User login
Clarity on torsemide vs. furosemide in HF: TRANSFORM-HF published
Survival and readmission risk were similar whether patients hospitalized with heart failure (HF) were discharged on furosemide or torsemide in a randomized trial.
The study, TRANSFORM-HF, helps fill a major gap in the sparse evidence base guiding diuretic therapy in patients with a history of HF hospitalization. In that setting, for example, results suggest that discharge on any appropriate loop diuretic is more important than which loop diuretic is chosen.
TRANSFORM-HF is no ordinary randomized trial. Designed as a pragmatic comparative effectiveness study, it featured a streamlined protocol and other adaptations that made it easier and cheaper to conduct but that have also complicated its interpretation, the trialists and some observers acknowledge.
Perceived torsemide advantages
Furosemide may be the most-prescribed loop diuretic in HF, but in practice – based on some limited evidence – clinicians often prefer torsemide for its perceived advantages that include greater bioavailability, potassium sparing, and potentially helpful pleiotropic effects.
TRANSFORM-HF, however, provides no evidence to support such a preference. The primary endpoint of all-cause mortality was about 26% over a median 17 months whether patients were assigned to an initial furosemide or torsemide-first strategy, regardless of ejection fraction. Composite rates of death or hospitalization at 12 months also weren’t significantly different, at about 49% and 47%, respectively.
The findings suggest that clinicians may safely continue to prescribe either loop diuretic at their discretion, now with the support of data from a randomized trial.
TRANSFORM-HF was published in the Journal of the American Medical Association, with lead author Robert J. Mentz, MD, Duke University School of Medicine, Durham, N.C.
Dr. Mentz had also presented the trial’s preliminary results at the November American Heart Association Scientific Sessions in Chicago. The findings unveiled at the meeting and those published in the journal are essentially the same.
Reflections of standard practice
With its pragmatic design, TRANSFORM-HF entered a diverse HF population broadly representative of actual clinical practice. Patients were managed with few restrictions in a protocol that allowed, for example, loop-diuretic crossovers and other discretionary diuretic changes.
Diuretic dosing also varied significantly between the groups, and there was an unexpectedly high prevalence of diuretic withdrawal, the published report notes. Those factors, it states, may have “diminished” the trial’s ability “to distinguish the hypothesized between-group differences.”
Still, the trial “should be celebrated for dispelling a long-standing myth, based on surrogate markers and small trials, of the superiority of torsemide over furosemide,” writes Michelle M. Kittleson, MD, PhD, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, in an accompanying editorial .
Now, she continues, “when faced with a patient with heart failure and congestive symptoms, clinicians can focus their energy on what really matters: Not the relative merits of different loop diuretics, but rather the initiation and optimization of evidence and guideline-based therapies to help their patients feel better and live longer.”
Trial design caveats
But that pragmatic design raises cautions, the editorial notes. “Pragmatic trials are more flexible and nimbler in design and execution, but this agility comes at a cost. An overly heterogeneous patient population can impact the trial’s ability to assess efficacy of therapies while minimally intensive follow-up precludes comprehensive outcome assessment.”
The study’s 2,859 patients hospitalized with HF were assigned to open-label treatment with furosemide or torsemide at more than 60 U.S. centers. Of the 1,428 and 1,431 patients, respectively, about 37% were women and 34% were African American.
The hazard ratio for all cause mortality across the 17.4-month follow-up, torsemide versus furosemide, was 1.02 (95% confidence interval, 0.89-1.18). The HR for death or hospitalization for any cause at 12 months was 0.92 (95% CI, 0.83-1.02). And the rate ratio for 12-month all-cause hospitalization was 0.94 (95% CI, 0.84-1.07).
“TRANSFORM-HF joins a catalog of cautionary tales in cardiology, whereby carefully executed negative trials have refuted the misleading promise of plausible surrogate end points and preliminary data,” Dr. Kittleson writes.
“The lesson: Clinicians should have a healthy suspicion for plausible pathophysiology, surrogate end points, and nonrandomized data as the sole basis of defining superiority of an intervention.”
TRANSFORM-HF was funded by the National Institutes of Health. Dr. Mentz reports receiving grants from American Regent and Novartis; personal fees from AstraZeneca, Boehringer Ingelheim/Eli Lilly, Cytokinetics, Bayer, Merck, and Pharmacosmos; and research support from Abbott, Amgen, Bayer, Boston Scientific, Fast BioMedical, Gilead, Innolife, Medtronic, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Disclosures for the other authors can be found with the original article. Dr. Kittleson reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Survival and readmission risk were similar whether patients hospitalized with heart failure (HF) were discharged on furosemide or torsemide in a randomized trial.
The study, TRANSFORM-HF, helps fill a major gap in the sparse evidence base guiding diuretic therapy in patients with a history of HF hospitalization. In that setting, for example, results suggest that discharge on any appropriate loop diuretic is more important than which loop diuretic is chosen.
TRANSFORM-HF is no ordinary randomized trial. Designed as a pragmatic comparative effectiveness study, it featured a streamlined protocol and other adaptations that made it easier and cheaper to conduct but that have also complicated its interpretation, the trialists and some observers acknowledge.
Perceived torsemide advantages
Furosemide may be the most-prescribed loop diuretic in HF, but in practice – based on some limited evidence – clinicians often prefer torsemide for its perceived advantages that include greater bioavailability, potassium sparing, and potentially helpful pleiotropic effects.
TRANSFORM-HF, however, provides no evidence to support such a preference. The primary endpoint of all-cause mortality was about 26% over a median 17 months whether patients were assigned to an initial furosemide or torsemide-first strategy, regardless of ejection fraction. Composite rates of death or hospitalization at 12 months also weren’t significantly different, at about 49% and 47%, respectively.
The findings suggest that clinicians may safely continue to prescribe either loop diuretic at their discretion, now with the support of data from a randomized trial.
TRANSFORM-HF was published in the Journal of the American Medical Association, with lead author Robert J. Mentz, MD, Duke University School of Medicine, Durham, N.C.
Dr. Mentz had also presented the trial’s preliminary results at the November American Heart Association Scientific Sessions in Chicago. The findings unveiled at the meeting and those published in the journal are essentially the same.
Reflections of standard practice
With its pragmatic design, TRANSFORM-HF entered a diverse HF population broadly representative of actual clinical practice. Patients were managed with few restrictions in a protocol that allowed, for example, loop-diuretic crossovers and other discretionary diuretic changes.
Diuretic dosing also varied significantly between the groups, and there was an unexpectedly high prevalence of diuretic withdrawal, the published report notes. Those factors, it states, may have “diminished” the trial’s ability “to distinguish the hypothesized between-group differences.”
Still, the trial “should be celebrated for dispelling a long-standing myth, based on surrogate markers and small trials, of the superiority of torsemide over furosemide,” writes Michelle M. Kittleson, MD, PhD, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, in an accompanying editorial .
Now, she continues, “when faced with a patient with heart failure and congestive symptoms, clinicians can focus their energy on what really matters: Not the relative merits of different loop diuretics, but rather the initiation and optimization of evidence and guideline-based therapies to help their patients feel better and live longer.”
Trial design caveats
But that pragmatic design raises cautions, the editorial notes. “Pragmatic trials are more flexible and nimbler in design and execution, but this agility comes at a cost. An overly heterogeneous patient population can impact the trial’s ability to assess efficacy of therapies while minimally intensive follow-up precludes comprehensive outcome assessment.”
The study’s 2,859 patients hospitalized with HF were assigned to open-label treatment with furosemide or torsemide at more than 60 U.S. centers. Of the 1,428 and 1,431 patients, respectively, about 37% were women and 34% were African American.
The hazard ratio for all cause mortality across the 17.4-month follow-up, torsemide versus furosemide, was 1.02 (95% confidence interval, 0.89-1.18). The HR for death or hospitalization for any cause at 12 months was 0.92 (95% CI, 0.83-1.02). And the rate ratio for 12-month all-cause hospitalization was 0.94 (95% CI, 0.84-1.07).
“TRANSFORM-HF joins a catalog of cautionary tales in cardiology, whereby carefully executed negative trials have refuted the misleading promise of plausible surrogate end points and preliminary data,” Dr. Kittleson writes.
“The lesson: Clinicians should have a healthy suspicion for plausible pathophysiology, surrogate end points, and nonrandomized data as the sole basis of defining superiority of an intervention.”
TRANSFORM-HF was funded by the National Institutes of Health. Dr. Mentz reports receiving grants from American Regent and Novartis; personal fees from AstraZeneca, Boehringer Ingelheim/Eli Lilly, Cytokinetics, Bayer, Merck, and Pharmacosmos; and research support from Abbott, Amgen, Bayer, Boston Scientific, Fast BioMedical, Gilead, Innolife, Medtronic, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Disclosures for the other authors can be found with the original article. Dr. Kittleson reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Survival and readmission risk were similar whether patients hospitalized with heart failure (HF) were discharged on furosemide or torsemide in a randomized trial.
The study, TRANSFORM-HF, helps fill a major gap in the sparse evidence base guiding diuretic therapy in patients with a history of HF hospitalization. In that setting, for example, results suggest that discharge on any appropriate loop diuretic is more important than which loop diuretic is chosen.
TRANSFORM-HF is no ordinary randomized trial. Designed as a pragmatic comparative effectiveness study, it featured a streamlined protocol and other adaptations that made it easier and cheaper to conduct but that have also complicated its interpretation, the trialists and some observers acknowledge.
Perceived torsemide advantages
Furosemide may be the most-prescribed loop diuretic in HF, but in practice – based on some limited evidence – clinicians often prefer torsemide for its perceived advantages that include greater bioavailability, potassium sparing, and potentially helpful pleiotropic effects.
TRANSFORM-HF, however, provides no evidence to support such a preference. The primary endpoint of all-cause mortality was about 26% over a median 17 months whether patients were assigned to an initial furosemide or torsemide-first strategy, regardless of ejection fraction. Composite rates of death or hospitalization at 12 months also weren’t significantly different, at about 49% and 47%, respectively.
The findings suggest that clinicians may safely continue to prescribe either loop diuretic at their discretion, now with the support of data from a randomized trial.
TRANSFORM-HF was published in the Journal of the American Medical Association, with lead author Robert J. Mentz, MD, Duke University School of Medicine, Durham, N.C.
Dr. Mentz had also presented the trial’s preliminary results at the November American Heart Association Scientific Sessions in Chicago. The findings unveiled at the meeting and those published in the journal are essentially the same.
Reflections of standard practice
With its pragmatic design, TRANSFORM-HF entered a diverse HF population broadly representative of actual clinical practice. Patients were managed with few restrictions in a protocol that allowed, for example, loop-diuretic crossovers and other discretionary diuretic changes.
Diuretic dosing also varied significantly between the groups, and there was an unexpectedly high prevalence of diuretic withdrawal, the published report notes. Those factors, it states, may have “diminished” the trial’s ability “to distinguish the hypothesized between-group differences.”
Still, the trial “should be celebrated for dispelling a long-standing myth, based on surrogate markers and small trials, of the superiority of torsemide over furosemide,” writes Michelle M. Kittleson, MD, PhD, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, in an accompanying editorial .
Now, she continues, “when faced with a patient with heart failure and congestive symptoms, clinicians can focus their energy on what really matters: Not the relative merits of different loop diuretics, but rather the initiation and optimization of evidence and guideline-based therapies to help their patients feel better and live longer.”
Trial design caveats
But that pragmatic design raises cautions, the editorial notes. “Pragmatic trials are more flexible and nimbler in design and execution, but this agility comes at a cost. An overly heterogeneous patient population can impact the trial’s ability to assess efficacy of therapies while minimally intensive follow-up precludes comprehensive outcome assessment.”
The study’s 2,859 patients hospitalized with HF were assigned to open-label treatment with furosemide or torsemide at more than 60 U.S. centers. Of the 1,428 and 1,431 patients, respectively, about 37% were women and 34% were African American.
The hazard ratio for all cause mortality across the 17.4-month follow-up, torsemide versus furosemide, was 1.02 (95% confidence interval, 0.89-1.18). The HR for death or hospitalization for any cause at 12 months was 0.92 (95% CI, 0.83-1.02). And the rate ratio for 12-month all-cause hospitalization was 0.94 (95% CI, 0.84-1.07).
“TRANSFORM-HF joins a catalog of cautionary tales in cardiology, whereby carefully executed negative trials have refuted the misleading promise of plausible surrogate end points and preliminary data,” Dr. Kittleson writes.
“The lesson: Clinicians should have a healthy suspicion for plausible pathophysiology, surrogate end points, and nonrandomized data as the sole basis of defining superiority of an intervention.”
TRANSFORM-HF was funded by the National Institutes of Health. Dr. Mentz reports receiving grants from American Regent and Novartis; personal fees from AstraZeneca, Boehringer Ingelheim/Eli Lilly, Cytokinetics, Bayer, Merck, and Pharmacosmos; and research support from Abbott, Amgen, Bayer, Boston Scientific, Fast BioMedical, Gilead, Innolife, Medtronic, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Disclosures for the other authors can be found with the original article. Dr. Kittleson reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
PCSK9 inhibitors for severe COVID? Pilot trial signals of benefit
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One in four cardiologists worldwide report mental health issues
ranging from anxiety or anger issues to major depression or other psychiatric disorders.
Such conditions varied in prevalence by cardiology subspecialty and years in the field, were more common in women than in men, and were closely linked to enduring hostile work environments and other strains of professional life.
The survey, conducted only months before the COVID-19 pandemic and with its share of limitations, still paints a picture that’s not pretty.
For example, mental health concerns were reported by about 42% of respondents who cited a hostile work environment, defined as workplace experience of discrimination based on age, sex, religion, race or ethnicity, or emotional or sexual harassment. Conversely, the prevalence of these concerns reached only 17% among those without such workplace conditions.
The study shows substantial overlap between cardiologists reporting hostility at work and those with mental health concerns, “and that was a significant finding,” Garima Sharma, MD, Johns Hopkins University, Baltimore, said in an interview.
Still, only 31% of male and 42% of female cardiologists (P < .001) reporting mental health concerns also said they had sought professional help either within or outside their own institutions.
That means “there is a lot of silent suffering” in the field, said Dr. Sharma, who is lead author on the study, published in the Journal of the American College of Cardiology.
Bringing back the conversation
The survey findings, she added, point to at least two potential ways the cardiology community can strive to diminish what may be a major underlying cause of the mental health concerns and their consequences.
“If you work towards reducing hostility at work and making mental health a priority for your workforce, then those experiencing these types of egregious conditions based on age, gender, race, ethnicity, or sexual orientation are less likely to be harmed.”
Mental health concerns among cardiologists are seldom openly discussed, so the current study can be “a way to bring them back into the conversation,” Dr. Sharma said. Clinician mental health “is extremely important because it directly impacts patient care and productivity.”
The survey’s reported mental health conditions “are an issue across the board in medicine, and amongst our medical students as well,” senior author Laxmi S. Mehta, MD, professor of internal medicine at Ohio State University, Columbus, said in an interview. The current study provides new details about their prevalence and predictors in cardiology and, she hopes, may improve the field’s awareness of and efforts to address the problem.
“We need to support those who have underlying mental health conditions, as well as improve the work environment to reduce contributory factors to mental illnesses. And we also need to work on reducing the stigma associated with seeking treatment and on reducing the barriers to receiving treatment,” said Dr. Mehta, who chairs the Workgroup on Clinician Well-Being of the ACC, which conducted the survey in 2019.
A global perspective
Cardiologists in Africa, the Americas, Asia, Europe, the Middle East, and Oceania – 5,890 in all – responded to mental health questions on the survey, which was novel for its global reach and insights across continents and cultures.
Respondents in South America and Central America reported the highest prevalences of mental health concerns, outliers at about 39% and 33%, respectively. Rates for most other geographic regions ranged narrowly from about 20% to 26%, the lowest reported in Asia and the Middle East.
Dr. Sharma acknowledged that the countries probably varied widely in social and cultural factors likely to influence survey responses, such as interpretation of the questionnaire’s mental health terminology or the degree to which the disorders are stigmatized.
“I think it’s hard to say how people may or may not respond culturally to a certain word or metric,” she said. But on the survey results, “whether you’re practicing in rural America, in rural India, or in the United Arab Emirates, Oceania, or Eastern Europe, there is a level of consistency, across the board, in what people are recognizing as mental health conditions.”
Junior vs. senior physicians
The global perspective “is a nice positive of the study, and the high rates in Central America and South America I think were something the field was not aware of and are an important contribution,” Srijan Sen, MD, PhD, said in an interview.
The psychological toll of hostile work environments is an issue throughout medicine, “but it seems greater in certain specialties, and cardiology may be one where it’s more of a problem,” observed Dr. Sen, who studies physician mental health at the University of Michigan, Ann Arbor, and wasn’t associated with the survey.
Mental health concerns in the survey were significantly more common among women than men (33.7% vs 26.3%), and for younger cardiologists, compared with older cardiologists (32.2% for those < 40 vs. 22.1% and 16.8% for those 55-69 and 70 or older, respectively).
Those findings seem to make sense, Dr. Sen observed. “Generally, cardiology and medicine broadly are hierarchical, so being more junior can be stressful.” And if there’s more hostility in the workplace, “it might fall on junior people.”
In other studies, moreover, “a high level of work-family conflict has been a real driver of depression and burnout, and that likely is affecting younger physicians, particularly young women physicians,” who may have smaller children and a greater burden of childcare than their seniors.
He pointed to the survey’s low response rate as an important limitation of the study. Of the 71,022 cardiologists invited to participate, only 5,890 (8.3%) responded and answered the queries on mental health.
With a response rate that low, a survey “can be biased in ways that we can’t predict,” Dr. Sen noted. Also, anyone concerned about the toxicity of their own workplace might be “more likely to respond to the survey than if they worked in a more pleasant place. That would provide a skewed sense of the overall experience of cardiologists.”
Those issues might not be a concern with the current survey, however, “because the results are consistent with other studies with higher response rates.”
‘Sobering report’
An accompanying editorial said Dr. Sharm and colleagues have provided “a sobering report on the global prevalence and potential contributors to mental health concerns” in the surveyed population.
Based on its lessons, Andrew J. Sauer, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., proposed several potential “interventions” the field could enact.
It could “selectively promote leaders who strive to mitigate implicit bias, discrimination, and harassment while advancing diversity, equity, and inclusion within the broad ranks of cardiologists.”
Also, he continued, “we must eliminate the stigmatization of mental illness among physicians. We need to handle mental health concerns with compassion and without blaming, like how we strive to treat our veterans who suffer from posttraumatic stress disorder.”
Lastly, Dr. Sauer wrote, “mentorship programs should be formalized to assist the cardiologist in transition zones from early to mid-career, with particular attention to women and those experiencing a simultaneously increased load of family burdens that compound existing workplace contributors to burnout and psychological distress.”
Years in practice
Of the cardiologists who responded to the survey’s mental health questions, 28% reported they have experienced mental health issues that could include alcohol/drug use disorder, suicidal tendencies, psychological distress (including anxiety, irritability, or anger), “other psychiatric disorders” (such as panic disorder, posttraumatic stress, or eating disorders) or major psychiatric disorders such as major depression, bipolar disorder, or schizophrenia.
Cardiologists with 5-10 years of practice post-training were more likely than cardiologists practicing for at least 20 years to have mental health concerns (31.9% vs. 22.6%, P < .001).
Mental health concerns were cited by 42% of respondents who cited “any type of discrimination” based on age, sex, race or ethnicity, or sexual orientation, the report noted.
Among those reporting any mental health concern, 2.7% considered suicide within the past year and 2.9% considered suicide more than 12 months previously. Women were more likely than men to consider suicide within the past year (3.8% vs. 2.3%) but were also more likely to seek help (42.3% vs. 31.1%; P < .001 for both differences), the authors wrote.
In multivariate analysis, predictors of mental health concerns included emotional harassment, 2.81 (odds ratio, 2.81; 95% confidence interval, 2.46-3.20), any discrimination (OR, 1.85; 95% CI, 1.61-2.12), being divorced (OR, 1.73; 95% CI, 1.26-2.36, age less than 55 years (OR, 1.43; 95% CI, 1.24-1.66), and being mid-career versus late (OR, 1.36; 95% CI, 1.14-1.62).
Because the survey was conducted from September to October 2019, before the pandemic’s traumatic effects unfolded on health care nearly everywhere, “I think there needs to be a follow-up at some point when everything has leveled out,” Dr. Sharma said. The current study is “a baseline, and not a healthy baseline,” for the field’s state of mental health that has likely grown worse during the pandemic.
But even without such a follow-up, the current study “is actionable enough that it forces us to do something about it right now.”
Dr. Sharma, Dr. Mehta, their coauthors, Dr. Sen, and Dr. Sauer reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
ranging from anxiety or anger issues to major depression or other psychiatric disorders.
Such conditions varied in prevalence by cardiology subspecialty and years in the field, were more common in women than in men, and were closely linked to enduring hostile work environments and other strains of professional life.
The survey, conducted only months before the COVID-19 pandemic and with its share of limitations, still paints a picture that’s not pretty.
For example, mental health concerns were reported by about 42% of respondents who cited a hostile work environment, defined as workplace experience of discrimination based on age, sex, religion, race or ethnicity, or emotional or sexual harassment. Conversely, the prevalence of these concerns reached only 17% among those without such workplace conditions.
The study shows substantial overlap between cardiologists reporting hostility at work and those with mental health concerns, “and that was a significant finding,” Garima Sharma, MD, Johns Hopkins University, Baltimore, said in an interview.
Still, only 31% of male and 42% of female cardiologists (P < .001) reporting mental health concerns also said they had sought professional help either within or outside their own institutions.
That means “there is a lot of silent suffering” in the field, said Dr. Sharma, who is lead author on the study, published in the Journal of the American College of Cardiology.
Bringing back the conversation
The survey findings, she added, point to at least two potential ways the cardiology community can strive to diminish what may be a major underlying cause of the mental health concerns and their consequences.
“If you work towards reducing hostility at work and making mental health a priority for your workforce, then those experiencing these types of egregious conditions based on age, gender, race, ethnicity, or sexual orientation are less likely to be harmed.”
Mental health concerns among cardiologists are seldom openly discussed, so the current study can be “a way to bring them back into the conversation,” Dr. Sharma said. Clinician mental health “is extremely important because it directly impacts patient care and productivity.”
The survey’s reported mental health conditions “are an issue across the board in medicine, and amongst our medical students as well,” senior author Laxmi S. Mehta, MD, professor of internal medicine at Ohio State University, Columbus, said in an interview. The current study provides new details about their prevalence and predictors in cardiology and, she hopes, may improve the field’s awareness of and efforts to address the problem.
“We need to support those who have underlying mental health conditions, as well as improve the work environment to reduce contributory factors to mental illnesses. And we also need to work on reducing the stigma associated with seeking treatment and on reducing the barriers to receiving treatment,” said Dr. Mehta, who chairs the Workgroup on Clinician Well-Being of the ACC, which conducted the survey in 2019.
A global perspective
Cardiologists in Africa, the Americas, Asia, Europe, the Middle East, and Oceania – 5,890 in all – responded to mental health questions on the survey, which was novel for its global reach and insights across continents and cultures.
Respondents in South America and Central America reported the highest prevalences of mental health concerns, outliers at about 39% and 33%, respectively. Rates for most other geographic regions ranged narrowly from about 20% to 26%, the lowest reported in Asia and the Middle East.
Dr. Sharma acknowledged that the countries probably varied widely in social and cultural factors likely to influence survey responses, such as interpretation of the questionnaire’s mental health terminology or the degree to which the disorders are stigmatized.
“I think it’s hard to say how people may or may not respond culturally to a certain word or metric,” she said. But on the survey results, “whether you’re practicing in rural America, in rural India, or in the United Arab Emirates, Oceania, or Eastern Europe, there is a level of consistency, across the board, in what people are recognizing as mental health conditions.”
Junior vs. senior physicians
The global perspective “is a nice positive of the study, and the high rates in Central America and South America I think were something the field was not aware of and are an important contribution,” Srijan Sen, MD, PhD, said in an interview.
The psychological toll of hostile work environments is an issue throughout medicine, “but it seems greater in certain specialties, and cardiology may be one where it’s more of a problem,” observed Dr. Sen, who studies physician mental health at the University of Michigan, Ann Arbor, and wasn’t associated with the survey.
Mental health concerns in the survey were significantly more common among women than men (33.7% vs 26.3%), and for younger cardiologists, compared with older cardiologists (32.2% for those < 40 vs. 22.1% and 16.8% for those 55-69 and 70 or older, respectively).
Those findings seem to make sense, Dr. Sen observed. “Generally, cardiology and medicine broadly are hierarchical, so being more junior can be stressful.” And if there’s more hostility in the workplace, “it might fall on junior people.”
In other studies, moreover, “a high level of work-family conflict has been a real driver of depression and burnout, and that likely is affecting younger physicians, particularly young women physicians,” who may have smaller children and a greater burden of childcare than their seniors.
He pointed to the survey’s low response rate as an important limitation of the study. Of the 71,022 cardiologists invited to participate, only 5,890 (8.3%) responded and answered the queries on mental health.
With a response rate that low, a survey “can be biased in ways that we can’t predict,” Dr. Sen noted. Also, anyone concerned about the toxicity of their own workplace might be “more likely to respond to the survey than if they worked in a more pleasant place. That would provide a skewed sense of the overall experience of cardiologists.”
Those issues might not be a concern with the current survey, however, “because the results are consistent with other studies with higher response rates.”
‘Sobering report’
An accompanying editorial said Dr. Sharm and colleagues have provided “a sobering report on the global prevalence and potential contributors to mental health concerns” in the surveyed population.
Based on its lessons, Andrew J. Sauer, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., proposed several potential “interventions” the field could enact.
It could “selectively promote leaders who strive to mitigate implicit bias, discrimination, and harassment while advancing diversity, equity, and inclusion within the broad ranks of cardiologists.”
Also, he continued, “we must eliminate the stigmatization of mental illness among physicians. We need to handle mental health concerns with compassion and without blaming, like how we strive to treat our veterans who suffer from posttraumatic stress disorder.”
Lastly, Dr. Sauer wrote, “mentorship programs should be formalized to assist the cardiologist in transition zones from early to mid-career, with particular attention to women and those experiencing a simultaneously increased load of family burdens that compound existing workplace contributors to burnout and psychological distress.”
Years in practice
Of the cardiologists who responded to the survey’s mental health questions, 28% reported they have experienced mental health issues that could include alcohol/drug use disorder, suicidal tendencies, psychological distress (including anxiety, irritability, or anger), “other psychiatric disorders” (such as panic disorder, posttraumatic stress, or eating disorders) or major psychiatric disorders such as major depression, bipolar disorder, or schizophrenia.
Cardiologists with 5-10 years of practice post-training were more likely than cardiologists practicing for at least 20 years to have mental health concerns (31.9% vs. 22.6%, P < .001).
Mental health concerns were cited by 42% of respondents who cited “any type of discrimination” based on age, sex, race or ethnicity, or sexual orientation, the report noted.
Among those reporting any mental health concern, 2.7% considered suicide within the past year and 2.9% considered suicide more than 12 months previously. Women were more likely than men to consider suicide within the past year (3.8% vs. 2.3%) but were also more likely to seek help (42.3% vs. 31.1%; P < .001 for both differences), the authors wrote.
In multivariate analysis, predictors of mental health concerns included emotional harassment, 2.81 (odds ratio, 2.81; 95% confidence interval, 2.46-3.20), any discrimination (OR, 1.85; 95% CI, 1.61-2.12), being divorced (OR, 1.73; 95% CI, 1.26-2.36, age less than 55 years (OR, 1.43; 95% CI, 1.24-1.66), and being mid-career versus late (OR, 1.36; 95% CI, 1.14-1.62).
Because the survey was conducted from September to October 2019, before the pandemic’s traumatic effects unfolded on health care nearly everywhere, “I think there needs to be a follow-up at some point when everything has leveled out,” Dr. Sharma said. The current study is “a baseline, and not a healthy baseline,” for the field’s state of mental health that has likely grown worse during the pandemic.
But even without such a follow-up, the current study “is actionable enough that it forces us to do something about it right now.”
Dr. Sharma, Dr. Mehta, their coauthors, Dr. Sen, and Dr. Sauer reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
ranging from anxiety or anger issues to major depression or other psychiatric disorders.
Such conditions varied in prevalence by cardiology subspecialty and years in the field, were more common in women than in men, and were closely linked to enduring hostile work environments and other strains of professional life.
The survey, conducted only months before the COVID-19 pandemic and with its share of limitations, still paints a picture that’s not pretty.
For example, mental health concerns were reported by about 42% of respondents who cited a hostile work environment, defined as workplace experience of discrimination based on age, sex, religion, race or ethnicity, or emotional or sexual harassment. Conversely, the prevalence of these concerns reached only 17% among those without such workplace conditions.
The study shows substantial overlap between cardiologists reporting hostility at work and those with mental health concerns, “and that was a significant finding,” Garima Sharma, MD, Johns Hopkins University, Baltimore, said in an interview.
Still, only 31% of male and 42% of female cardiologists (P < .001) reporting mental health concerns also said they had sought professional help either within or outside their own institutions.
That means “there is a lot of silent suffering” in the field, said Dr. Sharma, who is lead author on the study, published in the Journal of the American College of Cardiology.
Bringing back the conversation
The survey findings, she added, point to at least two potential ways the cardiology community can strive to diminish what may be a major underlying cause of the mental health concerns and their consequences.
“If you work towards reducing hostility at work and making mental health a priority for your workforce, then those experiencing these types of egregious conditions based on age, gender, race, ethnicity, or sexual orientation are less likely to be harmed.”
Mental health concerns among cardiologists are seldom openly discussed, so the current study can be “a way to bring them back into the conversation,” Dr. Sharma said. Clinician mental health “is extremely important because it directly impacts patient care and productivity.”
The survey’s reported mental health conditions “are an issue across the board in medicine, and amongst our medical students as well,” senior author Laxmi S. Mehta, MD, professor of internal medicine at Ohio State University, Columbus, said in an interview. The current study provides new details about their prevalence and predictors in cardiology and, she hopes, may improve the field’s awareness of and efforts to address the problem.
“We need to support those who have underlying mental health conditions, as well as improve the work environment to reduce contributory factors to mental illnesses. And we also need to work on reducing the stigma associated with seeking treatment and on reducing the barriers to receiving treatment,” said Dr. Mehta, who chairs the Workgroup on Clinician Well-Being of the ACC, which conducted the survey in 2019.
A global perspective
Cardiologists in Africa, the Americas, Asia, Europe, the Middle East, and Oceania – 5,890 in all – responded to mental health questions on the survey, which was novel for its global reach and insights across continents and cultures.
Respondents in South America and Central America reported the highest prevalences of mental health concerns, outliers at about 39% and 33%, respectively. Rates for most other geographic regions ranged narrowly from about 20% to 26%, the lowest reported in Asia and the Middle East.
Dr. Sharma acknowledged that the countries probably varied widely in social and cultural factors likely to influence survey responses, such as interpretation of the questionnaire’s mental health terminology or the degree to which the disorders are stigmatized.
“I think it’s hard to say how people may or may not respond culturally to a certain word or metric,” she said. But on the survey results, “whether you’re practicing in rural America, in rural India, or in the United Arab Emirates, Oceania, or Eastern Europe, there is a level of consistency, across the board, in what people are recognizing as mental health conditions.”
Junior vs. senior physicians
The global perspective “is a nice positive of the study, and the high rates in Central America and South America I think were something the field was not aware of and are an important contribution,” Srijan Sen, MD, PhD, said in an interview.
The psychological toll of hostile work environments is an issue throughout medicine, “but it seems greater in certain specialties, and cardiology may be one where it’s more of a problem,” observed Dr. Sen, who studies physician mental health at the University of Michigan, Ann Arbor, and wasn’t associated with the survey.
Mental health concerns in the survey were significantly more common among women than men (33.7% vs 26.3%), and for younger cardiologists, compared with older cardiologists (32.2% for those < 40 vs. 22.1% and 16.8% for those 55-69 and 70 or older, respectively).
Those findings seem to make sense, Dr. Sen observed. “Generally, cardiology and medicine broadly are hierarchical, so being more junior can be stressful.” And if there’s more hostility in the workplace, “it might fall on junior people.”
In other studies, moreover, “a high level of work-family conflict has been a real driver of depression and burnout, and that likely is affecting younger physicians, particularly young women physicians,” who may have smaller children and a greater burden of childcare than their seniors.
He pointed to the survey’s low response rate as an important limitation of the study. Of the 71,022 cardiologists invited to participate, only 5,890 (8.3%) responded and answered the queries on mental health.
With a response rate that low, a survey “can be biased in ways that we can’t predict,” Dr. Sen noted. Also, anyone concerned about the toxicity of their own workplace might be “more likely to respond to the survey than if they worked in a more pleasant place. That would provide a skewed sense of the overall experience of cardiologists.”
Those issues might not be a concern with the current survey, however, “because the results are consistent with other studies with higher response rates.”
‘Sobering report’
An accompanying editorial said Dr. Sharm and colleagues have provided “a sobering report on the global prevalence and potential contributors to mental health concerns” in the surveyed population.
Based on its lessons, Andrew J. Sauer, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., proposed several potential “interventions” the field could enact.
It could “selectively promote leaders who strive to mitigate implicit bias, discrimination, and harassment while advancing diversity, equity, and inclusion within the broad ranks of cardiologists.”
Also, he continued, “we must eliminate the stigmatization of mental illness among physicians. We need to handle mental health concerns with compassion and without blaming, like how we strive to treat our veterans who suffer from posttraumatic stress disorder.”
Lastly, Dr. Sauer wrote, “mentorship programs should be formalized to assist the cardiologist in transition zones from early to mid-career, with particular attention to women and those experiencing a simultaneously increased load of family burdens that compound existing workplace contributors to burnout and psychological distress.”
Years in practice
Of the cardiologists who responded to the survey’s mental health questions, 28% reported they have experienced mental health issues that could include alcohol/drug use disorder, suicidal tendencies, psychological distress (including anxiety, irritability, or anger), “other psychiatric disorders” (such as panic disorder, posttraumatic stress, or eating disorders) or major psychiatric disorders such as major depression, bipolar disorder, or schizophrenia.
Cardiologists with 5-10 years of practice post-training were more likely than cardiologists practicing for at least 20 years to have mental health concerns (31.9% vs. 22.6%, P < .001).
Mental health concerns were cited by 42% of respondents who cited “any type of discrimination” based on age, sex, race or ethnicity, or sexual orientation, the report noted.
Among those reporting any mental health concern, 2.7% considered suicide within the past year and 2.9% considered suicide more than 12 months previously. Women were more likely than men to consider suicide within the past year (3.8% vs. 2.3%) but were also more likely to seek help (42.3% vs. 31.1%; P < .001 for both differences), the authors wrote.
In multivariate analysis, predictors of mental health concerns included emotional harassment, 2.81 (odds ratio, 2.81; 95% confidence interval, 2.46-3.20), any discrimination (OR, 1.85; 95% CI, 1.61-2.12), being divorced (OR, 1.73; 95% CI, 1.26-2.36, age less than 55 years (OR, 1.43; 95% CI, 1.24-1.66), and being mid-career versus late (OR, 1.36; 95% CI, 1.14-1.62).
Because the survey was conducted from September to October 2019, before the pandemic’s traumatic effects unfolded on health care nearly everywhere, “I think there needs to be a follow-up at some point when everything has leveled out,” Dr. Sharma said. The current study is “a baseline, and not a healthy baseline,” for the field’s state of mental health that has likely grown worse during the pandemic.
But even without such a follow-up, the current study “is actionable enough that it forces us to do something about it right now.”
Dr. Sharma, Dr. Mehta, their coauthors, Dr. Sen, and Dr. Sauer reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Bempedoic acid cuts CV risk in the statin-intolerant: CLEAR top-line results
The randomized, placebo-controlled CLEAR Outcomes trial has shown a significant reduction in risk for a composite cardiovascular (CV) endpoint among its patients treated with the lipid-lowering agent bempedoic acid (Nexletol), the drug’s owner, Esperion, announced today.
The trial marks the first time an ATP-citrate lyase inhibitor has shown significant and “clinically meaningful” benefit for patients not adequately managed with standard lipid-modifying agents, Esperion president and CEO Sheldon Koenig said in a press release.
The brief statement provided only top-line results, without P values or other evidence of the magnitude of benefit in the active-therapy group. The company expects to present more complete results “at a key medical conference in the first quarter of 2023.”
CLEAR Outcomes had entered 14,014 patients with a history of or at high risk for CV disease events, elevated low-density lipoprotein cholesterol (LDL-C) levels, and demonstrated intolerance to at least two statins.
They were randomly assigned to bempedoic acid 180 mg once daily or placebo and followed for the primary endpoint of CV death, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization. The trial, conducted in 32 countries, launched in December 2016.
Bempedoic acid is currently approved for adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease on maximally tolerated statins who require additional LDL-C lowering, the company states.
Concomitant use of bempedoic acid with simvastatin or pravastatin, the press release says, may lead to increased statin concentrations and risk for “simvastatin- or pravastatin-related myopathy.” Therefore, “use with greater than 20 mg of simvastatin or 40 mg of pravastatin should be avoided.”
A version of this article first appeared on Medscape.com.
The randomized, placebo-controlled CLEAR Outcomes trial has shown a significant reduction in risk for a composite cardiovascular (CV) endpoint among its patients treated with the lipid-lowering agent bempedoic acid (Nexletol), the drug’s owner, Esperion, announced today.
The trial marks the first time an ATP-citrate lyase inhibitor has shown significant and “clinically meaningful” benefit for patients not adequately managed with standard lipid-modifying agents, Esperion president and CEO Sheldon Koenig said in a press release.
The brief statement provided only top-line results, without P values or other evidence of the magnitude of benefit in the active-therapy group. The company expects to present more complete results “at a key medical conference in the first quarter of 2023.”
CLEAR Outcomes had entered 14,014 patients with a history of or at high risk for CV disease events, elevated low-density lipoprotein cholesterol (LDL-C) levels, and demonstrated intolerance to at least two statins.
They were randomly assigned to bempedoic acid 180 mg once daily or placebo and followed for the primary endpoint of CV death, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization. The trial, conducted in 32 countries, launched in December 2016.
Bempedoic acid is currently approved for adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease on maximally tolerated statins who require additional LDL-C lowering, the company states.
Concomitant use of bempedoic acid with simvastatin or pravastatin, the press release says, may lead to increased statin concentrations and risk for “simvastatin- or pravastatin-related myopathy.” Therefore, “use with greater than 20 mg of simvastatin or 40 mg of pravastatin should be avoided.”
A version of this article first appeared on Medscape.com.
The randomized, placebo-controlled CLEAR Outcomes trial has shown a significant reduction in risk for a composite cardiovascular (CV) endpoint among its patients treated with the lipid-lowering agent bempedoic acid (Nexletol), the drug’s owner, Esperion, announced today.
The trial marks the first time an ATP-citrate lyase inhibitor has shown significant and “clinically meaningful” benefit for patients not adequately managed with standard lipid-modifying agents, Esperion president and CEO Sheldon Koenig said in a press release.
The brief statement provided only top-line results, without P values or other evidence of the magnitude of benefit in the active-therapy group. The company expects to present more complete results “at a key medical conference in the first quarter of 2023.”
CLEAR Outcomes had entered 14,014 patients with a history of or at high risk for CV disease events, elevated low-density lipoprotein cholesterol (LDL-C) levels, and demonstrated intolerance to at least two statins.
They were randomly assigned to bempedoic acid 180 mg once daily or placebo and followed for the primary endpoint of CV death, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization. The trial, conducted in 32 countries, launched in December 2016.
Bempedoic acid is currently approved for adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease on maximally tolerated statins who require additional LDL-C lowering, the company states.
Concomitant use of bempedoic acid with simvastatin or pravastatin, the press release says, may lead to increased statin concentrations and risk for “simvastatin- or pravastatin-related myopathy.” Therefore, “use with greater than 20 mg of simvastatin or 40 mg of pravastatin should be avoided.”
A version of this article first appeared on Medscape.com.
EHR alerts to both doc and patient may boost statin prescribing
Automated alerts to aid clinical decision-making are designed with the best of intentions but can be easy to ignore or overlook. But a randomized trial testing such electronic alerts or “nudges” for promoting statin prescribing may have identified a few design features that help their success, researchers say.
In the trial’s primary finding, for example, reminders displayed to primary care physicians in the electronic health record worked best when the system also reached out to the patient.
Reminders sent only to the clinician also boosted statin prescribing, but not as well, and nudging only the patient didn’t work at all, compared to a nudge-free usual care approach. The patient-only nudges consisted of text messages explaining why a statin prescription may figure in their upcoming appointment.
Nudge trustworthiness
Importantly, the clinician nudges were more than simply reminders to consider a statin prescription, Mitesh S. Patel, MD, MBA, Ascension Health, St. Louis, told this news organization. They also displayed the patient’s atherosclerotic cardiovascular disease (ASCVD) 10-year risk score and explained why a statin may be appropriate. He thinks that information, often left out of such clinical decision support alerts, increases physician trust in them.
In another key feature, Dr. Patel said, the EHR nudges themselves were actionable – that is, they were functional in ways that streamlined the prescribing process. In particular, they include checkbox shortcuts to prescribing statins at appropriate patient-specific dosages, making the entire process “faster and easier,” said Dr. Patel, who is senior author on the study published in JAMA Cardiology with lead author Srinath Adusumalli, MD, University of Pennsylvania, Philadelphia.
The timing may matter as well, he observed. In previous iterations of the study’s EHR nudge system, the nudge would appear “when you open the chart,” he said. “Now, it’s when you go to the orders section, which is when you’re going to be in the mindset of ordering prescriptions and tests.”
Prescription rates were higher with the doctor-patient nudges than with the doctor-only approach, Dr. Patel speculates, largely because the decision process for initiating statins is shared. “The most effective intervention is going to recognize that and try to bring the two groups together.”
Two text messages
The trial, with 158 participating physicians in 28 primary care practices, randomly assigned 4,131 patients to three intervention groups and one control group. Nudges were sent only to the physician, only to the patient, or to both physician and patient; and there was a no-nudge usual-care group.
Patient nudges consisted of two text messages, one 4 days and another 15 minutes before the appointment, announcing that prescription of a statin “to reduce the chance of a heart attack” would be discussed with the physician, the report states.
Statins are grossly underprescribed nationally, it notes, and that was reflected in prescription rates seen during the study’s initial 12-month, no-intervention period of observation. Rates ranged from only 4.7% up to 6% of patients across the four assignment groups.
During the subsequent 6-month intervention period, however, the rates climbed in the doctor-only and doctor-plus-patient nudge groups compared with usual care, by 5.5 (P = .01) and 7.2 (P = .001) absolute percentage points, respectively.
The overall cohort’s mean age was 65.5. About half were male, 29% were Black, 66% were White, and 22.6% already had a cardiovascular disease diagnosis. The analysis was adjusted for calendar month and preintervention statin prescribing rates. Further adjustment for demographics, insurance type, household income, and comorbidities yielded results similar to the primary analysis, the report states.
The results in context
“Although the differences in the combined clinician and patient and clinician-only arms were small, this outcome needs to be interpreted in the context of the population in which the study was performed,” an editorial accompanying the published report states.
For example, “the majority of untreated patients were candidates for primary, not secondary, prevention, making this group of patients particularly challenging for seeing large effect sizes of interventions.”
Moreover, “There was a high baseline prescription rate of statins in the statin-eligible population (approximately 70%) and a high rate of already established patients,” write Faraz S. Ahmad, MD, and Stephen D. Persell, MD, of Northwestern University, Chicago.
Among the approximately 30% of patients who had not previously been prescribed statins, the true target of the nudge interventions, the published trial report states, about 98% were not seeing the physician for the first time.
So “this may not have been the first opportunity to discuss statins,” they write. “It is possible that many of these patients were resistant to statins in the past, which could have created a ceiling effect for prescribing rates.”
Dr. Patel reports owning and receiving personal fees from Catalyst Health and serving on an advisory board for and receiving personal fees from Humana. Dr. Adusumalli reports having been employed by CVS Health. Dr. Ahmad reports receiving consulting fees from Teladoc Livongo and Pfizer. Dr. Persell discloses receiving grants from Omron Healthcare.
A version of this article first appeared on Medscape.com.
Automated alerts to aid clinical decision-making are designed with the best of intentions but can be easy to ignore or overlook. But a randomized trial testing such electronic alerts or “nudges” for promoting statin prescribing may have identified a few design features that help their success, researchers say.
In the trial’s primary finding, for example, reminders displayed to primary care physicians in the electronic health record worked best when the system also reached out to the patient.
Reminders sent only to the clinician also boosted statin prescribing, but not as well, and nudging only the patient didn’t work at all, compared to a nudge-free usual care approach. The patient-only nudges consisted of text messages explaining why a statin prescription may figure in their upcoming appointment.
Nudge trustworthiness
Importantly, the clinician nudges were more than simply reminders to consider a statin prescription, Mitesh S. Patel, MD, MBA, Ascension Health, St. Louis, told this news organization. They also displayed the patient’s atherosclerotic cardiovascular disease (ASCVD) 10-year risk score and explained why a statin may be appropriate. He thinks that information, often left out of such clinical decision support alerts, increases physician trust in them.
In another key feature, Dr. Patel said, the EHR nudges themselves were actionable – that is, they were functional in ways that streamlined the prescribing process. In particular, they include checkbox shortcuts to prescribing statins at appropriate patient-specific dosages, making the entire process “faster and easier,” said Dr. Patel, who is senior author on the study published in JAMA Cardiology with lead author Srinath Adusumalli, MD, University of Pennsylvania, Philadelphia.
The timing may matter as well, he observed. In previous iterations of the study’s EHR nudge system, the nudge would appear “when you open the chart,” he said. “Now, it’s when you go to the orders section, which is when you’re going to be in the mindset of ordering prescriptions and tests.”
Prescription rates were higher with the doctor-patient nudges than with the doctor-only approach, Dr. Patel speculates, largely because the decision process for initiating statins is shared. “The most effective intervention is going to recognize that and try to bring the two groups together.”
Two text messages
The trial, with 158 participating physicians in 28 primary care practices, randomly assigned 4,131 patients to three intervention groups and one control group. Nudges were sent only to the physician, only to the patient, or to both physician and patient; and there was a no-nudge usual-care group.
Patient nudges consisted of two text messages, one 4 days and another 15 minutes before the appointment, announcing that prescription of a statin “to reduce the chance of a heart attack” would be discussed with the physician, the report states.
Statins are grossly underprescribed nationally, it notes, and that was reflected in prescription rates seen during the study’s initial 12-month, no-intervention period of observation. Rates ranged from only 4.7% up to 6% of patients across the four assignment groups.
During the subsequent 6-month intervention period, however, the rates climbed in the doctor-only and doctor-plus-patient nudge groups compared with usual care, by 5.5 (P = .01) and 7.2 (P = .001) absolute percentage points, respectively.
The overall cohort’s mean age was 65.5. About half were male, 29% were Black, 66% were White, and 22.6% already had a cardiovascular disease diagnosis. The analysis was adjusted for calendar month and preintervention statin prescribing rates. Further adjustment for demographics, insurance type, household income, and comorbidities yielded results similar to the primary analysis, the report states.
The results in context
“Although the differences in the combined clinician and patient and clinician-only arms were small, this outcome needs to be interpreted in the context of the population in which the study was performed,” an editorial accompanying the published report states.
For example, “the majority of untreated patients were candidates for primary, not secondary, prevention, making this group of patients particularly challenging for seeing large effect sizes of interventions.”
Moreover, “There was a high baseline prescription rate of statins in the statin-eligible population (approximately 70%) and a high rate of already established patients,” write Faraz S. Ahmad, MD, and Stephen D. Persell, MD, of Northwestern University, Chicago.
Among the approximately 30% of patients who had not previously been prescribed statins, the true target of the nudge interventions, the published trial report states, about 98% were not seeing the physician for the first time.
So “this may not have been the first opportunity to discuss statins,” they write. “It is possible that many of these patients were resistant to statins in the past, which could have created a ceiling effect for prescribing rates.”
Dr. Patel reports owning and receiving personal fees from Catalyst Health and serving on an advisory board for and receiving personal fees from Humana. Dr. Adusumalli reports having been employed by CVS Health. Dr. Ahmad reports receiving consulting fees from Teladoc Livongo and Pfizer. Dr. Persell discloses receiving grants from Omron Healthcare.
A version of this article first appeared on Medscape.com.
Automated alerts to aid clinical decision-making are designed with the best of intentions but can be easy to ignore or overlook. But a randomized trial testing such electronic alerts or “nudges” for promoting statin prescribing may have identified a few design features that help their success, researchers say.
In the trial’s primary finding, for example, reminders displayed to primary care physicians in the electronic health record worked best when the system also reached out to the patient.
Reminders sent only to the clinician also boosted statin prescribing, but not as well, and nudging only the patient didn’t work at all, compared to a nudge-free usual care approach. The patient-only nudges consisted of text messages explaining why a statin prescription may figure in their upcoming appointment.
Nudge trustworthiness
Importantly, the clinician nudges were more than simply reminders to consider a statin prescription, Mitesh S. Patel, MD, MBA, Ascension Health, St. Louis, told this news organization. They also displayed the patient’s atherosclerotic cardiovascular disease (ASCVD) 10-year risk score and explained why a statin may be appropriate. He thinks that information, often left out of such clinical decision support alerts, increases physician trust in them.
In another key feature, Dr. Patel said, the EHR nudges themselves were actionable – that is, they were functional in ways that streamlined the prescribing process. In particular, they include checkbox shortcuts to prescribing statins at appropriate patient-specific dosages, making the entire process “faster and easier,” said Dr. Patel, who is senior author on the study published in JAMA Cardiology with lead author Srinath Adusumalli, MD, University of Pennsylvania, Philadelphia.
The timing may matter as well, he observed. In previous iterations of the study’s EHR nudge system, the nudge would appear “when you open the chart,” he said. “Now, it’s when you go to the orders section, which is when you’re going to be in the mindset of ordering prescriptions and tests.”
Prescription rates were higher with the doctor-patient nudges than with the doctor-only approach, Dr. Patel speculates, largely because the decision process for initiating statins is shared. “The most effective intervention is going to recognize that and try to bring the two groups together.”
Two text messages
The trial, with 158 participating physicians in 28 primary care practices, randomly assigned 4,131 patients to three intervention groups and one control group. Nudges were sent only to the physician, only to the patient, or to both physician and patient; and there was a no-nudge usual-care group.
Patient nudges consisted of two text messages, one 4 days and another 15 minutes before the appointment, announcing that prescription of a statin “to reduce the chance of a heart attack” would be discussed with the physician, the report states.
Statins are grossly underprescribed nationally, it notes, and that was reflected in prescription rates seen during the study’s initial 12-month, no-intervention period of observation. Rates ranged from only 4.7% up to 6% of patients across the four assignment groups.
During the subsequent 6-month intervention period, however, the rates climbed in the doctor-only and doctor-plus-patient nudge groups compared with usual care, by 5.5 (P = .01) and 7.2 (P = .001) absolute percentage points, respectively.
The overall cohort’s mean age was 65.5. About half were male, 29% were Black, 66% were White, and 22.6% already had a cardiovascular disease diagnosis. The analysis was adjusted for calendar month and preintervention statin prescribing rates. Further adjustment for demographics, insurance type, household income, and comorbidities yielded results similar to the primary analysis, the report states.
The results in context
“Although the differences in the combined clinician and patient and clinician-only arms were small, this outcome needs to be interpreted in the context of the population in which the study was performed,” an editorial accompanying the published report states.
For example, “the majority of untreated patients were candidates for primary, not secondary, prevention, making this group of patients particularly challenging for seeing large effect sizes of interventions.”
Moreover, “There was a high baseline prescription rate of statins in the statin-eligible population (approximately 70%) and a high rate of already established patients,” write Faraz S. Ahmad, MD, and Stephen D. Persell, MD, of Northwestern University, Chicago.
Among the approximately 30% of patients who had not previously been prescribed statins, the true target of the nudge interventions, the published trial report states, about 98% were not seeing the physician for the first time.
So “this may not have been the first opportunity to discuss statins,” they write. “It is possible that many of these patients were resistant to statins in the past, which could have created a ceiling effect for prescribing rates.”
Dr. Patel reports owning and receiving personal fees from Catalyst Health and serving on an advisory board for and receiving personal fees from Humana. Dr. Adusumalli reports having been employed by CVS Health. Dr. Ahmad reports receiving consulting fees from Teladoc Livongo and Pfizer. Dr. Persell discloses receiving grants from Omron Healthcare.
A version of this article first appeared on Medscape.com.
FDA tweaks Impella indications on basis of postapproval study
The U.S. Food and Drug Administration has updated the Abiomed Impella RP System’s approved indications in a way that “better reflects the characteristics of the patients who may benefit the most from treatment with the device,” the agency has announced.
The revised language reflects the final results of a postapproval study in which survival rates for patients who met the premarket-study entry criteria were comparable to rates seen in the premarket studies, the FDA observed.
The postapproval study “further confirms that the device is safe and effective when used for the currently approved indication.” The indication’s added words, however, tighten the description of eligible patients in a way that more precisely reflects the premarket-study population.
The update states that the Impella RP System is “indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥ 1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury.”
The FDA “believes that when the device is used for the currently approved indication in appropriately selected patients, the benefits of the Impella RP System continue to outweigh the risks.”
The reworded indication is the latest among several updates to the agency’s February 2019 letter to clinicians noting a signal of increased mortality associated with the Impella RP device in an interim analysis of the same postapproval study. Ultimately, no such signal has emerged among the subset of postapproval patients who would have been eligible for the premarket study.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has updated the Abiomed Impella RP System’s approved indications in a way that “better reflects the characteristics of the patients who may benefit the most from treatment with the device,” the agency has announced.
The revised language reflects the final results of a postapproval study in which survival rates for patients who met the premarket-study entry criteria were comparable to rates seen in the premarket studies, the FDA observed.
The postapproval study “further confirms that the device is safe and effective when used for the currently approved indication.” The indication’s added words, however, tighten the description of eligible patients in a way that more precisely reflects the premarket-study population.
The update states that the Impella RP System is “indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥ 1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury.”
The FDA “believes that when the device is used for the currently approved indication in appropriately selected patients, the benefits of the Impella RP System continue to outweigh the risks.”
The reworded indication is the latest among several updates to the agency’s February 2019 letter to clinicians noting a signal of increased mortality associated with the Impella RP device in an interim analysis of the same postapproval study. Ultimately, no such signal has emerged among the subset of postapproval patients who would have been eligible for the premarket study.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has updated the Abiomed Impella RP System’s approved indications in a way that “better reflects the characteristics of the patients who may benefit the most from treatment with the device,” the agency has announced.
The revised language reflects the final results of a postapproval study in which survival rates for patients who met the premarket-study entry criteria were comparable to rates seen in the premarket studies, the FDA observed.
The postapproval study “further confirms that the device is safe and effective when used for the currently approved indication.” The indication’s added words, however, tighten the description of eligible patients in a way that more precisely reflects the premarket-study population.
The update states that the Impella RP System is “indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥ 1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury.”
The FDA “believes that when the device is used for the currently approved indication in appropriately selected patients, the benefits of the Impella RP System continue to outweigh the risks.”
The reworded indication is the latest among several updates to the agency’s February 2019 letter to clinicians noting a signal of increased mortality associated with the Impella RP device in an interim analysis of the same postapproval study. Ultimately, no such signal has emerged among the subset of postapproval patients who would have been eligible for the premarket study.
A version of this article first appeared on Medscape.com.
FDA expands list of Getinge IABP system and component shortages
The U.S. Food and Drug Administration issued a letter to health care providers describing a current shortage of Getinge intra-aortic balloon pump (IABP) catheters and other components.
Earlier, the agency announced shortages of the company’s Maquet/Datascope IAB catheters, new Cardiosave IABP devices, and Cardiosave IABP parts. The new notification adds Getinge Maquet/Datascope IABP systems to the list.
The company’s letter explains that “ongoing supply chain issues have significantly impacted our ability to build intra-aortic balloon pumps, intra-aortic balloon catheters, and spare parts due to raw material shortages.”
It also offers guidance on maintaining Cardiosave Safety Disks and lithium-ion batteries in the face of the shortages. “In the event that you need a replacement pump while your IABP is undergoing service, please contact your local sales representative who may be able to assist with a temporary IABP.”
Providers are instructed to inform the company through its sales representatives “if you have any underutilized Maquet/Datascope IAB catheters or IABPs and are willing to share them with hospitals in need.”
The shortages are expected to continue into 2023, the FDA states in its letter.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration issued a letter to health care providers describing a current shortage of Getinge intra-aortic balloon pump (IABP) catheters and other components.
Earlier, the agency announced shortages of the company’s Maquet/Datascope IAB catheters, new Cardiosave IABP devices, and Cardiosave IABP parts. The new notification adds Getinge Maquet/Datascope IABP systems to the list.
The company’s letter explains that “ongoing supply chain issues have significantly impacted our ability to build intra-aortic balloon pumps, intra-aortic balloon catheters, and spare parts due to raw material shortages.”
It also offers guidance on maintaining Cardiosave Safety Disks and lithium-ion batteries in the face of the shortages. “In the event that you need a replacement pump while your IABP is undergoing service, please contact your local sales representative who may be able to assist with a temporary IABP.”
Providers are instructed to inform the company through its sales representatives “if you have any underutilized Maquet/Datascope IAB catheters or IABPs and are willing to share them with hospitals in need.”
The shortages are expected to continue into 2023, the FDA states in its letter.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration issued a letter to health care providers describing a current shortage of Getinge intra-aortic balloon pump (IABP) catheters and other components.
Earlier, the agency announced shortages of the company’s Maquet/Datascope IAB catheters, new Cardiosave IABP devices, and Cardiosave IABP parts. The new notification adds Getinge Maquet/Datascope IABP systems to the list.
The company’s letter explains that “ongoing supply chain issues have significantly impacted our ability to build intra-aortic balloon pumps, intra-aortic balloon catheters, and spare parts due to raw material shortages.”
It also offers guidance on maintaining Cardiosave Safety Disks and lithium-ion batteries in the face of the shortages. “In the event that you need a replacement pump while your IABP is undergoing service, please contact your local sales representative who may be able to assist with a temporary IABP.”
Providers are instructed to inform the company through its sales representatives “if you have any underutilized Maquet/Datascope IAB catheters or IABPs and are willing to share them with hospitals in need.”
The shortages are expected to continue into 2023, the FDA states in its letter.
A version of this article first appeared on Medscape.com.
IRONMAN galvanizes case for IV iron repletion in heart failure
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.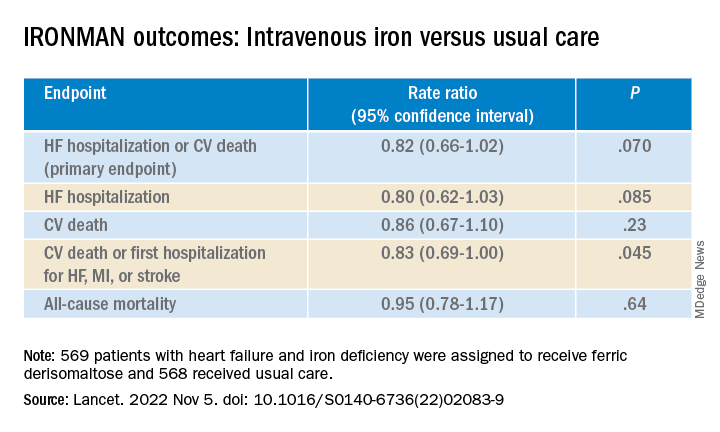
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Be aware, mindfulness training can lower systolic BP: MB-BP
CHICAGO – It’s been said that one can observe a lot just by watching. Turning such observation inward, new evidence suggests, might lead to blood pressure (BP) reductions that approach what’s possible from an antihypertensive agent.
Systolic BP fell over 6 months by almost 6 mm Hg, on average, in people with elevated BP who participated in an 8-week mindful awareness program as part of a randomized trial that included a usual-care control group.
The program taught established mindfulness-training techniques aimed at modifying behaviors regarding diet, exercise, and other controllable influences on the success of antihypertensive therapy.
Participants in the program, called Mindfulness-Based Blood Pressure Reduction (MB-BP), also the name of the single-center study, “showed potentially clinically relevant reductions in systolic blood pressure,” said principal investigator Eric B. Loucks, PhD, Brown University, Providence, R.I.
The phase 2 trial has some limitations, he observed, including on generalizability. For example, it entered about 200 mostly White, college-educated adults from one metropolitan area.
But if these findings are replicated in further studies, “preferably by other research groups, in a larger and broader population, and with longer follow-up,” Dr. Loucks said, the MB-BP intervention could become “an appealing approach to help control blood pressure.”
Dr. Loucks made the comments at a press conference prior to his formal presentation of MB-BP Nov. 6 at American Heart Association (AHA) Scientific Sessions 2022, held in Chicago and virtually.
Mindfulness-based interventions for elevated BP have not been widely studied, “so this is exactly what we need: a well-done trial with a control group to show that it actually works,” Amit Khera, MD, not connected with MB-BP, told this news organization.
The trial is “really important for proof of concept, but it had only 200 people. You need a larger one, and you need longer-term data,” agreed Dr. Khera, who directs the preventive cardiology program at the University of Texas Southwestern Medical Center, in Dallas. “Six months is good, but we want to see if it’s durable.”
Rhian M. Touyz, MBBCh, also not part of MB-BP, agreed that the nearly 6 mm Hg mean systolic BP reduction among program participants is clinically relevant. “I think in the context of global risk and reduction of target organ damage and cardiovascular events, it is significant in terms of events at a population level,” Dr. Touyz, McGill University Health Centre, Montreal, told this news organization.
Many patients on antihypertensive therapy that’s falling short resist the addition of another such agent, she observed, and instead might show further BP reduction from mindfulness training. The intervention probably also “would benefit health in general.” Mindfulness-based approaches could therefore be useful additions to treatment protocols for elevated BP, Dr. Touyz said.
How the training works
The MB-BP program used validated mindfulness-based stress-management techniques, adapted to address elevated BP, that included “personalized feedback and education about hypertension risk factors, mindful awareness training of participants’ relationships with hypertension risk factors, and support for behavior change,” Dr. Loucks and colleagues reported.
Participants were trained in mindfulness skills that included “self-awareness and emotion regulation,” Dr. Loucks said, which they then could apply to their “relationships with the things that we know influence blood pressure, like physical activity, diet, antihypertensive medication adherence, or alcohol consumption.”
One goal is to promote greater “attention control,” he said, “so that there’s some self-awareness that arises in terms of how we feel the next day, after a lot of alcohol consumption, for example, or lack of physical activity.” The process can provide insights that inspire patients to modify behaviors and risk factors that elevate BP, Dr. Loucks explained.
Effects on medication use
Systolic BP responses led some program participants to be managed on fewer or reduced dosages of antihypertensive meds, he told this news organization. Physicians seen outside of the trial could adjust their prescriptions, intensifying or pulling back on meds depending on their assessments of the patient. Any prescription changes would be documented by the researchers at the patient’s next class or trial-clinic visit.
The group that did the training, Dr. Loucks said, was 33% less likely to increase and 30% more likely to decrease their use of BP-lowering medications compared with the control group.
Elevated BP is so common and undertreated that “there is a need for every possible level of intervention, starting from the population level to the individual and everything else in between,” nephrologist Janani Rangaswami, MD, George Washington University, Washington, said at the press conference.
Therefore, “this mindfulness-based approach, in addition to standard of care with pharmacotherapy, is a really welcome addition to the hypertension literature,” said Dr. Rangaswami, who directs her center’s cardiorenal program. The systolic BP reduction seen in the intervention group, she agreed, was “clinically important and meaningful.”
Blinded assessments
The trial entered 201 patients with systolic and diastolic BP greater than 120 mm Hg and 80 mm Hg, respectively; 58.7% were women, 81% were White, and 73% were college-educated, Dr. Loucks reported.
The 100 assigned to the “enhanced usual care” control group received educational materials on controlling high BP. They and the 101 who followed the mindfulness-based program were given and trained on a home BP-monitoring device. They were then followed for the primary endpoint of change in systolic BP at 6 months.
Data management and outcomes assessments were conducted by trialists not involved in the training intervention who were blinded to randomization assignment.
In a prespecified unadjusted analysis by intention-to-treat, systolic BP in the intervention group dropped by a mean of 5.9 mm Hg (P < .001) compared with baseline and 4.5 mm Hg (P = .045), compared with the control group.
A post hoc analysis adjusted for sex and baseline BP showed an average 4.3 mm Hg reduction (P = .056) in those following the MB-BP program, compared with controls.
There were no observed significant effects on diastolic BP.
The study offered clues to how engagement in the MB-BP program might promote reductions in systolic BP, Dr. Loucks observed. For example, it may have led to increased activity levels, reduced sodium intake, and other dietary improvements.
Indeed, program participants averaged about 351 minutes less sedentary time (P = .02) and showed a 0.32-point improvement in Dietary Approaches to Stop Hypertension scores (P = .08), compared with the control group, Dr. Loucks reported. Other modifiable risk factors for elevated BP that could have responded to the mindfulness-based training, he proposed, include obesity, alcohol intake, and reaction to stress.
Dr. Loucks reports that he developed the MB-BP training and was a program instructor but did not receive related financial compensation; he had no other disclosures. Dr. Khera, Dr. Touyz, and Dr. Rangaswami had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – It’s been said that one can observe a lot just by watching. Turning such observation inward, new evidence suggests, might lead to blood pressure (BP) reductions that approach what’s possible from an antihypertensive agent.
Systolic BP fell over 6 months by almost 6 mm Hg, on average, in people with elevated BP who participated in an 8-week mindful awareness program as part of a randomized trial that included a usual-care control group.
The program taught established mindfulness-training techniques aimed at modifying behaviors regarding diet, exercise, and other controllable influences on the success of antihypertensive therapy.
Participants in the program, called Mindfulness-Based Blood Pressure Reduction (MB-BP), also the name of the single-center study, “showed potentially clinically relevant reductions in systolic blood pressure,” said principal investigator Eric B. Loucks, PhD, Brown University, Providence, R.I.
The phase 2 trial has some limitations, he observed, including on generalizability. For example, it entered about 200 mostly White, college-educated adults from one metropolitan area.
But if these findings are replicated in further studies, “preferably by other research groups, in a larger and broader population, and with longer follow-up,” Dr. Loucks said, the MB-BP intervention could become “an appealing approach to help control blood pressure.”
Dr. Loucks made the comments at a press conference prior to his formal presentation of MB-BP Nov. 6 at American Heart Association (AHA) Scientific Sessions 2022, held in Chicago and virtually.
Mindfulness-based interventions for elevated BP have not been widely studied, “so this is exactly what we need: a well-done trial with a control group to show that it actually works,” Amit Khera, MD, not connected with MB-BP, told this news organization.
The trial is “really important for proof of concept, but it had only 200 people. You need a larger one, and you need longer-term data,” agreed Dr. Khera, who directs the preventive cardiology program at the University of Texas Southwestern Medical Center, in Dallas. “Six months is good, but we want to see if it’s durable.”
Rhian M. Touyz, MBBCh, also not part of MB-BP, agreed that the nearly 6 mm Hg mean systolic BP reduction among program participants is clinically relevant. “I think in the context of global risk and reduction of target organ damage and cardiovascular events, it is significant in terms of events at a population level,” Dr. Touyz, McGill University Health Centre, Montreal, told this news organization.
Many patients on antihypertensive therapy that’s falling short resist the addition of another such agent, she observed, and instead might show further BP reduction from mindfulness training. The intervention probably also “would benefit health in general.” Mindfulness-based approaches could therefore be useful additions to treatment protocols for elevated BP, Dr. Touyz said.
How the training works
The MB-BP program used validated mindfulness-based stress-management techniques, adapted to address elevated BP, that included “personalized feedback and education about hypertension risk factors, mindful awareness training of participants’ relationships with hypertension risk factors, and support for behavior change,” Dr. Loucks and colleagues reported.
Participants were trained in mindfulness skills that included “self-awareness and emotion regulation,” Dr. Loucks said, which they then could apply to their “relationships with the things that we know influence blood pressure, like physical activity, diet, antihypertensive medication adherence, or alcohol consumption.”
One goal is to promote greater “attention control,” he said, “so that there’s some self-awareness that arises in terms of how we feel the next day, after a lot of alcohol consumption, for example, or lack of physical activity.” The process can provide insights that inspire patients to modify behaviors and risk factors that elevate BP, Dr. Loucks explained.
Effects on medication use
Systolic BP responses led some program participants to be managed on fewer or reduced dosages of antihypertensive meds, he told this news organization. Physicians seen outside of the trial could adjust their prescriptions, intensifying or pulling back on meds depending on their assessments of the patient. Any prescription changes would be documented by the researchers at the patient’s next class or trial-clinic visit.
The group that did the training, Dr. Loucks said, was 33% less likely to increase and 30% more likely to decrease their use of BP-lowering medications compared with the control group.
Elevated BP is so common and undertreated that “there is a need for every possible level of intervention, starting from the population level to the individual and everything else in between,” nephrologist Janani Rangaswami, MD, George Washington University, Washington, said at the press conference.
Therefore, “this mindfulness-based approach, in addition to standard of care with pharmacotherapy, is a really welcome addition to the hypertension literature,” said Dr. Rangaswami, who directs her center’s cardiorenal program. The systolic BP reduction seen in the intervention group, she agreed, was “clinically important and meaningful.”
Blinded assessments
The trial entered 201 patients with systolic and diastolic BP greater than 120 mm Hg and 80 mm Hg, respectively; 58.7% were women, 81% were White, and 73% were college-educated, Dr. Loucks reported.
The 100 assigned to the “enhanced usual care” control group received educational materials on controlling high BP. They and the 101 who followed the mindfulness-based program were given and trained on a home BP-monitoring device. They were then followed for the primary endpoint of change in systolic BP at 6 months.
Data management and outcomes assessments were conducted by trialists not involved in the training intervention who were blinded to randomization assignment.
In a prespecified unadjusted analysis by intention-to-treat, systolic BP in the intervention group dropped by a mean of 5.9 mm Hg (P < .001) compared with baseline and 4.5 mm Hg (P = .045), compared with the control group.
A post hoc analysis adjusted for sex and baseline BP showed an average 4.3 mm Hg reduction (P = .056) in those following the MB-BP program, compared with controls.
There were no observed significant effects on diastolic BP.
The study offered clues to how engagement in the MB-BP program might promote reductions in systolic BP, Dr. Loucks observed. For example, it may have led to increased activity levels, reduced sodium intake, and other dietary improvements.
Indeed, program participants averaged about 351 minutes less sedentary time (P = .02) and showed a 0.32-point improvement in Dietary Approaches to Stop Hypertension scores (P = .08), compared with the control group, Dr. Loucks reported. Other modifiable risk factors for elevated BP that could have responded to the mindfulness-based training, he proposed, include obesity, alcohol intake, and reaction to stress.
Dr. Loucks reports that he developed the MB-BP training and was a program instructor but did not receive related financial compensation; he had no other disclosures. Dr. Khera, Dr. Touyz, and Dr. Rangaswami had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – It’s been said that one can observe a lot just by watching. Turning such observation inward, new evidence suggests, might lead to blood pressure (BP) reductions that approach what’s possible from an antihypertensive agent.
Systolic BP fell over 6 months by almost 6 mm Hg, on average, in people with elevated BP who participated in an 8-week mindful awareness program as part of a randomized trial that included a usual-care control group.
The program taught established mindfulness-training techniques aimed at modifying behaviors regarding diet, exercise, and other controllable influences on the success of antihypertensive therapy.
Participants in the program, called Mindfulness-Based Blood Pressure Reduction (MB-BP), also the name of the single-center study, “showed potentially clinically relevant reductions in systolic blood pressure,” said principal investigator Eric B. Loucks, PhD, Brown University, Providence, R.I.
The phase 2 trial has some limitations, he observed, including on generalizability. For example, it entered about 200 mostly White, college-educated adults from one metropolitan area.
But if these findings are replicated in further studies, “preferably by other research groups, in a larger and broader population, and with longer follow-up,” Dr. Loucks said, the MB-BP intervention could become “an appealing approach to help control blood pressure.”
Dr. Loucks made the comments at a press conference prior to his formal presentation of MB-BP Nov. 6 at American Heart Association (AHA) Scientific Sessions 2022, held in Chicago and virtually.
Mindfulness-based interventions for elevated BP have not been widely studied, “so this is exactly what we need: a well-done trial with a control group to show that it actually works,” Amit Khera, MD, not connected with MB-BP, told this news organization.
The trial is “really important for proof of concept, but it had only 200 people. You need a larger one, and you need longer-term data,” agreed Dr. Khera, who directs the preventive cardiology program at the University of Texas Southwestern Medical Center, in Dallas. “Six months is good, but we want to see if it’s durable.”
Rhian M. Touyz, MBBCh, also not part of MB-BP, agreed that the nearly 6 mm Hg mean systolic BP reduction among program participants is clinically relevant. “I think in the context of global risk and reduction of target organ damage and cardiovascular events, it is significant in terms of events at a population level,” Dr. Touyz, McGill University Health Centre, Montreal, told this news organization.
Many patients on antihypertensive therapy that’s falling short resist the addition of another such agent, she observed, and instead might show further BP reduction from mindfulness training. The intervention probably also “would benefit health in general.” Mindfulness-based approaches could therefore be useful additions to treatment protocols for elevated BP, Dr. Touyz said.
How the training works
The MB-BP program used validated mindfulness-based stress-management techniques, adapted to address elevated BP, that included “personalized feedback and education about hypertension risk factors, mindful awareness training of participants’ relationships with hypertension risk factors, and support for behavior change,” Dr. Loucks and colleagues reported.
Participants were trained in mindfulness skills that included “self-awareness and emotion regulation,” Dr. Loucks said, which they then could apply to their “relationships with the things that we know influence blood pressure, like physical activity, diet, antihypertensive medication adherence, or alcohol consumption.”
One goal is to promote greater “attention control,” he said, “so that there’s some self-awareness that arises in terms of how we feel the next day, after a lot of alcohol consumption, for example, or lack of physical activity.” The process can provide insights that inspire patients to modify behaviors and risk factors that elevate BP, Dr. Loucks explained.
Effects on medication use
Systolic BP responses led some program participants to be managed on fewer or reduced dosages of antihypertensive meds, he told this news organization. Physicians seen outside of the trial could adjust their prescriptions, intensifying or pulling back on meds depending on their assessments of the patient. Any prescription changes would be documented by the researchers at the patient’s next class or trial-clinic visit.
The group that did the training, Dr. Loucks said, was 33% less likely to increase and 30% more likely to decrease their use of BP-lowering medications compared with the control group.
Elevated BP is so common and undertreated that “there is a need for every possible level of intervention, starting from the population level to the individual and everything else in between,” nephrologist Janani Rangaswami, MD, George Washington University, Washington, said at the press conference.
Therefore, “this mindfulness-based approach, in addition to standard of care with pharmacotherapy, is a really welcome addition to the hypertension literature,” said Dr. Rangaswami, who directs her center’s cardiorenal program. The systolic BP reduction seen in the intervention group, she agreed, was “clinically important and meaningful.”
Blinded assessments
The trial entered 201 patients with systolic and diastolic BP greater than 120 mm Hg and 80 mm Hg, respectively; 58.7% were women, 81% were White, and 73% were college-educated, Dr. Loucks reported.
The 100 assigned to the “enhanced usual care” control group received educational materials on controlling high BP. They and the 101 who followed the mindfulness-based program were given and trained on a home BP-monitoring device. They were then followed for the primary endpoint of change in systolic BP at 6 months.
Data management and outcomes assessments were conducted by trialists not involved in the training intervention who were blinded to randomization assignment.
In a prespecified unadjusted analysis by intention-to-treat, systolic BP in the intervention group dropped by a mean of 5.9 mm Hg (P < .001) compared with baseline and 4.5 mm Hg (P = .045), compared with the control group.
A post hoc analysis adjusted for sex and baseline BP showed an average 4.3 mm Hg reduction (P = .056) in those following the MB-BP program, compared with controls.
There were no observed significant effects on diastolic BP.
The study offered clues to how engagement in the MB-BP program might promote reductions in systolic BP, Dr. Loucks observed. For example, it may have led to increased activity levels, reduced sodium intake, and other dietary improvements.
Indeed, program participants averaged about 351 minutes less sedentary time (P = .02) and showed a 0.32-point improvement in Dietary Approaches to Stop Hypertension scores (P = .08), compared with the control group, Dr. Loucks reported. Other modifiable risk factors for elevated BP that could have responded to the mindfulness-based training, he proposed, include obesity, alcohol intake, and reaction to stress.
Dr. Loucks reports that he developed the MB-BP training and was a program instructor but did not receive related financial compensation; he had no other disclosures. Dr. Khera, Dr. Touyz, and Dr. Rangaswami had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Optimize HF meds rapidly and fully after hospital discharge: STRONG-HF
CHICAGO – Clinicians who prescribe heart failure meds are holding the best hand they’ve ever had, but with so much underuse and suboptimal dosing in actual practice, it seems many may not appreciate the value of their cards. But a major randomized trial that has captured the field’s attention may embolden them to go all in.
Results showed that a strategy of early, rapid up-titration of multiple guideline-directed meds in patients hospitalized with heart failure, compared with a usual-care approach, cut their 6-month risk for death or HF readmission by a steep 34% (P = .002).
The drugs had been started and partly up-titrated in the hospital with the goal of full up-titration within 2 weeks after discharge.
Patients well tolerated the high-intensity approach, researchers said. Their quality-of-life scores improved (P < .0001) compared with the usual-care group, and adverse events were considered few and manageable in the international trial with more than 1,000 patients.
Safety on the high-intensity strategy depended on close patient monitoring at frequently planned clinic visits along with guidance for the up-titrations from clinical signs and natriuretic peptide levels, observed Alexandre Mebazaa, MD, PhD, University of Paris and Public Hospitals of Paris.
Dr. Mebazaa is principal investigator on the trial, called STRONG-HF, which he presented at the American Heart Association scientific sessions, held in Chicago and virtually. He is also lead author on the study’s same-day publication in the Lancet.
The high-intensity strategy’s superiority emerged early in the trial, which was halted early on the data safety monitoring board’s recommendation, with about 90% of follow-ups completed. The board “felt it was unethical to keep patients in usual care,” Dr. Mebazaa said at a press conference.
A dramatic change
The next step, he said, will be to educate the heart failure community on the high-intensity care technique so it can swiftly enter clinical practice. Currently in acute heart failure, “very few patients are monitored after discharge and treated with full doses of heart failure therapies.”
Adoption of the strategy “would be a dramatic change from what’s currently being done,” said Martin B. Leon, MD, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, who moderated the press conference.
Only an estimated 5% of patients with HF in the United States receive full guideline-directed medical therapy, Dr. Leon said, “so the generalizability of this strategy, with careful follow-up that has safety involved in it, is absolutely crucial.”
But the potential impact of this high-intensity approach on resource use is unknown, raising questions about how widely and consistently it could be implemented, said Dr. Leon, who is not connected with STRONG-HF.
The trial called for in-hospital initiation of the three distinct drug classes that, at the time, were the core of guideline-directed HF therapy, with up-titration to 50% of recommended dosage by hospital discharge, and then to 100% within 2 weeks later.
The meds included a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system inhibitor (RASI). The latter could be an ACE inhibitor, angiotensin-receptor blocker (ARB), or angiotensin receptor-neprilysin inhibitor (ARNI).
How about a fourth drug?
Conspicuously absent from the list, for contemporary practice, was an SGLT2 inhibitor, a class that entered the HF guidelines well after STRONG-HF was designed. They would undoubtedly join the other three agents were the high-intensity strategy to enter practice, potentially changing its complexity and safety profile.
But Dr. Mebazaa and other experts don’t see that as a big challenge and would expect a smooth transition to a high-intensity approach that also includes the SGLT2 inhibitors.
STRONG-HF was necessary in part because many clinicians have been “reluctant” to take full advantage of three agents that had been the basis of guideline-directed therapy, he told this news organization.
That reluctance stemmed from concerns that beta-blockers might worsen the heart failure, ACE inhibitors could hurt the kidneys, or MRAs might cause hyperkalemia, Dr. Mebazaa said. The STRONG-HF high-intensity regimen, therefore, demanded multiple clinic visits for close follow-up.
But the SGLT2 inhibitors “are known to be rather safe drugs, at least much safer than the three others,” he said. So, it seems unlikely that their addition to a beta-blocker, RASI, and MRA in patients with HF would worsen the risk of adverse events.
John G.F. Cleland, MD, PhD, agrees. With addition of the fourth agent, “You may need to be a little bit more careful with renal function, just in that first couple of weeks,” he told this news organization. “But I think it would be easy to add an SGLT2 inhibitor into this regimen. And in general, there’s no titration with an SGLT2 inhibitor, so they’ll all be on full dose predischarge.”
Given the drugs’ diuretic-like action, moreover, some patients might be able to pull back on their loop diuretics, speculated Dr. Cleland, from the University of Glasgow’s School of Health and Wellbeing.
The prospect of a high-intensity strategy’s wide implementation in practice presents both “challenges and opportunities,” Amanda R. Vest, MBBS, MPH, Tufts University, Boston, told this news organization.
“There may be additional challenges in terms of ensuring we avoid hypotension or acute kidney injury in the up-titration phase,” said Dr. Vest, who is medical director of her center’s cardiac transplantation program but not connected with STRONG-HF.
“But it also gives us opportunities,” she added, “because there are some patients, especially in that vulnerable postdischarge phase, who are actually much more able to tolerate introduction of an SGLT2 inhibitor than, for example, an ACE inhibitor, ARB, or ARNI – or maybe a beta-blocker if they’ve been in a low cardiac-output state.” Effective dosing would depend on “the personalization and skill of the clinician in optimizing the medications in their correct sequence,” Dr. Vest said.
“It’s challenging to think that we would ever get to 100% up-titration,” she added, “and even in this excellent study, they didn’t get to 100%.” But as clinicians gain experience with the high-intensity strategy, especially as the SGLT2 inhibitors are included, “I think we can reasonably expect more progress to be made in these up-titration skills.”
No restrictions on LVEF
The researchers entered 1,078 patients hospitalized with acute HF in 14 countries across Africa, Europe, the Middle East, and South America, and randomly assigned them to the high-intensity management strategy or usual care.
About 60% of the patients were male and 77% were White. There were no entry restrictions based on left ventricular ejection fraction (LVEF), which exceeded 40% in almost a third of cases.
In the high-intensity care group’s 542 patients, the three agents were up-titrated to 50% of the maximum guideline-recommended dosage prior to hospital discharge, and to 100% within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were monitored closely at four planned clinical visits over the following 6 weeks.
The 536 patients assigned to usual care were discharged and managed according to local standards, with their meds handled by their own primary care doctors or cardiologists, the published report notes. They were reevaluated by STRONG-HF clinicians 90 days after discharge.
The number of clinic visits in the first 90 postdischarge days averaged 4.8 in the high-intensity care group and 1.0 for those receiving usual care. Full up-titration was far more likely in the high-intensity care group: 55% vs. 2% for RASI agents, 49% vs. 4% for beta-blockers, and 84% vs. 46% for MRAs.
They also fared significantly better on all measured parameters associated with decongestion, including weight, prevalence of peripheral edema, jugular venous pressure, NYHA functional class, and natriuretic peptide levels, the researchers said.
The primary endpoint of 180-day death from any cause or HF readmission was met by 15.2% of the high-intensity care group and 23.3% of usual-care patients, for an adjusted risk ratio (RR) of 0.66 (95% CI, 0.50-0.86; P = .0021).
Subgroup analyses saw no significant interactions by age, sex, race, geography, or baseline blood pressure, renal function, or LVEF. Patients with higher vs. lower baseline natriuretic peptide levels trend toward better responses to high-intensity care (P = .08)
The COVID effect
The group performed a sensitivity analysis that excluded deaths attributed to COVID-19 in STRONG-HF, which launched prior to the pandemic. The high-intensity strategy’s benefit for the primary endpoint grew, with an adjusted RR of 0.61 (95% CI, 0.46-0.82; P = .0005). There was no corresponding effect on death from any cause (P = .15).
Treatment-related adverse effects in the overall trial were seen in 41.1% of the high-intensity care group and in 29.5% of those assigned to usual care.
The higher rate in the high-intensity care arm “may be related to their higher number of [clinic] visits compared to usual care,” Dr. Mebazaa said. “However, serious adverse events and fatal adverse events were similar in both arms.”
Cardiac failure was the most common adverse event, developing in about 15% in both groups. It was followed by hypotension, hyperkalemia, and renal impairment, according to the published report.
Dr. Cleland cautioned that the risk of adverse events would potentially be higher should the high-intensity strategy become common clinical practice. The median age in STRONG-HF was 63, which is “10-15 years younger, on average, than the population with recently admitted heart failure that we see. There’s no doubt that older people have more multimorbidity.”
STRONG-HF was funded by Roche Diagnostics. Dr. Mebazaa discloses receiving grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and Merck, Sharp & Dohme; and consulting for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and to being a co-inventor on a patent involving combination therapy for patients having acute or persistent dyspnea.
Dr. Vest reports modest relationships with Boehringer Ingelheim, Corvia, and CareDx; and receiving research grants from the American Heart Association and the National Institutes of Health. Dr. Cleland discloses receiving honoraria from Idorsia; and research grants from Vifor Pharma, Medtronic, Bayer, and Bristol-Myers Squibb. Dr. Leon had no disclosures.
A version of this article first appeared on Medscape.com.
CHICAGO – Clinicians who prescribe heart failure meds are holding the best hand they’ve ever had, but with so much underuse and suboptimal dosing in actual practice, it seems many may not appreciate the value of their cards. But a major randomized trial that has captured the field’s attention may embolden them to go all in.
Results showed that a strategy of early, rapid up-titration of multiple guideline-directed meds in patients hospitalized with heart failure, compared with a usual-care approach, cut their 6-month risk for death or HF readmission by a steep 34% (P = .002).
The drugs had been started and partly up-titrated in the hospital with the goal of full up-titration within 2 weeks after discharge.
Patients well tolerated the high-intensity approach, researchers said. Their quality-of-life scores improved (P < .0001) compared with the usual-care group, and adverse events were considered few and manageable in the international trial with more than 1,000 patients.
Safety on the high-intensity strategy depended on close patient monitoring at frequently planned clinic visits along with guidance for the up-titrations from clinical signs and natriuretic peptide levels, observed Alexandre Mebazaa, MD, PhD, University of Paris and Public Hospitals of Paris.
Dr. Mebazaa is principal investigator on the trial, called STRONG-HF, which he presented at the American Heart Association scientific sessions, held in Chicago and virtually. He is also lead author on the study’s same-day publication in the Lancet.
The high-intensity strategy’s superiority emerged early in the trial, which was halted early on the data safety monitoring board’s recommendation, with about 90% of follow-ups completed. The board “felt it was unethical to keep patients in usual care,” Dr. Mebazaa said at a press conference.
A dramatic change
The next step, he said, will be to educate the heart failure community on the high-intensity care technique so it can swiftly enter clinical practice. Currently in acute heart failure, “very few patients are monitored after discharge and treated with full doses of heart failure therapies.”
Adoption of the strategy “would be a dramatic change from what’s currently being done,” said Martin B. Leon, MD, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, who moderated the press conference.
Only an estimated 5% of patients with HF in the United States receive full guideline-directed medical therapy, Dr. Leon said, “so the generalizability of this strategy, with careful follow-up that has safety involved in it, is absolutely crucial.”
But the potential impact of this high-intensity approach on resource use is unknown, raising questions about how widely and consistently it could be implemented, said Dr. Leon, who is not connected with STRONG-HF.
The trial called for in-hospital initiation of the three distinct drug classes that, at the time, were the core of guideline-directed HF therapy, with up-titration to 50% of recommended dosage by hospital discharge, and then to 100% within 2 weeks later.
The meds included a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system inhibitor (RASI). The latter could be an ACE inhibitor, angiotensin-receptor blocker (ARB), or angiotensin receptor-neprilysin inhibitor (ARNI).
How about a fourth drug?
Conspicuously absent from the list, for contemporary practice, was an SGLT2 inhibitor, a class that entered the HF guidelines well after STRONG-HF was designed. They would undoubtedly join the other three agents were the high-intensity strategy to enter practice, potentially changing its complexity and safety profile.
But Dr. Mebazaa and other experts don’t see that as a big challenge and would expect a smooth transition to a high-intensity approach that also includes the SGLT2 inhibitors.
STRONG-HF was necessary in part because many clinicians have been “reluctant” to take full advantage of three agents that had been the basis of guideline-directed therapy, he told this news organization.
That reluctance stemmed from concerns that beta-blockers might worsen the heart failure, ACE inhibitors could hurt the kidneys, or MRAs might cause hyperkalemia, Dr. Mebazaa said. The STRONG-HF high-intensity regimen, therefore, demanded multiple clinic visits for close follow-up.
But the SGLT2 inhibitors “are known to be rather safe drugs, at least much safer than the three others,” he said. So, it seems unlikely that their addition to a beta-blocker, RASI, and MRA in patients with HF would worsen the risk of adverse events.
John G.F. Cleland, MD, PhD, agrees. With addition of the fourth agent, “You may need to be a little bit more careful with renal function, just in that first couple of weeks,” he told this news organization. “But I think it would be easy to add an SGLT2 inhibitor into this regimen. And in general, there’s no titration with an SGLT2 inhibitor, so they’ll all be on full dose predischarge.”
Given the drugs’ diuretic-like action, moreover, some patients might be able to pull back on their loop diuretics, speculated Dr. Cleland, from the University of Glasgow’s School of Health and Wellbeing.
The prospect of a high-intensity strategy’s wide implementation in practice presents both “challenges and opportunities,” Amanda R. Vest, MBBS, MPH, Tufts University, Boston, told this news organization.
“There may be additional challenges in terms of ensuring we avoid hypotension or acute kidney injury in the up-titration phase,” said Dr. Vest, who is medical director of her center’s cardiac transplantation program but not connected with STRONG-HF.
“But it also gives us opportunities,” she added, “because there are some patients, especially in that vulnerable postdischarge phase, who are actually much more able to tolerate introduction of an SGLT2 inhibitor than, for example, an ACE inhibitor, ARB, or ARNI – or maybe a beta-blocker if they’ve been in a low cardiac-output state.” Effective dosing would depend on “the personalization and skill of the clinician in optimizing the medications in their correct sequence,” Dr. Vest said.
“It’s challenging to think that we would ever get to 100% up-titration,” she added, “and even in this excellent study, they didn’t get to 100%.” But as clinicians gain experience with the high-intensity strategy, especially as the SGLT2 inhibitors are included, “I think we can reasonably expect more progress to be made in these up-titration skills.”
No restrictions on LVEF
The researchers entered 1,078 patients hospitalized with acute HF in 14 countries across Africa, Europe, the Middle East, and South America, and randomly assigned them to the high-intensity management strategy or usual care.
About 60% of the patients were male and 77% were White. There were no entry restrictions based on left ventricular ejection fraction (LVEF), which exceeded 40% in almost a third of cases.
In the high-intensity care group’s 542 patients, the three agents were up-titrated to 50% of the maximum guideline-recommended dosage prior to hospital discharge, and to 100% within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were monitored closely at four planned clinical visits over the following 6 weeks.
The 536 patients assigned to usual care were discharged and managed according to local standards, with their meds handled by their own primary care doctors or cardiologists, the published report notes. They were reevaluated by STRONG-HF clinicians 90 days after discharge.
The number of clinic visits in the first 90 postdischarge days averaged 4.8 in the high-intensity care group and 1.0 for those receiving usual care. Full up-titration was far more likely in the high-intensity care group: 55% vs. 2% for RASI agents, 49% vs. 4% for beta-blockers, and 84% vs. 46% for MRAs.
They also fared significantly better on all measured parameters associated with decongestion, including weight, prevalence of peripheral edema, jugular venous pressure, NYHA functional class, and natriuretic peptide levels, the researchers said.
The primary endpoint of 180-day death from any cause or HF readmission was met by 15.2% of the high-intensity care group and 23.3% of usual-care patients, for an adjusted risk ratio (RR) of 0.66 (95% CI, 0.50-0.86; P = .0021).
Subgroup analyses saw no significant interactions by age, sex, race, geography, or baseline blood pressure, renal function, or LVEF. Patients with higher vs. lower baseline natriuretic peptide levels trend toward better responses to high-intensity care (P = .08)
The COVID effect
The group performed a sensitivity analysis that excluded deaths attributed to COVID-19 in STRONG-HF, which launched prior to the pandemic. The high-intensity strategy’s benefit for the primary endpoint grew, with an adjusted RR of 0.61 (95% CI, 0.46-0.82; P = .0005). There was no corresponding effect on death from any cause (P = .15).
Treatment-related adverse effects in the overall trial were seen in 41.1% of the high-intensity care group and in 29.5% of those assigned to usual care.
The higher rate in the high-intensity care arm “may be related to their higher number of [clinic] visits compared to usual care,” Dr. Mebazaa said. “However, serious adverse events and fatal adverse events were similar in both arms.”
Cardiac failure was the most common adverse event, developing in about 15% in both groups. It was followed by hypotension, hyperkalemia, and renal impairment, according to the published report.
Dr. Cleland cautioned that the risk of adverse events would potentially be higher should the high-intensity strategy become common clinical practice. The median age in STRONG-HF was 63, which is “10-15 years younger, on average, than the population with recently admitted heart failure that we see. There’s no doubt that older people have more multimorbidity.”
STRONG-HF was funded by Roche Diagnostics. Dr. Mebazaa discloses receiving grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and Merck, Sharp & Dohme; and consulting for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and to being a co-inventor on a patent involving combination therapy for patients having acute or persistent dyspnea.
Dr. Vest reports modest relationships with Boehringer Ingelheim, Corvia, and CareDx; and receiving research grants from the American Heart Association and the National Institutes of Health. Dr. Cleland discloses receiving honoraria from Idorsia; and research grants from Vifor Pharma, Medtronic, Bayer, and Bristol-Myers Squibb. Dr. Leon had no disclosures.
A version of this article first appeared on Medscape.com.
CHICAGO – Clinicians who prescribe heart failure meds are holding the best hand they’ve ever had, but with so much underuse and suboptimal dosing in actual practice, it seems many may not appreciate the value of their cards. But a major randomized trial that has captured the field’s attention may embolden them to go all in.
Results showed that a strategy of early, rapid up-titration of multiple guideline-directed meds in patients hospitalized with heart failure, compared with a usual-care approach, cut their 6-month risk for death or HF readmission by a steep 34% (P = .002).
The drugs had been started and partly up-titrated in the hospital with the goal of full up-titration within 2 weeks after discharge.
Patients well tolerated the high-intensity approach, researchers said. Their quality-of-life scores improved (P < .0001) compared with the usual-care group, and adverse events were considered few and manageable in the international trial with more than 1,000 patients.
Safety on the high-intensity strategy depended on close patient monitoring at frequently planned clinic visits along with guidance for the up-titrations from clinical signs and natriuretic peptide levels, observed Alexandre Mebazaa, MD, PhD, University of Paris and Public Hospitals of Paris.
Dr. Mebazaa is principal investigator on the trial, called STRONG-HF, which he presented at the American Heart Association scientific sessions, held in Chicago and virtually. He is also lead author on the study’s same-day publication in the Lancet.
The high-intensity strategy’s superiority emerged early in the trial, which was halted early on the data safety monitoring board’s recommendation, with about 90% of follow-ups completed. The board “felt it was unethical to keep patients in usual care,” Dr. Mebazaa said at a press conference.
A dramatic change
The next step, he said, will be to educate the heart failure community on the high-intensity care technique so it can swiftly enter clinical practice. Currently in acute heart failure, “very few patients are monitored after discharge and treated with full doses of heart failure therapies.”
Adoption of the strategy “would be a dramatic change from what’s currently being done,” said Martin B. Leon, MD, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, who moderated the press conference.
Only an estimated 5% of patients with HF in the United States receive full guideline-directed medical therapy, Dr. Leon said, “so the generalizability of this strategy, with careful follow-up that has safety involved in it, is absolutely crucial.”
But the potential impact of this high-intensity approach on resource use is unknown, raising questions about how widely and consistently it could be implemented, said Dr. Leon, who is not connected with STRONG-HF.
The trial called for in-hospital initiation of the three distinct drug classes that, at the time, were the core of guideline-directed HF therapy, with up-titration to 50% of recommended dosage by hospital discharge, and then to 100% within 2 weeks later.
The meds included a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system inhibitor (RASI). The latter could be an ACE inhibitor, angiotensin-receptor blocker (ARB), or angiotensin receptor-neprilysin inhibitor (ARNI).
How about a fourth drug?
Conspicuously absent from the list, for contemporary practice, was an SGLT2 inhibitor, a class that entered the HF guidelines well after STRONG-HF was designed. They would undoubtedly join the other three agents were the high-intensity strategy to enter practice, potentially changing its complexity and safety profile.
But Dr. Mebazaa and other experts don’t see that as a big challenge and would expect a smooth transition to a high-intensity approach that also includes the SGLT2 inhibitors.
STRONG-HF was necessary in part because many clinicians have been “reluctant” to take full advantage of three agents that had been the basis of guideline-directed therapy, he told this news organization.
That reluctance stemmed from concerns that beta-blockers might worsen the heart failure, ACE inhibitors could hurt the kidneys, or MRAs might cause hyperkalemia, Dr. Mebazaa said. The STRONG-HF high-intensity regimen, therefore, demanded multiple clinic visits for close follow-up.
But the SGLT2 inhibitors “are known to be rather safe drugs, at least much safer than the three others,” he said. So, it seems unlikely that their addition to a beta-blocker, RASI, and MRA in patients with HF would worsen the risk of adverse events.
John G.F. Cleland, MD, PhD, agrees. With addition of the fourth agent, “You may need to be a little bit more careful with renal function, just in that first couple of weeks,” he told this news organization. “But I think it would be easy to add an SGLT2 inhibitor into this regimen. And in general, there’s no titration with an SGLT2 inhibitor, so they’ll all be on full dose predischarge.”
Given the drugs’ diuretic-like action, moreover, some patients might be able to pull back on their loop diuretics, speculated Dr. Cleland, from the University of Glasgow’s School of Health and Wellbeing.
The prospect of a high-intensity strategy’s wide implementation in practice presents both “challenges and opportunities,” Amanda R. Vest, MBBS, MPH, Tufts University, Boston, told this news organization.
“There may be additional challenges in terms of ensuring we avoid hypotension or acute kidney injury in the up-titration phase,” said Dr. Vest, who is medical director of her center’s cardiac transplantation program but not connected with STRONG-HF.
“But it also gives us opportunities,” she added, “because there are some patients, especially in that vulnerable postdischarge phase, who are actually much more able to tolerate introduction of an SGLT2 inhibitor than, for example, an ACE inhibitor, ARB, or ARNI – or maybe a beta-blocker if they’ve been in a low cardiac-output state.” Effective dosing would depend on “the personalization and skill of the clinician in optimizing the medications in their correct sequence,” Dr. Vest said.
“It’s challenging to think that we would ever get to 100% up-titration,” she added, “and even in this excellent study, they didn’t get to 100%.” But as clinicians gain experience with the high-intensity strategy, especially as the SGLT2 inhibitors are included, “I think we can reasonably expect more progress to be made in these up-titration skills.”
No restrictions on LVEF
The researchers entered 1,078 patients hospitalized with acute HF in 14 countries across Africa, Europe, the Middle East, and South America, and randomly assigned them to the high-intensity management strategy or usual care.
About 60% of the patients were male and 77% were White. There were no entry restrictions based on left ventricular ejection fraction (LVEF), which exceeded 40% in almost a third of cases.
In the high-intensity care group’s 542 patients, the three agents were up-titrated to 50% of the maximum guideline-recommended dosage prior to hospital discharge, and to 100% within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were monitored closely at four planned clinical visits over the following 6 weeks.
The 536 patients assigned to usual care were discharged and managed according to local standards, with their meds handled by their own primary care doctors or cardiologists, the published report notes. They were reevaluated by STRONG-HF clinicians 90 days after discharge.
The number of clinic visits in the first 90 postdischarge days averaged 4.8 in the high-intensity care group and 1.0 for those receiving usual care. Full up-titration was far more likely in the high-intensity care group: 55% vs. 2% for RASI agents, 49% vs. 4% for beta-blockers, and 84% vs. 46% for MRAs.
They also fared significantly better on all measured parameters associated with decongestion, including weight, prevalence of peripheral edema, jugular venous pressure, NYHA functional class, and natriuretic peptide levels, the researchers said.
The primary endpoint of 180-day death from any cause or HF readmission was met by 15.2% of the high-intensity care group and 23.3% of usual-care patients, for an adjusted risk ratio (RR) of 0.66 (95% CI, 0.50-0.86; P = .0021).
Subgroup analyses saw no significant interactions by age, sex, race, geography, or baseline blood pressure, renal function, or LVEF. Patients with higher vs. lower baseline natriuretic peptide levels trend toward better responses to high-intensity care (P = .08)
The COVID effect
The group performed a sensitivity analysis that excluded deaths attributed to COVID-19 in STRONG-HF, which launched prior to the pandemic. The high-intensity strategy’s benefit for the primary endpoint grew, with an adjusted RR of 0.61 (95% CI, 0.46-0.82; P = .0005). There was no corresponding effect on death from any cause (P = .15).
Treatment-related adverse effects in the overall trial were seen in 41.1% of the high-intensity care group and in 29.5% of those assigned to usual care.
The higher rate in the high-intensity care arm “may be related to their higher number of [clinic] visits compared to usual care,” Dr. Mebazaa said. “However, serious adverse events and fatal adverse events were similar in both arms.”
Cardiac failure was the most common adverse event, developing in about 15% in both groups. It was followed by hypotension, hyperkalemia, and renal impairment, according to the published report.
Dr. Cleland cautioned that the risk of adverse events would potentially be higher should the high-intensity strategy become common clinical practice. The median age in STRONG-HF was 63, which is “10-15 years younger, on average, than the population with recently admitted heart failure that we see. There’s no doubt that older people have more multimorbidity.”
STRONG-HF was funded by Roche Diagnostics. Dr. Mebazaa discloses receiving grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and Merck, Sharp & Dohme; and consulting for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and to being a co-inventor on a patent involving combination therapy for patients having acute or persistent dyspnea.
Dr. Vest reports modest relationships with Boehringer Ingelheim, Corvia, and CareDx; and receiving research grants from the American Heart Association and the National Institutes of Health. Dr. Cleland discloses receiving honoraria from Idorsia; and research grants from Vifor Pharma, Medtronic, Bayer, and Bristol-Myers Squibb. Dr. Leon had no disclosures.
A version of this article first appeared on Medscape.com.
AT AHA 2022



