User login
‘Cautious’ DOAC underdosing in AFib may push mortality higher
The risk for death goes up for patients with atrial fibrillation (AFib) who are put on direct oral anticoagulants (DOAC) at dosages other than those approved for stroke prevention, whether higher or lower than doses specified in the labeling, suggests a large registry study.
A quarter of more than 10,000 patients in the registry took the drugs at such nonrecommended higher or lower dosages. Overwhelmingly it was the latter, perhaps reflecting caution on the part of some practitioners looking to minimize the risk of bleeding complications.
The risk of major bleeding indeed dropped sharply for those taking DOACs at lower-than-recommended levels, but at the cost of a 25% jump in all-cause mortality over 2 years, report investigators from their analysis of patients in the GARFIELD-AF registry published Sept. 14 in the Journal of the American College of Cardiology.
Risks of major bleeding and of stroke or systemic embolism didn’t climb significantly for patients either under- or overdosed.
In general, “physicians are worried about giving too much anticoagulant, and they tend to favor erring on the low-dose side,” lead author A. John Camm, MD, St. George’s University of London, said in an interview. That’s how it was when an oral anticoagulation meant a vitamin K antagonist (VKA) and underdosing was frequent; and it remains an issue in the DOAC era. “It’s not just a little problem. It’s a very big problem.”
Today, clinicians may prescribe DOACs similar to how they prescribed VKAs, by cautiously choosing a lower dosage for selected patients based on their risk profile, Dr. Camm observed. But in contrast to the VKAs, the DOACs “were studied with different dose-reduction strategies, and their labeling requires them to be prescribed according to different parameters.”
They variously base dosage reductions on age, body weight, renal function, or drug-drug interactions, for example, but some clinicians “tend to think that all of those factors should be applied in every instance, with every drug,” he said.
“So I think there’s some confusion and a lot of caution that physicians use with anticoagulants, and they often forget that the purpose of the anticoagulant is to prevent strokes and adverse outcomes such as mortality,” Dr. Camm said. “But by reducing the dose, they expose their patients to these other major cardiovascular events.”
Numerically, the excess mortality among underdosed patients appeared to be driven by death from heart failure or myocardial infarction. There was little or no contribution from sudden death, fatal strokes, or noncardiovascular death.
The findings “remind clinicians to dose DOACs properly and that there are consequences of dosing errors,” observes Gerald V. Naccarelli, MD, of Penn State University and the Milton S. Hershey Medical Center, Hershey, in an accompanying editorial.
Based on the major clinical trials that established the drugs as mainstream stroke-preventive therapy in AFib, as well as extensive regulatory review, each DOAC’s label-recommended dosing “is a guidance of the truth to achieve the highest efficacy and most safety in our patients,” Dr. Naccarelli wrote. “As clinicians are risk adverse, underdosing might result in lower major bleeding rates, and physicians are blamed for bleeding but not necessarily for allowing embolic strokes to occur. These data raise the issue that underdosing is associated with worse patient outcomes.”
The GARFIELD-AF analysis covered 10,426 adults with nonvalvular AFib in 35 countries who initiated a DOAC from 2013 to 2016. The drugs were prescribed at dosages consistent with recommendations in each respective country’s labeling for stroke prevention in AFib in 72.9% of the cohort. Most full and adjusted dose levels approved by the European Medicines Agency, Food and Drug Administration, and regulators in Japan were the same or similar.
But there were a few exceptions. All dosing criteria across the three regulatory domains were the same for apixaban (Eliquis). But variations included lower dosage options for rivaroxaban (Xarelto) and edoxaban (Savaysa, Lixiana) in Japan, and a uniquely low dabigatran (Pradaxa) dosage option in the United States.
The DOAC used least often was the one most frequently underdosed. More than half of patients prescribed edoxaban were given it at a lower-than-recommended dosage.
The adjusted hazard ratio for all-cause mortality at 2 years for DOAC under- or overdosing, compared with dosing at recommended levels, was 1.24 (95% confidence interval, 1.04-1.48). The difference was driven by underdosing, for which the HR was 1.25 (95% CI, 1.04-1.50). The HR for over-dosing was only 1.19 (95% CI, 0.83-1.71).
Multivariate adjustment accounted for age, sex, and ethnicity; type of AFib; diabetes; hypertension; history of bleeding; prior stroke, transient ischemic attack, or systemic embolism; heart failure; vascular disease; smoking; and heavy alcohol consumption.
The risk of stroke or systemic embolism didn’t go up or down significantly for either overdosed or underdosed patients. Neither group showed an increased risk for major bleeding; however, the HR for major bleeding in underdosed patients fell to 0.50 (95% CI, 0.28-0.88).
Underdosing was more common in some world regions than others. The rate exceeded 30% in all Latin American countries except Argentina, the report stated, and in all Asian countries except Singapore.
Japanese patients have long received oral anticoagulation at lower dosages than are used in the West, Dr. Camm observed. When VKAs were the only choice, for example, international normalized ratio targets were consistently a bit lower in Japan than in, for example, North America or Europe.
“And when [novel] OACs were developed, again, the Japanese took the view that their patients are more vulnerable to bleeding, and therefore a lower dose would be appropriate. In some instances, lower-dose regimens have been specifically studied in the Japanese,” Dr. Camm said. “Having said that, this concept of bleeding being more problematic in Asian patients has expanded well beyond Japan, and therefore in many Asian communities, lower doses of [novel] OACs are chosen.”
Many other factors may contribute to DOAC underdosing, including differences in dosing strategies between primary care practitioners and specialists, or between hospital-based and office-based clinicians, for example.
“It might also be argued that a physician who fails to treat a patient adequately in one arena may also be failing to treat the patient well in other aspects of their care,” Dr. Camm proposed. “Therefore you could have increased mortality due to other cardiovascular causes, or even noncardiovascular events, through absence of good quality care. Our study did not address that specifically. But it might be the case, speculatively.”
The study was supported by a grant from Bayer to the Thrombosis Research Institute, “which sponsors the GARFIELD-AF registry.” Dr. Camm discloses receiving grants and personal fees from Bayer, Boehringer Ingelheim, Pfizer/Bristol-Myers Squibb, and Daiichi Sankyo. Disclosures for the other authors are in the report. Dr. Naccarelli disclosed consulting and participating in research for Janssen and serving as a consultant for Milestone, Sanofi, Omeicos, and Acesion Pharma.
A version of this article originally appeared on Medscape.com.
The risk for death goes up for patients with atrial fibrillation (AFib) who are put on direct oral anticoagulants (DOAC) at dosages other than those approved for stroke prevention, whether higher or lower than doses specified in the labeling, suggests a large registry study.
A quarter of more than 10,000 patients in the registry took the drugs at such nonrecommended higher or lower dosages. Overwhelmingly it was the latter, perhaps reflecting caution on the part of some practitioners looking to minimize the risk of bleeding complications.
The risk of major bleeding indeed dropped sharply for those taking DOACs at lower-than-recommended levels, but at the cost of a 25% jump in all-cause mortality over 2 years, report investigators from their analysis of patients in the GARFIELD-AF registry published Sept. 14 in the Journal of the American College of Cardiology.
Risks of major bleeding and of stroke or systemic embolism didn’t climb significantly for patients either under- or overdosed.
In general, “physicians are worried about giving too much anticoagulant, and they tend to favor erring on the low-dose side,” lead author A. John Camm, MD, St. George’s University of London, said in an interview. That’s how it was when an oral anticoagulation meant a vitamin K antagonist (VKA) and underdosing was frequent; and it remains an issue in the DOAC era. “It’s not just a little problem. It’s a very big problem.”
Today, clinicians may prescribe DOACs similar to how they prescribed VKAs, by cautiously choosing a lower dosage for selected patients based on their risk profile, Dr. Camm observed. But in contrast to the VKAs, the DOACs “were studied with different dose-reduction strategies, and their labeling requires them to be prescribed according to different parameters.”
They variously base dosage reductions on age, body weight, renal function, or drug-drug interactions, for example, but some clinicians “tend to think that all of those factors should be applied in every instance, with every drug,” he said.
“So I think there’s some confusion and a lot of caution that physicians use with anticoagulants, and they often forget that the purpose of the anticoagulant is to prevent strokes and adverse outcomes such as mortality,” Dr. Camm said. “But by reducing the dose, they expose their patients to these other major cardiovascular events.”
Numerically, the excess mortality among underdosed patients appeared to be driven by death from heart failure or myocardial infarction. There was little or no contribution from sudden death, fatal strokes, or noncardiovascular death.
The findings “remind clinicians to dose DOACs properly and that there are consequences of dosing errors,” observes Gerald V. Naccarelli, MD, of Penn State University and the Milton S. Hershey Medical Center, Hershey, in an accompanying editorial.
Based on the major clinical trials that established the drugs as mainstream stroke-preventive therapy in AFib, as well as extensive regulatory review, each DOAC’s label-recommended dosing “is a guidance of the truth to achieve the highest efficacy and most safety in our patients,” Dr. Naccarelli wrote. “As clinicians are risk adverse, underdosing might result in lower major bleeding rates, and physicians are blamed for bleeding but not necessarily for allowing embolic strokes to occur. These data raise the issue that underdosing is associated with worse patient outcomes.”
The GARFIELD-AF analysis covered 10,426 adults with nonvalvular AFib in 35 countries who initiated a DOAC from 2013 to 2016. The drugs were prescribed at dosages consistent with recommendations in each respective country’s labeling for stroke prevention in AFib in 72.9% of the cohort. Most full and adjusted dose levels approved by the European Medicines Agency, Food and Drug Administration, and regulators in Japan were the same or similar.
But there were a few exceptions. All dosing criteria across the three regulatory domains were the same for apixaban (Eliquis). But variations included lower dosage options for rivaroxaban (Xarelto) and edoxaban (Savaysa, Lixiana) in Japan, and a uniquely low dabigatran (Pradaxa) dosage option in the United States.
The DOAC used least often was the one most frequently underdosed. More than half of patients prescribed edoxaban were given it at a lower-than-recommended dosage.
The adjusted hazard ratio for all-cause mortality at 2 years for DOAC under- or overdosing, compared with dosing at recommended levels, was 1.24 (95% confidence interval, 1.04-1.48). The difference was driven by underdosing, for which the HR was 1.25 (95% CI, 1.04-1.50). The HR for over-dosing was only 1.19 (95% CI, 0.83-1.71).
Multivariate adjustment accounted for age, sex, and ethnicity; type of AFib; diabetes; hypertension; history of bleeding; prior stroke, transient ischemic attack, or systemic embolism; heart failure; vascular disease; smoking; and heavy alcohol consumption.
The risk of stroke or systemic embolism didn’t go up or down significantly for either overdosed or underdosed patients. Neither group showed an increased risk for major bleeding; however, the HR for major bleeding in underdosed patients fell to 0.50 (95% CI, 0.28-0.88).
Underdosing was more common in some world regions than others. The rate exceeded 30% in all Latin American countries except Argentina, the report stated, and in all Asian countries except Singapore.
Japanese patients have long received oral anticoagulation at lower dosages than are used in the West, Dr. Camm observed. When VKAs were the only choice, for example, international normalized ratio targets were consistently a bit lower in Japan than in, for example, North America or Europe.
“And when [novel] OACs were developed, again, the Japanese took the view that their patients are more vulnerable to bleeding, and therefore a lower dose would be appropriate. In some instances, lower-dose regimens have been specifically studied in the Japanese,” Dr. Camm said. “Having said that, this concept of bleeding being more problematic in Asian patients has expanded well beyond Japan, and therefore in many Asian communities, lower doses of [novel] OACs are chosen.”
Many other factors may contribute to DOAC underdosing, including differences in dosing strategies between primary care practitioners and specialists, or between hospital-based and office-based clinicians, for example.
“It might also be argued that a physician who fails to treat a patient adequately in one arena may also be failing to treat the patient well in other aspects of their care,” Dr. Camm proposed. “Therefore you could have increased mortality due to other cardiovascular causes, or even noncardiovascular events, through absence of good quality care. Our study did not address that specifically. But it might be the case, speculatively.”
The study was supported by a grant from Bayer to the Thrombosis Research Institute, “which sponsors the GARFIELD-AF registry.” Dr. Camm discloses receiving grants and personal fees from Bayer, Boehringer Ingelheim, Pfizer/Bristol-Myers Squibb, and Daiichi Sankyo. Disclosures for the other authors are in the report. Dr. Naccarelli disclosed consulting and participating in research for Janssen and serving as a consultant for Milestone, Sanofi, Omeicos, and Acesion Pharma.
A version of this article originally appeared on Medscape.com.
The risk for death goes up for patients with atrial fibrillation (AFib) who are put on direct oral anticoagulants (DOAC) at dosages other than those approved for stroke prevention, whether higher or lower than doses specified in the labeling, suggests a large registry study.
A quarter of more than 10,000 patients in the registry took the drugs at such nonrecommended higher or lower dosages. Overwhelmingly it was the latter, perhaps reflecting caution on the part of some practitioners looking to minimize the risk of bleeding complications.
The risk of major bleeding indeed dropped sharply for those taking DOACs at lower-than-recommended levels, but at the cost of a 25% jump in all-cause mortality over 2 years, report investigators from their analysis of patients in the GARFIELD-AF registry published Sept. 14 in the Journal of the American College of Cardiology.
Risks of major bleeding and of stroke or systemic embolism didn’t climb significantly for patients either under- or overdosed.
In general, “physicians are worried about giving too much anticoagulant, and they tend to favor erring on the low-dose side,” lead author A. John Camm, MD, St. George’s University of London, said in an interview. That’s how it was when an oral anticoagulation meant a vitamin K antagonist (VKA) and underdosing was frequent; and it remains an issue in the DOAC era. “It’s not just a little problem. It’s a very big problem.”
Today, clinicians may prescribe DOACs similar to how they prescribed VKAs, by cautiously choosing a lower dosage for selected patients based on their risk profile, Dr. Camm observed. But in contrast to the VKAs, the DOACs “were studied with different dose-reduction strategies, and their labeling requires them to be prescribed according to different parameters.”
They variously base dosage reductions on age, body weight, renal function, or drug-drug interactions, for example, but some clinicians “tend to think that all of those factors should be applied in every instance, with every drug,” he said.
“So I think there’s some confusion and a lot of caution that physicians use with anticoagulants, and they often forget that the purpose of the anticoagulant is to prevent strokes and adverse outcomes such as mortality,” Dr. Camm said. “But by reducing the dose, they expose their patients to these other major cardiovascular events.”
Numerically, the excess mortality among underdosed patients appeared to be driven by death from heart failure or myocardial infarction. There was little or no contribution from sudden death, fatal strokes, or noncardiovascular death.
The findings “remind clinicians to dose DOACs properly and that there are consequences of dosing errors,” observes Gerald V. Naccarelli, MD, of Penn State University and the Milton S. Hershey Medical Center, Hershey, in an accompanying editorial.
Based on the major clinical trials that established the drugs as mainstream stroke-preventive therapy in AFib, as well as extensive regulatory review, each DOAC’s label-recommended dosing “is a guidance of the truth to achieve the highest efficacy and most safety in our patients,” Dr. Naccarelli wrote. “As clinicians are risk adverse, underdosing might result in lower major bleeding rates, and physicians are blamed for bleeding but not necessarily for allowing embolic strokes to occur. These data raise the issue that underdosing is associated with worse patient outcomes.”
The GARFIELD-AF analysis covered 10,426 adults with nonvalvular AFib in 35 countries who initiated a DOAC from 2013 to 2016. The drugs were prescribed at dosages consistent with recommendations in each respective country’s labeling for stroke prevention in AFib in 72.9% of the cohort. Most full and adjusted dose levels approved by the European Medicines Agency, Food and Drug Administration, and regulators in Japan were the same or similar.
But there were a few exceptions. All dosing criteria across the three regulatory domains were the same for apixaban (Eliquis). But variations included lower dosage options for rivaroxaban (Xarelto) and edoxaban (Savaysa, Lixiana) in Japan, and a uniquely low dabigatran (Pradaxa) dosage option in the United States.
The DOAC used least often was the one most frequently underdosed. More than half of patients prescribed edoxaban were given it at a lower-than-recommended dosage.
The adjusted hazard ratio for all-cause mortality at 2 years for DOAC under- or overdosing, compared with dosing at recommended levels, was 1.24 (95% confidence interval, 1.04-1.48). The difference was driven by underdosing, for which the HR was 1.25 (95% CI, 1.04-1.50). The HR for over-dosing was only 1.19 (95% CI, 0.83-1.71).
Multivariate adjustment accounted for age, sex, and ethnicity; type of AFib; diabetes; hypertension; history of bleeding; prior stroke, transient ischemic attack, or systemic embolism; heart failure; vascular disease; smoking; and heavy alcohol consumption.
The risk of stroke or systemic embolism didn’t go up or down significantly for either overdosed or underdosed patients. Neither group showed an increased risk for major bleeding; however, the HR for major bleeding in underdosed patients fell to 0.50 (95% CI, 0.28-0.88).
Underdosing was more common in some world regions than others. The rate exceeded 30% in all Latin American countries except Argentina, the report stated, and in all Asian countries except Singapore.
Japanese patients have long received oral anticoagulation at lower dosages than are used in the West, Dr. Camm observed. When VKAs were the only choice, for example, international normalized ratio targets were consistently a bit lower in Japan than in, for example, North America or Europe.
“And when [novel] OACs were developed, again, the Japanese took the view that their patients are more vulnerable to bleeding, and therefore a lower dose would be appropriate. In some instances, lower-dose regimens have been specifically studied in the Japanese,” Dr. Camm said. “Having said that, this concept of bleeding being more problematic in Asian patients has expanded well beyond Japan, and therefore in many Asian communities, lower doses of [novel] OACs are chosen.”
Many other factors may contribute to DOAC underdosing, including differences in dosing strategies between primary care practitioners and specialists, or between hospital-based and office-based clinicians, for example.
“It might also be argued that a physician who fails to treat a patient adequately in one arena may also be failing to treat the patient well in other aspects of their care,” Dr. Camm proposed. “Therefore you could have increased mortality due to other cardiovascular causes, or even noncardiovascular events, through absence of good quality care. Our study did not address that specifically. But it might be the case, speculatively.”
The study was supported by a grant from Bayer to the Thrombosis Research Institute, “which sponsors the GARFIELD-AF registry.” Dr. Camm discloses receiving grants and personal fees from Bayer, Boehringer Ingelheim, Pfizer/Bristol-Myers Squibb, and Daiichi Sankyo. Disclosures for the other authors are in the report. Dr. Naccarelli disclosed consulting and participating in research for Janssen and serving as a consultant for Milestone, Sanofi, Omeicos, and Acesion Pharma.
A version of this article originally appeared on Medscape.com.
Minidose edoxaban may safely cut AFib stroke risk in the frail, very elderly
suggests a randomized trial conducted in Japan.
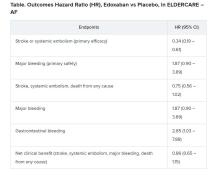
Many of the study’s 984 mostly octogenarian patients were objectively frail with poor renal function, low body weight, a history of serious bleeding, or other conditions that made them poor candidates for regular-dose oral anticoagulation. Yet those who took the factor Xa inhibitor edoxaban (Savaysa) at the off-label dosage of 15 mg once daily showed a two-thirds drop in risk for stroke or systemic embolism (P < .001), compared with patients who received placebo. There were no fatal bleeds and virtually no intracranial hemorrhages.
For such high-risk patients with nonvalvular AFib who otherwise would not be given an OAC, edoxaban 15 mg “can be an acceptable treatment option in decreasing the risk of devastating stroke”; however, “it may increase the risk of gastrointestinal bleeding, so care should be given in every patient,” said Ken Okumura, MD, PhD. Indeed, the rate of gastrointestinal bleeding tripled among the patients who received edoxaban, compared with those given placebo, at about 2.3% per year versus 0.8% per year.
Although their 87% increased risk for major bleeding did not reach significance, it hit close, with a P value of .09 in the trial, called Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF).
Dr. Okumura, of Saiseikai Kumamoto (Japan) Hospital, presented the study August 30 during the virtual annual congress of the European Society of Cardiology. He is lead author of an article describing the study, which was simultaneously published in the New England Journal of Medicine.
Many patients with AFib suffer strokes if they are not given oral anticoagulation because of “fear of major bleeding caused by standard OAC therapy,” Dr. Okumura noted. Others are inappropriately administered antiplatelets or anticoagulants at conventional dosages. “There is no standard of practice in Japan for patients like those in the present trial,” Dr. Okumura said. “However, I believe the present study opens a new possible path of thromboprophylaxis in such high-risk patients.”
Even with its relatively few bleeding events, ELDERCARE-AF “does suggest that the risk of the worst types of bleeds is not that high,” said Daniel E. Singer, MD, of Massachusetts General Hospital, Boston. “Gastrointestinal bleeding is annoying, and it will probably stop people from taking their edoxaban, but for the most part it doesn’t kill people.”
Moreover, he added, the trial suggests that low-dose edoxaban, in exchange for a steep reduction in thromboembolic risk, “doesn’t add to your risk of intracranial hemorrhage!”
ELDERCARE-AF may give practitioners “yet another reason to rethink” whether a low-dose DOAC such as edoxaban 15 mg/day may well be a good approach for such patients with AFib who are not receiving standard-dose OAC because of a perceived high risk for serious bleeding, said Dr. Singer, who was not involved in the study.
The trial randomly and evenly assigned 984 patients with AF in Japan to take either edoxaban 15 mg/day or placebo. The patients, who were at least 80 years old and had a CHADS2 score of 2 or higher, were judged inappropriate candidates for OAC at dosages approved for stroke prevention.
The mean age of the patients was 86.6, more than a decade older than patients “in the previous landmark clinical trials of direct oral anticoagulants,” and were 5-10 years older than the general AFib population, reported Dr. Okumura and colleagues.
Their mean weight was 52 kg, and mean creatinine clearance was 36.3 mL/min; 41% were classified as frail according to validated assessment tools.
Of the 303 patients who did not complete the trial, 158 voluntarily withdrew for various reasons. The withdrawal rate was similar in the two treatment arms. Outcomes were analyzed by intention to treat, the report noted.
The annualized rate of stroke or systemic embolism, the primary efficacy endpoint, was 2.3% for those who received edoxaban and 6.7% for the control group. Corresponding rates for the primary safety endpoint, major bleeding as determined by International Society on Thrombosis and Hemostasis criteria, were 3.3% and 1.8%, respectively.
“The question is, can the Food and Drug Administration act on this information? I doubt it can. What will be needed is to reproduce the study in a U.S. population to see if it holds,” Dr. Singer proposed.
“Edoxaban isn’t used much in the U.S. This could heighten interest. And who knows, there may be a gold rush,” he said, if the strategy were to pan out for the other DOACs, rivaroxaban (Xarelto), apixaban (Eliquis), and dabigatran (Pradaxa).
ELDERCARE-AF was funded by Daiichi Sankyo, from which Dr. Okumura reported receiving grants and personal fees; he also disclosed personal fees from Daiichi Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Johnson & Johnson, and Bayer.
A version of this article originally appeared on Medscape.com.
suggests a randomized trial conducted in Japan.
Many of the study’s 984 mostly octogenarian patients were objectively frail with poor renal function, low body weight, a history of serious bleeding, or other conditions that made them poor candidates for regular-dose oral anticoagulation. Yet those who took the factor Xa inhibitor edoxaban (Savaysa) at the off-label dosage of 15 mg once daily showed a two-thirds drop in risk for stroke or systemic embolism (P < .001), compared with patients who received placebo. There were no fatal bleeds and virtually no intracranial hemorrhages.
For such high-risk patients with nonvalvular AFib who otherwise would not be given an OAC, edoxaban 15 mg “can be an acceptable treatment option in decreasing the risk of devastating stroke”; however, “it may increase the risk of gastrointestinal bleeding, so care should be given in every patient,” said Ken Okumura, MD, PhD. Indeed, the rate of gastrointestinal bleeding tripled among the patients who received edoxaban, compared with those given placebo, at about 2.3% per year versus 0.8% per year.
Although their 87% increased risk for major bleeding did not reach significance, it hit close, with a P value of .09 in the trial, called Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF).
Dr. Okumura, of Saiseikai Kumamoto (Japan) Hospital, presented the study August 30 during the virtual annual congress of the European Society of Cardiology. He is lead author of an article describing the study, which was simultaneously published in the New England Journal of Medicine.
Many patients with AFib suffer strokes if they are not given oral anticoagulation because of “fear of major bleeding caused by standard OAC therapy,” Dr. Okumura noted. Others are inappropriately administered antiplatelets or anticoagulants at conventional dosages. “There is no standard of practice in Japan for patients like those in the present trial,” Dr. Okumura said. “However, I believe the present study opens a new possible path of thromboprophylaxis in such high-risk patients.”
Even with its relatively few bleeding events, ELDERCARE-AF “does suggest that the risk of the worst types of bleeds is not that high,” said Daniel E. Singer, MD, of Massachusetts General Hospital, Boston. “Gastrointestinal bleeding is annoying, and it will probably stop people from taking their edoxaban, but for the most part it doesn’t kill people.”
Moreover, he added, the trial suggests that low-dose edoxaban, in exchange for a steep reduction in thromboembolic risk, “doesn’t add to your risk of intracranial hemorrhage!”
ELDERCARE-AF may give practitioners “yet another reason to rethink” whether a low-dose DOAC such as edoxaban 15 mg/day may well be a good approach for such patients with AFib who are not receiving standard-dose OAC because of a perceived high risk for serious bleeding, said Dr. Singer, who was not involved in the study.
The trial randomly and evenly assigned 984 patients with AF in Japan to take either edoxaban 15 mg/day or placebo. The patients, who were at least 80 years old and had a CHADS2 score of 2 or higher, were judged inappropriate candidates for OAC at dosages approved for stroke prevention.
The mean age of the patients was 86.6, more than a decade older than patients “in the previous landmark clinical trials of direct oral anticoagulants,” and were 5-10 years older than the general AFib population, reported Dr. Okumura and colleagues.
Their mean weight was 52 kg, and mean creatinine clearance was 36.3 mL/min; 41% were classified as frail according to validated assessment tools.
Of the 303 patients who did not complete the trial, 158 voluntarily withdrew for various reasons. The withdrawal rate was similar in the two treatment arms. Outcomes were analyzed by intention to treat, the report noted.
The annualized rate of stroke or systemic embolism, the primary efficacy endpoint, was 2.3% for those who received edoxaban and 6.7% for the control group. Corresponding rates for the primary safety endpoint, major bleeding as determined by International Society on Thrombosis and Hemostasis criteria, were 3.3% and 1.8%, respectively.
“The question is, can the Food and Drug Administration act on this information? I doubt it can. What will be needed is to reproduce the study in a U.S. population to see if it holds,” Dr. Singer proposed.
“Edoxaban isn’t used much in the U.S. This could heighten interest. And who knows, there may be a gold rush,” he said, if the strategy were to pan out for the other DOACs, rivaroxaban (Xarelto), apixaban (Eliquis), and dabigatran (Pradaxa).
ELDERCARE-AF was funded by Daiichi Sankyo, from which Dr. Okumura reported receiving grants and personal fees; he also disclosed personal fees from Daiichi Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Johnson & Johnson, and Bayer.
A version of this article originally appeared on Medscape.com.
suggests a randomized trial conducted in Japan.
Many of the study’s 984 mostly octogenarian patients were objectively frail with poor renal function, low body weight, a history of serious bleeding, or other conditions that made them poor candidates for regular-dose oral anticoagulation. Yet those who took the factor Xa inhibitor edoxaban (Savaysa) at the off-label dosage of 15 mg once daily showed a two-thirds drop in risk for stroke or systemic embolism (P < .001), compared with patients who received placebo. There were no fatal bleeds and virtually no intracranial hemorrhages.
For such high-risk patients with nonvalvular AFib who otherwise would not be given an OAC, edoxaban 15 mg “can be an acceptable treatment option in decreasing the risk of devastating stroke”; however, “it may increase the risk of gastrointestinal bleeding, so care should be given in every patient,” said Ken Okumura, MD, PhD. Indeed, the rate of gastrointestinal bleeding tripled among the patients who received edoxaban, compared with those given placebo, at about 2.3% per year versus 0.8% per year.
Although their 87% increased risk for major bleeding did not reach significance, it hit close, with a P value of .09 in the trial, called Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF).
Dr. Okumura, of Saiseikai Kumamoto (Japan) Hospital, presented the study August 30 during the virtual annual congress of the European Society of Cardiology. He is lead author of an article describing the study, which was simultaneously published in the New England Journal of Medicine.
Many patients with AFib suffer strokes if they are not given oral anticoagulation because of “fear of major bleeding caused by standard OAC therapy,” Dr. Okumura noted. Others are inappropriately administered antiplatelets or anticoagulants at conventional dosages. “There is no standard of practice in Japan for patients like those in the present trial,” Dr. Okumura said. “However, I believe the present study opens a new possible path of thromboprophylaxis in such high-risk patients.”
Even with its relatively few bleeding events, ELDERCARE-AF “does suggest that the risk of the worst types of bleeds is not that high,” said Daniel E. Singer, MD, of Massachusetts General Hospital, Boston. “Gastrointestinal bleeding is annoying, and it will probably stop people from taking their edoxaban, but for the most part it doesn’t kill people.”
Moreover, he added, the trial suggests that low-dose edoxaban, in exchange for a steep reduction in thromboembolic risk, “doesn’t add to your risk of intracranial hemorrhage!”
ELDERCARE-AF may give practitioners “yet another reason to rethink” whether a low-dose DOAC such as edoxaban 15 mg/day may well be a good approach for such patients with AFib who are not receiving standard-dose OAC because of a perceived high risk for serious bleeding, said Dr. Singer, who was not involved in the study.
The trial randomly and evenly assigned 984 patients with AF in Japan to take either edoxaban 15 mg/day or placebo. The patients, who were at least 80 years old and had a CHADS2 score of 2 or higher, were judged inappropriate candidates for OAC at dosages approved for stroke prevention.
The mean age of the patients was 86.6, more than a decade older than patients “in the previous landmark clinical trials of direct oral anticoagulants,” and were 5-10 years older than the general AFib population, reported Dr. Okumura and colleagues.
Their mean weight was 52 kg, and mean creatinine clearance was 36.3 mL/min; 41% were classified as frail according to validated assessment tools.
Of the 303 patients who did not complete the trial, 158 voluntarily withdrew for various reasons. The withdrawal rate was similar in the two treatment arms. Outcomes were analyzed by intention to treat, the report noted.
The annualized rate of stroke or systemic embolism, the primary efficacy endpoint, was 2.3% for those who received edoxaban and 6.7% for the control group. Corresponding rates for the primary safety endpoint, major bleeding as determined by International Society on Thrombosis and Hemostasis criteria, were 3.3% and 1.8%, respectively.
“The question is, can the Food and Drug Administration act on this information? I doubt it can. What will be needed is to reproduce the study in a U.S. population to see if it holds,” Dr. Singer proposed.
“Edoxaban isn’t used much in the U.S. This could heighten interest. And who knows, there may be a gold rush,” he said, if the strategy were to pan out for the other DOACs, rivaroxaban (Xarelto), apixaban (Eliquis), and dabigatran (Pradaxa).
ELDERCARE-AF was funded by Daiichi Sankyo, from which Dr. Okumura reported receiving grants and personal fees; he also disclosed personal fees from Daiichi Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Johnson & Johnson, and Bayer.
A version of this article originally appeared on Medscape.com.
FROM ESC CONGRESS 2020
EXPLORER trial hints at potential new drug option in obstructive hypertrophic cardiomyopathy
An investigational drug that targets part of the molecular machinery underlying obstructive hypertrophic cardiomyopathy (HCM) can improve both symptoms and functional status in patients with the genetic disorder, suggests a placebo-controlled phase 3 trial.
Treatment with mavacamten (MyoKardia) worked partly by alleviating high-pressure gradients in the left ventricular outflow tract (LVOT), a key characteristic of obstructive HCM. Its effects appeared consistent across a wide range of objective and patient-assessed endpoints.
Mavacamten is “the first potential medical therapy addressing the underlying biology of symptoms in hypertrophic obstructive cardiomyopathy,” observed Iacopo Olivotto, MD, Careggi University Hospital, Florence, Italy.
Patients in the EXPLORER-HCM trial who took the new drug showed improvements in “every aspect of objective performance and subjective well-being,” Dr. Olivotto said at a preview for journalists before his formal online presentation of the results during the virtual European Society of Cardiology Congress 2020, staged in lieu of the traditional annual meeting because of the COVID-19 pandemic.
Dr. Olivotto, also lead author on the study’s same-day publication in The Lancet, was exuberant about the findings. “It is really hard to convey what this actually means for a scientific and clinical community that has spent over 60 years trying to understand and cure hypertrophic cardiomyopathy.”
MyoKardia released abbreviated top-line results of EXPLORER-HCM in May, which were reported by theheart.org | Medscape Cardiology at the time.
“I think it’s pretty exciting. We certainly need more and better drugs for this patient population,” Arnon Adler, MD, who is not associated with the trial but follows HCM at the Peter Munk Cardiac Centre, Toronto General Hospital, said in an interview.
The trial compared the new drug to placebo rather than full contemporary drug therapy for obstructive HCM, Adler cautioned, and had a fairly short follow-up time. But he was impressed that mavacamten’s apparent benefits seemed consistent not only for endpoints like change in New York Heart Association (NYHA) functional class and quality of life but also for more objective measures like peak VO2 and LVOT gradients.
“I think the results were promising across the board,” he told.
Unique mechanism of action
Mavacamten is described as a first-in-class, small-molecule, selective allosteric inhibitor of cardiac myosin adenosine triphosphatase that addresses the underlying pathophysiology of HCM by reducing actin–myosin cross-bridge formation. It thereby inhibits the excessive myocyte contractility that is a key mechanism of the disorder’s tell-tale hypertrophy, something the available HCM drug therapies don’t do.
Almost three-fourths of patients in the trial were initially in NYHA class 2. Such patients in practice tend to be treated pharmacologically, with more invasive but generally effective surgical myectomy and alcohol septal ablation performed more often for patients in NYHA class 3.
“In the EXPLORER-HCM trial, patients enrolled did not have any immediate indication for surgery,” although many of them in NYHA class 2 would likely progress to NYHA 3, Dr. Olivotto said in an interview.
Based on the trial, he said, it’s possible that mavacamten could lead to “earlier and broader treatment of obstruction symptoms in patients who would never have qualified for surgery in the first place because their symptoms may not be severe enough, but they are still limited.”
Notably, the published report notes, 27% of patients taking mavacamten achieved what was defined as a complete response – that is, a reduction of all LVOT gradients to less than 30 mm Hg in the total absence of symptoms.
Only 1% of patients in the placebo-treated control group met that goal, “showing that mavacamten might be capable of achieving marked relief of symptoms and LVOT obstruction,” the report states.
In the trial, “treatment with mavacamten led to clinically meaningful improvements in hemodynamic status, functional capacity, and subjective well-being in patients with obstructive hypertrophic cardiomyopathy,” agrees an editorial accompanying the EXPLORER-HCM publication.
Mavacamten might even compare favorably to surgery and ablative therapy, speculated the editorialists, Michael Papadakis, MBBS, MD, and colleagues of St. George’s University Hospitals NHS Foundation Trust, London. The drug appeared to reduce the peak LVOT gradient “to less than the guideline-based threshold for septal reduction therapy, 50 mm Hg, in 74% of patients, compared with 21% in the placebo group, indicating that mavacamten could represent a valid alternative to highly specialized invasive therapy,” they wrote.
Standard drug therapy
“There are approved drugs for obstructive hypertrophic cardiomyopathy, but they are ancient and were developed for other diseases,” observed Dr. Olivotto at the media briefing. Those drug options – primarily beta-blockers, nondihydropyridine calcium-channel blockers, and the sodium-channel blocker disopyramide – are often ineffective or cause onerous side effects, he said.
Notably in EXPLORER-HCM, patients in both the mavacamten and placebo groups could also be receiving beta-blockers and calcium-channel blockers, but no one in the trial could be receiving disopyramide, which can prolong the QT interval.
“By design,” mavacamten wasn’t compared to disopyramide, “a much more potent drug for lowering gradient and improving symptoms than beta-blockers or calcium-channel blockers,” said Martin S. Maron, MD, medical director at the Hypertrophic Cardiomyopathy Center and Research Institute, Tufts Medical Center, Boston.
Therefore, the trial’s results can’t be extrapolated to conclude that the new drug is superior to disopyramide “or the gold standard, surgical myectomy,” he said in an interview.
Dr. Adler agreed that observational studies suggest a benefit from disopyramide that may rival the apparent effect of mavacamten. “But of course, you can’t make direct comparisons because we never had a study like this for disopyramide.” Because it has many side effects and limitations, “it’s not a drug that I like using, but it is beneficial for some patients and I do use it quite a bit.”
What EXPLORER-HCM does seem to show, Dr. Maron said, “is that the mechanism of action of the drug does seem to play out. It lowers gradients in a pretty reliable and powerful way, and that translates into clinical improvement in many patients. So it starts to support the idea that this drug and the class of drugs, myosin inhibitors, may represent another medical therapy option for symptomatic obstructive HCM.”
And, he pointed out, about one-fifth of patients with obstructive HCM don’t respond to disopyramide with fewer symptoms, and in others the drug “starts to lose efficacy over time.” So disopyramide has limitations, and EXPLORER-HCM “provides the possibility of an additional drug option.”
EXPLORER-HCM randomly assigned 251 adults with obstructive HCM in 13 countries to receive mavacamten, titrated from a starting dosage of 5 mg/day to a possible 15 mg/day or to placebo for 30 weeks.
The patients were required to have a peak LVOT gradient at least 50 mm Hg, a left ventricular ejection fraction (LVEF) of at least 55%, and symptoms indicating NYHA class 2 or 3; ultimately, 73% started the trial in NYHA class 2.
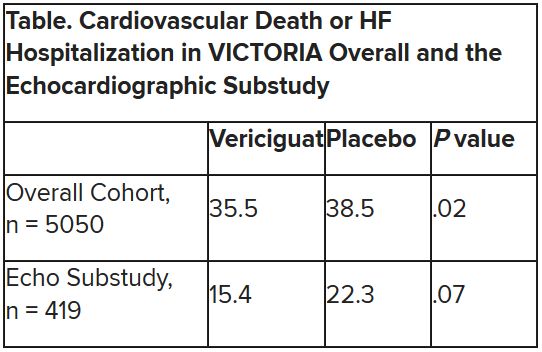
In the intention-to-treat analysis, 36.6% of patients receiving mavacamten and 17.2% of control patients met the composite primary endpoint (P = .0005), which consisted of either a 1.5–mL/kg per minute or greater improvement in peak oxygen consumption (pVO2) and at least a one-step reduction in NYHA functional class or at least a 3.0–mL/kg per minute pVO2 increase without deterioration in NYHA class, by week 30.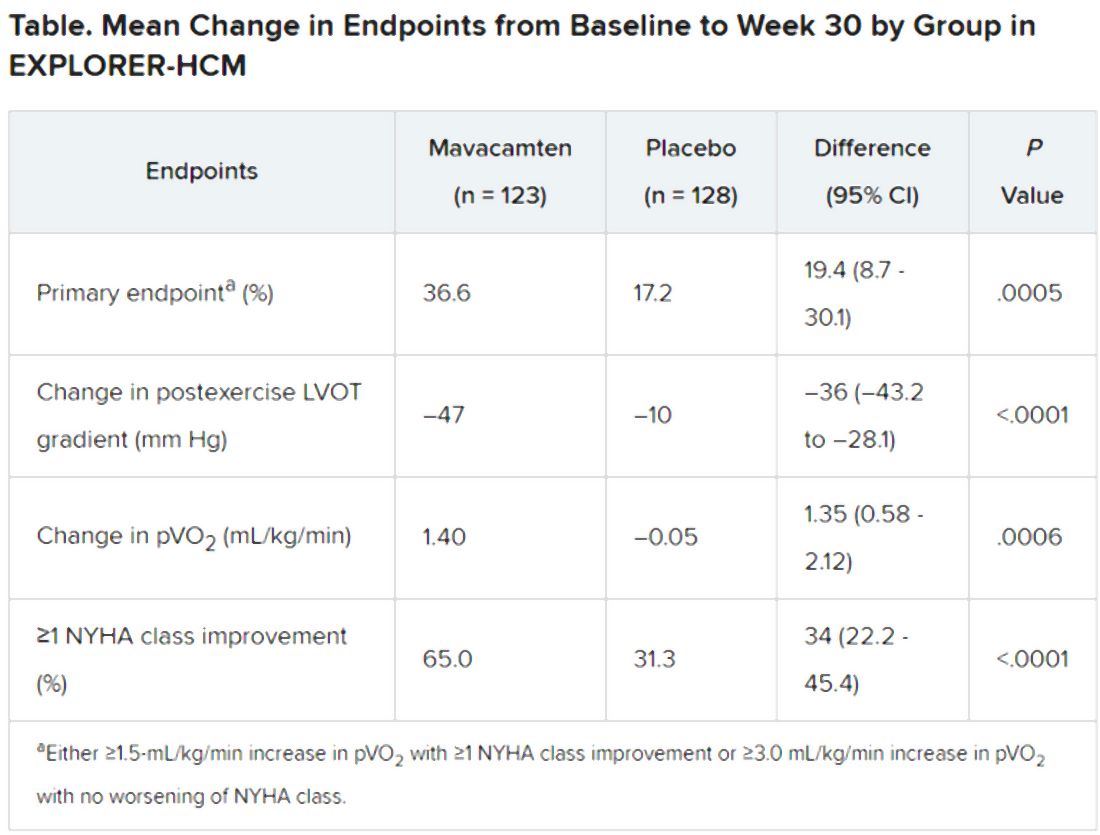
Patients receiving mavacamten also showed greater improvement in the individual endpoints of postexercise LVOT gradient, NYHA class, and two score-based symptom assessments – the Kansas City Cardiomyopathy Questionnaire-Clinical Summary and Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness-of-Breath domain – compared with control patients.
Safety and tolerability issues were similar in both groups, the reports notes. Ten patients in the mavacamten group reported 11 serious adverse events, compared with 20 such events reported by 11 patients in the control group.
“We can say from these results that mavacamten is a promising drug for symptom relief and functional class improvement associated with outflow gradient reduction in selected patients with hypertrophic obstructive cardiomyopathy,” and that, on the basis of this trial, it has potential as a drug of first choice, Franco Cecchi, MD, University of Florence (Italy), said as an invited discussant following Dr. Olivotto’s formal presentation of the trial.
Although serious adverse events were few, it was noteworthy that seven patients receiving mavacamten but only two patients receiving placebo showed LVEF reductions to below the 50% threshold during the trial, Dr. Cecchi observed. The LVEFs normalized once the drug was discontinued, but still, it may mean that mavacamten should be carefully uptitrated according to LVEF, he said.
Those LVEF reductions raise questions about “the reliability of being able to dose patients safely in the outpatient setting,” Dr. Maron said. “You have to ask, ‘Can this be extrapolated to the general practicing community without patients dropping their ejection fractions too much?’ ”
In addition, “we don’t have any idea about long-term efficacy for this drug, and we can draw very limited information about long-term safety here as well. That’s another other question mark,” Dr. Maron said.
“If I could have patients really become asymptomatic or mildly symptomatic without any surgery on a drug that is safe and can be taken for a prolonged period of time, that would be great,” Dr. Adler added. He noted that long-term follow-up of patients taking mavacamten in various trials has been underway and should help answer safety and efficacy questions about chronic therapy.
“Should mavacamten prove to be clinically effective and safe following long-term therapy in a larger and more diverse population, it would represent a much anticipated development in the treatment of hypertrophic cardiomyopathy,” the accompanying editorial states.
“Were the drug to realise its potential as a disease-modifying therapy in younger individuals, it would represent a great milestone in the area of inherited cardiomyopathies.”
MyoKardia funded EXPLORER-HCM. Dr. Olivotto discloses receiving grants from MyoKardia, Sanofi-Genzyme, Shire, Amicus, and Bayer; honoraria from Sanofi-Genzyme, Shire, and Bayer; and payments for consulting from MyoKardia. Disclosures for the other authors are in the report. Dr. Papadakis and the other editorialists report that they have no competing interests. Dr. Adler had no disclosures. Dr. Maron discloses consulting for and serving on a trial steering committee for Cytokinetics, sponsor of the 60-patient phase 2 placebo-controlled trial REDWOOD-HCM of patients with obstructive HCM treated with CK-3773274, a drug that works by a mechanism similar to that of mavacamten.
A version of this article originally appeared on Medscape.com.
An investigational drug that targets part of the molecular machinery underlying obstructive hypertrophic cardiomyopathy (HCM) can improve both symptoms and functional status in patients with the genetic disorder, suggests a placebo-controlled phase 3 trial.
Treatment with mavacamten (MyoKardia) worked partly by alleviating high-pressure gradients in the left ventricular outflow tract (LVOT), a key characteristic of obstructive HCM. Its effects appeared consistent across a wide range of objective and patient-assessed endpoints.
Mavacamten is “the first potential medical therapy addressing the underlying biology of symptoms in hypertrophic obstructive cardiomyopathy,” observed Iacopo Olivotto, MD, Careggi University Hospital, Florence, Italy.
Patients in the EXPLORER-HCM trial who took the new drug showed improvements in “every aspect of objective performance and subjective well-being,” Dr. Olivotto said at a preview for journalists before his formal online presentation of the results during the virtual European Society of Cardiology Congress 2020, staged in lieu of the traditional annual meeting because of the COVID-19 pandemic.
Dr. Olivotto, also lead author on the study’s same-day publication in The Lancet, was exuberant about the findings. “It is really hard to convey what this actually means for a scientific and clinical community that has spent over 60 years trying to understand and cure hypertrophic cardiomyopathy.”
MyoKardia released abbreviated top-line results of EXPLORER-HCM in May, which were reported by theheart.org | Medscape Cardiology at the time.
“I think it’s pretty exciting. We certainly need more and better drugs for this patient population,” Arnon Adler, MD, who is not associated with the trial but follows HCM at the Peter Munk Cardiac Centre, Toronto General Hospital, said in an interview.
The trial compared the new drug to placebo rather than full contemporary drug therapy for obstructive HCM, Adler cautioned, and had a fairly short follow-up time. But he was impressed that mavacamten’s apparent benefits seemed consistent not only for endpoints like change in New York Heart Association (NYHA) functional class and quality of life but also for more objective measures like peak VO2 and LVOT gradients.
“I think the results were promising across the board,” he told.
Unique mechanism of action
Mavacamten is described as a first-in-class, small-molecule, selective allosteric inhibitor of cardiac myosin adenosine triphosphatase that addresses the underlying pathophysiology of HCM by reducing actin–myosin cross-bridge formation. It thereby inhibits the excessive myocyte contractility that is a key mechanism of the disorder’s tell-tale hypertrophy, something the available HCM drug therapies don’t do.
Almost three-fourths of patients in the trial were initially in NYHA class 2. Such patients in practice tend to be treated pharmacologically, with more invasive but generally effective surgical myectomy and alcohol septal ablation performed more often for patients in NYHA class 3.
“In the EXPLORER-HCM trial, patients enrolled did not have any immediate indication for surgery,” although many of them in NYHA class 2 would likely progress to NYHA 3, Dr. Olivotto said in an interview.
Based on the trial, he said, it’s possible that mavacamten could lead to “earlier and broader treatment of obstruction symptoms in patients who would never have qualified for surgery in the first place because their symptoms may not be severe enough, but they are still limited.”
Notably, the published report notes, 27% of patients taking mavacamten achieved what was defined as a complete response – that is, a reduction of all LVOT gradients to less than 30 mm Hg in the total absence of symptoms.
Only 1% of patients in the placebo-treated control group met that goal, “showing that mavacamten might be capable of achieving marked relief of symptoms and LVOT obstruction,” the report states.
In the trial, “treatment with mavacamten led to clinically meaningful improvements in hemodynamic status, functional capacity, and subjective well-being in patients with obstructive hypertrophic cardiomyopathy,” agrees an editorial accompanying the EXPLORER-HCM publication.
Mavacamten might even compare favorably to surgery and ablative therapy, speculated the editorialists, Michael Papadakis, MBBS, MD, and colleagues of St. George’s University Hospitals NHS Foundation Trust, London. The drug appeared to reduce the peak LVOT gradient “to less than the guideline-based threshold for septal reduction therapy, 50 mm Hg, in 74% of patients, compared with 21% in the placebo group, indicating that mavacamten could represent a valid alternative to highly specialized invasive therapy,” they wrote.
Standard drug therapy
“There are approved drugs for obstructive hypertrophic cardiomyopathy, but they are ancient and were developed for other diseases,” observed Dr. Olivotto at the media briefing. Those drug options – primarily beta-blockers, nondihydropyridine calcium-channel blockers, and the sodium-channel blocker disopyramide – are often ineffective or cause onerous side effects, he said.
Notably in EXPLORER-HCM, patients in both the mavacamten and placebo groups could also be receiving beta-blockers and calcium-channel blockers, but no one in the trial could be receiving disopyramide, which can prolong the QT interval.
“By design,” mavacamten wasn’t compared to disopyramide, “a much more potent drug for lowering gradient and improving symptoms than beta-blockers or calcium-channel blockers,” said Martin S. Maron, MD, medical director at the Hypertrophic Cardiomyopathy Center and Research Institute, Tufts Medical Center, Boston.
Therefore, the trial’s results can’t be extrapolated to conclude that the new drug is superior to disopyramide “or the gold standard, surgical myectomy,” he said in an interview.
Dr. Adler agreed that observational studies suggest a benefit from disopyramide that may rival the apparent effect of mavacamten. “But of course, you can’t make direct comparisons because we never had a study like this for disopyramide.” Because it has many side effects and limitations, “it’s not a drug that I like using, but it is beneficial for some patients and I do use it quite a bit.”
What EXPLORER-HCM does seem to show, Dr. Maron said, “is that the mechanism of action of the drug does seem to play out. It lowers gradients in a pretty reliable and powerful way, and that translates into clinical improvement in many patients. So it starts to support the idea that this drug and the class of drugs, myosin inhibitors, may represent another medical therapy option for symptomatic obstructive HCM.”
And, he pointed out, about one-fifth of patients with obstructive HCM don’t respond to disopyramide with fewer symptoms, and in others the drug “starts to lose efficacy over time.” So disopyramide has limitations, and EXPLORER-HCM “provides the possibility of an additional drug option.”
EXPLORER-HCM randomly assigned 251 adults with obstructive HCM in 13 countries to receive mavacamten, titrated from a starting dosage of 5 mg/day to a possible 15 mg/day or to placebo for 30 weeks.
The patients were required to have a peak LVOT gradient at least 50 mm Hg, a left ventricular ejection fraction (LVEF) of at least 55%, and symptoms indicating NYHA class 2 or 3; ultimately, 73% started the trial in NYHA class 2.
In the intention-to-treat analysis, 36.6% of patients receiving mavacamten and 17.2% of control patients met the composite primary endpoint (P = .0005), which consisted of either a 1.5–mL/kg per minute or greater improvement in peak oxygen consumption (pVO2) and at least a one-step reduction in NYHA functional class or at least a 3.0–mL/kg per minute pVO2 increase without deterioration in NYHA class, by week 30.
Patients receiving mavacamten also showed greater improvement in the individual endpoints of postexercise LVOT gradient, NYHA class, and two score-based symptom assessments – the Kansas City Cardiomyopathy Questionnaire-Clinical Summary and Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness-of-Breath domain – compared with control patients.
Safety and tolerability issues were similar in both groups, the reports notes. Ten patients in the mavacamten group reported 11 serious adverse events, compared with 20 such events reported by 11 patients in the control group.
“We can say from these results that mavacamten is a promising drug for symptom relief and functional class improvement associated with outflow gradient reduction in selected patients with hypertrophic obstructive cardiomyopathy,” and that, on the basis of this trial, it has potential as a drug of first choice, Franco Cecchi, MD, University of Florence (Italy), said as an invited discussant following Dr. Olivotto’s formal presentation of the trial.
Although serious adverse events were few, it was noteworthy that seven patients receiving mavacamten but only two patients receiving placebo showed LVEF reductions to below the 50% threshold during the trial, Dr. Cecchi observed. The LVEFs normalized once the drug was discontinued, but still, it may mean that mavacamten should be carefully uptitrated according to LVEF, he said.
Those LVEF reductions raise questions about “the reliability of being able to dose patients safely in the outpatient setting,” Dr. Maron said. “You have to ask, ‘Can this be extrapolated to the general practicing community without patients dropping their ejection fractions too much?’ ”
In addition, “we don’t have any idea about long-term efficacy for this drug, and we can draw very limited information about long-term safety here as well. That’s another other question mark,” Dr. Maron said.
“If I could have patients really become asymptomatic or mildly symptomatic without any surgery on a drug that is safe and can be taken for a prolonged period of time, that would be great,” Dr. Adler added. He noted that long-term follow-up of patients taking mavacamten in various trials has been underway and should help answer safety and efficacy questions about chronic therapy.
“Should mavacamten prove to be clinically effective and safe following long-term therapy in a larger and more diverse population, it would represent a much anticipated development in the treatment of hypertrophic cardiomyopathy,” the accompanying editorial states.
“Were the drug to realise its potential as a disease-modifying therapy in younger individuals, it would represent a great milestone in the area of inherited cardiomyopathies.”
MyoKardia funded EXPLORER-HCM. Dr. Olivotto discloses receiving grants from MyoKardia, Sanofi-Genzyme, Shire, Amicus, and Bayer; honoraria from Sanofi-Genzyme, Shire, and Bayer; and payments for consulting from MyoKardia. Disclosures for the other authors are in the report. Dr. Papadakis and the other editorialists report that they have no competing interests. Dr. Adler had no disclosures. Dr. Maron discloses consulting for and serving on a trial steering committee for Cytokinetics, sponsor of the 60-patient phase 2 placebo-controlled trial REDWOOD-HCM of patients with obstructive HCM treated with CK-3773274, a drug that works by a mechanism similar to that of mavacamten.
A version of this article originally appeared on Medscape.com.
An investigational drug that targets part of the molecular machinery underlying obstructive hypertrophic cardiomyopathy (HCM) can improve both symptoms and functional status in patients with the genetic disorder, suggests a placebo-controlled phase 3 trial.
Treatment with mavacamten (MyoKardia) worked partly by alleviating high-pressure gradients in the left ventricular outflow tract (LVOT), a key characteristic of obstructive HCM. Its effects appeared consistent across a wide range of objective and patient-assessed endpoints.
Mavacamten is “the first potential medical therapy addressing the underlying biology of symptoms in hypertrophic obstructive cardiomyopathy,” observed Iacopo Olivotto, MD, Careggi University Hospital, Florence, Italy.
Patients in the EXPLORER-HCM trial who took the new drug showed improvements in “every aspect of objective performance and subjective well-being,” Dr. Olivotto said at a preview for journalists before his formal online presentation of the results during the virtual European Society of Cardiology Congress 2020, staged in lieu of the traditional annual meeting because of the COVID-19 pandemic.
Dr. Olivotto, also lead author on the study’s same-day publication in The Lancet, was exuberant about the findings. “It is really hard to convey what this actually means for a scientific and clinical community that has spent over 60 years trying to understand and cure hypertrophic cardiomyopathy.”
MyoKardia released abbreviated top-line results of EXPLORER-HCM in May, which were reported by theheart.org | Medscape Cardiology at the time.
“I think it’s pretty exciting. We certainly need more and better drugs for this patient population,” Arnon Adler, MD, who is not associated with the trial but follows HCM at the Peter Munk Cardiac Centre, Toronto General Hospital, said in an interview.
The trial compared the new drug to placebo rather than full contemporary drug therapy for obstructive HCM, Adler cautioned, and had a fairly short follow-up time. But he was impressed that mavacamten’s apparent benefits seemed consistent not only for endpoints like change in New York Heart Association (NYHA) functional class and quality of life but also for more objective measures like peak VO2 and LVOT gradients.
“I think the results were promising across the board,” he told.
Unique mechanism of action
Mavacamten is described as a first-in-class, small-molecule, selective allosteric inhibitor of cardiac myosin adenosine triphosphatase that addresses the underlying pathophysiology of HCM by reducing actin–myosin cross-bridge formation. It thereby inhibits the excessive myocyte contractility that is a key mechanism of the disorder’s tell-tale hypertrophy, something the available HCM drug therapies don’t do.
Almost three-fourths of patients in the trial were initially in NYHA class 2. Such patients in practice tend to be treated pharmacologically, with more invasive but generally effective surgical myectomy and alcohol septal ablation performed more often for patients in NYHA class 3.
“In the EXPLORER-HCM trial, patients enrolled did not have any immediate indication for surgery,” although many of them in NYHA class 2 would likely progress to NYHA 3, Dr. Olivotto said in an interview.
Based on the trial, he said, it’s possible that mavacamten could lead to “earlier and broader treatment of obstruction symptoms in patients who would never have qualified for surgery in the first place because their symptoms may not be severe enough, but they are still limited.”
Notably, the published report notes, 27% of patients taking mavacamten achieved what was defined as a complete response – that is, a reduction of all LVOT gradients to less than 30 mm Hg in the total absence of symptoms.
Only 1% of patients in the placebo-treated control group met that goal, “showing that mavacamten might be capable of achieving marked relief of symptoms and LVOT obstruction,” the report states.
In the trial, “treatment with mavacamten led to clinically meaningful improvements in hemodynamic status, functional capacity, and subjective well-being in patients with obstructive hypertrophic cardiomyopathy,” agrees an editorial accompanying the EXPLORER-HCM publication.
Mavacamten might even compare favorably to surgery and ablative therapy, speculated the editorialists, Michael Papadakis, MBBS, MD, and colleagues of St. George’s University Hospitals NHS Foundation Trust, London. The drug appeared to reduce the peak LVOT gradient “to less than the guideline-based threshold for septal reduction therapy, 50 mm Hg, in 74% of patients, compared with 21% in the placebo group, indicating that mavacamten could represent a valid alternative to highly specialized invasive therapy,” they wrote.
Standard drug therapy
“There are approved drugs for obstructive hypertrophic cardiomyopathy, but they are ancient and were developed for other diseases,” observed Dr. Olivotto at the media briefing. Those drug options – primarily beta-blockers, nondihydropyridine calcium-channel blockers, and the sodium-channel blocker disopyramide – are often ineffective or cause onerous side effects, he said.
Notably in EXPLORER-HCM, patients in both the mavacamten and placebo groups could also be receiving beta-blockers and calcium-channel blockers, but no one in the trial could be receiving disopyramide, which can prolong the QT interval.
“By design,” mavacamten wasn’t compared to disopyramide, “a much more potent drug for lowering gradient and improving symptoms than beta-blockers or calcium-channel blockers,” said Martin S. Maron, MD, medical director at the Hypertrophic Cardiomyopathy Center and Research Institute, Tufts Medical Center, Boston.
Therefore, the trial’s results can’t be extrapolated to conclude that the new drug is superior to disopyramide “or the gold standard, surgical myectomy,” he said in an interview.
Dr. Adler agreed that observational studies suggest a benefit from disopyramide that may rival the apparent effect of mavacamten. “But of course, you can’t make direct comparisons because we never had a study like this for disopyramide.” Because it has many side effects and limitations, “it’s not a drug that I like using, but it is beneficial for some patients and I do use it quite a bit.”
What EXPLORER-HCM does seem to show, Dr. Maron said, “is that the mechanism of action of the drug does seem to play out. It lowers gradients in a pretty reliable and powerful way, and that translates into clinical improvement in many patients. So it starts to support the idea that this drug and the class of drugs, myosin inhibitors, may represent another medical therapy option for symptomatic obstructive HCM.”
And, he pointed out, about one-fifth of patients with obstructive HCM don’t respond to disopyramide with fewer symptoms, and in others the drug “starts to lose efficacy over time.” So disopyramide has limitations, and EXPLORER-HCM “provides the possibility of an additional drug option.”
EXPLORER-HCM randomly assigned 251 adults with obstructive HCM in 13 countries to receive mavacamten, titrated from a starting dosage of 5 mg/day to a possible 15 mg/day or to placebo for 30 weeks.
The patients were required to have a peak LVOT gradient at least 50 mm Hg, a left ventricular ejection fraction (LVEF) of at least 55%, and symptoms indicating NYHA class 2 or 3; ultimately, 73% started the trial in NYHA class 2.
In the intention-to-treat analysis, 36.6% of patients receiving mavacamten and 17.2% of control patients met the composite primary endpoint (P = .0005), which consisted of either a 1.5–mL/kg per minute or greater improvement in peak oxygen consumption (pVO2) and at least a one-step reduction in NYHA functional class or at least a 3.0–mL/kg per minute pVO2 increase without deterioration in NYHA class, by week 30.
Patients receiving mavacamten also showed greater improvement in the individual endpoints of postexercise LVOT gradient, NYHA class, and two score-based symptom assessments – the Kansas City Cardiomyopathy Questionnaire-Clinical Summary and Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness-of-Breath domain – compared with control patients.
Safety and tolerability issues were similar in both groups, the reports notes. Ten patients in the mavacamten group reported 11 serious adverse events, compared with 20 such events reported by 11 patients in the control group.
“We can say from these results that mavacamten is a promising drug for symptom relief and functional class improvement associated with outflow gradient reduction in selected patients with hypertrophic obstructive cardiomyopathy,” and that, on the basis of this trial, it has potential as a drug of first choice, Franco Cecchi, MD, University of Florence (Italy), said as an invited discussant following Dr. Olivotto’s formal presentation of the trial.
Although serious adverse events were few, it was noteworthy that seven patients receiving mavacamten but only two patients receiving placebo showed LVEF reductions to below the 50% threshold during the trial, Dr. Cecchi observed. The LVEFs normalized once the drug was discontinued, but still, it may mean that mavacamten should be carefully uptitrated according to LVEF, he said.
Those LVEF reductions raise questions about “the reliability of being able to dose patients safely in the outpatient setting,” Dr. Maron said. “You have to ask, ‘Can this be extrapolated to the general practicing community without patients dropping their ejection fractions too much?’ ”
In addition, “we don’t have any idea about long-term efficacy for this drug, and we can draw very limited information about long-term safety here as well. That’s another other question mark,” Dr. Maron said.
“If I could have patients really become asymptomatic or mildly symptomatic without any surgery on a drug that is safe and can be taken for a prolonged period of time, that would be great,” Dr. Adler added. He noted that long-term follow-up of patients taking mavacamten in various trials has been underway and should help answer safety and efficacy questions about chronic therapy.
“Should mavacamten prove to be clinically effective and safe following long-term therapy in a larger and more diverse population, it would represent a much anticipated development in the treatment of hypertrophic cardiomyopathy,” the accompanying editorial states.
“Were the drug to realise its potential as a disease-modifying therapy in younger individuals, it would represent a great milestone in the area of inherited cardiomyopathies.”
MyoKardia funded EXPLORER-HCM. Dr. Olivotto discloses receiving grants from MyoKardia, Sanofi-Genzyme, Shire, Amicus, and Bayer; honoraria from Sanofi-Genzyme, Shire, and Bayer; and payments for consulting from MyoKardia. Disclosures for the other authors are in the report. Dr. Papadakis and the other editorialists report that they have no competing interests. Dr. Adler had no disclosures. Dr. Maron discloses consulting for and serving on a trial steering committee for Cytokinetics, sponsor of the 60-patient phase 2 placebo-controlled trial REDWOOD-HCM of patients with obstructive HCM treated with CK-3773274, a drug that works by a mechanism similar to that of mavacamten.
A version of this article originally appeared on Medscape.com.
Vast underdiagnosis of monogenic CV disease seen in cath referrals
Monogenic disorders with heart and vascular effects are each pretty rare in clinical practice but collectively can make up a fair proportion of the patients cardiologists see. Still, the diagnosis is missed more often than not, even when the clinical signs are there, suggests an observational study, supporting broader genetic testing in cardiovascular patients.
In a cohort of more than 8,000 patients referred for cardiac catheterization, diagnosis of such a monogenic cardiovascular disease (MCVD) was made in only 35% of those with one related gene variant and signs of phenotypic expression in the electronic health record.
The findings are novel for measuring the field’s “burden of missed diagnoses” in patients with MCVD, which “represent a missed opportunity that could be addressed by genetic screening,” contended the study report, published in the Aug. 18 issue of the Journal of the American College of Cardiology.
“The underrecognition of these diseases underscores the importance of including monogenic diseases in the treating physician’s differential diagnosis,” say the authors, led by Jawan W. Abdulrahim, MD, Duke University, Durham, N.C.
Diagnosis of MCVDs can be important, the group wrote, because many, including familial transthyretin amyloidosis (TTR) and other disorders that pose an increased risk for sudden death, have evidence-based treatment modalities available or are clinically actionable. “Identification of patients with MCVD variants” is also “important for cascade screening of family members who are at risk of inheriting the pathogenic mutations.”
“We tend to ignore these monogenic diseases because they are so rare individually but, in aggregate, monogenic diseases are actually quite common,” senior author Svati H. Shah, MD, MHS, also of Duke University, said in an interview.
The results “support that the cardiology community over time adopt a genotype-forward approach,” one in which every patient presenting to a cardiovascular clinic is genotyped, she said.
One implication of such an approach, Dr. Shah agreed, is that “we would be able to pick these people up earlier in their disease, especially in the context of therapies that could improve certainly their progression, but even their survival.”
For now, she said, the study suggests that “these disorders are more frequent than perhaps all cardiologists are aware of, and we just need to keep our eyes open and know when to refer patients to a cardiovascular genetics clinic, which maybe has more time and can deal with all the nuances that go along with genetic testing.”
In the total cohort, 4.5% were found to carry a gene variant known or believed to cause such diseases. The most frequently represented conditions were familial TTR, hereditary hemochromatosis, heterozygous familial hypercholesterolemia, and various cardiomyopathies.
Of those patients, 52 received a clinical diagnosis of the monogenic disorder after an EHR review. Of the 290 without such a diagnosis, two-thirds showed no evidence in their EHR of the variant’s phenotypic signs. But the records of the other third featured at least some signs that the disease had manifested clinically.
“These data serve as a reminder that monogenic Mendelian disease, including heart and vascular disease, varies in penetrance from individual to individual and may not always present with clinically detectable phenotypes,” noted an editorial accompanying the report.
They also “provide a compelling basis for expanding the role of targeted genetic testing in patients with more traditional forms of heart and vascular disease,” wrote Scott M. Damrauer, MD, University of Pennsylvania, Philadelphia, and William S. Weintraub, MD, Medstar Washington Hospital Center and Georgetown University, Washington.
“Based on the current report, the number needed to screen in a complex cardiovascular patient population to detect 1 case of undiagnosed monogenic cardiovascular disease is 85,” they wrote. “This places targeted genetic testing well within what is considered to be efficacious for most screening programs and in the range of that of other common cardiovascular diseases and cancers.”
Among the 342 patients with a variant predicting a single MCVD – in addition to the 52 who received a diagnosis – 193 had records with no indication of phenotypic expression and so did not receive a diagnosis.
But the 97 patients without a diagnosis who nevertheless had documented signs of some phenotypic expression were deemed, on the basis of extent of expression, to represent a possibly, probably, or definitely missed diagnosis.
Familial TTR made up about 45% of such potentially missed diagnoses, the report noted.
Broader screening of patients with cardiovascular disease, Dr. Shah speculated, “might actually be not only a clinically useful endeavor, but – when we think about the aggregate burden of these monogenic disorders – potentially even cost-effective.”
As the price of genetic sequencing drops, she said, “I think we’ll start to see even more health systems wanting to incorporate the genotype-forward approach.”
Dr. Shah reports serving as primary investigator for research sponsored by Verily Life Sciences and AstraZeneca. Dr. Abdulrahim reports no relevant relationships. Disclosures for the other authors are in the report. Dr. Damrauer discloses receiving research support from RenalytixAI and consulting fees from Calico Labs. Dr. Weintraub had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Monogenic disorders with heart and vascular effects are each pretty rare in clinical practice but collectively can make up a fair proportion of the patients cardiologists see. Still, the diagnosis is missed more often than not, even when the clinical signs are there, suggests an observational study, supporting broader genetic testing in cardiovascular patients.
In a cohort of more than 8,000 patients referred for cardiac catheterization, diagnosis of such a monogenic cardiovascular disease (MCVD) was made in only 35% of those with one related gene variant and signs of phenotypic expression in the electronic health record.
The findings are novel for measuring the field’s “burden of missed diagnoses” in patients with MCVD, which “represent a missed opportunity that could be addressed by genetic screening,” contended the study report, published in the Aug. 18 issue of the Journal of the American College of Cardiology.
“The underrecognition of these diseases underscores the importance of including monogenic diseases in the treating physician’s differential diagnosis,” say the authors, led by Jawan W. Abdulrahim, MD, Duke University, Durham, N.C.
Diagnosis of MCVDs can be important, the group wrote, because many, including familial transthyretin amyloidosis (TTR) and other disorders that pose an increased risk for sudden death, have evidence-based treatment modalities available or are clinically actionable. “Identification of patients with MCVD variants” is also “important for cascade screening of family members who are at risk of inheriting the pathogenic mutations.”
“We tend to ignore these monogenic diseases because they are so rare individually but, in aggregate, monogenic diseases are actually quite common,” senior author Svati H. Shah, MD, MHS, also of Duke University, said in an interview.
The results “support that the cardiology community over time adopt a genotype-forward approach,” one in which every patient presenting to a cardiovascular clinic is genotyped, she said.
One implication of such an approach, Dr. Shah agreed, is that “we would be able to pick these people up earlier in their disease, especially in the context of therapies that could improve certainly their progression, but even their survival.”
For now, she said, the study suggests that “these disorders are more frequent than perhaps all cardiologists are aware of, and we just need to keep our eyes open and know when to refer patients to a cardiovascular genetics clinic, which maybe has more time and can deal with all the nuances that go along with genetic testing.”
In the total cohort, 4.5% were found to carry a gene variant known or believed to cause such diseases. The most frequently represented conditions were familial TTR, hereditary hemochromatosis, heterozygous familial hypercholesterolemia, and various cardiomyopathies.
Of those patients, 52 received a clinical diagnosis of the monogenic disorder after an EHR review. Of the 290 without such a diagnosis, two-thirds showed no evidence in their EHR of the variant’s phenotypic signs. But the records of the other third featured at least some signs that the disease had manifested clinically.
“These data serve as a reminder that monogenic Mendelian disease, including heart and vascular disease, varies in penetrance from individual to individual and may not always present with clinically detectable phenotypes,” noted an editorial accompanying the report.
They also “provide a compelling basis for expanding the role of targeted genetic testing in patients with more traditional forms of heart and vascular disease,” wrote Scott M. Damrauer, MD, University of Pennsylvania, Philadelphia, and William S. Weintraub, MD, Medstar Washington Hospital Center and Georgetown University, Washington.
“Based on the current report, the number needed to screen in a complex cardiovascular patient population to detect 1 case of undiagnosed monogenic cardiovascular disease is 85,” they wrote. “This places targeted genetic testing well within what is considered to be efficacious for most screening programs and in the range of that of other common cardiovascular diseases and cancers.”
Among the 342 patients with a variant predicting a single MCVD – in addition to the 52 who received a diagnosis – 193 had records with no indication of phenotypic expression and so did not receive a diagnosis.
But the 97 patients without a diagnosis who nevertheless had documented signs of some phenotypic expression were deemed, on the basis of extent of expression, to represent a possibly, probably, or definitely missed diagnosis.
Familial TTR made up about 45% of such potentially missed diagnoses, the report noted.
Broader screening of patients with cardiovascular disease, Dr. Shah speculated, “might actually be not only a clinically useful endeavor, but – when we think about the aggregate burden of these monogenic disorders – potentially even cost-effective.”
As the price of genetic sequencing drops, she said, “I think we’ll start to see even more health systems wanting to incorporate the genotype-forward approach.”
Dr. Shah reports serving as primary investigator for research sponsored by Verily Life Sciences and AstraZeneca. Dr. Abdulrahim reports no relevant relationships. Disclosures for the other authors are in the report. Dr. Damrauer discloses receiving research support from RenalytixAI and consulting fees from Calico Labs. Dr. Weintraub had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Monogenic disorders with heart and vascular effects are each pretty rare in clinical practice but collectively can make up a fair proportion of the patients cardiologists see. Still, the diagnosis is missed more often than not, even when the clinical signs are there, suggests an observational study, supporting broader genetic testing in cardiovascular patients.
In a cohort of more than 8,000 patients referred for cardiac catheterization, diagnosis of such a monogenic cardiovascular disease (MCVD) was made in only 35% of those with one related gene variant and signs of phenotypic expression in the electronic health record.
The findings are novel for measuring the field’s “burden of missed diagnoses” in patients with MCVD, which “represent a missed opportunity that could be addressed by genetic screening,” contended the study report, published in the Aug. 18 issue of the Journal of the American College of Cardiology.
“The underrecognition of these diseases underscores the importance of including monogenic diseases in the treating physician’s differential diagnosis,” say the authors, led by Jawan W. Abdulrahim, MD, Duke University, Durham, N.C.
Diagnosis of MCVDs can be important, the group wrote, because many, including familial transthyretin amyloidosis (TTR) and other disorders that pose an increased risk for sudden death, have evidence-based treatment modalities available or are clinically actionable. “Identification of patients with MCVD variants” is also “important for cascade screening of family members who are at risk of inheriting the pathogenic mutations.”
“We tend to ignore these monogenic diseases because they are so rare individually but, in aggregate, monogenic diseases are actually quite common,” senior author Svati H. Shah, MD, MHS, also of Duke University, said in an interview.
The results “support that the cardiology community over time adopt a genotype-forward approach,” one in which every patient presenting to a cardiovascular clinic is genotyped, she said.
One implication of such an approach, Dr. Shah agreed, is that “we would be able to pick these people up earlier in their disease, especially in the context of therapies that could improve certainly their progression, but even their survival.”
For now, she said, the study suggests that “these disorders are more frequent than perhaps all cardiologists are aware of, and we just need to keep our eyes open and know when to refer patients to a cardiovascular genetics clinic, which maybe has more time and can deal with all the nuances that go along with genetic testing.”
In the total cohort, 4.5% were found to carry a gene variant known or believed to cause such diseases. The most frequently represented conditions were familial TTR, hereditary hemochromatosis, heterozygous familial hypercholesterolemia, and various cardiomyopathies.
Of those patients, 52 received a clinical diagnosis of the monogenic disorder after an EHR review. Of the 290 without such a diagnosis, two-thirds showed no evidence in their EHR of the variant’s phenotypic signs. But the records of the other third featured at least some signs that the disease had manifested clinically.
“These data serve as a reminder that monogenic Mendelian disease, including heart and vascular disease, varies in penetrance from individual to individual and may not always present with clinically detectable phenotypes,” noted an editorial accompanying the report.
They also “provide a compelling basis for expanding the role of targeted genetic testing in patients with more traditional forms of heart and vascular disease,” wrote Scott M. Damrauer, MD, University of Pennsylvania, Philadelphia, and William S. Weintraub, MD, Medstar Washington Hospital Center and Georgetown University, Washington.
“Based on the current report, the number needed to screen in a complex cardiovascular patient population to detect 1 case of undiagnosed monogenic cardiovascular disease is 85,” they wrote. “This places targeted genetic testing well within what is considered to be efficacious for most screening programs and in the range of that of other common cardiovascular diseases and cancers.”
Among the 342 patients with a variant predicting a single MCVD – in addition to the 52 who received a diagnosis – 193 had records with no indication of phenotypic expression and so did not receive a diagnosis.
But the 97 patients without a diagnosis who nevertheless had documented signs of some phenotypic expression were deemed, on the basis of extent of expression, to represent a possibly, probably, or definitely missed diagnosis.
Familial TTR made up about 45% of such potentially missed diagnoses, the report noted.
Broader screening of patients with cardiovascular disease, Dr. Shah speculated, “might actually be not only a clinically useful endeavor, but – when we think about the aggregate burden of these monogenic disorders – potentially even cost-effective.”
As the price of genetic sequencing drops, she said, “I think we’ll start to see even more health systems wanting to incorporate the genotype-forward approach.”
Dr. Shah reports serving as primary investigator for research sponsored by Verily Life Sciences and AstraZeneca. Dr. Abdulrahim reports no relevant relationships. Disclosures for the other authors are in the report. Dr. Damrauer discloses receiving research support from RenalytixAI and consulting fees from Calico Labs. Dr. Weintraub had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
All NSAIDs raise post-MI risk but some are safer than others: Next chapter
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Heart damage even after COVID-19 ‘recovery’ evokes specter of later heart failure
Evidence that the heart can take a major hit in patients hospitalized with COVID-19, especially those already with cardiovascular disease (CV) or its risk factors, has been sadly apparent from the pandemic’s earliest days.
Less clear from case studies and small series to date has been whether SARS-CoV-2 directly attacks the heart and whether acute cardiac effects of the illness may lead to some kind of lingering cardiomyopathy.
The field’s grasp of those issues advanced a bit in two new reports published July 27 in JAMA Cardiology that seem to validate concerns the virus can infect the myocardium, without necessarily causing myocarditis and the possibility that some “recovered” patients may be left with persisting myocardial injury and inflammation that potentially could later manifest as heart failure.
Persisting inflammation by cardiac magnetic resonance
A prospective cohort study with 100 patients recovered from a recent bout of the disease showed evidence of ventricular dysfunction, greater ventricular mass, and in 78% of the cohort, signs of myocardial inflammation by cardiac magnetic resonance (CMR) imaging. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
Two-thirds of the cohort, whose acute COVID-19 severity had “ranged from asymptomatic to minor-to-moderate symptoms,” had recovered at home, whereas the remaining “severely unwell patients” had been hospitalized, wrote the authors, led by Valentina O. Püntmann, MD, PhD, University Hospital Frankfurt (Germany).
None of the patients had a history of heart failure or cardiomyopathy, although some had hypertension, diabetes, or evidence of coronary disease.
“Our findings demonstrate that participants with a relative paucity of preexisting cardiovascular condition and with mostly home-based recovery had frequent cardiac inflammatory involvement, which was similar to the hospitalized subgroup with regards to severity and extent,” the group noted.
“There is a considerable ongoing myocardial inflammation in the heart muscle weeks after recovery from COVID-19 illness. This finding is important because it may herald a considerable burden of heart failure in a few years down the line,” Dr. Püntmann said in an interview.
Early diagnosis would offer “a good chance that early treatment could reduce the relentless course of inflammatory damage or even halt it,” she said.
“The relatively clear onset of COVID-19 illness provides an opportunity, which we often do not have with other conditions, to take a proactive action and to look for heart involvement early, within a few weeks of recovery.”
The study’s CMR evidence of inflammation edema, scarring, and pericardial effusion are among “the major diagnostic criteria for inflammatory and viral myocarditis,” observed Biykem Bozkurt, MD, PhD, from Baylor College of Medicine, Houston, who wasn’t part of either new study.
The findings suggest – consistent with previous evidence – that some patients with recent COVID-19 may be left with ongoing myocardial inflammation, and this study further adds that it could potentially become subacute or even chronic and in some may not be totally reversible, she said in an interview. How long the effects are likely to persist “remains to be determined. We need longer-term outcomes data.”
Viral presence without myocarditis
The accompanying report featured a postmortem analysis of hearts from 39 patients with mostly severe COVID-19 that pointed to a significant SARS-CoV-2 presence and signs that the virus vigorously replicated in the myocardium.
But there was no evidence that the infection led to fulminant myocarditis. Rather, the virus had apparently infiltrated the heart by localizing in interstitial cells or in macrophages that took up in the myocardium without actually entering myocytes, concluded the report’s authors, led by Diana Lindner, PhD, from the University Heart and Vascular Centre, Hamburg (Germany).
The findings suggest “that the presence of SARS-CoV-2 in cardiac tissue does not necessarily cause an inflammatory reaction consistent with clinical myocarditis,” the group wrote.
Previously in the literature, in “cases in which myocardial inflammation was present, there was also evidence of clinical myocarditis, and therefore the current cases underlie a different pathophysiology,” they concluded.
No evidence of the virus was seen in 15 cases, about 61% of the group. In 16 of the remaining 24 hearts, the viral load exceeded 1,000 copies per mcg of RNA, a substantial presence. Those 16 showed increased expression of inflammatory cytokines but no inflammatory cell infiltrates or changes in leukocyte counts, the researchers noted.
“Findings of suggested viral replication in the cases with a very high viral load are showing that we need to do more studies to find out long-term consequences, which we do not know right now,” senior author Dirk Westermann, MD, also from the University Heart and Vascular Centre, Hamburg, said.
Implications for heart failure
The postmortem findings from Dr. Lindner and associates “provide intriguing evidence that COVID-19 is associated with at least some component of myocardial injury, perhaps as the result of direct viral infection of the heart,” wrote Clyde W. Yancy, MD, MSc, from Northwestern University, Chicago, and Gregg C. Fonarow, MD, from the University of California, Los Angeles, in an editorial accompanying both reports.
The CMR study from Dr. Püntmann and colleagues – on the backdrop of earlier COVID-19 observations – suggests the potential for “residual left ventricular dysfunction and ongoing inflammation” in the months following a COVID-19 diagnosis. Both developments may be “of sufficient concern to represent a nidus for new-onset heart failure and other cardiovascular complications,” contend Dr. Yancy and Dr. Fonarow.
“When added to the postmortem pathological findings from Lindner et al, we see the plot thickening and we are inclined to raise a new and very evident concern that cardiomyopathy and heart failure related to COVID-19 may potentially evolve as the natural history of this infection becomes clearer,” they wrote.
Some patients, having recovered from the acute illness, may be left with a chronic inflammatory state that probably puts them at increased risk for future heart failure, agreed Dr. Bozkurt when interviewed. “They could show further decline in cardiac function, and their recovery might take longer than with the usual viral illnesses that we see,” she said.
“There could also be a risk of sudden death. Inflammation sometimes gives rise to sudden death and ventricular arrhythmia, which I would be very worried about, especially if the myocardium is stressed,” Dr. Bozkurt said. “So competitive sports in those patients potentially could be risky.”
COVID-19 cohort vs. matched control subjects
The CMR study from Dr. Püntmann and colleagues prospectively entered 100 patients recently recovered from an acute bout of COVID-19, either at home or at a hospital, who were followed in a registry based at University Hospital Frankfurt. Their median age was 49 years; 47% were female. They were compared with 50 age- and sex-matched control patients and 50 apparently healthy volunteers matched for risk factors, the group noted.
On the same day as the CMR assessment, the recently recovered patients, compared with the healthy control subjects and risk-factor matched control subjects, respectively, showed (P ≤ .001 in each case):
- A reduced left ventricular (LV) ejection fraction: 56% vs. 60% and 61%.
- A higher LV end-diastolic volume index: 86 mL/m2 vs. 80 mL/m2 and 75 mL/m2.
- A greater LV mass index: 51 g/m2 vs. 47 g/m2 and 53 g/m2.
- A higher hs-TnT level: 5.6 pg/mL vs. 3.2 pg/mL and 3.9 pg/mL.
- A greater prevalence of hs-TnT levels 3 pg/mL or more: 71% vs. 11% and 31%.
At CMR, 78% of the recovered COVID-19 patients showed abnormalities that included raised myocardial native T1 and T2 mapping, which is suggestive of fibrosis and edema from inflammation, compared with the two control groups (P < .001 for all differences), “independent of preexisting conditions, severity and overall course of the acute illness, and the time from the original diagnosis,” the group wrote. Native T1 and T2 mapping correlated significantly with hs-TnT.
“We now have the diagnostic means to detect cardiac inflammation early, and we need make every effort to apply them in every day practice,”Dr. Püntmann said in the interview.
“Using cardiac MRI will allow us to raise our game against COVID-19 and proactively develop efficient cardioprotective treatments,” she said. “Until we have effective means of protecting from the infection, that is vaccination, we must act swiftly and within the means at hand.”
The analysis evokes several other ways patients with COVID-19 might be screened for significant myocardial involvement.
“Strategies could include checking troponins, not only at admission but maybe at discharge and perhaps even those individuals who are at home and are not necessarily requiring care,” Dr. Bozkurt said.
“Biomarker profiling and screening for ongoing inflammation probably are going to be important components of COVID-19, especially for those with subclinical risk and disease.”
Dr. Westermann proposed that troponin elevations at discharge “might be a good starting point” for selecting COVID-19 patients for functional testing or imaging to screen for cardiac sequelae. Performing such tests routinely now “would be overwhelming given the massive increase in patients we still see today.”
Dr. Püntmann had no disclosures; statements of potential conflict for the other authors are in the report. Dr. Bozkurt has previously disclosed receiving consultant fees or honoraria from Bayer Healthcare, Bristol-Myers Squibb, Lantheus Medical Imaging, and Respicardia; serving on a data safety monitoring board for LivaNova USA ; and having unspecified relationships with Abbott Laboratories. Dr. Lindner had no disclosures; Dr. Westermann reported receiving personal fees from AstraZeneca, Bayer, Novartis, and Medtronic. Dr. Yancy is a deputy editor and Dr. Fonarow a section editor for JAMA Cardiology. Dr. Yancy had no other disclosures. Dr. Fonarow reported receiving personal fees from Abbott Laboratories, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards Lifesciences, Janssen, Medtronic, Merck, and Novartis.
A version of this article originally appeared on Medscape.com.
Evidence that the heart can take a major hit in patients hospitalized with COVID-19, especially those already with cardiovascular disease (CV) or its risk factors, has been sadly apparent from the pandemic’s earliest days.
Less clear from case studies and small series to date has been whether SARS-CoV-2 directly attacks the heart and whether acute cardiac effects of the illness may lead to some kind of lingering cardiomyopathy.
The field’s grasp of those issues advanced a bit in two new reports published July 27 in JAMA Cardiology that seem to validate concerns the virus can infect the myocardium, without necessarily causing myocarditis and the possibility that some “recovered” patients may be left with persisting myocardial injury and inflammation that potentially could later manifest as heart failure.
Persisting inflammation by cardiac magnetic resonance
A prospective cohort study with 100 patients recovered from a recent bout of the disease showed evidence of ventricular dysfunction, greater ventricular mass, and in 78% of the cohort, signs of myocardial inflammation by cardiac magnetic resonance (CMR) imaging. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
Two-thirds of the cohort, whose acute COVID-19 severity had “ranged from asymptomatic to minor-to-moderate symptoms,” had recovered at home, whereas the remaining “severely unwell patients” had been hospitalized, wrote the authors, led by Valentina O. Püntmann, MD, PhD, University Hospital Frankfurt (Germany).
None of the patients had a history of heart failure or cardiomyopathy, although some had hypertension, diabetes, or evidence of coronary disease.
“Our findings demonstrate that participants with a relative paucity of preexisting cardiovascular condition and with mostly home-based recovery had frequent cardiac inflammatory involvement, which was similar to the hospitalized subgroup with regards to severity and extent,” the group noted.
“There is a considerable ongoing myocardial inflammation in the heart muscle weeks after recovery from COVID-19 illness. This finding is important because it may herald a considerable burden of heart failure in a few years down the line,” Dr. Püntmann said in an interview.
Early diagnosis would offer “a good chance that early treatment could reduce the relentless course of inflammatory damage or even halt it,” she said.
“The relatively clear onset of COVID-19 illness provides an opportunity, which we often do not have with other conditions, to take a proactive action and to look for heart involvement early, within a few weeks of recovery.”
The study’s CMR evidence of inflammation edema, scarring, and pericardial effusion are among “the major diagnostic criteria for inflammatory and viral myocarditis,” observed Biykem Bozkurt, MD, PhD, from Baylor College of Medicine, Houston, who wasn’t part of either new study.
The findings suggest – consistent with previous evidence – that some patients with recent COVID-19 may be left with ongoing myocardial inflammation, and this study further adds that it could potentially become subacute or even chronic and in some may not be totally reversible, she said in an interview. How long the effects are likely to persist “remains to be determined. We need longer-term outcomes data.”
Viral presence without myocarditis
The accompanying report featured a postmortem analysis of hearts from 39 patients with mostly severe COVID-19 that pointed to a significant SARS-CoV-2 presence and signs that the virus vigorously replicated in the myocardium.
But there was no evidence that the infection led to fulminant myocarditis. Rather, the virus had apparently infiltrated the heart by localizing in interstitial cells or in macrophages that took up in the myocardium without actually entering myocytes, concluded the report’s authors, led by Diana Lindner, PhD, from the University Heart and Vascular Centre, Hamburg (Germany).
The findings suggest “that the presence of SARS-CoV-2 in cardiac tissue does not necessarily cause an inflammatory reaction consistent with clinical myocarditis,” the group wrote.
Previously in the literature, in “cases in which myocardial inflammation was present, there was also evidence of clinical myocarditis, and therefore the current cases underlie a different pathophysiology,” they concluded.
No evidence of the virus was seen in 15 cases, about 61% of the group. In 16 of the remaining 24 hearts, the viral load exceeded 1,000 copies per mcg of RNA, a substantial presence. Those 16 showed increased expression of inflammatory cytokines but no inflammatory cell infiltrates or changes in leukocyte counts, the researchers noted.
“Findings of suggested viral replication in the cases with a very high viral load are showing that we need to do more studies to find out long-term consequences, which we do not know right now,” senior author Dirk Westermann, MD, also from the University Heart and Vascular Centre, Hamburg, said.
Implications for heart failure
The postmortem findings from Dr. Lindner and associates “provide intriguing evidence that COVID-19 is associated with at least some component of myocardial injury, perhaps as the result of direct viral infection of the heart,” wrote Clyde W. Yancy, MD, MSc, from Northwestern University, Chicago, and Gregg C. Fonarow, MD, from the University of California, Los Angeles, in an editorial accompanying both reports.
The CMR study from Dr. Püntmann and colleagues – on the backdrop of earlier COVID-19 observations – suggests the potential for “residual left ventricular dysfunction and ongoing inflammation” in the months following a COVID-19 diagnosis. Both developments may be “of sufficient concern to represent a nidus for new-onset heart failure and other cardiovascular complications,” contend Dr. Yancy and Dr. Fonarow.
“When added to the postmortem pathological findings from Lindner et al, we see the plot thickening and we are inclined to raise a new and very evident concern that cardiomyopathy and heart failure related to COVID-19 may potentially evolve as the natural history of this infection becomes clearer,” they wrote.
Some patients, having recovered from the acute illness, may be left with a chronic inflammatory state that probably puts them at increased risk for future heart failure, agreed Dr. Bozkurt when interviewed. “They could show further decline in cardiac function, and their recovery might take longer than with the usual viral illnesses that we see,” she said.
“There could also be a risk of sudden death. Inflammation sometimes gives rise to sudden death and ventricular arrhythmia, which I would be very worried about, especially if the myocardium is stressed,” Dr. Bozkurt said. “So competitive sports in those patients potentially could be risky.”
COVID-19 cohort vs. matched control subjects
The CMR study from Dr. Püntmann and colleagues prospectively entered 100 patients recently recovered from an acute bout of COVID-19, either at home or at a hospital, who were followed in a registry based at University Hospital Frankfurt. Their median age was 49 years; 47% were female. They were compared with 50 age- and sex-matched control patients and 50 apparently healthy volunteers matched for risk factors, the group noted.
On the same day as the CMR assessment, the recently recovered patients, compared with the healthy control subjects and risk-factor matched control subjects, respectively, showed (P ≤ .001 in each case):
- A reduced left ventricular (LV) ejection fraction: 56% vs. 60% and 61%.
- A higher LV end-diastolic volume index: 86 mL/m2 vs. 80 mL/m2 and 75 mL/m2.
- A greater LV mass index: 51 g/m2 vs. 47 g/m2 and 53 g/m2.
- A higher hs-TnT level: 5.6 pg/mL vs. 3.2 pg/mL and 3.9 pg/mL.
- A greater prevalence of hs-TnT levels 3 pg/mL or more: 71% vs. 11% and 31%.
At CMR, 78% of the recovered COVID-19 patients showed abnormalities that included raised myocardial native T1 and T2 mapping, which is suggestive of fibrosis and edema from inflammation, compared with the two control groups (P < .001 for all differences), “independent of preexisting conditions, severity and overall course of the acute illness, and the time from the original diagnosis,” the group wrote. Native T1 and T2 mapping correlated significantly with hs-TnT.
“We now have the diagnostic means to detect cardiac inflammation early, and we need make every effort to apply them in every day practice,”Dr. Püntmann said in the interview.
“Using cardiac MRI will allow us to raise our game against COVID-19 and proactively develop efficient cardioprotective treatments,” she said. “Until we have effective means of protecting from the infection, that is vaccination, we must act swiftly and within the means at hand.”
The analysis evokes several other ways patients with COVID-19 might be screened for significant myocardial involvement.
“Strategies could include checking troponins, not only at admission but maybe at discharge and perhaps even those individuals who are at home and are not necessarily requiring care,” Dr. Bozkurt said.
“Biomarker profiling and screening for ongoing inflammation probably are going to be important components of COVID-19, especially for those with subclinical risk and disease.”
Dr. Westermann proposed that troponin elevations at discharge “might be a good starting point” for selecting COVID-19 patients for functional testing or imaging to screen for cardiac sequelae. Performing such tests routinely now “would be overwhelming given the massive increase in patients we still see today.”
Dr. Püntmann had no disclosures; statements of potential conflict for the other authors are in the report. Dr. Bozkurt has previously disclosed receiving consultant fees or honoraria from Bayer Healthcare, Bristol-Myers Squibb, Lantheus Medical Imaging, and Respicardia; serving on a data safety monitoring board for LivaNova USA ; and having unspecified relationships with Abbott Laboratories. Dr. Lindner had no disclosures; Dr. Westermann reported receiving personal fees from AstraZeneca, Bayer, Novartis, and Medtronic. Dr. Yancy is a deputy editor and Dr. Fonarow a section editor for JAMA Cardiology. Dr. Yancy had no other disclosures. Dr. Fonarow reported receiving personal fees from Abbott Laboratories, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards Lifesciences, Janssen, Medtronic, Merck, and Novartis.
A version of this article originally appeared on Medscape.com.
Evidence that the heart can take a major hit in patients hospitalized with COVID-19, especially those already with cardiovascular disease (CV) or its risk factors, has been sadly apparent from the pandemic’s earliest days.
Less clear from case studies and small series to date has been whether SARS-CoV-2 directly attacks the heart and whether acute cardiac effects of the illness may lead to some kind of lingering cardiomyopathy.
The field’s grasp of those issues advanced a bit in two new reports published July 27 in JAMA Cardiology that seem to validate concerns the virus can infect the myocardium, without necessarily causing myocarditis and the possibility that some “recovered” patients may be left with persisting myocardial injury and inflammation that potentially could later manifest as heart failure.
Persisting inflammation by cardiac magnetic resonance
A prospective cohort study with 100 patients recovered from a recent bout of the disease showed evidence of ventricular dysfunction, greater ventricular mass, and in 78% of the cohort, signs of myocardial inflammation by cardiac magnetic resonance (CMR) imaging. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
Two-thirds of the cohort, whose acute COVID-19 severity had “ranged from asymptomatic to minor-to-moderate symptoms,” had recovered at home, whereas the remaining “severely unwell patients” had been hospitalized, wrote the authors, led by Valentina O. Püntmann, MD, PhD, University Hospital Frankfurt (Germany).
None of the patients had a history of heart failure or cardiomyopathy, although some had hypertension, diabetes, or evidence of coronary disease.
“Our findings demonstrate that participants with a relative paucity of preexisting cardiovascular condition and with mostly home-based recovery had frequent cardiac inflammatory involvement, which was similar to the hospitalized subgroup with regards to severity and extent,” the group noted.
“There is a considerable ongoing myocardial inflammation in the heart muscle weeks after recovery from COVID-19 illness. This finding is important because it may herald a considerable burden of heart failure in a few years down the line,” Dr. Püntmann said in an interview.
Early diagnosis would offer “a good chance that early treatment could reduce the relentless course of inflammatory damage or even halt it,” she said.
“The relatively clear onset of COVID-19 illness provides an opportunity, which we often do not have with other conditions, to take a proactive action and to look for heart involvement early, within a few weeks of recovery.”
The study’s CMR evidence of inflammation edema, scarring, and pericardial effusion are among “the major diagnostic criteria for inflammatory and viral myocarditis,” observed Biykem Bozkurt, MD, PhD, from Baylor College of Medicine, Houston, who wasn’t part of either new study.
The findings suggest – consistent with previous evidence – that some patients with recent COVID-19 may be left with ongoing myocardial inflammation, and this study further adds that it could potentially become subacute or even chronic and in some may not be totally reversible, she said in an interview. How long the effects are likely to persist “remains to be determined. We need longer-term outcomes data.”
Viral presence without myocarditis
The accompanying report featured a postmortem analysis of hearts from 39 patients with mostly severe COVID-19 that pointed to a significant SARS-CoV-2 presence and signs that the virus vigorously replicated in the myocardium.
But there was no evidence that the infection led to fulminant myocarditis. Rather, the virus had apparently infiltrated the heart by localizing in interstitial cells or in macrophages that took up in the myocardium without actually entering myocytes, concluded the report’s authors, led by Diana Lindner, PhD, from the University Heart and Vascular Centre, Hamburg (Germany).
The findings suggest “that the presence of SARS-CoV-2 in cardiac tissue does not necessarily cause an inflammatory reaction consistent with clinical myocarditis,” the group wrote.
Previously in the literature, in “cases in which myocardial inflammation was present, there was also evidence of clinical myocarditis, and therefore the current cases underlie a different pathophysiology,” they concluded.
No evidence of the virus was seen in 15 cases, about 61% of the group. In 16 of the remaining 24 hearts, the viral load exceeded 1,000 copies per mcg of RNA, a substantial presence. Those 16 showed increased expression of inflammatory cytokines but no inflammatory cell infiltrates or changes in leukocyte counts, the researchers noted.
“Findings of suggested viral replication in the cases with a very high viral load are showing that we need to do more studies to find out long-term consequences, which we do not know right now,” senior author Dirk Westermann, MD, also from the University Heart and Vascular Centre, Hamburg, said.
Implications for heart failure
The postmortem findings from Dr. Lindner and associates “provide intriguing evidence that COVID-19 is associated with at least some component of myocardial injury, perhaps as the result of direct viral infection of the heart,” wrote Clyde W. Yancy, MD, MSc, from Northwestern University, Chicago, and Gregg C. Fonarow, MD, from the University of California, Los Angeles, in an editorial accompanying both reports.
The CMR study from Dr. Püntmann and colleagues – on the backdrop of earlier COVID-19 observations – suggests the potential for “residual left ventricular dysfunction and ongoing inflammation” in the months following a COVID-19 diagnosis. Both developments may be “of sufficient concern to represent a nidus for new-onset heart failure and other cardiovascular complications,” contend Dr. Yancy and Dr. Fonarow.
“When added to the postmortem pathological findings from Lindner et al, we see the plot thickening and we are inclined to raise a new and very evident concern that cardiomyopathy and heart failure related to COVID-19 may potentially evolve as the natural history of this infection becomes clearer,” they wrote.
Some patients, having recovered from the acute illness, may be left with a chronic inflammatory state that probably puts them at increased risk for future heart failure, agreed Dr. Bozkurt when interviewed. “They could show further decline in cardiac function, and their recovery might take longer than with the usual viral illnesses that we see,” she said.
“There could also be a risk of sudden death. Inflammation sometimes gives rise to sudden death and ventricular arrhythmia, which I would be very worried about, especially if the myocardium is stressed,” Dr. Bozkurt said. “So competitive sports in those patients potentially could be risky.”
COVID-19 cohort vs. matched control subjects
The CMR study from Dr. Püntmann and colleagues prospectively entered 100 patients recently recovered from an acute bout of COVID-19, either at home or at a hospital, who were followed in a registry based at University Hospital Frankfurt. Their median age was 49 years; 47% were female. They were compared with 50 age- and sex-matched control patients and 50 apparently healthy volunteers matched for risk factors, the group noted.
On the same day as the CMR assessment, the recently recovered patients, compared with the healthy control subjects and risk-factor matched control subjects, respectively, showed (P ≤ .001 in each case):
- A reduced left ventricular (LV) ejection fraction: 56% vs. 60% and 61%.
- A higher LV end-diastolic volume index: 86 mL/m2 vs. 80 mL/m2 and 75 mL/m2.
- A greater LV mass index: 51 g/m2 vs. 47 g/m2 and 53 g/m2.
- A higher hs-TnT level: 5.6 pg/mL vs. 3.2 pg/mL and 3.9 pg/mL.
- A greater prevalence of hs-TnT levels 3 pg/mL or more: 71% vs. 11% and 31%.
At CMR, 78% of the recovered COVID-19 patients showed abnormalities that included raised myocardial native T1 and T2 mapping, which is suggestive of fibrosis and edema from inflammation, compared with the two control groups (P < .001 for all differences), “independent of preexisting conditions, severity and overall course of the acute illness, and the time from the original diagnosis,” the group wrote. Native T1 and T2 mapping correlated significantly with hs-TnT.
“We now have the diagnostic means to detect cardiac inflammation early, and we need make every effort to apply them in every day practice,”Dr. Püntmann said in the interview.
“Using cardiac MRI will allow us to raise our game against COVID-19 and proactively develop efficient cardioprotective treatments,” she said. “Until we have effective means of protecting from the infection, that is vaccination, we must act swiftly and within the means at hand.”
The analysis evokes several other ways patients with COVID-19 might be screened for significant myocardial involvement.
“Strategies could include checking troponins, not only at admission but maybe at discharge and perhaps even those individuals who are at home and are not necessarily requiring care,” Dr. Bozkurt said.
“Biomarker profiling and screening for ongoing inflammation probably are going to be important components of COVID-19, especially for those with subclinical risk and disease.”
Dr. Westermann proposed that troponin elevations at discharge “might be a good starting point” for selecting COVID-19 patients for functional testing or imaging to screen for cardiac sequelae. Performing such tests routinely now “would be overwhelming given the massive increase in patients we still see today.”
Dr. Püntmann had no disclosures; statements of potential conflict for the other authors are in the report. Dr. Bozkurt has previously disclosed receiving consultant fees or honoraria from Bayer Healthcare, Bristol-Myers Squibb, Lantheus Medical Imaging, and Respicardia; serving on a data safety monitoring board for LivaNova USA ; and having unspecified relationships with Abbott Laboratories. Dr. Lindner had no disclosures; Dr. Westermann reported receiving personal fees from AstraZeneca, Bayer, Novartis, and Medtronic. Dr. Yancy is a deputy editor and Dr. Fonarow a section editor for JAMA Cardiology. Dr. Yancy had no other disclosures. Dr. Fonarow reported receiving personal fees from Abbott Laboratories, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards Lifesciences, Janssen, Medtronic, Merck, and Novartis.
A version of this article originally appeared on Medscape.com.
SCD-HeFT 10-year results: Primary-prevention ICD insights in nonischemic heart failure
A 10-year follow-up analysis based on one of cardiology’s most influential trials has shed further light on one of its key issues: how to sharpen selection of patients most likely to benefit from a primary prevention implantable cardioverter-defibrillator (ICD).
In a new report from SCD-HeFT, the survival advantage in patients with heart failure seen 5 years after receiving ICDs, compared with a non-ICD control group, narrowed a bit but remained significant after an additional 5 years. But not all patients with devices shared in that long-term ICD benefit. Patients with either ischemic disease or nonischemic cardiomyopathy (NICM) with devices showed a similar mortality risk reduction in the trial’s previously reported 5-year outcomes. That advantage, compared with non-ICD control patients, persisted throughout the subsequent 5 years for ischemic patients but tapered to nil for those with NICM.
The NICM patients “had what appears to be some accrual of benefit maybe out to about 6 years, and then the curves appear to come together where there’s no apparent further benefit after 6 years,” Jeanne E. Poole, MD, of the University of Washington, Seattle, said in an interview.
In both the 10-year analysis and the earlier results, ICD survival gains went preferentially to patients who enrolled with New York Heart Association (NYHA) functional class II symptoms. Patients who entered in NYHA class III “didn’t appear to have any benefit whatsoever” in either period, Dr. Poole said.
“The simple message is that the same groups of patients that benefited strongly from the ICD in the original SCD-HeFT – the NYHA class 2 patients and those with ischemic cardiomyopathy – were really the ones who benefited the greatest over the long term,” she said.
Dr. Poole is lead author on the SCD-HeFT 10-year analysis, which was published in the July 28 issue of the Journal of the American College of Cardiology.
Why the ICD survival effect disappeared midway in patients with NICM “is hard to sort out,” she said. Many in the control group were offered such devices after the trial concluded. Among those, it’s possible that disproportionately more control patients with NICM, compared with patients with ischemic disease, were fitted with ICDs that were also cardiac resynchronization therapy (CRT) devices, Dr. Poole and her colleagues speculated. That could have shifted their late outcomes to be more in line with patients who had received ICDs when the trial started.
Or “it is possible that the intermediate-term benefit of ICD therapy in NICM is overwhelmed by nonarrhythmic death in extended follow-up” given that ICDs prolong survival only by preventing arrhythmic death, noted an editorial accompanying the new SCD-HeFT publication.
Another possibility: Because NICM is a heterogeneous disorder with many potential causes, perhaps “the absence of long-term mortality benefit among SCD-HeFT participants with NICM was due to an unintended but preferential enrollment of subtypes at relatively lower risk for arrhythmic death in the longer term,” proposed Eric C. Stecker, MD, MPH, Oregon Health & Science University, Portland, and coauthors in their editorial.
“What are the take-away messages from the current analysis by Poole et al?” they asked. “These findings strongly support the clinical efficacy and cost-effectiveness of ICD therapy for the majority of patients with severe but mildly symptomatic ischemic cardiomyopathy who do not have an excessive comorbidity burden.”
But “the implications for patients with NICM are less clear,” they wrote. “Given evidence for intermediate-term benefit and the limitations inherent to assessing longer-term benefit, we do not believe it is appropriate to walk back guideline recommendations regarding ICD implantation for NICM patients.”
The findings in nonischemic patients invite comparison with the randomized DANISH trial, which entered only patients with NICM and, over more than 5 years, saw no primary-prevention ICD advantage for the end point of all-cause mortality.
But patients who received ICDs showed a reduction in arrhythmic death, a secondary end point. And mortality in the trial showed a significant interaction with patient age; survival went up sharply with ICDs for those younger than 60 years.
Also in DANISH, “the ICD treatment effect appears to vary over time, with an earlier phase showing possible survival benefit and a later phase showing attenuation of that benefit,” similar to what was seen long-term in SCD-HeFT, in which the interaction between mortality and time since implantation was significant at P = .0015, observe Dr. Poole and colleagues.
However, Dr. Poole cautioned when interviewed, patient management in DANISH, conducted exclusively in Denmark, may not have been representative of the rest of the world, complicating comparisons with other studies. For example, nearly 60% of all patients in DANISH had defibrillating CRT devices. Virtually everyone was on ACE inhibitors or angiotensin-receptor blockers, and almost 60% were taking aldosterone inhibitors.
“DANISH is an unusually high bar and probably does not reflect all patients with heart failure, and certainly does not reflect patients in the United States in terms of those high levels of guideline-directed medical therapy,” Dr. Poole said. The message from DANISH, she said, seems to be that patients with NICM who are definitely on goal-directed heart failure medications with CRT devices “probably don’t have a meaningful benefit from an ICD, on total mortality, because their sudden death rates are simply so low.”
SCD-HeFT had originally assigned 2,521 patients with heart failure of NYHA class II or III and an left ventricular ejection fraction of less than 35% to receive an ICD, amiodarone without an ICD, or an amiodarone placebo and no ICD; patients in the latter cohorts made up the non-ICD control group.
Those who received an ICD, compared with the non-ICD control patients, showed a 23% drop in all-cause mortality over a median of 45.5 months ending on October 31, 2003, Dr. Poole and colleagues noted in their current report. The trial’s primary results were unveiled 2005.
The current analysis, based on data collected in 2010 and 2011, followed the 1,855 patients alive at the trial’s official conclusion and combined outcomes before and after that time for a median follow-up of 11 years, Dr. Poole and colleagues reported.
In the ICD group, the overall hazard ratio for mortality by intention-to-treat was 0.87 (95% confidence interval, 0.76-0.98; P = .028), compared with the non-ICD control group.
In their report, Poole and associates clarified one of the foremost potential confounders in the current analysis: device implantations after the trial in patients who had been in the non-ICD groups. From partial clinical data collected after the trial, they wrote, the estimated rate of subsequent ICD implantation in non-ICD control patients was about 55%. Such a low number is consistent with clinical practice in the United States, where “a surprisingly low number of patients who are eligible actually end up getting devices,” Dr. Poole said.
Subsequent ICD use in the former non-ICD control patients presumably boosted their survival over the long term, narrowing the gap between their all-cause mortality and that of the original ICD patients, Dr. Poole observed. Despite that, the ICD-group’s late survival advantage remained significant.
SCD-HeFT was sponsored by Medtronic, Wyeth Pharmaceuticals, and the National Heart, Lung, and Blood Institute. The current analysis was partially supported by a grant from St. Jude Medical. Dr. Poole disclosed receiving research support from Medtronic, Biotronik, AtriCure, and Kestra; serving as a speaker for Boston Scientific, Medtronic, and MediaSphere Medical and on an advisory board for Boston Scientific; serving on a committee for Medtronic and on a data and safety monitoring board for EBR Systems; and receiving royalties from Elsevier and compensation from the Heart Rhythm Society for serving as editor in chief for the Heart Rhythm O2 journal. Disclosures for the other authors are in the report. Dr. Stecker and coauthors disclosed that they have no relevant relationships.
A version of this article originally appeared on Medscape.com.
A 10-year follow-up analysis based on one of cardiology’s most influential trials has shed further light on one of its key issues: how to sharpen selection of patients most likely to benefit from a primary prevention implantable cardioverter-defibrillator (ICD).
In a new report from SCD-HeFT, the survival advantage in patients with heart failure seen 5 years after receiving ICDs, compared with a non-ICD control group, narrowed a bit but remained significant after an additional 5 years. But not all patients with devices shared in that long-term ICD benefit. Patients with either ischemic disease or nonischemic cardiomyopathy (NICM) with devices showed a similar mortality risk reduction in the trial’s previously reported 5-year outcomes. That advantage, compared with non-ICD control patients, persisted throughout the subsequent 5 years for ischemic patients but tapered to nil for those with NICM.
The NICM patients “had what appears to be some accrual of benefit maybe out to about 6 years, and then the curves appear to come together where there’s no apparent further benefit after 6 years,” Jeanne E. Poole, MD, of the University of Washington, Seattle, said in an interview.
In both the 10-year analysis and the earlier results, ICD survival gains went preferentially to patients who enrolled with New York Heart Association (NYHA) functional class II symptoms. Patients who entered in NYHA class III “didn’t appear to have any benefit whatsoever” in either period, Dr. Poole said.
“The simple message is that the same groups of patients that benefited strongly from the ICD in the original SCD-HeFT – the NYHA class 2 patients and those with ischemic cardiomyopathy – were really the ones who benefited the greatest over the long term,” she said.
Dr. Poole is lead author on the SCD-HeFT 10-year analysis, which was published in the July 28 issue of the Journal of the American College of Cardiology.
Why the ICD survival effect disappeared midway in patients with NICM “is hard to sort out,” she said. Many in the control group were offered such devices after the trial concluded. Among those, it’s possible that disproportionately more control patients with NICM, compared with patients with ischemic disease, were fitted with ICDs that were also cardiac resynchronization therapy (CRT) devices, Dr. Poole and her colleagues speculated. That could have shifted their late outcomes to be more in line with patients who had received ICDs when the trial started.
Or “it is possible that the intermediate-term benefit of ICD therapy in NICM is overwhelmed by nonarrhythmic death in extended follow-up” given that ICDs prolong survival only by preventing arrhythmic death, noted an editorial accompanying the new SCD-HeFT publication.
Another possibility: Because NICM is a heterogeneous disorder with many potential causes, perhaps “the absence of long-term mortality benefit among SCD-HeFT participants with NICM was due to an unintended but preferential enrollment of subtypes at relatively lower risk for arrhythmic death in the longer term,” proposed Eric C. Stecker, MD, MPH, Oregon Health & Science University, Portland, and coauthors in their editorial.
“What are the take-away messages from the current analysis by Poole et al?” they asked. “These findings strongly support the clinical efficacy and cost-effectiveness of ICD therapy for the majority of patients with severe but mildly symptomatic ischemic cardiomyopathy who do not have an excessive comorbidity burden.”
But “the implications for patients with NICM are less clear,” they wrote. “Given evidence for intermediate-term benefit and the limitations inherent to assessing longer-term benefit, we do not believe it is appropriate to walk back guideline recommendations regarding ICD implantation for NICM patients.”
The findings in nonischemic patients invite comparison with the randomized DANISH trial, which entered only patients with NICM and, over more than 5 years, saw no primary-prevention ICD advantage for the end point of all-cause mortality.
But patients who received ICDs showed a reduction in arrhythmic death, a secondary end point. And mortality in the trial showed a significant interaction with patient age; survival went up sharply with ICDs for those younger than 60 years.
Also in DANISH, “the ICD treatment effect appears to vary over time, with an earlier phase showing possible survival benefit and a later phase showing attenuation of that benefit,” similar to what was seen long-term in SCD-HeFT, in which the interaction between mortality and time since implantation was significant at P = .0015, observe Dr. Poole and colleagues.
However, Dr. Poole cautioned when interviewed, patient management in DANISH, conducted exclusively in Denmark, may not have been representative of the rest of the world, complicating comparisons with other studies. For example, nearly 60% of all patients in DANISH had defibrillating CRT devices. Virtually everyone was on ACE inhibitors or angiotensin-receptor blockers, and almost 60% were taking aldosterone inhibitors.
“DANISH is an unusually high bar and probably does not reflect all patients with heart failure, and certainly does not reflect patients in the United States in terms of those high levels of guideline-directed medical therapy,” Dr. Poole said. The message from DANISH, she said, seems to be that patients with NICM who are definitely on goal-directed heart failure medications with CRT devices “probably don’t have a meaningful benefit from an ICD, on total mortality, because their sudden death rates are simply so low.”
SCD-HeFT had originally assigned 2,521 patients with heart failure of NYHA class II or III and an left ventricular ejection fraction of less than 35% to receive an ICD, amiodarone without an ICD, or an amiodarone placebo and no ICD; patients in the latter cohorts made up the non-ICD control group.
Those who received an ICD, compared with the non-ICD control patients, showed a 23% drop in all-cause mortality over a median of 45.5 months ending on October 31, 2003, Dr. Poole and colleagues noted in their current report. The trial’s primary results were unveiled 2005.
The current analysis, based on data collected in 2010 and 2011, followed the 1,855 patients alive at the trial’s official conclusion and combined outcomes before and after that time for a median follow-up of 11 years, Dr. Poole and colleagues reported.
In the ICD group, the overall hazard ratio for mortality by intention-to-treat was 0.87 (95% confidence interval, 0.76-0.98; P = .028), compared with the non-ICD control group.
In their report, Poole and associates clarified one of the foremost potential confounders in the current analysis: device implantations after the trial in patients who had been in the non-ICD groups. From partial clinical data collected after the trial, they wrote, the estimated rate of subsequent ICD implantation in non-ICD control patients was about 55%. Such a low number is consistent with clinical practice in the United States, where “a surprisingly low number of patients who are eligible actually end up getting devices,” Dr. Poole said.
Subsequent ICD use in the former non-ICD control patients presumably boosted their survival over the long term, narrowing the gap between their all-cause mortality and that of the original ICD patients, Dr. Poole observed. Despite that, the ICD-group’s late survival advantage remained significant.
SCD-HeFT was sponsored by Medtronic, Wyeth Pharmaceuticals, and the National Heart, Lung, and Blood Institute. The current analysis was partially supported by a grant from St. Jude Medical. Dr. Poole disclosed receiving research support from Medtronic, Biotronik, AtriCure, and Kestra; serving as a speaker for Boston Scientific, Medtronic, and MediaSphere Medical and on an advisory board for Boston Scientific; serving on a committee for Medtronic and on a data and safety monitoring board for EBR Systems; and receiving royalties from Elsevier and compensation from the Heart Rhythm Society for serving as editor in chief for the Heart Rhythm O2 journal. Disclosures for the other authors are in the report. Dr. Stecker and coauthors disclosed that they have no relevant relationships.
A version of this article originally appeared on Medscape.com.
A 10-year follow-up analysis based on one of cardiology’s most influential trials has shed further light on one of its key issues: how to sharpen selection of patients most likely to benefit from a primary prevention implantable cardioverter-defibrillator (ICD).
In a new report from SCD-HeFT, the survival advantage in patients with heart failure seen 5 years after receiving ICDs, compared with a non-ICD control group, narrowed a bit but remained significant after an additional 5 years. But not all patients with devices shared in that long-term ICD benefit. Patients with either ischemic disease or nonischemic cardiomyopathy (NICM) with devices showed a similar mortality risk reduction in the trial’s previously reported 5-year outcomes. That advantage, compared with non-ICD control patients, persisted throughout the subsequent 5 years for ischemic patients but tapered to nil for those with NICM.
The NICM patients “had what appears to be some accrual of benefit maybe out to about 6 years, and then the curves appear to come together where there’s no apparent further benefit after 6 years,” Jeanne E. Poole, MD, of the University of Washington, Seattle, said in an interview.
In both the 10-year analysis and the earlier results, ICD survival gains went preferentially to patients who enrolled with New York Heart Association (NYHA) functional class II symptoms. Patients who entered in NYHA class III “didn’t appear to have any benefit whatsoever” in either period, Dr. Poole said.
“The simple message is that the same groups of patients that benefited strongly from the ICD in the original SCD-HeFT – the NYHA class 2 patients and those with ischemic cardiomyopathy – were really the ones who benefited the greatest over the long term,” she said.
Dr. Poole is lead author on the SCD-HeFT 10-year analysis, which was published in the July 28 issue of the Journal of the American College of Cardiology.
Why the ICD survival effect disappeared midway in patients with NICM “is hard to sort out,” she said. Many in the control group were offered such devices after the trial concluded. Among those, it’s possible that disproportionately more control patients with NICM, compared with patients with ischemic disease, were fitted with ICDs that were also cardiac resynchronization therapy (CRT) devices, Dr. Poole and her colleagues speculated. That could have shifted their late outcomes to be more in line with patients who had received ICDs when the trial started.
Or “it is possible that the intermediate-term benefit of ICD therapy in NICM is overwhelmed by nonarrhythmic death in extended follow-up” given that ICDs prolong survival only by preventing arrhythmic death, noted an editorial accompanying the new SCD-HeFT publication.
Another possibility: Because NICM is a heterogeneous disorder with many potential causes, perhaps “the absence of long-term mortality benefit among SCD-HeFT participants with NICM was due to an unintended but preferential enrollment of subtypes at relatively lower risk for arrhythmic death in the longer term,” proposed Eric C. Stecker, MD, MPH, Oregon Health & Science University, Portland, and coauthors in their editorial.
“What are the take-away messages from the current analysis by Poole et al?” they asked. “These findings strongly support the clinical efficacy and cost-effectiveness of ICD therapy for the majority of patients with severe but mildly symptomatic ischemic cardiomyopathy who do not have an excessive comorbidity burden.”
But “the implications for patients with NICM are less clear,” they wrote. “Given evidence for intermediate-term benefit and the limitations inherent to assessing longer-term benefit, we do not believe it is appropriate to walk back guideline recommendations regarding ICD implantation for NICM patients.”
The findings in nonischemic patients invite comparison with the randomized DANISH trial, which entered only patients with NICM and, over more than 5 years, saw no primary-prevention ICD advantage for the end point of all-cause mortality.
But patients who received ICDs showed a reduction in arrhythmic death, a secondary end point. And mortality in the trial showed a significant interaction with patient age; survival went up sharply with ICDs for those younger than 60 years.
Also in DANISH, “the ICD treatment effect appears to vary over time, with an earlier phase showing possible survival benefit and a later phase showing attenuation of that benefit,” similar to what was seen long-term in SCD-HeFT, in which the interaction between mortality and time since implantation was significant at P = .0015, observe Dr. Poole and colleagues.
However, Dr. Poole cautioned when interviewed, patient management in DANISH, conducted exclusively in Denmark, may not have been representative of the rest of the world, complicating comparisons with other studies. For example, nearly 60% of all patients in DANISH had defibrillating CRT devices. Virtually everyone was on ACE inhibitors or angiotensin-receptor blockers, and almost 60% were taking aldosterone inhibitors.
“DANISH is an unusually high bar and probably does not reflect all patients with heart failure, and certainly does not reflect patients in the United States in terms of those high levels of guideline-directed medical therapy,” Dr. Poole said. The message from DANISH, she said, seems to be that patients with NICM who are definitely on goal-directed heart failure medications with CRT devices “probably don’t have a meaningful benefit from an ICD, on total mortality, because their sudden death rates are simply so low.”
SCD-HeFT had originally assigned 2,521 patients with heart failure of NYHA class II or III and an left ventricular ejection fraction of less than 35% to receive an ICD, amiodarone without an ICD, or an amiodarone placebo and no ICD; patients in the latter cohorts made up the non-ICD control group.
Those who received an ICD, compared with the non-ICD control patients, showed a 23% drop in all-cause mortality over a median of 45.5 months ending on October 31, 2003, Dr. Poole and colleagues noted in their current report. The trial’s primary results were unveiled 2005.
The current analysis, based on data collected in 2010 and 2011, followed the 1,855 patients alive at the trial’s official conclusion and combined outcomes before and after that time for a median follow-up of 11 years, Dr. Poole and colleagues reported.
In the ICD group, the overall hazard ratio for mortality by intention-to-treat was 0.87 (95% confidence interval, 0.76-0.98; P = .028), compared with the non-ICD control group.
In their report, Poole and associates clarified one of the foremost potential confounders in the current analysis: device implantations after the trial in patients who had been in the non-ICD groups. From partial clinical data collected after the trial, they wrote, the estimated rate of subsequent ICD implantation in non-ICD control patients was about 55%. Such a low number is consistent with clinical practice in the United States, where “a surprisingly low number of patients who are eligible actually end up getting devices,” Dr. Poole said.
Subsequent ICD use in the former non-ICD control patients presumably boosted their survival over the long term, narrowing the gap between their all-cause mortality and that of the original ICD patients, Dr. Poole observed. Despite that, the ICD-group’s late survival advantage remained significant.
SCD-HeFT was sponsored by Medtronic, Wyeth Pharmaceuticals, and the National Heart, Lung, and Blood Institute. The current analysis was partially supported by a grant from St. Jude Medical. Dr. Poole disclosed receiving research support from Medtronic, Biotronik, AtriCure, and Kestra; serving as a speaker for Boston Scientific, Medtronic, and MediaSphere Medical and on an advisory board for Boston Scientific; serving on a committee for Medtronic and on a data and safety monitoring board for EBR Systems; and receiving royalties from Elsevier and compensation from the Heart Rhythm Society for serving as editor in chief for the Heart Rhythm O2 journal. Disclosures for the other authors are in the report. Dr. Stecker and coauthors disclosed that they have no relevant relationships.
A version of this article originally appeared on Medscape.com.
Dapagliflozin benefits low-EF heart failure regardless of diuretic dose: DAPA-HF
The DAPA-HF trial has already changed cardiology in opening up a new class of drugs to patients with heart failure (HF), whether or not they have diabetes. Now the trial is yielding clues as to how it benefits them. For now, it’s doing so by process of elimination.
A new analysis suggests that dapagliflozin (Farxiga, AstraZeneca) didn’t need help from loop diuretics to cut the risk for clinical events in patients with HF with reduced ejection fraction (HFrEF), a benefit seen across the spectrum of glycosylated hemoglobin levels and without compromising renal function, said DAPA-HF investigators. Also, use of dapagliflozin and its clinical effects were not associated with changes in loop diuretic dosage. Those findings and others suggest the drug helps in HFrEF at least partly by some other mechanism than its own diuretic effect, the researchers say.
Such insights will likely be important to case-by-case decisions on whether to use the drug, a sodium-glucose cotransporter 2 (SGLT2) inhibitor once reserved for patients with diabetes, given the recently broader landscape of HF treatment options.
As previously reported from DAPA-HF, with more than 4,700 patients, those who received dapagliflozin showed significant reductions in the primary end point, a composite of cardiovascular (CV) death, HF hospitalization, and urgent HF visit requiring IV therapy over about 18 months. The 45% of patients with and 55% without type 2 diabetes enjoyed about equal benefit in the placebo-controlled trial for that end point, as well as for all-cause mortality.
SGLT2 inhibitors work in diabetes by promoting urinary glucose excretion. That had led some to speculate that its benefit in HFrEF comes primarily from a diuretic effect; the current findings largely put that question to rest.
“Our findings show that treatment with dapagliflozin was effective regardless of diuretic use or diuretic dose. They also show that dapagliflozin did not lead to an increase in renal adverse events or discontinuation of therapy in patients treated with a diuretic,” trialist Alice M. Jackson, MB, ChB, said in an interview.
“In fact, renal adverse events were generally less common in patients treated with dapagliflozin, across the diuretic categories,” said Dr. Jackson, from the University of Glasgow.
Dr. Jackson presented the new analysis at a Late-Breaking Science Session during the European Society of Cardiology Heart Failure Discoveries virtual meeting. The HFA sessions were conducted virtually this year due to the COVID-19 pandemic.
At baseline, 84% of patients were on conventional diuretics. The post hoc analysis broke out all patients by loop-diuretic dosage level: none; less than 40 mg furosemide equivalents (FE); 40 mg FE; or more than 40 mg FE. Clinical outcomes were similar across the four groups.
Clinicians in the trial “were not given specific advice about adjusting diuretic doses, but were encouraged to assess volume status and make changes to medical therapy based on this, if necessary,” Dr. Jackson said. “This suggests that, for most patients, starting dapagliflozin will not necessitate a change in diuretic dose.”
With the caveat that the event rate was low in the relatively few patients not prescribed loop diuretics, she said, “the magnitude of the benefit from dapagliflozin appeared to be larger in patients not treated with a diuretic.”
There was no suggestion of a diuretic dose–response effect or statistical interaction between diuretic use and clinical outcomes on dapagliflozin, Dr. Jackson observed in the interview.
Of note in the analysis, hematocrit levels shot up soon after patients started active therapy, but they didn’t rise much in the placebo group. The sustained hematocrit elevation on dapagliflozin, seen at all diuretic dosage levels, persisted even after dosage reductions at 6 months, she said.
“Dapagliflozin is effective in HFrEF irrespective of background diuretic therapy; therefore, it is almost certainly not purely acting as a diuretic,” Andrew J. Coats, MD, DSc, MBA, said in an interview.
The findings also “lessen the concern that dapagliflozin’s beneficial effects are only seen only in patients without effective diuretic dosing,” said Dr. Coats, from University of Warwick, Coventry, England.
“Altogether, these data give further reassurance that dapagliflozin can safely be used in heart failure, and has a beneficial effect independent of the use of diuretic drugs,” invited discussant Wolfram Doehner, MD, PhD, Charité-Universitätsmedizin Berlin, said after Dr. Jackson’s presentation of the analysis.
He made special mention of the sustained hematocrit elevation on dapagliflozin. “While this effect may likely relate to the mild reduction in plasma volume secondary to dapagliflozin therapy, it is noted that the increase in hematocrit was independent of any change of the diuretic dose,” Doehner said. “If additional mechanisms have a role for this observed increase in hematocrit, it may be of interest in further investigations.”
Dr. Jackson pointed to several observations that suggest the hematocrit finding isn’t explained by hemoconcentration from reduced plasma volume, at least not entirely.
For example, hematocrit levels rose “without any suggestion of a relationship between diuretic dose and degree of hematocrit elevation with dapagliflozin,” she said.
The elevations persisted even with diuretic dose reductions at 6 and 12 months, “which should have led to a decrease in hemoconcentration if it was caused by volume contraction.”
Also, she said, “among patients not taking a diuretic, volume depletion occurred less frequently in the dapagliflozin group than in the placebo group, but there was still a similar rise in hematocrit with dapagliflozin.”
Both Dr. Jackson and Dr. Coats said the sustained elevation in hematocrit on the drug is unlikely to pose a major hazard.
Dr. Coats said that, theoretically, “increased hematocrit could reduce peripheral vessel blood flow, making ischemia and thrombosis more likely. But the size of the effect is small and unlikely to be clinically important.”
A diuretic dose could not be determined for 128 of the trial’s 4,744 randomized patients with HFrEF, so the post hoc analysis was limited to the remaining 4,616. Of those, 746 were not on diuretics at baseline, 1,311 were on loop diuretics at less than 40 mg FE or on non-loop diuretics only, 1,365 were taking 40 mg FE, and 1,204 were on higher doses of loop diuretics.
The mean baseline dosage was 60 mg FE, which rose slightly throughout the trial. But the baseline dosage and the increases were both similar in the placebo and dapagliflozin groups. Dr. Jackson said 84% and 83% of patients on dapagliflozin and placebo, respectively, maintained their baseline dose at 6 months and about 77% in both groups at 12 months.
The overall trial’s significant primary endpoint reduction for dapagliflozin versus placebo applied similarly to patients not on a diuretics and to those on any dose of diuretic, with an interaction P value of .23 for the effect of diuretic use. The hazard ratios (95% confidence interval) were 0.57 (0.36-0.92) for patients not on diuretics, 0.78 (0.68-0.90) for patients on any diuretic dosage, and 0.74 (0.65-0.85) overall
Dr. Jackson said during her formal online presentation that patients on diuretics showed a “tendency toward slightly more volume depletion in those on dapagliflozin than in those on placebo, but the excess was small and not greater than approximately 3% in those taking 40 mg furosemide equivalent diuretic. And fortunately, this did not result in an increase in frequency in renal adverse events nor of discontinuation of study drug.”
Renal adverse events were similarly prevalent in the two treatment groups, as were such events leading to treatment discontinuation. But serious renal events were less common in the dapagliflozin group (1.6% vs 2.7%; P = .009), as was investigator-reported serious acute kidney injury (1.0% vs 1.9%; P = .007).
“Overall, renal events were infrequent,” Dr. Jackson said, and “because of the small number of events, it is very difficult to draw conclusions about the impact of dapagliflozin on renal function according to diuretic-dose subgroups.”
Still, she said, worsening renal function was less common on dapagliflozin in three of the four groups by diuretic dosage; the exception was the less than 40 mg FE group, “but the absolute difference in this group was only two events.”
There seem to be dapagliflozin mechanisms “underneath the surface that need to be unraveled,” Dr. Doehner said as discussant, processes that are favorable for the treatment of HFrEF in which “diuretics play no big role.”
Dr. Jackson has no disclosures. Dr. Coats has disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor. Dr. Doener has recently disclosed receiving grants and personal fees from Vifor, Pfizer, Boehringer Ingelheim, Sphingotec, ZS Pharma, Bayer, and Medtronic.
A version of this article originally appeared on Medscape.com.
The DAPA-HF trial has already changed cardiology in opening up a new class of drugs to patients with heart failure (HF), whether or not they have diabetes. Now the trial is yielding clues as to how it benefits them. For now, it’s doing so by process of elimination.
A new analysis suggests that dapagliflozin (Farxiga, AstraZeneca) didn’t need help from loop diuretics to cut the risk for clinical events in patients with HF with reduced ejection fraction (HFrEF), a benefit seen across the spectrum of glycosylated hemoglobin levels and without compromising renal function, said DAPA-HF investigators. Also, use of dapagliflozin and its clinical effects were not associated with changes in loop diuretic dosage. Those findings and others suggest the drug helps in HFrEF at least partly by some other mechanism than its own diuretic effect, the researchers say.
Such insights will likely be important to case-by-case decisions on whether to use the drug, a sodium-glucose cotransporter 2 (SGLT2) inhibitor once reserved for patients with diabetes, given the recently broader landscape of HF treatment options.
As previously reported from DAPA-HF, with more than 4,700 patients, those who received dapagliflozin showed significant reductions in the primary end point, a composite of cardiovascular (CV) death, HF hospitalization, and urgent HF visit requiring IV therapy over about 18 months. The 45% of patients with and 55% without type 2 diabetes enjoyed about equal benefit in the placebo-controlled trial for that end point, as well as for all-cause mortality.
SGLT2 inhibitors work in diabetes by promoting urinary glucose excretion. That had led some to speculate that its benefit in HFrEF comes primarily from a diuretic effect; the current findings largely put that question to rest.
“Our findings show that treatment with dapagliflozin was effective regardless of diuretic use or diuretic dose. They also show that dapagliflozin did not lead to an increase in renal adverse events or discontinuation of therapy in patients treated with a diuretic,” trialist Alice M. Jackson, MB, ChB, said in an interview.
“In fact, renal adverse events were generally less common in patients treated with dapagliflozin, across the diuretic categories,” said Dr. Jackson, from the University of Glasgow.
Dr. Jackson presented the new analysis at a Late-Breaking Science Session during the European Society of Cardiology Heart Failure Discoveries virtual meeting. The HFA sessions were conducted virtually this year due to the COVID-19 pandemic.
At baseline, 84% of patients were on conventional diuretics. The post hoc analysis broke out all patients by loop-diuretic dosage level: none; less than 40 mg furosemide equivalents (FE); 40 mg FE; or more than 40 mg FE. Clinical outcomes were similar across the four groups.
Clinicians in the trial “were not given specific advice about adjusting diuretic doses, but were encouraged to assess volume status and make changes to medical therapy based on this, if necessary,” Dr. Jackson said. “This suggests that, for most patients, starting dapagliflozin will not necessitate a change in diuretic dose.”
With the caveat that the event rate was low in the relatively few patients not prescribed loop diuretics, she said, “the magnitude of the benefit from dapagliflozin appeared to be larger in patients not treated with a diuretic.”
There was no suggestion of a diuretic dose–response effect or statistical interaction between diuretic use and clinical outcomes on dapagliflozin, Dr. Jackson observed in the interview.
Of note in the analysis, hematocrit levels shot up soon after patients started active therapy, but they didn’t rise much in the placebo group. The sustained hematocrit elevation on dapagliflozin, seen at all diuretic dosage levels, persisted even after dosage reductions at 6 months, she said.
“Dapagliflozin is effective in HFrEF irrespective of background diuretic therapy; therefore, it is almost certainly not purely acting as a diuretic,” Andrew J. Coats, MD, DSc, MBA, said in an interview.
The findings also “lessen the concern that dapagliflozin’s beneficial effects are only seen only in patients without effective diuretic dosing,” said Dr. Coats, from University of Warwick, Coventry, England.
“Altogether, these data give further reassurance that dapagliflozin can safely be used in heart failure, and has a beneficial effect independent of the use of diuretic drugs,” invited discussant Wolfram Doehner, MD, PhD, Charité-Universitätsmedizin Berlin, said after Dr. Jackson’s presentation of the analysis.
He made special mention of the sustained hematocrit elevation on dapagliflozin. “While this effect may likely relate to the mild reduction in plasma volume secondary to dapagliflozin therapy, it is noted that the increase in hematocrit was independent of any change of the diuretic dose,” Doehner said. “If additional mechanisms have a role for this observed increase in hematocrit, it may be of interest in further investigations.”
Dr. Jackson pointed to several observations that suggest the hematocrit finding isn’t explained by hemoconcentration from reduced plasma volume, at least not entirely.
For example, hematocrit levels rose “without any suggestion of a relationship between diuretic dose and degree of hematocrit elevation with dapagliflozin,” she said.
The elevations persisted even with diuretic dose reductions at 6 and 12 months, “which should have led to a decrease in hemoconcentration if it was caused by volume contraction.”
Also, she said, “among patients not taking a diuretic, volume depletion occurred less frequently in the dapagliflozin group than in the placebo group, but there was still a similar rise in hematocrit with dapagliflozin.”
Both Dr. Jackson and Dr. Coats said the sustained elevation in hematocrit on the drug is unlikely to pose a major hazard.
Dr. Coats said that, theoretically, “increased hematocrit could reduce peripheral vessel blood flow, making ischemia and thrombosis more likely. But the size of the effect is small and unlikely to be clinically important.”
A diuretic dose could not be determined for 128 of the trial’s 4,744 randomized patients with HFrEF, so the post hoc analysis was limited to the remaining 4,616. Of those, 746 were not on diuretics at baseline, 1,311 were on loop diuretics at less than 40 mg FE or on non-loop diuretics only, 1,365 were taking 40 mg FE, and 1,204 were on higher doses of loop diuretics.
The mean baseline dosage was 60 mg FE, which rose slightly throughout the trial. But the baseline dosage and the increases were both similar in the placebo and dapagliflozin groups. Dr. Jackson said 84% and 83% of patients on dapagliflozin and placebo, respectively, maintained their baseline dose at 6 months and about 77% in both groups at 12 months.
The overall trial’s significant primary endpoint reduction for dapagliflozin versus placebo applied similarly to patients not on a diuretics and to those on any dose of diuretic, with an interaction P value of .23 for the effect of diuretic use. The hazard ratios (95% confidence interval) were 0.57 (0.36-0.92) for patients not on diuretics, 0.78 (0.68-0.90) for patients on any diuretic dosage, and 0.74 (0.65-0.85) overall
Dr. Jackson said during her formal online presentation that patients on diuretics showed a “tendency toward slightly more volume depletion in those on dapagliflozin than in those on placebo, but the excess was small and not greater than approximately 3% in those taking 40 mg furosemide equivalent diuretic. And fortunately, this did not result in an increase in frequency in renal adverse events nor of discontinuation of study drug.”
Renal adverse events were similarly prevalent in the two treatment groups, as were such events leading to treatment discontinuation. But serious renal events were less common in the dapagliflozin group (1.6% vs 2.7%; P = .009), as was investigator-reported serious acute kidney injury (1.0% vs 1.9%; P = .007).
“Overall, renal events were infrequent,” Dr. Jackson said, and “because of the small number of events, it is very difficult to draw conclusions about the impact of dapagliflozin on renal function according to diuretic-dose subgroups.”
Still, she said, worsening renal function was less common on dapagliflozin in three of the four groups by diuretic dosage; the exception was the less than 40 mg FE group, “but the absolute difference in this group was only two events.”
There seem to be dapagliflozin mechanisms “underneath the surface that need to be unraveled,” Dr. Doehner said as discussant, processes that are favorable for the treatment of HFrEF in which “diuretics play no big role.”
Dr. Jackson has no disclosures. Dr. Coats has disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor. Dr. Doener has recently disclosed receiving grants and personal fees from Vifor, Pfizer, Boehringer Ingelheim, Sphingotec, ZS Pharma, Bayer, and Medtronic.
A version of this article originally appeared on Medscape.com.
The DAPA-HF trial has already changed cardiology in opening up a new class of drugs to patients with heart failure (HF), whether or not they have diabetes. Now the trial is yielding clues as to how it benefits them. For now, it’s doing so by process of elimination.
A new analysis suggests that dapagliflozin (Farxiga, AstraZeneca) didn’t need help from loop diuretics to cut the risk for clinical events in patients with HF with reduced ejection fraction (HFrEF), a benefit seen across the spectrum of glycosylated hemoglobin levels and without compromising renal function, said DAPA-HF investigators. Also, use of dapagliflozin and its clinical effects were not associated with changes in loop diuretic dosage. Those findings and others suggest the drug helps in HFrEF at least partly by some other mechanism than its own diuretic effect, the researchers say.
Such insights will likely be important to case-by-case decisions on whether to use the drug, a sodium-glucose cotransporter 2 (SGLT2) inhibitor once reserved for patients with diabetes, given the recently broader landscape of HF treatment options.
As previously reported from DAPA-HF, with more than 4,700 patients, those who received dapagliflozin showed significant reductions in the primary end point, a composite of cardiovascular (CV) death, HF hospitalization, and urgent HF visit requiring IV therapy over about 18 months. The 45% of patients with and 55% without type 2 diabetes enjoyed about equal benefit in the placebo-controlled trial for that end point, as well as for all-cause mortality.
SGLT2 inhibitors work in diabetes by promoting urinary glucose excretion. That had led some to speculate that its benefit in HFrEF comes primarily from a diuretic effect; the current findings largely put that question to rest.
“Our findings show that treatment with dapagliflozin was effective regardless of diuretic use or diuretic dose. They also show that dapagliflozin did not lead to an increase in renal adverse events or discontinuation of therapy in patients treated with a diuretic,” trialist Alice M. Jackson, MB, ChB, said in an interview.
“In fact, renal adverse events were generally less common in patients treated with dapagliflozin, across the diuretic categories,” said Dr. Jackson, from the University of Glasgow.
Dr. Jackson presented the new analysis at a Late-Breaking Science Session during the European Society of Cardiology Heart Failure Discoveries virtual meeting. The HFA sessions were conducted virtually this year due to the COVID-19 pandemic.
At baseline, 84% of patients were on conventional diuretics. The post hoc analysis broke out all patients by loop-diuretic dosage level: none; less than 40 mg furosemide equivalents (FE); 40 mg FE; or more than 40 mg FE. Clinical outcomes were similar across the four groups.
Clinicians in the trial “were not given specific advice about adjusting diuretic doses, but were encouraged to assess volume status and make changes to medical therapy based on this, if necessary,” Dr. Jackson said. “This suggests that, for most patients, starting dapagliflozin will not necessitate a change in diuretic dose.”
With the caveat that the event rate was low in the relatively few patients not prescribed loop diuretics, she said, “the magnitude of the benefit from dapagliflozin appeared to be larger in patients not treated with a diuretic.”
There was no suggestion of a diuretic dose–response effect or statistical interaction between diuretic use and clinical outcomes on dapagliflozin, Dr. Jackson observed in the interview.
Of note in the analysis, hematocrit levels shot up soon after patients started active therapy, but they didn’t rise much in the placebo group. The sustained hematocrit elevation on dapagliflozin, seen at all diuretic dosage levels, persisted even after dosage reductions at 6 months, she said.
“Dapagliflozin is effective in HFrEF irrespective of background diuretic therapy; therefore, it is almost certainly not purely acting as a diuretic,” Andrew J. Coats, MD, DSc, MBA, said in an interview.
The findings also “lessen the concern that dapagliflozin’s beneficial effects are only seen only in patients without effective diuretic dosing,” said Dr. Coats, from University of Warwick, Coventry, England.
“Altogether, these data give further reassurance that dapagliflozin can safely be used in heart failure, and has a beneficial effect independent of the use of diuretic drugs,” invited discussant Wolfram Doehner, MD, PhD, Charité-Universitätsmedizin Berlin, said after Dr. Jackson’s presentation of the analysis.
He made special mention of the sustained hematocrit elevation on dapagliflozin. “While this effect may likely relate to the mild reduction in plasma volume secondary to dapagliflozin therapy, it is noted that the increase in hematocrit was independent of any change of the diuretic dose,” Doehner said. “If additional mechanisms have a role for this observed increase in hematocrit, it may be of interest in further investigations.”
Dr. Jackson pointed to several observations that suggest the hematocrit finding isn’t explained by hemoconcentration from reduced plasma volume, at least not entirely.
For example, hematocrit levels rose “without any suggestion of a relationship between diuretic dose and degree of hematocrit elevation with dapagliflozin,” she said.
The elevations persisted even with diuretic dose reductions at 6 and 12 months, “which should have led to a decrease in hemoconcentration if it was caused by volume contraction.”
Also, she said, “among patients not taking a diuretic, volume depletion occurred less frequently in the dapagliflozin group than in the placebo group, but there was still a similar rise in hematocrit with dapagliflozin.”
Both Dr. Jackson and Dr. Coats said the sustained elevation in hematocrit on the drug is unlikely to pose a major hazard.
Dr. Coats said that, theoretically, “increased hematocrit could reduce peripheral vessel blood flow, making ischemia and thrombosis more likely. But the size of the effect is small and unlikely to be clinically important.”
A diuretic dose could not be determined for 128 of the trial’s 4,744 randomized patients with HFrEF, so the post hoc analysis was limited to the remaining 4,616. Of those, 746 were not on diuretics at baseline, 1,311 were on loop diuretics at less than 40 mg FE or on non-loop diuretics only, 1,365 were taking 40 mg FE, and 1,204 were on higher doses of loop diuretics.
The mean baseline dosage was 60 mg FE, which rose slightly throughout the trial. But the baseline dosage and the increases were both similar in the placebo and dapagliflozin groups. Dr. Jackson said 84% and 83% of patients on dapagliflozin and placebo, respectively, maintained their baseline dose at 6 months and about 77% in both groups at 12 months.
The overall trial’s significant primary endpoint reduction for dapagliflozin versus placebo applied similarly to patients not on a diuretics and to those on any dose of diuretic, with an interaction P value of .23 for the effect of diuretic use. The hazard ratios (95% confidence interval) were 0.57 (0.36-0.92) for patients not on diuretics, 0.78 (0.68-0.90) for patients on any diuretic dosage, and 0.74 (0.65-0.85) overall
Dr. Jackson said during her formal online presentation that patients on diuretics showed a “tendency toward slightly more volume depletion in those on dapagliflozin than in those on placebo, but the excess was small and not greater than approximately 3% in those taking 40 mg furosemide equivalent diuretic. And fortunately, this did not result in an increase in frequency in renal adverse events nor of discontinuation of study drug.”
Renal adverse events were similarly prevalent in the two treatment groups, as were such events leading to treatment discontinuation. But serious renal events were less common in the dapagliflozin group (1.6% vs 2.7%; P = .009), as was investigator-reported serious acute kidney injury (1.0% vs 1.9%; P = .007).
“Overall, renal events were infrequent,” Dr. Jackson said, and “because of the small number of events, it is very difficult to draw conclusions about the impact of dapagliflozin on renal function according to diuretic-dose subgroups.”
Still, she said, worsening renal function was less common on dapagliflozin in three of the four groups by diuretic dosage; the exception was the less than 40 mg FE group, “but the absolute difference in this group was only two events.”
There seem to be dapagliflozin mechanisms “underneath the surface that need to be unraveled,” Dr. Doehner said as discussant, processes that are favorable for the treatment of HFrEF in which “diuretics play no big role.”
Dr. Jackson has no disclosures. Dr. Coats has disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor. Dr. Doener has recently disclosed receiving grants and personal fees from Vifor, Pfizer, Boehringer Ingelheim, Sphingotec, ZS Pharma, Bayer, and Medtronic.
A version of this article originally appeared on Medscape.com.
FROM ESC HEART FAILURE 2020
VICTORIA results deepen mystery of vericiguat in low-EF heart failure
Although clinical outcomes improved for patients with high-risk heart failure (HF) who received vericiguat (Merck/Bayer) on top of standard therapy in a major randomized trial, a subgroup study failed to show any corresponding gains in ventricular function.
The discordant results from the 5,050-patient VICTORIA trial and its echocardiographic substudy highlight something of a mystery as to the mechanism of the investigational oral soluble guanylate cyclase stimulator’s clinical effects. In the overall trial, they included a drop in risk of cardiovascular (CV) death or first HF hospitalization, the primary endpoint.
In the echo substudy, which assessed patients with evaluable echocardiograms at both baseline and 8 months, vericiguat, compared with placebo, had no significant effect on two measures of left ventricular (LV) function. Patients in the prospectively conducted substudy made up less than 10% of the total trial population.
Both LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI) significantly improved in the vericiguat and control groups, but vericiguat “had no additional significant effect,” said Burkert Pieske, MD, of Charité University Medicine Berlin.
Still, he said, there was “evidence of a lower risk of events, evidence of a clinical benefit,” for those who received vericiguat, although it fell slightly short of significance in the substudy cohort of fewer than 500 patients.
Dr. Pieske reported the VICTORIA echo substudy results June 5 in a Late-Breaking Science Session during HFA Discoveries, the online backup for the Heart Failure Association of the European Society of Cardiology annual scientific meeting.
The traditional live HFA meeting had been scheduled for Barcelona but was canceled this year as a result of the COVID-19 pandemic.
Pointing to the significant echo improvements in both treatment groups, invited discussant Rudolf A. de Boer, MD, PhD, University of Groningen (the Netherlands), said the substudy shows that HF in high-risk patients “is associated with a transient deterioration of LV function and geometry, which can to a certain extent be reversed over time.”
That the effect apparently wasn’t influenced by vericiguat “may be explained by the fact that, in randomized controlled trials, patients – including those on placebo – tend to be treated very well.” In clinical practice, he said, “less complete reverse remodeling may be expected.”
Dr. de Boer also pointed to likely survivor bias in the study, in that only patients who survived to at least 8 months were included. That meant, among other things, that they were likely at lower overall risk than the total VICTORIA population, leaving less room for any treatment effect.
“Further, likely because of the play of chance in this substudy, the LV volumes were smaller in the vericiguat group at baseline, creating less of an opportunity for vericiguat to make a difference,” he said. “It could be speculated that, with larger volumes, the window of opportunity for vericiguat would have been wider.”
But “most strikingly,” the lack of vericiguat effect on echo parameters contrasts with the clinical benefits associated with the drug in the main trial, and possibly in the echo substudy, Dr. de Boer said, “creating a dissociation between the surrogate echo parameters and the clinical hard endpoints. And it could be imagined that the rather crude echo measures presented here, LVEF and LV volume, miss a more subtle effect of vericiguat.”
For example, it’s possible that the drug’s clinical effect in heart failure does not depend on any improvements in ventricular function, Dr. de Boer said, adding that vericiguat “may potentially also have important effects on pulmonary and peripheral vasculature,” so he recommended future studies look for any changes in arterial and right ventricular function from the drug.
VICTORIA enrolled only patients with HF and reduced ejection fraction who had previously experienced a decompensation event, usually only within the last 3 months, as it turned out. Those assigned to vericiguat on top of standard drug and device therapies showed a modest 10% decline in adjusted relative risk (P = .019) for the trial’s primary endpoint, CV death or first HF hospitalization.
But when the results were unveiled at a meeting, trialists and observers were more enthused about the drug’s effect in absolute terms, which by one measure was 4.2 fewer events on vericiguat per 100 patient-years. That translated to a number to treat of 24 to prevent one event, said to be impressive, given that the study’s patients were so high risk.
The echo substudy included 419 prospectively selected patients, 208 on vericiguat and 211 assigned to placebo, who had evaluable echocardiograms at both baseline and 8 months, as assessed at the VICTORIA echo core lab. They averaged 64.5 years in age with a mean baseline LVEF of 29%; about 27% were women.
Their clinical outcomes paralleled the overall study, with lower event rates overall and a difference between treatment groups that fell short of significance.
Neither of the study’s primary endpoints, the two echo parameters, responded differently to vericiguat, compared with placebo.
The overall VICTORIA trial “showed a modest but useful benefit in the combined endpoint of hospitalizations and mortality, but all due to fewer hospitalizations,” Andrew J. Coats, MD, DSc, MBA, told this news organization.
“The echo substudy was smaller, and many drugs that reduce hospitalization do not do it through effects on LV function,” said Dr. Coats of the University of Warwick, Coventry, England, who wasn’t a part of VICTORIA. “Other mechanisms may be via improved peripheral vascular or renal effects.”
VICTORIA and the echocardiographic substudy were supported by Merck Sharp & Dohme and Bayer AG. Dr. Pieske disclosed serving on a speakers bureau, advisory board, or committee for Bayer Healthcare, Merck, Novartis, AstraZeneca, Stealth, Servier, Daiichi-Sankyo, Biotronic, Abbott Vascular, and Bristol-Myers Squibb. Dr. de Boer disclosed receiving speaker fees from Abbott, AstraZeneca, Novartis, and Roche. Dr. Coats disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor.
A version of this article originally appeared on Medscape.com.
Although clinical outcomes improved for patients with high-risk heart failure (HF) who received vericiguat (Merck/Bayer) on top of standard therapy in a major randomized trial, a subgroup study failed to show any corresponding gains in ventricular function.
The discordant results from the 5,050-patient VICTORIA trial and its echocardiographic substudy highlight something of a mystery as to the mechanism of the investigational oral soluble guanylate cyclase stimulator’s clinical effects. In the overall trial, they included a drop in risk of cardiovascular (CV) death or first HF hospitalization, the primary endpoint.
In the echo substudy, which assessed patients with evaluable echocardiograms at both baseline and 8 months, vericiguat, compared with placebo, had no significant effect on two measures of left ventricular (LV) function. Patients in the prospectively conducted substudy made up less than 10% of the total trial population.
Both LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI) significantly improved in the vericiguat and control groups, but vericiguat “had no additional significant effect,” said Burkert Pieske, MD, of Charité University Medicine Berlin.
Still, he said, there was “evidence of a lower risk of events, evidence of a clinical benefit,” for those who received vericiguat, although it fell slightly short of significance in the substudy cohort of fewer than 500 patients.
Dr. Pieske reported the VICTORIA echo substudy results June 5 in a Late-Breaking Science Session during HFA Discoveries, the online backup for the Heart Failure Association of the European Society of Cardiology annual scientific meeting.
The traditional live HFA meeting had been scheduled for Barcelona but was canceled this year as a result of the COVID-19 pandemic.
Pointing to the significant echo improvements in both treatment groups, invited discussant Rudolf A. de Boer, MD, PhD, University of Groningen (the Netherlands), said the substudy shows that HF in high-risk patients “is associated with a transient deterioration of LV function and geometry, which can to a certain extent be reversed over time.”
That the effect apparently wasn’t influenced by vericiguat “may be explained by the fact that, in randomized controlled trials, patients – including those on placebo – tend to be treated very well.” In clinical practice, he said, “less complete reverse remodeling may be expected.”
Dr. de Boer also pointed to likely survivor bias in the study, in that only patients who survived to at least 8 months were included. That meant, among other things, that they were likely at lower overall risk than the total VICTORIA population, leaving less room for any treatment effect.
“Further, likely because of the play of chance in this substudy, the LV volumes were smaller in the vericiguat group at baseline, creating less of an opportunity for vericiguat to make a difference,” he said. “It could be speculated that, with larger volumes, the window of opportunity for vericiguat would have been wider.”
But “most strikingly,” the lack of vericiguat effect on echo parameters contrasts with the clinical benefits associated with the drug in the main trial, and possibly in the echo substudy, Dr. de Boer said, “creating a dissociation between the surrogate echo parameters and the clinical hard endpoints. And it could be imagined that the rather crude echo measures presented here, LVEF and LV volume, miss a more subtle effect of vericiguat.”
For example, it’s possible that the drug’s clinical effect in heart failure does not depend on any improvements in ventricular function, Dr. de Boer said, adding that vericiguat “may potentially also have important effects on pulmonary and peripheral vasculature,” so he recommended future studies look for any changes in arterial and right ventricular function from the drug.
VICTORIA enrolled only patients with HF and reduced ejection fraction who had previously experienced a decompensation event, usually only within the last 3 months, as it turned out. Those assigned to vericiguat on top of standard drug and device therapies showed a modest 10% decline in adjusted relative risk (P = .019) for the trial’s primary endpoint, CV death or first HF hospitalization.
But when the results were unveiled at a meeting, trialists and observers were more enthused about the drug’s effect in absolute terms, which by one measure was 4.2 fewer events on vericiguat per 100 patient-years. That translated to a number to treat of 24 to prevent one event, said to be impressive, given that the study’s patients were so high risk.
The echo substudy included 419 prospectively selected patients, 208 on vericiguat and 211 assigned to placebo, who had evaluable echocardiograms at both baseline and 8 months, as assessed at the VICTORIA echo core lab. They averaged 64.5 years in age with a mean baseline LVEF of 29%; about 27% were women.
Their clinical outcomes paralleled the overall study, with lower event rates overall and a difference between treatment groups that fell short of significance.
Neither of the study’s primary endpoints, the two echo parameters, responded differently to vericiguat, compared with placebo.
The overall VICTORIA trial “showed a modest but useful benefit in the combined endpoint of hospitalizations and mortality, but all due to fewer hospitalizations,” Andrew J. Coats, MD, DSc, MBA, told this news organization.
“The echo substudy was smaller, and many drugs that reduce hospitalization do not do it through effects on LV function,” said Dr. Coats of the University of Warwick, Coventry, England, who wasn’t a part of VICTORIA. “Other mechanisms may be via improved peripheral vascular or renal effects.”
VICTORIA and the echocardiographic substudy were supported by Merck Sharp & Dohme and Bayer AG. Dr. Pieske disclosed serving on a speakers bureau, advisory board, or committee for Bayer Healthcare, Merck, Novartis, AstraZeneca, Stealth, Servier, Daiichi-Sankyo, Biotronic, Abbott Vascular, and Bristol-Myers Squibb. Dr. de Boer disclosed receiving speaker fees from Abbott, AstraZeneca, Novartis, and Roche. Dr. Coats disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor.
A version of this article originally appeared on Medscape.com.
Although clinical outcomes improved for patients with high-risk heart failure (HF) who received vericiguat (Merck/Bayer) on top of standard therapy in a major randomized trial, a subgroup study failed to show any corresponding gains in ventricular function.
The discordant results from the 5,050-patient VICTORIA trial and its echocardiographic substudy highlight something of a mystery as to the mechanism of the investigational oral soluble guanylate cyclase stimulator’s clinical effects. In the overall trial, they included a drop in risk of cardiovascular (CV) death or first HF hospitalization, the primary endpoint.
In the echo substudy, which assessed patients with evaluable echocardiograms at both baseline and 8 months, vericiguat, compared with placebo, had no significant effect on two measures of left ventricular (LV) function. Patients in the prospectively conducted substudy made up less than 10% of the total trial population.
Both LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI) significantly improved in the vericiguat and control groups, but vericiguat “had no additional significant effect,” said Burkert Pieske, MD, of Charité University Medicine Berlin.
Still, he said, there was “evidence of a lower risk of events, evidence of a clinical benefit,” for those who received vericiguat, although it fell slightly short of significance in the substudy cohort of fewer than 500 patients.
Dr. Pieske reported the VICTORIA echo substudy results June 5 in a Late-Breaking Science Session during HFA Discoveries, the online backup for the Heart Failure Association of the European Society of Cardiology annual scientific meeting.
The traditional live HFA meeting had been scheduled for Barcelona but was canceled this year as a result of the COVID-19 pandemic.
Pointing to the significant echo improvements in both treatment groups, invited discussant Rudolf A. de Boer, MD, PhD, University of Groningen (the Netherlands), said the substudy shows that HF in high-risk patients “is associated with a transient deterioration of LV function and geometry, which can to a certain extent be reversed over time.”
That the effect apparently wasn’t influenced by vericiguat “may be explained by the fact that, in randomized controlled trials, patients – including those on placebo – tend to be treated very well.” In clinical practice, he said, “less complete reverse remodeling may be expected.”
Dr. de Boer also pointed to likely survivor bias in the study, in that only patients who survived to at least 8 months were included. That meant, among other things, that they were likely at lower overall risk than the total VICTORIA population, leaving less room for any treatment effect.
“Further, likely because of the play of chance in this substudy, the LV volumes were smaller in the vericiguat group at baseline, creating less of an opportunity for vericiguat to make a difference,” he said. “It could be speculated that, with larger volumes, the window of opportunity for vericiguat would have been wider.”
But “most strikingly,” the lack of vericiguat effect on echo parameters contrasts with the clinical benefits associated with the drug in the main trial, and possibly in the echo substudy, Dr. de Boer said, “creating a dissociation between the surrogate echo parameters and the clinical hard endpoints. And it could be imagined that the rather crude echo measures presented here, LVEF and LV volume, miss a more subtle effect of vericiguat.”
For example, it’s possible that the drug’s clinical effect in heart failure does not depend on any improvements in ventricular function, Dr. de Boer said, adding that vericiguat “may potentially also have important effects on pulmonary and peripheral vasculature,” so he recommended future studies look for any changes in arterial and right ventricular function from the drug.
VICTORIA enrolled only patients with HF and reduced ejection fraction who had previously experienced a decompensation event, usually only within the last 3 months, as it turned out. Those assigned to vericiguat on top of standard drug and device therapies showed a modest 10% decline in adjusted relative risk (P = .019) for the trial’s primary endpoint, CV death or first HF hospitalization.
But when the results were unveiled at a meeting, trialists and observers were more enthused about the drug’s effect in absolute terms, which by one measure was 4.2 fewer events on vericiguat per 100 patient-years. That translated to a number to treat of 24 to prevent one event, said to be impressive, given that the study’s patients were so high risk.
The echo substudy included 419 prospectively selected patients, 208 on vericiguat and 211 assigned to placebo, who had evaluable echocardiograms at both baseline and 8 months, as assessed at the VICTORIA echo core lab. They averaged 64.5 years in age with a mean baseline LVEF of 29%; about 27% were women.
Their clinical outcomes paralleled the overall study, with lower event rates overall and a difference between treatment groups that fell short of significance.
Neither of the study’s primary endpoints, the two echo parameters, responded differently to vericiguat, compared with placebo.
The overall VICTORIA trial “showed a modest but useful benefit in the combined endpoint of hospitalizations and mortality, but all due to fewer hospitalizations,” Andrew J. Coats, MD, DSc, MBA, told this news organization.
“The echo substudy was smaller, and many drugs that reduce hospitalization do not do it through effects on LV function,” said Dr. Coats of the University of Warwick, Coventry, England, who wasn’t a part of VICTORIA. “Other mechanisms may be via improved peripheral vascular or renal effects.”
VICTORIA and the echocardiographic substudy were supported by Merck Sharp & Dohme and Bayer AG. Dr. Pieske disclosed serving on a speakers bureau, advisory board, or committee for Bayer Healthcare, Merck, Novartis, AstraZeneca, Stealth, Servier, Daiichi-Sankyo, Biotronic, Abbott Vascular, and Bristol-Myers Squibb. Dr. de Boer disclosed receiving speaker fees from Abbott, AstraZeneca, Novartis, and Roche. Dr. Coats disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor.
A version of this article originally appeared on Medscape.com.
FROM ESC HEART FAILURE 2020
Novel agent for obstructive HCM nets functional gains; top-line results
Patients with obstructive hypertrophic cardiomyopathy (HCM) who took an investigational agent that targets cardiac myosin over about 7 months showed across-the-board improvements in functional capacity, symptoms, and left ventricular outflow obstruction in a randomized, controlled trial.
Treatment with the oral drug mavacamten (or MYK-461) was well tolerated and showed no untoward safety issues, compared with placebo in the phase 3 EXPLORER-HCM trial, its developer, MyoKardia, announced in a press release. The top-line trial results were made public in advance of a more expansive presentation at a later date.
The company describes mavacamten as an allosteric modulator of cardiac myosin that “reduces cardiac muscle contractility by inhibiting excessive myosin-actin cross-bridge formation that results in hypercontractility, left ventricular hypertrophy and reduced compliance.”
In the EXPLORER-HCM trial, with its 251 patients with symptomatic obstructive HCM, 37% of those randomly assigned to receive once-daily mavacamten and 17% of those given placebo (P =.0005) reached the functional primary endpoint by 30 weeks, the company reported.
The primary endpoint was a composite of either a ≥1.5 mL/kg per min improvement in peak VO2 along with symptomatic improvement or ≥3.0 mL/kg per min improvement without deterioration of symptom status.
Patients taking mavacamten also showed significant improvement in the secondary endpoints of left ventricular outflow tract peak gradient after exercise, NYHA functional class, Kansas City Cardiomyopathy Questionnaire Clinical Summary scores, and HCM Symptom Questionnaire Shortness of Breath Domain score, all at P = .0001, and peak VO2 at P = .0006, MyoKardia reported.
The company said beyond its bid to have the drug approved for obstructive HCM, based on its mechanism of action it foresees the drug as a potential treatment for nonobstructive HCM and for some patients with heart failure with preserved ejection fraction.
This article first appeared on Medscape.com.
Patients with obstructive hypertrophic cardiomyopathy (HCM) who took an investigational agent that targets cardiac myosin over about 7 months showed across-the-board improvements in functional capacity, symptoms, and left ventricular outflow obstruction in a randomized, controlled trial.
Treatment with the oral drug mavacamten (or MYK-461) was well tolerated and showed no untoward safety issues, compared with placebo in the phase 3 EXPLORER-HCM trial, its developer, MyoKardia, announced in a press release. The top-line trial results were made public in advance of a more expansive presentation at a later date.
The company describes mavacamten as an allosteric modulator of cardiac myosin that “reduces cardiac muscle contractility by inhibiting excessive myosin-actin cross-bridge formation that results in hypercontractility, left ventricular hypertrophy and reduced compliance.”
In the EXPLORER-HCM trial, with its 251 patients with symptomatic obstructive HCM, 37% of those randomly assigned to receive once-daily mavacamten and 17% of those given placebo (P =.0005) reached the functional primary endpoint by 30 weeks, the company reported.
The primary endpoint was a composite of either a ≥1.5 mL/kg per min improvement in peak VO2 along with symptomatic improvement or ≥3.0 mL/kg per min improvement without deterioration of symptom status.
Patients taking mavacamten also showed significant improvement in the secondary endpoints of left ventricular outflow tract peak gradient after exercise, NYHA functional class, Kansas City Cardiomyopathy Questionnaire Clinical Summary scores, and HCM Symptom Questionnaire Shortness of Breath Domain score, all at P = .0001, and peak VO2 at P = .0006, MyoKardia reported.
The company said beyond its bid to have the drug approved for obstructive HCM, based on its mechanism of action it foresees the drug as a potential treatment for nonobstructive HCM and for some patients with heart failure with preserved ejection fraction.
This article first appeared on Medscape.com.
Patients with obstructive hypertrophic cardiomyopathy (HCM) who took an investigational agent that targets cardiac myosin over about 7 months showed across-the-board improvements in functional capacity, symptoms, and left ventricular outflow obstruction in a randomized, controlled trial.
Treatment with the oral drug mavacamten (or MYK-461) was well tolerated and showed no untoward safety issues, compared with placebo in the phase 3 EXPLORER-HCM trial, its developer, MyoKardia, announced in a press release. The top-line trial results were made public in advance of a more expansive presentation at a later date.
The company describes mavacamten as an allosteric modulator of cardiac myosin that “reduces cardiac muscle contractility by inhibiting excessive myosin-actin cross-bridge formation that results in hypercontractility, left ventricular hypertrophy and reduced compliance.”
In the EXPLORER-HCM trial, with its 251 patients with symptomatic obstructive HCM, 37% of those randomly assigned to receive once-daily mavacamten and 17% of those given placebo (P =.0005) reached the functional primary endpoint by 30 weeks, the company reported.
The primary endpoint was a composite of either a ≥1.5 mL/kg per min improvement in peak VO2 along with symptomatic improvement or ≥3.0 mL/kg per min improvement without deterioration of symptom status.
Patients taking mavacamten also showed significant improvement in the secondary endpoints of left ventricular outflow tract peak gradient after exercise, NYHA functional class, Kansas City Cardiomyopathy Questionnaire Clinical Summary scores, and HCM Symptom Questionnaire Shortness of Breath Domain score, all at P = .0001, and peak VO2 at P = .0006, MyoKardia reported.
The company said beyond its bid to have the drug approved for obstructive HCM, based on its mechanism of action it foresees the drug as a potential treatment for nonobstructive HCM and for some patients with heart failure with preserved ejection fraction.
This article first appeared on Medscape.com.