriate revascularization,” whether percutaneous coronary intervention (PCI) or coronary bypass surgery, Dr. Bhatt said.
At a median follow-up of 5 years in the extension study, the rates of death from any cause were 40.6% for patients in the invasive-strategy group and 37.4% for those in the conservative-strategy group. That yielded a hazard ratio of 1.12 (95% confidence interval, 0.89-1.41; P = .32) after adjustment for age, sex, diabetes status, EF, dialysis status, and – for patients not on dialysis – baseline estimated glomerular filtration rate.
The rates of CV death were 29% for patients managed invasively and 27% for those initially managed conservatively, for a similarly adjusted HR of 1.04 (95% CI, 0.80-1.37; P = .75).
In subgroup analyses, Dr. Bangalore reported, there were no significant differences in all-cause or CV mortality by diabetes status, by severity of baseline ischemia, or by whether the patient had recently experienced new or more frequent angina at study entry, was on guideline-directed medical therapy at baseline, or was on dialysis.
Among the contributions of ISCHEMIA-CKD and its 5-year extension study, Dr. Bangalore told this news organization, is that the relative safety of revascularization they showed may help to counter “renalism,” that is, the aversion to invasive intervention in patients with advanced CKD in clinical practice.
For example, if a patient with advanced CKD presents with an acute myocardial infarction, “people are hesitant to take them to the cath lab,” Dr. Bangalore said. But “if you follow protocols, if you follow strategies to minimize the risk, you can safely go ahead and do it.”
But in patients with stable CAD, as the ISCHEMIA-CKD studies show, “routinely revascularizing them may not have significant benefits.”
ISCHEMIC-CKD and its extension study were funded by the National Heart, Lung, and Blood Institute. Dr. Bangalore discloses receiving research grants from NHLBI and serving as a consultant for Abbott Vascular, Biotronik, Boston Scientific, Amgen, Pfizer, Merck, and Reata. Dr. Bhatt has disclosed grants and/or personal fees from many companies; personal fees from WebMD and other publications or organizations; and having other relationships with Medscape Cardiology and other publications or organizations.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Vintage drug atop IV loop diuretics boosts decongestion in ADHF: ADVOR
A decades-old drug, added to standard loop diuretics, could potentially help more volume-overloaded patients with acute decompensated heart failure (ADHF) to be discharged from the hospital ‘dry,’ a randomized trial suggests.
Those who received intravenous acetazolamide, compared with placebo, on top of a usual-care IV loop diuretic in the multicenter study were 46% more likely to achieve “successful” decongestion – that is, to leave the hospital without lingering signs of volume overload.
The trial, with more than 500 patients, is the first “to unequivocally show benefit of any drug, namely acetazolamide, on major heart failure outcomes in patients with acute decompensated heart failure,” said Wilfried Mullens, MD, PhD, Hospital Oost-Limburg, Genk, Belgium, at a media briefing during the annual congress of the European Society of Cardiology, Barcelona.
The patients who received acetazolamide “also had a shorter hospital stay, having a major impact on not only quality of life, but also health care expenditures,” said Dr. Mullens, who leads the steering committee of the trial conducted in Belgium. He presented the results of Acetazolamide in Decompensated Heart Failure with Volume Overload (ADVOR) at ESC 2022 and is lead author on its same-day publication in the New England Journal of Medicine.
Complementary effects?
Current guidelines on managing volume-overloaded patients with ADHF owe a lot to the 2011 DOSE trial, which provided some of the first randomized-trial evidence in an arena led largely by clinical tradition. The advantages it saw with the high-dose furosemide strategy helped it enter into clinical practice, but even in DOSE, the strategy fell short of achieving full decongestion for many patients.
The ADVOR report describes acetazolamide as a carbonic anhydrase inhibitor that reduces sodium recovery in the proximal tubule, similar to the function of loop diuretics in the loop of Henle. Acting in different segments of the nephron, acetazolamide and loop diuretics like furosemide may potentially have complementary effects that improve diuretic “efficiency.”
The difference in decongestion effect between the acetazolamide and placebo groups grew consistently from baseline to day 3. “There was an increase in treatment effect over consecutive days,” Dr. Mullens said. “This highlights the importance of treating congestion both early and aggressively. You cannot catch up,” he said. “If you don’t treat them aggressively initially, you can never get them dry.”
Of the trial’s 519 patients, 42.2% of those assigned to acetazolamide and 30.5% of those in the control group were judged to have had successful decongestion at 3 days, the primary endpoint. Successful decongestion meant they had no remaining signs of volume overload, such as edema, pleural effusion, or ascites.
Although the trial wasn’t powered for clinical outcomes, the 3-month rates of death from any cause or rehospitalization for heart failure were similar at 29.7% in the acetazolamide group and 27.8% for the control group. All-cause mortality in an exploratory analysis was also statistically comparable at 15.2% and 12%, respectively.
Decongestion and clinical outcomes
The study is noteworthy “because it tests a readily available diuretic, acetazolamide, that is not used widely for ADHF,” and showed a benefit at 3 days from adding the drug to a prescribed loop diuretic regimen, Mark H. Drazner, MD, University of Texas Southwestern Medical Center, Dallas, told this news organization.
The benefit didn’t translate into improved clinical outcomes; indeed, mortality at 3 months was numerically higher in the acetazolamide group, observed Dr. Drazner, who is unaffiliated with the study.
Although ADVOR isn’t powered for mortality, he acknowledged, “one would expect the enhanced decongestion would have led to improved outcomes,” or at least a signal of such improvement.
It’s worth noting, Dr. Drazner added, “that the strategy tested was to add acetazolamide up front, on day 1, before loop diuretics were maximized.”
Indeed, the published report says all patients received IV loop diuretics at double the oral maintenance dose, given the first day in a single bolus immediately on randomization. The dose was split into two doses, given at least 6 hours apart, on day 2 and day 3. “The bolus of acetazolamide or matching placebo was administered simultaneously with the first dose of loop diuretics each day,” it states.
Although the protocol called for one loop diuretic dose on day 1, typically in practice patients would be dosed twice or three times each day, Dr. Drazner observed. Once-daily IV diuretic dosing may be less effective than a 2- or 3-times-per-day schedule, he said. As a result, acetazolamide might achieve faster decongestion after it is added to the loop diuretic, a benefit that would not otherwise be available in practice.
Messages for practice
Before this trial, Dr. Drazner said, he would usually add the thiazide diuretic metolazone as needed “to augment diuresis beyond maximum loop diuretics.” After ADVOR, “I’d be willing to try acetazolamide in that setting, recognizing I also don’t know the impact of metolazone on outcomes.”
Still, he would restrict either drug to patients who fail on maximal loop diuretics “rather than adding it routinely and up front, before the loop diuretics are maximized.”
More data are needed, Dr. Drazner said, including from a larger clinical-outcomes trial to confirm the strategy’s safety, before “the up-front addition of acetazolamide to submaximal loop diuretics doses” could become part of standard practice in ADHF.
Given the data so far, including those from ADVOR, “treatment with loop diuretics alone is probably sufficient for successful decongestion” among patients likely to respond to the drugs: that is, “those who are younger, those who have less severe or new-onset heart failure, and those who have normal kidney function,” states an editorial accompanying the published report.
“However, for the large group of patients who have some degree of diuretic resistance, or for those who have an inadequate initial response to loop-diuretic therapy, these data suggest the use of acetazolamide as a reasonable adjunct to achieving more rapid decongestion,” writes G. Michael Felker, MD, Duke University School of Medicine, Durham, N.C. Dr. Felker was lead author on the DOSE primary publication.
ADVOR entered volume-overloaded patients with ADHF and elevated natriuretic peptide levels who had been on oral maintenance with at least 40 mg of furosemide, or equivalent doses of other loop diuretics, for at least a month before randomization.
They were assigned to either IV acetazolamide at 500 mg once daily (n = 259) or placebo (n = 260) on top of an IV loop diuretic, at 27 centers in Belgium.
The risk ratio for the primary endpoint, successful decongestion after 3 days, was 1.46 (95% confidence interval, 1.17-1.82, P < .001) for the acetazolamide versus placebo groups.
In exploratory analyses, acetazolamide versus placebo, the RR for successful decongestion among patients who survived to discharge was increased at 1.27 (95% CI, 1.13-1.43). The hazard ratio for death from any cause at 3 months was not significant at 1.28 (95% CI, 0.78-2.05), nor was the HR for heart-failure rehospitalization at 3 months, 1.07 (95% CI, 0.71-1.59).
The role of SGLT2 inhibitors
ADVOR, understandably but maybe problematically, excluded patients taking SGLT2 inhibitors. The drugs have diuretic effects, among other useful properties, and became core therapy for a range of heart failure types after the trial was designed.
“This exclusion presents a conundrum for applying these results in contemporary clinical care,” Dr. Felker writes. For example, “the data supporting the efficacy and safety of SGLT2 inhibitors across the broad spectrum of patients with heart failure are now overwhelming, and most patients who are hospitalized for heart failure have a clear indication for these agents.”
Given that no ADVOR patients were on the drugs, his editorial states, “we can only speculate as to the efficacy of acetazolamide in patients treated with background SGLT2 inhibitors, which could potentially be additive, subadditive, or synergistic.”
The SGLT2 inhibitors will likely “be used in ADHF in the future, based on studies such as EMPULSE. It will be important to know whether SGLT2 inhibitors change the risk-benefit of also giving acetazolamide,” Dr. Drazner said when interviewed.
“I don’t think there’s any safety issue with regards to the combination of SGLT2 inhibitors and acetazolamide,” Dr. Mullens said. Their diuretic effects are likely to be additive, he proposed.
“Although SGLT2 inhibitors and acetazolamide both exert natriuretic and diuretic effects on the proximal tubules, their mode of action and potency differ substantially,” the published report states.
ADVOR is funded by the Belgian Health Care Knowledge Center. Dr. Mullens discloses receiving fees for speaking from Abbott Fund, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Medtronic, and Novartis. Disclosures for the other authors are at NEJM.org. Dr. Drazner has reported no relevant financial relationships. Dr. Felker discloses serving as a consultant for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Cardionomic, Cytokinetics, Novartis, Reprieve, and Sequana.
A version of this article first appeared on Medscape.com.
A decades-old drug, added to standard loop diuretics, could potentially help more volume-overloaded patients with acute decompensated heart failure (ADHF) to be discharged from the hospital ‘dry,’ a randomized trial suggests.
Those who received intravenous acetazolamide, compared with placebo, on top of a usual-care IV loop diuretic in the multicenter study were 46% more likely to achieve “successful” decongestion – that is, to leave the hospital without lingering signs of volume overload.
The trial, with more than 500 patients, is the first “to unequivocally show benefit of any drug, namely acetazolamide, on major heart failure outcomes in patients with acute decompensated heart failure,” said Wilfried Mullens, MD, PhD, Hospital Oost-Limburg, Genk, Belgium, at a media briefing during the annual congress of the European Society of Cardiology, Barcelona.
The patients who received acetazolamide “also had a shorter hospital stay, having a major impact on not only quality of life, but also health care expenditures,” said Dr. Mullens, who leads the steering committee of the trial conducted in Belgium. He presented the results of Acetazolamide in Decompensated Heart Failure with Volume Overload (ADVOR) at ESC 2022 and is lead author on its same-day publication in the New England Journal of Medicine.
Complementary effects?
Current guidelines on managing volume-overloaded patients with ADHF owe a lot to the 2011 DOSE trial, which provided some of the first randomized-trial evidence in an arena led largely by clinical tradition. The advantages it saw with the high-dose furosemide strategy helped it enter into clinical practice, but even in DOSE, the strategy fell short of achieving full decongestion for many patients.
The ADVOR report describes acetazolamide as a carbonic anhydrase inhibitor that reduces sodium recovery in the proximal tubule, similar to the function of loop diuretics in the loop of Henle. Acting in different segments of the nephron, acetazolamide and loop diuretics like furosemide may potentially have complementary effects that improve diuretic “efficiency.”
The difference in decongestion effect between the acetazolamide and placebo groups grew consistently from baseline to day 3. “There was an increase in treatment effect over consecutive days,” Dr. Mullens said. “This highlights the importance of treating congestion both early and aggressively. You cannot catch up,” he said. “If you don’t treat them aggressively initially, you can never get them dry.”
Of the trial’s 519 patients, 42.2% of those assigned to acetazolamide and 30.5% of those in the control group were judged to have had successful decongestion at 3 days, the primary endpoint. Successful decongestion meant they had no remaining signs of volume overload, such as edema, pleural effusion, or ascites.
Although the trial wasn’t powered for clinical outcomes, the 3-month rates of death from any cause or rehospitalization for heart failure were similar at 29.7% in the acetazolamide group and 27.8% for the control group. All-cause mortality in an exploratory analysis was also statistically comparable at 15.2% and 12%, respectively.
Decongestion and clinical outcomes
The study is noteworthy “because it tests a readily available diuretic, acetazolamide, that is not used widely for ADHF,” and showed a benefit at 3 days from adding the drug to a prescribed loop diuretic regimen, Mark H. Drazner, MD, University of Texas Southwestern Medical Center, Dallas, told this news organization.
The benefit didn’t translate into improved clinical outcomes; indeed, mortality at 3 months was numerically higher in the acetazolamide group, observed Dr. Drazner, who is unaffiliated with the study.
Although ADVOR isn’t powered for mortality, he acknowledged, “one would expect the enhanced decongestion would have led to improved outcomes,” or at least a signal of such improvement.
It’s worth noting, Dr. Drazner added, “that the strategy tested was to add acetazolamide up front, on day 1, before loop diuretics were maximized.”
Indeed, the published report says all patients received IV loop diuretics at double the oral maintenance dose, given the first day in a single bolus immediately on randomization. The dose was split into two doses, given at least 6 hours apart, on day 2 and day 3. “The bolus of acetazolamide or matching placebo was administered simultaneously with the first dose of loop diuretics each day,” it states.
Although the protocol called for one loop diuretic dose on day 1, typically in practice patients would be dosed twice or three times each day, Dr. Drazner observed. Once-daily IV diuretic dosing may be less effective than a 2- or 3-times-per-day schedule, he said. As a result, acetazolamide might achieve faster decongestion after it is added to the loop diuretic, a benefit that would not otherwise be available in practice.
Messages for practice
Before this trial, Dr. Drazner said, he would usually add the thiazide diuretic metolazone as needed “to augment diuresis beyond maximum loop diuretics.” After ADVOR, “I’d be willing to try acetazolamide in that setting, recognizing I also don’t know the impact of metolazone on outcomes.”
Still, he would restrict either drug to patients who fail on maximal loop diuretics “rather than adding it routinely and up front, before the loop diuretics are maximized.”
More data are needed, Dr. Drazner said, including from a larger clinical-outcomes trial to confirm the strategy’s safety, before “the up-front addition of acetazolamide to submaximal loop diuretics doses” could become part of standard practice in ADHF.
Given the data so far, including those from ADVOR, “treatment with loop diuretics alone is probably sufficient for successful decongestion” among patients likely to respond to the drugs: that is, “those who are younger, those who have less severe or new-onset heart failure, and those who have normal kidney function,” states an editorial accompanying the published report.
“However, for the large group of patients who have some degree of diuretic resistance, or for those who have an inadequate initial response to loop-diuretic therapy, these data suggest the use of acetazolamide as a reasonable adjunct to achieving more rapid decongestion,” writes G. Michael Felker, MD, Duke University School of Medicine, Durham, N.C. Dr. Felker was lead author on the DOSE primary publication.
ADVOR entered volume-overloaded patients with ADHF and elevated natriuretic peptide levels who had been on oral maintenance with at least 40 mg of furosemide, or equivalent doses of other loop diuretics, for at least a month before randomization.
They were assigned to either IV acetazolamide at 500 mg once daily (n = 259) or placebo (n = 260) on top of an IV loop diuretic, at 27 centers in Belgium.
The risk ratio for the primary endpoint, successful decongestion after 3 days, was 1.46 (95% confidence interval, 1.17-1.82, P < .001) for the acetazolamide versus placebo groups.
In exploratory analyses, acetazolamide versus placebo, the RR for successful decongestion among patients who survived to discharge was increased at 1.27 (95% CI, 1.13-1.43). The hazard ratio for death from any cause at 3 months was not significant at 1.28 (95% CI, 0.78-2.05), nor was the HR for heart-failure rehospitalization at 3 months, 1.07 (95% CI, 0.71-1.59).
The role of SGLT2 inhibitors
ADVOR, understandably but maybe problematically, excluded patients taking SGLT2 inhibitors. The drugs have diuretic effects, among other useful properties, and became core therapy for a range of heart failure types after the trial was designed.
“This exclusion presents a conundrum for applying these results in contemporary clinical care,” Dr. Felker writes. For example, “the data supporting the efficacy and safety of SGLT2 inhibitors across the broad spectrum of patients with heart failure are now overwhelming, and most patients who are hospitalized for heart failure have a clear indication for these agents.”
Given that no ADVOR patients were on the drugs, his editorial states, “we can only speculate as to the efficacy of acetazolamide in patients treated with background SGLT2 inhibitors, which could potentially be additive, subadditive, or synergistic.”
The SGLT2 inhibitors will likely “be used in ADHF in the future, based on studies such as EMPULSE. It will be important to know whether SGLT2 inhibitors change the risk-benefit of also giving acetazolamide,” Dr. Drazner said when interviewed.
“I don’t think there’s any safety issue with regards to the combination of SGLT2 inhibitors and acetazolamide,” Dr. Mullens said. Their diuretic effects are likely to be additive, he proposed.
“Although SGLT2 inhibitors and acetazolamide both exert natriuretic and diuretic effects on the proximal tubules, their mode of action and potency differ substantially,” the published report states.
ADVOR is funded by the Belgian Health Care Knowledge Center. Dr. Mullens discloses receiving fees for speaking from Abbott Fund, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Medtronic, and Novartis. Disclosures for the other authors are at NEJM.org. Dr. Drazner has reported no relevant financial relationships. Dr. Felker discloses serving as a consultant for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Cardionomic, Cytokinetics, Novartis, Reprieve, and Sequana.
A version of this article first appeared on Medscape.com.
A decades-old drug, added to standard loop diuretics, could potentially help more volume-overloaded patients with acute decompensated heart failure (ADHF) to be discharged from the hospital ‘dry,’ a randomized trial suggests.
Those who received intravenous acetazolamide, compared with placebo, on top of a usual-care IV loop diuretic in the multicenter study were 46% more likely to achieve “successful” decongestion – that is, to leave the hospital without lingering signs of volume overload.
The trial, with more than 500 patients, is the first “to unequivocally show benefit of any drug, namely acetazolamide, on major heart failure outcomes in patients with acute decompensated heart failure,” said Wilfried Mullens, MD, PhD, Hospital Oost-Limburg, Genk, Belgium, at a media briefing during the annual congress of the European Society of Cardiology, Barcelona.
The patients who received acetazolamide “also had a shorter hospital stay, having a major impact on not only quality of life, but also health care expenditures,” said Dr. Mullens, who leads the steering committee of the trial conducted in Belgium. He presented the results of Acetazolamide in Decompensated Heart Failure with Volume Overload (ADVOR) at ESC 2022 and is lead author on its same-day publication in the New England Journal of Medicine.
Complementary effects?
Current guidelines on managing volume-overloaded patients with ADHF owe a lot to the 2011 DOSE trial, which provided some of the first randomized-trial evidence in an arena led largely by clinical tradition. The advantages it saw with the high-dose furosemide strategy helped it enter into clinical practice, but even in DOSE, the strategy fell short of achieving full decongestion for many patients.
The ADVOR report describes acetazolamide as a carbonic anhydrase inhibitor that reduces sodium recovery in the proximal tubule, similar to the function of loop diuretics in the loop of Henle. Acting in different segments of the nephron, acetazolamide and loop diuretics like furosemide may potentially have complementary effects that improve diuretic “efficiency.”
The difference in decongestion effect between the acetazolamide and placebo groups grew consistently from baseline to day 3. “There was an increase in treatment effect over consecutive days,” Dr. Mullens said. “This highlights the importance of treating congestion both early and aggressively. You cannot catch up,” he said. “If you don’t treat them aggressively initially, you can never get them dry.”
Of the trial’s 519 patients, 42.2% of those assigned to acetazolamide and 30.5% of those in the control group were judged to have had successful decongestion at 3 days, the primary endpoint. Successful decongestion meant they had no remaining signs of volume overload, such as edema, pleural effusion, or ascites.
Although the trial wasn’t powered for clinical outcomes, the 3-month rates of death from any cause or rehospitalization for heart failure were similar at 29.7% in the acetazolamide group and 27.8% for the control group. All-cause mortality in an exploratory analysis was also statistically comparable at 15.2% and 12%, respectively.
Decongestion and clinical outcomes
The study is noteworthy “because it tests a readily available diuretic, acetazolamide, that is not used widely for ADHF,” and showed a benefit at 3 days from adding the drug to a prescribed loop diuretic regimen, Mark H. Drazner, MD, University of Texas Southwestern Medical Center, Dallas, told this news organization.
The benefit didn’t translate into improved clinical outcomes; indeed, mortality at 3 months was numerically higher in the acetazolamide group, observed Dr. Drazner, who is unaffiliated with the study.
Although ADVOR isn’t powered for mortality, he acknowledged, “one would expect the enhanced decongestion would have led to improved outcomes,” or at least a signal of such improvement.
It’s worth noting, Dr. Drazner added, “that the strategy tested was to add acetazolamide up front, on day 1, before loop diuretics were maximized.”
Indeed, the published report says all patients received IV loop diuretics at double the oral maintenance dose, given the first day in a single bolus immediately on randomization. The dose was split into two doses, given at least 6 hours apart, on day 2 and day 3. “The bolus of acetazolamide or matching placebo was administered simultaneously with the first dose of loop diuretics each day,” it states.
Although the protocol called for one loop diuretic dose on day 1, typically in practice patients would be dosed twice or three times each day, Dr. Drazner observed. Once-daily IV diuretic dosing may be less effective than a 2- or 3-times-per-day schedule, he said. As a result, acetazolamide might achieve faster decongestion after it is added to the loop diuretic, a benefit that would not otherwise be available in practice.
Messages for practice
Before this trial, Dr. Drazner said, he would usually add the thiazide diuretic metolazone as needed “to augment diuresis beyond maximum loop diuretics.” After ADVOR, “I’d be willing to try acetazolamide in that setting, recognizing I also don’t know the impact of metolazone on outcomes.”
Still, he would restrict either drug to patients who fail on maximal loop diuretics “rather than adding it routinely and up front, before the loop diuretics are maximized.”
More data are needed, Dr. Drazner said, including from a larger clinical-outcomes trial to confirm the strategy’s safety, before “the up-front addition of acetazolamide to submaximal loop diuretics doses” could become part of standard practice in ADHF.
Given the data so far, including those from ADVOR, “treatment with loop diuretics alone is probably sufficient for successful decongestion” among patients likely to respond to the drugs: that is, “those who are younger, those who have less severe or new-onset heart failure, and those who have normal kidney function,” states an editorial accompanying the published report.
“However, for the large group of patients who have some degree of diuretic resistance, or for those who have an inadequate initial response to loop-diuretic therapy, these data suggest the use of acetazolamide as a reasonable adjunct to achieving more rapid decongestion,” writes G. Michael Felker, MD, Duke University School of Medicine, Durham, N.C. Dr. Felker was lead author on the DOSE primary publication.
ADVOR entered volume-overloaded patients with ADHF and elevated natriuretic peptide levels who had been on oral maintenance with at least 40 mg of furosemide, or equivalent doses of other loop diuretics, for at least a month before randomization.
They were assigned to either IV acetazolamide at 500 mg once daily (n = 259) or placebo (n = 260) on top of an IV loop diuretic, at 27 centers in Belgium.
The risk ratio for the primary endpoint, successful decongestion after 3 days, was 1.46 (95% confidence interval, 1.17-1.82, P < .001) for the acetazolamide versus placebo groups.
In exploratory analyses, acetazolamide versus placebo, the RR for successful decongestion among patients who survived to discharge was increased at 1.27 (95% CI, 1.13-1.43). The hazard ratio for death from any cause at 3 months was not significant at 1.28 (95% CI, 0.78-2.05), nor was the HR for heart-failure rehospitalization at 3 months, 1.07 (95% CI, 0.71-1.59).
The role of SGLT2 inhibitors
ADVOR, understandably but maybe problematically, excluded patients taking SGLT2 inhibitors. The drugs have diuretic effects, among other useful properties, and became core therapy for a range of heart failure types after the trial was designed.
“This exclusion presents a conundrum for applying these results in contemporary clinical care,” Dr. Felker writes. For example, “the data supporting the efficacy and safety of SGLT2 inhibitors across the broad spectrum of patients with heart failure are now overwhelming, and most patients who are hospitalized for heart failure have a clear indication for these agents.”
Given that no ADVOR patients were on the drugs, his editorial states, “we can only speculate as to the efficacy of acetazolamide in patients treated with background SGLT2 inhibitors, which could potentially be additive, subadditive, or synergistic.”
The SGLT2 inhibitors will likely “be used in ADHF in the future, based on studies such as EMPULSE. It will be important to know whether SGLT2 inhibitors change the risk-benefit of also giving acetazolamide,” Dr. Drazner said when interviewed.
“I don’t think there’s any safety issue with regards to the combination of SGLT2 inhibitors and acetazolamide,” Dr. Mullens said. Their diuretic effects are likely to be additive, he proposed.
“Although SGLT2 inhibitors and acetazolamide both exert natriuretic and diuretic effects on the proximal tubules, their mode of action and potency differ substantially,” the published report states.
ADVOR is funded by the Belgian Health Care Knowledge Center. Dr. Mullens discloses receiving fees for speaking from Abbott Fund, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Medtronic, and Novartis. Disclosures for the other authors are at NEJM.org. Dr. Drazner has reported no relevant financial relationships. Dr. Felker discloses serving as a consultant for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Cardionomic, Cytokinetics, Novartis, Reprieve, and Sequana.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
‘Conservative’ USPSTF primary prevention statin guidance finalized
Questions about how to prescribe statins for primary prevention abound more than 3 decades after the drugs swept into clinical practice to become a first-line medical approach to cutting cardiovascular (CV) risk. Statin usage recommendations from different bodies can vary in ways both limited and fundamental, spurring the kind of debate that accompanies such a document newly issued by the United States Preventive Services Task Force.
The document, little changed from the draft guidance released for public comment in February, was published online Aug. 23 in JAMA and the USPSTF website. It replaces a similar document issued by the task force in 2016.
The guidance has much in common with, but also sharp differences from, the influential 2018 guidelines on blood cholesterol management developed by the American College of Cardiology, American Heart Association, and 10 other medical societies.
And it is provocative enough to elicit at least four editorials issued the same day across the JAMA family of journals. They highlight key differences between the two documents, among them the USPSTF guidance’s consistent, narrow reliance on 7.5% and 10% cut points for 10-year risk levels as estimated from the ACC/AHA pooled cohort equations (PCE).
The guidance pairs the 10-year risk metric with at least one of only four prescribed CV risk factors to arrive at a limited choice of statin therapy recommendations. But its decision process isn’t bolstered by coronary artery calcium (CAC) scores or the prespecified “risk enhancers” that allowed the ACC/AHA-multisociety guidelines to be applied broadly and still be closely personalized. Those guidelines provide more PCE-based risk tiers for greater discrimination of risk and allow statins to be considered across a broader age group.
The USPSTF guidance’s evidence base consists of 23 clinical trials and three observational studies that directly compared a statin to either placebo or no statin, task force member John B. Wong, MD, Tufts University School of Medicine, Boston, told this news organization.
“In either kind of study, we found that the vast majority of patients had one or more of four risk factors – dyslipidemia, hypertension, diabetes, or smoking. So, when we categorized high risk or increased risk, we included the presence of one or more of those risk factors,” said Dr. Wong, who is director of comparative effectiveness research at Tufts Clinical Translational Science Institute.
‘Sensible and practical’
The USPSTF guidance applies only to adults aged 40-75 without CV signs or symptoms and recommends a statin prescription for persons at “high risk,” that is with an estimated 10-year PCE-based risk for death or CV events of 10% or higher plus at least one of the four risk factors, a level B recommendation.
It recommends that “clinicians selectively offer a statin” to such persons at “increased risk,” who have at least one of the risk factors and an estimated 10-year risk for death or CV events of 7.5% to less than 10%, a level C recommendation. “The likelihood of benefit is smaller in this group” than in persons at high risk, the document states.
“These recommendations from the USPSTF are sensible and practical,” states Salim S. Virani, MD, PhD, DeBakey Veterans Affairs Medical Center, Houston, in a related editorial published the same day in JAMA Network Open. He calls the former B-level recommendation “a conservative approach” and the latter C-level recommendation a “nuanced approach.”
Both are “understandable” given that some studies suggest that the PCE may overestimate the CV risk, Dr. Virani observes. “On the other hand, statin therapy has been shown to be efficacious” at 10-year CV-risk levels down to about 5%.
The USPSTF document “I think is going to perpetuate a problem that we have in this country, which is vast undertreatment of lipids,” Eric D. Peterson, MD, MPH, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“We have a ton of good drugs that can lower cholesterol like crazy. If you lower cholesterol a lot, you improve outcomes,” he said. Dyslipidemia needs to be more widely and consistently treated, but “right now we have a pool of people in primary prevention who undertreat lipids and wait until disease happens – and then cardiologists get engaged. That’s an avoidable miss,” Dr. Peterson adds. He and JAMA Cardiology associate editor Ann Marie Navar, MD, PhD, provided JAMA with an editorial that accompanies the USPSTF guidance.
“My own personal bias would be that the [ACC/AHA-multisociety guidelines] are closer to being right,” Dr. Peterson said. They – unlike the USPSTF guidance – cover people with risk levels below 7.5%, down to at least 5%. They allow risk enhancers like metabolic syndrome, inflammatory diseases, or family history into the decision process. “And they’re more aggressive in diabetes and more aggressive in older people,” he said.
Higher threshold for therapy
The USPSTF guidance also explicitly omits some high-risk groups and makes little accommodation for others who might especially benefit from statins, several of the editorials contend. For example, states a related JAMA Cardiology editorial published the same day, “The USPSTF does not comment on familial hypercholesterolemia or an LDL-C level of 190 mg/dL or higher,” yet they are covered by the ACC/AHA-multispecialty guidelines.
In addition, write the editorialists, led by Neil J. Stone, MD, Northwestern University, Chicago, “the USPSTF uses a slightly higher threshold for initiation of statin therapy” than was used in the ACC/AHA-multisociety guidelines. USPSTF, for example, calls for 10-year risk to reach 10% before recommending a statin prescription.
“One concern about the USPSTF setting the bar higher for statin initiation is that it reduces the number of young patients (age 40-50 years) at risk for premature myocardial infarction considered for treatment,” write Dr. Stone and colleagues.
That may be related to a weakness of the PCE-based decision process. “Because the PCE estimates of 10-year CV disease risk rely so heavily on age, sex, and race, use of these estimates to identify candidates for statins results in significant skewing of the population recommended for statins,” write Dr. Navar and Dr. Peterson in their JAMA editorial.
The risk enhancers in the ACC/AHA-multispecialty guidelines, about a dozen of them, compensate for that limitation to some extent. But the PCE-dominated USPSTF risk estimates will likely miss some groups that could potentially benefit from statin therapy, Dr. Peterson agreed in an interview.
For example, younger adults facing years of high LDL-cholesterol levels could easily have PCE-based 10-year risk below 10%. “Having a high LDL over a lifetime puts you at really high risk,” he said. “Young people are missed even though their longitudinal risk is high.” So, by waiting for the lofty 10% level of risk over 10 years, “we limit the use of medicine that’s pretty cheap and highly effective.”
Dose intensity, adverse events
Also at variance from the ACC/AHA-multispecialty guidelines, the USPSTF states that, “Based on available evidence, use of moderate-intensity statin therapy seems reasonable for the primary prevention of CV disease in most persons.”
The task force specifically explored whether evidence supports some use of high-intensity vs. moderate-intensity statins, Tufts University’s Dr. Wong said. “We found only one study that looked at that particular question, and it didn’t give us a strong answer.” An elevated rosuvastatin-related diabetes risk was apparent in the JUPITER trial, “but for the other studies, we did not find that association.”
Most of the studies that explored statins for reducing risk for a first stroke or myocardial infarction used a moderate-dose statin, Dr. Wong said. “So that’s what we would usually recommend.”
But, Dr. Virani writes, consistent with the ACC/AHA-multispecialty guidelines, “clinicians should consider titrating the intensity of therapy to the risk of the individual.” Persons in certain high-risk primary prevention groups, such as those with end-organ injury from diabetes or LDL cholesterol at least 190 mg/dL, “may derive further benefit from the use of high-intensity statin therapy.”
Low-intensity statins are another potential option, but “in contrast with its 2016 recommendations, the USPSTF no longer recommends use of low-intensity statins in certain situations,” observes a fourth editorial published the same day in JAMA Internal Medicine, with lead author Anand R. Habib, MD, MPhil, and senior author Rita F. Redberg, MD, MSc, both of the University of California, San Francisco. Dr. Redberg is the journal’s editor and has long expressed cautions about statin safety.
“While it is understandable that the Task Force was limited by lack of data on dosing, this change is unfortunate for patients because the frequency of adverse effects increases as the statin dose increases,” the editorial states. Although USPSTF did not find statistically significant harm from the drugs, “in clinical practice, adverse events are commonly reported with use of statins.”
It continues: “At present, there are further reasons to curb our enthusiasm about the use of statins for primary prevention of CV disease.” To illustrate, the editorial questioned primary-prevention statins’ balance of risk vs. clinically meaningful benefit, not benefit that is merely statistically significant.
“The purported benefits of statins in terms of relative risk reduction are fairly constant across baseline lipid levels and cardiovascular risk score categories for primary prevention,” the editorial states.
“Therefore, the absolute benefit for those in lower-risk categories is likely small given that their baseline absolute risk is low, while the chance of adverse effects is constant across risk categories.”
However, USPSTF states, “In pooled analyses of trial data, statin therapy was not associated with increased risk of study withdrawal due to adverse events or serious adverse events.” Nor did it find significant associations with cancers, liver enzyme abnormalities, or diabetes, including new-onset diabetes.
And, the USPSTF adds, “Evidence on the association between statins and renal or cognitive harms is very limited but does not indicate increased risk.”
USPSTF is supported by the U.S. Agency for Healthcare Research and Quality. Dr. Virani discloses receiving grants from the Department of Veterans Affairs, National Institutes of Health, and the World Heart Federation; and personal fees from the American College of Cardiology. Dr. Peterson discloses serving on the JAMA editorial board and receiving research support to his institution from Amgen, Bristol-Myers Squibb, Esperion, and Janssen; and consulting fees from Novo Nordisk, Bayer, and Novartis. Dr. Navar discloses receiving research support to her institution from Amgen, Bristol-Myers Squibb, Esperion, and Janssen; and receiving honoraria and consulting fees from AstraZeneca, Boehringer Ingelheim, Bayer, Janssen, Lilly, Novo Nordisk, Novartis, New Amsterdam, and Pfizer. Dr. Stone discloses receiving an honorarium from Knowledge to Practice, an educational company not associated with the pharmaceutical industry; disclosures for the other authors are in the report. Dr. Redberg discloses receiving research funding from the Arnold Ventures Foundation and the Greenwall Foundation.
A version of this article first appeared on Medscape.com.
Questions about how to prescribe statins for primary prevention abound more than 3 decades after the drugs swept into clinical practice to become a first-line medical approach to cutting cardiovascular (CV) risk. Statin usage recommendations from different bodies can vary in ways both limited and fundamental, spurring the kind of debate that accompanies such a document newly issued by the United States Preventive Services Task Force.
The document, little changed from the draft guidance released for public comment in February, was published online Aug. 23 in JAMA and the USPSTF website. It replaces a similar document issued by the task force in 2016.
The guidance has much in common with, but also sharp differences from, the influential 2018 guidelines on blood cholesterol management developed by the American College of Cardiology, American Heart Association, and 10 other medical societies.
And it is provocative enough to elicit at least four editorials issued the same day across the JAMA family of journals. They highlight key differences between the two documents, among them the USPSTF guidance’s consistent, narrow reliance on 7.5% and 10% cut points for 10-year risk levels as estimated from the ACC/AHA pooled cohort equations (PCE).
The guidance pairs the 10-year risk metric with at least one of only four prescribed CV risk factors to arrive at a limited choice of statin therapy recommendations. But its decision process isn’t bolstered by coronary artery calcium (CAC) scores or the prespecified “risk enhancers” that allowed the ACC/AHA-multisociety guidelines to be applied broadly and still be closely personalized. Those guidelines provide more PCE-based risk tiers for greater discrimination of risk and allow statins to be considered across a broader age group.
The USPSTF guidance’s evidence base consists of 23 clinical trials and three observational studies that directly compared a statin to either placebo or no statin, task force member John B. Wong, MD, Tufts University School of Medicine, Boston, told this news organization.
“In either kind of study, we found that the vast majority of patients had one or more of four risk factors – dyslipidemia, hypertension, diabetes, or smoking. So, when we categorized high risk or increased risk, we included the presence of one or more of those risk factors,” said Dr. Wong, who is director of comparative effectiveness research at Tufts Clinical Translational Science Institute.
‘Sensible and practical’
The USPSTF guidance applies only to adults aged 40-75 without CV signs or symptoms and recommends a statin prescription for persons at “high risk,” that is with an estimated 10-year PCE-based risk for death or CV events of 10% or higher plus at least one of the four risk factors, a level B recommendation.
It recommends that “clinicians selectively offer a statin” to such persons at “increased risk,” who have at least one of the risk factors and an estimated 10-year risk for death or CV events of 7.5% to less than 10%, a level C recommendation. “The likelihood of benefit is smaller in this group” than in persons at high risk, the document states.
“These recommendations from the USPSTF are sensible and practical,” states Salim S. Virani, MD, PhD, DeBakey Veterans Affairs Medical Center, Houston, in a related editorial published the same day in JAMA Network Open. He calls the former B-level recommendation “a conservative approach” and the latter C-level recommendation a “nuanced approach.”
Both are “understandable” given that some studies suggest that the PCE may overestimate the CV risk, Dr. Virani observes. “On the other hand, statin therapy has been shown to be efficacious” at 10-year CV-risk levels down to about 5%.
The USPSTF document “I think is going to perpetuate a problem that we have in this country, which is vast undertreatment of lipids,” Eric D. Peterson, MD, MPH, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“We have a ton of good drugs that can lower cholesterol like crazy. If you lower cholesterol a lot, you improve outcomes,” he said. Dyslipidemia needs to be more widely and consistently treated, but “right now we have a pool of people in primary prevention who undertreat lipids and wait until disease happens – and then cardiologists get engaged. That’s an avoidable miss,” Dr. Peterson adds. He and JAMA Cardiology associate editor Ann Marie Navar, MD, PhD, provided JAMA with an editorial that accompanies the USPSTF guidance.
“My own personal bias would be that the [ACC/AHA-multisociety guidelines] are closer to being right,” Dr. Peterson said. They – unlike the USPSTF guidance – cover people with risk levels below 7.5%, down to at least 5%. They allow risk enhancers like metabolic syndrome, inflammatory diseases, or family history into the decision process. “And they’re more aggressive in diabetes and more aggressive in older people,” he said.
Higher threshold for therapy
The USPSTF guidance also explicitly omits some high-risk groups and makes little accommodation for others who might especially benefit from statins, several of the editorials contend. For example, states a related JAMA Cardiology editorial published the same day, “The USPSTF does not comment on familial hypercholesterolemia or an LDL-C level of 190 mg/dL or higher,” yet they are covered by the ACC/AHA-multispecialty guidelines.
In addition, write the editorialists, led by Neil J. Stone, MD, Northwestern University, Chicago, “the USPSTF uses a slightly higher threshold for initiation of statin therapy” than was used in the ACC/AHA-multisociety guidelines. USPSTF, for example, calls for 10-year risk to reach 10% before recommending a statin prescription.
“One concern about the USPSTF setting the bar higher for statin initiation is that it reduces the number of young patients (age 40-50 years) at risk for premature myocardial infarction considered for treatment,” write Dr. Stone and colleagues.
That may be related to a weakness of the PCE-based decision process. “Because the PCE estimates of 10-year CV disease risk rely so heavily on age, sex, and race, use of these estimates to identify candidates for statins results in significant skewing of the population recommended for statins,” write Dr. Navar and Dr. Peterson in their JAMA editorial.
The risk enhancers in the ACC/AHA-multispecialty guidelines, about a dozen of them, compensate for that limitation to some extent. But the PCE-dominated USPSTF risk estimates will likely miss some groups that could potentially benefit from statin therapy, Dr. Peterson agreed in an interview.
For example, younger adults facing years of high LDL-cholesterol levels could easily have PCE-based 10-year risk below 10%. “Having a high LDL over a lifetime puts you at really high risk,” he said. “Young people are missed even though their longitudinal risk is high.” So, by waiting for the lofty 10% level of risk over 10 years, “we limit the use of medicine that’s pretty cheap and highly effective.”
Dose intensity, adverse events
Also at variance from the ACC/AHA-multispecialty guidelines, the USPSTF states that, “Based on available evidence, use of moderate-intensity statin therapy seems reasonable for the primary prevention of CV disease in most persons.”
The task force specifically explored whether evidence supports some use of high-intensity vs. moderate-intensity statins, Tufts University’s Dr. Wong said. “We found only one study that looked at that particular question, and it didn’t give us a strong answer.” An elevated rosuvastatin-related diabetes risk was apparent in the JUPITER trial, “but for the other studies, we did not find that association.”
Most of the studies that explored statins for reducing risk for a first stroke or myocardial infarction used a moderate-dose statin, Dr. Wong said. “So that’s what we would usually recommend.”
But, Dr. Virani writes, consistent with the ACC/AHA-multispecialty guidelines, “clinicians should consider titrating the intensity of therapy to the risk of the individual.” Persons in certain high-risk primary prevention groups, such as those with end-organ injury from diabetes or LDL cholesterol at least 190 mg/dL, “may derive further benefit from the use of high-intensity statin therapy.”
Low-intensity statins are another potential option, but “in contrast with its 2016 recommendations, the USPSTF no longer recommends use of low-intensity statins in certain situations,” observes a fourth editorial published the same day in JAMA Internal Medicine, with lead author Anand R. Habib, MD, MPhil, and senior author Rita F. Redberg, MD, MSc, both of the University of California, San Francisco. Dr. Redberg is the journal’s editor and has long expressed cautions about statin safety.
“While it is understandable that the Task Force was limited by lack of data on dosing, this change is unfortunate for patients because the frequency of adverse effects increases as the statin dose increases,” the editorial states. Although USPSTF did not find statistically significant harm from the drugs, “in clinical practice, adverse events are commonly reported with use of statins.”
It continues: “At present, there are further reasons to curb our enthusiasm about the use of statins for primary prevention of CV disease.” To illustrate, the editorial questioned primary-prevention statins’ balance of risk vs. clinically meaningful benefit, not benefit that is merely statistically significant.
“The purported benefits of statins in terms of relative risk reduction are fairly constant across baseline lipid levels and cardiovascular risk score categories for primary prevention,” the editorial states.
“Therefore, the absolute benefit for those in lower-risk categories is likely small given that their baseline absolute risk is low, while the chance of adverse effects is constant across risk categories.”
However, USPSTF states, “In pooled analyses of trial data, statin therapy was not associated with increased risk of study withdrawal due to adverse events or serious adverse events.” Nor did it find significant associations with cancers, liver enzyme abnormalities, or diabetes, including new-onset diabetes.
And, the USPSTF adds, “Evidence on the association between statins and renal or cognitive harms is very limited but does not indicate increased risk.”
USPSTF is supported by the U.S. Agency for Healthcare Research and Quality. Dr. Virani discloses receiving grants from the Department of Veterans Affairs, National Institutes of Health, and the World Heart Federation; and personal fees from the American College of Cardiology. Dr. Peterson discloses serving on the JAMA editorial board and receiving research support to his institution from Amgen, Bristol-Myers Squibb, Esperion, and Janssen; and consulting fees from Novo Nordisk, Bayer, and Novartis. Dr. Navar discloses receiving research support to her institution from Amgen, Bristol-Myers Squibb, Esperion, and Janssen; and receiving honoraria and consulting fees from AstraZeneca, Boehringer Ingelheim, Bayer, Janssen, Lilly, Novo Nordisk, Novartis, New Amsterdam, and Pfizer. Dr. Stone discloses receiving an honorarium from Knowledge to Practice, an educational company not associated with the pharmaceutical industry; disclosures for the other authors are in the report. Dr. Redberg discloses receiving research funding from the Arnold Ventures Foundation and the Greenwall Foundation.
A version of this article first appeared on Medscape.com.
Questions about how to prescribe statins for primary prevention abound more than 3 decades after the drugs swept into clinical practice to become a first-line medical approach to cutting cardiovascular (CV) risk. Statin usage recommendations from different bodies can vary in ways both limited and fundamental, spurring the kind of debate that accompanies such a document newly issued by the United States Preventive Services Task Force.
The document, little changed from the draft guidance released for public comment in February, was published online Aug. 23 in JAMA and the USPSTF website. It replaces a similar document issued by the task force in 2016.
The guidance has much in common with, but also sharp differences from, the influential 2018 guidelines on blood cholesterol management developed by the American College of Cardiology, American Heart Association, and 10 other medical societies.
And it is provocative enough to elicit at least four editorials issued the same day across the JAMA family of journals. They highlight key differences between the two documents, among them the USPSTF guidance’s consistent, narrow reliance on 7.5% and 10% cut points for 10-year risk levels as estimated from the ACC/AHA pooled cohort equations (PCE).
The guidance pairs the 10-year risk metric with at least one of only four prescribed CV risk factors to arrive at a limited choice of statin therapy recommendations. But its decision process isn’t bolstered by coronary artery calcium (CAC) scores or the prespecified “risk enhancers” that allowed the ACC/AHA-multisociety guidelines to be applied broadly and still be closely personalized. Those guidelines provide more PCE-based risk tiers for greater discrimination of risk and allow statins to be considered across a broader age group.
The USPSTF guidance’s evidence base consists of 23 clinical trials and three observational studies that directly compared a statin to either placebo or no statin, task force member John B. Wong, MD, Tufts University School of Medicine, Boston, told this news organization.
“In either kind of study, we found that the vast majority of patients had one or more of four risk factors – dyslipidemia, hypertension, diabetes, or smoking. So, when we categorized high risk or increased risk, we included the presence of one or more of those risk factors,” said Dr. Wong, who is director of comparative effectiveness research at Tufts Clinical Translational Science Institute.
‘Sensible and practical’
The USPSTF guidance applies only to adults aged 40-75 without CV signs or symptoms and recommends a statin prescription for persons at “high risk,” that is with an estimated 10-year PCE-based risk for death or CV events of 10% or higher plus at least one of the four risk factors, a level B recommendation.
It recommends that “clinicians selectively offer a statin” to such persons at “increased risk,” who have at least one of the risk factors and an estimated 10-year risk for death or CV events of 7.5% to less than 10%, a level C recommendation. “The likelihood of benefit is smaller in this group” than in persons at high risk, the document states.
“These recommendations from the USPSTF are sensible and practical,” states Salim S. Virani, MD, PhD, DeBakey Veterans Affairs Medical Center, Houston, in a related editorial published the same day in JAMA Network Open. He calls the former B-level recommendation “a conservative approach” and the latter C-level recommendation a “nuanced approach.”
Both are “understandable” given that some studies suggest that the PCE may overestimate the CV risk, Dr. Virani observes. “On the other hand, statin therapy has been shown to be efficacious” at 10-year CV-risk levels down to about 5%.
The USPSTF document “I think is going to perpetuate a problem that we have in this country, which is vast undertreatment of lipids,” Eric D. Peterson, MD, MPH, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“We have a ton of good drugs that can lower cholesterol like crazy. If you lower cholesterol a lot, you improve outcomes,” he said. Dyslipidemia needs to be more widely and consistently treated, but “right now we have a pool of people in primary prevention who undertreat lipids and wait until disease happens – and then cardiologists get engaged. That’s an avoidable miss,” Dr. Peterson adds. He and JAMA Cardiology associate editor Ann Marie Navar, MD, PhD, provided JAMA with an editorial that accompanies the USPSTF guidance.
“My own personal bias would be that the [ACC/AHA-multisociety guidelines] are closer to being right,” Dr. Peterson said. They – unlike the USPSTF guidance – cover people with risk levels below 7.5%, down to at least 5%. They allow risk enhancers like metabolic syndrome, inflammatory diseases, or family history into the decision process. “And they’re more aggressive in diabetes and more aggressive in older people,” he said.
Higher threshold for therapy
The USPSTF guidance also explicitly omits some high-risk groups and makes little accommodation for others who might especially benefit from statins, several of the editorials contend. For example, states a related JAMA Cardiology editorial published the same day, “The USPSTF does not comment on familial hypercholesterolemia or an LDL-C level of 190 mg/dL or higher,” yet they are covered by the ACC/AHA-multispecialty guidelines.
In addition, write the editorialists, led by Neil J. Stone, MD, Northwestern University, Chicago, “the USPSTF uses a slightly higher threshold for initiation of statin therapy” than was used in the ACC/AHA-multisociety guidelines. USPSTF, for example, calls for 10-year risk to reach 10% before recommending a statin prescription.
“One concern about the USPSTF setting the bar higher for statin initiation is that it reduces the number of young patients (age 40-50 years) at risk for premature myocardial infarction considered for treatment,” write Dr. Stone and colleagues.
That may be related to a weakness of the PCE-based decision process. “Because the PCE estimates of 10-year CV disease risk rely so heavily on age, sex, and race, use of these estimates to identify candidates for statins results in significant skewing of the population recommended for statins,” write Dr. Navar and Dr. Peterson in their JAMA editorial.
The risk enhancers in the ACC/AHA-multispecialty guidelines, about a dozen of them, compensate for that limitation to some extent. But the PCE-dominated USPSTF risk estimates will likely miss some groups that could potentially benefit from statin therapy, Dr. Peterson agreed in an interview.
For example, younger adults facing years of high LDL-cholesterol levels could easily have PCE-based 10-year risk below 10%. “Having a high LDL over a lifetime puts you at really high risk,” he said. “Young people are missed even though their longitudinal risk is high.” So, by waiting for the lofty 10% level of risk over 10 years, “we limit the use of medicine that’s pretty cheap and highly effective.”
Dose intensity, adverse events
Also at variance from the ACC/AHA-multispecialty guidelines, the USPSTF states that, “Based on available evidence, use of moderate-intensity statin therapy seems reasonable for the primary prevention of CV disease in most persons.”
The task force specifically explored whether evidence supports some use of high-intensity vs. moderate-intensity statins, Tufts University’s Dr. Wong said. “We found only one study that looked at that particular question, and it didn’t give us a strong answer.” An elevated rosuvastatin-related diabetes risk was apparent in the JUPITER trial, “but for the other studies, we did not find that association.”
Most of the studies that explored statins for reducing risk for a first stroke or myocardial infarction used a moderate-dose statin, Dr. Wong said. “So that’s what we would usually recommend.”
But, Dr. Virani writes, consistent with the ACC/AHA-multispecialty guidelines, “clinicians should consider titrating the intensity of therapy to the risk of the individual.” Persons in certain high-risk primary prevention groups, such as those with end-organ injury from diabetes or LDL cholesterol at least 190 mg/dL, “may derive further benefit from the use of high-intensity statin therapy.”
Low-intensity statins are another potential option, but “in contrast with its 2016 recommendations, the USPSTF no longer recommends use of low-intensity statins in certain situations,” observes a fourth editorial published the same day in JAMA Internal Medicine, with lead author Anand R. Habib, MD, MPhil, and senior author Rita F. Redberg, MD, MSc, both of the University of California, San Francisco. Dr. Redberg is the journal’s editor and has long expressed cautions about statin safety.
“While it is understandable that the Task Force was limited by lack of data on dosing, this change is unfortunate for patients because the frequency of adverse effects increases as the statin dose increases,” the editorial states. Although USPSTF did not find statistically significant harm from the drugs, “in clinical practice, adverse events are commonly reported with use of statins.”
It continues: “At present, there are further reasons to curb our enthusiasm about the use of statins for primary prevention of CV disease.” To illustrate, the editorial questioned primary-prevention statins’ balance of risk vs. clinically meaningful benefit, not benefit that is merely statistically significant.
“The purported benefits of statins in terms of relative risk reduction are fairly constant across baseline lipid levels and cardiovascular risk score categories for primary prevention,” the editorial states.
“Therefore, the absolute benefit for those in lower-risk categories is likely small given that their baseline absolute risk is low, while the chance of adverse effects is constant across risk categories.”
However, USPSTF states, “In pooled analyses of trial data, statin therapy was not associated with increased risk of study withdrawal due to adverse events or serious adverse events.” Nor did it find significant associations with cancers, liver enzyme abnormalities, or diabetes, including new-onset diabetes.
And, the USPSTF adds, “Evidence on the association between statins and renal or cognitive harms is very limited but does not indicate increased risk.”
USPSTF is supported by the U.S. Agency for Healthcare Research and Quality. Dr. Virani discloses receiving grants from the Department of Veterans Affairs, National Institutes of Health, and the World Heart Federation; and personal fees from the American College of Cardiology. Dr. Peterson discloses serving on the JAMA editorial board and receiving research support to his institution from Amgen, Bristol-Myers Squibb, Esperion, and Janssen; and consulting fees from Novo Nordisk, Bayer, and Novartis. Dr. Navar discloses receiving research support to her institution from Amgen, Bristol-Myers Squibb, Esperion, and Janssen; and receiving honoraria and consulting fees from AstraZeneca, Boehringer Ingelheim, Bayer, Janssen, Lilly, Novo Nordisk, Novartis, New Amsterdam, and Pfizer. Dr. Stone discloses receiving an honorarium from Knowledge to Practice, an educational company not associated with the pharmaceutical industry; disclosures for the other authors are in the report. Dr. Redberg discloses receiving research funding from the Arnold Ventures Foundation and the Greenwall Foundation.
A version of this article first appeared on Medscape.com.
FROM JAMA
‘Obesity paradox’ in AFib challenged as mortality climbs with BMI
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
FROM OPEN HEART
Hot weather risk for nonfatal MI hinted for antiplatelets, beta-blockers
Patients who take beta-blockers or antiplatelet agents are lowering their risk for cardiovascular events, but the protection may fall short for those who spend time outdoors on hot summer days, hints a limited analysis published as a letter in Nature Cardiovascular Research.
Patients taking either a beta-blocker or antiplatelet, or both medications together, appeared at elevated risk for nonfatal acute MI specifically on days when the weather turned hot, suggests the registry cohort study that covered 14 years of clinical and meteorologic data.
“The take-away message is not that patients should stop using these two medications, by no means. We’re raising cautions for patients taking them, to watch out for themselves during high-heat days,” lead author Kai Chen, PhD, Yale University, New Haven, Conn., said in an interview.
“We’re not giving the message that these drugs have harmful effects” because the nature of the links between the medications and MI in the study, with its potential for confounding, remain unknown, said Dr. Chen, from the department of environmental health sciences and Yale Center on Climate Change and Health.
For example, patients who take beta-blockers or antiplatelets tend to be sicker than patients not on the drugs, which could make heat-related MI more likely, and the drugs wrongly appear to be culprits, he observed. The analysis contained signals that could support either scenario.
The study is based on cases of nonfatal MI in Augsburg, Germany, that are part of the MONICA-KORA MI registry. The odds of a heat-related nonfatal MI, it suggests, were increased 63% among patients taking antiplatelets and by 65% among those on beta-blockers, compared with those not on these drugs. The odds went up by 75% among those on both drug classes, but the risks weren’t raised in patients not taking them.
Rising heat-related MI
Chen said analysis was inspired by a 2019 report – also based on MONICA-KORA, from many of the same authors and using similar methods to track events by daily air temperature – that showed a rising trend for heat-related MI and declining rate for MI related to cold weather from 1987 to 2014. A next step, he figured, would be to determine whether the MI risk trends were associated with any cardiovascular medications.
The current study’s signal of risk related to antiplatelets and beta-blockers did not emerge for ACE inhibitors, calcium-channel blockers, or diuretics. Statins showed a link to increased nonfatal MI risk, but solely among participants aged younger than 60 years, who were also far less likely to have pre-existing coronary heart disease (CHD). He and his colleagues chose not to highlight that finding, Dr. Chen said, because the age subgroup analysis was grossly underpowered.
The overall analysis involved 2,494 cases of nonfatal MI that occurred during the warmer months – May to September – from 2001 to 2014. It was limited to nonfatal cases – those with at least a month of survival after hospital admission – because of insufficient data on medication use associated with fatal MIs, the report states.
Nonfatal MIs were defined as heat-related if they struck on days reaching the 95th percentile for temperature across the 14 years, in this case 24.2 °C (about 75.6 °F), relative to the average temperature of lowest nonfatal MI risk across the cohort, 7.5 °C (about 45.5 °F).
Patients served as both cases and their own controls, in that air temperature exposures on the day of their MI (case day) were compared with the remaining same days of the week in the same calendar month (control days). That approach, the report stated, “automatically controls for long-term time trends, seasonality, day of the week, and time-invariant confounders (for example, pre-existing cardiovascular disease).”
The odds ratio for heat-related MI for patients on antiplatelets was 1.63 (95% confidence interval, 1.07-2.46), and for antiplatelet nonusers was 0.94 (95% CI, 0.68-1.29). The difference between the two ratios was significant (P = .04).
The corresponding OR for patients taking beta-blockers was 1.65 (95% CI, 1.11-2.45), and for nonusers of beta-blockers was 0.90 (95% CI, 0.64-1.26). Again, the OR difference was significant (P = .02).
The ORs for users of both medication classes and nonusers of either med class, respectively, were 1.75 (95% CI, 1.12-2.73) and 0.84 (95% CI, 0.59-1.19). The latter OR was significantly lower than former (P = .01).
In a sign that antiplatelet and beta-blocker use might have been just a marker for sicker patients who were more vulnerable to heat-related MI, Chen said, the nonfatal MI risk was significantly elevated (OR, 2.17; 95% CI, 1.40-3.38) among patients with pre-existing CHD, but not among those free of pre-existing CHD (OR, 0.88; 95% CI, 0.65-1.20); the odds difference was P < .01.
That signal of confounding by indication is somewhat countered, the report states, by variations in nonfatal MI risk by age group. The increased chances of an event seen overall in relation to beta-blockers and antiplatelets were more pronounced among the 39% of patients aged 25-59 years (P < .01). That’s in spite that group’s lower CHD prevalence. The risk elevation solely among the older patients was attenuated and rendered nonsignificant, even with their greater CHD burden, the report noted.
The report speculates on a potential mechanism by which beta-blockers, at least, might conceivably raise the risk for heat-related MI. “Beta-receptor blockers inhibit skin vasodilation, resulting in reduced heat dissipation through convection and, at the same time, could intensify the blood-pressure-lowering effect of other antihypertensive drugs, which then could lead to syncope.”
Beta-blockers, Dr. Chen said, “can mechanistically make people more vulnerable to heat. That’s one potential explanation. Or it could be that these people taking the medications are just sicker. Whatever the reasons, the phenomenon we observed is that these patients taking these two medications are at higher risk during high-temperature days.”
Dr. Chen and the other authors declare no competing interests.
A version of this article first appeared on Medscape.com.
Patients who take beta-blockers or antiplatelet agents are lowering their risk for cardiovascular events, but the protection may fall short for those who spend time outdoors on hot summer days, hints a limited analysis published as a letter in Nature Cardiovascular Research.
Patients taking either a beta-blocker or antiplatelet, or both medications together, appeared at elevated risk for nonfatal acute MI specifically on days when the weather turned hot, suggests the registry cohort study that covered 14 years of clinical and meteorologic data.
“The take-away message is not that patients should stop using these two medications, by no means. We’re raising cautions for patients taking them, to watch out for themselves during high-heat days,” lead author Kai Chen, PhD, Yale University, New Haven, Conn., said in an interview.
“We’re not giving the message that these drugs have harmful effects” because the nature of the links between the medications and MI in the study, with its potential for confounding, remain unknown, said Dr. Chen, from the department of environmental health sciences and Yale Center on Climate Change and Health.
For example, patients who take beta-blockers or antiplatelets tend to be sicker than patients not on the drugs, which could make heat-related MI more likely, and the drugs wrongly appear to be culprits, he observed. The analysis contained signals that could support either scenario.
The study is based on cases of nonfatal MI in Augsburg, Germany, that are part of the MONICA-KORA MI registry. The odds of a heat-related nonfatal MI, it suggests, were increased 63% among patients taking antiplatelets and by 65% among those on beta-blockers, compared with those not on these drugs. The odds went up by 75% among those on both drug classes, but the risks weren’t raised in patients not taking them.
Rising heat-related MI
Chen said analysis was inspired by a 2019 report – also based on MONICA-KORA, from many of the same authors and using similar methods to track events by daily air temperature – that showed a rising trend for heat-related MI and declining rate for MI related to cold weather from 1987 to 2014. A next step, he figured, would be to determine whether the MI risk trends were associated with any cardiovascular medications.
The current study’s signal of risk related to antiplatelets and beta-blockers did not emerge for ACE inhibitors, calcium-channel blockers, or diuretics. Statins showed a link to increased nonfatal MI risk, but solely among participants aged younger than 60 years, who were also far less likely to have pre-existing coronary heart disease (CHD). He and his colleagues chose not to highlight that finding, Dr. Chen said, because the age subgroup analysis was grossly underpowered.
The overall analysis involved 2,494 cases of nonfatal MI that occurred during the warmer months – May to September – from 2001 to 2014. It was limited to nonfatal cases – those with at least a month of survival after hospital admission – because of insufficient data on medication use associated with fatal MIs, the report states.
Nonfatal MIs were defined as heat-related if they struck on days reaching the 95th percentile for temperature across the 14 years, in this case 24.2 °C (about 75.6 °F), relative to the average temperature of lowest nonfatal MI risk across the cohort, 7.5 °C (about 45.5 °F).
Patients served as both cases and their own controls, in that air temperature exposures on the day of their MI (case day) were compared with the remaining same days of the week in the same calendar month (control days). That approach, the report stated, “automatically controls for long-term time trends, seasonality, day of the week, and time-invariant confounders (for example, pre-existing cardiovascular disease).”
The odds ratio for heat-related MI for patients on antiplatelets was 1.63 (95% confidence interval, 1.07-2.46), and for antiplatelet nonusers was 0.94 (95% CI, 0.68-1.29). The difference between the two ratios was significant (P = .04).
The corresponding OR for patients taking beta-blockers was 1.65 (95% CI, 1.11-2.45), and for nonusers of beta-blockers was 0.90 (95% CI, 0.64-1.26). Again, the OR difference was significant (P = .02).
The ORs for users of both medication classes and nonusers of either med class, respectively, were 1.75 (95% CI, 1.12-2.73) and 0.84 (95% CI, 0.59-1.19). The latter OR was significantly lower than former (P = .01).
In a sign that antiplatelet and beta-blocker use might have been just a marker for sicker patients who were more vulnerable to heat-related MI, Chen said, the nonfatal MI risk was significantly elevated (OR, 2.17; 95% CI, 1.40-3.38) among patients with pre-existing CHD, but not among those free of pre-existing CHD (OR, 0.88; 95% CI, 0.65-1.20); the odds difference was P < .01.
That signal of confounding by indication is somewhat countered, the report states, by variations in nonfatal MI risk by age group. The increased chances of an event seen overall in relation to beta-blockers and antiplatelets were more pronounced among the 39% of patients aged 25-59 years (P < .01). That’s in spite that group’s lower CHD prevalence. The risk elevation solely among the older patients was attenuated and rendered nonsignificant, even with their greater CHD burden, the report noted.
The report speculates on a potential mechanism by which beta-blockers, at least, might conceivably raise the risk for heat-related MI. “Beta-receptor blockers inhibit skin vasodilation, resulting in reduced heat dissipation through convection and, at the same time, could intensify the blood-pressure-lowering effect of other antihypertensive drugs, which then could lead to syncope.”
Beta-blockers, Dr. Chen said, “can mechanistically make people more vulnerable to heat. That’s one potential explanation. Or it could be that these people taking the medications are just sicker. Whatever the reasons, the phenomenon we observed is that these patients taking these two medications are at higher risk during high-temperature days.”
Dr. Chen and the other authors declare no competing interests.
A version of this article first appeared on Medscape.com.
Patients who take beta-blockers or antiplatelet agents are lowering their risk for cardiovascular events, but the protection may fall short for those who spend time outdoors on hot summer days, hints a limited analysis published as a letter in Nature Cardiovascular Research.
Patients taking either a beta-blocker or antiplatelet, or both medications together, appeared at elevated risk for nonfatal acute MI specifically on days when the weather turned hot, suggests the registry cohort study that covered 14 years of clinical and meteorologic data.
“The take-away message is not that patients should stop using these two medications, by no means. We’re raising cautions for patients taking them, to watch out for themselves during high-heat days,” lead author Kai Chen, PhD, Yale University, New Haven, Conn., said in an interview.
“We’re not giving the message that these drugs have harmful effects” because the nature of the links between the medications and MI in the study, with its potential for confounding, remain unknown, said Dr. Chen, from the department of environmental health sciences and Yale Center on Climate Change and Health.
For example, patients who take beta-blockers or antiplatelets tend to be sicker than patients not on the drugs, which could make heat-related MI more likely, and the drugs wrongly appear to be culprits, he observed. The analysis contained signals that could support either scenario.
The study is based on cases of nonfatal MI in Augsburg, Germany, that are part of the MONICA-KORA MI registry. The odds of a heat-related nonfatal MI, it suggests, were increased 63% among patients taking antiplatelets and by 65% among those on beta-blockers, compared with those not on these drugs. The odds went up by 75% among those on both drug classes, but the risks weren’t raised in patients not taking them.
Rising heat-related MI
Chen said analysis was inspired by a 2019 report – also based on MONICA-KORA, from many of the same authors and using similar methods to track events by daily air temperature – that showed a rising trend for heat-related MI and declining rate for MI related to cold weather from 1987 to 2014. A next step, he figured, would be to determine whether the MI risk trends were associated with any cardiovascular medications.
The current study’s signal of risk related to antiplatelets and beta-blockers did not emerge for ACE inhibitors, calcium-channel blockers, or diuretics. Statins showed a link to increased nonfatal MI risk, but solely among participants aged younger than 60 years, who were also far less likely to have pre-existing coronary heart disease (CHD). He and his colleagues chose not to highlight that finding, Dr. Chen said, because the age subgroup analysis was grossly underpowered.
The overall analysis involved 2,494 cases of nonfatal MI that occurred during the warmer months – May to September – from 2001 to 2014. It was limited to nonfatal cases – those with at least a month of survival after hospital admission – because of insufficient data on medication use associated with fatal MIs, the report states.
Nonfatal MIs were defined as heat-related if they struck on days reaching the 95th percentile for temperature across the 14 years, in this case 24.2 °C (about 75.6 °F), relative to the average temperature of lowest nonfatal MI risk across the cohort, 7.5 °C (about 45.5 °F).
Patients served as both cases and their own controls, in that air temperature exposures on the day of their MI (case day) were compared with the remaining same days of the week in the same calendar month (control days). That approach, the report stated, “automatically controls for long-term time trends, seasonality, day of the week, and time-invariant confounders (for example, pre-existing cardiovascular disease).”
The odds ratio for heat-related MI for patients on antiplatelets was 1.63 (95% confidence interval, 1.07-2.46), and for antiplatelet nonusers was 0.94 (95% CI, 0.68-1.29). The difference between the two ratios was significant (P = .04).
The corresponding OR for patients taking beta-blockers was 1.65 (95% CI, 1.11-2.45), and for nonusers of beta-blockers was 0.90 (95% CI, 0.64-1.26). Again, the OR difference was significant (P = .02).
The ORs for users of both medication classes and nonusers of either med class, respectively, were 1.75 (95% CI, 1.12-2.73) and 0.84 (95% CI, 0.59-1.19). The latter OR was significantly lower than former (P = .01).
In a sign that antiplatelet and beta-blocker use might have been just a marker for sicker patients who were more vulnerable to heat-related MI, Chen said, the nonfatal MI risk was significantly elevated (OR, 2.17; 95% CI, 1.40-3.38) among patients with pre-existing CHD, but not among those free of pre-existing CHD (OR, 0.88; 95% CI, 0.65-1.20); the odds difference was P < .01.
That signal of confounding by indication is somewhat countered, the report states, by variations in nonfatal MI risk by age group. The increased chances of an event seen overall in relation to beta-blockers and antiplatelets were more pronounced among the 39% of patients aged 25-59 years (P < .01). That’s in spite that group’s lower CHD prevalence. The risk elevation solely among the older patients was attenuated and rendered nonsignificant, even with their greater CHD burden, the report noted.
The report speculates on a potential mechanism by which beta-blockers, at least, might conceivably raise the risk for heat-related MI. “Beta-receptor blockers inhibit skin vasodilation, resulting in reduced heat dissipation through convection and, at the same time, could intensify the blood-pressure-lowering effect of other antihypertensive drugs, which then could lead to syncope.”
Beta-blockers, Dr. Chen said, “can mechanistically make people more vulnerable to heat. That’s one potential explanation. Or it could be that these people taking the medications are just sicker. Whatever the reasons, the phenomenon we observed is that these patients taking these two medications are at higher risk during high-temperature days.”
Dr. Chen and the other authors declare no competing interests.
A version of this article first appeared on Medscape.com.
FROM NATURE CARDIOVASCULAR RESEARCH
Gout flares linked to transient jump in MI, stroke risk
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.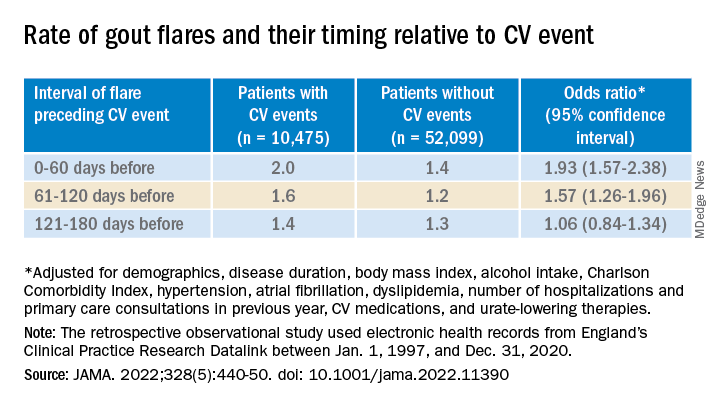
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
FROM JAMA









